User login
Given clinical and imaging outcomes, as well as results on IHC assay, this patient is diagnosed with triple-negative breast cancer and is referred for further consultation with a multidisciplinary care team.
Triple-negative breast cancer accounts for 15% of all female breast cancer cases. The term "triple-negative" refers to the absence of estrogen receptor (ER) and progesterone receptor (PR) targets and the HER2 target for treatment. Otherwise, triple-negative breast cancer is highly heterogeneous and includes multiple molecular subtypes. Typically, triple-negative breast cancer occurs in younger patients, has a higher grade, a greater tumor size, a higher clinical stage at diagnosis, and a poorer prognosis than non–triple-negative breast cancers.
Biopsy samples from patients with a new primary or newly metastatic breast cancer diagnosis should undergo HR testing, both ER and PR, as well as HER2 receptor testing. Results of ER and PR testing are negative if a validated IHC assay shows 0% to < 1% of nuclei stain, according to the latest American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) HR testing guideline. HER2 results are negative if a validated IHC assay shows weak to moderate complete membrane staining in < 10% of tumor cells, according to ASCO/CAP guidelines. If HER2 results of a primary tumor are negative and specific clinical criteria suggest further testing, the oncologist may choose to order another IHC assay or a validated dual probe in situ hybridization (ISH) assay to aid in clinical decision-making.
Pathologic classification of triple-negative breast cancers includes multiple subgroups determined by therapeutic possibilities: low-risk histologic types (salivary gland–like carcinomas, fibromatosis-like carcinoma, low-grade adenosquamous breast carcinoma), immune activation (tumor-infiltrating lymphocytes, CD8, programmed death–ligand 1, tumor mutational burden), HER2-low status (IHC score 1+/2+ nonamplified), associated germline BRCA mutations, and other targets (trophoblast cell-surface antigen 2, HER3).
Family history is the most common risk factor for developing breast cancer. If a person's mother or sister had breast cancer, the lifetime risk for that individual is four times greater than that of the general population. Risk for breast cancer grows even more if two or more first-degree relatives are diagnosed, or if one first-degree relative was diagnosed with breast cancer at ≤ 50 years of age. About 25% of triple-negative breast cancer cases have accompanying germline BRCA mutations. A recent study by Ahearn and colleagues found that 85 variants were linked to one or more tumor feature of ER-negative disease, and 32 of those variants were associated with triple-negative disease.
For a breast cancer diagnosis, the National Comprehensive Cancer Network recommends multidisciplinary care as well as the development of a personalized survivorship treatment plan, which includes a customized summary of possible long-term treatment toxicities. In addition, multidisciplinary care coordination encourages close follow-up that helps patients adhere to their medications and stay current with ongoing screening.
Because triple-negative breast cancer lacks HR and HER2 therapeutic targets, it can be difficult to treat. Standard therapy for high-risk triple-negative tumors includes chemotherapy with taxanes (paclitaxel or docetaxel) plus anthracycline-based treatment (cyclophosphamide plus doxorubicin). Neoadjuvant chemotherapy is recommended for patients with stage 2 and 3 tumors to reduce locoregional surgical extension and to allow for personalized treatment based on pathologic response. In 2018, two poly ADP ribose polymerase inhibitors, olaparib and talazoparib, were approved for use in select patients with triple-negative breast cancer, significantly changing the treatment paradigm for this disease. Other promising treatments have emerged recently in clinical trials for triple-negative disease, including immune checkpoint inhibitors, PI3K/AKT pathway inhibitors, and cytotoxin-conjugated antibodies.
Daniel S. Schwartz, MD, is Medical Director of Thoracic Oncology, St. Catherine of Siena Medical Center, Catholic Health Services, Smithtown, New York.
Dr. Schwartz serve(d) as a member of the following medical societies:
American College of Chest Physicians, American College of Surgeons, Society of Thoracic Surgeons, Western Thoracic Surgical Association.
Disclosure: Daniel S. Schwartz, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given clinical and imaging outcomes, as well as results on IHC assay, this patient is diagnosed with triple-negative breast cancer and is referred for further consultation with a multidisciplinary care team.
Triple-negative breast cancer accounts for 15% of all female breast cancer cases. The term "triple-negative" refers to the absence of estrogen receptor (ER) and progesterone receptor (PR) targets and the HER2 target for treatment. Otherwise, triple-negative breast cancer is highly heterogeneous and includes multiple molecular subtypes. Typically, triple-negative breast cancer occurs in younger patients, has a higher grade, a greater tumor size, a higher clinical stage at diagnosis, and a poorer prognosis than non–triple-negative breast cancers.
Biopsy samples from patients with a new primary or newly metastatic breast cancer diagnosis should undergo HR testing, both ER and PR, as well as HER2 receptor testing. Results of ER and PR testing are negative if a validated IHC assay shows 0% to < 1% of nuclei stain, according to the latest American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) HR testing guideline. HER2 results are negative if a validated IHC assay shows weak to moderate complete membrane staining in < 10% of tumor cells, according to ASCO/CAP guidelines. If HER2 results of a primary tumor are negative and specific clinical criteria suggest further testing, the oncologist may choose to order another IHC assay or a validated dual probe in situ hybridization (ISH) assay to aid in clinical decision-making.
Pathologic classification of triple-negative breast cancers includes multiple subgroups determined by therapeutic possibilities: low-risk histologic types (salivary gland–like carcinomas, fibromatosis-like carcinoma, low-grade adenosquamous breast carcinoma), immune activation (tumor-infiltrating lymphocytes, CD8, programmed death–ligand 1, tumor mutational burden), HER2-low status (IHC score 1+/2+ nonamplified), associated germline BRCA mutations, and other targets (trophoblast cell-surface antigen 2, HER3).
Family history is the most common risk factor for developing breast cancer. If a person's mother or sister had breast cancer, the lifetime risk for that individual is four times greater than that of the general population. Risk for breast cancer grows even more if two or more first-degree relatives are diagnosed, or if one first-degree relative was diagnosed with breast cancer at ≤ 50 years of age. About 25% of triple-negative breast cancer cases have accompanying germline BRCA mutations. A recent study by Ahearn and colleagues found that 85 variants were linked to one or more tumor feature of ER-negative disease, and 32 of those variants were associated with triple-negative disease.
For a breast cancer diagnosis, the National Comprehensive Cancer Network recommends multidisciplinary care as well as the development of a personalized survivorship treatment plan, which includes a customized summary of possible long-term treatment toxicities. In addition, multidisciplinary care coordination encourages close follow-up that helps patients adhere to their medications and stay current with ongoing screening.
Because triple-negative breast cancer lacks HR and HER2 therapeutic targets, it can be difficult to treat. Standard therapy for high-risk triple-negative tumors includes chemotherapy with taxanes (paclitaxel or docetaxel) plus anthracycline-based treatment (cyclophosphamide plus doxorubicin). Neoadjuvant chemotherapy is recommended for patients with stage 2 and 3 tumors to reduce locoregional surgical extension and to allow for personalized treatment based on pathologic response. In 2018, two poly ADP ribose polymerase inhibitors, olaparib and talazoparib, were approved for use in select patients with triple-negative breast cancer, significantly changing the treatment paradigm for this disease. Other promising treatments have emerged recently in clinical trials for triple-negative disease, including immune checkpoint inhibitors, PI3K/AKT pathway inhibitors, and cytotoxin-conjugated antibodies.
Daniel S. Schwartz, MD, is Medical Director of Thoracic Oncology, St. Catherine of Siena Medical Center, Catholic Health Services, Smithtown, New York.
Dr. Schwartz serve(d) as a member of the following medical societies:
American College of Chest Physicians, American College of Surgeons, Society of Thoracic Surgeons, Western Thoracic Surgical Association.
Disclosure: Daniel S. Schwartz, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given clinical and imaging outcomes, as well as results on IHC assay, this patient is diagnosed with triple-negative breast cancer and is referred for further consultation with a multidisciplinary care team.
Triple-negative breast cancer accounts for 15% of all female breast cancer cases. The term "triple-negative" refers to the absence of estrogen receptor (ER) and progesterone receptor (PR) targets and the HER2 target for treatment. Otherwise, triple-negative breast cancer is highly heterogeneous and includes multiple molecular subtypes. Typically, triple-negative breast cancer occurs in younger patients, has a higher grade, a greater tumor size, a higher clinical stage at diagnosis, and a poorer prognosis than non–triple-negative breast cancers.
Biopsy samples from patients with a new primary or newly metastatic breast cancer diagnosis should undergo HR testing, both ER and PR, as well as HER2 receptor testing. Results of ER and PR testing are negative if a validated IHC assay shows 0% to < 1% of nuclei stain, according to the latest American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) HR testing guideline. HER2 results are negative if a validated IHC assay shows weak to moderate complete membrane staining in < 10% of tumor cells, according to ASCO/CAP guidelines. If HER2 results of a primary tumor are negative and specific clinical criteria suggest further testing, the oncologist may choose to order another IHC assay or a validated dual probe in situ hybridization (ISH) assay to aid in clinical decision-making.
Pathologic classification of triple-negative breast cancers includes multiple subgroups determined by therapeutic possibilities: low-risk histologic types (salivary gland–like carcinomas, fibromatosis-like carcinoma, low-grade adenosquamous breast carcinoma), immune activation (tumor-infiltrating lymphocytes, CD8, programmed death–ligand 1, tumor mutational burden), HER2-low status (IHC score 1+/2+ nonamplified), associated germline BRCA mutations, and other targets (trophoblast cell-surface antigen 2, HER3).
Family history is the most common risk factor for developing breast cancer. If a person's mother or sister had breast cancer, the lifetime risk for that individual is four times greater than that of the general population. Risk for breast cancer grows even more if two or more first-degree relatives are diagnosed, or if one first-degree relative was diagnosed with breast cancer at ≤ 50 years of age. About 25% of triple-negative breast cancer cases have accompanying germline BRCA mutations. A recent study by Ahearn and colleagues found that 85 variants were linked to one or more tumor feature of ER-negative disease, and 32 of those variants were associated with triple-negative disease.
For a breast cancer diagnosis, the National Comprehensive Cancer Network recommends multidisciplinary care as well as the development of a personalized survivorship treatment plan, which includes a customized summary of possible long-term treatment toxicities. In addition, multidisciplinary care coordination encourages close follow-up that helps patients adhere to their medications and stay current with ongoing screening.
Because triple-negative breast cancer lacks HR and HER2 therapeutic targets, it can be difficult to treat. Standard therapy for high-risk triple-negative tumors includes chemotherapy with taxanes (paclitaxel or docetaxel) plus anthracycline-based treatment (cyclophosphamide plus doxorubicin). Neoadjuvant chemotherapy is recommended for patients with stage 2 and 3 tumors to reduce locoregional surgical extension and to allow for personalized treatment based on pathologic response. In 2018, two poly ADP ribose polymerase inhibitors, olaparib and talazoparib, were approved for use in select patients with triple-negative breast cancer, significantly changing the treatment paradigm for this disease. Other promising treatments have emerged recently in clinical trials for triple-negative disease, including immune checkpoint inhibitors, PI3K/AKT pathway inhibitors, and cytotoxin-conjugated antibodies.
Daniel S. Schwartz, MD, is Medical Director of Thoracic Oncology, St. Catherine of Siena Medical Center, Catholic Health Services, Smithtown, New York.
Dr. Schwartz serve(d) as a member of the following medical societies:
American College of Chest Physicians, American College of Surgeons, Society of Thoracic Surgeons, Western Thoracic Surgical Association.
Disclosure: Daniel S. Schwartz, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
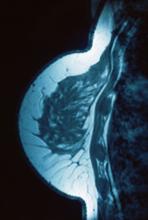
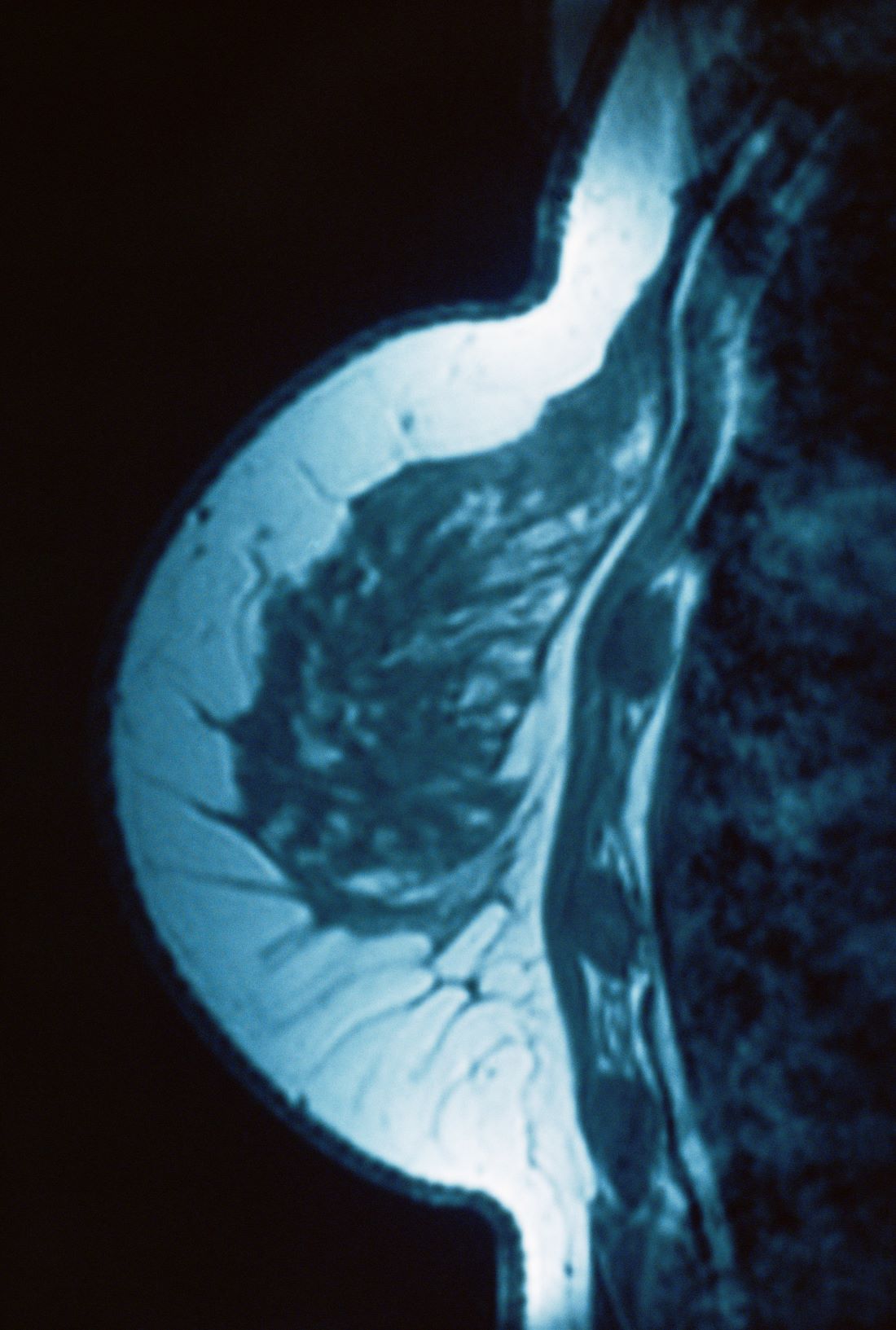
A 34-year-old woman visits her primary care physician after detecting a lump in her right breast. The physician conducts a physical exam and finds a hard, poorly mobile mass in the outer upper quadrant. Ultrasonography reveals an irregular hypoechoic mass of 22.1 x 15.1 mm with several abnormal axillary lymph nodes. MRI of the right breast shows an irregularly shaped and lobulated mass in the outer upper quadrant with enlarged axillary lymph nodes. On further radiologic examination, there is no detectable metastatic spread to the brain, chest, or upper abdomen. The patient undergoes a core needle biopsy of the tumor, which confirms ductal carcinoma and right axillary lymph node metastasis. Immunohistochemistry (IHC) assay of the sample is negative for hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2).