User login
Therapeutic Approaches in Advanced Breast Cancer
Skin changes and pain
The history and findings in this case are suggestive of inflammatory breast cancer.
Breast cancer is the leading life-threatening cancer diagnosed and the second-leading cause of cancer-related deaths in women worldwide. In the United States, estimates suggest that 287,850 new cases of invasive breast cancer were diagnosed in 2022 and 43,250 women died of the disease. Globally, approximately 2.3 million new diagnoses and 685,000 breast cancer–related deaths were reported in 2020.
Inflammatory breast cancer is a rare and highly aggressive subtype of locally advanced breast cancer. In the United States, inflammatory breast cancer accounts for approximately 2%-4% of breast cancer cases. Although its incidence is rare, 7% of breast cancer caused mortality is attributed to inflammatory breast cancer. Cases of inflammatory breast cancer tend to be diagnosed at a younger age compared with noninflammatory breast cancer cases. Risk factors include African-American race and obesity.
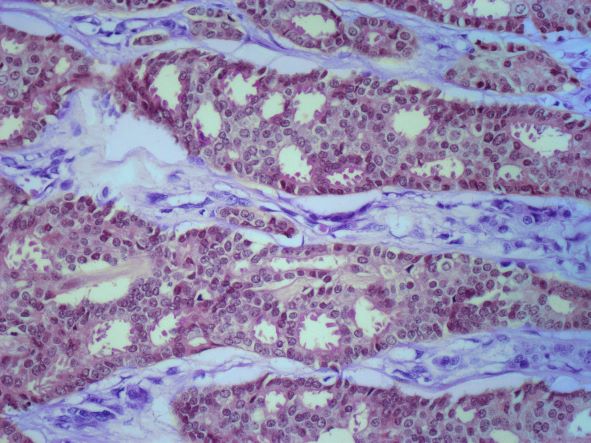
The symptoms of inflammatory breast cancer can vary broadly, ranging from subtle skin erythema to diffuse breast involvement with skin dimpling and nipple inversion. Diagnostic criteria include erythema occupying at least one third of the breast, edema, peau d'orange, and/or warmth, with or without an underlying mass; rapid onset (< 3 months); and pathologic confirmation of invasive breast carcinoma. Histologic findings include florid tumor emboli that obstruct dermal lymphatics, which results in swelling and inflammation of the affected breast.
Inflammatory breast cancer has been associated with a poor prognosis. However, treatment advances are helping to improve outcomes. Currently, 5-year survival rates are reported to be 40%-70%, with a median survival of 2-4 years. According to 2023 guidelines from the National Comprehensive Cancer Network (NCCN), the first-line treatment of inflammatory breast cancer involves neoadjuvant chemotherapy, modified radical mastectomy, and adjuvant radiation to the chest wall and regional nodes. Endocrine treatment should also be given to patients who are ER-positive and/or PR-positive (sequential chemotherapy followed by endocrine therapy). For patients who are HER2-positive, up to 1 year of HER2-targeted therapy should be given. HER2-targeted therapies can be administered concurrently with radiation and with endocrine therapy if indicated.
Delayed reconstruction after mastectomy remains the clinical standard for inflammatory breast cancer. This is because the need to resect involved skin negates the benefit of skin-sparing mastectomy for immediate reconstruction. Moreover, high rates of local and distant recurrence warrant comprehensive regional node irradiation in a timely fashion, which may be more challenging or subject to delay after immediate reconstruction. Rarely, the extent of skin excision at the time of mastectomy prohibits primary or local closure. In such cases, reconstruction of the chest wall defect with autologous tissue is required, and concomitant immediate reconstruction may be undertaken.
Detailed guidance on the treatment of inflammatory breast cancer, in the first line and beyond, are available from the NCCN.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of inflammatory breast cancer.
Breast cancer is the leading life-threatening cancer diagnosed and the second-leading cause of cancer-related deaths in women worldwide. In the United States, estimates suggest that 287,850 new cases of invasive breast cancer were diagnosed in 2022 and 43,250 women died of the disease. Globally, approximately 2.3 million new diagnoses and 685,000 breast cancer–related deaths were reported in 2020.
Inflammatory breast cancer is a rare and highly aggressive subtype of locally advanced breast cancer. In the United States, inflammatory breast cancer accounts for approximately 2%-4% of breast cancer cases. Although its incidence is rare, 7% of breast cancer caused mortality is attributed to inflammatory breast cancer. Cases of inflammatory breast cancer tend to be diagnosed at a younger age compared with noninflammatory breast cancer cases. Risk factors include African-American race and obesity.
The symptoms of inflammatory breast cancer can vary broadly, ranging from subtle skin erythema to diffuse breast involvement with skin dimpling and nipple inversion. Diagnostic criteria include erythema occupying at least one third of the breast, edema, peau d'orange, and/or warmth, with or without an underlying mass; rapid onset (< 3 months); and pathologic confirmation of invasive breast carcinoma. Histologic findings include florid tumor emboli that obstruct dermal lymphatics, which results in swelling and inflammation of the affected breast.
Inflammatory breast cancer has been associated with a poor prognosis. However, treatment advances are helping to improve outcomes. Currently, 5-year survival rates are reported to be 40%-70%, with a median survival of 2-4 years. According to 2023 guidelines from the National Comprehensive Cancer Network (NCCN), the first-line treatment of inflammatory breast cancer involves neoadjuvant chemotherapy, modified radical mastectomy, and adjuvant radiation to the chest wall and regional nodes. Endocrine treatment should also be given to patients who are ER-positive and/or PR-positive (sequential chemotherapy followed by endocrine therapy). For patients who are HER2-positive, up to 1 year of HER2-targeted therapy should be given. HER2-targeted therapies can be administered concurrently with radiation and with endocrine therapy if indicated.
Delayed reconstruction after mastectomy remains the clinical standard for inflammatory breast cancer. This is because the need to resect involved skin negates the benefit of skin-sparing mastectomy for immediate reconstruction. Moreover, high rates of local and distant recurrence warrant comprehensive regional node irradiation in a timely fashion, which may be more challenging or subject to delay after immediate reconstruction. Rarely, the extent of skin excision at the time of mastectomy prohibits primary or local closure. In such cases, reconstruction of the chest wall defect with autologous tissue is required, and concomitant immediate reconstruction may be undertaken.
Detailed guidance on the treatment of inflammatory breast cancer, in the first line and beyond, are available from the NCCN.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of inflammatory breast cancer.
Breast cancer is the leading life-threatening cancer diagnosed and the second-leading cause of cancer-related deaths in women worldwide. In the United States, estimates suggest that 287,850 new cases of invasive breast cancer were diagnosed in 2022 and 43,250 women died of the disease. Globally, approximately 2.3 million new diagnoses and 685,000 breast cancer–related deaths were reported in 2020.
Inflammatory breast cancer is a rare and highly aggressive subtype of locally advanced breast cancer. In the United States, inflammatory breast cancer accounts for approximately 2%-4% of breast cancer cases. Although its incidence is rare, 7% of breast cancer caused mortality is attributed to inflammatory breast cancer. Cases of inflammatory breast cancer tend to be diagnosed at a younger age compared with noninflammatory breast cancer cases. Risk factors include African-American race and obesity.
The symptoms of inflammatory breast cancer can vary broadly, ranging from subtle skin erythema to diffuse breast involvement with skin dimpling and nipple inversion. Diagnostic criteria include erythema occupying at least one third of the breast, edema, peau d'orange, and/or warmth, with or without an underlying mass; rapid onset (< 3 months); and pathologic confirmation of invasive breast carcinoma. Histologic findings include florid tumor emboli that obstruct dermal lymphatics, which results in swelling and inflammation of the affected breast.
Inflammatory breast cancer has been associated with a poor prognosis. However, treatment advances are helping to improve outcomes. Currently, 5-year survival rates are reported to be 40%-70%, with a median survival of 2-4 years. According to 2023 guidelines from the National Comprehensive Cancer Network (NCCN), the first-line treatment of inflammatory breast cancer involves neoadjuvant chemotherapy, modified radical mastectomy, and adjuvant radiation to the chest wall and regional nodes. Endocrine treatment should also be given to patients who are ER-positive and/or PR-positive (sequential chemotherapy followed by endocrine therapy). For patients who are HER2-positive, up to 1 year of HER2-targeted therapy should be given. HER2-targeted therapies can be administered concurrently with radiation and with endocrine therapy if indicated.
Delayed reconstruction after mastectomy remains the clinical standard for inflammatory breast cancer. This is because the need to resect involved skin negates the benefit of skin-sparing mastectomy for immediate reconstruction. Moreover, high rates of local and distant recurrence warrant comprehensive regional node irradiation in a timely fashion, which may be more challenging or subject to delay after immediate reconstruction. Rarely, the extent of skin excision at the time of mastectomy prohibits primary or local closure. In such cases, reconstruction of the chest wall defect with autologous tissue is required, and concomitant immediate reconstruction may be undertaken.
Detailed guidance on the treatment of inflammatory breast cancer, in the first line and beyond, are available from the NCCN.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
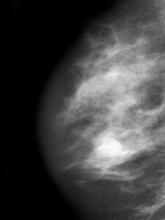
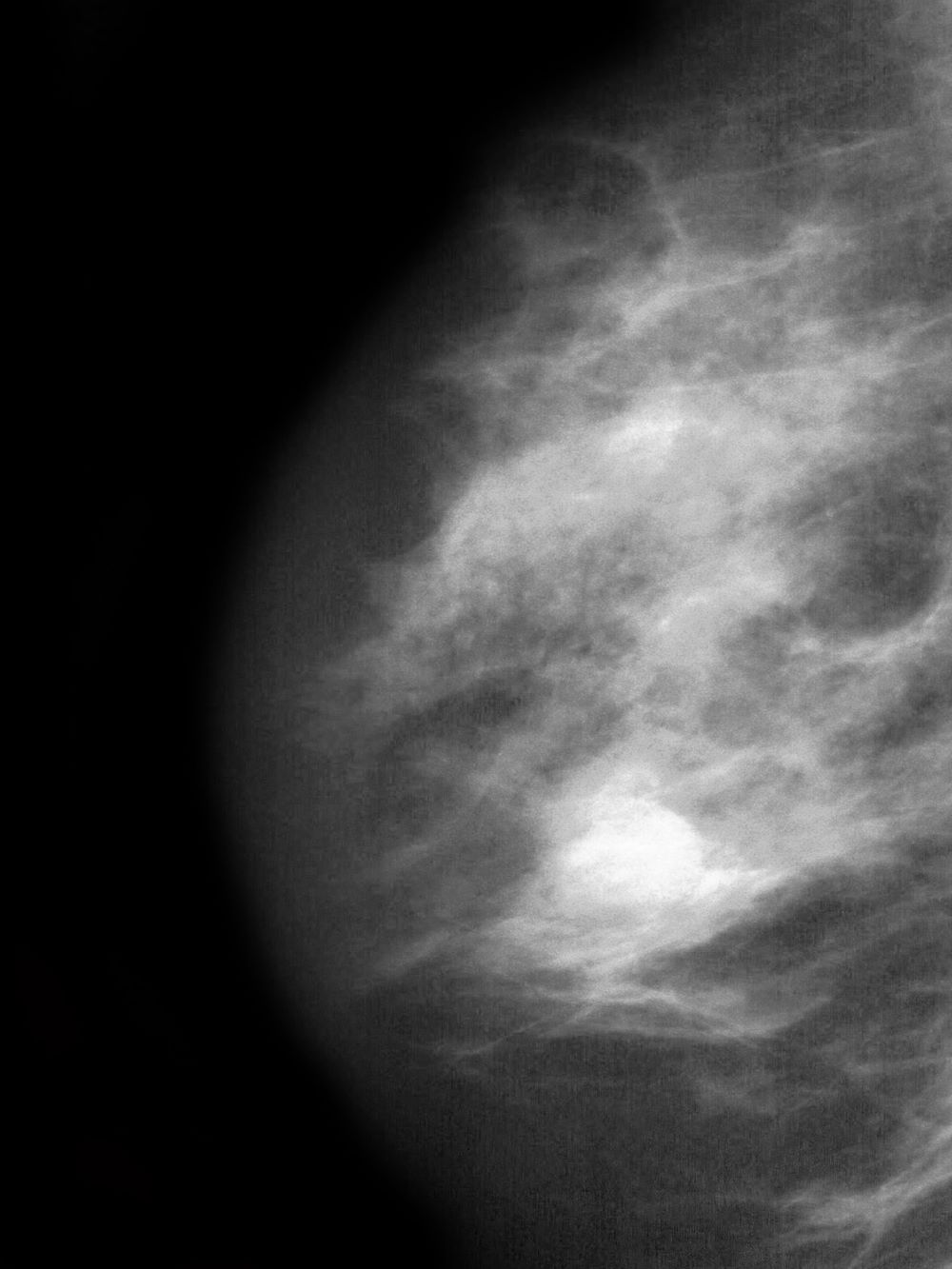
A 51-year-old nonsmoking Black woman presents with a lump in her left breast, as well as associated skin changes and pain of approximately 3 months' duration. The patient last underwent routine screening breast imaging 2 years earlier. The patient is 5 ft 7 in and weighs 200 lb (BMI 31.3). Previous medical history is unremarkable. There is a family history of breast cancer (maternal aunt) and lung cancer (maternal uncle). Physical examination reveals a palpable abnormality in the left breast with edema, skin thickening, and peau d'orange. More than one third of the breast is erythematous. A bilateral mammography reveals an irregular mass and calcifications in the upper outer quadrant of the left breast as well as numerous additional masses and focal asymmetries involving the upper outer and lower outer quadrant of the left breast that extend into the inner left breast. A 1.6-cm mass in the upper left breast is noted, with total abnormality spanning 12.7 cm. Left axillary lymphadenopathy is also observed. Skin punch biopsy of the affected breast reveals dermal lymphatic invasion by tumor cells and tumor emboli. Left axial fine-needle aspiration biopsy reveals malignant cells.
Advanced Breast Cancer Pathophysiology
Progressive back pain
The history and findings in this case are suggestive of advanced/metastatic breast cancer.
Breast cancer is the most frequently diagnosed life-threatening cancer and the second-leading cause of cancer-related deaths in women worldwide. In the United States, an estimated 287,850 new cases of invasive breast cancer were diagnosed in 2022 and 43,250 women died of the disease. Globally, approximately 2.3 million new diagnoses and 685,000 breast cancer–related deaths were reported in 2020.
Tumor size, nodal spread, and distant metastases (TNM) at the time of diagnosis are key prognostic factors. Immunohistochemistry tumor markers (ie, estrogen receptor [ER], progesterone receptor [PR], and HER2), as well as grade and Ki-67 expression, have also been shown to be independent predictors of breast cancer death and are used together with TNM to guide treatment decisions.
Despite advances in breast cancer diagnosis and treatment, metastatic recurrence remains a significant problem. Although the incidence of distance relapse is declining and survival times for patients with recurrent disease are improving, 20%-30% of patients with early breast cancer still die of metastatic disease. Metastatic breast cancer recurrence can arise months to decades after initial diagnosis and treatment.
According to the National Comprehensive Cancer Network (NCCN) guidelines, biopsy is a critical component of the workup for patients with recurrent or stage IV disease. This is because biopsy ensures accurate determination of metastatic/recurrent disease and tumor histology and enables biomarker determination and selection of appropriate treatment. Soft-tissue tumor biopsy is preferred over bone sites unless a portion of the biopsy can be protected from harsh decalcification solution to preserve more accurate evaluation of biomarkers. Determination of HR status (ER and PR) and HER2 status should be repeated in all cases when diagnostic tissue is obtained because ER and PR assays may be falsely negative or falsely positive, and there may be discordance between the primary and metastatic tumors. According to the NCCN panel, re-testing the receptor status of recurrent disease should be performed, particularly when it was previously unknown, originally negative, or not overexpressed.
Additionally, the staging evaluation of patients who present with recurrent or stage IV breast cancer should include history and physical exam; a complete blood cell count, liver function tests, chest diagnostic CT, bone scan, and radiography of any long or weight-bearing bones that are painful or appear abnormal on bone scan; diagnostic CT of the abdomen (with or without diagnostic CT of the pelvis) or MRI of the abdomen; and biopsy documentation of first recurrence whenever possible. The use of sodium fluoride PET or PET/CT for evaluating patients with recurrent disease is generally discouraged.
Presently, metastatic breast cancer remains incurable. However, in recent years, the treatment landscape for metastatic breast cancer has significantly advanced in all breast cancer subtypes, leading to improvements in progression-free survival and even overall survival in some cases. For example, newer, targeted approaches directly address mutation drivers and allow precise delivery of chemotherapeutic agents. Detailed guidance on the treatment of breast cancer can be found here and in the full NCCN guidelines.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of advanced/metastatic breast cancer.
Breast cancer is the most frequently diagnosed life-threatening cancer and the second-leading cause of cancer-related deaths in women worldwide. In the United States, an estimated 287,850 new cases of invasive breast cancer were diagnosed in 2022 and 43,250 women died of the disease. Globally, approximately 2.3 million new diagnoses and 685,000 breast cancer–related deaths were reported in 2020.
Tumor size, nodal spread, and distant metastases (TNM) at the time of diagnosis are key prognostic factors. Immunohistochemistry tumor markers (ie, estrogen receptor [ER], progesterone receptor [PR], and HER2), as well as grade and Ki-67 expression, have also been shown to be independent predictors of breast cancer death and are used together with TNM to guide treatment decisions.
Despite advances in breast cancer diagnosis and treatment, metastatic recurrence remains a significant problem. Although the incidence of distance relapse is declining and survival times for patients with recurrent disease are improving, 20%-30% of patients with early breast cancer still die of metastatic disease. Metastatic breast cancer recurrence can arise months to decades after initial diagnosis and treatment.
According to the National Comprehensive Cancer Network (NCCN) guidelines, biopsy is a critical component of the workup for patients with recurrent or stage IV disease. This is because biopsy ensures accurate determination of metastatic/recurrent disease and tumor histology and enables biomarker determination and selection of appropriate treatment. Soft-tissue tumor biopsy is preferred over bone sites unless a portion of the biopsy can be protected from harsh decalcification solution to preserve more accurate evaluation of biomarkers. Determination of HR status (ER and PR) and HER2 status should be repeated in all cases when diagnostic tissue is obtained because ER and PR assays may be falsely negative or falsely positive, and there may be discordance between the primary and metastatic tumors. According to the NCCN panel, re-testing the receptor status of recurrent disease should be performed, particularly when it was previously unknown, originally negative, or not overexpressed.
Additionally, the staging evaluation of patients who present with recurrent or stage IV breast cancer should include history and physical exam; a complete blood cell count, liver function tests, chest diagnostic CT, bone scan, and radiography of any long or weight-bearing bones that are painful or appear abnormal on bone scan; diagnostic CT of the abdomen (with or without diagnostic CT of the pelvis) or MRI of the abdomen; and biopsy documentation of first recurrence whenever possible. The use of sodium fluoride PET or PET/CT for evaluating patients with recurrent disease is generally discouraged.
Presently, metastatic breast cancer remains incurable. However, in recent years, the treatment landscape for metastatic breast cancer has significantly advanced in all breast cancer subtypes, leading to improvements in progression-free survival and even overall survival in some cases. For example, newer, targeted approaches directly address mutation drivers and allow precise delivery of chemotherapeutic agents. Detailed guidance on the treatment of breast cancer can be found here and in the full NCCN guidelines.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of advanced/metastatic breast cancer.
Breast cancer is the most frequently diagnosed life-threatening cancer and the second-leading cause of cancer-related deaths in women worldwide. In the United States, an estimated 287,850 new cases of invasive breast cancer were diagnosed in 2022 and 43,250 women died of the disease. Globally, approximately 2.3 million new diagnoses and 685,000 breast cancer–related deaths were reported in 2020.
Tumor size, nodal spread, and distant metastases (TNM) at the time of diagnosis are key prognostic factors. Immunohistochemistry tumor markers (ie, estrogen receptor [ER], progesterone receptor [PR], and HER2), as well as grade and Ki-67 expression, have also been shown to be independent predictors of breast cancer death and are used together with TNM to guide treatment decisions.
Despite advances in breast cancer diagnosis and treatment, metastatic recurrence remains a significant problem. Although the incidence of distance relapse is declining and survival times for patients with recurrent disease are improving, 20%-30% of patients with early breast cancer still die of metastatic disease. Metastatic breast cancer recurrence can arise months to decades after initial diagnosis and treatment.
According to the National Comprehensive Cancer Network (NCCN) guidelines, biopsy is a critical component of the workup for patients with recurrent or stage IV disease. This is because biopsy ensures accurate determination of metastatic/recurrent disease and tumor histology and enables biomarker determination and selection of appropriate treatment. Soft-tissue tumor biopsy is preferred over bone sites unless a portion of the biopsy can be protected from harsh decalcification solution to preserve more accurate evaluation of biomarkers. Determination of HR status (ER and PR) and HER2 status should be repeated in all cases when diagnostic tissue is obtained because ER and PR assays may be falsely negative or falsely positive, and there may be discordance between the primary and metastatic tumors. According to the NCCN panel, re-testing the receptor status of recurrent disease should be performed, particularly when it was previously unknown, originally negative, or not overexpressed.
Additionally, the staging evaluation of patients who present with recurrent or stage IV breast cancer should include history and physical exam; a complete blood cell count, liver function tests, chest diagnostic CT, bone scan, and radiography of any long or weight-bearing bones that are painful or appear abnormal on bone scan; diagnostic CT of the abdomen (with or without diagnostic CT of the pelvis) or MRI of the abdomen; and biopsy documentation of first recurrence whenever possible. The use of sodium fluoride PET or PET/CT for evaluating patients with recurrent disease is generally discouraged.
Presently, metastatic breast cancer remains incurable. However, in recent years, the treatment landscape for metastatic breast cancer has significantly advanced in all breast cancer subtypes, leading to improvements in progression-free survival and even overall survival in some cases. For example, newer, targeted approaches directly address mutation drivers and allow precise delivery of chemotherapeutic agents. Detailed guidance on the treatment of breast cancer can be found here and in the full NCCN guidelines.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 62-year-old nonsmoking woman presents with progressive moderate to severe back pain. The patient has a history of endometriosis and node-positive invasive ductal breast cancer, which was diagnosed 15 years ago. The tumor was hormone receptor (HR)–positive and human epidermal growth factor receptor 2 (HER2)–negative. After a lumpectomy, she received adjuvant chemotherapy, followed by radiation therapy and 5 years of adjuvant oral endocrine therapy. Physical examination reveals several large palpable nodes in the right axillary region; no abnormalities are noted in either breast or the left axillary region.
The patient is 5 ft 7 in and weighs 152 lb (BMI, 23.8). At her last visit, 3 years earlier, she weighed 176 lb. She states her weight loss has been unintentional and began about 6 months ago. The patient denies any respiratory or abdominal symptoms; she does report increasing fatigue, which she attributes to her back pain. Complete blood cell count values are within normal range, except for an elevated alkaline phosphatase level (215 IU/L).
A subsequent axillary lymph node ultrasound reveals several irregular hypoechoic masses in the right axilla of various sizes, the largest being 2.4 cm. PET, CT, and a bone scan were also performed and revealed multiple suspicious lesions in the spine and several pulmonary nodules.
Metastatic Breast Cancer Workup
Complaints of mass in breast
The history and findings in this case are consistent with advanced infiltrating ductal carcinoma.
Breast cancer is the most commonly diagnosed life-threatening cancer and the leading cause of cancer death among women worldwide. In the United States, it is estimated that 287,850 new cases of invasive breast cancer were diagnosed in 2022; in addition, 43,250 deaths because of breast cancer are expected to occur.
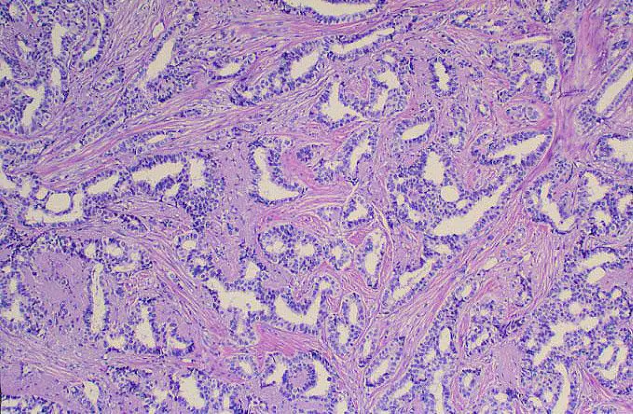
Infiltrating ductal carcinoma is the most common type of breast tumor. It accounts for 75% of breast cancers and has a propensity to metastasize via lymphatic vessels. This lesion has no specific histologic characteristics apart from invasion through the basement membrane. This enables it to be differentiated from ductal carcinoma in situ, which remains inside the duct.
All newly diagnosed cases of invasive breast cancers are tested for estrogen receptors, progesterone receptors, and HER2 status. The presence of estrogen and progesterone receptors is tested by immunohistochemistry, whereas HER2 can be tested by either immunohistochemistry or in situ hybridization (usually fluorescent or fluorescence in situ hybridization). Testing results have important treatment implications and prognostic significance.
Surgical treatment of invasive breast cancer consists of either lumpectomy or total mastectomy, followed by radiation therapy. Surgical resection is performed in all patients with nonmetastatic disease.
Systemic therapy options include endocrine therapy, cytotoxic chemotherapy, targeted therapy, and immunotherapy. The choice of systemic therapy is largely determined by subtype. For example, patients with hormone receptor–positive tumors receive endocrine therapy; a minority of these patients may receive chemotherapy as well. Patients with HER2-positive tumors receive HER2–targeted antibody or small-molecule inhibitor therapy combined with chemotherapy. For patients with triple-negative tumors, chemotherapy alone is often used; newer targeted therapies however may improve outcomes. In both the adjuvant and neoadjuvant setting, the primary goal of treatment is to eradicate or control undiscovered distant metastases. In the metastatic setting, the primary goal of treatment is to extend life and alleviate symptoms.
Depending on the individual patient, systemic therapy may be used in the adjuvant or neoadjuvant setting. Systemic therapy is chosen according to breast cancer subtype and recurrence risk, with the treatment for low-risk patients being de-escalated, whereas high-risk patients receive aggressive systemic treatment. When systemic therapy is used in the neoadjuvant setting, treatment response is the most important factor for predicting outcomes and selecting the optimal adjuvant therapy. Novel biological markers enable the selection of appropriate targeted therapy, which can achieve optimal efficacy.
Up-to-date, evidence-based recommendations for pre- and postoperative treatment of breast cancer, including advanced breast cancer, are provided by the National Comprehensive Cancer Network.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are consistent with advanced infiltrating ductal carcinoma.
Breast cancer is the most commonly diagnosed life-threatening cancer and the leading cause of cancer death among women worldwide. In the United States, it is estimated that 287,850 new cases of invasive breast cancer were diagnosed in 2022; in addition, 43,250 deaths because of breast cancer are expected to occur.
Infiltrating ductal carcinoma is the most common type of breast tumor. It accounts for 75% of breast cancers and has a propensity to metastasize via lymphatic vessels. This lesion has no specific histologic characteristics apart from invasion through the basement membrane. This enables it to be differentiated from ductal carcinoma in situ, which remains inside the duct.
All newly diagnosed cases of invasive breast cancers are tested for estrogen receptors, progesterone receptors, and HER2 status. The presence of estrogen and progesterone receptors is tested by immunohistochemistry, whereas HER2 can be tested by either immunohistochemistry or in situ hybridization (usually fluorescent or fluorescence in situ hybridization). Testing results have important treatment implications and prognostic significance.
Surgical treatment of invasive breast cancer consists of either lumpectomy or total mastectomy, followed by radiation therapy. Surgical resection is performed in all patients with nonmetastatic disease.
Systemic therapy options include endocrine therapy, cytotoxic chemotherapy, targeted therapy, and immunotherapy. The choice of systemic therapy is largely determined by subtype. For example, patients with hormone receptor–positive tumors receive endocrine therapy; a minority of these patients may receive chemotherapy as well. Patients with HER2-positive tumors receive HER2–targeted antibody or small-molecule inhibitor therapy combined with chemotherapy. For patients with triple-negative tumors, chemotherapy alone is often used; newer targeted therapies however may improve outcomes. In both the adjuvant and neoadjuvant setting, the primary goal of treatment is to eradicate or control undiscovered distant metastases. In the metastatic setting, the primary goal of treatment is to extend life and alleviate symptoms.
Depending on the individual patient, systemic therapy may be used in the adjuvant or neoadjuvant setting. Systemic therapy is chosen according to breast cancer subtype and recurrence risk, with the treatment for low-risk patients being de-escalated, whereas high-risk patients receive aggressive systemic treatment. When systemic therapy is used in the neoadjuvant setting, treatment response is the most important factor for predicting outcomes and selecting the optimal adjuvant therapy. Novel biological markers enable the selection of appropriate targeted therapy, which can achieve optimal efficacy.
Up-to-date, evidence-based recommendations for pre- and postoperative treatment of breast cancer, including advanced breast cancer, are provided by the National Comprehensive Cancer Network.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are consistent with advanced infiltrating ductal carcinoma.
Breast cancer is the most commonly diagnosed life-threatening cancer and the leading cause of cancer death among women worldwide. In the United States, it is estimated that 287,850 new cases of invasive breast cancer were diagnosed in 2022; in addition, 43,250 deaths because of breast cancer are expected to occur.
Infiltrating ductal carcinoma is the most common type of breast tumor. It accounts for 75% of breast cancers and has a propensity to metastasize via lymphatic vessels. This lesion has no specific histologic characteristics apart from invasion through the basement membrane. This enables it to be differentiated from ductal carcinoma in situ, which remains inside the duct.
All newly diagnosed cases of invasive breast cancers are tested for estrogen receptors, progesterone receptors, and HER2 status. The presence of estrogen and progesterone receptors is tested by immunohistochemistry, whereas HER2 can be tested by either immunohistochemistry or in situ hybridization (usually fluorescent or fluorescence in situ hybridization). Testing results have important treatment implications and prognostic significance.
Surgical treatment of invasive breast cancer consists of either lumpectomy or total mastectomy, followed by radiation therapy. Surgical resection is performed in all patients with nonmetastatic disease.
Systemic therapy options include endocrine therapy, cytotoxic chemotherapy, targeted therapy, and immunotherapy. The choice of systemic therapy is largely determined by subtype. For example, patients with hormone receptor–positive tumors receive endocrine therapy; a minority of these patients may receive chemotherapy as well. Patients with HER2-positive tumors receive HER2–targeted antibody or small-molecule inhibitor therapy combined with chemotherapy. For patients with triple-negative tumors, chemotherapy alone is often used; newer targeted therapies however may improve outcomes. In both the adjuvant and neoadjuvant setting, the primary goal of treatment is to eradicate or control undiscovered distant metastases. In the metastatic setting, the primary goal of treatment is to extend life and alleviate symptoms.
Depending on the individual patient, systemic therapy may be used in the adjuvant or neoadjuvant setting. Systemic therapy is chosen according to breast cancer subtype and recurrence risk, with the treatment for low-risk patients being de-escalated, whereas high-risk patients receive aggressive systemic treatment. When systemic therapy is used in the neoadjuvant setting, treatment response is the most important factor for predicting outcomes and selecting the optimal adjuvant therapy. Novel biological markers enable the selection of appropriate targeted therapy, which can achieve optimal efficacy.
Up-to-date, evidence-based recommendations for pre- and postoperative treatment of breast cancer, including advanced breast cancer, are provided by the National Comprehensive Cancer Network.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 40-year-old woman presents with a left breast mass that she discovered while showering. The patient has no significant medical history and does not take any medications. She has not yet undergone any routine mammography screening. Clinical breast examination reveals a large palpable immobile mass in the inner quadrant of the left breast. The nipple is retracted. Left axial lymphadenopathy is also detected. There are no palpable abnormalities in the right breast. The remainder of the physical examination is unremarkable. Laboratory findings are all within normal range, apart from C-reactive protein, which is elevated (8 mg/L). The patient is 5 ft 4 in and weighs 142 lb.
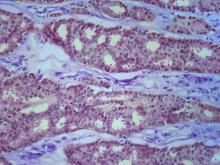
The patient is sent for a mammogram and needle biopsy of the mass and axial lymph nodes. Mammogram findings include an irregular mass 4.2 cm in size in the left breast with heterogeneous echotexture, abrupt interface, and intramass calcifications as well as overlying skin thickening. The mass is predominately in the inner left breast and is extending into the outside quadrant. Multiple morphologically abnormal lymph nodes are noted in the left axilla. Biopsy results include atypical glands infiltrating the surrounding stroma in an irregular pattern. Immunohistochemistry results show p53 expression.
Pulling sensation in chest
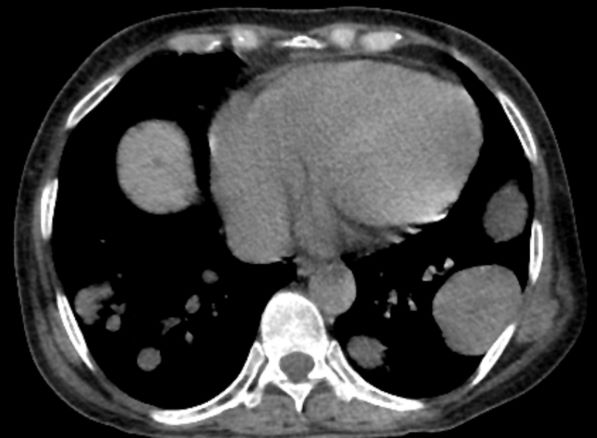
The history and findings in this case are suggestive of breast cancer with metastatic spread to the chest wall and lungs.
Globally, breast cancer is the most frequently diagnosed life-threatening cancer and the leading cause of cancer death among women. In the United States, an estimated 287,850 new cases of invasive breast cancer will be diagnosed in 2022; in addition, 43,250 deaths because of breast cancer are expected to occur. Despite advances in adjuvant treatment strategies, such as tamoxifen for patients with ER-positive breast cancer, many patients with early breast cancer still experience disease recurrence after primary therapy. Because of its systemic nature and inevitable resistance to therapy, metastatic breast cancer is largely incurable.
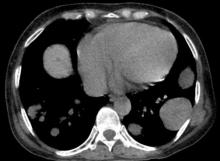
Approximately 5%-35% of patients with breast cancer develop locoregional recurrence either alone or with distant metastases. The lung is a frequent site of breast cancer metastasis. In addition, approximately 11% of patients have persistent chest wall progression. Recurrent breast cancer in the chest wall is considered a marker of poor prognosis and is normally accompanied by or a precursor to distant metastases.
Risk factors for chest wall recurrence include primary tumor size, primary stage, and lymph node involvement; in addition, the risk is increased in patients aged 40 years or younger and in those with gross multifocal or multicentric disease. Histopathological risk factors include positive margin status, DCIS, extensive intraductal component, high grade, lymphovascular invasion, tumor oncogene, and tumor suppressor gene expression (eg, p53 and HER2), and ER status.
According to the National Comprehensive Cancer Network (NCCN) 2022 guidelines, the staging evaluation of patients who present with recurrent or stage IV breast cancer should include:
• History and physical exam
• Complete blood count and liver function tests
• Chest diagnostic CT
• Bone scan
• Radiographs of any long or weight-bearing bones that are painful or appear abnormal on bone scan
• Diagnostic CT of the abdomen (with or without diagnostic CT of the pelvis) or MRI of the abdomen
• Biopsy documentation of first recurrence, when possible
The use of sodium fluoride PET or PET-CT for the evaluation of patients with recurrent disease is largely discouraged.
Determination of hormone receptor status (ER and progesterone receptor [PR]) as well as HER2 status should be repeated because ER and PR assays may be falsely negative or falsely positive and there may be discordance between the primary and metastatic tumors.
In the metastatic setting, genetic testing results may have therapeutic implications; specifically, germline mutations in BRCA1/BRCA2 have demonstrated clinical utility and therapeutic impact. Thus, the NCCN panel recommends that germline BRCA1/BRCA2 mutations be evaluated in all patients with recurrent or metastatic breast cancer to identify candidates for appropriate targeted therapies (eg, poly adenosine diphosphate ribose polymerase–inhibitor therapy).
In patients with recurrence of breast cancer to the chest wall, complete chest wall resection and appropriate reconstruction may prolong overall survival, although appropriate patient selection is essential for optimal outcomes. Patients with tumors that display a more aggressive phenotype (eg, triple-negative or HER2-positive disease) may not benefit from this approach and supportive care may be more appropriate.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of breast cancer with metastatic spread to the chest wall and lungs.
Globally, breast cancer is the most frequently diagnosed life-threatening cancer and the leading cause of cancer death among women. In the United States, an estimated 287,850 new cases of invasive breast cancer will be diagnosed in 2022; in addition, 43,250 deaths because of breast cancer are expected to occur. Despite advances in adjuvant treatment strategies, such as tamoxifen for patients with ER-positive breast cancer, many patients with early breast cancer still experience disease recurrence after primary therapy. Because of its systemic nature and inevitable resistance to therapy, metastatic breast cancer is largely incurable.
Approximately 5%-35% of patients with breast cancer develop locoregional recurrence either alone or with distant metastases. The lung is a frequent site of breast cancer metastasis. In addition, approximately 11% of patients have persistent chest wall progression. Recurrent breast cancer in the chest wall is considered a marker of poor prognosis and is normally accompanied by or a precursor to distant metastases.
Risk factors for chest wall recurrence include primary tumor size, primary stage, and lymph node involvement; in addition, the risk is increased in patients aged 40 years or younger and in those with gross multifocal or multicentric disease. Histopathological risk factors include positive margin status, DCIS, extensive intraductal component, high grade, lymphovascular invasion, tumor oncogene, and tumor suppressor gene expression (eg, p53 and HER2), and ER status.
According to the National Comprehensive Cancer Network (NCCN) 2022 guidelines, the staging evaluation of patients who present with recurrent or stage IV breast cancer should include:
• History and physical exam
• Complete blood count and liver function tests
• Chest diagnostic CT
• Bone scan
• Radiographs of any long or weight-bearing bones that are painful or appear abnormal on bone scan
• Diagnostic CT of the abdomen (with or without diagnostic CT of the pelvis) or MRI of the abdomen
• Biopsy documentation of first recurrence, when possible
The use of sodium fluoride PET or PET-CT for the evaluation of patients with recurrent disease is largely discouraged.
Determination of hormone receptor status (ER and progesterone receptor [PR]) as well as HER2 status should be repeated because ER and PR assays may be falsely negative or falsely positive and there may be discordance between the primary and metastatic tumors.
In the metastatic setting, genetic testing results may have therapeutic implications; specifically, germline mutations in BRCA1/BRCA2 have demonstrated clinical utility and therapeutic impact. Thus, the NCCN panel recommends that germline BRCA1/BRCA2 mutations be evaluated in all patients with recurrent or metastatic breast cancer to identify candidates for appropriate targeted therapies (eg, poly adenosine diphosphate ribose polymerase–inhibitor therapy).
In patients with recurrence of breast cancer to the chest wall, complete chest wall resection and appropriate reconstruction may prolong overall survival, although appropriate patient selection is essential for optimal outcomes. Patients with tumors that display a more aggressive phenotype (eg, triple-negative or HER2-positive disease) may not benefit from this approach and supportive care may be more appropriate.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of breast cancer with metastatic spread to the chest wall and lungs.
Globally, breast cancer is the most frequently diagnosed life-threatening cancer and the leading cause of cancer death among women. In the United States, an estimated 287,850 new cases of invasive breast cancer will be diagnosed in 2022; in addition, 43,250 deaths because of breast cancer are expected to occur. Despite advances in adjuvant treatment strategies, such as tamoxifen for patients with ER-positive breast cancer, many patients with early breast cancer still experience disease recurrence after primary therapy. Because of its systemic nature and inevitable resistance to therapy, metastatic breast cancer is largely incurable.
Approximately 5%-35% of patients with breast cancer develop locoregional recurrence either alone or with distant metastases. The lung is a frequent site of breast cancer metastasis. In addition, approximately 11% of patients have persistent chest wall progression. Recurrent breast cancer in the chest wall is considered a marker of poor prognosis and is normally accompanied by or a precursor to distant metastases.
Risk factors for chest wall recurrence include primary tumor size, primary stage, and lymph node involvement; in addition, the risk is increased in patients aged 40 years or younger and in those with gross multifocal or multicentric disease. Histopathological risk factors include positive margin status, DCIS, extensive intraductal component, high grade, lymphovascular invasion, tumor oncogene, and tumor suppressor gene expression (eg, p53 and HER2), and ER status.
According to the National Comprehensive Cancer Network (NCCN) 2022 guidelines, the staging evaluation of patients who present with recurrent or stage IV breast cancer should include:
• History and physical exam
• Complete blood count and liver function tests
• Chest diagnostic CT
• Bone scan
• Radiographs of any long or weight-bearing bones that are painful or appear abnormal on bone scan
• Diagnostic CT of the abdomen (with or without diagnostic CT of the pelvis) or MRI of the abdomen
• Biopsy documentation of first recurrence, when possible
The use of sodium fluoride PET or PET-CT for the evaluation of patients with recurrent disease is largely discouraged.
Determination of hormone receptor status (ER and progesterone receptor [PR]) as well as HER2 status should be repeated because ER and PR assays may be falsely negative or falsely positive and there may be discordance between the primary and metastatic tumors.
In the metastatic setting, genetic testing results may have therapeutic implications; specifically, germline mutations in BRCA1/BRCA2 have demonstrated clinical utility and therapeutic impact. Thus, the NCCN panel recommends that germline BRCA1/BRCA2 mutations be evaluated in all patients with recurrent or metastatic breast cancer to identify candidates for appropriate targeted therapies (eg, poly adenosine diphosphate ribose polymerase–inhibitor therapy).
In patients with recurrence of breast cancer to the chest wall, complete chest wall resection and appropriate reconstruction may prolong overall survival, although appropriate patient selection is essential for optimal outcomes. Patients with tumors that display a more aggressive phenotype (eg, triple-negative or HER2-positive disease) may not benefit from this approach and supportive care may be more appropriate.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 39-year-old nonsmoking woman in the United States presents with a history of a painful, pulling sensation in her chest that she originally attributed to a change in her exercise regimen. Six years earlier, the patient was diagnosed with estrogen receptor (ER)–positive ductal carcinoma in situ (DCIS) in her left breast. She opted for mastectomy and immediate reconstruction, followed by adjuvant therapy with tamoxifen (20 mg/d for 5 years). Physical examination reveals a palpable mass in the medial half of her left breast with several hard, painful nodules in the left axilla. Mild wheezing throughout the upper lungs is heard on auscultation. Abdominal examination does not reveal any abnormalities. Laboratory findings are all within normal range, apart from C-reactive protein, which is elevated. The patient is 5 ft 7 in and weighs 133 lb.