User login
FDA approves RSV monoclonal antibody for all infants
The monoclonal antibody Beyfortus (nirsevimab-alip), which already is approved for use in Europe and Canada, is indicated for newborns and infants born during or entering their first RSV season, and for children up to 24 months of age who are vulnerable to severe RSV through their second RSV season.
As many as 80,000 children under age 5 years are hospitalized with an RSV infection annually in the United States. Most cases are mild, but infants under 6 months, those born prematurely, and children with weakened immune systems or neuromuscular disorders are at an increased risk for severe illness, according to the Centers for Disease Control and Prevention.
The highly contagious virus is also a concern for immunocompromised adults and older people with underlying health conditions, who are at increased risk for severe disease.
Sanofi and AstraZeneca, which jointly developed the injectable agent, said in a press release that the companies plan to make it available by the fall of 2023. The long-acting antibody is given as a single intramuscular injection.
Beyfortus was approved in part based on data from the phase 3 MELODY trial, which found the shot reduced the incidence of medically attended lower respiratory tract infections associated with RSV by 74.9% versus placebo (95% confidence interval, 50.6-87.3; P < .001).
The phase 2/3 MEDLEY trial, conducted between July 2019 and May 2021, compared Beyfortus with palivizumab, another RSV antibody injection with more limited indications. The trial included more than 900 preterm infants less than 35 weeks’ gestational age and infants with congenital heart disease. Results were similar to the phase 3 MELODY trial, according to the manufacturers.
“Today’s approval marks an unprecedented moment for protecting infant health in the United States, following an RSV season that took a record toll on infants, their families, and the U.S. health care system,” said Thomas Triomphe, executive vice president for vaccines at Sanofi, in a press release about the FDA decision. “Beyfortus is the only monoclonal antibody approved for passive immunization to provide safe and effective protection for all infants during their first RSV season.”
A version of this article first appeared on Medscape.com.
The monoclonal antibody Beyfortus (nirsevimab-alip), which already is approved for use in Europe and Canada, is indicated for newborns and infants born during or entering their first RSV season, and for children up to 24 months of age who are vulnerable to severe RSV through their second RSV season.
As many as 80,000 children under age 5 years are hospitalized with an RSV infection annually in the United States. Most cases are mild, but infants under 6 months, those born prematurely, and children with weakened immune systems or neuromuscular disorders are at an increased risk for severe illness, according to the Centers for Disease Control and Prevention.
The highly contagious virus is also a concern for immunocompromised adults and older people with underlying health conditions, who are at increased risk for severe disease.
Sanofi and AstraZeneca, which jointly developed the injectable agent, said in a press release that the companies plan to make it available by the fall of 2023. The long-acting antibody is given as a single intramuscular injection.
Beyfortus was approved in part based on data from the phase 3 MELODY trial, which found the shot reduced the incidence of medically attended lower respiratory tract infections associated with RSV by 74.9% versus placebo (95% confidence interval, 50.6-87.3; P < .001).
The phase 2/3 MEDLEY trial, conducted between July 2019 and May 2021, compared Beyfortus with palivizumab, another RSV antibody injection with more limited indications. The trial included more than 900 preterm infants less than 35 weeks’ gestational age and infants with congenital heart disease. Results were similar to the phase 3 MELODY trial, according to the manufacturers.
“Today’s approval marks an unprecedented moment for protecting infant health in the United States, following an RSV season that took a record toll on infants, their families, and the U.S. health care system,” said Thomas Triomphe, executive vice president for vaccines at Sanofi, in a press release about the FDA decision. “Beyfortus is the only monoclonal antibody approved for passive immunization to provide safe and effective protection for all infants during their first RSV season.”
A version of this article first appeared on Medscape.com.
The monoclonal antibody Beyfortus (nirsevimab-alip), which already is approved for use in Europe and Canada, is indicated for newborns and infants born during or entering their first RSV season, and for children up to 24 months of age who are vulnerable to severe RSV through their second RSV season.
As many as 80,000 children under age 5 years are hospitalized with an RSV infection annually in the United States. Most cases are mild, but infants under 6 months, those born prematurely, and children with weakened immune systems or neuromuscular disorders are at an increased risk for severe illness, according to the Centers for Disease Control and Prevention.
The highly contagious virus is also a concern for immunocompromised adults and older people with underlying health conditions, who are at increased risk for severe disease.
Sanofi and AstraZeneca, which jointly developed the injectable agent, said in a press release that the companies plan to make it available by the fall of 2023. The long-acting antibody is given as a single intramuscular injection.
Beyfortus was approved in part based on data from the phase 3 MELODY trial, which found the shot reduced the incidence of medically attended lower respiratory tract infections associated with RSV by 74.9% versus placebo (95% confidence interval, 50.6-87.3; P < .001).
The phase 2/3 MEDLEY trial, conducted between July 2019 and May 2021, compared Beyfortus with palivizumab, another RSV antibody injection with more limited indications. The trial included more than 900 preterm infants less than 35 weeks’ gestational age and infants with congenital heart disease. Results were similar to the phase 3 MELODY trial, according to the manufacturers.
“Today’s approval marks an unprecedented moment for protecting infant health in the United States, following an RSV season that took a record toll on infants, their families, and the U.S. health care system,” said Thomas Triomphe, executive vice president for vaccines at Sanofi, in a press release about the FDA decision. “Beyfortus is the only monoclonal antibody approved for passive immunization to provide safe and effective protection for all infants during their first RSV season.”
A version of this article first appeared on Medscape.com.
Long COVID and vaccines: Separating facts from falsehoods
The COVID-19 vaccines have been a game changer for millions of people worldwide in preventing death or disability from the virus. Research suggests that they offer significant protection against long COVID.
False and unfounded claims made by some antivaccine groups that the vaccines themselves may cause long COVID persist and serve as barriers to vaccination.
To help separate the facts from falsehoods, here’s a checklist for doctors on what scientific studies have determined about vaccination and long COVID.
What the research shows
Doctors who work in long COVID clinics have for years suspected that vaccination may help protect against the development of long COVID, noted Lawrence Purpura, MD, MPH, an infectious disease specialist at New York–Presbyterian/Columbia University Irving Medical Center, who treats patients with long COVID in his clinic.
Over the past year, several large, well-conducted studies have borne out that theory, including the following studies:
- In the RECOVER study, published in May in the journal Nature Communications, researchers examined the electronic health records of more than 5 million people who had been diagnosed with COVID and found that vaccination reduced the risk that they would develop long COVID. Although the researchers didn’t compare the effects of having boosters to being fully vaccinated without them, experts have suggested that having a full round of recommended shots may offer the most protection. “My thoughts are that more shots are better, and other work has shown compelling evidence that the protective effect of vaccination on COVID-19 wanes over time,” said study coauthor Daniel Brannock, MS, a research scientist at RTI International in Research Triangle Park, N.C. “It stands to reason that the same is true for long COVID.”
- A review published in February in BMJ Medicine concluded that 10 studies showed a significant reduction in the incidence of long COVID among vaccinated patients. Even one dose of a vaccine was protective.
- A meta-analysis of six studies published last December in Antimicrobial Stewardship and Healthcare Epidemiology found that one or more doses of a COVID-19 vaccine were 29% effective in preventing symptoms of long COVID.
- In a June meta-analysis published in JAMA Internal Medicine, researchers analyzed more than 40 studies that included 860,000 patients and found that two doses of a COVID-19 vaccine reduced the risk of long COVID by almost half.
The message? COVID vaccination is very effective in reducing the risk of long COVID.
“It’s important to emphasize that many of the risk factors [for long COVID] cannot be changed, or at least cannot be changed easily, but vaccination is a decision that can be taken by everyone,” said Vassilios Vassiliou, MBBS, PhD, clinical professor of cardiac medicine at Norwich Medical School in England, who coauthored the article in JAMA Internal Medicine.
Why vaccines may be protective
The COVID-19 vaccines work well to prevent serious illness from the virus, noted Aaron Friedberg, MD, clinical coleader of the Post COVID Recovery Program at the Ohio State University Wexner Medical Center. That may be a clue to why the vaccines help prevent long COVID symptoms.
“When you get COVID and you’ve been vaccinated, the virus may still attach in your nose and respiratory tract, but it’s less likely to spread throughout your body,” he explained. “It’s like a forest fire – if the ground is wet or it starts to rain, it’s less likely to create a great blaze. As a result, your body is less likely to experience inflammation and damage that makes it more likely that you’ll develop long COVID.”
Dr. Friedberg stressed that even for patients who have had COVID, it’s important to get vaccinated – a message he consistently delivers to his own patients.
“There is some protection that comes from having COVID before, but for some people, that’s not enough,” he said. “It’s true that after infection, your body creates antibodies that help protect you against the virus. But I explain to patients that these may be like old Velcro: They barely grab on enough to stay on for the moment, but they don’t last long term. You’re much more likely to get a reliable immune response from the vaccine.”
In addition, a second or third bout of COVID could be the one that gives patients long COVID, Dr. Friedberg adds.
“I have a number of patients in my clinic who were fine after their first bout of COVID but experienced debilitating long COVID symptoms after they developed COVID again,” he said. “Why leave it to chance?”
Vaccines and ‘long vax’
The COVID vaccines are considered very safe but have been linked to very rare side effects, such as blood clots and heart inflammation. There have also been anecdotal reports of symptoms that resemble long COVID – a syndrome that has come to be known as “long Vax” – an extremely rare condition that may or may not be tied to vaccination.
“I have seen people in my clinic who developed symptoms suggestive of long COVID that linger for months – brain fog, fatigue, heart palpitations – soon after they got the COVID-19 vaccine,” said Dr. Purpura. But no published studies have suggested a link, he cautions.
A study called LISTEN is being organized at Yale in an effort to better understand postvaccine adverse events and a potential link to long COVID.
Talking to patients
Discussions of vaccination with patients, including those with COVID or long COVID, are often fraught and challenging, said Dr. Purpura.
“There’s a lot of fear that they will have a worsening of their symptoms,” he explained. The conversation he has with his patients mirrors the conversation all physicians should have with their patients about COVID-19 vaccination, even if they don’t have long COVID. He stresses the importance of highlighting the following components:
- Show compassion and empathy. “A lot of people have strongly held opinions – it’s worth it to try to find out why they feel the way that they do,” said Dr. Friedberg.
- Walk them through side effects. “Many people are afraid of the side effects of the vaccine, especially if they already have long COVID,” explained Dr. Purpura. Such patients can be asked how they felt after their last vaccination, such a shingles or flu shot. Then explain that the COVID-19 vaccine is not much different and that they may experience temporary side effects such as fatigue, headache, or a mild fever for 24-48 hours.
- Explain the benefits. Eighty-five percent of people say their health care provider is a trusted source of information on COVID-19 vaccines, according to the Kaiser Family Foundation. That trust is conducive to talks about the vaccine’s benefits, including its ability to protect against long COVID.
Other ways to reduce risk of long COVID
Vaccines can lower the chances of a patient’s developing long COVID. So can the antiviral medication nirmatrelvir (Paxlovid). A March 2023 study published in JAMA Internal Medicine included more than 280,000 people with COVID. The researchers found that vaccination reduced the risk for developing the condition by about 25%.
“I mention that study to all of my long COVID patients who become reinfected with the virus,” said Dr. Purpura. “It not only appears protective against long COVID, but since it lowers levels of virus circulating in their body, it seems to help prevent a flare-up of symptoms.”
Another treatment that may help is the diabetes drug metformin, he added.
A June 2023 study published in The Lancet Infectious Diseases found that when metformin was given within 3 days of symptom onset, the incidence of long COVID was reduced by about 41%.
“We’re still trying to wrap our brains around this one, but the thought is it may help to lower inflammation, which plays a role in long COVID,” Dr. Purpura explained. More studies need to be conducted, though, before recommending its use.
A version of this article first appeared on Medscape.com.
The COVID-19 vaccines have been a game changer for millions of people worldwide in preventing death or disability from the virus. Research suggests that they offer significant protection against long COVID.
False and unfounded claims made by some antivaccine groups that the vaccines themselves may cause long COVID persist and serve as barriers to vaccination.
To help separate the facts from falsehoods, here’s a checklist for doctors on what scientific studies have determined about vaccination and long COVID.
What the research shows
Doctors who work in long COVID clinics have for years suspected that vaccination may help protect against the development of long COVID, noted Lawrence Purpura, MD, MPH, an infectious disease specialist at New York–Presbyterian/Columbia University Irving Medical Center, who treats patients with long COVID in his clinic.
Over the past year, several large, well-conducted studies have borne out that theory, including the following studies:
- In the RECOVER study, published in May in the journal Nature Communications, researchers examined the electronic health records of more than 5 million people who had been diagnosed with COVID and found that vaccination reduced the risk that they would develop long COVID. Although the researchers didn’t compare the effects of having boosters to being fully vaccinated without them, experts have suggested that having a full round of recommended shots may offer the most protection. “My thoughts are that more shots are better, and other work has shown compelling evidence that the protective effect of vaccination on COVID-19 wanes over time,” said study coauthor Daniel Brannock, MS, a research scientist at RTI International in Research Triangle Park, N.C. “It stands to reason that the same is true for long COVID.”
- A review published in February in BMJ Medicine concluded that 10 studies showed a significant reduction in the incidence of long COVID among vaccinated patients. Even one dose of a vaccine was protective.
- A meta-analysis of six studies published last December in Antimicrobial Stewardship and Healthcare Epidemiology found that one or more doses of a COVID-19 vaccine were 29% effective in preventing symptoms of long COVID.
- In a June meta-analysis published in JAMA Internal Medicine, researchers analyzed more than 40 studies that included 860,000 patients and found that two doses of a COVID-19 vaccine reduced the risk of long COVID by almost half.
The message? COVID vaccination is very effective in reducing the risk of long COVID.
“It’s important to emphasize that many of the risk factors [for long COVID] cannot be changed, or at least cannot be changed easily, but vaccination is a decision that can be taken by everyone,” said Vassilios Vassiliou, MBBS, PhD, clinical professor of cardiac medicine at Norwich Medical School in England, who coauthored the article in JAMA Internal Medicine.
Why vaccines may be protective
The COVID-19 vaccines work well to prevent serious illness from the virus, noted Aaron Friedberg, MD, clinical coleader of the Post COVID Recovery Program at the Ohio State University Wexner Medical Center. That may be a clue to why the vaccines help prevent long COVID symptoms.
“When you get COVID and you’ve been vaccinated, the virus may still attach in your nose and respiratory tract, but it’s less likely to spread throughout your body,” he explained. “It’s like a forest fire – if the ground is wet or it starts to rain, it’s less likely to create a great blaze. As a result, your body is less likely to experience inflammation and damage that makes it more likely that you’ll develop long COVID.”
Dr. Friedberg stressed that even for patients who have had COVID, it’s important to get vaccinated – a message he consistently delivers to his own patients.
“There is some protection that comes from having COVID before, but for some people, that’s not enough,” he said. “It’s true that after infection, your body creates antibodies that help protect you against the virus. But I explain to patients that these may be like old Velcro: They barely grab on enough to stay on for the moment, but they don’t last long term. You’re much more likely to get a reliable immune response from the vaccine.”
In addition, a second or third bout of COVID could be the one that gives patients long COVID, Dr. Friedberg adds.
“I have a number of patients in my clinic who were fine after their first bout of COVID but experienced debilitating long COVID symptoms after they developed COVID again,” he said. “Why leave it to chance?”
Vaccines and ‘long vax’
The COVID vaccines are considered very safe but have been linked to very rare side effects, such as blood clots and heart inflammation. There have also been anecdotal reports of symptoms that resemble long COVID – a syndrome that has come to be known as “long Vax” – an extremely rare condition that may or may not be tied to vaccination.
“I have seen people in my clinic who developed symptoms suggestive of long COVID that linger for months – brain fog, fatigue, heart palpitations – soon after they got the COVID-19 vaccine,” said Dr. Purpura. But no published studies have suggested a link, he cautions.
A study called LISTEN is being organized at Yale in an effort to better understand postvaccine adverse events and a potential link to long COVID.
Talking to patients
Discussions of vaccination with patients, including those with COVID or long COVID, are often fraught and challenging, said Dr. Purpura.
“There’s a lot of fear that they will have a worsening of their symptoms,” he explained. The conversation he has with his patients mirrors the conversation all physicians should have with their patients about COVID-19 vaccination, even if they don’t have long COVID. He stresses the importance of highlighting the following components:
- Show compassion and empathy. “A lot of people have strongly held opinions – it’s worth it to try to find out why they feel the way that they do,” said Dr. Friedberg.
- Walk them through side effects. “Many people are afraid of the side effects of the vaccine, especially if they already have long COVID,” explained Dr. Purpura. Such patients can be asked how they felt after their last vaccination, such a shingles or flu shot. Then explain that the COVID-19 vaccine is not much different and that they may experience temporary side effects such as fatigue, headache, or a mild fever for 24-48 hours.
- Explain the benefits. Eighty-five percent of people say their health care provider is a trusted source of information on COVID-19 vaccines, according to the Kaiser Family Foundation. That trust is conducive to talks about the vaccine’s benefits, including its ability to protect against long COVID.
Other ways to reduce risk of long COVID
Vaccines can lower the chances of a patient’s developing long COVID. So can the antiviral medication nirmatrelvir (Paxlovid). A March 2023 study published in JAMA Internal Medicine included more than 280,000 people with COVID. The researchers found that vaccination reduced the risk for developing the condition by about 25%.
“I mention that study to all of my long COVID patients who become reinfected with the virus,” said Dr. Purpura. “It not only appears protective against long COVID, but since it lowers levels of virus circulating in their body, it seems to help prevent a flare-up of symptoms.”
Another treatment that may help is the diabetes drug metformin, he added.
A June 2023 study published in The Lancet Infectious Diseases found that when metformin was given within 3 days of symptom onset, the incidence of long COVID was reduced by about 41%.
“We’re still trying to wrap our brains around this one, but the thought is it may help to lower inflammation, which plays a role in long COVID,” Dr. Purpura explained. More studies need to be conducted, though, before recommending its use.
A version of this article first appeared on Medscape.com.
The COVID-19 vaccines have been a game changer for millions of people worldwide in preventing death or disability from the virus. Research suggests that they offer significant protection against long COVID.
False and unfounded claims made by some antivaccine groups that the vaccines themselves may cause long COVID persist and serve as barriers to vaccination.
To help separate the facts from falsehoods, here’s a checklist for doctors on what scientific studies have determined about vaccination and long COVID.
What the research shows
Doctors who work in long COVID clinics have for years suspected that vaccination may help protect against the development of long COVID, noted Lawrence Purpura, MD, MPH, an infectious disease specialist at New York–Presbyterian/Columbia University Irving Medical Center, who treats patients with long COVID in his clinic.
Over the past year, several large, well-conducted studies have borne out that theory, including the following studies:
- In the RECOVER study, published in May in the journal Nature Communications, researchers examined the electronic health records of more than 5 million people who had been diagnosed with COVID and found that vaccination reduced the risk that they would develop long COVID. Although the researchers didn’t compare the effects of having boosters to being fully vaccinated without them, experts have suggested that having a full round of recommended shots may offer the most protection. “My thoughts are that more shots are better, and other work has shown compelling evidence that the protective effect of vaccination on COVID-19 wanes over time,” said study coauthor Daniel Brannock, MS, a research scientist at RTI International in Research Triangle Park, N.C. “It stands to reason that the same is true for long COVID.”
- A review published in February in BMJ Medicine concluded that 10 studies showed a significant reduction in the incidence of long COVID among vaccinated patients. Even one dose of a vaccine was protective.
- A meta-analysis of six studies published last December in Antimicrobial Stewardship and Healthcare Epidemiology found that one or more doses of a COVID-19 vaccine were 29% effective in preventing symptoms of long COVID.
- In a June meta-analysis published in JAMA Internal Medicine, researchers analyzed more than 40 studies that included 860,000 patients and found that two doses of a COVID-19 vaccine reduced the risk of long COVID by almost half.
The message? COVID vaccination is very effective in reducing the risk of long COVID.
“It’s important to emphasize that many of the risk factors [for long COVID] cannot be changed, or at least cannot be changed easily, but vaccination is a decision that can be taken by everyone,” said Vassilios Vassiliou, MBBS, PhD, clinical professor of cardiac medicine at Norwich Medical School in England, who coauthored the article in JAMA Internal Medicine.
Why vaccines may be protective
The COVID-19 vaccines work well to prevent serious illness from the virus, noted Aaron Friedberg, MD, clinical coleader of the Post COVID Recovery Program at the Ohio State University Wexner Medical Center. That may be a clue to why the vaccines help prevent long COVID symptoms.
“When you get COVID and you’ve been vaccinated, the virus may still attach in your nose and respiratory tract, but it’s less likely to spread throughout your body,” he explained. “It’s like a forest fire – if the ground is wet or it starts to rain, it’s less likely to create a great blaze. As a result, your body is less likely to experience inflammation and damage that makes it more likely that you’ll develop long COVID.”
Dr. Friedberg stressed that even for patients who have had COVID, it’s important to get vaccinated – a message he consistently delivers to his own patients.
“There is some protection that comes from having COVID before, but for some people, that’s not enough,” he said. “It’s true that after infection, your body creates antibodies that help protect you against the virus. But I explain to patients that these may be like old Velcro: They barely grab on enough to stay on for the moment, but they don’t last long term. You’re much more likely to get a reliable immune response from the vaccine.”
In addition, a second or third bout of COVID could be the one that gives patients long COVID, Dr. Friedberg adds.
“I have a number of patients in my clinic who were fine after their first bout of COVID but experienced debilitating long COVID symptoms after they developed COVID again,” he said. “Why leave it to chance?”
Vaccines and ‘long vax’
The COVID vaccines are considered very safe but have been linked to very rare side effects, such as blood clots and heart inflammation. There have also been anecdotal reports of symptoms that resemble long COVID – a syndrome that has come to be known as “long Vax” – an extremely rare condition that may or may not be tied to vaccination.
“I have seen people in my clinic who developed symptoms suggestive of long COVID that linger for months – brain fog, fatigue, heart palpitations – soon after they got the COVID-19 vaccine,” said Dr. Purpura. But no published studies have suggested a link, he cautions.
A study called LISTEN is being organized at Yale in an effort to better understand postvaccine adverse events and a potential link to long COVID.
Talking to patients
Discussions of vaccination with patients, including those with COVID or long COVID, are often fraught and challenging, said Dr. Purpura.
“There’s a lot of fear that they will have a worsening of their symptoms,” he explained. The conversation he has with his patients mirrors the conversation all physicians should have with their patients about COVID-19 vaccination, even if they don’t have long COVID. He stresses the importance of highlighting the following components:
- Show compassion and empathy. “A lot of people have strongly held opinions – it’s worth it to try to find out why they feel the way that they do,” said Dr. Friedberg.
- Walk them through side effects. “Many people are afraid of the side effects of the vaccine, especially if they already have long COVID,” explained Dr. Purpura. Such patients can be asked how they felt after their last vaccination, such a shingles or flu shot. Then explain that the COVID-19 vaccine is not much different and that they may experience temporary side effects such as fatigue, headache, or a mild fever for 24-48 hours.
- Explain the benefits. Eighty-five percent of people say their health care provider is a trusted source of information on COVID-19 vaccines, according to the Kaiser Family Foundation. That trust is conducive to talks about the vaccine’s benefits, including its ability to protect against long COVID.
Other ways to reduce risk of long COVID
Vaccines can lower the chances of a patient’s developing long COVID. So can the antiviral medication nirmatrelvir (Paxlovid). A March 2023 study published in JAMA Internal Medicine included more than 280,000 people with COVID. The researchers found that vaccination reduced the risk for developing the condition by about 25%.
“I mention that study to all of my long COVID patients who become reinfected with the virus,” said Dr. Purpura. “It not only appears protective against long COVID, but since it lowers levels of virus circulating in their body, it seems to help prevent a flare-up of symptoms.”
Another treatment that may help is the diabetes drug metformin, he added.
A June 2023 study published in The Lancet Infectious Diseases found that when metformin was given within 3 days of symptom onset, the incidence of long COVID was reduced by about 41%.
“We’re still trying to wrap our brains around this one, but the thought is it may help to lower inflammation, which plays a role in long COVID,” Dr. Purpura explained. More studies need to be conducted, though, before recommending its use.
A version of this article first appeared on Medscape.com.
HPV rates skyrocket despite safe, effective vaccine
An epidemic of sexually transmitted HPV is now swirling around the United States and the United Kingdom, with some serious cases leading to oropharyngeal cancer, which can affect the back of the throat, tonsils, and tongue.
HPV is the leading cause (70%) of this oropharyngeal cancer, according to the CDC. It is the most common sexually transmitted disease in the nation, and around 3.6% of women and 10% of men report oral HPV specifically. But over the past decade, oropharyngeal cases have been steadily falling a little under 4% and 2%, respectively, according to the National Cancer Institute.
HPV is often undetectable and can clear up within a few months. But unfortunately for some, serious disease, such as throat cancer, can develop.
Studies show the HPV vaccine to be extremely effective in lowering sexually transmitted HPV cases. Yet, only 54.5% of young people aged 13-15 have taken the recommended two to three doses, according to the National Cancer Institute.
Why aren’t more young people taking the vaccine?
Low public awareness of the dangers of HPV may be behind young people’s poor vaccination rates, according to Teresa Lee, MD, of the Fox Chase Cancer Center in Philadelphia. “For example, while the link with head and neck cancers has been well-studied, the FDA labeling was not changed to reflect this as an indication until 2020,” she said.
Other reasons can include one’s socioeconomic background, poor health literacy, cultural or religious stigmas around vaccines, and lack of quality, low-cost health care, says Emmanuel Aguh, MD, a board-certified family medicine physician. “Some individuals and families are still resistant to vaccines and the noted lack of uptake.”
Doctors and other health care professionals should also be sure to tell patients of all ages about the risks of HPV infection and how well the vaccine works, Dr. Lee said. “Not everyone who is now eligible may have been offered the vaccine as a child, and the first time young adults may receive counseling on this subject may not be until they are entering a very busy period of their lives with many responsibilities – when it may be hard to fit in things like health maintenance.”
How safe is the HPV vaccine?
The Food and Drug Administration and Centers for Disease Control and Prevention have studied the HPV vaccine for years to find out how safe it is and how well it works, Dr. Aguh said. No major side effects have been reported, and the most common side effect is soreness where you get the shot (which is normal after most vaccines). Some dizziness and fainting in adolescents can also occur, so young people are usually asked to sit or lie down during the shot and for 15 minutes afterward, he said.
“Serious adverse events have not been reported at higher rates than expected following HPV vaccination, meaning there is no clear evidence they are related to the vaccine,” Dr. Lee said. “The vaccine is highly effective in decreasing rates of detectable infection with the high-risk HPV strains responsible for HPV-associated cancers.”
The HPV vaccine is largely recommended for people aged 9-26, and sometimes up to age 45, depending on the individual, Dr. Aguh said. If you are over 26, talk to your doctor about whether you should consider getting the vaccine.
“It is usually given in two doses for complete protection if taken before the 15th birthday,” Dr. Aguh said. “If taken afterward, or in those with a weak immune system, they might require three doses to be fully protected.”
The vaccine produces antibodies that can stop HPV from infecting cells and lowers your chances of catching an HPV-related cancer, such as throat cancer or cancer of the cervix, he said.
While the vaccine is not guaranteed to protect you from the more than 100 strains of HPV, it can protect you from HPV 16 and HPV 18 – two high-risk strains that cause around 70% of cervical cancers.
What is fueling the rise of HPV cases?
A misconception that oral sex is somehow a “safe and risk-free” alternative to anal or vaginal sex could be one reason, Dr. Aguh said.
“It is important to know that, with oral sex, you are exposed to many of the risks associated with vaginal intercourse, especially if you do not take any measures to protect yourself and/or your partner,” Dr. Aguh said. “[With oral sex] it is possible to end up contracting an infection like chlamydia, gonorrhea, and even HPV, leading to an increased risk of HPV-associated oropharyngeal cancers.”
A lack of public awareness of what can cause throat cancer could also explain this phenomenon. The number of people you have oral sex with, along with the age you begin sexual activity, can greatly determine your risk of the disease, according to Dr. Lee. She echoes a report by Hisham Mehanna, PhD, in The Conversation.
“For oropharyngeal cancer, the main risk factor is the number of lifetime sexual partners, especially oral sex,” wrote Dr. Mehanna, a professor at the Institute of Cancer and Genomic Sciences at the University of Birmingham (England). “Those with six or more lifetime oral-sex partners are 8.5 times more likely to develop oropharyngeal cancer than those who do not practice oral sex.”
What are symptoms of oropharyngeal cancer?
Labored breathing or swallowing, a cough that won’t go away, and crackling or hoarseness of your voice could all be signs of throat cancer. Other symptoms include earaches, swelling of the head or neck, and enlarged lymph nodes, among others, Dr. Aguh said.
“The signs and symptoms of HPV-related throat cancers can be difficult to identify and recognize, as they can be vague and are also associated with other medical conditions. Sometimes, there are no signs at all, or they are not easily noticeable due to the location,” he said.
You should go see your doctor if you have any of these ailments for an extended period.
How to reduce your risk
In addition to having six or more oral-sex partners, smoking and drinking heavily could also raise your risk of throat cancer, said Dr. Lee. Proper dental health – like seeing your dentist regularly and practicing proper oral hygiene – can also shave your risk.
“[Good dental health] can help not just with head and neck cancer risk, but with many other inflammation-related diseases,” Dr. Lee said.
Using dental dams and condoms can also be a good method of protection, Dr. Aguh said. A dental dam is a stretchy sheet of latex, or polyurethane plastic, in the shape of a square that is made for blocking body fluid to lower your risk of contracting an STD via oral sex.
Keep in mind: Even with these protections, make sure you and your partner discuss each other’s sexual history, any prior or current STDs and their preferred protection from STDs, said Dr. Aguh.
If you or your partner is being treated for an STD, consider opting out of oral sex and consulting a doctor.
The HPV vaccine is another common method of protection. The shot is “approved for prevention of nine of the most high-risk strains of HPV,” or those that are most commonly linked to cancer, according to Dr. Lee. The vaccine “reduces the frequency of infection” with these viruses, which can ultimately lower the risk of cancers linked to HPV, including cervical, anal, and vulvar and vaginal cancers, she said.
“The best time to receive treatment for prevention of disease is prior to onset of sexual intercourse,” said Dr. Lee.
To get your HPV vaccine, head to your family doctor, school- or community-based health center, or state health department, suggests the CDC.
A version of this article originally appeared on WebMD.com.
An epidemic of sexually transmitted HPV is now swirling around the United States and the United Kingdom, with some serious cases leading to oropharyngeal cancer, which can affect the back of the throat, tonsils, and tongue.
HPV is the leading cause (70%) of this oropharyngeal cancer, according to the CDC. It is the most common sexually transmitted disease in the nation, and around 3.6% of women and 10% of men report oral HPV specifically. But over the past decade, oropharyngeal cases have been steadily falling a little under 4% and 2%, respectively, according to the National Cancer Institute.
HPV is often undetectable and can clear up within a few months. But unfortunately for some, serious disease, such as throat cancer, can develop.
Studies show the HPV vaccine to be extremely effective in lowering sexually transmitted HPV cases. Yet, only 54.5% of young people aged 13-15 have taken the recommended two to three doses, according to the National Cancer Institute.
Why aren’t more young people taking the vaccine?
Low public awareness of the dangers of HPV may be behind young people’s poor vaccination rates, according to Teresa Lee, MD, of the Fox Chase Cancer Center in Philadelphia. “For example, while the link with head and neck cancers has been well-studied, the FDA labeling was not changed to reflect this as an indication until 2020,” she said.
Other reasons can include one’s socioeconomic background, poor health literacy, cultural or religious stigmas around vaccines, and lack of quality, low-cost health care, says Emmanuel Aguh, MD, a board-certified family medicine physician. “Some individuals and families are still resistant to vaccines and the noted lack of uptake.”
Doctors and other health care professionals should also be sure to tell patients of all ages about the risks of HPV infection and how well the vaccine works, Dr. Lee said. “Not everyone who is now eligible may have been offered the vaccine as a child, and the first time young adults may receive counseling on this subject may not be until they are entering a very busy period of their lives with many responsibilities – when it may be hard to fit in things like health maintenance.”
How safe is the HPV vaccine?
The Food and Drug Administration and Centers for Disease Control and Prevention have studied the HPV vaccine for years to find out how safe it is and how well it works, Dr. Aguh said. No major side effects have been reported, and the most common side effect is soreness where you get the shot (which is normal after most vaccines). Some dizziness and fainting in adolescents can also occur, so young people are usually asked to sit or lie down during the shot and for 15 minutes afterward, he said.
“Serious adverse events have not been reported at higher rates than expected following HPV vaccination, meaning there is no clear evidence they are related to the vaccine,” Dr. Lee said. “The vaccine is highly effective in decreasing rates of detectable infection with the high-risk HPV strains responsible for HPV-associated cancers.”
The HPV vaccine is largely recommended for people aged 9-26, and sometimes up to age 45, depending on the individual, Dr. Aguh said. If you are over 26, talk to your doctor about whether you should consider getting the vaccine.
“It is usually given in two doses for complete protection if taken before the 15th birthday,” Dr. Aguh said. “If taken afterward, or in those with a weak immune system, they might require three doses to be fully protected.”
The vaccine produces antibodies that can stop HPV from infecting cells and lowers your chances of catching an HPV-related cancer, such as throat cancer or cancer of the cervix, he said.
While the vaccine is not guaranteed to protect you from the more than 100 strains of HPV, it can protect you from HPV 16 and HPV 18 – two high-risk strains that cause around 70% of cervical cancers.
What is fueling the rise of HPV cases?
A misconception that oral sex is somehow a “safe and risk-free” alternative to anal or vaginal sex could be one reason, Dr. Aguh said.
“It is important to know that, with oral sex, you are exposed to many of the risks associated with vaginal intercourse, especially if you do not take any measures to protect yourself and/or your partner,” Dr. Aguh said. “[With oral sex] it is possible to end up contracting an infection like chlamydia, gonorrhea, and even HPV, leading to an increased risk of HPV-associated oropharyngeal cancers.”
A lack of public awareness of what can cause throat cancer could also explain this phenomenon. The number of people you have oral sex with, along with the age you begin sexual activity, can greatly determine your risk of the disease, according to Dr. Lee. She echoes a report by Hisham Mehanna, PhD, in The Conversation.
“For oropharyngeal cancer, the main risk factor is the number of lifetime sexual partners, especially oral sex,” wrote Dr. Mehanna, a professor at the Institute of Cancer and Genomic Sciences at the University of Birmingham (England). “Those with six or more lifetime oral-sex partners are 8.5 times more likely to develop oropharyngeal cancer than those who do not practice oral sex.”
What are symptoms of oropharyngeal cancer?
Labored breathing or swallowing, a cough that won’t go away, and crackling or hoarseness of your voice could all be signs of throat cancer. Other symptoms include earaches, swelling of the head or neck, and enlarged lymph nodes, among others, Dr. Aguh said.
“The signs and symptoms of HPV-related throat cancers can be difficult to identify and recognize, as they can be vague and are also associated with other medical conditions. Sometimes, there are no signs at all, or they are not easily noticeable due to the location,” he said.
You should go see your doctor if you have any of these ailments for an extended period.
How to reduce your risk
In addition to having six or more oral-sex partners, smoking and drinking heavily could also raise your risk of throat cancer, said Dr. Lee. Proper dental health – like seeing your dentist regularly and practicing proper oral hygiene – can also shave your risk.
“[Good dental health] can help not just with head and neck cancer risk, but with many other inflammation-related diseases,” Dr. Lee said.
Using dental dams and condoms can also be a good method of protection, Dr. Aguh said. A dental dam is a stretchy sheet of latex, or polyurethane plastic, in the shape of a square that is made for blocking body fluid to lower your risk of contracting an STD via oral sex.
Keep in mind: Even with these protections, make sure you and your partner discuss each other’s sexual history, any prior or current STDs and their preferred protection from STDs, said Dr. Aguh.
If you or your partner is being treated for an STD, consider opting out of oral sex and consulting a doctor.
The HPV vaccine is another common method of protection. The shot is “approved for prevention of nine of the most high-risk strains of HPV,” or those that are most commonly linked to cancer, according to Dr. Lee. The vaccine “reduces the frequency of infection” with these viruses, which can ultimately lower the risk of cancers linked to HPV, including cervical, anal, and vulvar and vaginal cancers, she said.
“The best time to receive treatment for prevention of disease is prior to onset of sexual intercourse,” said Dr. Lee.
To get your HPV vaccine, head to your family doctor, school- or community-based health center, or state health department, suggests the CDC.
A version of this article originally appeared on WebMD.com.
An epidemic of sexually transmitted HPV is now swirling around the United States and the United Kingdom, with some serious cases leading to oropharyngeal cancer, which can affect the back of the throat, tonsils, and tongue.
HPV is the leading cause (70%) of this oropharyngeal cancer, according to the CDC. It is the most common sexually transmitted disease in the nation, and around 3.6% of women and 10% of men report oral HPV specifically. But over the past decade, oropharyngeal cases have been steadily falling a little under 4% and 2%, respectively, according to the National Cancer Institute.
HPV is often undetectable and can clear up within a few months. But unfortunately for some, serious disease, such as throat cancer, can develop.
Studies show the HPV vaccine to be extremely effective in lowering sexually transmitted HPV cases. Yet, only 54.5% of young people aged 13-15 have taken the recommended two to three doses, according to the National Cancer Institute.
Why aren’t more young people taking the vaccine?
Low public awareness of the dangers of HPV may be behind young people’s poor vaccination rates, according to Teresa Lee, MD, of the Fox Chase Cancer Center in Philadelphia. “For example, while the link with head and neck cancers has been well-studied, the FDA labeling was not changed to reflect this as an indication until 2020,” she said.
Other reasons can include one’s socioeconomic background, poor health literacy, cultural or religious stigmas around vaccines, and lack of quality, low-cost health care, says Emmanuel Aguh, MD, a board-certified family medicine physician. “Some individuals and families are still resistant to vaccines and the noted lack of uptake.”
Doctors and other health care professionals should also be sure to tell patients of all ages about the risks of HPV infection and how well the vaccine works, Dr. Lee said. “Not everyone who is now eligible may have been offered the vaccine as a child, and the first time young adults may receive counseling on this subject may not be until they are entering a very busy period of their lives with many responsibilities – when it may be hard to fit in things like health maintenance.”
How safe is the HPV vaccine?
The Food and Drug Administration and Centers for Disease Control and Prevention have studied the HPV vaccine for years to find out how safe it is and how well it works, Dr. Aguh said. No major side effects have been reported, and the most common side effect is soreness where you get the shot (which is normal after most vaccines). Some dizziness and fainting in adolescents can also occur, so young people are usually asked to sit or lie down during the shot and for 15 minutes afterward, he said.
“Serious adverse events have not been reported at higher rates than expected following HPV vaccination, meaning there is no clear evidence they are related to the vaccine,” Dr. Lee said. “The vaccine is highly effective in decreasing rates of detectable infection with the high-risk HPV strains responsible for HPV-associated cancers.”
The HPV vaccine is largely recommended for people aged 9-26, and sometimes up to age 45, depending on the individual, Dr. Aguh said. If you are over 26, talk to your doctor about whether you should consider getting the vaccine.
“It is usually given in two doses for complete protection if taken before the 15th birthday,” Dr. Aguh said. “If taken afterward, or in those with a weak immune system, they might require three doses to be fully protected.”
The vaccine produces antibodies that can stop HPV from infecting cells and lowers your chances of catching an HPV-related cancer, such as throat cancer or cancer of the cervix, he said.
While the vaccine is not guaranteed to protect you from the more than 100 strains of HPV, it can protect you from HPV 16 and HPV 18 – two high-risk strains that cause around 70% of cervical cancers.
What is fueling the rise of HPV cases?
A misconception that oral sex is somehow a “safe and risk-free” alternative to anal or vaginal sex could be one reason, Dr. Aguh said.
“It is important to know that, with oral sex, you are exposed to many of the risks associated with vaginal intercourse, especially if you do not take any measures to protect yourself and/or your partner,” Dr. Aguh said. “[With oral sex] it is possible to end up contracting an infection like chlamydia, gonorrhea, and even HPV, leading to an increased risk of HPV-associated oropharyngeal cancers.”
A lack of public awareness of what can cause throat cancer could also explain this phenomenon. The number of people you have oral sex with, along with the age you begin sexual activity, can greatly determine your risk of the disease, according to Dr. Lee. She echoes a report by Hisham Mehanna, PhD, in The Conversation.
“For oropharyngeal cancer, the main risk factor is the number of lifetime sexual partners, especially oral sex,” wrote Dr. Mehanna, a professor at the Institute of Cancer and Genomic Sciences at the University of Birmingham (England). “Those with six or more lifetime oral-sex partners are 8.5 times more likely to develop oropharyngeal cancer than those who do not practice oral sex.”
What are symptoms of oropharyngeal cancer?
Labored breathing or swallowing, a cough that won’t go away, and crackling or hoarseness of your voice could all be signs of throat cancer. Other symptoms include earaches, swelling of the head or neck, and enlarged lymph nodes, among others, Dr. Aguh said.
“The signs and symptoms of HPV-related throat cancers can be difficult to identify and recognize, as they can be vague and are also associated with other medical conditions. Sometimes, there are no signs at all, or they are not easily noticeable due to the location,” he said.
You should go see your doctor if you have any of these ailments for an extended period.
How to reduce your risk
In addition to having six or more oral-sex partners, smoking and drinking heavily could also raise your risk of throat cancer, said Dr. Lee. Proper dental health – like seeing your dentist regularly and practicing proper oral hygiene – can also shave your risk.
“[Good dental health] can help not just with head and neck cancer risk, but with many other inflammation-related diseases,” Dr. Lee said.
Using dental dams and condoms can also be a good method of protection, Dr. Aguh said. A dental dam is a stretchy sheet of latex, or polyurethane plastic, in the shape of a square that is made for blocking body fluid to lower your risk of contracting an STD via oral sex.
Keep in mind: Even with these protections, make sure you and your partner discuss each other’s sexual history, any prior or current STDs and their preferred protection from STDs, said Dr. Aguh.
If you or your partner is being treated for an STD, consider opting out of oral sex and consulting a doctor.
The HPV vaccine is another common method of protection. The shot is “approved for prevention of nine of the most high-risk strains of HPV,” or those that are most commonly linked to cancer, according to Dr. Lee. The vaccine “reduces the frequency of infection” with these viruses, which can ultimately lower the risk of cancers linked to HPV, including cervical, anal, and vulvar and vaginal cancers, she said.
“The best time to receive treatment for prevention of disease is prior to onset of sexual intercourse,” said Dr. Lee.
To get your HPV vaccine, head to your family doctor, school- or community-based health center, or state health department, suggests the CDC.
A version of this article originally appeared on WebMD.com.
CDC signs off on RSV vaccine for older adults
The Centers for Disease Control and Prevention has given a green light to two new vaccines to protect against respiratory syncytial virus, or RSV, in older adults.
CDC Director Rochelle P. Walensky, MD, MPH, agreed with and endorsed the recommendations made earlier by CDC advisors that people age 60 and over may get one of two new vaccines for RSV. Decisions should be made based on discussions with one’s health care provider about whether the vaccine is right for them, the federal health agency said.
The new vaccines, the first licensed in the United States to protect against the respiratory illness, are expected to be available this fall.
On June 21, the CDC’s Advisory Committee on Immunization Practices (ACIP), an independent panel, stopped short of recommending the vaccines for everyone age 65 and above, which was the original question the committee was to consider. The experts amended that question, changing it to whether the panel should recommend the vaccine for those 65 and above if the person and their doctor agreed. The committee voted 9 to 5 in favor.
RSV vaccines
RSV leads to 6,000 to 10,000 deaths a year in the United States among those age 65 and older and 60,000 to 160,000 hospitalizations in that group. Seniors and infants are among the most vulnerable to the lower respiratory infection, marked by runny nose, wheezing, sneezing, decreased appetite, and fever.
The FDA in May approved two vaccines — GSK’s Arexvy and Pfizer’s Abrysvo — for adults age 60 and above.
The vote recommending shared decision-making about the vaccine, instead of a routine vaccination recommended for all, “is a weaker recommendation,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center in Nashville and medical director of the National Foundation for Infectious Diseases. Dr. Schaffner is a non-voting member of ACIP. He attended the meeting.
He said the experts voiced concern about a number of issues, including what some saw as a lack of sufficient data from trials on the most vulnerable groups, such as nursing home residents.
Experts also wanted more information about the duration of protection and exactly when a second dose might be needed. At the meeting, a GSK official said its vaccine was 84.6% effective after one and a half seasons, down from 94.1% after one season. A Pfizer official said its vaccine decreased the risk of RSV with three or more symptoms by 78.6% after a season and a half, down from 88.9% after one season.
The panel also wanted more data on whether the RSV vaccines could be administered at the same time as other vaccines recommended for adults.
Both companies gave a range of cost estimates. Pfizer expects its vaccine to cost $180 to $270 but said it could not guarantee that range. GSK said it expects a price of $200 to $295. Under the Inflation Reduction Act, recommended vaccines are covered under Medicare for those with Part D plans, which 51 million of 65 million Medicare patients have. Commercial insurance is likely to cover the vaccines if the CDC recommends them.
A version of this article first appeared on WebMD.com.
This article was updated 7/5/23.
The Centers for Disease Control and Prevention has given a green light to two new vaccines to protect against respiratory syncytial virus, or RSV, in older adults.
CDC Director Rochelle P. Walensky, MD, MPH, agreed with and endorsed the recommendations made earlier by CDC advisors that people age 60 and over may get one of two new vaccines for RSV. Decisions should be made based on discussions with one’s health care provider about whether the vaccine is right for them, the federal health agency said.
The new vaccines, the first licensed in the United States to protect against the respiratory illness, are expected to be available this fall.
On June 21, the CDC’s Advisory Committee on Immunization Practices (ACIP), an independent panel, stopped short of recommending the vaccines for everyone age 65 and above, which was the original question the committee was to consider. The experts amended that question, changing it to whether the panel should recommend the vaccine for those 65 and above if the person and their doctor agreed. The committee voted 9 to 5 in favor.
RSV vaccines
RSV leads to 6,000 to 10,000 deaths a year in the United States among those age 65 and older and 60,000 to 160,000 hospitalizations in that group. Seniors and infants are among the most vulnerable to the lower respiratory infection, marked by runny nose, wheezing, sneezing, decreased appetite, and fever.
The FDA in May approved two vaccines — GSK’s Arexvy and Pfizer’s Abrysvo — for adults age 60 and above.
The vote recommending shared decision-making about the vaccine, instead of a routine vaccination recommended for all, “is a weaker recommendation,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center in Nashville and medical director of the National Foundation for Infectious Diseases. Dr. Schaffner is a non-voting member of ACIP. He attended the meeting.
He said the experts voiced concern about a number of issues, including what some saw as a lack of sufficient data from trials on the most vulnerable groups, such as nursing home residents.
Experts also wanted more information about the duration of protection and exactly when a second dose might be needed. At the meeting, a GSK official said its vaccine was 84.6% effective after one and a half seasons, down from 94.1% after one season. A Pfizer official said its vaccine decreased the risk of RSV with three or more symptoms by 78.6% after a season and a half, down from 88.9% after one season.
The panel also wanted more data on whether the RSV vaccines could be administered at the same time as other vaccines recommended for adults.
Both companies gave a range of cost estimates. Pfizer expects its vaccine to cost $180 to $270 but said it could not guarantee that range. GSK said it expects a price of $200 to $295. Under the Inflation Reduction Act, recommended vaccines are covered under Medicare for those with Part D plans, which 51 million of 65 million Medicare patients have. Commercial insurance is likely to cover the vaccines if the CDC recommends them.
A version of this article first appeared on WebMD.com.
This article was updated 7/5/23.
The Centers for Disease Control and Prevention has given a green light to two new vaccines to protect against respiratory syncytial virus, or RSV, in older adults.
CDC Director Rochelle P. Walensky, MD, MPH, agreed with and endorsed the recommendations made earlier by CDC advisors that people age 60 and over may get one of two new vaccines for RSV. Decisions should be made based on discussions with one’s health care provider about whether the vaccine is right for them, the federal health agency said.
The new vaccines, the first licensed in the United States to protect against the respiratory illness, are expected to be available this fall.
On June 21, the CDC’s Advisory Committee on Immunization Practices (ACIP), an independent panel, stopped short of recommending the vaccines for everyone age 65 and above, which was the original question the committee was to consider. The experts amended that question, changing it to whether the panel should recommend the vaccine for those 65 and above if the person and their doctor agreed. The committee voted 9 to 5 in favor.
RSV vaccines
RSV leads to 6,000 to 10,000 deaths a year in the United States among those age 65 and older and 60,000 to 160,000 hospitalizations in that group. Seniors and infants are among the most vulnerable to the lower respiratory infection, marked by runny nose, wheezing, sneezing, decreased appetite, and fever.
The FDA in May approved two vaccines — GSK’s Arexvy and Pfizer’s Abrysvo — for adults age 60 and above.
The vote recommending shared decision-making about the vaccine, instead of a routine vaccination recommended for all, “is a weaker recommendation,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center in Nashville and medical director of the National Foundation for Infectious Diseases. Dr. Schaffner is a non-voting member of ACIP. He attended the meeting.
He said the experts voiced concern about a number of issues, including what some saw as a lack of sufficient data from trials on the most vulnerable groups, such as nursing home residents.
Experts also wanted more information about the duration of protection and exactly when a second dose might be needed. At the meeting, a GSK official said its vaccine was 84.6% effective after one and a half seasons, down from 94.1% after one season. A Pfizer official said its vaccine decreased the risk of RSV with three or more symptoms by 78.6% after a season and a half, down from 88.9% after one season.
The panel also wanted more data on whether the RSV vaccines could be administered at the same time as other vaccines recommended for adults.
Both companies gave a range of cost estimates. Pfizer expects its vaccine to cost $180 to $270 but said it could not guarantee that range. GSK said it expects a price of $200 to $295. Under the Inflation Reduction Act, recommended vaccines are covered under Medicare for those with Part D plans, which 51 million of 65 million Medicare patients have. Commercial insurance is likely to cover the vaccines if the CDC recommends them.
A version of this article first appeared on WebMD.com.
This article was updated 7/5/23.
FDA panel backs new COVID booster focusing only on variants
but questioned whether the population as a whole needs booster shots and how often they should be given.
The Vaccines and Related Biological Products Advisory Committee of the FDA voted 21-0 in favor of the recommendation about the strain to be used in the next crop of vaccines.
In the briefing document for the meeting, FDA staff said the available evidence suggests that a monovalent (single-strain) XBB-lineage vaccine “is warranted” for the 2023-2024 vaccination campaign and would replace the current bivalent vaccine, which targets the original version of the virus and two strains from the Omicron variant.
FDA staff also noted how such a shift would be in line with the World Health Organization toward targeting the XBB family of subvariants. European regulators have done this as well.
The FDA is not obligated to act on the panel’s recommendations. But the agency often does and is highly likely to do so in this case. Vaccine companies will need the recommendation from the FDA to begin making vaccines for the fall.
New shot every year?
The FDA asked its expert panel to vote only on the question about the makeup of future vaccines in terms of which strain to include.
But panelists also raised other questions during the meeting, including concerns about moves toward tying COVID vaccinations into the model of annual flu shots.
Paul Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, argued for greater focus on the response of T cells after vaccination, even in light of the already recognized waning of antibody protection.
In a recent Substack article, Dr. Offit called T cells the “unsung hero” of the pandemic. They take longer to develop after infection or vaccination than the antibodies that first attack the virus, but immune memory cells called B and T cells “are long-lived,” and their “protection against severe disease often lasts for years and sometimes decades.”
Dr. Offit said he was concerned about using a blanket approach for future recommendations for COVID vaccinations, following the one now in place for influenza vaccines.
The Centers for Disease Control and Prevention recommends flu shots for everyone 6 months and older, with rare exceptions.
“We need to continue to define who those high-risk groups are and not make this a recommendation for everybody every season,” he said.
Dr. Offit offered his own experience as an example. While he had been vaccinated against the virus’s early Wuhan strain, he still was infected, most likely with a variant that emerged later.
“That was a drifted virus. That’s why I had a mild infection but I didn’t have a severe infection, because presumably I had T cells which prevented that severe infection, which may last for years,” Dr. Offit said.
Pfizer and Moderna, the two companies that make mRNA-based COVID vaccines, are working on experimental products meant to protect against both flu and SARS-COv-2 in one shot. Novavax, maker of a more traditional protein-based COVID shot, is doing the same.
The idea of these combination products is to make it more convenient for people to protect against both viruses, while also offering companies some marketing advantages.
But without referring to these drugmakers’ plans for future combo flu-COVID shots, members of the FDA panel raised objections to an assumption of routine annual vaccines against variants of SARS-CoV-2.
Among the panelists who expressed concerns was Henry H. Bernstein, DO, a former member of the CDC’s Advisory Committee on Immunization Practices.
Bernstein questioned the approach of dubbing these the “2023-2024 formulas,” as this approach conveyed a sense of an expectation for a need for annual vaccines, as happens with flu.
“It’s not clear to me that this is a seasonal virus yet,” said Dr. Bernstein, who is also a professor of pediatrics at Hofstra University, Hempstead, N.Y..
In response to Dr. Bernstein’s point, Arnold Monto, MD, the acting chair of the FDA panel, suggested such a pattern could emerge, while also agreeing that it’s too soon to say for sure.
A professor emeritus at the University of Michigan, Ann Arbor, Dr. Monto’s career included pandemic planning and emergency response to virus outbreaks, including the 1968 Hong Kong influenza pandemic, avian influenza, and the original SARS.
“I think it’s premature to say that this virus will not become seasonal,” Dr. Monto said about SARS-CoV-2. “I agree. We’re not there yet, but we may be.”
At the end of the meeting, Dr. Monto recapped the meeting’s key points, noting that there was a general consensus that the XBB.1.5 subvariant would be the best to use in future COVID shots.
He also noted that Novavax, which makes the more traditional protein-based vaccine, along with Pfizer and Moderna, already have honed in on this subvariant, which would allow for rapid development of updated COVID vaccines.
“The fact that most of the manufacturers are ready to work on an XBB 1.5 [vaccine] is an added reason to select this strain or this variant, given the immunologic data,” Dr. Monto said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said the demands involved in manufacturing vaccines tilts toward annual changes.
“Practically, we’re going to have one update per year, barring a heroic effort to deal with a strain that pops up that is essentially so different that it requires us to mobilize tremendous resources to address that strain change,” he said.
Dr. Marks questioned the panelists’ concerns about likening flu and COVID vaccination practices. The FDA staff’s intent was to try to help the public understand the need for follow-on vaccination.
“I’m really having trouble understanding that committee’s need to bristle against something that’s similar to influenza. People understand a yearly influenza vaccine,” Dr. Marks said.
And it’s not certain when another major change in the COVID virus will follow the XBB subvariant, but it’s likely one will – and soon, Dr. Marks said.
“It looks like, probably by next fall, there’ll be further drift from this,” he said.
Informing the public
Dr. Marks also stressed the need to better convey the benefits of vaccination to people in the United States.
CDC data estimate that 70% of the U.S. population completed an initial series of the original monovalent vaccines, with only 17% then getting bivalent shots. There’s even a decline among people ages 65 and older. CDC estimates 94% of this group completed their primary series, but only 43% got the bivalent booster dose.
“We have to do better because we have not done a good job today communicating to the American public what’s going on here,” Marks said.
Researchers also are still trying to determine the best timing for people to get additional COVID shots. Finding the “sweet spot” where people can maximize additional protection is tricky, with people most protected if they happen to get shot near the beginning of an uptick in viral spread, the CDC’s Ruth Link-Gelles, PhD, MPH, told the panel during a presentation.
“You’re going to get the best incremental benefit if it’s been longer since your last vaccine,” she said. “But of course, if you wait too long since your last vaccine, you’re left with very little protection, and so you’re at higher risk of severe illness.”
Like Dr. Marks, Dr. Link-Gelles stressed the need for persuading more people to get follow-on vaccines.
“Most Americans, at this point, haven’t even received the bivalent and so are a year or more out from their monovalent dose and so have relatively little protection left,” she said.
A version of this article first appeared on WebMD.com.
but questioned whether the population as a whole needs booster shots and how often they should be given.
The Vaccines and Related Biological Products Advisory Committee of the FDA voted 21-0 in favor of the recommendation about the strain to be used in the next crop of vaccines.
In the briefing document for the meeting, FDA staff said the available evidence suggests that a monovalent (single-strain) XBB-lineage vaccine “is warranted” for the 2023-2024 vaccination campaign and would replace the current bivalent vaccine, which targets the original version of the virus and two strains from the Omicron variant.
FDA staff also noted how such a shift would be in line with the World Health Organization toward targeting the XBB family of subvariants. European regulators have done this as well.
The FDA is not obligated to act on the panel’s recommendations. But the agency often does and is highly likely to do so in this case. Vaccine companies will need the recommendation from the FDA to begin making vaccines for the fall.
New shot every year?
The FDA asked its expert panel to vote only on the question about the makeup of future vaccines in terms of which strain to include.
But panelists also raised other questions during the meeting, including concerns about moves toward tying COVID vaccinations into the model of annual flu shots.
Paul Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, argued for greater focus on the response of T cells after vaccination, even in light of the already recognized waning of antibody protection.
In a recent Substack article, Dr. Offit called T cells the “unsung hero” of the pandemic. They take longer to develop after infection or vaccination than the antibodies that first attack the virus, but immune memory cells called B and T cells “are long-lived,” and their “protection against severe disease often lasts for years and sometimes decades.”
Dr. Offit said he was concerned about using a blanket approach for future recommendations for COVID vaccinations, following the one now in place for influenza vaccines.
The Centers for Disease Control and Prevention recommends flu shots for everyone 6 months and older, with rare exceptions.
“We need to continue to define who those high-risk groups are and not make this a recommendation for everybody every season,” he said.
Dr. Offit offered his own experience as an example. While he had been vaccinated against the virus’s early Wuhan strain, he still was infected, most likely with a variant that emerged later.
“That was a drifted virus. That’s why I had a mild infection but I didn’t have a severe infection, because presumably I had T cells which prevented that severe infection, which may last for years,” Dr. Offit said.
Pfizer and Moderna, the two companies that make mRNA-based COVID vaccines, are working on experimental products meant to protect against both flu and SARS-COv-2 in one shot. Novavax, maker of a more traditional protein-based COVID shot, is doing the same.
The idea of these combination products is to make it more convenient for people to protect against both viruses, while also offering companies some marketing advantages.
But without referring to these drugmakers’ plans for future combo flu-COVID shots, members of the FDA panel raised objections to an assumption of routine annual vaccines against variants of SARS-CoV-2.
Among the panelists who expressed concerns was Henry H. Bernstein, DO, a former member of the CDC’s Advisory Committee on Immunization Practices.
Bernstein questioned the approach of dubbing these the “2023-2024 formulas,” as this approach conveyed a sense of an expectation for a need for annual vaccines, as happens with flu.
“It’s not clear to me that this is a seasonal virus yet,” said Dr. Bernstein, who is also a professor of pediatrics at Hofstra University, Hempstead, N.Y..
In response to Dr. Bernstein’s point, Arnold Monto, MD, the acting chair of the FDA panel, suggested such a pattern could emerge, while also agreeing that it’s too soon to say for sure.
A professor emeritus at the University of Michigan, Ann Arbor, Dr. Monto’s career included pandemic planning and emergency response to virus outbreaks, including the 1968 Hong Kong influenza pandemic, avian influenza, and the original SARS.
“I think it’s premature to say that this virus will not become seasonal,” Dr. Monto said about SARS-CoV-2. “I agree. We’re not there yet, but we may be.”
At the end of the meeting, Dr. Monto recapped the meeting’s key points, noting that there was a general consensus that the XBB.1.5 subvariant would be the best to use in future COVID shots.
He also noted that Novavax, which makes the more traditional protein-based vaccine, along with Pfizer and Moderna, already have honed in on this subvariant, which would allow for rapid development of updated COVID vaccines.
“The fact that most of the manufacturers are ready to work on an XBB 1.5 [vaccine] is an added reason to select this strain or this variant, given the immunologic data,” Dr. Monto said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said the demands involved in manufacturing vaccines tilts toward annual changes.
“Practically, we’re going to have one update per year, barring a heroic effort to deal with a strain that pops up that is essentially so different that it requires us to mobilize tremendous resources to address that strain change,” he said.
Dr. Marks questioned the panelists’ concerns about likening flu and COVID vaccination practices. The FDA staff’s intent was to try to help the public understand the need for follow-on vaccination.
“I’m really having trouble understanding that committee’s need to bristle against something that’s similar to influenza. People understand a yearly influenza vaccine,” Dr. Marks said.
And it’s not certain when another major change in the COVID virus will follow the XBB subvariant, but it’s likely one will – and soon, Dr. Marks said.
“It looks like, probably by next fall, there’ll be further drift from this,” he said.
Informing the public
Dr. Marks also stressed the need to better convey the benefits of vaccination to people in the United States.
CDC data estimate that 70% of the U.S. population completed an initial series of the original monovalent vaccines, with only 17% then getting bivalent shots. There’s even a decline among people ages 65 and older. CDC estimates 94% of this group completed their primary series, but only 43% got the bivalent booster dose.
“We have to do better because we have not done a good job today communicating to the American public what’s going on here,” Marks said.
Researchers also are still trying to determine the best timing for people to get additional COVID shots. Finding the “sweet spot” where people can maximize additional protection is tricky, with people most protected if they happen to get shot near the beginning of an uptick in viral spread, the CDC’s Ruth Link-Gelles, PhD, MPH, told the panel during a presentation.
“You’re going to get the best incremental benefit if it’s been longer since your last vaccine,” she said. “But of course, if you wait too long since your last vaccine, you’re left with very little protection, and so you’re at higher risk of severe illness.”
Like Dr. Marks, Dr. Link-Gelles stressed the need for persuading more people to get follow-on vaccines.
“Most Americans, at this point, haven’t even received the bivalent and so are a year or more out from their monovalent dose and so have relatively little protection left,” she said.
A version of this article first appeared on WebMD.com.
but questioned whether the population as a whole needs booster shots and how often they should be given.
The Vaccines and Related Biological Products Advisory Committee of the FDA voted 21-0 in favor of the recommendation about the strain to be used in the next crop of vaccines.
In the briefing document for the meeting, FDA staff said the available evidence suggests that a monovalent (single-strain) XBB-lineage vaccine “is warranted” for the 2023-2024 vaccination campaign and would replace the current bivalent vaccine, which targets the original version of the virus and two strains from the Omicron variant.
FDA staff also noted how such a shift would be in line with the World Health Organization toward targeting the XBB family of subvariants. European regulators have done this as well.
The FDA is not obligated to act on the panel’s recommendations. But the agency often does and is highly likely to do so in this case. Vaccine companies will need the recommendation from the FDA to begin making vaccines for the fall.
New shot every year?
The FDA asked its expert panel to vote only on the question about the makeup of future vaccines in terms of which strain to include.
But panelists also raised other questions during the meeting, including concerns about moves toward tying COVID vaccinations into the model of annual flu shots.
Paul Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, argued for greater focus on the response of T cells after vaccination, even in light of the already recognized waning of antibody protection.
In a recent Substack article, Dr. Offit called T cells the “unsung hero” of the pandemic. They take longer to develop after infection or vaccination than the antibodies that first attack the virus, but immune memory cells called B and T cells “are long-lived,” and their “protection against severe disease often lasts for years and sometimes decades.”
Dr. Offit said he was concerned about using a blanket approach for future recommendations for COVID vaccinations, following the one now in place for influenza vaccines.
The Centers for Disease Control and Prevention recommends flu shots for everyone 6 months and older, with rare exceptions.
“We need to continue to define who those high-risk groups are and not make this a recommendation for everybody every season,” he said.
Dr. Offit offered his own experience as an example. While he had been vaccinated against the virus’s early Wuhan strain, he still was infected, most likely with a variant that emerged later.
“That was a drifted virus. That’s why I had a mild infection but I didn’t have a severe infection, because presumably I had T cells which prevented that severe infection, which may last for years,” Dr. Offit said.
Pfizer and Moderna, the two companies that make mRNA-based COVID vaccines, are working on experimental products meant to protect against both flu and SARS-COv-2 in one shot. Novavax, maker of a more traditional protein-based COVID shot, is doing the same.
The idea of these combination products is to make it more convenient for people to protect against both viruses, while also offering companies some marketing advantages.
But without referring to these drugmakers’ plans for future combo flu-COVID shots, members of the FDA panel raised objections to an assumption of routine annual vaccines against variants of SARS-CoV-2.
Among the panelists who expressed concerns was Henry H. Bernstein, DO, a former member of the CDC’s Advisory Committee on Immunization Practices.
Bernstein questioned the approach of dubbing these the “2023-2024 formulas,” as this approach conveyed a sense of an expectation for a need for annual vaccines, as happens with flu.
“It’s not clear to me that this is a seasonal virus yet,” said Dr. Bernstein, who is also a professor of pediatrics at Hofstra University, Hempstead, N.Y..
In response to Dr. Bernstein’s point, Arnold Monto, MD, the acting chair of the FDA panel, suggested such a pattern could emerge, while also agreeing that it’s too soon to say for sure.
A professor emeritus at the University of Michigan, Ann Arbor, Dr. Monto’s career included pandemic planning and emergency response to virus outbreaks, including the 1968 Hong Kong influenza pandemic, avian influenza, and the original SARS.
“I think it’s premature to say that this virus will not become seasonal,” Dr. Monto said about SARS-CoV-2. “I agree. We’re not there yet, but we may be.”
At the end of the meeting, Dr. Monto recapped the meeting’s key points, noting that there was a general consensus that the XBB.1.5 subvariant would be the best to use in future COVID shots.
He also noted that Novavax, which makes the more traditional protein-based vaccine, along with Pfizer and Moderna, already have honed in on this subvariant, which would allow for rapid development of updated COVID vaccines.
“The fact that most of the manufacturers are ready to work on an XBB 1.5 [vaccine] is an added reason to select this strain or this variant, given the immunologic data,” Dr. Monto said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said the demands involved in manufacturing vaccines tilts toward annual changes.
“Practically, we’re going to have one update per year, barring a heroic effort to deal with a strain that pops up that is essentially so different that it requires us to mobilize tremendous resources to address that strain change,” he said.
Dr. Marks questioned the panelists’ concerns about likening flu and COVID vaccination practices. The FDA staff’s intent was to try to help the public understand the need for follow-on vaccination.
“I’m really having trouble understanding that committee’s need to bristle against something that’s similar to influenza. People understand a yearly influenza vaccine,” Dr. Marks said.
And it’s not certain when another major change in the COVID virus will follow the XBB subvariant, but it’s likely one will – and soon, Dr. Marks said.
“It looks like, probably by next fall, there’ll be further drift from this,” he said.
Informing the public
Dr. Marks also stressed the need to better convey the benefits of vaccination to people in the United States.
CDC data estimate that 70% of the U.S. population completed an initial series of the original monovalent vaccines, with only 17% then getting bivalent shots. There’s even a decline among people ages 65 and older. CDC estimates 94% of this group completed their primary series, but only 43% got the bivalent booster dose.
“We have to do better because we have not done a good job today communicating to the American public what’s going on here,” Marks said.
Researchers also are still trying to determine the best timing for people to get additional COVID shots. Finding the “sweet spot” where people can maximize additional protection is tricky, with people most protected if they happen to get shot near the beginning of an uptick in viral spread, the CDC’s Ruth Link-Gelles, PhD, MPH, told the panel during a presentation.
“You’re going to get the best incremental benefit if it’s been longer since your last vaccine,” she said. “But of course, if you wait too long since your last vaccine, you’re left with very little protection, and so you’re at higher risk of severe illness.”
Like Dr. Marks, Dr. Link-Gelles stressed the need for persuading more people to get follow-on vaccines.
“Most Americans, at this point, haven’t even received the bivalent and so are a year or more out from their monovalent dose and so have relatively little protection left,” she said.
A version of this article first appeared on WebMD.com.
Latest data: COVID vaccine safety, protection, and breakthrough infections in inflammatory, autoimmune diseases
MILAN – The impact of the COVID-19 pandemic on patients with rheumatic and nonrheumatic autoimmune diseases is ongoing and not yet fully comprehended. New data presented at the annual European Congress of Rheumatology, primarily derived from the global COVID-19 in Autoimmune Diseases (COVAD) survey but not limited to it, provide reassurance regarding the protection and safety of COVID-19 vaccines for older and younger adults, as well as for pregnant and breastfeeding women. These data also explore the influence of underlying diseases and medications on breakthrough SARS-CoV-2 infections and infection outcomes.
Safety of vaccines in patients with autoimmune or immune-mediated diseases
Following vaccination, even with low levels of antibodies, the risk of severe COVID-19 remains relatively low for patients who receive immunosuppressive therapy for various immune-mediated inflammatory diseases (IMIDs). This encouraging finding comes from the Nor-vaC study, presented by Hilde Ørbo, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Diakonhjemmet Hospital, Oslo.
During the presentation, Dr. Ørbo stated: “We did not find any specific diagnosis or medication associated with a significantly higher risk of hospitalization.” Receiving booster doses of the vaccine, having high levels of anti-spike antibodies after vaccination, and achieving hybrid immunity are correlated with further reductions in the risk of breakthrough SARS-CoV-2 infections.
Between Feb. 15, 2021, and Feb. 15, 2023, COVID-19 affected a similar proportion among the 729 patients and 350 healthy control persons (67% and 68%, respectively). Among the patients, 22 reported severe COVID-19, whereas none of the healthy control persons did. However, there were no fatalities among the patients. The study cohort consisted of patients with various IMIDs; 70% had an inflammatory joint disease. The use of immunosuppressive medications also varied, with 63% of patients using tumor necrosis factor inhibitors, either as monotherapy or in combination with other treatments, and other patients taking medications such as methotrexate, interleukin inhibitors, Janus kinase inhibitors, vedolizumab (Entyvio), and others.
While being older than 70 years and the presence of comorbidities were identified as risk factors for severe COVID-19, there was a significant reduction in risk with each additional vaccine dose. These results support the protective role of repeated COVID-19 vaccination for patients with IMIDs who are receiving immunosuppressive therapies; they yield a favorable prognosis even with the Omicron variant.
The study further compared the risk of severe COVID-19 between a group with hybrid immunity (having received three vaccine doses and experiencing breakthrough infection with the Omicron variant) and a group that received a fourth vaccine dose within the same time frame. The difference was striking: Hybrid immunity was associated with a 5.8-fold decrease in risk, compared with four-dose vaccination (P < .0001).
The level of antibodies, measured 2-4 weeks after the last vaccination, was predictive of the risk of breakthrough COVID-19. An antibody level above 6000 binding antibody units/mL after vaccination was significantly associated with a reduction in risk. “We can conclude that patients who receive multiple vaccine doses have a lower risk of COVID-19,” Dr. Ørbo said. “In patients who recently experienced breakthrough infections, the administration of a booster vaccine dose might be delayed.”
“The virus has undergone changes throughout the pandemic, while the vaccines have remained relatively stable. Are we anticipating more infections over time?” asked Hendrik Schulze-Koops, MD, PhD, of Ludwig Maximilians University of Munich (Germany), the session moderator. In response, Dr. Ørbo stated that 85% of the recorded infections in the study occurred after the emergence of the Omicron variant, and time was considered a covariable in the analysis.
These data shed light on a topic discussed by Pedro Machado, MD, PhD, professor and consultant in rheumatology and neuromuscular diseases at University College London, during his scientific session talk entitled, “Unsolved Issues of COVID Vaccination and Re-vaccination.” Dr. Machado referred to the VROOM study published in 2022, which examined the interruption of methotrexate for 2 weeks following booster administration. Both groups demonstrated a significant antibody response, but the group that stopped taking methotrexate showed double the antibody titers.
However, he emphasized, “what remains unknown is the clinical relevance of these differences in terms of severe infection, hospitalization, or even death. The potential benefit of increased immunogenicity by interrupting conventional synthetic disease-modifying antirheumatic drugs [csDMARDs] such as methotrexate before or after vaccination needs to be balanced against the potential risk of disease flare. Ultimately, decision-making should be individualized based on factors such as comorbidities, disease activity, and other considerations.” The results presented by Dr. Ørbo suggest that, while there may be a clinical difference in terms of severe infection, the overall prognosis for vaccinated patients is reasonably good.
Regarding other DMARDs, such as biologics, the approach may differ. Dr. Machado suggested: “In patients using rituximab or other B cell–depleting therapies, SARS-CoV-2 vaccination should be scheduled in a way that optimizes vaccine immunogenicity. A minimum of 10 B cells/mcL of blood is likely a relevant threshold above which a sufficient cellular and immune response is established.”
COVID vaccines are safe for pregnant and breastfeeding women
According to data from the COVAD study, which comprised two global cross-sectional surveys conducted in 2021 and 2022, the COVID-19 vaccine appeared safe for pregnant and breastfeeding women with autoimmune diseases (AID).
Presenter Laura Andreoli, MD, PhD, of the University of Brescia (Italy), said that, although pregnant patients with AID reported more adverse events related to vaccination, these rates were not significantly higher than those among pregnant, healthy control persons who were without AID. No difference in adverse events was observed between breastfeeding women and healthy control persons, and the incidence of disease flares did not significantly differ among all groups.
“In summary, this study provides initial insights into the safety of COVID-19 vaccination during the gestational and postpartum periods in women with autoimmune diseases. These reassuring observations will hopefully improve clinician-patient communication and address hesitancy towards COVID-19 vaccination, as the benefits for the mother and fetus through passive immunization appear to outweigh potential risks,” Dr. Andreoli said in an interview.
“The large number of participants and the global geographical spread of the COVAD survey were very beneficial in gaining access to this important subset of patients,” added Dr. Andreoli. However, she acknowledged that patients with low socioeconomic status and/or high disability were likely underrepresented. While no data on pregnancy outcomes have been collected thus far, Dr. Andreoli expressed the desire to include them in the study’s follow-up.
The COVAD survey data also indicate that, in general, vaccine hesitancy among patients with AID is decreasing; from 2021 to 2022, it declined from 16.5% to 5.1%, as Dr. Machado indicated in his presentation.
Multiple factors contribute to breakthrough infections
The risk of breakthrough SARS-CoV-2 infections after vaccination varies among patients with rheumatoid arthritis and rheumatic or nonrheumatic autoimmune diseases, primarily depending on the underlying condition rather than the immunosuppressive medication. Environmental factors also appear to play a role. This complex landscape emerges from a further analysis of the COVAD survey dataset.
Alessia Alunno, MD, PhD, of the University of L’Aquila (Italy), presented a detailed and occasionally counterintuitive picture of similarities and differences among young adult patients (aged 18-35 years), mostly women, with various rheumatic and nonrheumatic diseases in relation to COVID-19. Most notably, the type of disease seemed to have more significance than the immunosuppression resulting from the treatment regimen. This held true for vaccine safety as well as for the risk of breakthrough COVID-19 and symptom profiles.
Patients with rheumatic disease (RMD) and nonrheumatic autoimmune disease (nr-AD) had significantly different therapeutic profiles on average. Before vaccination, 45% of patients with RMD used glucocorticoids (GC), and 91% used immunosuppressants (IS). In contrast, only 9.5% of nr-AD patients used GC, and 21% were taking IS.
Interestingly, the overall prevalence of reported SARS-CoV-2 infections was not influenced by medication and was practically identical (25% to 28%) across all groups. However, there were intriguing differences in the occurrence of infections before and after vaccination between disease groups. Prevaccine infections were less frequent among patients with RMD compared with healthy control persons (adjusted odds ratio, 0.6), while the rates were similar among patients with nr-AD and healthy control persons. On the other hand, breakthrough infections were more frequent in patients with RMD (aOR, 2.7), whereas the rate was similar between healthy control persons and patients with nr-AD.
Despite a much lower rate of GC/IS use, patients with nr-AD experienced repeated infections more frequently. In contrast, patients with RMD were less prone to multiple infections, even compared with healthy control persons (aOR, 0.5).
Regarding the disease profile, fewer than 5% of all infected patients required advanced therapies for SARS-CoV-2 infection. Notably, all SARS-CoV-2 infections in patients with nr-AD were symptomatic, whereas among patients with RMD and healthy control persons, the incidence of asymptomatic infections was 3%. The rate of hospital admissions was 4% for patients with RMD, compared with 2% for patients with nr-AD and 1% for control persons. The RMD group exhibited some differences between prevaccine infections and breakthrough infections, including a significantly lower frequency of loss of smell and taste during breakthrough infections. Overall, patients with RMD and COVID-19 experienced cough, runny nose, throat pain, nausea, and vomiting more frequently. In contrast, patients with nr-AD had a much higher risk of skin rashes during breakthrough infections (aOR, 8.7).
Vaccine adverse events (AEs) were also influenced by the underlying disease. Patients with RMD and those with nr-AD were more likely to experience mild AEs after the first or second dose, compared with healthy control persons (adjusted OR, 2.4 and 2.0, respectively). The most common early, mild AEs across all groups were injection-site pain, headache, and fatigue, but they occurred more frequently in the nr-AD group than in the RMD or healthy control group. Additionally, fever and chills occurred more frequently among the nr-AD group. Late, mild AEs and severe AEs were rare and affected all groups equally.
“The overall incidence of AEs was very low. Our results certainly do not undermine the safety of vaccines,” Dr. Alunno said.
Disease flares were more common after vaccination (10% with RMD and 7% with nr-AD) than after infection (5% with RMD and 1.5% with nr-AD). Furthermore, in many cases, after vaccination, flares required a change of medications, particularly for patients with RMD.
Additional results from the COVAD survey from January to July 2022, presented by Naveen Ravichandran, MD, DM, of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, revealed a higher prevalence (OR, 1.2; P = .001) of breakthrough infections among patients with RA. A total of 22.6% of patients with RA experienced breakthrough infections, compared with 20.6% for patients with other autoimmune rheumatic diseases and 18.4% of healthy control persons. Hospitalizations and the need for advanced treatment were also more common among patients with RA (30.9%) than among healthy control persons (13.9%). Patients with RA who had breakthrough infections tended to be older (closer to 50 years of age on average) and female, and they were more likely to have comorbidities and mental disorders. The human development index of the patient’s country of residence also played a role. Further research is necessary to understand how breakthrough infection outcomes are affected by a patient’s socioeconomic situation.
According to Dr. Ravichandran, medication was not a significant factor, except for the use of steroids and rituximab, which were associated with a higher risk of severe COVID-19 and hospitalization. Patients using rituximab, in particular, faced significantly increased odds for hospitalization (OR, 3.4) and severe breakthrough COVID-19 (OR, 3.0).
Session moderator Kim Lauper, MD, of the University of Geneva, cautioned: “The roles of disease and medication are challenging to separate. Some diseases require a more aggressive immunosuppressive regimen. It’s possible that different diseases affect the immune system differently, but it is not easy to demonstrate.”
The complications observed in the data warrant further study, as mentioned by Dr. Schulze-Koops: “We have a problem tied to the time line of the pandemic, where we had different viruses, different population behaviors, different treatments, and different standards of care over time. We also have differences between ethnic communities and regions of the world. But most importantly, we have different viruses: From the original strain to Delta to Omicron, we know they have very different clinical outcomes. I believe we need more scientific research to unravel these factors.”
Dr. Ørbo, Dr. Ravichandran, Dr. Andreoli, and Dr. Alunno reported no relevant financial relationships. Dr. Machado has received grants and/or honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Orphazyme, Pfizer, Roche, and UCB.
A version of this article originally appeared on Medscape.com.
MILAN – The impact of the COVID-19 pandemic on patients with rheumatic and nonrheumatic autoimmune diseases is ongoing and not yet fully comprehended. New data presented at the annual European Congress of Rheumatology, primarily derived from the global COVID-19 in Autoimmune Diseases (COVAD) survey but not limited to it, provide reassurance regarding the protection and safety of COVID-19 vaccines for older and younger adults, as well as for pregnant and breastfeeding women. These data also explore the influence of underlying diseases and medications on breakthrough SARS-CoV-2 infections and infection outcomes.
Safety of vaccines in patients with autoimmune or immune-mediated diseases
Following vaccination, even with low levels of antibodies, the risk of severe COVID-19 remains relatively low for patients who receive immunosuppressive therapy for various immune-mediated inflammatory diseases (IMIDs). This encouraging finding comes from the Nor-vaC study, presented by Hilde Ørbo, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Diakonhjemmet Hospital, Oslo.
During the presentation, Dr. Ørbo stated: “We did not find any specific diagnosis or medication associated with a significantly higher risk of hospitalization.” Receiving booster doses of the vaccine, having high levels of anti-spike antibodies after vaccination, and achieving hybrid immunity are correlated with further reductions in the risk of breakthrough SARS-CoV-2 infections.
Between Feb. 15, 2021, and Feb. 15, 2023, COVID-19 affected a similar proportion among the 729 patients and 350 healthy control persons (67% and 68%, respectively). Among the patients, 22 reported severe COVID-19, whereas none of the healthy control persons did. However, there were no fatalities among the patients. The study cohort consisted of patients with various IMIDs; 70% had an inflammatory joint disease. The use of immunosuppressive medications also varied, with 63% of patients using tumor necrosis factor inhibitors, either as monotherapy or in combination with other treatments, and other patients taking medications such as methotrexate, interleukin inhibitors, Janus kinase inhibitors, vedolizumab (Entyvio), and others.
While being older than 70 years and the presence of comorbidities were identified as risk factors for severe COVID-19, there was a significant reduction in risk with each additional vaccine dose. These results support the protective role of repeated COVID-19 vaccination for patients with IMIDs who are receiving immunosuppressive therapies; they yield a favorable prognosis even with the Omicron variant.
The study further compared the risk of severe COVID-19 between a group with hybrid immunity (having received three vaccine doses and experiencing breakthrough infection with the Omicron variant) and a group that received a fourth vaccine dose within the same time frame. The difference was striking: Hybrid immunity was associated with a 5.8-fold decrease in risk, compared with four-dose vaccination (P < .0001).
The level of antibodies, measured 2-4 weeks after the last vaccination, was predictive of the risk of breakthrough COVID-19. An antibody level above 6000 binding antibody units/mL after vaccination was significantly associated with a reduction in risk. “We can conclude that patients who receive multiple vaccine doses have a lower risk of COVID-19,” Dr. Ørbo said. “In patients who recently experienced breakthrough infections, the administration of a booster vaccine dose might be delayed.”
“The virus has undergone changes throughout the pandemic, while the vaccines have remained relatively stable. Are we anticipating more infections over time?” asked Hendrik Schulze-Koops, MD, PhD, of Ludwig Maximilians University of Munich (Germany), the session moderator. In response, Dr. Ørbo stated that 85% of the recorded infections in the study occurred after the emergence of the Omicron variant, and time was considered a covariable in the analysis.
These data shed light on a topic discussed by Pedro Machado, MD, PhD, professor and consultant in rheumatology and neuromuscular diseases at University College London, during his scientific session talk entitled, “Unsolved Issues of COVID Vaccination and Re-vaccination.” Dr. Machado referred to the VROOM study published in 2022, which examined the interruption of methotrexate for 2 weeks following booster administration. Both groups demonstrated a significant antibody response, but the group that stopped taking methotrexate showed double the antibody titers.
However, he emphasized, “what remains unknown is the clinical relevance of these differences in terms of severe infection, hospitalization, or even death. The potential benefit of increased immunogenicity by interrupting conventional synthetic disease-modifying antirheumatic drugs [csDMARDs] such as methotrexate before or after vaccination needs to be balanced against the potential risk of disease flare. Ultimately, decision-making should be individualized based on factors such as comorbidities, disease activity, and other considerations.” The results presented by Dr. Ørbo suggest that, while there may be a clinical difference in terms of severe infection, the overall prognosis for vaccinated patients is reasonably good.
Regarding other DMARDs, such as biologics, the approach may differ. Dr. Machado suggested: “In patients using rituximab or other B cell–depleting therapies, SARS-CoV-2 vaccination should be scheduled in a way that optimizes vaccine immunogenicity. A minimum of 10 B cells/mcL of blood is likely a relevant threshold above which a sufficient cellular and immune response is established.”
COVID vaccines are safe for pregnant and breastfeeding women
According to data from the COVAD study, which comprised two global cross-sectional surveys conducted in 2021 and 2022, the COVID-19 vaccine appeared safe for pregnant and breastfeeding women with autoimmune diseases (AID).
Presenter Laura Andreoli, MD, PhD, of the University of Brescia (Italy), said that, although pregnant patients with AID reported more adverse events related to vaccination, these rates were not significantly higher than those among pregnant, healthy control persons who were without AID. No difference in adverse events was observed between breastfeeding women and healthy control persons, and the incidence of disease flares did not significantly differ among all groups.
“In summary, this study provides initial insights into the safety of COVID-19 vaccination during the gestational and postpartum periods in women with autoimmune diseases. These reassuring observations will hopefully improve clinician-patient communication and address hesitancy towards COVID-19 vaccination, as the benefits for the mother and fetus through passive immunization appear to outweigh potential risks,” Dr. Andreoli said in an interview.
“The large number of participants and the global geographical spread of the COVAD survey were very beneficial in gaining access to this important subset of patients,” added Dr. Andreoli. However, she acknowledged that patients with low socioeconomic status and/or high disability were likely underrepresented. While no data on pregnancy outcomes have been collected thus far, Dr. Andreoli expressed the desire to include them in the study’s follow-up.
The COVAD survey data also indicate that, in general, vaccine hesitancy among patients with AID is decreasing; from 2021 to 2022, it declined from 16.5% to 5.1%, as Dr. Machado indicated in his presentation.
Multiple factors contribute to breakthrough infections
The risk of breakthrough SARS-CoV-2 infections after vaccination varies among patients with rheumatoid arthritis and rheumatic or nonrheumatic autoimmune diseases, primarily depending on the underlying condition rather than the immunosuppressive medication. Environmental factors also appear to play a role. This complex landscape emerges from a further analysis of the COVAD survey dataset.
Alessia Alunno, MD, PhD, of the University of L’Aquila (Italy), presented a detailed and occasionally counterintuitive picture of similarities and differences among young adult patients (aged 18-35 years), mostly women, with various rheumatic and nonrheumatic diseases in relation to COVID-19. Most notably, the type of disease seemed to have more significance than the immunosuppression resulting from the treatment regimen. This held true for vaccine safety as well as for the risk of breakthrough COVID-19 and symptom profiles.
Patients with rheumatic disease (RMD) and nonrheumatic autoimmune disease (nr-AD) had significantly different therapeutic profiles on average. Before vaccination, 45% of patients with RMD used glucocorticoids (GC), and 91% used immunosuppressants (IS). In contrast, only 9.5% of nr-AD patients used GC, and 21% were taking IS.
Interestingly, the overall prevalence of reported SARS-CoV-2 infections was not influenced by medication and was practically identical (25% to 28%) across all groups. However, there were intriguing differences in the occurrence of infections before and after vaccination between disease groups. Prevaccine infections were less frequent among patients with RMD compared with healthy control persons (adjusted odds ratio, 0.6), while the rates were similar among patients with nr-AD and healthy control persons. On the other hand, breakthrough infections were more frequent in patients with RMD (aOR, 2.7), whereas the rate was similar between healthy control persons and patients with nr-AD.
Despite a much lower rate of GC/IS use, patients with nr-AD experienced repeated infections more frequently. In contrast, patients with RMD were less prone to multiple infections, even compared with healthy control persons (aOR, 0.5).
Regarding the disease profile, fewer than 5% of all infected patients required advanced therapies for SARS-CoV-2 infection. Notably, all SARS-CoV-2 infections in patients with nr-AD were symptomatic, whereas among patients with RMD and healthy control persons, the incidence of asymptomatic infections was 3%. The rate of hospital admissions was 4% for patients with RMD, compared with 2% for patients with nr-AD and 1% for control persons. The RMD group exhibited some differences between prevaccine infections and breakthrough infections, including a significantly lower frequency of loss of smell and taste during breakthrough infections. Overall, patients with RMD and COVID-19 experienced cough, runny nose, throat pain, nausea, and vomiting more frequently. In contrast, patients with nr-AD had a much higher risk of skin rashes during breakthrough infections (aOR, 8.7).
Vaccine adverse events (AEs) were also influenced by the underlying disease. Patients with RMD and those with nr-AD were more likely to experience mild AEs after the first or second dose, compared with healthy control persons (adjusted OR, 2.4 and 2.0, respectively). The most common early, mild AEs across all groups were injection-site pain, headache, and fatigue, but they occurred more frequently in the nr-AD group than in the RMD or healthy control group. Additionally, fever and chills occurred more frequently among the nr-AD group. Late, mild AEs and severe AEs were rare and affected all groups equally.
“The overall incidence of AEs was very low. Our results certainly do not undermine the safety of vaccines,” Dr. Alunno said.
Disease flares were more common after vaccination (10% with RMD and 7% with nr-AD) than after infection (5% with RMD and 1.5% with nr-AD). Furthermore, in many cases, after vaccination, flares required a change of medications, particularly for patients with RMD.
Additional results from the COVAD survey from January to July 2022, presented by Naveen Ravichandran, MD, DM, of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, revealed a higher prevalence (OR, 1.2; P = .001) of breakthrough infections among patients with RA. A total of 22.6% of patients with RA experienced breakthrough infections, compared with 20.6% for patients with other autoimmune rheumatic diseases and 18.4% of healthy control persons. Hospitalizations and the need for advanced treatment were also more common among patients with RA (30.9%) than among healthy control persons (13.9%). Patients with RA who had breakthrough infections tended to be older (closer to 50 years of age on average) and female, and they were more likely to have comorbidities and mental disorders. The human development index of the patient’s country of residence also played a role. Further research is necessary to understand how breakthrough infection outcomes are affected by a patient’s socioeconomic situation.
According to Dr. Ravichandran, medication was not a significant factor, except for the use of steroids and rituximab, which were associated with a higher risk of severe COVID-19 and hospitalization. Patients using rituximab, in particular, faced significantly increased odds for hospitalization (OR, 3.4) and severe breakthrough COVID-19 (OR, 3.0).
Session moderator Kim Lauper, MD, of the University of Geneva, cautioned: “The roles of disease and medication are challenging to separate. Some diseases require a more aggressive immunosuppressive regimen. It’s possible that different diseases affect the immune system differently, but it is not easy to demonstrate.”
The complications observed in the data warrant further study, as mentioned by Dr. Schulze-Koops: “We have a problem tied to the time line of the pandemic, where we had different viruses, different population behaviors, different treatments, and different standards of care over time. We also have differences between ethnic communities and regions of the world. But most importantly, we have different viruses: From the original strain to Delta to Omicron, we know they have very different clinical outcomes. I believe we need more scientific research to unravel these factors.”
Dr. Ørbo, Dr. Ravichandran, Dr. Andreoli, and Dr. Alunno reported no relevant financial relationships. Dr. Machado has received grants and/or honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Orphazyme, Pfizer, Roche, and UCB.
A version of this article originally appeared on Medscape.com.
MILAN – The impact of the COVID-19 pandemic on patients with rheumatic and nonrheumatic autoimmune diseases is ongoing and not yet fully comprehended. New data presented at the annual European Congress of Rheumatology, primarily derived from the global COVID-19 in Autoimmune Diseases (COVAD) survey but not limited to it, provide reassurance regarding the protection and safety of COVID-19 vaccines for older and younger adults, as well as for pregnant and breastfeeding women. These data also explore the influence of underlying diseases and medications on breakthrough SARS-CoV-2 infections and infection outcomes.
Safety of vaccines in patients with autoimmune or immune-mediated diseases
Following vaccination, even with low levels of antibodies, the risk of severe COVID-19 remains relatively low for patients who receive immunosuppressive therapy for various immune-mediated inflammatory diseases (IMIDs). This encouraging finding comes from the Nor-vaC study, presented by Hilde Ørbo, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Diakonhjemmet Hospital, Oslo.
During the presentation, Dr. Ørbo stated: “We did not find any specific diagnosis or medication associated with a significantly higher risk of hospitalization.” Receiving booster doses of the vaccine, having high levels of anti-spike antibodies after vaccination, and achieving hybrid immunity are correlated with further reductions in the risk of breakthrough SARS-CoV-2 infections.
Between Feb. 15, 2021, and Feb. 15, 2023, COVID-19 affected a similar proportion among the 729 patients and 350 healthy control persons (67% and 68%, respectively). Among the patients, 22 reported severe COVID-19, whereas none of the healthy control persons did. However, there were no fatalities among the patients. The study cohort consisted of patients with various IMIDs; 70% had an inflammatory joint disease. The use of immunosuppressive medications also varied, with 63% of patients using tumor necrosis factor inhibitors, either as monotherapy or in combination with other treatments, and other patients taking medications such as methotrexate, interleukin inhibitors, Janus kinase inhibitors, vedolizumab (Entyvio), and others.
While being older than 70 years and the presence of comorbidities were identified as risk factors for severe COVID-19, there was a significant reduction in risk with each additional vaccine dose. These results support the protective role of repeated COVID-19 vaccination for patients with IMIDs who are receiving immunosuppressive therapies; they yield a favorable prognosis even with the Omicron variant.
The study further compared the risk of severe COVID-19 between a group with hybrid immunity (having received three vaccine doses and experiencing breakthrough infection with the Omicron variant) and a group that received a fourth vaccine dose within the same time frame. The difference was striking: Hybrid immunity was associated with a 5.8-fold decrease in risk, compared with four-dose vaccination (P < .0001).
The level of antibodies, measured 2-4 weeks after the last vaccination, was predictive of the risk of breakthrough COVID-19. An antibody level above 6000 binding antibody units/mL after vaccination was significantly associated with a reduction in risk. “We can conclude that patients who receive multiple vaccine doses have a lower risk of COVID-19,” Dr. Ørbo said. “In patients who recently experienced breakthrough infections, the administration of a booster vaccine dose might be delayed.”
“The virus has undergone changes throughout the pandemic, while the vaccines have remained relatively stable. Are we anticipating more infections over time?” asked Hendrik Schulze-Koops, MD, PhD, of Ludwig Maximilians University of Munich (Germany), the session moderator. In response, Dr. Ørbo stated that 85% of the recorded infections in the study occurred after the emergence of the Omicron variant, and time was considered a covariable in the analysis.
These data shed light on a topic discussed by Pedro Machado, MD, PhD, professor and consultant in rheumatology and neuromuscular diseases at University College London, during his scientific session talk entitled, “Unsolved Issues of COVID Vaccination and Re-vaccination.” Dr. Machado referred to the VROOM study published in 2022, which examined the interruption of methotrexate for 2 weeks following booster administration. Both groups demonstrated a significant antibody response, but the group that stopped taking methotrexate showed double the antibody titers.
However, he emphasized, “what remains unknown is the clinical relevance of these differences in terms of severe infection, hospitalization, or even death. The potential benefit of increased immunogenicity by interrupting conventional synthetic disease-modifying antirheumatic drugs [csDMARDs] such as methotrexate before or after vaccination needs to be balanced against the potential risk of disease flare. Ultimately, decision-making should be individualized based on factors such as comorbidities, disease activity, and other considerations.” The results presented by Dr. Ørbo suggest that, while there may be a clinical difference in terms of severe infection, the overall prognosis for vaccinated patients is reasonably good.
Regarding other DMARDs, such as biologics, the approach may differ. Dr. Machado suggested: “In patients using rituximab or other B cell–depleting therapies, SARS-CoV-2 vaccination should be scheduled in a way that optimizes vaccine immunogenicity. A minimum of 10 B cells/mcL of blood is likely a relevant threshold above which a sufficient cellular and immune response is established.”
COVID vaccines are safe for pregnant and breastfeeding women
According to data from the COVAD study, which comprised two global cross-sectional surveys conducted in 2021 and 2022, the COVID-19 vaccine appeared safe for pregnant and breastfeeding women with autoimmune diseases (AID).
Presenter Laura Andreoli, MD, PhD, of the University of Brescia (Italy), said that, although pregnant patients with AID reported more adverse events related to vaccination, these rates were not significantly higher than those among pregnant, healthy control persons who were without AID. No difference in adverse events was observed between breastfeeding women and healthy control persons, and the incidence of disease flares did not significantly differ among all groups.
“In summary, this study provides initial insights into the safety of COVID-19 vaccination during the gestational and postpartum periods in women with autoimmune diseases. These reassuring observations will hopefully improve clinician-patient communication and address hesitancy towards COVID-19 vaccination, as the benefits for the mother and fetus through passive immunization appear to outweigh potential risks,” Dr. Andreoli said in an interview.
“The large number of participants and the global geographical spread of the COVAD survey were very beneficial in gaining access to this important subset of patients,” added Dr. Andreoli. However, she acknowledged that patients with low socioeconomic status and/or high disability were likely underrepresented. While no data on pregnancy outcomes have been collected thus far, Dr. Andreoli expressed the desire to include them in the study’s follow-up.
The COVAD survey data also indicate that, in general, vaccine hesitancy among patients with AID is decreasing; from 2021 to 2022, it declined from 16.5% to 5.1%, as Dr. Machado indicated in his presentation.
Multiple factors contribute to breakthrough infections
The risk of breakthrough SARS-CoV-2 infections after vaccination varies among patients with rheumatoid arthritis and rheumatic or nonrheumatic autoimmune diseases, primarily depending on the underlying condition rather than the immunosuppressive medication. Environmental factors also appear to play a role. This complex landscape emerges from a further analysis of the COVAD survey dataset.
Alessia Alunno, MD, PhD, of the University of L’Aquila (Italy), presented a detailed and occasionally counterintuitive picture of similarities and differences among young adult patients (aged 18-35 years), mostly women, with various rheumatic and nonrheumatic diseases in relation to COVID-19. Most notably, the type of disease seemed to have more significance than the immunosuppression resulting from the treatment regimen. This held true for vaccine safety as well as for the risk of breakthrough COVID-19 and symptom profiles.
Patients with rheumatic disease (RMD) and nonrheumatic autoimmune disease (nr-AD) had significantly different therapeutic profiles on average. Before vaccination, 45% of patients with RMD used glucocorticoids (GC), and 91% used immunosuppressants (IS). In contrast, only 9.5% of nr-AD patients used GC, and 21% were taking IS.
Interestingly, the overall prevalence of reported SARS-CoV-2 infections was not influenced by medication and was practically identical (25% to 28%) across all groups. However, there were intriguing differences in the occurrence of infections before and after vaccination between disease groups. Prevaccine infections were less frequent among patients with RMD compared with healthy control persons (adjusted odds ratio, 0.6), while the rates were similar among patients with nr-AD and healthy control persons. On the other hand, breakthrough infections were more frequent in patients with RMD (aOR, 2.7), whereas the rate was similar between healthy control persons and patients with nr-AD.
Despite a much lower rate of GC/IS use, patients with nr-AD experienced repeated infections more frequently. In contrast, patients with RMD were less prone to multiple infections, even compared with healthy control persons (aOR, 0.5).
Regarding the disease profile, fewer than 5% of all infected patients required advanced therapies for SARS-CoV-2 infection. Notably, all SARS-CoV-2 infections in patients with nr-AD were symptomatic, whereas among patients with RMD and healthy control persons, the incidence of asymptomatic infections was 3%. The rate of hospital admissions was 4% for patients with RMD, compared with 2% for patients with nr-AD and 1% for control persons. The RMD group exhibited some differences between prevaccine infections and breakthrough infections, including a significantly lower frequency of loss of smell and taste during breakthrough infections. Overall, patients with RMD and COVID-19 experienced cough, runny nose, throat pain, nausea, and vomiting more frequently. In contrast, patients with nr-AD had a much higher risk of skin rashes during breakthrough infections (aOR, 8.7).
Vaccine adverse events (AEs) were also influenced by the underlying disease. Patients with RMD and those with nr-AD were more likely to experience mild AEs after the first or second dose, compared with healthy control persons (adjusted OR, 2.4 and 2.0, respectively). The most common early, mild AEs across all groups were injection-site pain, headache, and fatigue, but they occurred more frequently in the nr-AD group than in the RMD or healthy control group. Additionally, fever and chills occurred more frequently among the nr-AD group. Late, mild AEs and severe AEs were rare and affected all groups equally.
“The overall incidence of AEs was very low. Our results certainly do not undermine the safety of vaccines,” Dr. Alunno said.
Disease flares were more common after vaccination (10% with RMD and 7% with nr-AD) than after infection (5% with RMD and 1.5% with nr-AD). Furthermore, in many cases, after vaccination, flares required a change of medications, particularly for patients with RMD.
Additional results from the COVAD survey from January to July 2022, presented by Naveen Ravichandran, MD, DM, of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, revealed a higher prevalence (OR, 1.2; P = .001) of breakthrough infections among patients with RA. A total of 22.6% of patients with RA experienced breakthrough infections, compared with 20.6% for patients with other autoimmune rheumatic diseases and 18.4% of healthy control persons. Hospitalizations and the need for advanced treatment were also more common among patients with RA (30.9%) than among healthy control persons (13.9%). Patients with RA who had breakthrough infections tended to be older (closer to 50 years of age on average) and female, and they were more likely to have comorbidities and mental disorders. The human development index of the patient’s country of residence also played a role. Further research is necessary to understand how breakthrough infection outcomes are affected by a patient’s socioeconomic situation.
According to Dr. Ravichandran, medication was not a significant factor, except for the use of steroids and rituximab, which were associated with a higher risk of severe COVID-19 and hospitalization. Patients using rituximab, in particular, faced significantly increased odds for hospitalization (OR, 3.4) and severe breakthrough COVID-19 (OR, 3.0).
Session moderator Kim Lauper, MD, of the University of Geneva, cautioned: “The roles of disease and medication are challenging to separate. Some diseases require a more aggressive immunosuppressive regimen. It’s possible that different diseases affect the immune system differently, but it is not easy to demonstrate.”
The complications observed in the data warrant further study, as mentioned by Dr. Schulze-Koops: “We have a problem tied to the time line of the pandemic, where we had different viruses, different population behaviors, different treatments, and different standards of care over time. We also have differences between ethnic communities and regions of the world. But most importantly, we have different viruses: From the original strain to Delta to Omicron, we know they have very different clinical outcomes. I believe we need more scientific research to unravel these factors.”
Dr. Ørbo, Dr. Ravichandran, Dr. Andreoli, and Dr. Alunno reported no relevant financial relationships. Dr. Machado has received grants and/or honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Orphazyme, Pfizer, Roche, and UCB.
A version of this article originally appeared on Medscape.com.
AT EULAR 2023
COVID vaccines safe for young children, study finds
TOPLINE:
COVID-19 vaccines from Moderna and Pfizer-BioNTech are safe for children under age 5 years, according to findings from a study funded by the Centers for Disease Control and Prevention.
METHODOLOGY:
- Data came from the Vaccine Safety Datalink, which gathers information from eight health systems in the United States.
- Analyzed data from 135,005 doses given to children age 4 and younger who received the Pfizer-BioNTech , and 112,006 doses given to children aged 5 and younger who received the Moderna version.
- Assessed for 23 safety outcomes, including myocarditis, pericarditis, and seizures.
TAKEAWAY:
- One case of hemorrhagic stroke and one case of pulmonary embolism occurred after vaccination but these were linked to preexisting congenital abnormalities.
IN PRACTICE:
“These results can provide reassurance to clinicians, parents, and policymakers alike.”
STUDY DETAILS:
The study was led by Kristin Goddard, MPH, a researcher at the Kaiser Permanente Vaccine Study Center in Oakland, Calif., and was funded by the Centers for Disease Control and Prevention.
LIMITATIONS:
The researchers reported low statistical power for early analysis, especially for rare outcomes. In addition, fewer than 25% of children in the database had received a vaccine at the time of analysis.
DISCLOSURES:
A coauthor reported receiving funding from Janssen Vaccines and Prevention for a study unrelated to COVID-19 vaccines. Another coauthor reported receiving grants from Pfizer in 2019 for clinical trials for coronavirus vaccines, and from Merck, GSK, and Sanofi Pasteur for unrelated research.
A version of this article first appeared on Medscape.com.
TOPLINE:
COVID-19 vaccines from Moderna and Pfizer-BioNTech are safe for children under age 5 years, according to findings from a study funded by the Centers for Disease Control and Prevention.
METHODOLOGY:
- Data came from the Vaccine Safety Datalink, which gathers information from eight health systems in the United States.
- Analyzed data from 135,005 doses given to children age 4 and younger who received the Pfizer-BioNTech , and 112,006 doses given to children aged 5 and younger who received the Moderna version.
- Assessed for 23 safety outcomes, including myocarditis, pericarditis, and seizures.
TAKEAWAY:
- One case of hemorrhagic stroke and one case of pulmonary embolism occurred after vaccination but these were linked to preexisting congenital abnormalities.
IN PRACTICE:
“These results can provide reassurance to clinicians, parents, and policymakers alike.”
STUDY DETAILS:
The study was led by Kristin Goddard, MPH, a researcher at the Kaiser Permanente Vaccine Study Center in Oakland, Calif., and was funded by the Centers for Disease Control and Prevention.
LIMITATIONS:
The researchers reported low statistical power for early analysis, especially for rare outcomes. In addition, fewer than 25% of children in the database had received a vaccine at the time of analysis.
DISCLOSURES:
A coauthor reported receiving funding from Janssen Vaccines and Prevention for a study unrelated to COVID-19 vaccines. Another coauthor reported receiving grants from Pfizer in 2019 for clinical trials for coronavirus vaccines, and from Merck, GSK, and Sanofi Pasteur for unrelated research.
A version of this article first appeared on Medscape.com.
TOPLINE:
COVID-19 vaccines from Moderna and Pfizer-BioNTech are safe for children under age 5 years, according to findings from a study funded by the Centers for Disease Control and Prevention.
METHODOLOGY:
- Data came from the Vaccine Safety Datalink, which gathers information from eight health systems in the United States.
- Analyzed data from 135,005 doses given to children age 4 and younger who received the Pfizer-BioNTech , and 112,006 doses given to children aged 5 and younger who received the Moderna version.
- Assessed for 23 safety outcomes, including myocarditis, pericarditis, and seizures.
TAKEAWAY:
- One case of hemorrhagic stroke and one case of pulmonary embolism occurred after vaccination but these were linked to preexisting congenital abnormalities.
IN PRACTICE:
“These results can provide reassurance to clinicians, parents, and policymakers alike.”
STUDY DETAILS:
The study was led by Kristin Goddard, MPH, a researcher at the Kaiser Permanente Vaccine Study Center in Oakland, Calif., and was funded by the Centers for Disease Control and Prevention.
LIMITATIONS:
The researchers reported low statistical power for early analysis, especially for rare outcomes. In addition, fewer than 25% of children in the database had received a vaccine at the time of analysis.
DISCLOSURES:
A coauthor reported receiving funding from Janssen Vaccines and Prevention for a study unrelated to COVID-19 vaccines. Another coauthor reported receiving grants from Pfizer in 2019 for clinical trials for coronavirus vaccines, and from Merck, GSK, and Sanofi Pasteur for unrelated research.
A version of this article first appeared on Medscape.com.
FROM PEDIATRICS
The new vaccine your patients may not want
Compared with the complicated and ever-changing recommended vaccine schedule for infants and children, vaccines for adults have been straightforward. Adults without compromised immunity who received all their childhood vaccinations are eligible for a tetanus and diphtheria (Td) or tetanus, diphtheria, and pertussis (Tdap) booster every 10 years, recombinant herpes zoster vaccine at age 50, and pneumococcal vaccines at age 65, along with annual influenza and (likely) COVID-19 vaccines. Last year, due to rising rates of acute hepatitis B, the Centers for Disease Control and Prevention first recommended universal hepatitis B vaccination for adults aged 19-59 years without a record of previous hepatitis B infection or vaccination.
An additional routine vaccine for adults is now on the horizon. The U.S. Food and Drug Administration recently approved Arexvy, a vaccine against respiratory syncytial virus (RSV) for adults aged 60 years or older. Two more RSV vaccines are in the final stages of development. Why should family physicians prioritize vaccinating older adults against RSV, and how can we incorporate this new vaccine into our practices and overcome patient hesitancy to receive yet another vaccine?
Clinicians tend to think of RSV as a serious disease in young children – which it is – but data suggest that in 2019, RSV infection led to more than 100,000 hospitalizations and 7,700 deaths in older adults in the United States. In a randomized controlled trial of 25,000 adults aged 60 years or older with a median of 6.7 months of follow-up, Arexvy reduced severe RSV disease by 94% and RSV-related acute respiratory infections by 71%, with similar effectiveness in adults with underlying health conditions. That’s considerably better protection than current influenza vaccines and comparable to COVID-19 mRNA vaccines before variants became widespread. Pain and fatigue were the most common side effects and usually resolved within 1-2 days.
Although the seasonal pattern of RSV shifted during the COVID-19 pandemic, RSV season historically begins in October, peaks in December, and ends in April. If the vaccine is recommended by the CDC and is widely available by fall, as the manufacturer, GSK, expects, it could be administered around the same time as influenza and COVID-19 vaccines.
The challenges of incorporating this new vaccine into practice will feel familiar: Many of our patients won’t have heard about it, may feel that they don’t need it, or may decline it because of concerns about side effects, real or imagined. (Of note, the FDA is requiring GSK to perform a postmarketing study to rule out associations with rare cases of Guillain-Barré syndrome and acute disseminated encephalomyelitis, and the company also plans to monitor the incidence of atrial fibrillation, which was slightly more common in the vaccine group than the placebo group.)
While a strong recommendation from a family physician is often enough to convince patients to accept vaccination, rampant misinformation during the pandemic may have worsened vaccine hesitancy for some. It may feel like a fruitless exercise to try to convince adults who have refused COVID-19 and influenza vaccines to accept a newer vaccine against a respiratory virus that causes less serious illness overall. But with other RSV vaccines and monoclonal antibodies for older adults and infants likely to be approved soon, it’s important for us to start laying the groundwork now by educating colleagues, staff, and patients about preventing serious illness caused by RSV.
Dr. Lin is an associate professor in the Department of Family Medicine at Georgetown University and a staff physician atMedStar Health Center, both in Washington. He has received income from UpToDate, Wiley-Blackwell, and the American Academy of Family Physicians.
A version of this article first appeared on Medscape.com.
Compared with the complicated and ever-changing recommended vaccine schedule for infants and children, vaccines for adults have been straightforward. Adults without compromised immunity who received all their childhood vaccinations are eligible for a tetanus and diphtheria (Td) or tetanus, diphtheria, and pertussis (Tdap) booster every 10 years, recombinant herpes zoster vaccine at age 50, and pneumococcal vaccines at age 65, along with annual influenza and (likely) COVID-19 vaccines. Last year, due to rising rates of acute hepatitis B, the Centers for Disease Control and Prevention first recommended universal hepatitis B vaccination for adults aged 19-59 years without a record of previous hepatitis B infection or vaccination.
An additional routine vaccine for adults is now on the horizon. The U.S. Food and Drug Administration recently approved Arexvy, a vaccine against respiratory syncytial virus (RSV) for adults aged 60 years or older. Two more RSV vaccines are in the final stages of development. Why should family physicians prioritize vaccinating older adults against RSV, and how can we incorporate this new vaccine into our practices and overcome patient hesitancy to receive yet another vaccine?
Clinicians tend to think of RSV as a serious disease in young children – which it is – but data suggest that in 2019, RSV infection led to more than 100,000 hospitalizations and 7,700 deaths in older adults in the United States. In a randomized controlled trial of 25,000 adults aged 60 years or older with a median of 6.7 months of follow-up, Arexvy reduced severe RSV disease by 94% and RSV-related acute respiratory infections by 71%, with similar effectiveness in adults with underlying health conditions. That’s considerably better protection than current influenza vaccines and comparable to COVID-19 mRNA vaccines before variants became widespread. Pain and fatigue were the most common side effects and usually resolved within 1-2 days.
Although the seasonal pattern of RSV shifted during the COVID-19 pandemic, RSV season historically begins in October, peaks in December, and ends in April. If the vaccine is recommended by the CDC and is widely available by fall, as the manufacturer, GSK, expects, it could be administered around the same time as influenza and COVID-19 vaccines.
The challenges of incorporating this new vaccine into practice will feel familiar: Many of our patients won’t have heard about it, may feel that they don’t need it, or may decline it because of concerns about side effects, real or imagined. (Of note, the FDA is requiring GSK to perform a postmarketing study to rule out associations with rare cases of Guillain-Barré syndrome and acute disseminated encephalomyelitis, and the company also plans to monitor the incidence of atrial fibrillation, which was slightly more common in the vaccine group than the placebo group.)
While a strong recommendation from a family physician is often enough to convince patients to accept vaccination, rampant misinformation during the pandemic may have worsened vaccine hesitancy for some. It may feel like a fruitless exercise to try to convince adults who have refused COVID-19 and influenza vaccines to accept a newer vaccine against a respiratory virus that causes less serious illness overall. But with other RSV vaccines and monoclonal antibodies for older adults and infants likely to be approved soon, it’s important for us to start laying the groundwork now by educating colleagues, staff, and patients about preventing serious illness caused by RSV.
Dr. Lin is an associate professor in the Department of Family Medicine at Georgetown University and a staff physician atMedStar Health Center, both in Washington. He has received income from UpToDate, Wiley-Blackwell, and the American Academy of Family Physicians.
A version of this article first appeared on Medscape.com.
Compared with the complicated and ever-changing recommended vaccine schedule for infants and children, vaccines for adults have been straightforward. Adults without compromised immunity who received all their childhood vaccinations are eligible for a tetanus and diphtheria (Td) or tetanus, diphtheria, and pertussis (Tdap) booster every 10 years, recombinant herpes zoster vaccine at age 50, and pneumococcal vaccines at age 65, along with annual influenza and (likely) COVID-19 vaccines. Last year, due to rising rates of acute hepatitis B, the Centers for Disease Control and Prevention first recommended universal hepatitis B vaccination for adults aged 19-59 years without a record of previous hepatitis B infection or vaccination.
An additional routine vaccine for adults is now on the horizon. The U.S. Food and Drug Administration recently approved Arexvy, a vaccine against respiratory syncytial virus (RSV) for adults aged 60 years or older. Two more RSV vaccines are in the final stages of development. Why should family physicians prioritize vaccinating older adults against RSV, and how can we incorporate this new vaccine into our practices and overcome patient hesitancy to receive yet another vaccine?
Clinicians tend to think of RSV as a serious disease in young children – which it is – but data suggest that in 2019, RSV infection led to more than 100,000 hospitalizations and 7,700 deaths in older adults in the United States. In a randomized controlled trial of 25,000 adults aged 60 years or older with a median of 6.7 months of follow-up, Arexvy reduced severe RSV disease by 94% and RSV-related acute respiratory infections by 71%, with similar effectiveness in adults with underlying health conditions. That’s considerably better protection than current influenza vaccines and comparable to COVID-19 mRNA vaccines before variants became widespread. Pain and fatigue were the most common side effects and usually resolved within 1-2 days.
Although the seasonal pattern of RSV shifted during the COVID-19 pandemic, RSV season historically begins in October, peaks in December, and ends in April. If the vaccine is recommended by the CDC and is widely available by fall, as the manufacturer, GSK, expects, it could be administered around the same time as influenza and COVID-19 vaccines.
The challenges of incorporating this new vaccine into practice will feel familiar: Many of our patients won’t have heard about it, may feel that they don’t need it, or may decline it because of concerns about side effects, real or imagined. (Of note, the FDA is requiring GSK to perform a postmarketing study to rule out associations with rare cases of Guillain-Barré syndrome and acute disseminated encephalomyelitis, and the company also plans to monitor the incidence of atrial fibrillation, which was slightly more common in the vaccine group than the placebo group.)
While a strong recommendation from a family physician is often enough to convince patients to accept vaccination, rampant misinformation during the pandemic may have worsened vaccine hesitancy for some. It may feel like a fruitless exercise to try to convince adults who have refused COVID-19 and influenza vaccines to accept a newer vaccine against a respiratory virus that causes less serious illness overall. But with other RSV vaccines and monoclonal antibodies for older adults and infants likely to be approved soon, it’s important for us to start laying the groundwork now by educating colleagues, staff, and patients about preventing serious illness caused by RSV.
Dr. Lin is an associate professor in the Department of Family Medicine at Georgetown University and a staff physician atMedStar Health Center, both in Washington. He has received income from UpToDate, Wiley-Blackwell, and the American Academy of Family Physicians.
A version of this article first appeared on Medscape.com.
Safety remains top parent concern for HPV vaccine
, according to a study published online in Pediatrics.
“Although HPV vaccination rates in the United States have steadily improved over the past decade, a sizable subset of parents remains highly hesitant about administering the vaccine to their adolescent children,” wrote Eric Adjei Boakye, PhD, of the departments of public health sciences and otolaryngology–head and neck surgery at the Henry Ford Health System, Detroit, and associates. But a silver lining in the study is the downward trend in parents not vaccinating their children against HPV because the child’s provider did not recommend it.
“Provider recommendation has been shown to be the single best predictor of HPV vaccine uptake and vaccine acceptability,” the authors wrote. They noted one previous study finding that provider recommendations for the vaccine had increased from 27% in 2012 to 49.3% in 2018.
Safety concerns increased while other concerns decreased
The findings were not surprising to Robert A. Bednarczyk, PhD, associate professor of global health at Emory University Rollins School of Public Health, Atlanta, who specializes in HPV vaccine research.
“We have seen over the years that vaccine safety concerns have been on the increase, notably recently in the context of the COVID-19 pandemic and vaccination program, but HPV vaccine safety, though well established, continues to be a major concern for parents,” Dr. Bednarczyk said in an interview. But he found it striking that parents’ other reasons for turning down the vaccine had declined. “This shows that the outreach around the need for HPV vaccination and efforts to improve provider recommendation strategies is likely having positive impacts on HPV vaccine attitudes.”
Top five reasons for not vaccinating
The researchers analyzed data from the National Immunization Survey–Teen for the years 2010 through 2020 to track the annual changes in the top five reasons cited for not planning to get the HPV vaccine. The data covered 119,695 teens aged 13-17.
The researchers identified parents’ five most commonly cited reasons for not planning to vaccinate their children against HPV: “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
Parents’ HPV vaccine hesitancy decreased by 5.5% each year from 2010 to 2012, but then it stagnated for the remaining years through 2020. Across most of that time, from 2010 to 2018, parents’ concerns about the vaccine’s safety and side effects increased by 15.6%. A major reason for this increase, the authors suggested, may include the widespread distribution of online misinformation, particularly given the 7.8 million increase in antivaccine social media accounts since 2019.
“Fear tactics are often used by antivaccine campaigners to dissuade parents from vaccinating their children. There have been several myths propagated about vaccines causing adverse reactions,” the authors wrote. “Although these myths have been scientifically debunked, they continue to circulate.”
In contrast to parents’ concerns, a study in 2021 found a downward trend in reports of nonserious adverse effects and no change in reports of serious adverse effects from the HPV vaccine between 2015 and 2018. Further, more than 95% of the adverse effect reports to the Vaccine Adverse Event Reporting System after HPV vaccination were nonserious.
Reducing perceived barriers
Meanwhile, however, parents’ other reasons for avoiding the vaccine became less prevalent throughout most of the study period. For each year between 2013 and 2020, the proportion of parents saying they didn’t intend to get their children the HPV vaccine because it was “not recommended” decreased by 6.8%.
Similarly, avoiding the vaccine due to “lack of knowledge” declined 9.9%, and avoidance because the child was “not sexually active” declined 5.9% each year from 2013 to 2020. No difference occurred during that time period regarding how frequently parents cited that the vaccine was “not necessary.”
“Decreases in the percentage of parents/guardians citing lack of provider recommendation, lack of knowledge, and child ‘not sexually active’ as the main reason for HPV vaccine hesitancy ... are encouraging and suggest that interventions have been successful in reducing perceived barriers to HPV vaccination,” the authors wrote.
Dr. Bednarczyk agreed that these findings were encouraging, underscoring that outreach and support for health care providers to give strong recommendations for the vaccine need to continue.
“But additionally, we need to find better ways to communicate about vaccine safety,” Dr. Bednarczyk said. “Seeing that the number of parents citing safety concerns as the primary barrier has not changed much between 2016 and 2020, but that the percent of parents having those concerns increased, likely means there is a stable part of the population with these safety concerns, and as more adolescents are getting vaccinated against HPV, the relative contribution of safety concerns is increasing.” A key way to address those concerns includes “engaging with our trusted community partners and giving them the tools to discuss the safety of HPV vaccination with members of the community,” he said.
Debunking misinformation
Like the authors, Dr. Bednarczyk pointed out several conditions that parents erroneously worry could be caused by the HPV vaccine, but he emphasized that simply telling parents those misconceptions are untrue is insufficient to allay fears.
“It’s important for both clinicians and community partners to recognize we cannot just present a list of facts and figures and statistics to parents to reassure them and hope that this works,” Dr. Bednarczyk said. “Effective communication, strong narratives to illustrate this knowledge, and engagement with not just clinicians but community partners and other trusted sources is needed.” Dr. Bednarczyk continues to support the evidence-based model of presumptive recommendations, which does not remove parental autonomy but simplifies vaccine messaging about what’s recommended, “but clinicians need to be prepared with both the data and effective ways to communicate it to address questions if they come up after the presumptive recommendation is given,” he added.
The researchers pointed out that their study data were collected before the pandemic, so “it is reasonable to expect that HPV vaccine–related safety concerns may continue to rise because of the plethora of misinformation surrounding coronavirus disease 2019 vaccination.”
Dr. Bednarczyk said it will be important to see in future research whether shifts in beliefs about the HPV vaccine have occurred in the midst of the pandemic and afterward.
“As the authors stated, it’s important to remember that HPV vaccination has consistently been shown to be safe and effective,” Dr. Bednarczyk said. “But those research findings are not seeming to resonate with parents, highlighting how we need to improve our outreach and communication work.”
The research did not receive external funding. A coauthor is a scientific adviser to Navigating Cancer. The other authors and Dr. Bednarczyk had no disclosures.
, according to a study published online in Pediatrics.
“Although HPV vaccination rates in the United States have steadily improved over the past decade, a sizable subset of parents remains highly hesitant about administering the vaccine to their adolescent children,” wrote Eric Adjei Boakye, PhD, of the departments of public health sciences and otolaryngology–head and neck surgery at the Henry Ford Health System, Detroit, and associates. But a silver lining in the study is the downward trend in parents not vaccinating their children against HPV because the child’s provider did not recommend it.
“Provider recommendation has been shown to be the single best predictor of HPV vaccine uptake and vaccine acceptability,” the authors wrote. They noted one previous study finding that provider recommendations for the vaccine had increased from 27% in 2012 to 49.3% in 2018.
Safety concerns increased while other concerns decreased
The findings were not surprising to Robert A. Bednarczyk, PhD, associate professor of global health at Emory University Rollins School of Public Health, Atlanta, who specializes in HPV vaccine research.
“We have seen over the years that vaccine safety concerns have been on the increase, notably recently in the context of the COVID-19 pandemic and vaccination program, but HPV vaccine safety, though well established, continues to be a major concern for parents,” Dr. Bednarczyk said in an interview. But he found it striking that parents’ other reasons for turning down the vaccine had declined. “This shows that the outreach around the need for HPV vaccination and efforts to improve provider recommendation strategies is likely having positive impacts on HPV vaccine attitudes.”
Top five reasons for not vaccinating
The researchers analyzed data from the National Immunization Survey–Teen for the years 2010 through 2020 to track the annual changes in the top five reasons cited for not planning to get the HPV vaccine. The data covered 119,695 teens aged 13-17.
The researchers identified parents’ five most commonly cited reasons for not planning to vaccinate their children against HPV: “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
Parents’ HPV vaccine hesitancy decreased by 5.5% each year from 2010 to 2012, but then it stagnated for the remaining years through 2020. Across most of that time, from 2010 to 2018, parents’ concerns about the vaccine’s safety and side effects increased by 15.6%. A major reason for this increase, the authors suggested, may include the widespread distribution of online misinformation, particularly given the 7.8 million increase in antivaccine social media accounts since 2019.
“Fear tactics are often used by antivaccine campaigners to dissuade parents from vaccinating their children. There have been several myths propagated about vaccines causing adverse reactions,” the authors wrote. “Although these myths have been scientifically debunked, they continue to circulate.”
In contrast to parents’ concerns, a study in 2021 found a downward trend in reports of nonserious adverse effects and no change in reports of serious adverse effects from the HPV vaccine between 2015 and 2018. Further, more than 95% of the adverse effect reports to the Vaccine Adverse Event Reporting System after HPV vaccination were nonserious.
Reducing perceived barriers
Meanwhile, however, parents’ other reasons for avoiding the vaccine became less prevalent throughout most of the study period. For each year between 2013 and 2020, the proportion of parents saying they didn’t intend to get their children the HPV vaccine because it was “not recommended” decreased by 6.8%.
Similarly, avoiding the vaccine due to “lack of knowledge” declined 9.9%, and avoidance because the child was “not sexually active” declined 5.9% each year from 2013 to 2020. No difference occurred during that time period regarding how frequently parents cited that the vaccine was “not necessary.”
“Decreases in the percentage of parents/guardians citing lack of provider recommendation, lack of knowledge, and child ‘not sexually active’ as the main reason for HPV vaccine hesitancy ... are encouraging and suggest that interventions have been successful in reducing perceived barriers to HPV vaccination,” the authors wrote.
Dr. Bednarczyk agreed that these findings were encouraging, underscoring that outreach and support for health care providers to give strong recommendations for the vaccine need to continue.
“But additionally, we need to find better ways to communicate about vaccine safety,” Dr. Bednarczyk said. “Seeing that the number of parents citing safety concerns as the primary barrier has not changed much between 2016 and 2020, but that the percent of parents having those concerns increased, likely means there is a stable part of the population with these safety concerns, and as more adolescents are getting vaccinated against HPV, the relative contribution of safety concerns is increasing.” A key way to address those concerns includes “engaging with our trusted community partners and giving them the tools to discuss the safety of HPV vaccination with members of the community,” he said.
Debunking misinformation
Like the authors, Dr. Bednarczyk pointed out several conditions that parents erroneously worry could be caused by the HPV vaccine, but he emphasized that simply telling parents those misconceptions are untrue is insufficient to allay fears.
“It’s important for both clinicians and community partners to recognize we cannot just present a list of facts and figures and statistics to parents to reassure them and hope that this works,” Dr. Bednarczyk said. “Effective communication, strong narratives to illustrate this knowledge, and engagement with not just clinicians but community partners and other trusted sources is needed.” Dr. Bednarczyk continues to support the evidence-based model of presumptive recommendations, which does not remove parental autonomy but simplifies vaccine messaging about what’s recommended, “but clinicians need to be prepared with both the data and effective ways to communicate it to address questions if they come up after the presumptive recommendation is given,” he added.
The researchers pointed out that their study data were collected before the pandemic, so “it is reasonable to expect that HPV vaccine–related safety concerns may continue to rise because of the plethora of misinformation surrounding coronavirus disease 2019 vaccination.”
Dr. Bednarczyk said it will be important to see in future research whether shifts in beliefs about the HPV vaccine have occurred in the midst of the pandemic and afterward.
“As the authors stated, it’s important to remember that HPV vaccination has consistently been shown to be safe and effective,” Dr. Bednarczyk said. “But those research findings are not seeming to resonate with parents, highlighting how we need to improve our outreach and communication work.”
The research did not receive external funding. A coauthor is a scientific adviser to Navigating Cancer. The other authors and Dr. Bednarczyk had no disclosures.
, according to a study published online in Pediatrics.
“Although HPV vaccination rates in the United States have steadily improved over the past decade, a sizable subset of parents remains highly hesitant about administering the vaccine to their adolescent children,” wrote Eric Adjei Boakye, PhD, of the departments of public health sciences and otolaryngology–head and neck surgery at the Henry Ford Health System, Detroit, and associates. But a silver lining in the study is the downward trend in parents not vaccinating their children against HPV because the child’s provider did not recommend it.
“Provider recommendation has been shown to be the single best predictor of HPV vaccine uptake and vaccine acceptability,” the authors wrote. They noted one previous study finding that provider recommendations for the vaccine had increased from 27% in 2012 to 49.3% in 2018.
Safety concerns increased while other concerns decreased
The findings were not surprising to Robert A. Bednarczyk, PhD, associate professor of global health at Emory University Rollins School of Public Health, Atlanta, who specializes in HPV vaccine research.
“We have seen over the years that vaccine safety concerns have been on the increase, notably recently in the context of the COVID-19 pandemic and vaccination program, but HPV vaccine safety, though well established, continues to be a major concern for parents,” Dr. Bednarczyk said in an interview. But he found it striking that parents’ other reasons for turning down the vaccine had declined. “This shows that the outreach around the need for HPV vaccination and efforts to improve provider recommendation strategies is likely having positive impacts on HPV vaccine attitudes.”
Top five reasons for not vaccinating
The researchers analyzed data from the National Immunization Survey–Teen for the years 2010 through 2020 to track the annual changes in the top five reasons cited for not planning to get the HPV vaccine. The data covered 119,695 teens aged 13-17.
The researchers identified parents’ five most commonly cited reasons for not planning to vaccinate their children against HPV: “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
Parents’ HPV vaccine hesitancy decreased by 5.5% each year from 2010 to 2012, but then it stagnated for the remaining years through 2020. Across most of that time, from 2010 to 2018, parents’ concerns about the vaccine’s safety and side effects increased by 15.6%. A major reason for this increase, the authors suggested, may include the widespread distribution of online misinformation, particularly given the 7.8 million increase in antivaccine social media accounts since 2019.
“Fear tactics are often used by antivaccine campaigners to dissuade parents from vaccinating their children. There have been several myths propagated about vaccines causing adverse reactions,” the authors wrote. “Although these myths have been scientifically debunked, they continue to circulate.”
In contrast to parents’ concerns, a study in 2021 found a downward trend in reports of nonserious adverse effects and no change in reports of serious adverse effects from the HPV vaccine between 2015 and 2018. Further, more than 95% of the adverse effect reports to the Vaccine Adverse Event Reporting System after HPV vaccination were nonserious.
Reducing perceived barriers
Meanwhile, however, parents’ other reasons for avoiding the vaccine became less prevalent throughout most of the study period. For each year between 2013 and 2020, the proportion of parents saying they didn’t intend to get their children the HPV vaccine because it was “not recommended” decreased by 6.8%.
Similarly, avoiding the vaccine due to “lack of knowledge” declined 9.9%, and avoidance because the child was “not sexually active” declined 5.9% each year from 2013 to 2020. No difference occurred during that time period regarding how frequently parents cited that the vaccine was “not necessary.”
“Decreases in the percentage of parents/guardians citing lack of provider recommendation, lack of knowledge, and child ‘not sexually active’ as the main reason for HPV vaccine hesitancy ... are encouraging and suggest that interventions have been successful in reducing perceived barriers to HPV vaccination,” the authors wrote.
Dr. Bednarczyk agreed that these findings were encouraging, underscoring that outreach and support for health care providers to give strong recommendations for the vaccine need to continue.
“But additionally, we need to find better ways to communicate about vaccine safety,” Dr. Bednarczyk said. “Seeing that the number of parents citing safety concerns as the primary barrier has not changed much between 2016 and 2020, but that the percent of parents having those concerns increased, likely means there is a stable part of the population with these safety concerns, and as more adolescents are getting vaccinated against HPV, the relative contribution of safety concerns is increasing.” A key way to address those concerns includes “engaging with our trusted community partners and giving them the tools to discuss the safety of HPV vaccination with members of the community,” he said.
Debunking misinformation
Like the authors, Dr. Bednarczyk pointed out several conditions that parents erroneously worry could be caused by the HPV vaccine, but he emphasized that simply telling parents those misconceptions are untrue is insufficient to allay fears.
“It’s important for both clinicians and community partners to recognize we cannot just present a list of facts and figures and statistics to parents to reassure them and hope that this works,” Dr. Bednarczyk said. “Effective communication, strong narratives to illustrate this knowledge, and engagement with not just clinicians but community partners and other trusted sources is needed.” Dr. Bednarczyk continues to support the evidence-based model of presumptive recommendations, which does not remove parental autonomy but simplifies vaccine messaging about what’s recommended, “but clinicians need to be prepared with both the data and effective ways to communicate it to address questions if they come up after the presumptive recommendation is given,” he added.
The researchers pointed out that their study data were collected before the pandemic, so “it is reasonable to expect that HPV vaccine–related safety concerns may continue to rise because of the plethora of misinformation surrounding coronavirus disease 2019 vaccination.”
Dr. Bednarczyk said it will be important to see in future research whether shifts in beliefs about the HPV vaccine have occurred in the midst of the pandemic and afterward.
“As the authors stated, it’s important to remember that HPV vaccination has consistently been shown to be safe and effective,” Dr. Bednarczyk said. “But those research findings are not seeming to resonate with parents, highlighting how we need to improve our outreach and communication work.”
The research did not receive external funding. A coauthor is a scientific adviser to Navigating Cancer. The other authors and Dr. Bednarczyk had no disclosures.
FROM PEDIATRICS
Severe rash after COVID-19 vaccination
A 41-year-old man presented for evaluation of an extensive skin rash that had erupted more than a month earlier. The patient had received 2 doses of the Pfizer COVID-19 vaccine 3 weeks apart. Ten days after his second dose, the patient developed a rash all over his body. He described the rash as burning, itchy, and uncomfortable. The patient denied any triggers such as recent or previous infections, stressors, or drugs. The patient had no personal or family history of dermatologic disorders; his general medical history was unremarkable. The patient smoked and drank alcohol occasionally.
On physical exam, the patient had a diffuse rash, which initially had manifested on both of his hands, including the palms, and then spread to 60% to 70% of his total body surface area, including his face, ears, anterior and posterior chest, upper and lower extremities, and buttocks. The rash consisted of 10- to 15-mm white scaly plaques that did not bleed.
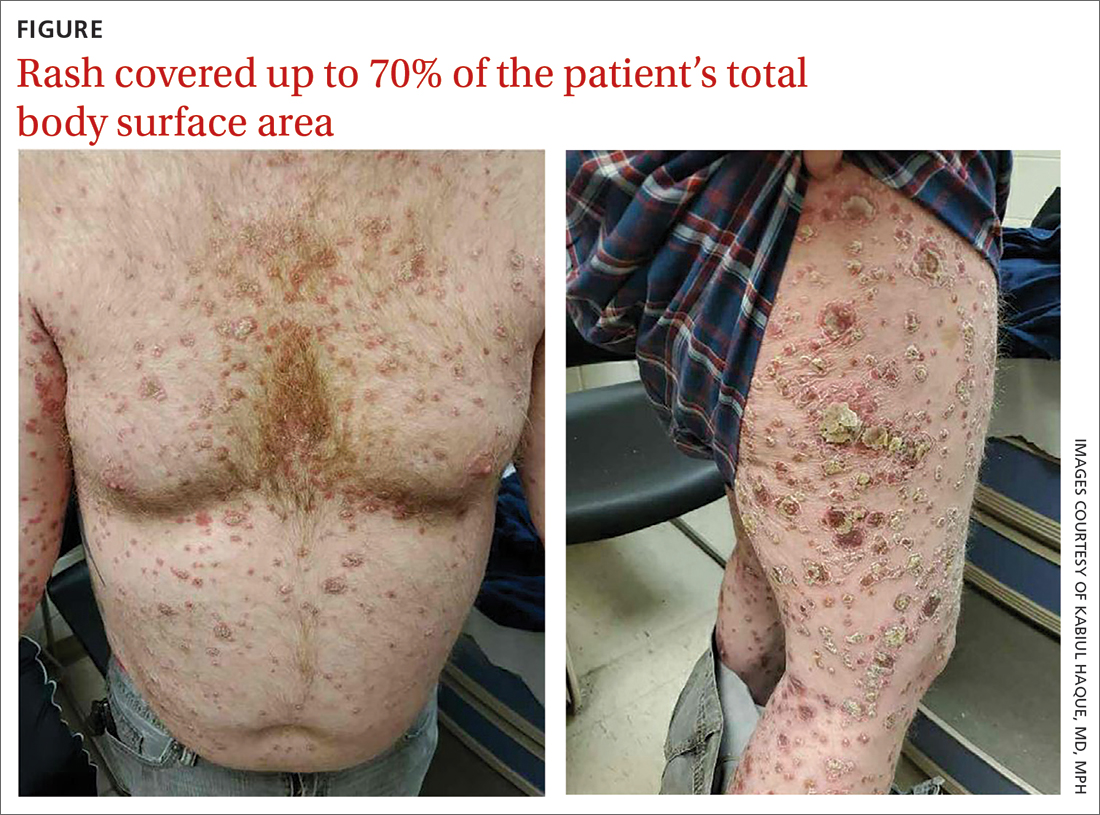
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
Punch biopsies were obtained, and histopathology revealed diffuse compact hyperkeratosis with broad zones of parakeratosis. There was attenuation of the granular layer and regular elongation of the rete ridges associated with thinning of the suprapapillary epidermis and mild spongiosis. These pathologic findings were consistent with a diagnosis of psoriasis. There were no drug-related skin eruption features, such as apoptotic keratinocytes, eosinophils, or interface dermatitis. Periodic acid-Schiff stains for fungal organisms were negative. The combined clinical presentation (itchy, teardrop-shaped, scaly lesions) and histologic impression were consistent with guttate psoriasis.
Psoriasis can be seen in various forms. Subtypes of psoriasis include guttate psoriasis, inverse psoriasis, erythrodermic psoriasis, nail psoriasis, and pustular psoriasis.1 Guttate psoriasis accounts for about 2% of psoriasis cases and usually is seen in patients younger than 30 years.2 Guttate psoriasis is characterized by 1- to 10-mm teardrop-shaped pink papules with fine scaling.3
Triggers for psoriasis. Vaccinations, medications, and infections (eg, group A beta-hemolytic streptococcal upper respiratory infections) can trigger guttate psoriasis.3 MRNA vaccines (eg, Moderna and Pfizer/BioNTech COVID-19 vaccines) have been associated with psoriasis episodes.1 Other vaccines such as influenza, rubella, bacillus Calmette-Guerin, tetanus-diphtheria, and pneumococcal polysaccharide also have been known to trigger psoriasis.4 Medications that can trigger psoriasis include beta-blockers, lithium, antimalarial drugs, and (in some cases) nonsteroidal anti-inflammatory drugs.5
The impact of COVID-19 vaccine. We are still learning about the incidence and prevalence of adverse effects (such as psoriasis) that can follow COVID-19 vaccination.
Psoriasis following vaccination. The pathologic mechanism for the new onset or flare of psoriasis after COVID-19 vaccination is unknown. What is known is that the dysregulation of Th-1 and Th-17 plays an important role in the pathogenesis of psoriasis.7 Previously, it was found that psoriasis can manifest after tetanus-diphtheria vaccines due to an increase in the production of Th-17 cells.7 Th-1 and Th-17 production also increases after influenza vaccine and can cause an onset or flare-up of psoriasis.8
Continue to: The differential includes syphilis and exfoliative dermatitis
The differential includes syphilis and exfoliative dermatitis
The differential diagnosis includes various forms of psoriasiform dermatitis, such as secondary syphilis, chronic spongiotic dermatitis, psoriasiform drug eruption, exfoliative dermatitis, and pityriasis rubra pilaris. A combination of clinical and histopathologic findings is used to zero in on the diagnosis. The summary below highlights the clinical findings.
Secondary syphilis manifests with symmetric papular eruptions primarily on the trunk and extremities with involvement on the palms and soles. Lesions are red or reddish brown, can be smooth, and are rarely pustular.
Chronic spongiotic dermatitis manifests with a shiny, glazed, cracked appearance and itchy reddish lesions on the soles.
Psoriasiform drug eruption manifests after drug administration with a psoriasis-like rash with erythematous, squamous, thick, dry, and plaque-type lesions.
Exfoliative dermatitis manifests with erythematous single or multiple pruritic patches on the trunk, head, and genitals.
Continue to: Pityriasis rubra pilaris
Pityriasis rubra pilaris manifests in various ways. Patients may have plaques that are erythematous, scaly, or follicular. Sometimes, it may manifest as erythroderma with an “island of sparing,” which is normal-looking skin in the affected areas.
How to make the diagnosis
Psoriasis can be diagnosed by physical examination. A skin biopsy is not usually necessary but can be helpful for complex cases.
There are no laboratory or genetic tests to confirm the diagnosis of psoriasis. Depending on the case, routine bloodwork (eg, complete blood count and metabolic panel) and infectious disease tests (eg, HIV, hepatitis panel, and
Starting with a low- to medium-potency steroid, such as betamethasone valerate 0.1% cream twice per day or triamcinolone acetonide 0.1% cream twice per day for 2 weeks, provides high safety and efficacy for localized disease.9 An appropriate-potency steroid should be chosen based on the disease severity, location, and patient’s preference and age. Topical vitamin D analogues often are used in conjunction with topical steroids to treat psoriasis.9
Depending on the severity, patient age, comorbidities, and availability of treatment, other treatment options for psoriasis include oral methotrexate (2.5 mg to 25 mg weekly, starting with a low dose), acitretin (10 mg to 50 mg daily), apremilast (10 mg daily, gradually increasing to 30 mg twice per day in a divided dose), biologics, and narrowband ultraviolet light.
In this case, betamethasone dipropionate 0.05% cream twice daily for 2 weeks was not sufficiently effective due to the extent of the psoriasis. Following consultation with a dermatologist, clobetasol propionate 0.05% cream twice per day and oral apremilast (10 mg once per day on the first day and 10 mg twice per day afterward) were prescribed for 2 weeks. The patient’s psoriasis improved somewhat after 2 weeks of the treatment, but many plaques remained. Therefore, apremilast was stopped and subcutaneous adalimumab was started (initial loading dose, 80 mg, then 40 mg every other week). The psoriasis lesions cleared over the next 2 to 3 months. The patient was maintained on the adalimumab to avoid a recurrence of lesions.
1. Wu PC, Huang IH, Wang CW, et al. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23:775-799. doi: 10.1007/s40257-022-00721-z
2. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. doi: 10.1016/j.jaad.2008.02.039
3. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
4. Wei N, Kresch M, Elbogen E, et al. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74-77. doi: 10.1016/j.jdcr.2021.11.016
5. Piérard-Franchimont C, Piérard GE. L’iatrogénie psoriasique [Drug-related psoriasis]. Rev Med Liege. 2012;67:139-142. French.
6. Huang Y, Tsai T. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 8:812010. doi: 10.3389/fmed.2021.812010
7. Pesque D, Lopez-Trujillo E, Marcantonio O, et al. New-onset and exacerbation of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36:e80-e157 doi: 10.1111/jdv.17690
8. Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. doi: 10.1155/2015/258430
9. Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi: 10.1016/j.jaad.2020.07.087
A 41-year-old man presented for evaluation of an extensive skin rash that had erupted more than a month earlier. The patient had received 2 doses of the Pfizer COVID-19 vaccine 3 weeks apart. Ten days after his second dose, the patient developed a rash all over his body. He described the rash as burning, itchy, and uncomfortable. The patient denied any triggers such as recent or previous infections, stressors, or drugs. The patient had no personal or family history of dermatologic disorders; his general medical history was unremarkable. The patient smoked and drank alcohol occasionally.
On physical exam, the patient had a diffuse rash, which initially had manifested on both of his hands, including the palms, and then spread to 60% to 70% of his total body surface area, including his face, ears, anterior and posterior chest, upper and lower extremities, and buttocks. The rash consisted of 10- to 15-mm white scaly plaques that did not bleed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
Punch biopsies were obtained, and histopathology revealed diffuse compact hyperkeratosis with broad zones of parakeratosis. There was attenuation of the granular layer and regular elongation of the rete ridges associated with thinning of the suprapapillary epidermis and mild spongiosis. These pathologic findings were consistent with a diagnosis of psoriasis. There were no drug-related skin eruption features, such as apoptotic keratinocytes, eosinophils, or interface dermatitis. Periodic acid-Schiff stains for fungal organisms were negative. The combined clinical presentation (itchy, teardrop-shaped, scaly lesions) and histologic impression were consistent with guttate psoriasis.
Psoriasis can be seen in various forms. Subtypes of psoriasis include guttate psoriasis, inverse psoriasis, erythrodermic psoriasis, nail psoriasis, and pustular psoriasis.1 Guttate psoriasis accounts for about 2% of psoriasis cases and usually is seen in patients younger than 30 years.2 Guttate psoriasis is characterized by 1- to 10-mm teardrop-shaped pink papules with fine scaling.3
Triggers for psoriasis. Vaccinations, medications, and infections (eg, group A beta-hemolytic streptococcal upper respiratory infections) can trigger guttate psoriasis.3 MRNA vaccines (eg, Moderna and Pfizer/BioNTech COVID-19 vaccines) have been associated with psoriasis episodes.1 Other vaccines such as influenza, rubella, bacillus Calmette-Guerin, tetanus-diphtheria, and pneumococcal polysaccharide also have been known to trigger psoriasis.4 Medications that can trigger psoriasis include beta-blockers, lithium, antimalarial drugs, and (in some cases) nonsteroidal anti-inflammatory drugs.5
The impact of COVID-19 vaccine. We are still learning about the incidence and prevalence of adverse effects (such as psoriasis) that can follow COVID-19 vaccination.
Psoriasis following vaccination. The pathologic mechanism for the new onset or flare of psoriasis after COVID-19 vaccination is unknown. What is known is that the dysregulation of Th-1 and Th-17 plays an important role in the pathogenesis of psoriasis.7 Previously, it was found that psoriasis can manifest after tetanus-diphtheria vaccines due to an increase in the production of Th-17 cells.7 Th-1 and Th-17 production also increases after influenza vaccine and can cause an onset or flare-up of psoriasis.8
Continue to: The differential includes syphilis and exfoliative dermatitis
The differential includes syphilis and exfoliative dermatitis
The differential diagnosis includes various forms of psoriasiform dermatitis, such as secondary syphilis, chronic spongiotic dermatitis, psoriasiform drug eruption, exfoliative dermatitis, and pityriasis rubra pilaris. A combination of clinical and histopathologic findings is used to zero in on the diagnosis. The summary below highlights the clinical findings.
Secondary syphilis manifests with symmetric papular eruptions primarily on the trunk and extremities with involvement on the palms and soles. Lesions are red or reddish brown, can be smooth, and are rarely pustular.
Chronic spongiotic dermatitis manifests with a shiny, glazed, cracked appearance and itchy reddish lesions on the soles.
Psoriasiform drug eruption manifests after drug administration with a psoriasis-like rash with erythematous, squamous, thick, dry, and plaque-type lesions.
Exfoliative dermatitis manifests with erythematous single or multiple pruritic patches on the trunk, head, and genitals.
Continue to: Pityriasis rubra pilaris
Pityriasis rubra pilaris manifests in various ways. Patients may have plaques that are erythematous, scaly, or follicular. Sometimes, it may manifest as erythroderma with an “island of sparing,” which is normal-looking skin in the affected areas.
How to make the diagnosis
Psoriasis can be diagnosed by physical examination. A skin biopsy is not usually necessary but can be helpful for complex cases.
There are no laboratory or genetic tests to confirm the diagnosis of psoriasis. Depending on the case, routine bloodwork (eg, complete blood count and metabolic panel) and infectious disease tests (eg, HIV, hepatitis panel, and
Starting with a low- to medium-potency steroid, such as betamethasone valerate 0.1% cream twice per day or triamcinolone acetonide 0.1% cream twice per day for 2 weeks, provides high safety and efficacy for localized disease.9 An appropriate-potency steroid should be chosen based on the disease severity, location, and patient’s preference and age. Topical vitamin D analogues often are used in conjunction with topical steroids to treat psoriasis.9
Depending on the severity, patient age, comorbidities, and availability of treatment, other treatment options for psoriasis include oral methotrexate (2.5 mg to 25 mg weekly, starting with a low dose), acitretin (10 mg to 50 mg daily), apremilast (10 mg daily, gradually increasing to 30 mg twice per day in a divided dose), biologics, and narrowband ultraviolet light.
In this case, betamethasone dipropionate 0.05% cream twice daily for 2 weeks was not sufficiently effective due to the extent of the psoriasis. Following consultation with a dermatologist, clobetasol propionate 0.05% cream twice per day and oral apremilast (10 mg once per day on the first day and 10 mg twice per day afterward) were prescribed for 2 weeks. The patient’s psoriasis improved somewhat after 2 weeks of the treatment, but many plaques remained. Therefore, apremilast was stopped and subcutaneous adalimumab was started (initial loading dose, 80 mg, then 40 mg every other week). The psoriasis lesions cleared over the next 2 to 3 months. The patient was maintained on the adalimumab to avoid a recurrence of lesions.
A 41-year-old man presented for evaluation of an extensive skin rash that had erupted more than a month earlier. The patient had received 2 doses of the Pfizer COVID-19 vaccine 3 weeks apart. Ten days after his second dose, the patient developed a rash all over his body. He described the rash as burning, itchy, and uncomfortable. The patient denied any triggers such as recent or previous infections, stressors, or drugs. The patient had no personal or family history of dermatologic disorders; his general medical history was unremarkable. The patient smoked and drank alcohol occasionally.
On physical exam, the patient had a diffuse rash, which initially had manifested on both of his hands, including the palms, and then spread to 60% to 70% of his total body surface area, including his face, ears, anterior and posterior chest, upper and lower extremities, and buttocks. The rash consisted of 10- to 15-mm white scaly plaques that did not bleed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
Punch biopsies were obtained, and histopathology revealed diffuse compact hyperkeratosis with broad zones of parakeratosis. There was attenuation of the granular layer and regular elongation of the rete ridges associated with thinning of the suprapapillary epidermis and mild spongiosis. These pathologic findings were consistent with a diagnosis of psoriasis. There were no drug-related skin eruption features, such as apoptotic keratinocytes, eosinophils, or interface dermatitis. Periodic acid-Schiff stains for fungal organisms were negative. The combined clinical presentation (itchy, teardrop-shaped, scaly lesions) and histologic impression were consistent with guttate psoriasis.
Psoriasis can be seen in various forms. Subtypes of psoriasis include guttate psoriasis, inverse psoriasis, erythrodermic psoriasis, nail psoriasis, and pustular psoriasis.1 Guttate psoriasis accounts for about 2% of psoriasis cases and usually is seen in patients younger than 30 years.2 Guttate psoriasis is characterized by 1- to 10-mm teardrop-shaped pink papules with fine scaling.3
Triggers for psoriasis. Vaccinations, medications, and infections (eg, group A beta-hemolytic streptococcal upper respiratory infections) can trigger guttate psoriasis.3 MRNA vaccines (eg, Moderna and Pfizer/BioNTech COVID-19 vaccines) have been associated with psoriasis episodes.1 Other vaccines such as influenza, rubella, bacillus Calmette-Guerin, tetanus-diphtheria, and pneumococcal polysaccharide also have been known to trigger psoriasis.4 Medications that can trigger psoriasis include beta-blockers, lithium, antimalarial drugs, and (in some cases) nonsteroidal anti-inflammatory drugs.5
The impact of COVID-19 vaccine. We are still learning about the incidence and prevalence of adverse effects (such as psoriasis) that can follow COVID-19 vaccination.
Psoriasis following vaccination. The pathologic mechanism for the new onset or flare of psoriasis after COVID-19 vaccination is unknown. What is known is that the dysregulation of Th-1 and Th-17 plays an important role in the pathogenesis of psoriasis.7 Previously, it was found that psoriasis can manifest after tetanus-diphtheria vaccines due to an increase in the production of Th-17 cells.7 Th-1 and Th-17 production also increases after influenza vaccine and can cause an onset or flare-up of psoriasis.8
Continue to: The differential includes syphilis and exfoliative dermatitis
The differential includes syphilis and exfoliative dermatitis
The differential diagnosis includes various forms of psoriasiform dermatitis, such as secondary syphilis, chronic spongiotic dermatitis, psoriasiform drug eruption, exfoliative dermatitis, and pityriasis rubra pilaris. A combination of clinical and histopathologic findings is used to zero in on the diagnosis. The summary below highlights the clinical findings.
Secondary syphilis manifests with symmetric papular eruptions primarily on the trunk and extremities with involvement on the palms and soles. Lesions are red or reddish brown, can be smooth, and are rarely pustular.
Chronic spongiotic dermatitis manifests with a shiny, glazed, cracked appearance and itchy reddish lesions on the soles.
Psoriasiform drug eruption manifests after drug administration with a psoriasis-like rash with erythematous, squamous, thick, dry, and plaque-type lesions.
Exfoliative dermatitis manifests with erythematous single or multiple pruritic patches on the trunk, head, and genitals.
Continue to: Pityriasis rubra pilaris
Pityriasis rubra pilaris manifests in various ways. Patients may have plaques that are erythematous, scaly, or follicular. Sometimes, it may manifest as erythroderma with an “island of sparing,” which is normal-looking skin in the affected areas.
How to make the diagnosis
Psoriasis can be diagnosed by physical examination. A skin biopsy is not usually necessary but can be helpful for complex cases.
There are no laboratory or genetic tests to confirm the diagnosis of psoriasis. Depending on the case, routine bloodwork (eg, complete blood count and metabolic panel) and infectious disease tests (eg, HIV, hepatitis panel, and
Starting with a low- to medium-potency steroid, such as betamethasone valerate 0.1% cream twice per day or triamcinolone acetonide 0.1% cream twice per day for 2 weeks, provides high safety and efficacy for localized disease.9 An appropriate-potency steroid should be chosen based on the disease severity, location, and patient’s preference and age. Topical vitamin D analogues often are used in conjunction with topical steroids to treat psoriasis.9
Depending on the severity, patient age, comorbidities, and availability of treatment, other treatment options for psoriasis include oral methotrexate (2.5 mg to 25 mg weekly, starting with a low dose), acitretin (10 mg to 50 mg daily), apremilast (10 mg daily, gradually increasing to 30 mg twice per day in a divided dose), biologics, and narrowband ultraviolet light.
In this case, betamethasone dipropionate 0.05% cream twice daily for 2 weeks was not sufficiently effective due to the extent of the psoriasis. Following consultation with a dermatologist, clobetasol propionate 0.05% cream twice per day and oral apremilast (10 mg once per day on the first day and 10 mg twice per day afterward) were prescribed for 2 weeks. The patient’s psoriasis improved somewhat after 2 weeks of the treatment, but many plaques remained. Therefore, apremilast was stopped and subcutaneous adalimumab was started (initial loading dose, 80 mg, then 40 mg every other week). The psoriasis lesions cleared over the next 2 to 3 months. The patient was maintained on the adalimumab to avoid a recurrence of lesions.
1. Wu PC, Huang IH, Wang CW, et al. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23:775-799. doi: 10.1007/s40257-022-00721-z
2. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. doi: 10.1016/j.jaad.2008.02.039
3. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
4. Wei N, Kresch M, Elbogen E, et al. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74-77. doi: 10.1016/j.jdcr.2021.11.016
5. Piérard-Franchimont C, Piérard GE. L’iatrogénie psoriasique [Drug-related psoriasis]. Rev Med Liege. 2012;67:139-142. French.
6. Huang Y, Tsai T. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 8:812010. doi: 10.3389/fmed.2021.812010
7. Pesque D, Lopez-Trujillo E, Marcantonio O, et al. New-onset and exacerbation of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36:e80-e157 doi: 10.1111/jdv.17690
8. Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. doi: 10.1155/2015/258430
9. Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi: 10.1016/j.jaad.2020.07.087
1. Wu PC, Huang IH, Wang CW, et al. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23:775-799. doi: 10.1007/s40257-022-00721-z
2. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. doi: 10.1016/j.jaad.2008.02.039
3. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
4. Wei N, Kresch M, Elbogen E, et al. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74-77. doi: 10.1016/j.jdcr.2021.11.016
5. Piérard-Franchimont C, Piérard GE. L’iatrogénie psoriasique [Drug-related psoriasis]. Rev Med Liege. 2012;67:139-142. French.
6. Huang Y, Tsai T. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 8:812010. doi: 10.3389/fmed.2021.812010
7. Pesque D, Lopez-Trujillo E, Marcantonio O, et al. New-onset and exacerbation of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36:e80-e157 doi: 10.1111/jdv.17690
8. Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. doi: 10.1155/2015/258430
9. Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi: 10.1016/j.jaad.2020.07.087