User login
ASCT may cure follicular lymphoma for some rituximab-naive patients
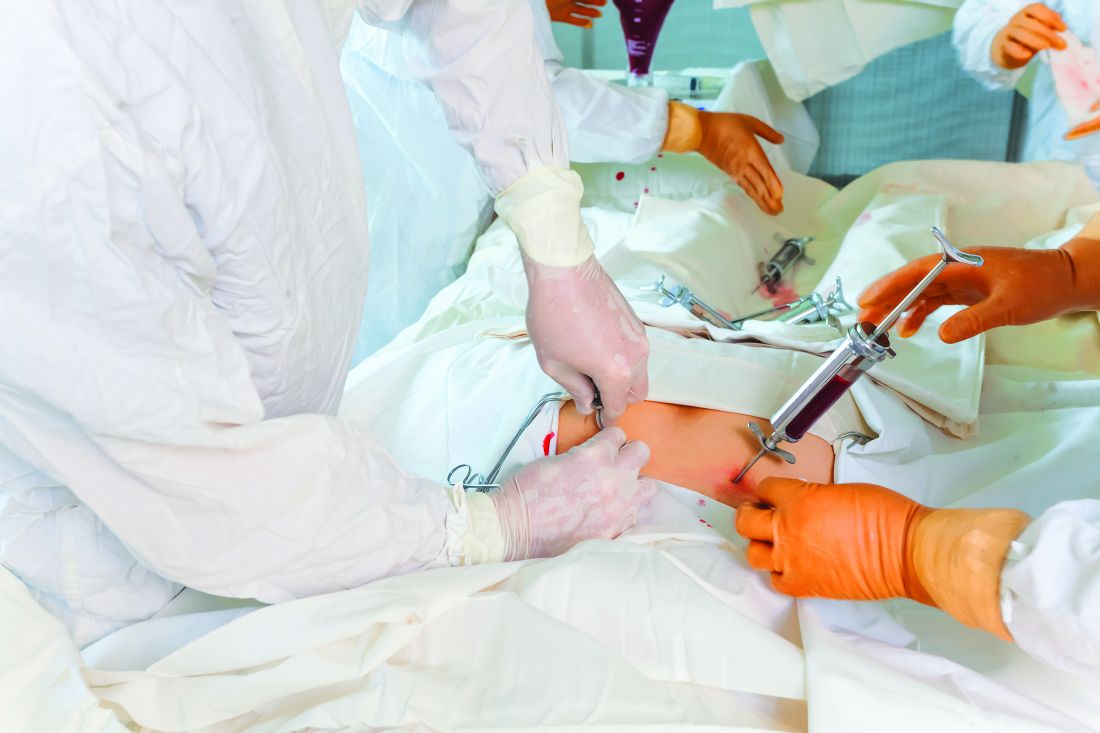
Prompt autologous stem cell transplantation (ASCT) is often curative in rituximab-naive patients with follicular lymphoma who have experienced early failure of first-line therapy and achieved a response to second-line therapy, suggest results from a registry-based study conducted by GELTAMO (the Spanish Lymphoma and Bone Marrow Transplant Group).
“Overall, our results suggest that, whereas some patients might benefit from more aggressive therapies, such as allogenic stem cell transplantations, or novel drugs, such as immunomodulatory agents, monoclonal antibodies, phosphoinositide 3-kinase inhibitors, or even the application of bispecific T-cell engagers and chimeric antigen receptor T cells, there are a considerable number of patients in this high-risk [early therapy failure] subgroup that can be cured with ASCT, even in the absence of rituximab,” Ana Jiménez-Ubieto, MD, PhD, of the Hospital Universitario, 12 de Octubre, Madrid, Spain, and colleagues wrote.
The results are more favorable when ASCT is performed in patients experiencing early therapy failure, with less than 1 year from first relapse after primary treatment to ASCT.
“Early ASCT could be a hopeful option in patients with difficult access to rituximab,” the researchers wrote in Hematology/Oncology and Stem Cell Therapy.
Patients with follicular lymphoma who experience relapse or progression during or soon after first-line therapy have poor overall survival, and there is no standard therapy for this population, according to the researchers. Previous research has shown that ASCT prolongs survival in those who have received rituximab before transplantation, but benefit in the absence of this agent is unknown.
Dr. Jiménez-Ubieto and colleagues conducted a multicenter registry-based retrospective cohort study of 134 patients with nontransformed follicular lymphoma who underwent ASCT during 1989-2007 while in second complete or partial response to rescue chemotherapy and had not received rituximab.
Overall, 65% of the patients had experienced early therapy failure (relapse or progression within 2 years of starting first-line chemotherapy). Within this group, 78% underwent ASCT within 1 year, and 67% underwent ASCT while in second complete response. Median posttransplantation follow-up for the entire study cohort was 13.4 years.
Study results showed that patients who had experienced early therapy failure versus who had not had poorer 5-year progression-free survival (43% vs. 57%; P = .048) but similar 5-year overall survival (69% vs. 77%; P = .4). However, those patients with early therapy failure who underwent ASCT within 1 year had a statistically indistinguishable 5-year progression-free survival relative to counterparts without early therapy failure (48% vs. 66%; P = .44).
Additionally, the 48% progression-free survival seen in this subset was almost identical to the 49% seen in a historical cohort of patients with early therapy failure who similarly underwent ASCT within 1 year of first relapse but received rituximab before transplantation (Hematol Oncol. 2018;36[5]:765-72). This suggests “that the possible synergistic effect of rituximab plus ASCT is not as relevant if ASCT is offered soon in the course of the disease,” the researchers wrote.
Patients who had experienced early therapy failure achieved better overall survival if they underwent ASCT while in second complete response, as opposed to second partial response. Notably, 56% of those who underwent ASCT while in second complete response were alive at 13.7 years of follow-up and remained so long term.
The study was funded by the Foundation Research Institute at the Hospital Universitario 12 de Octubre. The researchers reported having no relevant conflicts of interest.
SOURCE: Jiménez-Ubieto A et al. Hematol Oncol Stem Cell Ther. 2019 Jul 9. doi: 10.1016/j.hemonc.2019.06.001.
Prompt autologous stem cell transplantation (ASCT) is often curative in rituximab-naive patients with follicular lymphoma who have experienced early failure of first-line therapy and achieved a response to second-line therapy, suggest results from a registry-based study conducted by GELTAMO (the Spanish Lymphoma and Bone Marrow Transplant Group).
“Overall, our results suggest that, whereas some patients might benefit from more aggressive therapies, such as allogenic stem cell transplantations, or novel drugs, such as immunomodulatory agents, monoclonal antibodies, phosphoinositide 3-kinase inhibitors, or even the application of bispecific T-cell engagers and chimeric antigen receptor T cells, there are a considerable number of patients in this high-risk [early therapy failure] subgroup that can be cured with ASCT, even in the absence of rituximab,” Ana Jiménez-Ubieto, MD, PhD, of the Hospital Universitario, 12 de Octubre, Madrid, Spain, and colleagues wrote.
The results are more favorable when ASCT is performed in patients experiencing early therapy failure, with less than 1 year from first relapse after primary treatment to ASCT.
“Early ASCT could be a hopeful option in patients with difficult access to rituximab,” the researchers wrote in Hematology/Oncology and Stem Cell Therapy.
Patients with follicular lymphoma who experience relapse or progression during or soon after first-line therapy have poor overall survival, and there is no standard therapy for this population, according to the researchers. Previous research has shown that ASCT prolongs survival in those who have received rituximab before transplantation, but benefit in the absence of this agent is unknown.
Dr. Jiménez-Ubieto and colleagues conducted a multicenter registry-based retrospective cohort study of 134 patients with nontransformed follicular lymphoma who underwent ASCT during 1989-2007 while in second complete or partial response to rescue chemotherapy and had not received rituximab.
Overall, 65% of the patients had experienced early therapy failure (relapse or progression within 2 years of starting first-line chemotherapy). Within this group, 78% underwent ASCT within 1 year, and 67% underwent ASCT while in second complete response. Median posttransplantation follow-up for the entire study cohort was 13.4 years.
Study results showed that patients who had experienced early therapy failure versus who had not had poorer 5-year progression-free survival (43% vs. 57%; P = .048) but similar 5-year overall survival (69% vs. 77%; P = .4). However, those patients with early therapy failure who underwent ASCT within 1 year had a statistically indistinguishable 5-year progression-free survival relative to counterparts without early therapy failure (48% vs. 66%; P = .44).
Additionally, the 48% progression-free survival seen in this subset was almost identical to the 49% seen in a historical cohort of patients with early therapy failure who similarly underwent ASCT within 1 year of first relapse but received rituximab before transplantation (Hematol Oncol. 2018;36[5]:765-72). This suggests “that the possible synergistic effect of rituximab plus ASCT is not as relevant if ASCT is offered soon in the course of the disease,” the researchers wrote.
Patients who had experienced early therapy failure achieved better overall survival if they underwent ASCT while in second complete response, as opposed to second partial response. Notably, 56% of those who underwent ASCT while in second complete response were alive at 13.7 years of follow-up and remained so long term.
The study was funded by the Foundation Research Institute at the Hospital Universitario 12 de Octubre. The researchers reported having no relevant conflicts of interest.
SOURCE: Jiménez-Ubieto A et al. Hematol Oncol Stem Cell Ther. 2019 Jul 9. doi: 10.1016/j.hemonc.2019.06.001.
Prompt autologous stem cell transplantation (ASCT) is often curative in rituximab-naive patients with follicular lymphoma who have experienced early failure of first-line therapy and achieved a response to second-line therapy, suggest results from a registry-based study conducted by GELTAMO (the Spanish Lymphoma and Bone Marrow Transplant Group).
“Overall, our results suggest that, whereas some patients might benefit from more aggressive therapies, such as allogenic stem cell transplantations, or novel drugs, such as immunomodulatory agents, monoclonal antibodies, phosphoinositide 3-kinase inhibitors, or even the application of bispecific T-cell engagers and chimeric antigen receptor T cells, there are a considerable number of patients in this high-risk [early therapy failure] subgroup that can be cured with ASCT, even in the absence of rituximab,” Ana Jiménez-Ubieto, MD, PhD, of the Hospital Universitario, 12 de Octubre, Madrid, Spain, and colleagues wrote.
The results are more favorable when ASCT is performed in patients experiencing early therapy failure, with less than 1 year from first relapse after primary treatment to ASCT.
“Early ASCT could be a hopeful option in patients with difficult access to rituximab,” the researchers wrote in Hematology/Oncology and Stem Cell Therapy.
Patients with follicular lymphoma who experience relapse or progression during or soon after first-line therapy have poor overall survival, and there is no standard therapy for this population, according to the researchers. Previous research has shown that ASCT prolongs survival in those who have received rituximab before transplantation, but benefit in the absence of this agent is unknown.
Dr. Jiménez-Ubieto and colleagues conducted a multicenter registry-based retrospective cohort study of 134 patients with nontransformed follicular lymphoma who underwent ASCT during 1989-2007 while in second complete or partial response to rescue chemotherapy and had not received rituximab.
Overall, 65% of the patients had experienced early therapy failure (relapse or progression within 2 years of starting first-line chemotherapy). Within this group, 78% underwent ASCT within 1 year, and 67% underwent ASCT while in second complete response. Median posttransplantation follow-up for the entire study cohort was 13.4 years.
Study results showed that patients who had experienced early therapy failure versus who had not had poorer 5-year progression-free survival (43% vs. 57%; P = .048) but similar 5-year overall survival (69% vs. 77%; P = .4). However, those patients with early therapy failure who underwent ASCT within 1 year had a statistically indistinguishable 5-year progression-free survival relative to counterparts without early therapy failure (48% vs. 66%; P = .44).
Additionally, the 48% progression-free survival seen in this subset was almost identical to the 49% seen in a historical cohort of patients with early therapy failure who similarly underwent ASCT within 1 year of first relapse but received rituximab before transplantation (Hematol Oncol. 2018;36[5]:765-72). This suggests “that the possible synergistic effect of rituximab plus ASCT is not as relevant if ASCT is offered soon in the course of the disease,” the researchers wrote.
Patients who had experienced early therapy failure achieved better overall survival if they underwent ASCT while in second complete response, as opposed to second partial response. Notably, 56% of those who underwent ASCT while in second complete response were alive at 13.7 years of follow-up and remained so long term.
The study was funded by the Foundation Research Institute at the Hospital Universitario 12 de Octubre. The researchers reported having no relevant conflicts of interest.
SOURCE: Jiménez-Ubieto A et al. Hematol Oncol Stem Cell Ther. 2019 Jul 9. doi: 10.1016/j.hemonc.2019.06.001.
FROM HEMATOLOGY/ONCOLOGY AND STEM CELL THERAPY
Could home care replace inpatient HSCT?
Can receiving all posttransplant care at home benefit patients undergoing hematopoietic stem cell transplant (HSCT)? Researchers are conducting phase 2 trials to find out.
Nelson Chao, MD, and colleagues at Duke University in Durham, N.C., completed a phase 1 trial that suggested post-HSCT care at home was feasible and safe (Blood. 2017;130:745).
Now, the team is conducting phase 2 trials – NCT01725022 and NCT02218151 – comparing patients who receive all posttransplant care at home with patients treated in the hospital or in the outpatient setting with daily visits to the clinic.
The main goal is to determine if allogeneic HSCT recipients treated at home can maintain their normal microbiome and, as a result, have a lower risk of graft-versus-host disease (GVHD). The researchers are also looking at other outcomes such as quality of life, treatment-related morbidities and mortality, and the cost of care for both allogeneic and autologous transplant recipients.
To be eligible for home care after HSCT, a patient must live within a 90-minute driving distance of Duke and have a caregiver available at home. The patient’s home must pass an inspection, showing it to be free of sources for potential infection, such as mold or pets that sleep in the patient’s bed.
When the time comes for treatment, the patient receives conditioning at the hospital but can return home the day before or the day of transplant. After discharge, the patient is visited by a nurse practitioner or physician assistant each morning for a physical examination and blood draw.
In the afternoon, the patient is visited by a clinic nurse who brings any necessary supplies or treatments, such as blood products or intravenous antibiotics. The patient also has daily video calls with an attending physician and can be admitted to the hospital for any events that cannot be managed in the home setting.
Patients can have visitors and spend time away from home, but precautions are necessary. Friends or family who are sick should not be allowed to visit, and patients should avoid crowds when they go out.

Initial findings
The Duke team has treated 41 HSCT recipients at home so far. Dr. Chao said it’s still too early to draw any conclusions about differences in outcomes between home care and inpatient/outpatient HSCT.
However, a preliminary analysis of costs suggests home care is cheaper than inpatient HSCT. The researchers found that, for the first several transplants, at day 60, the cost of home care was roughly half that of inpatient HSCT.
In addition, patients seem to be happy with posttransplant care at home.
“The patients love being at home, in their own environment, with their families,” Dr. Chao said. “Almost every single patient [in the phase 1 trial] said that he or she liked it much better. There was one patient in the phase 1 that felt a little isolated, and I can see why because we say, ‘You can stay home, but don’t have a whole lot of people in.’ ”
One patient’s experience
Beth Vanderkin said it was “a blessing” to receive care at home after undergoing HSCT at Duke.
Ms. Vanderkin was diagnosed with diffuse large B-cell lymphoma in 2014. After two chemotherapy regimens failed to shrink the tumor in her chest, she underwent radiotherapy and responded well. When a PET scan revealed the tumor had gone completely, she proceeded to transplant.
She received a haploidentical HSCT using cells donated by her eldest daughter, Hannah Eichhorst. Ms. Vanderkin received the transplant in the hospital, and for 2 weeks after that, she made daily visits to the transplant clinic.
After those 2 weeks, Ms. Vanderkin continued her treatment at home. Like other patients eligible for home care, Ms. Vanderkin lived close to Duke, had a caregiver available, and had passed a home inspection. The Duke team shipped the needed medical supplies to her house and arranged twice-daily visits from nurses and daily video calls with a doctor.
Ms. Vanderkin said receiving care at home was “a game changer.” She derived comfort from recovering in her own environment, could spend more time with her family, and didn’t have to miss special events. While receiving care at home, Ms. Vanderkin attended the homecoming event where her son, Josiah, was part of the court. Wearing a face mask and carrying a portable pump in her purse, Ms. Vanderkin joined other mothers in escorting their children onto the football field.
“I got to escort my son out onto the field, and he was crowned king that night,” Ms. Vanderkin said. “I didn’t do a lot of things [while receiving care at home], but there were things I didn’t have to miss because I was at home and not in the hospital.”
Ms. Vanderkin said home care was also beneficial for her husband, who was her caregiver. Thomas Vanderkin was able to work from home while caring for his wife, and the daily nurses’ visits allowed him to run errands without having to leave Ms. Vanderkin alone.
Since her experience with home care, Ms. Vanderkin has spent many more days in the hospital and clinic. She experienced a relapse after the transplant and went on to receive more chemotherapy as well as ipilimumab. She responded to that treatment and has now been cancer-free for 3 years.
The ipilimumab did cause side effects, including intestinal problems that resulted in the need for parenteral nutrition. This side effect was made more bearable, Ms. Vanderkin said, because she was able to receive the parenteral nutrition at home. She and her husband were comfortable with additional home care because of their positive experience with posttransplant care.
“I think we’re conditioned to think that, to receive the best care, we have to be sitting in a hospital room or a clinic, but I think there’s a lot of things we can probably do at home,” Ms. Vanderkin said. “And we might fare a lot better as patients if we’re in an environment that we feel comfortable in.”
Experience at other centers
The team at Duke is not the first to study HSCT care at home. In fact, researchers in Sweden have been studying posttransplant home care since 1998.
A pilot trial the group published in 2000 suggested that home care was safe and, in some ways, superior to inpatient HSCT (Bone Marrow Transplant. 2000 Nov;26[10]:1057-60). Patients treated at home had a lower rate of bacteremia, fewer days of total parenteral nutrition, fewer erythrocyte transfusions, and fewer days on antibiotics and analgesics. Rates of fever, engraftment time, and acute GVHD were similar between the inpatient and home-care groups.
A study published by the same researchers in 2002 showed that patients who received home care had lower rates of grade 2-4 acute GVHD and transplant-related mortality compared to inpatients (Blood. 2002 Dec 15;100[13]:4317-24). Two-year overall survival was superior with home care as well.
On the other hand, a study the group published in 2013 showed no significant differences in 5-year survival, transplant-related mortality, relapse, or chronic GVHD between inpatients and those who received care at home (Biol Blood Marrow Transplant. 2013. doi: 10.1016/j.bbmt.2012.11.5189).
The phase 2 trials at Duke should provide more insight into patient outcomes, but results probably won’t be available for 2 more years, Dr. Chao said.
In the meantime, other U.S. researchers are studying home care as well. Memorial Sloan Kettering Cancer Center is conducting a pilot study to determine if HSCT care at home is feasible (NCT02671448).
Dr. Chao said home care should be possible for other centers, particularly those that already perform outpatient HSCT.
“Having the outpatient infrastructure to support these patients is a big step,” he said. “And I think we were able to do that mainly because we do most of our transplants in the outpatient setting already. So that jump to the home is a little less compared to a center that does no outpatient transplants.”
He added, “There’s a certain amount of inertia to overcome and a certain amount of apprehension from the caregivers initially because [patients aren’t] sitting in your unit all the time, but I don’t see this as a huge barrier.”
In fact, Dr. Chao said, if results with home care are favorable, it could potentially replace inpatient HSCT for certain patients.
Dr. Chao’s research is supported by Duke University, and he reported having no relevant financial disclosures.
Can receiving all posttransplant care at home benefit patients undergoing hematopoietic stem cell transplant (HSCT)? Researchers are conducting phase 2 trials to find out.
Nelson Chao, MD, and colleagues at Duke University in Durham, N.C., completed a phase 1 trial that suggested post-HSCT care at home was feasible and safe (Blood. 2017;130:745).
Now, the team is conducting phase 2 trials – NCT01725022 and NCT02218151 – comparing patients who receive all posttransplant care at home with patients treated in the hospital or in the outpatient setting with daily visits to the clinic.
The main goal is to determine if allogeneic HSCT recipients treated at home can maintain their normal microbiome and, as a result, have a lower risk of graft-versus-host disease (GVHD). The researchers are also looking at other outcomes such as quality of life, treatment-related morbidities and mortality, and the cost of care for both allogeneic and autologous transplant recipients.
To be eligible for home care after HSCT, a patient must live within a 90-minute driving distance of Duke and have a caregiver available at home. The patient’s home must pass an inspection, showing it to be free of sources for potential infection, such as mold or pets that sleep in the patient’s bed.
When the time comes for treatment, the patient receives conditioning at the hospital but can return home the day before or the day of transplant. After discharge, the patient is visited by a nurse practitioner or physician assistant each morning for a physical examination and blood draw.
In the afternoon, the patient is visited by a clinic nurse who brings any necessary supplies or treatments, such as blood products or intravenous antibiotics. The patient also has daily video calls with an attending physician and can be admitted to the hospital for any events that cannot be managed in the home setting.
Patients can have visitors and spend time away from home, but precautions are necessary. Friends or family who are sick should not be allowed to visit, and patients should avoid crowds when they go out.

Initial findings
The Duke team has treated 41 HSCT recipients at home so far. Dr. Chao said it’s still too early to draw any conclusions about differences in outcomes between home care and inpatient/outpatient HSCT.
However, a preliminary analysis of costs suggests home care is cheaper than inpatient HSCT. The researchers found that, for the first several transplants, at day 60, the cost of home care was roughly half that of inpatient HSCT.
In addition, patients seem to be happy with posttransplant care at home.
“The patients love being at home, in their own environment, with their families,” Dr. Chao said. “Almost every single patient [in the phase 1 trial] said that he or she liked it much better. There was one patient in the phase 1 that felt a little isolated, and I can see why because we say, ‘You can stay home, but don’t have a whole lot of people in.’ ”
One patient’s experience
Beth Vanderkin said it was “a blessing” to receive care at home after undergoing HSCT at Duke.
Ms. Vanderkin was diagnosed with diffuse large B-cell lymphoma in 2014. After two chemotherapy regimens failed to shrink the tumor in her chest, she underwent radiotherapy and responded well. When a PET scan revealed the tumor had gone completely, she proceeded to transplant.
She received a haploidentical HSCT using cells donated by her eldest daughter, Hannah Eichhorst. Ms. Vanderkin received the transplant in the hospital, and for 2 weeks after that, she made daily visits to the transplant clinic.
After those 2 weeks, Ms. Vanderkin continued her treatment at home. Like other patients eligible for home care, Ms. Vanderkin lived close to Duke, had a caregiver available, and had passed a home inspection. The Duke team shipped the needed medical supplies to her house and arranged twice-daily visits from nurses and daily video calls with a doctor.
Ms. Vanderkin said receiving care at home was “a game changer.” She derived comfort from recovering in her own environment, could spend more time with her family, and didn’t have to miss special events. While receiving care at home, Ms. Vanderkin attended the homecoming event where her son, Josiah, was part of the court. Wearing a face mask and carrying a portable pump in her purse, Ms. Vanderkin joined other mothers in escorting their children onto the football field.
“I got to escort my son out onto the field, and he was crowned king that night,” Ms. Vanderkin said. “I didn’t do a lot of things [while receiving care at home], but there were things I didn’t have to miss because I was at home and not in the hospital.”
Ms. Vanderkin said home care was also beneficial for her husband, who was her caregiver. Thomas Vanderkin was able to work from home while caring for his wife, and the daily nurses’ visits allowed him to run errands without having to leave Ms. Vanderkin alone.
Since her experience with home care, Ms. Vanderkin has spent many more days in the hospital and clinic. She experienced a relapse after the transplant and went on to receive more chemotherapy as well as ipilimumab. She responded to that treatment and has now been cancer-free for 3 years.
The ipilimumab did cause side effects, including intestinal problems that resulted in the need for parenteral nutrition. This side effect was made more bearable, Ms. Vanderkin said, because she was able to receive the parenteral nutrition at home. She and her husband were comfortable with additional home care because of their positive experience with posttransplant care.
“I think we’re conditioned to think that, to receive the best care, we have to be sitting in a hospital room or a clinic, but I think there’s a lot of things we can probably do at home,” Ms. Vanderkin said. “And we might fare a lot better as patients if we’re in an environment that we feel comfortable in.”
Experience at other centers
The team at Duke is not the first to study HSCT care at home. In fact, researchers in Sweden have been studying posttransplant home care since 1998.
A pilot trial the group published in 2000 suggested that home care was safe and, in some ways, superior to inpatient HSCT (Bone Marrow Transplant. 2000 Nov;26[10]:1057-60). Patients treated at home had a lower rate of bacteremia, fewer days of total parenteral nutrition, fewer erythrocyte transfusions, and fewer days on antibiotics and analgesics. Rates of fever, engraftment time, and acute GVHD were similar between the inpatient and home-care groups.
A study published by the same researchers in 2002 showed that patients who received home care had lower rates of grade 2-4 acute GVHD and transplant-related mortality compared to inpatients (Blood. 2002 Dec 15;100[13]:4317-24). Two-year overall survival was superior with home care as well.
On the other hand, a study the group published in 2013 showed no significant differences in 5-year survival, transplant-related mortality, relapse, or chronic GVHD between inpatients and those who received care at home (Biol Blood Marrow Transplant. 2013. doi: 10.1016/j.bbmt.2012.11.5189).
The phase 2 trials at Duke should provide more insight into patient outcomes, but results probably won’t be available for 2 more years, Dr. Chao said.
In the meantime, other U.S. researchers are studying home care as well. Memorial Sloan Kettering Cancer Center is conducting a pilot study to determine if HSCT care at home is feasible (NCT02671448).
Dr. Chao said home care should be possible for other centers, particularly those that already perform outpatient HSCT.
“Having the outpatient infrastructure to support these patients is a big step,” he said. “And I think we were able to do that mainly because we do most of our transplants in the outpatient setting already. So that jump to the home is a little less compared to a center that does no outpatient transplants.”
He added, “There’s a certain amount of inertia to overcome and a certain amount of apprehension from the caregivers initially because [patients aren’t] sitting in your unit all the time, but I don’t see this as a huge barrier.”
In fact, Dr. Chao said, if results with home care are favorable, it could potentially replace inpatient HSCT for certain patients.
Dr. Chao’s research is supported by Duke University, and he reported having no relevant financial disclosures.
Can receiving all posttransplant care at home benefit patients undergoing hematopoietic stem cell transplant (HSCT)? Researchers are conducting phase 2 trials to find out.
Nelson Chao, MD, and colleagues at Duke University in Durham, N.C., completed a phase 1 trial that suggested post-HSCT care at home was feasible and safe (Blood. 2017;130:745).
Now, the team is conducting phase 2 trials – NCT01725022 and NCT02218151 – comparing patients who receive all posttransplant care at home with patients treated in the hospital or in the outpatient setting with daily visits to the clinic.
The main goal is to determine if allogeneic HSCT recipients treated at home can maintain their normal microbiome and, as a result, have a lower risk of graft-versus-host disease (GVHD). The researchers are also looking at other outcomes such as quality of life, treatment-related morbidities and mortality, and the cost of care for both allogeneic and autologous transplant recipients.
To be eligible for home care after HSCT, a patient must live within a 90-minute driving distance of Duke and have a caregiver available at home. The patient’s home must pass an inspection, showing it to be free of sources for potential infection, such as mold or pets that sleep in the patient’s bed.
When the time comes for treatment, the patient receives conditioning at the hospital but can return home the day before or the day of transplant. After discharge, the patient is visited by a nurse practitioner or physician assistant each morning for a physical examination and blood draw.
In the afternoon, the patient is visited by a clinic nurse who brings any necessary supplies or treatments, such as blood products or intravenous antibiotics. The patient also has daily video calls with an attending physician and can be admitted to the hospital for any events that cannot be managed in the home setting.
Patients can have visitors and spend time away from home, but precautions are necessary. Friends or family who are sick should not be allowed to visit, and patients should avoid crowds when they go out.

Initial findings
The Duke team has treated 41 HSCT recipients at home so far. Dr. Chao said it’s still too early to draw any conclusions about differences in outcomes between home care and inpatient/outpatient HSCT.
However, a preliminary analysis of costs suggests home care is cheaper than inpatient HSCT. The researchers found that, for the first several transplants, at day 60, the cost of home care was roughly half that of inpatient HSCT.
In addition, patients seem to be happy with posttransplant care at home.
“The patients love being at home, in their own environment, with their families,” Dr. Chao said. “Almost every single patient [in the phase 1 trial] said that he or she liked it much better. There was one patient in the phase 1 that felt a little isolated, and I can see why because we say, ‘You can stay home, but don’t have a whole lot of people in.’ ”
One patient’s experience
Beth Vanderkin said it was “a blessing” to receive care at home after undergoing HSCT at Duke.
Ms. Vanderkin was diagnosed with diffuse large B-cell lymphoma in 2014. After two chemotherapy regimens failed to shrink the tumor in her chest, she underwent radiotherapy and responded well. When a PET scan revealed the tumor had gone completely, she proceeded to transplant.
She received a haploidentical HSCT using cells donated by her eldest daughter, Hannah Eichhorst. Ms. Vanderkin received the transplant in the hospital, and for 2 weeks after that, she made daily visits to the transplant clinic.
After those 2 weeks, Ms. Vanderkin continued her treatment at home. Like other patients eligible for home care, Ms. Vanderkin lived close to Duke, had a caregiver available, and had passed a home inspection. The Duke team shipped the needed medical supplies to her house and arranged twice-daily visits from nurses and daily video calls with a doctor.
Ms. Vanderkin said receiving care at home was “a game changer.” She derived comfort from recovering in her own environment, could spend more time with her family, and didn’t have to miss special events. While receiving care at home, Ms. Vanderkin attended the homecoming event where her son, Josiah, was part of the court. Wearing a face mask and carrying a portable pump in her purse, Ms. Vanderkin joined other mothers in escorting their children onto the football field.
“I got to escort my son out onto the field, and he was crowned king that night,” Ms. Vanderkin said. “I didn’t do a lot of things [while receiving care at home], but there were things I didn’t have to miss because I was at home and not in the hospital.”
Ms. Vanderkin said home care was also beneficial for her husband, who was her caregiver. Thomas Vanderkin was able to work from home while caring for his wife, and the daily nurses’ visits allowed him to run errands without having to leave Ms. Vanderkin alone.
Since her experience with home care, Ms. Vanderkin has spent many more days in the hospital and clinic. She experienced a relapse after the transplant and went on to receive more chemotherapy as well as ipilimumab. She responded to that treatment and has now been cancer-free for 3 years.
The ipilimumab did cause side effects, including intestinal problems that resulted in the need for parenteral nutrition. This side effect was made more bearable, Ms. Vanderkin said, because she was able to receive the parenteral nutrition at home. She and her husband were comfortable with additional home care because of their positive experience with posttransplant care.
“I think we’re conditioned to think that, to receive the best care, we have to be sitting in a hospital room or a clinic, but I think there’s a lot of things we can probably do at home,” Ms. Vanderkin said. “And we might fare a lot better as patients if we’re in an environment that we feel comfortable in.”
Experience at other centers
The team at Duke is not the first to study HSCT care at home. In fact, researchers in Sweden have been studying posttransplant home care since 1998.
A pilot trial the group published in 2000 suggested that home care was safe and, in some ways, superior to inpatient HSCT (Bone Marrow Transplant. 2000 Nov;26[10]:1057-60). Patients treated at home had a lower rate of bacteremia, fewer days of total parenteral nutrition, fewer erythrocyte transfusions, and fewer days on antibiotics and analgesics. Rates of fever, engraftment time, and acute GVHD were similar between the inpatient and home-care groups.
A study published by the same researchers in 2002 showed that patients who received home care had lower rates of grade 2-4 acute GVHD and transplant-related mortality compared to inpatients (Blood. 2002 Dec 15;100[13]:4317-24). Two-year overall survival was superior with home care as well.
On the other hand, a study the group published in 2013 showed no significant differences in 5-year survival, transplant-related mortality, relapse, or chronic GVHD between inpatients and those who received care at home (Biol Blood Marrow Transplant. 2013. doi: 10.1016/j.bbmt.2012.11.5189).
The phase 2 trials at Duke should provide more insight into patient outcomes, but results probably won’t be available for 2 more years, Dr. Chao said.
In the meantime, other U.S. researchers are studying home care as well. Memorial Sloan Kettering Cancer Center is conducting a pilot study to determine if HSCT care at home is feasible (NCT02671448).
Dr. Chao said home care should be possible for other centers, particularly those that already perform outpatient HSCT.
“Having the outpatient infrastructure to support these patients is a big step,” he said. “And I think we were able to do that mainly because we do most of our transplants in the outpatient setting already. So that jump to the home is a little less compared to a center that does no outpatient transplants.”
He added, “There’s a certain amount of inertia to overcome and a certain amount of apprehension from the caregivers initially because [patients aren’t] sitting in your unit all the time, but I don’t see this as a huge barrier.”
In fact, Dr. Chao said, if results with home care are favorable, it could potentially replace inpatient HSCT for certain patients.
Dr. Chao’s research is supported by Duke University, and he reported having no relevant financial disclosures.
VRD pretransplant induction deepens responses in myeloma
Pretransplant induction therapy with subcutaneous bortezomib, lenalidomide, and dexamethasone (VRD) deepened responses in patients with newly diagnosed multiple myeloma, according to an interim analysis of a phase 3 study.
Overall, the regimen was well tolerated, with a minimal number of patients discontinuing treatment because of treatment-emergent adverse events.
The ongoing, open-label, randomized, phase 3 study is designed to compare two transplant-conditioning regimens – intravenous busulfan plus melphalan versus melphalan – in patients who received VRD induction and consolidation, wrote Laura Rosiñol, MD, PhD, of the August Pi i Sunyer Biomedical Research Institute in Barcelona, and colleagues. The findings were published in Blood.
The PETHEMA/GEM2012 study included 458 patients with newly diagnosed multiple myeloma who were eligible for autologous stem cell transplantation. Study patients were previously untreated and aged younger than 65 years.
All patients received VRD induction, which consisted of subcutaneous bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 of each cycle; lenalidomide 25 mg/day on days 1-21; and dexamethasone 40 mg on days 1-4 and 9-12 at 4-week intervals for six cycles. Posttransplant consolidation consisted of two cycles of VRD.
The researchers conducted a grouped-response analysis of three different treatment phases: induction, transplant, and consolidation.
After analysis, the researchers found that responses deepened over the duration of treatment. In patients who started the sixth induction cycle, the response rates were 55.6%, 63.8%, 68.3%, and 70.4% after cycles 3, 4, 5, and post induction, respectively.
After six cycles of induction, the complete response rate was 33.4%, with a rate of undetectable minimal residual disease of 28.8%, which further increased at transplant (42.1%), and consolidation (45.2%).
With respect to safety, the most frequently reported grade 3 or higher treatment-emergent adverse events were neutropenia (12.9%) and infection (9.2%). The rate of grade 2 or higher peripheral neuropathy throughout induction was 17.0%, with lower rates of grade 3 (3.7%) and 4 (0.2%) toxicities.
“The regimen [used in the present study] has the highest lenalidomide and dexamethasone dose intensity per cycle and a lower bortezomib dose intensity per cycle than the 21-day regimens, which may offer high activity with low levels of toxicity, thereby enabling delivery of all planned induction cycles,” the researchers wrote, adding that “these results confirm that VRD is an effective pretransplant induction regimen and may be considered a new standard of care.”
The study was supported by Celgene, Janssen, Pierre Fabré, and the Instituto de Salud Carlos III. The authors reported financial affiliations with Celgene, Janssen, and several other companies.
SOURCE: Rosiñol L et al. Blood. 2019 Sep 4. doi: 10.1182/blood.2019000241.
Pretransplant induction therapy with subcutaneous bortezomib, lenalidomide, and dexamethasone (VRD) deepened responses in patients with newly diagnosed multiple myeloma, according to an interim analysis of a phase 3 study.
Overall, the regimen was well tolerated, with a minimal number of patients discontinuing treatment because of treatment-emergent adverse events.
The ongoing, open-label, randomized, phase 3 study is designed to compare two transplant-conditioning regimens – intravenous busulfan plus melphalan versus melphalan – in patients who received VRD induction and consolidation, wrote Laura Rosiñol, MD, PhD, of the August Pi i Sunyer Biomedical Research Institute in Barcelona, and colleagues. The findings were published in Blood.
The PETHEMA/GEM2012 study included 458 patients with newly diagnosed multiple myeloma who were eligible for autologous stem cell transplantation. Study patients were previously untreated and aged younger than 65 years.
All patients received VRD induction, which consisted of subcutaneous bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 of each cycle; lenalidomide 25 mg/day on days 1-21; and dexamethasone 40 mg on days 1-4 and 9-12 at 4-week intervals for six cycles. Posttransplant consolidation consisted of two cycles of VRD.
The researchers conducted a grouped-response analysis of three different treatment phases: induction, transplant, and consolidation.
After analysis, the researchers found that responses deepened over the duration of treatment. In patients who started the sixth induction cycle, the response rates were 55.6%, 63.8%, 68.3%, and 70.4% after cycles 3, 4, 5, and post induction, respectively.
After six cycles of induction, the complete response rate was 33.4%, with a rate of undetectable minimal residual disease of 28.8%, which further increased at transplant (42.1%), and consolidation (45.2%).
With respect to safety, the most frequently reported grade 3 or higher treatment-emergent adverse events were neutropenia (12.9%) and infection (9.2%). The rate of grade 2 or higher peripheral neuropathy throughout induction was 17.0%, with lower rates of grade 3 (3.7%) and 4 (0.2%) toxicities.
“The regimen [used in the present study] has the highest lenalidomide and dexamethasone dose intensity per cycle and a lower bortezomib dose intensity per cycle than the 21-day regimens, which may offer high activity with low levels of toxicity, thereby enabling delivery of all planned induction cycles,” the researchers wrote, adding that “these results confirm that VRD is an effective pretransplant induction regimen and may be considered a new standard of care.”
The study was supported by Celgene, Janssen, Pierre Fabré, and the Instituto de Salud Carlos III. The authors reported financial affiliations with Celgene, Janssen, and several other companies.
SOURCE: Rosiñol L et al. Blood. 2019 Sep 4. doi: 10.1182/blood.2019000241.
Pretransplant induction therapy with subcutaneous bortezomib, lenalidomide, and dexamethasone (VRD) deepened responses in patients with newly diagnosed multiple myeloma, according to an interim analysis of a phase 3 study.
Overall, the regimen was well tolerated, with a minimal number of patients discontinuing treatment because of treatment-emergent adverse events.
The ongoing, open-label, randomized, phase 3 study is designed to compare two transplant-conditioning regimens – intravenous busulfan plus melphalan versus melphalan – in patients who received VRD induction and consolidation, wrote Laura Rosiñol, MD, PhD, of the August Pi i Sunyer Biomedical Research Institute in Barcelona, and colleagues. The findings were published in Blood.
The PETHEMA/GEM2012 study included 458 patients with newly diagnosed multiple myeloma who were eligible for autologous stem cell transplantation. Study patients were previously untreated and aged younger than 65 years.
All patients received VRD induction, which consisted of subcutaneous bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 of each cycle; lenalidomide 25 mg/day on days 1-21; and dexamethasone 40 mg on days 1-4 and 9-12 at 4-week intervals for six cycles. Posttransplant consolidation consisted of two cycles of VRD.
The researchers conducted a grouped-response analysis of three different treatment phases: induction, transplant, and consolidation.
After analysis, the researchers found that responses deepened over the duration of treatment. In patients who started the sixth induction cycle, the response rates were 55.6%, 63.8%, 68.3%, and 70.4% after cycles 3, 4, 5, and post induction, respectively.
After six cycles of induction, the complete response rate was 33.4%, with a rate of undetectable minimal residual disease of 28.8%, which further increased at transplant (42.1%), and consolidation (45.2%).
With respect to safety, the most frequently reported grade 3 or higher treatment-emergent adverse events were neutropenia (12.9%) and infection (9.2%). The rate of grade 2 or higher peripheral neuropathy throughout induction was 17.0%, with lower rates of grade 3 (3.7%) and 4 (0.2%) toxicities.
“The regimen [used in the present study] has the highest lenalidomide and dexamethasone dose intensity per cycle and a lower bortezomib dose intensity per cycle than the 21-day regimens, which may offer high activity with low levels of toxicity, thereby enabling delivery of all planned induction cycles,” the researchers wrote, adding that “these results confirm that VRD is an effective pretransplant induction regimen and may be considered a new standard of care.”
The study was supported by Celgene, Janssen, Pierre Fabré, and the Instituto de Salud Carlos III. The authors reported financial affiliations with Celgene, Janssen, and several other companies.
SOURCE: Rosiñol L et al. Blood. 2019 Sep 4. doi: 10.1182/blood.2019000241.
FROM BLOOD
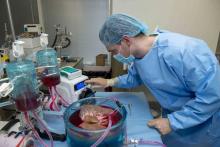
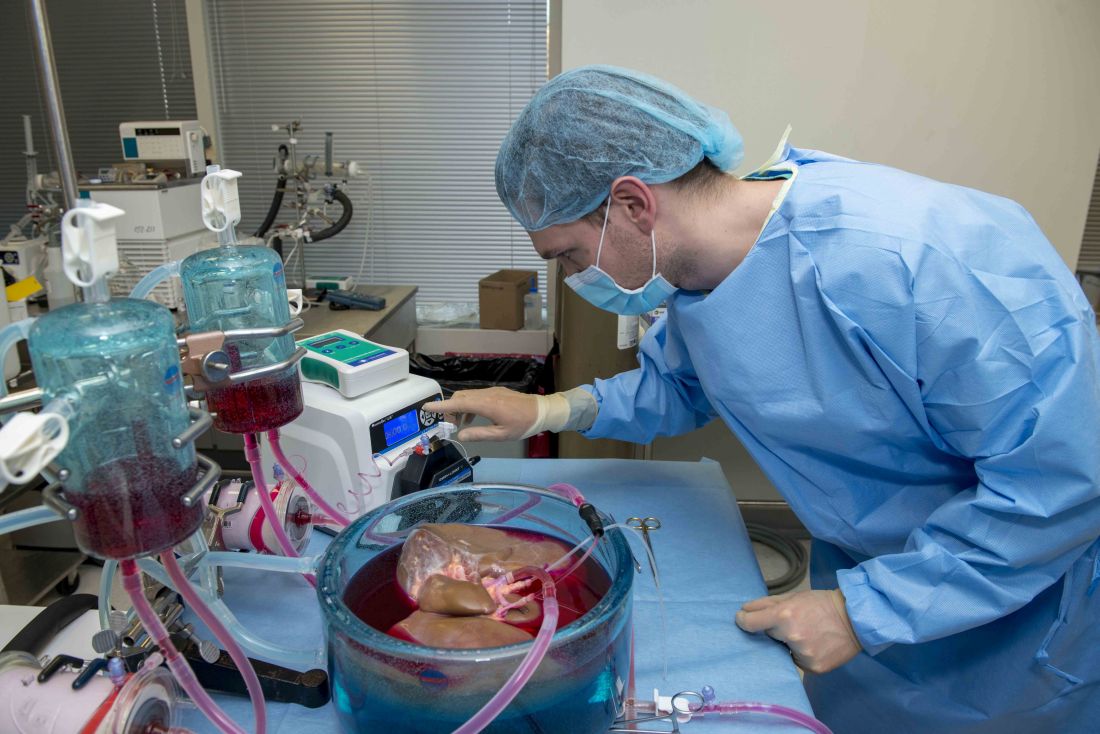
Supercooling extends donor liver viability by 27 hours
Standard cooling to 4°C provides just 12 hours of organ preservation, but laboratory testing showed that supercooling to –4°C added 27 hours of viability, reported lead author Reinier J. de Vries, MD, of Harvard Medical School and Massachusetts General Hospital in Boston, and colleagues.
“The absence of technology to preserve organs for more than a few hours is one of the fundamental causes of the donor organ–shortage crisis,” the investigators wrote in Nature Biotechnology.
Supercooling organs to high-subzero temperatures has been shown to prolong organ life while avoiding ice-mediated injury, but techniques that are successful for rat livers have been difficult to translate to human livers because of their larger size, which increases the risk of ice formation, the investigators explained.
Three strategies were employed to overcome this problem: minimization of air-liquid interfaces, development of a new supercooling-preservation solution, and hypothermic machine perfusion to more evenly distribute preservation solution throughout the liver tissue. For recovery of organs after supercooling, the investigators used subnormothermic machine perfusion, which has been used effectively in rat transplants.
In order to measure the impact of this process on organ viability, the investigators first measured adenylate energy content, both before supercooling and after recovery.
“Adenylate energy content, and, particularly, the organ’s ability to recover it during (re)perfusion, is considered the most representative metric for liver viability,” they wrote.
The difference between pre- and postsupercooling energy charge was less than 20%; in comparison, failed liver transplants in large animals and clinical trials have typically involved an energy-charge loss of 40% or more.
To further test organ viability, the investigators measured pre- and postsupercooling levels of bile production, oxygen uptake, and vascular resistance. All of these parameters have been shown to predict transplant success in rats, and bile production has additional precedent from human studies.
On average, bile production, portal resistance, and arterial resistance were not significantly affected by supercooling. Although portal vein resistance was 20% higher after supercooling, this compared favorably with increases of 100%-150% that have been measured in nonviable livers. Similarly, oxygen uptake increased by a mean of 17%, but this was three times lower than changes that have been observed in livers with impaired viability, at 51%.
Additional measures of hepatocellular injury, including AST and ALT, were also supportive of viability after supercooling. Histopathology confirmed these findings by showing preserved tissue architecture.
“In summary, we find that the human livers tested displayed no substantial difference in viability before and after extended subzero supercooling preservation,” the investigators wrote.
To simulate transplantation, the investigators reperfused the organs with blood at a normal temperature, including platelets, complement, and white blood cells, which are drivers of ischemia reperfusion injury. During this process, energy charge remained stable, which indicates preserved mitochondrial function. While energy charge held steady, lactate metabolism increased with bile and urea production, suggesting increased liver function. Bile pH and HCO3– levels fell within range for viability. Although bile glucose exceeded proposed criteria, the investigators pointed out that levels still fell within parameters for research-quality livers. Lactate levels also rose within the first hour of reperfusion, but the investigators suggested that this finding should be interpreted with appropriate context.
“It should be considered that the livers in this study were initially rejected for transplantation,” they wrote, “and the confidence intervals of the lactate concentration at the end of reperfusion largely overlap with time-matched values reported by others during [normothermic machine perfusion] of rejected human livers.”
Hepatocellular injury and histology also were evaluated during and after simulated transplantation, respectively, with favorable results. Although sites of preexisting hepatic injury were aggravated by the process, and rates of apoptosis increased, the investigators considered these changes were clinically insignificant.
Looking to the future, the investigators suggested that further refinement of the process could facilitate even-lower storage temperatures while better preserving liver viability.
“The use of human livers makes this study clinically relevant and promotes the translation of subzero organ preservation to the clinic,” the investigators concluded. “However, long-term survival experiments of transplanted supercooled livers in swine or an alternative large animal model will be needed before clinical translation.”
The study was funded by the National Institutes of Health and the Department of Defense. Dr. de Vries and four other coauthors have provisional patent applications related to the study, and one coauthor disclosed a financial relationship with Organ Solutions.
SOURCE: de Vries RJ et al. Nature Biotechnol. 2019 Sep 9. doi: 10.1038/s41587-019-0223-y.
Standard cooling to 4°C provides just 12 hours of organ preservation, but laboratory testing showed that supercooling to –4°C added 27 hours of viability, reported lead author Reinier J. de Vries, MD, of Harvard Medical School and Massachusetts General Hospital in Boston, and colleagues.
“The absence of technology to preserve organs for more than a few hours is one of the fundamental causes of the donor organ–shortage crisis,” the investigators wrote in Nature Biotechnology.
Supercooling organs to high-subzero temperatures has been shown to prolong organ life while avoiding ice-mediated injury, but techniques that are successful for rat livers have been difficult to translate to human livers because of their larger size, which increases the risk of ice formation, the investigators explained.
Three strategies were employed to overcome this problem: minimization of air-liquid interfaces, development of a new supercooling-preservation solution, and hypothermic machine perfusion to more evenly distribute preservation solution throughout the liver tissue. For recovery of organs after supercooling, the investigators used subnormothermic machine perfusion, which has been used effectively in rat transplants.
In order to measure the impact of this process on organ viability, the investigators first measured adenylate energy content, both before supercooling and after recovery.
“Adenylate energy content, and, particularly, the organ’s ability to recover it during (re)perfusion, is considered the most representative metric for liver viability,” they wrote.
The difference between pre- and postsupercooling energy charge was less than 20%; in comparison, failed liver transplants in large animals and clinical trials have typically involved an energy-charge loss of 40% or more.
To further test organ viability, the investigators measured pre- and postsupercooling levels of bile production, oxygen uptake, and vascular resistance. All of these parameters have been shown to predict transplant success in rats, and bile production has additional precedent from human studies.
On average, bile production, portal resistance, and arterial resistance were not significantly affected by supercooling. Although portal vein resistance was 20% higher after supercooling, this compared favorably with increases of 100%-150% that have been measured in nonviable livers. Similarly, oxygen uptake increased by a mean of 17%, but this was three times lower than changes that have been observed in livers with impaired viability, at 51%.
Additional measures of hepatocellular injury, including AST and ALT, were also supportive of viability after supercooling. Histopathology confirmed these findings by showing preserved tissue architecture.
“In summary, we find that the human livers tested displayed no substantial difference in viability before and after extended subzero supercooling preservation,” the investigators wrote.
To simulate transplantation, the investigators reperfused the organs with blood at a normal temperature, including platelets, complement, and white blood cells, which are drivers of ischemia reperfusion injury. During this process, energy charge remained stable, which indicates preserved mitochondrial function. While energy charge held steady, lactate metabolism increased with bile and urea production, suggesting increased liver function. Bile pH and HCO3– levels fell within range for viability. Although bile glucose exceeded proposed criteria, the investigators pointed out that levels still fell within parameters for research-quality livers. Lactate levels also rose within the first hour of reperfusion, but the investigators suggested that this finding should be interpreted with appropriate context.
“It should be considered that the livers in this study were initially rejected for transplantation,” they wrote, “and the confidence intervals of the lactate concentration at the end of reperfusion largely overlap with time-matched values reported by others during [normothermic machine perfusion] of rejected human livers.”
Hepatocellular injury and histology also were evaluated during and after simulated transplantation, respectively, with favorable results. Although sites of preexisting hepatic injury were aggravated by the process, and rates of apoptosis increased, the investigators considered these changes were clinically insignificant.
Looking to the future, the investigators suggested that further refinement of the process could facilitate even-lower storage temperatures while better preserving liver viability.
“The use of human livers makes this study clinically relevant and promotes the translation of subzero organ preservation to the clinic,” the investigators concluded. “However, long-term survival experiments of transplanted supercooled livers in swine or an alternative large animal model will be needed before clinical translation.”
The study was funded by the National Institutes of Health and the Department of Defense. Dr. de Vries and four other coauthors have provisional patent applications related to the study, and one coauthor disclosed a financial relationship with Organ Solutions.
SOURCE: de Vries RJ et al. Nature Biotechnol. 2019 Sep 9. doi: 10.1038/s41587-019-0223-y.
Standard cooling to 4°C provides just 12 hours of organ preservation, but laboratory testing showed that supercooling to –4°C added 27 hours of viability, reported lead author Reinier J. de Vries, MD, of Harvard Medical School and Massachusetts General Hospital in Boston, and colleagues.
“The absence of technology to preserve organs for more than a few hours is one of the fundamental causes of the donor organ–shortage crisis,” the investigators wrote in Nature Biotechnology.
Supercooling organs to high-subzero temperatures has been shown to prolong organ life while avoiding ice-mediated injury, but techniques that are successful for rat livers have been difficult to translate to human livers because of their larger size, which increases the risk of ice formation, the investigators explained.
Three strategies were employed to overcome this problem: minimization of air-liquid interfaces, development of a new supercooling-preservation solution, and hypothermic machine perfusion to more evenly distribute preservation solution throughout the liver tissue. For recovery of organs after supercooling, the investigators used subnormothermic machine perfusion, which has been used effectively in rat transplants.
In order to measure the impact of this process on organ viability, the investigators first measured adenylate energy content, both before supercooling and after recovery.
“Adenylate energy content, and, particularly, the organ’s ability to recover it during (re)perfusion, is considered the most representative metric for liver viability,” they wrote.
The difference between pre- and postsupercooling energy charge was less than 20%; in comparison, failed liver transplants in large animals and clinical trials have typically involved an energy-charge loss of 40% or more.
To further test organ viability, the investigators measured pre- and postsupercooling levels of bile production, oxygen uptake, and vascular resistance. All of these parameters have been shown to predict transplant success in rats, and bile production has additional precedent from human studies.
On average, bile production, portal resistance, and arterial resistance were not significantly affected by supercooling. Although portal vein resistance was 20% higher after supercooling, this compared favorably with increases of 100%-150% that have been measured in nonviable livers. Similarly, oxygen uptake increased by a mean of 17%, but this was three times lower than changes that have been observed in livers with impaired viability, at 51%.
Additional measures of hepatocellular injury, including AST and ALT, were also supportive of viability after supercooling. Histopathology confirmed these findings by showing preserved tissue architecture.
“In summary, we find that the human livers tested displayed no substantial difference in viability before and after extended subzero supercooling preservation,” the investigators wrote.
To simulate transplantation, the investigators reperfused the organs with blood at a normal temperature, including platelets, complement, and white blood cells, which are drivers of ischemia reperfusion injury. During this process, energy charge remained stable, which indicates preserved mitochondrial function. While energy charge held steady, lactate metabolism increased with bile and urea production, suggesting increased liver function. Bile pH and HCO3– levels fell within range for viability. Although bile glucose exceeded proposed criteria, the investigators pointed out that levels still fell within parameters for research-quality livers. Lactate levels also rose within the first hour of reperfusion, but the investigators suggested that this finding should be interpreted with appropriate context.
“It should be considered that the livers in this study were initially rejected for transplantation,” they wrote, “and the confidence intervals of the lactate concentration at the end of reperfusion largely overlap with time-matched values reported by others during [normothermic machine perfusion] of rejected human livers.”
Hepatocellular injury and histology also were evaluated during and after simulated transplantation, respectively, with favorable results. Although sites of preexisting hepatic injury were aggravated by the process, and rates of apoptosis increased, the investigators considered these changes were clinically insignificant.
Looking to the future, the investigators suggested that further refinement of the process could facilitate even-lower storage temperatures while better preserving liver viability.
“The use of human livers makes this study clinically relevant and promotes the translation of subzero organ preservation to the clinic,” the investigators concluded. “However, long-term survival experiments of transplanted supercooled livers in swine or an alternative large animal model will be needed before clinical translation.”
The study was funded by the National Institutes of Health and the Department of Defense. Dr. de Vries and four other coauthors have provisional patent applications related to the study, and one coauthor disclosed a financial relationship with Organ Solutions.
SOURCE: de Vries RJ et al. Nature Biotechnol. 2019 Sep 9. doi: 10.1038/s41587-019-0223-y.
FROM NATURE BIOTECHNOLOGY
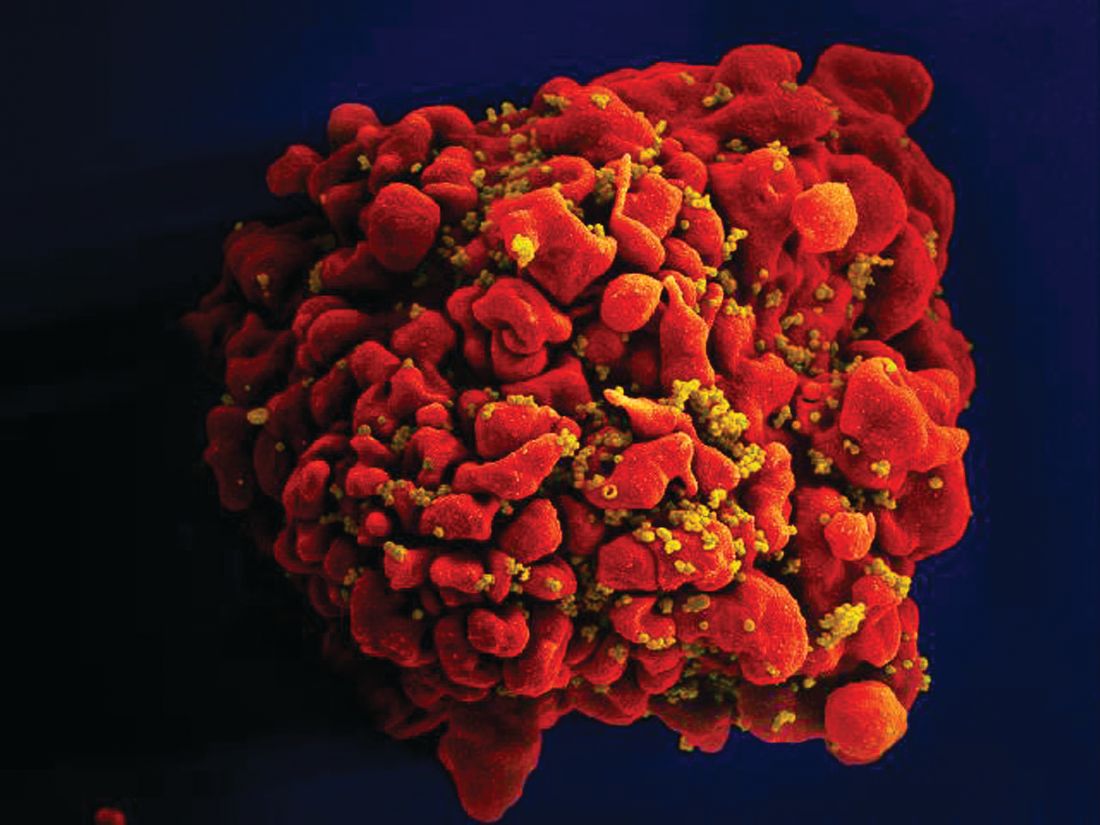
Stem cells gene edited to be HIV resistant treat ALL, but not HIV
Gene editing of donor stem cells prior to transplantation into a patient with both HIV infection and acute lymphoblastic leukemia (ALL) was safe and effectively treated the patient’s leukemia, but failed to resolve his HIV, investigators reported.
The 27-year-old man received an HLA-matched transplant of hematopoietic stem and progenitor cells (HSPCs) that had been genetically engineered to lack CCR5, a key gateway for HIV entry into cells.
Although the transplant resulted in complete remission of leukemia with full donor chimerism, only about 9% of the posttransplant lymphocytes showed disruption of CCR5, and during a brief trial of antiretroviral therapy interruption his HIV viral load rebounded, reported Hongkui Deng, PhD, and colleagues from Peking University in China.
Although the experiment did not meet its goal of a drug-free HIV remission, it serves as a proof of concept for the use of CRISPR-Cas9 (clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9) gene editing to treat HIV infection, the authors contend.
“These results show the proof of principle that transplantation and long-term engraftment of CRISPR-edited allogeneic HSPCs can be achieved; however, the efficiency of the response was not adequate to achieve the target of cure of HIV-1 infection,” they wrote in a brief report published in the New England Journal of Medicine.
As previously reported, other research groups have investigated genetic editing to mimic a naturally occurring mutation that effectively disables the CCR5 HIV coreceptor, preventing the retrovirus from entering healthy cells. The mutation was first identified in a man named Timothy Brown who came to be known as “the Berlin patient” after he was apparently cured of HIV infection after a bone marrow transplant from a donor who had the mutation.
Dr. Deng and colleagues took advantage of HSPC transplantation, a standard therapy for ALL to see whether it could also have beneficial effects on concomitant HIV infection.
They treated donor HSPCs with CRISPR-Cas9 to ablate CCR5 and then delivered them to the patient along with additional CD34-depleted donor cells from mobilized peripheral blood.
The transplant was a success, with neutrophil engraftment on day 13 and platelet engraftment on day 27, and the leukemia was in morphologic complete remission at week 4 following transplantation. The patient remained in complete remission from leukemia throughout the 19-month follow-up period, with full donor chimerism .
However, when a planned interruption of antiretroviral therapy was carried out at 7 months post transplant, the serum viral load increased to 3 × 107 copies/ml at week 4 following interruption, and the patient was restarted on the drug. His viral levels gradually decreased to undetectable level during the subsequent months.
The investigators noted that 2 weeks after the drug interruption trial was started there was a small increase in the percentage of CCR5 insertion/deletions.
“The low efficiency of gene editing in the patient may be due to the competitive engraftment of the coinfused HSPCs in CD34-depleted cells and the persistence of donor T cells. To further clarify the anti-HIV effect of CCR5-ablated HSPCs, it will be essential to increase the gene-editing efficiency of our CRISPR-Cas9 system and improve the transplantation protocol,” they wrote.
The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
SOURCE: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
Gene editing of donor stem cells prior to transplantation into a patient with both HIV infection and acute lymphoblastic leukemia (ALL) was safe and effectively treated the patient’s leukemia, but failed to resolve his HIV, investigators reported.
The 27-year-old man received an HLA-matched transplant of hematopoietic stem and progenitor cells (HSPCs) that had been genetically engineered to lack CCR5, a key gateway for HIV entry into cells.
Although the transplant resulted in complete remission of leukemia with full donor chimerism, only about 9% of the posttransplant lymphocytes showed disruption of CCR5, and during a brief trial of antiretroviral therapy interruption his HIV viral load rebounded, reported Hongkui Deng, PhD, and colleagues from Peking University in China.
Although the experiment did not meet its goal of a drug-free HIV remission, it serves as a proof of concept for the use of CRISPR-Cas9 (clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9) gene editing to treat HIV infection, the authors contend.
“These results show the proof of principle that transplantation and long-term engraftment of CRISPR-edited allogeneic HSPCs can be achieved; however, the efficiency of the response was not adequate to achieve the target of cure of HIV-1 infection,” they wrote in a brief report published in the New England Journal of Medicine.
As previously reported, other research groups have investigated genetic editing to mimic a naturally occurring mutation that effectively disables the CCR5 HIV coreceptor, preventing the retrovirus from entering healthy cells. The mutation was first identified in a man named Timothy Brown who came to be known as “the Berlin patient” after he was apparently cured of HIV infection after a bone marrow transplant from a donor who had the mutation.
Dr. Deng and colleagues took advantage of HSPC transplantation, a standard therapy for ALL to see whether it could also have beneficial effects on concomitant HIV infection.
They treated donor HSPCs with CRISPR-Cas9 to ablate CCR5 and then delivered them to the patient along with additional CD34-depleted donor cells from mobilized peripheral blood.
The transplant was a success, with neutrophil engraftment on day 13 and platelet engraftment on day 27, and the leukemia was in morphologic complete remission at week 4 following transplantation. The patient remained in complete remission from leukemia throughout the 19-month follow-up period, with full donor chimerism .
However, when a planned interruption of antiretroviral therapy was carried out at 7 months post transplant, the serum viral load increased to 3 × 107 copies/ml at week 4 following interruption, and the patient was restarted on the drug. His viral levels gradually decreased to undetectable level during the subsequent months.
The investigators noted that 2 weeks after the drug interruption trial was started there was a small increase in the percentage of CCR5 insertion/deletions.
“The low efficiency of gene editing in the patient may be due to the competitive engraftment of the coinfused HSPCs in CD34-depleted cells and the persistence of donor T cells. To further clarify the anti-HIV effect of CCR5-ablated HSPCs, it will be essential to increase the gene-editing efficiency of our CRISPR-Cas9 system and improve the transplantation protocol,” they wrote.
The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
SOURCE: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
Gene editing of donor stem cells prior to transplantation into a patient with both HIV infection and acute lymphoblastic leukemia (ALL) was safe and effectively treated the patient’s leukemia, but failed to resolve his HIV, investigators reported.
The 27-year-old man received an HLA-matched transplant of hematopoietic stem and progenitor cells (HSPCs) that had been genetically engineered to lack CCR5, a key gateway for HIV entry into cells.
Although the transplant resulted in complete remission of leukemia with full donor chimerism, only about 9% of the posttransplant lymphocytes showed disruption of CCR5, and during a brief trial of antiretroviral therapy interruption his HIV viral load rebounded, reported Hongkui Deng, PhD, and colleagues from Peking University in China.
Although the experiment did not meet its goal of a drug-free HIV remission, it serves as a proof of concept for the use of CRISPR-Cas9 (clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9) gene editing to treat HIV infection, the authors contend.
“These results show the proof of principle that transplantation and long-term engraftment of CRISPR-edited allogeneic HSPCs can be achieved; however, the efficiency of the response was not adequate to achieve the target of cure of HIV-1 infection,” they wrote in a brief report published in the New England Journal of Medicine.
As previously reported, other research groups have investigated genetic editing to mimic a naturally occurring mutation that effectively disables the CCR5 HIV coreceptor, preventing the retrovirus from entering healthy cells. The mutation was first identified in a man named Timothy Brown who came to be known as “the Berlin patient” after he was apparently cured of HIV infection after a bone marrow transplant from a donor who had the mutation.
Dr. Deng and colleagues took advantage of HSPC transplantation, a standard therapy for ALL to see whether it could also have beneficial effects on concomitant HIV infection.
They treated donor HSPCs with CRISPR-Cas9 to ablate CCR5 and then delivered them to the patient along with additional CD34-depleted donor cells from mobilized peripheral blood.
The transplant was a success, with neutrophil engraftment on day 13 and platelet engraftment on day 27, and the leukemia was in morphologic complete remission at week 4 following transplantation. The patient remained in complete remission from leukemia throughout the 19-month follow-up period, with full donor chimerism .
However, when a planned interruption of antiretroviral therapy was carried out at 7 months post transplant, the serum viral load increased to 3 × 107 copies/ml at week 4 following interruption, and the patient was restarted on the drug. His viral levels gradually decreased to undetectable level during the subsequent months.
The investigators noted that 2 weeks after the drug interruption trial was started there was a small increase in the percentage of CCR5 insertion/deletions.
“The low efficiency of gene editing in the patient may be due to the competitive engraftment of the coinfused HSPCs in CD34-depleted cells and the persistence of donor T cells. To further clarify the anti-HIV effect of CCR5-ablated HSPCs, it will be essential to increase the gene-editing efficiency of our CRISPR-Cas9 system and improve the transplantation protocol,” they wrote.
The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
SOURCE: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Donor cells depleted of the HIV coreceptor CCR5 effectively treated ALL, but not HIV.
Major finding: The patient had a sustained complete remission of ALL, but HIV persisted after transplantation.
Study details: Case report of a 27-year-old man with ALL and HIV.
Disclosures: The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
Source: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
Nonmyeloablative conditioning carries lowers infection risk in patients with AML
For patients with acute myeloid leukemia (AML) in need of allogeneic hematopoietic cell transplantation (alloHCT), reduced-intensity/nonmyeloablative conditioning (RIC/NMA) offers a lower risk of infection than myeloablative conditioning (MAC), based on a retrospective study involving more than 1,700 patients.
Within 100 days of treatment, patients who underwent MAC were significantly more likely to develop a bacterial infection, and develop it at an earlier date, than patients who had undergone RIC/NMA, reported lead author Celalettin Ustun, MD, of Rush University in Chicago, and colleagues.
“The incidence of infections, a common and often severe complication of alloHCT, is expected to be lower after RIC/NMA compared with MAC and thus contribute to the decreased [nonrelapse mortality],” the investigators wrote in Blood Advances, noting that this hypothesis has previously lacked supporting data, prompting the present study.
The retrospective analysis involved 1,755 patients with AML who were in first complete remission. Data were drawn from the Center for International Blood and Marrow Transplant Research (CIBMTR). The primary end point was incidence of infection within 100 days after T-cell replete alloHCT in patients receiving MAC (n = 978) versus those who underwent RIC/NMA (n = 777). Secondary end points included comparisons of infection types and infection density.
Patients who received RIC/NMA were generally older and more likely to have myelodysplastic syndrome than patients in the MAC group; the groups were otherwise similar, based on comorbidities, cytogenetic risks, and Karnofsky performance scores.
The proportion of patients who developed at least one infection was comparable between groups: 61% of MAC patients versus 58% of RIC/NMA patients (P = .21), but further analysis showed that MAC was in fact associated with some relatively increased risks. For instance, patients in the MAC group tended to develop infections sooner than patients treated with RIC/NMA (21 vs. 15 days), and more patients treated with MAC had at least one bacterial infection by day 100 (46% vs. 37%).
Although the proportion of patients developing at least one viral infection was slightly lower in the MAC group than the RIC/NMA group (34% vs. 39%), overall infection density was higher, which takes into account multiple infections.
The increased bacterial infections after MAC were caused by gram-positive bacteria, while the increased viral infections with RIC/NMA were caused by cytomegalovirus, the investigators reported.
“RIC/NMA alloHCT is associated with a decreased risk of any infection and particularly early bacterial infections,” the investigators wrote. “The risk of viral and fungal infections per days at risk is similar.”
The Center for International Blood and Marrow Transplant Research is supported by grants from the U.S. government and several pharmaceutical companies. The investigators reported having no conflicts of interest.
SOURCE: Ustun C et al. Blood Adv. 2019 Sep 10;3(17):2525-36.
For patients with acute myeloid leukemia (AML) in need of allogeneic hematopoietic cell transplantation (alloHCT), reduced-intensity/nonmyeloablative conditioning (RIC/NMA) offers a lower risk of infection than myeloablative conditioning (MAC), based on a retrospective study involving more than 1,700 patients.
Within 100 days of treatment, patients who underwent MAC were significantly more likely to develop a bacterial infection, and develop it at an earlier date, than patients who had undergone RIC/NMA, reported lead author Celalettin Ustun, MD, of Rush University in Chicago, and colleagues.
“The incidence of infections, a common and often severe complication of alloHCT, is expected to be lower after RIC/NMA compared with MAC and thus contribute to the decreased [nonrelapse mortality],” the investigators wrote in Blood Advances, noting that this hypothesis has previously lacked supporting data, prompting the present study.
The retrospective analysis involved 1,755 patients with AML who were in first complete remission. Data were drawn from the Center for International Blood and Marrow Transplant Research (CIBMTR). The primary end point was incidence of infection within 100 days after T-cell replete alloHCT in patients receiving MAC (n = 978) versus those who underwent RIC/NMA (n = 777). Secondary end points included comparisons of infection types and infection density.
Patients who received RIC/NMA were generally older and more likely to have myelodysplastic syndrome than patients in the MAC group; the groups were otherwise similar, based on comorbidities, cytogenetic risks, and Karnofsky performance scores.
The proportion of patients who developed at least one infection was comparable between groups: 61% of MAC patients versus 58% of RIC/NMA patients (P = .21), but further analysis showed that MAC was in fact associated with some relatively increased risks. For instance, patients in the MAC group tended to develop infections sooner than patients treated with RIC/NMA (21 vs. 15 days), and more patients treated with MAC had at least one bacterial infection by day 100 (46% vs. 37%).
Although the proportion of patients developing at least one viral infection was slightly lower in the MAC group than the RIC/NMA group (34% vs. 39%), overall infection density was higher, which takes into account multiple infections.
The increased bacterial infections after MAC were caused by gram-positive bacteria, while the increased viral infections with RIC/NMA were caused by cytomegalovirus, the investigators reported.
“RIC/NMA alloHCT is associated with a decreased risk of any infection and particularly early bacterial infections,” the investigators wrote. “The risk of viral and fungal infections per days at risk is similar.”
The Center for International Blood and Marrow Transplant Research is supported by grants from the U.S. government and several pharmaceutical companies. The investigators reported having no conflicts of interest.
SOURCE: Ustun C et al. Blood Adv. 2019 Sep 10;3(17):2525-36.
For patients with acute myeloid leukemia (AML) in need of allogeneic hematopoietic cell transplantation (alloHCT), reduced-intensity/nonmyeloablative conditioning (RIC/NMA) offers a lower risk of infection than myeloablative conditioning (MAC), based on a retrospective study involving more than 1,700 patients.
Within 100 days of treatment, patients who underwent MAC were significantly more likely to develop a bacterial infection, and develop it at an earlier date, than patients who had undergone RIC/NMA, reported lead author Celalettin Ustun, MD, of Rush University in Chicago, and colleagues.
“The incidence of infections, a common and often severe complication of alloHCT, is expected to be lower after RIC/NMA compared with MAC and thus contribute to the decreased [nonrelapse mortality],” the investigators wrote in Blood Advances, noting that this hypothesis has previously lacked supporting data, prompting the present study.
The retrospective analysis involved 1,755 patients with AML who were in first complete remission. Data were drawn from the Center for International Blood and Marrow Transplant Research (CIBMTR). The primary end point was incidence of infection within 100 days after T-cell replete alloHCT in patients receiving MAC (n = 978) versus those who underwent RIC/NMA (n = 777). Secondary end points included comparisons of infection types and infection density.
Patients who received RIC/NMA were generally older and more likely to have myelodysplastic syndrome than patients in the MAC group; the groups were otherwise similar, based on comorbidities, cytogenetic risks, and Karnofsky performance scores.
The proportion of patients who developed at least one infection was comparable between groups: 61% of MAC patients versus 58% of RIC/NMA patients (P = .21), but further analysis showed that MAC was in fact associated with some relatively increased risks. For instance, patients in the MAC group tended to develop infections sooner than patients treated with RIC/NMA (21 vs. 15 days), and more patients treated with MAC had at least one bacterial infection by day 100 (46% vs. 37%).
Although the proportion of patients developing at least one viral infection was slightly lower in the MAC group than the RIC/NMA group (34% vs. 39%), overall infection density was higher, which takes into account multiple infections.
The increased bacterial infections after MAC were caused by gram-positive bacteria, while the increased viral infections with RIC/NMA were caused by cytomegalovirus, the investigators reported.
“RIC/NMA alloHCT is associated with a decreased risk of any infection and particularly early bacterial infections,” the investigators wrote. “The risk of viral and fungal infections per days at risk is similar.”
The Center for International Blood and Marrow Transplant Research is supported by grants from the U.S. government and several pharmaceutical companies. The investigators reported having no conflicts of interest.
SOURCE: Ustun C et al. Blood Adv. 2019 Sep 10;3(17):2525-36.
FROM BLOOD ADVANCES
Key clinical point: For patients with acute myeloid leukemia (AML) in need of allogeneic hematopoietic cell transplantation (alloHCT), reduced-intensity/nonmyeloablative conditioning (RIC/NMA) offers a lower risk of infection than myeloablative conditioning (MAC).
Major finding: By day 100, 37% of patients who received RIC/NMA had at least one bacterial infection, compared with 46% of patients who underwent MAC (P = .0004).
Study details: A retrospective study involving 1,755 patients with AML in first complete remission.
Disclosures: The Center for International Blood and Marrow Transplant Research is supported by grants from the U.S. government and several pharmaceutical companies. The investigators reported having no conflicts of interest.
Source: Ustun C et al. Blood Adv. 2019 Sep 3(17):2525-36.
Pediatric HSCT recipients still risking sunburn
Young people who have received allogeneic hematopoietic stem cell transplants (HSCTs) are more likely to wear hats, sunscreen and other sun protection, but still intentionally tan and experience sunburn at the same rate as their peers, new research suggests.
In a survey‐based, cross‐sectional cohort study, researchers compared sun-protection behaviors and sun exposure in 85 children aged 21 years and younger who had undergone HSCT and 85 age-, sex-, and skin type–matched controls. The findings were published in Pediatric Dermatology.
HSCT recipients have a higher risk of long-term complications such as skin cancer, for which sun exposure is a major modifiable environmental risk factor.
“Therefore, consistent sun avoidance and protection as well as regular dermatologic evaluations are important for HSCT recipients,” wrote Edward B. Li, PhD, from Harvard Medical School, Boston, and coauthors.
The survey found no significant difference between the transplant and control group in the amount of intentional sun exposure, such as the amount of time spent outside on weekdays and weekends during the peak sun intensity hours of 10 a.m. and 4 p.m. More than one in five transplant recipients (21.2%) reported spending at least 3 hours a day outside between 10 a.m. and 4 p.m. on weekdays, as did 36.5% of transplant recipients on weekends.
There were also no significant differences between the two groups in terms of time spent tanning, either in the sun or in a tanning bed. Additionally, a similar number of transplant recipients and controls experienced one or more red or painful sunburns in the past year (25.9% vs. 27.1%).
However, transplant patients did practice better sun protection behaviors than did the control group, with 60% reporting that they always wore sunscreen, compared with 29.4% of controls. The transplant recipients were also significantly more likely to wear sunglasses and a hat and to stay in the shade or use an umbrella.
“While these data may reflect that HSCT patients are not practicing adequate sun avoidance, it may also suggest that these long‐term survivors are able to enjoy being outdoors as much as their peers and have a similar desire to have a tanned appearance,” the researchers wrote. “While a healthy and active lifestyle should be encouraged for all children, our results emphasize the need for pediatric HSCT survivors to be educated on their increased risk for UV‐related skin cancers, counseled on avoidance of intentional tanning, and advised on the importance of sun protection behaviors in an effort to improve long-term outcomes.”
The researchers noted that transplant recipients were significantly more likely to have had a full body skin exam from a health care professional than were individuals in the control group (61.2% vs. 4.7%) and were more likely to have done a self-check or been checked by a partner in the previous year.
The study was supported by the Society for Pediatric Dermatology, the Dermatology Foundation, the National Institutes of Health, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. One author declared a financial interest in a company developing a dermatological product. No other conflicts of interest were declared.
SOURCE: Li EB et al. Pediatr Dermatol. 2019 Aug 13. doi: 10.1111/pde.13984.
Young people who have received allogeneic hematopoietic stem cell transplants (HSCTs) are more likely to wear hats, sunscreen and other sun protection, but still intentionally tan and experience sunburn at the same rate as their peers, new research suggests.
In a survey‐based, cross‐sectional cohort study, researchers compared sun-protection behaviors and sun exposure in 85 children aged 21 years and younger who had undergone HSCT and 85 age-, sex-, and skin type–matched controls. The findings were published in Pediatric Dermatology.
HSCT recipients have a higher risk of long-term complications such as skin cancer, for which sun exposure is a major modifiable environmental risk factor.
“Therefore, consistent sun avoidance and protection as well as regular dermatologic evaluations are important for HSCT recipients,” wrote Edward B. Li, PhD, from Harvard Medical School, Boston, and coauthors.
The survey found no significant difference between the transplant and control group in the amount of intentional sun exposure, such as the amount of time spent outside on weekdays and weekends during the peak sun intensity hours of 10 a.m. and 4 p.m. More than one in five transplant recipients (21.2%) reported spending at least 3 hours a day outside between 10 a.m. and 4 p.m. on weekdays, as did 36.5% of transplant recipients on weekends.
There were also no significant differences between the two groups in terms of time spent tanning, either in the sun or in a tanning bed. Additionally, a similar number of transplant recipients and controls experienced one or more red or painful sunburns in the past year (25.9% vs. 27.1%).
However, transplant patients did practice better sun protection behaviors than did the control group, with 60% reporting that they always wore sunscreen, compared with 29.4% of controls. The transplant recipients were also significantly more likely to wear sunglasses and a hat and to stay in the shade or use an umbrella.
“While these data may reflect that HSCT patients are not practicing adequate sun avoidance, it may also suggest that these long‐term survivors are able to enjoy being outdoors as much as their peers and have a similar desire to have a tanned appearance,” the researchers wrote. “While a healthy and active lifestyle should be encouraged for all children, our results emphasize the need for pediatric HSCT survivors to be educated on their increased risk for UV‐related skin cancers, counseled on avoidance of intentional tanning, and advised on the importance of sun protection behaviors in an effort to improve long-term outcomes.”
The researchers noted that transplant recipients were significantly more likely to have had a full body skin exam from a health care professional than were individuals in the control group (61.2% vs. 4.7%) and were more likely to have done a self-check or been checked by a partner in the previous year.
The study was supported by the Society for Pediatric Dermatology, the Dermatology Foundation, the National Institutes of Health, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. One author declared a financial interest in a company developing a dermatological product. No other conflicts of interest were declared.
SOURCE: Li EB et al. Pediatr Dermatol. 2019 Aug 13. doi: 10.1111/pde.13984.
Young people who have received allogeneic hematopoietic stem cell transplants (HSCTs) are more likely to wear hats, sunscreen and other sun protection, but still intentionally tan and experience sunburn at the same rate as their peers, new research suggests.
In a survey‐based, cross‐sectional cohort study, researchers compared sun-protection behaviors and sun exposure in 85 children aged 21 years and younger who had undergone HSCT and 85 age-, sex-, and skin type–matched controls. The findings were published in Pediatric Dermatology.
HSCT recipients have a higher risk of long-term complications such as skin cancer, for which sun exposure is a major modifiable environmental risk factor.
“Therefore, consistent sun avoidance and protection as well as regular dermatologic evaluations are important for HSCT recipients,” wrote Edward B. Li, PhD, from Harvard Medical School, Boston, and coauthors.
The survey found no significant difference between the transplant and control group in the amount of intentional sun exposure, such as the amount of time spent outside on weekdays and weekends during the peak sun intensity hours of 10 a.m. and 4 p.m. More than one in five transplant recipients (21.2%) reported spending at least 3 hours a day outside between 10 a.m. and 4 p.m. on weekdays, as did 36.5% of transplant recipients on weekends.
There were also no significant differences between the two groups in terms of time spent tanning, either in the sun or in a tanning bed. Additionally, a similar number of transplant recipients and controls experienced one or more red or painful sunburns in the past year (25.9% vs. 27.1%).
However, transplant patients did practice better sun protection behaviors than did the control group, with 60% reporting that they always wore sunscreen, compared with 29.4% of controls. The transplant recipients were also significantly more likely to wear sunglasses and a hat and to stay in the shade or use an umbrella.
“While these data may reflect that HSCT patients are not practicing adequate sun avoidance, it may also suggest that these long‐term survivors are able to enjoy being outdoors as much as their peers and have a similar desire to have a tanned appearance,” the researchers wrote. “While a healthy and active lifestyle should be encouraged for all children, our results emphasize the need for pediatric HSCT survivors to be educated on their increased risk for UV‐related skin cancers, counseled on avoidance of intentional tanning, and advised on the importance of sun protection behaviors in an effort to improve long-term outcomes.”
The researchers noted that transplant recipients were significantly more likely to have had a full body skin exam from a health care professional than were individuals in the control group (61.2% vs. 4.7%) and were more likely to have done a self-check or been checked by a partner in the previous year.
The study was supported by the Society for Pediatric Dermatology, the Dermatology Foundation, the National Institutes of Health, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. One author declared a financial interest in a company developing a dermatological product. No other conflicts of interest were declared.
SOURCE: Li EB et al. Pediatr Dermatol. 2019 Aug 13. doi: 10.1111/pde.13984.
FROM PEDIATRIC DERMATOLOGY
Progressive myeloma after induction? Go straight to transplant
Patients with multiple myeloma who don’t respond to induction therapy may be better off advancing straight to autologous stem cell therapy, rather than undergoing salvage therapy before transplant, according to findings of an analysis that included both real-world and clinical trial patients.
Joanna Blocka, MD, of the University Hospital of Heidelberg (Germany) and colleagues found similar progression-free and overall survival rates for patients who had progressive disease and underwent autologous stem cell therapy (ASCT), compared with patients who underwent salvage therapy and improved to at least stable disease before proceeding to transplant. The findings were published in Leukemia & Lymphoma.
The real-world analysis included 1,599 patients with multiple myeloma who had undergone ASCT between 1991 and 2016. More than half of the patients (58%) were not enrolled in clinical trials. The remainder were split between the German-Speaking Myeloma Multicenter Group (GMMG)-HD3 and GMMG-HD4 trials, which compared various induction regimens.
Just 23 patients in the analysis received salvage therapy because of progressive disease and deepened their response before ASCT. Of these patients, 12 received novel agents in induction therapy and 11 received older medications.
Looking across all 1,599 patients, 5.3% achieved complete remission before first ASCT. Most patients (71.8%) achieved partial remission, 9.7% had a minimal response, and 5.7% had stable disease. A group of 120 patients (7.5%) progressed between the last course of induction and ASCT.
The researchers compared the progression-free and overall survival rates of patients with progressive disease versus those who had stable disease or better before their first transplant. Both univariable and multivariable analysis showed no statistically significant differences in either survival outcome between the two groups.
In the multivariable analysis, there was a hazard ratio of 1.23 (95% confidence interval, 0.98-1.56) for progression-free survival for patients with progressive disease versus those who responded to induction therapy. Similarly, the HR for overall survival between the two groups was 1.24 (95% CI, 0.93-1.65).
The researchers also analyzed the groups based on whether they received novel or older agents during induction.
Patients with progressive disease who received novel agents had significantly worse progression-free survival (22.2 months), compared with patients who responded to treatment with novel agents (22.2 months vs. 29.1 months; P = .03). The same trend was seen with overall survival in these groups (54.4 months vs. 97.5 months; P less than .001).
Rates of survival were similar for patients with progressive disease and responders who had received older medications at induction.
“This might be explained by a prognostically disadvantageous disease biology in patients nonresponsive to novel agents,” the researchers wrote.
The researchers also compared survival outcomes for the 120 patients who underwent ASCT with progressive disease versus the 23 patients who received salvage therapy and improved their response to at least stable disease before transplant. Univariable analysis showed that salvage patients actually did worse than those with progressive disease who proceeded straight to transplant – 12.1 months versus 22.9 months of progression-free survival (P = .04) and 33.1 versus 69.5 months of overall survival (P = .08). But on multivariable analysis, there was no significant difference between the two groups for progression-free survival (HR, 0.71; 95% CI, 0.28-1.80; P = .5) or overall survival (HR, 0.77; 95% CI, 0.30-1.95; P = .6). The use of novel agents did not appear to affect the survival outcomes in these patients.
The worse outcomes seen among salvage patients observed in univariable analysis “might be due to a cumulative toxic effect of salvage therapy,” the researchers suggested. “An alternative explanation could be that the patients who were offered salvage therapy might have had more aggressive disease than those who did not undergo salvage therapy.”
Dr. Blocka reported having no relevant financial disclosures. Other coauthors reported relationships with Janssen, Amgen, Bristol-Myers Squibb, Celgene, and others.
SOURCE: Blocka J et al. Leuk Lymphoma. 2019 Aug 19. doi: 10.1080/10428194.2019.1646905.
Patients with multiple myeloma who don’t respond to induction therapy may be better off advancing straight to autologous stem cell therapy, rather than undergoing salvage therapy before transplant, according to findings of an analysis that included both real-world and clinical trial patients.
Joanna Blocka, MD, of the University Hospital of Heidelberg (Germany) and colleagues found similar progression-free and overall survival rates for patients who had progressive disease and underwent autologous stem cell therapy (ASCT), compared with patients who underwent salvage therapy and improved to at least stable disease before proceeding to transplant. The findings were published in Leukemia & Lymphoma.
The real-world analysis included 1,599 patients with multiple myeloma who had undergone ASCT between 1991 and 2016. More than half of the patients (58%) were not enrolled in clinical trials. The remainder were split between the German-Speaking Myeloma Multicenter Group (GMMG)-HD3 and GMMG-HD4 trials, which compared various induction regimens.
Just 23 patients in the analysis received salvage therapy because of progressive disease and deepened their response before ASCT. Of these patients, 12 received novel agents in induction therapy and 11 received older medications.
Looking across all 1,599 patients, 5.3% achieved complete remission before first ASCT. Most patients (71.8%) achieved partial remission, 9.7% had a minimal response, and 5.7% had stable disease. A group of 120 patients (7.5%) progressed between the last course of induction and ASCT.
The researchers compared the progression-free and overall survival rates of patients with progressive disease versus those who had stable disease or better before their first transplant. Both univariable and multivariable analysis showed no statistically significant differences in either survival outcome between the two groups.
In the multivariable analysis, there was a hazard ratio of 1.23 (95% confidence interval, 0.98-1.56) for progression-free survival for patients with progressive disease versus those who responded to induction therapy. Similarly, the HR for overall survival between the two groups was 1.24 (95% CI, 0.93-1.65).
The researchers also analyzed the groups based on whether they received novel or older agents during induction.
Patients with progressive disease who received novel agents had significantly worse progression-free survival (22.2 months), compared with patients who responded to treatment with novel agents (22.2 months vs. 29.1 months; P = .03). The same trend was seen with overall survival in these groups (54.4 months vs. 97.5 months; P less than .001).
Rates of survival were similar for patients with progressive disease and responders who had received older medications at induction.
“This might be explained by a prognostically disadvantageous disease biology in patients nonresponsive to novel agents,” the researchers wrote.
The researchers also compared survival outcomes for the 120 patients who underwent ASCT with progressive disease versus the 23 patients who received salvage therapy and improved their response to at least stable disease before transplant. Univariable analysis showed that salvage patients actually did worse than those with progressive disease who proceeded straight to transplant – 12.1 months versus 22.9 months of progression-free survival (P = .04) and 33.1 versus 69.5 months of overall survival (P = .08). But on multivariable analysis, there was no significant difference between the two groups for progression-free survival (HR, 0.71; 95% CI, 0.28-1.80; P = .5) or overall survival (HR, 0.77; 95% CI, 0.30-1.95; P = .6). The use of novel agents did not appear to affect the survival outcomes in these patients.
The worse outcomes seen among salvage patients observed in univariable analysis “might be due to a cumulative toxic effect of salvage therapy,” the researchers suggested. “An alternative explanation could be that the patients who were offered salvage therapy might have had more aggressive disease than those who did not undergo salvage therapy.”
Dr. Blocka reported having no relevant financial disclosures. Other coauthors reported relationships with Janssen, Amgen, Bristol-Myers Squibb, Celgene, and others.
SOURCE: Blocka J et al. Leuk Lymphoma. 2019 Aug 19. doi: 10.1080/10428194.2019.1646905.
Patients with multiple myeloma who don’t respond to induction therapy may be better off advancing straight to autologous stem cell therapy, rather than undergoing salvage therapy before transplant, according to findings of an analysis that included both real-world and clinical trial patients.
Joanna Blocka, MD, of the University Hospital of Heidelberg (Germany) and colleagues found similar progression-free and overall survival rates for patients who had progressive disease and underwent autologous stem cell therapy (ASCT), compared with patients who underwent salvage therapy and improved to at least stable disease before proceeding to transplant. The findings were published in Leukemia & Lymphoma.
The real-world analysis included 1,599 patients with multiple myeloma who had undergone ASCT between 1991 and 2016. More than half of the patients (58%) were not enrolled in clinical trials. The remainder were split between the German-Speaking Myeloma Multicenter Group (GMMG)-HD3 and GMMG-HD4 trials, which compared various induction regimens.
Just 23 patients in the analysis received salvage therapy because of progressive disease and deepened their response before ASCT. Of these patients, 12 received novel agents in induction therapy and 11 received older medications.
Looking across all 1,599 patients, 5.3% achieved complete remission before first ASCT. Most patients (71.8%) achieved partial remission, 9.7% had a minimal response, and 5.7% had stable disease. A group of 120 patients (7.5%) progressed between the last course of induction and ASCT.
The researchers compared the progression-free and overall survival rates of patients with progressive disease versus those who had stable disease or better before their first transplant. Both univariable and multivariable analysis showed no statistically significant differences in either survival outcome between the two groups.
In the multivariable analysis, there was a hazard ratio of 1.23 (95% confidence interval, 0.98-1.56) for progression-free survival for patients with progressive disease versus those who responded to induction therapy. Similarly, the HR for overall survival between the two groups was 1.24 (95% CI, 0.93-1.65).
The researchers also analyzed the groups based on whether they received novel or older agents during induction.
Patients with progressive disease who received novel agents had significantly worse progression-free survival (22.2 months), compared with patients who responded to treatment with novel agents (22.2 months vs. 29.1 months; P = .03). The same trend was seen with overall survival in these groups (54.4 months vs. 97.5 months; P less than .001).
Rates of survival were similar for patients with progressive disease and responders who had received older medications at induction.
“This might be explained by a prognostically disadvantageous disease biology in patients nonresponsive to novel agents,” the researchers wrote.
The researchers also compared survival outcomes for the 120 patients who underwent ASCT with progressive disease versus the 23 patients who received salvage therapy and improved their response to at least stable disease before transplant. Univariable analysis showed that salvage patients actually did worse than those with progressive disease who proceeded straight to transplant – 12.1 months versus 22.9 months of progression-free survival (P = .04) and 33.1 versus 69.5 months of overall survival (P = .08). But on multivariable analysis, there was no significant difference between the two groups for progression-free survival (HR, 0.71; 95% CI, 0.28-1.80; P = .5) or overall survival (HR, 0.77; 95% CI, 0.30-1.95; P = .6). The use of novel agents did not appear to affect the survival outcomes in these patients.
The worse outcomes seen among salvage patients observed in univariable analysis “might be due to a cumulative toxic effect of salvage therapy,” the researchers suggested. “An alternative explanation could be that the patients who were offered salvage therapy might have had more aggressive disease than those who did not undergo salvage therapy.”
Dr. Blocka reported having no relevant financial disclosures. Other coauthors reported relationships with Janssen, Amgen, Bristol-Myers Squibb, Celgene, and others.
SOURCE: Blocka J et al. Leuk Lymphoma. 2019 Aug 19. doi: 10.1080/10428194.2019.1646905.
FROM LEUKEMIA & LYMPHOMA
Key clinical point:
Major finding: There was no difference between patients with progressive disease who went straight to ASCT and patients who received salvage therapy, both in terms of progression-free survival (hazard ratio, 0.71; 95% confidence interval, 0.28-1.80; P = .5) and overall survival (HR, 0.77; 95% CI, 0.30-1.95; P = .6).
Study details: An analysis of 1,599 patients with multiple myeloma who underwent ASCT. A subanalysis compared 120 patients with progressive disease before ASCT with 23 patients who received salvage treatment before ASCT.
Disclosures: Dr. Blocka reported having no relevant financial disclosures. Other coauthors reported relationships with Janssen, Amgen, Bristol-Myers Squibb, Celgene, and others.
Source: Blocka J et al. Leuk Lymphoma. 2019 Aug 19. doi: 10.1080/10428194.2019.1646905.
Novel conditioning regimen shows benefit for beta-thalassemia major
A novel transplant protocol (WZ-14-TM) improved survival outcomes and rates of graft-versus-host disease (GVHD) in patients with beta-thalassemia major undergoing hematopoietic stem cell transplant (HSCT) from an unrelated donor, according to findings from a single-center study.
“In August 2014, we began using WZ-14-TM in hopes of lowering the [graft failure] rate and transplant-related mortality,” Lan Sun, MD, of Wenzhou (China) Medical University and colleagues wrote in Biology of Blood and Marrow Transplantation.
The study cohort included 48 patients (aged 2-11 years) with beta-thalassemia major who underwent unrelated-donor HSCT from August 2014 to June 2018. Prior to transplantation, all participants received iron chelation therapy and regular red blood cell transfusions.
The original busulfan/cyclophosphamide–based conditioning regimen was modified to include antithymocyte globulin and fludarabine in order to reduce the risk of graft failure.
Additionally, the team lowered the cumulative dose of cyclophosphamide from 200 mg/kg to 100 mg/kg in an effort to lessen treatment-related toxicity.
After analysis, the researchers reported that the rates of thalassemia-free and overall survival were both 100%, while the incidence rates of acute (grade 2-4) and chronic GVHD were both 8.3%. In prior studies, the incidence rates of acute (grade 2-4) and chronic GVHD were 37%-42% and 14%-27%, respectively.
Neutrophil engraftment was achieved in a median duration of 13 days, while the median hemoglobin and platelet recovery times were 11 days and 12 days, respectively.
The low incidence of GVHD in their study may be related to the combination of antithymocyte globulin, cyclosporine A, mycophenolate mofetil, and methotrexate for GVHD prophylaxis, the researchers wrote.
They acknowledged two key limitations of the study were the small sample size and its single-center design. Accordingly, the findings should be validated in future studies.
The results suggest that the WZ-14-TM protocol is a “feasible and safe” conditioning regimen for patients with beta-thalassemia major undergoing unrelated-donor HSCT, they concluded.
The study was funded by the Public Welfare Science and Technology Project of Wenzhou, the Natural Science Foundation of Zhejiang Province, and the National Natural Science Foundation of China. The authors reported having no conflicts of interest.
SOURCE: Sun L et al. Biol Blood Marrow Transplant. 2019; 25(8):1592-6.
A novel transplant protocol (WZ-14-TM) improved survival outcomes and rates of graft-versus-host disease (GVHD) in patients with beta-thalassemia major undergoing hematopoietic stem cell transplant (HSCT) from an unrelated donor, according to findings from a single-center study.
“In August 2014, we began using WZ-14-TM in hopes of lowering the [graft failure] rate and transplant-related mortality,” Lan Sun, MD, of Wenzhou (China) Medical University and colleagues wrote in Biology of Blood and Marrow Transplantation.
The study cohort included 48 patients (aged 2-11 years) with beta-thalassemia major who underwent unrelated-donor HSCT from August 2014 to June 2018. Prior to transplantation, all participants received iron chelation therapy and regular red blood cell transfusions.
The original busulfan/cyclophosphamide–based conditioning regimen was modified to include antithymocyte globulin and fludarabine in order to reduce the risk of graft failure.
Additionally, the team lowered the cumulative dose of cyclophosphamide from 200 mg/kg to 100 mg/kg in an effort to lessen treatment-related toxicity.
After analysis, the researchers reported that the rates of thalassemia-free and overall survival were both 100%, while the incidence rates of acute (grade 2-4) and chronic GVHD were both 8.3%. In prior studies, the incidence rates of acute (grade 2-4) and chronic GVHD were 37%-42% and 14%-27%, respectively.
Neutrophil engraftment was achieved in a median duration of 13 days, while the median hemoglobin and platelet recovery times were 11 days and 12 days, respectively.
The low incidence of GVHD in their study may be related to the combination of antithymocyte globulin, cyclosporine A, mycophenolate mofetil, and methotrexate for GVHD prophylaxis, the researchers wrote.
They acknowledged two key limitations of the study were the small sample size and its single-center design. Accordingly, the findings should be validated in future studies.
The results suggest that the WZ-14-TM protocol is a “feasible and safe” conditioning regimen for patients with beta-thalassemia major undergoing unrelated-donor HSCT, they concluded.
The study was funded by the Public Welfare Science and Technology Project of Wenzhou, the Natural Science Foundation of Zhejiang Province, and the National Natural Science Foundation of China. The authors reported having no conflicts of interest.
SOURCE: Sun L et al. Biol Blood Marrow Transplant. 2019; 25(8):1592-6.
A novel transplant protocol (WZ-14-TM) improved survival outcomes and rates of graft-versus-host disease (GVHD) in patients with beta-thalassemia major undergoing hematopoietic stem cell transplant (HSCT) from an unrelated donor, according to findings from a single-center study.
“In August 2014, we began using WZ-14-TM in hopes of lowering the [graft failure] rate and transplant-related mortality,” Lan Sun, MD, of Wenzhou (China) Medical University and colleagues wrote in Biology of Blood and Marrow Transplantation.
The study cohort included 48 patients (aged 2-11 years) with beta-thalassemia major who underwent unrelated-donor HSCT from August 2014 to June 2018. Prior to transplantation, all participants received iron chelation therapy and regular red blood cell transfusions.
The original busulfan/cyclophosphamide–based conditioning regimen was modified to include antithymocyte globulin and fludarabine in order to reduce the risk of graft failure.
Additionally, the team lowered the cumulative dose of cyclophosphamide from 200 mg/kg to 100 mg/kg in an effort to lessen treatment-related toxicity.
After analysis, the researchers reported that the rates of thalassemia-free and overall survival were both 100%, while the incidence rates of acute (grade 2-4) and chronic GVHD were both 8.3%. In prior studies, the incidence rates of acute (grade 2-4) and chronic GVHD were 37%-42% and 14%-27%, respectively.
Neutrophil engraftment was achieved in a median duration of 13 days, while the median hemoglobin and platelet recovery times were 11 days and 12 days, respectively.
The low incidence of GVHD in their study may be related to the combination of antithymocyte globulin, cyclosporine A, mycophenolate mofetil, and methotrexate for GVHD prophylaxis, the researchers wrote.
They acknowledged two key limitations of the study were the small sample size and its single-center design. Accordingly, the findings should be validated in future studies.
The results suggest that the WZ-14-TM protocol is a “feasible and safe” conditioning regimen for patients with beta-thalassemia major undergoing unrelated-donor HSCT, they concluded.
The study was funded by the Public Welfare Science and Technology Project of Wenzhou, the Natural Science Foundation of Zhejiang Province, and the National Natural Science Foundation of China. The authors reported having no conflicts of interest.
SOURCE: Sun L et al. Biol Blood Marrow Transplant. 2019; 25(8):1592-6.
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION
Guidelines update donor selection criteria for HSCT
Newly updated guidelines can inform the selection of adult donors and cord blood units for allogeneic hematopoietic stem cell transplant.
The evidence-based guidelines suggest high-resolution human leukocyte antigen (HLA) matching and donor age are important when selecting adult donors, while HLA matching, cell dose, and banking practices should be considered when selecting cord blood units.
The guidelines were developed by the National Marrow Donor Program (NMDP) and Center for International Blood and Marrow Transplant Research (CIBMTR) and were recently published in Blood.
Adult donors
The guidelines recommend high-resolution HLA typing for adult donors and patients. This means typing for HLA-A, -B, -C, and -DRB1, at minimum. Typing at other loci – DPB1, DQB1, DRB3/4/5, DQA1, and DPA1 – is “optional but often helpful.”
An 8/8 HLA-matched donor is considered optimal. If only 7/8-matched donors are available, select a donor with a single allele mismatched at the patient’s homozygous locus if possible, and select an HLA-C*03:03 mismatch over an HLA-C*03:04 mismatch where applicable.
For both 8/8- and 7/8-matched donors, try to avoid mismatches at DQB1 and DRB3/4/5, and select DPB1 mismatches based on the DPB1 T-cell epitope algorithm. Mismatches of allotypes targeted by donor-specific HLA antibodies (DSA), including DQA1 and DPA1, should be avoided.
The guidelines recommend pursuing multiple donors because not all potential donors will be available. Younger donors should be prioritized over older donors. Other factors – such as sex or cytomegalovirus serostatus – should not affect donor selection.
Cord blood
For cord blood donations, testing attached segment identity is mandatory, red blood cell–replete units are not recommended, and both unit cryovolume and year of cryopreservation should be taken into consideration. The guidelines note that “some expert centers” favor red blood cell–depleted units with a postcryopreservation volume of about 25 ml/bag, and units banked more recently “may be linked to optimal banking practices.”
The guidelines recommend a minimum of eight high-resolution HLA typing for cord blood units and patients. A 4/6 match (HLA-A, -B, -DRB1) is acceptable, as is a 4/8 match (HLA-A, -B, -C, and -DRB1) or greater. In the case of a double-unit transplant, there is no need to match the units to each other.
“DSA must be considered on a case-by-case basis,” according to the guidelines. The patient’s diagnosis, prior immunosuppressive therapy, planned conditioning regimen, and DSA number/titer/specificity/complement fixation should be taken into consideration. DSA-targeted units should be avoided in patients with nonmalignant conditions and used with caution in patients with hematologic malignancies.
For single–cord blood units, the total nucleated cell dose should be at least 2.5 x 107/kg, and the number of CD34+ cells should be at least 1.5 x 105/kg. For double-unit transplants, the total nucleated cell dose should be at least 1.5 x 107/kg for each unit, and the number of CD34+ cells should be at least 1.0 x 105/kg for each unit.
The guidelines note that additional research is needed to inform how to balance cell dose against HLA match. However, cell dose should often take priority over HLA match for adults and larger pediatric patients, and HLA match can take priority in children, smaller adults, or patients with common HLA typing who have multiple units with a high cell dose.
The guidelines’ authors reported relationships with MolMed, NexImmune, AbbVie, Bellicum, Incyte, Medigene, Merck, Nektar, Novartis, Servier, Miltenyi, and the U.S. government/military.
SOURCE: Dehn J et al. Blood. 2019 Jul 10. doi: 10.1182/blood.2019001212.
Newly updated guidelines can inform the selection of adult donors and cord blood units for allogeneic hematopoietic stem cell transplant.
The evidence-based guidelines suggest high-resolution human leukocyte antigen (HLA) matching and donor age are important when selecting adult donors, while HLA matching, cell dose, and banking practices should be considered when selecting cord blood units.
The guidelines were developed by the National Marrow Donor Program (NMDP) and Center for International Blood and Marrow Transplant Research (CIBMTR) and were recently published in Blood.
Adult donors
The guidelines recommend high-resolution HLA typing for adult donors and patients. This means typing for HLA-A, -B, -C, and -DRB1, at minimum. Typing at other loci – DPB1, DQB1, DRB3/4/5, DQA1, and DPA1 – is “optional but often helpful.”
An 8/8 HLA-matched donor is considered optimal. If only 7/8-matched donors are available, select a donor with a single allele mismatched at the patient’s homozygous locus if possible, and select an HLA-C*03:03 mismatch over an HLA-C*03:04 mismatch where applicable.
For both 8/8- and 7/8-matched donors, try to avoid mismatches at DQB1 and DRB3/4/5, and select DPB1 mismatches based on the DPB1 T-cell epitope algorithm. Mismatches of allotypes targeted by donor-specific HLA antibodies (DSA), including DQA1 and DPA1, should be avoided.
The guidelines recommend pursuing multiple donors because not all potential donors will be available. Younger donors should be prioritized over older donors. Other factors – such as sex or cytomegalovirus serostatus – should not affect donor selection.
Cord blood
For cord blood donations, testing attached segment identity is mandatory, red blood cell–replete units are not recommended, and both unit cryovolume and year of cryopreservation should be taken into consideration. The guidelines note that “some expert centers” favor red blood cell–depleted units with a postcryopreservation volume of about 25 ml/bag, and units banked more recently “may be linked to optimal banking practices.”
The guidelines recommend a minimum of eight high-resolution HLA typing for cord blood units and patients. A 4/6 match (HLA-A, -B, -DRB1) is acceptable, as is a 4/8 match (HLA-A, -B, -C, and -DRB1) or greater. In the case of a double-unit transplant, there is no need to match the units to each other.
“DSA must be considered on a case-by-case basis,” according to the guidelines. The patient’s diagnosis, prior immunosuppressive therapy, planned conditioning regimen, and DSA number/titer/specificity/complement fixation should be taken into consideration. DSA-targeted units should be avoided in patients with nonmalignant conditions and used with caution in patients with hematologic malignancies.
For single–cord blood units, the total nucleated cell dose should be at least 2.5 x 107/kg, and the number of CD34+ cells should be at least 1.5 x 105/kg. For double-unit transplants, the total nucleated cell dose should be at least 1.5 x 107/kg for each unit, and the number of CD34+ cells should be at least 1.0 x 105/kg for each unit.
The guidelines note that additional research is needed to inform how to balance cell dose against HLA match. However, cell dose should often take priority over HLA match for adults and larger pediatric patients, and HLA match can take priority in children, smaller adults, or patients with common HLA typing who have multiple units with a high cell dose.
The guidelines’ authors reported relationships with MolMed, NexImmune, AbbVie, Bellicum, Incyte, Medigene, Merck, Nektar, Novartis, Servier, Miltenyi, and the U.S. government/military.
SOURCE: Dehn J et al. Blood. 2019 Jul 10. doi: 10.1182/blood.2019001212.
Newly updated guidelines can inform the selection of adult donors and cord blood units for allogeneic hematopoietic stem cell transplant.
The evidence-based guidelines suggest high-resolution human leukocyte antigen (HLA) matching and donor age are important when selecting adult donors, while HLA matching, cell dose, and banking practices should be considered when selecting cord blood units.
The guidelines were developed by the National Marrow Donor Program (NMDP) and Center for International Blood and Marrow Transplant Research (CIBMTR) and were recently published in Blood.
Adult donors
The guidelines recommend high-resolution HLA typing for adult donors and patients. This means typing for HLA-A, -B, -C, and -DRB1, at minimum. Typing at other loci – DPB1, DQB1, DRB3/4/5, DQA1, and DPA1 – is “optional but often helpful.”
An 8/8 HLA-matched donor is considered optimal. If only 7/8-matched donors are available, select a donor with a single allele mismatched at the patient’s homozygous locus if possible, and select an HLA-C*03:03 mismatch over an HLA-C*03:04 mismatch where applicable.
For both 8/8- and 7/8-matched donors, try to avoid mismatches at DQB1 and DRB3/4/5, and select DPB1 mismatches based on the DPB1 T-cell epitope algorithm. Mismatches of allotypes targeted by donor-specific HLA antibodies (DSA), including DQA1 and DPA1, should be avoided.
The guidelines recommend pursuing multiple donors because not all potential donors will be available. Younger donors should be prioritized over older donors. Other factors – such as sex or cytomegalovirus serostatus – should not affect donor selection.
Cord blood
For cord blood donations, testing attached segment identity is mandatory, red blood cell–replete units are not recommended, and both unit cryovolume and year of cryopreservation should be taken into consideration. The guidelines note that “some expert centers” favor red blood cell–depleted units with a postcryopreservation volume of about 25 ml/bag, and units banked more recently “may be linked to optimal banking practices.”
The guidelines recommend a minimum of eight high-resolution HLA typing for cord blood units and patients. A 4/6 match (HLA-A, -B, -DRB1) is acceptable, as is a 4/8 match (HLA-A, -B, -C, and -DRB1) or greater. In the case of a double-unit transplant, there is no need to match the units to each other.
“DSA must be considered on a case-by-case basis,” according to the guidelines. The patient’s diagnosis, prior immunosuppressive therapy, planned conditioning regimen, and DSA number/titer/specificity/complement fixation should be taken into consideration. DSA-targeted units should be avoided in patients with nonmalignant conditions and used with caution in patients with hematologic malignancies.
For single–cord blood units, the total nucleated cell dose should be at least 2.5 x 107/kg, and the number of CD34+ cells should be at least 1.5 x 105/kg. For double-unit transplants, the total nucleated cell dose should be at least 1.5 x 107/kg for each unit, and the number of CD34+ cells should be at least 1.0 x 105/kg for each unit.
The guidelines note that additional research is needed to inform how to balance cell dose against HLA match. However, cell dose should often take priority over HLA match for adults and larger pediatric patients, and HLA match can take priority in children, smaller adults, or patients with common HLA typing who have multiple units with a high cell dose.
The guidelines’ authors reported relationships with MolMed, NexImmune, AbbVie, Bellicum, Incyte, Medigene, Merck, Nektar, Novartis, Servier, Miltenyi, and the U.S. government/military.
SOURCE: Dehn J et al. Blood. 2019 Jul 10. doi: 10.1182/blood.2019001212.
FROM BLOOD