User login
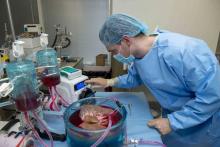
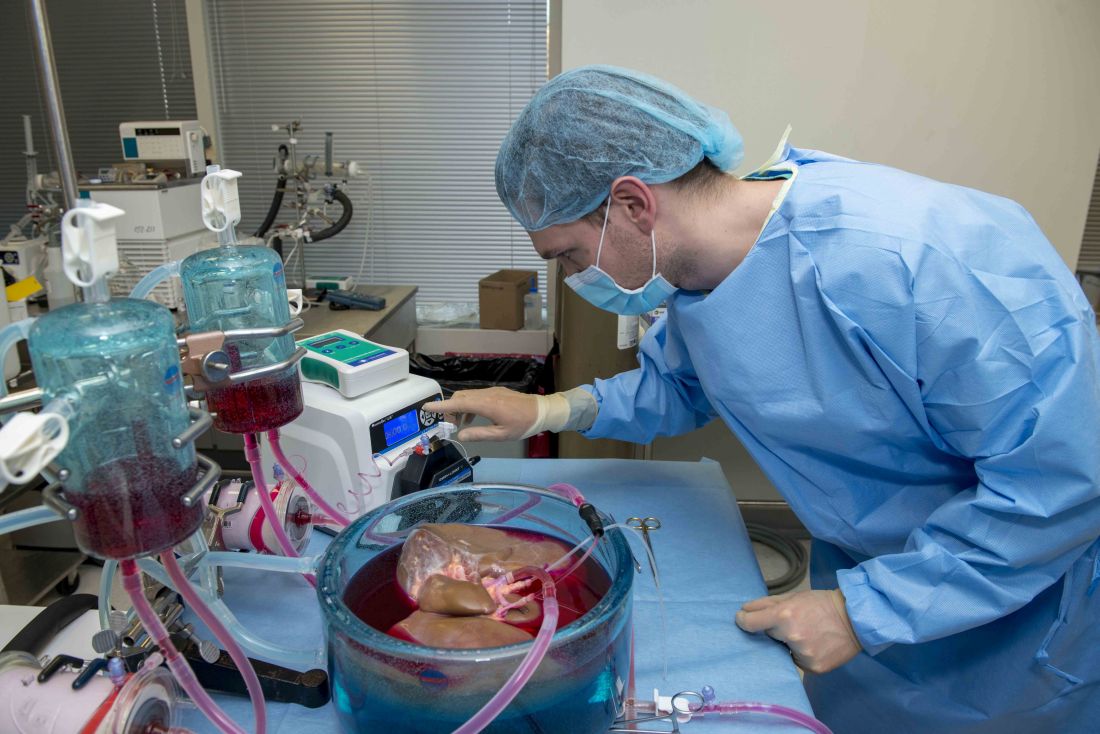
Standard cooling to 4°C provides just 12 hours of organ preservation, but laboratory testing showed that supercooling to –4°C added 27 hours of viability, reported lead author Reinier J. de Vries, MD, of Harvard Medical School and Massachusetts General Hospital in Boston, and colleagues.
“The absence of technology to preserve organs for more than a few hours is one of the fundamental causes of the donor organ–shortage crisis,” the investigators wrote in Nature Biotechnology.
Supercooling organs to high-subzero temperatures has been shown to prolong organ life while avoiding ice-mediated injury, but techniques that are successful for rat livers have been difficult to translate to human livers because of their larger size, which increases the risk of ice formation, the investigators explained.
Three strategies were employed to overcome this problem: minimization of air-liquid interfaces, development of a new supercooling-preservation solution, and hypothermic machine perfusion to more evenly distribute preservation solution throughout the liver tissue. For recovery of organs after supercooling, the investigators used subnormothermic machine perfusion, which has been used effectively in rat transplants.
In order to measure the impact of this process on organ viability, the investigators first measured adenylate energy content, both before supercooling and after recovery.
“Adenylate energy content, and, particularly, the organ’s ability to recover it during (re)perfusion, is considered the most representative metric for liver viability,” they wrote.
The difference between pre- and postsupercooling energy charge was less than 20%; in comparison, failed liver transplants in large animals and clinical trials have typically involved an energy-charge loss of 40% or more.
To further test organ viability, the investigators measured pre- and postsupercooling levels of bile production, oxygen uptake, and vascular resistance. All of these parameters have been shown to predict transplant success in rats, and bile production has additional precedent from human studies.
On average, bile production, portal resistance, and arterial resistance were not significantly affected by supercooling. Although portal vein resistance was 20% higher after supercooling, this compared favorably with increases of 100%-150% that have been measured in nonviable livers. Similarly, oxygen uptake increased by a mean of 17%, but this was three times lower than changes that have been observed in livers with impaired viability, at 51%.
Additional measures of hepatocellular injury, including AST and ALT, were also supportive of viability after supercooling. Histopathology confirmed these findings by showing preserved tissue architecture.
“In summary, we find that the human livers tested displayed no substantial difference in viability before and after extended subzero supercooling preservation,” the investigators wrote.
To simulate transplantation, the investigators reperfused the organs with blood at a normal temperature, including platelets, complement, and white blood cells, which are drivers of ischemia reperfusion injury. During this process, energy charge remained stable, which indicates preserved mitochondrial function. While energy charge held steady, lactate metabolism increased with bile and urea production, suggesting increased liver function. Bile pH and HCO3– levels fell within range for viability. Although bile glucose exceeded proposed criteria, the investigators pointed out that levels still fell within parameters for research-quality livers. Lactate levels also rose within the first hour of reperfusion, but the investigators suggested that this finding should be interpreted with appropriate context.
“It should be considered that the livers in this study were initially rejected for transplantation,” they wrote, “and the confidence intervals of the lactate concentration at the end of reperfusion largely overlap with time-matched values reported by others during [normothermic machine perfusion] of rejected human livers.”
Hepatocellular injury and histology also were evaluated during and after simulated transplantation, respectively, with favorable results. Although sites of preexisting hepatic injury were aggravated by the process, and rates of apoptosis increased, the investigators considered these changes were clinically insignificant.
Looking to the future, the investigators suggested that further refinement of the process could facilitate even-lower storage temperatures while better preserving liver viability.
“The use of human livers makes this study clinically relevant and promotes the translation of subzero organ preservation to the clinic,” the investigators concluded. “However, long-term survival experiments of transplanted supercooled livers in swine or an alternative large animal model will be needed before clinical translation.”
The study was funded by the National Institutes of Health and the Department of Defense. Dr. de Vries and four other coauthors have provisional patent applications related to the study, and one coauthor disclosed a financial relationship with Organ Solutions.
SOURCE: de Vries RJ et al. Nature Biotechnol. 2019 Sep 9. doi: 10.1038/s41587-019-0223-y.
Standard cooling to 4°C provides just 12 hours of organ preservation, but laboratory testing showed that supercooling to –4°C added 27 hours of viability, reported lead author Reinier J. de Vries, MD, of Harvard Medical School and Massachusetts General Hospital in Boston, and colleagues.
“The absence of technology to preserve organs for more than a few hours is one of the fundamental causes of the donor organ–shortage crisis,” the investigators wrote in Nature Biotechnology.
Supercooling organs to high-subzero temperatures has been shown to prolong organ life while avoiding ice-mediated injury, but techniques that are successful for rat livers have been difficult to translate to human livers because of their larger size, which increases the risk of ice formation, the investigators explained.
Three strategies were employed to overcome this problem: minimization of air-liquid interfaces, development of a new supercooling-preservation solution, and hypothermic machine perfusion to more evenly distribute preservation solution throughout the liver tissue. For recovery of organs after supercooling, the investigators used subnormothermic machine perfusion, which has been used effectively in rat transplants.
In order to measure the impact of this process on organ viability, the investigators first measured adenylate energy content, both before supercooling and after recovery.
“Adenylate energy content, and, particularly, the organ’s ability to recover it during (re)perfusion, is considered the most representative metric for liver viability,” they wrote.
The difference between pre- and postsupercooling energy charge was less than 20%; in comparison, failed liver transplants in large animals and clinical trials have typically involved an energy-charge loss of 40% or more.
To further test organ viability, the investigators measured pre- and postsupercooling levels of bile production, oxygen uptake, and vascular resistance. All of these parameters have been shown to predict transplant success in rats, and bile production has additional precedent from human studies.
On average, bile production, portal resistance, and arterial resistance were not significantly affected by supercooling. Although portal vein resistance was 20% higher after supercooling, this compared favorably with increases of 100%-150% that have been measured in nonviable livers. Similarly, oxygen uptake increased by a mean of 17%, but this was three times lower than changes that have been observed in livers with impaired viability, at 51%.
Additional measures of hepatocellular injury, including AST and ALT, were also supportive of viability after supercooling. Histopathology confirmed these findings by showing preserved tissue architecture.
“In summary, we find that the human livers tested displayed no substantial difference in viability before and after extended subzero supercooling preservation,” the investigators wrote.
To simulate transplantation, the investigators reperfused the organs with blood at a normal temperature, including platelets, complement, and white blood cells, which are drivers of ischemia reperfusion injury. During this process, energy charge remained stable, which indicates preserved mitochondrial function. While energy charge held steady, lactate metabolism increased with bile and urea production, suggesting increased liver function. Bile pH and HCO3– levels fell within range for viability. Although bile glucose exceeded proposed criteria, the investigators pointed out that levels still fell within parameters for research-quality livers. Lactate levels also rose within the first hour of reperfusion, but the investigators suggested that this finding should be interpreted with appropriate context.
“It should be considered that the livers in this study were initially rejected for transplantation,” they wrote, “and the confidence intervals of the lactate concentration at the end of reperfusion largely overlap with time-matched values reported by others during [normothermic machine perfusion] of rejected human livers.”
Hepatocellular injury and histology also were evaluated during and after simulated transplantation, respectively, with favorable results. Although sites of preexisting hepatic injury were aggravated by the process, and rates of apoptosis increased, the investigators considered these changes were clinically insignificant.
Looking to the future, the investigators suggested that further refinement of the process could facilitate even-lower storage temperatures while better preserving liver viability.
“The use of human livers makes this study clinically relevant and promotes the translation of subzero organ preservation to the clinic,” the investigators concluded. “However, long-term survival experiments of transplanted supercooled livers in swine or an alternative large animal model will be needed before clinical translation.”
The study was funded by the National Institutes of Health and the Department of Defense. Dr. de Vries and four other coauthors have provisional patent applications related to the study, and one coauthor disclosed a financial relationship with Organ Solutions.
SOURCE: de Vries RJ et al. Nature Biotechnol. 2019 Sep 9. doi: 10.1038/s41587-019-0223-y.
Standard cooling to 4°C provides just 12 hours of organ preservation, but laboratory testing showed that supercooling to –4°C added 27 hours of viability, reported lead author Reinier J. de Vries, MD, of Harvard Medical School and Massachusetts General Hospital in Boston, and colleagues.
“The absence of technology to preserve organs for more than a few hours is one of the fundamental causes of the donor organ–shortage crisis,” the investigators wrote in Nature Biotechnology.
Supercooling organs to high-subzero temperatures has been shown to prolong organ life while avoiding ice-mediated injury, but techniques that are successful for rat livers have been difficult to translate to human livers because of their larger size, which increases the risk of ice formation, the investigators explained.
Three strategies were employed to overcome this problem: minimization of air-liquid interfaces, development of a new supercooling-preservation solution, and hypothermic machine perfusion to more evenly distribute preservation solution throughout the liver tissue. For recovery of organs after supercooling, the investigators used subnormothermic machine perfusion, which has been used effectively in rat transplants.
In order to measure the impact of this process on organ viability, the investigators first measured adenylate energy content, both before supercooling and after recovery.
“Adenylate energy content, and, particularly, the organ’s ability to recover it during (re)perfusion, is considered the most representative metric for liver viability,” they wrote.
The difference between pre- and postsupercooling energy charge was less than 20%; in comparison, failed liver transplants in large animals and clinical trials have typically involved an energy-charge loss of 40% or more.
To further test organ viability, the investigators measured pre- and postsupercooling levels of bile production, oxygen uptake, and vascular resistance. All of these parameters have been shown to predict transplant success in rats, and bile production has additional precedent from human studies.
On average, bile production, portal resistance, and arterial resistance were not significantly affected by supercooling. Although portal vein resistance was 20% higher after supercooling, this compared favorably with increases of 100%-150% that have been measured in nonviable livers. Similarly, oxygen uptake increased by a mean of 17%, but this was three times lower than changes that have been observed in livers with impaired viability, at 51%.
Additional measures of hepatocellular injury, including AST and ALT, were also supportive of viability after supercooling. Histopathology confirmed these findings by showing preserved tissue architecture.
“In summary, we find that the human livers tested displayed no substantial difference in viability before and after extended subzero supercooling preservation,” the investigators wrote.
To simulate transplantation, the investigators reperfused the organs with blood at a normal temperature, including platelets, complement, and white blood cells, which are drivers of ischemia reperfusion injury. During this process, energy charge remained stable, which indicates preserved mitochondrial function. While energy charge held steady, lactate metabolism increased with bile and urea production, suggesting increased liver function. Bile pH and HCO3– levels fell within range for viability. Although bile glucose exceeded proposed criteria, the investigators pointed out that levels still fell within parameters for research-quality livers. Lactate levels also rose within the first hour of reperfusion, but the investigators suggested that this finding should be interpreted with appropriate context.
“It should be considered that the livers in this study were initially rejected for transplantation,” they wrote, “and the confidence intervals of the lactate concentration at the end of reperfusion largely overlap with time-matched values reported by others during [normothermic machine perfusion] of rejected human livers.”
Hepatocellular injury and histology also were evaluated during and after simulated transplantation, respectively, with favorable results. Although sites of preexisting hepatic injury were aggravated by the process, and rates of apoptosis increased, the investigators considered these changes were clinically insignificant.
Looking to the future, the investigators suggested that further refinement of the process could facilitate even-lower storage temperatures while better preserving liver viability.
“The use of human livers makes this study clinically relevant and promotes the translation of subzero organ preservation to the clinic,” the investigators concluded. “However, long-term survival experiments of transplanted supercooled livers in swine or an alternative large animal model will be needed before clinical translation.”
The study was funded by the National Institutes of Health and the Department of Defense. Dr. de Vries and four other coauthors have provisional patent applications related to the study, and one coauthor disclosed a financial relationship with Organ Solutions.
SOURCE: de Vries RJ et al. Nature Biotechnol. 2019 Sep 9. doi: 10.1038/s41587-019-0223-y.
FROM NATURE BIOTECHNOLOGY