User login
Immediate skin-to-skin contact after cesarean section improves outcomes for parent, newborn
Birth parents are typically separated from their newborns following a cesarean section. However, a recent study published in the journal Nursing Open suggests immediate skin-to-skin contact may accelerate uterine contractions, reduce maternal blood loss, reduce newborn crying, improve patient satisfaction and comfort, and increase the rate of breastfeeding.
“[O]ur study contributes to scientific knowledge with key information to reduce maternal morbidity and mortality rates in mothers who have undergone scheduled cesarean sections,” José Miguel Pérez-Jiménez, MD, of the faculty of nursing, physiotherapy, and podiatry at Hospital Universitario Virgen Macarena, University of Sevilla, Spain, and colleagues wrote in their study. It promotes greater stability in the mothers by reducing the risk of postpartum hemorrhage, making it better to not separate mother and child in the first hours after this surgery, he said.
Dr. Pérez-Jiménez and colleagues evaluated 83 women who underwent a scheduled cesarean section in an unblinded, randomized controlled trial. The women were randomized to receive skin-to-skin contact in the operating room that continued in the postpartum unit, or the normal protocol after cesarean section that consisted of having the mother transferred to the postanesthesia recovery room while the newborn was sent to a maternity room with a parent or companion. Researchers assessed variables such as plasma hemoglobin, uterine contractions, breastfeeding, and postoperative pain, as well as subjective measures such as maternal satisfaction, comfort, previous cesarean section experience, and newborn crying.
Women who received usual care following cesarean section were more likely to have uterine contractions at the umbilical level compared with the skin-to-skin contact group (70% vs. 3%; P ≤ .0001), while the skin-to-skin group was more likely to have uterine contractions at the infraumbilical level (92.5% vs. 22.5%; P ≤ .0001). There was a statistically significant decrease in predischarge hemoglobin in the control group compared with the skin-to-skin group (10.522 vs. 11.075 g/dL; P ≤ .017); the level of hemoglobin reduction favored the skin-to-skin group (1.01 vs. 2.265 g/dL; P ≤ .0001). Women in the skin-to-skin group were more likely to report mild pain on a 10-point visual analog scale (VAS) after being transferred to the recovery room (1.48 vs. 6.23 points; P ≤ .0001) and being transferred to a maternity room or room in the postpartum unit (0.60 vs. 5.23 points; P ≤ .0001). Breastfeeding at birth was significantly higher among patients with immediate skin-to-skin contact compared with the control group (92.5% vs. 32.5%; P ≤ .0001), and continued at 1 month after birth (92.5% vs. 12.5%; P ≤ .0001). Newborns of mothers in the skin-to-skin group were significantly less likely to cry compared with newborns in the control group (90% vs. 55%; P ≤ .001).
When asked to rate their satisfaction on a 10-point Likert scale, women in the skin-to-skin contact group rated their experience significantly higher than did the control group (9.98 vs. 6.5; P ≤ .0001), and all women who had previously had a cesarean section in the skin-to-skin group (30%) rated their experience at 10 points compared with their previous cesarean section without skin-to-skin contact.
Implementing skin-to-skin contact after cesarean section
Betsy M. Collins, MD, MPH, assistant professor of obstetrics and gynecology at Emory University, Atlanta, said in an interview that while some of the findings were largely unsurprising and “confirmed a lot of the things that we already know about skin-to-skin [contact],” one major finding was the “stark difference” in the percentage of new birth parents who started breastfeeding after skin-to-skin contact and were still breastfeeding at 1 month postpartum compared with birth parents in the control group. She was not involved with the study and noted that the results complement recommendations from the World Health Organization on starting breastfeeding within the first hour after birth and continuing breastfeeding through the first 6 months of life.
“That was likely one of the greatest take-home points from the study ... that early skin-to-skin really promoted initiation of breastfeeding,” Dr. Collins said.
Two reasons why skin-to-skin contact after cesarean section isn’t regularly provided is that it can be difficult for personnel and safety reasons to have an extra nurse to continue monitoring the health of the newborn in the operating room, and there is a lack of culture supporting of skin-to-skin contact in the OR, Dr. Collins explained.
“Just like anything else, if it’s built into your standard operating procedure, then you have everything set up in place to do that initial assessment of the infant and then get the baby skin-to-skin as quickly as possible,” she said. If it’s your standard operating procedure to not provide skin-to-skin contact, she said, then there is a little bit more inertia to overcome to start providing it as a standard procedure.
At her center, Dr. Collins said skin-to-skin contact is initiated as soon as possible after birth, even in the operating room. The steps to implementing that policy involved getting the anesthesiology department on board with supporting the policy in the OR and training the circulating nursing staff to ensure a that nurse is available to monitor the newborn.
“I think the most important thing to know is that it’s absolutely doable and that you just have to have a champion just like any other quality initiative,” she said. One of the best ways to do that is to have the patients themselves request it, she noted, compared with its being requested by a physician or nurse.
“I think some patients are disappointed when they have to undergo cesarean delivery or feel like they’re missing out if they can’t have a vaginal delivery,” Dr. Collins said. Immediate skin-to-skin contact is “very good for not only physiology, as we read about in this paper – all the things they said about the benefits of skin-to-skin [contact] are true – but it’s really good for mental health. That bonding begins right away.”
As a birth parent, being separated from your newborn for several hours after a cesarean section, on the other hand, can be “pretty devastating,” Dr. Collins said.
“I think this is something that, once it becomes a standard of care, it will be expected that most hospitals should be doing this,” she said.
The authors and Dr. Collins report no relevant conflicts of interest.
Birth parents are typically separated from their newborns following a cesarean section. However, a recent study published in the journal Nursing Open suggests immediate skin-to-skin contact may accelerate uterine contractions, reduce maternal blood loss, reduce newborn crying, improve patient satisfaction and comfort, and increase the rate of breastfeeding.
“[O]ur study contributes to scientific knowledge with key information to reduce maternal morbidity and mortality rates in mothers who have undergone scheduled cesarean sections,” José Miguel Pérez-Jiménez, MD, of the faculty of nursing, physiotherapy, and podiatry at Hospital Universitario Virgen Macarena, University of Sevilla, Spain, and colleagues wrote in their study. It promotes greater stability in the mothers by reducing the risk of postpartum hemorrhage, making it better to not separate mother and child in the first hours after this surgery, he said.
Dr. Pérez-Jiménez and colleagues evaluated 83 women who underwent a scheduled cesarean section in an unblinded, randomized controlled trial. The women were randomized to receive skin-to-skin contact in the operating room that continued in the postpartum unit, or the normal protocol after cesarean section that consisted of having the mother transferred to the postanesthesia recovery room while the newborn was sent to a maternity room with a parent or companion. Researchers assessed variables such as plasma hemoglobin, uterine contractions, breastfeeding, and postoperative pain, as well as subjective measures such as maternal satisfaction, comfort, previous cesarean section experience, and newborn crying.
Women who received usual care following cesarean section were more likely to have uterine contractions at the umbilical level compared with the skin-to-skin contact group (70% vs. 3%; P ≤ .0001), while the skin-to-skin group was more likely to have uterine contractions at the infraumbilical level (92.5% vs. 22.5%; P ≤ .0001). There was a statistically significant decrease in predischarge hemoglobin in the control group compared with the skin-to-skin group (10.522 vs. 11.075 g/dL; P ≤ .017); the level of hemoglobin reduction favored the skin-to-skin group (1.01 vs. 2.265 g/dL; P ≤ .0001). Women in the skin-to-skin group were more likely to report mild pain on a 10-point visual analog scale (VAS) after being transferred to the recovery room (1.48 vs. 6.23 points; P ≤ .0001) and being transferred to a maternity room or room in the postpartum unit (0.60 vs. 5.23 points; P ≤ .0001). Breastfeeding at birth was significantly higher among patients with immediate skin-to-skin contact compared with the control group (92.5% vs. 32.5%; P ≤ .0001), and continued at 1 month after birth (92.5% vs. 12.5%; P ≤ .0001). Newborns of mothers in the skin-to-skin group were significantly less likely to cry compared with newborns in the control group (90% vs. 55%; P ≤ .001).
When asked to rate their satisfaction on a 10-point Likert scale, women in the skin-to-skin contact group rated their experience significantly higher than did the control group (9.98 vs. 6.5; P ≤ .0001), and all women who had previously had a cesarean section in the skin-to-skin group (30%) rated their experience at 10 points compared with their previous cesarean section without skin-to-skin contact.
Implementing skin-to-skin contact after cesarean section
Betsy M. Collins, MD, MPH, assistant professor of obstetrics and gynecology at Emory University, Atlanta, said in an interview that while some of the findings were largely unsurprising and “confirmed a lot of the things that we already know about skin-to-skin [contact],” one major finding was the “stark difference” in the percentage of new birth parents who started breastfeeding after skin-to-skin contact and were still breastfeeding at 1 month postpartum compared with birth parents in the control group. She was not involved with the study and noted that the results complement recommendations from the World Health Organization on starting breastfeeding within the first hour after birth and continuing breastfeeding through the first 6 months of life.
“That was likely one of the greatest take-home points from the study ... that early skin-to-skin really promoted initiation of breastfeeding,” Dr. Collins said.
Two reasons why skin-to-skin contact after cesarean section isn’t regularly provided is that it can be difficult for personnel and safety reasons to have an extra nurse to continue monitoring the health of the newborn in the operating room, and there is a lack of culture supporting of skin-to-skin contact in the OR, Dr. Collins explained.
“Just like anything else, if it’s built into your standard operating procedure, then you have everything set up in place to do that initial assessment of the infant and then get the baby skin-to-skin as quickly as possible,” she said. If it’s your standard operating procedure to not provide skin-to-skin contact, she said, then there is a little bit more inertia to overcome to start providing it as a standard procedure.
At her center, Dr. Collins said skin-to-skin contact is initiated as soon as possible after birth, even in the operating room. The steps to implementing that policy involved getting the anesthesiology department on board with supporting the policy in the OR and training the circulating nursing staff to ensure a that nurse is available to monitor the newborn.
“I think the most important thing to know is that it’s absolutely doable and that you just have to have a champion just like any other quality initiative,” she said. One of the best ways to do that is to have the patients themselves request it, she noted, compared with its being requested by a physician or nurse.
“I think some patients are disappointed when they have to undergo cesarean delivery or feel like they’re missing out if they can’t have a vaginal delivery,” Dr. Collins said. Immediate skin-to-skin contact is “very good for not only physiology, as we read about in this paper – all the things they said about the benefits of skin-to-skin [contact] are true – but it’s really good for mental health. That bonding begins right away.”
As a birth parent, being separated from your newborn for several hours after a cesarean section, on the other hand, can be “pretty devastating,” Dr. Collins said.
“I think this is something that, once it becomes a standard of care, it will be expected that most hospitals should be doing this,” she said.
The authors and Dr. Collins report no relevant conflicts of interest.
Birth parents are typically separated from their newborns following a cesarean section. However, a recent study published in the journal Nursing Open suggests immediate skin-to-skin contact may accelerate uterine contractions, reduce maternal blood loss, reduce newborn crying, improve patient satisfaction and comfort, and increase the rate of breastfeeding.
“[O]ur study contributes to scientific knowledge with key information to reduce maternal morbidity and mortality rates in mothers who have undergone scheduled cesarean sections,” José Miguel Pérez-Jiménez, MD, of the faculty of nursing, physiotherapy, and podiatry at Hospital Universitario Virgen Macarena, University of Sevilla, Spain, and colleagues wrote in their study. It promotes greater stability in the mothers by reducing the risk of postpartum hemorrhage, making it better to not separate mother and child in the first hours after this surgery, he said.
Dr. Pérez-Jiménez and colleagues evaluated 83 women who underwent a scheduled cesarean section in an unblinded, randomized controlled trial. The women were randomized to receive skin-to-skin contact in the operating room that continued in the postpartum unit, or the normal protocol after cesarean section that consisted of having the mother transferred to the postanesthesia recovery room while the newborn was sent to a maternity room with a parent or companion. Researchers assessed variables such as plasma hemoglobin, uterine contractions, breastfeeding, and postoperative pain, as well as subjective measures such as maternal satisfaction, comfort, previous cesarean section experience, and newborn crying.
Women who received usual care following cesarean section were more likely to have uterine contractions at the umbilical level compared with the skin-to-skin contact group (70% vs. 3%; P ≤ .0001), while the skin-to-skin group was more likely to have uterine contractions at the infraumbilical level (92.5% vs. 22.5%; P ≤ .0001). There was a statistically significant decrease in predischarge hemoglobin in the control group compared with the skin-to-skin group (10.522 vs. 11.075 g/dL; P ≤ .017); the level of hemoglobin reduction favored the skin-to-skin group (1.01 vs. 2.265 g/dL; P ≤ .0001). Women in the skin-to-skin group were more likely to report mild pain on a 10-point visual analog scale (VAS) after being transferred to the recovery room (1.48 vs. 6.23 points; P ≤ .0001) and being transferred to a maternity room or room in the postpartum unit (0.60 vs. 5.23 points; P ≤ .0001). Breastfeeding at birth was significantly higher among patients with immediate skin-to-skin contact compared with the control group (92.5% vs. 32.5%; P ≤ .0001), and continued at 1 month after birth (92.5% vs. 12.5%; P ≤ .0001). Newborns of mothers in the skin-to-skin group were significantly less likely to cry compared with newborns in the control group (90% vs. 55%; P ≤ .001).
When asked to rate their satisfaction on a 10-point Likert scale, women in the skin-to-skin contact group rated their experience significantly higher than did the control group (9.98 vs. 6.5; P ≤ .0001), and all women who had previously had a cesarean section in the skin-to-skin group (30%) rated their experience at 10 points compared with their previous cesarean section without skin-to-skin contact.
Implementing skin-to-skin contact after cesarean section
Betsy M. Collins, MD, MPH, assistant professor of obstetrics and gynecology at Emory University, Atlanta, said in an interview that while some of the findings were largely unsurprising and “confirmed a lot of the things that we already know about skin-to-skin [contact],” one major finding was the “stark difference” in the percentage of new birth parents who started breastfeeding after skin-to-skin contact and were still breastfeeding at 1 month postpartum compared with birth parents in the control group. She was not involved with the study and noted that the results complement recommendations from the World Health Organization on starting breastfeeding within the first hour after birth and continuing breastfeeding through the first 6 months of life.
“That was likely one of the greatest take-home points from the study ... that early skin-to-skin really promoted initiation of breastfeeding,” Dr. Collins said.
Two reasons why skin-to-skin contact after cesarean section isn’t regularly provided is that it can be difficult for personnel and safety reasons to have an extra nurse to continue monitoring the health of the newborn in the operating room, and there is a lack of culture supporting of skin-to-skin contact in the OR, Dr. Collins explained.
“Just like anything else, if it’s built into your standard operating procedure, then you have everything set up in place to do that initial assessment of the infant and then get the baby skin-to-skin as quickly as possible,” she said. If it’s your standard operating procedure to not provide skin-to-skin contact, she said, then there is a little bit more inertia to overcome to start providing it as a standard procedure.
At her center, Dr. Collins said skin-to-skin contact is initiated as soon as possible after birth, even in the operating room. The steps to implementing that policy involved getting the anesthesiology department on board with supporting the policy in the OR and training the circulating nursing staff to ensure a that nurse is available to monitor the newborn.
“I think the most important thing to know is that it’s absolutely doable and that you just have to have a champion just like any other quality initiative,” she said. One of the best ways to do that is to have the patients themselves request it, she noted, compared with its being requested by a physician or nurse.
“I think some patients are disappointed when they have to undergo cesarean delivery or feel like they’re missing out if they can’t have a vaginal delivery,” Dr. Collins said. Immediate skin-to-skin contact is “very good for not only physiology, as we read about in this paper – all the things they said about the benefits of skin-to-skin [contact] are true – but it’s really good for mental health. That bonding begins right away.”
As a birth parent, being separated from your newborn for several hours after a cesarean section, on the other hand, can be “pretty devastating,” Dr. Collins said.
“I think this is something that, once it becomes a standard of care, it will be expected that most hospitals should be doing this,” she said.
The authors and Dr. Collins report no relevant conflicts of interest.
FROM NURSING OPEN
Docs used permanent, not temporary stitches; lawsuits result
The first in what have come to be known as the “wrong stitches” cases has been settled, a story in The Ledger reports.
The former plaintiff in the now-settled suit is Carrie Monk, a Lakeland, Fla., resident who underwent total laparoscopic hysterectomy at Lakeland Regional Health Medical Center several years ago. (The medical center is managed by Lakeland Regional Health Systems.) D As a result, over the next 19 months, she experienced abdominal pain and constant bleeding, which in turn affected her personal life as well as her work as a nurse in the intensive care unit. She underwent follow-up surgery to have the permanent sutures removed, but two could not be identified and excised.
In July 2020, Ms. Monk filed a medical malpractice claim against Lakeland Regional Health, its medical center, and the ob-gyns who had performed her surgery. She was among the first of the women who had received the permanent sutures to do so.
On February 28, 2021, The Ledger ran a story on Ms. Monk’s suit. Less than 2 weeks later, Lakeland Regional Health sent letters to patients who had undergone “wrong stitch” surgeries, cautioning of possible postsurgical complications. The company reportedly kept secret how many letters it had sent out.
Since then, at least nine similar suits have been filed against Lakeland Regional Health, bringing the total number of such suits to 12. Four of these suits have been settled, including Ms. Monk’s. Of the remaining eight cases, several are in various pretrial stages.
Under the terms of her settlement, neither Ms. Monk nor her attorney may disclose what financial compensation or other awards she’s received. The attorney, however, referred to the settlement as “amicable.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
The first in what have come to be known as the “wrong stitches” cases has been settled, a story in The Ledger reports.
The former plaintiff in the now-settled suit is Carrie Monk, a Lakeland, Fla., resident who underwent total laparoscopic hysterectomy at Lakeland Regional Health Medical Center several years ago. (The medical center is managed by Lakeland Regional Health Systems.) D As a result, over the next 19 months, she experienced abdominal pain and constant bleeding, which in turn affected her personal life as well as her work as a nurse in the intensive care unit. She underwent follow-up surgery to have the permanent sutures removed, but two could not be identified and excised.
In July 2020, Ms. Monk filed a medical malpractice claim against Lakeland Regional Health, its medical center, and the ob-gyns who had performed her surgery. She was among the first of the women who had received the permanent sutures to do so.
On February 28, 2021, The Ledger ran a story on Ms. Monk’s suit. Less than 2 weeks later, Lakeland Regional Health sent letters to patients who had undergone “wrong stitch” surgeries, cautioning of possible postsurgical complications. The company reportedly kept secret how many letters it had sent out.
Since then, at least nine similar suits have been filed against Lakeland Regional Health, bringing the total number of such suits to 12. Four of these suits have been settled, including Ms. Monk’s. Of the remaining eight cases, several are in various pretrial stages.
Under the terms of her settlement, neither Ms. Monk nor her attorney may disclose what financial compensation or other awards she’s received. The attorney, however, referred to the settlement as “amicable.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
The first in what have come to be known as the “wrong stitches” cases has been settled, a story in The Ledger reports.
The former plaintiff in the now-settled suit is Carrie Monk, a Lakeland, Fla., resident who underwent total laparoscopic hysterectomy at Lakeland Regional Health Medical Center several years ago. (The medical center is managed by Lakeland Regional Health Systems.) D As a result, over the next 19 months, she experienced abdominal pain and constant bleeding, which in turn affected her personal life as well as her work as a nurse in the intensive care unit. She underwent follow-up surgery to have the permanent sutures removed, but two could not be identified and excised.
In July 2020, Ms. Monk filed a medical malpractice claim against Lakeland Regional Health, its medical center, and the ob-gyns who had performed her surgery. She was among the first of the women who had received the permanent sutures to do so.
On February 28, 2021, The Ledger ran a story on Ms. Monk’s suit. Less than 2 weeks later, Lakeland Regional Health sent letters to patients who had undergone “wrong stitch” surgeries, cautioning of possible postsurgical complications. The company reportedly kept secret how many letters it had sent out.
Since then, at least nine similar suits have been filed against Lakeland Regional Health, bringing the total number of such suits to 12. Four of these suits have been settled, including Ms. Monk’s. Of the remaining eight cases, several are in various pretrial stages.
Under the terms of her settlement, neither Ms. Monk nor her attorney may disclose what financial compensation or other awards she’s received. The attorney, however, referred to the settlement as “amicable.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
Chest reconstruction surgeries up nearly fourfold among adolescents
The number of chest reconstruction surgeries performed for adolescents rose nearly fourfold between 2016 and 2019, researchers report in a study published in JAMA Pediatrics.
“To our knowledge, this study is the largest investigation to date of gender-affirming chest reconstruction in a pediatric population. The results demonstrate substantial increases in gender-affirming chest reconstruction for adolescents,” the authors report.
The researchers, from Vanderbilt University School of Medicine, Nashville, Tenn., used the Nationwide Ambulatory Surgery Sample to identify youth with gender dysphoria who underwent top surgery to remove, or, in rare cases, to add breasts.
The authors identified 829 chest surgeries. They adjusted the number to a weighted figure of 1,130 patients who underwent chest reconstruction during the study period. Of those, 98.6% underwent masculinizing surgery to remove breasts, and 1.4% underwent feminizing surgery. Roughly 100 individuals received gender-affirming chest surgeries in 2016. In 2019, the number had risen to 489 – a 389% increase, the authors reported.
Approximately 44% of the patients in the study were aged 17 years at the time of surgery, while 5.5% were younger than 14.
Around 78% of the individuals who underwent chest surgeries in 2019 were White, 2.7% were Black, 12.2% were Hispanic, and 2.5% were Asian or Pacific Islander. Half of the patients who underwent surgery had a household income of $82,000 or more, according to the researchers.
“Most transgender adolescents had either public or private health insurance coverage for these procedures, contrasting with the predominance of self-payers reported in earlier studies on transgender adults,” write the researchers, citing a 2018 study of trends in transgender surgery.
Masculinizing chest reconstruction, such as mastectomy, and feminizing chest reconstruction, such as augmentation mammaplasty, can be performed as outpatient procedures or as ambulatory surgeries, according to another study .
The study was supported by a grant from the National Center for Advancing Translational Sciences Clinical and Translational Science Awards Program. One author has reported receiving grant funding from Merck.
A version of this article first appeared on Medscape.com.
The number of chest reconstruction surgeries performed for adolescents rose nearly fourfold between 2016 and 2019, researchers report in a study published in JAMA Pediatrics.
“To our knowledge, this study is the largest investigation to date of gender-affirming chest reconstruction in a pediatric population. The results demonstrate substantial increases in gender-affirming chest reconstruction for adolescents,” the authors report.
The researchers, from Vanderbilt University School of Medicine, Nashville, Tenn., used the Nationwide Ambulatory Surgery Sample to identify youth with gender dysphoria who underwent top surgery to remove, or, in rare cases, to add breasts.
The authors identified 829 chest surgeries. They adjusted the number to a weighted figure of 1,130 patients who underwent chest reconstruction during the study period. Of those, 98.6% underwent masculinizing surgery to remove breasts, and 1.4% underwent feminizing surgery. Roughly 100 individuals received gender-affirming chest surgeries in 2016. In 2019, the number had risen to 489 – a 389% increase, the authors reported.
Approximately 44% of the patients in the study were aged 17 years at the time of surgery, while 5.5% were younger than 14.
Around 78% of the individuals who underwent chest surgeries in 2019 were White, 2.7% were Black, 12.2% were Hispanic, and 2.5% were Asian or Pacific Islander. Half of the patients who underwent surgery had a household income of $82,000 or more, according to the researchers.
“Most transgender adolescents had either public or private health insurance coverage for these procedures, contrasting with the predominance of self-payers reported in earlier studies on transgender adults,” write the researchers, citing a 2018 study of trends in transgender surgery.
Masculinizing chest reconstruction, such as mastectomy, and feminizing chest reconstruction, such as augmentation mammaplasty, can be performed as outpatient procedures or as ambulatory surgeries, according to another study .
The study was supported by a grant from the National Center for Advancing Translational Sciences Clinical and Translational Science Awards Program. One author has reported receiving grant funding from Merck.
A version of this article first appeared on Medscape.com.
The number of chest reconstruction surgeries performed for adolescents rose nearly fourfold between 2016 and 2019, researchers report in a study published in JAMA Pediatrics.
“To our knowledge, this study is the largest investigation to date of gender-affirming chest reconstruction in a pediatric population. The results demonstrate substantial increases in gender-affirming chest reconstruction for adolescents,” the authors report.
The researchers, from Vanderbilt University School of Medicine, Nashville, Tenn., used the Nationwide Ambulatory Surgery Sample to identify youth with gender dysphoria who underwent top surgery to remove, or, in rare cases, to add breasts.
The authors identified 829 chest surgeries. They adjusted the number to a weighted figure of 1,130 patients who underwent chest reconstruction during the study period. Of those, 98.6% underwent masculinizing surgery to remove breasts, and 1.4% underwent feminizing surgery. Roughly 100 individuals received gender-affirming chest surgeries in 2016. In 2019, the number had risen to 489 – a 389% increase, the authors reported.
Approximately 44% of the patients in the study were aged 17 years at the time of surgery, while 5.5% were younger than 14.
Around 78% of the individuals who underwent chest surgeries in 2019 were White, 2.7% were Black, 12.2% were Hispanic, and 2.5% were Asian or Pacific Islander. Half of the patients who underwent surgery had a household income of $82,000 or more, according to the researchers.
“Most transgender adolescents had either public or private health insurance coverage for these procedures, contrasting with the predominance of self-payers reported in earlier studies on transgender adults,” write the researchers, citing a 2018 study of trends in transgender surgery.
Masculinizing chest reconstruction, such as mastectomy, and feminizing chest reconstruction, such as augmentation mammaplasty, can be performed as outpatient procedures or as ambulatory surgeries, according to another study .
The study was supported by a grant from the National Center for Advancing Translational Sciences Clinical and Translational Science Awards Program. One author has reported receiving grant funding from Merck.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
Options and outcomes for uterine preservation at the time of prolapse surgery
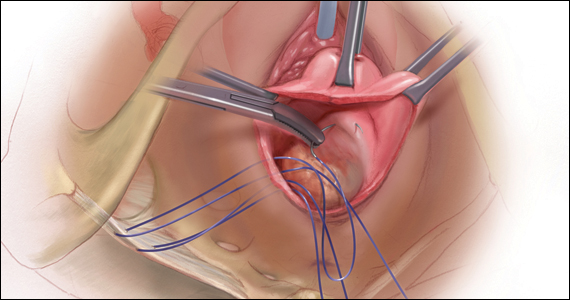
CASE Patient desires prolapse repair
A 65-year-old postmenopausal patient (G3P3) presents to your office with symptoms of a vaginal bulge for more than 1 year. She has no urinary incontinence symptoms and no bowel dysfunction symptoms. On examination, you diagnose stage 2 uterovaginal prolapse with both anterior and apical defects. The patient declines expectant and pessary management and desires surgery, but she states that she feels her uterus “is important for me to keep, as my babies grew in there and it is part of me.” She denies any family or personal history of breast, endometrial, or ovarian cancer and has no history of abnormal cervical cancer screening or postmenopausal bleeding. What are the options for this patient?
Who is the appropriate hysteropexy patient, and how do we counsel her?
Uterine prolapse is the third leading cause of benign hysterectomy, with approximately 70,000 procedures performed each year in the United States. It has long been acknowledged that the uterus is a passive bystander to the prolapse process,1 but modern practice often involves a hysterectomy as part of addressing apical prolapse. However, more and more uterine-preserving surgeries are being performed, with one study showing an increase from 1.8% to 5% from 2002 and 2012.2
When presented with the option to keep or remove their uterus during the time of prolapse surgery, 36% of patients indicated that they would prefer to keep their uterus with similar outcomes while 21% would still prefer uterine preservation even if outcomes were inferior compared with hysterectomy.3 Another study showed that 60% of patients would decline concurrent hysterectomy if there were equal surgical outcomes,4 and popular platforms, such as Health magazine (www.health.com) and AARP magazine (www.aarp.org), have listed benign hysterectomy as a “top surgery to avoid.”
Patients desire uterine preservation for many reasons, including concerns about sexual function and pleasure, the uterus being important to their sense of identity or womanhood, and concerns around menopausal symptoms. Early patient counseling and discussion of surgical goals can help clinicians fully understand a patient’s thoughts toward uterine preservation. Women who identified their uterus as important to their sense of self had a 28.2-times chance of preferring uterine preservation.3 Frequently, concerns about menopausal symptoms are more directly related to hormones and ovary removal, not uterus removal, but clinicians should be careful to also counsel patients on the increased risk of menopause in the 5 years after hysterectomy, even with ovarian preservation.5
There are some patients for whom experts do not recommend uterine preservation.6 Patients with an increased risk of cervical or endometrial pathology should be counseled on the benefits of hysterectomy. Additionally, patients who have abnormal uterine bleeding from benign pathology should consider hysterectomy to treat these issues and avoid future workups (TABLE). For postmenopausal patients with recent postmenopausal bleeding, we encourage hysterectomy. A study of patients undergoing hysterectomy at the time of prolapse repair found a rate of 13% unanticipated endometrial pathology with postmenopausal bleeding and negative preoperative workup.7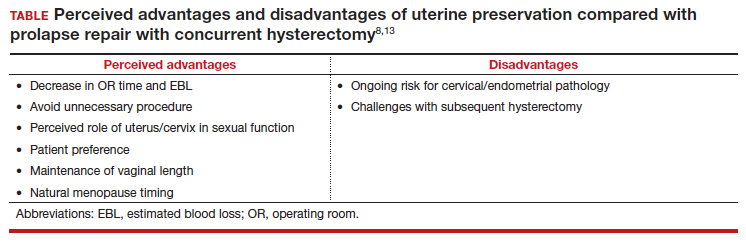
At this time, a majority of clinicians consider the desire for future fertility to be a relative contraindication to surgical prolapse repair and advise conservative management with pessary until childbearing is complete. This is reasonable, given the paucity of safety data in subsequent pregnancies as well as the lack of prolapse outcomes after those pregnancies.8,9 Lastly, cervical elongation is considered a relative contraindication, as it represents a risk for surgical failure.10,11 This may be counteracted with trachelectomy at the time of hysteropexy or surgeries such as the Manchester repair, which involve a trachelectomy routinely,12 but currently there is no strong evidence for this as routine practice.
Continue to: Uterine preservation surgical techniques and outcomes...
Uterine preservation surgical techniques and outcomes
Le Fort colpocleisis
First described in 1840 by Neugebauer of Poland and later by Le Fort in Paris in 1877, the Le Fort colpocleisis repair technique remains the most reliable prolapse surgery to date.14 The uterus is left in place while the vagina is narrowed and shortened. It typically also is performed with a levator plication to reduce the genital hiatus.
This procedure is quick and effective, with a 90% to 95% success rate. If necessary, it can be performed under local or regional anesthesia, making it a good option for medically frail patients. It is not an option for everyone, however, as penetrative intercourse is no longer an option after surgery. Studies suggest an approximately 13% dissatisfaction rate after the procedure, with most of that coming from postoperative urinary symptoms, such as urgency or stress incontinence,15 and some studies show a dissatisfaction rate as low as 0% in a well-counseled patient population.16,17
Vaginal native tissue hysteropexy
Many patients who elect for uterine preservation at the time of prolapse surgery are “minimalists,” meaning that a vaginal native tissue procedure appeals to them due to the lack of abdominal incisions, decreased operating room time, and lack of permanent graft materials.
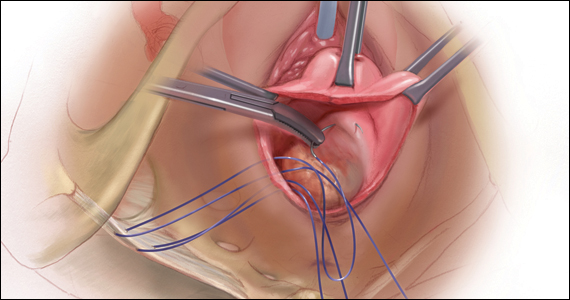
Of all the hysteropexy procedures, sacrospinous hysteropexy (SSHP) has the most robust data available. The approach to SSHP can be tailored to the patient’s anatomy and it is performed in a manner similar to posthysterectomy sacrospinous ligament fixation. The traditional posterior approach can be used with predominantly posterior prolapse, while an apical approach through a semilunar paracervical incision can be used for predominantly apical prolapse. Expert surgeons agree that one key to success is anchoring the suspension sutures through the cervical stroma, not just the vaginal epithelium.
Researchers in the Netherlands published the 5-year outcomes of a randomized trial that compared SSHP with vaginal hysterectomy with uterosacral ligament suspension.18 Their data showed no difference between groups in composite failure, reoperation rates, quality of life measures, and postoperative sexual function. Adverse events were very similar to those reported for posthysterectomy sacrospinous ligament fixation, including 15% transient buttock pain. Of note, the same authors explored risk factors for recurrence after SSHP and found that higher body mass index, smoking, and a large point Ba measurement were risk factors for prolapse recurrence.19
A randomized, controlled trial in the United Kingdom (the VUE trial) compared vaginal hysterectomy with apical suspension to uterine preservation with a variety of apical suspension techniques, mostly SSHP, and demonstrated no significant differences in outcomes.20 Overall, SSHP is an excellent option for many patients interested in uterine preservation.
Uterosacral ligament hysteropexy (USHP), when performed vaginally, is very similar to uterosacral ligament suspension at the time of vaginal hysterectomy, with entry into the peritoneal cavity through a posterior colpotomy. The uterosacral ligaments are grasped and delayed absorbable suture placed through the ligaments and anchored into the posterior cervical stroma. Given the maintenance of the normal axis of the vagina, USHP is a good technique for patients with isolated apical defects. Unfortunately, the least amount of quality data is available for USHP at this time. Currently, evidence suggests that complications are rare and that the procedure may offer acceptable anatomic and symptomatic outcomes.21 Some surgeons approach the uterosacral suspension laparoscopically, which also has mixed results in the literature, with failure rates between 8% and 27% and few robust studies.22–24
The Manchester-Fothergill operation, currently not common in the United States but popular in Europe, primarily is considered a treatment for cervical elongation when the uterosacral ligaments are intact. In this procedure, trachelectomy is performed and the uterosacral ligaments are plicated to the uterine body. Sturmdorf sutures are frequently placed to close off the endometrial canal, which can lead to hematometra and other complications of cervical stenosis. Previous unmatched studies have shown similar outcomes with the Manchester procedure compared with vaginal hysterectomy.25,26
The largest study currently available is a registry study from Denmark, with matched cohort populations, that compared the Manchester procedure, SSHP, and total vaginal hysterectomy with uterosacral ligament suspension.27 This study indicated less morbidity related to the Manchester procedure, decreased anterior recurrence compared with SSHP, and a 7% reoperation rate.27 The same authors also established better cost-effectiveness with the Manchester procedure as opposed to vaginal hysterectomy with uterosacral ligament suspension.28
Continue to: Vaginal mesh hysteropexy...
Vaginal mesh hysteropexy
Hysteropexy using vaginal mesh is limited in the United States given the removal of vaginal mesh kits from the market by the US Food and Drug Administration in 2019. However, a Pelvic Floor Disorders Network randomized trial compared vaginal mesh hysteropexy using the Uphold LITE transvaginal mesh support system (Boston Scientific) and vaginal hysterectomy with uterosacral ligament suspension.29 At 5 years, mesh hysteropexy had fewer failures than hysterectomy (37% vs 54%) and there was no difference in retreatment (9% vs 13%). The authors noted an 8% mesh exposure rate in the mesh hysteropexy group but 12% granulation tissue and 21% suture exposure rate in the hysterectomy group.29
While vaginal mesh hysteropexy was effective in the treatment of apical prolapse, the elevated mesh exposure rate and postoperative complications ultimately led to its removal from the market.
Sacrohysteropexy
Lastly, prolapse surgery with uterine preservation may be accomplished abdominally, most commonly laparoscopically with or without robotic assistance.
Sacrohysteropexy (SHP) involves the attachment of permanent synthetic mesh posteriorly to the posterior vagina and cervix with or without the additional placement of mesh to the anterior vagina and cervix. When the anterior mesh is placed, the arms are typically routed through the broad ligament bilaterally and joined with the posterior mesh for attachment to the anterior longitudinal ligament, overlying the sacrum.
Proponents of this technique endorse the use of mesh to augment already failing native tissues and propose similarities to the durability of sacrocolpopexy. While no randomized controlled trials have compared hysterectomy with sacrocolpopexy or supracervical hysterectomy with sacrocolpopexy to sacrohysteropexy, a meta-analysis suggests that sacrohysteropexy may have a decreased risk of mesh exposure but a higher reoperation rate with lower anatomic success.9 Randomized trials that compared abdominal sacrohysteropexy with vaginal hysterectomy and suspension indicate that apical support may be improved with sacrohysteropexy,30 but reoperations, postoperative pain and disability, and urinary dysfunction was higher with SHP.31,32
What further research is needed?
With the increasing patient and clinician interest in uterine preservation, more research is needed to improve patient counseling and surgical planning. Much of the current research compares hysteropexy outcomes with those of traditional prolapse repairs with hysterectomy, with only a few randomized trials. We are lacking robust, prospective comparison studies between hysteropexy methods, especially vaginal native tissue techniques, long-term follow-up on the prevalence of uterine or cervical pathology after hysteropexy, and pregnancy or postpartum outcomes following uterine preservation surgery.
Currently, work is underway to validate and test the effectiveness of a questionnaire to evaluate the uterus’s importance to the patient seeking prolapse surgery in order to optimize counseling. The VUE trial, which randomizes women to vaginal hysterectomy with suspension versus various prolapse surgeries with uterine preservation, is continuing its 6-year follow-up.20 In the Netherlands, an ongoing randomized, controlled trial (the SAM trial) is comparing the Manchester procedure with sacrospinous hysteropexy and will follow patients up to 24 months.33 Fortunately, both of these trials are rigorously assessing both objective and patient-centered outcomes.
CASE Counseling helps the patient weigh surgical options
After thorough review of her surgical options, the patient elects for a uterine-preserving prolapse repair. She would like to have the most minimally invasive procedure and does not want any permanent mesh used. You suggest, and she agrees to, a sacrospinous ligament hysteropexy, as it is the current technique with the most robust data. ●
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 pt 1):1717-1724; discussion 1724-1728. doi:10.1016/0002-9378(92)91562-o.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23:365-371. doi:10.1097/SPV.0000000000000426.
- Korbly NB, Kassis NC, Good MM, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol. 2013;209:470.e16. doi:10.1016/j.ajog.2013.08.003.
- Frick AC, Barber MD, Paraiso MF, et al. Attitudes toward hysterectomy in women undergoing evaluation for uterovaginal prolapse. Female Pelvic Med Reconstr Surg. 2013;19:103-109. doi:10.1097/SPV.0b013e31827d8667.
- Farquhar CM, Sadler L, Harvey SA, et al. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962. doi:10.1111/j.1471-0528.2005.00696.x
- Gutman R, Maher C. Uterine-preserving POP surgery. Int Urogynecol J. 2013;24:1803-1813. doi:10.1007/s00192-0132171-2.
- Frick AC, Walters MD, Larkin KS, et al. Risk of unanticipated abnormal gynecologic pathology at the time of hysterectomy for uterovaginal prolapse. Am J Obstet Gynecol. 2010;202:507. e1-4. doi:10.1016/j.ajog.2010.01.077.
- Meriwether KV, Balk EM, Antosh DD, et al. Uterine-preserving surgeries for the repair of pelvic organ prolapse: a systematic review with meta-analysis and clinical practice guidelines. Int Urogynecol J. 2019;30:505-522. doi:10.1007/s00192-01903876-2.
- Meriwether KV, Antosh DD, Olivera CK, et al. Uterine preservation vs hysterectomy in pelvic organ prolapse surgery: a systematic review with meta-analysis and clinical practice guidelines. Am J Obstet Gynecol. 2018;219:129-146. e2. doi:10.1016/j.ajog.2018.01.018.
- Lin TY, Su TH, Wang YL, et al. Risk factors for failure of transvaginal sacrospinous uterine suspension in the treatment of uterovaginal prolapse. J Formos Med Assoc. 2005;104:249-253.
- Hyakutake MT, Cundiff GW, Geoffrion R. Cervical elongation following sacrospinous hysteropexy: a case series. Int Urogynecol J. 2014;25:851-854. doi:10.1007/s00192-013-2258-9.
- Thys SD, Coolen AL, Martens IR, et al. A comparison of long-term outcome between Manchester Fothergill and vaginal hysterectomy as treatment for uterine descent. Int Urogynecol J. 2011;22:1171-1178. doi:10.1007/s00192-011-1422-3.
- Ridgeway BM, Meriwether KV. Uterine preservation in pelvic organ prolapse surgery. In: Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier, Inc; 2022:358-373.
- FitzGerald MP, Richter HE, Siddique S, et al; for the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271. doi:10.1007/s00192005-1339-9.
- Winkelman WD, Haviland MJ, Elkadry EA. Long-term pelvic f loor symptoms, recurrence, satisfaction, and regret following colpocleisis. Female Pelvic Med Reconstr Surg. 2020;26:558562. doi:10.1097/SPV.000000000000602.
- Lu M, Zeng W, Ju R, et al. Long-term clinical outcomes, recurrence, satisfaction, and regret after total colpocleisis with concomitant vaginal hysterectomy: a retrospective single-center study. Female Pelvic Med Reconstr Surg. 2021;27(4):e510-e515. doi:10.1097/SPV.0000000000000900.
- Wang X, Chen Y, Hua K. Pelvic symptoms, body image, and regret after LeFort colpocleisis: a long-term follow-up. J Minim Invasive Gynecol. 2017;24:415-419. doi:10.1016/j. jmig.2016.12.015.
- Schulten SFM, Detollenaere RJ, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with uterosacral ligament suspension in women with uterine prolapse stage 2 or higher: observational followup of a multicentre randomised trial. BMJ. 2019;366:I5149. doi:10.1136/bmj.l5149.
- Schulten SF, Detollenaere RJ, IntHout J, et al. Risk factors for pelvic organ prolapse recurrence after sacrospinous hysteropexy or vaginal hysterectomy with uterosacral ligament suspension. Am J Obstet Gynecol. 2022;227:252.e1252.e9. doi:10.1016/j.ajog.2022.04.017.
- Hemming C, Constable L, Goulao B, et al. Surgical interventions for uterine prolapse and for vault prolapse: the two VUE RCTs. Health Technol Assess. 2020;24:1-220. doi:10.3310/hta24130.
- Romanzi LJ, Tyagi R. Hysteropexy compared to hysterectomy for uterine prolapse surgery: does durability differ? Int Urogynecol J. 2012;23:625-631. doi:10.1007/s00192-011-1635-5.
- Rosen DM, Shukla A, Cario GM, et al. Is hysterectomy necessary for laparoscopic pelvic floor repair? A prospective study. J Minim Invasive Gynecol. 2008;15:729-734. doi:10.1016/j.jmig.2008.08.010.
- Bedford ND, Seman EI, O’Shea RT, et al. Effect of uterine preservation on outcome of laparoscopic uterosacral suspension. J Minim Invasive Gynecol. 2013;20(2):172-177. doi:10.1016/j.jmig.2012.10.014.
- Diwan A, Rardin CR, Strohsnitter WC, et al. Laparoscopic uterosacral ligament uterine suspension compared with vaginal hysterectomy with vaginal vault suspension for uterovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:79-83. doi:10.1007/s00192-005-1346-x.
- de Boer TA, Milani AL, Kluivers KB, et al. The effectiveness of surgical correction of uterine prolapse: cervical amputation with uterosacral ligament plication (modified Manchester) versus vaginal hysterectomy with high uterosacral ligament plication. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:13131319. doi:10.1007/s00192-009-0945-3.
- Thomas AG, Brodman ML, Dottino PR, et al. Manchester procedure vs. vaginal hysterectomy for uterine prolapse. A comparison. J Reprod Med. 1995;40:299-304.
- Husby KR, Larsen MD, Lose G, et al. Surgical treatment of primary uterine prolapse: a comparison of vaginal native tissue surgical techniques. Int Urogynecol J. 2019;30:18871893. doi:10.1007/s00192-019-03950-9.
- Husby KR, Tolstrup CK, Lose G, et al. Manchester-Fothergill procedure versus vaginal hysterectomy with uterosacral ligament suspension: an activity-based costing analysis. Int Urogynecol J. 2018;29:1161-1171. doi:10.1007/s00192-0183575-9.
- Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153.e1-153.e31. doi:10.1016/j. ajog.2021.03.012.
- Rahmanou P, Price N, Jackson SR. Laparoscopic hysteropexy versus vaginal hysterectomy for the treatment of uterovaginal prolapse: a prospective randomized pilot study. Int Urogynecol J. 2015;26:1687-1694. doi:10.1007/s00192-0152761-2.
- Roovers JP, van der Vaart CH, van der Bom JG, et al. A randomised controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. BJOG. 2004;111:50-56. doi:10.1111/j.1471-0528.2004.00001.x.
- Roovers JP, van der Bom JG, van der Vaart CH, et al. A randomized comparison of post-operative pain, quality of life, and physical performance during the first 6 weeks after abdominal or vaginal surgical correction of descensus uteri. Neurourol Urodyn. 2005;24:334-340. doi:10.1002/nau.20104.
- Schulten SFM, Enklaar RA, Kluivers KB, et al. Evaluation of two vaginal, uterus sparing operations for pelvic organ prolapse: modified Manchester operation (MM) and sacrospinous hysteropexy (SSH), a study protocol for a multicentre randomized non-inferiority trial (the SAM study). BMC Womens Health. 20192;19:49. doi:10.1186/ s12905-019-0749-7.

CASE Patient desires prolapse repair
A 65-year-old postmenopausal patient (G3P3) presents to your office with symptoms of a vaginal bulge for more than 1 year. She has no urinary incontinence symptoms and no bowel dysfunction symptoms. On examination, you diagnose stage 2 uterovaginal prolapse with both anterior and apical defects. The patient declines expectant and pessary management and desires surgery, but she states that she feels her uterus “is important for me to keep, as my babies grew in there and it is part of me.” She denies any family or personal history of breast, endometrial, or ovarian cancer and has no history of abnormal cervical cancer screening or postmenopausal bleeding. What are the options for this patient?
Who is the appropriate hysteropexy patient, and how do we counsel her?
Uterine prolapse is the third leading cause of benign hysterectomy, with approximately 70,000 procedures performed each year in the United States. It has long been acknowledged that the uterus is a passive bystander to the prolapse process,1 but modern practice often involves a hysterectomy as part of addressing apical prolapse. However, more and more uterine-preserving surgeries are being performed, with one study showing an increase from 1.8% to 5% from 2002 and 2012.2
When presented with the option to keep or remove their uterus during the time of prolapse surgery, 36% of patients indicated that they would prefer to keep their uterus with similar outcomes while 21% would still prefer uterine preservation even if outcomes were inferior compared with hysterectomy.3 Another study showed that 60% of patients would decline concurrent hysterectomy if there were equal surgical outcomes,4 and popular platforms, such as Health magazine (www.health.com) and AARP magazine (www.aarp.org), have listed benign hysterectomy as a “top surgery to avoid.”
Patients desire uterine preservation for many reasons, including concerns about sexual function and pleasure, the uterus being important to their sense of identity or womanhood, and concerns around menopausal symptoms. Early patient counseling and discussion of surgical goals can help clinicians fully understand a patient’s thoughts toward uterine preservation. Women who identified their uterus as important to their sense of self had a 28.2-times chance of preferring uterine preservation.3 Frequently, concerns about menopausal symptoms are more directly related to hormones and ovary removal, not uterus removal, but clinicians should be careful to also counsel patients on the increased risk of menopause in the 5 years after hysterectomy, even with ovarian preservation.5
There are some patients for whom experts do not recommend uterine preservation.6 Patients with an increased risk of cervical or endometrial pathology should be counseled on the benefits of hysterectomy. Additionally, patients who have abnormal uterine bleeding from benign pathology should consider hysterectomy to treat these issues and avoid future workups (TABLE). For postmenopausal patients with recent postmenopausal bleeding, we encourage hysterectomy. A study of patients undergoing hysterectomy at the time of prolapse repair found a rate of 13% unanticipated endometrial pathology with postmenopausal bleeding and negative preoperative workup.7
At this time, a majority of clinicians consider the desire for future fertility to be a relative contraindication to surgical prolapse repair and advise conservative management with pessary until childbearing is complete. This is reasonable, given the paucity of safety data in subsequent pregnancies as well as the lack of prolapse outcomes after those pregnancies.8,9 Lastly, cervical elongation is considered a relative contraindication, as it represents a risk for surgical failure.10,11 This may be counteracted with trachelectomy at the time of hysteropexy or surgeries such as the Manchester repair, which involve a trachelectomy routinely,12 but currently there is no strong evidence for this as routine practice.
Continue to: Uterine preservation surgical techniques and outcomes...
Uterine preservation surgical techniques and outcomes
Le Fort colpocleisis
First described in 1840 by Neugebauer of Poland and later by Le Fort in Paris in 1877, the Le Fort colpocleisis repair technique remains the most reliable prolapse surgery to date.14 The uterus is left in place while the vagina is narrowed and shortened. It typically also is performed with a levator plication to reduce the genital hiatus.
This procedure is quick and effective, with a 90% to 95% success rate. If necessary, it can be performed under local or regional anesthesia, making it a good option for medically frail patients. It is not an option for everyone, however, as penetrative intercourse is no longer an option after surgery. Studies suggest an approximately 13% dissatisfaction rate after the procedure, with most of that coming from postoperative urinary symptoms, such as urgency or stress incontinence,15 and some studies show a dissatisfaction rate as low as 0% in a well-counseled patient population.16,17
Vaginal native tissue hysteropexy
Many patients who elect for uterine preservation at the time of prolapse surgery are “minimalists,” meaning that a vaginal native tissue procedure appeals to them due to the lack of abdominal incisions, decreased operating room time, and lack of permanent graft materials.
Of all the hysteropexy procedures, sacrospinous hysteropexy (SSHP) has the most robust data available. The approach to SSHP can be tailored to the patient’s anatomy and it is performed in a manner similar to posthysterectomy sacrospinous ligament fixation. The traditional posterior approach can be used with predominantly posterior prolapse, while an apical approach through a semilunar paracervical incision can be used for predominantly apical prolapse. Expert surgeons agree that one key to success is anchoring the suspension sutures through the cervical stroma, not just the vaginal epithelium.
Researchers in the Netherlands published the 5-year outcomes of a randomized trial that compared SSHP with vaginal hysterectomy with uterosacral ligament suspension.18 Their data showed no difference between groups in composite failure, reoperation rates, quality of life measures, and postoperative sexual function. Adverse events were very similar to those reported for posthysterectomy sacrospinous ligament fixation, including 15% transient buttock pain. Of note, the same authors explored risk factors for recurrence after SSHP and found that higher body mass index, smoking, and a large point Ba measurement were risk factors for prolapse recurrence.19
A randomized, controlled trial in the United Kingdom (the VUE trial) compared vaginal hysterectomy with apical suspension to uterine preservation with a variety of apical suspension techniques, mostly SSHP, and demonstrated no significant differences in outcomes.20 Overall, SSHP is an excellent option for many patients interested in uterine preservation.
Uterosacral ligament hysteropexy (USHP), when performed vaginally, is very similar to uterosacral ligament suspension at the time of vaginal hysterectomy, with entry into the peritoneal cavity through a posterior colpotomy. The uterosacral ligaments are grasped and delayed absorbable suture placed through the ligaments and anchored into the posterior cervical stroma. Given the maintenance of the normal axis of the vagina, USHP is a good technique for patients with isolated apical defects. Unfortunately, the least amount of quality data is available for USHP at this time. Currently, evidence suggests that complications are rare and that the procedure may offer acceptable anatomic and symptomatic outcomes.21 Some surgeons approach the uterosacral suspension laparoscopically, which also has mixed results in the literature, with failure rates between 8% and 27% and few robust studies.22–24
The Manchester-Fothergill operation, currently not common in the United States but popular in Europe, primarily is considered a treatment for cervical elongation when the uterosacral ligaments are intact. In this procedure, trachelectomy is performed and the uterosacral ligaments are plicated to the uterine body. Sturmdorf sutures are frequently placed to close off the endometrial canal, which can lead to hematometra and other complications of cervical stenosis. Previous unmatched studies have shown similar outcomes with the Manchester procedure compared with vaginal hysterectomy.25,26
The largest study currently available is a registry study from Denmark, with matched cohort populations, that compared the Manchester procedure, SSHP, and total vaginal hysterectomy with uterosacral ligament suspension.27 This study indicated less morbidity related to the Manchester procedure, decreased anterior recurrence compared with SSHP, and a 7% reoperation rate.27 The same authors also established better cost-effectiveness with the Manchester procedure as opposed to vaginal hysterectomy with uterosacral ligament suspension.28
Continue to: Vaginal mesh hysteropexy...
Vaginal mesh hysteropexy
Hysteropexy using vaginal mesh is limited in the United States given the removal of vaginal mesh kits from the market by the US Food and Drug Administration in 2019. However, a Pelvic Floor Disorders Network randomized trial compared vaginal mesh hysteropexy using the Uphold LITE transvaginal mesh support system (Boston Scientific) and vaginal hysterectomy with uterosacral ligament suspension.29 At 5 years, mesh hysteropexy had fewer failures than hysterectomy (37% vs 54%) and there was no difference in retreatment (9% vs 13%). The authors noted an 8% mesh exposure rate in the mesh hysteropexy group but 12% granulation tissue and 21% suture exposure rate in the hysterectomy group.29
While vaginal mesh hysteropexy was effective in the treatment of apical prolapse, the elevated mesh exposure rate and postoperative complications ultimately led to its removal from the market.
Sacrohysteropexy
Lastly, prolapse surgery with uterine preservation may be accomplished abdominally, most commonly laparoscopically with or without robotic assistance.
Sacrohysteropexy (SHP) involves the attachment of permanent synthetic mesh posteriorly to the posterior vagina and cervix with or without the additional placement of mesh to the anterior vagina and cervix. When the anterior mesh is placed, the arms are typically routed through the broad ligament bilaterally and joined with the posterior mesh for attachment to the anterior longitudinal ligament, overlying the sacrum.
Proponents of this technique endorse the use of mesh to augment already failing native tissues and propose similarities to the durability of sacrocolpopexy. While no randomized controlled trials have compared hysterectomy with sacrocolpopexy or supracervical hysterectomy with sacrocolpopexy to sacrohysteropexy, a meta-analysis suggests that sacrohysteropexy may have a decreased risk of mesh exposure but a higher reoperation rate with lower anatomic success.9 Randomized trials that compared abdominal sacrohysteropexy with vaginal hysterectomy and suspension indicate that apical support may be improved with sacrohysteropexy,30 but reoperations, postoperative pain and disability, and urinary dysfunction was higher with SHP.31,32
What further research is needed?
With the increasing patient and clinician interest in uterine preservation, more research is needed to improve patient counseling and surgical planning. Much of the current research compares hysteropexy outcomes with those of traditional prolapse repairs with hysterectomy, with only a few randomized trials. We are lacking robust, prospective comparison studies between hysteropexy methods, especially vaginal native tissue techniques, long-term follow-up on the prevalence of uterine or cervical pathology after hysteropexy, and pregnancy or postpartum outcomes following uterine preservation surgery.
Currently, work is underway to validate and test the effectiveness of a questionnaire to evaluate the uterus’s importance to the patient seeking prolapse surgery in order to optimize counseling. The VUE trial, which randomizes women to vaginal hysterectomy with suspension versus various prolapse surgeries with uterine preservation, is continuing its 6-year follow-up.20 In the Netherlands, an ongoing randomized, controlled trial (the SAM trial) is comparing the Manchester procedure with sacrospinous hysteropexy and will follow patients up to 24 months.33 Fortunately, both of these trials are rigorously assessing both objective and patient-centered outcomes.
CASE Counseling helps the patient weigh surgical options
After thorough review of her surgical options, the patient elects for a uterine-preserving prolapse repair. She would like to have the most minimally invasive procedure and does not want any permanent mesh used. You suggest, and she agrees to, a sacrospinous ligament hysteropexy, as it is the current technique with the most robust data. ●

CASE Patient desires prolapse repair
A 65-year-old postmenopausal patient (G3P3) presents to your office with symptoms of a vaginal bulge for more than 1 year. She has no urinary incontinence symptoms and no bowel dysfunction symptoms. On examination, you diagnose stage 2 uterovaginal prolapse with both anterior and apical defects. The patient declines expectant and pessary management and desires surgery, but she states that she feels her uterus “is important for me to keep, as my babies grew in there and it is part of me.” She denies any family or personal history of breast, endometrial, or ovarian cancer and has no history of abnormal cervical cancer screening or postmenopausal bleeding. What are the options for this patient?
Who is the appropriate hysteropexy patient, and how do we counsel her?
Uterine prolapse is the third leading cause of benign hysterectomy, with approximately 70,000 procedures performed each year in the United States. It has long been acknowledged that the uterus is a passive bystander to the prolapse process,1 but modern practice often involves a hysterectomy as part of addressing apical prolapse. However, more and more uterine-preserving surgeries are being performed, with one study showing an increase from 1.8% to 5% from 2002 and 2012.2
When presented with the option to keep or remove their uterus during the time of prolapse surgery, 36% of patients indicated that they would prefer to keep their uterus with similar outcomes while 21% would still prefer uterine preservation even if outcomes were inferior compared with hysterectomy.3 Another study showed that 60% of patients would decline concurrent hysterectomy if there were equal surgical outcomes,4 and popular platforms, such as Health magazine (www.health.com) and AARP magazine (www.aarp.org), have listed benign hysterectomy as a “top surgery to avoid.”
Patients desire uterine preservation for many reasons, including concerns about sexual function and pleasure, the uterus being important to their sense of identity or womanhood, and concerns around menopausal symptoms. Early patient counseling and discussion of surgical goals can help clinicians fully understand a patient’s thoughts toward uterine preservation. Women who identified their uterus as important to their sense of self had a 28.2-times chance of preferring uterine preservation.3 Frequently, concerns about menopausal symptoms are more directly related to hormones and ovary removal, not uterus removal, but clinicians should be careful to also counsel patients on the increased risk of menopause in the 5 years after hysterectomy, even with ovarian preservation.5
There are some patients for whom experts do not recommend uterine preservation.6 Patients with an increased risk of cervical or endometrial pathology should be counseled on the benefits of hysterectomy. Additionally, patients who have abnormal uterine bleeding from benign pathology should consider hysterectomy to treat these issues and avoid future workups (TABLE). For postmenopausal patients with recent postmenopausal bleeding, we encourage hysterectomy. A study of patients undergoing hysterectomy at the time of prolapse repair found a rate of 13% unanticipated endometrial pathology with postmenopausal bleeding and negative preoperative workup.7
At this time, a majority of clinicians consider the desire for future fertility to be a relative contraindication to surgical prolapse repair and advise conservative management with pessary until childbearing is complete. This is reasonable, given the paucity of safety data in subsequent pregnancies as well as the lack of prolapse outcomes after those pregnancies.8,9 Lastly, cervical elongation is considered a relative contraindication, as it represents a risk for surgical failure.10,11 This may be counteracted with trachelectomy at the time of hysteropexy or surgeries such as the Manchester repair, which involve a trachelectomy routinely,12 but currently there is no strong evidence for this as routine practice.
Continue to: Uterine preservation surgical techniques and outcomes...
Uterine preservation surgical techniques and outcomes
Le Fort colpocleisis
First described in 1840 by Neugebauer of Poland and later by Le Fort in Paris in 1877, the Le Fort colpocleisis repair technique remains the most reliable prolapse surgery to date.14 The uterus is left in place while the vagina is narrowed and shortened. It typically also is performed with a levator plication to reduce the genital hiatus.
This procedure is quick and effective, with a 90% to 95% success rate. If necessary, it can be performed under local or regional anesthesia, making it a good option for medically frail patients. It is not an option for everyone, however, as penetrative intercourse is no longer an option after surgery. Studies suggest an approximately 13% dissatisfaction rate after the procedure, with most of that coming from postoperative urinary symptoms, such as urgency or stress incontinence,15 and some studies show a dissatisfaction rate as low as 0% in a well-counseled patient population.16,17
Vaginal native tissue hysteropexy
Many patients who elect for uterine preservation at the time of prolapse surgery are “minimalists,” meaning that a vaginal native tissue procedure appeals to them due to the lack of abdominal incisions, decreased operating room time, and lack of permanent graft materials.
Of all the hysteropexy procedures, sacrospinous hysteropexy (SSHP) has the most robust data available. The approach to SSHP can be tailored to the patient’s anatomy and it is performed in a manner similar to posthysterectomy sacrospinous ligament fixation. The traditional posterior approach can be used with predominantly posterior prolapse, while an apical approach through a semilunar paracervical incision can be used for predominantly apical prolapse. Expert surgeons agree that one key to success is anchoring the suspension sutures through the cervical stroma, not just the vaginal epithelium.
Researchers in the Netherlands published the 5-year outcomes of a randomized trial that compared SSHP with vaginal hysterectomy with uterosacral ligament suspension.18 Their data showed no difference between groups in composite failure, reoperation rates, quality of life measures, and postoperative sexual function. Adverse events were very similar to those reported for posthysterectomy sacrospinous ligament fixation, including 15% transient buttock pain. Of note, the same authors explored risk factors for recurrence after SSHP and found that higher body mass index, smoking, and a large point Ba measurement were risk factors for prolapse recurrence.19
A randomized, controlled trial in the United Kingdom (the VUE trial) compared vaginal hysterectomy with apical suspension to uterine preservation with a variety of apical suspension techniques, mostly SSHP, and demonstrated no significant differences in outcomes.20 Overall, SSHP is an excellent option for many patients interested in uterine preservation.
Uterosacral ligament hysteropexy (USHP), when performed vaginally, is very similar to uterosacral ligament suspension at the time of vaginal hysterectomy, with entry into the peritoneal cavity through a posterior colpotomy. The uterosacral ligaments are grasped and delayed absorbable suture placed through the ligaments and anchored into the posterior cervical stroma. Given the maintenance of the normal axis of the vagina, USHP is a good technique for patients with isolated apical defects. Unfortunately, the least amount of quality data is available for USHP at this time. Currently, evidence suggests that complications are rare and that the procedure may offer acceptable anatomic and symptomatic outcomes.21 Some surgeons approach the uterosacral suspension laparoscopically, which also has mixed results in the literature, with failure rates between 8% and 27% and few robust studies.22–24
The Manchester-Fothergill operation, currently not common in the United States but popular in Europe, primarily is considered a treatment for cervical elongation when the uterosacral ligaments are intact. In this procedure, trachelectomy is performed and the uterosacral ligaments are plicated to the uterine body. Sturmdorf sutures are frequently placed to close off the endometrial canal, which can lead to hematometra and other complications of cervical stenosis. Previous unmatched studies have shown similar outcomes with the Manchester procedure compared with vaginal hysterectomy.25,26
The largest study currently available is a registry study from Denmark, with matched cohort populations, that compared the Manchester procedure, SSHP, and total vaginal hysterectomy with uterosacral ligament suspension.27 This study indicated less morbidity related to the Manchester procedure, decreased anterior recurrence compared with SSHP, and a 7% reoperation rate.27 The same authors also established better cost-effectiveness with the Manchester procedure as opposed to vaginal hysterectomy with uterosacral ligament suspension.28
Continue to: Vaginal mesh hysteropexy...
Vaginal mesh hysteropexy
Hysteropexy using vaginal mesh is limited in the United States given the removal of vaginal mesh kits from the market by the US Food and Drug Administration in 2019. However, a Pelvic Floor Disorders Network randomized trial compared vaginal mesh hysteropexy using the Uphold LITE transvaginal mesh support system (Boston Scientific) and vaginal hysterectomy with uterosacral ligament suspension.29 At 5 years, mesh hysteropexy had fewer failures than hysterectomy (37% vs 54%) and there was no difference in retreatment (9% vs 13%). The authors noted an 8% mesh exposure rate in the mesh hysteropexy group but 12% granulation tissue and 21% suture exposure rate in the hysterectomy group.29
While vaginal mesh hysteropexy was effective in the treatment of apical prolapse, the elevated mesh exposure rate and postoperative complications ultimately led to its removal from the market.
Sacrohysteropexy
Lastly, prolapse surgery with uterine preservation may be accomplished abdominally, most commonly laparoscopically with or without robotic assistance.
Sacrohysteropexy (SHP) involves the attachment of permanent synthetic mesh posteriorly to the posterior vagina and cervix with or without the additional placement of mesh to the anterior vagina and cervix. When the anterior mesh is placed, the arms are typically routed through the broad ligament bilaterally and joined with the posterior mesh for attachment to the anterior longitudinal ligament, overlying the sacrum.
Proponents of this technique endorse the use of mesh to augment already failing native tissues and propose similarities to the durability of sacrocolpopexy. While no randomized controlled trials have compared hysterectomy with sacrocolpopexy or supracervical hysterectomy with sacrocolpopexy to sacrohysteropexy, a meta-analysis suggests that sacrohysteropexy may have a decreased risk of mesh exposure but a higher reoperation rate with lower anatomic success.9 Randomized trials that compared abdominal sacrohysteropexy with vaginal hysterectomy and suspension indicate that apical support may be improved with sacrohysteropexy,30 but reoperations, postoperative pain and disability, and urinary dysfunction was higher with SHP.31,32
What further research is needed?
With the increasing patient and clinician interest in uterine preservation, more research is needed to improve patient counseling and surgical planning. Much of the current research compares hysteropexy outcomes with those of traditional prolapse repairs with hysterectomy, with only a few randomized trials. We are lacking robust, prospective comparison studies between hysteropexy methods, especially vaginal native tissue techniques, long-term follow-up on the prevalence of uterine or cervical pathology after hysteropexy, and pregnancy or postpartum outcomes following uterine preservation surgery.
Currently, work is underway to validate and test the effectiveness of a questionnaire to evaluate the uterus’s importance to the patient seeking prolapse surgery in order to optimize counseling. The VUE trial, which randomizes women to vaginal hysterectomy with suspension versus various prolapse surgeries with uterine preservation, is continuing its 6-year follow-up.20 In the Netherlands, an ongoing randomized, controlled trial (the SAM trial) is comparing the Manchester procedure with sacrospinous hysteropexy and will follow patients up to 24 months.33 Fortunately, both of these trials are rigorously assessing both objective and patient-centered outcomes.
CASE Counseling helps the patient weigh surgical options
After thorough review of her surgical options, the patient elects for a uterine-preserving prolapse repair. She would like to have the most minimally invasive procedure and does not want any permanent mesh used. You suggest, and she agrees to, a sacrospinous ligament hysteropexy, as it is the current technique with the most robust data. ●
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 pt 1):1717-1724; discussion 1724-1728. doi:10.1016/0002-9378(92)91562-o.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23:365-371. doi:10.1097/SPV.0000000000000426.
- Korbly NB, Kassis NC, Good MM, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol. 2013;209:470.e16. doi:10.1016/j.ajog.2013.08.003.
- Frick AC, Barber MD, Paraiso MF, et al. Attitudes toward hysterectomy in women undergoing evaluation for uterovaginal prolapse. Female Pelvic Med Reconstr Surg. 2013;19:103-109. doi:10.1097/SPV.0b013e31827d8667.
- Farquhar CM, Sadler L, Harvey SA, et al. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962. doi:10.1111/j.1471-0528.2005.00696.x
- Gutman R, Maher C. Uterine-preserving POP surgery. Int Urogynecol J. 2013;24:1803-1813. doi:10.1007/s00192-0132171-2.
- Frick AC, Walters MD, Larkin KS, et al. Risk of unanticipated abnormal gynecologic pathology at the time of hysterectomy for uterovaginal prolapse. Am J Obstet Gynecol. 2010;202:507. e1-4. doi:10.1016/j.ajog.2010.01.077.
- Meriwether KV, Balk EM, Antosh DD, et al. Uterine-preserving surgeries for the repair of pelvic organ prolapse: a systematic review with meta-analysis and clinical practice guidelines. Int Urogynecol J. 2019;30:505-522. doi:10.1007/s00192-01903876-2.
- Meriwether KV, Antosh DD, Olivera CK, et al. Uterine preservation vs hysterectomy in pelvic organ prolapse surgery: a systematic review with meta-analysis and clinical practice guidelines. Am J Obstet Gynecol. 2018;219:129-146. e2. doi:10.1016/j.ajog.2018.01.018.
- Lin TY, Su TH, Wang YL, et al. Risk factors for failure of transvaginal sacrospinous uterine suspension in the treatment of uterovaginal prolapse. J Formos Med Assoc. 2005;104:249-253.
- Hyakutake MT, Cundiff GW, Geoffrion R. Cervical elongation following sacrospinous hysteropexy: a case series. Int Urogynecol J. 2014;25:851-854. doi:10.1007/s00192-013-2258-9.
- Thys SD, Coolen AL, Martens IR, et al. A comparison of long-term outcome between Manchester Fothergill and vaginal hysterectomy as treatment for uterine descent. Int Urogynecol J. 2011;22:1171-1178. doi:10.1007/s00192-011-1422-3.
- Ridgeway BM, Meriwether KV. Uterine preservation in pelvic organ prolapse surgery. In: Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier, Inc; 2022:358-373.
- FitzGerald MP, Richter HE, Siddique S, et al; for the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271. doi:10.1007/s00192005-1339-9.
- Winkelman WD, Haviland MJ, Elkadry EA. Long-term pelvic f loor symptoms, recurrence, satisfaction, and regret following colpocleisis. Female Pelvic Med Reconstr Surg. 2020;26:558562. doi:10.1097/SPV.000000000000602.
- Lu M, Zeng W, Ju R, et al. Long-term clinical outcomes, recurrence, satisfaction, and regret after total colpocleisis with concomitant vaginal hysterectomy: a retrospective single-center study. Female Pelvic Med Reconstr Surg. 2021;27(4):e510-e515. doi:10.1097/SPV.0000000000000900.
- Wang X, Chen Y, Hua K. Pelvic symptoms, body image, and regret after LeFort colpocleisis: a long-term follow-up. J Minim Invasive Gynecol. 2017;24:415-419. doi:10.1016/j. jmig.2016.12.015.
- Schulten SFM, Detollenaere RJ, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with uterosacral ligament suspension in women with uterine prolapse stage 2 or higher: observational followup of a multicentre randomised trial. BMJ. 2019;366:I5149. doi:10.1136/bmj.l5149.
- Schulten SF, Detollenaere RJ, IntHout J, et al. Risk factors for pelvic organ prolapse recurrence after sacrospinous hysteropexy or vaginal hysterectomy with uterosacral ligament suspension. Am J Obstet Gynecol. 2022;227:252.e1252.e9. doi:10.1016/j.ajog.2022.04.017.
- Hemming C, Constable L, Goulao B, et al. Surgical interventions for uterine prolapse and for vault prolapse: the two VUE RCTs. Health Technol Assess. 2020;24:1-220. doi:10.3310/hta24130.
- Romanzi LJ, Tyagi R. Hysteropexy compared to hysterectomy for uterine prolapse surgery: does durability differ? Int Urogynecol J. 2012;23:625-631. doi:10.1007/s00192-011-1635-5.
- Rosen DM, Shukla A, Cario GM, et al. Is hysterectomy necessary for laparoscopic pelvic floor repair? A prospective study. J Minim Invasive Gynecol. 2008;15:729-734. doi:10.1016/j.jmig.2008.08.010.
- Bedford ND, Seman EI, O’Shea RT, et al. Effect of uterine preservation on outcome of laparoscopic uterosacral suspension. J Minim Invasive Gynecol. 2013;20(2):172-177. doi:10.1016/j.jmig.2012.10.014.
- Diwan A, Rardin CR, Strohsnitter WC, et al. Laparoscopic uterosacral ligament uterine suspension compared with vaginal hysterectomy with vaginal vault suspension for uterovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:79-83. doi:10.1007/s00192-005-1346-x.
- de Boer TA, Milani AL, Kluivers KB, et al. The effectiveness of surgical correction of uterine prolapse: cervical amputation with uterosacral ligament plication (modified Manchester) versus vaginal hysterectomy with high uterosacral ligament plication. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:13131319. doi:10.1007/s00192-009-0945-3.
- Thomas AG, Brodman ML, Dottino PR, et al. Manchester procedure vs. vaginal hysterectomy for uterine prolapse. A comparison. J Reprod Med. 1995;40:299-304.
- Husby KR, Larsen MD, Lose G, et al. Surgical treatment of primary uterine prolapse: a comparison of vaginal native tissue surgical techniques. Int Urogynecol J. 2019;30:18871893. doi:10.1007/s00192-019-03950-9.
- Husby KR, Tolstrup CK, Lose G, et al. Manchester-Fothergill procedure versus vaginal hysterectomy with uterosacral ligament suspension: an activity-based costing analysis. Int Urogynecol J. 2018;29:1161-1171. doi:10.1007/s00192-0183575-9.
- Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153.e1-153.e31. doi:10.1016/j. ajog.2021.03.012.
- Rahmanou P, Price N, Jackson SR. Laparoscopic hysteropexy versus vaginal hysterectomy for the treatment of uterovaginal prolapse: a prospective randomized pilot study. Int Urogynecol J. 2015;26:1687-1694. doi:10.1007/s00192-0152761-2.
- Roovers JP, van der Vaart CH, van der Bom JG, et al. A randomised controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. BJOG. 2004;111:50-56. doi:10.1111/j.1471-0528.2004.00001.x.
- Roovers JP, van der Bom JG, van der Vaart CH, et al. A randomized comparison of post-operative pain, quality of life, and physical performance during the first 6 weeks after abdominal or vaginal surgical correction of descensus uteri. Neurourol Urodyn. 2005;24:334-340. doi:10.1002/nau.20104.
- Schulten SFM, Enklaar RA, Kluivers KB, et al. Evaluation of two vaginal, uterus sparing operations for pelvic organ prolapse: modified Manchester operation (MM) and sacrospinous hysteropexy (SSH), a study protocol for a multicentre randomized non-inferiority trial (the SAM study). BMC Womens Health. 20192;19:49. doi:10.1186/ s12905-019-0749-7.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 pt 1):1717-1724; discussion 1724-1728. doi:10.1016/0002-9378(92)91562-o.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23:365-371. doi:10.1097/SPV.0000000000000426.
- Korbly NB, Kassis NC, Good MM, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol. 2013;209:470.e16. doi:10.1016/j.ajog.2013.08.003.
- Frick AC, Barber MD, Paraiso MF, et al. Attitudes toward hysterectomy in women undergoing evaluation for uterovaginal prolapse. Female Pelvic Med Reconstr Surg. 2013;19:103-109. doi:10.1097/SPV.0b013e31827d8667.
- Farquhar CM, Sadler L, Harvey SA, et al. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962. doi:10.1111/j.1471-0528.2005.00696.x
- Gutman R, Maher C. Uterine-preserving POP surgery. Int Urogynecol J. 2013;24:1803-1813. doi:10.1007/s00192-0132171-2.
- Frick AC, Walters MD, Larkin KS, et al. Risk of unanticipated abnormal gynecologic pathology at the time of hysterectomy for uterovaginal prolapse. Am J Obstet Gynecol. 2010;202:507. e1-4. doi:10.1016/j.ajog.2010.01.077.
- Meriwether KV, Balk EM, Antosh DD, et al. Uterine-preserving surgeries for the repair of pelvic organ prolapse: a systematic review with meta-analysis and clinical practice guidelines. Int Urogynecol J. 2019;30:505-522. doi:10.1007/s00192-01903876-2.
- Meriwether KV, Antosh DD, Olivera CK, et al. Uterine preservation vs hysterectomy in pelvic organ prolapse surgery: a systematic review with meta-analysis and clinical practice guidelines. Am J Obstet Gynecol. 2018;219:129-146. e2. doi:10.1016/j.ajog.2018.01.018.
- Lin TY, Su TH, Wang YL, et al. Risk factors for failure of transvaginal sacrospinous uterine suspension in the treatment of uterovaginal prolapse. J Formos Med Assoc. 2005;104:249-253.
- Hyakutake MT, Cundiff GW, Geoffrion R. Cervical elongation following sacrospinous hysteropexy: a case series. Int Urogynecol J. 2014;25:851-854. doi:10.1007/s00192-013-2258-9.
- Thys SD, Coolen AL, Martens IR, et al. A comparison of long-term outcome between Manchester Fothergill and vaginal hysterectomy as treatment for uterine descent. Int Urogynecol J. 2011;22:1171-1178. doi:10.1007/s00192-011-1422-3.
- Ridgeway BM, Meriwether KV. Uterine preservation in pelvic organ prolapse surgery. In: Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier, Inc; 2022:358-373.
- FitzGerald MP, Richter HE, Siddique S, et al; for the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271. doi:10.1007/s00192005-1339-9.
- Winkelman WD, Haviland MJ, Elkadry EA. Long-term pelvic f loor symptoms, recurrence, satisfaction, and regret following colpocleisis. Female Pelvic Med Reconstr Surg. 2020;26:558562. doi:10.1097/SPV.000000000000602.
- Lu M, Zeng W, Ju R, et al. Long-term clinical outcomes, recurrence, satisfaction, and regret after total colpocleisis with concomitant vaginal hysterectomy: a retrospective single-center study. Female Pelvic Med Reconstr Surg. 2021;27(4):e510-e515. doi:10.1097/SPV.0000000000000900.
- Wang X, Chen Y, Hua K. Pelvic symptoms, body image, and regret after LeFort colpocleisis: a long-term follow-up. J Minim Invasive Gynecol. 2017;24:415-419. doi:10.1016/j. jmig.2016.12.015.
- Schulten SFM, Detollenaere RJ, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with uterosacral ligament suspension in women with uterine prolapse stage 2 or higher: observational followup of a multicentre randomised trial. BMJ. 2019;366:I5149. doi:10.1136/bmj.l5149.
- Schulten SF, Detollenaere RJ, IntHout J, et al. Risk factors for pelvic organ prolapse recurrence after sacrospinous hysteropexy or vaginal hysterectomy with uterosacral ligament suspension. Am J Obstet Gynecol. 2022;227:252.e1252.e9. doi:10.1016/j.ajog.2022.04.017.
- Hemming C, Constable L, Goulao B, et al. Surgical interventions for uterine prolapse and for vault prolapse: the two VUE RCTs. Health Technol Assess. 2020;24:1-220. doi:10.3310/hta24130.
- Romanzi LJ, Tyagi R. Hysteropexy compared to hysterectomy for uterine prolapse surgery: does durability differ? Int Urogynecol J. 2012;23:625-631. doi:10.1007/s00192-011-1635-5.
- Rosen DM, Shukla A, Cario GM, et al. Is hysterectomy necessary for laparoscopic pelvic floor repair? A prospective study. J Minim Invasive Gynecol. 2008;15:729-734. doi:10.1016/j.jmig.2008.08.010.
- Bedford ND, Seman EI, O’Shea RT, et al. Effect of uterine preservation on outcome of laparoscopic uterosacral suspension. J Minim Invasive Gynecol. 2013;20(2):172-177. doi:10.1016/j.jmig.2012.10.014.
- Diwan A, Rardin CR, Strohsnitter WC, et al. Laparoscopic uterosacral ligament uterine suspension compared with vaginal hysterectomy with vaginal vault suspension for uterovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:79-83. doi:10.1007/s00192-005-1346-x.
- de Boer TA, Milani AL, Kluivers KB, et al. The effectiveness of surgical correction of uterine prolapse: cervical amputation with uterosacral ligament plication (modified Manchester) versus vaginal hysterectomy with high uterosacral ligament plication. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:13131319. doi:10.1007/s00192-009-0945-3.
- Thomas AG, Brodman ML, Dottino PR, et al. Manchester procedure vs. vaginal hysterectomy for uterine prolapse. A comparison. J Reprod Med. 1995;40:299-304.
- Husby KR, Larsen MD, Lose G, et al. Surgical treatment of primary uterine prolapse: a comparison of vaginal native tissue surgical techniques. Int Urogynecol J. 2019;30:18871893. doi:10.1007/s00192-019-03950-9.
- Husby KR, Tolstrup CK, Lose G, et al. Manchester-Fothergill procedure versus vaginal hysterectomy with uterosacral ligament suspension: an activity-based costing analysis. Int Urogynecol J. 2018;29:1161-1171. doi:10.1007/s00192-0183575-9.
- Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153.e1-153.e31. doi:10.1016/j. ajog.2021.03.012.
- Rahmanou P, Price N, Jackson SR. Laparoscopic hysteropexy versus vaginal hysterectomy for the treatment of uterovaginal prolapse: a prospective randomized pilot study. Int Urogynecol J. 2015;26:1687-1694. doi:10.1007/s00192-0152761-2.
- Roovers JP, van der Vaart CH, van der Bom JG, et al. A randomised controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. BJOG. 2004;111:50-56. doi:10.1111/j.1471-0528.2004.00001.x.
- Roovers JP, van der Bom JG, van der Vaart CH, et al. A randomized comparison of post-operative pain, quality of life, and physical performance during the first 6 weeks after abdominal or vaginal surgical correction of descensus uteri. Neurourol Urodyn. 2005;24:334-340. doi:10.1002/nau.20104.
- Schulten SFM, Enklaar RA, Kluivers KB, et al. Evaluation of two vaginal, uterus sparing operations for pelvic organ prolapse: modified Manchester operation (MM) and sacrospinous hysteropexy (SSH), a study protocol for a multicentre randomized non-inferiority trial (the SAM study). BMC Womens Health. 20192;19:49. doi:10.1186/ s12905-019-0749-7.
Gender-affirming mastectomy boosts image and quality of life in gender-diverse youth
Adolescents and young adults who undergo “top surgery” for gender dysphoria overwhelmingly report being satisfied with the procedure in the near-term, new research shows.
The results of the prospective cohort study, reported recently in JAMA Pediatrics, suggest that the surgery can help facilitate gender congruence and comfort with body image for transmasculine and nonbinary youth. The authors, from Northwestern University, Chicago, said the findings may “help dispel misconceptions that gender-affirming treatment is experimental and support evidence-based practices of top surgery.”
Sumanas Jordan, MD, PhD, assistant professor of plastic surgery at Northwestern University, Chicago, and a coauthor of the study, said the study was the first prospective, matched cohort analysis showing that chest surgery improves outcomes in this age group.
“We focused our study on chest dysphoria, the distress due to the presence of breasts, and gender congruence, the feeling of alignment between identity and physical characteristics,” Dr. Jordan said. “We will continue to study the effect of surgery in other areas of health, such as physical functioning and quality of life, and follow our patients longer term.”
As many as 9% of adolescents and young adults identify as transgender or nonbinary - a group underrepresented in the pediatric literature, Dr. Jordan’s group said. Chest dysphoria often is associated with psychosocial issues such as depression and anxiety.
“Dysphoria can lead to a range of negative physical and emotional consequences, such as avoidance of exercise and sports, harmful chest-binding practices, functional limitations, and suicidal ideation, said M. Brett Cooper, MD, MEd, assistant professor of pediatrics, and adolescent and young adult medicine, at UT Southwestern Medical Center/Children’s Health, Dallas. “These young people often bind for several hours a day to reduce the presence of their chest.”
The study
The Northwestern team recruited 81 patients with a mean age of 18.6 years whose sex at birth was assigned female. Patients were overwhelmingly White (89%), and the majority (59%) were transgender male, the remaining patients nonbinary.
The population sample included patients aged 13-24 who underwent top surgery from December 2019 to April 2021 and a matched control group of those who did not have surgery.
Outcomes measures were assessed preoperatively and 3 months after surgery.
Thirty-six surgical patients and 34 of those in the control arm completed the outcomes measures. Surgical complications were minimal. Propensity analyses suggested an association between surgery and substantial improvements in scores on the following study endpoints:
- Chest dysphoria measure (–25.58 points, 95% confidence interval [CI], –29.18 to –21.98).
- Transgender congruence scale (7.78 points, 95%: CI, 6.06-9.50)
- Body image scale (–7.20 points, 95% CI, –11.68 to –2.72).
The patients who underwent top surgery reported significant improvements in scores of chest dysphoria, transgender congruence, and body image. The results for patients younger than age 18 paralleled those for older participants in the study.
While the results corroborate other studies showing that gender-affirming therapy improves mental health and quality of life among these young people, the researchers cautioned that some insurers require testosterone therapy for 1 year before their plans will cover the costs of gender-affirming surgery.
This may negatively affect those nonbinary patients who do not undergo hormone therapy,” the researchers wrote. They are currently collecting 1-year follow-up data to determine the long-term effects of top surgery on chest dysphoria, gender congruence, and body image.
As surgical patients progress through adult life, does the risk of regret increase? “We did not address regret in this short-term study,” Dr. Jordan said. “However, previous studies have shown very low levels of regret.”
An accompanying editorial concurred that top surgery is effective and medically necessary in this population of young people.
Calling the study “an important milestone in gender affirmation research,” Kishan M. Thadikonda, MD, and Katherine M. Gast, MD, MS, of the school of medicine and public health at the University of Wisconsin in Madison, said it will be important to follow this young cohort to prove these benefits will endure as patients age.
They cautioned, however, that nonbinary patients represented just 13% of the patient total and only 8% of the surgical cohort. Nonbinary patients are not well understood as a patient population when it comes to gender-affirmation surgery and are often included in studies with transgender patients despite clear differences, they noted.
Current setbacks
According to Dr. Cooper, politics is already affecting care in Texas. “Due to the sociopolitical climate in my state in regard to gender-affirming care, I have also seen a few young people have their surgeries either canceled or postponed by their parents,” he said. “This has led to a worsening of mental health in these patients.”
Dr. Cooper stressed the need for more research on the perspective of non-White and socioeconomically disadvantaged youth.
“This study also highlights the disparity between patients who have commercial insurance versus those who are on Medicaid,” he said. “Medicaid plans often do not cover this, so those patients usually have to continue to suffer or pay for this surgery out of their own pocket.”
This study was supported by the Northwestern University Clinical and Translational Sciences Institute, funded in part by the National Institutes of Health. Funding also came from the Plastic Surgery Foundation and American Association of Pediatric Plastic Surgery. Dr. Jordan received grants from the Plastic Surgery Foundation during the study. One coauthor reported consultant fees from CVS Caremark for consulting outside the submitted work, and another reported grants from the National Institutes of Health outside the submitted work. Dr. Cooper disclosed no competing interests relevant to his comments. The editorial commentators disclosed no conflicts of interest.
Adolescents and young adults who undergo “top surgery” for gender dysphoria overwhelmingly report being satisfied with the procedure in the near-term, new research shows.
The results of the prospective cohort study, reported recently in JAMA Pediatrics, suggest that the surgery can help facilitate gender congruence and comfort with body image for transmasculine and nonbinary youth. The authors, from Northwestern University, Chicago, said the findings may “help dispel misconceptions that gender-affirming treatment is experimental and support evidence-based practices of top surgery.”
Sumanas Jordan, MD, PhD, assistant professor of plastic surgery at Northwestern University, Chicago, and a coauthor of the study, said the study was the first prospective, matched cohort analysis showing that chest surgery improves outcomes in this age group.
“We focused our study on chest dysphoria, the distress due to the presence of breasts, and gender congruence, the feeling of alignment between identity and physical characteristics,” Dr. Jordan said. “We will continue to study the effect of surgery in other areas of health, such as physical functioning and quality of life, and follow our patients longer term.”
As many as 9% of adolescents and young adults identify as transgender or nonbinary - a group underrepresented in the pediatric literature, Dr. Jordan’s group said. Chest dysphoria often is associated with psychosocial issues such as depression and anxiety.
“Dysphoria can lead to a range of negative physical and emotional consequences, such as avoidance of exercise and sports, harmful chest-binding practices, functional limitations, and suicidal ideation, said M. Brett Cooper, MD, MEd, assistant professor of pediatrics, and adolescent and young adult medicine, at UT Southwestern Medical Center/Children’s Health, Dallas. “These young people often bind for several hours a day to reduce the presence of their chest.”
The study
The Northwestern team recruited 81 patients with a mean age of 18.6 years whose sex at birth was assigned female. Patients were overwhelmingly White (89%), and the majority (59%) were transgender male, the remaining patients nonbinary.
The population sample included patients aged 13-24 who underwent top surgery from December 2019 to April 2021 and a matched control group of those who did not have surgery.
Outcomes measures were assessed preoperatively and 3 months after surgery.
Thirty-six surgical patients and 34 of those in the control arm completed the outcomes measures. Surgical complications were minimal. Propensity analyses suggested an association between surgery and substantial improvements in scores on the following study endpoints:
- Chest dysphoria measure (–25.58 points, 95% confidence interval [CI], –29.18 to –21.98).
- Transgender congruence scale (7.78 points, 95%: CI, 6.06-9.50)
- Body image scale (–7.20 points, 95% CI, –11.68 to –2.72).
The patients who underwent top surgery reported significant improvements in scores of chest dysphoria, transgender congruence, and body image. The results for patients younger than age 18 paralleled those for older participants in the study.
While the results corroborate other studies showing that gender-affirming therapy improves mental health and quality of life among these young people, the researchers cautioned that some insurers require testosterone therapy for 1 year before their plans will cover the costs of gender-affirming surgery.
This may negatively affect those nonbinary patients who do not undergo hormone therapy,” the researchers wrote. They are currently collecting 1-year follow-up data to determine the long-term effects of top surgery on chest dysphoria, gender congruence, and body image.
As surgical patients progress through adult life, does the risk of regret increase? “We did not address regret in this short-term study,” Dr. Jordan said. “However, previous studies have shown very low levels of regret.”
An accompanying editorial concurred that top surgery is effective and medically necessary in this population of young people.
Calling the study “an important milestone in gender affirmation research,” Kishan M. Thadikonda, MD, and Katherine M. Gast, MD, MS, of the school of medicine and public health at the University of Wisconsin in Madison, said it will be important to follow this young cohort to prove these benefits will endure as patients age.
They cautioned, however, that nonbinary patients represented just 13% of the patient total and only 8% of the surgical cohort. Nonbinary patients are not well understood as a patient population when it comes to gender-affirmation surgery and are often included in studies with transgender patients despite clear differences, they noted.
Current setbacks
According to Dr. Cooper, politics is already affecting care in Texas. “Due to the sociopolitical climate in my state in regard to gender-affirming care, I have also seen a few young people have their surgeries either canceled or postponed by their parents,” he said. “This has led to a worsening of mental health in these patients.”
Dr. Cooper stressed the need for more research on the perspective of non-White and socioeconomically disadvantaged youth.
“This study also highlights the disparity between patients who have commercial insurance versus those who are on Medicaid,” he said. “Medicaid plans often do not cover this, so those patients usually have to continue to suffer or pay for this surgery out of their own pocket.”
This study was supported by the Northwestern University Clinical and Translational Sciences Institute, funded in part by the National Institutes of Health. Funding also came from the Plastic Surgery Foundation and American Association of Pediatric Plastic Surgery. Dr. Jordan received grants from the Plastic Surgery Foundation during the study. One coauthor reported consultant fees from CVS Caremark for consulting outside the submitted work, and another reported grants from the National Institutes of Health outside the submitted work. Dr. Cooper disclosed no competing interests relevant to his comments. The editorial commentators disclosed no conflicts of interest.
Adolescents and young adults who undergo “top surgery” for gender dysphoria overwhelmingly report being satisfied with the procedure in the near-term, new research shows.
The results of the prospective cohort study, reported recently in JAMA Pediatrics, suggest that the surgery can help facilitate gender congruence and comfort with body image for transmasculine and nonbinary youth. The authors, from Northwestern University, Chicago, said the findings may “help dispel misconceptions that gender-affirming treatment is experimental and support evidence-based practices of top surgery.”
Sumanas Jordan, MD, PhD, assistant professor of plastic surgery at Northwestern University, Chicago, and a coauthor of the study, said the study was the first prospective, matched cohort analysis showing that chest surgery improves outcomes in this age group.
“We focused our study on chest dysphoria, the distress due to the presence of breasts, and gender congruence, the feeling of alignment between identity and physical characteristics,” Dr. Jordan said. “We will continue to study the effect of surgery in other areas of health, such as physical functioning and quality of life, and follow our patients longer term.”
As many as 9% of adolescents and young adults identify as transgender or nonbinary - a group underrepresented in the pediatric literature, Dr. Jordan’s group said. Chest dysphoria often is associated with psychosocial issues such as depression and anxiety.
“Dysphoria can lead to a range of negative physical and emotional consequences, such as avoidance of exercise and sports, harmful chest-binding practices, functional limitations, and suicidal ideation, said M. Brett Cooper, MD, MEd, assistant professor of pediatrics, and adolescent and young adult medicine, at UT Southwestern Medical Center/Children’s Health, Dallas. “These young people often bind for several hours a day to reduce the presence of their chest.”
The study
The Northwestern team recruited 81 patients with a mean age of 18.6 years whose sex at birth was assigned female. Patients were overwhelmingly White (89%), and the majority (59%) were transgender male, the remaining patients nonbinary.
The population sample included patients aged 13-24 who underwent top surgery from December 2019 to April 2021 and a matched control group of those who did not have surgery.
Outcomes measures were assessed preoperatively and 3 months after surgery.
Thirty-six surgical patients and 34 of those in the control arm completed the outcomes measures. Surgical complications were minimal. Propensity analyses suggested an association between surgery and substantial improvements in scores on the following study endpoints:
- Chest dysphoria measure (–25.58 points, 95% confidence interval [CI], –29.18 to –21.98).
- Transgender congruence scale (7.78 points, 95%: CI, 6.06-9.50)
- Body image scale (–7.20 points, 95% CI, –11.68 to –2.72).
The patients who underwent top surgery reported significant improvements in scores of chest dysphoria, transgender congruence, and body image. The results for patients younger than age 18 paralleled those for older participants in the study.
While the results corroborate other studies showing that gender-affirming therapy improves mental health and quality of life among these young people, the researchers cautioned that some insurers require testosterone therapy for 1 year before their plans will cover the costs of gender-affirming surgery.
This may negatively affect those nonbinary patients who do not undergo hormone therapy,” the researchers wrote. They are currently collecting 1-year follow-up data to determine the long-term effects of top surgery on chest dysphoria, gender congruence, and body image.
As surgical patients progress through adult life, does the risk of regret increase? “We did not address regret in this short-term study,” Dr. Jordan said. “However, previous studies have shown very low levels of regret.”
An accompanying editorial concurred that top surgery is effective and medically necessary in this population of young people.
Calling the study “an important milestone in gender affirmation research,” Kishan M. Thadikonda, MD, and Katherine M. Gast, MD, MS, of the school of medicine and public health at the University of Wisconsin in Madison, said it will be important to follow this young cohort to prove these benefits will endure as patients age.
They cautioned, however, that nonbinary patients represented just 13% of the patient total and only 8% of the surgical cohort. Nonbinary patients are not well understood as a patient population when it comes to gender-affirmation surgery and are often included in studies with transgender patients despite clear differences, they noted.
Current setbacks
According to Dr. Cooper, politics is already affecting care in Texas. “Due to the sociopolitical climate in my state in regard to gender-affirming care, I have also seen a few young people have their surgeries either canceled or postponed by their parents,” he said. “This has led to a worsening of mental health in these patients.”
Dr. Cooper stressed the need for more research on the perspective of non-White and socioeconomically disadvantaged youth.
“This study also highlights the disparity between patients who have commercial insurance versus those who are on Medicaid,” he said. “Medicaid plans often do not cover this, so those patients usually have to continue to suffer or pay for this surgery out of their own pocket.”
This study was supported by the Northwestern University Clinical and Translational Sciences Institute, funded in part by the National Institutes of Health. Funding also came from the Plastic Surgery Foundation and American Association of Pediatric Plastic Surgery. Dr. Jordan received grants from the Plastic Surgery Foundation during the study. One coauthor reported consultant fees from CVS Caremark for consulting outside the submitted work, and another reported grants from the National Institutes of Health outside the submitted work. Dr. Cooper disclosed no competing interests relevant to his comments. The editorial commentators disclosed no conflicts of interest.
FROM JAMA PEDIATRICS
Postpartum sexual enjoyment: Does mode of delivery matter?
For some parents, resuming sexual intimacy after having a baby is a top priority. For others, not so much – and late-night feedings and diaper changes may not be the only hang-ups.
Dyspareunia – pain during sex – occurs in a substantial number of women after childbirth, and recent research sheds light on how psychological and biomedical factors relate to this condition.
Mode of delivery, for instance, may have less of an effect on sexual well-being than some people suspect.
Despite a perception that cesarean delivery might affect sexual function less than vaginal delivery does, how mothers delivered did not affect how often they had sex postpartum or the amount of enjoyment they got from it, according to research published in BJOG.
Eleven years after delivery, however, cesarean delivery was associated with a 74% increased likelihood of pain in the vagina during sex, compared with vaginal delivery, the researchers found (odds ratio, 1.74; 95% confidence interval, 1.46-2.08).
The results suggest that cesarean delivery “may not help protect against sexual dysfunction, as previously thought,” Flo Martin, a PhD student in epidemiology at the University of Bristol, United Kingdom, and lead author of the study, said in a news release.
For their study, Ms. Martin and her colleagues analyzed data from more than 10,300 participants in the Avon Longitudinal Study of Parents and Children, which recruited women in the United Kingdom who were pregnant in 1991 and 1992.
The researchers had data about pain during sex at 11 years. They had data about sexual enjoyment and frequency at 33 months, 5 years, 12 years, and 18 years after delivery.
If women experienced pain during sex years after cesarean delivery, uterine scarring might have been a cause, Ms. Martin and colleagues suggested. Alternatively, women with dyspareunia before delivery may be more likely to have cesarean surgery, which also could explain the association.
Other studies have likewise found that different modes of delivery generally lead to similar outcomes of sexual well-being after birth.
“Several of my own longitudinal studies have shown limited associations between mode of delivery and various aspects of sexual well-being, including sexual satisfaction, sexual function, and sexual desire,” said Natalie O. Rosen, PhD, director of the Couples and Sexual Health Laboratory at Dalhousie University, Halifax, N.S.
Nevertheless, other published studies have yielded conflicting results, so the question warrants further study, she said.
Pain catastrophizing
One study by Dr. Rosen’s group, published in Obstetrics & Gynecology, tracked sexual pain in 582 people from mid-pregnancy to 2 years postpartum.
About 21% of participants experienced moderate pain during sex, as determined by an average pain score greater than 4 on scale of 0-10 points. The rest were classified as having “minimal dyspareunia.”
Pain tended to peak at 3 months postpartum and then steadily decrease in both the moderate and minimal pain groups.
Mode of delivery did not affect the odds that a participant would have moderate dyspareunia. Neither did breastfeeding or prior chronic pain.
“But we did find one key thing to look out for: Those who reported a lot of negative thoughts and feelings about pain, something called pain catastrophizing, were more likely to experience moderate persistent pain during sex,” the researchers said in a video about their findings.
Pain catastrophizing 3 months after delivery was associated with significantly increased odds of following a moderate pain trajectory (odds ratio, 1.09; 95% confidence interval, 1.04-1.15).
Let’s talk about #postbabyhankypanky
Caring for a newborn while maintaining a romantic relationship can be challenging, and “there is a lack of evidence-based research aimed at helping couples prevent and navigate changes to their sexual well-being postpartum,” Dr. Rosen said.
During the 2-year study, a growing number of participants reported having sex less often over time. The percentage of women who had engaged in sexual activity in the past 4 weeks was 99% at baseline (20-24 weeks of gestation), 83.5% at 32 weeks of gestation, 73.9% at 3 months postpartum, and 69.6% at 2 years postpartum.
“One crucial way that couples sustain their connection is through their sexuality,” Dr. Rosen said. “Unfortunately, most new parents experience significant disruptions to their sexual function,” such as lower sexual desire or more pain during intercourse.
Dr. Rosen’s group has created a series of videos related to this topic dubbed #postbabyhankypanky to facilitate communication about sex postpartum. She encourages women with dyspareunia to talk with a health care provider because treatments such as cognitive-behavioral therapy, pelvic floor physical therapy, and topical medications can help manage pain.
‘Reassuring’ data
Veronica Gillispie-Bell, MD, MAS, director of quality for women’s services at the Ochsner Health System, New Orleans, said that she sees patients with postpartum sexual pain frequently.
Patients typically are instructed to have pelvic rest from delivery until 6 weeks after.
At the 6-week appointment, she tells patients to make sure that they are using lots of lubrication, because vaginal dryness related to hormonal changes during pregnancy and breastfeeding can make sex more painful, regardless of mode of delivery.
For many patients, she also recommends pelvic floor physical therapy.
As the medical director for the Louisiana Perinatal Quality Collaborative – a network of care providers, public health officials, and advocates that aims to improve outcomes for birthing persons, families, and newborns – Dr. Gillispie-Bell also is focused on reducing the rate of cesarean deliveries in the state. The BJOG study showing an increased risk for dyspareunia after a cesarean surgery serves as a reminder that there may be “long-term effects of having a C-section that may not be as obvious,” she said.
“C-sections are life-saving procedures, but they are not without risk,” Dr. Gillispie-Bell said.
Leila Frodsham, MBChB, a spokesperson for the Royal College of Obstetricians and Gynaecologists, told Medscape UK that it was “reassuring” to see “no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via cesarean section and those who delivered vaginally.”
“Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences,” Dr. Frodsham added.
Clinicians should also remain aware that sexual pain is also common during periods of subfertility, perimenopause, and initiation of sexual activity.
Combinations of biological, psychological, and social factors can influence pain during sex, and there is an interpersonal element to keep in mind as well, Dr. Rosen noted.
“Pain during sex is typically elicited in the context of a partnered relationship,” Dr. Rosen said. “This means that this is an inherently interpersonal issue – let’s not forget about the partner who is both impacted by and can impact the pain through their own responses.”
A version of this article first appeared on Medscape.com.
For some parents, resuming sexual intimacy after having a baby is a top priority. For others, not so much – and late-night feedings and diaper changes may not be the only hang-ups.
Dyspareunia – pain during sex – occurs in a substantial number of women after childbirth, and recent research sheds light on how psychological and biomedical factors relate to this condition.
Mode of delivery, for instance, may have less of an effect on sexual well-being than some people suspect.
Despite a perception that cesarean delivery might affect sexual function less than vaginal delivery does, how mothers delivered did not affect how often they had sex postpartum or the amount of enjoyment they got from it, according to research published in BJOG.
Eleven years after delivery, however, cesarean delivery was associated with a 74% increased likelihood of pain in the vagina during sex, compared with vaginal delivery, the researchers found (odds ratio, 1.74; 95% confidence interval, 1.46-2.08).
The results suggest that cesarean delivery “may not help protect against sexual dysfunction, as previously thought,” Flo Martin, a PhD student in epidemiology at the University of Bristol, United Kingdom, and lead author of the study, said in a news release.
For their study, Ms. Martin and her colleagues analyzed data from more than 10,300 participants in the Avon Longitudinal Study of Parents and Children, which recruited women in the United Kingdom who were pregnant in 1991 and 1992.
The researchers had data about pain during sex at 11 years. They had data about sexual enjoyment and frequency at 33 months, 5 years, 12 years, and 18 years after delivery.
If women experienced pain during sex years after cesarean delivery, uterine scarring might have been a cause, Ms. Martin and colleagues suggested. Alternatively, women with dyspareunia before delivery may be more likely to have cesarean surgery, which also could explain the association.
Other studies have likewise found that different modes of delivery generally lead to similar outcomes of sexual well-being after birth.
“Several of my own longitudinal studies have shown limited associations between mode of delivery and various aspects of sexual well-being, including sexual satisfaction, sexual function, and sexual desire,” said Natalie O. Rosen, PhD, director of the Couples and Sexual Health Laboratory at Dalhousie University, Halifax, N.S.
Nevertheless, other published studies have yielded conflicting results, so the question warrants further study, she said.
Pain catastrophizing
One study by Dr. Rosen’s group, published in Obstetrics & Gynecology, tracked sexual pain in 582 people from mid-pregnancy to 2 years postpartum.
About 21% of participants experienced moderate pain during sex, as determined by an average pain score greater than 4 on scale of 0-10 points. The rest were classified as having “minimal dyspareunia.”
Pain tended to peak at 3 months postpartum and then steadily decrease in both the moderate and minimal pain groups.
Mode of delivery did not affect the odds that a participant would have moderate dyspareunia. Neither did breastfeeding or prior chronic pain.
“But we did find one key thing to look out for: Those who reported a lot of negative thoughts and feelings about pain, something called pain catastrophizing, were more likely to experience moderate persistent pain during sex,” the researchers said in a video about their findings.
Pain catastrophizing 3 months after delivery was associated with significantly increased odds of following a moderate pain trajectory (odds ratio, 1.09; 95% confidence interval, 1.04-1.15).
Let’s talk about #postbabyhankypanky
Caring for a newborn while maintaining a romantic relationship can be challenging, and “there is a lack of evidence-based research aimed at helping couples prevent and navigate changes to their sexual well-being postpartum,” Dr. Rosen said.
During the 2-year study, a growing number of participants reported having sex less often over time. The percentage of women who had engaged in sexual activity in the past 4 weeks was 99% at baseline (20-24 weeks of gestation), 83.5% at 32 weeks of gestation, 73.9% at 3 months postpartum, and 69.6% at 2 years postpartum.
“One crucial way that couples sustain their connection is through their sexuality,” Dr. Rosen said. “Unfortunately, most new parents experience significant disruptions to their sexual function,” such as lower sexual desire or more pain during intercourse.
Dr. Rosen’s group has created a series of videos related to this topic dubbed #postbabyhankypanky to facilitate communication about sex postpartum. She encourages women with dyspareunia to talk with a health care provider because treatments such as cognitive-behavioral therapy, pelvic floor physical therapy, and topical medications can help manage pain.
‘Reassuring’ data
Veronica Gillispie-Bell, MD, MAS, director of quality for women’s services at the Ochsner Health System, New Orleans, said that she sees patients with postpartum sexual pain frequently.
Patients typically are instructed to have pelvic rest from delivery until 6 weeks after.
At the 6-week appointment, she tells patients to make sure that they are using lots of lubrication, because vaginal dryness related to hormonal changes during pregnancy and breastfeeding can make sex more painful, regardless of mode of delivery.
For many patients, she also recommends pelvic floor physical therapy.
As the medical director for the Louisiana Perinatal Quality Collaborative – a network of care providers, public health officials, and advocates that aims to improve outcomes for birthing persons, families, and newborns – Dr. Gillispie-Bell also is focused on reducing the rate of cesarean deliveries in the state. The BJOG study showing an increased risk for dyspareunia after a cesarean surgery serves as a reminder that there may be “long-term effects of having a C-section that may not be as obvious,” she said.
“C-sections are life-saving procedures, but they are not without risk,” Dr. Gillispie-Bell said.
Leila Frodsham, MBChB, a spokesperson for the Royal College of Obstetricians and Gynaecologists, told Medscape UK that it was “reassuring” to see “no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via cesarean section and those who delivered vaginally.”
“Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences,” Dr. Frodsham added.
Clinicians should also remain aware that sexual pain is also common during periods of subfertility, perimenopause, and initiation of sexual activity.
Combinations of biological, psychological, and social factors can influence pain during sex, and there is an interpersonal element to keep in mind as well, Dr. Rosen noted.
“Pain during sex is typically elicited in the context of a partnered relationship,” Dr. Rosen said. “This means that this is an inherently interpersonal issue – let’s not forget about the partner who is both impacted by and can impact the pain through their own responses.”
A version of this article first appeared on Medscape.com.
For some parents, resuming sexual intimacy after having a baby is a top priority. For others, not so much – and late-night feedings and diaper changes may not be the only hang-ups.
Dyspareunia – pain during sex – occurs in a substantial number of women after childbirth, and recent research sheds light on how psychological and biomedical factors relate to this condition.
Mode of delivery, for instance, may have less of an effect on sexual well-being than some people suspect.
Despite a perception that cesarean delivery might affect sexual function less than vaginal delivery does, how mothers delivered did not affect how often they had sex postpartum or the amount of enjoyment they got from it, according to research published in BJOG.
Eleven years after delivery, however, cesarean delivery was associated with a 74% increased likelihood of pain in the vagina during sex, compared with vaginal delivery, the researchers found (odds ratio, 1.74; 95% confidence interval, 1.46-2.08).
The results suggest that cesarean delivery “may not help protect against sexual dysfunction, as previously thought,” Flo Martin, a PhD student in epidemiology at the University of Bristol, United Kingdom, and lead author of the study, said in a news release.
For their study, Ms. Martin and her colleagues analyzed data from more than 10,300 participants in the Avon Longitudinal Study of Parents and Children, which recruited women in the United Kingdom who were pregnant in 1991 and 1992.
The researchers had data about pain during sex at 11 years. They had data about sexual enjoyment and frequency at 33 months, 5 years, 12 years, and 18 years after delivery.
If women experienced pain during sex years after cesarean delivery, uterine scarring might have been a cause, Ms. Martin and colleagues suggested. Alternatively, women with dyspareunia before delivery may be more likely to have cesarean surgery, which also could explain the association.
Other studies have likewise found that different modes of delivery generally lead to similar outcomes of sexual well-being after birth.
“Several of my own longitudinal studies have shown limited associations between mode of delivery and various aspects of sexual well-being, including sexual satisfaction, sexual function, and sexual desire,” said Natalie O. Rosen, PhD, director of the Couples and Sexual Health Laboratory at Dalhousie University, Halifax, N.S.
Nevertheless, other published studies have yielded conflicting results, so the question warrants further study, she said.
Pain catastrophizing
One study by Dr. Rosen’s group, published in Obstetrics & Gynecology, tracked sexual pain in 582 people from mid-pregnancy to 2 years postpartum.
About 21% of participants experienced moderate pain during sex, as determined by an average pain score greater than 4 on scale of 0-10 points. The rest were classified as having “minimal dyspareunia.”
Pain tended to peak at 3 months postpartum and then steadily decrease in both the moderate and minimal pain groups.
Mode of delivery did not affect the odds that a participant would have moderate dyspareunia. Neither did breastfeeding or prior chronic pain.
“But we did find one key thing to look out for: Those who reported a lot of negative thoughts and feelings about pain, something called pain catastrophizing, were more likely to experience moderate persistent pain during sex,” the researchers said in a video about their findings.
Pain catastrophizing 3 months after delivery was associated with significantly increased odds of following a moderate pain trajectory (odds ratio, 1.09; 95% confidence interval, 1.04-1.15).
Let’s talk about #postbabyhankypanky
Caring for a newborn while maintaining a romantic relationship can be challenging, and “there is a lack of evidence-based research aimed at helping couples prevent and navigate changes to their sexual well-being postpartum,” Dr. Rosen said.
During the 2-year study, a growing number of participants reported having sex less often over time. The percentage of women who had engaged in sexual activity in the past 4 weeks was 99% at baseline (20-24 weeks of gestation), 83.5% at 32 weeks of gestation, 73.9% at 3 months postpartum, and 69.6% at 2 years postpartum.
“One crucial way that couples sustain their connection is through their sexuality,” Dr. Rosen said. “Unfortunately, most new parents experience significant disruptions to their sexual function,” such as lower sexual desire or more pain during intercourse.
Dr. Rosen’s group has created a series of videos related to this topic dubbed #postbabyhankypanky to facilitate communication about sex postpartum. She encourages women with dyspareunia to talk with a health care provider because treatments such as cognitive-behavioral therapy, pelvic floor physical therapy, and topical medications can help manage pain.
‘Reassuring’ data
Veronica Gillispie-Bell, MD, MAS, director of quality for women’s services at the Ochsner Health System, New Orleans, said that she sees patients with postpartum sexual pain frequently.
Patients typically are instructed to have pelvic rest from delivery until 6 weeks after.
At the 6-week appointment, she tells patients to make sure that they are using lots of lubrication, because vaginal dryness related to hormonal changes during pregnancy and breastfeeding can make sex more painful, regardless of mode of delivery.
For many patients, she also recommends pelvic floor physical therapy.
As the medical director for the Louisiana Perinatal Quality Collaborative – a network of care providers, public health officials, and advocates that aims to improve outcomes for birthing persons, families, and newborns – Dr. Gillispie-Bell also is focused on reducing the rate of cesarean deliveries in the state. The BJOG study showing an increased risk for dyspareunia after a cesarean surgery serves as a reminder that there may be “long-term effects of having a C-section that may not be as obvious,” she said.
“C-sections are life-saving procedures, but they are not without risk,” Dr. Gillispie-Bell said.
Leila Frodsham, MBChB, a spokesperson for the Royal College of Obstetricians and Gynaecologists, told Medscape UK that it was “reassuring” to see “no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via cesarean section and those who delivered vaginally.”
“Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences,” Dr. Frodsham added.
Clinicians should also remain aware that sexual pain is also common during periods of subfertility, perimenopause, and initiation of sexual activity.
Combinations of biological, psychological, and social factors can influence pain during sex, and there is an interpersonal element to keep in mind as well, Dr. Rosen noted.
“Pain during sex is typically elicited in the context of a partnered relationship,” Dr. Rosen said. “This means that this is an inherently interpersonal issue – let’s not forget about the partner who is both impacted by and can impact the pain through their own responses.”
A version of this article first appeared on Medscape.com.
Surgical techniques for excision of juvenile cystic adenomyoma
‘Dr. Caveman’ had a leg up on amputation
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
Tips for navigating the altered retroperitoneum
Increased risk of dyspareunia following cesarean section
There is no evidence to support postulated associations between mode of delivery and subsequent maternal sexual enjoyment or frequency of intercourse, according to a new study from the University of Bristol (England). However, cesarean section was shown to be associated with a 74% increased risk of dyspareunia, and this was not necessarily due to abdominal scarring, the researchers said.
A team from the University of Bristol and the Karolinska Institutet in Sweden used data from participants in the Avon Longitudinal Study of Parents and Children, a prospective longitudinal birth cohort study also dubbed “Children of the 90s” and involving more than 14,000 women in the United Kingdom who were pregnant in 1991 and 1992. The study has been following the health and development of the parents, their children, and now their grandchildren in detail ever since.
The new study, published in BJOG, aimed to assess whether cesarean section maintains sexual well-being compared with vaginal delivery, as has been suggested to occur because of the reduced risk of genital damage – less chance of tearing – and the maintenance of vaginal tone. There is some evidence that cesarean section is associated with an increased risk of sexual problems such as dyspareunia, but few studies have looked at the postbirth period long term.
Mode of delivery was abstracted from routine obstetric records and recorded as one of either spontaneous vaginal delivery (SVD), cesarean section, assisted breech, breech extraction, forceps, or vacuum extraction. Women whose records showed “other” as mode of delivery or whose notes contained conflicting modes of delivery were excluded.
Self-reported questionnaires asking about general health and lifestyle and including questions relating to sexual enjoyment and frequency were collected at 33 months and at 5, 12, and 18 years postpartum. Women were asked if they enjoyed sexual intercourse, with possible responses of:
- Yes, very much.
- Yes, somewhat.
- No, not a lot.
- No, not at all.
- No sex at the moment.
Possible sexual frequency responses were:
- Not at all.
- Less than once a month.
- 1-3 times a month.
- About once a week.
- 2-4 times a week.
- 5 or more times a week.
First study to look at sexual frequency
The team noted that theirs is the first study investigating the association of mode of delivery with sexual frequency. “Although it may be less important for well-being than sexual enjoyment or sex-related pain, it is an important measure to observe alongside other sexual outcomes,” they said.
Separately, sex-related pain, in the vagina during sex or elsewhere after sex, was assessed once, at 11 years post partum.
The data showed that women who had a cesarean section (11% of the sample) tended to be older than those who had vaginal delivery, with a higher mean body mass index (24.2 versus 22.8 kg/m2), and were more likely to be nulliparous at the time of the index pregnancy (54% versus 44%).
There was no significant difference between cesarean section and vaginal delivery in terms of responses for sexual enjoyment or frequency at any time after childbirth, the authors said. Nor, in adjusted models, was there evidence of associations between the type of vaginal delivery and sexual enjoyment or frequency outcomes.
Pain during sex increased more than a decade after cesarean
However, while the majority of respondents reported no intercourse-related pain, those who delivered via cesarean were more likely than those who gave birth vaginally to report sex-related pain at 11 years post partum. This was specifically an elevated incidence of pain in the vagina during sex, with an odds ratio of 1.74 (95% confidence interval, 1.46-2.08) in the adjusted model. This finding was consistent for emergency and elective cesarean section separately – both types were associated with increased dyspareunia, compared with vaginal delivery.
The dataset did not include measures of individual prenatal sex-related pain and, therefore, “it is unknown from this study whether Caesarean section causes sex-related pain, as suggested by the findings, or whether prenatal sex-related pain predicts both Caesarean section and postnatal sex-related pain,” the researchers said.
“Longitudinal data on sex-related pain need to be collected both before and after parturition,” they recommend, to clarify the direction of a possible effect between cesarean section and dyspareunia.
Cesarean does not protect against sexual dysfunction
Meanwhile, “For women considering a planned Caesarean section in an uncomplicated pregnancy, evidence suggesting that Caesarean section may not protect against sexual dysfunction may help inform their decision-making in the antenatal period.”
Lead author Flo Martin, a PhD student in epidemiology at the University of Bristol, said: “Rates of Caesarean section have been rising over the last 20 years due to many contributing factors and, importantly, it has been suggested that Caesarean section maintains sexual wellbeing compared with vaginal delivery. It is crucial that a whole range of maternal and foetal outcomes following Caesarean section are investigated, including sexual wellbeing, to appropriately inform decision-making both pre- and postnatally.
“This research provides expectant mothers, as well as women who have given birth, with really important information and demonstrates that there was no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via Caesarean section and those who delivered vaginally. It also suggests that Caesarean section may not help protect against sexual dysfunction, as previously thought, where sex-related pain was higher among women who gave birth via Caesarean section more than 10 years postpartum.”
Asked to comment on the research, Dr. Leila Frodsham, consultant gynecologist and spokesperson for the Royal College of Obstetricians and Gynaecologists, told this news organization: “Sexual pain disorders affect 7.5% of women of all ages, but there are peaks: during the start of sexual activity, if subfertility is an issue, after childbirth, and in the peri/menopause. It can be up to three times more prevalent at these peak times.
“Many women with sexual pain are worried when they consider starting a family and request a Caesarean birth to reduce risk of worsening their pain. However, this study has demonstrated that a Caesarean birth is associated with increased sexual pain longer term, which is very useful for helping women to plan their births.
“While more research about postpartum sexual wellbeing is needed, the findings of this study are reassuring to those who are pregnant as it found no difference in the enjoyment or frequency of sex in the years after a vaginal or a Caesarean birth.
“Most women in the U.K. recover well whether they have a vaginal or a Caesarean birth. Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences.”
A version of this article first appeared on Medscape UK.
There is no evidence to support postulated associations between mode of delivery and subsequent maternal sexual enjoyment or frequency of intercourse, according to a new study from the University of Bristol (England). However, cesarean section was shown to be associated with a 74% increased risk of dyspareunia, and this was not necessarily due to abdominal scarring, the researchers said.
A team from the University of Bristol and the Karolinska Institutet in Sweden used data from participants in the Avon Longitudinal Study of Parents and Children, a prospective longitudinal birth cohort study also dubbed “Children of the 90s” and involving more than 14,000 women in the United Kingdom who were pregnant in 1991 and 1992. The study has been following the health and development of the parents, their children, and now their grandchildren in detail ever since.
The new study, published in BJOG, aimed to assess whether cesarean section maintains sexual well-being compared with vaginal delivery, as has been suggested to occur because of the reduced risk of genital damage – less chance of tearing – and the maintenance of vaginal tone. There is some evidence that cesarean section is associated with an increased risk of sexual problems such as dyspareunia, but few studies have looked at the postbirth period long term.
Mode of delivery was abstracted from routine obstetric records and recorded as one of either spontaneous vaginal delivery (SVD), cesarean section, assisted breech, breech extraction, forceps, or vacuum extraction. Women whose records showed “other” as mode of delivery or whose notes contained conflicting modes of delivery were excluded.
Self-reported questionnaires asking about general health and lifestyle and including questions relating to sexual enjoyment and frequency were collected at 33 months and at 5, 12, and 18 years postpartum. Women were asked if they enjoyed sexual intercourse, with possible responses of:
- Yes, very much.
- Yes, somewhat.
- No, not a lot.
- No, not at all.
- No sex at the moment.
Possible sexual frequency responses were:
- Not at all.
- Less than once a month.
- 1-3 times a month.
- About once a week.
- 2-4 times a week.
- 5 or more times a week.
First study to look at sexual frequency
The team noted that theirs is the first study investigating the association of mode of delivery with sexual frequency. “Although it may be less important for well-being than sexual enjoyment or sex-related pain, it is an important measure to observe alongside other sexual outcomes,” they said.
Separately, sex-related pain, in the vagina during sex or elsewhere after sex, was assessed once, at 11 years post partum.
The data showed that women who had a cesarean section (11% of the sample) tended to be older than those who had vaginal delivery, with a higher mean body mass index (24.2 versus 22.8 kg/m2), and were more likely to be nulliparous at the time of the index pregnancy (54% versus 44%).
There was no significant difference between cesarean section and vaginal delivery in terms of responses for sexual enjoyment or frequency at any time after childbirth, the authors said. Nor, in adjusted models, was there evidence of associations between the type of vaginal delivery and sexual enjoyment or frequency outcomes.
Pain during sex increased more than a decade after cesarean
However, while the majority of respondents reported no intercourse-related pain, those who delivered via cesarean were more likely than those who gave birth vaginally to report sex-related pain at 11 years post partum. This was specifically an elevated incidence of pain in the vagina during sex, with an odds ratio of 1.74 (95% confidence interval, 1.46-2.08) in the adjusted model. This finding was consistent for emergency and elective cesarean section separately – both types were associated with increased dyspareunia, compared with vaginal delivery.
The dataset did not include measures of individual prenatal sex-related pain and, therefore, “it is unknown from this study whether Caesarean section causes sex-related pain, as suggested by the findings, or whether prenatal sex-related pain predicts both Caesarean section and postnatal sex-related pain,” the researchers said.
“Longitudinal data on sex-related pain need to be collected both before and after parturition,” they recommend, to clarify the direction of a possible effect between cesarean section and dyspareunia.
Cesarean does not protect against sexual dysfunction
Meanwhile, “For women considering a planned Caesarean section in an uncomplicated pregnancy, evidence suggesting that Caesarean section may not protect against sexual dysfunction may help inform their decision-making in the antenatal period.”
Lead author Flo Martin, a PhD student in epidemiology at the University of Bristol, said: “Rates of Caesarean section have been rising over the last 20 years due to many contributing factors and, importantly, it has been suggested that Caesarean section maintains sexual wellbeing compared with vaginal delivery. It is crucial that a whole range of maternal and foetal outcomes following Caesarean section are investigated, including sexual wellbeing, to appropriately inform decision-making both pre- and postnatally.
“This research provides expectant mothers, as well as women who have given birth, with really important information and demonstrates that there was no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via Caesarean section and those who delivered vaginally. It also suggests that Caesarean section may not help protect against sexual dysfunction, as previously thought, where sex-related pain was higher among women who gave birth via Caesarean section more than 10 years postpartum.”
Asked to comment on the research, Dr. Leila Frodsham, consultant gynecologist and spokesperson for the Royal College of Obstetricians and Gynaecologists, told this news organization: “Sexual pain disorders affect 7.5% of women of all ages, but there are peaks: during the start of sexual activity, if subfertility is an issue, after childbirth, and in the peri/menopause. It can be up to three times more prevalent at these peak times.
“Many women with sexual pain are worried when they consider starting a family and request a Caesarean birth to reduce risk of worsening their pain. However, this study has demonstrated that a Caesarean birth is associated with increased sexual pain longer term, which is very useful for helping women to plan their births.
“While more research about postpartum sexual wellbeing is needed, the findings of this study are reassuring to those who are pregnant as it found no difference in the enjoyment or frequency of sex in the years after a vaginal or a Caesarean birth.
“Most women in the U.K. recover well whether they have a vaginal or a Caesarean birth. Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences.”
A version of this article first appeared on Medscape UK.
There is no evidence to support postulated associations between mode of delivery and subsequent maternal sexual enjoyment or frequency of intercourse, according to a new study from the University of Bristol (England). However, cesarean section was shown to be associated with a 74% increased risk of dyspareunia, and this was not necessarily due to abdominal scarring, the researchers said.
A team from the University of Bristol and the Karolinska Institutet in Sweden used data from participants in the Avon Longitudinal Study of Parents and Children, a prospective longitudinal birth cohort study also dubbed “Children of the 90s” and involving more than 14,000 women in the United Kingdom who were pregnant in 1991 and 1992. The study has been following the health and development of the parents, their children, and now their grandchildren in detail ever since.
The new study, published in BJOG, aimed to assess whether cesarean section maintains sexual well-being compared with vaginal delivery, as has been suggested to occur because of the reduced risk of genital damage – less chance of tearing – and the maintenance of vaginal tone. There is some evidence that cesarean section is associated with an increased risk of sexual problems such as dyspareunia, but few studies have looked at the postbirth period long term.
Mode of delivery was abstracted from routine obstetric records and recorded as one of either spontaneous vaginal delivery (SVD), cesarean section, assisted breech, breech extraction, forceps, or vacuum extraction. Women whose records showed “other” as mode of delivery or whose notes contained conflicting modes of delivery were excluded.
Self-reported questionnaires asking about general health and lifestyle and including questions relating to sexual enjoyment and frequency were collected at 33 months and at 5, 12, and 18 years postpartum. Women were asked if they enjoyed sexual intercourse, with possible responses of:
- Yes, very much.
- Yes, somewhat.
- No, not a lot.
- No, not at all.
- No sex at the moment.
Possible sexual frequency responses were:
- Not at all.
- Less than once a month.
- 1-3 times a month.
- About once a week.
- 2-4 times a week.
- 5 or more times a week.
First study to look at sexual frequency
The team noted that theirs is the first study investigating the association of mode of delivery with sexual frequency. “Although it may be less important for well-being than sexual enjoyment or sex-related pain, it is an important measure to observe alongside other sexual outcomes,” they said.
Separately, sex-related pain, in the vagina during sex or elsewhere after sex, was assessed once, at 11 years post partum.
The data showed that women who had a cesarean section (11% of the sample) tended to be older than those who had vaginal delivery, with a higher mean body mass index (24.2 versus 22.8 kg/m2), and were more likely to be nulliparous at the time of the index pregnancy (54% versus 44%).
There was no significant difference between cesarean section and vaginal delivery in terms of responses for sexual enjoyment or frequency at any time after childbirth, the authors said. Nor, in adjusted models, was there evidence of associations between the type of vaginal delivery and sexual enjoyment or frequency outcomes.
Pain during sex increased more than a decade after cesarean
However, while the majority of respondents reported no intercourse-related pain, those who delivered via cesarean were more likely than those who gave birth vaginally to report sex-related pain at 11 years post partum. This was specifically an elevated incidence of pain in the vagina during sex, with an odds ratio of 1.74 (95% confidence interval, 1.46-2.08) in the adjusted model. This finding was consistent for emergency and elective cesarean section separately – both types were associated with increased dyspareunia, compared with vaginal delivery.
The dataset did not include measures of individual prenatal sex-related pain and, therefore, “it is unknown from this study whether Caesarean section causes sex-related pain, as suggested by the findings, or whether prenatal sex-related pain predicts both Caesarean section and postnatal sex-related pain,” the researchers said.
“Longitudinal data on sex-related pain need to be collected both before and after parturition,” they recommend, to clarify the direction of a possible effect between cesarean section and dyspareunia.
Cesarean does not protect against sexual dysfunction
Meanwhile, “For women considering a planned Caesarean section in an uncomplicated pregnancy, evidence suggesting that Caesarean section may not protect against sexual dysfunction may help inform their decision-making in the antenatal period.”
Lead author Flo Martin, a PhD student in epidemiology at the University of Bristol, said: “Rates of Caesarean section have been rising over the last 20 years due to many contributing factors and, importantly, it has been suggested that Caesarean section maintains sexual wellbeing compared with vaginal delivery. It is crucial that a whole range of maternal and foetal outcomes following Caesarean section are investigated, including sexual wellbeing, to appropriately inform decision-making both pre- and postnatally.
“This research provides expectant mothers, as well as women who have given birth, with really important information and demonstrates that there was no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via Caesarean section and those who delivered vaginally. It also suggests that Caesarean section may not help protect against sexual dysfunction, as previously thought, where sex-related pain was higher among women who gave birth via Caesarean section more than 10 years postpartum.”
Asked to comment on the research, Dr. Leila Frodsham, consultant gynecologist and spokesperson for the Royal College of Obstetricians and Gynaecologists, told this news organization: “Sexual pain disorders affect 7.5% of women of all ages, but there are peaks: during the start of sexual activity, if subfertility is an issue, after childbirth, and in the peri/menopause. It can be up to three times more prevalent at these peak times.
“Many women with sexual pain are worried when they consider starting a family and request a Caesarean birth to reduce risk of worsening their pain. However, this study has demonstrated that a Caesarean birth is associated with increased sexual pain longer term, which is very useful for helping women to plan their births.
“While more research about postpartum sexual wellbeing is needed, the findings of this study are reassuring to those who are pregnant as it found no difference in the enjoyment or frequency of sex in the years after a vaginal or a Caesarean birth.
“Most women in the U.K. recover well whether they have a vaginal or a Caesarean birth. Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences.”
A version of this article first appeared on Medscape UK.