User login
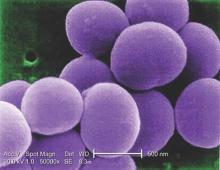
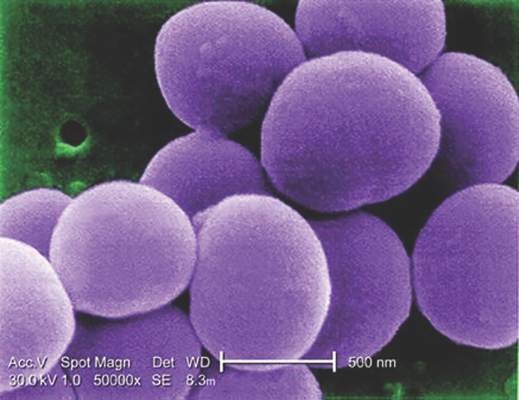
Glucocorticoids increase risk of S. aureus bacteremia
Use of systemic glucocorticoids significantly increased risk for community-acquired Staphylococcus aureus bacteremia (CA-SAB) in a dose-dependent fashion, based on data from a large Danish registry.
On average, current users of systemic glucocorticoids had an adjusted 2.5-fold increased risk of CA-SAB, compared with nonusers. The risk was most pronounced in long-term users of glucocorticoids, including patients with connective tissue disease and patients with chronic pulmonary disease. Among new users of glucocorticoids, the risk of CA-SAB was highest for patients with cancer, in the retrospective, case-control study published by Mayo Clinic Proceedings.
Dr. Jesper Smit of Aalborg (Denmark) University and his colleagues, looked at all 2,638 patients admitted with first-time CA-SAB and 26,379 matched population controls in Northern Denmark medical databases between January 1, 2000, and December 31, 2011.
New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Risk of CA-SAB rose in a dose-dependent fashion as 90-day cumulative doses increased. For subjects taking a cumulative dose of 150 mg or less, the adjusted OR for CA-SAB was 1.3. At a cumulative dose of 500-1000 mg, OR rose to 2.4. At a cumulative dose greater than 1000 mg, OR was 6.2.
Risk did not differ based on individuals’ sex, age group, or the severity of any comorbidity.
“This is the first study to specifically investigate whether the use of glucocorticoids is associated with increased risk of CA-SAB,” the authors concluded, adding that “these results extend the current knowledge of risk factors for CA-SAB and may serve as a reminder for clinicians to carefully weigh the elevated risk against the potential beneficial effect of glucocorticoid therapy, particularly in patients with concomitant CA-SAB risk factors.”
This study was supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
Use of systemic glucocorticoids significantly increased risk for community-acquired Staphylococcus aureus bacteremia (CA-SAB) in a dose-dependent fashion, based on data from a large Danish registry.
On average, current users of systemic glucocorticoids had an adjusted 2.5-fold increased risk of CA-SAB, compared with nonusers. The risk was most pronounced in long-term users of glucocorticoids, including patients with connective tissue disease and patients with chronic pulmonary disease. Among new users of glucocorticoids, the risk of CA-SAB was highest for patients with cancer, in the retrospective, case-control study published by Mayo Clinic Proceedings.
Dr. Jesper Smit of Aalborg (Denmark) University and his colleagues, looked at all 2,638 patients admitted with first-time CA-SAB and 26,379 matched population controls in Northern Denmark medical databases between January 1, 2000, and December 31, 2011.
New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Risk of CA-SAB rose in a dose-dependent fashion as 90-day cumulative doses increased. For subjects taking a cumulative dose of 150 mg or less, the adjusted OR for CA-SAB was 1.3. At a cumulative dose of 500-1000 mg, OR rose to 2.4. At a cumulative dose greater than 1000 mg, OR was 6.2.
Risk did not differ based on individuals’ sex, age group, or the severity of any comorbidity.
“This is the first study to specifically investigate whether the use of glucocorticoids is associated with increased risk of CA-SAB,” the authors concluded, adding that “these results extend the current knowledge of risk factors for CA-SAB and may serve as a reminder for clinicians to carefully weigh the elevated risk against the potential beneficial effect of glucocorticoid therapy, particularly in patients with concomitant CA-SAB risk factors.”
This study was supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
Use of systemic glucocorticoids significantly increased risk for community-acquired Staphylococcus aureus bacteremia (CA-SAB) in a dose-dependent fashion, based on data from a large Danish registry.
On average, current users of systemic glucocorticoids had an adjusted 2.5-fold increased risk of CA-SAB, compared with nonusers. The risk was most pronounced in long-term users of glucocorticoids, including patients with connective tissue disease and patients with chronic pulmonary disease. Among new users of glucocorticoids, the risk of CA-SAB was highest for patients with cancer, in the retrospective, case-control study published by Mayo Clinic Proceedings.
Dr. Jesper Smit of Aalborg (Denmark) University and his colleagues, looked at all 2,638 patients admitted with first-time CA-SAB and 26,379 matched population controls in Northern Denmark medical databases between January 1, 2000, and December 31, 2011.
New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Risk of CA-SAB rose in a dose-dependent fashion as 90-day cumulative doses increased. For subjects taking a cumulative dose of 150 mg or less, the adjusted OR for CA-SAB was 1.3. At a cumulative dose of 500-1000 mg, OR rose to 2.4. At a cumulative dose greater than 1000 mg, OR was 6.2.
Risk did not differ based on individuals’ sex, age group, or the severity of any comorbidity.
“This is the first study to specifically investigate whether the use of glucocorticoids is associated with increased risk of CA-SAB,” the authors concluded, adding that “these results extend the current knowledge of risk factors for CA-SAB and may serve as a reminder for clinicians to carefully weigh the elevated risk against the potential beneficial effect of glucocorticoid therapy, particularly in patients with concomitant CA-SAB risk factors.”
This study was supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
FROM MAYO CLINIC PROCEEDINGS
Key clinical point: Taking glucocorticoids can significantly increase the risk of contracting community-acquired Staphylococcus aureus bacteremia (CA-SAB).
Major finding: New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Data source: Retrospective, case-control study of all adults with first-time CA-SAB in Northern Denmark medical registries between 2000 and 2011.
Disclosures: Study supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
VIDEO: EULAR guidance on DMARD use in RA made ‘more concise’
LONDON – The European League Against Rheumatism guidelines on the use of disease-modifying antirheumatic drugs for the treatment of rheumatoid arthritis have been updated in line with current evidence and made more concise.
Dr. Josef S. Smolen of the department of rheumatology at the Medical University of Vienna who presented the 2016 guidelines at the European Congress of Rheumatology, noted that they now consist of 12 rather than the 14 recommendations that were included in the 2013 update (Ann Rheum Dis. 2014;73:492-509) and the 15 recommendations that were in the original 2010 version.
These 12 recommendations cover treatment targets and general approaches in the management of rheumatoid arthritis that incorporate disease-modifying antirheumatic drugs (DMARDs) and the use of glucocorticoids, and present treatment options as a hierarchy to help guide clinicians through appropriate procedures when initial and subsequent treatment fails. All DMARDs are considered in the recommendations, from the long-standing conventional synthetic (cs)DMARDs, such as methotrexate, sulfasalazine, and leflunomide, and the newer biologic DMARDs, such as the anti–tumor necrosis factor (TNF)–targeting drugs, to the newer biosimilar DMARDs, and targeted synthetic (ts)DMARDS, such as the Janus kinase (JAK) inhibitors tofacitinib and baricitinib.
The recommendations have been developed in accordance with EULAR’s standard operating procedure for the development of guidelines, Dr. Smolen observed, and involved three systematic literature reviews and expert opinion garnered from a task force of 50 experts and patients.
“This was the largest task force I have ever convened,” Dr. Smolen said, noting that rheumatologists from outside Europe had been invited to contribute their expertise and knowledge for the first time. Altogether 42 rheumatologists, three clinical fellows, two health professionals, and three patients were involved in revising the recommendations.
There are now four rather than three overarching principles, two of which are shared with early inflammatory arthritis recommendations that were also presented at the congress. The first two principles state that shared decision making is key to optimizing care and that rheumatologists should be the primary specialists looking after patients. The third principle recognizes the high burden that RA can have not only on an individual level but also on health care systems and society in general, which rheumatologists should be aware of. The fourth and final principle states that treatment decisions should be based on patients’ disease activity but that other factors, such as patients’ age, risk for progression, coexisting disease, and likely tolerance of treatment should also be kept in mind.
In an interview, Dr. Smolen highlighted that the EULAR recommendations cover three main phases of DMARD treatment: First is the DMARD-naive group of patients, who may be at an early or late stage of their disease. Second is the group in whom initial treatment has failed, and third is the group for whom subsequent treatment has not worked.
“In all these phases, we have some changes,” Dr. Smolen said. As an example, he noted that in the DMARD-naive setting, the use of csDMARDs has always been recommended but that the prior advice to consider combination csDMARD treatment has been edited out.
“We now say methotrexate should be part of the first treatment strategy, and the treatment strategy encompasses the use of additional, at least conventional synthetic, DMARDs.” Glucocorticoids are also more strongly recommended as part of the initial treatment strategy in combination with methotrexate, he said, although there is the proviso to use these for as short a time as possible.
In situations where patients do not respond to methotrexate plus glucocorticoids or they cannot tolerate methotrexate, then the recommendations advise stratifying patients into two groups. Those with poor prognostic factors might be switched to a biologic therapy, such as an anti-TNF agent or a tsDMARD. In regard to the latter, there is now more evidence behind the use of JAK inhibitors, notably tofacitinib, Dr. Smolen observed. Biologic DMARDs should be combined with csDMARDs, but if the latter is not tolerated then there is the option to use an IL-6 pathway inhibitor.
“There is now compelling evidence that all biologic DMARDs, including tocilizumab, convey better clinical, functional, and structural outcomes in combination with conventional synthetic DMARDs, especially methotrexate,” Dr. Smolen observed during his presentation of the recommendations. This may not be the case for the JAK inhibitors based on the current evidence.
When asked how the EULAR recommendations match up to those issued earlier this year by the American College of Rheumatology (Arthritis Care Res. 2016;68:1-25), Dr. Smolen observed that the two had become “much closer.” There remain differences in recommendations on glucocorticoid use, which are “somewhat clearer” in the European than in the American guidelines, and EULAR proposes combining biologic DMARDs with csDMARDs rather than using them as monotherapy. The EULAR recommendations also do not distinguish patients by disease duration but by treatment phase, and use prognostic factors for stratification.
The recommendations are currently in draft format and once finalized they will be published in Annals of the Rheumatic Diseases and also made freely available via the EULAR website, joining the organization’s many other recommendations for the management of rheumatic diseases. Dr. Smolen noted that these are intended as a template to provide national societies, health systems, and regulatory bodies a guide to the best evidence-based use of DMARDS in RA throughout Europe.
Dr. Smolen has received grant support and/or honoraria for consultations and/or for presentations from: AbbVie, Amgen, AstraZeneca, Astro-Pharma, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, ILTOO Pharma, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis-Sandoz, Pfizer, Roche-Chugai, Samsung, and UCB.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – The European League Against Rheumatism guidelines on the use of disease-modifying antirheumatic drugs for the treatment of rheumatoid arthritis have been updated in line with current evidence and made more concise.
Dr. Josef S. Smolen of the department of rheumatology at the Medical University of Vienna who presented the 2016 guidelines at the European Congress of Rheumatology, noted that they now consist of 12 rather than the 14 recommendations that were included in the 2013 update (Ann Rheum Dis. 2014;73:492-509) and the 15 recommendations that were in the original 2010 version.
These 12 recommendations cover treatment targets and general approaches in the management of rheumatoid arthritis that incorporate disease-modifying antirheumatic drugs (DMARDs) and the use of glucocorticoids, and present treatment options as a hierarchy to help guide clinicians through appropriate procedures when initial and subsequent treatment fails. All DMARDs are considered in the recommendations, from the long-standing conventional synthetic (cs)DMARDs, such as methotrexate, sulfasalazine, and leflunomide, and the newer biologic DMARDs, such as the anti–tumor necrosis factor (TNF)–targeting drugs, to the newer biosimilar DMARDs, and targeted synthetic (ts)DMARDS, such as the Janus kinase (JAK) inhibitors tofacitinib and baricitinib.
The recommendations have been developed in accordance with EULAR’s standard operating procedure for the development of guidelines, Dr. Smolen observed, and involved three systematic literature reviews and expert opinion garnered from a task force of 50 experts and patients.
“This was the largest task force I have ever convened,” Dr. Smolen said, noting that rheumatologists from outside Europe had been invited to contribute their expertise and knowledge for the first time. Altogether 42 rheumatologists, three clinical fellows, two health professionals, and three patients were involved in revising the recommendations.
There are now four rather than three overarching principles, two of which are shared with early inflammatory arthritis recommendations that were also presented at the congress. The first two principles state that shared decision making is key to optimizing care and that rheumatologists should be the primary specialists looking after patients. The third principle recognizes the high burden that RA can have not only on an individual level but also on health care systems and society in general, which rheumatologists should be aware of. The fourth and final principle states that treatment decisions should be based on patients’ disease activity but that other factors, such as patients’ age, risk for progression, coexisting disease, and likely tolerance of treatment should also be kept in mind.
In an interview, Dr. Smolen highlighted that the EULAR recommendations cover three main phases of DMARD treatment: First is the DMARD-naive group of patients, who may be at an early or late stage of their disease. Second is the group in whom initial treatment has failed, and third is the group for whom subsequent treatment has not worked.
“In all these phases, we have some changes,” Dr. Smolen said. As an example, he noted that in the DMARD-naive setting, the use of csDMARDs has always been recommended but that the prior advice to consider combination csDMARD treatment has been edited out.
“We now say methotrexate should be part of the first treatment strategy, and the treatment strategy encompasses the use of additional, at least conventional synthetic, DMARDs.” Glucocorticoids are also more strongly recommended as part of the initial treatment strategy in combination with methotrexate, he said, although there is the proviso to use these for as short a time as possible.
In situations where patients do not respond to methotrexate plus glucocorticoids or they cannot tolerate methotrexate, then the recommendations advise stratifying patients into two groups. Those with poor prognostic factors might be switched to a biologic therapy, such as an anti-TNF agent or a tsDMARD. In regard to the latter, there is now more evidence behind the use of JAK inhibitors, notably tofacitinib, Dr. Smolen observed. Biologic DMARDs should be combined with csDMARDs, but if the latter is not tolerated then there is the option to use an IL-6 pathway inhibitor.
“There is now compelling evidence that all biologic DMARDs, including tocilizumab, convey better clinical, functional, and structural outcomes in combination with conventional synthetic DMARDs, especially methotrexate,” Dr. Smolen observed during his presentation of the recommendations. This may not be the case for the JAK inhibitors based on the current evidence.
When asked how the EULAR recommendations match up to those issued earlier this year by the American College of Rheumatology (Arthritis Care Res. 2016;68:1-25), Dr. Smolen observed that the two had become “much closer.” There remain differences in recommendations on glucocorticoid use, which are “somewhat clearer” in the European than in the American guidelines, and EULAR proposes combining biologic DMARDs with csDMARDs rather than using them as monotherapy. The EULAR recommendations also do not distinguish patients by disease duration but by treatment phase, and use prognostic factors for stratification.
The recommendations are currently in draft format and once finalized they will be published in Annals of the Rheumatic Diseases and also made freely available via the EULAR website, joining the organization’s many other recommendations for the management of rheumatic diseases. Dr. Smolen noted that these are intended as a template to provide national societies, health systems, and regulatory bodies a guide to the best evidence-based use of DMARDS in RA throughout Europe.
Dr. Smolen has received grant support and/or honoraria for consultations and/or for presentations from: AbbVie, Amgen, AstraZeneca, Astro-Pharma, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, ILTOO Pharma, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis-Sandoz, Pfizer, Roche-Chugai, Samsung, and UCB.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – The European League Against Rheumatism guidelines on the use of disease-modifying antirheumatic drugs for the treatment of rheumatoid arthritis have been updated in line with current evidence and made more concise.
Dr. Josef S. Smolen of the department of rheumatology at the Medical University of Vienna who presented the 2016 guidelines at the European Congress of Rheumatology, noted that they now consist of 12 rather than the 14 recommendations that were included in the 2013 update (Ann Rheum Dis. 2014;73:492-509) and the 15 recommendations that were in the original 2010 version.
These 12 recommendations cover treatment targets and general approaches in the management of rheumatoid arthritis that incorporate disease-modifying antirheumatic drugs (DMARDs) and the use of glucocorticoids, and present treatment options as a hierarchy to help guide clinicians through appropriate procedures when initial and subsequent treatment fails. All DMARDs are considered in the recommendations, from the long-standing conventional synthetic (cs)DMARDs, such as methotrexate, sulfasalazine, and leflunomide, and the newer biologic DMARDs, such as the anti–tumor necrosis factor (TNF)–targeting drugs, to the newer biosimilar DMARDs, and targeted synthetic (ts)DMARDS, such as the Janus kinase (JAK) inhibitors tofacitinib and baricitinib.
The recommendations have been developed in accordance with EULAR’s standard operating procedure for the development of guidelines, Dr. Smolen observed, and involved three systematic literature reviews and expert opinion garnered from a task force of 50 experts and patients.
“This was the largest task force I have ever convened,” Dr. Smolen said, noting that rheumatologists from outside Europe had been invited to contribute their expertise and knowledge for the first time. Altogether 42 rheumatologists, three clinical fellows, two health professionals, and three patients were involved in revising the recommendations.
There are now four rather than three overarching principles, two of which are shared with early inflammatory arthritis recommendations that were also presented at the congress. The first two principles state that shared decision making is key to optimizing care and that rheumatologists should be the primary specialists looking after patients. The third principle recognizes the high burden that RA can have not only on an individual level but also on health care systems and society in general, which rheumatologists should be aware of. The fourth and final principle states that treatment decisions should be based on patients’ disease activity but that other factors, such as patients’ age, risk for progression, coexisting disease, and likely tolerance of treatment should also be kept in mind.
In an interview, Dr. Smolen highlighted that the EULAR recommendations cover three main phases of DMARD treatment: First is the DMARD-naive group of patients, who may be at an early or late stage of their disease. Second is the group in whom initial treatment has failed, and third is the group for whom subsequent treatment has not worked.
“In all these phases, we have some changes,” Dr. Smolen said. As an example, he noted that in the DMARD-naive setting, the use of csDMARDs has always been recommended but that the prior advice to consider combination csDMARD treatment has been edited out.
“We now say methotrexate should be part of the first treatment strategy, and the treatment strategy encompasses the use of additional, at least conventional synthetic, DMARDs.” Glucocorticoids are also more strongly recommended as part of the initial treatment strategy in combination with methotrexate, he said, although there is the proviso to use these for as short a time as possible.
In situations where patients do not respond to methotrexate plus glucocorticoids or they cannot tolerate methotrexate, then the recommendations advise stratifying patients into two groups. Those with poor prognostic factors might be switched to a biologic therapy, such as an anti-TNF agent or a tsDMARD. In regard to the latter, there is now more evidence behind the use of JAK inhibitors, notably tofacitinib, Dr. Smolen observed. Biologic DMARDs should be combined with csDMARDs, but if the latter is not tolerated then there is the option to use an IL-6 pathway inhibitor.
“There is now compelling evidence that all biologic DMARDs, including tocilizumab, convey better clinical, functional, and structural outcomes in combination with conventional synthetic DMARDs, especially methotrexate,” Dr. Smolen observed during his presentation of the recommendations. This may not be the case for the JAK inhibitors based on the current evidence.
When asked how the EULAR recommendations match up to those issued earlier this year by the American College of Rheumatology (Arthritis Care Res. 2016;68:1-25), Dr. Smolen observed that the two had become “much closer.” There remain differences in recommendations on glucocorticoid use, which are “somewhat clearer” in the European than in the American guidelines, and EULAR proposes combining biologic DMARDs with csDMARDs rather than using them as monotherapy. The EULAR recommendations also do not distinguish patients by disease duration but by treatment phase, and use prognostic factors for stratification.
The recommendations are currently in draft format and once finalized they will be published in Annals of the Rheumatic Diseases and also made freely available via the EULAR website, joining the organization’s many other recommendations for the management of rheumatic diseases. Dr. Smolen noted that these are intended as a template to provide national societies, health systems, and regulatory bodies a guide to the best evidence-based use of DMARDS in RA throughout Europe.
Dr. Smolen has received grant support and/or honoraria for consultations and/or for presentations from: AbbVie, Amgen, AstraZeneca, Astro-Pharma, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, ILTOO Pharma, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis-Sandoz, Pfizer, Roche-Chugai, Samsung, and UCB.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2016 CONGRESS
Simplify cardiac risk assessment for rheumatologic conditions
LONDON – Cardiovascular disease (CVD) risk assessment for patients with rheumatic diseases can be simple and integrated into general practice or rheumatology clinics, experts said during an Outcomes Science Session at the European Congress of Rheumatology
Patients with rheumatoid arthritis have a 50% higher risk of heart disease than do their counterparts without the disease, but “just having RA on its own isn’t sufficient to render that individual at high risk,” Dr. Naveed Sattar, professor of metabolic medicine at the University of Glasgow’s Institute of Cardiovascular and Medical Sciences in Scotland, said in an interview.
It’s simple enough to use traditional CVD risk factors in an RA population by including a patient’s age, gender, smoking status, and family history of heart disease, in addition to measuring blood pressure and blood lipid levels. Most risk scores will compile those features into a 10-year risk of a fatal CVD event. To account for the contribution of RA, Dr. Sattar said, simply multiply that score by 1.5.
While “there’s a fixation in some parts of Europe for [measuring] fasting lipids,” it is not necessary, Dr. Sattar said. The two lipid parameters that go in risk scores tend to be cholesterol and HDL cholesterol, he said, which change only minimally in fasting versus nonfasting states.
“The evidence overwhelmingly shows that nonfasting lipids, which can be done easily on the same sample as other clinic tests, are just as predictive of CVD risk as fasting lipids,” he said. “That really matters because many of our patients with RA or other conditions come to the hospital when they’re not fasting, and we shouldn’t be sending them away to come back fasting to do risk scores for CVD. That just doesn’t make sense.”
Updated guidelines from the European Society of Cardiology and guidelines soon to be released from the European League Against Rheumatism suggest that risk scores can be calculated every 5 years for most patients, a change from previous recommendations to calculate risk annually. Risk scoring is not perfect, however, and there is some debate about whether additional blood tests or ultrasound scanning of the carotid artery could augment the ability to predict heart disease risk. “We’re not quite there yet,” Dr. Sattar said. “I think we should do the simple things first and do them well.”
CV risk raised in all inflammatory arthritic diseases
During the same session, Dr. Paola de Pablo of the University of Birmingham, England, focused on how immune-mediated diseases predispose to premature, accelerated atherosclerosis and subsequent increased cardiovascular morbidity and mortality.
Cardiovascular risk is not only elevated in those with RA, she observed, but also in those with systemic lupus erythematosus, ankylosing spondylitis, psoriatic arthritis, vasculitides, and inflammatory myopathies. The risk varies but as a rule is more than 50% higher than the rate seen in the general population.
The underlying mechanisms are not clear, but chronic inflammation is closely linked with atherosclerosis, which in turn ups the risk for myocardial infarction and cerebrovascular accident.
Despite treatment, the risk often remains, Dr. de Pablo said. She highlighted how treatment with methotrexate and anti–tumor necrosis factor (TNF)–alpha drugs in RA had been associated with a reduction in the risk for heart attack of 20% and 40%, respectively (Ann Rheum Dis. 2014;74:480–89) so targeting inflammation with these drugs may have positive effects, at least in RA.
Managing traditional cardiovascular risk factors remains important, Dr. de Pablo said. That was a sentiment echoed by Dr. Sattar and by rheumatologist Dr. Michael Nurmohamed of the VU Medical Center in Amsterdam. This includes controlling blood pressure with antihypertensive medications and blood lipids with statins, and advocating smoking cessation and perhaps other appropriate lifestyle changes such as increasing physical exercise and controlling weight.
Dr. Nurmohamed, who was involved in the 2015 update of the EULAR recommendations on cardiovascular risk management, noted that traditional risk factor management in patients with arthritis in current clinical practice is often poor and that strategies to address this were urgently needed.
Although treating to-target and preventing disease flares in the rheumatic diseases is important, it lowers but does not normalize cardiovascular risk. “This appears to be irrespective of the drug used,” Dr. Nurmohamed said. Rheumatologists need to be careful when tapering medication, particularly the biologics, as these are perhaps helping to temper cardiovascular inflammation, which could worsen when doses are reduced. “Antirheumatic treatment only is not good enough to decrease or normalize the cardiovascular risk of our patients”, he emphasized.
Norwegian project shows how to integrate CVD assessment into routine practice
In a separate presentation, Dr. Eirik Ikdahl, a PhD student at Diakonhjemmet Hospital in Oslo, discussed how some rheumatology clinics in Norway are successfully incorporating CVD risk screening.
Through the Norwegian Collaboration on Atherosclerotic disease in patients with Rheumatic joint diseases (NOCAR), which started in April 2014, annual cardiovascular disease risk evaluations of patients with inflammatory joint diseases are being implemented into the practices of 11 rheumatology outpatient clinics. While waiting for clinic appointments, patients are given electronic devices through which they can report CVD risk factors via an electronic patient journal program called GoTreatIt Rheuma. From there, the clinic can order nonfasting lipid measurements and nurses can record patients’ blood pressure.
Then, using the ESC Systematic Coronary Risk Evaluation (SCORE) algorithm, the program automatically calculates a patient’s 10-year risk of a fatal CVD event. If the SCORE estimate is 5% or greater, the rheumatologist forwards a note to the patient’s primary care physician or cardiologist saying there is an indication for initiation of CVD-preventive measures such as medication or lifestyle changes. Rheumatologists and rheumatology nurses also deliver brief advice regarding smoking cessation and healthy diet.
“The main aim of the project is to raise awareness of the cardiovascular burden that these patients experience, and to ensure that patients with inflammatory joint diseases receive guideline-recommended cardiovascular preventive treatment,” Dr. Ikdahl said.
Of 6,150 patients defined as eligible for the NOCAR project in three of the centers, 41% (n = 2,519) received a CVD risk assessment during the first year and a half of the program, officers found in a recent review. Of those, 1,569 had RA, 418 had ankylosing spondylitis, 350 had psoriatic arthritis, and 122 had other spondyloarthritides.
Through the program, “a large number of high-risk patients have received screening that they would not otherwise have been offered,” Dr. Ikdahl said.
The major obstacles to successful implementation were time scarcity, defining a date for annual CVD risk assessment among patients who visit the clinics multiple times per year, and making sure lipids were measured before seeing the rheumatologist, he said. “We acknowledge there is room for improvement. It is challenging to implement new work tasks in an already busy rheumatology outpatient clinic, and since the project does not offer financial incentives to the participating centers, we rely on a collective effort and voluntary work based on resources already available.”
Remember CVD, but don’t forget other comorbidities
Other research presented by Dr. Laure Gossec, professor of rheumatology at Pitie-Salpétriere Hospital and Pierre & Marie Curie University in Paris highlighted the importance of identifying all comorbidities and their risk factors in patients with rheumatic diseases, and not just cardiovascular disease.
Dr. Gossec presented the results of an initiative aiming to make the collection and management of comorbidities easier in routine rheumatologic practice. The aim was to develop a simple, more pragmatic form that could be used to help rheumatologists manage selected comorbidities, and know when to refer for other specialist assessment. The focus was on ischemic cardiovascular disease, malignancies, infections such as chronic bronchitis, gastrointestinal disease such as diverticulitis, osteoporosis, and depression.
A committee of 18 experts, both physicians and nurses, was convened to examine the results of a systematic literature review of recommendations on comorbidity management and come up with concise recommendations for rheumatologists. Each of their recommendations covered whether or not the comorbidity was present (yes/no/don’t know) and if screening had been undertaken, such as measurement of blood lipids, and when this had occurred if known. There was then guidance on how to interpret these findings, calculate risk, and what to do if findings were abnormal.
The project is ongoing, and so far the expert panel has developed a pragmatic document with forms to help collect, report, and manage each specific comorbidity and its known risk factors. But it is still perhaps too long to be feasibly used in everyday practice, Dr. Gossec conceded. So the aim is to create a short, 2-page form that could summarize the recommendations briefly, and also develop a questionnaire for the patient to fill out and understand how to self-manage some comorbidities.
“We feel that this is a way to disseminate and adapt to the national context for France the EULAR comorbidities initiative,” Dr. Gossec said. “It also defines exactly what rheumatologists should be doing and when they should refer, hopefully to the benefit of our patients.”
Dr. Sattar has participated in advisory boards for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lily and UCB. He has also consulted for Merck and is a member of Roche’s speakers’ bureau. Dr. de Paolo and Dr. Nurmohamed reported no relevant financial disclosures. Dr. Ikdahl has received speaker’s honoraria from Pfizer. Dr. Gossec and coauthors have received honoraria from Abbvie France.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Cardiovascular disease (CVD) risk assessment for patients with rheumatic diseases can be simple and integrated into general practice or rheumatology clinics, experts said during an Outcomes Science Session at the European Congress of Rheumatology
Patients with rheumatoid arthritis have a 50% higher risk of heart disease than do their counterparts without the disease, but “just having RA on its own isn’t sufficient to render that individual at high risk,” Dr. Naveed Sattar, professor of metabolic medicine at the University of Glasgow’s Institute of Cardiovascular and Medical Sciences in Scotland, said in an interview.
It’s simple enough to use traditional CVD risk factors in an RA population by including a patient’s age, gender, smoking status, and family history of heart disease, in addition to measuring blood pressure and blood lipid levels. Most risk scores will compile those features into a 10-year risk of a fatal CVD event. To account for the contribution of RA, Dr. Sattar said, simply multiply that score by 1.5.
While “there’s a fixation in some parts of Europe for [measuring] fasting lipids,” it is not necessary, Dr. Sattar said. The two lipid parameters that go in risk scores tend to be cholesterol and HDL cholesterol, he said, which change only minimally in fasting versus nonfasting states.
“The evidence overwhelmingly shows that nonfasting lipids, which can be done easily on the same sample as other clinic tests, are just as predictive of CVD risk as fasting lipids,” he said. “That really matters because many of our patients with RA or other conditions come to the hospital when they’re not fasting, and we shouldn’t be sending them away to come back fasting to do risk scores for CVD. That just doesn’t make sense.”
Updated guidelines from the European Society of Cardiology and guidelines soon to be released from the European League Against Rheumatism suggest that risk scores can be calculated every 5 years for most patients, a change from previous recommendations to calculate risk annually. Risk scoring is not perfect, however, and there is some debate about whether additional blood tests or ultrasound scanning of the carotid artery could augment the ability to predict heart disease risk. “We’re not quite there yet,” Dr. Sattar said. “I think we should do the simple things first and do them well.”
CV risk raised in all inflammatory arthritic diseases
During the same session, Dr. Paola de Pablo of the University of Birmingham, England, focused on how immune-mediated diseases predispose to premature, accelerated atherosclerosis and subsequent increased cardiovascular morbidity and mortality.
Cardiovascular risk is not only elevated in those with RA, she observed, but also in those with systemic lupus erythematosus, ankylosing spondylitis, psoriatic arthritis, vasculitides, and inflammatory myopathies. The risk varies but as a rule is more than 50% higher than the rate seen in the general population.
The underlying mechanisms are not clear, but chronic inflammation is closely linked with atherosclerosis, which in turn ups the risk for myocardial infarction and cerebrovascular accident.
Despite treatment, the risk often remains, Dr. de Pablo said. She highlighted how treatment with methotrexate and anti–tumor necrosis factor (TNF)–alpha drugs in RA had been associated with a reduction in the risk for heart attack of 20% and 40%, respectively (Ann Rheum Dis. 2014;74:480–89) so targeting inflammation with these drugs may have positive effects, at least in RA.
Managing traditional cardiovascular risk factors remains important, Dr. de Pablo said. That was a sentiment echoed by Dr. Sattar and by rheumatologist Dr. Michael Nurmohamed of the VU Medical Center in Amsterdam. This includes controlling blood pressure with antihypertensive medications and blood lipids with statins, and advocating smoking cessation and perhaps other appropriate lifestyle changes such as increasing physical exercise and controlling weight.
Dr. Nurmohamed, who was involved in the 2015 update of the EULAR recommendations on cardiovascular risk management, noted that traditional risk factor management in patients with arthritis in current clinical practice is often poor and that strategies to address this were urgently needed.
Although treating to-target and preventing disease flares in the rheumatic diseases is important, it lowers but does not normalize cardiovascular risk. “This appears to be irrespective of the drug used,” Dr. Nurmohamed said. Rheumatologists need to be careful when tapering medication, particularly the biologics, as these are perhaps helping to temper cardiovascular inflammation, which could worsen when doses are reduced. “Antirheumatic treatment only is not good enough to decrease or normalize the cardiovascular risk of our patients”, he emphasized.
Norwegian project shows how to integrate CVD assessment into routine practice
In a separate presentation, Dr. Eirik Ikdahl, a PhD student at Diakonhjemmet Hospital in Oslo, discussed how some rheumatology clinics in Norway are successfully incorporating CVD risk screening.
Through the Norwegian Collaboration on Atherosclerotic disease in patients with Rheumatic joint diseases (NOCAR), which started in April 2014, annual cardiovascular disease risk evaluations of patients with inflammatory joint diseases are being implemented into the practices of 11 rheumatology outpatient clinics. While waiting for clinic appointments, patients are given electronic devices through which they can report CVD risk factors via an electronic patient journal program called GoTreatIt Rheuma. From there, the clinic can order nonfasting lipid measurements and nurses can record patients’ blood pressure.
Then, using the ESC Systematic Coronary Risk Evaluation (SCORE) algorithm, the program automatically calculates a patient’s 10-year risk of a fatal CVD event. If the SCORE estimate is 5% or greater, the rheumatologist forwards a note to the patient’s primary care physician or cardiologist saying there is an indication for initiation of CVD-preventive measures such as medication or lifestyle changes. Rheumatologists and rheumatology nurses also deliver brief advice regarding smoking cessation and healthy diet.
“The main aim of the project is to raise awareness of the cardiovascular burden that these patients experience, and to ensure that patients with inflammatory joint diseases receive guideline-recommended cardiovascular preventive treatment,” Dr. Ikdahl said.
Of 6,150 patients defined as eligible for the NOCAR project in three of the centers, 41% (n = 2,519) received a CVD risk assessment during the first year and a half of the program, officers found in a recent review. Of those, 1,569 had RA, 418 had ankylosing spondylitis, 350 had psoriatic arthritis, and 122 had other spondyloarthritides.
Through the program, “a large number of high-risk patients have received screening that they would not otherwise have been offered,” Dr. Ikdahl said.
The major obstacles to successful implementation were time scarcity, defining a date for annual CVD risk assessment among patients who visit the clinics multiple times per year, and making sure lipids were measured before seeing the rheumatologist, he said. “We acknowledge there is room for improvement. It is challenging to implement new work tasks in an already busy rheumatology outpatient clinic, and since the project does not offer financial incentives to the participating centers, we rely on a collective effort and voluntary work based on resources already available.”
Remember CVD, but don’t forget other comorbidities
Other research presented by Dr. Laure Gossec, professor of rheumatology at Pitie-Salpétriere Hospital and Pierre & Marie Curie University in Paris highlighted the importance of identifying all comorbidities and their risk factors in patients with rheumatic diseases, and not just cardiovascular disease.
Dr. Gossec presented the results of an initiative aiming to make the collection and management of comorbidities easier in routine rheumatologic practice. The aim was to develop a simple, more pragmatic form that could be used to help rheumatologists manage selected comorbidities, and know when to refer for other specialist assessment. The focus was on ischemic cardiovascular disease, malignancies, infections such as chronic bronchitis, gastrointestinal disease such as diverticulitis, osteoporosis, and depression.
A committee of 18 experts, both physicians and nurses, was convened to examine the results of a systematic literature review of recommendations on comorbidity management and come up with concise recommendations for rheumatologists. Each of their recommendations covered whether or not the comorbidity was present (yes/no/don’t know) and if screening had been undertaken, such as measurement of blood lipids, and when this had occurred if known. There was then guidance on how to interpret these findings, calculate risk, and what to do if findings were abnormal.
The project is ongoing, and so far the expert panel has developed a pragmatic document with forms to help collect, report, and manage each specific comorbidity and its known risk factors. But it is still perhaps too long to be feasibly used in everyday practice, Dr. Gossec conceded. So the aim is to create a short, 2-page form that could summarize the recommendations briefly, and also develop a questionnaire for the patient to fill out and understand how to self-manage some comorbidities.
“We feel that this is a way to disseminate and adapt to the national context for France the EULAR comorbidities initiative,” Dr. Gossec said. “It also defines exactly what rheumatologists should be doing and when they should refer, hopefully to the benefit of our patients.”
Dr. Sattar has participated in advisory boards for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lily and UCB. He has also consulted for Merck and is a member of Roche’s speakers’ bureau. Dr. de Paolo and Dr. Nurmohamed reported no relevant financial disclosures. Dr. Ikdahl has received speaker’s honoraria from Pfizer. Dr. Gossec and coauthors have received honoraria from Abbvie France.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Cardiovascular disease (CVD) risk assessment for patients with rheumatic diseases can be simple and integrated into general practice or rheumatology clinics, experts said during an Outcomes Science Session at the European Congress of Rheumatology
Patients with rheumatoid arthritis have a 50% higher risk of heart disease than do their counterparts without the disease, but “just having RA on its own isn’t sufficient to render that individual at high risk,” Dr. Naveed Sattar, professor of metabolic medicine at the University of Glasgow’s Institute of Cardiovascular and Medical Sciences in Scotland, said in an interview.
It’s simple enough to use traditional CVD risk factors in an RA population by including a patient’s age, gender, smoking status, and family history of heart disease, in addition to measuring blood pressure and blood lipid levels. Most risk scores will compile those features into a 10-year risk of a fatal CVD event. To account for the contribution of RA, Dr. Sattar said, simply multiply that score by 1.5.
While “there’s a fixation in some parts of Europe for [measuring] fasting lipids,” it is not necessary, Dr. Sattar said. The two lipid parameters that go in risk scores tend to be cholesterol and HDL cholesterol, he said, which change only minimally in fasting versus nonfasting states.
“The evidence overwhelmingly shows that nonfasting lipids, which can be done easily on the same sample as other clinic tests, are just as predictive of CVD risk as fasting lipids,” he said. “That really matters because many of our patients with RA or other conditions come to the hospital when they’re not fasting, and we shouldn’t be sending them away to come back fasting to do risk scores for CVD. That just doesn’t make sense.”
Updated guidelines from the European Society of Cardiology and guidelines soon to be released from the European League Against Rheumatism suggest that risk scores can be calculated every 5 years for most patients, a change from previous recommendations to calculate risk annually. Risk scoring is not perfect, however, and there is some debate about whether additional blood tests or ultrasound scanning of the carotid artery could augment the ability to predict heart disease risk. “We’re not quite there yet,” Dr. Sattar said. “I think we should do the simple things first and do them well.”
CV risk raised in all inflammatory arthritic diseases
During the same session, Dr. Paola de Pablo of the University of Birmingham, England, focused on how immune-mediated diseases predispose to premature, accelerated atherosclerosis and subsequent increased cardiovascular morbidity and mortality.
Cardiovascular risk is not only elevated in those with RA, she observed, but also in those with systemic lupus erythematosus, ankylosing spondylitis, psoriatic arthritis, vasculitides, and inflammatory myopathies. The risk varies but as a rule is more than 50% higher than the rate seen in the general population.
The underlying mechanisms are not clear, but chronic inflammation is closely linked with atherosclerosis, which in turn ups the risk for myocardial infarction and cerebrovascular accident.
Despite treatment, the risk often remains, Dr. de Pablo said. She highlighted how treatment with methotrexate and anti–tumor necrosis factor (TNF)–alpha drugs in RA had been associated with a reduction in the risk for heart attack of 20% and 40%, respectively (Ann Rheum Dis. 2014;74:480–89) so targeting inflammation with these drugs may have positive effects, at least in RA.
Managing traditional cardiovascular risk factors remains important, Dr. de Pablo said. That was a sentiment echoed by Dr. Sattar and by rheumatologist Dr. Michael Nurmohamed of the VU Medical Center in Amsterdam. This includes controlling blood pressure with antihypertensive medications and blood lipids with statins, and advocating smoking cessation and perhaps other appropriate lifestyle changes such as increasing physical exercise and controlling weight.
Dr. Nurmohamed, who was involved in the 2015 update of the EULAR recommendations on cardiovascular risk management, noted that traditional risk factor management in patients with arthritis in current clinical practice is often poor and that strategies to address this were urgently needed.
Although treating to-target and preventing disease flares in the rheumatic diseases is important, it lowers but does not normalize cardiovascular risk. “This appears to be irrespective of the drug used,” Dr. Nurmohamed said. Rheumatologists need to be careful when tapering medication, particularly the biologics, as these are perhaps helping to temper cardiovascular inflammation, which could worsen when doses are reduced. “Antirheumatic treatment only is not good enough to decrease or normalize the cardiovascular risk of our patients”, he emphasized.
Norwegian project shows how to integrate CVD assessment into routine practice
In a separate presentation, Dr. Eirik Ikdahl, a PhD student at Diakonhjemmet Hospital in Oslo, discussed how some rheumatology clinics in Norway are successfully incorporating CVD risk screening.
Through the Norwegian Collaboration on Atherosclerotic disease in patients with Rheumatic joint diseases (NOCAR), which started in April 2014, annual cardiovascular disease risk evaluations of patients with inflammatory joint diseases are being implemented into the practices of 11 rheumatology outpatient clinics. While waiting for clinic appointments, patients are given electronic devices through which they can report CVD risk factors via an electronic patient journal program called GoTreatIt Rheuma. From there, the clinic can order nonfasting lipid measurements and nurses can record patients’ blood pressure.
Then, using the ESC Systematic Coronary Risk Evaluation (SCORE) algorithm, the program automatically calculates a patient’s 10-year risk of a fatal CVD event. If the SCORE estimate is 5% or greater, the rheumatologist forwards a note to the patient’s primary care physician or cardiologist saying there is an indication for initiation of CVD-preventive measures such as medication or lifestyle changes. Rheumatologists and rheumatology nurses also deliver brief advice regarding smoking cessation and healthy diet.
“The main aim of the project is to raise awareness of the cardiovascular burden that these patients experience, and to ensure that patients with inflammatory joint diseases receive guideline-recommended cardiovascular preventive treatment,” Dr. Ikdahl said.
Of 6,150 patients defined as eligible for the NOCAR project in three of the centers, 41% (n = 2,519) received a CVD risk assessment during the first year and a half of the program, officers found in a recent review. Of those, 1,569 had RA, 418 had ankylosing spondylitis, 350 had psoriatic arthritis, and 122 had other spondyloarthritides.
Through the program, “a large number of high-risk patients have received screening that they would not otherwise have been offered,” Dr. Ikdahl said.
The major obstacles to successful implementation were time scarcity, defining a date for annual CVD risk assessment among patients who visit the clinics multiple times per year, and making sure lipids were measured before seeing the rheumatologist, he said. “We acknowledge there is room for improvement. It is challenging to implement new work tasks in an already busy rheumatology outpatient clinic, and since the project does not offer financial incentives to the participating centers, we rely on a collective effort and voluntary work based on resources already available.”
Remember CVD, but don’t forget other comorbidities
Other research presented by Dr. Laure Gossec, professor of rheumatology at Pitie-Salpétriere Hospital and Pierre & Marie Curie University in Paris highlighted the importance of identifying all comorbidities and their risk factors in patients with rheumatic diseases, and not just cardiovascular disease.
Dr. Gossec presented the results of an initiative aiming to make the collection and management of comorbidities easier in routine rheumatologic practice. The aim was to develop a simple, more pragmatic form that could be used to help rheumatologists manage selected comorbidities, and know when to refer for other specialist assessment. The focus was on ischemic cardiovascular disease, malignancies, infections such as chronic bronchitis, gastrointestinal disease such as diverticulitis, osteoporosis, and depression.
A committee of 18 experts, both physicians and nurses, was convened to examine the results of a systematic literature review of recommendations on comorbidity management and come up with concise recommendations for rheumatologists. Each of their recommendations covered whether or not the comorbidity was present (yes/no/don’t know) and if screening had been undertaken, such as measurement of blood lipids, and when this had occurred if known. There was then guidance on how to interpret these findings, calculate risk, and what to do if findings were abnormal.
The project is ongoing, and so far the expert panel has developed a pragmatic document with forms to help collect, report, and manage each specific comorbidity and its known risk factors. But it is still perhaps too long to be feasibly used in everyday practice, Dr. Gossec conceded. So the aim is to create a short, 2-page form that could summarize the recommendations briefly, and also develop a questionnaire for the patient to fill out and understand how to self-manage some comorbidities.
“We feel that this is a way to disseminate and adapt to the national context for France the EULAR comorbidities initiative,” Dr. Gossec said. “It also defines exactly what rheumatologists should be doing and when they should refer, hopefully to the benefit of our patients.”
Dr. Sattar has participated in advisory boards for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lily and UCB. He has also consulted for Merck and is a member of Roche’s speakers’ bureau. Dr. de Paolo and Dr. Nurmohamed reported no relevant financial disclosures. Dr. Ikdahl has received speaker’s honoraria from Pfizer. Dr. Gossec and coauthors have received honoraria from Abbvie France.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2016 CONGRESS
TNF blocker safety may differ in RA and psoriasis patients
Rheumatoid arthritis patients on anti–tumor necrosis factor medications had approximately twice the rate of serious adverse events as did psoriasis patients on the same medications, based on data from a pair of prospective studies of about 4,000 adults.
The findings were published online in the British Journal of Dermatology.
“Current trends are to extrapolate the abundant literature existing on the safety of TNF antagonists in RA to define safety management for psoriasis,” wrote Dr. Ignacio García-Doval of the Fundación Academia Española de Dermatología y Venereología, Madrid, and colleagues. However, data on the similarity of risk associated with anti-TNF medications in RA and psoriasis are limited, the investigators said (BJD. 2016. doi: 10.1111/bjd.14776).
The researchers compared data from two similarly designed, overlapping prospective safety registry studies of anti-TNF medications in RA patients (the BIOBADASER study) and psoriasis patients (the BIOBADADERM study).
In the cohort of 3,171 RA patients, the researchers identified 1,248 serious or fatal adverse events during 16,230 person-years of follow-up; in the cohort of 946 psoriasis patients, they identified 124 serious or fatal adverse events during 2,760 person-years of follow-up. The resulting incidence rate ratio of psoriasis to RA was 0.6. The increased risk of serious adverse events for RA patients compared with psoriasis patients remained after the investigators controlled for confounding factors including age, sex, treatments, and comorbid conditions including hypertension, diabetes, hypercholesterolemia, and methotrexate therapy, for a hazard ratio of 0.54.
Moreover, the types of serious adverse events were different between the RA and psoriasis groups. Among those with RA, the rates of serious infections, cardiac disorders, respiratory disorders, and infusion reactions were significantly greater among those with RA, while psoriatic patients “had more skin and subcutaneous tissue disorders and hepatobiliary disorders,” the researchers noted.
By contrast, “rates of nonserious adverse events cannot be compared between the two cohorts,” because of differences in definitions, they pointed out. These differences resulted in a nonserious adverse event rate that was more than twice as high in the psoriasis group as in the RA group (582.2 events per 1,000 patient-years vs. 242.8 events per 1,000 patient-years).
Based on the findings, “published safety results of TNF-antagonists in RA cannot be fully extrapolated to psoriasis, as patients with RA have a higher risk and a different pattern of serious adverse events,” they concluded.
The BIOBADADERM project is promoted by the Fundación Academia Española de Dermatología y Venereología, which is supported by the Spanish Medicines and Health Products Agency and by multiple pharmaceutical companies. BIOBADASER received funding from Fundacion Española de Reumatología and the Spanish Medicines and Health Products Agency and grants from numerous pharmaceutical companies. Lead author Dr. Garcia-Doval disclosed travel grants for congresses from Merck/Schering-Plough Pharmaceuticals, Pfizer, and Janssen; the remaining two authors disclosed being a speaker and/or a consultant for several companies, including AbbVie.
Rheumatoid arthritis patients on anti–tumor necrosis factor medications had approximately twice the rate of serious adverse events as did psoriasis patients on the same medications, based on data from a pair of prospective studies of about 4,000 adults.
The findings were published online in the British Journal of Dermatology.
“Current trends are to extrapolate the abundant literature existing on the safety of TNF antagonists in RA to define safety management for psoriasis,” wrote Dr. Ignacio García-Doval of the Fundación Academia Española de Dermatología y Venereología, Madrid, and colleagues. However, data on the similarity of risk associated with anti-TNF medications in RA and psoriasis are limited, the investigators said (BJD. 2016. doi: 10.1111/bjd.14776).
The researchers compared data from two similarly designed, overlapping prospective safety registry studies of anti-TNF medications in RA patients (the BIOBADASER study) and psoriasis patients (the BIOBADADERM study).
In the cohort of 3,171 RA patients, the researchers identified 1,248 serious or fatal adverse events during 16,230 person-years of follow-up; in the cohort of 946 psoriasis patients, they identified 124 serious or fatal adverse events during 2,760 person-years of follow-up. The resulting incidence rate ratio of psoriasis to RA was 0.6. The increased risk of serious adverse events for RA patients compared with psoriasis patients remained after the investigators controlled for confounding factors including age, sex, treatments, and comorbid conditions including hypertension, diabetes, hypercholesterolemia, and methotrexate therapy, for a hazard ratio of 0.54.
Moreover, the types of serious adverse events were different between the RA and psoriasis groups. Among those with RA, the rates of serious infections, cardiac disorders, respiratory disorders, and infusion reactions were significantly greater among those with RA, while psoriatic patients “had more skin and subcutaneous tissue disorders and hepatobiliary disorders,” the researchers noted.
By contrast, “rates of nonserious adverse events cannot be compared between the two cohorts,” because of differences in definitions, they pointed out. These differences resulted in a nonserious adverse event rate that was more than twice as high in the psoriasis group as in the RA group (582.2 events per 1,000 patient-years vs. 242.8 events per 1,000 patient-years).
Based on the findings, “published safety results of TNF-antagonists in RA cannot be fully extrapolated to psoriasis, as patients with RA have a higher risk and a different pattern of serious adverse events,” they concluded.
The BIOBADADERM project is promoted by the Fundación Academia Española de Dermatología y Venereología, which is supported by the Spanish Medicines and Health Products Agency and by multiple pharmaceutical companies. BIOBADASER received funding from Fundacion Española de Reumatología and the Spanish Medicines and Health Products Agency and grants from numerous pharmaceutical companies. Lead author Dr. Garcia-Doval disclosed travel grants for congresses from Merck/Schering-Plough Pharmaceuticals, Pfizer, and Janssen; the remaining two authors disclosed being a speaker and/or a consultant for several companies, including AbbVie.
Rheumatoid arthritis patients on anti–tumor necrosis factor medications had approximately twice the rate of serious adverse events as did psoriasis patients on the same medications, based on data from a pair of prospective studies of about 4,000 adults.
The findings were published online in the British Journal of Dermatology.
“Current trends are to extrapolate the abundant literature existing on the safety of TNF antagonists in RA to define safety management for psoriasis,” wrote Dr. Ignacio García-Doval of the Fundación Academia Española de Dermatología y Venereología, Madrid, and colleagues. However, data on the similarity of risk associated with anti-TNF medications in RA and psoriasis are limited, the investigators said (BJD. 2016. doi: 10.1111/bjd.14776).
The researchers compared data from two similarly designed, overlapping prospective safety registry studies of anti-TNF medications in RA patients (the BIOBADASER study) and psoriasis patients (the BIOBADADERM study).
In the cohort of 3,171 RA patients, the researchers identified 1,248 serious or fatal adverse events during 16,230 person-years of follow-up; in the cohort of 946 psoriasis patients, they identified 124 serious or fatal adverse events during 2,760 person-years of follow-up. The resulting incidence rate ratio of psoriasis to RA was 0.6. The increased risk of serious adverse events for RA patients compared with psoriasis patients remained after the investigators controlled for confounding factors including age, sex, treatments, and comorbid conditions including hypertension, diabetes, hypercholesterolemia, and methotrexate therapy, for a hazard ratio of 0.54.
Moreover, the types of serious adverse events were different between the RA and psoriasis groups. Among those with RA, the rates of serious infections, cardiac disorders, respiratory disorders, and infusion reactions were significantly greater among those with RA, while psoriatic patients “had more skin and subcutaneous tissue disorders and hepatobiliary disorders,” the researchers noted.
By contrast, “rates of nonserious adverse events cannot be compared between the two cohorts,” because of differences in definitions, they pointed out. These differences resulted in a nonserious adverse event rate that was more than twice as high in the psoriasis group as in the RA group (582.2 events per 1,000 patient-years vs. 242.8 events per 1,000 patient-years).
Based on the findings, “published safety results of TNF-antagonists in RA cannot be fully extrapolated to psoriasis, as patients with RA have a higher risk and a different pattern of serious adverse events,” they concluded.
The BIOBADADERM project is promoted by the Fundación Academia Española de Dermatología y Venereología, which is supported by the Spanish Medicines and Health Products Agency and by multiple pharmaceutical companies. BIOBADASER received funding from Fundacion Española de Reumatología and the Spanish Medicines and Health Products Agency and grants from numerous pharmaceutical companies. Lead author Dr. Garcia-Doval disclosed travel grants for congresses from Merck/Schering-Plough Pharmaceuticals, Pfizer, and Janssen; the remaining two authors disclosed being a speaker and/or a consultant for several companies, including AbbVie.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: TNF-antagonists provoke different adverse events in patients with RA than in those with psoriasis, and safety data should be extrapolated with caution.
Major finding: The risk of serious adverse events associated with anti-TNF therapy was approximately twice as high in RA patients as in psoriasis patients (hazard ratio, 0.54).
Data source: A pair of prospective studies including 4,117 adults with RA or psoriasis who received anti-TNF agents.
Disclosures: The BIOBADADERM project is promoted by the Fundación Academia Española de Dermatología y Venereología, which is supported by the Spanish Medicines and Health Products Agency and by multiple pharmaceutical companies. BIOBADASER received funding from Fundacion Española de Reumatología and the Spanish Medicines and Health Products Agency and grants from numerous pharmaceutical companies. Lead author Dr. Garcia-Doval disclosed travel grants for congresses from Merck/Schering-Plough Pharmaceuticals, Pfizer, and Janssen; the remaining two authors disclosed being a speaker and/or a consultant for several companies, including AbbVie.
Early identification, treatment still key to early arthritis guidelines
LONDON, ENGLAND – Prompt referral to a rheumatologist and early initiation of treatment remain 2 of the 12 key recommendations in the updated European League Against Rheumatism guidelines for early arthritis.
There are now three specific recommendations dealing with referral and diagnosis; four that cover initial drug treatment with disease-modifying antirheumatic drugs (DMARDs), nonsteroidal anti-inflammatory drugs (NSAIDs), and glucocorticoids; two that cover management strategy and monitoring; and three that cover nonpharmacologic interventions, prevention, and patient information and education.
While many of the recommendations have not radically changed, there have been revisions to the wording. In line with other EULAR recommendations for the management of rheumatic disease, the updated early arthritis guidelines also now contain three overarching principles. The first states that the management of early arthritis should aim to achieve the best possible care and emphasizes the need for shared decision making between rheumatologist and patient. The second states that a rheumatologist should be the main specialist looking after a patient with early arthritis, and the third states that a definitive diagnosis should be made only after a careful medical history and clinical examination have been undertaken.
“[The EULAR] recommendations deal especially with early-stage inflammatory arthritis,” said Dr. Bernard Combe of Hôpital Lapeyronie in Montpellier, France, who was the convener of the task force behind the updated guidelines. As such, they are universal for all rheumatologic arthritis conditions when diagnosed early, before they differentiate into more specifics, he said in an interview. That includes progression from early inflammatory arthritis to rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis.
Dr. Combe, who is also professor of rheumatology at Montpellier University and head of the bone and joint diseases department at Montpellier University Hospital, presented the updated EULAR guidance at the European Congress of Rheumatology. He noted that the guidelines were first written almost 10 years ago (Ann Rheum Dis. 2007 Jan;66(1):34-45) and so were in need of an update. A task force of 20 rheumatologists, two patients, and one health care professional representing 12 European countries were involved in the update that adhered to EULAR standard operating procedure of developing guidelines.
Although EULAR had published guidance on the management of arthritis in the intervening years, he said, this had focused more on management, and the aim of the early arthritis guidelines was to cover the “entire spectrum of the management of early inflammatory arthritis.”
The updated recommendations start and end with patient-centered statements, he observed. The first notes that patients with any joint swelling associated with pain or stiffness should be referred to and seen by a rheumatologist within 6 weeks of the onset of symptoms. The final recommendation deals with patient information and education about the disease, and programs aiming to help patients cope with pain, disability, and ensure their continued ability to work and participate in their usual social activities.
The concept of early identification and treatment is not new, Dr. Combe observed, but there is so much more evidence in support of initiating DMARD therapy within the first 3 months of referral, even if patients do not fulfill classification criteria for a specific inflammatory rheumatic disease.
In terms of diagnosis, the recommendations now hinge on performing a thorough clinical examination and using ultrasonography to confirm the presence of arthritis if needed. Magnetic resonance imaging (MRI) is no longer recommended in this initial diagnostic work up. This is due to the cost and often lack of widespread access in all European countries, Dr. Combe said. MRI might be considered later, however, if a diagnosis cannot be reached. Assessment of the number of swollen joints, acute phase reactants, and antibody tests (rheumatoid factor and anti-citrullinated protein antibody) also might be of use at this point.
Among the various DMARDs, methotrexate is recommended as the “anchor drug”; it should be used as part of the first treatment strategy in patients who are at risk of persistent disease, unless it contraindicated. The goal of treatment with DMARDs is to achieve clinical remission. Regular monitoring of disease activity, side effects, and comorbidities should be performed alongside their use. Regular monitoring of all pharmacologic therapy should include assessment of tender and swollen joint counts, global health assessments by the patient and the physician, and acute phase reactants.
As for NSAIDs, they are recommended for symptomatic relief, but “at the minimum effective dose for the shortest time possible.” The risks for gastrointestinal, renal, and cardiovascular complications should be carefully weighed against the likely benefits. Glucocorticoids also are recommended for reducing pain and swelling, and structural progression, but again these need to be used at the lowest possible dose and for no more than 6 months to avoid potential long-term side effects.
A recommendation on nonpharmacologic interventions also is included, which states that physical exercise and occupational therapy should be considered as adjunctive therapy.
In addition, the task force came up with a list of 10 research questions that need to be answered, which included items on risk prediction, optimal treatment combinations, and dosing regimens.
As with other EULAR guidelines, a flow chart is included that summarizes the recommendations to help guide physicians on when and how to treat, when to adapt dosing or change medication, and other treatments and approaches to consider.
There is a new recommendation on prevention highlighting the importance of smoking cessation, dental care, weight control, vaccination, and managing comorbidities.
Once the guidelines have been finalized, they will be published in the EULAR journal, Annals of the Rheumatic Diseases, later in the year.
Dr. Combe has received research grants and honoraria from Pfizer, Roche-Chugai, and UCB, and honoraria from Bristol-Myers Squibb, Janssen, Eli Lilly, Merck Sharpe and Dohme, and Novartis.
LONDON, ENGLAND – Prompt referral to a rheumatologist and early initiation of treatment remain 2 of the 12 key recommendations in the updated European League Against Rheumatism guidelines for early arthritis.
There are now three specific recommendations dealing with referral and diagnosis; four that cover initial drug treatment with disease-modifying antirheumatic drugs (DMARDs), nonsteroidal anti-inflammatory drugs (NSAIDs), and glucocorticoids; two that cover management strategy and monitoring; and three that cover nonpharmacologic interventions, prevention, and patient information and education.
While many of the recommendations have not radically changed, there have been revisions to the wording. In line with other EULAR recommendations for the management of rheumatic disease, the updated early arthritis guidelines also now contain three overarching principles. The first states that the management of early arthritis should aim to achieve the best possible care and emphasizes the need for shared decision making between rheumatologist and patient. The second states that a rheumatologist should be the main specialist looking after a patient with early arthritis, and the third states that a definitive diagnosis should be made only after a careful medical history and clinical examination have been undertaken.
“[The EULAR] recommendations deal especially with early-stage inflammatory arthritis,” said Dr. Bernard Combe of Hôpital Lapeyronie in Montpellier, France, who was the convener of the task force behind the updated guidelines. As such, they are universal for all rheumatologic arthritis conditions when diagnosed early, before they differentiate into more specifics, he said in an interview. That includes progression from early inflammatory arthritis to rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis.
Dr. Combe, who is also professor of rheumatology at Montpellier University and head of the bone and joint diseases department at Montpellier University Hospital, presented the updated EULAR guidance at the European Congress of Rheumatology. He noted that the guidelines were first written almost 10 years ago (Ann Rheum Dis. 2007 Jan;66(1):34-45) and so were in need of an update. A task force of 20 rheumatologists, two patients, and one health care professional representing 12 European countries were involved in the update that adhered to EULAR standard operating procedure of developing guidelines.
Although EULAR had published guidance on the management of arthritis in the intervening years, he said, this had focused more on management, and the aim of the early arthritis guidelines was to cover the “entire spectrum of the management of early inflammatory arthritis.”
The updated recommendations start and end with patient-centered statements, he observed. The first notes that patients with any joint swelling associated with pain or stiffness should be referred to and seen by a rheumatologist within 6 weeks of the onset of symptoms. The final recommendation deals with patient information and education about the disease, and programs aiming to help patients cope with pain, disability, and ensure their continued ability to work and participate in their usual social activities.
The concept of early identification and treatment is not new, Dr. Combe observed, but there is so much more evidence in support of initiating DMARD therapy within the first 3 months of referral, even if patients do not fulfill classification criteria for a specific inflammatory rheumatic disease.
In terms of diagnosis, the recommendations now hinge on performing a thorough clinical examination and using ultrasonography to confirm the presence of arthritis if needed. Magnetic resonance imaging (MRI) is no longer recommended in this initial diagnostic work up. This is due to the cost and often lack of widespread access in all European countries, Dr. Combe said. MRI might be considered later, however, if a diagnosis cannot be reached. Assessment of the number of swollen joints, acute phase reactants, and antibody tests (rheumatoid factor and anti-citrullinated protein antibody) also might be of use at this point.
Among the various DMARDs, methotrexate is recommended as the “anchor drug”; it should be used as part of the first treatment strategy in patients who are at risk of persistent disease, unless it contraindicated. The goal of treatment with DMARDs is to achieve clinical remission. Regular monitoring of disease activity, side effects, and comorbidities should be performed alongside their use. Regular monitoring of all pharmacologic therapy should include assessment of tender and swollen joint counts, global health assessments by the patient and the physician, and acute phase reactants.
As for NSAIDs, they are recommended for symptomatic relief, but “at the minimum effective dose for the shortest time possible.” The risks for gastrointestinal, renal, and cardiovascular complications should be carefully weighed against the likely benefits. Glucocorticoids also are recommended for reducing pain and swelling, and structural progression, but again these need to be used at the lowest possible dose and for no more than 6 months to avoid potential long-term side effects.
A recommendation on nonpharmacologic interventions also is included, which states that physical exercise and occupational therapy should be considered as adjunctive therapy.
In addition, the task force came up with a list of 10 research questions that need to be answered, which included items on risk prediction, optimal treatment combinations, and dosing regimens.
As with other EULAR guidelines, a flow chart is included that summarizes the recommendations to help guide physicians on when and how to treat, when to adapt dosing or change medication, and other treatments and approaches to consider.
There is a new recommendation on prevention highlighting the importance of smoking cessation, dental care, weight control, vaccination, and managing comorbidities.
Once the guidelines have been finalized, they will be published in the EULAR journal, Annals of the Rheumatic Diseases, later in the year.
Dr. Combe has received research grants and honoraria from Pfizer, Roche-Chugai, and UCB, and honoraria from Bristol-Myers Squibb, Janssen, Eli Lilly, Merck Sharpe and Dohme, and Novartis.
LONDON, ENGLAND – Prompt referral to a rheumatologist and early initiation of treatment remain 2 of the 12 key recommendations in the updated European League Against Rheumatism guidelines for early arthritis.
There are now three specific recommendations dealing with referral and diagnosis; four that cover initial drug treatment with disease-modifying antirheumatic drugs (DMARDs), nonsteroidal anti-inflammatory drugs (NSAIDs), and glucocorticoids; two that cover management strategy and monitoring; and three that cover nonpharmacologic interventions, prevention, and patient information and education.
While many of the recommendations have not radically changed, there have been revisions to the wording. In line with other EULAR recommendations for the management of rheumatic disease, the updated early arthritis guidelines also now contain three overarching principles. The first states that the management of early arthritis should aim to achieve the best possible care and emphasizes the need for shared decision making between rheumatologist and patient. The second states that a rheumatologist should be the main specialist looking after a patient with early arthritis, and the third states that a definitive diagnosis should be made only after a careful medical history and clinical examination have been undertaken.
“[The EULAR] recommendations deal especially with early-stage inflammatory arthritis,” said Dr. Bernard Combe of Hôpital Lapeyronie in Montpellier, France, who was the convener of the task force behind the updated guidelines. As such, they are universal for all rheumatologic arthritis conditions when diagnosed early, before they differentiate into more specifics, he said in an interview. That includes progression from early inflammatory arthritis to rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis.
Dr. Combe, who is also professor of rheumatology at Montpellier University and head of the bone and joint diseases department at Montpellier University Hospital, presented the updated EULAR guidance at the European Congress of Rheumatology. He noted that the guidelines were first written almost 10 years ago (Ann Rheum Dis. 2007 Jan;66(1):34-45) and so were in need of an update. A task force of 20 rheumatologists, two patients, and one health care professional representing 12 European countries were involved in the update that adhered to EULAR standard operating procedure of developing guidelines.
Although EULAR had published guidance on the management of arthritis in the intervening years, he said, this had focused more on management, and the aim of the early arthritis guidelines was to cover the “entire spectrum of the management of early inflammatory arthritis.”
The updated recommendations start and end with patient-centered statements, he observed. The first notes that patients with any joint swelling associated with pain or stiffness should be referred to and seen by a rheumatologist within 6 weeks of the onset of symptoms. The final recommendation deals with patient information and education about the disease, and programs aiming to help patients cope with pain, disability, and ensure their continued ability to work and participate in their usual social activities.
The concept of early identification and treatment is not new, Dr. Combe observed, but there is so much more evidence in support of initiating DMARD therapy within the first 3 months of referral, even if patients do not fulfill classification criteria for a specific inflammatory rheumatic disease.
In terms of diagnosis, the recommendations now hinge on performing a thorough clinical examination and using ultrasonography to confirm the presence of arthritis if needed. Magnetic resonance imaging (MRI) is no longer recommended in this initial diagnostic work up. This is due to the cost and often lack of widespread access in all European countries, Dr. Combe said. MRI might be considered later, however, if a diagnosis cannot be reached. Assessment of the number of swollen joints, acute phase reactants, and antibody tests (rheumatoid factor and anti-citrullinated protein antibody) also might be of use at this point.
Among the various DMARDs, methotrexate is recommended as the “anchor drug”; it should be used as part of the first treatment strategy in patients who are at risk of persistent disease, unless it contraindicated. The goal of treatment with DMARDs is to achieve clinical remission. Regular monitoring of disease activity, side effects, and comorbidities should be performed alongside their use. Regular monitoring of all pharmacologic therapy should include assessment of tender and swollen joint counts, global health assessments by the patient and the physician, and acute phase reactants.
As for NSAIDs, they are recommended for symptomatic relief, but “at the minimum effective dose for the shortest time possible.” The risks for gastrointestinal, renal, and cardiovascular complications should be carefully weighed against the likely benefits. Glucocorticoids also are recommended for reducing pain and swelling, and structural progression, but again these need to be used at the lowest possible dose and for no more than 6 months to avoid potential long-term side effects.
A recommendation on nonpharmacologic interventions also is included, which states that physical exercise and occupational therapy should be considered as adjunctive therapy.
In addition, the task force came up with a list of 10 research questions that need to be answered, which included items on risk prediction, optimal treatment combinations, and dosing regimens.
As with other EULAR guidelines, a flow chart is included that summarizes the recommendations to help guide physicians on when and how to treat, when to adapt dosing or change medication, and other treatments and approaches to consider.
There is a new recommendation on prevention highlighting the importance of smoking cessation, dental care, weight control, vaccination, and managing comorbidities.
Once the guidelines have been finalized, they will be published in the EULAR journal, Annals of the Rheumatic Diseases, later in the year.
Dr. Combe has received research grants and honoraria from Pfizer, Roche-Chugai, and UCB, and honoraria from Bristol-Myers Squibb, Janssen, Eli Lilly, Merck Sharpe and Dohme, and Novartis.
AT THE EULAR 2016 CONGRESS
VIDEO: Smoking, excess weight hinder sustained remission in early RA
LONDON – Tobacco use and excess weight can make it harder to achieve sustained remission in the treatment of early rheumatoid arthritis, according to findings from more than 1,000 patients in the Canadian Early Arthritis Cohort (CATCH) multicenter, prospective study.
Aggressive treatment that starts soon after diagnosis of rheumatoid arthritis (RA) is important for the absence of disease activity, which is the hallmark of sustained remission. But the reality is a success rate of less than 50% in the first 3 years with physical deterioration continuing thereafter. “Excess weight and smoking are two risk factors for developing RA. We were interested in seeing if they might also affect how well people responded to treatment,” said Susan Bartlett, Ph.D., a clinical psychologist at McGill University in Montreal.
At the annual European Congress of Rheumatology, Dr. Bartlett and colleagues reported on a cohort of 1,008 early RA patients who were enrolled in the Canadian Early Arthritis Cohort (CATCH) multicenter, prospective study and followed from around the time of diagnosis through the first 3 years of treatment to estimate the time it took until they achieved sustained remission, defined as having a 28-joint Disease Activity Score less than 2.6 for two consecutive visits.
Mean age of the patients (72% female, 81% white) was early 50s. Overall, 30% of females and 47% of males were overweight, one-third of both genders were obese, and 15%-20% smoked. Treatment at entry included methotrexate in mono- or combination therapy in about three-quarters of the patients, with steroids used in about half and biologics used sparingly.
The proportion of patients in sustained remission was 38% at 3 years, with a median time to remission of 11.3 months. “That finding wasn’t surprising because that is generally what is found in most studies of early RA. However, when we looked more closely at who was and wasn’t achieving remission, we found that people who smoked and those who were overweight or obese were much less likely than their nonsmoking, normal-weight peers to be in sustained remission,” Dr. Bartlett said in a pre-congress interview.
After adjustment for factors that could affect response to treatment – including age, race, disability status, pain, and early medications used – smoking (P = .046) and excess weight (P = .003) were associated with a poorer likelihood of achieving sustained remission. While more men than women were overweight or obese, the effects of weight and smoking appeared to be more problematic for women (P = .02).
An average nonsmoking male with a healthy body mass index (BMI; 25 kg/m2 or less) had about a 41% probability of achieving sustained remission within 3 years, compared with 15% for an obese male smoker. A nonsmoking female with a healthy BMI had a 27% probability of achieving sustained remission within 3 years, compared with 10% for an obese female smoker. Probabilities of sustained remission were also lower for overweight men and women, Dr. Bartlett reported.
Smoking and obesity have already been linked with an increased likelihood of developing RA, which in turn increases the risk of cardiovascular disease and premature death. The latest data suggest that both smoking and extra weight – including overweight and obese as defined by BMI – may also independently influence the success of treatment. “Our data suggest that if you have RA, it’s important to take the medications that your doctor has prescribed. If you smoke, you need to stop. And if you’re carrying extra weight, not only is that placing a greater demand on already vulnerable joints, it may also be making your RA treatment less effective,” Dr. Bartlett said.
These lifestyle modifications can be challenging for some people with RA, she said in the interview. Clinicians can help by considering lifestyle behaviors that lead to chronic diseases and poorer outcomes in addition to their more traditional view of diagnosis and treatment, she said, adding that patients and clinicians should know that even a small amount of weight loss can improve health and may improve response to therapy.
Well controlled clinical trials will be needed to better understand the benefits of weight control and smoking cessation on response to RA treatment. Also, why women who smoke and are overweight are at more of a disadvantage than their male counterparts is unknown. “As we begin putting these pieces together, we may learn valuable information that helps us to better control and ultimately cure RA,” said Dr. Bartlett.
The researchers had no conflicts of interest to declare.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Tobacco use and excess weight can make it harder to achieve sustained remission in the treatment of early rheumatoid arthritis, according to findings from more than 1,000 patients in the Canadian Early Arthritis Cohort (CATCH) multicenter, prospective study.
Aggressive treatment that starts soon after diagnosis of rheumatoid arthritis (RA) is important for the absence of disease activity, which is the hallmark of sustained remission. But the reality is a success rate of less than 50% in the first 3 years with physical deterioration continuing thereafter. “Excess weight and smoking are two risk factors for developing RA. We were interested in seeing if they might also affect how well people responded to treatment,” said Susan Bartlett, Ph.D., a clinical psychologist at McGill University in Montreal.
At the annual European Congress of Rheumatology, Dr. Bartlett and colleagues reported on a cohort of 1,008 early RA patients who were enrolled in the Canadian Early Arthritis Cohort (CATCH) multicenter, prospective study and followed from around the time of diagnosis through the first 3 years of treatment to estimate the time it took until they achieved sustained remission, defined as having a 28-joint Disease Activity Score less than 2.6 for two consecutive visits.
Mean age of the patients (72% female, 81% white) was early 50s. Overall, 30% of females and 47% of males were overweight, one-third of both genders were obese, and 15%-20% smoked. Treatment at entry included methotrexate in mono- or combination therapy in about three-quarters of the patients, with steroids used in about half and biologics used sparingly.
The proportion of patients in sustained remission was 38% at 3 years, with a median time to remission of 11.3 months. “That finding wasn’t surprising because that is generally what is found in most studies of early RA. However, when we looked more closely at who was and wasn’t achieving remission, we found that people who smoked and those who were overweight or obese were much less likely than their nonsmoking, normal-weight peers to be in sustained remission,” Dr. Bartlett said in a pre-congress interview.
After adjustment for factors that could affect response to treatment – including age, race, disability status, pain, and early medications used – smoking (P = .046) and excess weight (P = .003) were associated with a poorer likelihood of achieving sustained remission. While more men than women were overweight or obese, the effects of weight and smoking appeared to be more problematic for women (P = .02).
An average nonsmoking male with a healthy body mass index (BMI; 25 kg/m2 or less) had about a 41% probability of achieving sustained remission within 3 years, compared with 15% for an obese male smoker. A nonsmoking female with a healthy BMI had a 27% probability of achieving sustained remission within 3 years, compared with 10% for an obese female smoker. Probabilities of sustained remission were also lower for overweight men and women, Dr. Bartlett reported.
Smoking and obesity have already been linked with an increased likelihood of developing RA, which in turn increases the risk of cardiovascular disease and premature death. The latest data suggest that both smoking and extra weight – including overweight and obese as defined by BMI – may also independently influence the success of treatment. “Our data suggest that if you have RA, it’s important to take the medications that your doctor has prescribed. If you smoke, you need to stop. And if you’re carrying extra weight, not only is that placing a greater demand on already vulnerable joints, it may also be making your RA treatment less effective,” Dr. Bartlett said.
These lifestyle modifications can be challenging for some people with RA, she said in the interview. Clinicians can help by considering lifestyle behaviors that lead to chronic diseases and poorer outcomes in addition to their more traditional view of diagnosis and treatment, she said, adding that patients and clinicians should know that even a small amount of weight loss can improve health and may improve response to therapy.
Well controlled clinical trials will be needed to better understand the benefits of weight control and smoking cessation on response to RA treatment. Also, why women who smoke and are overweight are at more of a disadvantage than their male counterparts is unknown. “As we begin putting these pieces together, we may learn valuable information that helps us to better control and ultimately cure RA,” said Dr. Bartlett.
The researchers had no conflicts of interest to declare.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Tobacco use and excess weight can make it harder to achieve sustained remission in the treatment of early rheumatoid arthritis, according to findings from more than 1,000 patients in the Canadian Early Arthritis Cohort (CATCH) multicenter, prospective study.
Aggressive treatment that starts soon after diagnosis of rheumatoid arthritis (RA) is important for the absence of disease activity, which is the hallmark of sustained remission. But the reality is a success rate of less than 50% in the first 3 years with physical deterioration continuing thereafter. “Excess weight and smoking are two risk factors for developing RA. We were interested in seeing if they might also affect how well people responded to treatment,” said Susan Bartlett, Ph.D., a clinical psychologist at McGill University in Montreal.
At the annual European Congress of Rheumatology, Dr. Bartlett and colleagues reported on a cohort of 1,008 early RA patients who were enrolled in the Canadian Early Arthritis Cohort (CATCH) multicenter, prospective study and followed from around the time of diagnosis through the first 3 years of treatment to estimate the time it took until they achieved sustained remission, defined as having a 28-joint Disease Activity Score less than 2.6 for two consecutive visits.
Mean age of the patients (72% female, 81% white) was early 50s. Overall, 30% of females and 47% of males were overweight, one-third of both genders were obese, and 15%-20% smoked. Treatment at entry included methotrexate in mono- or combination therapy in about three-quarters of the patients, with steroids used in about half and biologics used sparingly.
The proportion of patients in sustained remission was 38% at 3 years, with a median time to remission of 11.3 months. “That finding wasn’t surprising because that is generally what is found in most studies of early RA. However, when we looked more closely at who was and wasn’t achieving remission, we found that people who smoked and those who were overweight or obese were much less likely than their nonsmoking, normal-weight peers to be in sustained remission,” Dr. Bartlett said in a pre-congress interview.
After adjustment for factors that could affect response to treatment – including age, race, disability status, pain, and early medications used – smoking (P = .046) and excess weight (P = .003) were associated with a poorer likelihood of achieving sustained remission. While more men than women were overweight or obese, the effects of weight and smoking appeared to be more problematic for women (P = .02).
An average nonsmoking male with a healthy body mass index (BMI; 25 kg/m2 or less) had about a 41% probability of achieving sustained remission within 3 years, compared with 15% for an obese male smoker. A nonsmoking female with a healthy BMI had a 27% probability of achieving sustained remission within 3 years, compared with 10% for an obese female smoker. Probabilities of sustained remission were also lower for overweight men and women, Dr. Bartlett reported.
Smoking and obesity have already been linked with an increased likelihood of developing RA, which in turn increases the risk of cardiovascular disease and premature death. The latest data suggest that both smoking and extra weight – including overweight and obese as defined by BMI – may also independently influence the success of treatment. “Our data suggest that if you have RA, it’s important to take the medications that your doctor has prescribed. If you smoke, you need to stop. And if you’re carrying extra weight, not only is that placing a greater demand on already vulnerable joints, it may also be making your RA treatment less effective,” Dr. Bartlett said.
These lifestyle modifications can be challenging for some people with RA, she said in the interview. Clinicians can help by considering lifestyle behaviors that lead to chronic diseases and poorer outcomes in addition to their more traditional view of diagnosis and treatment, she said, adding that patients and clinicians should know that even a small amount of weight loss can improve health and may improve response to therapy.
Well controlled clinical trials will be needed to better understand the benefits of weight control and smoking cessation on response to RA treatment. Also, why women who smoke and are overweight are at more of a disadvantage than their male counterparts is unknown. “As we begin putting these pieces together, we may learn valuable information that helps us to better control and ultimately cure RA,” said Dr. Bartlett.
The researchers had no conflicts of interest to declare.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR CONGRESS 2016
Key clinical point: The latest data suggest that both smoking and extra weight – including overweight and obese as defined by BMI – may independently influence the success of treatment for rheumatoid arthritis.
Major finding: A nonsmoking female with a healthy BMI had a 27% probability of achieving sustained remission within 3 years, compared with 10% for an obese female smoker.
Data source: A prospective study of 1,008 patients with early RA in the prospective, multicenter CATCH study.
Disclosures: The researchers had no conflicts of interest to declare.
Hydroxychloroquine, abatacept linked with reduced type 2 diabetes
LONDON – U.S. rheumatoid arthritis patients had a significantly reduced incidence of type 2 diabetes when on treatment with either hydroxychloroquine or abatacept in an analysis of more than 13,000 patients enrolled in a U.S. national registry during 2000-2015.
The same analysis also showed statistically significant increases in the risk for new-onset type 2 diabetes in rheumatoid arthritis (RA) patients treated with a glucocorticoid or a statin, Kaleb Michaud, Ph.D., reported in a poster at the European Congress of Rheumatology.
“Our findings can inform clinicians about determining appropriate treatment decisions in rheumatoid arthritis patients at increased risk for developing type 2 diabetes,” said Dr. Michaud, an epidemiologist at the University of Nebraska in Omaha and also codirector of the National Data Bank for Rheumatic Diseases in Wichita, Kan., the source of the data used in his study.
Hydroxychloroquine, a relatively inexpensive drug that’s often used in RA patients in combination with other drugs, might be a good agent to consider adding to the therapeutic mix of RA patients at risk for developing type 2 diabetes, he said in an interview. Although the implications of this finding for the use of abatacept (Orencia) are less clear, it is a signal of a novel benefit from the drug that merits further study, Dr. Michaud said.
The findings also suggest that, when rheumatoid arthritis patients receive statin treatment, they are candidates for more intensive monitoring of diabetes onset, he added.
His analysis focused on the 13,669 RA patients who participated in the National Data Bank for Rheumatic Diseases for at least a year during 2000-2015 and had not been diagnosed as having diabetes of any type at the time they entered the registry. During a median 4.6 years of follow-up, 1,139 patients either self-reported receiving a new diagnosis of type 2 diabetes or began treatment with a hypoglycemic medication. Patients averaged about 59 years old at the time they entered the registry, and about 80% were women.
Dr. Michaud and his associates assessed the incidence rate of type 2 diabetes according to six categories of RA treatment: methotrexate monotherapy, which they used as the reference group; any treatment with abatacept with or without methotrexate; any treatment with hydroxychloroquine; any treatment with a glucocorticoid; treatment with any other disease-modifying antirheumatic drug (DMARD) with methotrexate; and treatment with any other DMARD without methotrexate or no DMARD treatment. A seventh treatment category was treatment with a statin.
A series of time-varying Cox proportional hazard models that adjusted for sociodemographic variables, comorbidities, body mass index, and measures of RA severity showed that, when compared with methotrexate monotherapy, patients treated with abatacept had a statistically significant 48% reduced rate of developing diabetes, and those treated with hydroxychloroquine had a statistically significant 33% reduced diabetes incidence, they reported.
In contrast, compared with methotrexate monotherapy, treatment with a glucocorticoid linked with a statistically significant 31% increased rate of type 2 diabetes, and treatment with a statin linked with a statistically significant 56% increased rate of diabetes. Other forms of DMARD treatment, including tumor necrosis factor inhibitors and other biologic DMARDs, had no statistically significant link with diabetes development.
Dr. Michaud also reported additional analyses that further defined the associations between hydroxychloroquine treatment and reduced diabetes incidence: The reduction in diabetes incidence became statistically significant in patients only when they had received the drug for at least 2 years. The link with reduced diabetes incidence seemed dose dependent, with a stronger protective effect in patients who received at least 400 mg/day. Also, the strength of the protection waned in patients who had discontinued hydroxychloroquine for at least 6 months, and the protective effect completely disappeared once patients were off hydroxychloroquine for at least 1 year. Finally, the link between hydroxychloroquine use and reduced diabetes incidence also was statistically significant in patients who concurrently received a glucocorticoid, but a significant protective association disappeared in patients who received a statin as well as hydroxychloroquine.
Dr. Michaud had no disclosures. The study received no commercial support.
On Twitter @mitchelzoler
LONDON – U.S. rheumatoid arthritis patients had a significantly reduced incidence of type 2 diabetes when on treatment with either hydroxychloroquine or abatacept in an analysis of more than 13,000 patients enrolled in a U.S. national registry during 2000-2015.
The same analysis also showed statistically significant increases in the risk for new-onset type 2 diabetes in rheumatoid arthritis (RA) patients treated with a glucocorticoid or a statin, Kaleb Michaud, Ph.D., reported in a poster at the European Congress of Rheumatology.
“Our findings can inform clinicians about determining appropriate treatment decisions in rheumatoid arthritis patients at increased risk for developing type 2 diabetes,” said Dr. Michaud, an epidemiologist at the University of Nebraska in Omaha and also codirector of the National Data Bank for Rheumatic Diseases in Wichita, Kan., the source of the data used in his study.
Hydroxychloroquine, a relatively inexpensive drug that’s often used in RA patients in combination with other drugs, might be a good agent to consider adding to the therapeutic mix of RA patients at risk for developing type 2 diabetes, he said in an interview. Although the implications of this finding for the use of abatacept (Orencia) are less clear, it is a signal of a novel benefit from the drug that merits further study, Dr. Michaud said.
The findings also suggest that, when rheumatoid arthritis patients receive statin treatment, they are candidates for more intensive monitoring of diabetes onset, he added.
His analysis focused on the 13,669 RA patients who participated in the National Data Bank for Rheumatic Diseases for at least a year during 2000-2015 and had not been diagnosed as having diabetes of any type at the time they entered the registry. During a median 4.6 years of follow-up, 1,139 patients either self-reported receiving a new diagnosis of type 2 diabetes or began treatment with a hypoglycemic medication. Patients averaged about 59 years old at the time they entered the registry, and about 80% were women.
Dr. Michaud and his associates assessed the incidence rate of type 2 diabetes according to six categories of RA treatment: methotrexate monotherapy, which they used as the reference group; any treatment with abatacept with or without methotrexate; any treatment with hydroxychloroquine; any treatment with a glucocorticoid; treatment with any other disease-modifying antirheumatic drug (DMARD) with methotrexate; and treatment with any other DMARD without methotrexate or no DMARD treatment. A seventh treatment category was treatment with a statin.
A series of time-varying Cox proportional hazard models that adjusted for sociodemographic variables, comorbidities, body mass index, and measures of RA severity showed that, when compared with methotrexate monotherapy, patients treated with abatacept had a statistically significant 48% reduced rate of developing diabetes, and those treated with hydroxychloroquine had a statistically significant 33% reduced diabetes incidence, they reported.
In contrast, compared with methotrexate monotherapy, treatment with a glucocorticoid linked with a statistically significant 31% increased rate of type 2 diabetes, and treatment with a statin linked with a statistically significant 56% increased rate of diabetes. Other forms of DMARD treatment, including tumor necrosis factor inhibitors and other biologic DMARDs, had no statistically significant link with diabetes development.
Dr. Michaud also reported additional analyses that further defined the associations between hydroxychloroquine treatment and reduced diabetes incidence: The reduction in diabetes incidence became statistically significant in patients only when they had received the drug for at least 2 years. The link with reduced diabetes incidence seemed dose dependent, with a stronger protective effect in patients who received at least 400 mg/day. Also, the strength of the protection waned in patients who had discontinued hydroxychloroquine for at least 6 months, and the protective effect completely disappeared once patients were off hydroxychloroquine for at least 1 year. Finally, the link between hydroxychloroquine use and reduced diabetes incidence also was statistically significant in patients who concurrently received a glucocorticoid, but a significant protective association disappeared in patients who received a statin as well as hydroxychloroquine.
Dr. Michaud had no disclosures. The study received no commercial support.
On Twitter @mitchelzoler
LONDON – U.S. rheumatoid arthritis patients had a significantly reduced incidence of type 2 diabetes when on treatment with either hydroxychloroquine or abatacept in an analysis of more than 13,000 patients enrolled in a U.S. national registry during 2000-2015.
The same analysis also showed statistically significant increases in the risk for new-onset type 2 diabetes in rheumatoid arthritis (RA) patients treated with a glucocorticoid or a statin, Kaleb Michaud, Ph.D., reported in a poster at the European Congress of Rheumatology.
“Our findings can inform clinicians about determining appropriate treatment decisions in rheumatoid arthritis patients at increased risk for developing type 2 diabetes,” said Dr. Michaud, an epidemiologist at the University of Nebraska in Omaha and also codirector of the National Data Bank for Rheumatic Diseases in Wichita, Kan., the source of the data used in his study.
Hydroxychloroquine, a relatively inexpensive drug that’s often used in RA patients in combination with other drugs, might be a good agent to consider adding to the therapeutic mix of RA patients at risk for developing type 2 diabetes, he said in an interview. Although the implications of this finding for the use of abatacept (Orencia) are less clear, it is a signal of a novel benefit from the drug that merits further study, Dr. Michaud said.
The findings also suggest that, when rheumatoid arthritis patients receive statin treatment, they are candidates for more intensive monitoring of diabetes onset, he added.
His analysis focused on the 13,669 RA patients who participated in the National Data Bank for Rheumatic Diseases for at least a year during 2000-2015 and had not been diagnosed as having diabetes of any type at the time they entered the registry. During a median 4.6 years of follow-up, 1,139 patients either self-reported receiving a new diagnosis of type 2 diabetes or began treatment with a hypoglycemic medication. Patients averaged about 59 years old at the time they entered the registry, and about 80% were women.
Dr. Michaud and his associates assessed the incidence rate of type 2 diabetes according to six categories of RA treatment: methotrexate monotherapy, which they used as the reference group; any treatment with abatacept with or without methotrexate; any treatment with hydroxychloroquine; any treatment with a glucocorticoid; treatment with any other disease-modifying antirheumatic drug (DMARD) with methotrexate; and treatment with any other DMARD without methotrexate or no DMARD treatment. A seventh treatment category was treatment with a statin.
A series of time-varying Cox proportional hazard models that adjusted for sociodemographic variables, comorbidities, body mass index, and measures of RA severity showed that, when compared with methotrexate monotherapy, patients treated with abatacept had a statistically significant 48% reduced rate of developing diabetes, and those treated with hydroxychloroquine had a statistically significant 33% reduced diabetes incidence, they reported.
In contrast, compared with methotrexate monotherapy, treatment with a glucocorticoid linked with a statistically significant 31% increased rate of type 2 diabetes, and treatment with a statin linked with a statistically significant 56% increased rate of diabetes. Other forms of DMARD treatment, including tumor necrosis factor inhibitors and other biologic DMARDs, had no statistically significant link with diabetes development.
Dr. Michaud also reported additional analyses that further defined the associations between hydroxychloroquine treatment and reduced diabetes incidence: The reduction in diabetes incidence became statistically significant in patients only when they had received the drug for at least 2 years. The link with reduced diabetes incidence seemed dose dependent, with a stronger protective effect in patients who received at least 400 mg/day. Also, the strength of the protection waned in patients who had discontinued hydroxychloroquine for at least 6 months, and the protective effect completely disappeared once patients were off hydroxychloroquine for at least 1 year. Finally, the link between hydroxychloroquine use and reduced diabetes incidence also was statistically significant in patients who concurrently received a glucocorticoid, but a significant protective association disappeared in patients who received a statin as well as hydroxychloroquine.
Dr. Michaud had no disclosures. The study received no commercial support.
On Twitter @mitchelzoler
AT THE EULAR 2016 CONGRESS
Key clinical point: U.S. rheumatoid arthritis patients on hydroxychloroquine or abatacept had a significantly reduced incidence of type 2 diabetes, compared with patients on methotrexate monotherapy.
Major finding: Hydroxychloroquine cut type 2 diabetes incidence by 33% and abatacept cut it by 48%, compared with patients on methotrexate monotherapy.
Data source: Analysis of data collected from 13,669 U.S. rheumatoid arthritis patients enrolled in a registry during 2000-2015.
Disclosures: Dr. Michaud had no disclosures. The study received no commercial support.
Methotrexate in RA: Too low, too short, too few subcutaneous doses?
CHARLOTTE, N.C. – Methotrexate’s role as the mainstay drug of choice to treat rheumatoid arthritis has never been challenged, but questions persist as to why many rheumatologists don’t seem to titrate the dose of the drug up for a long enough period to see significant improvement in disease activity, or don’t instead start with or switch to subcutaneous administration before adding a biologic.
The issue is likely to come to greater prominence soon because of the rise of value-based care, according to Dr. James O’Dell, chief of the division of rheumatology at the University of Nebraska Medical Center, Omaha.
“Rheumatologists will ultimately be held accountable for providing value-based care. We’ll be measured on how well patients are doing, hopefully, and how expensive it is for you to take care of them. When rheumatologists are scored in that way, they will look for ways to provide quality care less expensively. And when they do that, they’ll use more methotrexate, they’ll use higher doses of methotrexate, they’ll use subcutaneous methotrexate, they’ll use more conventional therapy,” he said in an interview.
Dr. O’Dell called for rheumatologists to give methotrexate a longer time to work and to give subcutaneous methotrexate a shot before moving on to biologics, based on analyses of the TEAR (Treatment of Early Aggressive Rheumatoid Arthritis) trial and a U.S. pharmaceutical claims database study of methotrexate-prescribing habits during 2009-2014 that he presented at the annual meeting of the North Carolina Rheumatology Association (NCRA).
Overall, 28% of the early, poor-prognosis RA patients in the TEAR trial who were randomized to receive only oral methotrexate achieved a 28-joint Disease Activity Score (DAS28) of less than 3.2 at 24 weeks. The weekly dose of methotrexate was escalated if there were any tender or swollen joints, rising from 10 mg to 15 mg at 6 weeks and from 15 mg to 20 mg at 24 weeks. Those patients who did well on methotrexate alone showed no clinically meaningful or statistically significant clinical or radiographic differences at week 48 to the end of the study at week 102, compared with patients who initially took methotrexate alone but were randomized to either triple therapy with methotrexate, sulfasalazine, and hydroxychloroquine or combination disease-modifying antirheumatic drug (DMARD) therapy with etanercept and methotrexate after having a DAS28 of 3.2 or higher at 24 weeks, as well as patients who were initially randomized to triple therapy or combination DMARD therapy at the start of the trial (Arthritis Rheum. 2013;65:1985-94).
“The TEAR trial clearly showed that there are many individual patients who do not require anything more than methotrexate alone,” Dr. O’Dell said in an interview. “It also showed that combinations of conventional therapy – methotrexate, sulfasalazine, hydroxychloroquine – are equally efficacious both clinically and radiographically to combinations of methotrexate and etanercept. One of the messages that the TEAR trial clearly showed us is that if you have a patient that needs to step up to a biologic, you can titrate methotrexate up and wait until the 6-month time point to make that decision. And if you wait until 6 months, the patient is not going to be harmed in terms of their ultimate clinical response, and they’re not going to be harmed in terms of radiographic progression.”
However, patients using methotrexate in the TEAR trial only went up to a maximum of 20 mg/week orally, and this itself may be improved on because of evidence for greater bioavailability of oral methotrexate when it is titrated up to even higher doses by 6 months. There also is evidence, although limited, for the superior efficacy and bioavailability of subcutaneous methotrexate, Dr. O’Dell said.
Some data are beginning to indicate the usefulness of going straight to subcutaneous rather than oral methotrexate, he said. Data from the prospective, observational Canadian Early Arthritis Cohort (CATCH) have shown that 55% of patients with early RA who used subcutaneous methotrexate alone as their initial treatment needed it only during the first year of treatment, compared with 23% who were treated initially with oral methotrexate alone. Lack of efficacy was the only statistically significant difference between the two groups that was cited as a reason for failure of the initial treatment, which was the case for 72% of oral users vs. 40% of subcutaneous users.
Another analysis from the CATCH study that was reported at the 2016 Congress of the European League Against Rheumatism (EULAR) suggested that use of subcutaneous methotrexate as an initial treatment for RA could reduce the use of biologics by 53% after adjustment for confounding variables, compared with use of oral methotrexate.
When asked to comment on this, Dr. Daniel E. Furst noted that “there could be an awfully large selection bias in this study. If you ask yourself, ‘Why do people go to subcutaneous in an observational trial?’ it is possible that they didn’t have enough response to the oral. They didn’t go to a higher dose, but, rather stopped methotrexate altogether. The result is that injection looks better. I think this study has a significant flaw, as is often unavoidable in observational studies. This study, per se, does not prove that s.c. methotrexate has an advantage over oral methotrexate in terms of delay of starting a biologic.”
The increased bioavailability of subcutaneous administration versus oral – which is about 17% – does not make it necessarily better for all patients, Dr. Furst contended, although it can certainly be a good option for those affected by gastrointestinal side effects with oral administration. “Given that a 17% increase in bioavailability with subcutaneous administration, this equals about 3-4 mg extra methotrexate. 20 mg/week s.c. methotrexate equals about 23 or 24 mg/week of oral methotrexate. My point is that if a patients tolerates it, giving a little higher dose of oral methotrexate may be the same as giving a slightly higher dose subcutaneously. It is probably true, incidentally, that subcutaneous methotrexate, when given as a generic is less expensive than the oral. A 25-mg/mL vial costs somewhere in the range of one-half to one-third of oral.”
Waiting for 6 months of treatment with oral methotrexate is ideal when a patient is having some response but may not be appropriate for all, according to Dr. Furst, the Carl Pearson Professor of Medicine in the division of rheumatology at the University of California, Los Angeles. He cited research showing 35% of patients who did not yet have an ACR 20–level response at 12 weeks of treatment with oral methotrexate alone at 7.5-20 mg/week can reach that level of response by 26 weeks (Rheumatology. 2010;49[6]:1201-3). “What I took away from that is, on a practical basis, if patients have absolutely no response, however you define it, by 12 weeks, then stop. If they’re having some response, then wait and see if they get more response before stopping,” he said.
The claims analysis that Dr. O’Dell discussed at the NCRA annual meeting gets straight to that point of whether rheumatologists are giving methotrexate a long enough time to work. What compounds the problem, according to Dr. O’Dell, is that too few people are giving oral methotrexate a long enough chance to work or trying subcutaneous dosing. The analysis of claims data from 35,640 RA patients during 2009-2014 that he originally presented at the 2015 American College of Rheumatology annual meeting showed that 44% of patients who started on oral methotrexate alone stayed on it throughout the study period and didn’t need anything else.
Prescribers, however, stopped oral methotrexate at a mean dose of 15.3 mg and moved 87% straight to a biologic without giving subcutaneous methotrexate a shot. They did that after a median of less than 6 months, and within 3 months in more than 40% of patients. Of the patients who were given subcutaneous methotrexate when their oral formulation wasn’t enough that’s all most of them needed, as 72% remained on subcutaneous methotrexate alone for the remainder of the study period. The rest moved on to a biologic, but after a median of almost a year, not a few months. When their time on oral and subcutaneous methotrexate was included, their median time to a biologic was more than 2 years. The same results applied to patients who started treatment in 2009 or 2012.
What the evidence from the claims data study does not say is why physicians are prescribing this way. Some might suggest that the influence of pharmaceutical companies might have something to do with it, although the notion is controversial. A recent systematic review and meta-analysis of randomized, controlled trials that involved a direct comparison of methotrexate monotherapy against methotrexate plus a biologic or biologic monotherapy to treat RA concluded that there is a dosing bias in the trials where methotrexate monotherapy is underdosed because none of the 13 trials that met criteria to be in the review used a methotrexate dose of 25 mg/week (Ann Rheum Dis. 2016 Apr 18. doi: 10.1136/annrheumdis-2016-209383). The investigators, led by Dr. Josefina Durán of Pontificia Universidad Católica de Chile, Santiago, said that they used 25 mg/week as the maximum recommended dose because it is recommended by EULAR and expert opinion.
The contention of bias in the trial due to underdosing was met with pointed questioning from Dr. Joel Kremer of the Center for Rheumatology and Albany (N.Y.) Medical College, and founder and chief executive officer of the Consortium of Rheumatology Researchers of North America (CORRONA). The fact that the authors relied on an expert consensus statement from 2009 to use as their basis for a maximal dose recommendation of 25 mg/week is not “clinical science,” he said in a commentary accompanying the report by Dr. Durán and colleagues (Ann Rheum Dis. 2016 Apr 20. doi: 10.1136/annrheumdis-2016-209505). No trials have shown that pushing the dose from 20 to 25 mg/week gives “significant further clinical improvement without experiencing some possible clinical or laboratory toxicity,” he said, noting that it doesn’t make sense to use a one-size-fits all approach to methotrexate dosing because “the balance between maximal efficacy and toxicity is highly variable and likely to be quite different in diverse genetic populations.” Also, because 7 of the 13 studies cited by the meta-analysis were from 2010 or before, they didn’t have the opportunity to incorporate the higher doses recommended by the expert panel into their designs. Rather than having any preplanned bias, he said, “the dosages and route of administration employed by these trials reflect the common empirical practice in vogue at the time those trials were conducted.”
“To me, the Durán study is significantly overstating the data,” Dr. Furst said. He pointed out that in some of the trials the patients “were actually allowed to use higher doses, but [the meta-analysis authors] said they didn’t. The data showed that they were using 20 mg/week as a mean, but some of them went higher because the standard deviation was 4.5 mg. So to say that it wasn’t the full dosing isn’t quite legit. They actually did allow the full dosing.”
“Dr. Kremer’s point is appropriate,” Dr. O’Dell said, “but it’s not the reality. His point is that there are not a lot of trials that say methotrexate gets better when you use 25 mg/week vs. 20 mg/week, or when you use it subcutaneously vs. orally, even though we know the bioavailability is substantially greater. He’s right in a strict sense, but the whole concept, everything we know, the common sense, tells us that pushing methotrexate to higher tolerable doses and using it subcutaneously is better for our patients. All the data that have looked at that supports that.”
Dr. O’Dell serves on advisory boards for AbbVie, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, Coherus, Antares, and Medac. Dr. Furst has received research support and has received honoraria or fees for serving as a consultant or on the speakers’ bureau for many pharmaceutical companies that manufacturer biologics.
CHARLOTTE, N.C. – Methotrexate’s role as the mainstay drug of choice to treat rheumatoid arthritis has never been challenged, but questions persist as to why many rheumatologists don’t seem to titrate the dose of the drug up for a long enough period to see significant improvement in disease activity, or don’t instead start with or switch to subcutaneous administration before adding a biologic.
The issue is likely to come to greater prominence soon because of the rise of value-based care, according to Dr. James O’Dell, chief of the division of rheumatology at the University of Nebraska Medical Center, Omaha.
“Rheumatologists will ultimately be held accountable for providing value-based care. We’ll be measured on how well patients are doing, hopefully, and how expensive it is for you to take care of them. When rheumatologists are scored in that way, they will look for ways to provide quality care less expensively. And when they do that, they’ll use more methotrexate, they’ll use higher doses of methotrexate, they’ll use subcutaneous methotrexate, they’ll use more conventional therapy,” he said in an interview.
Dr. O’Dell called for rheumatologists to give methotrexate a longer time to work and to give subcutaneous methotrexate a shot before moving on to biologics, based on analyses of the TEAR (Treatment of Early Aggressive Rheumatoid Arthritis) trial and a U.S. pharmaceutical claims database study of methotrexate-prescribing habits during 2009-2014 that he presented at the annual meeting of the North Carolina Rheumatology Association (NCRA).
Overall, 28% of the early, poor-prognosis RA patients in the TEAR trial who were randomized to receive only oral methotrexate achieved a 28-joint Disease Activity Score (DAS28) of less than 3.2 at 24 weeks. The weekly dose of methotrexate was escalated if there were any tender or swollen joints, rising from 10 mg to 15 mg at 6 weeks and from 15 mg to 20 mg at 24 weeks. Those patients who did well on methotrexate alone showed no clinically meaningful or statistically significant clinical or radiographic differences at week 48 to the end of the study at week 102, compared with patients who initially took methotrexate alone but were randomized to either triple therapy with methotrexate, sulfasalazine, and hydroxychloroquine or combination disease-modifying antirheumatic drug (DMARD) therapy with etanercept and methotrexate after having a DAS28 of 3.2 or higher at 24 weeks, as well as patients who were initially randomized to triple therapy or combination DMARD therapy at the start of the trial (Arthritis Rheum. 2013;65:1985-94).
“The TEAR trial clearly showed that there are many individual patients who do not require anything more than methotrexate alone,” Dr. O’Dell said in an interview. “It also showed that combinations of conventional therapy – methotrexate, sulfasalazine, hydroxychloroquine – are equally efficacious both clinically and radiographically to combinations of methotrexate and etanercept. One of the messages that the TEAR trial clearly showed us is that if you have a patient that needs to step up to a biologic, you can titrate methotrexate up and wait until the 6-month time point to make that decision. And if you wait until 6 months, the patient is not going to be harmed in terms of their ultimate clinical response, and they’re not going to be harmed in terms of radiographic progression.”
However, patients using methotrexate in the TEAR trial only went up to a maximum of 20 mg/week orally, and this itself may be improved on because of evidence for greater bioavailability of oral methotrexate when it is titrated up to even higher doses by 6 months. There also is evidence, although limited, for the superior efficacy and bioavailability of subcutaneous methotrexate, Dr. O’Dell said.
Some data are beginning to indicate the usefulness of going straight to subcutaneous rather than oral methotrexate, he said. Data from the prospective, observational Canadian Early Arthritis Cohort (CATCH) have shown that 55% of patients with early RA who used subcutaneous methotrexate alone as their initial treatment needed it only during the first year of treatment, compared with 23% who were treated initially with oral methotrexate alone. Lack of efficacy was the only statistically significant difference between the two groups that was cited as a reason for failure of the initial treatment, which was the case for 72% of oral users vs. 40% of subcutaneous users.
Another analysis from the CATCH study that was reported at the 2016 Congress of the European League Against Rheumatism (EULAR) suggested that use of subcutaneous methotrexate as an initial treatment for RA could reduce the use of biologics by 53% after adjustment for confounding variables, compared with use of oral methotrexate.
When asked to comment on this, Dr. Daniel E. Furst noted that “there could be an awfully large selection bias in this study. If you ask yourself, ‘Why do people go to subcutaneous in an observational trial?’ it is possible that they didn’t have enough response to the oral. They didn’t go to a higher dose, but, rather stopped methotrexate altogether. The result is that injection looks better. I think this study has a significant flaw, as is often unavoidable in observational studies. This study, per se, does not prove that s.c. methotrexate has an advantage over oral methotrexate in terms of delay of starting a biologic.”
The increased bioavailability of subcutaneous administration versus oral – which is about 17% – does not make it necessarily better for all patients, Dr. Furst contended, although it can certainly be a good option for those affected by gastrointestinal side effects with oral administration. “Given that a 17% increase in bioavailability with subcutaneous administration, this equals about 3-4 mg extra methotrexate. 20 mg/week s.c. methotrexate equals about 23 or 24 mg/week of oral methotrexate. My point is that if a patients tolerates it, giving a little higher dose of oral methotrexate may be the same as giving a slightly higher dose subcutaneously. It is probably true, incidentally, that subcutaneous methotrexate, when given as a generic is less expensive than the oral. A 25-mg/mL vial costs somewhere in the range of one-half to one-third of oral.”
Waiting for 6 months of treatment with oral methotrexate is ideal when a patient is having some response but may not be appropriate for all, according to Dr. Furst, the Carl Pearson Professor of Medicine in the division of rheumatology at the University of California, Los Angeles. He cited research showing 35% of patients who did not yet have an ACR 20–level response at 12 weeks of treatment with oral methotrexate alone at 7.5-20 mg/week can reach that level of response by 26 weeks (Rheumatology. 2010;49[6]:1201-3). “What I took away from that is, on a practical basis, if patients have absolutely no response, however you define it, by 12 weeks, then stop. If they’re having some response, then wait and see if they get more response before stopping,” he said.
The claims analysis that Dr. O’Dell discussed at the NCRA annual meeting gets straight to that point of whether rheumatologists are giving methotrexate a long enough time to work. What compounds the problem, according to Dr. O’Dell, is that too few people are giving oral methotrexate a long enough chance to work or trying subcutaneous dosing. The analysis of claims data from 35,640 RA patients during 2009-2014 that he originally presented at the 2015 American College of Rheumatology annual meeting showed that 44% of patients who started on oral methotrexate alone stayed on it throughout the study period and didn’t need anything else.
Prescribers, however, stopped oral methotrexate at a mean dose of 15.3 mg and moved 87% straight to a biologic without giving subcutaneous methotrexate a shot. They did that after a median of less than 6 months, and within 3 months in more than 40% of patients. Of the patients who were given subcutaneous methotrexate when their oral formulation wasn’t enough that’s all most of them needed, as 72% remained on subcutaneous methotrexate alone for the remainder of the study period. The rest moved on to a biologic, but after a median of almost a year, not a few months. When their time on oral and subcutaneous methotrexate was included, their median time to a biologic was more than 2 years. The same results applied to patients who started treatment in 2009 or 2012.
What the evidence from the claims data study does not say is why physicians are prescribing this way. Some might suggest that the influence of pharmaceutical companies might have something to do with it, although the notion is controversial. A recent systematic review and meta-analysis of randomized, controlled trials that involved a direct comparison of methotrexate monotherapy against methotrexate plus a biologic or biologic monotherapy to treat RA concluded that there is a dosing bias in the trials where methotrexate monotherapy is underdosed because none of the 13 trials that met criteria to be in the review used a methotrexate dose of 25 mg/week (Ann Rheum Dis. 2016 Apr 18. doi: 10.1136/annrheumdis-2016-209383). The investigators, led by Dr. Josefina Durán of Pontificia Universidad Católica de Chile, Santiago, said that they used 25 mg/week as the maximum recommended dose because it is recommended by EULAR and expert opinion.
The contention of bias in the trial due to underdosing was met with pointed questioning from Dr. Joel Kremer of the Center for Rheumatology and Albany (N.Y.) Medical College, and founder and chief executive officer of the Consortium of Rheumatology Researchers of North America (CORRONA). The fact that the authors relied on an expert consensus statement from 2009 to use as their basis for a maximal dose recommendation of 25 mg/week is not “clinical science,” he said in a commentary accompanying the report by Dr. Durán and colleagues (Ann Rheum Dis. 2016 Apr 20. doi: 10.1136/annrheumdis-2016-209505). No trials have shown that pushing the dose from 20 to 25 mg/week gives “significant further clinical improvement without experiencing some possible clinical or laboratory toxicity,” he said, noting that it doesn’t make sense to use a one-size-fits all approach to methotrexate dosing because “the balance between maximal efficacy and toxicity is highly variable and likely to be quite different in diverse genetic populations.” Also, because 7 of the 13 studies cited by the meta-analysis were from 2010 or before, they didn’t have the opportunity to incorporate the higher doses recommended by the expert panel into their designs. Rather than having any preplanned bias, he said, “the dosages and route of administration employed by these trials reflect the common empirical practice in vogue at the time those trials were conducted.”
“To me, the Durán study is significantly overstating the data,” Dr. Furst said. He pointed out that in some of the trials the patients “were actually allowed to use higher doses, but [the meta-analysis authors] said they didn’t. The data showed that they were using 20 mg/week as a mean, but some of them went higher because the standard deviation was 4.5 mg. So to say that it wasn’t the full dosing isn’t quite legit. They actually did allow the full dosing.”
“Dr. Kremer’s point is appropriate,” Dr. O’Dell said, “but it’s not the reality. His point is that there are not a lot of trials that say methotrexate gets better when you use 25 mg/week vs. 20 mg/week, or when you use it subcutaneously vs. orally, even though we know the bioavailability is substantially greater. He’s right in a strict sense, but the whole concept, everything we know, the common sense, tells us that pushing methotrexate to higher tolerable doses and using it subcutaneously is better for our patients. All the data that have looked at that supports that.”
Dr. O’Dell serves on advisory boards for AbbVie, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, Coherus, Antares, and Medac. Dr. Furst has received research support and has received honoraria or fees for serving as a consultant or on the speakers’ bureau for many pharmaceutical companies that manufacturer biologics.
CHARLOTTE, N.C. – Methotrexate’s role as the mainstay drug of choice to treat rheumatoid arthritis has never been challenged, but questions persist as to why many rheumatologists don’t seem to titrate the dose of the drug up for a long enough period to see significant improvement in disease activity, or don’t instead start with or switch to subcutaneous administration before adding a biologic.
The issue is likely to come to greater prominence soon because of the rise of value-based care, according to Dr. James O’Dell, chief of the division of rheumatology at the University of Nebraska Medical Center, Omaha.
“Rheumatologists will ultimately be held accountable for providing value-based care. We’ll be measured on how well patients are doing, hopefully, and how expensive it is for you to take care of them. When rheumatologists are scored in that way, they will look for ways to provide quality care less expensively. And when they do that, they’ll use more methotrexate, they’ll use higher doses of methotrexate, they’ll use subcutaneous methotrexate, they’ll use more conventional therapy,” he said in an interview.
Dr. O’Dell called for rheumatologists to give methotrexate a longer time to work and to give subcutaneous methotrexate a shot before moving on to biologics, based on analyses of the TEAR (Treatment of Early Aggressive Rheumatoid Arthritis) trial and a U.S. pharmaceutical claims database study of methotrexate-prescribing habits during 2009-2014 that he presented at the annual meeting of the North Carolina Rheumatology Association (NCRA).
Overall, 28% of the early, poor-prognosis RA patients in the TEAR trial who were randomized to receive only oral methotrexate achieved a 28-joint Disease Activity Score (DAS28) of less than 3.2 at 24 weeks. The weekly dose of methotrexate was escalated if there were any tender or swollen joints, rising from 10 mg to 15 mg at 6 weeks and from 15 mg to 20 mg at 24 weeks. Those patients who did well on methotrexate alone showed no clinically meaningful or statistically significant clinical or radiographic differences at week 48 to the end of the study at week 102, compared with patients who initially took methotrexate alone but were randomized to either triple therapy with methotrexate, sulfasalazine, and hydroxychloroquine or combination disease-modifying antirheumatic drug (DMARD) therapy with etanercept and methotrexate after having a DAS28 of 3.2 or higher at 24 weeks, as well as patients who were initially randomized to triple therapy or combination DMARD therapy at the start of the trial (Arthritis Rheum. 2013;65:1985-94).
“The TEAR trial clearly showed that there are many individual patients who do not require anything more than methotrexate alone,” Dr. O’Dell said in an interview. “It also showed that combinations of conventional therapy – methotrexate, sulfasalazine, hydroxychloroquine – are equally efficacious both clinically and radiographically to combinations of methotrexate and etanercept. One of the messages that the TEAR trial clearly showed us is that if you have a patient that needs to step up to a biologic, you can titrate methotrexate up and wait until the 6-month time point to make that decision. And if you wait until 6 months, the patient is not going to be harmed in terms of their ultimate clinical response, and they’re not going to be harmed in terms of radiographic progression.”
However, patients using methotrexate in the TEAR trial only went up to a maximum of 20 mg/week orally, and this itself may be improved on because of evidence for greater bioavailability of oral methotrexate when it is titrated up to even higher doses by 6 months. There also is evidence, although limited, for the superior efficacy and bioavailability of subcutaneous methotrexate, Dr. O’Dell said.
Some data are beginning to indicate the usefulness of going straight to subcutaneous rather than oral methotrexate, he said. Data from the prospective, observational Canadian Early Arthritis Cohort (CATCH) have shown that 55% of patients with early RA who used subcutaneous methotrexate alone as their initial treatment needed it only during the first year of treatment, compared with 23% who were treated initially with oral methotrexate alone. Lack of efficacy was the only statistically significant difference between the two groups that was cited as a reason for failure of the initial treatment, which was the case for 72% of oral users vs. 40% of subcutaneous users.
Another analysis from the CATCH study that was reported at the 2016 Congress of the European League Against Rheumatism (EULAR) suggested that use of subcutaneous methotrexate as an initial treatment for RA could reduce the use of biologics by 53% after adjustment for confounding variables, compared with use of oral methotrexate.
When asked to comment on this, Dr. Daniel E. Furst noted that “there could be an awfully large selection bias in this study. If you ask yourself, ‘Why do people go to subcutaneous in an observational trial?’ it is possible that they didn’t have enough response to the oral. They didn’t go to a higher dose, but, rather stopped methotrexate altogether. The result is that injection looks better. I think this study has a significant flaw, as is often unavoidable in observational studies. This study, per se, does not prove that s.c. methotrexate has an advantage over oral methotrexate in terms of delay of starting a biologic.”
The increased bioavailability of subcutaneous administration versus oral – which is about 17% – does not make it necessarily better for all patients, Dr. Furst contended, although it can certainly be a good option for those affected by gastrointestinal side effects with oral administration. “Given that a 17% increase in bioavailability with subcutaneous administration, this equals about 3-4 mg extra methotrexate. 20 mg/week s.c. methotrexate equals about 23 or 24 mg/week of oral methotrexate. My point is that if a patients tolerates it, giving a little higher dose of oral methotrexate may be the same as giving a slightly higher dose subcutaneously. It is probably true, incidentally, that subcutaneous methotrexate, when given as a generic is less expensive than the oral. A 25-mg/mL vial costs somewhere in the range of one-half to one-third of oral.”
Waiting for 6 months of treatment with oral methotrexate is ideal when a patient is having some response but may not be appropriate for all, according to Dr. Furst, the Carl Pearson Professor of Medicine in the division of rheumatology at the University of California, Los Angeles. He cited research showing 35% of patients who did not yet have an ACR 20–level response at 12 weeks of treatment with oral methotrexate alone at 7.5-20 mg/week can reach that level of response by 26 weeks (Rheumatology. 2010;49[6]:1201-3). “What I took away from that is, on a practical basis, if patients have absolutely no response, however you define it, by 12 weeks, then stop. If they’re having some response, then wait and see if they get more response before stopping,” he said.
The claims analysis that Dr. O’Dell discussed at the NCRA annual meeting gets straight to that point of whether rheumatologists are giving methotrexate a long enough time to work. What compounds the problem, according to Dr. O’Dell, is that too few people are giving oral methotrexate a long enough chance to work or trying subcutaneous dosing. The analysis of claims data from 35,640 RA patients during 2009-2014 that he originally presented at the 2015 American College of Rheumatology annual meeting showed that 44% of patients who started on oral methotrexate alone stayed on it throughout the study period and didn’t need anything else.
Prescribers, however, stopped oral methotrexate at a mean dose of 15.3 mg and moved 87% straight to a biologic without giving subcutaneous methotrexate a shot. They did that after a median of less than 6 months, and within 3 months in more than 40% of patients. Of the patients who were given subcutaneous methotrexate when their oral formulation wasn’t enough that’s all most of them needed, as 72% remained on subcutaneous methotrexate alone for the remainder of the study period. The rest moved on to a biologic, but after a median of almost a year, not a few months. When their time on oral and subcutaneous methotrexate was included, their median time to a biologic was more than 2 years. The same results applied to patients who started treatment in 2009 or 2012.
What the evidence from the claims data study does not say is why physicians are prescribing this way. Some might suggest that the influence of pharmaceutical companies might have something to do with it, although the notion is controversial. A recent systematic review and meta-analysis of randomized, controlled trials that involved a direct comparison of methotrexate monotherapy against methotrexate plus a biologic or biologic monotherapy to treat RA concluded that there is a dosing bias in the trials where methotrexate monotherapy is underdosed because none of the 13 trials that met criteria to be in the review used a methotrexate dose of 25 mg/week (Ann Rheum Dis. 2016 Apr 18. doi: 10.1136/annrheumdis-2016-209383). The investigators, led by Dr. Josefina Durán of Pontificia Universidad Católica de Chile, Santiago, said that they used 25 mg/week as the maximum recommended dose because it is recommended by EULAR and expert opinion.
The contention of bias in the trial due to underdosing was met with pointed questioning from Dr. Joel Kremer of the Center for Rheumatology and Albany (N.Y.) Medical College, and founder and chief executive officer of the Consortium of Rheumatology Researchers of North America (CORRONA). The fact that the authors relied on an expert consensus statement from 2009 to use as their basis for a maximal dose recommendation of 25 mg/week is not “clinical science,” he said in a commentary accompanying the report by Dr. Durán and colleagues (Ann Rheum Dis. 2016 Apr 20. doi: 10.1136/annrheumdis-2016-209505). No trials have shown that pushing the dose from 20 to 25 mg/week gives “significant further clinical improvement without experiencing some possible clinical or laboratory toxicity,” he said, noting that it doesn’t make sense to use a one-size-fits all approach to methotrexate dosing because “the balance between maximal efficacy and toxicity is highly variable and likely to be quite different in diverse genetic populations.” Also, because 7 of the 13 studies cited by the meta-analysis were from 2010 or before, they didn’t have the opportunity to incorporate the higher doses recommended by the expert panel into their designs. Rather than having any preplanned bias, he said, “the dosages and route of administration employed by these trials reflect the common empirical practice in vogue at the time those trials were conducted.”
“To me, the Durán study is significantly overstating the data,” Dr. Furst said. He pointed out that in some of the trials the patients “were actually allowed to use higher doses, but [the meta-analysis authors] said they didn’t. The data showed that they were using 20 mg/week as a mean, but some of them went higher because the standard deviation was 4.5 mg. So to say that it wasn’t the full dosing isn’t quite legit. They actually did allow the full dosing.”
“Dr. Kremer’s point is appropriate,” Dr. O’Dell said, “but it’s not the reality. His point is that there are not a lot of trials that say methotrexate gets better when you use 25 mg/week vs. 20 mg/week, or when you use it subcutaneously vs. orally, even though we know the bioavailability is substantially greater. He’s right in a strict sense, but the whole concept, everything we know, the common sense, tells us that pushing methotrexate to higher tolerable doses and using it subcutaneously is better for our patients. All the data that have looked at that supports that.”
Dr. O’Dell serves on advisory boards for AbbVie, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, Coherus, Antares, and Medac. Dr. Furst has received research support and has received honoraria or fees for serving as a consultant or on the speakers’ bureau for many pharmaceutical companies that manufacturer biologics.
Anti-TNF certolizumab pegol effective in early RA
Anti-TNF drug certolizumab pegol in combination with methotrexate achieves significantly better outcomes in early active rheumatoid arthritis than does methotrexate alone, according to researchers.
They conducted a randomized, double-blind, placebo-controlled phase III trial of certolizumab pegol (CZP) in combination with dose-optimized methotrexate (MTX) versus dose-optimized methotrexate and placebo (PBO) in 879 treatment-naive patients with moderate to severe, active, progressive RA, and with poor prognostic factors.
After 1 year of treatment, 28.9% of the 660 patients who received the CZP and MTX combination had achieved sustained remission, compared with 15% of the 219 patients in the PBO+MTX group (P less than .001) (Ann Rheum Dis. 2016 May 10. doi: 10.1136/annrheumdis-2015-209057).
The study also found that 43.8% of patients in the treatment arm achieved sustained low disease activity, compared with 28.6% of the control arm (P less than .001). Adjustment for withdrawals in each arm did not significantly bias the results.
CZP with MTX has shown efficacy both in patients with established RA who have shown an insufficient response to MTX alone, and in MTX-naive individuals with early RA, which the authors said justified further study in recently-diagnosed individuals.
“These data demonstrate that CZP+MTX combination therapy results in a significantly higher proportion of patients achieving sREM [sustained remission] than those treated with PBO+MTX, even when using a ‘treat-to-tolerance’ dosing strategy for MTX,” wrote Dr. Paul Emery of Leeds Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds, England, and coauthors.
“Consequently, for patients with poor prognostic factors for severe disease progression, treating early and aggressively may represent a unique opportunity to achieve maximal clinical benefit.”
Significantly more patients treated with CZP and MTX than with PBO achieved an ACR50 response (61.8% vs. 52.6%, P = .023), signifying at least a 50% improvement in the number of tender and swollen joints, and in the patient assessments of disease status, pain, and function.
Patients in the active group also showed greater improvements in physical function, as measured by the Health Assessment Questionnaire–Disability Index, and significantly less radiographic progression. Researchers noted that patients in the active arm of the study showed less joint erosion and less joint space narrowing compared with those in the placebo arm.
“The analysis of radiographic data in C-EARLY demonstrates that CZP+MTX therapy can inhibit structural damage significantly more than MTX alone – the percentage of patients with radiographic nonprogression was significantly higher in the CZP+MTX group compared with the PBO+MTX group,” the authors reported.
The incidence of adverse events was similar in both groups, with nausea, upper respiratory tract infection, urinary tract infection, nasopharyngitis, headache, and increased levels of alanine aminotransferase the most commonly reported events in the active arm.
One death in a patient taking CZP and MTX was caused by disseminated, noncharacterized, mycobacterium infection, which the investigators considered to be related to the study medication. However, the overall rates of serious adverse events and withdrawals due to adverse events were similar between both arms of the study.
Patients given CZP began on a dose of 400 mg at baseline, week 2 and week 4, then 200 mg every 2 weeks until week 52, while the dose of oral MTX used in the study was titrated up from an initial dose of 10 mg/week by 5 mg every 2 weeks, to a maximum of 25 mg/week by week 8.
The authors suggested that the methotrexate titration was an important part of the treatment because it ensured each patient received the maximum-tolerated dose within 8 weeks.
“To our knowledge, there are no previous studies in MTX-naive or DMARD-naive patients with RA where MTX doses have been optimized per-protocol to the levels achieved in C-EARLY,” they wrote. “This optimization may have been responsible, in part, for the response observed for the PBO+MTX and CZP+MTX arms.”
The study and manuscript development was sponsored by UCB Pharma, which also signed off on the manuscript after a review. The authors declared consultancy, speaker’s fees, grants and other funding from a range of pharmaceutical companies including UCB Pharma, and five authors were employees of UCB Pharma.
Anti-TNF drug certolizumab pegol in combination with methotrexate achieves significantly better outcomes in early active rheumatoid arthritis than does methotrexate alone, according to researchers.
They conducted a randomized, double-blind, placebo-controlled phase III trial of certolizumab pegol (CZP) in combination with dose-optimized methotrexate (MTX) versus dose-optimized methotrexate and placebo (PBO) in 879 treatment-naive patients with moderate to severe, active, progressive RA, and with poor prognostic factors.
After 1 year of treatment, 28.9% of the 660 patients who received the CZP and MTX combination had achieved sustained remission, compared with 15% of the 219 patients in the PBO+MTX group (P less than .001) (Ann Rheum Dis. 2016 May 10. doi: 10.1136/annrheumdis-2015-209057).
The study also found that 43.8% of patients in the treatment arm achieved sustained low disease activity, compared with 28.6% of the control arm (P less than .001). Adjustment for withdrawals in each arm did not significantly bias the results.
CZP with MTX has shown efficacy both in patients with established RA who have shown an insufficient response to MTX alone, and in MTX-naive individuals with early RA, which the authors said justified further study in recently-diagnosed individuals.
“These data demonstrate that CZP+MTX combination therapy results in a significantly higher proportion of patients achieving sREM [sustained remission] than those treated with PBO+MTX, even when using a ‘treat-to-tolerance’ dosing strategy for MTX,” wrote Dr. Paul Emery of Leeds Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds, England, and coauthors.
“Consequently, for patients with poor prognostic factors for severe disease progression, treating early and aggressively may represent a unique opportunity to achieve maximal clinical benefit.”
Significantly more patients treated with CZP and MTX than with PBO achieved an ACR50 response (61.8% vs. 52.6%, P = .023), signifying at least a 50% improvement in the number of tender and swollen joints, and in the patient assessments of disease status, pain, and function.
Patients in the active group also showed greater improvements in physical function, as measured by the Health Assessment Questionnaire–Disability Index, and significantly less radiographic progression. Researchers noted that patients in the active arm of the study showed less joint erosion and less joint space narrowing compared with those in the placebo arm.
“The analysis of radiographic data in C-EARLY demonstrates that CZP+MTX therapy can inhibit structural damage significantly more than MTX alone – the percentage of patients with radiographic nonprogression was significantly higher in the CZP+MTX group compared with the PBO+MTX group,” the authors reported.
The incidence of adverse events was similar in both groups, with nausea, upper respiratory tract infection, urinary tract infection, nasopharyngitis, headache, and increased levels of alanine aminotransferase the most commonly reported events in the active arm.
One death in a patient taking CZP and MTX was caused by disseminated, noncharacterized, mycobacterium infection, which the investigators considered to be related to the study medication. However, the overall rates of serious adverse events and withdrawals due to adverse events were similar between both arms of the study.
Patients given CZP began on a dose of 400 mg at baseline, week 2 and week 4, then 200 mg every 2 weeks until week 52, while the dose of oral MTX used in the study was titrated up from an initial dose of 10 mg/week by 5 mg every 2 weeks, to a maximum of 25 mg/week by week 8.
The authors suggested that the methotrexate titration was an important part of the treatment because it ensured each patient received the maximum-tolerated dose within 8 weeks.
“To our knowledge, there are no previous studies in MTX-naive or DMARD-naive patients with RA where MTX doses have been optimized per-protocol to the levels achieved in C-EARLY,” they wrote. “This optimization may have been responsible, in part, for the response observed for the PBO+MTX and CZP+MTX arms.”
The study and manuscript development was sponsored by UCB Pharma, which also signed off on the manuscript after a review. The authors declared consultancy, speaker’s fees, grants and other funding from a range of pharmaceutical companies including UCB Pharma, and five authors were employees of UCB Pharma.
Anti-TNF drug certolizumab pegol in combination with methotrexate achieves significantly better outcomes in early active rheumatoid arthritis than does methotrexate alone, according to researchers.
They conducted a randomized, double-blind, placebo-controlled phase III trial of certolizumab pegol (CZP) in combination with dose-optimized methotrexate (MTX) versus dose-optimized methotrexate and placebo (PBO) in 879 treatment-naive patients with moderate to severe, active, progressive RA, and with poor prognostic factors.
After 1 year of treatment, 28.9% of the 660 patients who received the CZP and MTX combination had achieved sustained remission, compared with 15% of the 219 patients in the PBO+MTX group (P less than .001) (Ann Rheum Dis. 2016 May 10. doi: 10.1136/annrheumdis-2015-209057).
The study also found that 43.8% of patients in the treatment arm achieved sustained low disease activity, compared with 28.6% of the control arm (P less than .001). Adjustment for withdrawals in each arm did not significantly bias the results.
CZP with MTX has shown efficacy both in patients with established RA who have shown an insufficient response to MTX alone, and in MTX-naive individuals with early RA, which the authors said justified further study in recently-diagnosed individuals.
“These data demonstrate that CZP+MTX combination therapy results in a significantly higher proportion of patients achieving sREM [sustained remission] than those treated with PBO+MTX, even when using a ‘treat-to-tolerance’ dosing strategy for MTX,” wrote Dr. Paul Emery of Leeds Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds, England, and coauthors.
“Consequently, for patients with poor prognostic factors for severe disease progression, treating early and aggressively may represent a unique opportunity to achieve maximal clinical benefit.”
Significantly more patients treated with CZP and MTX than with PBO achieved an ACR50 response (61.8% vs. 52.6%, P = .023), signifying at least a 50% improvement in the number of tender and swollen joints, and in the patient assessments of disease status, pain, and function.
Patients in the active group also showed greater improvements in physical function, as measured by the Health Assessment Questionnaire–Disability Index, and significantly less radiographic progression. Researchers noted that patients in the active arm of the study showed less joint erosion and less joint space narrowing compared with those in the placebo arm.
“The analysis of radiographic data in C-EARLY demonstrates that CZP+MTX therapy can inhibit structural damage significantly more than MTX alone – the percentage of patients with radiographic nonprogression was significantly higher in the CZP+MTX group compared with the PBO+MTX group,” the authors reported.
The incidence of adverse events was similar in both groups, with nausea, upper respiratory tract infection, urinary tract infection, nasopharyngitis, headache, and increased levels of alanine aminotransferase the most commonly reported events in the active arm.
One death in a patient taking CZP and MTX was caused by disseminated, noncharacterized, mycobacterium infection, which the investigators considered to be related to the study medication. However, the overall rates of serious adverse events and withdrawals due to adverse events were similar between both arms of the study.
Patients given CZP began on a dose of 400 mg at baseline, week 2 and week 4, then 200 mg every 2 weeks until week 52, while the dose of oral MTX used in the study was titrated up from an initial dose of 10 mg/week by 5 mg every 2 weeks, to a maximum of 25 mg/week by week 8.
The authors suggested that the methotrexate titration was an important part of the treatment because it ensured each patient received the maximum-tolerated dose within 8 weeks.
“To our knowledge, there are no previous studies in MTX-naive or DMARD-naive patients with RA where MTX doses have been optimized per-protocol to the levels achieved in C-EARLY,” they wrote. “This optimization may have been responsible, in part, for the response observed for the PBO+MTX and CZP+MTX arms.”
The study and manuscript development was sponsored by UCB Pharma, which also signed off on the manuscript after a review. The authors declared consultancy, speaker’s fees, grants and other funding from a range of pharmaceutical companies including UCB Pharma, and five authors were employees of UCB Pharma.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Certolizumab pegol and methotrexate is more efficacious than methotrexate alone in treatment-naive individuals with early active rheumatoid arthritis.
Major finding: After 1 year of treatment, 28.9% of the 660 patients who received the certolizumab pegol and methotrexate combination had achieved sustained remission, compared with 15% of the 219 patients in the methotrexate and placebo group.
Data source: A randomized, double-blind, placebo-controlled phase III trial of 879 treatment-naive patients with moderate to severe, active, progressive rheumatoid arthritis.
Disclosures: The study and manuscript development was sponsored by UCB Pharma, which also signed off on the manuscript after a review. The authors declared consultancy, speaker fees, grants, and other funding from a range of pharmaceutical companies including UCB Pharma, and five authors were employees of UCB Pharma.
Doctors ask Congress to stop Part B drug payment test
WASHINGTON – Physician organizations are calling on Congress to stop a proposed federal regulation that would test how Medicare pays for drugs administered in a physician’s office.
Subcommittee member Rep. Larry Bucshon (R-Ind.), a cardiothoracic surgeon, called the premise of the proposed rule – that physicians are making prescribing decisions based on the cost of drugs – “almost an insult to the medical profession.” He has introduced legislation, H.R. 5122, that would require the Centers for Medicare & Medicaid Services to rescind the proposed rule.
CMS argues in the proposed rule that the current payment formula – average sales price (ASP) plus 6% – incentivizes the use of expensive drugs over lower-cost alternatives. Therefore, the agency seeks to run a test – half of doctors would continue to receive ASP plus 6%, while others would receive ASP plus 2.5% and a flat fee of $16.80. The proposed rule also would test other value-based tools. At the May 17 hearing, physicians presented their concerns regarding the proposed rule.
“CMS has yet to produce any evidence indicating that physician prescribing patterns show any correlation to that of choosing higher priced drugs as opposed to appropriate therapeutic treatment for patients,” said Dr. Debra A. Patt, vice president of Texas Oncology. Dr. Patt testified on behalf of the American Society of Clinical Oncology, the Community Oncology Alliance, and the U.S. Oncology Network. “Additionally, there is no evidence that the payment changes contemplated by CMS’s model will improve the quality of care, or for that matter, ensure patients have access to the same level of care they are currently receiving.”Dr. Michael Schweitz, national advocacy chair of the Coalition of State Rheumatology Organizations (CSRO), cited CMS’s admission that this rule will likely have no impact on ASP.
“While we appreciate CMS’s attention to the topic of drug costs, we feel that this proposal is misguided,” Dr. Schweitz testified. “As CMS acknowledges in the rule, the proposed approach ‘does not directly address the manufacturer’s ASP, which is a more significant driver of drug expenditures than the add-on payment amount for Part B drugs.’ Given that a slash to the ASP add-on is unlikely to actually lower costs for patients ... and may jeopardize access, we have requested that CMS withdraw the model and we urge the committee to do the same.”
Dr. Patt also questioned ASP, noting that it is simply an average, and the prices actually paid by rural and small group providers could be higher than larger group and hospital practices – and that difference puts the smaller and rural practices at a potentially significant financial disadvantage.
“Average sales price is by its very nature an average,” Dr. Patt testified. “Some people will pay higher amounts for procurement and some people will pay lower amounts. Larger hospital systems and larger practices have the ability to have contracting arrangements where they purchase at a lower price. … Smaller practices disproportionately pay a higher amount.”
Small practices that are slated to receive ASP plus 2.5% and that flat fee “could be losing money on all of the drugs that they buy. It will be impossible for smaller practices in rural areas to be open,” she added, noting that such a situation could cause care to be shifted to hospital outpatient departments, raising costs as well as access issues. “Cutting provider reimbursement without addressing the ASP, the actual cost of the drug in the first place, is just the wrong approach,” Rep. Bucshon said.
According to the proposed rule, whether or not a practice is in the test or control group would be based on ZIP code. Dr. Patt also noted that if she were in a test ZIP code and a neighboring ZIP code was not, she might have to refer patients to that area still receiving ASP plus 6% and “not at my center.”
Witnesses at the hearing also condemned the way CMS devised the proposed tests. Unlike the recent work on the Medicare Access and CHIP Reauthorization Act regulations and more specialized programs like the Oncology Care Model, Dr. Schweitz and Dr. Patt both testified that CMS made no outreach to the provider community with regards to getting their input before issuing the proposed rule.
They also took exception to the scope of the proposed test, which covers 49 of the 50 states (Maryland is excluded) and provides no mechanism for physicians to opt out under the proposed rule.
“I do see this as an experiment, but we conduct clinical research at our cancer and patients have informed consent,” Dr Patt said.
Dr. Schweitz agreed. “When you look at the goals of this plan, initially it appeared that it was to direct a way to save costs. But in meeting with [the Center for Medicare & Medicaid Innovation], we were advised that this is budget neutral. … So the goal of the program is to collect information which makes it a study, a test. So if the goal is to collect information, and the patients are part of that process, they should be signing informed consent.”
Several physician organizations have called on CMS to withdraw the proposal.
“We are deeply concerned that because the new methodology will frequently not properly cover the cost of physician administration of infused drugs, they will be forced to stop offering patients the ability to receive infusion treatments,” the American College of Rheumatology wrote in comments submitted on the proposed rule. Likewise, CSRO “must oppose the Part B drug payment model as it suffers from serious procedural and substantive flaws that we believe render it unworkable – and it does nothing to actually address drug prices,” according its comments.
While the proposal has garnered backlash from several directions, rheumatologists are seeing it as particularly burdensome because of the high price of medications with very limited options to substitute for lower-cost alternatives.
“Although we certainly seek to control costs for patients and Medicare whenever possible, the proposed new methodology does not adequately consider the higher average cost many of our physicians have acquiring, handling, administering, and billing for drugs and biologics,” according to the comments submitted by the ACR.
Indeed, comments from CSRO point out that when factoring in budget sequestration, the actual reimbursement physicians are receiving is ASP plus 4.4%, and doctors are actually losing money on certain drug purchases.
Of additional concern is that the proposed rule does not address ASP itself.
“A far greater concern than the add-on percentage is the underlying ASP, and the steep, fast price increases that these medications show each quarter, according to comments from the CSRO.
From 2007 to 2016, first-quarter ASP for infliximab rose from $53.73 to $79.90; ASP for abatacept rose from $18.70 to $39.44, according to CSRO comments. “These ASP increases are unsustainable for both the Medicare program and its beneficiaries, and we would like to work with CMS to explore actual solutions to stem the increases in those underlying prices.”
In its comments, ACR proposed a number of potential paths forward, starting with certain practices that should be exempted from the proposed demonstration: physician groups with 25 or fewer physicians; physician-owned practices that are located in rural and medically underserved areas; reimbursement changes for drugs and biologics that do not have an alternative with more than a 20% ASP differential; and drugs and biologics where there are three or fewer members of the drug class or biologics.
ACR also proposed altering the add-on formula that takes into account the costs of storing and administering supplies.
“For example, CMS could use a formula for reimbursement of ASP plus 6% or $500 (whichever is lower),” ACR said in its comments. “This formula would allow CMS to effectively target spending on expensive drugs, while leaving in place reimbursement rates for cheaper drugs.”
Additionally, ACR called for CMS to delay the testing of more value-based tools until it understands the impact of the ASP changes that are to be tested under this proposal.
CSRO does not have any specific policy recommendations to replace or modify the proposed rule, but rather calls for CMS to bring together all stakeholders, including patients, providers, payers, and manufacturers to devise a system that would work to the benefit of all while ensuring the best outcomes for patients, Dr. Schweitz said in an interview.
WASHINGTON – Physician organizations are calling on Congress to stop a proposed federal regulation that would test how Medicare pays for drugs administered in a physician’s office.
Subcommittee member Rep. Larry Bucshon (R-Ind.), a cardiothoracic surgeon, called the premise of the proposed rule – that physicians are making prescribing decisions based on the cost of drugs – “almost an insult to the medical profession.” He has introduced legislation, H.R. 5122, that would require the Centers for Medicare & Medicaid Services to rescind the proposed rule.
CMS argues in the proposed rule that the current payment formula – average sales price (ASP) plus 6% – incentivizes the use of expensive drugs over lower-cost alternatives. Therefore, the agency seeks to run a test – half of doctors would continue to receive ASP plus 6%, while others would receive ASP plus 2.5% and a flat fee of $16.80. The proposed rule also would test other value-based tools. At the May 17 hearing, physicians presented their concerns regarding the proposed rule.
“CMS has yet to produce any evidence indicating that physician prescribing patterns show any correlation to that of choosing higher priced drugs as opposed to appropriate therapeutic treatment for patients,” said Dr. Debra A. Patt, vice president of Texas Oncology. Dr. Patt testified on behalf of the American Society of Clinical Oncology, the Community Oncology Alliance, and the U.S. Oncology Network. “Additionally, there is no evidence that the payment changes contemplated by CMS’s model will improve the quality of care, or for that matter, ensure patients have access to the same level of care they are currently receiving.”Dr. Michael Schweitz, national advocacy chair of the Coalition of State Rheumatology Organizations (CSRO), cited CMS’s admission that this rule will likely have no impact on ASP.
“While we appreciate CMS’s attention to the topic of drug costs, we feel that this proposal is misguided,” Dr. Schweitz testified. “As CMS acknowledges in the rule, the proposed approach ‘does not directly address the manufacturer’s ASP, which is a more significant driver of drug expenditures than the add-on payment amount for Part B drugs.’ Given that a slash to the ASP add-on is unlikely to actually lower costs for patients ... and may jeopardize access, we have requested that CMS withdraw the model and we urge the committee to do the same.”
Dr. Patt also questioned ASP, noting that it is simply an average, and the prices actually paid by rural and small group providers could be higher than larger group and hospital practices – and that difference puts the smaller and rural practices at a potentially significant financial disadvantage.
“Average sales price is by its very nature an average,” Dr. Patt testified. “Some people will pay higher amounts for procurement and some people will pay lower amounts. Larger hospital systems and larger practices have the ability to have contracting arrangements where they purchase at a lower price. … Smaller practices disproportionately pay a higher amount.”
Small practices that are slated to receive ASP plus 2.5% and that flat fee “could be losing money on all of the drugs that they buy. It will be impossible for smaller practices in rural areas to be open,” she added, noting that such a situation could cause care to be shifted to hospital outpatient departments, raising costs as well as access issues. “Cutting provider reimbursement without addressing the ASP, the actual cost of the drug in the first place, is just the wrong approach,” Rep. Bucshon said.
According to the proposed rule, whether or not a practice is in the test or control group would be based on ZIP code. Dr. Patt also noted that if she were in a test ZIP code and a neighboring ZIP code was not, she might have to refer patients to that area still receiving ASP plus 6% and “not at my center.”
Witnesses at the hearing also condemned the way CMS devised the proposed tests. Unlike the recent work on the Medicare Access and CHIP Reauthorization Act regulations and more specialized programs like the Oncology Care Model, Dr. Schweitz and Dr. Patt both testified that CMS made no outreach to the provider community with regards to getting their input before issuing the proposed rule.
They also took exception to the scope of the proposed test, which covers 49 of the 50 states (Maryland is excluded) and provides no mechanism for physicians to opt out under the proposed rule.
“I do see this as an experiment, but we conduct clinical research at our cancer and patients have informed consent,” Dr Patt said.
Dr. Schweitz agreed. “When you look at the goals of this plan, initially it appeared that it was to direct a way to save costs. But in meeting with [the Center for Medicare & Medicaid Innovation], we were advised that this is budget neutral. … So the goal of the program is to collect information which makes it a study, a test. So if the goal is to collect information, and the patients are part of that process, they should be signing informed consent.”
Several physician organizations have called on CMS to withdraw the proposal.
“We are deeply concerned that because the new methodology will frequently not properly cover the cost of physician administration of infused drugs, they will be forced to stop offering patients the ability to receive infusion treatments,” the American College of Rheumatology wrote in comments submitted on the proposed rule. Likewise, CSRO “must oppose the Part B drug payment model as it suffers from serious procedural and substantive flaws that we believe render it unworkable – and it does nothing to actually address drug prices,” according its comments.
While the proposal has garnered backlash from several directions, rheumatologists are seeing it as particularly burdensome because of the high price of medications with very limited options to substitute for lower-cost alternatives.
“Although we certainly seek to control costs for patients and Medicare whenever possible, the proposed new methodology does not adequately consider the higher average cost many of our physicians have acquiring, handling, administering, and billing for drugs and biologics,” according to the comments submitted by the ACR.
Indeed, comments from CSRO point out that when factoring in budget sequestration, the actual reimbursement physicians are receiving is ASP plus 4.4%, and doctors are actually losing money on certain drug purchases.
Of additional concern is that the proposed rule does not address ASP itself.
“A far greater concern than the add-on percentage is the underlying ASP, and the steep, fast price increases that these medications show each quarter, according to comments from the CSRO.
From 2007 to 2016, first-quarter ASP for infliximab rose from $53.73 to $79.90; ASP for abatacept rose from $18.70 to $39.44, according to CSRO comments. “These ASP increases are unsustainable for both the Medicare program and its beneficiaries, and we would like to work with CMS to explore actual solutions to stem the increases in those underlying prices.”
In its comments, ACR proposed a number of potential paths forward, starting with certain practices that should be exempted from the proposed demonstration: physician groups with 25 or fewer physicians; physician-owned practices that are located in rural and medically underserved areas; reimbursement changes for drugs and biologics that do not have an alternative with more than a 20% ASP differential; and drugs and biologics where there are three or fewer members of the drug class or biologics.
ACR also proposed altering the add-on formula that takes into account the costs of storing and administering supplies.
“For example, CMS could use a formula for reimbursement of ASP plus 6% or $500 (whichever is lower),” ACR said in its comments. “This formula would allow CMS to effectively target spending on expensive drugs, while leaving in place reimbursement rates for cheaper drugs.”
Additionally, ACR called for CMS to delay the testing of more value-based tools until it understands the impact of the ASP changes that are to be tested under this proposal.
CSRO does not have any specific policy recommendations to replace or modify the proposed rule, but rather calls for CMS to bring together all stakeholders, including patients, providers, payers, and manufacturers to devise a system that would work to the benefit of all while ensuring the best outcomes for patients, Dr. Schweitz said in an interview.
WASHINGTON – Physician organizations are calling on Congress to stop a proposed federal regulation that would test how Medicare pays for drugs administered in a physician’s office.
Subcommittee member Rep. Larry Bucshon (R-Ind.), a cardiothoracic surgeon, called the premise of the proposed rule – that physicians are making prescribing decisions based on the cost of drugs – “almost an insult to the medical profession.” He has introduced legislation, H.R. 5122, that would require the Centers for Medicare & Medicaid Services to rescind the proposed rule.
CMS argues in the proposed rule that the current payment formula – average sales price (ASP) plus 6% – incentivizes the use of expensive drugs over lower-cost alternatives. Therefore, the agency seeks to run a test – half of doctors would continue to receive ASP plus 6%, while others would receive ASP plus 2.5% and a flat fee of $16.80. The proposed rule also would test other value-based tools. At the May 17 hearing, physicians presented their concerns regarding the proposed rule.
“CMS has yet to produce any evidence indicating that physician prescribing patterns show any correlation to that of choosing higher priced drugs as opposed to appropriate therapeutic treatment for patients,” said Dr. Debra A. Patt, vice president of Texas Oncology. Dr. Patt testified on behalf of the American Society of Clinical Oncology, the Community Oncology Alliance, and the U.S. Oncology Network. “Additionally, there is no evidence that the payment changes contemplated by CMS’s model will improve the quality of care, or for that matter, ensure patients have access to the same level of care they are currently receiving.”Dr. Michael Schweitz, national advocacy chair of the Coalition of State Rheumatology Organizations (CSRO), cited CMS’s admission that this rule will likely have no impact on ASP.
“While we appreciate CMS’s attention to the topic of drug costs, we feel that this proposal is misguided,” Dr. Schweitz testified. “As CMS acknowledges in the rule, the proposed approach ‘does not directly address the manufacturer’s ASP, which is a more significant driver of drug expenditures than the add-on payment amount for Part B drugs.’ Given that a slash to the ASP add-on is unlikely to actually lower costs for patients ... and may jeopardize access, we have requested that CMS withdraw the model and we urge the committee to do the same.”
Dr. Patt also questioned ASP, noting that it is simply an average, and the prices actually paid by rural and small group providers could be higher than larger group and hospital practices – and that difference puts the smaller and rural practices at a potentially significant financial disadvantage.
“Average sales price is by its very nature an average,” Dr. Patt testified. “Some people will pay higher amounts for procurement and some people will pay lower amounts. Larger hospital systems and larger practices have the ability to have contracting arrangements where they purchase at a lower price. … Smaller practices disproportionately pay a higher amount.”
Small practices that are slated to receive ASP plus 2.5% and that flat fee “could be losing money on all of the drugs that they buy. It will be impossible for smaller practices in rural areas to be open,” she added, noting that such a situation could cause care to be shifted to hospital outpatient departments, raising costs as well as access issues. “Cutting provider reimbursement without addressing the ASP, the actual cost of the drug in the first place, is just the wrong approach,” Rep. Bucshon said.
According to the proposed rule, whether or not a practice is in the test or control group would be based on ZIP code. Dr. Patt also noted that if she were in a test ZIP code and a neighboring ZIP code was not, she might have to refer patients to that area still receiving ASP plus 6% and “not at my center.”
Witnesses at the hearing also condemned the way CMS devised the proposed tests. Unlike the recent work on the Medicare Access and CHIP Reauthorization Act regulations and more specialized programs like the Oncology Care Model, Dr. Schweitz and Dr. Patt both testified that CMS made no outreach to the provider community with regards to getting their input before issuing the proposed rule.
They also took exception to the scope of the proposed test, which covers 49 of the 50 states (Maryland is excluded) and provides no mechanism for physicians to opt out under the proposed rule.
“I do see this as an experiment, but we conduct clinical research at our cancer and patients have informed consent,” Dr Patt said.
Dr. Schweitz agreed. “When you look at the goals of this plan, initially it appeared that it was to direct a way to save costs. But in meeting with [the Center for Medicare & Medicaid Innovation], we were advised that this is budget neutral. … So the goal of the program is to collect information which makes it a study, a test. So if the goal is to collect information, and the patients are part of that process, they should be signing informed consent.”
Several physician organizations have called on CMS to withdraw the proposal.
“We are deeply concerned that because the new methodology will frequently not properly cover the cost of physician administration of infused drugs, they will be forced to stop offering patients the ability to receive infusion treatments,” the American College of Rheumatology wrote in comments submitted on the proposed rule. Likewise, CSRO “must oppose the Part B drug payment model as it suffers from serious procedural and substantive flaws that we believe render it unworkable – and it does nothing to actually address drug prices,” according its comments.
While the proposal has garnered backlash from several directions, rheumatologists are seeing it as particularly burdensome because of the high price of medications with very limited options to substitute for lower-cost alternatives.
“Although we certainly seek to control costs for patients and Medicare whenever possible, the proposed new methodology does not adequately consider the higher average cost many of our physicians have acquiring, handling, administering, and billing for drugs and biologics,” according to the comments submitted by the ACR.
Indeed, comments from CSRO point out that when factoring in budget sequestration, the actual reimbursement physicians are receiving is ASP plus 4.4%, and doctors are actually losing money on certain drug purchases.
Of additional concern is that the proposed rule does not address ASP itself.
“A far greater concern than the add-on percentage is the underlying ASP, and the steep, fast price increases that these medications show each quarter, according to comments from the CSRO.
From 2007 to 2016, first-quarter ASP for infliximab rose from $53.73 to $79.90; ASP for abatacept rose from $18.70 to $39.44, according to CSRO comments. “These ASP increases are unsustainable for both the Medicare program and its beneficiaries, and we would like to work with CMS to explore actual solutions to stem the increases in those underlying prices.”
In its comments, ACR proposed a number of potential paths forward, starting with certain practices that should be exempted from the proposed demonstration: physician groups with 25 or fewer physicians; physician-owned practices that are located in rural and medically underserved areas; reimbursement changes for drugs and biologics that do not have an alternative with more than a 20% ASP differential; and drugs and biologics where there are three or fewer members of the drug class or biologics.
ACR also proposed altering the add-on formula that takes into account the costs of storing and administering supplies.
“For example, CMS could use a formula for reimbursement of ASP plus 6% or $500 (whichever is lower),” ACR said in its comments. “This formula would allow CMS to effectively target spending on expensive drugs, while leaving in place reimbursement rates for cheaper drugs.”
Additionally, ACR called for CMS to delay the testing of more value-based tools until it understands the impact of the ASP changes that are to be tested under this proposal.
CSRO does not have any specific policy recommendations to replace or modify the proposed rule, but rather calls for CMS to bring together all stakeholders, including patients, providers, payers, and manufacturers to devise a system that would work to the benefit of all while ensuring the best outcomes for patients, Dr. Schweitz said in an interview.
AT HOUSE ENERGY AND COMMERCE HEALTH SUBCOMMITTEE HEARING