User login
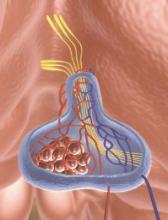
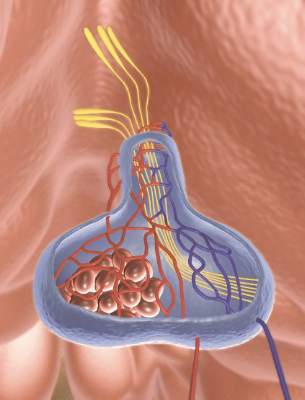
Thyroid surgery on the rise
There was a steady rise in the number of endocrine procedures being performed in the United States over the past decade, according to a new publication from the Endocrine Society.
Thyroid disease treatment costs in U.S. women alone totaled $4.3 billion in 2008, representing a cost of $343 per patient, according to the report, authored by the Endocrine Society under the guidance of an advisory committee chaired by Dr. Robert A. Vigersky, head of the Diabetes Institute at Walter Reed National Military Medical Center in Bethesda, Md.
The number of endocrine procedures also has increased steadily, mainly because of new and improved surgical techniques, but the annual case number is predicted to go as high as 173,000 by 2020.
Thyroid conditions affect five times as many women as men, particularly in the case of Hashimoto’s disease – the incidence in women is more than eight times that in men – and Graves’ disease, where women have a nearly sixfold higher incidence than men, according to the report, which is available online.
The most common thyroid disease is thyroid nodules, which international studies estimate affect up to 60% of the population. In the United States, findings from a study using chest radiography found nearly one in four adult outpatients had thyroid nodules.
The diagnosis of thyroid nodules has increased as imaging technologies such as CT scanning, ultrasound, and MRI improve on the traditional diagnostic method of physical examination. The increase in thyroid surgery for this condition has raised questions about whether this has led to improved outcomes.
Hyperthyroidism and hypothyroidism have a lower prevalence, although estimates suggest as much as 13% of the U.S. population has subclinical hypothyroidism.
This has implications particularly in pregnancy, as subclinical hypothyroidism may increase the risk of pregnancy complications, including preeclampsia, placental abruption, preterm birth, and neonatal mortality.
One study found that 12.4% of healthy pregnant women with no known thyroid disease had subclinical hypothyroidism. Overt hypothyroidism affects around 0.8% of adults, while overt hyperthyroidism affects just 0.5% of adults, the report stated.
Graves’ disease is one form of hyperthyroidism, and it has a prevalence in the U.S. population of 0.63%-1.49%, mostly affecting women, African Americans, and Asian/Pacific Islanders.
This condition is most commonly treated with antithyroid drugs or radioactive iodine therapy, although around 0.7% of patients undergo thyroidectomies.
Another group of thyroid diseases, grouped under the umbrella term of thyroiditis, includes a common element of inflammation of the thyroid gland. Among them is the autoimmune condition Hashimoto’s thyroiditis, which is thought to affect around 4.6% of the population.
Postpartum thyroiditis – an inflammatory autoimmune condition that develops in the first year after delivery – is estimated to have a prevalence around 4.5%, according to one review.
“Evidence is emerging that as women age subclinical hypothyroidism – as a sequel of postpartum thyroiditis – predisposes them to cardiovascular disease,” wrote Dr. Vigersky, also of the Uniformed Services University of the Health Sciences, and the other panel members.
“Hence, postpartum thyroiditis is no longer considered a mild and transient disorder.”
The report was produced by the Endocrine Society. There were no conflicts of interest declared.
There was a steady rise in the number of endocrine procedures being performed in the United States over the past decade, according to a new publication from the Endocrine Society.
Thyroid disease treatment costs in U.S. women alone totaled $4.3 billion in 2008, representing a cost of $343 per patient, according to the report, authored by the Endocrine Society under the guidance of an advisory committee chaired by Dr. Robert A. Vigersky, head of the Diabetes Institute at Walter Reed National Military Medical Center in Bethesda, Md.
The number of endocrine procedures also has increased steadily, mainly because of new and improved surgical techniques, but the annual case number is predicted to go as high as 173,000 by 2020.
Thyroid conditions affect five times as many women as men, particularly in the case of Hashimoto’s disease – the incidence in women is more than eight times that in men – and Graves’ disease, where women have a nearly sixfold higher incidence than men, according to the report, which is available online.
The most common thyroid disease is thyroid nodules, which international studies estimate affect up to 60% of the population. In the United States, findings from a study using chest radiography found nearly one in four adult outpatients had thyroid nodules.
The diagnosis of thyroid nodules has increased as imaging technologies such as CT scanning, ultrasound, and MRI improve on the traditional diagnostic method of physical examination. The increase in thyroid surgery for this condition has raised questions about whether this has led to improved outcomes.
Hyperthyroidism and hypothyroidism have a lower prevalence, although estimates suggest as much as 13% of the U.S. population has subclinical hypothyroidism.
This has implications particularly in pregnancy, as subclinical hypothyroidism may increase the risk of pregnancy complications, including preeclampsia, placental abruption, preterm birth, and neonatal mortality.
One study found that 12.4% of healthy pregnant women with no known thyroid disease had subclinical hypothyroidism. Overt hypothyroidism affects around 0.8% of adults, while overt hyperthyroidism affects just 0.5% of adults, the report stated.
Graves’ disease is one form of hyperthyroidism, and it has a prevalence in the U.S. population of 0.63%-1.49%, mostly affecting women, African Americans, and Asian/Pacific Islanders.
This condition is most commonly treated with antithyroid drugs or radioactive iodine therapy, although around 0.7% of patients undergo thyroidectomies.
Another group of thyroid diseases, grouped under the umbrella term of thyroiditis, includes a common element of inflammation of the thyroid gland. Among them is the autoimmune condition Hashimoto’s thyroiditis, which is thought to affect around 4.6% of the population.
Postpartum thyroiditis – an inflammatory autoimmune condition that develops in the first year after delivery – is estimated to have a prevalence around 4.5%, according to one review.
“Evidence is emerging that as women age subclinical hypothyroidism – as a sequel of postpartum thyroiditis – predisposes them to cardiovascular disease,” wrote Dr. Vigersky, also of the Uniformed Services University of the Health Sciences, and the other panel members.
“Hence, postpartum thyroiditis is no longer considered a mild and transient disorder.”
The report was produced by the Endocrine Society. There were no conflicts of interest declared.
There was a steady rise in the number of endocrine procedures being performed in the United States over the past decade, according to a new publication from the Endocrine Society.
Thyroid disease treatment costs in U.S. women alone totaled $4.3 billion in 2008, representing a cost of $343 per patient, according to the report, authored by the Endocrine Society under the guidance of an advisory committee chaired by Dr. Robert A. Vigersky, head of the Diabetes Institute at Walter Reed National Military Medical Center in Bethesda, Md.
The number of endocrine procedures also has increased steadily, mainly because of new and improved surgical techniques, but the annual case number is predicted to go as high as 173,000 by 2020.
Thyroid conditions affect five times as many women as men, particularly in the case of Hashimoto’s disease – the incidence in women is more than eight times that in men – and Graves’ disease, where women have a nearly sixfold higher incidence than men, according to the report, which is available online.
The most common thyroid disease is thyroid nodules, which international studies estimate affect up to 60% of the population. In the United States, findings from a study using chest radiography found nearly one in four adult outpatients had thyroid nodules.
The diagnosis of thyroid nodules has increased as imaging technologies such as CT scanning, ultrasound, and MRI improve on the traditional diagnostic method of physical examination. The increase in thyroid surgery for this condition has raised questions about whether this has led to improved outcomes.
Hyperthyroidism and hypothyroidism have a lower prevalence, although estimates suggest as much as 13% of the U.S. population has subclinical hypothyroidism.
This has implications particularly in pregnancy, as subclinical hypothyroidism may increase the risk of pregnancy complications, including preeclampsia, placental abruption, preterm birth, and neonatal mortality.
One study found that 12.4% of healthy pregnant women with no known thyroid disease had subclinical hypothyroidism. Overt hypothyroidism affects around 0.8% of adults, while overt hyperthyroidism affects just 0.5% of adults, the report stated.
Graves’ disease is one form of hyperthyroidism, and it has a prevalence in the U.S. population of 0.63%-1.49%, mostly affecting women, African Americans, and Asian/Pacific Islanders.
This condition is most commonly treated with antithyroid drugs or radioactive iodine therapy, although around 0.7% of patients undergo thyroidectomies.
Another group of thyroid diseases, grouped under the umbrella term of thyroiditis, includes a common element of inflammation of the thyroid gland. Among them is the autoimmune condition Hashimoto’s thyroiditis, which is thought to affect around 4.6% of the population.
Postpartum thyroiditis – an inflammatory autoimmune condition that develops in the first year after delivery – is estimated to have a prevalence around 4.5%, according to one review.
“Evidence is emerging that as women age subclinical hypothyroidism – as a sequel of postpartum thyroiditis – predisposes them to cardiovascular disease,” wrote Dr. Vigersky, also of the Uniformed Services University of the Health Sciences, and the other panel members.
“Hence, postpartum thyroiditis is no longer considered a mild and transient disorder.”
The report was produced by the Endocrine Society. There were no conflicts of interest declared.
Key clinical point: There was a steady rise in the number of endocrine procedures performed in the United States over the past decade.
Major finding: In 2008, thyroid disease treatment costs in women alone totaled $4.3 billion.
Data source: A publication of the Endocrine Society.
Disclosures: The report was produced by the Endocrine Society. There were no conflicts of interest declared.
Subclinical hyperthyroidism linked to higher fracture risk
Individuals with subclinical hyperthyroidism are at increased risk of hip and other fractures, according to the authors of a meta-analysis.
Researchers examined data from 70,298 individuals – 4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism – enrolled in 13 prospective cohort studies.
After adjusting for age, sex, and other fracture risk factors, the researchers found that individuals with subclinical hyperthyroidism had a 28% increase in the risk of any fracture and a 36% increased risk of hip fracture compared to individuals with normal thyroid function.
Subclinical hyperthyroidism – defined as a thyroid-stimulating hormone (TSH) level of less than 0.45 mIU/L with normal FT4 levels – was also associated with a 16% increase in the risk of nonspine fracture, according to a paper published online in the May 26 edition of JAMA.
Men with subclinical hyperthyroidism had a more than 3.5-fold increased in the risk of spine fracture, but the increase was not significant in women.
Lower TSH was associated with higher fracture rates, and the analysis showed a 61% increase in the risk of hip fracture and more than a 3.5-fold increase in spine fracture risk among individuals with a TSH less than 0.10 mIU/L.
The analysis yielded no link between subclinical hypothyroidism and fracture risk, and a comparison of fracture risk between individuals treated with thyroxine at baseline and untreated participants also showed no impact of thyroxine therapy on fracture outcomes (JAMA 2015, May 26 [doi:10.1001/jama.2015.5161].
“In prospective cohort studies, data about the association between subclinical thyroid dysfunction and fracture risk are in conflict because of inclusion of participants with overt thyroid disease and small sample sizes of participants with thyroid dysfunction or fracture events,” wrote Dr. Manuel R. Blum of Bern University Hospital, Switzerland, and an international team of coauthors.
They proposed three mechanisms by which thyroid dysfunction may affect fracture risk.
“First, thyroid hormones have been shown to have effects on osteoclasts and osteoblasts, with thyroid status in the upper normal range or excess thyroid hormones leading to accelerated bone turnover with bone loss and increased fracture risk,” they wrote.
Subclinical hyperthyroidism may also increase the risk of falls by affecting muscle strength and coordination, and thyroxine supplementation was also suggested as impacting fracture risk.
“Endogenous subclinical hyperthyroidism may be undetected for years because symptoms of subclinical hyperthyroidism are often nonspecific or absent,” the authors wrote. “This phenomenon has the potential to lead to a greater length of time for adverse associations with bone metabolism.”
The authors stressed the limitations of the observational data; for example, that thyroid function was assessed only at baseline and that some individuals may have progressed to overt thyroid dysfunction over the course of the study, and a lack of uniform definition of fracture type across the cohorts.
They said their findings supported current guideline recommendations that anyone aged 65 years or older, with subclinical hyperthyroidism and a TSH persistently lower than 0.1 mIU/L, should be treated, and treatment should be considered in those individuals with low TSH but still above 0.1 mIU/L.
The Swiss National Science Foundation and Swiss Heart Foundation supported the study. Some authors disclosed personal fees, grants and funding from a range of pharmaceutical companies.
Individuals with subclinical hyperthyroidism are at increased risk of hip and other fractures, according to the authors of a meta-analysis.
Researchers examined data from 70,298 individuals – 4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism – enrolled in 13 prospective cohort studies.
After adjusting for age, sex, and other fracture risk factors, the researchers found that individuals with subclinical hyperthyroidism had a 28% increase in the risk of any fracture and a 36% increased risk of hip fracture compared to individuals with normal thyroid function.
Subclinical hyperthyroidism – defined as a thyroid-stimulating hormone (TSH) level of less than 0.45 mIU/L with normal FT4 levels – was also associated with a 16% increase in the risk of nonspine fracture, according to a paper published online in the May 26 edition of JAMA.
Men with subclinical hyperthyroidism had a more than 3.5-fold increased in the risk of spine fracture, but the increase was not significant in women.
Lower TSH was associated with higher fracture rates, and the analysis showed a 61% increase in the risk of hip fracture and more than a 3.5-fold increase in spine fracture risk among individuals with a TSH less than 0.10 mIU/L.
The analysis yielded no link between subclinical hypothyroidism and fracture risk, and a comparison of fracture risk between individuals treated with thyroxine at baseline and untreated participants also showed no impact of thyroxine therapy on fracture outcomes (JAMA 2015, May 26 [doi:10.1001/jama.2015.5161].
“In prospective cohort studies, data about the association between subclinical thyroid dysfunction and fracture risk are in conflict because of inclusion of participants with overt thyroid disease and small sample sizes of participants with thyroid dysfunction or fracture events,” wrote Dr. Manuel R. Blum of Bern University Hospital, Switzerland, and an international team of coauthors.
They proposed three mechanisms by which thyroid dysfunction may affect fracture risk.
“First, thyroid hormones have been shown to have effects on osteoclasts and osteoblasts, with thyroid status in the upper normal range or excess thyroid hormones leading to accelerated bone turnover with bone loss and increased fracture risk,” they wrote.
Subclinical hyperthyroidism may also increase the risk of falls by affecting muscle strength and coordination, and thyroxine supplementation was also suggested as impacting fracture risk.
“Endogenous subclinical hyperthyroidism may be undetected for years because symptoms of subclinical hyperthyroidism are often nonspecific or absent,” the authors wrote. “This phenomenon has the potential to lead to a greater length of time for adverse associations with bone metabolism.”
The authors stressed the limitations of the observational data; for example, that thyroid function was assessed only at baseline and that some individuals may have progressed to overt thyroid dysfunction over the course of the study, and a lack of uniform definition of fracture type across the cohorts.
They said their findings supported current guideline recommendations that anyone aged 65 years or older, with subclinical hyperthyroidism and a TSH persistently lower than 0.1 mIU/L, should be treated, and treatment should be considered in those individuals with low TSH but still above 0.1 mIU/L.
The Swiss National Science Foundation and Swiss Heart Foundation supported the study. Some authors disclosed personal fees, grants and funding from a range of pharmaceutical companies.
Individuals with subclinical hyperthyroidism are at increased risk of hip and other fractures, according to the authors of a meta-analysis.
Researchers examined data from 70,298 individuals – 4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism – enrolled in 13 prospective cohort studies.
After adjusting for age, sex, and other fracture risk factors, the researchers found that individuals with subclinical hyperthyroidism had a 28% increase in the risk of any fracture and a 36% increased risk of hip fracture compared to individuals with normal thyroid function.
Subclinical hyperthyroidism – defined as a thyroid-stimulating hormone (TSH) level of less than 0.45 mIU/L with normal FT4 levels – was also associated with a 16% increase in the risk of nonspine fracture, according to a paper published online in the May 26 edition of JAMA.
Men with subclinical hyperthyroidism had a more than 3.5-fold increased in the risk of spine fracture, but the increase was not significant in women.
Lower TSH was associated with higher fracture rates, and the analysis showed a 61% increase in the risk of hip fracture and more than a 3.5-fold increase in spine fracture risk among individuals with a TSH less than 0.10 mIU/L.
The analysis yielded no link between subclinical hypothyroidism and fracture risk, and a comparison of fracture risk between individuals treated with thyroxine at baseline and untreated participants also showed no impact of thyroxine therapy on fracture outcomes (JAMA 2015, May 26 [doi:10.1001/jama.2015.5161].
“In prospective cohort studies, data about the association between subclinical thyroid dysfunction and fracture risk are in conflict because of inclusion of participants with overt thyroid disease and small sample sizes of participants with thyroid dysfunction or fracture events,” wrote Dr. Manuel R. Blum of Bern University Hospital, Switzerland, and an international team of coauthors.
They proposed three mechanisms by which thyroid dysfunction may affect fracture risk.
“First, thyroid hormones have been shown to have effects on osteoclasts and osteoblasts, with thyroid status in the upper normal range or excess thyroid hormones leading to accelerated bone turnover with bone loss and increased fracture risk,” they wrote.
Subclinical hyperthyroidism may also increase the risk of falls by affecting muscle strength and coordination, and thyroxine supplementation was also suggested as impacting fracture risk.
“Endogenous subclinical hyperthyroidism may be undetected for years because symptoms of subclinical hyperthyroidism are often nonspecific or absent,” the authors wrote. “This phenomenon has the potential to lead to a greater length of time for adverse associations with bone metabolism.”
The authors stressed the limitations of the observational data; for example, that thyroid function was assessed only at baseline and that some individuals may have progressed to overt thyroid dysfunction over the course of the study, and a lack of uniform definition of fracture type across the cohorts.
They said their findings supported current guideline recommendations that anyone aged 65 years or older, with subclinical hyperthyroidism and a TSH persistently lower than 0.1 mIU/L, should be treated, and treatment should be considered in those individuals with low TSH but still above 0.1 mIU/L.
The Swiss National Science Foundation and Swiss Heart Foundation supported the study. Some authors disclosed personal fees, grants and funding from a range of pharmaceutical companies.
FROM JAMA
Key clinical point: Subclinical hyperthyroidism is associated with an increased risk of hip and other fractures.
Major finding: Individuals with subclinical hyperthyroidism had a 28% increase in their risk of any fracture compared to individuals with normal thyroid function.
Data source: A meta-analysis of 13 prospective cohort studies comprising 70,298 individuals.
Disclosures: The Swiss National Science Foundation and Swiss Heart Foundation supported the study. Some authors disclosed personal fees, grants, and funding from a range of pharmaceutical companies.
AACE: Artificial sweeteners tentatively linked to Hashimoto’s thyroiditis
NASHVILLE, TENN.– You might want to discourage your patients with Hashimoto’s thyroiditis from using artificial sweeteners, according to investigators from Mount Sinai Hospital in New York.
They found that of 100 patients with antibody-confirmed Hashimoto’s thyroiditis (HT), 53 reported using the equivalent of 3.5 packets of artificial sweetener per day – mostly aspartame (Equal, NutraSweet) or sucralose (Splenda) – as estimated from a questionnaire about their daily intake of sugar-free foods. The investigators also found a weak but significant correlation between daily consumption of sugar substitutes and elevated levels of TSH (r = 0.23, P = 0.05).
They checked those results against 125 controls who were referred for Hashimoto’s work up but turned out to be antibody negative; 15 (12%) regularly used artificial sweeteners, 110 (88%) did not.
Perhaps artificial sweeteners, which are widely used in diet soda, yogurt, gum, ice cream, and other products, somehow amp up the immune system to attack the thyroid in some people. “I think there is something to this,” said lead investigator Dr. Issac Sachmechi, an associate professor of medicine, endocrinology, diabetes, and bone disease at Mount Sinai.
“When patients come to me for Hashimoto’s and I diagnose them, I ask them if they use artificial sweeteners. If they do, I mention this study and highly suggest they stop using them,” he said at the annual meeting of the American Association of Clinical Endocrinologists.
So far, three have taken his advice. Two had a complete reversal of disease, going from antibody positive to antibody negative and no longer needing thyroid replacements. Quitting artificial sweeteners had no effect on the third person. Her mother also had autoimmune thyroiditis, so perhaps there was a genetic component that went beyond any possible impact of artificial sweeteners, Dr. Sachmechi said.
The link, however, is far from proven; spontaneous remission has been reported before in the medical literature. Dr. Sachmechi said he plans to continue looking into the issue.
There is, however, biological plausibility for a connection. Aspartame, for instance, is metabolized to formaldehyde, which has been associated with type 4 delayed hypersensitivity reactions. There’s also been suggestions that sucralose may have negative effects on the thymus and spleen, and possible associations with autoimmune disorders, Dr. Sachmechi said.
The idea to look into the issue came “about 3 years ago, when I got a consult from a neurologist about a young woman with paresthesia. He looked at her thyroid function, and her TSH was elevated, so he sent her to me. I put her on Synthroid, 125 mcg. During her follow up, I saw that her requirement for Synthroid was going down, and eventually I had to stop it. I asked her if she was doing anything differently, and she told me she had read that artificial sweeteners may actually cause weight gain, so she stopped them to lose weight.” That was the only change, Dr. Sachmechi said.
There was no outside funding for the work and Dr. Sachmechi had no relevant disclosures.
NASHVILLE, TENN.– You might want to discourage your patients with Hashimoto’s thyroiditis from using artificial sweeteners, according to investigators from Mount Sinai Hospital in New York.
They found that of 100 patients with antibody-confirmed Hashimoto’s thyroiditis (HT), 53 reported using the equivalent of 3.5 packets of artificial sweetener per day – mostly aspartame (Equal, NutraSweet) or sucralose (Splenda) – as estimated from a questionnaire about their daily intake of sugar-free foods. The investigators also found a weak but significant correlation between daily consumption of sugar substitutes and elevated levels of TSH (r = 0.23, P = 0.05).
They checked those results against 125 controls who were referred for Hashimoto’s work up but turned out to be antibody negative; 15 (12%) regularly used artificial sweeteners, 110 (88%) did not.
Perhaps artificial sweeteners, which are widely used in diet soda, yogurt, gum, ice cream, and other products, somehow amp up the immune system to attack the thyroid in some people. “I think there is something to this,” said lead investigator Dr. Issac Sachmechi, an associate professor of medicine, endocrinology, diabetes, and bone disease at Mount Sinai.
“When patients come to me for Hashimoto’s and I diagnose them, I ask them if they use artificial sweeteners. If they do, I mention this study and highly suggest they stop using them,” he said at the annual meeting of the American Association of Clinical Endocrinologists.
So far, three have taken his advice. Two had a complete reversal of disease, going from antibody positive to antibody negative and no longer needing thyroid replacements. Quitting artificial sweeteners had no effect on the third person. Her mother also had autoimmune thyroiditis, so perhaps there was a genetic component that went beyond any possible impact of artificial sweeteners, Dr. Sachmechi said.
The link, however, is far from proven; spontaneous remission has been reported before in the medical literature. Dr. Sachmechi said he plans to continue looking into the issue.
There is, however, biological plausibility for a connection. Aspartame, for instance, is metabolized to formaldehyde, which has been associated with type 4 delayed hypersensitivity reactions. There’s also been suggestions that sucralose may have negative effects on the thymus and spleen, and possible associations with autoimmune disorders, Dr. Sachmechi said.
The idea to look into the issue came “about 3 years ago, when I got a consult from a neurologist about a young woman with paresthesia. He looked at her thyroid function, and her TSH was elevated, so he sent her to me. I put her on Synthroid, 125 mcg. During her follow up, I saw that her requirement for Synthroid was going down, and eventually I had to stop it. I asked her if she was doing anything differently, and she told me she had read that artificial sweeteners may actually cause weight gain, so she stopped them to lose weight.” That was the only change, Dr. Sachmechi said.
There was no outside funding for the work and Dr. Sachmechi had no relevant disclosures.
NASHVILLE, TENN.– You might want to discourage your patients with Hashimoto’s thyroiditis from using artificial sweeteners, according to investigators from Mount Sinai Hospital in New York.
They found that of 100 patients with antibody-confirmed Hashimoto’s thyroiditis (HT), 53 reported using the equivalent of 3.5 packets of artificial sweetener per day – mostly aspartame (Equal, NutraSweet) or sucralose (Splenda) – as estimated from a questionnaire about their daily intake of sugar-free foods. The investigators also found a weak but significant correlation between daily consumption of sugar substitutes and elevated levels of TSH (r = 0.23, P = 0.05).
They checked those results against 125 controls who were referred for Hashimoto’s work up but turned out to be antibody negative; 15 (12%) regularly used artificial sweeteners, 110 (88%) did not.
Perhaps artificial sweeteners, which are widely used in diet soda, yogurt, gum, ice cream, and other products, somehow amp up the immune system to attack the thyroid in some people. “I think there is something to this,” said lead investigator Dr. Issac Sachmechi, an associate professor of medicine, endocrinology, diabetes, and bone disease at Mount Sinai.
“When patients come to me for Hashimoto’s and I diagnose them, I ask them if they use artificial sweeteners. If they do, I mention this study and highly suggest they stop using them,” he said at the annual meeting of the American Association of Clinical Endocrinologists.
So far, three have taken his advice. Two had a complete reversal of disease, going from antibody positive to antibody negative and no longer needing thyroid replacements. Quitting artificial sweeteners had no effect on the third person. Her mother also had autoimmune thyroiditis, so perhaps there was a genetic component that went beyond any possible impact of artificial sweeteners, Dr. Sachmechi said.
The link, however, is far from proven; spontaneous remission has been reported before in the medical literature. Dr. Sachmechi said he plans to continue looking into the issue.
There is, however, biological plausibility for a connection. Aspartame, for instance, is metabolized to formaldehyde, which has been associated with type 4 delayed hypersensitivity reactions. There’s also been suggestions that sucralose may have negative effects on the thymus and spleen, and possible associations with autoimmune disorders, Dr. Sachmechi said.
The idea to look into the issue came “about 3 years ago, when I got a consult from a neurologist about a young woman with paresthesia. He looked at her thyroid function, and her TSH was elevated, so he sent her to me. I put her on Synthroid, 125 mcg. During her follow up, I saw that her requirement for Synthroid was going down, and eventually I had to stop it. I asked her if she was doing anything differently, and she told me she had read that artificial sweeteners may actually cause weight gain, so she stopped them to lose weight.” That was the only change, Dr. Sachmechi said.
There was no outside funding for the work and Dr. Sachmechi had no relevant disclosures.
AT AACE 2015
Key clinical point: Discourage Hashimoto’s patients from using sugar substitutes.
Major finding: In 100 patients with the disease, daily consumption of artificial sweeteners correlated with elevated levels of TSH (r = 0.23, P = 0.05).
Data source: Observational study of 225 patients.
Disclosures: There was no outside funding for the work, and the lead investigator has no relevant disclosures.
AACE: Free testosterone, prolactin levels signal MRI need in men with secondary hypogonadism
NASHVILLE, TENN.– Checking free testosterone and prolactin levels can help identify which men with secondary hypogonadism should get brain MRIs.
If the levels are normal, it’s unlikely that hypothalamic-pituitary imaging will reveal a medically significant pituitary abnormality. “However, MRI is warranted in men with high prolactin or very low free testosterone [because] both are associated with pituitary structural abnormalities,” said lead investigator and medical resident Dr. Cong Santoso of Wright-Patterson Air Force Base Medical Center in Dayton, Ohio.
Dr. Santoso and her associate, Dr. Thomas Koroscil of Wright State University, Dayton, reviewed the charts of 88 men with secondary hypogonadism, all of whom had gotten an MRI, which in many places was once a routine investigation for the problem. Men with known pituitary lesions, pituitary apoplexy, infiltrative diseases, infections, or glucocorticoid use were among those excluded from the study.
A total of 16 men (18%) had abnormal MRIs. Adenomas were found in nine (10%) and empty sellas in seven (8%). Men with pituitary adenomas had significantly lower free testosterone (FT), compared with those who had normal MRIs (18.7 pg/mL vs. 36.4 pg/mL). Men with empty sella syndrome had significantly higher prolactin (PRL), compared with men who had normal MRIs (21.4 ng/mL vs. 11.2 ng/mL), Dr. Santoso reported.
There was no difference in levels of follicle-stimulating hormone or luteinizing hormone. There was a trend towards lower total testosterone in men with structural abnormalities, but it was not significant, probably because of the low numbers in the study, Dr. Santoso said at the annual meeting of the American Association of Clinical Endocrinologists.
At 16%, the incidence of pituitary imaging abnormalities was not greater than the prevalence of abnormalities in the general population, “indicating that there is little value to routinely obtain MRI in the evaluation of men with secondary hypogonadism” when FT and PRL are normal, she said.
Endocrine Society guidelines for secondary hypogonadism in men note that “the diagnostic yield of pituitary imaging to exclude pituitary and/or hypothalamic tumor can be improved by performing [MRIs] in men with serum testosterone less than 150 ng/dL, panhypopituitarism, persistent hyperprolactinemia, or symptoms of tumor mass effect.”
The findings from Dr. Santoso’s study add to that advice by suggesting a role for FT and by pinning high PRL and low FT to particular structural abnormalities. “We are adding to the guidelines to make them” stronger, she said.
About 70 men (80%) had type 2 diabetes or metabolic syndrome, both of which are associated with secondary hypogonadism, but they had no endocrinologic differences, compared with the other men.
Endocrinologists at Dr. Santoso’s institutions have moved away from routine MRIs in men with secondary hypogonadism and are relying more on lab values to indicate when MRIs are needed, she said.
There was no outside funding for the work, and Dr. Santoso had no disclosures.
NASHVILLE, TENN.– Checking free testosterone and prolactin levels can help identify which men with secondary hypogonadism should get brain MRIs.
If the levels are normal, it’s unlikely that hypothalamic-pituitary imaging will reveal a medically significant pituitary abnormality. “However, MRI is warranted in men with high prolactin or very low free testosterone [because] both are associated with pituitary structural abnormalities,” said lead investigator and medical resident Dr. Cong Santoso of Wright-Patterson Air Force Base Medical Center in Dayton, Ohio.
Dr. Santoso and her associate, Dr. Thomas Koroscil of Wright State University, Dayton, reviewed the charts of 88 men with secondary hypogonadism, all of whom had gotten an MRI, which in many places was once a routine investigation for the problem. Men with known pituitary lesions, pituitary apoplexy, infiltrative diseases, infections, or glucocorticoid use were among those excluded from the study.
A total of 16 men (18%) had abnormal MRIs. Adenomas were found in nine (10%) and empty sellas in seven (8%). Men with pituitary adenomas had significantly lower free testosterone (FT), compared with those who had normal MRIs (18.7 pg/mL vs. 36.4 pg/mL). Men with empty sella syndrome had significantly higher prolactin (PRL), compared with men who had normal MRIs (21.4 ng/mL vs. 11.2 ng/mL), Dr. Santoso reported.
There was no difference in levels of follicle-stimulating hormone or luteinizing hormone. There was a trend towards lower total testosterone in men with structural abnormalities, but it was not significant, probably because of the low numbers in the study, Dr. Santoso said at the annual meeting of the American Association of Clinical Endocrinologists.
At 16%, the incidence of pituitary imaging abnormalities was not greater than the prevalence of abnormalities in the general population, “indicating that there is little value to routinely obtain MRI in the evaluation of men with secondary hypogonadism” when FT and PRL are normal, she said.
Endocrine Society guidelines for secondary hypogonadism in men note that “the diagnostic yield of pituitary imaging to exclude pituitary and/or hypothalamic tumor can be improved by performing [MRIs] in men with serum testosterone less than 150 ng/dL, panhypopituitarism, persistent hyperprolactinemia, or symptoms of tumor mass effect.”
The findings from Dr. Santoso’s study add to that advice by suggesting a role for FT and by pinning high PRL and low FT to particular structural abnormalities. “We are adding to the guidelines to make them” stronger, she said.
About 70 men (80%) had type 2 diabetes or metabolic syndrome, both of which are associated with secondary hypogonadism, but they had no endocrinologic differences, compared with the other men.
Endocrinologists at Dr. Santoso’s institutions have moved away from routine MRIs in men with secondary hypogonadism and are relying more on lab values to indicate when MRIs are needed, she said.
There was no outside funding for the work, and Dr. Santoso had no disclosures.
NASHVILLE, TENN.– Checking free testosterone and prolactin levels can help identify which men with secondary hypogonadism should get brain MRIs.
If the levels are normal, it’s unlikely that hypothalamic-pituitary imaging will reveal a medically significant pituitary abnormality. “However, MRI is warranted in men with high prolactin or very low free testosterone [because] both are associated with pituitary structural abnormalities,” said lead investigator and medical resident Dr. Cong Santoso of Wright-Patterson Air Force Base Medical Center in Dayton, Ohio.
Dr. Santoso and her associate, Dr. Thomas Koroscil of Wright State University, Dayton, reviewed the charts of 88 men with secondary hypogonadism, all of whom had gotten an MRI, which in many places was once a routine investigation for the problem. Men with known pituitary lesions, pituitary apoplexy, infiltrative diseases, infections, or glucocorticoid use were among those excluded from the study.
A total of 16 men (18%) had abnormal MRIs. Adenomas were found in nine (10%) and empty sellas in seven (8%). Men with pituitary adenomas had significantly lower free testosterone (FT), compared with those who had normal MRIs (18.7 pg/mL vs. 36.4 pg/mL). Men with empty sella syndrome had significantly higher prolactin (PRL), compared with men who had normal MRIs (21.4 ng/mL vs. 11.2 ng/mL), Dr. Santoso reported.
There was no difference in levels of follicle-stimulating hormone or luteinizing hormone. There was a trend towards lower total testosterone in men with structural abnormalities, but it was not significant, probably because of the low numbers in the study, Dr. Santoso said at the annual meeting of the American Association of Clinical Endocrinologists.
At 16%, the incidence of pituitary imaging abnormalities was not greater than the prevalence of abnormalities in the general population, “indicating that there is little value to routinely obtain MRI in the evaluation of men with secondary hypogonadism” when FT and PRL are normal, she said.
Endocrine Society guidelines for secondary hypogonadism in men note that “the diagnostic yield of pituitary imaging to exclude pituitary and/or hypothalamic tumor can be improved by performing [MRIs] in men with serum testosterone less than 150 ng/dL, panhypopituitarism, persistent hyperprolactinemia, or symptoms of tumor mass effect.”
The findings from Dr. Santoso’s study add to that advice by suggesting a role for FT and by pinning high PRL and low FT to particular structural abnormalities. “We are adding to the guidelines to make them” stronger, she said.
About 70 men (80%) had type 2 diabetes or metabolic syndrome, both of which are associated with secondary hypogonadism, but they had no endocrinologic differences, compared with the other men.
Endocrinologists at Dr. Santoso’s institutions have moved away from routine MRIs in men with secondary hypogonadism and are relying more on lab values to indicate when MRIs are needed, she said.
There was no outside funding for the work, and Dr. Santoso had no disclosures.
AT AACE 2015
Key clinical point: Lab values can guide imaging decisions in men with pituitary adenomas.
Major finding: Men with pituitary adenomas had significantly lower free testosterone, compared with those who had normal MRIs (18.7 pg/mL vs. 36.4 pg/mL). Men with empty sella syndrome had significantly higher prolactin, compared with men who had normal MRIs (21.4 ng/mL vs. 11.2 ng/mL).
Data source: Review of<b/>88 men with secondary hypogonadism.
Disclosures: There was no outside funding for the work, and the lead investigator had no disclosures.
Copeptin levels predicted diabetes insipidus after pituitary surgery
Copeptin levels of less than 2.5 pmol/L reliably identified diabetes insipidus after pituitary surgery, while levels of more than 30 pmol/L ruled out the condition, investigators reported online in the Journal of Clinical Endocrinology and Metabolism.
“Copeptin represents a new, early, and reliable single marker for postoperative diabetes insipidus in the post–pituitary surgery setting, where no such marker currently is known,” said Dr. Bettina Winzeler at University Hospital of Basel (Switzerland) and her associates. Postoperative measures of copeptin levels might help clinicians to differentiate patients who need closer postsurgical inpatient observation from those who can safely be discharged.
About 16%-34% of patients who undergo surgery in the sellar region develop central diabetes insipidus, which, without prompt rehydration, can cause severe hypernatremia, Dr. Winzeler and her associates noted. Risk factors for postoperative diabetes insipidus are not useful markers; however, copeptin, a surrogate of arginine vasopressin, is stable and can be measured quickly and reliably.
The researchers hypothesized that patients with postoperative diabetes insipidus would not have the markedly elevated copeptin levels that surgical stress normally triggers when posterior pituitary function is adequate. To test that theory, they measured daily postoperative copeptin levels for 205 consecutive patients who underwent surgeries for sellar lesions or tumors near the hypothalamus or pituitary gland (J. Clin. Endocrinol. Metab. 2015 Apr. 29 [doi:10.1210/jc.2014-4527]).
One day after surgery, 22 (81.5%) of the 27 patients whose copeptin levels were less than 2.5 pmol/L had diabetes insipidus (positive predictive value, 81%; specificity, 97%), said the researchers. In contrast, one of 40 (2.5%) patients with copeptin levels greater than 30 pmol/L had diabetes insipidus (negative predictive value 95%; sensitivity, 94%). Patients with diabetes insipidus also had significantly lower median and interquartile (25th-75th percentile) copeptin levels, compared with other patients (median for patients with diabetes insipidus, 2.9 pmol/L; interquartile range, 1.9-7.9 pmol/L; median for other patients, 10.8 pmol/L; IQR, 5.2-30.4 pmol/L; P less than .001). In the multivariate analysis, low postoperative copeptin levels remained associated with diabetes insipidus after adjusting for age, gender, body-mass index, tumor size and type of surgery, history of surgery or radiotherapy, cerebrospinal fluid leakage, and serum sodium concentration, the investigators reported (odds ratio, 1.62; 95% confidence interval, 1.25-2.10; P less than .001).
The study did not use standardized diagnostic criteria for diabetes insipidus, and postoperative blood samples were obtained as part of daily care and not at fixed time points, Dr. Winzeler and her associates noted. “Absence of standardized sampling for copeptin measurement at a defined early time point presumably decreased copeptin accuracy in this study, rendering our findings conservative,” they cautioned.
The Swiss National Foundation partially funded the research. Three coauthors reported having received speaker honoraria from Thermo Scientific Biomarkers, which develops and makes the copeptin assay.
Copeptin levels of less than 2.5 pmol/L reliably identified diabetes insipidus after pituitary surgery, while levels of more than 30 pmol/L ruled out the condition, investigators reported online in the Journal of Clinical Endocrinology and Metabolism.
“Copeptin represents a new, early, and reliable single marker for postoperative diabetes insipidus in the post–pituitary surgery setting, where no such marker currently is known,” said Dr. Bettina Winzeler at University Hospital of Basel (Switzerland) and her associates. Postoperative measures of copeptin levels might help clinicians to differentiate patients who need closer postsurgical inpatient observation from those who can safely be discharged.
About 16%-34% of patients who undergo surgery in the sellar region develop central diabetes insipidus, which, without prompt rehydration, can cause severe hypernatremia, Dr. Winzeler and her associates noted. Risk factors for postoperative diabetes insipidus are not useful markers; however, copeptin, a surrogate of arginine vasopressin, is stable and can be measured quickly and reliably.
The researchers hypothesized that patients with postoperative diabetes insipidus would not have the markedly elevated copeptin levels that surgical stress normally triggers when posterior pituitary function is adequate. To test that theory, they measured daily postoperative copeptin levels for 205 consecutive patients who underwent surgeries for sellar lesions or tumors near the hypothalamus or pituitary gland (J. Clin. Endocrinol. Metab. 2015 Apr. 29 [doi:10.1210/jc.2014-4527]).
One day after surgery, 22 (81.5%) of the 27 patients whose copeptin levels were less than 2.5 pmol/L had diabetes insipidus (positive predictive value, 81%; specificity, 97%), said the researchers. In contrast, one of 40 (2.5%) patients with copeptin levels greater than 30 pmol/L had diabetes insipidus (negative predictive value 95%; sensitivity, 94%). Patients with diabetes insipidus also had significantly lower median and interquartile (25th-75th percentile) copeptin levels, compared with other patients (median for patients with diabetes insipidus, 2.9 pmol/L; interquartile range, 1.9-7.9 pmol/L; median for other patients, 10.8 pmol/L; IQR, 5.2-30.4 pmol/L; P less than .001). In the multivariate analysis, low postoperative copeptin levels remained associated with diabetes insipidus after adjusting for age, gender, body-mass index, tumor size and type of surgery, history of surgery or radiotherapy, cerebrospinal fluid leakage, and serum sodium concentration, the investigators reported (odds ratio, 1.62; 95% confidence interval, 1.25-2.10; P less than .001).
The study did not use standardized diagnostic criteria for diabetes insipidus, and postoperative blood samples were obtained as part of daily care and not at fixed time points, Dr. Winzeler and her associates noted. “Absence of standardized sampling for copeptin measurement at a defined early time point presumably decreased copeptin accuracy in this study, rendering our findings conservative,” they cautioned.
The Swiss National Foundation partially funded the research. Three coauthors reported having received speaker honoraria from Thermo Scientific Biomarkers, which develops and makes the copeptin assay.
Copeptin levels of less than 2.5 pmol/L reliably identified diabetes insipidus after pituitary surgery, while levels of more than 30 pmol/L ruled out the condition, investigators reported online in the Journal of Clinical Endocrinology and Metabolism.
“Copeptin represents a new, early, and reliable single marker for postoperative diabetes insipidus in the post–pituitary surgery setting, where no such marker currently is known,” said Dr. Bettina Winzeler at University Hospital of Basel (Switzerland) and her associates. Postoperative measures of copeptin levels might help clinicians to differentiate patients who need closer postsurgical inpatient observation from those who can safely be discharged.
About 16%-34% of patients who undergo surgery in the sellar region develop central diabetes insipidus, which, without prompt rehydration, can cause severe hypernatremia, Dr. Winzeler and her associates noted. Risk factors for postoperative diabetes insipidus are not useful markers; however, copeptin, a surrogate of arginine vasopressin, is stable and can be measured quickly and reliably.
The researchers hypothesized that patients with postoperative diabetes insipidus would not have the markedly elevated copeptin levels that surgical stress normally triggers when posterior pituitary function is adequate. To test that theory, they measured daily postoperative copeptin levels for 205 consecutive patients who underwent surgeries for sellar lesions or tumors near the hypothalamus or pituitary gland (J. Clin. Endocrinol. Metab. 2015 Apr. 29 [doi:10.1210/jc.2014-4527]).
One day after surgery, 22 (81.5%) of the 27 patients whose copeptin levels were less than 2.5 pmol/L had diabetes insipidus (positive predictive value, 81%; specificity, 97%), said the researchers. In contrast, one of 40 (2.5%) patients with copeptin levels greater than 30 pmol/L had diabetes insipidus (negative predictive value 95%; sensitivity, 94%). Patients with diabetes insipidus also had significantly lower median and interquartile (25th-75th percentile) copeptin levels, compared with other patients (median for patients with diabetes insipidus, 2.9 pmol/L; interquartile range, 1.9-7.9 pmol/L; median for other patients, 10.8 pmol/L; IQR, 5.2-30.4 pmol/L; P less than .001). In the multivariate analysis, low postoperative copeptin levels remained associated with diabetes insipidus after adjusting for age, gender, body-mass index, tumor size and type of surgery, history of surgery or radiotherapy, cerebrospinal fluid leakage, and serum sodium concentration, the investigators reported (odds ratio, 1.62; 95% confidence interval, 1.25-2.10; P less than .001).
The study did not use standardized diagnostic criteria for diabetes insipidus, and postoperative blood samples were obtained as part of daily care and not at fixed time points, Dr. Winzeler and her associates noted. “Absence of standardized sampling for copeptin measurement at a defined early time point presumably decreased copeptin accuracy in this study, rendering our findings conservative,” they cautioned.
The Swiss National Foundation partially funded the research. Three coauthors reported having received speaker honoraria from Thermo Scientific Biomarkers, which develops and makes the copeptin assay.
Key clinical point: Low copeptin levels reliably predicted diabetes insipidus after pituitary surgery.
Major finding: A day after surgery, 81.5% of patients with copeptin levels less than 2.5 pmol/L had diabetes insipidus, compared with 2.5% of patients with levels greater than 30 pmol/L.
Data source: Multicenter, prospective, observational cohort study of 205 consecutive pituitary surgery patients.
Disclosures: The Swiss National Foundation partially funded the research. Three coauthors reported having received speaker honoraria from Thermo Scientific Biomarkers, which develops and makes the copeptin assay.
Nonfunctioning pituitary adenoma linked to higher mortality in women
Mortality among patients with nonfunctioning pituitary adenomas (NFPA) was higher in women than in man as well as in patients with a young age at diagnosis and those diagnosed with diabetes insipidus, according to a nationwide population-based study in Sweden.
Dr. Daniel S. Olsson of the University of Gothenburg and Sahlgrenska University Hospital in Gothenburg, Sweden, and his associates identified 2,795 unique patients with NFPA from the the Swedish National Patient Registry who had been diagnosed between 1987 and 2011. The mean age at diagnosis was 58 years, and the mean follow-up time was 7 years.
Standardized mortality ratio was increased in women with NFPA (1.29; 95% confidence interval, 1.11-1.48) but not in men (1.00; 95% CI, 0.88-1.12). Women with a diagnosis of hypopituitarism and/or diabetes insipidus had increased mortality ratio, but men did not (J. Clin. Endocrinol. Metab. 2015 May 7 [doi: 10.1210/jc.2015-1475]).
“We found an association between increased mortality and the presence of hypopituitarism in women with NFPA, which has been indicated in previous studies, but not clearly demonstrated. In contrast, men with hypopituitarism had no excess mortality,” the authors wrote.
Other predictors of excess mortality included being diagnosed under 40 years of age, as those patients had an increased SMR of 2.68 (95% CI, 1.23-5.09). Patients with hypopituitarism in combination with NFPA had an SMR of 1.06 (95% CI, 0.94-1.19) and for patients with diabetes insipidus the SMR was 1.71 (95% CI, 1.07-2.58).
Though the exact mechanisms behind the increased mortality ratios are unclear, the investigators noted that “adrenal insufficiency and its treatment in hypopituitary patients contributed to the excess mortality seen in these patients, although other pituitary hormone deficiencies, such as growth hormone deficiency may also play an important role.”
The authors noted that their study is the first to report the frequency of hypopituitarism (54%) in a large cohort of unselected patients with NFPA.
Dr. Olsen has received lecture fees from Pfizer and has been a consultant for Ipsen.
Mortality among patients with nonfunctioning pituitary adenomas (NFPA) was higher in women than in man as well as in patients with a young age at diagnosis and those diagnosed with diabetes insipidus, according to a nationwide population-based study in Sweden.
Dr. Daniel S. Olsson of the University of Gothenburg and Sahlgrenska University Hospital in Gothenburg, Sweden, and his associates identified 2,795 unique patients with NFPA from the the Swedish National Patient Registry who had been diagnosed between 1987 and 2011. The mean age at diagnosis was 58 years, and the mean follow-up time was 7 years.
Standardized mortality ratio was increased in women with NFPA (1.29; 95% confidence interval, 1.11-1.48) but not in men (1.00; 95% CI, 0.88-1.12). Women with a diagnosis of hypopituitarism and/or diabetes insipidus had increased mortality ratio, but men did not (J. Clin. Endocrinol. Metab. 2015 May 7 [doi: 10.1210/jc.2015-1475]).
“We found an association between increased mortality and the presence of hypopituitarism in women with NFPA, which has been indicated in previous studies, but not clearly demonstrated. In contrast, men with hypopituitarism had no excess mortality,” the authors wrote.
Other predictors of excess mortality included being diagnosed under 40 years of age, as those patients had an increased SMR of 2.68 (95% CI, 1.23-5.09). Patients with hypopituitarism in combination with NFPA had an SMR of 1.06 (95% CI, 0.94-1.19) and for patients with diabetes insipidus the SMR was 1.71 (95% CI, 1.07-2.58).
Though the exact mechanisms behind the increased mortality ratios are unclear, the investigators noted that “adrenal insufficiency and its treatment in hypopituitary patients contributed to the excess mortality seen in these patients, although other pituitary hormone deficiencies, such as growth hormone deficiency may also play an important role.”
The authors noted that their study is the first to report the frequency of hypopituitarism (54%) in a large cohort of unselected patients with NFPA.
Dr. Olsen has received lecture fees from Pfizer and has been a consultant for Ipsen.
Mortality among patients with nonfunctioning pituitary adenomas (NFPA) was higher in women than in man as well as in patients with a young age at diagnosis and those diagnosed with diabetes insipidus, according to a nationwide population-based study in Sweden.
Dr. Daniel S. Olsson of the University of Gothenburg and Sahlgrenska University Hospital in Gothenburg, Sweden, and his associates identified 2,795 unique patients with NFPA from the the Swedish National Patient Registry who had been diagnosed between 1987 and 2011. The mean age at diagnosis was 58 years, and the mean follow-up time was 7 years.
Standardized mortality ratio was increased in women with NFPA (1.29; 95% confidence interval, 1.11-1.48) but not in men (1.00; 95% CI, 0.88-1.12). Women with a diagnosis of hypopituitarism and/or diabetes insipidus had increased mortality ratio, but men did not (J. Clin. Endocrinol. Metab. 2015 May 7 [doi: 10.1210/jc.2015-1475]).
“We found an association between increased mortality and the presence of hypopituitarism in women with NFPA, which has been indicated in previous studies, but not clearly demonstrated. In contrast, men with hypopituitarism had no excess mortality,” the authors wrote.
Other predictors of excess mortality included being diagnosed under 40 years of age, as those patients had an increased SMR of 2.68 (95% CI, 1.23-5.09). Patients with hypopituitarism in combination with NFPA had an SMR of 1.06 (95% CI, 0.94-1.19) and for patients with diabetes insipidus the SMR was 1.71 (95% CI, 1.07-2.58).
Though the exact mechanisms behind the increased mortality ratios are unclear, the investigators noted that “adrenal insufficiency and its treatment in hypopituitary patients contributed to the excess mortality seen in these patients, although other pituitary hormone deficiencies, such as growth hormone deficiency may also play an important role.”
The authors noted that their study is the first to report the frequency of hypopituitarism (54%) in a large cohort of unselected patients with NFPA.
Dr. Olsen has received lecture fees from Pfizer and has been a consultant for Ipsen.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Key clinical point: Patients diagnosed with nonfunctioning pituitary adenoma may be at increased risk of a poor outcome if they are female, if they were diagnosed early in life, and if they were diagnosed with diabetes insipidus.
Major finding: Standardized mortality ratios were elevated in patients with hypopituitarism (SMR 1.06) and diabetes insipidus (SMR 1.71), and in women with NFPA (SMR 1.29) but not men (SMR 1.00).
Data source: A population-based study of 2,795 patients from nationwide health registries in Sweden.
Disclosures: Dr. Olsen has received lecture fees from Pfizer and has been a consultant for Ipsen.
New guidelines address hypopituitarism in TBI
New guidelines on traumatic brain injury and neuroendocrine dysfunction ask clinicians to be mindful that hypopituitarism in TBI survivors can develop following injuries classified as mild and can be slow to develop.
The guidelines – published online May 7, 2015,in Endocrine Reviews (doi:10.1210/er.2014-1065) and based on a review of 16 studies conducted between 2014 and 2011 – emphasize early detection and treatment of hypopituitarism in adults with TBI; recognition can be difficult because symptoms can progress slowly and be nonspecific. Deficiencies in pituitary hormones have been linked to elevated cardiovascular, metabolic, and cognitive risks, though studies in TBI populations are scant.About 12% of people with TBI will show persistent neuroendocrine dysfunction on repeat testing, according to Dr. Fatih Tanriverdi of Erciyes University in Kayseri, Turkey, and his associates. Although growth hormone (GH) deficiencies are the most common type of hormone deficiency after TBI, adrenocorticotropic hormone (ACTH), gonadotropins (FSH and LH), and thyroid-stimulating hormone (TSH) can all be diminished.
Dr. Tanriverdi and his colleagues noted that much more research is needed to determine the precise pathways by which TBI can cause pituitary dysfunction and whether hormone replacement can bring about functional or cognitive improvement in TBI patients with deficiencies. In addition to severity of injury, genetic factors, inflammation, and autoimmunity appear to bear on the risk of hypopituitarism after TBI, they said.
The guidelines recommend routine screening and early treatment for people with complicated mild TBI – a Glasgow coma score of 13-15 with some abnormalities on CT or MRI, hospitalization longer than 24 hours, or acute pituitary hormonal changes after injury – and for moderate and severe TBI. People with uncomplicated mild TBI (GCS of 13-15 but no screening abnormalities) are considered at low risk of developing pituitary dysfunction and do not need to be followed.
Patients with complicated mild TBI should be screened for ACTH deficiency immediately after injury and at hospital discharge and treated if necessary. At 6 months they should be reassessed for ACTH, TSH, and FSH/LH deficiencies and treated with hormone replacement therapy as needed. At a year post injury, patients should be assessed for GH deficiency and treated for this as well, if necessary, with annual clinical and hormonal evaluations continuing 5 years.
If no hormone deficiencies are found at 12 months, patients should still be reassessed annually through the fifth year for hypopituitarism, as hormone deficiencies can still develop.
Patients with moderate and severe TBI should be assessed and treated according to the same algorithm, the guidelines say, except that they do not require further screening beyond 1 year in the absence of symptoms. Patients should instead be coached to recognize symptoms of hypopituitarism and present for screening if symptoms appear.The guideline authors did not report outside funding and disclosed no conflicts of interest related to their recommendations.
New guidelines on traumatic brain injury and neuroendocrine dysfunction ask clinicians to be mindful that hypopituitarism in TBI survivors can develop following injuries classified as mild and can be slow to develop.
The guidelines – published online May 7, 2015,in Endocrine Reviews (doi:10.1210/er.2014-1065) and based on a review of 16 studies conducted between 2014 and 2011 – emphasize early detection and treatment of hypopituitarism in adults with TBI; recognition can be difficult because symptoms can progress slowly and be nonspecific. Deficiencies in pituitary hormones have been linked to elevated cardiovascular, metabolic, and cognitive risks, though studies in TBI populations are scant.About 12% of people with TBI will show persistent neuroendocrine dysfunction on repeat testing, according to Dr. Fatih Tanriverdi of Erciyes University in Kayseri, Turkey, and his associates. Although growth hormone (GH) deficiencies are the most common type of hormone deficiency after TBI, adrenocorticotropic hormone (ACTH), gonadotropins (FSH and LH), and thyroid-stimulating hormone (TSH) can all be diminished.
Dr. Tanriverdi and his colleagues noted that much more research is needed to determine the precise pathways by which TBI can cause pituitary dysfunction and whether hormone replacement can bring about functional or cognitive improvement in TBI patients with deficiencies. In addition to severity of injury, genetic factors, inflammation, and autoimmunity appear to bear on the risk of hypopituitarism after TBI, they said.
The guidelines recommend routine screening and early treatment for people with complicated mild TBI – a Glasgow coma score of 13-15 with some abnormalities on CT or MRI, hospitalization longer than 24 hours, or acute pituitary hormonal changes after injury – and for moderate and severe TBI. People with uncomplicated mild TBI (GCS of 13-15 but no screening abnormalities) are considered at low risk of developing pituitary dysfunction and do not need to be followed.
Patients with complicated mild TBI should be screened for ACTH deficiency immediately after injury and at hospital discharge and treated if necessary. At 6 months they should be reassessed for ACTH, TSH, and FSH/LH deficiencies and treated with hormone replacement therapy as needed. At a year post injury, patients should be assessed for GH deficiency and treated for this as well, if necessary, with annual clinical and hormonal evaluations continuing 5 years.
If no hormone deficiencies are found at 12 months, patients should still be reassessed annually through the fifth year for hypopituitarism, as hormone deficiencies can still develop.
Patients with moderate and severe TBI should be assessed and treated according to the same algorithm, the guidelines say, except that they do not require further screening beyond 1 year in the absence of symptoms. Patients should instead be coached to recognize symptoms of hypopituitarism and present for screening if symptoms appear.The guideline authors did not report outside funding and disclosed no conflicts of interest related to their recommendations.
New guidelines on traumatic brain injury and neuroendocrine dysfunction ask clinicians to be mindful that hypopituitarism in TBI survivors can develop following injuries classified as mild and can be slow to develop.
The guidelines – published online May 7, 2015,in Endocrine Reviews (doi:10.1210/er.2014-1065) and based on a review of 16 studies conducted between 2014 and 2011 – emphasize early detection and treatment of hypopituitarism in adults with TBI; recognition can be difficult because symptoms can progress slowly and be nonspecific. Deficiencies in pituitary hormones have been linked to elevated cardiovascular, metabolic, and cognitive risks, though studies in TBI populations are scant.About 12% of people with TBI will show persistent neuroendocrine dysfunction on repeat testing, according to Dr. Fatih Tanriverdi of Erciyes University in Kayseri, Turkey, and his associates. Although growth hormone (GH) deficiencies are the most common type of hormone deficiency after TBI, adrenocorticotropic hormone (ACTH), gonadotropins (FSH and LH), and thyroid-stimulating hormone (TSH) can all be diminished.
Dr. Tanriverdi and his colleagues noted that much more research is needed to determine the precise pathways by which TBI can cause pituitary dysfunction and whether hormone replacement can bring about functional or cognitive improvement in TBI patients with deficiencies. In addition to severity of injury, genetic factors, inflammation, and autoimmunity appear to bear on the risk of hypopituitarism after TBI, they said.
The guidelines recommend routine screening and early treatment for people with complicated mild TBI – a Glasgow coma score of 13-15 with some abnormalities on CT or MRI, hospitalization longer than 24 hours, or acute pituitary hormonal changes after injury – and for moderate and severe TBI. People with uncomplicated mild TBI (GCS of 13-15 but no screening abnormalities) are considered at low risk of developing pituitary dysfunction and do not need to be followed.
Patients with complicated mild TBI should be screened for ACTH deficiency immediately after injury and at hospital discharge and treated if necessary. At 6 months they should be reassessed for ACTH, TSH, and FSH/LH deficiencies and treated with hormone replacement therapy as needed. At a year post injury, patients should be assessed for GH deficiency and treated for this as well, if necessary, with annual clinical and hormonal evaluations continuing 5 years.
If no hormone deficiencies are found at 12 months, patients should still be reassessed annually through the fifth year for hypopituitarism, as hormone deficiencies can still develop.
Patients with moderate and severe TBI should be assessed and treated according to the same algorithm, the guidelines say, except that they do not require further screening beyond 1 year in the absence of symptoms. Patients should instead be coached to recognize symptoms of hypopituitarism and present for screening if symptoms appear.The guideline authors did not report outside funding and disclosed no conflicts of interest related to their recommendations.
Key clinical point: Screening for and treating pituitary hormone deficiencies is recommended for people with complicated mild traumatic brain injury and more severe forms of TBI.
Major finding: Patients with complicated mild TBI should be screened for up to 5 years following injury for pituitary hormone deficiencies and treated as necessary.
Data source: A systematic review of 16 prospective and cross-sectional observational studies (2004-2011) of anterior pituitary hormone dysfunction after TBI in adult patients (n = 1,203).
Disclosures: The guideline authors did not report outside funding and disclosed no conflicts of interest related to their recommendations.
Overactive androgens probably don’t cause women’s severe acne
Women with isolated postadolescent severe acne do not have increased levels of adrenal androgens, either basally or in response to corticotropin stimulation, reported Dr. N. Cinar of Hacettepe University in Ankara, Turkey, and colleagues. However, women with severe acne have secretion patterns of serum 17-hydroxyprogesterone similar to those of polycystic ovary syndrome patients, suggesting the two may have an unexplored connection.
To investigate the role of androgens in the pathogenesis of acne, the researchers compared 32 women with postadolescent severe acne and 32 women with polycystic ovary syndrome (PCOS) with 32 age- and body mass index–matched healthy controls (aged 17-34 years; BMI, 20.8 ± 1.9 kg/m2). They found basal testosterone, free androgen index, and dehydroepiandrosterone sulphate levels for androstenedione (used as metrics for determining adrenocortical production) were significantly higher in the PCOS group than in the women with acne and the controls (P < .05 for all).
In addition, women with PCOS and those with severe acne had significantly and similarly higher area under the curve values of serum 17-hydroxyprogesterone, compared with controls (P < .05), the investigators noted.
Read the entire article here: Journal of the European Academy of Dermatology and Venereology, (2015;29: 875-880 ([doi:10.1111/jdv.12696]).
Women with isolated postadolescent severe acne do not have increased levels of adrenal androgens, either basally or in response to corticotropin stimulation, reported Dr. N. Cinar of Hacettepe University in Ankara, Turkey, and colleagues. However, women with severe acne have secretion patterns of serum 17-hydroxyprogesterone similar to those of polycystic ovary syndrome patients, suggesting the two may have an unexplored connection.
To investigate the role of androgens in the pathogenesis of acne, the researchers compared 32 women with postadolescent severe acne and 32 women with polycystic ovary syndrome (PCOS) with 32 age- and body mass index–matched healthy controls (aged 17-34 years; BMI, 20.8 ± 1.9 kg/m2). They found basal testosterone, free androgen index, and dehydroepiandrosterone sulphate levels for androstenedione (used as metrics for determining adrenocortical production) were significantly higher in the PCOS group than in the women with acne and the controls (P < .05 for all).
In addition, women with PCOS and those with severe acne had significantly and similarly higher area under the curve values of serum 17-hydroxyprogesterone, compared with controls (P < .05), the investigators noted.
Read the entire article here: Journal of the European Academy of Dermatology and Venereology, (2015;29: 875-880 ([doi:10.1111/jdv.12696]).
Women with isolated postadolescent severe acne do not have increased levels of adrenal androgens, either basally or in response to corticotropin stimulation, reported Dr. N. Cinar of Hacettepe University in Ankara, Turkey, and colleagues. However, women with severe acne have secretion patterns of serum 17-hydroxyprogesterone similar to those of polycystic ovary syndrome patients, suggesting the two may have an unexplored connection.
To investigate the role of androgens in the pathogenesis of acne, the researchers compared 32 women with postadolescent severe acne and 32 women with polycystic ovary syndrome (PCOS) with 32 age- and body mass index–matched healthy controls (aged 17-34 years; BMI, 20.8 ± 1.9 kg/m2). They found basal testosterone, free androgen index, and dehydroepiandrosterone sulphate levels for androstenedione (used as metrics for determining adrenocortical production) were significantly higher in the PCOS group than in the women with acne and the controls (P < .05 for all).
In addition, women with PCOS and those with severe acne had significantly and similarly higher area under the curve values of serum 17-hydroxyprogesterone, compared with controls (P < .05), the investigators noted.
Read the entire article here: Journal of the European Academy of Dermatology and Venereology, (2015;29: 875-880 ([doi:10.1111/jdv.12696]).
Add baseline DHEAS when screening adrenal incidentalomas for subclinical hypercortisolism
SAN DIEGO – A single baseline measurement of dehydroepiandrosterone sulfate (DHEAS) outperforms overnight dexamethasone suppression and other standard tests for the detection of subclinical hypercortisolism in patients with adrenal incidentalomas, according to a prospective, blinded study of 185 consecutive patients at Cambridge University in England.
It’s an important finding because 1-mg overnight dexamethasone suppression testing is the current screening standard for detection of the condition, said Dr. Michael Dennedy, a former endocrinology fellow at Cambridge and now a senior lecturer at the National University of Ireland (NUI), Galway.
“On the basis of these data, we advocate the use of a single measurement of DHEAS as a convenient, reliable, and robust test for screening of SH [subclinical hypercortisolism] in the context of adrenal incidentalomas,” Dr. Dennedy said at the meeting of the Endocrine Society.
DHEAS avoids the sampling problems and high false positives of older tests and “reliably differentiates between SH and non-SH etiologies of adrenal nodules. We use it now as a standard test for patients who have adrenal nodules” at Cambridge and NUI Galway, he said.
The theory for checking DHEAS is straightforward. Like DHEA, it’s regulated by pituitary ACTH; sustained suppression of central ACTH leads to reductions in both. However, DHEA does not work for screening because it has a short half-life – about 25 minutes – and has circadian secretion patterns similar to ACTH. The half-life of DHEAS, on the other hand, is 10-16 hours and levels stay relatively stable throughout the day, which makes it a more attractive marker for detecting chronically suppressed ACTH.
At baseline, the team measured DHEAS by immunoassay and then put their 185 subjects through standard workups for newly diagnosed adrenal incidentalomas, including plasma metanephrines, 1-mg overnight dexamethasone suppression, 24-hour urinary free cortisol, and paired renin and aldosterone measurements. They then unblinded their DHEAS measurements to see how they fared against the standard approaches.
Because DHEAS levels are determined by a person’s age and gender, the investigators used a ratio of the actual baseline measurement divided by the lower limit of the appropriate reference range; for instance, 1.2 mmol/L in younger patients and 0.4 mmol/L in older patients.
A baseline DHEAS ratio at or below 1.12 was 100% sensitive and 92% specific for the diagnosis of SH in patients with adrenal incidentalomas. In contrast, a cortisol cutoff of 1.9 mcg/dL following 1-mg dexamethasone suppression was 100% sensitive but 82.9% specific and 24-hour urinary free cortisol was 69% sensitive and 68% specific.
The patients were aged 25 years to over 85, with an average age of about 65 years; 29 (16%) were diagnosed with SH. Most had nonfunctional adenomas, and a few had adrenal cortical carcinomas.
“Once upon a time, if there was a positive overnight dexamethasone or a positive urinary free cortisol, or both, we would send” patients on for a full inpatient Cushing’s workup. “Now we have a third criterion,” he said: suppressed DHEAS. Also, “in the presence of a high DHEAS with a failed dexamethasone suppression test, you would worry about adrenal corticocarcinoma, pituitary Cushing’s, or adrenal metastasis,” Dr. Dennedy said.
The investigators had no relevant disclosures, and there was no outside funding for the research.
SAN DIEGO – A single baseline measurement of dehydroepiandrosterone sulfate (DHEAS) outperforms overnight dexamethasone suppression and other standard tests for the detection of subclinical hypercortisolism in patients with adrenal incidentalomas, according to a prospective, blinded study of 185 consecutive patients at Cambridge University in England.
It’s an important finding because 1-mg overnight dexamethasone suppression testing is the current screening standard for detection of the condition, said Dr. Michael Dennedy, a former endocrinology fellow at Cambridge and now a senior lecturer at the National University of Ireland (NUI), Galway.
“On the basis of these data, we advocate the use of a single measurement of DHEAS as a convenient, reliable, and robust test for screening of SH [subclinical hypercortisolism] in the context of adrenal incidentalomas,” Dr. Dennedy said at the meeting of the Endocrine Society.
DHEAS avoids the sampling problems and high false positives of older tests and “reliably differentiates between SH and non-SH etiologies of adrenal nodules. We use it now as a standard test for patients who have adrenal nodules” at Cambridge and NUI Galway, he said.
The theory for checking DHEAS is straightforward. Like DHEA, it’s regulated by pituitary ACTH; sustained suppression of central ACTH leads to reductions in both. However, DHEA does not work for screening because it has a short half-life – about 25 minutes – and has circadian secretion patterns similar to ACTH. The half-life of DHEAS, on the other hand, is 10-16 hours and levels stay relatively stable throughout the day, which makes it a more attractive marker for detecting chronically suppressed ACTH.
At baseline, the team measured DHEAS by immunoassay and then put their 185 subjects through standard workups for newly diagnosed adrenal incidentalomas, including plasma metanephrines, 1-mg overnight dexamethasone suppression, 24-hour urinary free cortisol, and paired renin and aldosterone measurements. They then unblinded their DHEAS measurements to see how they fared against the standard approaches.
Because DHEAS levels are determined by a person’s age and gender, the investigators used a ratio of the actual baseline measurement divided by the lower limit of the appropriate reference range; for instance, 1.2 mmol/L in younger patients and 0.4 mmol/L in older patients.
A baseline DHEAS ratio at or below 1.12 was 100% sensitive and 92% specific for the diagnosis of SH in patients with adrenal incidentalomas. In contrast, a cortisol cutoff of 1.9 mcg/dL following 1-mg dexamethasone suppression was 100% sensitive but 82.9% specific and 24-hour urinary free cortisol was 69% sensitive and 68% specific.
The patients were aged 25 years to over 85, with an average age of about 65 years; 29 (16%) were diagnosed with SH. Most had nonfunctional adenomas, and a few had adrenal cortical carcinomas.
“Once upon a time, if there was a positive overnight dexamethasone or a positive urinary free cortisol, or both, we would send” patients on for a full inpatient Cushing’s workup. “Now we have a third criterion,” he said: suppressed DHEAS. Also, “in the presence of a high DHEAS with a failed dexamethasone suppression test, you would worry about adrenal corticocarcinoma, pituitary Cushing’s, or adrenal metastasis,” Dr. Dennedy said.
The investigators had no relevant disclosures, and there was no outside funding for the research.
SAN DIEGO – A single baseline measurement of dehydroepiandrosterone sulfate (DHEAS) outperforms overnight dexamethasone suppression and other standard tests for the detection of subclinical hypercortisolism in patients with adrenal incidentalomas, according to a prospective, blinded study of 185 consecutive patients at Cambridge University in England.
It’s an important finding because 1-mg overnight dexamethasone suppression testing is the current screening standard for detection of the condition, said Dr. Michael Dennedy, a former endocrinology fellow at Cambridge and now a senior lecturer at the National University of Ireland (NUI), Galway.
“On the basis of these data, we advocate the use of a single measurement of DHEAS as a convenient, reliable, and robust test for screening of SH [subclinical hypercortisolism] in the context of adrenal incidentalomas,” Dr. Dennedy said at the meeting of the Endocrine Society.
DHEAS avoids the sampling problems and high false positives of older tests and “reliably differentiates between SH and non-SH etiologies of adrenal nodules. We use it now as a standard test for patients who have adrenal nodules” at Cambridge and NUI Galway, he said.
The theory for checking DHEAS is straightforward. Like DHEA, it’s regulated by pituitary ACTH; sustained suppression of central ACTH leads to reductions in both. However, DHEA does not work for screening because it has a short half-life – about 25 minutes – and has circadian secretion patterns similar to ACTH. The half-life of DHEAS, on the other hand, is 10-16 hours and levels stay relatively stable throughout the day, which makes it a more attractive marker for detecting chronically suppressed ACTH.
At baseline, the team measured DHEAS by immunoassay and then put their 185 subjects through standard workups for newly diagnosed adrenal incidentalomas, including plasma metanephrines, 1-mg overnight dexamethasone suppression, 24-hour urinary free cortisol, and paired renin and aldosterone measurements. They then unblinded their DHEAS measurements to see how they fared against the standard approaches.
Because DHEAS levels are determined by a person’s age and gender, the investigators used a ratio of the actual baseline measurement divided by the lower limit of the appropriate reference range; for instance, 1.2 mmol/L in younger patients and 0.4 mmol/L in older patients.
A baseline DHEAS ratio at or below 1.12 was 100% sensitive and 92% specific for the diagnosis of SH in patients with adrenal incidentalomas. In contrast, a cortisol cutoff of 1.9 mcg/dL following 1-mg dexamethasone suppression was 100% sensitive but 82.9% specific and 24-hour urinary free cortisol was 69% sensitive and 68% specific.
The patients were aged 25 years to over 85, with an average age of about 65 years; 29 (16%) were diagnosed with SH. Most had nonfunctional adenomas, and a few had adrenal cortical carcinomas.
“Once upon a time, if there was a positive overnight dexamethasone or a positive urinary free cortisol, or both, we would send” patients on for a full inpatient Cushing’s workup. “Now we have a third criterion,” he said: suppressed DHEAS. Also, “in the presence of a high DHEAS with a failed dexamethasone suppression test, you would worry about adrenal corticocarcinoma, pituitary Cushing’s, or adrenal metastasis,” Dr. Dennedy said.
The investigators had no relevant disclosures, and there was no outside funding for the research.
AT ENDO 2015
Key clinical point: A single baseline measurement of dehydroepiandrosterone sulfate (DHEAS) beats 1-mg overnight dexamethasone suppression testing for detecting subclinical hypercortisolism.
Major finding: A baseline DHEAS ratio at or below 1.12 was 100% sensitive and 92% specific for the diagnosis of SH in patients with adrenal incidentalomas. In contrast, 1-mg dexamethasone suppression testing was 100% sensitive but 82.9% specific and 24-hour urinary free cortisol was 69% sensitive and 68% specific.
Data source: Prospective, blinded study of 185 patients worked up for adrenal incidentalomas.
Disclosures: The investigators had no relevant disclosures, and there was no outside funding for the work.
Pamphlet helped Graves’ patients find peace with treatment choices
San Diego – Graves’ disease patients are more comfortable with their treatment decisions when doctors walk them through the options with a short pamphlet being developed at the Mayo Clinic in Rochester, Minn.
None of the three treatment options for Graves’ – thyroidectomy, radioactive iodine, and antithyroid drugs – is clearly superior to the others. That usually means that patients end up getting whatever treatment their institution prefers, and not so much making the choice themselves, said lead investigator Dr. Juan Brito, an assistant endocrinology professor at the Mayo Clinic.
That approach isn’t going to work much longer as patient involvement and satisfaction come online as quality metrics, Dr. Brito noted.
Shared decision making has been a theme at Mayo for a while now, he said, and the institution has developed and validated a range of “decision-making tools” for patients with various diseases. The Graves’ diseasepamphlet is among its latest efforts.
“We don’t really care if patients make a decision for surgery, medicine, or radioactive iodine,” said Dr. Brito. “What we care about is that patients know what they are doing, are more involved in the process, and more satisfied with their decisions.
“We want patients to decide, not the institution,” added Dr. Brito, who noted that Mayo has traditionally been a radioactive iodine institution.
The Graves’ decision pamphlet lays out the pros and cons of all three options – effectiveness, costs, number of follow-up visits, likelihood of thyroid replacement, eye complications, and the like – using five easy-to-understand graphics. The goal is for doctors to use the pamphlet during consultations to ensure that all the bases are covered and patients understand what they hear. The pamphlet doesn’t make consults longer, Dr. Brito noted – if anything, it shortens them a bit.
To assess the pamphlet’s effectiveness, Dr. Brito and his team surveyed patients after their encounters.
The 19 patients guided through their options with the “Graves’ Disease Treatment Choice” pamphlet were more involved with decisions than the 33 patients who were not, as measured by the OPTION [observing patient involvement in decision making] scale (mean score 38.5 points versus 30.8, with 100 points indicating the greatest involvement, P = 0.02).
Pamphlet patients also were more certain of their decisions, as assessed by the Decisional Conflict Scale (19 points versus 31 points, with 0 points being complete certainty, P = 0.19). The length of the discussion was slightly shorter with the pamphlet (37 minutes versus 39 minutes), but the difference was not significant.
Pamphlet patients also seemed more knowledgeable about treatment side effects, and were more likely to choose radioactive iodine and less likely to choose antithyroid drugs; but the differences in what they opted for were also not significant.
On average, patients were in their early 40s, and three-quarters were women.
The decision tool “improves patient involvement in decision making about treatment options without increasing the length of the consultation,” Dr. Brito explained. “This tool helps the patient and clinician navigate through the options and enhances the quality of the process.”
The investigators have no disclosures and no outside funding for their work.
San Diego – Graves’ disease patients are more comfortable with their treatment decisions when doctors walk them through the options with a short pamphlet being developed at the Mayo Clinic in Rochester, Minn.
None of the three treatment options for Graves’ – thyroidectomy, radioactive iodine, and antithyroid drugs – is clearly superior to the others. That usually means that patients end up getting whatever treatment their institution prefers, and not so much making the choice themselves, said lead investigator Dr. Juan Brito, an assistant endocrinology professor at the Mayo Clinic.
That approach isn’t going to work much longer as patient involvement and satisfaction come online as quality metrics, Dr. Brito noted.
Shared decision making has been a theme at Mayo for a while now, he said, and the institution has developed and validated a range of “decision-making tools” for patients with various diseases. The Graves’ diseasepamphlet is among its latest efforts.
“We don’t really care if patients make a decision for surgery, medicine, or radioactive iodine,” said Dr. Brito. “What we care about is that patients know what they are doing, are more involved in the process, and more satisfied with their decisions.
“We want patients to decide, not the institution,” added Dr. Brito, who noted that Mayo has traditionally been a radioactive iodine institution.
The Graves’ decision pamphlet lays out the pros and cons of all three options – effectiveness, costs, number of follow-up visits, likelihood of thyroid replacement, eye complications, and the like – using five easy-to-understand graphics. The goal is for doctors to use the pamphlet during consultations to ensure that all the bases are covered and patients understand what they hear. The pamphlet doesn’t make consults longer, Dr. Brito noted – if anything, it shortens them a bit.
To assess the pamphlet’s effectiveness, Dr. Brito and his team surveyed patients after their encounters.
The 19 patients guided through their options with the “Graves’ Disease Treatment Choice” pamphlet were more involved with decisions than the 33 patients who were not, as measured by the OPTION [observing patient involvement in decision making] scale (mean score 38.5 points versus 30.8, with 100 points indicating the greatest involvement, P = 0.02).
Pamphlet patients also were more certain of their decisions, as assessed by the Decisional Conflict Scale (19 points versus 31 points, with 0 points being complete certainty, P = 0.19). The length of the discussion was slightly shorter with the pamphlet (37 minutes versus 39 minutes), but the difference was not significant.
Pamphlet patients also seemed more knowledgeable about treatment side effects, and were more likely to choose radioactive iodine and less likely to choose antithyroid drugs; but the differences in what they opted for were also not significant.
On average, patients were in their early 40s, and three-quarters were women.
The decision tool “improves patient involvement in decision making about treatment options without increasing the length of the consultation,” Dr. Brito explained. “This tool helps the patient and clinician navigate through the options and enhances the quality of the process.”
The investigators have no disclosures and no outside funding for their work.
San Diego – Graves’ disease patients are more comfortable with their treatment decisions when doctors walk them through the options with a short pamphlet being developed at the Mayo Clinic in Rochester, Minn.
None of the three treatment options for Graves’ – thyroidectomy, radioactive iodine, and antithyroid drugs – is clearly superior to the others. That usually means that patients end up getting whatever treatment their institution prefers, and not so much making the choice themselves, said lead investigator Dr. Juan Brito, an assistant endocrinology professor at the Mayo Clinic.
That approach isn’t going to work much longer as patient involvement and satisfaction come online as quality metrics, Dr. Brito noted.
Shared decision making has been a theme at Mayo for a while now, he said, and the institution has developed and validated a range of “decision-making tools” for patients with various diseases. The Graves’ diseasepamphlet is among its latest efforts.
“We don’t really care if patients make a decision for surgery, medicine, or radioactive iodine,” said Dr. Brito. “What we care about is that patients know what they are doing, are more involved in the process, and more satisfied with their decisions.
“We want patients to decide, not the institution,” added Dr. Brito, who noted that Mayo has traditionally been a radioactive iodine institution.
The Graves’ decision pamphlet lays out the pros and cons of all three options – effectiveness, costs, number of follow-up visits, likelihood of thyroid replacement, eye complications, and the like – using five easy-to-understand graphics. The goal is for doctors to use the pamphlet during consultations to ensure that all the bases are covered and patients understand what they hear. The pamphlet doesn’t make consults longer, Dr. Brito noted – if anything, it shortens them a bit.
To assess the pamphlet’s effectiveness, Dr. Brito and his team surveyed patients after their encounters.
The 19 patients guided through their options with the “Graves’ Disease Treatment Choice” pamphlet were more involved with decisions than the 33 patients who were not, as measured by the OPTION [observing patient involvement in decision making] scale (mean score 38.5 points versus 30.8, with 100 points indicating the greatest involvement, P = 0.02).
Pamphlet patients also were more certain of their decisions, as assessed by the Decisional Conflict Scale (19 points versus 31 points, with 0 points being complete certainty, P = 0.19). The length of the discussion was slightly shorter with the pamphlet (37 minutes versus 39 minutes), but the difference was not significant.
Pamphlet patients also seemed more knowledgeable about treatment side effects, and were more likely to choose radioactive iodine and less likely to choose antithyroid drugs; but the differences in what they opted for were also not significant.
On average, patients were in their early 40s, and three-quarters were women.
The decision tool “improves patient involvement in decision making about treatment options without increasing the length of the consultation,” Dr. Brito explained. “This tool helps the patient and clinician navigate through the options and enhances the quality of the process.”
The investigators have no disclosures and no outside funding for their work.
AT ENDO 2015
Key clinical point: It’s possible to increase patient involvement in treatment decisions without increasing length of consultations.
Major finding: The 19 Graves’ patients guided through treatment options with a Mayo Clinic Graves’ pamphlet were more involved with decision making than the 33 who were not, as measured by the OPTION scale (mean score 38.5 points versus 30.8, with 100 points indicating the greatest involvement, P = 0.02).
Data source: Surveys of 55 Graves’ disease patients.
Disclosures: The investigators have no disclosures and no outside funding for their work.