User login
USPSTF: No thyroid cancer screening for asymptomatic adults
The U.S. Preventive Services Task Force recommends against screening asymptomatic adults for thyroid cancer, because the harms of such screening outweigh the benefits, according to a recommendation statement published May 9 in JAMA.
The USPSTF makes recommendations about the effectiveness of specific health care services for patients who don’t have related signs or symptoms. In this case, the recommendation statement addresses screening of adults who have no signs or symptoms of thyroid cancer by using neck palpation or ultrasonography, said Kirsten Bibbins-Domingo, MD, PhD, chair of the organization and lead author of the recommendation statement, and her associates (JAMA. 2017 May 9;317[18]:1882-7).
This document is an update of the previous USPSTF recommendation statement issued in 1996 and was undertaken because there have been several major advances related to the disease since that time.
However, despite a comprehensive review of the current literature, the group found no direct evidence supporting a change to their original advice against such screening.
They emphasized that this applies only to asymptomatic adults, not to those who have hoarseness, throat pain, difficulty swallowing, lumps in the neck, swelling, or asymmetry of the neck; nor to those who have a history of exposure to ionizing radiation, a family history of thyroid cancer, or a genetic susceptibility to the disease.
The results of the literature review were summarized in an evidence report by Jennifer S. Lin, MD, of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates. They examined 67 studies involving nearly 584,000 patients, including 10 studies that addressed screening test performance, 3 that addressed the possible harms of screening, 2 that addressed treatment benefits, and 52 that addressed treatment harms.
No good-quality studies assessed the net benefit of thyroid cancer screening, nor whether “early” treatment of screen-detected cancers improved patient outcomes. However, the preponderance of evidence suggested that most thyroid cancers detected by screening are indolent.
So, treatment is likely unnecessary but exposes patients to “nontrivial” harms, including an increased risk of second primary malignancy, permanent adverse effects on the salivary glands, laryngeal nerve injury, hypoparathyroidism, and the need for lifelong thyroid replacement therapy and monitoring for cancer recurrence, Dr. Lin and her associates said in their evidence report (JAMA. 2017 May 9;317[18]:1888-1903).
The task force noted that no professional medical society currently recommends population-based screening for thyroid cancer. The American Cancer Society, American Thyroid Association, American Association of Clinical Endocrinologists, and American College of Endocrinology all have no specific recommendations for screening asymptomatic patients, while the American Academy of Family Physicians recommends against such screening, said Dr. Bibbins-Domingo, who is also a professor of medicine at the University of California, San Francisco, and her associates.
Further information regarding the recommendation statement and the evidence report is available at www.uspreventiveservicestaskforce.org.
The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
The rationale for recommending against screening asymptomatic people for thyroid cancer is compelling, and the evidence clearly points to the harms outweighing the benefits.
But that doesn’t mean that the conversation about screening should stop. What the field needs is not a better means to detect thyroid nodules, but a noninvasive measure to distinguish nodules whose cells will leave the thyroid capsule and cause morbidity from nodules whose cells will not. That will spare patients with indolent cancers from unnecessary treatment, while steering the minority of patients with more aggressive cancers to early treatment.
Anne R. Cappola, MD, is in the division of endocrinology, diabetes, and metabolism at the University of Pennsylvania, Philadelphia, and is an associate editor of JAMA. She reported having no relevant financial disclosures. Dr. Cappola made these remarks in an editorial accompanying the recommendation statement and the evidence report (JAMA. 2017 May 9;317[18]:1840-1).
The rationale for recommending against screening asymptomatic people for thyroid cancer is compelling, and the evidence clearly points to the harms outweighing the benefits.
But that doesn’t mean that the conversation about screening should stop. What the field needs is not a better means to detect thyroid nodules, but a noninvasive measure to distinguish nodules whose cells will leave the thyroid capsule and cause morbidity from nodules whose cells will not. That will spare patients with indolent cancers from unnecessary treatment, while steering the minority of patients with more aggressive cancers to early treatment.
Anne R. Cappola, MD, is in the division of endocrinology, diabetes, and metabolism at the University of Pennsylvania, Philadelphia, and is an associate editor of JAMA. She reported having no relevant financial disclosures. Dr. Cappola made these remarks in an editorial accompanying the recommendation statement and the evidence report (JAMA. 2017 May 9;317[18]:1840-1).
The rationale for recommending against screening asymptomatic people for thyroid cancer is compelling, and the evidence clearly points to the harms outweighing the benefits.
But that doesn’t mean that the conversation about screening should stop. What the field needs is not a better means to detect thyroid nodules, but a noninvasive measure to distinguish nodules whose cells will leave the thyroid capsule and cause morbidity from nodules whose cells will not. That will spare patients with indolent cancers from unnecessary treatment, while steering the minority of patients with more aggressive cancers to early treatment.
Anne R. Cappola, MD, is in the division of endocrinology, diabetes, and metabolism at the University of Pennsylvania, Philadelphia, and is an associate editor of JAMA. She reported having no relevant financial disclosures. Dr. Cappola made these remarks in an editorial accompanying the recommendation statement and the evidence report (JAMA. 2017 May 9;317[18]:1840-1).
The U.S. Preventive Services Task Force recommends against screening asymptomatic adults for thyroid cancer, because the harms of such screening outweigh the benefits, according to a recommendation statement published May 9 in JAMA.
The USPSTF makes recommendations about the effectiveness of specific health care services for patients who don’t have related signs or symptoms. In this case, the recommendation statement addresses screening of adults who have no signs or symptoms of thyroid cancer by using neck palpation or ultrasonography, said Kirsten Bibbins-Domingo, MD, PhD, chair of the organization and lead author of the recommendation statement, and her associates (JAMA. 2017 May 9;317[18]:1882-7).
This document is an update of the previous USPSTF recommendation statement issued in 1996 and was undertaken because there have been several major advances related to the disease since that time.
However, despite a comprehensive review of the current literature, the group found no direct evidence supporting a change to their original advice against such screening.
They emphasized that this applies only to asymptomatic adults, not to those who have hoarseness, throat pain, difficulty swallowing, lumps in the neck, swelling, or asymmetry of the neck; nor to those who have a history of exposure to ionizing radiation, a family history of thyroid cancer, or a genetic susceptibility to the disease.
The results of the literature review were summarized in an evidence report by Jennifer S. Lin, MD, of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates. They examined 67 studies involving nearly 584,000 patients, including 10 studies that addressed screening test performance, 3 that addressed the possible harms of screening, 2 that addressed treatment benefits, and 52 that addressed treatment harms.
No good-quality studies assessed the net benefit of thyroid cancer screening, nor whether “early” treatment of screen-detected cancers improved patient outcomes. However, the preponderance of evidence suggested that most thyroid cancers detected by screening are indolent.
So, treatment is likely unnecessary but exposes patients to “nontrivial” harms, including an increased risk of second primary malignancy, permanent adverse effects on the salivary glands, laryngeal nerve injury, hypoparathyroidism, and the need for lifelong thyroid replacement therapy and monitoring for cancer recurrence, Dr. Lin and her associates said in their evidence report (JAMA. 2017 May 9;317[18]:1888-1903).
The task force noted that no professional medical society currently recommends population-based screening for thyroid cancer. The American Cancer Society, American Thyroid Association, American Association of Clinical Endocrinologists, and American College of Endocrinology all have no specific recommendations for screening asymptomatic patients, while the American Academy of Family Physicians recommends against such screening, said Dr. Bibbins-Domingo, who is also a professor of medicine at the University of California, San Francisco, and her associates.
Further information regarding the recommendation statement and the evidence report is available at www.uspreventiveservicestaskforce.org.
The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
The U.S. Preventive Services Task Force recommends against screening asymptomatic adults for thyroid cancer, because the harms of such screening outweigh the benefits, according to a recommendation statement published May 9 in JAMA.
The USPSTF makes recommendations about the effectiveness of specific health care services for patients who don’t have related signs or symptoms. In this case, the recommendation statement addresses screening of adults who have no signs or symptoms of thyroid cancer by using neck palpation or ultrasonography, said Kirsten Bibbins-Domingo, MD, PhD, chair of the organization and lead author of the recommendation statement, and her associates (JAMA. 2017 May 9;317[18]:1882-7).
This document is an update of the previous USPSTF recommendation statement issued in 1996 and was undertaken because there have been several major advances related to the disease since that time.
However, despite a comprehensive review of the current literature, the group found no direct evidence supporting a change to their original advice against such screening.
They emphasized that this applies only to asymptomatic adults, not to those who have hoarseness, throat pain, difficulty swallowing, lumps in the neck, swelling, or asymmetry of the neck; nor to those who have a history of exposure to ionizing radiation, a family history of thyroid cancer, or a genetic susceptibility to the disease.
The results of the literature review were summarized in an evidence report by Jennifer S. Lin, MD, of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates. They examined 67 studies involving nearly 584,000 patients, including 10 studies that addressed screening test performance, 3 that addressed the possible harms of screening, 2 that addressed treatment benefits, and 52 that addressed treatment harms.
No good-quality studies assessed the net benefit of thyroid cancer screening, nor whether “early” treatment of screen-detected cancers improved patient outcomes. However, the preponderance of evidence suggested that most thyroid cancers detected by screening are indolent.
So, treatment is likely unnecessary but exposes patients to “nontrivial” harms, including an increased risk of second primary malignancy, permanent adverse effects on the salivary glands, laryngeal nerve injury, hypoparathyroidism, and the need for lifelong thyroid replacement therapy and monitoring for cancer recurrence, Dr. Lin and her associates said in their evidence report (JAMA. 2017 May 9;317[18]:1888-1903).
The task force noted that no professional medical society currently recommends population-based screening for thyroid cancer. The American Cancer Society, American Thyroid Association, American Association of Clinical Endocrinologists, and American College of Endocrinology all have no specific recommendations for screening asymptomatic patients, while the American Academy of Family Physicians recommends against such screening, said Dr. Bibbins-Domingo, who is also a professor of medicine at the University of California, San Francisco, and her associates.
Further information regarding the recommendation statement and the evidence report is available at www.uspreventiveservicestaskforce.org.
The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
FROM JAMA
Key clinical point: The USPSTF recommends against screening asymptomatic adults for thyroid cancer because the harms outweigh the benefits.
Major finding: None of the 67 studies reviewed in the evidence report directly assessed the net benefit of thyroid cancer screening, nor whether “early” treatment of screen-detected cancers improved patient outcomes.
Data source: A recommendation statement based on a review of 67 studies published during 1996-2016 involving 583,914 patients.
Disclosures: The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
No increase in hand osteoarthritis seen in Sjögren’s syndrome
LAS VEGAS – Patients with Sjögren’s syndrome do not have an increased prevalence of hand osteoarthritis, but they are strongly predisposed to have a history of hypothyroidism, Jeremie Sellam, MD, reported at the World Congress on Osteoarthritis.
Both of these findings in his small case-control study were unexpected, he added at the congress sponsored by the Osteoarthritis Research Society International.
The study included 34 women with primary Sjögren’s syndrome according to the 2002 American-European Consensus Group criteria and 54 female controls with sicca syndrome but no autoantibodies and no Sjögren’s syndrome. All subjects were evaluated at a specialized tertiary Sjögren’s syndrome clinic. The controls were referred there to ascertain whether they had Sjögren’s syndrome.
Among the Sjögren’s syndrome patients, 41% had radiographic evidence of hand osteoarthritis, 12% had symptomatic hand osteoarthritis, and 9% had erosive hand osteoarthritis. In the sicca syndrome–only patients, the rates were similar at 52%, 28%, and 9%, respectively.
Looking for commonalities and differences between the Sjögren’s syndrome patients and controls, Dr. Sellam and his coinvestigators noted that the Sjögren’s syndrome patients were significantly older, with an average age of 64 years, compared with 48.5 years in the controls.
Impressively, two-thirds of the 15 Sjögren’s syndrome patients with hand osteoarthritis had a history of hypothyroidism, compared with just 15% of the 27 non-autoimmune sicca syndrome patients with hand osteoarthritis and one-quarter of the Sjögren’s syndrome patients without hand osteoarthritis. This suggests a possible interaction between Sjögren’s syndrome, hand osteoarthritis, and a history of hypothyroidism which merits further study, according to the rheumatologist.
Because of the relatively small patient numbers in the French study, Dr. Sellam and coworkers ran a crosscheck with data from the Framingham Osteoarthritis Study and found hand osteoarthritis prevalence rates comparable to their own findings. For example, the prevalences of radiographic and erosive hand osteoarthritis in the French Sjögren’s syndrome and non-autoimmune sicca syndrome groups were similar to the 44% and 10% figures, respectively, in the general population of age-matched Framingham women (Ann Rheum Dis. 2011 Sep;70[9]:1581-6).
He reported having no financial conflicts regarding his study, which was conducted free of commercial support.
LAS VEGAS – Patients with Sjögren’s syndrome do not have an increased prevalence of hand osteoarthritis, but they are strongly predisposed to have a history of hypothyroidism, Jeremie Sellam, MD, reported at the World Congress on Osteoarthritis.
Both of these findings in his small case-control study were unexpected, he added at the congress sponsored by the Osteoarthritis Research Society International.
The study included 34 women with primary Sjögren’s syndrome according to the 2002 American-European Consensus Group criteria and 54 female controls with sicca syndrome but no autoantibodies and no Sjögren’s syndrome. All subjects were evaluated at a specialized tertiary Sjögren’s syndrome clinic. The controls were referred there to ascertain whether they had Sjögren’s syndrome.
Among the Sjögren’s syndrome patients, 41% had radiographic evidence of hand osteoarthritis, 12% had symptomatic hand osteoarthritis, and 9% had erosive hand osteoarthritis. In the sicca syndrome–only patients, the rates were similar at 52%, 28%, and 9%, respectively.
Looking for commonalities and differences between the Sjögren’s syndrome patients and controls, Dr. Sellam and his coinvestigators noted that the Sjögren’s syndrome patients were significantly older, with an average age of 64 years, compared with 48.5 years in the controls.
Impressively, two-thirds of the 15 Sjögren’s syndrome patients with hand osteoarthritis had a history of hypothyroidism, compared with just 15% of the 27 non-autoimmune sicca syndrome patients with hand osteoarthritis and one-quarter of the Sjögren’s syndrome patients without hand osteoarthritis. This suggests a possible interaction between Sjögren’s syndrome, hand osteoarthritis, and a history of hypothyroidism which merits further study, according to the rheumatologist.
Because of the relatively small patient numbers in the French study, Dr. Sellam and coworkers ran a crosscheck with data from the Framingham Osteoarthritis Study and found hand osteoarthritis prevalence rates comparable to their own findings. For example, the prevalences of radiographic and erosive hand osteoarthritis in the French Sjögren’s syndrome and non-autoimmune sicca syndrome groups were similar to the 44% and 10% figures, respectively, in the general population of age-matched Framingham women (Ann Rheum Dis. 2011 Sep;70[9]:1581-6).
He reported having no financial conflicts regarding his study, which was conducted free of commercial support.
LAS VEGAS – Patients with Sjögren’s syndrome do not have an increased prevalence of hand osteoarthritis, but they are strongly predisposed to have a history of hypothyroidism, Jeremie Sellam, MD, reported at the World Congress on Osteoarthritis.
Both of these findings in his small case-control study were unexpected, he added at the congress sponsored by the Osteoarthritis Research Society International.
The study included 34 women with primary Sjögren’s syndrome according to the 2002 American-European Consensus Group criteria and 54 female controls with sicca syndrome but no autoantibodies and no Sjögren’s syndrome. All subjects were evaluated at a specialized tertiary Sjögren’s syndrome clinic. The controls were referred there to ascertain whether they had Sjögren’s syndrome.
Among the Sjögren’s syndrome patients, 41% had radiographic evidence of hand osteoarthritis, 12% had symptomatic hand osteoarthritis, and 9% had erosive hand osteoarthritis. In the sicca syndrome–only patients, the rates were similar at 52%, 28%, and 9%, respectively.
Looking for commonalities and differences between the Sjögren’s syndrome patients and controls, Dr. Sellam and his coinvestigators noted that the Sjögren’s syndrome patients were significantly older, with an average age of 64 years, compared with 48.5 years in the controls.
Impressively, two-thirds of the 15 Sjögren’s syndrome patients with hand osteoarthritis had a history of hypothyroidism, compared with just 15% of the 27 non-autoimmune sicca syndrome patients with hand osteoarthritis and one-quarter of the Sjögren’s syndrome patients without hand osteoarthritis. This suggests a possible interaction between Sjögren’s syndrome, hand osteoarthritis, and a history of hypothyroidism which merits further study, according to the rheumatologist.
Because of the relatively small patient numbers in the French study, Dr. Sellam and coworkers ran a crosscheck with data from the Framingham Osteoarthritis Study and found hand osteoarthritis prevalence rates comparable to their own findings. For example, the prevalences of radiographic and erosive hand osteoarthritis in the French Sjögren’s syndrome and non-autoimmune sicca syndrome groups were similar to the 44% and 10% figures, respectively, in the general population of age-matched Framingham women (Ann Rheum Dis. 2011 Sep;70[9]:1581-6).
He reported having no financial conflicts regarding his study, which was conducted free of commercial support.
AT OARSI 2017
Key clinical point:
Major finding: Nine percent of women with Sjögren’s syndrome had erosive hand osteoarthritis, a prevalence identical to that in a group with non-autoimmune sicca syndrome only.
Data source: This case-control study included 34 women with Sjögren’s syndrome and 54 women with non-autoimmune sicca syndrome.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was conducted free of commercial support.
One in four practitioners doing FNAs are endocrinologists
AT ENDO 2017
ORLANDO – Endocrinologists made up about one in four of the practitioners performing fine needle aspiration (FNA) biopsies between 2012 and 2014, according to a review of data from the Centers for Medicare & Medicaid Services.
Similarly, endocrinologists performed 25% of image-guided thyroid biopsies.
Endocrine surgeons represent only a small percentage of all practitioners performing head and neck ultrasound exams and image-guided FNA, lead author Mamoona Khokhar, MD, said during a poster presentation at the annual meeting of the Endocrine Society. This is true even though the more portable nature of ultrasound has made it easier for motivated surgeons to incorporate its use into their practice, she said.
Examining 3 years of data from a provider utilization and payment database, Dr. Khokhar and her colleagues identified the types of practitioners who performed head and neck ultrasound, as well as image-guided FNA.
In their analysis, the researchers broadly divided practitioners into surgeons and nonsurgeons. Overall, of the 14,750 median annual practitioners performing head and neck ultrasound between 2012 and 2014, 97.2% were nonsurgeon practitioners, reported Dr. Khokhar, an endocrine surgery fellow at Columbia University Medical Center in New York.
Of all practitioners performing head and neck ultrasound, most (81%) were radiologists. Endocrinologists made up 8% of the overall pool performing ultrasounds.
Breaking the surgeon group down further showed that endocrine surgeons represented 14.7% of surgeons performing head and neck ultrasound, meaning that they made up just 0.4% of the practitioner pool for this procedure. Just over half (52%) of the surgeons performing ultrasounds were otolaryngologists.
The number of practitioners performing image-guided FNA was smaller, at a median 3,695 per year during the study period. Surgeons made up 10.7% of this number. Of the surgeons who performed image-guided FNA, 10.5% were endocrine surgeons. Endocrine surgeons made up 1.1% of all practitioners who billed for FNA.
Again, radiologists made up the majority (58%) of the practitioners performing FNA, and one in four (25%) of practitioners performing FNAs were endocrinologists. Just 5% of the practitioners performing FNAs were otolaryngologists.
More endocrine surgeons performed ultrasound than advanced practice providers (nurse practitioners or physician assistants, 0.2%), pathologists (0.1%), and surgical oncologists (0.04%; P for all, less than .0001). However, advanced practice providers and pathologists both performed significantly more FNAs than did endocrine surgeons (2.1% and 1.8%, P less than .0001).
Although the raw proportion of endocrine surgeons billing for these procedures increased during the study period, the increases were not statistically significant. Dr. Khokhar and her colleagues found that the proportion of American Association of Endocrine Surgeons members who performed head and neck ultrasound grew from 59% in 2012 to 72% in 2014 (P = 0.37), while the proportion performing FNA also increased, from 36% in 2012 to 46% in 2014 (P = .40).
Surgeons, however, may face a number of obstacles in setting up office-based ultrasound, which can lead to underutilization by surgeons, Dr. Khokhar noted, adding that “the results of this study suggest that endocrine surgeons may not be fully utilizing this critical tool in their clinical practice.”
The authors reported no outside sources of funding, and had no relevant conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
AT ENDO 2017
ORLANDO – Endocrinologists made up about one in four of the practitioners performing fine needle aspiration (FNA) biopsies between 2012 and 2014, according to a review of data from the Centers for Medicare & Medicaid Services.
Similarly, endocrinologists performed 25% of image-guided thyroid biopsies.
Endocrine surgeons represent only a small percentage of all practitioners performing head and neck ultrasound exams and image-guided FNA, lead author Mamoona Khokhar, MD, said during a poster presentation at the annual meeting of the Endocrine Society. This is true even though the more portable nature of ultrasound has made it easier for motivated surgeons to incorporate its use into their practice, she said.
Examining 3 years of data from a provider utilization and payment database, Dr. Khokhar and her colleagues identified the types of practitioners who performed head and neck ultrasound, as well as image-guided FNA.
In their analysis, the researchers broadly divided practitioners into surgeons and nonsurgeons. Overall, of the 14,750 median annual practitioners performing head and neck ultrasound between 2012 and 2014, 97.2% were nonsurgeon practitioners, reported Dr. Khokhar, an endocrine surgery fellow at Columbia University Medical Center in New York.
Of all practitioners performing head and neck ultrasound, most (81%) were radiologists. Endocrinologists made up 8% of the overall pool performing ultrasounds.
Breaking the surgeon group down further showed that endocrine surgeons represented 14.7% of surgeons performing head and neck ultrasound, meaning that they made up just 0.4% of the practitioner pool for this procedure. Just over half (52%) of the surgeons performing ultrasounds were otolaryngologists.
The number of practitioners performing image-guided FNA was smaller, at a median 3,695 per year during the study period. Surgeons made up 10.7% of this number. Of the surgeons who performed image-guided FNA, 10.5% were endocrine surgeons. Endocrine surgeons made up 1.1% of all practitioners who billed for FNA.
Again, radiologists made up the majority (58%) of the practitioners performing FNA, and one in four (25%) of practitioners performing FNAs were endocrinologists. Just 5% of the practitioners performing FNAs were otolaryngologists.
More endocrine surgeons performed ultrasound than advanced practice providers (nurse practitioners or physician assistants, 0.2%), pathologists (0.1%), and surgical oncologists (0.04%; P for all, less than .0001). However, advanced practice providers and pathologists both performed significantly more FNAs than did endocrine surgeons (2.1% and 1.8%, P less than .0001).
Although the raw proportion of endocrine surgeons billing for these procedures increased during the study period, the increases were not statistically significant. Dr. Khokhar and her colleagues found that the proportion of American Association of Endocrine Surgeons members who performed head and neck ultrasound grew from 59% in 2012 to 72% in 2014 (P = 0.37), while the proportion performing FNA also increased, from 36% in 2012 to 46% in 2014 (P = .40).
Surgeons, however, may face a number of obstacles in setting up office-based ultrasound, which can lead to underutilization by surgeons, Dr. Khokhar noted, adding that “the results of this study suggest that endocrine surgeons may not be fully utilizing this critical tool in their clinical practice.”
The authors reported no outside sources of funding, and had no relevant conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
AT ENDO 2017
ORLANDO – Endocrinologists made up about one in four of the practitioners performing fine needle aspiration (FNA) biopsies between 2012 and 2014, according to a review of data from the Centers for Medicare & Medicaid Services.
Similarly, endocrinologists performed 25% of image-guided thyroid biopsies.
Endocrine surgeons represent only a small percentage of all practitioners performing head and neck ultrasound exams and image-guided FNA, lead author Mamoona Khokhar, MD, said during a poster presentation at the annual meeting of the Endocrine Society. This is true even though the more portable nature of ultrasound has made it easier for motivated surgeons to incorporate its use into their practice, she said.
Examining 3 years of data from a provider utilization and payment database, Dr. Khokhar and her colleagues identified the types of practitioners who performed head and neck ultrasound, as well as image-guided FNA.
In their analysis, the researchers broadly divided practitioners into surgeons and nonsurgeons. Overall, of the 14,750 median annual practitioners performing head and neck ultrasound between 2012 and 2014, 97.2% were nonsurgeon practitioners, reported Dr. Khokhar, an endocrine surgery fellow at Columbia University Medical Center in New York.
Of all practitioners performing head and neck ultrasound, most (81%) were radiologists. Endocrinologists made up 8% of the overall pool performing ultrasounds.
Breaking the surgeon group down further showed that endocrine surgeons represented 14.7% of surgeons performing head and neck ultrasound, meaning that they made up just 0.4% of the practitioner pool for this procedure. Just over half (52%) of the surgeons performing ultrasounds were otolaryngologists.
The number of practitioners performing image-guided FNA was smaller, at a median 3,695 per year during the study period. Surgeons made up 10.7% of this number. Of the surgeons who performed image-guided FNA, 10.5% were endocrine surgeons. Endocrine surgeons made up 1.1% of all practitioners who billed for FNA.
Again, radiologists made up the majority (58%) of the practitioners performing FNA, and one in four (25%) of practitioners performing FNAs were endocrinologists. Just 5% of the practitioners performing FNAs were otolaryngologists.
More endocrine surgeons performed ultrasound than advanced practice providers (nurse practitioners or physician assistants, 0.2%), pathologists (0.1%), and surgical oncologists (0.04%; P for all, less than .0001). However, advanced practice providers and pathologists both performed significantly more FNAs than did endocrine surgeons (2.1% and 1.8%, P less than .0001).
Although the raw proportion of endocrine surgeons billing for these procedures increased during the study period, the increases were not statistically significant. Dr. Khokhar and her colleagues found that the proportion of American Association of Endocrine Surgeons members who performed head and neck ultrasound grew from 59% in 2012 to 72% in 2014 (P = 0.37), while the proportion performing FNA also increased, from 36% in 2012 to 46% in 2014 (P = .40).
Surgeons, however, may face a number of obstacles in setting up office-based ultrasound, which can lead to underutilization by surgeons, Dr. Khokhar noted, adding that “the results of this study suggest that endocrine surgeons may not be fully utilizing this critical tool in their clinical practice.”
The authors reported no outside sources of funding, and had no relevant conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
Key clinical point:
Major finding: Endocrinologists made up 25% of the practitioners performing FNAs.
Data source: A retrospective analysis of 3 years’ worth of data on head and neck ultrasound and fine needle aspiration from the Centers for Medicare & Medicaid Services.
Disclosures: None of the study authors reported relevant disclosures, and no external source of funding was reported.
Dulera inhaler linked to adrenocorticotropic suppression in small case series
ORLANDO – A combination corticosteroid asthma inhaler has, for the first time, been associated with growth delay and adrenocorticotropic suppression in children.
The single-center case series is small, but the results highlight the need to regularly monitor growth and adrenal function in children using inhaled mometasone furoate/formoterol fumarate (Dulera; Merck), investigators said at the annual meeting of the Endocrine Society.
“We are hoping to raise awareness of this risk in our pediatric endocrinology colleagues, as well as among allergists, pulmonologists, and pediatricians who treat these children,” said Fadi Al Muhaisen, MD. “These kids should be regularly screened for growth delay and adrenal insufficiency and have their growth plotted at every visit as well.”
Dulera was approved in the United States in 2010 as a maintenance therapy for chronic asthma in adults and children aged 12 years and older. Mometasone furoate is a potent corticosteroid, and formoterol fumarate is a long-acting beta2-adrenergic agonist. The prescribing information says that mometasone furoate exerts less effect on the hypothalamic-pituitary-adrenal axis than other inhaled corticosteroids, and that adrenal suppression is unlikely to occur when used at recommended dosages. These range from a low of 100 mcg/5 mcg, two puffs daily to a maximum dose of 800 mcg/20 mcg daily.
The review involved 18 children, all of whom were seen in the endocrinology clinic for growth failure or short stature and were receiving Dulera for management of their asthma. Of these, eight (44%) had a full adrenal evaluation. Six had biochemical evidence of adrenal suppression and two had normal adrenal function. The remaining 10 patients had not undergone an adrenal evaluation. None of them were on any other inhaled corticosteroid. The six children diagnosed with adrenal insufficiency had a mean age of 9.7 years, but ranged in age from 7 to 12 years. They had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids in the preceding 6 months before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at the time of diagnosis but had been using the higher dose for the preceding 18 months. Three were using concomitant nasal steroids.
The six children evaluated had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids during the 2 years before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at time of diagnosis, but had been using the higher dose for 18 months before that. Three were using concomitant nasal steroids.
All presented with growth failure, with bone age 1-3 years behind chronological age. One child was referred to the clinic after an emergency department visit for headache, nausea, diarrhea, and fatigue – symptoms of adrenal failure. That child had an adrenocroticotropin (ACTH) level of 10 pg/mL. Both his random peak cortisol measures after ACTH stimulation were less than 1 mcg/mL.
ACTH levels in four of the children were less than 5-6 pg/ml, with random and peak stimulated cortisols of around 1 mcg/mL. One patient had an ACTH level of 68 pg/mL, a random cortisol of less than 1 mcg/mL, and a peak stimulated cortisol of 8.7 mcg/mL.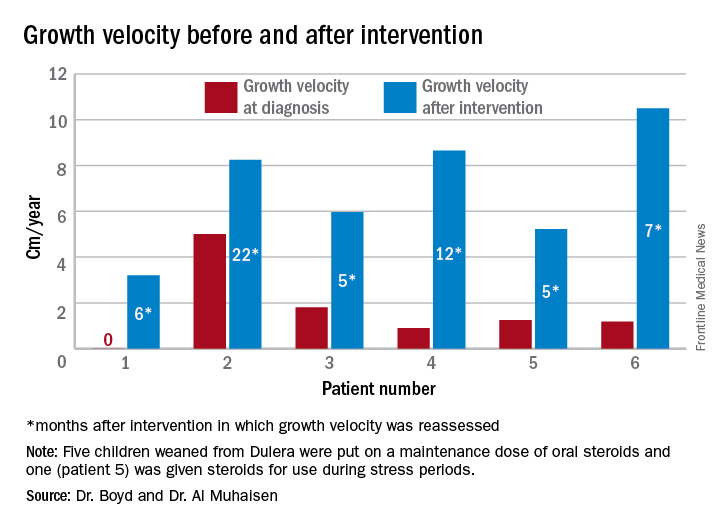
The results were all normal in the four subjects who had repeat adrenal function evaluation after intervention. Adrenal recovery took a mean of 20 months (5-30 months).
Growth accelerated rapidly after intervention, which was either initiation of maintenance oral steroids and discontinuation of Dulera or, in one patient, after Dulera was weaned. At time of adrenal insufficiency diagnosis, four patients had grown 1-2 cm in the prior year; one had not grown at all, and one had grown about 4.5 cm. After discontinuing or weaning the medication, all experienced growth spurts: 3 cm/year in 6 months; 8 cm/year in 22 months; 6 cm/year in 5 months; 8 cm/year in 12 months; 5 cm/year in 5 months; and 10 cm/year in 7 months.
There were no exacerbations in asthma, despite discontinuing the inhaled medication, Dr. Al Muhaisen said. Changing the asthma treatment required some open discussion between the investigators and the treating pulmonologists, he noted.
“We had some back-and-forth discussions, being very frank that we thought the adrenal insufficiency was directly related to this medication and that we needed to wean it and stop it as soon as possible.”
Neither Dr. Al Muhaisen nor Dr. Boyd had any financial disclosures.
ORLANDO – A combination corticosteroid asthma inhaler has, for the first time, been associated with growth delay and adrenocorticotropic suppression in children.
The single-center case series is small, but the results highlight the need to regularly monitor growth and adrenal function in children using inhaled mometasone furoate/formoterol fumarate (Dulera; Merck), investigators said at the annual meeting of the Endocrine Society.
“We are hoping to raise awareness of this risk in our pediatric endocrinology colleagues, as well as among allergists, pulmonologists, and pediatricians who treat these children,” said Fadi Al Muhaisen, MD. “These kids should be regularly screened for growth delay and adrenal insufficiency and have their growth plotted at every visit as well.”
Dulera was approved in the United States in 2010 as a maintenance therapy for chronic asthma in adults and children aged 12 years and older. Mometasone furoate is a potent corticosteroid, and formoterol fumarate is a long-acting beta2-adrenergic agonist. The prescribing information says that mometasone furoate exerts less effect on the hypothalamic-pituitary-adrenal axis than other inhaled corticosteroids, and that adrenal suppression is unlikely to occur when used at recommended dosages. These range from a low of 100 mcg/5 mcg, two puffs daily to a maximum dose of 800 mcg/20 mcg daily.
The review involved 18 children, all of whom were seen in the endocrinology clinic for growth failure or short stature and were receiving Dulera for management of their asthma. Of these, eight (44%) had a full adrenal evaluation. Six had biochemical evidence of adrenal suppression and two had normal adrenal function. The remaining 10 patients had not undergone an adrenal evaluation. None of them were on any other inhaled corticosteroid. The six children diagnosed with adrenal insufficiency had a mean age of 9.7 years, but ranged in age from 7 to 12 years. They had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids in the preceding 6 months before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at the time of diagnosis but had been using the higher dose for the preceding 18 months. Three were using concomitant nasal steroids.
The six children evaluated had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids during the 2 years before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at time of diagnosis, but had been using the higher dose for 18 months before that. Three were using concomitant nasal steroids.
All presented with growth failure, with bone age 1-3 years behind chronological age. One child was referred to the clinic after an emergency department visit for headache, nausea, diarrhea, and fatigue – symptoms of adrenal failure. That child had an adrenocroticotropin (ACTH) level of 10 pg/mL. Both his random peak cortisol measures after ACTH stimulation were less than 1 mcg/mL.
ACTH levels in four of the children were less than 5-6 pg/ml, with random and peak stimulated cortisols of around 1 mcg/mL. One patient had an ACTH level of 68 pg/mL, a random cortisol of less than 1 mcg/mL, and a peak stimulated cortisol of 8.7 mcg/mL.
The results were all normal in the four subjects who had repeat adrenal function evaluation after intervention. Adrenal recovery took a mean of 20 months (5-30 months).
Growth accelerated rapidly after intervention, which was either initiation of maintenance oral steroids and discontinuation of Dulera or, in one patient, after Dulera was weaned. At time of adrenal insufficiency diagnosis, four patients had grown 1-2 cm in the prior year; one had not grown at all, and one had grown about 4.5 cm. After discontinuing or weaning the medication, all experienced growth spurts: 3 cm/year in 6 months; 8 cm/year in 22 months; 6 cm/year in 5 months; 8 cm/year in 12 months; 5 cm/year in 5 months; and 10 cm/year in 7 months.
There were no exacerbations in asthma, despite discontinuing the inhaled medication, Dr. Al Muhaisen said. Changing the asthma treatment required some open discussion between the investigators and the treating pulmonologists, he noted.
“We had some back-and-forth discussions, being very frank that we thought the adrenal insufficiency was directly related to this medication and that we needed to wean it and stop it as soon as possible.”
Neither Dr. Al Muhaisen nor Dr. Boyd had any financial disclosures.
ORLANDO – A combination corticosteroid asthma inhaler has, for the first time, been associated with growth delay and adrenocorticotropic suppression in children.
The single-center case series is small, but the results highlight the need to regularly monitor growth and adrenal function in children using inhaled mometasone furoate/formoterol fumarate (Dulera; Merck), investigators said at the annual meeting of the Endocrine Society.
“We are hoping to raise awareness of this risk in our pediatric endocrinology colleagues, as well as among allergists, pulmonologists, and pediatricians who treat these children,” said Fadi Al Muhaisen, MD. “These kids should be regularly screened for growth delay and adrenal insufficiency and have their growth plotted at every visit as well.”
Dulera was approved in the United States in 2010 as a maintenance therapy for chronic asthma in adults and children aged 12 years and older. Mometasone furoate is a potent corticosteroid, and formoterol fumarate is a long-acting beta2-adrenergic agonist. The prescribing information says that mometasone furoate exerts less effect on the hypothalamic-pituitary-adrenal axis than other inhaled corticosteroids, and that adrenal suppression is unlikely to occur when used at recommended dosages. These range from a low of 100 mcg/5 mcg, two puffs daily to a maximum dose of 800 mcg/20 mcg daily.
The review involved 18 children, all of whom were seen in the endocrinology clinic for growth failure or short stature and were receiving Dulera for management of their asthma. Of these, eight (44%) had a full adrenal evaluation. Six had biochemical evidence of adrenal suppression and two had normal adrenal function. The remaining 10 patients had not undergone an adrenal evaluation. None of them were on any other inhaled corticosteroid. The six children diagnosed with adrenal insufficiency had a mean age of 9.7 years, but ranged in age from 7 to 12 years. They had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids in the preceding 6 months before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at the time of diagnosis but had been using the higher dose for the preceding 18 months. Three were using concomitant nasal steroids.
The six children evaluated had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids during the 2 years before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at time of diagnosis, but had been using the higher dose for 18 months before that. Three were using concomitant nasal steroids.
All presented with growth failure, with bone age 1-3 years behind chronological age. One child was referred to the clinic after an emergency department visit for headache, nausea, diarrhea, and fatigue – symptoms of adrenal failure. That child had an adrenocroticotropin (ACTH) level of 10 pg/mL. Both his random peak cortisol measures after ACTH stimulation were less than 1 mcg/mL.
ACTH levels in four of the children were less than 5-6 pg/ml, with random and peak stimulated cortisols of around 1 mcg/mL. One patient had an ACTH level of 68 pg/mL, a random cortisol of less than 1 mcg/mL, and a peak stimulated cortisol of 8.7 mcg/mL.
The results were all normal in the four subjects who had repeat adrenal function evaluation after intervention. Adrenal recovery took a mean of 20 months (5-30 months).
Growth accelerated rapidly after intervention, which was either initiation of maintenance oral steroids and discontinuation of Dulera or, in one patient, after Dulera was weaned. At time of adrenal insufficiency diagnosis, four patients had grown 1-2 cm in the prior year; one had not grown at all, and one had grown about 4.5 cm. After discontinuing or weaning the medication, all experienced growth spurts: 3 cm/year in 6 months; 8 cm/year in 22 months; 6 cm/year in 5 months; 8 cm/year in 12 months; 5 cm/year in 5 months; and 10 cm/year in 7 months.
There were no exacerbations in asthma, despite discontinuing the inhaled medication, Dr. Al Muhaisen said. Changing the asthma treatment required some open discussion between the investigators and the treating pulmonologists, he noted.
“We had some back-and-forth discussions, being very frank that we thought the adrenal insufficiency was directly related to this medication and that we needed to wean it and stop it as soon as possible.”
Neither Dr. Al Muhaisen nor Dr. Boyd had any financial disclosures.
AT ENDO 2017
Key clinical point:
Major finding: Of eight children who had an adrenal workup at an endocrinology clinic, six had adrenal suppression.
Data source: The case series comprised 18 children taking Dulera who presented with growth failure.
Disclosures: Neither Dr. Al Muhaisen nor Dr. Boyd had any financial disclosures.
Osilodrostat maintained cortisol control in Cushing’s syndrome
ORLANDO – Osilodrostat, a drug that normalized cortisol in 89% of patients with Cushing’s syndrome who took it during a phase II study, continued to exert a sustained benefit during a 31-month extension phase.
In an intent-to-treat analysis, all of the 16 patients who entered the LINC-2 extension study responded well to the medication, with no lapse in cortisol control, Rosario Pivonello, MD, said at the annual meeting of the Endocrine Society.
Osilodrostat, made by Novartis, is an oral inhibitor of 11 beta–hydroxylase. The enzyme catalyzes the last step of cortisol synthesis in the adrenal cortex. The drug was granted orphan status in 2014 by the European Medicines Agency.
In the LINC-2 study, 19 patients took osilodrostat at an initial dose of either 4 mg/day or 10 mg/day, if baseline urinary-free cortisol exceeded three times the upper normal limit. The dose was escalated every 2 weeks to up to 60 mg/day, until cortisol levels were at or below the upper limit of normal. In this study, the main efficacy endpoint was normalization of cortisol, or at least a 50% decrease from baseline at weeks 10 and 22.
Overall response was 89%. Osilodrostat treatment reduced urinary-free cortisol in all patients, and 79% had normal cortisol levels at week 22. The most common adverse events were nausea, diarrhea, asthenia, and adrenal insufficiency. New or worsening hirsutism and/or acne were reported among four female patients, all of whom had increased testosterone levels.
The LINC-2 extension study enrolled 16 patients from the phase II cohort, all of whom had responded to the medication. They were allowed to continue on their existing effective dose through the 31-month period.
Dr. Pivonello presented response curves that tracked cortisol levels from treatment initiation in the LINC-2 study. The median baseline cortisol level was about 1,500 nmol per 24 hours. By the fourth week of treatment, this had normalized in all of the patients who entered the extension phase. The response curve showed continued, stable cortisol suppression throughout the entire 31-month period.
Four patients dropped out during the course of the study. Dr. Pivonello didn’t discuss the reasons for these dropouts. He did break down the results by response, imputing the missing data from these four patients. In this analysis, the majority (87.5%) were fully controlled, with urinary-free cortisol in the normal range. The remainder were partially controlled, experiencing at least a 50% decrease in cortisol from their baseline levels. These responses were stable, with no patient experiencing loss of control over the follow-up period.
The 12 remaining patients are still taking the medication, and they experienced other clinical improvements as well. Systolic blood pressure decreased by a mean of 2.2% (from 130 mm Hg to 127 mm Hg). Diastolic blood pressure also improved, by 6% (from 85 mm Hg to 80 mm Hg).
Fasting plasma glucose dropped from a mean of 89 mg/dL to 82 mg/dL. Weight decreased from a mean of 84 kg to 74 kg, with a corresponding decrease in body mass index, from 29.6 kg/m2 to 26.2 kg/m2.
Serum aldosterone decreased along with cortisol, dropping from a mean of 168 pmol/L to just 19 pmol/L. Adrenocorticotropic hormone increased, as did 11-deoxycortisol, 11-deoxycorticosterone, and testosterone.
Pituitary tumor size was measured in six patients. It increased in three and decreased in three. Dr. Pivonello didn’t discuss why this might have occurred.
The most common adverse events were asthenia, adrenal insufficiency, diarrhea, fatigue, headache, nausea, and acne. These moderated over time in both number and severity.
However, there were eight serious adverse events among three patients, including prolonged Q-T interval on electrocardiogram, food poisoning, gastroenteritis, headache, noncardiac chest pain, symptoms related to pituitary tumor (two patients), and uncontrolled Cushing’s syndrome.
Two patients experienced hypokalemia. Six experienced mild events related to hypocortisolism.
Novartis is pursuing the drug with two placebo-controlled phase III studies (LINC-3 and LINC-4), Dr. Pivonello said. An additional phase II study is being conducted in Japan.
Dr. Pivonello has received consulting fees and honoraria from Novartis, which sponsored the study.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
ORLANDO – Osilodrostat, a drug that normalized cortisol in 89% of patients with Cushing’s syndrome who took it during a phase II study, continued to exert a sustained benefit during a 31-month extension phase.
In an intent-to-treat analysis, all of the 16 patients who entered the LINC-2 extension study responded well to the medication, with no lapse in cortisol control, Rosario Pivonello, MD, said at the annual meeting of the Endocrine Society.
Osilodrostat, made by Novartis, is an oral inhibitor of 11 beta–hydroxylase. The enzyme catalyzes the last step of cortisol synthesis in the adrenal cortex. The drug was granted orphan status in 2014 by the European Medicines Agency.
In the LINC-2 study, 19 patients took osilodrostat at an initial dose of either 4 mg/day or 10 mg/day, if baseline urinary-free cortisol exceeded three times the upper normal limit. The dose was escalated every 2 weeks to up to 60 mg/day, until cortisol levels were at or below the upper limit of normal. In this study, the main efficacy endpoint was normalization of cortisol, or at least a 50% decrease from baseline at weeks 10 and 22.
Overall response was 89%. Osilodrostat treatment reduced urinary-free cortisol in all patients, and 79% had normal cortisol levels at week 22. The most common adverse events were nausea, diarrhea, asthenia, and adrenal insufficiency. New or worsening hirsutism and/or acne were reported among four female patients, all of whom had increased testosterone levels.
The LINC-2 extension study enrolled 16 patients from the phase II cohort, all of whom had responded to the medication. They were allowed to continue on their existing effective dose through the 31-month period.
Dr. Pivonello presented response curves that tracked cortisol levels from treatment initiation in the LINC-2 study. The median baseline cortisol level was about 1,500 nmol per 24 hours. By the fourth week of treatment, this had normalized in all of the patients who entered the extension phase. The response curve showed continued, stable cortisol suppression throughout the entire 31-month period.
Four patients dropped out during the course of the study. Dr. Pivonello didn’t discuss the reasons for these dropouts. He did break down the results by response, imputing the missing data from these four patients. In this analysis, the majority (87.5%) were fully controlled, with urinary-free cortisol in the normal range. The remainder were partially controlled, experiencing at least a 50% decrease in cortisol from their baseline levels. These responses were stable, with no patient experiencing loss of control over the follow-up period.
The 12 remaining patients are still taking the medication, and they experienced other clinical improvements as well. Systolic blood pressure decreased by a mean of 2.2% (from 130 mm Hg to 127 mm Hg). Diastolic blood pressure also improved, by 6% (from 85 mm Hg to 80 mm Hg).
Fasting plasma glucose dropped from a mean of 89 mg/dL to 82 mg/dL. Weight decreased from a mean of 84 kg to 74 kg, with a corresponding decrease in body mass index, from 29.6 kg/m2 to 26.2 kg/m2.
Serum aldosterone decreased along with cortisol, dropping from a mean of 168 pmol/L to just 19 pmol/L. Adrenocorticotropic hormone increased, as did 11-deoxycortisol, 11-deoxycorticosterone, and testosterone.
Pituitary tumor size was measured in six patients. It increased in three and decreased in three. Dr. Pivonello didn’t discuss why this might have occurred.
The most common adverse events were asthenia, adrenal insufficiency, diarrhea, fatigue, headache, nausea, and acne. These moderated over time in both number and severity.
However, there were eight serious adverse events among three patients, including prolonged Q-T interval on electrocardiogram, food poisoning, gastroenteritis, headache, noncardiac chest pain, symptoms related to pituitary tumor (two patients), and uncontrolled Cushing’s syndrome.
Two patients experienced hypokalemia. Six experienced mild events related to hypocortisolism.
Novartis is pursuing the drug with two placebo-controlled phase III studies (LINC-3 and LINC-4), Dr. Pivonello said. An additional phase II study is being conducted in Japan.
Dr. Pivonello has received consulting fees and honoraria from Novartis, which sponsored the study.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
ORLANDO – Osilodrostat, a drug that normalized cortisol in 89% of patients with Cushing’s syndrome who took it during a phase II study, continued to exert a sustained benefit during a 31-month extension phase.
In an intent-to-treat analysis, all of the 16 patients who entered the LINC-2 extension study responded well to the medication, with no lapse in cortisol control, Rosario Pivonello, MD, said at the annual meeting of the Endocrine Society.
Osilodrostat, made by Novartis, is an oral inhibitor of 11 beta–hydroxylase. The enzyme catalyzes the last step of cortisol synthesis in the adrenal cortex. The drug was granted orphan status in 2014 by the European Medicines Agency.
In the LINC-2 study, 19 patients took osilodrostat at an initial dose of either 4 mg/day or 10 mg/day, if baseline urinary-free cortisol exceeded three times the upper normal limit. The dose was escalated every 2 weeks to up to 60 mg/day, until cortisol levels were at or below the upper limit of normal. In this study, the main efficacy endpoint was normalization of cortisol, or at least a 50% decrease from baseline at weeks 10 and 22.
Overall response was 89%. Osilodrostat treatment reduced urinary-free cortisol in all patients, and 79% had normal cortisol levels at week 22. The most common adverse events were nausea, diarrhea, asthenia, and adrenal insufficiency. New or worsening hirsutism and/or acne were reported among four female patients, all of whom had increased testosterone levels.
The LINC-2 extension study enrolled 16 patients from the phase II cohort, all of whom had responded to the medication. They were allowed to continue on their existing effective dose through the 31-month period.
Dr. Pivonello presented response curves that tracked cortisol levels from treatment initiation in the LINC-2 study. The median baseline cortisol level was about 1,500 nmol per 24 hours. By the fourth week of treatment, this had normalized in all of the patients who entered the extension phase. The response curve showed continued, stable cortisol suppression throughout the entire 31-month period.
Four patients dropped out during the course of the study. Dr. Pivonello didn’t discuss the reasons for these dropouts. He did break down the results by response, imputing the missing data from these four patients. In this analysis, the majority (87.5%) were fully controlled, with urinary-free cortisol in the normal range. The remainder were partially controlled, experiencing at least a 50% decrease in cortisol from their baseline levels. These responses were stable, with no patient experiencing loss of control over the follow-up period.
The 12 remaining patients are still taking the medication, and they experienced other clinical improvements as well. Systolic blood pressure decreased by a mean of 2.2% (from 130 mm Hg to 127 mm Hg). Diastolic blood pressure also improved, by 6% (from 85 mm Hg to 80 mm Hg).
Fasting plasma glucose dropped from a mean of 89 mg/dL to 82 mg/dL. Weight decreased from a mean of 84 kg to 74 kg, with a corresponding decrease in body mass index, from 29.6 kg/m2 to 26.2 kg/m2.
Serum aldosterone decreased along with cortisol, dropping from a mean of 168 pmol/L to just 19 pmol/L. Adrenocorticotropic hormone increased, as did 11-deoxycortisol, 11-deoxycorticosterone, and testosterone.
Pituitary tumor size was measured in six patients. It increased in three and decreased in three. Dr. Pivonello didn’t discuss why this might have occurred.
The most common adverse events were asthenia, adrenal insufficiency, diarrhea, fatigue, headache, nausea, and acne. These moderated over time in both number and severity.
However, there were eight serious adverse events among three patients, including prolonged Q-T interval on electrocardiogram, food poisoning, gastroenteritis, headache, noncardiac chest pain, symptoms related to pituitary tumor (two patients), and uncontrolled Cushing’s syndrome.
Two patients experienced hypokalemia. Six experienced mild events related to hypocortisolism.
Novartis is pursuing the drug with two placebo-controlled phase III studies (LINC-3 and LINC-4), Dr. Pivonello said. An additional phase II study is being conducted in Japan.
Dr. Pivonello has received consulting fees and honoraria from Novartis, which sponsored the study.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT ENDO 2017
Key clinical point:
Major finding: Every patient in the study responded either fully (87.5%) or partially, with a decrease of at least 50% in cortisol from baseline (12.5%), with no loss of control.
Data source: The 31-month extension study comprised 16 patients who had responded well to the drug in a phase II trial.
Disclosures: Dr. Pivonello has received consulting fees and honoraria from Novartis, which sponsored the study.
Long-acting growth hormone moves forward based on positive phase II data
ORLANDO – An extended-release human growth hormone formulation proved safe and effective in both children and adults, offering the prospect of a less-rigorous dosing schedule and potentially better patient compliance with treatment.
The two phase II studies examined somavaratan, which is being developed by Versartis of Menlo Park, Calif. Kevin Yuen, MD, an endocrinologist at the Swedish Medical Center, Seattle, and Wayne V. Moore, MD, a pediatric endocrinologist at Children’s Mercy Hospital, Kansas City, Mo., presented the data at the annual meeting of the Endocrine Society.
“Despite the fact that human growth hormone is a proven treatment for growth hormone deficiency, daily use of our current formulations can be a factor that affects compliance,” said Dr. Yuen. He cited a 2008 study of 158 men taking growth hormone, which found that only one-third were highly compliant (Endocr Pract. 2008 Mar;14[2]:143-54). “And even among this group, there were 21 doses missed over just a 3-month period.”
The group of 55 international experts described several strategies for creating long-acting growth hormone formulations, including depot formulations, pegylation, prodrugs, noncovalent albumin binding growth hormone compounds, and growth hormone fusion proteins. These preparations are currently in various stages of development, with some already approved in Europe and Asia.
Somavaratan (VRS-317) is a fusion protein produced in Escherichia coli. The active portion is recombinant human growth hormone, which is bound to long chains of hydrophilic amino acids. This reduces renal filtration, Dr. Yuen said. The growth hormone loses some potency in this construct, but its delayed clearance, with a half-life 30-60 times longer than recombinant human growth hormone (rhGH) allows it to exert a prolonged effect in target tissue.
Of the two phase II studies of the molecule, one was conducted in 64 prepubertal children who were naive to any growth hormone treatment, and one in 36 adults with adult-onset growth hormone deficiency. The company also has made these presentations available online.
The pediatric study reported 3-year data on the cohort, which began treatment of children at a mean age of 7 years. At baseline, the children were about 2.6 standard deviations (SDs) below their expected height, and their mean IGF-1 levels, about 1.7 SDs below. They showed a mean maximum stimulated growth hormone level of 5.4 ng/mL. Although they were a mean of 7.8 years chronologically at the study’s outset, their mean bone age was 6.4 years.
The first 12 months of the study consisted of dose-ranging trials, with initial doses of 5 mg/kg each month, then 2.5 mg/kg twice a month, and then 1.15 mg/kg weekly. During the last 2 years of the study, all children were taking 3.5 mg/kg, once a month.
Within the first year, all children taking the 3.5-mg/kg dose had achieved normal IGF-1 levels, which were consistent with levels achieved in the ANSWER registry dataset of somatropin (rDNA origin) injection (Norditropin) recombinant human growth hormone (Clin Epidemiol. 2013;5:119-27).
Height velocity improved, as did height standard deviation. By year 3, patients were a mean of 1.25 SDs below expected height – a significant improvement over baseline. These findings were almost superimposable with those in the ANSWER registry. Bone age and chronological age came into alignment within the first year and that association was maintained throughout the study – again, in almost superimposable curves with the registry data.
Somavaratan exerted no untoward metabolic effects. There were no adverse changes in body mass index. At baseline, the mean hemoglobin A1c was 5.2%; this was unchanged at 3 years. No patient developed diabetes. The most commonly reported adverse event was injection site pain (48%). Injection site erythema was reported in 5% of patients, but no injection site nodules occurred.
Other adverse events were headache, extremity pain, arthralgia, and musculoskeletal pain. Although the numbers were small overall, reports did increase after all the children were switched to the 3.5-mg/kg dose. However, they occurred in 5% or less of the patient group. There were no withdrawals due to adverse events.
The second trial was a dose-ranging study conducted in 49 adults aged 23-70 years. They all had been diagnosed with adult-onset growth hormone deficiency, but had stable pituitary function. If they were on any growth hormone therapy, they underwent a 14-day washout period.
The subjects were divided and dosed by age and gender. All subjects received one injection per month for 5 months.
Cohort A comprised 21 men and women aged 35 years or older, who took 0.6 mg/kg per month. Cohort B comprised six men and women younger than 35 years, who took 0.8 mg/kg per month. Cohort C comprised eight women taking oral estrogen contraceptives. These women received 1 mg/kg per month.
The cohorts were similar in body mass index and weight, but they did differ significantly in baseline IGF-1 levels. In cohort A, the level was 0.52 SDs below normal. In cohort B, it was 2.89 SDs below normal, and in cohort C, it was 2.29 SDs below.
Overall, somavaratan induced a rapid and dramatic increase in IGF-1 that tailed off over 30 days. By day 8 after injection, IGF-1 had risen from a mean baseline of -1 SDs to more than 2 SDs above. By day 22, it had returned to baseline levels. The response to the fifth injection was identical to that of the first, Dr. Yuen said.
Response varied somewhat by cohort, with the younger, mixed-gender group responding the most dramatically, with a mean increase of about 4 SDs from baseline. This put the group above the maximum response target of 1.5 SDs.
The older, mixed-gender cohort experienced about a 3 SDs increase – also above the target level. The women taking estrogen experienced the flattest response, gaining about 2 SDs. However, the response curve was nearly identical, with a rapid, sharp increase in IGF-1 within the first week, followed by a gradual decline to baseline by 22 days.
The adverse event profile was not quite as benign as it was in the pediatric study. Virtually all patients experienced at least one adverse event. A third were mild and 58% moderate. The rest were serious, with one severe and one life-threatening event. Dr. Yuen did not discuss adverse events; these were, however, included in supplementary slides available on the Versartis Inc. slide set.
The finding of a predictable, 3-week tailing-off of efficacy, combined with the fact that patients responded so dramatically, exceeding the maximum target of a 1.5 SDs increase in IGF-1, has prompted a new dosing protocol for the somavaratan open-label extension study, which includes all the phase II completers, plus an additional 40 adult patients.
Doses will be titrated to each subject’s individual IGF-1 responses, based on the IGF-I level 7 days post dose until a maintenance dose is reached. Subjects receiving somavaratan in a previous somavaratan study will have their dose decreased by half (minimum dose of 20 mg, or 40 mg for women on estrogen).
Dr. Yuen is a member of the Versartis advisory board. Dr. Moore has received research support from the company.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
ORLANDO – An extended-release human growth hormone formulation proved safe and effective in both children and adults, offering the prospect of a less-rigorous dosing schedule and potentially better patient compliance with treatment.
The two phase II studies examined somavaratan, which is being developed by Versartis of Menlo Park, Calif. Kevin Yuen, MD, an endocrinologist at the Swedish Medical Center, Seattle, and Wayne V. Moore, MD, a pediatric endocrinologist at Children’s Mercy Hospital, Kansas City, Mo., presented the data at the annual meeting of the Endocrine Society.
“Despite the fact that human growth hormone is a proven treatment for growth hormone deficiency, daily use of our current formulations can be a factor that affects compliance,” said Dr. Yuen. He cited a 2008 study of 158 men taking growth hormone, which found that only one-third were highly compliant (Endocr Pract. 2008 Mar;14[2]:143-54). “And even among this group, there were 21 doses missed over just a 3-month period.”
The group of 55 international experts described several strategies for creating long-acting growth hormone formulations, including depot formulations, pegylation, prodrugs, noncovalent albumin binding growth hormone compounds, and growth hormone fusion proteins. These preparations are currently in various stages of development, with some already approved in Europe and Asia.
Somavaratan (VRS-317) is a fusion protein produced in Escherichia coli. The active portion is recombinant human growth hormone, which is bound to long chains of hydrophilic amino acids. This reduces renal filtration, Dr. Yuen said. The growth hormone loses some potency in this construct, but its delayed clearance, with a half-life 30-60 times longer than recombinant human growth hormone (rhGH) allows it to exert a prolonged effect in target tissue.
Of the two phase II studies of the molecule, one was conducted in 64 prepubertal children who were naive to any growth hormone treatment, and one in 36 adults with adult-onset growth hormone deficiency. The company also has made these presentations available online.
The pediatric study reported 3-year data on the cohort, which began treatment of children at a mean age of 7 years. At baseline, the children were about 2.6 standard deviations (SDs) below their expected height, and their mean IGF-1 levels, about 1.7 SDs below. They showed a mean maximum stimulated growth hormone level of 5.4 ng/mL. Although they were a mean of 7.8 years chronologically at the study’s outset, their mean bone age was 6.4 years.
The first 12 months of the study consisted of dose-ranging trials, with initial doses of 5 mg/kg each month, then 2.5 mg/kg twice a month, and then 1.15 mg/kg weekly. During the last 2 years of the study, all children were taking 3.5 mg/kg, once a month.
Within the first year, all children taking the 3.5-mg/kg dose had achieved normal IGF-1 levels, which were consistent with levels achieved in the ANSWER registry dataset of somatropin (rDNA origin) injection (Norditropin) recombinant human growth hormone (Clin Epidemiol. 2013;5:119-27).
Height velocity improved, as did height standard deviation. By year 3, patients were a mean of 1.25 SDs below expected height – a significant improvement over baseline. These findings were almost superimposable with those in the ANSWER registry. Bone age and chronological age came into alignment within the first year and that association was maintained throughout the study – again, in almost superimposable curves with the registry data.
Somavaratan exerted no untoward metabolic effects. There were no adverse changes in body mass index. At baseline, the mean hemoglobin A1c was 5.2%; this was unchanged at 3 years. No patient developed diabetes. The most commonly reported adverse event was injection site pain (48%). Injection site erythema was reported in 5% of patients, but no injection site nodules occurred.
Other adverse events were headache, extremity pain, arthralgia, and musculoskeletal pain. Although the numbers were small overall, reports did increase after all the children were switched to the 3.5-mg/kg dose. However, they occurred in 5% or less of the patient group. There were no withdrawals due to adverse events.
The second trial was a dose-ranging study conducted in 49 adults aged 23-70 years. They all had been diagnosed with adult-onset growth hormone deficiency, but had stable pituitary function. If they were on any growth hormone therapy, they underwent a 14-day washout period.
The subjects were divided and dosed by age and gender. All subjects received one injection per month for 5 months.
Cohort A comprised 21 men and women aged 35 years or older, who took 0.6 mg/kg per month. Cohort B comprised six men and women younger than 35 years, who took 0.8 mg/kg per month. Cohort C comprised eight women taking oral estrogen contraceptives. These women received 1 mg/kg per month.
The cohorts were similar in body mass index and weight, but they did differ significantly in baseline IGF-1 levels. In cohort A, the level was 0.52 SDs below normal. In cohort B, it was 2.89 SDs below normal, and in cohort C, it was 2.29 SDs below.
Overall, somavaratan induced a rapid and dramatic increase in IGF-1 that tailed off over 30 days. By day 8 after injection, IGF-1 had risen from a mean baseline of -1 SDs to more than 2 SDs above. By day 22, it had returned to baseline levels. The response to the fifth injection was identical to that of the first, Dr. Yuen said.
Response varied somewhat by cohort, with the younger, mixed-gender group responding the most dramatically, with a mean increase of about 4 SDs from baseline. This put the group above the maximum response target of 1.5 SDs.
The older, mixed-gender cohort experienced about a 3 SDs increase – also above the target level. The women taking estrogen experienced the flattest response, gaining about 2 SDs. However, the response curve was nearly identical, with a rapid, sharp increase in IGF-1 within the first week, followed by a gradual decline to baseline by 22 days.
The adverse event profile was not quite as benign as it was in the pediatric study. Virtually all patients experienced at least one adverse event. A third were mild and 58% moderate. The rest were serious, with one severe and one life-threatening event. Dr. Yuen did not discuss adverse events; these were, however, included in supplementary slides available on the Versartis Inc. slide set.
The finding of a predictable, 3-week tailing-off of efficacy, combined with the fact that patients responded so dramatically, exceeding the maximum target of a 1.5 SDs increase in IGF-1, has prompted a new dosing protocol for the somavaratan open-label extension study, which includes all the phase II completers, plus an additional 40 adult patients.
Doses will be titrated to each subject’s individual IGF-1 responses, based on the IGF-I level 7 days post dose until a maintenance dose is reached. Subjects receiving somavaratan in a previous somavaratan study will have their dose decreased by half (minimum dose of 20 mg, or 40 mg for women on estrogen).
Dr. Yuen is a member of the Versartis advisory board. Dr. Moore has received research support from the company.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
ORLANDO – An extended-release human growth hormone formulation proved safe and effective in both children and adults, offering the prospect of a less-rigorous dosing schedule and potentially better patient compliance with treatment.
The two phase II studies examined somavaratan, which is being developed by Versartis of Menlo Park, Calif. Kevin Yuen, MD, an endocrinologist at the Swedish Medical Center, Seattle, and Wayne V. Moore, MD, a pediatric endocrinologist at Children’s Mercy Hospital, Kansas City, Mo., presented the data at the annual meeting of the Endocrine Society.
“Despite the fact that human growth hormone is a proven treatment for growth hormone deficiency, daily use of our current formulations can be a factor that affects compliance,” said Dr. Yuen. He cited a 2008 study of 158 men taking growth hormone, which found that only one-third were highly compliant (Endocr Pract. 2008 Mar;14[2]:143-54). “And even among this group, there were 21 doses missed over just a 3-month period.”
The group of 55 international experts described several strategies for creating long-acting growth hormone formulations, including depot formulations, pegylation, prodrugs, noncovalent albumin binding growth hormone compounds, and growth hormone fusion proteins. These preparations are currently in various stages of development, with some already approved in Europe and Asia.
Somavaratan (VRS-317) is a fusion protein produced in Escherichia coli. The active portion is recombinant human growth hormone, which is bound to long chains of hydrophilic amino acids. This reduces renal filtration, Dr. Yuen said. The growth hormone loses some potency in this construct, but its delayed clearance, with a half-life 30-60 times longer than recombinant human growth hormone (rhGH) allows it to exert a prolonged effect in target tissue.
Of the two phase II studies of the molecule, one was conducted in 64 prepubertal children who were naive to any growth hormone treatment, and one in 36 adults with adult-onset growth hormone deficiency. The company also has made these presentations available online.
The pediatric study reported 3-year data on the cohort, which began treatment of children at a mean age of 7 years. At baseline, the children were about 2.6 standard deviations (SDs) below their expected height, and their mean IGF-1 levels, about 1.7 SDs below. They showed a mean maximum stimulated growth hormone level of 5.4 ng/mL. Although they were a mean of 7.8 years chronologically at the study’s outset, their mean bone age was 6.4 years.
The first 12 months of the study consisted of dose-ranging trials, with initial doses of 5 mg/kg each month, then 2.5 mg/kg twice a month, and then 1.15 mg/kg weekly. During the last 2 years of the study, all children were taking 3.5 mg/kg, once a month.
Within the first year, all children taking the 3.5-mg/kg dose had achieved normal IGF-1 levels, which were consistent with levels achieved in the ANSWER registry dataset of somatropin (rDNA origin) injection (Norditropin) recombinant human growth hormone (Clin Epidemiol. 2013;5:119-27).
Height velocity improved, as did height standard deviation. By year 3, patients were a mean of 1.25 SDs below expected height – a significant improvement over baseline. These findings were almost superimposable with those in the ANSWER registry. Bone age and chronological age came into alignment within the first year and that association was maintained throughout the study – again, in almost superimposable curves with the registry data.
Somavaratan exerted no untoward metabolic effects. There were no adverse changes in body mass index. At baseline, the mean hemoglobin A1c was 5.2%; this was unchanged at 3 years. No patient developed diabetes. The most commonly reported adverse event was injection site pain (48%). Injection site erythema was reported in 5% of patients, but no injection site nodules occurred.
Other adverse events were headache, extremity pain, arthralgia, and musculoskeletal pain. Although the numbers were small overall, reports did increase after all the children were switched to the 3.5-mg/kg dose. However, they occurred in 5% or less of the patient group. There were no withdrawals due to adverse events.
The second trial was a dose-ranging study conducted in 49 adults aged 23-70 years. They all had been diagnosed with adult-onset growth hormone deficiency, but had stable pituitary function. If they were on any growth hormone therapy, they underwent a 14-day washout period.
The subjects were divided and dosed by age and gender. All subjects received one injection per month for 5 months.
Cohort A comprised 21 men and women aged 35 years or older, who took 0.6 mg/kg per month. Cohort B comprised six men and women younger than 35 years, who took 0.8 mg/kg per month. Cohort C comprised eight women taking oral estrogen contraceptives. These women received 1 mg/kg per month.
The cohorts were similar in body mass index and weight, but they did differ significantly in baseline IGF-1 levels. In cohort A, the level was 0.52 SDs below normal. In cohort B, it was 2.89 SDs below normal, and in cohort C, it was 2.29 SDs below.
Overall, somavaratan induced a rapid and dramatic increase in IGF-1 that tailed off over 30 days. By day 8 after injection, IGF-1 had risen from a mean baseline of -1 SDs to more than 2 SDs above. By day 22, it had returned to baseline levels. The response to the fifth injection was identical to that of the first, Dr. Yuen said.
Response varied somewhat by cohort, with the younger, mixed-gender group responding the most dramatically, with a mean increase of about 4 SDs from baseline. This put the group above the maximum response target of 1.5 SDs.
The older, mixed-gender cohort experienced about a 3 SDs increase – also above the target level. The women taking estrogen experienced the flattest response, gaining about 2 SDs. However, the response curve was nearly identical, with a rapid, sharp increase in IGF-1 within the first week, followed by a gradual decline to baseline by 22 days.
The adverse event profile was not quite as benign as it was in the pediatric study. Virtually all patients experienced at least one adverse event. A third were mild and 58% moderate. The rest were serious, with one severe and one life-threatening event. Dr. Yuen did not discuss adverse events; these were, however, included in supplementary slides available on the Versartis Inc. slide set.
The finding of a predictable, 3-week tailing-off of efficacy, combined with the fact that patients responded so dramatically, exceeding the maximum target of a 1.5 SDs increase in IGF-1, has prompted a new dosing protocol for the somavaratan open-label extension study, which includes all the phase II completers, plus an additional 40 adult patients.
Doses will be titrated to each subject’s individual IGF-1 responses, based on the IGF-I level 7 days post dose until a maintenance dose is reached. Subjects receiving somavaratan in a previous somavaratan study will have their dose decreased by half (minimum dose of 20 mg, or 40 mg for women on estrogen).
Dr. Yuen is a member of the Versartis advisory board. Dr. Moore has received research support from the company.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
AT ENDO 2017
Key clinical point:
Major finding: In the 64-patient pediatric study, the molecule raised growth velocity and IGF-1 levels by several standard deviations. In the 48-patient adult study, it raised IGF-1 levels by up to 4 standard deviations.
Data source: A 64-patient pediatric dose-ranging study and a 48-patient adult study.
Disclosures: Versartis sponsored the studies. Dr. Yuen is on the company’s advisory board. Dr. Moore has received research support from the company.
Levothyroxine: No benefit for subclinical hypothyroidism in elderly
Levothyroxine provided no benefits in older patients with subclinical hypothyroidism in the first large randomized clinical trial of the treatment for this indication, which was presented at the annual meeting of the Endocrine Society.
An estimated 8%-18% of people aged 65 and older have subclinical hypothyroidism, which is defined as an elevated serum thyrotropin level and a serum free-thyroxine level within the reference range. The condition is considered to be a possible contributor to many problems affecting numerous physiologic systems including the vascular tree, the heart, the brain, skeletal muscle, and bone, said David J. Stott, MD, of the academic section of geriatric medicine, Institute of Cardiovascular and Medical Sciences, University of Glasgow.
The mean age of the study participants was 74 years, and they were followed for a median of 18 months after initiating treatment. The active intervention did boost thyroid function as expected, compared with placebo.
The two primary outcome measures were change between baseline and 1 year in hypothyroid symptoms and in tiredness scores on the 100-point ThyPRO (Thyroid-Related Quality of Life Patient-Reported Outcome) measure. There were no significant differences between the two study groups in changes in either of these scores. The mean 1-year score for hypothyroid symptoms was 16.6 for levothyroxine and 16.7 for placebo, and the mean 1-year score for tiredness was 28.7 for levothyroxine and 28.6 for placebo.
Secondary outcomes also did not differ significantly between the two study groups, including general health-related quality of life; hand-grip strength (reflecting possible effects on skeletal muscle); executive cognitive function (reflecting possible effects on the brain); blood pressure, weight, body mass index, and waist circumference (reflecting possible effects on cardiovascular health); or activities of daily living. In addition, both fatal and nonfatal cardiovascular events were similar between the two study groups at 1 year and at extended 3-year follow-up.
Further analyses did not identify any subgroup of adults who benefited from active treatment. The lack of benefit extended across all older age groups, both genders, and all serum thyrotropin levels at baseline. In addition, all the sensitivity analyses confirmed the results of the main analysis.
Adverse events also were not significantly different between the two study groups. This included four serious adverse events of special interest: new-onset atrial fibrillation, heart failure, fracture, or osteoporosis. There also was no increase in symptoms of hyperthyroidism in the active-treatment group.
Levothyroxine provided no benefits in older patients with subclinical hypothyroidism in the first large randomized clinical trial of the treatment for this indication, which was presented at the annual meeting of the Endocrine Society.
An estimated 8%-18% of people aged 65 and older have subclinical hypothyroidism, which is defined as an elevated serum thyrotropin level and a serum free-thyroxine level within the reference range. The condition is considered to be a possible contributor to many problems affecting numerous physiologic systems including the vascular tree, the heart, the brain, skeletal muscle, and bone, said David J. Stott, MD, of the academic section of geriatric medicine, Institute of Cardiovascular and Medical Sciences, University of Glasgow.
The mean age of the study participants was 74 years, and they were followed for a median of 18 months after initiating treatment. The active intervention did boost thyroid function as expected, compared with placebo.
The two primary outcome measures were change between baseline and 1 year in hypothyroid symptoms and in tiredness scores on the 100-point ThyPRO (Thyroid-Related Quality of Life Patient-Reported Outcome) measure. There were no significant differences between the two study groups in changes in either of these scores. The mean 1-year score for hypothyroid symptoms was 16.6 for levothyroxine and 16.7 for placebo, and the mean 1-year score for tiredness was 28.7 for levothyroxine and 28.6 for placebo.
Secondary outcomes also did not differ significantly between the two study groups, including general health-related quality of life; hand-grip strength (reflecting possible effects on skeletal muscle); executive cognitive function (reflecting possible effects on the brain); blood pressure, weight, body mass index, and waist circumference (reflecting possible effects on cardiovascular health); or activities of daily living. In addition, both fatal and nonfatal cardiovascular events were similar between the two study groups at 1 year and at extended 3-year follow-up.
Further analyses did not identify any subgroup of adults who benefited from active treatment. The lack of benefit extended across all older age groups, both genders, and all serum thyrotropin levels at baseline. In addition, all the sensitivity analyses confirmed the results of the main analysis.
Adverse events also were not significantly different between the two study groups. This included four serious adverse events of special interest: new-onset atrial fibrillation, heart failure, fracture, or osteoporosis. There also was no increase in symptoms of hyperthyroidism in the active-treatment group.
Levothyroxine provided no benefits in older patients with subclinical hypothyroidism in the first large randomized clinical trial of the treatment for this indication, which was presented at the annual meeting of the Endocrine Society.
An estimated 8%-18% of people aged 65 and older have subclinical hypothyroidism, which is defined as an elevated serum thyrotropin level and a serum free-thyroxine level within the reference range. The condition is considered to be a possible contributor to many problems affecting numerous physiologic systems including the vascular tree, the heart, the brain, skeletal muscle, and bone, said David J. Stott, MD, of the academic section of geriatric medicine, Institute of Cardiovascular and Medical Sciences, University of Glasgow.
The mean age of the study participants was 74 years, and they were followed for a median of 18 months after initiating treatment. The active intervention did boost thyroid function as expected, compared with placebo.
The two primary outcome measures were change between baseline and 1 year in hypothyroid symptoms and in tiredness scores on the 100-point ThyPRO (Thyroid-Related Quality of Life Patient-Reported Outcome) measure. There were no significant differences between the two study groups in changes in either of these scores. The mean 1-year score for hypothyroid symptoms was 16.6 for levothyroxine and 16.7 for placebo, and the mean 1-year score for tiredness was 28.7 for levothyroxine and 28.6 for placebo.
Secondary outcomes also did not differ significantly between the two study groups, including general health-related quality of life; hand-grip strength (reflecting possible effects on skeletal muscle); executive cognitive function (reflecting possible effects on the brain); blood pressure, weight, body mass index, and waist circumference (reflecting possible effects on cardiovascular health); or activities of daily living. In addition, both fatal and nonfatal cardiovascular events were similar between the two study groups at 1 year and at extended 3-year follow-up.
Further analyses did not identify any subgroup of adults who benefited from active treatment. The lack of benefit extended across all older age groups, both genders, and all serum thyrotropin levels at baseline. In addition, all the sensitivity analyses confirmed the results of the main analysis.
Adverse events also were not significantly different between the two study groups. This included four serious adverse events of special interest: new-onset atrial fibrillation, heart failure, fracture, or osteoporosis. There also was no increase in symptoms of hyperthyroidism in the active-treatment group.
FROM ENDO 2017
Key clinical point: Levothyroxine provided no benefits in older patients with subclinical hypothyroidism in the first large randomized clinical trial of the therapy.
Major finding: The mean score for hypothyroid symptoms was 16.6 for levothyroxine and 16.7 for placebo after 1 year of treatment, and the mean score for tiredness was 28.7 for levothyroxine and 28.6 for placebo.
Data source: An international randomized double-blind placebo-controlled clinical trial involving 737 older patients with subclinical hypothyroidism.
Disclosures: This study was supported by the European Union and several foundations. The levothyroxine and matching placebo were provided free of charge by Merck, which played no role in the design, analysis, or reporting of the study. Dr. Stott reported having no relevant financial disclosures; one of his associates reported ties to Bristol/Myers Squibb, Pfizer, and Bayer.
Temozolomide may help half of patients with aggressive pituitary tumors
ORLANDO – Temozolomide, an alkylating agent approved for glioblastoma, improved long-term survival in about half of patients who took it for aggressive pituitary tumors, a retrospective study has determined.
The study, conducted by members of the French Society of Endocrinology, comprised 43 patients. Of the 51% who responded to the treatment, the median overall survival time was 44 months, compared to just 16 months for patients who didn’t respond, Gérald Raverot, MD, said at the annual meeting of the Endocrine Society.
“Despite the very good response we saw in some patients, we also saw a high risk of recurrence, with a median of about 30 months,” for relapse, he noted. “And a second course of temozolomide always failed.”
When used for aggressive pituitary tumors, temozolomide is usually given in a conventional scheme of up to 12 cycles. It’s typically reserved for tumors that have responded poorly to other treatment regimens, Dr. Raverot said.
The drug has not been widely studied in patients with aggressive pituitary tumors, although there have been a number of case reports suggesting that can be beneficial. Data on about 90 patients have been published. The largest series to date appeared in 2015 and comprised 24 patients. It found about a 50% response rate to the drug. Two patients had a complete regression and seven patients had a partial regression of tumor mass. Tumor mass shrunk to less than 30% in three patients, less than 50% in three, and less than 75% in one.
Because of both the promise temozolomide shows in these very tough cases, and the paucity of descriptive and clinical data, Dr. Raverot and his colleagues conducted a multi-center study that spanned 21 facilities in France and comprised 43 patients who were treated from 2006-2016. The intent was to evaluate efficacy at the end of treatment, or at last follow-up in the case of those who were still being treated. Tumor response was defined as a decrease of more than 30% in the largest tumor diameter; hormonal response was more than a 50% decrease in baseline hormone levels. The endpoint was overall survival and relapse-free survival.
Of the 43 patients, 29 were men. The group’s mean age at diagnosis was 43 years, and the mean age at temozolomide treatment, 53 years. Fourteen of the tumors were carcinomas and 12 were silent or initially silent.
About half of the tumors (23) were adrenocorticotropic hormone-producing. Other tumor types were prolactin-secreting (13) and growth hormone-secreting (3); an additional three tumors secreted both prolactin and growth hormone.
Most patients (36) underwent a typical temozolomide protocol. This consisted of at least one 5-day cycle of 150 mg/m2/day every 28 days, followed by 250 mg/m2/day thereafter. The median number of cycles was 6.5, but this ranged from 1-24 cycles.
Six patients were treated according to the Stupp protocol for temozolomide in glioblastoma. This consists of daily temozolomide 75 mg/m2 with concomitant radiotherapy for 6 weeks, followed by a standard temozolomide protocol. Four patients underwent 6 cycles; one patient 12 cycles, and one patient, 17 cycles.
An additional four patients had concomitant radiotherapy within 4 months of their temozolomide treatment.
The overall response rate was 51% (22 patients). Dr. Raverot attempted to identify clinical characteristics predictive of response. There was no association with gender, age at diagnosis or age at temozolomide treatment, tumor type, whether or not the tumor was a carcinoma, or what type of hormone it secreted. Nor was there a response associated with hypermethylation of the O6-methylguanine-DNA-methyltransferase (MGMT) gene.
Dr. Raverot found only one positive association with response. Tumors that were silent or initially silent (12) were much less likely to respond than secreting tumors. Of the 21 nonresponsive tumors, 10 were silent (45%). Of the 22 responsive tumors, only 2 were silent (9%).
Dr. Raverot also analyzed response by protocol and found intriguing results. Of the 10 patients who had concomitant radiotherapy, seven responded and three did not. Patients who underwent the Stupp protocol also tended to do better, he said. “Of the six who had this, five responded, so this is interesting.”
However, he cautioned, both of these positive associations are based on such small numbers that it’s impossible to draw firm conclusions.
Dr. Raverot had survival data on 38 patients with a median follow-up of 16 months after the end of treatment. Of these, 20 were responders and 18 were non-responders. Death had occurred in 13 of the nonresponders and five responders.
Of the 20 responders, 10 were still controlled at the time of last follow-up, and 10 had relapsed at a median of 5 months after treatment cessation. Five of these patients had a second course of temozolomide, but none of them responded to it, Dr. Raverot said. Three of these patients have died and two are still living.
“We looked at other salvage treatments for them, but none of these therapies could control the disease. Unfortunately, we just don’t have good treatment options for these patients. And even among those with good treatment response, there is a risk of early recurrence, with a median time of 30 months to relapse. The second course of temozolomide always fails. So we have now some questions about who we should maintain on treatment. We don’t have this answered yet, and we need to.”
Dr. Raverot had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
ORLANDO – Temozolomide, an alkylating agent approved for glioblastoma, improved long-term survival in about half of patients who took it for aggressive pituitary tumors, a retrospective study has determined.
The study, conducted by members of the French Society of Endocrinology, comprised 43 patients. Of the 51% who responded to the treatment, the median overall survival time was 44 months, compared to just 16 months for patients who didn’t respond, Gérald Raverot, MD, said at the annual meeting of the Endocrine Society.
“Despite the very good response we saw in some patients, we also saw a high risk of recurrence, with a median of about 30 months,” for relapse, he noted. “And a second course of temozolomide always failed.”
When used for aggressive pituitary tumors, temozolomide is usually given in a conventional scheme of up to 12 cycles. It’s typically reserved for tumors that have responded poorly to other treatment regimens, Dr. Raverot said.
The drug has not been widely studied in patients with aggressive pituitary tumors, although there have been a number of case reports suggesting that can be beneficial. Data on about 90 patients have been published. The largest series to date appeared in 2015 and comprised 24 patients. It found about a 50% response rate to the drug. Two patients had a complete regression and seven patients had a partial regression of tumor mass. Tumor mass shrunk to less than 30% in three patients, less than 50% in three, and less than 75% in one.
Because of both the promise temozolomide shows in these very tough cases, and the paucity of descriptive and clinical data, Dr. Raverot and his colleagues conducted a multi-center study that spanned 21 facilities in France and comprised 43 patients who were treated from 2006-2016. The intent was to evaluate efficacy at the end of treatment, or at last follow-up in the case of those who were still being treated. Tumor response was defined as a decrease of more than 30% in the largest tumor diameter; hormonal response was more than a 50% decrease in baseline hormone levels. The endpoint was overall survival and relapse-free survival.
Of the 43 patients, 29 were men. The group’s mean age at diagnosis was 43 years, and the mean age at temozolomide treatment, 53 years. Fourteen of the tumors were carcinomas and 12 were silent or initially silent.
About half of the tumors (23) were adrenocorticotropic hormone-producing. Other tumor types were prolactin-secreting (13) and growth hormone-secreting (3); an additional three tumors secreted both prolactin and growth hormone.
Most patients (36) underwent a typical temozolomide protocol. This consisted of at least one 5-day cycle of 150 mg/m2/day every 28 days, followed by 250 mg/m2/day thereafter. The median number of cycles was 6.5, but this ranged from 1-24 cycles.
Six patients were treated according to the Stupp protocol for temozolomide in glioblastoma. This consists of daily temozolomide 75 mg/m2 with concomitant radiotherapy for 6 weeks, followed by a standard temozolomide protocol. Four patients underwent 6 cycles; one patient 12 cycles, and one patient, 17 cycles.
An additional four patients had concomitant radiotherapy within 4 months of their temozolomide treatment.
The overall response rate was 51% (22 patients). Dr. Raverot attempted to identify clinical characteristics predictive of response. There was no association with gender, age at diagnosis or age at temozolomide treatment, tumor type, whether or not the tumor was a carcinoma, or what type of hormone it secreted. Nor was there a response associated with hypermethylation of the O6-methylguanine-DNA-methyltransferase (MGMT) gene.
Dr. Raverot found only one positive association with response. Tumors that were silent or initially silent (12) were much less likely to respond than secreting tumors. Of the 21 nonresponsive tumors, 10 were silent (45%). Of the 22 responsive tumors, only 2 were silent (9%).
Dr. Raverot also analyzed response by protocol and found intriguing results. Of the 10 patients who had concomitant radiotherapy, seven responded and three did not. Patients who underwent the Stupp protocol also tended to do better, he said. “Of the six who had this, five responded, so this is interesting.”
However, he cautioned, both of these positive associations are based on such small numbers that it’s impossible to draw firm conclusions.
Dr. Raverot had survival data on 38 patients with a median follow-up of 16 months after the end of treatment. Of these, 20 were responders and 18 were non-responders. Death had occurred in 13 of the nonresponders and five responders.
Of the 20 responders, 10 were still controlled at the time of last follow-up, and 10 had relapsed at a median of 5 months after treatment cessation. Five of these patients had a second course of temozolomide, but none of them responded to it, Dr. Raverot said. Three of these patients have died and two are still living.
“We looked at other salvage treatments for them, but none of these therapies could control the disease. Unfortunately, we just don’t have good treatment options for these patients. And even among those with good treatment response, there is a risk of early recurrence, with a median time of 30 months to relapse. The second course of temozolomide always fails. So we have now some questions about who we should maintain on treatment. We don’t have this answered yet, and we need to.”
Dr. Raverot had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
ORLANDO – Temozolomide, an alkylating agent approved for glioblastoma, improved long-term survival in about half of patients who took it for aggressive pituitary tumors, a retrospective study has determined.
The study, conducted by members of the French Society of Endocrinology, comprised 43 patients. Of the 51% who responded to the treatment, the median overall survival time was 44 months, compared to just 16 months for patients who didn’t respond, Gérald Raverot, MD, said at the annual meeting of the Endocrine Society.
“Despite the very good response we saw in some patients, we also saw a high risk of recurrence, with a median of about 30 months,” for relapse, he noted. “And a second course of temozolomide always failed.”
When used for aggressive pituitary tumors, temozolomide is usually given in a conventional scheme of up to 12 cycles. It’s typically reserved for tumors that have responded poorly to other treatment regimens, Dr. Raverot said.
The drug has not been widely studied in patients with aggressive pituitary tumors, although there have been a number of case reports suggesting that can be beneficial. Data on about 90 patients have been published. The largest series to date appeared in 2015 and comprised 24 patients. It found about a 50% response rate to the drug. Two patients had a complete regression and seven patients had a partial regression of tumor mass. Tumor mass shrunk to less than 30% in three patients, less than 50% in three, and less than 75% in one.
Because of both the promise temozolomide shows in these very tough cases, and the paucity of descriptive and clinical data, Dr. Raverot and his colleagues conducted a multi-center study that spanned 21 facilities in France and comprised 43 patients who were treated from 2006-2016. The intent was to evaluate efficacy at the end of treatment, or at last follow-up in the case of those who were still being treated. Tumor response was defined as a decrease of more than 30% in the largest tumor diameter; hormonal response was more than a 50% decrease in baseline hormone levels. The endpoint was overall survival and relapse-free survival.
Of the 43 patients, 29 were men. The group’s mean age at diagnosis was 43 years, and the mean age at temozolomide treatment, 53 years. Fourteen of the tumors were carcinomas and 12 were silent or initially silent.
About half of the tumors (23) were adrenocorticotropic hormone-producing. Other tumor types were prolactin-secreting (13) and growth hormone-secreting (3); an additional three tumors secreted both prolactin and growth hormone.
Most patients (36) underwent a typical temozolomide protocol. This consisted of at least one 5-day cycle of 150 mg/m2/day every 28 days, followed by 250 mg/m2/day thereafter. The median number of cycles was 6.5, but this ranged from 1-24 cycles.
Six patients were treated according to the Stupp protocol for temozolomide in glioblastoma. This consists of daily temozolomide 75 mg/m2 with concomitant radiotherapy for 6 weeks, followed by a standard temozolomide protocol. Four patients underwent 6 cycles; one patient 12 cycles, and one patient, 17 cycles.
An additional four patients had concomitant radiotherapy within 4 months of their temozolomide treatment.
The overall response rate was 51% (22 patients). Dr. Raverot attempted to identify clinical characteristics predictive of response. There was no association with gender, age at diagnosis or age at temozolomide treatment, tumor type, whether or not the tumor was a carcinoma, or what type of hormone it secreted. Nor was there a response associated with hypermethylation of the O6-methylguanine-DNA-methyltransferase (MGMT) gene.
Dr. Raverot found only one positive association with response. Tumors that were silent or initially silent (12) were much less likely to respond than secreting tumors. Of the 21 nonresponsive tumors, 10 were silent (45%). Of the 22 responsive tumors, only 2 were silent (9%).
Dr. Raverot also analyzed response by protocol and found intriguing results. Of the 10 patients who had concomitant radiotherapy, seven responded and three did not. Patients who underwent the Stupp protocol also tended to do better, he said. “Of the six who had this, five responded, so this is interesting.”
However, he cautioned, both of these positive associations are based on such small numbers that it’s impossible to draw firm conclusions.
Dr. Raverot had survival data on 38 patients with a median follow-up of 16 months after the end of treatment. Of these, 20 were responders and 18 were non-responders. Death had occurred in 13 of the nonresponders and five responders.
Of the 20 responders, 10 were still controlled at the time of last follow-up, and 10 had relapsed at a median of 5 months after treatment cessation. Five of these patients had a second course of temozolomide, but none of them responded to it, Dr. Raverot said. Three of these patients have died and two are still living.
“We looked at other salvage treatments for them, but none of these therapies could control the disease. Unfortunately, we just don’t have good treatment options for these patients. And even among those with good treatment response, there is a risk of early recurrence, with a median time of 30 months to relapse. The second course of temozolomide always fails. So we have now some questions about who we should maintain on treatment. We don’t have this answered yet, and we need to.”
Dr. Raverot had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
AT ENDO 2017
Key clinical point:
Major finding: Of the 51% who responded to the treatment, the median overall survival time was 44 months, compared to just 16 months for patients who didn’t respond.
Data source: The retrospective study comprised 43 patients treated in France.
Disclosures: Dr. Raverot had no financial disclosures.
Thyroid cancer incidence: It’s not all good news
ORLANDO – The incidence of thyroid cancer in the United States between 2000-2013 has dropped in whites while increasing in blacks and Hispanics, Anupam Kotwal, MBBS, said during a press briefing at the annual meeting of the Endocrine Society.
Other recently reported data have shown a steady gradual incidence in thyroid cancer between 1974-2013 (JAMA. 2017 Mar 31. doi:10.1001/jama.2017.2719).
From 2000 to 2013, the incidence of thyroid cancer as a whole increased from 7.4 to 14.5 cases per 100,000 population with an annual percent increase of 6.7% from 2000-2009 (P less than .05) and 2.4% from 2010 to 2013 (P less than .05). In Hispanics and African-Americans, thyroid cancer incidence has continuously increased, with an annual percent increase of 4.7% (P less than .05) and 5.1% (P less than .05) respectively, whereas for non-Hispanic whites, the annual percent increase decelerated from 7.1% (P less than .05) before 2009 to 2.2% after 2009.
Looking at changes to incidence by age, non-Hispanic white women over the age of 75 are the only ones to see a decrease, from 6.5 cases per 100,000 in 2010 to 2.4 cases per 100,000 population in 2014. The investigations reported the same acceleration of incidence among everyone under the age of 20 years.
These findings are consistent with recent reports demonstrating that thyroid cancer is the 2nd most common cancer among Hispanic females, female adolescents and young adults.
Dr. Kotwal reported that he had no relevant conflicts of interest.
ORLANDO – The incidence of thyroid cancer in the United States between 2000-2013 has dropped in whites while increasing in blacks and Hispanics, Anupam Kotwal, MBBS, said during a press briefing at the annual meeting of the Endocrine Society.
Other recently reported data have shown a steady gradual incidence in thyroid cancer between 1974-2013 (JAMA. 2017 Mar 31. doi:10.1001/jama.2017.2719).
From 2000 to 2013, the incidence of thyroid cancer as a whole increased from 7.4 to 14.5 cases per 100,000 population with an annual percent increase of 6.7% from 2000-2009 (P less than .05) and 2.4% from 2010 to 2013 (P less than .05). In Hispanics and African-Americans, thyroid cancer incidence has continuously increased, with an annual percent increase of 4.7% (P less than .05) and 5.1% (P less than .05) respectively, whereas for non-Hispanic whites, the annual percent increase decelerated from 7.1% (P less than .05) before 2009 to 2.2% after 2009.
Looking at changes to incidence by age, non-Hispanic white women over the age of 75 are the only ones to see a decrease, from 6.5 cases per 100,000 in 2010 to 2.4 cases per 100,000 population in 2014. The investigations reported the same acceleration of incidence among everyone under the age of 20 years.
These findings are consistent with recent reports demonstrating that thyroid cancer is the 2nd most common cancer among Hispanic females, female adolescents and young adults.
Dr. Kotwal reported that he had no relevant conflicts of interest.
ORLANDO – The incidence of thyroid cancer in the United States between 2000-2013 has dropped in whites while increasing in blacks and Hispanics, Anupam Kotwal, MBBS, said during a press briefing at the annual meeting of the Endocrine Society.
Other recently reported data have shown a steady gradual incidence in thyroid cancer between 1974-2013 (JAMA. 2017 Mar 31. doi:10.1001/jama.2017.2719).
From 2000 to 2013, the incidence of thyroid cancer as a whole increased from 7.4 to 14.5 cases per 100,000 population with an annual percent increase of 6.7% from 2000-2009 (P less than .05) and 2.4% from 2010 to 2013 (P less than .05). In Hispanics and African-Americans, thyroid cancer incidence has continuously increased, with an annual percent increase of 4.7% (P less than .05) and 5.1% (P less than .05) respectively, whereas for non-Hispanic whites, the annual percent increase decelerated from 7.1% (P less than .05) before 2009 to 2.2% after 2009.
Looking at changes to incidence by age, non-Hispanic white women over the age of 75 are the only ones to see a decrease, from 6.5 cases per 100,000 in 2010 to 2.4 cases per 100,000 population in 2014. The investigations reported the same acceleration of incidence among everyone under the age of 20 years.
These findings are consistent with recent reports demonstrating that thyroid cancer is the 2nd most common cancer among Hispanic females, female adolescents and young adults.
Dr. Kotwal reported that he had no relevant conflicts of interest.
AT ENDO 2017
Key clinical point:
Major finding: The incidence of thyroid cancer has dropped from 7 cases per 100,000 in 2000 to 2.2 cases per 100,000 in 2013 among whites. Among blacks it has increased from 5 cases to 7 cases per 100,000 over that time frame and in Hispanics from 7 cases to 12 cases per 100,000.
Data source: Data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results data base.
Disclosures: The study received no external funding. Dr. Kotwal reported he had no relevant financial conflicts of interest.
Rotterdam Study: High T4 levels increased the risk for atherosclerotic events, death
ORLANDO – Elevated serum levels of free T4 are associated with a greater risk of atherosclerotic events and, in some cases, death, among adults in their 60s, Arjola Bano, MD, said at a press briefing at the annual meeting of the Endocrine Society.
Earlier research has shown that high FT4 levels are associated with a greater likelihood of development of atherosclerosis. But no one has looked beyond that to see if an excess of FT4 can change the course of the disease.
The investigators used electron beam computed tomography to measure coronary artery calcification. Confounders such as age, sex, smoking, alcohol intake, body mass index, total cholesterol, triglycerides, systolic blood pressure, diabetes, and use of anti-hypertension or lipid lowering medications were controlled for using multivariable-adjusted Cox proportional and logistic regression models.
Dr. Bano reported that during a median follow-up of 8.8 years (range, 4.5-11.8 years), there were 580 ASCV deaths and 1,130 first-time hard ASCV events. The risk of ASCV mortality increased with higher FT4 levels (hazard ratio, 2.35; 95% confidence interval, 1.61-3.41 per 1 ng/dL) and lower TSH levels (HR, 0.92; 95% CI, 0.84-1.00/1 logTSH), predominantly among participants with prevalent ASCV disease (HR, 5.76; 95% CI, 2.79-11.89 for FT4; HR, 0.81; 95% CI, 0.69-0.95 for TSH).
In addition, higher FT4 levels were associated with higher risk of first-time hard ASCV event (HR, 1.87; 95% CI, 1.34-2.59). Also, FT4 levels were positively associated with having a high CAC score (OR, 2.34; 95% CI, 1.36-4.04).
It is noteworthy that results remained similar after restricting the analyses to participants with thyroid function within reference ranges, she stressed.
How to explain these findings? These data suggest that the link between thyroid function and atherosclerosis is mediated through yet unexplored cardiovascular risk factors or via alternative pathways.
Dr. Bano and her associates all report that they have no relevant financial conflicts of interest.
ORLANDO – Elevated serum levels of free T4 are associated with a greater risk of atherosclerotic events and, in some cases, death, among adults in their 60s, Arjola Bano, MD, said at a press briefing at the annual meeting of the Endocrine Society.
Earlier research has shown that high FT4 levels are associated with a greater likelihood of development of atherosclerosis. But no one has looked beyond that to see if an excess of FT4 can change the course of the disease.
The investigators used electron beam computed tomography to measure coronary artery calcification. Confounders such as age, sex, smoking, alcohol intake, body mass index, total cholesterol, triglycerides, systolic blood pressure, diabetes, and use of anti-hypertension or lipid lowering medications were controlled for using multivariable-adjusted Cox proportional and logistic regression models.
Dr. Bano reported that during a median follow-up of 8.8 years (range, 4.5-11.8 years), there were 580 ASCV deaths and 1,130 first-time hard ASCV events. The risk of ASCV mortality increased with higher FT4 levels (hazard ratio, 2.35; 95% confidence interval, 1.61-3.41 per 1 ng/dL) and lower TSH levels (HR, 0.92; 95% CI, 0.84-1.00/1 logTSH), predominantly among participants with prevalent ASCV disease (HR, 5.76; 95% CI, 2.79-11.89 for FT4; HR, 0.81; 95% CI, 0.69-0.95 for TSH).
In addition, higher FT4 levels were associated with higher risk of first-time hard ASCV event (HR, 1.87; 95% CI, 1.34-2.59). Also, FT4 levels were positively associated with having a high CAC score (OR, 2.34; 95% CI, 1.36-4.04).
It is noteworthy that results remained similar after restricting the analyses to participants with thyroid function within reference ranges, she stressed.
How to explain these findings? These data suggest that the link between thyroid function and atherosclerosis is mediated through yet unexplored cardiovascular risk factors or via alternative pathways.
Dr. Bano and her associates all report that they have no relevant financial conflicts of interest.
ORLANDO – Elevated serum levels of free T4 are associated with a greater risk of atherosclerotic events and, in some cases, death, among adults in their 60s, Arjola Bano, MD, said at a press briefing at the annual meeting of the Endocrine Society.
Earlier research has shown that high FT4 levels are associated with a greater likelihood of development of atherosclerosis. But no one has looked beyond that to see if an excess of FT4 can change the course of the disease.
The investigators used electron beam computed tomography to measure coronary artery calcification. Confounders such as age, sex, smoking, alcohol intake, body mass index, total cholesterol, triglycerides, systolic blood pressure, diabetes, and use of anti-hypertension or lipid lowering medications were controlled for using multivariable-adjusted Cox proportional and logistic regression models.
Dr. Bano reported that during a median follow-up of 8.8 years (range, 4.5-11.8 years), there were 580 ASCV deaths and 1,130 first-time hard ASCV events. The risk of ASCV mortality increased with higher FT4 levels (hazard ratio, 2.35; 95% confidence interval, 1.61-3.41 per 1 ng/dL) and lower TSH levels (HR, 0.92; 95% CI, 0.84-1.00/1 logTSH), predominantly among participants with prevalent ASCV disease (HR, 5.76; 95% CI, 2.79-11.89 for FT4; HR, 0.81; 95% CI, 0.69-0.95 for TSH).
In addition, higher FT4 levels were associated with higher risk of first-time hard ASCV event (HR, 1.87; 95% CI, 1.34-2.59). Also, FT4 levels were positively associated with having a high CAC score (OR, 2.34; 95% CI, 1.36-4.04).
It is noteworthy that results remained similar after restricting the analyses to participants with thyroid function within reference ranges, she stressed.
How to explain these findings? These data suggest that the link between thyroid function and atherosclerosis is mediated through yet unexplored cardiovascular risk factors or via alternative pathways.
Dr. Bano and her associates all report that they have no relevant financial conflicts of interest.
AT ENDO 2017
Key clinical point: Major finding: The odds of an atherosclerotic event were 2.28 times higher in adults with elevated FT4 levels than those with high TSH, even when the high levels were still within the normal range.
Data source: Prospective study of 9,231 adults followed over 8.8 years.
Disclosures: Dr. Bano and her associates all report that they have no relevant financial conflicts of interest.















