User login
Use of levoketoconazole improved several clinical features of Cushing’s disease
LOS ANGELES – including acne, hirsutism, and peripheral edema, as did patient-reported quality of life and symptoms of depression.
The findings come from an analysis of secondary endpoints among patients enrolled in SONICS, an open-label, phase 3 study of levoketoconazole as a treatment for endogenous Cushing’s disease (CD) that enrolled 94 patients at centers in North America, Europe and the Middle East. An investigational cortisol synthesis inhibitor, levoketoconazole is being developed by Strongbridge Biopharma and is not yet approved by the Food and Drug Administration.
“Despite the availability of approved treatments, the medical needs in Cushing’s [disease] remain very high,” the study’s principal investigator, Maria Fleseriu, MD, FACE, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists, where the data were presented. “This study demonstrates that levoketoconazole has the potential to address several clinical features of Cushing’s, owing to its clinically translated novel mechanism of action to suppress both cortisol and androgen syntheses (the latter elevated in many women with CD). Interestingly, there was no evidence of clinically important free-T reduction in men, and more studies are needed to elucidate this mechanism.”
In SONICS, adults with confirmed CD and mean 24-hour urinary free-cortisol (mUFC) value at least 1.5 times the upper limit of normal were treated with levoketoconazole in three phases: 2- to 21-week dose-titration phase (150-600 mg BID, as needed, to target mUFC normalization); 6-month maintenance phase (primary endpoint); and 6-month extended evaluation phase. The end of maintenance phase findings that focused on reductions in mUFC and safety had been previously reported. The current analysis focused on secondary endpoints, including changes from baseline to end of maintenance in investigator-assessed CD clinical signs and symptoms (acne score [range: 0-44]; hirsutism score [women only; range: 0-36]; and peripheral edema score [range: 0-12]), and patient-reported outcomes of quality of life (Cushing QoL questionnaire score [range: 0-100]) and depression (Beck Depression Inventory II score [range: 0-63]). The researchers also assessed hormones including free testosterone levels, and they used paired t-tests to infer statistical significance of the mean changes from baseline to end of maintenance for all measures.
Of the 94 patients enrolled in SONICS, 77 entered the maintenance phase, said Dr. Fleseriu, professor of medicine and neurological surgery and director of the pituitary center at Oregon Health and Science University, Portland. The patients’ mean age was 44 years and mean baseline mUFC was 243.3 mcg/day; 82% of patients were female, and 96% were white. Between baseline and the end of maintenance, the researchers observed significant mean improvements in acne scores (from 2.8 to –1.8, respectively; P = .0063), hirsutism scores (women only, from 7.8 to –2.6; P = .0008), and peripheral edema scores (from 1.0 to –0.4; P = .0295). They also observed significant mean improvements in quality of life and depression scores between baseline and end of maintenance (P less than .0001 and P = .0043, respectively). Mean free-testosterone levels increased nonsignificantly between baseline and end of maintenance in men (from 5.1 to 5.8 ng/dL) yet decreased significantly in women (from 0.3 to 0.1 ng/dL; P less than 0.0001; reference). Overall, 33 patients (35%) discontinued taking levoketoconazole by the end of the maintenance phase. Twelve (13%) discontinued because of adverse events.
“I wasn’t necessarily surprised with any of the data in this poster as I had experience with the drug in clinical trials, but I was definitely pleased to see overall significant improvements in acne score, hirsutism score in women, and peripheral edema score,” Dr. Fleseriu said. “Those are benefits that could potentially increase long-term adherence to treatment and quality-of-life improvements, particularly in women. It’s also exciting to see that quality of life and depression improved in these patients as well.”
These types of patient-reported outcomes are so important to how our patients feel about their disease and should be more of a focus for us physicians; it’s important to look at efficacy, safety and patient reported outcomes when we decide for an individualized treatment for each patient,” she noted.
Dr. Fleseriu reported that she has received research funding for Oregon Health and Science University from Novartis, Millendo, and Strongbridge. She has also received scientific consulting fees from Novartis and Strongbridge.
LOS ANGELES – including acne, hirsutism, and peripheral edema, as did patient-reported quality of life and symptoms of depression.
The findings come from an analysis of secondary endpoints among patients enrolled in SONICS, an open-label, phase 3 study of levoketoconazole as a treatment for endogenous Cushing’s disease (CD) that enrolled 94 patients at centers in North America, Europe and the Middle East. An investigational cortisol synthesis inhibitor, levoketoconazole is being developed by Strongbridge Biopharma and is not yet approved by the Food and Drug Administration.
“Despite the availability of approved treatments, the medical needs in Cushing’s [disease] remain very high,” the study’s principal investigator, Maria Fleseriu, MD, FACE, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists, where the data were presented. “This study demonstrates that levoketoconazole has the potential to address several clinical features of Cushing’s, owing to its clinically translated novel mechanism of action to suppress both cortisol and androgen syntheses (the latter elevated in many women with CD). Interestingly, there was no evidence of clinically important free-T reduction in men, and more studies are needed to elucidate this mechanism.”
In SONICS, adults with confirmed CD and mean 24-hour urinary free-cortisol (mUFC) value at least 1.5 times the upper limit of normal were treated with levoketoconazole in three phases: 2- to 21-week dose-titration phase (150-600 mg BID, as needed, to target mUFC normalization); 6-month maintenance phase (primary endpoint); and 6-month extended evaluation phase. The end of maintenance phase findings that focused on reductions in mUFC and safety had been previously reported. The current analysis focused on secondary endpoints, including changes from baseline to end of maintenance in investigator-assessed CD clinical signs and symptoms (acne score [range: 0-44]; hirsutism score [women only; range: 0-36]; and peripheral edema score [range: 0-12]), and patient-reported outcomes of quality of life (Cushing QoL questionnaire score [range: 0-100]) and depression (Beck Depression Inventory II score [range: 0-63]). The researchers also assessed hormones including free testosterone levels, and they used paired t-tests to infer statistical significance of the mean changes from baseline to end of maintenance for all measures.
Of the 94 patients enrolled in SONICS, 77 entered the maintenance phase, said Dr. Fleseriu, professor of medicine and neurological surgery and director of the pituitary center at Oregon Health and Science University, Portland. The patients’ mean age was 44 years and mean baseline mUFC was 243.3 mcg/day; 82% of patients were female, and 96% were white. Between baseline and the end of maintenance, the researchers observed significant mean improvements in acne scores (from 2.8 to –1.8, respectively; P = .0063), hirsutism scores (women only, from 7.8 to –2.6; P = .0008), and peripheral edema scores (from 1.0 to –0.4; P = .0295). They also observed significant mean improvements in quality of life and depression scores between baseline and end of maintenance (P less than .0001 and P = .0043, respectively). Mean free-testosterone levels increased nonsignificantly between baseline and end of maintenance in men (from 5.1 to 5.8 ng/dL) yet decreased significantly in women (from 0.3 to 0.1 ng/dL; P less than 0.0001; reference). Overall, 33 patients (35%) discontinued taking levoketoconazole by the end of the maintenance phase. Twelve (13%) discontinued because of adverse events.
“I wasn’t necessarily surprised with any of the data in this poster as I had experience with the drug in clinical trials, but I was definitely pleased to see overall significant improvements in acne score, hirsutism score in women, and peripheral edema score,” Dr. Fleseriu said. “Those are benefits that could potentially increase long-term adherence to treatment and quality-of-life improvements, particularly in women. It’s also exciting to see that quality of life and depression improved in these patients as well.”
These types of patient-reported outcomes are so important to how our patients feel about their disease and should be more of a focus for us physicians; it’s important to look at efficacy, safety and patient reported outcomes when we decide for an individualized treatment for each patient,” she noted.
Dr. Fleseriu reported that she has received research funding for Oregon Health and Science University from Novartis, Millendo, and Strongbridge. She has also received scientific consulting fees from Novartis and Strongbridge.
LOS ANGELES – including acne, hirsutism, and peripheral edema, as did patient-reported quality of life and symptoms of depression.
The findings come from an analysis of secondary endpoints among patients enrolled in SONICS, an open-label, phase 3 study of levoketoconazole as a treatment for endogenous Cushing’s disease (CD) that enrolled 94 patients at centers in North America, Europe and the Middle East. An investigational cortisol synthesis inhibitor, levoketoconazole is being developed by Strongbridge Biopharma and is not yet approved by the Food and Drug Administration.
“Despite the availability of approved treatments, the medical needs in Cushing’s [disease] remain very high,” the study’s principal investigator, Maria Fleseriu, MD, FACE, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists, where the data were presented. “This study demonstrates that levoketoconazole has the potential to address several clinical features of Cushing’s, owing to its clinically translated novel mechanism of action to suppress both cortisol and androgen syntheses (the latter elevated in many women with CD). Interestingly, there was no evidence of clinically important free-T reduction in men, and more studies are needed to elucidate this mechanism.”
In SONICS, adults with confirmed CD and mean 24-hour urinary free-cortisol (mUFC) value at least 1.5 times the upper limit of normal were treated with levoketoconazole in three phases: 2- to 21-week dose-titration phase (150-600 mg BID, as needed, to target mUFC normalization); 6-month maintenance phase (primary endpoint); and 6-month extended evaluation phase. The end of maintenance phase findings that focused on reductions in mUFC and safety had been previously reported. The current analysis focused on secondary endpoints, including changes from baseline to end of maintenance in investigator-assessed CD clinical signs and symptoms (acne score [range: 0-44]; hirsutism score [women only; range: 0-36]; and peripheral edema score [range: 0-12]), and patient-reported outcomes of quality of life (Cushing QoL questionnaire score [range: 0-100]) and depression (Beck Depression Inventory II score [range: 0-63]). The researchers also assessed hormones including free testosterone levels, and they used paired t-tests to infer statistical significance of the mean changes from baseline to end of maintenance for all measures.
Of the 94 patients enrolled in SONICS, 77 entered the maintenance phase, said Dr. Fleseriu, professor of medicine and neurological surgery and director of the pituitary center at Oregon Health and Science University, Portland. The patients’ mean age was 44 years and mean baseline mUFC was 243.3 mcg/day; 82% of patients were female, and 96% were white. Between baseline and the end of maintenance, the researchers observed significant mean improvements in acne scores (from 2.8 to –1.8, respectively; P = .0063), hirsutism scores (women only, from 7.8 to –2.6; P = .0008), and peripheral edema scores (from 1.0 to –0.4; P = .0295). They also observed significant mean improvements in quality of life and depression scores between baseline and end of maintenance (P less than .0001 and P = .0043, respectively). Mean free-testosterone levels increased nonsignificantly between baseline and end of maintenance in men (from 5.1 to 5.8 ng/dL) yet decreased significantly in women (from 0.3 to 0.1 ng/dL; P less than 0.0001; reference). Overall, 33 patients (35%) discontinued taking levoketoconazole by the end of the maintenance phase. Twelve (13%) discontinued because of adverse events.
“I wasn’t necessarily surprised with any of the data in this poster as I had experience with the drug in clinical trials, but I was definitely pleased to see overall significant improvements in acne score, hirsutism score in women, and peripheral edema score,” Dr. Fleseriu said. “Those are benefits that could potentially increase long-term adherence to treatment and quality-of-life improvements, particularly in women. It’s also exciting to see that quality of life and depression improved in these patients as well.”
These types of patient-reported outcomes are so important to how our patients feel about their disease and should be more of a focus for us physicians; it’s important to look at efficacy, safety and patient reported outcomes when we decide for an individualized treatment for each patient,” she noted.
Dr. Fleseriu reported that she has received research funding for Oregon Health and Science University from Novartis, Millendo, and Strongbridge. She has also received scientific consulting fees from Novartis and Strongbridge.
REPORTING FROM AACE 2019
Key clinical point: Several clinical features of Cushing’s disease improved following 6 months of treatment with the investigational agent levoketoconazole.
Major finding: Between baseline and the end of maintenance, the researchers observed significant mean improvements in acne scores (from 2.8 to –1.8, respectively; P = .0063), hirsutism scores (women only, from 7.8 to –2.6; P = .0008), and peripheral edema scores (from 1.0 to –0.4; P = .0295).
Study details: An analysis of secondary endpoints among 77 patients enrolled in SONICS.
Disclosures: Dr. Fleseriu reported that she has received research funding for Oregon Health and Science University from Novartis, Millendo, and Strongbridge. She has also received scientific consulting fees from Novartis and Strongbridge.
ATA risk stratification for DTC performs well in real-world cohort
NEW ORLEANS – The 2015 American Thyroid Association risk stratification system for patients with differentiated thyroid cancer performed well in a real-world cohort with a high proportion of high-risk patients, according to a study presented at the annual meeting of the Endocrine Society.
“The 2015 ATA Risk Stratification System is an excellent predictor of both persisting disease and survival,” wrote Evert F.S. van Velsen, MD, and his colleagues at Erasmus Medical Center, Rotterdam, the Netherlands, in a poster accompanying the presentation.
Among a group of 236 patients with differentiated thyroid cancer (DTC), Dr. van Velsen and his coauthors looked at how the ATA high-risk criteria influenced patient response to therapy. By the end of the 14-year study period, initial gross extrathyroidal disease extension meant patients were much less likely to have an excellent response (odds ratio, 0.26; P less than .001), and much more likely to have persistent disease (OR, 2.57; P = .001).
Odds of having an excellent response were reduced by having high postoperative thyroglobulin levels (OR, 0.21; P less than .001), and persistent disease was more likely (OR, 2.39; P = .002).
Other high-risk criteria associated with significantly lower odds of excellent response included distant metastases (OR, 0.36), incomplete resection (OR, 0.51), and having follicular thyroid carcinoma (FTC) with extensive vascular invasion (OR, 0.27). All these risk factors also were associated with higher odds of persistent disease.
“Recurrence after no evidence of disease occurred in 14%” of the study population, said Dr. van Velsen and his coauthors, adding, “Clinicians should be aware of the relatively high recurrence risk, even after an excellent response to therapy.”
The study aimed to evaluate the 2015 ATA risk stratification system’s prognostic value in a population that included a relatively large proportion of high-risk DTC patients, to include many FTC patients. This work, they noted, augments previous assessments of the risk stratification system in lower-risk populations.
The authors noted that, in addition to predicting disease recurrence, the risk stratification system also worked as a predictor of disease-specific survival. Patients with structural incomplete response fared the worst, with a survival probability below 0.5 at 200 months on a Kaplan-Meier curve of disease-specific survival. Survival probability remained at 1.0 for patients with excellent response after first therapy and was intermediate for those with indeterminate response and biochemical incomplete response.
Overall mortality was higher in FTC patients. Over the study period, 31 of the 76 FTC patients (41%) died, compared with 39 of the PTC patients (24%; P = .010). In all, 28% of the FTC patients and 18% of the PTC patients died of thyroid cancer, but this difference didn’t reach statistical significance.
The retrospective study included adults with DTC meeting the 2015 ATA high-risk criteria who were diagnosed and/or treated at Erasmus Medical Center over a 13-year span ending in December 2015.
Overall, the investigators found 236 patients meeting inclusion criteria; 160 had papillary thyroid cancer (PTC), and the remaining 76 had FTC. The latter group were significantly older at baseline than PTC patients (64 versus 53 years), and were significantly less likely to undergo neck dissection (22% versus 55%).
In the full cohort, 96 patients (41%) had one high-risk factor, and an additional 74 (31%) had two risk factors. The remaining patients had three or more risk factors.
There was no between-group difference in the likelihood of receiving radioactive iodine treatment, but those with FTC had a lower cumulative radiation dose (195 versus 298 mCi; P less than .001).
More than half of patients (58%) had persistent disease after completing their first therapy. Of these, 51% had structural incomplete response and 7% had biochemical incomplete response. The response was indeterminate for about a quarter of the cohort, and the remaining 17% had an excellent initial response.
By the end of the study period, 55% of patients had persistent disease, and 51% had structural incomplete response (a more likely result for those with FTC than PTC). Just 4% had a biochemical incomplete response, and the response was indeterminate for 16%. Response was judged excellent for 29% of patients.
Dr. van Velsen and his coauthors reported that they had no relevant disclosures.
SOURCE: van Velsen EFS et al. ENDO 2019, Abstract MON-549.
NEW ORLEANS – The 2015 American Thyroid Association risk stratification system for patients with differentiated thyroid cancer performed well in a real-world cohort with a high proportion of high-risk patients, according to a study presented at the annual meeting of the Endocrine Society.
“The 2015 ATA Risk Stratification System is an excellent predictor of both persisting disease and survival,” wrote Evert F.S. van Velsen, MD, and his colleagues at Erasmus Medical Center, Rotterdam, the Netherlands, in a poster accompanying the presentation.
Among a group of 236 patients with differentiated thyroid cancer (DTC), Dr. van Velsen and his coauthors looked at how the ATA high-risk criteria influenced patient response to therapy. By the end of the 14-year study period, initial gross extrathyroidal disease extension meant patients were much less likely to have an excellent response (odds ratio, 0.26; P less than .001), and much more likely to have persistent disease (OR, 2.57; P = .001).
Odds of having an excellent response were reduced by having high postoperative thyroglobulin levels (OR, 0.21; P less than .001), and persistent disease was more likely (OR, 2.39; P = .002).
Other high-risk criteria associated with significantly lower odds of excellent response included distant metastases (OR, 0.36), incomplete resection (OR, 0.51), and having follicular thyroid carcinoma (FTC) with extensive vascular invasion (OR, 0.27). All these risk factors also were associated with higher odds of persistent disease.
“Recurrence after no evidence of disease occurred in 14%” of the study population, said Dr. van Velsen and his coauthors, adding, “Clinicians should be aware of the relatively high recurrence risk, even after an excellent response to therapy.”
The study aimed to evaluate the 2015 ATA risk stratification system’s prognostic value in a population that included a relatively large proportion of high-risk DTC patients, to include many FTC patients. This work, they noted, augments previous assessments of the risk stratification system in lower-risk populations.
The authors noted that, in addition to predicting disease recurrence, the risk stratification system also worked as a predictor of disease-specific survival. Patients with structural incomplete response fared the worst, with a survival probability below 0.5 at 200 months on a Kaplan-Meier curve of disease-specific survival. Survival probability remained at 1.0 for patients with excellent response after first therapy and was intermediate for those with indeterminate response and biochemical incomplete response.
Overall mortality was higher in FTC patients. Over the study period, 31 of the 76 FTC patients (41%) died, compared with 39 of the PTC patients (24%; P = .010). In all, 28% of the FTC patients and 18% of the PTC patients died of thyroid cancer, but this difference didn’t reach statistical significance.
The retrospective study included adults with DTC meeting the 2015 ATA high-risk criteria who were diagnosed and/or treated at Erasmus Medical Center over a 13-year span ending in December 2015.
Overall, the investigators found 236 patients meeting inclusion criteria; 160 had papillary thyroid cancer (PTC), and the remaining 76 had FTC. The latter group were significantly older at baseline than PTC patients (64 versus 53 years), and were significantly less likely to undergo neck dissection (22% versus 55%).
In the full cohort, 96 patients (41%) had one high-risk factor, and an additional 74 (31%) had two risk factors. The remaining patients had three or more risk factors.
There was no between-group difference in the likelihood of receiving radioactive iodine treatment, but those with FTC had a lower cumulative radiation dose (195 versus 298 mCi; P less than .001).
More than half of patients (58%) had persistent disease after completing their first therapy. Of these, 51% had structural incomplete response and 7% had biochemical incomplete response. The response was indeterminate for about a quarter of the cohort, and the remaining 17% had an excellent initial response.
By the end of the study period, 55% of patients had persistent disease, and 51% had structural incomplete response (a more likely result for those with FTC than PTC). Just 4% had a biochemical incomplete response, and the response was indeterminate for 16%. Response was judged excellent for 29% of patients.
Dr. van Velsen and his coauthors reported that they had no relevant disclosures.
SOURCE: van Velsen EFS et al. ENDO 2019, Abstract MON-549.
NEW ORLEANS – The 2015 American Thyroid Association risk stratification system for patients with differentiated thyroid cancer performed well in a real-world cohort with a high proportion of high-risk patients, according to a study presented at the annual meeting of the Endocrine Society.
“The 2015 ATA Risk Stratification System is an excellent predictor of both persisting disease and survival,” wrote Evert F.S. van Velsen, MD, and his colleagues at Erasmus Medical Center, Rotterdam, the Netherlands, in a poster accompanying the presentation.
Among a group of 236 patients with differentiated thyroid cancer (DTC), Dr. van Velsen and his coauthors looked at how the ATA high-risk criteria influenced patient response to therapy. By the end of the 14-year study period, initial gross extrathyroidal disease extension meant patients were much less likely to have an excellent response (odds ratio, 0.26; P less than .001), and much more likely to have persistent disease (OR, 2.57; P = .001).
Odds of having an excellent response were reduced by having high postoperative thyroglobulin levels (OR, 0.21; P less than .001), and persistent disease was more likely (OR, 2.39; P = .002).
Other high-risk criteria associated with significantly lower odds of excellent response included distant metastases (OR, 0.36), incomplete resection (OR, 0.51), and having follicular thyroid carcinoma (FTC) with extensive vascular invasion (OR, 0.27). All these risk factors also were associated with higher odds of persistent disease.
“Recurrence after no evidence of disease occurred in 14%” of the study population, said Dr. van Velsen and his coauthors, adding, “Clinicians should be aware of the relatively high recurrence risk, even after an excellent response to therapy.”
The study aimed to evaluate the 2015 ATA risk stratification system’s prognostic value in a population that included a relatively large proportion of high-risk DTC patients, to include many FTC patients. This work, they noted, augments previous assessments of the risk stratification system in lower-risk populations.
The authors noted that, in addition to predicting disease recurrence, the risk stratification system also worked as a predictor of disease-specific survival. Patients with structural incomplete response fared the worst, with a survival probability below 0.5 at 200 months on a Kaplan-Meier curve of disease-specific survival. Survival probability remained at 1.0 for patients with excellent response after first therapy and was intermediate for those with indeterminate response and biochemical incomplete response.
Overall mortality was higher in FTC patients. Over the study period, 31 of the 76 FTC patients (41%) died, compared with 39 of the PTC patients (24%; P = .010). In all, 28% of the FTC patients and 18% of the PTC patients died of thyroid cancer, but this difference didn’t reach statistical significance.
The retrospective study included adults with DTC meeting the 2015 ATA high-risk criteria who were diagnosed and/or treated at Erasmus Medical Center over a 13-year span ending in December 2015.
Overall, the investigators found 236 patients meeting inclusion criteria; 160 had papillary thyroid cancer (PTC), and the remaining 76 had FTC. The latter group were significantly older at baseline than PTC patients (64 versus 53 years), and were significantly less likely to undergo neck dissection (22% versus 55%).
In the full cohort, 96 patients (41%) had one high-risk factor, and an additional 74 (31%) had two risk factors. The remaining patients had three or more risk factors.
There was no between-group difference in the likelihood of receiving radioactive iodine treatment, but those with FTC had a lower cumulative radiation dose (195 versus 298 mCi; P less than .001).
More than half of patients (58%) had persistent disease after completing their first therapy. Of these, 51% had structural incomplete response and 7% had biochemical incomplete response. The response was indeterminate for about a quarter of the cohort, and the remaining 17% had an excellent initial response.
By the end of the study period, 55% of patients had persistent disease, and 51% had structural incomplete response (a more likely result for those with FTC than PTC). Just 4% had a biochemical incomplete response, and the response was indeterminate for 16%. Response was judged excellent for 29% of patients.
Dr. van Velsen and his coauthors reported that they had no relevant disclosures.
SOURCE: van Velsen EFS et al. ENDO 2019, Abstract MON-549.
REPORTING FROM ENDO 2019
Key clinical point: in a cohort of high-risk patients.
Major finding: Gross extrathyroidal disease extension and high postoperative thyroglobulin levels predicted poor response (OR for excellent response, 0.26 and 0.21, respectively).
Study details: Retrospective single-center study of 236 patients with DTC meeting American Thyroid Association criteria for high risk.
Disclosures: The authors reported no external sources of funding and that they had no conflicts of interest.
Source: van Velsen EFS et al. ENDO 2019, Abstract MON-549.
No birth rate gains from levothyroxine in pregnancy
Treatment with levothyroxine does not improve the live birth rate in women with thyroid peroxidase antibodies before conception, according to data presented at the annual meeting of the Endocrine Society.
Until now, the evidence for the use of levothyroxine in pregnant women with thyroid peroxidase antibodies but normal thyroid function has been inconclusive, Rima K. Dhillon-Smith, MBChB, PhD, of the University of Birmingham (England), and her coauthors said in a paper published simultaneously with the meeting presentation March 23 in the New England Journal of Medicine.
Previous studies have shown that women with thyroid peroxidase antibodies but normal thyroid function have a nearly fourfold higher risk of miscarriage and twofold higher risk of preterm birth, compared with women who don’t have the antibodies.
In the new double-blind study, 952 women with thyroid peroxidase antibodies, normal thyroid function, and a history of miscarriage or infertility were randomized either to daily 50 mcg levothyroxine or placebo, taken from conception to the end of pregnancy.
The rate of pregnancy was similar in the levothyroxine and placebo groups (56.6% vs. 58.3%, respectively), as was the live birth rate (37.4% vs. 37.9%), despite the observation that the levothyroxine group had consistently lower serum thyrotropin and higher free T4 concentrations than did the placebo group.
There were also no significant differences between the two groups in secondary outcomes of miscarriage, preterm birth, or neonatal outcomes such as birth weight.
Researchers also saw no statistically significant differences in the rate of serious adverse events or in the number of women who showed abnormal results on thyroid function tests.
The authors noted that the dosage of levothyroxine used in the study was fixed, leaving the possibility that “the dose may need to be adjusted depending on the participant’s body weight, thyroid peroxidase antibody level, or thyrotropin concentration.”
Existing guidelines from the American Thyroid Association acknowledge the lack of evidence in favor of levothyroxine decreasing the risk of pregnancy loss. However, the guidelines also state that it can be considered in antibody-positive, euthyroid women with a history of loss, “given its potential benefits in comparison with its minimal risk.”
The study was supported by the National Institute for Health Research. No conflicts of interest were declared.
SOURCE: Dhillon-Smith R et al. N Engl J Med. 2019 March 23. doi: 10.1056/NEJMoa1812537
Treatment with levothyroxine does not improve the live birth rate in women with thyroid peroxidase antibodies before conception, according to data presented at the annual meeting of the Endocrine Society.
Until now, the evidence for the use of levothyroxine in pregnant women with thyroid peroxidase antibodies but normal thyroid function has been inconclusive, Rima K. Dhillon-Smith, MBChB, PhD, of the University of Birmingham (England), and her coauthors said in a paper published simultaneously with the meeting presentation March 23 in the New England Journal of Medicine.
Previous studies have shown that women with thyroid peroxidase antibodies but normal thyroid function have a nearly fourfold higher risk of miscarriage and twofold higher risk of preterm birth, compared with women who don’t have the antibodies.
In the new double-blind study, 952 women with thyroid peroxidase antibodies, normal thyroid function, and a history of miscarriage or infertility were randomized either to daily 50 mcg levothyroxine or placebo, taken from conception to the end of pregnancy.
The rate of pregnancy was similar in the levothyroxine and placebo groups (56.6% vs. 58.3%, respectively), as was the live birth rate (37.4% vs. 37.9%), despite the observation that the levothyroxine group had consistently lower serum thyrotropin and higher free T4 concentrations than did the placebo group.
There were also no significant differences between the two groups in secondary outcomes of miscarriage, preterm birth, or neonatal outcomes such as birth weight.
Researchers also saw no statistically significant differences in the rate of serious adverse events or in the number of women who showed abnormal results on thyroid function tests.
The authors noted that the dosage of levothyroxine used in the study was fixed, leaving the possibility that “the dose may need to be adjusted depending on the participant’s body weight, thyroid peroxidase antibody level, or thyrotropin concentration.”
Existing guidelines from the American Thyroid Association acknowledge the lack of evidence in favor of levothyroxine decreasing the risk of pregnancy loss. However, the guidelines also state that it can be considered in antibody-positive, euthyroid women with a history of loss, “given its potential benefits in comparison with its minimal risk.”
The study was supported by the National Institute for Health Research. No conflicts of interest were declared.
SOURCE: Dhillon-Smith R et al. N Engl J Med. 2019 March 23. doi: 10.1056/NEJMoa1812537
Treatment with levothyroxine does not improve the live birth rate in women with thyroid peroxidase antibodies before conception, according to data presented at the annual meeting of the Endocrine Society.
Until now, the evidence for the use of levothyroxine in pregnant women with thyroid peroxidase antibodies but normal thyroid function has been inconclusive, Rima K. Dhillon-Smith, MBChB, PhD, of the University of Birmingham (England), and her coauthors said in a paper published simultaneously with the meeting presentation March 23 in the New England Journal of Medicine.
Previous studies have shown that women with thyroid peroxidase antibodies but normal thyroid function have a nearly fourfold higher risk of miscarriage and twofold higher risk of preterm birth, compared with women who don’t have the antibodies.
In the new double-blind study, 952 women with thyroid peroxidase antibodies, normal thyroid function, and a history of miscarriage or infertility were randomized either to daily 50 mcg levothyroxine or placebo, taken from conception to the end of pregnancy.
The rate of pregnancy was similar in the levothyroxine and placebo groups (56.6% vs. 58.3%, respectively), as was the live birth rate (37.4% vs. 37.9%), despite the observation that the levothyroxine group had consistently lower serum thyrotropin and higher free T4 concentrations than did the placebo group.
There were also no significant differences between the two groups in secondary outcomes of miscarriage, preterm birth, or neonatal outcomes such as birth weight.
Researchers also saw no statistically significant differences in the rate of serious adverse events or in the number of women who showed abnormal results on thyroid function tests.
The authors noted that the dosage of levothyroxine used in the study was fixed, leaving the possibility that “the dose may need to be adjusted depending on the participant’s body weight, thyroid peroxidase antibody level, or thyrotropin concentration.”
Existing guidelines from the American Thyroid Association acknowledge the lack of evidence in favor of levothyroxine decreasing the risk of pregnancy loss. However, the guidelines also state that it can be considered in antibody-positive, euthyroid women with a history of loss, “given its potential benefits in comparison with its minimal risk.”
The study was supported by the National Institute for Health Research. No conflicts of interest were declared.
SOURCE: Dhillon-Smith R et al. N Engl J Med. 2019 March 23. doi: 10.1056/NEJMoa1812537
FROM ENDO 2019
Key clinical point:
Major finding: Pregnancy rates and outcomes were similar in women treated with levothyroxine and those treated with placebo.
Study details: Double-blind, randomized, placebo-controlled trial in 952 women.
Disclosures: The study was supported by the National Institute for Health Research. No conflicts of interest were declared.
Source: Dhillon-Smith R et al. N Engl J Med. 2019 March 23. doi: 10.1056/NEJMoa1812537
Subclinical hypothyroidism boosts immediate risk of heart failure
CHICAGO – The short-term risk of developing heart failure in patients with newly identified hypothyroidism, be it overt or subclinical, is double that of euthyroid individuals, Caroline H. Noergaard, MD, reported at the American Heart Association scientific sessions.
“This is really important clinically. The association with heart failure has previously been shown in both overt and subclinical hyperthyroidism, but it’s actually new knowledge that hypothyroidism is associated with immediate risk of heart failure. And a lot of people have subclinical hypothyroidism,” said Dr. Noergaard, a PhD student in epidemiology at Aalborg (Denmark) University.
Also at the meeting, Jeffrey L. Anderson, MD, reported that free thyroxine levels within the normal reference range were associated in graded fashion with an increased prevalence and incidence of atrial fibrillation in a large Utah study, a finding that provides independent confirmation of an earlier report by investigators from the population-based Rotterdam Study.
“These findings validate those of the Rotterdam Study in a much larger dataset and may have important clinical implications, including a redefinition of the reference range and the target-free T4 levels for thyroxine replacement therapy,” observed Dr. Anderson, professor of internal medicine at the University of Utah, Salt Lake City, and a research cardiologist at the Intermountain Medical Center Heart Institute.
Hypothyroidism and heart failure
Dr. Noergaard presented a retrospective study of over 1 million Copenhagen-area adults (mean age, 50 years) with no history of heart failure, who had their first thyroid function test. She and her coinvestigators turned to comprehensive Danish national health care registries to determine how many of these individuals were diagnosed with new-onset heart failure within 90 days after their thyroid function test.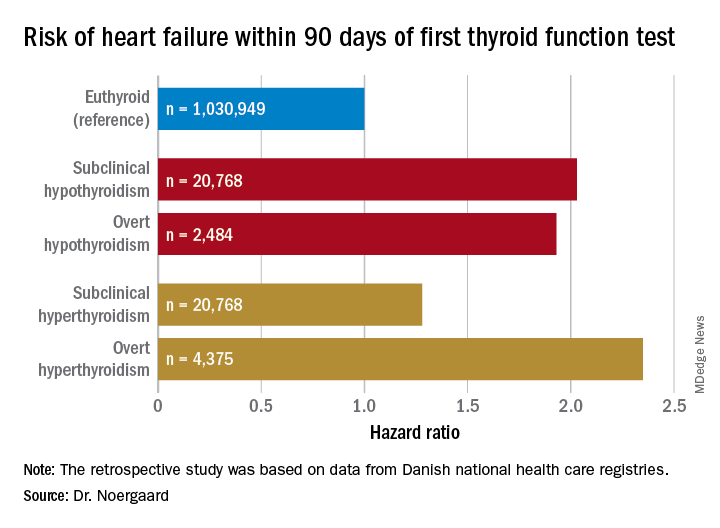
Subclinical hypothyroidism was defined by a thyroid-stimulating hormone level greater than 5 mIU/L and a free T4 of 9-22 pmol/L. Overt hypothyroidism required a TSH greater than 5 mIU/L with a free T4 less than 9 pmol/L.
Free T4 predicts atrial fibrillation risk
Dr. Anderson presented a retrospective analysis of 174,914 adult patients in the Intermountain Healthcare EMR database, none of whom were on thyroid replacement at entry. The patients, who were a mean age of 64 years and 65% women, were followed for an average of 6.3 years. Of these, 88.4% had a free T4 within the normal reference range of 0.75-1.5 ng/dL, 7.4% had a value below the cutoff for normal, and 4.2% had a free T4 above the reference range.
Upon dividing the patients within the normal range into quartiles based upon their free T4 level, he and his coinvestigators found that the baseline prevalence of atrial fibrillation was 8.7% in those in quartile 1, 9.3% in quartile 2, 10.5% in quartile 3, and 12.6% in quartile 4. In a multivariate analysis adjusted for potential confounders, the risk of prevalent atrial fibrillation was increased by 11% for patients in quartile 2, compared with those in the first quartile, by 22% in quartile 3, and by 40% in quartile 4.
The incidence of new-onset atrial fibrillation during 3 years of follow-up was 4.1% in patients in normal-range quartile 1, 4.3% in quartile 2, 4.5% in quartile 3, and 5.2% in the top normal-range quartile. The odds of developing atrial fibrillation were increased by 8% and 16% in quartiles 3 and 4, compared with quartile 1.
Serum TSH and free T3 levels showed no consistent relationship with atrial fibrillation.
The Utah findings confirm in a large U.S. population the earlier report from the Rotterdam Study (J Clin Endocrinol Metab. 2015 Oct;100(10):3718-24).
Dr. Noergaard and Dr. Anderson reported having no financial conflicts regarding their studies, which were carried out free of commercial support.
CHICAGO – The short-term risk of developing heart failure in patients with newly identified hypothyroidism, be it overt or subclinical, is double that of euthyroid individuals, Caroline H. Noergaard, MD, reported at the American Heart Association scientific sessions.
“This is really important clinically. The association with heart failure has previously been shown in both overt and subclinical hyperthyroidism, but it’s actually new knowledge that hypothyroidism is associated with immediate risk of heart failure. And a lot of people have subclinical hypothyroidism,” said Dr. Noergaard, a PhD student in epidemiology at Aalborg (Denmark) University.
Also at the meeting, Jeffrey L. Anderson, MD, reported that free thyroxine levels within the normal reference range were associated in graded fashion with an increased prevalence and incidence of atrial fibrillation in a large Utah study, a finding that provides independent confirmation of an earlier report by investigators from the population-based Rotterdam Study.
“These findings validate those of the Rotterdam Study in a much larger dataset and may have important clinical implications, including a redefinition of the reference range and the target-free T4 levels for thyroxine replacement therapy,” observed Dr. Anderson, professor of internal medicine at the University of Utah, Salt Lake City, and a research cardiologist at the Intermountain Medical Center Heart Institute.
Hypothyroidism and heart failure
Dr. Noergaard presented a retrospective study of over 1 million Copenhagen-area adults (mean age, 50 years) with no history of heart failure, who had their first thyroid function test. She and her coinvestigators turned to comprehensive Danish national health care registries to determine how many of these individuals were diagnosed with new-onset heart failure within 90 days after their thyroid function test.
Subclinical hypothyroidism was defined by a thyroid-stimulating hormone level greater than 5 mIU/L and a free T4 of 9-22 pmol/L. Overt hypothyroidism required a TSH greater than 5 mIU/L with a free T4 less than 9 pmol/L.
Free T4 predicts atrial fibrillation risk
Dr. Anderson presented a retrospective analysis of 174,914 adult patients in the Intermountain Healthcare EMR database, none of whom were on thyroid replacement at entry. The patients, who were a mean age of 64 years and 65% women, were followed for an average of 6.3 years. Of these, 88.4% had a free T4 within the normal reference range of 0.75-1.5 ng/dL, 7.4% had a value below the cutoff for normal, and 4.2% had a free T4 above the reference range.
Upon dividing the patients within the normal range into quartiles based upon their free T4 level, he and his coinvestigators found that the baseline prevalence of atrial fibrillation was 8.7% in those in quartile 1, 9.3% in quartile 2, 10.5% in quartile 3, and 12.6% in quartile 4. In a multivariate analysis adjusted for potential confounders, the risk of prevalent atrial fibrillation was increased by 11% for patients in quartile 2, compared with those in the first quartile, by 22% in quartile 3, and by 40% in quartile 4.
The incidence of new-onset atrial fibrillation during 3 years of follow-up was 4.1% in patients in normal-range quartile 1, 4.3% in quartile 2, 4.5% in quartile 3, and 5.2% in the top normal-range quartile. The odds of developing atrial fibrillation were increased by 8% and 16% in quartiles 3 and 4, compared with quartile 1.
Serum TSH and free T3 levels showed no consistent relationship with atrial fibrillation.
The Utah findings confirm in a large U.S. population the earlier report from the Rotterdam Study (J Clin Endocrinol Metab. 2015 Oct;100(10):3718-24).
Dr. Noergaard and Dr. Anderson reported having no financial conflicts regarding their studies, which were carried out free of commercial support.
CHICAGO – The short-term risk of developing heart failure in patients with newly identified hypothyroidism, be it overt or subclinical, is double that of euthyroid individuals, Caroline H. Noergaard, MD, reported at the American Heart Association scientific sessions.
“This is really important clinically. The association with heart failure has previously been shown in both overt and subclinical hyperthyroidism, but it’s actually new knowledge that hypothyroidism is associated with immediate risk of heart failure. And a lot of people have subclinical hypothyroidism,” said Dr. Noergaard, a PhD student in epidemiology at Aalborg (Denmark) University.
Also at the meeting, Jeffrey L. Anderson, MD, reported that free thyroxine levels within the normal reference range were associated in graded fashion with an increased prevalence and incidence of atrial fibrillation in a large Utah study, a finding that provides independent confirmation of an earlier report by investigators from the population-based Rotterdam Study.
“These findings validate those of the Rotterdam Study in a much larger dataset and may have important clinical implications, including a redefinition of the reference range and the target-free T4 levels for thyroxine replacement therapy,” observed Dr. Anderson, professor of internal medicine at the University of Utah, Salt Lake City, and a research cardiologist at the Intermountain Medical Center Heart Institute.
Hypothyroidism and heart failure
Dr. Noergaard presented a retrospective study of over 1 million Copenhagen-area adults (mean age, 50 years) with no history of heart failure, who had their first thyroid function test. She and her coinvestigators turned to comprehensive Danish national health care registries to determine how many of these individuals were diagnosed with new-onset heart failure within 90 days after their thyroid function test.
Subclinical hypothyroidism was defined by a thyroid-stimulating hormone level greater than 5 mIU/L and a free T4 of 9-22 pmol/L. Overt hypothyroidism required a TSH greater than 5 mIU/L with a free T4 less than 9 pmol/L.
Free T4 predicts atrial fibrillation risk
Dr. Anderson presented a retrospective analysis of 174,914 adult patients in the Intermountain Healthcare EMR database, none of whom were on thyroid replacement at entry. The patients, who were a mean age of 64 years and 65% women, were followed for an average of 6.3 years. Of these, 88.4% had a free T4 within the normal reference range of 0.75-1.5 ng/dL, 7.4% had a value below the cutoff for normal, and 4.2% had a free T4 above the reference range.
Upon dividing the patients within the normal range into quartiles based upon their free T4 level, he and his coinvestigators found that the baseline prevalence of atrial fibrillation was 8.7% in those in quartile 1, 9.3% in quartile 2, 10.5% in quartile 3, and 12.6% in quartile 4. In a multivariate analysis adjusted for potential confounders, the risk of prevalent atrial fibrillation was increased by 11% for patients in quartile 2, compared with those in the first quartile, by 22% in quartile 3, and by 40% in quartile 4.
The incidence of new-onset atrial fibrillation during 3 years of follow-up was 4.1% in patients in normal-range quartile 1, 4.3% in quartile 2, 4.5% in quartile 3, and 5.2% in the top normal-range quartile. The odds of developing atrial fibrillation were increased by 8% and 16% in quartiles 3 and 4, compared with quartile 1.
Serum TSH and free T3 levels showed no consistent relationship with atrial fibrillation.
The Utah findings confirm in a large U.S. population the earlier report from the Rotterdam Study (J Clin Endocrinol Metab. 2015 Oct;100(10):3718-24).
Dr. Noergaard and Dr. Anderson reported having no financial conflicts regarding their studies, which were carried out free of commercial support.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Both subclinical and overt hypothyroidism are associated with a 100% increased risk of being diagnosed with heart failure, compared with euthyroid individuals.
Study details: This was a retrospective study of the association between free thyroxine levels and short-term risk of developing heart failure in more than 1 million Copenhagen-area patients.
Disclosures: The presenter reported having no financial conflicts regarding the Danish study, conducted free of commercial support.
Low-normal thyroid function tied to advanced fibrosis
Advanced fibrosis affected 5.9% of adults with low-normal thyroid function or subclinical hypothyroidism – more than double the prevalence among adults with strict-normal thyroid function (2.8%; P less than .001), according to the results of a large survey study.
Based on these findings, therapy to improve low thyroid function might help prevent advanced fibrosis secondary to nonalcoholic fatty liver disease, wrote Donghee Kim, MD, PhD, of Stanford University (Calif.), together with his associates in Clinical Gastroenterology and Hepatology.
Prior research has linked low-normal thyroid function with obesity, cardiometabolic diseases, and fractures. For this study, Dr. Kim and his coinvestigators analyzed data from 7,259 adults who lacked major etiologies of chronic liver disease and were included in the National Health and Nutrition Examination Survey between 2007 and 2012.
After accounting for demographic, socioeconomic, and clinical variables, the odds of biopsy-confirmed advanced fibrosis were 100% higher in adults with low-normal thyroid function or subclinical hypothyroidism, compared with adults with strict-normal thyroid function (odds ratio, 2.0; 95% confidence interval, 1.2-3.3). The prevalence and odds of advanced fibrosis was similar in each of these two subgroups. Furthermore, low thyroid function remained strongly linked with advanced fibrosis after accounting for insulin resistance using data from fasting subjects (OR, 2.3; 95% CI, 1.2-4.4).
Previously, Dr. Kim and his coinvestigators found a strong link between biopsy-proven advanced fibrosis and low-normal thyroid function or subclinical hypothyroidism among adults in Korea. “These [new] results are consistent with our previous observations in [an] Asian population, and show their generalizability to the Western world across all ethnicities.”
The researchers did not acknowledge external funding sources. They reported having no conflicts of interest.
SOURCE: Kim D et al. Clin Gastroenterol Hepatol. 2018 Nov 17. doi: 10.1016/j.cgh.2018.11.024.
Advanced fibrosis affected 5.9% of adults with low-normal thyroid function or subclinical hypothyroidism – more than double the prevalence among adults with strict-normal thyroid function (2.8%; P less than .001), according to the results of a large survey study.
Based on these findings, therapy to improve low thyroid function might help prevent advanced fibrosis secondary to nonalcoholic fatty liver disease, wrote Donghee Kim, MD, PhD, of Stanford University (Calif.), together with his associates in Clinical Gastroenterology and Hepatology.
Prior research has linked low-normal thyroid function with obesity, cardiometabolic diseases, and fractures. For this study, Dr. Kim and his coinvestigators analyzed data from 7,259 adults who lacked major etiologies of chronic liver disease and were included in the National Health and Nutrition Examination Survey between 2007 and 2012.
After accounting for demographic, socioeconomic, and clinical variables, the odds of biopsy-confirmed advanced fibrosis were 100% higher in adults with low-normal thyroid function or subclinical hypothyroidism, compared with adults with strict-normal thyroid function (odds ratio, 2.0; 95% confidence interval, 1.2-3.3). The prevalence and odds of advanced fibrosis was similar in each of these two subgroups. Furthermore, low thyroid function remained strongly linked with advanced fibrosis after accounting for insulin resistance using data from fasting subjects (OR, 2.3; 95% CI, 1.2-4.4).
Previously, Dr. Kim and his coinvestigators found a strong link between biopsy-proven advanced fibrosis and low-normal thyroid function or subclinical hypothyroidism among adults in Korea. “These [new] results are consistent with our previous observations in [an] Asian population, and show their generalizability to the Western world across all ethnicities.”
The researchers did not acknowledge external funding sources. They reported having no conflicts of interest.
SOURCE: Kim D et al. Clin Gastroenterol Hepatol. 2018 Nov 17. doi: 10.1016/j.cgh.2018.11.024.
Advanced fibrosis affected 5.9% of adults with low-normal thyroid function or subclinical hypothyroidism – more than double the prevalence among adults with strict-normal thyroid function (2.8%; P less than .001), according to the results of a large survey study.
Based on these findings, therapy to improve low thyroid function might help prevent advanced fibrosis secondary to nonalcoholic fatty liver disease, wrote Donghee Kim, MD, PhD, of Stanford University (Calif.), together with his associates in Clinical Gastroenterology and Hepatology.
Prior research has linked low-normal thyroid function with obesity, cardiometabolic diseases, and fractures. For this study, Dr. Kim and his coinvestigators analyzed data from 7,259 adults who lacked major etiologies of chronic liver disease and were included in the National Health and Nutrition Examination Survey between 2007 and 2012.
After accounting for demographic, socioeconomic, and clinical variables, the odds of biopsy-confirmed advanced fibrosis were 100% higher in adults with low-normal thyroid function or subclinical hypothyroidism, compared with adults with strict-normal thyroid function (odds ratio, 2.0; 95% confidence interval, 1.2-3.3). The prevalence and odds of advanced fibrosis was similar in each of these two subgroups. Furthermore, low thyroid function remained strongly linked with advanced fibrosis after accounting for insulin resistance using data from fasting subjects (OR, 2.3; 95% CI, 1.2-4.4).
Previously, Dr. Kim and his coinvestigators found a strong link between biopsy-proven advanced fibrosis and low-normal thyroid function or subclinical hypothyroidism among adults in Korea. “These [new] results are consistent with our previous observations in [an] Asian population, and show their generalizability to the Western world across all ethnicities.”
The researchers did not acknowledge external funding sources. They reported having no conflicts of interest.
SOURCE: Kim D et al. Clin Gastroenterol Hepatol. 2018 Nov 17. doi: 10.1016/j.cgh.2018.11.024.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Low-normal thyroid function correlates significantly with advanced fibrosis.
Major finding: In all, 5.9% of adults with low-normal thyroid function had advanced fibrosis, compared with 2.8% of individuals with strict-normal thyroid function (P less than .001).
Study details: A study of 7,259 adults from the National Health and Nutrition Examination Survey (2007-2012).
Disclosures: The investigators did not acknowledge funding sources. They reported having no conflicts of interest.
Source: Kim D et al. Clin Gastroenterol Hepatol. 2018 Nov 17. doi: 10.1016/j.cgh.2018.11.024.
Initial screening not enough to catch all cases of preterm congenital hypothyroidism
reported Niamh McGrath, MD, of the department of pediatric endocrinology at Children’s University Hospital, Dublin, and her associates in the Journal of Pediatrics.
In a population-based prospective review of 898,424 records between 2004 and 2016, Dr. McGrath and her associates identified all preterm infants less than 33 weeks diagnosed with congenital hypothyroidism and receiving treatment with levothyroxine. Of the infants screened, just 53 were selected to participate in the study, including 26 who were diagnosed at the first thyroid-stimulating hormone (TSH) screening and 27 who had delayed TSH elevation.
Gestational age ranged from 23 to 33 weeks, median birth weight measured 1.2 kg, median serum TSH concentration at the time of diagnosis was 78.3 mU/L, and median free thyroxine concentration was 8.9 pmol/L.
For half of the infants ultimately diagnosed, congenital hypothyroidism was not detected during the initial newborn screening. The authors also noted that 25% of patients with delayed TSH elevation had been exposed to iodine while undergoing surgery for necrotizing enterocolitis; after age 28 days, four of these infants were found to have elevated TSH. They cautioned that while this finding emphasizes the need for close monitoring and repeat screening of infants who have been exposed to iodine for up to 1 month following exposure, they could not be certain that the iodine exposure was responsible for the transient hypothyroidism in these patients.
Dr. McGrath and her associates emphasized the importance of repeat TSH screening for all preterm infants, noting that standard protocol screenings conducted just once at age 2 weeks or 4 weeks are not sufficient to effectively identify all cases of congenital hypothyroidism in which TSH elevation is delayed. Instead, they recommended measuring TSH on days 3-5 and at 1 week, 2 weeks, 4 weeks, and term-corrected gestational age.
“Our data are consistent with studies showing a high incidence of delayed TSH rise, particularly in very-low-birth-weight infants,” the authors wrote. They speculated that delays in detecting primary congenital hypothyroidism could be caused by the suppression of TSH secretion as a result of “hypothalamic-pituitary immaturity, medication administration, and effects of serious neonatal illness.” In fact, had standard, recommended 2-week-only screening protocols been followed with their patient population, fully 48% of infants with delayed TSH elevation would have been overlooked; half of these patients were later found to have decompensated hypothyroidism.
Neurodevelopmental disability caused by congenital hypothyroidism, which affects roughly 1 in every 2,000-4,000 births, is increasingly being prevented with newborn screenings that identify the condition early, but the incidence of congenital hypothyroidism has increased considerably in the past 20 years. The authors attribute this increase to the gradual change in screening cutoff levels and the rise in number of preterm infants who are surviving.
The need for a second screening, as well as appropriate timing and optimal TSH cutoff, are “subjects of active debate,” the authors wrote. The latest European screening guidelines recommend second screenings for preterm and low-birth-weight infants at either 2 weeks of age or 2 weeks following preliminary screening. Although they make no recommendations regarding additional screenings, American Academy of Pediatrics guidelines, published in 2006, cite a “disproportionate incidence” of delayed increase in TSH and congenital hypothyroidism in infants with very low birth weight. Dr. McGrath and her associates speculated that not all screening programs have adopted repeat screening of preterm infants, perhaps because of the low yield results, “the transient nature of most cases” detected, as well as conflicting long-term data on neurodevelopmental outcomes.
Dr. McGrath receives funding from the Children’s Fund for Health. The authors declared no conflicts of interest.
SOURCE: McGrath N et al. J Pediatr. 2018 Oct 24. doi: 10.1016/j.jpeds.2018.09.044.
reported Niamh McGrath, MD, of the department of pediatric endocrinology at Children’s University Hospital, Dublin, and her associates in the Journal of Pediatrics.
In a population-based prospective review of 898,424 records between 2004 and 2016, Dr. McGrath and her associates identified all preterm infants less than 33 weeks diagnosed with congenital hypothyroidism and receiving treatment with levothyroxine. Of the infants screened, just 53 were selected to participate in the study, including 26 who were diagnosed at the first thyroid-stimulating hormone (TSH) screening and 27 who had delayed TSH elevation.
Gestational age ranged from 23 to 33 weeks, median birth weight measured 1.2 kg, median serum TSH concentration at the time of diagnosis was 78.3 mU/L, and median free thyroxine concentration was 8.9 pmol/L.
For half of the infants ultimately diagnosed, congenital hypothyroidism was not detected during the initial newborn screening. The authors also noted that 25% of patients with delayed TSH elevation had been exposed to iodine while undergoing surgery for necrotizing enterocolitis; after age 28 days, four of these infants were found to have elevated TSH. They cautioned that while this finding emphasizes the need for close monitoring and repeat screening of infants who have been exposed to iodine for up to 1 month following exposure, they could not be certain that the iodine exposure was responsible for the transient hypothyroidism in these patients.
Dr. McGrath and her associates emphasized the importance of repeat TSH screening for all preterm infants, noting that standard protocol screenings conducted just once at age 2 weeks or 4 weeks are not sufficient to effectively identify all cases of congenital hypothyroidism in which TSH elevation is delayed. Instead, they recommended measuring TSH on days 3-5 and at 1 week, 2 weeks, 4 weeks, and term-corrected gestational age.
“Our data are consistent with studies showing a high incidence of delayed TSH rise, particularly in very-low-birth-weight infants,” the authors wrote. They speculated that delays in detecting primary congenital hypothyroidism could be caused by the suppression of TSH secretion as a result of “hypothalamic-pituitary immaturity, medication administration, and effects of serious neonatal illness.” In fact, had standard, recommended 2-week-only screening protocols been followed with their patient population, fully 48% of infants with delayed TSH elevation would have been overlooked; half of these patients were later found to have decompensated hypothyroidism.
Neurodevelopmental disability caused by congenital hypothyroidism, which affects roughly 1 in every 2,000-4,000 births, is increasingly being prevented with newborn screenings that identify the condition early, but the incidence of congenital hypothyroidism has increased considerably in the past 20 years. The authors attribute this increase to the gradual change in screening cutoff levels and the rise in number of preterm infants who are surviving.
The need for a second screening, as well as appropriate timing and optimal TSH cutoff, are “subjects of active debate,” the authors wrote. The latest European screening guidelines recommend second screenings for preterm and low-birth-weight infants at either 2 weeks of age or 2 weeks following preliminary screening. Although they make no recommendations regarding additional screenings, American Academy of Pediatrics guidelines, published in 2006, cite a “disproportionate incidence” of delayed increase in TSH and congenital hypothyroidism in infants with very low birth weight. Dr. McGrath and her associates speculated that not all screening programs have adopted repeat screening of preterm infants, perhaps because of the low yield results, “the transient nature of most cases” detected, as well as conflicting long-term data on neurodevelopmental outcomes.
Dr. McGrath receives funding from the Children’s Fund for Health. The authors declared no conflicts of interest.
SOURCE: McGrath N et al. J Pediatr. 2018 Oct 24. doi: 10.1016/j.jpeds.2018.09.044.
reported Niamh McGrath, MD, of the department of pediatric endocrinology at Children’s University Hospital, Dublin, and her associates in the Journal of Pediatrics.
In a population-based prospective review of 898,424 records between 2004 and 2016, Dr. McGrath and her associates identified all preterm infants less than 33 weeks diagnosed with congenital hypothyroidism and receiving treatment with levothyroxine. Of the infants screened, just 53 were selected to participate in the study, including 26 who were diagnosed at the first thyroid-stimulating hormone (TSH) screening and 27 who had delayed TSH elevation.
Gestational age ranged from 23 to 33 weeks, median birth weight measured 1.2 kg, median serum TSH concentration at the time of diagnosis was 78.3 mU/L, and median free thyroxine concentration was 8.9 pmol/L.
For half of the infants ultimately diagnosed, congenital hypothyroidism was not detected during the initial newborn screening. The authors also noted that 25% of patients with delayed TSH elevation had been exposed to iodine while undergoing surgery for necrotizing enterocolitis; after age 28 days, four of these infants were found to have elevated TSH. They cautioned that while this finding emphasizes the need for close monitoring and repeat screening of infants who have been exposed to iodine for up to 1 month following exposure, they could not be certain that the iodine exposure was responsible for the transient hypothyroidism in these patients.
Dr. McGrath and her associates emphasized the importance of repeat TSH screening for all preterm infants, noting that standard protocol screenings conducted just once at age 2 weeks or 4 weeks are not sufficient to effectively identify all cases of congenital hypothyroidism in which TSH elevation is delayed. Instead, they recommended measuring TSH on days 3-5 and at 1 week, 2 weeks, 4 weeks, and term-corrected gestational age.
“Our data are consistent with studies showing a high incidence of delayed TSH rise, particularly in very-low-birth-weight infants,” the authors wrote. They speculated that delays in detecting primary congenital hypothyroidism could be caused by the suppression of TSH secretion as a result of “hypothalamic-pituitary immaturity, medication administration, and effects of serious neonatal illness.” In fact, had standard, recommended 2-week-only screening protocols been followed with their patient population, fully 48% of infants with delayed TSH elevation would have been overlooked; half of these patients were later found to have decompensated hypothyroidism.
Neurodevelopmental disability caused by congenital hypothyroidism, which affects roughly 1 in every 2,000-4,000 births, is increasingly being prevented with newborn screenings that identify the condition early, but the incidence of congenital hypothyroidism has increased considerably in the past 20 years. The authors attribute this increase to the gradual change in screening cutoff levels and the rise in number of preterm infants who are surviving.
The need for a second screening, as well as appropriate timing and optimal TSH cutoff, are “subjects of active debate,” the authors wrote. The latest European screening guidelines recommend second screenings for preterm and low-birth-weight infants at either 2 weeks of age or 2 weeks following preliminary screening. Although they make no recommendations regarding additional screenings, American Academy of Pediatrics guidelines, published in 2006, cite a “disproportionate incidence” of delayed increase in TSH and congenital hypothyroidism in infants with very low birth weight. Dr. McGrath and her associates speculated that not all screening programs have adopted repeat screening of preterm infants, perhaps because of the low yield results, “the transient nature of most cases” detected, as well as conflicting long-term data on neurodevelopmental outcomes.
Dr. McGrath receives funding from the Children’s Fund for Health. The authors declared no conflicts of interest.
SOURCE: McGrath N et al. J Pediatr. 2018 Oct 24. doi: 10.1016/j.jpeds.2018.09.044.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point: Periodic screenings are key to preventing permanent, decompensated hypothyroidism.
Major finding: High incidence of delayed TSH rise is common, especially in very-low-birth-weight infants.
Study details: A population-based prospective review of 898,424 records.
Disclosures: Dr. McGrath receives funding from the Children’s Fund for Health. The authors declared no conflicts of interest.
Source: McGrath N et al. J Pediatr. 2018 Oct 24. doi: 10.1016/j.jpeds.2018.09.044.
Endocrine Society updates guidelines for congenital adrenal hyperplasia
recently updated by the Endocrine Society.
The guidelines are an update to the 2010 Endocrine Society Clinical Practice Guideline on congenital adrenal hyperplasia (CAH) due to steroid 21-hydroxylase deficiency. They were published in The Journal of Clinical Endocrinology and Metabolism.
Richard J. Auchus, MD, PhD, of the University of Michigan, Ann Arbor, and coauthor of the 2018 guidelines, said many of the guidelines remain the same, such as use of neonatal screening. However, neonatal diagnosis methods should use gestational age and birth weight or liquid chromatography–tandem mass spectrometry for secondary screening. The authors also noted that the addition of commercially available serum 21-deoxycortisol measurements, while untested, could potentially help identify CAH carriers.
Changes in genital reconstructive surgery were also addressed in the new guidelines, and a recent systematic review and meta-analysis found a “favorable benefit to risk ratio” for both early and late genital reconstructive surgery. Dr. Auchus said the timing of the surgery remains controversial and that there were “downsides of both approaches.”
“I wish there was a straightforward and perfect solution, but I don’t think there is,” he said in an interview.
Dexamethasone for the prenatal treatment of CAH, and prenatal therapy in general is still regarded as experimental and is not recommended, Dr. Auchus said. The authors encouraged pregnant women who are considering prenatal treatment of CAH to go through Institutional Review Board–approved centers that can obtain outcomes. Pregnant women should not receive a glucocorticoid that traverses the placenta, such as dexamethasone.
Classical CAH should be treated with hydrocortisone maintenance therapy, while nonclassic CAH patients should receive glucocorticoid treatment, such as in cases of early onset and rapid progression of pubarche or bone age in children and overt virilization in adolescents.
Dr. Auchus said the new guidelines have been reorganized so information is easier to find, with recommendations beginning at birth before transitioning into recommendations for childhood and adulthood.
“I think the pediatric endocrinologists are familiar with the management of this disease, but I think a lot of the internal medicine endocrinologists don’t get much training in fellowships, and I think it will be easy for them now to find the information,” Dr. Auchus said. “[I]n the previous set of guidelines, it would’ve been difficult for them to find the information that’s scattered throughout.”
However, Dr. Auchus noted, the guidelines were careful to avoid recommendations of specific levels for analyzing biomarkers for monitoring treatment and specific doses. “[W]e gave some general ideas about ranges: that they should be low, they should be normal, they should be not very high, but it’s okay if it’s a little bit high,” he added.
Also, the evidence for the recommendations is limited to best practice guidelines because of a lack of randomized controlled trials, he noted.
“We certainly do need additional long-term data on these patients,” Dr. Auchus said. “[I]t’s our hope that with some of the networks that have been developed for studying adrenal diseases that we can collect that information in a minimally intrusive way for the benefit of all the current and future patients.”
The guidelines were funded by the Intramural Research Program of the National Institutes of Health. The authors report various personal and organizational financial interests in the form of paid consultancies, researcher support positions, advisory board memberships and investigator roles. See the full study for a complete list of disclosures.
SOURCE: Speiser PW et al. J Clin Endocrinol Metab. 2018 Sep 27. doi: 10.1210/jc.2018-01865.
recently updated by the Endocrine Society.
The guidelines are an update to the 2010 Endocrine Society Clinical Practice Guideline on congenital adrenal hyperplasia (CAH) due to steroid 21-hydroxylase deficiency. They were published in The Journal of Clinical Endocrinology and Metabolism.
Richard J. Auchus, MD, PhD, of the University of Michigan, Ann Arbor, and coauthor of the 2018 guidelines, said many of the guidelines remain the same, such as use of neonatal screening. However, neonatal diagnosis methods should use gestational age and birth weight or liquid chromatography–tandem mass spectrometry for secondary screening. The authors also noted that the addition of commercially available serum 21-deoxycortisol measurements, while untested, could potentially help identify CAH carriers.
Changes in genital reconstructive surgery were also addressed in the new guidelines, and a recent systematic review and meta-analysis found a “favorable benefit to risk ratio” for both early and late genital reconstructive surgery. Dr. Auchus said the timing of the surgery remains controversial and that there were “downsides of both approaches.”
“I wish there was a straightforward and perfect solution, but I don’t think there is,” he said in an interview.
Dexamethasone for the prenatal treatment of CAH, and prenatal therapy in general is still regarded as experimental and is not recommended, Dr. Auchus said. The authors encouraged pregnant women who are considering prenatal treatment of CAH to go through Institutional Review Board–approved centers that can obtain outcomes. Pregnant women should not receive a glucocorticoid that traverses the placenta, such as dexamethasone.
Classical CAH should be treated with hydrocortisone maintenance therapy, while nonclassic CAH patients should receive glucocorticoid treatment, such as in cases of early onset and rapid progression of pubarche or bone age in children and overt virilization in adolescents.
Dr. Auchus said the new guidelines have been reorganized so information is easier to find, with recommendations beginning at birth before transitioning into recommendations for childhood and adulthood.
“I think the pediatric endocrinologists are familiar with the management of this disease, but I think a lot of the internal medicine endocrinologists don’t get much training in fellowships, and I think it will be easy for them now to find the information,” Dr. Auchus said. “[I]n the previous set of guidelines, it would’ve been difficult for them to find the information that’s scattered throughout.”
However, Dr. Auchus noted, the guidelines were careful to avoid recommendations of specific levels for analyzing biomarkers for monitoring treatment and specific doses. “[W]e gave some general ideas about ranges: that they should be low, they should be normal, they should be not very high, but it’s okay if it’s a little bit high,” he added.
Also, the evidence for the recommendations is limited to best practice guidelines because of a lack of randomized controlled trials, he noted.
“We certainly do need additional long-term data on these patients,” Dr. Auchus said. “[I]t’s our hope that with some of the networks that have been developed for studying adrenal diseases that we can collect that information in a minimally intrusive way for the benefit of all the current and future patients.”
The guidelines were funded by the Intramural Research Program of the National Institutes of Health. The authors report various personal and organizational financial interests in the form of paid consultancies, researcher support positions, advisory board memberships and investigator roles. See the full study for a complete list of disclosures.
SOURCE: Speiser PW et al. J Clin Endocrinol Metab. 2018 Sep 27. doi: 10.1210/jc.2018-01865.
recently updated by the Endocrine Society.
The guidelines are an update to the 2010 Endocrine Society Clinical Practice Guideline on congenital adrenal hyperplasia (CAH) due to steroid 21-hydroxylase deficiency. They were published in The Journal of Clinical Endocrinology and Metabolism.
Richard J. Auchus, MD, PhD, of the University of Michigan, Ann Arbor, and coauthor of the 2018 guidelines, said many of the guidelines remain the same, such as use of neonatal screening. However, neonatal diagnosis methods should use gestational age and birth weight or liquid chromatography–tandem mass spectrometry for secondary screening. The authors also noted that the addition of commercially available serum 21-deoxycortisol measurements, while untested, could potentially help identify CAH carriers.
Changes in genital reconstructive surgery were also addressed in the new guidelines, and a recent systematic review and meta-analysis found a “favorable benefit to risk ratio” for both early and late genital reconstructive surgery. Dr. Auchus said the timing of the surgery remains controversial and that there were “downsides of both approaches.”
“I wish there was a straightforward and perfect solution, but I don’t think there is,” he said in an interview.
Dexamethasone for the prenatal treatment of CAH, and prenatal therapy in general is still regarded as experimental and is not recommended, Dr. Auchus said. The authors encouraged pregnant women who are considering prenatal treatment of CAH to go through Institutional Review Board–approved centers that can obtain outcomes. Pregnant women should not receive a glucocorticoid that traverses the placenta, such as dexamethasone.
Classical CAH should be treated with hydrocortisone maintenance therapy, while nonclassic CAH patients should receive glucocorticoid treatment, such as in cases of early onset and rapid progression of pubarche or bone age in children and overt virilization in adolescents.
Dr. Auchus said the new guidelines have been reorganized so information is easier to find, with recommendations beginning at birth before transitioning into recommendations for childhood and adulthood.
“I think the pediatric endocrinologists are familiar with the management of this disease, but I think a lot of the internal medicine endocrinologists don’t get much training in fellowships, and I think it will be easy for them now to find the information,” Dr. Auchus said. “[I]n the previous set of guidelines, it would’ve been difficult for them to find the information that’s scattered throughout.”
However, Dr. Auchus noted, the guidelines were careful to avoid recommendations of specific levels for analyzing biomarkers for monitoring treatment and specific doses. “[W]e gave some general ideas about ranges: that they should be low, they should be normal, they should be not very high, but it’s okay if it’s a little bit high,” he added.
Also, the evidence for the recommendations is limited to best practice guidelines because of a lack of randomized controlled trials, he noted.
“We certainly do need additional long-term data on these patients,” Dr. Auchus said. “[I]t’s our hope that with some of the networks that have been developed for studying adrenal diseases that we can collect that information in a minimally intrusive way for the benefit of all the current and future patients.”
The guidelines were funded by the Intramural Research Program of the National Institutes of Health. The authors report various personal and organizational financial interests in the form of paid consultancies, researcher support positions, advisory board memberships and investigator roles. See the full study for a complete list of disclosures.
SOURCE: Speiser PW et al. J Clin Endocrinol Metab. 2018 Sep 27. doi: 10.1210/jc.2018-01865.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Thyroid markers linked to risk of gestational diabetes
Thyroid dysfunction early in pregnancy may increase risk of gestational diabetes, results of a longitudinal study suggest.
Increased levels of free triiodothyronine (fT3) and the ratio of fT3 to free thyroxine (fT4) were associated with increased risk of this common metabolic complication of pregnancy, study authors reported in the Journal of Clinical Endocrinology & Metabolism.
“To our knowledge, this is the first study to identify fT3 and the fT3:fT4 ratio measured early in pregnancy as independent risk factors of gestational diabetes,” wrote Shristi Rawal, PhD, of the National Institute of Child Health and Human Development (NICHD) , and her colleagues.
Although routine thyroid function screening during pregnancy remains controversial, Dr. Rawal and colleagues said their results support the “potential benefits” of the practice, particularly in light of other recent evidence suggesting thyroid-related adverse pregnancy outcomes.
The current case control study by Dr. Rawal and her coinvestigators included 107 women with gestational diabetes and 214 nongestational diabetes controls selected from a 12-center pregnancy cohort, which included 2,802 women aged between 18 and 40 years. The thyroid markers fT3, fT4, and thyroid-stimulating hormone (TSH) were measured at four pregnancy visits, including first trimester (weeks 10-14) and second trimester (weeks 15-26).
The fT3:fT4 ratio had the strongest association with gestational diabetes. In the second trimester measurement, women in the highest quartile had an almost 14-fold increase in risk when compared to the lowest quartile, after adjusting for potential confounders including prepregnancy body mass index and diabetes family history (adjusted odds ratio, 13.60; 95% confidence interval, 3.97-46.30), Dr. Rawal and her colleagues reported. The ratio of fT3:fT4 at the first trimester was also associated with increased risk (aOR, 8.63; 95% CI, 2.87-26.00).
Similarly, fT3 was positively associated with gestational diabetes at the first trimester (aOR, 4.25; 95% CI, 1.67-10.80) and second trimester (aOR, 3.89; 95% CI, 1.50-10.10), investigators reported.
By contrast, there was no association between fT4 or TSH and gestational diabetes, they found.
“These findings, in combination with previous evidence of thyroid-related adverse pregnancy outcomes, support the benefits of thyroid screening among pregnant women in early to mid pregnancy,” senior author Cuilin Zhang, MD, MPH, PhD, of the NICHD, said in a press statement.
Thyroid function abnormalities are relatively common in pregnant women and have been associated with obstetric complications such as pregnancy loss and premature delivery, investigators noted.
Previous evidence is sparse regarding a potential link between thyroid dysfunction and gestational diabetes. There are some prospective studies that show women with hypothyroidism have an increased incidence of gestational diabetes, Dr. Rawal and her colleagues wrote. Isolated hypothyroxinema, or normal TSH and low fT4, has also been linked to increased risk in some studies, but not in others, they added.
Support for the study came from NICHD and the American Recovery and Reinvestment Act research grants. The authors reported no conflicts of interest.
SOURCE: Rawal S et al. J Clin Endocrinol Metab. 2018 Jun 7. doi: 10.1210/jc.2017-024421.
Thyroid dysfunction early in pregnancy may increase risk of gestational diabetes, results of a longitudinal study suggest.
Increased levels of free triiodothyronine (fT3) and the ratio of fT3 to free thyroxine (fT4) were associated with increased risk of this common metabolic complication of pregnancy, study authors reported in the Journal of Clinical Endocrinology & Metabolism.
“To our knowledge, this is the first study to identify fT3 and the fT3:fT4 ratio measured early in pregnancy as independent risk factors of gestational diabetes,” wrote Shristi Rawal, PhD, of the National Institute of Child Health and Human Development (NICHD) , and her colleagues.
Although routine thyroid function screening during pregnancy remains controversial, Dr. Rawal and colleagues said their results support the “potential benefits” of the practice, particularly in light of other recent evidence suggesting thyroid-related adverse pregnancy outcomes.
The current case control study by Dr. Rawal and her coinvestigators included 107 women with gestational diabetes and 214 nongestational diabetes controls selected from a 12-center pregnancy cohort, which included 2,802 women aged between 18 and 40 years. The thyroid markers fT3, fT4, and thyroid-stimulating hormone (TSH) were measured at four pregnancy visits, including first trimester (weeks 10-14) and second trimester (weeks 15-26).
The fT3:fT4 ratio had the strongest association with gestational diabetes. In the second trimester measurement, women in the highest quartile had an almost 14-fold increase in risk when compared to the lowest quartile, after adjusting for potential confounders including prepregnancy body mass index and diabetes family history (adjusted odds ratio, 13.60; 95% confidence interval, 3.97-46.30), Dr. Rawal and her colleagues reported. The ratio of fT3:fT4 at the first trimester was also associated with increased risk (aOR, 8.63; 95% CI, 2.87-26.00).
Similarly, fT3 was positively associated with gestational diabetes at the first trimester (aOR, 4.25; 95% CI, 1.67-10.80) and second trimester (aOR, 3.89; 95% CI, 1.50-10.10), investigators reported.
By contrast, there was no association between fT4 or TSH and gestational diabetes, they found.
“These findings, in combination with previous evidence of thyroid-related adverse pregnancy outcomes, support the benefits of thyroid screening among pregnant women in early to mid pregnancy,” senior author Cuilin Zhang, MD, MPH, PhD, of the NICHD, said in a press statement.
Thyroid function abnormalities are relatively common in pregnant women and have been associated with obstetric complications such as pregnancy loss and premature delivery, investigators noted.
Previous evidence is sparse regarding a potential link between thyroid dysfunction and gestational diabetes. There are some prospective studies that show women with hypothyroidism have an increased incidence of gestational diabetes, Dr. Rawal and her colleagues wrote. Isolated hypothyroxinema, or normal TSH and low fT4, has also been linked to increased risk in some studies, but not in others, they added.
Support for the study came from NICHD and the American Recovery and Reinvestment Act research grants. The authors reported no conflicts of interest.
SOURCE: Rawal S et al. J Clin Endocrinol Metab. 2018 Jun 7. doi: 10.1210/jc.2017-024421.
Thyroid dysfunction early in pregnancy may increase risk of gestational diabetes, results of a longitudinal study suggest.
Increased levels of free triiodothyronine (fT3) and the ratio of fT3 to free thyroxine (fT4) were associated with increased risk of this common metabolic complication of pregnancy, study authors reported in the Journal of Clinical Endocrinology & Metabolism.
“To our knowledge, this is the first study to identify fT3 and the fT3:fT4 ratio measured early in pregnancy as independent risk factors of gestational diabetes,” wrote Shristi Rawal, PhD, of the National Institute of Child Health and Human Development (NICHD) , and her colleagues.
Although routine thyroid function screening during pregnancy remains controversial, Dr. Rawal and colleagues said their results support the “potential benefits” of the practice, particularly in light of other recent evidence suggesting thyroid-related adverse pregnancy outcomes.
The current case control study by Dr. Rawal and her coinvestigators included 107 women with gestational diabetes and 214 nongestational diabetes controls selected from a 12-center pregnancy cohort, which included 2,802 women aged between 18 and 40 years. The thyroid markers fT3, fT4, and thyroid-stimulating hormone (TSH) were measured at four pregnancy visits, including first trimester (weeks 10-14) and second trimester (weeks 15-26).
The fT3:fT4 ratio had the strongest association with gestational diabetes. In the second trimester measurement, women in the highest quartile had an almost 14-fold increase in risk when compared to the lowest quartile, after adjusting for potential confounders including prepregnancy body mass index and diabetes family history (adjusted odds ratio, 13.60; 95% confidence interval, 3.97-46.30), Dr. Rawal and her colleagues reported. The ratio of fT3:fT4 at the first trimester was also associated with increased risk (aOR, 8.63; 95% CI, 2.87-26.00).
Similarly, fT3 was positively associated with gestational diabetes at the first trimester (aOR, 4.25; 95% CI, 1.67-10.80) and second trimester (aOR, 3.89; 95% CI, 1.50-10.10), investigators reported.
By contrast, there was no association between fT4 or TSH and gestational diabetes, they found.
“These findings, in combination with previous evidence of thyroid-related adverse pregnancy outcomes, support the benefits of thyroid screening among pregnant women in early to mid pregnancy,” senior author Cuilin Zhang, MD, MPH, PhD, of the NICHD, said in a press statement.
Thyroid function abnormalities are relatively common in pregnant women and have been associated with obstetric complications such as pregnancy loss and premature delivery, investigators noted.
Previous evidence is sparse regarding a potential link between thyroid dysfunction and gestational diabetes. There are some prospective studies that show women with hypothyroidism have an increased incidence of gestational diabetes, Dr. Rawal and her colleagues wrote. Isolated hypothyroxinema, or normal TSH and low fT4, has also been linked to increased risk in some studies, but not in others, they added.
Support for the study came from NICHD and the American Recovery and Reinvestment Act research grants. The authors reported no conflicts of interest.
SOURCE: Rawal S et al. J Clin Endocrinol Metab. 2018 Jun 7. doi: 10.1210/jc.2017-024421.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM.
Key clinical point: Thyroid dysfunction early in pregnancy may increase risk of gestational diabetes.
Major finding: The triiodothyronine to thyroxine ratio in the second trimester had the strongest association with gestational diabetes (adjusted odds ratio, 13.60; 95% confidence interval, 3.97-46.30).
Study details: A case control study including 107 gestational diabetes cases and 214 nongestational diabetes controls.
Disclosures: The authors had no disclosures. Support for the study came from the National Institute of Child Health and Human Development and the American Recovery and Reinvestment Act research grants.
Source: Rawal S et al. J Clin Endocrinol Metab. 2018 Jun 7. doi: 10.1210/jc.2017-024421.
Primary hPTH often goes unnoticed
SAN FRANCISCO – Primary hyperparathyroidism was detected in 7% of 742 patients with recurrent kidney stones at a single tertiary care clinic, and the patients’ primary care physicians may have missed the diagnosis because several affected patients’ calcium levels were in the high normal range.
Of the 53 patients diagnosed with primary hyperparathyroidism (hPTH), 72% had high normal serum calcium levels. After examining the charts of those patients, researchers found that 11 of the 53 patients (21%) had been tested for parathyroid hormone and serum calcium levels and could have been identified by their primary care physicians.
None of the 742 patients with kidney stones in the study had vitamin D deficiency or gastrointestinal malabsorption. All were tested for serum calcium and intact serum PTH, and those with hypercalcemia or high normal calcium (greater than 10 mg/dL) and elevated intact serum PTH were diagnosed with primary hPTH.
The findings emphasize “the importance of [looking] for not just outright primary hyperparathyroidism, but the ratio between PTH and calcium levels,” said Mr. Boyd.
The study received no funding. Mr. Boyd declared no relevant financial relationships.
SOURCE: Boyd C et al. AUA 2018, Abstract MP13-03.
SAN FRANCISCO – Primary hyperparathyroidism was detected in 7% of 742 patients with recurrent kidney stones at a single tertiary care clinic, and the patients’ primary care physicians may have missed the diagnosis because several affected patients’ calcium levels were in the high normal range.
Of the 53 patients diagnosed with primary hyperparathyroidism (hPTH), 72% had high normal serum calcium levels. After examining the charts of those patients, researchers found that 11 of the 53 patients (21%) had been tested for parathyroid hormone and serum calcium levels and could have been identified by their primary care physicians.
None of the 742 patients with kidney stones in the study had vitamin D deficiency or gastrointestinal malabsorption. All were tested for serum calcium and intact serum PTH, and those with hypercalcemia or high normal calcium (greater than 10 mg/dL) and elevated intact serum PTH were diagnosed with primary hPTH.
The findings emphasize “the importance of [looking] for not just outright primary hyperparathyroidism, but the ratio between PTH and calcium levels,” said Mr. Boyd.
The study received no funding. Mr. Boyd declared no relevant financial relationships.
SOURCE: Boyd C et al. AUA 2018, Abstract MP13-03.
SAN FRANCISCO – Primary hyperparathyroidism was detected in 7% of 742 patients with recurrent kidney stones at a single tertiary care clinic, and the patients’ primary care physicians may have missed the diagnosis because several affected patients’ calcium levels were in the high normal range.
Of the 53 patients diagnosed with primary hyperparathyroidism (hPTH), 72% had high normal serum calcium levels. After examining the charts of those patients, researchers found that 11 of the 53 patients (21%) had been tested for parathyroid hormone and serum calcium levels and could have been identified by their primary care physicians.
None of the 742 patients with kidney stones in the study had vitamin D deficiency or gastrointestinal malabsorption. All were tested for serum calcium and intact serum PTH, and those with hypercalcemia or high normal calcium (greater than 10 mg/dL) and elevated intact serum PTH were diagnosed with primary hPTH.
The findings emphasize “the importance of [looking] for not just outright primary hyperparathyroidism, but the ratio between PTH and calcium levels,” said Mr. Boyd.
The study received no funding. Mr. Boyd declared no relevant financial relationships.
SOURCE: Boyd C et al. AUA 2018, Abstract MP13-03.
REPORTING FROM THE AUA ANNUAL MEETING
Key clinical point: Calcium levels in the high normal range may be confounding diagnoses.
Major finding: About 20% of primary hyperparathyroidism cases could have been spotted by the primary care physician based on tests that had been ordered.
Study details: A retrospective analysis of 742 patients at a tertiary care kidney stone clinic.
Disclosures: The study received no funding. Mr. Boyd declared no relevant financial relationships.
Source: Boyd C et al. AUA 2018, Abstract MP13-03.
VIDEO: Acromegaly study reveals gender-specific differences
BOSTON – , according to study results presented at the annual meeting of the American Association of Clinical Endocrinologists.
The study was based on data for 112 patients (54 male, 58 female) operated on by one neurosurgeon between 1994 and 2016. The mean age at surgery was 43.6 years in men and 48.7 in women (P = .04), according to Talin Handa, Emory University, Atlanta, who presented the retrospective analysis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Men had higher mean IGF-1 levels (874 ng/mL vs. 716 ng/mL for women; P less than .01), and were more likely to have hypopituitarism.
Adjuvant treatment for acromegaly was needed in 57% of men and 49% of women. Following adjuvant treatment, 72% of men maintained surgical remission or achieved normal IGF-1 levels, compared with 89% of women (P = .03). Mean follow-up was shorter in men, 3.6 years, versus 5.2 years for women (P = .02), the researchers reported.
Six-year event-free survival was higher in women (P less than .01), according to the researchers.
For more study findings, watch our video interview.
SOURCE: Handa T et al. AACE 2018. Abstract #824.
BOSTON – , according to study results presented at the annual meeting of the American Association of Clinical Endocrinologists.
The study was based on data for 112 patients (54 male, 58 female) operated on by one neurosurgeon between 1994 and 2016. The mean age at surgery was 43.6 years in men and 48.7 in women (P = .04), according to Talin Handa, Emory University, Atlanta, who presented the retrospective analysis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Men had higher mean IGF-1 levels (874 ng/mL vs. 716 ng/mL for women; P less than .01), and were more likely to have hypopituitarism.
Adjuvant treatment for acromegaly was needed in 57% of men and 49% of women. Following adjuvant treatment, 72% of men maintained surgical remission or achieved normal IGF-1 levels, compared with 89% of women (P = .03). Mean follow-up was shorter in men, 3.6 years, versus 5.2 years for women (P = .02), the researchers reported.
Six-year event-free survival was higher in women (P less than .01), according to the researchers.
For more study findings, watch our video interview.
SOURCE: Handa T et al. AACE 2018. Abstract #824.
BOSTON – , according to study results presented at the annual meeting of the American Association of Clinical Endocrinologists.
The study was based on data for 112 patients (54 male, 58 female) operated on by one neurosurgeon between 1994 and 2016. The mean age at surgery was 43.6 years in men and 48.7 in women (P = .04), according to Talin Handa, Emory University, Atlanta, who presented the retrospective analysis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Men had higher mean IGF-1 levels (874 ng/mL vs. 716 ng/mL for women; P less than .01), and were more likely to have hypopituitarism.
Adjuvant treatment for acromegaly was needed in 57% of men and 49% of women. Following adjuvant treatment, 72% of men maintained surgical remission or achieved normal IGF-1 levels, compared with 89% of women (P = .03). Mean follow-up was shorter in men, 3.6 years, versus 5.2 years for women (P = .02), the researchers reported.
Six-year event-free survival was higher in women (P less than .01), according to the researchers.
For more study findings, watch our video interview.
SOURCE: Handa T et al. AACE 2018. Abstract #824.
REPORTING FROM AACE 2018
Key clinical point: Men with acromegaly present at a younger age, have higher IGF-1 levels, and achieve lower biochemical control rates than do women with the disorder.
Major finding: Men were less likely than women to have surgical remissions or normal IGF-1 levels after adjuvant treatment (72% for men and 89% for women; P = .03).
Study details: A retrospective analysis of 112 patients (54 male, 58 female) operated on by one neurosurgeon during 1994-2016.
Disclosures: The presenter had no disclosures related to the presentation.
Source: Handa T et al. AACE 2018. Abstract #824.







