User login
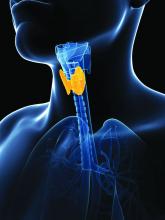
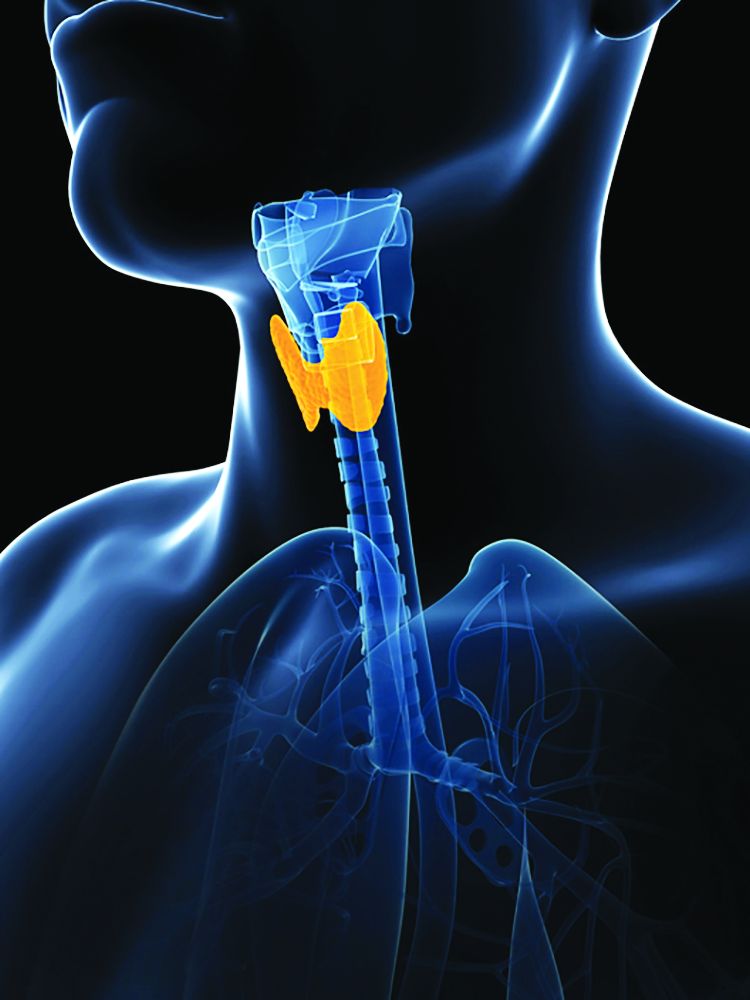
Global thyroid cancer overdiagnosis in children and adolescents
Global patterns of the incidence of thyroid cancer in children and adolescents closely correspond to the increases seen in recent decades in adults. The patterns point to the same culprit in both groups – overdiagnosis. The finding underscores recommendations to limit screening.
“Our findings suggest that recommendations against screening for thyroid cancer in the asymptomatic adult population who are free from risk factors should be extended to explicitly recommend against screening for thyroid cancer in similar populations of children and adolescents,” say the authors, led by Salvatore Vaccarella, PhD, of the International Agency for Research on Cancer, in Lyon, France.
The study was published online Jan. 19 in The Lancet Diabetes and Endocrinology.
In an accompanying comment, Livia Lamartina and colleagues from the department of nuclear medicine and endocrine oncology, Institut Gustave Roussy and the University Paris-Saclay, Villejuif, France, emphasize that unnecessary screening of thyroid cancer in children can have substantial implications.
“Overdiagnosis might transform a child into a thyroid cancer patient for the rest of their life, and overtreatment might induce complications and possibly lead to the requirement of lifelong thyroid hormone treatment,” they write.
“Therefore, screening with ultrasonography should not be recommended in asymptomatic children and adolescents,” they conclude.
Study findings
For the study, Dr. Vaccarella and colleagues evaluated the incidence of thyroid cancer in 49 countries and territories and mortality in 27 countries, using the most up-to-date data from the International Incidence of Childhood Cancer Volume 3 study, the Cancer in Five Continents database, and the World Health Organization mortality database.
Although there was considerable variability between countries, the incidence of thyroid cancer in children and adolescents aged 0-19 years increased rapidly between 1998 and 2002 and again between 2008 and 2012 in nearly all countries.
Country-specific incidence rates strongly correlated with rates in adults (r > 0.8), including the temporal aspects of the incidence rates (r > .0.6).
Of the 8049 thyroid cancers that were detected, 6935 (86.2%) were papillary carcinomas, 682 (8.5%) were follicular carcinomas, and 307 (3.8%) were medullary carcinomas, as determined on the basis of the WHO classification of thyroid carcinomas. Sixty-four tumors (0.8%) were of unspecified subtype. As is commonly observed in adults, rates were higher in girls than in boys and increased with older age for both sexes.
The strong correlation between children and adults in the timing of the increases in incidence was especially notable in countries where overdiagnosis has been identified as having a major role in the increasing thyroid cancer rates. Those countries are South Korea, the United States, Italy, France, and Australia, where 60%-90% of thyroid cancer diagnoses are attributable to overdiagnosis. Overall, the incidence of thyroid cancer was less than 1.5 per one million person-years in children younger than 10 years. There were small variations by country and sex.
Thyroid cancer mortalities remain low
Overall, the rate of thyroid cancer mortality among those younger than 20 years in each country was less than 0.1 per 10 million person-years, “corresponding to less than 10 deaths per year in all of the included countries collectively,” note Dr. Vaccarella and colleagues.
“The epidemiological pattern seen in children and adolescents mirrored that seen in adults. These findings suggest that, in affected countries and territories, there might be overdiagnosis in children and adolescents, as has been observed in adults,” they write.
The incidence of thyroid cancer in children and adolescents between 2008 and 2012 ranged from 0.4 per one million person-years in Uganda and Kenya and 13.4 per 1 million person-years in Belarus, where the increase is believed to be related to the Chernobyl nuclear power plant accident and to increased screening in the years following the accident.
Subclinical discoveries may lead to unnecessary measures
Thyroid cancer was once a rare condition. Rates began to increase steadily in the 1990s, corresponding with rapid advances in noninvasive diagnostic imaging. Currently, thyroid cancer is the fifth most diagnosed cancer worldwide in adult women and the third most common in women aged 50 years and younger.
Diagnostic measures ranging from ultrasound and MRI to fine-needle aspiration biopsy have played a large role in the increase in diagnoses. The diagnostic techniques are revealing subclinical cancers in thyroid glands that previously went undetected and that usually do not cause harm over a person’s lifetime. According to Dr. Vaccarella and colleagues, such discoveries can open the door to a wide range of unnecessary measures.
The possible consequences of overdiagnosis include unnecessary treatments, the need to undergo lifelong medical care, and potential adverse effects, which could negatively affect quality of life.
Recent research from the International Agency for Research on Cancer has indicated that there has been an “epidemic of overdiagnosis” of thyroid cancer. The pattern has even reached less affluent regions as diagnostic technologies have become widely available.
“What is surprising is the magnitude of this,” Dr. Vaccarella said in an interview.
“Without overdiagnosis, thyroid cancer would probably still be a relatively rare cancer,” he said.
The study authors have disclosed no relevant financial relationships. Dr. Lamartina has received personal, advisory board, and clinical trial principal investigator fees from Bayer, personal fees from Eisai, and clinical trial principal investigator fees from AstraZeneca. The other editorialists’ financial relationships are listed in the original article.
A version of this article first appeared on Medscape.com.
Global patterns of the incidence of thyroid cancer in children and adolescents closely correspond to the increases seen in recent decades in adults. The patterns point to the same culprit in both groups – overdiagnosis. The finding underscores recommendations to limit screening.
“Our findings suggest that recommendations against screening for thyroid cancer in the asymptomatic adult population who are free from risk factors should be extended to explicitly recommend against screening for thyroid cancer in similar populations of children and adolescents,” say the authors, led by Salvatore Vaccarella, PhD, of the International Agency for Research on Cancer, in Lyon, France.
The study was published online Jan. 19 in The Lancet Diabetes and Endocrinology.
In an accompanying comment, Livia Lamartina and colleagues from the department of nuclear medicine and endocrine oncology, Institut Gustave Roussy and the University Paris-Saclay, Villejuif, France, emphasize that unnecessary screening of thyroid cancer in children can have substantial implications.
“Overdiagnosis might transform a child into a thyroid cancer patient for the rest of their life, and overtreatment might induce complications and possibly lead to the requirement of lifelong thyroid hormone treatment,” they write.
“Therefore, screening with ultrasonography should not be recommended in asymptomatic children and adolescents,” they conclude.
Study findings
For the study, Dr. Vaccarella and colleagues evaluated the incidence of thyroid cancer in 49 countries and territories and mortality in 27 countries, using the most up-to-date data from the International Incidence of Childhood Cancer Volume 3 study, the Cancer in Five Continents database, and the World Health Organization mortality database.
Although there was considerable variability between countries, the incidence of thyroid cancer in children and adolescents aged 0-19 years increased rapidly between 1998 and 2002 and again between 2008 and 2012 in nearly all countries.
Country-specific incidence rates strongly correlated with rates in adults (r > 0.8), including the temporal aspects of the incidence rates (r > .0.6).
Of the 8049 thyroid cancers that were detected, 6935 (86.2%) were papillary carcinomas, 682 (8.5%) were follicular carcinomas, and 307 (3.8%) were medullary carcinomas, as determined on the basis of the WHO classification of thyroid carcinomas. Sixty-four tumors (0.8%) were of unspecified subtype. As is commonly observed in adults, rates were higher in girls than in boys and increased with older age for both sexes.
The strong correlation between children and adults in the timing of the increases in incidence was especially notable in countries where overdiagnosis has been identified as having a major role in the increasing thyroid cancer rates. Those countries are South Korea, the United States, Italy, France, and Australia, where 60%-90% of thyroid cancer diagnoses are attributable to overdiagnosis. Overall, the incidence of thyroid cancer was less than 1.5 per one million person-years in children younger than 10 years. There were small variations by country and sex.
Thyroid cancer mortalities remain low
Overall, the rate of thyroid cancer mortality among those younger than 20 years in each country was less than 0.1 per 10 million person-years, “corresponding to less than 10 deaths per year in all of the included countries collectively,” note Dr. Vaccarella and colleagues.
“The epidemiological pattern seen in children and adolescents mirrored that seen in adults. These findings suggest that, in affected countries and territories, there might be overdiagnosis in children and adolescents, as has been observed in adults,” they write.
The incidence of thyroid cancer in children and adolescents between 2008 and 2012 ranged from 0.4 per one million person-years in Uganda and Kenya and 13.4 per 1 million person-years in Belarus, where the increase is believed to be related to the Chernobyl nuclear power plant accident and to increased screening in the years following the accident.
Subclinical discoveries may lead to unnecessary measures
Thyroid cancer was once a rare condition. Rates began to increase steadily in the 1990s, corresponding with rapid advances in noninvasive diagnostic imaging. Currently, thyroid cancer is the fifth most diagnosed cancer worldwide in adult women and the third most common in women aged 50 years and younger.
Diagnostic measures ranging from ultrasound and MRI to fine-needle aspiration biopsy have played a large role in the increase in diagnoses. The diagnostic techniques are revealing subclinical cancers in thyroid glands that previously went undetected and that usually do not cause harm over a person’s lifetime. According to Dr. Vaccarella and colleagues, such discoveries can open the door to a wide range of unnecessary measures.
The possible consequences of overdiagnosis include unnecessary treatments, the need to undergo lifelong medical care, and potential adverse effects, which could negatively affect quality of life.
Recent research from the International Agency for Research on Cancer has indicated that there has been an “epidemic of overdiagnosis” of thyroid cancer. The pattern has even reached less affluent regions as diagnostic technologies have become widely available.
“What is surprising is the magnitude of this,” Dr. Vaccarella said in an interview.
“Without overdiagnosis, thyroid cancer would probably still be a relatively rare cancer,” he said.
The study authors have disclosed no relevant financial relationships. Dr. Lamartina has received personal, advisory board, and clinical trial principal investigator fees from Bayer, personal fees from Eisai, and clinical trial principal investigator fees from AstraZeneca. The other editorialists’ financial relationships are listed in the original article.
A version of this article first appeared on Medscape.com.
Global patterns of the incidence of thyroid cancer in children and adolescents closely correspond to the increases seen in recent decades in adults. The patterns point to the same culprit in both groups – overdiagnosis. The finding underscores recommendations to limit screening.
“Our findings suggest that recommendations against screening for thyroid cancer in the asymptomatic adult population who are free from risk factors should be extended to explicitly recommend against screening for thyroid cancer in similar populations of children and adolescents,” say the authors, led by Salvatore Vaccarella, PhD, of the International Agency for Research on Cancer, in Lyon, France.
The study was published online Jan. 19 in The Lancet Diabetes and Endocrinology.
In an accompanying comment, Livia Lamartina and colleagues from the department of nuclear medicine and endocrine oncology, Institut Gustave Roussy and the University Paris-Saclay, Villejuif, France, emphasize that unnecessary screening of thyroid cancer in children can have substantial implications.
“Overdiagnosis might transform a child into a thyroid cancer patient for the rest of their life, and overtreatment might induce complications and possibly lead to the requirement of lifelong thyroid hormone treatment,” they write.
“Therefore, screening with ultrasonography should not be recommended in asymptomatic children and adolescents,” they conclude.
Study findings
For the study, Dr. Vaccarella and colleagues evaluated the incidence of thyroid cancer in 49 countries and territories and mortality in 27 countries, using the most up-to-date data from the International Incidence of Childhood Cancer Volume 3 study, the Cancer in Five Continents database, and the World Health Organization mortality database.
Although there was considerable variability between countries, the incidence of thyroid cancer in children and adolescents aged 0-19 years increased rapidly between 1998 and 2002 and again between 2008 and 2012 in nearly all countries.
Country-specific incidence rates strongly correlated with rates in adults (r > 0.8), including the temporal aspects of the incidence rates (r > .0.6).
Of the 8049 thyroid cancers that were detected, 6935 (86.2%) were papillary carcinomas, 682 (8.5%) were follicular carcinomas, and 307 (3.8%) were medullary carcinomas, as determined on the basis of the WHO classification of thyroid carcinomas. Sixty-four tumors (0.8%) were of unspecified subtype. As is commonly observed in adults, rates were higher in girls than in boys and increased with older age for both sexes.
The strong correlation between children and adults in the timing of the increases in incidence was especially notable in countries where overdiagnosis has been identified as having a major role in the increasing thyroid cancer rates. Those countries are South Korea, the United States, Italy, France, and Australia, where 60%-90% of thyroid cancer diagnoses are attributable to overdiagnosis. Overall, the incidence of thyroid cancer was less than 1.5 per one million person-years in children younger than 10 years. There were small variations by country and sex.
Thyroid cancer mortalities remain low
Overall, the rate of thyroid cancer mortality among those younger than 20 years in each country was less than 0.1 per 10 million person-years, “corresponding to less than 10 deaths per year in all of the included countries collectively,” note Dr. Vaccarella and colleagues.
“The epidemiological pattern seen in children and adolescents mirrored that seen in adults. These findings suggest that, in affected countries and territories, there might be overdiagnosis in children and adolescents, as has been observed in adults,” they write.
The incidence of thyroid cancer in children and adolescents between 2008 and 2012 ranged from 0.4 per one million person-years in Uganda and Kenya and 13.4 per 1 million person-years in Belarus, where the increase is believed to be related to the Chernobyl nuclear power plant accident and to increased screening in the years following the accident.
Subclinical discoveries may lead to unnecessary measures
Thyroid cancer was once a rare condition. Rates began to increase steadily in the 1990s, corresponding with rapid advances in noninvasive diagnostic imaging. Currently, thyroid cancer is the fifth most diagnosed cancer worldwide in adult women and the third most common in women aged 50 years and younger.
Diagnostic measures ranging from ultrasound and MRI to fine-needle aspiration biopsy have played a large role in the increase in diagnoses. The diagnostic techniques are revealing subclinical cancers in thyroid glands that previously went undetected and that usually do not cause harm over a person’s lifetime. According to Dr. Vaccarella and colleagues, such discoveries can open the door to a wide range of unnecessary measures.
The possible consequences of overdiagnosis include unnecessary treatments, the need to undergo lifelong medical care, and potential adverse effects, which could negatively affect quality of life.
Recent research from the International Agency for Research on Cancer has indicated that there has been an “epidemic of overdiagnosis” of thyroid cancer. The pattern has even reached less affluent regions as diagnostic technologies have become widely available.
“What is surprising is the magnitude of this,” Dr. Vaccarella said in an interview.
“Without overdiagnosis, thyroid cancer would probably still be a relatively rare cancer,” he said.
The study authors have disclosed no relevant financial relationships. Dr. Lamartina has received personal, advisory board, and clinical trial principal investigator fees from Bayer, personal fees from Eisai, and clinical trial principal investigator fees from AstraZeneca. The other editorialists’ financial relationships are listed in the original article.
A version of this article first appeared on Medscape.com.
Adding liothyronine for hypothyroidism doesn’t up breast cancer risk
“An increasing number of patients ask their physicians for a prescription of combination therapy, often causing tensions. Thus, the question of whether combination therapy does any harm to patients is crucial,” say Tereza Planck, MD, PhD, of Skane University Hospital, Malmo, Sweden, and colleagues, in their article published online Jan. 5 in Thyroid.
“Our data provide reassuring evidence regarding the risk of cancer and mortality,” they stress.
Asked to comment, Caroline T. Nguyen, MD, agrees that the study results are welcome in light of some previous evidence.
“The findings of these [prior] studies were concerning as they suggested an association between T3 and breast cancer, breast cancer-specific mortality, and poorer prognosis with potential estrogen-like activity of T3 on the estrogen receptor,” Dr. Nguyen of the division of endocrinology, diabetes & metabolism at Keck Medical Center of University of Southern California, Los Angeles, told this news organization.
“Therefore, the findings of this paper provide some reassurance, which is important because, as the paper states, the use of T3 is becoming increasingly common.”
Many patients with hypothyroidism opt to add liothyronine
Although the standard treatment for hypothyroidism, levothyroxine, increases free thyroxine (T4) to high-normal levels, it may potentially lower triiodothyronine (T3) to relatively low levels. There is speculation that the imbalance in a subset of patients could explain why some fail to have an adequate reduction of symptoms with levothyroxine alone.
To offset the effect, some add liothyronine (a synthetic version of T3) to levothyroxine treatment as so-called “combination therapy.” However, a long-term study conducted in Scotland showed a borderline significant increase in breast cancer risk with the combination, raising concern.
To further investigate, Dr. Planck and coauthors used Swedish adult population data, identifying 575,461 individuals who had made at least three purchases of thyroid hormone therapy between July 2005 and December 2017, and had no history of breast cancer at the time of their first prescription.
Among the individuals, 11,147 had made at least three purchases of LT3, including combinations with LT4. LT4-only users were an average age of 54.4 years, and the average age of those who also took LT3 was 44.7 years.
Over a median follow-up of 8.1 years, there was no significantly increased risk of breast cancer among women treated with LT3 plus LT4 versus LT4 alone (hazard ratio, 0.93), after adjusting for differences in age, sex, previous thyroid cancer, previous other cancer, use of antithyroid preparations, use of sex hormones, and dose.
Further evaluation of women as well as men showed those treated with LT3 also had no increased incidence of any cancer (HR, 0.97).
In dose-adjusted models, LT3 treatment did, surprisingly, appear to have a protective effect in terms of all-cause mortality (HR, 0.69) and any cancer mortality (HR, 0.78) for men and women.
However, the implications of these latter results remain uncertain, first author Dr. Planck said in an interview.
“We think the data on reduced mortality should be interpreted with caution, as we only observe the differences in the models adjusting for dose,” she noted.
LT3 treatment still considered ‘experimental’
Despite the dramatic increase in LT3 prescribing in recent years noted by the authors, as many as five systematic reviews/meta-analyses have shown no superiority of combination therapy over LT4 alone in terms of hypothyroid symptoms, quality of life, or patient preference.
As a result, many international guidelines still consider the combination-treatment approach to be experimental.
Other trials that have raised concerns about the combination include previous large, prospective Swedish studies that have linked higher endogenous T3 levels to breast cancer in postmenopausal women.
As for the mechanism, some small experimental studies have suggested an estrogenlike effect whereby T3 could enhance the proliferation of breast cancer cells.
On a broader level, thyroid hormones, in general, have been extensively studied in cancer research as possibly promoting cancer cell proliferation in a variety of cancer types.
However, the current findings should lay some of those concerns to rest, Dr. Planck reiterated: “Our data provide reassuring evidence regarding the risk of cancer and mortality.”
“We did not identify any increase in breast cancer incidence, any cancer incidence, all-cause mortality, any cancer mortality, or breast cancer mortality between individuals using LT3 and LT4 treatment.”
The authors and Nguyen have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“An increasing number of patients ask their physicians for a prescription of combination therapy, often causing tensions. Thus, the question of whether combination therapy does any harm to patients is crucial,” say Tereza Planck, MD, PhD, of Skane University Hospital, Malmo, Sweden, and colleagues, in their article published online Jan. 5 in Thyroid.
“Our data provide reassuring evidence regarding the risk of cancer and mortality,” they stress.
Asked to comment, Caroline T. Nguyen, MD, agrees that the study results are welcome in light of some previous evidence.
“The findings of these [prior] studies were concerning as they suggested an association between T3 and breast cancer, breast cancer-specific mortality, and poorer prognosis with potential estrogen-like activity of T3 on the estrogen receptor,” Dr. Nguyen of the division of endocrinology, diabetes & metabolism at Keck Medical Center of University of Southern California, Los Angeles, told this news organization.
“Therefore, the findings of this paper provide some reassurance, which is important because, as the paper states, the use of T3 is becoming increasingly common.”
Many patients with hypothyroidism opt to add liothyronine
Although the standard treatment for hypothyroidism, levothyroxine, increases free thyroxine (T4) to high-normal levels, it may potentially lower triiodothyronine (T3) to relatively low levels. There is speculation that the imbalance in a subset of patients could explain why some fail to have an adequate reduction of symptoms with levothyroxine alone.
To offset the effect, some add liothyronine (a synthetic version of T3) to levothyroxine treatment as so-called “combination therapy.” However, a long-term study conducted in Scotland showed a borderline significant increase in breast cancer risk with the combination, raising concern.
To further investigate, Dr. Planck and coauthors used Swedish adult population data, identifying 575,461 individuals who had made at least three purchases of thyroid hormone therapy between July 2005 and December 2017, and had no history of breast cancer at the time of their first prescription.
Among the individuals, 11,147 had made at least three purchases of LT3, including combinations with LT4. LT4-only users were an average age of 54.4 years, and the average age of those who also took LT3 was 44.7 years.
Over a median follow-up of 8.1 years, there was no significantly increased risk of breast cancer among women treated with LT3 plus LT4 versus LT4 alone (hazard ratio, 0.93), after adjusting for differences in age, sex, previous thyroid cancer, previous other cancer, use of antithyroid preparations, use of sex hormones, and dose.
Further evaluation of women as well as men showed those treated with LT3 also had no increased incidence of any cancer (HR, 0.97).
In dose-adjusted models, LT3 treatment did, surprisingly, appear to have a protective effect in terms of all-cause mortality (HR, 0.69) and any cancer mortality (HR, 0.78) for men and women.
However, the implications of these latter results remain uncertain, first author Dr. Planck said in an interview.
“We think the data on reduced mortality should be interpreted with caution, as we only observe the differences in the models adjusting for dose,” she noted.
LT3 treatment still considered ‘experimental’
Despite the dramatic increase in LT3 prescribing in recent years noted by the authors, as many as five systematic reviews/meta-analyses have shown no superiority of combination therapy over LT4 alone in terms of hypothyroid symptoms, quality of life, or patient preference.
As a result, many international guidelines still consider the combination-treatment approach to be experimental.
Other trials that have raised concerns about the combination include previous large, prospective Swedish studies that have linked higher endogenous T3 levels to breast cancer in postmenopausal women.
As for the mechanism, some small experimental studies have suggested an estrogenlike effect whereby T3 could enhance the proliferation of breast cancer cells.
On a broader level, thyroid hormones, in general, have been extensively studied in cancer research as possibly promoting cancer cell proliferation in a variety of cancer types.
However, the current findings should lay some of those concerns to rest, Dr. Planck reiterated: “Our data provide reassuring evidence regarding the risk of cancer and mortality.”
“We did not identify any increase in breast cancer incidence, any cancer incidence, all-cause mortality, any cancer mortality, or breast cancer mortality between individuals using LT3 and LT4 treatment.”
The authors and Nguyen have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“An increasing number of patients ask their physicians for a prescription of combination therapy, often causing tensions. Thus, the question of whether combination therapy does any harm to patients is crucial,” say Tereza Planck, MD, PhD, of Skane University Hospital, Malmo, Sweden, and colleagues, in their article published online Jan. 5 in Thyroid.
“Our data provide reassuring evidence regarding the risk of cancer and mortality,” they stress.
Asked to comment, Caroline T. Nguyen, MD, agrees that the study results are welcome in light of some previous evidence.
“The findings of these [prior] studies were concerning as they suggested an association between T3 and breast cancer, breast cancer-specific mortality, and poorer prognosis with potential estrogen-like activity of T3 on the estrogen receptor,” Dr. Nguyen of the division of endocrinology, diabetes & metabolism at Keck Medical Center of University of Southern California, Los Angeles, told this news organization.
“Therefore, the findings of this paper provide some reassurance, which is important because, as the paper states, the use of T3 is becoming increasingly common.”
Many patients with hypothyroidism opt to add liothyronine
Although the standard treatment for hypothyroidism, levothyroxine, increases free thyroxine (T4) to high-normal levels, it may potentially lower triiodothyronine (T3) to relatively low levels. There is speculation that the imbalance in a subset of patients could explain why some fail to have an adequate reduction of symptoms with levothyroxine alone.
To offset the effect, some add liothyronine (a synthetic version of T3) to levothyroxine treatment as so-called “combination therapy.” However, a long-term study conducted in Scotland showed a borderline significant increase in breast cancer risk with the combination, raising concern.
To further investigate, Dr. Planck and coauthors used Swedish adult population data, identifying 575,461 individuals who had made at least three purchases of thyroid hormone therapy between July 2005 and December 2017, and had no history of breast cancer at the time of their first prescription.
Among the individuals, 11,147 had made at least three purchases of LT3, including combinations with LT4. LT4-only users were an average age of 54.4 years, and the average age of those who also took LT3 was 44.7 years.
Over a median follow-up of 8.1 years, there was no significantly increased risk of breast cancer among women treated with LT3 plus LT4 versus LT4 alone (hazard ratio, 0.93), after adjusting for differences in age, sex, previous thyroid cancer, previous other cancer, use of antithyroid preparations, use of sex hormones, and dose.
Further evaluation of women as well as men showed those treated with LT3 also had no increased incidence of any cancer (HR, 0.97).
In dose-adjusted models, LT3 treatment did, surprisingly, appear to have a protective effect in terms of all-cause mortality (HR, 0.69) and any cancer mortality (HR, 0.78) for men and women.
However, the implications of these latter results remain uncertain, first author Dr. Planck said in an interview.
“We think the data on reduced mortality should be interpreted with caution, as we only observe the differences in the models adjusting for dose,” she noted.
LT3 treatment still considered ‘experimental’
Despite the dramatic increase in LT3 prescribing in recent years noted by the authors, as many as five systematic reviews/meta-analyses have shown no superiority of combination therapy over LT4 alone in terms of hypothyroid symptoms, quality of life, or patient preference.
As a result, many international guidelines still consider the combination-treatment approach to be experimental.
Other trials that have raised concerns about the combination include previous large, prospective Swedish studies that have linked higher endogenous T3 levels to breast cancer in postmenopausal women.
As for the mechanism, some small experimental studies have suggested an estrogenlike effect whereby T3 could enhance the proliferation of breast cancer cells.
On a broader level, thyroid hormones, in general, have been extensively studied in cancer research as possibly promoting cancer cell proliferation in a variety of cancer types.
However, the current findings should lay some of those concerns to rest, Dr. Planck reiterated: “Our data provide reassuring evidence regarding the risk of cancer and mortality.”
“We did not identify any increase in breast cancer incidence, any cancer incidence, all-cause mortality, any cancer mortality, or breast cancer mortality between individuals using LT3 and LT4 treatment.”
The authors and Nguyen have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Don’t miss postpartum thyroiditis
All patients with postpartum depression should be screened for thyroid dysfunction, as postpartum thyroiditis is often missed and misdiagnosed, according to Christine Kessler, CNS, ANP.
Postpartum thyroiditis (PPT) is “an inflammatory, autoimmune thyroid condition,” Ms. Kessler said at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education. This dysfunction can involve high or low thyroid-stimulating hormone and may occur during the first postpartum year in women who were euthyroid prior to pregnancy. Women with PPT will be thyroid peroxidase (TPO) antibody positive. Postpartum thyroiditis also can occur after a miscarriage.
PPT can occur when the immune system rebounds after pregnancy following immune suppression during pregnancy. “Autoimmune destruction of the thyroid gland leads to initial release of stored thyroid hormone,” Ms. Kessler said. Notably, “patients with a predisposition for Hashimoto’s will have an attack on the thyroid gland. Don’t miss this in your patients.”
PPT is the most common endocrine disease in premenopausal women, with an incidence of 8%-14% in the United States, noted Ms. Kessler, a nurse practitioner in private practice in Virginia. However, the symptoms are often attributed to anxiety, depression, or the stress of new motherhood.
Women with PPT have positive thyroid peroxidase antibodies, said Ms. Kessler, and the higher the antibody, the higher the risk for PPT. Other risk factors include the presence of autoimmune disorders prior to pregnancy, a patient or family history of thyroid dysfunction, and a history of PPT.
Roughly one-third of women with PPT present with hyperthyroidism alone, another third present with hypothyroidism alone, and another third have the classic presentation of PPT, which starts with a transient hyperthyroid phase that usually occurs 1-4 months post partum, followed by a hypothyroid phase and euthyroid phase that is usually achieved within the first 12-18 months post partum, she said.
Patients presenting with PPT in the hyperthyroid phase display symptoms including insomnia, anxiety, irritability, heat intolerance, fatigue, and palpitations, Ms. Kessler said. These women “are often told they have postpartum depression; they aren’t sleeping well, and they feel like they are failing as a mom.”
Patients in the hypothyroid phase may present with fatigue, depression, cold intolerance, dry skin, impaired concentration, and paresthesias, she noted.
Treatment for PPT depends on the stage patients are in when they present. For patients in the hyperthyroid phase, Ms. Kessler recommended beta-blockers for relief of symptoms including tremor and palpitations, but these should be tapered as symptoms decrease. “There is no need for antithyroid drugs for women in the hyperthyroid phase.”
For patients presenting in the hypothyroid phase, Ms. Kessler recommended levothyroxine for 6-12 months if needed, but the drug should be tapered and discontinued after PPT, as about 80% of patients will become euthyroid. However, approximately 50% of women with PPT will develop hypothyroidism in 2-10 years, so ongoing follow-up is essential for these patients.
Ms. Kessler disclosed serving as an adviser/speaker for Novo Nordisk, serving as a speaker for Salix and Acella, and serving as National Study Chair of probiotic use with antibiotics for Clarion Brand. Global Academy and this news organization are owned by the same parent company.
All patients with postpartum depression should be screened for thyroid dysfunction, as postpartum thyroiditis is often missed and misdiagnosed, according to Christine Kessler, CNS, ANP.
Postpartum thyroiditis (PPT) is “an inflammatory, autoimmune thyroid condition,” Ms. Kessler said at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education. This dysfunction can involve high or low thyroid-stimulating hormone and may occur during the first postpartum year in women who were euthyroid prior to pregnancy. Women with PPT will be thyroid peroxidase (TPO) antibody positive. Postpartum thyroiditis also can occur after a miscarriage.
PPT can occur when the immune system rebounds after pregnancy following immune suppression during pregnancy. “Autoimmune destruction of the thyroid gland leads to initial release of stored thyroid hormone,” Ms. Kessler said. Notably, “patients with a predisposition for Hashimoto’s will have an attack on the thyroid gland. Don’t miss this in your patients.”
PPT is the most common endocrine disease in premenopausal women, with an incidence of 8%-14% in the United States, noted Ms. Kessler, a nurse practitioner in private practice in Virginia. However, the symptoms are often attributed to anxiety, depression, or the stress of new motherhood.
Women with PPT have positive thyroid peroxidase antibodies, said Ms. Kessler, and the higher the antibody, the higher the risk for PPT. Other risk factors include the presence of autoimmune disorders prior to pregnancy, a patient or family history of thyroid dysfunction, and a history of PPT.
Roughly one-third of women with PPT present with hyperthyroidism alone, another third present with hypothyroidism alone, and another third have the classic presentation of PPT, which starts with a transient hyperthyroid phase that usually occurs 1-4 months post partum, followed by a hypothyroid phase and euthyroid phase that is usually achieved within the first 12-18 months post partum, she said.
Patients presenting with PPT in the hyperthyroid phase display symptoms including insomnia, anxiety, irritability, heat intolerance, fatigue, and palpitations, Ms. Kessler said. These women “are often told they have postpartum depression; they aren’t sleeping well, and they feel like they are failing as a mom.”
Patients in the hypothyroid phase may present with fatigue, depression, cold intolerance, dry skin, impaired concentration, and paresthesias, she noted.
Treatment for PPT depends on the stage patients are in when they present. For patients in the hyperthyroid phase, Ms. Kessler recommended beta-blockers for relief of symptoms including tremor and palpitations, but these should be tapered as symptoms decrease. “There is no need for antithyroid drugs for women in the hyperthyroid phase.”
For patients presenting in the hypothyroid phase, Ms. Kessler recommended levothyroxine for 6-12 months if needed, but the drug should be tapered and discontinued after PPT, as about 80% of patients will become euthyroid. However, approximately 50% of women with PPT will develop hypothyroidism in 2-10 years, so ongoing follow-up is essential for these patients.
Ms. Kessler disclosed serving as an adviser/speaker for Novo Nordisk, serving as a speaker for Salix and Acella, and serving as National Study Chair of probiotic use with antibiotics for Clarion Brand. Global Academy and this news organization are owned by the same parent company.
All patients with postpartum depression should be screened for thyroid dysfunction, as postpartum thyroiditis is often missed and misdiagnosed, according to Christine Kessler, CNS, ANP.
Postpartum thyroiditis (PPT) is “an inflammatory, autoimmune thyroid condition,” Ms. Kessler said at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education. This dysfunction can involve high or low thyroid-stimulating hormone and may occur during the first postpartum year in women who were euthyroid prior to pregnancy. Women with PPT will be thyroid peroxidase (TPO) antibody positive. Postpartum thyroiditis also can occur after a miscarriage.
PPT can occur when the immune system rebounds after pregnancy following immune suppression during pregnancy. “Autoimmune destruction of the thyroid gland leads to initial release of stored thyroid hormone,” Ms. Kessler said. Notably, “patients with a predisposition for Hashimoto’s will have an attack on the thyroid gland. Don’t miss this in your patients.”
PPT is the most common endocrine disease in premenopausal women, with an incidence of 8%-14% in the United States, noted Ms. Kessler, a nurse practitioner in private practice in Virginia. However, the symptoms are often attributed to anxiety, depression, or the stress of new motherhood.
Women with PPT have positive thyroid peroxidase antibodies, said Ms. Kessler, and the higher the antibody, the higher the risk for PPT. Other risk factors include the presence of autoimmune disorders prior to pregnancy, a patient or family history of thyroid dysfunction, and a history of PPT.
Roughly one-third of women with PPT present with hyperthyroidism alone, another third present with hypothyroidism alone, and another third have the classic presentation of PPT, which starts with a transient hyperthyroid phase that usually occurs 1-4 months post partum, followed by a hypothyroid phase and euthyroid phase that is usually achieved within the first 12-18 months post partum, she said.
Patients presenting with PPT in the hyperthyroid phase display symptoms including insomnia, anxiety, irritability, heat intolerance, fatigue, and palpitations, Ms. Kessler said. These women “are often told they have postpartum depression; they aren’t sleeping well, and they feel like they are failing as a mom.”
Patients in the hypothyroid phase may present with fatigue, depression, cold intolerance, dry skin, impaired concentration, and paresthesias, she noted.
Treatment for PPT depends on the stage patients are in when they present. For patients in the hyperthyroid phase, Ms. Kessler recommended beta-blockers for relief of symptoms including tremor and palpitations, but these should be tapered as symptoms decrease. “There is no need for antithyroid drugs for women in the hyperthyroid phase.”
For patients presenting in the hypothyroid phase, Ms. Kessler recommended levothyroxine for 6-12 months if needed, but the drug should be tapered and discontinued after PPT, as about 80% of patients will become euthyroid. However, approximately 50% of women with PPT will develop hypothyroidism in 2-10 years, so ongoing follow-up is essential for these patients.
Ms. Kessler disclosed serving as an adviser/speaker for Novo Nordisk, serving as a speaker for Salix and Acella, and serving as National Study Chair of probiotic use with antibiotics for Clarion Brand. Global Academy and this news organization are owned by the same parent company.
FROM MEDS 2020
New eGFR equation ‘less biased’ by age, kidney function; some disagree
The European Kidney Function Consortium (EKFC) equation surpasses existing equations by “resulting in generally lower bias across the spectrum of age and kidney function,” its developers wrote in an article published online Nov. 9 in Annals of Internal Medicine.
“The new EKFC equation may have helpful properties and perform better in estimating GFR, compared with the current KDIGO [Kidney Disease: Improving Global Outcomes]-recommended equations,” they added.
The primary KDIGO-recommended equation in its most recent guideline was the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, designed for adults, and a companion equation, the CKiD, covers children and adolescents.
“Key in our [new] equation is the adjustment for differences in serum creatinine generation between children and adults, or between men and women,” lead author Hans Pottel, PhD, KU Leuven (Belgium), said in an interview.
In an accompanying editorial, Andrew M. Levey, MD, and associates wrote: “We agree that a single eGFR equation that can be used in children and adults and performs well in the transition from adolescence to young adulthood is a worthy goal.”
“But the claim of equivalent or superior performance, compared with the CKD-EPI equation is not conclusive,” claimed Dr. Levey, who led the research team that developed the CKD-EPI equation, and coauthors.
Dr. Levey is professor of medicine at Tufts University, Boston.
What’s new is Q
Dr. Pottel and codevelopers devised what they call Q values: age- and sex-dependent median creatinine levels in normal individuals.
Q values act to “normalize or rescale creatinine before entering it into the equation, because we know that creatinine generation is different” based on factors that include age, sex, and muscle mass.
The EKFC equation extends the CKD-EPI equation and first eGFR equation by using Q values and applying across age ranges, like the full-age spectrum (FAS) equation, first reported in 2016 by a team led by Dr. Pottel.
“Although the FAS equation was designed to overcome the challenge in measuring GFR in patients transitioning from adolescence to adult nephrology care, it also underestimates GFR at low serum creatinine values and in patients with chronic kidney disease,” wrote Dr. Pottel and coauthors.
Hence, their intent to tweak the FAS equation to overcome this limitation and create the EKFC equation.
“The new equation combines the strengths of the CKD-EPI and FAS equations,” they woite.
However, “we acknowledge that lack of precision is still a major problem with all eGFR equations,” including the new EKFC, they added.
Editorialists dispute better performance of EKFC over CKD-EPI
In their editorial, Dr. Levey and coauthors noted the EKFC equations and other adapted equations in development “represent a conceptual advance over the FAS equations,” but they dispute the claims of better performance, compared with the CKD-EPI.
“We compared the performance of the EKFC and CKD-EPI equations in a different, large external validation population of Black and non-Black adults,” the external population used to validate the CKD-EPI equation, the editorialists reported.
The upshot was “our results did not confirm the author’s conclusions” about the EKFC equation.
In response, Dr. Pottel highlighted that the EKFC equation is currently not designed for use in Black patients.
“With its derivation and validation now reported in the new article, the EKFC equation is fully validated and ready for routine use in Whites,” he said. “We plan to evaluate and possibly fine tune our equation for its application in other ethnicities.”
Regarding the inferior performance, compared with the CKD-EPI equation in the non-Black population tested by the editorialists, Dr. Pottel cited “calibration issues for serum creatinine” that some experts have found in the datasets compiled by developers of the CKI-EPI equation that could limit the utility of these data.
Still room for improvement; app hopefully coming next year
Dr. Pottel and coauthors developed and validated the EKFC equation with data from 19,629 patients drawn from 13 cohorts. This included 11,251 patients from seven cohorts for development and internal validation, and 8378 from six cohorts for external validation. The EKFC effort received endorsement from the European Renal Association–European Dialysis and Transplant Association.
However, “We acknowledge that there is still room for improvement,” Dr. Pottel said.
Although the new report presents the EKFC equations (actually two slightly different equations depending on whether a patient’s serum creatinine is higher or lower than the relevant Q value), most potential users will likely find the equations easier to work with once they’re in an app form that allows someone to simply plug in age, sex, and serum creatinine level. That app currently doesn’t exist but is coming soon, promised Dr. Pottel.
“I hope to have an electronic tool by the beginning of 2021,” he said. “I have to find a programmer who can do this for me.”
The EKFC project has received no commercial funding. Dr. Pottel reported no relevant financial relationships. Dr. Levey has reported receiving research funding from AstraZeneca.
A version of this article originally appeared on Medscape.com.
The European Kidney Function Consortium (EKFC) equation surpasses existing equations by “resulting in generally lower bias across the spectrum of age and kidney function,” its developers wrote in an article published online Nov. 9 in Annals of Internal Medicine.
“The new EKFC equation may have helpful properties and perform better in estimating GFR, compared with the current KDIGO [Kidney Disease: Improving Global Outcomes]-recommended equations,” they added.
The primary KDIGO-recommended equation in its most recent guideline was the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, designed for adults, and a companion equation, the CKiD, covers children and adolescents.
“Key in our [new] equation is the adjustment for differences in serum creatinine generation between children and adults, or between men and women,” lead author Hans Pottel, PhD, KU Leuven (Belgium), said in an interview.
In an accompanying editorial, Andrew M. Levey, MD, and associates wrote: “We agree that a single eGFR equation that can be used in children and adults and performs well in the transition from adolescence to young adulthood is a worthy goal.”
“But the claim of equivalent or superior performance, compared with the CKD-EPI equation is not conclusive,” claimed Dr. Levey, who led the research team that developed the CKD-EPI equation, and coauthors.
Dr. Levey is professor of medicine at Tufts University, Boston.
What’s new is Q
Dr. Pottel and codevelopers devised what they call Q values: age- and sex-dependent median creatinine levels in normal individuals.
Q values act to “normalize or rescale creatinine before entering it into the equation, because we know that creatinine generation is different” based on factors that include age, sex, and muscle mass.
The EKFC equation extends the CKD-EPI equation and first eGFR equation by using Q values and applying across age ranges, like the full-age spectrum (FAS) equation, first reported in 2016 by a team led by Dr. Pottel.
“Although the FAS equation was designed to overcome the challenge in measuring GFR in patients transitioning from adolescence to adult nephrology care, it also underestimates GFR at low serum creatinine values and in patients with chronic kidney disease,” wrote Dr. Pottel and coauthors.
Hence, their intent to tweak the FAS equation to overcome this limitation and create the EKFC equation.
“The new equation combines the strengths of the CKD-EPI and FAS equations,” they woite.
However, “we acknowledge that lack of precision is still a major problem with all eGFR equations,” including the new EKFC, they added.
Editorialists dispute better performance of EKFC over CKD-EPI
In their editorial, Dr. Levey and coauthors noted the EKFC equations and other adapted equations in development “represent a conceptual advance over the FAS equations,” but they dispute the claims of better performance, compared with the CKD-EPI.
“We compared the performance of the EKFC and CKD-EPI equations in a different, large external validation population of Black and non-Black adults,” the external population used to validate the CKD-EPI equation, the editorialists reported.
The upshot was “our results did not confirm the author’s conclusions” about the EKFC equation.
In response, Dr. Pottel highlighted that the EKFC equation is currently not designed for use in Black patients.
“With its derivation and validation now reported in the new article, the EKFC equation is fully validated and ready for routine use in Whites,” he said. “We plan to evaluate and possibly fine tune our equation for its application in other ethnicities.”
Regarding the inferior performance, compared with the CKD-EPI equation in the non-Black population tested by the editorialists, Dr. Pottel cited “calibration issues for serum creatinine” that some experts have found in the datasets compiled by developers of the CKI-EPI equation that could limit the utility of these data.
Still room for improvement; app hopefully coming next year
Dr. Pottel and coauthors developed and validated the EKFC equation with data from 19,629 patients drawn from 13 cohorts. This included 11,251 patients from seven cohorts for development and internal validation, and 8378 from six cohorts for external validation. The EKFC effort received endorsement from the European Renal Association–European Dialysis and Transplant Association.
However, “We acknowledge that there is still room for improvement,” Dr. Pottel said.
Although the new report presents the EKFC equations (actually two slightly different equations depending on whether a patient’s serum creatinine is higher or lower than the relevant Q value), most potential users will likely find the equations easier to work with once they’re in an app form that allows someone to simply plug in age, sex, and serum creatinine level. That app currently doesn’t exist but is coming soon, promised Dr. Pottel.
“I hope to have an electronic tool by the beginning of 2021,” he said. “I have to find a programmer who can do this for me.”
The EKFC project has received no commercial funding. Dr. Pottel reported no relevant financial relationships. Dr. Levey has reported receiving research funding from AstraZeneca.
A version of this article originally appeared on Medscape.com.
The European Kidney Function Consortium (EKFC) equation surpasses existing equations by “resulting in generally lower bias across the spectrum of age and kidney function,” its developers wrote in an article published online Nov. 9 in Annals of Internal Medicine.
“The new EKFC equation may have helpful properties and perform better in estimating GFR, compared with the current KDIGO [Kidney Disease: Improving Global Outcomes]-recommended equations,” they added.
The primary KDIGO-recommended equation in its most recent guideline was the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, designed for adults, and a companion equation, the CKiD, covers children and adolescents.
“Key in our [new] equation is the adjustment for differences in serum creatinine generation between children and adults, or between men and women,” lead author Hans Pottel, PhD, KU Leuven (Belgium), said in an interview.
In an accompanying editorial, Andrew M. Levey, MD, and associates wrote: “We agree that a single eGFR equation that can be used in children and adults and performs well in the transition from adolescence to young adulthood is a worthy goal.”
“But the claim of equivalent or superior performance, compared with the CKD-EPI equation is not conclusive,” claimed Dr. Levey, who led the research team that developed the CKD-EPI equation, and coauthors.
Dr. Levey is professor of medicine at Tufts University, Boston.
What’s new is Q
Dr. Pottel and codevelopers devised what they call Q values: age- and sex-dependent median creatinine levels in normal individuals.
Q values act to “normalize or rescale creatinine before entering it into the equation, because we know that creatinine generation is different” based on factors that include age, sex, and muscle mass.
The EKFC equation extends the CKD-EPI equation and first eGFR equation by using Q values and applying across age ranges, like the full-age spectrum (FAS) equation, first reported in 2016 by a team led by Dr. Pottel.
“Although the FAS equation was designed to overcome the challenge in measuring GFR in patients transitioning from adolescence to adult nephrology care, it also underestimates GFR at low serum creatinine values and in patients with chronic kidney disease,” wrote Dr. Pottel and coauthors.
Hence, their intent to tweak the FAS equation to overcome this limitation and create the EKFC equation.
“The new equation combines the strengths of the CKD-EPI and FAS equations,” they woite.
However, “we acknowledge that lack of precision is still a major problem with all eGFR equations,” including the new EKFC, they added.
Editorialists dispute better performance of EKFC over CKD-EPI
In their editorial, Dr. Levey and coauthors noted the EKFC equations and other adapted equations in development “represent a conceptual advance over the FAS equations,” but they dispute the claims of better performance, compared with the CKD-EPI.
“We compared the performance of the EKFC and CKD-EPI equations in a different, large external validation population of Black and non-Black adults,” the external population used to validate the CKD-EPI equation, the editorialists reported.
The upshot was “our results did not confirm the author’s conclusions” about the EKFC equation.
In response, Dr. Pottel highlighted that the EKFC equation is currently not designed for use in Black patients.
“With its derivation and validation now reported in the new article, the EKFC equation is fully validated and ready for routine use in Whites,” he said. “We plan to evaluate and possibly fine tune our equation for its application in other ethnicities.”
Regarding the inferior performance, compared with the CKD-EPI equation in the non-Black population tested by the editorialists, Dr. Pottel cited “calibration issues for serum creatinine” that some experts have found in the datasets compiled by developers of the CKI-EPI equation that could limit the utility of these data.
Still room for improvement; app hopefully coming next year
Dr. Pottel and coauthors developed and validated the EKFC equation with data from 19,629 patients drawn from 13 cohorts. This included 11,251 patients from seven cohorts for development and internal validation, and 8378 from six cohorts for external validation. The EKFC effort received endorsement from the European Renal Association–European Dialysis and Transplant Association.
However, “We acknowledge that there is still room for improvement,” Dr. Pottel said.
Although the new report presents the EKFC equations (actually two slightly different equations depending on whether a patient’s serum creatinine is higher or lower than the relevant Q value), most potential users will likely find the equations easier to work with once they’re in an app form that allows someone to simply plug in age, sex, and serum creatinine level. That app currently doesn’t exist but is coming soon, promised Dr. Pottel.
“I hope to have an electronic tool by the beginning of 2021,” he said. “I have to find a programmer who can do this for me.”
The EKFC project has received no commercial funding. Dr. Pottel reported no relevant financial relationships. Dr. Levey has reported receiving research funding from AstraZeneca.
A version of this article originally appeared on Medscape.com.
No link shown between thyroid dysfunction and heart failure
Thyroid dysfunction had virtually no independent impact on survival in a retrospective study of nearly 5,000 English patients with chronic heart failure, adding to evidence that subclinical thyroid disorders in these patients requires no special management beyond ongoing monitoring.
“Although thyroid dysfunction is related to outcome in patients with chronic heart failure, the association disappears when adjustment is made for established prognostic variables, such as age, NT-proBNP [N-terminal of the prohormone brain natriuretic peptide], and [New York Heart Association] class,” wrote Nathan A. Samuel, MBChB, and coauthors in the American Journal of Cardiology.
Results from several earlier studies had shown evidence for reduced survival in heart failure patients with thyroid dysfunction, but in analyses that did not adjust for heart failure severity, such as a 2013 report that used data from the Sudden Cardiac Death in Heart Failure Trial SCD-HeFT. Other studies that adjusted for heart failure severity based on serum level of natriuretic peptides did not show significant associations between thyroid function and mortality, and when those results couple with the new report they together minimize the immediate risk from subclinical thyroid dysfunction faced by heart failure patients, wrote the authors of the new report.
Don’t treat subclinical thyroid dysfunction
“Our results suggest that subclinical thyroid disease has little impact on outcomes, and that we should not treat subclinical hypothyroidism in the expectation of improving outlook,” said Andrew L. Clark, MD, senior author on the new report and professor and head of the department of academic cardiology at Hull (England) York Medical School.
“Both hyper-and hypothyroidism can cause heart failure, so thyroid function should always be checked in patients when they present with heart failure. A small proportion of patients have heart failure that is potentially reversible” with thyroid-directed treatment, Dr. Clark said in an interview.
But “subclinical disease should probably not be treated, although we have not conducted a clinical trial that proves this assertion. We speculate, based on our findings, that such a trial is unlikely to be positive.”
Patients with subclinical thyroid disorders, particularly subclinical hypothyroidism, “need to be followed and treated should they develop clinical disease,” he maintained. “Except in extreme circumstances, such as the handful of patients who might have gross myxedema and may be near coma, thyroid replacement therapy for those [with heart failure] who have clinical hypothyroidism should follow standard lines.”
It is important to monitor thyroid function,” agreed Dr. Samuel, a researcher in the department of academic cardiology at Hull York Medical School. “We found that thyroxine use was most common among patients with hyperthyroidism, suggesting that they were previously hypothyroid and had received inappropriate treatment.”
Confounder adjustment mitigates the thyroid link
The new analysis used data collected from 6,782 consecutive heart failure patients enrolled during 2000-2018 at a community heart failure clinic that serves patients in the region of Hull, England. The researchers identified 4,992 of these patients with confirmed heart failure and adequate data for their analyses, including 2,997 (60%) with heart failure with reduced ejection fraction (HFrEF) and 1,995 (40%) with heart failure with normal ejection fraction (HFnEF, the term used by the authors but often called heart failure with preserved ejection fraction).
Thyroid hormone levels showed that 90% of these patients were euthyroid, 6% were hyperthyroid, and 4% were hypothyroid, rates consistent with prior reports for both the general population and heart failure patients. Only 12 patients (0.2%) had overt hypothyroidism, and fewer that 1% (about 45 patients) had overt hyperthyroidism. Patients averaged about 73 years of age, and during a median 4.6 years of follow-up 58% died.
Both the hypo- and hyperthyroid patients showed significantly higher mortality rates than euthyroid patients in a univariate analysis. But the patients with thyroid dysfunction also had more comorbidities, more severe heart failure symptoms measured by NYHA functional class, and more severe heart failure measured as higher serum levels of NT-proBNP.
In a multivariate analysis that adjusted for these factors, the significant differences disappeared among the entire group of heart failure patients for the outcomes of all-cause mortality, and mortality or hospitalization with heart failure. The multivariate analysis also showed no significant association between higher levels of thyroid-stimulating hormone (TSH) and all-cause death or death plus heart failure hospitalization among the patients with HFrEF.
Among patients with HFnEF, the multivariate adjusted analysis showed a small increase in both mortality and mortality plus hospitalization for heart failure, a 2% rise for each of these two endpoints for each 1 mIU/L increase in TSH, the authors reported. Although the P value for each of these two significant differences among patients with HFnEF was .02, the 95% confidence interval included 1.00 and ranged from 1.00 to 1.04.
The multivariate analysis identified three variables with the strongest associations with all-cause mortality: older age, higher levels of NT-proBNP, and higher NYHA class indicating greater functional impairment.
The results support the hypothesis that “worsening heart failure can lead to down-regulation of thyroid hormone signaling,” the authors suggested. Their study is also “the first to examine the prognostic significance of thyroid dysfunction in a large population of patients with HFnEF.” This analysis showed a “weak but significant association between increasing TSH and both mortality and the composite endpoint in patients with HFnEF.”
“HFnEF is a heterogeneous group of conditions that are difficult to diagnose in many cases. Therefore, future studies are needed to provide further clarity on the effect of thyroid dysfunction in these patients,” Dr. Samuel said.
The study received no commercial funding. Dr. Samuel and Dr. Clark had no disclosures.
SOURCE: Samuel NA et al. Am J Cardiol. 2020 Oct 24. doi: 10.1016/j.amjcard.2020.10.034.
Thyroid dysfunction had virtually no independent impact on survival in a retrospective study of nearly 5,000 English patients with chronic heart failure, adding to evidence that subclinical thyroid disorders in these patients requires no special management beyond ongoing monitoring.
“Although thyroid dysfunction is related to outcome in patients with chronic heart failure, the association disappears when adjustment is made for established prognostic variables, such as age, NT-proBNP [N-terminal of the prohormone brain natriuretic peptide], and [New York Heart Association] class,” wrote Nathan A. Samuel, MBChB, and coauthors in the American Journal of Cardiology.
Results from several earlier studies had shown evidence for reduced survival in heart failure patients with thyroid dysfunction, but in analyses that did not adjust for heart failure severity, such as a 2013 report that used data from the Sudden Cardiac Death in Heart Failure Trial SCD-HeFT. Other studies that adjusted for heart failure severity based on serum level of natriuretic peptides did not show significant associations between thyroid function and mortality, and when those results couple with the new report they together minimize the immediate risk from subclinical thyroid dysfunction faced by heart failure patients, wrote the authors of the new report.
Don’t treat subclinical thyroid dysfunction
“Our results suggest that subclinical thyroid disease has little impact on outcomes, and that we should not treat subclinical hypothyroidism in the expectation of improving outlook,” said Andrew L. Clark, MD, senior author on the new report and professor and head of the department of academic cardiology at Hull (England) York Medical School.
“Both hyper-and hypothyroidism can cause heart failure, so thyroid function should always be checked in patients when they present with heart failure. A small proportion of patients have heart failure that is potentially reversible” with thyroid-directed treatment, Dr. Clark said in an interview.
But “subclinical disease should probably not be treated, although we have not conducted a clinical trial that proves this assertion. We speculate, based on our findings, that such a trial is unlikely to be positive.”
Patients with subclinical thyroid disorders, particularly subclinical hypothyroidism, “need to be followed and treated should they develop clinical disease,” he maintained. “Except in extreme circumstances, such as the handful of patients who might have gross myxedema and may be near coma, thyroid replacement therapy for those [with heart failure] who have clinical hypothyroidism should follow standard lines.”
It is important to monitor thyroid function,” agreed Dr. Samuel, a researcher in the department of academic cardiology at Hull York Medical School. “We found that thyroxine use was most common among patients with hyperthyroidism, suggesting that they were previously hypothyroid and had received inappropriate treatment.”
Confounder adjustment mitigates the thyroid link
The new analysis used data collected from 6,782 consecutive heart failure patients enrolled during 2000-2018 at a community heart failure clinic that serves patients in the region of Hull, England. The researchers identified 4,992 of these patients with confirmed heart failure and adequate data for their analyses, including 2,997 (60%) with heart failure with reduced ejection fraction (HFrEF) and 1,995 (40%) with heart failure with normal ejection fraction (HFnEF, the term used by the authors but often called heart failure with preserved ejection fraction).
Thyroid hormone levels showed that 90% of these patients were euthyroid, 6% were hyperthyroid, and 4% were hypothyroid, rates consistent with prior reports for both the general population and heart failure patients. Only 12 patients (0.2%) had overt hypothyroidism, and fewer that 1% (about 45 patients) had overt hyperthyroidism. Patients averaged about 73 years of age, and during a median 4.6 years of follow-up 58% died.
Both the hypo- and hyperthyroid patients showed significantly higher mortality rates than euthyroid patients in a univariate analysis. But the patients with thyroid dysfunction also had more comorbidities, more severe heart failure symptoms measured by NYHA functional class, and more severe heart failure measured as higher serum levels of NT-proBNP.
In a multivariate analysis that adjusted for these factors, the significant differences disappeared among the entire group of heart failure patients for the outcomes of all-cause mortality, and mortality or hospitalization with heart failure. The multivariate analysis also showed no significant association between higher levels of thyroid-stimulating hormone (TSH) and all-cause death or death plus heart failure hospitalization among the patients with HFrEF.
Among patients with HFnEF, the multivariate adjusted analysis showed a small increase in both mortality and mortality plus hospitalization for heart failure, a 2% rise for each of these two endpoints for each 1 mIU/L increase in TSH, the authors reported. Although the P value for each of these two significant differences among patients with HFnEF was .02, the 95% confidence interval included 1.00 and ranged from 1.00 to 1.04.
The multivariate analysis identified three variables with the strongest associations with all-cause mortality: older age, higher levels of NT-proBNP, and higher NYHA class indicating greater functional impairment.
The results support the hypothesis that “worsening heart failure can lead to down-regulation of thyroid hormone signaling,” the authors suggested. Their study is also “the first to examine the prognostic significance of thyroid dysfunction in a large population of patients with HFnEF.” This analysis showed a “weak but significant association between increasing TSH and both mortality and the composite endpoint in patients with HFnEF.”
“HFnEF is a heterogeneous group of conditions that are difficult to diagnose in many cases. Therefore, future studies are needed to provide further clarity on the effect of thyroid dysfunction in these patients,” Dr. Samuel said.
The study received no commercial funding. Dr. Samuel and Dr. Clark had no disclosures.
SOURCE: Samuel NA et al. Am J Cardiol. 2020 Oct 24. doi: 10.1016/j.amjcard.2020.10.034.
Thyroid dysfunction had virtually no independent impact on survival in a retrospective study of nearly 5,000 English patients with chronic heart failure, adding to evidence that subclinical thyroid disorders in these patients requires no special management beyond ongoing monitoring.
“Although thyroid dysfunction is related to outcome in patients with chronic heart failure, the association disappears when adjustment is made for established prognostic variables, such as age, NT-proBNP [N-terminal of the prohormone brain natriuretic peptide], and [New York Heart Association] class,” wrote Nathan A. Samuel, MBChB, and coauthors in the American Journal of Cardiology.
Results from several earlier studies had shown evidence for reduced survival in heart failure patients with thyroid dysfunction, but in analyses that did not adjust for heart failure severity, such as a 2013 report that used data from the Sudden Cardiac Death in Heart Failure Trial SCD-HeFT. Other studies that adjusted for heart failure severity based on serum level of natriuretic peptides did not show significant associations between thyroid function and mortality, and when those results couple with the new report they together minimize the immediate risk from subclinical thyroid dysfunction faced by heart failure patients, wrote the authors of the new report.
Don’t treat subclinical thyroid dysfunction
“Our results suggest that subclinical thyroid disease has little impact on outcomes, and that we should not treat subclinical hypothyroidism in the expectation of improving outlook,” said Andrew L. Clark, MD, senior author on the new report and professor and head of the department of academic cardiology at Hull (England) York Medical School.
“Both hyper-and hypothyroidism can cause heart failure, so thyroid function should always be checked in patients when they present with heart failure. A small proportion of patients have heart failure that is potentially reversible” with thyroid-directed treatment, Dr. Clark said in an interview.
But “subclinical disease should probably not be treated, although we have not conducted a clinical trial that proves this assertion. We speculate, based on our findings, that such a trial is unlikely to be positive.”
Patients with subclinical thyroid disorders, particularly subclinical hypothyroidism, “need to be followed and treated should they develop clinical disease,” he maintained. “Except in extreme circumstances, such as the handful of patients who might have gross myxedema and may be near coma, thyroid replacement therapy for those [with heart failure] who have clinical hypothyroidism should follow standard lines.”
It is important to monitor thyroid function,” agreed Dr. Samuel, a researcher in the department of academic cardiology at Hull York Medical School. “We found that thyroxine use was most common among patients with hyperthyroidism, suggesting that they were previously hypothyroid and had received inappropriate treatment.”
Confounder adjustment mitigates the thyroid link
The new analysis used data collected from 6,782 consecutive heart failure patients enrolled during 2000-2018 at a community heart failure clinic that serves patients in the region of Hull, England. The researchers identified 4,992 of these patients with confirmed heart failure and adequate data for their analyses, including 2,997 (60%) with heart failure with reduced ejection fraction (HFrEF) and 1,995 (40%) with heart failure with normal ejection fraction (HFnEF, the term used by the authors but often called heart failure with preserved ejection fraction).
Thyroid hormone levels showed that 90% of these patients were euthyroid, 6% were hyperthyroid, and 4% were hypothyroid, rates consistent with prior reports for both the general population and heart failure patients. Only 12 patients (0.2%) had overt hypothyroidism, and fewer that 1% (about 45 patients) had overt hyperthyroidism. Patients averaged about 73 years of age, and during a median 4.6 years of follow-up 58% died.
Both the hypo- and hyperthyroid patients showed significantly higher mortality rates than euthyroid patients in a univariate analysis. But the patients with thyroid dysfunction also had more comorbidities, more severe heart failure symptoms measured by NYHA functional class, and more severe heart failure measured as higher serum levels of NT-proBNP.
In a multivariate analysis that adjusted for these factors, the significant differences disappeared among the entire group of heart failure patients for the outcomes of all-cause mortality, and mortality or hospitalization with heart failure. The multivariate analysis also showed no significant association between higher levels of thyroid-stimulating hormone (TSH) and all-cause death or death plus heart failure hospitalization among the patients with HFrEF.
Among patients with HFnEF, the multivariate adjusted analysis showed a small increase in both mortality and mortality plus hospitalization for heart failure, a 2% rise for each of these two endpoints for each 1 mIU/L increase in TSH, the authors reported. Although the P value for each of these two significant differences among patients with HFnEF was .02, the 95% confidence interval included 1.00 and ranged from 1.00 to 1.04.
The multivariate analysis identified three variables with the strongest associations with all-cause mortality: older age, higher levels of NT-proBNP, and higher NYHA class indicating greater functional impairment.
The results support the hypothesis that “worsening heart failure can lead to down-regulation of thyroid hormone signaling,” the authors suggested. Their study is also “the first to examine the prognostic significance of thyroid dysfunction in a large population of patients with HFnEF.” This analysis showed a “weak but significant association between increasing TSH and both mortality and the composite endpoint in patients with HFnEF.”
“HFnEF is a heterogeneous group of conditions that are difficult to diagnose in many cases. Therefore, future studies are needed to provide further clarity on the effect of thyroid dysfunction in these patients,” Dr. Samuel said.
The study received no commercial funding. Dr. Samuel and Dr. Clark had no disclosures.
SOURCE: Samuel NA et al. Am J Cardiol. 2020 Oct 24. doi: 10.1016/j.amjcard.2020.10.034.
FROM THE AMERICAN JOURNAL OF CARDIOLOGY
First-of-its kind guideline on lipid monitoring in endocrine diseases
Endocrine diseases of any type – not just diabetes – can represent a cardiovascular risk and patients with those disorders should be screened for high cholesterol, according to a new clinical practice guideline from the Endocrine Society.
“The simple recommendation to check a lipid panel in patients with endocrine diseases and calculate cardiovascular risk may be practice changing because that is not done routinely,” Connie Newman, MD, chair of the Endocrine Society committee that developed the guideline, said in an interview.
“Usually the focus is on assessment and treatment of the endocrine disease, rather than on assessment and treatment of atherosclerotic cardiovascular disease risk,” said Newman, an adjunct professor of medicine in the department of medicine, division of endocrinology, diabetes & metabolism, at New York University.
Whereas diabetes, well-known for its increased cardiovascular risk profile, is commonly addressed in other cardiovascular and cholesterol practice management guidelines, the array of other endocrine diseases are not typically included.
“This guideline is the first of its kind,” Dr. Newman said. “The Endocrine Society has not previously issued a guideline on lipid management in endocrine disorders [and] other organizations have not written guidelines on this topic.
“Rather, guidelines have been written on cholesterol management, but these do not describe cholesterol management in patients with endocrine diseases such as thyroid disease [hypothyroidism and hyperthyroidism], Cushing’s syndrome, acromegaly, growth hormone deficiency, menopause, male hypogonadism, and obesity,” she noted.
But these conditions carry a host of cardiovascular risk factors that may require careful monitoring and management.
“Although endocrine hormones, such as thyroid hormone, cortisol, estrogen, testosterone, growth hormone, and insulin, affect pathways for lipid metabolism, physicians lack guidance on lipid abnormalities, cardiovascular risk, and treatment to reduce lipids and cardiovascular risk in patients with endocrine diseases,” she explained.
Vinaya Simha, MD, an internal medicine specialist at the Mayo Clinic in Rochester, Minn., agrees that the guideline is notable in addressing an unmet need.
Recommendations that stand out to Dr. Simha include the suggestion of adding eicosapentaenoic acid (EPA) ethyl ester to reduce the risk of cardiovascular disease in adults with diabetes or atherosclerotic cardiovascular disease who have elevated triglyceride levels despite statin treatment.
James L. Rosenzweig, MD, an endocrinologist at Hebrew SeniorLife in Boston, agreed that this is an important addition to an area that needs more guidance.
“Many of these clinical situations can exacerbate dyslipidemia and some also increase the cardiovascular risk to a greater extent in combination with elevated cholesterol and/or triglycerides,” he said in an interview.
“In many cases, treatment of the underlying disorder appropriately can have an important impact in resolving the lipid disorder. In others, more aggressive pharmacological treatment is indicated,” he said.
“I think that this will be a valuable resource, especially for endocrinologists, but it can be used as well by providers in other disciplines.”
Key recommendations for different endocrine conditions
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, details those risks and provides evidence-based recommendations on their management and treatment.
Key recommendations include:
- Obtain a lipid panel and evaluate cardiovascular risk factors in all adults with endocrine disorders.
- In patients with and risk factors for cardiovascular disease, start statin therapy in addition to lifestyle modification to reduce cardiovascular risk. “This could mean earlier treatment because other guidelines recommend consideration of therapy at age 40,” Dr. Newman said.
- Statin therapy is also recommended for adults over 40 with with a duration of diabetes of more than 20 years and/or microvascular complications, regardless of their cardiovascular risk score. “This means earlier treatment of patients with type 1 diabetes with statins in order to reduce cardiovascular disease risk,” Dr. Newman noted.
- In patients with hyperlipidemia, rule out as the cause before treating with lipid-lowering medications. And among patients who are found to have hypothyroidism, reevaluate the lipid profile when the patient has thyroid hormone levels in the normal range.
- Adults with persistent endogenous Cushing’s syndrome should have their lipid profile monitored. Statin therapy should be considered in addition to lifestyle modifications, irrespective of the cardiovascular risk score.
- In postmenopausal women, high cholesterol or triglycerides should be treated with statins rather than hormone therapy.
- Evaluate and treat lipids and other cardiovascular risk factors in women who enter menopause early (before the age of 40-45 years).
Nice summary of ‘risk-enhancing’ endocrine disorders
Dr. Simha said in an interview that the new guideline is “probably the first comprehensive statement addressing lipid treatment in patients with a broad range of endocrine disorders besides diabetes.”
“Most of the treatment recommendations are congruent with other current guidelines such as the American College of Cardiology/American Heart Association [guidelines], but there is specific mention of which endocrine disorders represent enhanced cardiovascular risk,” she explained.
The new recommendations are notable for including “a nice summary of how different endocrine disorders affect lipid values, and also which endocrine disorders need to be considered as ‘risk-enhancing factors,’ ” Dr. Simha noted.
“The use of EPA in patients with hypertriglyceridemia is novel, compared to the ACC/AHA recommendation. This reflects new data which is now available,” she added.
The American Association of Clinical Endocrinologists also just issued a new algorithm on lipid management and prevention of cardiovascular disease in which treatment of hypertriglyceridemia is emphasized.
In addition, the new Endocrine Society guideline “also mentions an LDL [cholesterol] treatment threshold of 70 mg/dL, and 55 mg/dL in some patient categories, which previous guidelines have not,” Dr. Simha noted.
Overall, Dr. Newman added that the goal of the guideline is to increase awareness of key issues with endocrine diseases that may not necessarily be on clinicians’ radars.
“We hope that it will make a lipid panel and cardiovascular risk evaluation routine in adults with endocrine diseases and cause a greater focus on therapies to reduce heart disease and stroke,” she said.
Dr. Newman, Dr. Simha, and Dr. Rosenzweig reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Endocrine diseases of any type – not just diabetes – can represent a cardiovascular risk and patients with those disorders should be screened for high cholesterol, according to a new clinical practice guideline from the Endocrine Society.
“The simple recommendation to check a lipid panel in patients with endocrine diseases and calculate cardiovascular risk may be practice changing because that is not done routinely,” Connie Newman, MD, chair of the Endocrine Society committee that developed the guideline, said in an interview.
“Usually the focus is on assessment and treatment of the endocrine disease, rather than on assessment and treatment of atherosclerotic cardiovascular disease risk,” said Newman, an adjunct professor of medicine in the department of medicine, division of endocrinology, diabetes & metabolism, at New York University.
Whereas diabetes, well-known for its increased cardiovascular risk profile, is commonly addressed in other cardiovascular and cholesterol practice management guidelines, the array of other endocrine diseases are not typically included.
“This guideline is the first of its kind,” Dr. Newman said. “The Endocrine Society has not previously issued a guideline on lipid management in endocrine disorders [and] other organizations have not written guidelines on this topic.
“Rather, guidelines have been written on cholesterol management, but these do not describe cholesterol management in patients with endocrine diseases such as thyroid disease [hypothyroidism and hyperthyroidism], Cushing’s syndrome, acromegaly, growth hormone deficiency, menopause, male hypogonadism, and obesity,” she noted.
But these conditions carry a host of cardiovascular risk factors that may require careful monitoring and management.
“Although endocrine hormones, such as thyroid hormone, cortisol, estrogen, testosterone, growth hormone, and insulin, affect pathways for lipid metabolism, physicians lack guidance on lipid abnormalities, cardiovascular risk, and treatment to reduce lipids and cardiovascular risk in patients with endocrine diseases,” she explained.
Vinaya Simha, MD, an internal medicine specialist at the Mayo Clinic in Rochester, Minn., agrees that the guideline is notable in addressing an unmet need.
Recommendations that stand out to Dr. Simha include the suggestion of adding eicosapentaenoic acid (EPA) ethyl ester to reduce the risk of cardiovascular disease in adults with diabetes or atherosclerotic cardiovascular disease who have elevated triglyceride levels despite statin treatment.
James L. Rosenzweig, MD, an endocrinologist at Hebrew SeniorLife in Boston, agreed that this is an important addition to an area that needs more guidance.
“Many of these clinical situations can exacerbate dyslipidemia and some also increase the cardiovascular risk to a greater extent in combination with elevated cholesterol and/or triglycerides,” he said in an interview.
“In many cases, treatment of the underlying disorder appropriately can have an important impact in resolving the lipid disorder. In others, more aggressive pharmacological treatment is indicated,” he said.
“I think that this will be a valuable resource, especially for endocrinologists, but it can be used as well by providers in other disciplines.”
Key recommendations for different endocrine conditions
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, details those risks and provides evidence-based recommendations on their management and treatment.
Key recommendations include:
- Obtain a lipid panel and evaluate cardiovascular risk factors in all adults with endocrine disorders.
- In patients with and risk factors for cardiovascular disease, start statin therapy in addition to lifestyle modification to reduce cardiovascular risk. “This could mean earlier treatment because other guidelines recommend consideration of therapy at age 40,” Dr. Newman said.
- Statin therapy is also recommended for adults over 40 with with a duration of diabetes of more than 20 years and/or microvascular complications, regardless of their cardiovascular risk score. “This means earlier treatment of patients with type 1 diabetes with statins in order to reduce cardiovascular disease risk,” Dr. Newman noted.
- In patients with hyperlipidemia, rule out as the cause before treating with lipid-lowering medications. And among patients who are found to have hypothyroidism, reevaluate the lipid profile when the patient has thyroid hormone levels in the normal range.
- Adults with persistent endogenous Cushing’s syndrome should have their lipid profile monitored. Statin therapy should be considered in addition to lifestyle modifications, irrespective of the cardiovascular risk score.
- In postmenopausal women, high cholesterol or triglycerides should be treated with statins rather than hormone therapy.
- Evaluate and treat lipids and other cardiovascular risk factors in women who enter menopause early (before the age of 40-45 years).
Nice summary of ‘risk-enhancing’ endocrine disorders
Dr. Simha said in an interview that the new guideline is “probably the first comprehensive statement addressing lipid treatment in patients with a broad range of endocrine disorders besides diabetes.”
“Most of the treatment recommendations are congruent with other current guidelines such as the American College of Cardiology/American Heart Association [guidelines], but there is specific mention of which endocrine disorders represent enhanced cardiovascular risk,” she explained.
The new recommendations are notable for including “a nice summary of how different endocrine disorders affect lipid values, and also which endocrine disorders need to be considered as ‘risk-enhancing factors,’ ” Dr. Simha noted.
“The use of EPA in patients with hypertriglyceridemia is novel, compared to the ACC/AHA recommendation. This reflects new data which is now available,” she added.
The American Association of Clinical Endocrinologists also just issued a new algorithm on lipid management and prevention of cardiovascular disease in which treatment of hypertriglyceridemia is emphasized.
In addition, the new Endocrine Society guideline “also mentions an LDL [cholesterol] treatment threshold of 70 mg/dL, and 55 mg/dL in some patient categories, which previous guidelines have not,” Dr. Simha noted.
Overall, Dr. Newman added that the goal of the guideline is to increase awareness of key issues with endocrine diseases that may not necessarily be on clinicians’ radars.
“We hope that it will make a lipid panel and cardiovascular risk evaluation routine in adults with endocrine diseases and cause a greater focus on therapies to reduce heart disease and stroke,” she said.
Dr. Newman, Dr. Simha, and Dr. Rosenzweig reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Endocrine diseases of any type – not just diabetes – can represent a cardiovascular risk and patients with those disorders should be screened for high cholesterol, according to a new clinical practice guideline from the Endocrine Society.
“The simple recommendation to check a lipid panel in patients with endocrine diseases and calculate cardiovascular risk may be practice changing because that is not done routinely,” Connie Newman, MD, chair of the Endocrine Society committee that developed the guideline, said in an interview.
“Usually the focus is on assessment and treatment of the endocrine disease, rather than on assessment and treatment of atherosclerotic cardiovascular disease risk,” said Newman, an adjunct professor of medicine in the department of medicine, division of endocrinology, diabetes & metabolism, at New York University.
Whereas diabetes, well-known for its increased cardiovascular risk profile, is commonly addressed in other cardiovascular and cholesterol practice management guidelines, the array of other endocrine diseases are not typically included.
“This guideline is the first of its kind,” Dr. Newman said. “The Endocrine Society has not previously issued a guideline on lipid management in endocrine disorders [and] other organizations have not written guidelines on this topic.
“Rather, guidelines have been written on cholesterol management, but these do not describe cholesterol management in patients with endocrine diseases such as thyroid disease [hypothyroidism and hyperthyroidism], Cushing’s syndrome, acromegaly, growth hormone deficiency, menopause, male hypogonadism, and obesity,” she noted.
But these conditions carry a host of cardiovascular risk factors that may require careful monitoring and management.
“Although endocrine hormones, such as thyroid hormone, cortisol, estrogen, testosterone, growth hormone, and insulin, affect pathways for lipid metabolism, physicians lack guidance on lipid abnormalities, cardiovascular risk, and treatment to reduce lipids and cardiovascular risk in patients with endocrine diseases,” she explained.
Vinaya Simha, MD, an internal medicine specialist at the Mayo Clinic in Rochester, Minn., agrees that the guideline is notable in addressing an unmet need.
Recommendations that stand out to Dr. Simha include the suggestion of adding eicosapentaenoic acid (EPA) ethyl ester to reduce the risk of cardiovascular disease in adults with diabetes or atherosclerotic cardiovascular disease who have elevated triglyceride levels despite statin treatment.
James L. Rosenzweig, MD, an endocrinologist at Hebrew SeniorLife in Boston, agreed that this is an important addition to an area that needs more guidance.
“Many of these clinical situations can exacerbate dyslipidemia and some also increase the cardiovascular risk to a greater extent in combination with elevated cholesterol and/or triglycerides,” he said in an interview.
“In many cases, treatment of the underlying disorder appropriately can have an important impact in resolving the lipid disorder. In others, more aggressive pharmacological treatment is indicated,” he said.
“I think that this will be a valuable resource, especially for endocrinologists, but it can be used as well by providers in other disciplines.”
Key recommendations for different endocrine conditions
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, details those risks and provides evidence-based recommendations on their management and treatment.
Key recommendations include:
- Obtain a lipid panel and evaluate cardiovascular risk factors in all adults with endocrine disorders.
- In patients with and risk factors for cardiovascular disease, start statin therapy in addition to lifestyle modification to reduce cardiovascular risk. “This could mean earlier treatment because other guidelines recommend consideration of therapy at age 40,” Dr. Newman said.
- Statin therapy is also recommended for adults over 40 with with a duration of diabetes of more than 20 years and/or microvascular complications, regardless of their cardiovascular risk score. “This means earlier treatment of patients with type 1 diabetes with statins in order to reduce cardiovascular disease risk,” Dr. Newman noted.
- In patients with hyperlipidemia, rule out as the cause before treating with lipid-lowering medications. And among patients who are found to have hypothyroidism, reevaluate the lipid profile when the patient has thyroid hormone levels in the normal range.
- Adults with persistent endogenous Cushing’s syndrome should have their lipid profile monitored. Statin therapy should be considered in addition to lifestyle modifications, irrespective of the cardiovascular risk score.
- In postmenopausal women, high cholesterol or triglycerides should be treated with statins rather than hormone therapy.
- Evaluate and treat lipids and other cardiovascular risk factors in women who enter menopause early (before the age of 40-45 years).
Nice summary of ‘risk-enhancing’ endocrine disorders
Dr. Simha said in an interview that the new guideline is “probably the first comprehensive statement addressing lipid treatment in patients with a broad range of endocrine disorders besides diabetes.”
“Most of the treatment recommendations are congruent with other current guidelines such as the American College of Cardiology/American Heart Association [guidelines], but there is specific mention of which endocrine disorders represent enhanced cardiovascular risk,” she explained.
The new recommendations are notable for including “a nice summary of how different endocrine disorders affect lipid values, and also which endocrine disorders need to be considered as ‘risk-enhancing factors,’ ” Dr. Simha noted.
“The use of EPA in patients with hypertriglyceridemia is novel, compared to the ACC/AHA recommendation. This reflects new data which is now available,” she added.
The American Association of Clinical Endocrinologists also just issued a new algorithm on lipid management and prevention of cardiovascular disease in which treatment of hypertriglyceridemia is emphasized.
In addition, the new Endocrine Society guideline “also mentions an LDL [cholesterol] treatment threshold of 70 mg/dL, and 55 mg/dL in some patient categories, which previous guidelines have not,” Dr. Simha noted.
Overall, Dr. Newman added that the goal of the guideline is to increase awareness of key issues with endocrine diseases that may not necessarily be on clinicians’ radars.
“We hope that it will make a lipid panel and cardiovascular risk evaluation routine in adults with endocrine diseases and cause a greater focus on therapies to reduce heart disease and stroke,” she said.
Dr. Newman, Dr. Simha, and Dr. Rosenzweig reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Avoid pituitary pitfalls in hyperprolactinemia
,” Ashlyn Smith, PA-C, of Endocrinology Associates, Scottsdale, Ariz., said in a presentation at the at the virtual meeting of the annual Metabolic and Endocrine Disease Summit held by Global Academy for Medical Education.
The most common demographic for pituitary disorders is women in their 30s and 40s, Ms. Smith said. Early red flags for pituitary problems include patients presenting with headaches and/or blurred or double vision, which could signal bitemporal hemianopsia, she said.
Roughly two-thirds of pituitary adenomas are functional, meaning that they secrete pituitary hormones and cause clinical syndromes, Ms. Smith said. The most common reason for hypersecretion is hyperprolactinemia, she said.
Hyperprolactinemia, like most pituitary conditions, is more common in women than men, Ms. Smith noted. However, symptoms may include not only galactorrhea, but also gynecomastia, and hypogonadism, which may be red flags in men, she noted.
“Prolactin inhibits the gonadal pathway, so we see low gonadal hormones. For example, if men present with atypical hypogonadism for their age, or women present with changes in the menstrual cycle, check the prolactin levels,” she said.
The etiologies of hyperprolactinemia include physiologic reasons such as breastfeeding and pregnancy, as well as intercourse and breast manipulation, stress, and sleep issues. Pathologic reasons for prolactin elevation include prolactinoma, gonad-hormone secreting tumor, hypothyroidism, and renal insufficiency, Ms. Smith said.
Evaluation of patients with suspected hyperprolactinemia includes screening for physiologic causes, renal function and thyroid function tests, and a thyroid-specific MRI. Ordering a dedicated MRI of the pituitary gland is important to help identify compression of the optic nerve, noted Ms. Smith.
A medication review also is essential in evaluating hyperprolactinemia, and especially in the setting of the COVID-19 pandemic, because patients may have made changes to psychiatric medications, said Ms. Smith. Neuroleptics and antipsychotics including risperidone, haloperidol, chlorpromazine, and thiothixene can be associated with hyperprolactinemia, as can benzodiazepines and various analgesics and antidepressants, she said.
Treatment in cases of medication-induced hyperprolactinemia can be challenging if the patients are unable to change a medication, said Ms. Smith. However, patients with hypogonadism or low bone mineral density who can’t change medications may benefit from exogenous gonadal hormones, she said.
Some patients with hyperprolactinemia benefit from treatment with dopamine agonists, which may ease symptoms and reduce the size of the prolactinoma, she explained. However, patients on dopamine agonists should be alert to side effects including constipation and orthostasis. Ms. Smith said she recommends that patients on dopamine agonists for hyperprolactinemia take the medication at night so they are lying down if orthostasis occurs.
Monitor prolactin levels at 1 month, and taper or discontinue if the prolactin returns to normal and the adenoma resolves, which can take approximately 2 years, she said. Ms. Smith then advised follow-up every 3 months for 1 year, then annual prolactin checks.
The risk of recurrence ranges from 26% to 69%, Ms. Smith said, and is higher in patients with higher prolactin levels and larger adenomas, she noted. Recurrence is most likely within a year of withdrawal from treatment, she said.
Ms. Smith disclosed serving as an adviser and speaker for Abbott Nutrition, a speaker for Xeris Pharmaceuticals, and an adviser for Sanofi and Radius.
Global Academy for Medical Education and this news organization are owned by the same parent company.
SOURCE: Smith A. MEDS 2020.
,” Ashlyn Smith, PA-C, of Endocrinology Associates, Scottsdale, Ariz., said in a presentation at the at the virtual meeting of the annual Metabolic and Endocrine Disease Summit held by Global Academy for Medical Education.
The most common demographic for pituitary disorders is women in their 30s and 40s, Ms. Smith said. Early red flags for pituitary problems include patients presenting with headaches and/or blurred or double vision, which could signal bitemporal hemianopsia, she said.
Roughly two-thirds of pituitary adenomas are functional, meaning that they secrete pituitary hormones and cause clinical syndromes, Ms. Smith said. The most common reason for hypersecretion is hyperprolactinemia, she said.
Hyperprolactinemia, like most pituitary conditions, is more common in women than men, Ms. Smith noted. However, symptoms may include not only galactorrhea, but also gynecomastia, and hypogonadism, which may be red flags in men, she noted.
“Prolactin inhibits the gonadal pathway, so we see low gonadal hormones. For example, if men present with atypical hypogonadism for their age, or women present with changes in the menstrual cycle, check the prolactin levels,” she said.
The etiologies of hyperprolactinemia include physiologic reasons such as breastfeeding and pregnancy, as well as intercourse and breast manipulation, stress, and sleep issues. Pathologic reasons for prolactin elevation include prolactinoma, gonad-hormone secreting tumor, hypothyroidism, and renal insufficiency, Ms. Smith said.
Evaluation of patients with suspected hyperprolactinemia includes screening for physiologic causes, renal function and thyroid function tests, and a thyroid-specific MRI. Ordering a dedicated MRI of the pituitary gland is important to help identify compression of the optic nerve, noted Ms. Smith.
A medication review also is essential in evaluating hyperprolactinemia, and especially in the setting of the COVID-19 pandemic, because patients may have made changes to psychiatric medications, said Ms. Smith. Neuroleptics and antipsychotics including risperidone, haloperidol, chlorpromazine, and thiothixene can be associated with hyperprolactinemia, as can benzodiazepines and various analgesics and antidepressants, she said.
Treatment in cases of medication-induced hyperprolactinemia can be challenging if the patients are unable to change a medication, said Ms. Smith. However, patients with hypogonadism or low bone mineral density who can’t change medications may benefit from exogenous gonadal hormones, she said.
Some patients with hyperprolactinemia benefit from treatment with dopamine agonists, which may ease symptoms and reduce the size of the prolactinoma, she explained. However, patients on dopamine agonists should be alert to side effects including constipation and orthostasis. Ms. Smith said she recommends that patients on dopamine agonists for hyperprolactinemia take the medication at night so they are lying down if orthostasis occurs.
Monitor prolactin levels at 1 month, and taper or discontinue if the prolactin returns to normal and the adenoma resolves, which can take approximately 2 years, she said. Ms. Smith then advised follow-up every 3 months for 1 year, then annual prolactin checks.
The risk of recurrence ranges from 26% to 69%, Ms. Smith said, and is higher in patients with higher prolactin levels and larger adenomas, she noted. Recurrence is most likely within a year of withdrawal from treatment, she said.
Ms. Smith disclosed serving as an adviser and speaker for Abbott Nutrition, a speaker for Xeris Pharmaceuticals, and an adviser for Sanofi and Radius.
Global Academy for Medical Education and this news organization are owned by the same parent company.
SOURCE: Smith A. MEDS 2020.
,” Ashlyn Smith, PA-C, of Endocrinology Associates, Scottsdale, Ariz., said in a presentation at the at the virtual meeting of the annual Metabolic and Endocrine Disease Summit held by Global Academy for Medical Education.
The most common demographic for pituitary disorders is women in their 30s and 40s, Ms. Smith said. Early red flags for pituitary problems include patients presenting with headaches and/or blurred or double vision, which could signal bitemporal hemianopsia, she said.
Roughly two-thirds of pituitary adenomas are functional, meaning that they secrete pituitary hormones and cause clinical syndromes, Ms. Smith said. The most common reason for hypersecretion is hyperprolactinemia, she said.
Hyperprolactinemia, like most pituitary conditions, is more common in women than men, Ms. Smith noted. However, symptoms may include not only galactorrhea, but also gynecomastia, and hypogonadism, which may be red flags in men, she noted.
“Prolactin inhibits the gonadal pathway, so we see low gonadal hormones. For example, if men present with atypical hypogonadism for their age, or women present with changes in the menstrual cycle, check the prolactin levels,” she said.
The etiologies of hyperprolactinemia include physiologic reasons such as breastfeeding and pregnancy, as well as intercourse and breast manipulation, stress, and sleep issues. Pathologic reasons for prolactin elevation include prolactinoma, gonad-hormone secreting tumor, hypothyroidism, and renal insufficiency, Ms. Smith said.
Evaluation of patients with suspected hyperprolactinemia includes screening for physiologic causes, renal function and thyroid function tests, and a thyroid-specific MRI. Ordering a dedicated MRI of the pituitary gland is important to help identify compression of the optic nerve, noted Ms. Smith.
A medication review also is essential in evaluating hyperprolactinemia, and especially in the setting of the COVID-19 pandemic, because patients may have made changes to psychiatric medications, said Ms. Smith. Neuroleptics and antipsychotics including risperidone, haloperidol, chlorpromazine, and thiothixene can be associated with hyperprolactinemia, as can benzodiazepines and various analgesics and antidepressants, she said.
Treatment in cases of medication-induced hyperprolactinemia can be challenging if the patients are unable to change a medication, said Ms. Smith. However, patients with hypogonadism or low bone mineral density who can’t change medications may benefit from exogenous gonadal hormones, she said.
Some patients with hyperprolactinemia benefit from treatment with dopamine agonists, which may ease symptoms and reduce the size of the prolactinoma, she explained. However, patients on dopamine agonists should be alert to side effects including constipation and orthostasis. Ms. Smith said she recommends that patients on dopamine agonists for hyperprolactinemia take the medication at night so they are lying down if orthostasis occurs.
Monitor prolactin levels at 1 month, and taper or discontinue if the prolactin returns to normal and the adenoma resolves, which can take approximately 2 years, she said. Ms. Smith then advised follow-up every 3 months for 1 year, then annual prolactin checks.
The risk of recurrence ranges from 26% to 69%, Ms. Smith said, and is higher in patients with higher prolactin levels and larger adenomas, she noted. Recurrence is most likely within a year of withdrawal from treatment, she said.
Ms. Smith disclosed serving as an adviser and speaker for Abbott Nutrition, a speaker for Xeris Pharmaceuticals, and an adviser for Sanofi and Radius.
Global Academy for Medical Education and this news organization are owned by the same parent company.
SOURCE: Smith A. MEDS 2020.
EXPERT ANALYSIS FROM MEDS 2020
Adrenal vein sampling looms as choke point for aldosteronism assessment of hypertensives
At a time when new evidence strongly suggests that roughly a fifth of patents with hypertension have primary aldosteronism as the cause, other recent findings suggest that many of these possibly tens of millions of patients with aldosterone-driven high blood pressure may as a consequence need an expensive and not-widely-available diagnostic test – adrenal vein sampling – to determine whether they are candidates for a definitive surgical cure to their aldosteronism.
Some endocrinologists worry the worldwide infrastructure for running adrenal vein sampling (AVS) isn’t close to being in place to deliver on this looming need for patients with primary aldosteronism (PA), especially given the burgeoning numbers now being cited for PA prevalence.
“The system could be overwhelmed,” warned Robert M. Carey, MD, a cardiovascular endocrinologist and professor of medicine at the University of Virginia in Charlottesville. “Right now, adrenal vein sampling [AVS] is the gold standard,” for distinguishing unilateral and bilateral excess aldosterone secretion, “but not every radiologist can do AVS. Until we find a surrogate biomarker that can distinguish unilateral and bilateral PA” many patients will need AVS, Dr. Carey said in an interview.
“AVS is important for accurate lateralization of aldosterone excess in patients, but it may not be feasible for all patients with PA to undergo AVS. If the prevalence of PA truly is on the order of 15% [of all patients with hypertension] then health systems would be stretched to offer all of them AVS, which is technically challenging and requires dedicated training and is therefore limited to expert centers,” commented Jun Yang, MBBS, a cardiovascular endocrinologist at the Hudson Institute of Medical Research and a hypertension researcher at Monash University, both in Melbourne. “At Monash, our interventional radiologists have increased their [AVS] success rate from 40% to more than 90% during the past 10 years, and our waiting list for patients scheduled for AVS is now 3-4 months long,” Dr. Yang said in an interview.
Finding a unilateral adrenal nodule as the cause of PA means that surgical removal is an option, a step that often fully resolves the PA and normalizes blood pressure. Patients with a bilateral source of the aldosterone are not candidates for surgical cure and must be managed with medical treatment, usually a mineralocorticoid receptor antagonist such as spironolactone that can neutralize or at least reduce the impact of hyperaldosteronism.
AVS finds unilateral adenomas when imaging can’t
The evidence that raised concerns about the reliability of imaging as an easier and noninvasive means to identify hypertensive patients with PA and a unilateral adrenal nodule that makes them candidates for surgical removal to resolve their PA and hypertension came out in May 2020 in a review of 174 PA patients who underwent AVS at a single center in Calgary, Alta., during 2006-2018.
The review included 366 patients with PA referred to the University of Calgary for assessment, of whom 179 had no adrenal nodule visible with either CT or MRI imaging, with 174 of these patients also undergoing successful AVS. The procedure revealed 70 patients (40%) had unilateral aldosterone secretion (Can J Cardiol. 2020 May 16. doi: 10.1016/j.cjca.2020.05.013).
In an editorial about this report that appeared a few weeks later, Ross D. Feldman, MD, a hypertension-management researcher and professor of medicine at the University of Manitoba in Winnipeg, Man., said the finding was “amazing,” and “confirms that lateralization of aldosterone secretion in a patient with PA but without an identifiable mass on that side is not a zebra,” but instead a presentation that “occurs in almost half of patients with PA and no discernible adenoma on the side that lateralizes.” (Can J. Cardiol. 2020 Jul 3. doi: 10.1016/j.cjca.2020.06.022).
Although this was just one center’s experience, the authors are not alone in making this finding, although prior reports seem to have been largely forgotten or ignored until now.
“The discordance between AVS and adrenal imaging has been documented by numerous groups, and in our own experience [in Melbourne] around 40% of patients with unilateral aldosterone excess do not have a distinct unilateral adenoma on CT,” said Dr. Yang.
“Here’s the problem,” summed up Dr. Feldman in an interview. “Nearly half of patients with hyperaldosteronism don’t localize based on a CT or MRI, so you have to do AVS, but AVS is not generally available; it’s only at tertiary centers; and you have to do a lot of them,” to do them well. “It’s a half-day procedure, and you have to hit the correct adrenal vein.”
AVS for millions?
Compounding the challenge is the other bit of bombshell news recently dropped on the endocrinology and hypertension communities: PA may be much more prevalent that previously suspected, occurring in roughly 20% of patients with hypertension, according to study results that also came out in 2020 (Ann Int Med. 2020 Jul 7;173[1]:10-20).
The upshot, according to Dr. Feldman and others, is that researchers will need to find reliable criteria besides imaging for identifying PA patients with an increased likelihood of having a lateralized source for their excess aldosterone production. That’s “the only hope,” said Dr. Feldman, “so we won’t have to do AVS on 20 million Americans.”
Unfortunately, the path toward a successful screen to winnow down candidates for AVS has been long and not especially fruitful, with efforts dating back at least 50 years, and with one of the most recent efforts at stratifying PA patients by certain laboratory measures getting dismissed as producing a benefit that “might not be substantial,” wrote Michael Stowasser, MBBS, in a published commentary (J Hypertension. 2020 Jul;38[7]:1259-61).
In contrast to Dr. Feldman, Dr. Stowasser was more optimistic about the prospects for avoiding an immediate crisis in AVS assessment of PA patients, mostly because so few patients with PA are now identified by clinicians. Given the poor record clinicians have historically rung up diagnosing PA, “it would seem unlikely that we are going to be flooded with AVS requests any time soon,” he wrote. There is also reason to hope that increased demand for AVS will help broaden availability, and innovative testing methods promise to speed up the procedure, said Dr. Stowasser, a professor of medicine at the University of Queensland in Brisbane, Australia and director of the Endocrine Hypertension Research Centre at Greenslopes and Princess Alexandra Hospitals in Brisbane, in an interview.
But regardless of whether AVS testing becomes more available or streamlined, recent events suggest there will be little way to avoid eventually having to run millions of these diagnostic procedures.
Patients with PA “who decide they will not want surgery do not need AVS. For all other patients with PA, you need AVS. The medical system will just have to respond,” Dr. Carey concluded.
Dr. Carey, Dr. Yang, Dr. Feldman, and Dr. Stowasser had no relevant disclosures.
At a time when new evidence strongly suggests that roughly a fifth of patents with hypertension have primary aldosteronism as the cause, other recent findings suggest that many of these possibly tens of millions of patients with aldosterone-driven high blood pressure may as a consequence need an expensive and not-widely-available diagnostic test – adrenal vein sampling – to determine whether they are candidates for a definitive surgical cure to their aldosteronism.
Some endocrinologists worry the worldwide infrastructure for running adrenal vein sampling (AVS) isn’t close to being in place to deliver on this looming need for patients with primary aldosteronism (PA), especially given the burgeoning numbers now being cited for PA prevalence.
“The system could be overwhelmed,” warned Robert M. Carey, MD, a cardiovascular endocrinologist and professor of medicine at the University of Virginia in Charlottesville. “Right now, adrenal vein sampling [AVS] is the gold standard,” for distinguishing unilateral and bilateral excess aldosterone secretion, “but not every radiologist can do AVS. Until we find a surrogate biomarker that can distinguish unilateral and bilateral PA” many patients will need AVS, Dr. Carey said in an interview.
“AVS is important for accurate lateralization of aldosterone excess in patients, but it may not be feasible for all patients with PA to undergo AVS. If the prevalence of PA truly is on the order of 15% [of all patients with hypertension] then health systems would be stretched to offer all of them AVS, which is technically challenging and requires dedicated training and is therefore limited to expert centers,” commented Jun Yang, MBBS, a cardiovascular endocrinologist at the Hudson Institute of Medical Research and a hypertension researcher at Monash University, both in Melbourne. “At Monash, our interventional radiologists have increased their [AVS] success rate from 40% to more than 90% during the past 10 years, and our waiting list for patients scheduled for AVS is now 3-4 months long,” Dr. Yang said in an interview.
Finding a unilateral adrenal nodule as the cause of PA means that surgical removal is an option, a step that often fully resolves the PA and normalizes blood pressure. Patients with a bilateral source of the aldosterone are not candidates for surgical cure and must be managed with medical treatment, usually a mineralocorticoid receptor antagonist such as spironolactone that can neutralize or at least reduce the impact of hyperaldosteronism.
AVS finds unilateral adenomas when imaging can’t
The evidence that raised concerns about the reliability of imaging as an easier and noninvasive means to identify hypertensive patients with PA and a unilateral adrenal nodule that makes them candidates for surgical removal to resolve their PA and hypertension came out in May 2020 in a review of 174 PA patients who underwent AVS at a single center in Calgary, Alta., during 2006-2018.
The review included 366 patients with PA referred to the University of Calgary for assessment, of whom 179 had no adrenal nodule visible with either CT or MRI imaging, with 174 of these patients also undergoing successful AVS. The procedure revealed 70 patients (40%) had unilateral aldosterone secretion (Can J Cardiol. 2020 May 16. doi: 10.1016/j.cjca.2020.05.013).
In an editorial about this report that appeared a few weeks later, Ross D. Feldman, MD, a hypertension-management researcher and professor of medicine at the University of Manitoba in Winnipeg, Man., said the finding was “amazing,” and “confirms that lateralization of aldosterone secretion in a patient with PA but without an identifiable mass on that side is not a zebra,” but instead a presentation that “occurs in almost half of patients with PA and no discernible adenoma on the side that lateralizes.” (Can J. Cardiol. 2020 Jul 3. doi: 10.1016/j.cjca.2020.06.022).
Although this was just one center’s experience, the authors are not alone in making this finding, although prior reports seem to have been largely forgotten or ignored until now.
“The discordance between AVS and adrenal imaging has been documented by numerous groups, and in our own experience [in Melbourne] around 40% of patients with unilateral aldosterone excess do not have a distinct unilateral adenoma on CT,” said Dr. Yang.
“Here’s the problem,” summed up Dr. Feldman in an interview. “Nearly half of patients with hyperaldosteronism don’t localize based on a CT or MRI, so you have to do AVS, but AVS is not generally available; it’s only at tertiary centers; and you have to do a lot of them,” to do them well. “It’s a half-day procedure, and you have to hit the correct adrenal vein.”
AVS for millions?
Compounding the challenge is the other bit of bombshell news recently dropped on the endocrinology and hypertension communities: PA may be much more prevalent that previously suspected, occurring in roughly 20% of patients with hypertension, according to study results that also came out in 2020 (Ann Int Med. 2020 Jul 7;173[1]:10-20).
The upshot, according to Dr. Feldman and others, is that researchers will need to find reliable criteria besides imaging for identifying PA patients with an increased likelihood of having a lateralized source for their excess aldosterone production. That’s “the only hope,” said Dr. Feldman, “so we won’t have to do AVS on 20 million Americans.”
Unfortunately, the path toward a successful screen to winnow down candidates for AVS has been long and not especially fruitful, with efforts dating back at least 50 years, and with one of the most recent efforts at stratifying PA patients by certain laboratory measures getting dismissed as producing a benefit that “might not be substantial,” wrote Michael Stowasser, MBBS, in a published commentary (J Hypertension. 2020 Jul;38[7]:1259-61).
In contrast to Dr. Feldman, Dr. Stowasser was more optimistic about the prospects for avoiding an immediate crisis in AVS assessment of PA patients, mostly because so few patients with PA are now identified by clinicians. Given the poor record clinicians have historically rung up diagnosing PA, “it would seem unlikely that we are going to be flooded with AVS requests any time soon,” he wrote. There is also reason to hope that increased demand for AVS will help broaden availability, and innovative testing methods promise to speed up the procedure, said Dr. Stowasser, a professor of medicine at the University of Queensland in Brisbane, Australia and director of the Endocrine Hypertension Research Centre at Greenslopes and Princess Alexandra Hospitals in Brisbane, in an interview.
But regardless of whether AVS testing becomes more available or streamlined, recent events suggest there will be little way to avoid eventually having to run millions of these diagnostic procedures.
Patients with PA “who decide they will not want surgery do not need AVS. For all other patients with PA, you need AVS. The medical system will just have to respond,” Dr. Carey concluded.
Dr. Carey, Dr. Yang, Dr. Feldman, and Dr. Stowasser had no relevant disclosures.
At a time when new evidence strongly suggests that roughly a fifth of patents with hypertension have primary aldosteronism as the cause, other recent findings suggest that many of these possibly tens of millions of patients with aldosterone-driven high blood pressure may as a consequence need an expensive and not-widely-available diagnostic test – adrenal vein sampling – to determine whether they are candidates for a definitive surgical cure to their aldosteronism.
Some endocrinologists worry the worldwide infrastructure for running adrenal vein sampling (AVS) isn’t close to being in place to deliver on this looming need for patients with primary aldosteronism (PA), especially given the burgeoning numbers now being cited for PA prevalence.
“The system could be overwhelmed,” warned Robert M. Carey, MD, a cardiovascular endocrinologist and professor of medicine at the University of Virginia in Charlottesville. “Right now, adrenal vein sampling [AVS] is the gold standard,” for distinguishing unilateral and bilateral excess aldosterone secretion, “but not every radiologist can do AVS. Until we find a surrogate biomarker that can distinguish unilateral and bilateral PA” many patients will need AVS, Dr. Carey said in an interview.
“AVS is important for accurate lateralization of aldosterone excess in patients, but it may not be feasible for all patients with PA to undergo AVS. If the prevalence of PA truly is on the order of 15% [of all patients with hypertension] then health systems would be stretched to offer all of them AVS, which is technically challenging and requires dedicated training and is therefore limited to expert centers,” commented Jun Yang, MBBS, a cardiovascular endocrinologist at the Hudson Institute of Medical Research and a hypertension researcher at Monash University, both in Melbourne. “At Monash, our interventional radiologists have increased their [AVS] success rate from 40% to more than 90% during the past 10 years, and our waiting list for patients scheduled for AVS is now 3-4 months long,” Dr. Yang said in an interview.
Finding a unilateral adrenal nodule as the cause of PA means that surgical removal is an option, a step that often fully resolves the PA and normalizes blood pressure. Patients with a bilateral source of the aldosterone are not candidates for surgical cure and must be managed with medical treatment, usually a mineralocorticoid receptor antagonist such as spironolactone that can neutralize or at least reduce the impact of hyperaldosteronism.
AVS finds unilateral adenomas when imaging can’t
The evidence that raised concerns about the reliability of imaging as an easier and noninvasive means to identify hypertensive patients with PA and a unilateral adrenal nodule that makes them candidates for surgical removal to resolve their PA and hypertension came out in May 2020 in a review of 174 PA patients who underwent AVS at a single center in Calgary, Alta., during 2006-2018.
The review included 366 patients with PA referred to the University of Calgary for assessment, of whom 179 had no adrenal nodule visible with either CT or MRI imaging, with 174 of these patients also undergoing successful AVS. The procedure revealed 70 patients (40%) had unilateral aldosterone secretion (Can J Cardiol. 2020 May 16. doi: 10.1016/j.cjca.2020.05.013).
In an editorial about this report that appeared a few weeks later, Ross D. Feldman, MD, a hypertension-management researcher and professor of medicine at the University of Manitoba in Winnipeg, Man., said the finding was “amazing,” and “confirms that lateralization of aldosterone secretion in a patient with PA but without an identifiable mass on that side is not a zebra,” but instead a presentation that “occurs in almost half of patients with PA and no discernible adenoma on the side that lateralizes.” (Can J. Cardiol. 2020 Jul 3. doi: 10.1016/j.cjca.2020.06.022).
Although this was just one center’s experience, the authors are not alone in making this finding, although prior reports seem to have been largely forgotten or ignored until now.
“The discordance between AVS and adrenal imaging has been documented by numerous groups, and in our own experience [in Melbourne] around 40% of patients with unilateral aldosterone excess do not have a distinct unilateral adenoma on CT,” said Dr. Yang.
“Here’s the problem,” summed up Dr. Feldman in an interview. “Nearly half of patients with hyperaldosteronism don’t localize based on a CT or MRI, so you have to do AVS, but AVS is not generally available; it’s only at tertiary centers; and you have to do a lot of them,” to do them well. “It’s a half-day procedure, and you have to hit the correct adrenal vein.”
AVS for millions?
Compounding the challenge is the other bit of bombshell news recently dropped on the endocrinology and hypertension communities: PA may be much more prevalent that previously suspected, occurring in roughly 20% of patients with hypertension, according to study results that also came out in 2020 (Ann Int Med. 2020 Jul 7;173[1]:10-20).
The upshot, according to Dr. Feldman and others, is that researchers will need to find reliable criteria besides imaging for identifying PA patients with an increased likelihood of having a lateralized source for their excess aldosterone production. That’s “the only hope,” said Dr. Feldman, “so we won’t have to do AVS on 20 million Americans.”
Unfortunately, the path toward a successful screen to winnow down candidates for AVS has been long and not especially fruitful, with efforts dating back at least 50 years, and with one of the most recent efforts at stratifying PA patients by certain laboratory measures getting dismissed as producing a benefit that “might not be substantial,” wrote Michael Stowasser, MBBS, in a published commentary (J Hypertension. 2020 Jul;38[7]:1259-61).
In contrast to Dr. Feldman, Dr. Stowasser was more optimistic about the prospects for avoiding an immediate crisis in AVS assessment of PA patients, mostly because so few patients with PA are now identified by clinicians. Given the poor record clinicians have historically rung up diagnosing PA, “it would seem unlikely that we are going to be flooded with AVS requests any time soon,” he wrote. There is also reason to hope that increased demand for AVS will help broaden availability, and innovative testing methods promise to speed up the procedure, said Dr. Stowasser, a professor of medicine at the University of Queensland in Brisbane, Australia and director of the Endocrine Hypertension Research Centre at Greenslopes and Princess Alexandra Hospitals in Brisbane, in an interview.
But regardless of whether AVS testing becomes more available or streamlined, recent events suggest there will be little way to avoid eventually having to run millions of these diagnostic procedures.
Patients with PA “who decide they will not want surgery do not need AVS. For all other patients with PA, you need AVS. The medical system will just have to respond,” Dr. Carey concluded.
Dr. Carey, Dr. Yang, Dr. Feldman, and Dr. Stowasser had no relevant disclosures.
New data challenge primary care’s inattention to aldosterone in hypertension
Jun Yang, MBBS, had watched as her father, who had battled hypertension for decades, ended up on four medications that still couldn’t bring his blood pressure to a healthy level. The cardiovascular endocrinologist then ran some tests, and soon thereafter her father had his blood pressure optimized on just one targeted medication.
Dr. Yang’s father was found to have a hormonal condition known as primary aldosteronism (PA) as the cause of his hypertension.
It turns out that PA is not as rare as once thought.
An eye-catching report in Annals of Internal Medicine this spring of an unexpectedly high prevalence of primary aldosteronism among a diverse cross section of U.S. patients with hypertension has raised issues that could dramatically change the way doctors in America, and elsewhere, assess and manage high blood pressure.
Foremost is the question of whether primary care physicians – the clinicians at the front line for diagnosing and initially treating most patients with hypertension – will absorb and act on this new evidence. For them, aldosteronism doesn’t automatically come to mind when they see high numbers on a BP monitor, and yet this latest research found that up to a third of all 726 patients in the study who were diagnosed with hypertension and with high urinary salt levels had PA.
That translates to a roughly three- to fivefold increase over standard prevalence estimates, and is a ”game changer” for how clinicians should approach hypertension management and PA diagnosis going forward, said John W. Funder, MD, in an editorial accompanying the Annals study.
Long considered relatively uncommon, hypertension driven by an excess of the hormone aldosterone, often because of an adenoma on the adrenal gland, is not the same as conventional “essential” hypertension. The former benefits from early diagnosis because its treatment is completely different – close to half of all PA patients can be treated definitively and quickly with surgical removal of an adenoma from one side of the adrenal gland.
For other PA patients, who have bilateral adrenal hyperplasia that is impossible to resolve surgically, treatment with drugs called mineralocorticoid receptor antagonists (MRAs), such as spironolactone, is needed because they target the hormonal cause of the high BP.
But what usually happens is that a patient with PA is mistakenly diagnosed with essential hypertension, in which the classic approach to treatment is to start with one regular antihypertensive drug, and add on further ones from different drug classes if blood pressure is not adequately controlled. When patients are taking three drugs, without adequate control, they are labeled as having “resistant hypertension.”
But in the case of PA, none of these conventional antihypertensives work, and the process of continuing to monitor and add different drugs wastes time, during which patients deteriorate.
“We need to change the culture of waiting for hypertension to be resistant and have patients riddled with end-organ damage,” due to years of persistently high BP and excess aldosterone “before we look for a secondary cause” like PA, declared Dr. Yang, of Hudson Institute of Medical Research and Monash University in Melbourne, during an interview.
So early diagnosis and prompt treatment of PA is key.
In addition to boosting the public health importance of early PA detection in hypertensive patients, the new up-sized PA prevalence numbers throw a spotlight on primary care physicians (PCPs) as key players who will need to apply the findings to practice on a public health scale.
These novel results create a need for “new guidelines, and a radically revised game plan with the key role of PCPs” emphasized in future management of patients with hypertension, said Dr. Funder, a professor of medicine at Monash University, in a second recent editorial in Hypertension.
“Buy-in by PCPs is essential,” agrees Robert M. Carey, MD, a cardiovascular endocrinologist and professor of medicine at the University of Virginia in Charlottesville, and a coauthor of the new study.
But he too acknowledges that this presents a major challenge. PCPs and internists, who diagnose a lot of hypertension, are “not used to thinking about aldosterone,” he said in an interview, encapsulating the key problem faced by proponents of earlier and more widespread PA assessment.
This dilemma looms as a “huge public health issue,” Dr. Carey warned.
‘We’re a long way from getting’ PCPs to buy in to PA screening
Will PCPs grow more comfortable with screening patients for PA themselves, or might they become more willing to refer hypertensive individuals for assessment at an expert center?
One skeptic is Ross D. Feldman, MD, a hypertension-management researcher and professor of medicine at the University of Manitoba in Winnipeg. The finding about high PA prevalence in patients with hypertension “is brand new, [and] the message needs to get to PCPs,” he said. But, “We’re a long way from getting it” to them. “I don’t know how to do that. It will be a tough sell.”
In addition, repositioning MRAs as an earlier option for many hypertensive patients won’t be easy either, because “we’ll never have outcome-trial data for MRAs,” given that they are now generic drugs, he noted.
“No clinical trial data show [MRAs] are first-line drugs,” said Dr. Feldman, who explained that, instead, MRAs are considered “go-to drugs” for patients with treatment-resistant hypertension, a niche therapeutic area. Results from the PATHWAY-2 trial published 5 years ago in Lancet showed “spironolactone was clearly the most effective treatment for the condition,” according to the report authors.
But even among patients with resistant hypertension, screening for PA dramatically lags despite being enshrined in guidelines.
“PCPs should start checking aldosterone-to-renin ratios [a widely used PA screen] in all patients with resistant hypertension or hypertension with hypokalemia, and then refer patients to specialists for testing and management,” said Jordana B. Cohen, MD, a nephrologist and hypertension researcher at the University of Pennsylvania in Philadelphia.
But recent studies of U.S. patient populations with clinical characteristics that meet existing criteria for PA screening showed that just 1%-2% of these individuals underwent an initial PA assessment, she noted, citing reports in the journals Surgery and Hypertension.
“We need to prioritize improving screening in these high-risk patients,” she stressed in an interview.
This illustrates that, in some respects, the new prevalence numbers are beside the point, because PA has been going unscreened and overlooked far too often even in the context of historical, lower prevalence rates, said Dr. Yang.
“The key point is that approximately 1 in 10 people with hypertension, and even more with resistant hypertension, have a form of the disease that is worse than essential hypertension but is routinely missed at present” and is also highly treatable.
“Evidence for the need for increased awareness of PA has been building for 2 decades,” stressed Dr. Yang, who has coauthored several commentaries and reviews that have bemoaned PA’s underappreciated status.
Interest in partnering with PCPs on guidance grows
One potential solution is to have endocrinologists and hypertension specialists’ partner with PCPs to come up with diagnostic and management recommendations. Both Dr. Funder and Dr. Carey are opinion leaders regarding the role of aldosterone in hypertension, and both were coauthors of the 2016 Endocrine Society guideline for PA assessment and management published in the Journal of Clinical Endocrinology & Metabolism , with Dr. Funder chairing the writing panel.
Now approaching its fifth year in effect, this guideline is “due for revision,” and “my hope is that we’ll be able to partner with one or more PCP organizations to come up with a version of the guideline targeted to PCPs,” Dr. Carey said.
He voiced interest in working on this with the American College of Physicians, which represents U.S. internal medicine physicians, and the American Academy of Family Physicians.
“We definitely need a partnership and educational efforts to get the word out from these organizations and not from a specialty society,” said Dr. Carey.
Dr. Funder said he has submitted a proposal to the Endocrine Society for a guidelines update he would chair with Dr. Carey’s assistance and with a diverse writing group that includes PCPs. Dr. Carey said that ideally this panel would write and release a revised guideline in 2021.
“Several of us are chomping at the bit to get this done,” he noted.
But participation by the ACP and AAFP remain uncertain as of September 2020. When approached about this, an ACP spokesperson said the organization had no comment. A spokesperson for the AAFP said, “It’s too early to tell if we will partner with any other organizations to develop guidelines specific to excess aldosterone, and how such guidelines might be received by our members.”
Recent history shows little cooperation between ACP, AAFP, and what might be termed the U.S. hypertension “establishment.” For example, when the American College of Cardiology and the American Heart Association released their most recent essential hypertension management guidelines in Hypertension in 2018, it was never adopted by ACP or AAFP.
The latter two organizations continue to endorse a higher BP threshold for diagnosing hypertension, and higher treatment targets set by alternative expert panels to those of the AHA/ACC.
Collaboration feasible, although PCPs overworked
Dr. Carey hopes that this episode will not preclude agreement over PA screening.
“I think it is still possible to partner with [the ACP and AAFP],” he observed, adding that he believes high PA prevalence among hypertensive patients and its consequences when unrecognized is “noncontentious.”
But he acknowledges that other, substantial hurdles also exist, notably the “overwhelming workload” that American PCPs already face.
David O’Gurek, MD, a family and community medicine physician at the Lewis Katz School of Medicine of Temple University in Philadelphia, agrees that a revamped approach to PA screening developed cooperatively between PCPs and specialists is an important goal and potentially feasible despite prior disagreements. “There has to be room for collaboration,” he said, but also emphasized the need for developing policies based on a systematic evidence review and a focus on patient-centered outcomes.
“We’re certainly missing patients with PA, but there needs to be greater clarity and standardization about the most appropriate screening approach and cutoff level” for flagging patients who need specialized assessment, Dr. O’Gurek said in an interview.
The current endocrinology literature also shows that experts remain divided on how best to accomplish this.
And some hypertension specialists question whether existing evidence is conclusive enough to warrant revised guidelines.
Dr. Cohen, the nephrologist and hypertension researcher, said that, while the recent prevalence report in Annals of Internal Medicine is “intriguing, hypothesis-generating information that suggests we are missing many cases of hyperaldosteronism in routine care,” she nevertheless believes that “we need additional data to be able to truly understand the breadth and implications of the findings.”
William C. Cushman, MD, a hypertension management specialist at the University of Tennessee Health Science Center in Memphis, agrees.
Changing existing practice guidelines “really needs randomized, controlled trials demonstrating a difference in long-term outcomes, ideally major cardiovascular outcomes,” that result from broader PA screening, he said.
Dr. Carey concurs that more evidence is needed to confirm the Annals report, but is confident this evidence will be in hand by the time a guideline-revision panel meets in 2021.
Australian model of PCPs screening for PA could be implemented in United States
An example of what might be possible when PCPs, endocrinologists, and hypertension specialists work together to make PA screening more accessible can be found in Melbourne, at the Endocrine Hypertension Service of Monash Health, in association with the Hudson Institute of Medical Research.
This began operating in July 2016, cofounded by Dr. Yang, whose experiences with her own father made her sensitive to the issue.
The service’s aim is to “address the underdiagnosis of PA, and to offer a streamlined diagnostic service for patients with hypertension,” with an “extensive outreach program” targeted to regional PCPs that, among other messages, encourages them to screen patients for PA when blood pressures exceed 140/90 mm Hg.
During its first 3 years of operation, the service saw 267 patients, with PA diagnosed in 135 and ruled out in 73 patients.
Notably, the proportion of these patients referred from PCPs jumped from 21% of 70 patients during the first year of operation to 47% of 70 patients during year 2, and 52% of 127 patients during the third year, ending in July 2019, said Dr. Yang, who continues to help run the service.
During the first year, a scant 3% of referred patients had recently diagnosed hypertension, but this rose to 14% during the second year, and to 19% during the most recent year with data available.
The median duration of diagnosed hypertension among referred patients fell from 11 years during year 1, to 7 years during year 3.
Service clinicians diagnosed 37 patients with unilateral adenomas, and removed them from 23 patients with four more awaiting surgery and the remaining 10 opting instead for medical management. Another 95 patients went on therapy with a MRA, and during the most recent year studied all patients who began a MRA regimen had a partial or complete clinical response.
Dr. Carey said the “creative program represents a model for implementation in U.S. practice.
Dr. Funder, Dr. Carey, Dr. Feldman, Dr. Yang, Dr. Cohen, and Dr. O’Gurek had no relevant disclosures. Dr. Cushman has been a consultant to Novartis, received personal fees from Sanofi, and research funding from Eli Lilly.
Jun Yang, MBBS, had watched as her father, who had battled hypertension for decades, ended up on four medications that still couldn’t bring his blood pressure to a healthy level. The cardiovascular endocrinologist then ran some tests, and soon thereafter her father had his blood pressure optimized on just one targeted medication.
Dr. Yang’s father was found to have a hormonal condition known as primary aldosteronism (PA) as the cause of his hypertension.
It turns out that PA is not as rare as once thought.
An eye-catching report in Annals of Internal Medicine this spring of an unexpectedly high prevalence of primary aldosteronism among a diverse cross section of U.S. patients with hypertension has raised issues that could dramatically change the way doctors in America, and elsewhere, assess and manage high blood pressure.
Foremost is the question of whether primary care physicians – the clinicians at the front line for diagnosing and initially treating most patients with hypertension – will absorb and act on this new evidence. For them, aldosteronism doesn’t automatically come to mind when they see high numbers on a BP monitor, and yet this latest research found that up to a third of all 726 patients in the study who were diagnosed with hypertension and with high urinary salt levels had PA.
That translates to a roughly three- to fivefold increase over standard prevalence estimates, and is a ”game changer” for how clinicians should approach hypertension management and PA diagnosis going forward, said John W. Funder, MD, in an editorial accompanying the Annals study.
Long considered relatively uncommon, hypertension driven by an excess of the hormone aldosterone, often because of an adenoma on the adrenal gland, is not the same as conventional “essential” hypertension. The former benefits from early diagnosis because its treatment is completely different – close to half of all PA patients can be treated definitively and quickly with surgical removal of an adenoma from one side of the adrenal gland.
For other PA patients, who have bilateral adrenal hyperplasia that is impossible to resolve surgically, treatment with drugs called mineralocorticoid receptor antagonists (MRAs), such as spironolactone, is needed because they target the hormonal cause of the high BP.
But what usually happens is that a patient with PA is mistakenly diagnosed with essential hypertension, in which the classic approach to treatment is to start with one regular antihypertensive drug, and add on further ones from different drug classes if blood pressure is not adequately controlled. When patients are taking three drugs, without adequate control, they are labeled as having “resistant hypertension.”
But in the case of PA, none of these conventional antihypertensives work, and the process of continuing to monitor and add different drugs wastes time, during which patients deteriorate.
“We need to change the culture of waiting for hypertension to be resistant and have patients riddled with end-organ damage,” due to years of persistently high BP and excess aldosterone “before we look for a secondary cause” like PA, declared Dr. Yang, of Hudson Institute of Medical Research and Monash University in Melbourne, during an interview.
So early diagnosis and prompt treatment of PA is key.
In addition to boosting the public health importance of early PA detection in hypertensive patients, the new up-sized PA prevalence numbers throw a spotlight on primary care physicians (PCPs) as key players who will need to apply the findings to practice on a public health scale.
These novel results create a need for “new guidelines, and a radically revised game plan with the key role of PCPs” emphasized in future management of patients with hypertension, said Dr. Funder, a professor of medicine at Monash University, in a second recent editorial in Hypertension.
“Buy-in by PCPs is essential,” agrees Robert M. Carey, MD, a cardiovascular endocrinologist and professor of medicine at the University of Virginia in Charlottesville, and a coauthor of the new study.
But he too acknowledges that this presents a major challenge. PCPs and internists, who diagnose a lot of hypertension, are “not used to thinking about aldosterone,” he said in an interview, encapsulating the key problem faced by proponents of earlier and more widespread PA assessment.
This dilemma looms as a “huge public health issue,” Dr. Carey warned.
‘We’re a long way from getting’ PCPs to buy in to PA screening
Will PCPs grow more comfortable with screening patients for PA themselves, or might they become more willing to refer hypertensive individuals for assessment at an expert center?
One skeptic is Ross D. Feldman, MD, a hypertension-management researcher and professor of medicine at the University of Manitoba in Winnipeg. The finding about high PA prevalence in patients with hypertension “is brand new, [and] the message needs to get to PCPs,” he said. But, “We’re a long way from getting it” to them. “I don’t know how to do that. It will be a tough sell.”
In addition, repositioning MRAs as an earlier option for many hypertensive patients won’t be easy either, because “we’ll never have outcome-trial data for MRAs,” given that they are now generic drugs, he noted.
“No clinical trial data show [MRAs] are first-line drugs,” said Dr. Feldman, who explained that, instead, MRAs are considered “go-to drugs” for patients with treatment-resistant hypertension, a niche therapeutic area. Results from the PATHWAY-2 trial published 5 years ago in Lancet showed “spironolactone was clearly the most effective treatment for the condition,” according to the report authors.
But even among patients with resistant hypertension, screening for PA dramatically lags despite being enshrined in guidelines.
“PCPs should start checking aldosterone-to-renin ratios [a widely used PA screen] in all patients with resistant hypertension or hypertension with hypokalemia, and then refer patients to specialists for testing and management,” said Jordana B. Cohen, MD, a nephrologist and hypertension researcher at the University of Pennsylvania in Philadelphia.
But recent studies of U.S. patient populations with clinical characteristics that meet existing criteria for PA screening showed that just 1%-2% of these individuals underwent an initial PA assessment, she noted, citing reports in the journals Surgery and Hypertension.
“We need to prioritize improving screening in these high-risk patients,” she stressed in an interview.
This illustrates that, in some respects, the new prevalence numbers are beside the point, because PA has been going unscreened and overlooked far too often even in the context of historical, lower prevalence rates, said Dr. Yang.
“The key point is that approximately 1 in 10 people with hypertension, and even more with resistant hypertension, have a form of the disease that is worse than essential hypertension but is routinely missed at present” and is also highly treatable.
“Evidence for the need for increased awareness of PA has been building for 2 decades,” stressed Dr. Yang, who has coauthored several commentaries and reviews that have bemoaned PA’s underappreciated status.
Interest in partnering with PCPs on guidance grows
One potential solution is to have endocrinologists and hypertension specialists’ partner with PCPs to come up with diagnostic and management recommendations. Both Dr. Funder and Dr. Carey are opinion leaders regarding the role of aldosterone in hypertension, and both were coauthors of the 2016 Endocrine Society guideline for PA assessment and management published in the Journal of Clinical Endocrinology & Metabolism , with Dr. Funder chairing the writing panel.
Now approaching its fifth year in effect, this guideline is “due for revision,” and “my hope is that we’ll be able to partner with one or more PCP organizations to come up with a version of the guideline targeted to PCPs,” Dr. Carey said.
He voiced interest in working on this with the American College of Physicians, which represents U.S. internal medicine physicians, and the American Academy of Family Physicians.
“We definitely need a partnership and educational efforts to get the word out from these organizations and not from a specialty society,” said Dr. Carey.
Dr. Funder said he has submitted a proposal to the Endocrine Society for a guidelines update he would chair with Dr. Carey’s assistance and with a diverse writing group that includes PCPs. Dr. Carey said that ideally this panel would write and release a revised guideline in 2021.
“Several of us are chomping at the bit to get this done,” he noted.
But participation by the ACP and AAFP remain uncertain as of September 2020. When approached about this, an ACP spokesperson said the organization had no comment. A spokesperson for the AAFP said, “It’s too early to tell if we will partner with any other organizations to develop guidelines specific to excess aldosterone, and how such guidelines might be received by our members.”
Recent history shows little cooperation between ACP, AAFP, and what might be termed the U.S. hypertension “establishment.” For example, when the American College of Cardiology and the American Heart Association released their most recent essential hypertension management guidelines in Hypertension in 2018, it was never adopted by ACP or AAFP.
The latter two organizations continue to endorse a higher BP threshold for diagnosing hypertension, and higher treatment targets set by alternative expert panels to those of the AHA/ACC.
Collaboration feasible, although PCPs overworked
Dr. Carey hopes that this episode will not preclude agreement over PA screening.
“I think it is still possible to partner with [the ACP and AAFP],” he observed, adding that he believes high PA prevalence among hypertensive patients and its consequences when unrecognized is “noncontentious.”
But he acknowledges that other, substantial hurdles also exist, notably the “overwhelming workload” that American PCPs already face.
David O’Gurek, MD, a family and community medicine physician at the Lewis Katz School of Medicine of Temple University in Philadelphia, agrees that a revamped approach to PA screening developed cooperatively between PCPs and specialists is an important goal and potentially feasible despite prior disagreements. “There has to be room for collaboration,” he said, but also emphasized the need for developing policies based on a systematic evidence review and a focus on patient-centered outcomes.
“We’re certainly missing patients with PA, but there needs to be greater clarity and standardization about the most appropriate screening approach and cutoff level” for flagging patients who need specialized assessment, Dr. O’Gurek said in an interview.
The current endocrinology literature also shows that experts remain divided on how best to accomplish this.
And some hypertension specialists question whether existing evidence is conclusive enough to warrant revised guidelines.
Dr. Cohen, the nephrologist and hypertension researcher, said that, while the recent prevalence report in Annals of Internal Medicine is “intriguing, hypothesis-generating information that suggests we are missing many cases of hyperaldosteronism in routine care,” she nevertheless believes that “we need additional data to be able to truly understand the breadth and implications of the findings.”
William C. Cushman, MD, a hypertension management specialist at the University of Tennessee Health Science Center in Memphis, agrees.
Changing existing practice guidelines “really needs randomized, controlled trials demonstrating a difference in long-term outcomes, ideally major cardiovascular outcomes,” that result from broader PA screening, he said.
Dr. Carey concurs that more evidence is needed to confirm the Annals report, but is confident this evidence will be in hand by the time a guideline-revision panel meets in 2021.
Australian model of PCPs screening for PA could be implemented in United States
An example of what might be possible when PCPs, endocrinologists, and hypertension specialists work together to make PA screening more accessible can be found in Melbourne, at the Endocrine Hypertension Service of Monash Health, in association with the Hudson Institute of Medical Research.
This began operating in July 2016, cofounded by Dr. Yang, whose experiences with her own father made her sensitive to the issue.
The service’s aim is to “address the underdiagnosis of PA, and to offer a streamlined diagnostic service for patients with hypertension,” with an “extensive outreach program” targeted to regional PCPs that, among other messages, encourages them to screen patients for PA when blood pressures exceed 140/90 mm Hg.
During its first 3 years of operation, the service saw 267 patients, with PA diagnosed in 135 and ruled out in 73 patients.
Notably, the proportion of these patients referred from PCPs jumped from 21% of 70 patients during the first year of operation to 47% of 70 patients during year 2, and 52% of 127 patients during the third year, ending in July 2019, said Dr. Yang, who continues to help run the service.
During the first year, a scant 3% of referred patients had recently diagnosed hypertension, but this rose to 14% during the second year, and to 19% during the most recent year with data available.
The median duration of diagnosed hypertension among referred patients fell from 11 years during year 1, to 7 years during year 3.
Service clinicians diagnosed 37 patients with unilateral adenomas, and removed them from 23 patients with four more awaiting surgery and the remaining 10 opting instead for medical management. Another 95 patients went on therapy with a MRA, and during the most recent year studied all patients who began a MRA regimen had a partial or complete clinical response.
Dr. Carey said the “creative program represents a model for implementation in U.S. practice.
Dr. Funder, Dr. Carey, Dr. Feldman, Dr. Yang, Dr. Cohen, and Dr. O’Gurek had no relevant disclosures. Dr. Cushman has been a consultant to Novartis, received personal fees from Sanofi, and research funding from Eli Lilly.
Jun Yang, MBBS, had watched as her father, who had battled hypertension for decades, ended up on four medications that still couldn’t bring his blood pressure to a healthy level. The cardiovascular endocrinologist then ran some tests, and soon thereafter her father had his blood pressure optimized on just one targeted medication.
Dr. Yang’s father was found to have a hormonal condition known as primary aldosteronism (PA) as the cause of his hypertension.
It turns out that PA is not as rare as once thought.
An eye-catching report in Annals of Internal Medicine this spring of an unexpectedly high prevalence of primary aldosteronism among a diverse cross section of U.S. patients with hypertension has raised issues that could dramatically change the way doctors in America, and elsewhere, assess and manage high blood pressure.
Foremost is the question of whether primary care physicians – the clinicians at the front line for diagnosing and initially treating most patients with hypertension – will absorb and act on this new evidence. For them, aldosteronism doesn’t automatically come to mind when they see high numbers on a BP monitor, and yet this latest research found that up to a third of all 726 patients in the study who were diagnosed with hypertension and with high urinary salt levels had PA.
That translates to a roughly three- to fivefold increase over standard prevalence estimates, and is a ”game changer” for how clinicians should approach hypertension management and PA diagnosis going forward, said John W. Funder, MD, in an editorial accompanying the Annals study.
Long considered relatively uncommon, hypertension driven by an excess of the hormone aldosterone, often because of an adenoma on the adrenal gland, is not the same as conventional “essential” hypertension. The former benefits from early diagnosis because its treatment is completely different – close to half of all PA patients can be treated definitively and quickly with surgical removal of an adenoma from one side of the adrenal gland.
For other PA patients, who have bilateral adrenal hyperplasia that is impossible to resolve surgically, treatment with drugs called mineralocorticoid receptor antagonists (MRAs), such as spironolactone, is needed because they target the hormonal cause of the high BP.
But what usually happens is that a patient with PA is mistakenly diagnosed with essential hypertension, in which the classic approach to treatment is to start with one regular antihypertensive drug, and add on further ones from different drug classes if blood pressure is not adequately controlled. When patients are taking three drugs, without adequate control, they are labeled as having “resistant hypertension.”
But in the case of PA, none of these conventional antihypertensives work, and the process of continuing to monitor and add different drugs wastes time, during which patients deteriorate.
“We need to change the culture of waiting for hypertension to be resistant and have patients riddled with end-organ damage,” due to years of persistently high BP and excess aldosterone “before we look for a secondary cause” like PA, declared Dr. Yang, of Hudson Institute of Medical Research and Monash University in Melbourne, during an interview.
So early diagnosis and prompt treatment of PA is key.
In addition to boosting the public health importance of early PA detection in hypertensive patients, the new up-sized PA prevalence numbers throw a spotlight on primary care physicians (PCPs) as key players who will need to apply the findings to practice on a public health scale.
These novel results create a need for “new guidelines, and a radically revised game plan with the key role of PCPs” emphasized in future management of patients with hypertension, said Dr. Funder, a professor of medicine at Monash University, in a second recent editorial in Hypertension.
“Buy-in by PCPs is essential,” agrees Robert M. Carey, MD, a cardiovascular endocrinologist and professor of medicine at the University of Virginia in Charlottesville, and a coauthor of the new study.
But he too acknowledges that this presents a major challenge. PCPs and internists, who diagnose a lot of hypertension, are “not used to thinking about aldosterone,” he said in an interview, encapsulating the key problem faced by proponents of earlier and more widespread PA assessment.
This dilemma looms as a “huge public health issue,” Dr. Carey warned.
‘We’re a long way from getting’ PCPs to buy in to PA screening
Will PCPs grow more comfortable with screening patients for PA themselves, or might they become more willing to refer hypertensive individuals for assessment at an expert center?
One skeptic is Ross D. Feldman, MD, a hypertension-management researcher and professor of medicine at the University of Manitoba in Winnipeg. The finding about high PA prevalence in patients with hypertension “is brand new, [and] the message needs to get to PCPs,” he said. But, “We’re a long way from getting it” to them. “I don’t know how to do that. It will be a tough sell.”
In addition, repositioning MRAs as an earlier option for many hypertensive patients won’t be easy either, because “we’ll never have outcome-trial data for MRAs,” given that they are now generic drugs, he noted.
“No clinical trial data show [MRAs] are first-line drugs,” said Dr. Feldman, who explained that, instead, MRAs are considered “go-to drugs” for patients with treatment-resistant hypertension, a niche therapeutic area. Results from the PATHWAY-2 trial published 5 years ago in Lancet showed “spironolactone was clearly the most effective treatment for the condition,” according to the report authors.
But even among patients with resistant hypertension, screening for PA dramatically lags despite being enshrined in guidelines.
“PCPs should start checking aldosterone-to-renin ratios [a widely used PA screen] in all patients with resistant hypertension or hypertension with hypokalemia, and then refer patients to specialists for testing and management,” said Jordana B. Cohen, MD, a nephrologist and hypertension researcher at the University of Pennsylvania in Philadelphia.
But recent studies of U.S. patient populations with clinical characteristics that meet existing criteria for PA screening showed that just 1%-2% of these individuals underwent an initial PA assessment, she noted, citing reports in the journals Surgery and Hypertension.
“We need to prioritize improving screening in these high-risk patients,” she stressed in an interview.
This illustrates that, in some respects, the new prevalence numbers are beside the point, because PA has been going unscreened and overlooked far too often even in the context of historical, lower prevalence rates, said Dr. Yang.
“The key point is that approximately 1 in 10 people with hypertension, and even more with resistant hypertension, have a form of the disease that is worse than essential hypertension but is routinely missed at present” and is also highly treatable.
“Evidence for the need for increased awareness of PA has been building for 2 decades,” stressed Dr. Yang, who has coauthored several commentaries and reviews that have bemoaned PA’s underappreciated status.
Interest in partnering with PCPs on guidance grows
One potential solution is to have endocrinologists and hypertension specialists’ partner with PCPs to come up with diagnostic and management recommendations. Both Dr. Funder and Dr. Carey are opinion leaders regarding the role of aldosterone in hypertension, and both were coauthors of the 2016 Endocrine Society guideline for PA assessment and management published in the Journal of Clinical Endocrinology & Metabolism , with Dr. Funder chairing the writing panel.
Now approaching its fifth year in effect, this guideline is “due for revision,” and “my hope is that we’ll be able to partner with one or more PCP organizations to come up with a version of the guideline targeted to PCPs,” Dr. Carey said.
He voiced interest in working on this with the American College of Physicians, which represents U.S. internal medicine physicians, and the American Academy of Family Physicians.
“We definitely need a partnership and educational efforts to get the word out from these organizations and not from a specialty society,” said Dr. Carey.
Dr. Funder said he has submitted a proposal to the Endocrine Society for a guidelines update he would chair with Dr. Carey’s assistance and with a diverse writing group that includes PCPs. Dr. Carey said that ideally this panel would write and release a revised guideline in 2021.
“Several of us are chomping at the bit to get this done,” he noted.
But participation by the ACP and AAFP remain uncertain as of September 2020. When approached about this, an ACP spokesperson said the organization had no comment. A spokesperson for the AAFP said, “It’s too early to tell if we will partner with any other organizations to develop guidelines specific to excess aldosterone, and how such guidelines might be received by our members.”
Recent history shows little cooperation between ACP, AAFP, and what might be termed the U.S. hypertension “establishment.” For example, when the American College of Cardiology and the American Heart Association released their most recent essential hypertension management guidelines in Hypertension in 2018, it was never adopted by ACP or AAFP.
The latter two organizations continue to endorse a higher BP threshold for diagnosing hypertension, and higher treatment targets set by alternative expert panels to those of the AHA/ACC.
Collaboration feasible, although PCPs overworked
Dr. Carey hopes that this episode will not preclude agreement over PA screening.
“I think it is still possible to partner with [the ACP and AAFP],” he observed, adding that he believes high PA prevalence among hypertensive patients and its consequences when unrecognized is “noncontentious.”
But he acknowledges that other, substantial hurdles also exist, notably the “overwhelming workload” that American PCPs already face.
David O’Gurek, MD, a family and community medicine physician at the Lewis Katz School of Medicine of Temple University in Philadelphia, agrees that a revamped approach to PA screening developed cooperatively between PCPs and specialists is an important goal and potentially feasible despite prior disagreements. “There has to be room for collaboration,” he said, but also emphasized the need for developing policies based on a systematic evidence review and a focus on patient-centered outcomes.
“We’re certainly missing patients with PA, but there needs to be greater clarity and standardization about the most appropriate screening approach and cutoff level” for flagging patients who need specialized assessment, Dr. O’Gurek said in an interview.
The current endocrinology literature also shows that experts remain divided on how best to accomplish this.
And some hypertension specialists question whether existing evidence is conclusive enough to warrant revised guidelines.
Dr. Cohen, the nephrologist and hypertension researcher, said that, while the recent prevalence report in Annals of Internal Medicine is “intriguing, hypothesis-generating information that suggests we are missing many cases of hyperaldosteronism in routine care,” she nevertheless believes that “we need additional data to be able to truly understand the breadth and implications of the findings.”
William C. Cushman, MD, a hypertension management specialist at the University of Tennessee Health Science Center in Memphis, agrees.
Changing existing practice guidelines “really needs randomized, controlled trials demonstrating a difference in long-term outcomes, ideally major cardiovascular outcomes,” that result from broader PA screening, he said.
Dr. Carey concurs that more evidence is needed to confirm the Annals report, but is confident this evidence will be in hand by the time a guideline-revision panel meets in 2021.
Australian model of PCPs screening for PA could be implemented in United States
An example of what might be possible when PCPs, endocrinologists, and hypertension specialists work together to make PA screening more accessible can be found in Melbourne, at the Endocrine Hypertension Service of Monash Health, in association with the Hudson Institute of Medical Research.
This began operating in July 2016, cofounded by Dr. Yang, whose experiences with her own father made her sensitive to the issue.
The service’s aim is to “address the underdiagnosis of PA, and to offer a streamlined diagnostic service for patients with hypertension,” with an “extensive outreach program” targeted to regional PCPs that, among other messages, encourages them to screen patients for PA when blood pressures exceed 140/90 mm Hg.
During its first 3 years of operation, the service saw 267 patients, with PA diagnosed in 135 and ruled out in 73 patients.
Notably, the proportion of these patients referred from PCPs jumped from 21% of 70 patients during the first year of operation to 47% of 70 patients during year 2, and 52% of 127 patients during the third year, ending in July 2019, said Dr. Yang, who continues to help run the service.
During the first year, a scant 3% of referred patients had recently diagnosed hypertension, but this rose to 14% during the second year, and to 19% during the most recent year with data available.
The median duration of diagnosed hypertension among referred patients fell from 11 years during year 1, to 7 years during year 3.
Service clinicians diagnosed 37 patients with unilateral adenomas, and removed them from 23 patients with four more awaiting surgery and the remaining 10 opting instead for medical management. Another 95 patients went on therapy with a MRA, and during the most recent year studied all patients who began a MRA regimen had a partial or complete clinical response.
Dr. Carey said the “creative program represents a model for implementation in U.S. practice.
Dr. Funder, Dr. Carey, Dr. Feldman, Dr. Yang, Dr. Cohen, and Dr. O’Gurek had no relevant disclosures. Dr. Cushman has been a consultant to Novartis, received personal fees from Sanofi, and research funding from Eli Lilly.
Keep desiccated thyroid as a treatment option for hypothyroidism
new research shows.
The findings are “unanticipated ... given concerns for variability between batches of desiccated thyroid cited by national guidelines,” wrote the authors of the study, which was published this month in the Annals of Family Medicine.
In the trial, patients who had been treated for hypothyroidism at Kaiser Permanente Colorado were matched retrospectively into groups of 450 patients each according to whether they were treated with desiccated thyroid or synthetic levothyroxine.
After a follow-up of 3 years, TSH values within normal ranges (0.320-5.500 uIU/mL) were seen at approximately the same rate among those treated with desiccated thyroid and those who received levothyroxine (79.1% vs. 79.3%; P = .905).
“This study showed that after 3 years TSH values in both groups remained within reference ranges approximately 80% of the time,” said Rolake Kuye, PharmD, and colleagues with Kaiser Permanente, in Denver, Colorado.
In an accompanying editorial, Jill Schneiderhan, MD, and Suzanna Zick, ND, MPH, of the University of Michigan, Ann Arbor, say the overall results indicate that the continued use of desiccated thyroid is warranted in some cases.
“Keeping desiccated thyroid medications as an option in our tool kit will allow for improved shared decision-making, while allowing for patient preference, and offer an option for those patients who remain symptomatic on levothyroxine monotherapy,” they advised.
Some variability still seen with desiccated thyroid
Desiccated thyroid (dehydrated porcine thyroid), which was long the standard of care, is still commonly used in the treatment of hypothyroidism, despite having been replaced beginning in the 1970s by synthetic levothyroxine in light of evidence that the former was associated with more variability in thyroid hormone levels.
Desiccated thyroid is still sold legally by prescription in the United States under the names Nature Thyroid, Thyroid USP, and Armour Thyroid and is currently used by up to 30% of patients with hypothyroidism, according to recent estimates.
Consistent with concerns about variability in thyroid hormone levels, the new study did show greater variability in TSH levels with desiccated thyroid when assessed on a visit-to-visit basis.
Dr. Kuye and coauthors therefore recommended that, “[f]or providers targeting a tighter TSH goal in certain patients, the decreased TSH variability with levothyroxine could be clinically meaningful.”
This long-term investigation is “much needed”
This new study adds important new insight to the ongoing debate over hypothyroidism treatment, said Dr. Schneiderhan and Dr. Zick in their editorial.
“[The study authors] begin a much-needed investigation into whether patients prescribed synthetic levothyroxine compared with desiccated thyroid had differences in TSH stability over the course of 3 years.
“Further prospective studies are needed to confirm these results and to explore differences in more diverse patient populations, such as Hashimoto’s thyroiditis, as well as on quality of life and other important patient-reported outcomes such as fatigue and weight gain,” the editorialists added.
“This study does, however, provide helpful information that desiccated thyroid products are a reasonable choice for treating some hypothyroid patients.”
For 60% of patients in both groups, TSH levels were within reference range for whole study
In the study, Dr. Kuye and colleagues matched patients (average age, 63 years; 90% women) in terms of characteristics such as race, comorbidities, and cholesterol levels.
Patients were excluded if they had been prescribed more than one agent for the treatment of hypothyroidism or if they had comorbid conditions, including a history of thyroid cancer or other related comorbidities, as well as pregnancy.
With respect to visit-to-visit TSH level variability, the lower rate among patients prescribed levothyroxine in comparison with patients prescribed desiccated thyroid was statistically significant (1.25 vs. 1.44; P = .015). Among 60% of patients in both groups, all TSH values measured during the study period were within reference ranges, however (P = .951).
The median number of TSH laboratory studies obtained during the study was four in the synthetic levothyroxine group and three for patients prescribed desiccated thyroid (P = .578).
There were some notable differences between the groups. Patients in the desiccated thyroid group had lower body mass index (P = .032), hemoglobin A1c levels (P = .041), and lower baseline TSH values (2.4 vs. 3.4 uIU/mL; P = .001). compared with those prescribed levothyroxine.
Limitations include the fact that the authors could not account for potentially important variables such as rates of adherence, differences in prescriber practice between agents, or the concurrent use of other medications.
Subjective outcomes not assessed: “One-size-fits-all approach doesn’t work”
The authors note they were not able to assess subjective outcomes, which, as noted by the editorialists, are particularly important in hypothyroidism.
“Emerging evidence shows that for many patients, symptoms persist despite normal TSH values,” Dr. Schneiderhan and Dr. Zick write.
They cite as an example a large study that found significant impairment in psychological well-being among patients treated with thyroxine replacement, despite their achieving normal TSH levels.
In addition, synthetic levothyroxine is associated with other uncertainties, such as complexities in the conversion of T4 to triiodothyronine (T3) that may disrupt thyroid metabolism in some patients.
In addition, there are differences in the amounts of thyroid replacement needed by certain groups, such as patients who have undergone thyroidectomies.
“The one-size-fits-all approach for treating hypothyroidism does not work ... for all patients,” they concluded.
The study authors and editorialists have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research shows.
The findings are “unanticipated ... given concerns for variability between batches of desiccated thyroid cited by national guidelines,” wrote the authors of the study, which was published this month in the Annals of Family Medicine.
In the trial, patients who had been treated for hypothyroidism at Kaiser Permanente Colorado were matched retrospectively into groups of 450 patients each according to whether they were treated with desiccated thyroid or synthetic levothyroxine.
After a follow-up of 3 years, TSH values within normal ranges (0.320-5.500 uIU/mL) were seen at approximately the same rate among those treated with desiccated thyroid and those who received levothyroxine (79.1% vs. 79.3%; P = .905).
“This study showed that after 3 years TSH values in both groups remained within reference ranges approximately 80% of the time,” said Rolake Kuye, PharmD, and colleagues with Kaiser Permanente, in Denver, Colorado.
In an accompanying editorial, Jill Schneiderhan, MD, and Suzanna Zick, ND, MPH, of the University of Michigan, Ann Arbor, say the overall results indicate that the continued use of desiccated thyroid is warranted in some cases.
“Keeping desiccated thyroid medications as an option in our tool kit will allow for improved shared decision-making, while allowing for patient preference, and offer an option for those patients who remain symptomatic on levothyroxine monotherapy,” they advised.
Some variability still seen with desiccated thyroid
Desiccated thyroid (dehydrated porcine thyroid), which was long the standard of care, is still commonly used in the treatment of hypothyroidism, despite having been replaced beginning in the 1970s by synthetic levothyroxine in light of evidence that the former was associated with more variability in thyroid hormone levels.
Desiccated thyroid is still sold legally by prescription in the United States under the names Nature Thyroid, Thyroid USP, and Armour Thyroid and is currently used by up to 30% of patients with hypothyroidism, according to recent estimates.
Consistent with concerns about variability in thyroid hormone levels, the new study did show greater variability in TSH levels with desiccated thyroid when assessed on a visit-to-visit basis.
Dr. Kuye and coauthors therefore recommended that, “[f]or providers targeting a tighter TSH goal in certain patients, the decreased TSH variability with levothyroxine could be clinically meaningful.”
This long-term investigation is “much needed”
This new study adds important new insight to the ongoing debate over hypothyroidism treatment, said Dr. Schneiderhan and Dr. Zick in their editorial.
“[The study authors] begin a much-needed investigation into whether patients prescribed synthetic levothyroxine compared with desiccated thyroid had differences in TSH stability over the course of 3 years.
“Further prospective studies are needed to confirm these results and to explore differences in more diverse patient populations, such as Hashimoto’s thyroiditis, as well as on quality of life and other important patient-reported outcomes such as fatigue and weight gain,” the editorialists added.
“This study does, however, provide helpful information that desiccated thyroid products are a reasonable choice for treating some hypothyroid patients.”
For 60% of patients in both groups, TSH levels were within reference range for whole study
In the study, Dr. Kuye and colleagues matched patients (average age, 63 years; 90% women) in terms of characteristics such as race, comorbidities, and cholesterol levels.
Patients were excluded if they had been prescribed more than one agent for the treatment of hypothyroidism or if they had comorbid conditions, including a history of thyroid cancer or other related comorbidities, as well as pregnancy.
With respect to visit-to-visit TSH level variability, the lower rate among patients prescribed levothyroxine in comparison with patients prescribed desiccated thyroid was statistically significant (1.25 vs. 1.44; P = .015). Among 60% of patients in both groups, all TSH values measured during the study period were within reference ranges, however (P = .951).
The median number of TSH laboratory studies obtained during the study was four in the synthetic levothyroxine group and three for patients prescribed desiccated thyroid (P = .578).
There were some notable differences between the groups. Patients in the desiccated thyroid group had lower body mass index (P = .032), hemoglobin A1c levels (P = .041), and lower baseline TSH values (2.4 vs. 3.4 uIU/mL; P = .001). compared with those prescribed levothyroxine.
Limitations include the fact that the authors could not account for potentially important variables such as rates of adherence, differences in prescriber practice between agents, or the concurrent use of other medications.
Subjective outcomes not assessed: “One-size-fits-all approach doesn’t work”
The authors note they were not able to assess subjective outcomes, which, as noted by the editorialists, are particularly important in hypothyroidism.
“Emerging evidence shows that for many patients, symptoms persist despite normal TSH values,” Dr. Schneiderhan and Dr. Zick write.
They cite as an example a large study that found significant impairment in psychological well-being among patients treated with thyroxine replacement, despite their achieving normal TSH levels.
In addition, synthetic levothyroxine is associated with other uncertainties, such as complexities in the conversion of T4 to triiodothyronine (T3) that may disrupt thyroid metabolism in some patients.
In addition, there are differences in the amounts of thyroid replacement needed by certain groups, such as patients who have undergone thyroidectomies.
“The one-size-fits-all approach for treating hypothyroidism does not work ... for all patients,” they concluded.
The study authors and editorialists have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research shows.
The findings are “unanticipated ... given concerns for variability between batches of desiccated thyroid cited by national guidelines,” wrote the authors of the study, which was published this month in the Annals of Family Medicine.
In the trial, patients who had been treated for hypothyroidism at Kaiser Permanente Colorado were matched retrospectively into groups of 450 patients each according to whether they were treated with desiccated thyroid or synthetic levothyroxine.
After a follow-up of 3 years, TSH values within normal ranges (0.320-5.500 uIU/mL) were seen at approximately the same rate among those treated with desiccated thyroid and those who received levothyroxine (79.1% vs. 79.3%; P = .905).
“This study showed that after 3 years TSH values in both groups remained within reference ranges approximately 80% of the time,” said Rolake Kuye, PharmD, and colleagues with Kaiser Permanente, in Denver, Colorado.
In an accompanying editorial, Jill Schneiderhan, MD, and Suzanna Zick, ND, MPH, of the University of Michigan, Ann Arbor, say the overall results indicate that the continued use of desiccated thyroid is warranted in some cases.
“Keeping desiccated thyroid medications as an option in our tool kit will allow for improved shared decision-making, while allowing for patient preference, and offer an option for those patients who remain symptomatic on levothyroxine monotherapy,” they advised.
Some variability still seen with desiccated thyroid
Desiccated thyroid (dehydrated porcine thyroid), which was long the standard of care, is still commonly used in the treatment of hypothyroidism, despite having been replaced beginning in the 1970s by synthetic levothyroxine in light of evidence that the former was associated with more variability in thyroid hormone levels.
Desiccated thyroid is still sold legally by prescription in the United States under the names Nature Thyroid, Thyroid USP, and Armour Thyroid and is currently used by up to 30% of patients with hypothyroidism, according to recent estimates.
Consistent with concerns about variability in thyroid hormone levels, the new study did show greater variability in TSH levels with desiccated thyroid when assessed on a visit-to-visit basis.
Dr. Kuye and coauthors therefore recommended that, “[f]or providers targeting a tighter TSH goal in certain patients, the decreased TSH variability with levothyroxine could be clinically meaningful.”
This long-term investigation is “much needed”
This new study adds important new insight to the ongoing debate over hypothyroidism treatment, said Dr. Schneiderhan and Dr. Zick in their editorial.
“[The study authors] begin a much-needed investigation into whether patients prescribed synthetic levothyroxine compared with desiccated thyroid had differences in TSH stability over the course of 3 years.
“Further prospective studies are needed to confirm these results and to explore differences in more diverse patient populations, such as Hashimoto’s thyroiditis, as well as on quality of life and other important patient-reported outcomes such as fatigue and weight gain,” the editorialists added.
“This study does, however, provide helpful information that desiccated thyroid products are a reasonable choice for treating some hypothyroid patients.”
For 60% of patients in both groups, TSH levels were within reference range for whole study
In the study, Dr. Kuye and colleagues matched patients (average age, 63 years; 90% women) in terms of characteristics such as race, comorbidities, and cholesterol levels.
Patients were excluded if they had been prescribed more than one agent for the treatment of hypothyroidism or if they had comorbid conditions, including a history of thyroid cancer or other related comorbidities, as well as pregnancy.
With respect to visit-to-visit TSH level variability, the lower rate among patients prescribed levothyroxine in comparison with patients prescribed desiccated thyroid was statistically significant (1.25 vs. 1.44; P = .015). Among 60% of patients in both groups, all TSH values measured during the study period were within reference ranges, however (P = .951).
The median number of TSH laboratory studies obtained during the study was four in the synthetic levothyroxine group and three for patients prescribed desiccated thyroid (P = .578).
There were some notable differences between the groups. Patients in the desiccated thyroid group had lower body mass index (P = .032), hemoglobin A1c levels (P = .041), and lower baseline TSH values (2.4 vs. 3.4 uIU/mL; P = .001). compared with those prescribed levothyroxine.
Limitations include the fact that the authors could not account for potentially important variables such as rates of adherence, differences in prescriber practice between agents, or the concurrent use of other medications.
Subjective outcomes not assessed: “One-size-fits-all approach doesn’t work”
The authors note they were not able to assess subjective outcomes, which, as noted by the editorialists, are particularly important in hypothyroidism.
“Emerging evidence shows that for many patients, symptoms persist despite normal TSH values,” Dr. Schneiderhan and Dr. Zick write.
They cite as an example a large study that found significant impairment in psychological well-being among patients treated with thyroxine replacement, despite their achieving normal TSH levels.
In addition, synthetic levothyroxine is associated with other uncertainties, such as complexities in the conversion of T4 to triiodothyronine (T3) that may disrupt thyroid metabolism in some patients.
In addition, there are differences in the amounts of thyroid replacement needed by certain groups, such as patients who have undergone thyroidectomies.
“The one-size-fits-all approach for treating hypothyroidism does not work ... for all patients,” they concluded.
The study authors and editorialists have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.