User login
SGLT2 inhibitors for diabetes: No link to fractures in older adults
Use of sodium-glucose cotransporter-2 (SGLT2) inhibitors does not appear to raise the risk for fractures in older adults, new research suggests.
The data come from a nationwide propensity score-matched study of U.S. Medicare recipients with type 2 diabetes who were new users of either an SGLT2 inhibitor, a dipeptidyl peptidase 4 (DPP-4) inhibitor, or a glucagon-like peptide (GLP-1) receptor agonist.
“The use of SGLT2 inhibitors was not associated with an increased risk of nontraumatic fractures compared with DPP-4 inhibitors or GLP-1 agonists. Results were consistent across categories of sex, frailty, age, and insulin use,” say Min Zhuo, MD, of Harvard Medical School, Boston, and colleagues, who published their work online October 27 in JAMA Network Open.
“Our results add to the evidence base evaluating the safety profile of SGLT2 inhibitors in older adults outside of [randomized controlled trials] and further characterize the risk-benefit balance of SGLT2 inhibitors in clinical practice,” they write.
Asked to comment, Simeon I. Taylor, MD, PhD, told this news organization, “This is a high-quality study that is generally reassuring that relatively short, less than 1 year, treatment with an SGLT2 inhibitor does not appear to significantly increase the risk of bone fractures.”
However, Dr. Taylor, of the Division of Endocrinology, Diabetes, and Nutrition, University of Maryland School of Medicine, Baltimore, also noted: “Notwithstanding these reassuring data, the paper also does a good job of pointing out important limitations.”
“Most importantly, these data do not address questions related to the risk of long-term chronic therapy. It is instructive to refer back to the published data demonstrating an approximately 2-year lag before a significant increase in the risk of fracture was observed in rosiglitazone-treated patients in the ADOPT study. The length of the lag is likely related to the baseline bone mineral density at the time drug therapy is initiated. These considerations may contribute to the observed variation in bone-related outcomes in different studies.”
Concern about SGLT2 inhibitors and fractures first arose in 2017 from the CANVAS study, in which the overall fracture risk with canagliflozin was a significant 26% higher than placebo. However, subsequent larger randomized trials of canagliflozin and other SGLT2 inhibitors did not find the same risk.
In addition, previous observational studies in younger adults have also not found use of SGLT2 inhibitors to be associated with increased fracture risk compared with DPP-4 inhibitors or GLP-1 agonists.
Understanding fracture risk with SGLT2 inhibitors is ‘critical’
Older adults with type 2 diabetes may benefit from reductions in atherosclerotic cardiovascular events, hospitalization for heart failure, end-stage kidney disease, and death associated with SGLT2 inhibitors, but the fact that aging may have negative effects on bone metabolism means “understanding the fracture risk associated with SGLT2 inhibitors in older adults with type 2 diabetes is critical,” say Dr. Zhuo and colleagues.
In the current study, they analyzed claims data for Medicare beneficiaries aged 66 years and older (1 year past Medicare eligibility) who were newly prescribed an SGLT2 inhibitor, DPP-4 inhibitor, or GLP-1 agonist between April 1, 2013 and Dec. 31, 2017.
A total of 45,889 patients from each treatment group were propensity-matched using 58 baseline characteristics, for a total of 137,667 patients.
After matching, there were 501 events of the primary composite outcome (nontraumatic pelvic fracture, hip fracture requiring surgery, or humerus, radius, or ulna fracture requiring intervention) within 30 days. By treatment group, fracture rates per 1,000 person-years were 4.69, 5.26, and 4.71 for SGLT2 inhibitors, DPP-4 inhibitors, and GLP-1 agonists respectively.
The differences between patients taking DPP-4 inhibitors or GLP-1 agonists compared with SGLT2 inhibitors were not significant, with hazard ratios of 0.90 and 1.00, respectively.
Results remained consistent in various sensitivity and subgroup analyses, including limiting the data to just the canagliflozin group. Overall, the fracture rate was greater with female sex, frailty, older age, and insulin use, consistent across drug classes.
The risks for falls and hypoglycemia were lower in the SGLT2 inhibitor versus matched DPP-4 inhibitor groups (hazard ratio, 0.82), and there was no difference in syncope. None of those differences were significant for the SGLT2 inhibitor group compared with the GLP-1 agonist group.
Consistent with previous data, the risk for diabetic ketoacidosis was higher with SGLT2 inhibitors versus DPP-4 inhibitors and GLP-1 agonists (HR, 1.29 and 1.58), and the risk for heart failure hospitalization was lower (HR, 0.42 and 0.69).
The study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, department of medicine, Brigham and Women’s Hospital, Harvard Medical School. Dr. Zhuo was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Taylor is a consultant for Ionis Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Use of sodium-glucose cotransporter-2 (SGLT2) inhibitors does not appear to raise the risk for fractures in older adults, new research suggests.
The data come from a nationwide propensity score-matched study of U.S. Medicare recipients with type 2 diabetes who were new users of either an SGLT2 inhibitor, a dipeptidyl peptidase 4 (DPP-4) inhibitor, or a glucagon-like peptide (GLP-1) receptor agonist.
“The use of SGLT2 inhibitors was not associated with an increased risk of nontraumatic fractures compared with DPP-4 inhibitors or GLP-1 agonists. Results were consistent across categories of sex, frailty, age, and insulin use,” say Min Zhuo, MD, of Harvard Medical School, Boston, and colleagues, who published their work online October 27 in JAMA Network Open.
“Our results add to the evidence base evaluating the safety profile of SGLT2 inhibitors in older adults outside of [randomized controlled trials] and further characterize the risk-benefit balance of SGLT2 inhibitors in clinical practice,” they write.
Asked to comment, Simeon I. Taylor, MD, PhD, told this news organization, “This is a high-quality study that is generally reassuring that relatively short, less than 1 year, treatment with an SGLT2 inhibitor does not appear to significantly increase the risk of bone fractures.”
However, Dr. Taylor, of the Division of Endocrinology, Diabetes, and Nutrition, University of Maryland School of Medicine, Baltimore, also noted: “Notwithstanding these reassuring data, the paper also does a good job of pointing out important limitations.”
“Most importantly, these data do not address questions related to the risk of long-term chronic therapy. It is instructive to refer back to the published data demonstrating an approximately 2-year lag before a significant increase in the risk of fracture was observed in rosiglitazone-treated patients in the ADOPT study. The length of the lag is likely related to the baseline bone mineral density at the time drug therapy is initiated. These considerations may contribute to the observed variation in bone-related outcomes in different studies.”
Concern about SGLT2 inhibitors and fractures first arose in 2017 from the CANVAS study, in which the overall fracture risk with canagliflozin was a significant 26% higher than placebo. However, subsequent larger randomized trials of canagliflozin and other SGLT2 inhibitors did not find the same risk.
In addition, previous observational studies in younger adults have also not found use of SGLT2 inhibitors to be associated with increased fracture risk compared with DPP-4 inhibitors or GLP-1 agonists.
Understanding fracture risk with SGLT2 inhibitors is ‘critical’
Older adults with type 2 diabetes may benefit from reductions in atherosclerotic cardiovascular events, hospitalization for heart failure, end-stage kidney disease, and death associated with SGLT2 inhibitors, but the fact that aging may have negative effects on bone metabolism means “understanding the fracture risk associated with SGLT2 inhibitors in older adults with type 2 diabetes is critical,” say Dr. Zhuo and colleagues.
In the current study, they analyzed claims data for Medicare beneficiaries aged 66 years and older (1 year past Medicare eligibility) who were newly prescribed an SGLT2 inhibitor, DPP-4 inhibitor, or GLP-1 agonist between April 1, 2013 and Dec. 31, 2017.
A total of 45,889 patients from each treatment group were propensity-matched using 58 baseline characteristics, for a total of 137,667 patients.
After matching, there were 501 events of the primary composite outcome (nontraumatic pelvic fracture, hip fracture requiring surgery, or humerus, radius, or ulna fracture requiring intervention) within 30 days. By treatment group, fracture rates per 1,000 person-years were 4.69, 5.26, and 4.71 for SGLT2 inhibitors, DPP-4 inhibitors, and GLP-1 agonists respectively.
The differences between patients taking DPP-4 inhibitors or GLP-1 agonists compared with SGLT2 inhibitors were not significant, with hazard ratios of 0.90 and 1.00, respectively.
Results remained consistent in various sensitivity and subgroup analyses, including limiting the data to just the canagliflozin group. Overall, the fracture rate was greater with female sex, frailty, older age, and insulin use, consistent across drug classes.
The risks for falls and hypoglycemia were lower in the SGLT2 inhibitor versus matched DPP-4 inhibitor groups (hazard ratio, 0.82), and there was no difference in syncope. None of those differences were significant for the SGLT2 inhibitor group compared with the GLP-1 agonist group.
Consistent with previous data, the risk for diabetic ketoacidosis was higher with SGLT2 inhibitors versus DPP-4 inhibitors and GLP-1 agonists (HR, 1.29 and 1.58), and the risk for heart failure hospitalization was lower (HR, 0.42 and 0.69).
The study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, department of medicine, Brigham and Women’s Hospital, Harvard Medical School. Dr. Zhuo was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Taylor is a consultant for Ionis Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Use of sodium-glucose cotransporter-2 (SGLT2) inhibitors does not appear to raise the risk for fractures in older adults, new research suggests.
The data come from a nationwide propensity score-matched study of U.S. Medicare recipients with type 2 diabetes who were new users of either an SGLT2 inhibitor, a dipeptidyl peptidase 4 (DPP-4) inhibitor, or a glucagon-like peptide (GLP-1) receptor agonist.
“The use of SGLT2 inhibitors was not associated with an increased risk of nontraumatic fractures compared with DPP-4 inhibitors or GLP-1 agonists. Results were consistent across categories of sex, frailty, age, and insulin use,” say Min Zhuo, MD, of Harvard Medical School, Boston, and colleagues, who published their work online October 27 in JAMA Network Open.
“Our results add to the evidence base evaluating the safety profile of SGLT2 inhibitors in older adults outside of [randomized controlled trials] and further characterize the risk-benefit balance of SGLT2 inhibitors in clinical practice,” they write.
Asked to comment, Simeon I. Taylor, MD, PhD, told this news organization, “This is a high-quality study that is generally reassuring that relatively short, less than 1 year, treatment with an SGLT2 inhibitor does not appear to significantly increase the risk of bone fractures.”
However, Dr. Taylor, of the Division of Endocrinology, Diabetes, and Nutrition, University of Maryland School of Medicine, Baltimore, also noted: “Notwithstanding these reassuring data, the paper also does a good job of pointing out important limitations.”
“Most importantly, these data do not address questions related to the risk of long-term chronic therapy. It is instructive to refer back to the published data demonstrating an approximately 2-year lag before a significant increase in the risk of fracture was observed in rosiglitazone-treated patients in the ADOPT study. The length of the lag is likely related to the baseline bone mineral density at the time drug therapy is initiated. These considerations may contribute to the observed variation in bone-related outcomes in different studies.”
Concern about SGLT2 inhibitors and fractures first arose in 2017 from the CANVAS study, in which the overall fracture risk with canagliflozin was a significant 26% higher than placebo. However, subsequent larger randomized trials of canagliflozin and other SGLT2 inhibitors did not find the same risk.
In addition, previous observational studies in younger adults have also not found use of SGLT2 inhibitors to be associated with increased fracture risk compared with DPP-4 inhibitors or GLP-1 agonists.
Understanding fracture risk with SGLT2 inhibitors is ‘critical’
Older adults with type 2 diabetes may benefit from reductions in atherosclerotic cardiovascular events, hospitalization for heart failure, end-stage kidney disease, and death associated with SGLT2 inhibitors, but the fact that aging may have negative effects on bone metabolism means “understanding the fracture risk associated with SGLT2 inhibitors in older adults with type 2 diabetes is critical,” say Dr. Zhuo and colleagues.
In the current study, they analyzed claims data for Medicare beneficiaries aged 66 years and older (1 year past Medicare eligibility) who were newly prescribed an SGLT2 inhibitor, DPP-4 inhibitor, or GLP-1 agonist between April 1, 2013 and Dec. 31, 2017.
A total of 45,889 patients from each treatment group were propensity-matched using 58 baseline characteristics, for a total of 137,667 patients.
After matching, there were 501 events of the primary composite outcome (nontraumatic pelvic fracture, hip fracture requiring surgery, or humerus, radius, or ulna fracture requiring intervention) within 30 days. By treatment group, fracture rates per 1,000 person-years were 4.69, 5.26, and 4.71 for SGLT2 inhibitors, DPP-4 inhibitors, and GLP-1 agonists respectively.
The differences between patients taking DPP-4 inhibitors or GLP-1 agonists compared with SGLT2 inhibitors were not significant, with hazard ratios of 0.90 and 1.00, respectively.
Results remained consistent in various sensitivity and subgroup analyses, including limiting the data to just the canagliflozin group. Overall, the fracture rate was greater with female sex, frailty, older age, and insulin use, consistent across drug classes.
The risks for falls and hypoglycemia were lower in the SGLT2 inhibitor versus matched DPP-4 inhibitor groups (hazard ratio, 0.82), and there was no difference in syncope. None of those differences were significant for the SGLT2 inhibitor group compared with the GLP-1 agonist group.
Consistent with previous data, the risk for diabetic ketoacidosis was higher with SGLT2 inhibitors versus DPP-4 inhibitors and GLP-1 agonists (HR, 1.29 and 1.58), and the risk for heart failure hospitalization was lower (HR, 0.42 and 0.69).
The study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, department of medicine, Brigham and Women’s Hospital, Harvard Medical School. Dr. Zhuo was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Taylor is a consultant for Ionis Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Free vitamin D no better at predicting death in men than standard testing
In the clinical assessment of vitamin D concentrations, free 25-hydroxyvitamin D shows little added benefit to the current standard of total 25(OH)D, with deficiencies in each associated with at least a twofold risk of all-cause mortality, new research shows.
“In this prospective, population-based study of middle-aged and older European men, total 25(OH)D levels below 20 mcg/L were independently associated with a twofold increased all-cause mortality,” the researchers reported.
“Lower concentrations of free 25(OH)D were also predictive of mortality, but did not provide any additional information,” they noted. “The data do not support routine measurement of free 25(OH)D or 1,25(OH)2D [1,25-dihydroxyvitamin D] over total 25(OH)D levels.”
Despite vitamin D deficiency being well established as playing a role in a wide range of adverse health effects, including cardiovascular disease and mortality, there has been a lack of consensus on the optimal concentration of total 25(OH)D, with studies showing inconsistent levels to define insufficiency and deficiency.
One aspect of the debate has focused on precisely how to measure the concentrations, with some evidence supporting the “free hormone hypothesis,” which suggests that free 25(OH)D could represent a better indicator than the standard total 25(OH)D of functional availability of vitamin D, and have stronger clinical utility.
To investigate both issues, Marian Dejaeger, MD, PhD, and colleagues evaluated prospective data on 1,915 men recruited from eight centers around Europe in the European Male Aging Study in a report published in the Journal of Clinical Endocrinology & Metabolism
The men, who were aged between 40 and 79 years, had a mean follow-up of 12.3 years; during that time, about a quarter (23.5%) of them died.
In addition to other factors, including being older, having a higher body mass index, and having at least two comorbidities, men who died had significantly lower levels of total 25(OH)D, total 1,25(OH)2D, free 25(OH)D, and free 1,25(OH)2D, as well as higher parathyroid hormone and creatinine values.
After adjustment for key confounders, including body mass index, smoking, alcohol consumption, kidney function, number of comorbidities at baseline and other factors, men with a total 25(OH)D below 20 mcg/L had a significantly increased risk of mortality, compared with those who had normal levels of vitamin D, defined as above 30 mcg/L (hazard ratio, 2.03; P < .001).
In terms of free 25(OH)D, the lowest three free 25(OH)D quintiles (under 4.43 ng/L) similarly had a significantly higher mortality risk, compared with the highest quintile (HR, 2.09; P < .01) after adjustment for the confounders.
Further observations of all quintiles of other measures of 1,25(OH)2D and vitamin D binding protein (DBP) showed no associations with mortality after adjusting for confounders.
Methods of measurement
An important caveat of the study is the type of method used to measure free 25(OH)D. The authors calculated free 25(OH)D using a formula, as opposed to the alternative of direct measurement with an enzyme-linked immunosorbent assay kit, and there can be important differences between the two approaches, said Daniel Bikle, MD, PhD, a professor of medicine and dermatology at the San Francisco Veterans Affairs Medical Center and University of California, San Francisco, in a comment on the research.
“The biggest problem is that calculating free 25(OH)D does not give an accurate estimate of the real free level, so making conclusions regarding its role in clinical situations is subject to error,” said Dr. Bikle, who recently authored a review of the free hormone hypothesis.
A calculation approach “depends heavily on the total 25(OH)D level, so in a population with reasonably normal DBP and albumin levels, the correlation with total 25(OH)D is very high, so I am not surprised by the results showing no additional value,” he said in an interview.
The authors addressed their use of the calculation over the direct measurement in the study, noting that there is a “high correlation between both methods.”
But they added that, “as no equilibrium analysis method is available for free 25(OH)D, nor for free 1,25(OH)2D, no method can be considered superior.”
Dr. Dejaeger, of the department of public health and primary care, Katholieke Universiteit Leuven (Belgium), added that she agreed that high or low DBP could potentially shift some correlations, but noted that other research has shown calculated and direct measures to match relatively well.
“So we partly agree [with Dr. Bikle] not being surprised that we did not find an added value because we also found little variation in DBP, but we are not convinced that a different measurement method could make the difference here.”
Another caveat of the study is that, despite half of the measurements being taken in the summer, more than 90% of subjects in the study’s cohort had vitamin D insufficiency, defined in the study as total 25(OH)D levels below 30 mcg/L, and as many as 70% had deficiency, with levels below 20 mcg/L.
Therefore, “as the number of participants with high levels of total 25(OH)D in our study is small, a true threshold concentration for optimal vitamin D status cannot be defined on basis of our data,” the authors noted.
Under current recommendations, the Endocrine Society indicates that concentrations below 30 mcg/L are insufficient, while other groups, including the Institute of Medicine, suggest concentrations of 20 mcg/L or above are adequate.
Free hormone hypothesis
Under the free hormone hypothesis, which is observed with thyroid hormones and sex steroids, the very small fraction of free hormones that are not bound to protein carriers can enter cells and help facilitate biologic activity.
The hypothesis of a role of free 25(OH)D in mortality was supported by a recent study, in which free 25(OH)D levels – but not total 25(OH)D levels, were found to be independently associated with an increased risk of all-cause and cardiovascular mortality among patients with coronary artery disease.
However, two other studies are more consistent with the new findings, including one study showing no added value of free 25(OH)D as a marker for bone mineral density in older women, and another study showing no value as a marker of metabolic variables in healthy children.
“Currently, there are no hard data to support routine measurements of free 25(OH)D or 1,25(OH)2D over total 25(OH)D, the current standard of assessing vitamin D status, as stated in guidelines from different scientific bodies,” Dr. Dejaeger said in an interview.
The study received support from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre. Dr. Dejaeger and Dr. Bikle had no disclosures to report.
In the clinical assessment of vitamin D concentrations, free 25-hydroxyvitamin D shows little added benefit to the current standard of total 25(OH)D, with deficiencies in each associated with at least a twofold risk of all-cause mortality, new research shows.
“In this prospective, population-based study of middle-aged and older European men, total 25(OH)D levels below 20 mcg/L were independently associated with a twofold increased all-cause mortality,” the researchers reported.
“Lower concentrations of free 25(OH)D were also predictive of mortality, but did not provide any additional information,” they noted. “The data do not support routine measurement of free 25(OH)D or 1,25(OH)2D [1,25-dihydroxyvitamin D] over total 25(OH)D levels.”
Despite vitamin D deficiency being well established as playing a role in a wide range of adverse health effects, including cardiovascular disease and mortality, there has been a lack of consensus on the optimal concentration of total 25(OH)D, with studies showing inconsistent levels to define insufficiency and deficiency.
One aspect of the debate has focused on precisely how to measure the concentrations, with some evidence supporting the “free hormone hypothesis,” which suggests that free 25(OH)D could represent a better indicator than the standard total 25(OH)D of functional availability of vitamin D, and have stronger clinical utility.
To investigate both issues, Marian Dejaeger, MD, PhD, and colleagues evaluated prospective data on 1,915 men recruited from eight centers around Europe in the European Male Aging Study in a report published in the Journal of Clinical Endocrinology & Metabolism
The men, who were aged between 40 and 79 years, had a mean follow-up of 12.3 years; during that time, about a quarter (23.5%) of them died.
In addition to other factors, including being older, having a higher body mass index, and having at least two comorbidities, men who died had significantly lower levels of total 25(OH)D, total 1,25(OH)2D, free 25(OH)D, and free 1,25(OH)2D, as well as higher parathyroid hormone and creatinine values.
After adjustment for key confounders, including body mass index, smoking, alcohol consumption, kidney function, number of comorbidities at baseline and other factors, men with a total 25(OH)D below 20 mcg/L had a significantly increased risk of mortality, compared with those who had normal levels of vitamin D, defined as above 30 mcg/L (hazard ratio, 2.03; P < .001).
In terms of free 25(OH)D, the lowest three free 25(OH)D quintiles (under 4.43 ng/L) similarly had a significantly higher mortality risk, compared with the highest quintile (HR, 2.09; P < .01) after adjustment for the confounders.
Further observations of all quintiles of other measures of 1,25(OH)2D and vitamin D binding protein (DBP) showed no associations with mortality after adjusting for confounders.
Methods of measurement
An important caveat of the study is the type of method used to measure free 25(OH)D. The authors calculated free 25(OH)D using a formula, as opposed to the alternative of direct measurement with an enzyme-linked immunosorbent assay kit, and there can be important differences between the two approaches, said Daniel Bikle, MD, PhD, a professor of medicine and dermatology at the San Francisco Veterans Affairs Medical Center and University of California, San Francisco, in a comment on the research.
“The biggest problem is that calculating free 25(OH)D does not give an accurate estimate of the real free level, so making conclusions regarding its role in clinical situations is subject to error,” said Dr. Bikle, who recently authored a review of the free hormone hypothesis.
A calculation approach “depends heavily on the total 25(OH)D level, so in a population with reasonably normal DBP and albumin levels, the correlation with total 25(OH)D is very high, so I am not surprised by the results showing no additional value,” he said in an interview.
The authors addressed their use of the calculation over the direct measurement in the study, noting that there is a “high correlation between both methods.”
But they added that, “as no equilibrium analysis method is available for free 25(OH)D, nor for free 1,25(OH)2D, no method can be considered superior.”
Dr. Dejaeger, of the department of public health and primary care, Katholieke Universiteit Leuven (Belgium), added that she agreed that high or low DBP could potentially shift some correlations, but noted that other research has shown calculated and direct measures to match relatively well.
“So we partly agree [with Dr. Bikle] not being surprised that we did not find an added value because we also found little variation in DBP, but we are not convinced that a different measurement method could make the difference here.”
Another caveat of the study is that, despite half of the measurements being taken in the summer, more than 90% of subjects in the study’s cohort had vitamin D insufficiency, defined in the study as total 25(OH)D levels below 30 mcg/L, and as many as 70% had deficiency, with levels below 20 mcg/L.
Therefore, “as the number of participants with high levels of total 25(OH)D in our study is small, a true threshold concentration for optimal vitamin D status cannot be defined on basis of our data,” the authors noted.
Under current recommendations, the Endocrine Society indicates that concentrations below 30 mcg/L are insufficient, while other groups, including the Institute of Medicine, suggest concentrations of 20 mcg/L or above are adequate.
Free hormone hypothesis
Under the free hormone hypothesis, which is observed with thyroid hormones and sex steroids, the very small fraction of free hormones that are not bound to protein carriers can enter cells and help facilitate biologic activity.
The hypothesis of a role of free 25(OH)D in mortality was supported by a recent study, in which free 25(OH)D levels – but not total 25(OH)D levels, were found to be independently associated with an increased risk of all-cause and cardiovascular mortality among patients with coronary artery disease.
However, two other studies are more consistent with the new findings, including one study showing no added value of free 25(OH)D as a marker for bone mineral density in older women, and another study showing no value as a marker of metabolic variables in healthy children.
“Currently, there are no hard data to support routine measurements of free 25(OH)D or 1,25(OH)2D over total 25(OH)D, the current standard of assessing vitamin D status, as stated in guidelines from different scientific bodies,” Dr. Dejaeger said in an interview.
The study received support from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre. Dr. Dejaeger and Dr. Bikle had no disclosures to report.
In the clinical assessment of vitamin D concentrations, free 25-hydroxyvitamin D shows little added benefit to the current standard of total 25(OH)D, with deficiencies in each associated with at least a twofold risk of all-cause mortality, new research shows.
“In this prospective, population-based study of middle-aged and older European men, total 25(OH)D levels below 20 mcg/L were independently associated with a twofold increased all-cause mortality,” the researchers reported.
“Lower concentrations of free 25(OH)D were also predictive of mortality, but did not provide any additional information,” they noted. “The data do not support routine measurement of free 25(OH)D or 1,25(OH)2D [1,25-dihydroxyvitamin D] over total 25(OH)D levels.”
Despite vitamin D deficiency being well established as playing a role in a wide range of adverse health effects, including cardiovascular disease and mortality, there has been a lack of consensus on the optimal concentration of total 25(OH)D, with studies showing inconsistent levels to define insufficiency and deficiency.
One aspect of the debate has focused on precisely how to measure the concentrations, with some evidence supporting the “free hormone hypothesis,” which suggests that free 25(OH)D could represent a better indicator than the standard total 25(OH)D of functional availability of vitamin D, and have stronger clinical utility.
To investigate both issues, Marian Dejaeger, MD, PhD, and colleagues evaluated prospective data on 1,915 men recruited from eight centers around Europe in the European Male Aging Study in a report published in the Journal of Clinical Endocrinology & Metabolism
The men, who were aged between 40 and 79 years, had a mean follow-up of 12.3 years; during that time, about a quarter (23.5%) of them died.
In addition to other factors, including being older, having a higher body mass index, and having at least two comorbidities, men who died had significantly lower levels of total 25(OH)D, total 1,25(OH)2D, free 25(OH)D, and free 1,25(OH)2D, as well as higher parathyroid hormone and creatinine values.
After adjustment for key confounders, including body mass index, smoking, alcohol consumption, kidney function, number of comorbidities at baseline and other factors, men with a total 25(OH)D below 20 mcg/L had a significantly increased risk of mortality, compared with those who had normal levels of vitamin D, defined as above 30 mcg/L (hazard ratio, 2.03; P < .001).
In terms of free 25(OH)D, the lowest three free 25(OH)D quintiles (under 4.43 ng/L) similarly had a significantly higher mortality risk, compared with the highest quintile (HR, 2.09; P < .01) after adjustment for the confounders.
Further observations of all quintiles of other measures of 1,25(OH)2D and vitamin D binding protein (DBP) showed no associations with mortality after adjusting for confounders.
Methods of measurement
An important caveat of the study is the type of method used to measure free 25(OH)D. The authors calculated free 25(OH)D using a formula, as opposed to the alternative of direct measurement with an enzyme-linked immunosorbent assay kit, and there can be important differences between the two approaches, said Daniel Bikle, MD, PhD, a professor of medicine and dermatology at the San Francisco Veterans Affairs Medical Center and University of California, San Francisco, in a comment on the research.
“The biggest problem is that calculating free 25(OH)D does not give an accurate estimate of the real free level, so making conclusions regarding its role in clinical situations is subject to error,” said Dr. Bikle, who recently authored a review of the free hormone hypothesis.
A calculation approach “depends heavily on the total 25(OH)D level, so in a population with reasonably normal DBP and albumin levels, the correlation with total 25(OH)D is very high, so I am not surprised by the results showing no additional value,” he said in an interview.
The authors addressed their use of the calculation over the direct measurement in the study, noting that there is a “high correlation between both methods.”
But they added that, “as no equilibrium analysis method is available for free 25(OH)D, nor for free 1,25(OH)2D, no method can be considered superior.”
Dr. Dejaeger, of the department of public health and primary care, Katholieke Universiteit Leuven (Belgium), added that she agreed that high or low DBP could potentially shift some correlations, but noted that other research has shown calculated and direct measures to match relatively well.
“So we partly agree [with Dr. Bikle] not being surprised that we did not find an added value because we also found little variation in DBP, but we are not convinced that a different measurement method could make the difference here.”
Another caveat of the study is that, despite half of the measurements being taken in the summer, more than 90% of subjects in the study’s cohort had vitamin D insufficiency, defined in the study as total 25(OH)D levels below 30 mcg/L, and as many as 70% had deficiency, with levels below 20 mcg/L.
Therefore, “as the number of participants with high levels of total 25(OH)D in our study is small, a true threshold concentration for optimal vitamin D status cannot be defined on basis of our data,” the authors noted.
Under current recommendations, the Endocrine Society indicates that concentrations below 30 mcg/L are insufficient, while other groups, including the Institute of Medicine, suggest concentrations of 20 mcg/L or above are adequate.
Free hormone hypothesis
Under the free hormone hypothesis, which is observed with thyroid hormones and sex steroids, the very small fraction of free hormones that are not bound to protein carriers can enter cells and help facilitate biologic activity.
The hypothesis of a role of free 25(OH)D in mortality was supported by a recent study, in which free 25(OH)D levels – but not total 25(OH)D levels, were found to be independently associated with an increased risk of all-cause and cardiovascular mortality among patients with coronary artery disease.
However, two other studies are more consistent with the new findings, including one study showing no added value of free 25(OH)D as a marker for bone mineral density in older women, and another study showing no value as a marker of metabolic variables in healthy children.
“Currently, there are no hard data to support routine measurements of free 25(OH)D or 1,25(OH)2D over total 25(OH)D, the current standard of assessing vitamin D status, as stated in guidelines from different scientific bodies,” Dr. Dejaeger said in an interview.
The study received support from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre. Dr. Dejaeger and Dr. Bikle had no disclosures to report.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Bone risk: Is time since menopause a better predictor than age?
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Guidelines for managing hypo- and hyperparathyroidism
A large international team of experts has developed two comprehensive guidelines for diagnosing, evaluating, and managing hypoparathyroidism and hyperparathyroidism, which replace guidelines issued 5 and 7 years ago.
Aliya A. Khan, MD, presented an overview of the hypoparathyroidism guidelines and John P. Bilezikian, MD, presented key aspects of the hyperparathyroidism guidelines at the American Society of Bone and Mineral Research (ASBMR) 2021 Annual Meeting.
The guidelines will be published as 17 articles in two issues of the society’s Journal of Bone and Mineral Research in 2022 – one on hypoparathyroidism and the other on hyperparathyroidism.
The work represents an “unprecedented effort” by more than 100 experts from 16 countries (United States, Canada, Australia, Brazil, China, Denmark, France, Germany, India, Italy, Israel, Lebanon, Singapore, Spain, Sweden, and the United Kingdom), Dr. Bilezikian told this news organization in an interview.
More than 100 international and national endocrine and osteoporosis organizations, societies, and patient advocacy groups from more than 50 countries have expressed interest in endorsing the guidelines.
Management of hypoparathyroidism
The new guidelines on hypoparathyroidism replace the guidelines issued in 2016 that were developed at the First International Conference on the Management of Hypoparathyroidism, Dr. Khan, from McMaster University, Hamilton, Ont., said in an email.
There was a need for new hypoparathyroidism guidelines, she explained, because of the better understanding of associated complications, how to predict who will develop hypoparathyroidism postoperatively (and how to prevent this), how and when to investigate a genetic cause further, when to consider parathyroid hormone (PTH) replacement therapy (and the benefits of the various molecules available today as well as those being evaluated in clinical research), and how to diagnose and manage hypoparathyroidism during pregnancy and lactation.
The experts in hypoparathyroidism were divided into four task forces that covered epidemiology and financial burden, etiology and pathophysiology, genetics and diagnosis, and patient evaluation and management.
The guidelines, developed over the past 18 months, provide detailed evidence-based graded (strong to weak) as well as ungraded (current practice) recommendations.
Summarizing a few key takeaways, Dr. Khan noted the guidelines recommend that clinicians treating patients with hypoparathyroidism should:
- Diagnose hypoparathyroidism if serum calcium corrected for albumin is low in the presence of a low or inappropriately normal PTH confirmed on two occasions 2 weeks apart (which may be supported by other specified abnormalities).
- Determine the cause for the hypoparathyroidism (which includes postsurgery, genetic variant, autoimmune, radiation, or idiopathic causes).
- Evaluate target organ damage.
- Try to achieve treatment goals and minimize risks for long-term complications.
- Consider PTH replacement therapy if patients have inadequate control, with symptoms of hypocalcemia or hypercalcemia, high phosphate, kidney disease, or high urine calcium, or poor quality of life.
The guideline strongly recommends using PTH measurements after total thyroidectomy to try to predict which patients will develop permanent postsurgical hypoparathyroidism.
It provides a clinical approach for establishing the genetic etiology of hypoparathyroidism.
A meta-analysis of 81 studies identified that the most common symptoms/complications of chronic hypoparathyroidism were, in descending order, cataract (24%), infection (18%), nephrolithiasis, renal insufficiency, seizures, depression, ischemic heart disease, and arrhythmias.
Based on the best available evidence, the guideline advises that “clinicians need to carefully determine why a patient has hypoparathyroidism and develop an individualized treatment plan with conventional therapy consisting of calcium, active vitamin D, hydrochlorothiazide, and plain vitamin D,” Dr. Khan continued.
“If a patient has poorly controlled hypoparathyroidism with many symptoms or is not doing well, then clinicians must consider PTH replacement therapy, since this will replace the missing hormone, lower the urine calcium losses, bring the serum calcium back up to the normal reference range, and lower phosphate (which appears to be associated with kidney calcification and may also contribute to basal ganglia calcification and calcium deposits in the eye),” she noted.
The guideline also discusses the optimal way to monitor and treat patients during pregnancy, delivery, and breastfeeding to optimize outcomes for mother and baby. The key points are closer patient monitoring with normalization of calcium, urine calcium, phosphate, and vitamin D.
Management of primary hyperparathyroidism
There was a need to update the previous 2014 guidelines developed at the Fourth International Workshop on the Management of Primary Hyperparathyroidism because, among other things, recent studies have provided new evidence about the different clinical phenotypes of primary hyperparathyroidism and ways the disease affects the skeleton and kidneys, Dr. Bilezikian, from the College of Physicians and Surgeons, Columbia University, New York, explained.
The experts in hyperparathyroidism were divided into four task forces that covered epidemiology, pathophysiology and genetics; classical and nonclassical disease manifestations; surgical aspects; and patient evaluation and management.
As part of these topics, the experts reviewed biochemical, skeletal, and renal findings, nonclassical features (such as neurocognitive complaints), nutritional and pharmacologic approaches, and disease course with or without surgical or medical intervention.
They made recommendations for diagnosis of hypercalcemic and normocalcemic phenotypes, differential diagnosis, evaluation of the skeleton and the kidney, indications for surgery, role of parathyroid imaging, indications for pharmacologic intervention, and monitoring.
“Consider the way this disease has appeared to change in the last 50 years,” said Dr. Bilezikian. In the 1940s, 50s, and 60s, patients with hyperparathyroidism were really sick and had severe bone disease and kidney disease. Then in the 70s, 80s, and 90s, the disease was more often discovered because of a screening test; high serum calcium was a hallmark of finding asymptomatic hyperparathyroidism.
In recent years, hyperparathyroidism is often discovered incidentally, when examining the skeleton or kidneys, he continued.
Primary hyperparathyroidism can now be subdivided into three types: patients who have target organ (kidney, bone) involvement, patients who don’t have this, and patients who have normocalcemic primary hyperparathyroidism.
The guideline discusses new medications that have become available for hyperparathyroidism, as well as surgery (the only cure), including how preoperative imaging can identify the overactive parathyroid gland, and the guidelines go into detail about how to monitor a patient and why a clinician would or would not recommend surgery, Dr. Bilezikian explained.
In the end, treatment is tailored to the individual.
Last, the guideline identifies eight areas where more research is needed.
The guidelines were funded by unrestricted educational grants from Amolyt, Ascendis, Calcilytix, and Takeda. Dr. Khan has reported participating on advisory boards for Alexion, Amgen, Amolyt, and Takeda, being a consultant for Amgen, receiving grants from Alexion, Amgen, Takeda, and Ascendis, being an investigator for Alexion, Amgen, Takeda, Ascendis, and Chugai, and being a speaker for Alexion, Amgen, Takeda, and Ultragenyx. Dr. Bilezikian has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large international team of experts has developed two comprehensive guidelines for diagnosing, evaluating, and managing hypoparathyroidism and hyperparathyroidism, which replace guidelines issued 5 and 7 years ago.
Aliya A. Khan, MD, presented an overview of the hypoparathyroidism guidelines and John P. Bilezikian, MD, presented key aspects of the hyperparathyroidism guidelines at the American Society of Bone and Mineral Research (ASBMR) 2021 Annual Meeting.
The guidelines will be published as 17 articles in two issues of the society’s Journal of Bone and Mineral Research in 2022 – one on hypoparathyroidism and the other on hyperparathyroidism.
The work represents an “unprecedented effort” by more than 100 experts from 16 countries (United States, Canada, Australia, Brazil, China, Denmark, France, Germany, India, Italy, Israel, Lebanon, Singapore, Spain, Sweden, and the United Kingdom), Dr. Bilezikian told this news organization in an interview.
More than 100 international and national endocrine and osteoporosis organizations, societies, and patient advocacy groups from more than 50 countries have expressed interest in endorsing the guidelines.
Management of hypoparathyroidism
The new guidelines on hypoparathyroidism replace the guidelines issued in 2016 that were developed at the First International Conference on the Management of Hypoparathyroidism, Dr. Khan, from McMaster University, Hamilton, Ont., said in an email.
There was a need for new hypoparathyroidism guidelines, she explained, because of the better understanding of associated complications, how to predict who will develop hypoparathyroidism postoperatively (and how to prevent this), how and when to investigate a genetic cause further, when to consider parathyroid hormone (PTH) replacement therapy (and the benefits of the various molecules available today as well as those being evaluated in clinical research), and how to diagnose and manage hypoparathyroidism during pregnancy and lactation.
The experts in hypoparathyroidism were divided into four task forces that covered epidemiology and financial burden, etiology and pathophysiology, genetics and diagnosis, and patient evaluation and management.
The guidelines, developed over the past 18 months, provide detailed evidence-based graded (strong to weak) as well as ungraded (current practice) recommendations.
Summarizing a few key takeaways, Dr. Khan noted the guidelines recommend that clinicians treating patients with hypoparathyroidism should:
- Diagnose hypoparathyroidism if serum calcium corrected for albumin is low in the presence of a low or inappropriately normal PTH confirmed on two occasions 2 weeks apart (which may be supported by other specified abnormalities).
- Determine the cause for the hypoparathyroidism (which includes postsurgery, genetic variant, autoimmune, radiation, or idiopathic causes).
- Evaluate target organ damage.
- Try to achieve treatment goals and minimize risks for long-term complications.
- Consider PTH replacement therapy if patients have inadequate control, with symptoms of hypocalcemia or hypercalcemia, high phosphate, kidney disease, or high urine calcium, or poor quality of life.
The guideline strongly recommends using PTH measurements after total thyroidectomy to try to predict which patients will develop permanent postsurgical hypoparathyroidism.
It provides a clinical approach for establishing the genetic etiology of hypoparathyroidism.
A meta-analysis of 81 studies identified that the most common symptoms/complications of chronic hypoparathyroidism were, in descending order, cataract (24%), infection (18%), nephrolithiasis, renal insufficiency, seizures, depression, ischemic heart disease, and arrhythmias.
Based on the best available evidence, the guideline advises that “clinicians need to carefully determine why a patient has hypoparathyroidism and develop an individualized treatment plan with conventional therapy consisting of calcium, active vitamin D, hydrochlorothiazide, and plain vitamin D,” Dr. Khan continued.
“If a patient has poorly controlled hypoparathyroidism with many symptoms or is not doing well, then clinicians must consider PTH replacement therapy, since this will replace the missing hormone, lower the urine calcium losses, bring the serum calcium back up to the normal reference range, and lower phosphate (which appears to be associated with kidney calcification and may also contribute to basal ganglia calcification and calcium deposits in the eye),” she noted.
The guideline also discusses the optimal way to monitor and treat patients during pregnancy, delivery, and breastfeeding to optimize outcomes for mother and baby. The key points are closer patient monitoring with normalization of calcium, urine calcium, phosphate, and vitamin D.
Management of primary hyperparathyroidism
There was a need to update the previous 2014 guidelines developed at the Fourth International Workshop on the Management of Primary Hyperparathyroidism because, among other things, recent studies have provided new evidence about the different clinical phenotypes of primary hyperparathyroidism and ways the disease affects the skeleton and kidneys, Dr. Bilezikian, from the College of Physicians and Surgeons, Columbia University, New York, explained.
The experts in hyperparathyroidism were divided into four task forces that covered epidemiology, pathophysiology and genetics; classical and nonclassical disease manifestations; surgical aspects; and patient evaluation and management.
As part of these topics, the experts reviewed biochemical, skeletal, and renal findings, nonclassical features (such as neurocognitive complaints), nutritional and pharmacologic approaches, and disease course with or without surgical or medical intervention.
They made recommendations for diagnosis of hypercalcemic and normocalcemic phenotypes, differential diagnosis, evaluation of the skeleton and the kidney, indications for surgery, role of parathyroid imaging, indications for pharmacologic intervention, and monitoring.
“Consider the way this disease has appeared to change in the last 50 years,” said Dr. Bilezikian. In the 1940s, 50s, and 60s, patients with hyperparathyroidism were really sick and had severe bone disease and kidney disease. Then in the 70s, 80s, and 90s, the disease was more often discovered because of a screening test; high serum calcium was a hallmark of finding asymptomatic hyperparathyroidism.
In recent years, hyperparathyroidism is often discovered incidentally, when examining the skeleton or kidneys, he continued.
Primary hyperparathyroidism can now be subdivided into three types: patients who have target organ (kidney, bone) involvement, patients who don’t have this, and patients who have normocalcemic primary hyperparathyroidism.
The guideline discusses new medications that have become available for hyperparathyroidism, as well as surgery (the only cure), including how preoperative imaging can identify the overactive parathyroid gland, and the guidelines go into detail about how to monitor a patient and why a clinician would or would not recommend surgery, Dr. Bilezikian explained.
In the end, treatment is tailored to the individual.
Last, the guideline identifies eight areas where more research is needed.
The guidelines were funded by unrestricted educational grants from Amolyt, Ascendis, Calcilytix, and Takeda. Dr. Khan has reported participating on advisory boards for Alexion, Amgen, Amolyt, and Takeda, being a consultant for Amgen, receiving grants from Alexion, Amgen, Takeda, and Ascendis, being an investigator for Alexion, Amgen, Takeda, Ascendis, and Chugai, and being a speaker for Alexion, Amgen, Takeda, and Ultragenyx. Dr. Bilezikian has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large international team of experts has developed two comprehensive guidelines for diagnosing, evaluating, and managing hypoparathyroidism and hyperparathyroidism, which replace guidelines issued 5 and 7 years ago.
Aliya A. Khan, MD, presented an overview of the hypoparathyroidism guidelines and John P. Bilezikian, MD, presented key aspects of the hyperparathyroidism guidelines at the American Society of Bone and Mineral Research (ASBMR) 2021 Annual Meeting.
The guidelines will be published as 17 articles in two issues of the society’s Journal of Bone and Mineral Research in 2022 – one on hypoparathyroidism and the other on hyperparathyroidism.
The work represents an “unprecedented effort” by more than 100 experts from 16 countries (United States, Canada, Australia, Brazil, China, Denmark, France, Germany, India, Italy, Israel, Lebanon, Singapore, Spain, Sweden, and the United Kingdom), Dr. Bilezikian told this news organization in an interview.
More than 100 international and national endocrine and osteoporosis organizations, societies, and patient advocacy groups from more than 50 countries have expressed interest in endorsing the guidelines.
Management of hypoparathyroidism
The new guidelines on hypoparathyroidism replace the guidelines issued in 2016 that were developed at the First International Conference on the Management of Hypoparathyroidism, Dr. Khan, from McMaster University, Hamilton, Ont., said in an email.
There was a need for new hypoparathyroidism guidelines, she explained, because of the better understanding of associated complications, how to predict who will develop hypoparathyroidism postoperatively (and how to prevent this), how and when to investigate a genetic cause further, when to consider parathyroid hormone (PTH) replacement therapy (and the benefits of the various molecules available today as well as those being evaluated in clinical research), and how to diagnose and manage hypoparathyroidism during pregnancy and lactation.
The experts in hypoparathyroidism were divided into four task forces that covered epidemiology and financial burden, etiology and pathophysiology, genetics and diagnosis, and patient evaluation and management.
The guidelines, developed over the past 18 months, provide detailed evidence-based graded (strong to weak) as well as ungraded (current practice) recommendations.
Summarizing a few key takeaways, Dr. Khan noted the guidelines recommend that clinicians treating patients with hypoparathyroidism should:
- Diagnose hypoparathyroidism if serum calcium corrected for albumin is low in the presence of a low or inappropriately normal PTH confirmed on two occasions 2 weeks apart (which may be supported by other specified abnormalities).
- Determine the cause for the hypoparathyroidism (which includes postsurgery, genetic variant, autoimmune, radiation, or idiopathic causes).
- Evaluate target organ damage.
- Try to achieve treatment goals and minimize risks for long-term complications.
- Consider PTH replacement therapy if patients have inadequate control, with symptoms of hypocalcemia or hypercalcemia, high phosphate, kidney disease, or high urine calcium, or poor quality of life.
The guideline strongly recommends using PTH measurements after total thyroidectomy to try to predict which patients will develop permanent postsurgical hypoparathyroidism.
It provides a clinical approach for establishing the genetic etiology of hypoparathyroidism.
A meta-analysis of 81 studies identified that the most common symptoms/complications of chronic hypoparathyroidism were, in descending order, cataract (24%), infection (18%), nephrolithiasis, renal insufficiency, seizures, depression, ischemic heart disease, and arrhythmias.
Based on the best available evidence, the guideline advises that “clinicians need to carefully determine why a patient has hypoparathyroidism and develop an individualized treatment plan with conventional therapy consisting of calcium, active vitamin D, hydrochlorothiazide, and plain vitamin D,” Dr. Khan continued.
“If a patient has poorly controlled hypoparathyroidism with many symptoms or is not doing well, then clinicians must consider PTH replacement therapy, since this will replace the missing hormone, lower the urine calcium losses, bring the serum calcium back up to the normal reference range, and lower phosphate (which appears to be associated with kidney calcification and may also contribute to basal ganglia calcification and calcium deposits in the eye),” she noted.
The guideline also discusses the optimal way to monitor and treat patients during pregnancy, delivery, and breastfeeding to optimize outcomes for mother and baby. The key points are closer patient monitoring with normalization of calcium, urine calcium, phosphate, and vitamin D.
Management of primary hyperparathyroidism
There was a need to update the previous 2014 guidelines developed at the Fourth International Workshop on the Management of Primary Hyperparathyroidism because, among other things, recent studies have provided new evidence about the different clinical phenotypes of primary hyperparathyroidism and ways the disease affects the skeleton and kidneys, Dr. Bilezikian, from the College of Physicians and Surgeons, Columbia University, New York, explained.
The experts in hyperparathyroidism were divided into four task forces that covered epidemiology, pathophysiology and genetics; classical and nonclassical disease manifestations; surgical aspects; and patient evaluation and management.
As part of these topics, the experts reviewed biochemical, skeletal, and renal findings, nonclassical features (such as neurocognitive complaints), nutritional and pharmacologic approaches, and disease course with or without surgical or medical intervention.
They made recommendations for diagnosis of hypercalcemic and normocalcemic phenotypes, differential diagnosis, evaluation of the skeleton and the kidney, indications for surgery, role of parathyroid imaging, indications for pharmacologic intervention, and monitoring.
“Consider the way this disease has appeared to change in the last 50 years,” said Dr. Bilezikian. In the 1940s, 50s, and 60s, patients with hyperparathyroidism were really sick and had severe bone disease and kidney disease. Then in the 70s, 80s, and 90s, the disease was more often discovered because of a screening test; high serum calcium was a hallmark of finding asymptomatic hyperparathyroidism.
In recent years, hyperparathyroidism is often discovered incidentally, when examining the skeleton or kidneys, he continued.
Primary hyperparathyroidism can now be subdivided into three types: patients who have target organ (kidney, bone) involvement, patients who don’t have this, and patients who have normocalcemic primary hyperparathyroidism.
The guideline discusses new medications that have become available for hyperparathyroidism, as well as surgery (the only cure), including how preoperative imaging can identify the overactive parathyroid gland, and the guidelines go into detail about how to monitor a patient and why a clinician would or would not recommend surgery, Dr. Bilezikian explained.
In the end, treatment is tailored to the individual.
Last, the guideline identifies eight areas where more research is needed.
The guidelines were funded by unrestricted educational grants from Amolyt, Ascendis, Calcilytix, and Takeda. Dr. Khan has reported participating on advisory boards for Alexion, Amgen, Amolyt, and Takeda, being a consultant for Amgen, receiving grants from Alexion, Amgen, Takeda, and Ascendis, being an investigator for Alexion, Amgen, Takeda, Ascendis, and Chugai, and being a speaker for Alexion, Amgen, Takeda, and Ultragenyx. Dr. Bilezikian has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Better bone builder: High-intensity exercise vs. Pilates
An 8-month high-intensity resistance and impact training program (HiRIT, Onero) led to greater gains in lumbar spine bone mineral density (BMD) and leg/back strength than a low-intensity Pilates-based program (Buff Bones).
These findings are from the Medication and Exercise for Osteoporosis (MEDEX-OP) trial, which included 115 postmenopausal women with low bone mass. Patients were randomly assigned to attend either the HiRIT or Pilates-based exercise program. The participants attended supervised 45-min sessions twice weekly.
HiRIT was better than the low-intensity Pilates-based exercise program for enhancing bone mass, muscle strength, functional performance, and stature, the researchers reported. The low-intensity program did improve function, but to a lesser extent
Of the 115 participants, most (86) were not taking osteoporosis medicine. For the 29 women who were receiving it, the medication appeared to enhance the effect of exercise.
Melanie Fischbacher, PhD candidate, Griffith University, Gold Coast, Australia, presented these findings in an oral session at the annual meeting of the American Society for Bone and Mineral Research; the study was also published in the Journal of Bone and Mineral Research.
The study’s senior author, Belinda R. Beck, PhD, director of the Bone Clinic in Brisbane, Australia, developed the Onero HiRIT program and has licensed it to others in Australia.
“It is a very effective program and we have shown it can be undertaken safely, but it must be supervised because of the heavy weights and high-risk clientele,” Beck stressed to this news organization.
“This is not a program you should just hand to a patient and tell them to do in a gym,” she said.
“Both forms of exercise in our study were beneficial for functional outcomes but Onero improved back extensor strength, mobility and stature considerably more than Buff Bones,” Ms. Fischbacher said in an interview.
Nevertheless, “the contribution of functional capacity to risk of falling and fracture cannot be overstated, and bone medications do not address function,” she noted.
“More trials combining bone medication and bone-targeted exercise are needed,” the researchers concluded.
Compliance stands out, study supports high-intensity exercise
Kristen M. Beavers, PhD, MPH, RD, who was not involved with this research, told this news organization that participant compliance in the study really stands out.
“Compliance to an 8-month, 2 day/week high-intensity resistance training program among older women with low bone mass was quite good in this study [>80%], with very few adverse events reported,” said Dr. Beavers, of the department of health and exercise science, Wake Forest University, Winston Salem, N.C.
“A lot of individuals wouldn’t even consider recommending this type/intensity of exercise to this population, because they are worried it is too risky and/or the uptake will be low,” she said.
Although the benefit in BMD and strength wasn’t seen universally across all bone/muscle outcomes assessed, the findings do reinforce the idea that high-intensity exercise is more efficacious for bone health than low-intensity exercise, she noted.
“The possible additive effect of high-intensity exercise when combined with medication is worth confirming in larger, adequately designed/powered studies,” according to Dr. Beavers.
“The general consensus in the field is that higher-intensity exercise is more osteogenic than low-intensity exercise, but improving muscle mass, quality, and function (including balance) are also important to reduce the risk of falls, which is a major contributor to incident fracture,” she noted.
Exercise, even low-intensity exercise, reduces the risk for falls, as shown in a recent meta-analysis, she added. This is something antiresorptive medications don’t do.
Building on the LIFTMOR and LIFTMOR-M Trials
Previously, the Australian group showed that HiRIT is efficacious and safe for bone formation in individuals with low to very low bone mass – in postmenopausal women in the LIFTMOR study (J Bone Miner Res. 2017 Oct 4 .doi: 10.1002/jbmr.3284), and in men in the LIFTMOR-M study.
The current study compared two exercise programs. The researchers randomly assigned 86 women who were not taking antiresorptive medication to the high-intensity (42) or low-intensity (44) exercise program. They also assigned 29 women who were receiving antiresorptive medication to the high-intensity (15) or low-intensity (14) exercise program.
In the high-intensity exercise plus medication subgroup, the women were taking denosumab (12), risedronate (2) or alendronate (1). In the low-intensity exercise plus medication subgroup, the women were taking denosumab (9), risedronate (1), alendronate (3), or zoledronic acid (1).
The mean age of the women was 64-68 years. The mean lumbar spine T score was –1.5 to –2.3, and the mean femoral neck T score was –1.7 to –2.0 (determined by dual-energy x-ray absorptiometry) .
The HiRIT training program consisted of three free-weight resistance training exercises (deadlift, back squat, overhead press), one high-impact exercise (jump drop), and two balance exercises. The exercises varied each session.
The low-intensity training consisted of bone-specific Pilates-based exercises performed on the mat; standing weight-bearing exercise with 1-kg dumbbells; and impact exercises, such as heel drops and stomping.
At 8 months, compared with women in the low-intensity exercise program, those in the HiRIT program demonstrated greater improvement in lumbar spine BMD (1.9% vs. 0.1%) and stature (0.2 cm vs. 0.0 cm), muscle strength, and functional performance.
Functional performance improved with both exercise programs, but the HiRIT program led to greater leg and back muscle strength and better results in the five times sit-to-stand test (P < .05).
HiRIT plus bone medication improved BMD at the femoral neck and total hip, whereas HiRIT alone did not. Low-intensity exercise plus bone medication improved BMD at the lumbar spine and total hip, whereas low-intensity exercise alone did not.
The retention rate was 90%. The rate of exercise compliance was 83% in the high-intensity group and 82% in the low-intensity group.
Thirty falls were reported by 24 participants (21%). One fracture occurred in each exercise group. Three adverse events occurred in the low-intensity group, and four occurred in the high-intensity group.
Dr. Beck owns the Bone Clinic and sells licenses to the Onero program. The other researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An 8-month high-intensity resistance and impact training program (HiRIT, Onero) led to greater gains in lumbar spine bone mineral density (BMD) and leg/back strength than a low-intensity Pilates-based program (Buff Bones).
These findings are from the Medication and Exercise for Osteoporosis (MEDEX-OP) trial, which included 115 postmenopausal women with low bone mass. Patients were randomly assigned to attend either the HiRIT or Pilates-based exercise program. The participants attended supervised 45-min sessions twice weekly.
HiRIT was better than the low-intensity Pilates-based exercise program for enhancing bone mass, muscle strength, functional performance, and stature, the researchers reported. The low-intensity program did improve function, but to a lesser extent
Of the 115 participants, most (86) were not taking osteoporosis medicine. For the 29 women who were receiving it, the medication appeared to enhance the effect of exercise.
Melanie Fischbacher, PhD candidate, Griffith University, Gold Coast, Australia, presented these findings in an oral session at the annual meeting of the American Society for Bone and Mineral Research; the study was also published in the Journal of Bone and Mineral Research.
The study’s senior author, Belinda R. Beck, PhD, director of the Bone Clinic in Brisbane, Australia, developed the Onero HiRIT program and has licensed it to others in Australia.
“It is a very effective program and we have shown it can be undertaken safely, but it must be supervised because of the heavy weights and high-risk clientele,” Beck stressed to this news organization.
“This is not a program you should just hand to a patient and tell them to do in a gym,” she said.
“Both forms of exercise in our study were beneficial for functional outcomes but Onero improved back extensor strength, mobility and stature considerably more than Buff Bones,” Ms. Fischbacher said in an interview.
Nevertheless, “the contribution of functional capacity to risk of falling and fracture cannot be overstated, and bone medications do not address function,” she noted.
“More trials combining bone medication and bone-targeted exercise are needed,” the researchers concluded.
Compliance stands out, study supports high-intensity exercise
Kristen M. Beavers, PhD, MPH, RD, who was not involved with this research, told this news organization that participant compliance in the study really stands out.
“Compliance to an 8-month, 2 day/week high-intensity resistance training program among older women with low bone mass was quite good in this study [>80%], with very few adverse events reported,” said Dr. Beavers, of the department of health and exercise science, Wake Forest University, Winston Salem, N.C.
“A lot of individuals wouldn’t even consider recommending this type/intensity of exercise to this population, because they are worried it is too risky and/or the uptake will be low,” she said.
Although the benefit in BMD and strength wasn’t seen universally across all bone/muscle outcomes assessed, the findings do reinforce the idea that high-intensity exercise is more efficacious for bone health than low-intensity exercise, she noted.
“The possible additive effect of high-intensity exercise when combined with medication is worth confirming in larger, adequately designed/powered studies,” according to Dr. Beavers.
“The general consensus in the field is that higher-intensity exercise is more osteogenic than low-intensity exercise, but improving muscle mass, quality, and function (including balance) are also important to reduce the risk of falls, which is a major contributor to incident fracture,” she noted.
Exercise, even low-intensity exercise, reduces the risk for falls, as shown in a recent meta-analysis, she added. This is something antiresorptive medications don’t do.
Building on the LIFTMOR and LIFTMOR-M Trials
Previously, the Australian group showed that HiRIT is efficacious and safe for bone formation in individuals with low to very low bone mass – in postmenopausal women in the LIFTMOR study (J Bone Miner Res. 2017 Oct 4 .doi: 10.1002/jbmr.3284), and in men in the LIFTMOR-M study.
The current study compared two exercise programs. The researchers randomly assigned 86 women who were not taking antiresorptive medication to the high-intensity (42) or low-intensity (44) exercise program. They also assigned 29 women who were receiving antiresorptive medication to the high-intensity (15) or low-intensity (14) exercise program.
In the high-intensity exercise plus medication subgroup, the women were taking denosumab (12), risedronate (2) or alendronate (1). In the low-intensity exercise plus medication subgroup, the women were taking denosumab (9), risedronate (1), alendronate (3), or zoledronic acid (1).
The mean age of the women was 64-68 years. The mean lumbar spine T score was –1.5 to –2.3, and the mean femoral neck T score was –1.7 to –2.0 (determined by dual-energy x-ray absorptiometry) .
The HiRIT training program consisted of three free-weight resistance training exercises (deadlift, back squat, overhead press), one high-impact exercise (jump drop), and two balance exercises. The exercises varied each session.
The low-intensity training consisted of bone-specific Pilates-based exercises performed on the mat; standing weight-bearing exercise with 1-kg dumbbells; and impact exercises, such as heel drops and stomping.
At 8 months, compared with women in the low-intensity exercise program, those in the HiRIT program demonstrated greater improvement in lumbar spine BMD (1.9% vs. 0.1%) and stature (0.2 cm vs. 0.0 cm), muscle strength, and functional performance.
Functional performance improved with both exercise programs, but the HiRIT program led to greater leg and back muscle strength and better results in the five times sit-to-stand test (P < .05).
HiRIT plus bone medication improved BMD at the femoral neck and total hip, whereas HiRIT alone did not. Low-intensity exercise plus bone medication improved BMD at the lumbar spine and total hip, whereas low-intensity exercise alone did not.
The retention rate was 90%. The rate of exercise compliance was 83% in the high-intensity group and 82% in the low-intensity group.
Thirty falls were reported by 24 participants (21%). One fracture occurred in each exercise group. Three adverse events occurred in the low-intensity group, and four occurred in the high-intensity group.
Dr. Beck owns the Bone Clinic and sells licenses to the Onero program. The other researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An 8-month high-intensity resistance and impact training program (HiRIT, Onero) led to greater gains in lumbar spine bone mineral density (BMD) and leg/back strength than a low-intensity Pilates-based program (Buff Bones).
These findings are from the Medication and Exercise for Osteoporosis (MEDEX-OP) trial, which included 115 postmenopausal women with low bone mass. Patients were randomly assigned to attend either the HiRIT or Pilates-based exercise program. The participants attended supervised 45-min sessions twice weekly.
HiRIT was better than the low-intensity Pilates-based exercise program for enhancing bone mass, muscle strength, functional performance, and stature, the researchers reported. The low-intensity program did improve function, but to a lesser extent
Of the 115 participants, most (86) were not taking osteoporosis medicine. For the 29 women who were receiving it, the medication appeared to enhance the effect of exercise.
Melanie Fischbacher, PhD candidate, Griffith University, Gold Coast, Australia, presented these findings in an oral session at the annual meeting of the American Society for Bone and Mineral Research; the study was also published in the Journal of Bone and Mineral Research.
The study’s senior author, Belinda R. Beck, PhD, director of the Bone Clinic in Brisbane, Australia, developed the Onero HiRIT program and has licensed it to others in Australia.
“It is a very effective program and we have shown it can be undertaken safely, but it must be supervised because of the heavy weights and high-risk clientele,” Beck stressed to this news organization.
“This is not a program you should just hand to a patient and tell them to do in a gym,” she said.
“Both forms of exercise in our study were beneficial for functional outcomes but Onero improved back extensor strength, mobility and stature considerably more than Buff Bones,” Ms. Fischbacher said in an interview.
Nevertheless, “the contribution of functional capacity to risk of falling and fracture cannot be overstated, and bone medications do not address function,” she noted.
“More trials combining bone medication and bone-targeted exercise are needed,” the researchers concluded.
Compliance stands out, study supports high-intensity exercise
Kristen M. Beavers, PhD, MPH, RD, who was not involved with this research, told this news organization that participant compliance in the study really stands out.
“Compliance to an 8-month, 2 day/week high-intensity resistance training program among older women with low bone mass was quite good in this study [>80%], with very few adverse events reported,” said Dr. Beavers, of the department of health and exercise science, Wake Forest University, Winston Salem, N.C.
“A lot of individuals wouldn’t even consider recommending this type/intensity of exercise to this population, because they are worried it is too risky and/or the uptake will be low,” she said.
Although the benefit in BMD and strength wasn’t seen universally across all bone/muscle outcomes assessed, the findings do reinforce the idea that high-intensity exercise is more efficacious for bone health than low-intensity exercise, she noted.
“The possible additive effect of high-intensity exercise when combined with medication is worth confirming in larger, adequately designed/powered studies,” according to Dr. Beavers.
“The general consensus in the field is that higher-intensity exercise is more osteogenic than low-intensity exercise, but improving muscle mass, quality, and function (including balance) are also important to reduce the risk of falls, which is a major contributor to incident fracture,” she noted.
Exercise, even low-intensity exercise, reduces the risk for falls, as shown in a recent meta-analysis, she added. This is something antiresorptive medications don’t do.
Building on the LIFTMOR and LIFTMOR-M Trials
Previously, the Australian group showed that HiRIT is efficacious and safe for bone formation in individuals with low to very low bone mass – in postmenopausal women in the LIFTMOR study (J Bone Miner Res. 2017 Oct 4 .doi: 10.1002/jbmr.3284), and in men in the LIFTMOR-M study.
The current study compared two exercise programs. The researchers randomly assigned 86 women who were not taking antiresorptive medication to the high-intensity (42) or low-intensity (44) exercise program. They also assigned 29 women who were receiving antiresorptive medication to the high-intensity (15) or low-intensity (14) exercise program.
In the high-intensity exercise plus medication subgroup, the women were taking denosumab (12), risedronate (2) or alendronate (1). In the low-intensity exercise plus medication subgroup, the women were taking denosumab (9), risedronate (1), alendronate (3), or zoledronic acid (1).
The mean age of the women was 64-68 years. The mean lumbar spine T score was –1.5 to –2.3, and the mean femoral neck T score was –1.7 to –2.0 (determined by dual-energy x-ray absorptiometry) .
The HiRIT training program consisted of three free-weight resistance training exercises (deadlift, back squat, overhead press), one high-impact exercise (jump drop), and two balance exercises. The exercises varied each session.
The low-intensity training consisted of bone-specific Pilates-based exercises performed on the mat; standing weight-bearing exercise with 1-kg dumbbells; and impact exercises, such as heel drops and stomping.
At 8 months, compared with women in the low-intensity exercise program, those in the HiRIT program demonstrated greater improvement in lumbar spine BMD (1.9% vs. 0.1%) and stature (0.2 cm vs. 0.0 cm), muscle strength, and functional performance.
Functional performance improved with both exercise programs, but the HiRIT program led to greater leg and back muscle strength and better results in the five times sit-to-stand test (P < .05).
HiRIT plus bone medication improved BMD at the femoral neck and total hip, whereas HiRIT alone did not. Low-intensity exercise plus bone medication improved BMD at the lumbar spine and total hip, whereas low-intensity exercise alone did not.
The retention rate was 90%. The rate of exercise compliance was 83% in the high-intensity group and 82% in the low-intensity group.
Thirty falls were reported by 24 participants (21%). One fracture occurred in each exercise group. Three adverse events occurred in the low-intensity group, and four occurred in the high-intensity group.
Dr. Beck owns the Bone Clinic and sells licenses to the Onero program. The other researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Lumbar epidural steroid jab lowers bone formation in older women
Among postmenopausal women who received an epidural steroid injection (ESI) in the lumbar spine to treat back and leg pain arising from a compressed nerve in the spine, levels of bone formation biomarkers were decreased. The decrease in levels persisted more than 12 weeks, results from a new study show.
In addition, serum cortisol levels decreased by 50% at week 1 after the ESI, indicating systemic absorption of the steroid.
“The extent and duration of these effects suggest that patients who receive multiple [ESIs in the lumbar spine] may be at particular risk for harmful skeletal consequences,” Shannon Clare reported in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research.
Further studies are needed of the relationship between these short-term changes in bone turnover and bone loss and the risk for fracture among the burgeoning population treated with ESIs, added Ms. Clare, of the Hospital for Special Surgery, New York.
The researchers examined changes in serum levels of bone formation and resorption markers and other analytes in 24 women who received a lumbar ESI for radicular back pain and in 8 other women from the hospital population who served as control persons.
Among the women who received ESI, 1 week after the injection, serum levels of two bone formation biomarkers – total procollagen type 1 N-terminal peptide (P1NP) and osteocalcin – were about 27% lower than at baseline. The suppression persisted beyond 12 weeks.
Serum levels of the bone resorption biomarker C-terminal telopeptide of type I collagen (CTX) did not differ significantly after ESI.
“Our results are notable because we found that the duration of suppression of bone formation extended beyond 12 weeks, a far longer duration than seen previously with intra-articular injections” of glucocorticoids, said Ms. Clare and senior author Emily M. Stein, MD, director of research for the Metabolic Bone Service and an endocrinologist at the Hospital for Special Surgery and is associate professor of medicine at Weill Cornell Medicine, both in New York.
The findings suggest that patients should not receive multiple doses within a 12-week period, they told this news organization in a joint email response.
Women are not typically screened for osteopenia or osteoporosis before ESI. However, “our results suggest that physicians should consider screening women for osteoporosis who receive ESI, particularly those who are treated with multiple doses,” said Ms. Clare and Dr. Stein. “Steroid exposure should be minimized as much as possible by having patients space injections as far as they can tolerate.”
Systemic absorption, negative impact on bone turnover markers
“The hypothesis that [ESIs] interfere with the vertebral osseous microenvironment and increase the risk of vertebral fractures has been supported with evidence in the literature,” Mohamad Bydon, MD, professor of neurosurgery, orthopedic surgery, and health services research at the Mayo Clinic, Rochester, Minn., said in an interview.
Prior studies have demonstrated a decrease in bone mineral density (BMD) and an increase in vertebral fractures following ESI, added Dr. Bydon, senior author of a 2018 review of the effect of ESI on BMD and vertebral fracture risk that was published in Pain Medicine. He was not involved with the current study.
“The article by Clare et al. provides evidence on the systemic absorption of glucocorticoids by demonstrating a drop in serum cortisol following ESI,” he noted. “The measurement of bone metabolism biomarkers offers molecular confirmation of clinical and radiological observations of previous studies” showing that ESI affects the vertebrae.
More than 9 million ESIs each year
Each year, more than 9 million ESIs are administered to patients in the United States to relieve radicular back and leg pain that may be caused by a herniated disc or spinal stenosis (a gradual narrowing of the open spaces in the spinal column, which is common in older adults), the researchers explained.
Some patients experience sufficient pain relief with ESIs. Others may not be eligible for surgery and may receive multiple ESIs annually for many years because they provide pain relief.
It is well established that oral and intravenous glucocorticoids profoundly suppress bone formation and transiently increase bone resorption, causing substantial bone loss and increased fracture risk within 3 months of administration, Ms. Clare explained in the session.
Long-term use of high-dose inhaled glucocorticoids has been associated with bone loss and fractures. However, the effect of ESIs on bone has been less well studied.
The researchers hypothesized that ESIs are systemically absorbed and cause suppression of bone formation without a compensatory decrease in bone resorption.
They enrolled 24 patients who had undergone lumbar ESIs and 8 control patients. The mean age of the patients in the two groups was 63 years and 68 years, respectively. Most patients were White (88% and 100%, respectively). The mean body mass index was 27 kg/m2 and 28 kg/m2, respectively. On average, the patients had entered menopause 12 and 16 years earlier, respectively.
In the group that received steroid injections, almost two-thirds (15 patients, 63%) received triamcinolone. The rest received dexamethasone (six patients, 25%) or betamethasone (three patients, 12%) at doses that were equivalent to 80 mg triamcinolone.
The patients’ baseline serum levels of 25-hydroxy vitamin D, parathyroid hormone, cortisol, P1NP, osteocalcin, and CTX were within the reference ranges and were similar in the two groups.
The researchers also determined serum levels of cortisol (to assess suppression of endogenous glucocorticoids), osteocalcin, P1NP, and CTX in the patients and control persons at 1, 4, 12, 26, and 52 weeks after patients had received the ESI.
The researchers acknowledged that the small sample is a study limitation. In addition, the first serum samples were taken 1 week after the injection, and so any earlier changes in analyte levels were not captured. The patients also received different types of steroids, although the doses were similar when converted to triamcinolone equivalents.
The study was supported by a Spine Service grant from the Hospital for Special Surgery. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among postmenopausal women who received an epidural steroid injection (ESI) in the lumbar spine to treat back and leg pain arising from a compressed nerve in the spine, levels of bone formation biomarkers were decreased. The decrease in levels persisted more than 12 weeks, results from a new study show.
In addition, serum cortisol levels decreased by 50% at week 1 after the ESI, indicating systemic absorption of the steroid.
“The extent and duration of these effects suggest that patients who receive multiple [ESIs in the lumbar spine] may be at particular risk for harmful skeletal consequences,” Shannon Clare reported in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research.
Further studies are needed of the relationship between these short-term changes in bone turnover and bone loss and the risk for fracture among the burgeoning population treated with ESIs, added Ms. Clare, of the Hospital for Special Surgery, New York.
The researchers examined changes in serum levels of bone formation and resorption markers and other analytes in 24 women who received a lumbar ESI for radicular back pain and in 8 other women from the hospital population who served as control persons.
Among the women who received ESI, 1 week after the injection, serum levels of two bone formation biomarkers – total procollagen type 1 N-terminal peptide (P1NP) and osteocalcin – were about 27% lower than at baseline. The suppression persisted beyond 12 weeks.
Serum levels of the bone resorption biomarker C-terminal telopeptide of type I collagen (CTX) did not differ significantly after ESI.
“Our results are notable because we found that the duration of suppression of bone formation extended beyond 12 weeks, a far longer duration than seen previously with intra-articular injections” of glucocorticoids, said Ms. Clare and senior author Emily M. Stein, MD, director of research for the Metabolic Bone Service and an endocrinologist at the Hospital for Special Surgery and is associate professor of medicine at Weill Cornell Medicine, both in New York.
The findings suggest that patients should not receive multiple doses within a 12-week period, they told this news organization in a joint email response.
Women are not typically screened for osteopenia or osteoporosis before ESI. However, “our results suggest that physicians should consider screening women for osteoporosis who receive ESI, particularly those who are treated with multiple doses,” said Ms. Clare and Dr. Stein. “Steroid exposure should be minimized as much as possible by having patients space injections as far as they can tolerate.”
Systemic absorption, negative impact on bone turnover markers
“The hypothesis that [ESIs] interfere with the vertebral osseous microenvironment and increase the risk of vertebral fractures has been supported with evidence in the literature,” Mohamad Bydon, MD, professor of neurosurgery, orthopedic surgery, and health services research at the Mayo Clinic, Rochester, Minn., said in an interview.
Prior studies have demonstrated a decrease in bone mineral density (BMD) and an increase in vertebral fractures following ESI, added Dr. Bydon, senior author of a 2018 review of the effect of ESI on BMD and vertebral fracture risk that was published in Pain Medicine. He was not involved with the current study.
“The article by Clare et al. provides evidence on the systemic absorption of glucocorticoids by demonstrating a drop in serum cortisol following ESI,” he noted. “The measurement of bone metabolism biomarkers offers molecular confirmation of clinical and radiological observations of previous studies” showing that ESI affects the vertebrae.
More than 9 million ESIs each year
Each year, more than 9 million ESIs are administered to patients in the United States to relieve radicular back and leg pain that may be caused by a herniated disc or spinal stenosis (a gradual narrowing of the open spaces in the spinal column, which is common in older adults), the researchers explained.
Some patients experience sufficient pain relief with ESIs. Others may not be eligible for surgery and may receive multiple ESIs annually for many years because they provide pain relief.
It is well established that oral and intravenous glucocorticoids profoundly suppress bone formation and transiently increase bone resorption, causing substantial bone loss and increased fracture risk within 3 months of administration, Ms. Clare explained in the session.
Long-term use of high-dose inhaled glucocorticoids has been associated with bone loss and fractures. However, the effect of ESIs on bone has been less well studied.
The researchers hypothesized that ESIs are systemically absorbed and cause suppression of bone formation without a compensatory decrease in bone resorption.
They enrolled 24 patients who had undergone lumbar ESIs and 8 control patients. The mean age of the patients in the two groups was 63 years and 68 years, respectively. Most patients were White (88% and 100%, respectively). The mean body mass index was 27 kg/m2 and 28 kg/m2, respectively. On average, the patients had entered menopause 12 and 16 years earlier, respectively.
In the group that received steroid injections, almost two-thirds (15 patients, 63%) received triamcinolone. The rest received dexamethasone (six patients, 25%) or betamethasone (three patients, 12%) at doses that were equivalent to 80 mg triamcinolone.
The patients’ baseline serum levels of 25-hydroxy vitamin D, parathyroid hormone, cortisol, P1NP, osteocalcin, and CTX were within the reference ranges and were similar in the two groups.
The researchers also determined serum levels of cortisol (to assess suppression of endogenous glucocorticoids), osteocalcin, P1NP, and CTX in the patients and control persons at 1, 4, 12, 26, and 52 weeks after patients had received the ESI.
The researchers acknowledged that the small sample is a study limitation. In addition, the first serum samples were taken 1 week after the injection, and so any earlier changes in analyte levels were not captured. The patients also received different types of steroids, although the doses were similar when converted to triamcinolone equivalents.
The study was supported by a Spine Service grant from the Hospital for Special Surgery. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among postmenopausal women who received an epidural steroid injection (ESI) in the lumbar spine to treat back and leg pain arising from a compressed nerve in the spine, levels of bone formation biomarkers were decreased. The decrease in levels persisted more than 12 weeks, results from a new study show.
In addition, serum cortisol levels decreased by 50% at week 1 after the ESI, indicating systemic absorption of the steroid.
“The extent and duration of these effects suggest that patients who receive multiple [ESIs in the lumbar spine] may be at particular risk for harmful skeletal consequences,” Shannon Clare reported in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research.
Further studies are needed of the relationship between these short-term changes in bone turnover and bone loss and the risk for fracture among the burgeoning population treated with ESIs, added Ms. Clare, of the Hospital for Special Surgery, New York.
The researchers examined changes in serum levels of bone formation and resorption markers and other analytes in 24 women who received a lumbar ESI for radicular back pain and in 8 other women from the hospital population who served as control persons.
Among the women who received ESI, 1 week after the injection, serum levels of two bone formation biomarkers – total procollagen type 1 N-terminal peptide (P1NP) and osteocalcin – were about 27% lower than at baseline. The suppression persisted beyond 12 weeks.
Serum levels of the bone resorption biomarker C-terminal telopeptide of type I collagen (CTX) did not differ significantly after ESI.
“Our results are notable because we found that the duration of suppression of bone formation extended beyond 12 weeks, a far longer duration than seen previously with intra-articular injections” of glucocorticoids, said Ms. Clare and senior author Emily M. Stein, MD, director of research for the Metabolic Bone Service and an endocrinologist at the Hospital for Special Surgery and is associate professor of medicine at Weill Cornell Medicine, both in New York.
The findings suggest that patients should not receive multiple doses within a 12-week period, they told this news organization in a joint email response.
Women are not typically screened for osteopenia or osteoporosis before ESI. However, “our results suggest that physicians should consider screening women for osteoporosis who receive ESI, particularly those who are treated with multiple doses,” said Ms. Clare and Dr. Stein. “Steroid exposure should be minimized as much as possible by having patients space injections as far as they can tolerate.”
Systemic absorption, negative impact on bone turnover markers
“The hypothesis that [ESIs] interfere with the vertebral osseous microenvironment and increase the risk of vertebral fractures has been supported with evidence in the literature,” Mohamad Bydon, MD, professor of neurosurgery, orthopedic surgery, and health services research at the Mayo Clinic, Rochester, Minn., said in an interview.
Prior studies have demonstrated a decrease in bone mineral density (BMD) and an increase in vertebral fractures following ESI, added Dr. Bydon, senior author of a 2018 review of the effect of ESI on BMD and vertebral fracture risk that was published in Pain Medicine. He was not involved with the current study.
“The article by Clare et al. provides evidence on the systemic absorption of glucocorticoids by demonstrating a drop in serum cortisol following ESI,” he noted. “The measurement of bone metabolism biomarkers offers molecular confirmation of clinical and radiological observations of previous studies” showing that ESI affects the vertebrae.
More than 9 million ESIs each year
Each year, more than 9 million ESIs are administered to patients in the United States to relieve radicular back and leg pain that may be caused by a herniated disc or spinal stenosis (a gradual narrowing of the open spaces in the spinal column, which is common in older adults), the researchers explained.
Some patients experience sufficient pain relief with ESIs. Others may not be eligible for surgery and may receive multiple ESIs annually for many years because they provide pain relief.
It is well established that oral and intravenous glucocorticoids profoundly suppress bone formation and transiently increase bone resorption, causing substantial bone loss and increased fracture risk within 3 months of administration, Ms. Clare explained in the session.
Long-term use of high-dose inhaled glucocorticoids has been associated with bone loss and fractures. However, the effect of ESIs on bone has been less well studied.
The researchers hypothesized that ESIs are systemically absorbed and cause suppression of bone formation without a compensatory decrease in bone resorption.
They enrolled 24 patients who had undergone lumbar ESIs and 8 control patients. The mean age of the patients in the two groups was 63 years and 68 years, respectively. Most patients were White (88% and 100%, respectively). The mean body mass index was 27 kg/m2 and 28 kg/m2, respectively. On average, the patients had entered menopause 12 and 16 years earlier, respectively.
In the group that received steroid injections, almost two-thirds (15 patients, 63%) received triamcinolone. The rest received dexamethasone (six patients, 25%) or betamethasone (three patients, 12%) at doses that were equivalent to 80 mg triamcinolone.
The patients’ baseline serum levels of 25-hydroxy vitamin D, parathyroid hormone, cortisol, P1NP, osteocalcin, and CTX were within the reference ranges and were similar in the two groups.
The researchers also determined serum levels of cortisol (to assess suppression of endogenous glucocorticoids), osteocalcin, P1NP, and CTX in the patients and control persons at 1, 4, 12, 26, and 52 weeks after patients had received the ESI.
The researchers acknowledged that the small sample is a study limitation. In addition, the first serum samples were taken 1 week after the injection, and so any earlier changes in analyte levels were not captured. The patients also received different types of steroids, although the doses were similar when converted to triamcinolone equivalents.
The study was supported by a Spine Service grant from the Hospital for Special Surgery. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Oral PTH shows promise for osteoporosis in early phase 2 study
An investigational oral form of parathyroid hormone (PTH 1-34), EB 613 (Entera Bio) met its primary efficacy outcome in a phase 2 dosing study involving postmenopausal women with low bone mineral density (BMD).
The adverse effect profile of the drug was similar to that of the injectable PTH 1-34 teriparatide (Forteo), which is approved for osteoporosis.
Arthur C. Santora, MD, chief medical officer, Entera Bio, presented 6-month findings from the study during an oral session at the annual meeting of the American Society of Bone and Mineral Research. The 3-month findings from the study were reported as a poster.
If the drug demonstrates efficacy and safety in larger phase 3 trials, it could be the first oral bone-building (anabolic) therapy for osteoporosis.
Clifford J. Rosen, MD, PhD, who was not involved with the research, told this news organization: “I think this is an intriguing study.” The most likely patients for oral PTH, he added, “are those that have osteoporosis, previous fracture, or very low BMD, particularly those unlikely or unwilling to take bisphosphonates.”
However, “this is very early in the process before this drug could come to market,” cautioned Dr. Rosen, who is director of the Center for Clinical and Translational Research, Maine Medical Research Institute, Scarborough.
“Much more data on efficacy are required at 12 and 24 months for phase 2, and then a full phase 3 [clinical trial] with high-risk fracture patients,” he said.
The company is seeking input from the Food and Drug Administration to develop the protocol for a phase 3 trial. They expect to start this trial in 2022 at sites in the United States, Europe, and Israel, Dr. Santora said.
Primary outcome met
The study randomly assigned 161 postmenopausal women with osteoporosis or low BMD to receive placebo or the investigational oral PTH for 6 months.
Compared with women who received placebo, those who received the study drug experienced a significantly greater increase in the bone formation marker procollagen type I N-terminal propeptide (P1NP) from baseline to 3 months, thereby meeting the study’s primary outcome.
In secondary outcomes, women who received the 2.5-mg/d dose experienced a similar 6-month increase in BMD at the spine and greater increases in BMD at the total hip and femoral neck than those who received injectable teriparatide, Dr. Santora reported.
“The study’s key takeaway is that a once-daily oral PTH [tablet] has the potential to produce the same BMD effects as subcutaneous injections of PTH,” he said in an interview.
Additionally, “the drug was well tolerated when the dose was titrated by adding additional tablets, which suggests that the dose can be tailored to each patient,” he said.
Other study findings
Injectable teriparatide reduces the risk for vertebral fractures by up to 80%, Dr. Santora noted, but the fact that the drug must be administered by injection may deter some older patients from using it.
The company developed an oral form of biosynthetic human PTH with a proprietary drug delivery.
The researchers conducted the phase 2 study at four sites in Israel between June 2019 and May 2021. They enrolled women aged 50 years and older who had entered menopause at least 3 years earlier and who had osteoporosis or low BMD.
Forty-three women received placebo, and the others received oral PTH at doses of 0.5 mg/d (n = 25), 1.0 mg/d (n = 29), 1.5 mg/d (n = 28), 2.5 mg/d (n = 19), or at a dose that was titrated up to 2.5 mg/d starting at 1.5 mg/d for month 1, then 2 mg/d for month 2, and then 2.5 mg/d for months 3 to 6 (n = 17).
The mean age of the patients was 61 years, the mean body mass index was 25-27 kg/m2, and the mean T score at the spine of –2.2 to –2.45.
Among the women who received 2.5 mg/d of oral PTH for the full 6 months, serum levels of the bone resorption marker C-terminal telopeptide of type I collagen (CTX) decreased 21% from baseline to 6 months, and serum levels of P1NP increased at month 1 and then decreased to baseline by month 6.
The women who received 2.5 mg/d of oral PTH for the full 6 months also demonstrated significantly greater increases in BMD at the lumbar spine (3.8%), total hip (1.4%), and femoral neck (2.4%), compared with women who received placebo.
The safety profile of oral PTH was consistent with that of subcutaneous PTH. Patients experienced headache, nausea, presyncope, and dizziness; there were no treatment-emergent hypercalcemia adverse events.
A few ‘unexpected findings’
Suzanne M. Jan De Beur, MD, outgoing ASBMR president, said, “Oral PTH appeared to increase BMD by [dual-energy x-ray absorptiometry] at the lumbar spine effectively and to a similar degree as teriparatide in previous studies.”
She identified two unexpected findings.
“There were increases in BMD by DXA at the femoral neck and total hip at 6 months that were [greater than those] seen in previous trials of teriparatide. Second, markers of bone resorption (CTX) decreased at 6 months, and this is in stark contrast to the increases observed with teriparatide treatment,” she noted in an interview.
Dr. Rosen also noted that “the decrease in CTX is very unusual for PTH and difficult to explain.” He added: “P1NP, a marker of bone formation, was not increased.”
Dr. Jan de Beur continued: “Teriparatide (PTH1-34) and abaloparatide are effective anabolic agents that we use to treat patients with high risk of osteoporotic fracture. Although effective, the burden of daily subcutaneous injection can be a barrier for older individuals, those with poor dexterity, and those that are averse to self-injection.
“Taken together, these results appear promising, that oral PTH may prove to be an effective anabolic agent for osteoporosis treatment,” she summarized.
She stressed that a larger phase 3 study is needed to demonstrate safety and efficacy.
The study was funded by Entera Bio. Dr. Santora is chief medical officer of Entera Bio.
A version of this article first appeared on Medscape.com .
An investigational oral form of parathyroid hormone (PTH 1-34), EB 613 (Entera Bio) met its primary efficacy outcome in a phase 2 dosing study involving postmenopausal women with low bone mineral density (BMD).
The adverse effect profile of the drug was similar to that of the injectable PTH 1-34 teriparatide (Forteo), which is approved for osteoporosis.
Arthur C. Santora, MD, chief medical officer, Entera Bio, presented 6-month findings from the study during an oral session at the annual meeting of the American Society of Bone and Mineral Research. The 3-month findings from the study were reported as a poster.
If the drug demonstrates efficacy and safety in larger phase 3 trials, it could be the first oral bone-building (anabolic) therapy for osteoporosis.
Clifford J. Rosen, MD, PhD, who was not involved with the research, told this news organization: “I think this is an intriguing study.” The most likely patients for oral PTH, he added, “are those that have osteoporosis, previous fracture, or very low BMD, particularly those unlikely or unwilling to take bisphosphonates.”
However, “this is very early in the process before this drug could come to market,” cautioned Dr. Rosen, who is director of the Center for Clinical and Translational Research, Maine Medical Research Institute, Scarborough.
“Much more data on efficacy are required at 12 and 24 months for phase 2, and then a full phase 3 [clinical trial] with high-risk fracture patients,” he said.
The company is seeking input from the Food and Drug Administration to develop the protocol for a phase 3 trial. They expect to start this trial in 2022 at sites in the United States, Europe, and Israel, Dr. Santora said.
Primary outcome met
The study randomly assigned 161 postmenopausal women with osteoporosis or low BMD to receive placebo or the investigational oral PTH for 6 months.
Compared with women who received placebo, those who received the study drug experienced a significantly greater increase in the bone formation marker procollagen type I N-terminal propeptide (P1NP) from baseline to 3 months, thereby meeting the study’s primary outcome.
In secondary outcomes, women who received the 2.5-mg/d dose experienced a similar 6-month increase in BMD at the spine and greater increases in BMD at the total hip and femoral neck than those who received injectable teriparatide, Dr. Santora reported.
“The study’s key takeaway is that a once-daily oral PTH [tablet] has the potential to produce the same BMD effects as subcutaneous injections of PTH,” he said in an interview.
Additionally, “the drug was well tolerated when the dose was titrated by adding additional tablets, which suggests that the dose can be tailored to each patient,” he said.
Other study findings
Injectable teriparatide reduces the risk for vertebral fractures by up to 80%, Dr. Santora noted, but the fact that the drug must be administered by injection may deter some older patients from using it.
The company developed an oral form of biosynthetic human PTH with a proprietary drug delivery.
The researchers conducted the phase 2 study at four sites in Israel between June 2019 and May 2021. They enrolled women aged 50 years and older who had entered menopause at least 3 years earlier and who had osteoporosis or low BMD.
Forty-three women received placebo, and the others received oral PTH at doses of 0.5 mg/d (n = 25), 1.0 mg/d (n = 29), 1.5 mg/d (n = 28), 2.5 mg/d (n = 19), or at a dose that was titrated up to 2.5 mg/d starting at 1.5 mg/d for month 1, then 2 mg/d for month 2, and then 2.5 mg/d for months 3 to 6 (n = 17).
The mean age of the patients was 61 years, the mean body mass index was 25-27 kg/m2, and the mean T score at the spine of –2.2 to –2.45.
Among the women who received 2.5 mg/d of oral PTH for the full 6 months, serum levels of the bone resorption marker C-terminal telopeptide of type I collagen (CTX) decreased 21% from baseline to 6 months, and serum levels of P1NP increased at month 1 and then decreased to baseline by month 6.
The women who received 2.5 mg/d of oral PTH for the full 6 months also demonstrated significantly greater increases in BMD at the lumbar spine (3.8%), total hip (1.4%), and femoral neck (2.4%), compared with women who received placebo.
The safety profile of oral PTH was consistent with that of subcutaneous PTH. Patients experienced headache, nausea, presyncope, and dizziness; there were no treatment-emergent hypercalcemia adverse events.
A few ‘unexpected findings’
Suzanne M. Jan De Beur, MD, outgoing ASBMR president, said, “Oral PTH appeared to increase BMD by [dual-energy x-ray absorptiometry] at the lumbar spine effectively and to a similar degree as teriparatide in previous studies.”
She identified two unexpected findings.
“There were increases in BMD by DXA at the femoral neck and total hip at 6 months that were [greater than those] seen in previous trials of teriparatide. Second, markers of bone resorption (CTX) decreased at 6 months, and this is in stark contrast to the increases observed with teriparatide treatment,” she noted in an interview.
Dr. Rosen also noted that “the decrease in CTX is very unusual for PTH and difficult to explain.” He added: “P1NP, a marker of bone formation, was not increased.”
Dr. Jan de Beur continued: “Teriparatide (PTH1-34) and abaloparatide are effective anabolic agents that we use to treat patients with high risk of osteoporotic fracture. Although effective, the burden of daily subcutaneous injection can be a barrier for older individuals, those with poor dexterity, and those that are averse to self-injection.
“Taken together, these results appear promising, that oral PTH may prove to be an effective anabolic agent for osteoporosis treatment,” she summarized.
She stressed that a larger phase 3 study is needed to demonstrate safety and efficacy.
The study was funded by Entera Bio. Dr. Santora is chief medical officer of Entera Bio.
A version of this article first appeared on Medscape.com .
An investigational oral form of parathyroid hormone (PTH 1-34), EB 613 (Entera Bio) met its primary efficacy outcome in a phase 2 dosing study involving postmenopausal women with low bone mineral density (BMD).
The adverse effect profile of the drug was similar to that of the injectable PTH 1-34 teriparatide (Forteo), which is approved for osteoporosis.
Arthur C. Santora, MD, chief medical officer, Entera Bio, presented 6-month findings from the study during an oral session at the annual meeting of the American Society of Bone and Mineral Research. The 3-month findings from the study were reported as a poster.
If the drug demonstrates efficacy and safety in larger phase 3 trials, it could be the first oral bone-building (anabolic) therapy for osteoporosis.
Clifford J. Rosen, MD, PhD, who was not involved with the research, told this news organization: “I think this is an intriguing study.” The most likely patients for oral PTH, he added, “are those that have osteoporosis, previous fracture, or very low BMD, particularly those unlikely or unwilling to take bisphosphonates.”
However, “this is very early in the process before this drug could come to market,” cautioned Dr. Rosen, who is director of the Center for Clinical and Translational Research, Maine Medical Research Institute, Scarborough.
“Much more data on efficacy are required at 12 and 24 months for phase 2, and then a full phase 3 [clinical trial] with high-risk fracture patients,” he said.
The company is seeking input from the Food and Drug Administration to develop the protocol for a phase 3 trial. They expect to start this trial in 2022 at sites in the United States, Europe, and Israel, Dr. Santora said.
Primary outcome met
The study randomly assigned 161 postmenopausal women with osteoporosis or low BMD to receive placebo or the investigational oral PTH for 6 months.
Compared with women who received placebo, those who received the study drug experienced a significantly greater increase in the bone formation marker procollagen type I N-terminal propeptide (P1NP) from baseline to 3 months, thereby meeting the study’s primary outcome.
In secondary outcomes, women who received the 2.5-mg/d dose experienced a similar 6-month increase in BMD at the spine and greater increases in BMD at the total hip and femoral neck than those who received injectable teriparatide, Dr. Santora reported.
“The study’s key takeaway is that a once-daily oral PTH [tablet] has the potential to produce the same BMD effects as subcutaneous injections of PTH,” he said in an interview.
Additionally, “the drug was well tolerated when the dose was titrated by adding additional tablets, which suggests that the dose can be tailored to each patient,” he said.
Other study findings
Injectable teriparatide reduces the risk for vertebral fractures by up to 80%, Dr. Santora noted, but the fact that the drug must be administered by injection may deter some older patients from using it.
The company developed an oral form of biosynthetic human PTH with a proprietary drug delivery.
The researchers conducted the phase 2 study at four sites in Israel between June 2019 and May 2021. They enrolled women aged 50 years and older who had entered menopause at least 3 years earlier and who had osteoporosis or low BMD.
Forty-three women received placebo, and the others received oral PTH at doses of 0.5 mg/d (n = 25), 1.0 mg/d (n = 29), 1.5 mg/d (n = 28), 2.5 mg/d (n = 19), or at a dose that was titrated up to 2.5 mg/d starting at 1.5 mg/d for month 1, then 2 mg/d for month 2, and then 2.5 mg/d for months 3 to 6 (n = 17).
The mean age of the patients was 61 years, the mean body mass index was 25-27 kg/m2, and the mean T score at the spine of –2.2 to –2.45.
Among the women who received 2.5 mg/d of oral PTH for the full 6 months, serum levels of the bone resorption marker C-terminal telopeptide of type I collagen (CTX) decreased 21% from baseline to 6 months, and serum levels of P1NP increased at month 1 and then decreased to baseline by month 6.
The women who received 2.5 mg/d of oral PTH for the full 6 months also demonstrated significantly greater increases in BMD at the lumbar spine (3.8%), total hip (1.4%), and femoral neck (2.4%), compared with women who received placebo.
The safety profile of oral PTH was consistent with that of subcutaneous PTH. Patients experienced headache, nausea, presyncope, and dizziness; there were no treatment-emergent hypercalcemia adverse events.
A few ‘unexpected findings’
Suzanne M. Jan De Beur, MD, outgoing ASBMR president, said, “Oral PTH appeared to increase BMD by [dual-energy x-ray absorptiometry] at the lumbar spine effectively and to a similar degree as teriparatide in previous studies.”
She identified two unexpected findings.
“There were increases in BMD by DXA at the femoral neck and total hip at 6 months that were [greater than those] seen in previous trials of teriparatide. Second, markers of bone resorption (CTX) decreased at 6 months, and this is in stark contrast to the increases observed with teriparatide treatment,” she noted in an interview.
Dr. Rosen also noted that “the decrease in CTX is very unusual for PTH and difficult to explain.” He added: “P1NP, a marker of bone formation, was not increased.”
Dr. Jan de Beur continued: “Teriparatide (PTH1-34) and abaloparatide are effective anabolic agents that we use to treat patients with high risk of osteoporotic fracture. Although effective, the burden of daily subcutaneous injection can be a barrier for older individuals, those with poor dexterity, and those that are averse to self-injection.
“Taken together, these results appear promising, that oral PTH may prove to be an effective anabolic agent for osteoporosis treatment,” she summarized.
She stressed that a larger phase 3 study is needed to demonstrate safety and efficacy.
The study was funded by Entera Bio. Dr. Santora is chief medical officer of Entera Bio.
A version of this article first appeared on Medscape.com .
Abaloparatide significantly reduced fractures, increased BMD in women at high fracture risk
Postmenopausal women at high or very high risk of fracture gained significantly more bone mineral density and were significantly less likely to experience a fracture when taking abaloparatide for 18 months, according to new research presented at the hybrid annual meeting of the North American Menopause Society.
“The findings showed that abaloparatide was better than teriparatide in a number of parameters important in osteoporosis treatment, and similar in others, in high-risk and very-high-risk postmenopausal women with osteoporosis,” Bart Clarke, MD, a professor of medicine at Mayo Clinic in Rochester, Minn., said in an interview. “Abaloparatide is safe and effective for use in high-risk or very-high-risk postmenopausal women,” as defined by the new American Association of Clinical Endocrinology/American College of Endocrinology osteoporosis guidelines.
Ricardo R. Correa, MD, of the department of endocrinology and director of diversity for graduate medical education at the University of Arizona, Phoenix, said that the study demonstrates that abaloparatide and teriparatide have a very similar effect with abaloparatide providing a slightly better absolute risk reduction in fracture. Dr. Correa was not involved in the research.
“What will drive my decision in what to prescribe will be the cost and insurance coverage,” Dr. Correa said. “At the Veterans Administration hospital, the option that we have is abaloparatide, so this is the option that we use.”
Among women at least 65 years old who have already had one fracture, 1 in 10 will experience another fracture within the next year, and 30% will have another fracture within the next 5 years, the authors noted in their background material. Since phase 3 ACTIVE study data in 2016 showed that abaloparatide reduces fracture risk while increasing bone mineral density, compared with placebo, the researchers reanalyzed that data to assess the drug’s efficacy in patients at high or very high risk for fracture.
The study involved 2,463 postmenopausal women with osteoporosis who received one of three interventions: 80 mcg abaloparatide daily, placebo, or 20 mcg subcutaneous teriparatide daily. Only the abaloparatide and placebo groups were double blinded.
“Teriparatide was used as the comparator drug because teriparatide was previously approved as the first anabolic drug for osteoporosis,” Dr. Clarke said in an interview. “The hope was to show that abaloparatide was a better anabolic drug.”
Women were considered at high or very high risk of fracture if they met at least one of the following four criteria from the 2020 American Association of Clinical Endocrinology guidelines:
- Fracture within the past 12 months or prevalent vertebral fracture.
- Very low T-score (less than –3.0) at baseline at any site.
- Multiple fractures at baseline since age 45.
- Very high fracture risk based on the Fracture Risk Assessment Tool (FRAX) (at least 30% for major osteoporotic fracture or at least 4.5% for hip fracture).
Among the 2,026 patients who met at least one of these criteria, 664 received abaloparatide, 685 received teriparatide, and 677 received placebo. Both the abaloparatide and teriparatide significantly reduced new vertebral fracture risk, compared with placebo. In the abaloparatide group, 0.72% of women had a new vertebral fracture, compared with 0.99% in the teriparatide group and 4.77% in the placebo group (P < .0001).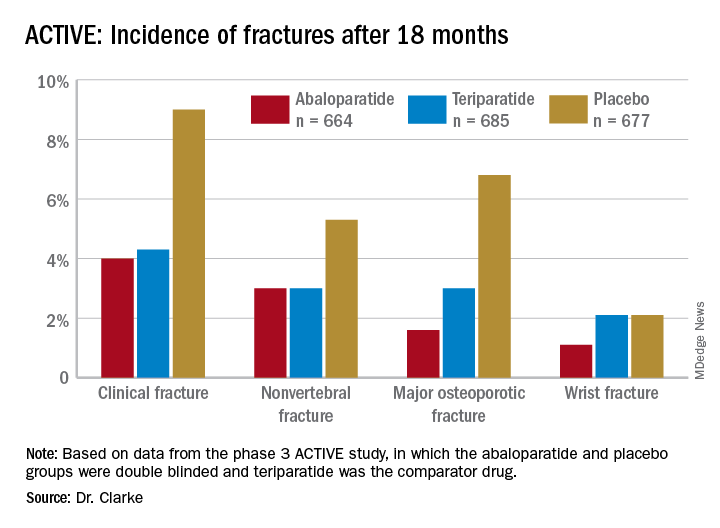
Abaloparatide and teriparatide also led to significant increases in lumbar spine, total hip, and femoral neck bone mineral density, compared with placebo (P < .0001).
The study was limited by its duration of 18 months and the Food and Drug Administration’s restriction on using abaloparatide for more than 2 years because of the theoretical risk of increasing osteosarcoma, although that risk has never been demonstrated in humans, Dr. Correa said. ”We need more data with abaloparitide in more than 2 years,” he added.
In determining which medication clinicians should first prescribe to manage osteoporosis, Dr. Correa said practitioners should consider the type of osteoporosis women have, their preferences, and their labs on kidney function.
With mild to moderate osteoporosis, bisphosphonates will be the first option while denosumab will be preferred for moderate to severe osteoporosis. Teriparatide and abaloparitide are the first-line options for severe osteoporosis, he said.
“If the glomerular filtration rate is low, we cannot use bisphosphonate and we will have to limit our use to denosumab,” he said. Route and frequency of delivery plays a role in patient preferences.
“If the patient prefers an infusion once a year or a pill, then bisphosphonate,” he said, but “if the patient is fine with an injection every 6 months, then denosumab.” Patients who need and can do an injection every day can take abaloparitide or teriparatide.
Failure of previous treatments also guide clinical decisions, he added. ”If the patient has been on one medication and has a fracture or the bone mineral density decreases, then we need to switch to another medication, usually teriparatide or abaloparitide, to build new bone.”
Contraindications for abaloparatide include a high serum calcium before therapy or prior allergic reactions to components in abaloparatide, Dr. Clarke said. No new safety signals showed up in the data analysis.
The research was funded by Radius Health. Dr. Clarke is an advisory board member of Amgen, and another author consults and speaks for Amgen and is a Radius Health Advisory Board member. Two other authors are Radius Health employees who own stock in the company. Dr Correa has no disclosures.
Postmenopausal women at high or very high risk of fracture gained significantly more bone mineral density and were significantly less likely to experience a fracture when taking abaloparatide for 18 months, according to new research presented at the hybrid annual meeting of the North American Menopause Society.
“The findings showed that abaloparatide was better than teriparatide in a number of parameters important in osteoporosis treatment, and similar in others, in high-risk and very-high-risk postmenopausal women with osteoporosis,” Bart Clarke, MD, a professor of medicine at Mayo Clinic in Rochester, Minn., said in an interview. “Abaloparatide is safe and effective for use in high-risk or very-high-risk postmenopausal women,” as defined by the new American Association of Clinical Endocrinology/American College of Endocrinology osteoporosis guidelines.
Ricardo R. Correa, MD, of the department of endocrinology and director of diversity for graduate medical education at the University of Arizona, Phoenix, said that the study demonstrates that abaloparatide and teriparatide have a very similar effect with abaloparatide providing a slightly better absolute risk reduction in fracture. Dr. Correa was not involved in the research.
“What will drive my decision in what to prescribe will be the cost and insurance coverage,” Dr. Correa said. “At the Veterans Administration hospital, the option that we have is abaloparatide, so this is the option that we use.”
Among women at least 65 years old who have already had one fracture, 1 in 10 will experience another fracture within the next year, and 30% will have another fracture within the next 5 years, the authors noted in their background material. Since phase 3 ACTIVE study data in 2016 showed that abaloparatide reduces fracture risk while increasing bone mineral density, compared with placebo, the researchers reanalyzed that data to assess the drug’s efficacy in patients at high or very high risk for fracture.
The study involved 2,463 postmenopausal women with osteoporosis who received one of three interventions: 80 mcg abaloparatide daily, placebo, or 20 mcg subcutaneous teriparatide daily. Only the abaloparatide and placebo groups were double blinded.
“Teriparatide was used as the comparator drug because teriparatide was previously approved as the first anabolic drug for osteoporosis,” Dr. Clarke said in an interview. “The hope was to show that abaloparatide was a better anabolic drug.”
Women were considered at high or very high risk of fracture if they met at least one of the following four criteria from the 2020 American Association of Clinical Endocrinology guidelines:
- Fracture within the past 12 months or prevalent vertebral fracture.
- Very low T-score (less than –3.0) at baseline at any site.
- Multiple fractures at baseline since age 45.
- Very high fracture risk based on the Fracture Risk Assessment Tool (FRAX) (at least 30% for major osteoporotic fracture or at least 4.5% for hip fracture).
Among the 2,026 patients who met at least one of these criteria, 664 received abaloparatide, 685 received teriparatide, and 677 received placebo. Both the abaloparatide and teriparatide significantly reduced new vertebral fracture risk, compared with placebo. In the abaloparatide group, 0.72% of women had a new vertebral fracture, compared with 0.99% in the teriparatide group and 4.77% in the placebo group (P < .0001).
Abaloparatide and teriparatide also led to significant increases in lumbar spine, total hip, and femoral neck bone mineral density, compared with placebo (P < .0001).
The study was limited by its duration of 18 months and the Food and Drug Administration’s restriction on using abaloparatide for more than 2 years because of the theoretical risk of increasing osteosarcoma, although that risk has never been demonstrated in humans, Dr. Correa said. ”We need more data with abaloparitide in more than 2 years,” he added.
In determining which medication clinicians should first prescribe to manage osteoporosis, Dr. Correa said practitioners should consider the type of osteoporosis women have, their preferences, and their labs on kidney function.
With mild to moderate osteoporosis, bisphosphonates will be the first option while denosumab will be preferred for moderate to severe osteoporosis. Teriparatide and abaloparitide are the first-line options for severe osteoporosis, he said.
“If the glomerular filtration rate is low, we cannot use bisphosphonate and we will have to limit our use to denosumab,” he said. Route and frequency of delivery plays a role in patient preferences.
“If the patient prefers an infusion once a year or a pill, then bisphosphonate,” he said, but “if the patient is fine with an injection every 6 months, then denosumab.” Patients who need and can do an injection every day can take abaloparitide or teriparatide.
Failure of previous treatments also guide clinical decisions, he added. ”If the patient has been on one medication and has a fracture or the bone mineral density decreases, then we need to switch to another medication, usually teriparatide or abaloparitide, to build new bone.”
Contraindications for abaloparatide include a high serum calcium before therapy or prior allergic reactions to components in abaloparatide, Dr. Clarke said. No new safety signals showed up in the data analysis.
The research was funded by Radius Health. Dr. Clarke is an advisory board member of Amgen, and another author consults and speaks for Amgen and is a Radius Health Advisory Board member. Two other authors are Radius Health employees who own stock in the company. Dr Correa has no disclosures.
Postmenopausal women at high or very high risk of fracture gained significantly more bone mineral density and were significantly less likely to experience a fracture when taking abaloparatide for 18 months, according to new research presented at the hybrid annual meeting of the North American Menopause Society.
“The findings showed that abaloparatide was better than teriparatide in a number of parameters important in osteoporosis treatment, and similar in others, in high-risk and very-high-risk postmenopausal women with osteoporosis,” Bart Clarke, MD, a professor of medicine at Mayo Clinic in Rochester, Minn., said in an interview. “Abaloparatide is safe and effective for use in high-risk or very-high-risk postmenopausal women,” as defined by the new American Association of Clinical Endocrinology/American College of Endocrinology osteoporosis guidelines.
Ricardo R. Correa, MD, of the department of endocrinology and director of diversity for graduate medical education at the University of Arizona, Phoenix, said that the study demonstrates that abaloparatide and teriparatide have a very similar effect with abaloparatide providing a slightly better absolute risk reduction in fracture. Dr. Correa was not involved in the research.
“What will drive my decision in what to prescribe will be the cost and insurance coverage,” Dr. Correa said. “At the Veterans Administration hospital, the option that we have is abaloparatide, so this is the option that we use.”
Among women at least 65 years old who have already had one fracture, 1 in 10 will experience another fracture within the next year, and 30% will have another fracture within the next 5 years, the authors noted in their background material. Since phase 3 ACTIVE study data in 2016 showed that abaloparatide reduces fracture risk while increasing bone mineral density, compared with placebo, the researchers reanalyzed that data to assess the drug’s efficacy in patients at high or very high risk for fracture.
The study involved 2,463 postmenopausal women with osteoporosis who received one of three interventions: 80 mcg abaloparatide daily, placebo, or 20 mcg subcutaneous teriparatide daily. Only the abaloparatide and placebo groups were double blinded.
“Teriparatide was used as the comparator drug because teriparatide was previously approved as the first anabolic drug for osteoporosis,” Dr. Clarke said in an interview. “The hope was to show that abaloparatide was a better anabolic drug.”
Women were considered at high or very high risk of fracture if they met at least one of the following four criteria from the 2020 American Association of Clinical Endocrinology guidelines:
- Fracture within the past 12 months or prevalent vertebral fracture.
- Very low T-score (less than –3.0) at baseline at any site.
- Multiple fractures at baseline since age 45.
- Very high fracture risk based on the Fracture Risk Assessment Tool (FRAX) (at least 30% for major osteoporotic fracture or at least 4.5% for hip fracture).
Among the 2,026 patients who met at least one of these criteria, 664 received abaloparatide, 685 received teriparatide, and 677 received placebo. Both the abaloparatide and teriparatide significantly reduced new vertebral fracture risk, compared with placebo. In the abaloparatide group, 0.72% of women had a new vertebral fracture, compared with 0.99% in the teriparatide group and 4.77% in the placebo group (P < .0001).
Abaloparatide and teriparatide also led to significant increases in lumbar spine, total hip, and femoral neck bone mineral density, compared with placebo (P < .0001).
The study was limited by its duration of 18 months and the Food and Drug Administration’s restriction on using abaloparatide for more than 2 years because of the theoretical risk of increasing osteosarcoma, although that risk has never been demonstrated in humans, Dr. Correa said. ”We need more data with abaloparitide in more than 2 years,” he added.
In determining which medication clinicians should first prescribe to manage osteoporosis, Dr. Correa said practitioners should consider the type of osteoporosis women have, their preferences, and their labs on kidney function.
With mild to moderate osteoporosis, bisphosphonates will be the first option while denosumab will be preferred for moderate to severe osteoporosis. Teriparatide and abaloparitide are the first-line options for severe osteoporosis, he said.
“If the glomerular filtration rate is low, we cannot use bisphosphonate and we will have to limit our use to denosumab,” he said. Route and frequency of delivery plays a role in patient preferences.
“If the patient prefers an infusion once a year or a pill, then bisphosphonate,” he said, but “if the patient is fine with an injection every 6 months, then denosumab.” Patients who need and can do an injection every day can take abaloparitide or teriparatide.
Failure of previous treatments also guide clinical decisions, he added. ”If the patient has been on one medication and has a fracture or the bone mineral density decreases, then we need to switch to another medication, usually teriparatide or abaloparitide, to build new bone.”
Contraindications for abaloparatide include a high serum calcium before therapy or prior allergic reactions to components in abaloparatide, Dr. Clarke said. No new safety signals showed up in the data analysis.
The research was funded by Radius Health. Dr. Clarke is an advisory board member of Amgen, and another author consults and speaks for Amgen and is a Radius Health Advisory Board member. Two other authors are Radius Health employees who own stock in the company. Dr Correa has no disclosures.
FROM NAMS 2021
Cut risedronate drug holiday to under 2 years in older patients
Any pause in taking the osteoporosis drug risedronate (Actonel) should last no longer than 2 years rather than the 2-3 years currently recommended for bisphosphonates, new research suggests.
In a cohort of patients aged 66 and older in Ontario, Canada, those who had been taking risedronate had a 34% greater risk of a hip fracture during year 2 to year 3 of a pause in taking the drug – a drug holiday – compared with those who had been taking alendronate (Fosamax).
The study showed that “risedronate, which has a shorter half-life, confers relatively less hip fracture protection than alendronate during drug holidays longer than 2 years and careful monitoring and follow-up after 2 years is likely warranted,” Kaley (Kaleen) N. Hayes, Pharm D, PhD, summarized in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research. Dr. Hayes is an assistant professor in the department of health services, policy, and practice at Brown University School of Public Health, Providence, R.I.
“Although alendronate and risedronate have similar effectiveness for preventing fractures on treatment, our findings suggest that older patients on a risedronate drug holiday may benefit from assessment to consider resuming therapy after 2 years to prevent hip fractures,” she elaborated in an email.
Juliet Compston, MD, identified this study as one of the meeting’s clinical science highlights.
“This is the first study to directly compare fracture incidence during a drug holiday after treatment with the two most commonly prescribed oral bisphosphonates, alendronate and risedronate,” she told this news organization in an email.
The difference in fracture incidence during the 3-year drug holiday is “consistent with the known difference in pharmacokinetic properties of the two drugs,” noted Dr. Compston, professor of bone medicine and honorary consultant physician at the University of Cambridge (England) School of Clinical Medicine.
Since the increased risk of fracture after stopping risedronate vs. alendronate was seen by 2 years, “reevaluation of risk in risedronate-treated patients should therefore be considered earlier than the recommended period of 2-3 years after discontinuation,” she said.
“The study does not provide information about the optimal duration of drug holiday for either risedronate or alendronate, but it supports a shorter duration for the former of up to 2 years,” according to Dr. Compston.
Study rationale and findings
“The question of whether people treated for osteoporosis with oral bisphosphonates should have drug holidays is controversial,” Dr. Compston noted, “but many guidelines recommend that in lower-risk individuals who have received bisphosphonates for 5 years, a break from treatment of 2-3 years should be considered.”
Five or more years of bisphosphonate treatment for osteoporosis has been associated with rare adverse effects such as atypical femoral fractures, and these drugs appear to have fracture protection effects that linger for a while, so a drug holiday is recommended for most patients, Dr. Hayes added.
Guidelines such as the 2016 ASBMR task force report on long-term bisphosphonates for osteoporosis, she continued, “acknowledge that evidence for this recommendation comes primarily from the extension trial for alendronate, and patients undergoing a risedronate drug holiday may need to be reassessed earlier because of risedronate’s shorter half-life.”
Compared with alendronate, risedronate accumulates less in the bone and is eliminated more quickly from the body, so its fracture protection during drug holidays may be shorter.
The researchers aimed to estimate the 3-year fracture risk after discontinuing long-term (3 or more years) risedronate vs. alendronate therapy among older adults in Ontario.
From health care administrative data, they identified 120,368 patients aged 66 years and older who had started taking risedronate or alendronate as initial therapy for osteoporosis during the period 2000-2016. They had taken the therapy for 3 or more years (with at least 80% adherence) before stopping it for 120 days or longer.
The researchers found that 45% of patients were taking risedronate and 55% were taking alendronate, which are the main bisphosphonates used in Ontario, Dr. Hayes noted. Etidronate (Didronel) is recommended as second-line therapy and accounts for less than 2% of patients starting oral bisphosphonate therapy.
In an earlier study, the researchers identified a shift toward greater use of risedronate than alendronate since 2008, likely related to newer formulations (for example, monthly and weekly delayed-release formulations of risedronate vs. only weekly alendronate formulations).
The researchers matched 25,077 patients taking alendronate with 25,077 patients taking risedronate, based on fracture risk–related characteristics, including demographics, diagnoses, medication use, and health care use.
The patients had a mean age of 74 when they started taking an oral bisphosphonate; 82% were women and most were White.
Most patients (78%) had received a prescription from a general practitioner and, on average, they took the bisphosphonate therapy for 5.9 years before the drug holiday.
The primary outcome of incident hip fracture during a 3-year drug holiday occurred in 915 patients. There were 12.4 events per 1,000 patients in the risedronate group vs. 10.6 events per 1,000 patients in the alendronate group (hazard ratio, 1.18; 95% confidence interval, 1.04-1.34).
The risks were not significantly higher during year 1 or year 2 of the drug holiday, but the curves began to diverge after 2 years, coauthor Suzanne Cadarette, PhD, of the Leslie Dan Faculty of Pharmacy at the University of Toronto, explained when replying to a question after the presentation. Dr. Cadarette supervised this PhD dissertation research by Dr. Hayes.
The researchers acknowledged that the limitations of their study include a lack of information about race or bone mineral density, and the findings may not apply to a younger, more racially diverse population.
The research was supported by the University of Toronto Dalla Lana School of Public Health and the Leslie Dan Faculty of Pharmacy, a Canadian Institutes of Health Research grant, and a doctoral research award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Any pause in taking the osteoporosis drug risedronate (Actonel) should last no longer than 2 years rather than the 2-3 years currently recommended for bisphosphonates, new research suggests.
In a cohort of patients aged 66 and older in Ontario, Canada, those who had been taking risedronate had a 34% greater risk of a hip fracture during year 2 to year 3 of a pause in taking the drug – a drug holiday – compared with those who had been taking alendronate (Fosamax).
The study showed that “risedronate, which has a shorter half-life, confers relatively less hip fracture protection than alendronate during drug holidays longer than 2 years and careful monitoring and follow-up after 2 years is likely warranted,” Kaley (Kaleen) N. Hayes, Pharm D, PhD, summarized in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research. Dr. Hayes is an assistant professor in the department of health services, policy, and practice at Brown University School of Public Health, Providence, R.I.
“Although alendronate and risedronate have similar effectiveness for preventing fractures on treatment, our findings suggest that older patients on a risedronate drug holiday may benefit from assessment to consider resuming therapy after 2 years to prevent hip fractures,” she elaborated in an email.
Juliet Compston, MD, identified this study as one of the meeting’s clinical science highlights.
“This is the first study to directly compare fracture incidence during a drug holiday after treatment with the two most commonly prescribed oral bisphosphonates, alendronate and risedronate,” she told this news organization in an email.
The difference in fracture incidence during the 3-year drug holiday is “consistent with the known difference in pharmacokinetic properties of the two drugs,” noted Dr. Compston, professor of bone medicine and honorary consultant physician at the University of Cambridge (England) School of Clinical Medicine.
Since the increased risk of fracture after stopping risedronate vs. alendronate was seen by 2 years, “reevaluation of risk in risedronate-treated patients should therefore be considered earlier than the recommended period of 2-3 years after discontinuation,” she said.
“The study does not provide information about the optimal duration of drug holiday for either risedronate or alendronate, but it supports a shorter duration for the former of up to 2 years,” according to Dr. Compston.
Study rationale and findings
“The question of whether people treated for osteoporosis with oral bisphosphonates should have drug holidays is controversial,” Dr. Compston noted, “but many guidelines recommend that in lower-risk individuals who have received bisphosphonates for 5 years, a break from treatment of 2-3 years should be considered.”
Five or more years of bisphosphonate treatment for osteoporosis has been associated with rare adverse effects such as atypical femoral fractures, and these drugs appear to have fracture protection effects that linger for a while, so a drug holiday is recommended for most patients, Dr. Hayes added.
Guidelines such as the 2016 ASBMR task force report on long-term bisphosphonates for osteoporosis, she continued, “acknowledge that evidence for this recommendation comes primarily from the extension trial for alendronate, and patients undergoing a risedronate drug holiday may need to be reassessed earlier because of risedronate’s shorter half-life.”
Compared with alendronate, risedronate accumulates less in the bone and is eliminated more quickly from the body, so its fracture protection during drug holidays may be shorter.
The researchers aimed to estimate the 3-year fracture risk after discontinuing long-term (3 or more years) risedronate vs. alendronate therapy among older adults in Ontario.
From health care administrative data, they identified 120,368 patients aged 66 years and older who had started taking risedronate or alendronate as initial therapy for osteoporosis during the period 2000-2016. They had taken the therapy for 3 or more years (with at least 80% adherence) before stopping it for 120 days or longer.
The researchers found that 45% of patients were taking risedronate and 55% were taking alendronate, which are the main bisphosphonates used in Ontario, Dr. Hayes noted. Etidronate (Didronel) is recommended as second-line therapy and accounts for less than 2% of patients starting oral bisphosphonate therapy.
In an earlier study, the researchers identified a shift toward greater use of risedronate than alendronate since 2008, likely related to newer formulations (for example, monthly and weekly delayed-release formulations of risedronate vs. only weekly alendronate formulations).
The researchers matched 25,077 patients taking alendronate with 25,077 patients taking risedronate, based on fracture risk–related characteristics, including demographics, diagnoses, medication use, and health care use.
The patients had a mean age of 74 when they started taking an oral bisphosphonate; 82% were women and most were White.
Most patients (78%) had received a prescription from a general practitioner and, on average, they took the bisphosphonate therapy for 5.9 years before the drug holiday.
The primary outcome of incident hip fracture during a 3-year drug holiday occurred in 915 patients. There were 12.4 events per 1,000 patients in the risedronate group vs. 10.6 events per 1,000 patients in the alendronate group (hazard ratio, 1.18; 95% confidence interval, 1.04-1.34).
The risks were not significantly higher during year 1 or year 2 of the drug holiday, but the curves began to diverge after 2 years, coauthor Suzanne Cadarette, PhD, of the Leslie Dan Faculty of Pharmacy at the University of Toronto, explained when replying to a question after the presentation. Dr. Cadarette supervised this PhD dissertation research by Dr. Hayes.
The researchers acknowledged that the limitations of their study include a lack of information about race or bone mineral density, and the findings may not apply to a younger, more racially diverse population.
The research was supported by the University of Toronto Dalla Lana School of Public Health and the Leslie Dan Faculty of Pharmacy, a Canadian Institutes of Health Research grant, and a doctoral research award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Any pause in taking the osteoporosis drug risedronate (Actonel) should last no longer than 2 years rather than the 2-3 years currently recommended for bisphosphonates, new research suggests.
In a cohort of patients aged 66 and older in Ontario, Canada, those who had been taking risedronate had a 34% greater risk of a hip fracture during year 2 to year 3 of a pause in taking the drug – a drug holiday – compared with those who had been taking alendronate (Fosamax).
The study showed that “risedronate, which has a shorter half-life, confers relatively less hip fracture protection than alendronate during drug holidays longer than 2 years and careful monitoring and follow-up after 2 years is likely warranted,” Kaley (Kaleen) N. Hayes, Pharm D, PhD, summarized in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research. Dr. Hayes is an assistant professor in the department of health services, policy, and practice at Brown University School of Public Health, Providence, R.I.
“Although alendronate and risedronate have similar effectiveness for preventing fractures on treatment, our findings suggest that older patients on a risedronate drug holiday may benefit from assessment to consider resuming therapy after 2 years to prevent hip fractures,” she elaborated in an email.
Juliet Compston, MD, identified this study as one of the meeting’s clinical science highlights.
“This is the first study to directly compare fracture incidence during a drug holiday after treatment with the two most commonly prescribed oral bisphosphonates, alendronate and risedronate,” she told this news organization in an email.
The difference in fracture incidence during the 3-year drug holiday is “consistent with the known difference in pharmacokinetic properties of the two drugs,” noted Dr. Compston, professor of bone medicine and honorary consultant physician at the University of Cambridge (England) School of Clinical Medicine.
Since the increased risk of fracture after stopping risedronate vs. alendronate was seen by 2 years, “reevaluation of risk in risedronate-treated patients should therefore be considered earlier than the recommended period of 2-3 years after discontinuation,” she said.
“The study does not provide information about the optimal duration of drug holiday for either risedronate or alendronate, but it supports a shorter duration for the former of up to 2 years,” according to Dr. Compston.
Study rationale and findings
“The question of whether people treated for osteoporosis with oral bisphosphonates should have drug holidays is controversial,” Dr. Compston noted, “but many guidelines recommend that in lower-risk individuals who have received bisphosphonates for 5 years, a break from treatment of 2-3 years should be considered.”
Five or more years of bisphosphonate treatment for osteoporosis has been associated with rare adverse effects such as atypical femoral fractures, and these drugs appear to have fracture protection effects that linger for a while, so a drug holiday is recommended for most patients, Dr. Hayes added.
Guidelines such as the 2016 ASBMR task force report on long-term bisphosphonates for osteoporosis, she continued, “acknowledge that evidence for this recommendation comes primarily from the extension trial for alendronate, and patients undergoing a risedronate drug holiday may need to be reassessed earlier because of risedronate’s shorter half-life.”
Compared with alendronate, risedronate accumulates less in the bone and is eliminated more quickly from the body, so its fracture protection during drug holidays may be shorter.
The researchers aimed to estimate the 3-year fracture risk after discontinuing long-term (3 or more years) risedronate vs. alendronate therapy among older adults in Ontario.
From health care administrative data, they identified 120,368 patients aged 66 years and older who had started taking risedronate or alendronate as initial therapy for osteoporosis during the period 2000-2016. They had taken the therapy for 3 or more years (with at least 80% adherence) before stopping it for 120 days or longer.
The researchers found that 45% of patients were taking risedronate and 55% were taking alendronate, which are the main bisphosphonates used in Ontario, Dr. Hayes noted. Etidronate (Didronel) is recommended as second-line therapy and accounts for less than 2% of patients starting oral bisphosphonate therapy.
In an earlier study, the researchers identified a shift toward greater use of risedronate than alendronate since 2008, likely related to newer formulations (for example, monthly and weekly delayed-release formulations of risedronate vs. only weekly alendronate formulations).
The researchers matched 25,077 patients taking alendronate with 25,077 patients taking risedronate, based on fracture risk–related characteristics, including demographics, diagnoses, medication use, and health care use.
The patients had a mean age of 74 when they started taking an oral bisphosphonate; 82% were women and most were White.
Most patients (78%) had received a prescription from a general practitioner and, on average, they took the bisphosphonate therapy for 5.9 years before the drug holiday.
The primary outcome of incident hip fracture during a 3-year drug holiday occurred in 915 patients. There were 12.4 events per 1,000 patients in the risedronate group vs. 10.6 events per 1,000 patients in the alendronate group (hazard ratio, 1.18; 95% confidence interval, 1.04-1.34).
The risks were not significantly higher during year 1 or year 2 of the drug holiday, but the curves began to diverge after 2 years, coauthor Suzanne Cadarette, PhD, of the Leslie Dan Faculty of Pharmacy at the University of Toronto, explained when replying to a question after the presentation. Dr. Cadarette supervised this PhD dissertation research by Dr. Hayes.
The researchers acknowledged that the limitations of their study include a lack of information about race or bone mineral density, and the findings may not apply to a younger, more racially diverse population.
The research was supported by the University of Toronto Dalla Lana School of Public Health and the Leslie Dan Faculty of Pharmacy, a Canadian Institutes of Health Research grant, and a doctoral research award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2021
Exercise appears to improve bone structure, not density
“Postmenopausal women with low bone mass should obtain adequate calcium and vitamin D and participate in bone-loading exercises,” researchers noted in a recent study published in Osteoporosis International.
“Additional use of bisphosphonates will increase bone mineral density (BMD), especially at the spine,” wrote Nancy Waltman, PhD, College of Nursing, University of Nebraska Medical Center, Omaha, and colleagues.
The findings are partial results from the Heartland Osteoporosis Prevention Study (HOPS), which randomized women who had entered menopause within the previous 6 months and had osteopenia (low bone mass, T score –1.0 to –2.49) to receive one of three treatments for 12 months:
- Bone-loading and resistance exercise plus calcium and vitamin D supplements.
- Risedronate plus calcium and vitamin D supplements.
- Calcium and vitamin D supplements alone (control).
At 1 year, “risedronate significantly increased BMD at the spine, compared to exercise and control, and serum biomarkers of bone turnover also significantly reduced in the risedronate group,” Laura Bilek, PT, PhD, said during an oral presentation of the research at the annual meeting of the American Society for Bone and Mineral Research.
However, the results also showed that, importantly, “in postmenopausal women, exercise appears to improve strength at the hip through changes in structure, not BMD,” stressed Dr. Bilek, of the College of Allied Health Professionals, University of Nebraska Medical Center.
Bone health is about more than just bone mineral density
“The key takeaway for clinicians is that bone health is about more than just density!” she noted in an email.
Current guidelines don’t recommend prescribing risedronate until a woman has overt osteoporosis, she said.
On the other hand, many studies have shown that, to be most effective, bone-loading exercises should be a lifelong habit and women should begin to do them at least during menopause and should not wait until bone loss occurs.
Other studies have shown that exercise changes bone structure (size or geometry), which improves bone strength. The current study supports both prior observations.
And exercise also improves muscle strength and decreases the risk of falls and fractures, Dr. Bilek noted.
Invited to comment, Pauline M. Camacho, MD, cochair of the task force for the American Association of Clinical Endocrinologists (AACE) guidelines for osteoporosis, noted that all three measures – pharmacotherapy, exercise, and calcium/vitamin D – are important in the successful management of osteoporosis.
This study showed that risedronate is superior to calcium/vitamin D supplementation as well as exercise for BMD and for bone turnover in these women with osteopenia, said Dr. Camacho, professor of medicine and director of the Osteoporosis and Metabolic Bone Disease Center, Loyola University Medical Center, Chicago.
“Most women with osteopenia do not receive pharmacologic therapy,” she noted, and receive it only “if there is a history of fractures or they have other features that change that diagnosis to osteoporosis.
“There is no downside to exercise, and this needs to be advised to all patients,” she said. “The other aspect of exercise that was not assessed in this study is its effect on balance. Patients who exercise will have improved balance, which should translate into fewer falls, and thus fewer fractures.”
How can women with osteopenia maintain bone health?
In their article, Dr. Waltman and colleagues say the Lifting Intervention for Training Muscle and Osteoporosis Rehabilitation (LIFTMOR) clinical trial is one of the first to address clinician concerns about the safety and effectiveness of exercise to improve bone health.
In that trial of 101 postmenopausal women with low bone mass, 8 months of 30-minute, twice-weekly, supervised high-intensity resistance and impact training was safe and BMD increased by 2.9% at the lumbar spine and 0.3% at the femoral neck.
“Our [HOPS] study,” Dr. Waltman and colleagues explained, “builds on the LIFTMOR clinical trial and adds further data to inform whether postmenopausal women with low bone mass can effectively maintain or even improve BMD with bone-loading exercises prior to prescriptions for medication.
“Our long-term goal is to contribute to the development of clinical practice guidelines for the prevention of fractures in postmenopausal women with low bone mass,” they said.
They randomized 276 postmenopausal women who were a mean age of 54 (range, 44-63); most were White (78%) or Hispanic (6%).
Women were excluded from the study if they had a diagnosis of osteoporosis (T-score < −2.5); had an increased risk of a major fracture or hip fracture; had been on bisphosphonates within the last 6 months; were currently on estrogen, tamoxifen, or aromatase inhibitors; had a serum vitamin D level < 10 mg/mL or > 100 mg/mL; had any conditions that prohibited prescriptions for calcium and vitamin D supplements, risedronate, or exercise; or weighed more than 300 pounds.
All women received 1,200 mg/day of calcium (from supplements or diet) and 1,000-3,000 IU/day of vitamin D supplements, based on their serum 25(OH) vitamin D levels.
The exercise program consisted of visiting a gym three times a week for 45 minutes of bone-loading exercise – jogging with a weighted vest – and resistance exercises, which were supervised by a trainer for the first 2 weeks.
Women in the risedronate group received a 150-mg tablet of risedronate every 4 weeks.
At baseline, 6 months, and 12 months, the women had DXA scans to determine BMD and hip structure, and had blood tests to determine levels of serum markers for bone formation (bone specific alkaline phosphatase [Alkphase B]) and bone resorption (N-terminal telopeptide [NTx]).
Compared with baseline, at 12 months, the women had the following changes in BMD at the following sites:
- Spine: +1.9%, +0.9%, and –0.4%, in the risedronate, exercise, and control groups.
- Total hip: +0.9%, +0.5%, and +0.5%, in the risedronate, exercise, and control groups.
- Femoral neck: +0.09%, –0.4%, and –0.5%, in the risedronate, exercise, and control groups.
These improvements in BMD were significantly greater in the risedronate group than in the exercise or control groups (P < .01 for both).
The decreases in serum levels of NtX and Alkphase B were also greater with risedronate than in the exercise or control groups (P < .01 for all).
The most frequent adverse effect with the calcium supplement was constipation (n = 4). Some women taking risedronate had gastrointestinal disturbances (n = 4), muscle or joint pain (n = 11), or chest pain and dizziness (n = 2). None of the women had adverse effects from vitamin D. A few women had muscle soreness from exercise that went away after the exercises were adapted. None of the women had a serious injury or fracture from exercise.
More women in the exercise group withdrew from the study (n = 20), with most citing lack of time as the reason; 13 women withdrew from the risedronate group, and 16 withdrew from the control group.
Of the 276 participants who completed the 12-month study, treatment adherence was 92% for calcium, 94% for vitamin D, 75% for risedronate, and 59% for exercise.
Exercise was associated with positive changes in intertrochanter hip structural analysis measures, which will be described in an upcoming study, Dr. Bilek said.
The study was funded by the National Institute of Nursing Research of the National Institutes of Health. The researchers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Postmenopausal women with low bone mass should obtain adequate calcium and vitamin D and participate in bone-loading exercises,” researchers noted in a recent study published in Osteoporosis International.
“Additional use of bisphosphonates will increase bone mineral density (BMD), especially at the spine,” wrote Nancy Waltman, PhD, College of Nursing, University of Nebraska Medical Center, Omaha, and colleagues.
The findings are partial results from the Heartland Osteoporosis Prevention Study (HOPS), which randomized women who had entered menopause within the previous 6 months and had osteopenia (low bone mass, T score –1.0 to –2.49) to receive one of three treatments for 12 months:
- Bone-loading and resistance exercise plus calcium and vitamin D supplements.
- Risedronate plus calcium and vitamin D supplements.
- Calcium and vitamin D supplements alone (control).
At 1 year, “risedronate significantly increased BMD at the spine, compared to exercise and control, and serum biomarkers of bone turnover also significantly reduced in the risedronate group,” Laura Bilek, PT, PhD, said during an oral presentation of the research at the annual meeting of the American Society for Bone and Mineral Research.
However, the results also showed that, importantly, “in postmenopausal women, exercise appears to improve strength at the hip through changes in structure, not BMD,” stressed Dr. Bilek, of the College of Allied Health Professionals, University of Nebraska Medical Center.
Bone health is about more than just bone mineral density
“The key takeaway for clinicians is that bone health is about more than just density!” she noted in an email.
Current guidelines don’t recommend prescribing risedronate until a woman has overt osteoporosis, she said.
On the other hand, many studies have shown that, to be most effective, bone-loading exercises should be a lifelong habit and women should begin to do them at least during menopause and should not wait until bone loss occurs.
Other studies have shown that exercise changes bone structure (size or geometry), which improves bone strength. The current study supports both prior observations.
And exercise also improves muscle strength and decreases the risk of falls and fractures, Dr. Bilek noted.
Invited to comment, Pauline M. Camacho, MD, cochair of the task force for the American Association of Clinical Endocrinologists (AACE) guidelines for osteoporosis, noted that all three measures – pharmacotherapy, exercise, and calcium/vitamin D – are important in the successful management of osteoporosis.
This study showed that risedronate is superior to calcium/vitamin D supplementation as well as exercise for BMD and for bone turnover in these women with osteopenia, said Dr. Camacho, professor of medicine and director of the Osteoporosis and Metabolic Bone Disease Center, Loyola University Medical Center, Chicago.
“Most women with osteopenia do not receive pharmacologic therapy,” she noted, and receive it only “if there is a history of fractures or they have other features that change that diagnosis to osteoporosis.
“There is no downside to exercise, and this needs to be advised to all patients,” she said. “The other aspect of exercise that was not assessed in this study is its effect on balance. Patients who exercise will have improved balance, which should translate into fewer falls, and thus fewer fractures.”
How can women with osteopenia maintain bone health?
In their article, Dr. Waltman and colleagues say the Lifting Intervention for Training Muscle and Osteoporosis Rehabilitation (LIFTMOR) clinical trial is one of the first to address clinician concerns about the safety and effectiveness of exercise to improve bone health.
In that trial of 101 postmenopausal women with low bone mass, 8 months of 30-minute, twice-weekly, supervised high-intensity resistance and impact training was safe and BMD increased by 2.9% at the lumbar spine and 0.3% at the femoral neck.
“Our [HOPS] study,” Dr. Waltman and colleagues explained, “builds on the LIFTMOR clinical trial and adds further data to inform whether postmenopausal women with low bone mass can effectively maintain or even improve BMD with bone-loading exercises prior to prescriptions for medication.
“Our long-term goal is to contribute to the development of clinical practice guidelines for the prevention of fractures in postmenopausal women with low bone mass,” they said.
They randomized 276 postmenopausal women who were a mean age of 54 (range, 44-63); most were White (78%) or Hispanic (6%).
Women were excluded from the study if they had a diagnosis of osteoporosis (T-score < −2.5); had an increased risk of a major fracture or hip fracture; had been on bisphosphonates within the last 6 months; were currently on estrogen, tamoxifen, or aromatase inhibitors; had a serum vitamin D level < 10 mg/mL or > 100 mg/mL; had any conditions that prohibited prescriptions for calcium and vitamin D supplements, risedronate, or exercise; or weighed more than 300 pounds.
All women received 1,200 mg/day of calcium (from supplements or diet) and 1,000-3,000 IU/day of vitamin D supplements, based on their serum 25(OH) vitamin D levels.
The exercise program consisted of visiting a gym three times a week for 45 minutes of bone-loading exercise – jogging with a weighted vest – and resistance exercises, which were supervised by a trainer for the first 2 weeks.
Women in the risedronate group received a 150-mg tablet of risedronate every 4 weeks.
At baseline, 6 months, and 12 months, the women had DXA scans to determine BMD and hip structure, and had blood tests to determine levels of serum markers for bone formation (bone specific alkaline phosphatase [Alkphase B]) and bone resorption (N-terminal telopeptide [NTx]).
Compared with baseline, at 12 months, the women had the following changes in BMD at the following sites:
- Spine: +1.9%, +0.9%, and –0.4%, in the risedronate, exercise, and control groups.
- Total hip: +0.9%, +0.5%, and +0.5%, in the risedronate, exercise, and control groups.
- Femoral neck: +0.09%, –0.4%, and –0.5%, in the risedronate, exercise, and control groups.
These improvements in BMD were significantly greater in the risedronate group than in the exercise or control groups (P < .01 for both).
The decreases in serum levels of NtX and Alkphase B were also greater with risedronate than in the exercise or control groups (P < .01 for all).
The most frequent adverse effect with the calcium supplement was constipation (n = 4). Some women taking risedronate had gastrointestinal disturbances (n = 4), muscle or joint pain (n = 11), or chest pain and dizziness (n = 2). None of the women had adverse effects from vitamin D. A few women had muscle soreness from exercise that went away after the exercises were adapted. None of the women had a serious injury or fracture from exercise.
More women in the exercise group withdrew from the study (n = 20), with most citing lack of time as the reason; 13 women withdrew from the risedronate group, and 16 withdrew from the control group.
Of the 276 participants who completed the 12-month study, treatment adherence was 92% for calcium, 94% for vitamin D, 75% for risedronate, and 59% for exercise.
Exercise was associated with positive changes in intertrochanter hip structural analysis measures, which will be described in an upcoming study, Dr. Bilek said.
The study was funded by the National Institute of Nursing Research of the National Institutes of Health. The researchers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Postmenopausal women with low bone mass should obtain adequate calcium and vitamin D and participate in bone-loading exercises,” researchers noted in a recent study published in Osteoporosis International.
“Additional use of bisphosphonates will increase bone mineral density (BMD), especially at the spine,” wrote Nancy Waltman, PhD, College of Nursing, University of Nebraska Medical Center, Omaha, and colleagues.
The findings are partial results from the Heartland Osteoporosis Prevention Study (HOPS), which randomized women who had entered menopause within the previous 6 months and had osteopenia (low bone mass, T score –1.0 to –2.49) to receive one of three treatments for 12 months:
- Bone-loading and resistance exercise plus calcium and vitamin D supplements.
- Risedronate plus calcium and vitamin D supplements.
- Calcium and vitamin D supplements alone (control).
At 1 year, “risedronate significantly increased BMD at the spine, compared to exercise and control, and serum biomarkers of bone turnover also significantly reduced in the risedronate group,” Laura Bilek, PT, PhD, said during an oral presentation of the research at the annual meeting of the American Society for Bone and Mineral Research.
However, the results also showed that, importantly, “in postmenopausal women, exercise appears to improve strength at the hip through changes in structure, not BMD,” stressed Dr. Bilek, of the College of Allied Health Professionals, University of Nebraska Medical Center.
Bone health is about more than just bone mineral density
“The key takeaway for clinicians is that bone health is about more than just density!” she noted in an email.
Current guidelines don’t recommend prescribing risedronate until a woman has overt osteoporosis, she said.
On the other hand, many studies have shown that, to be most effective, bone-loading exercises should be a lifelong habit and women should begin to do them at least during menopause and should not wait until bone loss occurs.
Other studies have shown that exercise changes bone structure (size or geometry), which improves bone strength. The current study supports both prior observations.
And exercise also improves muscle strength and decreases the risk of falls and fractures, Dr. Bilek noted.
Invited to comment, Pauline M. Camacho, MD, cochair of the task force for the American Association of Clinical Endocrinologists (AACE) guidelines for osteoporosis, noted that all three measures – pharmacotherapy, exercise, and calcium/vitamin D – are important in the successful management of osteoporosis.
This study showed that risedronate is superior to calcium/vitamin D supplementation as well as exercise for BMD and for bone turnover in these women with osteopenia, said Dr. Camacho, professor of medicine and director of the Osteoporosis and Metabolic Bone Disease Center, Loyola University Medical Center, Chicago.
“Most women with osteopenia do not receive pharmacologic therapy,” she noted, and receive it only “if there is a history of fractures or they have other features that change that diagnosis to osteoporosis.
“There is no downside to exercise, and this needs to be advised to all patients,” she said. “The other aspect of exercise that was not assessed in this study is its effect on balance. Patients who exercise will have improved balance, which should translate into fewer falls, and thus fewer fractures.”
How can women with osteopenia maintain bone health?
In their article, Dr. Waltman and colleagues say the Lifting Intervention for Training Muscle and Osteoporosis Rehabilitation (LIFTMOR) clinical trial is one of the first to address clinician concerns about the safety and effectiveness of exercise to improve bone health.
In that trial of 101 postmenopausal women with low bone mass, 8 months of 30-minute, twice-weekly, supervised high-intensity resistance and impact training was safe and BMD increased by 2.9% at the lumbar spine and 0.3% at the femoral neck.
“Our [HOPS] study,” Dr. Waltman and colleagues explained, “builds on the LIFTMOR clinical trial and adds further data to inform whether postmenopausal women with low bone mass can effectively maintain or even improve BMD with bone-loading exercises prior to prescriptions for medication.
“Our long-term goal is to contribute to the development of clinical practice guidelines for the prevention of fractures in postmenopausal women with low bone mass,” they said.
They randomized 276 postmenopausal women who were a mean age of 54 (range, 44-63); most were White (78%) or Hispanic (6%).
Women were excluded from the study if they had a diagnosis of osteoporosis (T-score < −2.5); had an increased risk of a major fracture or hip fracture; had been on bisphosphonates within the last 6 months; were currently on estrogen, tamoxifen, or aromatase inhibitors; had a serum vitamin D level < 10 mg/mL or > 100 mg/mL; had any conditions that prohibited prescriptions for calcium and vitamin D supplements, risedronate, or exercise; or weighed more than 300 pounds.
All women received 1,200 mg/day of calcium (from supplements or diet) and 1,000-3,000 IU/day of vitamin D supplements, based on their serum 25(OH) vitamin D levels.
The exercise program consisted of visiting a gym three times a week for 45 minutes of bone-loading exercise – jogging with a weighted vest – and resistance exercises, which were supervised by a trainer for the first 2 weeks.
Women in the risedronate group received a 150-mg tablet of risedronate every 4 weeks.
At baseline, 6 months, and 12 months, the women had DXA scans to determine BMD and hip structure, and had blood tests to determine levels of serum markers for bone formation (bone specific alkaline phosphatase [Alkphase B]) and bone resorption (N-terminal telopeptide [NTx]).
Compared with baseline, at 12 months, the women had the following changes in BMD at the following sites:
- Spine: +1.9%, +0.9%, and –0.4%, in the risedronate, exercise, and control groups.
- Total hip: +0.9%, +0.5%, and +0.5%, in the risedronate, exercise, and control groups.
- Femoral neck: +0.09%, –0.4%, and –0.5%, in the risedronate, exercise, and control groups.
These improvements in BMD were significantly greater in the risedronate group than in the exercise or control groups (P < .01 for both).
The decreases in serum levels of NtX and Alkphase B were also greater with risedronate than in the exercise or control groups (P < .01 for all).
The most frequent adverse effect with the calcium supplement was constipation (n = 4). Some women taking risedronate had gastrointestinal disturbances (n = 4), muscle or joint pain (n = 11), or chest pain and dizziness (n = 2). None of the women had adverse effects from vitamin D. A few women had muscle soreness from exercise that went away after the exercises were adapted. None of the women had a serious injury or fracture from exercise.
More women in the exercise group withdrew from the study (n = 20), with most citing lack of time as the reason; 13 women withdrew from the risedronate group, and 16 withdrew from the control group.
Of the 276 participants who completed the 12-month study, treatment adherence was 92% for calcium, 94% for vitamin D, 75% for risedronate, and 59% for exercise.
Exercise was associated with positive changes in intertrochanter hip structural analysis measures, which will be described in an upcoming study, Dr. Bilek said.
The study was funded by the National Institute of Nursing Research of the National Institutes of Health. The researchers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2021











