User login
USPSTF Recommends Exercise To Prevent Falls in Older Adults
Exercise interventions are recommended to help prevent falls and fall-related morbidity in community-dwelling adults aged 65 years and older who are at increased risk of falls, according to a new recommendation statement from the U.S. Preventive Services Task Force (USPSTF) (JAMA. 2024 Jun 4. doi: 10.1001/jama.2024.8481).
Falls remain the leading cause of injury-related morbidity and mortality among older adults in the United States, with approximately 27% of community-dwelling individuals aged 65 years and older reporting at least one fall in the past year, wrote lead author Wanda K. Nicholson, MD, of George Washington University, Washington, and colleagues.
The task force concluded with moderate certainty that exercise interventions yielded a moderate benefit in fall reduction among older adults at risk (grade B recommendation).
The decision to offer multifactorial fall prevention interventions to older adults at risk for falls should be individualized based on assessment of potential risks and benefits of these interventions, including circumstances of prior falls, presence of comorbid medical conditions, and the patient’s values and preferences (grade C recommendation), the authors wrote.
The exercise intervention could include individual or group activity, although most of the studies in the systematic review involved group exercise, the authors noted.
The recommendation was based on data from a systematic evidence review published in JAMA (2024 Jun 4. doi: 10.1001/jama.2024.4166). The task force reviewed data from 83 randomized trials published between January 1, 2016, and May 8, 2023, deemed fair to good quality that examined six types of fall prevention interventions in a total of 48,839 individuals. Of these, 28 studies involved multifactorial interventions and 27 involved exercise interventions.
Overall, multifactorial interventions and exercise interventions were associated with a significant reduction in falls (incidence rate ratio 0.84 and 0.85, respectively).
Exercise interventions were significantly associated with reduced individual risk of one or more falls and injurious falls, but not with reduced individual risk of injurious falls. However, multifactorial interventions were not significantly associated with reductions in risk of one or more falls, injurious falls, fall-related fractures, individual risk of injurious falls, or individual risk of fall-related fractures.
Although teasing out the specific exercise components that are most effective for fall prevention is challenging, the most commonly studied components associated with reduced risk of falls included gait training, balance training, and functional training, followed by strength and resistance training, the task force noted.
Duration of exercise interventions in the reviewed studies ranged from 2 to 30 months and the most common frequency of sessions was 2 to 3 per week.
Based on these findings, the task force found that exercise had the most consistent benefits for reduced risk across several fall-related outcomes. Although individuals in the studies of multifactorial interventions were at increased risk for falls, the multistep process of interventions to address an individual’s multiple risk factors limited their effectiveness, in part because of logistical challenges and inconsistent adherence, the authors wrote.
The results of the review were limited by several factors, including the focus on studies with a primary or secondary aim of fall prevention, the fact that the recommendation does not apply to many subgroups of older adults, and the lack of data on health outcomes unrelated to falls that were associated with the interventions, the authors noted.
The new recommendation is consistent with and replaces the 2018 USPSTF recommendation on interventions for fall prevention in community-dwelling older adults, but without the recommendation against vitamin D supplementation as a fall prevention intervention. The new recommendation does not address vitamin D use; evidence will be examined in a separate recommendation, the task force wrote.
How to Get Older Adults Moving
“The biggest obstacle to exercise is patient inertia and choice to engage in other sedentary activities,” David B. Reuben, MD, and David A. Ganz, MD, both of the University of California, Los Angeles, wrote in an accompanying editorial (JAMA. 2024 Jun 4. doi: 10.1001/jama.2024.9063).
“Given the demonstrated benefits of exercise for cardiovascular disease, cognitive function, and favorable associations with all-cause, cardiovascular, and cancer mortality, specific fall prevention exercise recommendations need to be considered in the context of universal exercise recommendations, including aerobic and muscle strengthening exercise,” the authors wrote. However, maintaining regular exercise is a challenge for many older adults, and more research is needed on factors that drive exercise initiation and adherence in this population, they said.
Multifactorial fall assessments in particular take time, and more fall prevention programs are needed that include multifactorial assessments and interventions, the editorialists said. “Even if primary care clinicians faithfully implement the USPSTF recommendations, a significant reduction in falls and their resulting injuries is still far off,” in part, because of the need for more programs and policies, and the need to improve access to exercise programs and provide insurance coverage for them, they noted.
“Above all, older persons need to be active participants in exercise and reduction of risk factors for falls,” the editorialists concluded.
The research for the recommendation was funded by the Agency for Healthcare Research and Quality (AHRQ). The authors had no financial conflicts to disclose. Dr. Ganz disclosed serving as an author of the 2022 World Guidelines for Falls Prevention and Management for Older Adults.
Exercise interventions are recommended to help prevent falls and fall-related morbidity in community-dwelling adults aged 65 years and older who are at increased risk of falls, according to a new recommendation statement from the U.S. Preventive Services Task Force (USPSTF) (JAMA. 2024 Jun 4. doi: 10.1001/jama.2024.8481).
Falls remain the leading cause of injury-related morbidity and mortality among older adults in the United States, with approximately 27% of community-dwelling individuals aged 65 years and older reporting at least one fall in the past year, wrote lead author Wanda K. Nicholson, MD, of George Washington University, Washington, and colleagues.
The task force concluded with moderate certainty that exercise interventions yielded a moderate benefit in fall reduction among older adults at risk (grade B recommendation).
The decision to offer multifactorial fall prevention interventions to older adults at risk for falls should be individualized based on assessment of potential risks and benefits of these interventions, including circumstances of prior falls, presence of comorbid medical conditions, and the patient’s values and preferences (grade C recommendation), the authors wrote.
The exercise intervention could include individual or group activity, although most of the studies in the systematic review involved group exercise, the authors noted.
The recommendation was based on data from a systematic evidence review published in JAMA (2024 Jun 4. doi: 10.1001/jama.2024.4166). The task force reviewed data from 83 randomized trials published between January 1, 2016, and May 8, 2023, deemed fair to good quality that examined six types of fall prevention interventions in a total of 48,839 individuals. Of these, 28 studies involved multifactorial interventions and 27 involved exercise interventions.
Overall, multifactorial interventions and exercise interventions were associated with a significant reduction in falls (incidence rate ratio 0.84 and 0.85, respectively).
Exercise interventions were significantly associated with reduced individual risk of one or more falls and injurious falls, but not with reduced individual risk of injurious falls. However, multifactorial interventions were not significantly associated with reductions in risk of one or more falls, injurious falls, fall-related fractures, individual risk of injurious falls, or individual risk of fall-related fractures.
Although teasing out the specific exercise components that are most effective for fall prevention is challenging, the most commonly studied components associated with reduced risk of falls included gait training, balance training, and functional training, followed by strength and resistance training, the task force noted.
Duration of exercise interventions in the reviewed studies ranged from 2 to 30 months and the most common frequency of sessions was 2 to 3 per week.
Based on these findings, the task force found that exercise had the most consistent benefits for reduced risk across several fall-related outcomes. Although individuals in the studies of multifactorial interventions were at increased risk for falls, the multistep process of interventions to address an individual’s multiple risk factors limited their effectiveness, in part because of logistical challenges and inconsistent adherence, the authors wrote.
The results of the review were limited by several factors, including the focus on studies with a primary or secondary aim of fall prevention, the fact that the recommendation does not apply to many subgroups of older adults, and the lack of data on health outcomes unrelated to falls that were associated with the interventions, the authors noted.
The new recommendation is consistent with and replaces the 2018 USPSTF recommendation on interventions for fall prevention in community-dwelling older adults, but without the recommendation against vitamin D supplementation as a fall prevention intervention. The new recommendation does not address vitamin D use; evidence will be examined in a separate recommendation, the task force wrote.
How to Get Older Adults Moving
“The biggest obstacle to exercise is patient inertia and choice to engage in other sedentary activities,” David B. Reuben, MD, and David A. Ganz, MD, both of the University of California, Los Angeles, wrote in an accompanying editorial (JAMA. 2024 Jun 4. doi: 10.1001/jama.2024.9063).
“Given the demonstrated benefits of exercise for cardiovascular disease, cognitive function, and favorable associations with all-cause, cardiovascular, and cancer mortality, specific fall prevention exercise recommendations need to be considered in the context of universal exercise recommendations, including aerobic and muscle strengthening exercise,” the authors wrote. However, maintaining regular exercise is a challenge for many older adults, and more research is needed on factors that drive exercise initiation and adherence in this population, they said.
Multifactorial fall assessments in particular take time, and more fall prevention programs are needed that include multifactorial assessments and interventions, the editorialists said. “Even if primary care clinicians faithfully implement the USPSTF recommendations, a significant reduction in falls and their resulting injuries is still far off,” in part, because of the need for more programs and policies, and the need to improve access to exercise programs and provide insurance coverage for them, they noted.
“Above all, older persons need to be active participants in exercise and reduction of risk factors for falls,” the editorialists concluded.
The research for the recommendation was funded by the Agency for Healthcare Research and Quality (AHRQ). The authors had no financial conflicts to disclose. Dr. Ganz disclosed serving as an author of the 2022 World Guidelines for Falls Prevention and Management for Older Adults.
Exercise interventions are recommended to help prevent falls and fall-related morbidity in community-dwelling adults aged 65 years and older who are at increased risk of falls, according to a new recommendation statement from the U.S. Preventive Services Task Force (USPSTF) (JAMA. 2024 Jun 4. doi: 10.1001/jama.2024.8481).
Falls remain the leading cause of injury-related morbidity and mortality among older adults in the United States, with approximately 27% of community-dwelling individuals aged 65 years and older reporting at least one fall in the past year, wrote lead author Wanda K. Nicholson, MD, of George Washington University, Washington, and colleagues.
The task force concluded with moderate certainty that exercise interventions yielded a moderate benefit in fall reduction among older adults at risk (grade B recommendation).
The decision to offer multifactorial fall prevention interventions to older adults at risk for falls should be individualized based on assessment of potential risks and benefits of these interventions, including circumstances of prior falls, presence of comorbid medical conditions, and the patient’s values and preferences (grade C recommendation), the authors wrote.
The exercise intervention could include individual or group activity, although most of the studies in the systematic review involved group exercise, the authors noted.
The recommendation was based on data from a systematic evidence review published in JAMA (2024 Jun 4. doi: 10.1001/jama.2024.4166). The task force reviewed data from 83 randomized trials published between January 1, 2016, and May 8, 2023, deemed fair to good quality that examined six types of fall prevention interventions in a total of 48,839 individuals. Of these, 28 studies involved multifactorial interventions and 27 involved exercise interventions.
Overall, multifactorial interventions and exercise interventions were associated with a significant reduction in falls (incidence rate ratio 0.84 and 0.85, respectively).
Exercise interventions were significantly associated with reduced individual risk of one or more falls and injurious falls, but not with reduced individual risk of injurious falls. However, multifactorial interventions were not significantly associated with reductions in risk of one or more falls, injurious falls, fall-related fractures, individual risk of injurious falls, or individual risk of fall-related fractures.
Although teasing out the specific exercise components that are most effective for fall prevention is challenging, the most commonly studied components associated with reduced risk of falls included gait training, balance training, and functional training, followed by strength and resistance training, the task force noted.
Duration of exercise interventions in the reviewed studies ranged from 2 to 30 months and the most common frequency of sessions was 2 to 3 per week.
Based on these findings, the task force found that exercise had the most consistent benefits for reduced risk across several fall-related outcomes. Although individuals in the studies of multifactorial interventions were at increased risk for falls, the multistep process of interventions to address an individual’s multiple risk factors limited their effectiveness, in part because of logistical challenges and inconsistent adherence, the authors wrote.
The results of the review were limited by several factors, including the focus on studies with a primary or secondary aim of fall prevention, the fact that the recommendation does not apply to many subgroups of older adults, and the lack of data on health outcomes unrelated to falls that were associated with the interventions, the authors noted.
The new recommendation is consistent with and replaces the 2018 USPSTF recommendation on interventions for fall prevention in community-dwelling older adults, but without the recommendation against vitamin D supplementation as a fall prevention intervention. The new recommendation does not address vitamin D use; evidence will be examined in a separate recommendation, the task force wrote.
How to Get Older Adults Moving
“The biggest obstacle to exercise is patient inertia and choice to engage in other sedentary activities,” David B. Reuben, MD, and David A. Ganz, MD, both of the University of California, Los Angeles, wrote in an accompanying editorial (JAMA. 2024 Jun 4. doi: 10.1001/jama.2024.9063).
“Given the demonstrated benefits of exercise for cardiovascular disease, cognitive function, and favorable associations with all-cause, cardiovascular, and cancer mortality, specific fall prevention exercise recommendations need to be considered in the context of universal exercise recommendations, including aerobic and muscle strengthening exercise,” the authors wrote. However, maintaining regular exercise is a challenge for many older adults, and more research is needed on factors that drive exercise initiation and adherence in this population, they said.
Multifactorial fall assessments in particular take time, and more fall prevention programs are needed that include multifactorial assessments and interventions, the editorialists said. “Even if primary care clinicians faithfully implement the USPSTF recommendations, a significant reduction in falls and their resulting injuries is still far off,” in part, because of the need for more programs and policies, and the need to improve access to exercise programs and provide insurance coverage for them, they noted.
“Above all, older persons need to be active participants in exercise and reduction of risk factors for falls,” the editorialists concluded.
The research for the recommendation was funded by the Agency for Healthcare Research and Quality (AHRQ). The authors had no financial conflicts to disclose. Dr. Ganz disclosed serving as an author of the 2022 World Guidelines for Falls Prevention and Management for Older Adults.
FROM JAMA
Are Secondary Osteoporosis Causes Under-Investigated?
NEW ORLEANS — Postmenopausal women with osteoporosis may not be receiving all the recommended tests to rule out secondary causes of bone loss prior to treatment initiation, new research found.
In a single-center chart review of 150 postmenopausal women who had been diagnosed and treated for osteoporosis, most had received a complete blood cell count, basic metabolic panel, thyroid screening, and vitamin D testing. However, one in four had not been tested for a parathyroid hormone (PTH) level, and in nearly two thirds, a 24-hour urine calcium collection had not been ordered.
Overall, less than a third had received the complete workup for secondary osteoporosis causes as recommended by the American Association of Clinical Endocrinologists (AACE) and the Endocrine Society.
“An appropriate evaluation for secondary causes of osteoporosis is essential because it impacts different treatment options and modalities. We discovered low rates of complete testing for secondary causes of osteoporosis in our patient population prior to treatment initiation,” said Kajol Manglani, MD, an internal medicine resident at Georgetown University/MedStar Washington Hospital Center, Washington, DC, and colleagues, in a poster at the American Association of Clinical Endocrinology (AACE) annual meeting held on May 9-12, 2024.
First author Sheetal Bulchandani, MD, said in an interview, “It depends a lot on clinical judgment, but there are certain things that everybody with osteoporosis should be evaluated for. We looked for the things that all the guidelines recommend.”
Studies have suggested that up to 30% of postmenopausal women with osteoporosis have secondary causes, noted Dr. Bulchandani, who conducted the study as a postdoctoral fellow with colleagues at Georgetown University/MedStar Washington Hospital and is now in private endocrine practice in Petersburg, Virginia.
“It’s important not to assume that every woman who walks in with osteoporosis has postmenopausal osteoporosis. I think it would be appropriate to at least discuss with the patients what would warrant certain kinds of clinical workup. … If you don’t figure out if there is an underlying cause, you may end up using an unnecessary medication,” Dr. Bulchandani said.
Are You Missing Something Treatable?
For example, she said, if the patient has underlying hyperparathyroidism and is treated with osteoporosis medications, “you might not see the desired or expected outcome in their bone density.”
Asked to comment, Rachel Pessah-Pollack, MD, clinical associate professor at the Holman Division of Endocrinology, Diabetes, and Metabolism at New York University School of Medicine, New York City, told this news organization, “Certainly, if you have patients who have osteoporosis, it’s important to take a good history and consider secondary causes of bone loss because you may find a treatable etiology that actually can improve their bone density without even starting on a medication.”
Dr. Pessah-Pollack, who was an author of the 2020 AACE/American College of Endocrinology 2020 Clinical Practice Guidelines for the Diagnosis and Treatment of Osteoporosis, said a 24-hour urine calcium collection, not a spot calcium check, is “super important because you’re looking to see if there’s any evidence of hypercalciuria or malabsorption that may be associated with higher rates of bone loss. … These may be a little more cumbersome and harder to get patients to do and more logistics to arrange. But clearly, if you pick up hypercalciuria, that is a potentially treatable etiology and can improve bone density as well.”
Another example, Dr. Pessah-Pollack said, is “if they have a low serum calcium level and high PTH, that would be a real reason to look for celiac disease. By not getting that PTH level, you may be missing that potential diagnosis. There is a wide range of additional causes of osteoporosis ranging from common conditions such as hyperthyroidism to rare conditions such as Cushing disease.”
Differences in Ordering Found Across Specialties
The 150 postmenopausal women were all receiving treatment with either alendronate, denosumab, or zoledronic acid. Their average age was 64.7 years, and 63% were seeing an endocrinologist.
Complete workups as per AACE and Endocrine Society guidelines had been performed in just 28% of those who saw an endocrinologist and 12.5% of patients seen by a rheumatologist, in contrast to 84% of those who saw the head of the hospital’s fracture prevention program.
Overall, across all specialties, just 28.67% had the complete recommended workup for secondary osteoporosis causes.
The most missed test was a 24-hour urine calcium collection, ordered for just 38% of the patients, while PTH was ordered for 73% and phosphorus for 80%. The rest were more commonly ordered: Thyroid-stimulating hormone level for 92.7%, complete blood cell count for 91.3%, basic metabolic panel for 100%, and vitamin D level for 96%.
The high rate of vitamin D testing is noteworthy, Dr. Pessah-Pollack said. “The fact that 96% of women are having vitamin D levels checked as part of an osteoporosis evaluation means that everybody’s aware about vitamin D deficiency, and people want to know what their vitamin D levels are. … That’s good because we want to identify vitamin D deficiency in our osteoporosis patients.”
But the low rate of complete secondary screening even by endocrinologists is concerning. “I look at this study as an opportunity for education that we can reinforce the importance of a secondary evaluation for our osteoporosis patients and really tailor which additional tests should be ordered for the individual patient,” Dr. Pessah-Pollack said.
In the poster, Dr. Bulchandani and colleagues wrote, “Further intervention will be aimed to ensure physicians undertake adequate evaluation before considering further treatment directions.” Possibilities that have been discussed include electronic health record alerts and educational materials for primary care providers, she told this news organization.
Dr. Manglani and Dr. Bulchandani had no disclosures. Dr. Pessah-Pollack is an advisor for Boehringer Ingelheim and Eli Lilly.
A version of this article appeared on Medscape.com.
NEW ORLEANS — Postmenopausal women with osteoporosis may not be receiving all the recommended tests to rule out secondary causes of bone loss prior to treatment initiation, new research found.
In a single-center chart review of 150 postmenopausal women who had been diagnosed and treated for osteoporosis, most had received a complete blood cell count, basic metabolic panel, thyroid screening, and vitamin D testing. However, one in four had not been tested for a parathyroid hormone (PTH) level, and in nearly two thirds, a 24-hour urine calcium collection had not been ordered.
Overall, less than a third had received the complete workup for secondary osteoporosis causes as recommended by the American Association of Clinical Endocrinologists (AACE) and the Endocrine Society.
“An appropriate evaluation for secondary causes of osteoporosis is essential because it impacts different treatment options and modalities. We discovered low rates of complete testing for secondary causes of osteoporosis in our patient population prior to treatment initiation,” said Kajol Manglani, MD, an internal medicine resident at Georgetown University/MedStar Washington Hospital Center, Washington, DC, and colleagues, in a poster at the American Association of Clinical Endocrinology (AACE) annual meeting held on May 9-12, 2024.
First author Sheetal Bulchandani, MD, said in an interview, “It depends a lot on clinical judgment, but there are certain things that everybody with osteoporosis should be evaluated for. We looked for the things that all the guidelines recommend.”
Studies have suggested that up to 30% of postmenopausal women with osteoporosis have secondary causes, noted Dr. Bulchandani, who conducted the study as a postdoctoral fellow with colleagues at Georgetown University/MedStar Washington Hospital and is now in private endocrine practice in Petersburg, Virginia.
“It’s important not to assume that every woman who walks in with osteoporosis has postmenopausal osteoporosis. I think it would be appropriate to at least discuss with the patients what would warrant certain kinds of clinical workup. … If you don’t figure out if there is an underlying cause, you may end up using an unnecessary medication,” Dr. Bulchandani said.
Are You Missing Something Treatable?
For example, she said, if the patient has underlying hyperparathyroidism and is treated with osteoporosis medications, “you might not see the desired or expected outcome in their bone density.”
Asked to comment, Rachel Pessah-Pollack, MD, clinical associate professor at the Holman Division of Endocrinology, Diabetes, and Metabolism at New York University School of Medicine, New York City, told this news organization, “Certainly, if you have patients who have osteoporosis, it’s important to take a good history and consider secondary causes of bone loss because you may find a treatable etiology that actually can improve their bone density without even starting on a medication.”
Dr. Pessah-Pollack, who was an author of the 2020 AACE/American College of Endocrinology 2020 Clinical Practice Guidelines for the Diagnosis and Treatment of Osteoporosis, said a 24-hour urine calcium collection, not a spot calcium check, is “super important because you’re looking to see if there’s any evidence of hypercalciuria or malabsorption that may be associated with higher rates of bone loss. … These may be a little more cumbersome and harder to get patients to do and more logistics to arrange. But clearly, if you pick up hypercalciuria, that is a potentially treatable etiology and can improve bone density as well.”
Another example, Dr. Pessah-Pollack said, is “if they have a low serum calcium level and high PTH, that would be a real reason to look for celiac disease. By not getting that PTH level, you may be missing that potential diagnosis. There is a wide range of additional causes of osteoporosis ranging from common conditions such as hyperthyroidism to rare conditions such as Cushing disease.”
Differences in Ordering Found Across Specialties
The 150 postmenopausal women were all receiving treatment with either alendronate, denosumab, or zoledronic acid. Their average age was 64.7 years, and 63% were seeing an endocrinologist.
Complete workups as per AACE and Endocrine Society guidelines had been performed in just 28% of those who saw an endocrinologist and 12.5% of patients seen by a rheumatologist, in contrast to 84% of those who saw the head of the hospital’s fracture prevention program.
Overall, across all specialties, just 28.67% had the complete recommended workup for secondary osteoporosis causes.
The most missed test was a 24-hour urine calcium collection, ordered for just 38% of the patients, while PTH was ordered for 73% and phosphorus for 80%. The rest were more commonly ordered: Thyroid-stimulating hormone level for 92.7%, complete blood cell count for 91.3%, basic metabolic panel for 100%, and vitamin D level for 96%.
The high rate of vitamin D testing is noteworthy, Dr. Pessah-Pollack said. “The fact that 96% of women are having vitamin D levels checked as part of an osteoporosis evaluation means that everybody’s aware about vitamin D deficiency, and people want to know what their vitamin D levels are. … That’s good because we want to identify vitamin D deficiency in our osteoporosis patients.”
But the low rate of complete secondary screening even by endocrinologists is concerning. “I look at this study as an opportunity for education that we can reinforce the importance of a secondary evaluation for our osteoporosis patients and really tailor which additional tests should be ordered for the individual patient,” Dr. Pessah-Pollack said.
In the poster, Dr. Bulchandani and colleagues wrote, “Further intervention will be aimed to ensure physicians undertake adequate evaluation before considering further treatment directions.” Possibilities that have been discussed include electronic health record alerts and educational materials for primary care providers, she told this news organization.
Dr. Manglani and Dr. Bulchandani had no disclosures. Dr. Pessah-Pollack is an advisor for Boehringer Ingelheim and Eli Lilly.
A version of this article appeared on Medscape.com.
NEW ORLEANS — Postmenopausal women with osteoporosis may not be receiving all the recommended tests to rule out secondary causes of bone loss prior to treatment initiation, new research found.
In a single-center chart review of 150 postmenopausal women who had been diagnosed and treated for osteoporosis, most had received a complete blood cell count, basic metabolic panel, thyroid screening, and vitamin D testing. However, one in four had not been tested for a parathyroid hormone (PTH) level, and in nearly two thirds, a 24-hour urine calcium collection had not been ordered.
Overall, less than a third had received the complete workup for secondary osteoporosis causes as recommended by the American Association of Clinical Endocrinologists (AACE) and the Endocrine Society.
“An appropriate evaluation for secondary causes of osteoporosis is essential because it impacts different treatment options and modalities. We discovered low rates of complete testing for secondary causes of osteoporosis in our patient population prior to treatment initiation,” said Kajol Manglani, MD, an internal medicine resident at Georgetown University/MedStar Washington Hospital Center, Washington, DC, and colleagues, in a poster at the American Association of Clinical Endocrinology (AACE) annual meeting held on May 9-12, 2024.
First author Sheetal Bulchandani, MD, said in an interview, “It depends a lot on clinical judgment, but there are certain things that everybody with osteoporosis should be evaluated for. We looked for the things that all the guidelines recommend.”
Studies have suggested that up to 30% of postmenopausal women with osteoporosis have secondary causes, noted Dr. Bulchandani, who conducted the study as a postdoctoral fellow with colleagues at Georgetown University/MedStar Washington Hospital and is now in private endocrine practice in Petersburg, Virginia.
“It’s important not to assume that every woman who walks in with osteoporosis has postmenopausal osteoporosis. I think it would be appropriate to at least discuss with the patients what would warrant certain kinds of clinical workup. … If you don’t figure out if there is an underlying cause, you may end up using an unnecessary medication,” Dr. Bulchandani said.
Are You Missing Something Treatable?
For example, she said, if the patient has underlying hyperparathyroidism and is treated with osteoporosis medications, “you might not see the desired or expected outcome in their bone density.”
Asked to comment, Rachel Pessah-Pollack, MD, clinical associate professor at the Holman Division of Endocrinology, Diabetes, and Metabolism at New York University School of Medicine, New York City, told this news organization, “Certainly, if you have patients who have osteoporosis, it’s important to take a good history and consider secondary causes of bone loss because you may find a treatable etiology that actually can improve their bone density without even starting on a medication.”
Dr. Pessah-Pollack, who was an author of the 2020 AACE/American College of Endocrinology 2020 Clinical Practice Guidelines for the Diagnosis and Treatment of Osteoporosis, said a 24-hour urine calcium collection, not a spot calcium check, is “super important because you’re looking to see if there’s any evidence of hypercalciuria or malabsorption that may be associated with higher rates of bone loss. … These may be a little more cumbersome and harder to get patients to do and more logistics to arrange. But clearly, if you pick up hypercalciuria, that is a potentially treatable etiology and can improve bone density as well.”
Another example, Dr. Pessah-Pollack said, is “if they have a low serum calcium level and high PTH, that would be a real reason to look for celiac disease. By not getting that PTH level, you may be missing that potential diagnosis. There is a wide range of additional causes of osteoporosis ranging from common conditions such as hyperthyroidism to rare conditions such as Cushing disease.”
Differences in Ordering Found Across Specialties
The 150 postmenopausal women were all receiving treatment with either alendronate, denosumab, or zoledronic acid. Their average age was 64.7 years, and 63% were seeing an endocrinologist.
Complete workups as per AACE and Endocrine Society guidelines had been performed in just 28% of those who saw an endocrinologist and 12.5% of patients seen by a rheumatologist, in contrast to 84% of those who saw the head of the hospital’s fracture prevention program.
Overall, across all specialties, just 28.67% had the complete recommended workup for secondary osteoporosis causes.
The most missed test was a 24-hour urine calcium collection, ordered for just 38% of the patients, while PTH was ordered for 73% and phosphorus for 80%. The rest were more commonly ordered: Thyroid-stimulating hormone level for 92.7%, complete blood cell count for 91.3%, basic metabolic panel for 100%, and vitamin D level for 96%.
The high rate of vitamin D testing is noteworthy, Dr. Pessah-Pollack said. “The fact that 96% of women are having vitamin D levels checked as part of an osteoporosis evaluation means that everybody’s aware about vitamin D deficiency, and people want to know what their vitamin D levels are. … That’s good because we want to identify vitamin D deficiency in our osteoporosis patients.”
But the low rate of complete secondary screening even by endocrinologists is concerning. “I look at this study as an opportunity for education that we can reinforce the importance of a secondary evaluation for our osteoporosis patients and really tailor which additional tests should be ordered for the individual patient,” Dr. Pessah-Pollack said.
In the poster, Dr. Bulchandani and colleagues wrote, “Further intervention will be aimed to ensure physicians undertake adequate evaluation before considering further treatment directions.” Possibilities that have been discussed include electronic health record alerts and educational materials for primary care providers, she told this news organization.
Dr. Manglani and Dr. Bulchandani had no disclosures. Dr. Pessah-Pollack is an advisor for Boehringer Ingelheim and Eli Lilly.
A version of this article appeared on Medscape.com.
Anti-Osteoporosis Drugs Found Just as Effective in Seniors
TOPLINE:
Anti-osteoporosis medications reduce fracture risk similarly, regardless of whether patients are younger or older than 70 years.
METHODOLOGY:
- Investigators conducted the study as part of a to assess bone mineral density as a surrogate marker for fracture risk.
- Analyses used individual patient data from 23 randomized placebo-controlled trials of anti-osteoporosis medications (11 of bisphosphonates, four of selective estrogen receptor modulators, three of anabolic medications, two of hormone replacement therapy, and one each of odanacatib, denosumab, and romosozumab).
- Overall, 43% of the included 123,164 patients were aged 70 years or older.
- The main outcomes were fractures and bone mineral density.
TAKEAWAY:
- There was a similar benefit regardless of age when it came to the reduction in risks for hip fracture (odds ratio, 0.65 vs 0.72; P for interaction = .50) and any fracture (odds ratio, 0.72 vs 0.70; P for interaction = .20).
- Findings were comparable in analyses restricted to bisphosphonate trials, except that the reduction in hip fracture risk was greater among the younger group (hazard ratio, 0.44 vs 0.79; P for interaction = .02).
- The benefit of anti-osteoporosis medication in increasing hip and spine bone mineral density at 24 months was significantly greater among the older patients.
IN PRACTICE:
Taken together, the study results “strongly support treatment in those over age 70,” the authors wrote. “These are important findings with potential impact in patient treatment since it goes against a common misconception that medications are less effective in older people,” they added.
SOURCE:
The study was led by Marian Schini, MD, PhD, FHEA, University of Sheffield, England, and was published online in the Journal of Bone and Mineral Research.
LIMITATIONS:
Limitations included a preponderance of female patients (99%), possible residual confounding, a lack of analysis of adverse effects, and potentially different findings using alternate age cutoffs.
DISCLOSURES:
The study was funded by the American Society for Bone Mineral Research. Some authors disclosed affiliations with companies that manufacture anti-osteoporosis drugs.
A version of this article appeared on Medscape.com.
TOPLINE:
Anti-osteoporosis medications reduce fracture risk similarly, regardless of whether patients are younger or older than 70 years.
METHODOLOGY:
- Investigators conducted the study as part of a to assess bone mineral density as a surrogate marker for fracture risk.
- Analyses used individual patient data from 23 randomized placebo-controlled trials of anti-osteoporosis medications (11 of bisphosphonates, four of selective estrogen receptor modulators, three of anabolic medications, two of hormone replacement therapy, and one each of odanacatib, denosumab, and romosozumab).
- Overall, 43% of the included 123,164 patients were aged 70 years or older.
- The main outcomes were fractures and bone mineral density.
TAKEAWAY:
- There was a similar benefit regardless of age when it came to the reduction in risks for hip fracture (odds ratio, 0.65 vs 0.72; P for interaction = .50) and any fracture (odds ratio, 0.72 vs 0.70; P for interaction = .20).
- Findings were comparable in analyses restricted to bisphosphonate trials, except that the reduction in hip fracture risk was greater among the younger group (hazard ratio, 0.44 vs 0.79; P for interaction = .02).
- The benefit of anti-osteoporosis medication in increasing hip and spine bone mineral density at 24 months was significantly greater among the older patients.
IN PRACTICE:
Taken together, the study results “strongly support treatment in those over age 70,” the authors wrote. “These are important findings with potential impact in patient treatment since it goes against a common misconception that medications are less effective in older people,” they added.
SOURCE:
The study was led by Marian Schini, MD, PhD, FHEA, University of Sheffield, England, and was published online in the Journal of Bone and Mineral Research.
LIMITATIONS:
Limitations included a preponderance of female patients (99%), possible residual confounding, a lack of analysis of adverse effects, and potentially different findings using alternate age cutoffs.
DISCLOSURES:
The study was funded by the American Society for Bone Mineral Research. Some authors disclosed affiliations with companies that manufacture anti-osteoporosis drugs.
A version of this article appeared on Medscape.com.
TOPLINE:
Anti-osteoporosis medications reduce fracture risk similarly, regardless of whether patients are younger or older than 70 years.
METHODOLOGY:
- Investigators conducted the study as part of a to assess bone mineral density as a surrogate marker for fracture risk.
- Analyses used individual patient data from 23 randomized placebo-controlled trials of anti-osteoporosis medications (11 of bisphosphonates, four of selective estrogen receptor modulators, three of anabolic medications, two of hormone replacement therapy, and one each of odanacatib, denosumab, and romosozumab).
- Overall, 43% of the included 123,164 patients were aged 70 years or older.
- The main outcomes were fractures and bone mineral density.
TAKEAWAY:
- There was a similar benefit regardless of age when it came to the reduction in risks for hip fracture (odds ratio, 0.65 vs 0.72; P for interaction = .50) and any fracture (odds ratio, 0.72 vs 0.70; P for interaction = .20).
- Findings were comparable in analyses restricted to bisphosphonate trials, except that the reduction in hip fracture risk was greater among the younger group (hazard ratio, 0.44 vs 0.79; P for interaction = .02).
- The benefit of anti-osteoporosis medication in increasing hip and spine bone mineral density at 24 months was significantly greater among the older patients.
IN PRACTICE:
Taken together, the study results “strongly support treatment in those over age 70,” the authors wrote. “These are important findings with potential impact in patient treatment since it goes against a common misconception that medications are less effective in older people,” they added.
SOURCE:
The study was led by Marian Schini, MD, PhD, FHEA, University of Sheffield, England, and was published online in the Journal of Bone and Mineral Research.
LIMITATIONS:
Limitations included a preponderance of female patients (99%), possible residual confounding, a lack of analysis of adverse effects, and potentially different findings using alternate age cutoffs.
DISCLOSURES:
The study was funded by the American Society for Bone Mineral Research. Some authors disclosed affiliations with companies that manufacture anti-osteoporosis drugs.
A version of this article appeared on Medscape.com.
Is It Possible to Reverse Osteoporosis?
Fractures, particularly hip and spine fractures, are a major cause of mortality and morbidity among older individuals. The term “osteoporosis” indicates increased porosity of bones resulting in low bone density; increased bone fragility; and an increased risk for fracture, often with minimal trauma.
During the adolescent years, bone accrues at a rapid rate, and optimal bone accrual during this time is essential to attain optimal peak bone mass, typically achieved in the third decade of life. Bone mass then stays stable until the 40s-50s, after which it starts to decline. One’s peak bone mass sets the stage for both immediate and future bone health. Individuals with lower peak bone mass tend to have less optimal bone health throughout their lives, and this becomes particularly problematic in older men and in the postmenopausal years for women.
One’s genes have a major impact on bone density and are currently not modifiable.
Modifiable factors include mechanical loading of bones through exercise activity, maintaining a normal body weight, and ensuring adequate intake of micronutrients (including calcium and vitamin D) and macronutrients. Medications such as glucocorticoids that have deleterious effects on bones should be limited as far as possible. Endocrine, gastrointestinal, renal, and rheumatologic conditions and others, such as cancer, which are known to be associated with reduced bone density and increased fracture risk, should be managed appropriately.
A deficiency of the gonadal hormones (estrogen and testosterone) and high blood concentrations of cortisol are particularly deleterious to bone. Hormone replacement therapy in those with gonadal hormone deficiency and strategies to reduce cortisol levels in those with hypercortisolemia are essential to prevent osteoporosis and also improve bone density over time. The same applies to management of conditions such as anorexia nervosa, relative energy deficiency in sports, inflammatory bowel disease, celiac disease, cystic fibrosis, chronic kidney disease, and chronic arthritis.
Once osteoporosis has developed, depending on the cause, these strategies may not be sufficient to completely reverse the condition, and pharmacologic therapy may be necessary to improve bone density and reduce fracture risk. This is particularly an issue with postmenopausal women and older men. In these individuals, medications that increase bone formation or reduce bone loss may be necessary.
Medications that reduce bone loss include bisphosphonates and denosumab; these are also called “antiresorptive medications” because they reduce bone resorption by cells called osteoclasts. Bisphosphonates include alendronate, risedronate, ibandronate, pamidronate, and zoledronic acid, and these medications have direct effects on osteoclasts, reducing their activity. Some bisphosphonates, such as alendronate and risedronate, are taken orally (daily, weekly, or monthly, depending on the medication and its strength), whereas others, such as pamidronate and zoledronic acid, are administered intravenously: every 3-4 months for pamidronate and every 6-12 months for zoledronic acid. Ibandronate is available both orally and intravenously.
Denosumab is a medication that inhibits the action of receptor activator of nuclear factor-kappa ligand 1 (RANKL), which otherwise increases osteoclast activity. It is administered as a subcutaneous injection every 6 months to treat osteoporosis. One concern with denosumab is a rapid increase in bone loss after its discontinuation.
Medications that increase bone formation are called bone anabolics and include teriparatide, abaloparatide, and romosozumab. Teriparatide is a synthetic form of parathyroid hormone (recombinant PTH1-34) administered daily for up to 2 years. Abaloparatide is a synthetic analog of parathyroid hormone–related peptide (PTHrP), which is also administered daily as a subcutaneous injection. Romosozumab inhibits sclerostin (a substance that otherwise reduces bone formation and increases bone resorption) and is administered as a subcutaneous injection once a month. Effects of these medications tend to be lost after they are discontinued.
In 2019, the Endocrine Society published guidelines for managing postmenopausal osteoporosis. The guidelines recommend lifestyle modifications, including attention to diet, calcium and vitamin D supplements, and weight-bearing exercise for all postmenopausal women. They also recommend assessing fracture risk using country-specific existing models.
Guidelines vary depending on whether fracture risk is low, moderate, or high. Patients at low risk are followed and reassessed every 2-4 years for fracture risk. Those at moderate risk may be followed similarly or prescribed bisphosphonates. Those at high risk are prescribed an antiresorptive, such as a bisphosphonate or denosumab, or a bone anabolic, such as teriparatide or abaloparatide (for up to 2 years) or romosozumab (for a year), with calcium and vitamin D and are reassessed at defined intervals for fracture risk; subsequent management then depends on the assessed fracture risk.
People who are on a bone anabolic should typically follow this with an antiresorptive medication to maintain the gains achieved with the former after that medication is discontinued. Patients who discontinue denosumab should be switched to bisphosphonates to prevent the increase in bone loss that typically occurs.
In postmenopausal women who are intolerant to or inappropriate for use of these medications, guidelines vary depending on age (younger or older than 60 years) and presence or absence of vasomotor symptoms (such as hot flashes). Options could include the use of calcium and vitamin D supplements; hormone replacement therapy with estrogen with or without a progestin; or selective estrogen receptor modulators (such as raloxifene or bazedoxifene), tibolone, or calcitonin.
It’s important to recognize that all pharmacologic therapy carries the risk for adverse events, and it’s essential to take the necessary steps to prevent, monitor for, and manage any adverse effects that may develop.
Managing osteoporosis in older men could include the use of bone anabolics and/or antiresorptives. In younger individuals, use of pharmacologic therapy is less common but sometimes necessary, particularly when bone density is very low and associated with a problematic fracture history — for example, in those with genetic conditions such as osteogenesis imperfecta. Furthermore, the occurrence of vertebral compression fractures often requires bisphosphonate treatment regardless of bone density, particularly in patients on chronic glucocorticoid therapy.
Preventing osteoporosis is best managed by paying attention to lifestyle; optimizing nutrition and calcium and vitamin D intake; and managing conditions and limiting the use of medications that reduce bone density.
However, in certain patients, these measures are not enough, and pharmacologic therapy with bone anabolics or antiresorptives may be necessary to improve bone density and reduce fracture risk.
Dr. Misra, of the University of Virginia and UVA Health Children’s Hospital, Charlottesville, disclosed ties with AbbVie, Sanofi, and Ipsen.
A version of this article appeared on Medscape.com.
Fractures, particularly hip and spine fractures, are a major cause of mortality and morbidity among older individuals. The term “osteoporosis” indicates increased porosity of bones resulting in low bone density; increased bone fragility; and an increased risk for fracture, often with minimal trauma.
During the adolescent years, bone accrues at a rapid rate, and optimal bone accrual during this time is essential to attain optimal peak bone mass, typically achieved in the third decade of life. Bone mass then stays stable until the 40s-50s, after which it starts to decline. One’s peak bone mass sets the stage for both immediate and future bone health. Individuals with lower peak bone mass tend to have less optimal bone health throughout their lives, and this becomes particularly problematic in older men and in the postmenopausal years for women.
One’s genes have a major impact on bone density and are currently not modifiable.
Modifiable factors include mechanical loading of bones through exercise activity, maintaining a normal body weight, and ensuring adequate intake of micronutrients (including calcium and vitamin D) and macronutrients. Medications such as glucocorticoids that have deleterious effects on bones should be limited as far as possible. Endocrine, gastrointestinal, renal, and rheumatologic conditions and others, such as cancer, which are known to be associated with reduced bone density and increased fracture risk, should be managed appropriately.
A deficiency of the gonadal hormones (estrogen and testosterone) and high blood concentrations of cortisol are particularly deleterious to bone. Hormone replacement therapy in those with gonadal hormone deficiency and strategies to reduce cortisol levels in those with hypercortisolemia are essential to prevent osteoporosis and also improve bone density over time. The same applies to management of conditions such as anorexia nervosa, relative energy deficiency in sports, inflammatory bowel disease, celiac disease, cystic fibrosis, chronic kidney disease, and chronic arthritis.
Once osteoporosis has developed, depending on the cause, these strategies may not be sufficient to completely reverse the condition, and pharmacologic therapy may be necessary to improve bone density and reduce fracture risk. This is particularly an issue with postmenopausal women and older men. In these individuals, medications that increase bone formation or reduce bone loss may be necessary.
Medications that reduce bone loss include bisphosphonates and denosumab; these are also called “antiresorptive medications” because they reduce bone resorption by cells called osteoclasts. Bisphosphonates include alendronate, risedronate, ibandronate, pamidronate, and zoledronic acid, and these medications have direct effects on osteoclasts, reducing their activity. Some bisphosphonates, such as alendronate and risedronate, are taken orally (daily, weekly, or monthly, depending on the medication and its strength), whereas others, such as pamidronate and zoledronic acid, are administered intravenously: every 3-4 months for pamidronate and every 6-12 months for zoledronic acid. Ibandronate is available both orally and intravenously.
Denosumab is a medication that inhibits the action of receptor activator of nuclear factor-kappa ligand 1 (RANKL), which otherwise increases osteoclast activity. It is administered as a subcutaneous injection every 6 months to treat osteoporosis. One concern with denosumab is a rapid increase in bone loss after its discontinuation.
Medications that increase bone formation are called bone anabolics and include teriparatide, abaloparatide, and romosozumab. Teriparatide is a synthetic form of parathyroid hormone (recombinant PTH1-34) administered daily for up to 2 years. Abaloparatide is a synthetic analog of parathyroid hormone–related peptide (PTHrP), which is also administered daily as a subcutaneous injection. Romosozumab inhibits sclerostin (a substance that otherwise reduces bone formation and increases bone resorption) and is administered as a subcutaneous injection once a month. Effects of these medications tend to be lost after they are discontinued.
In 2019, the Endocrine Society published guidelines for managing postmenopausal osteoporosis. The guidelines recommend lifestyle modifications, including attention to diet, calcium and vitamin D supplements, and weight-bearing exercise for all postmenopausal women. They also recommend assessing fracture risk using country-specific existing models.
Guidelines vary depending on whether fracture risk is low, moderate, or high. Patients at low risk are followed and reassessed every 2-4 years for fracture risk. Those at moderate risk may be followed similarly or prescribed bisphosphonates. Those at high risk are prescribed an antiresorptive, such as a bisphosphonate or denosumab, or a bone anabolic, such as teriparatide or abaloparatide (for up to 2 years) or romosozumab (for a year), with calcium and vitamin D and are reassessed at defined intervals for fracture risk; subsequent management then depends on the assessed fracture risk.
People who are on a bone anabolic should typically follow this with an antiresorptive medication to maintain the gains achieved with the former after that medication is discontinued. Patients who discontinue denosumab should be switched to bisphosphonates to prevent the increase in bone loss that typically occurs.
In postmenopausal women who are intolerant to or inappropriate for use of these medications, guidelines vary depending on age (younger or older than 60 years) and presence or absence of vasomotor symptoms (such as hot flashes). Options could include the use of calcium and vitamin D supplements; hormone replacement therapy with estrogen with or without a progestin; or selective estrogen receptor modulators (such as raloxifene or bazedoxifene), tibolone, or calcitonin.
It’s important to recognize that all pharmacologic therapy carries the risk for adverse events, and it’s essential to take the necessary steps to prevent, monitor for, and manage any adverse effects that may develop.
Managing osteoporosis in older men could include the use of bone anabolics and/or antiresorptives. In younger individuals, use of pharmacologic therapy is less common but sometimes necessary, particularly when bone density is very low and associated with a problematic fracture history — for example, in those with genetic conditions such as osteogenesis imperfecta. Furthermore, the occurrence of vertebral compression fractures often requires bisphosphonate treatment regardless of bone density, particularly in patients on chronic glucocorticoid therapy.
Preventing osteoporosis is best managed by paying attention to lifestyle; optimizing nutrition and calcium and vitamin D intake; and managing conditions and limiting the use of medications that reduce bone density.
However, in certain patients, these measures are not enough, and pharmacologic therapy with bone anabolics or antiresorptives may be necessary to improve bone density and reduce fracture risk.
Dr. Misra, of the University of Virginia and UVA Health Children’s Hospital, Charlottesville, disclosed ties with AbbVie, Sanofi, and Ipsen.
A version of this article appeared on Medscape.com.
Fractures, particularly hip and spine fractures, are a major cause of mortality and morbidity among older individuals. The term “osteoporosis” indicates increased porosity of bones resulting in low bone density; increased bone fragility; and an increased risk for fracture, often with minimal trauma.
During the adolescent years, bone accrues at a rapid rate, and optimal bone accrual during this time is essential to attain optimal peak bone mass, typically achieved in the third decade of life. Bone mass then stays stable until the 40s-50s, after which it starts to decline. One’s peak bone mass sets the stage for both immediate and future bone health. Individuals with lower peak bone mass tend to have less optimal bone health throughout their lives, and this becomes particularly problematic in older men and in the postmenopausal years for women.
One’s genes have a major impact on bone density and are currently not modifiable.
Modifiable factors include mechanical loading of bones through exercise activity, maintaining a normal body weight, and ensuring adequate intake of micronutrients (including calcium and vitamin D) and macronutrients. Medications such as glucocorticoids that have deleterious effects on bones should be limited as far as possible. Endocrine, gastrointestinal, renal, and rheumatologic conditions and others, such as cancer, which are known to be associated with reduced bone density and increased fracture risk, should be managed appropriately.
A deficiency of the gonadal hormones (estrogen and testosterone) and high blood concentrations of cortisol are particularly deleterious to bone. Hormone replacement therapy in those with gonadal hormone deficiency and strategies to reduce cortisol levels in those with hypercortisolemia are essential to prevent osteoporosis and also improve bone density over time. The same applies to management of conditions such as anorexia nervosa, relative energy deficiency in sports, inflammatory bowel disease, celiac disease, cystic fibrosis, chronic kidney disease, and chronic arthritis.
Once osteoporosis has developed, depending on the cause, these strategies may not be sufficient to completely reverse the condition, and pharmacologic therapy may be necessary to improve bone density and reduce fracture risk. This is particularly an issue with postmenopausal women and older men. In these individuals, medications that increase bone formation or reduce bone loss may be necessary.
Medications that reduce bone loss include bisphosphonates and denosumab; these are also called “antiresorptive medications” because they reduce bone resorption by cells called osteoclasts. Bisphosphonates include alendronate, risedronate, ibandronate, pamidronate, and zoledronic acid, and these medications have direct effects on osteoclasts, reducing their activity. Some bisphosphonates, such as alendronate and risedronate, are taken orally (daily, weekly, or monthly, depending on the medication and its strength), whereas others, such as pamidronate and zoledronic acid, are administered intravenously: every 3-4 months for pamidronate and every 6-12 months for zoledronic acid. Ibandronate is available both orally and intravenously.
Denosumab is a medication that inhibits the action of receptor activator of nuclear factor-kappa ligand 1 (RANKL), which otherwise increases osteoclast activity. It is administered as a subcutaneous injection every 6 months to treat osteoporosis. One concern with denosumab is a rapid increase in bone loss after its discontinuation.
Medications that increase bone formation are called bone anabolics and include teriparatide, abaloparatide, and romosozumab. Teriparatide is a synthetic form of parathyroid hormone (recombinant PTH1-34) administered daily for up to 2 years. Abaloparatide is a synthetic analog of parathyroid hormone–related peptide (PTHrP), which is also administered daily as a subcutaneous injection. Romosozumab inhibits sclerostin (a substance that otherwise reduces bone formation and increases bone resorption) and is administered as a subcutaneous injection once a month. Effects of these medications tend to be lost after they are discontinued.
In 2019, the Endocrine Society published guidelines for managing postmenopausal osteoporosis. The guidelines recommend lifestyle modifications, including attention to diet, calcium and vitamin D supplements, and weight-bearing exercise for all postmenopausal women. They also recommend assessing fracture risk using country-specific existing models.
Guidelines vary depending on whether fracture risk is low, moderate, or high. Patients at low risk are followed and reassessed every 2-4 years for fracture risk. Those at moderate risk may be followed similarly or prescribed bisphosphonates. Those at high risk are prescribed an antiresorptive, such as a bisphosphonate or denosumab, or a bone anabolic, such as teriparatide or abaloparatide (for up to 2 years) or romosozumab (for a year), with calcium and vitamin D and are reassessed at defined intervals for fracture risk; subsequent management then depends on the assessed fracture risk.
People who are on a bone anabolic should typically follow this with an antiresorptive medication to maintain the gains achieved with the former after that medication is discontinued. Patients who discontinue denosumab should be switched to bisphosphonates to prevent the increase in bone loss that typically occurs.
In postmenopausal women who are intolerant to or inappropriate for use of these medications, guidelines vary depending on age (younger or older than 60 years) and presence or absence of vasomotor symptoms (such as hot flashes). Options could include the use of calcium and vitamin D supplements; hormone replacement therapy with estrogen with or without a progestin; or selective estrogen receptor modulators (such as raloxifene or bazedoxifene), tibolone, or calcitonin.
It’s important to recognize that all pharmacologic therapy carries the risk for adverse events, and it’s essential to take the necessary steps to prevent, monitor for, and manage any adverse effects that may develop.
Managing osteoporosis in older men could include the use of bone anabolics and/or antiresorptives. In younger individuals, use of pharmacologic therapy is less common but sometimes necessary, particularly when bone density is very low and associated with a problematic fracture history — for example, in those with genetic conditions such as osteogenesis imperfecta. Furthermore, the occurrence of vertebral compression fractures often requires bisphosphonate treatment regardless of bone density, particularly in patients on chronic glucocorticoid therapy.
Preventing osteoporosis is best managed by paying attention to lifestyle; optimizing nutrition and calcium and vitamin D intake; and managing conditions and limiting the use of medications that reduce bone density.
However, in certain patients, these measures are not enough, and pharmacologic therapy with bone anabolics or antiresorptives may be necessary to improve bone density and reduce fracture risk.
Dr. Misra, of the University of Virginia and UVA Health Children’s Hospital, Charlottesville, disclosed ties with AbbVie, Sanofi, and Ipsen.
A version of this article appeared on Medscape.com.
Vitamin D Supplements May Be a Double-Edged Sword
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
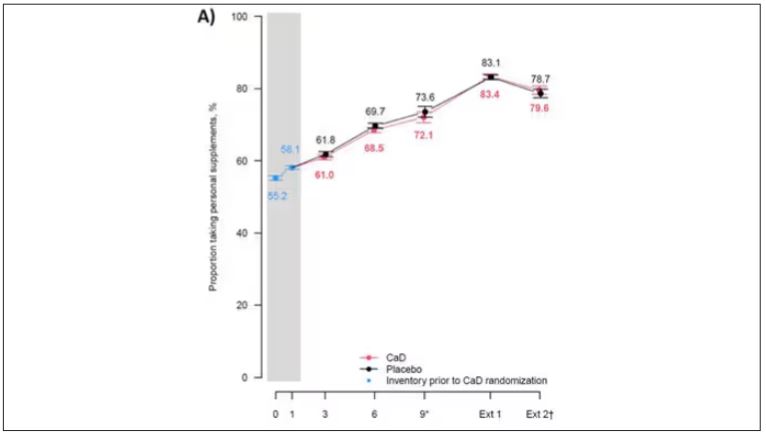
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
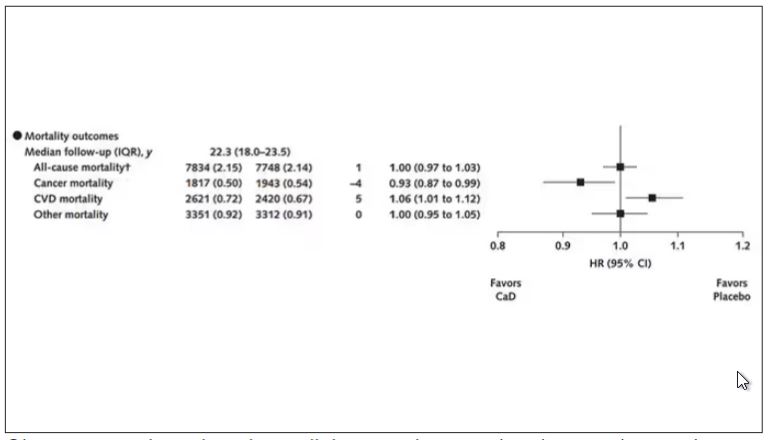
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
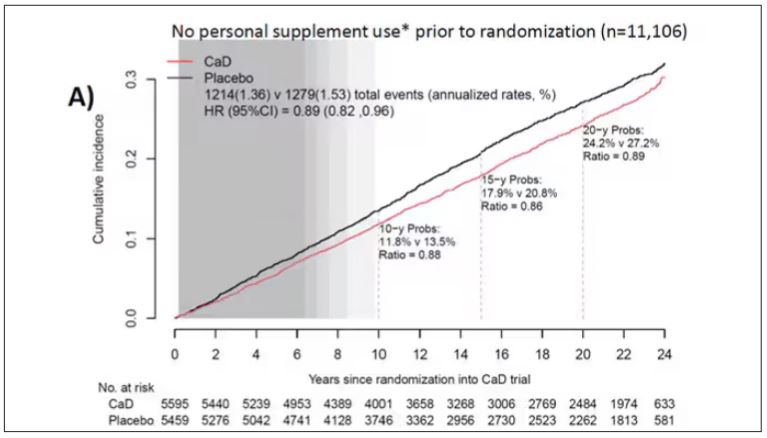
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
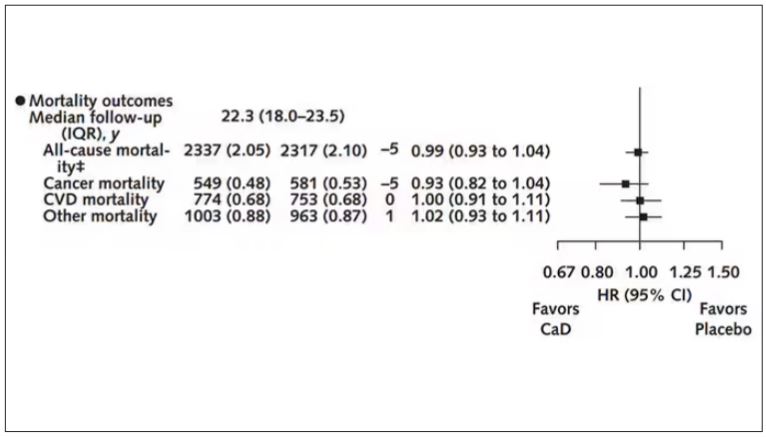
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
Long-Term Calcium and Vitamin D: Cancer Deaths Down, CVD Deaths Up in Older Women?
Some doctors may be scratching their heads over a new analysis reporting that combined calcium and vitamin D (CaD) supplements appear to be associated with a slight 6% increase in cardiovascular (CVD) mortality, a slight 7% decrease in cancer risk, and no effect on osteoporotic fracture in postmenopausal women.
The study, in Annals of Internal Medicine, found no effect of supplementation on all-cause mortality.
The findings emerged from an analysis of more than 20 years’ follow-up data on a randomized trial in postmenopausal women conducted as part of the Women’s Health Initiative (WHI).
Cynthia A. Thomson, PhD, RD, first author and cancer prevention scientist at the Arizona Cancer Center and a professor of health promotion sciences at the University of Arizona in Tucson said the findings recommend individualized assessment of the need for supplements for older women as they consider them in hopes of preventing fractures.
“Evaluate your patients individually and understand that there are some who may benefit from supplementation, for example, in terms of reducing colorectal cancer mortality,” Dr. Thomson said in an interview. The approach should be nuanced. “If you check the adequacy of vitamin D and calcium in their diets, supplementation may not be needed.” She added that supplementation is best considered in the context of a woman’s overall health profile, including risk factors for fracture, heart disease, and cancer, especially colorectal cancer (CRC).
Study Details
The investigators conducted postintervention follow-up of the WHI’s 7-year multicenter randomized intervention trial of CaD vs placebo.
Since existing evidence of long-term health outcomes was limited, the trial, begun in 1999 and closed in 2005, enrolled 36,282 postmenopausal women (mean age 62) with no history of breast or colorectal cancer. They were randomly assigned 1:1 to supplementation with 1000 mg of calcium carbonate (400 mg elemental calcium) plus 400 IU of vitamin D3 daily or placebo, taken twice daily in half doses.
Study outcomes were incidence of CRC, total and invasive breast cancer; disease-specific and all-cause mortality; total CVD; and hip fracture measured through December 2020, with analyses stratified by personal supplement usage.
Cancer. CaD was associated with reduced incident total cancer, CRC, and invasive breast cancer — notably among participants not taking CaD before randomization. Cancer incidence estimates varied widely, the authors noted, when stratified by supplement use before randomization. Noting that CaD seemed to have more cancer-related impact in those without prior supplementation, the authors suggested supplementation may affect cancer biology primarily by augmenting nutrient insufficiency.
An estimated 7% reduction in cancer mortality was observed after a median cumulative follow-up of 22.3 years: 1817 vs 1943 deaths (hazard ratio, 0.93; 95% CI, 0.87-0.99).
CVD. An estimated 6% increase in CVD mortality was seen in the CaD group: 2621 vs 2420 deaths (HR, 1.06; 95% CI, 1.01-1.12). Pretrial supplement users were found to be at higher CVD risk.
Hip fracture. No effect on hip fracture risk was measured, but the authors cautioned that hip fracture and CVD outcomes were available only for a subset of participants, and the effects of calcium alone vs vitamin D alone vs the combination could not be disentangled.
In a small subgroup analysis, some CaD users were seen to respond in terms of bone mineral density but since only 4 of the study’s 40 sites collected such information, the study was underpowered to examine the effect. ”Many other studies, however, show a response to supplementation in women who already have bone mineral deficits,” Dr. Thomson said.
The Calcification Question
One of the possible mechanisms of harm is that high-dose calcium supplements can increase the rate of blood coagulation and promote vascular calcification, said Emma Laing, PhD, RD, director of dietetics at the University of Georgia in Athens and a spokesperson for the Chicago-based Academy of Nutrition and Dietetics.
“Other factors that should be considered when determining a patient’s CVD risk are race, genetic predisposition, medical and social history, response to stress, and lifestyle behaviors, as well as the length of time supplements have been consumed,” added Dr. Laing, who was not involved in the WHI analysis.
“We asked ourselves if CaD supplements might contribute to calcification of the coronary arteries, since some believe this to be the case, although the literature is mixed,” said Dr. Thomson.
“So we did a shorter ancillary study in a small sample of several hundred [women] to see if there was any increase in calcification” and no difference was seen on imaging across the two arms. “However, women who were already on supplements before entering the study seemed to be at higher CVD risk,” she said.
Added study coauthor JoAnn E. Manson, MD, DrPH, chief of the division of preventive medicine at Brigham and Women’s Hospital and professor of women’s health at Harvard Medical School, both in Boston: “With no increase or decrease in coronary artery calcium at the end of the trial, we don’t believe starting or continuing calcium/vitamin D supplements should require screening for coronary artery disease.”
Some randomized trials and systematic reviews, however, have observed an increased risk of CVD in healthy patients on calcium supplements, with one Korean meta-analysis reporting a 15% increase in CVD risk in healthy postmenopausal women taking calcium supplements. Another meta-analysis found a link between calcium supplements and a greater risk of various cardiovascular outcomes, especially myocardial infarction.
Vitamin D Supplementation
As for vitamin D only supplementation, an updated meta-analysis including more than 83,000 individuals showed that it confers no cardiovascular protection and is therefore not indicated for this purpose.
Practice Considerations
Offering an outsider’s perspective, Sarah G. Candler, MD, MPH, an internist in Houston specializing in primary care for older high-risk adults, said: “Unfortunately, this latest study continues the trend of creating more questions than answers. If the adverse outcome of CVD death is a result of supplementation, it is unclear if this is due to the vitamin D, the calcium, or both. And it is unclear if this is dose dependent, time dependent, or due to concurrent risk factors unique to certain populations.
“It is recommended that patients at risk of osteoporosis based on age, sex, medications, and lifestyle be screened for osteoporosis and treated accordingly, including supplementation with CaD,” Dr. Candler said. “It remains unclear whether supplementation with CaD in the absence of osteoporosis and osteopenia is net beneficial or harmful, and at this time I would not recommend it to my patients.”
Added Dr. Manson: “The very small increase seen in cardiovascular mortality wouldn’t be a reason to discontinue supplementation among women who have been advised by their healthcare providers to take these supplements for bone health or other purposes.
“Among those at usual risk of fracture, we recommend trying to obtain adequate calcium and vitamin D from food sources first and to use supplements only for the purpose of filling gaps in intake,” Dr. Manson continued. Overall, the findings support the national recommended dietary allowances for daily calcium intake of 1200 mg and daily vitamin D intake of 600-800 IU among postmenopausal women for maintenance of bone health, she said.
While a 2022 study found that vitamin D supplementation alone did not prevent fractures in healthy adults, other research has shown that a calcium/vitamin D combination is more likely to protect the skeleton.
“Patients at risk for fractures will probably benefit from calcium and/or vitamin D supplementation if they do not meet dietary intake requirements, have malabsorption syndromes, are taking medications that affect nutrient absorption, or if they are older and not regularly exposed to sunlight,” said Dr. Laing. “A combination of biochemical, imaging, functional, and dietary intake data can help determine if a supplement is warranted.”
She stressed that additional research is needed in more diverse populations before changing practice guidelines. “However, doctors should continue to weigh the risks and benefits of prescribing supplements for each patient.”
The WHI program is funded by the National Heart, Lung, and Blood Institute. Dr. Thomson disclosed no competing interests. Dr. Manson reported a relationship with Mars Edge. Multiple authors reported grant support from government funding agencies. The outside commentators had no relevant competing interests to disclose.
Some doctors may be scratching their heads over a new analysis reporting that combined calcium and vitamin D (CaD) supplements appear to be associated with a slight 6% increase in cardiovascular (CVD) mortality, a slight 7% decrease in cancer risk, and no effect on osteoporotic fracture in postmenopausal women.
The study, in Annals of Internal Medicine, found no effect of supplementation on all-cause mortality.
The findings emerged from an analysis of more than 20 years’ follow-up data on a randomized trial in postmenopausal women conducted as part of the Women’s Health Initiative (WHI).
Cynthia A. Thomson, PhD, RD, first author and cancer prevention scientist at the Arizona Cancer Center and a professor of health promotion sciences at the University of Arizona in Tucson said the findings recommend individualized assessment of the need for supplements for older women as they consider them in hopes of preventing fractures.
“Evaluate your patients individually and understand that there are some who may benefit from supplementation, for example, in terms of reducing colorectal cancer mortality,” Dr. Thomson said in an interview. The approach should be nuanced. “If you check the adequacy of vitamin D and calcium in their diets, supplementation may not be needed.” She added that supplementation is best considered in the context of a woman’s overall health profile, including risk factors for fracture, heart disease, and cancer, especially colorectal cancer (CRC).
Study Details
The investigators conducted postintervention follow-up of the WHI’s 7-year multicenter randomized intervention trial of CaD vs placebo.
Since existing evidence of long-term health outcomes was limited, the trial, begun in 1999 and closed in 2005, enrolled 36,282 postmenopausal women (mean age 62) with no history of breast or colorectal cancer. They were randomly assigned 1:1 to supplementation with 1000 mg of calcium carbonate (400 mg elemental calcium) plus 400 IU of vitamin D3 daily or placebo, taken twice daily in half doses.
Study outcomes were incidence of CRC, total and invasive breast cancer; disease-specific and all-cause mortality; total CVD; and hip fracture measured through December 2020, with analyses stratified by personal supplement usage.
Cancer. CaD was associated with reduced incident total cancer, CRC, and invasive breast cancer — notably among participants not taking CaD before randomization. Cancer incidence estimates varied widely, the authors noted, when stratified by supplement use before randomization. Noting that CaD seemed to have more cancer-related impact in those without prior supplementation, the authors suggested supplementation may affect cancer biology primarily by augmenting nutrient insufficiency.
An estimated 7% reduction in cancer mortality was observed after a median cumulative follow-up of 22.3 years: 1817 vs 1943 deaths (hazard ratio, 0.93; 95% CI, 0.87-0.99).
CVD. An estimated 6% increase in CVD mortality was seen in the CaD group: 2621 vs 2420 deaths (HR, 1.06; 95% CI, 1.01-1.12). Pretrial supplement users were found to be at higher CVD risk.
Hip fracture. No effect on hip fracture risk was measured, but the authors cautioned that hip fracture and CVD outcomes were available only for a subset of participants, and the effects of calcium alone vs vitamin D alone vs the combination could not be disentangled.
In a small subgroup analysis, some CaD users were seen to respond in terms of bone mineral density but since only 4 of the study’s 40 sites collected such information, the study was underpowered to examine the effect. ”Many other studies, however, show a response to supplementation in women who already have bone mineral deficits,” Dr. Thomson said.
The Calcification Question
One of the possible mechanisms of harm is that high-dose calcium supplements can increase the rate of blood coagulation and promote vascular calcification, said Emma Laing, PhD, RD, director of dietetics at the University of Georgia in Athens and a spokesperson for the Chicago-based Academy of Nutrition and Dietetics.
“Other factors that should be considered when determining a patient’s CVD risk are race, genetic predisposition, medical and social history, response to stress, and lifestyle behaviors, as well as the length of time supplements have been consumed,” added Dr. Laing, who was not involved in the WHI analysis.
“We asked ourselves if CaD supplements might contribute to calcification of the coronary arteries, since some believe this to be the case, although the literature is mixed,” said Dr. Thomson.
“So we did a shorter ancillary study in a small sample of several hundred [women] to see if there was any increase in calcification” and no difference was seen on imaging across the two arms. “However, women who were already on supplements before entering the study seemed to be at higher CVD risk,” she said.
Added study coauthor JoAnn E. Manson, MD, DrPH, chief of the division of preventive medicine at Brigham and Women’s Hospital and professor of women’s health at Harvard Medical School, both in Boston: “With no increase or decrease in coronary artery calcium at the end of the trial, we don’t believe starting or continuing calcium/vitamin D supplements should require screening for coronary artery disease.”
Some randomized trials and systematic reviews, however, have observed an increased risk of CVD in healthy patients on calcium supplements, with one Korean meta-analysis reporting a 15% increase in CVD risk in healthy postmenopausal women taking calcium supplements. Another meta-analysis found a link between calcium supplements and a greater risk of various cardiovascular outcomes, especially myocardial infarction.
Vitamin D Supplementation
As for vitamin D only supplementation, an updated meta-analysis including more than 83,000 individuals showed that it confers no cardiovascular protection and is therefore not indicated for this purpose.
Practice Considerations
Offering an outsider’s perspective, Sarah G. Candler, MD, MPH, an internist in Houston specializing in primary care for older high-risk adults, said: “Unfortunately, this latest study continues the trend of creating more questions than answers. If the adverse outcome of CVD death is a result of supplementation, it is unclear if this is due to the vitamin D, the calcium, or both. And it is unclear if this is dose dependent, time dependent, or due to concurrent risk factors unique to certain populations.
“It is recommended that patients at risk of osteoporosis based on age, sex, medications, and lifestyle be screened for osteoporosis and treated accordingly, including supplementation with CaD,” Dr. Candler said. “It remains unclear whether supplementation with CaD in the absence of osteoporosis and osteopenia is net beneficial or harmful, and at this time I would not recommend it to my patients.”
Added Dr. Manson: “The very small increase seen in cardiovascular mortality wouldn’t be a reason to discontinue supplementation among women who have been advised by their healthcare providers to take these supplements for bone health or other purposes.
“Among those at usual risk of fracture, we recommend trying to obtain adequate calcium and vitamin D from food sources first and to use supplements only for the purpose of filling gaps in intake,” Dr. Manson continued. Overall, the findings support the national recommended dietary allowances for daily calcium intake of 1200 mg and daily vitamin D intake of 600-800 IU among postmenopausal women for maintenance of bone health, she said.
While a 2022 study found that vitamin D supplementation alone did not prevent fractures in healthy adults, other research has shown that a calcium/vitamin D combination is more likely to protect the skeleton.
“Patients at risk for fractures will probably benefit from calcium and/or vitamin D supplementation if they do not meet dietary intake requirements, have malabsorption syndromes, are taking medications that affect nutrient absorption, or if they are older and not regularly exposed to sunlight,” said Dr. Laing. “A combination of biochemical, imaging, functional, and dietary intake data can help determine if a supplement is warranted.”
She stressed that additional research is needed in more diverse populations before changing practice guidelines. “However, doctors should continue to weigh the risks and benefits of prescribing supplements for each patient.”
The WHI program is funded by the National Heart, Lung, and Blood Institute. Dr. Thomson disclosed no competing interests. Dr. Manson reported a relationship with Mars Edge. Multiple authors reported grant support from government funding agencies. The outside commentators had no relevant competing interests to disclose.
Some doctors may be scratching their heads over a new analysis reporting that combined calcium and vitamin D (CaD) supplements appear to be associated with a slight 6% increase in cardiovascular (CVD) mortality, a slight 7% decrease in cancer risk, and no effect on osteoporotic fracture in postmenopausal women.
The study, in Annals of Internal Medicine, found no effect of supplementation on all-cause mortality.
The findings emerged from an analysis of more than 20 years’ follow-up data on a randomized trial in postmenopausal women conducted as part of the Women’s Health Initiative (WHI).
Cynthia A. Thomson, PhD, RD, first author and cancer prevention scientist at the Arizona Cancer Center and a professor of health promotion sciences at the University of Arizona in Tucson said the findings recommend individualized assessment of the need for supplements for older women as they consider them in hopes of preventing fractures.
“Evaluate your patients individually and understand that there are some who may benefit from supplementation, for example, in terms of reducing colorectal cancer mortality,” Dr. Thomson said in an interview. The approach should be nuanced. “If you check the adequacy of vitamin D and calcium in their diets, supplementation may not be needed.” She added that supplementation is best considered in the context of a woman’s overall health profile, including risk factors for fracture, heart disease, and cancer, especially colorectal cancer (CRC).
Study Details
The investigators conducted postintervention follow-up of the WHI’s 7-year multicenter randomized intervention trial of CaD vs placebo.
Since existing evidence of long-term health outcomes was limited, the trial, begun in 1999 and closed in 2005, enrolled 36,282 postmenopausal women (mean age 62) with no history of breast or colorectal cancer. They were randomly assigned 1:1 to supplementation with 1000 mg of calcium carbonate (400 mg elemental calcium) plus 400 IU of vitamin D3 daily or placebo, taken twice daily in half doses.
Study outcomes were incidence of CRC, total and invasive breast cancer; disease-specific and all-cause mortality; total CVD; and hip fracture measured through December 2020, with analyses stratified by personal supplement usage.
Cancer. CaD was associated with reduced incident total cancer, CRC, and invasive breast cancer — notably among participants not taking CaD before randomization. Cancer incidence estimates varied widely, the authors noted, when stratified by supplement use before randomization. Noting that CaD seemed to have more cancer-related impact in those without prior supplementation, the authors suggested supplementation may affect cancer biology primarily by augmenting nutrient insufficiency.
An estimated 7% reduction in cancer mortality was observed after a median cumulative follow-up of 22.3 years: 1817 vs 1943 deaths (hazard ratio, 0.93; 95% CI, 0.87-0.99).
CVD. An estimated 6% increase in CVD mortality was seen in the CaD group: 2621 vs 2420 deaths (HR, 1.06; 95% CI, 1.01-1.12). Pretrial supplement users were found to be at higher CVD risk.
Hip fracture. No effect on hip fracture risk was measured, but the authors cautioned that hip fracture and CVD outcomes were available only for a subset of participants, and the effects of calcium alone vs vitamin D alone vs the combination could not be disentangled.
In a small subgroup analysis, some CaD users were seen to respond in terms of bone mineral density but since only 4 of the study’s 40 sites collected such information, the study was underpowered to examine the effect. ”Many other studies, however, show a response to supplementation in women who already have bone mineral deficits,” Dr. Thomson said.
The Calcification Question
One of the possible mechanisms of harm is that high-dose calcium supplements can increase the rate of blood coagulation and promote vascular calcification, said Emma Laing, PhD, RD, director of dietetics at the University of Georgia in Athens and a spokesperson for the Chicago-based Academy of Nutrition and Dietetics.
“Other factors that should be considered when determining a patient’s CVD risk are race, genetic predisposition, medical and social history, response to stress, and lifestyle behaviors, as well as the length of time supplements have been consumed,” added Dr. Laing, who was not involved in the WHI analysis.
“We asked ourselves if CaD supplements might contribute to calcification of the coronary arteries, since some believe this to be the case, although the literature is mixed,” said Dr. Thomson.
“So we did a shorter ancillary study in a small sample of several hundred [women] to see if there was any increase in calcification” and no difference was seen on imaging across the two arms. “However, women who were already on supplements before entering the study seemed to be at higher CVD risk,” she said.
Added study coauthor JoAnn E. Manson, MD, DrPH, chief of the division of preventive medicine at Brigham and Women’s Hospital and professor of women’s health at Harvard Medical School, both in Boston: “With no increase or decrease in coronary artery calcium at the end of the trial, we don’t believe starting or continuing calcium/vitamin D supplements should require screening for coronary artery disease.”
Some randomized trials and systematic reviews, however, have observed an increased risk of CVD in healthy patients on calcium supplements, with one Korean meta-analysis reporting a 15% increase in CVD risk in healthy postmenopausal women taking calcium supplements. Another meta-analysis found a link between calcium supplements and a greater risk of various cardiovascular outcomes, especially myocardial infarction.
Vitamin D Supplementation
As for vitamin D only supplementation, an updated meta-analysis including more than 83,000 individuals showed that it confers no cardiovascular protection and is therefore not indicated for this purpose.
Practice Considerations
Offering an outsider’s perspective, Sarah G. Candler, MD, MPH, an internist in Houston specializing in primary care for older high-risk adults, said: “Unfortunately, this latest study continues the trend of creating more questions than answers. If the adverse outcome of CVD death is a result of supplementation, it is unclear if this is due to the vitamin D, the calcium, or both. And it is unclear if this is dose dependent, time dependent, or due to concurrent risk factors unique to certain populations.
“It is recommended that patients at risk of osteoporosis based on age, sex, medications, and lifestyle be screened for osteoporosis and treated accordingly, including supplementation with CaD,” Dr. Candler said. “It remains unclear whether supplementation with CaD in the absence of osteoporosis and osteopenia is net beneficial or harmful, and at this time I would not recommend it to my patients.”
Added Dr. Manson: “The very small increase seen in cardiovascular mortality wouldn’t be a reason to discontinue supplementation among women who have been advised by their healthcare providers to take these supplements for bone health or other purposes.
“Among those at usual risk of fracture, we recommend trying to obtain adequate calcium and vitamin D from food sources first and to use supplements only for the purpose of filling gaps in intake,” Dr. Manson continued. Overall, the findings support the national recommended dietary allowances for daily calcium intake of 1200 mg and daily vitamin D intake of 600-800 IU among postmenopausal women for maintenance of bone health, she said.
While a 2022 study found that vitamin D supplementation alone did not prevent fractures in healthy adults, other research has shown that a calcium/vitamin D combination is more likely to protect the skeleton.
“Patients at risk for fractures will probably benefit from calcium and/or vitamin D supplementation if they do not meet dietary intake requirements, have malabsorption syndromes, are taking medications that affect nutrient absorption, or if they are older and not regularly exposed to sunlight,” said Dr. Laing. “A combination of biochemical, imaging, functional, and dietary intake data can help determine if a supplement is warranted.”
She stressed that additional research is needed in more diverse populations before changing practice guidelines. “However, doctors should continue to weigh the risks and benefits of prescribing supplements for each patient.”
The WHI program is funded by the National Heart, Lung, and Blood Institute. Dr. Thomson disclosed no competing interests. Dr. Manson reported a relationship with Mars Edge. Multiple authors reported grant support from government funding agencies. The outside commentators had no relevant competing interests to disclose.
FROM ANNALS OF INTERNAL MEDICINE
First Denosumab Biosimilar Approved in Two Different Formulations
The US Food and Drug Administration (FDA) has approved the first biosimilar to denosumab, denosumab-bddz (Wyost/Jubbonti).
The biosimilar was also granted interchangeability status, which allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). Sandoz announced the approval on March 5, 2024. The lower dosage of denosumab-bddz, marketed as Jubbonti, was also approved by Health Canada in February.
The FDA approval “is based on robust clinical studies and accompanied by labeling with safety warnings,” according to the press release. Like the reference products Prolia and Xgeva, denosumab-bddz is approved for two indications at separate doses.
Wyost (120-mg/1.7-mL injection) is approved to:
- Prevent skeletal-related events in patients with multiple myeloma and in patients with bone metastases from solid tumors
- Treat adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity
- Treat hypercalcemia of cancer that is refractory to bisphosphonate therapy
Jubbonti (60-mg/1-mL injection) is approved to:
- Treat postmenopausal women with osteoporosis who are at high risk for fracture
- Increase bone mass in men with osteoporosis who are at high risk for fracture
- Treat glucocorticoid-induced osteoporosis in men and women who are at high risk for fracture
- Increase bone mass in men who are at high risk for fracture who are receiving androgen deprivation therapy for nonmetastatic prostate cancer
- Increase bone mass in women who are at high risk for fracture who are receiving adjuvant aromatase inhibitor therapy for breast cancer.
Both doses are contraindicated for hypocalcemia and known clinically significant hypersensitivity to denosumab products. Exposure to denosumab products during pregnancy can cause fetal harm, so women of reproductive potential should be advised to use effective contraception during therapy and for at least 5 months after the last dose of denosumab-bddz.
Sandoz did not provide information on US launch details, citing “ongoing patent litigation around these products.”
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved the first biosimilar to denosumab, denosumab-bddz (Wyost/Jubbonti).
The biosimilar was also granted interchangeability status, which allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). Sandoz announced the approval on March 5, 2024. The lower dosage of denosumab-bddz, marketed as Jubbonti, was also approved by Health Canada in February.
The FDA approval “is based on robust clinical studies and accompanied by labeling with safety warnings,” according to the press release. Like the reference products Prolia and Xgeva, denosumab-bddz is approved for two indications at separate doses.
Wyost (120-mg/1.7-mL injection) is approved to:
- Prevent skeletal-related events in patients with multiple myeloma and in patients with bone metastases from solid tumors
- Treat adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity
- Treat hypercalcemia of cancer that is refractory to bisphosphonate therapy
Jubbonti (60-mg/1-mL injection) is approved to:
- Treat postmenopausal women with osteoporosis who are at high risk for fracture
- Increase bone mass in men with osteoporosis who are at high risk for fracture
- Treat glucocorticoid-induced osteoporosis in men and women who are at high risk for fracture
- Increase bone mass in men who are at high risk for fracture who are receiving androgen deprivation therapy for nonmetastatic prostate cancer
- Increase bone mass in women who are at high risk for fracture who are receiving adjuvant aromatase inhibitor therapy for breast cancer.
Both doses are contraindicated for hypocalcemia and known clinically significant hypersensitivity to denosumab products. Exposure to denosumab products during pregnancy can cause fetal harm, so women of reproductive potential should be advised to use effective contraception during therapy and for at least 5 months after the last dose of denosumab-bddz.
Sandoz did not provide information on US launch details, citing “ongoing patent litigation around these products.”
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved the first biosimilar to denosumab, denosumab-bddz (Wyost/Jubbonti).
The biosimilar was also granted interchangeability status, which allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). Sandoz announced the approval on March 5, 2024. The lower dosage of denosumab-bddz, marketed as Jubbonti, was also approved by Health Canada in February.
The FDA approval “is based on robust clinical studies and accompanied by labeling with safety warnings,” according to the press release. Like the reference products Prolia and Xgeva, denosumab-bddz is approved for two indications at separate doses.
Wyost (120-mg/1.7-mL injection) is approved to:
- Prevent skeletal-related events in patients with multiple myeloma and in patients with bone metastases from solid tumors
- Treat adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity
- Treat hypercalcemia of cancer that is refractory to bisphosphonate therapy
Jubbonti (60-mg/1-mL injection) is approved to:
- Treat postmenopausal women with osteoporosis who are at high risk for fracture
- Increase bone mass in men with osteoporosis who are at high risk for fracture
- Treat glucocorticoid-induced osteoporosis in men and women who are at high risk for fracture
- Increase bone mass in men who are at high risk for fracture who are receiving androgen deprivation therapy for nonmetastatic prostate cancer
- Increase bone mass in women who are at high risk for fracture who are receiving adjuvant aromatase inhibitor therapy for breast cancer.
Both doses are contraindicated for hypocalcemia and known clinically significant hypersensitivity to denosumab products. Exposure to denosumab products during pregnancy can cause fetal harm, so women of reproductive potential should be advised to use effective contraception during therapy and for at least 5 months after the last dose of denosumab-bddz.
Sandoz did not provide information on US launch details, citing “ongoing patent litigation around these products.”
A version of this article appeared on Medscape.com.
Osteoporosis Drug Denosumab May Confer Lower Risk for Diabetes
TOPLINE:
Continued denosumab treatment is associated with a lower risk for diabetes in adults with osteoporosis older than 65 years, found a large-scale cohort study in Taiwan.
METHODOLOGY:
- Denosumab, used in osteoporosis treatment, has been suggested to improve glycemic parameters, but clinical evidence of its effects on diabetes risk is limited and inconsistent.
- Using data from Taiwan’s National Health Insurance Research Database (NHIRD), the study asked if continued denosumab treatment (60 mg) for osteoporosis reduced the risk for diabetes compared to those who discontinued denosumab.
- Researchers included all new users of denosumab between 2012 and 2019 who had no prior history of malignant neoplasms, Paget disease, or diabetes requiring antidiabetic medication.
- Patients in the treatment group (n = 34,255), who received a second dose of denosumab within 225 days, were 1:1 propensity matched with a control group (n = 34,255) of patients who had discontinued denosumab after the first dose.
- The 68,510 patients (mean age, 77.7 years; 84.3% women) were followed up for a mean of 1.9 years. The primary outcome was new-onset diabetes that required treatment with any antidiabetic drug.
TAKEAWAY:
- Continued denosumab treatment vs its discontinuation was associated with a lower risk for incident diabetes (hazard ratio [HR], 0.84; 95% CI, 0.78-0.90).
- In patients aged 65 years or older who were on continued treatment of denosumab, the risk for diabetes was lower (HR, 0.80; 95% CI, 0.75-0.85) but not among those younger than 65 years.
- A reduced risk for diabetes with continued denosumab treatment was observed in both men (HR, 0.85; 95% CI, 0.73-0.97) and women (HR, 0.81; 95% CI, 0.76-0.86).
- Lower diabetes risk with continued denosumab treatment was observed regardless of comorbidities, such as dyslipidemia, hypertension, ischemic heart disease, or kidney failure.
IN PRACTICE:
“Given the high osteoporosis prevalence, the extensive use of antiosteoporosis medications, and the negative effect of diabetes on both patient health and healthcare system burdens in the global aging population, our findings possess substantial clinical and public health significance,” the authors wrote.
SOURCE:
This study was led by Huei-Kai Huang, MD, Department of Family Medicine and Department of Medical Research, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Hualien, Taiwan, and published online in JAMA Network Open.
LIMITATIONS:
The research used claims-based data, so some clinical details, such as lifestyle, substance use, prediabetes weight status, and laboratory results, were not included. Owing to the anonymity policy of the NHIRD, patients could not be directly evaluated to validate incident diabetes. The study included the Taiwanese population, so the findings may not be generalizable to other populations. In Taiwan, the threshold for reimbursement of initiating denosumab treatment for osteoporosis includes below-normal bone density scores and a hip or vertebral fracture.
DISCLOSURES:
This study was supported by grants from the National Science and Technology Council of Taiwan and the National Health Research Institutes of Taiwan and a grant from the Buddhist Tzu Chi Medical Foundation. The corresponding author and a coauthor disclosed receiving funds from Amgen, Novartis, Pfizer, Sanofi, Takeda, and AbbVie, all outside the submitted work.
A version of this article appeared on Medscape.com.
TOPLINE:
Continued denosumab treatment is associated with a lower risk for diabetes in adults with osteoporosis older than 65 years, found a large-scale cohort study in Taiwan.
METHODOLOGY:
- Denosumab, used in osteoporosis treatment, has been suggested to improve glycemic parameters, but clinical evidence of its effects on diabetes risk is limited and inconsistent.
- Using data from Taiwan’s National Health Insurance Research Database (NHIRD), the study asked if continued denosumab treatment (60 mg) for osteoporosis reduced the risk for diabetes compared to those who discontinued denosumab.
- Researchers included all new users of denosumab between 2012 and 2019 who had no prior history of malignant neoplasms, Paget disease, or diabetes requiring antidiabetic medication.
- Patients in the treatment group (n = 34,255), who received a second dose of denosumab within 225 days, were 1:1 propensity matched with a control group (n = 34,255) of patients who had discontinued denosumab after the first dose.
- The 68,510 patients (mean age, 77.7 years; 84.3% women) were followed up for a mean of 1.9 years. The primary outcome was new-onset diabetes that required treatment with any antidiabetic drug.
TAKEAWAY:
- Continued denosumab treatment vs its discontinuation was associated with a lower risk for incident diabetes (hazard ratio [HR], 0.84; 95% CI, 0.78-0.90).
- In patients aged 65 years or older who were on continued treatment of denosumab, the risk for diabetes was lower (HR, 0.80; 95% CI, 0.75-0.85) but not among those younger than 65 years.
- A reduced risk for diabetes with continued denosumab treatment was observed in both men (HR, 0.85; 95% CI, 0.73-0.97) and women (HR, 0.81; 95% CI, 0.76-0.86).
- Lower diabetes risk with continued denosumab treatment was observed regardless of comorbidities, such as dyslipidemia, hypertension, ischemic heart disease, or kidney failure.
IN PRACTICE:
“Given the high osteoporosis prevalence, the extensive use of antiosteoporosis medications, and the negative effect of diabetes on both patient health and healthcare system burdens in the global aging population, our findings possess substantial clinical and public health significance,” the authors wrote.
SOURCE:
This study was led by Huei-Kai Huang, MD, Department of Family Medicine and Department of Medical Research, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Hualien, Taiwan, and published online in JAMA Network Open.
LIMITATIONS:
The research used claims-based data, so some clinical details, such as lifestyle, substance use, prediabetes weight status, and laboratory results, were not included. Owing to the anonymity policy of the NHIRD, patients could not be directly evaluated to validate incident diabetes. The study included the Taiwanese population, so the findings may not be generalizable to other populations. In Taiwan, the threshold for reimbursement of initiating denosumab treatment for osteoporosis includes below-normal bone density scores and a hip or vertebral fracture.
DISCLOSURES:
This study was supported by grants from the National Science and Technology Council of Taiwan and the National Health Research Institutes of Taiwan and a grant from the Buddhist Tzu Chi Medical Foundation. The corresponding author and a coauthor disclosed receiving funds from Amgen, Novartis, Pfizer, Sanofi, Takeda, and AbbVie, all outside the submitted work.
A version of this article appeared on Medscape.com.
TOPLINE:
Continued denosumab treatment is associated with a lower risk for diabetes in adults with osteoporosis older than 65 years, found a large-scale cohort study in Taiwan.
METHODOLOGY:
- Denosumab, used in osteoporosis treatment, has been suggested to improve glycemic parameters, but clinical evidence of its effects on diabetes risk is limited and inconsistent.
- Using data from Taiwan’s National Health Insurance Research Database (NHIRD), the study asked if continued denosumab treatment (60 mg) for osteoporosis reduced the risk for diabetes compared to those who discontinued denosumab.
- Researchers included all new users of denosumab between 2012 and 2019 who had no prior history of malignant neoplasms, Paget disease, or diabetes requiring antidiabetic medication.
- Patients in the treatment group (n = 34,255), who received a second dose of denosumab within 225 days, were 1:1 propensity matched with a control group (n = 34,255) of patients who had discontinued denosumab after the first dose.
- The 68,510 patients (mean age, 77.7 years; 84.3% women) were followed up for a mean of 1.9 years. The primary outcome was new-onset diabetes that required treatment with any antidiabetic drug.
TAKEAWAY:
- Continued denosumab treatment vs its discontinuation was associated with a lower risk for incident diabetes (hazard ratio [HR], 0.84; 95% CI, 0.78-0.90).
- In patients aged 65 years or older who were on continued treatment of denosumab, the risk for diabetes was lower (HR, 0.80; 95% CI, 0.75-0.85) but not among those younger than 65 years.
- A reduced risk for diabetes with continued denosumab treatment was observed in both men (HR, 0.85; 95% CI, 0.73-0.97) and women (HR, 0.81; 95% CI, 0.76-0.86).
- Lower diabetes risk with continued denosumab treatment was observed regardless of comorbidities, such as dyslipidemia, hypertension, ischemic heart disease, or kidney failure.
IN PRACTICE:
“Given the high osteoporosis prevalence, the extensive use of antiosteoporosis medications, and the negative effect of diabetes on both patient health and healthcare system burdens in the global aging population, our findings possess substantial clinical and public health significance,” the authors wrote.
SOURCE:
This study was led by Huei-Kai Huang, MD, Department of Family Medicine and Department of Medical Research, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Hualien, Taiwan, and published online in JAMA Network Open.
LIMITATIONS:
The research used claims-based data, so some clinical details, such as lifestyle, substance use, prediabetes weight status, and laboratory results, were not included. Owing to the anonymity policy of the NHIRD, patients could not be directly evaluated to validate incident diabetes. The study included the Taiwanese population, so the findings may not be generalizable to other populations. In Taiwan, the threshold for reimbursement of initiating denosumab treatment for osteoporosis includes below-normal bone density scores and a hip or vertebral fracture.
DISCLOSURES:
This study was supported by grants from the National Science and Technology Council of Taiwan and the National Health Research Institutes of Taiwan and a grant from the Buddhist Tzu Chi Medical Foundation. The corresponding author and a coauthor disclosed receiving funds from Amgen, Novartis, Pfizer, Sanofi, Takeda, and AbbVie, all outside the submitted work.
A version of this article appeared on Medscape.com.
Study: Healthy Plant-Based Diets Do Not Raise Hip Fracture Risk
Long-term adherence to a plant-based diet was not tied to a greater risk of hip fracture and some plant-based regimens may actually reduce the risk, a large cohort study of postmenopausal women in the United States suggested.
Not all plant-centered regimens are healthful, however, and this study factored dietary quality into risk.
Writing in JAMA Network Open, the study authors compared the lowest to highest quintiles of Plant-Based Diet Index scores. They found the most recent intake of a healthy plant-based diet (hPDI) to be associated with a somewhat lower (21%) risk of fracture while the most recent intake of its unhealthy counterpart (uPDI) was linked to a somewhat higher (28%) risk.
“In addition, higher baseline scores in the uPDI were associated with higher risk of hip fracture,” wrote the researchers, led by Mercedes Sotos Prieto, PhD, a nutritional epidemiologist in the Department of Preventive Medicine and Public Health at the Autonomous University of Madrid.
Plant-based diets, characterized by higher consumption of plant foods and lower or no intake of animal foods, have raised concerns about their potential harm to bone health. In a recent meta-analysis, vegetarians, but particularly vegans with no consumption of any animal food, had a higher fracture risk and lower bone mineral density compared with omnivores.
Another study found that compared with meat eaters, fish eaters and vegetarians had a higher risk of hip fractures. These analyses, however, did not assess the quality of the plant-based diets.
“We hypothesized that the differences in the quality of the plant-based diets — whole grains, fruits, and vegetables vs refined carbohydrates or snacks, which are both plant-based but very different, would be important in the association for the risk of hip fracture,” Dr. Sotos Prieto said in an interview.
Study details
Her study drew on data from 70,285 postmenopausal White women who were in the US Nurses’ Health Study from 1984 through 2014; data were analyzed from Jan. 1 to July 31, 2023.
The mean age of the nurses was 54.92 years, and 2038 cases of hip fracture were reported during the study over as long as 30 years of follow-up.
Healthy plant foods included whole grains, fruits, vegetables, nuts, legumes, vegetable oils, and tea or coffee and received positive scores, whereas less healthy plant foods such as fruit juices, sweetened beverages, refined grains, potatoes, sweets, or desserts and animal foods received reversed scores. Dietary and lifestyle information was collected by self-reported questionnaires.
Individuals with higher hPDI scores were leaner, more physically active, less likely to be smokers, and more likely to use vitamin and calcium supplements. Not surprisingly, they also had higher intakes of dietary calcium and healthy plant foods and had lower intake of less healthy plant foods. “It’s plausible that reverse causation may account for the risk associations, as individuals with underlying health conditions that predisposed them to higher fracture risk may have changed their diet,” Dr. Sotos Prieto said. “In addition, baseline diet may reflect diet early on, which could be an important predictor of bone mineral density when there was more active bone turnover.”
Lack of information precluded adjustment for the use of anti-osteoporotic medication.
Neither the hPDI, with a hazard ratio (HR) for highest vs lowest quintile of 0.97 (95% confidence interval, 0.83-1.14) nor the uPDI, with an HR for highest vs lowest quintile of 1.02 (95% CI, 0.87-1.20) for diet adherence over the long term was associated with hip fracture risk.
For recent dietary intake in the highest vs lowest quintiles, however, the hPDI was associated with a 21% lower risk of hip fracture: HR, 0.79 (95% CI, 0.68-0.92; P = .02 for trend). In contrast, the uPDI was associated with a 28% higher risk: HR, 1.28 (95% CI, 1.09-1.51; P = .008 for trend).
Future studies in other populations are needed to confirm the results and enhance their generalizability, Dr. Sotos Prieto said. “Investigating the temporal dynamics of dietary patterns and their effects by examining how recent dietary changes may impact health outcomes over different timeframes is important.” In the meantime, people wishing to follow a plant-based diet should make sure it features high-quality foods.
This work was supported by Instituto de Salud Carlos III, State Secretary of Research, Development and Innovation of Spain, and the European Research Funds and European Social Fund, the Agencia Estatal de Investigación, the National Institutes of Health, and a Ramón y Cajal contract from the Ministry of Science, Innovation, and Universities. A coauthor reported a patent pending. No other disclosures were reported.
Long-term adherence to a plant-based diet was not tied to a greater risk of hip fracture and some plant-based regimens may actually reduce the risk, a large cohort study of postmenopausal women in the United States suggested.
Not all plant-centered regimens are healthful, however, and this study factored dietary quality into risk.
Writing in JAMA Network Open, the study authors compared the lowest to highest quintiles of Plant-Based Diet Index scores. They found the most recent intake of a healthy plant-based diet (hPDI) to be associated with a somewhat lower (21%) risk of fracture while the most recent intake of its unhealthy counterpart (uPDI) was linked to a somewhat higher (28%) risk.
“In addition, higher baseline scores in the uPDI were associated with higher risk of hip fracture,” wrote the researchers, led by Mercedes Sotos Prieto, PhD, a nutritional epidemiologist in the Department of Preventive Medicine and Public Health at the Autonomous University of Madrid.
Plant-based diets, characterized by higher consumption of plant foods and lower or no intake of animal foods, have raised concerns about their potential harm to bone health. In a recent meta-analysis, vegetarians, but particularly vegans with no consumption of any animal food, had a higher fracture risk and lower bone mineral density compared with omnivores.
Another study found that compared with meat eaters, fish eaters and vegetarians had a higher risk of hip fractures. These analyses, however, did not assess the quality of the plant-based diets.
“We hypothesized that the differences in the quality of the plant-based diets — whole grains, fruits, and vegetables vs refined carbohydrates or snacks, which are both plant-based but very different, would be important in the association for the risk of hip fracture,” Dr. Sotos Prieto said in an interview.
Study details
Her study drew on data from 70,285 postmenopausal White women who were in the US Nurses’ Health Study from 1984 through 2014; data were analyzed from Jan. 1 to July 31, 2023.
The mean age of the nurses was 54.92 years, and 2038 cases of hip fracture were reported during the study over as long as 30 years of follow-up.
Healthy plant foods included whole grains, fruits, vegetables, nuts, legumes, vegetable oils, and tea or coffee and received positive scores, whereas less healthy plant foods such as fruit juices, sweetened beverages, refined grains, potatoes, sweets, or desserts and animal foods received reversed scores. Dietary and lifestyle information was collected by self-reported questionnaires.
Individuals with higher hPDI scores were leaner, more physically active, less likely to be smokers, and more likely to use vitamin and calcium supplements. Not surprisingly, they also had higher intakes of dietary calcium and healthy plant foods and had lower intake of less healthy plant foods. “It’s plausible that reverse causation may account for the risk associations, as individuals with underlying health conditions that predisposed them to higher fracture risk may have changed their diet,” Dr. Sotos Prieto said. “In addition, baseline diet may reflect diet early on, which could be an important predictor of bone mineral density when there was more active bone turnover.”
Lack of information precluded adjustment for the use of anti-osteoporotic medication.
Neither the hPDI, with a hazard ratio (HR) for highest vs lowest quintile of 0.97 (95% confidence interval, 0.83-1.14) nor the uPDI, with an HR for highest vs lowest quintile of 1.02 (95% CI, 0.87-1.20) for diet adherence over the long term was associated with hip fracture risk.
For recent dietary intake in the highest vs lowest quintiles, however, the hPDI was associated with a 21% lower risk of hip fracture: HR, 0.79 (95% CI, 0.68-0.92; P = .02 for trend). In contrast, the uPDI was associated with a 28% higher risk: HR, 1.28 (95% CI, 1.09-1.51; P = .008 for trend).
Future studies in other populations are needed to confirm the results and enhance their generalizability, Dr. Sotos Prieto said. “Investigating the temporal dynamics of dietary patterns and their effects by examining how recent dietary changes may impact health outcomes over different timeframes is important.” In the meantime, people wishing to follow a plant-based diet should make sure it features high-quality foods.
This work was supported by Instituto de Salud Carlos III, State Secretary of Research, Development and Innovation of Spain, and the European Research Funds and European Social Fund, the Agencia Estatal de Investigación, the National Institutes of Health, and a Ramón y Cajal contract from the Ministry of Science, Innovation, and Universities. A coauthor reported a patent pending. No other disclosures were reported.
Long-term adherence to a plant-based diet was not tied to a greater risk of hip fracture and some plant-based regimens may actually reduce the risk, a large cohort study of postmenopausal women in the United States suggested.
Not all plant-centered regimens are healthful, however, and this study factored dietary quality into risk.
Writing in JAMA Network Open, the study authors compared the lowest to highest quintiles of Plant-Based Diet Index scores. They found the most recent intake of a healthy plant-based diet (hPDI) to be associated with a somewhat lower (21%) risk of fracture while the most recent intake of its unhealthy counterpart (uPDI) was linked to a somewhat higher (28%) risk.
“In addition, higher baseline scores in the uPDI were associated with higher risk of hip fracture,” wrote the researchers, led by Mercedes Sotos Prieto, PhD, a nutritional epidemiologist in the Department of Preventive Medicine and Public Health at the Autonomous University of Madrid.
Plant-based diets, characterized by higher consumption of plant foods and lower or no intake of animal foods, have raised concerns about their potential harm to bone health. In a recent meta-analysis, vegetarians, but particularly vegans with no consumption of any animal food, had a higher fracture risk and lower bone mineral density compared with omnivores.
Another study found that compared with meat eaters, fish eaters and vegetarians had a higher risk of hip fractures. These analyses, however, did not assess the quality of the plant-based diets.
“We hypothesized that the differences in the quality of the plant-based diets — whole grains, fruits, and vegetables vs refined carbohydrates or snacks, which are both plant-based but very different, would be important in the association for the risk of hip fracture,” Dr. Sotos Prieto said in an interview.
Study details
Her study drew on data from 70,285 postmenopausal White women who were in the US Nurses’ Health Study from 1984 through 2014; data were analyzed from Jan. 1 to July 31, 2023.
The mean age of the nurses was 54.92 years, and 2038 cases of hip fracture were reported during the study over as long as 30 years of follow-up.
Healthy plant foods included whole grains, fruits, vegetables, nuts, legumes, vegetable oils, and tea or coffee and received positive scores, whereas less healthy plant foods such as fruit juices, sweetened beverages, refined grains, potatoes, sweets, or desserts and animal foods received reversed scores. Dietary and lifestyle information was collected by self-reported questionnaires.
Individuals with higher hPDI scores were leaner, more physically active, less likely to be smokers, and more likely to use vitamin and calcium supplements. Not surprisingly, they also had higher intakes of dietary calcium and healthy plant foods and had lower intake of less healthy plant foods. “It’s plausible that reverse causation may account for the risk associations, as individuals with underlying health conditions that predisposed them to higher fracture risk may have changed their diet,” Dr. Sotos Prieto said. “In addition, baseline diet may reflect diet early on, which could be an important predictor of bone mineral density when there was more active bone turnover.”
Lack of information precluded adjustment for the use of anti-osteoporotic medication.
Neither the hPDI, with a hazard ratio (HR) for highest vs lowest quintile of 0.97 (95% confidence interval, 0.83-1.14) nor the uPDI, with an HR for highest vs lowest quintile of 1.02 (95% CI, 0.87-1.20) for diet adherence over the long term was associated with hip fracture risk.
For recent dietary intake in the highest vs lowest quintiles, however, the hPDI was associated with a 21% lower risk of hip fracture: HR, 0.79 (95% CI, 0.68-0.92; P = .02 for trend). In contrast, the uPDI was associated with a 28% higher risk: HR, 1.28 (95% CI, 1.09-1.51; P = .008 for trend).
Future studies in other populations are needed to confirm the results and enhance their generalizability, Dr. Sotos Prieto said. “Investigating the temporal dynamics of dietary patterns and their effects by examining how recent dietary changes may impact health outcomes over different timeframes is important.” In the meantime, people wishing to follow a plant-based diet should make sure it features high-quality foods.
This work was supported by Instituto de Salud Carlos III, State Secretary of Research, Development and Innovation of Spain, and the European Research Funds and European Social Fund, the Agencia Estatal de Investigación, the National Institutes of Health, and a Ramón y Cajal contract from the Ministry of Science, Innovation, and Universities. A coauthor reported a patent pending. No other disclosures were reported.
FROM JAMA NETWORK OPEN
Calcium Pyrophosphate Deposition Disease Nearly Doubles Fracture Risk
Patients with calcium pyrophosphate deposition (CPPD) disease, also known as pseudogout, have an 80% higher risk for fracture than individuals who do not have the disease, according to a new analysis.
This trend was driven by wrist fractures, where there was a more than threefold increased risk.
Previous studies identified an association between CPPD and low bone mineral density, and there is growing evidence suggesting that the dysregulation of osteoprotegerin — a molecule that is important in the regulation of osteoclasts — may be associated with early-onset CPPD, noted Sara K. Tedeschi, MD, MPH, the lead author of the study and head of crystal-induced arthritic diseases at Brigham and Women’s Hospital, Boston, Massachusetts.
However, CPPD’s association with fracture risk has yet to be explored.
In the study, Dr. Tedeschi and colleagues used Mass General Brigham electronic health record (EHR) data from 1991 to 2023 to identify 1148 individuals with acute calcium pyrophosphate (CPP) crystal arthritis. The index date was defined as the first documentation of pseudogout or synovial fluid CPP crystals. These patients were matched to 3730 comparators based on healthcare encounters within 30 days of the index date of a patient with CPPD. Patients were also matched based on the year of their first EHR encounter. Patients with a fracture documented prior to the index date were excluded from the analysis.
The primary outcome was the first fracture of the humerus, knee, wrist, hip, or pelvis, detected via published algorithms using diagnostic and procedural codes.
The research was published on January 14 in Arthritis & Rheumatology.
Although participants were not matched on age or sex, the average age was 73, and most participants were female. In total, 83.1% of participants in the CPPD group and 80.0% of those in the control group were White.
After adjustment for confounding factors including age, sex, comorbidities, and glucocorticoid use, CPPD was associated with an 80% higher risk for any fracture (hazard risk [HR], 1.8). Fracture risk was highest for the wrist (HR, 3.6).
Patients with CPPD had a 40% higher risk to experience a humerus or pelvis fracture and a 30% higher risk for hip fractures, but the results were not statistically significant.
The results were similar for sensitivity analyses that excluded patients who were prescribed glucocorticoids, treatment for osteoporosis, or had a diagnosis of rheumatoid arthritis.
Asked to comment, John D. FitzGerald, MD, PhD, clinical chief of rheumatology at the University of California, Los Angeles, noted that these associations were “convincing and strong. I thought it was a very good study and important work. CPPD is common and osteoporosis is common, so better understanding the connection is important.”
It’s still not clear why the risk for wrist fractures was highest, but Dr. Tedeschi had two hypotheses. The researchers were unable to assess for falls in this dataset, but it’s possible that patients with CPPD experiencing joint pain could fall and try to brace themselves with an outstretched arm, leading to a wrist fracture.
CPPD also commonly affects the wrist, “so it’s possible that if CPPD is affecting the wrist and if there is an association between CPPD and low bone density, maybe there’s particularly low bone density at the wrist,” she said.
Dr. FitzGerald agreed that both hypotheses were plausible, but “with the retrospective study, there could be a lot of things that are unobserved or unexplained,” he added.
Dr. Tedeschi is interested in exploring what could be causing the association with an increased fracture risk in future research.
“I hope this draws attention to the fact that people with CPPD can have related medical problems that are outside of their joints,” added Dr. Tedeschi. “Thinking about routine screening for osteopenia and osteoporosis could be a good first step in patients with CPPD.”
The study was funded by grants from the US National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Tedeschi has worked as a consultant for Novartis. Dr. FitzGerald reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Patients with calcium pyrophosphate deposition (CPPD) disease, also known as pseudogout, have an 80% higher risk for fracture than individuals who do not have the disease, according to a new analysis.
This trend was driven by wrist fractures, where there was a more than threefold increased risk.
Previous studies identified an association between CPPD and low bone mineral density, and there is growing evidence suggesting that the dysregulation of osteoprotegerin — a molecule that is important in the regulation of osteoclasts — may be associated with early-onset CPPD, noted Sara K. Tedeschi, MD, MPH, the lead author of the study and head of crystal-induced arthritic diseases at Brigham and Women’s Hospital, Boston, Massachusetts.
However, CPPD’s association with fracture risk has yet to be explored.
In the study, Dr. Tedeschi and colleagues used Mass General Brigham electronic health record (EHR) data from 1991 to 2023 to identify 1148 individuals with acute calcium pyrophosphate (CPP) crystal arthritis. The index date was defined as the first documentation of pseudogout or synovial fluid CPP crystals. These patients were matched to 3730 comparators based on healthcare encounters within 30 days of the index date of a patient with CPPD. Patients were also matched based on the year of their first EHR encounter. Patients with a fracture documented prior to the index date were excluded from the analysis.
The primary outcome was the first fracture of the humerus, knee, wrist, hip, or pelvis, detected via published algorithms using diagnostic and procedural codes.
The research was published on January 14 in Arthritis & Rheumatology.
Although participants were not matched on age or sex, the average age was 73, and most participants were female. In total, 83.1% of participants in the CPPD group and 80.0% of those in the control group were White.
After adjustment for confounding factors including age, sex, comorbidities, and glucocorticoid use, CPPD was associated with an 80% higher risk for any fracture (hazard risk [HR], 1.8). Fracture risk was highest for the wrist (HR, 3.6).
Patients with CPPD had a 40% higher risk to experience a humerus or pelvis fracture and a 30% higher risk for hip fractures, but the results were not statistically significant.
The results were similar for sensitivity analyses that excluded patients who were prescribed glucocorticoids, treatment for osteoporosis, or had a diagnosis of rheumatoid arthritis.
Asked to comment, John D. FitzGerald, MD, PhD, clinical chief of rheumatology at the University of California, Los Angeles, noted that these associations were “convincing and strong. I thought it was a very good study and important work. CPPD is common and osteoporosis is common, so better understanding the connection is important.”
It’s still not clear why the risk for wrist fractures was highest, but Dr. Tedeschi had two hypotheses. The researchers were unable to assess for falls in this dataset, but it’s possible that patients with CPPD experiencing joint pain could fall and try to brace themselves with an outstretched arm, leading to a wrist fracture.
CPPD also commonly affects the wrist, “so it’s possible that if CPPD is affecting the wrist and if there is an association between CPPD and low bone density, maybe there’s particularly low bone density at the wrist,” she said.
Dr. FitzGerald agreed that both hypotheses were plausible, but “with the retrospective study, there could be a lot of things that are unobserved or unexplained,” he added.
Dr. Tedeschi is interested in exploring what could be causing the association with an increased fracture risk in future research.
“I hope this draws attention to the fact that people with CPPD can have related medical problems that are outside of their joints,” added Dr. Tedeschi. “Thinking about routine screening for osteopenia and osteoporosis could be a good first step in patients with CPPD.”
The study was funded by grants from the US National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Tedeschi has worked as a consultant for Novartis. Dr. FitzGerald reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Patients with calcium pyrophosphate deposition (CPPD) disease, also known as pseudogout, have an 80% higher risk for fracture than individuals who do not have the disease, according to a new analysis.
This trend was driven by wrist fractures, where there was a more than threefold increased risk.
Previous studies identified an association between CPPD and low bone mineral density, and there is growing evidence suggesting that the dysregulation of osteoprotegerin — a molecule that is important in the regulation of osteoclasts — may be associated with early-onset CPPD, noted Sara K. Tedeschi, MD, MPH, the lead author of the study and head of crystal-induced arthritic diseases at Brigham and Women’s Hospital, Boston, Massachusetts.
However, CPPD’s association with fracture risk has yet to be explored.
In the study, Dr. Tedeschi and colleagues used Mass General Brigham electronic health record (EHR) data from 1991 to 2023 to identify 1148 individuals with acute calcium pyrophosphate (CPP) crystal arthritis. The index date was defined as the first documentation of pseudogout or synovial fluid CPP crystals. These patients were matched to 3730 comparators based on healthcare encounters within 30 days of the index date of a patient with CPPD. Patients were also matched based on the year of their first EHR encounter. Patients with a fracture documented prior to the index date were excluded from the analysis.
The primary outcome was the first fracture of the humerus, knee, wrist, hip, or pelvis, detected via published algorithms using diagnostic and procedural codes.
The research was published on January 14 in Arthritis & Rheumatology.
Although participants were not matched on age or sex, the average age was 73, and most participants were female. In total, 83.1% of participants in the CPPD group and 80.0% of those in the control group were White.
After adjustment for confounding factors including age, sex, comorbidities, and glucocorticoid use, CPPD was associated with an 80% higher risk for any fracture (hazard risk [HR], 1.8). Fracture risk was highest for the wrist (HR, 3.6).
Patients with CPPD had a 40% higher risk to experience a humerus or pelvis fracture and a 30% higher risk for hip fractures, but the results were not statistically significant.
The results were similar for sensitivity analyses that excluded patients who were prescribed glucocorticoids, treatment for osteoporosis, or had a diagnosis of rheumatoid arthritis.
Asked to comment, John D. FitzGerald, MD, PhD, clinical chief of rheumatology at the University of California, Los Angeles, noted that these associations were “convincing and strong. I thought it was a very good study and important work. CPPD is common and osteoporosis is common, so better understanding the connection is important.”
It’s still not clear why the risk for wrist fractures was highest, but Dr. Tedeschi had two hypotheses. The researchers were unable to assess for falls in this dataset, but it’s possible that patients with CPPD experiencing joint pain could fall and try to brace themselves with an outstretched arm, leading to a wrist fracture.
CPPD also commonly affects the wrist, “so it’s possible that if CPPD is affecting the wrist and if there is an association between CPPD and low bone density, maybe there’s particularly low bone density at the wrist,” she said.
Dr. FitzGerald agreed that both hypotheses were plausible, but “with the retrospective study, there could be a lot of things that are unobserved or unexplained,” he added.
Dr. Tedeschi is interested in exploring what could be causing the association with an increased fracture risk in future research.
“I hope this draws attention to the fact that people with CPPD can have related medical problems that are outside of their joints,” added Dr. Tedeschi. “Thinking about routine screening for osteopenia and osteoporosis could be a good first step in patients with CPPD.”
The study was funded by grants from the US National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Tedeschi has worked as a consultant for Novartis. Dr. FitzGerald reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY