User login
Good news, bad news for buprenorphine in opioid use disorder
Misuse of buprenorphine in the United States by patients with opioid use disorder (OUD) dropped sharply between 2015 and 2019, new research shows.
Analyses of data from the National Survey on Drug Use and Health also showed that about 50% of the patients with OUD were not receiving substance use treatment – and that some may be misusing buprenorphine in an effort to self-treat their addiction.
Interestingly, there was no association between buprenorphine misuse and income among those with OUD or with race, ethnicity, or insurance status regardless of OUD status, which bucks commonly held perceptions of those with the disorder.
Overall, the findings “underscore the need to pursue actions that expand access to buprenorphine-based OUD treatment, to develop strategies to monitor and reduce buprenorphine misuse, and to address associated conditions,” the investigators, led by Beth Han, MD, PhD, National Institute on Drug Abuse (NIDA), write.
The study was published online October 15 in JAMA Network Open.
Opioid deaths
Centers for Disease Control and Prevention data Of those deaths, 69,710 involved opioids.
Buprenorphine, a medication approved by the U.S. Food and Drug Administration to treat OUD, has been shown to reduce opioid cravings and withdrawal symptoms and lower overdose risk.
The new survey included responses from 214,505 adults. Of these, 51.7% were women, 45.5% were age 50 years or older, and 63.9% were non-Hispanic White.
Responses were collected between 2015-2019 as part of an annual survey administered annually by the Substance Abuse and Mental Health Services Administration.
Misuse was defined as any use outside the prescribed amount, frequency, duration, or indication.
In 2019, hydrocodone, oxycodone, codeine, and tramadol were the most misused prescription opioid products. An estimated 2.4 million adults used buprenorphine, with 1.7 million reporting no misuse in the past 12 months.
While buprenorphine misuse was stable between 2015 and 2019 among individuals without OUD, misuse declined significantly among those with OUD – from 20.5% in 2015 to 15.9% in 2019 (P = .04).
A different picture of misuse
The demographic data reveals a picture of buprenorphine misuse that researchers note is quite different from common perceptions about people with substance use.
Those with OUD who misused buprenorphine were more likely to be non-Hispanic White (82.9% vs. 73.6%, respectively) and less likely to live in large metropolitan areas (47.7% vs. 58.1%).
Among participants with OUD, buprenorphine misuse was significantly associated with age, especially in those between 24 and 34 years (adjusted odds ratio [aOR], 2.9; 95% confidence interval, 1.4-5.8) and between 35 and 49 years (aOR, 2.3; 95% CI, 1.2-4.5).
It was also significantly associated with living in nonmetropolitan areas (aOR, 1.8; 95% CI, 1.0-3.0) and having past-year polysubstance use and use disorders (aOR, 3.9; 95% CI, 1.3-11.2); but negatively associated with past-year treatment for illicit drug use–only treatment (aOR, 0.4; 95% CI, 0.3-0.7).
There was no significant association between buprenorphine misuse and income in participants with OUD or with race, ethnicity, or insurance status, regardless of OUD status.
“Perceptions that persons of racial and ethnic minority groups and people living in poverty are more likely to misuse their medication are incorrect,” the researchers write.
“Nevertheless, these factors have been found to be important factors associated with opioid harms and receipt of buprenorphine treatment,” they add.
Between 2015 and 2017, the largest increase in opioid-related drug overdose deaths was among Black people aged 25 to 34, and the largest increase involving synthetic opioids was among Hispanic individuals aged 45 to 54. At the same time, White people were more likely to receive buprenorphine treatment for OUD.
‘Don’t exaggerate concerns’
Among survey participants with OUD, 57% of those who had misused buprenorphine in the past year had received no substance use treatment. Among those with OUD who had not misused the drug in the past year, 49% had received no treatment for their addiction.
The most common reason for buprenorphine misuse cited by those with OUD was “because I am hooked” (27.3%), which researchers said suggests people may be taking buprenorphine without a prescription to self-treat their OUD.
The investigators note that although buprenorphine is inexpensive and effective, clinicians currently must receive a federal waiver to prescribe it to more than 30 patients at a time.
Concern over potential misuse may be one reason some clinicians have been reluctant to complete the training process. However, the study results showed misuse rates of other opioids, including oxycodone and hydrocodone, were higher than those reported for buprenorphine.
“Many other prescription opioids are misused at much higher rates,” co-investigator Wilson Compton, MD, MPE, deputy director of NIDA, told this news organization.
“While there are concerns about all of them, we want to make sure that people don’t exaggerate the concerns – and understanding that oxycodone and hydrocodone are so much more frequently misused is important,” added Dr. Compton.
Symptom of inadequate access?
Commenting on the research, Bobby Mukkamala, MD, chair of the American Medical Association Board of Trustees, said individuals who misuse buprenorphine “commonly do so to alleviate uncontrolled pain or symptoms of withdrawal.”
“So-called misuse of buprenorphine is a symptom of inadequate access to physicians to treat opioid use disorder,” said Dr. Mukkamala, who also chairs the AMA Substance Use and Pain Care Task Force.
A 2020 study from the U.S. Department of Health & Human Services showed 40% of U.S. counties have no clinicians with a federal waiver permitting them to prescribe buprenorphine in an office setting.
In April, the HHS released new practice guidelines that allow certain practitioners licensed under state law who have a valid Drug Enforcement Administration registration to treat up to 30 patients with buprenorphine without having to complete requirements related to training, counseling, and other ancillary services known as an “X-waiver.”
The move was welcomed by many in the field, but Dr. Mukkamala said the agency did not go far enough.
“The AMA supports removing the federal X-waiver requirement to help destigmatize the provision of buprenorphine as well as remove the many administrative barriers that come with the federal requirement,” he said.
The study was funded by the National Institute on Drug Abuse. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Misuse of buprenorphine in the United States by patients with opioid use disorder (OUD) dropped sharply between 2015 and 2019, new research shows.
Analyses of data from the National Survey on Drug Use and Health also showed that about 50% of the patients with OUD were not receiving substance use treatment – and that some may be misusing buprenorphine in an effort to self-treat their addiction.
Interestingly, there was no association between buprenorphine misuse and income among those with OUD or with race, ethnicity, or insurance status regardless of OUD status, which bucks commonly held perceptions of those with the disorder.
Overall, the findings “underscore the need to pursue actions that expand access to buprenorphine-based OUD treatment, to develop strategies to monitor and reduce buprenorphine misuse, and to address associated conditions,” the investigators, led by Beth Han, MD, PhD, National Institute on Drug Abuse (NIDA), write.
The study was published online October 15 in JAMA Network Open.
Opioid deaths
Centers for Disease Control and Prevention data Of those deaths, 69,710 involved opioids.
Buprenorphine, a medication approved by the U.S. Food and Drug Administration to treat OUD, has been shown to reduce opioid cravings and withdrawal symptoms and lower overdose risk.
The new survey included responses from 214,505 adults. Of these, 51.7% were women, 45.5% were age 50 years or older, and 63.9% were non-Hispanic White.
Responses were collected between 2015-2019 as part of an annual survey administered annually by the Substance Abuse and Mental Health Services Administration.
Misuse was defined as any use outside the prescribed amount, frequency, duration, or indication.
In 2019, hydrocodone, oxycodone, codeine, and tramadol were the most misused prescription opioid products. An estimated 2.4 million adults used buprenorphine, with 1.7 million reporting no misuse in the past 12 months.
While buprenorphine misuse was stable between 2015 and 2019 among individuals without OUD, misuse declined significantly among those with OUD – from 20.5% in 2015 to 15.9% in 2019 (P = .04).
A different picture of misuse
The demographic data reveals a picture of buprenorphine misuse that researchers note is quite different from common perceptions about people with substance use.
Those with OUD who misused buprenorphine were more likely to be non-Hispanic White (82.9% vs. 73.6%, respectively) and less likely to live in large metropolitan areas (47.7% vs. 58.1%).
Among participants with OUD, buprenorphine misuse was significantly associated with age, especially in those between 24 and 34 years (adjusted odds ratio [aOR], 2.9; 95% confidence interval, 1.4-5.8) and between 35 and 49 years (aOR, 2.3; 95% CI, 1.2-4.5).
It was also significantly associated with living in nonmetropolitan areas (aOR, 1.8; 95% CI, 1.0-3.0) and having past-year polysubstance use and use disorders (aOR, 3.9; 95% CI, 1.3-11.2); but negatively associated with past-year treatment for illicit drug use–only treatment (aOR, 0.4; 95% CI, 0.3-0.7).
There was no significant association between buprenorphine misuse and income in participants with OUD or with race, ethnicity, or insurance status, regardless of OUD status.
“Perceptions that persons of racial and ethnic minority groups and people living in poverty are more likely to misuse their medication are incorrect,” the researchers write.
“Nevertheless, these factors have been found to be important factors associated with opioid harms and receipt of buprenorphine treatment,” they add.
Between 2015 and 2017, the largest increase in opioid-related drug overdose deaths was among Black people aged 25 to 34, and the largest increase involving synthetic opioids was among Hispanic individuals aged 45 to 54. At the same time, White people were more likely to receive buprenorphine treatment for OUD.
‘Don’t exaggerate concerns’
Among survey participants with OUD, 57% of those who had misused buprenorphine in the past year had received no substance use treatment. Among those with OUD who had not misused the drug in the past year, 49% had received no treatment for their addiction.
The most common reason for buprenorphine misuse cited by those with OUD was “because I am hooked” (27.3%), which researchers said suggests people may be taking buprenorphine without a prescription to self-treat their OUD.
The investigators note that although buprenorphine is inexpensive and effective, clinicians currently must receive a federal waiver to prescribe it to more than 30 patients at a time.
Concern over potential misuse may be one reason some clinicians have been reluctant to complete the training process. However, the study results showed misuse rates of other opioids, including oxycodone and hydrocodone, were higher than those reported for buprenorphine.
“Many other prescription opioids are misused at much higher rates,” co-investigator Wilson Compton, MD, MPE, deputy director of NIDA, told this news organization.
“While there are concerns about all of them, we want to make sure that people don’t exaggerate the concerns – and understanding that oxycodone and hydrocodone are so much more frequently misused is important,” added Dr. Compton.
Symptom of inadequate access?
Commenting on the research, Bobby Mukkamala, MD, chair of the American Medical Association Board of Trustees, said individuals who misuse buprenorphine “commonly do so to alleviate uncontrolled pain or symptoms of withdrawal.”
“So-called misuse of buprenorphine is a symptom of inadequate access to physicians to treat opioid use disorder,” said Dr. Mukkamala, who also chairs the AMA Substance Use and Pain Care Task Force.
A 2020 study from the U.S. Department of Health & Human Services showed 40% of U.S. counties have no clinicians with a federal waiver permitting them to prescribe buprenorphine in an office setting.
In April, the HHS released new practice guidelines that allow certain practitioners licensed under state law who have a valid Drug Enforcement Administration registration to treat up to 30 patients with buprenorphine without having to complete requirements related to training, counseling, and other ancillary services known as an “X-waiver.”
The move was welcomed by many in the field, but Dr. Mukkamala said the agency did not go far enough.
“The AMA supports removing the federal X-waiver requirement to help destigmatize the provision of buprenorphine as well as remove the many administrative barriers that come with the federal requirement,” he said.
The study was funded by the National Institute on Drug Abuse. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Misuse of buprenorphine in the United States by patients with opioid use disorder (OUD) dropped sharply between 2015 and 2019, new research shows.
Analyses of data from the National Survey on Drug Use and Health also showed that about 50% of the patients with OUD were not receiving substance use treatment – and that some may be misusing buprenorphine in an effort to self-treat their addiction.
Interestingly, there was no association between buprenorphine misuse and income among those with OUD or with race, ethnicity, or insurance status regardless of OUD status, which bucks commonly held perceptions of those with the disorder.
Overall, the findings “underscore the need to pursue actions that expand access to buprenorphine-based OUD treatment, to develop strategies to monitor and reduce buprenorphine misuse, and to address associated conditions,” the investigators, led by Beth Han, MD, PhD, National Institute on Drug Abuse (NIDA), write.
The study was published online October 15 in JAMA Network Open.
Opioid deaths
Centers for Disease Control and Prevention data Of those deaths, 69,710 involved opioids.
Buprenorphine, a medication approved by the U.S. Food and Drug Administration to treat OUD, has been shown to reduce opioid cravings and withdrawal symptoms and lower overdose risk.
The new survey included responses from 214,505 adults. Of these, 51.7% were women, 45.5% were age 50 years or older, and 63.9% were non-Hispanic White.
Responses were collected between 2015-2019 as part of an annual survey administered annually by the Substance Abuse and Mental Health Services Administration.
Misuse was defined as any use outside the prescribed amount, frequency, duration, or indication.
In 2019, hydrocodone, oxycodone, codeine, and tramadol were the most misused prescription opioid products. An estimated 2.4 million adults used buprenorphine, with 1.7 million reporting no misuse in the past 12 months.
While buprenorphine misuse was stable between 2015 and 2019 among individuals without OUD, misuse declined significantly among those with OUD – from 20.5% in 2015 to 15.9% in 2019 (P = .04).
A different picture of misuse
The demographic data reveals a picture of buprenorphine misuse that researchers note is quite different from common perceptions about people with substance use.
Those with OUD who misused buprenorphine were more likely to be non-Hispanic White (82.9% vs. 73.6%, respectively) and less likely to live in large metropolitan areas (47.7% vs. 58.1%).
Among participants with OUD, buprenorphine misuse was significantly associated with age, especially in those between 24 and 34 years (adjusted odds ratio [aOR], 2.9; 95% confidence interval, 1.4-5.8) and between 35 and 49 years (aOR, 2.3; 95% CI, 1.2-4.5).
It was also significantly associated with living in nonmetropolitan areas (aOR, 1.8; 95% CI, 1.0-3.0) and having past-year polysubstance use and use disorders (aOR, 3.9; 95% CI, 1.3-11.2); but negatively associated with past-year treatment for illicit drug use–only treatment (aOR, 0.4; 95% CI, 0.3-0.7).
There was no significant association between buprenorphine misuse and income in participants with OUD or with race, ethnicity, or insurance status, regardless of OUD status.
“Perceptions that persons of racial and ethnic minority groups and people living in poverty are more likely to misuse their medication are incorrect,” the researchers write.
“Nevertheless, these factors have been found to be important factors associated with opioid harms and receipt of buprenorphine treatment,” they add.
Between 2015 and 2017, the largest increase in opioid-related drug overdose deaths was among Black people aged 25 to 34, and the largest increase involving synthetic opioids was among Hispanic individuals aged 45 to 54. At the same time, White people were more likely to receive buprenorphine treatment for OUD.
‘Don’t exaggerate concerns’
Among survey participants with OUD, 57% of those who had misused buprenorphine in the past year had received no substance use treatment. Among those with OUD who had not misused the drug in the past year, 49% had received no treatment for their addiction.
The most common reason for buprenorphine misuse cited by those with OUD was “because I am hooked” (27.3%), which researchers said suggests people may be taking buprenorphine without a prescription to self-treat their OUD.
The investigators note that although buprenorphine is inexpensive and effective, clinicians currently must receive a federal waiver to prescribe it to more than 30 patients at a time.
Concern over potential misuse may be one reason some clinicians have been reluctant to complete the training process. However, the study results showed misuse rates of other opioids, including oxycodone and hydrocodone, were higher than those reported for buprenorphine.
“Many other prescription opioids are misused at much higher rates,” co-investigator Wilson Compton, MD, MPE, deputy director of NIDA, told this news organization.
“While there are concerns about all of them, we want to make sure that people don’t exaggerate the concerns – and understanding that oxycodone and hydrocodone are so much more frequently misused is important,” added Dr. Compton.
Symptom of inadequate access?
Commenting on the research, Bobby Mukkamala, MD, chair of the American Medical Association Board of Trustees, said individuals who misuse buprenorphine “commonly do so to alleviate uncontrolled pain or symptoms of withdrawal.”
“So-called misuse of buprenorphine is a symptom of inadequate access to physicians to treat opioid use disorder,” said Dr. Mukkamala, who also chairs the AMA Substance Use and Pain Care Task Force.
A 2020 study from the U.S. Department of Health & Human Services showed 40% of U.S. counties have no clinicians with a federal waiver permitting them to prescribe buprenorphine in an office setting.
In April, the HHS released new practice guidelines that allow certain practitioners licensed under state law who have a valid Drug Enforcement Administration registration to treat up to 30 patients with buprenorphine without having to complete requirements related to training, counseling, and other ancillary services known as an “X-waiver.”
The move was welcomed by many in the field, but Dr. Mukkamala said the agency did not go far enough.
“The AMA supports removing the federal X-waiver requirement to help destigmatize the provision of buprenorphine as well as remove the many administrative barriers that come with the federal requirement,” he said.
The study was funded by the National Institute on Drug Abuse. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Opioid prescribing mapped: Alabama highest, New York lowest
Medicare beneficiaries in Alabama were more likely to get a prescription for an opioid than in any other state in 2019, based on newly released data.
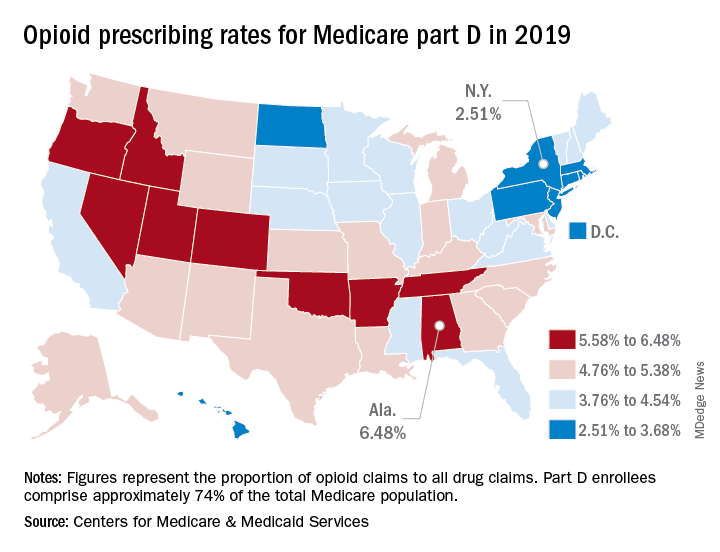
That year, opioids represented 6.48% of all drug claims for part D enrollees in the state, just ahead of Utah at 6.41%. Idaho, at 6.07%, was the only other state with an opioid prescribing rate over 6%, while Oklahoma came in at an even 6.0%, according to the latest update of the Centers for Medicare & Medicaid Services’ dataset.
The lowest rate in 2019 belonged to New York, where 2.51% of drug claims, including original prescriptions and refills, involved an opioid. Rhode Island was next at 2.87%, followed by New Jersey (3.23%), Massachusetts (3.26%), and North Dakota (3.39%),
Altogether, Medicare part D processed 1.5 billion drug claims in 2019, of which 66.1 million, or 4.41%, involved opioids. Both of the opioid numbers were down from 2018, when opioids represented 4.68% (70.2 million) of the 1.5 billion total claims, and from 2014, when opioids were involved in 5.73% (81,026,831) of the 1.41 billion drug claims, the CMS data show. That works out to 5.77% fewer opioids in 2019, compared with 2014, despite the increase in total volume.
from 2014 to 2019, with Hawaii showing the smallest decline as it slipped 0.41 percentage points from 3.9% to 3.49%, according to the CMS.
In 2019, part D beneficiaries in Vermont were the most likely to receive a long-acting opioid, which accounted for 20.14% of all opioid prescriptions in the state, while Kentucky had the lowest share of prescriptions written for long-acting forms at 6.41%. The national average was 11.02%, dropping from 11.79% in 2018 and 12.75% in 2014, the CMS reported.
Medicare beneficiaries in Alabama were more likely to get a prescription for an opioid than in any other state in 2019, based on newly released data.

That year, opioids represented 6.48% of all drug claims for part D enrollees in the state, just ahead of Utah at 6.41%. Idaho, at 6.07%, was the only other state with an opioid prescribing rate over 6%, while Oklahoma came in at an even 6.0%, according to the latest update of the Centers for Medicare & Medicaid Services’ dataset.
The lowest rate in 2019 belonged to New York, where 2.51% of drug claims, including original prescriptions and refills, involved an opioid. Rhode Island was next at 2.87%, followed by New Jersey (3.23%), Massachusetts (3.26%), and North Dakota (3.39%),
Altogether, Medicare part D processed 1.5 billion drug claims in 2019, of which 66.1 million, or 4.41%, involved opioids. Both of the opioid numbers were down from 2018, when opioids represented 4.68% (70.2 million) of the 1.5 billion total claims, and from 2014, when opioids were involved in 5.73% (81,026,831) of the 1.41 billion drug claims, the CMS data show. That works out to 5.77% fewer opioids in 2019, compared with 2014, despite the increase in total volume.
from 2014 to 2019, with Hawaii showing the smallest decline as it slipped 0.41 percentage points from 3.9% to 3.49%, according to the CMS.
In 2019, part D beneficiaries in Vermont were the most likely to receive a long-acting opioid, which accounted for 20.14% of all opioid prescriptions in the state, while Kentucky had the lowest share of prescriptions written for long-acting forms at 6.41%. The national average was 11.02%, dropping from 11.79% in 2018 and 12.75% in 2014, the CMS reported.
Medicare beneficiaries in Alabama were more likely to get a prescription for an opioid than in any other state in 2019, based on newly released data.

That year, opioids represented 6.48% of all drug claims for part D enrollees in the state, just ahead of Utah at 6.41%. Idaho, at 6.07%, was the only other state with an opioid prescribing rate over 6%, while Oklahoma came in at an even 6.0%, according to the latest update of the Centers for Medicare & Medicaid Services’ dataset.
The lowest rate in 2019 belonged to New York, where 2.51% of drug claims, including original prescriptions and refills, involved an opioid. Rhode Island was next at 2.87%, followed by New Jersey (3.23%), Massachusetts (3.26%), and North Dakota (3.39%),
Altogether, Medicare part D processed 1.5 billion drug claims in 2019, of which 66.1 million, or 4.41%, involved opioids. Both of the opioid numbers were down from 2018, when opioids represented 4.68% (70.2 million) of the 1.5 billion total claims, and from 2014, when opioids were involved in 5.73% (81,026,831) of the 1.41 billion drug claims, the CMS data show. That works out to 5.77% fewer opioids in 2019, compared with 2014, despite the increase in total volume.
from 2014 to 2019, with Hawaii showing the smallest decline as it slipped 0.41 percentage points from 3.9% to 3.49%, according to the CMS.
In 2019, part D beneficiaries in Vermont were the most likely to receive a long-acting opioid, which accounted for 20.14% of all opioid prescriptions in the state, while Kentucky had the lowest share of prescriptions written for long-acting forms at 6.41%. The national average was 11.02%, dropping from 11.79% in 2018 and 12.75% in 2014, the CMS reported.
Opioid overdoses tied to lasting cognitive impairment
Opioid overdoses usually aren’t fatal, but a new review of numerous studies, mostly case reports and case series, suggests that they can have long-lasting effects on cognition, possibly because of hypoxia resulting from respiratory depression.
Erin L. Winstanley, PhD, MA, and associates noted in the review that opioids cause about 80% of worldwide deaths from illicit drug use, and the Centers for Disease Control and Prevention’s provisional August 2021 number of more than 88,000 opioid-caused deaths in the United States is the highest ever recorded – a 27% increase over what was reported last December. That number suggests that the opioid epidemic continues to rage, but the study results also show that the neurological consequences of nonfatal overdoses are an important public health problem.
And that’s something that may be overlooked, according to Mark S. Gold, MD, who was not involved with the study and was asked to comment on the review, which was published in the Journal of Addiction Science.
“Assuming that an overdose has no effect on the brain, mood, and behavior is not supported by experience or the literature. He is a University of Florida, Gainesville, Emeritus Eminent Scholar, adjunct professor of psychiatry at Washington University in St. Louis, and a member of the clinical council of Washington University’s Public Health Institute.
A common pattern among patients with opioid use disorder (OUD) is that they undergo treatment with medication-assisted therapy (MAT), only to drop out of treatment and then repeat the treatment at a later date. That suggests that physicians should take a harder look at the limitations of MAT and other treatments, Dr. Gold said.
Although the review found some associations between neurocognitive deficits and opioid overdose, the authors point out that it is difficult to make direct comparisons because of biases and differences in methodology among the included studies. They were not able to reach conclusions about the prevalence of brain injuries following nonfatal opioid overdoses. Few included studies controlled for confounding factors that might contribute to or explain neurocognitive impairments, reported Dr. Winstanley, associate professor in the department of behavioral medicine and psychiatry at the University of West Virginia, Morgantown, and associates.
Still, distinct patterns emerged from the analysis of almost 3,500 subjects in 79 studies in 21 countries. Twenty-nine studies reported diagnoses of leukoencephalopathy, which affects white matter. Spongiform leukoencephalopathy is known to occur secondarily after exposure to a variety of toxic agents, including carbon monoxide poisoning and drugs of abuse. The damage can lead to erosion of higher cerebral function. The condition can occur from 2 to 180 days after a hypoxic brain injury, potentially complicating efforts to attribute it specifically to an opioid overdose. Amnestic syndrome was also reported in some studies. One study found that about 39% of people seeking buprenorphine treatment suffered from neurocognitive impairment.
Dr. Gold called the study’s findings novel and of public health importance. “Each overdose takes a toll on the body, and especially the brain,” he said.
Better documentation needed
The variability in symptoms, as well as their timing, present challenges to initial treatment, which often occur before a patient reaches the hospital. This is a vital window because the length of time of inadequate respiration because of opioid overdose is likely to predict the extent of brain injury. The duration of inadequate respiration may not be captured in electronic medical records, and emergency departments don’t typically collect toxicology information, which may lead health care providers to attribute neurocognitive impairments to ongoing drug use rather than an acute anoxic or hypoxic episode. Further neurocognitive damage may have a delayed onset, and better documentation of these events could help physicians determine whether those symptoms stem from the acute event.
Dr. Winstanley and associates called for more research, including prospective case-control studies to identify brain changes following opioid-related overdose.
The authors also suggested that physicians might want to consider screening patients who experience prolonged anoxia or hypoxia for neurocognitive impairments and brain injuries. Dr. Gold agreed.
“Clinicians working with OUD patients should take these data to heart and take a comprehensive history of previous overdoses, loss of consciousness, head trauma, and following up on the history with neuropsychological and other tests of brain function,” Dr. Gold said. “After an assessment, rehabilitation and treatment might then be more personalized and effective.”
Dr. Gold had no relevant financial disclosures.
Opioid overdoses usually aren’t fatal, but a new review of numerous studies, mostly case reports and case series, suggests that they can have long-lasting effects on cognition, possibly because of hypoxia resulting from respiratory depression.
Erin L. Winstanley, PhD, MA, and associates noted in the review that opioids cause about 80% of worldwide deaths from illicit drug use, and the Centers for Disease Control and Prevention’s provisional August 2021 number of more than 88,000 opioid-caused deaths in the United States is the highest ever recorded – a 27% increase over what was reported last December. That number suggests that the opioid epidemic continues to rage, but the study results also show that the neurological consequences of nonfatal overdoses are an important public health problem.
And that’s something that may be overlooked, according to Mark S. Gold, MD, who was not involved with the study and was asked to comment on the review, which was published in the Journal of Addiction Science.
“Assuming that an overdose has no effect on the brain, mood, and behavior is not supported by experience or the literature. He is a University of Florida, Gainesville, Emeritus Eminent Scholar, adjunct professor of psychiatry at Washington University in St. Louis, and a member of the clinical council of Washington University’s Public Health Institute.
A common pattern among patients with opioid use disorder (OUD) is that they undergo treatment with medication-assisted therapy (MAT), only to drop out of treatment and then repeat the treatment at a later date. That suggests that physicians should take a harder look at the limitations of MAT and other treatments, Dr. Gold said.
Although the review found some associations between neurocognitive deficits and opioid overdose, the authors point out that it is difficult to make direct comparisons because of biases and differences in methodology among the included studies. They were not able to reach conclusions about the prevalence of brain injuries following nonfatal opioid overdoses. Few included studies controlled for confounding factors that might contribute to or explain neurocognitive impairments, reported Dr. Winstanley, associate professor in the department of behavioral medicine and psychiatry at the University of West Virginia, Morgantown, and associates.
Still, distinct patterns emerged from the analysis of almost 3,500 subjects in 79 studies in 21 countries. Twenty-nine studies reported diagnoses of leukoencephalopathy, which affects white matter. Spongiform leukoencephalopathy is known to occur secondarily after exposure to a variety of toxic agents, including carbon monoxide poisoning and drugs of abuse. The damage can lead to erosion of higher cerebral function. The condition can occur from 2 to 180 days after a hypoxic brain injury, potentially complicating efforts to attribute it specifically to an opioid overdose. Amnestic syndrome was also reported in some studies. One study found that about 39% of people seeking buprenorphine treatment suffered from neurocognitive impairment.
Dr. Gold called the study’s findings novel and of public health importance. “Each overdose takes a toll on the body, and especially the brain,” he said.
Better documentation needed
The variability in symptoms, as well as their timing, present challenges to initial treatment, which often occur before a patient reaches the hospital. This is a vital window because the length of time of inadequate respiration because of opioid overdose is likely to predict the extent of brain injury. The duration of inadequate respiration may not be captured in electronic medical records, and emergency departments don’t typically collect toxicology information, which may lead health care providers to attribute neurocognitive impairments to ongoing drug use rather than an acute anoxic or hypoxic episode. Further neurocognitive damage may have a delayed onset, and better documentation of these events could help physicians determine whether those symptoms stem from the acute event.
Dr. Winstanley and associates called for more research, including prospective case-control studies to identify brain changes following opioid-related overdose.
The authors also suggested that physicians might want to consider screening patients who experience prolonged anoxia or hypoxia for neurocognitive impairments and brain injuries. Dr. Gold agreed.
“Clinicians working with OUD patients should take these data to heart and take a comprehensive history of previous overdoses, loss of consciousness, head trauma, and following up on the history with neuropsychological and other tests of brain function,” Dr. Gold said. “After an assessment, rehabilitation and treatment might then be more personalized and effective.”
Dr. Gold had no relevant financial disclosures.
Opioid overdoses usually aren’t fatal, but a new review of numerous studies, mostly case reports and case series, suggests that they can have long-lasting effects on cognition, possibly because of hypoxia resulting from respiratory depression.
Erin L. Winstanley, PhD, MA, and associates noted in the review that opioids cause about 80% of worldwide deaths from illicit drug use, and the Centers for Disease Control and Prevention’s provisional August 2021 number of more than 88,000 opioid-caused deaths in the United States is the highest ever recorded – a 27% increase over what was reported last December. That number suggests that the opioid epidemic continues to rage, but the study results also show that the neurological consequences of nonfatal overdoses are an important public health problem.
And that’s something that may be overlooked, according to Mark S. Gold, MD, who was not involved with the study and was asked to comment on the review, which was published in the Journal of Addiction Science.
“Assuming that an overdose has no effect on the brain, mood, and behavior is not supported by experience or the literature. He is a University of Florida, Gainesville, Emeritus Eminent Scholar, adjunct professor of psychiatry at Washington University in St. Louis, and a member of the clinical council of Washington University’s Public Health Institute.
A common pattern among patients with opioid use disorder (OUD) is that they undergo treatment with medication-assisted therapy (MAT), only to drop out of treatment and then repeat the treatment at a later date. That suggests that physicians should take a harder look at the limitations of MAT and other treatments, Dr. Gold said.
Although the review found some associations between neurocognitive deficits and opioid overdose, the authors point out that it is difficult to make direct comparisons because of biases and differences in methodology among the included studies. They were not able to reach conclusions about the prevalence of brain injuries following nonfatal opioid overdoses. Few included studies controlled for confounding factors that might contribute to or explain neurocognitive impairments, reported Dr. Winstanley, associate professor in the department of behavioral medicine and psychiatry at the University of West Virginia, Morgantown, and associates.
Still, distinct patterns emerged from the analysis of almost 3,500 subjects in 79 studies in 21 countries. Twenty-nine studies reported diagnoses of leukoencephalopathy, which affects white matter. Spongiform leukoencephalopathy is known to occur secondarily after exposure to a variety of toxic agents, including carbon monoxide poisoning and drugs of abuse. The damage can lead to erosion of higher cerebral function. The condition can occur from 2 to 180 days after a hypoxic brain injury, potentially complicating efforts to attribute it specifically to an opioid overdose. Amnestic syndrome was also reported in some studies. One study found that about 39% of people seeking buprenorphine treatment suffered from neurocognitive impairment.
Dr. Gold called the study’s findings novel and of public health importance. “Each overdose takes a toll on the body, and especially the brain,” he said.
Better documentation needed
The variability in symptoms, as well as their timing, present challenges to initial treatment, which often occur before a patient reaches the hospital. This is a vital window because the length of time of inadequate respiration because of opioid overdose is likely to predict the extent of brain injury. The duration of inadequate respiration may not be captured in electronic medical records, and emergency departments don’t typically collect toxicology information, which may lead health care providers to attribute neurocognitive impairments to ongoing drug use rather than an acute anoxic or hypoxic episode. Further neurocognitive damage may have a delayed onset, and better documentation of these events could help physicians determine whether those symptoms stem from the acute event.
Dr. Winstanley and associates called for more research, including prospective case-control studies to identify brain changes following opioid-related overdose.
The authors also suggested that physicians might want to consider screening patients who experience prolonged anoxia or hypoxia for neurocognitive impairments and brain injuries. Dr. Gold agreed.
“Clinicians working with OUD patients should take these data to heart and take a comprehensive history of previous overdoses, loss of consciousness, head trauma, and following up on the history with neuropsychological and other tests of brain function,” Dr. Gold said. “After an assessment, rehabilitation and treatment might then be more personalized and effective.”
Dr. Gold had no relevant financial disclosures.
FROM THE JOURNAL OF ADDICTION SCIENCE
Growing proportion of cardiac arrests in U.S. considered opioid related
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
FROM ESC 2021
EDs saw more benzodiazepine overdoses, but fewer patients overall, in 2020
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.
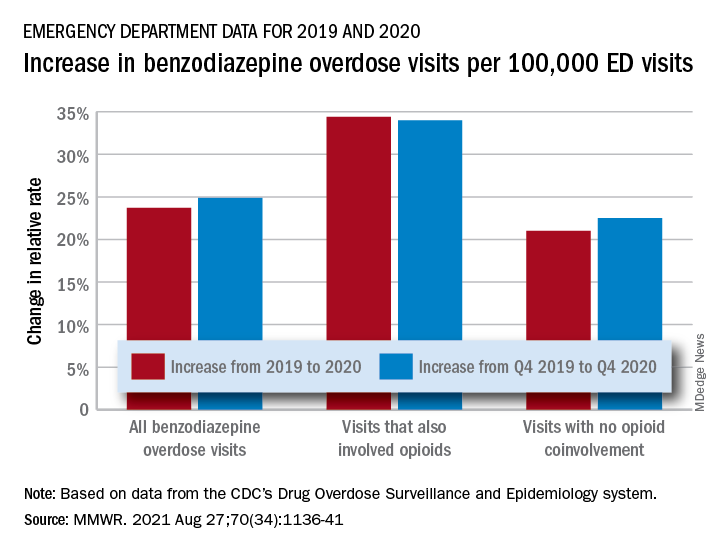
The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.

The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.

The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
FROM MMWR
Opioid prescribing laws having an impact
State laws capping initial opioid prescriptions to 7 days or less have led to a reduction in opioid prescribing, a new analysis of Medicare data shows.
While overall opioid prescribing has decreased, the reduction in states with legislation restricting opioid prescribing was “significantly greater than in states without such legislation,” study investigator Michael Brenner, MD, University of Michigan, Ann Arbor, said in an interview.
The study was published online August 9 in JAMA Internal Medicine.
Significant but limited effect
Because of rising concern around the opioid crisis, 23 states representing 43% of the U.S. population passed laws from 2016 through 2018 limiting initial opioid prescription to 7 days or less.
Using Medicare data from 2013 through 2018, Dr. Brenner and colleagues conducted a before-and-after study to assess the effect of these laws.
They found that on average, the number of days an opioid was prescribed for each Medicare beneficiary decreased by 11.6 days (from 44.2 days in 2013 to 32.7 days in 2018) in states that imposed duration limits, compared with 10.1 days in states without these laws (from 43.4 days in 2013 to 33.3 days in 2018).
Prior to the start of duration limits in 2016, days an opioid was prescribed were comparable among states.
After adjusting for state-level differences in race, urbanization, median income, tobacco and alcohol use, serious mental illness, and other factors, state laws limiting opioid prescriptions to 7 days or less were associated with a reduction in prescribing of 1.7 days per enrollee, “suggesting a significant but limited outcome” for these laws, the researchers note.
, but this was not significantly different in states with limit laws versus those without. However, state laws limiting duration led to a significant reduction in days of opioid prescribed among surgeons, dentists, pain specialists, and other specialists.
Inadequate pain control?
The researchers note the study was limited to Medicare beneficiaries; however, excess opioid prescribing is prevalent across all patient populations.
In addition, it’s not possible to tell from the data whether acute pain was adequately controlled with fewer pills.
“The question of adequacy of pain control is a crucial one that has been investigated extensively in prior work but was not possible to evaluate in this particular study,” said Dr. Brenner.
However, “ample evidence supports a role for reducing opioid prescribing and that such reduction can be achieved while ensuring that pain is adequately controlled with fewer pills,” he noted.
“A persistent misconception is that opioids are uniquely powerful and effective for controlling pain. Patients may perceive that effective analgesia is being withheld when opioids are not included in a regimen,” Dr. Brenner added.
“Yet, the evidence from meta-analyses derived from large numbers of randomized clinical trials finds that [nonsteroidal anti-inflammatory drugs] NSAIDS combined with acetaminophen provide similar or improved acute pain when compared to commonly prescribed opioid regimens, based on number-needed-to-treat analyses,” he added.
In a related editorial, Deborah Grady, MD, MPH, with University of California, San Francisco, and Mitchell H. Katz, MD, president and CEO of NYC Health + Hospitals, say the decrease in opioid prescribing with duration limits was “small but probably meaningful.”
Restricting initial prescriptions to seven or fewer days is “reasonable because patients with new onset of pain should be re-evaluated in a week if the pain continues,” they write.
However, Dr. Grady and Dr. Katz “worry” that restricting initial prescriptions to shorter periods, such as 3 or 5 days, as has occurred in six states, “may result in patients with acute pain going untreated or having to go to extraordinary effort to obtain adequate pain relief.”
In their view, the data from this study suggest that limiting initial prescriptions to seven or fewer days is “helpful, but we would not restrict any further given that we do not know how it affected patients with acute pain.”
The study had no specific funding. Dr. Brenner, Dr. Grady, and Dr. Katz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
State laws capping initial opioid prescriptions to 7 days or less have led to a reduction in opioid prescribing, a new analysis of Medicare data shows.
While overall opioid prescribing has decreased, the reduction in states with legislation restricting opioid prescribing was “significantly greater than in states without such legislation,” study investigator Michael Brenner, MD, University of Michigan, Ann Arbor, said in an interview.
The study was published online August 9 in JAMA Internal Medicine.
Significant but limited effect
Because of rising concern around the opioid crisis, 23 states representing 43% of the U.S. population passed laws from 2016 through 2018 limiting initial opioid prescription to 7 days or less.
Using Medicare data from 2013 through 2018, Dr. Brenner and colleagues conducted a before-and-after study to assess the effect of these laws.
They found that on average, the number of days an opioid was prescribed for each Medicare beneficiary decreased by 11.6 days (from 44.2 days in 2013 to 32.7 days in 2018) in states that imposed duration limits, compared with 10.1 days in states without these laws (from 43.4 days in 2013 to 33.3 days in 2018).
Prior to the start of duration limits in 2016, days an opioid was prescribed were comparable among states.
After adjusting for state-level differences in race, urbanization, median income, tobacco and alcohol use, serious mental illness, and other factors, state laws limiting opioid prescriptions to 7 days or less were associated with a reduction in prescribing of 1.7 days per enrollee, “suggesting a significant but limited outcome” for these laws, the researchers note.
, but this was not significantly different in states with limit laws versus those without. However, state laws limiting duration led to a significant reduction in days of opioid prescribed among surgeons, dentists, pain specialists, and other specialists.
Inadequate pain control?
The researchers note the study was limited to Medicare beneficiaries; however, excess opioid prescribing is prevalent across all patient populations.
In addition, it’s not possible to tell from the data whether acute pain was adequately controlled with fewer pills.
“The question of adequacy of pain control is a crucial one that has been investigated extensively in prior work but was not possible to evaluate in this particular study,” said Dr. Brenner.
However, “ample evidence supports a role for reducing opioid prescribing and that such reduction can be achieved while ensuring that pain is adequately controlled with fewer pills,” he noted.
“A persistent misconception is that opioids are uniquely powerful and effective for controlling pain. Patients may perceive that effective analgesia is being withheld when opioids are not included in a regimen,” Dr. Brenner added.
“Yet, the evidence from meta-analyses derived from large numbers of randomized clinical trials finds that [nonsteroidal anti-inflammatory drugs] NSAIDS combined with acetaminophen provide similar or improved acute pain when compared to commonly prescribed opioid regimens, based on number-needed-to-treat analyses,” he added.
In a related editorial, Deborah Grady, MD, MPH, with University of California, San Francisco, and Mitchell H. Katz, MD, president and CEO of NYC Health + Hospitals, say the decrease in opioid prescribing with duration limits was “small but probably meaningful.”
Restricting initial prescriptions to seven or fewer days is “reasonable because patients with new onset of pain should be re-evaluated in a week if the pain continues,” they write.
However, Dr. Grady and Dr. Katz “worry” that restricting initial prescriptions to shorter periods, such as 3 or 5 days, as has occurred in six states, “may result in patients with acute pain going untreated or having to go to extraordinary effort to obtain adequate pain relief.”
In their view, the data from this study suggest that limiting initial prescriptions to seven or fewer days is “helpful, but we would not restrict any further given that we do not know how it affected patients with acute pain.”
The study had no specific funding. Dr. Brenner, Dr. Grady, and Dr. Katz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
State laws capping initial opioid prescriptions to 7 days or less have led to a reduction in opioid prescribing, a new analysis of Medicare data shows.
While overall opioid prescribing has decreased, the reduction in states with legislation restricting opioid prescribing was “significantly greater than in states without such legislation,” study investigator Michael Brenner, MD, University of Michigan, Ann Arbor, said in an interview.
The study was published online August 9 in JAMA Internal Medicine.
Significant but limited effect
Because of rising concern around the opioid crisis, 23 states representing 43% of the U.S. population passed laws from 2016 through 2018 limiting initial opioid prescription to 7 days or less.
Using Medicare data from 2013 through 2018, Dr. Brenner and colleagues conducted a before-and-after study to assess the effect of these laws.
They found that on average, the number of days an opioid was prescribed for each Medicare beneficiary decreased by 11.6 days (from 44.2 days in 2013 to 32.7 days in 2018) in states that imposed duration limits, compared with 10.1 days in states without these laws (from 43.4 days in 2013 to 33.3 days in 2018).
Prior to the start of duration limits in 2016, days an opioid was prescribed were comparable among states.
After adjusting for state-level differences in race, urbanization, median income, tobacco and alcohol use, serious mental illness, and other factors, state laws limiting opioid prescriptions to 7 days or less were associated with a reduction in prescribing of 1.7 days per enrollee, “suggesting a significant but limited outcome” for these laws, the researchers note.
, but this was not significantly different in states with limit laws versus those without. However, state laws limiting duration led to a significant reduction in days of opioid prescribed among surgeons, dentists, pain specialists, and other specialists.
Inadequate pain control?
The researchers note the study was limited to Medicare beneficiaries; however, excess opioid prescribing is prevalent across all patient populations.
In addition, it’s not possible to tell from the data whether acute pain was adequately controlled with fewer pills.
“The question of adequacy of pain control is a crucial one that has been investigated extensively in prior work but was not possible to evaluate in this particular study,” said Dr. Brenner.
However, “ample evidence supports a role for reducing opioid prescribing and that such reduction can be achieved while ensuring that pain is adequately controlled with fewer pills,” he noted.
“A persistent misconception is that opioids are uniquely powerful and effective for controlling pain. Patients may perceive that effective analgesia is being withheld when opioids are not included in a regimen,” Dr. Brenner added.
“Yet, the evidence from meta-analyses derived from large numbers of randomized clinical trials finds that [nonsteroidal anti-inflammatory drugs] NSAIDS combined with acetaminophen provide similar or improved acute pain when compared to commonly prescribed opioid regimens, based on number-needed-to-treat analyses,” he added.
In a related editorial, Deborah Grady, MD, MPH, with University of California, San Francisco, and Mitchell H. Katz, MD, president and CEO of NYC Health + Hospitals, say the decrease in opioid prescribing with duration limits was “small but probably meaningful.”
Restricting initial prescriptions to seven or fewer days is “reasonable because patients with new onset of pain should be re-evaluated in a week if the pain continues,” they write.
However, Dr. Grady and Dr. Katz “worry” that restricting initial prescriptions to shorter periods, such as 3 or 5 days, as has occurred in six states, “may result in patients with acute pain going untreated or having to go to extraordinary effort to obtain adequate pain relief.”
In their view, the data from this study suggest that limiting initial prescriptions to seven or fewer days is “helpful, but we would not restrict any further given that we do not know how it affected patients with acute pain.”
The study had no specific funding. Dr. Brenner, Dr. Grady, and Dr. Katz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sharp decrease in opioid access for dying U.S. cancer patients
There has been a sharp decrease in access to opioids during the past decade, and many patients are going to emergency departments for pain treatment.
Overall, during the study period (2007-2017), there was a 34% reduction in the number of opioid prescriptions filled per patient and a 38% reduction in the total dose of opioids filled near the end of life.
There was a dramatic drop in the use of long-acting opioids, which can provide patients with more consistent pain relief and are important for managing severe cancer pain. The investigators’ results show that during the study period, the number of long-acting opioid prescriptions filled per patient fell by 50%.
“We do believe that the decline in cancer patients’ access to opioids near the end of life is likely attributable to the efforts to curtail opioid misuse,” commented lead author Andrea Enzinger, MD, a medical oncologist at Dana-Farber Cancer Institute, Boston.
The study was published online July 22 in the Journal of Clinical Oncology.
“The study provides fascinating data that support our clinical observations,” said Marcin Chwistek, MD, FAAHPM, director of the supportive oncology and palliative care program at Fox Chase Cancer Center, Philadelphia, who was asked for comment. “Primarily, we have noticed a heightened reluctance on the parts of patients with cancer, including those with advanced cancer, to take opioids in general.”
Many factors involved
The crisis of opioid misuse and abuse led to the implementation of regulations to curb inappropriate prescribing. But these restrictions on opioid prescribing may have unintended consequences for patients with advanced, incurable malignancies who are experiencing pain.
“Many but not all opioid regulations specifically exclude cancer patients,” said Dr. Enzinger. “However, the cumulative effect of these regulations may have had a chilling effect on providers’ comfort or willingness to prescribe opioids, even for cancer pain.”
She said in an interview that the prescribing of opioids has become much more difficult. Prescribers are often required to sign an opioid agreement with patients prior to providing them with opioids. Health care professionals may need to use a two-factor authentication to prescribe, and prescribers in 49 of 50 U.S. states are required to check electronic prescription drug monitoring programs prior to providing the prescription.
“After the medications are prescribed, insurance companies require prior-authorization paperwork before filling the medications, particularly for long-acting opioids or high-dose opioids,” Dr. Enzinger said. “These barriers pile up and make the whole process onerous and time consuming.”
Patient factors may also have contributed to the decline in use.
“Cancer patients are often very hesitant to use opioids to treat their pain, as they worry about becoming addicted or being labeled a ‘pill seeker,’” she explained. “Also, the added regulations, such as requirements for prior authorization paperwork, signing opioid agreements, and so on, may add to the stigma of opioid therapy and send a message to patients that these medications are inherently dangerous.”
Dr. Enzinger added that there are legitimate reasons why patients may not want to use opioids and that these should be respected. “But addiction risk should really not weigh into the decisions about pain management for patients who are dying from cancer,” she said.
Decline in opioid dose and prescriptions
Dr. Enzinger and colleagues used administrative data from the Centers for Medicare & Medicaid Services to identify 270,632 Medicare fee-for-service patients who had cancers that were associated with poor prognoses and who died from 2007 to 2017. During this period, the opioid crisis was first recognized. There followed legislative reforms and subsequent declines in population-based opioid prescribing.
Among the patients in the study, the most common cancers were lung, colorectal, pancreatic, prostate, and breast cancers; 166,962 patients (61.7%) were enrolled in hospice before death. This percentage increased from 57.1% in 2007 to 66.2% in 2017 (P for trend < .001).
From 2007 to 2017, the proportion of patients filling greater than or equal to 1 opioid prescriptions declined from 42.0% to 35.5%. The proportion declined faster from 2012-2017 than from 2007-2011.
The proportion of patients who filled prescriptions for long-acting opioids dropped from 18.1% to 11.5%. Here again, the decline was faster from 2012-2017 than from 2007-2011. Prescriptions for strong short-acting opioids declined from 31.7% to 28.5%. Prescribing was initially stable from 2007-2011 and began to decline in 2012. Conversely, prescriptions for weak short-acting opioids dropped from 8.4% to 6.5% from 2007-2011 and then stabilized after 2012.
The mean daily dose fell 24.5%, from 85.6 morphine milligram equivalents per day (MMED) to 64.6 MMED. Overall, the total amount of opioids prescribed per decedent fell 38.0%, from 1,075 MMEs per person to 666 MMEs.
At the same time, the proportion of patients who visited EDs increased 50.8%, from 13.2% to 19.9%.
Experts weigh in
Approached for an independent comment, Amit Barochia, MD, a hematologist/oncologist with Health First Medical Group, Titusville, Fla., commented that the decline could be due, in part, to greater vigilance and awareness by physicians in light of more stringent requirements and of federal and state regulations. “Some physicians are avoiding prescribing opioids due to more regulations and requirements as well, which is routing patients to the ER for pain relief,” he said.
Dr. Barochia agreed that some of the decline could be due to patient factors. “I do think that some of the patients are hesitant about considering opioid use for better pain relief, in part due to fear of addiction as well as complications arising from their use,” he said. “This is likely resulting from more awareness in the community about their adverse effects.
“That awareness could come from aggressive media coverage as well as social media,” he continued. “It is also true that there is a difficulty in getting authorization for certain opioid products, which is delaying the onset of a proper pain regimen that would help to provide adequate pain relief early on.”
For patients with advanced cancer, earlier referral to palliative care would be beneficial, Dr. Barochia pointed out, because this would allow for a more in-depth discussion about pain in addition to addressing the physical and mental symptoms associated with cancer.
Fox Chase Cancer Center’s Dr. Chwistek noted that patients and their caregivers are often apprehensive about the potential adverse effects of opioids, because they often hear about community-based opioid overdoses and are fearful of taking the medications. “Additionally, it has become increasingly challenging to fill opioid prescriptions at local pharmacies, due to quantity limitations, ubiquitous need for prior authorizations, and stigma,” he said.
The fear of addiction is often brought up by the patients during clinic visits, and insurers and pharmacies have imposed many limits on opioid prescribing. “Most of these can be overcome with prior authorizations, but not always, and prior authorizations are time consuming, confusing, and very frustrating for patients,” he said in an interview.
These findings suggest that not enough patients are getting optimal palliative care. “One of the primary tenets of palliative care is optimal symptom control, including pain,” said Dr. Chwistek. “Palliative care teams have the experience and insight needed to help patients overcome the barriers to appropriate pain control. Education, support, and advocacy are critical to ensure that patients’ pain is appropriately addressed.”
The study was funded by a grant from the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services.
A version of this article first appeared on Medscape.com.
There has been a sharp decrease in access to opioids during the past decade, and many patients are going to emergency departments for pain treatment.
Overall, during the study period (2007-2017), there was a 34% reduction in the number of opioid prescriptions filled per patient and a 38% reduction in the total dose of opioids filled near the end of life.
There was a dramatic drop in the use of long-acting opioids, which can provide patients with more consistent pain relief and are important for managing severe cancer pain. The investigators’ results show that during the study period, the number of long-acting opioid prescriptions filled per patient fell by 50%.
“We do believe that the decline in cancer patients’ access to opioids near the end of life is likely attributable to the efforts to curtail opioid misuse,” commented lead author Andrea Enzinger, MD, a medical oncologist at Dana-Farber Cancer Institute, Boston.
The study was published online July 22 in the Journal of Clinical Oncology.
“The study provides fascinating data that support our clinical observations,” said Marcin Chwistek, MD, FAAHPM, director of the supportive oncology and palliative care program at Fox Chase Cancer Center, Philadelphia, who was asked for comment. “Primarily, we have noticed a heightened reluctance on the parts of patients with cancer, including those with advanced cancer, to take opioids in general.”
Many factors involved
The crisis of opioid misuse and abuse led to the implementation of regulations to curb inappropriate prescribing. But these restrictions on opioid prescribing may have unintended consequences for patients with advanced, incurable malignancies who are experiencing pain.
“Many but not all opioid regulations specifically exclude cancer patients,” said Dr. Enzinger. “However, the cumulative effect of these regulations may have had a chilling effect on providers’ comfort or willingness to prescribe opioids, even for cancer pain.”
She said in an interview that the prescribing of opioids has become much more difficult. Prescribers are often required to sign an opioid agreement with patients prior to providing them with opioids. Health care professionals may need to use a two-factor authentication to prescribe, and prescribers in 49 of 50 U.S. states are required to check electronic prescription drug monitoring programs prior to providing the prescription.
“After the medications are prescribed, insurance companies require prior-authorization paperwork before filling the medications, particularly for long-acting opioids or high-dose opioids,” Dr. Enzinger said. “These barriers pile up and make the whole process onerous and time consuming.”
Patient factors may also have contributed to the decline in use.
“Cancer patients are often very hesitant to use opioids to treat their pain, as they worry about becoming addicted or being labeled a ‘pill seeker,’” she explained. “Also, the added regulations, such as requirements for prior authorization paperwork, signing opioid agreements, and so on, may add to the stigma of opioid therapy and send a message to patients that these medications are inherently dangerous.”
Dr. Enzinger added that there are legitimate reasons why patients may not want to use opioids and that these should be respected. “But addiction risk should really not weigh into the decisions about pain management for patients who are dying from cancer,” she said.
Decline in opioid dose and prescriptions
Dr. Enzinger and colleagues used administrative data from the Centers for Medicare & Medicaid Services to identify 270,632 Medicare fee-for-service patients who had cancers that were associated with poor prognoses and who died from 2007 to 2017. During this period, the opioid crisis was first recognized. There followed legislative reforms and subsequent declines in population-based opioid prescribing.
Among the patients in the study, the most common cancers were lung, colorectal, pancreatic, prostate, and breast cancers; 166,962 patients (61.7%) were enrolled in hospice before death. This percentage increased from 57.1% in 2007 to 66.2% in 2017 (P for trend < .001).
From 2007 to 2017, the proportion of patients filling greater than or equal to 1 opioid prescriptions declined from 42.0% to 35.5%. The proportion declined faster from 2012-2017 than from 2007-2011.
The proportion of patients who filled prescriptions for long-acting opioids dropped from 18.1% to 11.5%. Here again, the decline was faster from 2012-2017 than from 2007-2011. Prescriptions for strong short-acting opioids declined from 31.7% to 28.5%. Prescribing was initially stable from 2007-2011 and began to decline in 2012. Conversely, prescriptions for weak short-acting opioids dropped from 8.4% to 6.5% from 2007-2011 and then stabilized after 2012.
The mean daily dose fell 24.5%, from 85.6 morphine milligram equivalents per day (MMED) to 64.6 MMED. Overall, the total amount of opioids prescribed per decedent fell 38.0%, from 1,075 MMEs per person to 666 MMEs.
At the same time, the proportion of patients who visited EDs increased 50.8%, from 13.2% to 19.9%.
Experts weigh in
Approached for an independent comment, Amit Barochia, MD, a hematologist/oncologist with Health First Medical Group, Titusville, Fla., commented that the decline could be due, in part, to greater vigilance and awareness by physicians in light of more stringent requirements and of federal and state regulations. “Some physicians are avoiding prescribing opioids due to more regulations and requirements as well, which is routing patients to the ER for pain relief,” he said.
Dr. Barochia agreed that some of the decline could be due to patient factors. “I do think that some of the patients are hesitant about considering opioid use for better pain relief, in part due to fear of addiction as well as complications arising from their use,” he said. “This is likely resulting from more awareness in the community about their adverse effects.
“That awareness could come from aggressive media coverage as well as social media,” he continued. “It is also true that there is a difficulty in getting authorization for certain opioid products, which is delaying the onset of a proper pain regimen that would help to provide adequate pain relief early on.”
For patients with advanced cancer, earlier referral to palliative care would be beneficial, Dr. Barochia pointed out, because this would allow for a more in-depth discussion about pain in addition to addressing the physical and mental symptoms associated with cancer.
Fox Chase Cancer Center’s Dr. Chwistek noted that patients and their caregivers are often apprehensive about the potential adverse effects of opioids, because they often hear about community-based opioid overdoses and are fearful of taking the medications. “Additionally, it has become increasingly challenging to fill opioid prescriptions at local pharmacies, due to quantity limitations, ubiquitous need for prior authorizations, and stigma,” he said.
The fear of addiction is often brought up by the patients during clinic visits, and insurers and pharmacies have imposed many limits on opioid prescribing. “Most of these can be overcome with prior authorizations, but not always, and prior authorizations are time consuming, confusing, and very frustrating for patients,” he said in an interview.
These findings suggest that not enough patients are getting optimal palliative care. “One of the primary tenets of palliative care is optimal symptom control, including pain,” said Dr. Chwistek. “Palliative care teams have the experience and insight needed to help patients overcome the barriers to appropriate pain control. Education, support, and advocacy are critical to ensure that patients’ pain is appropriately addressed.”
The study was funded by a grant from the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services.
A version of this article first appeared on Medscape.com.
There has been a sharp decrease in access to opioids during the past decade, and many patients are going to emergency departments for pain treatment.
Overall, during the study period (2007-2017), there was a 34% reduction in the number of opioid prescriptions filled per patient and a 38% reduction in the total dose of opioids filled near the end of life.
There was a dramatic drop in the use of long-acting opioids, which can provide patients with more consistent pain relief and are important for managing severe cancer pain. The investigators’ results show that during the study period, the number of long-acting opioid prescriptions filled per patient fell by 50%.
“We do believe that the decline in cancer patients’ access to opioids near the end of life is likely attributable to the efforts to curtail opioid misuse,” commented lead author Andrea Enzinger, MD, a medical oncologist at Dana-Farber Cancer Institute, Boston.
The study was published online July 22 in the Journal of Clinical Oncology.
“The study provides fascinating data that support our clinical observations,” said Marcin Chwistek, MD, FAAHPM, director of the supportive oncology and palliative care program at Fox Chase Cancer Center, Philadelphia, who was asked for comment. “Primarily, we have noticed a heightened reluctance on the parts of patients with cancer, including those with advanced cancer, to take opioids in general.”
Many factors involved
The crisis of opioid misuse and abuse led to the implementation of regulations to curb inappropriate prescribing. But these restrictions on opioid prescribing may have unintended consequences for patients with advanced, incurable malignancies who are experiencing pain.
“Many but not all opioid regulations specifically exclude cancer patients,” said Dr. Enzinger. “However, the cumulative effect of these regulations may have had a chilling effect on providers’ comfort or willingness to prescribe opioids, even for cancer pain.”
She said in an interview that the prescribing of opioids has become much more difficult. Prescribers are often required to sign an opioid agreement with patients prior to providing them with opioids. Health care professionals may need to use a two-factor authentication to prescribe, and prescribers in 49 of 50 U.S. states are required to check electronic prescription drug monitoring programs prior to providing the prescription.
“After the medications are prescribed, insurance companies require prior-authorization paperwork before filling the medications, particularly for long-acting opioids or high-dose opioids,” Dr. Enzinger said. “These barriers pile up and make the whole process onerous and time consuming.”
Patient factors may also have contributed to the decline in use.
“Cancer patients are often very hesitant to use opioids to treat their pain, as they worry about becoming addicted or being labeled a ‘pill seeker,’” she explained. “Also, the added regulations, such as requirements for prior authorization paperwork, signing opioid agreements, and so on, may add to the stigma of opioid therapy and send a message to patients that these medications are inherently dangerous.”
Dr. Enzinger added that there are legitimate reasons why patients may not want to use opioids and that these should be respected. “But addiction risk should really not weigh into the decisions about pain management for patients who are dying from cancer,” she said.
Decline in opioid dose and prescriptions
Dr. Enzinger and colleagues used administrative data from the Centers for Medicare & Medicaid Services to identify 270,632 Medicare fee-for-service patients who had cancers that were associated with poor prognoses and who died from 2007 to 2017. During this period, the opioid crisis was first recognized. There followed legislative reforms and subsequent declines in population-based opioid prescribing.
Among the patients in the study, the most common cancers were lung, colorectal, pancreatic, prostate, and breast cancers; 166,962 patients (61.7%) were enrolled in hospice before death. This percentage increased from 57.1% in 2007 to 66.2% in 2017 (P for trend < .001).
From 2007 to 2017, the proportion of patients filling greater than or equal to 1 opioid prescriptions declined from 42.0% to 35.5%. The proportion declined faster from 2012-2017 than from 2007-2011.
The proportion of patients who filled prescriptions for long-acting opioids dropped from 18.1% to 11.5%. Here again, the decline was faster from 2012-2017 than from 2007-2011. Prescriptions for strong short-acting opioids declined from 31.7% to 28.5%. Prescribing was initially stable from 2007-2011 and began to decline in 2012. Conversely, prescriptions for weak short-acting opioids dropped from 8.4% to 6.5% from 2007-2011 and then stabilized after 2012.
The mean daily dose fell 24.5%, from 85.6 morphine milligram equivalents per day (MMED) to 64.6 MMED. Overall, the total amount of opioids prescribed per decedent fell 38.0%, from 1,075 MMEs per person to 666 MMEs.
At the same time, the proportion of patients who visited EDs increased 50.8%, from 13.2% to 19.9%.
Experts weigh in
Approached for an independent comment, Amit Barochia, MD, a hematologist/oncologist with Health First Medical Group, Titusville, Fla., commented that the decline could be due, in part, to greater vigilance and awareness by physicians in light of more stringent requirements and of federal and state regulations. “Some physicians are avoiding prescribing opioids due to more regulations and requirements as well, which is routing patients to the ER for pain relief,” he said.
Dr. Barochia agreed that some of the decline could be due to patient factors. “I do think that some of the patients are hesitant about considering opioid use for better pain relief, in part due to fear of addiction as well as complications arising from their use,” he said. “This is likely resulting from more awareness in the community about their adverse effects.
“That awareness could come from aggressive media coverage as well as social media,” he continued. “It is also true that there is a difficulty in getting authorization for certain opioid products, which is delaying the onset of a proper pain regimen that would help to provide adequate pain relief early on.”
For patients with advanced cancer, earlier referral to palliative care would be beneficial, Dr. Barochia pointed out, because this would allow for a more in-depth discussion about pain in addition to addressing the physical and mental symptoms associated with cancer.
Fox Chase Cancer Center’s Dr. Chwistek noted that patients and their caregivers are often apprehensive about the potential adverse effects of opioids, because they often hear about community-based opioid overdoses and are fearful of taking the medications. “Additionally, it has become increasingly challenging to fill opioid prescriptions at local pharmacies, due to quantity limitations, ubiquitous need for prior authorizations, and stigma,” he said.
The fear of addiction is often brought up by the patients during clinic visits, and insurers and pharmacies have imposed many limits on opioid prescribing. “Most of these can be overcome with prior authorizations, but not always, and prior authorizations are time consuming, confusing, and very frustrating for patients,” he said in an interview.
These findings suggest that not enough patients are getting optimal palliative care. “One of the primary tenets of palliative care is optimal symptom control, including pain,” said Dr. Chwistek. “Palliative care teams have the experience and insight needed to help patients overcome the barriers to appropriate pain control. Education, support, and advocacy are critical to ensure that patients’ pain is appropriately addressed.”
The study was funded by a grant from the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services.
A version of this article first appeared on Medscape.com.
Record number of U.S. drug overdoses in 2020
More Americans died from drug overdoses in 2020 than in any other year, the CDC said July 14.
, according to the provisional data the National Center for Health Statistics reported.
The spikes are largely attributed to the rise in use of fentanyl and other synthetic opioids.
The Washington Post reported that more than 69,000 overdose deaths involved opioids, up from 50,963 in 2019.
Amid the crush of overdoses, the White House announced that President Joe Biden has nominated Rahul Gupta, MD, to lead the White House Office of National Drug Control Policy.
Dr. Gupta is a former health commissioner of West Virginia, and is chief medical and health officer for the March of Dimes.
“Dr. Gupta led efforts in West Virginia to address the opioid crisis, gaining national prominence as a leader in tackling this issue,” March of Dimes President and CEO Stacey Stewart said in a statement. “At March of Dimes, he has advocated for policies and programs to prevent and treat substance use, with a focus on the safety and care of pregnant women and infants.”
Healthday contributed to this report. A version of this article first appeared on WebMD.com.
More Americans died from drug overdoses in 2020 than in any other year, the CDC said July 14.
, according to the provisional data the National Center for Health Statistics reported.
The spikes are largely attributed to the rise in use of fentanyl and other synthetic opioids.
The Washington Post reported that more than 69,000 overdose deaths involved opioids, up from 50,963 in 2019.
Amid the crush of overdoses, the White House announced that President Joe Biden has nominated Rahul Gupta, MD, to lead the White House Office of National Drug Control Policy.
Dr. Gupta is a former health commissioner of West Virginia, and is chief medical and health officer for the March of Dimes.
“Dr. Gupta led efforts in West Virginia to address the opioid crisis, gaining national prominence as a leader in tackling this issue,” March of Dimes President and CEO Stacey Stewart said in a statement. “At March of Dimes, he has advocated for policies and programs to prevent and treat substance use, with a focus on the safety and care of pregnant women and infants.”
Healthday contributed to this report. A version of this article first appeared on WebMD.com.
More Americans died from drug overdoses in 2020 than in any other year, the CDC said July 14.
, according to the provisional data the National Center for Health Statistics reported.
The spikes are largely attributed to the rise in use of fentanyl and other synthetic opioids.
The Washington Post reported that more than 69,000 overdose deaths involved opioids, up from 50,963 in 2019.
Amid the crush of overdoses, the White House announced that President Joe Biden has nominated Rahul Gupta, MD, to lead the White House Office of National Drug Control Policy.
Dr. Gupta is a former health commissioner of West Virginia, and is chief medical and health officer for the March of Dimes.
“Dr. Gupta led efforts in West Virginia to address the opioid crisis, gaining national prominence as a leader in tackling this issue,” March of Dimes President and CEO Stacey Stewart said in a statement. “At March of Dimes, he has advocated for policies and programs to prevent and treat substance use, with a focus on the safety and care of pregnant women and infants.”
Healthday contributed to this report. A version of this article first appeared on WebMD.com.
Opioid addiction meds may curb growing problem of kratom dependence
Medications typically used to treat opioid use disorder (OUD) may* also be effective for the growing public health problem of kratom addiction, new research shows.
Results of a comprehensive literature review and an expert survey suggest buprenorphine, naltrexone, and methadone may be effective for patients seeking help for kratom addiction, and if further research confirms these findings, the indication for OUD medications could potentially be expanded to include moderate-to-severe kratom addiction, study investigator Saeed Ahmed, MD, medical director of West Ridge Center at Rutland Regional Medical Center, Rutland, Vermont, said in an interview.
Dr. Ahmed, who practices general psychiatry and addiction psychiatry, presented the findings at the virtual American Psychiatric Association 2021 Annual Meeting.
Emerging public health problem
Kratom can be ingested in pill or capsule form or as an extract. Its leaves can be chewed or dried and powdered to make a tea. It can also be incorporated into topical creams, balms, or tinctures.
Products containing the substance are “readily available and legal for sale in many states and cities in the U.S.,” said Dr. Ahmed, adding that it can be purchased online or at local smoke shops and is increasingly used by individuals to self-treat a variety of conditions including pain, anxiety, and mood conditions and as an opioid substitute.
As reported by this news organization, a 2018 analysis conducted by the U.S. Food and Drug Administration showed kratom is, in fact, an opioid, a finding that garnered significant push-back from the American Kratom Association.
Kratom addiction is an “emerging public health problem,” said Dr. Ahmed, adding that in recent years the number of calls to poison control centers across the country has increased 52-fold – from one per month to two per day. He believes misinformation through social media has helped fuel its use.
Kratom use, the investigators note, can lead to muscle pain, weight loss, insomnia, hallucinations and, in some cases (particularly when combined with synthetic opioids or benzodiazepines), it can lead to respiratory depression, seizures, coma, and death.
In addition,
To investigate, the researchers conducted a systematic literature search for cases pertaining to maintenance treatment for kratom dependence. They also tapped into case reports and scientific posters from reliable online sources and conference proceedings. In addition, they conducted a survey of members from the American Society of Addiction Medicine (ASAM).
The researchers found 14 reports of long-term management of kratom addiction, half of which did not involve an OUD. It’s important to exclude OUDs to avoid possible confounding.
In most cases, buprenorphine was used, but in a few cases naltrexone or methadone were prescribed. All cases had a favorable outcome. Dr. Ahmed noted that buprenorphine maintenance doses appear to be lower than those required to effectively treat OUD.
With a response rate of 11.5% (82 respondents) the ASAM survey results showed 82.6% of respondents (n = 57) had experience managing KUD, including 27.5% (n = 19) who had kratom addiction only. Of these, 89.5% (n = 17-19), used buprenorphine to manage KUD and of these, 6 combined it with talk therapy.
Dr. Ahmed cautioned that the included cases varied significantly in terms of relevant data, including kratom dose and route of administration, toxicology screening used to monitor abstinence, and duration of maintenance follow-up.
Despite these limitations, the review and survey underscore the importance of including moderate to severe kratom dependence as an indication for current OUD medications, the researchers note.
Including kratom addiction as an indication for these medications is important, especially for patients who are heavily addicted, to meet DSM-5 diagnostic criteria for moderate or severe SUD, they add.
In addition, the researchers recommend that clinicians consider referring patients with moderate to severe kratom dependence for counseling or enrollment in 12-step addiction treatment programs.
A separate diagnosis?
Dr. Ahmed said he would like to see kratom dependence included in the DSM-5 as a separate entity because it is a botanical with properties similar to, but different from, traditional opioids.
“This will not only help to better inform clinicians about a diagnostic criteria encompassing problematic use and facilitate screening, but it will also pave the way for treatments to be explored for this diagnosable condition,” he said. Dr. Ahmed pointed to a review published in the Wisconsin Medical Journal earlier this year that explored potential treatments for kratom dependence.
Commenting on the study for an interview, Petros Levounis, MD, professor and chair, department of psychiatry, and associate dean for professional development, Rutgers New Jersey Medical School, Newark, said the authors “have done a great job reviewing the literature and asking experts” about kratom addiction treatment.
“The punchline of their study is that kratom behaves very much like an opioid and is treated like an opioid.”
Dr. Levounis noted that kratom dependence is so new that experts don’t know much about it. However, he added, emerging evidence suggests that kratom “should be considered an opioid more than anything else,” but specified that he does not believe it warrants its own diagnosis.
He noted that individual opioids don’t have their own diagnostic category and that opioid use disorder is an umbrella term that covers all of these drugs.
Dr. Ahmed and Dr. Levounis have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*Updated 5/18/2021
Medications typically used to treat opioid use disorder (OUD) may* also be effective for the growing public health problem of kratom addiction, new research shows.
Results of a comprehensive literature review and an expert survey suggest buprenorphine, naltrexone, and methadone may be effective for patients seeking help for kratom addiction, and if further research confirms these findings, the indication for OUD medications could potentially be expanded to include moderate-to-severe kratom addiction, study investigator Saeed Ahmed, MD, medical director of West Ridge Center at Rutland Regional Medical Center, Rutland, Vermont, said in an interview.
Dr. Ahmed, who practices general psychiatry and addiction psychiatry, presented the findings at the virtual American Psychiatric Association 2021 Annual Meeting.
Emerging public health problem
Kratom can be ingested in pill or capsule form or as an extract. Its leaves can be chewed or dried and powdered to make a tea. It can also be incorporated into topical creams, balms, or tinctures.
Products containing the substance are “readily available and legal for sale in many states and cities in the U.S.,” said Dr. Ahmed, adding that it can be purchased online or at local smoke shops and is increasingly used by individuals to self-treat a variety of conditions including pain, anxiety, and mood conditions and as an opioid substitute.
As reported by this news organization, a 2018 analysis conducted by the U.S. Food and Drug Administration showed kratom is, in fact, an opioid, a finding that garnered significant push-back from the American Kratom Association.
Kratom addiction is an “emerging public health problem,” said Dr. Ahmed, adding that in recent years the number of calls to poison control centers across the country has increased 52-fold – from one per month to two per day. He believes misinformation through social media has helped fuel its use.
Kratom use, the investigators note, can lead to muscle pain, weight loss, insomnia, hallucinations and, in some cases (particularly when combined with synthetic opioids or benzodiazepines), it can lead to respiratory depression, seizures, coma, and death.
In addition,
To investigate, the researchers conducted a systematic literature search for cases pertaining to maintenance treatment for kratom dependence. They also tapped into case reports and scientific posters from reliable online sources and conference proceedings. In addition, they conducted a survey of members from the American Society of Addiction Medicine (ASAM).
The researchers found 14 reports of long-term management of kratom addiction, half of which did not involve an OUD. It’s important to exclude OUDs to avoid possible confounding.
In most cases, buprenorphine was used, but in a few cases naltrexone or methadone were prescribed. All cases had a favorable outcome. Dr. Ahmed noted that buprenorphine maintenance doses appear to be lower than those required to effectively treat OUD.
With a response rate of 11.5% (82 respondents) the ASAM survey results showed 82.6% of respondents (n = 57) had experience managing KUD, including 27.5% (n = 19) who had kratom addiction only. Of these, 89.5% (n = 17-19), used buprenorphine to manage KUD and of these, 6 combined it with talk therapy.
Dr. Ahmed cautioned that the included cases varied significantly in terms of relevant data, including kratom dose and route of administration, toxicology screening used to monitor abstinence, and duration of maintenance follow-up.
Despite these limitations, the review and survey underscore the importance of including moderate to severe kratom dependence as an indication for current OUD medications, the researchers note.
Including kratom addiction as an indication for these medications is important, especially for patients who are heavily addicted, to meet DSM-5 diagnostic criteria for moderate or severe SUD, they add.
In addition, the researchers recommend that clinicians consider referring patients with moderate to severe kratom dependence for counseling or enrollment in 12-step addiction treatment programs.
A separate diagnosis?
Dr. Ahmed said he would like to see kratom dependence included in the DSM-5 as a separate entity because it is a botanical with properties similar to, but different from, traditional opioids.
“This will not only help to better inform clinicians about a diagnostic criteria encompassing problematic use and facilitate screening, but it will also pave the way for treatments to be explored for this diagnosable condition,” he said. Dr. Ahmed pointed to a review published in the Wisconsin Medical Journal earlier this year that explored potential treatments for kratom dependence.
Commenting on the study for an interview, Petros Levounis, MD, professor and chair, department of psychiatry, and associate dean for professional development, Rutgers New Jersey Medical School, Newark, said the authors “have done a great job reviewing the literature and asking experts” about kratom addiction treatment.
“The punchline of their study is that kratom behaves very much like an opioid and is treated like an opioid.”
Dr. Levounis noted that kratom dependence is so new that experts don’t know much about it. However, he added, emerging evidence suggests that kratom “should be considered an opioid more than anything else,” but specified that he does not believe it warrants its own diagnosis.
He noted that individual opioids don’t have their own diagnostic category and that opioid use disorder is an umbrella term that covers all of these drugs.
Dr. Ahmed and Dr. Levounis have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*Updated 5/18/2021
Medications typically used to treat opioid use disorder (OUD) may* also be effective for the growing public health problem of kratom addiction, new research shows.
Results of a comprehensive literature review and an expert survey suggest buprenorphine, naltrexone, and methadone may be effective for patients seeking help for kratom addiction, and if further research confirms these findings, the indication for OUD medications could potentially be expanded to include moderate-to-severe kratom addiction, study investigator Saeed Ahmed, MD, medical director of West Ridge Center at Rutland Regional Medical Center, Rutland, Vermont, said in an interview.
Dr. Ahmed, who practices general psychiatry and addiction psychiatry, presented the findings at the virtual American Psychiatric Association 2021 Annual Meeting.
Emerging public health problem
Kratom can be ingested in pill or capsule form or as an extract. Its leaves can be chewed or dried and powdered to make a tea. It can also be incorporated into topical creams, balms, or tinctures.
Products containing the substance are “readily available and legal for sale in many states and cities in the U.S.,” said Dr. Ahmed, adding that it can be purchased online or at local smoke shops and is increasingly used by individuals to self-treat a variety of conditions including pain, anxiety, and mood conditions and as an opioid substitute.
As reported by this news organization, a 2018 analysis conducted by the U.S. Food and Drug Administration showed kratom is, in fact, an opioid, a finding that garnered significant push-back from the American Kratom Association.
Kratom addiction is an “emerging public health problem,” said Dr. Ahmed, adding that in recent years the number of calls to poison control centers across the country has increased 52-fold – from one per month to two per day. He believes misinformation through social media has helped fuel its use.
Kratom use, the investigators note, can lead to muscle pain, weight loss, insomnia, hallucinations and, in some cases (particularly when combined with synthetic opioids or benzodiazepines), it can lead to respiratory depression, seizures, coma, and death.
In addition,
To investigate, the researchers conducted a systematic literature search for cases pertaining to maintenance treatment for kratom dependence. They also tapped into case reports and scientific posters from reliable online sources and conference proceedings. In addition, they conducted a survey of members from the American Society of Addiction Medicine (ASAM).
The researchers found 14 reports of long-term management of kratom addiction, half of which did not involve an OUD. It’s important to exclude OUDs to avoid possible confounding.
In most cases, buprenorphine was used, but in a few cases naltrexone or methadone were prescribed. All cases had a favorable outcome. Dr. Ahmed noted that buprenorphine maintenance doses appear to be lower than those required to effectively treat OUD.
With a response rate of 11.5% (82 respondents) the ASAM survey results showed 82.6% of respondents (n = 57) had experience managing KUD, including 27.5% (n = 19) who had kratom addiction only. Of these, 89.5% (n = 17-19), used buprenorphine to manage KUD and of these, 6 combined it with talk therapy.
Dr. Ahmed cautioned that the included cases varied significantly in terms of relevant data, including kratom dose and route of administration, toxicology screening used to monitor abstinence, and duration of maintenance follow-up.
Despite these limitations, the review and survey underscore the importance of including moderate to severe kratom dependence as an indication for current OUD medications, the researchers note.
Including kratom addiction as an indication for these medications is important, especially for patients who are heavily addicted, to meet DSM-5 diagnostic criteria for moderate or severe SUD, they add.
In addition, the researchers recommend that clinicians consider referring patients with moderate to severe kratom dependence for counseling or enrollment in 12-step addiction treatment programs.
A separate diagnosis?
Dr. Ahmed said he would like to see kratom dependence included in the DSM-5 as a separate entity because it is a botanical with properties similar to, but different from, traditional opioids.
“This will not only help to better inform clinicians about a diagnostic criteria encompassing problematic use and facilitate screening, but it will also pave the way for treatments to be explored for this diagnosable condition,” he said. Dr. Ahmed pointed to a review published in the Wisconsin Medical Journal earlier this year that explored potential treatments for kratom dependence.
Commenting on the study for an interview, Petros Levounis, MD, professor and chair, department of psychiatry, and associate dean for professional development, Rutgers New Jersey Medical School, Newark, said the authors “have done a great job reviewing the literature and asking experts” about kratom addiction treatment.
“The punchline of their study is that kratom behaves very much like an opioid and is treated like an opioid.”
Dr. Levounis noted that kratom dependence is so new that experts don’t know much about it. However, he added, emerging evidence suggests that kratom “should be considered an opioid more than anything else,” but specified that he does not believe it warrants its own diagnosis.
He noted that individual opioids don’t have their own diagnostic category and that opioid use disorder is an umbrella term that covers all of these drugs.
Dr. Ahmed and Dr. Levounis have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*Updated 5/18/2021
Adulterants in street drugs could increase susceptibility to COVID
The composition of street drugs like heroin and cocaine are changing. According to a new analysis, almost all contain at least one toxic adulterant, and many contain a plethora. Most adulterants have pharmacologic activities and toxicities. Their presence has added impact in the context of the COVID-19 pandemic, since some may cause a drastic drop in white blood cells that could leave drug users more vulnerable to infection.
“It’s remarkable that we just forgot to notice, in the horrendous transition from prescription opioid epidemic to the illicit opioid and psychostimulant epidemics, that we would have to pay special attention to what the medications are in the drugs that the person was exposed to – and for how long,” said Mark S. Gold, MD, a coauthor of the review.
The analysis showed that adulterants include new psychoactive substances, industrial compounds, fungicides, veterinary medications, and various impurities. In addition, other various medications are being found in street drugs, such as antipsychotics, antidepressants, anxiolytics, antihistamines, anthelmintics, anesthetics, anti-inflammatory agents, antipyretics, analgesics, antispasmodics, antiarrhythmics, antimalarials, bronchodilators, decongestants, expectorants, muscle relaxers, natural/synthetic hallucinogens, and sedatives.
Illicit drugs are by nature manufactured without Food and Drug Administration oversight, and it is becoming increasingly common that substances like leftover medicines and other active drugs are added to illicit drug batches to add weight, said Dr. Gold, a professor at Washington University,St. Louis. The study appeared in Current Psychopharmacology.
Effects of adulterants ‘terrifying’
The findings of adulterants and their consequences are concerning, according to Jean Lud Cadet, MD, who was asked to comment on the findings. “The blood dysplasia, the pulmonary problems that some of those adulterants can cause – it’s actually terrifying, to put it bluntly,” said Dr. Cadet, who is a senior investigator and chief of the Molecular Neuropsychiatry Research Branch at the National Institute on Drug Abuse.
Before 2000, street drugs were generally diluted with comparatively benign substances such as caffeine, sugars, or lidocaine. Drugs like phenacetin, levamisole, acetaminophen, and diltiazem began to appear in heroin and cocaine in the late 1990s, and by 2010, more powerful adulterants like fentanyl, ketamine, and quetiapine became common.
In 2015, the U.S. Department of State partnered with the Colombo Plan, an international organization based in Sri Lanka, to use field spectroscopy to detect toxins directly in cocaine and heroin samples found in Argentina, Brazil, Ecuador, Peru, Sri Lanka, Thailand, Honduras, Guatemala, Mexico, Colombia, and South Africa. They found a range of adulterants such as aminopyrine, diltiazem, metamizole, levamisole, and phenacetin.
A similar project with 431 heroin and cocaine samples from Vermont and Kentucky found that 69% of samples had five or more controlled drugs, toxic adulterants, or impurities. About 15% had nine or more, and 95% of samples had at least one toxic adulterant.
In the midst of the COVID-19 pandemic, these adulterants take on even greater significance. Individuals with substance use disorders often have other health conditions that can make them more vulnerable to viral infections, and this could be exacerbated by the effects of adulterants on white blood cells or other systems. The pandemic has also had an indirect effect by causing a shortage of street drugs. During production shortages, traffickers might boost potency by adding more cutting agents and adulterants. As a result, COVID-19 and opioid addiction tend to reinforce each other.
“The clinical message would be that our [substance use] patients will contract infectious disease and need to be prioritized for [COVID-19] vaccination,” said Dr. Gold.
The findings came as a surprise to Dr. Cadet, and that illustrates a need to publicize the presence of adulterants in street drugs.
“If I wasn’t aware of many of these, then the general public is also not going to be aware of them,” Dr. Cadet said. “Scientists, including myself, and government agencies need to do a better job [of communicating this issue].”
The study references individuals with substance use disorder, but Dr. Cadet cautioned that anyone who uses street drugs, even once or twice, could be a victim of adulterants. “You don’t need to have met criteria for diagnosis in order to suffer the consequences.”
The study had no funding. Dr. Gold and Dr. Cadet have no relevant financial disclosures.
The composition of street drugs like heroin and cocaine are changing. According to a new analysis, almost all contain at least one toxic adulterant, and many contain a plethora. Most adulterants have pharmacologic activities and toxicities. Their presence has added impact in the context of the COVID-19 pandemic, since some may cause a drastic drop in white blood cells that could leave drug users more vulnerable to infection.
“It’s remarkable that we just forgot to notice, in the horrendous transition from prescription opioid epidemic to the illicit opioid and psychostimulant epidemics, that we would have to pay special attention to what the medications are in the drugs that the person was exposed to – and for how long,” said Mark S. Gold, MD, a coauthor of the review.
The analysis showed that adulterants include new psychoactive substances, industrial compounds, fungicides, veterinary medications, and various impurities. In addition, other various medications are being found in street drugs, such as antipsychotics, antidepressants, anxiolytics, antihistamines, anthelmintics, anesthetics, anti-inflammatory agents, antipyretics, analgesics, antispasmodics, antiarrhythmics, antimalarials, bronchodilators, decongestants, expectorants, muscle relaxers, natural/synthetic hallucinogens, and sedatives.
Illicit drugs are by nature manufactured without Food and Drug Administration oversight, and it is becoming increasingly common that substances like leftover medicines and other active drugs are added to illicit drug batches to add weight, said Dr. Gold, a professor at Washington University,St. Louis. The study appeared in Current Psychopharmacology.
Effects of adulterants ‘terrifying’
The findings of adulterants and their consequences are concerning, according to Jean Lud Cadet, MD, who was asked to comment on the findings. “The blood dysplasia, the pulmonary problems that some of those adulterants can cause – it’s actually terrifying, to put it bluntly,” said Dr. Cadet, who is a senior investigator and chief of the Molecular Neuropsychiatry Research Branch at the National Institute on Drug Abuse.
Before 2000, street drugs were generally diluted with comparatively benign substances such as caffeine, sugars, or lidocaine. Drugs like phenacetin, levamisole, acetaminophen, and diltiazem began to appear in heroin and cocaine in the late 1990s, and by 2010, more powerful adulterants like fentanyl, ketamine, and quetiapine became common.
In 2015, the U.S. Department of State partnered with the Colombo Plan, an international organization based in Sri Lanka, to use field spectroscopy to detect toxins directly in cocaine and heroin samples found in Argentina, Brazil, Ecuador, Peru, Sri Lanka, Thailand, Honduras, Guatemala, Mexico, Colombia, and South Africa. They found a range of adulterants such as aminopyrine, diltiazem, metamizole, levamisole, and phenacetin.
A similar project with 431 heroin and cocaine samples from Vermont and Kentucky found that 69% of samples had five or more controlled drugs, toxic adulterants, or impurities. About 15% had nine or more, and 95% of samples had at least one toxic adulterant.
In the midst of the COVID-19 pandemic, these adulterants take on even greater significance. Individuals with substance use disorders often have other health conditions that can make them more vulnerable to viral infections, and this could be exacerbated by the effects of adulterants on white blood cells or other systems. The pandemic has also had an indirect effect by causing a shortage of street drugs. During production shortages, traffickers might boost potency by adding more cutting agents and adulterants. As a result, COVID-19 and opioid addiction tend to reinforce each other.
“The clinical message would be that our [substance use] patients will contract infectious disease and need to be prioritized for [COVID-19] vaccination,” said Dr. Gold.
The findings came as a surprise to Dr. Cadet, and that illustrates a need to publicize the presence of adulterants in street drugs.
“If I wasn’t aware of many of these, then the general public is also not going to be aware of them,” Dr. Cadet said. “Scientists, including myself, and government agencies need to do a better job [of communicating this issue].”
The study references individuals with substance use disorder, but Dr. Cadet cautioned that anyone who uses street drugs, even once or twice, could be a victim of adulterants. “You don’t need to have met criteria for diagnosis in order to suffer the consequences.”
The study had no funding. Dr. Gold and Dr. Cadet have no relevant financial disclosures.
The composition of street drugs like heroin and cocaine are changing. According to a new analysis, almost all contain at least one toxic adulterant, and many contain a plethora. Most adulterants have pharmacologic activities and toxicities. Their presence has added impact in the context of the COVID-19 pandemic, since some may cause a drastic drop in white blood cells that could leave drug users more vulnerable to infection.
“It’s remarkable that we just forgot to notice, in the horrendous transition from prescription opioid epidemic to the illicit opioid and psychostimulant epidemics, that we would have to pay special attention to what the medications are in the drugs that the person was exposed to – and for how long,” said Mark S. Gold, MD, a coauthor of the review.
The analysis showed that adulterants include new psychoactive substances, industrial compounds, fungicides, veterinary medications, and various impurities. In addition, other various medications are being found in street drugs, such as antipsychotics, antidepressants, anxiolytics, antihistamines, anthelmintics, anesthetics, anti-inflammatory agents, antipyretics, analgesics, antispasmodics, antiarrhythmics, antimalarials, bronchodilators, decongestants, expectorants, muscle relaxers, natural/synthetic hallucinogens, and sedatives.
Illicit drugs are by nature manufactured without Food and Drug Administration oversight, and it is becoming increasingly common that substances like leftover medicines and other active drugs are added to illicit drug batches to add weight, said Dr. Gold, a professor at Washington University,St. Louis. The study appeared in Current Psychopharmacology.
Effects of adulterants ‘terrifying’
The findings of adulterants and their consequences are concerning, according to Jean Lud Cadet, MD, who was asked to comment on the findings. “The blood dysplasia, the pulmonary problems that some of those adulterants can cause – it’s actually terrifying, to put it bluntly,” said Dr. Cadet, who is a senior investigator and chief of the Molecular Neuropsychiatry Research Branch at the National Institute on Drug Abuse.
Before 2000, street drugs were generally diluted with comparatively benign substances such as caffeine, sugars, or lidocaine. Drugs like phenacetin, levamisole, acetaminophen, and diltiazem began to appear in heroin and cocaine in the late 1990s, and by 2010, more powerful adulterants like fentanyl, ketamine, and quetiapine became common.
In 2015, the U.S. Department of State partnered with the Colombo Plan, an international organization based in Sri Lanka, to use field spectroscopy to detect toxins directly in cocaine and heroin samples found in Argentina, Brazil, Ecuador, Peru, Sri Lanka, Thailand, Honduras, Guatemala, Mexico, Colombia, and South Africa. They found a range of adulterants such as aminopyrine, diltiazem, metamizole, levamisole, and phenacetin.
A similar project with 431 heroin and cocaine samples from Vermont and Kentucky found that 69% of samples had five or more controlled drugs, toxic adulterants, or impurities. About 15% had nine or more, and 95% of samples had at least one toxic adulterant.
In the midst of the COVID-19 pandemic, these adulterants take on even greater significance. Individuals with substance use disorders often have other health conditions that can make them more vulnerable to viral infections, and this could be exacerbated by the effects of adulterants on white blood cells or other systems. The pandemic has also had an indirect effect by causing a shortage of street drugs. During production shortages, traffickers might boost potency by adding more cutting agents and adulterants. As a result, COVID-19 and opioid addiction tend to reinforce each other.
“The clinical message would be that our [substance use] patients will contract infectious disease and need to be prioritized for [COVID-19] vaccination,” said Dr. Gold.
The findings came as a surprise to Dr. Cadet, and that illustrates a need to publicize the presence of adulterants in street drugs.
“If I wasn’t aware of many of these, then the general public is also not going to be aware of them,” Dr. Cadet said. “Scientists, including myself, and government agencies need to do a better job [of communicating this issue].”
The study references individuals with substance use disorder, but Dr. Cadet cautioned that anyone who uses street drugs, even once or twice, could be a victim of adulterants. “You don’t need to have met criteria for diagnosis in order to suffer the consequences.”
The study had no funding. Dr. Gold and Dr. Cadet have no relevant financial disclosures.
FROM CURRENT PSYCHOPHARMACOLOGY



