User login
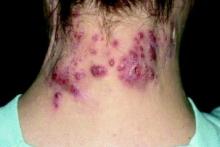
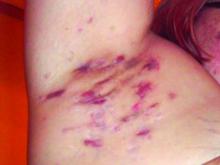
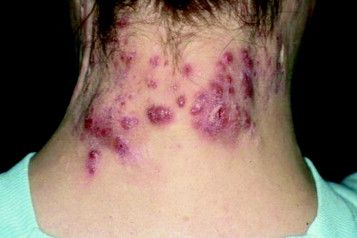
Patient-Reported Outcomes Predict Mortality in Cutaneous Chronic GVHD
. Independent of potential confounders, these PROs moreover predicted non-relapse mortality for all three disease subtypes, making PROs potentially useful adjuncts for risk stratification and treatment decisions, the study authors said.
“These two findings highlight the importance of patient-reported outcomes in measuring this disease,” lead author Emily Baumrin, MD, MSCE, assistant professor of dermatology and medicine at the University of Pennsylvania, Philadelphia, told this news organization. The study was published online February 28 in JAMA Dermatology.
Symptoms and QOL
The investigators monitored 436 patients from the Chronic GVHD Consortium until December 2020. The Lee Symptom Scale (LSS) skin subscale was used to evaluate symptom burden and the Functional Assessment of Cancer Therapy–Bone Marrow Transplantation (FACT-BMT) was used to measure quality of life.
Patients with sclerotic GVHD and combination disease at diagnosis had significantly worse median LSS scores than did those with epidermal disease (25, 35, and 20 points, respectively; P = .01). Patients with sclerotic disease had worse median FACT-BMT scores versus those with epidermal involvement (104 versus 109 points, respectively; P = .08).
Although these scores improved with all skin subtypes, LSS skin subscale and FACT-BMT scores remained significantly worse (by 9.0 points and 6.1 points, respectively) for patients with combination and sclerotic disease versus those with epidermal disease after adjusting for potential confounders.
Regarding mortality, every 7-point worsening (clinically meaningful difference) in FACT-BMT score at diagnosis of skin chronic GVHD conferred 9.1% increases in odds of both all-cause mortality and non-relapse mortality, after adjustment for factors such as age and sex. Likewise, for every 11 points worsening (clinically meaningful difference) in LSS skin subscale scores at diagnosis, researchers observed odds increases of 10% in all-cause mortality and 16.4% in non-relapse mortality.
Because patients with combination disease had only slightly more epidermal body surface area (BSA) involvement but significantly higher symptom burden than the other subtypes, the authors added, combination disease may represent a distinct phenotype. “Since we’ve also shown that the severity of patient-reported outcomes is associated with mortality,” Dr. Baumrin said in the interview, “perhaps these patients are at the highest risk of mortality as well.”
A growing population
Although many might think of chronic GVHD as rare, she noted, the number of allogeneic hematopoietic cell transplant (HCT) survivors living in the United States is growing. In a modeling study published in October of 2013 in Biology of Blood and Marrow Transplantation, authors predicted that by 2030, this figure will reach 502,000 — about half of whom will develop chronic GVHD, she said.
With more HCTs being performed each year and ongoing improvements in supportive care, patients are living longer post transplant. “Therefore, many transplant survivors are being taken care of in the community outside of transplant centers.”
Accordingly, Dr. Baumrin said, study findings are relevant to dermatologists in academic and transplant centers and the community who provide skin cancer screenings or other dermatologic care for transplant recipients. “Upon diagnosis of chronic GVHD, the evaluation of disease burden by patient-reported outcome measures may assist in assessing disease severity and response to treatments over time — and to stratify patients at higher risk for mortality and communicate that back to transplant physicians.”
Incorporating PROs into clinical practice might prove especially helpful for patients with sclerotic chronic cutaneous GVHD. Currently, clinicians assess cutaneous GVHD clinically, using parameters including skin thickness. The National Institutes of Health (NIH) Skin Score, used in clinical trials, also measures BSA.
“The issue with sclerosis is, it’s hard to determine clinical severity based on physical examination alone,” Dr. Baumrin said. It can be difficult to quantify skin thickness and changes over time. “So it’s hard to detect improvements, which are often slow. Patient-reported outcome measures may be a more sensitive way to detect response to treatment than our clinical assessments, which are often crude for sclerotic disease.”
In a secondary analysis of the phase 2 clinical trial of belumosudil, a treatment for chronic GVHD, published in October 2022 in Transplantation and Cellular Therapy, response rate was around 30% measured by NIH Skin Score and 77% by PROs. “Our clinical examination in sclerotic type disease falls short in terms of determining therapeutic benefit. PROs might complement those clinical measures,” she said.
Future research will involve determining and validating which PROs matter most clinically and to patients, added Dr. Baumrin. Although widely used in evaluating transplant patients, LSS skin subscale and FACT-BMT scores may not represent patients’ experience of living with cutaneous chronic GVHD as effectively as might other tools such as the Dermatology Life Quality Index (DLQI) or Patient-Reported Outcomes Measurement Information System (PROMIS) measures, she explained.
Study strengths included authors’ use of well-validated PROs rather than novel unvalidated measures, Sandra A. Mitchell, PhD, CRNP, of the National Cancer Institute, Rockville, Maryland, and Edward W. Cowen, MD, MHSc, of the Dermatology Branch at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), Bethesda, Maryland, wrote in an accompanying editorial in JAMA Dermatology. However, they added, incorporating causes of death might have revealed that the excess mortality associated with sclerotic disease stemmed at least partly from adverse effects of prolonged immunosuppression, particularly infection.
If future studies establish this to be the case, said Dr. Baumrin, reducing immunosuppression might be warranted for these patients. “And if death is primarily due to chronic GVHD itself, maybe we should treat more aggressively. PROs can help guide this decision.”
The study was supported by the NIH/NIAMS and the University of Pennsylvania. Dr. Baumrin and three coauthors report no relevant financial relationships; other authors had disclosures related to several pharmaceutical companies. Dr. Mitchell and Dr. Cowen had no disclosures.
. Independent of potential confounders, these PROs moreover predicted non-relapse mortality for all three disease subtypes, making PROs potentially useful adjuncts for risk stratification and treatment decisions, the study authors said.
“These two findings highlight the importance of patient-reported outcomes in measuring this disease,” lead author Emily Baumrin, MD, MSCE, assistant professor of dermatology and medicine at the University of Pennsylvania, Philadelphia, told this news organization. The study was published online February 28 in JAMA Dermatology.
Symptoms and QOL
The investigators monitored 436 patients from the Chronic GVHD Consortium until December 2020. The Lee Symptom Scale (LSS) skin subscale was used to evaluate symptom burden and the Functional Assessment of Cancer Therapy–Bone Marrow Transplantation (FACT-BMT) was used to measure quality of life.
Patients with sclerotic GVHD and combination disease at diagnosis had significantly worse median LSS scores than did those with epidermal disease (25, 35, and 20 points, respectively; P = .01). Patients with sclerotic disease had worse median FACT-BMT scores versus those with epidermal involvement (104 versus 109 points, respectively; P = .08).
Although these scores improved with all skin subtypes, LSS skin subscale and FACT-BMT scores remained significantly worse (by 9.0 points and 6.1 points, respectively) for patients with combination and sclerotic disease versus those with epidermal disease after adjusting for potential confounders.
Regarding mortality, every 7-point worsening (clinically meaningful difference) in FACT-BMT score at diagnosis of skin chronic GVHD conferred 9.1% increases in odds of both all-cause mortality and non-relapse mortality, after adjustment for factors such as age and sex. Likewise, for every 11 points worsening (clinically meaningful difference) in LSS skin subscale scores at diagnosis, researchers observed odds increases of 10% in all-cause mortality and 16.4% in non-relapse mortality.
Because patients with combination disease had only slightly more epidermal body surface area (BSA) involvement but significantly higher symptom burden than the other subtypes, the authors added, combination disease may represent a distinct phenotype. “Since we’ve also shown that the severity of patient-reported outcomes is associated with mortality,” Dr. Baumrin said in the interview, “perhaps these patients are at the highest risk of mortality as well.”
A growing population
Although many might think of chronic GVHD as rare, she noted, the number of allogeneic hematopoietic cell transplant (HCT) survivors living in the United States is growing. In a modeling study published in October of 2013 in Biology of Blood and Marrow Transplantation, authors predicted that by 2030, this figure will reach 502,000 — about half of whom will develop chronic GVHD, she said.
With more HCTs being performed each year and ongoing improvements in supportive care, patients are living longer post transplant. “Therefore, many transplant survivors are being taken care of in the community outside of transplant centers.”
Accordingly, Dr. Baumrin said, study findings are relevant to dermatologists in academic and transplant centers and the community who provide skin cancer screenings or other dermatologic care for transplant recipients. “Upon diagnosis of chronic GVHD, the evaluation of disease burden by patient-reported outcome measures may assist in assessing disease severity and response to treatments over time — and to stratify patients at higher risk for mortality and communicate that back to transplant physicians.”
Incorporating PROs into clinical practice might prove especially helpful for patients with sclerotic chronic cutaneous GVHD. Currently, clinicians assess cutaneous GVHD clinically, using parameters including skin thickness. The National Institutes of Health (NIH) Skin Score, used in clinical trials, also measures BSA.
“The issue with sclerosis is, it’s hard to determine clinical severity based on physical examination alone,” Dr. Baumrin said. It can be difficult to quantify skin thickness and changes over time. “So it’s hard to detect improvements, which are often slow. Patient-reported outcome measures may be a more sensitive way to detect response to treatment than our clinical assessments, which are often crude for sclerotic disease.”
In a secondary analysis of the phase 2 clinical trial of belumosudil, a treatment for chronic GVHD, published in October 2022 in Transplantation and Cellular Therapy, response rate was around 30% measured by NIH Skin Score and 77% by PROs. “Our clinical examination in sclerotic type disease falls short in terms of determining therapeutic benefit. PROs might complement those clinical measures,” she said.
Future research will involve determining and validating which PROs matter most clinically and to patients, added Dr. Baumrin. Although widely used in evaluating transplant patients, LSS skin subscale and FACT-BMT scores may not represent patients’ experience of living with cutaneous chronic GVHD as effectively as might other tools such as the Dermatology Life Quality Index (DLQI) or Patient-Reported Outcomes Measurement Information System (PROMIS) measures, she explained.
Study strengths included authors’ use of well-validated PROs rather than novel unvalidated measures, Sandra A. Mitchell, PhD, CRNP, of the National Cancer Institute, Rockville, Maryland, and Edward W. Cowen, MD, MHSc, of the Dermatology Branch at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), Bethesda, Maryland, wrote in an accompanying editorial in JAMA Dermatology. However, they added, incorporating causes of death might have revealed that the excess mortality associated with sclerotic disease stemmed at least partly from adverse effects of prolonged immunosuppression, particularly infection.
If future studies establish this to be the case, said Dr. Baumrin, reducing immunosuppression might be warranted for these patients. “And if death is primarily due to chronic GVHD itself, maybe we should treat more aggressively. PROs can help guide this decision.”
The study was supported by the NIH/NIAMS and the University of Pennsylvania. Dr. Baumrin and three coauthors report no relevant financial relationships; other authors had disclosures related to several pharmaceutical companies. Dr. Mitchell and Dr. Cowen had no disclosures.
. Independent of potential confounders, these PROs moreover predicted non-relapse mortality for all three disease subtypes, making PROs potentially useful adjuncts for risk stratification and treatment decisions, the study authors said.
“These two findings highlight the importance of patient-reported outcomes in measuring this disease,” lead author Emily Baumrin, MD, MSCE, assistant professor of dermatology and medicine at the University of Pennsylvania, Philadelphia, told this news organization. The study was published online February 28 in JAMA Dermatology.
Symptoms and QOL
The investigators monitored 436 patients from the Chronic GVHD Consortium until December 2020. The Lee Symptom Scale (LSS) skin subscale was used to evaluate symptom burden and the Functional Assessment of Cancer Therapy–Bone Marrow Transplantation (FACT-BMT) was used to measure quality of life.
Patients with sclerotic GVHD and combination disease at diagnosis had significantly worse median LSS scores than did those with epidermal disease (25, 35, and 20 points, respectively; P = .01). Patients with sclerotic disease had worse median FACT-BMT scores versus those with epidermal involvement (104 versus 109 points, respectively; P = .08).
Although these scores improved with all skin subtypes, LSS skin subscale and FACT-BMT scores remained significantly worse (by 9.0 points and 6.1 points, respectively) for patients with combination and sclerotic disease versus those with epidermal disease after adjusting for potential confounders.
Regarding mortality, every 7-point worsening (clinically meaningful difference) in FACT-BMT score at diagnosis of skin chronic GVHD conferred 9.1% increases in odds of both all-cause mortality and non-relapse mortality, after adjustment for factors such as age and sex. Likewise, for every 11 points worsening (clinically meaningful difference) in LSS skin subscale scores at diagnosis, researchers observed odds increases of 10% in all-cause mortality and 16.4% in non-relapse mortality.
Because patients with combination disease had only slightly more epidermal body surface area (BSA) involvement but significantly higher symptom burden than the other subtypes, the authors added, combination disease may represent a distinct phenotype. “Since we’ve also shown that the severity of patient-reported outcomes is associated with mortality,” Dr. Baumrin said in the interview, “perhaps these patients are at the highest risk of mortality as well.”
A growing population
Although many might think of chronic GVHD as rare, she noted, the number of allogeneic hematopoietic cell transplant (HCT) survivors living in the United States is growing. In a modeling study published in October of 2013 in Biology of Blood and Marrow Transplantation, authors predicted that by 2030, this figure will reach 502,000 — about half of whom will develop chronic GVHD, she said.
With more HCTs being performed each year and ongoing improvements in supportive care, patients are living longer post transplant. “Therefore, many transplant survivors are being taken care of in the community outside of transplant centers.”
Accordingly, Dr. Baumrin said, study findings are relevant to dermatologists in academic and transplant centers and the community who provide skin cancer screenings or other dermatologic care for transplant recipients. “Upon diagnosis of chronic GVHD, the evaluation of disease burden by patient-reported outcome measures may assist in assessing disease severity and response to treatments over time — and to stratify patients at higher risk for mortality and communicate that back to transplant physicians.”
Incorporating PROs into clinical practice might prove especially helpful for patients with sclerotic chronic cutaneous GVHD. Currently, clinicians assess cutaneous GVHD clinically, using parameters including skin thickness. The National Institutes of Health (NIH) Skin Score, used in clinical trials, also measures BSA.
“The issue with sclerosis is, it’s hard to determine clinical severity based on physical examination alone,” Dr. Baumrin said. It can be difficult to quantify skin thickness and changes over time. “So it’s hard to detect improvements, which are often slow. Patient-reported outcome measures may be a more sensitive way to detect response to treatment than our clinical assessments, which are often crude for sclerotic disease.”
In a secondary analysis of the phase 2 clinical trial of belumosudil, a treatment for chronic GVHD, published in October 2022 in Transplantation and Cellular Therapy, response rate was around 30% measured by NIH Skin Score and 77% by PROs. “Our clinical examination in sclerotic type disease falls short in terms of determining therapeutic benefit. PROs might complement those clinical measures,” she said.
Future research will involve determining and validating which PROs matter most clinically and to patients, added Dr. Baumrin. Although widely used in evaluating transplant patients, LSS skin subscale and FACT-BMT scores may not represent patients’ experience of living with cutaneous chronic GVHD as effectively as might other tools such as the Dermatology Life Quality Index (DLQI) or Patient-Reported Outcomes Measurement Information System (PROMIS) measures, she explained.
Study strengths included authors’ use of well-validated PROs rather than novel unvalidated measures, Sandra A. Mitchell, PhD, CRNP, of the National Cancer Institute, Rockville, Maryland, and Edward W. Cowen, MD, MHSc, of the Dermatology Branch at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), Bethesda, Maryland, wrote in an accompanying editorial in JAMA Dermatology. However, they added, incorporating causes of death might have revealed that the excess mortality associated with sclerotic disease stemmed at least partly from adverse effects of prolonged immunosuppression, particularly infection.
If future studies establish this to be the case, said Dr. Baumrin, reducing immunosuppression might be warranted for these patients. “And if death is primarily due to chronic GVHD itself, maybe we should treat more aggressively. PROs can help guide this decision.”
The study was supported by the NIH/NIAMS and the University of Pennsylvania. Dr. Baumrin and three coauthors report no relevant financial relationships; other authors had disclosures related to several pharmaceutical companies. Dr. Mitchell and Dr. Cowen had no disclosures.
FROM JAMA DERMATOLOGY
Study Eyes Longer IV Ertapenem for Recalcitrant Hidradenitis Suppurativa
at the completion of treatment, a retrospective study showed.
“These findings suggest a course of 12 to 16 weeks of ertapenem may be appropriate as a new standard length of therapy in HS patients, which is at least twice the current recommendation of the North American treatment guidelines,” wrote corresponding author Steven R. Cohen, MD, MPH, of the departments of dermatology at Weill Cornell Medicine and Albert Einstein College of Medicine, New York, and his coauthors. The results were published online February 14, 2024, in JAMA Dermatology.
In an earlier study , some of the same researchers evaluated the efficacy of daily IV ertapenem for 6 weeks in seven patients with HS. The patients experienced “notable remediation of disease that was rapidly lost within 1 month of withdrawal.”
Treatment guidelines published in 2019 recommend ertapenem as a highly effective third-line therapy limited to one 6-week course “as rescue therapy or during surgical planning, given the practical barriers to home infusions and concerns about antibiotic resistance” .
For the current analysis, Dr. Cohen and colleagues explored the effects of a longer duration of treatment with ertapenem in this patient population. They retrospectively reviewed the medical records of 98 patients with HS who received care at Albert Einstein College of Medicine’s Montefiore HS Center between 2018 and 2022. Each patient used an elastomeric pump to self-administer 1 g IV ertapenem daily for 12-16 weeks.
Key outcome measures of interest were the HS Physician Global Assessment (PGA) score (a 6-point scale ranging from clear to very severe) and a numerical rating scale (NRS) for pain (an 11-point scale in which a score of 0 indicates no pain and a score of 10 indicates the worst possible pain) and markers of inflammation such as leukocytes, erythrocyte sedimentation rate, C-reactive protein (CRP), and interleukin (IL)-6. The researchers measured these outcomes at baseline, the midcourse of IV ertapenem treatment, at the end of the course, and post therapy.
The mean age of the patients was 35.8 years, 62.2% were female, and 60.2% were Black. The mean treatment duration was 13.1 weeks and the mean posttherapy follow-up occurred after a mean of 7.8 weeks.
Between baseline and posttherapy follow-up, the HS PGA scores dropped from a mean of 3.9 to 2.7 and the NRS for pain dropped from 4.2 to 1.8 (P < .001 for both associations). Markers of inflammation also dropped between baseline and post therapy.
Specifically, values for CRP dropped from 5.4 to 2.4 mg/dL; IL-6 dropped from 25.2 to 13.7, and leukocytes dropped from 11.3 to 10.0 (P < .001 for all associations). Among the 76 patients who participated in a follow-up telephone survey, 63 (80.3%) reported medium to high satisfaction with their course of ertapenem, and 69 (90.8%) said they would recommend the treatment to other patients with HS.
The authors noted certain limitations of their study, including its retrospective, single-center design, the lack of a control group, and the fact that the HS-PGA scores at each visit did not meet the threshold of a 2-point decrease that is considered a clinically meaningful in the medical literature.
The definitive mechanism of ertapenem efficacy remains elusive, the authors pointed out. “Although oral antibiotics are generally accepted as a core therapeutic approach to HS, much less is known about the efficacy of IV antibiotics, especially ertapenem, a parenteral carbapenem possessing activity against many gram-positive bacteria, gram-negative bacteria, and anaerobic organisms,” they wrote.
In an accompanying editorial, Haley B. Naik, MD, MHSc, a dermatologist at the University of California, San Francisco, said that adopting prolonged courses of ertapenem treatment “comes with substantial individual and public health considerations”.
“Even though HS is a noninfectious disease, microbes might play a role in inciting HS immune dysregulation, prompting the inclusion of antimicrobial therapy in treatment regimens. However, broad-spectrum antibiotics for HS are associated with high levels of antibiotic resistance,” she wrote. Prolonged use of ertapenem and other carbapenems in HS treatment “will likely increase antimicrobial resistance, thereby limiting management of both HS and comorbid infections.”
Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, who was asked to comment on the study, said that, despite significant advances in the management of HS over the past decade, there are still patients who do not respond adequately to standard treatments.
For these patients, IV ertapenem can serve as a valuable bridge to a longer-term therapeutic option, “be it surgery or escalated immunomodulation,” such as dual biologic therapy, she said. “In my personal experience, IV ertapenem, which like the authors I also typically use for a 12-week course, delivers impressive and fast results even in the worst disease cases.
“It can be difficult to maintain the therapeutic benefit of ertapenem after it is discontinued, which is why patients should be on concomitant medications as they were in this study and have a post-ertapenem treatment plan in place,” said Dr. Hsiao, who was not involved with the study. “Hopefully, we will be able to one day understand why ertapenem is so effective for HS and be able to harness that benefit for patients without concern for antimicrobial resistance.”
Dr. Cohen reported receiving personal fees from Verrica Pharmaceuticals and belonging to the Board of Trustees of the American Skin Association outside the submitted work. No other disclosures were reported. Dr. Naik reported having received grants from AbbVie and the National Institutes of Health; personal fees from Novartis, UCB, Boehringer Ingelheim, 23andMe, Aristea Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, and Pfizer; and shares from Radera during the conduct of the study. She is a board member of the Hidradenitis Suppurativa Foundation. Dr. Hsiao disclosed that she is a member of the board of directors for the Hidradenitis Suppurativa Foundation. She has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, UCB, as a speaker for AbbVie, Novartis, and UCB, and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
at the completion of treatment, a retrospective study showed.
“These findings suggest a course of 12 to 16 weeks of ertapenem may be appropriate as a new standard length of therapy in HS patients, which is at least twice the current recommendation of the North American treatment guidelines,” wrote corresponding author Steven R. Cohen, MD, MPH, of the departments of dermatology at Weill Cornell Medicine and Albert Einstein College of Medicine, New York, and his coauthors. The results were published online February 14, 2024, in JAMA Dermatology.
In an earlier study , some of the same researchers evaluated the efficacy of daily IV ertapenem for 6 weeks in seven patients with HS. The patients experienced “notable remediation of disease that was rapidly lost within 1 month of withdrawal.”
Treatment guidelines published in 2019 recommend ertapenem as a highly effective third-line therapy limited to one 6-week course “as rescue therapy or during surgical planning, given the practical barriers to home infusions and concerns about antibiotic resistance” .
For the current analysis, Dr. Cohen and colleagues explored the effects of a longer duration of treatment with ertapenem in this patient population. They retrospectively reviewed the medical records of 98 patients with HS who received care at Albert Einstein College of Medicine’s Montefiore HS Center between 2018 and 2022. Each patient used an elastomeric pump to self-administer 1 g IV ertapenem daily for 12-16 weeks.
Key outcome measures of interest were the HS Physician Global Assessment (PGA) score (a 6-point scale ranging from clear to very severe) and a numerical rating scale (NRS) for pain (an 11-point scale in which a score of 0 indicates no pain and a score of 10 indicates the worst possible pain) and markers of inflammation such as leukocytes, erythrocyte sedimentation rate, C-reactive protein (CRP), and interleukin (IL)-6. The researchers measured these outcomes at baseline, the midcourse of IV ertapenem treatment, at the end of the course, and post therapy.
The mean age of the patients was 35.8 years, 62.2% were female, and 60.2% were Black. The mean treatment duration was 13.1 weeks and the mean posttherapy follow-up occurred after a mean of 7.8 weeks.
Between baseline and posttherapy follow-up, the HS PGA scores dropped from a mean of 3.9 to 2.7 and the NRS for pain dropped from 4.2 to 1.8 (P < .001 for both associations). Markers of inflammation also dropped between baseline and post therapy.
Specifically, values for CRP dropped from 5.4 to 2.4 mg/dL; IL-6 dropped from 25.2 to 13.7, and leukocytes dropped from 11.3 to 10.0 (P < .001 for all associations). Among the 76 patients who participated in a follow-up telephone survey, 63 (80.3%) reported medium to high satisfaction with their course of ertapenem, and 69 (90.8%) said they would recommend the treatment to other patients with HS.
The authors noted certain limitations of their study, including its retrospective, single-center design, the lack of a control group, and the fact that the HS-PGA scores at each visit did not meet the threshold of a 2-point decrease that is considered a clinically meaningful in the medical literature.
The definitive mechanism of ertapenem efficacy remains elusive, the authors pointed out. “Although oral antibiotics are generally accepted as a core therapeutic approach to HS, much less is known about the efficacy of IV antibiotics, especially ertapenem, a parenteral carbapenem possessing activity against many gram-positive bacteria, gram-negative bacteria, and anaerobic organisms,” they wrote.
In an accompanying editorial, Haley B. Naik, MD, MHSc, a dermatologist at the University of California, San Francisco, said that adopting prolonged courses of ertapenem treatment “comes with substantial individual and public health considerations”.
“Even though HS is a noninfectious disease, microbes might play a role in inciting HS immune dysregulation, prompting the inclusion of antimicrobial therapy in treatment regimens. However, broad-spectrum antibiotics for HS are associated with high levels of antibiotic resistance,” she wrote. Prolonged use of ertapenem and other carbapenems in HS treatment “will likely increase antimicrobial resistance, thereby limiting management of both HS and comorbid infections.”
Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, who was asked to comment on the study, said that, despite significant advances in the management of HS over the past decade, there are still patients who do not respond adequately to standard treatments.
For these patients, IV ertapenem can serve as a valuable bridge to a longer-term therapeutic option, “be it surgery or escalated immunomodulation,” such as dual biologic therapy, she said. “In my personal experience, IV ertapenem, which like the authors I also typically use for a 12-week course, delivers impressive and fast results even in the worst disease cases.
“It can be difficult to maintain the therapeutic benefit of ertapenem after it is discontinued, which is why patients should be on concomitant medications as they were in this study and have a post-ertapenem treatment plan in place,” said Dr. Hsiao, who was not involved with the study. “Hopefully, we will be able to one day understand why ertapenem is so effective for HS and be able to harness that benefit for patients without concern for antimicrobial resistance.”
Dr. Cohen reported receiving personal fees from Verrica Pharmaceuticals and belonging to the Board of Trustees of the American Skin Association outside the submitted work. No other disclosures were reported. Dr. Naik reported having received grants from AbbVie and the National Institutes of Health; personal fees from Novartis, UCB, Boehringer Ingelheim, 23andMe, Aristea Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, and Pfizer; and shares from Radera during the conduct of the study. She is a board member of the Hidradenitis Suppurativa Foundation. Dr. Hsiao disclosed that she is a member of the board of directors for the Hidradenitis Suppurativa Foundation. She has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, UCB, as a speaker for AbbVie, Novartis, and UCB, and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
at the completion of treatment, a retrospective study showed.
“These findings suggest a course of 12 to 16 weeks of ertapenem may be appropriate as a new standard length of therapy in HS patients, which is at least twice the current recommendation of the North American treatment guidelines,” wrote corresponding author Steven R. Cohen, MD, MPH, of the departments of dermatology at Weill Cornell Medicine and Albert Einstein College of Medicine, New York, and his coauthors. The results were published online February 14, 2024, in JAMA Dermatology.
In an earlier study , some of the same researchers evaluated the efficacy of daily IV ertapenem for 6 weeks in seven patients with HS. The patients experienced “notable remediation of disease that was rapidly lost within 1 month of withdrawal.”
Treatment guidelines published in 2019 recommend ertapenem as a highly effective third-line therapy limited to one 6-week course “as rescue therapy or during surgical planning, given the practical barriers to home infusions and concerns about antibiotic resistance” .
For the current analysis, Dr. Cohen and colleagues explored the effects of a longer duration of treatment with ertapenem in this patient population. They retrospectively reviewed the medical records of 98 patients with HS who received care at Albert Einstein College of Medicine’s Montefiore HS Center between 2018 and 2022. Each patient used an elastomeric pump to self-administer 1 g IV ertapenem daily for 12-16 weeks.
Key outcome measures of interest were the HS Physician Global Assessment (PGA) score (a 6-point scale ranging from clear to very severe) and a numerical rating scale (NRS) for pain (an 11-point scale in which a score of 0 indicates no pain and a score of 10 indicates the worst possible pain) and markers of inflammation such as leukocytes, erythrocyte sedimentation rate, C-reactive protein (CRP), and interleukin (IL)-6. The researchers measured these outcomes at baseline, the midcourse of IV ertapenem treatment, at the end of the course, and post therapy.
The mean age of the patients was 35.8 years, 62.2% were female, and 60.2% were Black. The mean treatment duration was 13.1 weeks and the mean posttherapy follow-up occurred after a mean of 7.8 weeks.
Between baseline and posttherapy follow-up, the HS PGA scores dropped from a mean of 3.9 to 2.7 and the NRS for pain dropped from 4.2 to 1.8 (P < .001 for both associations). Markers of inflammation also dropped between baseline and post therapy.
Specifically, values for CRP dropped from 5.4 to 2.4 mg/dL; IL-6 dropped from 25.2 to 13.7, and leukocytes dropped from 11.3 to 10.0 (P < .001 for all associations). Among the 76 patients who participated in a follow-up telephone survey, 63 (80.3%) reported medium to high satisfaction with their course of ertapenem, and 69 (90.8%) said they would recommend the treatment to other patients with HS.
The authors noted certain limitations of their study, including its retrospective, single-center design, the lack of a control group, and the fact that the HS-PGA scores at each visit did not meet the threshold of a 2-point decrease that is considered a clinically meaningful in the medical literature.
The definitive mechanism of ertapenem efficacy remains elusive, the authors pointed out. “Although oral antibiotics are generally accepted as a core therapeutic approach to HS, much less is known about the efficacy of IV antibiotics, especially ertapenem, a parenteral carbapenem possessing activity against many gram-positive bacteria, gram-negative bacteria, and anaerobic organisms,” they wrote.
In an accompanying editorial, Haley B. Naik, MD, MHSc, a dermatologist at the University of California, San Francisco, said that adopting prolonged courses of ertapenem treatment “comes with substantial individual and public health considerations”.
“Even though HS is a noninfectious disease, microbes might play a role in inciting HS immune dysregulation, prompting the inclusion of antimicrobial therapy in treatment regimens. However, broad-spectrum antibiotics for HS are associated with high levels of antibiotic resistance,” she wrote. Prolonged use of ertapenem and other carbapenems in HS treatment “will likely increase antimicrobial resistance, thereby limiting management of both HS and comorbid infections.”
Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, who was asked to comment on the study, said that, despite significant advances in the management of HS over the past decade, there are still patients who do not respond adequately to standard treatments.
For these patients, IV ertapenem can serve as a valuable bridge to a longer-term therapeutic option, “be it surgery or escalated immunomodulation,” such as dual biologic therapy, she said. “In my personal experience, IV ertapenem, which like the authors I also typically use for a 12-week course, delivers impressive and fast results even in the worst disease cases.
“It can be difficult to maintain the therapeutic benefit of ertapenem after it is discontinued, which is why patients should be on concomitant medications as they were in this study and have a post-ertapenem treatment plan in place,” said Dr. Hsiao, who was not involved with the study. “Hopefully, we will be able to one day understand why ertapenem is so effective for HS and be able to harness that benefit for patients without concern for antimicrobial resistance.”
Dr. Cohen reported receiving personal fees from Verrica Pharmaceuticals and belonging to the Board of Trustees of the American Skin Association outside the submitted work. No other disclosures were reported. Dr. Naik reported having received grants from AbbVie and the National Institutes of Health; personal fees from Novartis, UCB, Boehringer Ingelheim, 23andMe, Aristea Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, and Pfizer; and shares from Radera during the conduct of the study. She is a board member of the Hidradenitis Suppurativa Foundation. Dr. Hsiao disclosed that she is a member of the board of directors for the Hidradenitis Suppurativa Foundation. She has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, UCB, as a speaker for AbbVie, Novartis, and UCB, and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
FROM JAMA DERMATOLOGY
What Skin Manifestations Are Associated With Pediatric IBD?
TOPLINE:
Skin conditions burden many children with inflammatory bowel disease (IBD), according to the authors of a single-center study.
METHODOLOGY:
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with (CD) or ulcerative (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with Crohn’s disease (CD) or ulcerative colitis (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
TAKEAWAY:
- The most common noninfectious dermatologic condition among the 425 children and adolescents was (30.8%), followed by eczema (15.8%) and perianal skin tags (14.6%).
- Angular cheilitis was more common among those with CD than those with UC (7.2% vs 2%, respectively; P = .024) as was keratosis pilaris (6.9% vs 0.7%; P = .003), and perianal skin complications such as skin tags (20.3% vs 4%), fistulas (13.4% vs 2.7%), and abscesses (13.4% vs 2%; P < .001 for all associations).
- Fungal skin infections were more frequently diagnosed in children with UC than those with CD (15.4% vs 8%; P = .017).
- The researchers observed that the severity of IBD correlated with a higher prevalence of perianal fistula (P = .003), perianal region abscess (P = .041), psoriasis (P < .001), and pyoderma gangrenosum (P = .003).
IN PRACTICE:
“Early identification of common dermatologic conditions in children and adolescents with IBD and recognizing their characteristic associations may alter management and improve skin-related outcomes in this patient population,” the authors wrote.
SOURCE:
Corresponding author Megha M. Tollefson, MD, of the Department of Dermatology at Mayo Clinic, Rochester, Minnesota, and colleagues conducted the research, which was published in Pediatric Dermatology.
LIMITATIONS:
The single-center design and the fact that database studies are subject to extraction error. There was no age- and sex-matched cohort to determine whether the prevalence of cutaneous infections, acne, eczema, and other inflammatory disorders was truly increased in IBD.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Skin conditions burden many children with inflammatory bowel disease (IBD), according to the authors of a single-center study.
METHODOLOGY:
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with (CD) or ulcerative (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with Crohn’s disease (CD) or ulcerative colitis (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
TAKEAWAY:
- The most common noninfectious dermatologic condition among the 425 children and adolescents was (30.8%), followed by eczema (15.8%) and perianal skin tags (14.6%).
- Angular cheilitis was more common among those with CD than those with UC (7.2% vs 2%, respectively; P = .024) as was keratosis pilaris (6.9% vs 0.7%; P = .003), and perianal skin complications such as skin tags (20.3% vs 4%), fistulas (13.4% vs 2.7%), and abscesses (13.4% vs 2%; P < .001 for all associations).
- Fungal skin infections were more frequently diagnosed in children with UC than those with CD (15.4% vs 8%; P = .017).
- The researchers observed that the severity of IBD correlated with a higher prevalence of perianal fistula (P = .003), perianal region abscess (P = .041), psoriasis (P < .001), and pyoderma gangrenosum (P = .003).
IN PRACTICE:
“Early identification of common dermatologic conditions in children and adolescents with IBD and recognizing their characteristic associations may alter management and improve skin-related outcomes in this patient population,” the authors wrote.
SOURCE:
Corresponding author Megha M. Tollefson, MD, of the Department of Dermatology at Mayo Clinic, Rochester, Minnesota, and colleagues conducted the research, which was published in Pediatric Dermatology.
LIMITATIONS:
The single-center design and the fact that database studies are subject to extraction error. There was no age- and sex-matched cohort to determine whether the prevalence of cutaneous infections, acne, eczema, and other inflammatory disorders was truly increased in IBD.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Skin conditions burden many children with inflammatory bowel disease (IBD), according to the authors of a single-center study.
METHODOLOGY:
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with (CD) or ulcerative (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with Crohn’s disease (CD) or ulcerative colitis (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
TAKEAWAY:
- The most common noninfectious dermatologic condition among the 425 children and adolescents was (30.8%), followed by eczema (15.8%) and perianal skin tags (14.6%).
- Angular cheilitis was more common among those with CD than those with UC (7.2% vs 2%, respectively; P = .024) as was keratosis pilaris (6.9% vs 0.7%; P = .003), and perianal skin complications such as skin tags (20.3% vs 4%), fistulas (13.4% vs 2.7%), and abscesses (13.4% vs 2%; P < .001 for all associations).
- Fungal skin infections were more frequently diagnosed in children with UC than those with CD (15.4% vs 8%; P = .017).
- The researchers observed that the severity of IBD correlated with a higher prevalence of perianal fistula (P = .003), perianal region abscess (P = .041), psoriasis (P < .001), and pyoderma gangrenosum (P = .003).
IN PRACTICE:
“Early identification of common dermatologic conditions in children and adolescents with IBD and recognizing their characteristic associations may alter management and improve skin-related outcomes in this patient population,” the authors wrote.
SOURCE:
Corresponding author Megha M. Tollefson, MD, of the Department of Dermatology at Mayo Clinic, Rochester, Minnesota, and colleagues conducted the research, which was published in Pediatric Dermatology.
LIMITATIONS:
The single-center design and the fact that database studies are subject to extraction error. There was no age- and sex-matched cohort to determine whether the prevalence of cutaneous infections, acne, eczema, and other inflammatory disorders was truly increased in IBD.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
Company Announces Regulatory Filing for Nemolizumab for Two Indications
On February 14, 2024, Galderma announced that .
A first-in-class investigational monoclonal antibody specifically designed to inhibit interleukin (IL) IL-31 signaling, nemolizumab has also been granted FDA Priority Review for prurigo nodularis, according to a press release from the company. The European Medicines Agency has also accepted Galderma’s Marketing Authorization Applications for nemolizumab for both prurigo nodularis and atopic dermatitis.
The regulatory developments follow data from the phase III OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in patients with prurigo nodularis (NCT04501679 and NCT04501666). According to the press release, in OLYMPIA 1 and 2, 58% and 56% of patients, respectively, achieved at least a least four-point reduction in itch intensity as measured by the peak-pruritus numerical rating scale (PP-NRS), compared with 17% and 21% in the placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the investigator’s global assessment (IGA) score, compared with 7% and 11% in the placebo groups (P < .0001).
On February 14, 2024, Galderma announced that .
A first-in-class investigational monoclonal antibody specifically designed to inhibit interleukin (IL) IL-31 signaling, nemolizumab has also been granted FDA Priority Review for prurigo nodularis, according to a press release from the company. The European Medicines Agency has also accepted Galderma’s Marketing Authorization Applications for nemolizumab for both prurigo nodularis and atopic dermatitis.
The regulatory developments follow data from the phase III OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in patients with prurigo nodularis (NCT04501679 and NCT04501666). According to the press release, in OLYMPIA 1 and 2, 58% and 56% of patients, respectively, achieved at least a least four-point reduction in itch intensity as measured by the peak-pruritus numerical rating scale (PP-NRS), compared with 17% and 21% in the placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the investigator’s global assessment (IGA) score, compared with 7% and 11% in the placebo groups (P < .0001).
On February 14, 2024, Galderma announced that .
A first-in-class investigational monoclonal antibody specifically designed to inhibit interleukin (IL) IL-31 signaling, nemolizumab has also been granted FDA Priority Review for prurigo nodularis, according to a press release from the company. The European Medicines Agency has also accepted Galderma’s Marketing Authorization Applications for nemolizumab for both prurigo nodularis and atopic dermatitis.
The regulatory developments follow data from the phase III OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in patients with prurigo nodularis (NCT04501679 and NCT04501666). According to the press release, in OLYMPIA 1 and 2, 58% and 56% of patients, respectively, achieved at least a least four-point reduction in itch intensity as measured by the peak-pruritus numerical rating scale (PP-NRS), compared with 17% and 21% in the placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the investigator’s global assessment (IGA) score, compared with 7% and 11% in the placebo groups (P < .0001).
Pretreatment Lab Testing for Chronic Skin Diseases Diverges From Guidelines
in a national commercial insurance claims database.
Because of concerns for the potential reactivation of tuberculosis or hepatitis B or C, or for an increased risk for infections, myelosuppression, and hepatoxicity in the wake of immunomodulator use, some medical societies recommend screening patients for hepatitis B, hepatitis C, and tuberculosis before starting these medications, wrote Maria C. Schneeweiss, MD, of Brigham and Women’s Hospital, Boston, Massachusetts, and colleagues.
“Conducting this study was crucial because of the increasing use of systemic immunomodulatory agents for chronic inflammatory skin diseases and the recognized need for pretreatment testing to prevent complications,” coauthor Denys Shay, a PhD candidate in population health sciences at Harvard University, Cambridge, Massachusetts, said in an interview.
“Despite recommendations from professional societies, there was a lack of clarity on how consistently these guidelines were being followed in the United States. This study aimed to fill that gap in knowledge, providing a comprehensive view of current practices and highlighting areas for improvement,” he said.
In the study, published online in JAMA Dermatology, he and his coauthors identified 122,308 adults in the United States with psoriasis, hidradenitis suppurativa, or atopic dermatitis who started an immunomodulatory agent, including methotrexate (28,684 patients), tumor necrosis factor (TNF)–alpha inhibitors (40,965), ustekinumab (12,841), interleukin (IL)-23 inhibitors (6116), IL-17A inhibitors (9799), dupilumab (7787), and apremilast (16,116). The data were from a commercial insurance claims database from December 31, 2002, to December 31, 2020.
The primary outcome was the proportion of patients who underwent recommended screening lab tests including tuberculosis, hepatitis, liver function, complete blood cell counts (CBCs), and lipid panels within 6 months before treatment initiation and during the first 2 years of treatment. The median age of the study population was 49 years, and 52.1% were male.
A CBC was the most common pretreatment test across treatments, performed in 41%-69% of patients before starting treatment. Tuberculosis screening occurred in 11%-59% of patients within 6 months of initiating treatment, and 3%-26% had updated tests after 1 year. Similarly, 13%-41% of patients underwent hepatitis screening prior to treatment.
The highest levels of pretreatment testing occurred for TNF-alpha inhibitors, ustekinumab, IL-17A inhibitors, and IL-23 inhibitors, with similar patterns, while the lowest levels of testing occurred with apremilast and dupilumab.
Testing prevalence before starting apremilast and after a year of treatment was 15%-45% and 9%-36%, respectively. Testing before initiation and a year into treatment with dupilumab was 11%-41% and 3%-25%, respectively.
The findings were limited by several factors including the descriptive design, which does not allow for evaluation of the testing practices, the researchers said.
However, the results show the extent of patients with chronic inflammatory skin diseases (CISDs) who do not undergo pretreatment testing, and research is needed to create testing practices on the basis of recommendations for each agent and incorporating each patient’s history and clinical profile, they concluded.
“The finding that less than 60% of patients received recommended pretreatment testing was initially somewhat surprising,” Shay said in the interview. “However, the context provided by higher rates of baseline testing within the 6-12 months before treatment initiation and the potential for additional testing not captured by the dataset — such as hospital stays — suggests that the gap may not be as large as this estimate,” he said.
“The key message for clinicians is that there are considerable variations in laboratory testing practices with regard to the initiation of systemic immunomodulatory agents in patients with CISDs,” Shay said. “This represents a divergence from existing testing guidelines.”
“Further research is needed to understand the reasons for the variations in pretreatment testing practices and whether this heterogeneity affects patient outcomes,” he added.
Resist Routine Testing
The study findings represent a call to action in the form of ongoing assessment of the safety, clinical utility, and cost-effectiveness of pretreatment testing, wrote Clinton W. Enos, MD, Ana Ormaza Vera, MD, and Abby S. Van Voorhees, MD, of the Department of Dermatology, Eastern Virginia Medical School, Norfolk, Virginia, in an accompanying editorial.
The data in the current study suggesting less frequent laboratory testing compared with current guidelines could stem from a high comfort level with many of the therapies that have been available and in use for many years, they noted. Clinicians’ lack of knowledge of the laboratory screening and monitoring guidelines also may play a role, they said.
However, the authors cautioned against routine checking of laboratory results “without purpose” and without attention to their clinical utility and cost. “A thorough medical history is essential and can serve as a sensitive indicator of which patients are more at risk for diseases such as TB or hepatitis, thereby allowing for more meaningful laboratory screening use,” they said.
Evidence supporting prescreening labs for the spectrum of systemic agents used in dermatology varies considerably, “some trapped in time and carried forward for decades until finally questioned, others rooted in treatment mechanism and clinical data,” Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington, DC, said in an interview.
The study elucidated the current state of clinical practice, said Friedman, who was not involved with the study. This includes screening even if the label says it is not necessary and letting screening slide when guidelines say otherwise — even if the guidelines are outdated and insurance requires certain metrics prior to approval, he said.
Looking ahead, “we need better consensus and even better communication/education on said guidance,” Dr. Friedman said. “Clear, concise, evidenced-based, and expert-validated guidance to ensure we are meaningfully using medical resources” is what is needed, he added. “It will certainly take a village, and close collaboration between the industry and practitioners is key to success.”
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Shay had no financial conflicts to disclose. Lead author Dr. Schneeweiss disclosed grants from UCB Pharma and AbbVie to Brigham and Women’s Hospital outside the submitted work. Other authors disclosed receiving personal fees from Aetion and grants from UCB Pharma and Takeda outside the submitted work; grants from Amarin, Kowa, Novartis, and Pfizer outside the submitted work; and personal fees from Hims & Hers, AbbVie, Sun Pharmaceuticals, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN, Boehringer Ingelheim, ACOM, Olaplex, and Legacy Healthcare during the study. No other disclosures were reported.
Editorial author Dr. Enos disclosed serving as an investigator for Amgen and Castle Biosciences and receiving grants from Arcutis Biotherapeutics outside the submitted work. Dr. Van Voorhees disclosed an honorarium outside the submitted work.
Dr. Friedman had no relevant financial conflicts to disclose.
A version of this article appeared on Medscape.com.
in a national commercial insurance claims database.
Because of concerns for the potential reactivation of tuberculosis or hepatitis B or C, or for an increased risk for infections, myelosuppression, and hepatoxicity in the wake of immunomodulator use, some medical societies recommend screening patients for hepatitis B, hepatitis C, and tuberculosis before starting these medications, wrote Maria C. Schneeweiss, MD, of Brigham and Women’s Hospital, Boston, Massachusetts, and colleagues.
“Conducting this study was crucial because of the increasing use of systemic immunomodulatory agents for chronic inflammatory skin diseases and the recognized need for pretreatment testing to prevent complications,” coauthor Denys Shay, a PhD candidate in population health sciences at Harvard University, Cambridge, Massachusetts, said in an interview.
“Despite recommendations from professional societies, there was a lack of clarity on how consistently these guidelines were being followed in the United States. This study aimed to fill that gap in knowledge, providing a comprehensive view of current practices and highlighting areas for improvement,” he said.
In the study, published online in JAMA Dermatology, he and his coauthors identified 122,308 adults in the United States with psoriasis, hidradenitis suppurativa, or atopic dermatitis who started an immunomodulatory agent, including methotrexate (28,684 patients), tumor necrosis factor (TNF)–alpha inhibitors (40,965), ustekinumab (12,841), interleukin (IL)-23 inhibitors (6116), IL-17A inhibitors (9799), dupilumab (7787), and apremilast (16,116). The data were from a commercial insurance claims database from December 31, 2002, to December 31, 2020.
The primary outcome was the proportion of patients who underwent recommended screening lab tests including tuberculosis, hepatitis, liver function, complete blood cell counts (CBCs), and lipid panels within 6 months before treatment initiation and during the first 2 years of treatment. The median age of the study population was 49 years, and 52.1% were male.
A CBC was the most common pretreatment test across treatments, performed in 41%-69% of patients before starting treatment. Tuberculosis screening occurred in 11%-59% of patients within 6 months of initiating treatment, and 3%-26% had updated tests after 1 year. Similarly, 13%-41% of patients underwent hepatitis screening prior to treatment.
The highest levels of pretreatment testing occurred for TNF-alpha inhibitors, ustekinumab, IL-17A inhibitors, and IL-23 inhibitors, with similar patterns, while the lowest levels of testing occurred with apremilast and dupilumab.
Testing prevalence before starting apremilast and after a year of treatment was 15%-45% and 9%-36%, respectively. Testing before initiation and a year into treatment with dupilumab was 11%-41% and 3%-25%, respectively.
The findings were limited by several factors including the descriptive design, which does not allow for evaluation of the testing practices, the researchers said.
However, the results show the extent of patients with chronic inflammatory skin diseases (CISDs) who do not undergo pretreatment testing, and research is needed to create testing practices on the basis of recommendations for each agent and incorporating each patient’s history and clinical profile, they concluded.
“The finding that less than 60% of patients received recommended pretreatment testing was initially somewhat surprising,” Shay said in the interview. “However, the context provided by higher rates of baseline testing within the 6-12 months before treatment initiation and the potential for additional testing not captured by the dataset — such as hospital stays — suggests that the gap may not be as large as this estimate,” he said.
“The key message for clinicians is that there are considerable variations in laboratory testing practices with regard to the initiation of systemic immunomodulatory agents in patients with CISDs,” Shay said. “This represents a divergence from existing testing guidelines.”
“Further research is needed to understand the reasons for the variations in pretreatment testing practices and whether this heterogeneity affects patient outcomes,” he added.
Resist Routine Testing
The study findings represent a call to action in the form of ongoing assessment of the safety, clinical utility, and cost-effectiveness of pretreatment testing, wrote Clinton W. Enos, MD, Ana Ormaza Vera, MD, and Abby S. Van Voorhees, MD, of the Department of Dermatology, Eastern Virginia Medical School, Norfolk, Virginia, in an accompanying editorial.
The data in the current study suggesting less frequent laboratory testing compared with current guidelines could stem from a high comfort level with many of the therapies that have been available and in use for many years, they noted. Clinicians’ lack of knowledge of the laboratory screening and monitoring guidelines also may play a role, they said.
However, the authors cautioned against routine checking of laboratory results “without purpose” and without attention to their clinical utility and cost. “A thorough medical history is essential and can serve as a sensitive indicator of which patients are more at risk for diseases such as TB or hepatitis, thereby allowing for more meaningful laboratory screening use,” they said.
Evidence supporting prescreening labs for the spectrum of systemic agents used in dermatology varies considerably, “some trapped in time and carried forward for decades until finally questioned, others rooted in treatment mechanism and clinical data,” Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington, DC, said in an interview.
The study elucidated the current state of clinical practice, said Friedman, who was not involved with the study. This includes screening even if the label says it is not necessary and letting screening slide when guidelines say otherwise — even if the guidelines are outdated and insurance requires certain metrics prior to approval, he said.
Looking ahead, “we need better consensus and even better communication/education on said guidance,” Dr. Friedman said. “Clear, concise, evidenced-based, and expert-validated guidance to ensure we are meaningfully using medical resources” is what is needed, he added. “It will certainly take a village, and close collaboration between the industry and practitioners is key to success.”
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Shay had no financial conflicts to disclose. Lead author Dr. Schneeweiss disclosed grants from UCB Pharma and AbbVie to Brigham and Women’s Hospital outside the submitted work. Other authors disclosed receiving personal fees from Aetion and grants from UCB Pharma and Takeda outside the submitted work; grants from Amarin, Kowa, Novartis, and Pfizer outside the submitted work; and personal fees from Hims & Hers, AbbVie, Sun Pharmaceuticals, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN, Boehringer Ingelheim, ACOM, Olaplex, and Legacy Healthcare during the study. No other disclosures were reported.
Editorial author Dr. Enos disclosed serving as an investigator for Amgen and Castle Biosciences and receiving grants from Arcutis Biotherapeutics outside the submitted work. Dr. Van Voorhees disclosed an honorarium outside the submitted work.
Dr. Friedman had no relevant financial conflicts to disclose.
A version of this article appeared on Medscape.com.
in a national commercial insurance claims database.
Because of concerns for the potential reactivation of tuberculosis or hepatitis B or C, or for an increased risk for infections, myelosuppression, and hepatoxicity in the wake of immunomodulator use, some medical societies recommend screening patients for hepatitis B, hepatitis C, and tuberculosis before starting these medications, wrote Maria C. Schneeweiss, MD, of Brigham and Women’s Hospital, Boston, Massachusetts, and colleagues.
“Conducting this study was crucial because of the increasing use of systemic immunomodulatory agents for chronic inflammatory skin diseases and the recognized need for pretreatment testing to prevent complications,” coauthor Denys Shay, a PhD candidate in population health sciences at Harvard University, Cambridge, Massachusetts, said in an interview.
“Despite recommendations from professional societies, there was a lack of clarity on how consistently these guidelines were being followed in the United States. This study aimed to fill that gap in knowledge, providing a comprehensive view of current practices and highlighting areas for improvement,” he said.
In the study, published online in JAMA Dermatology, he and his coauthors identified 122,308 adults in the United States with psoriasis, hidradenitis suppurativa, or atopic dermatitis who started an immunomodulatory agent, including methotrexate (28,684 patients), tumor necrosis factor (TNF)–alpha inhibitors (40,965), ustekinumab (12,841), interleukin (IL)-23 inhibitors (6116), IL-17A inhibitors (9799), dupilumab (7787), and apremilast (16,116). The data were from a commercial insurance claims database from December 31, 2002, to December 31, 2020.
The primary outcome was the proportion of patients who underwent recommended screening lab tests including tuberculosis, hepatitis, liver function, complete blood cell counts (CBCs), and lipid panels within 6 months before treatment initiation and during the first 2 years of treatment. The median age of the study population was 49 years, and 52.1% were male.
A CBC was the most common pretreatment test across treatments, performed in 41%-69% of patients before starting treatment. Tuberculosis screening occurred in 11%-59% of patients within 6 months of initiating treatment, and 3%-26% had updated tests after 1 year. Similarly, 13%-41% of patients underwent hepatitis screening prior to treatment.
The highest levels of pretreatment testing occurred for TNF-alpha inhibitors, ustekinumab, IL-17A inhibitors, and IL-23 inhibitors, with similar patterns, while the lowest levels of testing occurred with apremilast and dupilumab.
Testing prevalence before starting apremilast and after a year of treatment was 15%-45% and 9%-36%, respectively. Testing before initiation and a year into treatment with dupilumab was 11%-41% and 3%-25%, respectively.
The findings were limited by several factors including the descriptive design, which does not allow for evaluation of the testing practices, the researchers said.
However, the results show the extent of patients with chronic inflammatory skin diseases (CISDs) who do not undergo pretreatment testing, and research is needed to create testing practices on the basis of recommendations for each agent and incorporating each patient’s history and clinical profile, they concluded.
“The finding that less than 60% of patients received recommended pretreatment testing was initially somewhat surprising,” Shay said in the interview. “However, the context provided by higher rates of baseline testing within the 6-12 months before treatment initiation and the potential for additional testing not captured by the dataset — such as hospital stays — suggests that the gap may not be as large as this estimate,” he said.
“The key message for clinicians is that there are considerable variations in laboratory testing practices with regard to the initiation of systemic immunomodulatory agents in patients with CISDs,” Shay said. “This represents a divergence from existing testing guidelines.”
“Further research is needed to understand the reasons for the variations in pretreatment testing practices and whether this heterogeneity affects patient outcomes,” he added.
Resist Routine Testing
The study findings represent a call to action in the form of ongoing assessment of the safety, clinical utility, and cost-effectiveness of pretreatment testing, wrote Clinton W. Enos, MD, Ana Ormaza Vera, MD, and Abby S. Van Voorhees, MD, of the Department of Dermatology, Eastern Virginia Medical School, Norfolk, Virginia, in an accompanying editorial.
The data in the current study suggesting less frequent laboratory testing compared with current guidelines could stem from a high comfort level with many of the therapies that have been available and in use for many years, they noted. Clinicians’ lack of knowledge of the laboratory screening and monitoring guidelines also may play a role, they said.
However, the authors cautioned against routine checking of laboratory results “without purpose” and without attention to their clinical utility and cost. “A thorough medical history is essential and can serve as a sensitive indicator of which patients are more at risk for diseases such as TB or hepatitis, thereby allowing for more meaningful laboratory screening use,” they said.
Evidence supporting prescreening labs for the spectrum of systemic agents used in dermatology varies considerably, “some trapped in time and carried forward for decades until finally questioned, others rooted in treatment mechanism and clinical data,” Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington, DC, said in an interview.
The study elucidated the current state of clinical practice, said Friedman, who was not involved with the study. This includes screening even if the label says it is not necessary and letting screening slide when guidelines say otherwise — even if the guidelines are outdated and insurance requires certain metrics prior to approval, he said.
Looking ahead, “we need better consensus and even better communication/education on said guidance,” Dr. Friedman said. “Clear, concise, evidenced-based, and expert-validated guidance to ensure we are meaningfully using medical resources” is what is needed, he added. “It will certainly take a village, and close collaboration between the industry and practitioners is key to success.”
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Shay had no financial conflicts to disclose. Lead author Dr. Schneeweiss disclosed grants from UCB Pharma and AbbVie to Brigham and Women’s Hospital outside the submitted work. Other authors disclosed receiving personal fees from Aetion and grants from UCB Pharma and Takeda outside the submitted work; grants from Amarin, Kowa, Novartis, and Pfizer outside the submitted work; and personal fees from Hims & Hers, AbbVie, Sun Pharmaceuticals, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN, Boehringer Ingelheim, ACOM, Olaplex, and Legacy Healthcare during the study. No other disclosures were reported.
Editorial author Dr. Enos disclosed serving as an investigator for Amgen and Castle Biosciences and receiving grants from Arcutis Biotherapeutics outside the submitted work. Dr. Van Voorhees disclosed an honorarium outside the submitted work.
Dr. Friedman had no relevant financial conflicts to disclose.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Survey: Dermatology Residents Shortchanged on Sensitive Skin Education
Although sensitive skin affects an estimated 40%-70% of the population, knowledge of the pathophysiology of sensitive skin is incomplete, and consensus is lacking as to the best diagnosis and treatment strategies, and the inclusion of sensitive skin education in dermatology curricula has not been examined, according to Erika T. McCormick, BS, and Adam Friedman, MD, of George Washington University, Washington, DC.
For the study, published in the Journal of Drugs in Dermatology, they developed a 26-question survey for dermatology residents that asked about sensitive skin in dermatology residency training. Participants came from the Orlando Dermatology, Aesthetic, and Surgical Conference email list.
Survey respondents included 214 residents at various levels of training at programs across the United States; 67.1% were female, 92.1% were aged 25-34 years, and 85.5% were in academic or university programs.
Overall, 99% of respondents believed that sensitive skin issues should be part of their residency training to some extent, and 84% reported experiences with patients for whom the chief presenting complaint was sensitive skin.
However, fewer than half (48%) of the residents reported specific resident education in sensitive skin, while 51% reported nonspecific education about sensitive skin education in the context of other skin diseases, and 1% reported no education about sensitive skin.
Less than one-quarter of the respondents who received any sensitive skin education reported feeling comfortable in their ability to diagnose, evaluate, and manage sensitive skin, while those with sensitive skin–specific education were significantly more likely to describe themselves as “very knowledgeable.”
As for treatment approaches, residents with specific sensitive skin education were more likely than were those without sensitive skin–specific training to ask patients about allergies and past reactions to skin products, and to counsel them about environmental triggers.
Notably, 96% of the respondents were not familiar with the Sensitive Skin (SS) Scale–10, a validated measure of sensitive skin severity.
The most common challenges in care of patients with sensitive skin were assessing improvement over time, reported by 25% of respondents, recommending products (23%), and prescribing/medical management (22%). The topics residents expressed most interest in learning about were product recommendations (78%), patient counseling (77%), reviewing research on sensitive skin (70%), diagnosing sensitive skin (67%), using the SS-10 (48%), and clinical research updates (40%).
The findings were limited by several factors including the reliance on self-reports, the researchers noted. However, the results highlight the lack of consensus in treatment of sensitive skin and the need to address this knowledge gap at the residency level, they said.
Improving Tools for Practice
“Many practice patterns and approaches are forged in the fires of training,” corresponding author Dr. Friedman, professor and chair of dermatology and residency program director at George Washington University, said in an interview. “Identifying gaps, especially for heavily prevalent issues, questions, and concerns such as sensitive skin that residents will encounter in practice is important to ensure an educated workforce,” he said.
Education on sensitive skin is lacking because, until recently, research and clinical guidance have been lacking, Dr. Friedman said. The root of the problem is that sensitive skin is mainly considered a symptom, rather than an independent condition, he explained. “Depending on the study, the prevalence of sensitive skin has been reported as high as 70%, with roughly 40% of these patients having no primary skin condition,” he said. This means sensitive skin can be both a symptom and a condition, which causes confusion for clinicians and patients, he added.
“Therefore, in order to overcome this gap, the condition itself at a minimum needs a standard definition and a way to diagnosis, which we fortunately have in the validated research tool known as the SS-10,” said Dr. Friedman.
Almost all residents surveyed in the current study had never heard of the SS-10, but more than half found it to be useful after learning of it through the study survey, he noted.
Looking ahead, greater elucidation of the pathophysiology of sensitive skin is needed to effectively pursue studies of products and treatments for these patients, but the SS-10 can be used to define and monitor the condition to evaluate improvement, he added.
The study was funded by an independent fellowship grant from Galderma. Ms. McCormick is supported by an unrestricted fellowship grant funded by Galderma. Dr. Friedman has served as a consultant for Galderma.
Although sensitive skin affects an estimated 40%-70% of the population, knowledge of the pathophysiology of sensitive skin is incomplete, and consensus is lacking as to the best diagnosis and treatment strategies, and the inclusion of sensitive skin education in dermatology curricula has not been examined, according to Erika T. McCormick, BS, and Adam Friedman, MD, of George Washington University, Washington, DC.
For the study, published in the Journal of Drugs in Dermatology, they developed a 26-question survey for dermatology residents that asked about sensitive skin in dermatology residency training. Participants came from the Orlando Dermatology, Aesthetic, and Surgical Conference email list.
Survey respondents included 214 residents at various levels of training at programs across the United States; 67.1% were female, 92.1% were aged 25-34 years, and 85.5% were in academic or university programs.
Overall, 99% of respondents believed that sensitive skin issues should be part of their residency training to some extent, and 84% reported experiences with patients for whom the chief presenting complaint was sensitive skin.
However, fewer than half (48%) of the residents reported specific resident education in sensitive skin, while 51% reported nonspecific education about sensitive skin education in the context of other skin diseases, and 1% reported no education about sensitive skin.
Less than one-quarter of the respondents who received any sensitive skin education reported feeling comfortable in their ability to diagnose, evaluate, and manage sensitive skin, while those with sensitive skin–specific education were significantly more likely to describe themselves as “very knowledgeable.”
As for treatment approaches, residents with specific sensitive skin education were more likely than were those without sensitive skin–specific training to ask patients about allergies and past reactions to skin products, and to counsel them about environmental triggers.
Notably, 96% of the respondents were not familiar with the Sensitive Skin (SS) Scale–10, a validated measure of sensitive skin severity.
The most common challenges in care of patients with sensitive skin were assessing improvement over time, reported by 25% of respondents, recommending products (23%), and prescribing/medical management (22%). The topics residents expressed most interest in learning about were product recommendations (78%), patient counseling (77%), reviewing research on sensitive skin (70%), diagnosing sensitive skin (67%), using the SS-10 (48%), and clinical research updates (40%).
The findings were limited by several factors including the reliance on self-reports, the researchers noted. However, the results highlight the lack of consensus in treatment of sensitive skin and the need to address this knowledge gap at the residency level, they said.
Improving Tools for Practice
“Many practice patterns and approaches are forged in the fires of training,” corresponding author Dr. Friedman, professor and chair of dermatology and residency program director at George Washington University, said in an interview. “Identifying gaps, especially for heavily prevalent issues, questions, and concerns such as sensitive skin that residents will encounter in practice is important to ensure an educated workforce,” he said.
Education on sensitive skin is lacking because, until recently, research and clinical guidance have been lacking, Dr. Friedman said. The root of the problem is that sensitive skin is mainly considered a symptom, rather than an independent condition, he explained. “Depending on the study, the prevalence of sensitive skin has been reported as high as 70%, with roughly 40% of these patients having no primary skin condition,” he said. This means sensitive skin can be both a symptom and a condition, which causes confusion for clinicians and patients, he added.
“Therefore, in order to overcome this gap, the condition itself at a minimum needs a standard definition and a way to diagnosis, which we fortunately have in the validated research tool known as the SS-10,” said Dr. Friedman.
Almost all residents surveyed in the current study had never heard of the SS-10, but more than half found it to be useful after learning of it through the study survey, he noted.
Looking ahead, greater elucidation of the pathophysiology of sensitive skin is needed to effectively pursue studies of products and treatments for these patients, but the SS-10 can be used to define and monitor the condition to evaluate improvement, he added.
The study was funded by an independent fellowship grant from Galderma. Ms. McCormick is supported by an unrestricted fellowship grant funded by Galderma. Dr. Friedman has served as a consultant for Galderma.
Although sensitive skin affects an estimated 40%-70% of the population, knowledge of the pathophysiology of sensitive skin is incomplete, and consensus is lacking as to the best diagnosis and treatment strategies, and the inclusion of sensitive skin education in dermatology curricula has not been examined, according to Erika T. McCormick, BS, and Adam Friedman, MD, of George Washington University, Washington, DC.
For the study, published in the Journal of Drugs in Dermatology, they developed a 26-question survey for dermatology residents that asked about sensitive skin in dermatology residency training. Participants came from the Orlando Dermatology, Aesthetic, and Surgical Conference email list.
Survey respondents included 214 residents at various levels of training at programs across the United States; 67.1% were female, 92.1% were aged 25-34 years, and 85.5% were in academic or university programs.
Overall, 99% of respondents believed that sensitive skin issues should be part of their residency training to some extent, and 84% reported experiences with patients for whom the chief presenting complaint was sensitive skin.
However, fewer than half (48%) of the residents reported specific resident education in sensitive skin, while 51% reported nonspecific education about sensitive skin education in the context of other skin diseases, and 1% reported no education about sensitive skin.
Less than one-quarter of the respondents who received any sensitive skin education reported feeling comfortable in their ability to diagnose, evaluate, and manage sensitive skin, while those with sensitive skin–specific education were significantly more likely to describe themselves as “very knowledgeable.”
As for treatment approaches, residents with specific sensitive skin education were more likely than were those without sensitive skin–specific training to ask patients about allergies and past reactions to skin products, and to counsel them about environmental triggers.
Notably, 96% of the respondents were not familiar with the Sensitive Skin (SS) Scale–10, a validated measure of sensitive skin severity.
The most common challenges in care of patients with sensitive skin were assessing improvement over time, reported by 25% of respondents, recommending products (23%), and prescribing/medical management (22%). The topics residents expressed most interest in learning about were product recommendations (78%), patient counseling (77%), reviewing research on sensitive skin (70%), diagnosing sensitive skin (67%), using the SS-10 (48%), and clinical research updates (40%).
The findings were limited by several factors including the reliance on self-reports, the researchers noted. However, the results highlight the lack of consensus in treatment of sensitive skin and the need to address this knowledge gap at the residency level, they said.
Improving Tools for Practice
“Many practice patterns and approaches are forged in the fires of training,” corresponding author Dr. Friedman, professor and chair of dermatology and residency program director at George Washington University, said in an interview. “Identifying gaps, especially for heavily prevalent issues, questions, and concerns such as sensitive skin that residents will encounter in practice is important to ensure an educated workforce,” he said.
Education on sensitive skin is lacking because, until recently, research and clinical guidance have been lacking, Dr. Friedman said. The root of the problem is that sensitive skin is mainly considered a symptom, rather than an independent condition, he explained. “Depending on the study, the prevalence of sensitive skin has been reported as high as 70%, with roughly 40% of these patients having no primary skin condition,” he said. This means sensitive skin can be both a symptom and a condition, which causes confusion for clinicians and patients, he added.
“Therefore, in order to overcome this gap, the condition itself at a minimum needs a standard definition and a way to diagnosis, which we fortunately have in the validated research tool known as the SS-10,” said Dr. Friedman.
Almost all residents surveyed in the current study had never heard of the SS-10, but more than half found it to be useful after learning of it through the study survey, he noted.
Looking ahead, greater elucidation of the pathophysiology of sensitive skin is needed to effectively pursue studies of products and treatments for these patients, but the SS-10 can be used to define and monitor the condition to evaluate improvement, he added.
The study was funded by an independent fellowship grant from Galderma. Ms. McCormick is supported by an unrestricted fellowship grant funded by Galderma. Dr. Friedman has served as a consultant for Galderma.
FROM THE JOURNAL OF DRUGS IN DERMATOLOGY
New Tools on the Horizon for Managing cSCC in Solid Organ Transplant Recipients
The patient had an advanced cutaneous squamous cell carcinoma (cSCC) on the face that seemed to be affecting the facial nerve, ruling out aggressive surgery. When Mohs surgery failed to clear the tumor, radiation was ordered. But the best option — an immune checkpoint inhibitor — could not be administered because the patient was a lung transplant recipient.
Although approved for metastatic cSCC, immune checkpoint inhibitors are associated with a higher potential for rejection of an organ transplant.
“The feeling is that the risk of rejection is just too great if we were to try to give an immune checkpoint inhibitor,” said Sean Christensen, MD, PhD, director of dermatologic surgery at Yale Dermatology–Branford, in Connecticut, who was treating the patient. Dr. Christensen consulted with the transplant team, and together they decided to switch the patient to sirolimus, an immunosuppressant that has been shown to have less risk of promoting skin cancer in those who take the medication. Sirolimus, however, is not as well tolerated as the usual first-line immunosuppressant, tacrolimus.
Organ transplant recipients have a 200-fold increased incidence of keratinocyte carcinoma compared with immunocompetent individuals, and cSCC accounts for 80% of skin cancers in those recipients, according to a 2022 paper published in Transplant International, by Matthew Bottomley, MRCP, and colleagues at the University of Oxford, England.
And in a 2017 JAMA Dermatology study on skin cancer in organ transplant recipients in the United States, Sarah Arron, MD, and colleagues, wrote that posttransplant cSCC has an incidence of 812 per 100,000 person-years. To put that in perspective, breast cancer has an incidence of 126 per 100,000 person-years and prostate cancer, an incidence of 112 per 100,000 person-years, according to data from the Surveillance, Epidemiology, and End Results (SEER) Program and the Centers for Disease Control and Prevention, respectively.
Once a transplant recipient has a single cSCC, he or she is at higher risk for developing multiple lesions and is at greatly increased risk for metastasis and death. Skin cancer-specific mortality in transplants patients is ninefold higher than for immunocompetent patients, reported Johns Hopkins dermatologist Kristin Page Bibee, MD, PhD, and colleagues in a 2020 paper in Oral Oncology.
Clinicians focus primarily on reducing patients’ sun exposure to prevent precancerous and cancerous lesions. While field therapy, such as topical 5-flourouracil, and systemic therapy, including acitretin, can be as effective in treating cSCCs as they are for immunocompetent patients, dermatologists are hoping for more tools.
Dr. Christensen, associate professor of dermatology, Yale University, told this news organization that immune checkpoint inhibitors might become more useful in the future as trials are exploring the feasibility of injecting them directly into the cancers. “That’s a really exciting area of research,” he said, noting that direct injection would lower the risk of transplant rejection.
In an interview, Dr. Bottomley said that he is excited about new techniques, such as high-resolution spatial transcriptomic and proteomic profiling. Those techniques will allow researchers “to identify new pathways and mechanisms that we can target to reduce cSCC risk in both immunocompetent and immunosuppressed patients, ideally without the increased risk of graft rejection that we see with immune checkpoint inhibitors,” said Dr. Bottomley, a consultant nephrologist in the Oxford Kidney and Transplant Unit at Churchill Hospital.
Reducing Risk Factors
Dr. Bottomley said that there’s also been renewed effort to identify how to reduce cSCC risk in transplant recipients through recently developed consensus guidelines and a proposed decision framework developed by Dr. Bottomley and colleagues. The evidence will help clinicians have “greater confidence in making early interventions,” he said.
Currently, solid organ transplant patients are told to reduce sun exposure, in part because the majority of cSCCs occur in sun-exposed areas, such as the head and neck, and ultraviolet radiation leads to mutations. “Sun protection is critical,” Dr. Christensen said. That’s especially true in younger transplant recipients, who may have decades of sun exposure, he said.
The immunosuppressive medications also increase cancer risk, for a variety of reasons. One of the more-commonly used immunosuppressants in the past, azathioprine, is itself carcinogenic. Other antirejection medications, such as tacrolimus and mycophenolate, may also induce mutagenic changes that give rise to malignancies, according to the paper by Dr. Bibee, assistant professor of dermatology at Johns Hopkins, Baltimore.
Both Dr. Bibee, in her paper, and Dr. Arron, in an interview, noted that voriconazole, an antifungal used to prevent Aspergillus infection after lung transplant, has been associated with an increase in cSCC in lung transplant recipients.
In addition, immunosuppression essentially “blocks the body’s immune system from recognizing that there are abnormal cancerous cells present,” Dr. Arron, a dermatologist in private practice in Burlingame, California, told this news organization.
Previously, while at the High-Risk Skin Cancer Program at University of California, San Francisco (UCSF), Dr. Arron and others studied whether human papillomavirus (HPV) might play a role in spurring the development of cSCC formation in the immunocompromised. HPV is highly prevalent on the skin, but the virus found on the skin tends to be composed of lower-risk strains.
“In our research, we did not find any biologic mechanism by which this virus might be driving these cancers,” said Dr. Arron, although she said that some researchers “feel very strongly that HPV must be in some way a driver.”
Dr. Bottomley believes that HPV’s role has not been completely determined. The excess incidence of cSCC suggests a virus might be involved, as has been seen with excess risk of lymphoma in patients with Epstein-Barr virus, he said.
Some of his research is focusing on whether advanced immune aging is an independent risk factor for subsequent cSCC development in solid organ transplant recipients. The immune system undergoes changes as people age, and the speed of this process varies from patient to patient, which means immune age can be different from chronological age, said Dr. Bottomley. “We’re still exploring why immune aging should predispose you to cSCC,” he said.
When to Intervene?
Transplant patients are followed by dermatologists at regular intervals. But guidelines are not consistent on the recommended timing of those intervals.
Dr. Arron and colleagues in 2019 created a risk prediction module that recommended frequency of follow-up based on low, medium, high, or very high risk. The tool is available to clinicians in an app called SUNTRAC, or the Skin and Ultraviolet Neoplasia Transplant Risk Assessment Calculator.
A question that Dr. Arron said dermatologists and transplant specialists have wrangled with: How early can they intervene to prevent further lesions?
In the 2022 decision framework paper in Transplant International, Dr. Bottomley and dermatology colleagues from around the world attempted to better delineate when and how clinicians should intervene when a cSCC is first detected. That first cSCC “should be regarded as a ‘red flag’ heralding an increased risk of further skin cancers and possibly internal malignancies,” the authors wrote. That moment is “a key opportunity to proactively consider secondary preventive strategies,” they wrote, but noted that the best interventions and “their sequencing remain unclear,” indicating the need for further research.
Coordinating With the Transplant Team
A key strategy to help prevent cSCC development — suggested in Dr. Bottomley’s paper, and by Dr. Arron and Dr. Christensen — is to consult with the transplant team on potentially changing a patient’s immunosuppressive medication or reducing the dose.
Dr. Arron said that a decade ago, it was somewhat of a novel concept, requiring data-sharing and making personal connections with the transplant team to forge trusting relationships. By the time she left UCSF a few years ago, she said, “the transplant program was very much on board with preventing and treating skin cancer and oftentimes they were making changes even before I would suggest them.”
Suggesting a change or dose reduction is not undertaken lightly. “Our transplant physician colleagues are balancing multiple problems in very sick patients, of which skin cancer might be one, but not the most pressing one in the setting of other transplant complications,” said Dr. Arron.
Dr. Bottomley said that “as transplant physicians, we very much respect and value the input of our dermatology colleagues,” but agreed that many factors “outside malignancy risk” must be weighed when considering changing an immunosuppressive regimen.
In a Delphi Consensus Statement on prevention of cSCC in organ transplant recipients, published in 2021 in JAMA Dermatology, the authors recommended having discussions about immunosuppression with transplant specialists, but did not make a recommendation on what strategy to use. The consensus panel said it preferred “to defer this decision to transplant physicians.”
Acitretin a Go, Nicotinamide Not So Much
Outside of changing an immunosuppressive regimen, among the interventions for secondary prevention are acitretin, the systemic retinoid, and nicotinamide, a form of niacin.
Dr. Christensen conducted a small retrospective investigation evaluating the effectiveness of acitretin in reducing cSCC in both immunocompromised and immunocompetent patients who had received care at Yale, which was recently published in the Journal of the American Academy of Dermatology. Acitretin reduced invasive cSCC by about 75% in both patient groups — a surprising result for the immunocompetent group, but well-established in patients who have had a solid organ transplant. But acitretin had no effect on cSCC in situ or basal cell carcinoma. “The benefit of acitretin is primarily in preventing the invasive SCC,” said Dr. Christensen, which is why he tends to reserve it for patients who have already had several cSCCs.
“It’s not a completely benign medication,” he said, noting the need for monitoring for cholesterol and liver function.
Several years ago, a study in immunocompetent patients, published in the New England Journal of Medicine, found that nicotinamide (also known as niacinamide) reduced the rate of nonmelonoma skin cancer by 23%, giving clinicians hope that it might also be a low-risk, low-cost cancer preventive for solid organ transplant patients. But enthusiasm has dampened since a 2023 study in the New England Journal of Medicine found that the vitamin did not reduce cSCCs in transplant recipients.
Dr. Christensen said he believes the most-recent study wasn’t powered to detect a 25% reduction in cancers. “It’s certainly possible that it still works exactly the same way in transplant patients that it does in immunocompetent patients,” he said. “There’s very little risk of recommending it to patients for general prevention. But it probably has a very modest effect in many,” he said.
Dr. Arron agreed, saying, “it may be that we simply need bigger studies to achieve that statistical significance.” Even so, she said she would not use the therapy “until there is more evidence supporting the use of nicotinamide in transplant recipients.”
Immune checkpoint inhibitors such as cemiplimab and pembrolizumab have been approved by the US Food and Drug Administration for advanced cSCC; nivolumab is another drug in the same class that has not yet been approved for cSCC. But “there’s always been a fear — and a legitimate fear — that if you gave those to organ transplant recipients they would reject their organ,” said Dr. Christensen.
Patients who take the checkpoint inhibitors may first have to stop taking their antirejection drugs, leaving them at risk. It also appears that the checkpoint inhibitors themselves contribute to organ rejection. Recent studies suggest that “the rate of organ rejection is only about 30% to 40%,” with the checkpoint inhibitors, said Dr. Christensen. “Obviously that’s still not an ideal outcome,” he said, but noted that with patients who have inoperable metastatic cSCC, “immune therapy can be a good option.”
Dr. Christensen reported no disclosures. Dr. Bottomley has previously received speaker fees and an educational grant from Astellas. Dr. Arron disclosed ties with Regeneron, Castle Biosciences, and Enspectra Health, not specific to transplantation.
The patient had an advanced cutaneous squamous cell carcinoma (cSCC) on the face that seemed to be affecting the facial nerve, ruling out aggressive surgery. When Mohs surgery failed to clear the tumor, radiation was ordered. But the best option — an immune checkpoint inhibitor — could not be administered because the patient was a lung transplant recipient.
Although approved for metastatic cSCC, immune checkpoint inhibitors are associated with a higher potential for rejection of an organ transplant.
“The feeling is that the risk of rejection is just too great if we were to try to give an immune checkpoint inhibitor,” said Sean Christensen, MD, PhD, director of dermatologic surgery at Yale Dermatology–Branford, in Connecticut, who was treating the patient. Dr. Christensen consulted with the transplant team, and together they decided to switch the patient to sirolimus, an immunosuppressant that has been shown to have less risk of promoting skin cancer in those who take the medication. Sirolimus, however, is not as well tolerated as the usual first-line immunosuppressant, tacrolimus.
Organ transplant recipients have a 200-fold increased incidence of keratinocyte carcinoma compared with immunocompetent individuals, and cSCC accounts for 80% of skin cancers in those recipients, according to a 2022 paper published in Transplant International, by Matthew Bottomley, MRCP, and colleagues at the University of Oxford, England.
And in a 2017 JAMA Dermatology study on skin cancer in organ transplant recipients in the United States, Sarah Arron, MD, and colleagues, wrote that posttransplant cSCC has an incidence of 812 per 100,000 person-years. To put that in perspective, breast cancer has an incidence of 126 per 100,000 person-years and prostate cancer, an incidence of 112 per 100,000 person-years, according to data from the Surveillance, Epidemiology, and End Results (SEER) Program and the Centers for Disease Control and Prevention, respectively.
Once a transplant recipient has a single cSCC, he or she is at higher risk for developing multiple lesions and is at greatly increased risk for metastasis and death. Skin cancer-specific mortality in transplants patients is ninefold higher than for immunocompetent patients, reported Johns Hopkins dermatologist Kristin Page Bibee, MD, PhD, and colleagues in a 2020 paper in Oral Oncology.
Clinicians focus primarily on reducing patients’ sun exposure to prevent precancerous and cancerous lesions. While field therapy, such as topical 5-flourouracil, and systemic therapy, including acitretin, can be as effective in treating cSCCs as they are for immunocompetent patients, dermatologists are hoping for more tools.
Dr. Christensen, associate professor of dermatology, Yale University, told this news organization that immune checkpoint inhibitors might become more useful in the future as trials are exploring the feasibility of injecting them directly into the cancers. “That’s a really exciting area of research,” he said, noting that direct injection would lower the risk of transplant rejection.
In an interview, Dr. Bottomley said that he is excited about new techniques, such as high-resolution spatial transcriptomic and proteomic profiling. Those techniques will allow researchers “to identify new pathways and mechanisms that we can target to reduce cSCC risk in both immunocompetent and immunosuppressed patients, ideally without the increased risk of graft rejection that we see with immune checkpoint inhibitors,” said Dr. Bottomley, a consultant nephrologist in the Oxford Kidney and Transplant Unit at Churchill Hospital.
Reducing Risk Factors
Dr. Bottomley said that there’s also been renewed effort to identify how to reduce cSCC risk in transplant recipients through recently developed consensus guidelines and a proposed decision framework developed by Dr. Bottomley and colleagues. The evidence will help clinicians have “greater confidence in making early interventions,” he said.
Currently, solid organ transplant patients are told to reduce sun exposure, in part because the majority of cSCCs occur in sun-exposed areas, such as the head and neck, and ultraviolet radiation leads to mutations. “Sun protection is critical,” Dr. Christensen said. That’s especially true in younger transplant recipients, who may have decades of sun exposure, he said.
The immunosuppressive medications also increase cancer risk, for a variety of reasons. One of the more-commonly used immunosuppressants in the past, azathioprine, is itself carcinogenic. Other antirejection medications, such as tacrolimus and mycophenolate, may also induce mutagenic changes that give rise to malignancies, according to the paper by Dr. Bibee, assistant professor of dermatology at Johns Hopkins, Baltimore.
Both Dr. Bibee, in her paper, and Dr. Arron, in an interview, noted that voriconazole, an antifungal used to prevent Aspergillus infection after lung transplant, has been associated with an increase in cSCC in lung transplant recipients.
In addition, immunosuppression essentially “blocks the body’s immune system from recognizing that there are abnormal cancerous cells present,” Dr. Arron, a dermatologist in private practice in Burlingame, California, told this news organization.
Previously, while at the High-Risk Skin Cancer Program at University of California, San Francisco (UCSF), Dr. Arron and others studied whether human papillomavirus (HPV) might play a role in spurring the development of cSCC formation in the immunocompromised. HPV is highly prevalent on the skin, but the virus found on the skin tends to be composed of lower-risk strains.
“In our research, we did not find any biologic mechanism by which this virus might be driving these cancers,” said Dr. Arron, although she said that some researchers “feel very strongly that HPV must be in some way a driver.”
Dr. Bottomley believes that HPV’s role has not been completely determined. The excess incidence of cSCC suggests a virus might be involved, as has been seen with excess risk of lymphoma in patients with Epstein-Barr virus, he said.
Some of his research is focusing on whether advanced immune aging is an independent risk factor for subsequent cSCC development in solid organ transplant recipients. The immune system undergoes changes as people age, and the speed of this process varies from patient to patient, which means immune age can be different from chronological age, said Dr. Bottomley. “We’re still exploring why immune aging should predispose you to cSCC,” he said.
When to Intervene?
Transplant patients are followed by dermatologists at regular intervals. But guidelines are not consistent on the recommended timing of those intervals.
Dr. Arron and colleagues in 2019 created a risk prediction module that recommended frequency of follow-up based on low, medium, high, or very high risk. The tool is available to clinicians in an app called SUNTRAC, or the Skin and Ultraviolet Neoplasia Transplant Risk Assessment Calculator.
A question that Dr. Arron said dermatologists and transplant specialists have wrangled with: How early can they intervene to prevent further lesions?
In the 2022 decision framework paper in Transplant International, Dr. Bottomley and dermatology colleagues from around the world attempted to better delineate when and how clinicians should intervene when a cSCC is first detected. That first cSCC “should be regarded as a ‘red flag’ heralding an increased risk of further skin cancers and possibly internal malignancies,” the authors wrote. That moment is “a key opportunity to proactively consider secondary preventive strategies,” they wrote, but noted that the best interventions and “their sequencing remain unclear,” indicating the need for further research.
Coordinating With the Transplant Team
A key strategy to help prevent cSCC development — suggested in Dr. Bottomley’s paper, and by Dr. Arron and Dr. Christensen — is to consult with the transplant team on potentially changing a patient’s immunosuppressive medication or reducing the dose.
Dr. Arron said that a decade ago, it was somewhat of a novel concept, requiring data-sharing and making personal connections with the transplant team to forge trusting relationships. By the time she left UCSF a few years ago, she said, “the transplant program was very much on board with preventing and treating skin cancer and oftentimes they were making changes even before I would suggest them.”
Suggesting a change or dose reduction is not undertaken lightly. “Our transplant physician colleagues are balancing multiple problems in very sick patients, of which skin cancer might be one, but not the most pressing one in the setting of other transplant complications,” said Dr. Arron.
Dr. Bottomley said that “as transplant physicians, we very much respect and value the input of our dermatology colleagues,” but agreed that many factors “outside malignancy risk” must be weighed when considering changing an immunosuppressive regimen.
In a Delphi Consensus Statement on prevention of cSCC in organ transplant recipients, published in 2021 in JAMA Dermatology, the authors recommended having discussions about immunosuppression with transplant specialists, but did not make a recommendation on what strategy to use. The consensus panel said it preferred “to defer this decision to transplant physicians.”
Acitretin a Go, Nicotinamide Not So Much
Outside of changing an immunosuppressive regimen, among the interventions for secondary prevention are acitretin, the systemic retinoid, and nicotinamide, a form of niacin.
Dr. Christensen conducted a small retrospective investigation evaluating the effectiveness of acitretin in reducing cSCC in both immunocompromised and immunocompetent patients who had received care at Yale, which was recently published in the Journal of the American Academy of Dermatology. Acitretin reduced invasive cSCC by about 75% in both patient groups — a surprising result for the immunocompetent group, but well-established in patients who have had a solid organ transplant. But acitretin had no effect on cSCC in situ or basal cell carcinoma. “The benefit of acitretin is primarily in preventing the invasive SCC,” said Dr. Christensen, which is why he tends to reserve it for patients who have already had several cSCCs.
“It’s not a completely benign medication,” he said, noting the need for monitoring for cholesterol and liver function.
Several years ago, a study in immunocompetent patients, published in the New England Journal of Medicine, found that nicotinamide (also known as niacinamide) reduced the rate of nonmelonoma skin cancer by 23%, giving clinicians hope that it might also be a low-risk, low-cost cancer preventive for solid organ transplant patients. But enthusiasm has dampened since a 2023 study in the New England Journal of Medicine found that the vitamin did not reduce cSCCs in transplant recipients.
Dr. Christensen said he believes the most-recent study wasn’t powered to detect a 25% reduction in cancers. “It’s certainly possible that it still works exactly the same way in transplant patients that it does in immunocompetent patients,” he said. “There’s very little risk of recommending it to patients for general prevention. But it probably has a very modest effect in many,” he said.
Dr. Arron agreed, saying, “it may be that we simply need bigger studies to achieve that statistical significance.” Even so, she said she would not use the therapy “until there is more evidence supporting the use of nicotinamide in transplant recipients.”
Immune checkpoint inhibitors such as cemiplimab and pembrolizumab have been approved by the US Food and Drug Administration for advanced cSCC; nivolumab is another drug in the same class that has not yet been approved for cSCC. But “there’s always been a fear — and a legitimate fear — that if you gave those to organ transplant recipients they would reject their organ,” said Dr. Christensen.
Patients who take the checkpoint inhibitors may first have to stop taking their antirejection drugs, leaving them at risk. It also appears that the checkpoint inhibitors themselves contribute to organ rejection. Recent studies suggest that “the rate of organ rejection is only about 30% to 40%,” with the checkpoint inhibitors, said Dr. Christensen. “Obviously that’s still not an ideal outcome,” he said, but noted that with patients who have inoperable metastatic cSCC, “immune therapy can be a good option.”
Dr. Christensen reported no disclosures. Dr. Bottomley has previously received speaker fees and an educational grant from Astellas. Dr. Arron disclosed ties with Regeneron, Castle Biosciences, and Enspectra Health, not specific to transplantation.
The patient had an advanced cutaneous squamous cell carcinoma (cSCC) on the face that seemed to be affecting the facial nerve, ruling out aggressive surgery. When Mohs surgery failed to clear the tumor, radiation was ordered. But the best option — an immune checkpoint inhibitor — could not be administered because the patient was a lung transplant recipient.
Although approved for metastatic cSCC, immune checkpoint inhibitors are associated with a higher potential for rejection of an organ transplant.
“The feeling is that the risk of rejection is just too great if we were to try to give an immune checkpoint inhibitor,” said Sean Christensen, MD, PhD, director of dermatologic surgery at Yale Dermatology–Branford, in Connecticut, who was treating the patient. Dr. Christensen consulted with the transplant team, and together they decided to switch the patient to sirolimus, an immunosuppressant that has been shown to have less risk of promoting skin cancer in those who take the medication. Sirolimus, however, is not as well tolerated as the usual first-line immunosuppressant, tacrolimus.
Organ transplant recipients have a 200-fold increased incidence of keratinocyte carcinoma compared with immunocompetent individuals, and cSCC accounts for 80% of skin cancers in those recipients, according to a 2022 paper published in Transplant International, by Matthew Bottomley, MRCP, and colleagues at the University of Oxford, England.
And in a 2017 JAMA Dermatology study on skin cancer in organ transplant recipients in the United States, Sarah Arron, MD, and colleagues, wrote that posttransplant cSCC has an incidence of 812 per 100,000 person-years. To put that in perspective, breast cancer has an incidence of 126 per 100,000 person-years and prostate cancer, an incidence of 112 per 100,000 person-years, according to data from the Surveillance, Epidemiology, and End Results (SEER) Program and the Centers for Disease Control and Prevention, respectively.
Once a transplant recipient has a single cSCC, he or she is at higher risk for developing multiple lesions and is at greatly increased risk for metastasis and death. Skin cancer-specific mortality in transplants patients is ninefold higher than for immunocompetent patients, reported Johns Hopkins dermatologist Kristin Page Bibee, MD, PhD, and colleagues in a 2020 paper in Oral Oncology.
Clinicians focus primarily on reducing patients’ sun exposure to prevent precancerous and cancerous lesions. While field therapy, such as topical 5-flourouracil, and systemic therapy, including acitretin, can be as effective in treating cSCCs as they are for immunocompetent patients, dermatologists are hoping for more tools.
Dr. Christensen, associate professor of dermatology, Yale University, told this news organization that immune checkpoint inhibitors might become more useful in the future as trials are exploring the feasibility of injecting them directly into the cancers. “That’s a really exciting area of research,” he said, noting that direct injection would lower the risk of transplant rejection.
In an interview, Dr. Bottomley said that he is excited about new techniques, such as high-resolution spatial transcriptomic and proteomic profiling. Those techniques will allow researchers “to identify new pathways and mechanisms that we can target to reduce cSCC risk in both immunocompetent and immunosuppressed patients, ideally without the increased risk of graft rejection that we see with immune checkpoint inhibitors,” said Dr. Bottomley, a consultant nephrologist in the Oxford Kidney and Transplant Unit at Churchill Hospital.
Reducing Risk Factors
Dr. Bottomley said that there’s also been renewed effort to identify how to reduce cSCC risk in transplant recipients through recently developed consensus guidelines and a proposed decision framework developed by Dr. Bottomley and colleagues. The evidence will help clinicians have “greater confidence in making early interventions,” he said.
Currently, solid organ transplant patients are told to reduce sun exposure, in part because the majority of cSCCs occur in sun-exposed areas, such as the head and neck, and ultraviolet radiation leads to mutations. “Sun protection is critical,” Dr. Christensen said. That’s especially true in younger transplant recipients, who may have decades of sun exposure, he said.
The immunosuppressive medications also increase cancer risk, for a variety of reasons. One of the more-commonly used immunosuppressants in the past, azathioprine, is itself carcinogenic. Other antirejection medications, such as tacrolimus and mycophenolate, may also induce mutagenic changes that give rise to malignancies, according to the paper by Dr. Bibee, assistant professor of dermatology at Johns Hopkins, Baltimore.
Both Dr. Bibee, in her paper, and Dr. Arron, in an interview, noted that voriconazole, an antifungal used to prevent Aspergillus infection after lung transplant, has been associated with an increase in cSCC in lung transplant recipients.
In addition, immunosuppression essentially “blocks the body’s immune system from recognizing that there are abnormal cancerous cells present,” Dr. Arron, a dermatologist in private practice in Burlingame, California, told this news organization.
Previously, while at the High-Risk Skin Cancer Program at University of California, San Francisco (UCSF), Dr. Arron and others studied whether human papillomavirus (HPV) might play a role in spurring the development of cSCC formation in the immunocompromised. HPV is highly prevalent on the skin, but the virus found on the skin tends to be composed of lower-risk strains.
“In our research, we did not find any biologic mechanism by which this virus might be driving these cancers,” said Dr. Arron, although she said that some researchers “feel very strongly that HPV must be in some way a driver.”
Dr. Bottomley believes that HPV’s role has not been completely determined. The excess incidence of cSCC suggests a virus might be involved, as has been seen with excess risk of lymphoma in patients with Epstein-Barr virus, he said.
Some of his research is focusing on whether advanced immune aging is an independent risk factor for subsequent cSCC development in solid organ transplant recipients. The immune system undergoes changes as people age, and the speed of this process varies from patient to patient, which means immune age can be different from chronological age, said Dr. Bottomley. “We’re still exploring why immune aging should predispose you to cSCC,” he said.
When to Intervene?
Transplant patients are followed by dermatologists at regular intervals. But guidelines are not consistent on the recommended timing of those intervals.
Dr. Arron and colleagues in 2019 created a risk prediction module that recommended frequency of follow-up based on low, medium, high, or very high risk. The tool is available to clinicians in an app called SUNTRAC, or the Skin and Ultraviolet Neoplasia Transplant Risk Assessment Calculator.
A question that Dr. Arron said dermatologists and transplant specialists have wrangled with: How early can they intervene to prevent further lesions?
In the 2022 decision framework paper in Transplant International, Dr. Bottomley and dermatology colleagues from around the world attempted to better delineate when and how clinicians should intervene when a cSCC is first detected. That first cSCC “should be regarded as a ‘red flag’ heralding an increased risk of further skin cancers and possibly internal malignancies,” the authors wrote. That moment is “a key opportunity to proactively consider secondary preventive strategies,” they wrote, but noted that the best interventions and “their sequencing remain unclear,” indicating the need for further research.
Coordinating With the Transplant Team
A key strategy to help prevent cSCC development — suggested in Dr. Bottomley’s paper, and by Dr. Arron and Dr. Christensen — is to consult with the transplant team on potentially changing a patient’s immunosuppressive medication or reducing the dose.
Dr. Arron said that a decade ago, it was somewhat of a novel concept, requiring data-sharing and making personal connections with the transplant team to forge trusting relationships. By the time she left UCSF a few years ago, she said, “the transplant program was very much on board with preventing and treating skin cancer and oftentimes they were making changes even before I would suggest them.”
Suggesting a change or dose reduction is not undertaken lightly. “Our transplant physician colleagues are balancing multiple problems in very sick patients, of which skin cancer might be one, but not the most pressing one in the setting of other transplant complications,” said Dr. Arron.
Dr. Bottomley said that “as transplant physicians, we very much respect and value the input of our dermatology colleagues,” but agreed that many factors “outside malignancy risk” must be weighed when considering changing an immunosuppressive regimen.
In a Delphi Consensus Statement on prevention of cSCC in organ transplant recipients, published in 2021 in JAMA Dermatology, the authors recommended having discussions about immunosuppression with transplant specialists, but did not make a recommendation on what strategy to use. The consensus panel said it preferred “to defer this decision to transplant physicians.”
Acitretin a Go, Nicotinamide Not So Much
Outside of changing an immunosuppressive regimen, among the interventions for secondary prevention are acitretin, the systemic retinoid, and nicotinamide, a form of niacin.
Dr. Christensen conducted a small retrospective investigation evaluating the effectiveness of acitretin in reducing cSCC in both immunocompromised and immunocompetent patients who had received care at Yale, which was recently published in the Journal of the American Academy of Dermatology. Acitretin reduced invasive cSCC by about 75% in both patient groups — a surprising result for the immunocompetent group, but well-established in patients who have had a solid organ transplant. But acitretin had no effect on cSCC in situ or basal cell carcinoma. “The benefit of acitretin is primarily in preventing the invasive SCC,” said Dr. Christensen, which is why he tends to reserve it for patients who have already had several cSCCs.
“It’s not a completely benign medication,” he said, noting the need for monitoring for cholesterol and liver function.
Several years ago, a study in immunocompetent patients, published in the New England Journal of Medicine, found that nicotinamide (also known as niacinamide) reduced the rate of nonmelonoma skin cancer by 23%, giving clinicians hope that it might also be a low-risk, low-cost cancer preventive for solid organ transplant patients. But enthusiasm has dampened since a 2023 study in the New England Journal of Medicine found that the vitamin did not reduce cSCCs in transplant recipients.
Dr. Christensen said he believes the most-recent study wasn’t powered to detect a 25% reduction in cancers. “It’s certainly possible that it still works exactly the same way in transplant patients that it does in immunocompetent patients,” he said. “There’s very little risk of recommending it to patients for general prevention. But it probably has a very modest effect in many,” he said.
Dr. Arron agreed, saying, “it may be that we simply need bigger studies to achieve that statistical significance.” Even so, she said she would not use the therapy “until there is more evidence supporting the use of nicotinamide in transplant recipients.”
Immune checkpoint inhibitors such as cemiplimab and pembrolizumab have been approved by the US Food and Drug Administration for advanced cSCC; nivolumab is another drug in the same class that has not yet been approved for cSCC. But “there’s always been a fear — and a legitimate fear — that if you gave those to organ transplant recipients they would reject their organ,” said Dr. Christensen.
Patients who take the checkpoint inhibitors may first have to stop taking their antirejection drugs, leaving them at risk. It also appears that the checkpoint inhibitors themselves contribute to organ rejection. Recent studies suggest that “the rate of organ rejection is only about 30% to 40%,” with the checkpoint inhibitors, said Dr. Christensen. “Obviously that’s still not an ideal outcome,” he said, but noted that with patients who have inoperable metastatic cSCC, “immune therapy can be a good option.”
Dr. Christensen reported no disclosures. Dr. Bottomley has previously received speaker fees and an educational grant from Astellas. Dr. Arron disclosed ties with Regeneron, Castle Biosciences, and Enspectra Health, not specific to transplantation.
Success with Sirolimus in Treating Skin Sarcoidosis Could Spur Studies in Other Organs
Sirolimus may be an effective treatment for patients with persistent cutaneous sarcoidosis.
In a small clinical trial, 7 of 10 patients treated with sirolimus via oral solution had improvements in skin lesions after 4 months, which was sustained for up to 2 years after the study concluded.
The results suggested that mechanistic target of rapamycin (mTOR) inhibition is a potential therapeutic avenue for sarcoidosis, which the authors said should be explored in larger clinical trials.
In the past decade, there has been a growing amount of evidence suggesting mTOR’s role in sarcoidosis. In 2017, researchers showed that activation of mTOR in macrophages could cause progressive sarcoidosis in mice. In additional studies, high levels of mTOR activity were detected in human sarcoidosis granulomas in various organs, including the skin, lung, and heart.
Three case reports also documented using the mTOR inhibitor sirolimus to effectively treat systemic sarcoidosis.
“Although all reports observed improvement of the disease following the treatment, no clinical trial investigating the efficacy and safety of sirolimus in patients with sarcoidosis had been published” prior to this study, wrote senior author Georg Stary, MD, of the Medical University of Vienna and the Research Center for Molecular Medicine of the Austrian Academy of Sciences, Vienna, Austria, and colleagues.
The findings were published in the The Lancet Rheumatology.
For the study, researchers recruited 16 individuals with persistent and glucocorticoid-refractory cutaneous sarcoidosis between September 2019 and June 2021. A total of 14 participants were randomly assigned to the topical phase of the study, whereas two immediately received systemic treatment. All treatment was conducted at Vienna General Hospital.
In the placebo-controlled, double-blinded topical treatment arm, patients received either 0.1% topical sirolimus in Vaseline or Vaseline alone (placebo) twice daily for 2 months. After a 1-month washout period, participants were switched to the alternate treatment arm for an additional 2 months.
Following this topical phase and an additional 1-month washout period, all remaining participants received systemic sirolimus via a 1-mg/mL solution, starting with a 6-mg loading dose and continuing with 2 mg once daily for 4 months. The primary outcome was change in Cutaneous Sarcoidosis Activity and Morphology Index (CSAMI) from baseline, with decrease of more than five points representing a response to treatment.
A total of 10 patients completed the trial.
There was no change in CSAMI in either topical treatment groups. In the systemic group, 70% of patients had clinical improvement in skin lesions, with three responders in this group having complete resolution of skin lesions. The median change in CSAMI was −7.0 points (P = .018).
This improvement persisted for 2 months following study conclusion, with more pronounced improvement from baseline after 2 years of drug-free follow-up (−11.5 points).
There were no serious adverse events reported during the study, but 42% of patients treated with systemic sirolimus reported mild skin reactions, such as acne and eczema. Other related adverse events were hypertriglyceridemia (17%), hyperglycemia (17%), and proteinuria (8%).
Compared with clinical outcomes with tofacitinib and tumor necrosis factor (TNF) inhibitors, “the strength of our study lies in the sustained treatment effect after drug withdrawal among all responders. This prolonged effect has not yet been explored with tofacitinib, whereas with TNF inhibitors disease relapse was seen in more than 50% of patients at 3-8 months,” the authors wrote.
The researchers also analyzed participants’ skin biopsies to gain a better understanding of how mTOR inhibition affected granuloma structures. They found that, at baseline, mTOR activity was significantly lower in the fibroblasts of treatment nonresponders than in responders. They speculated that lower expression of mTOR could make these granuloma-associated cells resistant to systemic sirolimus.
These promising findings combine “clinical response with a molecular analysis,” Avrom Caplan, MD, co-director of the Sarcoidosis Program at NYU Langone in New York City, told this news organization. He was not involved with the research. Adding molecular information to clinical outcome data “helps solidify that [the mTOR] pathway has relevance in the sarcoid granuloma formation.”
The study had a limited sample size — a challenge for many clinical trials of rare diseases, Dr. Caplan said. Larger clinical trials are necessary to explore mTOR inhibition in sarcoidosis, both he and the authors agreed. A larger trial could also include greater heterogeneity of patients, including varied sarcoid presentation and demographics, Dr. Caplan noted. In this study, all but one participants were White individuals, and 63% of participants were female.
Larger studies could also address important questions on ideal length of therapy, dosing, and where this therapy “would fall within the therapeutic step ladder,” Dr. Caplan continued.
Whether mTOR inhibition could be effective at treating individuals with sarcoidosis in other organs beyond the skin is also unknown.
“If the pathogenesis of sarcoid granuloma formation does include mTOR upregulation, which they are showing here…then you could hypothesize that, yes, using this therapy could benefit other organs,” he said. “But that has to be investigated in larger trials.”
The study was funded in part by a Vienna Science and Technology Fund project. Several authors report receiving grants from the Austrian Science Fund and one from the Ann Theodore Foundation Breakthrough Sarcoidosis Initiative. Dr. Caplan reported no relevant financial relationships.
A version of this article appeared on Medscape.com .
Sirolimus may be an effective treatment for patients with persistent cutaneous sarcoidosis.
In a small clinical trial, 7 of 10 patients treated with sirolimus via oral solution had improvements in skin lesions after 4 months, which was sustained for up to 2 years after the study concluded.
The results suggested that mechanistic target of rapamycin (mTOR) inhibition is a potential therapeutic avenue for sarcoidosis, which the authors said should be explored in larger clinical trials.
In the past decade, there has been a growing amount of evidence suggesting mTOR’s role in sarcoidosis. In 2017, researchers showed that activation of mTOR in macrophages could cause progressive sarcoidosis in mice. In additional studies, high levels of mTOR activity were detected in human sarcoidosis granulomas in various organs, including the skin, lung, and heart.
Three case reports also documented using the mTOR inhibitor sirolimus to effectively treat systemic sarcoidosis.
“Although all reports observed improvement of the disease following the treatment, no clinical trial investigating the efficacy and safety of sirolimus in patients with sarcoidosis had been published” prior to this study, wrote senior author Georg Stary, MD, of the Medical University of Vienna and the Research Center for Molecular Medicine of the Austrian Academy of Sciences, Vienna, Austria, and colleagues.
The findings were published in the The Lancet Rheumatology.
For the study, researchers recruited 16 individuals with persistent and glucocorticoid-refractory cutaneous sarcoidosis between September 2019 and June 2021. A total of 14 participants were randomly assigned to the topical phase of the study, whereas two immediately received systemic treatment. All treatment was conducted at Vienna General Hospital.
In the placebo-controlled, double-blinded topical treatment arm, patients received either 0.1% topical sirolimus in Vaseline or Vaseline alone (placebo) twice daily for 2 months. After a 1-month washout period, participants were switched to the alternate treatment arm for an additional 2 months.
Following this topical phase and an additional 1-month washout period, all remaining participants received systemic sirolimus via a 1-mg/mL solution, starting with a 6-mg loading dose and continuing with 2 mg once daily for 4 months. The primary outcome was change in Cutaneous Sarcoidosis Activity and Morphology Index (CSAMI) from baseline, with decrease of more than five points representing a response to treatment.
A total of 10 patients completed the trial.
There was no change in CSAMI in either topical treatment groups. In the systemic group, 70% of patients had clinical improvement in skin lesions, with three responders in this group having complete resolution of skin lesions. The median change in CSAMI was −7.0 points (P = .018).
This improvement persisted for 2 months following study conclusion, with more pronounced improvement from baseline after 2 years of drug-free follow-up (−11.5 points).
There were no serious adverse events reported during the study, but 42% of patients treated with systemic sirolimus reported mild skin reactions, such as acne and eczema. Other related adverse events were hypertriglyceridemia (17%), hyperglycemia (17%), and proteinuria (8%).
Compared with clinical outcomes with tofacitinib and tumor necrosis factor (TNF) inhibitors, “the strength of our study lies in the sustained treatment effect after drug withdrawal among all responders. This prolonged effect has not yet been explored with tofacitinib, whereas with TNF inhibitors disease relapse was seen in more than 50% of patients at 3-8 months,” the authors wrote.
The researchers also analyzed participants’ skin biopsies to gain a better understanding of how mTOR inhibition affected granuloma structures. They found that, at baseline, mTOR activity was significantly lower in the fibroblasts of treatment nonresponders than in responders. They speculated that lower expression of mTOR could make these granuloma-associated cells resistant to systemic sirolimus.
These promising findings combine “clinical response with a molecular analysis,” Avrom Caplan, MD, co-director of the Sarcoidosis Program at NYU Langone in New York City, told this news organization. He was not involved with the research. Adding molecular information to clinical outcome data “helps solidify that [the mTOR] pathway has relevance in the sarcoid granuloma formation.”
The study had a limited sample size — a challenge for many clinical trials of rare diseases, Dr. Caplan said. Larger clinical trials are necessary to explore mTOR inhibition in sarcoidosis, both he and the authors agreed. A larger trial could also include greater heterogeneity of patients, including varied sarcoid presentation and demographics, Dr. Caplan noted. In this study, all but one participants were White individuals, and 63% of participants were female.
Larger studies could also address important questions on ideal length of therapy, dosing, and where this therapy “would fall within the therapeutic step ladder,” Dr. Caplan continued.
Whether mTOR inhibition could be effective at treating individuals with sarcoidosis in other organs beyond the skin is also unknown.
“If the pathogenesis of sarcoid granuloma formation does include mTOR upregulation, which they are showing here…then you could hypothesize that, yes, using this therapy could benefit other organs,” he said. “But that has to be investigated in larger trials.”
The study was funded in part by a Vienna Science and Technology Fund project. Several authors report receiving grants from the Austrian Science Fund and one from the Ann Theodore Foundation Breakthrough Sarcoidosis Initiative. Dr. Caplan reported no relevant financial relationships.
A version of this article appeared on Medscape.com .
Sirolimus may be an effective treatment for patients with persistent cutaneous sarcoidosis.
In a small clinical trial, 7 of 10 patients treated with sirolimus via oral solution had improvements in skin lesions after 4 months, which was sustained for up to 2 years after the study concluded.
The results suggested that mechanistic target of rapamycin (mTOR) inhibition is a potential therapeutic avenue for sarcoidosis, which the authors said should be explored in larger clinical trials.
In the past decade, there has been a growing amount of evidence suggesting mTOR’s role in sarcoidosis. In 2017, researchers showed that activation of mTOR in macrophages could cause progressive sarcoidosis in mice. In additional studies, high levels of mTOR activity were detected in human sarcoidosis granulomas in various organs, including the skin, lung, and heart.
Three case reports also documented using the mTOR inhibitor sirolimus to effectively treat systemic sarcoidosis.
“Although all reports observed improvement of the disease following the treatment, no clinical trial investigating the efficacy and safety of sirolimus in patients with sarcoidosis had been published” prior to this study, wrote senior author Georg Stary, MD, of the Medical University of Vienna and the Research Center for Molecular Medicine of the Austrian Academy of Sciences, Vienna, Austria, and colleagues.
The findings were published in the The Lancet Rheumatology.
For the study, researchers recruited 16 individuals with persistent and glucocorticoid-refractory cutaneous sarcoidosis between September 2019 and June 2021. A total of 14 participants were randomly assigned to the topical phase of the study, whereas two immediately received systemic treatment. All treatment was conducted at Vienna General Hospital.
In the placebo-controlled, double-blinded topical treatment arm, patients received either 0.1% topical sirolimus in Vaseline or Vaseline alone (placebo) twice daily for 2 months. After a 1-month washout period, participants were switched to the alternate treatment arm for an additional 2 months.
Following this topical phase and an additional 1-month washout period, all remaining participants received systemic sirolimus via a 1-mg/mL solution, starting with a 6-mg loading dose and continuing with 2 mg once daily for 4 months. The primary outcome was change in Cutaneous Sarcoidosis Activity and Morphology Index (CSAMI) from baseline, with decrease of more than five points representing a response to treatment.
A total of 10 patients completed the trial.
There was no change in CSAMI in either topical treatment groups. In the systemic group, 70% of patients had clinical improvement in skin lesions, with three responders in this group having complete resolution of skin lesions. The median change in CSAMI was −7.0 points (P = .018).
This improvement persisted for 2 months following study conclusion, with more pronounced improvement from baseline after 2 years of drug-free follow-up (−11.5 points).
There were no serious adverse events reported during the study, but 42% of patients treated with systemic sirolimus reported mild skin reactions, such as acne and eczema. Other related adverse events were hypertriglyceridemia (17%), hyperglycemia (17%), and proteinuria (8%).
Compared with clinical outcomes with tofacitinib and tumor necrosis factor (TNF) inhibitors, “the strength of our study lies in the sustained treatment effect after drug withdrawal among all responders. This prolonged effect has not yet been explored with tofacitinib, whereas with TNF inhibitors disease relapse was seen in more than 50% of patients at 3-8 months,” the authors wrote.
The researchers also analyzed participants’ skin biopsies to gain a better understanding of how mTOR inhibition affected granuloma structures. They found that, at baseline, mTOR activity was significantly lower in the fibroblasts of treatment nonresponders than in responders. They speculated that lower expression of mTOR could make these granuloma-associated cells resistant to systemic sirolimus.
These promising findings combine “clinical response with a molecular analysis,” Avrom Caplan, MD, co-director of the Sarcoidosis Program at NYU Langone in New York City, told this news organization. He was not involved with the research. Adding molecular information to clinical outcome data “helps solidify that [the mTOR] pathway has relevance in the sarcoid granuloma formation.”
The study had a limited sample size — a challenge for many clinical trials of rare diseases, Dr. Caplan said. Larger clinical trials are necessary to explore mTOR inhibition in sarcoidosis, both he and the authors agreed. A larger trial could also include greater heterogeneity of patients, including varied sarcoid presentation and demographics, Dr. Caplan noted. In this study, all but one participants were White individuals, and 63% of participants were female.
Larger studies could also address important questions on ideal length of therapy, dosing, and where this therapy “would fall within the therapeutic step ladder,” Dr. Caplan continued.
Whether mTOR inhibition could be effective at treating individuals with sarcoidosis in other organs beyond the skin is also unknown.
“If the pathogenesis of sarcoid granuloma formation does include mTOR upregulation, which they are showing here…then you could hypothesize that, yes, using this therapy could benefit other organs,” he said. “But that has to be investigated in larger trials.”
The study was funded in part by a Vienna Science and Technology Fund project. Several authors report receiving grants from the Austrian Science Fund and one from the Ann Theodore Foundation Breakthrough Sarcoidosis Initiative. Dr. Caplan reported no relevant financial relationships.
A version of this article appeared on Medscape.com .
FROM THE LANCET RHEUMATOLOGY
Rituximab Results in Sustained Remission for Pemphigus, Study Found
TOPLINE:
, an analysis showed.
METHODOLOGY:
- The short-term efficacy and safety of first-line treatment with rituximab for pemphigus were demonstrated in the Ritux 3 trial, but the rates of long-term remission are unknown.
- French investigators from 25 dermatology departments evaluated 83 patients from the Ritux 3 trial between January 1, 2010, and December 31, 2015.
- They used Kaplan-Meir curves to determine the 5- and 7-year rates of disease-free survival (DFS) without corticosteroids.
TAKEAWAY:
- Of the 83 patients, 44 were in the rituximab-plus-prednisone group and 39 were in the prednisone-only group, with a median follow-up of 87.3 months (7.3 years).
- Among patients in the rituximab plus prednisone group, 43 (93.5%) achieved complete remission without corticosteroids at any time during follow-up, compared with 17 patients (39%) in the prednisone-only group.
- DFS (without corticosteroid therapy) statistically favored patients in the rituximab plus prednisone group compared with patients in the prednisone-only group at follow-up times of 5 years (76.7% vs 35.3%, respectively) and 7 years (72.1% vs 35.3%; P < .001 for both associations).
- In another finding, 31 patients in the rituximab plus prednisone group reported fewer serious adverse events (SAEs) than 58 patients in the prednisone-only group, which corresponds to 0.67 and 1.32 SAEs per patient, respectively (P = .003).
IN PRACTICE:
The study findings demonstrated “the superiority of rituximab over a standard corticosteroids regimen, both in the short term and the long term,” the authors wrote.
SOURCE:
Corresponding author Billal Tedbirt, MD, of the Department of Dermatology at CHU Rouen in France, led the study, which was published online on January 24, 2024, in JAMA Dermatology.
LIMITATIONS:
Nearly 8% of patients did not attend the end of follow-up visit. Also, serum samples used to predict relapse were drawn at month 36, but the researchers said that a window of every 4-6 months might provide higher accuracy of relapses.
DISCLOSURES:
Dr. Tedbirt reported having no disclosures. Four of the study authors reported being investigators for and/or receiving personal fees from several pharmaceutical companies. The study was supported by a grant from the French Society of Dermatology.
A version of this article appeared on Medscape.com.
TOPLINE:
, an analysis showed.
METHODOLOGY:
- The short-term efficacy and safety of first-line treatment with rituximab for pemphigus were demonstrated in the Ritux 3 trial, but the rates of long-term remission are unknown.
- French investigators from 25 dermatology departments evaluated 83 patients from the Ritux 3 trial between January 1, 2010, and December 31, 2015.
- They used Kaplan-Meir curves to determine the 5- and 7-year rates of disease-free survival (DFS) without corticosteroids.
TAKEAWAY:
- Of the 83 patients, 44 were in the rituximab-plus-prednisone group and 39 were in the prednisone-only group, with a median follow-up of 87.3 months (7.3 years).
- Among patients in the rituximab plus prednisone group, 43 (93.5%) achieved complete remission without corticosteroids at any time during follow-up, compared with 17 patients (39%) in the prednisone-only group.
- DFS (without corticosteroid therapy) statistically favored patients in the rituximab plus prednisone group compared with patients in the prednisone-only group at follow-up times of 5 years (76.7% vs 35.3%, respectively) and 7 years (72.1% vs 35.3%; P < .001 for both associations).
- In another finding, 31 patients in the rituximab plus prednisone group reported fewer serious adverse events (SAEs) than 58 patients in the prednisone-only group, which corresponds to 0.67 and 1.32 SAEs per patient, respectively (P = .003).
IN PRACTICE:
The study findings demonstrated “the superiority of rituximab over a standard corticosteroids regimen, both in the short term and the long term,” the authors wrote.
SOURCE:
Corresponding author Billal Tedbirt, MD, of the Department of Dermatology at CHU Rouen in France, led the study, which was published online on January 24, 2024, in JAMA Dermatology.
LIMITATIONS:
Nearly 8% of patients did not attend the end of follow-up visit. Also, serum samples used to predict relapse were drawn at month 36, but the researchers said that a window of every 4-6 months might provide higher accuracy of relapses.
DISCLOSURES:
Dr. Tedbirt reported having no disclosures. Four of the study authors reported being investigators for and/or receiving personal fees from several pharmaceutical companies. The study was supported by a grant from the French Society of Dermatology.
A version of this article appeared on Medscape.com.
TOPLINE:
, an analysis showed.
METHODOLOGY:
- The short-term efficacy and safety of first-line treatment with rituximab for pemphigus were demonstrated in the Ritux 3 trial, but the rates of long-term remission are unknown.
- French investigators from 25 dermatology departments evaluated 83 patients from the Ritux 3 trial between January 1, 2010, and December 31, 2015.
- They used Kaplan-Meir curves to determine the 5- and 7-year rates of disease-free survival (DFS) without corticosteroids.
TAKEAWAY:
- Of the 83 patients, 44 were in the rituximab-plus-prednisone group and 39 were in the prednisone-only group, with a median follow-up of 87.3 months (7.3 years).
- Among patients in the rituximab plus prednisone group, 43 (93.5%) achieved complete remission without corticosteroids at any time during follow-up, compared with 17 patients (39%) in the prednisone-only group.
- DFS (without corticosteroid therapy) statistically favored patients in the rituximab plus prednisone group compared with patients in the prednisone-only group at follow-up times of 5 years (76.7% vs 35.3%, respectively) and 7 years (72.1% vs 35.3%; P < .001 for both associations).
- In another finding, 31 patients in the rituximab plus prednisone group reported fewer serious adverse events (SAEs) than 58 patients in the prednisone-only group, which corresponds to 0.67 and 1.32 SAEs per patient, respectively (P = .003).
IN PRACTICE:
The study findings demonstrated “the superiority of rituximab over a standard corticosteroids regimen, both in the short term and the long term,” the authors wrote.
SOURCE:
Corresponding author Billal Tedbirt, MD, of the Department of Dermatology at CHU Rouen in France, led the study, which was published online on January 24, 2024, in JAMA Dermatology.
LIMITATIONS:
Nearly 8% of patients did not attend the end of follow-up visit. Also, serum samples used to predict relapse were drawn at month 36, but the researchers said that a window of every 4-6 months might provide higher accuracy of relapses.
DISCLOSURES:
Dr. Tedbirt reported having no disclosures. Four of the study authors reported being investigators for and/or receiving personal fees from several pharmaceutical companies. The study was supported by a grant from the French Society of Dermatology.
A version of this article appeared on Medscape.com.
Cutaneous lupus, dermatomyositis: Excitement growing around emerging therapies
ORLANDO, FLORIDA — Advances in treating medical conditions rarely emerge in a straight line. Oftentimes, progress comes in fits and starts, and therapies to treat cutaneous lupus erythematosus (CLE) and dermatomyositis are no exception.
Beyond approved treatments that deserve more attention, like belimumab, approved by the Food and Drug Administration (FDA) for systemic lupus erythematosus (SLE) in 2011, and Octagam 10%, an intravenous immune globulin (IVIG) preparation approved for dermatomyositis in 2021, anticipation is growing for emerging therapies and their potential to provide relief to patients, Anthony Fernandez, MD, PhD, said at the ODAC Dermatology, Aesthetic & Surgical Conference. The tyrosine kinase 2 (TYK2) inhibitor deucravacitinib, Janus kinase (JAK) inhibitors brepocitinib and baricitinib, and the monoclonal antibody anifrolumab, he noted, are prime examples.
“. In my opinion, this is the start of what will be the most exciting decade in the history of these two diseases,” said Dr. Fernandez, director of medical dermatology at the Cleveland Clinic.
Emerging Treatments for Cutaneous Lupus
Although SLE can involve many organ systems, the skin is one of the most affected. There are specific cutaneous lesions categorized as either acute cutaneous lupus, subacute cutaneous lupus, or chronic cutaneous lupus.
The oral TYK2 inhibitor deucravacitinib, for example, should be able to dampen interleukin responses in people with CLE, Dr. Fernandez said. Deucravacitinib was approved by the FDA to treat psoriasis in September 2022.
A phase 2 study published in 2023 focused on this agent for relief of systemic lupus. Improvements in cutaneous disease were a secondary endpoint. The trial demonstrated that the patients treated with deucravacitinib achieved a 56%-70% CLASI-50 response, depending on dosing, compared with a 17% response among those on placebo at week 48.
Based on the trial results, recruitment has begun for a phase 2 trial to evaluate deucravacitinib, compared with placebo, in patients with discoid and/or subacute cutaneous lupus. “This may be another medicine we have available to give to any of our patients with cutaneous lupus,” Dr. Fernandez said.
Anifrolumab Appears Promising
The FDA approval of anifrolumab, a type I interferon (IFN) receptor antagonist, for treating moderate to severe SLE in July 2021, for example, is good news for dermatologists and their patients, added Dr. Fernandez. “Almost immediately after approval, case studies showed marked improvement in patients with refractory cutaneous lupus.” While the therapy was approved for treating systemic lupus, it allows for off-label treatment of the cutaneous predominant form of the disease, he said.
Furthermore, the manufacturer of anifrolumab, AstraZeneca, is launching the LAVENDER clinical trial to assess the monoclonal antibody specifically for treating CLE. “This is a big deal because we may be able to prescribe anifrolumab for our cutaneous lupus patients who don’t have systemic lupus,” Dr. Fernandez said.
Phase 3 data supported use the of anifrolumab in systemic lupus, including the TULIP-2 trial, which demonstrated its superiority to placebo for reducing severity of systemic disease and lowering corticosteroid use. A study published in March 2023 of 11 patients showed that they had a “very fast response” to the agent, Dr. Fernandez said, with a 50% or greater improvement in the Cutaneous Lupus Erythematosus Disease Area and Severity Index activity score reached by all participants at week 16. Improvements of 50% or more in this scoring system are considered clinically meaningful, he added.
Upcoming Dermatomyositis Treatments
Why highlight emerging therapies for CLE and dermatomyositis in the same ODAC presentation? Although distinct conditions, these autoimmune conditions are both mediated by type 1 IFN inflammation.
Dermatomyositis is a relatively rare immune-mediated disease that most commonly affects the skin and muscle. Doctors score disease presentation, activity, and clinical improvements on a scale similar to CLASI for cutaneous lupus, the CDASI or Cutaneous Dermatomyositis Disease Area and Severity Index. Among people with CDASI activity scores of at least 14, which is the threshold for moderate to severe disease, a 20% improvement is clinically meaningful, Dr. Fernandez said. In addition, a 40% or greater improvement correlates with significant improvements in quality of life.
There is now more evidence for the use of IVIG to treat dermatomyositis. “Among those of us who treat dermatomyositis on a regular basis, we believe IVIG is the most potent treatment. We’ve known that for a long time,” Dr. Fernandez said.
Despite this tenet, for years, there was only one placebo-controlled trial, published in 1993, that evaluated IVIG treatment for dermatomyositis, and it included only 15 participants. That was until October 2022, he said, when the New England Journal of Medicine published a study comparing a specific brand of IVIG (Octagam) with placebo in 95 people with dermatomyositis.
In the study, 79% of participants treated with IVIG had a total improvement score of at least 20 (minimal improvement), the primary endpoint, at 16 weeks, compared with 44% of those receiving a placebo. Those treated with IVIG also had significant improvements in the CDASI score, a secondary endpoint, compared with those on placebo, he said.
Based on results of this trial, the FDA approved Octagam 10% for dermatomyositis in adults. Dr. Fernandez noted the approval is restricted to the brand of IVIG in the trial, not all IVIG products. However, “the FDA approval is most important to us because it gives us ammunition to fight for insurers to approve IVIG when we feel our patients with dermatomyositis need it,” regardless of the brand.
The Potential of JAK1 Inhibitors
An open-label study of the JAK inhibitor tofacitinib, published in December 2020, showed that mean changes in CDASI activity scores at 12 weeks were statistically significant compared with baseline in 10 people with dermatomyositis. “The importance of this study is that it is proof of concept that JAK inhibition can be effective for treating dermatomyositis, especially with active skin disease,” Dr. Fernandez said.
In addition, two large phase 3 trials are evaluating JAK inhibitor safety and efficacy for treating dermatomyositis. One is the VALOR trial, currently recruiting people with recalcitrant dermatomyositis to evaluate treatment with brepocitinib. Researchers in France are looking at another JAK inhibitor, baricitinib, for treating relapsing or treatment-naive dermatomyositis. Recruitment for the BIRD clinical trial is ongoing.
Monoclonal Antibody Showing Promise
“When it comes to looking specifically at dermatomyositis cutaneous disease, it’s been found that the levels of IFN beta correlate best with not only lesional skin type 1 IFN inflammatory signatures but also overall clinical disease activity,” Dr. Fernandez said. This correlation is stronger than for any other IFN-1-type cytokine active in the disorder.
“Perhaps blocking IFN beta might be best way to get control of dermatomyositis activity,” he added.
With that in mind, a phase 2 trial of dazukibart presented at the American Academy of Dermatology 2023 annual meeting highlighted the promise of this agent that targets type 1 IFN beta.
The primary endpoint was improvement in CDASI at 12 weeks. “This medication has remarkable efficacy,” Dr. Fernandez said. “We were one of the sites for this trial. Despite being blinded, there was no question about who was receiving drug and who was receiving placebo.”
“A minimal clinical improvement in disease activity was seen in more than 90%, so almost every patient who received this medication had meaningful improvement,” he added.
Based on the results, the manufacturer, Pfizer, is recruiting participants for a phase 3 trial to further assess dazukibart in dermatomyositis and polymyositis. Dr. Fernandez said, “This is a story you should pay attention to if you treat any dermatomyositis patients at all.”
Regarding these emerging therapies for CLE and dermatomyositis, “This looks very much like the early days of psoriasis, in the early 2000s, when there was a lot of activity developing treatments,” Dr. Fernandez said. “I will predict that within 10 years, we will have multiple novel agents available that will probably work better than anything we have today.”
Dr. Fernandez reported receiving grant and/or research support from Alexion, Incyte, Mallinckrodt Pharmaceuticals, Novartis, Pfizer, and Priovant Therapeutics; acting as a consultant or advisory board member for AbbVie, Biogen, Mallinckrodt Pharmaceuticals; and being a member of the speaker bureau or receiving honoraria for non-CME from AbbVie, Kyowa Kirin, and Mallinckrodt Pharmaceuticals.
A version of this article appeared on Medscape.com.
ORLANDO, FLORIDA — Advances in treating medical conditions rarely emerge in a straight line. Oftentimes, progress comes in fits and starts, and therapies to treat cutaneous lupus erythematosus (CLE) and dermatomyositis are no exception.
Beyond approved treatments that deserve more attention, like belimumab, approved by the Food and Drug Administration (FDA) for systemic lupus erythematosus (SLE) in 2011, and Octagam 10%, an intravenous immune globulin (IVIG) preparation approved for dermatomyositis in 2021, anticipation is growing for emerging therapies and their potential to provide relief to patients, Anthony Fernandez, MD, PhD, said at the ODAC Dermatology, Aesthetic & Surgical Conference. The tyrosine kinase 2 (TYK2) inhibitor deucravacitinib, Janus kinase (JAK) inhibitors brepocitinib and baricitinib, and the monoclonal antibody anifrolumab, he noted, are prime examples.
“. In my opinion, this is the start of what will be the most exciting decade in the history of these two diseases,” said Dr. Fernandez, director of medical dermatology at the Cleveland Clinic.
Emerging Treatments for Cutaneous Lupus
Although SLE can involve many organ systems, the skin is one of the most affected. There are specific cutaneous lesions categorized as either acute cutaneous lupus, subacute cutaneous lupus, or chronic cutaneous lupus.
The oral TYK2 inhibitor deucravacitinib, for example, should be able to dampen interleukin responses in people with CLE, Dr. Fernandez said. Deucravacitinib was approved by the FDA to treat psoriasis in September 2022.
A phase 2 study published in 2023 focused on this agent for relief of systemic lupus. Improvements in cutaneous disease were a secondary endpoint. The trial demonstrated that the patients treated with deucravacitinib achieved a 56%-70% CLASI-50 response, depending on dosing, compared with a 17% response among those on placebo at week 48.
Based on the trial results, recruitment has begun for a phase 2 trial to evaluate deucravacitinib, compared with placebo, in patients with discoid and/or subacute cutaneous lupus. “This may be another medicine we have available to give to any of our patients with cutaneous lupus,” Dr. Fernandez said.
Anifrolumab Appears Promising
The FDA approval of anifrolumab, a type I interferon (IFN) receptor antagonist, for treating moderate to severe SLE in July 2021, for example, is good news for dermatologists and their patients, added Dr. Fernandez. “Almost immediately after approval, case studies showed marked improvement in patients with refractory cutaneous lupus.” While the therapy was approved for treating systemic lupus, it allows for off-label treatment of the cutaneous predominant form of the disease, he said.
Furthermore, the manufacturer of anifrolumab, AstraZeneca, is launching the LAVENDER clinical trial to assess the monoclonal antibody specifically for treating CLE. “This is a big deal because we may be able to prescribe anifrolumab for our cutaneous lupus patients who don’t have systemic lupus,” Dr. Fernandez said.
Phase 3 data supported use the of anifrolumab in systemic lupus, including the TULIP-2 trial, which demonstrated its superiority to placebo for reducing severity of systemic disease and lowering corticosteroid use. A study published in March 2023 of 11 patients showed that they had a “very fast response” to the agent, Dr. Fernandez said, with a 50% or greater improvement in the Cutaneous Lupus Erythematosus Disease Area and Severity Index activity score reached by all participants at week 16. Improvements of 50% or more in this scoring system are considered clinically meaningful, he added.
Upcoming Dermatomyositis Treatments
Why highlight emerging therapies for CLE and dermatomyositis in the same ODAC presentation? Although distinct conditions, these autoimmune conditions are both mediated by type 1 IFN inflammation.
Dermatomyositis is a relatively rare immune-mediated disease that most commonly affects the skin and muscle. Doctors score disease presentation, activity, and clinical improvements on a scale similar to CLASI for cutaneous lupus, the CDASI or Cutaneous Dermatomyositis Disease Area and Severity Index. Among people with CDASI activity scores of at least 14, which is the threshold for moderate to severe disease, a 20% improvement is clinically meaningful, Dr. Fernandez said. In addition, a 40% or greater improvement correlates with significant improvements in quality of life.
There is now more evidence for the use of IVIG to treat dermatomyositis. “Among those of us who treat dermatomyositis on a regular basis, we believe IVIG is the most potent treatment. We’ve known that for a long time,” Dr. Fernandez said.
Despite this tenet, for years, there was only one placebo-controlled trial, published in 1993, that evaluated IVIG treatment for dermatomyositis, and it included only 15 participants. That was until October 2022, he said, when the New England Journal of Medicine published a study comparing a specific brand of IVIG (Octagam) with placebo in 95 people with dermatomyositis.
In the study, 79% of participants treated with IVIG had a total improvement score of at least 20 (minimal improvement), the primary endpoint, at 16 weeks, compared with 44% of those receiving a placebo. Those treated with IVIG also had significant improvements in the CDASI score, a secondary endpoint, compared with those on placebo, he said.
Based on results of this trial, the FDA approved Octagam 10% for dermatomyositis in adults. Dr. Fernandez noted the approval is restricted to the brand of IVIG in the trial, not all IVIG products. However, “the FDA approval is most important to us because it gives us ammunition to fight for insurers to approve IVIG when we feel our patients with dermatomyositis need it,” regardless of the brand.
The Potential of JAK1 Inhibitors
An open-label study of the JAK inhibitor tofacitinib, published in December 2020, showed that mean changes in CDASI activity scores at 12 weeks were statistically significant compared with baseline in 10 people with dermatomyositis. “The importance of this study is that it is proof of concept that JAK inhibition can be effective for treating dermatomyositis, especially with active skin disease,” Dr. Fernandez said.
In addition, two large phase 3 trials are evaluating JAK inhibitor safety and efficacy for treating dermatomyositis. One is the VALOR trial, currently recruiting people with recalcitrant dermatomyositis to evaluate treatment with brepocitinib. Researchers in France are looking at another JAK inhibitor, baricitinib, for treating relapsing or treatment-naive dermatomyositis. Recruitment for the BIRD clinical trial is ongoing.
Monoclonal Antibody Showing Promise
“When it comes to looking specifically at dermatomyositis cutaneous disease, it’s been found that the levels of IFN beta correlate best with not only lesional skin type 1 IFN inflammatory signatures but also overall clinical disease activity,” Dr. Fernandez said. This correlation is stronger than for any other IFN-1-type cytokine active in the disorder.
“Perhaps blocking IFN beta might be best way to get control of dermatomyositis activity,” he added.
With that in mind, a phase 2 trial of dazukibart presented at the American Academy of Dermatology 2023 annual meeting highlighted the promise of this agent that targets type 1 IFN beta.
The primary endpoint was improvement in CDASI at 12 weeks. “This medication has remarkable efficacy,” Dr. Fernandez said. “We were one of the sites for this trial. Despite being blinded, there was no question about who was receiving drug and who was receiving placebo.”
“A minimal clinical improvement in disease activity was seen in more than 90%, so almost every patient who received this medication had meaningful improvement,” he added.
Based on the results, the manufacturer, Pfizer, is recruiting participants for a phase 3 trial to further assess dazukibart in dermatomyositis and polymyositis. Dr. Fernandez said, “This is a story you should pay attention to if you treat any dermatomyositis patients at all.”
Regarding these emerging therapies for CLE and dermatomyositis, “This looks very much like the early days of psoriasis, in the early 2000s, when there was a lot of activity developing treatments,” Dr. Fernandez said. “I will predict that within 10 years, we will have multiple novel agents available that will probably work better than anything we have today.”
Dr. Fernandez reported receiving grant and/or research support from Alexion, Incyte, Mallinckrodt Pharmaceuticals, Novartis, Pfizer, and Priovant Therapeutics; acting as a consultant or advisory board member for AbbVie, Biogen, Mallinckrodt Pharmaceuticals; and being a member of the speaker bureau or receiving honoraria for non-CME from AbbVie, Kyowa Kirin, and Mallinckrodt Pharmaceuticals.
A version of this article appeared on Medscape.com.
ORLANDO, FLORIDA — Advances in treating medical conditions rarely emerge in a straight line. Oftentimes, progress comes in fits and starts, and therapies to treat cutaneous lupus erythematosus (CLE) and dermatomyositis are no exception.
Beyond approved treatments that deserve more attention, like belimumab, approved by the Food and Drug Administration (FDA) for systemic lupus erythematosus (SLE) in 2011, and Octagam 10%, an intravenous immune globulin (IVIG) preparation approved for dermatomyositis in 2021, anticipation is growing for emerging therapies and their potential to provide relief to patients, Anthony Fernandez, MD, PhD, said at the ODAC Dermatology, Aesthetic & Surgical Conference. The tyrosine kinase 2 (TYK2) inhibitor deucravacitinib, Janus kinase (JAK) inhibitors brepocitinib and baricitinib, and the monoclonal antibody anifrolumab, he noted, are prime examples.
“. In my opinion, this is the start of what will be the most exciting decade in the history of these two diseases,” said Dr. Fernandez, director of medical dermatology at the Cleveland Clinic.
Emerging Treatments for Cutaneous Lupus
Although SLE can involve many organ systems, the skin is one of the most affected. There are specific cutaneous lesions categorized as either acute cutaneous lupus, subacute cutaneous lupus, or chronic cutaneous lupus.
The oral TYK2 inhibitor deucravacitinib, for example, should be able to dampen interleukin responses in people with CLE, Dr. Fernandez said. Deucravacitinib was approved by the FDA to treat psoriasis in September 2022.
A phase 2 study published in 2023 focused on this agent for relief of systemic lupus. Improvements in cutaneous disease were a secondary endpoint. The trial demonstrated that the patients treated with deucravacitinib achieved a 56%-70% CLASI-50 response, depending on dosing, compared with a 17% response among those on placebo at week 48.
Based on the trial results, recruitment has begun for a phase 2 trial to evaluate deucravacitinib, compared with placebo, in patients with discoid and/or subacute cutaneous lupus. “This may be another medicine we have available to give to any of our patients with cutaneous lupus,” Dr. Fernandez said.
Anifrolumab Appears Promising
The FDA approval of anifrolumab, a type I interferon (IFN) receptor antagonist, for treating moderate to severe SLE in July 2021, for example, is good news for dermatologists and their patients, added Dr. Fernandez. “Almost immediately after approval, case studies showed marked improvement in patients with refractory cutaneous lupus.” While the therapy was approved for treating systemic lupus, it allows for off-label treatment of the cutaneous predominant form of the disease, he said.
Furthermore, the manufacturer of anifrolumab, AstraZeneca, is launching the LAVENDER clinical trial to assess the monoclonal antibody specifically for treating CLE. “This is a big deal because we may be able to prescribe anifrolumab for our cutaneous lupus patients who don’t have systemic lupus,” Dr. Fernandez said.
Phase 3 data supported use the of anifrolumab in systemic lupus, including the TULIP-2 trial, which demonstrated its superiority to placebo for reducing severity of systemic disease and lowering corticosteroid use. A study published in March 2023 of 11 patients showed that they had a “very fast response” to the agent, Dr. Fernandez said, with a 50% or greater improvement in the Cutaneous Lupus Erythematosus Disease Area and Severity Index activity score reached by all participants at week 16. Improvements of 50% or more in this scoring system are considered clinically meaningful, he added.
Upcoming Dermatomyositis Treatments
Why highlight emerging therapies for CLE and dermatomyositis in the same ODAC presentation? Although distinct conditions, these autoimmune conditions are both mediated by type 1 IFN inflammation.
Dermatomyositis is a relatively rare immune-mediated disease that most commonly affects the skin and muscle. Doctors score disease presentation, activity, and clinical improvements on a scale similar to CLASI for cutaneous lupus, the CDASI or Cutaneous Dermatomyositis Disease Area and Severity Index. Among people with CDASI activity scores of at least 14, which is the threshold for moderate to severe disease, a 20% improvement is clinically meaningful, Dr. Fernandez said. In addition, a 40% or greater improvement correlates with significant improvements in quality of life.
There is now more evidence for the use of IVIG to treat dermatomyositis. “Among those of us who treat dermatomyositis on a regular basis, we believe IVIG is the most potent treatment. We’ve known that for a long time,” Dr. Fernandez said.
Despite this tenet, for years, there was only one placebo-controlled trial, published in 1993, that evaluated IVIG treatment for dermatomyositis, and it included only 15 participants. That was until October 2022, he said, when the New England Journal of Medicine published a study comparing a specific brand of IVIG (Octagam) with placebo in 95 people with dermatomyositis.
In the study, 79% of participants treated with IVIG had a total improvement score of at least 20 (minimal improvement), the primary endpoint, at 16 weeks, compared with 44% of those receiving a placebo. Those treated with IVIG also had significant improvements in the CDASI score, a secondary endpoint, compared with those on placebo, he said.
Based on results of this trial, the FDA approved Octagam 10% for dermatomyositis in adults. Dr. Fernandez noted the approval is restricted to the brand of IVIG in the trial, not all IVIG products. However, “the FDA approval is most important to us because it gives us ammunition to fight for insurers to approve IVIG when we feel our patients with dermatomyositis need it,” regardless of the brand.
The Potential of JAK1 Inhibitors
An open-label study of the JAK inhibitor tofacitinib, published in December 2020, showed that mean changes in CDASI activity scores at 12 weeks were statistically significant compared with baseline in 10 people with dermatomyositis. “The importance of this study is that it is proof of concept that JAK inhibition can be effective for treating dermatomyositis, especially with active skin disease,” Dr. Fernandez said.
In addition, two large phase 3 trials are evaluating JAK inhibitor safety and efficacy for treating dermatomyositis. One is the VALOR trial, currently recruiting people with recalcitrant dermatomyositis to evaluate treatment with brepocitinib. Researchers in France are looking at another JAK inhibitor, baricitinib, for treating relapsing or treatment-naive dermatomyositis. Recruitment for the BIRD clinical trial is ongoing.
Monoclonal Antibody Showing Promise
“When it comes to looking specifically at dermatomyositis cutaneous disease, it’s been found that the levels of IFN beta correlate best with not only lesional skin type 1 IFN inflammatory signatures but also overall clinical disease activity,” Dr. Fernandez said. This correlation is stronger than for any other IFN-1-type cytokine active in the disorder.
“Perhaps blocking IFN beta might be best way to get control of dermatomyositis activity,” he added.
With that in mind, a phase 2 trial of dazukibart presented at the American Academy of Dermatology 2023 annual meeting highlighted the promise of this agent that targets type 1 IFN beta.
The primary endpoint was improvement in CDASI at 12 weeks. “This medication has remarkable efficacy,” Dr. Fernandez said. “We were one of the sites for this trial. Despite being blinded, there was no question about who was receiving drug and who was receiving placebo.”
“A minimal clinical improvement in disease activity was seen in more than 90%, so almost every patient who received this medication had meaningful improvement,” he added.
Based on the results, the manufacturer, Pfizer, is recruiting participants for a phase 3 trial to further assess dazukibart in dermatomyositis and polymyositis. Dr. Fernandez said, “This is a story you should pay attention to if you treat any dermatomyositis patients at all.”
Regarding these emerging therapies for CLE and dermatomyositis, “This looks very much like the early days of psoriasis, in the early 2000s, when there was a lot of activity developing treatments,” Dr. Fernandez said. “I will predict that within 10 years, we will have multiple novel agents available that will probably work better than anything we have today.”
Dr. Fernandez reported receiving grant and/or research support from Alexion, Incyte, Mallinckrodt Pharmaceuticals, Novartis, Pfizer, and Priovant Therapeutics; acting as a consultant or advisory board member for AbbVie, Biogen, Mallinckrodt Pharmaceuticals; and being a member of the speaker bureau or receiving honoraria for non-CME from AbbVie, Kyowa Kirin, and Mallinckrodt Pharmaceuticals.
A version of this article appeared on Medscape.com.
FROM ODAC 2024