User login
New tool may provide point-of-care differentiation between bacterial, viral infections
The World Health Organization estimates that 14.9 million of 57 million annual deaths worldwide (25%) are related directly to diseases caused by bacterial and/or viral infections.
The first crucial step in order to build a successful surveillance system is to accurately identify and diagnose disease, Ivana Pennisi reminded the audience at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. A problem, particularly in primary care, is differentiating between patients with bacterial infections who might benefit from antibiotics and those with viral infections where supportive treatment is generally required. One solution might a rapid point-of-care tool.
Ms. Pennisi described early experiences of using microchip technology to detect RNA biomarkers in the blood rather than look for the pathogen itself. Early results suggest high diagnostic accuracy at low cost.
It is known that when a bacteria or virus enters the body, it stimulates the immune system in a unique way leading to the expression of different genes in the host blood. As part of the Personalized Management of Febrile Illnesses study, researchers have demonstrated a number of high correlated transcripts. Of current interest are two genes which are upregulated in childhood febrile illnesses.
Ms. Pennisi, a PhD student working as part of a multidisciplinary at the department of infectious disease and Centre for Bioinspired Technology at Imperial College, London, developed loop-mediated isothermal amplification (LAMP) assays to detect for the first time host RNA signatures on a nucleic acid–based point-of-care handheld system to discriminate bacterial from viral infection. The amplification reaction is then combined with microchip technology in the well of a portable point-of-care device named Lacewing. It translates the nucleic acid amplification signal into a quantitative electrochemical signal without the need for a thermal cycler.
The combination of genomic expertise in the section of paediatrics lead by Michael Levin, PhD, and microchip-based technologies in the department of electrical and electronic engineering under the guidance of Pantelis Georgiou, PhD, enabled the team overcome many clinical challenges.
Ms. Pennisi presented her team’s early experiences with clinical samples from 455 febrile children. First, transcription isothermal amplification techniques were employed to confirm bacterial and viral infections. Results were then validated using standard fluorescent-based quantitative polymerase chain reaction (PCR) instruments. In order to define a decision boundary between bacterial and viral patients, cutoff levels were determined using multivariate logistic regression analysis. Results then were evaluated using microarrays, reverse transcriptase PCR (RT-PCR), and the eLAMP to confirm comparability with preferred techniques.
In conclusion, Ms. Pennisi reported that the two-gene signature combined with the use of eLAMP technology in She outlined her vision for the future: “The patient sample and reagent are loaded into a disposable cartridge. This is then placed into a device to monitor in real time the reaction and share all the data via a Bluetooth to a dedicated app on a smart phone. All data and location of the outbreak are then stored in [the] cloud, making it easier for epidemiological studies and tracking of new outbreaks. We hope that by enhancing the capability of our platform, we contribute to better patient care.”
“Distinguishing between bacterial and viral infections remains one of the key questions in the daily pediatric acute care,” commented Lauri Ivaska, MD, from the department of pediatrics and adolescent medicine at Turku (Finland) University Hospital. “One of the most promising laboratory methods to do this is by measuring quantities of two specific host RNA transcripts from a blood sample. It would be of great importance if this could be done reliably by using a fast and cheap method as presented here by Ivana Pennisi.”
Ms. Pennisi had no relevant financial disclosures.
The World Health Organization estimates that 14.9 million of 57 million annual deaths worldwide (25%) are related directly to diseases caused by bacterial and/or viral infections.
The first crucial step in order to build a successful surveillance system is to accurately identify and diagnose disease, Ivana Pennisi reminded the audience at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. A problem, particularly in primary care, is differentiating between patients with bacterial infections who might benefit from antibiotics and those with viral infections where supportive treatment is generally required. One solution might a rapid point-of-care tool.
Ms. Pennisi described early experiences of using microchip technology to detect RNA biomarkers in the blood rather than look for the pathogen itself. Early results suggest high diagnostic accuracy at low cost.
It is known that when a bacteria or virus enters the body, it stimulates the immune system in a unique way leading to the expression of different genes in the host blood. As part of the Personalized Management of Febrile Illnesses study, researchers have demonstrated a number of high correlated transcripts. Of current interest are two genes which are upregulated in childhood febrile illnesses.
Ms. Pennisi, a PhD student working as part of a multidisciplinary at the department of infectious disease and Centre for Bioinspired Technology at Imperial College, London, developed loop-mediated isothermal amplification (LAMP) assays to detect for the first time host RNA signatures on a nucleic acid–based point-of-care handheld system to discriminate bacterial from viral infection. The amplification reaction is then combined with microchip technology in the well of a portable point-of-care device named Lacewing. It translates the nucleic acid amplification signal into a quantitative electrochemical signal without the need for a thermal cycler.
The combination of genomic expertise in the section of paediatrics lead by Michael Levin, PhD, and microchip-based technologies in the department of electrical and electronic engineering under the guidance of Pantelis Georgiou, PhD, enabled the team overcome many clinical challenges.
Ms. Pennisi presented her team’s early experiences with clinical samples from 455 febrile children. First, transcription isothermal amplification techniques were employed to confirm bacterial and viral infections. Results were then validated using standard fluorescent-based quantitative polymerase chain reaction (PCR) instruments. In order to define a decision boundary between bacterial and viral patients, cutoff levels were determined using multivariate logistic regression analysis. Results then were evaluated using microarrays, reverse transcriptase PCR (RT-PCR), and the eLAMP to confirm comparability with preferred techniques.
In conclusion, Ms. Pennisi reported that the two-gene signature combined with the use of eLAMP technology in She outlined her vision for the future: “The patient sample and reagent are loaded into a disposable cartridge. This is then placed into a device to monitor in real time the reaction and share all the data via a Bluetooth to a dedicated app on a smart phone. All data and location of the outbreak are then stored in [the] cloud, making it easier for epidemiological studies and tracking of new outbreaks. We hope that by enhancing the capability of our platform, we contribute to better patient care.”
“Distinguishing between bacterial and viral infections remains one of the key questions in the daily pediatric acute care,” commented Lauri Ivaska, MD, from the department of pediatrics and adolescent medicine at Turku (Finland) University Hospital. “One of the most promising laboratory methods to do this is by measuring quantities of two specific host RNA transcripts from a blood sample. It would be of great importance if this could be done reliably by using a fast and cheap method as presented here by Ivana Pennisi.”
Ms. Pennisi had no relevant financial disclosures.
The World Health Organization estimates that 14.9 million of 57 million annual deaths worldwide (25%) are related directly to diseases caused by bacterial and/or viral infections.
The first crucial step in order to build a successful surveillance system is to accurately identify and diagnose disease, Ivana Pennisi reminded the audience at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. A problem, particularly in primary care, is differentiating between patients with bacterial infections who might benefit from antibiotics and those with viral infections where supportive treatment is generally required. One solution might a rapid point-of-care tool.
Ms. Pennisi described early experiences of using microchip technology to detect RNA biomarkers in the blood rather than look for the pathogen itself. Early results suggest high diagnostic accuracy at low cost.
It is known that when a bacteria or virus enters the body, it stimulates the immune system in a unique way leading to the expression of different genes in the host blood. As part of the Personalized Management of Febrile Illnesses study, researchers have demonstrated a number of high correlated transcripts. Of current interest are two genes which are upregulated in childhood febrile illnesses.
Ms. Pennisi, a PhD student working as part of a multidisciplinary at the department of infectious disease and Centre for Bioinspired Technology at Imperial College, London, developed loop-mediated isothermal amplification (LAMP) assays to detect for the first time host RNA signatures on a nucleic acid–based point-of-care handheld system to discriminate bacterial from viral infection. The amplification reaction is then combined with microchip technology in the well of a portable point-of-care device named Lacewing. It translates the nucleic acid amplification signal into a quantitative electrochemical signal without the need for a thermal cycler.
The combination of genomic expertise in the section of paediatrics lead by Michael Levin, PhD, and microchip-based technologies in the department of electrical and electronic engineering under the guidance of Pantelis Georgiou, PhD, enabled the team overcome many clinical challenges.
Ms. Pennisi presented her team’s early experiences with clinical samples from 455 febrile children. First, transcription isothermal amplification techniques were employed to confirm bacterial and viral infections. Results were then validated using standard fluorescent-based quantitative polymerase chain reaction (PCR) instruments. In order to define a decision boundary between bacterial and viral patients, cutoff levels were determined using multivariate logistic regression analysis. Results then were evaluated using microarrays, reverse transcriptase PCR (RT-PCR), and the eLAMP to confirm comparability with preferred techniques.
In conclusion, Ms. Pennisi reported that the two-gene signature combined with the use of eLAMP technology in She outlined her vision for the future: “The patient sample and reagent are loaded into a disposable cartridge. This is then placed into a device to monitor in real time the reaction and share all the data via a Bluetooth to a dedicated app on a smart phone. All data and location of the outbreak are then stored in [the] cloud, making it easier for epidemiological studies and tracking of new outbreaks. We hope that by enhancing the capability of our platform, we contribute to better patient care.”
“Distinguishing between bacterial and viral infections remains one of the key questions in the daily pediatric acute care,” commented Lauri Ivaska, MD, from the department of pediatrics and adolescent medicine at Turku (Finland) University Hospital. “One of the most promising laboratory methods to do this is by measuring quantities of two specific host RNA transcripts from a blood sample. It would be of great importance if this could be done reliably by using a fast and cheap method as presented here by Ivana Pennisi.”
Ms. Pennisi had no relevant financial disclosures.
FROM ESPID 2020
Three genes could predict congenital Zika infection susceptibility
Dr. Irene Rivero-Calle, MD, shared at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
ZIKV, an emerging flavivirus, is responsible for one the most critical pandemic emergencies of the last decade and has been associated with severe neonatal brain disabilities, declared Dr. Rivero-Calle, of the Hospital Clínico Universitario de Santiago de Compostela in Santiago de Compostela, Spain. “We think that understanding the genomic background could explain some of the most relevant symptoms of congenital Zika syndrome (CZS) and could be essential to better comprehend this disease.”
To achieve this understanding, Dr. Rivero-Calle and her colleagues conducted a study aiming to analyze any genetic factors that could explain the variation in phenotypes in newborns from mothers who had a Zika infection during their pregnancy. Additionally, they strove to “elucidate if the possible genetic association is specific to mothers or their newborns, and to check if this genomic background or any genomic ancestry pattern could be related with the phenotype,” she explained.
In their study, Dr. Rivero-Calle and her team analyzed 80 samples, comprising 40 samples from mothers who had been infected by ZIKV during their pregnancy and 40 from their newborns. Of those descendants, 20 were asymptomatic and 20 were symptomatic (13 had CZS, 3 had microcephaly, 2 had a pathologic MRI, 1 had hearing loss, and 1 was born preterm).
Population stratification, which Dr. Rivero-Calle explained “lets us know if the population is African, European, or Native American looking at the genes,” did not show any relation with the phenotype. We had a mixture of population genomics among all samples.”
Dr. Rivero-Calle and her team then performed three analyses: genotype analysis, an allelic test, and gene analysis. The allelic test and gene-collapsing method highlighted three genes (PANO1, PIDD1, and SLC25A22) as potential determinants of the varying phenotypes in the newborns from ZIKV-infected mothers. Overrepresentation analysis of gene ontology terms shows that PIDD1 and PANO1 are related to apoptosis and cell death, which is closely related to early infantile epilepsy. This could explain the most severe complications of CZS: seizures, brain damage, microcephaly, and detrimental neurodevelopmental growth. Regarding reactome and KEGG analysis, gene PIID1 is related with p53 pathway, which correlates with cell’s death and apoptosis, and with microcephaly, a typical phenotypic feature of CZS.
“So, in conclusion, we found three genes which could predict susceptibility to congenital Zika infection; we saw that the functionality of these genes seems to be deeply related with mechanisms which could explain the different phenotypes; and we saw that these three genes only appear in the children’s cohort, so there is no candidate gene in the mother’s genomic background which can help predict the phenotype of the newborn,” Dr. Rivero-Calle declared. “Finally, there is no ancestry pattern associated with disabilities caused by Zika infection.”
Dr. Rivero-Calle reported that this project (ZikAction) has received funding from the European Union’s Horizon 2020 research and innovation program, under grant agreement 734857.
Dr. Irene Rivero-Calle, MD, shared at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
ZIKV, an emerging flavivirus, is responsible for one the most critical pandemic emergencies of the last decade and has been associated with severe neonatal brain disabilities, declared Dr. Rivero-Calle, of the Hospital Clínico Universitario de Santiago de Compostela in Santiago de Compostela, Spain. “We think that understanding the genomic background could explain some of the most relevant symptoms of congenital Zika syndrome (CZS) and could be essential to better comprehend this disease.”
To achieve this understanding, Dr. Rivero-Calle and her colleagues conducted a study aiming to analyze any genetic factors that could explain the variation in phenotypes in newborns from mothers who had a Zika infection during their pregnancy. Additionally, they strove to “elucidate if the possible genetic association is specific to mothers or their newborns, and to check if this genomic background or any genomic ancestry pattern could be related with the phenotype,” she explained.
In their study, Dr. Rivero-Calle and her team analyzed 80 samples, comprising 40 samples from mothers who had been infected by ZIKV during their pregnancy and 40 from their newborns. Of those descendants, 20 were asymptomatic and 20 were symptomatic (13 had CZS, 3 had microcephaly, 2 had a pathologic MRI, 1 had hearing loss, and 1 was born preterm).
Population stratification, which Dr. Rivero-Calle explained “lets us know if the population is African, European, or Native American looking at the genes,” did not show any relation with the phenotype. We had a mixture of population genomics among all samples.”
Dr. Rivero-Calle and her team then performed three analyses: genotype analysis, an allelic test, and gene analysis. The allelic test and gene-collapsing method highlighted three genes (PANO1, PIDD1, and SLC25A22) as potential determinants of the varying phenotypes in the newborns from ZIKV-infected mothers. Overrepresentation analysis of gene ontology terms shows that PIDD1 and PANO1 are related to apoptosis and cell death, which is closely related to early infantile epilepsy. This could explain the most severe complications of CZS: seizures, brain damage, microcephaly, and detrimental neurodevelopmental growth. Regarding reactome and KEGG analysis, gene PIID1 is related with p53 pathway, which correlates with cell’s death and apoptosis, and with microcephaly, a typical phenotypic feature of CZS.
“So, in conclusion, we found three genes which could predict susceptibility to congenital Zika infection; we saw that the functionality of these genes seems to be deeply related with mechanisms which could explain the different phenotypes; and we saw that these three genes only appear in the children’s cohort, so there is no candidate gene in the mother’s genomic background which can help predict the phenotype of the newborn,” Dr. Rivero-Calle declared. “Finally, there is no ancestry pattern associated with disabilities caused by Zika infection.”
Dr. Rivero-Calle reported that this project (ZikAction) has received funding from the European Union’s Horizon 2020 research and innovation program, under grant agreement 734857.
Dr. Irene Rivero-Calle, MD, shared at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
ZIKV, an emerging flavivirus, is responsible for one the most critical pandemic emergencies of the last decade and has been associated with severe neonatal brain disabilities, declared Dr. Rivero-Calle, of the Hospital Clínico Universitario de Santiago de Compostela in Santiago de Compostela, Spain. “We think that understanding the genomic background could explain some of the most relevant symptoms of congenital Zika syndrome (CZS) and could be essential to better comprehend this disease.”
To achieve this understanding, Dr. Rivero-Calle and her colleagues conducted a study aiming to analyze any genetic factors that could explain the variation in phenotypes in newborns from mothers who had a Zika infection during their pregnancy. Additionally, they strove to “elucidate if the possible genetic association is specific to mothers or their newborns, and to check if this genomic background or any genomic ancestry pattern could be related with the phenotype,” she explained.
In their study, Dr. Rivero-Calle and her team analyzed 80 samples, comprising 40 samples from mothers who had been infected by ZIKV during their pregnancy and 40 from their newborns. Of those descendants, 20 were asymptomatic and 20 were symptomatic (13 had CZS, 3 had microcephaly, 2 had a pathologic MRI, 1 had hearing loss, and 1 was born preterm).
Population stratification, which Dr. Rivero-Calle explained “lets us know if the population is African, European, or Native American looking at the genes,” did not show any relation with the phenotype. We had a mixture of population genomics among all samples.”
Dr. Rivero-Calle and her team then performed three analyses: genotype analysis, an allelic test, and gene analysis. The allelic test and gene-collapsing method highlighted three genes (PANO1, PIDD1, and SLC25A22) as potential determinants of the varying phenotypes in the newborns from ZIKV-infected mothers. Overrepresentation analysis of gene ontology terms shows that PIDD1 and PANO1 are related to apoptosis and cell death, which is closely related to early infantile epilepsy. This could explain the most severe complications of CZS: seizures, brain damage, microcephaly, and detrimental neurodevelopmental growth. Regarding reactome and KEGG analysis, gene PIID1 is related with p53 pathway, which correlates with cell’s death and apoptosis, and with microcephaly, a typical phenotypic feature of CZS.
“So, in conclusion, we found three genes which could predict susceptibility to congenital Zika infection; we saw that the functionality of these genes seems to be deeply related with mechanisms which could explain the different phenotypes; and we saw that these three genes only appear in the children’s cohort, so there is no candidate gene in the mother’s genomic background which can help predict the phenotype of the newborn,” Dr. Rivero-Calle declared. “Finally, there is no ancestry pattern associated with disabilities caused by Zika infection.”
Dr. Rivero-Calle reported that this project (ZikAction) has received funding from the European Union’s Horizon 2020 research and innovation program, under grant agreement 734857.
FROM ESPID 2020
C. difficile control could require integrated approach
Clostridioides difficile (C. diff) infection (CDI) is a pathogen of both humans and animals, and to control it will require an integrated approach that encompasses human health care, veterinary health care, environmental regulation, and public policy. That is the conclusion of a group led by Su-Chen Lim, MD, and Tom Riley, MD, of Edith Cowan University in Australia, who published a review in Clinical Microbiology and Infection.
CDI was generally considered a nuisance infection until the early 21st century, when a hypervirulent fluoroquinolone-resistant strain emerged in North America. The strain is now documented In the United States, Canada, and most countries in Europe.
Another new feature of CDI is increased evidence of community transmission, which was previously rare. This is defined as cases where the patient experienced symptom onset outside the hospital, and had no history of hospitalization in the previous 12 weeks or symptom onset within 48 hours of hospital admission. Community-associated CDI now accounts for 41% of U.S. cases, nearly 30% of Australian cases, and about 14% in Europe, according to recent studies.
Several features of CDI suggest a need for an integrated management plan. The preferred habitat of C. diff is the gastrointestinal track of mammals, and likely colonizes all mammalian neonates. Over time, colonization by other microbes likely crowd it out and prevent overgrowth. But widespread use of antimicrobials in animal production can lead to the creation of an environment resembling that of the neonate, allowing C. diff to expand. That has led to food animals becoming a major C. diff reservoir, and whole-genome studies showed that strains found in humans, food, animals, and the environment are closely related and sometimes genetically indistinguishable, suggesting transmission between humans and animals that may be attributable to contaminated food and environments.
The authors suggest that C. diff infection control should be guided by the One Health initiative, which seeks cooperation between physicians, osteopathic physicians, veterinarians, dentists, nurses, and other scientific and environmental disciplines. The goal is to enhance surveillance and interdisciplinary communication, as well as integrated policies. The authors note that C. diff is often thought of by physicians as primarily a hospital problem, who may be unaware of the increased prevalence of community-acquired disease. It is also a significant problem in agriculture, since as many as 50% of piglets succumb to the disease. Other studies have recently shown that asymptomatic carriers of toxigenic strains are likely to transmit the bacteria to C. diff-negative patients. Asymptomatic carriers cluster with symptomatic patients. In one Cleveland hospital, more than 25% of hospital-associated CDI cases were found to have been colonized prior to admission, suggesting that these were not true hospital-associated cases.
C. diff has been isolated from a wide range of sources, including food animals, meat, seafood, vegetables, household environments, and natural environments like rivers, lakes, and soil. About 20% of calves and 70% of piglets are colonized with C. diff. It has a high prevalence in meat products in the United States, but lower in the Europe, possibly because of different slaughtering practices.
The authors suggest that zoonotic C. diff spread is unlikely to be confined to any geographic region or population, and that widespread C. diff contamination is occurring through food or the environment. This could be occurring because spores can withstand cooking temperatures and disseminate through the air, and even through manure from food animals made into compost or fertilizer.
Veterinary efforts mimicking hospital measures have reduced animal CDI, but there are no rapid diagnostic tests for CDI in animals, making it challenging to control its spread in this context.
The authors call for enhanced antimicrobial stewardship in both human and animal settings, including banning of antimicrobial agents as growth promoters. This has been done in the United States and Europe, but not in Brazil, China, Canada, India, and Australia. They also call for research on inactivation of C. diff spores during waste treatment.
Even better, the authors suggest that vaccines should be developed and employed in both animals and humans. No such vaccine exists in animals, but Pfizer has one for humans in a phase 3 clinical trial, but it does not prevent colonization. Others are in development.
The epidemiology of CDI is an ongoing challenge, with emerging new strains and changing social and environmental conditions. “However, it is with the collaborative efforts of industry partners, policymakers, veterinarians, clinicians, and researchers that CDI needs to be approached, a perfect example of One Health. Opening an interdisciplinary dialogue to address CDI and One Health issues has to be the focus of future studies,” the authors concluded.
SOURCE: SC Lim et al. Clinical Microbiology and Infection. 2020;26:85-863.
Clostridioides difficile (C. diff) infection (CDI) is a pathogen of both humans and animals, and to control it will require an integrated approach that encompasses human health care, veterinary health care, environmental regulation, and public policy. That is the conclusion of a group led by Su-Chen Lim, MD, and Tom Riley, MD, of Edith Cowan University in Australia, who published a review in Clinical Microbiology and Infection.
CDI was generally considered a nuisance infection until the early 21st century, when a hypervirulent fluoroquinolone-resistant strain emerged in North America. The strain is now documented In the United States, Canada, and most countries in Europe.
Another new feature of CDI is increased evidence of community transmission, which was previously rare. This is defined as cases where the patient experienced symptom onset outside the hospital, and had no history of hospitalization in the previous 12 weeks or symptom onset within 48 hours of hospital admission. Community-associated CDI now accounts for 41% of U.S. cases, nearly 30% of Australian cases, and about 14% in Europe, according to recent studies.
Several features of CDI suggest a need for an integrated management plan. The preferred habitat of C. diff is the gastrointestinal track of mammals, and likely colonizes all mammalian neonates. Over time, colonization by other microbes likely crowd it out and prevent overgrowth. But widespread use of antimicrobials in animal production can lead to the creation of an environment resembling that of the neonate, allowing C. diff to expand. That has led to food animals becoming a major C. diff reservoir, and whole-genome studies showed that strains found in humans, food, animals, and the environment are closely related and sometimes genetically indistinguishable, suggesting transmission between humans and animals that may be attributable to contaminated food and environments.
The authors suggest that C. diff infection control should be guided by the One Health initiative, which seeks cooperation between physicians, osteopathic physicians, veterinarians, dentists, nurses, and other scientific and environmental disciplines. The goal is to enhance surveillance and interdisciplinary communication, as well as integrated policies. The authors note that C. diff is often thought of by physicians as primarily a hospital problem, who may be unaware of the increased prevalence of community-acquired disease. It is also a significant problem in agriculture, since as many as 50% of piglets succumb to the disease. Other studies have recently shown that asymptomatic carriers of toxigenic strains are likely to transmit the bacteria to C. diff-negative patients. Asymptomatic carriers cluster with symptomatic patients. In one Cleveland hospital, more than 25% of hospital-associated CDI cases were found to have been colonized prior to admission, suggesting that these were not true hospital-associated cases.
C. diff has been isolated from a wide range of sources, including food animals, meat, seafood, vegetables, household environments, and natural environments like rivers, lakes, and soil. About 20% of calves and 70% of piglets are colonized with C. diff. It has a high prevalence in meat products in the United States, but lower in the Europe, possibly because of different slaughtering practices.
The authors suggest that zoonotic C. diff spread is unlikely to be confined to any geographic region or population, and that widespread C. diff contamination is occurring through food or the environment. This could be occurring because spores can withstand cooking temperatures and disseminate through the air, and even through manure from food animals made into compost or fertilizer.
Veterinary efforts mimicking hospital measures have reduced animal CDI, but there are no rapid diagnostic tests for CDI in animals, making it challenging to control its spread in this context.
The authors call for enhanced antimicrobial stewardship in both human and animal settings, including banning of antimicrobial agents as growth promoters. This has been done in the United States and Europe, but not in Brazil, China, Canada, India, and Australia. They also call for research on inactivation of C. diff spores during waste treatment.
Even better, the authors suggest that vaccines should be developed and employed in both animals and humans. No such vaccine exists in animals, but Pfizer has one for humans in a phase 3 clinical trial, but it does not prevent colonization. Others are in development.
The epidemiology of CDI is an ongoing challenge, with emerging new strains and changing social and environmental conditions. “However, it is with the collaborative efforts of industry partners, policymakers, veterinarians, clinicians, and researchers that CDI needs to be approached, a perfect example of One Health. Opening an interdisciplinary dialogue to address CDI and One Health issues has to be the focus of future studies,” the authors concluded.
SOURCE: SC Lim et al. Clinical Microbiology and Infection. 2020;26:85-863.
Clostridioides difficile (C. diff) infection (CDI) is a pathogen of both humans and animals, and to control it will require an integrated approach that encompasses human health care, veterinary health care, environmental regulation, and public policy. That is the conclusion of a group led by Su-Chen Lim, MD, and Tom Riley, MD, of Edith Cowan University in Australia, who published a review in Clinical Microbiology and Infection.
CDI was generally considered a nuisance infection until the early 21st century, when a hypervirulent fluoroquinolone-resistant strain emerged in North America. The strain is now documented In the United States, Canada, and most countries in Europe.
Another new feature of CDI is increased evidence of community transmission, which was previously rare. This is defined as cases where the patient experienced symptom onset outside the hospital, and had no history of hospitalization in the previous 12 weeks or symptom onset within 48 hours of hospital admission. Community-associated CDI now accounts for 41% of U.S. cases, nearly 30% of Australian cases, and about 14% in Europe, according to recent studies.
Several features of CDI suggest a need for an integrated management plan. The preferred habitat of C. diff is the gastrointestinal track of mammals, and likely colonizes all mammalian neonates. Over time, colonization by other microbes likely crowd it out and prevent overgrowth. But widespread use of antimicrobials in animal production can lead to the creation of an environment resembling that of the neonate, allowing C. diff to expand. That has led to food animals becoming a major C. diff reservoir, and whole-genome studies showed that strains found in humans, food, animals, and the environment are closely related and sometimes genetically indistinguishable, suggesting transmission between humans and animals that may be attributable to contaminated food and environments.
The authors suggest that C. diff infection control should be guided by the One Health initiative, which seeks cooperation between physicians, osteopathic physicians, veterinarians, dentists, nurses, and other scientific and environmental disciplines. The goal is to enhance surveillance and interdisciplinary communication, as well as integrated policies. The authors note that C. diff is often thought of by physicians as primarily a hospital problem, who may be unaware of the increased prevalence of community-acquired disease. It is also a significant problem in agriculture, since as many as 50% of piglets succumb to the disease. Other studies have recently shown that asymptomatic carriers of toxigenic strains are likely to transmit the bacteria to C. diff-negative patients. Asymptomatic carriers cluster with symptomatic patients. In one Cleveland hospital, more than 25% of hospital-associated CDI cases were found to have been colonized prior to admission, suggesting that these were not true hospital-associated cases.
C. diff has been isolated from a wide range of sources, including food animals, meat, seafood, vegetables, household environments, and natural environments like rivers, lakes, and soil. About 20% of calves and 70% of piglets are colonized with C. diff. It has a high prevalence in meat products in the United States, but lower in the Europe, possibly because of different slaughtering practices.
The authors suggest that zoonotic C. diff spread is unlikely to be confined to any geographic region or population, and that widespread C. diff contamination is occurring through food or the environment. This could be occurring because spores can withstand cooking temperatures and disseminate through the air, and even through manure from food animals made into compost or fertilizer.
Veterinary efforts mimicking hospital measures have reduced animal CDI, but there are no rapid diagnostic tests for CDI in animals, making it challenging to control its spread in this context.
The authors call for enhanced antimicrobial stewardship in both human and animal settings, including banning of antimicrobial agents as growth promoters. This has been done in the United States and Europe, but not in Brazil, China, Canada, India, and Australia. They also call for research on inactivation of C. diff spores during waste treatment.
Even better, the authors suggest that vaccines should be developed and employed in both animals and humans. No such vaccine exists in animals, but Pfizer has one for humans in a phase 3 clinical trial, but it does not prevent colonization. Others are in development.
The epidemiology of CDI is an ongoing challenge, with emerging new strains and changing social and environmental conditions. “However, it is with the collaborative efforts of industry partners, policymakers, veterinarians, clinicians, and researchers that CDI needs to be approached, a perfect example of One Health. Opening an interdisciplinary dialogue to address CDI and One Health issues has to be the focus of future studies,” the authors concluded.
SOURCE: SC Lim et al. Clinical Microbiology and Infection. 2020;26:85-863.
FROM CLINICAL MICROBIOLOGY AND INFECTION
Meningococcal transmission risk appears low among pediatric health care professionals
at a university – lower than expected for all age groups, Lisa-Maria Steurer, MD, said regarding study findings reported at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
“This implicates that the risk of horizontal meningococcal transmission via this health care professional cohort seems to be low,” said Dr. Steurer, of the Medical University of Vienna.
Her data were based on a survey conducted between April and October 2018 at the department of paediatrics and adolescent medicine at the tertiary university pediatric hospital. The study aimed to determine colonization rates of Neisseria meningitidis and the serogroup distribution of carried meningococcal isolates in asymptomatic health care professionals employed there, reported Dr. Steurer. Her research team also sought to identify what factors increased risk of N. meningitidis carriage.
“We who work in pediatrics and adolescent medicine are exposed to those patient cohorts with the highest risk for meningococcal carriage, but also to those patients who have the highest risk for serious, invasive meningococcal disease, which peaks at the extremities of age,” declared Dr. Steurer. “But currently, there is no surveillance of asymptomatic carriers in this health care professional cohort.”
A total of 437 oropharyngeal swabs were collected from enrolled nurses, pediatricians, and medical students working in the department and immediately plated onto selective agar plates. Conventional culture was used to identify bacteria, and meningococcal isolates were characterized further through whole-genome sequencing. Sociodemographic data and information on participants’ vaccination status were collected via questionnaire.
The main finding was an overall meningococcal prevalence of 1.14%. Among the participants, the median age was 33 years, and the highest rate of carriage, 4.4%, was observed in those aged 18-25 years. None of the carriers were older than 35 years. There was a negative association found between carriage and participants’ age and time employed in the field, Dr. Steurer said.
“Risk-factor analysis found an inverse correlation with meningococcal carriage for age and timespan working in pediatrics. On the contrary, no correlations with carriage could be found for all other factors evaluated,” she said. These factors included recent contact with an immunodeficient patient, respiratory tract infection, smoking, vaccination against any meningococcal serogroup, different professions, main work settings, month of swab collection, and living with children or adolescents in the same household.
Of the study population, 29% reported that they had been vaccinated against at least one meningococcal serogroup. “Interestingly, while more than 50% of doctors and medical students had a vaccination against at least one meningococcal serogroup, only 17% of nurses were vaccinated,” Dr. Steurer remarked.
The study was financially supported by Pfizer. Dr. Steurer had no other relevant financial disclosures.
at a university – lower than expected for all age groups, Lisa-Maria Steurer, MD, said regarding study findings reported at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
“This implicates that the risk of horizontal meningococcal transmission via this health care professional cohort seems to be low,” said Dr. Steurer, of the Medical University of Vienna.
Her data were based on a survey conducted between April and October 2018 at the department of paediatrics and adolescent medicine at the tertiary university pediatric hospital. The study aimed to determine colonization rates of Neisseria meningitidis and the serogroup distribution of carried meningococcal isolates in asymptomatic health care professionals employed there, reported Dr. Steurer. Her research team also sought to identify what factors increased risk of N. meningitidis carriage.
“We who work in pediatrics and adolescent medicine are exposed to those patient cohorts with the highest risk for meningococcal carriage, but also to those patients who have the highest risk for serious, invasive meningococcal disease, which peaks at the extremities of age,” declared Dr. Steurer. “But currently, there is no surveillance of asymptomatic carriers in this health care professional cohort.”
A total of 437 oropharyngeal swabs were collected from enrolled nurses, pediatricians, and medical students working in the department and immediately plated onto selective agar plates. Conventional culture was used to identify bacteria, and meningococcal isolates were characterized further through whole-genome sequencing. Sociodemographic data and information on participants’ vaccination status were collected via questionnaire.
The main finding was an overall meningococcal prevalence of 1.14%. Among the participants, the median age was 33 years, and the highest rate of carriage, 4.4%, was observed in those aged 18-25 years. None of the carriers were older than 35 years. There was a negative association found between carriage and participants’ age and time employed in the field, Dr. Steurer said.
“Risk-factor analysis found an inverse correlation with meningococcal carriage for age and timespan working in pediatrics. On the contrary, no correlations with carriage could be found for all other factors evaluated,” she said. These factors included recent contact with an immunodeficient patient, respiratory tract infection, smoking, vaccination against any meningococcal serogroup, different professions, main work settings, month of swab collection, and living with children or adolescents in the same household.
Of the study population, 29% reported that they had been vaccinated against at least one meningococcal serogroup. “Interestingly, while more than 50% of doctors and medical students had a vaccination against at least one meningococcal serogroup, only 17% of nurses were vaccinated,” Dr. Steurer remarked.
The study was financially supported by Pfizer. Dr. Steurer had no other relevant financial disclosures.
at a university – lower than expected for all age groups, Lisa-Maria Steurer, MD, said regarding study findings reported at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
“This implicates that the risk of horizontal meningococcal transmission via this health care professional cohort seems to be low,” said Dr. Steurer, of the Medical University of Vienna.
Her data were based on a survey conducted between April and October 2018 at the department of paediatrics and adolescent medicine at the tertiary university pediatric hospital. The study aimed to determine colonization rates of Neisseria meningitidis and the serogroup distribution of carried meningococcal isolates in asymptomatic health care professionals employed there, reported Dr. Steurer. Her research team also sought to identify what factors increased risk of N. meningitidis carriage.
“We who work in pediatrics and adolescent medicine are exposed to those patient cohorts with the highest risk for meningococcal carriage, but also to those patients who have the highest risk for serious, invasive meningococcal disease, which peaks at the extremities of age,” declared Dr. Steurer. “But currently, there is no surveillance of asymptomatic carriers in this health care professional cohort.”
A total of 437 oropharyngeal swabs were collected from enrolled nurses, pediatricians, and medical students working in the department and immediately plated onto selective agar plates. Conventional culture was used to identify bacteria, and meningococcal isolates were characterized further through whole-genome sequencing. Sociodemographic data and information on participants’ vaccination status were collected via questionnaire.
The main finding was an overall meningococcal prevalence of 1.14%. Among the participants, the median age was 33 years, and the highest rate of carriage, 4.4%, was observed in those aged 18-25 years. None of the carriers were older than 35 years. There was a negative association found between carriage and participants’ age and time employed in the field, Dr. Steurer said.
“Risk-factor analysis found an inverse correlation with meningococcal carriage for age and timespan working in pediatrics. On the contrary, no correlations with carriage could be found for all other factors evaluated,” she said. These factors included recent contact with an immunodeficient patient, respiratory tract infection, smoking, vaccination against any meningococcal serogroup, different professions, main work settings, month of swab collection, and living with children or adolescents in the same household.
Of the study population, 29% reported that they had been vaccinated against at least one meningococcal serogroup. “Interestingly, while more than 50% of doctors and medical students had a vaccination against at least one meningococcal serogroup, only 17% of nurses were vaccinated,” Dr. Steurer remarked.
The study was financially supported by Pfizer. Dr. Steurer had no other relevant financial disclosures.
FROM ESPID 2020
Children and school during the pandemic: What’s the answer?
Countries across the world are in the process of closing and reopening schools to contain the spread of COVID-19. Should there be universal testing and quarantining of sick school children and their classmates?
In a lively debate at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Andreea M. Panciu, MD, from the National Institute of Infectious Diseases in Bucharest, argued for routine testing and quarantining of all school children. Her opposite number, Danilo Buonsenso, MD, from the Centre for Global Health Research and Studies, Fondazione Policlinico Universitario Agostino Gemelli Istituto di Ricovero e Cura a Carattere Scientifico, Rome, made the case for a more selective approach.
Should children be sent to school?
stated Dr. Panciu as she started the debate by explaining the challenges faced by schools in adhering to key mitigation strategies. The U.S. Centers for Disease Control and Prevention recommends that students keep 1.8 m (6 feet) distance from one another. “In many school settings this is not feasible without drastically limiting the number of students,” she explained. “This is a massive challenge for many schools that are already overcrowded.”
The use of facemasks also is a challenge in classrooms. Children have a lower tolerance or may not be able to use the mask properly. There also are concerns regarding impaired learning, speech development, social development, and facial recognition. “We need to look at the evidence; preventive measures work,” responded Dr. Buonsenso. If distance can be implemented, the more distance the lower the transmission of infection, with 1.5-2 meters having the best effects. “Distance can be difficult when school buildings do not allow it, however, governments have had time to plan, and this should not be a limitation to education for kids.”
A recent review clearly showed that children and adolescents aged under 20 years have a much lower risk of susceptibility to COVID-19 infection, compared with adults. This is especially the case for children younger than 14 years. “There is no excuse, let’s bring the children back to school,” argued Dr. Buonsenso.
Dr. Panciu responded with several studies that have tried to quantify the amount of SARS-CoV-2 virus that is carried by infected children. Viral load in the nasopharynx in children under 5 years with mild to moderate COVID-19 symptoms was higher than that of both children over 5 as well as adults. The viral load in young children did not seem to differ by age or symptom severity. “There doesn’t appear to be a significant difference in viral load between symptomatic children and symptomatic adults,” she stated.
“But the question is: ‘How infectious are children?’ ” reacted Dr. Buonsenso. Data from South Korea showed that, for children, particularly those under 10 years, the number of secondary cases of contacts was very low, suggesting that children are rarely spreading the virus.
Dr. Buonsenso and colleagues assessed 30 households containing children aged under 18 years where an adult had been infected with COVID-19 in Rome during the peak of the pandemic. In no cases was it found that a child was the index case. This was supported by data from China, also obtained during the peak of the pandemic, which showed that the number of children infected was very low, but more importantly the number of secondary attacks from contact with children was also very low.
What about children who are sick at school?
The debate moved to discussing what should be done when a child is sick at school. Dr. Panciu clarified recommendations by the CDC regarding what steps to take if a student displays signs of infection consistent with COVID-19: Should they test positive, they are to stay at home for 10 days from the time signs and symptoms first appeared. Further, any teachers or students identified as close contacts are advised to stay at home for 14 days. (Since the ESPID meeting, the CDC has made changes in quarantine times for COVID-19. People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.)
A significant problem is the overlap between COVID-19 symptoms and those associated with other common illnesses because of a range of viruses. This is particularly true in younger children who often suffer from viral infections. “It is common for children to have up to eight respiratory illnesses a year,” explained Dr. Panciu, “and some may have symptoms so mild that they don’t notice them.”
“We need to be a little bit more children focused, otherwise we are going to be isolating children all the time,” said Dr. Buonsenso. The Royal College of Paediatrics and Child Health state that a child with a simple runny nose or sporadic cough without a fever, who would have attended school in other times, should not be tested for COVID-19. He moved on to then cite several studies that show little or no evidence of COVID-19 transmission between school children. This included a prospective cohort study in Australia showing that child-to-child transmission occurred in 0.3%. “To date, the advantages from routine quarantine and over testing seem too low to balance the social consequences on children and families,” he concluded.
As the debate drew to a close, Dr. Panciu reported several studies that did demonstrate transmission between school-age children. Data from an overnight camp in Georgia where the median age was 12 years showed the attack rate was 44% for ages 11-17 years and 51% for ages 6-10 years. Similar conclusions were reached in an Israeli study looking at a large COVID-19 outbreak in a school. This occurred 10 days after reopening, in spite of preventive measures being in place. “Opening safely isn’t just about the adjustments a school makes,” she said, “it’s also about how much of the virus is circulating in the community, which affects the likelihood that students and staff will bring COVID-19 into their classrooms.”
Damian Roland, consultant and honorary associate professor in pediatric emergency medicine at the University of Leicester (England), commented: “Maximizing educational potential while reducing the spread of COVID19 is a challenge laden with scientific equipoise while simultaneously infused with emotion. The evidence of transmission between, and infectivity from, children is not complete, as this debate has demonstrated. It is important scientists, clinicians, educators, and policy makers make collaborative decisions, aware there is not one perfect answer, and willing to understand and incorporate others views and objectives rather than holding onto single beliefs or approaches.”
No financial conflicts of interest were declared.
Countries across the world are in the process of closing and reopening schools to contain the spread of COVID-19. Should there be universal testing and quarantining of sick school children and their classmates?
In a lively debate at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Andreea M. Panciu, MD, from the National Institute of Infectious Diseases in Bucharest, argued for routine testing and quarantining of all school children. Her opposite number, Danilo Buonsenso, MD, from the Centre for Global Health Research and Studies, Fondazione Policlinico Universitario Agostino Gemelli Istituto di Ricovero e Cura a Carattere Scientifico, Rome, made the case for a more selective approach.
Should children be sent to school?
stated Dr. Panciu as she started the debate by explaining the challenges faced by schools in adhering to key mitigation strategies. The U.S. Centers for Disease Control and Prevention recommends that students keep 1.8 m (6 feet) distance from one another. “In many school settings this is not feasible without drastically limiting the number of students,” she explained. “This is a massive challenge for many schools that are already overcrowded.”
The use of facemasks also is a challenge in classrooms. Children have a lower tolerance or may not be able to use the mask properly. There also are concerns regarding impaired learning, speech development, social development, and facial recognition. “We need to look at the evidence; preventive measures work,” responded Dr. Buonsenso. If distance can be implemented, the more distance the lower the transmission of infection, with 1.5-2 meters having the best effects. “Distance can be difficult when school buildings do not allow it, however, governments have had time to plan, and this should not be a limitation to education for kids.”
A recent review clearly showed that children and adolescents aged under 20 years have a much lower risk of susceptibility to COVID-19 infection, compared with adults. This is especially the case for children younger than 14 years. “There is no excuse, let’s bring the children back to school,” argued Dr. Buonsenso.
Dr. Panciu responded with several studies that have tried to quantify the amount of SARS-CoV-2 virus that is carried by infected children. Viral load in the nasopharynx in children under 5 years with mild to moderate COVID-19 symptoms was higher than that of both children over 5 as well as adults. The viral load in young children did not seem to differ by age or symptom severity. “There doesn’t appear to be a significant difference in viral load between symptomatic children and symptomatic adults,” she stated.
“But the question is: ‘How infectious are children?’ ” reacted Dr. Buonsenso. Data from South Korea showed that, for children, particularly those under 10 years, the number of secondary cases of contacts was very low, suggesting that children are rarely spreading the virus.
Dr. Buonsenso and colleagues assessed 30 households containing children aged under 18 years where an adult had been infected with COVID-19 in Rome during the peak of the pandemic. In no cases was it found that a child was the index case. This was supported by data from China, also obtained during the peak of the pandemic, which showed that the number of children infected was very low, but more importantly the number of secondary attacks from contact with children was also very low.
What about children who are sick at school?
The debate moved to discussing what should be done when a child is sick at school. Dr. Panciu clarified recommendations by the CDC regarding what steps to take if a student displays signs of infection consistent with COVID-19: Should they test positive, they are to stay at home for 10 days from the time signs and symptoms first appeared. Further, any teachers or students identified as close contacts are advised to stay at home for 14 days. (Since the ESPID meeting, the CDC has made changes in quarantine times for COVID-19. People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.)
A significant problem is the overlap between COVID-19 symptoms and those associated with other common illnesses because of a range of viruses. This is particularly true in younger children who often suffer from viral infections. “It is common for children to have up to eight respiratory illnesses a year,” explained Dr. Panciu, “and some may have symptoms so mild that they don’t notice them.”
“We need to be a little bit more children focused, otherwise we are going to be isolating children all the time,” said Dr. Buonsenso. The Royal College of Paediatrics and Child Health state that a child with a simple runny nose or sporadic cough without a fever, who would have attended school in other times, should not be tested for COVID-19. He moved on to then cite several studies that show little or no evidence of COVID-19 transmission between school children. This included a prospective cohort study in Australia showing that child-to-child transmission occurred in 0.3%. “To date, the advantages from routine quarantine and over testing seem too low to balance the social consequences on children and families,” he concluded.
As the debate drew to a close, Dr. Panciu reported several studies that did demonstrate transmission between school-age children. Data from an overnight camp in Georgia where the median age was 12 years showed the attack rate was 44% for ages 11-17 years and 51% for ages 6-10 years. Similar conclusions were reached in an Israeli study looking at a large COVID-19 outbreak in a school. This occurred 10 days after reopening, in spite of preventive measures being in place. “Opening safely isn’t just about the adjustments a school makes,” she said, “it’s also about how much of the virus is circulating in the community, which affects the likelihood that students and staff will bring COVID-19 into their classrooms.”
Damian Roland, consultant and honorary associate professor in pediatric emergency medicine at the University of Leicester (England), commented: “Maximizing educational potential while reducing the spread of COVID19 is a challenge laden with scientific equipoise while simultaneously infused with emotion. The evidence of transmission between, and infectivity from, children is not complete, as this debate has demonstrated. It is important scientists, clinicians, educators, and policy makers make collaborative decisions, aware there is not one perfect answer, and willing to understand and incorporate others views and objectives rather than holding onto single beliefs or approaches.”
No financial conflicts of interest were declared.
Countries across the world are in the process of closing and reopening schools to contain the spread of COVID-19. Should there be universal testing and quarantining of sick school children and their classmates?
In a lively debate at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Andreea M. Panciu, MD, from the National Institute of Infectious Diseases in Bucharest, argued for routine testing and quarantining of all school children. Her opposite number, Danilo Buonsenso, MD, from the Centre for Global Health Research and Studies, Fondazione Policlinico Universitario Agostino Gemelli Istituto di Ricovero e Cura a Carattere Scientifico, Rome, made the case for a more selective approach.
Should children be sent to school?
stated Dr. Panciu as she started the debate by explaining the challenges faced by schools in adhering to key mitigation strategies. The U.S. Centers for Disease Control and Prevention recommends that students keep 1.8 m (6 feet) distance from one another. “In many school settings this is not feasible without drastically limiting the number of students,” she explained. “This is a massive challenge for many schools that are already overcrowded.”
The use of facemasks also is a challenge in classrooms. Children have a lower tolerance or may not be able to use the mask properly. There also are concerns regarding impaired learning, speech development, social development, and facial recognition. “We need to look at the evidence; preventive measures work,” responded Dr. Buonsenso. If distance can be implemented, the more distance the lower the transmission of infection, with 1.5-2 meters having the best effects. “Distance can be difficult when school buildings do not allow it, however, governments have had time to plan, and this should not be a limitation to education for kids.”
A recent review clearly showed that children and adolescents aged under 20 years have a much lower risk of susceptibility to COVID-19 infection, compared with adults. This is especially the case for children younger than 14 years. “There is no excuse, let’s bring the children back to school,” argued Dr. Buonsenso.
Dr. Panciu responded with several studies that have tried to quantify the amount of SARS-CoV-2 virus that is carried by infected children. Viral load in the nasopharynx in children under 5 years with mild to moderate COVID-19 symptoms was higher than that of both children over 5 as well as adults. The viral load in young children did not seem to differ by age or symptom severity. “There doesn’t appear to be a significant difference in viral load between symptomatic children and symptomatic adults,” she stated.
“But the question is: ‘How infectious are children?’ ” reacted Dr. Buonsenso. Data from South Korea showed that, for children, particularly those under 10 years, the number of secondary cases of contacts was very low, suggesting that children are rarely spreading the virus.
Dr. Buonsenso and colleagues assessed 30 households containing children aged under 18 years where an adult had been infected with COVID-19 in Rome during the peak of the pandemic. In no cases was it found that a child was the index case. This was supported by data from China, also obtained during the peak of the pandemic, which showed that the number of children infected was very low, but more importantly the number of secondary attacks from contact with children was also very low.
What about children who are sick at school?
The debate moved to discussing what should be done when a child is sick at school. Dr. Panciu clarified recommendations by the CDC regarding what steps to take if a student displays signs of infection consistent with COVID-19: Should they test positive, they are to stay at home for 10 days from the time signs and symptoms first appeared. Further, any teachers or students identified as close contacts are advised to stay at home for 14 days. (Since the ESPID meeting, the CDC has made changes in quarantine times for COVID-19. People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.)
A significant problem is the overlap between COVID-19 symptoms and those associated with other common illnesses because of a range of viruses. This is particularly true in younger children who often suffer from viral infections. “It is common for children to have up to eight respiratory illnesses a year,” explained Dr. Panciu, “and some may have symptoms so mild that they don’t notice them.”
“We need to be a little bit more children focused, otherwise we are going to be isolating children all the time,” said Dr. Buonsenso. The Royal College of Paediatrics and Child Health state that a child with a simple runny nose or sporadic cough without a fever, who would have attended school in other times, should not be tested for COVID-19. He moved on to then cite several studies that show little or no evidence of COVID-19 transmission between school children. This included a prospective cohort study in Australia showing that child-to-child transmission occurred in 0.3%. “To date, the advantages from routine quarantine and over testing seem too low to balance the social consequences on children and families,” he concluded.
As the debate drew to a close, Dr. Panciu reported several studies that did demonstrate transmission between school-age children. Data from an overnight camp in Georgia where the median age was 12 years showed the attack rate was 44% for ages 11-17 years and 51% for ages 6-10 years. Similar conclusions were reached in an Israeli study looking at a large COVID-19 outbreak in a school. This occurred 10 days after reopening, in spite of preventive measures being in place. “Opening safely isn’t just about the adjustments a school makes,” she said, “it’s also about how much of the virus is circulating in the community, which affects the likelihood that students and staff will bring COVID-19 into their classrooms.”
Damian Roland, consultant and honorary associate professor in pediatric emergency medicine at the University of Leicester (England), commented: “Maximizing educational potential while reducing the spread of COVID19 is a challenge laden with scientific equipoise while simultaneously infused with emotion. The evidence of transmission between, and infectivity from, children is not complete, as this debate has demonstrated. It is important scientists, clinicians, educators, and policy makers make collaborative decisions, aware there is not one perfect answer, and willing to understand and incorporate others views and objectives rather than holding onto single beliefs or approaches.”
No financial conflicts of interest were declared.
FROM ESPID 2020
New child COVID-19 cases down in last weekly count
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 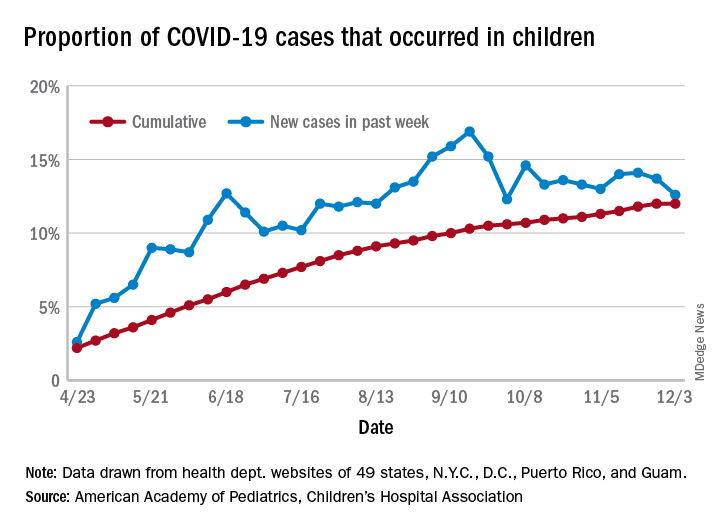
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
Joint guidelines favor antibody testing for certain Lyme disease manifestations
New clinical practice guidelines on Lyme disease place a strong emphasis on antibody testing to assess for rheumatologic and neurologic syndromes. “Diagnostically, we recommend testing via antibodies, and an index of antibodies in cerebrospinal fluid [CSF] versus serum. Importantly, we recommend against using polymerase chain reaction [PCR] in CSF,” Jeffrey A. Rumbaugh, MD, PhD, a coauthor of the guidelines and a member of the American Academy of Neurology, said in an interview.
The Infectious Diseases Society of America, AAN, and the American College of Rheumatology convened a multidisciplinary panel to develop the 43 recommendations, seeking input from 12 additional medical specialties, and patients. The panel conducted a systematic review of available evidence on preventing, diagnosing, and treating Lyme disease, using the Grading of Recommendations Assessment, Development and Evaluation model to evaluate clinical evidence and strength of recommendations. The guidelines were simultaneous published in Clinical Infectious Diseases, Neurology, Arthritis & Rheumatology, and Arthritis Care & Research.
This is the first time these organizations have collaborated on joint Lyme disease guidelines, which focus mainly on neurologic, cardiac, and rheumatologic manifestations.
“We are very excited to provide these updated guidelines to assist clinicians working in numerous medical specialties around the country, and even the world, as they care for patients suffering from Lyme disease,” Dr. Rumbaugh said.
When to use and not to use PCR
Guideline authors called for specific testing regimens depending on presentation of symptoms. Generally, they advised that individuals with a skin rash suggestive of early disease seek a clinical diagnosis instead of laboratory testing.
Recommendations on Lyme arthritis support previous IDSA guidelines published in 2006, Linda K. Bockenstedt, MD, professor of medicine at Yale University, New Haven, Conn., and a coauthor of the guidelines, said in an interview.
To evaluate for potential Lyme arthritis, clinicians should choose serum antibody testing over PCR or culture of blood or synovial fluid/tissue. However, if a doctor is assessing a seropositive patient for Lyme arthritis diagnosis but needs more information for treatment decisions, the authors recommended PCR applied to synovial fluid or tissue over Borrelia culture.
“Synovial fluid can be analyzed by PCR, but sensitivity is generally lower than serology,” Dr. Bockenstedt explained. Additionally, culture of joint fluid or synovial tissue for Lyme spirochetes has 0% sensitivity in multiple studies. “For these reasons, we recommend serum antibody testing over PCR of joint fluid or other methods for an initial diagnosis.”
Serum antibody testing over PCR or culture is also recommended for identifying Lyme neuroborreliosis in the peripheral nervous system (PNS) or CNS.
Despite the recent popularity of Lyme PCR testing in hospitals and labs, “with Lyme at least, antibodies are better in the CSF,” Dr. Rumbaugh said. Studies have shown that “most patients with even early neurologic Lyme disease are seropositive by conventional antibody testing at time of initial clinical presentation, and that intrathecal antibody production, as demonstrated by an elevated CSF:serum index, is highly specific for CNS involvement.”
If done correctly, antibody testing is both sensitive and specific for neurologic Lyme disease. “On the other hand, sensitivity of Lyme PCR performed on CSF has been only in the 5%-17% range in studies. Incidentally, Lyme PCR on blood is also not sensitive and therefore not recommended,” Dr. Rumbaugh said.
Guideline authors recommended testing in patients with the following conditions: acute neurologic disorders such as meningitis, painful radiculoneuritis, mononeuropathy multiplex; evidence of spinal cord or brain inflammation; and acute myocarditis/pericarditis of unknown cause in an appropriate epidemiologic setting.
They did not recommend testing in patients with typical amyotrophic lateral sclerosis; relapsing remitting multiple sclerosis; Parkinson’s disease, dementia, or cognitive decline; new-onset seizures; other neurologic syndromes or those lacking clinical or epidemiologic history that would support a diagnosis of Lyme disease; and patients with chronic cardiomyopathy of unknown cause.
The authors also called for judicious use of electrocardiogram to screen for Lyme carditis, recommending it only in patients signs or symptoms of this condition. However, patients at risk for or showing signs of severe cardiac complications of Lyme disease should be hospitalized and monitored via ECG.
Timelines for antibiotics
Most patients with Lyme disease should receive oral antibiotics, although duration times vary depending on the disease state. “We recommend that prophylactic antibiotic therapy be given to adults and children only within 72 hours of removal of an identified high-risk tick bite, but not for bites that are equivocal risk or low risk,” according to the guideline authors.
Specific antibiotic treatment regimens by condition are as follows: 10-14 days for early-stage disease, 14 days for Lyme carditis, 14-21 days for neurologic Lyme disease, and 28 days for late Lyme arthritis.
“Despite arthritis occurring late in the course of infection, treatment with a 28-day course of oral antibiotic is effective, although the rates of complete resolution of joint swelling can vary,” Dr. Bockenstedt said. Clinicians may consider a second 28-day course of oral antibiotics or a 2- to 4-week course of ceftriaxone in patients with persistent swelling, after an initial course of oral antibiotics.
Citing knowledge gaps, the authors made no recommendation on secondary antibiotic treatment for unresolved Lyme arthritis. Rheumatologists can play an important role in the care of this small subset of patients, Dr. Bockenstedt noted. “Studies of patients with ‘postantibiotic Lyme arthritis’ show that they can be treated successfully with intra-articular steroids, nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, biologic response modifiers, and even synovectomy with successful outcomes.” Some of these therapies also work in cases where first courses of oral and intravenous antibiotics are unsuccessful.
“Antibiotic therapy for longer than 8 weeks is not expected to provide additional benefit to patients with persistent arthritis if that treatment has included one course of IV therapy,” the authors clarified.
For patients with Lyme disease–associated meningitis, cranial neuropathy, radiculoneuropathy, or other PNS manifestations, the authors recommended intravenous ceftriaxone, cefotaxime, penicillin G, or oral doxycycline over other antimicrobials.
“For most neurologic presentations, oral doxycycline is just as effective as appropriate IV antibiotics,” Dr. Rumbaugh said. “The exception is the relatively rare situation where the patient is felt to have parenchymal involvement of brain or spinal cord, in which case the guidelines recommend IV antibiotics over oral antibiotics.” In the studies, there was no statistically significant difference between oral or intravenous regimens in response rate or risk of adverse effects.
Patients with nonspecific symptoms such as fatigue, pain, or cognitive impairment following treatment should not receive additional antibiotic therapy if there’s no evidence of treatment failure or infection. These two markers “would include objective signs of disease activity, such as arthritis, meningitis, or neuropathy,” the guideline authors wrote in comments accompanying the recommendation.
Clinicians caring for patients with symptomatic bradycardia caused by Lyme carditis should consider temporary pacing measures instead of a permanent pacemaker. For patients hospitalized with Lyme carditis, “we suggest initially using IV ceftriaxone over oral antibiotics until there is evidence of clinical improvement, then switching to oral antibiotics to complete treatment,” they advised. Outpatients with this condition should receive oral antibiotics instead of intravenous antibiotics.
Advice on antibodies testing ‘particularly cogent’
For individuals without expertise in these areas, the recommendations are clear and useful, Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and research professor at the University of Florence (Italy), said in an interview.
“As a rheumatologist, I would have appreciated literature references for some of the recommendations but, nevertheless, find these useful. I applaud the care with which the evidence was gathered and the general formatting, which tried to review multiple possible scenarios surrounding Lyme arthritis,” said Dr. Furst, offering a third-party perspective.
The advice on using antibodies tests to make a diagnosis of Lyme arthritis “is particularly cogent and more useful than trying to culture these fastidious organisms,” he added.
The IDSA, AAN, and ACR provided support for the guideline. Dr. Bockenstedt reported receiving research funding from the National Institutes of Health and the Gordon and the Llura Gund Foundation and remuneration from L2 Diagnostics for investigator-initiated NIH-sponsored research. Dr. Rumbaugh had no conflicts of interest to disclose. Dr. Furst reported no conflicts of interest in commenting on these guidelines.
SOURCE: Rumbaugh JA et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1215.
New clinical practice guidelines on Lyme disease place a strong emphasis on antibody testing to assess for rheumatologic and neurologic syndromes. “Diagnostically, we recommend testing via antibodies, and an index of antibodies in cerebrospinal fluid [CSF] versus serum. Importantly, we recommend against using polymerase chain reaction [PCR] in CSF,” Jeffrey A. Rumbaugh, MD, PhD, a coauthor of the guidelines and a member of the American Academy of Neurology, said in an interview.
The Infectious Diseases Society of America, AAN, and the American College of Rheumatology convened a multidisciplinary panel to develop the 43 recommendations, seeking input from 12 additional medical specialties, and patients. The panel conducted a systematic review of available evidence on preventing, diagnosing, and treating Lyme disease, using the Grading of Recommendations Assessment, Development and Evaluation model to evaluate clinical evidence and strength of recommendations. The guidelines were simultaneous published in Clinical Infectious Diseases, Neurology, Arthritis & Rheumatology, and Arthritis Care & Research.
This is the first time these organizations have collaborated on joint Lyme disease guidelines, which focus mainly on neurologic, cardiac, and rheumatologic manifestations.
“We are very excited to provide these updated guidelines to assist clinicians working in numerous medical specialties around the country, and even the world, as they care for patients suffering from Lyme disease,” Dr. Rumbaugh said.
When to use and not to use PCR
Guideline authors called for specific testing regimens depending on presentation of symptoms. Generally, they advised that individuals with a skin rash suggestive of early disease seek a clinical diagnosis instead of laboratory testing.
Recommendations on Lyme arthritis support previous IDSA guidelines published in 2006, Linda K. Bockenstedt, MD, professor of medicine at Yale University, New Haven, Conn., and a coauthor of the guidelines, said in an interview.
To evaluate for potential Lyme arthritis, clinicians should choose serum antibody testing over PCR or culture of blood or synovial fluid/tissue. However, if a doctor is assessing a seropositive patient for Lyme arthritis diagnosis but needs more information for treatment decisions, the authors recommended PCR applied to synovial fluid or tissue over Borrelia culture.
“Synovial fluid can be analyzed by PCR, but sensitivity is generally lower than serology,” Dr. Bockenstedt explained. Additionally, culture of joint fluid or synovial tissue for Lyme spirochetes has 0% sensitivity in multiple studies. “For these reasons, we recommend serum antibody testing over PCR of joint fluid or other methods for an initial diagnosis.”
Serum antibody testing over PCR or culture is also recommended for identifying Lyme neuroborreliosis in the peripheral nervous system (PNS) or CNS.
Despite the recent popularity of Lyme PCR testing in hospitals and labs, “with Lyme at least, antibodies are better in the CSF,” Dr. Rumbaugh said. Studies have shown that “most patients with even early neurologic Lyme disease are seropositive by conventional antibody testing at time of initial clinical presentation, and that intrathecal antibody production, as demonstrated by an elevated CSF:serum index, is highly specific for CNS involvement.”
If done correctly, antibody testing is both sensitive and specific for neurologic Lyme disease. “On the other hand, sensitivity of Lyme PCR performed on CSF has been only in the 5%-17% range in studies. Incidentally, Lyme PCR on blood is also not sensitive and therefore not recommended,” Dr. Rumbaugh said.
Guideline authors recommended testing in patients with the following conditions: acute neurologic disorders such as meningitis, painful radiculoneuritis, mononeuropathy multiplex; evidence of spinal cord or brain inflammation; and acute myocarditis/pericarditis of unknown cause in an appropriate epidemiologic setting.
They did not recommend testing in patients with typical amyotrophic lateral sclerosis; relapsing remitting multiple sclerosis; Parkinson’s disease, dementia, or cognitive decline; new-onset seizures; other neurologic syndromes or those lacking clinical or epidemiologic history that would support a diagnosis of Lyme disease; and patients with chronic cardiomyopathy of unknown cause.
The authors also called for judicious use of electrocardiogram to screen for Lyme carditis, recommending it only in patients signs or symptoms of this condition. However, patients at risk for or showing signs of severe cardiac complications of Lyme disease should be hospitalized and monitored via ECG.
Timelines for antibiotics
Most patients with Lyme disease should receive oral antibiotics, although duration times vary depending on the disease state. “We recommend that prophylactic antibiotic therapy be given to adults and children only within 72 hours of removal of an identified high-risk tick bite, but not for bites that are equivocal risk or low risk,” according to the guideline authors.
Specific antibiotic treatment regimens by condition are as follows: 10-14 days for early-stage disease, 14 days for Lyme carditis, 14-21 days for neurologic Lyme disease, and 28 days for late Lyme arthritis.
“Despite arthritis occurring late in the course of infection, treatment with a 28-day course of oral antibiotic is effective, although the rates of complete resolution of joint swelling can vary,” Dr. Bockenstedt said. Clinicians may consider a second 28-day course of oral antibiotics or a 2- to 4-week course of ceftriaxone in patients with persistent swelling, after an initial course of oral antibiotics.
Citing knowledge gaps, the authors made no recommendation on secondary antibiotic treatment for unresolved Lyme arthritis. Rheumatologists can play an important role in the care of this small subset of patients, Dr. Bockenstedt noted. “Studies of patients with ‘postantibiotic Lyme arthritis’ show that they can be treated successfully with intra-articular steroids, nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, biologic response modifiers, and even synovectomy with successful outcomes.” Some of these therapies also work in cases where first courses of oral and intravenous antibiotics are unsuccessful.
“Antibiotic therapy for longer than 8 weeks is not expected to provide additional benefit to patients with persistent arthritis if that treatment has included one course of IV therapy,” the authors clarified.
For patients with Lyme disease–associated meningitis, cranial neuropathy, radiculoneuropathy, or other PNS manifestations, the authors recommended intravenous ceftriaxone, cefotaxime, penicillin G, or oral doxycycline over other antimicrobials.
“For most neurologic presentations, oral doxycycline is just as effective as appropriate IV antibiotics,” Dr. Rumbaugh said. “The exception is the relatively rare situation where the patient is felt to have parenchymal involvement of brain or spinal cord, in which case the guidelines recommend IV antibiotics over oral antibiotics.” In the studies, there was no statistically significant difference between oral or intravenous regimens in response rate or risk of adverse effects.
Patients with nonspecific symptoms such as fatigue, pain, or cognitive impairment following treatment should not receive additional antibiotic therapy if there’s no evidence of treatment failure or infection. These two markers “would include objective signs of disease activity, such as arthritis, meningitis, or neuropathy,” the guideline authors wrote in comments accompanying the recommendation.
Clinicians caring for patients with symptomatic bradycardia caused by Lyme carditis should consider temporary pacing measures instead of a permanent pacemaker. For patients hospitalized with Lyme carditis, “we suggest initially using IV ceftriaxone over oral antibiotics until there is evidence of clinical improvement, then switching to oral antibiotics to complete treatment,” they advised. Outpatients with this condition should receive oral antibiotics instead of intravenous antibiotics.
Advice on antibodies testing ‘particularly cogent’
For individuals without expertise in these areas, the recommendations are clear and useful, Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and research professor at the University of Florence (Italy), said in an interview.
“As a rheumatologist, I would have appreciated literature references for some of the recommendations but, nevertheless, find these useful. I applaud the care with which the evidence was gathered and the general formatting, which tried to review multiple possible scenarios surrounding Lyme arthritis,” said Dr. Furst, offering a third-party perspective.
The advice on using antibodies tests to make a diagnosis of Lyme arthritis “is particularly cogent and more useful than trying to culture these fastidious organisms,” he added.
The IDSA, AAN, and ACR provided support for the guideline. Dr. Bockenstedt reported receiving research funding from the National Institutes of Health and the Gordon and the Llura Gund Foundation and remuneration from L2 Diagnostics for investigator-initiated NIH-sponsored research. Dr. Rumbaugh had no conflicts of interest to disclose. Dr. Furst reported no conflicts of interest in commenting on these guidelines.
SOURCE: Rumbaugh JA et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1215.
New clinical practice guidelines on Lyme disease place a strong emphasis on antibody testing to assess for rheumatologic and neurologic syndromes. “Diagnostically, we recommend testing via antibodies, and an index of antibodies in cerebrospinal fluid [CSF] versus serum. Importantly, we recommend against using polymerase chain reaction [PCR] in CSF,” Jeffrey A. Rumbaugh, MD, PhD, a coauthor of the guidelines and a member of the American Academy of Neurology, said in an interview.
The Infectious Diseases Society of America, AAN, and the American College of Rheumatology convened a multidisciplinary panel to develop the 43 recommendations, seeking input from 12 additional medical specialties, and patients. The panel conducted a systematic review of available evidence on preventing, diagnosing, and treating Lyme disease, using the Grading of Recommendations Assessment, Development and Evaluation model to evaluate clinical evidence and strength of recommendations. The guidelines were simultaneous published in Clinical Infectious Diseases, Neurology, Arthritis & Rheumatology, and Arthritis Care & Research.
This is the first time these organizations have collaborated on joint Lyme disease guidelines, which focus mainly on neurologic, cardiac, and rheumatologic manifestations.
“We are very excited to provide these updated guidelines to assist clinicians working in numerous medical specialties around the country, and even the world, as they care for patients suffering from Lyme disease,” Dr. Rumbaugh said.
When to use and not to use PCR
Guideline authors called for specific testing regimens depending on presentation of symptoms. Generally, they advised that individuals with a skin rash suggestive of early disease seek a clinical diagnosis instead of laboratory testing.
Recommendations on Lyme arthritis support previous IDSA guidelines published in 2006, Linda K. Bockenstedt, MD, professor of medicine at Yale University, New Haven, Conn., and a coauthor of the guidelines, said in an interview.
To evaluate for potential Lyme arthritis, clinicians should choose serum antibody testing over PCR or culture of blood or synovial fluid/tissue. However, if a doctor is assessing a seropositive patient for Lyme arthritis diagnosis but needs more information for treatment decisions, the authors recommended PCR applied to synovial fluid or tissue over Borrelia culture.
“Synovial fluid can be analyzed by PCR, but sensitivity is generally lower than serology,” Dr. Bockenstedt explained. Additionally, culture of joint fluid or synovial tissue for Lyme spirochetes has 0% sensitivity in multiple studies. “For these reasons, we recommend serum antibody testing over PCR of joint fluid or other methods for an initial diagnosis.”
Serum antibody testing over PCR or culture is also recommended for identifying Lyme neuroborreliosis in the peripheral nervous system (PNS) or CNS.
Despite the recent popularity of Lyme PCR testing in hospitals and labs, “with Lyme at least, antibodies are better in the CSF,” Dr. Rumbaugh said. Studies have shown that “most patients with even early neurologic Lyme disease are seropositive by conventional antibody testing at time of initial clinical presentation, and that intrathecal antibody production, as demonstrated by an elevated CSF:serum index, is highly specific for CNS involvement.”
If done correctly, antibody testing is both sensitive and specific for neurologic Lyme disease. “On the other hand, sensitivity of Lyme PCR performed on CSF has been only in the 5%-17% range in studies. Incidentally, Lyme PCR on blood is also not sensitive and therefore not recommended,” Dr. Rumbaugh said.
Guideline authors recommended testing in patients with the following conditions: acute neurologic disorders such as meningitis, painful radiculoneuritis, mononeuropathy multiplex; evidence of spinal cord or brain inflammation; and acute myocarditis/pericarditis of unknown cause in an appropriate epidemiologic setting.
They did not recommend testing in patients with typical amyotrophic lateral sclerosis; relapsing remitting multiple sclerosis; Parkinson’s disease, dementia, or cognitive decline; new-onset seizures; other neurologic syndromes or those lacking clinical or epidemiologic history that would support a diagnosis of Lyme disease; and patients with chronic cardiomyopathy of unknown cause.
The authors also called for judicious use of electrocardiogram to screen for Lyme carditis, recommending it only in patients signs or symptoms of this condition. However, patients at risk for or showing signs of severe cardiac complications of Lyme disease should be hospitalized and monitored via ECG.
Timelines for antibiotics
Most patients with Lyme disease should receive oral antibiotics, although duration times vary depending on the disease state. “We recommend that prophylactic antibiotic therapy be given to adults and children only within 72 hours of removal of an identified high-risk tick bite, but not for bites that are equivocal risk or low risk,” according to the guideline authors.
Specific antibiotic treatment regimens by condition are as follows: 10-14 days for early-stage disease, 14 days for Lyme carditis, 14-21 days for neurologic Lyme disease, and 28 days for late Lyme arthritis.
“Despite arthritis occurring late in the course of infection, treatment with a 28-day course of oral antibiotic is effective, although the rates of complete resolution of joint swelling can vary,” Dr. Bockenstedt said. Clinicians may consider a second 28-day course of oral antibiotics or a 2- to 4-week course of ceftriaxone in patients with persistent swelling, after an initial course of oral antibiotics.
Citing knowledge gaps, the authors made no recommendation on secondary antibiotic treatment for unresolved Lyme arthritis. Rheumatologists can play an important role in the care of this small subset of patients, Dr. Bockenstedt noted. “Studies of patients with ‘postantibiotic Lyme arthritis’ show that they can be treated successfully with intra-articular steroids, nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, biologic response modifiers, and even synovectomy with successful outcomes.” Some of these therapies also work in cases where first courses of oral and intravenous antibiotics are unsuccessful.
“Antibiotic therapy for longer than 8 weeks is not expected to provide additional benefit to patients with persistent arthritis if that treatment has included one course of IV therapy,” the authors clarified.
For patients with Lyme disease–associated meningitis, cranial neuropathy, radiculoneuropathy, or other PNS manifestations, the authors recommended intravenous ceftriaxone, cefotaxime, penicillin G, or oral doxycycline over other antimicrobials.
“For most neurologic presentations, oral doxycycline is just as effective as appropriate IV antibiotics,” Dr. Rumbaugh said. “The exception is the relatively rare situation where the patient is felt to have parenchymal involvement of brain or spinal cord, in which case the guidelines recommend IV antibiotics over oral antibiotics.” In the studies, there was no statistically significant difference between oral or intravenous regimens in response rate or risk of adverse effects.
Patients with nonspecific symptoms such as fatigue, pain, or cognitive impairment following treatment should not receive additional antibiotic therapy if there’s no evidence of treatment failure or infection. These two markers “would include objective signs of disease activity, such as arthritis, meningitis, or neuropathy,” the guideline authors wrote in comments accompanying the recommendation.
Clinicians caring for patients with symptomatic bradycardia caused by Lyme carditis should consider temporary pacing measures instead of a permanent pacemaker. For patients hospitalized with Lyme carditis, “we suggest initially using IV ceftriaxone over oral antibiotics until there is evidence of clinical improvement, then switching to oral antibiotics to complete treatment,” they advised. Outpatients with this condition should receive oral antibiotics instead of intravenous antibiotics.
Advice on antibodies testing ‘particularly cogent’
For individuals without expertise in these areas, the recommendations are clear and useful, Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and research professor at the University of Florence (Italy), said in an interview.
“As a rheumatologist, I would have appreciated literature references for some of the recommendations but, nevertheless, find these useful. I applaud the care with which the evidence was gathered and the general formatting, which tried to review multiple possible scenarios surrounding Lyme arthritis,” said Dr. Furst, offering a third-party perspective.
The advice on using antibodies tests to make a diagnosis of Lyme arthritis “is particularly cogent and more useful than trying to culture these fastidious organisms,” he added.
The IDSA, AAN, and ACR provided support for the guideline. Dr. Bockenstedt reported receiving research funding from the National Institutes of Health and the Gordon and the Llura Gund Foundation and remuneration from L2 Diagnostics for investigator-initiated NIH-sponsored research. Dr. Rumbaugh had no conflicts of interest to disclose. Dr. Furst reported no conflicts of interest in commenting on these guidelines.
SOURCE: Rumbaugh JA et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1215.
FROM CLINICAL INFECTIOUS DISEASES
COVID-19 and risk of clotting: ‘Be proactive about prevention’
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
Excess antibiotics and adverse events in patients with pneumonia
Background: Past surveys of providers revealed a tendency to select longer durations of antibiotics to reduce disease recurrence, but recent studies have shown that shorter courses of antibiotics are safe and equally effective in treatment for pneumonia. In addition, there has been a renewed focus on reducing unnecessary use of antibiotics to decrease adverse effects.
Study design: Retrospective cohort study.
Setting: 43 hospitals in the Michigan Hospital Medicine Safety Consortium.
Synopsis: A retrospective chart review of 6,481 patients hospitalized with pneumonia revealed that 67.8% of patients received excessive days of antibiotic treatment. On average, patients received 2 days of excessive treatment and 93.2% of the additional days came in the form of antibiotics prescribed at discharge.
Excessive treatment was defined as more than 5 days for community-acquired pneumonia (CAP) and more than 7 days for health care–associated pneumonia, methicillin-resistant Staphylococcus aureus, or gram-negative organisms. The authors adjusted for time to clinical stability when defining the expected duration of treatment.
After statistical adjustment, excess antibiotic days were not associated with increased rates of C. diff infection, emergency department visits, readmission, or 30-day mortality. Additional treatment was associated with increased patient-reported adverse effects including diarrhea, gastrointestinal distress, and mucosal candidiasis.
The impact of this study is limited by a few factors. The study was observational and relied on provider documentation and patient reporting of adverse events. Also, it was published prior to updates to the Infectious Diseases Society of America CAP guidelines, which may affect how it will be interpreted once those guidelines are released.
Bottom line: Adherence to the shortest effective duration of antibiotic treatment for pneumonia may lead to a reduction in the rates of patient reported adverse effects while not impacting treatment success.
Citation: Vaughn VM et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019 Aug 6;171(3):153-63.
Dr. Purdy is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.
Background: Past surveys of providers revealed a tendency to select longer durations of antibiotics to reduce disease recurrence, but recent studies have shown that shorter courses of antibiotics are safe and equally effective in treatment for pneumonia. In addition, there has been a renewed focus on reducing unnecessary use of antibiotics to decrease adverse effects.
Study design: Retrospective cohort study.
Setting: 43 hospitals in the Michigan Hospital Medicine Safety Consortium.
Synopsis: A retrospective chart review of 6,481 patients hospitalized with pneumonia revealed that 67.8% of patients received excessive days of antibiotic treatment. On average, patients received 2 days of excessive treatment and 93.2% of the additional days came in the form of antibiotics prescribed at discharge.
Excessive treatment was defined as more than 5 days for community-acquired pneumonia (CAP) and more than 7 days for health care–associated pneumonia, methicillin-resistant Staphylococcus aureus, or gram-negative organisms. The authors adjusted for time to clinical stability when defining the expected duration of treatment.
After statistical adjustment, excess antibiotic days were not associated with increased rates of C. diff infection, emergency department visits, readmission, or 30-day mortality. Additional treatment was associated with increased patient-reported adverse effects including diarrhea, gastrointestinal distress, and mucosal candidiasis.
The impact of this study is limited by a few factors. The study was observational and relied on provider documentation and patient reporting of adverse events. Also, it was published prior to updates to the Infectious Diseases Society of America CAP guidelines, which may affect how it will be interpreted once those guidelines are released.
Bottom line: Adherence to the shortest effective duration of antibiotic treatment for pneumonia may lead to a reduction in the rates of patient reported adverse effects while not impacting treatment success.
Citation: Vaughn VM et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019 Aug 6;171(3):153-63.
Dr. Purdy is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.
Background: Past surveys of providers revealed a tendency to select longer durations of antibiotics to reduce disease recurrence, but recent studies have shown that shorter courses of antibiotics are safe and equally effective in treatment for pneumonia. In addition, there has been a renewed focus on reducing unnecessary use of antibiotics to decrease adverse effects.
Study design: Retrospective cohort study.
Setting: 43 hospitals in the Michigan Hospital Medicine Safety Consortium.
Synopsis: A retrospective chart review of 6,481 patients hospitalized with pneumonia revealed that 67.8% of patients received excessive days of antibiotic treatment. On average, patients received 2 days of excessive treatment and 93.2% of the additional days came in the form of antibiotics prescribed at discharge.
Excessive treatment was defined as more than 5 days for community-acquired pneumonia (CAP) and more than 7 days for health care–associated pneumonia, methicillin-resistant Staphylococcus aureus, or gram-negative organisms. The authors adjusted for time to clinical stability when defining the expected duration of treatment.
After statistical adjustment, excess antibiotic days were not associated with increased rates of C. diff infection, emergency department visits, readmission, or 30-day mortality. Additional treatment was associated with increased patient-reported adverse effects including diarrhea, gastrointestinal distress, and mucosal candidiasis.
The impact of this study is limited by a few factors. The study was observational and relied on provider documentation and patient reporting of adverse events. Also, it was published prior to updates to the Infectious Diseases Society of America CAP guidelines, which may affect how it will be interpreted once those guidelines are released.
Bottom line: Adherence to the shortest effective duration of antibiotic treatment for pneumonia may lead to a reduction in the rates of patient reported adverse effects while not impacting treatment success.
Citation: Vaughn VM et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019 Aug 6;171(3):153-63.
Dr. Purdy is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.
Assessing the impact of glucocorticoids on COVID-19 mortality
Clinical question: Is early glucocorticoid therapy associated with reduced mortality or need for mechanical ventilation in hospitalized patients with SARS-CoV-2 infection?
Background: Glucocorticoids have been used as adjunctive treatment in some infections with inflammatory responses, but their efficacy in COVID-19 infections had not been entirely clear. The RECOVERY trial found a subset of patients with COVID-19 who may benefit from treatment with glucocorticoids. The ideal role of steroids in this infection, and who the subset of patients might be for whom they would benefit, is so far unclear.
Study design: Retrospective cohort analysis.
Setting: Large academic health center in New York.
Synopsis: Researchers analyzed admissions of COVID-19 positive patients hospitalized between March 11, 2020 and April 13, 2020 who did not die or become mechanically ventilated within the first 48 hours of admission. Patients treated with glucocorticoids within 48 hours of admission were compared with patients who were not treated with glucocorticoids during this time frame. In total, 2,998 patients were examined, of whom 1,806 met inclusion criteria, and 140 (7.7%) were treated with glucocorticoids within 48 hours of admission. These treated patients were more likely to have an underlying pulmonary or rheumatologic comorbidity. Early use of glucocorticoids was not associated with in-hospital mortality or mechanical ventilation in either adjusted or unadjusted models. However, if the initial C-reactive protein (CRP) was >20mg/dL, this was associated with a reduced risk of mortality or mechanical ventilation in unadjusted (odds ratio, 0.23; 95% confidence interval, 0.08-0.70) and adjusted analyses for clinical characteristics (adjusted OR, 0.20; 95% CI, 0.06-0.67). Conversely, treatment in patients with CRP <10mg/dL was associated with significantly increased risk of mortality or ventilation during analysis.
Bottom line: Glucocorticoids can benefit patients with significantly elevated CRP but may be harmful to those with lower CRPs.
Citation: Keller MJ et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;8;489-493. Published online first. 2020 Jul 22. doi:10.12788/jhm.3497.
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
Clinical question: Is early glucocorticoid therapy associated with reduced mortality or need for mechanical ventilation in hospitalized patients with SARS-CoV-2 infection?
Background: Glucocorticoids have been used as adjunctive treatment in some infections with inflammatory responses, but their efficacy in COVID-19 infections had not been entirely clear. The RECOVERY trial found a subset of patients with COVID-19 who may benefit from treatment with glucocorticoids. The ideal role of steroids in this infection, and who the subset of patients might be for whom they would benefit, is so far unclear.
Study design: Retrospective cohort analysis.
Setting: Large academic health center in New York.
Synopsis: Researchers analyzed admissions of COVID-19 positive patients hospitalized between March 11, 2020 and April 13, 2020 who did not die or become mechanically ventilated within the first 48 hours of admission. Patients treated with glucocorticoids within 48 hours of admission were compared with patients who were not treated with glucocorticoids during this time frame. In total, 2,998 patients were examined, of whom 1,806 met inclusion criteria, and 140 (7.7%) were treated with glucocorticoids within 48 hours of admission. These treated patients were more likely to have an underlying pulmonary or rheumatologic comorbidity. Early use of glucocorticoids was not associated with in-hospital mortality or mechanical ventilation in either adjusted or unadjusted models. However, if the initial C-reactive protein (CRP) was >20mg/dL, this was associated with a reduced risk of mortality or mechanical ventilation in unadjusted (odds ratio, 0.23; 95% confidence interval, 0.08-0.70) and adjusted analyses for clinical characteristics (adjusted OR, 0.20; 95% CI, 0.06-0.67). Conversely, treatment in patients with CRP <10mg/dL was associated with significantly increased risk of mortality or ventilation during analysis.
Bottom line: Glucocorticoids can benefit patients with significantly elevated CRP but may be harmful to those with lower CRPs.
Citation: Keller MJ et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;8;489-493. Published online first. 2020 Jul 22. doi:10.12788/jhm.3497.
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
Clinical question: Is early glucocorticoid therapy associated with reduced mortality or need for mechanical ventilation in hospitalized patients with SARS-CoV-2 infection?
Background: Glucocorticoids have been used as adjunctive treatment in some infections with inflammatory responses, but their efficacy in COVID-19 infections had not been entirely clear. The RECOVERY trial found a subset of patients with COVID-19 who may benefit from treatment with glucocorticoids. The ideal role of steroids in this infection, and who the subset of patients might be for whom they would benefit, is so far unclear.
Study design: Retrospective cohort analysis.
Setting: Large academic health center in New York.
Synopsis: Researchers analyzed admissions of COVID-19 positive patients hospitalized between March 11, 2020 and April 13, 2020 who did not die or become mechanically ventilated within the first 48 hours of admission. Patients treated with glucocorticoids within 48 hours of admission were compared with patients who were not treated with glucocorticoids during this time frame. In total, 2,998 patients were examined, of whom 1,806 met inclusion criteria, and 140 (7.7%) were treated with glucocorticoids within 48 hours of admission. These treated patients were more likely to have an underlying pulmonary or rheumatologic comorbidity. Early use of glucocorticoids was not associated with in-hospital mortality or mechanical ventilation in either adjusted or unadjusted models. However, if the initial C-reactive protein (CRP) was >20mg/dL, this was associated with a reduced risk of mortality or mechanical ventilation in unadjusted (odds ratio, 0.23; 95% confidence interval, 0.08-0.70) and adjusted analyses for clinical characteristics (adjusted OR, 0.20; 95% CI, 0.06-0.67). Conversely, treatment in patients with CRP <10mg/dL was associated with significantly increased risk of mortality or ventilation during analysis.
Bottom line: Glucocorticoids can benefit patients with significantly elevated CRP but may be harmful to those with lower CRPs.
Citation: Keller MJ et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;8;489-493. Published online first. 2020 Jul 22. doi:10.12788/jhm.3497.
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
FROM THE JOURNAL OF HOSPITAL MEDICINE