User login
AI-Identified Vascular Healing Can Predict Clinical Relapse in Ulcerative Colitis
STOCKHOLM — , according to data from the study of a novel investigational tool.
Clinical relapse was predicted in 3% of patients identified as having vascular healing in all segments compared with 23.9% in those with vascular activity (ie, one or more segments were active), reported Yasuharu Maeda, MD, gastroenterologist from Showa University Northern Yokohama Hospital, Digestive Disease Center, Yokohama, Japan.
In patients with a Mayo Endoscopic Score (MES) ≤ 1, the clinical relapse rate was 3% and 18.6% in the vascular healing and vascular active groups, respectively, he said.
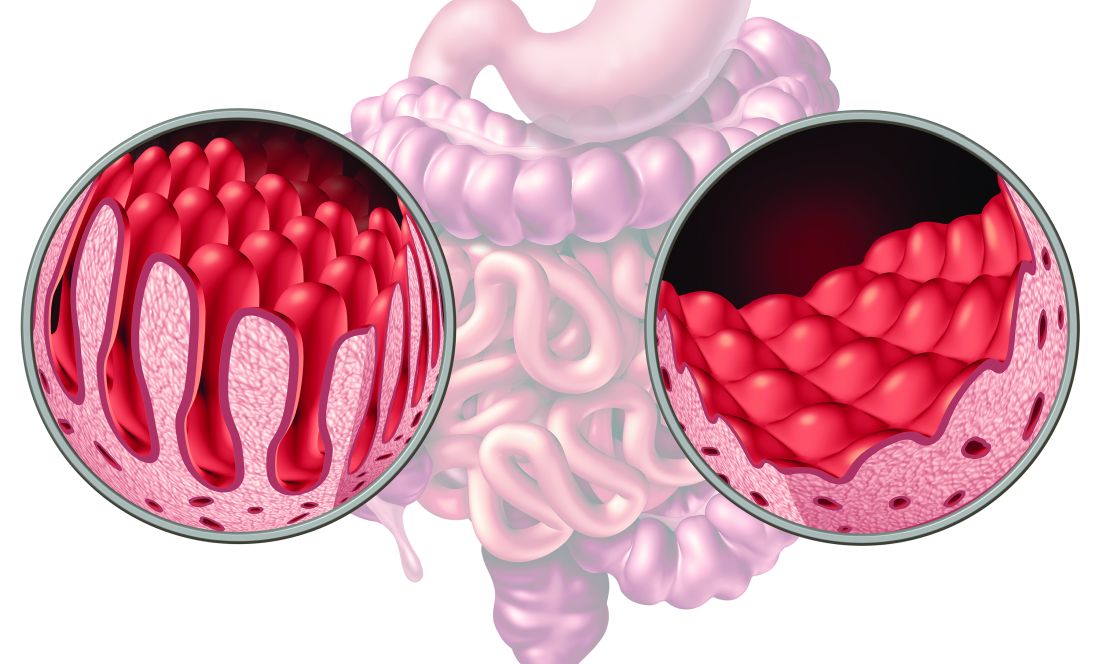
Endoscopic remission is a crucial treat-to-target goal in patients with UC, and image-enhanced endoscopy is spreading in routine practice as a way to detect inflammation and to predict outcomes, Dr. Maeda said.
“Image-enhanced vascular findings lead to a stronger correlation with histological activities and long-term prognosis compared with white light endoscopy assessment,” he explained. “It also means that assessment can be done on-site without biopsy, pathologist effort, and associated costs; however, specialist training is required to achieve a high accuracy in outputs.”
Dr. Maeda presented the data (Abstract OP16) at the annual congress of the European Crohn’s and Colitis Organisation.
Stratifying the Relapse Risk
Dr. Maeda and colleagues developed a novel AI-based narrow-band imaging system, training it by using 8853 images from 167 patients with UC.
The AI system, EndoBRAIN-UC (Cybernet System Corp, Tokyo), is in use and currently adapted for only one endoscope, the Endocyto CFH290EC (Olympus EMEA, Tokyo), but for the purpose of this study, it was trained on images from five different scopes.
“By combining narrow-band imaging and AI, we developed a system where we can differentiate between vascular activity and vascular healing. This allows us to predict relapse,” Dr. Maeda said.
In an open-label, prospective cohort study, they tested the system with the aim of assessing the efficacy of AI-identified vascular healing to stratify the relapse risk in 100 patients showing clinical remission of UC (ie, partial MES ≤ 1).
Patient characteristics were similar between both groups with an average disease duration of 10 years.
In the vascular healing group (n = 33), the average age was 52 years, 20% were men, 58% had extensive colitis, and 52% had a MES score of 0.
In the vascular active group (n = 67), the average age was 56 years, 32% were men, 61% had extensive colitis, and 25% had a MES score of 0.
Colonoscopy was performed using the AI system to identify mucosa as healing or active for six colorectal segments of each patient. The MES and histologic assessment for these segments were also recorded. Patients were then followed for up to 12 months and assessed for clinical relapse.
The clinical relapse rate was higher in the vascular active group than in the vascular healing group as identified by AI.
“We only evaluated the diagnostic output of the AI but obtained white light endoscopies and biopsies for contrast studies,” Dr. Maeda noted.
They also looked at whether the endoscopist’s level of experience (ie, trainee or expert) was important but found that clinical relapse predictive values were independent of the endoscopist’s experience.
Still in the Early Stages
AI-assisted colonoscopy work is still at an early stage , said session co-moderator, Monika Ferlitsch, MD, head of Internal Medicine Department II, gastroenterology and hepatology, Evangelical Hospital, in Vienna, Austria.
We now have initial results, but “I suspect it will take 10-20 years for implementation into routine clinical practice,” she said.
The best outcome for our patients is to be able to predict response to therapy and recurrence rates, “and we see this is possible now with AI. But of course, we need more clinical data to support it,” Dr. Ferlitsch said.
Dr. Maeda and Dr. Ferlitsch have declared no financial disclosures.
A version of this article appeared on Medscape.com.
STOCKHOLM — , according to data from the study of a novel investigational tool.
Clinical relapse was predicted in 3% of patients identified as having vascular healing in all segments compared with 23.9% in those with vascular activity (ie, one or more segments were active), reported Yasuharu Maeda, MD, gastroenterologist from Showa University Northern Yokohama Hospital, Digestive Disease Center, Yokohama, Japan.
In patients with a Mayo Endoscopic Score (MES) ≤ 1, the clinical relapse rate was 3% and 18.6% in the vascular healing and vascular active groups, respectively, he said.
Endoscopic remission is a crucial treat-to-target goal in patients with UC, and image-enhanced endoscopy is spreading in routine practice as a way to detect inflammation and to predict outcomes, Dr. Maeda said.
“Image-enhanced vascular findings lead to a stronger correlation with histological activities and long-term prognosis compared with white light endoscopy assessment,” he explained. “It also means that assessment can be done on-site without biopsy, pathologist effort, and associated costs; however, specialist training is required to achieve a high accuracy in outputs.”
Dr. Maeda presented the data (Abstract OP16) at the annual congress of the European Crohn’s and Colitis Organisation.
Stratifying the Relapse Risk
Dr. Maeda and colleagues developed a novel AI-based narrow-band imaging system, training it by using 8853 images from 167 patients with UC.
The AI system, EndoBRAIN-UC (Cybernet System Corp, Tokyo), is in use and currently adapted for only one endoscope, the Endocyto CFH290EC (Olympus EMEA, Tokyo), but for the purpose of this study, it was trained on images from five different scopes.
“By combining narrow-band imaging and AI, we developed a system where we can differentiate between vascular activity and vascular healing. This allows us to predict relapse,” Dr. Maeda said.
In an open-label, prospective cohort study, they tested the system with the aim of assessing the efficacy of AI-identified vascular healing to stratify the relapse risk in 100 patients showing clinical remission of UC (ie, partial MES ≤ 1).
Patient characteristics were similar between both groups with an average disease duration of 10 years.
In the vascular healing group (n = 33), the average age was 52 years, 20% were men, 58% had extensive colitis, and 52% had a MES score of 0.
In the vascular active group (n = 67), the average age was 56 years, 32% were men, 61% had extensive colitis, and 25% had a MES score of 0.
Colonoscopy was performed using the AI system to identify mucosa as healing or active for six colorectal segments of each patient. The MES and histologic assessment for these segments were also recorded. Patients were then followed for up to 12 months and assessed for clinical relapse.
The clinical relapse rate was higher in the vascular active group than in the vascular healing group as identified by AI.
“We only evaluated the diagnostic output of the AI but obtained white light endoscopies and biopsies for contrast studies,” Dr. Maeda noted.
They also looked at whether the endoscopist’s level of experience (ie, trainee or expert) was important but found that clinical relapse predictive values were independent of the endoscopist’s experience.
Still in the Early Stages
AI-assisted colonoscopy work is still at an early stage , said session co-moderator, Monika Ferlitsch, MD, head of Internal Medicine Department II, gastroenterology and hepatology, Evangelical Hospital, in Vienna, Austria.
We now have initial results, but “I suspect it will take 10-20 years for implementation into routine clinical practice,” she said.
The best outcome for our patients is to be able to predict response to therapy and recurrence rates, “and we see this is possible now with AI. But of course, we need more clinical data to support it,” Dr. Ferlitsch said.
Dr. Maeda and Dr. Ferlitsch have declared no financial disclosures.
A version of this article appeared on Medscape.com.
STOCKHOLM — , according to data from the study of a novel investigational tool.
Clinical relapse was predicted in 3% of patients identified as having vascular healing in all segments compared with 23.9% in those with vascular activity (ie, one or more segments were active), reported Yasuharu Maeda, MD, gastroenterologist from Showa University Northern Yokohama Hospital, Digestive Disease Center, Yokohama, Japan.
In patients with a Mayo Endoscopic Score (MES) ≤ 1, the clinical relapse rate was 3% and 18.6% in the vascular healing and vascular active groups, respectively, he said.
Endoscopic remission is a crucial treat-to-target goal in patients with UC, and image-enhanced endoscopy is spreading in routine practice as a way to detect inflammation and to predict outcomes, Dr. Maeda said.
“Image-enhanced vascular findings lead to a stronger correlation with histological activities and long-term prognosis compared with white light endoscopy assessment,” he explained. “It also means that assessment can be done on-site without biopsy, pathologist effort, and associated costs; however, specialist training is required to achieve a high accuracy in outputs.”
Dr. Maeda presented the data (Abstract OP16) at the annual congress of the European Crohn’s and Colitis Organisation.
Stratifying the Relapse Risk
Dr. Maeda and colleagues developed a novel AI-based narrow-band imaging system, training it by using 8853 images from 167 patients with UC.
The AI system, EndoBRAIN-UC (Cybernet System Corp, Tokyo), is in use and currently adapted for only one endoscope, the Endocyto CFH290EC (Olympus EMEA, Tokyo), but for the purpose of this study, it was trained on images from five different scopes.
“By combining narrow-band imaging and AI, we developed a system where we can differentiate between vascular activity and vascular healing. This allows us to predict relapse,” Dr. Maeda said.
In an open-label, prospective cohort study, they tested the system with the aim of assessing the efficacy of AI-identified vascular healing to stratify the relapse risk in 100 patients showing clinical remission of UC (ie, partial MES ≤ 1).
Patient characteristics were similar between both groups with an average disease duration of 10 years.
In the vascular healing group (n = 33), the average age was 52 years, 20% were men, 58% had extensive colitis, and 52% had a MES score of 0.
In the vascular active group (n = 67), the average age was 56 years, 32% were men, 61% had extensive colitis, and 25% had a MES score of 0.
Colonoscopy was performed using the AI system to identify mucosa as healing or active for six colorectal segments of each patient. The MES and histologic assessment for these segments were also recorded. Patients were then followed for up to 12 months and assessed for clinical relapse.
The clinical relapse rate was higher in the vascular active group than in the vascular healing group as identified by AI.
“We only evaluated the diagnostic output of the AI but obtained white light endoscopies and biopsies for contrast studies,” Dr. Maeda noted.
They also looked at whether the endoscopist’s level of experience (ie, trainee or expert) was important but found that clinical relapse predictive values were independent of the endoscopist’s experience.
Still in the Early Stages
AI-assisted colonoscopy work is still at an early stage , said session co-moderator, Monika Ferlitsch, MD, head of Internal Medicine Department II, gastroenterology and hepatology, Evangelical Hospital, in Vienna, Austria.
We now have initial results, but “I suspect it will take 10-20 years for implementation into routine clinical practice,” she said.
The best outcome for our patients is to be able to predict response to therapy and recurrence rates, “and we see this is possible now with AI. But of course, we need more clinical data to support it,” Dr. Ferlitsch said.
Dr. Maeda and Dr. Ferlitsch have declared no financial disclosures.
A version of this article appeared on Medscape.com.
FROM ECCO 2024
Novel Biotherapeutic to Be Tested in Ulcerative Colitis
STOCKHOLM —
The eight-strain live biotherapeutic product (MB310, Microbiotica) is due to enter its first clinical trial later this year.
In patients with UC who showed clinical benefit after fecal microbiota transplantation (FMT), these eight specific bacterial strains were shown to be consistently elevated, reported Ron Carter, MD, chief medical officer at Microbiotica, Cambridge, UK, the company developing the product.
In addition, “in lab studies, we’ve found they exhibit anti-inflammatory effects when incubated with different cells of the immune system,” he explained. With the biotherapeutic product, “we want to prompt the immune system to realign the gut inflammation” in UC.
On February 23, at the annual congress of the European Crohn’s and Colitis Organisation, Dr. Carter presented the work-to-date and the near-term plans for a phase 1b randomized, placebo-controlled, double-blind trial to be conducted across roughly 20 sites in Europe (Abstract DOP 67). The trial, COMPOSER-1, will investigate the engraftment and initial signs of clinical activity of MB310 in 30 patients with active, mild to moderate UC (modified Mayo score 4-7; endoscopic subscore ≥ 2).
Calming Inflammation With Specific Bacteria
To date, the researchers have focused on identifying bacterial species that correlate with clinical outcomes in individual patients with UC after treatment with FMT. Dr. Carter and colleagues worked in collaboration with Sam Costello, MBBS, gastroenterologist at The Queen Elizabeth Hospital in Adelaide, Australia.
The initial research involved analyzing stool samples from a round 70 patients diagnosed with mild to moderate UC, who were randomly assigned to receive either pooled, anaerobically prepared, healthy donor FMT, or their own autologous FMT as a comparative control. FMT was delivered via colonoscopy and two enemas over 7 days. The primary endpoint of the study was achieving steroid-free remission of UC on the basis of specific Mayo scores, at the end of week 8.
“Results indicated that the group receiving the pooled donor FMT showed a 32% success rate with respect to the primary outcome compared with only 9% of the control group,” said Dr. Carter. “This suggested a promising therapeutic strategy.”
Based on these outcomes, Microbiotica then decided to conduct a local study in which they analyzed fecal samples from FMT donors and from pre-FMT and post-FMT recipients in order to precisely identify which bacterial species were associated with clinical response.
“With our precision microbiome analysis platform, we determined eight bacterial species that were consistently elevated in FMT responders, but absent or not elevated in non-responders,” reported Dr. Carter.
Next, they carried out subspecies-level bioinformatic analysis and identified one bacterial strain from each of the eight species, which were then cultured individually, freeze-dried, and combined into a custom delayed-release capsule.
The researchers tested the resulting live biotherapeutic product in cellular assays using human cell lines to confirm that they effectively modulated immune functions.
The cellular assays showed that in epithelial cell monolayer tests, MB310 not only enhanced barrier integrity but also protected the cellular barrier from inflammation.
The eight bacterial strain combination also exhibited anti-inflammatory effects when incubated with different primary innate immune cells, dendritic cells, and M1 macrophages.
And, Dr. Carter emphasized, the specific MB310 bacterial strains were found to regulate T-cells directly or via metabolites.
Moving in the Right Direction
Julien Kirchgesner, MD, Saint-Antoine Hospital and Sorbonne University, Paris, France, who co-moderated the session where the research was presented, welcomed the work.
“Many more trials need to be done before we see this enter the clinic, but this is moving in the right direction,” Dr. Kirchgesner noted.
“We need to find only the functionally active bacterial strains, rather than just take the fecal transplant alone [as is done with FMT],” he said. That is what is being done here.
“The researchers want to identify which bacterial strains will impact the effectiveness in patients, and to select for these strains before industrializing the process and producing them at the optimal strength, at scale,” Dr. Kirchgesner said.
Dr. Carter is chief medical officer at Microbiotica and Dr Kirshgensner has no financial disclosures.
A version of this article appeared on Medscape.com.
STOCKHOLM —
The eight-strain live biotherapeutic product (MB310, Microbiotica) is due to enter its first clinical trial later this year.
In patients with UC who showed clinical benefit after fecal microbiota transplantation (FMT), these eight specific bacterial strains were shown to be consistently elevated, reported Ron Carter, MD, chief medical officer at Microbiotica, Cambridge, UK, the company developing the product.
In addition, “in lab studies, we’ve found they exhibit anti-inflammatory effects when incubated with different cells of the immune system,” he explained. With the biotherapeutic product, “we want to prompt the immune system to realign the gut inflammation” in UC.
On February 23, at the annual congress of the European Crohn’s and Colitis Organisation, Dr. Carter presented the work-to-date and the near-term plans for a phase 1b randomized, placebo-controlled, double-blind trial to be conducted across roughly 20 sites in Europe (Abstract DOP 67). The trial, COMPOSER-1, will investigate the engraftment and initial signs of clinical activity of MB310 in 30 patients with active, mild to moderate UC (modified Mayo score 4-7; endoscopic subscore ≥ 2).
Calming Inflammation With Specific Bacteria
To date, the researchers have focused on identifying bacterial species that correlate with clinical outcomes in individual patients with UC after treatment with FMT. Dr. Carter and colleagues worked in collaboration with Sam Costello, MBBS, gastroenterologist at The Queen Elizabeth Hospital in Adelaide, Australia.
The initial research involved analyzing stool samples from a round 70 patients diagnosed with mild to moderate UC, who were randomly assigned to receive either pooled, anaerobically prepared, healthy donor FMT, or their own autologous FMT as a comparative control. FMT was delivered via colonoscopy and two enemas over 7 days. The primary endpoint of the study was achieving steroid-free remission of UC on the basis of specific Mayo scores, at the end of week 8.
“Results indicated that the group receiving the pooled donor FMT showed a 32% success rate with respect to the primary outcome compared with only 9% of the control group,” said Dr. Carter. “This suggested a promising therapeutic strategy.”
Based on these outcomes, Microbiotica then decided to conduct a local study in which they analyzed fecal samples from FMT donors and from pre-FMT and post-FMT recipients in order to precisely identify which bacterial species were associated with clinical response.
“With our precision microbiome analysis platform, we determined eight bacterial species that were consistently elevated in FMT responders, but absent or not elevated in non-responders,” reported Dr. Carter.
Next, they carried out subspecies-level bioinformatic analysis and identified one bacterial strain from each of the eight species, which were then cultured individually, freeze-dried, and combined into a custom delayed-release capsule.
The researchers tested the resulting live biotherapeutic product in cellular assays using human cell lines to confirm that they effectively modulated immune functions.
The cellular assays showed that in epithelial cell monolayer tests, MB310 not only enhanced barrier integrity but also protected the cellular barrier from inflammation.
The eight bacterial strain combination also exhibited anti-inflammatory effects when incubated with different primary innate immune cells, dendritic cells, and M1 macrophages.
And, Dr. Carter emphasized, the specific MB310 bacterial strains were found to regulate T-cells directly or via metabolites.
Moving in the Right Direction
Julien Kirchgesner, MD, Saint-Antoine Hospital and Sorbonne University, Paris, France, who co-moderated the session where the research was presented, welcomed the work.
“Many more trials need to be done before we see this enter the clinic, but this is moving in the right direction,” Dr. Kirchgesner noted.
“We need to find only the functionally active bacterial strains, rather than just take the fecal transplant alone [as is done with FMT],” he said. That is what is being done here.
“The researchers want to identify which bacterial strains will impact the effectiveness in patients, and to select for these strains before industrializing the process and producing them at the optimal strength, at scale,” Dr. Kirchgesner said.
Dr. Carter is chief medical officer at Microbiotica and Dr Kirshgensner has no financial disclosures.
A version of this article appeared on Medscape.com.
STOCKHOLM —
The eight-strain live biotherapeutic product (MB310, Microbiotica) is due to enter its first clinical trial later this year.
In patients with UC who showed clinical benefit after fecal microbiota transplantation (FMT), these eight specific bacterial strains were shown to be consistently elevated, reported Ron Carter, MD, chief medical officer at Microbiotica, Cambridge, UK, the company developing the product.
In addition, “in lab studies, we’ve found they exhibit anti-inflammatory effects when incubated with different cells of the immune system,” he explained. With the biotherapeutic product, “we want to prompt the immune system to realign the gut inflammation” in UC.
On February 23, at the annual congress of the European Crohn’s and Colitis Organisation, Dr. Carter presented the work-to-date and the near-term plans for a phase 1b randomized, placebo-controlled, double-blind trial to be conducted across roughly 20 sites in Europe (Abstract DOP 67). The trial, COMPOSER-1, will investigate the engraftment and initial signs of clinical activity of MB310 in 30 patients with active, mild to moderate UC (modified Mayo score 4-7; endoscopic subscore ≥ 2).
Calming Inflammation With Specific Bacteria
To date, the researchers have focused on identifying bacterial species that correlate with clinical outcomes in individual patients with UC after treatment with FMT. Dr. Carter and colleagues worked in collaboration with Sam Costello, MBBS, gastroenterologist at The Queen Elizabeth Hospital in Adelaide, Australia.
The initial research involved analyzing stool samples from a round 70 patients diagnosed with mild to moderate UC, who were randomly assigned to receive either pooled, anaerobically prepared, healthy donor FMT, or their own autologous FMT as a comparative control. FMT was delivered via colonoscopy and two enemas over 7 days. The primary endpoint of the study was achieving steroid-free remission of UC on the basis of specific Mayo scores, at the end of week 8.
“Results indicated that the group receiving the pooled donor FMT showed a 32% success rate with respect to the primary outcome compared with only 9% of the control group,” said Dr. Carter. “This suggested a promising therapeutic strategy.”
Based on these outcomes, Microbiotica then decided to conduct a local study in which they analyzed fecal samples from FMT donors and from pre-FMT and post-FMT recipients in order to precisely identify which bacterial species were associated with clinical response.
“With our precision microbiome analysis platform, we determined eight bacterial species that were consistently elevated in FMT responders, but absent or not elevated in non-responders,” reported Dr. Carter.
Next, they carried out subspecies-level bioinformatic analysis and identified one bacterial strain from each of the eight species, which were then cultured individually, freeze-dried, and combined into a custom delayed-release capsule.
The researchers tested the resulting live biotherapeutic product in cellular assays using human cell lines to confirm that they effectively modulated immune functions.
The cellular assays showed that in epithelial cell monolayer tests, MB310 not only enhanced barrier integrity but also protected the cellular barrier from inflammation.
The eight bacterial strain combination also exhibited anti-inflammatory effects when incubated with different primary innate immune cells, dendritic cells, and M1 macrophages.
And, Dr. Carter emphasized, the specific MB310 bacterial strains were found to regulate T-cells directly or via metabolites.
Moving in the Right Direction
Julien Kirchgesner, MD, Saint-Antoine Hospital and Sorbonne University, Paris, France, who co-moderated the session where the research was presented, welcomed the work.
“Many more trials need to be done before we see this enter the clinic, but this is moving in the right direction,” Dr. Kirchgesner noted.
“We need to find only the functionally active bacterial strains, rather than just take the fecal transplant alone [as is done with FMT],” he said. That is what is being done here.
“The researchers want to identify which bacterial strains will impact the effectiveness in patients, and to select for these strains before industrializing the process and producing them at the optimal strength, at scale,” Dr. Kirchgesner said.
Dr. Carter is chief medical officer at Microbiotica and Dr Kirshgensner has no financial disclosures.
A version of this article appeared on Medscape.com.
FROM ECCO 2024
Risankizumab in Crohn’s Disease: Clinical, Endoscopic Outcomes Remain Stable for up to 3 Years
STOCKHOLM — , according to results of the FORTIFY extension study.
“For me, most striking are the endoscopic endpoints,” Marc Ferrante, MD, PhD, AGAF, said in an interview. In the most conservative analysis, “you see a benefit the longer you follow the patients ... We haven’t seen this with many — if any — other compounds before.”
Dr. Ferrante, from University Hospitals Leuven in Belgium, added that patients showed less antibody formation in response to risankizumab, an anti-interleukin (IL)–23 p19 inhibitor, compared with anti–tumor necrosis factor agents.
“Most patients seemed to continue on treatment without the formation of antibodies to risankizumab becoming a problem,” he said. Also, for patients who achieve a good response to risankizumab, the effects were the same whether “they received this biologic first line, or only after failing other compounds.”
Generally, “I think we all have the impression that the IL-23 inhibitors have good efficacy, probably even better than other compounds available,” said Dr. Ferrante. “And, importantly, this is true without any increased adverse effects.”
“Now, with these new long-term data in risankizumab, we see the benefit-risk ratio continues to be favorable,” he added.
Dr. Ferrante presented the data (Abstract DOP 53) on February 23 at the annual congress of the European Crohn’s and Colitis Organisation.
Open-Label Extension up to 152 Weeks
The ongoing FORTIFY maintenance open-label extension study is evaluating the long-term efficacy and safety of risankizumab in patients with moderate to severe CD.
These data follow the initial 52-week study published in 2022 showing that subcutaneous risankizumab was a safe and efficacious treatment for maintenance of remission in patients with moderately to severely active CD. Dr. Ferrante also led that study.
Participants in this open-label extension study who had already completed 52-weeks maintenance dosing received 180-mg subcutaneous risankizumab every 8 weeks (n = 872) at week 56. Those who had received prior rescue therapy, a single 1200-mg intravenous risankizumab dose followed by 360-mg subcutaneously every 8 weeks, continued with this latter regimen (n = 275). Data for analysis were pooled from both treatment groups (risankizumab 180 mg and 360 mg), and clinical outcomes were evaluated every 6 months.
Data for the population, after patients who received rescue treatment were imputed as nonresponders, showed Clinical Disease Activity Index (CDAI) clinical response of 84.9% at week 56 and 52.7% at week 152. CDAI clinical remission was 66.7% at week 56 and 47.2% at week 152 for this population.
For endoscopic outcomes, additional benefit was seen over time. Endoscopic response, considered to be the best available predictor of long-term outcomes, was 50.8% at week 56 and 52.5% at week 152. Endoscopic remission was 35.8% at week 56 and 41.8% at week 152, and ulcer-free endoscopy was seen in 28.6% patients at week 56 and 35.5% at week 152.
The safety profile of risankizumab is consistent and supports long-term treatment, Dr. Ferrante said.
Treatment emergent adverse events included major adverse cardiovascular events in five patients on risankizumab and 50 serious infections.
‘Effective and Durable Option’
Providing comment, Tim Raine, MD, a consultant gastroenterologist & IBD lead at Cambridge University Hospitals, United Kingdom, remarked on the value of long-term extension studies for understanding the impact of continued drug administration beyond the typical 1-year time horizon of registrational clinical trials given that CD is currently incurable.
“However, there are limitations with long-term extension studies,” he said in an interview. In particular, the patients who remain in the study tend to be “those who have had a good experience with the drug and remain motivated to take part in ongoing monitoring.”
But “patients will drop out of a long-term extension study for reasons that may or may not reflect loss of benefit from the drug, and this can be problematic with handling of missing data,” he explained.
In light of this issue, “the investigators have used the most stringent way of handling these patients, regarding all who drop out as instances of failure of the drug. This offers the most robust assessment of durability of response that may slightly underestimate the true long-term efficacy,” said Dr. Raine.
“Nevertheless, the response and remission rates for clinical endpoints suggest good durability of effect out to 3 years of follow-up. The endoscopic data are also encouraging,” he asserted.
“Taken together, these data suggest that risankizumab can offer an effective and durable option for some patients with Crohn’s disease and is associated with a favorable safety profile.”
Dr. Ferrante disclosed ties with AbbVie, Agomab Therapeutics, Amgen, Biogen, Boehringer Ingelheim, Celgene, Celltrion, EG, Eli Lilly, Falk, Ferring, Janssen, Janssen-Cilag, Lamepro, Medtronic, MRM, MSD, Pfizer, Regeneron, Samsung Bioepis, Sandoz, Takeda, ThermoFisher, Truvion Healthcare, and Viatris. Dr. Raine disclosed ties with AbbVie, Arena, Aslan, AstraZeneca, Boehringer-Ingelheim, BMS, Celgene, Ferring, Galapagos, Gilead, GSK, Heptares, LabGenius, Janssen, Mylan, MSD, Novartis, Pfizer, Sandoz, Takeda, and UCB.
A version of this article appeared on Medscape.com.
STOCKHOLM — , according to results of the FORTIFY extension study.
“For me, most striking are the endoscopic endpoints,” Marc Ferrante, MD, PhD, AGAF, said in an interview. In the most conservative analysis, “you see a benefit the longer you follow the patients ... We haven’t seen this with many — if any — other compounds before.”
Dr. Ferrante, from University Hospitals Leuven in Belgium, added that patients showed less antibody formation in response to risankizumab, an anti-interleukin (IL)–23 p19 inhibitor, compared with anti–tumor necrosis factor agents.
“Most patients seemed to continue on treatment without the formation of antibodies to risankizumab becoming a problem,” he said. Also, for patients who achieve a good response to risankizumab, the effects were the same whether “they received this biologic first line, or only after failing other compounds.”
Generally, “I think we all have the impression that the IL-23 inhibitors have good efficacy, probably even better than other compounds available,” said Dr. Ferrante. “And, importantly, this is true without any increased adverse effects.”
“Now, with these new long-term data in risankizumab, we see the benefit-risk ratio continues to be favorable,” he added.
Dr. Ferrante presented the data (Abstract DOP 53) on February 23 at the annual congress of the European Crohn’s and Colitis Organisation.
Open-Label Extension up to 152 Weeks
The ongoing FORTIFY maintenance open-label extension study is evaluating the long-term efficacy and safety of risankizumab in patients with moderate to severe CD.
These data follow the initial 52-week study published in 2022 showing that subcutaneous risankizumab was a safe and efficacious treatment for maintenance of remission in patients with moderately to severely active CD. Dr. Ferrante also led that study.
Participants in this open-label extension study who had already completed 52-weeks maintenance dosing received 180-mg subcutaneous risankizumab every 8 weeks (n = 872) at week 56. Those who had received prior rescue therapy, a single 1200-mg intravenous risankizumab dose followed by 360-mg subcutaneously every 8 weeks, continued with this latter regimen (n = 275). Data for analysis were pooled from both treatment groups (risankizumab 180 mg and 360 mg), and clinical outcomes were evaluated every 6 months.
Data for the population, after patients who received rescue treatment were imputed as nonresponders, showed Clinical Disease Activity Index (CDAI) clinical response of 84.9% at week 56 and 52.7% at week 152. CDAI clinical remission was 66.7% at week 56 and 47.2% at week 152 for this population.
For endoscopic outcomes, additional benefit was seen over time. Endoscopic response, considered to be the best available predictor of long-term outcomes, was 50.8% at week 56 and 52.5% at week 152. Endoscopic remission was 35.8% at week 56 and 41.8% at week 152, and ulcer-free endoscopy was seen in 28.6% patients at week 56 and 35.5% at week 152.
The safety profile of risankizumab is consistent and supports long-term treatment, Dr. Ferrante said.
Treatment emergent adverse events included major adverse cardiovascular events in five patients on risankizumab and 50 serious infections.
‘Effective and Durable Option’
Providing comment, Tim Raine, MD, a consultant gastroenterologist & IBD lead at Cambridge University Hospitals, United Kingdom, remarked on the value of long-term extension studies for understanding the impact of continued drug administration beyond the typical 1-year time horizon of registrational clinical trials given that CD is currently incurable.
“However, there are limitations with long-term extension studies,” he said in an interview. In particular, the patients who remain in the study tend to be “those who have had a good experience with the drug and remain motivated to take part in ongoing monitoring.”
But “patients will drop out of a long-term extension study for reasons that may or may not reflect loss of benefit from the drug, and this can be problematic with handling of missing data,” he explained.
In light of this issue, “the investigators have used the most stringent way of handling these patients, regarding all who drop out as instances of failure of the drug. This offers the most robust assessment of durability of response that may slightly underestimate the true long-term efficacy,” said Dr. Raine.
“Nevertheless, the response and remission rates for clinical endpoints suggest good durability of effect out to 3 years of follow-up. The endoscopic data are also encouraging,” he asserted.
“Taken together, these data suggest that risankizumab can offer an effective and durable option for some patients with Crohn’s disease and is associated with a favorable safety profile.”
Dr. Ferrante disclosed ties with AbbVie, Agomab Therapeutics, Amgen, Biogen, Boehringer Ingelheim, Celgene, Celltrion, EG, Eli Lilly, Falk, Ferring, Janssen, Janssen-Cilag, Lamepro, Medtronic, MRM, MSD, Pfizer, Regeneron, Samsung Bioepis, Sandoz, Takeda, ThermoFisher, Truvion Healthcare, and Viatris. Dr. Raine disclosed ties with AbbVie, Arena, Aslan, AstraZeneca, Boehringer-Ingelheim, BMS, Celgene, Ferring, Galapagos, Gilead, GSK, Heptares, LabGenius, Janssen, Mylan, MSD, Novartis, Pfizer, Sandoz, Takeda, and UCB.
A version of this article appeared on Medscape.com.
STOCKHOLM — , according to results of the FORTIFY extension study.
“For me, most striking are the endoscopic endpoints,” Marc Ferrante, MD, PhD, AGAF, said in an interview. In the most conservative analysis, “you see a benefit the longer you follow the patients ... We haven’t seen this with many — if any — other compounds before.”
Dr. Ferrante, from University Hospitals Leuven in Belgium, added that patients showed less antibody formation in response to risankizumab, an anti-interleukin (IL)–23 p19 inhibitor, compared with anti–tumor necrosis factor agents.
“Most patients seemed to continue on treatment without the formation of antibodies to risankizumab becoming a problem,” he said. Also, for patients who achieve a good response to risankizumab, the effects were the same whether “they received this biologic first line, or only after failing other compounds.”
Generally, “I think we all have the impression that the IL-23 inhibitors have good efficacy, probably even better than other compounds available,” said Dr. Ferrante. “And, importantly, this is true without any increased adverse effects.”
“Now, with these new long-term data in risankizumab, we see the benefit-risk ratio continues to be favorable,” he added.
Dr. Ferrante presented the data (Abstract DOP 53) on February 23 at the annual congress of the European Crohn’s and Colitis Organisation.
Open-Label Extension up to 152 Weeks
The ongoing FORTIFY maintenance open-label extension study is evaluating the long-term efficacy and safety of risankizumab in patients with moderate to severe CD.
These data follow the initial 52-week study published in 2022 showing that subcutaneous risankizumab was a safe and efficacious treatment for maintenance of remission in patients with moderately to severely active CD. Dr. Ferrante also led that study.
Participants in this open-label extension study who had already completed 52-weeks maintenance dosing received 180-mg subcutaneous risankizumab every 8 weeks (n = 872) at week 56. Those who had received prior rescue therapy, a single 1200-mg intravenous risankizumab dose followed by 360-mg subcutaneously every 8 weeks, continued with this latter regimen (n = 275). Data for analysis were pooled from both treatment groups (risankizumab 180 mg and 360 mg), and clinical outcomes were evaluated every 6 months.
Data for the population, after patients who received rescue treatment were imputed as nonresponders, showed Clinical Disease Activity Index (CDAI) clinical response of 84.9% at week 56 and 52.7% at week 152. CDAI clinical remission was 66.7% at week 56 and 47.2% at week 152 for this population.
For endoscopic outcomes, additional benefit was seen over time. Endoscopic response, considered to be the best available predictor of long-term outcomes, was 50.8% at week 56 and 52.5% at week 152. Endoscopic remission was 35.8% at week 56 and 41.8% at week 152, and ulcer-free endoscopy was seen in 28.6% patients at week 56 and 35.5% at week 152.
The safety profile of risankizumab is consistent and supports long-term treatment, Dr. Ferrante said.
Treatment emergent adverse events included major adverse cardiovascular events in five patients on risankizumab and 50 serious infections.
‘Effective and Durable Option’
Providing comment, Tim Raine, MD, a consultant gastroenterologist & IBD lead at Cambridge University Hospitals, United Kingdom, remarked on the value of long-term extension studies for understanding the impact of continued drug administration beyond the typical 1-year time horizon of registrational clinical trials given that CD is currently incurable.
“However, there are limitations with long-term extension studies,” he said in an interview. In particular, the patients who remain in the study tend to be “those who have had a good experience with the drug and remain motivated to take part in ongoing monitoring.”
But “patients will drop out of a long-term extension study for reasons that may or may not reflect loss of benefit from the drug, and this can be problematic with handling of missing data,” he explained.
In light of this issue, “the investigators have used the most stringent way of handling these patients, regarding all who drop out as instances of failure of the drug. This offers the most robust assessment of durability of response that may slightly underestimate the true long-term efficacy,” said Dr. Raine.
“Nevertheless, the response and remission rates for clinical endpoints suggest good durability of effect out to 3 years of follow-up. The endoscopic data are also encouraging,” he asserted.
“Taken together, these data suggest that risankizumab can offer an effective and durable option for some patients with Crohn’s disease and is associated with a favorable safety profile.”
Dr. Ferrante disclosed ties with AbbVie, Agomab Therapeutics, Amgen, Biogen, Boehringer Ingelheim, Celgene, Celltrion, EG, Eli Lilly, Falk, Ferring, Janssen, Janssen-Cilag, Lamepro, Medtronic, MRM, MSD, Pfizer, Regeneron, Samsung Bioepis, Sandoz, Takeda, ThermoFisher, Truvion Healthcare, and Viatris. Dr. Raine disclosed ties with AbbVie, Arena, Aslan, AstraZeneca, Boehringer-Ingelheim, BMS, Celgene, Ferring, Galapagos, Gilead, GSK, Heptares, LabGenius, Janssen, Mylan, MSD, Novartis, Pfizer, Sandoz, Takeda, and UCB.
A version of this article appeared on Medscape.com.
FROM ECCO 2024
Capsule Endoscopy–Guided Treatment Reduces Flares in Crohn’s Disease Compared With Standard Care
STOCKHOLM — , a new study showed.
Capsule endoscopy reveals small-intestinal inflammation in over 70% of patients with CD who are in clinical remission, said Shomron Ben-Horin, MD, from Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel. The question remains however, which patients would benefit from treatment intensification.
The randomized controlled CURE-CD trial showed that patients who have high inflammatory activity — a Lewis score ≥ 350 as seen in video capsule endoscopy findings — experienced statistically significantly fewer relapses during the 2-year follow-up vs patients with the same amount of inflammation who continued their standard treatment, he said.
Dr. Ben-Horin presented the results at the annual congress of the European Crohn’s and Colitis Organisation on behalf of the Israeli IBD Research Nucleus.
He and his colleagues aimed to examine the value of video capsule endoscopy to guide proactive treat-to-target strategy in patients with CD who are in clinical remission, adding onto their previous work published in 2019 that established the benefit of video capsule endoscopy over calprotectin in predicting relapse.
The prospective randomized controlled trial recruited 60 patients with CD involving the small bowel who were in clinical remission (Crohn’s Disease Activity Index [CDAI] < 150). Patients underwent clinical, biomarker, and imaging checks as well as video capsule endoscopies at baseline and every 6 months thereafter for up to 24 months.
A total of 40 patients with a Lewis score ≥ 350 and who were considered high-risk were randomized to either proactive treatment optimization (targeting video capsule endoscopy mucosal healing, n = 20) or to continued standard care (n = 20) for 24 months. Patients with a Lewis score < 350 who were considered to be low-risk continued standard care (n = 20) which may or may not have been biologic.
The primary outcome was the rate of clinical relapse (disease exacerbation comprised of a CDAI increase > 70 points or hospitalization/surgery) by 24 months in high-risk patients who received standard care vs proactive care.
Secondary outcomes included risk for flare in the low-risk group (all on standard care) vs the high-risk group also on standard care, predictive profiles for flare of calprotectin, MRI, intestinal ultrasound, and Lewis score over the 24 months.
A Nearly Threefold Difference
Treatment intensification in the high-risk proactive strategy group was split between therapeutic drug monitoring–based biologic dose-escalation (n = 11 in 20), starting a biologic (8 in 20), or swapping a biologic (1 in 20).
By 24 months, clinical flare occurred in 5 in 20 (25%) of the high-risk proactive group vs 14 (70%) of the high-risk standard-care group (odds ratio [OR], 0.14; 95% CI, 0.04-0.57; P = .006), Dr. Ben-Horin reported.
The data also showed that low-risk patients had a lower incidence of clinical flare at approximately 45% compared with 70% in high-risk patients also on standard care (P = .11 by intention-to-treat analysis; P = .06 by per-protocol analysis).
In an interview, Dr. Ben-Horin said that despite the results, there remained a “big dilemma” about who should be treated if they show mucosal inflammation on video capsule endoscopy.
“Crohn’s disease is a progressive disease, and we don’t want flares down the road in a patient that currently feels okay but has underlying inflammation,” he said. “On the other hand, it’s important to consider that our therapies are sometimes associated with adverse events, they can be very costly, and become a huge burden to our healthcare systems and to some of the patients.”
It is important to ask whether we should or treat patients in remission more aggressively “because not everyone will progress and some of them may never flare,” he explained.
The findings offer three layers of added evidence to the field, Dr. Ben-Horin said. First, it offers further support to the treat-to-target approach if tailored to a high-risk group. Second, the study suggests that treat-to-target studies should be looking only at the high-risk patients and not the entire study population. “This is the first study of its kind to take this approach,” he pointed out.
“Thirdly, our results build on our preceding trial to show that using video capsule endoscopy enables us to stratify the risk of patients’ with small-bowel Crohn’s disease and tailor their treatment accordingly, probably more accurately than calprotectin or C-reactive protein.”
Asked to comment on the results, Maria Abreu, MD, AGAF, a gastroenterologist who specializes in inflammatory bowel disease at the University of Miami, Miami, Florida, said that though it was a small study, it highlights that patients need to be closely monitored with objective, quantifiable tests for the best outcomes.
She continued: “Capsule endoscopy is certainly the most sensitive test we have and lends itself in short order to artificial intelligence interpretation and quantification to make it even more robust.”
“We now need to nuance who needs a capsule vs intestinal ultrasound vs a colonoscopy, and that is likely to depend on disease location and manifestations of the disease,” ie, mucosa-only or transmural-predominant disease, she noted.
Dr. Ben-Horin has declared that the trial was funded by the Helmsley Charitable Trust. The VCE was partly provided by Medtronic. He disclosed advisory board fees and/or consulting and/or research support from Janssen, AbbVie, CellTrion, Takeda, Schering Plough, Pfizer, Ferring, Falk Pharma, GlaxoSmithKline, Novartis, Roche, Galmed, EviNature, Galmed, PredictaMed, NeoPharm.
Dr. Abreu has served as a consultant and scientific advisory board member for AbbVie, Arena Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly Pharmaceuticals, Gilead, Janssen Biotech, and Prometheus Biosciences, University of California, Berkeley; a speaker for Alimentiv; and has had projects funded by Pfizer, Prometheus Laboratories, and Takeda Pharmaceuticals.
A version of this article appeared on Medscape.com.
STOCKHOLM — , a new study showed.
Capsule endoscopy reveals small-intestinal inflammation in over 70% of patients with CD who are in clinical remission, said Shomron Ben-Horin, MD, from Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel. The question remains however, which patients would benefit from treatment intensification.
The randomized controlled CURE-CD trial showed that patients who have high inflammatory activity — a Lewis score ≥ 350 as seen in video capsule endoscopy findings — experienced statistically significantly fewer relapses during the 2-year follow-up vs patients with the same amount of inflammation who continued their standard treatment, he said.
Dr. Ben-Horin presented the results at the annual congress of the European Crohn’s and Colitis Organisation on behalf of the Israeli IBD Research Nucleus.
He and his colleagues aimed to examine the value of video capsule endoscopy to guide proactive treat-to-target strategy in patients with CD who are in clinical remission, adding onto their previous work published in 2019 that established the benefit of video capsule endoscopy over calprotectin in predicting relapse.
The prospective randomized controlled trial recruited 60 patients with CD involving the small bowel who were in clinical remission (Crohn’s Disease Activity Index [CDAI] < 150). Patients underwent clinical, biomarker, and imaging checks as well as video capsule endoscopies at baseline and every 6 months thereafter for up to 24 months.
A total of 40 patients with a Lewis score ≥ 350 and who were considered high-risk were randomized to either proactive treatment optimization (targeting video capsule endoscopy mucosal healing, n = 20) or to continued standard care (n = 20) for 24 months. Patients with a Lewis score < 350 who were considered to be low-risk continued standard care (n = 20) which may or may not have been biologic.
The primary outcome was the rate of clinical relapse (disease exacerbation comprised of a CDAI increase > 70 points or hospitalization/surgery) by 24 months in high-risk patients who received standard care vs proactive care.
Secondary outcomes included risk for flare in the low-risk group (all on standard care) vs the high-risk group also on standard care, predictive profiles for flare of calprotectin, MRI, intestinal ultrasound, and Lewis score over the 24 months.
A Nearly Threefold Difference
Treatment intensification in the high-risk proactive strategy group was split between therapeutic drug monitoring–based biologic dose-escalation (n = 11 in 20), starting a biologic (8 in 20), or swapping a biologic (1 in 20).
By 24 months, clinical flare occurred in 5 in 20 (25%) of the high-risk proactive group vs 14 (70%) of the high-risk standard-care group (odds ratio [OR], 0.14; 95% CI, 0.04-0.57; P = .006), Dr. Ben-Horin reported.
The data also showed that low-risk patients had a lower incidence of clinical flare at approximately 45% compared with 70% in high-risk patients also on standard care (P = .11 by intention-to-treat analysis; P = .06 by per-protocol analysis).
In an interview, Dr. Ben-Horin said that despite the results, there remained a “big dilemma” about who should be treated if they show mucosal inflammation on video capsule endoscopy.
“Crohn’s disease is a progressive disease, and we don’t want flares down the road in a patient that currently feels okay but has underlying inflammation,” he said. “On the other hand, it’s important to consider that our therapies are sometimes associated with adverse events, they can be very costly, and become a huge burden to our healthcare systems and to some of the patients.”
It is important to ask whether we should or treat patients in remission more aggressively “because not everyone will progress and some of them may never flare,” he explained.
The findings offer three layers of added evidence to the field, Dr. Ben-Horin said. First, it offers further support to the treat-to-target approach if tailored to a high-risk group. Second, the study suggests that treat-to-target studies should be looking only at the high-risk patients and not the entire study population. “This is the first study of its kind to take this approach,” he pointed out.
“Thirdly, our results build on our preceding trial to show that using video capsule endoscopy enables us to stratify the risk of patients’ with small-bowel Crohn’s disease and tailor their treatment accordingly, probably more accurately than calprotectin or C-reactive protein.”
Asked to comment on the results, Maria Abreu, MD, AGAF, a gastroenterologist who specializes in inflammatory bowel disease at the University of Miami, Miami, Florida, said that though it was a small study, it highlights that patients need to be closely monitored with objective, quantifiable tests for the best outcomes.
She continued: “Capsule endoscopy is certainly the most sensitive test we have and lends itself in short order to artificial intelligence interpretation and quantification to make it even more robust.”
“We now need to nuance who needs a capsule vs intestinal ultrasound vs a colonoscopy, and that is likely to depend on disease location and manifestations of the disease,” ie, mucosa-only or transmural-predominant disease, she noted.
Dr. Ben-Horin has declared that the trial was funded by the Helmsley Charitable Trust. The VCE was partly provided by Medtronic. He disclosed advisory board fees and/or consulting and/or research support from Janssen, AbbVie, CellTrion, Takeda, Schering Plough, Pfizer, Ferring, Falk Pharma, GlaxoSmithKline, Novartis, Roche, Galmed, EviNature, Galmed, PredictaMed, NeoPharm.
Dr. Abreu has served as a consultant and scientific advisory board member for AbbVie, Arena Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly Pharmaceuticals, Gilead, Janssen Biotech, and Prometheus Biosciences, University of California, Berkeley; a speaker for Alimentiv; and has had projects funded by Pfizer, Prometheus Laboratories, and Takeda Pharmaceuticals.
A version of this article appeared on Medscape.com.
STOCKHOLM — , a new study showed.
Capsule endoscopy reveals small-intestinal inflammation in over 70% of patients with CD who are in clinical remission, said Shomron Ben-Horin, MD, from Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel. The question remains however, which patients would benefit from treatment intensification.
The randomized controlled CURE-CD trial showed that patients who have high inflammatory activity — a Lewis score ≥ 350 as seen in video capsule endoscopy findings — experienced statistically significantly fewer relapses during the 2-year follow-up vs patients with the same amount of inflammation who continued their standard treatment, he said.
Dr. Ben-Horin presented the results at the annual congress of the European Crohn’s and Colitis Organisation on behalf of the Israeli IBD Research Nucleus.
He and his colleagues aimed to examine the value of video capsule endoscopy to guide proactive treat-to-target strategy in patients with CD who are in clinical remission, adding onto their previous work published in 2019 that established the benefit of video capsule endoscopy over calprotectin in predicting relapse.
The prospective randomized controlled trial recruited 60 patients with CD involving the small bowel who were in clinical remission (Crohn’s Disease Activity Index [CDAI] < 150). Patients underwent clinical, biomarker, and imaging checks as well as video capsule endoscopies at baseline and every 6 months thereafter for up to 24 months.
A total of 40 patients with a Lewis score ≥ 350 and who were considered high-risk were randomized to either proactive treatment optimization (targeting video capsule endoscopy mucosal healing, n = 20) or to continued standard care (n = 20) for 24 months. Patients with a Lewis score < 350 who were considered to be low-risk continued standard care (n = 20) which may or may not have been biologic.
The primary outcome was the rate of clinical relapse (disease exacerbation comprised of a CDAI increase > 70 points or hospitalization/surgery) by 24 months in high-risk patients who received standard care vs proactive care.
Secondary outcomes included risk for flare in the low-risk group (all on standard care) vs the high-risk group also on standard care, predictive profiles for flare of calprotectin, MRI, intestinal ultrasound, and Lewis score over the 24 months.
A Nearly Threefold Difference
Treatment intensification in the high-risk proactive strategy group was split between therapeutic drug monitoring–based biologic dose-escalation (n = 11 in 20), starting a biologic (8 in 20), or swapping a biologic (1 in 20).
By 24 months, clinical flare occurred in 5 in 20 (25%) of the high-risk proactive group vs 14 (70%) of the high-risk standard-care group (odds ratio [OR], 0.14; 95% CI, 0.04-0.57; P = .006), Dr. Ben-Horin reported.
The data also showed that low-risk patients had a lower incidence of clinical flare at approximately 45% compared with 70% in high-risk patients also on standard care (P = .11 by intention-to-treat analysis; P = .06 by per-protocol analysis).
In an interview, Dr. Ben-Horin said that despite the results, there remained a “big dilemma” about who should be treated if they show mucosal inflammation on video capsule endoscopy.
“Crohn’s disease is a progressive disease, and we don’t want flares down the road in a patient that currently feels okay but has underlying inflammation,” he said. “On the other hand, it’s important to consider that our therapies are sometimes associated with adverse events, they can be very costly, and become a huge burden to our healthcare systems and to some of the patients.”
It is important to ask whether we should or treat patients in remission more aggressively “because not everyone will progress and some of them may never flare,” he explained.
The findings offer three layers of added evidence to the field, Dr. Ben-Horin said. First, it offers further support to the treat-to-target approach if tailored to a high-risk group. Second, the study suggests that treat-to-target studies should be looking only at the high-risk patients and not the entire study population. “This is the first study of its kind to take this approach,” he pointed out.
“Thirdly, our results build on our preceding trial to show that using video capsule endoscopy enables us to stratify the risk of patients’ with small-bowel Crohn’s disease and tailor their treatment accordingly, probably more accurately than calprotectin or C-reactive protein.”
Asked to comment on the results, Maria Abreu, MD, AGAF, a gastroenterologist who specializes in inflammatory bowel disease at the University of Miami, Miami, Florida, said that though it was a small study, it highlights that patients need to be closely monitored with objective, quantifiable tests for the best outcomes.
She continued: “Capsule endoscopy is certainly the most sensitive test we have and lends itself in short order to artificial intelligence interpretation and quantification to make it even more robust.”
“We now need to nuance who needs a capsule vs intestinal ultrasound vs a colonoscopy, and that is likely to depend on disease location and manifestations of the disease,” ie, mucosa-only or transmural-predominant disease, she noted.
Dr. Ben-Horin has declared that the trial was funded by the Helmsley Charitable Trust. The VCE was partly provided by Medtronic. He disclosed advisory board fees and/or consulting and/or research support from Janssen, AbbVie, CellTrion, Takeda, Schering Plough, Pfizer, Ferring, Falk Pharma, GlaxoSmithKline, Novartis, Roche, Galmed, EviNature, Galmed, PredictaMed, NeoPharm.
Dr. Abreu has served as a consultant and scientific advisory board member for AbbVie, Arena Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly Pharmaceuticals, Gilead, Janssen Biotech, and Prometheus Biosciences, University of California, Berkeley; a speaker for Alimentiv; and has had projects funded by Pfizer, Prometheus Laboratories, and Takeda Pharmaceuticals.
A version of this article appeared on Medscape.com.
FROM ECCO 2024
No-Biopsy Approach to Celiac Disease Diagnosis Appears Effective for Select Adult Patients
Select adult patients with immunoglobulin A-tissue transglutaminase antibody levels (IgA-tTG) greater than or equal to 10 times the upper limit of normal (ULN) and a moderate-to-high pretest probability of celiac disease could be diagnosed without undergoing invasive endoscopy and duodenal biopsy, according to a new study.
, the authors wrote.
“Our study confirms the high accuracy of serology-based diagnosis of coeliac disease in select adult patients,” said Mohamed G. Shiha, MBBCh, MRCP, lead author and a clinical research fellow in gastroenterology at Sheffield Teaching Hospitals in the United Kingdom.
“This no-biopsy approach could lead to a shorter time to diagnosis, increased patient satisfaction, and reduced healthcare costs,” he said.
The study was published online in Gastroenterology.
Evaluating the No-Biopsy Approach
Dr. Shiha and colleagues conducted a systematic review and meta-analysis to evaluate to the accuracy of a no-biopsy approach for diagnosing celiac disease in adults. They looked for studies that reported the sensitivity and specificity of IgA-tTG ≥10xULN compared with duodenal biopsies (with a Marsh grade ≥2) in adults with suspected celiac disease.
The research team used a bivariate random-effects model to calculate the summary estimates of sensitivity, specificity, and positive and negative likelihood ratios. Then the positive and negative likelihood ratios were used to calculate the positive predictive value (PPV) of the no-biopsy approach across different pretest probabilities of celiac disease.
Among 18 studies with 12,103 participants from 15 countries, the pooled prevalence of biopsy-proven celiac disease was 62%. The proportion of patients with IgA-tTG ≥10xULN was 32%.
The summary sensitivity of IgA-tTG ≥10xULN was 51%, and the summary specificity was 100% for the diagnosis of celiac disease. The positive and negative likelihood ratios were 183.42 and .49, respectively. The area under the summary receiver operating characteristic curve was .83.
Overall, the PPV of IgA-tTG ≥10xULN to identify patients with celiac disease was 98%, which varied according to pretest probability of celiac disease in the studied population. Specifically, the PPV was 65%, 88%, 95%, and 99% if celiac disease prevalence was 1%, 4%, 10%, and 40%, respectively. The 40% prevalence represents the lower confidence interval of the pooled prevalence from the included studies, the authors noted.
“We provided PPV estimates of IgA-tTG ≥10xULN for common pretest probabilities of coeliac disease to aid clinicians and patients in reaching an informed decision on a no-biopsy diagnosis based on the best available evidence,” the authors wrote.
Considering Additional Factors
Due to the increased accuracy of serological tests, pediatric guidelines have adopted a no-biopsy approach, the authors wrote. Children with IgA-tTG ≥10xULN and positive serum endomysial antibodies (EMA) can be diagnosed with celiac disease without biopsy.
However, the no-biopsy approach remains controversial for diagnosing adult patients and requires additional study, the authors wrote. They noted a limitation that all included studies were conducted in secondary and tertiary care settings and excluded patients with known celiac disease or on a gluten-free diet, so the results may not be generalizable to primary care settings.
In addition, relying on serology testing alone could lead to potential false-positive diagnoses, unnecessary dietary restriction, and negative effects on patients’ quality of life, the authors wrote.
At the same time, duodenal biopsy may not always be accurate due to inadequate sampling and could result in false-negative histology. The no-biopsy approach could mitigate this potential risk, the authors noted.
“This study systematically collates the growing data supporting the accuracy of antibody testing to diagnose celiac disease,” said Benjamin Lebwohl, MD, AGAF, professor of medicine and epidemiology at Columbia University Medical Center and director of clinical research for the Celiac Disease Center at Columbia University, New York. Dr. Lebwohl wasn’t involved with this study.
“We have historically relied on duodenal biopsy to confirm the diagnosis of celiac disease, and the biopsy will still have a central role in most cases in the foreseeable future,” he said. “But as we hone our understanding of antibody testing, one day we may be able to accept or even recommend a biopsy-free approach in select patients.”
Two authors reported grant support from the National Institute for Health and Care Research and National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Shiha reported speaker honorarium from Thermo Fisher. Dr. Lebwohl reported no relevant disclosures.
Select adult patients with immunoglobulin A-tissue transglutaminase antibody levels (IgA-tTG) greater than or equal to 10 times the upper limit of normal (ULN) and a moderate-to-high pretest probability of celiac disease could be diagnosed without undergoing invasive endoscopy and duodenal biopsy, according to a new study.
, the authors wrote.
“Our study confirms the high accuracy of serology-based diagnosis of coeliac disease in select adult patients,” said Mohamed G. Shiha, MBBCh, MRCP, lead author and a clinical research fellow in gastroenterology at Sheffield Teaching Hospitals in the United Kingdom.
“This no-biopsy approach could lead to a shorter time to diagnosis, increased patient satisfaction, and reduced healthcare costs,” he said.
The study was published online in Gastroenterology.
Evaluating the No-Biopsy Approach
Dr. Shiha and colleagues conducted a systematic review and meta-analysis to evaluate to the accuracy of a no-biopsy approach for diagnosing celiac disease in adults. They looked for studies that reported the sensitivity and specificity of IgA-tTG ≥10xULN compared with duodenal biopsies (with a Marsh grade ≥2) in adults with suspected celiac disease.
The research team used a bivariate random-effects model to calculate the summary estimates of sensitivity, specificity, and positive and negative likelihood ratios. Then the positive and negative likelihood ratios were used to calculate the positive predictive value (PPV) of the no-biopsy approach across different pretest probabilities of celiac disease.
Among 18 studies with 12,103 participants from 15 countries, the pooled prevalence of biopsy-proven celiac disease was 62%. The proportion of patients with IgA-tTG ≥10xULN was 32%.
The summary sensitivity of IgA-tTG ≥10xULN was 51%, and the summary specificity was 100% for the diagnosis of celiac disease. The positive and negative likelihood ratios were 183.42 and .49, respectively. The area under the summary receiver operating characteristic curve was .83.
Overall, the PPV of IgA-tTG ≥10xULN to identify patients with celiac disease was 98%, which varied according to pretest probability of celiac disease in the studied population. Specifically, the PPV was 65%, 88%, 95%, and 99% if celiac disease prevalence was 1%, 4%, 10%, and 40%, respectively. The 40% prevalence represents the lower confidence interval of the pooled prevalence from the included studies, the authors noted.
“We provided PPV estimates of IgA-tTG ≥10xULN for common pretest probabilities of coeliac disease to aid clinicians and patients in reaching an informed decision on a no-biopsy diagnosis based on the best available evidence,” the authors wrote.
Considering Additional Factors
Due to the increased accuracy of serological tests, pediatric guidelines have adopted a no-biopsy approach, the authors wrote. Children with IgA-tTG ≥10xULN and positive serum endomysial antibodies (EMA) can be diagnosed with celiac disease without biopsy.
However, the no-biopsy approach remains controversial for diagnosing adult patients and requires additional study, the authors wrote. They noted a limitation that all included studies were conducted in secondary and tertiary care settings and excluded patients with known celiac disease or on a gluten-free diet, so the results may not be generalizable to primary care settings.
In addition, relying on serology testing alone could lead to potential false-positive diagnoses, unnecessary dietary restriction, and negative effects on patients’ quality of life, the authors wrote.
At the same time, duodenal biopsy may not always be accurate due to inadequate sampling and could result in false-negative histology. The no-biopsy approach could mitigate this potential risk, the authors noted.
“This study systematically collates the growing data supporting the accuracy of antibody testing to diagnose celiac disease,” said Benjamin Lebwohl, MD, AGAF, professor of medicine and epidemiology at Columbia University Medical Center and director of clinical research for the Celiac Disease Center at Columbia University, New York. Dr. Lebwohl wasn’t involved with this study.
“We have historically relied on duodenal biopsy to confirm the diagnosis of celiac disease, and the biopsy will still have a central role in most cases in the foreseeable future,” he said. “But as we hone our understanding of antibody testing, one day we may be able to accept or even recommend a biopsy-free approach in select patients.”
Two authors reported grant support from the National Institute for Health and Care Research and National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Shiha reported speaker honorarium from Thermo Fisher. Dr. Lebwohl reported no relevant disclosures.
Select adult patients with immunoglobulin A-tissue transglutaminase antibody levels (IgA-tTG) greater than or equal to 10 times the upper limit of normal (ULN) and a moderate-to-high pretest probability of celiac disease could be diagnosed without undergoing invasive endoscopy and duodenal biopsy, according to a new study.
, the authors wrote.
“Our study confirms the high accuracy of serology-based diagnosis of coeliac disease in select adult patients,” said Mohamed G. Shiha, MBBCh, MRCP, lead author and a clinical research fellow in gastroenterology at Sheffield Teaching Hospitals in the United Kingdom.
“This no-biopsy approach could lead to a shorter time to diagnosis, increased patient satisfaction, and reduced healthcare costs,” he said.
The study was published online in Gastroenterology.
Evaluating the No-Biopsy Approach
Dr. Shiha and colleagues conducted a systematic review and meta-analysis to evaluate to the accuracy of a no-biopsy approach for diagnosing celiac disease in adults. They looked for studies that reported the sensitivity and specificity of IgA-tTG ≥10xULN compared with duodenal biopsies (with a Marsh grade ≥2) in adults with suspected celiac disease.
The research team used a bivariate random-effects model to calculate the summary estimates of sensitivity, specificity, and positive and negative likelihood ratios. Then the positive and negative likelihood ratios were used to calculate the positive predictive value (PPV) of the no-biopsy approach across different pretest probabilities of celiac disease.
Among 18 studies with 12,103 participants from 15 countries, the pooled prevalence of biopsy-proven celiac disease was 62%. The proportion of patients with IgA-tTG ≥10xULN was 32%.
The summary sensitivity of IgA-tTG ≥10xULN was 51%, and the summary specificity was 100% for the diagnosis of celiac disease. The positive and negative likelihood ratios were 183.42 and .49, respectively. The area under the summary receiver operating characteristic curve was .83.
Overall, the PPV of IgA-tTG ≥10xULN to identify patients with celiac disease was 98%, which varied according to pretest probability of celiac disease in the studied population. Specifically, the PPV was 65%, 88%, 95%, and 99% if celiac disease prevalence was 1%, 4%, 10%, and 40%, respectively. The 40% prevalence represents the lower confidence interval of the pooled prevalence from the included studies, the authors noted.
“We provided PPV estimates of IgA-tTG ≥10xULN for common pretest probabilities of coeliac disease to aid clinicians and patients in reaching an informed decision on a no-biopsy diagnosis based on the best available evidence,” the authors wrote.
Considering Additional Factors
Due to the increased accuracy of serological tests, pediatric guidelines have adopted a no-biopsy approach, the authors wrote. Children with IgA-tTG ≥10xULN and positive serum endomysial antibodies (EMA) can be diagnosed with celiac disease without biopsy.
However, the no-biopsy approach remains controversial for diagnosing adult patients and requires additional study, the authors wrote. They noted a limitation that all included studies were conducted in secondary and tertiary care settings and excluded patients with known celiac disease or on a gluten-free diet, so the results may not be generalizable to primary care settings.
In addition, relying on serology testing alone could lead to potential false-positive diagnoses, unnecessary dietary restriction, and negative effects on patients’ quality of life, the authors wrote.
At the same time, duodenal biopsy may not always be accurate due to inadequate sampling and could result in false-negative histology. The no-biopsy approach could mitigate this potential risk, the authors noted.
“This study systematically collates the growing data supporting the accuracy of antibody testing to diagnose celiac disease,” said Benjamin Lebwohl, MD, AGAF, professor of medicine and epidemiology at Columbia University Medical Center and director of clinical research for the Celiac Disease Center at Columbia University, New York. Dr. Lebwohl wasn’t involved with this study.
“We have historically relied on duodenal biopsy to confirm the diagnosis of celiac disease, and the biopsy will still have a central role in most cases in the foreseeable future,” he said. “But as we hone our understanding of antibody testing, one day we may be able to accept or even recommend a biopsy-free approach in select patients.”
Two authors reported grant support from the National Institute for Health and Care Research and National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Shiha reported speaker honorarium from Thermo Fisher. Dr. Lebwohl reported no relevant disclosures.
FROM GASTROENTEROLOGY
Mood Interventions May Reduce IBD Inflammation
, according to a new study.
“IBD is a distressing condition, and current medication that reduces inflammation is expensive and can have side effects,” said Natasha Seaton, first author and a PhD student at the Institute of Psychiatry, Psychology and Neuroscience (IoPPN) at King’s College London.
“Our study showed that interventions that treat mental health reduce levels of inflammation in the body,” she said. “This indicates that mood interventions could be a valuable tool in our approach to help those with IBD.”
The study was published online in eBioMedicine.
Analyzing Mood Interventions
Ms. Seaton and colleagues conducted a systematic review and meta-analysis of randomized controlled trials in adults with IBD that measured inflammatory biomarker levels and tested a mood intervention, including those aimed at reducing depression, anxiety, stress, or distress or improving emotional well-being.
Looking at data from 28 randomized controlled trials with 1789 participants, the research team evaluated whether mood interventions affected IBD inflammation, particularly IBD indicators such as C-reactive protein and fecal calprotectin, and other general inflammatory biomarkers.
The researchers found mood interventions significantly reduced levels of inflammatory biomarkers, compared with controls, corresponding to an 18% reduction in inflammatory biomarkers.
Psychological therapies had the best outcomes related to IBD inflammation, compared with antidepressants or exercise. These therapies included cognitive behavioral therapy, acceptance and commitment therapy, and mindfulness-based stress reduction.
Individual analyses of IBD-specific inflammatory markers found small but statistically significant reductions in C-reactive protein and fecal calprotectin after a mood intervention. This could mean mood treatments have positive effects on both inflammation and disease-specific biomarkers, the authors wrote.
In addition, interventions that had a larger positive effect on mood had a greater effect in reducing inflammatory biomarkers. This suggests that a better mood could reduce IBD inflammation, they noted.
“We know stress-related feelings can increase inflammation, and the findings suggest that by improving mood, we can reduce this type of inflammation,” said Valeria Mondelli, MD, PhD, clinical professor of psychoneuroimmunology at King’s IoPPN.
“This adds to the growing body of research demonstrating the role of inflammation in mental health and suggests that interventions working to improve mood could also have direct physical effects on levels of inflammation,” she said. “However, more research is needed to understand exact mechanisms in IBD.”
Cost Benefit
Many IBD interventions and medications can be expensive for patients, have significant negative side effects, and have a lower long-term treatment response, the authors noted. Mood interventions, whether psychological therapy or medication, could potentially reduce costs and improve both mood and inflammation.
Previous studies have indicated that psychosocial factors, as well as mood disorders such as anxiety and depression, affect IBD symptom severity and progression, the authors wrote. However, researchers still need to understand the mechanisms behind this connection, including gut-brain dynamics.
Future research should focus on interventions that have been effective at improving mood in patients with IBD, assess inflammation and disease activity at numerous timepoints, and include potential variables related to illness self-management, the authors wrote.
In addition, implementation of mood interventions for IBD management may require better continuity of care and healthcare integration.
“Integrated mental health support, alongside pharmacological treatments, may offer a more holistic approach to IBD care, potentially leading to reduced disease and healthcare costs,” said Rona Moss-Morris, PhD, senior author and professor of psychology at King’s IoPPN.
Medications taken to reduce inflammation can be costly compared with psychological therapies, she said. “Given this, including psychological interventions, such as cost-effective digital interventions, within IBD management might reduce the need for anti-inflammatory medication, resulting in an overall cost benefit.”
The study was funded by the Medical Research Council (MRC) and National Institute for Health and Care Research Maudsley Biomedical Research Centre, which is hosted by South London and Maudsley NHS Foundation Trust in partnership with King’s College London. Ms. Seaton was funded by an MRC Doctoral Training Partnership. No other interests were declared.
A version of this article appeared on Medscape.com.
, according to a new study.
“IBD is a distressing condition, and current medication that reduces inflammation is expensive and can have side effects,” said Natasha Seaton, first author and a PhD student at the Institute of Psychiatry, Psychology and Neuroscience (IoPPN) at King’s College London.
“Our study showed that interventions that treat mental health reduce levels of inflammation in the body,” she said. “This indicates that mood interventions could be a valuable tool in our approach to help those with IBD.”
The study was published online in eBioMedicine.
Analyzing Mood Interventions
Ms. Seaton and colleagues conducted a systematic review and meta-analysis of randomized controlled trials in adults with IBD that measured inflammatory biomarker levels and tested a mood intervention, including those aimed at reducing depression, anxiety, stress, or distress or improving emotional well-being.
Looking at data from 28 randomized controlled trials with 1789 participants, the research team evaluated whether mood interventions affected IBD inflammation, particularly IBD indicators such as C-reactive protein and fecal calprotectin, and other general inflammatory biomarkers.
The researchers found mood interventions significantly reduced levels of inflammatory biomarkers, compared with controls, corresponding to an 18% reduction in inflammatory biomarkers.
Psychological therapies had the best outcomes related to IBD inflammation, compared with antidepressants or exercise. These therapies included cognitive behavioral therapy, acceptance and commitment therapy, and mindfulness-based stress reduction.
Individual analyses of IBD-specific inflammatory markers found small but statistically significant reductions in C-reactive protein and fecal calprotectin after a mood intervention. This could mean mood treatments have positive effects on both inflammation and disease-specific biomarkers, the authors wrote.
In addition, interventions that had a larger positive effect on mood had a greater effect in reducing inflammatory biomarkers. This suggests that a better mood could reduce IBD inflammation, they noted.
“We know stress-related feelings can increase inflammation, and the findings suggest that by improving mood, we can reduce this type of inflammation,” said Valeria Mondelli, MD, PhD, clinical professor of psychoneuroimmunology at King’s IoPPN.
“This adds to the growing body of research demonstrating the role of inflammation in mental health and suggests that interventions working to improve mood could also have direct physical effects on levels of inflammation,” she said. “However, more research is needed to understand exact mechanisms in IBD.”
Cost Benefit
Many IBD interventions and medications can be expensive for patients, have significant negative side effects, and have a lower long-term treatment response, the authors noted. Mood interventions, whether psychological therapy or medication, could potentially reduce costs and improve both mood and inflammation.
Previous studies have indicated that psychosocial factors, as well as mood disorders such as anxiety and depression, affect IBD symptom severity and progression, the authors wrote. However, researchers still need to understand the mechanisms behind this connection, including gut-brain dynamics.
Future research should focus on interventions that have been effective at improving mood in patients with IBD, assess inflammation and disease activity at numerous timepoints, and include potential variables related to illness self-management, the authors wrote.
In addition, implementation of mood interventions for IBD management may require better continuity of care and healthcare integration.
“Integrated mental health support, alongside pharmacological treatments, may offer a more holistic approach to IBD care, potentially leading to reduced disease and healthcare costs,” said Rona Moss-Morris, PhD, senior author and professor of psychology at King’s IoPPN.
Medications taken to reduce inflammation can be costly compared with psychological therapies, she said. “Given this, including psychological interventions, such as cost-effective digital interventions, within IBD management might reduce the need for anti-inflammatory medication, resulting in an overall cost benefit.”
The study was funded by the Medical Research Council (MRC) and National Institute for Health and Care Research Maudsley Biomedical Research Centre, which is hosted by South London and Maudsley NHS Foundation Trust in partnership with King’s College London. Ms. Seaton was funded by an MRC Doctoral Training Partnership. No other interests were declared.
A version of this article appeared on Medscape.com.
, according to a new study.
“IBD is a distressing condition, and current medication that reduces inflammation is expensive and can have side effects,” said Natasha Seaton, first author and a PhD student at the Institute of Psychiatry, Psychology and Neuroscience (IoPPN) at King’s College London.
“Our study showed that interventions that treat mental health reduce levels of inflammation in the body,” she said. “This indicates that mood interventions could be a valuable tool in our approach to help those with IBD.”
The study was published online in eBioMedicine.
Analyzing Mood Interventions
Ms. Seaton and colleagues conducted a systematic review and meta-analysis of randomized controlled trials in adults with IBD that measured inflammatory biomarker levels and tested a mood intervention, including those aimed at reducing depression, anxiety, stress, or distress or improving emotional well-being.
Looking at data from 28 randomized controlled trials with 1789 participants, the research team evaluated whether mood interventions affected IBD inflammation, particularly IBD indicators such as C-reactive protein and fecal calprotectin, and other general inflammatory biomarkers.
The researchers found mood interventions significantly reduced levels of inflammatory biomarkers, compared with controls, corresponding to an 18% reduction in inflammatory biomarkers.
Psychological therapies had the best outcomes related to IBD inflammation, compared with antidepressants or exercise. These therapies included cognitive behavioral therapy, acceptance and commitment therapy, and mindfulness-based stress reduction.
Individual analyses of IBD-specific inflammatory markers found small but statistically significant reductions in C-reactive protein and fecal calprotectin after a mood intervention. This could mean mood treatments have positive effects on both inflammation and disease-specific biomarkers, the authors wrote.
In addition, interventions that had a larger positive effect on mood had a greater effect in reducing inflammatory biomarkers. This suggests that a better mood could reduce IBD inflammation, they noted.
“We know stress-related feelings can increase inflammation, and the findings suggest that by improving mood, we can reduce this type of inflammation,” said Valeria Mondelli, MD, PhD, clinical professor of psychoneuroimmunology at King’s IoPPN.
“This adds to the growing body of research demonstrating the role of inflammation in mental health and suggests that interventions working to improve mood could also have direct physical effects on levels of inflammation,” she said. “However, more research is needed to understand exact mechanisms in IBD.”
Cost Benefit
Many IBD interventions and medications can be expensive for patients, have significant negative side effects, and have a lower long-term treatment response, the authors noted. Mood interventions, whether psychological therapy or medication, could potentially reduce costs and improve both mood and inflammation.
Previous studies have indicated that psychosocial factors, as well as mood disorders such as anxiety and depression, affect IBD symptom severity and progression, the authors wrote. However, researchers still need to understand the mechanisms behind this connection, including gut-brain dynamics.
Future research should focus on interventions that have been effective at improving mood in patients with IBD, assess inflammation and disease activity at numerous timepoints, and include potential variables related to illness self-management, the authors wrote.
In addition, implementation of mood interventions for IBD management may require better continuity of care and healthcare integration.
“Integrated mental health support, alongside pharmacological treatments, may offer a more holistic approach to IBD care, potentially leading to reduced disease and healthcare costs,” said Rona Moss-Morris, PhD, senior author and professor of psychology at King’s IoPPN.
Medications taken to reduce inflammation can be costly compared with psychological therapies, she said. “Given this, including psychological interventions, such as cost-effective digital interventions, within IBD management might reduce the need for anti-inflammatory medication, resulting in an overall cost benefit.”
The study was funded by the Medical Research Council (MRC) and National Institute for Health and Care Research Maudsley Biomedical Research Centre, which is hosted by South London and Maudsley NHS Foundation Trust in partnership with King’s College London. Ms. Seaton was funded by an MRC Doctoral Training Partnership. No other interests were declared.
A version of this article appeared on Medscape.com.
Biosimilars Have Driven Down Cost of Infliximab
LAS VEGAS — , according to two new retrospective analyses that separately looked at private insurance and Medicare patients.
“It is interesting to see that biosimilar use is increasing in patients with Medicare insurance in the U.S. since introduction into the market. This shows that clinicians are getting more comfortable with their use in clinical practice,” said Sara Horst, MD, associate professor of gastroenterology, hepatology, and nutrition at Vanderbilt University Medical Center in Nashville, Tennessee. That “is a continued important question in use, especially in the older population,” Dr. Horst said at the poster session where the two studies were presented, at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
One study examined Medicare part D participants. The researchers included infliximab prescriptions between 2013 and 2021, with a break in 2017 when biosimilars were first introduced. Between 2013 and 2017, there was a 93.7% annual increase in infliximab cost, and a 29.0% increase in annual claims. Between 2017 and 2021, those numbers declined to 23.9% and 12.1%, respectively. The researchers also examined trends by individual states and found a wide range of results. “Nationwide, it seems to be doing exactly what you’d expect, and I’d say it’s encouraging. This is exactly what you want with the introduction of biosimilars, and this is what you want for the patient population. But there are some states that have not really kept up with the rest of the country, and may need to look further into what types of trends are there,” said Modan Goldman, who presented the study. He is a medical student at Carle Illinois College of Medicine in Urbana, Illinois.
The other study looked at use of infliximab versus a biosimilar between 2015 and 2021 in 42,009 patients 64 or younger, drawing data from Merative Marketscan Commercial Claims and Encounters Database. They excluded government-funded insurance. Between January 2015 and December 2017, the cost of a vial of infliximab increased by $6.31 per month, reaching a maxim cost of $1,491 per vial. In January 2018, the cost decreased by $62 per vial, with a downward trend after that of $12.93 per vial per month.
“[Getting] access to these drugs, especially for pediatric IBD, has been an uphill battle with insurance companies and getting the medication and the doses we want,” said Samantha Paglinco, DO, who presented the Marketscan study.
The findings demonstrated clear cost reductions. “At the beginning of our period, infliximab originator Remicade was a little over $1,200 per vial, and at the end of our period of 2021, we expected it to be around $1,800 per vial. However, with the introduction of multiple infliximab biosimilars, we did see it come down to $800 per vial, which generated a savings of $1,000, which is pretty significant. We’re hoping that by generating overall cost savings to insurance companies, this will allow for greater access, because we know infliximab is such a good medication for pediatric IBD and for many other autoimmune conditions,” said Dr. Paglinco, a pediatric GI fellow at Nationwide Children’s Hospital in Columbus, Ohio.
The decrease in the cost of medication per vial in both the originator and biosimilar is an important finding in light of cost containment strategies, although Dr. Horst pointed out that the study didn’t determine if there were any savings to patients. “That is what clinicians and patients care about the most. I worry that while these savings were seen at insurance and health care organizational levels, patients may not have experienced any cost savings, and this is something we need to consider in the future,” she said.
The FDA created the biosimilar regulatory pathway in 2010 in an effort to reduce costs. “And they have. The cost savings however, has not directly translated to the individual patient in their copays or necessarily in changes to access when prescribing. Nor has it allowed dose escalation more easily, which is frequently needed,” said David T. Rubin, MD, AGAF, who was asked for comment.
Although the changes in prescribing patterns may reflect cost savings to patients, there are some limits to this interpretation, according to Dr. Rubin, of the University of Chicago. Payers are often the ones who determine a switch to biosimilar. The study also does not account for disease activity or phenotype, which can be an important confounder. Newer biologics that have come on to the market in recent years may also have affected the prescribing patterns for infliximab.
Nevertheless, an association between introduction of biosimilars and declining cost in the originator product (Remicade) is welcome, according to Dr. Rubin. “This is nice to see and we have seen it in our practices,” he said.
Unfortunately, “it has also not been seen to translate into reduced costs for patients nor has it been associated with increased access or changes in payer policies to allow these drugs to be used, or dose escalations to occur,” Dr. Rubin added.
Dr. Paglinco and Mr. Goldman have no relevant financial disclosures. Dr. Horst has consulted for Janssen, Takeda, Bristol-Myers Squibb, and Abbvie. Dr. Rubin has received grant support from Takeda and has consulted for Abbvie, Bristol-Myers Squibb, Janssen Pharmaceuticals, Lilly, Pfizer, and Takeda.
LAS VEGAS — , according to two new retrospective analyses that separately looked at private insurance and Medicare patients.
“It is interesting to see that biosimilar use is increasing in patients with Medicare insurance in the U.S. since introduction into the market. This shows that clinicians are getting more comfortable with their use in clinical practice,” said Sara Horst, MD, associate professor of gastroenterology, hepatology, and nutrition at Vanderbilt University Medical Center in Nashville, Tennessee. That “is a continued important question in use, especially in the older population,” Dr. Horst said at the poster session where the two studies were presented, at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
One study examined Medicare part D participants. The researchers included infliximab prescriptions between 2013 and 2021, with a break in 2017 when biosimilars were first introduced. Between 2013 and 2017, there was a 93.7% annual increase in infliximab cost, and a 29.0% increase in annual claims. Between 2017 and 2021, those numbers declined to 23.9% and 12.1%, respectively. The researchers also examined trends by individual states and found a wide range of results. “Nationwide, it seems to be doing exactly what you’d expect, and I’d say it’s encouraging. This is exactly what you want with the introduction of biosimilars, and this is what you want for the patient population. But there are some states that have not really kept up with the rest of the country, and may need to look further into what types of trends are there,” said Modan Goldman, who presented the study. He is a medical student at Carle Illinois College of Medicine in Urbana, Illinois.
The other study looked at use of infliximab versus a biosimilar between 2015 and 2021 in 42,009 patients 64 or younger, drawing data from Merative Marketscan Commercial Claims and Encounters Database. They excluded government-funded insurance. Between January 2015 and December 2017, the cost of a vial of infliximab increased by $6.31 per month, reaching a maxim cost of $1,491 per vial. In January 2018, the cost decreased by $62 per vial, with a downward trend after that of $12.93 per vial per month.
“[Getting] access to these drugs, especially for pediatric IBD, has been an uphill battle with insurance companies and getting the medication and the doses we want,” said Samantha Paglinco, DO, who presented the Marketscan study.
The findings demonstrated clear cost reductions. “At the beginning of our period, infliximab originator Remicade was a little over $1,200 per vial, and at the end of our period of 2021, we expected it to be around $1,800 per vial. However, with the introduction of multiple infliximab biosimilars, we did see it come down to $800 per vial, which generated a savings of $1,000, which is pretty significant. We’re hoping that by generating overall cost savings to insurance companies, this will allow for greater access, because we know infliximab is such a good medication for pediatric IBD and for many other autoimmune conditions,” said Dr. Paglinco, a pediatric GI fellow at Nationwide Children’s Hospital in Columbus, Ohio.
The decrease in the cost of medication per vial in both the originator and biosimilar is an important finding in light of cost containment strategies, although Dr. Horst pointed out that the study didn’t determine if there were any savings to patients. “That is what clinicians and patients care about the most. I worry that while these savings were seen at insurance and health care organizational levels, patients may not have experienced any cost savings, and this is something we need to consider in the future,” she said.
The FDA created the biosimilar regulatory pathway in 2010 in an effort to reduce costs. “And they have. The cost savings however, has not directly translated to the individual patient in their copays or necessarily in changes to access when prescribing. Nor has it allowed dose escalation more easily, which is frequently needed,” said David T. Rubin, MD, AGAF, who was asked for comment.
Although the changes in prescribing patterns may reflect cost savings to patients, there are some limits to this interpretation, according to Dr. Rubin, of the University of Chicago. Payers are often the ones who determine a switch to biosimilar. The study also does not account for disease activity or phenotype, which can be an important confounder. Newer biologics that have come on to the market in recent years may also have affected the prescribing patterns for infliximab.
Nevertheless, an association between introduction of biosimilars and declining cost in the originator product (Remicade) is welcome, according to Dr. Rubin. “This is nice to see and we have seen it in our practices,” he said.
Unfortunately, “it has also not been seen to translate into reduced costs for patients nor has it been associated with increased access or changes in payer policies to allow these drugs to be used, or dose escalations to occur,” Dr. Rubin added.
Dr. Paglinco and Mr. Goldman have no relevant financial disclosures. Dr. Horst has consulted for Janssen, Takeda, Bristol-Myers Squibb, and Abbvie. Dr. Rubin has received grant support from Takeda and has consulted for Abbvie, Bristol-Myers Squibb, Janssen Pharmaceuticals, Lilly, Pfizer, and Takeda.
LAS VEGAS — , according to two new retrospective analyses that separately looked at private insurance and Medicare patients.
“It is interesting to see that biosimilar use is increasing in patients with Medicare insurance in the U.S. since introduction into the market. This shows that clinicians are getting more comfortable with their use in clinical practice,” said Sara Horst, MD, associate professor of gastroenterology, hepatology, and nutrition at Vanderbilt University Medical Center in Nashville, Tennessee. That “is a continued important question in use, especially in the older population,” Dr. Horst said at the poster session where the two studies were presented, at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
One study examined Medicare part D participants. The researchers included infliximab prescriptions between 2013 and 2021, with a break in 2017 when biosimilars were first introduced. Between 2013 and 2017, there was a 93.7% annual increase in infliximab cost, and a 29.0% increase in annual claims. Between 2017 and 2021, those numbers declined to 23.9% and 12.1%, respectively. The researchers also examined trends by individual states and found a wide range of results. “Nationwide, it seems to be doing exactly what you’d expect, and I’d say it’s encouraging. This is exactly what you want with the introduction of biosimilars, and this is what you want for the patient population. But there are some states that have not really kept up with the rest of the country, and may need to look further into what types of trends are there,” said Modan Goldman, who presented the study. He is a medical student at Carle Illinois College of Medicine in Urbana, Illinois.
The other study looked at use of infliximab versus a biosimilar between 2015 and 2021 in 42,009 patients 64 or younger, drawing data from Merative Marketscan Commercial Claims and Encounters Database. They excluded government-funded insurance. Between January 2015 and December 2017, the cost of a vial of infliximab increased by $6.31 per month, reaching a maxim cost of $1,491 per vial. In January 2018, the cost decreased by $62 per vial, with a downward trend after that of $12.93 per vial per month.
“[Getting] access to these drugs, especially for pediatric IBD, has been an uphill battle with insurance companies and getting the medication and the doses we want,” said Samantha Paglinco, DO, who presented the Marketscan study.
The findings demonstrated clear cost reductions. “At the beginning of our period, infliximab originator Remicade was a little over $1,200 per vial, and at the end of our period of 2021, we expected it to be around $1,800 per vial. However, with the introduction of multiple infliximab biosimilars, we did see it come down to $800 per vial, which generated a savings of $1,000, which is pretty significant. We’re hoping that by generating overall cost savings to insurance companies, this will allow for greater access, because we know infliximab is such a good medication for pediatric IBD and for many other autoimmune conditions,” said Dr. Paglinco, a pediatric GI fellow at Nationwide Children’s Hospital in Columbus, Ohio.
The decrease in the cost of medication per vial in both the originator and biosimilar is an important finding in light of cost containment strategies, although Dr. Horst pointed out that the study didn’t determine if there were any savings to patients. “That is what clinicians and patients care about the most. I worry that while these savings were seen at insurance and health care organizational levels, patients may not have experienced any cost savings, and this is something we need to consider in the future,” she said.
The FDA created the biosimilar regulatory pathway in 2010 in an effort to reduce costs. “And they have. The cost savings however, has not directly translated to the individual patient in their copays or necessarily in changes to access when prescribing. Nor has it allowed dose escalation more easily, which is frequently needed,” said David T. Rubin, MD, AGAF, who was asked for comment.
Although the changes in prescribing patterns may reflect cost savings to patients, there are some limits to this interpretation, according to Dr. Rubin, of the University of Chicago. Payers are often the ones who determine a switch to biosimilar. The study also does not account for disease activity or phenotype, which can be an important confounder. Newer biologics that have come on to the market in recent years may also have affected the prescribing patterns for infliximab.
Nevertheless, an association between introduction of biosimilars and declining cost in the originator product (Remicade) is welcome, according to Dr. Rubin. “This is nice to see and we have seen it in our practices,” he said.
Unfortunately, “it has also not been seen to translate into reduced costs for patients nor has it been associated with increased access or changes in payer policies to allow these drugs to be used, or dose escalations to occur,” Dr. Rubin added.
Dr. Paglinco and Mr. Goldman have no relevant financial disclosures. Dr. Horst has consulted for Janssen, Takeda, Bristol-Myers Squibb, and Abbvie. Dr. Rubin has received grant support from Takeda and has consulted for Abbvie, Bristol-Myers Squibb, Janssen Pharmaceuticals, Lilly, Pfizer, and Takeda.
FROM CROHN’S AND COLITIS CONGRESS
In Refractory IBD, Combination Therapies Appear Safe, Effective
LAS VEGAS — . The study updates a meta-analysis published in 2022, which included 13 studies. The new work included 23 studies that looked at 8 different combinations.
There is a potential concern that the high adverse event rates seen in biologics could be compounded when they are used in combination, according to Ali Osman, MD, who presented the results at a poster session at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “Theoretically, you should have more side effects or more serious side effects, but interestingly we didn’t find major side effects. I think the key message is that the combinations of biologic agents are promising in terms of efficacy. In terms of adverse events, it doesn’t lead to major adverse events,” said Dr. Osman, an instructor at Washington University School of Medicine in St. Louis.
Although the study did not directly compare the combinations, it did find potential differences in efficacy. “Our most effective [combination] in terms of response and remission was a combination of ustekinumab and an anti-TNF agent with a combined rate of 81.6%. Our lowest adverse events rate were [with the combination of] tofacitinib and vedolizumab,” said Dr. Osman.
The research is a useful update, according to David T. Rubin, MD, AGAF, who was asked for comment. “This has been explored before, but this is a nice effort to describe and try to compare studies of combination biological therapies or biologicals combined with [the JAK inhibitor]. This is to further explore the efforts being made to break the therapeutic ceiling by combining mechanisms, treat IBD and extra-intestinal manifestations with multiple agents simultaneously, and to explore novel treatment strategies,” said Dr. Rubin, professor of medicine and director of the Inflammatory Bowel Disease Center at University of Chicago Medicine.
He noted that the meta-analysis is limited by heterogeneity among the studies, many of which were case series that had been re-analyzed. The update included some prospective proof-of-concept studies of interest that were not in the earlier meta-analysis, including VEGA (anti-IL13 guselkumab plus anti-TNF golimumab versus either drug alone), and EXPLORER (vedolizumab, adalimumab, methotrexate), as well as a study of infliximab combined with natalizumab.
“We await the ongoing prospective trials of dual targeted therapies and novel designs for a future that will undoubtedly include thoughtful and rational combinations,” said Dr. Rubin.
The review included 23 studies that had a minimum of two patients who were treated with a combination of two biologics or a biologic and tofacitinib. The biologics included the anti-TNF antibodies adalimumab, certolizumab pegol, golimumab, and infliximab; as well as guselkumab, natalizumab, ustekinumab, and vedolizumab. Overall, the studies included 531 patients who underwent 543 therapeutic trials, using 8 different combinations.
The highest pooled clinical response observed was 81.6% with ustekinumab combined with an anti-TNF agent (P = .04, 9 studies, 44 therapeutic trials), which also had the highest remission rate of 64.2% (P = .03).
For the treatment of Crohn’s disease, the highest pooled clinical response and remission rates were also seen with ustekinumab combined with an anti-TNF agent (8 studies, 29 therapeutic trials), at 91.6% (P = .28). In ulcerative colitis, vedolizumab plus ustekinumab had the highest pooled clinical response rate at 100.0% (P = 1.00; 4 studies, 4 treatment trials) and ustekinumab plus an anti-TNF agent F at 100.0% (P = 1.00; 4 studies, 5 treatment trials).
Tofacitinib combined with vedolizumab had the lower adverse event rate (12.5%; P = .10; 8 studies, 76 treatment trials) followed by ustekinumab and an anti-TNF agent (12.7%; P = .08; 9 studies, 43 treatment trials) and tofacitinib plus anti-TNF (13.0%; 6 studies, 27 treatment trials).
Other combinations included guselkumab plus ant-TNF (1 study; clinical response, 69.0%), natalizumab plus an anti-TNF agent (1 study, clinical response, 36.5%), tofacitinib plus an anti-TNF agent (5 studies, clinical response, 71.6%), tofacitinib plus ustekinumab (5 studies, clinical response, 70.8%), tofacitinib plus vedolizumab (8 studies, clinical response, 52.7%), vedolizumab plus an anti-TNF agent (13 studies, clinical response, 62.8%), and vedolizumab plus ustekinumab (12 studies, clinical response, 79.3%).
Dr. Osman has no relevant financial conflicts of interest. Dr. Rubin has received grant support from Takeda, and has served as a consultant for Abbvie, Bristol-Myers Squibb, Janssen Pharmaceuticals, Lilly, Pfizer, and Takeda.
LAS VEGAS — . The study updates a meta-analysis published in 2022, which included 13 studies. The new work included 23 studies that looked at 8 different combinations.
There is a potential concern that the high adverse event rates seen in biologics could be compounded when they are used in combination, according to Ali Osman, MD, who presented the results at a poster session at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “Theoretically, you should have more side effects or more serious side effects, but interestingly we didn’t find major side effects. I think the key message is that the combinations of biologic agents are promising in terms of efficacy. In terms of adverse events, it doesn’t lead to major adverse events,” said Dr. Osman, an instructor at Washington University School of Medicine in St. Louis.
Although the study did not directly compare the combinations, it did find potential differences in efficacy. “Our most effective [combination] in terms of response and remission was a combination of ustekinumab and an anti-TNF agent with a combined rate of 81.6%. Our lowest adverse events rate were [with the combination of] tofacitinib and vedolizumab,” said Dr. Osman.
The research is a useful update, according to David T. Rubin, MD, AGAF, who was asked for comment. “This has been explored before, but this is a nice effort to describe and try to compare studies of combination biological therapies or biologicals combined with [the JAK inhibitor]. This is to further explore the efforts being made to break the therapeutic ceiling by combining mechanisms, treat IBD and extra-intestinal manifestations with multiple agents simultaneously, and to explore novel treatment strategies,” said Dr. Rubin, professor of medicine and director of the Inflammatory Bowel Disease Center at University of Chicago Medicine.
He noted that the meta-analysis is limited by heterogeneity among the studies, many of which were case series that had been re-analyzed. The update included some prospective proof-of-concept studies of interest that were not in the earlier meta-analysis, including VEGA (anti-IL13 guselkumab plus anti-TNF golimumab versus either drug alone), and EXPLORER (vedolizumab, adalimumab, methotrexate), as well as a study of infliximab combined with natalizumab.
“We await the ongoing prospective trials of dual targeted therapies and novel designs for a future that will undoubtedly include thoughtful and rational combinations,” said Dr. Rubin.
The review included 23 studies that had a minimum of two patients who were treated with a combination of two biologics or a biologic and tofacitinib. The biologics included the anti-TNF antibodies adalimumab, certolizumab pegol, golimumab, and infliximab; as well as guselkumab, natalizumab, ustekinumab, and vedolizumab. Overall, the studies included 531 patients who underwent 543 therapeutic trials, using 8 different combinations.
The highest pooled clinical response observed was 81.6% with ustekinumab combined with an anti-TNF agent (P = .04, 9 studies, 44 therapeutic trials), which also had the highest remission rate of 64.2% (P = .03).
For the treatment of Crohn’s disease, the highest pooled clinical response and remission rates were also seen with ustekinumab combined with an anti-TNF agent (8 studies, 29 therapeutic trials), at 91.6% (P = .28). In ulcerative colitis, vedolizumab plus ustekinumab had the highest pooled clinical response rate at 100.0% (P = 1.00; 4 studies, 4 treatment trials) and ustekinumab plus an anti-TNF agent F at 100.0% (P = 1.00; 4 studies, 5 treatment trials).
Tofacitinib combined with vedolizumab had the lower adverse event rate (12.5%; P = .10; 8 studies, 76 treatment trials) followed by ustekinumab and an anti-TNF agent (12.7%; P = .08; 9 studies, 43 treatment trials) and tofacitinib plus anti-TNF (13.0%; 6 studies, 27 treatment trials).
Other combinations included guselkumab plus ant-TNF (1 study; clinical response, 69.0%), natalizumab plus an anti-TNF agent (1 study, clinical response, 36.5%), tofacitinib plus an anti-TNF agent (5 studies, clinical response, 71.6%), tofacitinib plus ustekinumab (5 studies, clinical response, 70.8%), tofacitinib plus vedolizumab (8 studies, clinical response, 52.7%), vedolizumab plus an anti-TNF agent (13 studies, clinical response, 62.8%), and vedolizumab plus ustekinumab (12 studies, clinical response, 79.3%).
Dr. Osman has no relevant financial conflicts of interest. Dr. Rubin has received grant support from Takeda, and has served as a consultant for Abbvie, Bristol-Myers Squibb, Janssen Pharmaceuticals, Lilly, Pfizer, and Takeda.
LAS VEGAS — . The study updates a meta-analysis published in 2022, which included 13 studies. The new work included 23 studies that looked at 8 different combinations.
There is a potential concern that the high adverse event rates seen in biologics could be compounded when they are used in combination, according to Ali Osman, MD, who presented the results at a poster session at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “Theoretically, you should have more side effects or more serious side effects, but interestingly we didn’t find major side effects. I think the key message is that the combinations of biologic agents are promising in terms of efficacy. In terms of adverse events, it doesn’t lead to major adverse events,” said Dr. Osman, an instructor at Washington University School of Medicine in St. Louis.
Although the study did not directly compare the combinations, it did find potential differences in efficacy. “Our most effective [combination] in terms of response and remission was a combination of ustekinumab and an anti-TNF agent with a combined rate of 81.6%. Our lowest adverse events rate were [with the combination of] tofacitinib and vedolizumab,” said Dr. Osman.
The research is a useful update, according to David T. Rubin, MD, AGAF, who was asked for comment. “This has been explored before, but this is a nice effort to describe and try to compare studies of combination biological therapies or biologicals combined with [the JAK inhibitor]. This is to further explore the efforts being made to break the therapeutic ceiling by combining mechanisms, treat IBD and extra-intestinal manifestations with multiple agents simultaneously, and to explore novel treatment strategies,” said Dr. Rubin, professor of medicine and director of the Inflammatory Bowel Disease Center at University of Chicago Medicine.
He noted that the meta-analysis is limited by heterogeneity among the studies, many of which were case series that had been re-analyzed. The update included some prospective proof-of-concept studies of interest that were not in the earlier meta-analysis, including VEGA (anti-IL13 guselkumab plus anti-TNF golimumab versus either drug alone), and EXPLORER (vedolizumab, adalimumab, methotrexate), as well as a study of infliximab combined with natalizumab.
“We await the ongoing prospective trials of dual targeted therapies and novel designs for a future that will undoubtedly include thoughtful and rational combinations,” said Dr. Rubin.
The review included 23 studies that had a minimum of two patients who were treated with a combination of two biologics or a biologic and tofacitinib. The biologics included the anti-TNF antibodies adalimumab, certolizumab pegol, golimumab, and infliximab; as well as guselkumab, natalizumab, ustekinumab, and vedolizumab. Overall, the studies included 531 patients who underwent 543 therapeutic trials, using 8 different combinations.
The highest pooled clinical response observed was 81.6% with ustekinumab combined with an anti-TNF agent (P = .04, 9 studies, 44 therapeutic trials), which also had the highest remission rate of 64.2% (P = .03).
For the treatment of Crohn’s disease, the highest pooled clinical response and remission rates were also seen with ustekinumab combined with an anti-TNF agent (8 studies, 29 therapeutic trials), at 91.6% (P = .28). In ulcerative colitis, vedolizumab plus ustekinumab had the highest pooled clinical response rate at 100.0% (P = 1.00; 4 studies, 4 treatment trials) and ustekinumab plus an anti-TNF agent F at 100.0% (P = 1.00; 4 studies, 5 treatment trials).
Tofacitinib combined with vedolizumab had the lower adverse event rate (12.5%; P = .10; 8 studies, 76 treatment trials) followed by ustekinumab and an anti-TNF agent (12.7%; P = .08; 9 studies, 43 treatment trials) and tofacitinib plus anti-TNF (13.0%; 6 studies, 27 treatment trials).
Other combinations included guselkumab plus ant-TNF (1 study; clinical response, 69.0%), natalizumab plus an anti-TNF agent (1 study, clinical response, 36.5%), tofacitinib plus an anti-TNF agent (5 studies, clinical response, 71.6%), tofacitinib plus ustekinumab (5 studies, clinical response, 70.8%), tofacitinib plus vedolizumab (8 studies, clinical response, 52.7%), vedolizumab plus an anti-TNF agent (13 studies, clinical response, 62.8%), and vedolizumab plus ustekinumab (12 studies, clinical response, 79.3%).
Dr. Osman has no relevant financial conflicts of interest. Dr. Rubin has received grant support from Takeda, and has served as a consultant for Abbvie, Bristol-Myers Squibb, Janssen Pharmaceuticals, Lilly, Pfizer, and Takeda.
FROM CROHN’S & COLITIS CONGRESS
Telephone Best for Switching Patients to New Colonoscopy Intervals
In an article published in Clinical Gastroenterology and Hepatology, a group led by Jeffrey K. Lee, MD, MPH, a gastroenterologist at Kaiser Permanente Medical Center in San Francisco, reported the following 60-day response rates for the three contact methods in potentially transitioning more than 600 post-polypectomy patients to the new interval:
- Telephone: 64.5%
- Secure messaging: 51.7%
- Mailed letter: 31.3%
Compared with letter outreach, overall rate differences were significant for telephone (18.1%) and secure message outreach (13.1%).
Such interventions are widely used, the authors noted , but have not been compared for efficacy terms of communicating updated colonoscopy intervals.
The trial’s aim was to inform low-risk patients of the recommended interval update from 5 years — used since the 1990s — to 7-10 years. Given a choice, more patients opted to transition to the 10-year surveillance interval in the telephone (37%) and secure messaging arms (32.%) compared with mailed-letter arm (18.9%).
In addition to telephone and secure messaging outreach, factors positively associated with adoption of the 10-year interval were a positive fecal immunochemical test–based index colonoscopy and increasing age. Patients with these characteristics may be biased toward avoiding colonoscopy if not medically necessary, the authors conjectured.
Inversely associated factors included Asian or Pacific Islander race (odds ratio .58), Hispanic ethnicity (OR .40), and a higher Charlson comorbidity score of 2 vs 0 (OR .43).
Possible explanations for the race and ethnicity associations include gaps in culturally component care, lack of engagement with the English-based outreach approaches, and medical mistrust, the authors said.
“In this study, we gave all our patients an option to either extend their surveillance interval to current guideline recommendations or continue with their old interval, and some chose to do that,” Dr. Lee said in an interview. “Patients really appreciated having a choice and to be informed about the latest guideline changes.”
“A critical challenge to health systems is how to effectively de-implement outdated surveillance recommendations for low-risk patients who have a 5-year follow-up interval and potentially transition them to the recommended 7- to 10-year interval,” Dr. Lee and colleagues wrote.
More than 5 million surveillance colonoscopies are performed annually in US patients with a history of adenomas, the main precursor lesion for colorectal cancer, the authors noted.
With the recent guidelines issued in 2020 by the US Multi-Society Task Force on Colorectal Cancer lengthening the follow-up interval to 7-10 years , physicians are being advised to reevaluate low-risk patients previously scheduled with 5-year surveillance and provide an updated recommendation for follow-up.
Study Details
The three-arm pragmatic randomized trial was conducted in low-risk patients 54-70 years of age with one or two small (< 10 mm) tubular adenomas at baseline colonoscopy. Participants due for 5-year surveillance in 2022 were randomly assigned to one of three outreach arms: telephone (n = 200], secure messaging (n = 203), and mailed letter (n = 201). Stratified by age, sex, race, and ethnicity, participants could change their assigned interval to 10 years or continue with their previously scheduled 5-year interval.
As to economic considerations, the authors said that telephone may be the costliest form of outreach in terms of staffing resources. “We don’t know because we did not conduct a formal cost-effectiveness analysis,” Dr. Lee said. “However, we do know phone outreach requires a lot of personnel effort, which is why we also explored the less costly option of secure messaging/email.”
But based on the findings, telephone outreach would be a reasonable approach to update patients on post-polypectomy surveillance guideline changes if secure messaging or text messaging isn’t available, he added.
Downsides to Retroactive Changes?
Commenting on the study but not involved in it, Nabil M. Mansour, MD, an assistant professor and director of the McNair General GI Clinic at Baylor College of Medicine in Houston, noted that unlike Kaiser Permanente, his center decided against an overall effort to switch patients colonoscopied before the release of the new guidelines over to the new interval.
“Several of our physicians may have chosen to recommend a 5-year interval specifically for a variety of reasons and we felt going back, and making a blanket change to everyone’s interval retrospectively might create confusion and frustration and might actually delay the colonoscopies of some patients for which their doctors had a very good, legitimate reason to recommend a 5-year interval,” he said in an interview.
Dr. Mansour added that no difficulties were encountered in getting patients to agree to a 10-year interval. In his view telephone communication or in-person clinic visits are likely the most effective ways but both are more labor-intensive than automated patient portal messages. “I do not think traditional snail mail is effective.” His clinic uses automatic EMR reminders.
Offering another perspective on the study, Aditya Sreenivasan, MD, a gastroenterologist at Northwell Health in New York City, said his center has not reached out to correct the old intervals. “When I see a patient who previously had a colonoscopy with another physician, I always follow the previous recommendation for when the next colonoscopy should be, regardless of whether or not it technically meets guideline recommendations,” he told this news organization. “I do this because I was not there during the procedure and am not aware of any circumstances that would require a shorter interval that may not be apparent from the report.”
While he agrees with the new guidelines, Dr. Sreenivasan is “not sure if retroactively changing intervals is beneficial to patients, as the presence of guidelines may subconsciously influence the behavior of the endoscopist at the time of the procedure. For example, if a patient has a technically challenging colonoscopy and the endoscopist is running late, the endoscopist may drop their guard once they find a polyp and miss 1-2 additional small polyps that they would have spent more time looking for if they knew their next one would be in 10 years instead of 5.”
As for notification method, despite the logistical downside of taking dedicated staff time to make telephone calls, Dr. Sreenivasan said, “I think having a conversation with the patient directly is a much better way to communicate this information as it allows the patient to ask and answer questions. Things like tone of voice can provide reassurance that one cannot get via email.” Looking to the future, the study authors acknowledged that combinations of initial and reminder outreach approaches — for example, a mailed letter followed by secure message or telephone call — could potentially yield higher response rates and/or adoption rates than they observed. And a longer follow-up period with additional reminders may have produced higher yields. Additional studies are needed to optimize outreach approaches and to understand patient barriers to adopting the new guideline recommendations in different healthcare settings.
The study was supported by a Delivery Science grant from the Kaiser Permanente Northern California.
The authors disclosed no conflicts of interest. Dr. Mansour and Dr. Sreenivasan disclosed no conflicts of interest relevant to their comments.
In an article published in Clinical Gastroenterology and Hepatology, a group led by Jeffrey K. Lee, MD, MPH, a gastroenterologist at Kaiser Permanente Medical Center in San Francisco, reported the following 60-day response rates for the three contact methods in potentially transitioning more than 600 post-polypectomy patients to the new interval:
- Telephone: 64.5%
- Secure messaging: 51.7%
- Mailed letter: 31.3%
Compared with letter outreach, overall rate differences were significant for telephone (18.1%) and secure message outreach (13.1%).
Such interventions are widely used, the authors noted , but have not been compared for efficacy terms of communicating updated colonoscopy intervals.
The trial’s aim was to inform low-risk patients of the recommended interval update from 5 years — used since the 1990s — to 7-10 years. Given a choice, more patients opted to transition to the 10-year surveillance interval in the telephone (37%) and secure messaging arms (32.%) compared with mailed-letter arm (18.9%).
In addition to telephone and secure messaging outreach, factors positively associated with adoption of the 10-year interval were a positive fecal immunochemical test–based index colonoscopy and increasing age. Patients with these characteristics may be biased toward avoiding colonoscopy if not medically necessary, the authors conjectured.
Inversely associated factors included Asian or Pacific Islander race (odds ratio .58), Hispanic ethnicity (OR .40), and a higher Charlson comorbidity score of 2 vs 0 (OR .43).
Possible explanations for the race and ethnicity associations include gaps in culturally component care, lack of engagement with the English-based outreach approaches, and medical mistrust, the authors said.
“In this study, we gave all our patients an option to either extend their surveillance interval to current guideline recommendations or continue with their old interval, and some chose to do that,” Dr. Lee said in an interview. “Patients really appreciated having a choice and to be informed about the latest guideline changes.”
“A critical challenge to health systems is how to effectively de-implement outdated surveillance recommendations for low-risk patients who have a 5-year follow-up interval and potentially transition them to the recommended 7- to 10-year interval,” Dr. Lee and colleagues wrote.
More than 5 million surveillance colonoscopies are performed annually in US patients with a history of adenomas, the main precursor lesion for colorectal cancer, the authors noted.
With the recent guidelines issued in 2020 by the US Multi-Society Task Force on Colorectal Cancer lengthening the follow-up interval to 7-10 years , physicians are being advised to reevaluate low-risk patients previously scheduled with 5-year surveillance and provide an updated recommendation for follow-up.
Study Details
The three-arm pragmatic randomized trial was conducted in low-risk patients 54-70 years of age with one or two small (< 10 mm) tubular adenomas at baseline colonoscopy. Participants due for 5-year surveillance in 2022 were randomly assigned to one of three outreach arms: telephone (n = 200], secure messaging (n = 203), and mailed letter (n = 201). Stratified by age, sex, race, and ethnicity, participants could change their assigned interval to 10 years or continue with their previously scheduled 5-year interval.
As to economic considerations, the authors said that telephone may be the costliest form of outreach in terms of staffing resources. “We don’t know because we did not conduct a formal cost-effectiveness analysis,” Dr. Lee said. “However, we do know phone outreach requires a lot of personnel effort, which is why we also explored the less costly option of secure messaging/email.”
But based on the findings, telephone outreach would be a reasonable approach to update patients on post-polypectomy surveillance guideline changes if secure messaging or text messaging isn’t available, he added.
Downsides to Retroactive Changes?
Commenting on the study but not involved in it, Nabil M. Mansour, MD, an assistant professor and director of the McNair General GI Clinic at Baylor College of Medicine in Houston, noted that unlike Kaiser Permanente, his center decided against an overall effort to switch patients colonoscopied before the release of the new guidelines over to the new interval.
“Several of our physicians may have chosen to recommend a 5-year interval specifically for a variety of reasons and we felt going back, and making a blanket change to everyone’s interval retrospectively might create confusion and frustration and might actually delay the colonoscopies of some patients for which their doctors had a very good, legitimate reason to recommend a 5-year interval,” he said in an interview.
Dr. Mansour added that no difficulties were encountered in getting patients to agree to a 10-year interval. In his view telephone communication or in-person clinic visits are likely the most effective ways but both are more labor-intensive than automated patient portal messages. “I do not think traditional snail mail is effective.” His clinic uses automatic EMR reminders.
Offering another perspective on the study, Aditya Sreenivasan, MD, a gastroenterologist at Northwell Health in New York City, said his center has not reached out to correct the old intervals. “When I see a patient who previously had a colonoscopy with another physician, I always follow the previous recommendation for when the next colonoscopy should be, regardless of whether or not it technically meets guideline recommendations,” he told this news organization. “I do this because I was not there during the procedure and am not aware of any circumstances that would require a shorter interval that may not be apparent from the report.”
While he agrees with the new guidelines, Dr. Sreenivasan is “not sure if retroactively changing intervals is beneficial to patients, as the presence of guidelines may subconsciously influence the behavior of the endoscopist at the time of the procedure. For example, if a patient has a technically challenging colonoscopy and the endoscopist is running late, the endoscopist may drop their guard once they find a polyp and miss 1-2 additional small polyps that they would have spent more time looking for if they knew their next one would be in 10 years instead of 5.”
As for notification method, despite the logistical downside of taking dedicated staff time to make telephone calls, Dr. Sreenivasan said, “I think having a conversation with the patient directly is a much better way to communicate this information as it allows the patient to ask and answer questions. Things like tone of voice can provide reassurance that one cannot get via email.” Looking to the future, the study authors acknowledged that combinations of initial and reminder outreach approaches — for example, a mailed letter followed by secure message or telephone call — could potentially yield higher response rates and/or adoption rates than they observed. And a longer follow-up period with additional reminders may have produced higher yields. Additional studies are needed to optimize outreach approaches and to understand patient barriers to adopting the new guideline recommendations in different healthcare settings.
The study was supported by a Delivery Science grant from the Kaiser Permanente Northern California.
The authors disclosed no conflicts of interest. Dr. Mansour and Dr. Sreenivasan disclosed no conflicts of interest relevant to their comments.
In an article published in Clinical Gastroenterology and Hepatology, a group led by Jeffrey K. Lee, MD, MPH, a gastroenterologist at Kaiser Permanente Medical Center in San Francisco, reported the following 60-day response rates for the three contact methods in potentially transitioning more than 600 post-polypectomy patients to the new interval:
- Telephone: 64.5%
- Secure messaging: 51.7%
- Mailed letter: 31.3%
Compared with letter outreach, overall rate differences were significant for telephone (18.1%) and secure message outreach (13.1%).
Such interventions are widely used, the authors noted , but have not been compared for efficacy terms of communicating updated colonoscopy intervals.
The trial’s aim was to inform low-risk patients of the recommended interval update from 5 years — used since the 1990s — to 7-10 years. Given a choice, more patients opted to transition to the 10-year surveillance interval in the telephone (37%) and secure messaging arms (32.%) compared with mailed-letter arm (18.9%).
In addition to telephone and secure messaging outreach, factors positively associated with adoption of the 10-year interval were a positive fecal immunochemical test–based index colonoscopy and increasing age. Patients with these characteristics may be biased toward avoiding colonoscopy if not medically necessary, the authors conjectured.
Inversely associated factors included Asian or Pacific Islander race (odds ratio .58), Hispanic ethnicity (OR .40), and a higher Charlson comorbidity score of 2 vs 0 (OR .43).
Possible explanations for the race and ethnicity associations include gaps in culturally component care, lack of engagement with the English-based outreach approaches, and medical mistrust, the authors said.
“In this study, we gave all our patients an option to either extend their surveillance interval to current guideline recommendations or continue with their old interval, and some chose to do that,” Dr. Lee said in an interview. “Patients really appreciated having a choice and to be informed about the latest guideline changes.”
“A critical challenge to health systems is how to effectively de-implement outdated surveillance recommendations for low-risk patients who have a 5-year follow-up interval and potentially transition them to the recommended 7- to 10-year interval,” Dr. Lee and colleagues wrote.
More than 5 million surveillance colonoscopies are performed annually in US patients with a history of adenomas, the main precursor lesion for colorectal cancer, the authors noted.
With the recent guidelines issued in 2020 by the US Multi-Society Task Force on Colorectal Cancer lengthening the follow-up interval to 7-10 years , physicians are being advised to reevaluate low-risk patients previously scheduled with 5-year surveillance and provide an updated recommendation for follow-up.
Study Details
The three-arm pragmatic randomized trial was conducted in low-risk patients 54-70 years of age with one or two small (< 10 mm) tubular adenomas at baseline colonoscopy. Participants due for 5-year surveillance in 2022 were randomly assigned to one of three outreach arms: telephone (n = 200], secure messaging (n = 203), and mailed letter (n = 201). Stratified by age, sex, race, and ethnicity, participants could change their assigned interval to 10 years or continue with their previously scheduled 5-year interval.
As to economic considerations, the authors said that telephone may be the costliest form of outreach in terms of staffing resources. “We don’t know because we did not conduct a formal cost-effectiveness analysis,” Dr. Lee said. “However, we do know phone outreach requires a lot of personnel effort, which is why we also explored the less costly option of secure messaging/email.”
But based on the findings, telephone outreach would be a reasonable approach to update patients on post-polypectomy surveillance guideline changes if secure messaging or text messaging isn’t available, he added.
Downsides to Retroactive Changes?
Commenting on the study but not involved in it, Nabil M. Mansour, MD, an assistant professor and director of the McNair General GI Clinic at Baylor College of Medicine in Houston, noted that unlike Kaiser Permanente, his center decided against an overall effort to switch patients colonoscopied before the release of the new guidelines over to the new interval.
“Several of our physicians may have chosen to recommend a 5-year interval specifically for a variety of reasons and we felt going back, and making a blanket change to everyone’s interval retrospectively might create confusion and frustration and might actually delay the colonoscopies of some patients for which their doctors had a very good, legitimate reason to recommend a 5-year interval,” he said in an interview.
Dr. Mansour added that no difficulties were encountered in getting patients to agree to a 10-year interval. In his view telephone communication or in-person clinic visits are likely the most effective ways but both are more labor-intensive than automated patient portal messages. “I do not think traditional snail mail is effective.” His clinic uses automatic EMR reminders.
Offering another perspective on the study, Aditya Sreenivasan, MD, a gastroenterologist at Northwell Health in New York City, said his center has not reached out to correct the old intervals. “When I see a patient who previously had a colonoscopy with another physician, I always follow the previous recommendation for when the next colonoscopy should be, regardless of whether or not it technically meets guideline recommendations,” he told this news organization. “I do this because I was not there during the procedure and am not aware of any circumstances that would require a shorter interval that may not be apparent from the report.”
While he agrees with the new guidelines, Dr. Sreenivasan is “not sure if retroactively changing intervals is beneficial to patients, as the presence of guidelines may subconsciously influence the behavior of the endoscopist at the time of the procedure. For example, if a patient has a technically challenging colonoscopy and the endoscopist is running late, the endoscopist may drop their guard once they find a polyp and miss 1-2 additional small polyps that they would have spent more time looking for if they knew their next one would be in 10 years instead of 5.”
As for notification method, despite the logistical downside of taking dedicated staff time to make telephone calls, Dr. Sreenivasan said, “I think having a conversation with the patient directly is a much better way to communicate this information as it allows the patient to ask and answer questions. Things like tone of voice can provide reassurance that one cannot get via email.” Looking to the future, the study authors acknowledged that combinations of initial and reminder outreach approaches — for example, a mailed letter followed by secure message or telephone call — could potentially yield higher response rates and/or adoption rates than they observed. And a longer follow-up period with additional reminders may have produced higher yields. Additional studies are needed to optimize outreach approaches and to understand patient barriers to adopting the new guideline recommendations in different healthcare settings.
The study was supported by a Delivery Science grant from the Kaiser Permanente Northern California.
The authors disclosed no conflicts of interest. Dr. Mansour and Dr. Sreenivasan disclosed no conflicts of interest relevant to their comments.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Working together
Dear Friends,
After 6 months in my first faculty position, I have come to appreciate the term “multidisciplinary approach” more than ever. Not only does this facilitate optimal patient care, but I have personally learned so much from experts in other fields. This theme resonates across this issue of The New Gastroenterologist, from treating complex gallbladder disease, to caring for sexual and gender minorities, and collaborating with the tech industry to advance patient care.
Our “In Focus” feature, written by Dr. Andrew Gilman and Dr. Todd Baron, is on endoscopic management of gallbladder disease. They review endoscopic treatment options in patients with benign gallbladder disease, with emphasis on working with surgical and interventional radiology colleagues, as well as relaying endoscopic tips and techniques to achieve success in these complicated procedures.
In the “Short Clinical Reviews” section, Dr. David Chiang and Dr. Victor Chedid highlight the gaps in research and clinical care and competency for sexual and gender minorities, particularly in patients with inflammatory bowel disease. They describe the creation of the Pride in IBD clinic at Mayo Clinic in Rochester, Minn., that creates a culturally sensitive space to care for this community.
As trainees transition to early faculty, becoming a mentor is a new role that can be very rewarding and daunting at the same time. Dr. Anna Lok, recipient of the AGA’s Distinguished Mentor Award, and Dr. Vincent Chen share invaluable experiences and advice on being a mentor from senior and early-career perspectives, respectively. Similarly in the transition to early faculty, Erin Anderson, CPA, answers five common financial questions that arise to better understand and manage a significant increase in salary.
Lastly, Dr. Shifa Umar describes her unique experience as part of the AGA’s annual Tech Summit Fellows Program, a cross-section of medicine, technology, and innovation.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu), or Jillian Schweitzer (jschweitzer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The concept of the clinicopathologic conference (CPC) was introduced by Dr. Walter B. Cannon as a medical student at Harvard Medical School.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
After 6 months in my first faculty position, I have come to appreciate the term “multidisciplinary approach” more than ever. Not only does this facilitate optimal patient care, but I have personally learned so much from experts in other fields. This theme resonates across this issue of The New Gastroenterologist, from treating complex gallbladder disease, to caring for sexual and gender minorities, and collaborating with the tech industry to advance patient care.
Our “In Focus” feature, written by Dr. Andrew Gilman and Dr. Todd Baron, is on endoscopic management of gallbladder disease. They review endoscopic treatment options in patients with benign gallbladder disease, with emphasis on working with surgical and interventional radiology colleagues, as well as relaying endoscopic tips and techniques to achieve success in these complicated procedures.
In the “Short Clinical Reviews” section, Dr. David Chiang and Dr. Victor Chedid highlight the gaps in research and clinical care and competency for sexual and gender minorities, particularly in patients with inflammatory bowel disease. They describe the creation of the Pride in IBD clinic at Mayo Clinic in Rochester, Minn., that creates a culturally sensitive space to care for this community.
As trainees transition to early faculty, becoming a mentor is a new role that can be very rewarding and daunting at the same time. Dr. Anna Lok, recipient of the AGA’s Distinguished Mentor Award, and Dr. Vincent Chen share invaluable experiences and advice on being a mentor from senior and early-career perspectives, respectively. Similarly in the transition to early faculty, Erin Anderson, CPA, answers five common financial questions that arise to better understand and manage a significant increase in salary.
Lastly, Dr. Shifa Umar describes her unique experience as part of the AGA’s annual Tech Summit Fellows Program, a cross-section of medicine, technology, and innovation.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu), or Jillian Schweitzer (jschweitzer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The concept of the clinicopathologic conference (CPC) was introduced by Dr. Walter B. Cannon as a medical student at Harvard Medical School.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
After 6 months in my first faculty position, I have come to appreciate the term “multidisciplinary approach” more than ever. Not only does this facilitate optimal patient care, but I have personally learned so much from experts in other fields. This theme resonates across this issue of The New Gastroenterologist, from treating complex gallbladder disease, to caring for sexual and gender minorities, and collaborating with the tech industry to advance patient care.
Our “In Focus” feature, written by Dr. Andrew Gilman and Dr. Todd Baron, is on endoscopic management of gallbladder disease. They review endoscopic treatment options in patients with benign gallbladder disease, with emphasis on working with surgical and interventional radiology colleagues, as well as relaying endoscopic tips and techniques to achieve success in these complicated procedures.
In the “Short Clinical Reviews” section, Dr. David Chiang and Dr. Victor Chedid highlight the gaps in research and clinical care and competency for sexual and gender minorities, particularly in patients with inflammatory bowel disease. They describe the creation of the Pride in IBD clinic at Mayo Clinic in Rochester, Minn., that creates a culturally sensitive space to care for this community.
As trainees transition to early faculty, becoming a mentor is a new role that can be very rewarding and daunting at the same time. Dr. Anna Lok, recipient of the AGA’s Distinguished Mentor Award, and Dr. Vincent Chen share invaluable experiences and advice on being a mentor from senior and early-career perspectives, respectively. Similarly in the transition to early faculty, Erin Anderson, CPA, answers five common financial questions that arise to better understand and manage a significant increase in salary.
Lastly, Dr. Shifa Umar describes her unique experience as part of the AGA’s annual Tech Summit Fellows Program, a cross-section of medicine, technology, and innovation.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu), or Jillian Schweitzer (jschweitzer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The concept of the clinicopathologic conference (CPC) was introduced by Dr. Walter B. Cannon as a medical student at Harvard Medical School.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis