User login
Comparison of Intravenous Low Molecular Weight Iron Dextran and Intravenous Iron Sucrose to Treat Iron Deficiency Anemia: A Single Center Experience
PURPOSE
To evaluate if low molecular weight iron dextran (LMWID) is a safe and effective alternative to iron sucrose for intravenous iron administration.
BACKGROUND
In recent years, intravenous iron administration has increased due to clinical indications and rapid iron repletion. Early IV iron formulations had safety concerns that precluded widespread use. High molecular weight iron dextran was removed from the US market in 2009 due to safety concerns. Since then, several new IV formulations including LMWID and iron sucrose have been approved with a favorable benefit risk profile. While recent evidence and guidelines indicate that LMWID and other iron formulations have comparable safety profiles, no head-to-head comparisons exist. Both iron sucrose and LMWID are used for the treatment of IDA in Veterans Affairs hospitals. Iron sucrose is given 200 mg weekly for 5 weeks, while LMWID is given as a single 1-gram dose over 3 hours. We conducted a retrospective crosssectional analysis to compare the safety and efficacy of IV LMWID to IV iron sucrose.
METHODS
We identified 129 patients (LMWID: n=29, iron sucrose: n=100) who received intravenous iron from 01/01/2022 to 03/03/2023. To match the sample size, we selected every 3rd patient from the iron sucrose group (n=33). We captured data on infusion-related reactions, history of asthma/inflammatory bowel disease/> 2 drug allergies, overall and ≥ 2 g/dL hemoglobin increase, and treatment cost. Descriptive statistics were used to describe the safety and efficacy parameters. An unpaired t-test was used to calculate statistical significance of the cost.
RESULTS
We found that 82.7% of the patients who received LMWID had an increase in hemoglobin vs. 60.6% in the iron sucrose group. 48.3% of patients in LMWID had ≥ 2 hemoglobin increases vs. 27.3% in the iron sucrose group. The cost for LMWID administration was $2016.10, compared to $2315.40 for administration of IV iron sucrose cost. Two-tailed p value < 0.0001 indicating the observed difference to be statistically significant. No infusion reactions were observed in both groups.
CONCLUSIONS
In this single center analysis, IV LMWID administered provided comparable safety, and improved effectiveness, and cost-effectiveness to iron sucrose.
PURPOSE
To evaluate if low molecular weight iron dextran (LMWID) is a safe and effective alternative to iron sucrose for intravenous iron administration.
BACKGROUND
In recent years, intravenous iron administration has increased due to clinical indications and rapid iron repletion. Early IV iron formulations had safety concerns that precluded widespread use. High molecular weight iron dextran was removed from the US market in 2009 due to safety concerns. Since then, several new IV formulations including LMWID and iron sucrose have been approved with a favorable benefit risk profile. While recent evidence and guidelines indicate that LMWID and other iron formulations have comparable safety profiles, no head-to-head comparisons exist. Both iron sucrose and LMWID are used for the treatment of IDA in Veterans Affairs hospitals. Iron sucrose is given 200 mg weekly for 5 weeks, while LMWID is given as a single 1-gram dose over 3 hours. We conducted a retrospective crosssectional analysis to compare the safety and efficacy of IV LMWID to IV iron sucrose.
METHODS
We identified 129 patients (LMWID: n=29, iron sucrose: n=100) who received intravenous iron from 01/01/2022 to 03/03/2023. To match the sample size, we selected every 3rd patient from the iron sucrose group (n=33). We captured data on infusion-related reactions, history of asthma/inflammatory bowel disease/> 2 drug allergies, overall and ≥ 2 g/dL hemoglobin increase, and treatment cost. Descriptive statistics were used to describe the safety and efficacy parameters. An unpaired t-test was used to calculate statistical significance of the cost.
RESULTS
We found that 82.7% of the patients who received LMWID had an increase in hemoglobin vs. 60.6% in the iron sucrose group. 48.3% of patients in LMWID had ≥ 2 hemoglobin increases vs. 27.3% in the iron sucrose group. The cost for LMWID administration was $2016.10, compared to $2315.40 for administration of IV iron sucrose cost. Two-tailed p value < 0.0001 indicating the observed difference to be statistically significant. No infusion reactions were observed in both groups.
CONCLUSIONS
In this single center analysis, IV LMWID administered provided comparable safety, and improved effectiveness, and cost-effectiveness to iron sucrose.
PURPOSE
To evaluate if low molecular weight iron dextran (LMWID) is a safe and effective alternative to iron sucrose for intravenous iron administration.
BACKGROUND
In recent years, intravenous iron administration has increased due to clinical indications and rapid iron repletion. Early IV iron formulations had safety concerns that precluded widespread use. High molecular weight iron dextran was removed from the US market in 2009 due to safety concerns. Since then, several new IV formulations including LMWID and iron sucrose have been approved with a favorable benefit risk profile. While recent evidence and guidelines indicate that LMWID and other iron formulations have comparable safety profiles, no head-to-head comparisons exist. Both iron sucrose and LMWID are used for the treatment of IDA in Veterans Affairs hospitals. Iron sucrose is given 200 mg weekly for 5 weeks, while LMWID is given as a single 1-gram dose over 3 hours. We conducted a retrospective crosssectional analysis to compare the safety and efficacy of IV LMWID to IV iron sucrose.
METHODS
We identified 129 patients (LMWID: n=29, iron sucrose: n=100) who received intravenous iron from 01/01/2022 to 03/03/2023. To match the sample size, we selected every 3rd patient from the iron sucrose group (n=33). We captured data on infusion-related reactions, history of asthma/inflammatory bowel disease/> 2 drug allergies, overall and ≥ 2 g/dL hemoglobin increase, and treatment cost. Descriptive statistics were used to describe the safety and efficacy parameters. An unpaired t-test was used to calculate statistical significance of the cost.
RESULTS
We found that 82.7% of the patients who received LMWID had an increase in hemoglobin vs. 60.6% in the iron sucrose group. 48.3% of patients in LMWID had ≥ 2 hemoglobin increases vs. 27.3% in the iron sucrose group. The cost for LMWID administration was $2016.10, compared to $2315.40 for administration of IV iron sucrose cost. Two-tailed p value < 0.0001 indicating the observed difference to be statistically significant. No infusion reactions were observed in both groups.
CONCLUSIONS
In this single center analysis, IV LMWID administered provided comparable safety, and improved effectiveness, and cost-effectiveness to iron sucrose.
Revision of a Massive Transfusion Protocol to Allow for Verbal Orders
PURPOSE
To improve the time to release of blood products for patients with severe or life-threatening bleeding.
BACKGROUND
Exsanguination, and the resultant coagulopathy, is the number one cause of trauma-related death. Massive transfusion protocols (MTP) improve mortality by shortening the time to transfusion and correcting coagulopathy. Many patients do not meet criteria for massive transfusion (> 10 units RBCs in 24 hours), yet present with clinical instability and require rapid release (RR) of uncrossmatched blood. A quality improvement initiative was performed to identify barriers to the MTP/RR protocol at a single institution.
METHODS/DATA
A multidisciplinary subcommittee was formed to evaluate the safety and efficacy of the current MTP/RR process. Timed mock-MTP/RR trials were conducted to identify areas of delay with a goal to achieve a blood to bedside (B2B) time of under 10 minutes.
RESULTS
Timed mock-MTP/RR trials were conducted, which revealed a baseline B2B time of approximately 30 minutes. We identified problems and categorized them in terms of ordering (phase 1) and processing (phase 2). We found significant delays in phase 1. Reasons for delay were varied and included difficulty logging into the computer, staff unavailable to place orders (involved in resuscitation efforts), orders entered incorrectly, etc. Once orders were received, the blood bank could process them quickly in phase 2. Using root cause analysis, we discovered a critical step was to remove the barrier of electronic ordering. For this, a new process was developed in which the blood bank could accept verbal orders to release uncrossmatched blood during a medical emergency. Over the course of one year, a new policy for MTP/RR was drafted, an education training video was recorded, informational flyers were printed, and training drills were conducted. A repeat mock-MTP/RR scenario was performed after the change showing the B2B time was reduced by 90% from pre-intervention values to under 3 minutes. Since implementation, no new safety signals have been received, and the staff have reported improved satisfaction with the MTP/RR process.
IMPLICATIONS
A critical piece of any MTP/RR is the immediate availability of blood. Allowing verbal orders for blood products reduced time to transfusion by 90%. Through multidisciplinary effort, safe and efficient release of uncrossmatched blood products for nontraumatic massive transfusion can be achieved.
PURPOSE
To improve the time to release of blood products for patients with severe or life-threatening bleeding.
BACKGROUND
Exsanguination, and the resultant coagulopathy, is the number one cause of trauma-related death. Massive transfusion protocols (MTP) improve mortality by shortening the time to transfusion and correcting coagulopathy. Many patients do not meet criteria for massive transfusion (> 10 units RBCs in 24 hours), yet present with clinical instability and require rapid release (RR) of uncrossmatched blood. A quality improvement initiative was performed to identify barriers to the MTP/RR protocol at a single institution.
METHODS/DATA
A multidisciplinary subcommittee was formed to evaluate the safety and efficacy of the current MTP/RR process. Timed mock-MTP/RR trials were conducted to identify areas of delay with a goal to achieve a blood to bedside (B2B) time of under 10 minutes.
RESULTS
Timed mock-MTP/RR trials were conducted, which revealed a baseline B2B time of approximately 30 minutes. We identified problems and categorized them in terms of ordering (phase 1) and processing (phase 2). We found significant delays in phase 1. Reasons for delay were varied and included difficulty logging into the computer, staff unavailable to place orders (involved in resuscitation efforts), orders entered incorrectly, etc. Once orders were received, the blood bank could process them quickly in phase 2. Using root cause analysis, we discovered a critical step was to remove the barrier of electronic ordering. For this, a new process was developed in which the blood bank could accept verbal orders to release uncrossmatched blood during a medical emergency. Over the course of one year, a new policy for MTP/RR was drafted, an education training video was recorded, informational flyers were printed, and training drills were conducted. A repeat mock-MTP/RR scenario was performed after the change showing the B2B time was reduced by 90% from pre-intervention values to under 3 minutes. Since implementation, no new safety signals have been received, and the staff have reported improved satisfaction with the MTP/RR process.
IMPLICATIONS
A critical piece of any MTP/RR is the immediate availability of blood. Allowing verbal orders for blood products reduced time to transfusion by 90%. Through multidisciplinary effort, safe and efficient release of uncrossmatched blood products for nontraumatic massive transfusion can be achieved.
PURPOSE
To improve the time to release of blood products for patients with severe or life-threatening bleeding.
BACKGROUND
Exsanguination, and the resultant coagulopathy, is the number one cause of trauma-related death. Massive transfusion protocols (MTP) improve mortality by shortening the time to transfusion and correcting coagulopathy. Many patients do not meet criteria for massive transfusion (> 10 units RBCs in 24 hours), yet present with clinical instability and require rapid release (RR) of uncrossmatched blood. A quality improvement initiative was performed to identify barriers to the MTP/RR protocol at a single institution.
METHODS/DATA
A multidisciplinary subcommittee was formed to evaluate the safety and efficacy of the current MTP/RR process. Timed mock-MTP/RR trials were conducted to identify areas of delay with a goal to achieve a blood to bedside (B2B) time of under 10 minutes.
RESULTS
Timed mock-MTP/RR trials were conducted, which revealed a baseline B2B time of approximately 30 minutes. We identified problems and categorized them in terms of ordering (phase 1) and processing (phase 2). We found significant delays in phase 1. Reasons for delay were varied and included difficulty logging into the computer, staff unavailable to place orders (involved in resuscitation efforts), orders entered incorrectly, etc. Once orders were received, the blood bank could process them quickly in phase 2. Using root cause analysis, we discovered a critical step was to remove the barrier of electronic ordering. For this, a new process was developed in which the blood bank could accept verbal orders to release uncrossmatched blood during a medical emergency. Over the course of one year, a new policy for MTP/RR was drafted, an education training video was recorded, informational flyers were printed, and training drills were conducted. A repeat mock-MTP/RR scenario was performed after the change showing the B2B time was reduced by 90% from pre-intervention values to under 3 minutes. Since implementation, no new safety signals have been received, and the staff have reported improved satisfaction with the MTP/RR process.
IMPLICATIONS
A critical piece of any MTP/RR is the immediate availability of blood. Allowing verbal orders for blood products reduced time to transfusion by 90%. Through multidisciplinary effort, safe and efficient release of uncrossmatched blood products for nontraumatic massive transfusion can be achieved.
Improving Germline Genetic Testing Among Veterans With High Risk, Very High Risk and Metastatic Prostate Cancer
PURPOSE
To improve germline genetic testing among Veterans with high risk, very high risk and metastatic prostate cancer.
BACKGROUND
During our Commission on Cancer survey in 2021, it was noted that the Detroit VA’s referrals for germline genetic testing and counseling were extremely low. In 2020, only 1 Veteran was referred for prostate germline genetic testing and counseling and only 8 Veterans were referred in 2021. It was felt that the need to refer Veterans outside of the Detroit VA may have contributed to these low numbers. Our Cancer Committee chose prostate cancer as a disease to focus on. We chose a timeline of one year to implement our process.
METHODS
We made testing and counseling locally accessible to Veterans and encouraged medical oncology providers to make it part of the care of Veterans with high risk, very high risk and metastatic prostate cancer. We sought the assistance of the VA’s National Precision Oncology Program and were able to secure financial and logistical support to perform germline molecular prostate panel testing at the Detroit VA. We were also able to identify a cancer genetic specialist at the Ann Arbor VA that would perform genetic counseling among this group of patients based on their test results. Our medical oncology providers identified Veterans meeting the criteria for testing. Education regarding germline testing, its benefits and implications were conducted with Veterans, and performed after obtaining their informed consent in collaboration with our pathology department. The specimen is then sent to a VA central laboratory for processing. Detroit VA providers are alerted by the local laboratory once results are available. Veterans are then referred to the genetic counseling specialist based on the results. Some of these counseling visits are done virtually for the Veteran’s convenience.
DATA ANALYSIS
A retrospective chart analysis was used to collect the data.
RESULTS
After the implementation of our initiative, 97 Veterans with high risk, very high risk or metastatic prostate cancer were educated on the benefits of germline genetic testing, 87 of whom agreed to be tested. As of 4/2/23, 48 tests have already been performed. Pathogenic variants were recorded on 2 Veterans so far. One was for BRCA2 and KDM6A, and the other was for ATM. Data collection and recording is on-going.
IMPLICATIONS
Improving accessibility and incorporating genetic testing and counseling in cancer care can improve their utilization.
PURPOSE
To improve germline genetic testing among Veterans with high risk, very high risk and metastatic prostate cancer.
BACKGROUND
During our Commission on Cancer survey in 2021, it was noted that the Detroit VA’s referrals for germline genetic testing and counseling were extremely low. In 2020, only 1 Veteran was referred for prostate germline genetic testing and counseling and only 8 Veterans were referred in 2021. It was felt that the need to refer Veterans outside of the Detroit VA may have contributed to these low numbers. Our Cancer Committee chose prostate cancer as a disease to focus on. We chose a timeline of one year to implement our process.
METHODS
We made testing and counseling locally accessible to Veterans and encouraged medical oncology providers to make it part of the care of Veterans with high risk, very high risk and metastatic prostate cancer. We sought the assistance of the VA’s National Precision Oncology Program and were able to secure financial and logistical support to perform germline molecular prostate panel testing at the Detroit VA. We were also able to identify a cancer genetic specialist at the Ann Arbor VA that would perform genetic counseling among this group of patients based on their test results. Our medical oncology providers identified Veterans meeting the criteria for testing. Education regarding germline testing, its benefits and implications were conducted with Veterans, and performed after obtaining their informed consent in collaboration with our pathology department. The specimen is then sent to a VA central laboratory for processing. Detroit VA providers are alerted by the local laboratory once results are available. Veterans are then referred to the genetic counseling specialist based on the results. Some of these counseling visits are done virtually for the Veteran’s convenience.
DATA ANALYSIS
A retrospective chart analysis was used to collect the data.
RESULTS
After the implementation of our initiative, 97 Veterans with high risk, very high risk or metastatic prostate cancer were educated on the benefits of germline genetic testing, 87 of whom agreed to be tested. As of 4/2/23, 48 tests have already been performed. Pathogenic variants were recorded on 2 Veterans so far. One was for BRCA2 and KDM6A, and the other was for ATM. Data collection and recording is on-going.
IMPLICATIONS
Improving accessibility and incorporating genetic testing and counseling in cancer care can improve their utilization.
PURPOSE
To improve germline genetic testing among Veterans with high risk, very high risk and metastatic prostate cancer.
BACKGROUND
During our Commission on Cancer survey in 2021, it was noted that the Detroit VA’s referrals for germline genetic testing and counseling were extremely low. In 2020, only 1 Veteran was referred for prostate germline genetic testing and counseling and only 8 Veterans were referred in 2021. It was felt that the need to refer Veterans outside of the Detroit VA may have contributed to these low numbers. Our Cancer Committee chose prostate cancer as a disease to focus on. We chose a timeline of one year to implement our process.
METHODS
We made testing and counseling locally accessible to Veterans and encouraged medical oncology providers to make it part of the care of Veterans with high risk, very high risk and metastatic prostate cancer. We sought the assistance of the VA’s National Precision Oncology Program and were able to secure financial and logistical support to perform germline molecular prostate panel testing at the Detroit VA. We were also able to identify a cancer genetic specialist at the Ann Arbor VA that would perform genetic counseling among this group of patients based on their test results. Our medical oncology providers identified Veterans meeting the criteria for testing. Education regarding germline testing, its benefits and implications were conducted with Veterans, and performed after obtaining their informed consent in collaboration with our pathology department. The specimen is then sent to a VA central laboratory for processing. Detroit VA providers are alerted by the local laboratory once results are available. Veterans are then referred to the genetic counseling specialist based on the results. Some of these counseling visits are done virtually for the Veteran’s convenience.
DATA ANALYSIS
A retrospective chart analysis was used to collect the data.
RESULTS
After the implementation of our initiative, 97 Veterans with high risk, very high risk or metastatic prostate cancer were educated on the benefits of germline genetic testing, 87 of whom agreed to be tested. As of 4/2/23, 48 tests have already been performed. Pathogenic variants were recorded on 2 Veterans so far. One was for BRCA2 and KDM6A, and the other was for ATM. Data collection and recording is on-going.
IMPLICATIONS
Improving accessibility and incorporating genetic testing and counseling in cancer care can improve their utilization.
My favorite iron pearls
A 45-year-old women presents for evaluation of fatigue. She has been tired for the past 6 months. She has had no problems with sleep and no other new symptoms. Her physical exam is unremarkable. Her Patient Health Questionnaire–9 score is 4. Lab results are as follows: hemoglobin, 13 g/dL; hematocrit, 39%; mean corpuscular volume, 90 fL; blood urea nitrogen, 10 mg/dL; Cr, 1.0 mg/dL; AST, 20 IU/L; ALT, 15 IU/L; ferritin, 35 mcg/mL; thyroid-stimulating hormone, 3.5 mIU/L.
What would you recommend?
A. Sertraline
B. Sleep study
C. Iron supplementation
I would treat this patient with iron. Verdon and colleagues conducted a randomized, double-blind placebo-controlled trial of iron treatment in nonanemic women.1 The women who received iron had a much greater reduction in fatigue score, compared with the women who did not (P < .004). Only women with ferritin levels less than 50 mcg/L benefited. Houston and colleagues performed a systematic review of the literature of iron supplementation for fatigue and concluded that iron should be considered for treatment of fatigue in nonanemic women.2 The key number for benefit was a ferritin level less than 50 mcg/L.
Hair thinning is a common concern for many women. Does iron deficiency have a possible role in this problem? A number of studies have correlated low ferritin levels with hair loss.3 There is less clear evidence of iron treatment being effective. Hard studied 140 women with diffuse hair loss, and found 19% had iron deficiency without anemia.4 All patients with iron deficiency were treated with oral iron and in all patients hair loss ceased, and hair regrowth occurred. The target ferritin goal for treatment is greater than 40 mcg/L.5
Iron deficiency is an important trigger for restless leg syndrome (RLS). All patients who present with RLS should have ferritin checked, and appropriate evaluation for the cause of iron deficiency if ferritin levels are low. Allen and colleagues published clinical practice guidelines for iron treatment of RLS.6 The guidelines conclude that ferric carboxymaltose (1,000 mg) is effective for treating moderate to severe RLS in those with serum ferritin less than 300 mcg/L and could be used as first-line therapy for RLS in adults, with oral iron (65 mg) possibly effective in patients with ferritin levels less than 75 mcg/L.
Pearl: Think of iron as therapy for fatigue in nonanemic women with a ferritin level less than 50 mcg/L, consider a trial of iron for thinning hair in women with ferritin levels less than 50 mcg/L, and a trial of iron in those with RLS with ferritin levels less than 75 mcg/L.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Verdon F et al. BMJ. 2003 May 24;326(7399):1124. .
2. Houston BL et al. BMJ Open. 2018 Apr 5;8(4):e019240.
3. Almohanna HM et al. Dermatol Ther (Heidelb). 2019 Mar;9(1):51-70. .
4. Hard S. Acta Derm Venereol. 1963;43:562-9.
5. Kantor J et al. J Invest Dermatol. 2003 Nov;121(5):985-8. .
6. Allen RP et al. Sleep Med. 2018 Jan;41:27-44. .
A 45-year-old women presents for evaluation of fatigue. She has been tired for the past 6 months. She has had no problems with sleep and no other new symptoms. Her physical exam is unremarkable. Her Patient Health Questionnaire–9 score is 4. Lab results are as follows: hemoglobin, 13 g/dL; hematocrit, 39%; mean corpuscular volume, 90 fL; blood urea nitrogen, 10 mg/dL; Cr, 1.0 mg/dL; AST, 20 IU/L; ALT, 15 IU/L; ferritin, 35 mcg/mL; thyroid-stimulating hormone, 3.5 mIU/L.
What would you recommend?
A. Sertraline
B. Sleep study
C. Iron supplementation
I would treat this patient with iron. Verdon and colleagues conducted a randomized, double-blind placebo-controlled trial of iron treatment in nonanemic women.1 The women who received iron had a much greater reduction in fatigue score, compared with the women who did not (P < .004). Only women with ferritin levels less than 50 mcg/L benefited. Houston and colleagues performed a systematic review of the literature of iron supplementation for fatigue and concluded that iron should be considered for treatment of fatigue in nonanemic women.2 The key number for benefit was a ferritin level less than 50 mcg/L.
Hair thinning is a common concern for many women. Does iron deficiency have a possible role in this problem? A number of studies have correlated low ferritin levels with hair loss.3 There is less clear evidence of iron treatment being effective. Hard studied 140 women with diffuse hair loss, and found 19% had iron deficiency without anemia.4 All patients with iron deficiency were treated with oral iron and in all patients hair loss ceased, and hair regrowth occurred. The target ferritin goal for treatment is greater than 40 mcg/L.5
Iron deficiency is an important trigger for restless leg syndrome (RLS). All patients who present with RLS should have ferritin checked, and appropriate evaluation for the cause of iron deficiency if ferritin levels are low. Allen and colleagues published clinical practice guidelines for iron treatment of RLS.6 The guidelines conclude that ferric carboxymaltose (1,000 mg) is effective for treating moderate to severe RLS in those with serum ferritin less than 300 mcg/L and could be used as first-line therapy for RLS in adults, with oral iron (65 mg) possibly effective in patients with ferritin levels less than 75 mcg/L.
Pearl: Think of iron as therapy for fatigue in nonanemic women with a ferritin level less than 50 mcg/L, consider a trial of iron for thinning hair in women with ferritin levels less than 50 mcg/L, and a trial of iron in those with RLS with ferritin levels less than 75 mcg/L.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Verdon F et al. BMJ. 2003 May 24;326(7399):1124. .
2. Houston BL et al. BMJ Open. 2018 Apr 5;8(4):e019240.
3. Almohanna HM et al. Dermatol Ther (Heidelb). 2019 Mar;9(1):51-70. .
4. Hard S. Acta Derm Venereol. 1963;43:562-9.
5. Kantor J et al. J Invest Dermatol. 2003 Nov;121(5):985-8. .
6. Allen RP et al. Sleep Med. 2018 Jan;41:27-44. .
A 45-year-old women presents for evaluation of fatigue. She has been tired for the past 6 months. She has had no problems with sleep and no other new symptoms. Her physical exam is unremarkable. Her Patient Health Questionnaire–9 score is 4. Lab results are as follows: hemoglobin, 13 g/dL; hematocrit, 39%; mean corpuscular volume, 90 fL; blood urea nitrogen, 10 mg/dL; Cr, 1.0 mg/dL; AST, 20 IU/L; ALT, 15 IU/L; ferritin, 35 mcg/mL; thyroid-stimulating hormone, 3.5 mIU/L.
What would you recommend?
A. Sertraline
B. Sleep study
C. Iron supplementation
I would treat this patient with iron. Verdon and colleagues conducted a randomized, double-blind placebo-controlled trial of iron treatment in nonanemic women.1 The women who received iron had a much greater reduction in fatigue score, compared with the women who did not (P < .004). Only women with ferritin levels less than 50 mcg/L benefited. Houston and colleagues performed a systematic review of the literature of iron supplementation for fatigue and concluded that iron should be considered for treatment of fatigue in nonanemic women.2 The key number for benefit was a ferritin level less than 50 mcg/L.
Hair thinning is a common concern for many women. Does iron deficiency have a possible role in this problem? A number of studies have correlated low ferritin levels with hair loss.3 There is less clear evidence of iron treatment being effective. Hard studied 140 women with diffuse hair loss, and found 19% had iron deficiency without anemia.4 All patients with iron deficiency were treated with oral iron and in all patients hair loss ceased, and hair regrowth occurred. The target ferritin goal for treatment is greater than 40 mcg/L.5
Iron deficiency is an important trigger for restless leg syndrome (RLS). All patients who present with RLS should have ferritin checked, and appropriate evaluation for the cause of iron deficiency if ferritin levels are low. Allen and colleagues published clinical practice guidelines for iron treatment of RLS.6 The guidelines conclude that ferric carboxymaltose (1,000 mg) is effective for treating moderate to severe RLS in those with serum ferritin less than 300 mcg/L and could be used as first-line therapy for RLS in adults, with oral iron (65 mg) possibly effective in patients with ferritin levels less than 75 mcg/L.
Pearl: Think of iron as therapy for fatigue in nonanemic women with a ferritin level less than 50 mcg/L, consider a trial of iron for thinning hair in women with ferritin levels less than 50 mcg/L, and a trial of iron in those with RLS with ferritin levels less than 75 mcg/L.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Verdon F et al. BMJ. 2003 May 24;326(7399):1124. .
2. Houston BL et al. BMJ Open. 2018 Apr 5;8(4):e019240.
3. Almohanna HM et al. Dermatol Ther (Heidelb). 2019 Mar;9(1):51-70. .
4. Hard S. Acta Derm Venereol. 1963;43:562-9.
5. Kantor J et al. J Invest Dermatol. 2003 Nov;121(5):985-8. .
6. Allen RP et al. Sleep Med. 2018 Jan;41:27-44. .
Rapid-onset ulcerative hand nodule
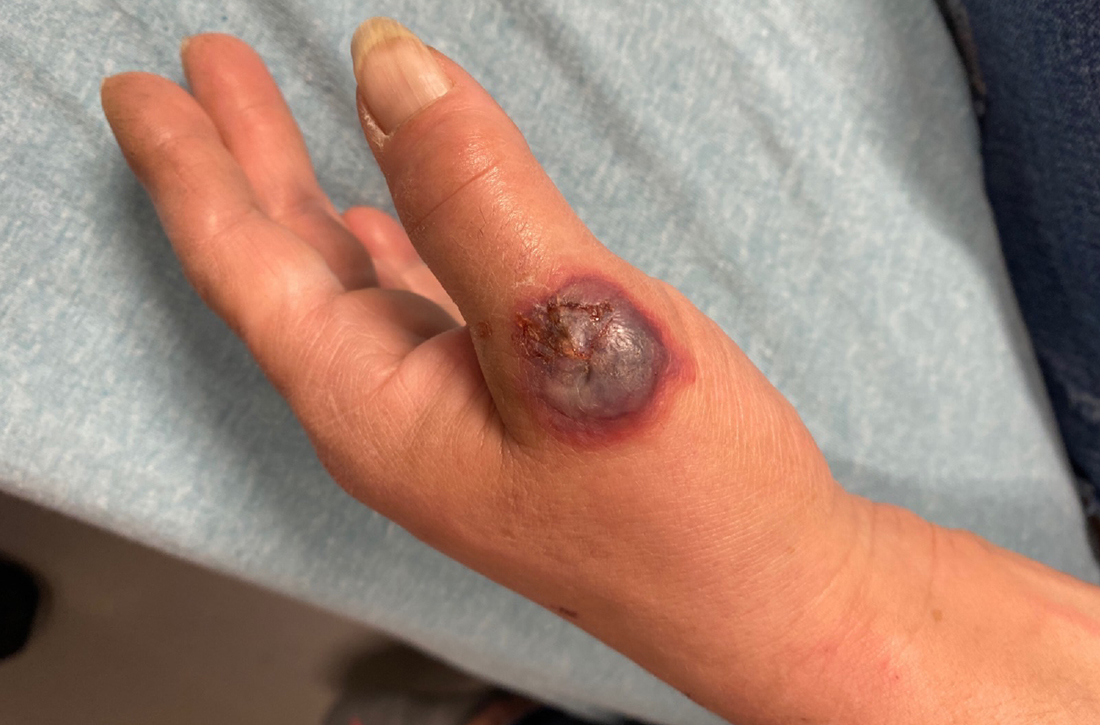
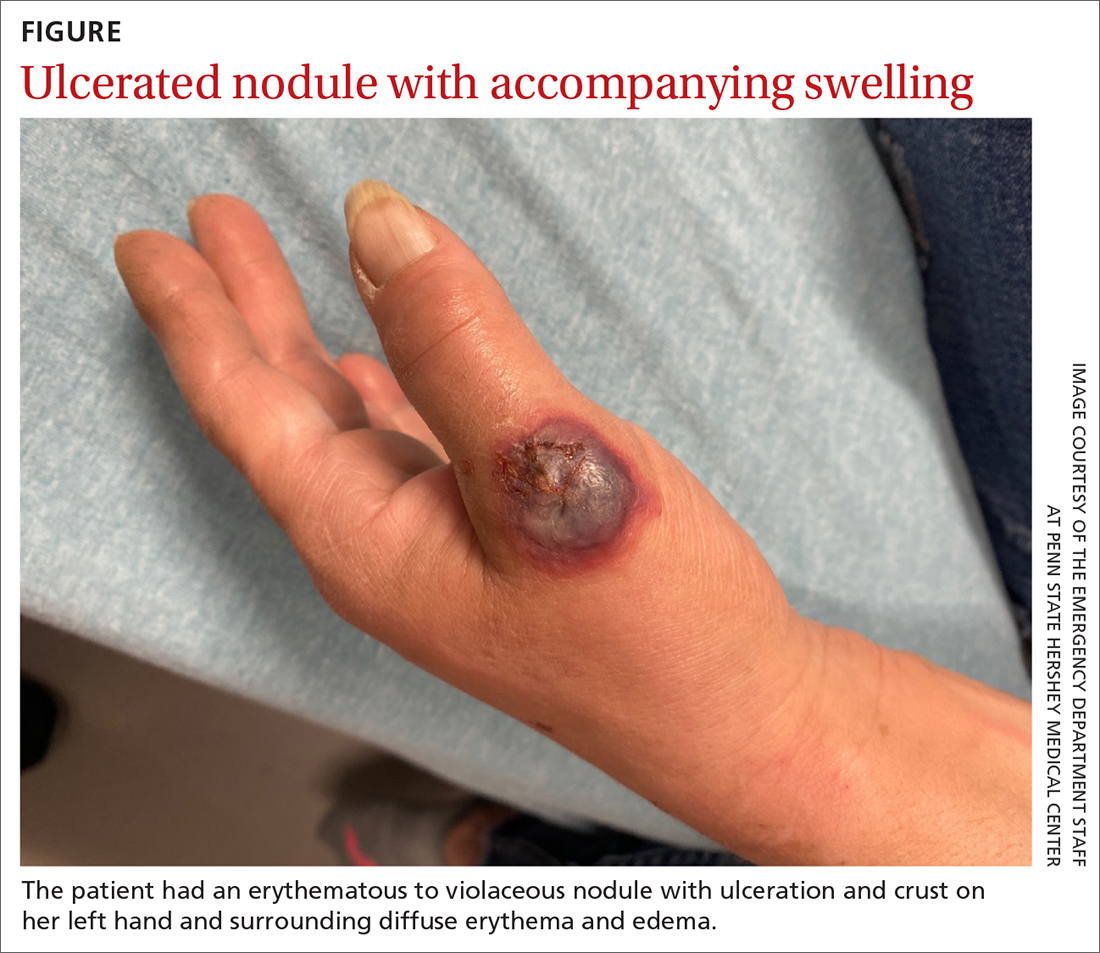
A 55-YEAR-OLD WOMAN developed a small red papule on her left hand that, over the course of a week, progressed rapidly into an ulcerated nodule with accompanying swelling and pain. She reported concomitant fatigue, unintentional weight loss, and swollen axillary lymph nodes. Past medical history included rheumatoid arthritis.
A physical examination of her left hand revealed a tender, erythematous to violaceous nodule with ulceration and crust and surrounding diffuse erythema and edema (FIGURE). She also had several enlarged, nontender right axillary lymph nodes. Initial lab evaluation was significant for leukocytosis (13.8 K/uL) with increased neutrophils, lymphocytes, and eosinophils. Two punch biopsies were performed and the samples submitted for hematoxylin and eosin (H&E) staining and tissue culture.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Neutrophilic dermatosis of the dorsal hands
The results of H&E were consistent with neutrophilic dermatosis of the dorsal hands (NDDH). Tissue culture was negative for fungus, bacteria, and atypical mycobacteria, confirming the diagnosis.
NDDH is a neutrophilic dermatosis and considered a localized variant of Sweet syndrome, manifesting on the dorsal hands as suppurative, erythematous to violaceous papules, plaques, or nodules that often undergo necrosis, blistering, and ulceration. The diagnosis can be made clinically, although a biopsy is usually performed for confirmation. It is characterized histologically by a dense dermal neutrophilic infiltrate along with dermal edema.1
The pathogenesis of NDDH is not fully known.2 It is often preceded by trauma and may be associated with recent infection (respiratory, gastrointestinal), inflammatory bowel disease, autoimmune disease (eg, rheumatoid arthritis), or malignancy.1 The most common associated malignancies are hematologic, such as myelodysplastic syndrome, leukemia, or lymphoma, although solid tumors also can be seen.1,3 Therefore, patients who receive a diagnosis of NDDH typically require further work-up to rule out these associated conditions. NDDH is a rare enough entity that incidence/prevalence data aren’t available or likely to be accurate.
The differential includes infection and neoplastic processes
NDDH often is mistaken for an infectious abscess and unsuccessfully treated with antimicrobial agents, such as those commonly used for staphylococcus and streptococcus skin and soft-tissue infections. Thus, wound or tissue culture may be considered to exclude infection from the differential diagnosis. In addition to infectious processes such as sporotrichosis or an atypical mycobacterial infection, the differential includes other neutrophilic dermatoses and neoplastic processes such as lymphoma or leukemia cutis.
Sporotrichosis is caused by Sporothrix schenckii and usually spreads proximally after entering through a wound or cut. Special stains on histology and culture are needed to make the diagnosis.
Continue to: Atypical mycobacterial infections
Atypical mycobacterial infections usually enter through an area of trauma and spread proximally after inoculation. Atypical mycobacterial infections can be diagnosed via biopsy with special stains, culture, and polymerase chain reaction of the tissue.
Neutrophilic dermatoses are a broad category of dermatoses that include NDDH, pyoderma gangrenosum, and Sweet syndrome. This category of dermatoses is differentiated by morphology and distribution of lesions.
Lymphoma can be primary cutaneous or secondary to a systemic lymphoma. A biopsy will show a collection of atypical lymphocytes.
Treatment begins with steroids
Treatment with topical (eg, 0.05% clobetasol ointment bid), intralesional (10 to 40 mg/mL triamcinolone acetonide), or systemic (eg, prednisone 0.5 to 1 mg/kg tapered over the course of 1-2 months) steroids is considered first-line therapy and often results in rapid clinical improvement. Agents such as dapsone (25 to 150 mg/d) and/or colchicine (0.6 mg bid to tid) may be used in recalcitrant cases or in patients for whom steroids are contraindicated.2
Our patient’s NDDH was treated with prednisone (~1.0 mg/kg daily tapered over the course of 6 weeks). She was referred to Hematology/Oncology for further work-up of her constitutional symptoms, lymphadenopathy, and leukocytosis. Ultimately, she received a diagnosis of concomitant chronic lymphocytic leukemia/small lymphocytic lymphoma. The patient required no immediate treatment for her indolent lymphoma and was advised that she would need to get blood work done on a regular basis and have annual check-ups.
1. Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
2. Micallef D, Bonnici M, Pisani D, et al. Neutrophilic dermatosis of the dorsal hands: a review of 123 Cases. J Am Acad Dermatol. 2019;S0190-9622(19)32678-7. doi: 10.1016/j.jaad.2019.08.070
3. Mobini N, Sadrolashrafi K, Michaels S. Neutrophilic dermatosis of the dorsal hands: report of a case and review of the literature. Case Rep Dermatol Med. 2019;2019:8301585. doi: 10.1155/2019/8301585
A 55-YEAR-OLD WOMAN developed a small red papule on her left hand that, over the course of a week, progressed rapidly into an ulcerated nodule with accompanying swelling and pain. She reported concomitant fatigue, unintentional weight loss, and swollen axillary lymph nodes. Past medical history included rheumatoid arthritis.
A physical examination of her left hand revealed a tender, erythematous to violaceous nodule with ulceration and crust and surrounding diffuse erythema and edema (FIGURE). She also had several enlarged, nontender right axillary lymph nodes. Initial lab evaluation was significant for leukocytosis (13.8 K/uL) with increased neutrophils, lymphocytes, and eosinophils. Two punch biopsies were performed and the samples submitted for hematoxylin and eosin (H&E) staining and tissue culture.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Neutrophilic dermatosis of the dorsal hands
The results of H&E were consistent with neutrophilic dermatosis of the dorsal hands (NDDH). Tissue culture was negative for fungus, bacteria, and atypical mycobacteria, confirming the diagnosis.
NDDH is a neutrophilic dermatosis and considered a localized variant of Sweet syndrome, manifesting on the dorsal hands as suppurative, erythematous to violaceous papules, plaques, or nodules that often undergo necrosis, blistering, and ulceration. The diagnosis can be made clinically, although a biopsy is usually performed for confirmation. It is characterized histologically by a dense dermal neutrophilic infiltrate along with dermal edema.1
The pathogenesis of NDDH is not fully known.2 It is often preceded by trauma and may be associated with recent infection (respiratory, gastrointestinal), inflammatory bowel disease, autoimmune disease (eg, rheumatoid arthritis), or malignancy.1 The most common associated malignancies are hematologic, such as myelodysplastic syndrome, leukemia, or lymphoma, although solid tumors also can be seen.1,3 Therefore, patients who receive a diagnosis of NDDH typically require further work-up to rule out these associated conditions. NDDH is a rare enough entity that incidence/prevalence data aren’t available or likely to be accurate.
The differential includes infection and neoplastic processes
NDDH often is mistaken for an infectious abscess and unsuccessfully treated with antimicrobial agents, such as those commonly used for staphylococcus and streptococcus skin and soft-tissue infections. Thus, wound or tissue culture may be considered to exclude infection from the differential diagnosis. In addition to infectious processes such as sporotrichosis or an atypical mycobacterial infection, the differential includes other neutrophilic dermatoses and neoplastic processes such as lymphoma or leukemia cutis.
Sporotrichosis is caused by Sporothrix schenckii and usually spreads proximally after entering through a wound or cut. Special stains on histology and culture are needed to make the diagnosis.
Continue to: Atypical mycobacterial infections
Atypical mycobacterial infections usually enter through an area of trauma and spread proximally after inoculation. Atypical mycobacterial infections can be diagnosed via biopsy with special stains, culture, and polymerase chain reaction of the tissue.
Neutrophilic dermatoses are a broad category of dermatoses that include NDDH, pyoderma gangrenosum, and Sweet syndrome. This category of dermatoses is differentiated by morphology and distribution of lesions.
Lymphoma can be primary cutaneous or secondary to a systemic lymphoma. A biopsy will show a collection of atypical lymphocytes.
Treatment begins with steroids
Treatment with topical (eg, 0.05% clobetasol ointment bid), intralesional (10 to 40 mg/mL triamcinolone acetonide), or systemic (eg, prednisone 0.5 to 1 mg/kg tapered over the course of 1-2 months) steroids is considered first-line therapy and often results in rapid clinical improvement. Agents such as dapsone (25 to 150 mg/d) and/or colchicine (0.6 mg bid to tid) may be used in recalcitrant cases or in patients for whom steroids are contraindicated.2
Our patient’s NDDH was treated with prednisone (~1.0 mg/kg daily tapered over the course of 6 weeks). She was referred to Hematology/Oncology for further work-up of her constitutional symptoms, lymphadenopathy, and leukocytosis. Ultimately, she received a diagnosis of concomitant chronic lymphocytic leukemia/small lymphocytic lymphoma. The patient required no immediate treatment for her indolent lymphoma and was advised that she would need to get blood work done on a regular basis and have annual check-ups.
A 55-YEAR-OLD WOMAN developed a small red papule on her left hand that, over the course of a week, progressed rapidly into an ulcerated nodule with accompanying swelling and pain. She reported concomitant fatigue, unintentional weight loss, and swollen axillary lymph nodes. Past medical history included rheumatoid arthritis.
A physical examination of her left hand revealed a tender, erythematous to violaceous nodule with ulceration and crust and surrounding diffuse erythema and edema (FIGURE). She also had several enlarged, nontender right axillary lymph nodes. Initial lab evaluation was significant for leukocytosis (13.8 K/uL) with increased neutrophils, lymphocytes, and eosinophils. Two punch biopsies were performed and the samples submitted for hematoxylin and eosin (H&E) staining and tissue culture.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Neutrophilic dermatosis of the dorsal hands
The results of H&E were consistent with neutrophilic dermatosis of the dorsal hands (NDDH). Tissue culture was negative for fungus, bacteria, and atypical mycobacteria, confirming the diagnosis.
NDDH is a neutrophilic dermatosis and considered a localized variant of Sweet syndrome, manifesting on the dorsal hands as suppurative, erythematous to violaceous papules, plaques, or nodules that often undergo necrosis, blistering, and ulceration. The diagnosis can be made clinically, although a biopsy is usually performed for confirmation. It is characterized histologically by a dense dermal neutrophilic infiltrate along with dermal edema.1
The pathogenesis of NDDH is not fully known.2 It is often preceded by trauma and may be associated with recent infection (respiratory, gastrointestinal), inflammatory bowel disease, autoimmune disease (eg, rheumatoid arthritis), or malignancy.1 The most common associated malignancies are hematologic, such as myelodysplastic syndrome, leukemia, or lymphoma, although solid tumors also can be seen.1,3 Therefore, patients who receive a diagnosis of NDDH typically require further work-up to rule out these associated conditions. NDDH is a rare enough entity that incidence/prevalence data aren’t available or likely to be accurate.
The differential includes infection and neoplastic processes
NDDH often is mistaken for an infectious abscess and unsuccessfully treated with antimicrobial agents, such as those commonly used for staphylococcus and streptococcus skin and soft-tissue infections. Thus, wound or tissue culture may be considered to exclude infection from the differential diagnosis. In addition to infectious processes such as sporotrichosis or an atypical mycobacterial infection, the differential includes other neutrophilic dermatoses and neoplastic processes such as lymphoma or leukemia cutis.
Sporotrichosis is caused by Sporothrix schenckii and usually spreads proximally after entering through a wound or cut. Special stains on histology and culture are needed to make the diagnosis.
Continue to: Atypical mycobacterial infections
Atypical mycobacterial infections usually enter through an area of trauma and spread proximally after inoculation. Atypical mycobacterial infections can be diagnosed via biopsy with special stains, culture, and polymerase chain reaction of the tissue.
Neutrophilic dermatoses are a broad category of dermatoses that include NDDH, pyoderma gangrenosum, and Sweet syndrome. This category of dermatoses is differentiated by morphology and distribution of lesions.
Lymphoma can be primary cutaneous or secondary to a systemic lymphoma. A biopsy will show a collection of atypical lymphocytes.
Treatment begins with steroids
Treatment with topical (eg, 0.05% clobetasol ointment bid), intralesional (10 to 40 mg/mL triamcinolone acetonide), or systemic (eg, prednisone 0.5 to 1 mg/kg tapered over the course of 1-2 months) steroids is considered first-line therapy and often results in rapid clinical improvement. Agents such as dapsone (25 to 150 mg/d) and/or colchicine (0.6 mg bid to tid) may be used in recalcitrant cases or in patients for whom steroids are contraindicated.2
Our patient’s NDDH was treated with prednisone (~1.0 mg/kg daily tapered over the course of 6 weeks). She was referred to Hematology/Oncology for further work-up of her constitutional symptoms, lymphadenopathy, and leukocytosis. Ultimately, she received a diagnosis of concomitant chronic lymphocytic leukemia/small lymphocytic lymphoma. The patient required no immediate treatment for her indolent lymphoma and was advised that she would need to get blood work done on a regular basis and have annual check-ups.
1. Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
2. Micallef D, Bonnici M, Pisani D, et al. Neutrophilic dermatosis of the dorsal hands: a review of 123 Cases. J Am Acad Dermatol. 2019;S0190-9622(19)32678-7. doi: 10.1016/j.jaad.2019.08.070
3. Mobini N, Sadrolashrafi K, Michaels S. Neutrophilic dermatosis of the dorsal hands: report of a case and review of the literature. Case Rep Dermatol Med. 2019;2019:8301585. doi: 10.1155/2019/8301585
1. Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
2. Micallef D, Bonnici M, Pisani D, et al. Neutrophilic dermatosis of the dorsal hands: a review of 123 Cases. J Am Acad Dermatol. 2019;S0190-9622(19)32678-7. doi: 10.1016/j.jaad.2019.08.070
3. Mobini N, Sadrolashrafi K, Michaels S. Neutrophilic dermatosis of the dorsal hands: report of a case and review of the literature. Case Rep Dermatol Med. 2019;2019:8301585. doi: 10.1155/2019/8301585
Young girls, women are at high risk of iron deficiency: Study
, which can lead to fatigue and increase the risk of many health problems, according to a new study.
Researchers also found that 6 in every 100 of the girls and young women had extremely low iron levels, known as iron-deficiency anemia, which impacts the blood’s ability to carry oxygen throughout the body.
The findings suggest that current screening guidelines for iron levels in girls and women may be flawed, resulting in missed chances to get a simple blood test that can diagnose the easy-to-treat condition. Iron supplements are often prescribed as a treatment.
The study was published in JAMA and included 12 years of data for a total of nearly 3,500 girls and women aged 12-21 years.
In addition to shortness of breath and fatigue, other symptoms of iron deficiency anemia are:
- Pale skin
- Cold hands and feet
- Feeling dizzy or lightheaded
- Unusual cravings for nonfood items such as ice, dirt, or paper.
The Cleveland Clinic says the most common causes of iron-deficiency anemia are those that involve blood loss, including heavy menstrual periods. The body gets iron from food, and not getting enough iron from food, as can happen from eating a vegan or vegetarian diet, can also lead to deficiency.
In this latest study, researchers found that young women and girls’ likelihood to have iron deficiency or iron-deficiency anemia were significantly linked to race and ethnicity, poverty status, access to sufficient or quality food (also called food insecurity), and body mass index. Black and Hispanic girls and women were more likely to have iron level problems, compared with White girls and women. Black girls and women were four times more likely to have iron-deficiency anemia, compared with White girls and women.
The authors did not discuss potential causes and suggested further study is needed to identify risk factors of iron deficiency in girls and young women.
A version of this article originally appeared on WebMD.com.
, which can lead to fatigue and increase the risk of many health problems, according to a new study.
Researchers also found that 6 in every 100 of the girls and young women had extremely low iron levels, known as iron-deficiency anemia, which impacts the blood’s ability to carry oxygen throughout the body.
The findings suggest that current screening guidelines for iron levels in girls and women may be flawed, resulting in missed chances to get a simple blood test that can diagnose the easy-to-treat condition. Iron supplements are often prescribed as a treatment.
The study was published in JAMA and included 12 years of data for a total of nearly 3,500 girls and women aged 12-21 years.
In addition to shortness of breath and fatigue, other symptoms of iron deficiency anemia are:
- Pale skin
- Cold hands and feet
- Feeling dizzy or lightheaded
- Unusual cravings for nonfood items such as ice, dirt, or paper.
The Cleveland Clinic says the most common causes of iron-deficiency anemia are those that involve blood loss, including heavy menstrual periods. The body gets iron from food, and not getting enough iron from food, as can happen from eating a vegan or vegetarian diet, can also lead to deficiency.
In this latest study, researchers found that young women and girls’ likelihood to have iron deficiency or iron-deficiency anemia were significantly linked to race and ethnicity, poverty status, access to sufficient or quality food (also called food insecurity), and body mass index. Black and Hispanic girls and women were more likely to have iron level problems, compared with White girls and women. Black girls and women were four times more likely to have iron-deficiency anemia, compared with White girls and women.
The authors did not discuss potential causes and suggested further study is needed to identify risk factors of iron deficiency in girls and young women.
A version of this article originally appeared on WebMD.com.
, which can lead to fatigue and increase the risk of many health problems, according to a new study.
Researchers also found that 6 in every 100 of the girls and young women had extremely low iron levels, known as iron-deficiency anemia, which impacts the blood’s ability to carry oxygen throughout the body.
The findings suggest that current screening guidelines for iron levels in girls and women may be flawed, resulting in missed chances to get a simple blood test that can diagnose the easy-to-treat condition. Iron supplements are often prescribed as a treatment.
The study was published in JAMA and included 12 years of data for a total of nearly 3,500 girls and women aged 12-21 years.
In addition to shortness of breath and fatigue, other symptoms of iron deficiency anemia are:
- Pale skin
- Cold hands and feet
- Feeling dizzy or lightheaded
- Unusual cravings for nonfood items such as ice, dirt, or paper.
The Cleveland Clinic says the most common causes of iron-deficiency anemia are those that involve blood loss, including heavy menstrual periods. The body gets iron from food, and not getting enough iron from food, as can happen from eating a vegan or vegetarian diet, can also lead to deficiency.
In this latest study, researchers found that young women and girls’ likelihood to have iron deficiency or iron-deficiency anemia were significantly linked to race and ethnicity, poverty status, access to sufficient or quality food (also called food insecurity), and body mass index. Black and Hispanic girls and women were more likely to have iron level problems, compared with White girls and women. Black girls and women were four times more likely to have iron-deficiency anemia, compared with White girls and women.
The authors did not discuss potential causes and suggested further study is needed to identify risk factors of iron deficiency in girls and young women.
A version of this article originally appeared on WebMD.com.
FROM JAMA
Consider mental health and social factors in management of sickle cell disease
Complications from sickle cell disease (SCD) can affect education and life opportunities, and these complications have been associated with social determinants of health such as socioeconomic status, depression, health literacy, and level of education, according to Kelly M. Harris, PhD, of Washington University in St. Louis, and colleagues.
Pain is a hallmark of SCD, and “the current climate around pain management and opioid use has specific implications for individuals with [SCD], especially youth,” Dr. Harris said in an interview.
In a study published in JAMA Network Open, the researchers analyzed 2,264 participants (average age, 27.9 years; 56.2% were female) in the Sickle Cell Disease Implementation Consortium a study that includes patient assessment, treatment, and creation of a longitudinal registry.
The participants completed the Adult Sickle Cell Quality of Life Measurement Information System to provide data on the frequency and severity of pain episodes related to SCD over the past 12 months. Multivariable regression analysis was used to examine the associations of education, employment, and mental health with pain frequency and severity.
Overall, 79.8% of participants reported severe pain, and 47.8% reported more than four episodes of pain in the past year.
Notably, 20% of the participants were diagnosed with depression, and increased pain frequency was significantly associated with depression, although no significant association appeared between pain severity and depression, the researchers said.
A total of 47% of the participants reported using pain medication and 49% reported using hydroxyurea. In addition, 628 participants (28.0%) underwent regular blood transfusions.
Neither education level nor income was associated with increased pain frequency or severity. Age younger than 18 years was significantly associated with both pain frequency and severity, as was daily used of pain medication. Unemployment and female sex also were associated with increased pain frequency.
The findings were limited by several factors including the cross-sectional design that prevents conclusions of causality, and by the reliance on patient reports of depression, which likely led to underreporting, the researchers noted.
However, the results are consistent with previous studies suggesting that pain and negative feelings were associated with reduced quality of life in SCD patients, especially younger patients, and support the need to screen SCD patients for depression, especially those who report more severe and/or more frequent pain, they said.
Take a comprehensive approach to a complex condition
“When treating pain, we cannot just rely on medication,” Dr. Harris said. “It is important that providers consider the full experiences of patients and pursue holistic and comprehensive treatment approaches to reducing pain. Screening for depression should be a regular practice, particularly for patients experiencing frequent and/or severe pain.
“Racial discrimination, stigma, and bias impact pain diagnosis and treatment for individuals with SCD,” said Dr. Harris. “Increasing awareness of the associations between depression and pain frequency and severity ... may help address these barriers.”
Data highlight treatment gaps
Alexander A. Boucher, MD, a member of the division of pediatric hematology and oncology at the University of Minnesota, Minneapolis, noted the researchers included patients as young as midadolescence, with a majority being under 35 years old. “The 18- to 30-year-old range is an especially high-risk age window for increased acute health care utilization, even compared with other chronic adolescent/young adult conditions. “The demographics in the study group also reasonably approximate those for young adults with SCD in urban centers. By taking a multicenter approach across a several-state region, I believe the findings offer better generalizability, since health care access and mental health access can vary state-by-state,” and the current results show a more standard experience.
“It was a bit surprising that female [sex] maintained such an association with pain across the different components of the study,” and that the pain peak was in the 25- to 34-year-old age range, said Dr. Boucher. However, anecdotally, the late teens and early 20s “can be laden with mental health concerns due to the life transitions that accompany most people at that time. The note that hydroxyurea use was associated with more pain and depression symptoms was interesting, and serves as a reminder that what is happening to the red blood cells and in the blood vessels, such as red blood cell breakdown, sickling, and vaso-occlusion are only a part of what causes pain, and hydroxyurea is not likely to play a role in mitigating mental health aspects of pain.”
The findings that overall pain frequency and related pain medication use were associated with higher depression rates “may in part reflect a blind spot for physicians and medical teams, who often resort back to physical pain-based heuristics.” Physicians may misunderstand chronic pain and its management and look for quick fixes for pain out of uncertainty or urgency, said Dr. Boucher. “This serves to diminish the perspectives of patients as people first (not embodiments of a disease) and can lead to missed opportunities to tackle mental health challenges.”
Barriers and limitations
There are barriers to mental health screening in hematology care,” Dr. Boucher said. First, most hematologists are not experts in mental health and while they may have some from their medical training in these disorders, it can be difficult to maintain the level of health literacy needed to stay up to date on treatments. Second, depression screening may not be part of regular patient intake and the Patient Health Questionnaire–2 or PHQ-9 offer only short-term (2-week) snapshots of depression.
“Perhaps most critically, even if we do successfully screen, the access to mental health specialists is severely limited, just as it is across the medical landscape, so intervention opportunities may be suboptimal,” said Dr. Boucher. The problem is magnified if, as the current study suggests, the rates of depression in SCD are approximately three times greater than the population overall.
In the current study, “the fact that only half of those who self-reported depression symptoms actually had depression documented as a diagnosis in their medical records suggests that we are missing a lot of patients affected by mental health disturbances.”
This study is limited in measurement of the contribution of social determinants of health, he said, as they were primarily focused on employment status and income. The study does not describe other factors like support systems, housing, and transportation.
“I would like to see studies that not only identify associated drivers of pain, but also offer evidence for successful interventions,” Dr. Boucher said, and these studies should include patient-centered interventions versus disease-centered interventions.
Undertreatment persists
Other concerns with sickle cell anemia include the underuse of hydroxyurea to reduce complications associated with the disease such as pain, stroke, and even early death. Another recent study in JAMA Network Open suggested that use of hydroxyurea remained low in children and youth despite the issuing of guidelines, and that underserved populations were especially affected. In that study, the researchers found that the patients’ annual days’ supply of hydroxyurea in New York state did not change significantly after the guideline update.
SCD also has been associated with increased risk of other poor outcomes, such as stillbirth and increased risk of poor COVID-19–related outcomes and COVID-19–related deaths.
The study by Dr. Harris and colleagues was supported by the National Institutes of Health through the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. Dr. Harris had no financial conflicts to disclose. The hydroxyurea study was supported by the Agency for Healthcare Research and Quality and the NHLBI. The researchers had no financial conflicts to disclose. Dr. Boucher disclosed conducting research with SCL Behring, but had no relevant financial conflicts.
Complications from sickle cell disease (SCD) can affect education and life opportunities, and these complications have been associated with social determinants of health such as socioeconomic status, depression, health literacy, and level of education, according to Kelly M. Harris, PhD, of Washington University in St. Louis, and colleagues.
Pain is a hallmark of SCD, and “the current climate around pain management and opioid use has specific implications for individuals with [SCD], especially youth,” Dr. Harris said in an interview.
In a study published in JAMA Network Open, the researchers analyzed 2,264 participants (average age, 27.9 years; 56.2% were female) in the Sickle Cell Disease Implementation Consortium a study that includes patient assessment, treatment, and creation of a longitudinal registry.
The participants completed the Adult Sickle Cell Quality of Life Measurement Information System to provide data on the frequency and severity of pain episodes related to SCD over the past 12 months. Multivariable regression analysis was used to examine the associations of education, employment, and mental health with pain frequency and severity.
Overall, 79.8% of participants reported severe pain, and 47.8% reported more than four episodes of pain in the past year.
Notably, 20% of the participants were diagnosed with depression, and increased pain frequency was significantly associated with depression, although no significant association appeared between pain severity and depression, the researchers said.
A total of 47% of the participants reported using pain medication and 49% reported using hydroxyurea. In addition, 628 participants (28.0%) underwent regular blood transfusions.
Neither education level nor income was associated with increased pain frequency or severity. Age younger than 18 years was significantly associated with both pain frequency and severity, as was daily used of pain medication. Unemployment and female sex also were associated with increased pain frequency.
The findings were limited by several factors including the cross-sectional design that prevents conclusions of causality, and by the reliance on patient reports of depression, which likely led to underreporting, the researchers noted.
However, the results are consistent with previous studies suggesting that pain and negative feelings were associated with reduced quality of life in SCD patients, especially younger patients, and support the need to screen SCD patients for depression, especially those who report more severe and/or more frequent pain, they said.
Take a comprehensive approach to a complex condition
“When treating pain, we cannot just rely on medication,” Dr. Harris said. “It is important that providers consider the full experiences of patients and pursue holistic and comprehensive treatment approaches to reducing pain. Screening for depression should be a regular practice, particularly for patients experiencing frequent and/or severe pain.
“Racial discrimination, stigma, and bias impact pain diagnosis and treatment for individuals with SCD,” said Dr. Harris. “Increasing awareness of the associations between depression and pain frequency and severity ... may help address these barriers.”
Data highlight treatment gaps
Alexander A. Boucher, MD, a member of the division of pediatric hematology and oncology at the University of Minnesota, Minneapolis, noted the researchers included patients as young as midadolescence, with a majority being under 35 years old. “The 18- to 30-year-old range is an especially high-risk age window for increased acute health care utilization, even compared with other chronic adolescent/young adult conditions. “The demographics in the study group also reasonably approximate those for young adults with SCD in urban centers. By taking a multicenter approach across a several-state region, I believe the findings offer better generalizability, since health care access and mental health access can vary state-by-state,” and the current results show a more standard experience.
“It was a bit surprising that female [sex] maintained such an association with pain across the different components of the study,” and that the pain peak was in the 25- to 34-year-old age range, said Dr. Boucher. However, anecdotally, the late teens and early 20s “can be laden with mental health concerns due to the life transitions that accompany most people at that time. The note that hydroxyurea use was associated with more pain and depression symptoms was interesting, and serves as a reminder that what is happening to the red blood cells and in the blood vessels, such as red blood cell breakdown, sickling, and vaso-occlusion are only a part of what causes pain, and hydroxyurea is not likely to play a role in mitigating mental health aspects of pain.”
The findings that overall pain frequency and related pain medication use were associated with higher depression rates “may in part reflect a blind spot for physicians and medical teams, who often resort back to physical pain-based heuristics.” Physicians may misunderstand chronic pain and its management and look for quick fixes for pain out of uncertainty or urgency, said Dr. Boucher. “This serves to diminish the perspectives of patients as people first (not embodiments of a disease) and can lead to missed opportunities to tackle mental health challenges.”
Barriers and limitations
There are barriers to mental health screening in hematology care,” Dr. Boucher said. First, most hematologists are not experts in mental health and while they may have some from their medical training in these disorders, it can be difficult to maintain the level of health literacy needed to stay up to date on treatments. Second, depression screening may not be part of regular patient intake and the Patient Health Questionnaire–2 or PHQ-9 offer only short-term (2-week) snapshots of depression.
“Perhaps most critically, even if we do successfully screen, the access to mental health specialists is severely limited, just as it is across the medical landscape, so intervention opportunities may be suboptimal,” said Dr. Boucher. The problem is magnified if, as the current study suggests, the rates of depression in SCD are approximately three times greater than the population overall.
In the current study, “the fact that only half of those who self-reported depression symptoms actually had depression documented as a diagnosis in their medical records suggests that we are missing a lot of patients affected by mental health disturbances.”
This study is limited in measurement of the contribution of social determinants of health, he said, as they were primarily focused on employment status and income. The study does not describe other factors like support systems, housing, and transportation.
“I would like to see studies that not only identify associated drivers of pain, but also offer evidence for successful interventions,” Dr. Boucher said, and these studies should include patient-centered interventions versus disease-centered interventions.
Undertreatment persists
Other concerns with sickle cell anemia include the underuse of hydroxyurea to reduce complications associated with the disease such as pain, stroke, and even early death. Another recent study in JAMA Network Open suggested that use of hydroxyurea remained low in children and youth despite the issuing of guidelines, and that underserved populations were especially affected. In that study, the researchers found that the patients’ annual days’ supply of hydroxyurea in New York state did not change significantly after the guideline update.
SCD also has been associated with increased risk of other poor outcomes, such as stillbirth and increased risk of poor COVID-19–related outcomes and COVID-19–related deaths.
The study by Dr. Harris and colleagues was supported by the National Institutes of Health through the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. Dr. Harris had no financial conflicts to disclose. The hydroxyurea study was supported by the Agency for Healthcare Research and Quality and the NHLBI. The researchers had no financial conflicts to disclose. Dr. Boucher disclosed conducting research with SCL Behring, but had no relevant financial conflicts.
Complications from sickle cell disease (SCD) can affect education and life opportunities, and these complications have been associated with social determinants of health such as socioeconomic status, depression, health literacy, and level of education, according to Kelly M. Harris, PhD, of Washington University in St. Louis, and colleagues.
Pain is a hallmark of SCD, and “the current climate around pain management and opioid use has specific implications for individuals with [SCD], especially youth,” Dr. Harris said in an interview.
In a study published in JAMA Network Open, the researchers analyzed 2,264 participants (average age, 27.9 years; 56.2% were female) in the Sickle Cell Disease Implementation Consortium a study that includes patient assessment, treatment, and creation of a longitudinal registry.
The participants completed the Adult Sickle Cell Quality of Life Measurement Information System to provide data on the frequency and severity of pain episodes related to SCD over the past 12 months. Multivariable regression analysis was used to examine the associations of education, employment, and mental health with pain frequency and severity.
Overall, 79.8% of participants reported severe pain, and 47.8% reported more than four episodes of pain in the past year.
Notably, 20% of the participants were diagnosed with depression, and increased pain frequency was significantly associated with depression, although no significant association appeared between pain severity and depression, the researchers said.
A total of 47% of the participants reported using pain medication and 49% reported using hydroxyurea. In addition, 628 participants (28.0%) underwent regular blood transfusions.
Neither education level nor income was associated with increased pain frequency or severity. Age younger than 18 years was significantly associated with both pain frequency and severity, as was daily used of pain medication. Unemployment and female sex also were associated with increased pain frequency.
The findings were limited by several factors including the cross-sectional design that prevents conclusions of causality, and by the reliance on patient reports of depression, which likely led to underreporting, the researchers noted.
However, the results are consistent with previous studies suggesting that pain and negative feelings were associated with reduced quality of life in SCD patients, especially younger patients, and support the need to screen SCD patients for depression, especially those who report more severe and/or more frequent pain, they said.
Take a comprehensive approach to a complex condition
“When treating pain, we cannot just rely on medication,” Dr. Harris said. “It is important that providers consider the full experiences of patients and pursue holistic and comprehensive treatment approaches to reducing pain. Screening for depression should be a regular practice, particularly for patients experiencing frequent and/or severe pain.
“Racial discrimination, stigma, and bias impact pain diagnosis and treatment for individuals with SCD,” said Dr. Harris. “Increasing awareness of the associations between depression and pain frequency and severity ... may help address these barriers.”
Data highlight treatment gaps
Alexander A. Boucher, MD, a member of the division of pediatric hematology and oncology at the University of Minnesota, Minneapolis, noted the researchers included patients as young as midadolescence, with a majority being under 35 years old. “The 18- to 30-year-old range is an especially high-risk age window for increased acute health care utilization, even compared with other chronic adolescent/young adult conditions. “The demographics in the study group also reasonably approximate those for young adults with SCD in urban centers. By taking a multicenter approach across a several-state region, I believe the findings offer better generalizability, since health care access and mental health access can vary state-by-state,” and the current results show a more standard experience.
“It was a bit surprising that female [sex] maintained such an association with pain across the different components of the study,” and that the pain peak was in the 25- to 34-year-old age range, said Dr. Boucher. However, anecdotally, the late teens and early 20s “can be laden with mental health concerns due to the life transitions that accompany most people at that time. The note that hydroxyurea use was associated with more pain and depression symptoms was interesting, and serves as a reminder that what is happening to the red blood cells and in the blood vessels, such as red blood cell breakdown, sickling, and vaso-occlusion are only a part of what causes pain, and hydroxyurea is not likely to play a role in mitigating mental health aspects of pain.”
The findings that overall pain frequency and related pain medication use were associated with higher depression rates “may in part reflect a blind spot for physicians and medical teams, who often resort back to physical pain-based heuristics.” Physicians may misunderstand chronic pain and its management and look for quick fixes for pain out of uncertainty or urgency, said Dr. Boucher. “This serves to diminish the perspectives of patients as people first (not embodiments of a disease) and can lead to missed opportunities to tackle mental health challenges.”
Barriers and limitations
There are barriers to mental health screening in hematology care,” Dr. Boucher said. First, most hematologists are not experts in mental health and while they may have some from their medical training in these disorders, it can be difficult to maintain the level of health literacy needed to stay up to date on treatments. Second, depression screening may not be part of regular patient intake and the Patient Health Questionnaire–2 or PHQ-9 offer only short-term (2-week) snapshots of depression.
“Perhaps most critically, even if we do successfully screen, the access to mental health specialists is severely limited, just as it is across the medical landscape, so intervention opportunities may be suboptimal,” said Dr. Boucher. The problem is magnified if, as the current study suggests, the rates of depression in SCD are approximately three times greater than the population overall.
In the current study, “the fact that only half of those who self-reported depression symptoms actually had depression documented as a diagnosis in their medical records suggests that we are missing a lot of patients affected by mental health disturbances.”
This study is limited in measurement of the contribution of social determinants of health, he said, as they were primarily focused on employment status and income. The study does not describe other factors like support systems, housing, and transportation.
“I would like to see studies that not only identify associated drivers of pain, but also offer evidence for successful interventions,” Dr. Boucher said, and these studies should include patient-centered interventions versus disease-centered interventions.
Undertreatment persists
Other concerns with sickle cell anemia include the underuse of hydroxyurea to reduce complications associated with the disease such as pain, stroke, and even early death. Another recent study in JAMA Network Open suggested that use of hydroxyurea remained low in children and youth despite the issuing of guidelines, and that underserved populations were especially affected. In that study, the researchers found that the patients’ annual days’ supply of hydroxyurea in New York state did not change significantly after the guideline update.
SCD also has been associated with increased risk of other poor outcomes, such as stillbirth and increased risk of poor COVID-19–related outcomes and COVID-19–related deaths.
The study by Dr. Harris and colleagues was supported by the National Institutes of Health through the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. Dr. Harris had no financial conflicts to disclose. The hydroxyurea study was supported by the Agency for Healthcare Research and Quality and the NHLBI. The researchers had no financial conflicts to disclose. Dr. Boucher disclosed conducting research with SCL Behring, but had no relevant financial conflicts.
FROM JAMA NETWORK OPEN
Aspirin warning: Anemia may increase with daily use
, according to results from a new randomized controlled trial.
In the study, which was published in Annals of Internal Medicine, investigators analyzed data from the Aspirin in Reducing Events in the Elderly (ASPREE) trial and examined hemoglobin concentrations among 19,114 healthy, community-dwelling older patients.
“We knew from large clinical trials, including our ASPREE trial, that daily low-dose aspirin increased the risk of clinically significant bleeding,” said Zoe McQuilten, MBBS, PhD, a hematologist at Monash University in Australia and the study’s lead author. “From our study, we found that low-dose aspirin also increased the risk of anemia during the trial, and this was most likely due to bleeding that was not clinically apparent.”
Anemia is common among elderly patients. It can cause fatigue, fast or irregular heartbeat, headache, chest pain, and pounding or whooshing sounds in the ear, according to the Cleveland Clinic. It can also worsen conditions such as heart failure, cognitive impairment, and depression in people aged 65 and older.
The U.S. Preventive Services Task Force changed its recommendation on aspirin for the primary prevention of cardiovascular disease in 2022, recommending against initiating low-dose aspirin for adults aged 60 years or older. For adults aged 40-59 who have a 10% or greater 10-year risk for cardiovascular disease, the agency recommends that patients and clinicians make the decision to initiate low-dose aspirin use on a case-by-case basis, as the net benefit is small.
Dr. McQuilten said she spent the last 5 years designing substages of anemia and conditions such as blood cancer. In many cases of anemia, doctors are unable to determine the underlying cause, she said. One study published in the Journal of American Geriatrics Society in 2021 found that in about one-third of anemia cases, the etiology was not clear.
About 50% of people older than 60 who were involved in the latest study took aspirin for prevention from 2011 to 2018. That number likely dropped after changes were made to the guidelines in 2022, according to Dr. McQuilten, but long-term use may have continued among older patients. The researchers also examined ferritin levels, which serve as a proxy for iron levels, at baseline and after 3 years.
The incidence of anemia was 51 events per 1,000 person-years in the aspirin group compared with 43 events per 1,000 person-years in the placebo group, according to the researchers. The estimated probability of experiencing anemia within 5 years was 23.5% (95% confidence interval [CI], 22.4%-24.6%) in the aspirin group and 20.3% (95% CI: 19.3% to 21.4%) in the placebo group. Aspirin therapy resulted in a 20% increase in the risk for anemia (95% CI, 1.12-1.29).
People who took aspirin were more likely to have lower serum levels of ferritin at the 3-year mark than were those who received placebo. The average decrease in ferritin among participants who took aspirin was 11.5% greater (95% CI, 9.3%-13.7%) than among those who took placebo.
Basil Eldadah, MD, PhD, supervisory medical officer at the National Institute on Aging, part of the National Institutes of Health, said the findings should encourage clinicians to pay closer attention to hemoglobin levels and have conversations with patients to discuss their need for taking aspirin.
“If somebody is already taking aspirin for any reason, keep an eye on hemoglobin,” said Dr. Eldadah, who was not involved in the study. “For somebody who’s taking aspirin and who’s older, and it’s not for an indication like cardiovascular disease, consider seriously whether that’s the best treatment option.”
The study did not examine the functional consequences of anemia on participants, which Dr. Eldadah said could be fodder for future research. The researchers said one limitation was that it was not clear whether anemia was sufficient to cause symptoms that affected participants’ quality of life or whether occult bleeding caused the anemia. The researchers also did not document whether patients saw their regular physicians and received treatment for anemia over the course of the trial.
The study was funded through grants from the National Health and Medical Research Council and the Bill and Melinda Gates Foundation. The authors reported receiving consulting fees, honoraria, and stock options, and have participated on data monitoring boards not related to the study for Vifor Pharma, ITL Biomedical, Pfizer, Boehringer Ingelheim, Bayer Healthcare, AbbVie, and Abbott Diagnostics.
A version of this article originally appeared on Medscape.com.
, according to results from a new randomized controlled trial.
In the study, which was published in Annals of Internal Medicine, investigators analyzed data from the Aspirin in Reducing Events in the Elderly (ASPREE) trial and examined hemoglobin concentrations among 19,114 healthy, community-dwelling older patients.
“We knew from large clinical trials, including our ASPREE trial, that daily low-dose aspirin increased the risk of clinically significant bleeding,” said Zoe McQuilten, MBBS, PhD, a hematologist at Monash University in Australia and the study’s lead author. “From our study, we found that low-dose aspirin also increased the risk of anemia during the trial, and this was most likely due to bleeding that was not clinically apparent.”
Anemia is common among elderly patients. It can cause fatigue, fast or irregular heartbeat, headache, chest pain, and pounding or whooshing sounds in the ear, according to the Cleveland Clinic. It can also worsen conditions such as heart failure, cognitive impairment, and depression in people aged 65 and older.
The U.S. Preventive Services Task Force changed its recommendation on aspirin for the primary prevention of cardiovascular disease in 2022, recommending against initiating low-dose aspirin for adults aged 60 years or older. For adults aged 40-59 who have a 10% or greater 10-year risk for cardiovascular disease, the agency recommends that patients and clinicians make the decision to initiate low-dose aspirin use on a case-by-case basis, as the net benefit is small.
Dr. McQuilten said she spent the last 5 years designing substages of anemia and conditions such as blood cancer. In many cases of anemia, doctors are unable to determine the underlying cause, she said. One study published in the Journal of American Geriatrics Society in 2021 found that in about one-third of anemia cases, the etiology was not clear.
About 50% of people older than 60 who were involved in the latest study took aspirin for prevention from 2011 to 2018. That number likely dropped after changes were made to the guidelines in 2022, according to Dr. McQuilten, but long-term use may have continued among older patients. The researchers also examined ferritin levels, which serve as a proxy for iron levels, at baseline and after 3 years.
The incidence of anemia was 51 events per 1,000 person-years in the aspirin group compared with 43 events per 1,000 person-years in the placebo group, according to the researchers. The estimated probability of experiencing anemia within 5 years was 23.5% (95% confidence interval [CI], 22.4%-24.6%) in the aspirin group and 20.3% (95% CI: 19.3% to 21.4%) in the placebo group. Aspirin therapy resulted in a 20% increase in the risk for anemia (95% CI, 1.12-1.29).
People who took aspirin were more likely to have lower serum levels of ferritin at the 3-year mark than were those who received placebo. The average decrease in ferritin among participants who took aspirin was 11.5% greater (95% CI, 9.3%-13.7%) than among those who took placebo.
Basil Eldadah, MD, PhD, supervisory medical officer at the National Institute on Aging, part of the National Institutes of Health, said the findings should encourage clinicians to pay closer attention to hemoglobin levels and have conversations with patients to discuss their need for taking aspirin.
“If somebody is already taking aspirin for any reason, keep an eye on hemoglobin,” said Dr. Eldadah, who was not involved in the study. “For somebody who’s taking aspirin and who’s older, and it’s not for an indication like cardiovascular disease, consider seriously whether that’s the best treatment option.”
The study did not examine the functional consequences of anemia on participants, which Dr. Eldadah said could be fodder for future research. The researchers said one limitation was that it was not clear whether anemia was sufficient to cause symptoms that affected participants’ quality of life or whether occult bleeding caused the anemia. The researchers also did not document whether patients saw their regular physicians and received treatment for anemia over the course of the trial.
The study was funded through grants from the National Health and Medical Research Council and the Bill and Melinda Gates Foundation. The authors reported receiving consulting fees, honoraria, and stock options, and have participated on data monitoring boards not related to the study for Vifor Pharma, ITL Biomedical, Pfizer, Boehringer Ingelheim, Bayer Healthcare, AbbVie, and Abbott Diagnostics.
A version of this article originally appeared on Medscape.com.
, according to results from a new randomized controlled trial.
In the study, which was published in Annals of Internal Medicine, investigators analyzed data from the Aspirin in Reducing Events in the Elderly (ASPREE) trial and examined hemoglobin concentrations among 19,114 healthy, community-dwelling older patients.
“We knew from large clinical trials, including our ASPREE trial, that daily low-dose aspirin increased the risk of clinically significant bleeding,” said Zoe McQuilten, MBBS, PhD, a hematologist at Monash University in Australia and the study’s lead author. “From our study, we found that low-dose aspirin also increased the risk of anemia during the trial, and this was most likely due to bleeding that was not clinically apparent.”
Anemia is common among elderly patients. It can cause fatigue, fast or irregular heartbeat, headache, chest pain, and pounding or whooshing sounds in the ear, according to the Cleveland Clinic. It can also worsen conditions such as heart failure, cognitive impairment, and depression in people aged 65 and older.
The U.S. Preventive Services Task Force changed its recommendation on aspirin for the primary prevention of cardiovascular disease in 2022, recommending against initiating low-dose aspirin for adults aged 60 years or older. For adults aged 40-59 who have a 10% or greater 10-year risk for cardiovascular disease, the agency recommends that patients and clinicians make the decision to initiate low-dose aspirin use on a case-by-case basis, as the net benefit is small.
Dr. McQuilten said she spent the last 5 years designing substages of anemia and conditions such as blood cancer. In many cases of anemia, doctors are unable to determine the underlying cause, she said. One study published in the Journal of American Geriatrics Society in 2021 found that in about one-third of anemia cases, the etiology was not clear.
About 50% of people older than 60 who were involved in the latest study took aspirin for prevention from 2011 to 2018. That number likely dropped after changes were made to the guidelines in 2022, according to Dr. McQuilten, but long-term use may have continued among older patients. The researchers also examined ferritin levels, which serve as a proxy for iron levels, at baseline and after 3 years.
The incidence of anemia was 51 events per 1,000 person-years in the aspirin group compared with 43 events per 1,000 person-years in the placebo group, according to the researchers. The estimated probability of experiencing anemia within 5 years was 23.5% (95% confidence interval [CI], 22.4%-24.6%) in the aspirin group and 20.3% (95% CI: 19.3% to 21.4%) in the placebo group. Aspirin therapy resulted in a 20% increase in the risk for anemia (95% CI, 1.12-1.29).
People who took aspirin were more likely to have lower serum levels of ferritin at the 3-year mark than were those who received placebo. The average decrease in ferritin among participants who took aspirin was 11.5% greater (95% CI, 9.3%-13.7%) than among those who took placebo.
Basil Eldadah, MD, PhD, supervisory medical officer at the National Institute on Aging, part of the National Institutes of Health, said the findings should encourage clinicians to pay closer attention to hemoglobin levels and have conversations with patients to discuss their need for taking aspirin.
“If somebody is already taking aspirin for any reason, keep an eye on hemoglobin,” said Dr. Eldadah, who was not involved in the study. “For somebody who’s taking aspirin and who’s older, and it’s not for an indication like cardiovascular disease, consider seriously whether that’s the best treatment option.”
The study did not examine the functional consequences of anemia on participants, which Dr. Eldadah said could be fodder for future research. The researchers said one limitation was that it was not clear whether anemia was sufficient to cause symptoms that affected participants’ quality of life or whether occult bleeding caused the anemia. The researchers also did not document whether patients saw their regular physicians and received treatment for anemia over the course of the trial.
The study was funded through grants from the National Health and Medical Research Council and the Bill and Melinda Gates Foundation. The authors reported receiving consulting fees, honoraria, and stock options, and have participated on data monitoring boards not related to the study for Vifor Pharma, ITL Biomedical, Pfizer, Boehringer Ingelheim, Bayer Healthcare, AbbVie, and Abbott Diagnostics.
A version of this article originally appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Guide explains nonsurgical management of major hemorrhage
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
“Terrific progress”: Adding blinatumomab for infant leukemia
.
Two-year disease-free and overall survival measures, as well as the percentage of children who had complete minimal residual disease (MRD) responses, were substantially higher among the 30 infants in the study than in historical controls treated with the same chemotherapy backbone in an earlier trial, Interfant-06.
“These outcome data are very promising, given the poor survival and lack of improvements in outcomes among infants with KMT2A-rearranged ALL in recent decades,” said the investigators, led by Inge M. van der Sluis, MD, PhD, a hematologist-oncologist at Princess Maxima Center for Pediatric Oncology in Utrecht, the Netherlands.
“The low incidence of relapse after treatment with blinatumomab is remarkable, given that in historical controls relapses occur frequently and early during therapy,” the investigators stated. Although the “follow-up time was relatively short” in the study, “it included the period historically defined” as being at high risk of relapse, they said.
The team suggested that future research should assess whether infants benefit from multiple courses of blinatumomab, rather than the one course used in the study, and whether blinatumomab plus chemotherapy can replace stem cell transplants for high-risk infants.
Pediatric community responds
There was excitement on Twitter about the results; a number of pediatric blood cancer specialists were impressed and posted the study on that platform. Comments included, “Wow! After years of stagnation, a huge step forward for infant leukemia” and “great news for infant lymphoblastic leukemia.”
Akshay Sharma, MBBS, a pediatric bone marrow transplant and cellular therapy specialist at St. Jude Children’s Research Hospital, Memphis, also posted. He said in an interview that the findings are “very exciting.”
The “outcomes of children diagnosed with leukemia in their infancy, particularly if they have a KMT2A rearrangement, have been dismal. This is terrific progress and a testament to the role that immunotherapy and novel agents will be playing in treatment of several malignant diseases in the decade to come,” he said.
Another poster, Pratik “Tik” Patel, MD, a pediatric hematology/oncology fellow at Emory University in Atlanta, told this news organization that the study “is welcome news to pediatric oncologists” and highlights “the success in incorporating newer immune-based therapeutics upfront in treatment rather than in relapsed/refractory settings.”
The National Cancer Institute–funded Children’s Oncology Group is thinking the same way. The group is launching a large, randomized trial to test if adding blinatumomab to chemotherapy upfront for B-cell acute lymphoblastic leukemia and lymphoblastic lymphoma improves outcomes in children and young adults aged 1-31 years. Results are due after 2029.
Study details
Blinatumomab is an expensive “T-cell engager” that helps cytotoxic CD3+T cells link to and destroy leukemic CD19+ B cells. Past studies have shown that it’s safe and works in older children and adults with B-lineage ALL after intensive chemotherapy, but until now the approach hadn’t been tested in infants, the investigators said.
The 30 subjects in the study were under a year old and newly diagnosed with KMT2A-rearranged ALL. They were treated with the Interfant-06 chemotherapy regimen – cytosine arabinoside and other agents – plus one postinduction course of blinatumomab at 15 micrograms/m2 per day as a 4-week continuous infusion. Eight of nine high-risk patients had allogeneic hematopoietic stem cell transplants.
Overall survival was 93.3% over a median follow up of 26.3 months, substantially higher than the 65.8% in the Interfant-06 trial. Two-year disease-free survival was 81.6% versus 49.4% in Interfant-06.
Sixteen patients (53%) were MRD negative after blinatumomab infusion and 12 (40%) had low levels of MRD. All of the children who continued chemotherapy went on to become MRD negative.
There were no permanent blinatumomab discontinuations and no treatment related deaths. Serious toxic effects were consistent with those in older patients and included four fevers, four infections, and one case each of hypertension and vomiting.
There were no cases of severe cytokine release syndrome (CRS) because of the low tumor burden of the subjects. Likewise, there were no obvious neurologic adverse events – like CRS, a particular concern with blinatumomab – but “we cannot rule out underreporting of mild neurologic symptoms that may have been unrecognized in infants,” the investigators said.
Patients who relapsed in the study had CNS involvement at relapse. “This underscores the need for adequate intrathecal chemotherapy during the blinatumomab infusion, because the efficacy of blinatumomab for the treatment of CNS leukemia may be limited,” they said.
The work was supported by Amgen, the maker of blinatumomab, as well as the Princess Maxima Center Foundation, the Danish Childhood Cancer Foundation, and others. Dr. Sluis is a consultant and researcher for Amgen. Five other authors were also consultants/advisers/researchers for the company. Dr. Sharma and Dr. Patel didn’t have any relevant disclosures.
.
Two-year disease-free and overall survival measures, as well as the percentage of children who had complete minimal residual disease (MRD) responses, were substantially higher among the 30 infants in the study than in historical controls treated with the same chemotherapy backbone in an earlier trial, Interfant-06.
“These outcome data are very promising, given the poor survival and lack of improvements in outcomes among infants with KMT2A-rearranged ALL in recent decades,” said the investigators, led by Inge M. van der Sluis, MD, PhD, a hematologist-oncologist at Princess Maxima Center for Pediatric Oncology in Utrecht, the Netherlands.
“The low incidence of relapse after treatment with blinatumomab is remarkable, given that in historical controls relapses occur frequently and early during therapy,” the investigators stated. Although the “follow-up time was relatively short” in the study, “it included the period historically defined” as being at high risk of relapse, they said.
The team suggested that future research should assess whether infants benefit from multiple courses of blinatumomab, rather than the one course used in the study, and whether blinatumomab plus chemotherapy can replace stem cell transplants for high-risk infants.
Pediatric community responds
There was excitement on Twitter about the results; a number of pediatric blood cancer specialists were impressed and posted the study on that platform. Comments included, “Wow! After years of stagnation, a huge step forward for infant leukemia” and “great news for infant lymphoblastic leukemia.”
Akshay Sharma, MBBS, a pediatric bone marrow transplant and cellular therapy specialist at St. Jude Children’s Research Hospital, Memphis, also posted. He said in an interview that the findings are “very exciting.”
The “outcomes of children diagnosed with leukemia in their infancy, particularly if they have a KMT2A rearrangement, have been dismal. This is terrific progress and a testament to the role that immunotherapy and novel agents will be playing in treatment of several malignant diseases in the decade to come,” he said.
Another poster, Pratik “Tik” Patel, MD, a pediatric hematology/oncology fellow at Emory University in Atlanta, told this news organization that the study “is welcome news to pediatric oncologists” and highlights “the success in incorporating newer immune-based therapeutics upfront in treatment rather than in relapsed/refractory settings.”
The National Cancer Institute–funded Children’s Oncology Group is thinking the same way. The group is launching a large, randomized trial to test if adding blinatumomab to chemotherapy upfront for B-cell acute lymphoblastic leukemia and lymphoblastic lymphoma improves outcomes in children and young adults aged 1-31 years. Results are due after 2029.
Study details
Blinatumomab is an expensive “T-cell engager” that helps cytotoxic CD3+T cells link to and destroy leukemic CD19+ B cells. Past studies have shown that it’s safe and works in older children and adults with B-lineage ALL after intensive chemotherapy, but until now the approach hadn’t been tested in infants, the investigators said.
The 30 subjects in the study were under a year old and newly diagnosed with KMT2A-rearranged ALL. They were treated with the Interfant-06 chemotherapy regimen – cytosine arabinoside and other agents – plus one postinduction course of blinatumomab at 15 micrograms/m2 per day as a 4-week continuous infusion. Eight of nine high-risk patients had allogeneic hematopoietic stem cell transplants.
Overall survival was 93.3% over a median follow up of 26.3 months, substantially higher than the 65.8% in the Interfant-06 trial. Two-year disease-free survival was 81.6% versus 49.4% in Interfant-06.
Sixteen patients (53%) were MRD negative after blinatumomab infusion and 12 (40%) had low levels of MRD. All of the children who continued chemotherapy went on to become MRD negative.
There were no permanent blinatumomab discontinuations and no treatment related deaths. Serious toxic effects were consistent with those in older patients and included four fevers, four infections, and one case each of hypertension and vomiting.
There were no cases of severe cytokine release syndrome (CRS) because of the low tumor burden of the subjects. Likewise, there were no obvious neurologic adverse events – like CRS, a particular concern with blinatumomab – but “we cannot rule out underreporting of mild neurologic symptoms that may have been unrecognized in infants,” the investigators said.
Patients who relapsed in the study had CNS involvement at relapse. “This underscores the need for adequate intrathecal chemotherapy during the blinatumomab infusion, because the efficacy of blinatumomab for the treatment of CNS leukemia may be limited,” they said.
The work was supported by Amgen, the maker of blinatumomab, as well as the Princess Maxima Center Foundation, the Danish Childhood Cancer Foundation, and others. Dr. Sluis is a consultant and researcher for Amgen. Five other authors were also consultants/advisers/researchers for the company. Dr. Sharma and Dr. Patel didn’t have any relevant disclosures.
.
Two-year disease-free and overall survival measures, as well as the percentage of children who had complete minimal residual disease (MRD) responses, were substantially higher among the 30 infants in the study than in historical controls treated with the same chemotherapy backbone in an earlier trial, Interfant-06.
“These outcome data are very promising, given the poor survival and lack of improvements in outcomes among infants with KMT2A-rearranged ALL in recent decades,” said the investigators, led by Inge M. van der Sluis, MD, PhD, a hematologist-oncologist at Princess Maxima Center for Pediatric Oncology in Utrecht, the Netherlands.
“The low incidence of relapse after treatment with blinatumomab is remarkable, given that in historical controls relapses occur frequently and early during therapy,” the investigators stated. Although the “follow-up time was relatively short” in the study, “it included the period historically defined” as being at high risk of relapse, they said.
The team suggested that future research should assess whether infants benefit from multiple courses of blinatumomab, rather than the one course used in the study, and whether blinatumomab plus chemotherapy can replace stem cell transplants for high-risk infants.
Pediatric community responds
There was excitement on Twitter about the results; a number of pediatric blood cancer specialists were impressed and posted the study on that platform. Comments included, “Wow! After years of stagnation, a huge step forward for infant leukemia” and “great news for infant lymphoblastic leukemia.”
Akshay Sharma, MBBS, a pediatric bone marrow transplant and cellular therapy specialist at St. Jude Children’s Research Hospital, Memphis, also posted. He said in an interview that the findings are “very exciting.”
The “outcomes of children diagnosed with leukemia in their infancy, particularly if they have a KMT2A rearrangement, have been dismal. This is terrific progress and a testament to the role that immunotherapy and novel agents will be playing in treatment of several malignant diseases in the decade to come,” he said.
Another poster, Pratik “Tik” Patel, MD, a pediatric hematology/oncology fellow at Emory University in Atlanta, told this news organization that the study “is welcome news to pediatric oncologists” and highlights “the success in incorporating newer immune-based therapeutics upfront in treatment rather than in relapsed/refractory settings.”
The National Cancer Institute–funded Children’s Oncology Group is thinking the same way. The group is launching a large, randomized trial to test if adding blinatumomab to chemotherapy upfront for B-cell acute lymphoblastic leukemia and lymphoblastic lymphoma improves outcomes in children and young adults aged 1-31 years. Results are due after 2029.
Study details
Blinatumomab is an expensive “T-cell engager” that helps cytotoxic CD3+T cells link to and destroy leukemic CD19+ B cells. Past studies have shown that it’s safe and works in older children and adults with B-lineage ALL after intensive chemotherapy, but until now the approach hadn’t been tested in infants, the investigators said.
The 30 subjects in the study were under a year old and newly diagnosed with KMT2A-rearranged ALL. They were treated with the Interfant-06 chemotherapy regimen – cytosine arabinoside and other agents – plus one postinduction course of blinatumomab at 15 micrograms/m2 per day as a 4-week continuous infusion. Eight of nine high-risk patients had allogeneic hematopoietic stem cell transplants.
Overall survival was 93.3% over a median follow up of 26.3 months, substantially higher than the 65.8% in the Interfant-06 trial. Two-year disease-free survival was 81.6% versus 49.4% in Interfant-06.
Sixteen patients (53%) were MRD negative after blinatumomab infusion and 12 (40%) had low levels of MRD. All of the children who continued chemotherapy went on to become MRD negative.
There were no permanent blinatumomab discontinuations and no treatment related deaths. Serious toxic effects were consistent with those in older patients and included four fevers, four infections, and one case each of hypertension and vomiting.
There were no cases of severe cytokine release syndrome (CRS) because of the low tumor burden of the subjects. Likewise, there were no obvious neurologic adverse events – like CRS, a particular concern with blinatumomab – but “we cannot rule out underreporting of mild neurologic symptoms that may have been unrecognized in infants,” the investigators said.
Patients who relapsed in the study had CNS involvement at relapse. “This underscores the need for adequate intrathecal chemotherapy during the blinatumomab infusion, because the efficacy of blinatumomab for the treatment of CNS leukemia may be limited,” they said.
The work was supported by Amgen, the maker of blinatumomab, as well as the Princess Maxima Center Foundation, the Danish Childhood Cancer Foundation, and others. Dr. Sluis is a consultant and researcher for Amgen. Five other authors were also consultants/advisers/researchers for the company. Dr. Sharma and Dr. Patel didn’t have any relevant disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE