User login
Alpha-Gal Syndrome: 5 Things to Know
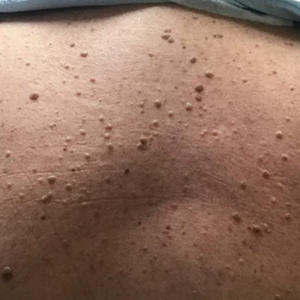
Alpha-gal syndrome (AGS), a tickborne disease commonly called “red meat allergy,” is a serious, potentially life-threatening allergy to the carbohydrate alpha-gal. The alpha-gal carbohydrate is found in most mammals, though it is not in humans, apes, or old-world monkeys. People with AGS can have allergic reactions when they consume mammalian meat, dairy products, or other products derived from mammals. People often live with this disease for years before receiving a correct diagnosis, greatly impacting their quality of life. The number of suspected cases is also rising.
More than 110,000 suspected AGS cases were identified between 2010 and 2022, according to a Centers for Disease Control and Prevention (CDC) report.1 However, because the diagnosis requires a positive test and a clinical exam and some people may not get tested, as many as 450,000 people might be affected by AGS in the United States. Additionally, a CDC survey found that nearly half (42%) of US healthcare providers had never heard of AGS.2 Among those who had, less than one third (29%) knew how to diagnose the condition.
Here are 5 things clinicians need to know about AGS.
1. People can develop AGS after being bitten by a tick, primarily the lone star tick (Amblyomma americanum), in the United States.
In the United States, AGS is primarily associated with the bite of a lone star tick, but other kinds of ticks have not been ruled out. The majority of suspected AGS cases in the United States were reported in parts of Arkansas, Delaware, Illinois, Indiana, Kansas, Kentucky, Maryland, Mississippi, Missouri, North Carolina, Oklahoma, Tennessee, and Virginia. The lone star tick is widely distributed with established populations in Alabama, Arkansas, Connecticut, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, New York, North Carolina, Ohio, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
While AGS is associated with tick bites, more research is needed to understand the role ticks play in starting this condition, and why certain people develop AGS. Anyone can develop AGS, but most cases have been reported in adults.
Know how to recognize the symptoms of AGS and be prepared to test, diagnose, and manage AGS, particularly in states where lone star ticks are found.
2. Tick bites are only one risk factor for developing AGS.
Many people are bitten by lone star ticks and will never develop AGS. Scientists are exploring the connection between other risk factors and developing AGS. A recent study has shown that people diagnosed with AGS may be more likely to have a family member who was also diagnosed with AGS, have another food allergy, have an allergy to stinging or biting insects, or have A or O blood types.3
Research has also shown that environmental risk factors could contribute to developing AGS,4 like living in an area with lone star ticks, remembering finding a tick on themselves, recalling multiple tick bites, living near a wooded forest, spending more time outside, or living in areas with deer, such as larger properties, wooded forests, and properties with shrubs and brush.
Ask your patient questions about other allergies and history of recent tick bites or outdoor exposure to help determine if testing for AGS is appropriate.
3. Symptoms of AGS are consistently inconsistent.
There is a spectrum of how sensitive AGS patients are to alpha-gal, and reactions are often different from person to person, which can make it difficult to diagnose. The first allergic reaction to AGS typically occurs between 1-6 months after a tick bite. Symptoms commonly appear 2-6 hours after being in contact with products containing alpha-gal, like red meat (beef, pork, lamb, venison, rabbit, or other meat from mammals), dairy, and some medications. Symptoms can range from mild to severe and include hives or itchy rash; swelling of the lips, throat, tongue, or eyelids; gastrointestinal symptoms such as nausea, vomiting, or diarrhea; heartburn or indigestion; cough, shortness of breath, or difficulty breathing; dizziness or a drop in blood pressure; or anaphylaxis.
Consider AGS if a patient reports waking up in the middle of the night with allergic symptoms after eating alpha-gal containing products for dinner, if allergic reactions are delayed, or if a patient has anaphylaxis of unknown cause, adult-onset allergy, or allergic symptoms and reports a recent tick bite.
4. Diagnosing AGS requires a combination of a blood test and a physical exam.
Diagnosing AGS requires a detailed patient history, physical exam, and a blood test to detect specific immunoglobulin E (IgE) antibodies specific to alpha-gal (alpha-gal sIgE). Tests for alpha-gal sIgE antibodies are available at several large commercial laboratories and some academic institutions. Skin tests to identify reactions to allergens like pork or beef may also be used to inform AGS diagnosis. However, a positive alpha-gal sIgE test or skin test does not mean a person has AGS. Many people, particularly those who live in regions with lone star ticks, have positive alpha-gal specific IgE tests without having AGS.
Consider the test results along with your patient’s symptoms and risk factors.
5. There is no treatment for AGS, but people can take prevention steps and AGS can be managed.
People can protect themselves and their family from AGS by preventing tick bites. Encourage your patients to use an Environmental Protection Agency–registered insect repellent outdoors, wear permethrin-treated clothing, and conduct thorough tick checks after outdoor activities.
Once a person is no longer exposed to alpha-gal containing products, they should no longer experience symptoms. People with AGS should also proactively prevent tick bites. Tick bites can trigger or reactivate AGS.
For patients who have AGS, help manage their symptoms and identify alpha-gal containing products to avoid.
Dr. Kersh is Chief of the Rickettsial Zoonoses Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, and disclosed no relevant conflicts of interest.
CDC resources:
About Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Testing and Diagnosis for Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Resources | Alpha-gal Syndrome | CDC
References
Thompson JM et al. MMWR Morb Mortal Wkly Rep. 2023;72:815-820.
Carpenter A et al. MMWR Morb Mortal Wkly Rep. 2023;72:809-814. Taylor ML et al. Ann Allergy, Asthma & Immunol. 2024 Jun;132(6):759.e2-764.e2. Kersh GJ et al. Ann Allergy, Asthma & Immunol. 2023 Apr;130(4):472-478.
Alpha-gal syndrome (AGS), a tickborne disease commonly called “red meat allergy,” is a serious, potentially life-threatening allergy to the carbohydrate alpha-gal. The alpha-gal carbohydrate is found in most mammals, though it is not in humans, apes, or old-world monkeys. People with AGS can have allergic reactions when they consume mammalian meat, dairy products, or other products derived from mammals. People often live with this disease for years before receiving a correct diagnosis, greatly impacting their quality of life. The number of suspected cases is also rising.
More than 110,000 suspected AGS cases were identified between 2010 and 2022, according to a Centers for Disease Control and Prevention (CDC) report.1 However, because the diagnosis requires a positive test and a clinical exam and some people may not get tested, as many as 450,000 people might be affected by AGS in the United States. Additionally, a CDC survey found that nearly half (42%) of US healthcare providers had never heard of AGS.2 Among those who had, less than one third (29%) knew how to diagnose the condition.
Here are 5 things clinicians need to know about AGS.
1. People can develop AGS after being bitten by a tick, primarily the lone star tick (Amblyomma americanum), in the United States.
In the United States, AGS is primarily associated with the bite of a lone star tick, but other kinds of ticks have not been ruled out. The majority of suspected AGS cases in the United States were reported in parts of Arkansas, Delaware, Illinois, Indiana, Kansas, Kentucky, Maryland, Mississippi, Missouri, North Carolina, Oklahoma, Tennessee, and Virginia. The lone star tick is widely distributed with established populations in Alabama, Arkansas, Connecticut, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, New York, North Carolina, Ohio, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
While AGS is associated with tick bites, more research is needed to understand the role ticks play in starting this condition, and why certain people develop AGS. Anyone can develop AGS, but most cases have been reported in adults.
Know how to recognize the symptoms of AGS and be prepared to test, diagnose, and manage AGS, particularly in states where lone star ticks are found.
2. Tick bites are only one risk factor for developing AGS.
Many people are bitten by lone star ticks and will never develop AGS. Scientists are exploring the connection between other risk factors and developing AGS. A recent study has shown that people diagnosed with AGS may be more likely to have a family member who was also diagnosed with AGS, have another food allergy, have an allergy to stinging or biting insects, or have A or O blood types.3
Research has also shown that environmental risk factors could contribute to developing AGS,4 like living in an area with lone star ticks, remembering finding a tick on themselves, recalling multiple tick bites, living near a wooded forest, spending more time outside, or living in areas with deer, such as larger properties, wooded forests, and properties with shrubs and brush.
Ask your patient questions about other allergies and history of recent tick bites or outdoor exposure to help determine if testing for AGS is appropriate.
3. Symptoms of AGS are consistently inconsistent.
There is a spectrum of how sensitive AGS patients are to alpha-gal, and reactions are often different from person to person, which can make it difficult to diagnose. The first allergic reaction to AGS typically occurs between 1-6 months after a tick bite. Symptoms commonly appear 2-6 hours after being in contact with products containing alpha-gal, like red meat (beef, pork, lamb, venison, rabbit, or other meat from mammals), dairy, and some medications. Symptoms can range from mild to severe and include hives or itchy rash; swelling of the lips, throat, tongue, or eyelids; gastrointestinal symptoms such as nausea, vomiting, or diarrhea; heartburn or indigestion; cough, shortness of breath, or difficulty breathing; dizziness or a drop in blood pressure; or anaphylaxis.
Consider AGS if a patient reports waking up in the middle of the night with allergic symptoms after eating alpha-gal containing products for dinner, if allergic reactions are delayed, or if a patient has anaphylaxis of unknown cause, adult-onset allergy, or allergic symptoms and reports a recent tick bite.
4. Diagnosing AGS requires a combination of a blood test and a physical exam.
Diagnosing AGS requires a detailed patient history, physical exam, and a blood test to detect specific immunoglobulin E (IgE) antibodies specific to alpha-gal (alpha-gal sIgE). Tests for alpha-gal sIgE antibodies are available at several large commercial laboratories and some academic institutions. Skin tests to identify reactions to allergens like pork or beef may also be used to inform AGS diagnosis. However, a positive alpha-gal sIgE test or skin test does not mean a person has AGS. Many people, particularly those who live in regions with lone star ticks, have positive alpha-gal specific IgE tests without having AGS.
Consider the test results along with your patient’s symptoms and risk factors.
5. There is no treatment for AGS, but people can take prevention steps and AGS can be managed.
People can protect themselves and their family from AGS by preventing tick bites. Encourage your patients to use an Environmental Protection Agency–registered insect repellent outdoors, wear permethrin-treated clothing, and conduct thorough tick checks after outdoor activities.
Once a person is no longer exposed to alpha-gal containing products, they should no longer experience symptoms. People with AGS should also proactively prevent tick bites. Tick bites can trigger or reactivate AGS.
For patients who have AGS, help manage their symptoms and identify alpha-gal containing products to avoid.
Dr. Kersh is Chief of the Rickettsial Zoonoses Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, and disclosed no relevant conflicts of interest.
CDC resources:
About Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Testing and Diagnosis for Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Resources | Alpha-gal Syndrome | CDC
References
Thompson JM et al. MMWR Morb Mortal Wkly Rep. 2023;72:815-820.
Carpenter A et al. MMWR Morb Mortal Wkly Rep. 2023;72:809-814. Taylor ML et al. Ann Allergy, Asthma & Immunol. 2024 Jun;132(6):759.e2-764.e2. Kersh GJ et al. Ann Allergy, Asthma & Immunol. 2023 Apr;130(4):472-478.
Alpha-gal syndrome (AGS), a tickborne disease commonly called “red meat allergy,” is a serious, potentially life-threatening allergy to the carbohydrate alpha-gal. The alpha-gal carbohydrate is found in most mammals, though it is not in humans, apes, or old-world monkeys. People with AGS can have allergic reactions when they consume mammalian meat, dairy products, or other products derived from mammals. People often live with this disease for years before receiving a correct diagnosis, greatly impacting their quality of life. The number of suspected cases is also rising.
More than 110,000 suspected AGS cases were identified between 2010 and 2022, according to a Centers for Disease Control and Prevention (CDC) report.1 However, because the diagnosis requires a positive test and a clinical exam and some people may not get tested, as many as 450,000 people might be affected by AGS in the United States. Additionally, a CDC survey found that nearly half (42%) of US healthcare providers had never heard of AGS.2 Among those who had, less than one third (29%) knew how to diagnose the condition.
Here are 5 things clinicians need to know about AGS.
1. People can develop AGS after being bitten by a tick, primarily the lone star tick (Amblyomma americanum), in the United States.
In the United States, AGS is primarily associated with the bite of a lone star tick, but other kinds of ticks have not been ruled out. The majority of suspected AGS cases in the United States were reported in parts of Arkansas, Delaware, Illinois, Indiana, Kansas, Kentucky, Maryland, Mississippi, Missouri, North Carolina, Oklahoma, Tennessee, and Virginia. The lone star tick is widely distributed with established populations in Alabama, Arkansas, Connecticut, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, New York, North Carolina, Ohio, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
While AGS is associated with tick bites, more research is needed to understand the role ticks play in starting this condition, and why certain people develop AGS. Anyone can develop AGS, but most cases have been reported in adults.
Know how to recognize the symptoms of AGS and be prepared to test, diagnose, and manage AGS, particularly in states where lone star ticks are found.
2. Tick bites are only one risk factor for developing AGS.
Many people are bitten by lone star ticks and will never develop AGS. Scientists are exploring the connection between other risk factors and developing AGS. A recent study has shown that people diagnosed with AGS may be more likely to have a family member who was also diagnosed with AGS, have another food allergy, have an allergy to stinging or biting insects, or have A or O blood types.3
Research has also shown that environmental risk factors could contribute to developing AGS,4 like living in an area with lone star ticks, remembering finding a tick on themselves, recalling multiple tick bites, living near a wooded forest, spending more time outside, or living in areas with deer, such as larger properties, wooded forests, and properties with shrubs and brush.
Ask your patient questions about other allergies and history of recent tick bites or outdoor exposure to help determine if testing for AGS is appropriate.
3. Symptoms of AGS are consistently inconsistent.
There is a spectrum of how sensitive AGS patients are to alpha-gal, and reactions are often different from person to person, which can make it difficult to diagnose. The first allergic reaction to AGS typically occurs between 1-6 months after a tick bite. Symptoms commonly appear 2-6 hours after being in contact with products containing alpha-gal, like red meat (beef, pork, lamb, venison, rabbit, or other meat from mammals), dairy, and some medications. Symptoms can range from mild to severe and include hives or itchy rash; swelling of the lips, throat, tongue, or eyelids; gastrointestinal symptoms such as nausea, vomiting, or diarrhea; heartburn or indigestion; cough, shortness of breath, or difficulty breathing; dizziness or a drop in blood pressure; or anaphylaxis.
Consider AGS if a patient reports waking up in the middle of the night with allergic symptoms after eating alpha-gal containing products for dinner, if allergic reactions are delayed, or if a patient has anaphylaxis of unknown cause, adult-onset allergy, or allergic symptoms and reports a recent tick bite.
4. Diagnosing AGS requires a combination of a blood test and a physical exam.
Diagnosing AGS requires a detailed patient history, physical exam, and a blood test to detect specific immunoglobulin E (IgE) antibodies specific to alpha-gal (alpha-gal sIgE). Tests for alpha-gal sIgE antibodies are available at several large commercial laboratories and some academic institutions. Skin tests to identify reactions to allergens like pork or beef may also be used to inform AGS diagnosis. However, a positive alpha-gal sIgE test or skin test does not mean a person has AGS. Many people, particularly those who live in regions with lone star ticks, have positive alpha-gal specific IgE tests without having AGS.
Consider the test results along with your patient’s symptoms and risk factors.
5. There is no treatment for AGS, but people can take prevention steps and AGS can be managed.
People can protect themselves and their family from AGS by preventing tick bites. Encourage your patients to use an Environmental Protection Agency–registered insect repellent outdoors, wear permethrin-treated clothing, and conduct thorough tick checks after outdoor activities.
Once a person is no longer exposed to alpha-gal containing products, they should no longer experience symptoms. People with AGS should also proactively prevent tick bites. Tick bites can trigger or reactivate AGS.
For patients who have AGS, help manage their symptoms and identify alpha-gal containing products to avoid.
Dr. Kersh is Chief of the Rickettsial Zoonoses Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, and disclosed no relevant conflicts of interest.
CDC resources:
About Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Testing and Diagnosis for Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Resources | Alpha-gal Syndrome | CDC
References
Thompson JM et al. MMWR Morb Mortal Wkly Rep. 2023;72:815-820.
Carpenter A et al. MMWR Morb Mortal Wkly Rep. 2023;72:809-814. Taylor ML et al. Ann Allergy, Asthma & Immunol. 2024 Jun;132(6):759.e2-764.e2. Kersh GJ et al. Ann Allergy, Asthma & Immunol. 2023 Apr;130(4):472-478.
Understanding of Hidradenitis Suppurativa Pathophysiology Advancing
NEW YORK, NY — , according to two investigators intimately involved in much of the recent progress.
“Success is being achieved by targeting multiple inflammatory axes in HS, and therapeutics are evolving rapidly,” reported James G. Krueger, MD, PhD, head of the Laboratory of Investigative Dermatology, Rockefeller University, New York, NY.
The activity of targeted anti-inflammatory therapies — bimekizumab just joined adalimumab and secukinumab as a third approved biologic for HS — is not news, but the degree to which inflammation is upregulated systemically, not just at areas of skin involvement, has changed the conceptualization of HS.
HS Is a Systemic Inflammatory Disease
Relative to psoriasis, for which there are many parallels, “HS is hugely more inflammatory in the systemic circulation,” Krueger said at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024. Yet, HS is also more complex involving additional pathways that appear to include dysbiosis. The concept of follicular occlusion, once a common explanation for HS, has been left far behind.
“Unlike psoriasis, which we can treat really well by inhibiting a single pathway target, HS is just not that simple,” Krueger said. Although largely an inflammatory process, the cascade of inflammatory factors for specific manifestations, such as tunnels, means that optimal therapy in one case might have little benefit in another.
The relatively new evidence that HS activity is not confined to lesional skin might be the most important recent step toward new strategies to target disease. These studies were performed by Kristina Navrazhina, MD, PhD, now a resident in dermatology at the Icahn School of Medicine at Mount Sinai, New York. She received her PhD while studying HS activity in non-lesional skin. Her work has led her to conclude that the best chance for better outcomes in HS is early diagnosis and treatment. Although this is generally true of any pathology, the changes in the HS phenotype once fistulae form includes a poor response to conventional therapies.
In fact, based on her work in evaluating HS activity in non-lesional skin, Navrazhina has shown that “many patients with modest lesions already have advanced disease.” Consistent with the premise that HS is a deeply systemic inflammatory process, nodules, considered an early manifestation, turn out to be “the tip of the iceberg.”
Non-Lesional HS Skin Is Inflamed
When she has employed RNA sequencing based on tape strip sampling from completely normal skin away from nodules, interleukin (IL)-17 and a broad array of other inflammatory markers were found to be upregulated. When she performed ultrasound to look for disease activity under the normal skin, she has often found tunnels already formed. Doppler ultrasound showed some of these tunnels were actively draining.
This might provide a partial explanation for why therapies are not always effective even when clinical signs of disease are modest.
“Are we missing the opportunity for intervening?” Navrazhina asked, noting that early intervention has been limited traditionally by extremely long diagnostic delays. Citing the literature, Navrazhina said the average delay is 7 years for HS versus 1 year for psoriasis. Patients often cycle through 3 or 4 providers before the diagnosis is made, she said.
Awakening first-line clinicians to the signs and symptoms of HS, whether in the emergency room or primary care, is a critical message because of the incrementally difficult task to control disease once fistulae have formed.
Krueger made the same appeal. For the neutrophilic inflammation that characterizes nodules, targeted therapies are often effective, but he agreed that available therapies are generally far less so once tunnels form.
Role Seen for Bacteria in HS Pathogenesis
One reason might be an interaction between anaerobic bacteria and the keratinocytes that form the tunnel walls, according to Krueger. Although HS is not typically considered an infectious disease, he reported that the interaction of these bacteria with keratinocytes is associated with expression of approximately 1000 inflammatory gene products. The process of tunnel formation is traced to how factors recruited by upregulated inflammation, such as chemokines, coordinate.
He described recent work pursing novel strategies such as highly targeted antibiotics or inhibitors of complement factor C5a, which has been proposed as a biomarker for HS, to intervene in preventing or reversing HS tunnels.
While this work progresses, one of the most Important unmet needs in HS is an accepted measure of clinically meaningful improvement in advanced disease, particularly the impact of therapy on HS tunnels, according to Krueger.
“There is no measure of tunnel activity that the FDA accepts in evaluating drugs,” he noted, which will be essential for approving therapies that offer this benefit.
A phase 3 trials program for one of the promising drugs, sonelokimab, was announced early in 2024. A nanobody that targets IL-17A/A, IL-17A/F, and IL-17F/F, the small size of this molecule permits exceptional tissue penetration while the broad anti-IL-17 activity has a high degree of theoretical potential in late-stage HS, according to Krueger.
There are numerous pieces of the HS puzzle that are still missing, but both Krueger and Navrazhina are enthusiastic about new targets and opportunities for disease control that are stemming from a better understanding of the underlying pathophysiology. Not least, both indicated that testing for inflammatory phenotypes will allow for individualized therapeutic choices with a maximum likelihood of response, particularly if earlier diagnosis permits earlier treatment.
“Due to the heterogeneity of HS, it is hard to know who will respond to which treatment or which treatment should be started first,” Navrazhina said. She thinks that early measures of the inflammatory profile in nodules or even non-lesional skin might provide that guidance.
Both Krueger and Navrazhina reported no financial relationships relevant to this work.
A version of this article appeared on Medscape.com.
NEW YORK, NY — , according to two investigators intimately involved in much of the recent progress.
“Success is being achieved by targeting multiple inflammatory axes in HS, and therapeutics are evolving rapidly,” reported James G. Krueger, MD, PhD, head of the Laboratory of Investigative Dermatology, Rockefeller University, New York, NY.
The activity of targeted anti-inflammatory therapies — bimekizumab just joined adalimumab and secukinumab as a third approved biologic for HS — is not news, but the degree to which inflammation is upregulated systemically, not just at areas of skin involvement, has changed the conceptualization of HS.
HS Is a Systemic Inflammatory Disease
Relative to psoriasis, for which there are many parallels, “HS is hugely more inflammatory in the systemic circulation,” Krueger said at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024. Yet, HS is also more complex involving additional pathways that appear to include dysbiosis. The concept of follicular occlusion, once a common explanation for HS, has been left far behind.
“Unlike psoriasis, which we can treat really well by inhibiting a single pathway target, HS is just not that simple,” Krueger said. Although largely an inflammatory process, the cascade of inflammatory factors for specific manifestations, such as tunnels, means that optimal therapy in one case might have little benefit in another.
The relatively new evidence that HS activity is not confined to lesional skin might be the most important recent step toward new strategies to target disease. These studies were performed by Kristina Navrazhina, MD, PhD, now a resident in dermatology at the Icahn School of Medicine at Mount Sinai, New York. She received her PhD while studying HS activity in non-lesional skin. Her work has led her to conclude that the best chance for better outcomes in HS is early diagnosis and treatment. Although this is generally true of any pathology, the changes in the HS phenotype once fistulae form includes a poor response to conventional therapies.
In fact, based on her work in evaluating HS activity in non-lesional skin, Navrazhina has shown that “many patients with modest lesions already have advanced disease.” Consistent with the premise that HS is a deeply systemic inflammatory process, nodules, considered an early manifestation, turn out to be “the tip of the iceberg.”
Non-Lesional HS Skin Is Inflamed
When she has employed RNA sequencing based on tape strip sampling from completely normal skin away from nodules, interleukin (IL)-17 and a broad array of other inflammatory markers were found to be upregulated. When she performed ultrasound to look for disease activity under the normal skin, she has often found tunnels already formed. Doppler ultrasound showed some of these tunnels were actively draining.
This might provide a partial explanation for why therapies are not always effective even when clinical signs of disease are modest.
“Are we missing the opportunity for intervening?” Navrazhina asked, noting that early intervention has been limited traditionally by extremely long diagnostic delays. Citing the literature, Navrazhina said the average delay is 7 years for HS versus 1 year for psoriasis. Patients often cycle through 3 or 4 providers before the diagnosis is made, she said.
Awakening first-line clinicians to the signs and symptoms of HS, whether in the emergency room or primary care, is a critical message because of the incrementally difficult task to control disease once fistulae have formed.
Krueger made the same appeal. For the neutrophilic inflammation that characterizes nodules, targeted therapies are often effective, but he agreed that available therapies are generally far less so once tunnels form.
Role Seen for Bacteria in HS Pathogenesis
One reason might be an interaction between anaerobic bacteria and the keratinocytes that form the tunnel walls, according to Krueger. Although HS is not typically considered an infectious disease, he reported that the interaction of these bacteria with keratinocytes is associated with expression of approximately 1000 inflammatory gene products. The process of tunnel formation is traced to how factors recruited by upregulated inflammation, such as chemokines, coordinate.
He described recent work pursing novel strategies such as highly targeted antibiotics or inhibitors of complement factor C5a, which has been proposed as a biomarker for HS, to intervene in preventing or reversing HS tunnels.
While this work progresses, one of the most Important unmet needs in HS is an accepted measure of clinically meaningful improvement in advanced disease, particularly the impact of therapy on HS tunnels, according to Krueger.
“There is no measure of tunnel activity that the FDA accepts in evaluating drugs,” he noted, which will be essential for approving therapies that offer this benefit.
A phase 3 trials program for one of the promising drugs, sonelokimab, was announced early in 2024. A nanobody that targets IL-17A/A, IL-17A/F, and IL-17F/F, the small size of this molecule permits exceptional tissue penetration while the broad anti-IL-17 activity has a high degree of theoretical potential in late-stage HS, according to Krueger.
There are numerous pieces of the HS puzzle that are still missing, but both Krueger and Navrazhina are enthusiastic about new targets and opportunities for disease control that are stemming from a better understanding of the underlying pathophysiology. Not least, both indicated that testing for inflammatory phenotypes will allow for individualized therapeutic choices with a maximum likelihood of response, particularly if earlier diagnosis permits earlier treatment.
“Due to the heterogeneity of HS, it is hard to know who will respond to which treatment or which treatment should be started first,” Navrazhina said. She thinks that early measures of the inflammatory profile in nodules or even non-lesional skin might provide that guidance.
Both Krueger and Navrazhina reported no financial relationships relevant to this work.
A version of this article appeared on Medscape.com.
NEW YORK, NY — , according to two investigators intimately involved in much of the recent progress.
“Success is being achieved by targeting multiple inflammatory axes in HS, and therapeutics are evolving rapidly,” reported James G. Krueger, MD, PhD, head of the Laboratory of Investigative Dermatology, Rockefeller University, New York, NY.
The activity of targeted anti-inflammatory therapies — bimekizumab just joined adalimumab and secukinumab as a third approved biologic for HS — is not news, but the degree to which inflammation is upregulated systemically, not just at areas of skin involvement, has changed the conceptualization of HS.
HS Is a Systemic Inflammatory Disease
Relative to psoriasis, for which there are many parallels, “HS is hugely more inflammatory in the systemic circulation,” Krueger said at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024. Yet, HS is also more complex involving additional pathways that appear to include dysbiosis. The concept of follicular occlusion, once a common explanation for HS, has been left far behind.
“Unlike psoriasis, which we can treat really well by inhibiting a single pathway target, HS is just not that simple,” Krueger said. Although largely an inflammatory process, the cascade of inflammatory factors for specific manifestations, such as tunnels, means that optimal therapy in one case might have little benefit in another.
The relatively new evidence that HS activity is not confined to lesional skin might be the most important recent step toward new strategies to target disease. These studies were performed by Kristina Navrazhina, MD, PhD, now a resident in dermatology at the Icahn School of Medicine at Mount Sinai, New York. She received her PhD while studying HS activity in non-lesional skin. Her work has led her to conclude that the best chance for better outcomes in HS is early diagnosis and treatment. Although this is generally true of any pathology, the changes in the HS phenotype once fistulae form includes a poor response to conventional therapies.
In fact, based on her work in evaluating HS activity in non-lesional skin, Navrazhina has shown that “many patients with modest lesions already have advanced disease.” Consistent with the premise that HS is a deeply systemic inflammatory process, nodules, considered an early manifestation, turn out to be “the tip of the iceberg.”
Non-Lesional HS Skin Is Inflamed
When she has employed RNA sequencing based on tape strip sampling from completely normal skin away from nodules, interleukin (IL)-17 and a broad array of other inflammatory markers were found to be upregulated. When she performed ultrasound to look for disease activity under the normal skin, she has often found tunnels already formed. Doppler ultrasound showed some of these tunnels were actively draining.
This might provide a partial explanation for why therapies are not always effective even when clinical signs of disease are modest.
“Are we missing the opportunity for intervening?” Navrazhina asked, noting that early intervention has been limited traditionally by extremely long diagnostic delays. Citing the literature, Navrazhina said the average delay is 7 years for HS versus 1 year for psoriasis. Patients often cycle through 3 or 4 providers before the diagnosis is made, she said.
Awakening first-line clinicians to the signs and symptoms of HS, whether in the emergency room or primary care, is a critical message because of the incrementally difficult task to control disease once fistulae have formed.
Krueger made the same appeal. For the neutrophilic inflammation that characterizes nodules, targeted therapies are often effective, but he agreed that available therapies are generally far less so once tunnels form.
Role Seen for Bacteria in HS Pathogenesis
One reason might be an interaction between anaerobic bacteria and the keratinocytes that form the tunnel walls, according to Krueger. Although HS is not typically considered an infectious disease, he reported that the interaction of these bacteria with keratinocytes is associated with expression of approximately 1000 inflammatory gene products. The process of tunnel formation is traced to how factors recruited by upregulated inflammation, such as chemokines, coordinate.
He described recent work pursing novel strategies such as highly targeted antibiotics or inhibitors of complement factor C5a, which has been proposed as a biomarker for HS, to intervene in preventing or reversing HS tunnels.
While this work progresses, one of the most Important unmet needs in HS is an accepted measure of clinically meaningful improvement in advanced disease, particularly the impact of therapy on HS tunnels, according to Krueger.
“There is no measure of tunnel activity that the FDA accepts in evaluating drugs,” he noted, which will be essential for approving therapies that offer this benefit.
A phase 3 trials program for one of the promising drugs, sonelokimab, was announced early in 2024. A nanobody that targets IL-17A/A, IL-17A/F, and IL-17F/F, the small size of this molecule permits exceptional tissue penetration while the broad anti-IL-17 activity has a high degree of theoretical potential in late-stage HS, according to Krueger.
There are numerous pieces of the HS puzzle that are still missing, but both Krueger and Navrazhina are enthusiastic about new targets and opportunities for disease control that are stemming from a better understanding of the underlying pathophysiology. Not least, both indicated that testing for inflammatory phenotypes will allow for individualized therapeutic choices with a maximum likelihood of response, particularly if earlier diagnosis permits earlier treatment.
“Due to the heterogeneity of HS, it is hard to know who will respond to which treatment or which treatment should be started first,” Navrazhina said. She thinks that early measures of the inflammatory profile in nodules or even non-lesional skin might provide that guidance.
Both Krueger and Navrazhina reported no financial relationships relevant to this work.
A version of this article appeared on Medscape.com.
Geriatric Dermatology: Q&A With Daniel C. Butler, MD
Daniel C. Butler, MD, is associate professor of dermatology and director of the new Inflammatory and Aging Skin Research Program in the Division of Dermatology at the University of Arizona College of Medicine, Tucson, Arizona. Before returning to Arizona, where he had attended medical school, Butler practiced and was a researcher at the University of California, San Francisco, and its geriatric dermatology clinic. He is a co-founder and continues to co-lead the American Academy of Dermatology (AAD) Geriatric Dermatology Expert Resource Group (ERG).
Butler’s interest in geriatric dermatology is rooted in his experience growing up with four grandparents and witnessing their wisdom, relationships, moments with loved ones, and other unique and desirable parts of growing old. “When I looked later at how aging was perceived in dermatology, I found it was a lot about ‘antiaging,’” he told this news organization. “I thought there was a needed voice in dermatology for healthy aging, for all the desirable things that only growing old can provide, along with all the incredible ‘antiaging’ things we can do.”
In interviews, Butler spoke about research priorities in geriatric dermatology, how the “4M” model of geriatrics should be applied within dermatology, how dermatologists can best work with older complex patients, and more. The conversation was edited for clarity and length.
What is geriatric dermatology? It is described by the AAD’s Geriatric Dermatology ERG as “an emerging subspecialty.” Yet it’s also viewed more broadly. Please speak about its various identities and meanings and its importance for dermatology.
If you’re a Mohs surgeon, you’re seeing a strong majority of over 65 patients. And in various specialty clinics, such as inflammatory skin disease, geriatric dermatology pertains to you. In many ways, it can be viewed as a mindset.
From a framework standpoint, and as a field, geriatric dermatology is a basic science initiative, a clinical initiative, an educational initiative, and an advocacy initiative. The goal is to be able to influence, grow, and learn in each of these categories for our older patients. This is happening: Research in this field has progressed, and education has progressed, which has driven some progress in clinical care.
How has research progressed in the basic science of aging skin? What are key questions for dermatology?
There has been a lot of basic science research on aging skin and on how an aging immune system, for instance, is reflected in conditions such as bullous pemphigoid, atopic dermatitis (AD), and chronic itch. But aging involves more than immunosenescence. I think of aging skin as a three-headed monster that involves changes in the skin barrier and the microbiome as well. But is there a primary piece of aging in the skin? What comes first or influences the other? More research on these questions can potentially influence our treatments.
With respect to the immune system, what we’re finding in the skin is that age-related change is not a decline in the immune system per se, but rather aberrance in response. Parts of the system tend to become overactive, with a skew toward overexpression of type 2 inflammation. This can be problematic, driving conditions such as chronic itch.
With respect to the skin barrier, we lose essential fatty acids, and we lose a lot of our recovery ability and our ability to respond quickly to environmental stressors. But are barrier changes triggering the immune system? Or is it the other way around?
The microbiome, which is a big focus of research, involves similar chicken-and-egg discussions. Is it the microbiome that changes and alters the barrier, which then entices the immune system? Which one happens first? We have a lot to learn, and there’s probably not one answer for every patient.
Please speak about research more broadly. What questions and issues need to be answered and addressed to improve the dermatologic care of older adults?
In general, research in dermatology is very disease-specific and not particularly conducive to looking at the larger demographic populations. We have a huge opportunity, therefore, to break the mold and grow geriatric dermatology as an area of population-based research — so that geriatric dermatology research encompasses not only the melanoma researcher who’s trying to understand how aging influences the melanocytes but also the epidemiologic researcher looking at how our diagnoses and coding and prescription practices are different in the 65-plus age group.
Clinically speaking, researchers want to better understand how aging influences the clinical presentations of our diseases. And there’s research to be done on best practices. For example, what are the best practices for treating basal cell carcinomas in patients with mild cognitive impairment? How should we consider the use of topicals in a patient who has severe arthritis or who lives alone? And then how should we teach practical approaches to help providers meet people where they are?
Looking at it from a healthcare system standpoint, there are many care delivery and access issues — practical pieces — to research, and we’re getting a lot better with this. We’re also advocating not only for more inclusion of older adults in clinical trials of treatments but also for the use of evaluations and outcomes that are relevant and important for older adults.
One piece of good news is that we’re seeing safer treatment options with tremendous efficacy that target known pathways for diseases like AD and chronic itch that affect older adults. Again, now we must find ways to improve access to these novel, safe options.
Our research program at the University of Arizona College of Medicine, which we’re just getting off the ground, aims to be dual-sided, looking both at the basic science of aging skin and at access and care delivery issues, such as how to ensure that patients on Medicare have access to medications that are at least on par with others with private insurance.
What are the most common dermatologic problems experienced by older adults?
Based on my experience and on research that we expect to be published soon, it’s absolutely nonmelanoma skin cancers, precancers like actinic keratoses — and on the inflammatory disease side, itch, AD, and psoriasis. Of course, also common are the age-related changes to the skin that we put in the benign category, such as solar lentigines.
How does age influence dermatologic diseases from a pathophysiological and clinical standpoint?
Diseases overall are very similar and respond to the same treatments, but age in and of itself does influence little pieces. For example, there is more crossover in the presentation of psoriasis and AD in older adults, leading to delays in the diagnosis of psoriasis.
With AD, we’ve found that itch is the predominant symptom for older adults rather than the red rash. We see higher or more severe itch scores in older adults with AD with less visual changes on the skin than in younger cohorts. And rash occurs in different locations than in young patients. Older adults typically present with it on their chest, back, and across the trunk, rather than in folded areas. They’re also more likely to get it on their legs in a nummular pattern as opposed to the more traditional flexural area presentation.
What unique considerations need to be made in treating older adults? How should the 4M model of geriatrics be applied to dermatologic care?
Our care model pushes us to be very algorithmic, but at the end of the day, what’s really important are the 4Ms: Mobility, medication, mentation, and “what matters most.” As you’re having your shared decision-making conversations with your patients and their families, these should be your priorities.
A patient with physical limitations, for instance, may not be able to apply a topical cream twice a day all over the body. They may have comorbidities and treatments for these comorbidities that may conflict with medications you’re considering.
And then mentation is so important. For a long time, we used antihistamines for older adults, but this has been proven to be bad for their mentation and risky in other ways. We need to be sure we’re prioritizing their ability to be clear mentally when we’re prescribing medications and even when we’re considering surgical approaches. Do they show capacity for that procedure or treatment, and how will they respond to that treatment later on?
Using the 4M model to drive conversations is a way to get all of us to connect to the patient and learn about what’s most important for them. In many ways, geriatrics is about taking a step back from your specialist skills and thinking about how you would want a family member treated.
We want to avoid treating just the lesion or the pathologic diagnosis. We want to avoid the “conveyor belt” from a biopsy to Mohs. I have 95-year-olds who say, “Heck yeah, if Mohs is the best treatment, that’s what I want.” And I have 70-year-olds who say, “I think I’ll go with another option,” and that’s the right decision for them. It’s having the conversation that matters.
In practice, given time constraints and other confines, how can dermatologists best work with more complex older patients? What are your practical tips?
People talk about having 45-minute “golden year” conversations with their older patients, but it doesn’t have to be this way. In pursuing geriatric dermatology, I decided early on that I wanted to make sure it was practical, so I’ve focused on maximizing shorter visits and on embracing the concept that relationships can be developed over time. Each time we meet with someone, we’re building equity to have bigger conversations later on.
I can have a 15-minute conversation about whether my patient may want to have Mohs surgery, for instance, or escalate treatment to a systemic agent for their chronic inflammatory disease. If that time isn’t enough, I can encourage further thought about treatment options, acknowledge that decisions aren’t necessarily easy, and schedule a follow-up or offer to call the patient after clinic to continue the conversation.
Sometimes, when I’m at an impasse and my patient is unsure how to proceed, I’ll use clear metrics relevant to older adults — sleep, activity level, and caregiver burden — to help my patient. If someone is not sleeping because of their lesion — if they’re so itchy or their inflammatory disease is uncontrolled, for instance — I’ll point out that the side effects of not sleeping are worse than the medications or surgery we’d pursue. If someone removes themselves from an activity due to their skin condition, that’s a red flag. And if the caregiver in the room is overwhelmed or frustrated by having to put cream on twice a day, I’ll use this to advance treatment.
What resources are available for dermatologists interested in improving their geriatric dermatology skills or advancing the area?
For those interested in investigating these issues or improving their practices, the AAD’s Geriatric Dermatology ERG is always welcoming of new members. The ERG will have an all-inclusive meeting at the 2025 annual AAD meeting in March.
The AAD also has educational modules on geriatric dermatology that were recently published as an initiative of our ERG. More information is available on the website. Also valuable is the ElderDerm conference hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC; the second such conference takes place in May 2025.
Butler reported that he had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Daniel C. Butler, MD, is associate professor of dermatology and director of the new Inflammatory and Aging Skin Research Program in the Division of Dermatology at the University of Arizona College of Medicine, Tucson, Arizona. Before returning to Arizona, where he had attended medical school, Butler practiced and was a researcher at the University of California, San Francisco, and its geriatric dermatology clinic. He is a co-founder and continues to co-lead the American Academy of Dermatology (AAD) Geriatric Dermatology Expert Resource Group (ERG).
Butler’s interest in geriatric dermatology is rooted in his experience growing up with four grandparents and witnessing their wisdom, relationships, moments with loved ones, and other unique and desirable parts of growing old. “When I looked later at how aging was perceived in dermatology, I found it was a lot about ‘antiaging,’” he told this news organization. “I thought there was a needed voice in dermatology for healthy aging, for all the desirable things that only growing old can provide, along with all the incredible ‘antiaging’ things we can do.”
In interviews, Butler spoke about research priorities in geriatric dermatology, how the “4M” model of geriatrics should be applied within dermatology, how dermatologists can best work with older complex patients, and more. The conversation was edited for clarity and length.
What is geriatric dermatology? It is described by the AAD’s Geriatric Dermatology ERG as “an emerging subspecialty.” Yet it’s also viewed more broadly. Please speak about its various identities and meanings and its importance for dermatology.
If you’re a Mohs surgeon, you’re seeing a strong majority of over 65 patients. And in various specialty clinics, such as inflammatory skin disease, geriatric dermatology pertains to you. In many ways, it can be viewed as a mindset.
From a framework standpoint, and as a field, geriatric dermatology is a basic science initiative, a clinical initiative, an educational initiative, and an advocacy initiative. The goal is to be able to influence, grow, and learn in each of these categories for our older patients. This is happening: Research in this field has progressed, and education has progressed, which has driven some progress in clinical care.
How has research progressed in the basic science of aging skin? What are key questions for dermatology?
There has been a lot of basic science research on aging skin and on how an aging immune system, for instance, is reflected in conditions such as bullous pemphigoid, atopic dermatitis (AD), and chronic itch. But aging involves more than immunosenescence. I think of aging skin as a three-headed monster that involves changes in the skin barrier and the microbiome as well. But is there a primary piece of aging in the skin? What comes first or influences the other? More research on these questions can potentially influence our treatments.
With respect to the immune system, what we’re finding in the skin is that age-related change is not a decline in the immune system per se, but rather aberrance in response. Parts of the system tend to become overactive, with a skew toward overexpression of type 2 inflammation. This can be problematic, driving conditions such as chronic itch.
With respect to the skin barrier, we lose essential fatty acids, and we lose a lot of our recovery ability and our ability to respond quickly to environmental stressors. But are barrier changes triggering the immune system? Or is it the other way around?
The microbiome, which is a big focus of research, involves similar chicken-and-egg discussions. Is it the microbiome that changes and alters the barrier, which then entices the immune system? Which one happens first? We have a lot to learn, and there’s probably not one answer for every patient.
Please speak about research more broadly. What questions and issues need to be answered and addressed to improve the dermatologic care of older adults?
In general, research in dermatology is very disease-specific and not particularly conducive to looking at the larger demographic populations. We have a huge opportunity, therefore, to break the mold and grow geriatric dermatology as an area of population-based research — so that geriatric dermatology research encompasses not only the melanoma researcher who’s trying to understand how aging influences the melanocytes but also the epidemiologic researcher looking at how our diagnoses and coding and prescription practices are different in the 65-plus age group.
Clinically speaking, researchers want to better understand how aging influences the clinical presentations of our diseases. And there’s research to be done on best practices. For example, what are the best practices for treating basal cell carcinomas in patients with mild cognitive impairment? How should we consider the use of topicals in a patient who has severe arthritis or who lives alone? And then how should we teach practical approaches to help providers meet people where they are?
Looking at it from a healthcare system standpoint, there are many care delivery and access issues — practical pieces — to research, and we’re getting a lot better with this. We’re also advocating not only for more inclusion of older adults in clinical trials of treatments but also for the use of evaluations and outcomes that are relevant and important for older adults.
One piece of good news is that we’re seeing safer treatment options with tremendous efficacy that target known pathways for diseases like AD and chronic itch that affect older adults. Again, now we must find ways to improve access to these novel, safe options.
Our research program at the University of Arizona College of Medicine, which we’re just getting off the ground, aims to be dual-sided, looking both at the basic science of aging skin and at access and care delivery issues, such as how to ensure that patients on Medicare have access to medications that are at least on par with others with private insurance.
What are the most common dermatologic problems experienced by older adults?
Based on my experience and on research that we expect to be published soon, it’s absolutely nonmelanoma skin cancers, precancers like actinic keratoses — and on the inflammatory disease side, itch, AD, and psoriasis. Of course, also common are the age-related changes to the skin that we put in the benign category, such as solar lentigines.
How does age influence dermatologic diseases from a pathophysiological and clinical standpoint?
Diseases overall are very similar and respond to the same treatments, but age in and of itself does influence little pieces. For example, there is more crossover in the presentation of psoriasis and AD in older adults, leading to delays in the diagnosis of psoriasis.
With AD, we’ve found that itch is the predominant symptom for older adults rather than the red rash. We see higher or more severe itch scores in older adults with AD with less visual changes on the skin than in younger cohorts. And rash occurs in different locations than in young patients. Older adults typically present with it on their chest, back, and across the trunk, rather than in folded areas. They’re also more likely to get it on their legs in a nummular pattern as opposed to the more traditional flexural area presentation.
What unique considerations need to be made in treating older adults? How should the 4M model of geriatrics be applied to dermatologic care?
Our care model pushes us to be very algorithmic, but at the end of the day, what’s really important are the 4Ms: Mobility, medication, mentation, and “what matters most.” As you’re having your shared decision-making conversations with your patients and their families, these should be your priorities.
A patient with physical limitations, for instance, may not be able to apply a topical cream twice a day all over the body. They may have comorbidities and treatments for these comorbidities that may conflict with medications you’re considering.
And then mentation is so important. For a long time, we used antihistamines for older adults, but this has been proven to be bad for their mentation and risky in other ways. We need to be sure we’re prioritizing their ability to be clear mentally when we’re prescribing medications and even when we’re considering surgical approaches. Do they show capacity for that procedure or treatment, and how will they respond to that treatment later on?
Using the 4M model to drive conversations is a way to get all of us to connect to the patient and learn about what’s most important for them. In many ways, geriatrics is about taking a step back from your specialist skills and thinking about how you would want a family member treated.
We want to avoid treating just the lesion or the pathologic diagnosis. We want to avoid the “conveyor belt” from a biopsy to Mohs. I have 95-year-olds who say, “Heck yeah, if Mohs is the best treatment, that’s what I want.” And I have 70-year-olds who say, “I think I’ll go with another option,” and that’s the right decision for them. It’s having the conversation that matters.
In practice, given time constraints and other confines, how can dermatologists best work with more complex older patients? What are your practical tips?
People talk about having 45-minute “golden year” conversations with their older patients, but it doesn’t have to be this way. In pursuing geriatric dermatology, I decided early on that I wanted to make sure it was practical, so I’ve focused on maximizing shorter visits and on embracing the concept that relationships can be developed over time. Each time we meet with someone, we’re building equity to have bigger conversations later on.
I can have a 15-minute conversation about whether my patient may want to have Mohs surgery, for instance, or escalate treatment to a systemic agent for their chronic inflammatory disease. If that time isn’t enough, I can encourage further thought about treatment options, acknowledge that decisions aren’t necessarily easy, and schedule a follow-up or offer to call the patient after clinic to continue the conversation.
Sometimes, when I’m at an impasse and my patient is unsure how to proceed, I’ll use clear metrics relevant to older adults — sleep, activity level, and caregiver burden — to help my patient. If someone is not sleeping because of their lesion — if they’re so itchy or their inflammatory disease is uncontrolled, for instance — I’ll point out that the side effects of not sleeping are worse than the medications or surgery we’d pursue. If someone removes themselves from an activity due to their skin condition, that’s a red flag. And if the caregiver in the room is overwhelmed or frustrated by having to put cream on twice a day, I’ll use this to advance treatment.
What resources are available for dermatologists interested in improving their geriatric dermatology skills or advancing the area?
For those interested in investigating these issues or improving their practices, the AAD’s Geriatric Dermatology ERG is always welcoming of new members. The ERG will have an all-inclusive meeting at the 2025 annual AAD meeting in March.
The AAD also has educational modules on geriatric dermatology that were recently published as an initiative of our ERG. More information is available on the website. Also valuable is the ElderDerm conference hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC; the second such conference takes place in May 2025.
Butler reported that he had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Daniel C. Butler, MD, is associate professor of dermatology and director of the new Inflammatory and Aging Skin Research Program in the Division of Dermatology at the University of Arizona College of Medicine, Tucson, Arizona. Before returning to Arizona, where he had attended medical school, Butler practiced and was a researcher at the University of California, San Francisco, and its geriatric dermatology clinic. He is a co-founder and continues to co-lead the American Academy of Dermatology (AAD) Geriatric Dermatology Expert Resource Group (ERG).
Butler’s interest in geriatric dermatology is rooted in his experience growing up with four grandparents and witnessing their wisdom, relationships, moments with loved ones, and other unique and desirable parts of growing old. “When I looked later at how aging was perceived in dermatology, I found it was a lot about ‘antiaging,’” he told this news organization. “I thought there was a needed voice in dermatology for healthy aging, for all the desirable things that only growing old can provide, along with all the incredible ‘antiaging’ things we can do.”
In interviews, Butler spoke about research priorities in geriatric dermatology, how the “4M” model of geriatrics should be applied within dermatology, how dermatologists can best work with older complex patients, and more. The conversation was edited for clarity and length.
What is geriatric dermatology? It is described by the AAD’s Geriatric Dermatology ERG as “an emerging subspecialty.” Yet it’s also viewed more broadly. Please speak about its various identities and meanings and its importance for dermatology.
If you’re a Mohs surgeon, you’re seeing a strong majority of over 65 patients. And in various specialty clinics, such as inflammatory skin disease, geriatric dermatology pertains to you. In many ways, it can be viewed as a mindset.
From a framework standpoint, and as a field, geriatric dermatology is a basic science initiative, a clinical initiative, an educational initiative, and an advocacy initiative. The goal is to be able to influence, grow, and learn in each of these categories for our older patients. This is happening: Research in this field has progressed, and education has progressed, which has driven some progress in clinical care.
How has research progressed in the basic science of aging skin? What are key questions for dermatology?
There has been a lot of basic science research on aging skin and on how an aging immune system, for instance, is reflected in conditions such as bullous pemphigoid, atopic dermatitis (AD), and chronic itch. But aging involves more than immunosenescence. I think of aging skin as a three-headed monster that involves changes in the skin barrier and the microbiome as well. But is there a primary piece of aging in the skin? What comes first or influences the other? More research on these questions can potentially influence our treatments.
With respect to the immune system, what we’re finding in the skin is that age-related change is not a decline in the immune system per se, but rather aberrance in response. Parts of the system tend to become overactive, with a skew toward overexpression of type 2 inflammation. This can be problematic, driving conditions such as chronic itch.
With respect to the skin barrier, we lose essential fatty acids, and we lose a lot of our recovery ability and our ability to respond quickly to environmental stressors. But are barrier changes triggering the immune system? Or is it the other way around?
The microbiome, which is a big focus of research, involves similar chicken-and-egg discussions. Is it the microbiome that changes and alters the barrier, which then entices the immune system? Which one happens first? We have a lot to learn, and there’s probably not one answer for every patient.
Please speak about research more broadly. What questions and issues need to be answered and addressed to improve the dermatologic care of older adults?
In general, research in dermatology is very disease-specific and not particularly conducive to looking at the larger demographic populations. We have a huge opportunity, therefore, to break the mold and grow geriatric dermatology as an area of population-based research — so that geriatric dermatology research encompasses not only the melanoma researcher who’s trying to understand how aging influences the melanocytes but also the epidemiologic researcher looking at how our diagnoses and coding and prescription practices are different in the 65-plus age group.
Clinically speaking, researchers want to better understand how aging influences the clinical presentations of our diseases. And there’s research to be done on best practices. For example, what are the best practices for treating basal cell carcinomas in patients with mild cognitive impairment? How should we consider the use of topicals in a patient who has severe arthritis or who lives alone? And then how should we teach practical approaches to help providers meet people where they are?
Looking at it from a healthcare system standpoint, there are many care delivery and access issues — practical pieces — to research, and we’re getting a lot better with this. We’re also advocating not only for more inclusion of older adults in clinical trials of treatments but also for the use of evaluations and outcomes that are relevant and important for older adults.
One piece of good news is that we’re seeing safer treatment options with tremendous efficacy that target known pathways for diseases like AD and chronic itch that affect older adults. Again, now we must find ways to improve access to these novel, safe options.
Our research program at the University of Arizona College of Medicine, which we’re just getting off the ground, aims to be dual-sided, looking both at the basic science of aging skin and at access and care delivery issues, such as how to ensure that patients on Medicare have access to medications that are at least on par with others with private insurance.
What are the most common dermatologic problems experienced by older adults?
Based on my experience and on research that we expect to be published soon, it’s absolutely nonmelanoma skin cancers, precancers like actinic keratoses — and on the inflammatory disease side, itch, AD, and psoriasis. Of course, also common are the age-related changes to the skin that we put in the benign category, such as solar lentigines.
How does age influence dermatologic diseases from a pathophysiological and clinical standpoint?
Diseases overall are very similar and respond to the same treatments, but age in and of itself does influence little pieces. For example, there is more crossover in the presentation of psoriasis and AD in older adults, leading to delays in the diagnosis of psoriasis.
With AD, we’ve found that itch is the predominant symptom for older adults rather than the red rash. We see higher or more severe itch scores in older adults with AD with less visual changes on the skin than in younger cohorts. And rash occurs in different locations than in young patients. Older adults typically present with it on their chest, back, and across the trunk, rather than in folded areas. They’re also more likely to get it on their legs in a nummular pattern as opposed to the more traditional flexural area presentation.
What unique considerations need to be made in treating older adults? How should the 4M model of geriatrics be applied to dermatologic care?
Our care model pushes us to be very algorithmic, but at the end of the day, what’s really important are the 4Ms: Mobility, medication, mentation, and “what matters most.” As you’re having your shared decision-making conversations with your patients and their families, these should be your priorities.
A patient with physical limitations, for instance, may not be able to apply a topical cream twice a day all over the body. They may have comorbidities and treatments for these comorbidities that may conflict with medications you’re considering.
And then mentation is so important. For a long time, we used antihistamines for older adults, but this has been proven to be bad for their mentation and risky in other ways. We need to be sure we’re prioritizing their ability to be clear mentally when we’re prescribing medications and even when we’re considering surgical approaches. Do they show capacity for that procedure or treatment, and how will they respond to that treatment later on?
Using the 4M model to drive conversations is a way to get all of us to connect to the patient and learn about what’s most important for them. In many ways, geriatrics is about taking a step back from your specialist skills and thinking about how you would want a family member treated.
We want to avoid treating just the lesion or the pathologic diagnosis. We want to avoid the “conveyor belt” from a biopsy to Mohs. I have 95-year-olds who say, “Heck yeah, if Mohs is the best treatment, that’s what I want.” And I have 70-year-olds who say, “I think I’ll go with another option,” and that’s the right decision for them. It’s having the conversation that matters.
In practice, given time constraints and other confines, how can dermatologists best work with more complex older patients? What are your practical tips?
People talk about having 45-minute “golden year” conversations with their older patients, but it doesn’t have to be this way. In pursuing geriatric dermatology, I decided early on that I wanted to make sure it was practical, so I’ve focused on maximizing shorter visits and on embracing the concept that relationships can be developed over time. Each time we meet with someone, we’re building equity to have bigger conversations later on.
I can have a 15-minute conversation about whether my patient may want to have Mohs surgery, for instance, or escalate treatment to a systemic agent for their chronic inflammatory disease. If that time isn’t enough, I can encourage further thought about treatment options, acknowledge that decisions aren’t necessarily easy, and schedule a follow-up or offer to call the patient after clinic to continue the conversation.
Sometimes, when I’m at an impasse and my patient is unsure how to proceed, I’ll use clear metrics relevant to older adults — sleep, activity level, and caregiver burden — to help my patient. If someone is not sleeping because of their lesion — if they’re so itchy or their inflammatory disease is uncontrolled, for instance — I’ll point out that the side effects of not sleeping are worse than the medications or surgery we’d pursue. If someone removes themselves from an activity due to their skin condition, that’s a red flag. And if the caregiver in the room is overwhelmed or frustrated by having to put cream on twice a day, I’ll use this to advance treatment.
What resources are available for dermatologists interested in improving their geriatric dermatology skills or advancing the area?
For those interested in investigating these issues or improving their practices, the AAD’s Geriatric Dermatology ERG is always welcoming of new members. The ERG will have an all-inclusive meeting at the 2025 annual AAD meeting in March.
The AAD also has educational modules on geriatric dermatology that were recently published as an initiative of our ERG. More information is available on the website. Also valuable is the ElderDerm conference hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC; the second such conference takes place in May 2025.
Butler reported that he had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Scalp Nodule With Copious Fluid
Scalp Nodule With Copious Fluid
The Diagnosis: Apocrine Hidrocystoma
Histopathology of the excised nodule revealed a partially collapsed, multiloculated dermal cyst lined with apocrine cells, which was consistent with a diagnosis of apocrine hidrocystoma. Apocrine hidrocystomas are cysts that range from flesh-colored to blue-black and most commonly manifest as solitary lesions on the face, particularly near the eyelids.1,2 Apocrine hidrocystomas typically range from 1 to 10 mm in diameter and contain fluid that can be colorless, yellow-brown, or blue-black.1,2 Apocrine hidrocystomas usually are reported between the ages of 30 and 70 years and have no sex predilection.3
Apocrine hidrocystomas are thought to develop from adenomatous growth of apocrine sweat gland coils.4 The term apocrine hidrocystoma has been used interchangeably with apocrine cystadenoma, though some investigators have recommended using the latter term only for lesions with true papillary projections.5 Definitive diagnosis is obtained through histopathology, which typically shows unilocular or multilocular cystic spaces in the dermis lined by an apocrine secretory epithelium. These secretory cells often demonstrate decapitation secretion and apical snouting. The cyst wall may send pseudopapillary projections into the cystic cavity.1,2 While apocrine and eccrine hidrocystomas previously were recognized as separate entities, it has been suggested that so-called eccrine hidrocystomas are truly apocrine in nature, with a cyst wall that is compressed by the cyst contents.4
Apocrine hidrocystomas are benign and do not require treatment; however, they may be removed for cosmetic purposes, most commonly via surgical excision. Lesions treated with needle puncture as monotherapy frequently recur. Other successful methods for removal include cyst puncture followed by hypertonic glucose sclerotherapy, trichloroacetic acid injection, botulinum toxin A injection, or CO2 laser treatment.3,6
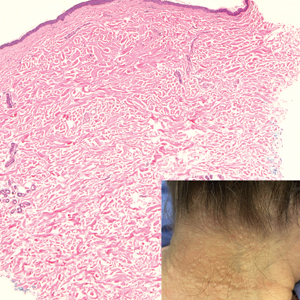
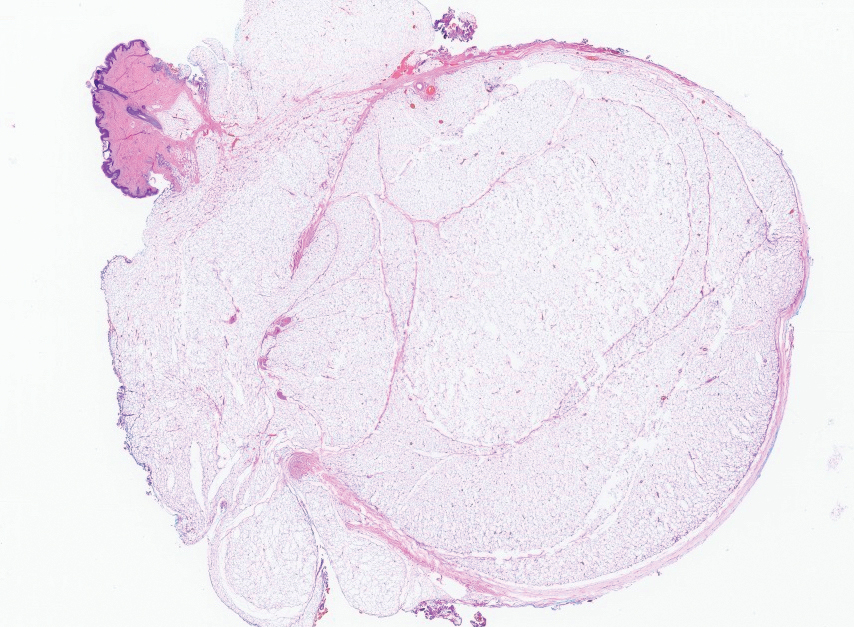
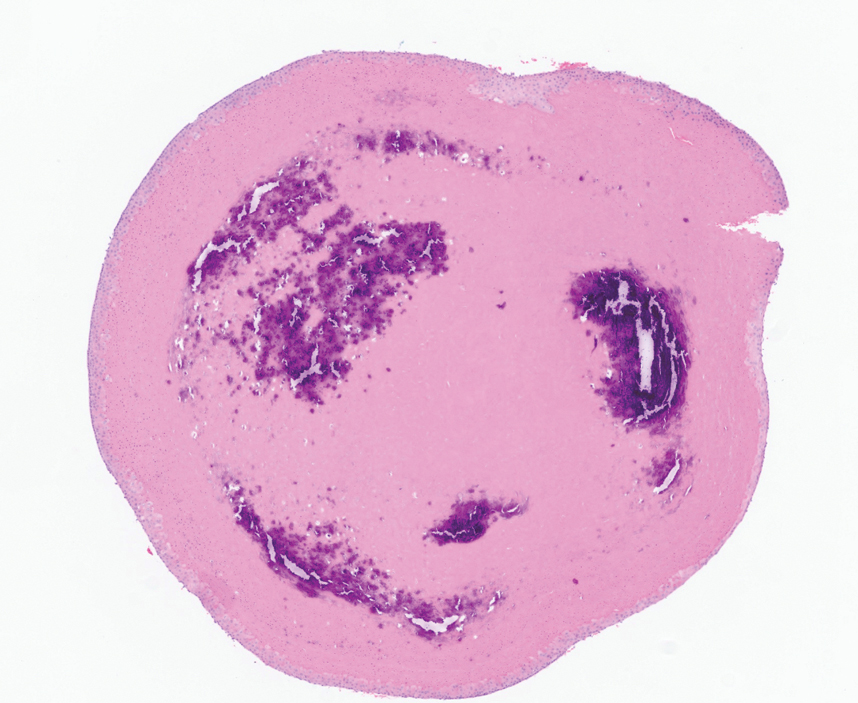
Several clinical and histopathologic findings can distinguish between apocrine hidrocystomas and other diagnoses in the differential. Lipomas are common benign tumors composed of mature fat that typically manifest as solitary, painless, soft nodules with a normal overlying epidermis. They frequently are distributed on the neck, arms, legs, and buttocks. While the differential for our patient initially included lipoma, these lesions do not contain or release fluid, which was present in our patient. On histopathology, lipoma shows a uniform population of mature fat cells with small, uniform, and eccentric nuclei (Figure 1).7
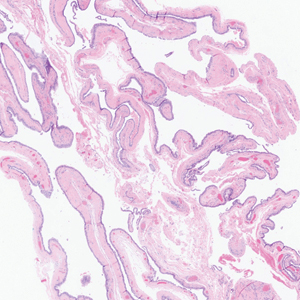
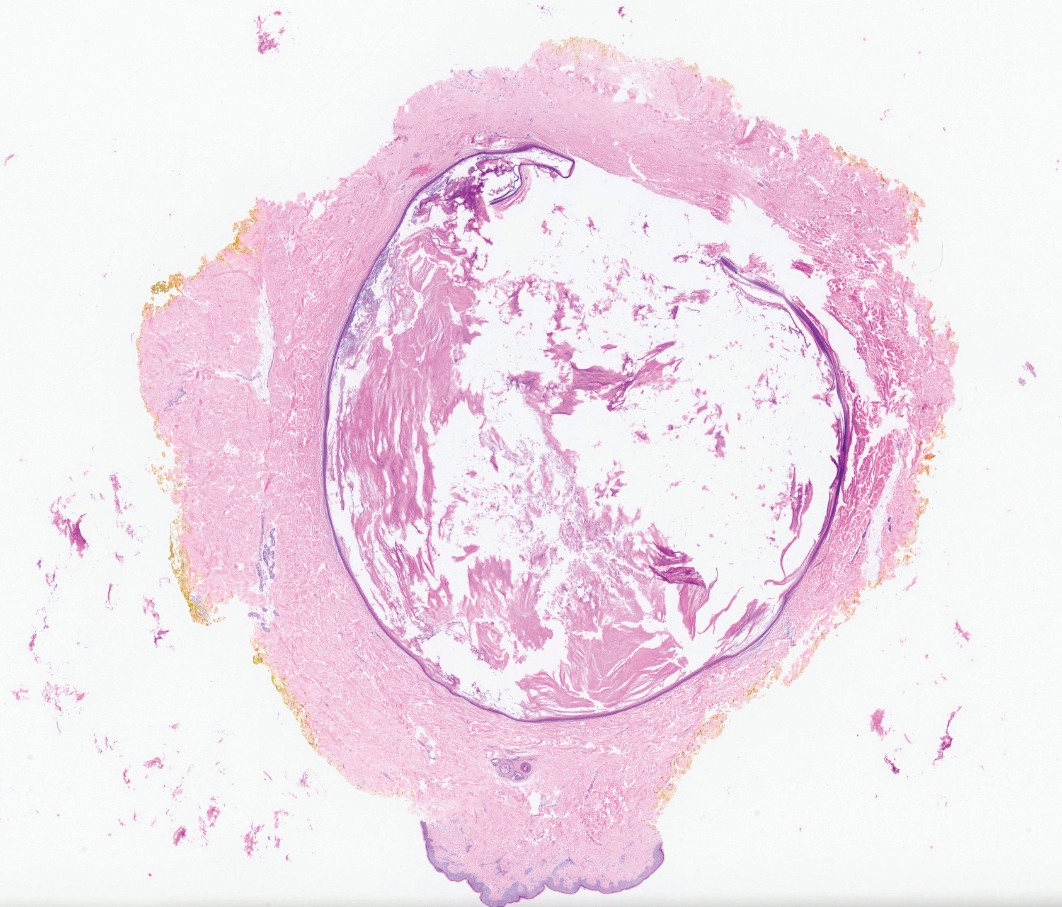
Epidermal inclusion cysts are derived from the follicular infundibulum and commonly are found on the face and upper trunk. They manifest as flesh-colored dermal nodules and may have a visible punctum. As opposed to the cystic cavities lined with apocrine cells seen in apocrine hidrocystomas, epidermal inclusion cysts are lined with a stratified squamous epithelium, are filled with laminated keratin, and have a visible granular layer (Figure 2).8
Pilar cysts, also known as trichilemmal cysts, clinically resemble epidermal inclusion cysts but are derived from the outer root sheath of hair follicles, manifesting as flesh-colored dermal nodules almost always found on the scalp. On histopathology, pilar cysts are lined with stratified squamous epithelial cells without a visible granular layer and are filled with compact eosinophilic keratin (Figure 3).8
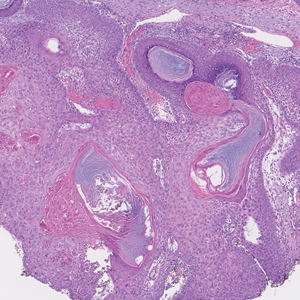
Tubular apocrine adenomas are benign neoplasms of the apocrine glands that manifest as smooth nodules. They are within the same spectrum as papillary eccrine adenomas, appearing more frequently on the legs and less frequently on the face and scalp.9 Histopathology generally demonstrates well-circumscribed lobules of tubular structures in the dermis. Similar to apocrine hidrocystomas, tubular apocrine adenomas will demonstrate an inner layer of columnar apocrine cells with decapitation secretion, but the tubular architecture helps differentiate it from other adnexal tumors (Figure 4).10
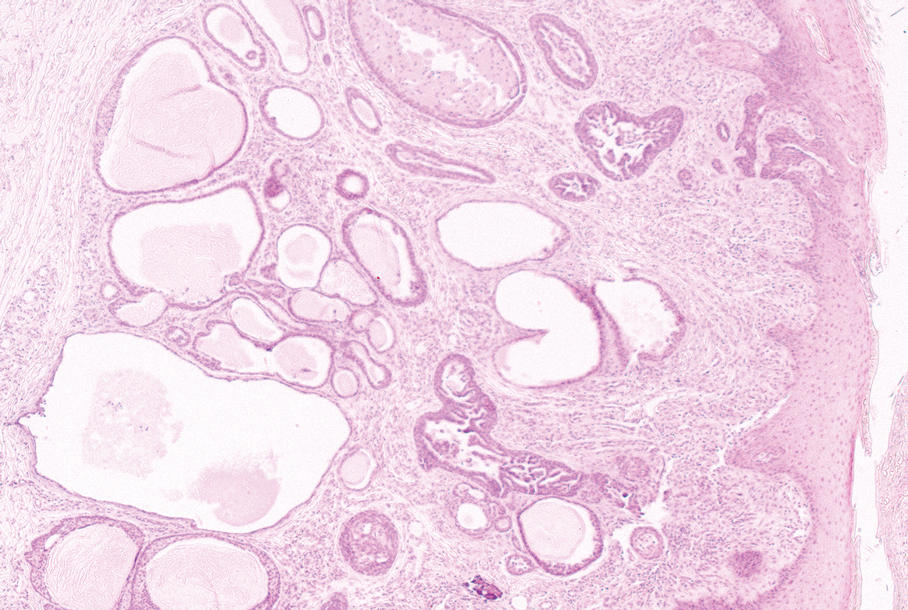
The clinical manifestation of the apocrine hidrocystoma in our patient was unusual due to its size and location. Apocrine hidrocystomas rarely are found on the scalp, with few other cases found in the literature. To our knowledge, this is the largest apocrine hidrocystoma found on the scalp to date, although there is at least 1 other published case of an apocrine hidrocystoma on the scalp measuring at least 3 cm in diameter.11 Our case highlights the importance of recognizing atypical manifestations of apocrine hidrocystomas, as a lesion on the midline scalp that discharges a thin fluid might raise initial concern for an intracranial connection. Awareness of atypical manifestations of common lesions can expand dermatologists’ differential diagnoses and help them to reassure patients.
- Smith JD. Apocrine hidrocystoma (cystadenoma). Arch Dermatol. 1974;109:700. doi:10.1001/archderm.1974.01630050046010
- Mehregan AH. Apocrine cystadenoma: a clinicopathologic study with special reference to the pigmented variety. Arch Dermatol. 1964;90:274. doi:10.1001/archderm.1964.01600030024005
- Hafsi W, Badri T, Shah F. Apocrine hidrocystoma. StatPearls [Internet]. Updated April 13, 2024. Accessed November 6, 2024. http://www.ncbi.nlm.nih.gov/books/NBK448109/
- de Viragh PA, Szeimies RM, Eckert F. Apocrine cystadenoma, apocrine hidrocystoma, and eccrine hidrocystoma: three distinct tumors defined by expression of keratins and human milk fat globulin 1. J Cutan Pathol. 1997;24:249-255. doi:10.1111/j.1600-0560.1997.tb01590.x
- Sugiyama A, Sugiura M, Piris A, et al. Apocrine cystadenoma and apocrine hidrocystoma: examination of 21 cases with emphasis on nomenclature according to proliferative features. J Cutan Pathol. 2007;34:912-917. doi:10.1111/j.1600-0560.2007.00757.x
- Bickley LK, Goldberg DJ, Imaeda S, et al. Treatment of multiple apocrine hidrocystomas with the carbon dioxide (CO2) laser. J Dermatol Surg Oncol. 1989;15:599-602. doi:10.1111/j.1524-4725.1989.tb03597.x
- Kaddu S. Smooth muscle, adipose and cartilage neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:2086-2101.
- Stone MS. Cysts. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1057-1074.
- Requena L, Sangüeza O. Tubular adenoma. In: Requena L, Sangüeza O, eds. Cutaneous Adnexal Neoplasms. Springer International Publishing; 2017:127-136. doi:10.1007/978-3-319-45704-8_12
- McCalmont TH, Pincus LB. Adnexal neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1057-1074.
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
The Diagnosis: Apocrine Hidrocystoma
Histopathology of the excised nodule revealed a partially collapsed, multiloculated dermal cyst lined with apocrine cells, which was consistent with a diagnosis of apocrine hidrocystoma. Apocrine hidrocystomas are cysts that range from flesh-colored to blue-black and most commonly manifest as solitary lesions on the face, particularly near the eyelids.1,2 Apocrine hidrocystomas typically range from 1 to 10 mm in diameter and contain fluid that can be colorless, yellow-brown, or blue-black.1,2 Apocrine hidrocystomas usually are reported between the ages of 30 and 70 years and have no sex predilection.3
Apocrine hidrocystomas are thought to develop from adenomatous growth of apocrine sweat gland coils.4 The term apocrine hidrocystoma has been used interchangeably with apocrine cystadenoma, though some investigators have recommended using the latter term only for lesions with true papillary projections.5 Definitive diagnosis is obtained through histopathology, which typically shows unilocular or multilocular cystic spaces in the dermis lined by an apocrine secretory epithelium. These secretory cells often demonstrate decapitation secretion and apical snouting. The cyst wall may send pseudopapillary projections into the cystic cavity.1,2 While apocrine and eccrine hidrocystomas previously were recognized as separate entities, it has been suggested that so-called eccrine hidrocystomas are truly apocrine in nature, with a cyst wall that is compressed by the cyst contents.4
Apocrine hidrocystomas are benign and do not require treatment; however, they may be removed for cosmetic purposes, most commonly via surgical excision. Lesions treated with needle puncture as monotherapy frequently recur. Other successful methods for removal include cyst puncture followed by hypertonic glucose sclerotherapy, trichloroacetic acid injection, botulinum toxin A injection, or CO2 laser treatment.3,6
Several clinical and histopathologic findings can distinguish between apocrine hidrocystomas and other diagnoses in the differential. Lipomas are common benign tumors composed of mature fat that typically manifest as solitary, painless, soft nodules with a normal overlying epidermis. They frequently are distributed on the neck, arms, legs, and buttocks. While the differential for our patient initially included lipoma, these lesions do not contain or release fluid, which was present in our patient. On histopathology, lipoma shows a uniform population of mature fat cells with small, uniform, and eccentric nuclei (Figure 1).7

Epidermal inclusion cysts are derived from the follicular infundibulum and commonly are found on the face and upper trunk. They manifest as flesh-colored dermal nodules and may have a visible punctum. As opposed to the cystic cavities lined with apocrine cells seen in apocrine hidrocystomas, epidermal inclusion cysts are lined with a stratified squamous epithelium, are filled with laminated keratin, and have a visible granular layer (Figure 2).8

Pilar cysts, also known as trichilemmal cysts, clinically resemble epidermal inclusion cysts but are derived from the outer root sheath of hair follicles, manifesting as flesh-colored dermal nodules almost always found on the scalp. On histopathology, pilar cysts are lined with stratified squamous epithelial cells without a visible granular layer and are filled with compact eosinophilic keratin (Figure 3).8

Tubular apocrine adenomas are benign neoplasms of the apocrine glands that manifest as smooth nodules. They are within the same spectrum as papillary eccrine adenomas, appearing more frequently on the legs and less frequently on the face and scalp.9 Histopathology generally demonstrates well-circumscribed lobules of tubular structures in the dermis. Similar to apocrine hidrocystomas, tubular apocrine adenomas will demonstrate an inner layer of columnar apocrine cells with decapitation secretion, but the tubular architecture helps differentiate it from other adnexal tumors (Figure 4).10

The clinical manifestation of the apocrine hidrocystoma in our patient was unusual due to its size and location. Apocrine hidrocystomas rarely are found on the scalp, with few other cases found in the literature. To our knowledge, this is the largest apocrine hidrocystoma found on the scalp to date, although there is at least 1 other published case of an apocrine hidrocystoma on the scalp measuring at least 3 cm in diameter.11 Our case highlights the importance of recognizing atypical manifestations of apocrine hidrocystomas, as a lesion on the midline scalp that discharges a thin fluid might raise initial concern for an intracranial connection. Awareness of atypical manifestations of common lesions can expand dermatologists’ differential diagnoses and help them to reassure patients.
The Diagnosis: Apocrine Hidrocystoma
Histopathology of the excised nodule revealed a partially collapsed, multiloculated dermal cyst lined with apocrine cells, which was consistent with a diagnosis of apocrine hidrocystoma. Apocrine hidrocystomas are cysts that range from flesh-colored to blue-black and most commonly manifest as solitary lesions on the face, particularly near the eyelids.1,2 Apocrine hidrocystomas typically range from 1 to 10 mm in diameter and contain fluid that can be colorless, yellow-brown, or blue-black.1,2 Apocrine hidrocystomas usually are reported between the ages of 30 and 70 years and have no sex predilection.3
Apocrine hidrocystomas are thought to develop from adenomatous growth of apocrine sweat gland coils.4 The term apocrine hidrocystoma has been used interchangeably with apocrine cystadenoma, though some investigators have recommended using the latter term only for lesions with true papillary projections.5 Definitive diagnosis is obtained through histopathology, which typically shows unilocular or multilocular cystic spaces in the dermis lined by an apocrine secretory epithelium. These secretory cells often demonstrate decapitation secretion and apical snouting. The cyst wall may send pseudopapillary projections into the cystic cavity.1,2 While apocrine and eccrine hidrocystomas previously were recognized as separate entities, it has been suggested that so-called eccrine hidrocystomas are truly apocrine in nature, with a cyst wall that is compressed by the cyst contents.4
Apocrine hidrocystomas are benign and do not require treatment; however, they may be removed for cosmetic purposes, most commonly via surgical excision. Lesions treated with needle puncture as monotherapy frequently recur. Other successful methods for removal include cyst puncture followed by hypertonic glucose sclerotherapy, trichloroacetic acid injection, botulinum toxin A injection, or CO2 laser treatment.3,6
Several clinical and histopathologic findings can distinguish between apocrine hidrocystomas and other diagnoses in the differential. Lipomas are common benign tumors composed of mature fat that typically manifest as solitary, painless, soft nodules with a normal overlying epidermis. They frequently are distributed on the neck, arms, legs, and buttocks. While the differential for our patient initially included lipoma, these lesions do not contain or release fluid, which was present in our patient. On histopathology, lipoma shows a uniform population of mature fat cells with small, uniform, and eccentric nuclei (Figure 1).7

Epidermal inclusion cysts are derived from the follicular infundibulum and commonly are found on the face and upper trunk. They manifest as flesh-colored dermal nodules and may have a visible punctum. As opposed to the cystic cavities lined with apocrine cells seen in apocrine hidrocystomas, epidermal inclusion cysts are lined with a stratified squamous epithelium, are filled with laminated keratin, and have a visible granular layer (Figure 2).8

Pilar cysts, also known as trichilemmal cysts, clinically resemble epidermal inclusion cysts but are derived from the outer root sheath of hair follicles, manifesting as flesh-colored dermal nodules almost always found on the scalp. On histopathology, pilar cysts are lined with stratified squamous epithelial cells without a visible granular layer and are filled with compact eosinophilic keratin (Figure 3).8

Tubular apocrine adenomas are benign neoplasms of the apocrine glands that manifest as smooth nodules. They are within the same spectrum as papillary eccrine adenomas, appearing more frequently on the legs and less frequently on the face and scalp.9 Histopathology generally demonstrates well-circumscribed lobules of tubular structures in the dermis. Similar to apocrine hidrocystomas, tubular apocrine adenomas will demonstrate an inner layer of columnar apocrine cells with decapitation secretion, but the tubular architecture helps differentiate it from other adnexal tumors (Figure 4).10

The clinical manifestation of the apocrine hidrocystoma in our patient was unusual due to its size and location. Apocrine hidrocystomas rarely are found on the scalp, with few other cases found in the literature. To our knowledge, this is the largest apocrine hidrocystoma found on the scalp to date, although there is at least 1 other published case of an apocrine hidrocystoma on the scalp measuring at least 3 cm in diameter.11 Our case highlights the importance of recognizing atypical manifestations of apocrine hidrocystomas, as a lesion on the midline scalp that discharges a thin fluid might raise initial concern for an intracranial connection. Awareness of atypical manifestations of common lesions can expand dermatologists’ differential diagnoses and help them to reassure patients.
- Smith JD. Apocrine hidrocystoma (cystadenoma). Arch Dermatol. 1974;109:700. doi:10.1001/archderm.1974.01630050046010
- Mehregan AH. Apocrine cystadenoma: a clinicopathologic study with special reference to the pigmented variety. Arch Dermatol. 1964;90:274. doi:10.1001/archderm.1964.01600030024005
- Hafsi W, Badri T, Shah F. Apocrine hidrocystoma. StatPearls [Internet]. Updated April 13, 2024. Accessed November 6, 2024. http://www.ncbi.nlm.nih.gov/books/NBK448109/
- de Viragh PA, Szeimies RM, Eckert F. Apocrine cystadenoma, apocrine hidrocystoma, and eccrine hidrocystoma: three distinct tumors defined by expression of keratins and human milk fat globulin 1. J Cutan Pathol. 1997;24:249-255. doi:10.1111/j.1600-0560.1997.tb01590.x
- Sugiyama A, Sugiura M, Piris A, et al. Apocrine cystadenoma and apocrine hidrocystoma: examination of 21 cases with emphasis on nomenclature according to proliferative features. J Cutan Pathol. 2007;34:912-917. doi:10.1111/j.1600-0560.2007.00757.x
- Bickley LK, Goldberg DJ, Imaeda S, et al. Treatment of multiple apocrine hidrocystomas with the carbon dioxide (CO2) laser. J Dermatol Surg Oncol. 1989;15:599-602. doi:10.1111/j.1524-4725.1989.tb03597.x
- Kaddu S. Smooth muscle, adipose and cartilage neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:2086-2101.
- Stone MS. Cysts. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1057-1074.
- Requena L, Sangüeza O. Tubular adenoma. In: Requena L, Sangüeza O, eds. Cutaneous Adnexal Neoplasms. Springer International Publishing; 2017:127-136. doi:10.1007/978-3-319-45704-8_12
- McCalmont TH, Pincus LB. Adnexal neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1057-1074.
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Smith JD. Apocrine hidrocystoma (cystadenoma). Arch Dermatol. 1974;109:700. doi:10.1001/archderm.1974.01630050046010
- Mehregan AH. Apocrine cystadenoma: a clinicopathologic study with special reference to the pigmented variety. Arch Dermatol. 1964;90:274. doi:10.1001/archderm.1964.01600030024005
- Hafsi W, Badri T, Shah F. Apocrine hidrocystoma. StatPearls [Internet]. Updated April 13, 2024. Accessed November 6, 2024. http://www.ncbi.nlm.nih.gov/books/NBK448109/
- de Viragh PA, Szeimies RM, Eckert F. Apocrine cystadenoma, apocrine hidrocystoma, and eccrine hidrocystoma: three distinct tumors defined by expression of keratins and human milk fat globulin 1. J Cutan Pathol. 1997;24:249-255. doi:10.1111/j.1600-0560.1997.tb01590.x
- Sugiyama A, Sugiura M, Piris A, et al. Apocrine cystadenoma and apocrine hidrocystoma: examination of 21 cases with emphasis on nomenclature according to proliferative features. J Cutan Pathol. 2007;34:912-917. doi:10.1111/j.1600-0560.2007.00757.x
- Bickley LK, Goldberg DJ, Imaeda S, et al. Treatment of multiple apocrine hidrocystomas with the carbon dioxide (CO2) laser. J Dermatol Surg Oncol. 1989;15:599-602. doi:10.1111/j.1524-4725.1989.tb03597.x
- Kaddu S. Smooth muscle, adipose and cartilage neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:2086-2101.
- Stone MS. Cysts. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1057-1074.
- Requena L, Sangüeza O. Tubular adenoma. In: Requena L, Sangüeza O, eds. Cutaneous Adnexal Neoplasms. Springer International Publishing; 2017:127-136. doi:10.1007/978-3-319-45704-8_12
- McCalmont TH, Pincus LB. Adnexal neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1057-1074.
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
Scalp Nodule With Copious Fluid
Scalp Nodule With Copious Fluid
A 48-year-old woman presented to the dermatology clinic with a suspected cyst on the occipital scalp. The patient noted that the lesion had been present for years and denied any pain, pruritus, or drainage from the site. Physical examination revealed a soft, flesh-colored, subcutaneous nodule measuring 4.2×3.2 cm on the midline occipital scalp. During excision, the lesion drained a copious amount of thin yellowish fluid.
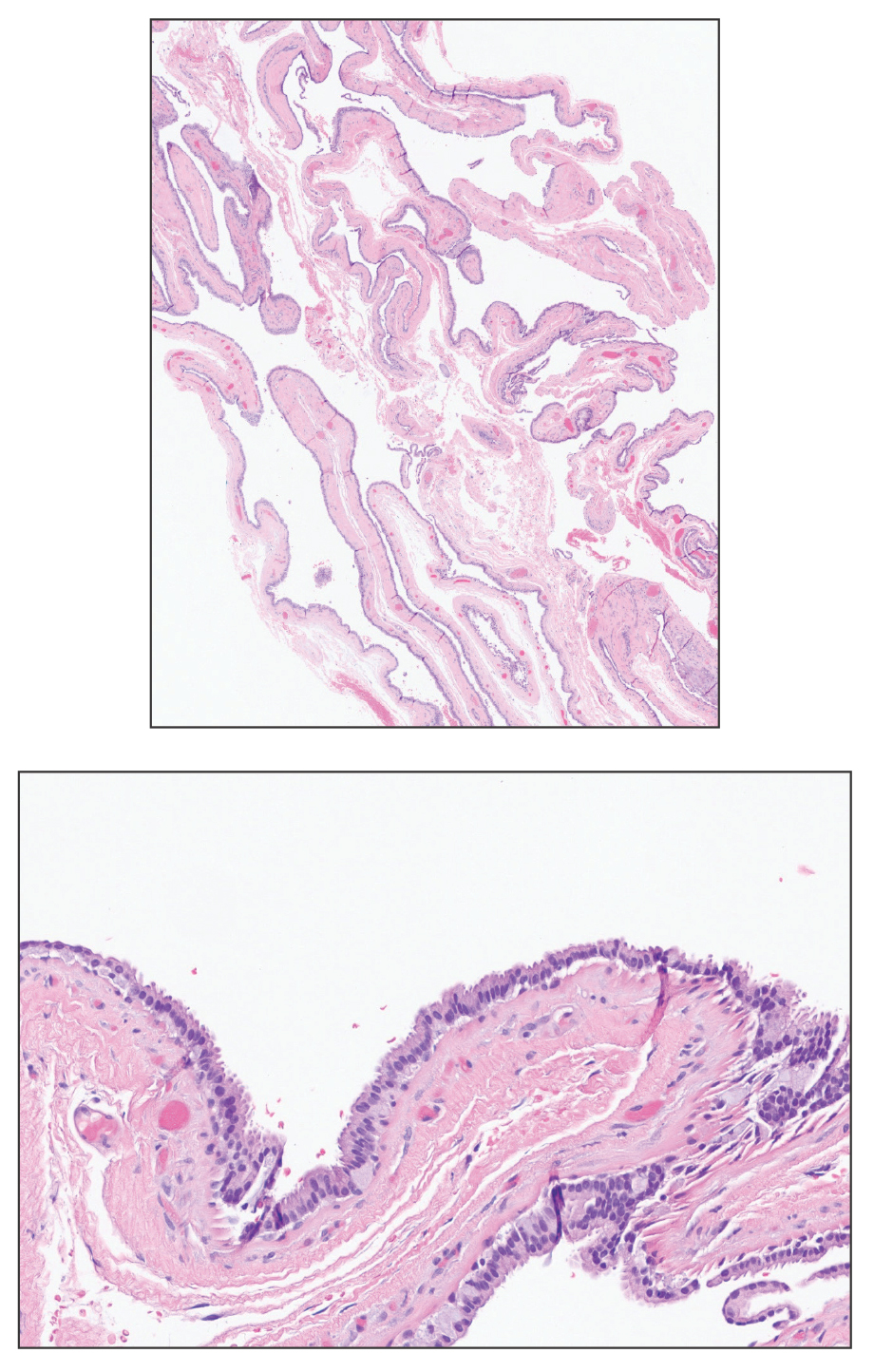
Asymptomatic Papules on the Neck
THE DIAGNOSIS: White Fibrous Papulosis
Given the histopathology findings, location on a sun-exposed site, lack of any additional systemic signs or symptoms, and no family history of similar lesions to suggest an underlying genetic condition, a diagnosis of white fibrous papulosis (WFP) was made. White fibrous papulosis is a relatively rare cutaneous disorder that was first reported by Shimizu et al1 in 1985. It is characterized by numerous grouped, 2- to 3-mm, white to flesh-colored papules that in most cases are confined to the neck in middle-aged to elderly individuals; however, cases involving the upper trunk and axillae also have been reported.1-3 The etiology of this condition is unclear but is thought to be related to aging and chronic exposure to UV light. Although treatment is not required, various modalities including tretinoin, excision, and laser therapy have been trialed with varying success.2,4 Our patient elected not to proceed with treatment.
Histologically, WFP may manifest similarly to connective tissue nevi; the overall architecture is nonspecific with focally thickened collagen and often elastic fibers that may be normal to reduced and/or fragmented, as well as an overall decrease in superficial dermal elastic tissue.3,5 Therefore, the differential diagnosis may include connective tissue nevi and require clinical correlation to make a correct diagnosis.
Pseudoxanthoma elasticum (PXE) is an autosomalrecessive disorder most commonly related to mutations in the ATP binding cassette subfamily C member 6 (ABCC6) gene that tends to manifest clinically on the neck and flexural extremities.6 This disease affects elastic fibers, which may become calcified over time. Pseudoxanthoma elasticum is associated with ocular complications relating to the Bruch membrane of the retina and angioid streaks; choroidal neovascularization involving the damaged Bruch membrane and episodes of acute retinopathy may result in vision loss in later stages of the disease.7 Involvement of the elastic laminae of arteries can be associated with cardiovascular and cerebrovascular complications such as stroke, coronary artery disease, claudication, and aneurysms. Involvement of the gastrointestinal or genitourinary tracts also may occur and most commonly manifests with bleeding. Pathologic alterations in the elastic fibers of the lungs also have been reported in patients with PXE.8 Histologically, PXE exhibits increased abnormally clumped and fragmented elastic fibers in the superficial dermis, often with calcification (Figure 1). Pseudo-PXE related to D-penicillamine use often lacks calcification and has a bramble bush appearance.9

Fibrofolliculomas may manifest alone or in association with an underlying condition such as Birt-Hogg-Dubé syndrome, in which lesions are most frequently seen scattered on the scalp, face, ears, neck, or upper trunk.10 This condition is related to a folliculin (FLCN) gene germline mutation. Birt-Hogg-Dubé syndrome also may be associated with acrochordons, trichodiscomas, renal cancer, and lung cysts with or without spontaneous pneumothorax. Less frequently noted findings include oral papules, epidermal cysts, angiofibromas, lipomas/angiolipomas, parotid gland tumors, and thyroid neoplasms. Connective tissue nevi/collagenomas can appear clinically similar to fibrofolliculomas; true connective tissue nevi are reported less commonly in Birt-Hogg-Dubé syndrome.11 Histologically, a fibrofolliculoma manifests with epidermal strands originating from a hair follicle associated with prominent surrounding connective tissue (Figure 2).
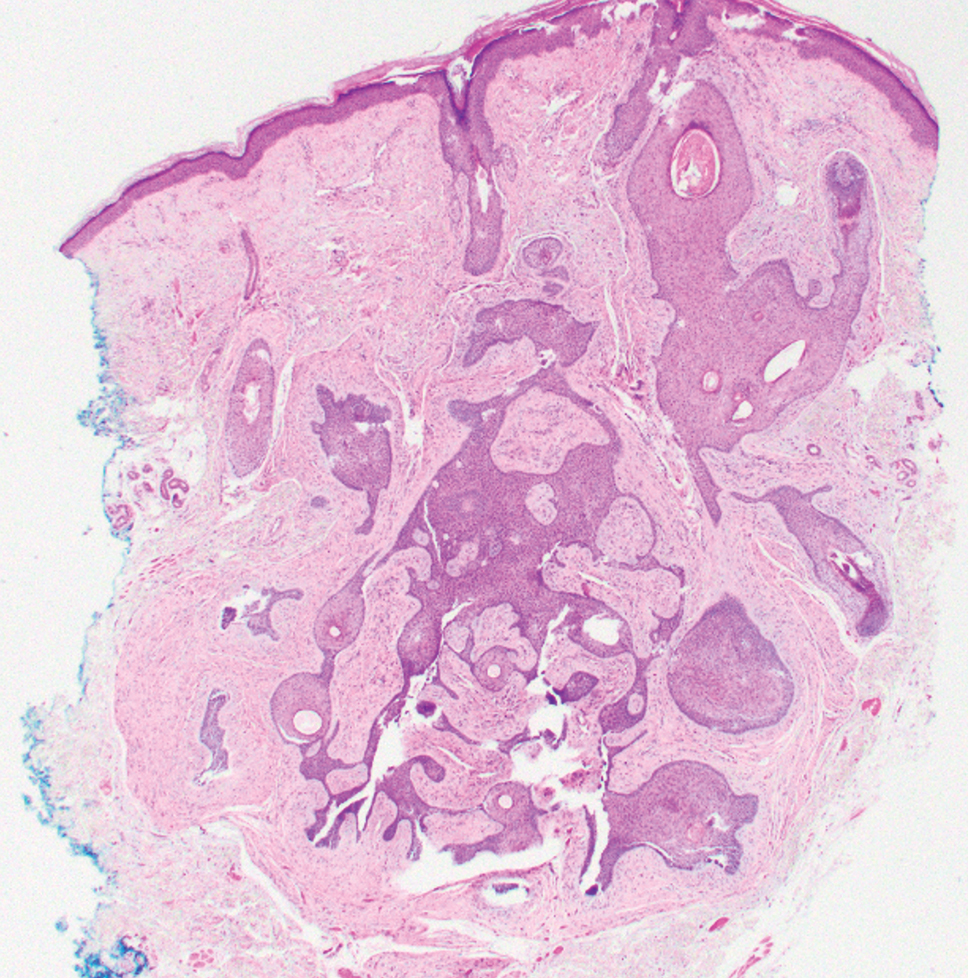
Elastofibroma dorsi is a benign tumor of connective tissue that most commonly manifests clinically as a solitary subcutaneous mass on the back near the inferior angle of the scapula; it typically develops below the rhomboid major and latissimus dorsi muscles.12 The pathogenesis is uncertain, but some patients have reported a family history of the condition or a history of repetitive shoulder movement/trauma prior to onset; the mass may be asymptomatic or associated with pain and/or swelling. Those affected tend to be older than 50 years.13 Histologically, thickened and rounded to beaded elastic fibers are seen admixed with collagen (Figure 3).
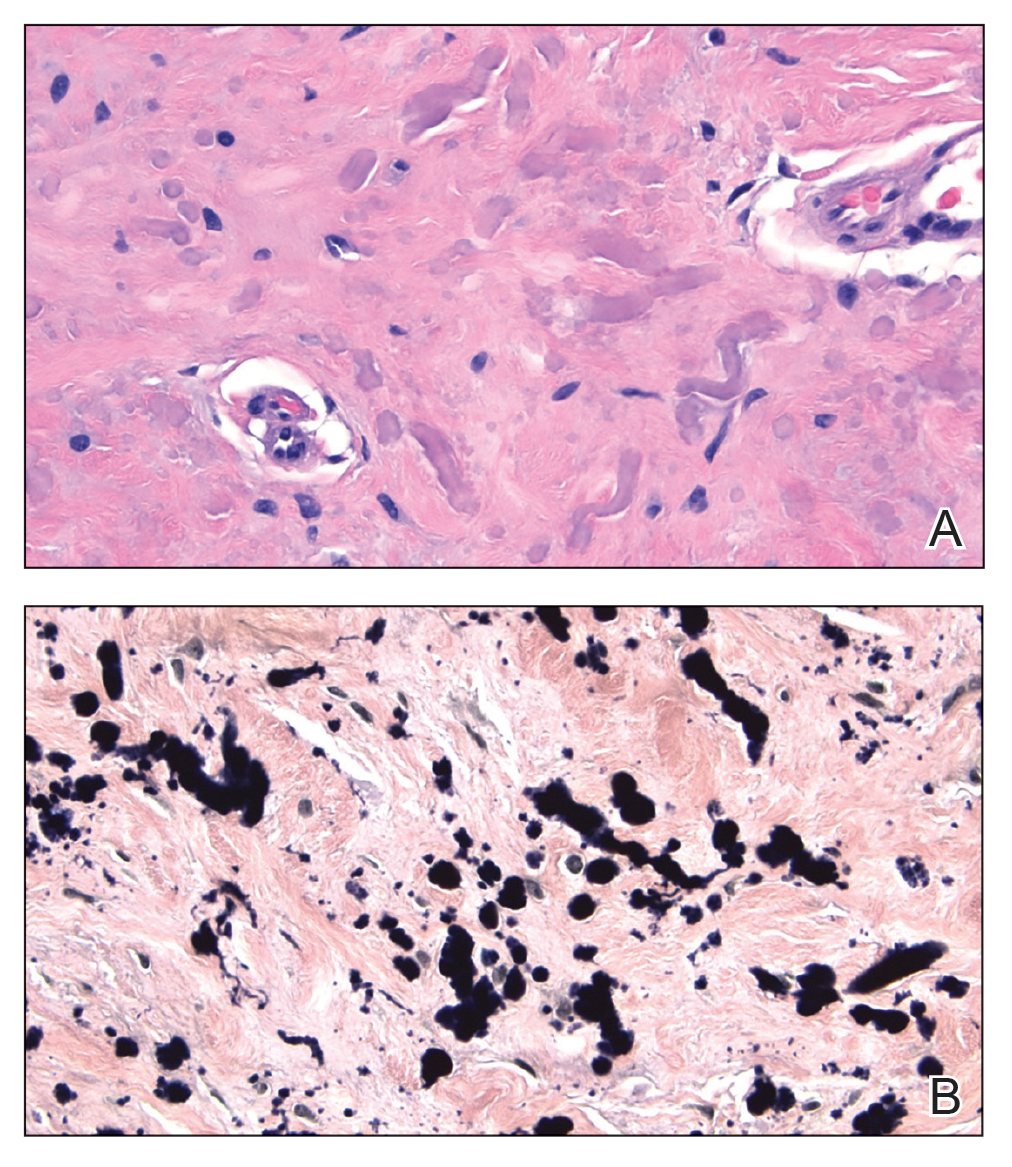
Actinic (solar) elastosis frequently is encountered in many skin biopsies and is caused by chronic photodamage. More hypertrophic variants, such as papular or nodular solar elastosis, may clinically manifest similarly to WFP.14 Histologically, actinic elastosis manifests as a considerable increase in elastic tissue in the papillary and superficial reticular dermis (Figure 4).
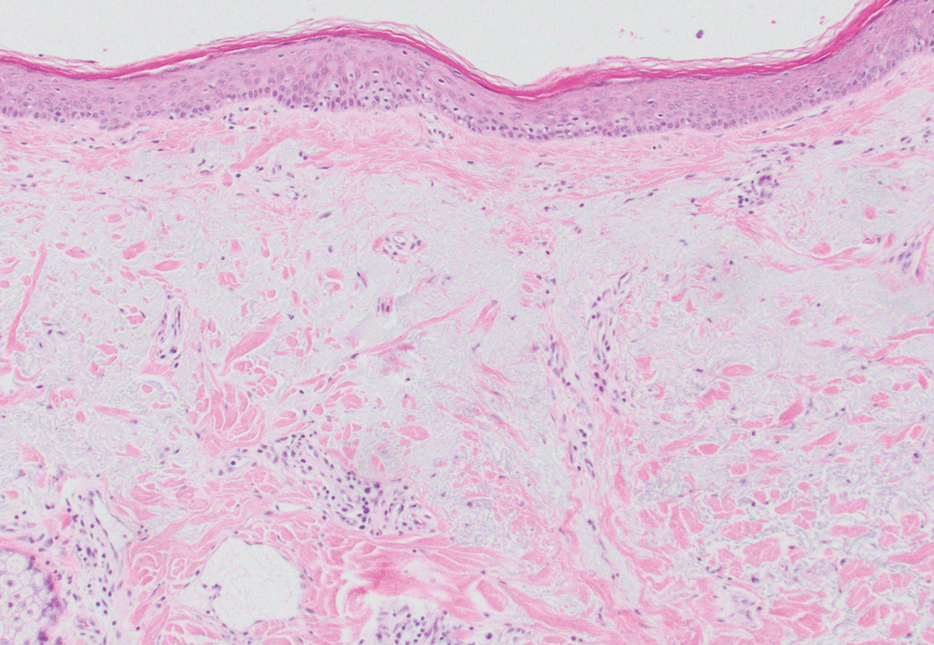
- Shimizu H, Nishikawa T, Kimura S. White fibrous papulosis of the neck: review of our 16 cases. Nihon Hifuka Gakkai Zasshi. 1985;95:1077-1084.
- Teo W, Pang S. White fibrous papulosis of the chest and back. J Am Acad Dermatol. 2012;66:AB33.
- Dokic Y, Tschen J. White fibrous papulosis of the axillae and neck. Cureus. 2020;12:E7635.
- Lueangarun S, Panchaprateep R. White fibrous papulosis of the neck treated with fractionated 1550-nm erbium glass laser: a case report. J Lasers Med Sci. 2016;7:256-258.
- Rios-Gomez M, Ramos-Garibay JA, Perez-Santana ME, et al. White fibrous papulosis of the neck: a case report. Cureus. 2022;14:E25661.
- Váradi A, Szabó Z, Pomozi V, et al. ABCC6 as a target in pseudoxanthoma elasticum. Curr Drug Targets. 2011;12:671-682.
- Gliem M, Birtel J, Müller PL, et al. Acute retinopathy in pseudoxanthoma elasticum. JAMA Ophthalmol. 2019;137:1165-1173.
- Germain DP. Pseudoxanthoma elasticum. Orphanet J Rare Dis. 2017;12:85. doi:10.1186/s13023-017-0639-8
- Chisti MA, Binamer Y, Alfadley A, et al. D-penicillamine-induced pseudo-pseudoxanthoma elasticum and extensive elastosis perforans serpiginosa with excellent response to acitretin. Ann Saudi Med. 2019;39:56-60.
- Criscito MC, Mu EW, Meehan SA, et al. Dermoscopic features of a solitary fibrofolliculoma on the left cheek. J Am Acad Dermatol. 2017;76(2 suppl 1):S8-S9.
- Sattler EC, Steinlein OK. Birt-Hogg-Dubé syndrome. In: Adam MP, Everman DB, Mirzaa GM, et al, eds. GeneReviews® [Internet]. Updated January 30, 2020. Accessed February 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK1522
- Patnayak R, Jena A, Settipalli S, et al. Elastofibroma: an uncommon tumor revisited. J Cutan Aesthet Surg. 2016;9:34-37. doi:10.4103/0974- 2077.178543
- Chandrasekar CR, Grimer RJ, Carter SR, et al. Elastofibroma dorsi: an uncommon benign pseudotumour. Sarcoma. 2008;2008:756565. doi:10.1155/2008/756565
- Kwittken J. Papular elastosis. Cutis. 2000;66:81-83.
THE DIAGNOSIS: White Fibrous Papulosis
Given the histopathology findings, location on a sun-exposed site, lack of any additional systemic signs or symptoms, and no family history of similar lesions to suggest an underlying genetic condition, a diagnosis of white fibrous papulosis (WFP) was made. White fibrous papulosis is a relatively rare cutaneous disorder that was first reported by Shimizu et al1 in 1985. It is characterized by numerous grouped, 2- to 3-mm, white to flesh-colored papules that in most cases are confined to the neck in middle-aged to elderly individuals; however, cases involving the upper trunk and axillae also have been reported.1-3 The etiology of this condition is unclear but is thought to be related to aging and chronic exposure to UV light. Although treatment is not required, various modalities including tretinoin, excision, and laser therapy have been trialed with varying success.2,4 Our patient elected not to proceed with treatment.
Histologically, WFP may manifest similarly to connective tissue nevi; the overall architecture is nonspecific with focally thickened collagen and often elastic fibers that may be normal to reduced and/or fragmented, as well as an overall decrease in superficial dermal elastic tissue.3,5 Therefore, the differential diagnosis may include connective tissue nevi and require clinical correlation to make a correct diagnosis.
Pseudoxanthoma elasticum (PXE) is an autosomalrecessive disorder most commonly related to mutations in the ATP binding cassette subfamily C member 6 (ABCC6) gene that tends to manifest clinically on the neck and flexural extremities.6 This disease affects elastic fibers, which may become calcified over time. Pseudoxanthoma elasticum is associated with ocular complications relating to the Bruch membrane of the retina and angioid streaks; choroidal neovascularization involving the damaged Bruch membrane and episodes of acute retinopathy may result in vision loss in later stages of the disease.7 Involvement of the elastic laminae of arteries can be associated with cardiovascular and cerebrovascular complications such as stroke, coronary artery disease, claudication, and aneurysms. Involvement of the gastrointestinal or genitourinary tracts also may occur and most commonly manifests with bleeding. Pathologic alterations in the elastic fibers of the lungs also have been reported in patients with PXE.8 Histologically, PXE exhibits increased abnormally clumped and fragmented elastic fibers in the superficial dermis, often with calcification (Figure 1). Pseudo-PXE related to D-penicillamine use often lacks calcification and has a bramble bush appearance.9

Fibrofolliculomas may manifest alone or in association with an underlying condition such as Birt-Hogg-Dubé syndrome, in which lesions are most frequently seen scattered on the scalp, face, ears, neck, or upper trunk.10 This condition is related to a folliculin (FLCN) gene germline mutation. Birt-Hogg-Dubé syndrome also may be associated with acrochordons, trichodiscomas, renal cancer, and lung cysts with or without spontaneous pneumothorax. Less frequently noted findings include oral papules, epidermal cysts, angiofibromas, lipomas/angiolipomas, parotid gland tumors, and thyroid neoplasms. Connective tissue nevi/collagenomas can appear clinically similar to fibrofolliculomas; true connective tissue nevi are reported less commonly in Birt-Hogg-Dubé syndrome.11 Histologically, a fibrofolliculoma manifests with epidermal strands originating from a hair follicle associated with prominent surrounding connective tissue (Figure 2).

Elastofibroma dorsi is a benign tumor of connective tissue that most commonly manifests clinically as a solitary subcutaneous mass on the back near the inferior angle of the scapula; it typically develops below the rhomboid major and latissimus dorsi muscles.12 The pathogenesis is uncertain, but some patients have reported a family history of the condition or a history of repetitive shoulder movement/trauma prior to onset; the mass may be asymptomatic or associated with pain and/or swelling. Those affected tend to be older than 50 years.13 Histologically, thickened and rounded to beaded elastic fibers are seen admixed with collagen (Figure 3).

Actinic (solar) elastosis frequently is encountered in many skin biopsies and is caused by chronic photodamage. More hypertrophic variants, such as papular or nodular solar elastosis, may clinically manifest similarly to WFP.14 Histologically, actinic elastosis manifests as a considerable increase in elastic tissue in the papillary and superficial reticular dermis (Figure 4).

THE DIAGNOSIS: White Fibrous Papulosis
Given the histopathology findings, location on a sun-exposed site, lack of any additional systemic signs or symptoms, and no family history of similar lesions to suggest an underlying genetic condition, a diagnosis of white fibrous papulosis (WFP) was made. White fibrous papulosis is a relatively rare cutaneous disorder that was first reported by Shimizu et al1 in 1985. It is characterized by numerous grouped, 2- to 3-mm, white to flesh-colored papules that in most cases are confined to the neck in middle-aged to elderly individuals; however, cases involving the upper trunk and axillae also have been reported.1-3 The etiology of this condition is unclear but is thought to be related to aging and chronic exposure to UV light. Although treatment is not required, various modalities including tretinoin, excision, and laser therapy have been trialed with varying success.2,4 Our patient elected not to proceed with treatment.
Histologically, WFP may manifest similarly to connective tissue nevi; the overall architecture is nonspecific with focally thickened collagen and often elastic fibers that may be normal to reduced and/or fragmented, as well as an overall decrease in superficial dermal elastic tissue.3,5 Therefore, the differential diagnosis may include connective tissue nevi and require clinical correlation to make a correct diagnosis.
Pseudoxanthoma elasticum (PXE) is an autosomalrecessive disorder most commonly related to mutations in the ATP binding cassette subfamily C member 6 (ABCC6) gene that tends to manifest clinically on the neck and flexural extremities.6 This disease affects elastic fibers, which may become calcified over time. Pseudoxanthoma elasticum is associated with ocular complications relating to the Bruch membrane of the retina and angioid streaks; choroidal neovascularization involving the damaged Bruch membrane and episodes of acute retinopathy may result in vision loss in later stages of the disease.7 Involvement of the elastic laminae of arteries can be associated with cardiovascular and cerebrovascular complications such as stroke, coronary artery disease, claudication, and aneurysms. Involvement of the gastrointestinal or genitourinary tracts also may occur and most commonly manifests with bleeding. Pathologic alterations in the elastic fibers of the lungs also have been reported in patients with PXE.8 Histologically, PXE exhibits increased abnormally clumped and fragmented elastic fibers in the superficial dermis, often with calcification (Figure 1). Pseudo-PXE related to D-penicillamine use often lacks calcification and has a bramble bush appearance.9

Fibrofolliculomas may manifest alone or in association with an underlying condition such as Birt-Hogg-Dubé syndrome, in which lesions are most frequently seen scattered on the scalp, face, ears, neck, or upper trunk.10 This condition is related to a folliculin (FLCN) gene germline mutation. Birt-Hogg-Dubé syndrome also may be associated with acrochordons, trichodiscomas, renal cancer, and lung cysts with or without spontaneous pneumothorax. Less frequently noted findings include oral papules, epidermal cysts, angiofibromas, lipomas/angiolipomas, parotid gland tumors, and thyroid neoplasms. Connective tissue nevi/collagenomas can appear clinically similar to fibrofolliculomas; true connective tissue nevi are reported less commonly in Birt-Hogg-Dubé syndrome.11 Histologically, a fibrofolliculoma manifests with epidermal strands originating from a hair follicle associated with prominent surrounding connective tissue (Figure 2).

Elastofibroma dorsi is a benign tumor of connective tissue that most commonly manifests clinically as a solitary subcutaneous mass on the back near the inferior angle of the scapula; it typically develops below the rhomboid major and latissimus dorsi muscles.12 The pathogenesis is uncertain, but some patients have reported a family history of the condition or a history of repetitive shoulder movement/trauma prior to onset; the mass may be asymptomatic or associated with pain and/or swelling. Those affected tend to be older than 50 years.13 Histologically, thickened and rounded to beaded elastic fibers are seen admixed with collagen (Figure 3).

Actinic (solar) elastosis frequently is encountered in many skin biopsies and is caused by chronic photodamage. More hypertrophic variants, such as papular or nodular solar elastosis, may clinically manifest similarly to WFP.14 Histologically, actinic elastosis manifests as a considerable increase in elastic tissue in the papillary and superficial reticular dermis (Figure 4).

- Shimizu H, Nishikawa T, Kimura S. White fibrous papulosis of the neck: review of our 16 cases. Nihon Hifuka Gakkai Zasshi. 1985;95:1077-1084.
- Teo W, Pang S. White fibrous papulosis of the chest and back. J Am Acad Dermatol. 2012;66:AB33.
- Dokic Y, Tschen J. White fibrous papulosis of the axillae and neck. Cureus. 2020;12:E7635.
- Lueangarun S, Panchaprateep R. White fibrous papulosis of the neck treated with fractionated 1550-nm erbium glass laser: a case report. J Lasers Med Sci. 2016;7:256-258.
- Rios-Gomez M, Ramos-Garibay JA, Perez-Santana ME, et al. White fibrous papulosis of the neck: a case report. Cureus. 2022;14:E25661.
- Váradi A, Szabó Z, Pomozi V, et al. ABCC6 as a target in pseudoxanthoma elasticum. Curr Drug Targets. 2011;12:671-682.
- Gliem M, Birtel J, Müller PL, et al. Acute retinopathy in pseudoxanthoma elasticum. JAMA Ophthalmol. 2019;137:1165-1173.
- Germain DP. Pseudoxanthoma elasticum. Orphanet J Rare Dis. 2017;12:85. doi:10.1186/s13023-017-0639-8
- Chisti MA, Binamer Y, Alfadley A, et al. D-penicillamine-induced pseudo-pseudoxanthoma elasticum and extensive elastosis perforans serpiginosa with excellent response to acitretin. Ann Saudi Med. 2019;39:56-60.
- Criscito MC, Mu EW, Meehan SA, et al. Dermoscopic features of a solitary fibrofolliculoma on the left cheek. J Am Acad Dermatol. 2017;76(2 suppl 1):S8-S9.
- Sattler EC, Steinlein OK. Birt-Hogg-Dubé syndrome. In: Adam MP, Everman DB, Mirzaa GM, et al, eds. GeneReviews® [Internet]. Updated January 30, 2020. Accessed February 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK1522
- Patnayak R, Jena A, Settipalli S, et al. Elastofibroma: an uncommon tumor revisited. J Cutan Aesthet Surg. 2016;9:34-37. doi:10.4103/0974- 2077.178543
- Chandrasekar CR, Grimer RJ, Carter SR, et al. Elastofibroma dorsi: an uncommon benign pseudotumour. Sarcoma. 2008;2008:756565. doi:10.1155/2008/756565
- Kwittken J. Papular elastosis. Cutis. 2000;66:81-83.
- Shimizu H, Nishikawa T, Kimura S. White fibrous papulosis of the neck: review of our 16 cases. Nihon Hifuka Gakkai Zasshi. 1985;95:1077-1084.
- Teo W, Pang S. White fibrous papulosis of the chest and back. J Am Acad Dermatol. 2012;66:AB33.
- Dokic Y, Tschen J. White fibrous papulosis of the axillae and neck. Cureus. 2020;12:E7635.
- Lueangarun S, Panchaprateep R. White fibrous papulosis of the neck treated with fractionated 1550-nm erbium glass laser: a case report. J Lasers Med Sci. 2016;7:256-258.
- Rios-Gomez M, Ramos-Garibay JA, Perez-Santana ME, et al. White fibrous papulosis of the neck: a case report. Cureus. 2022;14:E25661.
- Váradi A, Szabó Z, Pomozi V, et al. ABCC6 as a target in pseudoxanthoma elasticum. Curr Drug Targets. 2011;12:671-682.
- Gliem M, Birtel J, Müller PL, et al. Acute retinopathy in pseudoxanthoma elasticum. JAMA Ophthalmol. 2019;137:1165-1173.
- Germain DP. Pseudoxanthoma elasticum. Orphanet J Rare Dis. 2017;12:85. doi:10.1186/s13023-017-0639-8
- Chisti MA, Binamer Y, Alfadley A, et al. D-penicillamine-induced pseudo-pseudoxanthoma elasticum and extensive elastosis perforans serpiginosa with excellent response to acitretin. Ann Saudi Med. 2019;39:56-60.
- Criscito MC, Mu EW, Meehan SA, et al. Dermoscopic features of a solitary fibrofolliculoma on the left cheek. J Am Acad Dermatol. 2017;76(2 suppl 1):S8-S9.
- Sattler EC, Steinlein OK. Birt-Hogg-Dubé syndrome. In: Adam MP, Everman DB, Mirzaa GM, et al, eds. GeneReviews® [Internet]. Updated January 30, 2020. Accessed February 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK1522
- Patnayak R, Jena A, Settipalli S, et al. Elastofibroma: an uncommon tumor revisited. J Cutan Aesthet Surg. 2016;9:34-37. doi:10.4103/0974- 2077.178543
- Chandrasekar CR, Grimer RJ, Carter SR, et al. Elastofibroma dorsi: an uncommon benign pseudotumour. Sarcoma. 2008;2008:756565. doi:10.1155/2008/756565
- Kwittken J. Papular elastosis. Cutis. 2000;66:81-83.
A 70-year-old woman with a history of osteoporosis and breast cancer presented for evaluation of asymptomatic, 2- to 3-mm, white to flesh-colored papules concentrated on the inferior occipital scalp and posterior neck (inset) for at least several months. She had no additional systemic signs or symptoms, and there was no family history of similar skin findings. A punch biopsy was performed.
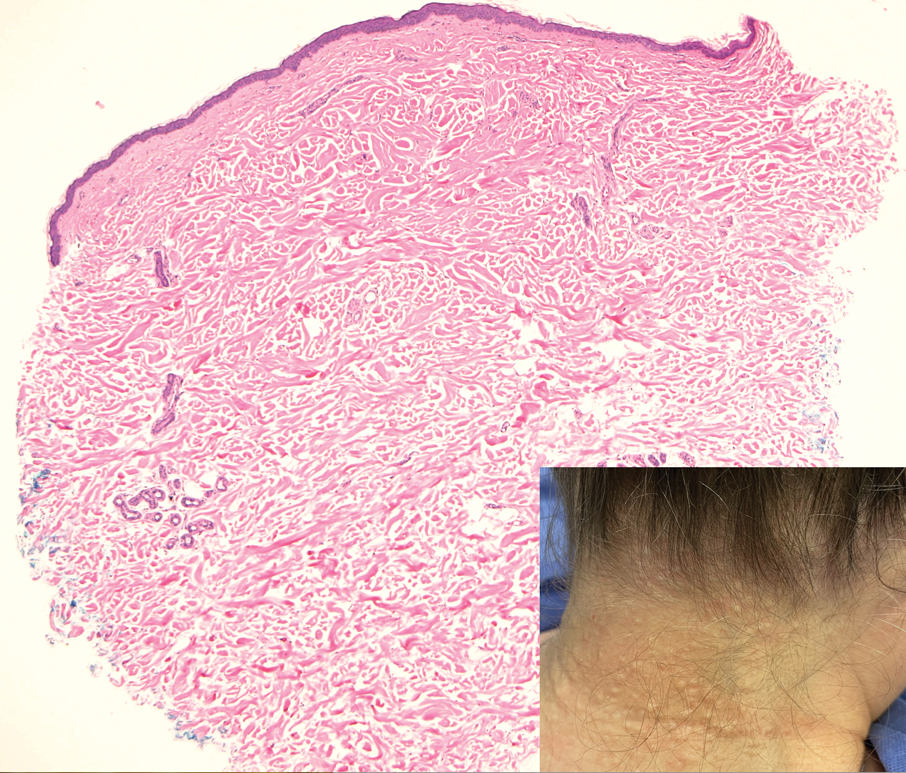
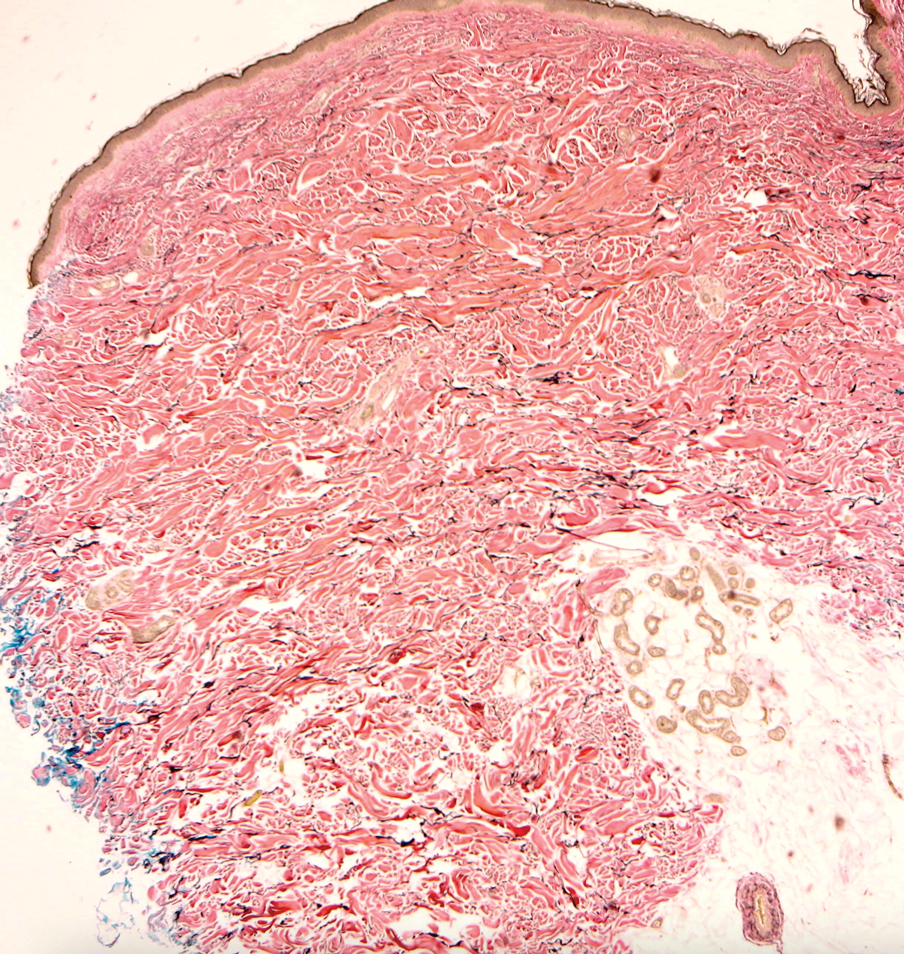
Multiple Painless Whitish Papules on the Vulva and Perianal Region
THE DIAGNOSIS: Papular Acantholytic Dyskeratosis
Histopathology of the lesion in our patient revealed hyperkeratosis, parakeratosis, dyskeratosis, and acantholysis of keratinocytes. The dermis showed variable chronic inflammatory cells. Corps ronds and grains in the acantholytic layer of the epidermis were identified. Hair follicles were not affected by acantholysis. Anti–desmoglein 1 and anti–desmoglein 3 serum antibodies were negative. Based on the combined clinical and histologic findings, the patient was diagnosed with papular acantholytic dyskeratosis (PAD) of the genitocrural area.
Although its typical histopathologic pattern mimics both Hailey-Hailey disease and Darier disease, PAD is a rare unique clinicopathologic entity recognized by dermatopathologists. It usually occurs in middle-aged women with no family history of similar conditions. The multiple localized, flesh-colored to whitish papules of PAD tend to coalesce into plaques in the anogenital and genitocrural regions. Plaques usually are asymptomatic but may be pruritic. Histopathologically, PAD will demonstrate hyperkeratosis, dyskeratosis, and acantholysis. Corps ronds and grains will be present in the acantholytic layer of the epidermis.1,2
The differential diagnosis for PAD includes pemphigus vegetans, Hailey-Hailey disease, Darier disease, and Grover disease. Patients usually develop pemphigus vegetans at an older age (typically 50–70 years).3 Histopathologically, it is characterized by pseudoepitheliomatous hyperplasia with an eosinophilic microabscess as well as acantholysis that involves the follicular epithelium (Figure 1),4 which were not seen in our patient. Direct immunofluorescence will show the intercellular pattern of the pemphigus group, and antidesmoglein antibodies can be detected by enzyme-linked immunosorbent assay.4,5
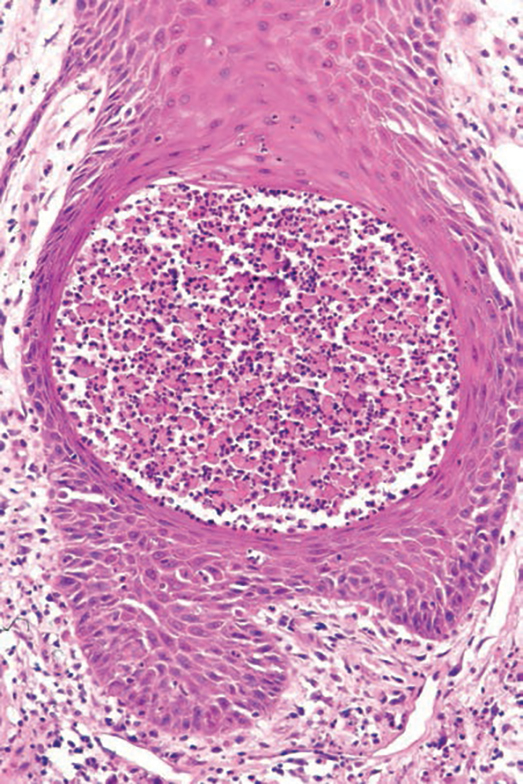
Hailey-Hailey disease (also known as benign familial pemphigus) typically manifests as itchy malodorous vesicles and erosions, especially in intertriginous areas. The most commonly affected sites are the groin, neck, under the breasts, and between the buttocks. In one study, two-thirds of affected patients reported a relevant family history.4 Histopathology will show minimal dyskeratosis and suprabasilar acantholysis with loss of intercellular bridges, classically described as resembling a dilapidated brick wall (Figure 2).4,5 There is no notable follicular involvement with acantholysis.4
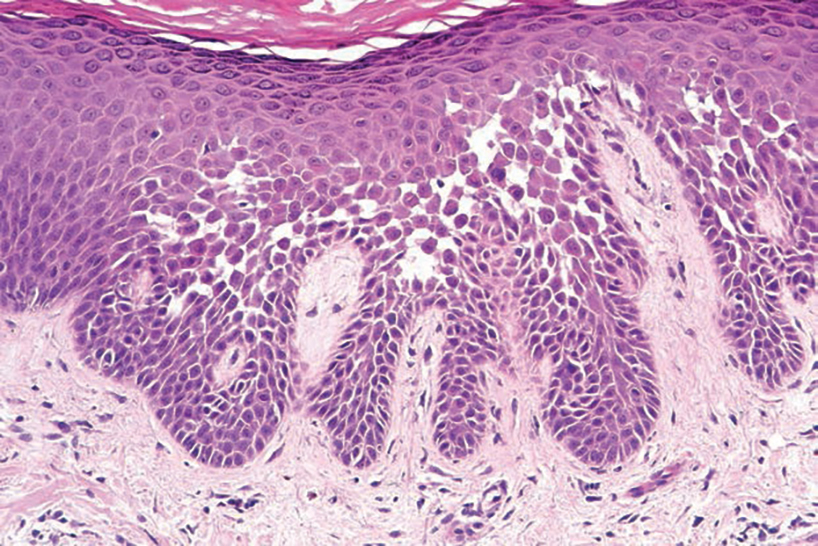
characteristic dilapidated brick wall appearance (H&E, original
magnification ×40).
Darier disease (also known as keratosis follicularis) typically is inherited in an autosomal-dominant pattern.4 It is found on the seborrheic areas such as the scalp, forehead, nasolabial folds, and upper chest. Characteristic features include distal notching of the nails, mucosal lesions, and palmoplantar papules. Histopathology will reveal acantholysis, dyskeratosis, suprabasilar acantholysis, and corps ronds and grains.4 Acantholysis in Darier disease can be in discrete foci and/or widespread (Figure 3).4 Darier disease demonstrates more dyskeratosis than Hailey-Hailey disease.4,5

Grover disease (also referred to as transient acantholytic dermatosis) is observed predominantly in individuals who are middle-aged or older, though occurrence in children has been rarely reported.4 It affects the trunk, neck, and proximal limbs but spares the genital area. Histopathology may reveal acantholysis (similar to Hailey-Hailey disease or pemphigus vulgaris), dyskeratosis (resembling Darier disease), spongiosis, parakeratosis, and a superficial perivascular lymphocytic infiltrate with eosinophils.4 A histologic clue to the diagnosis is small lesion size (1–3 mm). Usually, only 1 or 2 small discrete lesions that span a few rete ridges are noted (Figure 4).4 Grover disease can cause follicular or acrosyringeal involvement.4
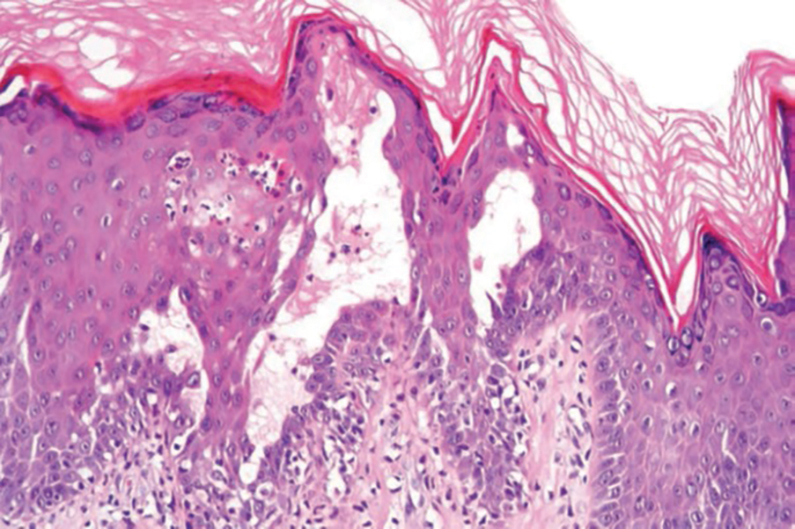
- Al-Muriesh M, Abdul-Fattah B, Wang X, et al. Papular acantholytic dyskeratosis of the anogenital and genitocrural area: case series and review of the literature. J Cutan Pathol. 2016;43:749-758. doi:10.1111/cup.12736
- Harrell J, Nielson C, Beers P, et al. Eruption on the vulva and groin. JAAD Case Reports. 2019;6:6-8. doi:10.1016/j.jdcr.2019.11.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed September 18, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229
- Acantholytic disorders. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin: With Clinical Correlations. Elsevier/ Saunders; 2012:171-200.
- Mohr MR, Erdag G, Shada AL, et al. Two patients with Hailey- Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211215. doi:10.1001/archdermatol.2010.445
THE DIAGNOSIS: Papular Acantholytic Dyskeratosis
Histopathology of the lesion in our patient revealed hyperkeratosis, parakeratosis, dyskeratosis, and acantholysis of keratinocytes. The dermis showed variable chronic inflammatory cells. Corps ronds and grains in the acantholytic layer of the epidermis were identified. Hair follicles were not affected by acantholysis. Anti–desmoglein 1 and anti–desmoglein 3 serum antibodies were negative. Based on the combined clinical and histologic findings, the patient was diagnosed with papular acantholytic dyskeratosis (PAD) of the genitocrural area.
Although its typical histopathologic pattern mimics both Hailey-Hailey disease and Darier disease, PAD is a rare unique clinicopathologic entity recognized by dermatopathologists. It usually occurs in middle-aged women with no family history of similar conditions. The multiple localized, flesh-colored to whitish papules of PAD tend to coalesce into plaques in the anogenital and genitocrural regions. Plaques usually are asymptomatic but may be pruritic. Histopathologically, PAD will demonstrate hyperkeratosis, dyskeratosis, and acantholysis. Corps ronds and grains will be present in the acantholytic layer of the epidermis.1,2
The differential diagnosis for PAD includes pemphigus vegetans, Hailey-Hailey disease, Darier disease, and Grover disease. Patients usually develop pemphigus vegetans at an older age (typically 50–70 years).3 Histopathologically, it is characterized by pseudoepitheliomatous hyperplasia with an eosinophilic microabscess as well as acantholysis that involves the follicular epithelium (Figure 1),4 which were not seen in our patient. Direct immunofluorescence will show the intercellular pattern of the pemphigus group, and antidesmoglein antibodies can be detected by enzyme-linked immunosorbent assay.4,5

Hailey-Hailey disease (also known as benign familial pemphigus) typically manifests as itchy malodorous vesicles and erosions, especially in intertriginous areas. The most commonly affected sites are the groin, neck, under the breasts, and between the buttocks. In one study, two-thirds of affected patients reported a relevant family history.4 Histopathology will show minimal dyskeratosis and suprabasilar acantholysis with loss of intercellular bridges, classically described as resembling a dilapidated brick wall (Figure 2).4,5 There is no notable follicular involvement with acantholysis.4

characteristic dilapidated brick wall appearance (H&E, original
magnification ×40).
Darier disease (also known as keratosis follicularis) typically is inherited in an autosomal-dominant pattern.4 It is found on the seborrheic areas such as the scalp, forehead, nasolabial folds, and upper chest. Characteristic features include distal notching of the nails, mucosal lesions, and palmoplantar papules. Histopathology will reveal acantholysis, dyskeratosis, suprabasilar acantholysis, and corps ronds and grains.4 Acantholysis in Darier disease can be in discrete foci and/or widespread (Figure 3).4 Darier disease demonstrates more dyskeratosis than Hailey-Hailey disease.4,5

Grover disease (also referred to as transient acantholytic dermatosis) is observed predominantly in individuals who are middle-aged or older, though occurrence in children has been rarely reported.4 It affects the trunk, neck, and proximal limbs but spares the genital area. Histopathology may reveal acantholysis (similar to Hailey-Hailey disease or pemphigus vulgaris), dyskeratosis (resembling Darier disease), spongiosis, parakeratosis, and a superficial perivascular lymphocytic infiltrate with eosinophils.4 A histologic clue to the diagnosis is small lesion size (1–3 mm). Usually, only 1 or 2 small discrete lesions that span a few rete ridges are noted (Figure 4).4 Grover disease can cause follicular or acrosyringeal involvement.4

THE DIAGNOSIS: Papular Acantholytic Dyskeratosis
Histopathology of the lesion in our patient revealed hyperkeratosis, parakeratosis, dyskeratosis, and acantholysis of keratinocytes. The dermis showed variable chronic inflammatory cells. Corps ronds and grains in the acantholytic layer of the epidermis were identified. Hair follicles were not affected by acantholysis. Anti–desmoglein 1 and anti–desmoglein 3 serum antibodies were negative. Based on the combined clinical and histologic findings, the patient was diagnosed with papular acantholytic dyskeratosis (PAD) of the genitocrural area.
Although its typical histopathologic pattern mimics both Hailey-Hailey disease and Darier disease, PAD is a rare unique clinicopathologic entity recognized by dermatopathologists. It usually occurs in middle-aged women with no family history of similar conditions. The multiple localized, flesh-colored to whitish papules of PAD tend to coalesce into plaques in the anogenital and genitocrural regions. Plaques usually are asymptomatic but may be pruritic. Histopathologically, PAD will demonstrate hyperkeratosis, dyskeratosis, and acantholysis. Corps ronds and grains will be present in the acantholytic layer of the epidermis.1,2
The differential diagnosis for PAD includes pemphigus vegetans, Hailey-Hailey disease, Darier disease, and Grover disease. Patients usually develop pemphigus vegetans at an older age (typically 50–70 years).3 Histopathologically, it is characterized by pseudoepitheliomatous hyperplasia with an eosinophilic microabscess as well as acantholysis that involves the follicular epithelium (Figure 1),4 which were not seen in our patient. Direct immunofluorescence will show the intercellular pattern of the pemphigus group, and antidesmoglein antibodies can be detected by enzyme-linked immunosorbent assay.4,5

Hailey-Hailey disease (also known as benign familial pemphigus) typically manifests as itchy malodorous vesicles and erosions, especially in intertriginous areas. The most commonly affected sites are the groin, neck, under the breasts, and between the buttocks. In one study, two-thirds of affected patients reported a relevant family history.4 Histopathology will show minimal dyskeratosis and suprabasilar acantholysis with loss of intercellular bridges, classically described as resembling a dilapidated brick wall (Figure 2).4,5 There is no notable follicular involvement with acantholysis.4

characteristic dilapidated brick wall appearance (H&E, original
magnification ×40).
Darier disease (also known as keratosis follicularis) typically is inherited in an autosomal-dominant pattern.4 It is found on the seborrheic areas such as the scalp, forehead, nasolabial folds, and upper chest. Characteristic features include distal notching of the nails, mucosal lesions, and palmoplantar papules. Histopathology will reveal acantholysis, dyskeratosis, suprabasilar acantholysis, and corps ronds and grains.4 Acantholysis in Darier disease can be in discrete foci and/or widespread (Figure 3).4 Darier disease demonstrates more dyskeratosis than Hailey-Hailey disease.4,5

Grover disease (also referred to as transient acantholytic dermatosis) is observed predominantly in individuals who are middle-aged or older, though occurrence in children has been rarely reported.4 It affects the trunk, neck, and proximal limbs but spares the genital area. Histopathology may reveal acantholysis (similar to Hailey-Hailey disease or pemphigus vulgaris), dyskeratosis (resembling Darier disease), spongiosis, parakeratosis, and a superficial perivascular lymphocytic infiltrate with eosinophils.4 A histologic clue to the diagnosis is small lesion size (1–3 mm). Usually, only 1 or 2 small discrete lesions that span a few rete ridges are noted (Figure 4).4 Grover disease can cause follicular or acrosyringeal involvement.4

- Al-Muriesh M, Abdul-Fattah B, Wang X, et al. Papular acantholytic dyskeratosis of the anogenital and genitocrural area: case series and review of the literature. J Cutan Pathol. 2016;43:749-758. doi:10.1111/cup.12736
- Harrell J, Nielson C, Beers P, et al. Eruption on the vulva and groin. JAAD Case Reports. 2019;6:6-8. doi:10.1016/j.jdcr.2019.11.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed September 18, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229
- Acantholytic disorders. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin: With Clinical Correlations. Elsevier/ Saunders; 2012:171-200.
- Mohr MR, Erdag G, Shada AL, et al. Two patients with Hailey- Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211215. doi:10.1001/archdermatol.2010.445
- Al-Muriesh M, Abdul-Fattah B, Wang X, et al. Papular acantholytic dyskeratosis of the anogenital and genitocrural area: case series and review of the literature. J Cutan Pathol. 2016;43:749-758. doi:10.1111/cup.12736
- Harrell J, Nielson C, Beers P, et al. Eruption on the vulva and groin. JAAD Case Reports. 2019;6:6-8. doi:10.1016/j.jdcr.2019.11.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed September 18, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229
- Acantholytic disorders. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin: With Clinical Correlations. Elsevier/ Saunders; 2012:171-200.
- Mohr MR, Erdag G, Shada AL, et al. Two patients with Hailey- Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211215. doi:10.1001/archdermatol.2010.445
A 21-year-old woman presented with a chronic eruption in the anogenital region of 4 years’ duration. Clinical examination revealed numerous painless, mildly itchy, malodorous, whitish papules on an erythematous base that were distributed on the vulva and perianal region. There were no erosions, and no other areas were involved. Routine laboratory tests were within reference range. The patient had no sexual partner and no family history of similar lesions. A skin biopsy was performed.
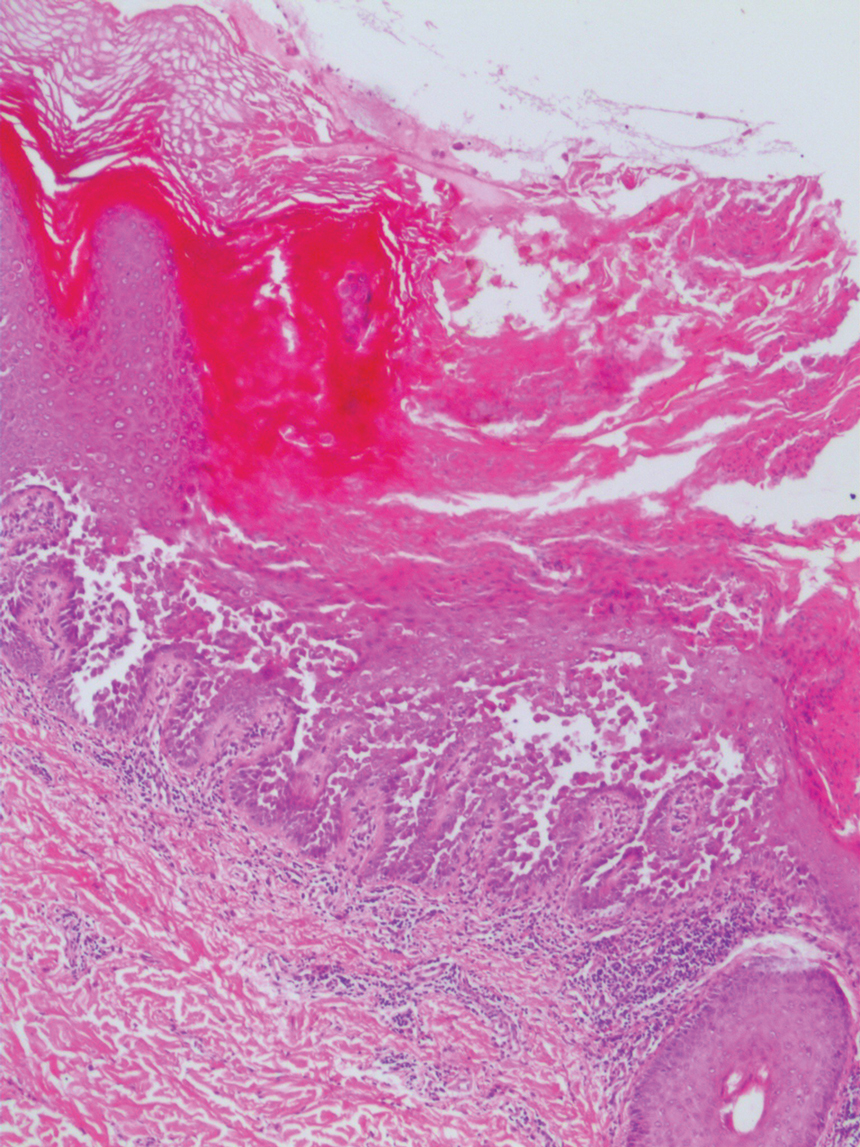
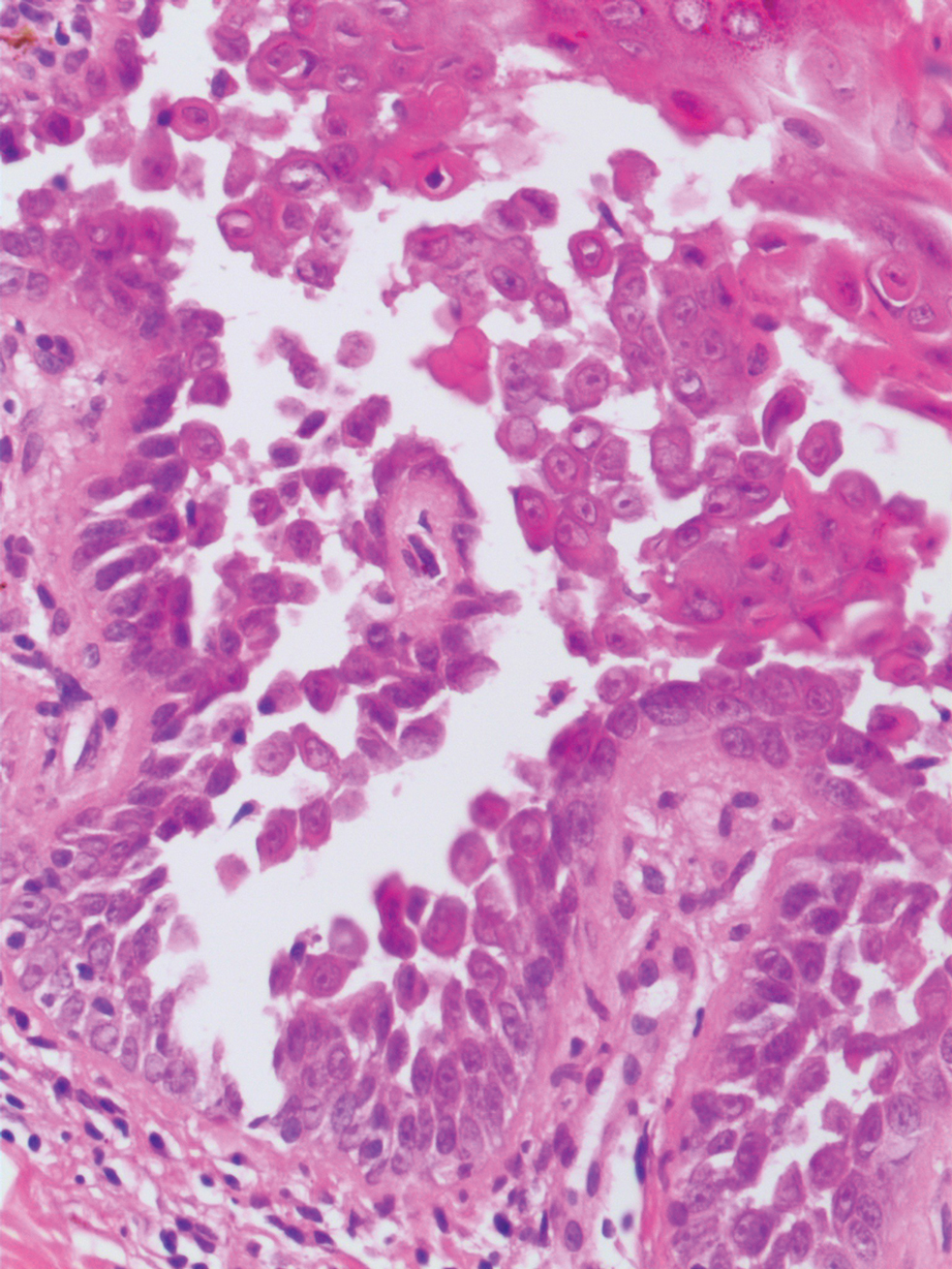
Purpuric Lesions on the Leg
THE DIAGNOSIS: Dengue Hemorrhagic Fever
The retiform purpura observed in our patient was suggestive of a vasculitic, thrombotic, or embolic etiology. Dengue IgM serologic testing performed based on her extensive travel history and recent return from a dengue-endemic area was positive, indicating acute infection. A clinical diagnosis of dengue hemorrhagic fever (DHF) was made based on the hemorrhagic appearance of the lesion. Histopathology revealed leukocytoclastic vasculitis (Figure). Anti–double-stranded DNA, antideoxyribonuclease, C3 and C4, CH50 (total hemolytic complement), antineutrophil cytoplasmic antibodies, HIV, and hepatitis B virus tests were normal. Direct immunofluorescence was negative.
Dengue virus is a single-stranded RNA virus transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is one of the most prevalent arthropod-borne viruses affecting humans today.1,2 Infection with the dengue virus generally is seen in travelers visiting tropical regions of Africa, Mexico, South America, South and Central Asia, Southeast Asia, and the Caribbean.1 The Table shows the global distribution of dengue serotypes from 2000 to 2014.3,4 There are 4 serotypes of the dengue virus: DENV-1 to DENV-4. Infection with 1 strain elicits longlasting immunity to that strain, but subsequent infection with another strain can result in severe DHF due to antibody cross-reaction.1
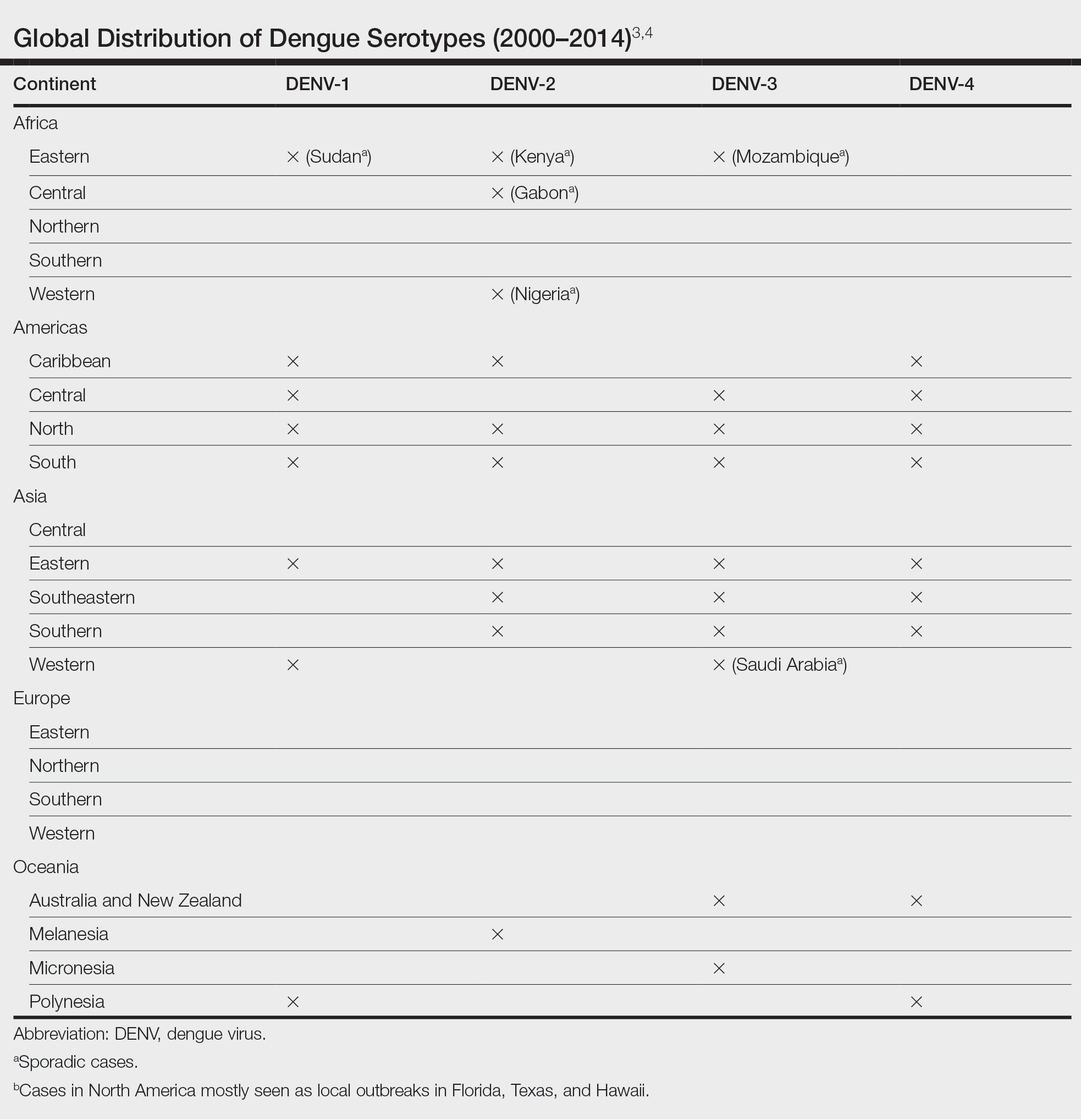
Dengue virus infection ranges from mildly symptomatic to a spectrum of increasingly severe conditions that comprise dengue fever (DF) and DHF, as well as dengue shock syndrome and brain stem hemorrhage, which may be fatal.2,5 Dengue fever manifests as severe myalgia, fever, headache (usually retro-orbital), arthralgia, erythema, and rubelliform exanthema.6 The frequency of skin eruptions in patients with DF varies with the virus strain and outbreaks.7 The lesions initially develop with the onset of fever and manifest as flushing or erythematous mottling of the face, neck, and chest areas.1,7 The morbilliform eruption develops 2 to 6 days after the onset of the fever, beginning on the trunk and spreading to the face and extremities.1,7 The rash may become confluent with characteristic sparing of small round areas of normal skin described as white islands in a sea of red.2 Verrucous papules on the ears also have been described and may resemble those seen in Cowden syndrome. In patients with prior infection with a different strain of the virus, hemorrhagic lesions may develop, including characteristic retiform purpura, a positive tourniquet test, and the appearance of petechiae on the lower legs. Pruritus and desquamation, especially on the palms and soles, may follow the termination of the eruption.7
The differential diagnosis of DF includes measles, rubella, enteroviruses, and influenza. Chikungunya and West Nile viruses in Asia and Africa and the O’nyong-nyong virus in Africa are also arboviruses that cause a clinical picture similar to DF but not DHF. Other diagnostic considerations include phases of scarlet fever, typhoid, malaria, leptospirosis, hepatitis A, and trypanosomal and rickettsial diseases.7 The differential diagnosis of DHF includes antineutrophil cytoplasmic antibody–associated vasculitis, rheumatoid vasculitis, and bacterial septic vasculitis.
Acute clinical diagnosis of DF can be challenging because of the nonspecific symptoms that can be seen in almost every infectious disease. Clinical presentation assessment should be confirmed with laboratory testing.6 Dengue virus infection usually is confirmed by the identification of viral genomic RNA, antigens, or the antibodies it elicits. Enzyme-linked immunosorbent assay–based serologic tests are cost-effective and easy to perform.5 IgM antibodies usually show cross-reactivity with platelets, but the antibody levels are not positively correlated with the severity of DF.8 Primary infection with the dengue virus is characterized by the elevation of specific IgM levels that usually occurs 3 to 5 days after symptom onset and persists during the postfebrile stage (up to 30 to 60 days). In secondary infections, the IgM levels usually rise more slowly and reach a lower level than in primary infections.9 For both primary and secondary infections, testing IgM levels after the febrile stage may be helpful with the laboratory diagnosis.
Currently, there is no antiviral drug available for dengue. Treatment of dengue infection is symptomatic and supportive.2
Dengue hemorrhagic fever is indicated by a rising hematocrit (≥20%) and a falling platelet count (>100,000/mm3) accompanying clinical signs of hemorrhage. Treatment includes intravenous fluid replacement and careful clinical monitoring of hematocrit levels, platelet count, vitals, urine output, and other signs of shock.5 For patients with a history of dengue infection, travel to areas with other serotypes is not recommended.
If any travel to a high-risk area is planned, countryspecific travel recommendations and warnings should be reviewed from the Centers for Disease Control and Prevention’s website (https://wwwnc.cdc.gov/travel/notices/level1/dengue-global). Use of an Environmental Protection Agency–registered insect repellent to avoid mosquito bites and acetaminophen for managing symptoms is advised. During travel, staying in places with window and door screens and using a bed net during sleep are suggested. Long-sleeved shirts and long pants also are preferred. Travelers should see a health care provider if they have symptoms of dengue.10
African tick bite fever (ATBF) is caused by Rickettsia africae transmitted by Amblyomma ticks. Skin findings in ATBF include erythematous, firm, tender papules with central eschars consistent with the feeding patterns of ticks.11 Histopathology of ATBF usually includes fibrinoid necrosis of vessels in the dermis with a perivascular inflammatory infiltrate and coagulation necrosis of the surrounding dermis consistent with eschar formation.12 The lack of an eschar weighs against this diagnosis.
African trypanosomiasis (also known as sleeping sickness) is caused by protozoa transmitted by the tsetse fly. A chancrelike, circumscribed, rubbery, indurated red or violaceous nodule measuring 2 to 5 cm in diameter often develops as the earliest cutaneous sign of the disease.13 Nonspecific histopathologic findings, such as infiltration of lymphocytes and macrophages and proliferation of endothelial cells and fibroblasts, may be observed.14 Extravascular parasites have been noted in skin biopsies.15 In later stages, skin lesions called trypanids may be observed as macular, papular, annular, targetoid, purpuric, and erythematous lesions, and histopathologic findings consistent with vasculitis also may be seen.13
Chikungunya virus infection is an acute-onset, mosquito-borne viral disease. Skin manifestations may start with nonspecific, generalized, morbilliform, maculopapular rashes coinciding with fever, which also may be seen initially with DHF. Skin hyperpigmentation, mostly centrofacial and involving the nose (chik sign); purpuric and ecchymotic lesions over the trunk and flexors of limbs in adults, often surmounted by subepidermal bullae and lesions resembling toxic epidermal necrolysis; and nonhealing ulcers in the genital and groin areas are common skin manifestations of chikungunya infection.16 Intraepithelial splitting with acantholysis and perivascular lymphohistiocytic infiltration may be observed in the histopathology of blistering lesions, which are not consistent with DHF.17
Zika virus infection is caused by an arbovirus within the Flaviviridae family, which also includes the dengue virus. Initial mucocutaneous findings of the Zika virus include nonspecific diffuse maculopapular eruptions. The eruption generally spares the palms and soles; however, various manifestations including involvement of the palms and soles have been reported.18 The morbilliform eruption begins on the face and extends to the trunk and extremities. Mild hemorrhagic manifestations, including petechiae and bleeding gums, may be observed. Distinguishing between dengue and Zika virus infection relies on the severity of symptoms and laboratory tests, including polymerase chain reaction or IgM antibody testing.19 The other conditions listed do not produce hemorrhagic fever.
- Pincus LB, Grossman ME, Fox LP. The exanthem of dengue fever: clinical features of two US tourists traveling abroad. J Am Acad Dermatol. 2008;58:308-316. doi:10.1016/j.jaad.2007.08.042
- Radakovic-Fijan S, Graninger W, Müller C, et al. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430-433. doi:10.1067/mjd.2002.111904
- Yamashita A, Sakamoto T, Sekizuka T, et al. DGV: dengue genographic viewer. Front Microbiol. 2016;7:875. doi:10.3389/fmicb.2016.00875
- Centers for Disease and Prevention. Dengue in the US states and territories. Updated October 7, 2020. Accessed September 30, 2024. https://www.cdc.gov/dengue/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/dengue/areaswithrisk/in-the-us.html
- Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. doi:10.1155/2016/6803098
- Muller DA, Depelsenaire AC, Young PR. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis. 2017;215(suppl 2):S89-S95. doi:10.1093/infdis/jiw649
- Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117-122. doi:10.1016/0738-081x(89)90034-5
- Lin CF, Lei HY, Liu CC, et al. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143-149. doi:10.1002/1096- 9071(20000201)63:2<143::AID-JMV1009>3.0.CO;2-L
- Tripathi NK, Shrivastava A, Dash PK, et al. Detection of dengue virus. Methods Mol Biol. 2011;665:51-64. doi:10.1007/978-1-60761-817-1_4
- Centers for Disease Control and Prevention. Plan for travel. Accessed September 30, 2024. https://wwwnc.cdc.gov/travel
- Mack I, Ritz N. African tick-bite fever. N Engl J Med. 2019;380:960. doi:10.1056/NEJMicm1810093
- Lepidi H, Fournier PE, Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12:1332- 1337. doi:10.3201/eid1209.051540
- McGovern TW, Williams W, Fitzpatrick JE, et al. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol. 1995;131:1178-1182.
- Kristensson K, Bentivoglio M. Pathology of African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, eds. Progress in Human African Trypanosomiasis, Sleeping Sickness. Springer; 1999:157-181.
- Capewell P, Cren-Travaillé C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi:10.7554/eLife.17716
- Singal A. Chikungunya and skin: current perspective. Indian Dermatol Online J. 2017;8:307-309. doi:10.4103/idoj.IDOJ_93_17
- Robin S, Ramful D, Zettor J, et al. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2009;169:67-72. doi:10.1007/s00431-009-0986-0
- Hussain A, Ali F, Latiwesh OB, et al. A comprehensive review of the manifestations and pathogenesis of Zika virus in neonates and adults. Cureus. 2018;10:E3290. doi:10.7759/cureus.3290
- Farahnik B, Beroukhim K, Blattner CM, et al. Cutaneous manifestations of the Zika virus. J Am Acad Dermatol. 2016;74:1286-1287. doi:10.1016/j.jaad.2016.02.1232
THE DIAGNOSIS: Dengue Hemorrhagic Fever
The retiform purpura observed in our patient was suggestive of a vasculitic, thrombotic, or embolic etiology. Dengue IgM serologic testing performed based on her extensive travel history and recent return from a dengue-endemic area was positive, indicating acute infection. A clinical diagnosis of dengue hemorrhagic fever (DHF) was made based on the hemorrhagic appearance of the lesion. Histopathology revealed leukocytoclastic vasculitis (Figure). Anti–double-stranded DNA, antideoxyribonuclease, C3 and C4, CH50 (total hemolytic complement), antineutrophil cytoplasmic antibodies, HIV, and hepatitis B virus tests were normal. Direct immunofluorescence was negative.

Dengue virus is a single-stranded RNA virus transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is one of the most prevalent arthropod-borne viruses affecting humans today.1,2 Infection with the dengue virus generally is seen in travelers visiting tropical regions of Africa, Mexico, South America, South and Central Asia, Southeast Asia, and the Caribbean.1 The Table shows the global distribution of dengue serotypes from 2000 to 2014.3,4 There are 4 serotypes of the dengue virus: DENV-1 to DENV-4. Infection with 1 strain elicits longlasting immunity to that strain, but subsequent infection with another strain can result in severe DHF due to antibody cross-reaction.1

Dengue virus infection ranges from mildly symptomatic to a spectrum of increasingly severe conditions that comprise dengue fever (DF) and DHF, as well as dengue shock syndrome and brain stem hemorrhage, which may be fatal.2,5 Dengue fever manifests as severe myalgia, fever, headache (usually retro-orbital), arthralgia, erythema, and rubelliform exanthema.6 The frequency of skin eruptions in patients with DF varies with the virus strain and outbreaks.7 The lesions initially develop with the onset of fever and manifest as flushing or erythematous mottling of the face, neck, and chest areas.1,7 The morbilliform eruption develops 2 to 6 days after the onset of the fever, beginning on the trunk and spreading to the face and extremities.1,7 The rash may become confluent with characteristic sparing of small round areas of normal skin described as white islands in a sea of red.2 Verrucous papules on the ears also have been described and may resemble those seen in Cowden syndrome. In patients with prior infection with a different strain of the virus, hemorrhagic lesions may develop, including characteristic retiform purpura, a positive tourniquet test, and the appearance of petechiae on the lower legs. Pruritus and desquamation, especially on the palms and soles, may follow the termination of the eruption.7
The differential diagnosis of DF includes measles, rubella, enteroviruses, and influenza. Chikungunya and West Nile viruses in Asia and Africa and the O’nyong-nyong virus in Africa are also arboviruses that cause a clinical picture similar to DF but not DHF. Other diagnostic considerations include phases of scarlet fever, typhoid, malaria, leptospirosis, hepatitis A, and trypanosomal and rickettsial diseases.7 The differential diagnosis of DHF includes antineutrophil cytoplasmic antibody–associated vasculitis, rheumatoid vasculitis, and bacterial septic vasculitis.
Acute clinical diagnosis of DF can be challenging because of the nonspecific symptoms that can be seen in almost every infectious disease. Clinical presentation assessment should be confirmed with laboratory testing.6 Dengue virus infection usually is confirmed by the identification of viral genomic RNA, antigens, or the antibodies it elicits. Enzyme-linked immunosorbent assay–based serologic tests are cost-effective and easy to perform.5 IgM antibodies usually show cross-reactivity with platelets, but the antibody levels are not positively correlated with the severity of DF.8 Primary infection with the dengue virus is characterized by the elevation of specific IgM levels that usually occurs 3 to 5 days after symptom onset and persists during the postfebrile stage (up to 30 to 60 days). In secondary infections, the IgM levels usually rise more slowly and reach a lower level than in primary infections.9 For both primary and secondary infections, testing IgM levels after the febrile stage may be helpful with the laboratory diagnosis.
Currently, there is no antiviral drug available for dengue. Treatment of dengue infection is symptomatic and supportive.2
Dengue hemorrhagic fever is indicated by a rising hematocrit (≥20%) and a falling platelet count (>100,000/mm3) accompanying clinical signs of hemorrhage. Treatment includes intravenous fluid replacement and careful clinical monitoring of hematocrit levels, platelet count, vitals, urine output, and other signs of shock.5 For patients with a history of dengue infection, travel to areas with other serotypes is not recommended.
If any travel to a high-risk area is planned, countryspecific travel recommendations and warnings should be reviewed from the Centers for Disease Control and Prevention’s website (https://wwwnc.cdc.gov/travel/notices/level1/dengue-global). Use of an Environmental Protection Agency–registered insect repellent to avoid mosquito bites and acetaminophen for managing symptoms is advised. During travel, staying in places with window and door screens and using a bed net during sleep are suggested. Long-sleeved shirts and long pants also are preferred. Travelers should see a health care provider if they have symptoms of dengue.10
African tick bite fever (ATBF) is caused by Rickettsia africae transmitted by Amblyomma ticks. Skin findings in ATBF include erythematous, firm, tender papules with central eschars consistent with the feeding patterns of ticks.11 Histopathology of ATBF usually includes fibrinoid necrosis of vessels in the dermis with a perivascular inflammatory infiltrate and coagulation necrosis of the surrounding dermis consistent with eschar formation.12 The lack of an eschar weighs against this diagnosis.
African trypanosomiasis (also known as sleeping sickness) is caused by protozoa transmitted by the tsetse fly. A chancrelike, circumscribed, rubbery, indurated red or violaceous nodule measuring 2 to 5 cm in diameter often develops as the earliest cutaneous sign of the disease.13 Nonspecific histopathologic findings, such as infiltration of lymphocytes and macrophages and proliferation of endothelial cells and fibroblasts, may be observed.14 Extravascular parasites have been noted in skin biopsies.15 In later stages, skin lesions called trypanids may be observed as macular, papular, annular, targetoid, purpuric, and erythematous lesions, and histopathologic findings consistent with vasculitis also may be seen.13
Chikungunya virus infection is an acute-onset, mosquito-borne viral disease. Skin manifestations may start with nonspecific, generalized, morbilliform, maculopapular rashes coinciding with fever, which also may be seen initially with DHF. Skin hyperpigmentation, mostly centrofacial and involving the nose (chik sign); purpuric and ecchymotic lesions over the trunk and flexors of limbs in adults, often surmounted by subepidermal bullae and lesions resembling toxic epidermal necrolysis; and nonhealing ulcers in the genital and groin areas are common skin manifestations of chikungunya infection.16 Intraepithelial splitting with acantholysis and perivascular lymphohistiocytic infiltration may be observed in the histopathology of blistering lesions, which are not consistent with DHF.17
Zika virus infection is caused by an arbovirus within the Flaviviridae family, which also includes the dengue virus. Initial mucocutaneous findings of the Zika virus include nonspecific diffuse maculopapular eruptions. The eruption generally spares the palms and soles; however, various manifestations including involvement of the palms and soles have been reported.18 The morbilliform eruption begins on the face and extends to the trunk and extremities. Mild hemorrhagic manifestations, including petechiae and bleeding gums, may be observed. Distinguishing between dengue and Zika virus infection relies on the severity of symptoms and laboratory tests, including polymerase chain reaction or IgM antibody testing.19 The other conditions listed do not produce hemorrhagic fever.
THE DIAGNOSIS: Dengue Hemorrhagic Fever
The retiform purpura observed in our patient was suggestive of a vasculitic, thrombotic, or embolic etiology. Dengue IgM serologic testing performed based on her extensive travel history and recent return from a dengue-endemic area was positive, indicating acute infection. A clinical diagnosis of dengue hemorrhagic fever (DHF) was made based on the hemorrhagic appearance of the lesion. Histopathology revealed leukocytoclastic vasculitis (Figure). Anti–double-stranded DNA, antideoxyribonuclease, C3 and C4, CH50 (total hemolytic complement), antineutrophil cytoplasmic antibodies, HIV, and hepatitis B virus tests were normal. Direct immunofluorescence was negative.

Dengue virus is a single-stranded RNA virus transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is one of the most prevalent arthropod-borne viruses affecting humans today.1,2 Infection with the dengue virus generally is seen in travelers visiting tropical regions of Africa, Mexico, South America, South and Central Asia, Southeast Asia, and the Caribbean.1 The Table shows the global distribution of dengue serotypes from 2000 to 2014.3,4 There are 4 serotypes of the dengue virus: DENV-1 to DENV-4. Infection with 1 strain elicits longlasting immunity to that strain, but subsequent infection with another strain can result in severe DHF due to antibody cross-reaction.1

Dengue virus infection ranges from mildly symptomatic to a spectrum of increasingly severe conditions that comprise dengue fever (DF) and DHF, as well as dengue shock syndrome and brain stem hemorrhage, which may be fatal.2,5 Dengue fever manifests as severe myalgia, fever, headache (usually retro-orbital), arthralgia, erythema, and rubelliform exanthema.6 The frequency of skin eruptions in patients with DF varies with the virus strain and outbreaks.7 The lesions initially develop with the onset of fever and manifest as flushing or erythematous mottling of the face, neck, and chest areas.1,7 The morbilliform eruption develops 2 to 6 days after the onset of the fever, beginning on the trunk and spreading to the face and extremities.1,7 The rash may become confluent with characteristic sparing of small round areas of normal skin described as white islands in a sea of red.2 Verrucous papules on the ears also have been described and may resemble those seen in Cowden syndrome. In patients with prior infection with a different strain of the virus, hemorrhagic lesions may develop, including characteristic retiform purpura, a positive tourniquet test, and the appearance of petechiae on the lower legs. Pruritus and desquamation, especially on the palms and soles, may follow the termination of the eruption.7
The differential diagnosis of DF includes measles, rubella, enteroviruses, and influenza. Chikungunya and West Nile viruses in Asia and Africa and the O’nyong-nyong virus in Africa are also arboviruses that cause a clinical picture similar to DF but not DHF. Other diagnostic considerations include phases of scarlet fever, typhoid, malaria, leptospirosis, hepatitis A, and trypanosomal and rickettsial diseases.7 The differential diagnosis of DHF includes antineutrophil cytoplasmic antibody–associated vasculitis, rheumatoid vasculitis, and bacterial septic vasculitis.
Acute clinical diagnosis of DF can be challenging because of the nonspecific symptoms that can be seen in almost every infectious disease. Clinical presentation assessment should be confirmed with laboratory testing.6 Dengue virus infection usually is confirmed by the identification of viral genomic RNA, antigens, or the antibodies it elicits. Enzyme-linked immunosorbent assay–based serologic tests are cost-effective and easy to perform.5 IgM antibodies usually show cross-reactivity with platelets, but the antibody levels are not positively correlated with the severity of DF.8 Primary infection with the dengue virus is characterized by the elevation of specific IgM levels that usually occurs 3 to 5 days after symptom onset and persists during the postfebrile stage (up to 30 to 60 days). In secondary infections, the IgM levels usually rise more slowly and reach a lower level than in primary infections.9 For both primary and secondary infections, testing IgM levels after the febrile stage may be helpful with the laboratory diagnosis.
Currently, there is no antiviral drug available for dengue. Treatment of dengue infection is symptomatic and supportive.2
Dengue hemorrhagic fever is indicated by a rising hematocrit (≥20%) and a falling platelet count (>100,000/mm3) accompanying clinical signs of hemorrhage. Treatment includes intravenous fluid replacement and careful clinical monitoring of hematocrit levels, platelet count, vitals, urine output, and other signs of shock.5 For patients with a history of dengue infection, travel to areas with other serotypes is not recommended.
If any travel to a high-risk area is planned, countryspecific travel recommendations and warnings should be reviewed from the Centers for Disease Control and Prevention’s website (https://wwwnc.cdc.gov/travel/notices/level1/dengue-global). Use of an Environmental Protection Agency–registered insect repellent to avoid mosquito bites and acetaminophen for managing symptoms is advised. During travel, staying in places with window and door screens and using a bed net during sleep are suggested. Long-sleeved shirts and long pants also are preferred. Travelers should see a health care provider if they have symptoms of dengue.10
African tick bite fever (ATBF) is caused by Rickettsia africae transmitted by Amblyomma ticks. Skin findings in ATBF include erythematous, firm, tender papules with central eschars consistent with the feeding patterns of ticks.11 Histopathology of ATBF usually includes fibrinoid necrosis of vessels in the dermis with a perivascular inflammatory infiltrate and coagulation necrosis of the surrounding dermis consistent with eschar formation.12 The lack of an eschar weighs against this diagnosis.
African trypanosomiasis (also known as sleeping sickness) is caused by protozoa transmitted by the tsetse fly. A chancrelike, circumscribed, rubbery, indurated red or violaceous nodule measuring 2 to 5 cm in diameter often develops as the earliest cutaneous sign of the disease.13 Nonspecific histopathologic findings, such as infiltration of lymphocytes and macrophages and proliferation of endothelial cells and fibroblasts, may be observed.14 Extravascular parasites have been noted in skin biopsies.15 In later stages, skin lesions called trypanids may be observed as macular, papular, annular, targetoid, purpuric, and erythematous lesions, and histopathologic findings consistent with vasculitis also may be seen.13
Chikungunya virus infection is an acute-onset, mosquito-borne viral disease. Skin manifestations may start with nonspecific, generalized, morbilliform, maculopapular rashes coinciding with fever, which also may be seen initially with DHF. Skin hyperpigmentation, mostly centrofacial and involving the nose (chik sign); purpuric and ecchymotic lesions over the trunk and flexors of limbs in adults, often surmounted by subepidermal bullae and lesions resembling toxic epidermal necrolysis; and nonhealing ulcers in the genital and groin areas are common skin manifestations of chikungunya infection.16 Intraepithelial splitting with acantholysis and perivascular lymphohistiocytic infiltration may be observed in the histopathology of blistering lesions, which are not consistent with DHF.17
Zika virus infection is caused by an arbovirus within the Flaviviridae family, which also includes the dengue virus. Initial mucocutaneous findings of the Zika virus include nonspecific diffuse maculopapular eruptions. The eruption generally spares the palms and soles; however, various manifestations including involvement of the palms and soles have been reported.18 The morbilliform eruption begins on the face and extends to the trunk and extremities. Mild hemorrhagic manifestations, including petechiae and bleeding gums, may be observed. Distinguishing between dengue and Zika virus infection relies on the severity of symptoms and laboratory tests, including polymerase chain reaction or IgM antibody testing.19 The other conditions listed do not produce hemorrhagic fever.
- Pincus LB, Grossman ME, Fox LP. The exanthem of dengue fever: clinical features of two US tourists traveling abroad. J Am Acad Dermatol. 2008;58:308-316. doi:10.1016/j.jaad.2007.08.042
- Radakovic-Fijan S, Graninger W, Müller C, et al. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430-433. doi:10.1067/mjd.2002.111904
- Yamashita A, Sakamoto T, Sekizuka T, et al. DGV: dengue genographic viewer. Front Microbiol. 2016;7:875. doi:10.3389/fmicb.2016.00875
- Centers for Disease and Prevention. Dengue in the US states and territories. Updated October 7, 2020. Accessed September 30, 2024. https://www.cdc.gov/dengue/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/dengue/areaswithrisk/in-the-us.html
- Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. doi:10.1155/2016/6803098
- Muller DA, Depelsenaire AC, Young PR. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis. 2017;215(suppl 2):S89-S95. doi:10.1093/infdis/jiw649
- Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117-122. doi:10.1016/0738-081x(89)90034-5
- Lin CF, Lei HY, Liu CC, et al. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143-149. doi:10.1002/1096- 9071(20000201)63:2<143::AID-JMV1009>3.0.CO;2-L
- Tripathi NK, Shrivastava A, Dash PK, et al. Detection of dengue virus. Methods Mol Biol. 2011;665:51-64. doi:10.1007/978-1-60761-817-1_4
- Centers for Disease Control and Prevention. Plan for travel. Accessed September 30, 2024. https://wwwnc.cdc.gov/travel
- Mack I, Ritz N. African tick-bite fever. N Engl J Med. 2019;380:960. doi:10.1056/NEJMicm1810093
- Lepidi H, Fournier PE, Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12:1332- 1337. doi:10.3201/eid1209.051540
- McGovern TW, Williams W, Fitzpatrick JE, et al. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol. 1995;131:1178-1182.
- Kristensson K, Bentivoglio M. Pathology of African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, eds. Progress in Human African Trypanosomiasis, Sleeping Sickness. Springer; 1999:157-181.
- Capewell P, Cren-Travaillé C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi:10.7554/eLife.17716
- Singal A. Chikungunya and skin: current perspective. Indian Dermatol Online J. 2017;8:307-309. doi:10.4103/idoj.IDOJ_93_17
- Robin S, Ramful D, Zettor J, et al. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2009;169:67-72. doi:10.1007/s00431-009-0986-0
- Hussain A, Ali F, Latiwesh OB, et al. A comprehensive review of the manifestations and pathogenesis of Zika virus in neonates and adults. Cureus. 2018;10:E3290. doi:10.7759/cureus.3290
- Farahnik B, Beroukhim K, Blattner CM, et al. Cutaneous manifestations of the Zika virus. J Am Acad Dermatol. 2016;74:1286-1287. doi:10.1016/j.jaad.2016.02.1232
- Pincus LB, Grossman ME, Fox LP. The exanthem of dengue fever: clinical features of two US tourists traveling abroad. J Am Acad Dermatol. 2008;58:308-316. doi:10.1016/j.jaad.2007.08.042
- Radakovic-Fijan S, Graninger W, Müller C, et al. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430-433. doi:10.1067/mjd.2002.111904
- Yamashita A, Sakamoto T, Sekizuka T, et al. DGV: dengue genographic viewer. Front Microbiol. 2016;7:875. doi:10.3389/fmicb.2016.00875
- Centers for Disease and Prevention. Dengue in the US states and territories. Updated October 7, 2020. Accessed September 30, 2024. https://www.cdc.gov/dengue/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/dengue/areaswithrisk/in-the-us.html
- Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. doi:10.1155/2016/6803098
- Muller DA, Depelsenaire AC, Young PR. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis. 2017;215(suppl 2):S89-S95. doi:10.1093/infdis/jiw649
- Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117-122. doi:10.1016/0738-081x(89)90034-5
- Lin CF, Lei HY, Liu CC, et al. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143-149. doi:10.1002/1096- 9071(20000201)63:2<143::AID-JMV1009>3.0.CO;2-L
- Tripathi NK, Shrivastava A, Dash PK, et al. Detection of dengue virus. Methods Mol Biol. 2011;665:51-64. doi:10.1007/978-1-60761-817-1_4
- Centers for Disease Control and Prevention. Plan for travel. Accessed September 30, 2024. https://wwwnc.cdc.gov/travel
- Mack I, Ritz N. African tick-bite fever. N Engl J Med. 2019;380:960. doi:10.1056/NEJMicm1810093
- Lepidi H, Fournier PE, Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12:1332- 1337. doi:10.3201/eid1209.051540
- McGovern TW, Williams W, Fitzpatrick JE, et al. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol. 1995;131:1178-1182.
- Kristensson K, Bentivoglio M. Pathology of African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, eds. Progress in Human African Trypanosomiasis, Sleeping Sickness. Springer; 1999:157-181.
- Capewell P, Cren-Travaillé C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi:10.7554/eLife.17716
- Singal A. Chikungunya and skin: current perspective. Indian Dermatol Online J. 2017;8:307-309. doi:10.4103/idoj.IDOJ_93_17
- Robin S, Ramful D, Zettor J, et al. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2009;169:67-72. doi:10.1007/s00431-009-0986-0
- Hussain A, Ali F, Latiwesh OB, et al. A comprehensive review of the manifestations and pathogenesis of Zika virus in neonates and adults. Cureus. 2018;10:E3290. doi:10.7759/cureus.3290
- Farahnik B, Beroukhim K, Blattner CM, et al. Cutaneous manifestations of the Zika virus. J Am Acad Dermatol. 2016;74:1286-1287. doi:10.1016/j.jaad.2016.02.1232
A 74-year-old woman who frequently traveled abroad presented to the dermatology department with retiform purpura of the lower leg along with gastrointestinal cramps, fatigue, and myalgia. The patient reported that the symptoms had started 10 days after returning from a recent trip to Africa.
Inspection of Deep Tumor Margins for Accurate Cutaneous Squamous Cell Carcinoma Staging
To the Editor:
Histopathologic analysis of debulk specimens in Mohs micrographic surgery (MMS) may augment identification of high-risk factors in cutaneous squamous cell carcinoma (cSCC), which may warrant tumor upstaging.1 Intratumor location has not been studied when looking at these high-risk factors. Herein, we report 4 cSCCs initially categorized as well differentiated that were reclassified as moderate to poorly differentiated on analysis of debulk specimens obtained via shave removal.
An 80-year-old man (patient 1) presented with a tender 2-cm erythematous plaque with dried hemorrhagic crusting on the frontal scalp. He had a history of nonmelanoma skin cancers. A biopsy revealed a well-differentiated cSCC, which was upgraded from a T2a tumor to T2b during MMS due to galea involvement. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells at the deep margin (Figure 1A). Given T2b staging, baseline imaging and radiation therapy were recommended.
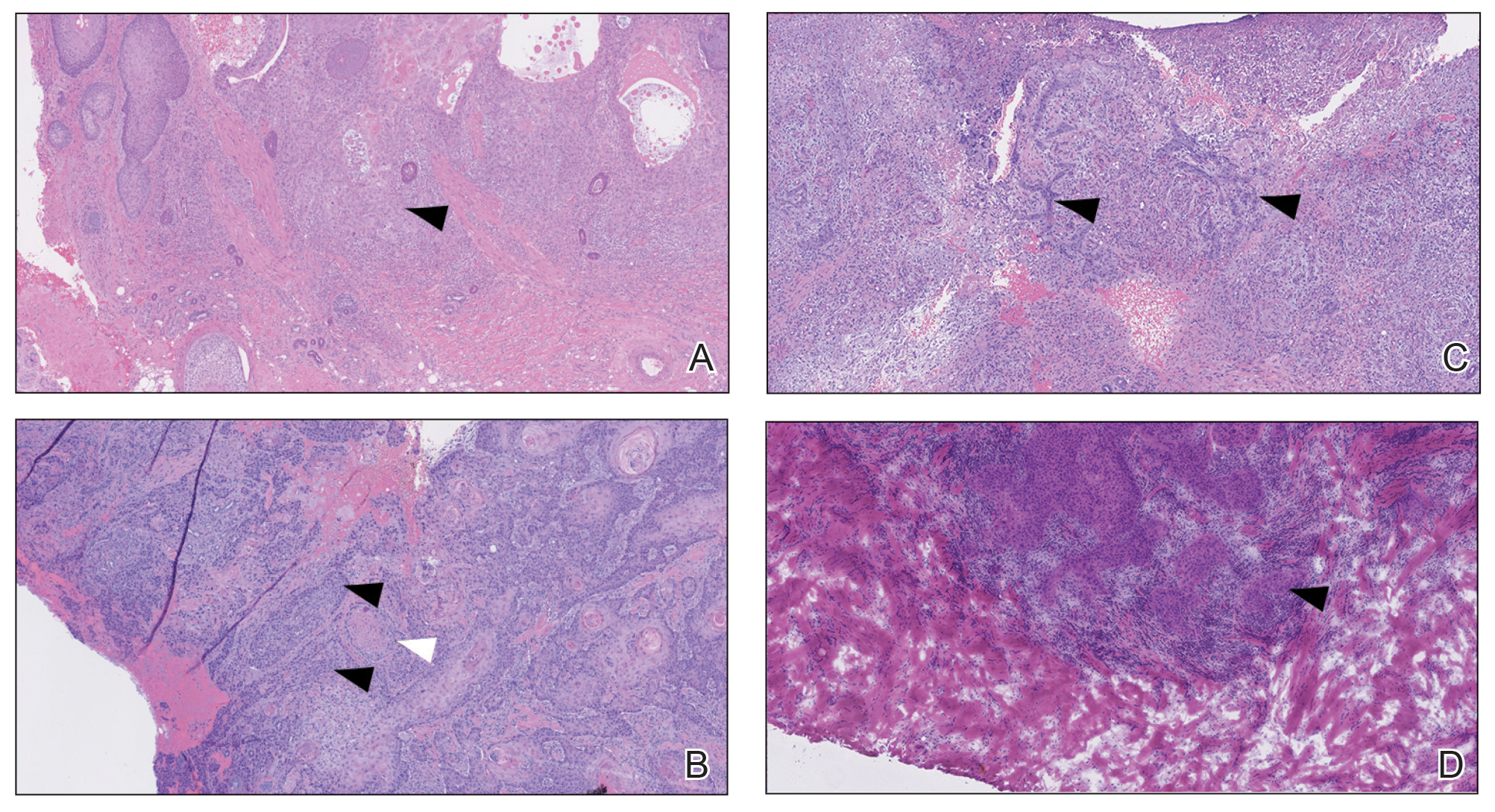
A 75-year-old man (patient 2) presented with a 2-cm erythematous plaque on the left vertex scalp with hemorrhagic crusting, yellow scale, and purulent drainage. He had a history of cSCCs. A biopsy revealed well-differentiated invasive cSCC, which was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Examination of the second Mohs stage revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, infiltration beyond the subcutaneous fat, and perineural invasion (Figure 1B). Given T2b staging, baseline imaging and radiation therapy were recommended.
An 86-year-old woman (patient 3) presented with a tender 2.4-cm plum-colored nodule on the right lower leg. She had a history of basal cell carcinoma. A biopsy revealed a well-differentiated invasive cSCC staged at T2a. Debulk analysis revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, though the staging remained the same (Figure 1C).
An 82-year-old man (patient 4) presented with a 2.7-cm ulcerated nodule with adjacent scaling on the vertex scalp. He had no history of skin cancer. A biopsy revealed a well-differentiated cSCC (Figure 2) that was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells with single-cell extension at the deep margin in the galea (Figure 1D). Given T2b staging, baseline imaging and radiation therapy were recommended.
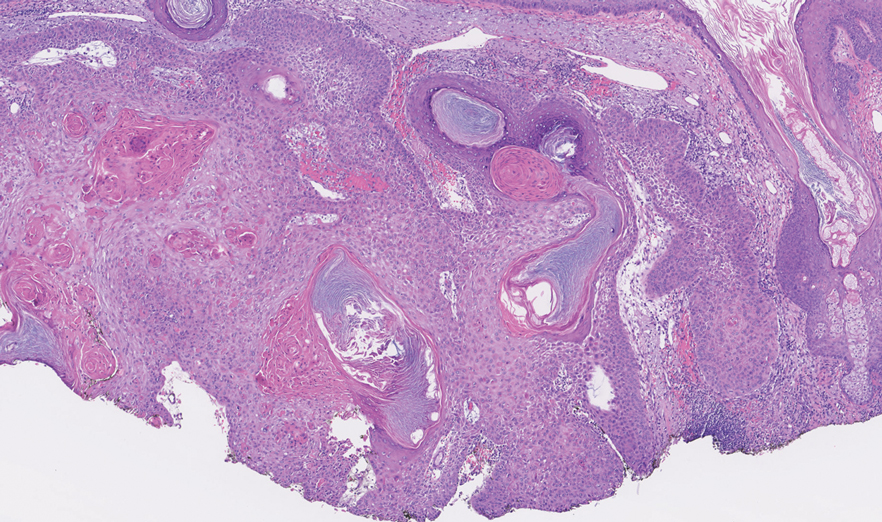
Tumor differentiation is a factor included in the Brigham and Women’s Hospital staging system, and intratumor variability can be clinically relevant for tumor staging.1 Specifically, cSCCs may exhibit intratumor heterogeneity in which predominantly well-differentiated tumors contain focal areas of poorer differentiation.2 This intratumor heterogeneity complicates estimation of tumor risk, as a well-differentiated tumor on biopsy may exhibit poor differentiation at a deeper margin. Our cases highlight that the cells at the deeper margin indeed can show poorer differentiation or other higher-risk tumor features. Thus, the most clinically relevant cells for tumor staging and prognostication may not be visible on initial biopsy, underscoring the utility of close examination of the deep layer of the debulk specimen and Mohs layer for comprehensive staging.
Genetic studies have attempted to identify gene expression patterns in cSCCs that predispose to invasion.3 Three of the top 6 genes in this “invasion signature gene set” were matrix metalloproteases; additionally, IL-24 messenger RNA was upregulated in both the cSCC invasion front and in situ cSCCs. IL-24 has been shown to upregulate the expression of matrix metalloprotease 7 in vitro, suggesting that it may influence tumor progression.3 Although gene expression was not included in this series, the identification of genetic variability in the most poorly differentiated cells residing in the deep margins is of great interest and may reveal mutations contributing to irregular cell morphology and cSCC invasiveness.
Prior studies have indicated that a proportion of cSCCs are histopathologically upgraded from the initial biopsy during MMS due to evidence of perineural invasion, bony invasion, or lesser differentiation noted during MMS stages or debulk analysis.1,4 However, the majority of Mohs surgeons report immediately discarding debulk specimens without further evaluation.5 Herein, we highlight 4 cSCC cases in which the deep margins of the debulk specimen contained the most dedifferentiated cells. Our findings emphasize the importance of thoroughly examining deep tumor margins for complete staging yet also highlight that identifying cells at these margins may not change patient management when high-risk criteria are already met.
- McIlwee BE, Abidi NY, Ravi M, et al. Utility of debulk specimens during Mohs micrographic surgery for cutaneous squamous cell carcinoma. Dermatol Surg. 2021;47:599-604.
- Ramón y Cajal S, Sesé M, Capdevila C, et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med. 2020;98:161-177.
- Mitsui H, Suárez-Fariñas M, Gulati N, et al. Gene expression profiling of the leading edge of cutaneous squamous cell carcinoma: IL-24-driven MMP-7. J Invest Dermatol. 2014;134:1418-1427.
- Chung E, Hoang S, McEvoy AM, et al. Histopathologic upgrading of cutaneous squamous cell carcinomas during Mohs micrographic surgery: a retrospective cohort study. J Am Acad Dermatol. 2021;85:923-930.
- Alniemi DT, Swanson AM, Lasarev M, et al. Tumor debulking trends for keratinocyte carcinomas among Mohs surgeons. Dermatol Surg. 2021;47:1660-1661.
To the Editor:
Histopathologic analysis of debulk specimens in Mohs micrographic surgery (MMS) may augment identification of high-risk factors in cutaneous squamous cell carcinoma (cSCC), which may warrant tumor upstaging.1 Intratumor location has not been studied when looking at these high-risk factors. Herein, we report 4 cSCCs initially categorized as well differentiated that were reclassified as moderate to poorly differentiated on analysis of debulk specimens obtained via shave removal.
An 80-year-old man (patient 1) presented with a tender 2-cm erythematous plaque with dried hemorrhagic crusting on the frontal scalp. He had a history of nonmelanoma skin cancers. A biopsy revealed a well-differentiated cSCC, which was upgraded from a T2a tumor to T2b during MMS due to galea involvement. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells at the deep margin (Figure 1A). Given T2b staging, baseline imaging and radiation therapy were recommended.

A 75-year-old man (patient 2) presented with a 2-cm erythematous plaque on the left vertex scalp with hemorrhagic crusting, yellow scale, and purulent drainage. He had a history of cSCCs. A biopsy revealed well-differentiated invasive cSCC, which was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Examination of the second Mohs stage revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, infiltration beyond the subcutaneous fat, and perineural invasion (Figure 1B). Given T2b staging, baseline imaging and radiation therapy were recommended.
An 86-year-old woman (patient 3) presented with a tender 2.4-cm plum-colored nodule on the right lower leg. She had a history of basal cell carcinoma. A biopsy revealed a well-differentiated invasive cSCC staged at T2a. Debulk analysis revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, though the staging remained the same (Figure 1C).
An 82-year-old man (patient 4) presented with a 2.7-cm ulcerated nodule with adjacent scaling on the vertex scalp. He had no history of skin cancer. A biopsy revealed a well-differentiated cSCC (Figure 2) that was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells with single-cell extension at the deep margin in the galea (Figure 1D). Given T2b staging, baseline imaging and radiation therapy were recommended.

Tumor differentiation is a factor included in the Brigham and Women’s Hospital staging system, and intratumor variability can be clinically relevant for tumor staging.1 Specifically, cSCCs may exhibit intratumor heterogeneity in which predominantly well-differentiated tumors contain focal areas of poorer differentiation.2 This intratumor heterogeneity complicates estimation of tumor risk, as a well-differentiated tumor on biopsy may exhibit poor differentiation at a deeper margin. Our cases highlight that the cells at the deeper margin indeed can show poorer differentiation or other higher-risk tumor features. Thus, the most clinically relevant cells for tumor staging and prognostication may not be visible on initial biopsy, underscoring the utility of close examination of the deep layer of the debulk specimen and Mohs layer for comprehensive staging.
Genetic studies have attempted to identify gene expression patterns in cSCCs that predispose to invasion.3 Three of the top 6 genes in this “invasion signature gene set” were matrix metalloproteases; additionally, IL-24 messenger RNA was upregulated in both the cSCC invasion front and in situ cSCCs. IL-24 has been shown to upregulate the expression of matrix metalloprotease 7 in vitro, suggesting that it may influence tumor progression.3 Although gene expression was not included in this series, the identification of genetic variability in the most poorly differentiated cells residing in the deep margins is of great interest and may reveal mutations contributing to irregular cell morphology and cSCC invasiveness.
Prior studies have indicated that a proportion of cSCCs are histopathologically upgraded from the initial biopsy during MMS due to evidence of perineural invasion, bony invasion, or lesser differentiation noted during MMS stages or debulk analysis.1,4 However, the majority of Mohs surgeons report immediately discarding debulk specimens without further evaluation.5 Herein, we highlight 4 cSCC cases in which the deep margins of the debulk specimen contained the most dedifferentiated cells. Our findings emphasize the importance of thoroughly examining deep tumor margins for complete staging yet also highlight that identifying cells at these margins may not change patient management when high-risk criteria are already met.
To the Editor:
Histopathologic analysis of debulk specimens in Mohs micrographic surgery (MMS) may augment identification of high-risk factors in cutaneous squamous cell carcinoma (cSCC), which may warrant tumor upstaging.1 Intratumor location has not been studied when looking at these high-risk factors. Herein, we report 4 cSCCs initially categorized as well differentiated that were reclassified as moderate to poorly differentiated on analysis of debulk specimens obtained via shave removal.
An 80-year-old man (patient 1) presented with a tender 2-cm erythematous plaque with dried hemorrhagic crusting on the frontal scalp. He had a history of nonmelanoma skin cancers. A biopsy revealed a well-differentiated cSCC, which was upgraded from a T2a tumor to T2b during MMS due to galea involvement. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells at the deep margin (Figure 1A). Given T2b staging, baseline imaging and radiation therapy were recommended.

A 75-year-old man (patient 2) presented with a 2-cm erythematous plaque on the left vertex scalp with hemorrhagic crusting, yellow scale, and purulent drainage. He had a history of cSCCs. A biopsy revealed well-differentiated invasive cSCC, which was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Examination of the second Mohs stage revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, infiltration beyond the subcutaneous fat, and perineural invasion (Figure 1B). Given T2b staging, baseline imaging and radiation therapy were recommended.
An 86-year-old woman (patient 3) presented with a tender 2.4-cm plum-colored nodule on the right lower leg. She had a history of basal cell carcinoma. A biopsy revealed a well-differentiated invasive cSCC staged at T2a. Debulk analysis revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, though the staging remained the same (Figure 1C).
An 82-year-old man (patient 4) presented with a 2.7-cm ulcerated nodule with adjacent scaling on the vertex scalp. He had no history of skin cancer. A biopsy revealed a well-differentiated cSCC (Figure 2) that was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells with single-cell extension at the deep margin in the galea (Figure 1D). Given T2b staging, baseline imaging and radiation therapy were recommended.

Tumor differentiation is a factor included in the Brigham and Women’s Hospital staging system, and intratumor variability can be clinically relevant for tumor staging.1 Specifically, cSCCs may exhibit intratumor heterogeneity in which predominantly well-differentiated tumors contain focal areas of poorer differentiation.2 This intratumor heterogeneity complicates estimation of tumor risk, as a well-differentiated tumor on biopsy may exhibit poor differentiation at a deeper margin. Our cases highlight that the cells at the deeper margin indeed can show poorer differentiation or other higher-risk tumor features. Thus, the most clinically relevant cells for tumor staging and prognostication may not be visible on initial biopsy, underscoring the utility of close examination of the deep layer of the debulk specimen and Mohs layer for comprehensive staging.
Genetic studies have attempted to identify gene expression patterns in cSCCs that predispose to invasion.3 Three of the top 6 genes in this “invasion signature gene set” were matrix metalloproteases; additionally, IL-24 messenger RNA was upregulated in both the cSCC invasion front and in situ cSCCs. IL-24 has been shown to upregulate the expression of matrix metalloprotease 7 in vitro, suggesting that it may influence tumor progression.3 Although gene expression was not included in this series, the identification of genetic variability in the most poorly differentiated cells residing in the deep margins is of great interest and may reveal mutations contributing to irregular cell morphology and cSCC invasiveness.
Prior studies have indicated that a proportion of cSCCs are histopathologically upgraded from the initial biopsy during MMS due to evidence of perineural invasion, bony invasion, or lesser differentiation noted during MMS stages or debulk analysis.1,4 However, the majority of Mohs surgeons report immediately discarding debulk specimens without further evaluation.5 Herein, we highlight 4 cSCC cases in which the deep margins of the debulk specimen contained the most dedifferentiated cells. Our findings emphasize the importance of thoroughly examining deep tumor margins for complete staging yet also highlight that identifying cells at these margins may not change patient management when high-risk criteria are already met.
- McIlwee BE, Abidi NY, Ravi M, et al. Utility of debulk specimens during Mohs micrographic surgery for cutaneous squamous cell carcinoma. Dermatol Surg. 2021;47:599-604.
- Ramón y Cajal S, Sesé M, Capdevila C, et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med. 2020;98:161-177.
- Mitsui H, Suárez-Fariñas M, Gulati N, et al. Gene expression profiling of the leading edge of cutaneous squamous cell carcinoma: IL-24-driven MMP-7. J Invest Dermatol. 2014;134:1418-1427.
- Chung E, Hoang S, McEvoy AM, et al. Histopathologic upgrading of cutaneous squamous cell carcinomas during Mohs micrographic surgery: a retrospective cohort study. J Am Acad Dermatol. 2021;85:923-930.
- Alniemi DT, Swanson AM, Lasarev M, et al. Tumor debulking trends for keratinocyte carcinomas among Mohs surgeons. Dermatol Surg. 2021;47:1660-1661.
- McIlwee BE, Abidi NY, Ravi M, et al. Utility of debulk specimens during Mohs micrographic surgery for cutaneous squamous cell carcinoma. Dermatol Surg. 2021;47:599-604.
- Ramón y Cajal S, Sesé M, Capdevila C, et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med. 2020;98:161-177.
- Mitsui H, Suárez-Fariñas M, Gulati N, et al. Gene expression profiling of the leading edge of cutaneous squamous cell carcinoma: IL-24-driven MMP-7. J Invest Dermatol. 2014;134:1418-1427.
- Chung E, Hoang S, McEvoy AM, et al. Histopathologic upgrading of cutaneous squamous cell carcinomas during Mohs micrographic surgery: a retrospective cohort study. J Am Acad Dermatol. 2021;85:923-930.
- Alniemi DT, Swanson AM, Lasarev M, et al. Tumor debulking trends for keratinocyte carcinomas among Mohs surgeons. Dermatol Surg. 2021;47:1660-1661.
Practice Points
- A proportion of cutaneous squamous cell carcinomas are upgraded from the initial biopsy during Mohs micrographic surgery due to evidence of perineural invasion, bony invasion, or lesser differentiation noted on Mohs stages or debulk analysis.
- Thorough inspection of the deep tumor margins may be required for accurate tumor staging and evaluation of metastatic risk. Cells at the deep margin of the tumor may demonstrate poorer differentiation and/or other higher-risk tumor features than those closer to the surface.
- Tumor staging may be incomplete until the deep margins are assessed to find the most dysplastic and likely clinically relevant cells, which may be missed without evaluation of the debulked tumor.
Transient Eruption of Verrucous Keratoses During Encorafenib Therapy: Adverse Event or Paraneoplastic Phenomenon?
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1 BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.
Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1 BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.


Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1 BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.


Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
Practice Points
- Verrucous keratoses are common cutaneous adverse events (AEs) associated with BRAF inhibitor therapy.
- Verrucous papules may be a paraneoplastic phenomenon and can be differentiated from a treatment-related AE based on the timing and progression in relation to tumor burden.
- Although treatment of particularly bothersome lesions with cryotherapy may be warranted, verrucous papules secondary to BRAF inhibitor therapy may resolve spontaneously.
Rare Case of Photodistributed Hyperpigmentation Linked to Kratom Consumption
To the Editor:
Kratom (Mitragyna speciosa) is an evergreen tree native to Southeast Asia.1 Its leaves contain psychoactive compounds including mitragynine and 7-hydroxymitragynine, which exert dose-dependent effects on the central nervous system through opioid and monoaminergic receptors.2,3 At low doses (1–5 g), kratom elicits mild stimulant effects such as increased sociability, alertness, and talkativeness. At high doses (5–15 g), kratom has depressant effects that can provide relief from pain and opioid-withdrawal symptoms.3
Traditionally, kratom has been used in Southeast Asia for recreational and ceremonial purposes, to ease opioid-withdrawal symptoms, and to reduce fatigue from manual labor.4 In the 21st century, availability of kratom expanded to Europe, Australia, and the United States, largely facilitated by widespread dissemination of deceitful marketing and unregulated sales on the internet.1 Although large-scale epidemiologic studies evaluating kratom’s prevalence are scarce, available evidence indicates rising worldwide usage, with a notable increase in kratom-related poison center calls between 2011 and 2017 in the United States.5 In July 2023, kratom made headlines due to the death of a woman in Florida following use of the substance.6
A cross-sectional study revealed that in the United States, kratom typically is used by White individuals for self-treatment of anxiety, depression, pain, and opioid withdrawal.7 However, the potential for severe adverse effects and dependence on kratom can outweigh the benefits.6,8 Reported adverse effects of kratom include tachycardia, hypercholesteremia, liver injury, hallucinations, respiratory depression, seizure, coma, and death.9,10 We present a case of kratom-induced photodistributed hyperpigmentation.
A 63-year-old man presented to the dermatology clinic with diffuse tender, pruritic, hyperpigmented skin lesions that developed over the course of 1 year. The lesions were distributed on sun-exposed areas, including the face, neck, and forearms (Figure 1). The patient reported no other major symptoms, and his health was otherwise unremarkable. He had a medical history of psoriasiform and spongiotic dermatitis consistent with eczema, psoriasis, hypercholesteremia, and hyperlipidemia. The patient was not taking any medications at the time of presentation. He had a family history of plaque psoriasis in his father. Five years prior to the current presentation, the patient was treated with adalimumab for steroid-resistant psoriasis; however, despite initial improvement, he experienced recurrence of scaly erythematous plaques and had discontinued adalimumab the year prior to presentation.
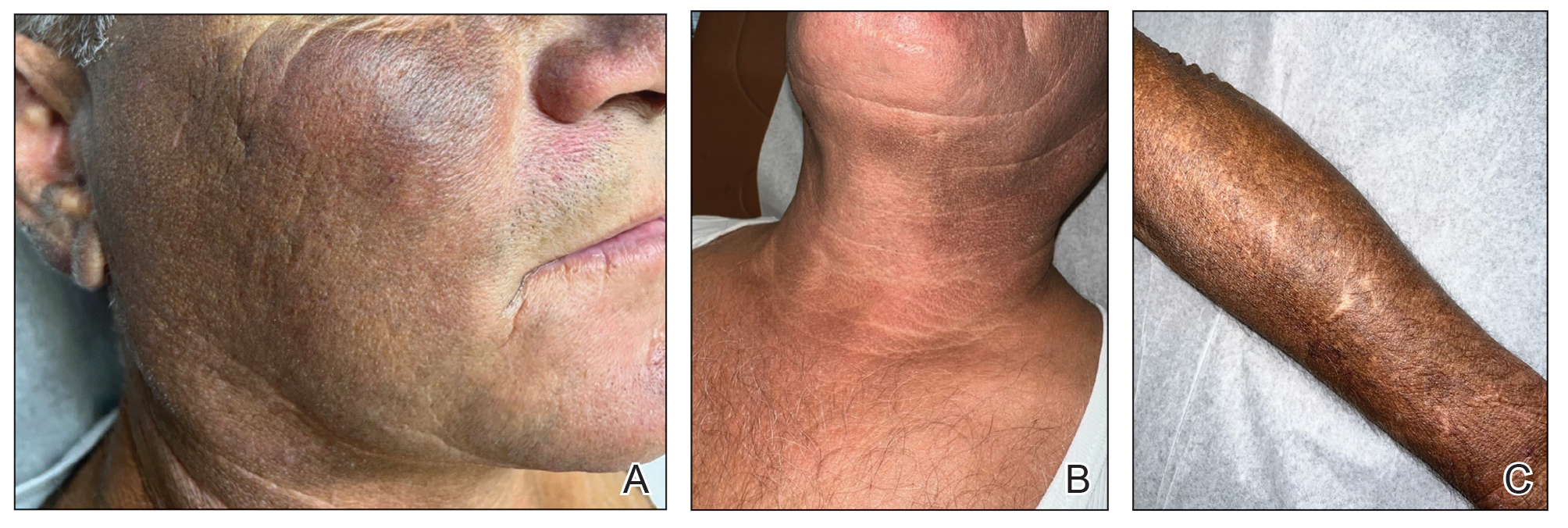
When adalimumab was discontinued, the patient sought alternative treatment for the skin symptoms and began self-administering kratom in an attempt to alleviate associated physical discomfort. He ingested approximately 3 bottles of liquid kratom per day, with each bottle containing 180 mg of mitragynine and less than 8 mg of 7-hydroxymitragynine. Although not scientifically proven, kratom has been colloquially advertised to improve psoriasis.11 The patient reported no other medication use or allergies.
Shave biopsies of hyperpigmented lesions on the right side of the neck, ear, and forearm were performed. Histopathology revealed a sparse superficial, perivascular, lymphocytic infiltrate accompanied by a prominent number of melanophages in the superficial dermis (Figure 2). Special stains further confirmed that the pigment was melanin; the specimens stained positive with Fontana-Masson stain (Figure 3) and negative with an iron stain (Figure 4).
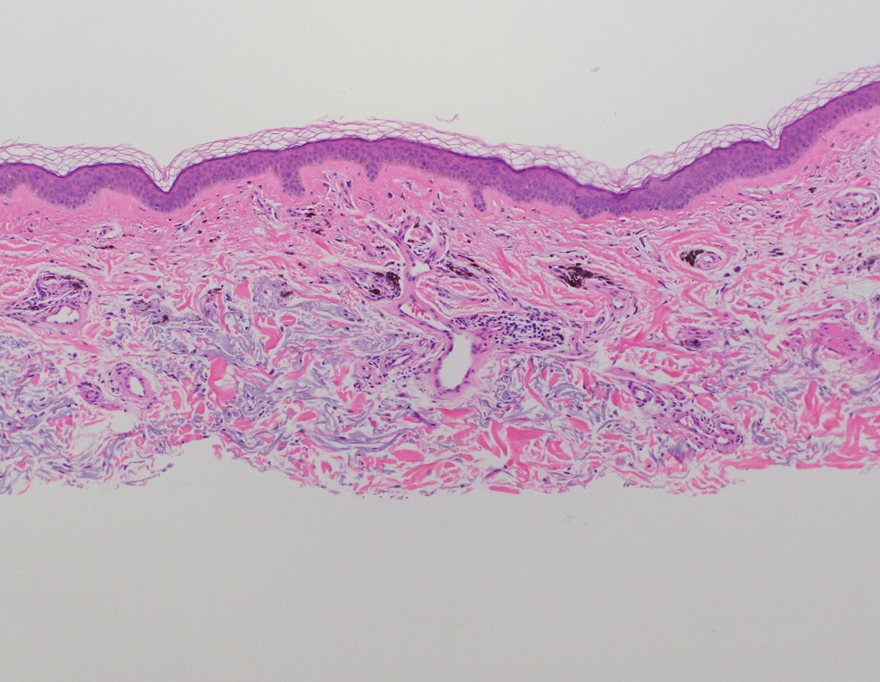
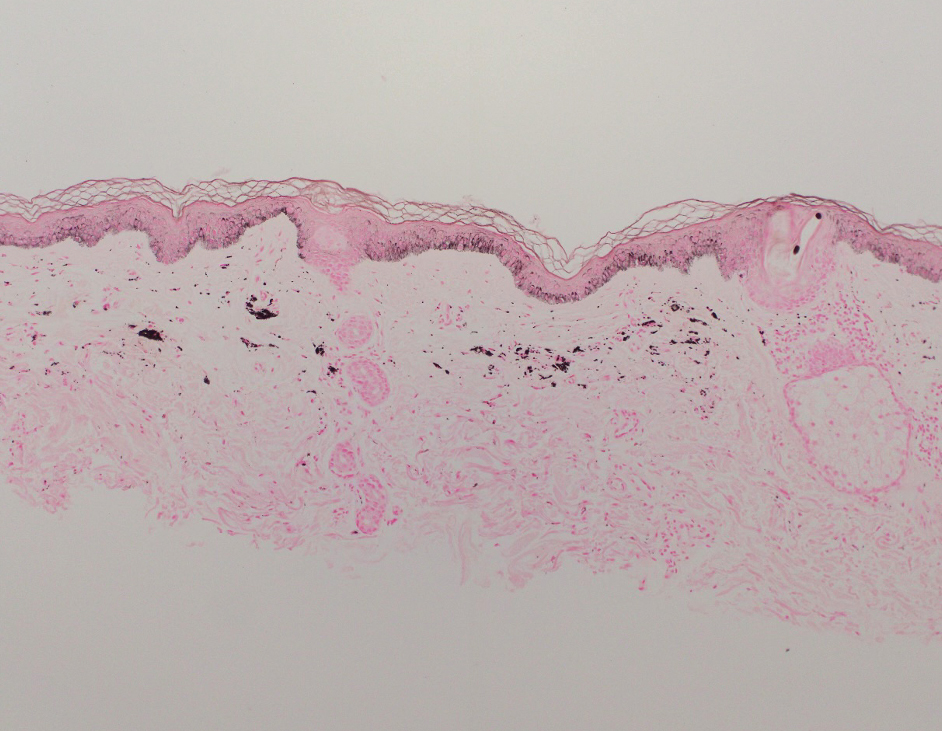
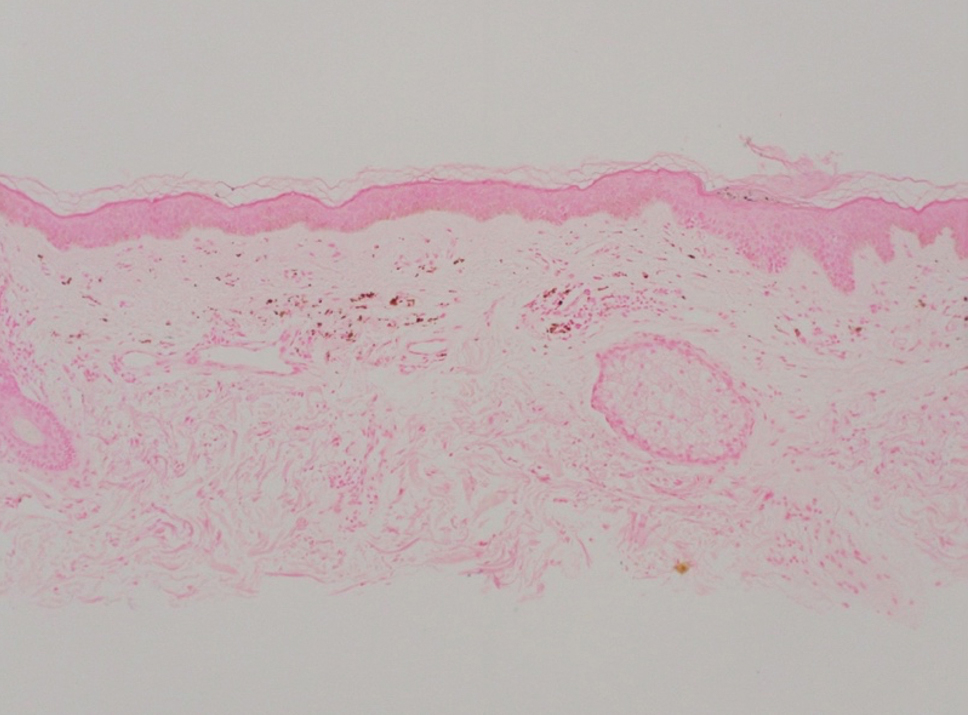
Adalimumab-induced hyperpigmentation was considered. A prior case of adalimumab-induced hyperpigmentation manifested on the face. Histopathology was consistent with a superficial, perivascular, lymphocytic infiltrate with melanophages in the dermis; however, hyperpigmentation was absent in the periorbital area, and affected areas faded 4 months after discontinuation of adalimumab.12 Our patient presented with hyperpigmentation 1 year after adalimumab cessation, and the hyperpigmented areas included the periorbital region. Because of the distinct temporal and clinical features, adalimumab-induced hyperpigmentation was eliminated from the differential diagnosis.
Based on the photodistributed pattern of hyperpigmentation, histopathology, and the temporal relationship between hyperpigmentation onset and kratom usage, a diagnosis of kratom-induced photodistributed hyperpigmentation was made. The patient was advised to discontinue kratom use and use sun protection to prevent further photodamage. The patient subsequently was lost to follow-up.
Kratom alkaloids bind all 3 opioid receptors—μOP, δOP, and κOPs—in a G-protein–biased manner with 7-hydroxymitragynine, the most pharmacologically active alkaloid, exhibiting a higher affinity for μ-opioid receptors.13,14 In human epidermal melanocytes, binding between μ-opioid receptors and β-endorphin, an endogenous opioid, is associated with increased melanin production. This melanogenesis has been linked to hyperpigmentation.15 Given the similarity between kratom alkaloids and β-endorphin in opioid-receptor binding, it is possible that kratom-induced hyperpigmentation may occur through a similar mechanism involving μ-opioid receptors and melanogenesis in epidermal melanocytes. Moreover, some researchers have theorized that sun exposure may result in free radical formation of certain drugs or their metabolites. These free radicals then can interact with cellular DNA, triggering the release of pigmentary mediators and resulting in hyperpigmentation.16 This theory may explain the photodistributed pattern of kratom-induced hyperpigmentation. Further studies are needed to understand the mechanism behind this adverse reaction and its implications for patient treatment.
Literature on kratom-induced hyperpigmentation is limited. Powell et al17 reported a similar case of kratom-induced photodistributed hyperpigmentation—a White man had taken kratom to reduce opioid use and subsequently developed hyperpigmented patches on the arms and face. Moreover, anonymous Reddit users have shared anecdotal reports of hyperpigmentation following kratom use.18
Physicians should be aware of hyperpigmentation as a potential adverse reaction of kratom use as its prevalence increases globally. Further research is warranted to elucidate the mechanism behind this adverse reaction and identify risk factors.
- Prozialeck WC, Avery BA, Boyer EW, et al. Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70-77. doi:10.1016/j.drugpo.2019.05.003
- Bergen-Cico D, MacClurg K. Kratom (Mitragyna speciosa) use, addiction potential, and legal status. In: Preedy VR, ed. Neuropathology of Drug Addictions and Substance Misuse. 2016:903-911. doi:10.1016/B978-0-12-800634-4.00089-5
- Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130:127-138. doi:10.1007/s00414-015-1279-y
- Transnational Institute. Kratom in Thailand: decriminalisation and community control? May 3, 2011. Accessed August 23, 2024. https://www.tni.org/en/publication/kratom-in-thailand-decriminalisation-and-community-control
- Eastlack SC, Cornett EM, Kaye AD. Kratom—pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9:55-69. doi:10.1007/s40122-020-00151-x
- Reyes R. Family of Florida mom who died from herbal substance kratom wins $11M suit. New York Post. July 30, 2023. Updated July 31, 2023. Accessed August 23, 2024. https://nypost.com/2023/07/30/family-of-florida-mom-who-died-from-herbal-substance-kratom-wins-11m-suit/
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. doi:10.1016/j.drugalcdep.2020.107849
- Mayo Clinic. Kratom: unsafe and ineffective. Accessed August 23, 2024. https://www.mayoclinic.org/healthy-lifestyle/consumer-health/in-depth/kratom/art-20402171
- Sethi R, Hoang N, Ravishankar DA, et al. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22:19nr02507.
- Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:775-777. doi:10.1002/phar.2280
- Qrius. 6 benefits of kratom you should know for healthy skin. March 21, 2023. Accessed August 23, 2024. https://qrius.com/6-benefits-of-kratom-you-should-know-for-healthy-skin/
- Blomberg M, Zachariae COC, Grønhøj F. Hyperpigmentation of the face following adalimumab treatment. Acta Derm Venereol. 2009;89:546-547. doi:10.2340/00015555-0697
- Matsumoto K, Hatori Y, Murayama T, et al. Involvement of μ-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549:63-70. doi:10.1016/j.ejphar.2006.08.013
- Jentsch MJ, Pippin MM. Kratom. In: StatPearls. StatPearls Publishing; 2023.
- Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, et al. Opioids and the skin—where do we stand? Exp Dermatol. 2009;18:424-430.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10. doi:10.5070/D33c97j4z5
- Powell LR, Ryser TJ, Morey GE, et al. Kratom as a novel cause of photodistributed hyperpigmentation. JAAD Case Rep. 2022;28:145-148. doi:10.1016/j.jdcr.2022.07.033
- Haccoon. Skin discoloring? Reddit. June 30, 2019. Accessed August 23, 2024. https://www.reddit.com/r/quittingkratom/comments/c7b1cm/skin_discoloring/
To the Editor:
Kratom (Mitragyna speciosa) is an evergreen tree native to Southeast Asia.1 Its leaves contain psychoactive compounds including mitragynine and 7-hydroxymitragynine, which exert dose-dependent effects on the central nervous system through opioid and monoaminergic receptors.2,3 At low doses (1–5 g), kratom elicits mild stimulant effects such as increased sociability, alertness, and talkativeness. At high doses (5–15 g), kratom has depressant effects that can provide relief from pain and opioid-withdrawal symptoms.3
Traditionally, kratom has been used in Southeast Asia for recreational and ceremonial purposes, to ease opioid-withdrawal symptoms, and to reduce fatigue from manual labor.4 In the 21st century, availability of kratom expanded to Europe, Australia, and the United States, largely facilitated by widespread dissemination of deceitful marketing and unregulated sales on the internet.1 Although large-scale epidemiologic studies evaluating kratom’s prevalence are scarce, available evidence indicates rising worldwide usage, with a notable increase in kratom-related poison center calls between 2011 and 2017 in the United States.5 In July 2023, kratom made headlines due to the death of a woman in Florida following use of the substance.6
A cross-sectional study revealed that in the United States, kratom typically is used by White individuals for self-treatment of anxiety, depression, pain, and opioid withdrawal.7 However, the potential for severe adverse effects and dependence on kratom can outweigh the benefits.6,8 Reported adverse effects of kratom include tachycardia, hypercholesteremia, liver injury, hallucinations, respiratory depression, seizure, coma, and death.9,10 We present a case of kratom-induced photodistributed hyperpigmentation.
A 63-year-old man presented to the dermatology clinic with diffuse tender, pruritic, hyperpigmented skin lesions that developed over the course of 1 year. The lesions were distributed on sun-exposed areas, including the face, neck, and forearms (Figure 1). The patient reported no other major symptoms, and his health was otherwise unremarkable. He had a medical history of psoriasiform and spongiotic dermatitis consistent with eczema, psoriasis, hypercholesteremia, and hyperlipidemia. The patient was not taking any medications at the time of presentation. He had a family history of plaque psoriasis in his father. Five years prior to the current presentation, the patient was treated with adalimumab for steroid-resistant psoriasis; however, despite initial improvement, he experienced recurrence of scaly erythematous plaques and had discontinued adalimumab the year prior to presentation.

When adalimumab was discontinued, the patient sought alternative treatment for the skin symptoms and began self-administering kratom in an attempt to alleviate associated physical discomfort. He ingested approximately 3 bottles of liquid kratom per day, with each bottle containing 180 mg of mitragynine and less than 8 mg of 7-hydroxymitragynine. Although not scientifically proven, kratom has been colloquially advertised to improve psoriasis.11 The patient reported no other medication use or allergies.
Shave biopsies of hyperpigmented lesions on the right side of the neck, ear, and forearm were performed. Histopathology revealed a sparse superficial, perivascular, lymphocytic infiltrate accompanied by a prominent number of melanophages in the superficial dermis (Figure 2). Special stains further confirmed that the pigment was melanin; the specimens stained positive with Fontana-Masson stain (Figure 3) and negative with an iron stain (Figure 4).



Adalimumab-induced hyperpigmentation was considered. A prior case of adalimumab-induced hyperpigmentation manifested on the face. Histopathology was consistent with a superficial, perivascular, lymphocytic infiltrate with melanophages in the dermis; however, hyperpigmentation was absent in the periorbital area, and affected areas faded 4 months after discontinuation of adalimumab.12 Our patient presented with hyperpigmentation 1 year after adalimumab cessation, and the hyperpigmented areas included the periorbital region. Because of the distinct temporal and clinical features, adalimumab-induced hyperpigmentation was eliminated from the differential diagnosis.
Based on the photodistributed pattern of hyperpigmentation, histopathology, and the temporal relationship between hyperpigmentation onset and kratom usage, a diagnosis of kratom-induced photodistributed hyperpigmentation was made. The patient was advised to discontinue kratom use and use sun protection to prevent further photodamage. The patient subsequently was lost to follow-up.
Kratom alkaloids bind all 3 opioid receptors—μOP, δOP, and κOPs—in a G-protein–biased manner with 7-hydroxymitragynine, the most pharmacologically active alkaloid, exhibiting a higher affinity for μ-opioid receptors.13,14 In human epidermal melanocytes, binding between μ-opioid receptors and β-endorphin, an endogenous opioid, is associated with increased melanin production. This melanogenesis has been linked to hyperpigmentation.15 Given the similarity between kratom alkaloids and β-endorphin in opioid-receptor binding, it is possible that kratom-induced hyperpigmentation may occur through a similar mechanism involving μ-opioid receptors and melanogenesis in epidermal melanocytes. Moreover, some researchers have theorized that sun exposure may result in free radical formation of certain drugs or their metabolites. These free radicals then can interact with cellular DNA, triggering the release of pigmentary mediators and resulting in hyperpigmentation.16 This theory may explain the photodistributed pattern of kratom-induced hyperpigmentation. Further studies are needed to understand the mechanism behind this adverse reaction and its implications for patient treatment.
Literature on kratom-induced hyperpigmentation is limited. Powell et al17 reported a similar case of kratom-induced photodistributed hyperpigmentation—a White man had taken kratom to reduce opioid use and subsequently developed hyperpigmented patches on the arms and face. Moreover, anonymous Reddit users have shared anecdotal reports of hyperpigmentation following kratom use.18
Physicians should be aware of hyperpigmentation as a potential adverse reaction of kratom use as its prevalence increases globally. Further research is warranted to elucidate the mechanism behind this adverse reaction and identify risk factors.
To the Editor:
Kratom (Mitragyna speciosa) is an evergreen tree native to Southeast Asia.1 Its leaves contain psychoactive compounds including mitragynine and 7-hydroxymitragynine, which exert dose-dependent effects on the central nervous system through opioid and monoaminergic receptors.2,3 At low doses (1–5 g), kratom elicits mild stimulant effects such as increased sociability, alertness, and talkativeness. At high doses (5–15 g), kratom has depressant effects that can provide relief from pain and opioid-withdrawal symptoms.3
Traditionally, kratom has been used in Southeast Asia for recreational and ceremonial purposes, to ease opioid-withdrawal symptoms, and to reduce fatigue from manual labor.4 In the 21st century, availability of kratom expanded to Europe, Australia, and the United States, largely facilitated by widespread dissemination of deceitful marketing and unregulated sales on the internet.1 Although large-scale epidemiologic studies evaluating kratom’s prevalence are scarce, available evidence indicates rising worldwide usage, with a notable increase in kratom-related poison center calls between 2011 and 2017 in the United States.5 In July 2023, kratom made headlines due to the death of a woman in Florida following use of the substance.6
A cross-sectional study revealed that in the United States, kratom typically is used by White individuals for self-treatment of anxiety, depression, pain, and opioid withdrawal.7 However, the potential for severe adverse effects and dependence on kratom can outweigh the benefits.6,8 Reported adverse effects of kratom include tachycardia, hypercholesteremia, liver injury, hallucinations, respiratory depression, seizure, coma, and death.9,10 We present a case of kratom-induced photodistributed hyperpigmentation.
A 63-year-old man presented to the dermatology clinic with diffuse tender, pruritic, hyperpigmented skin lesions that developed over the course of 1 year. The lesions were distributed on sun-exposed areas, including the face, neck, and forearms (Figure 1). The patient reported no other major symptoms, and his health was otherwise unremarkable. He had a medical history of psoriasiform and spongiotic dermatitis consistent with eczema, psoriasis, hypercholesteremia, and hyperlipidemia. The patient was not taking any medications at the time of presentation. He had a family history of plaque psoriasis in his father. Five years prior to the current presentation, the patient was treated with adalimumab for steroid-resistant psoriasis; however, despite initial improvement, he experienced recurrence of scaly erythematous plaques and had discontinued adalimumab the year prior to presentation.

When adalimumab was discontinued, the patient sought alternative treatment for the skin symptoms and began self-administering kratom in an attempt to alleviate associated physical discomfort. He ingested approximately 3 bottles of liquid kratom per day, with each bottle containing 180 mg of mitragynine and less than 8 mg of 7-hydroxymitragynine. Although not scientifically proven, kratom has been colloquially advertised to improve psoriasis.11 The patient reported no other medication use or allergies.
Shave biopsies of hyperpigmented lesions on the right side of the neck, ear, and forearm were performed. Histopathology revealed a sparse superficial, perivascular, lymphocytic infiltrate accompanied by a prominent number of melanophages in the superficial dermis (Figure 2). Special stains further confirmed that the pigment was melanin; the specimens stained positive with Fontana-Masson stain (Figure 3) and negative with an iron stain (Figure 4).



Adalimumab-induced hyperpigmentation was considered. A prior case of adalimumab-induced hyperpigmentation manifested on the face. Histopathology was consistent with a superficial, perivascular, lymphocytic infiltrate with melanophages in the dermis; however, hyperpigmentation was absent in the periorbital area, and affected areas faded 4 months after discontinuation of adalimumab.12 Our patient presented with hyperpigmentation 1 year after adalimumab cessation, and the hyperpigmented areas included the periorbital region. Because of the distinct temporal and clinical features, adalimumab-induced hyperpigmentation was eliminated from the differential diagnosis.
Based on the photodistributed pattern of hyperpigmentation, histopathology, and the temporal relationship between hyperpigmentation onset and kratom usage, a diagnosis of kratom-induced photodistributed hyperpigmentation was made. The patient was advised to discontinue kratom use and use sun protection to prevent further photodamage. The patient subsequently was lost to follow-up.
Kratom alkaloids bind all 3 opioid receptors—μOP, δOP, and κOPs—in a G-protein–biased manner with 7-hydroxymitragynine, the most pharmacologically active alkaloid, exhibiting a higher affinity for μ-opioid receptors.13,14 In human epidermal melanocytes, binding between μ-opioid receptors and β-endorphin, an endogenous opioid, is associated with increased melanin production. This melanogenesis has been linked to hyperpigmentation.15 Given the similarity between kratom alkaloids and β-endorphin in opioid-receptor binding, it is possible that kratom-induced hyperpigmentation may occur through a similar mechanism involving μ-opioid receptors and melanogenesis in epidermal melanocytes. Moreover, some researchers have theorized that sun exposure may result in free radical formation of certain drugs or their metabolites. These free radicals then can interact with cellular DNA, triggering the release of pigmentary mediators and resulting in hyperpigmentation.16 This theory may explain the photodistributed pattern of kratom-induced hyperpigmentation. Further studies are needed to understand the mechanism behind this adverse reaction and its implications for patient treatment.
Literature on kratom-induced hyperpigmentation is limited. Powell et al17 reported a similar case of kratom-induced photodistributed hyperpigmentation—a White man had taken kratom to reduce opioid use and subsequently developed hyperpigmented patches on the arms and face. Moreover, anonymous Reddit users have shared anecdotal reports of hyperpigmentation following kratom use.18
Physicians should be aware of hyperpigmentation as a potential adverse reaction of kratom use as its prevalence increases globally. Further research is warranted to elucidate the mechanism behind this adverse reaction and identify risk factors.
- Prozialeck WC, Avery BA, Boyer EW, et al. Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70-77. doi:10.1016/j.drugpo.2019.05.003
- Bergen-Cico D, MacClurg K. Kratom (Mitragyna speciosa) use, addiction potential, and legal status. In: Preedy VR, ed. Neuropathology of Drug Addictions and Substance Misuse. 2016:903-911. doi:10.1016/B978-0-12-800634-4.00089-5
- Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130:127-138. doi:10.1007/s00414-015-1279-y
- Transnational Institute. Kratom in Thailand: decriminalisation and community control? May 3, 2011. Accessed August 23, 2024. https://www.tni.org/en/publication/kratom-in-thailand-decriminalisation-and-community-control
- Eastlack SC, Cornett EM, Kaye AD. Kratom—pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9:55-69. doi:10.1007/s40122-020-00151-x
- Reyes R. Family of Florida mom who died from herbal substance kratom wins $11M suit. New York Post. July 30, 2023. Updated July 31, 2023. Accessed August 23, 2024. https://nypost.com/2023/07/30/family-of-florida-mom-who-died-from-herbal-substance-kratom-wins-11m-suit/
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. doi:10.1016/j.drugalcdep.2020.107849
- Mayo Clinic. Kratom: unsafe and ineffective. Accessed August 23, 2024. https://www.mayoclinic.org/healthy-lifestyle/consumer-health/in-depth/kratom/art-20402171
- Sethi R, Hoang N, Ravishankar DA, et al. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22:19nr02507.
- Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:775-777. doi:10.1002/phar.2280
- Qrius. 6 benefits of kratom you should know for healthy skin. March 21, 2023. Accessed August 23, 2024. https://qrius.com/6-benefits-of-kratom-you-should-know-for-healthy-skin/
- Blomberg M, Zachariae COC, Grønhøj F. Hyperpigmentation of the face following adalimumab treatment. Acta Derm Venereol. 2009;89:546-547. doi:10.2340/00015555-0697
- Matsumoto K, Hatori Y, Murayama T, et al. Involvement of μ-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549:63-70. doi:10.1016/j.ejphar.2006.08.013
- Jentsch MJ, Pippin MM. Kratom. In: StatPearls. StatPearls Publishing; 2023.
- Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, et al. Opioids and the skin—where do we stand? Exp Dermatol. 2009;18:424-430.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10. doi:10.5070/D33c97j4z5
- Powell LR, Ryser TJ, Morey GE, et al. Kratom as a novel cause of photodistributed hyperpigmentation. JAAD Case Rep. 2022;28:145-148. doi:10.1016/j.jdcr.2022.07.033
- Haccoon. Skin discoloring? Reddit. June 30, 2019. Accessed August 23, 2024. https://www.reddit.com/r/quittingkratom/comments/c7b1cm/skin_discoloring/
- Prozialeck WC, Avery BA, Boyer EW, et al. Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70-77. doi:10.1016/j.drugpo.2019.05.003
- Bergen-Cico D, MacClurg K. Kratom (Mitragyna speciosa) use, addiction potential, and legal status. In: Preedy VR, ed. Neuropathology of Drug Addictions and Substance Misuse. 2016:903-911. doi:10.1016/B978-0-12-800634-4.00089-5
- Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130:127-138. doi:10.1007/s00414-015-1279-y
- Transnational Institute. Kratom in Thailand: decriminalisation and community control? May 3, 2011. Accessed August 23, 2024. https://www.tni.org/en/publication/kratom-in-thailand-decriminalisation-and-community-control
- Eastlack SC, Cornett EM, Kaye AD. Kratom—pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9:55-69. doi:10.1007/s40122-020-00151-x
- Reyes R. Family of Florida mom who died from herbal substance kratom wins $11M suit. New York Post. July 30, 2023. Updated July 31, 2023. Accessed August 23, 2024. https://nypost.com/2023/07/30/family-of-florida-mom-who-died-from-herbal-substance-kratom-wins-11m-suit/
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. doi:10.1016/j.drugalcdep.2020.107849
- Mayo Clinic. Kratom: unsafe and ineffective. Accessed August 23, 2024. https://www.mayoclinic.org/healthy-lifestyle/consumer-health/in-depth/kratom/art-20402171
- Sethi R, Hoang N, Ravishankar DA, et al. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22:19nr02507.
- Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:775-777. doi:10.1002/phar.2280
- Qrius. 6 benefits of kratom you should know for healthy skin. March 21, 2023. Accessed August 23, 2024. https://qrius.com/6-benefits-of-kratom-you-should-know-for-healthy-skin/
- Blomberg M, Zachariae COC, Grønhøj F. Hyperpigmentation of the face following adalimumab treatment. Acta Derm Venereol. 2009;89:546-547. doi:10.2340/00015555-0697
- Matsumoto K, Hatori Y, Murayama T, et al. Involvement of μ-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549:63-70. doi:10.1016/j.ejphar.2006.08.013
- Jentsch MJ, Pippin MM. Kratom. In: StatPearls. StatPearls Publishing; 2023.
- Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, et al. Opioids and the skin—where do we stand? Exp Dermatol. 2009;18:424-430.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10. doi:10.5070/D33c97j4z5
- Powell LR, Ryser TJ, Morey GE, et al. Kratom as a novel cause of photodistributed hyperpigmentation. JAAD Case Rep. 2022;28:145-148. doi:10.1016/j.jdcr.2022.07.033
- Haccoon. Skin discoloring? Reddit. June 30, 2019. Accessed August 23, 2024. https://www.reddit.com/r/quittingkratom/comments/c7b1cm/skin_discoloring/
Practice Points
- Clinicians should be aware of photodistributed hyperpigmentation as a potential adverse effect of kratom usage.
- Kratom-induced photodistributed hyperpigmentation should be suspected in patients with hyperpigmented lesions in sun-exposed areas of the skin following kratom use. A biopsy of lesions should be obtained to confirm the diagnosis.
- Cessation of kratom should be recommended.