User login
Irritable temperament predicts bipolar disorder risk
When German psychiatrist Emil Kraepelin (1856-1926) studied emotions in patients with affective disorders, he identified four temperaments: the depressive (DT), the hyperthymic (HT), the irritable (IT), and the cyclothymic (CT). Subsequent researchers later identified an anxious temperament (AT).
“The notion that temperaments can be useful in predicting bipolar disorders sparked a plethora of research,” wrote Elie G. Karam, MD, of Saint George Hospital, Beirut, and colleagues. In particular, the cyclothymic (CT) and irritable (IT) temperament types have been targeted in studies of patients with bipolar disorders, but previous studies of temperament and bipolar have been limited by methodological issues, they said.
In a study published in European Psychiatry, the researchers reviewed data from 1,723 consecutive adult outpatients who presented to a university-based mental health clinic with various symptoms between January 2014 and September 2019.
Patients were assessed using the Hypomania Checklist-32 (HCL-32) and the Temperament Evaluation of Memphis, Pisa, Paris, and San Diego-Auto-questionnaire (TEMPS-A), then were diagnosed by psychiatrists using DSM-5 criteria. Patients with any bipolar types as defined by the DSM-5 underwent simple and multiple binary logistic regression analyses. The analysis included continuous scores and categorical normalized z-scores.
A total of 369 patients had confirmed DSM-5 diagnosis of bipolar disorder (52 with type I, 176 with type II, 102 with other specified bipolar and related disorder, and 39 with substance- or medication-induced bipolar disorder. The mean age of the participants was 38 years, and 54% were female.
In a bivariate analysis, all continuous temperament scores were significant predictors of bipolar disorder; all except AT remained significant in multivariate analysis. Increasing scores of IT, CT, and HT were associated with bipolar disorder, but increasing scores of DT were reflective of lower chance of bipolar disorder, the researchers noted.
In multivariate analysis of categorical normalized z-scores, IT and CT were significant predictors of bipolar disorder. At the highest point, CT was the stronger predictor, compared with IT (odds ratio, 3.84 vs. 2.55); having a higher DT score significantly reduced the odds of bipolar disorder (OR, 0.50).
However, “after adjusting for the presence of all temperaments as well as age and gender, only IT remained a significant predictor of patients with bipolar I disorder with adjusted OR of 1.19,” the researchers wrote.
“Correlations among temperaments were solid whether looking at patients with bipolarity or not, further emphasizing the necessity of controlling for them,” the researchers wrote in their discussion.
The findings were limited by several factors including the lack of structured interviews, the use of an outpatient-only sample, and the small number of bipolar I patients, the researchers noted.
However, the result suggest that IT can serve as a predictor of bipolar I and bipolar II disorders they said. Given the underdiagnosis of bipolar disorder in many studies, the incorporation of temperaments into the assessment of patients and research participants alike is likely to help us detect the presence of bipolarity more readily and quite importantly help us in our quest to understand their genesis,” they concluded.
The study was supported in part by anonymous private unrestricted donations to IDRAAC, Lebanon, and by Eli Lilly. The researchers had no financial conflicts to disclose.
When German psychiatrist Emil Kraepelin (1856-1926) studied emotions in patients with affective disorders, he identified four temperaments: the depressive (DT), the hyperthymic (HT), the irritable (IT), and the cyclothymic (CT). Subsequent researchers later identified an anxious temperament (AT).
“The notion that temperaments can be useful in predicting bipolar disorders sparked a plethora of research,” wrote Elie G. Karam, MD, of Saint George Hospital, Beirut, and colleagues. In particular, the cyclothymic (CT) and irritable (IT) temperament types have been targeted in studies of patients with bipolar disorders, but previous studies of temperament and bipolar have been limited by methodological issues, they said.
In a study published in European Psychiatry, the researchers reviewed data from 1,723 consecutive adult outpatients who presented to a university-based mental health clinic with various symptoms between January 2014 and September 2019.
Patients were assessed using the Hypomania Checklist-32 (HCL-32) and the Temperament Evaluation of Memphis, Pisa, Paris, and San Diego-Auto-questionnaire (TEMPS-A), then were diagnosed by psychiatrists using DSM-5 criteria. Patients with any bipolar types as defined by the DSM-5 underwent simple and multiple binary logistic regression analyses. The analysis included continuous scores and categorical normalized z-scores.
A total of 369 patients had confirmed DSM-5 diagnosis of bipolar disorder (52 with type I, 176 with type II, 102 with other specified bipolar and related disorder, and 39 with substance- or medication-induced bipolar disorder. The mean age of the participants was 38 years, and 54% were female.
In a bivariate analysis, all continuous temperament scores were significant predictors of bipolar disorder; all except AT remained significant in multivariate analysis. Increasing scores of IT, CT, and HT were associated with bipolar disorder, but increasing scores of DT were reflective of lower chance of bipolar disorder, the researchers noted.
In multivariate analysis of categorical normalized z-scores, IT and CT were significant predictors of bipolar disorder. At the highest point, CT was the stronger predictor, compared with IT (odds ratio, 3.84 vs. 2.55); having a higher DT score significantly reduced the odds of bipolar disorder (OR, 0.50).
However, “after adjusting for the presence of all temperaments as well as age and gender, only IT remained a significant predictor of patients with bipolar I disorder with adjusted OR of 1.19,” the researchers wrote.
“Correlations among temperaments were solid whether looking at patients with bipolarity or not, further emphasizing the necessity of controlling for them,” the researchers wrote in their discussion.
The findings were limited by several factors including the lack of structured interviews, the use of an outpatient-only sample, and the small number of bipolar I patients, the researchers noted.
However, the result suggest that IT can serve as a predictor of bipolar I and bipolar II disorders they said. Given the underdiagnosis of bipolar disorder in many studies, the incorporation of temperaments into the assessment of patients and research participants alike is likely to help us detect the presence of bipolarity more readily and quite importantly help us in our quest to understand their genesis,” they concluded.
The study was supported in part by anonymous private unrestricted donations to IDRAAC, Lebanon, and by Eli Lilly. The researchers had no financial conflicts to disclose.
When German psychiatrist Emil Kraepelin (1856-1926) studied emotions in patients with affective disorders, he identified four temperaments: the depressive (DT), the hyperthymic (HT), the irritable (IT), and the cyclothymic (CT). Subsequent researchers later identified an anxious temperament (AT).
“The notion that temperaments can be useful in predicting bipolar disorders sparked a plethora of research,” wrote Elie G. Karam, MD, of Saint George Hospital, Beirut, and colleagues. In particular, the cyclothymic (CT) and irritable (IT) temperament types have been targeted in studies of patients with bipolar disorders, but previous studies of temperament and bipolar have been limited by methodological issues, they said.
In a study published in European Psychiatry, the researchers reviewed data from 1,723 consecutive adult outpatients who presented to a university-based mental health clinic with various symptoms between January 2014 and September 2019.
Patients were assessed using the Hypomania Checklist-32 (HCL-32) and the Temperament Evaluation of Memphis, Pisa, Paris, and San Diego-Auto-questionnaire (TEMPS-A), then were diagnosed by psychiatrists using DSM-5 criteria. Patients with any bipolar types as defined by the DSM-5 underwent simple and multiple binary logistic regression analyses. The analysis included continuous scores and categorical normalized z-scores.
A total of 369 patients had confirmed DSM-5 diagnosis of bipolar disorder (52 with type I, 176 with type II, 102 with other specified bipolar and related disorder, and 39 with substance- or medication-induced bipolar disorder. The mean age of the participants was 38 years, and 54% were female.
In a bivariate analysis, all continuous temperament scores were significant predictors of bipolar disorder; all except AT remained significant in multivariate analysis. Increasing scores of IT, CT, and HT were associated with bipolar disorder, but increasing scores of DT were reflective of lower chance of bipolar disorder, the researchers noted.
In multivariate analysis of categorical normalized z-scores, IT and CT were significant predictors of bipolar disorder. At the highest point, CT was the stronger predictor, compared with IT (odds ratio, 3.84 vs. 2.55); having a higher DT score significantly reduced the odds of bipolar disorder (OR, 0.50).
However, “after adjusting for the presence of all temperaments as well as age and gender, only IT remained a significant predictor of patients with bipolar I disorder with adjusted OR of 1.19,” the researchers wrote.
“Correlations among temperaments were solid whether looking at patients with bipolarity or not, further emphasizing the necessity of controlling for them,” the researchers wrote in their discussion.
The findings were limited by several factors including the lack of structured interviews, the use of an outpatient-only sample, and the small number of bipolar I patients, the researchers noted.
However, the result suggest that IT can serve as a predictor of bipolar I and bipolar II disorders they said. Given the underdiagnosis of bipolar disorder in many studies, the incorporation of temperaments into the assessment of patients and research participants alike is likely to help us detect the presence of bipolarity more readily and quite importantly help us in our quest to understand their genesis,” they concluded.
The study was supported in part by anonymous private unrestricted donations to IDRAAC, Lebanon, and by Eli Lilly. The researchers had no financial conflicts to disclose.
FROM EUROPEAN PSYCHIATRY
Dialectical behavior therapy decreased suicide attempts in bipolar teens
, based on data from 100 individuals aged 12-18 years.
Bipolar spectrum disorder (BP) is known to substantially increase the risk for suicide in youth, but no psychosocial intervention for this population has targeted suicidal behavior in particular, wrote Tina R. Goldstein, PhD, of the University of Pittsburgh, and colleagues.
Dialectical behavior therapy (DBT) had shown effectiveness for decreasing suicide attempts in adults with borderline personality disorder, and previous studies of DBT have shown reduced suicidal ideation, self-harm, and suicide attempts in suicidal adolescents, but these studies have mainly excluded BP teens, the researchers said.
In a study published in JAMA Psychiatry, the researchers recruited adolescents aged 12-18 years with a diagnosis of BP who were treated at an outpatient clinic between November 2014 and September 2019. Of these, 47 were randomized to 1 year of DBT (a total of 36 sessions) and 53 to standard of care (SOC) psychotherapy. All participants also received medication using a flexible algorithm.
The primary outcomes were suicide attempts over a 1-year period and measurements of mood symptoms and states, specifically depression and hypomania/mania. Secondary analyses included the effect of DBT on individuals with a history of suicide attempt and on improving emotion dysregulation. The mean age of the participants was 16.1 years; 85 were female, and 74% were White.
Participants in both DBT and SOC groups reported similar rates of suicide attempt rates at study enrollment based on the Adolescent Longitudinal Follow-Up Evaluation (ALIFE) with a mean of 2.0 and 1.8 attempts, respectively (P = .80). Based on the Columbia–Suicide Severity Rating Scale Pediatric Version (C-SSRS), participants in the DBT group had slightly more suicide attempts than the SOC group at study enrollment, with a mean of 1.4 and 0.6 attempts, respectively (P = .02).
Controlling for baseline attempts, participants in the DBT group had significantly fewer suicide attempts over the study period, compared with the SOC group as measured by both ALIFE (mean 0.2 vs. 1.1) and C-SSRS (mean 0.04 vs. 0.10, P = .03 for both measures). The incidence rate ratios for reduced suicide attempts were 0.32 for ALIFE and 0.13 for C-SSRS, both significant in favor of DBT, compared with SOC.
Overall, both groups showed similarly significant improvement on measures of mood symptoms and episodes over the study period. The standardized depression rating scale slope was –0.17 and the standardized mania rating scale slope was –0.24.
DBT was significantly more effective than SOC psychotherapy at decreasing suicide attempts over 1 year (ALIFE: incidence rate ratio, 0.32; 95% CI, 0.11-0.96; C-SSRS: IRR, 0.13; 95% CI, 0.02-0.78).
On further analysis, the decrease in suicide attempts in the DBT group was greater over time and among those with a lifetime history of suicide attempts (IRR, 0.23). “Decreased risk of suicide attempt in DBT was mediated by improvement in emotion dysregulation, particularly for those with high baseline emotion dysregulation,” the researchers wrote in their discussion.
The findings were limited by several factors including the mainly female, non-Hispanic White study population, and controlled clinical setting, the researchers noted. Data from a forthcoming community implementation field trial will address some generalizability issues, although more work is needed to address disparities in BP diagnosis and treatment, they added.
However, the results support the potential of DBT for mood management and for reducing suicide attempts in a high-risk adolescent population, especially those with high levels of emotional dysregulation, on par with other established psychosocial treatments, the researchers concluded.
More options needed to manage increased risk
“It was important to conduct this study at this time because, while still relatively rare, bipolar spectrum disorders in adolescents confer increased risk for suicide,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview. The complexity of BP and the increased risk of suicide in these patients challenge clinicians to identify robust evidence-based interventions beyond pharmacotherapy that mitigate this risk, said Dr. Loper, who is triple board certified in pediatrics, general psychiatry, and child & adolescent psychiatry, but was not involved in the study.
The current study findings were not surprising, because DBT has proven effective in decreasing suicidal ideation and suicide attempts in other high-risk adolescent patient populations, Dr. Loper said. “Given the therapeutic content of DBT, with emphasis on mindfulness, distress tolerance, social skills, and emotional regulation, I think it is reasonable to hypothesize that DBT might be a globally applicable intervention, independent of mental health diagnosis or etiology of suicidal ideation,” he said.
The take-home message for clinicians is that the results support the efficacy of DBT as an intervention for adolescents with BP and suicidal ideation, self-injurious behavior, or suicide attempts, said Dr. Loper. For these patients, given their increased suicide risk, “DBT should certainly be recommended as a component of their treatment plan,” he said.
However, barriers to the use of DBT in clinical practice exist, notably access and cost, Dr. Loper noted. “I think that the most prominent barrier in accessing DBT in clinical practice is the availability of certified, structured DBT treatment programs, and particularly those willing to provide services to adolescents,” he said. “Additionally, certified DBT programs, which are the gold standard, are often not covered by third-party payers, making cost yet another potential barrier.”
Looking ahead, Dr. Loper agreed with the study authors that additional research with a more diverse patient population representative of adolescents with bipolar spectrum disorder “is a crucial area of focus.”
The study was funded by the National Institutes of Mental Health through a grant to Dr. Goldstein, who also disclosed royalties from Guilford Press unrelated to the current study. Dr. Loper had no financial conflicts to disclose.
, based on data from 100 individuals aged 12-18 years.
Bipolar spectrum disorder (BP) is known to substantially increase the risk for suicide in youth, but no psychosocial intervention for this population has targeted suicidal behavior in particular, wrote Tina R. Goldstein, PhD, of the University of Pittsburgh, and colleagues.
Dialectical behavior therapy (DBT) had shown effectiveness for decreasing suicide attempts in adults with borderline personality disorder, and previous studies of DBT have shown reduced suicidal ideation, self-harm, and suicide attempts in suicidal adolescents, but these studies have mainly excluded BP teens, the researchers said.
In a study published in JAMA Psychiatry, the researchers recruited adolescents aged 12-18 years with a diagnosis of BP who were treated at an outpatient clinic between November 2014 and September 2019. Of these, 47 were randomized to 1 year of DBT (a total of 36 sessions) and 53 to standard of care (SOC) psychotherapy. All participants also received medication using a flexible algorithm.
The primary outcomes were suicide attempts over a 1-year period and measurements of mood symptoms and states, specifically depression and hypomania/mania. Secondary analyses included the effect of DBT on individuals with a history of suicide attempt and on improving emotion dysregulation. The mean age of the participants was 16.1 years; 85 were female, and 74% were White.
Participants in both DBT and SOC groups reported similar rates of suicide attempt rates at study enrollment based on the Adolescent Longitudinal Follow-Up Evaluation (ALIFE) with a mean of 2.0 and 1.8 attempts, respectively (P = .80). Based on the Columbia–Suicide Severity Rating Scale Pediatric Version (C-SSRS), participants in the DBT group had slightly more suicide attempts than the SOC group at study enrollment, with a mean of 1.4 and 0.6 attempts, respectively (P = .02).
Controlling for baseline attempts, participants in the DBT group had significantly fewer suicide attempts over the study period, compared with the SOC group as measured by both ALIFE (mean 0.2 vs. 1.1) and C-SSRS (mean 0.04 vs. 0.10, P = .03 for both measures). The incidence rate ratios for reduced suicide attempts were 0.32 for ALIFE and 0.13 for C-SSRS, both significant in favor of DBT, compared with SOC.
Overall, both groups showed similarly significant improvement on measures of mood symptoms and episodes over the study period. The standardized depression rating scale slope was –0.17 and the standardized mania rating scale slope was –0.24.
DBT was significantly more effective than SOC psychotherapy at decreasing suicide attempts over 1 year (ALIFE: incidence rate ratio, 0.32; 95% CI, 0.11-0.96; C-SSRS: IRR, 0.13; 95% CI, 0.02-0.78).
On further analysis, the decrease in suicide attempts in the DBT group was greater over time and among those with a lifetime history of suicide attempts (IRR, 0.23). “Decreased risk of suicide attempt in DBT was mediated by improvement in emotion dysregulation, particularly for those with high baseline emotion dysregulation,” the researchers wrote in their discussion.
The findings were limited by several factors including the mainly female, non-Hispanic White study population, and controlled clinical setting, the researchers noted. Data from a forthcoming community implementation field trial will address some generalizability issues, although more work is needed to address disparities in BP diagnosis and treatment, they added.
However, the results support the potential of DBT for mood management and for reducing suicide attempts in a high-risk adolescent population, especially those with high levels of emotional dysregulation, on par with other established psychosocial treatments, the researchers concluded.
More options needed to manage increased risk
“It was important to conduct this study at this time because, while still relatively rare, bipolar spectrum disorders in adolescents confer increased risk for suicide,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview. The complexity of BP and the increased risk of suicide in these patients challenge clinicians to identify robust evidence-based interventions beyond pharmacotherapy that mitigate this risk, said Dr. Loper, who is triple board certified in pediatrics, general psychiatry, and child & adolescent psychiatry, but was not involved in the study.
The current study findings were not surprising, because DBT has proven effective in decreasing suicidal ideation and suicide attempts in other high-risk adolescent patient populations, Dr. Loper said. “Given the therapeutic content of DBT, with emphasis on mindfulness, distress tolerance, social skills, and emotional regulation, I think it is reasonable to hypothesize that DBT might be a globally applicable intervention, independent of mental health diagnosis or etiology of suicidal ideation,” he said.
The take-home message for clinicians is that the results support the efficacy of DBT as an intervention for adolescents with BP and suicidal ideation, self-injurious behavior, or suicide attempts, said Dr. Loper. For these patients, given their increased suicide risk, “DBT should certainly be recommended as a component of their treatment plan,” he said.
However, barriers to the use of DBT in clinical practice exist, notably access and cost, Dr. Loper noted. “I think that the most prominent barrier in accessing DBT in clinical practice is the availability of certified, structured DBT treatment programs, and particularly those willing to provide services to adolescents,” he said. “Additionally, certified DBT programs, which are the gold standard, are often not covered by third-party payers, making cost yet another potential barrier.”
Looking ahead, Dr. Loper agreed with the study authors that additional research with a more diverse patient population representative of adolescents with bipolar spectrum disorder “is a crucial area of focus.”
The study was funded by the National Institutes of Mental Health through a grant to Dr. Goldstein, who also disclosed royalties from Guilford Press unrelated to the current study. Dr. Loper had no financial conflicts to disclose.
, based on data from 100 individuals aged 12-18 years.
Bipolar spectrum disorder (BP) is known to substantially increase the risk for suicide in youth, but no psychosocial intervention for this population has targeted suicidal behavior in particular, wrote Tina R. Goldstein, PhD, of the University of Pittsburgh, and colleagues.
Dialectical behavior therapy (DBT) had shown effectiveness for decreasing suicide attempts in adults with borderline personality disorder, and previous studies of DBT have shown reduced suicidal ideation, self-harm, and suicide attempts in suicidal adolescents, but these studies have mainly excluded BP teens, the researchers said.
In a study published in JAMA Psychiatry, the researchers recruited adolescents aged 12-18 years with a diagnosis of BP who were treated at an outpatient clinic between November 2014 and September 2019. Of these, 47 were randomized to 1 year of DBT (a total of 36 sessions) and 53 to standard of care (SOC) psychotherapy. All participants also received medication using a flexible algorithm.
The primary outcomes were suicide attempts over a 1-year period and measurements of mood symptoms and states, specifically depression and hypomania/mania. Secondary analyses included the effect of DBT on individuals with a history of suicide attempt and on improving emotion dysregulation. The mean age of the participants was 16.1 years; 85 were female, and 74% were White.
Participants in both DBT and SOC groups reported similar rates of suicide attempt rates at study enrollment based on the Adolescent Longitudinal Follow-Up Evaluation (ALIFE) with a mean of 2.0 and 1.8 attempts, respectively (P = .80). Based on the Columbia–Suicide Severity Rating Scale Pediatric Version (C-SSRS), participants in the DBT group had slightly more suicide attempts than the SOC group at study enrollment, with a mean of 1.4 and 0.6 attempts, respectively (P = .02).
Controlling for baseline attempts, participants in the DBT group had significantly fewer suicide attempts over the study period, compared with the SOC group as measured by both ALIFE (mean 0.2 vs. 1.1) and C-SSRS (mean 0.04 vs. 0.10, P = .03 for both measures). The incidence rate ratios for reduced suicide attempts were 0.32 for ALIFE and 0.13 for C-SSRS, both significant in favor of DBT, compared with SOC.
Overall, both groups showed similarly significant improvement on measures of mood symptoms and episodes over the study period. The standardized depression rating scale slope was –0.17 and the standardized mania rating scale slope was –0.24.
DBT was significantly more effective than SOC psychotherapy at decreasing suicide attempts over 1 year (ALIFE: incidence rate ratio, 0.32; 95% CI, 0.11-0.96; C-SSRS: IRR, 0.13; 95% CI, 0.02-0.78).
On further analysis, the decrease in suicide attempts in the DBT group was greater over time and among those with a lifetime history of suicide attempts (IRR, 0.23). “Decreased risk of suicide attempt in DBT was mediated by improvement in emotion dysregulation, particularly for those with high baseline emotion dysregulation,” the researchers wrote in their discussion.
The findings were limited by several factors including the mainly female, non-Hispanic White study population, and controlled clinical setting, the researchers noted. Data from a forthcoming community implementation field trial will address some generalizability issues, although more work is needed to address disparities in BP diagnosis and treatment, they added.
However, the results support the potential of DBT for mood management and for reducing suicide attempts in a high-risk adolescent population, especially those with high levels of emotional dysregulation, on par with other established psychosocial treatments, the researchers concluded.
More options needed to manage increased risk
“It was important to conduct this study at this time because, while still relatively rare, bipolar spectrum disorders in adolescents confer increased risk for suicide,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview. The complexity of BP and the increased risk of suicide in these patients challenge clinicians to identify robust evidence-based interventions beyond pharmacotherapy that mitigate this risk, said Dr. Loper, who is triple board certified in pediatrics, general psychiatry, and child & adolescent psychiatry, but was not involved in the study.
The current study findings were not surprising, because DBT has proven effective in decreasing suicidal ideation and suicide attempts in other high-risk adolescent patient populations, Dr. Loper said. “Given the therapeutic content of DBT, with emphasis on mindfulness, distress tolerance, social skills, and emotional regulation, I think it is reasonable to hypothesize that DBT might be a globally applicable intervention, independent of mental health diagnosis or etiology of suicidal ideation,” he said.
The take-home message for clinicians is that the results support the efficacy of DBT as an intervention for adolescents with BP and suicidal ideation, self-injurious behavior, or suicide attempts, said Dr. Loper. For these patients, given their increased suicide risk, “DBT should certainly be recommended as a component of their treatment plan,” he said.
However, barriers to the use of DBT in clinical practice exist, notably access and cost, Dr. Loper noted. “I think that the most prominent barrier in accessing DBT in clinical practice is the availability of certified, structured DBT treatment programs, and particularly those willing to provide services to adolescents,” he said. “Additionally, certified DBT programs, which are the gold standard, are often not covered by third-party payers, making cost yet another potential barrier.”
Looking ahead, Dr. Loper agreed with the study authors that additional research with a more diverse patient population representative of adolescents with bipolar spectrum disorder “is a crucial area of focus.”
The study was funded by the National Institutes of Mental Health through a grant to Dr. Goldstein, who also disclosed royalties from Guilford Press unrelated to the current study. Dr. Loper had no financial conflicts to disclose.
FROM JAMA PSYCHIATRY
The cult of the suicide risk assessment
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Screen bipolar patients for eating disorders
Previous research of bipolar disorder (BD) shows a high rate of comorbidities with other psychiatric disorders, including eating disorders (EDs), Valentin Flaudias, PhD, of Nantes (France) University and colleagues wrote.
“There is growing evidence that, compared with individuals with BD alone, individuals with both BD and EDs have a more severe clinical profile, including increased mood instability, alcohol use disorders, anxiety disorders, more depressive episodes, more rapid cycling, increased suicidality, and poorer response to medication,” but studies of BD type-specific ED prevalence have been inconsistent, they said.
In a study published in the Journal of Affective Disorders, the researchers reviewed data from 2,929 outpatients who underwent assessments for BD at 1 of 12 psychiatric centers in France. Of these, 1,505 met criteria for type I and 1,424 met criteria for type II. The post hoc analysis included identification of lifetime prevalence of ED. Diagnosis was based on the DSM-4-TR and the researchers considered three ED types: anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED). Subtypes of BD were type I and type II. DSM not otherwise specified diagnoses for BD and EDs were excluded. The mean age of the participants was 40.5 years, and 61% were women.
A total of 479 individuals met criteria for comorbid EDs (16.4%). ED prevalence was significantly higher in BD type II patients than in BD type I patients (20.6 % vs. 12.4 %, P < .001). The overall breakdown according to ED subtype was 30% for AN, 13% for BN, and 56% for BED. The researchers found no significant differences in patients with AN, BN, or BED according to BD subtype.
In a multivariate analysis, BD patients with ED were more likely than those without ED to be women (77% vs. 55%), especially those with AN (95% vs. 82%).
BD patients with ED also tended to be younger than those without ED (37 years vs. 41 years) and reported more frequent suicide attempts (50% vs. 35%). Younger age and more frequent suicide attempts were further significant among BD patients with AN, compared with those with BED, but BD patients with BED reported higher levels of childhood trauma.
BD patients with ED also reported higher levels of depressive symptoms than those without ED, although history of psychosis was less frequent among BD patients with AN and BED compared with BD patients without EDs.
Overall, “after controlling for other variables, the independent factors differentiating BD patients with versus without ED were primarily younger age, female gender, abnormal BMI, increased affective lability and higher comorbidity with anxiety disorders,” the researchers wrote. In addition, presence of EDs except for AN was associated with decreased current functioning.
The findings were limited by several factors including the cross-sectional design, lack of a control group of non-BD individuals, and the consideration of ED over a lifetime, and small number of BN cases, the researchers noted.
However, the results suggest a high prevalence of ED in BD patients and highlight the need to screen BD patients for ED and provide integrated care. More research is needed to explore the evolution of the two conditions as comorbidities and to examine subtypes and of both conditions and their interactions, they concluded.
The study was supported by the FondaMental Foundation, French National Institute for Health and Medical Research, Public Hospitals of Paris, and the French National Research Agency’s Investment for the Future program. The researchers had no financial conflicts to disclose.
Previous research of bipolar disorder (BD) shows a high rate of comorbidities with other psychiatric disorders, including eating disorders (EDs), Valentin Flaudias, PhD, of Nantes (France) University and colleagues wrote.
“There is growing evidence that, compared with individuals with BD alone, individuals with both BD and EDs have a more severe clinical profile, including increased mood instability, alcohol use disorders, anxiety disorders, more depressive episodes, more rapid cycling, increased suicidality, and poorer response to medication,” but studies of BD type-specific ED prevalence have been inconsistent, they said.
In a study published in the Journal of Affective Disorders, the researchers reviewed data from 2,929 outpatients who underwent assessments for BD at 1 of 12 psychiatric centers in France. Of these, 1,505 met criteria for type I and 1,424 met criteria for type II. The post hoc analysis included identification of lifetime prevalence of ED. Diagnosis was based on the DSM-4-TR and the researchers considered three ED types: anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED). Subtypes of BD were type I and type II. DSM not otherwise specified diagnoses for BD and EDs were excluded. The mean age of the participants was 40.5 years, and 61% were women.
A total of 479 individuals met criteria for comorbid EDs (16.4%). ED prevalence was significantly higher in BD type II patients than in BD type I patients (20.6 % vs. 12.4 %, P < .001). The overall breakdown according to ED subtype was 30% for AN, 13% for BN, and 56% for BED. The researchers found no significant differences in patients with AN, BN, or BED according to BD subtype.
In a multivariate analysis, BD patients with ED were more likely than those without ED to be women (77% vs. 55%), especially those with AN (95% vs. 82%).
BD patients with ED also tended to be younger than those without ED (37 years vs. 41 years) and reported more frequent suicide attempts (50% vs. 35%). Younger age and more frequent suicide attempts were further significant among BD patients with AN, compared with those with BED, but BD patients with BED reported higher levels of childhood trauma.
BD patients with ED also reported higher levels of depressive symptoms than those without ED, although history of psychosis was less frequent among BD patients with AN and BED compared with BD patients without EDs.
Overall, “after controlling for other variables, the independent factors differentiating BD patients with versus without ED were primarily younger age, female gender, abnormal BMI, increased affective lability and higher comorbidity with anxiety disorders,” the researchers wrote. In addition, presence of EDs except for AN was associated with decreased current functioning.
The findings were limited by several factors including the cross-sectional design, lack of a control group of non-BD individuals, and the consideration of ED over a lifetime, and small number of BN cases, the researchers noted.
However, the results suggest a high prevalence of ED in BD patients and highlight the need to screen BD patients for ED and provide integrated care. More research is needed to explore the evolution of the two conditions as comorbidities and to examine subtypes and of both conditions and their interactions, they concluded.
The study was supported by the FondaMental Foundation, French National Institute for Health and Medical Research, Public Hospitals of Paris, and the French National Research Agency’s Investment for the Future program. The researchers had no financial conflicts to disclose.
Previous research of bipolar disorder (BD) shows a high rate of comorbidities with other psychiatric disorders, including eating disorders (EDs), Valentin Flaudias, PhD, of Nantes (France) University and colleagues wrote.
“There is growing evidence that, compared with individuals with BD alone, individuals with both BD and EDs have a more severe clinical profile, including increased mood instability, alcohol use disorders, anxiety disorders, more depressive episodes, more rapid cycling, increased suicidality, and poorer response to medication,” but studies of BD type-specific ED prevalence have been inconsistent, they said.
In a study published in the Journal of Affective Disorders, the researchers reviewed data from 2,929 outpatients who underwent assessments for BD at 1 of 12 psychiatric centers in France. Of these, 1,505 met criteria for type I and 1,424 met criteria for type II. The post hoc analysis included identification of lifetime prevalence of ED. Diagnosis was based on the DSM-4-TR and the researchers considered three ED types: anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED). Subtypes of BD were type I and type II. DSM not otherwise specified diagnoses for BD and EDs were excluded. The mean age of the participants was 40.5 years, and 61% were women.
A total of 479 individuals met criteria for comorbid EDs (16.4%). ED prevalence was significantly higher in BD type II patients than in BD type I patients (20.6 % vs. 12.4 %, P < .001). The overall breakdown according to ED subtype was 30% for AN, 13% for BN, and 56% for BED. The researchers found no significant differences in patients with AN, BN, or BED according to BD subtype.
In a multivariate analysis, BD patients with ED were more likely than those without ED to be women (77% vs. 55%), especially those with AN (95% vs. 82%).
BD patients with ED also tended to be younger than those without ED (37 years vs. 41 years) and reported more frequent suicide attempts (50% vs. 35%). Younger age and more frequent suicide attempts were further significant among BD patients with AN, compared with those with BED, but BD patients with BED reported higher levels of childhood trauma.
BD patients with ED also reported higher levels of depressive symptoms than those without ED, although history of psychosis was less frequent among BD patients with AN and BED compared with BD patients without EDs.
Overall, “after controlling for other variables, the independent factors differentiating BD patients with versus without ED were primarily younger age, female gender, abnormal BMI, increased affective lability and higher comorbidity with anxiety disorders,” the researchers wrote. In addition, presence of EDs except for AN was associated with decreased current functioning.
The findings were limited by several factors including the cross-sectional design, lack of a control group of non-BD individuals, and the consideration of ED over a lifetime, and small number of BN cases, the researchers noted.
However, the results suggest a high prevalence of ED in BD patients and highlight the need to screen BD patients for ED and provide integrated care. More research is needed to explore the evolution of the two conditions as comorbidities and to examine subtypes and of both conditions and their interactions, they concluded.
The study was supported by the FondaMental Foundation, French National Institute for Health and Medical Research, Public Hospitals of Paris, and the French National Research Agency’s Investment for the Future program. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Brain volume patterns vary across psychiatric disorders
A large brain imaging study of adults with six different psychiatric illnesses shows that heterogeneity in regional gray matter volume deviations is a general feature of psychiatric illness, but that these regionally heterogeneous areas are often embedded within common functional circuits and networks.
study investigator Ashlea Segal said in an email.
The findings also suggest that it’s “unlikely that a single cause or mechanism of a given disorder exists, and that a ‘one-size-fits-all’ approach to treatment is likely only appropriate for a small subset of individuals. In fact, one size doesn’t fit all. It probably doesn’t even fit most,” said Ms. Segal, a PhD candidate with the Turner Institute for Brain and Mental Health’s Neural Systems and Behaviour Lab at Monash University in Melbourne.
“Focusing on brain alterations at an individual level allows us to develop more personally tailored treatments,” Ms. Segal added.
Regional heterogeneity, the authors write, “thus offers a plausible explanation for the well-described clinical heterogeneity observed in psychiatric disorders, while circuit- and network-level aggregation of deviations is a putative neural substrate for phenotypic similarities between patients assigned the same diagnosis.”
The study was published online in Nature Neuroscience
Beyond group averages
For decades, researchers have mapped brain areas showing reduced gray matter volume (GMV) in people diagnosed with a variety of mental illnesses, but these maps have only been generated at the level of group averages, Ms. Segal explained.
“This means that we understand how the brains of people with, say, schizophrenia, differ from those without schizophrenia on average, but we can’t really say much about individual people,” Ms. Segal said.
For their study, the researchers used new statistical techniques developed by Andre Marquand, PhD, who co-led the project, to characterize the heterogeneity of GMV differences in 1,294 individuals diagnosed with one of six psychiatric conditions and 1,465 matched controls. Dr. Marquand is affiliated with the Donders Institute for Brain, Cognition, and Behavior in Nijmegen, the Netherlands.
These techniques “allow us to benchmark the size of over 1,000 different brain regions in any given person relative to what we should expect to see in the general population. In this way, we can identify, for any person, brain regions showing unusually small or large volumes, given that person’s age and sex,” Ms. Segal told this news organization.
The clinical sample included 202 individuals with autism spectrum disorder, 153 with attention-deficit/hyperactivity disorder (ADHD), 228 with bipolar disorder, 161 with major depressive disorder, 167 with obsessive-compulsive disorder, and 383 individuals with schizophrenia.
Confirming earlier findings, those with psychiatric illness showed more GMV deviations than healthy controls, the researchers found.
However, at the individual level, deviations from population expectations for regional gray matter volumes were “highly heterogeneous,” affecting the same area in less than 7% of people with the same diagnosis, they note. “This result means that it is difficult to pinpoint treatment targets or causal mechanisms by focusing on group averages alone,” Alex Fornito, PhD, of Monash University, who led the research team, said in a statement.
“It may also explain why people with the same diagnosis show wide variability in their symptom profiles and treatment outcomes,” Dr. Fornito added.
Yet, despite considerable heterogeneity at the regional level across different diagnoses, these deviations were embedded within common functional circuits and networks in up to 56% of cases.
The salience-ventral attention network, for example, which plays a central role in cognitive control, interoceptive awareness, and switching between internally and externally focused attention, was implicated across diagnoses, with other neural networks selectively involved in depression, bipolar disorder, schizophrenia, and ADHD.
The researchers say the approach they developed opens new opportunities for mapping brain changes in mental illness.
“The framework we have developed allows us to understand the diversity of brain changes in people with mental illness at different levels, from individual regions through to more widespread brain circuits and networks, offering a deeper insight into how the brain is affected in individual people,” Dr. Fornito said in a statement.
The study had no commercial funding. Ms. Segal, Dr. Fornito, and Dr. Marquand report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large brain imaging study of adults with six different psychiatric illnesses shows that heterogeneity in regional gray matter volume deviations is a general feature of psychiatric illness, but that these regionally heterogeneous areas are often embedded within common functional circuits and networks.
study investigator Ashlea Segal said in an email.
The findings also suggest that it’s “unlikely that a single cause or mechanism of a given disorder exists, and that a ‘one-size-fits-all’ approach to treatment is likely only appropriate for a small subset of individuals. In fact, one size doesn’t fit all. It probably doesn’t even fit most,” said Ms. Segal, a PhD candidate with the Turner Institute for Brain and Mental Health’s Neural Systems and Behaviour Lab at Monash University in Melbourne.
“Focusing on brain alterations at an individual level allows us to develop more personally tailored treatments,” Ms. Segal added.
Regional heterogeneity, the authors write, “thus offers a plausible explanation for the well-described clinical heterogeneity observed in psychiatric disorders, while circuit- and network-level aggregation of deviations is a putative neural substrate for phenotypic similarities between patients assigned the same diagnosis.”
The study was published online in Nature Neuroscience
Beyond group averages
For decades, researchers have mapped brain areas showing reduced gray matter volume (GMV) in people diagnosed with a variety of mental illnesses, but these maps have only been generated at the level of group averages, Ms. Segal explained.
“This means that we understand how the brains of people with, say, schizophrenia, differ from those without schizophrenia on average, but we can’t really say much about individual people,” Ms. Segal said.
For their study, the researchers used new statistical techniques developed by Andre Marquand, PhD, who co-led the project, to characterize the heterogeneity of GMV differences in 1,294 individuals diagnosed with one of six psychiatric conditions and 1,465 matched controls. Dr. Marquand is affiliated with the Donders Institute for Brain, Cognition, and Behavior in Nijmegen, the Netherlands.
These techniques “allow us to benchmark the size of over 1,000 different brain regions in any given person relative to what we should expect to see in the general population. In this way, we can identify, for any person, brain regions showing unusually small or large volumes, given that person’s age and sex,” Ms. Segal told this news organization.
The clinical sample included 202 individuals with autism spectrum disorder, 153 with attention-deficit/hyperactivity disorder (ADHD), 228 with bipolar disorder, 161 with major depressive disorder, 167 with obsessive-compulsive disorder, and 383 individuals with schizophrenia.
Confirming earlier findings, those with psychiatric illness showed more GMV deviations than healthy controls, the researchers found.
However, at the individual level, deviations from population expectations for regional gray matter volumes were “highly heterogeneous,” affecting the same area in less than 7% of people with the same diagnosis, they note. “This result means that it is difficult to pinpoint treatment targets or causal mechanisms by focusing on group averages alone,” Alex Fornito, PhD, of Monash University, who led the research team, said in a statement.
“It may also explain why people with the same diagnosis show wide variability in their symptom profiles and treatment outcomes,” Dr. Fornito added.
Yet, despite considerable heterogeneity at the regional level across different diagnoses, these deviations were embedded within common functional circuits and networks in up to 56% of cases.
The salience-ventral attention network, for example, which plays a central role in cognitive control, interoceptive awareness, and switching between internally and externally focused attention, was implicated across diagnoses, with other neural networks selectively involved in depression, bipolar disorder, schizophrenia, and ADHD.
The researchers say the approach they developed opens new opportunities for mapping brain changes in mental illness.
“The framework we have developed allows us to understand the diversity of brain changes in people with mental illness at different levels, from individual regions through to more widespread brain circuits and networks, offering a deeper insight into how the brain is affected in individual people,” Dr. Fornito said in a statement.
The study had no commercial funding. Ms. Segal, Dr. Fornito, and Dr. Marquand report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large brain imaging study of adults with six different psychiatric illnesses shows that heterogeneity in regional gray matter volume deviations is a general feature of psychiatric illness, but that these regionally heterogeneous areas are often embedded within common functional circuits and networks.
study investigator Ashlea Segal said in an email.
The findings also suggest that it’s “unlikely that a single cause or mechanism of a given disorder exists, and that a ‘one-size-fits-all’ approach to treatment is likely only appropriate for a small subset of individuals. In fact, one size doesn’t fit all. It probably doesn’t even fit most,” said Ms. Segal, a PhD candidate with the Turner Institute for Brain and Mental Health’s Neural Systems and Behaviour Lab at Monash University in Melbourne.
“Focusing on brain alterations at an individual level allows us to develop more personally tailored treatments,” Ms. Segal added.
Regional heterogeneity, the authors write, “thus offers a plausible explanation for the well-described clinical heterogeneity observed in psychiatric disorders, while circuit- and network-level aggregation of deviations is a putative neural substrate for phenotypic similarities between patients assigned the same diagnosis.”
The study was published online in Nature Neuroscience
Beyond group averages
For decades, researchers have mapped brain areas showing reduced gray matter volume (GMV) in people diagnosed with a variety of mental illnesses, but these maps have only been generated at the level of group averages, Ms. Segal explained.
“This means that we understand how the brains of people with, say, schizophrenia, differ from those without schizophrenia on average, but we can’t really say much about individual people,” Ms. Segal said.
For their study, the researchers used new statistical techniques developed by Andre Marquand, PhD, who co-led the project, to characterize the heterogeneity of GMV differences in 1,294 individuals diagnosed with one of six psychiatric conditions and 1,465 matched controls. Dr. Marquand is affiliated with the Donders Institute for Brain, Cognition, and Behavior in Nijmegen, the Netherlands.
These techniques “allow us to benchmark the size of over 1,000 different brain regions in any given person relative to what we should expect to see in the general population. In this way, we can identify, for any person, brain regions showing unusually small or large volumes, given that person’s age and sex,” Ms. Segal told this news organization.
The clinical sample included 202 individuals with autism spectrum disorder, 153 with attention-deficit/hyperactivity disorder (ADHD), 228 with bipolar disorder, 161 with major depressive disorder, 167 with obsessive-compulsive disorder, and 383 individuals with schizophrenia.
Confirming earlier findings, those with psychiatric illness showed more GMV deviations than healthy controls, the researchers found.
However, at the individual level, deviations from population expectations for regional gray matter volumes were “highly heterogeneous,” affecting the same area in less than 7% of people with the same diagnosis, they note. “This result means that it is difficult to pinpoint treatment targets or causal mechanisms by focusing on group averages alone,” Alex Fornito, PhD, of Monash University, who led the research team, said in a statement.
“It may also explain why people with the same diagnosis show wide variability in their symptom profiles and treatment outcomes,” Dr. Fornito added.
Yet, despite considerable heterogeneity at the regional level across different diagnoses, these deviations were embedded within common functional circuits and networks in up to 56% of cases.
The salience-ventral attention network, for example, which plays a central role in cognitive control, interoceptive awareness, and switching between internally and externally focused attention, was implicated across diagnoses, with other neural networks selectively involved in depression, bipolar disorder, schizophrenia, and ADHD.
The researchers say the approach they developed opens new opportunities for mapping brain changes in mental illness.
“The framework we have developed allows us to understand the diversity of brain changes in people with mental illness at different levels, from individual regions through to more widespread brain circuits and networks, offering a deeper insight into how the brain is affected in individual people,” Dr. Fornito said in a statement.
The study had no commercial funding. Ms. Segal, Dr. Fornito, and Dr. Marquand report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE NEUROSCIENCE
Controversial issue of maintenance therapy for bipolar depression still unresolved
Continuing antidepressant therapy for 52 weeks, as opposed to stopping it at 8 weeks, was not more beneficial with regard to the primary outcome of occurrence of any mood episode.
However, a prespecified sensitivity analysis of the primary outcome and of the secondary analyses suggests that continuing antidepressant therapy for 52 weeks may prolong the time to a depressive relapse.
“Because the primary outcome is negative and the prespecified sensitivity analysis is positive and the secondary outcomes are positive, some clinicians will pick the position that they work and some that they don’t work,” lead investigator Lakshmi Yatham, MBBS, with University of British Columbia, Vancouver, told this news organization.
The study was published online in the New England Journal of Medicine.
Controversial issue
Adjunctive antidepressant therapy – alongside mood stabilizers and/or second-generation antipsychotic medications – are often used to treat acute depressive episodes in patients with bipolar I disorder.
Currently, the Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) advise discontinuing antidepressant treatment 8 weeks after remission of depression.
Yet, the duration of antidepressant therapy for bipolar depression is “highly controversial,” due to a lack of evidence and concerns that antidepressants may induce mania, mixed states, or rapid cycling between mania and depression, Dr. Yatham said.
Dr. Yatham and colleagues assessed the safety and efficacy of continuing adjunctive antidepressant treatment (escitalopram or bupropion XL) for 52 weeks after remission, compared with discontinuing antidepressant therapy at 8 weeks after remission.
The final analysis included 177 patients (mean age 41 years, 48% men) with bipolar I disorder who had remission of depression; 90 patients continued treatment with an antidepressant for 52 weeks and 87 were switched to placebo at 8 weeks. All were taking a mood stabilizer or a second-generation antipsychotic or both.
The primary outcome, assessed in a time-to-event analysis, was any mood episode, as defined by scores on scales measuring symptoms of hypomania or mania, depression, suicidality, and mood-episode severity; additional treatment or hospitalization for mood symptoms; or attempted or completed suicide.
At 52 weeks, 28 patients (31%) in the 52-week group had experienced any mood episode (primary outcome), compared with 40 patients (46%) in the 8-week group.
The primary outcome did not reach statistical significance (hazard ratio, 0.68; 95% confidence interval, 0.43-1.10; P = .12).
The researchers note that the decision by the study team to include relapses that occurred during the first 6 weeks of the study may have affected the primary outcome.
“During the first 6 weeks, both groups were getting the same treatment, and we thought there shouldn’t be any difference in relapse, but sadly, there were more relapses in the 52-week group even though the treatments were identical,” Dr. Yatham said.
However, in a sensitivity analysis of the primary outcome after week 6, when treatment between the two groups differed, patients continuing antidepressant treatment were 40% less likely to experience a relapse of any mood event (HR, 0.60) and 59% less likely to experience a depressive episode (HR, 0.41) relative to the placebo group.
“From the point where the two groups began receiving different treatments, we see a significant benefit for patients who continued treatment with antidepressants,” Dr. Yatham said in a news release.
“Treating depression in bipolar disorder is challenging. Reducing the risk of relapse is important because it can provide patients with a great deal of stability that ultimately lets them get back to the activities they enjoy and can greatly improve their quality of life,” he added.
Although fewer patients in the 52-week group than 8-week group had a depressive episode within 52 weeks (17% vs. 40%; HR, 0.43), more had a manic or hypomanic event (12% vs. 6%; HR, 2.28).
The estimated probability of remaining free of a depressive episode at 52 weeks was 72% in the 52-week group versus 53% in the 8-week group. The estimated probability of remaining free of a manic episode at 52 weeks was 81% and 92%, respectively.
The incidence of adverse events was similar in the two groups, with a low rate of discontinuation due to adverse events and no serious adverse events. Clinically significant weight gain (≥ 7% increase in body weight) was observed in 14% of patients in the 52-week group and 7% of patients in the 8-week group.
Limitations of the trial include the fact that it was stopped early, before the planned sample size was reached, owing to slow recruitment and funding issues.
Other limitations include a lack of ethnic diversity (only 12% were White and < 1% Black) and overrepresentation of patients from India, which may limit generalizability.
In addition, the findings may not be applicable to treatment with antidepressants other than escitalopram and buproprion XL. Finally, the study population was also enriched for patients who responded to these antidepressants.
Need for an individualized approach
Commenting on the study, Roger McIntyre, MD, professor of psychiatry in pharmacology, University of Toronto, noted the study was not easy to conduct, and the investigators should be credited for conducting a maintenance study in bipolar depression.
“Although the study reports, as it should, that there is no evidence of maintenance effect, the secondary analysis, which was not adjusted for multiplicity, does suggest that there is a benefit,” said Dr. McIntyre, who was not associated with this research.
“However, the authors are also correct in stating that one cannot draw a conclusion because it was not the primary question and was not adjusted for multiplicity,” he added.
“If anything,” said Dr. McIntyre, “what these results do support is the notion that antidepressants are unlikely to destabilize all patients. Instead, the risk of destabilization seems to be largely limited to some persons, and there is a suggestion, based on the secondary outcome of this study, that maintenance antidepressant benefits can be seen in some people. But again that’s a testable hypothesis.”
Also weighing in on the research, Madhukar H. Trivedi, MD, professor of psychiatry and director, Center for Depression Research and Clinical Care, University of Texas Southwestern Medical Center, Dallas, said the study is “interesting,” adding that it was “unfortunate that the researchers had to curtail recruitment and reduce the size of the trial.”
“But the main finding is indeed that there was no significant advantage with 52 [weeks] continuation, except maybe increasing time to relapse. There are indeed a number of interesting findings in the secondary analyses, but sample size may have limited certainty,” Dr. Trivedi said.
“It seems that the results would not suggest a change in the current guidelines, and yet, we have to also mention that, for now, one has to make individual decisions and maybe recommend a more definitive complete trial,” added Dr. Trivedi, who was not involved in the study.
The study was supported by the Canadian Institutes of Health Research. Bausch Health (formerly Valeant), Lundbeck, and Lupin provided trial medications but were not involved in the design or conduct of the trial, data collection or analyses, writing of the manuscript, or decision to submit the manuscript for publication. Disclosures for authors are available at the conclusion of the original article.
A version of this article first appeared on Medscape.com.
Continuing antidepressant therapy for 52 weeks, as opposed to stopping it at 8 weeks, was not more beneficial with regard to the primary outcome of occurrence of any mood episode.
However, a prespecified sensitivity analysis of the primary outcome and of the secondary analyses suggests that continuing antidepressant therapy for 52 weeks may prolong the time to a depressive relapse.
“Because the primary outcome is negative and the prespecified sensitivity analysis is positive and the secondary outcomes are positive, some clinicians will pick the position that they work and some that they don’t work,” lead investigator Lakshmi Yatham, MBBS, with University of British Columbia, Vancouver, told this news organization.
The study was published online in the New England Journal of Medicine.
Controversial issue
Adjunctive antidepressant therapy – alongside mood stabilizers and/or second-generation antipsychotic medications – are often used to treat acute depressive episodes in patients with bipolar I disorder.
Currently, the Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) advise discontinuing antidepressant treatment 8 weeks after remission of depression.
Yet, the duration of antidepressant therapy for bipolar depression is “highly controversial,” due to a lack of evidence and concerns that antidepressants may induce mania, mixed states, or rapid cycling between mania and depression, Dr. Yatham said.
Dr. Yatham and colleagues assessed the safety and efficacy of continuing adjunctive antidepressant treatment (escitalopram or bupropion XL) for 52 weeks after remission, compared with discontinuing antidepressant therapy at 8 weeks after remission.
The final analysis included 177 patients (mean age 41 years, 48% men) with bipolar I disorder who had remission of depression; 90 patients continued treatment with an antidepressant for 52 weeks and 87 were switched to placebo at 8 weeks. All were taking a mood stabilizer or a second-generation antipsychotic or both.
The primary outcome, assessed in a time-to-event analysis, was any mood episode, as defined by scores on scales measuring symptoms of hypomania or mania, depression, suicidality, and mood-episode severity; additional treatment or hospitalization for mood symptoms; or attempted or completed suicide.
At 52 weeks, 28 patients (31%) in the 52-week group had experienced any mood episode (primary outcome), compared with 40 patients (46%) in the 8-week group.
The primary outcome did not reach statistical significance (hazard ratio, 0.68; 95% confidence interval, 0.43-1.10; P = .12).
The researchers note that the decision by the study team to include relapses that occurred during the first 6 weeks of the study may have affected the primary outcome.
“During the first 6 weeks, both groups were getting the same treatment, and we thought there shouldn’t be any difference in relapse, but sadly, there were more relapses in the 52-week group even though the treatments were identical,” Dr. Yatham said.
However, in a sensitivity analysis of the primary outcome after week 6, when treatment between the two groups differed, patients continuing antidepressant treatment were 40% less likely to experience a relapse of any mood event (HR, 0.60) and 59% less likely to experience a depressive episode (HR, 0.41) relative to the placebo group.
“From the point where the two groups began receiving different treatments, we see a significant benefit for patients who continued treatment with antidepressants,” Dr. Yatham said in a news release.
“Treating depression in bipolar disorder is challenging. Reducing the risk of relapse is important because it can provide patients with a great deal of stability that ultimately lets them get back to the activities they enjoy and can greatly improve their quality of life,” he added.
Although fewer patients in the 52-week group than 8-week group had a depressive episode within 52 weeks (17% vs. 40%; HR, 0.43), more had a manic or hypomanic event (12% vs. 6%; HR, 2.28).
The estimated probability of remaining free of a depressive episode at 52 weeks was 72% in the 52-week group versus 53% in the 8-week group. The estimated probability of remaining free of a manic episode at 52 weeks was 81% and 92%, respectively.
The incidence of adverse events was similar in the two groups, with a low rate of discontinuation due to adverse events and no serious adverse events. Clinically significant weight gain (≥ 7% increase in body weight) was observed in 14% of patients in the 52-week group and 7% of patients in the 8-week group.
Limitations of the trial include the fact that it was stopped early, before the planned sample size was reached, owing to slow recruitment and funding issues.
Other limitations include a lack of ethnic diversity (only 12% were White and < 1% Black) and overrepresentation of patients from India, which may limit generalizability.
In addition, the findings may not be applicable to treatment with antidepressants other than escitalopram and buproprion XL. Finally, the study population was also enriched for patients who responded to these antidepressants.
Need for an individualized approach
Commenting on the study, Roger McIntyre, MD, professor of psychiatry in pharmacology, University of Toronto, noted the study was not easy to conduct, and the investigators should be credited for conducting a maintenance study in bipolar depression.
“Although the study reports, as it should, that there is no evidence of maintenance effect, the secondary analysis, which was not adjusted for multiplicity, does suggest that there is a benefit,” said Dr. McIntyre, who was not associated with this research.
“However, the authors are also correct in stating that one cannot draw a conclusion because it was not the primary question and was not adjusted for multiplicity,” he added.
“If anything,” said Dr. McIntyre, “what these results do support is the notion that antidepressants are unlikely to destabilize all patients. Instead, the risk of destabilization seems to be largely limited to some persons, and there is a suggestion, based on the secondary outcome of this study, that maintenance antidepressant benefits can be seen in some people. But again that’s a testable hypothesis.”
Also weighing in on the research, Madhukar H. Trivedi, MD, professor of psychiatry and director, Center for Depression Research and Clinical Care, University of Texas Southwestern Medical Center, Dallas, said the study is “interesting,” adding that it was “unfortunate that the researchers had to curtail recruitment and reduce the size of the trial.”
“But the main finding is indeed that there was no significant advantage with 52 [weeks] continuation, except maybe increasing time to relapse. There are indeed a number of interesting findings in the secondary analyses, but sample size may have limited certainty,” Dr. Trivedi said.
“It seems that the results would not suggest a change in the current guidelines, and yet, we have to also mention that, for now, one has to make individual decisions and maybe recommend a more definitive complete trial,” added Dr. Trivedi, who was not involved in the study.
The study was supported by the Canadian Institutes of Health Research. Bausch Health (formerly Valeant), Lundbeck, and Lupin provided trial medications but were not involved in the design or conduct of the trial, data collection or analyses, writing of the manuscript, or decision to submit the manuscript for publication. Disclosures for authors are available at the conclusion of the original article.
A version of this article first appeared on Medscape.com.
Continuing antidepressant therapy for 52 weeks, as opposed to stopping it at 8 weeks, was not more beneficial with regard to the primary outcome of occurrence of any mood episode.
However, a prespecified sensitivity analysis of the primary outcome and of the secondary analyses suggests that continuing antidepressant therapy for 52 weeks may prolong the time to a depressive relapse.
“Because the primary outcome is negative and the prespecified sensitivity analysis is positive and the secondary outcomes are positive, some clinicians will pick the position that they work and some that they don’t work,” lead investigator Lakshmi Yatham, MBBS, with University of British Columbia, Vancouver, told this news organization.
The study was published online in the New England Journal of Medicine.
Controversial issue
Adjunctive antidepressant therapy – alongside mood stabilizers and/or second-generation antipsychotic medications – are often used to treat acute depressive episodes in patients with bipolar I disorder.
Currently, the Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) advise discontinuing antidepressant treatment 8 weeks after remission of depression.
Yet, the duration of antidepressant therapy for bipolar depression is “highly controversial,” due to a lack of evidence and concerns that antidepressants may induce mania, mixed states, or rapid cycling between mania and depression, Dr. Yatham said.
Dr. Yatham and colleagues assessed the safety and efficacy of continuing adjunctive antidepressant treatment (escitalopram or bupropion XL) for 52 weeks after remission, compared with discontinuing antidepressant therapy at 8 weeks after remission.
The final analysis included 177 patients (mean age 41 years, 48% men) with bipolar I disorder who had remission of depression; 90 patients continued treatment with an antidepressant for 52 weeks and 87 were switched to placebo at 8 weeks. All were taking a mood stabilizer or a second-generation antipsychotic or both.
The primary outcome, assessed in a time-to-event analysis, was any mood episode, as defined by scores on scales measuring symptoms of hypomania or mania, depression, suicidality, and mood-episode severity; additional treatment or hospitalization for mood symptoms; or attempted or completed suicide.
At 52 weeks, 28 patients (31%) in the 52-week group had experienced any mood episode (primary outcome), compared with 40 patients (46%) in the 8-week group.
The primary outcome did not reach statistical significance (hazard ratio, 0.68; 95% confidence interval, 0.43-1.10; P = .12).
The researchers note that the decision by the study team to include relapses that occurred during the first 6 weeks of the study may have affected the primary outcome.
“During the first 6 weeks, both groups were getting the same treatment, and we thought there shouldn’t be any difference in relapse, but sadly, there were more relapses in the 52-week group even though the treatments were identical,” Dr. Yatham said.
However, in a sensitivity analysis of the primary outcome after week 6, when treatment between the two groups differed, patients continuing antidepressant treatment were 40% less likely to experience a relapse of any mood event (HR, 0.60) and 59% less likely to experience a depressive episode (HR, 0.41) relative to the placebo group.
“From the point where the two groups began receiving different treatments, we see a significant benefit for patients who continued treatment with antidepressants,” Dr. Yatham said in a news release.
“Treating depression in bipolar disorder is challenging. Reducing the risk of relapse is important because it can provide patients with a great deal of stability that ultimately lets them get back to the activities they enjoy and can greatly improve their quality of life,” he added.
Although fewer patients in the 52-week group than 8-week group had a depressive episode within 52 weeks (17% vs. 40%; HR, 0.43), more had a manic or hypomanic event (12% vs. 6%; HR, 2.28).
The estimated probability of remaining free of a depressive episode at 52 weeks was 72% in the 52-week group versus 53% in the 8-week group. The estimated probability of remaining free of a manic episode at 52 weeks was 81% and 92%, respectively.
The incidence of adverse events was similar in the two groups, with a low rate of discontinuation due to adverse events and no serious adverse events. Clinically significant weight gain (≥ 7% increase in body weight) was observed in 14% of patients in the 52-week group and 7% of patients in the 8-week group.
Limitations of the trial include the fact that it was stopped early, before the planned sample size was reached, owing to slow recruitment and funding issues.
Other limitations include a lack of ethnic diversity (only 12% were White and < 1% Black) and overrepresentation of patients from India, which may limit generalizability.
In addition, the findings may not be applicable to treatment with antidepressants other than escitalopram and buproprion XL. Finally, the study population was also enriched for patients who responded to these antidepressants.
Need for an individualized approach
Commenting on the study, Roger McIntyre, MD, professor of psychiatry in pharmacology, University of Toronto, noted the study was not easy to conduct, and the investigators should be credited for conducting a maintenance study in bipolar depression.
“Although the study reports, as it should, that there is no evidence of maintenance effect, the secondary analysis, which was not adjusted for multiplicity, does suggest that there is a benefit,” said Dr. McIntyre, who was not associated with this research.
“However, the authors are also correct in stating that one cannot draw a conclusion because it was not the primary question and was not adjusted for multiplicity,” he added.
“If anything,” said Dr. McIntyre, “what these results do support is the notion that antidepressants are unlikely to destabilize all patients. Instead, the risk of destabilization seems to be largely limited to some persons, and there is a suggestion, based on the secondary outcome of this study, that maintenance antidepressant benefits can be seen in some people. But again that’s a testable hypothesis.”
Also weighing in on the research, Madhukar H. Trivedi, MD, professor of psychiatry and director, Center for Depression Research and Clinical Care, University of Texas Southwestern Medical Center, Dallas, said the study is “interesting,” adding that it was “unfortunate that the researchers had to curtail recruitment and reduce the size of the trial.”
“But the main finding is indeed that there was no significant advantage with 52 [weeks] continuation, except maybe increasing time to relapse. There are indeed a number of interesting findings in the secondary analyses, but sample size may have limited certainty,” Dr. Trivedi said.
“It seems that the results would not suggest a change in the current guidelines, and yet, we have to also mention that, for now, one has to make individual decisions and maybe recommend a more definitive complete trial,” added Dr. Trivedi, who was not involved in the study.
The study was supported by the Canadian Institutes of Health Research. Bausch Health (formerly Valeant), Lundbeck, and Lupin provided trial medications but were not involved in the design or conduct of the trial, data collection or analyses, writing of the manuscript, or decision to submit the manuscript for publication. Disclosures for authors are available at the conclusion of the original article.
A version of this article first appeared on Medscape.com.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Ancestry may predict bipolar patients’ response to lithium
Lithium remains the first-line treatment for BPD, but clinical improvement occurs in less than one-third of patients, and factors that might affect response, especially genetic factors, have not been well studied, wrote Ana M. Díaz-Zuluaga, MD, of University of Antioquia, Medellín, Colombia, and colleagues.
Previous genetic research identified four linked single nucleotide polymorphisms (SNPs) in a single locus on chromosome 21 that were associated with lithium response, but the study was limited to individuals with European and Asian ancestry, the researchers said.
In a study published in the Journal of Affective Disorders, the researchers identified 172 adults aged 18 and older with a diagnosis of BPD I or II based on the DSM-IV-TR criteria. Participants had been taking lithium continuously for at least 6 months. Lithium response was defined using the Retrospective Criteria of Long-Term Treatment Response in Research Subjects with BD, also known as the Alda scale. Total Alda scale scores of 7 or higher indicated a responder phenotype; scores less than 7 were considered nonresponders.
Ancestry was determined using DNA samples and the software Structure Version 2.2, and participants were classified as Amerindian, African, or European.
The overall response rate to lithium was 15.11% (26 of 172 patients). In a univariate analysis, no significant differences emerged between responders and nonresponders in demographics or clinical characteristics. However, patients responsive to lithium were significantly less likely of African ancestry, compared with nonresponders (0.1 vs. 0.2, P = .005) and more likely of European ancestry (0.5 vs. 0.3, P = .024), and had fewer depressive episodes (2 vs. 3.9, P = .002). The difference in responders vs. nonresponders of Amerindian ancestry was not statistically significant (0.4 vs. 0.5, P = .204).
The researchers then used machine learning based on Advanced Recursive Partitioning Approaches (ARPAs) to create classification trees with and without ancestry components for predicting response to lithium. “Variable importance analysis shows that the most important predictor is the probability of Amerindian ancestry component, followed by the Amerindian and European ancestral components individual variances, and then by the African and European ancestry components,” the researchers wrote.
Without the ancestry component, the sensitivity and specificity for predicting a treatment response to lithium were 50% and 94.5% respectively, with an area under the curve of 72.2%.
“However, when ancestral components are included in the model, the sensitivity and specificity are 93 % and 84 %, respectively,” with an AUC of 89.2%, the researchers said.
Clinical predictors of treatment response included disease duration, number of depressive episodes, total number of affective episodes, and number of manic episodes.
The findings were limited by several factors including the cross-sectional design and potential impact of other psychotropic drugs, the researchers noted. A replication of the study in an independent dataset is needed to validate the findings, they said.
However, the study is the first known to explore the effect of ancestry on bipolar patients’ response to lithium, and suggests that ancestry components have potential predictive value in the clinical setting that could support a more personalized approach to treatment, the researchers said.
The study was supported by PRISMA U.T., Colciencias, Invitaci
Lithium remains the first-line treatment for BPD, but clinical improvement occurs in less than one-third of patients, and factors that might affect response, especially genetic factors, have not been well studied, wrote Ana M. Díaz-Zuluaga, MD, of University of Antioquia, Medellín, Colombia, and colleagues.
Previous genetic research identified four linked single nucleotide polymorphisms (SNPs) in a single locus on chromosome 21 that were associated with lithium response, but the study was limited to individuals with European and Asian ancestry, the researchers said.
In a study published in the Journal of Affective Disorders, the researchers identified 172 adults aged 18 and older with a diagnosis of BPD I or II based on the DSM-IV-TR criteria. Participants had been taking lithium continuously for at least 6 months. Lithium response was defined using the Retrospective Criteria of Long-Term Treatment Response in Research Subjects with BD, also known as the Alda scale. Total Alda scale scores of 7 or higher indicated a responder phenotype; scores less than 7 were considered nonresponders.
Ancestry was determined using DNA samples and the software Structure Version 2.2, and participants were classified as Amerindian, African, or European.
The overall response rate to lithium was 15.11% (26 of 172 patients). In a univariate analysis, no significant differences emerged between responders and nonresponders in demographics or clinical characteristics. However, patients responsive to lithium were significantly less likely of African ancestry, compared with nonresponders (0.1 vs. 0.2, P = .005) and more likely of European ancestry (0.5 vs. 0.3, P = .024), and had fewer depressive episodes (2 vs. 3.9, P = .002). The difference in responders vs. nonresponders of Amerindian ancestry was not statistically significant (0.4 vs. 0.5, P = .204).
The researchers then used machine learning based on Advanced Recursive Partitioning Approaches (ARPAs) to create classification trees with and without ancestry components for predicting response to lithium. “Variable importance analysis shows that the most important predictor is the probability of Amerindian ancestry component, followed by the Amerindian and European ancestral components individual variances, and then by the African and European ancestry components,” the researchers wrote.
Without the ancestry component, the sensitivity and specificity for predicting a treatment response to lithium were 50% and 94.5% respectively, with an area under the curve of 72.2%.
“However, when ancestral components are included in the model, the sensitivity and specificity are 93 % and 84 %, respectively,” with an AUC of 89.2%, the researchers said.
Clinical predictors of treatment response included disease duration, number of depressive episodes, total number of affective episodes, and number of manic episodes.
The findings were limited by several factors including the cross-sectional design and potential impact of other psychotropic drugs, the researchers noted. A replication of the study in an independent dataset is needed to validate the findings, they said.
However, the study is the first known to explore the effect of ancestry on bipolar patients’ response to lithium, and suggests that ancestry components have potential predictive value in the clinical setting that could support a more personalized approach to treatment, the researchers said.
The study was supported by PRISMA U.T., Colciencias, Invitaci
Lithium remains the first-line treatment for BPD, but clinical improvement occurs in less than one-third of patients, and factors that might affect response, especially genetic factors, have not been well studied, wrote Ana M. Díaz-Zuluaga, MD, of University of Antioquia, Medellín, Colombia, and colleagues.
Previous genetic research identified four linked single nucleotide polymorphisms (SNPs) in a single locus on chromosome 21 that were associated with lithium response, but the study was limited to individuals with European and Asian ancestry, the researchers said.
In a study published in the Journal of Affective Disorders, the researchers identified 172 adults aged 18 and older with a diagnosis of BPD I or II based on the DSM-IV-TR criteria. Participants had been taking lithium continuously for at least 6 months. Lithium response was defined using the Retrospective Criteria of Long-Term Treatment Response in Research Subjects with BD, also known as the Alda scale. Total Alda scale scores of 7 or higher indicated a responder phenotype; scores less than 7 were considered nonresponders.
Ancestry was determined using DNA samples and the software Structure Version 2.2, and participants were classified as Amerindian, African, or European.
The overall response rate to lithium was 15.11% (26 of 172 patients). In a univariate analysis, no significant differences emerged between responders and nonresponders in demographics or clinical characteristics. However, patients responsive to lithium were significantly less likely of African ancestry, compared with nonresponders (0.1 vs. 0.2, P = .005) and more likely of European ancestry (0.5 vs. 0.3, P = .024), and had fewer depressive episodes (2 vs. 3.9, P = .002). The difference in responders vs. nonresponders of Amerindian ancestry was not statistically significant (0.4 vs. 0.5, P = .204).
The researchers then used machine learning based on Advanced Recursive Partitioning Approaches (ARPAs) to create classification trees with and without ancestry components for predicting response to lithium. “Variable importance analysis shows that the most important predictor is the probability of Amerindian ancestry component, followed by the Amerindian and European ancestral components individual variances, and then by the African and European ancestry components,” the researchers wrote.
Without the ancestry component, the sensitivity and specificity for predicting a treatment response to lithium were 50% and 94.5% respectively, with an area under the curve of 72.2%.
“However, when ancestral components are included in the model, the sensitivity and specificity are 93 % and 84 %, respectively,” with an AUC of 89.2%, the researchers said.
Clinical predictors of treatment response included disease duration, number of depressive episodes, total number of affective episodes, and number of manic episodes.
The findings were limited by several factors including the cross-sectional design and potential impact of other psychotropic drugs, the researchers noted. A replication of the study in an independent dataset is needed to validate the findings, they said.
However, the study is the first known to explore the effect of ancestry on bipolar patients’ response to lithium, and suggests that ancestry components have potential predictive value in the clinical setting that could support a more personalized approach to treatment, the researchers said.
The study was supported by PRISMA U.T., Colciencias, Invitaci
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Bipolar disorder tied to a sixfold increased risk of early death
In addition, patients with BD are three times more likely to die prematurely of all causes, compared with the general population, with alcohol-related diseases contributing to more premature deaths than cardiovascular disease (CVD), diabetes, and cancer.
The study results emphasize the need for personalized approaches to risk prediction and prevention of premature cause-specific mortality over the life-course of individuals with BD, lead investigator Tapio Paljärvi, PhD, an epidemiologist at Niuvanniemi Hospital in Kuopio, Finland, told this news organization.
The findings were published online in BMJ Mental Health.
Alcohol a major contributor to early death
A number of studies have established that those with BD have twice the risk of dying prematurely, compared with those without the disorder.
To learn more about the factors contributing to early death in this patient population, the investigators analyzed data from nationwide Finnish medical and insurance registries. They identified and tracked the health of 47,000 patients, aged 15-64 years, with BD between 2004 and 2018.
The average age at the beginning of the monitoring period was 38 years, and 57% of the cohort were women.
To determine the excess deaths directly attributable to BD, the researchers compared the ratio of deaths observed over the monitoring period in those with BD to the number expected to die in the general population, also known as the standard mortality ratio.
Of the group with BD, 3,300 died during the monitoring period. The average age at death was 50, and almost two-thirds (65%, or 2,137) of those who died were men.
Investigators grouped excess deaths in BD patients into two categories – somatic and external.
Of those with BD who died from somatic or disease-related causes, alcohol caused the highest rate of death (29%). The second-leading cause was heart disease and stroke (27%), followed by cancer (22%), respiratory diseases (4%), and diabetes (2%).
Among the 595 patients with BD who died because of alcohol consumption, liver disease was the leading cause of death (48%). The second cause was accidental alcohol poisoning (28%), followed by alcohol dependence (10%).
The leading cause of death from external causes in BD patients was suicide (58%, or 740), nearly half of which (48%) were from an overdose with prescribed psychotropic medications.
Overall, 64%, or 2,104, of the deaths in BD patients from any cause were considered excess deaths, that is, the number of deaths above those expected for those without BD of comparable age and sex.
Most of the excess deaths from somatic illness were either from alcohol-related causes (40%) – a rate three times higher than that of the general population – CVD (26%), or cancer (10%).
High suicide rate
When the team examined excess deaths from external causes, they found that 61% (651) were attributable to suicide, a rate eight times higher than that of the general population.
“In terms of absolute numbers, somatic causes of death represented the majority of all deaths in BD, as also reported in previous research,” Dr. Paljärvi said.
“However, this finding reflects the fact that in many high-income countries most of the deaths are due to somatic causes; with CVD, cancers, and diseases of the nervous system as the leading causes of death in the older age groups,” he added.
Dr. Paljärvi advised that clinicians treating patients with BD balance therapeutic response with potentially serious long-term medication side effects, to prevent premature deaths.
A stronger emphasis on identifying and treating comorbid substance abuse is also warranted, he noted.
Dr. Paljärvi noted that the underlying causes of the excess somatic mortality in people with BD are not fully understood, but may result from the “complex interaction between various established risk factors, including tobacco use, alcohol abuse, physical inactivity, unhealthy diet, obesity, hypertension, etc.”
Regarding the generalizability of the findings, he said many previous studies have been based only on inpatient data and noted that the current study included individuals from various sources including inpatient and outpatient registries as well as social insurance registries.
“While the reported excess all-cause mortality rates are strikingly similar across populations globally, there is a paucity of more detailed cause-specific analyses of excess mortality in BD,” said Dr. Paljärvi, adding that these findings should be replicated in other countries, including the United States.
Chronic inflammation
Commenting on the findings, Benjamin Goldstein, MD, PhD, professor of psychiatry and pharmacology at the University of Toronto, noted that there are clear disparities in access to, and quality of care among, patients with BD and other serious mental illnesses.
“Taking heart disease as an example, disparities exist at virtually every point of contact, ranging from the point of preventive care to the time it takes to be assessed in the ER, to the likelihood of receiving cardiac catheterization, to the quality of postdischarge care,” said Dr. Goldstein.
He also noted that CVD occurs in patients with BD, on average, 10-15 years earlier than the general population. However, he added, “there is important evidence that when people with BD receive the same standard of care as those without BD their cardiovascular outcomes are similar.”
Dr. Goldstein also noted that inflammation, which is a driver of cardiovascular risk, is elevated among patients with BD, particularly during mania and depression.
“Given that the average person with BD has some degree of mood symptoms about 40% of the time, chronically elevated inflammation likely contributes in part to the excess risk of heart disease in bipolar disorder,” he said.
Dr. Goldstein’s team’s research focuses on microvessels. “We have found that microvessel function in both the heart and the brain, determined by MRI, is reduced among teens with BD,” he said.
His team has also found that endothelial function in fingertip microvessels, an indicator of future heart disease risk, varies according to mood states.
“Collectively, these findings suggest the microvascular problems may explain, in part, the extra risk of heart disease beyond traditional risk factors in BD,” he added.
The study was funded by a Wellcome Trust Senior Clinical Research Fellowship and by the Oxford Health Biomedical Research Centre. Dr. Paljärvi and Dr. Goldstein report no relevant financial relationships.
A version of this article appeared on Medscape.com.
In addition, patients with BD are three times more likely to die prematurely of all causes, compared with the general population, with alcohol-related diseases contributing to more premature deaths than cardiovascular disease (CVD), diabetes, and cancer.
The study results emphasize the need for personalized approaches to risk prediction and prevention of premature cause-specific mortality over the life-course of individuals with BD, lead investigator Tapio Paljärvi, PhD, an epidemiologist at Niuvanniemi Hospital in Kuopio, Finland, told this news organization.
The findings were published online in BMJ Mental Health.
Alcohol a major contributor to early death
A number of studies have established that those with BD have twice the risk of dying prematurely, compared with those without the disorder.
To learn more about the factors contributing to early death in this patient population, the investigators analyzed data from nationwide Finnish medical and insurance registries. They identified and tracked the health of 47,000 patients, aged 15-64 years, with BD between 2004 and 2018.
The average age at the beginning of the monitoring period was 38 years, and 57% of the cohort were women.
To determine the excess deaths directly attributable to BD, the researchers compared the ratio of deaths observed over the monitoring period in those with BD to the number expected to die in the general population, also known as the standard mortality ratio.
Of the group with BD, 3,300 died during the monitoring period. The average age at death was 50, and almost two-thirds (65%, or 2,137) of those who died were men.
Investigators grouped excess deaths in BD patients into two categories – somatic and external.
Of those with BD who died from somatic or disease-related causes, alcohol caused the highest rate of death (29%). The second-leading cause was heart disease and stroke (27%), followed by cancer (22%), respiratory diseases (4%), and diabetes (2%).
Among the 595 patients with BD who died because of alcohol consumption, liver disease was the leading cause of death (48%). The second cause was accidental alcohol poisoning (28%), followed by alcohol dependence (10%).
The leading cause of death from external causes in BD patients was suicide (58%, or 740), nearly half of which (48%) were from an overdose with prescribed psychotropic medications.
Overall, 64%, or 2,104, of the deaths in BD patients from any cause were considered excess deaths, that is, the number of deaths above those expected for those without BD of comparable age and sex.
Most of the excess deaths from somatic illness were either from alcohol-related causes (40%) – a rate three times higher than that of the general population – CVD (26%), or cancer (10%).
High suicide rate
When the team examined excess deaths from external causes, they found that 61% (651) were attributable to suicide, a rate eight times higher than that of the general population.
“In terms of absolute numbers, somatic causes of death represented the majority of all deaths in BD, as also reported in previous research,” Dr. Paljärvi said.
“However, this finding reflects the fact that in many high-income countries most of the deaths are due to somatic causes; with CVD, cancers, and diseases of the nervous system as the leading causes of death in the older age groups,” he added.
Dr. Paljärvi advised that clinicians treating patients with BD balance therapeutic response with potentially serious long-term medication side effects, to prevent premature deaths.
A stronger emphasis on identifying and treating comorbid substance abuse is also warranted, he noted.
Dr. Paljärvi noted that the underlying causes of the excess somatic mortality in people with BD are not fully understood, but may result from the “complex interaction between various established risk factors, including tobacco use, alcohol abuse, physical inactivity, unhealthy diet, obesity, hypertension, etc.”
Regarding the generalizability of the findings, he said many previous studies have been based only on inpatient data and noted that the current study included individuals from various sources including inpatient and outpatient registries as well as social insurance registries.
“While the reported excess all-cause mortality rates are strikingly similar across populations globally, there is a paucity of more detailed cause-specific analyses of excess mortality in BD,” said Dr. Paljärvi, adding that these findings should be replicated in other countries, including the United States.
Chronic inflammation
Commenting on the findings, Benjamin Goldstein, MD, PhD, professor of psychiatry and pharmacology at the University of Toronto, noted that there are clear disparities in access to, and quality of care among, patients with BD and other serious mental illnesses.
“Taking heart disease as an example, disparities exist at virtually every point of contact, ranging from the point of preventive care to the time it takes to be assessed in the ER, to the likelihood of receiving cardiac catheterization, to the quality of postdischarge care,” said Dr. Goldstein.
He also noted that CVD occurs in patients with BD, on average, 10-15 years earlier than the general population. However, he added, “there is important evidence that when people with BD receive the same standard of care as those without BD their cardiovascular outcomes are similar.”
Dr. Goldstein also noted that inflammation, which is a driver of cardiovascular risk, is elevated among patients with BD, particularly during mania and depression.
“Given that the average person with BD has some degree of mood symptoms about 40% of the time, chronically elevated inflammation likely contributes in part to the excess risk of heart disease in bipolar disorder,” he said.
Dr. Goldstein’s team’s research focuses on microvessels. “We have found that microvessel function in both the heart and the brain, determined by MRI, is reduced among teens with BD,” he said.
His team has also found that endothelial function in fingertip microvessels, an indicator of future heart disease risk, varies according to mood states.
“Collectively, these findings suggest the microvascular problems may explain, in part, the extra risk of heart disease beyond traditional risk factors in BD,” he added.
The study was funded by a Wellcome Trust Senior Clinical Research Fellowship and by the Oxford Health Biomedical Research Centre. Dr. Paljärvi and Dr. Goldstein report no relevant financial relationships.
A version of this article appeared on Medscape.com.
In addition, patients with BD are three times more likely to die prematurely of all causes, compared with the general population, with alcohol-related diseases contributing to more premature deaths than cardiovascular disease (CVD), diabetes, and cancer.
The study results emphasize the need for personalized approaches to risk prediction and prevention of premature cause-specific mortality over the life-course of individuals with BD, lead investigator Tapio Paljärvi, PhD, an epidemiologist at Niuvanniemi Hospital in Kuopio, Finland, told this news organization.
The findings were published online in BMJ Mental Health.
Alcohol a major contributor to early death
A number of studies have established that those with BD have twice the risk of dying prematurely, compared with those without the disorder.
To learn more about the factors contributing to early death in this patient population, the investigators analyzed data from nationwide Finnish medical and insurance registries. They identified and tracked the health of 47,000 patients, aged 15-64 years, with BD between 2004 and 2018.
The average age at the beginning of the monitoring period was 38 years, and 57% of the cohort were women.
To determine the excess deaths directly attributable to BD, the researchers compared the ratio of deaths observed over the monitoring period in those with BD to the number expected to die in the general population, also known as the standard mortality ratio.
Of the group with BD, 3,300 died during the monitoring period. The average age at death was 50, and almost two-thirds (65%, or 2,137) of those who died were men.
Investigators grouped excess deaths in BD patients into two categories – somatic and external.
Of those with BD who died from somatic or disease-related causes, alcohol caused the highest rate of death (29%). The second-leading cause was heart disease and stroke (27%), followed by cancer (22%), respiratory diseases (4%), and diabetes (2%).
Among the 595 patients with BD who died because of alcohol consumption, liver disease was the leading cause of death (48%). The second cause was accidental alcohol poisoning (28%), followed by alcohol dependence (10%).
The leading cause of death from external causes in BD patients was suicide (58%, or 740), nearly half of which (48%) were from an overdose with prescribed psychotropic medications.
Overall, 64%, or 2,104, of the deaths in BD patients from any cause were considered excess deaths, that is, the number of deaths above those expected for those without BD of comparable age and sex.
Most of the excess deaths from somatic illness were either from alcohol-related causes (40%) – a rate three times higher than that of the general population – CVD (26%), or cancer (10%).
High suicide rate
When the team examined excess deaths from external causes, they found that 61% (651) were attributable to suicide, a rate eight times higher than that of the general population.
“In terms of absolute numbers, somatic causes of death represented the majority of all deaths in BD, as also reported in previous research,” Dr. Paljärvi said.
“However, this finding reflects the fact that in many high-income countries most of the deaths are due to somatic causes; with CVD, cancers, and diseases of the nervous system as the leading causes of death in the older age groups,” he added.
Dr. Paljärvi advised that clinicians treating patients with BD balance therapeutic response with potentially serious long-term medication side effects, to prevent premature deaths.
A stronger emphasis on identifying and treating comorbid substance abuse is also warranted, he noted.
Dr. Paljärvi noted that the underlying causes of the excess somatic mortality in people with BD are not fully understood, but may result from the “complex interaction between various established risk factors, including tobacco use, alcohol abuse, physical inactivity, unhealthy diet, obesity, hypertension, etc.”
Regarding the generalizability of the findings, he said many previous studies have been based only on inpatient data and noted that the current study included individuals from various sources including inpatient and outpatient registries as well as social insurance registries.
“While the reported excess all-cause mortality rates are strikingly similar across populations globally, there is a paucity of more detailed cause-specific analyses of excess mortality in BD,” said Dr. Paljärvi, adding that these findings should be replicated in other countries, including the United States.
Chronic inflammation
Commenting on the findings, Benjamin Goldstein, MD, PhD, professor of psychiatry and pharmacology at the University of Toronto, noted that there are clear disparities in access to, and quality of care among, patients with BD and other serious mental illnesses.
“Taking heart disease as an example, disparities exist at virtually every point of contact, ranging from the point of preventive care to the time it takes to be assessed in the ER, to the likelihood of receiving cardiac catheterization, to the quality of postdischarge care,” said Dr. Goldstein.
He also noted that CVD occurs in patients with BD, on average, 10-15 years earlier than the general population. However, he added, “there is important evidence that when people with BD receive the same standard of care as those without BD their cardiovascular outcomes are similar.”
Dr. Goldstein also noted that inflammation, which is a driver of cardiovascular risk, is elevated among patients with BD, particularly during mania and depression.
“Given that the average person with BD has some degree of mood symptoms about 40% of the time, chronically elevated inflammation likely contributes in part to the excess risk of heart disease in bipolar disorder,” he said.
Dr. Goldstein’s team’s research focuses on microvessels. “We have found that microvessel function in both the heart and the brain, determined by MRI, is reduced among teens with BD,” he said.
His team has also found that endothelial function in fingertip microvessels, an indicator of future heart disease risk, varies according to mood states.
“Collectively, these findings suggest the microvascular problems may explain, in part, the extra risk of heart disease beyond traditional risk factors in BD,” he added.
The study was funded by a Wellcome Trust Senior Clinical Research Fellowship and by the Oxford Health Biomedical Research Centre. Dr. Paljärvi and Dr. Goldstein report no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM BMJ MENTAL HEALTH
Brain damage from recurrent relapses of bipolar mania: A call for early LAI use
Bipolar disorder (BD) is a psychotic mood disorder. Like schizophrenia, it has been shown to be associated with significant degeneration and structural brain abnormalities with multiple relapses.1,2
Just as I have always advocated preventing recurrences in schizophrenia by using long-acting injectable (LAI) antipsychotic formulations immediately after the first episode to prevent psychotic relapses and progressive brain damage,3 I strongly recommend using LAIs right after hospital discharge from the first manic episode. It is the most rational management approach for bipolar mania given the grave consequences of multiple episodes, which are so common in this psychotic mood disorder due to poor medication adherence.
In contrast to the depressive episodes of BD I, where patients have insight into their depression and seek psychiatric treatment, during a manic episode patients often have no insight (anosognosia) that they suffer from a serious brain disorder, and refuse treatment.4 In addition, young patients with BD I frequently discontinue their oral mood stabilizer or second-generation antipsychotic (which are approved for mania) because they miss the blissful euphoria and the buoyant physical and mental energy of their manic episodes. They are completely oblivious to (and uninformed about) the grave neurobiological damage of further manic episodes, which can condemn them to clinical, functional, and cognitive deterioration. These patients are also likely to become treatment-resistant, which has been labeled as “the malignant transformation of bipolar disorder.”5
The evidence for progressive brain tissue loss, clinical deterioration, functional decline, and treatment resistance is abundant.6 I was the lead investigator of the first study to report ventricular dilatation (which is a proxy for cortical atrophy) in bipolar mania,7 a discovery that was subsequently replicated by 2 dozen researchers. This was followed by numerous neuroimaging studies reporting a loss of volume across multiple brain regions, including the frontal lobe, temporal lobe, cerebellum, thalamus, hippocampus, and basal ganglia. BD is heterogeneous8 with 4 stages (Table 19), and patients experience progressively worse brain structure and function with each stage.
Many patients with bipolar mania end up with poor clinical and functional outcomes, even when they respond well to initial treatment with lithium, anticonvulsant mood stabilizers, or second-generation antipsychotics. With their intentional nonadherence to oral medications leading to multiple recurrent relapses, these patients are at serious risk for neuroprogression and brain atrophic changes driven by multiple factors: inflammatory cytokines, increased cortical steroids, decreased neurotrophins, deceased neurogenesis, increased oxidative stress, and mitochondrial energy dysfunction. The consequences include progressive shortening of the interval between episodes with every relapse and loss of responsiveness to pharmacotherapy as the illness progresses.6,10 Predictors of a downhill progression include genetic vulnerability, perinatal complication during fetal life, childhood trauma (physical, sexual, emotional, or neglect), substance use, stress, psychiatric/medial comorbidities, and especially the number of episodes.9,11
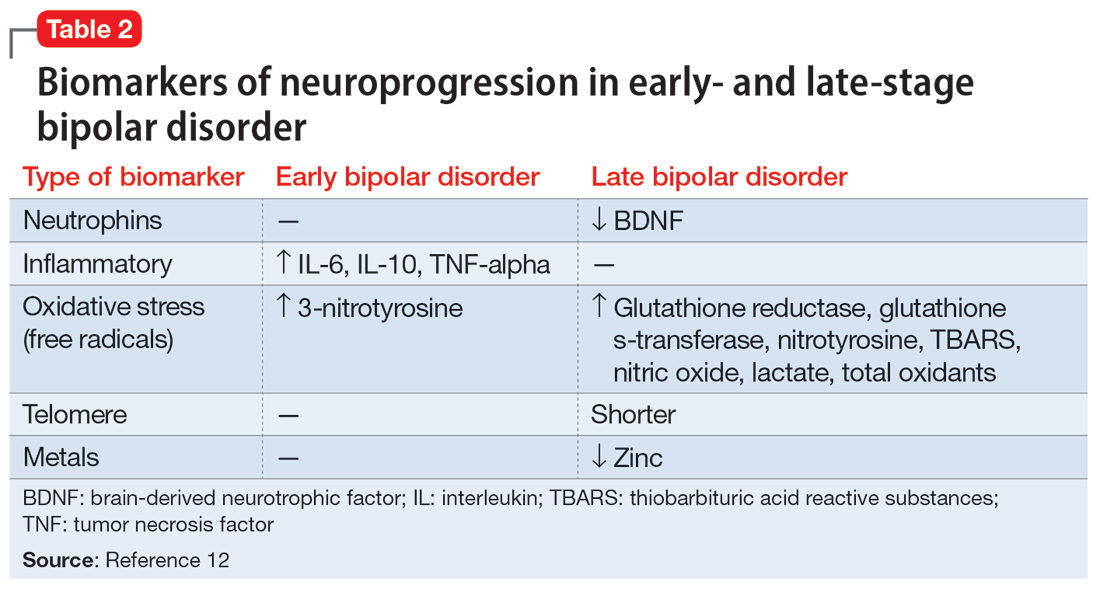
Biomarkers have been reported in both the early and late stages of BD (Table 212) as well as in postmortem studies (Table 38,13). They reflect the progressive neurodegenerative nature of recurrent BD I episodes as the disorder moves to the advanced stages. I summarize these stages in Table 19 and Table 212 for the benefit of psychiatric clinicians who do not have access to the neuroscience journals where such findings are usually published.
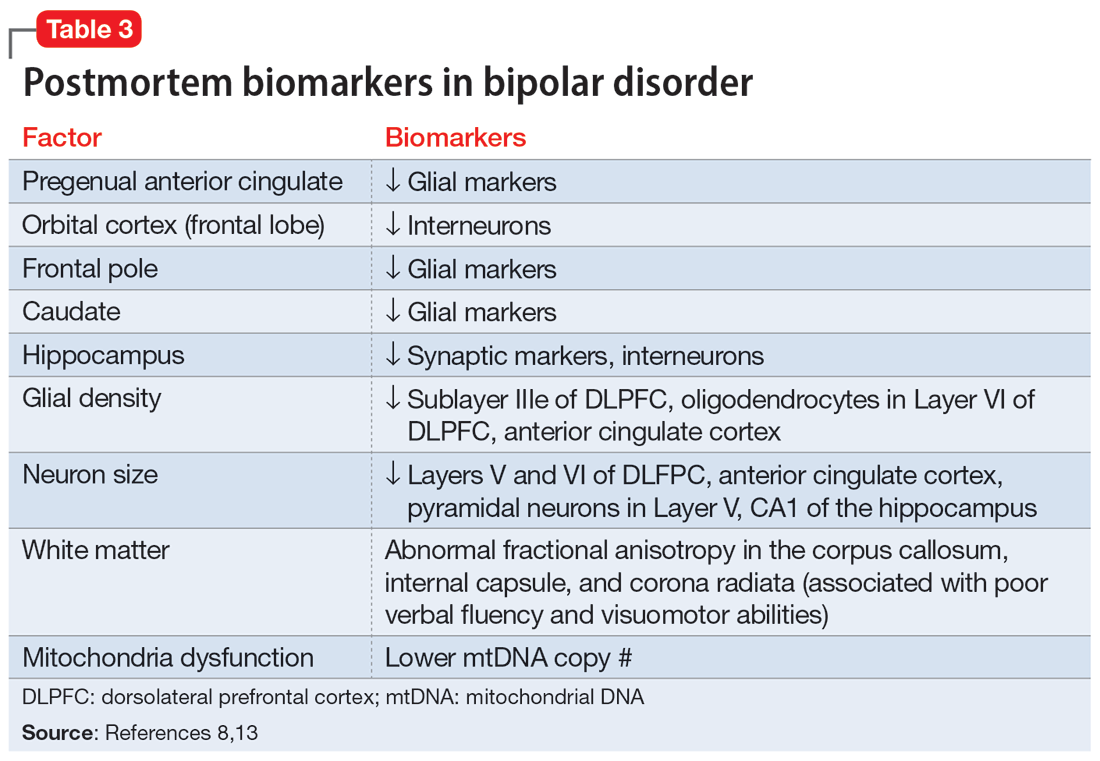
BD I is also believed to be associated with accelerated aging14,15 and an increased risk for dementia16 or cognitive deterioration.17 There is also an emerging hypothesis that neuroprogression and treatment resistance in BD is frequently associated with insulin resistance,18 peripheral inflammation,19 and blood-brain barrier permeability dysfunction.20
The bottom line is that like patients with schizophrenia, where relapses lead to devastating consequences,21 those with BD are at a similar high risk for neuroprogression, which includes atrophy in several brain regions, treatment resistance, and functional disability. This underscores the urgency for implementing LAI therapy early in the illness, when the first manic episode (Stage 2) emerges after the prodrome (Stage 1). This is the best strategy to preserve brain health in persons with BD22 and to allow them to remain functional with their many intellectual gifts, such as eloquence, poetry, artistic talents, humor, and social skills. It is unfortunate that the combination of patients’ and clinicians’ reluctance to use an LAI early in the illness dooms many patients with BD to a potentially avoidable malignant outcome.
1. Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10(1):105-106.
2. Kapezinski NS, Mwangi B, Cassidy RM, et al. Neuroprogression and illness trajectories in bipolar disorder. Expert Rev Neurother. 2017;17(3):277-285.
3. Nasrallah HA. Errors of omission and commission in psychiatric practice. Current Psychiatry. 2017;16(11):4,6,8.
4. Nasrallah HA. Is anosognosia a delusion, a negative symptom, or a cognitive deficit? Current Psychiatry. 2022;21(1):6-8,14.
5. Post RM. Preventing the malignant transformation of bipolar disorder. JAMA. 2018;319(12):1197-1198.
6. Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35(3):804-817.
7. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cerebral ventricular enlargement in young manic males. A controlled CT study. J Affective Dis. 1982;4(1):15-19.
8. Maletic V, Raison C. Integrated neurobiology of bipolar disorder. Front Psychiatry. 2014;5:98.
9. Berk M. Neuroprogression: pathways to progressive brain changes in bipolar disorder. Int J Neuropsychopharmacol. 2009;12(4):441-445.
10. Berk M, Conus P, Kapczinski F, et al. From neuroprogression to neuroprotection: implications for clinical care. Med J Aust. 2010;193(S4):S36-S40.
11. Passos IC, Mwangi B, Vieta E, et al. Areas of controversy in neuroprogression in bipolar disorder. Acta Psychiatr Scand. 2016;134(2):91-103.
12. Fries GR, Pfaffenseller B, Stertz L, et al. Staging and neuroprogression in bipolar disorder. Curr Psychiatry Rep. 2012;14(6):667-675.
13. Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7(5):541-547.
14. Fries GR, Zamzow MJ, Andrews T, et al. Accelerated aging in bipolar disorder: a comprehensive review of molecular findings and their clinical implications. Neurosci Biobehav Rev. 2020;112:107-116.
15. Fries GR, Bauer IE, Scaini G, et al. Accelerated hippocampal biological aging in bipolar disorder. Bipolar Dis. 2020;22(5):498-507.
16. Diniz BS, Teixeira AL, Cao F, et al. History of bipolar disorder and the risk of dementia: a systematic review and meta-analysis. Am J Geriatr Psychiatry. 2017;25(4):357-362.
17. Bauer IE, Ouyang A, Mwangi B, et al. Reduced white matter integrity and verbal fluency impairment in young adults with bipolar disorder: a diffusion tensor imaging study. J Psychiatr Res. 2015;62:115-122.
18. Calkin CV. Insulin resistance takes center stage: a new paradigm in the progression of bipolar disorder. Ann Med. 2019;51(5-6):281-293.
19. Grewal S, McKinlay S, Kapczinski F, et al. Biomarkers of neuroprogression and late staging in bipolar disorder: a systematic review. Aust N Z J Psychiatry. 2023;57(3):328-343.
20. Calkin C, McClelland C, Cairns K, et al. Insulin resistance and blood-brain barrier dysfunction underlie neuroprogression in bipolar disorder. Front Psychiatry. 2021;12:636174.
21. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
22. Berk M, Hallam K, Malhi GS, et al. Evidence and implications for early intervention in bipolar disorder. J Ment Health. 2010;19(2):113-126.
Bipolar disorder (BD) is a psychotic mood disorder. Like schizophrenia, it has been shown to be associated with significant degeneration and structural brain abnormalities with multiple relapses.1,2
Just as I have always advocated preventing recurrences in schizophrenia by using long-acting injectable (LAI) antipsychotic formulations immediately after the first episode to prevent psychotic relapses and progressive brain damage,3 I strongly recommend using LAIs right after hospital discharge from the first manic episode. It is the most rational management approach for bipolar mania given the grave consequences of multiple episodes, which are so common in this psychotic mood disorder due to poor medication adherence.
In contrast to the depressive episodes of BD I, where patients have insight into their depression and seek psychiatric treatment, during a manic episode patients often have no insight (anosognosia) that they suffer from a serious brain disorder, and refuse treatment.4 In addition, young patients with BD I frequently discontinue their oral mood stabilizer or second-generation antipsychotic (which are approved for mania) because they miss the blissful euphoria and the buoyant physical and mental energy of their manic episodes. They are completely oblivious to (and uninformed about) the grave neurobiological damage of further manic episodes, which can condemn them to clinical, functional, and cognitive deterioration. These patients are also likely to become treatment-resistant, which has been labeled as “the malignant transformation of bipolar disorder.”5
The evidence for progressive brain tissue loss, clinical deterioration, functional decline, and treatment resistance is abundant.6 I was the lead investigator of the first study to report ventricular dilatation (which is a proxy for cortical atrophy) in bipolar mania,7 a discovery that was subsequently replicated by 2 dozen researchers. This was followed by numerous neuroimaging studies reporting a loss of volume across multiple brain regions, including the frontal lobe, temporal lobe, cerebellum, thalamus, hippocampus, and basal ganglia. BD is heterogeneous8 with 4 stages (Table 19), and patients experience progressively worse brain structure and function with each stage.
Many patients with bipolar mania end up with poor clinical and functional outcomes, even when they respond well to initial treatment with lithium, anticonvulsant mood stabilizers, or second-generation antipsychotics. With their intentional nonadherence to oral medications leading to multiple recurrent relapses, these patients are at serious risk for neuroprogression and brain atrophic changes driven by multiple factors: inflammatory cytokines, increased cortical steroids, decreased neurotrophins, deceased neurogenesis, increased oxidative stress, and mitochondrial energy dysfunction. The consequences include progressive shortening of the interval between episodes with every relapse and loss of responsiveness to pharmacotherapy as the illness progresses.6,10 Predictors of a downhill progression include genetic vulnerability, perinatal complication during fetal life, childhood trauma (physical, sexual, emotional, or neglect), substance use, stress, psychiatric/medial comorbidities, and especially the number of episodes.9,11

Biomarkers have been reported in both the early and late stages of BD (Table 212) as well as in postmortem studies (Table 38,13). They reflect the progressive neurodegenerative nature of recurrent BD I episodes as the disorder moves to the advanced stages. I summarize these stages in Table 19 and Table 212 for the benefit of psychiatric clinicians who do not have access to the neuroscience journals where such findings are usually published.

BD I is also believed to be associated with accelerated aging14,15 and an increased risk for dementia16 or cognitive deterioration.17 There is also an emerging hypothesis that neuroprogression and treatment resistance in BD is frequently associated with insulin resistance,18 peripheral inflammation,19 and blood-brain barrier permeability dysfunction.20
The bottom line is that like patients with schizophrenia, where relapses lead to devastating consequences,21 those with BD are at a similar high risk for neuroprogression, which includes atrophy in several brain regions, treatment resistance, and functional disability. This underscores the urgency for implementing LAI therapy early in the illness, when the first manic episode (Stage 2) emerges after the prodrome (Stage 1). This is the best strategy to preserve brain health in persons with BD22 and to allow them to remain functional with their many intellectual gifts, such as eloquence, poetry, artistic talents, humor, and social skills. It is unfortunate that the combination of patients’ and clinicians’ reluctance to use an LAI early in the illness dooms many patients with BD to a potentially avoidable malignant outcome.
Bipolar disorder (BD) is a psychotic mood disorder. Like schizophrenia, it has been shown to be associated with significant degeneration and structural brain abnormalities with multiple relapses.1,2
Just as I have always advocated preventing recurrences in schizophrenia by using long-acting injectable (LAI) antipsychotic formulations immediately after the first episode to prevent psychotic relapses and progressive brain damage,3 I strongly recommend using LAIs right after hospital discharge from the first manic episode. It is the most rational management approach for bipolar mania given the grave consequences of multiple episodes, which are so common in this psychotic mood disorder due to poor medication adherence.
In contrast to the depressive episodes of BD I, where patients have insight into their depression and seek psychiatric treatment, during a manic episode patients often have no insight (anosognosia) that they suffer from a serious brain disorder, and refuse treatment.4 In addition, young patients with BD I frequently discontinue their oral mood stabilizer or second-generation antipsychotic (which are approved for mania) because they miss the blissful euphoria and the buoyant physical and mental energy of their manic episodes. They are completely oblivious to (and uninformed about) the grave neurobiological damage of further manic episodes, which can condemn them to clinical, functional, and cognitive deterioration. These patients are also likely to become treatment-resistant, which has been labeled as “the malignant transformation of bipolar disorder.”5
The evidence for progressive brain tissue loss, clinical deterioration, functional decline, and treatment resistance is abundant.6 I was the lead investigator of the first study to report ventricular dilatation (which is a proxy for cortical atrophy) in bipolar mania,7 a discovery that was subsequently replicated by 2 dozen researchers. This was followed by numerous neuroimaging studies reporting a loss of volume across multiple brain regions, including the frontal lobe, temporal lobe, cerebellum, thalamus, hippocampus, and basal ganglia. BD is heterogeneous8 with 4 stages (Table 19), and patients experience progressively worse brain structure and function with each stage.
Many patients with bipolar mania end up with poor clinical and functional outcomes, even when they respond well to initial treatment with lithium, anticonvulsant mood stabilizers, or second-generation antipsychotics. With their intentional nonadherence to oral medications leading to multiple recurrent relapses, these patients are at serious risk for neuroprogression and brain atrophic changes driven by multiple factors: inflammatory cytokines, increased cortical steroids, decreased neurotrophins, deceased neurogenesis, increased oxidative stress, and mitochondrial energy dysfunction. The consequences include progressive shortening of the interval between episodes with every relapse and loss of responsiveness to pharmacotherapy as the illness progresses.6,10 Predictors of a downhill progression include genetic vulnerability, perinatal complication during fetal life, childhood trauma (physical, sexual, emotional, or neglect), substance use, stress, psychiatric/medial comorbidities, and especially the number of episodes.9,11

Biomarkers have been reported in both the early and late stages of BD (Table 212) as well as in postmortem studies (Table 38,13). They reflect the progressive neurodegenerative nature of recurrent BD I episodes as the disorder moves to the advanced stages. I summarize these stages in Table 19 and Table 212 for the benefit of psychiatric clinicians who do not have access to the neuroscience journals where such findings are usually published.

BD I is also believed to be associated with accelerated aging14,15 and an increased risk for dementia16 or cognitive deterioration.17 There is also an emerging hypothesis that neuroprogression and treatment resistance in BD is frequently associated with insulin resistance,18 peripheral inflammation,19 and blood-brain barrier permeability dysfunction.20
The bottom line is that like patients with schizophrenia, where relapses lead to devastating consequences,21 those with BD are at a similar high risk for neuroprogression, which includes atrophy in several brain regions, treatment resistance, and functional disability. This underscores the urgency for implementing LAI therapy early in the illness, when the first manic episode (Stage 2) emerges after the prodrome (Stage 1). This is the best strategy to preserve brain health in persons with BD22 and to allow them to remain functional with their many intellectual gifts, such as eloquence, poetry, artistic talents, humor, and social skills. It is unfortunate that the combination of patients’ and clinicians’ reluctance to use an LAI early in the illness dooms many patients with BD to a potentially avoidable malignant outcome.
1. Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10(1):105-106.
2. Kapezinski NS, Mwangi B, Cassidy RM, et al. Neuroprogression and illness trajectories in bipolar disorder. Expert Rev Neurother. 2017;17(3):277-285.
3. Nasrallah HA. Errors of omission and commission in psychiatric practice. Current Psychiatry. 2017;16(11):4,6,8.
4. Nasrallah HA. Is anosognosia a delusion, a negative symptom, or a cognitive deficit? Current Psychiatry. 2022;21(1):6-8,14.
5. Post RM. Preventing the malignant transformation of bipolar disorder. JAMA. 2018;319(12):1197-1198.
6. Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35(3):804-817.
7. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cerebral ventricular enlargement in young manic males. A controlled CT study. J Affective Dis. 1982;4(1):15-19.
8. Maletic V, Raison C. Integrated neurobiology of bipolar disorder. Front Psychiatry. 2014;5:98.
9. Berk M. Neuroprogression: pathways to progressive brain changes in bipolar disorder. Int J Neuropsychopharmacol. 2009;12(4):441-445.
10. Berk M, Conus P, Kapczinski F, et al. From neuroprogression to neuroprotection: implications for clinical care. Med J Aust. 2010;193(S4):S36-S40.
11. Passos IC, Mwangi B, Vieta E, et al. Areas of controversy in neuroprogression in bipolar disorder. Acta Psychiatr Scand. 2016;134(2):91-103.
12. Fries GR, Pfaffenseller B, Stertz L, et al. Staging and neuroprogression in bipolar disorder. Curr Psychiatry Rep. 2012;14(6):667-675.
13. Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7(5):541-547.
14. Fries GR, Zamzow MJ, Andrews T, et al. Accelerated aging in bipolar disorder: a comprehensive review of molecular findings and their clinical implications. Neurosci Biobehav Rev. 2020;112:107-116.
15. Fries GR, Bauer IE, Scaini G, et al. Accelerated hippocampal biological aging in bipolar disorder. Bipolar Dis. 2020;22(5):498-507.
16. Diniz BS, Teixeira AL, Cao F, et al. History of bipolar disorder and the risk of dementia: a systematic review and meta-analysis. Am J Geriatr Psychiatry. 2017;25(4):357-362.
17. Bauer IE, Ouyang A, Mwangi B, et al. Reduced white matter integrity and verbal fluency impairment in young adults with bipolar disorder: a diffusion tensor imaging study. J Psychiatr Res. 2015;62:115-122.
18. Calkin CV. Insulin resistance takes center stage: a new paradigm in the progression of bipolar disorder. Ann Med. 2019;51(5-6):281-293.
19. Grewal S, McKinlay S, Kapczinski F, et al. Biomarkers of neuroprogression and late staging in bipolar disorder: a systematic review. Aust N Z J Psychiatry. 2023;57(3):328-343.
20. Calkin C, McClelland C, Cairns K, et al. Insulin resistance and blood-brain barrier dysfunction underlie neuroprogression in bipolar disorder. Front Psychiatry. 2021;12:636174.
21. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
22. Berk M, Hallam K, Malhi GS, et al. Evidence and implications for early intervention in bipolar disorder. J Ment Health. 2010;19(2):113-126.
1. Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10(1):105-106.
2. Kapezinski NS, Mwangi B, Cassidy RM, et al. Neuroprogression and illness trajectories in bipolar disorder. Expert Rev Neurother. 2017;17(3):277-285.
3. Nasrallah HA. Errors of omission and commission in psychiatric practice. Current Psychiatry. 2017;16(11):4,6,8.
4. Nasrallah HA. Is anosognosia a delusion, a negative symptom, or a cognitive deficit? Current Psychiatry. 2022;21(1):6-8,14.
5. Post RM. Preventing the malignant transformation of bipolar disorder. JAMA. 2018;319(12):1197-1198.
6. Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35(3):804-817.
7. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cerebral ventricular enlargement in young manic males. A controlled CT study. J Affective Dis. 1982;4(1):15-19.
8. Maletic V, Raison C. Integrated neurobiology of bipolar disorder. Front Psychiatry. 2014;5:98.
9. Berk M. Neuroprogression: pathways to progressive brain changes in bipolar disorder. Int J Neuropsychopharmacol. 2009;12(4):441-445.
10. Berk M, Conus P, Kapczinski F, et al. From neuroprogression to neuroprotection: implications for clinical care. Med J Aust. 2010;193(S4):S36-S40.
11. Passos IC, Mwangi B, Vieta E, et al. Areas of controversy in neuroprogression in bipolar disorder. Acta Psychiatr Scand. 2016;134(2):91-103.
12. Fries GR, Pfaffenseller B, Stertz L, et al. Staging and neuroprogression in bipolar disorder. Curr Psychiatry Rep. 2012;14(6):667-675.
13. Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7(5):541-547.
14. Fries GR, Zamzow MJ, Andrews T, et al. Accelerated aging in bipolar disorder: a comprehensive review of molecular findings and their clinical implications. Neurosci Biobehav Rev. 2020;112:107-116.
15. Fries GR, Bauer IE, Scaini G, et al. Accelerated hippocampal biological aging in bipolar disorder. Bipolar Dis. 2020;22(5):498-507.
16. Diniz BS, Teixeira AL, Cao F, et al. History of bipolar disorder and the risk of dementia: a systematic review and meta-analysis. Am J Geriatr Psychiatry. 2017;25(4):357-362.
17. Bauer IE, Ouyang A, Mwangi B, et al. Reduced white matter integrity and verbal fluency impairment in young adults with bipolar disorder: a diffusion tensor imaging study. J Psychiatr Res. 2015;62:115-122.
18. Calkin CV. Insulin resistance takes center stage: a new paradigm in the progression of bipolar disorder. Ann Med. 2019;51(5-6):281-293.
19. Grewal S, McKinlay S, Kapczinski F, et al. Biomarkers of neuroprogression and late staging in bipolar disorder: a systematic review. Aust N Z J Psychiatry. 2023;57(3):328-343.
20. Calkin C, McClelland C, Cairns K, et al. Insulin resistance and blood-brain barrier dysfunction underlie neuroprogression in bipolar disorder. Front Psychiatry. 2021;12:636174.
21. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
22. Berk M, Hallam K, Malhi GS, et al. Evidence and implications for early intervention in bipolar disorder. J Ment Health. 2010;19(2):113-126.
Lamotrigine interactions with oral contraceptives
Ms. A, age 20, presents to the clinic after experiencing difficulty sleeping, depressed mood, fatigue, and difficulty concentrating. Her psychiatric history includes bipolar II disorder (BD II), predominantly with depressive episodes. Ms. A’s current medications include a combination of lamotrigine 200 mg/d and bupropion extended-release 450 mg/d, and her symptoms were well maintained until 2 weeks ago. When her psychiatrist performs a medication reconciliation at her medication management appointment, Ms. A indicates she started taking an oral contraceptive, ethinyl estradiol and norgestimate, approximately 1 month ago for management of endometriosis symptoms. She is not currently taking any other medications or supplements.
Lamotrigine is indicated for epilepsy and as maintenance treatment for BD I. It is also used off-label to treat other mood disorders. After oral administration, lamotrigine is rapidly and fully absorbed with a high bioavailability (98%).The principal metabolic pathway is via glucuronic acid conjugation, leading to the major inactive metabolite 2-N-glucuronide. Minor metabolites include 5-N-glucuronide and a 2-N-glucuronide metabolite.1
Combined oral contraceptives contain an estrogen component, typically ethinyl estradiol, and a progestin component, which varies based on the specific formulation. The metabolism of ethinyl estradiol occurs through cytochrome P450 (CYP)3A4, CYP2C9, sulfation, and glucuronidation. For progestin—the second component of combined oral contraceptives and the lone component of progestin-only oral contraceptives—metabolism occurs via CYP3A4 and conjugation reactions.2 This article focuses on lamotrigine interactions specifically with oral contraceptives, but it is important to note that other formulations of combined hormonal contraceptives, such as the combined contraceptive patch (Ortho Evra) and vaginal ring (NuvaRing), would be expected to interact in the same way as oral formulations.3
Bidirectional interaction
While many antiseizure medications are known to interact with and potentially decrease the efficacy of oral contraceptives (Table 13-6), the interactions between lamotrigine and oral contraceptives is uniquely bidirectional. Combined oral contraceptives are thought to interact with lamotrigine primarily via the estrogen component, which causes increased metabolism of lamotrigine through induction of glucuronidation. This drug interaction decreases the plasma concentrations of lamotrigine in the body by up to 2-fold, resulting in an increased risk of seizures or inadequate mood stabilization.1 This effect on metabolism is very rapid, resulting in decreases in lamotrigine concentrations within 1 week.4,7 A recent study suggested that certain progestins may also contribute to decreased plasma levels of lamotrigine, but the mechanism for this is unknown (Table 23-7).8
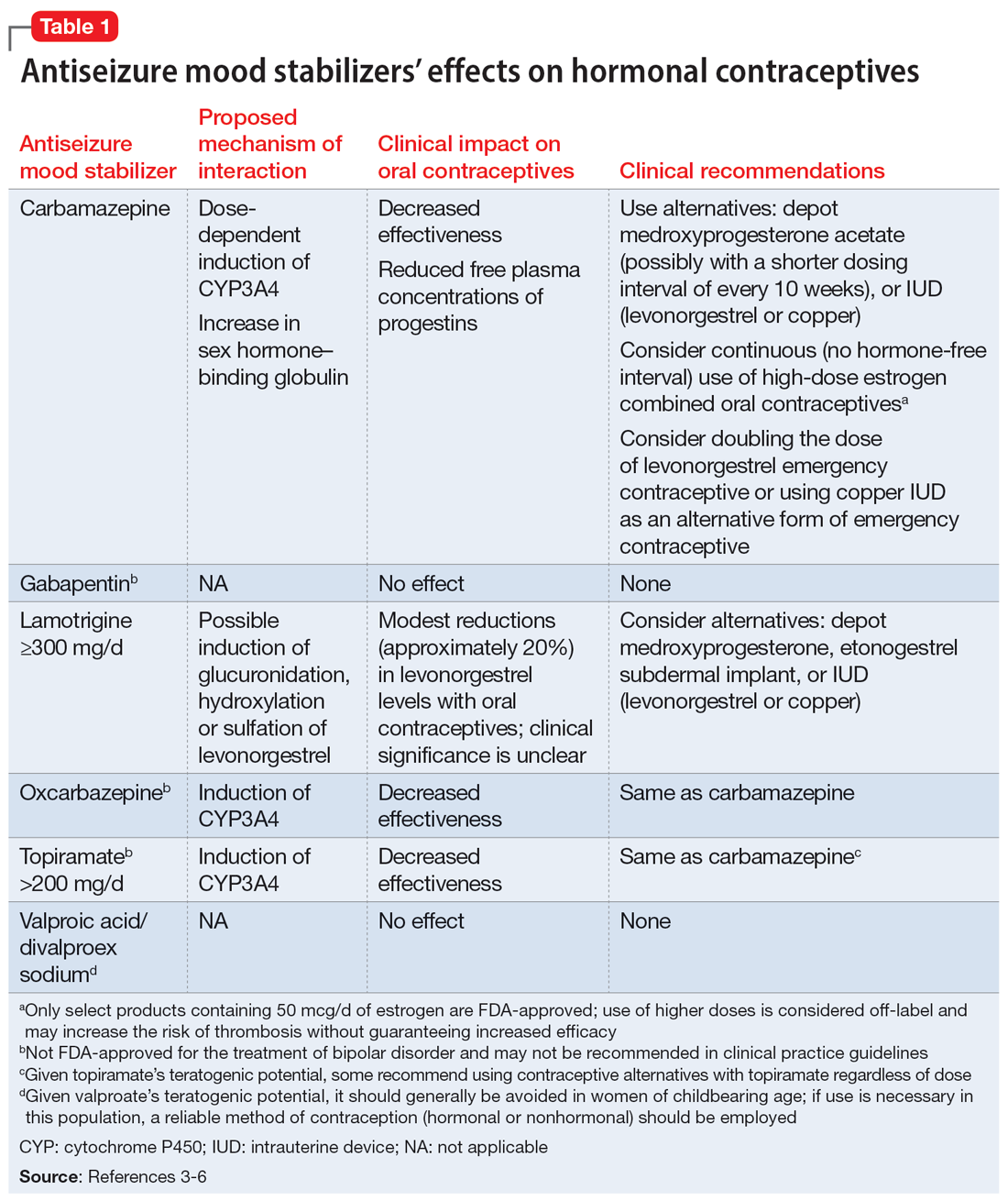
Clinicians should consider increasing the lamotrigine dose (potentially as much as 2-fold) in a patient who initiates treatment with a combined hormonal contraceptive. Dose increases should not be >50 to 100 mg/d every week.1 Collect lamotrigine blood levels before starting a hormonal contraceptive and during dose titration. While there is not a well-established therapeutic range for lamotrigine in BD, expert consensus recommends a range of 1 to 6 mcg/mL.8
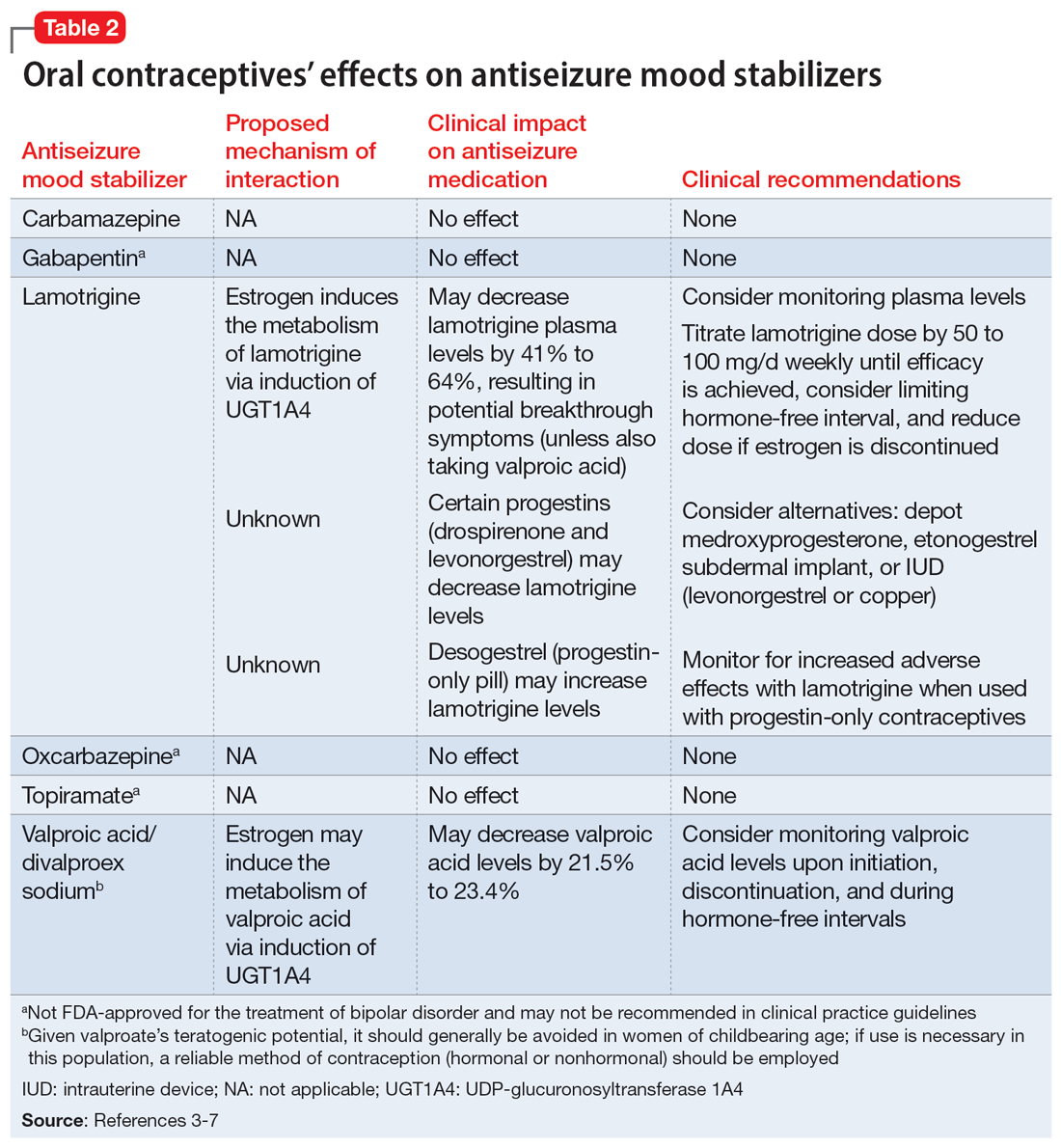
The lamotrigine dose should be decreased if combined hormonal contraceptives are discontinued. Dose decreases should not exceed 25% of the total daily dose per week.1 Desogestrel, a progestin-only medication, may increase exposure to lamotrigine, but this has not been observed in research with other progestins.5,9 When starting a progestin-only pill, monitor patients for signs of lamotrigine toxicity (ataxia, diplopia, dizziness) and consider monitoring their blood levels.
An important consideration to note with combined oral contraceptives is the hormone-free interval, also known as the pill-free week. Due to the rapid effect of estrogens, the lamotrigine concentrations have been shown to rise, even double, during this hormone-free interval, so patients should be closely monitored for adverse effects.3 Some recommend use of an extended cycle regimen (with a limited hormone-free interval), or continuous cycle regimen (with no hormone-free interval) to avoid fluctuations in lamotrigine levels.3,5 Additionally, data suggest that in patients taking lamotrigine and valproate, which inhibits glucuronidation, oral contraceptives do not cause reductions in lamotrigine concentrations.2,5 In these instances, dose increases of lamotrigine are not needed.
Continue to: The metabolism of ethinyl estradiol...
The metabolism of ethinyl estradiol and progestin are susceptible to CYP3A4 induction and increased glucuronidation. Serum concentrations may be reduced by ≥50% when used concomitantly with CYP enzyme–inducing medications, which could possibly result in subtherapeutic levels and unplanned pregnancy.3 CYP3A4 induction occurs for up to 4 weeks after discontinuation of an enzyme-inducing agent, pointing to the need for alternative or backup contraception during this time.3 Lamotrigine is not a CYP enzyme–inducing medication; it is unlikely to affect the efficacy of oral contraceptives in the same manner as other antiseizure medications. However, a study of lamotrigine and the combined hormonal contraceptive ethinyl estradiol and levonorgestrel demonstrated reduced exposure to levonorgestrel, resulting in breakthrough bleeding.5
In a study on the coadministration of lamotrigine and combined oral contraceptives, Sidhu et al4 observed a small mean reduction (20%) in progestin concentrations when lamotrigine was used at a dose of 300 mg/d. Although there is no research suggesting decreased effectiveness in preventing pregnancy when lamotrigine is used with combined oral contraceptives, progestin-only oral contraceptives, or progestin implants, additional or alternative contraceptive methods may be considered based on this pharmacokinetic data, particularly in patients who require lamotrigine doses ≥300 mg/d.5
CASE CONTINUED
Given when Ms. A started the oral contraceptive, the treatment team determines it is likely that an interaction with lamotrigine is causing her resurgence of depressive symptoms. Her care team decides to titrate the lamotrigine gradually to 300 mg/d, then 400 mg/d if needed, while carefully monitoring for signs of a serious rash. This dosage increase may help Ms. A achieve symptom remission. Monitoring plasma levels may be considered, although it is unknown what plasma level was effective for Ms. A before she started the oral contraceptive. Ms. A would need to be counseled regarding the effect of higher doses of lamotrigine on the effectiveness of the oral contraceptive.
Although it does not appear Ms. A is using the oral contraceptive specifically to prevent pregnancy, the team informs her about the possibility of unintended pregnancy with this medication combination. If Ms. A was also using the medication for this indication, alternative contraceptive options would include medroxyprogesterone acetate, levonorgestrel implants, or an intrauterine device (levonorgestrel or copper, though copper would not be effective for endometriosis symptom management). Ms. A should consult with her gynecologist regarding the most appropriate option for her endometriosis. If the decision is made to discontinue her oral contraceptive in the future, the lamotrigine dose should be decreased to her previously effective dose of 200 mg/d.
Related Resources
- Makino KK, Hatters Friedman S, Amin J. Emergency contraception for psychiatric patients. Current Psychiatry. 2022;21(11):34-39,44-45. doi:10.12788/cp.0300
- MGH Center for Women’s Mental Health. You asked: is there an interaction between lamotrigine and oral contraceptives? September 29, 2015. https://womensmentalhealth.org/posts/you-asked-is-there-an-interaction-between-lamotrigine-andoral-contraceptives/
Drug Brand Names
Bupropion extended-release • Wellbutrin XL
Carbamazepine • Equetro, Tegretol
Desogestrel • Cerazette
Divalproex sodium • Depakote
Ethinyl estradiol and etonogestrel • NuvaRing
Ethinyl estradiol and norelgestromin • Ortho Evra
Ethinyl estradiol and norgestimate • Ortho Tri-Cyclen, TriNessa, others
Etonogestrel • Implanon, Nexplanon
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levonorgestrel emergency contraceptive pill • AfterPill, Plan B
Levonorgestrel intrauterine device • Mirena, Skyla
Medroxyprogesterone acetate • Depo-Provera
Oxcarbazepine • Trileptal
Topiramate • Topamax
Valproic acid • Depakene
1. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2020.
2. Lee CR. Drug interactions and hormonal contraception. Trends in Urology Gynaecology & Sexual Health. 2009;14(3):23-26.
3. Williams D. Antiepileptic drugs and contraception. US Pharm. 2014;39(1):39-42.
4. Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61(2):191-199. doi:10.1111/j.1365-2125.2005.02539.x
5. Faculty of Sexual & Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Published May 9, 2022. Accessed September 28, 2022. https://www.fsrh.org/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/
6. Johnston CA, Crawford PM. Anti-epileptic drugs and hormonal treatments. Curr Treat Options Neurol. 2014;16(5):288. doi:10.1007/s11940-014-0288-3
7. Christensen J, Petrenaite V, Atterman J, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia. 2007;48(3):484-489. doi:10.1111/j.1528-1167.2007.00997.x
8. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
9. Rauchenzauner M, Deichmann S, Pittschieler, et al. Bidirectional interaction between oral contraception and lamotrigine in women with epilepsy – role of progestins. Seizure. 2020;74:89-92. doi:10.1016/j.seizure.2019.11.011
Ms. A, age 20, presents to the clinic after experiencing difficulty sleeping, depressed mood, fatigue, and difficulty concentrating. Her psychiatric history includes bipolar II disorder (BD II), predominantly with depressive episodes. Ms. A’s current medications include a combination of lamotrigine 200 mg/d and bupropion extended-release 450 mg/d, and her symptoms were well maintained until 2 weeks ago. When her psychiatrist performs a medication reconciliation at her medication management appointment, Ms. A indicates she started taking an oral contraceptive, ethinyl estradiol and norgestimate, approximately 1 month ago for management of endometriosis symptoms. She is not currently taking any other medications or supplements.
Lamotrigine is indicated for epilepsy and as maintenance treatment for BD I. It is also used off-label to treat other mood disorders. After oral administration, lamotrigine is rapidly and fully absorbed with a high bioavailability (98%).The principal metabolic pathway is via glucuronic acid conjugation, leading to the major inactive metabolite 2-N-glucuronide. Minor metabolites include 5-N-glucuronide and a 2-N-glucuronide metabolite.1
Combined oral contraceptives contain an estrogen component, typically ethinyl estradiol, and a progestin component, which varies based on the specific formulation. The metabolism of ethinyl estradiol occurs through cytochrome P450 (CYP)3A4, CYP2C9, sulfation, and glucuronidation. For progestin—the second component of combined oral contraceptives and the lone component of progestin-only oral contraceptives—metabolism occurs via CYP3A4 and conjugation reactions.2 This article focuses on lamotrigine interactions specifically with oral contraceptives, but it is important to note that other formulations of combined hormonal contraceptives, such as the combined contraceptive patch (Ortho Evra) and vaginal ring (NuvaRing), would be expected to interact in the same way as oral formulations.3
Bidirectional interaction
While many antiseizure medications are known to interact with and potentially decrease the efficacy of oral contraceptives (Table 13-6), the interactions between lamotrigine and oral contraceptives is uniquely bidirectional. Combined oral contraceptives are thought to interact with lamotrigine primarily via the estrogen component, which causes increased metabolism of lamotrigine through induction of glucuronidation. This drug interaction decreases the plasma concentrations of lamotrigine in the body by up to 2-fold, resulting in an increased risk of seizures or inadequate mood stabilization.1 This effect on metabolism is very rapid, resulting in decreases in lamotrigine concentrations within 1 week.4,7 A recent study suggested that certain progestins may also contribute to decreased plasma levels of lamotrigine, but the mechanism for this is unknown (Table 23-7).8

Clinicians should consider increasing the lamotrigine dose (potentially as much as 2-fold) in a patient who initiates treatment with a combined hormonal contraceptive. Dose increases should not be >50 to 100 mg/d every week.1 Collect lamotrigine blood levels before starting a hormonal contraceptive and during dose titration. While there is not a well-established therapeutic range for lamotrigine in BD, expert consensus recommends a range of 1 to 6 mcg/mL.8

The lamotrigine dose should be decreased if combined hormonal contraceptives are discontinued. Dose decreases should not exceed 25% of the total daily dose per week.1 Desogestrel, a progestin-only medication, may increase exposure to lamotrigine, but this has not been observed in research with other progestins.5,9 When starting a progestin-only pill, monitor patients for signs of lamotrigine toxicity (ataxia, diplopia, dizziness) and consider monitoring their blood levels.
An important consideration to note with combined oral contraceptives is the hormone-free interval, also known as the pill-free week. Due to the rapid effect of estrogens, the lamotrigine concentrations have been shown to rise, even double, during this hormone-free interval, so patients should be closely monitored for adverse effects.3 Some recommend use of an extended cycle regimen (with a limited hormone-free interval), or continuous cycle regimen (with no hormone-free interval) to avoid fluctuations in lamotrigine levels.3,5 Additionally, data suggest that in patients taking lamotrigine and valproate, which inhibits glucuronidation, oral contraceptives do not cause reductions in lamotrigine concentrations.2,5 In these instances, dose increases of lamotrigine are not needed.
Continue to: The metabolism of ethinyl estradiol...
The metabolism of ethinyl estradiol and progestin are susceptible to CYP3A4 induction and increased glucuronidation. Serum concentrations may be reduced by ≥50% when used concomitantly with CYP enzyme–inducing medications, which could possibly result in subtherapeutic levels and unplanned pregnancy.3 CYP3A4 induction occurs for up to 4 weeks after discontinuation of an enzyme-inducing agent, pointing to the need for alternative or backup contraception during this time.3 Lamotrigine is not a CYP enzyme–inducing medication; it is unlikely to affect the efficacy of oral contraceptives in the same manner as other antiseizure medications. However, a study of lamotrigine and the combined hormonal contraceptive ethinyl estradiol and levonorgestrel demonstrated reduced exposure to levonorgestrel, resulting in breakthrough bleeding.5
In a study on the coadministration of lamotrigine and combined oral contraceptives, Sidhu et al4 observed a small mean reduction (20%) in progestin concentrations when lamotrigine was used at a dose of 300 mg/d. Although there is no research suggesting decreased effectiveness in preventing pregnancy when lamotrigine is used with combined oral contraceptives, progestin-only oral contraceptives, or progestin implants, additional or alternative contraceptive methods may be considered based on this pharmacokinetic data, particularly in patients who require lamotrigine doses ≥300 mg/d.5
CASE CONTINUED
Given when Ms. A started the oral contraceptive, the treatment team determines it is likely that an interaction with lamotrigine is causing her resurgence of depressive symptoms. Her care team decides to titrate the lamotrigine gradually to 300 mg/d, then 400 mg/d if needed, while carefully monitoring for signs of a serious rash. This dosage increase may help Ms. A achieve symptom remission. Monitoring plasma levels may be considered, although it is unknown what plasma level was effective for Ms. A before she started the oral contraceptive. Ms. A would need to be counseled regarding the effect of higher doses of lamotrigine on the effectiveness of the oral contraceptive.
Although it does not appear Ms. A is using the oral contraceptive specifically to prevent pregnancy, the team informs her about the possibility of unintended pregnancy with this medication combination. If Ms. A was also using the medication for this indication, alternative contraceptive options would include medroxyprogesterone acetate, levonorgestrel implants, or an intrauterine device (levonorgestrel or copper, though copper would not be effective for endometriosis symptom management). Ms. A should consult with her gynecologist regarding the most appropriate option for her endometriosis. If the decision is made to discontinue her oral contraceptive in the future, the lamotrigine dose should be decreased to her previously effective dose of 200 mg/d.
Related Resources
- Makino KK, Hatters Friedman S, Amin J. Emergency contraception for psychiatric patients. Current Psychiatry. 2022;21(11):34-39,44-45. doi:10.12788/cp.0300
- MGH Center for Women’s Mental Health. You asked: is there an interaction between lamotrigine and oral contraceptives? September 29, 2015. https://womensmentalhealth.org/posts/you-asked-is-there-an-interaction-between-lamotrigine-andoral-contraceptives/
Drug Brand Names
Bupropion extended-release • Wellbutrin XL
Carbamazepine • Equetro, Tegretol
Desogestrel • Cerazette
Divalproex sodium • Depakote
Ethinyl estradiol and etonogestrel • NuvaRing
Ethinyl estradiol and norelgestromin • Ortho Evra
Ethinyl estradiol and norgestimate • Ortho Tri-Cyclen, TriNessa, others
Etonogestrel • Implanon, Nexplanon
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levonorgestrel emergency contraceptive pill • AfterPill, Plan B
Levonorgestrel intrauterine device • Mirena, Skyla
Medroxyprogesterone acetate • Depo-Provera
Oxcarbazepine • Trileptal
Topiramate • Topamax
Valproic acid • Depakene
Ms. A, age 20, presents to the clinic after experiencing difficulty sleeping, depressed mood, fatigue, and difficulty concentrating. Her psychiatric history includes bipolar II disorder (BD II), predominantly with depressive episodes. Ms. A’s current medications include a combination of lamotrigine 200 mg/d and bupropion extended-release 450 mg/d, and her symptoms were well maintained until 2 weeks ago. When her psychiatrist performs a medication reconciliation at her medication management appointment, Ms. A indicates she started taking an oral contraceptive, ethinyl estradiol and norgestimate, approximately 1 month ago for management of endometriosis symptoms. She is not currently taking any other medications or supplements.
Lamotrigine is indicated for epilepsy and as maintenance treatment for BD I. It is also used off-label to treat other mood disorders. After oral administration, lamotrigine is rapidly and fully absorbed with a high bioavailability (98%).The principal metabolic pathway is via glucuronic acid conjugation, leading to the major inactive metabolite 2-N-glucuronide. Minor metabolites include 5-N-glucuronide and a 2-N-glucuronide metabolite.1
Combined oral contraceptives contain an estrogen component, typically ethinyl estradiol, and a progestin component, which varies based on the specific formulation. The metabolism of ethinyl estradiol occurs through cytochrome P450 (CYP)3A4, CYP2C9, sulfation, and glucuronidation. For progestin—the second component of combined oral contraceptives and the lone component of progestin-only oral contraceptives—metabolism occurs via CYP3A4 and conjugation reactions.2 This article focuses on lamotrigine interactions specifically with oral contraceptives, but it is important to note that other formulations of combined hormonal contraceptives, such as the combined contraceptive patch (Ortho Evra) and vaginal ring (NuvaRing), would be expected to interact in the same way as oral formulations.3
Bidirectional interaction
While many antiseizure medications are known to interact with and potentially decrease the efficacy of oral contraceptives (Table 13-6), the interactions between lamotrigine and oral contraceptives is uniquely bidirectional. Combined oral contraceptives are thought to interact with lamotrigine primarily via the estrogen component, which causes increased metabolism of lamotrigine through induction of glucuronidation. This drug interaction decreases the plasma concentrations of lamotrigine in the body by up to 2-fold, resulting in an increased risk of seizures or inadequate mood stabilization.1 This effect on metabolism is very rapid, resulting in decreases in lamotrigine concentrations within 1 week.4,7 A recent study suggested that certain progestins may also contribute to decreased plasma levels of lamotrigine, but the mechanism for this is unknown (Table 23-7).8

Clinicians should consider increasing the lamotrigine dose (potentially as much as 2-fold) in a patient who initiates treatment with a combined hormonal contraceptive. Dose increases should not be >50 to 100 mg/d every week.1 Collect lamotrigine blood levels before starting a hormonal contraceptive and during dose titration. While there is not a well-established therapeutic range for lamotrigine in BD, expert consensus recommends a range of 1 to 6 mcg/mL.8

The lamotrigine dose should be decreased if combined hormonal contraceptives are discontinued. Dose decreases should not exceed 25% of the total daily dose per week.1 Desogestrel, a progestin-only medication, may increase exposure to lamotrigine, but this has not been observed in research with other progestins.5,9 When starting a progestin-only pill, monitor patients for signs of lamotrigine toxicity (ataxia, diplopia, dizziness) and consider monitoring their blood levels.
An important consideration to note with combined oral contraceptives is the hormone-free interval, also known as the pill-free week. Due to the rapid effect of estrogens, the lamotrigine concentrations have been shown to rise, even double, during this hormone-free interval, so patients should be closely monitored for adverse effects.3 Some recommend use of an extended cycle regimen (with a limited hormone-free interval), or continuous cycle regimen (with no hormone-free interval) to avoid fluctuations in lamotrigine levels.3,5 Additionally, data suggest that in patients taking lamotrigine and valproate, which inhibits glucuronidation, oral contraceptives do not cause reductions in lamotrigine concentrations.2,5 In these instances, dose increases of lamotrigine are not needed.
Continue to: The metabolism of ethinyl estradiol...
The metabolism of ethinyl estradiol and progestin are susceptible to CYP3A4 induction and increased glucuronidation. Serum concentrations may be reduced by ≥50% when used concomitantly with CYP enzyme–inducing medications, which could possibly result in subtherapeutic levels and unplanned pregnancy.3 CYP3A4 induction occurs for up to 4 weeks after discontinuation of an enzyme-inducing agent, pointing to the need for alternative or backup contraception during this time.3 Lamotrigine is not a CYP enzyme–inducing medication; it is unlikely to affect the efficacy of oral contraceptives in the same manner as other antiseizure medications. However, a study of lamotrigine and the combined hormonal contraceptive ethinyl estradiol and levonorgestrel demonstrated reduced exposure to levonorgestrel, resulting in breakthrough bleeding.5
In a study on the coadministration of lamotrigine and combined oral contraceptives, Sidhu et al4 observed a small mean reduction (20%) in progestin concentrations when lamotrigine was used at a dose of 300 mg/d. Although there is no research suggesting decreased effectiveness in preventing pregnancy when lamotrigine is used with combined oral contraceptives, progestin-only oral contraceptives, or progestin implants, additional or alternative contraceptive methods may be considered based on this pharmacokinetic data, particularly in patients who require lamotrigine doses ≥300 mg/d.5
CASE CONTINUED
Given when Ms. A started the oral contraceptive, the treatment team determines it is likely that an interaction with lamotrigine is causing her resurgence of depressive symptoms. Her care team decides to titrate the lamotrigine gradually to 300 mg/d, then 400 mg/d if needed, while carefully monitoring for signs of a serious rash. This dosage increase may help Ms. A achieve symptom remission. Monitoring plasma levels may be considered, although it is unknown what plasma level was effective for Ms. A before she started the oral contraceptive. Ms. A would need to be counseled regarding the effect of higher doses of lamotrigine on the effectiveness of the oral contraceptive.
Although it does not appear Ms. A is using the oral contraceptive specifically to prevent pregnancy, the team informs her about the possibility of unintended pregnancy with this medication combination. If Ms. A was also using the medication for this indication, alternative contraceptive options would include medroxyprogesterone acetate, levonorgestrel implants, or an intrauterine device (levonorgestrel or copper, though copper would not be effective for endometriosis symptom management). Ms. A should consult with her gynecologist regarding the most appropriate option for her endometriosis. If the decision is made to discontinue her oral contraceptive in the future, the lamotrigine dose should be decreased to her previously effective dose of 200 mg/d.
Related Resources
- Makino KK, Hatters Friedman S, Amin J. Emergency contraception for psychiatric patients. Current Psychiatry. 2022;21(11):34-39,44-45. doi:10.12788/cp.0300
- MGH Center for Women’s Mental Health. You asked: is there an interaction between lamotrigine and oral contraceptives? September 29, 2015. https://womensmentalhealth.org/posts/you-asked-is-there-an-interaction-between-lamotrigine-andoral-contraceptives/
Drug Brand Names
Bupropion extended-release • Wellbutrin XL
Carbamazepine • Equetro, Tegretol
Desogestrel • Cerazette
Divalproex sodium • Depakote
Ethinyl estradiol and etonogestrel • NuvaRing
Ethinyl estradiol and norelgestromin • Ortho Evra
Ethinyl estradiol and norgestimate • Ortho Tri-Cyclen, TriNessa, others
Etonogestrel • Implanon, Nexplanon
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levonorgestrel emergency contraceptive pill • AfterPill, Plan B
Levonorgestrel intrauterine device • Mirena, Skyla
Medroxyprogesterone acetate • Depo-Provera
Oxcarbazepine • Trileptal
Topiramate • Topamax
Valproic acid • Depakene
1. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2020.
2. Lee CR. Drug interactions and hormonal contraception. Trends in Urology Gynaecology & Sexual Health. 2009;14(3):23-26.
3. Williams D. Antiepileptic drugs and contraception. US Pharm. 2014;39(1):39-42.
4. Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61(2):191-199. doi:10.1111/j.1365-2125.2005.02539.x
5. Faculty of Sexual & Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Published May 9, 2022. Accessed September 28, 2022. https://www.fsrh.org/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/
6. Johnston CA, Crawford PM. Anti-epileptic drugs and hormonal treatments. Curr Treat Options Neurol. 2014;16(5):288. doi:10.1007/s11940-014-0288-3
7. Christensen J, Petrenaite V, Atterman J, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia. 2007;48(3):484-489. doi:10.1111/j.1528-1167.2007.00997.x
8. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
9. Rauchenzauner M, Deichmann S, Pittschieler, et al. Bidirectional interaction between oral contraception and lamotrigine in women with epilepsy – role of progestins. Seizure. 2020;74:89-92. doi:10.1016/j.seizure.2019.11.011
1. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2020.
2. Lee CR. Drug interactions and hormonal contraception. Trends in Urology Gynaecology & Sexual Health. 2009;14(3):23-26.
3. Williams D. Antiepileptic drugs and contraception. US Pharm. 2014;39(1):39-42.
4. Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61(2):191-199. doi:10.1111/j.1365-2125.2005.02539.x
5. Faculty of Sexual & Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Published May 9, 2022. Accessed September 28, 2022. https://www.fsrh.org/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/
6. Johnston CA, Crawford PM. Anti-epileptic drugs and hormonal treatments. Curr Treat Options Neurol. 2014;16(5):288. doi:10.1007/s11940-014-0288-3
7. Christensen J, Petrenaite V, Atterman J, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia. 2007;48(3):484-489. doi:10.1111/j.1528-1167.2007.00997.x
8. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
9. Rauchenzauner M, Deichmann S, Pittschieler, et al. Bidirectional interaction between oral contraception and lamotrigine in women with epilepsy – role of progestins. Seizure. 2020;74:89-92. doi:10.1016/j.seizure.2019.11.011