User login
SCAI issues guidelines for PFO management, makes case for expansion
The first-ever guidelines for interventional cardiologists using percutaneous patent foramen ovale closure recommend expanding the use of the procedure beyond the Food and Drug Administration–approved indication following PFO-associated ischemic stroke, adding clarification about the use of PFO with anticoagulation and hedging against abuse and overuse of the procedure, said the chair of the guideline writing committee.
“The most important things surrounding these guidelines are to help clinicians and policymakers – third-party payers – to address PFO in patient subsets that were not included in the large randomized clinical trials that led to FDA approval,” said writing group chair Clifford J. Kavinsky, MD, PhD, chief of structural and interventional cardiology at Rush University Medical Center, Chicago.
The Society for Cardiovascular Angiography & Interventions issued the guidelines at its annual scientific sessions meeting in Atlanta and published them simultaneously in the society’s journal.
The guidelines issue strong and conditional recommendations. The former means clinicians should order the intervention for most patients; the latter means decisionmaking is more nuanced and should consider contributing factors.
The guidelines clarify patient selection for PFO closure outside the “pretty narrow” indication the FDA approved, Dr. Kavinsky said, which is for PFO-associated ischemic stroke in patients aged 18-60 years.
“So what about patients who are older than 60? What about patients who had their stroke 10 years ago?” Dr. Kavinsky asked. “Those are issues that were unanswered in the randomized clinical trials.”
The guidelines also refine recommendations about anticoagulation in these patients, including its use after PFO closure in selected patients, Dr. Kavinsky noted. “It’s the opinion of the panel that although anticoagulants may be effective, because of issues of noncompliance, because of issues of interruption of therapy by physicians for a variety of reasons, including surgery or noncompliance, that it is preferable to do a PFO device closure to giving anticoagulant therapy.”
Many of the recommendations cover PFO closure alongside antiplatelet or anticoagulation therapy. Key conditional recommendations for patients who haven’t had a PFO-related stroke are:
- Avoiding its routine use in patients with chronic migraines, prior decompression illness (DCI), thrombophilia, atrial septal aneurysm, transient ischemic attack (TIA), or deep vein thrombosis (DVT).
- Considering PFO closure in patients with platypnea-orthodeoxia syndrome (POS) with no other discernible cause of hypoxia or systemic embolism in whom other embolic causes have been ruled out.
In patients who’ve had a PFO-related stroke, the guidelines strongly recommend PFO closure versus antiplatelet therapy alone, but conditionally, not in patients with atrial fibrillation who’ve had an ischemic stroke. They also conditionally suggest PFO closure rather than long-term antiplatelet therapy alone in PFO stroke patients aged 60 and older, as well as those with thrombophilia already on antiplatelet therapy but not anticoagulation. However, the guidelines make no recommendation on PFO closure based on how much time has passed since the previous stroke.
“Furthermore,” Dr. Kavinsky said, “in patients who require lifelong anticoagulation because of recurrent DVT or recurrent pulmonary emboli or thrombopenia, if they’ve had a PFO-mediated stroke, then it’s our opinion that they should have their PFO closed in addition to taking lifelong anticoagulation because of the same issues of noncompliance and interruption of therapy.” Those are conditional recommendations.
The guideline also checks a box in the FDA labeling that mandated agreement between cardiology and neurology in patient selection. The American Academy of Neurology (AAN) issued its own guideline in 2020 for patients with stroke and PFO. In Europe, the European Society of Cardiology issued two position papers on expanded applications of PFO closure.
The recommendations on when PFO closure shouldn’t be done are noteworthy, Dr. Kavinsky said. “PFOs are present in 25% of the adult population, so the number of patients with PFO is huge and the indication for the FDA is really narrow: to reduce the risk of recurrent stroke in patients with PFO-mediated stroke. So, there’s the tremendous potential for abuse out there, of excessive procedures, of doing unnecessary procedures.”
The guidelines are a follow-up to the operator institutional requirements document SCAI issued in 2019 that set requirements for hospital offering and physicians performing PFO closure, Dr. Kavinsky added.
In an editorial accompanying the published guideline, Robert J. Sommer, MD, and Jamil A. Aboulhosn, MD, wrote that they support the recommendations “which help spotlight and clarify the growing list of potential indications for PFO closure.” They noted that the guidelines panel’s “strong” recommendations were for indications validated by randomized trials and that “conditional” recommendations were based on panelists’ experience and observational data.
“It is critical to recognize that most of these guidelines represent consensus opinion only,” wrote Dr. Sommer, who specializes in adult congenital and pediatric cardiology at Columbia University Irving Medical Center, New York, and Dr. Aboulhosn, an interventional cardiologist at Ronald Reagan University of California, Los Angeles, Medical Center. They emphasized the guidelines’ “heavy emphasis” on shared decisionmaking with patients.
Dr. Kavinsky is a principal investigator for Edwards Lifesciences, W.L. Gore and Associates, Medtronic, and Abbott. Dr. Sommer is a principal investigator and investigator in studies sponsored by W.L. Gore & Associates. Dr. Aboulhosn is a consultant to Abbott Medical.
The first-ever guidelines for interventional cardiologists using percutaneous patent foramen ovale closure recommend expanding the use of the procedure beyond the Food and Drug Administration–approved indication following PFO-associated ischemic stroke, adding clarification about the use of PFO with anticoagulation and hedging against abuse and overuse of the procedure, said the chair of the guideline writing committee.
“The most important things surrounding these guidelines are to help clinicians and policymakers – third-party payers – to address PFO in patient subsets that were not included in the large randomized clinical trials that led to FDA approval,” said writing group chair Clifford J. Kavinsky, MD, PhD, chief of structural and interventional cardiology at Rush University Medical Center, Chicago.
The Society for Cardiovascular Angiography & Interventions issued the guidelines at its annual scientific sessions meeting in Atlanta and published them simultaneously in the society’s journal.
The guidelines issue strong and conditional recommendations. The former means clinicians should order the intervention for most patients; the latter means decisionmaking is more nuanced and should consider contributing factors.
The guidelines clarify patient selection for PFO closure outside the “pretty narrow” indication the FDA approved, Dr. Kavinsky said, which is for PFO-associated ischemic stroke in patients aged 18-60 years.
“So what about patients who are older than 60? What about patients who had their stroke 10 years ago?” Dr. Kavinsky asked. “Those are issues that were unanswered in the randomized clinical trials.”
The guidelines also refine recommendations about anticoagulation in these patients, including its use after PFO closure in selected patients, Dr. Kavinsky noted. “It’s the opinion of the panel that although anticoagulants may be effective, because of issues of noncompliance, because of issues of interruption of therapy by physicians for a variety of reasons, including surgery or noncompliance, that it is preferable to do a PFO device closure to giving anticoagulant therapy.”
Many of the recommendations cover PFO closure alongside antiplatelet or anticoagulation therapy. Key conditional recommendations for patients who haven’t had a PFO-related stroke are:
- Avoiding its routine use in patients with chronic migraines, prior decompression illness (DCI), thrombophilia, atrial septal aneurysm, transient ischemic attack (TIA), or deep vein thrombosis (DVT).
- Considering PFO closure in patients with platypnea-orthodeoxia syndrome (POS) with no other discernible cause of hypoxia or systemic embolism in whom other embolic causes have been ruled out.
In patients who’ve had a PFO-related stroke, the guidelines strongly recommend PFO closure versus antiplatelet therapy alone, but conditionally, not in patients with atrial fibrillation who’ve had an ischemic stroke. They also conditionally suggest PFO closure rather than long-term antiplatelet therapy alone in PFO stroke patients aged 60 and older, as well as those with thrombophilia already on antiplatelet therapy but not anticoagulation. However, the guidelines make no recommendation on PFO closure based on how much time has passed since the previous stroke.
“Furthermore,” Dr. Kavinsky said, “in patients who require lifelong anticoagulation because of recurrent DVT or recurrent pulmonary emboli or thrombopenia, if they’ve had a PFO-mediated stroke, then it’s our opinion that they should have their PFO closed in addition to taking lifelong anticoagulation because of the same issues of noncompliance and interruption of therapy.” Those are conditional recommendations.
The guideline also checks a box in the FDA labeling that mandated agreement between cardiology and neurology in patient selection. The American Academy of Neurology (AAN) issued its own guideline in 2020 for patients with stroke and PFO. In Europe, the European Society of Cardiology issued two position papers on expanded applications of PFO closure.
The recommendations on when PFO closure shouldn’t be done are noteworthy, Dr. Kavinsky said. “PFOs are present in 25% of the adult population, so the number of patients with PFO is huge and the indication for the FDA is really narrow: to reduce the risk of recurrent stroke in patients with PFO-mediated stroke. So, there’s the tremendous potential for abuse out there, of excessive procedures, of doing unnecessary procedures.”
The guidelines are a follow-up to the operator institutional requirements document SCAI issued in 2019 that set requirements for hospital offering and physicians performing PFO closure, Dr. Kavinsky added.
In an editorial accompanying the published guideline, Robert J. Sommer, MD, and Jamil A. Aboulhosn, MD, wrote that they support the recommendations “which help spotlight and clarify the growing list of potential indications for PFO closure.” They noted that the guidelines panel’s “strong” recommendations were for indications validated by randomized trials and that “conditional” recommendations were based on panelists’ experience and observational data.
“It is critical to recognize that most of these guidelines represent consensus opinion only,” wrote Dr. Sommer, who specializes in adult congenital and pediatric cardiology at Columbia University Irving Medical Center, New York, and Dr. Aboulhosn, an interventional cardiologist at Ronald Reagan University of California, Los Angeles, Medical Center. They emphasized the guidelines’ “heavy emphasis” on shared decisionmaking with patients.
Dr. Kavinsky is a principal investigator for Edwards Lifesciences, W.L. Gore and Associates, Medtronic, and Abbott. Dr. Sommer is a principal investigator and investigator in studies sponsored by W.L. Gore & Associates. Dr. Aboulhosn is a consultant to Abbott Medical.
The first-ever guidelines for interventional cardiologists using percutaneous patent foramen ovale closure recommend expanding the use of the procedure beyond the Food and Drug Administration–approved indication following PFO-associated ischemic stroke, adding clarification about the use of PFO with anticoagulation and hedging against abuse and overuse of the procedure, said the chair of the guideline writing committee.
“The most important things surrounding these guidelines are to help clinicians and policymakers – third-party payers – to address PFO in patient subsets that were not included in the large randomized clinical trials that led to FDA approval,” said writing group chair Clifford J. Kavinsky, MD, PhD, chief of structural and interventional cardiology at Rush University Medical Center, Chicago.
The Society for Cardiovascular Angiography & Interventions issued the guidelines at its annual scientific sessions meeting in Atlanta and published them simultaneously in the society’s journal.
The guidelines issue strong and conditional recommendations. The former means clinicians should order the intervention for most patients; the latter means decisionmaking is more nuanced and should consider contributing factors.
The guidelines clarify patient selection for PFO closure outside the “pretty narrow” indication the FDA approved, Dr. Kavinsky said, which is for PFO-associated ischemic stroke in patients aged 18-60 years.
“So what about patients who are older than 60? What about patients who had their stroke 10 years ago?” Dr. Kavinsky asked. “Those are issues that were unanswered in the randomized clinical trials.”
The guidelines also refine recommendations about anticoagulation in these patients, including its use after PFO closure in selected patients, Dr. Kavinsky noted. “It’s the opinion of the panel that although anticoagulants may be effective, because of issues of noncompliance, because of issues of interruption of therapy by physicians for a variety of reasons, including surgery or noncompliance, that it is preferable to do a PFO device closure to giving anticoagulant therapy.”
Many of the recommendations cover PFO closure alongside antiplatelet or anticoagulation therapy. Key conditional recommendations for patients who haven’t had a PFO-related stroke are:
- Avoiding its routine use in patients with chronic migraines, prior decompression illness (DCI), thrombophilia, atrial septal aneurysm, transient ischemic attack (TIA), or deep vein thrombosis (DVT).
- Considering PFO closure in patients with platypnea-orthodeoxia syndrome (POS) with no other discernible cause of hypoxia or systemic embolism in whom other embolic causes have been ruled out.
In patients who’ve had a PFO-related stroke, the guidelines strongly recommend PFO closure versus antiplatelet therapy alone, but conditionally, not in patients with atrial fibrillation who’ve had an ischemic stroke. They also conditionally suggest PFO closure rather than long-term antiplatelet therapy alone in PFO stroke patients aged 60 and older, as well as those with thrombophilia already on antiplatelet therapy but not anticoagulation. However, the guidelines make no recommendation on PFO closure based on how much time has passed since the previous stroke.
“Furthermore,” Dr. Kavinsky said, “in patients who require lifelong anticoagulation because of recurrent DVT or recurrent pulmonary emboli or thrombopenia, if they’ve had a PFO-mediated stroke, then it’s our opinion that they should have their PFO closed in addition to taking lifelong anticoagulation because of the same issues of noncompliance and interruption of therapy.” Those are conditional recommendations.
The guideline also checks a box in the FDA labeling that mandated agreement between cardiology and neurology in patient selection. The American Academy of Neurology (AAN) issued its own guideline in 2020 for patients with stroke and PFO. In Europe, the European Society of Cardiology issued two position papers on expanded applications of PFO closure.
The recommendations on when PFO closure shouldn’t be done are noteworthy, Dr. Kavinsky said. “PFOs are present in 25% of the adult population, so the number of patients with PFO is huge and the indication for the FDA is really narrow: to reduce the risk of recurrent stroke in patients with PFO-mediated stroke. So, there’s the tremendous potential for abuse out there, of excessive procedures, of doing unnecessary procedures.”
The guidelines are a follow-up to the operator institutional requirements document SCAI issued in 2019 that set requirements for hospital offering and physicians performing PFO closure, Dr. Kavinsky added.
In an editorial accompanying the published guideline, Robert J. Sommer, MD, and Jamil A. Aboulhosn, MD, wrote that they support the recommendations “which help spotlight and clarify the growing list of potential indications for PFO closure.” They noted that the guidelines panel’s “strong” recommendations were for indications validated by randomized trials and that “conditional” recommendations were based on panelists’ experience and observational data.
“It is critical to recognize that most of these guidelines represent consensus opinion only,” wrote Dr. Sommer, who specializes in adult congenital and pediatric cardiology at Columbia University Irving Medical Center, New York, and Dr. Aboulhosn, an interventional cardiologist at Ronald Reagan University of California, Los Angeles, Medical Center. They emphasized the guidelines’ “heavy emphasis” on shared decisionmaking with patients.
Dr. Kavinsky is a principal investigator for Edwards Lifesciences, W.L. Gore and Associates, Medtronic, and Abbott. Dr. Sommer is a principal investigator and investigator in studies sponsored by W.L. Gore & Associates. Dr. Aboulhosn is a consultant to Abbott Medical.
FROM SCAI 2022
Keeping thyroid hormone treatment on target is key for the heart
A new study highlights the importance of avoiding both exogenous hyperthyroidism and exogenous hypothyroidism to decrease cardiovascular risk and death among patients receiving thyroid hormone treatment.
“Our findings suggest that clinicians should make every effort to maintain euthyroidism in patients on thyroid hormone treatment, regardless of underlying cardiovascular risk, particularly in vulnerable populations, such as older adults,” senior author Maria Papaleontiou, MD, said in an interview.
Commenting on the study, David S. Cooper, MD, of Johns Hopkins University School of Medicine, Baltimore, agreed that the findings are significant.
“Both undertreatment and overtreatment were associated with adverse cardiovascular outcomes, meaning that patients’ thyroid function needs to be monitored, and levothyroxine adjusted if need be, on an ongoing basis,” he told this news organization.
Getting the balance right: a tricky task
Variations in thyroid hormone levels falling above or below target ranges are common with thyroid hormone therapy, as a wide array of factors can prompt the need to regularly adjust dosing to maintain “index” levels. And while guidelines from the American Thyroid Association (ATA) recommend maintaining serum thyroid stimulating hormone (TSH) levels in the normal ranges during treatment, the task is tricky.
“Despite these [ATA] guidelines, prior studies in adults with hypothyroidism have shown that up to 30% are undertreated and up to 48% are overtreated,” said Dr. Papaleontiou, an assistant professor in the Division of Metabolism, Endocrinology at the University of Michigan, Ann Arbor.
In a previous study, Dr. Papaleontiou and colleagues showed that the intensity of thyroid hormone treatment is a modifiable risk factor for incident atrial fibrillation and stroke, however, less is understood about the association with cardiovascular mortality.
For the new study, published in JAMA Network Open, Josh M. Evron, MD, of the University of North Carolina, Chapel Hill, and colleagues further investigated the issue in a large, retrospective cohort of 705,307 adults in the Veterans Health Administration Corporate Data Warehouse treated with thyroid hormone during 2004-2017 who had a median follow-up of 4 years.
They investigated the roles of TSH as well as free thyroxine (FT4) levels among 701,929 adults in the group with data on TSH and 373,981 patients with FT4 measurements.
The mean age of participants was 67 years and 88.7% were male.
Over the course of the study, 10.8% of patients (75,963) died of cardiovascular causes.
Compared with patients with normal thyroid levels, those with exogenous hyperthyroidism related to thyroid hormone treatment had an increased risk of cardiovascular mortality, specifically including when TSH levels were below 0.1 mIU/L (adjusted hazard ratio, 1.39) and when FT4 levels were above 1.9 ng/dL (AHR, 1.29), independent of factors including age, sex, and traditional cardiovascular risk factors, including hypertension, smoking, and previous cardiovascular disease or arrhythmia.
In addition, the increased risk of cardiovascular mortality was observed with exogenous hypothyroidism, specifically among those with TSH levels above 20 mIU/L (AHR, 2.67) and FT4 levels below 0.7 ng/dL (AHR, 1.56), after multivariate adjustment.
Of note, the risk of cardiovascular mortality was dose-dependent, with the risk increasing progressively with the lower and higher TSH levels, compared with normal levels.
The increased mortality risk in relation to TSH levels was more pronounced among older patients, compared with FT4 associations, the authors note.
“From a clinical perspective, older adults, and particularly the oldest old (aged 85 years), appear to be the most vulnerable, with increased risk of cardiovascular mortality with both exogenous hyperthyroidism and hypothyroidism,” they report.
Among key limitations is that women, who make up the majority of patients with thyroid disease, are under-represented in the predominantly male population of the Veterans Health Administration.
Nevertheless, “because the risk of cardiovascular disease is higher for men than for women, and because more than 70,000 women were included in this cohort, the results of this study are highly clinically relevant,” the authors note.
Addressing over- and under-treatment will avoid harm
The results are also important considering the status of levothyroxine (for hypothyroidism) as consistently ranking among the top three prescription medications in the United States.
And with the common occurrence of exogenous hyperthyroidism or hypothyroidism, the findings have important implications.
“Addressing over- and under-treatment of hypothyroidism promptly will help reduce patient harm, particularly in vulnerable populations such as older adults who are at higher risk for adverse effects,” Dr. Papaleontiou said.
Dr. Cooper further commented that the findings underscore the need to be aware of treatment adjustments and targets that may vary according to patient age.
“In older persons, over 65-70, the target TSH may be higher [for example, 2-4 mIU/L] than in younger persons, and in patients above ages 70 or 80, serum TSH levels may be allowed to rise even further into the 4-6 mIU/L range,” he explained.
“The older the patient, the higher the chance for an adverse cardiovascular outcome if the TSH is subnormal due to iatrogenic thyrotoxicosis,” Dr. Cooper explained.
“In contrast, in younger individuals, an elevated TSH, indicating mild [subclinical] hypothyroidism may be associated with increased cardiovascular risk, especially with serum TSH levels greater than 7 mIU/L.”
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study highlights the importance of avoiding both exogenous hyperthyroidism and exogenous hypothyroidism to decrease cardiovascular risk and death among patients receiving thyroid hormone treatment.
“Our findings suggest that clinicians should make every effort to maintain euthyroidism in patients on thyroid hormone treatment, regardless of underlying cardiovascular risk, particularly in vulnerable populations, such as older adults,” senior author Maria Papaleontiou, MD, said in an interview.
Commenting on the study, David S. Cooper, MD, of Johns Hopkins University School of Medicine, Baltimore, agreed that the findings are significant.
“Both undertreatment and overtreatment were associated with adverse cardiovascular outcomes, meaning that patients’ thyroid function needs to be monitored, and levothyroxine adjusted if need be, on an ongoing basis,” he told this news organization.
Getting the balance right: a tricky task
Variations in thyroid hormone levels falling above or below target ranges are common with thyroid hormone therapy, as a wide array of factors can prompt the need to regularly adjust dosing to maintain “index” levels. And while guidelines from the American Thyroid Association (ATA) recommend maintaining serum thyroid stimulating hormone (TSH) levels in the normal ranges during treatment, the task is tricky.
“Despite these [ATA] guidelines, prior studies in adults with hypothyroidism have shown that up to 30% are undertreated and up to 48% are overtreated,” said Dr. Papaleontiou, an assistant professor in the Division of Metabolism, Endocrinology at the University of Michigan, Ann Arbor.
In a previous study, Dr. Papaleontiou and colleagues showed that the intensity of thyroid hormone treatment is a modifiable risk factor for incident atrial fibrillation and stroke, however, less is understood about the association with cardiovascular mortality.
For the new study, published in JAMA Network Open, Josh M. Evron, MD, of the University of North Carolina, Chapel Hill, and colleagues further investigated the issue in a large, retrospective cohort of 705,307 adults in the Veterans Health Administration Corporate Data Warehouse treated with thyroid hormone during 2004-2017 who had a median follow-up of 4 years.
They investigated the roles of TSH as well as free thyroxine (FT4) levels among 701,929 adults in the group with data on TSH and 373,981 patients with FT4 measurements.
The mean age of participants was 67 years and 88.7% were male.
Over the course of the study, 10.8% of patients (75,963) died of cardiovascular causes.
Compared with patients with normal thyroid levels, those with exogenous hyperthyroidism related to thyroid hormone treatment had an increased risk of cardiovascular mortality, specifically including when TSH levels were below 0.1 mIU/L (adjusted hazard ratio, 1.39) and when FT4 levels were above 1.9 ng/dL (AHR, 1.29), independent of factors including age, sex, and traditional cardiovascular risk factors, including hypertension, smoking, and previous cardiovascular disease or arrhythmia.
In addition, the increased risk of cardiovascular mortality was observed with exogenous hypothyroidism, specifically among those with TSH levels above 20 mIU/L (AHR, 2.67) and FT4 levels below 0.7 ng/dL (AHR, 1.56), after multivariate adjustment.
Of note, the risk of cardiovascular mortality was dose-dependent, with the risk increasing progressively with the lower and higher TSH levels, compared with normal levels.
The increased mortality risk in relation to TSH levels was more pronounced among older patients, compared with FT4 associations, the authors note.
“From a clinical perspective, older adults, and particularly the oldest old (aged 85 years), appear to be the most vulnerable, with increased risk of cardiovascular mortality with both exogenous hyperthyroidism and hypothyroidism,” they report.
Among key limitations is that women, who make up the majority of patients with thyroid disease, are under-represented in the predominantly male population of the Veterans Health Administration.
Nevertheless, “because the risk of cardiovascular disease is higher for men than for women, and because more than 70,000 women were included in this cohort, the results of this study are highly clinically relevant,” the authors note.
Addressing over- and under-treatment will avoid harm
The results are also important considering the status of levothyroxine (for hypothyroidism) as consistently ranking among the top three prescription medications in the United States.
And with the common occurrence of exogenous hyperthyroidism or hypothyroidism, the findings have important implications.
“Addressing over- and under-treatment of hypothyroidism promptly will help reduce patient harm, particularly in vulnerable populations such as older adults who are at higher risk for adverse effects,” Dr. Papaleontiou said.
Dr. Cooper further commented that the findings underscore the need to be aware of treatment adjustments and targets that may vary according to patient age.
“In older persons, over 65-70, the target TSH may be higher [for example, 2-4 mIU/L] than in younger persons, and in patients above ages 70 or 80, serum TSH levels may be allowed to rise even further into the 4-6 mIU/L range,” he explained.
“The older the patient, the higher the chance for an adverse cardiovascular outcome if the TSH is subnormal due to iatrogenic thyrotoxicosis,” Dr. Cooper explained.
“In contrast, in younger individuals, an elevated TSH, indicating mild [subclinical] hypothyroidism may be associated with increased cardiovascular risk, especially with serum TSH levels greater than 7 mIU/L.”
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study highlights the importance of avoiding both exogenous hyperthyroidism and exogenous hypothyroidism to decrease cardiovascular risk and death among patients receiving thyroid hormone treatment.
“Our findings suggest that clinicians should make every effort to maintain euthyroidism in patients on thyroid hormone treatment, regardless of underlying cardiovascular risk, particularly in vulnerable populations, such as older adults,” senior author Maria Papaleontiou, MD, said in an interview.
Commenting on the study, David S. Cooper, MD, of Johns Hopkins University School of Medicine, Baltimore, agreed that the findings are significant.
“Both undertreatment and overtreatment were associated with adverse cardiovascular outcomes, meaning that patients’ thyroid function needs to be monitored, and levothyroxine adjusted if need be, on an ongoing basis,” he told this news organization.
Getting the balance right: a tricky task
Variations in thyroid hormone levels falling above or below target ranges are common with thyroid hormone therapy, as a wide array of factors can prompt the need to regularly adjust dosing to maintain “index” levels. And while guidelines from the American Thyroid Association (ATA) recommend maintaining serum thyroid stimulating hormone (TSH) levels in the normal ranges during treatment, the task is tricky.
“Despite these [ATA] guidelines, prior studies in adults with hypothyroidism have shown that up to 30% are undertreated and up to 48% are overtreated,” said Dr. Papaleontiou, an assistant professor in the Division of Metabolism, Endocrinology at the University of Michigan, Ann Arbor.
In a previous study, Dr. Papaleontiou and colleagues showed that the intensity of thyroid hormone treatment is a modifiable risk factor for incident atrial fibrillation and stroke, however, less is understood about the association with cardiovascular mortality.
For the new study, published in JAMA Network Open, Josh M. Evron, MD, of the University of North Carolina, Chapel Hill, and colleagues further investigated the issue in a large, retrospective cohort of 705,307 adults in the Veterans Health Administration Corporate Data Warehouse treated with thyroid hormone during 2004-2017 who had a median follow-up of 4 years.
They investigated the roles of TSH as well as free thyroxine (FT4) levels among 701,929 adults in the group with data on TSH and 373,981 patients with FT4 measurements.
The mean age of participants was 67 years and 88.7% were male.
Over the course of the study, 10.8% of patients (75,963) died of cardiovascular causes.
Compared with patients with normal thyroid levels, those with exogenous hyperthyroidism related to thyroid hormone treatment had an increased risk of cardiovascular mortality, specifically including when TSH levels were below 0.1 mIU/L (adjusted hazard ratio, 1.39) and when FT4 levels were above 1.9 ng/dL (AHR, 1.29), independent of factors including age, sex, and traditional cardiovascular risk factors, including hypertension, smoking, and previous cardiovascular disease or arrhythmia.
In addition, the increased risk of cardiovascular mortality was observed with exogenous hypothyroidism, specifically among those with TSH levels above 20 mIU/L (AHR, 2.67) and FT4 levels below 0.7 ng/dL (AHR, 1.56), after multivariate adjustment.
Of note, the risk of cardiovascular mortality was dose-dependent, with the risk increasing progressively with the lower and higher TSH levels, compared with normal levels.
The increased mortality risk in relation to TSH levels was more pronounced among older patients, compared with FT4 associations, the authors note.
“From a clinical perspective, older adults, and particularly the oldest old (aged 85 years), appear to be the most vulnerable, with increased risk of cardiovascular mortality with both exogenous hyperthyroidism and hypothyroidism,” they report.
Among key limitations is that women, who make up the majority of patients with thyroid disease, are under-represented in the predominantly male population of the Veterans Health Administration.
Nevertheless, “because the risk of cardiovascular disease is higher for men than for women, and because more than 70,000 women were included in this cohort, the results of this study are highly clinically relevant,” the authors note.
Addressing over- and under-treatment will avoid harm
The results are also important considering the status of levothyroxine (for hypothyroidism) as consistently ranking among the top three prescription medications in the United States.
And with the common occurrence of exogenous hyperthyroidism or hypothyroidism, the findings have important implications.
“Addressing over- and under-treatment of hypothyroidism promptly will help reduce patient harm, particularly in vulnerable populations such as older adults who are at higher risk for adverse effects,” Dr. Papaleontiou said.
Dr. Cooper further commented that the findings underscore the need to be aware of treatment adjustments and targets that may vary according to patient age.
“In older persons, over 65-70, the target TSH may be higher [for example, 2-4 mIU/L] than in younger persons, and in patients above ages 70 or 80, serum TSH levels may be allowed to rise even further into the 4-6 mIU/L range,” he explained.
“The older the patient, the higher the chance for an adverse cardiovascular outcome if the TSH is subnormal due to iatrogenic thyrotoxicosis,” Dr. Cooper explained.
“In contrast, in younger individuals, an elevated TSH, indicating mild [subclinical] hypothyroidism may be associated with increased cardiovascular risk, especially with serum TSH levels greater than 7 mIU/L.”
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Taking cardiac pacing from boring to super cool
For the past 2 decades, catheter ablation stole most of the excitement in electrophysiology. Cardiac pacing was seen as necessary but boring. His-bundle pacing earned only modest attention.
But at the annual scientific sessions of the Heart Rhythm Society, cardiac pacing consolidated its comeback and entered the super-cool category.
Not one but three late-breaking clinical trials considered the role of pacing the heart’s conduction system for both preventive and therapeutic purposes. Conduction system pacing, or CSP as we call it, includes pacing the His bundle or the left bundle branch. Left bundle–branch pacing has now largely replaced His-bundle pacing.
Before I tell you about the studies, let’s review why CSP disrupts the status quo.
The core idea goes back to basic physiology: After the impulse leaves the atrioventricular node, the heart’s specialized conduction system allows rapid and synchronous conduction to both the right and left ventricles.
Standard cardiac pacing means fixing a pacing lead into the muscle of the right ventricle. From that spot, conduction spreads via slower muscle-to-muscle conduction, which leads to a wide QRS complex and the right ventricle contracts before the left ventricle.
While such dyssynchronous contraction is better than no contraction, this approach leads to a pacing-induced cardiomyopathy in a substantial number of cases. (The incidence reported in many studies varies widely.)
The most disruptive effect of conduction system pacing is that it is a form of cardiac resynchronization therapy (CRT). And that is nifty because, until recently, resynchronizing the ventricles required placing two ventricular leads: one in the right ventricle and the other in the coronary sinus to pace the left ventricle.
Left bundle-branch pacing vs. biventricular pacing
The first of the three HRS studies is the LBBP-RESYNC randomized controlled trial led by Jiangang Zou, MD, PhD, and performed in multiple centers in China. It compared the efficacy of left bundle–branch pacing (LBBP) with that of conventional biventricular pacing in 40 patients with heart failure who were eligible for CRT. The primary endpoint was the change in left ventricular ejection fraction (LVEF) from baseline to 6-month follow-up.
The results favored LBBP. Although both pacing techniques improved LVEF from baseline, the between-group difference in LVEF was greater in the LBBP arm than the biventricular pacing arm by a statistically significant 5.6% (95% confidence interval, 0.3%-10.9%). Secondary endpoints, such as reductions in left ventricular end-systolic volume, N-terminal of the prohormone brain natriuretic peptide, and QRS duration, also favored LBBP.
Conduction system pacing vs. biventricular pacing
A second late-breaking study, from the Geisinger group, led by Pugazhendhi Vijayaraman, MD, was simultaneously published in Heart Rhythm.
This nonrandomized observational study compared nearly 500 patients eligible for CRT treated at two health systems. One group favors conduction system pacing and the other does traditional biventricular pacing, which set up a two-armed comparison.
CSP was accomplished by LBBP (65%) and His-bundle pacing (35%).
The primary endpoint of death or first hospitalization for heart failure occurred in 28.3% of patients in the CSP arm versus 38.4% of the biventricular arm (hazard ratio, 1.52; 95% CI, 1.08-2.09). QRS duration and LVEF also improved from baseline in both groups.
LBB area pacing as a bailout for failed CRT
The Geisinger group also presented and published an international multicenter study that assessed the feasibility of LBBP as a bailout when standard biventricular pacing did not work – because of inadequate coronary sinus anatomy or CRT nonresponse, defined as lack of clinical or echocardiographic improvement.
This series included 212 patients in whom CRT failed and who underwent attempted LBBP pacing. The bailout was successful in 200 patients (91%). The primary endpoint was defined as an increase in LVEF above 5% on echocardiography.
During 12-month follow-up, 61% of patients had an improvement in LVEF above 5% and nearly 30% had a “super-response,” defined as a 20% or greater increase or normalization of LVEF. Similar to the previous studies, LBBP resulted in shorter QRS duration and improved echocardiography parameters.
Am I persuaded?
I was an early adopter of His-bundle pacing. When successful, it delivered both aesthetically pleasing QRS complexes and clinical efficacy. But there were many challenges: it is technically difficult, and capture thresholds are often high at implant and get higher over time, which leads to shorter battery life.
Pacing the left bundle branch mitigates these challenges. Here, the operator approaches from the right side and screws the lead a few millimeters into the septum, so the tip of the lead can capture the left bundle or one of its branches. This allows activation of the heart’s specialized conduction system and thus synchronizes right and left ventricle contraction.
Although there is a learning curve, LBBP is technically easier than His-bundle pacing and ultimately results in far better pacing and sensing parameters. What’s more, the preferred lead for LBBP has a stellar efficacy record – over years.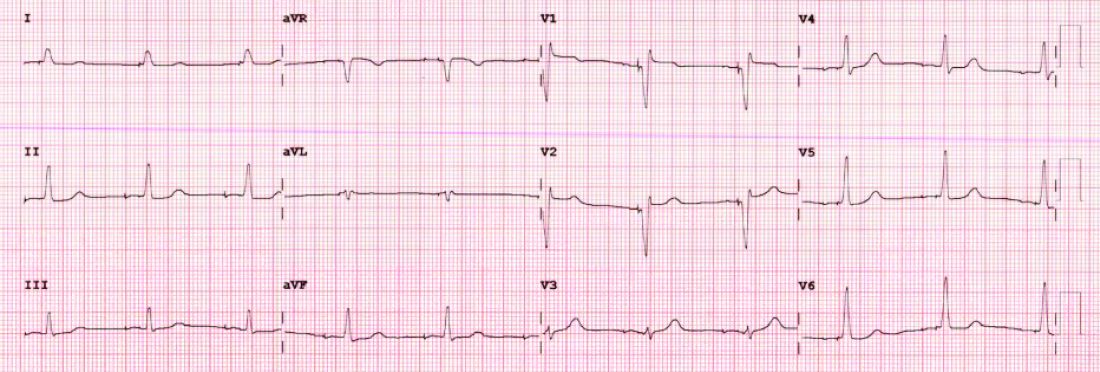
I have become enthralled by the gorgeous QRS complexes from LBBP. The ability to pace the heart without creating dyssynchrony infuses me with joy. I chose cardiology largely because of the beauty of the ECG.
But as a medical conservative who is cautious about unproven therapies, I have questions. How is LBBP defined? Is left septal pacing good enough, or do you need actual left bundle capture? What about long-term performance of a lead in the septum?
Biventricular pacing has set a high bar because it has been proven effective for reducing hard clinical outcomes in large randomized controlled trials.
The studies at HRS begin to answer these questions. The randomized controlled trial from China supports the notion that effective LBBP (the investigators rigorously defined left bundle capture) leads to favorable effects on cardiac contraction. The two observational studies reported similarly encouraging findings on cardiac function.
The three studies therefore tentatively support the notion that LBBP actually produces favorable cardiac performance.
Whether LBBP leads to better clinical outcomes remains uncertain. The nonrandomized comparison study, which found better hard outcomes in the CSP arm, cannot be used to infer causality. There is too much risk for selection bias.
But the LBBP bailout study does suggest that this strategy is reasonable when coronary sinus leads fail – especially since the alternative is surgical placement of an epicardial lead on the left ventricle.
At minimum, the HRS studies persuade me that LBBP will likely prevent pacing-induced cardiomyopathy. If I or a family member required a pacemaker, I’d surely want the operator to be skilled at placing a left bundle lead.
While I am confident that conduction system pacing will become a transformative advance in cardiac pacing, aesthetically pleasing ECG patterns are not enough. There remains much to learn with this nascent approach.
The barriers to getting more CSP trials
The challenge going forward will be funding new trials. CSP stands to prevent pacing-induced cardiomyopathy and offer less costly alternatives to standard biventricular pacing for CRT. This is great for patients, but it would mean that fewer higher-cost CRT devices will be sold.
Heart rhythm research is largely industry-funded because in most cases better therapies for patients mean more profits for industry. In the case of CSP, there is no such confluence of interests.
Conduction system pacing has come about because of the efforts of a few tireless champions who not only published extensively but were also skilled at using social media to spread the excitement. Trials have been small and often self-funded.
The data presented at HRS provides enough equipoise to support a large outcomes-based randomized controlled trial. Imagine if our CSP champions were able to find public-funding sources for such future trials.
Now that would be super cool.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky., and is a writer and podcaster for Medscape. He participates in clinical research and writes often about the state of medical evidence. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
For the past 2 decades, catheter ablation stole most of the excitement in electrophysiology. Cardiac pacing was seen as necessary but boring. His-bundle pacing earned only modest attention.
But at the annual scientific sessions of the Heart Rhythm Society, cardiac pacing consolidated its comeback and entered the super-cool category.
Not one but three late-breaking clinical trials considered the role of pacing the heart’s conduction system for both preventive and therapeutic purposes. Conduction system pacing, or CSP as we call it, includes pacing the His bundle or the left bundle branch. Left bundle–branch pacing has now largely replaced His-bundle pacing.
Before I tell you about the studies, let’s review why CSP disrupts the status quo.
The core idea goes back to basic physiology: After the impulse leaves the atrioventricular node, the heart’s specialized conduction system allows rapid and synchronous conduction to both the right and left ventricles.
Standard cardiac pacing means fixing a pacing lead into the muscle of the right ventricle. From that spot, conduction spreads via slower muscle-to-muscle conduction, which leads to a wide QRS complex and the right ventricle contracts before the left ventricle.
While such dyssynchronous contraction is better than no contraction, this approach leads to a pacing-induced cardiomyopathy in a substantial number of cases. (The incidence reported in many studies varies widely.)
The most disruptive effect of conduction system pacing is that it is a form of cardiac resynchronization therapy (CRT). And that is nifty because, until recently, resynchronizing the ventricles required placing two ventricular leads: one in the right ventricle and the other in the coronary sinus to pace the left ventricle.
Left bundle-branch pacing vs. biventricular pacing
The first of the three HRS studies is the LBBP-RESYNC randomized controlled trial led by Jiangang Zou, MD, PhD, and performed in multiple centers in China. It compared the efficacy of left bundle–branch pacing (LBBP) with that of conventional biventricular pacing in 40 patients with heart failure who were eligible for CRT. The primary endpoint was the change in left ventricular ejection fraction (LVEF) from baseline to 6-month follow-up.
The results favored LBBP. Although both pacing techniques improved LVEF from baseline, the between-group difference in LVEF was greater in the LBBP arm than the biventricular pacing arm by a statistically significant 5.6% (95% confidence interval, 0.3%-10.9%). Secondary endpoints, such as reductions in left ventricular end-systolic volume, N-terminal of the prohormone brain natriuretic peptide, and QRS duration, also favored LBBP.
Conduction system pacing vs. biventricular pacing
A second late-breaking study, from the Geisinger group, led by Pugazhendhi Vijayaraman, MD, was simultaneously published in Heart Rhythm.
This nonrandomized observational study compared nearly 500 patients eligible for CRT treated at two health systems. One group favors conduction system pacing and the other does traditional biventricular pacing, which set up a two-armed comparison.
CSP was accomplished by LBBP (65%) and His-bundle pacing (35%).
The primary endpoint of death or first hospitalization for heart failure occurred in 28.3% of patients in the CSP arm versus 38.4% of the biventricular arm (hazard ratio, 1.52; 95% CI, 1.08-2.09). QRS duration and LVEF also improved from baseline in both groups.
LBB area pacing as a bailout for failed CRT
The Geisinger group also presented and published an international multicenter study that assessed the feasibility of LBBP as a bailout when standard biventricular pacing did not work – because of inadequate coronary sinus anatomy or CRT nonresponse, defined as lack of clinical or echocardiographic improvement.
This series included 212 patients in whom CRT failed and who underwent attempted LBBP pacing. The bailout was successful in 200 patients (91%). The primary endpoint was defined as an increase in LVEF above 5% on echocardiography.
During 12-month follow-up, 61% of patients had an improvement in LVEF above 5% and nearly 30% had a “super-response,” defined as a 20% or greater increase or normalization of LVEF. Similar to the previous studies, LBBP resulted in shorter QRS duration and improved echocardiography parameters.
Am I persuaded?
I was an early adopter of His-bundle pacing. When successful, it delivered both aesthetically pleasing QRS complexes and clinical efficacy. But there were many challenges: it is technically difficult, and capture thresholds are often high at implant and get higher over time, which leads to shorter battery life.
Pacing the left bundle branch mitigates these challenges. Here, the operator approaches from the right side and screws the lead a few millimeters into the septum, so the tip of the lead can capture the left bundle or one of its branches. This allows activation of the heart’s specialized conduction system and thus synchronizes right and left ventricle contraction.
Although there is a learning curve, LBBP is technically easier than His-bundle pacing and ultimately results in far better pacing and sensing parameters. What’s more, the preferred lead for LBBP has a stellar efficacy record – over years.
I have become enthralled by the gorgeous QRS complexes from LBBP. The ability to pace the heart without creating dyssynchrony infuses me with joy. I chose cardiology largely because of the beauty of the ECG.
But as a medical conservative who is cautious about unproven therapies, I have questions. How is LBBP defined? Is left septal pacing good enough, or do you need actual left bundle capture? What about long-term performance of a lead in the septum?
Biventricular pacing has set a high bar because it has been proven effective for reducing hard clinical outcomes in large randomized controlled trials.
The studies at HRS begin to answer these questions. The randomized controlled trial from China supports the notion that effective LBBP (the investigators rigorously defined left bundle capture) leads to favorable effects on cardiac contraction. The two observational studies reported similarly encouraging findings on cardiac function.
The three studies therefore tentatively support the notion that LBBP actually produces favorable cardiac performance.
Whether LBBP leads to better clinical outcomes remains uncertain. The nonrandomized comparison study, which found better hard outcomes in the CSP arm, cannot be used to infer causality. There is too much risk for selection bias.
But the LBBP bailout study does suggest that this strategy is reasonable when coronary sinus leads fail – especially since the alternative is surgical placement of an epicardial lead on the left ventricle.
At minimum, the HRS studies persuade me that LBBP will likely prevent pacing-induced cardiomyopathy. If I or a family member required a pacemaker, I’d surely want the operator to be skilled at placing a left bundle lead.
While I am confident that conduction system pacing will become a transformative advance in cardiac pacing, aesthetically pleasing ECG patterns are not enough. There remains much to learn with this nascent approach.
The barriers to getting more CSP trials
The challenge going forward will be funding new trials. CSP stands to prevent pacing-induced cardiomyopathy and offer less costly alternatives to standard biventricular pacing for CRT. This is great for patients, but it would mean that fewer higher-cost CRT devices will be sold.
Heart rhythm research is largely industry-funded because in most cases better therapies for patients mean more profits for industry. In the case of CSP, there is no such confluence of interests.
Conduction system pacing has come about because of the efforts of a few tireless champions who not only published extensively but were also skilled at using social media to spread the excitement. Trials have been small and often self-funded.
The data presented at HRS provides enough equipoise to support a large outcomes-based randomized controlled trial. Imagine if our CSP champions were able to find public-funding sources for such future trials.
Now that would be super cool.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky., and is a writer and podcaster for Medscape. He participates in clinical research and writes often about the state of medical evidence. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
For the past 2 decades, catheter ablation stole most of the excitement in electrophysiology. Cardiac pacing was seen as necessary but boring. His-bundle pacing earned only modest attention.
But at the annual scientific sessions of the Heart Rhythm Society, cardiac pacing consolidated its comeback and entered the super-cool category.
Not one but three late-breaking clinical trials considered the role of pacing the heart’s conduction system for both preventive and therapeutic purposes. Conduction system pacing, or CSP as we call it, includes pacing the His bundle or the left bundle branch. Left bundle–branch pacing has now largely replaced His-bundle pacing.
Before I tell you about the studies, let’s review why CSP disrupts the status quo.
The core idea goes back to basic physiology: After the impulse leaves the atrioventricular node, the heart’s specialized conduction system allows rapid and synchronous conduction to both the right and left ventricles.
Standard cardiac pacing means fixing a pacing lead into the muscle of the right ventricle. From that spot, conduction spreads via slower muscle-to-muscle conduction, which leads to a wide QRS complex and the right ventricle contracts before the left ventricle.
While such dyssynchronous contraction is better than no contraction, this approach leads to a pacing-induced cardiomyopathy in a substantial number of cases. (The incidence reported in many studies varies widely.)
The most disruptive effect of conduction system pacing is that it is a form of cardiac resynchronization therapy (CRT). And that is nifty because, until recently, resynchronizing the ventricles required placing two ventricular leads: one in the right ventricle and the other in the coronary sinus to pace the left ventricle.
Left bundle-branch pacing vs. biventricular pacing
The first of the three HRS studies is the LBBP-RESYNC randomized controlled trial led by Jiangang Zou, MD, PhD, and performed in multiple centers in China. It compared the efficacy of left bundle–branch pacing (LBBP) with that of conventional biventricular pacing in 40 patients with heart failure who were eligible for CRT. The primary endpoint was the change in left ventricular ejection fraction (LVEF) from baseline to 6-month follow-up.
The results favored LBBP. Although both pacing techniques improved LVEF from baseline, the between-group difference in LVEF was greater in the LBBP arm than the biventricular pacing arm by a statistically significant 5.6% (95% confidence interval, 0.3%-10.9%). Secondary endpoints, such as reductions in left ventricular end-systolic volume, N-terminal of the prohormone brain natriuretic peptide, and QRS duration, also favored LBBP.
Conduction system pacing vs. biventricular pacing
A second late-breaking study, from the Geisinger group, led by Pugazhendhi Vijayaraman, MD, was simultaneously published in Heart Rhythm.
This nonrandomized observational study compared nearly 500 patients eligible for CRT treated at two health systems. One group favors conduction system pacing and the other does traditional biventricular pacing, which set up a two-armed comparison.
CSP was accomplished by LBBP (65%) and His-bundle pacing (35%).
The primary endpoint of death or first hospitalization for heart failure occurred in 28.3% of patients in the CSP arm versus 38.4% of the biventricular arm (hazard ratio, 1.52; 95% CI, 1.08-2.09). QRS duration and LVEF also improved from baseline in both groups.
LBB area pacing as a bailout for failed CRT
The Geisinger group also presented and published an international multicenter study that assessed the feasibility of LBBP as a bailout when standard biventricular pacing did not work – because of inadequate coronary sinus anatomy or CRT nonresponse, defined as lack of clinical or echocardiographic improvement.
This series included 212 patients in whom CRT failed and who underwent attempted LBBP pacing. The bailout was successful in 200 patients (91%). The primary endpoint was defined as an increase in LVEF above 5% on echocardiography.
During 12-month follow-up, 61% of patients had an improvement in LVEF above 5% and nearly 30% had a “super-response,” defined as a 20% or greater increase or normalization of LVEF. Similar to the previous studies, LBBP resulted in shorter QRS duration and improved echocardiography parameters.
Am I persuaded?
I was an early adopter of His-bundle pacing. When successful, it delivered both aesthetically pleasing QRS complexes and clinical efficacy. But there were many challenges: it is technically difficult, and capture thresholds are often high at implant and get higher over time, which leads to shorter battery life.
Pacing the left bundle branch mitigates these challenges. Here, the operator approaches from the right side and screws the lead a few millimeters into the septum, so the tip of the lead can capture the left bundle or one of its branches. This allows activation of the heart’s specialized conduction system and thus synchronizes right and left ventricle contraction.
Although there is a learning curve, LBBP is technically easier than His-bundle pacing and ultimately results in far better pacing and sensing parameters. What’s more, the preferred lead for LBBP has a stellar efficacy record – over years.
I have become enthralled by the gorgeous QRS complexes from LBBP. The ability to pace the heart without creating dyssynchrony infuses me with joy. I chose cardiology largely because of the beauty of the ECG.
But as a medical conservative who is cautious about unproven therapies, I have questions. How is LBBP defined? Is left septal pacing good enough, or do you need actual left bundle capture? What about long-term performance of a lead in the septum?
Biventricular pacing has set a high bar because it has been proven effective for reducing hard clinical outcomes in large randomized controlled trials.
The studies at HRS begin to answer these questions. The randomized controlled trial from China supports the notion that effective LBBP (the investigators rigorously defined left bundle capture) leads to favorable effects on cardiac contraction. The two observational studies reported similarly encouraging findings on cardiac function.
The three studies therefore tentatively support the notion that LBBP actually produces favorable cardiac performance.
Whether LBBP leads to better clinical outcomes remains uncertain. The nonrandomized comparison study, which found better hard outcomes in the CSP arm, cannot be used to infer causality. There is too much risk for selection bias.
But the LBBP bailout study does suggest that this strategy is reasonable when coronary sinus leads fail – especially since the alternative is surgical placement of an epicardial lead on the left ventricle.
At minimum, the HRS studies persuade me that LBBP will likely prevent pacing-induced cardiomyopathy. If I or a family member required a pacemaker, I’d surely want the operator to be skilled at placing a left bundle lead.
While I am confident that conduction system pacing will become a transformative advance in cardiac pacing, aesthetically pleasing ECG patterns are not enough. There remains much to learn with this nascent approach.
The barriers to getting more CSP trials
The challenge going forward will be funding new trials. CSP stands to prevent pacing-induced cardiomyopathy and offer less costly alternatives to standard biventricular pacing for CRT. This is great for patients, but it would mean that fewer higher-cost CRT devices will be sold.
Heart rhythm research is largely industry-funded because in most cases better therapies for patients mean more profits for industry. In the case of CSP, there is no such confluence of interests.
Conduction system pacing has come about because of the efforts of a few tireless champions who not only published extensively but were also skilled at using social media to spread the excitement. Trials have been small and often self-funded.
The data presented at HRS provides enough equipoise to support a large outcomes-based randomized controlled trial. Imagine if our CSP champions were able to find public-funding sources for such future trials.
Now that would be super cool.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky., and is a writer and podcaster for Medscape. He participates in clinical research and writes often about the state of medical evidence. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Antithrombotic therapies shifting for Watchman LAA occlusion
A new study finds clinicians are shifting away from the U.S. Food and Drug Administration–approved combination of warfarin and aspirin after left atrial appendage occlusion (LAAO) with the Watchman device and that adverse events, particularly bleeding, are lower when aspirin is dropped.
Of 31,994 patients successfully implanted with the Watchman 2.5 device in the 3 years after its March 2015 approval, only 1 in 10 received the full postprocedure protocol studied in pivotal trials and codified into the FDA-device approval.
The protocol consisted of aspirin (81-325 mg) indefinitely and warfarin for 45 days. Following transesophageal echocardiography, patients were then maintained on warfarin and aspirin if there was a peridevice leak greater than 5 mm or switched to clopidogrel 75 mg for 6 months if a peridevice leak was ruled out or was 5 mm or less.
Based on the results, drawn from the National Cardiovascular Data Registry (NCDR) LAAO Registry, the most common discharge medications were warfarin and aspirin in 36.9% of patients, followed by a direct oral anticoagulant (DOAC) and aspirin (20.8%), warfarin alone (13.5%), DOAC only (12.3%), and dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor (5%).
“There’s a little bit of practice leading the science in this space,” lead author James V. Freeman, MD, MPH, Yale School of Medicine, New Haven, Conn., told this news organization.
Patients who couldn’t tolerate long-term anticoagulation were excluded from the pivotal trials but are now the patients in whom the device is most often used, because of the Centers for Medicare & Medicaid reimbursement mandate for a relative or absolute contraindication to long-term anticoagulation, he noted.
Not surprisingly, 70% of patients in the registry had history of clinically relevant bleeding, the mean CHA2DS2-VASc score was 4.6, and mean HAS-BLED score was 3. At an average age of 76, they were also older, by years, than those in the clinical trials.
Secular trends at the time also saw the ascendancy of the DOACs relative to warfarin, observed Dr. Freeman. “So I think it’s pretty reasonable for physicians to be considering DOACs rather than warfarin in this context.”
Aspirin takes another hit
Results, published May 2 in the Journal of the American College of Cardiology, showed that any adverse event occurred at 45 days in 5.7% of patients discharged on warfarin and aspirin, 4% on warfarin alone, 5.2% on DOAC and aspirin, 3.8% on DOAC only, and 5.5% on DAPT.
Rates of any major adverse event were 4.4%, 3.3%, 4.3%, 3.1%, and 4.2% respectively, and for major bleeding were 3%, 1.8%, 2.8%, 1.7%, and 2.2% respectively. Although patients were similar across treatment groups, those treated with DAPT were slightly older and had more comorbidities, Dr. Freeman said.
In Cox frailty regression, the adjusted risk of any adverse event at 45 days was significantly lower when patients were discharged on warfarin alone (hazard ratio, 0.692; 95% confidence interval, 0.56-0.84) and a DOAC alone (HR, 0.731; 95% CI, 0.57-0.93), compared with warfarin and aspirin. There were no differences among the other groups.
The risk of any major adverse event was also significantly lower with warfarin alone (HR, 0.658; 95% CI, 0.53-0.80) and DOAC alone (HR, 0.767; 95% CI, 0.59-0.98).
At 6 months, rates of any adverse event (HR, 0.814; 95% CI, 0.72-0.93) and any major adverse event (HR, 0.840; 95% CI, 0.73-0.95) were significantly lower only in patients treated with warfarin alone.
“I think if there’s a take-home [message] here, it’s that for a lot of patients there’s good data now to suggest getting rid of the aspirin is a very reasonable thing to do,” Dr. Freeman said.
Further studies are needed in the space, but the results are consistent with those from transcatheter aortic valve replacement studies showing discharge on warfarin or DOAC anticoagulation alone reduces major adverse events without increasing thrombotic events, he said.
“I do think if there’s a strong indication for aspirin – someone has terrible coronary disease – there may be a role for using it,” Dr. Freeman said. But for a lot of these patients, anticoagulation alone without aspirin “may present a big opportunity to mitigate morbidity associated with this procedure.”
Dr. Freeman said he doesn’t expect the findings would be dramatically different with the second-generation Watchman FLX device but noted that randomized data will be forthcoming, as Boston Scientific changed the CHAMPION-AF trial protocol to include DOAC alone without aspirin.
Commenting for this news organization, Domenico Della Rocca, MD, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Austin, said the study is a useful overview of post-LAAO therapies in a large population – but not surprising.
“Practice has changed over the years. More and more we are adopting and trusting the DOACs,” he said. “And, we are realizing that dual antiplatelet therapy is so aggressive and antiplatelet therapy alone maybe is not the best choice based on data on activation of coagulation.”
Commenting further, he said “I think it’s too early to suggest being too keen to completely drop aspirin,” noting that 20%-25% of patients have clopidogrel resistance and that the combination of two antiplatelets may be too aggressive a strategy for others.
Dr. Della Rocca and colleagues recently reported favorable long-term results with half-dose DOAC therapy after Watchman implantation and said the team is launching a randomized trial in more than 500 LAAO patients in the United States and Europe later this year. The trial will be comparing a DOAC-based strategy with low-dose apixaban long-term versus clopidogrel and aspirin initially and then switching to 100 mg aspirin long-term.
“We hope that in the next 2-3 years we will have some better answers, but at this point I would say that clopidogrel is kind of an obsolete strategy for appendage closure,” Dr. Della Rocca said.
In an accompanying editorial, David R. Holmes Jr., MD, Mayo Clinic, Rochester, Minn., says “the cornucopia of these specific strategies can be expected to change as practices evolve, as instructions for use broaden and, hopefully, with the results of well-done, scientifically performed trials. This current LAAO Registry report, however, serves as a useful benchmark.”
He cautioned that this is an observational cohort study and that unmeasured imbalances still may affect the ability to identify an unbiased treatment signal. The use of DAPT was also infrequent during the study and “conclusions based on this information are soft.”
The study was funded by the American College of Cardiology National Cardiovascular Data Registry (NCDR), and the National Heart, Lung, and Blood Institute (NHLBI) grants. Dr. Freeman has received salary support from the ACC NCDR and the NHLBI and has received consulting/advisory board fees from Boston Scientific, Medtronic, Janssen Pharmaceuticals, and Biosense Webster.
A version of this article first appeared on Medscape.com.
A new study finds clinicians are shifting away from the U.S. Food and Drug Administration–approved combination of warfarin and aspirin after left atrial appendage occlusion (LAAO) with the Watchman device and that adverse events, particularly bleeding, are lower when aspirin is dropped.
Of 31,994 patients successfully implanted with the Watchman 2.5 device in the 3 years after its March 2015 approval, only 1 in 10 received the full postprocedure protocol studied in pivotal trials and codified into the FDA-device approval.
The protocol consisted of aspirin (81-325 mg) indefinitely and warfarin for 45 days. Following transesophageal echocardiography, patients were then maintained on warfarin and aspirin if there was a peridevice leak greater than 5 mm or switched to clopidogrel 75 mg for 6 months if a peridevice leak was ruled out or was 5 mm or less.
Based on the results, drawn from the National Cardiovascular Data Registry (NCDR) LAAO Registry, the most common discharge medications were warfarin and aspirin in 36.9% of patients, followed by a direct oral anticoagulant (DOAC) and aspirin (20.8%), warfarin alone (13.5%), DOAC only (12.3%), and dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor (5%).
“There’s a little bit of practice leading the science in this space,” lead author James V. Freeman, MD, MPH, Yale School of Medicine, New Haven, Conn., told this news organization.
Patients who couldn’t tolerate long-term anticoagulation were excluded from the pivotal trials but are now the patients in whom the device is most often used, because of the Centers for Medicare & Medicaid reimbursement mandate for a relative or absolute contraindication to long-term anticoagulation, he noted.
Not surprisingly, 70% of patients in the registry had history of clinically relevant bleeding, the mean CHA2DS2-VASc score was 4.6, and mean HAS-BLED score was 3. At an average age of 76, they were also older, by years, than those in the clinical trials.
Secular trends at the time also saw the ascendancy of the DOACs relative to warfarin, observed Dr. Freeman. “So I think it’s pretty reasonable for physicians to be considering DOACs rather than warfarin in this context.”
Aspirin takes another hit
Results, published May 2 in the Journal of the American College of Cardiology, showed that any adverse event occurred at 45 days in 5.7% of patients discharged on warfarin and aspirin, 4% on warfarin alone, 5.2% on DOAC and aspirin, 3.8% on DOAC only, and 5.5% on DAPT.
Rates of any major adverse event were 4.4%, 3.3%, 4.3%, 3.1%, and 4.2% respectively, and for major bleeding were 3%, 1.8%, 2.8%, 1.7%, and 2.2% respectively. Although patients were similar across treatment groups, those treated with DAPT were slightly older and had more comorbidities, Dr. Freeman said.
In Cox frailty regression, the adjusted risk of any adverse event at 45 days was significantly lower when patients were discharged on warfarin alone (hazard ratio, 0.692; 95% confidence interval, 0.56-0.84) and a DOAC alone (HR, 0.731; 95% CI, 0.57-0.93), compared with warfarin and aspirin. There were no differences among the other groups.
The risk of any major adverse event was also significantly lower with warfarin alone (HR, 0.658; 95% CI, 0.53-0.80) and DOAC alone (HR, 0.767; 95% CI, 0.59-0.98).
At 6 months, rates of any adverse event (HR, 0.814; 95% CI, 0.72-0.93) and any major adverse event (HR, 0.840; 95% CI, 0.73-0.95) were significantly lower only in patients treated with warfarin alone.
“I think if there’s a take-home [message] here, it’s that for a lot of patients there’s good data now to suggest getting rid of the aspirin is a very reasonable thing to do,” Dr. Freeman said.
Further studies are needed in the space, but the results are consistent with those from transcatheter aortic valve replacement studies showing discharge on warfarin or DOAC anticoagulation alone reduces major adverse events without increasing thrombotic events, he said.
“I do think if there’s a strong indication for aspirin – someone has terrible coronary disease – there may be a role for using it,” Dr. Freeman said. But for a lot of these patients, anticoagulation alone without aspirin “may present a big opportunity to mitigate morbidity associated with this procedure.”
Dr. Freeman said he doesn’t expect the findings would be dramatically different with the second-generation Watchman FLX device but noted that randomized data will be forthcoming, as Boston Scientific changed the CHAMPION-AF trial protocol to include DOAC alone without aspirin.
Commenting for this news organization, Domenico Della Rocca, MD, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Austin, said the study is a useful overview of post-LAAO therapies in a large population – but not surprising.
“Practice has changed over the years. More and more we are adopting and trusting the DOACs,” he said. “And, we are realizing that dual antiplatelet therapy is so aggressive and antiplatelet therapy alone maybe is not the best choice based on data on activation of coagulation.”
Commenting further, he said “I think it’s too early to suggest being too keen to completely drop aspirin,” noting that 20%-25% of patients have clopidogrel resistance and that the combination of two antiplatelets may be too aggressive a strategy for others.
Dr. Della Rocca and colleagues recently reported favorable long-term results with half-dose DOAC therapy after Watchman implantation and said the team is launching a randomized trial in more than 500 LAAO patients in the United States and Europe later this year. The trial will be comparing a DOAC-based strategy with low-dose apixaban long-term versus clopidogrel and aspirin initially and then switching to 100 mg aspirin long-term.
“We hope that in the next 2-3 years we will have some better answers, but at this point I would say that clopidogrel is kind of an obsolete strategy for appendage closure,” Dr. Della Rocca said.
In an accompanying editorial, David R. Holmes Jr., MD, Mayo Clinic, Rochester, Minn., says “the cornucopia of these specific strategies can be expected to change as practices evolve, as instructions for use broaden and, hopefully, with the results of well-done, scientifically performed trials. This current LAAO Registry report, however, serves as a useful benchmark.”
He cautioned that this is an observational cohort study and that unmeasured imbalances still may affect the ability to identify an unbiased treatment signal. The use of DAPT was also infrequent during the study and “conclusions based on this information are soft.”
The study was funded by the American College of Cardiology National Cardiovascular Data Registry (NCDR), and the National Heart, Lung, and Blood Institute (NHLBI) grants. Dr. Freeman has received salary support from the ACC NCDR and the NHLBI and has received consulting/advisory board fees from Boston Scientific, Medtronic, Janssen Pharmaceuticals, and Biosense Webster.
A version of this article first appeared on Medscape.com.
A new study finds clinicians are shifting away from the U.S. Food and Drug Administration–approved combination of warfarin and aspirin after left atrial appendage occlusion (LAAO) with the Watchman device and that adverse events, particularly bleeding, are lower when aspirin is dropped.
Of 31,994 patients successfully implanted with the Watchman 2.5 device in the 3 years after its March 2015 approval, only 1 in 10 received the full postprocedure protocol studied in pivotal trials and codified into the FDA-device approval.
The protocol consisted of aspirin (81-325 mg) indefinitely and warfarin for 45 days. Following transesophageal echocardiography, patients were then maintained on warfarin and aspirin if there was a peridevice leak greater than 5 mm or switched to clopidogrel 75 mg for 6 months if a peridevice leak was ruled out or was 5 mm or less.
Based on the results, drawn from the National Cardiovascular Data Registry (NCDR) LAAO Registry, the most common discharge medications were warfarin and aspirin in 36.9% of patients, followed by a direct oral anticoagulant (DOAC) and aspirin (20.8%), warfarin alone (13.5%), DOAC only (12.3%), and dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor (5%).
“There’s a little bit of practice leading the science in this space,” lead author James V. Freeman, MD, MPH, Yale School of Medicine, New Haven, Conn., told this news organization.
Patients who couldn’t tolerate long-term anticoagulation were excluded from the pivotal trials but are now the patients in whom the device is most often used, because of the Centers for Medicare & Medicaid reimbursement mandate for a relative or absolute contraindication to long-term anticoagulation, he noted.
Not surprisingly, 70% of patients in the registry had history of clinically relevant bleeding, the mean CHA2DS2-VASc score was 4.6, and mean HAS-BLED score was 3. At an average age of 76, they were also older, by years, than those in the clinical trials.
Secular trends at the time also saw the ascendancy of the DOACs relative to warfarin, observed Dr. Freeman. “So I think it’s pretty reasonable for physicians to be considering DOACs rather than warfarin in this context.”
Aspirin takes another hit
Results, published May 2 in the Journal of the American College of Cardiology, showed that any adverse event occurred at 45 days in 5.7% of patients discharged on warfarin and aspirin, 4% on warfarin alone, 5.2% on DOAC and aspirin, 3.8% on DOAC only, and 5.5% on DAPT.
Rates of any major adverse event were 4.4%, 3.3%, 4.3%, 3.1%, and 4.2% respectively, and for major bleeding were 3%, 1.8%, 2.8%, 1.7%, and 2.2% respectively. Although patients were similar across treatment groups, those treated with DAPT were slightly older and had more comorbidities, Dr. Freeman said.
In Cox frailty regression, the adjusted risk of any adverse event at 45 days was significantly lower when patients were discharged on warfarin alone (hazard ratio, 0.692; 95% confidence interval, 0.56-0.84) and a DOAC alone (HR, 0.731; 95% CI, 0.57-0.93), compared with warfarin and aspirin. There were no differences among the other groups.
The risk of any major adverse event was also significantly lower with warfarin alone (HR, 0.658; 95% CI, 0.53-0.80) and DOAC alone (HR, 0.767; 95% CI, 0.59-0.98).
At 6 months, rates of any adverse event (HR, 0.814; 95% CI, 0.72-0.93) and any major adverse event (HR, 0.840; 95% CI, 0.73-0.95) were significantly lower only in patients treated with warfarin alone.
“I think if there’s a take-home [message] here, it’s that for a lot of patients there’s good data now to suggest getting rid of the aspirin is a very reasonable thing to do,” Dr. Freeman said.
Further studies are needed in the space, but the results are consistent with those from transcatheter aortic valve replacement studies showing discharge on warfarin or DOAC anticoagulation alone reduces major adverse events without increasing thrombotic events, he said.
“I do think if there’s a strong indication for aspirin – someone has terrible coronary disease – there may be a role for using it,” Dr. Freeman said. But for a lot of these patients, anticoagulation alone without aspirin “may present a big opportunity to mitigate morbidity associated with this procedure.”
Dr. Freeman said he doesn’t expect the findings would be dramatically different with the second-generation Watchman FLX device but noted that randomized data will be forthcoming, as Boston Scientific changed the CHAMPION-AF trial protocol to include DOAC alone without aspirin.
Commenting for this news organization, Domenico Della Rocca, MD, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Austin, said the study is a useful overview of post-LAAO therapies in a large population – but not surprising.
“Practice has changed over the years. More and more we are adopting and trusting the DOACs,” he said. “And, we are realizing that dual antiplatelet therapy is so aggressive and antiplatelet therapy alone maybe is not the best choice based on data on activation of coagulation.”
Commenting further, he said “I think it’s too early to suggest being too keen to completely drop aspirin,” noting that 20%-25% of patients have clopidogrel resistance and that the combination of two antiplatelets may be too aggressive a strategy for others.
Dr. Della Rocca and colleagues recently reported favorable long-term results with half-dose DOAC therapy after Watchman implantation and said the team is launching a randomized trial in more than 500 LAAO patients in the United States and Europe later this year. The trial will be comparing a DOAC-based strategy with low-dose apixaban long-term versus clopidogrel and aspirin initially and then switching to 100 mg aspirin long-term.
“We hope that in the next 2-3 years we will have some better answers, but at this point I would say that clopidogrel is kind of an obsolete strategy for appendage closure,” Dr. Della Rocca said.
In an accompanying editorial, David R. Holmes Jr., MD, Mayo Clinic, Rochester, Minn., says “the cornucopia of these specific strategies can be expected to change as practices evolve, as instructions for use broaden and, hopefully, with the results of well-done, scientifically performed trials. This current LAAO Registry report, however, serves as a useful benchmark.”
He cautioned that this is an observational cohort study and that unmeasured imbalances still may affect the ability to identify an unbiased treatment signal. The use of DAPT was also infrequent during the study and “conclusions based on this information are soft.”
The study was funded by the American College of Cardiology National Cardiovascular Data Registry (NCDR), and the National Heart, Lung, and Blood Institute (NHLBI) grants. Dr. Freeman has received salary support from the ACC NCDR and the NHLBI and has received consulting/advisory board fees from Boston Scientific, Medtronic, Janssen Pharmaceuticals, and Biosense Webster.
A version of this article first appeared on Medscape.com.
Cutting dementia risk in AFib: Does rhythm control strategy matter?
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. However, a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD-only at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, told this news organization. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings April 30 at the Heart Rhythm Society 2022 Scientific Sessions, conducted virtually and live in San Francisco.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ontario, who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin-system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler discloses consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. However, a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD-only at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, told this news organization. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings April 30 at the Heart Rhythm Society 2022 Scientific Sessions, conducted virtually and live in San Francisco.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ontario, who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin-system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler discloses consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. However, a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD-only at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, told this news organization. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings April 30 at the Heart Rhythm Society 2022 Scientific Sessions, conducted virtually and live in San Francisco.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ontario, who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin-system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler discloses consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
Cutting dementia risk in atrial fibrillation: Does rhythm control strategy matter?
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. But a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD alone at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said in an interview. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings at the annual scientific sessions of the Heart Rhythm Society.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ont., who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler disclosed consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. But a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD alone at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said in an interview. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings at the annual scientific sessions of the Heart Rhythm Society.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ont., who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler disclosed consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. But a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD alone at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said in an interview. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings at the annual scientific sessions of the Heart Rhythm Society.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ont., who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler disclosed consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
HEART RHYTHM 2022
Will you have cardiac arrest? New tech may predict if and when
Deaths from COVID-19 may have caught more attention lately, but heart disease remains the leading cause of death in the United States.
More than 300,000 Americans will die this year of sudden cardiac arrest (also called sudden cardiac death, or SCD), when the heart abruptly stops working.
These events happen suddenly and often without warning, making them nearly impossible to predict. But that may be changing, thanks to 3D imaging and artificial intelligence (AI) technology under study at Johns Hopkins University, Baltimore.
There, researchers are working to create more accurate and personalized models of the heart – and not just any heart, your heart, if you have heart disease.
“Right now, a clinician can only say whether a patient is at risk or not at risk for sudden death,” says Dan Popescu, PhD, a Johns Hopkins research scientist and first author of a new study on AI’s ability to predict sudden cardiac arrest. “With this new technology, you can have much more nuanced predictions of probability of an event over time.”
Put another way: With AI, clinicians may be able not only to predict if someone is at risk for sudden cardiac arrest, but also when it is most likely to happen. They can do this using a much clearer and more personalized look at the electrical “wiring” of your heart.
Your heart, the conductor
Your heart isn’t just a metronome responsible for keeping a steady stream of blood pumping to tissues with every beat. It’s also a conductor through which vital energy flows.
To make the heart beat, electrical impulses flow from the top to the bottom of the organ. Healthy heart cells relay this electricity seamlessly. But in a heart damaged by inflammation or a past heart attack, scar tissue will block the energy flow.
When an electrical impulse encounters a scarred area, the signal can become erratic, disrupting the set top-to-bottom path and causing irregular heartbeats (arrhythmias), which increase someone’s danger of sudden cardiac death.
Seeing the heart in 3D
Today’s tests offer some insights into the heart’s makeup. For example, MRI scans can reveal damaged areas. PET scans can show inflammation. And EKGs can record the heart’s electrical signals from beat to beat.
But all these technologies offer only a snapshot, showing heart health at a moment in time. They can’t predict the future. That’s why scientists at Johns Hopkins are going further to develop 3D digital replicas of a person’s heart, known as computational heart models.
Computational models are computer-simulated replicas that combine mathematics, physics, and computer science. These models have been around for a long time and are used in many fields, ranging from manufacturing to economics.
In heart medicine, these models are populated with digital “cells,” which imitate living cells and can be programmed with different electrical properties, depending on whether they are healthy or diseased.
“Currently available imaging and testing (MRIs, PETs, EKGs) give some representation of the scarring, but you cannot translate that to what is going to happen over time,” says Natalia Trayanova, PhD, of the Johns Hopkins department of biomedical engineering.
“With computational heart models, we create a dynamic digital image of the heart. We can then give the digital image an electrical stimulus and assess how the heart is able to respond. Then you can better predict what is going to happen.”
The computerized 3D models also mean better, more accurate treatment for heart conditions.
For example, a common treatment for a type of arrhythmia known as atrial fibrillation is ablation, or burning some heart tissue. Ablation stops the erratic electrical impulses causing the arrhythmia, but it can also damage otherwise healthy heart cells.
A personalized computational heart model could allow doctors to see more accurately what areas should and shouldn’t be treated for a specific patient.
Using deep learning AI to predict health outcomes
Dr. Trayanova’s colleague Dr. Popescu is applying deep learning and AI to do more with computerized heart models to predict the future.
In a recent paper in Nature Cardiovascular Research, the research team showed their algorithm assessed the health of 269 patients and was able to predict the chance of sudden cardiac arrest up to 10 years in advance.
“This is really the first time ever, as far as we know, where deep learning technology has been proven to analyze scarring of the heart in a successful way,” Dr. Popescu says.
Dr. Popescu and Dr. Trayanova say the AI algorithm gathers information from the 3D computational heart models with patient data like MRIs, ethnicity, age, lifestyle, and other clinical information. Analyzing all these data can produce accurate and consistent estimates about how long patients might live if they are at risk for sudden death.
“You can’t afford to be wrong. If you are wrong, you can actually impact a patient’s quality of life dramatically,” Dr. Popescu says. “Having clinicians use this technology in the decision-making process will provide confidence in a better diagnosis and prognosis.”
While the current study was specifically about patients with a particular type of heart disease, Dr. Popescu says his algorithm can also be trained to assess other health conditions.
So when might you see this being used outside of a research study? Dr. Trayanova predicts 3D imaging of heart models could be available in 2 years, but first the technique must be tested in more clinical trials – some of which are happening right now.
Adding AI to the heart models will require more studies and Food and Drug Administration approval, so the timeline is less clear. But perhaps the biggest hurdle is that after approval the technologies would need to be adopted and used by clinicians and caregivers.
“The much harder question to answer is, ‘When will doctors be perfectly comfortable with AI tools?’ And I don’t know the answer,” Dr. Popescu says. “How to use AI as an aid in the decision-making process is something that’s not currently taught.”
A version of this article first appeared on WebMD.com.
Deaths from COVID-19 may have caught more attention lately, but heart disease remains the leading cause of death in the United States.
More than 300,000 Americans will die this year of sudden cardiac arrest (also called sudden cardiac death, or SCD), when the heart abruptly stops working.
These events happen suddenly and often without warning, making them nearly impossible to predict. But that may be changing, thanks to 3D imaging and artificial intelligence (AI) technology under study at Johns Hopkins University, Baltimore.
There, researchers are working to create more accurate and personalized models of the heart – and not just any heart, your heart, if you have heart disease.
“Right now, a clinician can only say whether a patient is at risk or not at risk for sudden death,” says Dan Popescu, PhD, a Johns Hopkins research scientist and first author of a new study on AI’s ability to predict sudden cardiac arrest. “With this new technology, you can have much more nuanced predictions of probability of an event over time.”
Put another way: With AI, clinicians may be able not only to predict if someone is at risk for sudden cardiac arrest, but also when it is most likely to happen. They can do this using a much clearer and more personalized look at the electrical “wiring” of your heart.
Your heart, the conductor
Your heart isn’t just a metronome responsible for keeping a steady stream of blood pumping to tissues with every beat. It’s also a conductor through which vital energy flows.
To make the heart beat, electrical impulses flow from the top to the bottom of the organ. Healthy heart cells relay this electricity seamlessly. But in a heart damaged by inflammation or a past heart attack, scar tissue will block the energy flow.
When an electrical impulse encounters a scarred area, the signal can become erratic, disrupting the set top-to-bottom path and causing irregular heartbeats (arrhythmias), which increase someone’s danger of sudden cardiac death.
Seeing the heart in 3D
Today’s tests offer some insights into the heart’s makeup. For example, MRI scans can reveal damaged areas. PET scans can show inflammation. And EKGs can record the heart’s electrical signals from beat to beat.
But all these technologies offer only a snapshot, showing heart health at a moment in time. They can’t predict the future. That’s why scientists at Johns Hopkins are going further to develop 3D digital replicas of a person’s heart, known as computational heart models.
Computational models are computer-simulated replicas that combine mathematics, physics, and computer science. These models have been around for a long time and are used in many fields, ranging from manufacturing to economics.
In heart medicine, these models are populated with digital “cells,” which imitate living cells and can be programmed with different electrical properties, depending on whether they are healthy or diseased.
“Currently available imaging and testing (MRIs, PETs, EKGs) give some representation of the scarring, but you cannot translate that to what is going to happen over time,” says Natalia Trayanova, PhD, of the Johns Hopkins department of biomedical engineering.
“With computational heart models, we create a dynamic digital image of the heart. We can then give the digital image an electrical stimulus and assess how the heart is able to respond. Then you can better predict what is going to happen.”
The computerized 3D models also mean better, more accurate treatment for heart conditions.
For example, a common treatment for a type of arrhythmia known as atrial fibrillation is ablation, or burning some heart tissue. Ablation stops the erratic electrical impulses causing the arrhythmia, but it can also damage otherwise healthy heart cells.
A personalized computational heart model could allow doctors to see more accurately what areas should and shouldn’t be treated for a specific patient.
Using deep learning AI to predict health outcomes
Dr. Trayanova’s colleague Dr. Popescu is applying deep learning and AI to do more with computerized heart models to predict the future.
In a recent paper in Nature Cardiovascular Research, the research team showed their algorithm assessed the health of 269 patients and was able to predict the chance of sudden cardiac arrest up to 10 years in advance.
“This is really the first time ever, as far as we know, where deep learning technology has been proven to analyze scarring of the heart in a successful way,” Dr. Popescu says.
Dr. Popescu and Dr. Trayanova say the AI algorithm gathers information from the 3D computational heart models with patient data like MRIs, ethnicity, age, lifestyle, and other clinical information. Analyzing all these data can produce accurate and consistent estimates about how long patients might live if they are at risk for sudden death.
“You can’t afford to be wrong. If you are wrong, you can actually impact a patient’s quality of life dramatically,” Dr. Popescu says. “Having clinicians use this technology in the decision-making process will provide confidence in a better diagnosis and prognosis.”
While the current study was specifically about patients with a particular type of heart disease, Dr. Popescu says his algorithm can also be trained to assess other health conditions.
So when might you see this being used outside of a research study? Dr. Trayanova predicts 3D imaging of heart models could be available in 2 years, but first the technique must be tested in more clinical trials – some of which are happening right now.
Adding AI to the heart models will require more studies and Food and Drug Administration approval, so the timeline is less clear. But perhaps the biggest hurdle is that after approval the technologies would need to be adopted and used by clinicians and caregivers.
“The much harder question to answer is, ‘When will doctors be perfectly comfortable with AI tools?’ And I don’t know the answer,” Dr. Popescu says. “How to use AI as an aid in the decision-making process is something that’s not currently taught.”
A version of this article first appeared on WebMD.com.
Deaths from COVID-19 may have caught more attention lately, but heart disease remains the leading cause of death in the United States.
More than 300,000 Americans will die this year of sudden cardiac arrest (also called sudden cardiac death, or SCD), when the heart abruptly stops working.
These events happen suddenly and often without warning, making them nearly impossible to predict. But that may be changing, thanks to 3D imaging and artificial intelligence (AI) technology under study at Johns Hopkins University, Baltimore.
There, researchers are working to create more accurate and personalized models of the heart – and not just any heart, your heart, if you have heart disease.
“Right now, a clinician can only say whether a patient is at risk or not at risk for sudden death,” says Dan Popescu, PhD, a Johns Hopkins research scientist and first author of a new study on AI’s ability to predict sudden cardiac arrest. “With this new technology, you can have much more nuanced predictions of probability of an event over time.”
Put another way: With AI, clinicians may be able not only to predict if someone is at risk for sudden cardiac arrest, but also when it is most likely to happen. They can do this using a much clearer and more personalized look at the electrical “wiring” of your heart.
Your heart, the conductor
Your heart isn’t just a metronome responsible for keeping a steady stream of blood pumping to tissues with every beat. It’s also a conductor through which vital energy flows.
To make the heart beat, electrical impulses flow from the top to the bottom of the organ. Healthy heart cells relay this electricity seamlessly. But in a heart damaged by inflammation or a past heart attack, scar tissue will block the energy flow.
When an electrical impulse encounters a scarred area, the signal can become erratic, disrupting the set top-to-bottom path and causing irregular heartbeats (arrhythmias), which increase someone’s danger of sudden cardiac death.
Seeing the heart in 3D
Today’s tests offer some insights into the heart’s makeup. For example, MRI scans can reveal damaged areas. PET scans can show inflammation. And EKGs can record the heart’s electrical signals from beat to beat.
But all these technologies offer only a snapshot, showing heart health at a moment in time. They can’t predict the future. That’s why scientists at Johns Hopkins are going further to develop 3D digital replicas of a person’s heart, known as computational heart models.
Computational models are computer-simulated replicas that combine mathematics, physics, and computer science. These models have been around for a long time and are used in many fields, ranging from manufacturing to economics.
In heart medicine, these models are populated with digital “cells,” which imitate living cells and can be programmed with different electrical properties, depending on whether they are healthy or diseased.
“Currently available imaging and testing (MRIs, PETs, EKGs) give some representation of the scarring, but you cannot translate that to what is going to happen over time,” says Natalia Trayanova, PhD, of the Johns Hopkins department of biomedical engineering.
“With computational heart models, we create a dynamic digital image of the heart. We can then give the digital image an electrical stimulus and assess how the heart is able to respond. Then you can better predict what is going to happen.”
The computerized 3D models also mean better, more accurate treatment for heart conditions.
For example, a common treatment for a type of arrhythmia known as atrial fibrillation is ablation, or burning some heart tissue. Ablation stops the erratic electrical impulses causing the arrhythmia, but it can also damage otherwise healthy heart cells.
A personalized computational heart model could allow doctors to see more accurately what areas should and shouldn’t be treated for a specific patient.
Using deep learning AI to predict health outcomes
Dr. Trayanova’s colleague Dr. Popescu is applying deep learning and AI to do more with computerized heart models to predict the future.
In a recent paper in Nature Cardiovascular Research, the research team showed their algorithm assessed the health of 269 patients and was able to predict the chance of sudden cardiac arrest up to 10 years in advance.
“This is really the first time ever, as far as we know, where deep learning technology has been proven to analyze scarring of the heart in a successful way,” Dr. Popescu says.
Dr. Popescu and Dr. Trayanova say the AI algorithm gathers information from the 3D computational heart models with patient data like MRIs, ethnicity, age, lifestyle, and other clinical information. Analyzing all these data can produce accurate and consistent estimates about how long patients might live if they are at risk for sudden death.
“You can’t afford to be wrong. If you are wrong, you can actually impact a patient’s quality of life dramatically,” Dr. Popescu says. “Having clinicians use this technology in the decision-making process will provide confidence in a better diagnosis and prognosis.”
While the current study was specifically about patients with a particular type of heart disease, Dr. Popescu says his algorithm can also be trained to assess other health conditions.
So when might you see this being used outside of a research study? Dr. Trayanova predicts 3D imaging of heart models could be available in 2 years, but first the technique must be tested in more clinical trials – some of which are happening right now.
Adding AI to the heart models will require more studies and Food and Drug Administration approval, so the timeline is less clear. But perhaps the biggest hurdle is that after approval the technologies would need to be adopted and used by clinicians and caregivers.
“The much harder question to answer is, ‘When will doctors be perfectly comfortable with AI tools?’ And I don’t know the answer,” Dr. Popescu says. “How to use AI as an aid in the decision-making process is something that’s not currently taught.”
A version of this article first appeared on WebMD.com.
Study points to causal role for Lp(a) in atrial fibrillation
Although lipoprotein(a) is causally related to coronary artery disease and aortic valve stenosis – two known risk factors for atrial fibrillation (AFib) – evidence linking Lp(a) to a causal role in the development of AFib has been lukewarm at best.
A recent Mendelian randomization study showed only a nominally significant effect of Lp(a) on AFib, whereas an ARIC substudy showed high levels of Lp(a) to be associated with elevated ischemic stroke risk but not incident AFib.
A new study that adds the heft of Mendelian randomization to large observational and genetic analyses, however, implicates Lp(a) as a potential causal mediator of AFib, independent of its known effects on atherosclerotic cardiovascular disease (ASCVD).
“Why this is exciting is because it shows that Lp(a) has effects beyond the arteries and beyond the aortic valve, and that provides two things,” senior author Guillaume Paré, MD, MSc, Population Health Research Institute, Hamilton, Ontario, told this news organization.
“First, it provides a potential means to decrease the risk, because there are all these Lp(a) inhibitors in development,” he said. “But I think the other thing is that it just points to a new pathway that leads to atrial fibrillation development that could potentially be targeted with other drugs when it’s better understood. We don’t pretend that we understand the biology there, but it opens this possibility.”
The results were published in the Journal of the American College of Cardiology.
Using data from 435,579 participants in the UK Biobank, the researchers identified 20,432 cases of incident AFib over a median of 11 years of follow-up. They also constructed a genetic risk score for Lp(a) using genetic variants within 500 kb of the LPA gene.
After common AFib risk factors were controlled for, results showed a 3% increased risk for incident AFib per 50 nmol/L increase in Lp(a) at enrollment (hazard ratio, 1.03; 95% confidence interval, 1.02-1.05).
A Mendelian randomization analysis showed a similar association between genetically predicted Lp(a) and AFib (odds ratio, 1.03; 95% CI, 1.02-1.05).
To replicate the results, the investigators performed separate Mendelian randomization analyses using publicly available genome-wide association study (GWAS) statistics from the largest GWAS of AFib involving more than 1 million participants and from the FinnGen cohort involving more than 114,000 Finnish residents.
The analyses showed a 3% increase in risk for AFib in the genome-wide study (OR, 1.03; 95% CI, 1.02-1.05) and an 8% increase in risk in the Finnish study (OR, 1.08; 95% CI, 1.04-1.12) per 50 nmol/L increase in Lp(a).
There was no evidence that the effect of observed or genetically predicted Lp(a) was modified by prevalent ischemic heart disease or aortic stenosis.
Further, MR analyses revealed no risk effect of low-density-lipoprotein cholesterol or triglycerides on AFib.
Notably, only 39% of Lp(a) was mediated through ASCVD, suggesting that Lp(a) partly influences AFib independent of its known effect on ASCVD.
“To me, the eureka moment is when we repeated the same analysis for LDL cholesterol and it had absolutely no association with AFib,” Dr. Paré said. “Because up to that point, there was always this lingering doubt that, well, it’s because of coronary artery disease, and that’s logical. But the signal is completely flat with LDL, and we see this strong signal with Lp(a).”
Another ‘red flag’
Erin D. Michos, MD, MHS, senior author of the ARIC substudy and associate director of preventive cardiology at Johns Hopkins School of Medicine, Baltimore, said the findings are “another red flag that lipoprotein(a) is a marker we need to pay attention to and potentially needs treatment.”
“The fact that it was Mendelian randomization does suggest that there’s a causal role,” she said. “I think the relationship is relatively modest compared to its known risk for stroke, ASCVD, coronary disease, and aortic stenosis, ... which may be why we didn’t see it in the ARIC cohort with 12,000 participants. You needed to have a million participants and 60,000 cases to see an effect here.”
Dr. Michos said she hopes the findings encourage increased testing, particularly with multiple potential treatments currently in the pipeline. She pointed out that the researchers estimated that the experimental antisense agent pelacarsen, which lowers Lp(a) by about 80%, would translate into about an 8% reduction in AFib risk, or “the same effect as 2 kg of weight loss or a 5 mm Hg reduction in blood pressure, which we do think are meaningful.”
Adding to this point in an accompanying editorial, Daniel Seung Kim, MD, PhD, and Abha Khandelwal, MD, MS, Stanford University School of Medicine, California, say that “moreover, reduction of Lp(a) levels would have multifactorial effects on CAD, cerebrovascular/peripheral artery disease, and AS risk.
“Therefore, approaches to reduce Lp(a) should be prioritized to further reduce the morbidity and mortality of a rapidly aging population,” they write.
The editorialists also join the researchers in calling for inclusion of AFib as a secondary outcome in ongoing Lp(a) trials, in addition to cerebrovascular disease and peripheral vascular disease.
Unanswered questions
As to what’s driving the risk effect of Lp(a), first author Pedrum Mohammadi-Shemirani, PhD, also from the Population Health Research Institute, explained that in aortic stenosis, “mechanical stress increases endothelial permeability, allowing Lp(a) to infiltrate valvular tissue and induce gene expression that results in microcalcifications and cell death.”
“So, in theory, a similar sort of mechanism could be at play in atrial tissue that may lead to damage and the electrical remodeling that causes atrial fibrillation,” he told this news organization.
Dr. Mohammadi-Shemirani also noted that Lp(a) has proinflammatory properties, but added that any potential mechanisms are “speculative and require further research to disentangle.”
Dr. Paré and colleagues say follow-up studies are also warranted, noting that generalizability of the results may be limited because AFib cases were found using electronic health records in the population-scale cohorts and because few UK Biobank participants were of non-European ancestry and Lp(a) levels vary among ethnic groups.
Another limitation is that the number of kringle IV type 2 domain repeats within the LPA gene, the largest contributor to genetic variation in Lp(a), could not be directly measured. Still, 71.4% of the variation in Lp(a) was explained using the genetic risk score alone, they say.
Dr. Paré holds the Canada Research Chair in Genetic and Molecular Epidemiology and Cisco Systems Professorship in Integrated Health Biosystems. Dr. Mohammadi-Shemirani is supported by the Frederick Banting and Charles Best Canada Graduate Scholarship from the Canadian Institute of Health Research. Dr. Michos reports consulting for Novartis and serving on advisory boards for Novartis, AstraZeneca, Bayer, and Boehringer Ingelheim. Dr. Kim reports grant support from the National Institutes of Health and the American Heart Association. Dr. Khandelwal serves on the advisory board of Amgen and has received funding from Novartis CTQJ and Akcea.
A version of this article first appeared on Medscape.com.
Although lipoprotein(a) is causally related to coronary artery disease and aortic valve stenosis – two known risk factors for atrial fibrillation (AFib) – evidence linking Lp(a) to a causal role in the development of AFib has been lukewarm at best.
A recent Mendelian randomization study showed only a nominally significant effect of Lp(a) on AFib, whereas an ARIC substudy showed high levels of Lp(a) to be associated with elevated ischemic stroke risk but not incident AFib.
A new study that adds the heft of Mendelian randomization to large observational and genetic analyses, however, implicates Lp(a) as a potential causal mediator of AFib, independent of its known effects on atherosclerotic cardiovascular disease (ASCVD).
“Why this is exciting is because it shows that Lp(a) has effects beyond the arteries and beyond the aortic valve, and that provides two things,” senior author Guillaume Paré, MD, MSc, Population Health Research Institute, Hamilton, Ontario, told this news organization.
“First, it provides a potential means to decrease the risk, because there are all these Lp(a) inhibitors in development,” he said. “But I think the other thing is that it just points to a new pathway that leads to atrial fibrillation development that could potentially be targeted with other drugs when it’s better understood. We don’t pretend that we understand the biology there, but it opens this possibility.”
The results were published in the Journal of the American College of Cardiology.
Using data from 435,579 participants in the UK Biobank, the researchers identified 20,432 cases of incident AFib over a median of 11 years of follow-up. They also constructed a genetic risk score for Lp(a) using genetic variants within 500 kb of the LPA gene.
After common AFib risk factors were controlled for, results showed a 3% increased risk for incident AFib per 50 nmol/L increase in Lp(a) at enrollment (hazard ratio, 1.03; 95% confidence interval, 1.02-1.05).
A Mendelian randomization analysis showed a similar association between genetically predicted Lp(a) and AFib (odds ratio, 1.03; 95% CI, 1.02-1.05).
To replicate the results, the investigators performed separate Mendelian randomization analyses using publicly available genome-wide association study (GWAS) statistics from the largest GWAS of AFib involving more than 1 million participants and from the FinnGen cohort involving more than 114,000 Finnish residents.
The analyses showed a 3% increase in risk for AFib in the genome-wide study (OR, 1.03; 95% CI, 1.02-1.05) and an 8% increase in risk in the Finnish study (OR, 1.08; 95% CI, 1.04-1.12) per 50 nmol/L increase in Lp(a).
There was no evidence that the effect of observed or genetically predicted Lp(a) was modified by prevalent ischemic heart disease or aortic stenosis.
Further, MR analyses revealed no risk effect of low-density-lipoprotein cholesterol or triglycerides on AFib.
Notably, only 39% of Lp(a) was mediated through ASCVD, suggesting that Lp(a) partly influences AFib independent of its known effect on ASCVD.
“To me, the eureka moment is when we repeated the same analysis for LDL cholesterol and it had absolutely no association with AFib,” Dr. Paré said. “Because up to that point, there was always this lingering doubt that, well, it’s because of coronary artery disease, and that’s logical. But the signal is completely flat with LDL, and we see this strong signal with Lp(a).”
Another ‘red flag’
Erin D. Michos, MD, MHS, senior author of the ARIC substudy and associate director of preventive cardiology at Johns Hopkins School of Medicine, Baltimore, said the findings are “another red flag that lipoprotein(a) is a marker we need to pay attention to and potentially needs treatment.”
“The fact that it was Mendelian randomization does suggest that there’s a causal role,” she said. “I think the relationship is relatively modest compared to its known risk for stroke, ASCVD, coronary disease, and aortic stenosis, ... which may be why we didn’t see it in the ARIC cohort with 12,000 participants. You needed to have a million participants and 60,000 cases to see an effect here.”
Dr. Michos said she hopes the findings encourage increased testing, particularly with multiple potential treatments currently in the pipeline. She pointed out that the researchers estimated that the experimental antisense agent pelacarsen, which lowers Lp(a) by about 80%, would translate into about an 8% reduction in AFib risk, or “the same effect as 2 kg of weight loss or a 5 mm Hg reduction in blood pressure, which we do think are meaningful.”
Adding to this point in an accompanying editorial, Daniel Seung Kim, MD, PhD, and Abha Khandelwal, MD, MS, Stanford University School of Medicine, California, say that “moreover, reduction of Lp(a) levels would have multifactorial effects on CAD, cerebrovascular/peripheral artery disease, and AS risk.
“Therefore, approaches to reduce Lp(a) should be prioritized to further reduce the morbidity and mortality of a rapidly aging population,” they write.
The editorialists also join the researchers in calling for inclusion of AFib as a secondary outcome in ongoing Lp(a) trials, in addition to cerebrovascular disease and peripheral vascular disease.
Unanswered questions
As to what’s driving the risk effect of Lp(a), first author Pedrum Mohammadi-Shemirani, PhD, also from the Population Health Research Institute, explained that in aortic stenosis, “mechanical stress increases endothelial permeability, allowing Lp(a) to infiltrate valvular tissue and induce gene expression that results in microcalcifications and cell death.”
“So, in theory, a similar sort of mechanism could be at play in atrial tissue that may lead to damage and the electrical remodeling that causes atrial fibrillation,” he told this news organization.
Dr. Mohammadi-Shemirani also noted that Lp(a) has proinflammatory properties, but added that any potential mechanisms are “speculative and require further research to disentangle.”
Dr. Paré and colleagues say follow-up studies are also warranted, noting that generalizability of the results may be limited because AFib cases were found using electronic health records in the population-scale cohorts and because few UK Biobank participants were of non-European ancestry and Lp(a) levels vary among ethnic groups.
Another limitation is that the number of kringle IV type 2 domain repeats within the LPA gene, the largest contributor to genetic variation in Lp(a), could not be directly measured. Still, 71.4% of the variation in Lp(a) was explained using the genetic risk score alone, they say.
Dr. Paré holds the Canada Research Chair in Genetic and Molecular Epidemiology and Cisco Systems Professorship in Integrated Health Biosystems. Dr. Mohammadi-Shemirani is supported by the Frederick Banting and Charles Best Canada Graduate Scholarship from the Canadian Institute of Health Research. Dr. Michos reports consulting for Novartis and serving on advisory boards for Novartis, AstraZeneca, Bayer, and Boehringer Ingelheim. Dr. Kim reports grant support from the National Institutes of Health and the American Heart Association. Dr. Khandelwal serves on the advisory board of Amgen and has received funding from Novartis CTQJ and Akcea.
A version of this article first appeared on Medscape.com.
Although lipoprotein(a) is causally related to coronary artery disease and aortic valve stenosis – two known risk factors for atrial fibrillation (AFib) – evidence linking Lp(a) to a causal role in the development of AFib has been lukewarm at best.
A recent Mendelian randomization study showed only a nominally significant effect of Lp(a) on AFib, whereas an ARIC substudy showed high levels of Lp(a) to be associated with elevated ischemic stroke risk but not incident AFib.
A new study that adds the heft of Mendelian randomization to large observational and genetic analyses, however, implicates Lp(a) as a potential causal mediator of AFib, independent of its known effects on atherosclerotic cardiovascular disease (ASCVD).
“Why this is exciting is because it shows that Lp(a) has effects beyond the arteries and beyond the aortic valve, and that provides two things,” senior author Guillaume Paré, MD, MSc, Population Health Research Institute, Hamilton, Ontario, told this news organization.
“First, it provides a potential means to decrease the risk, because there are all these Lp(a) inhibitors in development,” he said. “But I think the other thing is that it just points to a new pathway that leads to atrial fibrillation development that could potentially be targeted with other drugs when it’s better understood. We don’t pretend that we understand the biology there, but it opens this possibility.”
The results were published in the Journal of the American College of Cardiology.
Using data from 435,579 participants in the UK Biobank, the researchers identified 20,432 cases of incident AFib over a median of 11 years of follow-up. They also constructed a genetic risk score for Lp(a) using genetic variants within 500 kb of the LPA gene.
After common AFib risk factors were controlled for, results showed a 3% increased risk for incident AFib per 50 nmol/L increase in Lp(a) at enrollment (hazard ratio, 1.03; 95% confidence interval, 1.02-1.05).
A Mendelian randomization analysis showed a similar association between genetically predicted Lp(a) and AFib (odds ratio, 1.03; 95% CI, 1.02-1.05).
To replicate the results, the investigators performed separate Mendelian randomization analyses using publicly available genome-wide association study (GWAS) statistics from the largest GWAS of AFib involving more than 1 million participants and from the FinnGen cohort involving more than 114,000 Finnish residents.
The analyses showed a 3% increase in risk for AFib in the genome-wide study (OR, 1.03; 95% CI, 1.02-1.05) and an 8% increase in risk in the Finnish study (OR, 1.08; 95% CI, 1.04-1.12) per 50 nmol/L increase in Lp(a).
There was no evidence that the effect of observed or genetically predicted Lp(a) was modified by prevalent ischemic heart disease or aortic stenosis.
Further, MR analyses revealed no risk effect of low-density-lipoprotein cholesterol or triglycerides on AFib.
Notably, only 39% of Lp(a) was mediated through ASCVD, suggesting that Lp(a) partly influences AFib independent of its known effect on ASCVD.
“To me, the eureka moment is when we repeated the same analysis for LDL cholesterol and it had absolutely no association with AFib,” Dr. Paré said. “Because up to that point, there was always this lingering doubt that, well, it’s because of coronary artery disease, and that’s logical. But the signal is completely flat with LDL, and we see this strong signal with Lp(a).”
Another ‘red flag’
Erin D. Michos, MD, MHS, senior author of the ARIC substudy and associate director of preventive cardiology at Johns Hopkins School of Medicine, Baltimore, said the findings are “another red flag that lipoprotein(a) is a marker we need to pay attention to and potentially needs treatment.”
“The fact that it was Mendelian randomization does suggest that there’s a causal role,” she said. “I think the relationship is relatively modest compared to its known risk for stroke, ASCVD, coronary disease, and aortic stenosis, ... which may be why we didn’t see it in the ARIC cohort with 12,000 participants. You needed to have a million participants and 60,000 cases to see an effect here.”
Dr. Michos said she hopes the findings encourage increased testing, particularly with multiple potential treatments currently in the pipeline. She pointed out that the researchers estimated that the experimental antisense agent pelacarsen, which lowers Lp(a) by about 80%, would translate into about an 8% reduction in AFib risk, or “the same effect as 2 kg of weight loss or a 5 mm Hg reduction in blood pressure, which we do think are meaningful.”
Adding to this point in an accompanying editorial, Daniel Seung Kim, MD, PhD, and Abha Khandelwal, MD, MS, Stanford University School of Medicine, California, say that “moreover, reduction of Lp(a) levels would have multifactorial effects on CAD, cerebrovascular/peripheral artery disease, and AS risk.
“Therefore, approaches to reduce Lp(a) should be prioritized to further reduce the morbidity and mortality of a rapidly aging population,” they write.
The editorialists also join the researchers in calling for inclusion of AFib as a secondary outcome in ongoing Lp(a) trials, in addition to cerebrovascular disease and peripheral vascular disease.
Unanswered questions
As to what’s driving the risk effect of Lp(a), first author Pedrum Mohammadi-Shemirani, PhD, also from the Population Health Research Institute, explained that in aortic stenosis, “mechanical stress increases endothelial permeability, allowing Lp(a) to infiltrate valvular tissue and induce gene expression that results in microcalcifications and cell death.”
“So, in theory, a similar sort of mechanism could be at play in atrial tissue that may lead to damage and the electrical remodeling that causes atrial fibrillation,” he told this news organization.
Dr. Mohammadi-Shemirani also noted that Lp(a) has proinflammatory properties, but added that any potential mechanisms are “speculative and require further research to disentangle.”
Dr. Paré and colleagues say follow-up studies are also warranted, noting that generalizability of the results may be limited because AFib cases were found using electronic health records in the population-scale cohorts and because few UK Biobank participants were of non-European ancestry and Lp(a) levels vary among ethnic groups.
Another limitation is that the number of kringle IV type 2 domain repeats within the LPA gene, the largest contributor to genetic variation in Lp(a), could not be directly measured. Still, 71.4% of the variation in Lp(a) was explained using the genetic risk score alone, they say.
Dr. Paré holds the Canada Research Chair in Genetic and Molecular Epidemiology and Cisco Systems Professorship in Integrated Health Biosystems. Dr. Mohammadi-Shemirani is supported by the Frederick Banting and Charles Best Canada Graduate Scholarship from the Canadian Institute of Health Research. Dr. Michos reports consulting for Novartis and serving on advisory boards for Novartis, AstraZeneca, Bayer, and Boehringer Ingelheim. Dr. Kim reports grant support from the National Institutes of Health and the American Heart Association. Dr. Khandelwal serves on the advisory board of Amgen and has received funding from Novartis CTQJ and Akcea.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Young and older athletes show similar arrhythmia patterns with fQRS
The prevalence of exercise-induced arrhythmias in young athletes with fragmented QRS (fQRS) patterns in lead V1 was 27%, similar to that seen in adult athletes, based on data from nearly 700 individuals.
Recent data suggest that fQRS complex in lead V1 (fQRSV1) in healthy athletes may promote arrhythmias in the context of training-induced right ventricular remodeling, but the prevalence and significance in young athletes has not been well studied, Guilia Quinto, MD, of the University of Padova (Italy) said in a presentation at the annual congress of the European Association of Preventive Cardiology.
Dr. Quinto and colleagues assessed data from of young athletes on ventricular arrhythmias during exercise tests.
The study population included 684 young athletes with a mean age of 15 years; 64% were male. Baseline data collection included medical history, physical exam, resting ECG, standardized maximum exercise tolerance, and echocardiography evaluation.
The overall prevalence of fQRSV1 was 27%. Individuals with fQRSV1 were significantly less likely than those without fQRSV1 to be female (22% vs. 43%), and to present with a lower resting heart rate (66.98 beats per minute vs. 70.08 beats per minute).
Echocardiographic data showed that individuals with fQRSV1 had significantly different morphological and functional right ventricular characteristics.
Notably, right ventricular end-diastolic diameter was 20.42 mm/m2 among individuals with fQRSV1 and 19.81 mm/m2 in those without, a significant difference (P = .019), Dr. Quinto said. Tricuspid annulus plain systolic excursion also differed significantly; 24.33 mm and 23.75 mm for individuals with and without fQRSV1, respectively (P = .013).
However, the individuals with fQRSV1 showed no increased occurrence of any type of exercise-induced arrhythmias regardless of morphology or complexity, said Dr. Quinto.
The prevalence of common and uncommon arrhythmias among individuals with and without fQRSV1 was 31% versus 34% and 13% versus 11%, respectively; these differences were not significant.
The study findings were limited by the relatively small size, but were strengthened by the review of echocardiographic data by two independent physicians, she said.
The results show that the overall prevalence of fQRSV1 in young athletes is comparable with patterns seen in studies of adult athletes, and no differences in exercise-induced arrhythmias occurred despite differences in right ventricular characteristics, she concluded.
Expanded insight into evaluation
The ECG pattern identified in the current study is often encountered in the evaluation of athletes, but its importance was unknown, Matthew Martinez, MD, a sports cardiologist at the Atlantic Health System in Morristown, N.J., said in an interview.
“Studies of ECG findings in athletes continues to inform us about which findings are important to evaluate. This study furthers our understanding of how to proceed,” and will serve as a guide for additional testing to reduce athlete risk, he said.
Looking ahead, “this study should guide clinicians about additional testing and evaluation when fQRS is present in adolescent athletes compared to adults,” Dr. Martinez noted. However, additional research is needed to determine which is the next best test, and whether the patient requires ongoing surveillance, or whether a single evaluation is sufficient, he said. “Further study should focus on best practices after fQRS is identified and whether outcomes can be linked to this finding.”
The study received no outside funding. Dr. Quinto and Dr. Martinez had no financial conflicts to disclose.
The prevalence of exercise-induced arrhythmias in young athletes with fragmented QRS (fQRS) patterns in lead V1 was 27%, similar to that seen in adult athletes, based on data from nearly 700 individuals.
Recent data suggest that fQRS complex in lead V1 (fQRSV1) in healthy athletes may promote arrhythmias in the context of training-induced right ventricular remodeling, but the prevalence and significance in young athletes has not been well studied, Guilia Quinto, MD, of the University of Padova (Italy) said in a presentation at the annual congress of the European Association of Preventive Cardiology.
Dr. Quinto and colleagues assessed data from of young athletes on ventricular arrhythmias during exercise tests.
The study population included 684 young athletes with a mean age of 15 years; 64% were male. Baseline data collection included medical history, physical exam, resting ECG, standardized maximum exercise tolerance, and echocardiography evaluation.
The overall prevalence of fQRSV1 was 27%. Individuals with fQRSV1 were significantly less likely than those without fQRSV1 to be female (22% vs. 43%), and to present with a lower resting heart rate (66.98 beats per minute vs. 70.08 beats per minute).
Echocardiographic data showed that individuals with fQRSV1 had significantly different morphological and functional right ventricular characteristics.
Notably, right ventricular end-diastolic diameter was 20.42 mm/m2 among individuals with fQRSV1 and 19.81 mm/m2 in those without, a significant difference (P = .019), Dr. Quinto said. Tricuspid annulus plain systolic excursion also differed significantly; 24.33 mm and 23.75 mm for individuals with and without fQRSV1, respectively (P = .013).
However, the individuals with fQRSV1 showed no increased occurrence of any type of exercise-induced arrhythmias regardless of morphology or complexity, said Dr. Quinto.
The prevalence of common and uncommon arrhythmias among individuals with and without fQRSV1 was 31% versus 34% and 13% versus 11%, respectively; these differences were not significant.
The study findings were limited by the relatively small size, but were strengthened by the review of echocardiographic data by two independent physicians, she said.
The results show that the overall prevalence of fQRSV1 in young athletes is comparable with patterns seen in studies of adult athletes, and no differences in exercise-induced arrhythmias occurred despite differences in right ventricular characteristics, she concluded.
Expanded insight into evaluation
The ECG pattern identified in the current study is often encountered in the evaluation of athletes, but its importance was unknown, Matthew Martinez, MD, a sports cardiologist at the Atlantic Health System in Morristown, N.J., said in an interview.
“Studies of ECG findings in athletes continues to inform us about which findings are important to evaluate. This study furthers our understanding of how to proceed,” and will serve as a guide for additional testing to reduce athlete risk, he said.
Looking ahead, “this study should guide clinicians about additional testing and evaluation when fQRS is present in adolescent athletes compared to adults,” Dr. Martinez noted. However, additional research is needed to determine which is the next best test, and whether the patient requires ongoing surveillance, or whether a single evaluation is sufficient, he said. “Further study should focus on best practices after fQRS is identified and whether outcomes can be linked to this finding.”
The study received no outside funding. Dr. Quinto and Dr. Martinez had no financial conflicts to disclose.
The prevalence of exercise-induced arrhythmias in young athletes with fragmented QRS (fQRS) patterns in lead V1 was 27%, similar to that seen in adult athletes, based on data from nearly 700 individuals.
Recent data suggest that fQRS complex in lead V1 (fQRSV1) in healthy athletes may promote arrhythmias in the context of training-induced right ventricular remodeling, but the prevalence and significance in young athletes has not been well studied, Guilia Quinto, MD, of the University of Padova (Italy) said in a presentation at the annual congress of the European Association of Preventive Cardiology.
Dr. Quinto and colleagues assessed data from of young athletes on ventricular arrhythmias during exercise tests.
The study population included 684 young athletes with a mean age of 15 years; 64% were male. Baseline data collection included medical history, physical exam, resting ECG, standardized maximum exercise tolerance, and echocardiography evaluation.
The overall prevalence of fQRSV1 was 27%. Individuals with fQRSV1 were significantly less likely than those without fQRSV1 to be female (22% vs. 43%), and to present with a lower resting heart rate (66.98 beats per minute vs. 70.08 beats per minute).
Echocardiographic data showed that individuals with fQRSV1 had significantly different morphological and functional right ventricular characteristics.
Notably, right ventricular end-diastolic diameter was 20.42 mm/m2 among individuals with fQRSV1 and 19.81 mm/m2 in those without, a significant difference (P = .019), Dr. Quinto said. Tricuspid annulus plain systolic excursion also differed significantly; 24.33 mm and 23.75 mm for individuals with and without fQRSV1, respectively (P = .013).
However, the individuals with fQRSV1 showed no increased occurrence of any type of exercise-induced arrhythmias regardless of morphology or complexity, said Dr. Quinto.
The prevalence of common and uncommon arrhythmias among individuals with and without fQRSV1 was 31% versus 34% and 13% versus 11%, respectively; these differences were not significant.
The study findings were limited by the relatively small size, but were strengthened by the review of echocardiographic data by two independent physicians, she said.
The results show that the overall prevalence of fQRSV1 in young athletes is comparable with patterns seen in studies of adult athletes, and no differences in exercise-induced arrhythmias occurred despite differences in right ventricular characteristics, she concluded.
Expanded insight into evaluation
The ECG pattern identified in the current study is often encountered in the evaluation of athletes, but its importance was unknown, Matthew Martinez, MD, a sports cardiologist at the Atlantic Health System in Morristown, N.J., said in an interview.
“Studies of ECG findings in athletes continues to inform us about which findings are important to evaluate. This study furthers our understanding of how to proceed,” and will serve as a guide for additional testing to reduce athlete risk, he said.
Looking ahead, “this study should guide clinicians about additional testing and evaluation when fQRS is present in adolescent athletes compared to adults,” Dr. Martinez noted. However, additional research is needed to determine which is the next best test, and whether the patient requires ongoing surveillance, or whether a single evaluation is sufficient, he said. “Further study should focus on best practices after fQRS is identified and whether outcomes can be linked to this finding.”
The study received no outside funding. Dr. Quinto and Dr. Martinez had no financial conflicts to disclose.
FROM ESC PREVENTIVE CARDIOLOGY 2022
New smart device shows highly accurate AFib detection: mAFA II
Screening for heart rhythm disorders with a smartphone app and a wearable device had a high rate of correctly detecting atrial fibrillation (AFib) in a large new study.
The mAFA II study, conducted in a mass low-risk population in China, showed that more than 93% of possible AFib episodes detected by the smartphone app were confirmed to be AFib on further monitoring.
The study also used the app to screen for obstructive sleep apnea and found that sleep apnea was the most common risk factor associated with increased AFib susceptibility, and those identified as having the most severe sleep apnea were 1.5 times more likely to have AFib than those who did not have this condition.
This suggests that tools suitable for detecting both AFib and sleep apnea can work synergistically to further enhance health monitoring, said lead author, Yutao Guo, MD, professor of internal medicine at Chinese PLA General Hospital, Beijing.
Dr. Guo presented the mAFA II study at the American College of Cardiology (ACC) 2022 Scientific Session held in Washington, D.C., and online.
The trial, which involved more than 2.8 million participants, is the largest study to date to demonstrate how wearable consumer technologies can be used to screen for heart problems during everyday activities, Dr. Guo noted.
“Consumer-led screening with these technologies could increase early diagnosis of AFib and facilitate an integrated approach to fully implement clustered risk management to reduce AFib burden and its related complications,” she concluded.
Discussant of the study at the ACC session at which it was presented, Jodie Hurwitz, MD, Director of the Electrophysiology Lab at Medical City Hospital, Dallas, called this “a pretty impressive study. To get a 93.8% confirmation of AFib with these devices is great.”
But Dr. Hurwitz pointed out that the age of patients in the study was relatively young (average 37 years), and the group who really need to use such a device is much older than that.
“The take-home messages from this study are that AFib wearable detection algorithms have the ability to detect true AFib and that they might also be able to detect risk factors (such as sleep apnea) that predispose to AFib possibly even before AFib is present,” Dr. Hurwitz commented.
Moderator of the session, Edward Fry, MD, cardiologist at Ascension St. Vincent Heart Center, Indianapolis, and incoming president of the ACC, described the area of AFib screening with smart devices as “fascinating, especially with the perspective of the scalability of these types of studies.”
The mAFA II study tracked more than 2.8 million people who used a Huawei phone app together with Huawei and Honor smart devices incorporating photoplethysmography (PPG) technology, a light-based method to monitor blood flow and pulse. If an abnormal rhythm was detected, the wearer would be contacted by a clinician to set up an appointment for a clinical assessment.
Over the course of 4 years of the study, 12,244 (0.4%) of users received a notification of suspected AFib. Among 5,227 people who chose to follow up with a clinician, AFib was confirmed in 93.8% of patients using standard AFib diagnostic tools, including clinical evaluation, an electrocardiogram, and 24-hour Holter monitoring.
In this study, a subset of the individuals screened for AFib were also screened for signs of sleep apnea using the same PPG technology to detect physiological changes in parameters including oxygenation and respiratory rates. The app is also able to determine whether the individual is awake or asleep. Dr. Guo noted that the PPG algorithm for obstructive sleep apnea risk has been validated, compared with polysomnography or home sleep apnea tests.
Using measurements of apnea (signalled by a reduced respiratory rate) and hypopnea (when oxygenation would decrease), the apnea–hypopnea index (AHI) is calculated to determine the severity of the sleep apnea.
Of the 961,931 participants screened for sleep apnea, about 18,000 were notified they may have the condition.
Obstructive sleep apnea was the most reported common risk factor associated with increased AFib susceptibility, and those individuals with the highest risk sleep apnea (more than 80% monitoring measures with AHI greater than or equal to 30 during sleep) resulted in a 1.5-fold increase in prevalent AFib, Dr. Guo reported.
The mAFA II is the latest of several studies to show that AFib can be detected with various smartphone apps and wearable devices. Previous studies have included the Fitbit Heart Study and the Apple Heart Study.
Dr. Hurwitz told this news organization that the electrophysiologist community is enthusiastic about this new smart device technology.
“I sent my sister one so she could determine if she develops AFib: That’s a pretty good endorsement,” she commented, but added that there are still concerns about the rate of false-positive results.
Dr. Hurwitz said she suspected that there will probably be meaningful differences between the different apps and devices, but the algorithms are all proprietary, and the use of photoplethysmography seems to make a big difference.
She noted that the detection of sleep apnea in the current study was a novel approach. “This is important, as sleep apnea is felt to contribute to AFib, and treating it is felt to decrease the frequency of AFib. Perhaps if patients with sleep apnea were treated before they had documented AFib, the AFib burden could be reduced,” she said.
She added that further studies were needed to fine tune the algorithms and to try and identify other factors or heart rate variabilities that may predict future risk of AFib.
The study was funded by the National Natural Science Foundation of China. Dr. Guo reports no disclosures.
A version of this article first appeared on Medscape.com.
Screening for heart rhythm disorders with a smartphone app and a wearable device had a high rate of correctly detecting atrial fibrillation (AFib) in a large new study.
The mAFA II study, conducted in a mass low-risk population in China, showed that more than 93% of possible AFib episodes detected by the smartphone app were confirmed to be AFib on further monitoring.
The study also used the app to screen for obstructive sleep apnea and found that sleep apnea was the most common risk factor associated with increased AFib susceptibility, and those identified as having the most severe sleep apnea were 1.5 times more likely to have AFib than those who did not have this condition.
This suggests that tools suitable for detecting both AFib and sleep apnea can work synergistically to further enhance health monitoring, said lead author, Yutao Guo, MD, professor of internal medicine at Chinese PLA General Hospital, Beijing.
Dr. Guo presented the mAFA II study at the American College of Cardiology (ACC) 2022 Scientific Session held in Washington, D.C., and online.
The trial, which involved more than 2.8 million participants, is the largest study to date to demonstrate how wearable consumer technologies can be used to screen for heart problems during everyday activities, Dr. Guo noted.
“Consumer-led screening with these technologies could increase early diagnosis of AFib and facilitate an integrated approach to fully implement clustered risk management to reduce AFib burden and its related complications,” she concluded.
Discussant of the study at the ACC session at which it was presented, Jodie Hurwitz, MD, Director of the Electrophysiology Lab at Medical City Hospital, Dallas, called this “a pretty impressive study. To get a 93.8% confirmation of AFib with these devices is great.”
But Dr. Hurwitz pointed out that the age of patients in the study was relatively young (average 37 years), and the group who really need to use such a device is much older than that.
“The take-home messages from this study are that AFib wearable detection algorithms have the ability to detect true AFib and that they might also be able to detect risk factors (such as sleep apnea) that predispose to AFib possibly even before AFib is present,” Dr. Hurwitz commented.
Moderator of the session, Edward Fry, MD, cardiologist at Ascension St. Vincent Heart Center, Indianapolis, and incoming president of the ACC, described the area of AFib screening with smart devices as “fascinating, especially with the perspective of the scalability of these types of studies.”
The mAFA II study tracked more than 2.8 million people who used a Huawei phone app together with Huawei and Honor smart devices incorporating photoplethysmography (PPG) technology, a light-based method to monitor blood flow and pulse. If an abnormal rhythm was detected, the wearer would be contacted by a clinician to set up an appointment for a clinical assessment.
Over the course of 4 years of the study, 12,244 (0.4%) of users received a notification of suspected AFib. Among 5,227 people who chose to follow up with a clinician, AFib was confirmed in 93.8% of patients using standard AFib diagnostic tools, including clinical evaluation, an electrocardiogram, and 24-hour Holter monitoring.
In this study, a subset of the individuals screened for AFib were also screened for signs of sleep apnea using the same PPG technology to detect physiological changes in parameters including oxygenation and respiratory rates. The app is also able to determine whether the individual is awake or asleep. Dr. Guo noted that the PPG algorithm for obstructive sleep apnea risk has been validated, compared with polysomnography or home sleep apnea tests.
Using measurements of apnea (signalled by a reduced respiratory rate) and hypopnea (when oxygenation would decrease), the apnea–hypopnea index (AHI) is calculated to determine the severity of the sleep apnea.
Of the 961,931 participants screened for sleep apnea, about 18,000 were notified they may have the condition.
Obstructive sleep apnea was the most reported common risk factor associated with increased AFib susceptibility, and those individuals with the highest risk sleep apnea (more than 80% monitoring measures with AHI greater than or equal to 30 during sleep) resulted in a 1.5-fold increase in prevalent AFib, Dr. Guo reported.
The mAFA II is the latest of several studies to show that AFib can be detected with various smartphone apps and wearable devices. Previous studies have included the Fitbit Heart Study and the Apple Heart Study.
Dr. Hurwitz told this news organization that the electrophysiologist community is enthusiastic about this new smart device technology.
“I sent my sister one so she could determine if she develops AFib: That’s a pretty good endorsement,” she commented, but added that there are still concerns about the rate of false-positive results.
Dr. Hurwitz said she suspected that there will probably be meaningful differences between the different apps and devices, but the algorithms are all proprietary, and the use of photoplethysmography seems to make a big difference.
She noted that the detection of sleep apnea in the current study was a novel approach. “This is important, as sleep apnea is felt to contribute to AFib, and treating it is felt to decrease the frequency of AFib. Perhaps if patients with sleep apnea were treated before they had documented AFib, the AFib burden could be reduced,” she said.
She added that further studies were needed to fine tune the algorithms and to try and identify other factors or heart rate variabilities that may predict future risk of AFib.
The study was funded by the National Natural Science Foundation of China. Dr. Guo reports no disclosures.
A version of this article first appeared on Medscape.com.
Screening for heart rhythm disorders with a smartphone app and a wearable device had a high rate of correctly detecting atrial fibrillation (AFib) in a large new study.
The mAFA II study, conducted in a mass low-risk population in China, showed that more than 93% of possible AFib episodes detected by the smartphone app were confirmed to be AFib on further monitoring.
The study also used the app to screen for obstructive sleep apnea and found that sleep apnea was the most common risk factor associated with increased AFib susceptibility, and those identified as having the most severe sleep apnea were 1.5 times more likely to have AFib than those who did not have this condition.
This suggests that tools suitable for detecting both AFib and sleep apnea can work synergistically to further enhance health monitoring, said lead author, Yutao Guo, MD, professor of internal medicine at Chinese PLA General Hospital, Beijing.
Dr. Guo presented the mAFA II study at the American College of Cardiology (ACC) 2022 Scientific Session held in Washington, D.C., and online.
The trial, which involved more than 2.8 million participants, is the largest study to date to demonstrate how wearable consumer technologies can be used to screen for heart problems during everyday activities, Dr. Guo noted.
“Consumer-led screening with these technologies could increase early diagnosis of AFib and facilitate an integrated approach to fully implement clustered risk management to reduce AFib burden and its related complications,” she concluded.
Discussant of the study at the ACC session at which it was presented, Jodie Hurwitz, MD, Director of the Electrophysiology Lab at Medical City Hospital, Dallas, called this “a pretty impressive study. To get a 93.8% confirmation of AFib with these devices is great.”
But Dr. Hurwitz pointed out that the age of patients in the study was relatively young (average 37 years), and the group who really need to use such a device is much older than that.
“The take-home messages from this study are that AFib wearable detection algorithms have the ability to detect true AFib and that they might also be able to detect risk factors (such as sleep apnea) that predispose to AFib possibly even before AFib is present,” Dr. Hurwitz commented.
Moderator of the session, Edward Fry, MD, cardiologist at Ascension St. Vincent Heart Center, Indianapolis, and incoming president of the ACC, described the area of AFib screening with smart devices as “fascinating, especially with the perspective of the scalability of these types of studies.”
The mAFA II study tracked more than 2.8 million people who used a Huawei phone app together with Huawei and Honor smart devices incorporating photoplethysmography (PPG) technology, a light-based method to monitor blood flow and pulse. If an abnormal rhythm was detected, the wearer would be contacted by a clinician to set up an appointment for a clinical assessment.
Over the course of 4 years of the study, 12,244 (0.4%) of users received a notification of suspected AFib. Among 5,227 people who chose to follow up with a clinician, AFib was confirmed in 93.8% of patients using standard AFib diagnostic tools, including clinical evaluation, an electrocardiogram, and 24-hour Holter monitoring.
In this study, a subset of the individuals screened for AFib were also screened for signs of sleep apnea using the same PPG technology to detect physiological changes in parameters including oxygenation and respiratory rates. The app is also able to determine whether the individual is awake or asleep. Dr. Guo noted that the PPG algorithm for obstructive sleep apnea risk has been validated, compared with polysomnography or home sleep apnea tests.
Using measurements of apnea (signalled by a reduced respiratory rate) and hypopnea (when oxygenation would decrease), the apnea–hypopnea index (AHI) is calculated to determine the severity of the sleep apnea.
Of the 961,931 participants screened for sleep apnea, about 18,000 were notified they may have the condition.
Obstructive sleep apnea was the most reported common risk factor associated with increased AFib susceptibility, and those individuals with the highest risk sleep apnea (more than 80% monitoring measures with AHI greater than or equal to 30 during sleep) resulted in a 1.5-fold increase in prevalent AFib, Dr. Guo reported.
The mAFA II is the latest of several studies to show that AFib can be detected with various smartphone apps and wearable devices. Previous studies have included the Fitbit Heart Study and the Apple Heart Study.
Dr. Hurwitz told this news organization that the electrophysiologist community is enthusiastic about this new smart device technology.
“I sent my sister one so she could determine if she develops AFib: That’s a pretty good endorsement,” she commented, but added that there are still concerns about the rate of false-positive results.
Dr. Hurwitz said she suspected that there will probably be meaningful differences between the different apps and devices, but the algorithms are all proprietary, and the use of photoplethysmography seems to make a big difference.
She noted that the detection of sleep apnea in the current study was a novel approach. “This is important, as sleep apnea is felt to contribute to AFib, and treating it is felt to decrease the frequency of AFib. Perhaps if patients with sleep apnea were treated before they had documented AFib, the AFib burden could be reduced,” she said.
She added that further studies were needed to fine tune the algorithms and to try and identify other factors or heart rate variabilities that may predict future risk of AFib.
The study was funded by the National Natural Science Foundation of China. Dr. Guo reports no disclosures.
A version of this article first appeared on Medscape.com.