User login
Macrolide monotherapy works in some NTM lung disease
Patients with cystic fibrosis or bronchiectasis and one form of Mycobacterium abscessus disease can be successfully treated with long-term oral macrolide monotherapy following short-term intravenous combination antibiotic therapy, a Korean research team has shown.
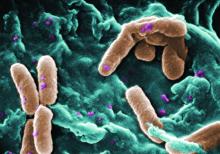
The M. abscessus complex is implicated in between a fifth and half of all cases of lung disease caused by nontuberculous mycobacteria (NTM). Though treatment is notoriously difficult and prolonged in all NTM lung disease, one subspecies of M. abscessus – M. massiliense – lacks the active gene needed for developing resistance to macrolide-based antibiotics, making it potentially more readily treated.
In research published in CHEST, Won-Jung Koh, MD, of Samsung Medical Center and Sungkyunkwan University in Seoul, South Korea, and colleagues, sought to determine the optimal treatment protocol for patients with massiliense disease (Chest. 2016 Dec;150[6]:1211-21). They identified 71 patients with massiliense disease who had initiated antibiotic treatment between January 2007 and December 2012. These patients were part of an ongoing prospective cohort study on NTM lung disease. The first 28 patients in the study were hospitalized for 4 weeks and treated with intravenous amikacin and cefoxitin along with oral clarithromycin and a fluoroquinolone. Following discharge these patients remained on the oral agents for 24 months.
Two years into the study, the protocol changed, and the next 43 patients were treated with a 2-week course of intravenous amikacin and cefoxitin along with the oral agents. In some patients, azithromycin, which came into use in Korea for NTM lung disease in 2011, replaced a fluoroquinolone. After discharge, all patients stayed on the oral macrolides (with seven also taking a fluoroquinolone) until their sputum cultures were negative for 12 months.
For the patients treated for 4 weeks, the response rates after 12 months of treatment were 89% for symptoms, 79% for computed tomography, and 100% for negative sputum cultures. In the patients treated for 2 weeks, they were 100%, 91%, and 91%, respectively. None of these differences between the two groups were statistically significant. Median total treatment duration, however, was significantly shorter – by nearly a year – in the 2-week plus macrolide monotherapy group than in the other group of patients (15.2 months vs. 23.9 months, P less than .001).
Acquired macrolide resistance developed in two patients in the group who received a 2-week course of intravenous amikacin and cefoxitin along with the oral agents, including one case of high-level clarithromycin resistance. Genotyping revealed reinfection with different strains of M. massiliense.
“[Oral] macrolide therapy after an initial 2-week course of combination antibiotics, rather than long-term parenteral antibiotics, might be effective in most patients with M. massiliense lung disease,” Dr. Koh and colleagues wrote, noting that their study’s nonrandomized single-site design was a limitation, and that multicenter randomized trials would be needed “to assess the efficacy” of the findings.
The Korean government funded Dr. Koh and colleagues’ study. None of the authors disclosed conflicts of interest.
“In this study by Koh et al., it is gratifying that most patients had a favorable microbiologic outcome. It is also somewhat surprising that only two patients developed acquired macrolide resistant M. abscessus subsp massiliense isolates. While the absolute number is low, for those two individuals, the consequences of developing macrolide resistance are far from trivial. They have transitioned from having a mycobacterial infection that is relatively easy to treat effectively to a mycobacterial infection that is not,” David E. Griffith, MD, FCCP, and Timothy R. Aksamit, MD, FCCP, wrote in an editorial published in the December issue of CHEST (Chest. 2016 Dec;150[6];1177-8).
The authors noted that they “enthusiastically applaud and acknowledge the prolific and consistently excellent work done by the group in South Korea, but we cannot endorse the widespread adoption of macrolide monotherapy for” this patient group. “In our view, the risk/benefit balance of this approach does not favor macrolide monotherapy even though the majority of patients in this study were adequately treated.”
Dr. Griffith is professor of medicine at University of Texas Health Science Center, Tyler, and Dr. Aksamit is a consultant on pulmonary disease and critical care medicine at the Mayo Clinic, Rochester, Minn. They disclosed no conflicts of interest.
“In this study by Koh et al., it is gratifying that most patients had a favorable microbiologic outcome. It is also somewhat surprising that only two patients developed acquired macrolide resistant M. abscessus subsp massiliense isolates. While the absolute number is low, for those two individuals, the consequences of developing macrolide resistance are far from trivial. They have transitioned from having a mycobacterial infection that is relatively easy to treat effectively to a mycobacterial infection that is not,” David E. Griffith, MD, FCCP, and Timothy R. Aksamit, MD, FCCP, wrote in an editorial published in the December issue of CHEST (Chest. 2016 Dec;150[6];1177-8).
The authors noted that they “enthusiastically applaud and acknowledge the prolific and consistently excellent work done by the group in South Korea, but we cannot endorse the widespread adoption of macrolide monotherapy for” this patient group. “In our view, the risk/benefit balance of this approach does not favor macrolide monotherapy even though the majority of patients in this study were adequately treated.”
Dr. Griffith is professor of medicine at University of Texas Health Science Center, Tyler, and Dr. Aksamit is a consultant on pulmonary disease and critical care medicine at the Mayo Clinic, Rochester, Minn. They disclosed no conflicts of interest.
“In this study by Koh et al., it is gratifying that most patients had a favorable microbiologic outcome. It is also somewhat surprising that only two patients developed acquired macrolide resistant M. abscessus subsp massiliense isolates. While the absolute number is low, for those two individuals, the consequences of developing macrolide resistance are far from trivial. They have transitioned from having a mycobacterial infection that is relatively easy to treat effectively to a mycobacterial infection that is not,” David E. Griffith, MD, FCCP, and Timothy R. Aksamit, MD, FCCP, wrote in an editorial published in the December issue of CHEST (Chest. 2016 Dec;150[6];1177-8).
The authors noted that they “enthusiastically applaud and acknowledge the prolific and consistently excellent work done by the group in South Korea, but we cannot endorse the widespread adoption of macrolide monotherapy for” this patient group. “In our view, the risk/benefit balance of this approach does not favor macrolide monotherapy even though the majority of patients in this study were adequately treated.”
Dr. Griffith is professor of medicine at University of Texas Health Science Center, Tyler, and Dr. Aksamit is a consultant on pulmonary disease and critical care medicine at the Mayo Clinic, Rochester, Minn. They disclosed no conflicts of interest.
Patients with cystic fibrosis or bronchiectasis and one form of Mycobacterium abscessus disease can be successfully treated with long-term oral macrolide monotherapy following short-term intravenous combination antibiotic therapy, a Korean research team has shown.
The M. abscessus complex is implicated in between a fifth and half of all cases of lung disease caused by nontuberculous mycobacteria (NTM). Though treatment is notoriously difficult and prolonged in all NTM lung disease, one subspecies of M. abscessus – M. massiliense – lacks the active gene needed for developing resistance to macrolide-based antibiotics, making it potentially more readily treated.
In research published in CHEST, Won-Jung Koh, MD, of Samsung Medical Center and Sungkyunkwan University in Seoul, South Korea, and colleagues, sought to determine the optimal treatment protocol for patients with massiliense disease (Chest. 2016 Dec;150[6]:1211-21). They identified 71 patients with massiliense disease who had initiated antibiotic treatment between January 2007 and December 2012. These patients were part of an ongoing prospective cohort study on NTM lung disease. The first 28 patients in the study were hospitalized for 4 weeks and treated with intravenous amikacin and cefoxitin along with oral clarithromycin and a fluoroquinolone. Following discharge these patients remained on the oral agents for 24 months.
Two years into the study, the protocol changed, and the next 43 patients were treated with a 2-week course of intravenous amikacin and cefoxitin along with the oral agents. In some patients, azithromycin, which came into use in Korea for NTM lung disease in 2011, replaced a fluoroquinolone. After discharge, all patients stayed on the oral macrolides (with seven also taking a fluoroquinolone) until their sputum cultures were negative for 12 months.
For the patients treated for 4 weeks, the response rates after 12 months of treatment were 89% for symptoms, 79% for computed tomography, and 100% for negative sputum cultures. In the patients treated for 2 weeks, they were 100%, 91%, and 91%, respectively. None of these differences between the two groups were statistically significant. Median total treatment duration, however, was significantly shorter – by nearly a year – in the 2-week plus macrolide monotherapy group than in the other group of patients (15.2 months vs. 23.9 months, P less than .001).
Acquired macrolide resistance developed in two patients in the group who received a 2-week course of intravenous amikacin and cefoxitin along with the oral agents, including one case of high-level clarithromycin resistance. Genotyping revealed reinfection with different strains of M. massiliense.
“[Oral] macrolide therapy after an initial 2-week course of combination antibiotics, rather than long-term parenteral antibiotics, might be effective in most patients with M. massiliense lung disease,” Dr. Koh and colleagues wrote, noting that their study’s nonrandomized single-site design was a limitation, and that multicenter randomized trials would be needed “to assess the efficacy” of the findings.
The Korean government funded Dr. Koh and colleagues’ study. None of the authors disclosed conflicts of interest.
Patients with cystic fibrosis or bronchiectasis and one form of Mycobacterium abscessus disease can be successfully treated with long-term oral macrolide monotherapy following short-term intravenous combination antibiotic therapy, a Korean research team has shown.
The M. abscessus complex is implicated in between a fifth and half of all cases of lung disease caused by nontuberculous mycobacteria (NTM). Though treatment is notoriously difficult and prolonged in all NTM lung disease, one subspecies of M. abscessus – M. massiliense – lacks the active gene needed for developing resistance to macrolide-based antibiotics, making it potentially more readily treated.
In research published in CHEST, Won-Jung Koh, MD, of Samsung Medical Center and Sungkyunkwan University in Seoul, South Korea, and colleagues, sought to determine the optimal treatment protocol for patients with massiliense disease (Chest. 2016 Dec;150[6]:1211-21). They identified 71 patients with massiliense disease who had initiated antibiotic treatment between January 2007 and December 2012. These patients were part of an ongoing prospective cohort study on NTM lung disease. The first 28 patients in the study were hospitalized for 4 weeks and treated with intravenous amikacin and cefoxitin along with oral clarithromycin and a fluoroquinolone. Following discharge these patients remained on the oral agents for 24 months.
Two years into the study, the protocol changed, and the next 43 patients were treated with a 2-week course of intravenous amikacin and cefoxitin along with the oral agents. In some patients, azithromycin, which came into use in Korea for NTM lung disease in 2011, replaced a fluoroquinolone. After discharge, all patients stayed on the oral macrolides (with seven also taking a fluoroquinolone) until their sputum cultures were negative for 12 months.
For the patients treated for 4 weeks, the response rates after 12 months of treatment were 89% for symptoms, 79% for computed tomography, and 100% for negative sputum cultures. In the patients treated for 2 weeks, they were 100%, 91%, and 91%, respectively. None of these differences between the two groups were statistically significant. Median total treatment duration, however, was significantly shorter – by nearly a year – in the 2-week plus macrolide monotherapy group than in the other group of patients (15.2 months vs. 23.9 months, P less than .001).
Acquired macrolide resistance developed in two patients in the group who received a 2-week course of intravenous amikacin and cefoxitin along with the oral agents, including one case of high-level clarithromycin resistance. Genotyping revealed reinfection with different strains of M. massiliense.
“[Oral] macrolide therapy after an initial 2-week course of combination antibiotics, rather than long-term parenteral antibiotics, might be effective in most patients with M. massiliense lung disease,” Dr. Koh and colleagues wrote, noting that their study’s nonrandomized single-site design was a limitation, and that multicenter randomized trials would be needed “to assess the efficacy” of the findings.
The Korean government funded Dr. Koh and colleagues’ study. None of the authors disclosed conflicts of interest.
FROM CHEST
Key clinical point: A short course of intravenous antibiotics followed by oral macrolides may be effective at treating lung disease caused by the massiliense subspecies of M. abscessus.
Major finding: Of 43 patients receiving 2 weeks of combination antibiotics followed by a year of oral macrolides, 39 (91%) converted to negative sputum cultures before 12 months.
Data source: A prospective cohort study enrolling 71 patients at a single treatment center in Korea.
Disclosures: The Korean government sponsored the study and investigators disclosed no conflicts of interest.
Rate of resistant P. aeruginosa in children rising steadily
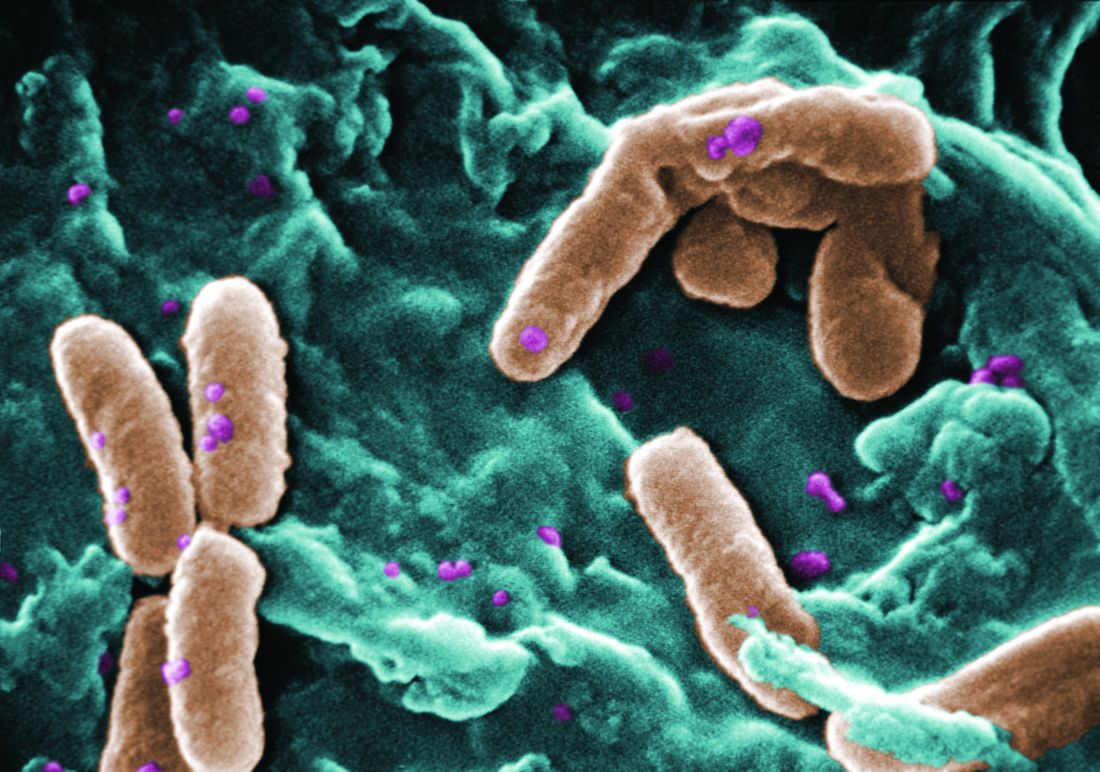
The rate of infection with resistant Pseudomonas aeruginosa among children has risen steadily about 4% per year since 1999, according to a report.
Infections with P aeruginosa in children occur most often associated with pulmonary disease in patients with cystic fibrosis, but healthy children also can experience a variety of P aeruginosa infection types.
They focused on 77,349 P. aeruginosa isolates that were tested for resistance for all five antibiotic classes: cephalosporins, beta-lactam and beta-lactamase inhibitors, carbapenems, fluoroquinolones, and aminoglycosides. The investigators were specifically interested in multidrug- and carbapenem-resistant P. aeruginosa. They described the organism as “arguably the most resistance-prone health care–related pathogen.”
The study samples were obtained from the respiratory tract, urinary tract, ear, sinuses, wounds, skin, and connective tissues of children aged 1-17 years who were treated in 1999-2012; 2012 was the last year for which this information was collected in this database. Patients were treated in ambulatory, inpatient, ICU, and long-term-care settings. The study excluded children with cystic fibrosis and children under 1 year of age, so as to improve the applicability of the results to the general pediatric population.
The proportion of multidrug-resistant P. aeruginosa samples rose from 15% to 26% during the 13-year study period, and the proportion of carbapenem-resistant P. aeruginosa samples rose from 9% to 20%. This represents an average annual increase of approximately 4% for both types of resistance, Dr. Logan and her associates said (J Ped Infect Dis. 2016 Nov 16. doi: 10.1093/jpids/piw064).
These increases were consistent across all but one age group and all but one treatment setting, the exception being inpatients aged 13-17 years. The prevalence of resistant P. aeruginosa was highest among adolescents in ambulatory, ICU, or long-term-care settings; their rates were three times higher than those in children aged 1 year and two times higher than those in children aged 5 years. It is possible that this pattern reflects “an increasing number of older children with a medically complex condition who have frequent exposure to the health care environment,” the investigators noted.
“The results of our study highlight the need for bacterial surveillance, strategies for implementing effective infection prevention, and antimicrobial stewardship programs.” In addition, all health care facilities should consider using rapid molecular diagnostic platforms to guide antibiotic treatment decisions, “to reduce the burden of the persistent and continually evolving global threat of extensively drug-resistant organisms,” Dr. Logan and her associates added.
This study was supported by the National Institute of Allergy and Infectious Diseases; the Global Antibiotic Resistance Partnership, funded by the Bill and Melinda Gates Foundation; and the Health Grand Challenges Program at Princeton University. Dr. Logan and her associates reported having no relevant financial disclosures.
The rate of infection with resistant Pseudomonas aeruginosa among children has risen steadily about 4% per year since 1999, according to a report.
Infections with P aeruginosa in children occur most often associated with pulmonary disease in patients with cystic fibrosis, but healthy children also can experience a variety of P aeruginosa infection types.
They focused on 77,349 P. aeruginosa isolates that were tested for resistance for all five antibiotic classes: cephalosporins, beta-lactam and beta-lactamase inhibitors, carbapenems, fluoroquinolones, and aminoglycosides. The investigators were specifically interested in multidrug- and carbapenem-resistant P. aeruginosa. They described the organism as “arguably the most resistance-prone health care–related pathogen.”
The study samples were obtained from the respiratory tract, urinary tract, ear, sinuses, wounds, skin, and connective tissues of children aged 1-17 years who were treated in 1999-2012; 2012 was the last year for which this information was collected in this database. Patients were treated in ambulatory, inpatient, ICU, and long-term-care settings. The study excluded children with cystic fibrosis and children under 1 year of age, so as to improve the applicability of the results to the general pediatric population.
The proportion of multidrug-resistant P. aeruginosa samples rose from 15% to 26% during the 13-year study period, and the proportion of carbapenem-resistant P. aeruginosa samples rose from 9% to 20%. This represents an average annual increase of approximately 4% for both types of resistance, Dr. Logan and her associates said (J Ped Infect Dis. 2016 Nov 16. doi: 10.1093/jpids/piw064).
These increases were consistent across all but one age group and all but one treatment setting, the exception being inpatients aged 13-17 years. The prevalence of resistant P. aeruginosa was highest among adolescents in ambulatory, ICU, or long-term-care settings; their rates were three times higher than those in children aged 1 year and two times higher than those in children aged 5 years. It is possible that this pattern reflects “an increasing number of older children with a medically complex condition who have frequent exposure to the health care environment,” the investigators noted.
“The results of our study highlight the need for bacterial surveillance, strategies for implementing effective infection prevention, and antimicrobial stewardship programs.” In addition, all health care facilities should consider using rapid molecular diagnostic platforms to guide antibiotic treatment decisions, “to reduce the burden of the persistent and continually evolving global threat of extensively drug-resistant organisms,” Dr. Logan and her associates added.
This study was supported by the National Institute of Allergy and Infectious Diseases; the Global Antibiotic Resistance Partnership, funded by the Bill and Melinda Gates Foundation; and the Health Grand Challenges Program at Princeton University. Dr. Logan and her associates reported having no relevant financial disclosures.
The rate of infection with resistant Pseudomonas aeruginosa among children has risen steadily about 4% per year since 1999, according to a report.
Infections with P aeruginosa in children occur most often associated with pulmonary disease in patients with cystic fibrosis, but healthy children also can experience a variety of P aeruginosa infection types.
They focused on 77,349 P. aeruginosa isolates that were tested for resistance for all five antibiotic classes: cephalosporins, beta-lactam and beta-lactamase inhibitors, carbapenems, fluoroquinolones, and aminoglycosides. The investigators were specifically interested in multidrug- and carbapenem-resistant P. aeruginosa. They described the organism as “arguably the most resistance-prone health care–related pathogen.”
The study samples were obtained from the respiratory tract, urinary tract, ear, sinuses, wounds, skin, and connective tissues of children aged 1-17 years who were treated in 1999-2012; 2012 was the last year for which this information was collected in this database. Patients were treated in ambulatory, inpatient, ICU, and long-term-care settings. The study excluded children with cystic fibrosis and children under 1 year of age, so as to improve the applicability of the results to the general pediatric population.
The proportion of multidrug-resistant P. aeruginosa samples rose from 15% to 26% during the 13-year study period, and the proportion of carbapenem-resistant P. aeruginosa samples rose from 9% to 20%. This represents an average annual increase of approximately 4% for both types of resistance, Dr. Logan and her associates said (J Ped Infect Dis. 2016 Nov 16. doi: 10.1093/jpids/piw064).
These increases were consistent across all but one age group and all but one treatment setting, the exception being inpatients aged 13-17 years. The prevalence of resistant P. aeruginosa was highest among adolescents in ambulatory, ICU, or long-term-care settings; their rates were three times higher than those in children aged 1 year and two times higher than those in children aged 5 years. It is possible that this pattern reflects “an increasing number of older children with a medically complex condition who have frequent exposure to the health care environment,” the investigators noted.
“The results of our study highlight the need for bacterial surveillance, strategies for implementing effective infection prevention, and antimicrobial stewardship programs.” In addition, all health care facilities should consider using rapid molecular diagnostic platforms to guide antibiotic treatment decisions, “to reduce the burden of the persistent and continually evolving global threat of extensively drug-resistant organisms,” Dr. Logan and her associates added.
This study was supported by the National Institute of Allergy and Infectious Diseases; the Global Antibiotic Resistance Partnership, funded by the Bill and Melinda Gates Foundation; and the Health Grand Challenges Program at Princeton University. Dr. Logan and her associates reported having no relevant financial disclosures.
FROM THE JOURNAL OF PEDIATRIC INFECTIOUS DISEASES
Key clinical point:
Major finding: The proportion of multidrug-resistant P. aeruginosa samples rose from 15% to 26% during the 13-year study period, and the proportion of carbapenem-resistant P. aeruginosa samples rose from 9% to 20%.
Data source: A longitudinal analysis of information in a nationally representative database of microbiology laboratories serving approximately 300 U.S. hospitals.
Disclosures: This study was supported by the National Institute of Allergy and Infectious Diseases; the Global Antibiotic Resistance Partnership, funded by the Bill and Melinda Gates Foundation; and the Health Grand Challenges Program at Princeton University. Dr. Logan and her associates reported having no relevant financial disclosures.
‘Skip phenomenon’ could explain fluctuating positivity for S. aureus bacteremia
NEW ORLEANS – A proportion of patients treated appropriately with antibiotics for Staphylococcus aureus bacteremia can generate a negative blood culture followed by a positive one, a new clinical entity researchers are calling the “skip phenomenon.”
“This pattern is really in cases where people have known Staph. aureus bacteremia; it seems to clear; they’re on appropriate antibiotic therapy; and despite that, we see that the blood cultures come back positive again several days later,” explained Justin A. Fiala, MD, of Mayo Clinic in Rochester, Minn.
In a video interview, Dr. Fiala outlined the findings of the first study to identify and characterize this phenomenon.
Certain patients with S. aureus bacteremia could be at higher risk for skip phenomenon, for example. The nested case-control study identified these higher-risk patients, a population that might warrant more clinical testing.
Dr. Fiala also discussed associations with patient outcomes, as well as the overall prevalence of skip phenomenon in his research, which included more than 900 patients with S. aureus bacteremia treated at Mayo Clinic.
Dr. Fiala had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – A proportion of patients treated appropriately with antibiotics for Staphylococcus aureus bacteremia can generate a negative blood culture followed by a positive one, a new clinical entity researchers are calling the “skip phenomenon.”
“This pattern is really in cases where people have known Staph. aureus bacteremia; it seems to clear; they’re on appropriate antibiotic therapy; and despite that, we see that the blood cultures come back positive again several days later,” explained Justin A. Fiala, MD, of Mayo Clinic in Rochester, Minn.
In a video interview, Dr. Fiala outlined the findings of the first study to identify and characterize this phenomenon.
Certain patients with S. aureus bacteremia could be at higher risk for skip phenomenon, for example. The nested case-control study identified these higher-risk patients, a population that might warrant more clinical testing.
Dr. Fiala also discussed associations with patient outcomes, as well as the overall prevalence of skip phenomenon in his research, which included more than 900 patients with S. aureus bacteremia treated at Mayo Clinic.
Dr. Fiala had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – A proportion of patients treated appropriately with antibiotics for Staphylococcus aureus bacteremia can generate a negative blood culture followed by a positive one, a new clinical entity researchers are calling the “skip phenomenon.”
“This pattern is really in cases where people have known Staph. aureus bacteremia; it seems to clear; they’re on appropriate antibiotic therapy; and despite that, we see that the blood cultures come back positive again several days later,” explained Justin A. Fiala, MD, of Mayo Clinic in Rochester, Minn.
In a video interview, Dr. Fiala outlined the findings of the first study to identify and characterize this phenomenon.
Certain patients with S. aureus bacteremia could be at higher risk for skip phenomenon, for example. The nested case-control study identified these higher-risk patients, a population that might warrant more clinical testing.
Dr. Fiala also discussed associations with patient outcomes, as well as the overall prevalence of skip phenomenon in his research, which included more than 900 patients with S. aureus bacteremia treated at Mayo Clinic.
Dr. Fiala had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
‘Fight the Resistance’ with Antibiotic Stewardship Mentored Implementation
In conjunction with the Centers for Disease Control & Prevention’s Get Smart about Antibiotics Week, SHM is committed to promoting improved antibiotic-prescribing behaviors among the nation’s hospitalists through its “Fight the Resistance” awareness campaign.
Display SHM’s three downloadable “Fight the Resistance” posters, available at www.fighttheresistance.org. Hang them in your break rooms, hallways, or other high-profile locations to help remind your colleagues about the dangers of antibiotic resistance. SHM will be launching a mentored implementation program on antibiotics in early 2017. To be notified when the program becomes available, visit www.hospitalmedicine.org/ABX16.
In conjunction with the Centers for Disease Control & Prevention’s Get Smart about Antibiotics Week, SHM is committed to promoting improved antibiotic-prescribing behaviors among the nation’s hospitalists through its “Fight the Resistance” awareness campaign.
Display SHM’s three downloadable “Fight the Resistance” posters, available at www.fighttheresistance.org. Hang them in your break rooms, hallways, or other high-profile locations to help remind your colleagues about the dangers of antibiotic resistance. SHM will be launching a mentored implementation program on antibiotics in early 2017. To be notified when the program becomes available, visit www.hospitalmedicine.org/ABX16.
In conjunction with the Centers for Disease Control & Prevention’s Get Smart about Antibiotics Week, SHM is committed to promoting improved antibiotic-prescribing behaviors among the nation’s hospitalists through its “Fight the Resistance” awareness campaign.
Display SHM’s three downloadable “Fight the Resistance” posters, available at www.fighttheresistance.org. Hang them in your break rooms, hallways, or other high-profile locations to help remind your colleagues about the dangers of antibiotic resistance. SHM will be launching a mentored implementation program on antibiotics in early 2017. To be notified when the program becomes available, visit www.hospitalmedicine.org/ABX16.
Infectious disease physicians: Antibiotic shortages are the new norm
NEW ORLEANS – Antibiotic shortages reported by the Emerging Infections Network (EIN) in 2011 persist in 2016, according to a web-based follow-up survey of infectious disease physicians.
Of 701 network members who responded to the EIN survey in early 2016, 70% reported needing to modify their antimicrobial choice because of a shortage in the past 2 years. They did so by using broader-spectrum agents (75% of respondents), more costly agents (58%), less effective second-line agents (45%), and more toxic agents (37%), Adi Gundlapalli, MD, PhD, reported at an annual scientific meeting on infectious diseases.
In addition, 73% of respondents reported that the shortages affected patient care or outcomes, reported Dr. Gundlapalli of the University of Utah, Salt Lake City.
The percentage of respondents reporting adverse patient outcomes related to shortages increased from 2011 to 2016 (51% vs.73%), he noted at the combined annual meetings of the Infectious Diseases Society of America, the Society of Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The top 10 antimicrobials they reported as being in short supply were piperacillin-tazobactam, ampicillin-sulbactam, meropenem, cefotaxime, cefepime, trimethoprim-sulfamethoxazole (TMP-SMX), doxycycline, imipenem, acyclovir, and amikacin. TMP-SMX and acyclovir were in short supply at both time points.
The most common ways respondents reported learning about drug shortages were from hospital notification (76%), from a colleague (56%), from a pharmacy that contacted them regarding a prescription for the agent (53%), or from the Food and Drug Administration website or another website on shortages (23%). The most common ways of learning about a shortage changed – from notification after trying to prescribe a drug in 2011, to proactive hospital/system (local) notification in 2016; 71% of respondents said that communications in 2016 were sufficient.
Most respondents (83%) reported that guidelines for dealing with shortages had been developed by an antimicrobial stewardship program (ASP) at their institution.
“This, I think, is one of the highlight results,” said Dr. Gundlapalli, who is also a staff physician at the VA Salt Lake City Health System. “In 2011, we had no specific question or comments received about [ASPs], and here in 2016, 83% of respondents’ institutions had developed guidelines related to drug shortages.”
Respondents also had the opportunity to submit free-text responses, and among the themes that emerged was concern regarding toxicity and adverse outcomes associated with increased use of aminoglycosides because of the shortage of piperacillin-tazobactam. Another – described as a blessing in disguise – was the shortage of meropenem, which led one ASP to “institute restrictions on its use, which have continued,” he said.
“Another theme was ‘simpler agents seem more likely to be in shortage,’ ” Dr. Gundlapalli said, noting ampicillin-sulbactam in 2016 and Pen-G as examples.
“And then, of course, the other theme across the board ... was our new asset,” he said, explaining that some respondents commented on the value of ASP pharmacists and programs to help with drug shortage issues.
The overall theme of this follow-up survey, in the context of prior surveys in 2001 and 2011, is that antibiotic shortages are the “new normal – a way of life,” Dr. Gundlapalli said.
“The concerns do persist, and we feel there is further work to be done here,” he said. He specifically noted that there is a need to inform and educate fellows and colleagues in hospitals, increase awareness generally, improve communication strategies, and conduct detailed studies on adverse effects and outcomes.
“And now, since ASPs are very pervasive ... maybe it’s time to formalize and delineate the role of ASPs in antimicrobial shortages,” he said.
The problem of antibiotic shortages “harkens back to the day when penicillin was recycled in the urine [of soldiers in World War II] to save this very scarce resource ... but that’s a very extreme measure to take,” noted Donald Graham, MD, of the Springfield (Ill.) Clinic, one of the study’s coauthors. “It seems like it’s time for the other federal arm – namely, the Food and Drug Administration – to do something about this.”
Dr. Graham said he believes the problem is in part because of economics, and in part because of “the higher standards that the FDA imposes upon these manufacturing concerns.” These drugs often are low-profit items, and it isn’t always in the financial best interest of a pharmaceutical company to upgrade their facilities.
“But they really have to recognize the importance of having availability of these simple agents,” he said, pleading with any FDA representatives in the audience to “maybe think about some of these very high standards.”
Dr. Gundlapalli reported having no disclosures. Dr. Graham disclosed relationships with Astellas and Theravance Biopharma.
NEW ORLEANS – Antibiotic shortages reported by the Emerging Infections Network (EIN) in 2011 persist in 2016, according to a web-based follow-up survey of infectious disease physicians.
Of 701 network members who responded to the EIN survey in early 2016, 70% reported needing to modify their antimicrobial choice because of a shortage in the past 2 years. They did so by using broader-spectrum agents (75% of respondents), more costly agents (58%), less effective second-line agents (45%), and more toxic agents (37%), Adi Gundlapalli, MD, PhD, reported at an annual scientific meeting on infectious diseases.
In addition, 73% of respondents reported that the shortages affected patient care or outcomes, reported Dr. Gundlapalli of the University of Utah, Salt Lake City.
The percentage of respondents reporting adverse patient outcomes related to shortages increased from 2011 to 2016 (51% vs.73%), he noted at the combined annual meetings of the Infectious Diseases Society of America, the Society of Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The top 10 antimicrobials they reported as being in short supply were piperacillin-tazobactam, ampicillin-sulbactam, meropenem, cefotaxime, cefepime, trimethoprim-sulfamethoxazole (TMP-SMX), doxycycline, imipenem, acyclovir, and amikacin. TMP-SMX and acyclovir were in short supply at both time points.
The most common ways respondents reported learning about drug shortages were from hospital notification (76%), from a colleague (56%), from a pharmacy that contacted them regarding a prescription for the agent (53%), or from the Food and Drug Administration website or another website on shortages (23%). The most common ways of learning about a shortage changed – from notification after trying to prescribe a drug in 2011, to proactive hospital/system (local) notification in 2016; 71% of respondents said that communications in 2016 were sufficient.
Most respondents (83%) reported that guidelines for dealing with shortages had been developed by an antimicrobial stewardship program (ASP) at their institution.
“This, I think, is one of the highlight results,” said Dr. Gundlapalli, who is also a staff physician at the VA Salt Lake City Health System. “In 2011, we had no specific question or comments received about [ASPs], and here in 2016, 83% of respondents’ institutions had developed guidelines related to drug shortages.”
Respondents also had the opportunity to submit free-text responses, and among the themes that emerged was concern regarding toxicity and adverse outcomes associated with increased use of aminoglycosides because of the shortage of piperacillin-tazobactam. Another – described as a blessing in disguise – was the shortage of meropenem, which led one ASP to “institute restrictions on its use, which have continued,” he said.
“Another theme was ‘simpler agents seem more likely to be in shortage,’ ” Dr. Gundlapalli said, noting ampicillin-sulbactam in 2016 and Pen-G as examples.
“And then, of course, the other theme across the board ... was our new asset,” he said, explaining that some respondents commented on the value of ASP pharmacists and programs to help with drug shortage issues.
The overall theme of this follow-up survey, in the context of prior surveys in 2001 and 2011, is that antibiotic shortages are the “new normal – a way of life,” Dr. Gundlapalli said.
“The concerns do persist, and we feel there is further work to be done here,” he said. He specifically noted that there is a need to inform and educate fellows and colleagues in hospitals, increase awareness generally, improve communication strategies, and conduct detailed studies on adverse effects and outcomes.
“And now, since ASPs are very pervasive ... maybe it’s time to formalize and delineate the role of ASPs in antimicrobial shortages,” he said.
The problem of antibiotic shortages “harkens back to the day when penicillin was recycled in the urine [of soldiers in World War II] to save this very scarce resource ... but that’s a very extreme measure to take,” noted Donald Graham, MD, of the Springfield (Ill.) Clinic, one of the study’s coauthors. “It seems like it’s time for the other federal arm – namely, the Food and Drug Administration – to do something about this.”
Dr. Graham said he believes the problem is in part because of economics, and in part because of “the higher standards that the FDA imposes upon these manufacturing concerns.” These drugs often are low-profit items, and it isn’t always in the financial best interest of a pharmaceutical company to upgrade their facilities.
“But they really have to recognize the importance of having availability of these simple agents,” he said, pleading with any FDA representatives in the audience to “maybe think about some of these very high standards.”
Dr. Gundlapalli reported having no disclosures. Dr. Graham disclosed relationships with Astellas and Theravance Biopharma.
NEW ORLEANS – Antibiotic shortages reported by the Emerging Infections Network (EIN) in 2011 persist in 2016, according to a web-based follow-up survey of infectious disease physicians.
Of 701 network members who responded to the EIN survey in early 2016, 70% reported needing to modify their antimicrobial choice because of a shortage in the past 2 years. They did so by using broader-spectrum agents (75% of respondents), more costly agents (58%), less effective second-line agents (45%), and more toxic agents (37%), Adi Gundlapalli, MD, PhD, reported at an annual scientific meeting on infectious diseases.
In addition, 73% of respondents reported that the shortages affected patient care or outcomes, reported Dr. Gundlapalli of the University of Utah, Salt Lake City.
The percentage of respondents reporting adverse patient outcomes related to shortages increased from 2011 to 2016 (51% vs.73%), he noted at the combined annual meetings of the Infectious Diseases Society of America, the Society of Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The top 10 antimicrobials they reported as being in short supply were piperacillin-tazobactam, ampicillin-sulbactam, meropenem, cefotaxime, cefepime, trimethoprim-sulfamethoxazole (TMP-SMX), doxycycline, imipenem, acyclovir, and amikacin. TMP-SMX and acyclovir were in short supply at both time points.
The most common ways respondents reported learning about drug shortages were from hospital notification (76%), from a colleague (56%), from a pharmacy that contacted them regarding a prescription for the agent (53%), or from the Food and Drug Administration website or another website on shortages (23%). The most common ways of learning about a shortage changed – from notification after trying to prescribe a drug in 2011, to proactive hospital/system (local) notification in 2016; 71% of respondents said that communications in 2016 were sufficient.
Most respondents (83%) reported that guidelines for dealing with shortages had been developed by an antimicrobial stewardship program (ASP) at their institution.
“This, I think, is one of the highlight results,” said Dr. Gundlapalli, who is also a staff physician at the VA Salt Lake City Health System. “In 2011, we had no specific question or comments received about [ASPs], and here in 2016, 83% of respondents’ institutions had developed guidelines related to drug shortages.”
Respondents also had the opportunity to submit free-text responses, and among the themes that emerged was concern regarding toxicity and adverse outcomes associated with increased use of aminoglycosides because of the shortage of piperacillin-tazobactam. Another – described as a blessing in disguise – was the shortage of meropenem, which led one ASP to “institute restrictions on its use, which have continued,” he said.
“Another theme was ‘simpler agents seem more likely to be in shortage,’ ” Dr. Gundlapalli said, noting ampicillin-sulbactam in 2016 and Pen-G as examples.
“And then, of course, the other theme across the board ... was our new asset,” he said, explaining that some respondents commented on the value of ASP pharmacists and programs to help with drug shortage issues.
The overall theme of this follow-up survey, in the context of prior surveys in 2001 and 2011, is that antibiotic shortages are the “new normal – a way of life,” Dr. Gundlapalli said.
“The concerns do persist, and we feel there is further work to be done here,” he said. He specifically noted that there is a need to inform and educate fellows and colleagues in hospitals, increase awareness generally, improve communication strategies, and conduct detailed studies on adverse effects and outcomes.
“And now, since ASPs are very pervasive ... maybe it’s time to formalize and delineate the role of ASPs in antimicrobial shortages,” he said.
The problem of antibiotic shortages “harkens back to the day when penicillin was recycled in the urine [of soldiers in World War II] to save this very scarce resource ... but that’s a very extreme measure to take,” noted Donald Graham, MD, of the Springfield (Ill.) Clinic, one of the study’s coauthors. “It seems like it’s time for the other federal arm – namely, the Food and Drug Administration – to do something about this.”
Dr. Graham said he believes the problem is in part because of economics, and in part because of “the higher standards that the FDA imposes upon these manufacturing concerns.” These drugs often are low-profit items, and it isn’t always in the financial best interest of a pharmaceutical company to upgrade their facilities.
“But they really have to recognize the importance of having availability of these simple agents,” he said, pleading with any FDA representatives in the audience to “maybe think about some of these very high standards.”
Dr. Gundlapalli reported having no disclosures. Dr. Graham disclosed relationships with Astellas and Theravance Biopharma.
AT IDWEEK 2016
Key clinical point:
Major finding: 70% of respondents reported needing to modify their antimicrobial choice because of a shortage in the past 2 years, and 73% said shortages affected patient care or outcomes.
Data source: A follow-up survey of 701 physicians.
Disclosures: Dr. Gundlapalli reported having no disclosures. Dr. Graham disclosed relationships with Astellas and Theravance Biopharma.
Behavioral interventions durably reduced inappropriate antibiotic prescribing
NEW ORLEANS – The benefits of an 18-month behavioral intervention to reduce inappropriate antibiotic prescribing in the primary care setting were maintained 18 months after the intervention ended, according to follow-up data from a cluster randomized clinical trial.
During the 18-month intervention period, physicians at 47 adult and pediatric practices that participated in the trial, which compared three behavioral interventions and intervention combinations, significantly reduced their inappropriate prescribing.
After 18 months, the results were durable – and particularly so in the groups that received interventions that used “social motivation,” Jeffrey Linder, MD, of Brigham & Women’s Hospital and Harvard Medical School, Boston, reported at an annual scientific meeting on infectious diseases.
A total of 16,959 antibiotic-inappropriate visits (visits for nonspecific upper respiratory tract infections, acute bronchitis, and influenza) were made to 248 clinicians during the 18-month intervention period, and 3,192 such visits were made to 224 clinicians during the postintervention period (JAMA. 2016 Feb 9;315[6]:562-70).
The interventions included “suggested alternatives,” which was an electronic health record-based approach that prompted the prescriber to answer whether a prescription was for an acute respiratory infection. A “yes” answer resulted in the prescriber receiving information about appropriate prescribing, along with a list of “easy nonantibiotic alternatives,” Dr. Linder explained, noting that the interventions involved “trying to make it easy to do the right thing.”
An “accountable justification” intervention used a similar process, but rather than suggesting alternative options, the program asked the prescriber to input a “tweet-length justification” of the prescription. The justification was then entered into the patient’s chart.
The third intervention involved “peer comparison.” Prescribers received monthly e-mail feedback regarding how their prescribing stacked up to that of their peers – specifically noting whether they were or were not “top performers.”
Some of the groups in the trial received combinations of these interventions, but the follow-up analysis showed that the latter two approaches, which involved “social motivation,” had the most durable effects.
For example, the inappropriate antibiotic prescribing rate for those in the “accountable justification” group decreased from 23.2% to 5.2% at the end of the 18-month intervention period (absolute difference, -18.1%) and increased to 9% at the end of follow-up.
The inappropriate prescribing rate decreased from about 20% to about 4% in the “peer comparison” group at the end of the intervention period (absolute difference of -16.3%), then increased to 5% at the end of follow-up, Dr. Linder said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
“The statistically best player here – the peer comparison group – went from 20% to 4% to 5%, so it only went back up 1% even after we turned the intervention off for 18 months,” he said.
Antibiotics often are inappropriately prescribed for acute respiratory infections in primary care. Such infections – including colds, sinusitis, strep throat, nonstrep pharyngitis, acute bronchitis, and influenza – make up only 10% of all ambulatory visits in the United States, but they account for 44% of all antibiotic prescribing, Dr. Linder said.
An estimated 50% of antibiotic prescriptions for acute respiratory infections are inappropriate, he added, noting that little success has been achieved with prior antibiotic stewardship efforts that focused largely on clinician education.
“So, we tried to tackle it a bit differently,” he said. “We saw a persistent significant change in antibiotic prescribing in the peer comparison intervention group. ... I would say that interventions that take advantage of social motivation appear to be effective or persistent.”
Dr. Linder reported having no relevant disclosures.
NEW ORLEANS – The benefits of an 18-month behavioral intervention to reduce inappropriate antibiotic prescribing in the primary care setting were maintained 18 months after the intervention ended, according to follow-up data from a cluster randomized clinical trial.
During the 18-month intervention period, physicians at 47 adult and pediatric practices that participated in the trial, which compared three behavioral interventions and intervention combinations, significantly reduced their inappropriate prescribing.
After 18 months, the results were durable – and particularly so in the groups that received interventions that used “social motivation,” Jeffrey Linder, MD, of Brigham & Women’s Hospital and Harvard Medical School, Boston, reported at an annual scientific meeting on infectious diseases.
A total of 16,959 antibiotic-inappropriate visits (visits for nonspecific upper respiratory tract infections, acute bronchitis, and influenza) were made to 248 clinicians during the 18-month intervention period, and 3,192 such visits were made to 224 clinicians during the postintervention period (JAMA. 2016 Feb 9;315[6]:562-70).
The interventions included “suggested alternatives,” which was an electronic health record-based approach that prompted the prescriber to answer whether a prescription was for an acute respiratory infection. A “yes” answer resulted in the prescriber receiving information about appropriate prescribing, along with a list of “easy nonantibiotic alternatives,” Dr. Linder explained, noting that the interventions involved “trying to make it easy to do the right thing.”
An “accountable justification” intervention used a similar process, but rather than suggesting alternative options, the program asked the prescriber to input a “tweet-length justification” of the prescription. The justification was then entered into the patient’s chart.
The third intervention involved “peer comparison.” Prescribers received monthly e-mail feedback regarding how their prescribing stacked up to that of their peers – specifically noting whether they were or were not “top performers.”
Some of the groups in the trial received combinations of these interventions, but the follow-up analysis showed that the latter two approaches, which involved “social motivation,” had the most durable effects.
For example, the inappropriate antibiotic prescribing rate for those in the “accountable justification” group decreased from 23.2% to 5.2% at the end of the 18-month intervention period (absolute difference, -18.1%) and increased to 9% at the end of follow-up.
The inappropriate prescribing rate decreased from about 20% to about 4% in the “peer comparison” group at the end of the intervention period (absolute difference of -16.3%), then increased to 5% at the end of follow-up, Dr. Linder said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
“The statistically best player here – the peer comparison group – went from 20% to 4% to 5%, so it only went back up 1% even after we turned the intervention off for 18 months,” he said.
Antibiotics often are inappropriately prescribed for acute respiratory infections in primary care. Such infections – including colds, sinusitis, strep throat, nonstrep pharyngitis, acute bronchitis, and influenza – make up only 10% of all ambulatory visits in the United States, but they account for 44% of all antibiotic prescribing, Dr. Linder said.
An estimated 50% of antibiotic prescriptions for acute respiratory infections are inappropriate, he added, noting that little success has been achieved with prior antibiotic stewardship efforts that focused largely on clinician education.
“So, we tried to tackle it a bit differently,” he said. “We saw a persistent significant change in antibiotic prescribing in the peer comparison intervention group. ... I would say that interventions that take advantage of social motivation appear to be effective or persistent.”
Dr. Linder reported having no relevant disclosures.
NEW ORLEANS – The benefits of an 18-month behavioral intervention to reduce inappropriate antibiotic prescribing in the primary care setting were maintained 18 months after the intervention ended, according to follow-up data from a cluster randomized clinical trial.
During the 18-month intervention period, physicians at 47 adult and pediatric practices that participated in the trial, which compared three behavioral interventions and intervention combinations, significantly reduced their inappropriate prescribing.
After 18 months, the results were durable – and particularly so in the groups that received interventions that used “social motivation,” Jeffrey Linder, MD, of Brigham & Women’s Hospital and Harvard Medical School, Boston, reported at an annual scientific meeting on infectious diseases.
A total of 16,959 antibiotic-inappropriate visits (visits for nonspecific upper respiratory tract infections, acute bronchitis, and influenza) were made to 248 clinicians during the 18-month intervention period, and 3,192 such visits were made to 224 clinicians during the postintervention period (JAMA. 2016 Feb 9;315[6]:562-70).
The interventions included “suggested alternatives,” which was an electronic health record-based approach that prompted the prescriber to answer whether a prescription was for an acute respiratory infection. A “yes” answer resulted in the prescriber receiving information about appropriate prescribing, along with a list of “easy nonantibiotic alternatives,” Dr. Linder explained, noting that the interventions involved “trying to make it easy to do the right thing.”
An “accountable justification” intervention used a similar process, but rather than suggesting alternative options, the program asked the prescriber to input a “tweet-length justification” of the prescription. The justification was then entered into the patient’s chart.
The third intervention involved “peer comparison.” Prescribers received monthly e-mail feedback regarding how their prescribing stacked up to that of their peers – specifically noting whether they were or were not “top performers.”
Some of the groups in the trial received combinations of these interventions, but the follow-up analysis showed that the latter two approaches, which involved “social motivation,” had the most durable effects.
For example, the inappropriate antibiotic prescribing rate for those in the “accountable justification” group decreased from 23.2% to 5.2% at the end of the 18-month intervention period (absolute difference, -18.1%) and increased to 9% at the end of follow-up.
The inappropriate prescribing rate decreased from about 20% to about 4% in the “peer comparison” group at the end of the intervention period (absolute difference of -16.3%), then increased to 5% at the end of follow-up, Dr. Linder said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
“The statistically best player here – the peer comparison group – went from 20% to 4% to 5%, so it only went back up 1% even after we turned the intervention off for 18 months,” he said.
Antibiotics often are inappropriately prescribed for acute respiratory infections in primary care. Such infections – including colds, sinusitis, strep throat, nonstrep pharyngitis, acute bronchitis, and influenza – make up only 10% of all ambulatory visits in the United States, but they account for 44% of all antibiotic prescribing, Dr. Linder said.
An estimated 50% of antibiotic prescriptions for acute respiratory infections are inappropriate, he added, noting that little success has been achieved with prior antibiotic stewardship efforts that focused largely on clinician education.
“So, we tried to tackle it a bit differently,” he said. “We saw a persistent significant change in antibiotic prescribing in the peer comparison intervention group. ... I would say that interventions that take advantage of social motivation appear to be effective or persistent.”
Dr. Linder reported having no relevant disclosures.
Key clinical point: The benefits of an 18-month behavioral intervention to reduce inappropriate antibiotic prescribing in the primary care setting were maintained 18 months after the intervention ended, according to follow-up data from a cluster randomized clinical trial.
Major finding: Inappropriate antibiotic prescribing increased only slightly, from 4% to 5%, during 18 months of follow-up in the “peer comparison” group.
Data source: Follow-up of a cluster randomized, controlled clinical trial involving nearly 3,200 patient visits with 224 clinicians.
Disclosures: Dr. Linder reported having no disclosures.
Novel antibiotic hits skin and soft tissue infections with one-two punch
NEW ORLEANS – A novel antibiotic in development fared well in terms of efficacy and safety for patients hospitalized for suspected or confirmed Gram-positive acute skin and soft tissue infections, reveals the first reported findings of a phase II, randomized study.
Investigators randomized 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis to three different dosing intravenous/oral regimens of gepotidacin (GlaxoSmithKline). Patients in the 750-mg/1,500-mg q12h and 1,000-mg/2,000-mg q8h groups met the primary efficacy endpoint of an 80% or greater clinical success (83% and 92%, respectively) within 2-3 days. A third group, randomized to 1,000-mg/2,000-mg q12h, had a 72% early success rate.
All three groups of patients achieved the primary safety outcome, defined as less than a 2.5% withdrawal rate due to drug-related adverse events during gepotidacin treatment. One patient in the 750-mg q12h group withdrew because of a migraine related to the study drug.
Gepotidacin cleaves bacterial DNA in two places to block replication. “Because of its dual mechanism, there are a lot of potential applications,” Dr. O’Riordan said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Gepotidacin is also being assessed in ongoing gonorrhea, complicated intra-abdominal infections, and urinary tract infection studies.
The researchers in the current study also measured clinical success at post therapy days 12-18. They found 90% of the 750-mg/1,500-mg q12h group, 82% of the 1,000-mg/2,000-mg q8h, and 84% of the 1,000-mg/2,000-mg q12h group achieved the composite efficacy endpoint.
Overall, 84 or 69% of study participants experienced an adverse event. Nausea, diarrhea, and vomiting were the most common mild-to-moderate adverse events associated with the 10 days of gepotidacin treatment. Two serious adverse events not related to treatment also occurred during the study.
The “low adverse events and reproducible resolution of skin infections” in this phase II study support further development of gepotidacin, Dr. O’Riordan said.
Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
NEW ORLEANS – A novel antibiotic in development fared well in terms of efficacy and safety for patients hospitalized for suspected or confirmed Gram-positive acute skin and soft tissue infections, reveals the first reported findings of a phase II, randomized study.
Investigators randomized 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis to three different dosing intravenous/oral regimens of gepotidacin (GlaxoSmithKline). Patients in the 750-mg/1,500-mg q12h and 1,000-mg/2,000-mg q8h groups met the primary efficacy endpoint of an 80% or greater clinical success (83% and 92%, respectively) within 2-3 days. A third group, randomized to 1,000-mg/2,000-mg q12h, had a 72% early success rate.
All three groups of patients achieved the primary safety outcome, defined as less than a 2.5% withdrawal rate due to drug-related adverse events during gepotidacin treatment. One patient in the 750-mg q12h group withdrew because of a migraine related to the study drug.
Gepotidacin cleaves bacterial DNA in two places to block replication. “Because of its dual mechanism, there are a lot of potential applications,” Dr. O’Riordan said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Gepotidacin is also being assessed in ongoing gonorrhea, complicated intra-abdominal infections, and urinary tract infection studies.
The researchers in the current study also measured clinical success at post therapy days 12-18. They found 90% of the 750-mg/1,500-mg q12h group, 82% of the 1,000-mg/2,000-mg q8h, and 84% of the 1,000-mg/2,000-mg q12h group achieved the composite efficacy endpoint.
Overall, 84 or 69% of study participants experienced an adverse event. Nausea, diarrhea, and vomiting were the most common mild-to-moderate adverse events associated with the 10 days of gepotidacin treatment. Two serious adverse events not related to treatment also occurred during the study.
The “low adverse events and reproducible resolution of skin infections” in this phase II study support further development of gepotidacin, Dr. O’Riordan said.
Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
NEW ORLEANS – A novel antibiotic in development fared well in terms of efficacy and safety for patients hospitalized for suspected or confirmed Gram-positive acute skin and soft tissue infections, reveals the first reported findings of a phase II, randomized study.
Investigators randomized 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis to three different dosing intravenous/oral regimens of gepotidacin (GlaxoSmithKline). Patients in the 750-mg/1,500-mg q12h and 1,000-mg/2,000-mg q8h groups met the primary efficacy endpoint of an 80% or greater clinical success (83% and 92%, respectively) within 2-3 days. A third group, randomized to 1,000-mg/2,000-mg q12h, had a 72% early success rate.
All three groups of patients achieved the primary safety outcome, defined as less than a 2.5% withdrawal rate due to drug-related adverse events during gepotidacin treatment. One patient in the 750-mg q12h group withdrew because of a migraine related to the study drug.
Gepotidacin cleaves bacterial DNA in two places to block replication. “Because of its dual mechanism, there are a lot of potential applications,” Dr. O’Riordan said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Gepotidacin is also being assessed in ongoing gonorrhea, complicated intra-abdominal infections, and urinary tract infection studies.
The researchers in the current study also measured clinical success at post therapy days 12-18. They found 90% of the 750-mg/1,500-mg q12h group, 82% of the 1,000-mg/2,000-mg q8h, and 84% of the 1,000-mg/2,000-mg q12h group achieved the composite efficacy endpoint.
Overall, 84 or 69% of study participants experienced an adverse event. Nausea, diarrhea, and vomiting were the most common mild-to-moderate adverse events associated with the 10 days of gepotidacin treatment. Two serious adverse events not related to treatment also occurred during the study.
The “low adverse events and reproducible resolution of skin infections” in this phase II study support further development of gepotidacin, Dr. O’Riordan said.
Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
AT IDWEEK 2016
Key clinical point: A dual-mechanism-of-action antibiotic in development shows good efficacy and a low adverse event rate in a phase II study.
Major finding: A total 71 of 122 adult patients achieved clinical success within 48 to 72 hours with gepotidacin treatment.
Data source: 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis.
Disclosures: Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
CDC: Seven cases of multidrug resistant C. auris have occurred in United States
The Centers for Disease Control and Prevention have reported the first cases of the multidrug-resistant fungal infection Candida auris in the United States, with evidence suggesting transmission may have occurred within U.S. health care facilities.
The report, published in the Nov. 4 edition of Morbidity and Mortality Weekly Report, described seven cases of patients infected with C. auris, which was isolated from blood in five cases, urine in one, and the ear in one. All the patients with bloodstream infections had central venous catheters at the time of diagnosis, and four of these patients died in the weeks and months after diagnosis of the infection.
Patients’ underlying conditions usually involved immune system suppression resulting from corticisteroid therapy, malignancty, short gut syndrome, or parapleglia with a long-term, indwelling Foley catheter.
C. auris was first isolated in 2009 in Japan, but has since been reported in countries including Colombia, India, South Africa, Israel, and the United Kingdom. Snigdha Vallabhaneni, MD, of the mycotic diseases branch of CDC’s division of food water and environmental diseases, and her coauthors, said its appearance in the United States is a cause for serious concern (MMWR. 2016 Nov 4. doi: 0.15585/mmwr.mm6544e1).
“First, many isolates are multidrug resistant, with some strains having elevated minimum inhibitory concentrations to drugs in all three major classes of antifungal medications, a feature not found in other clinically relevant Candida species,” the authors wrote. All the patients with bloodstream infections were treated with antifungal echinocandins, and one also received liposomal amphotericin B.
“Second, C. auris is challenging to identify, requiring specialized methods such as matrix-assisted laser desorption/ionization time-of-flight or molecular identification based on sequencing the D1-D2 region of the 28s ribosomal DNA.”
They also highlighted that C. auris is known to cause outbreaks in health care settings. Samples taken from the mattress, bedside table, bed rail, chair, and windowsill in the room of one patient all tested positive for C. auris.
The authors also sequenced the genome of the isolates and found that isolates taken from patients admitted to the same hospital in New Jersey or the same Illinois hospital were nearly identical.
“Facilities should ensure thorough daily and terminal cleaning of rooms of patients with C. auris infections, including use of an [Environmental Protection Agency]–registered disinfectant with a fungal claim,” the authors wrote, stressing that facilities and laboratories should continue to report cases and forward suspicious unidentified Candida isolates to state or local health authorities and the CDC.
No conflicts of interest were declared.
The Centers for Disease Control and Prevention have reported the first cases of the multidrug-resistant fungal infection Candida auris in the United States, with evidence suggesting transmission may have occurred within U.S. health care facilities.
The report, published in the Nov. 4 edition of Morbidity and Mortality Weekly Report, described seven cases of patients infected with C. auris, which was isolated from blood in five cases, urine in one, and the ear in one. All the patients with bloodstream infections had central venous catheters at the time of diagnosis, and four of these patients died in the weeks and months after diagnosis of the infection.
Patients’ underlying conditions usually involved immune system suppression resulting from corticisteroid therapy, malignancty, short gut syndrome, or parapleglia with a long-term, indwelling Foley catheter.
C. auris was first isolated in 2009 in Japan, but has since been reported in countries including Colombia, India, South Africa, Israel, and the United Kingdom. Snigdha Vallabhaneni, MD, of the mycotic diseases branch of CDC’s division of food water and environmental diseases, and her coauthors, said its appearance in the United States is a cause for serious concern (MMWR. 2016 Nov 4. doi: 0.15585/mmwr.mm6544e1).
“First, many isolates are multidrug resistant, with some strains having elevated minimum inhibitory concentrations to drugs in all three major classes of antifungal medications, a feature not found in other clinically relevant Candida species,” the authors wrote. All the patients with bloodstream infections were treated with antifungal echinocandins, and one also received liposomal amphotericin B.
“Second, C. auris is challenging to identify, requiring specialized methods such as matrix-assisted laser desorption/ionization time-of-flight or molecular identification based on sequencing the D1-D2 region of the 28s ribosomal DNA.”
They also highlighted that C. auris is known to cause outbreaks in health care settings. Samples taken from the mattress, bedside table, bed rail, chair, and windowsill in the room of one patient all tested positive for C. auris.
The authors also sequenced the genome of the isolates and found that isolates taken from patients admitted to the same hospital in New Jersey or the same Illinois hospital were nearly identical.
“Facilities should ensure thorough daily and terminal cleaning of rooms of patients with C. auris infections, including use of an [Environmental Protection Agency]–registered disinfectant with a fungal claim,” the authors wrote, stressing that facilities and laboratories should continue to report cases and forward suspicious unidentified Candida isolates to state or local health authorities and the CDC.
No conflicts of interest were declared.
The Centers for Disease Control and Prevention have reported the first cases of the multidrug-resistant fungal infection Candida auris in the United States, with evidence suggesting transmission may have occurred within U.S. health care facilities.
The report, published in the Nov. 4 edition of Morbidity and Mortality Weekly Report, described seven cases of patients infected with C. auris, which was isolated from blood in five cases, urine in one, and the ear in one. All the patients with bloodstream infections had central venous catheters at the time of diagnosis, and four of these patients died in the weeks and months after diagnosis of the infection.
Patients’ underlying conditions usually involved immune system suppression resulting from corticisteroid therapy, malignancty, short gut syndrome, or parapleglia with a long-term, indwelling Foley catheter.
C. auris was first isolated in 2009 in Japan, but has since been reported in countries including Colombia, India, South Africa, Israel, and the United Kingdom. Snigdha Vallabhaneni, MD, of the mycotic diseases branch of CDC’s division of food water and environmental diseases, and her coauthors, said its appearance in the United States is a cause for serious concern (MMWR. 2016 Nov 4. doi: 0.15585/mmwr.mm6544e1).
“First, many isolates are multidrug resistant, with some strains having elevated minimum inhibitory concentrations to drugs in all three major classes of antifungal medications, a feature not found in other clinically relevant Candida species,” the authors wrote. All the patients with bloodstream infections were treated with antifungal echinocandins, and one also received liposomal amphotericin B.
“Second, C. auris is challenging to identify, requiring specialized methods such as matrix-assisted laser desorption/ionization time-of-flight or molecular identification based on sequencing the D1-D2 region of the 28s ribosomal DNA.”
They also highlighted that C. auris is known to cause outbreaks in health care settings. Samples taken from the mattress, bedside table, bed rail, chair, and windowsill in the room of one patient all tested positive for C. auris.
The authors also sequenced the genome of the isolates and found that isolates taken from patients admitted to the same hospital in New Jersey or the same Illinois hospital were nearly identical.
“Facilities should ensure thorough daily and terminal cleaning of rooms of patients with C. auris infections, including use of an [Environmental Protection Agency]–registered disinfectant with a fungal claim,” the authors wrote, stressing that facilities and laboratories should continue to report cases and forward suspicious unidentified Candida isolates to state or local health authorities and the CDC.
No conflicts of interest were declared.
Key clinical point: The first cases of the multidrug-resistant fungal infection C. auris have been reported in the United States.
Major finding: Seven cases of infection with the multidrug-resistant emerging fungal infection C. auris have been reported in the United States, five of which were bloodstream infections.
Data source: Case series.
Disclosures: No conflicts of interest were declared.
Treated bacteremia that clears, then recurs, termed ‘skip phenomenon’
NEW ORLEANS – When Mayo Clinic physicians noticed some patients on appropriate antibiotic treatment for Staphylococcus aureus bacteremia cleared the infection, only to see it recur a few days later, Justin A. Fiala, MD and his colleagues grew curious.
Dr. Fiala, an infectious diseases internist at Mayo Clinic, Rochester, Minn., was intrigued by the possibility of fluctuating blood culture positivity in this subset of bacteremia patients.
“We wanted first to see whether or not this is a real entity and determine the prevalence of this ‘skip pattern,’” Dr. Fiala said at IDWeek 2016, the annual combined meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association and the Pediatric Infectious Diseases Society. He said identifying predictors and finding any differences in clinical outcomes compared to control S. aureus bacteremia (SAB) patients were additional aims.
Dr. Fiala and his colleagues assessed a hospitalized cohort of 726 adults with SAB at Mayo Clinic between July 2006 and June 2011. Patients with one or more negative blood cultures followed by a positive culture were identified within this group, and compared with 2 to 4 patients matched for age, sex and duration of bacteremia who served as controls.
The investigators found 29 patients – or 4% – of the 726 had this ‘skip pattern’ of infection, clearance, and reinfection. Those with the phenomenon were 90% male and tended to be older, with a mean age of 69 years, compared to the controls. They had index bacteremia about two days longer than controls. The study also revealed a significant difference in mean number of central venous catheters: 2.7 in the skip phenomenon group versus 1.7 in controls.
Given the predominance of the skip phenomenon in older, immunosuppressed males, “the takeaway … is that serial negative blood cultures may be warranted in these patient groups,” Dr. Fiala said.
The groups did not differ significantly by presence of implants or foreign bodies or by whether SAB was nosocomial or acquired in the community. “We thought it was interesting that 90% had immune suppression, although it was not statistically significant,” Dr. Fiala said.
With no prior reports in the medical literature, the researchers named this clinical entity “skip phenomenon.” Dr. Fiala noted that published studies have assessed recurrence of SAB after completion of antibiotics, but not specifically during treatment.
“We think this is a topic that is quite clinically prevalent and applicable,” Dr. Fiala said. He pointed out that SAB is common, accounting for about 20% of all nosocomial bacteremia cases. SAB also highly virulent with a mortality rate estimated between 20% and 35%. Although the study did not reveal significant mortality differences in the subgroup with skip phenomenon, “we can say there is increased morbidity.”
The most recent IDSA guidelines state that a single set of negative blood cultures is sufficient to demonstrate clearance of SAB, Dr. Fiala said. “Could this be falsely reassuring if Staphylococcus aureus does have a tendency to exhibit this fluctuating pattern?”
The retrospective design of the study and the relatively small number of patients with the skip phenomenon were limitations, the investigators acknowledged. Dr. Fiala had no relevant disclosures.
NEW ORLEANS – When Mayo Clinic physicians noticed some patients on appropriate antibiotic treatment for Staphylococcus aureus bacteremia cleared the infection, only to see it recur a few days later, Justin A. Fiala, MD and his colleagues grew curious.
Dr. Fiala, an infectious diseases internist at Mayo Clinic, Rochester, Minn., was intrigued by the possibility of fluctuating blood culture positivity in this subset of bacteremia patients.
“We wanted first to see whether or not this is a real entity and determine the prevalence of this ‘skip pattern,’” Dr. Fiala said at IDWeek 2016, the annual combined meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association and the Pediatric Infectious Diseases Society. He said identifying predictors and finding any differences in clinical outcomes compared to control S. aureus bacteremia (SAB) patients were additional aims.
Dr. Fiala and his colleagues assessed a hospitalized cohort of 726 adults with SAB at Mayo Clinic between July 2006 and June 2011. Patients with one or more negative blood cultures followed by a positive culture were identified within this group, and compared with 2 to 4 patients matched for age, sex and duration of bacteremia who served as controls.
The investigators found 29 patients – or 4% – of the 726 had this ‘skip pattern’ of infection, clearance, and reinfection. Those with the phenomenon were 90% male and tended to be older, with a mean age of 69 years, compared to the controls. They had index bacteremia about two days longer than controls. The study also revealed a significant difference in mean number of central venous catheters: 2.7 in the skip phenomenon group versus 1.7 in controls.
Given the predominance of the skip phenomenon in older, immunosuppressed males, “the takeaway … is that serial negative blood cultures may be warranted in these patient groups,” Dr. Fiala said.
The groups did not differ significantly by presence of implants or foreign bodies or by whether SAB was nosocomial or acquired in the community. “We thought it was interesting that 90% had immune suppression, although it was not statistically significant,” Dr. Fiala said.
With no prior reports in the medical literature, the researchers named this clinical entity “skip phenomenon.” Dr. Fiala noted that published studies have assessed recurrence of SAB after completion of antibiotics, but not specifically during treatment.
“We think this is a topic that is quite clinically prevalent and applicable,” Dr. Fiala said. He pointed out that SAB is common, accounting for about 20% of all nosocomial bacteremia cases. SAB also highly virulent with a mortality rate estimated between 20% and 35%. Although the study did not reveal significant mortality differences in the subgroup with skip phenomenon, “we can say there is increased morbidity.”
The most recent IDSA guidelines state that a single set of negative blood cultures is sufficient to demonstrate clearance of SAB, Dr. Fiala said. “Could this be falsely reassuring if Staphylococcus aureus does have a tendency to exhibit this fluctuating pattern?”
The retrospective design of the study and the relatively small number of patients with the skip phenomenon were limitations, the investigators acknowledged. Dr. Fiala had no relevant disclosures.
NEW ORLEANS – When Mayo Clinic physicians noticed some patients on appropriate antibiotic treatment for Staphylococcus aureus bacteremia cleared the infection, only to see it recur a few days later, Justin A. Fiala, MD and his colleagues grew curious.
Dr. Fiala, an infectious diseases internist at Mayo Clinic, Rochester, Minn., was intrigued by the possibility of fluctuating blood culture positivity in this subset of bacteremia patients.
“We wanted first to see whether or not this is a real entity and determine the prevalence of this ‘skip pattern,’” Dr. Fiala said at IDWeek 2016, the annual combined meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association and the Pediatric Infectious Diseases Society. He said identifying predictors and finding any differences in clinical outcomes compared to control S. aureus bacteremia (SAB) patients were additional aims.
Dr. Fiala and his colleagues assessed a hospitalized cohort of 726 adults with SAB at Mayo Clinic between July 2006 and June 2011. Patients with one or more negative blood cultures followed by a positive culture were identified within this group, and compared with 2 to 4 patients matched for age, sex and duration of bacteremia who served as controls.
The investigators found 29 patients – or 4% – of the 726 had this ‘skip pattern’ of infection, clearance, and reinfection. Those with the phenomenon were 90% male and tended to be older, with a mean age of 69 years, compared to the controls. They had index bacteremia about two days longer than controls. The study also revealed a significant difference in mean number of central venous catheters: 2.7 in the skip phenomenon group versus 1.7 in controls.
Given the predominance of the skip phenomenon in older, immunosuppressed males, “the takeaway … is that serial negative blood cultures may be warranted in these patient groups,” Dr. Fiala said.
The groups did not differ significantly by presence of implants or foreign bodies or by whether SAB was nosocomial or acquired in the community. “We thought it was interesting that 90% had immune suppression, although it was not statistically significant,” Dr. Fiala said.
With no prior reports in the medical literature, the researchers named this clinical entity “skip phenomenon.” Dr. Fiala noted that published studies have assessed recurrence of SAB after completion of antibiotics, but not specifically during treatment.
“We think this is a topic that is quite clinically prevalent and applicable,” Dr. Fiala said. He pointed out that SAB is common, accounting for about 20% of all nosocomial bacteremia cases. SAB also highly virulent with a mortality rate estimated between 20% and 35%. Although the study did not reveal significant mortality differences in the subgroup with skip phenomenon, “we can say there is increased morbidity.”
The most recent IDSA guidelines state that a single set of negative blood cultures is sufficient to demonstrate clearance of SAB, Dr. Fiala said. “Could this be falsely reassuring if Staphylococcus aureus does have a tendency to exhibit this fluctuating pattern?”
The retrospective design of the study and the relatively small number of patients with the skip phenomenon were limitations, the investigators acknowledged. Dr. Fiala had no relevant disclosures.
Key clinical point:
Major finding: About 4% of S. aureus bacteremia cases may not clear completely, as judged by one negative blood culture, contrary to recent IDSA guidelines.
Data source: Nested case-control study of 726 adult inpatients at Mayo Clinic between July 2006 and June 2011 with ≥3 days of S. aureus bacteremia.
Disclosures: Dr. Fiala had no relevant disclosures.
CDC study finds worrisome trends in hospital antibiotic use
U.S. hospitals have not cut overall antibiotic use and have significantly increased the use of several broad-spectrum agents, according to a first-in-kind analysis of national hospital administrative data.
“We identified significant changes in specific antibiotic classes and regional variation that may have important implications for reducing antibiotic-resistant infections,” James Baggs, PhD, and colleagues from the Centers for Disease Control and Prevention, Atlanta, reported in the study, published online on September 19 in JAMA Internal Medicine.
The retrospective study included approximately 300 acute care hospitals in the Truven Health MarketScan Hospital Drug Database, which covered 34 million pediatric and adult patient discharges equating to 166 million patient-daysIn all, 55% of patients received at least one antibiotic dose while in the hospital, and for every 1,000 patient-days, 755 days included antibiotic therapy, the investigators said. Overall antibiotic use rose during the study period by only 5.6 average days of therapy per 1,000 patient-days, which was not statistically significant.
However, the use of third and fourth-generation cephalosporins rose by a mean of 10.3 days of therapy per 1,000 patient-days (95% confidence interval, 3.1 to 17.5), and hospitals also used significantly more macrolides (mean rise, 4.8 days of therapy per 1,000 patient-days; 95% confidence interval, 2.0 to 7.6 days), glycopeptides, (22.4; 17.5 to 27.3); β-lactam/β-lactamase inhibitor combinations (18.0; 13.3 to 22.6), carbapenems (7.4; 4.6 to 10.2), and tetracyclines (3.3; 2.0 to 4.7)
Inpatient antibiotic use also varied significantly by region, the investigators said. Hospitals in rural areas used about 16 more days of antibiotic therapy per 1,000 patient-days compared with those in urban areas. Hospitals in Mid-Atlantic states (New Jersey, New York, Pennsylvania) and Pacific Coast states (Alaska, California, Hawaii, Oregon, and Washington) used the least antibiotics (649 and 665 days per 1,000 patient-days, respectively), while Southwest Central states (Arkansas, Louisiana, Oklahoma, and Texas) used the most (823 days).
The CDC provided funding for the study. The researchers had no disclosures.
The dramatic variation in antibiotic prescribing across individual clinicians, regions in the United States, and internationally indicates great potential for improvement. ... In the article by Baggs et al, inpatient antibiotic prescribing in some regions of the United States is roughly 20% lower than other regions. On a per capita basis, Swedes consume less than half the antibiotics per capita than Americans.
Growing patterns of antibiotic resistance have driven calls for more physician education and new diagnostics. While these efforts may help, it is important to recognize that many emotionally salient factors are driving physicians to inappropriately prescribe antibiotics. Future interventions need to counterbalance these factors using tools from behavioral science to reduce the use of inappropriate antibiotics.
Ateev Mehrotra, MD, MPH, and Jeffrey A. Linder, MD, MPH, are at Harvard University, Boston. They had no disclosures. These comments are from an editorial that accompanied the study ( JAMA Intern Med. 2016 Sept 19. doi: 10.1001/jamainternmed.2016.6254).
The dramatic variation in antibiotic prescribing across individual clinicians, regions in the United States, and internationally indicates great potential for improvement. ... In the article by Baggs et al, inpatient antibiotic prescribing in some regions of the United States is roughly 20% lower than other regions. On a per capita basis, Swedes consume less than half the antibiotics per capita than Americans.
Growing patterns of antibiotic resistance have driven calls for more physician education and new diagnostics. While these efforts may help, it is important to recognize that many emotionally salient factors are driving physicians to inappropriately prescribe antibiotics. Future interventions need to counterbalance these factors using tools from behavioral science to reduce the use of inappropriate antibiotics.
Ateev Mehrotra, MD, MPH, and Jeffrey A. Linder, MD, MPH, are at Harvard University, Boston. They had no disclosures. These comments are from an editorial that accompanied the study ( JAMA Intern Med. 2016 Sept 19. doi: 10.1001/jamainternmed.2016.6254).
The dramatic variation in antibiotic prescribing across individual clinicians, regions in the United States, and internationally indicates great potential for improvement. ... In the article by Baggs et al, inpatient antibiotic prescribing in some regions of the United States is roughly 20% lower than other regions. On a per capita basis, Swedes consume less than half the antibiotics per capita than Americans.
Growing patterns of antibiotic resistance have driven calls for more physician education and new diagnostics. While these efforts may help, it is important to recognize that many emotionally salient factors are driving physicians to inappropriately prescribe antibiotics. Future interventions need to counterbalance these factors using tools from behavioral science to reduce the use of inappropriate antibiotics.
Ateev Mehrotra, MD, MPH, and Jeffrey A. Linder, MD, MPH, are at Harvard University, Boston. They had no disclosures. These comments are from an editorial that accompanied the study ( JAMA Intern Med. 2016 Sept 19. doi: 10.1001/jamainternmed.2016.6254).
U.S. hospitals have not cut overall antibiotic use and have significantly increased the use of several broad-spectrum agents, according to a first-in-kind analysis of national hospital administrative data.
“We identified significant changes in specific antibiotic classes and regional variation that may have important implications for reducing antibiotic-resistant infections,” James Baggs, PhD, and colleagues from the Centers for Disease Control and Prevention, Atlanta, reported in the study, published online on September 19 in JAMA Internal Medicine.
The retrospective study included approximately 300 acute care hospitals in the Truven Health MarketScan Hospital Drug Database, which covered 34 million pediatric and adult patient discharges equating to 166 million patient-daysIn all, 55% of patients received at least one antibiotic dose while in the hospital, and for every 1,000 patient-days, 755 days included antibiotic therapy, the investigators said. Overall antibiotic use rose during the study period by only 5.6 average days of therapy per 1,000 patient-days, which was not statistically significant.
However, the use of third and fourth-generation cephalosporins rose by a mean of 10.3 days of therapy per 1,000 patient-days (95% confidence interval, 3.1 to 17.5), and hospitals also used significantly more macrolides (mean rise, 4.8 days of therapy per 1,000 patient-days; 95% confidence interval, 2.0 to 7.6 days), glycopeptides, (22.4; 17.5 to 27.3); β-lactam/β-lactamase inhibitor combinations (18.0; 13.3 to 22.6), carbapenems (7.4; 4.6 to 10.2), and tetracyclines (3.3; 2.0 to 4.7)
Inpatient antibiotic use also varied significantly by region, the investigators said. Hospitals in rural areas used about 16 more days of antibiotic therapy per 1,000 patient-days compared with those in urban areas. Hospitals in Mid-Atlantic states (New Jersey, New York, Pennsylvania) and Pacific Coast states (Alaska, California, Hawaii, Oregon, and Washington) used the least antibiotics (649 and 665 days per 1,000 patient-days, respectively), while Southwest Central states (Arkansas, Louisiana, Oklahoma, and Texas) used the most (823 days).
The CDC provided funding for the study. The researchers had no disclosures.
U.S. hospitals have not cut overall antibiotic use and have significantly increased the use of several broad-spectrum agents, according to a first-in-kind analysis of national hospital administrative data.
“We identified significant changes in specific antibiotic classes and regional variation that may have important implications for reducing antibiotic-resistant infections,” James Baggs, PhD, and colleagues from the Centers for Disease Control and Prevention, Atlanta, reported in the study, published online on September 19 in JAMA Internal Medicine.
The retrospective study included approximately 300 acute care hospitals in the Truven Health MarketScan Hospital Drug Database, which covered 34 million pediatric and adult patient discharges equating to 166 million patient-daysIn all, 55% of patients received at least one antibiotic dose while in the hospital, and for every 1,000 patient-days, 755 days included antibiotic therapy, the investigators said. Overall antibiotic use rose during the study period by only 5.6 average days of therapy per 1,000 patient-days, which was not statistically significant.
However, the use of third and fourth-generation cephalosporins rose by a mean of 10.3 days of therapy per 1,000 patient-days (95% confidence interval, 3.1 to 17.5), and hospitals also used significantly more macrolides (mean rise, 4.8 days of therapy per 1,000 patient-days; 95% confidence interval, 2.0 to 7.6 days), glycopeptides, (22.4; 17.5 to 27.3); β-lactam/β-lactamase inhibitor combinations (18.0; 13.3 to 22.6), carbapenems (7.4; 4.6 to 10.2), and tetracyclines (3.3; 2.0 to 4.7)
Inpatient antibiotic use also varied significantly by region, the investigators said. Hospitals in rural areas used about 16 more days of antibiotic therapy per 1,000 patient-days compared with those in urban areas. Hospitals in Mid-Atlantic states (New Jersey, New York, Pennsylvania) and Pacific Coast states (Alaska, California, Hawaii, Oregon, and Washington) used the least antibiotics (649 and 665 days per 1,000 patient-days, respectively), while Southwest Central states (Arkansas, Louisiana, Oklahoma, and Texas) used the most (823 days).
The CDC provided funding for the study. The researchers had no disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Inpatient antibiotic use did not decrease between 2006 and 2012, and the use of several broad-spectrum agents rose significantly.
Major finding: Hospitals significantly decreased their use of fluoroquinolones and first- and second-generation cephalosporins, but these trends were offset by significant rises in the use of vancomycin, carbapenem, third- and fourth-generation cephalosporins, and β-lactam/β- lactamase inhibitor combinations.
Data source: A retrospective study of administrative hospital discharge data for about 300 US hospitals from 2006 through 2012.
Disclosures: The Centers for Disease Control and Prevention provided funding. The researchers had no disclosures.