User login
Leukemia research pioneer dies at 92
James F. Holland, MD, passed away last week, at the age of 92, due to complications of cardiovascular disease.
Dr Holland has been called a pioneer in the field of leukemia research.
He and his colleagues are credited with using combination chemotherapy to transform pediatric acute lymphoblastic leukemia from an incurable illness to one with a high survival rate.
Dr Holland and his colleagues also developed the 7+3 regimen—3 daily injections of daunorubicin and 7 days of intravenous cytarabine—for patients with acute myeloid leukemia.
Dr Holland was born on May 16, 1925, in Morristown, New Jersey. He graduated from Princeton University in 1944 and earned his medical degree from Columbia University College of Physicians and Surgeons in 1947.
Dr Holland was a captain in the US Army Medical Corps from 1949 to 1951. After that, he worked at Francis Delafield Hospital (which closed in 1975) in New York, New York. He joined the National Cancer Institute (NCI) in 1953. Two years later, he began working at Roswell Park Cancer Institute in Buffalo, New York.
Dr Holland became Roswell Park’s chief of medicine and director of the Cancer Clinical Research Center. But he continued to work with the NCI, conducting research as part of Acute Leukemia Group B, which later became Cancer and Leukemia Group B.
After spending a year on an oncology exchange program in the Soviet Union, Dr Holland started at Mount Sinai in New York, New York, in 1973. While there, he established the Department of Neoplastic Diseases at The Tisch Cancer Institute, Icahn School of Medicine.
Most recently, Dr Holland was a distinguished professor of neoplastic diseases at Mount Sinai. He saw patients but also conducted research on the human mammary tumor virus.
Dr Holland collaborated with Emil Frei III to publish the textbook Cancer Medicine, which is now in its ninth edition. Dr Holland served as president of the American Association for Cancer Research and the American Society of Clinical Oncology. He was a co-founder of the African Organization for Research and Training in Cancer as well.
Dr Holland was married to Jimmie C. Holland, MD, who is credited with founding the field of psycho-oncology. Jimmie passed away in December 2017. The couple is survived by 6 children and 9 grandchildren.
James F. Holland, MD, passed away last week, at the age of 92, due to complications of cardiovascular disease.
Dr Holland has been called a pioneer in the field of leukemia research.
He and his colleagues are credited with using combination chemotherapy to transform pediatric acute lymphoblastic leukemia from an incurable illness to one with a high survival rate.
Dr Holland and his colleagues also developed the 7+3 regimen—3 daily injections of daunorubicin and 7 days of intravenous cytarabine—for patients with acute myeloid leukemia.
Dr Holland was born on May 16, 1925, in Morristown, New Jersey. He graduated from Princeton University in 1944 and earned his medical degree from Columbia University College of Physicians and Surgeons in 1947.
Dr Holland was a captain in the US Army Medical Corps from 1949 to 1951. After that, he worked at Francis Delafield Hospital (which closed in 1975) in New York, New York. He joined the National Cancer Institute (NCI) in 1953. Two years later, he began working at Roswell Park Cancer Institute in Buffalo, New York.
Dr Holland became Roswell Park’s chief of medicine and director of the Cancer Clinical Research Center. But he continued to work with the NCI, conducting research as part of Acute Leukemia Group B, which later became Cancer and Leukemia Group B.
After spending a year on an oncology exchange program in the Soviet Union, Dr Holland started at Mount Sinai in New York, New York, in 1973. While there, he established the Department of Neoplastic Diseases at The Tisch Cancer Institute, Icahn School of Medicine.
Most recently, Dr Holland was a distinguished professor of neoplastic diseases at Mount Sinai. He saw patients but also conducted research on the human mammary tumor virus.
Dr Holland collaborated with Emil Frei III to publish the textbook Cancer Medicine, which is now in its ninth edition. Dr Holland served as president of the American Association for Cancer Research and the American Society of Clinical Oncology. He was a co-founder of the African Organization for Research and Training in Cancer as well.
Dr Holland was married to Jimmie C. Holland, MD, who is credited with founding the field of psycho-oncology. Jimmie passed away in December 2017. The couple is survived by 6 children and 9 grandchildren.
James F. Holland, MD, passed away last week, at the age of 92, due to complications of cardiovascular disease.
Dr Holland has been called a pioneer in the field of leukemia research.
He and his colleagues are credited with using combination chemotherapy to transform pediatric acute lymphoblastic leukemia from an incurable illness to one with a high survival rate.
Dr Holland and his colleagues also developed the 7+3 regimen—3 daily injections of daunorubicin and 7 days of intravenous cytarabine—for patients with acute myeloid leukemia.
Dr Holland was born on May 16, 1925, in Morristown, New Jersey. He graduated from Princeton University in 1944 and earned his medical degree from Columbia University College of Physicians and Surgeons in 1947.
Dr Holland was a captain in the US Army Medical Corps from 1949 to 1951. After that, he worked at Francis Delafield Hospital (which closed in 1975) in New York, New York. He joined the National Cancer Institute (NCI) in 1953. Two years later, he began working at Roswell Park Cancer Institute in Buffalo, New York.
Dr Holland became Roswell Park’s chief of medicine and director of the Cancer Clinical Research Center. But he continued to work with the NCI, conducting research as part of Acute Leukemia Group B, which later became Cancer and Leukemia Group B.
After spending a year on an oncology exchange program in the Soviet Union, Dr Holland started at Mount Sinai in New York, New York, in 1973. While there, he established the Department of Neoplastic Diseases at The Tisch Cancer Institute, Icahn School of Medicine.
Most recently, Dr Holland was a distinguished professor of neoplastic diseases at Mount Sinai. He saw patients but also conducted research on the human mammary tumor virus.
Dr Holland collaborated with Emil Frei III to publish the textbook Cancer Medicine, which is now in its ninth edition. Dr Holland served as president of the American Association for Cancer Research and the American Society of Clinical Oncology. He was a co-founder of the African Organization for Research and Training in Cancer as well.
Dr Holland was married to Jimmie C. Holland, MD, who is credited with founding the field of psycho-oncology. Jimmie passed away in December 2017. The couple is survived by 6 children and 9 grandchildren.
Chemotherapy, metabolic pathway may affect CAR T-cell potential
Two critical factors – prior exposure to chemotherapy and a glycolytic metabolism – appear to degrade the potential of T cells to become chimeric antigen receptor–T cells.
Chemotherapy, especially with cyclophosphamide and doxorubicin, seems particularly toxic to T cells, damaging the mitochondria and decreasing the cells’ spare respiratory capacity – a measure of mitochondrial health, David Barrett, MD, said during a press briefing held in advance of the annual meeting of the American Association for Cancer Research.
These new findings may help explain why children with acute lymphoblastic leukemia (ALL) tend to respond so vigorously to CAR T treatment, and why T cells from patients with solid tumors simply don’t grow, or die soon after patient infusion, he said in an interview. They also suggest a benefit of harvesting T cells before any chemotherapy, a procedure Dr. Barrett and his colleagues have advocated.
“Based on these data we have altered our practice for T-cell therapy in high-risk leukemia patients. If we have a patient who may have a poor prognosis, we try to collect the cells early and store them before proceeding, because we know chemotherapy will progressively degrade them.”
There still is no successful CAR T-cell protocol for solid tumors, but Dr. Barrett said these findings eventually may help such patients, particularly if more advanced experiments in manipulating the cells’ metabolism prove successful.
He and his colleagues investigated why T cells from some patients result in a poor clinical product that either fails manufacture or does not proliferate in the patient. They examined T cells from 157 pediatric patients with a variety of cancers, including ALL, non-Hodgkin lymphoma, neuroblastoma, osteosarcoma, rhabdomyosarcoma, Wilms tumor, Hodgkin disease, chronic myelogenous leukemia, and Ewing sarcoma. The team obtained cells at diagnosis and after each cycle of chemotherapy.
They examined how well the cells grew in the transformation and expansion process. A “pass” was considered a fivefold expansion in response to CD3/CD28 exposure for 7 days. Normal donor cells typically expand 20- to 30-fold in this time.
Only T cells taken from ALL and Wilms tumor patients before chemotherapy achieved a pass, Dr. Barrett said. Most of the ALL expansions (80%) and half of the Wilms tumor expansions passed. “We noted very poor CAR T-cell potential in all the other tumor types – less than a 30% pass. We noted a decline in potential with cumulative chemotherapy in all cases, though this was particularly significant in children less than 3 years old.”
The team also used RNA profiling to look at the cells’ metabolic pathways. Dr. Barrett noted that T cells are highly metabolically adaptable, capable of using several different fuel types and switching from one to another. Glucose and fatty acids are frequent fuels. Most of the cells from patients with solid tumors exhibited a glycolytic metabolism, while cells from patients with ALL and Wilms tumor appeared to rely more on fatty acids.
“One is not inherently worse than the other,” he said. “But glycolysis appears to be a bad thing when we’re trying to turn them into CAR T cells. Those T cells were too exhausted to do anything.”
However, Dr. Barrett encouraged the cells to switch fuels by treating them in vitro with palmitic acid, the most common fatty acid in plants and animals.
“We were growing the cells in a media containing sugar, fatty acids, and amino acids,” he explained. “We just started overloading them with palmitic acid, which has a natural transporter on the T-cell surface, so it already had a good pathway to get into the cell. It helped restore some of the performance of these T cells in some assays, although it wasn’t a complete reversal. But it was encouraging that something as simple as providing an alternate fuel was enough to get some positive effect. Whether or not we would also have to block glucose use to get it to really work is something we continue to study.”
T cells that had been exposed to chemotherapy also did poorly. Cyclophosphamide and doxorubicin seemed particularly toxic. Cells with exposure to these two agents had severely depleted CAR T cell potential with very poor spare respiratory capacity. This is a marker of mitochondrial injury, Dr. Barrett said. “That wasn’t a huge surprise. We already knew that cyclophosphamide is very toxic to T cells.”
But the finding did suggest the simple intervention of harvesting T cells before chemotherapy, which is what Dr. Barrett and his colleagues now do in their high-risk ALL patients. Whether or not this would improve response in patients with solid tumors is still unknown.
He had no financial disclosures. This study was supported by the AACR, the Doris Duke Charitable Foundation Clinical Science Development Award, the Jeffrey Pride Foundation Research Award, and the St. Baldrick’s Foundation Scholar Award.
SOURCE: Barrett DM et al. AACR 2018, Abstract 1631.
Two critical factors – prior exposure to chemotherapy and a glycolytic metabolism – appear to degrade the potential of T cells to become chimeric antigen receptor–T cells.
Chemotherapy, especially with cyclophosphamide and doxorubicin, seems particularly toxic to T cells, damaging the mitochondria and decreasing the cells’ spare respiratory capacity – a measure of mitochondrial health, David Barrett, MD, said during a press briefing held in advance of the annual meeting of the American Association for Cancer Research.
These new findings may help explain why children with acute lymphoblastic leukemia (ALL) tend to respond so vigorously to CAR T treatment, and why T cells from patients with solid tumors simply don’t grow, or die soon after patient infusion, he said in an interview. They also suggest a benefit of harvesting T cells before any chemotherapy, a procedure Dr. Barrett and his colleagues have advocated.
“Based on these data we have altered our practice for T-cell therapy in high-risk leukemia patients. If we have a patient who may have a poor prognosis, we try to collect the cells early and store them before proceeding, because we know chemotherapy will progressively degrade them.”
There still is no successful CAR T-cell protocol for solid tumors, but Dr. Barrett said these findings eventually may help such patients, particularly if more advanced experiments in manipulating the cells’ metabolism prove successful.
He and his colleagues investigated why T cells from some patients result in a poor clinical product that either fails manufacture or does not proliferate in the patient. They examined T cells from 157 pediatric patients with a variety of cancers, including ALL, non-Hodgkin lymphoma, neuroblastoma, osteosarcoma, rhabdomyosarcoma, Wilms tumor, Hodgkin disease, chronic myelogenous leukemia, and Ewing sarcoma. The team obtained cells at diagnosis and after each cycle of chemotherapy.
They examined how well the cells grew in the transformation and expansion process. A “pass” was considered a fivefold expansion in response to CD3/CD28 exposure for 7 days. Normal donor cells typically expand 20- to 30-fold in this time.
Only T cells taken from ALL and Wilms tumor patients before chemotherapy achieved a pass, Dr. Barrett said. Most of the ALL expansions (80%) and half of the Wilms tumor expansions passed. “We noted very poor CAR T-cell potential in all the other tumor types – less than a 30% pass. We noted a decline in potential with cumulative chemotherapy in all cases, though this was particularly significant in children less than 3 years old.”
The team also used RNA profiling to look at the cells’ metabolic pathways. Dr. Barrett noted that T cells are highly metabolically adaptable, capable of using several different fuel types and switching from one to another. Glucose and fatty acids are frequent fuels. Most of the cells from patients with solid tumors exhibited a glycolytic metabolism, while cells from patients with ALL and Wilms tumor appeared to rely more on fatty acids.
“One is not inherently worse than the other,” he said. “But glycolysis appears to be a bad thing when we’re trying to turn them into CAR T cells. Those T cells were too exhausted to do anything.”
However, Dr. Barrett encouraged the cells to switch fuels by treating them in vitro with palmitic acid, the most common fatty acid in plants and animals.
“We were growing the cells in a media containing sugar, fatty acids, and amino acids,” he explained. “We just started overloading them with palmitic acid, which has a natural transporter on the T-cell surface, so it already had a good pathway to get into the cell. It helped restore some of the performance of these T cells in some assays, although it wasn’t a complete reversal. But it was encouraging that something as simple as providing an alternate fuel was enough to get some positive effect. Whether or not we would also have to block glucose use to get it to really work is something we continue to study.”
T cells that had been exposed to chemotherapy also did poorly. Cyclophosphamide and doxorubicin seemed particularly toxic. Cells with exposure to these two agents had severely depleted CAR T cell potential with very poor spare respiratory capacity. This is a marker of mitochondrial injury, Dr. Barrett said. “That wasn’t a huge surprise. We already knew that cyclophosphamide is very toxic to T cells.”
But the finding did suggest the simple intervention of harvesting T cells before chemotherapy, which is what Dr. Barrett and his colleagues now do in their high-risk ALL patients. Whether or not this would improve response in patients with solid tumors is still unknown.
He had no financial disclosures. This study was supported by the AACR, the Doris Duke Charitable Foundation Clinical Science Development Award, the Jeffrey Pride Foundation Research Award, and the St. Baldrick’s Foundation Scholar Award.
SOURCE: Barrett DM et al. AACR 2018, Abstract 1631.
Two critical factors – prior exposure to chemotherapy and a glycolytic metabolism – appear to degrade the potential of T cells to become chimeric antigen receptor–T cells.
Chemotherapy, especially with cyclophosphamide and doxorubicin, seems particularly toxic to T cells, damaging the mitochondria and decreasing the cells’ spare respiratory capacity – a measure of mitochondrial health, David Barrett, MD, said during a press briefing held in advance of the annual meeting of the American Association for Cancer Research.
These new findings may help explain why children with acute lymphoblastic leukemia (ALL) tend to respond so vigorously to CAR T treatment, and why T cells from patients with solid tumors simply don’t grow, or die soon after patient infusion, he said in an interview. They also suggest a benefit of harvesting T cells before any chemotherapy, a procedure Dr. Barrett and his colleagues have advocated.
“Based on these data we have altered our practice for T-cell therapy in high-risk leukemia patients. If we have a patient who may have a poor prognosis, we try to collect the cells early and store them before proceeding, because we know chemotherapy will progressively degrade them.”
There still is no successful CAR T-cell protocol for solid tumors, but Dr. Barrett said these findings eventually may help such patients, particularly if more advanced experiments in manipulating the cells’ metabolism prove successful.
He and his colleagues investigated why T cells from some patients result in a poor clinical product that either fails manufacture or does not proliferate in the patient. They examined T cells from 157 pediatric patients with a variety of cancers, including ALL, non-Hodgkin lymphoma, neuroblastoma, osteosarcoma, rhabdomyosarcoma, Wilms tumor, Hodgkin disease, chronic myelogenous leukemia, and Ewing sarcoma. The team obtained cells at diagnosis and after each cycle of chemotherapy.
They examined how well the cells grew in the transformation and expansion process. A “pass” was considered a fivefold expansion in response to CD3/CD28 exposure for 7 days. Normal donor cells typically expand 20- to 30-fold in this time.
Only T cells taken from ALL and Wilms tumor patients before chemotherapy achieved a pass, Dr. Barrett said. Most of the ALL expansions (80%) and half of the Wilms tumor expansions passed. “We noted very poor CAR T-cell potential in all the other tumor types – less than a 30% pass. We noted a decline in potential with cumulative chemotherapy in all cases, though this was particularly significant in children less than 3 years old.”
The team also used RNA profiling to look at the cells’ metabolic pathways. Dr. Barrett noted that T cells are highly metabolically adaptable, capable of using several different fuel types and switching from one to another. Glucose and fatty acids are frequent fuels. Most of the cells from patients with solid tumors exhibited a glycolytic metabolism, while cells from patients with ALL and Wilms tumor appeared to rely more on fatty acids.
“One is not inherently worse than the other,” he said. “But glycolysis appears to be a bad thing when we’re trying to turn them into CAR T cells. Those T cells were too exhausted to do anything.”
However, Dr. Barrett encouraged the cells to switch fuels by treating them in vitro with palmitic acid, the most common fatty acid in plants and animals.
“We were growing the cells in a media containing sugar, fatty acids, and amino acids,” he explained. “We just started overloading them with palmitic acid, which has a natural transporter on the T-cell surface, so it already had a good pathway to get into the cell. It helped restore some of the performance of these T cells in some assays, although it wasn’t a complete reversal. But it was encouraging that something as simple as providing an alternate fuel was enough to get some positive effect. Whether or not we would also have to block glucose use to get it to really work is something we continue to study.”
T cells that had been exposed to chemotherapy also did poorly. Cyclophosphamide and doxorubicin seemed particularly toxic. Cells with exposure to these two agents had severely depleted CAR T cell potential with very poor spare respiratory capacity. This is a marker of mitochondrial injury, Dr. Barrett said. “That wasn’t a huge surprise. We already knew that cyclophosphamide is very toxic to T cells.”
But the finding did suggest the simple intervention of harvesting T cells before chemotherapy, which is what Dr. Barrett and his colleagues now do in their high-risk ALL patients. Whether or not this would improve response in patients with solid tumors is still unknown.
He had no financial disclosures. This study was supported by the AACR, the Doris Duke Charitable Foundation Clinical Science Development Award, the Jeffrey Pride Foundation Research Award, and the St. Baldrick’s Foundation Scholar Award.
SOURCE: Barrett DM et al. AACR 2018, Abstract 1631.
FROM AACR 2018
Key clinical point: Prior exposure to chemotherapy may degrade the potential of T cells to become CAR T cells, suggesting a benefit of harvesting T cells before any chemotherapy.
Major finding: Only T cells taken from ALL and Wilm’s tumor patients before chemotherapy achieved a fivefold expansion in response to CD3/CD28 exposure for 7 days.
Study details: An examination of T cells from 157 pediatric patients with a variety of cancers at diagnosis and after each cycle of chemotherapy.
Disclosures: The study was supported by the American Association of Cancer Research, the Doris Duke Charitable Foundation Clinical Science Development Award, the Jeffrey Pride Foundation Research Award, and the St. Baldrick’s Foundation Scholar Award. Dr. Barrett and his coauthors had no financial disclosures.
Source: Barrett DM et al. AACR 2018, Abstract 1631.
Manufactured graft deemed safe in blood cancer patients
LISBON—Phase 1 results suggest a programmed cellular therapy is safe for use in patients with hematologic malignancies.
The therapy, ProTmune, is being developed as a next-generation allogeneic graft intended to reduce the incidence and severity of acute graft-versus-host disease (GVHD) after hematopoietic stem cell transplant (HSCT).
Three of 7 patients who received ProTmune in this trial did develop acute GVHD, and 2 patients died.
However, the remaining 5 patients were still alive and disease-free at last follow-up.
There were no serious adverse events (AEs) attributed to ProTmune. The most common AEs were nausea, vomiting, and chest pain.
These results were presented at the 44th Annual Meeting of the EBMT (abstract A401*).
The trial, known as PROTECT, is sponsored by Fate Therapeutics, the company developing ProTmune.
The phase 1 portion of PROTECT enrolled 7 adults with hematologic malignancies—1 with myelodysplastic syndrome, 3 with acute lymphoblastic leukemia, and 3 with acute myeloid leukemia.
Patients were set to undergo matched, unrelated donor HSCT and received ProTmune as the graft. ProTmune is manufactured by modulating a mobilized peripheral blood graft with 2 small molecules, FT1050 and FT4145.
The patients ranged in age from 34 to 69, and most (n=5) were female. For conditioning, patients received fludarabine/busulfan (n=1), busulfan/cyclophosphamide (n=1), fludarabine/melphalan (n=3), or cyclophosphamide/total body irradiation (n=2).
Results
The data cut-off was February 26, 2018. The median time on study was 228 days (range, 151 to 353).
None of the patients had graft failure. The median time to neutrophil engraftment was 18 days (range, 14 to 22).
Three patients had acute GVHD at day 100 after HSCT. Two patients had grade 2 skin GVHD, and 1 had grade 3 GVHD in the skin and gut.
All 3 patients responded to steroid treatment. GVHD resolved in 5 days for the patient with grade 3 GVHD. For the grade 2 patients, GVHD resolved in 7 days and 8 days, respectively.
None of the patients relapsed, but 2 died—1 of pulmonary edema and 1 of atrial fibrillation.
AEs related to ProTmune included grade 1 vomiting (n=2), grade 2 nausea (n=2), and grade 2 chest pain (n=1).
Phase 2
The phase 2 portion of PROTECT is ongoing. This is a randomized, controlled, double-blinded trial designed to assess the safety and efficacy of ProTmune in up to 60 adults with hematologic malignancies undergoing matched, unrelated donor HSCT following myeloablative conditioning.
Patients are being randomized, in a 1:1 ratio, to receive either ProTmune or a conventional, mobilized peripheral blood cell graft from a matched, unrelated donor.
The primary efficacy endpoint is the cumulative incidence of grade 2-4 acute GVHD by day 100 post-HSCT. Rates of chronic GVHD, cancer relapse, disease-free survival, and overall survival are also being assessed.
*Some data in the abstract differ from the presentation.
LISBON—Phase 1 results suggest a programmed cellular therapy is safe for use in patients with hematologic malignancies.
The therapy, ProTmune, is being developed as a next-generation allogeneic graft intended to reduce the incidence and severity of acute graft-versus-host disease (GVHD) after hematopoietic stem cell transplant (HSCT).
Three of 7 patients who received ProTmune in this trial did develop acute GVHD, and 2 patients died.
However, the remaining 5 patients were still alive and disease-free at last follow-up.
There were no serious adverse events (AEs) attributed to ProTmune. The most common AEs were nausea, vomiting, and chest pain.
These results were presented at the 44th Annual Meeting of the EBMT (abstract A401*).
The trial, known as PROTECT, is sponsored by Fate Therapeutics, the company developing ProTmune.
The phase 1 portion of PROTECT enrolled 7 adults with hematologic malignancies—1 with myelodysplastic syndrome, 3 with acute lymphoblastic leukemia, and 3 with acute myeloid leukemia.
Patients were set to undergo matched, unrelated donor HSCT and received ProTmune as the graft. ProTmune is manufactured by modulating a mobilized peripheral blood graft with 2 small molecules, FT1050 and FT4145.
The patients ranged in age from 34 to 69, and most (n=5) were female. For conditioning, patients received fludarabine/busulfan (n=1), busulfan/cyclophosphamide (n=1), fludarabine/melphalan (n=3), or cyclophosphamide/total body irradiation (n=2).
Results
The data cut-off was February 26, 2018. The median time on study was 228 days (range, 151 to 353).
None of the patients had graft failure. The median time to neutrophil engraftment was 18 days (range, 14 to 22).
Three patients had acute GVHD at day 100 after HSCT. Two patients had grade 2 skin GVHD, and 1 had grade 3 GVHD in the skin and gut.
All 3 patients responded to steroid treatment. GVHD resolved in 5 days for the patient with grade 3 GVHD. For the grade 2 patients, GVHD resolved in 7 days and 8 days, respectively.
None of the patients relapsed, but 2 died—1 of pulmonary edema and 1 of atrial fibrillation.
AEs related to ProTmune included grade 1 vomiting (n=2), grade 2 nausea (n=2), and grade 2 chest pain (n=1).
Phase 2
The phase 2 portion of PROTECT is ongoing. This is a randomized, controlled, double-blinded trial designed to assess the safety and efficacy of ProTmune in up to 60 adults with hematologic malignancies undergoing matched, unrelated donor HSCT following myeloablative conditioning.
Patients are being randomized, in a 1:1 ratio, to receive either ProTmune or a conventional, mobilized peripheral blood cell graft from a matched, unrelated donor.
The primary efficacy endpoint is the cumulative incidence of grade 2-4 acute GVHD by day 100 post-HSCT. Rates of chronic GVHD, cancer relapse, disease-free survival, and overall survival are also being assessed.
*Some data in the abstract differ from the presentation.
LISBON—Phase 1 results suggest a programmed cellular therapy is safe for use in patients with hematologic malignancies.
The therapy, ProTmune, is being developed as a next-generation allogeneic graft intended to reduce the incidence and severity of acute graft-versus-host disease (GVHD) after hematopoietic stem cell transplant (HSCT).
Three of 7 patients who received ProTmune in this trial did develop acute GVHD, and 2 patients died.
However, the remaining 5 patients were still alive and disease-free at last follow-up.
There were no serious adverse events (AEs) attributed to ProTmune. The most common AEs were nausea, vomiting, and chest pain.
These results were presented at the 44th Annual Meeting of the EBMT (abstract A401*).
The trial, known as PROTECT, is sponsored by Fate Therapeutics, the company developing ProTmune.
The phase 1 portion of PROTECT enrolled 7 adults with hematologic malignancies—1 with myelodysplastic syndrome, 3 with acute lymphoblastic leukemia, and 3 with acute myeloid leukemia.
Patients were set to undergo matched, unrelated donor HSCT and received ProTmune as the graft. ProTmune is manufactured by modulating a mobilized peripheral blood graft with 2 small molecules, FT1050 and FT4145.
The patients ranged in age from 34 to 69, and most (n=5) were female. For conditioning, patients received fludarabine/busulfan (n=1), busulfan/cyclophosphamide (n=1), fludarabine/melphalan (n=3), or cyclophosphamide/total body irradiation (n=2).
Results
The data cut-off was February 26, 2018. The median time on study was 228 days (range, 151 to 353).
None of the patients had graft failure. The median time to neutrophil engraftment was 18 days (range, 14 to 22).
Three patients had acute GVHD at day 100 after HSCT. Two patients had grade 2 skin GVHD, and 1 had grade 3 GVHD in the skin and gut.
All 3 patients responded to steroid treatment. GVHD resolved in 5 days for the patient with grade 3 GVHD. For the grade 2 patients, GVHD resolved in 7 days and 8 days, respectively.
None of the patients relapsed, but 2 died—1 of pulmonary edema and 1 of atrial fibrillation.
AEs related to ProTmune included grade 1 vomiting (n=2), grade 2 nausea (n=2), and grade 2 chest pain (n=1).
Phase 2
The phase 2 portion of PROTECT is ongoing. This is a randomized, controlled, double-blinded trial designed to assess the safety and efficacy of ProTmune in up to 60 adults with hematologic malignancies undergoing matched, unrelated donor HSCT following myeloablative conditioning.
Patients are being randomized, in a 1:1 ratio, to receive either ProTmune or a conventional, mobilized peripheral blood cell graft from a matched, unrelated donor.
The primary efficacy endpoint is the cumulative incidence of grade 2-4 acute GVHD by day 100 post-HSCT. Rates of chronic GVHD, cancer relapse, disease-free survival, and overall survival are also being assessed.
*Some data in the abstract differ from the presentation.
Metabolic changes in T cells may limit CAR potential in kids
Researchers analyzed peripheral blood T cells from 157 pediatric cancer patients at diagnosis and after chemotherapy and found the potential to produce effective chimeric antigen receptor (CAR) T cells declined with each cycle of chemotherapy.
This was also true for acute lymphoblastic leukemia (ALL) and Wilms’ tumor, which had high CAR T-cell manufacturing potential in the pre-chemotherapy samples.
Children younger than 3 years particularly showed a significant decline in CAR T-cell potential with cumulative cycles of chemotherapy.
“Everybody knows that chemotherapy is really bad for your T cells, and the more chemo you get, the less likely you are to have healthy T cells,” David M. Barrett, MD, PhD, of Children’s Hospital of Philadelphia in Pennsylvania, said at a press preview of research to be presented at the AACR Annual Meeting 2018.
“We know a lot about what a highly active, highly successful CAR T cell looks like right before it goes back into the patient after it’s finished manufacturing,” Dr Barrett added.
But he and his colleagues wanted to determine what goes into producing high-quality cells from a patient and the difference between cells that were good starting material and cells that weren’t.
The investigators analyzed blood samples from pediatric patients with ALL, non-Hodgkin lymphoma, neuroblastoma, osteosarcoma, rhabdomyosarcoma, Wilms’ tumor, Hodgkin lymphoma, chronic myeloid leukemia, and Ewing sarcoma. The team collected samples at diagnosis and after every cycle of chemotherapy.
Using flow cytometry, they quantified the CD3+ cell population and expanded the T cells using CD3 and CD28 stimulatory beads, “the backbone of pretty much every center’s way to make CAR T cells in the lab,” Dr Barrett said.
And the researchers found poor CAR T-cell manufacturing potential in all tumor types at diagnosis except for ALL and Wilms’ tumor. In standard-risk and high-risk ALL, more than 90% of patients had high-quality T cells at diagnosis.
The team report the findings in abstract 1631, which is scheduled to be presented at the AACR Annual Meeting on April 15.
“This may have played into why pediatric ALL is one of the great successes with CAR T-cell therapy,” Dr Barrett explained. “We may have actually been working with uniquely well-suited, good starting material to build a CAR T cell.”
T cells from lymphoma patients—Burkitt lymphoma, diffuse large B-cell lymphoma, primary mediastinal B-cell lymphoma, and Hodgkin lymphoma—were actually quite poor in their potential to become good CAR T cells, Dr Barrett noted.
“This may be reflected clinically in pediatrics at Children’s Hospital of Philadelphia,” he said. “We’ve only been able to successfully treat 3 children with lymphoma, as opposed to more than 200 children with leukemia.”
The only other type of tumor that seemed to have good CAR T potential was Wilms’ tumor.
“I don’t have a CAR T cell for Wilms’ tumor yet,” Dr Barrett said, “but, if I wanted to make one, I would at least have a degree of confidence that the cells gotten from a patient would at least be able to be successfully made into a highly functional T cell that can go back into a patient.”
The investigators also observed that cumulative chemotherapy alters the metabolic profile in T cells, “gradually turning them by cycle 6 into something that doesn’t work anymore,” Dr Barrett said.
The researchers then looked into what differences there were in the quality of collected T cells and found that metabolic changes varied with tumor and treatment.
T cells with poor CAR T-cell potential were biased toward using glycolysis as their energy source instead of using fatty acids.
“Normal, healthy donor T cells cluster together in terms of metabolic pathways that are active or inactive,” Dr Barrett explained.
“[P]atients who had leukemia and the Wilms’ tumor patients could make successful CAR T cells from those samples. On the other hand, solid tumors and a Hodgkin disease patient look like they have a very different metabolic profile. And that is associated with failure to make a good CAR T cell.”
The investigators were able to get the T cells to shift metabolic pathways by “essentially force-feeding T cells things like fatty acids so they don’t use as much glucose,” Dr Barrett said.
“We’ve had some success in force-feeding them essentially neutral amino acids and others like arginine. And so you can actually potentially provide a T cell with an attractive alternative fuel source.”
Dr Barrett noted that the findings have already altered practice for children at his institution.
They now collect T cells early even if the patient is not eligible for a CAR trial, “simply because we know that cumulative chemotherapy is going to progressively deteriorate the likelihood that those cells will make a functional CAR product, and we’ve been recommending that to other centers,” Dr Barrett said.
“We’re trying to understand what goes into making the best starting material so that we can alter our approaches to make sure that we make a highly functional CAR T-cell product not only for kids with leukemia and CART19, but also potentially for solid tumor CARs as we try to develop those in the future.”
Researchers analyzed peripheral blood T cells from 157 pediatric cancer patients at diagnosis and after chemotherapy and found the potential to produce effective chimeric antigen receptor (CAR) T cells declined with each cycle of chemotherapy.
This was also true for acute lymphoblastic leukemia (ALL) and Wilms’ tumor, which had high CAR T-cell manufacturing potential in the pre-chemotherapy samples.
Children younger than 3 years particularly showed a significant decline in CAR T-cell potential with cumulative cycles of chemotherapy.
“Everybody knows that chemotherapy is really bad for your T cells, and the more chemo you get, the less likely you are to have healthy T cells,” David M. Barrett, MD, PhD, of Children’s Hospital of Philadelphia in Pennsylvania, said at a press preview of research to be presented at the AACR Annual Meeting 2018.
“We know a lot about what a highly active, highly successful CAR T cell looks like right before it goes back into the patient after it’s finished manufacturing,” Dr Barrett added.
But he and his colleagues wanted to determine what goes into producing high-quality cells from a patient and the difference between cells that were good starting material and cells that weren’t.
The investigators analyzed blood samples from pediatric patients with ALL, non-Hodgkin lymphoma, neuroblastoma, osteosarcoma, rhabdomyosarcoma, Wilms’ tumor, Hodgkin lymphoma, chronic myeloid leukemia, and Ewing sarcoma. The team collected samples at diagnosis and after every cycle of chemotherapy.
Using flow cytometry, they quantified the CD3+ cell population and expanded the T cells using CD3 and CD28 stimulatory beads, “the backbone of pretty much every center’s way to make CAR T cells in the lab,” Dr Barrett said.
And the researchers found poor CAR T-cell manufacturing potential in all tumor types at diagnosis except for ALL and Wilms’ tumor. In standard-risk and high-risk ALL, more than 90% of patients had high-quality T cells at diagnosis.
The team report the findings in abstract 1631, which is scheduled to be presented at the AACR Annual Meeting on April 15.
“This may have played into why pediatric ALL is one of the great successes with CAR T-cell therapy,” Dr Barrett explained. “We may have actually been working with uniquely well-suited, good starting material to build a CAR T cell.”
T cells from lymphoma patients—Burkitt lymphoma, diffuse large B-cell lymphoma, primary mediastinal B-cell lymphoma, and Hodgkin lymphoma—were actually quite poor in their potential to become good CAR T cells, Dr Barrett noted.
“This may be reflected clinically in pediatrics at Children’s Hospital of Philadelphia,” he said. “We’ve only been able to successfully treat 3 children with lymphoma, as opposed to more than 200 children with leukemia.”
The only other type of tumor that seemed to have good CAR T potential was Wilms’ tumor.
“I don’t have a CAR T cell for Wilms’ tumor yet,” Dr Barrett said, “but, if I wanted to make one, I would at least have a degree of confidence that the cells gotten from a patient would at least be able to be successfully made into a highly functional T cell that can go back into a patient.”
The investigators also observed that cumulative chemotherapy alters the metabolic profile in T cells, “gradually turning them by cycle 6 into something that doesn’t work anymore,” Dr Barrett said.
The researchers then looked into what differences there were in the quality of collected T cells and found that metabolic changes varied with tumor and treatment.
T cells with poor CAR T-cell potential were biased toward using glycolysis as their energy source instead of using fatty acids.
“Normal, healthy donor T cells cluster together in terms of metabolic pathways that are active or inactive,” Dr Barrett explained.
“[P]atients who had leukemia and the Wilms’ tumor patients could make successful CAR T cells from those samples. On the other hand, solid tumors and a Hodgkin disease patient look like they have a very different metabolic profile. And that is associated with failure to make a good CAR T cell.”
The investigators were able to get the T cells to shift metabolic pathways by “essentially force-feeding T cells things like fatty acids so they don’t use as much glucose,” Dr Barrett said.
“We’ve had some success in force-feeding them essentially neutral amino acids and others like arginine. And so you can actually potentially provide a T cell with an attractive alternative fuel source.”
Dr Barrett noted that the findings have already altered practice for children at his institution.
They now collect T cells early even if the patient is not eligible for a CAR trial, “simply because we know that cumulative chemotherapy is going to progressively deteriorate the likelihood that those cells will make a functional CAR product, and we’ve been recommending that to other centers,” Dr Barrett said.
“We’re trying to understand what goes into making the best starting material so that we can alter our approaches to make sure that we make a highly functional CAR T-cell product not only for kids with leukemia and CART19, but also potentially for solid tumor CARs as we try to develop those in the future.”
Researchers analyzed peripheral blood T cells from 157 pediatric cancer patients at diagnosis and after chemotherapy and found the potential to produce effective chimeric antigen receptor (CAR) T cells declined with each cycle of chemotherapy.
This was also true for acute lymphoblastic leukemia (ALL) and Wilms’ tumor, which had high CAR T-cell manufacturing potential in the pre-chemotherapy samples.
Children younger than 3 years particularly showed a significant decline in CAR T-cell potential with cumulative cycles of chemotherapy.
“Everybody knows that chemotherapy is really bad for your T cells, and the more chemo you get, the less likely you are to have healthy T cells,” David M. Barrett, MD, PhD, of Children’s Hospital of Philadelphia in Pennsylvania, said at a press preview of research to be presented at the AACR Annual Meeting 2018.
“We know a lot about what a highly active, highly successful CAR T cell looks like right before it goes back into the patient after it’s finished manufacturing,” Dr Barrett added.
But he and his colleagues wanted to determine what goes into producing high-quality cells from a patient and the difference between cells that were good starting material and cells that weren’t.
The investigators analyzed blood samples from pediatric patients with ALL, non-Hodgkin lymphoma, neuroblastoma, osteosarcoma, rhabdomyosarcoma, Wilms’ tumor, Hodgkin lymphoma, chronic myeloid leukemia, and Ewing sarcoma. The team collected samples at diagnosis and after every cycle of chemotherapy.
Using flow cytometry, they quantified the CD3+ cell population and expanded the T cells using CD3 and CD28 stimulatory beads, “the backbone of pretty much every center’s way to make CAR T cells in the lab,” Dr Barrett said.
And the researchers found poor CAR T-cell manufacturing potential in all tumor types at diagnosis except for ALL and Wilms’ tumor. In standard-risk and high-risk ALL, more than 90% of patients had high-quality T cells at diagnosis.
The team report the findings in abstract 1631, which is scheduled to be presented at the AACR Annual Meeting on April 15.
“This may have played into why pediatric ALL is one of the great successes with CAR T-cell therapy,” Dr Barrett explained. “We may have actually been working with uniquely well-suited, good starting material to build a CAR T cell.”
T cells from lymphoma patients—Burkitt lymphoma, diffuse large B-cell lymphoma, primary mediastinal B-cell lymphoma, and Hodgkin lymphoma—were actually quite poor in their potential to become good CAR T cells, Dr Barrett noted.
“This may be reflected clinically in pediatrics at Children’s Hospital of Philadelphia,” he said. “We’ve only been able to successfully treat 3 children with lymphoma, as opposed to more than 200 children with leukemia.”
The only other type of tumor that seemed to have good CAR T potential was Wilms’ tumor.
“I don’t have a CAR T cell for Wilms’ tumor yet,” Dr Barrett said, “but, if I wanted to make one, I would at least have a degree of confidence that the cells gotten from a patient would at least be able to be successfully made into a highly functional T cell that can go back into a patient.”
The investigators also observed that cumulative chemotherapy alters the metabolic profile in T cells, “gradually turning them by cycle 6 into something that doesn’t work anymore,” Dr Barrett said.
The researchers then looked into what differences there were in the quality of collected T cells and found that metabolic changes varied with tumor and treatment.
T cells with poor CAR T-cell potential were biased toward using glycolysis as their energy source instead of using fatty acids.
“Normal, healthy donor T cells cluster together in terms of metabolic pathways that are active or inactive,” Dr Barrett explained.
“[P]atients who had leukemia and the Wilms’ tumor patients could make successful CAR T cells from those samples. On the other hand, solid tumors and a Hodgkin disease patient look like they have a very different metabolic profile. And that is associated with failure to make a good CAR T cell.”
The investigators were able to get the T cells to shift metabolic pathways by “essentially force-feeding T cells things like fatty acids so they don’t use as much glucose,” Dr Barrett said.
“We’ve had some success in force-feeding them essentially neutral amino acids and others like arginine. And so you can actually potentially provide a T cell with an attractive alternative fuel source.”
Dr Barrett noted that the findings have already altered practice for children at his institution.
They now collect T cells early even if the patient is not eligible for a CAR trial, “simply because we know that cumulative chemotherapy is going to progressively deteriorate the likelihood that those cells will make a functional CAR product, and we’ve been recommending that to other centers,” Dr Barrett said.
“We’re trying to understand what goes into making the best starting material so that we can alter our approaches to make sure that we make a highly functional CAR T-cell product not only for kids with leukemia and CART19, but also potentially for solid tumor CARs as we try to develop those in the future.”
A global snapshot of leukemia incidence
, according to an analysis of World Health Organization cancer databases.
Incidence also is generally higher in males, with a global male to female ratio of 1.4. For men, the highest regional leukemia rate – estimated at 11.3 per 100,000 population for 2012 – was found in Australia and New Zealand, with northern America (the United States and Canada) next at 10.5 per 100,000. Australia/New Zealand and northern America had the highest rate for women at 7.2 per 100,000, followed by western Europe and northern Europe at 6.0 per 100,000, reported Adalberto Miranda-Filho, PhD, of the WHO’s International Agency for Research on Cancer in Lyon, France, and his associates.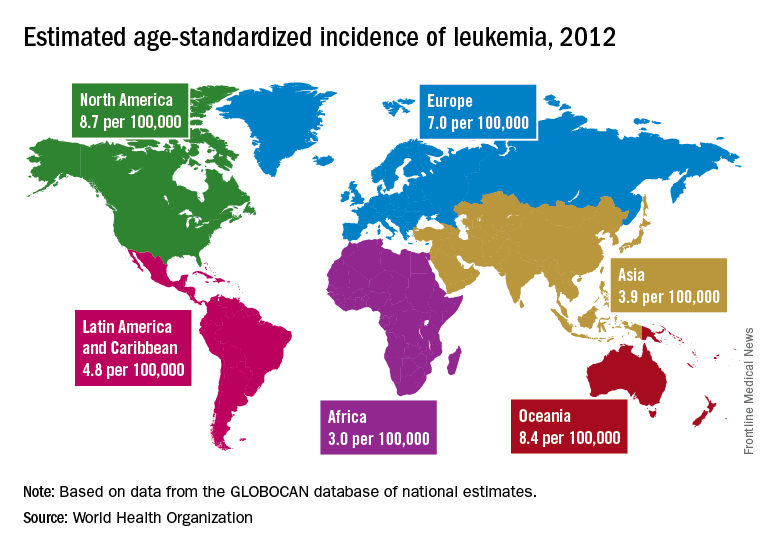
The lowest regional rates for women were found in western Africa (1.2 per 100,000), middle Africa (1.8), and Micronesia/Polynesia (2.1). For men, leukemia incidence was lowest in western Africa (1.4 per 100,000), middle Africa (2.6), and south-central Asia (3.4), according to data from the WHO’s GLOBOCAN database. The report was published in The Lancet Haematology.
Estimates for leukemia subtypes in 2003-2007 – calculated for 54 countries, not regions – also showed a great deal of variation. For acute lymphoblastic leukemia, Ecuador had the highest rates for both males (2.8 per 100,000) and females (3.3), with high rates seen in Costa Rica, Columbia, and Cyprus. Rates in the United States were near the top: 2.1 for males and 1.6 for females. Rates were lowest for men in Jamaica (0.4) and Serbia (0.6) and for women in India (0.5) and Serbia and Cuba (0.6), Dr. Miranda-Filho and his associates said.
Incidence rates for acute myeloid leukemia were highest in Australia for men (2.8 per 100,000) and Austria for women (2.2), with the United States near the top for both men (2.6) and women (1.9). The lowest rates occurred in Cuba and Egypt for men (0.9 per 100,000) and Cuba for women (0.4), data from the WHO’s Cancer Incidence in Five Continents Volume X show.
Chronic lymphocytic leukemia incidence was highest for men in Canada (4.5 per 100,000), Ireland and Lithuania (4.4), and Slovakia (4.3). The incidence was highest for women in Lithuania (2.5), Canada (2.3), and Slovakia and Denmark (2.1). Incidence in the United States was 3.5 for men and 1.8 for women. At the other end of the scale, the lowest rates for both men and women were in Japan and Malaysia (0.1), the investigators’ analysis showed.
Chronic myeloid leukemia rates were the lowest of the subtypes, and Tunisia was the lowest for men at 0.4 per 100,000 and tied for lowest with Serbia, Slovenia, and Puerto Rico for women at 0.3. Incidence was highest for men in Australia at 1.8 per 100,000 and highest for women in Uruguay at 1.1. Rates in the United States were 1.3 for men and 0.8 for women, Dr. Miranda-Filho and his associates said.
“The higher incidence of acute lymphoblastic leukaemia in parts of South America, as well as of chronic lymphocytic leukaemia in populations across North America and Oceania, alongside a lower incidence in Asia, might be important markers for further epidemiological study, and a means to better understand the underlying factors to support future cancer prevention strategies,” the investigators wrote.
SOURCE: Miranda-Filho A et al. Lancet Haematol. 2018;5:e14-24.
, according to an analysis of World Health Organization cancer databases.
Incidence also is generally higher in males, with a global male to female ratio of 1.4. For men, the highest regional leukemia rate – estimated at 11.3 per 100,000 population for 2012 – was found in Australia and New Zealand, with northern America (the United States and Canada) next at 10.5 per 100,000. Australia/New Zealand and northern America had the highest rate for women at 7.2 per 100,000, followed by western Europe and northern Europe at 6.0 per 100,000, reported Adalberto Miranda-Filho, PhD, of the WHO’s International Agency for Research on Cancer in Lyon, France, and his associates.
The lowest regional rates for women were found in western Africa (1.2 per 100,000), middle Africa (1.8), and Micronesia/Polynesia (2.1). For men, leukemia incidence was lowest in western Africa (1.4 per 100,000), middle Africa (2.6), and south-central Asia (3.4), according to data from the WHO’s GLOBOCAN database. The report was published in The Lancet Haematology.
Estimates for leukemia subtypes in 2003-2007 – calculated for 54 countries, not regions – also showed a great deal of variation. For acute lymphoblastic leukemia, Ecuador had the highest rates for both males (2.8 per 100,000) and females (3.3), with high rates seen in Costa Rica, Columbia, and Cyprus. Rates in the United States were near the top: 2.1 for males and 1.6 for females. Rates were lowest for men in Jamaica (0.4) and Serbia (0.6) and for women in India (0.5) and Serbia and Cuba (0.6), Dr. Miranda-Filho and his associates said.
Incidence rates for acute myeloid leukemia were highest in Australia for men (2.8 per 100,000) and Austria for women (2.2), with the United States near the top for both men (2.6) and women (1.9). The lowest rates occurred in Cuba and Egypt for men (0.9 per 100,000) and Cuba for women (0.4), data from the WHO’s Cancer Incidence in Five Continents Volume X show.
Chronic lymphocytic leukemia incidence was highest for men in Canada (4.5 per 100,000), Ireland and Lithuania (4.4), and Slovakia (4.3). The incidence was highest for women in Lithuania (2.5), Canada (2.3), and Slovakia and Denmark (2.1). Incidence in the United States was 3.5 for men and 1.8 for women. At the other end of the scale, the lowest rates for both men and women were in Japan and Malaysia (0.1), the investigators’ analysis showed.
Chronic myeloid leukemia rates were the lowest of the subtypes, and Tunisia was the lowest for men at 0.4 per 100,000 and tied for lowest with Serbia, Slovenia, and Puerto Rico for women at 0.3. Incidence was highest for men in Australia at 1.8 per 100,000 and highest for women in Uruguay at 1.1. Rates in the United States were 1.3 for men and 0.8 for women, Dr. Miranda-Filho and his associates said.
“The higher incidence of acute lymphoblastic leukaemia in parts of South America, as well as of chronic lymphocytic leukaemia in populations across North America and Oceania, alongside a lower incidence in Asia, might be important markers for further epidemiological study, and a means to better understand the underlying factors to support future cancer prevention strategies,” the investigators wrote.
SOURCE: Miranda-Filho A et al. Lancet Haematol. 2018;5:e14-24.
, according to an analysis of World Health Organization cancer databases.
Incidence also is generally higher in males, with a global male to female ratio of 1.4. For men, the highest regional leukemia rate – estimated at 11.3 per 100,000 population for 2012 – was found in Australia and New Zealand, with northern America (the United States and Canada) next at 10.5 per 100,000. Australia/New Zealand and northern America had the highest rate for women at 7.2 per 100,000, followed by western Europe and northern Europe at 6.0 per 100,000, reported Adalberto Miranda-Filho, PhD, of the WHO’s International Agency for Research on Cancer in Lyon, France, and his associates.
The lowest regional rates for women were found in western Africa (1.2 per 100,000), middle Africa (1.8), and Micronesia/Polynesia (2.1). For men, leukemia incidence was lowest in western Africa (1.4 per 100,000), middle Africa (2.6), and south-central Asia (3.4), according to data from the WHO’s GLOBOCAN database. The report was published in The Lancet Haematology.
Estimates for leukemia subtypes in 2003-2007 – calculated for 54 countries, not regions – also showed a great deal of variation. For acute lymphoblastic leukemia, Ecuador had the highest rates for both males (2.8 per 100,000) and females (3.3), with high rates seen in Costa Rica, Columbia, and Cyprus. Rates in the United States were near the top: 2.1 for males and 1.6 for females. Rates were lowest for men in Jamaica (0.4) and Serbia (0.6) and for women in India (0.5) and Serbia and Cuba (0.6), Dr. Miranda-Filho and his associates said.
Incidence rates for acute myeloid leukemia were highest in Australia for men (2.8 per 100,000) and Austria for women (2.2), with the United States near the top for both men (2.6) and women (1.9). The lowest rates occurred in Cuba and Egypt for men (0.9 per 100,000) and Cuba for women (0.4), data from the WHO’s Cancer Incidence in Five Continents Volume X show.
Chronic lymphocytic leukemia incidence was highest for men in Canada (4.5 per 100,000), Ireland and Lithuania (4.4), and Slovakia (4.3). The incidence was highest for women in Lithuania (2.5), Canada (2.3), and Slovakia and Denmark (2.1). Incidence in the United States was 3.5 for men and 1.8 for women. At the other end of the scale, the lowest rates for both men and women were in Japan and Malaysia (0.1), the investigators’ analysis showed.
Chronic myeloid leukemia rates were the lowest of the subtypes, and Tunisia was the lowest for men at 0.4 per 100,000 and tied for lowest with Serbia, Slovenia, and Puerto Rico for women at 0.3. Incidence was highest for men in Australia at 1.8 per 100,000 and highest for women in Uruguay at 1.1. Rates in the United States were 1.3 for men and 0.8 for women, Dr. Miranda-Filho and his associates said.
“The higher incidence of acute lymphoblastic leukaemia in parts of South America, as well as of chronic lymphocytic leukaemia in populations across North America and Oceania, alongside a lower incidence in Asia, might be important markers for further epidemiological study, and a means to better understand the underlying factors to support future cancer prevention strategies,” the investigators wrote.
SOURCE: Miranda-Filho A et al. Lancet Haematol. 2018;5:e14-24.
FROM THE LANCET HAEMATOLOGY
Consider steroid-induced hypertension when treating pediatric ALL
Nearly 15% of children undergoing induction therapy for acute lymphoblastic leukemia (ALL) developed and were treated for steroid-induced hypertension, according to a study conducted by researchers at the Ohio State University in Columbus.
Ian Bakk and his associates performed a retrospective review of data from the Pediatric Health Information System, a database of the Child Health Corporation of America consisting of inpatient information from 40 free-standing children’s hospitals in the United States. They looked at new cases of ALL from the period of 2009-2013 and analyzed data from 5,578 children who received induction chemotherapy for ALL. In all, .
An adjusted regression analysis showed that infants less than 1 year of age had the highest odds of developing steroid-induced hypertension (adjusted odds ratio, 4.05), followed by children with abnormal glucose (aOR, 2.09), those with secondary diabetes mellitus (aOR, 1.67), and obese patients (aOR, 1.63).
“These findings can help physicians identify patients at high risk for [hypertension] at the time of ALL diagnosis,” the researchers wrote.
mschneider@frontlinemedcom.com
SOURCE: Bakk, I et al. Am J Pediatr Hematol Oncol. 2018;40:27-30.
Nearly 15% of children undergoing induction therapy for acute lymphoblastic leukemia (ALL) developed and were treated for steroid-induced hypertension, according to a study conducted by researchers at the Ohio State University in Columbus.
Ian Bakk and his associates performed a retrospective review of data from the Pediatric Health Information System, a database of the Child Health Corporation of America consisting of inpatient information from 40 free-standing children’s hospitals in the United States. They looked at new cases of ALL from the period of 2009-2013 and analyzed data from 5,578 children who received induction chemotherapy for ALL. In all, .
An adjusted regression analysis showed that infants less than 1 year of age had the highest odds of developing steroid-induced hypertension (adjusted odds ratio, 4.05), followed by children with abnormal glucose (aOR, 2.09), those with secondary diabetes mellitus (aOR, 1.67), and obese patients (aOR, 1.63).
“These findings can help physicians identify patients at high risk for [hypertension] at the time of ALL diagnosis,” the researchers wrote.
mschneider@frontlinemedcom.com
SOURCE: Bakk, I et al. Am J Pediatr Hematol Oncol. 2018;40:27-30.
Nearly 15% of children undergoing induction therapy for acute lymphoblastic leukemia (ALL) developed and were treated for steroid-induced hypertension, according to a study conducted by researchers at the Ohio State University in Columbus.
Ian Bakk and his associates performed a retrospective review of data from the Pediatric Health Information System, a database of the Child Health Corporation of America consisting of inpatient information from 40 free-standing children’s hospitals in the United States. They looked at new cases of ALL from the period of 2009-2013 and analyzed data from 5,578 children who received induction chemotherapy for ALL. In all, .
An adjusted regression analysis showed that infants less than 1 year of age had the highest odds of developing steroid-induced hypertension (adjusted odds ratio, 4.05), followed by children with abnormal glucose (aOR, 2.09), those with secondary diabetes mellitus (aOR, 1.67), and obese patients (aOR, 1.63).
“These findings can help physicians identify patients at high risk for [hypertension] at the time of ALL diagnosis,” the researchers wrote.
mschneider@frontlinemedcom.com
SOURCE: Bakk, I et al. Am J Pediatr Hematol Oncol. 2018;40:27-30.
Method may predict relapse at BCP-ALL diagnosis
Researchers say they have developed a technique that can help them determine, at diagnosis, whether children with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) will relapse after treatment.
The method involves examining individual leukemia cells using mass cytometry.
In looking at the cells’ stage of development and signaling behavior, the researchers were able to identify a subset of malignant cells that predispose a patient to relapse.
The team described this method, which they termed “developmentally dependent predictor of relapse (DDPR),” in Nature Medicine.
Prior research suggested relapse may be driven by treatment-resistant cells that are present from the beginning of disease development.
“We wondered, can we identify those cells at the time the patient first presents to the clinic, and can we treat patients with a specific therapy to target them?” said study author Kara Davis, DO, of Stanford University in California.
Dr Davis and her colleagues used mass cytometry to analyze diagnostic bone marrow samples from 60 patients with BCP-ALL.
To pinpoint the problematic cells among the millions of cells in each patient’s sample, the researchers had to figure out how to organize the data.
“Every patient has vastly different features to their cancer,” Dr Davis said, “and we had to ask, ‘Is there any common thread between them?’”
The solution, the researchers found, was to match BCP-ALL cells and healthy B cells according to their developmental states, comparing the leukemic cells to the healthy cells.
The comparison revealed 6 features of leukemic cell populations that were associated with relapse.
Broadly, the features suggested that pro-BII cells with activated mTOR signaling were associated with relapse, as were pre-BI cells with activated and unresponsive pre-B-cell receptor signaling.
“We do not understand the mechanisms by which malignant cells from the pro-BII and pre-BI stages of development resist treatment,” Dr Davis noted.
However, she and her colleagues were able to show the leukemic cell features identified by DDPR could predict relapse in the BCP-ALL patients.
Of the 60 patients analyzed, there were 54 with at least 3 years of follow-up. The researchers divided these patients into a training cohort (n=44) and a validation cohort (n=10).
The team used an integrated cumulative/dynamic area under the curve (iAUC) and a C-statistic to assess DDPR performance in both cohorts.
In the training cohort, DDPR had an iAUC value of 0.92 and a C-statistic of 0.87. In the validation cohort, DDPR had an iAUC value of 0.85 and a C-statistic of 0.87.
The researchers also said DDPR “performed well” in predicting relapse-free survival in a retrospective analysis of both cohorts (P = 2.8 × 10−7).
Now, the researchers plan to validate DDPR in a larger number of patients and evaluate whether the same general approach could predict relapse in other cancers.
Researchers say they have developed a technique that can help them determine, at diagnosis, whether children with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) will relapse after treatment.
The method involves examining individual leukemia cells using mass cytometry.
In looking at the cells’ stage of development and signaling behavior, the researchers were able to identify a subset of malignant cells that predispose a patient to relapse.
The team described this method, which they termed “developmentally dependent predictor of relapse (DDPR),” in Nature Medicine.
Prior research suggested relapse may be driven by treatment-resistant cells that are present from the beginning of disease development.
“We wondered, can we identify those cells at the time the patient first presents to the clinic, and can we treat patients with a specific therapy to target them?” said study author Kara Davis, DO, of Stanford University in California.
Dr Davis and her colleagues used mass cytometry to analyze diagnostic bone marrow samples from 60 patients with BCP-ALL.
To pinpoint the problematic cells among the millions of cells in each patient’s sample, the researchers had to figure out how to organize the data.
“Every patient has vastly different features to their cancer,” Dr Davis said, “and we had to ask, ‘Is there any common thread between them?’”
The solution, the researchers found, was to match BCP-ALL cells and healthy B cells according to their developmental states, comparing the leukemic cells to the healthy cells.
The comparison revealed 6 features of leukemic cell populations that were associated with relapse.
Broadly, the features suggested that pro-BII cells with activated mTOR signaling were associated with relapse, as were pre-BI cells with activated and unresponsive pre-B-cell receptor signaling.
“We do not understand the mechanisms by which malignant cells from the pro-BII and pre-BI stages of development resist treatment,” Dr Davis noted.
However, she and her colleagues were able to show the leukemic cell features identified by DDPR could predict relapse in the BCP-ALL patients.
Of the 60 patients analyzed, there were 54 with at least 3 years of follow-up. The researchers divided these patients into a training cohort (n=44) and a validation cohort (n=10).
The team used an integrated cumulative/dynamic area under the curve (iAUC) and a C-statistic to assess DDPR performance in both cohorts.
In the training cohort, DDPR had an iAUC value of 0.92 and a C-statistic of 0.87. In the validation cohort, DDPR had an iAUC value of 0.85 and a C-statistic of 0.87.
The researchers also said DDPR “performed well” in predicting relapse-free survival in a retrospective analysis of both cohorts (P = 2.8 × 10−7).
Now, the researchers plan to validate DDPR in a larger number of patients and evaluate whether the same general approach could predict relapse in other cancers.
Researchers say they have developed a technique that can help them determine, at diagnosis, whether children with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) will relapse after treatment.
The method involves examining individual leukemia cells using mass cytometry.
In looking at the cells’ stage of development and signaling behavior, the researchers were able to identify a subset of malignant cells that predispose a patient to relapse.
The team described this method, which they termed “developmentally dependent predictor of relapse (DDPR),” in Nature Medicine.
Prior research suggested relapse may be driven by treatment-resistant cells that are present from the beginning of disease development.
“We wondered, can we identify those cells at the time the patient first presents to the clinic, and can we treat patients with a specific therapy to target them?” said study author Kara Davis, DO, of Stanford University in California.
Dr Davis and her colleagues used mass cytometry to analyze diagnostic bone marrow samples from 60 patients with BCP-ALL.
To pinpoint the problematic cells among the millions of cells in each patient’s sample, the researchers had to figure out how to organize the data.
“Every patient has vastly different features to their cancer,” Dr Davis said, “and we had to ask, ‘Is there any common thread between them?’”
The solution, the researchers found, was to match BCP-ALL cells and healthy B cells according to their developmental states, comparing the leukemic cells to the healthy cells.
The comparison revealed 6 features of leukemic cell populations that were associated with relapse.
Broadly, the features suggested that pro-BII cells with activated mTOR signaling were associated with relapse, as were pre-BI cells with activated and unresponsive pre-B-cell receptor signaling.
“We do not understand the mechanisms by which malignant cells from the pro-BII and pre-BI stages of development resist treatment,” Dr Davis noted.
However, she and her colleagues were able to show the leukemic cell features identified by DDPR could predict relapse in the BCP-ALL patients.
Of the 60 patients analyzed, there were 54 with at least 3 years of follow-up. The researchers divided these patients into a training cohort (n=44) and a validation cohort (n=10).
The team used an integrated cumulative/dynamic area under the curve (iAUC) and a C-statistic to assess DDPR performance in both cohorts.
In the training cohort, DDPR had an iAUC value of 0.92 and a C-statistic of 0.87. In the validation cohort, DDPR had an iAUC value of 0.85 and a C-statistic of 0.87.
The researchers also said DDPR “performed well” in predicting relapse-free survival in a retrospective analysis of both cohorts (P = 2.8 × 10−7).
Now, the researchers plan to validate DDPR in a larger number of patients and evaluate whether the same general approach could predict relapse in other cancers.
Phase 2 study tests low-dose maintenance therapy for ALL
Researchers at MD Anderson Cancer Center in Houston are studying the safety and clinical effectiveness of low-dose inotuzumab ozogamicin in controlling acute lymphocytic leukemia (ALL).
The phase 2 study (NCT03441061), which was launched on Feb. 15, will include up to 40 adult patients with B-cell ALL who are in complete remission with molecular failure or molecular relapse at any point after 3 months of frontline therapy. The study is looking first at relapse-free survival, and secondarily at overall survival and minimal residual disease negativity rate overall and after the first cycle. In addition, the researchers will consider the safety of the drug in this setting.
Inotuzumab ozogamicin (Besponsa) was approved by the Food and Drug Administration in August 2017 for the treatment of adults with relapsed or refractory B-cell precursor ALL.
During the open-label, single-arm study, patients can receive up to six cycles of the drug and each cycle is 28 (plus or minus 7) days. Eligible patients will receive inotuzumab ozogamicin on days 1,8, and 15 of cycle 1 and days 1 and 8 of cycles 2-6.
If a patient chooses to undergo a stem cell transplant from a donor, that patient will receive only two to three cycles of the drug. Study participants may also be taken off treatment after cycle 2 if the disease has had no response.
The study is being conducted at MD Anderson Cancer Center and is expected to be completed in February 2023. The study is sponsored by Pfizer.
Researchers at MD Anderson Cancer Center in Houston are studying the safety and clinical effectiveness of low-dose inotuzumab ozogamicin in controlling acute lymphocytic leukemia (ALL).
The phase 2 study (NCT03441061), which was launched on Feb. 15, will include up to 40 adult patients with B-cell ALL who are in complete remission with molecular failure or molecular relapse at any point after 3 months of frontline therapy. The study is looking first at relapse-free survival, and secondarily at overall survival and minimal residual disease negativity rate overall and after the first cycle. In addition, the researchers will consider the safety of the drug in this setting.
Inotuzumab ozogamicin (Besponsa) was approved by the Food and Drug Administration in August 2017 for the treatment of adults with relapsed or refractory B-cell precursor ALL.
During the open-label, single-arm study, patients can receive up to six cycles of the drug and each cycle is 28 (plus or minus 7) days. Eligible patients will receive inotuzumab ozogamicin on days 1,8, and 15 of cycle 1 and days 1 and 8 of cycles 2-6.
If a patient chooses to undergo a stem cell transplant from a donor, that patient will receive only two to three cycles of the drug. Study participants may also be taken off treatment after cycle 2 if the disease has had no response.
The study is being conducted at MD Anderson Cancer Center and is expected to be completed in February 2023. The study is sponsored by Pfizer.
Researchers at MD Anderson Cancer Center in Houston are studying the safety and clinical effectiveness of low-dose inotuzumab ozogamicin in controlling acute lymphocytic leukemia (ALL).
The phase 2 study (NCT03441061), which was launched on Feb. 15, will include up to 40 adult patients with B-cell ALL who are in complete remission with molecular failure or molecular relapse at any point after 3 months of frontline therapy. The study is looking first at relapse-free survival, and secondarily at overall survival and minimal residual disease negativity rate overall and after the first cycle. In addition, the researchers will consider the safety of the drug in this setting.
Inotuzumab ozogamicin (Besponsa) was approved by the Food and Drug Administration in August 2017 for the treatment of adults with relapsed or refractory B-cell precursor ALL.
During the open-label, single-arm study, patients can receive up to six cycles of the drug and each cycle is 28 (plus or minus 7) days. Eligible patients will receive inotuzumab ozogamicin on days 1,8, and 15 of cycle 1 and days 1 and 8 of cycles 2-6.
If a patient chooses to undergo a stem cell transplant from a donor, that patient will receive only two to three cycles of the drug. Study participants may also be taken off treatment after cycle 2 if the disease has had no response.
The study is being conducted at MD Anderson Cancer Center and is expected to be completed in February 2023. The study is sponsored by Pfizer.
FROM CLINICALTRIALS.GOV
Expanded UCB product can stand alone
SALT LAKE CITY—The expanded umbilical cord blood (UCB) product NiCord can be used as a stand-alone graft, according to research presented at the 2018 BMT Tandem Meetings.
Researchers found that a single NiCord unit provided “robust” engraftment in a phase 1/2 study of patients with high-risk hematologic malignancies.
NiCord recipients had quicker neutrophil and platelet engraftment than matched control subjects who received standard myeloablative UCB transplant (single or double).
Mitchell Horwitz, MD, of the Duke University Medical Center in Durham, North Carolina, presented these results at the meeting as abstract 49.* The research was sponsored by Gamida Cell, the company developing NiCord.
“[NiCord] is an ex vivo expanded cell product that’s derived from an entire unit of umbilical cord blood,” Dr Horwitz explained. “It’s manufactured starting with a CD133-positive selection, which is the progenitor cell population that’s cultured, and a T-cell containing CD133-negative fraction that is provided also at the time of transplant.”
“The culture system contains nicotinamide—that’s the active ingredient in the culture. And that’s supplemented with cytokines—thrombopoietin, IL-6, FLT-3 ligand, and stem cell factor. The culture is 21 days.”
Previous research showed that double UCB transplant including a NiCord unit could provide benefits over standard double UCB transplant. This led Dr Horwitz and his colleagues to wonder if NiCord could be used as a stand-alone graft.
So the team evaluated the safety and efficacy of NiCord alone in 36 adolescents/adults with high-risk hematologic malignancies.
Patients had acute myelogenous leukemia (n=17), acute lymphoblastic leukemia (n=9), myelodysplastic syndrome (n=7), chronic myelogenous leukemia (n=2), and Hodgkin lymphoma (n=1).
Most patients had intermediate (n=15) or high-risk (n=13) disease. They had a median age of 44 (range, 13-63) and a median weight of 75 kg (range, 41-125).
Treatment
For conditioning, 19 patients received thiotepa, busulfan, and fludarabine. Fifteen patients received total body irradiation and fludarabine with or without cyclophosphamide or thiotepa. And 2 patients received clofarabine, fludarabine, and busulfan.
Most patients had a 4/6 human leukocyte antigen (HLA) match (n=26), 8 had a 5/6 HLA match, and 2 had a 6/6 HLA match.
The median total nucleated cell dose was 2.4 x 107/kg prior to expansion of the UCB unit and 3.7 x 107/kg after expansion. The median CD34+ cell dose was 0.2 x 106/kg and 6.3 x 106/kg, respectively.
“CD34 cells were expanded 33-fold in the 3-week culture system,” Dr Horwitz noted. “That translated to a median CD34 dose of 6.3 x 106/kg, a dose comparable to what would be obtained from an adult donor graft.”
Engraftment
There was 1 case of primary graft failure and 2 cases of secondary graft failure. One case of secondary graft failure was associated with an HHV-6 infection, and the other was due to a lethal adenovirus infection.
Of those patients who engrafted, 97% achieved full donor chimerism, and 3% had mixed chimerism.
Dr Horwitz and his colleagues compared engraftment results in the NiCord recipients to results in a cohort of patients from the CIBMTR registry who underwent UCB transplants from 2010 to 2013. They had similar characteristics as the NiCord patients—age, conditioning regimen, disease status, etc.
In total, there were 148 CIBMTR registry patients, 20% of whom received a single UCB unit.
The median time to neutrophil engraftment was 11.5 days (range, 6-26) with NiCord and 21 days in the CIBMTR matched control cohort (P<0.001). The cumulative incidence of neutrophil engraftment was 94.4% and 89.7%, respectively.
The median time to platelet engraftment was 34 days (range, 25-96) with NiCord and 46 days in the CIBMTR controls (P<0.001). The cumulative incidence of platelet engraftment was 80.6% and 67.1%, respectively.
“There’s a median 10-day reduction in neutrophil recovery [and] 12-day reduction in time to platelet recovery [with NiCord],” Dr Horwitz noted. “There is evidence of robust and durable engraftment with a NiCord unit, with one patient now over 7 years from his first transplant on the pilot trial.”
Relapse, survival, and GVHD
Dr Horwitz reported other outcomes in the NiCord recipients without making comparisons to the CIBMTR matched controls.
The estimated 2-year rate of non-relapse mortality in NiCord recipients was 23.8%, and the estimated 2-year incidence of relapse was 33.2%.
The estimated disease-free survival was 49.1% at 1 year and 43.0% at 2 years. The estimated overall survival was 51.2% at 1 year and 2 years.
At 100 days, the rate of grade 2-4 acute GVHD was 44.0%, and the rate of grade 3-4 acute GVHD was 11.1%.
The estimated 1-year rate of mild to severe chronic GVHD was 40.5%, and the estimated 2-year rate of moderate to severe chronic GVHD was 9.8%.
Dr Horwitz said these “promising results” have led to the launch of a phase 3 registration trial in which researchers are comparing NiCord to standard single or double UCB transplant. The trial is open for accrual.
*Information in the abstract differs from the presentation.
SALT LAKE CITY—The expanded umbilical cord blood (UCB) product NiCord can be used as a stand-alone graft, according to research presented at the 2018 BMT Tandem Meetings.
Researchers found that a single NiCord unit provided “robust” engraftment in a phase 1/2 study of patients with high-risk hematologic malignancies.
NiCord recipients had quicker neutrophil and platelet engraftment than matched control subjects who received standard myeloablative UCB transplant (single or double).
Mitchell Horwitz, MD, of the Duke University Medical Center in Durham, North Carolina, presented these results at the meeting as abstract 49.* The research was sponsored by Gamida Cell, the company developing NiCord.
“[NiCord] is an ex vivo expanded cell product that’s derived from an entire unit of umbilical cord blood,” Dr Horwitz explained. “It’s manufactured starting with a CD133-positive selection, which is the progenitor cell population that’s cultured, and a T-cell containing CD133-negative fraction that is provided also at the time of transplant.”
“The culture system contains nicotinamide—that’s the active ingredient in the culture. And that’s supplemented with cytokines—thrombopoietin, IL-6, FLT-3 ligand, and stem cell factor. The culture is 21 days.”
Previous research showed that double UCB transplant including a NiCord unit could provide benefits over standard double UCB transplant. This led Dr Horwitz and his colleagues to wonder if NiCord could be used as a stand-alone graft.
So the team evaluated the safety and efficacy of NiCord alone in 36 adolescents/adults with high-risk hematologic malignancies.
Patients had acute myelogenous leukemia (n=17), acute lymphoblastic leukemia (n=9), myelodysplastic syndrome (n=7), chronic myelogenous leukemia (n=2), and Hodgkin lymphoma (n=1).
Most patients had intermediate (n=15) or high-risk (n=13) disease. They had a median age of 44 (range, 13-63) and a median weight of 75 kg (range, 41-125).
Treatment
For conditioning, 19 patients received thiotepa, busulfan, and fludarabine. Fifteen patients received total body irradiation and fludarabine with or without cyclophosphamide or thiotepa. And 2 patients received clofarabine, fludarabine, and busulfan.
Most patients had a 4/6 human leukocyte antigen (HLA) match (n=26), 8 had a 5/6 HLA match, and 2 had a 6/6 HLA match.
The median total nucleated cell dose was 2.4 x 107/kg prior to expansion of the UCB unit and 3.7 x 107/kg after expansion. The median CD34+ cell dose was 0.2 x 106/kg and 6.3 x 106/kg, respectively.
“CD34 cells were expanded 33-fold in the 3-week culture system,” Dr Horwitz noted. “That translated to a median CD34 dose of 6.3 x 106/kg, a dose comparable to what would be obtained from an adult donor graft.”
Engraftment
There was 1 case of primary graft failure and 2 cases of secondary graft failure. One case of secondary graft failure was associated with an HHV-6 infection, and the other was due to a lethal adenovirus infection.
Of those patients who engrafted, 97% achieved full donor chimerism, and 3% had mixed chimerism.
Dr Horwitz and his colleagues compared engraftment results in the NiCord recipients to results in a cohort of patients from the CIBMTR registry who underwent UCB transplants from 2010 to 2013. They had similar characteristics as the NiCord patients—age, conditioning regimen, disease status, etc.
In total, there were 148 CIBMTR registry patients, 20% of whom received a single UCB unit.
The median time to neutrophil engraftment was 11.5 days (range, 6-26) with NiCord and 21 days in the CIBMTR matched control cohort (P<0.001). The cumulative incidence of neutrophil engraftment was 94.4% and 89.7%, respectively.
The median time to platelet engraftment was 34 days (range, 25-96) with NiCord and 46 days in the CIBMTR controls (P<0.001). The cumulative incidence of platelet engraftment was 80.6% and 67.1%, respectively.
“There’s a median 10-day reduction in neutrophil recovery [and] 12-day reduction in time to platelet recovery [with NiCord],” Dr Horwitz noted. “There is evidence of robust and durable engraftment with a NiCord unit, with one patient now over 7 years from his first transplant on the pilot trial.”
Relapse, survival, and GVHD
Dr Horwitz reported other outcomes in the NiCord recipients without making comparisons to the CIBMTR matched controls.
The estimated 2-year rate of non-relapse mortality in NiCord recipients was 23.8%, and the estimated 2-year incidence of relapse was 33.2%.
The estimated disease-free survival was 49.1% at 1 year and 43.0% at 2 years. The estimated overall survival was 51.2% at 1 year and 2 years.
At 100 days, the rate of grade 2-4 acute GVHD was 44.0%, and the rate of grade 3-4 acute GVHD was 11.1%.
The estimated 1-year rate of mild to severe chronic GVHD was 40.5%, and the estimated 2-year rate of moderate to severe chronic GVHD was 9.8%.
Dr Horwitz said these “promising results” have led to the launch of a phase 3 registration trial in which researchers are comparing NiCord to standard single or double UCB transplant. The trial is open for accrual.
*Information in the abstract differs from the presentation.
SALT LAKE CITY—The expanded umbilical cord blood (UCB) product NiCord can be used as a stand-alone graft, according to research presented at the 2018 BMT Tandem Meetings.
Researchers found that a single NiCord unit provided “robust” engraftment in a phase 1/2 study of patients with high-risk hematologic malignancies.
NiCord recipients had quicker neutrophil and platelet engraftment than matched control subjects who received standard myeloablative UCB transplant (single or double).
Mitchell Horwitz, MD, of the Duke University Medical Center in Durham, North Carolina, presented these results at the meeting as abstract 49.* The research was sponsored by Gamida Cell, the company developing NiCord.
“[NiCord] is an ex vivo expanded cell product that’s derived from an entire unit of umbilical cord blood,” Dr Horwitz explained. “It’s manufactured starting with a CD133-positive selection, which is the progenitor cell population that’s cultured, and a T-cell containing CD133-negative fraction that is provided also at the time of transplant.”
“The culture system contains nicotinamide—that’s the active ingredient in the culture. And that’s supplemented with cytokines—thrombopoietin, IL-6, FLT-3 ligand, and stem cell factor. The culture is 21 days.”
Previous research showed that double UCB transplant including a NiCord unit could provide benefits over standard double UCB transplant. This led Dr Horwitz and his colleagues to wonder if NiCord could be used as a stand-alone graft.
So the team evaluated the safety and efficacy of NiCord alone in 36 adolescents/adults with high-risk hematologic malignancies.
Patients had acute myelogenous leukemia (n=17), acute lymphoblastic leukemia (n=9), myelodysplastic syndrome (n=7), chronic myelogenous leukemia (n=2), and Hodgkin lymphoma (n=1).
Most patients had intermediate (n=15) or high-risk (n=13) disease. They had a median age of 44 (range, 13-63) and a median weight of 75 kg (range, 41-125).
Treatment
For conditioning, 19 patients received thiotepa, busulfan, and fludarabine. Fifteen patients received total body irradiation and fludarabine with or without cyclophosphamide or thiotepa. And 2 patients received clofarabine, fludarabine, and busulfan.
Most patients had a 4/6 human leukocyte antigen (HLA) match (n=26), 8 had a 5/6 HLA match, and 2 had a 6/6 HLA match.
The median total nucleated cell dose was 2.4 x 107/kg prior to expansion of the UCB unit and 3.7 x 107/kg after expansion. The median CD34+ cell dose was 0.2 x 106/kg and 6.3 x 106/kg, respectively.
“CD34 cells were expanded 33-fold in the 3-week culture system,” Dr Horwitz noted. “That translated to a median CD34 dose of 6.3 x 106/kg, a dose comparable to what would be obtained from an adult donor graft.”
Engraftment
There was 1 case of primary graft failure and 2 cases of secondary graft failure. One case of secondary graft failure was associated with an HHV-6 infection, and the other was due to a lethal adenovirus infection.
Of those patients who engrafted, 97% achieved full donor chimerism, and 3% had mixed chimerism.
Dr Horwitz and his colleagues compared engraftment results in the NiCord recipients to results in a cohort of patients from the CIBMTR registry who underwent UCB transplants from 2010 to 2013. They had similar characteristics as the NiCord patients—age, conditioning regimen, disease status, etc.
In total, there were 148 CIBMTR registry patients, 20% of whom received a single UCB unit.
The median time to neutrophil engraftment was 11.5 days (range, 6-26) with NiCord and 21 days in the CIBMTR matched control cohort (P<0.001). The cumulative incidence of neutrophil engraftment was 94.4% and 89.7%, respectively.
The median time to platelet engraftment was 34 days (range, 25-96) with NiCord and 46 days in the CIBMTR controls (P<0.001). The cumulative incidence of platelet engraftment was 80.6% and 67.1%, respectively.
“There’s a median 10-day reduction in neutrophil recovery [and] 12-day reduction in time to platelet recovery [with NiCord],” Dr Horwitz noted. “There is evidence of robust and durable engraftment with a NiCord unit, with one patient now over 7 years from his first transplant on the pilot trial.”
Relapse, survival, and GVHD
Dr Horwitz reported other outcomes in the NiCord recipients without making comparisons to the CIBMTR matched controls.
The estimated 2-year rate of non-relapse mortality in NiCord recipients was 23.8%, and the estimated 2-year incidence of relapse was 33.2%.
The estimated disease-free survival was 49.1% at 1 year and 43.0% at 2 years. The estimated overall survival was 51.2% at 1 year and 2 years.
At 100 days, the rate of grade 2-4 acute GVHD was 44.0%, and the rate of grade 3-4 acute GVHD was 11.1%.
The estimated 1-year rate of mild to severe chronic GVHD was 40.5%, and the estimated 2-year rate of moderate to severe chronic GVHD was 9.8%.
Dr Horwitz said these “promising results” have led to the launch of a phase 3 registration trial in which researchers are comparing NiCord to standard single or double UCB transplant. The trial is open for accrual.
*Information in the abstract differs from the presentation.
Disease burden impacts survival, toxicity after CAR T-cell therapy
Adults with relapsed or refractory acute lymphoblastic leukemia (ALL) have better outcomes if they have a low disease burden when receiving chimeric antigen receptor (CAR) T-cell therapy, according to research published in NEJM.
The final analysis of a phase 1 trial showed that patients with a low disease burden at baseline had superior event-free survival (EFS) and overall survival (OS) after therapy, compared to patients with a high disease burden.
Patients with a low disease burden also had a lower rate of cytokine release syndrome (CRS) and neurotoxic events.
“This is the longest follow-up study of people with ALL treated with CAR therapy,” said study author Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York, New York.
“With the long follow-up, we were able to demonstrate, for the first time, that patients with a lower disease burden benefited the most from CAR therapy, with significantly improved survival and reduced toxicity.”
The study included 53 adults with ALL who had a median age of 44 (range, 23-74).
They were heavily pretreated, with 68% receiving CAR T-cell therapy as a third or later salvage treatment. Thirty-six percent of patients had received an allogeneic transplant, and 23% had primary refractory disease.
In this trial, patients received a single infusion of 19-28z CAR T cells after conditioning chemotherapy. The maximum follow-up time was 5.5 years, with a median follow-up of 29 months.
In all, 83% of patients achieved a complete response. The median EFS was 6.1 months, and the median OS was 12.9 months.
The median EFS was significantly longer for patients with a low disease burden (<5% bone marrow blasts) compared to a high disease burden (≥5% bone marrow blasts or extramedullary disease)—10.6 months and 5.6 months, respectively (P=0.01).
The same was true for the median OS, which was 20.1 months in patients with a low disease burden and 12.4 months in those with a high disease burden (P=0.02).
For the entire study population, the rate of CRS was 85%, and 26% of patients had severe CRS. One patient died of severe CRS and multi-organ failure before the researchers began modifying the dose of CAR T cells according to the pretreatment disease burden.
Severe CRS occurred in 41% of patients with a high disease burden and 5% of those with a low disease burden.
In the entire study population, 2% of patients had grade 2 neurotoxic effects, 36% had grade 3, 6% had grade 4, and none had grade 5. The rate of severe neurotoxicity was 42%.
Neurotoxic effects occurred in 59% of patients with a high disease burden and 14% of those with a low disease burden.
“Among all of the clinical and disease factors we examined, pretreatment disease burden was the strongest predictor of long-term outcome after CAR therapy,” Dr Park said. “Our data supports the incorporation of CAR therapy in an earlier treatment setting in ALL, when the disease volume is small, so as to achieve the greatest long-term efficacy and lowest toxicity.”
This work was supported by Juno Therapeutics, the National Institutes of Health, the Carson Family Charitable Trust, the Emerald Foundation, the Mr. and Mrs. Goodwyn Commonwealth Fund, the Terry Fox Run for Cancer Research organized by the Canadian Association of New York, Kate’s Team, William Laurence and Blanche Hughes Foundation, the Center for Experimental Therapeutics at Memorial Sloan Kettering, and the Lake Road Foundation. ![]()
Adults with relapsed or refractory acute lymphoblastic leukemia (ALL) have better outcomes if they have a low disease burden when receiving chimeric antigen receptor (CAR) T-cell therapy, according to research published in NEJM.
The final analysis of a phase 1 trial showed that patients with a low disease burden at baseline had superior event-free survival (EFS) and overall survival (OS) after therapy, compared to patients with a high disease burden.
Patients with a low disease burden also had a lower rate of cytokine release syndrome (CRS) and neurotoxic events.
“This is the longest follow-up study of people with ALL treated with CAR therapy,” said study author Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York, New York.
“With the long follow-up, we were able to demonstrate, for the first time, that patients with a lower disease burden benefited the most from CAR therapy, with significantly improved survival and reduced toxicity.”
The study included 53 adults with ALL who had a median age of 44 (range, 23-74).
They were heavily pretreated, with 68% receiving CAR T-cell therapy as a third or later salvage treatment. Thirty-six percent of patients had received an allogeneic transplant, and 23% had primary refractory disease.
In this trial, patients received a single infusion of 19-28z CAR T cells after conditioning chemotherapy. The maximum follow-up time was 5.5 years, with a median follow-up of 29 months.
In all, 83% of patients achieved a complete response. The median EFS was 6.1 months, and the median OS was 12.9 months.
The median EFS was significantly longer for patients with a low disease burden (<5% bone marrow blasts) compared to a high disease burden (≥5% bone marrow blasts or extramedullary disease)—10.6 months and 5.6 months, respectively (P=0.01).
The same was true for the median OS, which was 20.1 months in patients with a low disease burden and 12.4 months in those with a high disease burden (P=0.02).
For the entire study population, the rate of CRS was 85%, and 26% of patients had severe CRS. One patient died of severe CRS and multi-organ failure before the researchers began modifying the dose of CAR T cells according to the pretreatment disease burden.
Severe CRS occurred in 41% of patients with a high disease burden and 5% of those with a low disease burden.
In the entire study population, 2% of patients had grade 2 neurotoxic effects, 36% had grade 3, 6% had grade 4, and none had grade 5. The rate of severe neurotoxicity was 42%.
Neurotoxic effects occurred in 59% of patients with a high disease burden and 14% of those with a low disease burden.
“Among all of the clinical and disease factors we examined, pretreatment disease burden was the strongest predictor of long-term outcome after CAR therapy,” Dr Park said. “Our data supports the incorporation of CAR therapy in an earlier treatment setting in ALL, when the disease volume is small, so as to achieve the greatest long-term efficacy and lowest toxicity.”
This work was supported by Juno Therapeutics, the National Institutes of Health, the Carson Family Charitable Trust, the Emerald Foundation, the Mr. and Mrs. Goodwyn Commonwealth Fund, the Terry Fox Run for Cancer Research organized by the Canadian Association of New York, Kate’s Team, William Laurence and Blanche Hughes Foundation, the Center for Experimental Therapeutics at Memorial Sloan Kettering, and the Lake Road Foundation. ![]()
Adults with relapsed or refractory acute lymphoblastic leukemia (ALL) have better outcomes if they have a low disease burden when receiving chimeric antigen receptor (CAR) T-cell therapy, according to research published in NEJM.
The final analysis of a phase 1 trial showed that patients with a low disease burden at baseline had superior event-free survival (EFS) and overall survival (OS) after therapy, compared to patients with a high disease burden.
Patients with a low disease burden also had a lower rate of cytokine release syndrome (CRS) and neurotoxic events.
“This is the longest follow-up study of people with ALL treated with CAR therapy,” said study author Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York, New York.
“With the long follow-up, we were able to demonstrate, for the first time, that patients with a lower disease burden benefited the most from CAR therapy, with significantly improved survival and reduced toxicity.”
The study included 53 adults with ALL who had a median age of 44 (range, 23-74).
They were heavily pretreated, with 68% receiving CAR T-cell therapy as a third or later salvage treatment. Thirty-six percent of patients had received an allogeneic transplant, and 23% had primary refractory disease.
In this trial, patients received a single infusion of 19-28z CAR T cells after conditioning chemotherapy. The maximum follow-up time was 5.5 years, with a median follow-up of 29 months.
In all, 83% of patients achieved a complete response. The median EFS was 6.1 months, and the median OS was 12.9 months.
The median EFS was significantly longer for patients with a low disease burden (<5% bone marrow blasts) compared to a high disease burden (≥5% bone marrow blasts or extramedullary disease)—10.6 months and 5.6 months, respectively (P=0.01).
The same was true for the median OS, which was 20.1 months in patients with a low disease burden and 12.4 months in those with a high disease burden (P=0.02).
For the entire study population, the rate of CRS was 85%, and 26% of patients had severe CRS. One patient died of severe CRS and multi-organ failure before the researchers began modifying the dose of CAR T cells according to the pretreatment disease burden.
Severe CRS occurred in 41% of patients with a high disease burden and 5% of those with a low disease burden.
In the entire study population, 2% of patients had grade 2 neurotoxic effects, 36% had grade 3, 6% had grade 4, and none had grade 5. The rate of severe neurotoxicity was 42%.
Neurotoxic effects occurred in 59% of patients with a high disease burden and 14% of those with a low disease burden.
“Among all of the clinical and disease factors we examined, pretreatment disease burden was the strongest predictor of long-term outcome after CAR therapy,” Dr Park said. “Our data supports the incorporation of CAR therapy in an earlier treatment setting in ALL, when the disease volume is small, so as to achieve the greatest long-term efficacy and lowest toxicity.”
This work was supported by Juno Therapeutics, the National Institutes of Health, the Carson Family Charitable Trust, the Emerald Foundation, the Mr. and Mrs. Goodwyn Commonwealth Fund, the Terry Fox Run for Cancer Research organized by the Canadian Association of New York, Kate’s Team, William Laurence and Blanche Hughes Foundation, the Center for Experimental Therapeutics at Memorial Sloan Kettering, and the Lake Road Foundation. ![]()