User login
Hispanic ALL patients face higher treatment toxicity
Hispanic pediatric patients undergoing treatment for acute lymphoblastic leukemia (ALL) had a risk of methotrexate toxicity that was more than twice that of non-Hispanic whites, according to results of a prospective multicenter study.
Methotrexate toxicity often led to treatment modification or delays, which may have increased relapse risk in the Hispanic patients, according to investigator Michael E. Scheurer, PhD, MPH, of Baylor College of Medicine, Houston, and his colleagues.
“We had observed that our Hispanic patients tended to experience neurotoxicity more often than other groups, but we were surprised to see the magnitude of the difference,” Dr. Scheurer said in statement.
The study, described in Clinical Cancer Research, involved 280 patients with newly diagnosed ALL enrolled at one of three major U.S. pediatric cancer treatment centers. Nearly half of the patients (48.2%) were Hispanic, and approximately 86% had a diagnosis of pre B-cell leukemia.
The patients, who had a mean age of 8.4 years at diagnosis, were treated with modern ALL protocols and were followed from diagnosis to the start of maintenance/continuation therapy.
Methotrexate toxicity was seen in 39 patients at the time of the analysis. Of those patients, 29 (74.4%) were Hispanic, Dr. Scheurer and his coauthors reported.
Compared with non-Hispanic whites, Hispanics had a high risk of methotrexate neurotoxicity, even after the researchers accounted for age, sex, ALL risk stratification, and other factors (adjusted hazard ratio, 2.43; 95% confidence interval, 1.06-5.58).
Among nine patients who experienced a second neurotoxic event, all were Hispanic.
Patients who had neurotoxicity received an average of 2.25 fewer doses of intrathecal methotrexate, and slightly lower intravenous methotrexate doses. About three-quarters of the patients experiencing methotrexate toxicity received leucovorin after intrathecal methotrexate, according to the investigators, who noted that leucovorin may interact with methotrexate and reduce efficacy.
“These findings may help us better understand what factors contribute to poorer survival among Hispanic patients with ALL,” wrote Dr. Scheurer and his coauthors.
Relapse occurred in 15.4% of patients with neurotoxicity (6 of 39 patients), and in 2.1% of patients with no neurotoxicity (13 of 241 patients).
Taken together, the findings add to the growing body of evidence that Hispanics and other minority pediatric patients with ALL experience “significant disparities” in treatment outcomes, according to the investigators.
That body of evidence includes several recent cases series that suggest Hispanic patients with ALL have a high prevalence of methotrexate neurotoxicity.
It remains unclear why Hispanic patients would have a higher risk of methotrexate toxicity, and that must be explored in future studies, the investigators said.
The research team is currently investigating biomarkers that may help identify patients at risk of methotrexate toxicity up front. “If we can identify these at-risk patients, we can potentially employ strategies to either fully prevent or mitigate these toxicities,” Dr. Scheurer said in a statement.
The research was supported by the National Institutes of Health and Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium, a St. Baldrick’s Foundation Consortium Research Grant. The researchers reported having no potential conflicts of interest.
SOURCE: Taylor OA et al. Clin Cancer Res. 2018 Sep 11. doi: 10.1158/1078-0432.CCR-18-0939.
Hispanic pediatric patients undergoing treatment for acute lymphoblastic leukemia (ALL) had a risk of methotrexate toxicity that was more than twice that of non-Hispanic whites, according to results of a prospective multicenter study.
Methotrexate toxicity often led to treatment modification or delays, which may have increased relapse risk in the Hispanic patients, according to investigator Michael E. Scheurer, PhD, MPH, of Baylor College of Medicine, Houston, and his colleagues.
“We had observed that our Hispanic patients tended to experience neurotoxicity more often than other groups, but we were surprised to see the magnitude of the difference,” Dr. Scheurer said in statement.
The study, described in Clinical Cancer Research, involved 280 patients with newly diagnosed ALL enrolled at one of three major U.S. pediatric cancer treatment centers. Nearly half of the patients (48.2%) were Hispanic, and approximately 86% had a diagnosis of pre B-cell leukemia.
The patients, who had a mean age of 8.4 years at diagnosis, were treated with modern ALL protocols and were followed from diagnosis to the start of maintenance/continuation therapy.
Methotrexate toxicity was seen in 39 patients at the time of the analysis. Of those patients, 29 (74.4%) were Hispanic, Dr. Scheurer and his coauthors reported.
Compared with non-Hispanic whites, Hispanics had a high risk of methotrexate neurotoxicity, even after the researchers accounted for age, sex, ALL risk stratification, and other factors (adjusted hazard ratio, 2.43; 95% confidence interval, 1.06-5.58).
Among nine patients who experienced a second neurotoxic event, all were Hispanic.
Patients who had neurotoxicity received an average of 2.25 fewer doses of intrathecal methotrexate, and slightly lower intravenous methotrexate doses. About three-quarters of the patients experiencing methotrexate toxicity received leucovorin after intrathecal methotrexate, according to the investigators, who noted that leucovorin may interact with methotrexate and reduce efficacy.
“These findings may help us better understand what factors contribute to poorer survival among Hispanic patients with ALL,” wrote Dr. Scheurer and his coauthors.
Relapse occurred in 15.4% of patients with neurotoxicity (6 of 39 patients), and in 2.1% of patients with no neurotoxicity (13 of 241 patients).
Taken together, the findings add to the growing body of evidence that Hispanics and other minority pediatric patients with ALL experience “significant disparities” in treatment outcomes, according to the investigators.
That body of evidence includes several recent cases series that suggest Hispanic patients with ALL have a high prevalence of methotrexate neurotoxicity.
It remains unclear why Hispanic patients would have a higher risk of methotrexate toxicity, and that must be explored in future studies, the investigators said.
The research team is currently investigating biomarkers that may help identify patients at risk of methotrexate toxicity up front. “If we can identify these at-risk patients, we can potentially employ strategies to either fully prevent or mitigate these toxicities,” Dr. Scheurer said in a statement.
The research was supported by the National Institutes of Health and Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium, a St. Baldrick’s Foundation Consortium Research Grant. The researchers reported having no potential conflicts of interest.
SOURCE: Taylor OA et al. Clin Cancer Res. 2018 Sep 11. doi: 10.1158/1078-0432.CCR-18-0939.
Hispanic pediatric patients undergoing treatment for acute lymphoblastic leukemia (ALL) had a risk of methotrexate toxicity that was more than twice that of non-Hispanic whites, according to results of a prospective multicenter study.
Methotrexate toxicity often led to treatment modification or delays, which may have increased relapse risk in the Hispanic patients, according to investigator Michael E. Scheurer, PhD, MPH, of Baylor College of Medicine, Houston, and his colleagues.
“We had observed that our Hispanic patients tended to experience neurotoxicity more often than other groups, but we were surprised to see the magnitude of the difference,” Dr. Scheurer said in statement.
The study, described in Clinical Cancer Research, involved 280 patients with newly diagnosed ALL enrolled at one of three major U.S. pediatric cancer treatment centers. Nearly half of the patients (48.2%) were Hispanic, and approximately 86% had a diagnosis of pre B-cell leukemia.
The patients, who had a mean age of 8.4 years at diagnosis, were treated with modern ALL protocols and were followed from diagnosis to the start of maintenance/continuation therapy.
Methotrexate toxicity was seen in 39 patients at the time of the analysis. Of those patients, 29 (74.4%) were Hispanic, Dr. Scheurer and his coauthors reported.
Compared with non-Hispanic whites, Hispanics had a high risk of methotrexate neurotoxicity, even after the researchers accounted for age, sex, ALL risk stratification, and other factors (adjusted hazard ratio, 2.43; 95% confidence interval, 1.06-5.58).
Among nine patients who experienced a second neurotoxic event, all were Hispanic.
Patients who had neurotoxicity received an average of 2.25 fewer doses of intrathecal methotrexate, and slightly lower intravenous methotrexate doses. About three-quarters of the patients experiencing methotrexate toxicity received leucovorin after intrathecal methotrexate, according to the investigators, who noted that leucovorin may interact with methotrexate and reduce efficacy.
“These findings may help us better understand what factors contribute to poorer survival among Hispanic patients with ALL,” wrote Dr. Scheurer and his coauthors.
Relapse occurred in 15.4% of patients with neurotoxicity (6 of 39 patients), and in 2.1% of patients with no neurotoxicity (13 of 241 patients).
Taken together, the findings add to the growing body of evidence that Hispanics and other minority pediatric patients with ALL experience “significant disparities” in treatment outcomes, according to the investigators.
That body of evidence includes several recent cases series that suggest Hispanic patients with ALL have a high prevalence of methotrexate neurotoxicity.
It remains unclear why Hispanic patients would have a higher risk of methotrexate toxicity, and that must be explored in future studies, the investigators said.
The research team is currently investigating biomarkers that may help identify patients at risk of methotrexate toxicity up front. “If we can identify these at-risk patients, we can potentially employ strategies to either fully prevent or mitigate these toxicities,” Dr. Scheurer said in a statement.
The research was supported by the National Institutes of Health and Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium, a St. Baldrick’s Foundation Consortium Research Grant. The researchers reported having no potential conflicts of interest.
SOURCE: Taylor OA et al. Clin Cancer Res. 2018 Sep 11. doi: 10.1158/1078-0432.CCR-18-0939.
FROM CLINICAL CANCER RESEARCH
Key clinical point:
Major finding: After researchers accounted for age, sex, ALL risk stratification, and other factors, the adjusted hazard ratio was 2.43 (95% CI, 1.06-5.58).
Study details: A prospective multicenter study of 280 patients with newly diagnosed ALL, nearly half of whom were Hispanic.
Disclosures: The research was supported by the National Institutes of Health and Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium, a St. Baldrick’s Foundation Consortium Research Grant. The study authors reported having no potential conflicts of interest.
Source: Taylor OA et al. Clin Cancer Res. 2018 Sep 11. doi: 10.1158/1078-0432.CCR-18-0939.
Novartis nabs first CAR T approval in Canada
the first chimeric antigen receptor (CAR) T-cell therapy to receive regulatory approval in Canada.
Tisagenlecleucel is approved to treat patients aged 3-25 years who have B-cell acute lymphoblastic leukemia (ALL) and relapsed after allogenic stem cell transplant (SCT) or are otherwise ineligible for SCT, have experienced second or later relapse, or have refractory disease.
Tisagenlecleucel is also approved in Canada to treat adults who have received two or more lines of systemic therapy and have relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high-grade B-cell lymphoma, or DLBCL arising from follicular lymphoma.
Novartis, the company marketing tisagenlecleucel, said it is working with qualified treatment centers in Canada to prepare for the delivery of tisagenlecleucel. Certification and training are underway at these centers and Novartis is enhancing manufacturing capacity to meet patient needs.
Tisagenlecleucel has been studied in a pair of phase 2 trials – JULIET and ELIANA.
JULIET enrolled 165 adults with relapsed/refractory DLBCL, 111 of whom received a single infusion of tisagenlecleucel.
The overall response rate was 52% and the complete response (CR) rate was 40%. The median duration of response was not reached with a median follow-up of 13.9 months. At last follow-up, none of the responders had gone on to SCT.
The 12-month overall survival (OS) rate was 49%; the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome.
These results were presented at the 2018 annual congress of the European Hematology Association in June.
The ELIANA trial included 75 children and young adults with relapsed/refractory ALL. All patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The overall remission rate was 81%, with 60% of patients achieving a CR and 21% achieving CR with incomplete hematologic recovery. All patients whose best response was CR with incomplete hematologic recovery were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to SCT while in remission. At last follow-up, four were still in remission, and four had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
About 95% of patients had adverse events thought to be related to tisagenlecleucel. The incidence of treatment-related grade 3/4 adverse eventss was 73% (N Engl J Med 2018; 378:439-48).
the first chimeric antigen receptor (CAR) T-cell therapy to receive regulatory approval in Canada.
Tisagenlecleucel is approved to treat patients aged 3-25 years who have B-cell acute lymphoblastic leukemia (ALL) and relapsed after allogenic stem cell transplant (SCT) or are otherwise ineligible for SCT, have experienced second or later relapse, or have refractory disease.
Tisagenlecleucel is also approved in Canada to treat adults who have received two or more lines of systemic therapy and have relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high-grade B-cell lymphoma, or DLBCL arising from follicular lymphoma.
Novartis, the company marketing tisagenlecleucel, said it is working with qualified treatment centers in Canada to prepare for the delivery of tisagenlecleucel. Certification and training are underway at these centers and Novartis is enhancing manufacturing capacity to meet patient needs.
Tisagenlecleucel has been studied in a pair of phase 2 trials – JULIET and ELIANA.
JULIET enrolled 165 adults with relapsed/refractory DLBCL, 111 of whom received a single infusion of tisagenlecleucel.
The overall response rate was 52% and the complete response (CR) rate was 40%. The median duration of response was not reached with a median follow-up of 13.9 months. At last follow-up, none of the responders had gone on to SCT.
The 12-month overall survival (OS) rate was 49%; the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome.
These results were presented at the 2018 annual congress of the European Hematology Association in June.
The ELIANA trial included 75 children and young adults with relapsed/refractory ALL. All patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The overall remission rate was 81%, with 60% of patients achieving a CR and 21% achieving CR with incomplete hematologic recovery. All patients whose best response was CR with incomplete hematologic recovery were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to SCT while in remission. At last follow-up, four were still in remission, and four had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
About 95% of patients had adverse events thought to be related to tisagenlecleucel. The incidence of treatment-related grade 3/4 adverse eventss was 73% (N Engl J Med 2018; 378:439-48).
the first chimeric antigen receptor (CAR) T-cell therapy to receive regulatory approval in Canada.
Tisagenlecleucel is approved to treat patients aged 3-25 years who have B-cell acute lymphoblastic leukemia (ALL) and relapsed after allogenic stem cell transplant (SCT) or are otherwise ineligible for SCT, have experienced second or later relapse, or have refractory disease.
Tisagenlecleucel is also approved in Canada to treat adults who have received two or more lines of systemic therapy and have relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high-grade B-cell lymphoma, or DLBCL arising from follicular lymphoma.
Novartis, the company marketing tisagenlecleucel, said it is working with qualified treatment centers in Canada to prepare for the delivery of tisagenlecleucel. Certification and training are underway at these centers and Novartis is enhancing manufacturing capacity to meet patient needs.
Tisagenlecleucel has been studied in a pair of phase 2 trials – JULIET and ELIANA.
JULIET enrolled 165 adults with relapsed/refractory DLBCL, 111 of whom received a single infusion of tisagenlecleucel.
The overall response rate was 52% and the complete response (CR) rate was 40%. The median duration of response was not reached with a median follow-up of 13.9 months. At last follow-up, none of the responders had gone on to SCT.
The 12-month overall survival (OS) rate was 49%; the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome.
These results were presented at the 2018 annual congress of the European Hematology Association in June.
The ELIANA trial included 75 children and young adults with relapsed/refractory ALL. All patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The overall remission rate was 81%, with 60% of patients achieving a CR and 21% achieving CR with incomplete hematologic recovery. All patients whose best response was CR with incomplete hematologic recovery were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to SCT while in remission. At last follow-up, four were still in remission, and four had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
About 95% of patients had adverse events thought to be related to tisagenlecleucel. The incidence of treatment-related grade 3/4 adverse eventss was 73% (N Engl J Med 2018; 378:439-48).
First CAR T-cell therapy approved in Canada
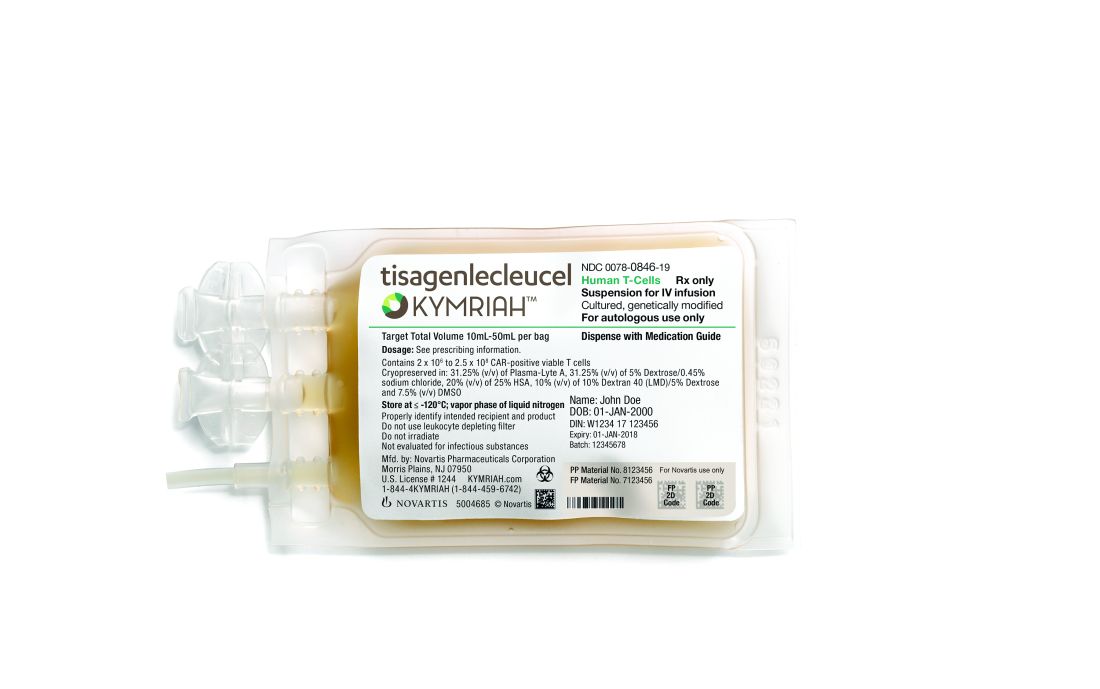
Health Canada has authorized use of tisagenlecleucel (Kymriah™), making it the first chimeric antigen receptor (CAR) T-cell therapy to receive regulatory approval in Canada.
Tisagenlecleucel (formerly CTL019) is approved to treat patients ages 3 to 25 with B-cell acute lymphoblastic leukemia (ALL) who have relapsed after allogeneic stem cell transplant (SCT) or are otherwise ineligible for SCT, have experienced second or later relapse, or have refractory disease.
Tisagenlecleucel is also approved in Canada to treat adults who have received two or more lines of systemic therapy and have relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma, or DLBCL arising from follicular lymphoma.
Novartis, the company marketing tisagenlecleucel, said it is working with qualified treatment centers in Canada to prepare for the delivery of tisagenlecleucel. Certification and training are underway at these centers, and Novartis is enhancing manufacturing capacity to meet patient needs.
Tisagenlecleucel has been studied in a pair of phase 2 trials—ELIANA and JULIET.
JULIET trial
JULIET enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The overall response rate was 52%, and the complete response (CR) rate was 40%. The median duration of response was not reached with a median follow-up of 13.9 months. At last follow-up, none of the responders had gone on to SCT.
The 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
These results were presented at the 23rd Annual Congress of the European Hematology Association in June (abstract S799).
ELIANA trial
ELIANA included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi. All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to SCT while in remission. At last follow-up, four were still in remission, and four had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
Ninety-five percent of patients had AEs thought to be related to tisagenlecleucel. The incidence of treatment-related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
These results were published in The New England Journal of Medicine in February.
Health Canada has authorized use of tisagenlecleucel (Kymriah™), making it the first chimeric antigen receptor (CAR) T-cell therapy to receive regulatory approval in Canada.
Tisagenlecleucel (formerly CTL019) is approved to treat patients ages 3 to 25 with B-cell acute lymphoblastic leukemia (ALL) who have relapsed after allogeneic stem cell transplant (SCT) or are otherwise ineligible for SCT, have experienced second or later relapse, or have refractory disease.
Tisagenlecleucel is also approved in Canada to treat adults who have received two or more lines of systemic therapy and have relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma, or DLBCL arising from follicular lymphoma.
Novartis, the company marketing tisagenlecleucel, said it is working with qualified treatment centers in Canada to prepare for the delivery of tisagenlecleucel. Certification and training are underway at these centers, and Novartis is enhancing manufacturing capacity to meet patient needs.
Tisagenlecleucel has been studied in a pair of phase 2 trials—ELIANA and JULIET.
JULIET trial
JULIET enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The overall response rate was 52%, and the complete response (CR) rate was 40%. The median duration of response was not reached with a median follow-up of 13.9 months. At last follow-up, none of the responders had gone on to SCT.
The 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
These results were presented at the 23rd Annual Congress of the European Hematology Association in June (abstract S799).
ELIANA trial
ELIANA included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi. All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to SCT while in remission. At last follow-up, four were still in remission, and four had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
Ninety-five percent of patients had AEs thought to be related to tisagenlecleucel. The incidence of treatment-related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
These results were published in The New England Journal of Medicine in February.
Health Canada has authorized use of tisagenlecleucel (Kymriah™), making it the first chimeric antigen receptor (CAR) T-cell therapy to receive regulatory approval in Canada.
Tisagenlecleucel (formerly CTL019) is approved to treat patients ages 3 to 25 with B-cell acute lymphoblastic leukemia (ALL) who have relapsed after allogeneic stem cell transplant (SCT) or are otherwise ineligible for SCT, have experienced second or later relapse, or have refractory disease.
Tisagenlecleucel is also approved in Canada to treat adults who have received two or more lines of systemic therapy and have relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma, or DLBCL arising from follicular lymphoma.
Novartis, the company marketing tisagenlecleucel, said it is working with qualified treatment centers in Canada to prepare for the delivery of tisagenlecleucel. Certification and training are underway at these centers, and Novartis is enhancing manufacturing capacity to meet patient needs.
Tisagenlecleucel has been studied in a pair of phase 2 trials—ELIANA and JULIET.
JULIET trial
JULIET enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The overall response rate was 52%, and the complete response (CR) rate was 40%. The median duration of response was not reached with a median follow-up of 13.9 months. At last follow-up, none of the responders had gone on to SCT.
The 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
These results were presented at the 23rd Annual Congress of the European Hematology Association in June (abstract S799).
ELIANA trial
ELIANA included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi. All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to SCT while in remission. At last follow-up, four were still in remission, and four had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
Ninety-five percent of patients had AEs thought to be related to tisagenlecleucel. The incidence of treatment-related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
These results were published in The New England Journal of Medicine in February.
England green-lights coverage of one CAR T-cell therapy
The National Health Service (NHS) of England has announced that tisagenlecleucel (Kymriah), a chimeric antigen receptor (CAR) T-cell therapy, will soon be available for certain leukemia patients.
, and patients could potentially begin receiving the treatment within weeks.
NHS England struck a deal with Novartis to lower the price of tisagenlecleucel, which costs around £282,000 per patient at its full list price. The discount offered to the NHS is confidential.
Tisagenlecleucel was recently approved by the European Commission (EC) to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
The EC also approved tisagenlecleucel to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
However, tisagenlecleucel will be available only for ALL patients in England, at least initially. A decision has not been made regarding funding for tisagenlecleucel in DLBCL, and Novartis previously decided to launch tisagenlecleucel in ALL first.
“It’s fantastic news for children and young people with this form of leukemia that CAR T-cell therapy will be made available on the NHS, making them the first in Europe to have routine access to this exciting new type of immunotherapy,” said Charles Swanton, Cancer Research UK’s chief clinician.
The first three NHS hospitals to go through the international accreditation process for the provision of tisagenlecleucel are in London, Manchester, and Newcastle. Subject to passing accreditation requirements, the first treatments could begin in a matter of weeks.
Another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta), has not fared as well as in England. The National Institute for Health and Care Excellence (NICE) recently issued draft guidance recommending against the use of axicabtagene ciloleucel in England.
Axicabtagene ciloleucel was approved by the EC to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy. However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. NICE also said the price of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of NHS resources, and the therapy does not meet the criteria for inclusion in the Cancer Drugs Fund.
The National Health Service (NHS) of England has announced that tisagenlecleucel (Kymriah), a chimeric antigen receptor (CAR) T-cell therapy, will soon be available for certain leukemia patients.
, and patients could potentially begin receiving the treatment within weeks.
NHS England struck a deal with Novartis to lower the price of tisagenlecleucel, which costs around £282,000 per patient at its full list price. The discount offered to the NHS is confidential.
Tisagenlecleucel was recently approved by the European Commission (EC) to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
The EC also approved tisagenlecleucel to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
However, tisagenlecleucel will be available only for ALL patients in England, at least initially. A decision has not been made regarding funding for tisagenlecleucel in DLBCL, and Novartis previously decided to launch tisagenlecleucel in ALL first.
“It’s fantastic news for children and young people with this form of leukemia that CAR T-cell therapy will be made available on the NHS, making them the first in Europe to have routine access to this exciting new type of immunotherapy,” said Charles Swanton, Cancer Research UK’s chief clinician.
The first three NHS hospitals to go through the international accreditation process for the provision of tisagenlecleucel are in London, Manchester, and Newcastle. Subject to passing accreditation requirements, the first treatments could begin in a matter of weeks.
Another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta), has not fared as well as in England. The National Institute for Health and Care Excellence (NICE) recently issued draft guidance recommending against the use of axicabtagene ciloleucel in England.
Axicabtagene ciloleucel was approved by the EC to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy. However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. NICE also said the price of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of NHS resources, and the therapy does not meet the criteria for inclusion in the Cancer Drugs Fund.
The National Health Service (NHS) of England has announced that tisagenlecleucel (Kymriah), a chimeric antigen receptor (CAR) T-cell therapy, will soon be available for certain leukemia patients.
, and patients could potentially begin receiving the treatment within weeks.
NHS England struck a deal with Novartis to lower the price of tisagenlecleucel, which costs around £282,000 per patient at its full list price. The discount offered to the NHS is confidential.
Tisagenlecleucel was recently approved by the European Commission (EC) to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
The EC also approved tisagenlecleucel to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
However, tisagenlecleucel will be available only for ALL patients in England, at least initially. A decision has not been made regarding funding for tisagenlecleucel in DLBCL, and Novartis previously decided to launch tisagenlecleucel in ALL first.
“It’s fantastic news for children and young people with this form of leukemia that CAR T-cell therapy will be made available on the NHS, making them the first in Europe to have routine access to this exciting new type of immunotherapy,” said Charles Swanton, Cancer Research UK’s chief clinician.
The first three NHS hospitals to go through the international accreditation process for the provision of tisagenlecleucel are in London, Manchester, and Newcastle. Subject to passing accreditation requirements, the first treatments could begin in a matter of weeks.
Another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta), has not fared as well as in England. The National Institute for Health and Care Excellence (NICE) recently issued draft guidance recommending against the use of axicabtagene ciloleucel in England.
Axicabtagene ciloleucel was approved by the EC to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy. However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. NICE also said the price of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of NHS resources, and the therapy does not meet the criteria for inclusion in the Cancer Drugs Fund.
Escalating MTX appears superior for T-ALL
Escalating methotrexate (MTX) may produce better outcomes than high-dose MTX in children and young adults with T-cell acute lymphoblastic leukemia (T-ALL), according to research published in the Journal of Clinical Oncology.
Researchers compared escalating and high-dose MTX given with the augmented Berlin-Frankfurt-Munster regimen in patients with T-ALL.
Disease-free survival (DFS) and overall survival (OS) rates were significantly higher among patients who received escalating MTX.
The improved survival outcomes in this trial, AALL0434, are the “opposite effect” of what was observed in a parallel trial, AALL0232. In that trial, high-dose MTX was superior to the escalating strategy in patients with B-cell acute lymphoblastic leukemia (B-ALL).
The parallel trial design was used because of the known differences between T-ALL and B-ALL in sensitivity to MTX and pegaspargase, according to investigator Stuart S. Winter, MD, of Children’s Minnesota Minneapolis Hospital, and his coauthors.
The AALL0434 study included 1,031 T-ALL patients between 1 and 31 years of age without CNS3 disease or testicular leukemia. They were randomized to post-induction therapy that included either escalating intravenous MTX or high-dose MTX.
The escalating regimen was superior to high-dose MTX, according to investigators.
The 5-year DFS rate was 91.5% with escalating MTX and 85.3% with high-dose MTX (P=0.005). The 5-year OS rate was 93.7% and 89.4%, respectively (P=0.036).
In contrast, the parallel AALL0232 study of B-ALL patients showed that high-dose MTX produced superior 5-year event-free survival and OS. This led Dr. Winter and his colleagues to speculate on how the findings could be reconciled.
Neither trial was a strict comparison of two different MTX schedules, due to differences in doses of pegaspargase, mercaptopurine, and vincristine between arms, as well as differences in the timing of cranial radiation therapy.
Of note, patients randomized to escalated MTX had two additional doses of pegaspargase. Enhanced asparagine depletion in that arm may have prevented relapse events, the investigators said.
They also said differences in adherence could have played a role, as the cost and time burden of the escalating MTX approach are “substantially less” than the high-dose approach.
The AALL0434 trial also included a second randomization to an addition of five, 6-day cycles of nelarabine versus no nelarabine. Results of that randomization, reported earlier this year, showed that nelarabine improved DFS.
AALL0434 was supported by grants from the National Institutes of Health and by St. Baldrick’s Foundation. Dr. Winter reported relationships with Amgen and Jazz Pharmaceuticals. His coauthors reported relationships with Novo Nordisk, Tandem, Pfizer, Novartis, and TypeZero Technologies, among others.
Escalating methotrexate (MTX) may produce better outcomes than high-dose MTX in children and young adults with T-cell acute lymphoblastic leukemia (T-ALL), according to research published in the Journal of Clinical Oncology.
Researchers compared escalating and high-dose MTX given with the augmented Berlin-Frankfurt-Munster regimen in patients with T-ALL.
Disease-free survival (DFS) and overall survival (OS) rates were significantly higher among patients who received escalating MTX.
The improved survival outcomes in this trial, AALL0434, are the “opposite effect” of what was observed in a parallel trial, AALL0232. In that trial, high-dose MTX was superior to the escalating strategy in patients with B-cell acute lymphoblastic leukemia (B-ALL).
The parallel trial design was used because of the known differences between T-ALL and B-ALL in sensitivity to MTX and pegaspargase, according to investigator Stuart S. Winter, MD, of Children’s Minnesota Minneapolis Hospital, and his coauthors.
The AALL0434 study included 1,031 T-ALL patients between 1 and 31 years of age without CNS3 disease or testicular leukemia. They were randomized to post-induction therapy that included either escalating intravenous MTX or high-dose MTX.
The escalating regimen was superior to high-dose MTX, according to investigators.
The 5-year DFS rate was 91.5% with escalating MTX and 85.3% with high-dose MTX (P=0.005). The 5-year OS rate was 93.7% and 89.4%, respectively (P=0.036).
In contrast, the parallel AALL0232 study of B-ALL patients showed that high-dose MTX produced superior 5-year event-free survival and OS. This led Dr. Winter and his colleagues to speculate on how the findings could be reconciled.
Neither trial was a strict comparison of two different MTX schedules, due to differences in doses of pegaspargase, mercaptopurine, and vincristine between arms, as well as differences in the timing of cranial radiation therapy.
Of note, patients randomized to escalated MTX had two additional doses of pegaspargase. Enhanced asparagine depletion in that arm may have prevented relapse events, the investigators said.
They also said differences in adherence could have played a role, as the cost and time burden of the escalating MTX approach are “substantially less” than the high-dose approach.
The AALL0434 trial also included a second randomization to an addition of five, 6-day cycles of nelarabine versus no nelarabine. Results of that randomization, reported earlier this year, showed that nelarabine improved DFS.
AALL0434 was supported by grants from the National Institutes of Health and by St. Baldrick’s Foundation. Dr. Winter reported relationships with Amgen and Jazz Pharmaceuticals. His coauthors reported relationships with Novo Nordisk, Tandem, Pfizer, Novartis, and TypeZero Technologies, among others.
Escalating methotrexate (MTX) may produce better outcomes than high-dose MTX in children and young adults with T-cell acute lymphoblastic leukemia (T-ALL), according to research published in the Journal of Clinical Oncology.
Researchers compared escalating and high-dose MTX given with the augmented Berlin-Frankfurt-Munster regimen in patients with T-ALL.
Disease-free survival (DFS) and overall survival (OS) rates were significantly higher among patients who received escalating MTX.
The improved survival outcomes in this trial, AALL0434, are the “opposite effect” of what was observed in a parallel trial, AALL0232. In that trial, high-dose MTX was superior to the escalating strategy in patients with B-cell acute lymphoblastic leukemia (B-ALL).
The parallel trial design was used because of the known differences between T-ALL and B-ALL in sensitivity to MTX and pegaspargase, according to investigator Stuart S. Winter, MD, of Children’s Minnesota Minneapolis Hospital, and his coauthors.
The AALL0434 study included 1,031 T-ALL patients between 1 and 31 years of age without CNS3 disease or testicular leukemia. They were randomized to post-induction therapy that included either escalating intravenous MTX or high-dose MTX.
The escalating regimen was superior to high-dose MTX, according to investigators.
The 5-year DFS rate was 91.5% with escalating MTX and 85.3% with high-dose MTX (P=0.005). The 5-year OS rate was 93.7% and 89.4%, respectively (P=0.036).
In contrast, the parallel AALL0232 study of B-ALL patients showed that high-dose MTX produced superior 5-year event-free survival and OS. This led Dr. Winter and his colleagues to speculate on how the findings could be reconciled.
Neither trial was a strict comparison of two different MTX schedules, due to differences in doses of pegaspargase, mercaptopurine, and vincristine between arms, as well as differences in the timing of cranial radiation therapy.
Of note, patients randomized to escalated MTX had two additional doses of pegaspargase. Enhanced asparagine depletion in that arm may have prevented relapse events, the investigators said.
They also said differences in adherence could have played a role, as the cost and time burden of the escalating MTX approach are “substantially less” than the high-dose approach.
The AALL0434 trial also included a second randomization to an addition of five, 6-day cycles of nelarabine versus no nelarabine. Results of that randomization, reported earlier this year, showed that nelarabine improved DFS.
AALL0434 was supported by grants from the National Institutes of Health and by St. Baldrick’s Foundation. Dr. Winter reported relationships with Amgen and Jazz Pharmaceuticals. His coauthors reported relationships with Novo Nordisk, Tandem, Pfizer, Novartis, and TypeZero Technologies, among others.
CAR T-cell therapy will soon be available in England, NHS says
The National Health Service (NHS) of England has announced that tisagenlecleucel (Kymriah®), a chimeric antigen receptor (CAR) T-cell therapy, will soon be available for certain leukemia patients.
Tisagenlecleucel will be made available through the Cancer Drugs Fund, and patients could potentially begin receiving the treatment within weeks.
NHS England struck a deal with Novartis to lower the price of tisagenlecleucel, which costs around £282,000 per patient at its full list price. The discount offered to the NHS is confidential.
Tisagenlecleucel was recently approved by the European Commission (EC) to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
The EC also approved tisagenlecleucel to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
However, tisagenlecleucel will only be available for ALL patients in England, at least initially. A decision has not been made regarding funding for tisagenlecleucel in DLBCL, and Novartis previously decided to launch tisagenlecleucel in ALL first.
“It’s fantastic news for children and young people with this form of leukemia that CAR T-cell therapy will be made available on the NHS, making them the first in Europe to have routine access to this exciting new type of immunotherapy,” said Charles Swanton, Cancer Research UK’s chief clinician.
The first three NHS hospitals to go through the international accreditation process for the provision of tisagenlecleucel are in London, Manchester, and Newcastle. Subject to passing accreditation requirements, the first treatments could begin in a matter of weeks.
Another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta®), has not fared as well as tisagenlecleucel. The National Institute for Health and Care Excellence (NICE) recently issued a draft guidance recommending against the use of axicabtagene ciloleucel in England.
Axicabtagene ciloleucel was approved by the EC to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. NICE also said the price of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of NHS resources, and axicabtagene ciloleucel does not meet the criteria for inclusion in the Cancer Drugs Fund.
The National Health Service (NHS) of England has announced that tisagenlecleucel (Kymriah®), a chimeric antigen receptor (CAR) T-cell therapy, will soon be available for certain leukemia patients.
Tisagenlecleucel will be made available through the Cancer Drugs Fund, and patients could potentially begin receiving the treatment within weeks.
NHS England struck a deal with Novartis to lower the price of tisagenlecleucel, which costs around £282,000 per patient at its full list price. The discount offered to the NHS is confidential.
Tisagenlecleucel was recently approved by the European Commission (EC) to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
The EC also approved tisagenlecleucel to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
However, tisagenlecleucel will only be available for ALL patients in England, at least initially. A decision has not been made regarding funding for tisagenlecleucel in DLBCL, and Novartis previously decided to launch tisagenlecleucel in ALL first.
“It’s fantastic news for children and young people with this form of leukemia that CAR T-cell therapy will be made available on the NHS, making them the first in Europe to have routine access to this exciting new type of immunotherapy,” said Charles Swanton, Cancer Research UK’s chief clinician.
The first three NHS hospitals to go through the international accreditation process for the provision of tisagenlecleucel are in London, Manchester, and Newcastle. Subject to passing accreditation requirements, the first treatments could begin in a matter of weeks.
Another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta®), has not fared as well as tisagenlecleucel. The National Institute for Health and Care Excellence (NICE) recently issued a draft guidance recommending against the use of axicabtagene ciloleucel in England.
Axicabtagene ciloleucel was approved by the EC to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. NICE also said the price of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of NHS resources, and axicabtagene ciloleucel does not meet the criteria for inclusion in the Cancer Drugs Fund.
The National Health Service (NHS) of England has announced that tisagenlecleucel (Kymriah®), a chimeric antigen receptor (CAR) T-cell therapy, will soon be available for certain leukemia patients.
Tisagenlecleucel will be made available through the Cancer Drugs Fund, and patients could potentially begin receiving the treatment within weeks.
NHS England struck a deal with Novartis to lower the price of tisagenlecleucel, which costs around £282,000 per patient at its full list price. The discount offered to the NHS is confidential.
Tisagenlecleucel was recently approved by the European Commission (EC) to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
The EC also approved tisagenlecleucel to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
However, tisagenlecleucel will only be available for ALL patients in England, at least initially. A decision has not been made regarding funding for tisagenlecleucel in DLBCL, and Novartis previously decided to launch tisagenlecleucel in ALL first.
“It’s fantastic news for children and young people with this form of leukemia that CAR T-cell therapy will be made available on the NHS, making them the first in Europe to have routine access to this exciting new type of immunotherapy,” said Charles Swanton, Cancer Research UK’s chief clinician.
The first three NHS hospitals to go through the international accreditation process for the provision of tisagenlecleucel are in London, Manchester, and Newcastle. Subject to passing accreditation requirements, the first treatments could begin in a matter of weeks.
Another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta®), has not fared as well as tisagenlecleucel. The National Institute for Health and Care Excellence (NICE) recently issued a draft guidance recommending against the use of axicabtagene ciloleucel in England.
Axicabtagene ciloleucel was approved by the EC to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. NICE also said the price of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of NHS resources, and axicabtagene ciloleucel does not meet the criteria for inclusion in the Cancer Drugs Fund.
Escalating methotrexate may improve survival in T-cell ALL
An escalating methotrexate strategy provided superior survival outcomes compared with high-dose methotrexate in a chemotherapy regimen for children and young adults with T-cell acute lymphoblastic leukemia (T-ALL), results of a large, randomized trial show.
There were also fewer relapses reported for escalating versus high-dose methotrexate in the study, which evaluated the effects of these two intensification strategies in patients receiving an augmented Berlin-Frankfurt-Muenster (ABFM) chemotherapy regimen.
These findings come from a report in the Journal of Clinical Oncology on the Children’s Oncology Group (COG) AALL0434 trial, which to the knowledge of the investigators is the largest T-ALL study ever conducted.
The improved survival outcomes in AALL0434 are the “opposite effect” of what was observed in a parallel trial, AALL0232, showing that high-dose methotrexate was superior to the escalating strategy in B-cell acute lymphoblastic leukemia (B-ALL), the authors reported.
The parallel trial design was in fact used because of the known differences between T-ALL and B-ALL in sensitivity to methotrexate and pegaspargase, according to investigator Stuart S. Winter, MD, of Children’s Minnesota Cancer and Blood Disorders Program, Minneapolis, and his coauthors.
“Although treatment intensification has improved survival for children with ALL, the best timing and sequence of key therapeutic interventions, such as asparaginase and methotrexate, which seem to be particularly important for T-ALL, remain unclear,” Dr. Winter and his colleagues said.
In the AALL0434 study, a total of 1,031 T-ALL patients between 1 and 31 years of age without CNS3 disease or testicular leukemia were randomized to postinduction therapy that included either the so-called Capizzi-style escalating intravenous methotrexate or high-dose methotrexate.
The escalating intravenous regimen was superior to high-dose methotrexate, according to investigators. Respectively, the 5-year rate of disease-free survival was 91.5% versus 85.3% (P = .005) and the 5-year rate of overall survival was 93.7% versus 89.4% (P = .036).
Relapses were observed in 32 patients receiving the escalating regimen, versus 59 for patients receiving high-dose methotrexate.
By contrast, the parallel AALL0232 study of B-ALL patients showed that high-dose methotrexate had superior 5-year event-free survival and overall survival, leading Dr. Winter and his colleagues to speculate on how the findings could be reconciled.
Neither trial was a strict comparison of two different methotrexate schedules, due to differences in doses of pegaspargase, 6-MP, and vincristine between arms, as well as differences in the timing of cranial radiation therapy.
Of note, patients randomized to escalated methotrexate had two additional doses of pegaspargase. As a result, enhanced asparagine depletion in that arm may have also prevented relapse events, the investigators said.
Differences in adherence could also have played a role, as the cost and time burden of the escalated approach are “substantially less” than the high-dose approach, they added.
The AALL0434 trial also included a second randomization to an addition of five, 6-day cycles of nelarabine versus no nelarabine. Results of that randomization, reported earlier this year, showed that nelarabine improved disease-free survival, including a 91% 4-year disease-free survival rate for patients receiving both nelarabine and escalating-dose methotrexate.
The study was supported by grants from the National Institutes of Health and by St. Baldrick’s Foundation. Dr. Winter reported relationships with Amgen and Jazz Pharmaceuticals. Study coauthors reported relationships with Novo Nordisk, Tandem, Pfizer, Novartis, and TypeZero Technologies, among others.
SOURCE: Winter SS et al. J Clin Oncol. 2018 Aug 23: doi: 10.1200/JCO.2018.77.7250.
An escalating methotrexate strategy provided superior survival outcomes compared with high-dose methotrexate in a chemotherapy regimen for children and young adults with T-cell acute lymphoblastic leukemia (T-ALL), results of a large, randomized trial show.
There were also fewer relapses reported for escalating versus high-dose methotrexate in the study, which evaluated the effects of these two intensification strategies in patients receiving an augmented Berlin-Frankfurt-Muenster (ABFM) chemotherapy regimen.
These findings come from a report in the Journal of Clinical Oncology on the Children’s Oncology Group (COG) AALL0434 trial, which to the knowledge of the investigators is the largest T-ALL study ever conducted.
The improved survival outcomes in AALL0434 are the “opposite effect” of what was observed in a parallel trial, AALL0232, showing that high-dose methotrexate was superior to the escalating strategy in B-cell acute lymphoblastic leukemia (B-ALL), the authors reported.
The parallel trial design was in fact used because of the known differences between T-ALL and B-ALL in sensitivity to methotrexate and pegaspargase, according to investigator Stuart S. Winter, MD, of Children’s Minnesota Cancer and Blood Disorders Program, Minneapolis, and his coauthors.
“Although treatment intensification has improved survival for children with ALL, the best timing and sequence of key therapeutic interventions, such as asparaginase and methotrexate, which seem to be particularly important for T-ALL, remain unclear,” Dr. Winter and his colleagues said.
In the AALL0434 study, a total of 1,031 T-ALL patients between 1 and 31 years of age without CNS3 disease or testicular leukemia were randomized to postinduction therapy that included either the so-called Capizzi-style escalating intravenous methotrexate or high-dose methotrexate.
The escalating intravenous regimen was superior to high-dose methotrexate, according to investigators. Respectively, the 5-year rate of disease-free survival was 91.5% versus 85.3% (P = .005) and the 5-year rate of overall survival was 93.7% versus 89.4% (P = .036).
Relapses were observed in 32 patients receiving the escalating regimen, versus 59 for patients receiving high-dose methotrexate.
By contrast, the parallel AALL0232 study of B-ALL patients showed that high-dose methotrexate had superior 5-year event-free survival and overall survival, leading Dr. Winter and his colleagues to speculate on how the findings could be reconciled.
Neither trial was a strict comparison of two different methotrexate schedules, due to differences in doses of pegaspargase, 6-MP, and vincristine between arms, as well as differences in the timing of cranial radiation therapy.
Of note, patients randomized to escalated methotrexate had two additional doses of pegaspargase. As a result, enhanced asparagine depletion in that arm may have also prevented relapse events, the investigators said.
Differences in adherence could also have played a role, as the cost and time burden of the escalated approach are “substantially less” than the high-dose approach, they added.
The AALL0434 trial also included a second randomization to an addition of five, 6-day cycles of nelarabine versus no nelarabine. Results of that randomization, reported earlier this year, showed that nelarabine improved disease-free survival, including a 91% 4-year disease-free survival rate for patients receiving both nelarabine and escalating-dose methotrexate.
The study was supported by grants from the National Institutes of Health and by St. Baldrick’s Foundation. Dr. Winter reported relationships with Amgen and Jazz Pharmaceuticals. Study coauthors reported relationships with Novo Nordisk, Tandem, Pfizer, Novartis, and TypeZero Technologies, among others.
SOURCE: Winter SS et al. J Clin Oncol. 2018 Aug 23: doi: 10.1200/JCO.2018.77.7250.
An escalating methotrexate strategy provided superior survival outcomes compared with high-dose methotrexate in a chemotherapy regimen for children and young adults with T-cell acute lymphoblastic leukemia (T-ALL), results of a large, randomized trial show.
There were also fewer relapses reported for escalating versus high-dose methotrexate in the study, which evaluated the effects of these two intensification strategies in patients receiving an augmented Berlin-Frankfurt-Muenster (ABFM) chemotherapy regimen.
These findings come from a report in the Journal of Clinical Oncology on the Children’s Oncology Group (COG) AALL0434 trial, which to the knowledge of the investigators is the largest T-ALL study ever conducted.
The improved survival outcomes in AALL0434 are the “opposite effect” of what was observed in a parallel trial, AALL0232, showing that high-dose methotrexate was superior to the escalating strategy in B-cell acute lymphoblastic leukemia (B-ALL), the authors reported.
The parallel trial design was in fact used because of the known differences between T-ALL and B-ALL in sensitivity to methotrexate and pegaspargase, according to investigator Stuart S. Winter, MD, of Children’s Minnesota Cancer and Blood Disorders Program, Minneapolis, and his coauthors.
“Although treatment intensification has improved survival for children with ALL, the best timing and sequence of key therapeutic interventions, such as asparaginase and methotrexate, which seem to be particularly important for T-ALL, remain unclear,” Dr. Winter and his colleagues said.
In the AALL0434 study, a total of 1,031 T-ALL patients between 1 and 31 years of age without CNS3 disease or testicular leukemia were randomized to postinduction therapy that included either the so-called Capizzi-style escalating intravenous methotrexate or high-dose methotrexate.
The escalating intravenous regimen was superior to high-dose methotrexate, according to investigators. Respectively, the 5-year rate of disease-free survival was 91.5% versus 85.3% (P = .005) and the 5-year rate of overall survival was 93.7% versus 89.4% (P = .036).
Relapses were observed in 32 patients receiving the escalating regimen, versus 59 for patients receiving high-dose methotrexate.
By contrast, the parallel AALL0232 study of B-ALL patients showed that high-dose methotrexate had superior 5-year event-free survival and overall survival, leading Dr. Winter and his colleagues to speculate on how the findings could be reconciled.
Neither trial was a strict comparison of two different methotrexate schedules, due to differences in doses of pegaspargase, 6-MP, and vincristine between arms, as well as differences in the timing of cranial radiation therapy.
Of note, patients randomized to escalated methotrexate had two additional doses of pegaspargase. As a result, enhanced asparagine depletion in that arm may have also prevented relapse events, the investigators said.
Differences in adherence could also have played a role, as the cost and time burden of the escalated approach are “substantially less” than the high-dose approach, they added.
The AALL0434 trial also included a second randomization to an addition of five, 6-day cycles of nelarabine versus no nelarabine. Results of that randomization, reported earlier this year, showed that nelarabine improved disease-free survival, including a 91% 4-year disease-free survival rate for patients receiving both nelarabine and escalating-dose methotrexate.
The study was supported by grants from the National Institutes of Health and by St. Baldrick’s Foundation. Dr. Winter reported relationships with Amgen and Jazz Pharmaceuticals. Study coauthors reported relationships with Novo Nordisk, Tandem, Pfizer, Novartis, and TypeZero Technologies, among others.
SOURCE: Winter SS et al. J Clin Oncol. 2018 Aug 23: doi: 10.1200/JCO.2018.77.7250.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: An (T-ALL).
Major finding: The 5-year disease-free survival rate was 91.5% versus 85.3% (P = .005) and overall survival was 93.7% versus 89.4% (P = .036), respectively, for the escalating and high-dose approaches.
Study details: Results after methotrexate randomization in 1,031 T-ALL patients without CNS3 disease or testicular leukemia in the Children’s Oncology Group (COG) AALL0434 trial.
Disclosures: The study was supported by grants from the National Institutes of Health and by St. Baldrick’s Foundation. The authors reported disclosures related to Amgen, Jazz Pharmaceuticals, Novo Nordisk, Tandem, Pfizer, Novartis, and TypeZero Technologies, among others.
Source: Winter SS et al. J Clin Oncol. 2018 Aug 23. doi: 10.1200/JCO.2018.77.7250.
Blinatumomab gains European approval in children
The a bispecific, CD19-directed, CD3 T-cell engager immunotherapy.
Blinatumomab is now approved as monotherapy for children aged 1 year or older who have relapsed/refractory, Philadelphia chromosome–negative, CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL). The patients must have received at least two prior therapies, or they must have relapsed after allogeneic hematopoietic stem cell transplant.
The European Commission’s (EC) new approval of blinatumomab extends to all countries in the European Union, as well as Norway, Iceland, and Liechtenstein.
In 2015, the EC approved blinatumomab to treat adults with Philadelphia chromosome–negative, relapsed/refractory B-cell precursor ALL.The EC’s approval of blinatumomab in pediatric patients is based on results from a phase 1/2 study published in the Journal of Clinical Oncology in 2016. The study included 93 pediatric patients with relapsed/refractory B-cell precursor ALL. Patients received blinatumomab as a continuous intravenous infusion – 49 patients in the phase 1 portion of the trial and 44 in phase 2. The patients were followed for 2 years.
There were four dose-limiting toxicities during the phase 1 portion of the trial, two of which were fatal. Three patients had grade 4 cytokine release syndrome (CRS), one had grade 5 cardiac failure (as well as grade 4 CRS), and one had grade 5 respiratory failure. Based on the dose-limiting toxicities, the maximum tolerated dose of blinatumomab was 15 mcg/m2 per day, but a stepwise dosage was recommended to reduce the risk of CRS.
The recommended dose was 5 mcg/m2 per day on days 1-7 and 15 mcg/m2 per day on days 8-28 for cycle 1, and 15 mcg/m2 per day on days 1-28 for subsequent cycles, according to the study results.Among the 70 patients who received the recommended dose of blinatumomab, 27 (39%) achieved a complete response within the first two cycles. A total of 14 of these patients (52%) achieved minimal residual disease negativity.
The a bispecific, CD19-directed, CD3 T-cell engager immunotherapy.
Blinatumomab is now approved as monotherapy for children aged 1 year or older who have relapsed/refractory, Philadelphia chromosome–negative, CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL). The patients must have received at least two prior therapies, or they must have relapsed after allogeneic hematopoietic stem cell transplant.
The European Commission’s (EC) new approval of blinatumomab extends to all countries in the European Union, as well as Norway, Iceland, and Liechtenstein.
In 2015, the EC approved blinatumomab to treat adults with Philadelphia chromosome–negative, relapsed/refractory B-cell precursor ALL.The EC’s approval of blinatumomab in pediatric patients is based on results from a phase 1/2 study published in the Journal of Clinical Oncology in 2016. The study included 93 pediatric patients with relapsed/refractory B-cell precursor ALL. Patients received blinatumomab as a continuous intravenous infusion – 49 patients in the phase 1 portion of the trial and 44 in phase 2. The patients were followed for 2 years.
There were four dose-limiting toxicities during the phase 1 portion of the trial, two of which were fatal. Three patients had grade 4 cytokine release syndrome (CRS), one had grade 5 cardiac failure (as well as grade 4 CRS), and one had grade 5 respiratory failure. Based on the dose-limiting toxicities, the maximum tolerated dose of blinatumomab was 15 mcg/m2 per day, but a stepwise dosage was recommended to reduce the risk of CRS.
The recommended dose was 5 mcg/m2 per day on days 1-7 and 15 mcg/m2 per day on days 8-28 for cycle 1, and 15 mcg/m2 per day on days 1-28 for subsequent cycles, according to the study results.Among the 70 patients who received the recommended dose of blinatumomab, 27 (39%) achieved a complete response within the first two cycles. A total of 14 of these patients (52%) achieved minimal residual disease negativity.
The a bispecific, CD19-directed, CD3 T-cell engager immunotherapy.
Blinatumomab is now approved as monotherapy for children aged 1 year or older who have relapsed/refractory, Philadelphia chromosome–negative, CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL). The patients must have received at least two prior therapies, or they must have relapsed after allogeneic hematopoietic stem cell transplant.
The European Commission’s (EC) new approval of blinatumomab extends to all countries in the European Union, as well as Norway, Iceland, and Liechtenstein.
In 2015, the EC approved blinatumomab to treat adults with Philadelphia chromosome–negative, relapsed/refractory B-cell precursor ALL.The EC’s approval of blinatumomab in pediatric patients is based on results from a phase 1/2 study published in the Journal of Clinical Oncology in 2016. The study included 93 pediatric patients with relapsed/refractory B-cell precursor ALL. Patients received blinatumomab as a continuous intravenous infusion – 49 patients in the phase 1 portion of the trial and 44 in phase 2. The patients were followed for 2 years.
There were four dose-limiting toxicities during the phase 1 portion of the trial, two of which were fatal. Three patients had grade 4 cytokine release syndrome (CRS), one had grade 5 cardiac failure (as well as grade 4 CRS), and one had grade 5 respiratory failure. Based on the dose-limiting toxicities, the maximum tolerated dose of blinatumomab was 15 mcg/m2 per day, but a stepwise dosage was recommended to reduce the risk of CRS.
The recommended dose was 5 mcg/m2 per day on days 1-7 and 15 mcg/m2 per day on days 8-28 for cycle 1, and 15 mcg/m2 per day on days 1-28 for subsequent cycles, according to the study results.Among the 70 patients who received the recommended dose of blinatumomab, 27 (39%) achieved a complete response within the first two cycles. A total of 14 of these patients (52%) achieved minimal residual disease negativity.
EC approves blinatumomab for kids
The European Commission (EC) has expanded the approved indication for blinatumomab (Blincyto®), a bispecific, CD19-directed, CD3 T-cell engager immunotherapy.
Blinatumomab is now approved as monotherapy for pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL).
The patients must have received at least two prior therapies, or they must have relapsed after allogeneic hematopoietic stem cell transplant.
This approval of blinatumomab extends to all countries in the European Union, as well as Norway, Iceland, and Liechtenstein.
The EC previously approved blinatumomab to treat adults with Philadelphia chromosome-negative, relapsed/refractory B-cell precursor ALL.
’205 study
The EC’s approval of blinatumomab in pediatric patients is based on results from the phase 1/2 ’205 study, which were published in the Journal of Clinical Oncology in 2016.
The study included 93 pediatric patients with relapsed/refractory B-cell precursor ALL. Patients received blinatumomab as a continuous intravenous infusion—49 patients in the phase 1 portion of the trial and 44 in phase 2. The patients were followed for 2 years.
There were 4 dose-limiting toxicities (DLTs) during the phase 1 portion of the trial, and 2 DLTs were fatal. Three patients had grade 4 cytokine release syndrome (CRS), one had grade 5 cardiac failure (as well as grade 4 CRS), and one had grade 5 respiratory failure.
Recommended dose
Based on the DLTs, the maximum-tolerated dose of blinatumomab was 15 µg/m2/day, but a step-wise dosage was recommended to reduce the risk of CRS. The recommended dose was 5 μg/m2/day on days 1-7 and 15 μg/m2/day on days 8-28 for cycle 1, and 15 μg/m2/day on days 1-28 for subsequent cycles.
Dose adjustment was possible in case of adverse events (AEs). Patients who responded to blinatumomab but later relapsed had the option to be retreated with blinatumomab.
Seventy patients received at least one infusion of blinatumomab at the recommended dose. The median number of treatment cycles was 1 (range, 1 to 5).
The patients’ median age was 8 years (range, 7 months to 17 years). Forty patients (57%) had undergone allogeneic transplant prior to receiving blinatumomab, and 39 (56%) had refractory disease.
Safety
The most common AEs among patients who received the recommended dose of blinatumomab were pyrexia (80%), anemia (41%), nausea (33%), and headache (30%).
The most frequent grade 3 or higher AEs were anemia (36%), thrombocytopenia (21%), febrile neutropenia (17%), hypokalemia (17%), and neutropenia (17%).
Eight patients developed CRS. Three had grade 3 and one had grade 4 CRS.
Ten patients (14%) had treatment interruptions due to AEs, and 4 (6%) discontinued treatment permanently because of AEs.
Six patients had fatal AEs. Three died after they went on to allogeneic transplant—one of multiorgan failure, one of sepsis, and one of respiratory failure. The three other deaths were due to fungal infection, multiorgan failure, and thrombocytopenia.
Response and follow-up
Among the 70 patients who received the recommended dose of blinatumomab, 27 (39%) achieved a complete response (CR) within the first 2 cycles. Fourteen of these patients (52%) achieved minimal residual disease negativity.
Thirteen of the 27 patients (48%) who achieved a CR went on to receive an allogeneic transplant.
At the end of the 2-year follow-up, 4 of the 27 complete responders were still in remission.
Two of the patients had relapsed but were still alive, three had withdrawn consent (one in CR and two after relapse), three had died in CR after transplant, and 15 had relapsed and died.
Of the 43 patients who did not achieve a CR within the first two treatment cycles, 8 were still alive at the end of the 2-year follow-up.
The European Commission (EC) has expanded the approved indication for blinatumomab (Blincyto®), a bispecific, CD19-directed, CD3 T-cell engager immunotherapy.
Blinatumomab is now approved as monotherapy for pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL).
The patients must have received at least two prior therapies, or they must have relapsed after allogeneic hematopoietic stem cell transplant.
This approval of blinatumomab extends to all countries in the European Union, as well as Norway, Iceland, and Liechtenstein.
The EC previously approved blinatumomab to treat adults with Philadelphia chromosome-negative, relapsed/refractory B-cell precursor ALL.
’205 study
The EC’s approval of blinatumomab in pediatric patients is based on results from the phase 1/2 ’205 study, which were published in the Journal of Clinical Oncology in 2016.
The study included 93 pediatric patients with relapsed/refractory B-cell precursor ALL. Patients received blinatumomab as a continuous intravenous infusion—49 patients in the phase 1 portion of the trial and 44 in phase 2. The patients were followed for 2 years.
There were 4 dose-limiting toxicities (DLTs) during the phase 1 portion of the trial, and 2 DLTs were fatal. Three patients had grade 4 cytokine release syndrome (CRS), one had grade 5 cardiac failure (as well as grade 4 CRS), and one had grade 5 respiratory failure.
Recommended dose
Based on the DLTs, the maximum-tolerated dose of blinatumomab was 15 µg/m2/day, but a step-wise dosage was recommended to reduce the risk of CRS. The recommended dose was 5 μg/m2/day on days 1-7 and 15 μg/m2/day on days 8-28 for cycle 1, and 15 μg/m2/day on days 1-28 for subsequent cycles.
Dose adjustment was possible in case of adverse events (AEs). Patients who responded to blinatumomab but later relapsed had the option to be retreated with blinatumomab.
Seventy patients received at least one infusion of blinatumomab at the recommended dose. The median number of treatment cycles was 1 (range, 1 to 5).
The patients’ median age was 8 years (range, 7 months to 17 years). Forty patients (57%) had undergone allogeneic transplant prior to receiving blinatumomab, and 39 (56%) had refractory disease.
Safety
The most common AEs among patients who received the recommended dose of blinatumomab were pyrexia (80%), anemia (41%), nausea (33%), and headache (30%).
The most frequent grade 3 or higher AEs were anemia (36%), thrombocytopenia (21%), febrile neutropenia (17%), hypokalemia (17%), and neutropenia (17%).
Eight patients developed CRS. Three had grade 3 and one had grade 4 CRS.
Ten patients (14%) had treatment interruptions due to AEs, and 4 (6%) discontinued treatment permanently because of AEs.
Six patients had fatal AEs. Three died after they went on to allogeneic transplant—one of multiorgan failure, one of sepsis, and one of respiratory failure. The three other deaths were due to fungal infection, multiorgan failure, and thrombocytopenia.
Response and follow-up
Among the 70 patients who received the recommended dose of blinatumomab, 27 (39%) achieved a complete response (CR) within the first 2 cycles. Fourteen of these patients (52%) achieved minimal residual disease negativity.
Thirteen of the 27 patients (48%) who achieved a CR went on to receive an allogeneic transplant.
At the end of the 2-year follow-up, 4 of the 27 complete responders were still in remission.
Two of the patients had relapsed but were still alive, three had withdrawn consent (one in CR and two after relapse), three had died in CR after transplant, and 15 had relapsed and died.
Of the 43 patients who did not achieve a CR within the first two treatment cycles, 8 were still alive at the end of the 2-year follow-up.
The European Commission (EC) has expanded the approved indication for blinatumomab (Blincyto®), a bispecific, CD19-directed, CD3 T-cell engager immunotherapy.
Blinatumomab is now approved as monotherapy for pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL).
The patients must have received at least two prior therapies, or they must have relapsed after allogeneic hematopoietic stem cell transplant.
This approval of blinatumomab extends to all countries in the European Union, as well as Norway, Iceland, and Liechtenstein.
The EC previously approved blinatumomab to treat adults with Philadelphia chromosome-negative, relapsed/refractory B-cell precursor ALL.
’205 study
The EC’s approval of blinatumomab in pediatric patients is based on results from the phase 1/2 ’205 study, which were published in the Journal of Clinical Oncology in 2016.
The study included 93 pediatric patients with relapsed/refractory B-cell precursor ALL. Patients received blinatumomab as a continuous intravenous infusion—49 patients in the phase 1 portion of the trial and 44 in phase 2. The patients were followed for 2 years.
There were 4 dose-limiting toxicities (DLTs) during the phase 1 portion of the trial, and 2 DLTs were fatal. Three patients had grade 4 cytokine release syndrome (CRS), one had grade 5 cardiac failure (as well as grade 4 CRS), and one had grade 5 respiratory failure.
Recommended dose
Based on the DLTs, the maximum-tolerated dose of blinatumomab was 15 µg/m2/day, but a step-wise dosage was recommended to reduce the risk of CRS. The recommended dose was 5 μg/m2/day on days 1-7 and 15 μg/m2/day on days 8-28 for cycle 1, and 15 μg/m2/day on days 1-28 for subsequent cycles.
Dose adjustment was possible in case of adverse events (AEs). Patients who responded to blinatumomab but later relapsed had the option to be retreated with blinatumomab.
Seventy patients received at least one infusion of blinatumomab at the recommended dose. The median number of treatment cycles was 1 (range, 1 to 5).
The patients’ median age was 8 years (range, 7 months to 17 years). Forty patients (57%) had undergone allogeneic transplant prior to receiving blinatumomab, and 39 (56%) had refractory disease.
Safety
The most common AEs among patients who received the recommended dose of blinatumomab were pyrexia (80%), anemia (41%), nausea (33%), and headache (30%).
The most frequent grade 3 or higher AEs were anemia (36%), thrombocytopenia (21%), febrile neutropenia (17%), hypokalemia (17%), and neutropenia (17%).
Eight patients developed CRS. Three had grade 3 and one had grade 4 CRS.
Ten patients (14%) had treatment interruptions due to AEs, and 4 (6%) discontinued treatment permanently because of AEs.
Six patients had fatal AEs. Three died after they went on to allogeneic transplant—one of multiorgan failure, one of sepsis, and one of respiratory failure. The three other deaths were due to fungal infection, multiorgan failure, and thrombocytopenia.
Response and follow-up
Among the 70 patients who received the recommended dose of blinatumomab, 27 (39%) achieved a complete response (CR) within the first 2 cycles. Fourteen of these patients (52%) achieved minimal residual disease negativity.
Thirteen of the 27 patients (48%) who achieved a CR went on to receive an allogeneic transplant.
At the end of the 2-year follow-up, 4 of the 27 complete responders were still in remission.
Two of the patients had relapsed but were still alive, three had withdrawn consent (one in CR and two after relapse), three had died in CR after transplant, and 15 had relapsed and died.
Of the 43 patients who did not achieve a CR within the first two treatment cycles, 8 were still alive at the end of the 2-year follow-up.
European Commission approves first CAR T-cell therapies
The , two chimeric antigen receptor (CAR) T-cell therapies.
Tisagenlecleucel is now approved for use in pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
Tisagenlecleucel is also approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
Axicabtagene ciloleucel is approved for adults with relapsed/refractory DLBCL and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy. The treatment is marketed by Kite, a Gilead company.
The axicabtagene ciloleucel approval is based on results from the single arm, ZUMA-1 trial. During the study of 101 patients who received a single infusion, 72% responded to therapy and 51% achieved a complete response. At 1 year, median overall survival had not been reached.
Novartis expects to launch tisagenlecleucel initially for pediatric ALL. The company said timing for tisagenlecleucel availability in each country will depend on multiple factors, including the onboarding of qualified treatment centers for the appropriate indications, as well as the completion of national reimbursement procedures.
The EC’s approval of tisagenlecleucel is based on results from the phase 2 JULIET and ELIANA trials.
Updated results from JULIET were presented at the annual congress of the European Hematology Association in June 2018. The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so because of disease progression or clinical deterioration.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. At the time of data cutoff, none of the responders had gone on to receive a stem cell transplant.
Updated results from ELIANA were published in New England Journal of Medicine (2018;378:439-48).
The trial included 75 children and young adults with relapsed/refractory ALL. The overall remission rate was 81% (61/75), with 60% of patients (n = 45) achieving a complete remission (CR) and 21% (n = 16) achieving a CR with incomplete hematologic recovery (CRi).
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
The , two chimeric antigen receptor (CAR) T-cell therapies.
Tisagenlecleucel is now approved for use in pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
Tisagenlecleucel is also approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
Axicabtagene ciloleucel is approved for adults with relapsed/refractory DLBCL and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy. The treatment is marketed by Kite, a Gilead company.
The axicabtagene ciloleucel approval is based on results from the single arm, ZUMA-1 trial. During the study of 101 patients who received a single infusion, 72% responded to therapy and 51% achieved a complete response. At 1 year, median overall survival had not been reached.
Novartis expects to launch tisagenlecleucel initially for pediatric ALL. The company said timing for tisagenlecleucel availability in each country will depend on multiple factors, including the onboarding of qualified treatment centers for the appropriate indications, as well as the completion of national reimbursement procedures.
The EC’s approval of tisagenlecleucel is based on results from the phase 2 JULIET and ELIANA trials.
Updated results from JULIET were presented at the annual congress of the European Hematology Association in June 2018. The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so because of disease progression or clinical deterioration.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. At the time of data cutoff, none of the responders had gone on to receive a stem cell transplant.
Updated results from ELIANA were published in New England Journal of Medicine (2018;378:439-48).
The trial included 75 children and young adults with relapsed/refractory ALL. The overall remission rate was 81% (61/75), with 60% of patients (n = 45) achieving a complete remission (CR) and 21% (n = 16) achieving a CR with incomplete hematologic recovery (CRi).
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
The , two chimeric antigen receptor (CAR) T-cell therapies.
Tisagenlecleucel is now approved for use in pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
Tisagenlecleucel is also approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
Axicabtagene ciloleucel is approved for adults with relapsed/refractory DLBCL and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy. The treatment is marketed by Kite, a Gilead company.
The axicabtagene ciloleucel approval is based on results from the single arm, ZUMA-1 trial. During the study of 101 patients who received a single infusion, 72% responded to therapy and 51% achieved a complete response. At 1 year, median overall survival had not been reached.
Novartis expects to launch tisagenlecleucel initially for pediatric ALL. The company said timing for tisagenlecleucel availability in each country will depend on multiple factors, including the onboarding of qualified treatment centers for the appropriate indications, as well as the completion of national reimbursement procedures.
The EC’s approval of tisagenlecleucel is based on results from the phase 2 JULIET and ELIANA trials.
Updated results from JULIET were presented at the annual congress of the European Hematology Association in June 2018. The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so because of disease progression or clinical deterioration.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. At the time of data cutoff, none of the responders had gone on to receive a stem cell transplant.
Updated results from ELIANA were published in New England Journal of Medicine (2018;378:439-48).
The trial included 75 children and young adults with relapsed/refractory ALL. The overall remission rate was 81% (61/75), with 60% of patients (n = 45) achieving a complete remission (CR) and 21% (n = 16) achieving a CR with incomplete hematologic recovery (CRi).
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.