User login
Product Update: Sureglide Cesarean Scalpel; Viveve Medical; NovaSure ADVANCED
SAFER CESAREAN SCALPEL
FOR MORE INFORMATION, VISIT: www.sureglide.info
RF ENERGY TO IMPROVE SEXUAL FUNCTION
According to Viveve Medical, results of the recent Viveve I multicenter, blinded, randomized, sham controlled study showed no serious adverse effects plus improvement in arousal and/or orgasm self-reported by 9 of 10 women who noted vaginal laxity and sexual dysfunction following vaginal childbirth.
FOR MORE INFORMATION, VISIT: www.viveve.com
NEXT-GENERATION ENDOMETRIAL ABLATION
FOR MORE INFORMATION, VISIT: www.novasure.com
SAFER CESAREAN SCALPEL
FOR MORE INFORMATION, VISIT: www.sureglide.info
RF ENERGY TO IMPROVE SEXUAL FUNCTION
According to Viveve Medical, results of the recent Viveve I multicenter, blinded, randomized, sham controlled study showed no serious adverse effects plus improvement in arousal and/or orgasm self-reported by 9 of 10 women who noted vaginal laxity and sexual dysfunction following vaginal childbirth.
FOR MORE INFORMATION, VISIT: www.viveve.com
NEXT-GENERATION ENDOMETRIAL ABLATION
FOR MORE INFORMATION, VISIT: www.novasure.com
SAFER CESAREAN SCALPEL
FOR MORE INFORMATION, VISIT: www.sureglide.info
RF ENERGY TO IMPROVE SEXUAL FUNCTION
According to Viveve Medical, results of the recent Viveve I multicenter, blinded, randomized, sham controlled study showed no serious adverse effects plus improvement in arousal and/or orgasm self-reported by 9 of 10 women who noted vaginal laxity and sexual dysfunction following vaginal childbirth.
FOR MORE INFORMATION, VISIT: www.viveve.com
NEXT-GENERATION ENDOMETRIAL ABLATION
FOR MORE INFORMATION, VISIT: www.novasure.com
Product News: January 2017
Enbrel
Amgen Inc announces that the US Food and Drug Administration has approved the supplemental Biologics License Application for the expanded use of Enbrel (etanercept) for the treatment of moderate to severe plaque psoriasis in pediatric patients (aged 4–17 years). Enbrel, a tumor necrosis factor blocker, was approved for the treatment of moderate to severe plaque psoriasis in adults in 2004. For more information, visit www.enbrel.com.
Eucrisa
Pfizer Inc announces US Food and Drug Administration approval of Eucrisa (crisaborole) ointment 2% for the treatment of mild to moderate atopic dermatitis in patients 2 years and older. Eucrisa is a nonsteroidal topical phosphodiesterase 4 inhibitor and is applied twice daily. This approval provides patients with atopic dermatitis another treatment alternative, as this community has not had a new prescription treatment for more than 10 years. For more information, visit www.pfizer.com.
Isdinceutics
Isdin based in Spain has launched the Isdinceutics line of physician-dispensed cosmeceuticals to the US market, which focuses on vitamins and hydrators rather than chemicals to rejuvenate the skin. Isdinceutics features a daily antioxidant routine with Flavo-C Ultraglican and Flavo-C Serum to reduce the appearance of microwrinkles and elevate the skin’s natural moisture production. Products to correct pigmentation problems as well as undereye circles and puffiness also are
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@frontlinemedcom.com.
Enbrel
Amgen Inc announces that the US Food and Drug Administration has approved the supplemental Biologics License Application for the expanded use of Enbrel (etanercept) for the treatment of moderate to severe plaque psoriasis in pediatric patients (aged 4–17 years). Enbrel, a tumor necrosis factor blocker, was approved for the treatment of moderate to severe plaque psoriasis in adults in 2004. For more information, visit www.enbrel.com.
Eucrisa
Pfizer Inc announces US Food and Drug Administration approval of Eucrisa (crisaborole) ointment 2% for the treatment of mild to moderate atopic dermatitis in patients 2 years and older. Eucrisa is a nonsteroidal topical phosphodiesterase 4 inhibitor and is applied twice daily. This approval provides patients with atopic dermatitis another treatment alternative, as this community has not had a new prescription treatment for more than 10 years. For more information, visit www.pfizer.com.
Isdinceutics
Isdin based in Spain has launched the Isdinceutics line of physician-dispensed cosmeceuticals to the US market, which focuses on vitamins and hydrators rather than chemicals to rejuvenate the skin. Isdinceutics features a daily antioxidant routine with Flavo-C Ultraglican and Flavo-C Serum to reduce the appearance of microwrinkles and elevate the skin’s natural moisture production. Products to correct pigmentation problems as well as undereye circles and puffiness also are
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@frontlinemedcom.com.
Enbrel
Amgen Inc announces that the US Food and Drug Administration has approved the supplemental Biologics License Application for the expanded use of Enbrel (etanercept) for the treatment of moderate to severe plaque psoriasis in pediatric patients (aged 4–17 years). Enbrel, a tumor necrosis factor blocker, was approved for the treatment of moderate to severe plaque psoriasis in adults in 2004. For more information, visit www.enbrel.com.
Eucrisa
Pfizer Inc announces US Food and Drug Administration approval of Eucrisa (crisaborole) ointment 2% for the treatment of mild to moderate atopic dermatitis in patients 2 years and older. Eucrisa is a nonsteroidal topical phosphodiesterase 4 inhibitor and is applied twice daily. This approval provides patients with atopic dermatitis another treatment alternative, as this community has not had a new prescription treatment for more than 10 years. For more information, visit www.pfizer.com.
Isdinceutics
Isdin based in Spain has launched the Isdinceutics line of physician-dispensed cosmeceuticals to the US market, which focuses on vitamins and hydrators rather than chemicals to rejuvenate the skin. Isdinceutics features a daily antioxidant routine with Flavo-C Ultraglican and Flavo-C Serum to reduce the appearance of microwrinkles and elevate the skin’s natural moisture production. Products to correct pigmentation problems as well as undereye circles and puffiness also are
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@frontlinemedcom.com.
Product Update: JUST…Love, pjur med
MASSAGE AND MOISTURING OIL
Just Pure Essentials suggests that JUST…Love be used to moisturize the face and body and for facial cleansing and shaving. It has been recommended by women’s health professionals as a lubricant and moisturizer to relieve vaginal dryness, says the manufacturer.
Just Pure Essentials products are 100% vegan; chemical and preservative free; and without alcohols, additives, or phytoestrogens. The base of each product is coconut medium-chain triglyceride (MCT) oil pressed from nongenetically modified coconut oil by a steam distillation process. The oil is blended with other organic oils and botanical ingredients, including green tea see oil, French plum oil, argan, and marshmallow leaf. It does not contain dyes, perfumes, or artificial flavors. Just Pure Essentials says its formulas do not dry up and turn sticky.
FOR MORE INFORMATION, VISIT: www.justpureessentials.com
SEXUAL HEALTH AND WELLBEING
pjur med says that PREMIUM glide is specially formulated for dry or highly sensitive genital mucous membranes and is made of non–pore-blocking, high-quality silicones. SENSITIVE glide is specifically developed for those with very sensitive genital mucous membranes, according to the manufacturer. Both products are available in 3.4 fl oz bottles. pjur med suggests its products are suitable for every skin type and maintains that they are dermatologically tested and paraben-, glycerin-, and preservative-free. In addition, pjur med says its formulas have been kept as neutral as possible and the ingredients have been put together in a way that microbial growth cannot occur.
FOR MORE INFORMATION, VISIT: www.pjurmed.com
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
MASSAGE AND MOISTURING OIL
Just Pure Essentials suggests that JUST…Love be used to moisturize the face and body and for facial cleansing and shaving. It has been recommended by women’s health professionals as a lubricant and moisturizer to relieve vaginal dryness, says the manufacturer.
Just Pure Essentials products are 100% vegan; chemical and preservative free; and without alcohols, additives, or phytoestrogens. The base of each product is coconut medium-chain triglyceride (MCT) oil pressed from nongenetically modified coconut oil by a steam distillation process. The oil is blended with other organic oils and botanical ingredients, including green tea see oil, French plum oil, argan, and marshmallow leaf. It does not contain dyes, perfumes, or artificial flavors. Just Pure Essentials says its formulas do not dry up and turn sticky.
FOR MORE INFORMATION, VISIT: www.justpureessentials.com
SEXUAL HEALTH AND WELLBEING
pjur med says that PREMIUM glide is specially formulated for dry or highly sensitive genital mucous membranes and is made of non–pore-blocking, high-quality silicones. SENSITIVE glide is specifically developed for those with very sensitive genital mucous membranes, according to the manufacturer. Both products are available in 3.4 fl oz bottles. pjur med suggests its products are suitable for every skin type and maintains that they are dermatologically tested and paraben-, glycerin-, and preservative-free. In addition, pjur med says its formulas have been kept as neutral as possible and the ingredients have been put together in a way that microbial growth cannot occur.
FOR MORE INFORMATION, VISIT: www.pjurmed.com
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
MASSAGE AND MOISTURING OIL
Just Pure Essentials suggests that JUST…Love be used to moisturize the face and body and for facial cleansing and shaving. It has been recommended by women’s health professionals as a lubricant and moisturizer to relieve vaginal dryness, says the manufacturer.
Just Pure Essentials products are 100% vegan; chemical and preservative free; and without alcohols, additives, or phytoestrogens. The base of each product is coconut medium-chain triglyceride (MCT) oil pressed from nongenetically modified coconut oil by a steam distillation process. The oil is blended with other organic oils and botanical ingredients, including green tea see oil, French plum oil, argan, and marshmallow leaf. It does not contain dyes, perfumes, or artificial flavors. Just Pure Essentials says its formulas do not dry up and turn sticky.
FOR MORE INFORMATION, VISIT: www.justpureessentials.com
SEXUAL HEALTH AND WELLBEING
pjur med says that PREMIUM glide is specially formulated for dry or highly sensitive genital mucous membranes and is made of non–pore-blocking, high-quality silicones. SENSITIVE glide is specifically developed for those with very sensitive genital mucous membranes, according to the manufacturer. Both products are available in 3.4 fl oz bottles. pjur med suggests its products are suitable for every skin type and maintains that they are dermatologically tested and paraben-, glycerin-, and preservative-free. In addition, pjur med says its formulas have been kept as neutral as possible and the ingredients have been put together in a way that microbial growth cannot occur.
FOR MORE INFORMATION, VISIT: www.pjurmed.com
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Product Update: Kyleena; LapCap2
NEW LNG IUS: GOOD FOR 5 YEARS
FOR MORE INFORMATION, VISIT:
https://hcp.kyleena-us.com/
EASIER LAPAROSCOPIC ACCESS
To use LapCap2, says Life Care, center it over the umbilicus, attach a hose to the suction port, and apply negative pressure. The abdomen immediately rises for Veress needle insertion. Inert gas is then delivered to replace the negative pressure, and the procedure can continue.
FOR MORE INFORMATION, VISIT:
http://www.lcmd.com/products/lapcap2
NEW LNG IUS: GOOD FOR 5 YEARS
FOR MORE INFORMATION, VISIT:
https://hcp.kyleena-us.com/
EASIER LAPAROSCOPIC ACCESS
To use LapCap2, says Life Care, center it over the umbilicus, attach a hose to the suction port, and apply negative pressure. The abdomen immediately rises for Veress needle insertion. Inert gas is then delivered to replace the negative pressure, and the procedure can continue.
FOR MORE INFORMATION, VISIT:
http://www.lcmd.com/products/lapcap2
NEW LNG IUS: GOOD FOR 5 YEARS
FOR MORE INFORMATION, VISIT:
https://hcp.kyleena-us.com/
EASIER LAPAROSCOPIC ACCESS
To use LapCap2, says Life Care, center it over the umbilicus, attach a hose to the suction port, and apply negative pressure. The abdomen immediately rises for Veress needle insertion. Inert gas is then delivered to replace the negative pressure, and the procedure can continue.
FOR MORE INFORMATION, VISIT:
http://www.lcmd.com/products/lapcap2
Product News: October 2016
Carmex Comfort Care
Carma Labs Inc introduces Carmex Comfort Care lip balm, a natural lip care product formulated with colloidal oatmeal and cold-pressed fruit oil to deliver soothing, long-lasting moisture and hydration. Colloidal oatmeal also possesses antioxidant and anti-inflammatory properties that benefit sensitive drug skin, especially on the lips. For more information, visit www.mycarmex.com.
Erelzi
Sandoz Inc, a Novartis Division, announces US Food and Drug Administration approval of Erelzi (etanercept-szzs) for all indications included in the reference product label: rheumatoid arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, and polyarticular juvenile idiopathic arthritis. Erelzi is the second approved biosimilar from Sandoz. For more information, visit www.erelzi.com.
Loprox Cream
Medimetriks Pharmaceuticals, Inc, announces the launch of Loprox (ciclopirox) Cream 0.77% and the Loprox Cream Kit. Loprox Cream is a broad-spectrum therapy that treats 5 different skin infections from 6 different pathogens. It is indicated for the topical treatment of tinea pedis, tinea cruris, and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, and Microsporum canis; candidiasis due to Candia albicans; and tinea (pityriasis) versicolor due to Malassezia furfur. Loprox Cream works quickly, usually within the first week (2 weeks for tinea versicolor), providing patients needed relief of pruritus and other symptoms. The Loprox Cream Kit includes Loprox Cream and Rehyla Hair + Body Cleanser for patient convenience. The cleanser hydrates and conditions and is gentle for daily use on the scalp and body. For more information, visit www.medimetriks.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@frontlinemedcom.com.
Carmex Comfort Care
Carma Labs Inc introduces Carmex Comfort Care lip balm, a natural lip care product formulated with colloidal oatmeal and cold-pressed fruit oil to deliver soothing, long-lasting moisture and hydration. Colloidal oatmeal also possesses antioxidant and anti-inflammatory properties that benefit sensitive drug skin, especially on the lips. For more information, visit www.mycarmex.com.
Erelzi
Sandoz Inc, a Novartis Division, announces US Food and Drug Administration approval of Erelzi (etanercept-szzs) for all indications included in the reference product label: rheumatoid arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, and polyarticular juvenile idiopathic arthritis. Erelzi is the second approved biosimilar from Sandoz. For more information, visit www.erelzi.com.
Loprox Cream
Medimetriks Pharmaceuticals, Inc, announces the launch of Loprox (ciclopirox) Cream 0.77% and the Loprox Cream Kit. Loprox Cream is a broad-spectrum therapy that treats 5 different skin infections from 6 different pathogens. It is indicated for the topical treatment of tinea pedis, tinea cruris, and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, and Microsporum canis; candidiasis due to Candia albicans; and tinea (pityriasis) versicolor due to Malassezia furfur. Loprox Cream works quickly, usually within the first week (2 weeks for tinea versicolor), providing patients needed relief of pruritus and other symptoms. The Loprox Cream Kit includes Loprox Cream and Rehyla Hair + Body Cleanser for patient convenience. The cleanser hydrates and conditions and is gentle for daily use on the scalp and body. For more information, visit www.medimetriks.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@frontlinemedcom.com.
Carmex Comfort Care
Carma Labs Inc introduces Carmex Comfort Care lip balm, a natural lip care product formulated with colloidal oatmeal and cold-pressed fruit oil to deliver soothing, long-lasting moisture and hydration. Colloidal oatmeal also possesses antioxidant and anti-inflammatory properties that benefit sensitive drug skin, especially on the lips. For more information, visit www.mycarmex.com.
Erelzi
Sandoz Inc, a Novartis Division, announces US Food and Drug Administration approval of Erelzi (etanercept-szzs) for all indications included in the reference product label: rheumatoid arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, and polyarticular juvenile idiopathic arthritis. Erelzi is the second approved biosimilar from Sandoz. For more information, visit www.erelzi.com.
Loprox Cream
Medimetriks Pharmaceuticals, Inc, announces the launch of Loprox (ciclopirox) Cream 0.77% and the Loprox Cream Kit. Loprox Cream is a broad-spectrum therapy that treats 5 different skin infections from 6 different pathogens. It is indicated for the topical treatment of tinea pedis, tinea cruris, and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, and Microsporum canis; candidiasis due to Candia albicans; and tinea (pityriasis) versicolor due to Malassezia furfur. Loprox Cream works quickly, usually within the first week (2 weeks for tinea versicolor), providing patients needed relief of pruritus and other symptoms. The Loprox Cream Kit includes Loprox Cream and Rehyla Hair + Body Cleanser for patient convenience. The cleanser hydrates and conditions and is gentle for daily use on the scalp and body. For more information, visit www.medimetriks.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@frontlinemedcom.com.
Product Update: SureSound+ device; LILETTA single-handed inserter
PRECISE PREABLATION MEASUREMENT
The SureSound + device is designed to allow a physician to measure cavity length with one click, unlike traditional sounding methods that require multiple calculations, says Hologic. Hologic indicates that the device is inserted through the cervical canal, the Malecot anchor is deployed on the internal os, and the inner probe’s polyethylene tip is extended to the fundus to gauge uterine cavity length. The single-use device also may reduce the risk of contamination, says Hologic.
The manufacturer indicates the NovaSure procedure is appropriate for treating premenopausal women with heavy periods due to benign causes who have finished childbearing.
FOR MORE INFORMATION, VISIT: http://www.novasure.com/hcp/suresound_plus
NEW LILETTA INSERTER
The companies indicate that the LILETTA IUD is for use by women wishing to prevent pregnancy for up to 3 years regardless of parity or body mass index, with a cumulative 3-year efficacy rate of 99.45%. Approval of the new LILETTA single-handed inserter was based on the ACCESS IUS study that enrolled 1,751 US women and also led to LILETTA’s approval. Product marketing materials state that, during the ACCESS IUS study, the success rate for insertions with the new inserter was 99.2%. The original inserter requires a 2-handed technique.
According to Allergan and Medicines360, the new inserter includes: an ergonomic design allowing for single-handed insertion that can be used with either hand, a bendable tube to accommodate the anatomy of the patient during insertion, and the ability to reload the device if needed before insertion.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
PRECISE PREABLATION MEASUREMENT
The SureSound + device is designed to allow a physician to measure cavity length with one click, unlike traditional sounding methods that require multiple calculations, says Hologic. Hologic indicates that the device is inserted through the cervical canal, the Malecot anchor is deployed on the internal os, and the inner probe’s polyethylene tip is extended to the fundus to gauge uterine cavity length. The single-use device also may reduce the risk of contamination, says Hologic.
The manufacturer indicates the NovaSure procedure is appropriate for treating premenopausal women with heavy periods due to benign causes who have finished childbearing.
FOR MORE INFORMATION, VISIT: http://www.novasure.com/hcp/suresound_plus
NEW LILETTA INSERTER
The companies indicate that the LILETTA IUD is for use by women wishing to prevent pregnancy for up to 3 years regardless of parity or body mass index, with a cumulative 3-year efficacy rate of 99.45%. Approval of the new LILETTA single-handed inserter was based on the ACCESS IUS study that enrolled 1,751 US women and also led to LILETTA’s approval. Product marketing materials state that, during the ACCESS IUS study, the success rate for insertions with the new inserter was 99.2%. The original inserter requires a 2-handed technique.
According to Allergan and Medicines360, the new inserter includes: an ergonomic design allowing for single-handed insertion that can be used with either hand, a bendable tube to accommodate the anatomy of the patient during insertion, and the ability to reload the device if needed before insertion.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
PRECISE PREABLATION MEASUREMENT
The SureSound + device is designed to allow a physician to measure cavity length with one click, unlike traditional sounding methods that require multiple calculations, says Hologic. Hologic indicates that the device is inserted through the cervical canal, the Malecot anchor is deployed on the internal os, and the inner probe’s polyethylene tip is extended to the fundus to gauge uterine cavity length. The single-use device also may reduce the risk of contamination, says Hologic.
The manufacturer indicates the NovaSure procedure is appropriate for treating premenopausal women with heavy periods due to benign causes who have finished childbearing.
FOR MORE INFORMATION, VISIT: http://www.novasure.com/hcp/suresound_plus
NEW LILETTA INSERTER
The companies indicate that the LILETTA IUD is for use by women wishing to prevent pregnancy for up to 3 years regardless of parity or body mass index, with a cumulative 3-year efficacy rate of 99.45%. Approval of the new LILETTA single-handed inserter was based on the ACCESS IUS study that enrolled 1,751 US women and also led to LILETTA’s approval. Product marketing materials state that, during the ACCESS IUS study, the success rate for insertions with the new inserter was 99.2%. The original inserter requires a 2-handed technique.
According to Allergan and Medicines360, the new inserter includes: an ergonomic design allowing for single-handed insertion that can be used with either hand, a bendable tube to accommodate the anatomy of the patient during insertion, and the ability to reload the device if needed before insertion.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
Collagen Meniscus Implant
Ivy Sports Medicine (http://www.ivysportsmed.com/en)
Collagen Meniscus Implant
The number of patients undergoing arthroscopic partial meniscectomy has continued to increase. However, this is potentially not a benign procedure, as there are increased contact pressures on the articular cartilage even with the removal of only a segment of the meniscus.
The Collagen Meniscus Implant (CMI, Ivy Sports Medicine) is a resorbable and biocompatible Type I collagen matrix that was developed to restore the segmental loss of meniscal tissue in the knee. It consists of a porous cross-linked matrix scaffold that allows for the ingrowth of the body’s own cells. The CMI is the only meniscal implant composed of purely biological materials and is available in an off-the-shelf supply.
The CMI is available in the United States for use in the restoration of segmental loss of the medial meniscus. The CMI can be utilized in either an acute or chronic situation. In the acute case, it would be indicated when the medial meniscus is irreparable, and that segment must be removed. In the chronic case, the patient would have had a previous partial meniscectomy and/or failed meniscus repair and had developed either pain or signs of early articular cartilage wear in the compartment. The procedure can be done arthroscopically and as an outpatient. The CMI can be kept on the shelf to be available as needed; it has a 2-year shelf life. There are specialized instruments for measuring the length of implant needed and for delivery of the implant.
The CMI has been utilized clinically for 18 years with excellent clinical results. Patients treated with CMI have benefited in over 80% of cases. Studies have demonstrated improved knee function, activity levels, and pain values from the pre- to postoperative periods.1,2 In addition, functional improvements have been maintained for over 10 years. The reoperation rate has been demonstrated to be 10% to 20%, which is comparable to the reoperation rate after meniscal repair.
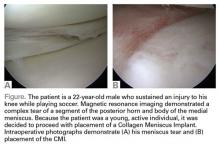
Surgical pearl: The surgical technique for insertion of the CMI is relatively uncomplicated (Figures A, B).
The second step is to measure the length of your meniscus defect with the measuring rod.
Once measured, you want to oversize the implant 10% to 15% (ie, if you measure 30 mm, you will cut at least 34 mm). Use the measuring rod to measure the length of the CMI and mark your length. Use a new scalpel blade to cut the CMI.
Place the measured CMI into the delivery clamp and insert through a mini-arthrotomy into the meniscal defect. The fixation technique of the CMI is entirely up to the implanting surgeon. Most surgeons have used a combination of all-inside and inside-out meniscus repair techniques. It is recommended to start fixing the CMI first posteriorly. The posterior stitch is usually an all-inside horizontal mattress stitch. Coming 1 cm anteriorly, place a vertical mattress stitch. Continue this method sequentially while moving anteriorly. The anterior suture is the surgeon’s choice for device, but it should be a horizontal mattress like the most posterior stitch. It is important while tightening your suture tension to apply the concept of “approximated and not strangulated.” Once completed, close wounds in typical fashion.
1. Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med. 2011;39(5):977-985
2. Bulgheroni P, Murena L, Ratti C, Bulgheroni E, Ronga M, Cherubino P. Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee. 2010;17(3):224-229.
Ivy Sports Medicine (http://www.ivysportsmed.com/en)
Collagen Meniscus Implant
The number of patients undergoing arthroscopic partial meniscectomy has continued to increase. However, this is potentially not a benign procedure, as there are increased contact pressures on the articular cartilage even with the removal of only a segment of the meniscus.
The Collagen Meniscus Implant (CMI, Ivy Sports Medicine) is a resorbable and biocompatible Type I collagen matrix that was developed to restore the segmental loss of meniscal tissue in the knee. It consists of a porous cross-linked matrix scaffold that allows for the ingrowth of the body’s own cells. The CMI is the only meniscal implant composed of purely biological materials and is available in an off-the-shelf supply.
The CMI is available in the United States for use in the restoration of segmental loss of the medial meniscus. The CMI can be utilized in either an acute or chronic situation. In the acute case, it would be indicated when the medial meniscus is irreparable, and that segment must be removed. In the chronic case, the patient would have had a previous partial meniscectomy and/or failed meniscus repair and had developed either pain or signs of early articular cartilage wear in the compartment. The procedure can be done arthroscopically and as an outpatient. The CMI can be kept on the shelf to be available as needed; it has a 2-year shelf life. There are specialized instruments for measuring the length of implant needed and for delivery of the implant.
The CMI has been utilized clinically for 18 years with excellent clinical results. Patients treated with CMI have benefited in over 80% of cases. Studies have demonstrated improved knee function, activity levels, and pain values from the pre- to postoperative periods.1,2 In addition, functional improvements have been maintained for over 10 years. The reoperation rate has been demonstrated to be 10% to 20%, which is comparable to the reoperation rate after meniscal repair.
Surgical pearl: The surgical technique for insertion of the CMI is relatively uncomplicated (Figures A, B).
The second step is to measure the length of your meniscus defect with the measuring rod.
Once measured, you want to oversize the implant 10% to 15% (ie, if you measure 30 mm, you will cut at least 34 mm). Use the measuring rod to measure the length of the CMI and mark your length. Use a new scalpel blade to cut the CMI.
Place the measured CMI into the delivery clamp and insert through a mini-arthrotomy into the meniscal defect. The fixation technique of the CMI is entirely up to the implanting surgeon. Most surgeons have used a combination of all-inside and inside-out meniscus repair techniques. It is recommended to start fixing the CMI first posteriorly. The posterior stitch is usually an all-inside horizontal mattress stitch. Coming 1 cm anteriorly, place a vertical mattress stitch. Continue this method sequentially while moving anteriorly. The anterior suture is the surgeon’s choice for device, but it should be a horizontal mattress like the most posterior stitch. It is important while tightening your suture tension to apply the concept of “approximated and not strangulated.” Once completed, close wounds in typical fashion.
Ivy Sports Medicine (http://www.ivysportsmed.com/en)
Collagen Meniscus Implant
The number of patients undergoing arthroscopic partial meniscectomy has continued to increase. However, this is potentially not a benign procedure, as there are increased contact pressures on the articular cartilage even with the removal of only a segment of the meniscus.
The Collagen Meniscus Implant (CMI, Ivy Sports Medicine) is a resorbable and biocompatible Type I collagen matrix that was developed to restore the segmental loss of meniscal tissue in the knee. It consists of a porous cross-linked matrix scaffold that allows for the ingrowth of the body’s own cells. The CMI is the only meniscal implant composed of purely biological materials and is available in an off-the-shelf supply.
The CMI is available in the United States for use in the restoration of segmental loss of the medial meniscus. The CMI can be utilized in either an acute or chronic situation. In the acute case, it would be indicated when the medial meniscus is irreparable, and that segment must be removed. In the chronic case, the patient would have had a previous partial meniscectomy and/or failed meniscus repair and had developed either pain or signs of early articular cartilage wear in the compartment. The procedure can be done arthroscopically and as an outpatient. The CMI can be kept on the shelf to be available as needed; it has a 2-year shelf life. There are specialized instruments for measuring the length of implant needed and for delivery of the implant.
The CMI has been utilized clinically for 18 years with excellent clinical results. Patients treated with CMI have benefited in over 80% of cases. Studies have demonstrated improved knee function, activity levels, and pain values from the pre- to postoperative periods.1,2 In addition, functional improvements have been maintained for over 10 years. The reoperation rate has been demonstrated to be 10% to 20%, which is comparable to the reoperation rate after meniscal repair.
Surgical pearl: The surgical technique for insertion of the CMI is relatively uncomplicated (Figures A, B).
The second step is to measure the length of your meniscus defect with the measuring rod.
Once measured, you want to oversize the implant 10% to 15% (ie, if you measure 30 mm, you will cut at least 34 mm). Use the measuring rod to measure the length of the CMI and mark your length. Use a new scalpel blade to cut the CMI.
Place the measured CMI into the delivery clamp and insert through a mini-arthrotomy into the meniscal defect. The fixation technique of the CMI is entirely up to the implanting surgeon. Most surgeons have used a combination of all-inside and inside-out meniscus repair techniques. It is recommended to start fixing the CMI first posteriorly. The posterior stitch is usually an all-inside horizontal mattress stitch. Coming 1 cm anteriorly, place a vertical mattress stitch. Continue this method sequentially while moving anteriorly. The anterior suture is the surgeon’s choice for device, but it should be a horizontal mattress like the most posterior stitch. It is important while tightening your suture tension to apply the concept of “approximated and not strangulated.” Once completed, close wounds in typical fashion.
1. Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med. 2011;39(5):977-985
2. Bulgheroni P, Murena L, Ratti C, Bulgheroni E, Ronga M, Cherubino P. Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee. 2010;17(3):224-229.
1. Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med. 2011;39(5):977-985
2. Bulgheroni P, Murena L, Ratti C, Bulgheroni E, Ronga M, Cherubino P. Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee. 2010;17(3):224-229.