User login
Organs of Metastasis Predominate with Age in Non-Small Cell Lung Cancer Subtypes: National Cancer Database Analysis
Background
Patients diagnosed with lung cancer are predominantly non-small cell lung cancer (NSCLC), a leading cause of cancer-related deaths. Thus, it is imperative to investigate and distinguish the differences present at diagnosis to possibly improve survival outcomes. NSCLC commonly metastasizes within older patients near the mean age of 71 years, but also in early onset patients which represents the patients younger than the earliest lung cancer screening age of 50.
Objective
To reveal differences in ratios of metastasis locations in squamous cell carcinoma (SCC), adenocarcinoma (ACC), and adenosquamous carcinoma (ASC).
Methods
The National Cancer Database (NCDB) was utilized to identify patients diagnosed with SCC, ACC, and ASC using the histology codes 8070, 8140, and 8560 from the ICD-O-3.2 from 2004 to 2022. Age groups were 70 years. Metastases located to the brain, liver, bone, and lung were included. Chi-Square tests were performed. The data was analyzed using R version 4.4.2 and statistical significance was set to α = 0.05.
Results
In this study, 1,445,119 patients were analyzed. Chi-Square tests identified significant differences in the ratios of organ metastasis locations between age groups in each subtype (p < 0.001). SCC in each age group similarly metastasized most to bone (36.3%, 34.7%, 34.5%), but notably more local lung metastasis was observed in the oldest group (33.6%). In ACC and ASC, the oldest group also had greater ratios of spread within the lungs (28.0%, 27.2%). Overall, the younger the age group, distant spread to the brain increased (ex. 29.0%, 24.4%, 17.5%). This suggests a widely heterogenous distribution of metastases at diagnosis of NSCLC subtypes and patient age.
Conclusions
This study demonstrated that patients with SCC, ACC, or ASC subtypes of NSCLC share similar predominant locations based in part on patient age, irrespective of cancer origin. NSCLC may more distantly metastasize in younger patients to the brain, while older patients may have locally metastatic cancer. Further analysis of key demographic variables as well as common undertaken treatment options may prove informative and reveal existing differences in survival outcomes.
Background
Patients diagnosed with lung cancer are predominantly non-small cell lung cancer (NSCLC), a leading cause of cancer-related deaths. Thus, it is imperative to investigate and distinguish the differences present at diagnosis to possibly improve survival outcomes. NSCLC commonly metastasizes within older patients near the mean age of 71 years, but also in early onset patients which represents the patients younger than the earliest lung cancer screening age of 50.
Objective
To reveal differences in ratios of metastasis locations in squamous cell carcinoma (SCC), adenocarcinoma (ACC), and adenosquamous carcinoma (ASC).
Methods
The National Cancer Database (NCDB) was utilized to identify patients diagnosed with SCC, ACC, and ASC using the histology codes 8070, 8140, and 8560 from the ICD-O-3.2 from 2004 to 2022. Age groups were 70 years. Metastases located to the brain, liver, bone, and lung were included. Chi-Square tests were performed. The data was analyzed using R version 4.4.2 and statistical significance was set to α = 0.05.
Results
In this study, 1,445,119 patients were analyzed. Chi-Square tests identified significant differences in the ratios of organ metastasis locations between age groups in each subtype (p < 0.001). SCC in each age group similarly metastasized most to bone (36.3%, 34.7%, 34.5%), but notably more local lung metastasis was observed in the oldest group (33.6%). In ACC and ASC, the oldest group also had greater ratios of spread within the lungs (28.0%, 27.2%). Overall, the younger the age group, distant spread to the brain increased (ex. 29.0%, 24.4%, 17.5%). This suggests a widely heterogenous distribution of metastases at diagnosis of NSCLC subtypes and patient age.
Conclusions
This study demonstrated that patients with SCC, ACC, or ASC subtypes of NSCLC share similar predominant locations based in part on patient age, irrespective of cancer origin. NSCLC may more distantly metastasize in younger patients to the brain, while older patients may have locally metastatic cancer. Further analysis of key demographic variables as well as common undertaken treatment options may prove informative and reveal existing differences in survival outcomes.
Background
Patients diagnosed with lung cancer are predominantly non-small cell lung cancer (NSCLC), a leading cause of cancer-related deaths. Thus, it is imperative to investigate and distinguish the differences present at diagnosis to possibly improve survival outcomes. NSCLC commonly metastasizes within older patients near the mean age of 71 years, but also in early onset patients which represents the patients younger than the earliest lung cancer screening age of 50.
Objective
To reveal differences in ratios of metastasis locations in squamous cell carcinoma (SCC), adenocarcinoma (ACC), and adenosquamous carcinoma (ASC).
Methods
The National Cancer Database (NCDB) was utilized to identify patients diagnosed with SCC, ACC, and ASC using the histology codes 8070, 8140, and 8560 from the ICD-O-3.2 from 2004 to 2022. Age groups were 70 years. Metastases located to the brain, liver, bone, and lung were included. Chi-Square tests were performed. The data was analyzed using R version 4.4.2 and statistical significance was set to α = 0.05.
Results
In this study, 1,445,119 patients were analyzed. Chi-Square tests identified significant differences in the ratios of organ metastasis locations between age groups in each subtype (p < 0.001). SCC in each age group similarly metastasized most to bone (36.3%, 34.7%, 34.5%), but notably more local lung metastasis was observed in the oldest group (33.6%). In ACC and ASC, the oldest group also had greater ratios of spread within the lungs (28.0%, 27.2%). Overall, the younger the age group, distant spread to the brain increased (ex. 29.0%, 24.4%, 17.5%). This suggests a widely heterogenous distribution of metastases at diagnosis of NSCLC subtypes and patient age.
Conclusions
This study demonstrated that patients with SCC, ACC, or ASC subtypes of NSCLC share similar predominant locations based in part on patient age, irrespective of cancer origin. NSCLC may more distantly metastasize in younger patients to the brain, while older patients may have locally metastatic cancer. Further analysis of key demographic variables as well as common undertaken treatment options may prove informative and reveal existing differences in survival outcomes.
Shifting Demographics: A Temporal Analysis of the Alarming Rise in Rectal Adenocarcinoma Among Young Adults
Background
Rectal adenocarcinoma has long been associated with older adults, with routine screening typically beginning at age 45 or older. However, recent data reveal a concerning rise in rectal cancer incidence among adults under 40. These early-onset cases often present at later stages and may have distinct biological features. While some research attributes this trend to genetic or environmental factors, the contribution of socioeconomic disparities and healthcare access has not been fully explored. Identifying these influences is essential to shaping targeted prevention and early detection strategies for younger populations.
Objective
To evaluate temporal trends in rectal adenocarcinoma among young adults and assess demographic and socioeconomic predictors of early-onset diagnosis.
Methods
Data were drawn from the National Cancer Database (NCDB) for patients diagnosed with rectal adenocarcinoma from 2004 to 2022. Among 440,316 cases, 17,842 (4.1%) occurred in individuals under 40. Linear regression assessed temporal trends, while logistic regression evaluated associations between early-onset diagnosis and variables including sex, race, insurance status, income level, Charlson-Deyo comorbidity score, and tumor stage. Statistical significance was defined as α = 0.05.
Results
The number of young adults diagnosed rose from 424 in 2004 to 937 in 2022—an increase of over 120%. Each year was associated with a 1.7% rise in odds of early diagnosis (OR = 1.017, p < 0.001). Male patients had 24.7% higher odds (OR = 1.247, p < 0.001), and Black patients had 59.3% higher odds compared to White patients (OR = 1.593, p < 0.001). Non-private insurance was linked to a 41.6% decrease in early diagnosis (OR = 0.584, p < 0.001). Income level was not significant (p = 0.426). Lower Charlson-Deyo scores and higher tumor stages were also associated with early-onset cases.
Conclusions
Rectal adenocarcinoma is increasingly affecting younger adults, with significant associations across demographic and insurance variables. These findings call for improved awareness, early diagnostic strategies, and further research into underlying causes to mitigate this growing public health concern.
Background
Rectal adenocarcinoma has long been associated with older adults, with routine screening typically beginning at age 45 or older. However, recent data reveal a concerning rise in rectal cancer incidence among adults under 40. These early-onset cases often present at later stages and may have distinct biological features. While some research attributes this trend to genetic or environmental factors, the contribution of socioeconomic disparities and healthcare access has not been fully explored. Identifying these influences is essential to shaping targeted prevention and early detection strategies for younger populations.
Objective
To evaluate temporal trends in rectal adenocarcinoma among young adults and assess demographic and socioeconomic predictors of early-onset diagnosis.
Methods
Data were drawn from the National Cancer Database (NCDB) for patients diagnosed with rectal adenocarcinoma from 2004 to 2022. Among 440,316 cases, 17,842 (4.1%) occurred in individuals under 40. Linear regression assessed temporal trends, while logistic regression evaluated associations between early-onset diagnosis and variables including sex, race, insurance status, income level, Charlson-Deyo comorbidity score, and tumor stage. Statistical significance was defined as α = 0.05.
Results
The number of young adults diagnosed rose from 424 in 2004 to 937 in 2022—an increase of over 120%. Each year was associated with a 1.7% rise in odds of early diagnosis (OR = 1.017, p < 0.001). Male patients had 24.7% higher odds (OR = 1.247, p < 0.001), and Black patients had 59.3% higher odds compared to White patients (OR = 1.593, p < 0.001). Non-private insurance was linked to a 41.6% decrease in early diagnosis (OR = 0.584, p < 0.001). Income level was not significant (p = 0.426). Lower Charlson-Deyo scores and higher tumor stages were also associated with early-onset cases.
Conclusions
Rectal adenocarcinoma is increasingly affecting younger adults, with significant associations across demographic and insurance variables. These findings call for improved awareness, early diagnostic strategies, and further research into underlying causes to mitigate this growing public health concern.
Background
Rectal adenocarcinoma has long been associated with older adults, with routine screening typically beginning at age 45 or older. However, recent data reveal a concerning rise in rectal cancer incidence among adults under 40. These early-onset cases often present at later stages and may have distinct biological features. While some research attributes this trend to genetic or environmental factors, the contribution of socioeconomic disparities and healthcare access has not been fully explored. Identifying these influences is essential to shaping targeted prevention and early detection strategies for younger populations.
Objective
To evaluate temporal trends in rectal adenocarcinoma among young adults and assess demographic and socioeconomic predictors of early-onset diagnosis.
Methods
Data were drawn from the National Cancer Database (NCDB) for patients diagnosed with rectal adenocarcinoma from 2004 to 2022. Among 440,316 cases, 17,842 (4.1%) occurred in individuals under 40. Linear regression assessed temporal trends, while logistic regression evaluated associations between early-onset diagnosis and variables including sex, race, insurance status, income level, Charlson-Deyo comorbidity score, and tumor stage. Statistical significance was defined as α = 0.05.
Results
The number of young adults diagnosed rose from 424 in 2004 to 937 in 2022—an increase of over 120%. Each year was associated with a 1.7% rise in odds of early diagnosis (OR = 1.017, p < 0.001). Male patients had 24.7% higher odds (OR = 1.247, p < 0.001), and Black patients had 59.3% higher odds compared to White patients (OR = 1.593, p < 0.001). Non-private insurance was linked to a 41.6% decrease in early diagnosis (OR = 0.584, p < 0.001). Income level was not significant (p = 0.426). Lower Charlson-Deyo scores and higher tumor stages were also associated with early-onset cases.
Conclusions
Rectal adenocarcinoma is increasingly affecting younger adults, with significant associations across demographic and insurance variables. These findings call for improved awareness, early diagnostic strategies, and further research into underlying causes to mitigate this growing public health concern.
Epidemiology and Survival of Parotid Gland Malignancies With Brain Metastases: A Population- Based Study
Background
Parotid gland malignancies are a rare subset of salivary gland tumors, comprising approximately 1–3% of all head and neck cancers. While distant metastases commonly involve the lungs, brain metastases are exceedingly rare and remain poorly characterized. Management typically includes stereotactic radiosurgery or whole-brain radiation. This study evaluates the incidence, clinicopathologic features, and survival outcomes of patients with parotid gland tumors and brain metastases using data from Surveillance, Epidemiology, and End Results (SEER) database.
Methods
SEER database (2010–2022) was queried for patients diagnosed with primary malignant neoplasms of the parotid gland (ICD-O-3 site code C07.9). Cases of brain metastases were identified using SEER metastatic site variables. Age-adjusted incidence rates (IR) per 100,000 population were calculated using SEER*Stat 8.4.5. Kaplan-Meier survival analyses were conducted using GraphPad Prism, and survival differences were assessed using the log-rank test.
Results
Among 12,951 patients diagnosed with parotid malignancy, 47 (0.36%) had brain metastases. The median age at diagnosis was 67 years, and 77.5% were male. The overall incidence rate (IR) of brain metastases was 0.00235 per 100,000 population, with a significantly higher rate observed in males compared to females (p < 0.0001). The most common histologic subtype associated with brain involvement was squamous cell carcinoma (SCC, n=10), followed by adenocarcinoma. Median overall survival (mOS) for patients with brain metastases was 2 months (hazard ratio [HR] 6.28; 95% CI: 2.71–14.55), compared to 131 months for those without brain involvement (p < 0.001). 1-year cancer-specific survival for patients with brain metastases was 38%. Among patients with parotid SCC and brain metastases, mOS was 3 months, compared to 39 months in those without brain involvement (HR 5.70; 95% CI: 1.09–29.68; p < 0.0001).
Conclusions
Brain metastases from parotid gland cancers, though rare, are associated with markedly poor outcomes. This highlights the importance of early neurologic assessment and brain imaging in high-risk patients, particularly with SCC histology. Prior studies have shown that TP53 mutations are common in parotid SCC, but their role in CNS spread remains unclear. Future research should explore molecular pathways underlying neurotropism in parotid cancers and investigate targeted systemic therapies with CNS penetration to improve outcomes.
Background
Parotid gland malignancies are a rare subset of salivary gland tumors, comprising approximately 1–3% of all head and neck cancers. While distant metastases commonly involve the lungs, brain metastases are exceedingly rare and remain poorly characterized. Management typically includes stereotactic radiosurgery or whole-brain radiation. This study evaluates the incidence, clinicopathologic features, and survival outcomes of patients with parotid gland tumors and brain metastases using data from Surveillance, Epidemiology, and End Results (SEER) database.
Methods
SEER database (2010–2022) was queried for patients diagnosed with primary malignant neoplasms of the parotid gland (ICD-O-3 site code C07.9). Cases of brain metastases were identified using SEER metastatic site variables. Age-adjusted incidence rates (IR) per 100,000 population were calculated using SEER*Stat 8.4.5. Kaplan-Meier survival analyses were conducted using GraphPad Prism, and survival differences were assessed using the log-rank test.
Results
Among 12,951 patients diagnosed with parotid malignancy, 47 (0.36%) had brain metastases. The median age at diagnosis was 67 years, and 77.5% were male. The overall incidence rate (IR) of brain metastases was 0.00235 per 100,000 population, with a significantly higher rate observed in males compared to females (p < 0.0001). The most common histologic subtype associated with brain involvement was squamous cell carcinoma (SCC, n=10), followed by adenocarcinoma. Median overall survival (mOS) for patients with brain metastases was 2 months (hazard ratio [HR] 6.28; 95% CI: 2.71–14.55), compared to 131 months for those without brain involvement (p < 0.001). 1-year cancer-specific survival for patients with brain metastases was 38%. Among patients with parotid SCC and brain metastases, mOS was 3 months, compared to 39 months in those without brain involvement (HR 5.70; 95% CI: 1.09–29.68; p < 0.0001).
Conclusions
Brain metastases from parotid gland cancers, though rare, are associated with markedly poor outcomes. This highlights the importance of early neurologic assessment and brain imaging in high-risk patients, particularly with SCC histology. Prior studies have shown that TP53 mutations are common in parotid SCC, but their role in CNS spread remains unclear. Future research should explore molecular pathways underlying neurotropism in parotid cancers and investigate targeted systemic therapies with CNS penetration to improve outcomes.
Background
Parotid gland malignancies are a rare subset of salivary gland tumors, comprising approximately 1–3% of all head and neck cancers. While distant metastases commonly involve the lungs, brain metastases are exceedingly rare and remain poorly characterized. Management typically includes stereotactic radiosurgery or whole-brain radiation. This study evaluates the incidence, clinicopathologic features, and survival outcomes of patients with parotid gland tumors and brain metastases using data from Surveillance, Epidemiology, and End Results (SEER) database.
Methods
SEER database (2010–2022) was queried for patients diagnosed with primary malignant neoplasms of the parotid gland (ICD-O-3 site code C07.9). Cases of brain metastases were identified using SEER metastatic site variables. Age-adjusted incidence rates (IR) per 100,000 population were calculated using SEER*Stat 8.4.5. Kaplan-Meier survival analyses were conducted using GraphPad Prism, and survival differences were assessed using the log-rank test.
Results
Among 12,951 patients diagnosed with parotid malignancy, 47 (0.36%) had brain metastases. The median age at diagnosis was 67 years, and 77.5% were male. The overall incidence rate (IR) of brain metastases was 0.00235 per 100,000 population, with a significantly higher rate observed in males compared to females (p < 0.0001). The most common histologic subtype associated with brain involvement was squamous cell carcinoma (SCC, n=10), followed by adenocarcinoma. Median overall survival (mOS) for patients with brain metastases was 2 months (hazard ratio [HR] 6.28; 95% CI: 2.71–14.55), compared to 131 months for those without brain involvement (p < 0.001). 1-year cancer-specific survival for patients with brain metastases was 38%. Among patients with parotid SCC and brain metastases, mOS was 3 months, compared to 39 months in those without brain involvement (HR 5.70; 95% CI: 1.09–29.68; p < 0.0001).
Conclusions
Brain metastases from parotid gland cancers, though rare, are associated with markedly poor outcomes. This highlights the importance of early neurologic assessment and brain imaging in high-risk patients, particularly with SCC histology. Prior studies have shown that TP53 mutations are common in parotid SCC, but their role in CNS spread remains unclear. Future research should explore molecular pathways underlying neurotropism in parotid cancers and investigate targeted systemic therapies with CNS penetration to improve outcomes.
Augmenting DNA Damage by Chemotherapy With CDK7 Inhibition to Disrupt PARP Expression in Cholangiocarcinoma
Papillary Cystadenocarcinoma: NCDB Insights on Outcomes and Socioeconomic Disparities
Background
Papillary cystadenocarcinoma is a rare, aggressive malignancy typically arising in the ovaries, often following malignant transformation of benign precursors. Characterized by local invasion and recurrence, it lacks standardized treatment protocols and comprehensive epidemiological data. Existing literature is limited to case reports and small series, leaving gaps in population-level data to guide clinical decision-making. This study uses the National Cancer Database (NCDB) to assess demographic, socioeconomic, and treatment patterns to identify disparities and inform management.
Methods
A retrospective cohort analysis of 345 patients with histologically confirmed papillary cystadenocarcinoma (ICD-O-3 code 8450) was conducted using the 2004–2020 NCDB. Demographic, treatment, and survival data were described; incidence trends were assessed via linear regression; and survival was analyzed using Kaplan-Meier curves.
Results
The cohort was predominantly female (97.1%), mean age 62.1 years (SD = 14.0), and 87.2% White. Most had private insurance (44.9%) or Medicare (40.9%). Over half (51.9%) resided in metropolitan areas >1 million. Primary tumor sites were ovarian (80.0%) and endometrial (5.2%), with 39.7% presenting at Stage III. Surgery was performed in 90.4% of cases, with 51.9% achieving negative margins. Most were treated at comprehensive community (41.0%) or academic/research programs (28.7%). Primary therapies included chemotherapy (62.3%), radiation (6.4%), and hormone therapy (1.7%). Thirty-day mortality was 1.9%, and 90-day mortality was 5.4%. Survival was 97.7% at 2 years, 94.2% at 5 years, and 88.6% at 10 years. Mean survival was 97.5 months (95% CI: 88.2–106.7).
Conclusions
This is the first NCDB-based analysis of papillary cystadenocarcinoma, offering insight into its clinical characteristics. Ovarian and endometrial origins were most common, reinforcing its gynecologic profile. High surgical rates and margin negativity suggest aggressive local treatment is central to management. Disparities emerged: patients were more likely to live in urban areas, hold private insurance, and receive care at community programs. These findings highlight the need for further investigation into socioeconomic inequities and may inform future guidelines to improve equitable care delivery across health systems, including community-based programs such as the VHA.
Background
Papillary cystadenocarcinoma is a rare, aggressive malignancy typically arising in the ovaries, often following malignant transformation of benign precursors. Characterized by local invasion and recurrence, it lacks standardized treatment protocols and comprehensive epidemiological data. Existing literature is limited to case reports and small series, leaving gaps in population-level data to guide clinical decision-making. This study uses the National Cancer Database (NCDB) to assess demographic, socioeconomic, and treatment patterns to identify disparities and inform management.
Methods
A retrospective cohort analysis of 345 patients with histologically confirmed papillary cystadenocarcinoma (ICD-O-3 code 8450) was conducted using the 2004–2020 NCDB. Demographic, treatment, and survival data were described; incidence trends were assessed via linear regression; and survival was analyzed using Kaplan-Meier curves.
Results
The cohort was predominantly female (97.1%), mean age 62.1 years (SD = 14.0), and 87.2% White. Most had private insurance (44.9%) or Medicare (40.9%). Over half (51.9%) resided in metropolitan areas >1 million. Primary tumor sites were ovarian (80.0%) and endometrial (5.2%), with 39.7% presenting at Stage III. Surgery was performed in 90.4% of cases, with 51.9% achieving negative margins. Most were treated at comprehensive community (41.0%) or academic/research programs (28.7%). Primary therapies included chemotherapy (62.3%), radiation (6.4%), and hormone therapy (1.7%). Thirty-day mortality was 1.9%, and 90-day mortality was 5.4%. Survival was 97.7% at 2 years, 94.2% at 5 years, and 88.6% at 10 years. Mean survival was 97.5 months (95% CI: 88.2–106.7).
Conclusions
This is the first NCDB-based analysis of papillary cystadenocarcinoma, offering insight into its clinical characteristics. Ovarian and endometrial origins were most common, reinforcing its gynecologic profile. High surgical rates and margin negativity suggest aggressive local treatment is central to management. Disparities emerged: patients were more likely to live in urban areas, hold private insurance, and receive care at community programs. These findings highlight the need for further investigation into socioeconomic inequities and may inform future guidelines to improve equitable care delivery across health systems, including community-based programs such as the VHA.
Background
Papillary cystadenocarcinoma is a rare, aggressive malignancy typically arising in the ovaries, often following malignant transformation of benign precursors. Characterized by local invasion and recurrence, it lacks standardized treatment protocols and comprehensive epidemiological data. Existing literature is limited to case reports and small series, leaving gaps in population-level data to guide clinical decision-making. This study uses the National Cancer Database (NCDB) to assess demographic, socioeconomic, and treatment patterns to identify disparities and inform management.
Methods
A retrospective cohort analysis of 345 patients with histologically confirmed papillary cystadenocarcinoma (ICD-O-3 code 8450) was conducted using the 2004–2020 NCDB. Demographic, treatment, and survival data were described; incidence trends were assessed via linear regression; and survival was analyzed using Kaplan-Meier curves.
Results
The cohort was predominantly female (97.1%), mean age 62.1 years (SD = 14.0), and 87.2% White. Most had private insurance (44.9%) or Medicare (40.9%). Over half (51.9%) resided in metropolitan areas >1 million. Primary tumor sites were ovarian (80.0%) and endometrial (5.2%), with 39.7% presenting at Stage III. Surgery was performed in 90.4% of cases, with 51.9% achieving negative margins. Most were treated at comprehensive community (41.0%) or academic/research programs (28.7%). Primary therapies included chemotherapy (62.3%), radiation (6.4%), and hormone therapy (1.7%). Thirty-day mortality was 1.9%, and 90-day mortality was 5.4%. Survival was 97.7% at 2 years, 94.2% at 5 years, and 88.6% at 10 years. Mean survival was 97.5 months (95% CI: 88.2–106.7).
Conclusions
This is the first NCDB-based analysis of papillary cystadenocarcinoma, offering insight into its clinical characteristics. Ovarian and endometrial origins were most common, reinforcing its gynecologic profile. High surgical rates and margin negativity suggest aggressive local treatment is central to management. Disparities emerged: patients were more likely to live in urban areas, hold private insurance, and receive care at community programs. These findings highlight the need for further investigation into socioeconomic inequities and may inform future guidelines to improve equitable care delivery across health systems, including community-based programs such as the VHA.
Assessing Geographical Trends in End-of-Life Cancer Care Using CDC WONDER’s Place of Death Data
Background
19.8% of all deaths in the US in 2023 were due to cancer. Despite its prevalence, there is minimal literature analyzing geographical trends in end-of-life care in cancer patients. This study aims to assess the evolution of end-of-life preferences in cancer patients, particularly during the COVID-19 pandemic, and account for geographical disparities to optimize palliative care delivery.
Methods
The CDC WONDER database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old that died with malignant neoplasms (ICD 10: C00- C97) in the US from 2003-2023. Deaths were stratified by region and urbanization. Proportional mortality was calculated, and statistically significant trends in mortality over time were identified using Joinpoint regression.
Results
There were 13,654,631 total deaths from malignant neoplasms over the study period. Home (40.3%) was the most common place of death followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decreased in proportion except for home which rose 7.0% from 41.7% to 48.7%. The South had the highest hospice rates (11.3%); 5.0% greater than the next highest region (Northeast; 8.3%). The West had the highest home rates (47.1%); 6.2% greater than the next closest region (South; 40.9%). The Northeast had the highest medical facility rates (36.0%); 5.5% higher than the next highest region (South, 30.5%). Nonmetro areas (< 50,000 population) had the lowest hospice (4.9%) and highest nursing home rates (15.8%). They also saw a substantial jump (+15.4%) in home deaths from 2019-21. All urbanizations saw a drop in medical facility deaths in 2020 but all have since climbed to surpass their 2019 rates except for nonmetro areas which have dropped 7.3% from 2020-2023.
Conclusion
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Geographical disparities persist in end-of-life care, particularly in nonmetro areas. This highlights the need to increase education and access to palliative care. Further research should aim at why the rural populations have failed to revert to pre-COVID trends like the other urbanization groups.
Background
19.8% of all deaths in the US in 2023 were due to cancer. Despite its prevalence, there is minimal literature analyzing geographical trends in end-of-life care in cancer patients. This study aims to assess the evolution of end-of-life preferences in cancer patients, particularly during the COVID-19 pandemic, and account for geographical disparities to optimize palliative care delivery.
Methods
The CDC WONDER database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old that died with malignant neoplasms (ICD 10: C00- C97) in the US from 2003-2023. Deaths were stratified by region and urbanization. Proportional mortality was calculated, and statistically significant trends in mortality over time were identified using Joinpoint regression.
Results
There were 13,654,631 total deaths from malignant neoplasms over the study period. Home (40.3%) was the most common place of death followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decreased in proportion except for home which rose 7.0% from 41.7% to 48.7%. The South had the highest hospice rates (11.3%); 5.0% greater than the next highest region (Northeast; 8.3%). The West had the highest home rates (47.1%); 6.2% greater than the next closest region (South; 40.9%). The Northeast had the highest medical facility rates (36.0%); 5.5% higher than the next highest region (South, 30.5%). Nonmetro areas (< 50,000 population) had the lowest hospice (4.9%) and highest nursing home rates (15.8%). They also saw a substantial jump (+15.4%) in home deaths from 2019-21. All urbanizations saw a drop in medical facility deaths in 2020 but all have since climbed to surpass their 2019 rates except for nonmetro areas which have dropped 7.3% from 2020-2023.
Conclusion
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Geographical disparities persist in end-of-life care, particularly in nonmetro areas. This highlights the need to increase education and access to palliative care. Further research should aim at why the rural populations have failed to revert to pre-COVID trends like the other urbanization groups.
Background
19.8% of all deaths in the US in 2023 were due to cancer. Despite its prevalence, there is minimal literature analyzing geographical trends in end-of-life care in cancer patients. This study aims to assess the evolution of end-of-life preferences in cancer patients, particularly during the COVID-19 pandemic, and account for geographical disparities to optimize palliative care delivery.
Methods
The CDC WONDER database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old that died with malignant neoplasms (ICD 10: C00- C97) in the US from 2003-2023. Deaths were stratified by region and urbanization. Proportional mortality was calculated, and statistically significant trends in mortality over time were identified using Joinpoint regression.
Results
There were 13,654,631 total deaths from malignant neoplasms over the study period. Home (40.3%) was the most common place of death followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decreased in proportion except for home which rose 7.0% from 41.7% to 48.7%. The South had the highest hospice rates (11.3%); 5.0% greater than the next highest region (Northeast; 8.3%). The West had the highest home rates (47.1%); 6.2% greater than the next closest region (South; 40.9%). The Northeast had the highest medical facility rates (36.0%); 5.5% higher than the next highest region (South, 30.5%). Nonmetro areas (< 50,000 population) had the lowest hospice (4.9%) and highest nursing home rates (15.8%). They also saw a substantial jump (+15.4%) in home deaths from 2019-21. All urbanizations saw a drop in medical facility deaths in 2020 but all have since climbed to surpass their 2019 rates except for nonmetro areas which have dropped 7.3% from 2020-2023.
Conclusion
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Geographical disparities persist in end-of-life care, particularly in nonmetro areas. This highlights the need to increase education and access to palliative care. Further research should aim at why the rural populations have failed to revert to pre-COVID trends like the other urbanization groups.
Demographical Trends in End-of-Life Care in Malignant Neoplasms: A CDC Wonder Analysis Using Place of Death
Background
In 2024, it was estimated that 2,001,140 new cases of cancer were diagnosed in the United States with 611,720 people succumbing to the disease. There is scant literature analyzing how the place of death in cancer patients has evolved over time, particularly during the COVID-19 pandemic, and how it varies demographically. This study aims to analyze the evolution of end-of-life preferences in cancer patients and assess for racial or sexual disparities to optimize palliative care and ensure it aligns with the patient’s wishes.
Methods
The CDC Wonder database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old who died with malignant neoplasms (ICD-10: C00-C97) in the US from 2003-2023. Deaths were stratified by sex and race. Proportional mortality was calculated, and statistically significant temporal trends in mortality were identified using Joinpoint regression.
Results
From 2003 to 2023, there were 13,654,631 total deaths from malignant cancer. Home deaths were the most common (40.3%) followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decrease in proportion except for home which rose 7.1%. From 2003-2023, home (+4.0%) and hospice (+10.0%) rose in frequency while medical facility (-10.9%) and nursing home (-6.8%) declined. Females died in nursing homes at a greater proportion than males (15.8% vs. 13.1%) while males died in medical facilities more frequently (32.4% vs. 28.8%). Black patients were the least likely to die at home (33.1%), 5.9% less than the next lowest (Asian/ Pacific Islander; 39.0%), while Hispanic patients were most likely (46.9%); 5.7% more than the next highest (White, 41.7%). White patients were the least likely to die in medical facilities (28.4%) but were also most likely to die in nursing homes (15.3%).
Conclusions
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Disparities persist in end-of-life care across both sex and racial groups. This highlights the need to increase education and access to palliative care. Further research should elucidate cultural and racial discrepancies surrounding end-of-life treatment and preferences to provide context for these differences.
Background
In 2024, it was estimated that 2,001,140 new cases of cancer were diagnosed in the United States with 611,720 people succumbing to the disease. There is scant literature analyzing how the place of death in cancer patients has evolved over time, particularly during the COVID-19 pandemic, and how it varies demographically. This study aims to analyze the evolution of end-of-life preferences in cancer patients and assess for racial or sexual disparities to optimize palliative care and ensure it aligns with the patient’s wishes.
Methods
The CDC Wonder database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old who died with malignant neoplasms (ICD-10: C00-C97) in the US from 2003-2023. Deaths were stratified by sex and race. Proportional mortality was calculated, and statistically significant temporal trends in mortality were identified using Joinpoint regression.
Results
From 2003 to 2023, there were 13,654,631 total deaths from malignant cancer. Home deaths were the most common (40.3%) followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decrease in proportion except for home which rose 7.1%. From 2003-2023, home (+4.0%) and hospice (+10.0%) rose in frequency while medical facility (-10.9%) and nursing home (-6.8%) declined. Females died in nursing homes at a greater proportion than males (15.8% vs. 13.1%) while males died in medical facilities more frequently (32.4% vs. 28.8%). Black patients were the least likely to die at home (33.1%), 5.9% less than the next lowest (Asian/ Pacific Islander; 39.0%), while Hispanic patients were most likely (46.9%); 5.7% more than the next highest (White, 41.7%). White patients were the least likely to die in medical facilities (28.4%) but were also most likely to die in nursing homes (15.3%).
Conclusions
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Disparities persist in end-of-life care across both sex and racial groups. This highlights the need to increase education and access to palliative care. Further research should elucidate cultural and racial discrepancies surrounding end-of-life treatment and preferences to provide context for these differences.
Background
In 2024, it was estimated that 2,001,140 new cases of cancer were diagnosed in the United States with 611,720 people succumbing to the disease. There is scant literature analyzing how the place of death in cancer patients has evolved over time, particularly during the COVID-19 pandemic, and how it varies demographically. This study aims to analyze the evolution of end-of-life preferences in cancer patients and assess for racial or sexual disparities to optimize palliative care and ensure it aligns with the patient’s wishes.
Methods
The CDC Wonder database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old who died with malignant neoplasms (ICD-10: C00-C97) in the US from 2003-2023. Deaths were stratified by sex and race. Proportional mortality was calculated, and statistically significant temporal trends in mortality were identified using Joinpoint regression.
Results
From 2003 to 2023, there were 13,654,631 total deaths from malignant cancer. Home deaths were the most common (40.3%) followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decrease in proportion except for home which rose 7.1%. From 2003-2023, home (+4.0%) and hospice (+10.0%) rose in frequency while medical facility (-10.9%) and nursing home (-6.8%) declined. Females died in nursing homes at a greater proportion than males (15.8% vs. 13.1%) while males died in medical facilities more frequently (32.4% vs. 28.8%). Black patients were the least likely to die at home (33.1%), 5.9% less than the next lowest (Asian/ Pacific Islander; 39.0%), while Hispanic patients were most likely (46.9%); 5.7% more than the next highest (White, 41.7%). White patients were the least likely to die in medical facilities (28.4%) but were also most likely to die in nursing homes (15.3%).
Conclusions
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Disparities persist in end-of-life care across both sex and racial groups. This highlights the need to increase education and access to palliative care. Further research should elucidate cultural and racial discrepancies surrounding end-of-life treatment and preferences to provide context for these differences.
Findings from (ImPaCT): Improving Patients With Prostate Cancer’s Access to Germline Testing
Background
With the onset of precision oncology, findings from germline mutational analysis have been helpful in treating patients with cancer and aids in cancer prevention, early detection, and improved overall outcomes. Germline genetic testing is now part of the standard of care for certain types of patients with prostate cancer. There is a very limited body of work that investigated demographic, disease- related and social factors that may be influencing Veterans’ participation in germline genetic testing. This study helps to identify whether certain factors may be influencing decisions on participation in prostate germline testing among Veterans with prostate malignancy.
Methods
The study was conducted using retrospective chart review. Data was collected from the periods of August 1, 2022 to December 31, 2023 among Veterans with prostate cancer who met criteria for germline genetic testing. Demographic and clinical information were collected including age, race, extent of disease (high risk, very high-risk or metastatic disease), significant co-morbidities, educational level, family and personal history of cancer, travel time, germline genetic test findings, impact on treatment approaches, referral for genetic counseling, and whether Veterans agreed or declined germline genetic testing. Data was analyzed using descriptive statistics. A total of 180 charts were reviewed, with 171 meeting the criteria for inclusion. The mean age of the participants is 73, with the youngest being 55 and the oldest being 101 years old. Majority of the participants were African American (77%).
Results
Only about two percent of those who met the inclusion criteria declined to undergo testing with the one living the farthest away from the testing hospital residing 18 miles away. Those who declined testing ranged in age from 67 to 88, majority had high risk prostate cancer and no family history of malignancy, and had 0-1 serious co-morbidity. None of their educational informational was available for review.
Conclusions
Participation in germline genetic testing can be enhanced with adequate patient education and availability of accessible resources, even among patient populations that are not always well-represented in clinical research. The presence of multiple serious co-morbidities and distance from a testing facility do not seem to contribute to hesitancy in germline genetic testing participation.
Background
With the onset of precision oncology, findings from germline mutational analysis have been helpful in treating patients with cancer and aids in cancer prevention, early detection, and improved overall outcomes. Germline genetic testing is now part of the standard of care for certain types of patients with prostate cancer. There is a very limited body of work that investigated demographic, disease- related and social factors that may be influencing Veterans’ participation in germline genetic testing. This study helps to identify whether certain factors may be influencing decisions on participation in prostate germline testing among Veterans with prostate malignancy.
Methods
The study was conducted using retrospective chart review. Data was collected from the periods of August 1, 2022 to December 31, 2023 among Veterans with prostate cancer who met criteria for germline genetic testing. Demographic and clinical information were collected including age, race, extent of disease (high risk, very high-risk or metastatic disease), significant co-morbidities, educational level, family and personal history of cancer, travel time, germline genetic test findings, impact on treatment approaches, referral for genetic counseling, and whether Veterans agreed or declined germline genetic testing. Data was analyzed using descriptive statistics. A total of 180 charts were reviewed, with 171 meeting the criteria for inclusion. The mean age of the participants is 73, with the youngest being 55 and the oldest being 101 years old. Majority of the participants were African American (77%).
Results
Only about two percent of those who met the inclusion criteria declined to undergo testing with the one living the farthest away from the testing hospital residing 18 miles away. Those who declined testing ranged in age from 67 to 88, majority had high risk prostate cancer and no family history of malignancy, and had 0-1 serious co-morbidity. None of their educational informational was available for review.
Conclusions
Participation in germline genetic testing can be enhanced with adequate patient education and availability of accessible resources, even among patient populations that are not always well-represented in clinical research. The presence of multiple serious co-morbidities and distance from a testing facility do not seem to contribute to hesitancy in germline genetic testing participation.
Background
With the onset of precision oncology, findings from germline mutational analysis have been helpful in treating patients with cancer and aids in cancer prevention, early detection, and improved overall outcomes. Germline genetic testing is now part of the standard of care for certain types of patients with prostate cancer. There is a very limited body of work that investigated demographic, disease- related and social factors that may be influencing Veterans’ participation in germline genetic testing. This study helps to identify whether certain factors may be influencing decisions on participation in prostate germline testing among Veterans with prostate malignancy.
Methods
The study was conducted using retrospective chart review. Data was collected from the periods of August 1, 2022 to December 31, 2023 among Veterans with prostate cancer who met criteria for germline genetic testing. Demographic and clinical information were collected including age, race, extent of disease (high risk, very high-risk or metastatic disease), significant co-morbidities, educational level, family and personal history of cancer, travel time, germline genetic test findings, impact on treatment approaches, referral for genetic counseling, and whether Veterans agreed or declined germline genetic testing. Data was analyzed using descriptive statistics. A total of 180 charts were reviewed, with 171 meeting the criteria for inclusion. The mean age of the participants is 73, with the youngest being 55 and the oldest being 101 years old. Majority of the participants were African American (77%).
Results
Only about two percent of those who met the inclusion criteria declined to undergo testing with the one living the farthest away from the testing hospital residing 18 miles away. Those who declined testing ranged in age from 67 to 88, majority had high risk prostate cancer and no family history of malignancy, and had 0-1 serious co-morbidity. None of their educational informational was available for review.
Conclusions
Participation in germline genetic testing can be enhanced with adequate patient education and availability of accessible resources, even among patient populations that are not always well-represented in clinical research. The presence of multiple serious co-morbidities and distance from a testing facility do not seem to contribute to hesitancy in germline genetic testing participation.
Advanced Imaging Techniques Use in Giant Cell Arteritis Diagnosis: The Experience at Walter Reed National Military Medical Center
Advanced Imaging Techniques Use in Giant Cell Arteritis Diagnosis: The Experience at Walter Reed National Military Medical Center
Giant cell arteritis (GCA), the most commonly diagnosed systemic vasculitis, is a large- and medium-vessel vasculitis that can lead to significant morbidity due to aneurysm formation or vascular occlusion if not diagnosed in a timely manner.1,2 Diagnosis is typically based on clinical history and inflammatory markers. Laboratory inflammatory markers may be normal in the early stages of GCA but can be abnormal due to other unrelated reasons leading to a false positive diagnosis.3 Delayed treatment may lead to visual loss, jaw or limb claudication, or ischemic stroke.2 Initial treatment typically includes high-dose steroids that can lead to significant adverse reactions such as hypothalamic-pituitary-adrenal axis dysfunction, metabolic syndrome, premature atherosclerosis, and increased risk of infection.4-6
The 1990 American College of Rheumatology (ACR) criteria for GCA are widely recognized (Table 1).7 The criteria focuses on clinical manifestations, including new onset headache, temporal artery tenderness, age ≥ 50 years, erythrocyte sedimentation rate (ESR) ≥ 50 mm/hr, and temporal artery biopsy with positive anatomical findings.8 When 3 of the 5 1990 ACR criteria are present, the sensitivity and specificity is estimated to be > 90% for GCA vs alternative vasculitides.7

Although the 1990 ACR criteria do not include imaging, modalities such as ultrasound, computed tomography angiography (CTA), 18F-FDG positron emission tomography (PET), and magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA) may be used in GCA diagnosis.8-10 These imaging modalities have been added to the proposed ACR classification criteria for GCA.11 For this updated point system standard, age ≥ 50 years is a requirement and includes a positive temporal artery biopsy or temporal artery halo sign on ultrasound (+5 points), an ESR ≥ 50 mm/h or C-reactive protein (CRP) ≥ 10 mg/L (+3 points), or sudden visual loss (+3 points). Scalp tenderness, jaw or tongue claudication, new temporal headache, morning stiffness in shoulders or neck, temporal artery abnormality on vascular examination, bilateral axillary vessel involvement on imaging, and 18F-FDG PET activity throughout the aorta are scored +2 points each. With these new criteria, a cumulative score ≥ 6 is classified as GCA. Diagnostic accuracy is further improved with imaging: ultrasonography (sensitivity 55% and specificity 95%) and 18F-FDG PET (sensitivity 69% and specificity 92%), CTA (sensitivity 71% and specificity 86%), and MRI/MRA (sensitivity 73% and specificity 88%).12-15
In recent years, clinicians have reported increased glucose uptake in arteries observed on PET imaging that suggests GCA.9,10,16-20 18F-FDG accumulates in cells with high metabolic activity rates, such as areas of inflammation. In assessing temporal arteries or other involved vasculature (eg, axillary or great vessels) for GCA, this modality indicates increased glucose uptake in the lining of vessel walls. The inflammation of vessel walls can then be visualized with PET. 18F-FDG PET presents a noninvasive imaging technique for evaluating GCA but its use has been limited in the United States due to its high cost.
Methods
Approval for a retrospective chart review of patients evaluated for suspected GCA was obtained from the Walter Reed National Military Medical Center (WRNMMC) Institutional Review Board. The review included patients who underwent diagnostic procedures such as ultrasound, MRI, CT angiogram, and PET studies from 2016 through 2022. International Classification of Diseases codes used for case identification included: M31.6, M31.5, I77.6, I77.8, I77.89, I67.7, and I68.2. The Current Procedural Terminology code used for temporal artery biopsy is 37609.
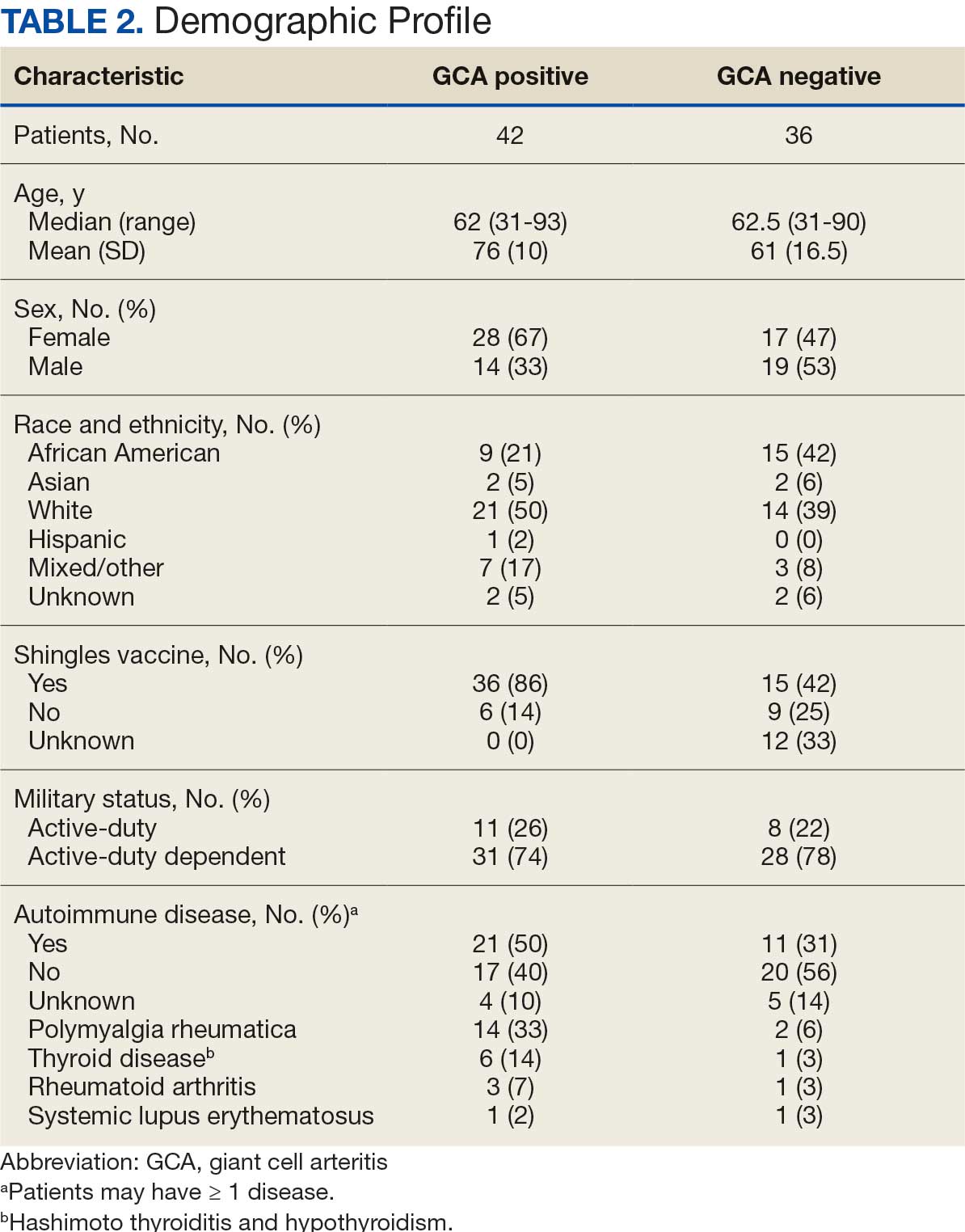
Results
Seventy-eight charts were reviewed and 42 patients (54%) were diagnosed with GCA (Table 2). This study sample had a much higher proportion of African American subjects (31%) when compared with the civilian population, likely reflecting the higher representation of African Americans in the armed forces. Twenty-eight females (67%) were GCA positive. The most common presenting symptoms included 27 patients (64%) with headache, 17 (40%) with scalp tenderness, and 14 (33%) with jaw pain. The mean 1990 ACR score was 3.8 (range, 2-5). With respect to the score criteria: 41 patients (98%) were aged ≥ 50 years, 31 (74%) had new onset headache, and 31 (74%) had elevated ESR (Table 3). Acute ischemic optic neuropathy was documented in 4 patients (10%) with confirmed GCA. The mean ESR and CRP values at diagnosis were 66.2 mm/h (range, 7-122 mm/h) and 8.711 μg/mL (range, 0.054 – 92.690 μg/mL), respectively. Twenty-seven patients (64%) underwent biopsy: 24 (89%) were unilateral and 3 (11%) were bilateral (Table 4). Four patients with GCA (10%) were missing biopsy data. Nineteen patients with GCA (70%) had biopsies with pathologic findings consistent with GCA.
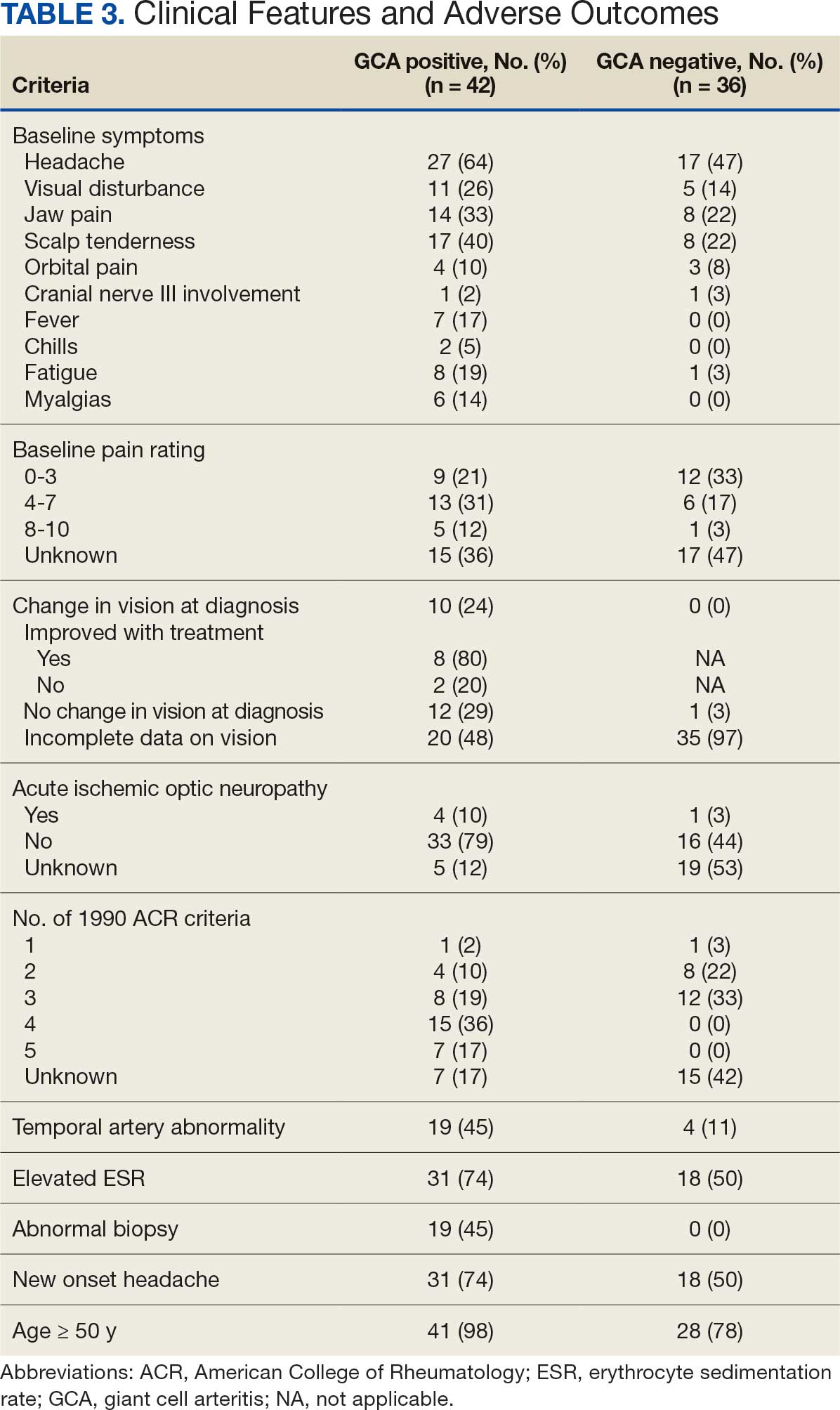
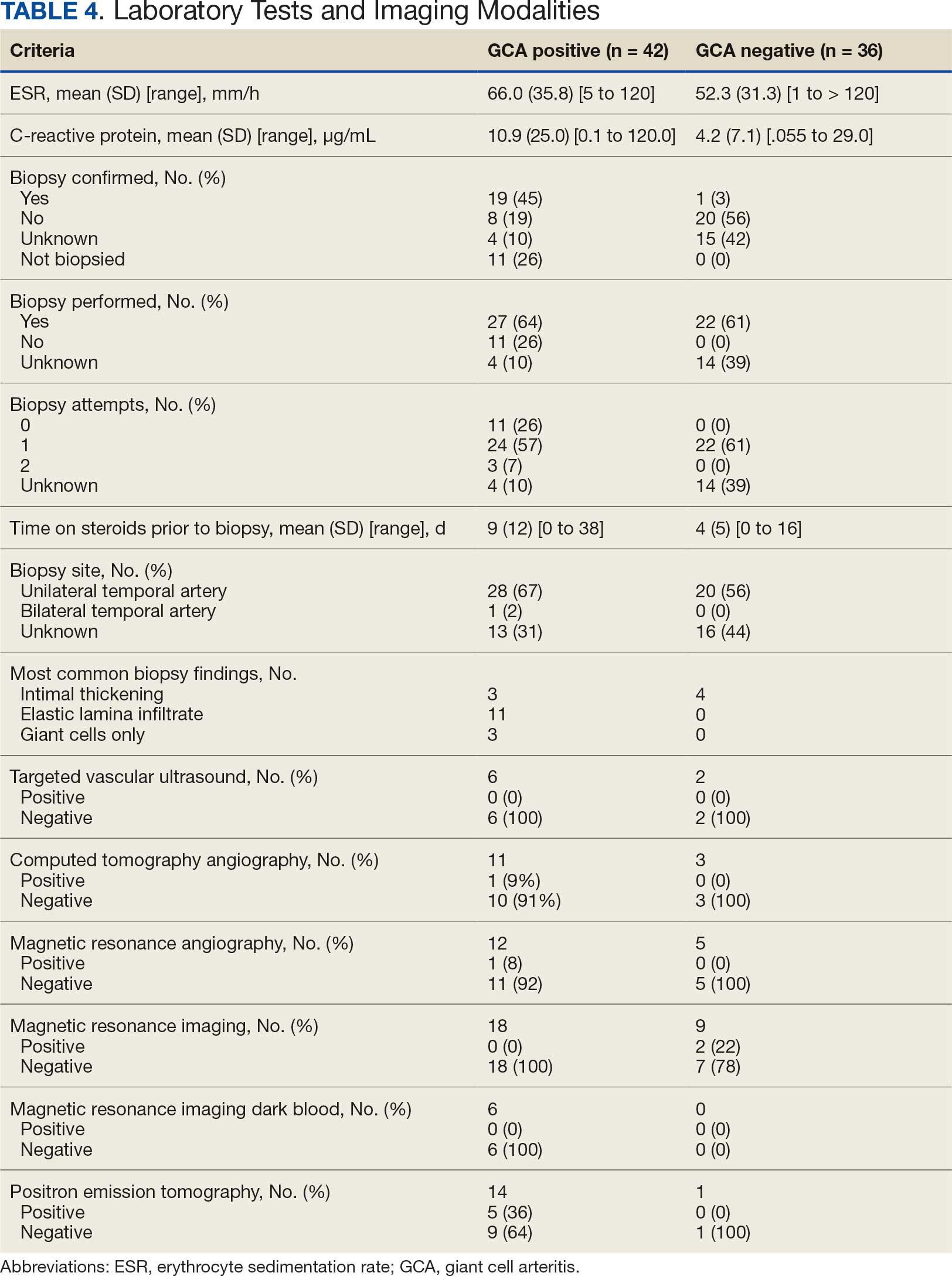
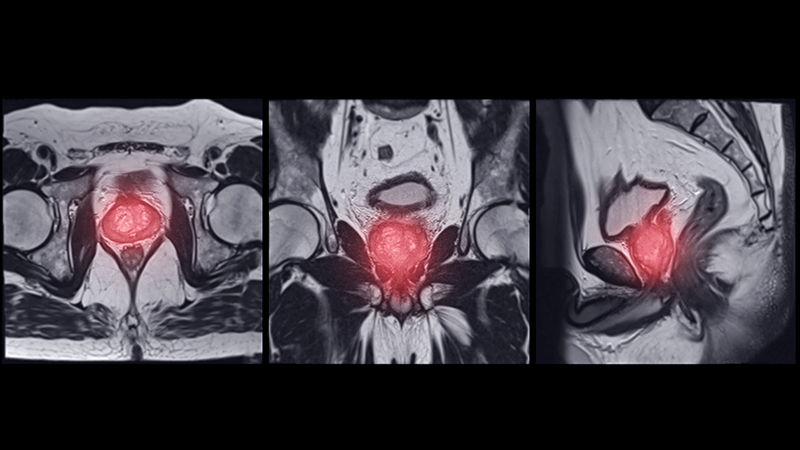
Twenty-five patients with GCA (60%) received ≥ 1 imaging modality. The most common imaging modality was MRI, which was used for 18 (43%) patients. Fourteen patients (33%) had 18F-FDG PET, 12 patients (29%) had MRA, and 11 patients (26%) had CTA. The small number of patients who underwent point-of-care ultrasound (POCUS), brain MRI, or dark blood MRI were negative for disease. Five patients who underwent 18F-FDG PET had findings consistent with GCA. One patient with GCA had CTA of the head and neck with radiographic findings supportive of GCA.
Discussion
The available evidence supports the use of additional screening tests to increase the temporal artery biopsy yield for GCA. Inflammatory laboratory markers demonstrate some sensitivity but are nonspecific for GCA. In this study, only 60% of patients with GCA underwent diagnostic imaging as part of the workup. There are multiple factors that may contribute to the underutilization of advanced imaging in the diagnosis of GCA, including outdated standardized diagnostic criteria, limited resources (direct access to modalities), and lack of clinician awareness of diagnostic testing options. In this retrospective review, 30 patients (71%) were diagnosed with GCA with a 1990 ACR GCA score ≤ 3. Of these 30 patients, 19 underwent confirmatory biopsy followed by prolonged courses of steroid therapy. In addition, only 25 patients underwent advanced imaging to increase diagnostic accuracy of the suspected syndrome.
A large meta-analysis demonstrated a sensitivity of 77.3% (95% CI, 71.8-81.9%) for temporal artery biopsy.21 The overall yield was 40% in the meta-analysis. Advanced noninvasive imaging represents an appropriate method of evaluating GCA.8-20 In our study, 18F-FDG PET demonstrated the highest sensitivity (36%) for the diagnosis of GCA. Ultrasonography is recommended as an initial screening tool to identify the noncompressible halo sign (a hypoechoic circumferential wall thickening due to edema) as a cost-effective and widely available technology.22 Other research has corroborated the beneficial use of ultrasonography in improving diagnostic accuracy by detecting the noncompressible halo sign in temporal arteries.22,23 GCA diagnostic performance has been significantly improved with the use of B-mode probes ≥ 15 MHz as well as proposals to incorporate a compression sign or interrogating the axillary vessels, showing a sensitivity of 54% to 77%.23,24
POCUS may reduce the risk of a false-negative biopsy and improve yield with more frequent utilization. However, ultrasonography may be limited by operator skills and visualization of the great vessels. The accuracy of ultrasonography is dependent on the experience and adeptness of the operator. Additional studies are needed to establish a systematic standard for POCUS training to ensure accurate interpretation and uniform interrogation procedure.24 Artificial intelligence (AI) may aid in interpreting results of POCUS and bridging the operator skill gap among operators.25,26 AI and machine learning techniques can assist in detecting the noncompressible halo sign and compression sign in temporal arteries and other affected vessels.
In comparing the WRNMMC patient population with other US civilian GCA cohorts, there are some differences and similarities. There was a high representation of African American patients in the study, which may reflect a greater severity of autoimmune disease expression in this population.27 We also observed a higher number of females and an association with polymyalgia rheumatica in the data, consistent with previous reports.28,29 The females in this study were primarily civilians and therefore more similar to the general population of individuals with GCA. In contrast, male patients were more likely to be active-duty service members or have prior service experience with increased exposure to novel environmental factors linked to increased risk of autoimmune disease. This includes an increased risk of Guillain-Barré syndrome and Graves disease among Vietnam veterans exposed to Agent Orange.30,31 Other studies have found that veterans with posttraumatic stress disorder are at increased risk for severe autoimmune diseases.32,33 As more women join the active-duty military, the impact of autoimmune disease in the military service population is expected to grow, requiring further research.
Conclusions
Early diagnosis and treatment of GCA are critical to preventing serious outcomes, such as visual loss, jaw or limb claudication, or ischemic stroke. The incidence of autoimmune disease is expected to rise in the armed forces and veteran populations due to exposure to novel environmental factors and the increasing representation of women in the military. The use of additional screening tools can aid in earlier diagnosis of GCA. The 2022 ACR classification criteria for GCA represent significant updates to the 1990 criteria, incorporating ancillary tests such as the temporal artery halo sign on ultrasound, bilateral axillary vessel screening on imaging, and 18F-FDG PET activity throughout the aorta. The updated criteria require further validation and supports the adoption of a multidisciplinary approach that includes ultrasonography, vascular MRI/CT, and 18F-FDG PET. Furthermore, AI may play a future key role in ultrasound interpretation and study interrogation procedure. Ultimately, ultrasonography is a noninvasive and promising technique for the early diagnosis of GCA. A target goal is to increase the yield of positive temporal artery biopsies to ≥ 70%.
- Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603-606. doi:10.1007/s10157-013-0869-6
- Kale N, Eggenberger E. Diagnosis and management of giant cell arteritis: a review. Curr Opin Ophthalmol. 2010;21:417-422. doi:10.1097/ICU.0b013e32833eae8b
- Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA. 2002;287:92-101.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23-43. doi:10.1016/s0163-7258(02)00297-8
- Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125-1133. doi:10.1002/art.22504
- Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and ß-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894. doi:10.1136/ard.2011.151464
- Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122-1128. doi:10.1002/art.1780330810
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford). 2017;56:506-515. doi:10.1093/rheumatology/kew273
- Slart RHJ, Nienhuis PH, Glaudemans AWJM, et al. Role of 18F-FDG PET/CT in large vessel vasculitis and polymyalgia rheumatica. J Nucl Med. 2023;64:515-521. doi:10.2967/jnumed.122.265016
- Shimol JB, Amital H, Lidar M, Domachevsky L, Shoenfeld Y, Davidson T. The utility of PET/CT in large vessel vasculitis. Sci Rep. 2020;10:17709. doi:10.1038/s41598-020-73818-2
- Ponte C, Grayson PC, Robson JC, et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Giant Cell Arteritis. Arthritis Rheumatol. 2022;74:1881-1889. doi:10.1002/art.42325
- He J, Williamson L, Ng B, et al. The diagnostic accuracy of temporal artery ultrasound and temporal artery biopsy in giant cell arteritis: a single center Australian experience over 10 years. Int J Rheum Dis. 2022;25:447-453. doi:10.1111/1756-185X.14288
- Stellingwerff MD, Brouwer E, Lensen KDF, et al. Different scoring methods of FDG PET/CT in giant cell arteritis: need for standardization. Medicine (Baltimore). 2015;94:e1542. doi:10.1097/MD.0000000000001542
- Conway R, Smyth AE, Kavanagh RG, et al. Diagnostic utility of computed tomographic angiography in giant-cell arteritis. Stroke. 2018;49:2233-2236. doi:10.1161/STROKEAHA.118.021995
- Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4:e000612. doi:10.1136/rmdopen-2017-000612
- Rehak Z, Vasina J, Ptacek J, et al. PET/CT in giant cell arteritis: high 18F-FDG uptake in the temporal, occipital and vertebral arteries. Rev Esp Med Nucl Imagen Mol. 2016;35:398-401. doi:10.1016/j.remn.2016.03.007
- Salvarani C, Soriano A, Muratore F, et al. Is PET/CT essential in the diagnosis and follow-up of temporal arteritis? Autoimmun Rev. 2017;16:1125-1130. doi:10.1016/j.autrev.2017.09.007
- Brodmann M, Lipp RW, Passath A, et al. The role of 2-18F-fluoro-2-deoxy-D-glucose positron emission tomography in the diagnosis of giant cell arteritis of the temporal arteries. Rheumatology (Oxford). 2004;43:241-242. doi:10.1093/rheumatology/keh025
- Flaus A, Granjon D, Habouzit V, Gaultier JB, Prevot-Bitot N. Unusual and diffuse hypermetabolism in routine 18F-FDG PET/CT of the supra-aortic vessels in biopsy-positive giant cell arteritis. Clin Nucl Med. 2018;43:e336-e337. doi:10.1097/RLU.0000000000002198
- Berger CT, Sommer G, Aschwanden M, et al. The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis. Swiss Med Wkly. 2018;148:w14661. doi:10.4414/smw.2018.14661
- Rubenstein E, Maldini C, Gonzalez-Chiappe S, et al. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: a systematic literature review and meta-analysis. Rheumatology (Oxford). 2020;59:1011-1020. doi:10.1093/rheumatology/kez385
- Tsivgoulis G, Heliopoulos I, Vadikolias K, et al. Teaching neuroimages: ultrasound findings in giant-cell arteritis. Neurology. 2010;75:e67-e68. doi:10.1212/WNL.0b013e3181f881e9
- Nakajima E, Moon FH, Canvas Jr N, et al. Accuracy of Doppler ultrasound in the diagnosis of giant cell arteritis: a systematic review and meta-analysis. Adv Rheumatol. 2023;63:5. doi:10.1186/s42358-023-00286-3
- Naumegni SR, Hoffmann C, Cornec D, et al. Temporal artery ultrasound to diagnose giant cell arteritis: a practical guide. Ultrasound Med Biol. 2021;47:201-213. doi:10.1016/j.ultrasmedbio.2020.10.004
- Kim YH. Artificial intelligence in medical ultrasonography: driving on an unpaved road. Ultrasonography. 2021;40:313-317. doi:10.14366/usg.21031
- Sultan LR, Mohamed MH, Andronikou S. ChatGPT-4: a breakthrough in ultrasound image analysis. Radiol Adv. 2024;1:umae006. doi:10.1093/radadv/umae006
- Cipriani VP, Klein S. Clinical characteristics of multiple sclerosis in African-Americans. Curr Neurol Neurosci Rep. 2019;19:87. doi:10.1007/s11910-019-1000-5
- Sturm A, Dechant C, Proft F, et al. Gender differences in giant cell arteritis: a case-control study. Clin Exp Rheumatol. 2016;34:S70-72.
- Li KJ, Semenov D, Turk M, et al. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Res Ther. 2021;23:82. doi:10.1186/s13075-021-02450-w
- Nelson L, Gormley R, Riddle MS, Tribble DR, Porter CK. The epidemiology of Guillain-Barré syndrome in U.S. military personnel: a case-control study. BMC Res Notes. 2009;2:171. doi:10.1186/1756-0500-2-171
- Spaulding SW. The possible roles of environmental factors and the aryl hydrocarbon receptor in the prevalence of thyroid diseases in Vietnam era veterans. Curr Opin Endocrinol Diabetes Obes. 2011;18:315-320.
- O’Donovan A, Cohen BE, Seal KH, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365-374. doi:10.1016/j.biopsych.2014.06.015
- Bookwalter DB, Roenfeldt KA, LeardMann CA, Kong SY, Riddle MS, Rull RP. Posttraumatic stress disorder and risk of selected autoimmune diseases among US military personnel. BMC Psychiatry. 2020;20:23. doi:10.1186/s12888-020-2432-9
Giant cell arteritis (GCA), the most commonly diagnosed systemic vasculitis, is a large- and medium-vessel vasculitis that can lead to significant morbidity due to aneurysm formation or vascular occlusion if not diagnosed in a timely manner.1,2 Diagnosis is typically based on clinical history and inflammatory markers. Laboratory inflammatory markers may be normal in the early stages of GCA but can be abnormal due to other unrelated reasons leading to a false positive diagnosis.3 Delayed treatment may lead to visual loss, jaw or limb claudication, or ischemic stroke.2 Initial treatment typically includes high-dose steroids that can lead to significant adverse reactions such as hypothalamic-pituitary-adrenal axis dysfunction, metabolic syndrome, premature atherosclerosis, and increased risk of infection.4-6
The 1990 American College of Rheumatology (ACR) criteria for GCA are widely recognized (Table 1).7 The criteria focuses on clinical manifestations, including new onset headache, temporal artery tenderness, age ≥ 50 years, erythrocyte sedimentation rate (ESR) ≥ 50 mm/hr, and temporal artery biopsy with positive anatomical findings.8 When 3 of the 5 1990 ACR criteria are present, the sensitivity and specificity is estimated to be > 90% for GCA vs alternative vasculitides.7

Although the 1990 ACR criteria do not include imaging, modalities such as ultrasound, computed tomography angiography (CTA), 18F-FDG positron emission tomography (PET), and magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA) may be used in GCA diagnosis.8-10 These imaging modalities have been added to the proposed ACR classification criteria for GCA.11 For this updated point system standard, age ≥ 50 years is a requirement and includes a positive temporal artery biopsy or temporal artery halo sign on ultrasound (+5 points), an ESR ≥ 50 mm/h or C-reactive protein (CRP) ≥ 10 mg/L (+3 points), or sudden visual loss (+3 points). Scalp tenderness, jaw or tongue claudication, new temporal headache, morning stiffness in shoulders or neck, temporal artery abnormality on vascular examination, bilateral axillary vessel involvement on imaging, and 18F-FDG PET activity throughout the aorta are scored +2 points each. With these new criteria, a cumulative score ≥ 6 is classified as GCA. Diagnostic accuracy is further improved with imaging: ultrasonography (sensitivity 55% and specificity 95%) and 18F-FDG PET (sensitivity 69% and specificity 92%), CTA (sensitivity 71% and specificity 86%), and MRI/MRA (sensitivity 73% and specificity 88%).12-15
In recent years, clinicians have reported increased glucose uptake in arteries observed on PET imaging that suggests GCA.9,10,16-20 18F-FDG accumulates in cells with high metabolic activity rates, such as areas of inflammation. In assessing temporal arteries or other involved vasculature (eg, axillary or great vessels) for GCA, this modality indicates increased glucose uptake in the lining of vessel walls. The inflammation of vessel walls can then be visualized with PET. 18F-FDG PET presents a noninvasive imaging technique for evaluating GCA but its use has been limited in the United States due to its high cost.
Methods
Approval for a retrospective chart review of patients evaluated for suspected GCA was obtained from the Walter Reed National Military Medical Center (WRNMMC) Institutional Review Board. The review included patients who underwent diagnostic procedures such as ultrasound, MRI, CT angiogram, and PET studies from 2016 through 2022. International Classification of Diseases codes used for case identification included: M31.6, M31.5, I77.6, I77.8, I77.89, I67.7, and I68.2. The Current Procedural Terminology code used for temporal artery biopsy is 37609.
Results
Seventy-eight charts were reviewed and 42 patients (54%) were diagnosed with GCA (Table 2). This study sample had a much higher proportion of African American subjects (31%) when compared with the civilian population, likely reflecting the higher representation of African Americans in the armed forces. Twenty-eight females (67%) were GCA positive. The most common presenting symptoms included 27 patients (64%) with headache, 17 (40%) with scalp tenderness, and 14 (33%) with jaw pain. The mean 1990 ACR score was 3.8 (range, 2-5). With respect to the score criteria: 41 patients (98%) were aged ≥ 50 years, 31 (74%) had new onset headache, and 31 (74%) had elevated ESR (Table 3). Acute ischemic optic neuropathy was documented in 4 patients (10%) with confirmed GCA. The mean ESR and CRP values at diagnosis were 66.2 mm/h (range, 7-122 mm/h) and 8.711 μg/mL (range, 0.054 – 92.690 μg/mL), respectively. Twenty-seven patients (64%) underwent biopsy: 24 (89%) were unilateral and 3 (11%) were bilateral (Table 4). Four patients with GCA (10%) were missing biopsy data. Nineteen patients with GCA (70%) had biopsies with pathologic findings consistent with GCA.



Twenty-five patients with GCA (60%) received ≥ 1 imaging modality. The most common imaging modality was MRI, which was used for 18 (43%) patients. Fourteen patients (33%) had 18F-FDG PET, 12 patients (29%) had MRA, and 11 patients (26%) had CTA. The small number of patients who underwent point-of-care ultrasound (POCUS), brain MRI, or dark blood MRI were negative for disease. Five patients who underwent 18F-FDG PET had findings consistent with GCA. One patient with GCA had CTA of the head and neck with radiographic findings supportive of GCA.
Discussion
The available evidence supports the use of additional screening tests to increase the temporal artery biopsy yield for GCA. Inflammatory laboratory markers demonstrate some sensitivity but are nonspecific for GCA. In this study, only 60% of patients with GCA underwent diagnostic imaging as part of the workup. There are multiple factors that may contribute to the underutilization of advanced imaging in the diagnosis of GCA, including outdated standardized diagnostic criteria, limited resources (direct access to modalities), and lack of clinician awareness of diagnostic testing options. In this retrospective review, 30 patients (71%) were diagnosed with GCA with a 1990 ACR GCA score ≤ 3. Of these 30 patients, 19 underwent confirmatory biopsy followed by prolonged courses of steroid therapy. In addition, only 25 patients underwent advanced imaging to increase diagnostic accuracy of the suspected syndrome.
A large meta-analysis demonstrated a sensitivity of 77.3% (95% CI, 71.8-81.9%) for temporal artery biopsy.21 The overall yield was 40% in the meta-analysis. Advanced noninvasive imaging represents an appropriate method of evaluating GCA.8-20 In our study, 18F-FDG PET demonstrated the highest sensitivity (36%) for the diagnosis of GCA. Ultrasonography is recommended as an initial screening tool to identify the noncompressible halo sign (a hypoechoic circumferential wall thickening due to edema) as a cost-effective and widely available technology.22 Other research has corroborated the beneficial use of ultrasonography in improving diagnostic accuracy by detecting the noncompressible halo sign in temporal arteries.22,23 GCA diagnostic performance has been significantly improved with the use of B-mode probes ≥ 15 MHz as well as proposals to incorporate a compression sign or interrogating the axillary vessels, showing a sensitivity of 54% to 77%.23,24
POCUS may reduce the risk of a false-negative biopsy and improve yield with more frequent utilization. However, ultrasonography may be limited by operator skills and visualization of the great vessels. The accuracy of ultrasonography is dependent on the experience and adeptness of the operator. Additional studies are needed to establish a systematic standard for POCUS training to ensure accurate interpretation and uniform interrogation procedure.24 Artificial intelligence (AI) may aid in interpreting results of POCUS and bridging the operator skill gap among operators.25,26 AI and machine learning techniques can assist in detecting the noncompressible halo sign and compression sign in temporal arteries and other affected vessels.
In comparing the WRNMMC patient population with other US civilian GCA cohorts, there are some differences and similarities. There was a high representation of African American patients in the study, which may reflect a greater severity of autoimmune disease expression in this population.27 We also observed a higher number of females and an association with polymyalgia rheumatica in the data, consistent with previous reports.28,29 The females in this study were primarily civilians and therefore more similar to the general population of individuals with GCA. In contrast, male patients were more likely to be active-duty service members or have prior service experience with increased exposure to novel environmental factors linked to increased risk of autoimmune disease. This includes an increased risk of Guillain-Barré syndrome and Graves disease among Vietnam veterans exposed to Agent Orange.30,31 Other studies have found that veterans with posttraumatic stress disorder are at increased risk for severe autoimmune diseases.32,33 As more women join the active-duty military, the impact of autoimmune disease in the military service population is expected to grow, requiring further research.
Conclusions
Early diagnosis and treatment of GCA are critical to preventing serious outcomes, such as visual loss, jaw or limb claudication, or ischemic stroke. The incidence of autoimmune disease is expected to rise in the armed forces and veteran populations due to exposure to novel environmental factors and the increasing representation of women in the military. The use of additional screening tools can aid in earlier diagnosis of GCA. The 2022 ACR classification criteria for GCA represent significant updates to the 1990 criteria, incorporating ancillary tests such as the temporal artery halo sign on ultrasound, bilateral axillary vessel screening on imaging, and 18F-FDG PET activity throughout the aorta. The updated criteria require further validation and supports the adoption of a multidisciplinary approach that includes ultrasonography, vascular MRI/CT, and 18F-FDG PET. Furthermore, AI may play a future key role in ultrasound interpretation and study interrogation procedure. Ultimately, ultrasonography is a noninvasive and promising technique for the early diagnosis of GCA. A target goal is to increase the yield of positive temporal artery biopsies to ≥ 70%.
Giant cell arteritis (GCA), the most commonly diagnosed systemic vasculitis, is a large- and medium-vessel vasculitis that can lead to significant morbidity due to aneurysm formation or vascular occlusion if not diagnosed in a timely manner.1,2 Diagnosis is typically based on clinical history and inflammatory markers. Laboratory inflammatory markers may be normal in the early stages of GCA but can be abnormal due to other unrelated reasons leading to a false positive diagnosis.3 Delayed treatment may lead to visual loss, jaw or limb claudication, or ischemic stroke.2 Initial treatment typically includes high-dose steroids that can lead to significant adverse reactions such as hypothalamic-pituitary-adrenal axis dysfunction, metabolic syndrome, premature atherosclerosis, and increased risk of infection.4-6
The 1990 American College of Rheumatology (ACR) criteria for GCA are widely recognized (Table 1).7 The criteria focuses on clinical manifestations, including new onset headache, temporal artery tenderness, age ≥ 50 years, erythrocyte sedimentation rate (ESR) ≥ 50 mm/hr, and temporal artery biopsy with positive anatomical findings.8 When 3 of the 5 1990 ACR criteria are present, the sensitivity and specificity is estimated to be > 90% for GCA vs alternative vasculitides.7

Although the 1990 ACR criteria do not include imaging, modalities such as ultrasound, computed tomography angiography (CTA), 18F-FDG positron emission tomography (PET), and magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA) may be used in GCA diagnosis.8-10 These imaging modalities have been added to the proposed ACR classification criteria for GCA.11 For this updated point system standard, age ≥ 50 years is a requirement and includes a positive temporal artery biopsy or temporal artery halo sign on ultrasound (+5 points), an ESR ≥ 50 mm/h or C-reactive protein (CRP) ≥ 10 mg/L (+3 points), or sudden visual loss (+3 points). Scalp tenderness, jaw or tongue claudication, new temporal headache, morning stiffness in shoulders or neck, temporal artery abnormality on vascular examination, bilateral axillary vessel involvement on imaging, and 18F-FDG PET activity throughout the aorta are scored +2 points each. With these new criteria, a cumulative score ≥ 6 is classified as GCA. Diagnostic accuracy is further improved with imaging: ultrasonography (sensitivity 55% and specificity 95%) and 18F-FDG PET (sensitivity 69% and specificity 92%), CTA (sensitivity 71% and specificity 86%), and MRI/MRA (sensitivity 73% and specificity 88%).12-15
In recent years, clinicians have reported increased glucose uptake in arteries observed on PET imaging that suggests GCA.9,10,16-20 18F-FDG accumulates in cells with high metabolic activity rates, such as areas of inflammation. In assessing temporal arteries or other involved vasculature (eg, axillary or great vessels) for GCA, this modality indicates increased glucose uptake in the lining of vessel walls. The inflammation of vessel walls can then be visualized with PET. 18F-FDG PET presents a noninvasive imaging technique for evaluating GCA but its use has been limited in the United States due to its high cost.
Methods
Approval for a retrospective chart review of patients evaluated for suspected GCA was obtained from the Walter Reed National Military Medical Center (WRNMMC) Institutional Review Board. The review included patients who underwent diagnostic procedures such as ultrasound, MRI, CT angiogram, and PET studies from 2016 through 2022. International Classification of Diseases codes used for case identification included: M31.6, M31.5, I77.6, I77.8, I77.89, I67.7, and I68.2. The Current Procedural Terminology code used for temporal artery biopsy is 37609.
Results
Seventy-eight charts were reviewed and 42 patients (54%) were diagnosed with GCA (Table 2). This study sample had a much higher proportion of African American subjects (31%) when compared with the civilian population, likely reflecting the higher representation of African Americans in the armed forces. Twenty-eight females (67%) were GCA positive. The most common presenting symptoms included 27 patients (64%) with headache, 17 (40%) with scalp tenderness, and 14 (33%) with jaw pain. The mean 1990 ACR score was 3.8 (range, 2-5). With respect to the score criteria: 41 patients (98%) were aged ≥ 50 years, 31 (74%) had new onset headache, and 31 (74%) had elevated ESR (Table 3). Acute ischemic optic neuropathy was documented in 4 patients (10%) with confirmed GCA. The mean ESR and CRP values at diagnosis were 66.2 mm/h (range, 7-122 mm/h) and 8.711 μg/mL (range, 0.054 – 92.690 μg/mL), respectively. Twenty-seven patients (64%) underwent biopsy: 24 (89%) were unilateral and 3 (11%) were bilateral (Table 4). Four patients with GCA (10%) were missing biopsy data. Nineteen patients with GCA (70%) had biopsies with pathologic findings consistent with GCA.



Twenty-five patients with GCA (60%) received ≥ 1 imaging modality. The most common imaging modality was MRI, which was used for 18 (43%) patients. Fourteen patients (33%) had 18F-FDG PET, 12 patients (29%) had MRA, and 11 patients (26%) had CTA. The small number of patients who underwent point-of-care ultrasound (POCUS), brain MRI, or dark blood MRI were negative for disease. Five patients who underwent 18F-FDG PET had findings consistent with GCA. One patient with GCA had CTA of the head and neck with radiographic findings supportive of GCA.
Discussion
The available evidence supports the use of additional screening tests to increase the temporal artery biopsy yield for GCA. Inflammatory laboratory markers demonstrate some sensitivity but are nonspecific for GCA. In this study, only 60% of patients with GCA underwent diagnostic imaging as part of the workup. There are multiple factors that may contribute to the underutilization of advanced imaging in the diagnosis of GCA, including outdated standardized diagnostic criteria, limited resources (direct access to modalities), and lack of clinician awareness of diagnostic testing options. In this retrospective review, 30 patients (71%) were diagnosed with GCA with a 1990 ACR GCA score ≤ 3. Of these 30 patients, 19 underwent confirmatory biopsy followed by prolonged courses of steroid therapy. In addition, only 25 patients underwent advanced imaging to increase diagnostic accuracy of the suspected syndrome.
A large meta-analysis demonstrated a sensitivity of 77.3% (95% CI, 71.8-81.9%) for temporal artery biopsy.21 The overall yield was 40% in the meta-analysis. Advanced noninvasive imaging represents an appropriate method of evaluating GCA.8-20 In our study, 18F-FDG PET demonstrated the highest sensitivity (36%) for the diagnosis of GCA. Ultrasonography is recommended as an initial screening tool to identify the noncompressible halo sign (a hypoechoic circumferential wall thickening due to edema) as a cost-effective and widely available technology.22 Other research has corroborated the beneficial use of ultrasonography in improving diagnostic accuracy by detecting the noncompressible halo sign in temporal arteries.22,23 GCA diagnostic performance has been significantly improved with the use of B-mode probes ≥ 15 MHz as well as proposals to incorporate a compression sign or interrogating the axillary vessels, showing a sensitivity of 54% to 77%.23,24
POCUS may reduce the risk of a false-negative biopsy and improve yield with more frequent utilization. However, ultrasonography may be limited by operator skills and visualization of the great vessels. The accuracy of ultrasonography is dependent on the experience and adeptness of the operator. Additional studies are needed to establish a systematic standard for POCUS training to ensure accurate interpretation and uniform interrogation procedure.24 Artificial intelligence (AI) may aid in interpreting results of POCUS and bridging the operator skill gap among operators.25,26 AI and machine learning techniques can assist in detecting the noncompressible halo sign and compression sign in temporal arteries and other affected vessels.
In comparing the WRNMMC patient population with other US civilian GCA cohorts, there are some differences and similarities. There was a high representation of African American patients in the study, which may reflect a greater severity of autoimmune disease expression in this population.27 We also observed a higher number of females and an association with polymyalgia rheumatica in the data, consistent with previous reports.28,29 The females in this study were primarily civilians and therefore more similar to the general population of individuals with GCA. In contrast, male patients were more likely to be active-duty service members or have prior service experience with increased exposure to novel environmental factors linked to increased risk of autoimmune disease. This includes an increased risk of Guillain-Barré syndrome and Graves disease among Vietnam veterans exposed to Agent Orange.30,31 Other studies have found that veterans with posttraumatic stress disorder are at increased risk for severe autoimmune diseases.32,33 As more women join the active-duty military, the impact of autoimmune disease in the military service population is expected to grow, requiring further research.
Conclusions
Early diagnosis and treatment of GCA are critical to preventing serious outcomes, such as visual loss, jaw or limb claudication, or ischemic stroke. The incidence of autoimmune disease is expected to rise in the armed forces and veteran populations due to exposure to novel environmental factors and the increasing representation of women in the military. The use of additional screening tools can aid in earlier diagnosis of GCA. The 2022 ACR classification criteria for GCA represent significant updates to the 1990 criteria, incorporating ancillary tests such as the temporal artery halo sign on ultrasound, bilateral axillary vessel screening on imaging, and 18F-FDG PET activity throughout the aorta. The updated criteria require further validation and supports the adoption of a multidisciplinary approach that includes ultrasonography, vascular MRI/CT, and 18F-FDG PET. Furthermore, AI may play a future key role in ultrasound interpretation and study interrogation procedure. Ultimately, ultrasonography is a noninvasive and promising technique for the early diagnosis of GCA. A target goal is to increase the yield of positive temporal artery biopsies to ≥ 70%.
- Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603-606. doi:10.1007/s10157-013-0869-6
- Kale N, Eggenberger E. Diagnosis and management of giant cell arteritis: a review. Curr Opin Ophthalmol. 2010;21:417-422. doi:10.1097/ICU.0b013e32833eae8b
- Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA. 2002;287:92-101.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23-43. doi:10.1016/s0163-7258(02)00297-8
- Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125-1133. doi:10.1002/art.22504
- Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and ß-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894. doi:10.1136/ard.2011.151464
- Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122-1128. doi:10.1002/art.1780330810
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford). 2017;56:506-515. doi:10.1093/rheumatology/kew273
- Slart RHJ, Nienhuis PH, Glaudemans AWJM, et al. Role of 18F-FDG PET/CT in large vessel vasculitis and polymyalgia rheumatica. J Nucl Med. 2023;64:515-521. doi:10.2967/jnumed.122.265016
- Shimol JB, Amital H, Lidar M, Domachevsky L, Shoenfeld Y, Davidson T. The utility of PET/CT in large vessel vasculitis. Sci Rep. 2020;10:17709. doi:10.1038/s41598-020-73818-2
- Ponte C, Grayson PC, Robson JC, et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Giant Cell Arteritis. Arthritis Rheumatol. 2022;74:1881-1889. doi:10.1002/art.42325
- He J, Williamson L, Ng B, et al. The diagnostic accuracy of temporal artery ultrasound and temporal artery biopsy in giant cell arteritis: a single center Australian experience over 10 years. Int J Rheum Dis. 2022;25:447-453. doi:10.1111/1756-185X.14288
- Stellingwerff MD, Brouwer E, Lensen KDF, et al. Different scoring methods of FDG PET/CT in giant cell arteritis: need for standardization. Medicine (Baltimore). 2015;94:e1542. doi:10.1097/MD.0000000000001542
- Conway R, Smyth AE, Kavanagh RG, et al. Diagnostic utility of computed tomographic angiography in giant-cell arteritis. Stroke. 2018;49:2233-2236. doi:10.1161/STROKEAHA.118.021995
- Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4:e000612. doi:10.1136/rmdopen-2017-000612
- Rehak Z, Vasina J, Ptacek J, et al. PET/CT in giant cell arteritis: high 18F-FDG uptake in the temporal, occipital and vertebral arteries. Rev Esp Med Nucl Imagen Mol. 2016;35:398-401. doi:10.1016/j.remn.2016.03.007
- Salvarani C, Soriano A, Muratore F, et al. Is PET/CT essential in the diagnosis and follow-up of temporal arteritis? Autoimmun Rev. 2017;16:1125-1130. doi:10.1016/j.autrev.2017.09.007
- Brodmann M, Lipp RW, Passath A, et al. The role of 2-18F-fluoro-2-deoxy-D-glucose positron emission tomography in the diagnosis of giant cell arteritis of the temporal arteries. Rheumatology (Oxford). 2004;43:241-242. doi:10.1093/rheumatology/keh025
- Flaus A, Granjon D, Habouzit V, Gaultier JB, Prevot-Bitot N. Unusual and diffuse hypermetabolism in routine 18F-FDG PET/CT of the supra-aortic vessels in biopsy-positive giant cell arteritis. Clin Nucl Med. 2018;43:e336-e337. doi:10.1097/RLU.0000000000002198
- Berger CT, Sommer G, Aschwanden M, et al. The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis. Swiss Med Wkly. 2018;148:w14661. doi:10.4414/smw.2018.14661
- Rubenstein E, Maldini C, Gonzalez-Chiappe S, et al. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: a systematic literature review and meta-analysis. Rheumatology (Oxford). 2020;59:1011-1020. doi:10.1093/rheumatology/kez385
- Tsivgoulis G, Heliopoulos I, Vadikolias K, et al. Teaching neuroimages: ultrasound findings in giant-cell arteritis. Neurology. 2010;75:e67-e68. doi:10.1212/WNL.0b013e3181f881e9
- Nakajima E, Moon FH, Canvas Jr N, et al. Accuracy of Doppler ultrasound in the diagnosis of giant cell arteritis: a systematic review and meta-analysis. Adv Rheumatol. 2023;63:5. doi:10.1186/s42358-023-00286-3
- Naumegni SR, Hoffmann C, Cornec D, et al. Temporal artery ultrasound to diagnose giant cell arteritis: a practical guide. Ultrasound Med Biol. 2021;47:201-213. doi:10.1016/j.ultrasmedbio.2020.10.004
- Kim YH. Artificial intelligence in medical ultrasonography: driving on an unpaved road. Ultrasonography. 2021;40:313-317. doi:10.14366/usg.21031
- Sultan LR, Mohamed MH, Andronikou S. ChatGPT-4: a breakthrough in ultrasound image analysis. Radiol Adv. 2024;1:umae006. doi:10.1093/radadv/umae006
- Cipriani VP, Klein S. Clinical characteristics of multiple sclerosis in African-Americans. Curr Neurol Neurosci Rep. 2019;19:87. doi:10.1007/s11910-019-1000-5
- Sturm A, Dechant C, Proft F, et al. Gender differences in giant cell arteritis: a case-control study. Clin Exp Rheumatol. 2016;34:S70-72.
- Li KJ, Semenov D, Turk M, et al. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Res Ther. 2021;23:82. doi:10.1186/s13075-021-02450-w
- Nelson L, Gormley R, Riddle MS, Tribble DR, Porter CK. The epidemiology of Guillain-Barré syndrome in U.S. military personnel: a case-control study. BMC Res Notes. 2009;2:171. doi:10.1186/1756-0500-2-171
- Spaulding SW. The possible roles of environmental factors and the aryl hydrocarbon receptor in the prevalence of thyroid diseases in Vietnam era veterans. Curr Opin Endocrinol Diabetes Obes. 2011;18:315-320.
- O’Donovan A, Cohen BE, Seal KH, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365-374. doi:10.1016/j.biopsych.2014.06.015
- Bookwalter DB, Roenfeldt KA, LeardMann CA, Kong SY, Riddle MS, Rull RP. Posttraumatic stress disorder and risk of selected autoimmune diseases among US military personnel. BMC Psychiatry. 2020;20:23. doi:10.1186/s12888-020-2432-9
- Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603-606. doi:10.1007/s10157-013-0869-6
- Kale N, Eggenberger E. Diagnosis and management of giant cell arteritis: a review. Curr Opin Ophthalmol. 2010;21:417-422. doi:10.1097/ICU.0b013e32833eae8b
- Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA. 2002;287:92-101.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23-43. doi:10.1016/s0163-7258(02)00297-8
- Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125-1133. doi:10.1002/art.22504
- Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and ß-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894. doi:10.1136/ard.2011.151464
- Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122-1128. doi:10.1002/art.1780330810
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford). 2017;56:506-515. doi:10.1093/rheumatology/kew273
- Slart RHJ, Nienhuis PH, Glaudemans AWJM, et al. Role of 18F-FDG PET/CT in large vessel vasculitis and polymyalgia rheumatica. J Nucl Med. 2023;64:515-521. doi:10.2967/jnumed.122.265016
- Shimol JB, Amital H, Lidar M, Domachevsky L, Shoenfeld Y, Davidson T. The utility of PET/CT in large vessel vasculitis. Sci Rep. 2020;10:17709. doi:10.1038/s41598-020-73818-2
- Ponte C, Grayson PC, Robson JC, et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Giant Cell Arteritis. Arthritis Rheumatol. 2022;74:1881-1889. doi:10.1002/art.42325
- He J, Williamson L, Ng B, et al. The diagnostic accuracy of temporal artery ultrasound and temporal artery biopsy in giant cell arteritis: a single center Australian experience over 10 years. Int J Rheum Dis. 2022;25:447-453. doi:10.1111/1756-185X.14288
- Stellingwerff MD, Brouwer E, Lensen KDF, et al. Different scoring methods of FDG PET/CT in giant cell arteritis: need for standardization. Medicine (Baltimore). 2015;94:e1542. doi:10.1097/MD.0000000000001542
- Conway R, Smyth AE, Kavanagh RG, et al. Diagnostic utility of computed tomographic angiography in giant-cell arteritis. Stroke. 2018;49:2233-2236. doi:10.1161/STROKEAHA.118.021995
- Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4:e000612. doi:10.1136/rmdopen-2017-000612
- Rehak Z, Vasina J, Ptacek J, et al. PET/CT in giant cell arteritis: high 18F-FDG uptake in the temporal, occipital and vertebral arteries. Rev Esp Med Nucl Imagen Mol. 2016;35:398-401. doi:10.1016/j.remn.2016.03.007
- Salvarani C, Soriano A, Muratore F, et al. Is PET/CT essential in the diagnosis and follow-up of temporal arteritis? Autoimmun Rev. 2017;16:1125-1130. doi:10.1016/j.autrev.2017.09.007
- Brodmann M, Lipp RW, Passath A, et al. The role of 2-18F-fluoro-2-deoxy-D-glucose positron emission tomography in the diagnosis of giant cell arteritis of the temporal arteries. Rheumatology (Oxford). 2004;43:241-242. doi:10.1093/rheumatology/keh025
- Flaus A, Granjon D, Habouzit V, Gaultier JB, Prevot-Bitot N. Unusual and diffuse hypermetabolism in routine 18F-FDG PET/CT of the supra-aortic vessels in biopsy-positive giant cell arteritis. Clin Nucl Med. 2018;43:e336-e337. doi:10.1097/RLU.0000000000002198
- Berger CT, Sommer G, Aschwanden M, et al. The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis. Swiss Med Wkly. 2018;148:w14661. doi:10.4414/smw.2018.14661
- Rubenstein E, Maldini C, Gonzalez-Chiappe S, et al. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: a systematic literature review and meta-analysis. Rheumatology (Oxford). 2020;59:1011-1020. doi:10.1093/rheumatology/kez385
- Tsivgoulis G, Heliopoulos I, Vadikolias K, et al. Teaching neuroimages: ultrasound findings in giant-cell arteritis. Neurology. 2010;75:e67-e68. doi:10.1212/WNL.0b013e3181f881e9
- Nakajima E, Moon FH, Canvas Jr N, et al. Accuracy of Doppler ultrasound in the diagnosis of giant cell arteritis: a systematic review and meta-analysis. Adv Rheumatol. 2023;63:5. doi:10.1186/s42358-023-00286-3
- Naumegni SR, Hoffmann C, Cornec D, et al. Temporal artery ultrasound to diagnose giant cell arteritis: a practical guide. Ultrasound Med Biol. 2021;47:201-213. doi:10.1016/j.ultrasmedbio.2020.10.004
- Kim YH. Artificial intelligence in medical ultrasonography: driving on an unpaved road. Ultrasonography. 2021;40:313-317. doi:10.14366/usg.21031
- Sultan LR, Mohamed MH, Andronikou S. ChatGPT-4: a breakthrough in ultrasound image analysis. Radiol Adv. 2024;1:umae006. doi:10.1093/radadv/umae006
- Cipriani VP, Klein S. Clinical characteristics of multiple sclerosis in African-Americans. Curr Neurol Neurosci Rep. 2019;19:87. doi:10.1007/s11910-019-1000-5
- Sturm A, Dechant C, Proft F, et al. Gender differences in giant cell arteritis: a case-control study. Clin Exp Rheumatol. 2016;34:S70-72.
- Li KJ, Semenov D, Turk M, et al. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Res Ther. 2021;23:82. doi:10.1186/s13075-021-02450-w
- Nelson L, Gormley R, Riddle MS, Tribble DR, Porter CK. The epidemiology of Guillain-Barré syndrome in U.S. military personnel: a case-control study. BMC Res Notes. 2009;2:171. doi:10.1186/1756-0500-2-171
- Spaulding SW. The possible roles of environmental factors and the aryl hydrocarbon receptor in the prevalence of thyroid diseases in Vietnam era veterans. Curr Opin Endocrinol Diabetes Obes. 2011;18:315-320.
- O’Donovan A, Cohen BE, Seal KH, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365-374. doi:10.1016/j.biopsych.2014.06.015
- Bookwalter DB, Roenfeldt KA, LeardMann CA, Kong SY, Riddle MS, Rull RP. Posttraumatic stress disorder and risk of selected autoimmune diseases among US military personnel. BMC Psychiatry. 2020;20:23. doi:10.1186/s12888-020-2432-9
Advanced Imaging Techniques Use in Giant Cell Arteritis Diagnosis: The Experience at Walter Reed National Military Medical Center
Advanced Imaging Techniques Use in Giant Cell Arteritis Diagnosis: The Experience at Walter Reed National Military Medical Center
Epidemiologic and Clinical Evaluation of the Bidirectional Link Between Molluscum Contagiosum and Atopic Dermatitis in Children
Epidemiologic and Clinical Evaluation of the Bidirectional Link Between Molluscum Contagiosum and Atopic Dermatitis in Children
Molluscum contagiosum (MC), which is caused by a DNA virus in the Poxviridae family, is a common viral skin infection that primarily affects children.1-4 The reported incidence and prevalence of MC exhibit notable geographic variation. Worldwide, annual incidence rates per 1000 individuals range from 3.1 to 25, and prevalence ranges from 0.27% to 34.6%.2-7
Molluscum contagiosum is diagnosed clinically and typically manifests as smooth, flesh-colored papules measuring 2 to 6 mm in diameter with central umbilication. It can manifest as a single lesion or multiple clustered lesions, or in a disseminated pattern. The primary mode of transmission is through contact with skin, lesions, or contaminated personal items, or via self-inoculation. The majority of cases are asymptomatic, but in some patients, MC may be associated with pruritus, tenderness, erythema, or irritation. When present, secondary bacterial infections can cause localized inflammation and pain.1,3,4 The pathogenesis hinges on MC virus replication within keratinocytes, disrupting cellular differentiation and keratinization. The virus persists in the host by influencing the immune response through various mechanisms, including interference with signaling pathways, apoptosis inhibition, and antigen presentation disruption.3,4
Molluscum contagiosum typically follows a self-limiting trajectory, resolving over several months to 2 years.3,4 The resolution timeframe is intricately linked to variables such as the patient’s immune profile, lesion burden, and treatment approach. For symptomatic lesions, a variety of treatment options have been described, including physical ablation (eg, cryotherapy, curettage) and topical agents such as potassium hydroxide, cantharidin, imiquimod, and salicylic acid.3,4,8,9
Atopic dermatitis (AD) is a common chronic relapsing inflammatory skin disorder. In the United States, its prevalence ranges from 15% to 30% in children and from 2% to 10% in adults, with ongoing evidence of a growing global incidence.10-14 While AD can emerge at any age, typical onset is during early childhood. The clinical manifestation of AD includes a spectrum of eczematous features, often accompanied by persistent itching. The pathogenesis is multifactorial, involving a complex interplay of genetic, immunologic, and environmental factors. Key contributors to this multifaceted process encompass a compromised epidermal barrier, alterations in the skin microbiome, and an immune dysregulation promoting a type 2 immune response. Epidermal barrier dysfunction can be attributed to various factors, including diminished ceramide production, altered lipid composition, the release of inflammatory mediators, and mechanical damage from the persistent itch-scratch cycle.10-13,15 These factors or their interplay may enhance the susceptibility of patients with AD to infections.
Several studies conducted across various geographic regions examining the relationship between MC and AD have reported variable findings.2,6,7,16-21 Published studies have reported a prevalence of AD in children with MC ranging from 13.2% to 43%.2,6,7,16-21 Although some studies suggest a higher rate of atopy in patients with MC, not all research has confirmed this association.16,21 Dohil et al2 reported a greater number of MC lesions in children with AD than those without an atopic background. Silverberg20 reported that in 10% (5/50) of children with MC, the onset of AD was triggered, and in 22% (11/50) MC was associated with flares of pre-existing AD.
In this study, we aimed to assess MC infection rates in children with AD, analyze the epidemiologic aspects and severity differences between atopic children with and without MC infection, and compare data from atopic and nonatopic children with MC.
Methods
In this retrospective cohort study, we analyzed the medical records of pediatric patients diagnosed with MC, AD, or both conditions at an outpatient dermatology practice in Netanya, HaSharon, Israel, from September 2013 to August 2022. Data were collected from the electronic medical records and included patient demographics, the clinical presentation of MC and/or AD at diagnosis, and the duration of both conditions. Only patients with complete data and at least 6 months of follow-up were included. Key epidemiologic characteristics assessed included patient sex, age at the initial visit, and age at the onset of MC and/or AD. Diagnoses of MC and AD were established through clinical examinations conducted by dermatologists. The clinical evaluation of AD encompassed the assessment of body surface area involvement (categorized as <5%, 5%-10%, or >10%). Atopic dermatitis severity was classified as mild, moderate, or severe using the validated Investigator Global Assessment Scale for Atopic Dermatitis.22 Clinical evaluation of MC included assessment of the number of lesions (categorized as ≤4, 5-9, or ≥10), presence of inflammatory lesions, and resolution times for individual lesions (categorized as <1 week, several weeks, or unknown), as well as the overall resolution time for all lesions (categorized as <6 months, 6-12 months, 13-18 months, or >18 months). The temporal relationship between the appearance of MC and AD also was assessed.
Statistical Analysis—Numbers and percentages were used for categorical variables. Continuous variables were represented by mean and standard deviation. Categorical variables were compared using the χ2 test, and continuous variables between groups were compared using the Student t test. All statistical tests were 2-sided, with statistical significance defined as P≤.05. Statistical analysis was performed using SPSS software version 28 (IBM).
Results
Study Population—A total of 610 children were included in the study; 263 (43%) were female and 347 (57%) were male. The patients ranged in age from 4 months to 10 years, with a mean (SD) age of 4.87 (1.82) years. Five hundred fifty-six (91%) patients had AD, and 336 (55%) had MC. Within this cohort, 274 (45%) children had AD only, 54 (9%) had MC only, and 282 (46%) had both AD and MC. Regarding the temporal sequence, among the 282 children who had both AD and MC, AD preceded MC in 203 (72%) cases, both conditions were diagnosed concomitantly in 43 (15%) cases, and MC preceded AD in 36 (13%) cases. For cases in which the MC diagnosis followed the diagnosis of AD, the mean (SD) time between each diagnosis was 3.17 (1.5) years.
Comparison of Atopic and Nonatopic Children With MC—Although a higher proportion of males were diagnosed with MC (with or without concurrent AD), the differences in sex distribution between the 2 groups did not reach statistical significance. Among all children with MC, the majority (81.5% [274/336]) were aged 1 to 6 years at presentation. Patients with MC as their sole diagnosis had a similar mean age compared with those with concurrent AD. However, a detailed age subgroup analysis revealed a notable distinction: in the group with MC as the sole diagnosis, the majority (95% [51/54]) were younger than 7 years. In contrast, in the combined MC and AD group, MC manifested across a wider age range, with 21% (58/282) of patients being older than 7 years. In MC cases associated with AD, a notably higher lesion count and increased local inflammatory response were observed compared to those without AD. The time for complete resolution of all MC lesions was substantially prolonged in patients with comorbid AD. Specifically, 93% (50/54) of patients with MC without comorbid AD achieved full resolution within 1 year, whereas 52% (146/282) of patients with comorbid AD required more than 1 year for resolution (eTable 1).
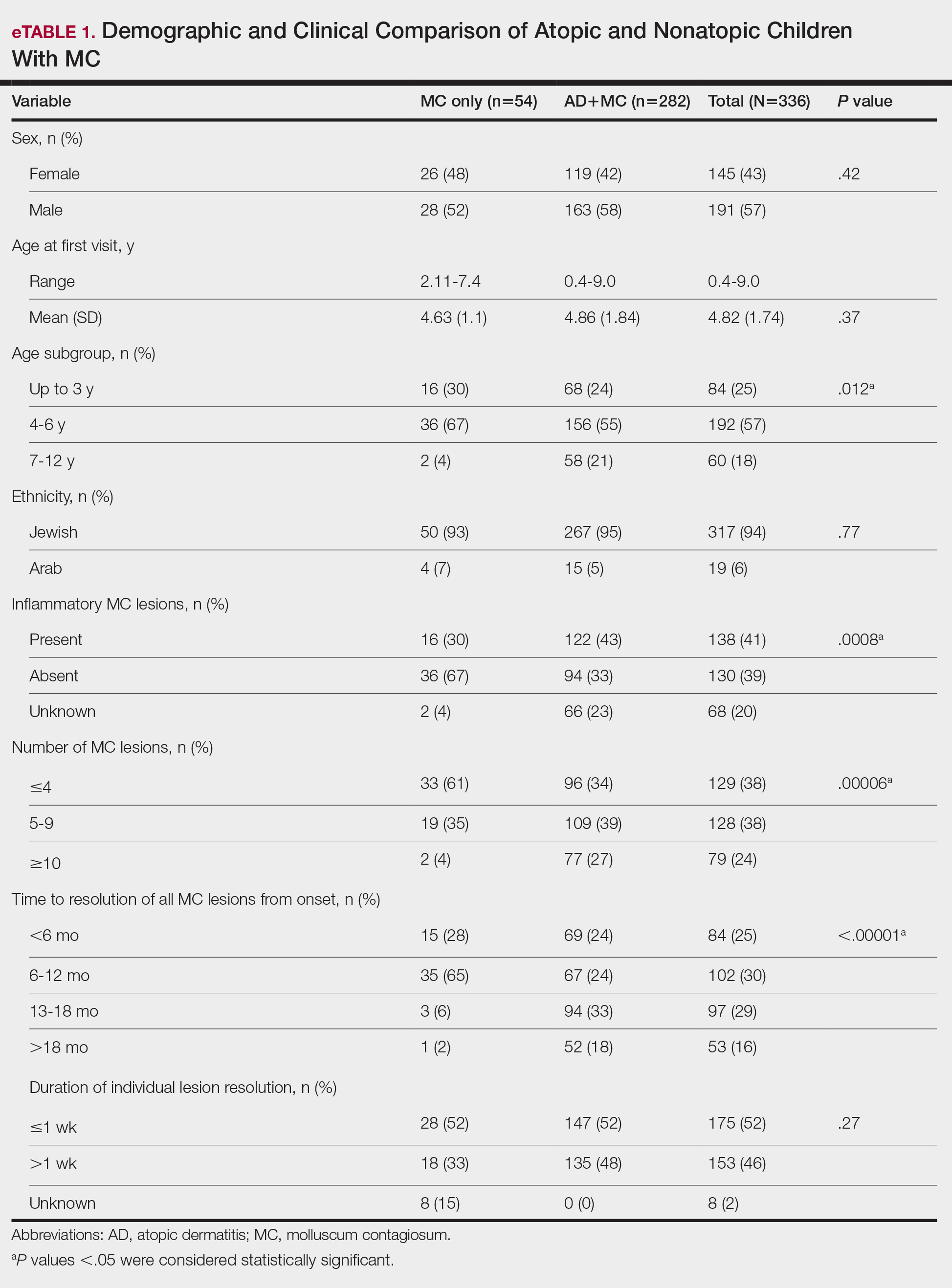
Comparison of Atopic Children With and Without MC—Sex, age distribution, and disease duration showed no differences between atopic patients with and without MC. Atopic patients with MC exhibited greater body surface area involvement and higher validated Investigator Global Assessment Scale for Atopic Dermatitis scores compared to atopic patients without MC (eTable 2).
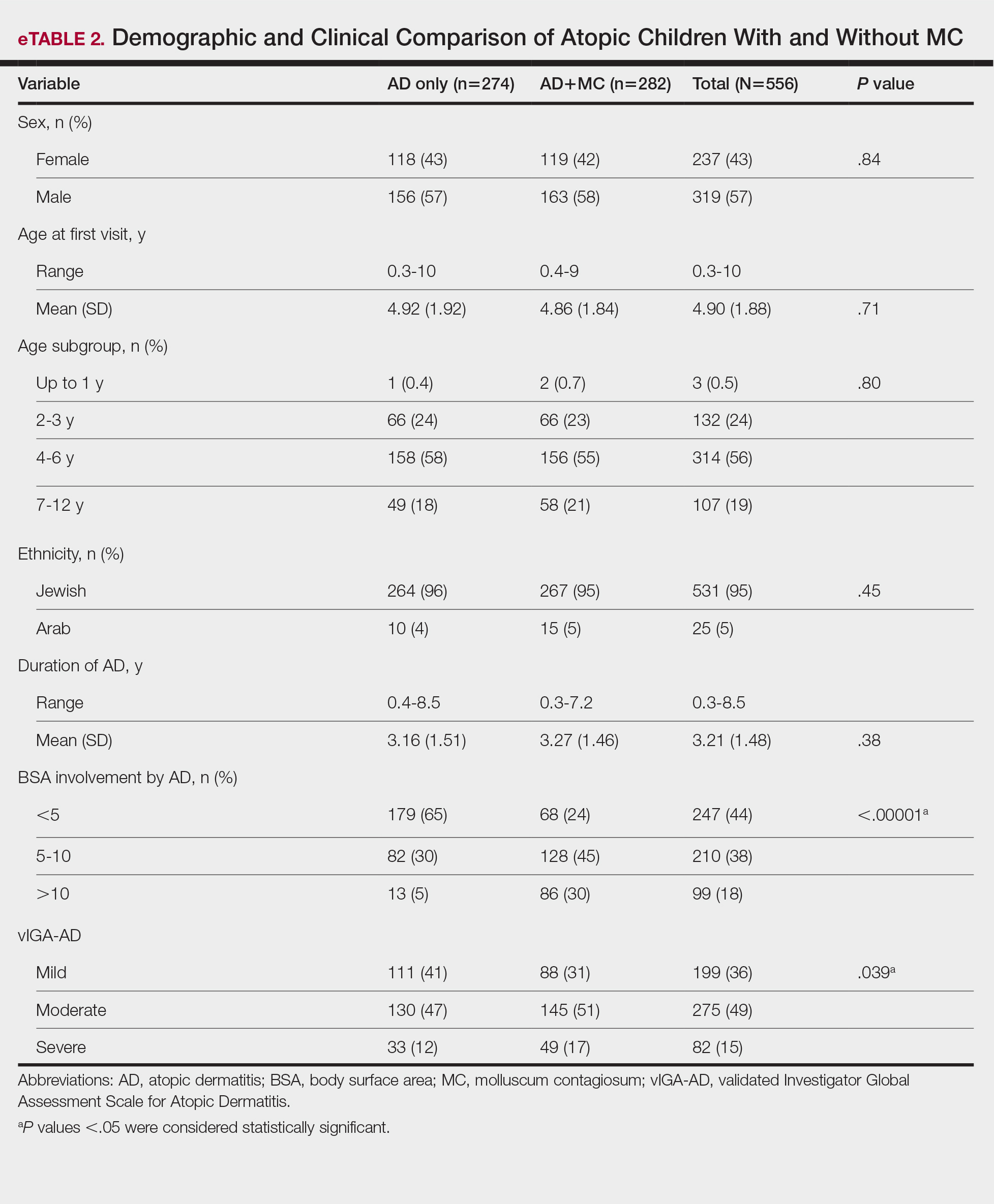
Comment
This study examined the relationship between MC and AD in pediatric patients, revealing a notable correlation and yielding valuable epidemiologic and clinical insights. Consistent with previous research, our study demonstrated a high prevalence of AD in children with MC.2,6,7,16-21 Previous studies indicated AD rates of 13% to 43% in pediatric patients with MC, whereas our study found a higher prevalence (84%), signifying a substantial majority of patients with MC in our cohort had AD. This discrepancy arises from factors such as demographic, genetic, and environmental differences, along with differences in access to medical care, referral practices, and diagnostic approaches across health care systems.14
Our temporal analysis of MC and AD diagnoses offers important insights. In the majority (72% [203/282]) of cases, the diagnosis of AD preceded MC, supporting previous research suggesting that the underlying pathophysiology of AD heightens susceptibility to MC.15,17-20 Less frequently, MC was diagnosed before or concurrently with AD, indicating that MC may occasionally trigger or exacerbate milder or undiagnosed AD, as previously proposed.20
A notable finding in our study was the expanded age range for MC onset in patients with AD, encompassing older age groups compared to patients with MC as their sole diagnosis, possibly due to persistent immune dysregulation. To the best of our knowledge, this specific observation has not been systematically reported or documented in prior cohort studies. Visible skin lesions of MC may have a psychological impact on patients, influencing self-consciousness and causing embarrassment and emotional distress. This may be more pronounced in older children, who are more aware of their appearance and social perceptions.23-25 These considerations should play a role in the management of MC.
Our study revealed that children with AD and MC displayed higher lesion counts, increased local inflammatory responses, and a more protracted resolution period compared to nonatopic children. In more than 50% of children with AD, MC took more than 1 year for resolution, whereas the majority of those without AD achieved resolution within 1 year. These findings may be attributed to AD-related immune dysregulation, influencing the natural course of MC. Consequently, it suggests that while nonatopic children with MC usually are managed through observation, atopic patients may benefit from an intervention-oriented approach.
Comparing atopic patients with and without MC showed a heightened occurrence of severe and extensive AD among those with concurrent MC. Several factors could contribute to this observation. On one hand, there could be a direct association between the extent and severity of AD, leading to an elevated susceptibility to MC. Conversely, MC might exacerbate immunologic dysregulation and intensify skin inflammation in atopic individuals.20 Additionally, itching related to both disorders may exacerbate inflammation and compromise the epidermal barrier, facilitating the spread of MC. This interplay suggests that each condition exacerbates the other in a self-reinforcing cycle. The importance of patient and caregiver education is underscored by recognizing these interactions. To manage both conditions effectively, health care providers should counsel patients and caregivers on maintaining proper skin care practices such as gentle cleansing with mild, fragrance-free products, regular moisturization, and avoidance of irritants, encourage them to avoid scratching, and recommend adopting an active treatment approach.
Our study had notable strengths. Firstly, a substantial sample size enhanced the statistical reliability of our findings. Additionally, valuable insights into the epidemiology and clinical aspects of AD and MC were obtained by utilizing real-world data from an outpatient dermatology practice. In our study, clinical evaluations covered body surface area involvement and disease severity for AD while also assessing lesion counts and the presence of inflammatory lesions for MC. This comprehensive approach facilitated a thorough analysis of both conditions. The extended data collection period not only allowed for observation of their clinical course and duration, but also enabled a detailed assessment of their interplay.
Our study also had several limitations. Primarily, its retrospective design relied on the accuracy and comprehensiveness of medical records, which may have introduced bias. The exclusion of some patients due to incomplete data further increased the potential for selection bias. Additionally, this study was conducted in a single outpatient dermatology practice in Israel, resulting in a study population composed predominantly of Jewish patients (94%), with a minority (6%) of Arab patients. Other ethnic groups, including Black, Asian, and Hispanic populations, were not represented. This reflects the country’s demographic composition rather than an intentional selection bias. However, the limited ethnic diversity reduces the generalizability of our findings. Differences in demographics, coding practices, health care utilization (eg, timeliness of seeking care, access to dermatology services), and treatment strategies also may impact the observed prevalence, clinical characteristics, and patient outcomes. Furthermore, while our study highlighted the potential advantages of a proactive treatment approach for atopic children with MC, it did not evaluate specific treatment protocols. Future research should aim to confirm the most efficacious therapeutic strategies for managing MC in atopic individuals and to include a more diverse population to better understand the applicability of findings across various ethnic groups.
Conclusion
Our study found a high prevalence of AD in children with MC and a strong bidirectional relationship between these conditions. Pediatric patients with AD display a broader age range for MC, greater lesion burden, increased local inflammatory responses, prolonged resolution times, and more extensive and severe AD.
Recognizing the interplay between MC and AD is crucial, highlighting the importance of health care providers educating patients and caregivers. Emphasizing skin hygiene, discouraging scratching, and implementing proactive treatment approaches can enhance the outcomes of both conditions. Further research into the underlying mechanisms of this association and effective therapeutic strategies for MC in atopic individuals is warranted.
Acknowledgments—The authors thank Zvi Segal, MD (Tel Hashomer, Israel) for his insightful contribution to the statistical analysis of the results. We would like to express our appreciation to the dedicated team of the dermatology practice in Netanya for the support throughout the performance of the study. Additionally, we thank all study participants and their parents for their participation and contribution to our research.
- Han H, Smythe C, Yousefian F, et al. Molluscum contagiosum virus evasion of immune surveillance: a review. J Drugs Dermatol. 2023;22182-189.
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54.
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305;E1;E2.
- Forbat E, Al-Niaimi F, Ali FR. Molluscum contagiosum: review and update on management. Pediatr Dermatol. 2017;34:504-515.
- Olsen JR, Gallacher J, Piguet V, et al. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31:130-136.
- Kakourou T, Zachariades A, Anastasiou T, et al. Molluscum contagiosum in Greek children: a case series. Int J Dermatol. 2005;44:221-223.
- Osio A, Deslandes E, Saada V, et al. Clinical characteristics of molluscum contagiosum in children in a private dermatology practice in the greater Paris area, France: a prospective study in 661 patients. Dermatology. 2011;222:314-320.
- Hebert AA, Bhatia N, Del Rosso JQ. Molluscum contagiosum: epidemiology, considerations, treatment options, and therapeutic gaps. J Clin Aesthet Dermatol. 2023;16(8 Suppl 1):S4-S11.
- Chao YC, Ko MJ, Tsai WC, et al. Comparative efficacy of treatments for molluscum contagiosum: a systematic review and network meta-analysis. J Dtsch Dermatol Ges. 2023;21:587-597.
- Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33:281-288.
- Hale G, Davies E, Grindlay DJC, et al. What’s new in atopic eczema? an analysis of systematic reviews published in 2017. part 2: epidemiology, etiology, and risk factors. Clin Exp Dermatol. 2019;44:868-873.
- Tracy A, Bhatti S, Eichenfield LF. Update on pediatric atopic dermatitis. Cutis. 2020;106:143-146.
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345-360.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Manti S, Amorini M, Cuppari C, et al. Filaggrin mutations and molluscum contagiosum skin infection in patients with atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119446-451.
- Seize M, Ianhez M, Cestari S. A study of the correlation between molluscum contagiosum and atopic dermatitis in children. An Bras Dermatol. 2011;86:663-668.
- Ren Z, Silverberg JI. Association of atopic dermatitis with bacterial, fungal, viral, and sexually transmitted skin infections. Dermatitis. 2020;31:157-164.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Han JH, Yoon JW, Yook HJ, et al. Evaluation of atopic dermatitis and cutaneous infectious disorders using sequential pattern mining: a nationwide population-based cohort study. J Clin Med. 2022;11:3422.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Hayashida S, Furusho N, Uchi H, et al. Are lifetime prevalence of impetigo, molluscum and herpes infection really increased in children having atopic dermatitis? J Dermatol Sci. 2010;60:173-178.
- Simpson E, Bissonnette R, Eichenfield LF, et al. The Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD): the development and reliability testing of a novel clinical outcome measurement instrument for the severity of atopic dermatitis. J Am Acad Dermatol. 2020;83:839-846.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195.
- Ðurovic´ MR, Jankovic´ J, Spiric´ VT, et al. Does age influence the quality of life in children with atopic dermatitis? PLoS One. 2019;14:E0224618.
- Chernyshov PV. Stigmatization and self-perception in children with atopic dermatitis. Clin Cosmet Investig Dermatol. 2016;9:159-166.
Molluscum contagiosum (MC), which is caused by a DNA virus in the Poxviridae family, is a common viral skin infection that primarily affects children.1-4 The reported incidence and prevalence of MC exhibit notable geographic variation. Worldwide, annual incidence rates per 1000 individuals range from 3.1 to 25, and prevalence ranges from 0.27% to 34.6%.2-7
Molluscum contagiosum is diagnosed clinically and typically manifests as smooth, flesh-colored papules measuring 2 to 6 mm in diameter with central umbilication. It can manifest as a single lesion or multiple clustered lesions, or in a disseminated pattern. The primary mode of transmission is through contact with skin, lesions, or contaminated personal items, or via self-inoculation. The majority of cases are asymptomatic, but in some patients, MC may be associated with pruritus, tenderness, erythema, or irritation. When present, secondary bacterial infections can cause localized inflammation and pain.1,3,4 The pathogenesis hinges on MC virus replication within keratinocytes, disrupting cellular differentiation and keratinization. The virus persists in the host by influencing the immune response through various mechanisms, including interference with signaling pathways, apoptosis inhibition, and antigen presentation disruption.3,4
Molluscum contagiosum typically follows a self-limiting trajectory, resolving over several months to 2 years.3,4 The resolution timeframe is intricately linked to variables such as the patient’s immune profile, lesion burden, and treatment approach. For symptomatic lesions, a variety of treatment options have been described, including physical ablation (eg, cryotherapy, curettage) and topical agents such as potassium hydroxide, cantharidin, imiquimod, and salicylic acid.3,4,8,9
Atopic dermatitis (AD) is a common chronic relapsing inflammatory skin disorder. In the United States, its prevalence ranges from 15% to 30% in children and from 2% to 10% in adults, with ongoing evidence of a growing global incidence.10-14 While AD can emerge at any age, typical onset is during early childhood. The clinical manifestation of AD includes a spectrum of eczematous features, often accompanied by persistent itching. The pathogenesis is multifactorial, involving a complex interplay of genetic, immunologic, and environmental factors. Key contributors to this multifaceted process encompass a compromised epidermal barrier, alterations in the skin microbiome, and an immune dysregulation promoting a type 2 immune response. Epidermal barrier dysfunction can be attributed to various factors, including diminished ceramide production, altered lipid composition, the release of inflammatory mediators, and mechanical damage from the persistent itch-scratch cycle.10-13,15 These factors or their interplay may enhance the susceptibility of patients with AD to infections.
Several studies conducted across various geographic regions examining the relationship between MC and AD have reported variable findings.2,6,7,16-21 Published studies have reported a prevalence of AD in children with MC ranging from 13.2% to 43%.2,6,7,16-21 Although some studies suggest a higher rate of atopy in patients with MC, not all research has confirmed this association.16,21 Dohil et al2 reported a greater number of MC lesions in children with AD than those without an atopic background. Silverberg20 reported that in 10% (5/50) of children with MC, the onset of AD was triggered, and in 22% (11/50) MC was associated with flares of pre-existing AD.
In this study, we aimed to assess MC infection rates in children with AD, analyze the epidemiologic aspects and severity differences between atopic children with and without MC infection, and compare data from atopic and nonatopic children with MC.
Methods
In this retrospective cohort study, we analyzed the medical records of pediatric patients diagnosed with MC, AD, or both conditions at an outpatient dermatology practice in Netanya, HaSharon, Israel, from September 2013 to August 2022. Data were collected from the electronic medical records and included patient demographics, the clinical presentation of MC and/or AD at diagnosis, and the duration of both conditions. Only patients with complete data and at least 6 months of follow-up were included. Key epidemiologic characteristics assessed included patient sex, age at the initial visit, and age at the onset of MC and/or AD. Diagnoses of MC and AD were established through clinical examinations conducted by dermatologists. The clinical evaluation of AD encompassed the assessment of body surface area involvement (categorized as <5%, 5%-10%, or >10%). Atopic dermatitis severity was classified as mild, moderate, or severe using the validated Investigator Global Assessment Scale for Atopic Dermatitis.22 Clinical evaluation of MC included assessment of the number of lesions (categorized as ≤4, 5-9, or ≥10), presence of inflammatory lesions, and resolution times for individual lesions (categorized as <1 week, several weeks, or unknown), as well as the overall resolution time for all lesions (categorized as <6 months, 6-12 months, 13-18 months, or >18 months). The temporal relationship between the appearance of MC and AD also was assessed.
Statistical Analysis—Numbers and percentages were used for categorical variables. Continuous variables were represented by mean and standard deviation. Categorical variables were compared using the χ2 test, and continuous variables between groups were compared using the Student t test. All statistical tests were 2-sided, with statistical significance defined as P≤.05. Statistical analysis was performed using SPSS software version 28 (IBM).
Results
Study Population—A total of 610 children were included in the study; 263 (43%) were female and 347 (57%) were male. The patients ranged in age from 4 months to 10 years, with a mean (SD) age of 4.87 (1.82) years. Five hundred fifty-six (91%) patients had AD, and 336 (55%) had MC. Within this cohort, 274 (45%) children had AD only, 54 (9%) had MC only, and 282 (46%) had both AD and MC. Regarding the temporal sequence, among the 282 children who had both AD and MC, AD preceded MC in 203 (72%) cases, both conditions were diagnosed concomitantly in 43 (15%) cases, and MC preceded AD in 36 (13%) cases. For cases in which the MC diagnosis followed the diagnosis of AD, the mean (SD) time between each diagnosis was 3.17 (1.5) years.
Comparison of Atopic and Nonatopic Children With MC—Although a higher proportion of males were diagnosed with MC (with or without concurrent AD), the differences in sex distribution between the 2 groups did not reach statistical significance. Among all children with MC, the majority (81.5% [274/336]) were aged 1 to 6 years at presentation. Patients with MC as their sole diagnosis had a similar mean age compared with those with concurrent AD. However, a detailed age subgroup analysis revealed a notable distinction: in the group with MC as the sole diagnosis, the majority (95% [51/54]) were younger than 7 years. In contrast, in the combined MC and AD group, MC manifested across a wider age range, with 21% (58/282) of patients being older than 7 years. In MC cases associated with AD, a notably higher lesion count and increased local inflammatory response were observed compared to those without AD. The time for complete resolution of all MC lesions was substantially prolonged in patients with comorbid AD. Specifically, 93% (50/54) of patients with MC without comorbid AD achieved full resolution within 1 year, whereas 52% (146/282) of patients with comorbid AD required more than 1 year for resolution (eTable 1).

Comparison of Atopic Children With and Without MC—Sex, age distribution, and disease duration showed no differences between atopic patients with and without MC. Atopic patients with MC exhibited greater body surface area involvement and higher validated Investigator Global Assessment Scale for Atopic Dermatitis scores compared to atopic patients without MC (eTable 2).

Comment
This study examined the relationship between MC and AD in pediatric patients, revealing a notable correlation and yielding valuable epidemiologic and clinical insights. Consistent with previous research, our study demonstrated a high prevalence of AD in children with MC.2,6,7,16-21 Previous studies indicated AD rates of 13% to 43% in pediatric patients with MC, whereas our study found a higher prevalence (84%), signifying a substantial majority of patients with MC in our cohort had AD. This discrepancy arises from factors such as demographic, genetic, and environmental differences, along with differences in access to medical care, referral practices, and diagnostic approaches across health care systems.14
Our temporal analysis of MC and AD diagnoses offers important insights. In the majority (72% [203/282]) of cases, the diagnosis of AD preceded MC, supporting previous research suggesting that the underlying pathophysiology of AD heightens susceptibility to MC.15,17-20 Less frequently, MC was diagnosed before or concurrently with AD, indicating that MC may occasionally trigger or exacerbate milder or undiagnosed AD, as previously proposed.20
A notable finding in our study was the expanded age range for MC onset in patients with AD, encompassing older age groups compared to patients with MC as their sole diagnosis, possibly due to persistent immune dysregulation. To the best of our knowledge, this specific observation has not been systematically reported or documented in prior cohort studies. Visible skin lesions of MC may have a psychological impact on patients, influencing self-consciousness and causing embarrassment and emotional distress. This may be more pronounced in older children, who are more aware of their appearance and social perceptions.23-25 These considerations should play a role in the management of MC.
Our study revealed that children with AD and MC displayed higher lesion counts, increased local inflammatory responses, and a more protracted resolution period compared to nonatopic children. In more than 50% of children with AD, MC took more than 1 year for resolution, whereas the majority of those without AD achieved resolution within 1 year. These findings may be attributed to AD-related immune dysregulation, influencing the natural course of MC. Consequently, it suggests that while nonatopic children with MC usually are managed through observation, atopic patients may benefit from an intervention-oriented approach.
Comparing atopic patients with and without MC showed a heightened occurrence of severe and extensive AD among those with concurrent MC. Several factors could contribute to this observation. On one hand, there could be a direct association between the extent and severity of AD, leading to an elevated susceptibility to MC. Conversely, MC might exacerbate immunologic dysregulation and intensify skin inflammation in atopic individuals.20 Additionally, itching related to both disorders may exacerbate inflammation and compromise the epidermal barrier, facilitating the spread of MC. This interplay suggests that each condition exacerbates the other in a self-reinforcing cycle. The importance of patient and caregiver education is underscored by recognizing these interactions. To manage both conditions effectively, health care providers should counsel patients and caregivers on maintaining proper skin care practices such as gentle cleansing with mild, fragrance-free products, regular moisturization, and avoidance of irritants, encourage them to avoid scratching, and recommend adopting an active treatment approach.
Our study had notable strengths. Firstly, a substantial sample size enhanced the statistical reliability of our findings. Additionally, valuable insights into the epidemiology and clinical aspects of AD and MC were obtained by utilizing real-world data from an outpatient dermatology practice. In our study, clinical evaluations covered body surface area involvement and disease severity for AD while also assessing lesion counts and the presence of inflammatory lesions for MC. This comprehensive approach facilitated a thorough analysis of both conditions. The extended data collection period not only allowed for observation of their clinical course and duration, but also enabled a detailed assessment of their interplay.
Our study also had several limitations. Primarily, its retrospective design relied on the accuracy and comprehensiveness of medical records, which may have introduced bias. The exclusion of some patients due to incomplete data further increased the potential for selection bias. Additionally, this study was conducted in a single outpatient dermatology practice in Israel, resulting in a study population composed predominantly of Jewish patients (94%), with a minority (6%) of Arab patients. Other ethnic groups, including Black, Asian, and Hispanic populations, were not represented. This reflects the country’s demographic composition rather than an intentional selection bias. However, the limited ethnic diversity reduces the generalizability of our findings. Differences in demographics, coding practices, health care utilization (eg, timeliness of seeking care, access to dermatology services), and treatment strategies also may impact the observed prevalence, clinical characteristics, and patient outcomes. Furthermore, while our study highlighted the potential advantages of a proactive treatment approach for atopic children with MC, it did not evaluate specific treatment protocols. Future research should aim to confirm the most efficacious therapeutic strategies for managing MC in atopic individuals and to include a more diverse population to better understand the applicability of findings across various ethnic groups.
Conclusion
Our study found a high prevalence of AD in children with MC and a strong bidirectional relationship between these conditions. Pediatric patients with AD display a broader age range for MC, greater lesion burden, increased local inflammatory responses, prolonged resolution times, and more extensive and severe AD.
Recognizing the interplay between MC and AD is crucial, highlighting the importance of health care providers educating patients and caregivers. Emphasizing skin hygiene, discouraging scratching, and implementing proactive treatment approaches can enhance the outcomes of both conditions. Further research into the underlying mechanisms of this association and effective therapeutic strategies for MC in atopic individuals is warranted.
Acknowledgments—The authors thank Zvi Segal, MD (Tel Hashomer, Israel) for his insightful contribution to the statistical analysis of the results. We would like to express our appreciation to the dedicated team of the dermatology practice in Netanya for the support throughout the performance of the study. Additionally, we thank all study participants and their parents for their participation and contribution to our research.
Molluscum contagiosum (MC), which is caused by a DNA virus in the Poxviridae family, is a common viral skin infection that primarily affects children.1-4 The reported incidence and prevalence of MC exhibit notable geographic variation. Worldwide, annual incidence rates per 1000 individuals range from 3.1 to 25, and prevalence ranges from 0.27% to 34.6%.2-7
Molluscum contagiosum is diagnosed clinically and typically manifests as smooth, flesh-colored papules measuring 2 to 6 mm in diameter with central umbilication. It can manifest as a single lesion or multiple clustered lesions, or in a disseminated pattern. The primary mode of transmission is through contact with skin, lesions, or contaminated personal items, or via self-inoculation. The majority of cases are asymptomatic, but in some patients, MC may be associated with pruritus, tenderness, erythema, or irritation. When present, secondary bacterial infections can cause localized inflammation and pain.1,3,4 The pathogenesis hinges on MC virus replication within keratinocytes, disrupting cellular differentiation and keratinization. The virus persists in the host by influencing the immune response through various mechanisms, including interference with signaling pathways, apoptosis inhibition, and antigen presentation disruption.3,4
Molluscum contagiosum typically follows a self-limiting trajectory, resolving over several months to 2 years.3,4 The resolution timeframe is intricately linked to variables such as the patient’s immune profile, lesion burden, and treatment approach. For symptomatic lesions, a variety of treatment options have been described, including physical ablation (eg, cryotherapy, curettage) and topical agents such as potassium hydroxide, cantharidin, imiquimod, and salicylic acid.3,4,8,9
Atopic dermatitis (AD) is a common chronic relapsing inflammatory skin disorder. In the United States, its prevalence ranges from 15% to 30% in children and from 2% to 10% in adults, with ongoing evidence of a growing global incidence.10-14 While AD can emerge at any age, typical onset is during early childhood. The clinical manifestation of AD includes a spectrum of eczematous features, often accompanied by persistent itching. The pathogenesis is multifactorial, involving a complex interplay of genetic, immunologic, and environmental factors. Key contributors to this multifaceted process encompass a compromised epidermal barrier, alterations in the skin microbiome, and an immune dysregulation promoting a type 2 immune response. Epidermal barrier dysfunction can be attributed to various factors, including diminished ceramide production, altered lipid composition, the release of inflammatory mediators, and mechanical damage from the persistent itch-scratch cycle.10-13,15 These factors or their interplay may enhance the susceptibility of patients with AD to infections.
Several studies conducted across various geographic regions examining the relationship between MC and AD have reported variable findings.2,6,7,16-21 Published studies have reported a prevalence of AD in children with MC ranging from 13.2% to 43%.2,6,7,16-21 Although some studies suggest a higher rate of atopy in patients with MC, not all research has confirmed this association.16,21 Dohil et al2 reported a greater number of MC lesions in children with AD than those without an atopic background. Silverberg20 reported that in 10% (5/50) of children with MC, the onset of AD was triggered, and in 22% (11/50) MC was associated with flares of pre-existing AD.
In this study, we aimed to assess MC infection rates in children with AD, analyze the epidemiologic aspects and severity differences between atopic children with and without MC infection, and compare data from atopic and nonatopic children with MC.
Methods
In this retrospective cohort study, we analyzed the medical records of pediatric patients diagnosed with MC, AD, or both conditions at an outpatient dermatology practice in Netanya, HaSharon, Israel, from September 2013 to August 2022. Data were collected from the electronic medical records and included patient demographics, the clinical presentation of MC and/or AD at diagnosis, and the duration of both conditions. Only patients with complete data and at least 6 months of follow-up were included. Key epidemiologic characteristics assessed included patient sex, age at the initial visit, and age at the onset of MC and/or AD. Diagnoses of MC and AD were established through clinical examinations conducted by dermatologists. The clinical evaluation of AD encompassed the assessment of body surface area involvement (categorized as <5%, 5%-10%, or >10%). Atopic dermatitis severity was classified as mild, moderate, or severe using the validated Investigator Global Assessment Scale for Atopic Dermatitis.22 Clinical evaluation of MC included assessment of the number of lesions (categorized as ≤4, 5-9, or ≥10), presence of inflammatory lesions, and resolution times for individual lesions (categorized as <1 week, several weeks, or unknown), as well as the overall resolution time for all lesions (categorized as <6 months, 6-12 months, 13-18 months, or >18 months). The temporal relationship between the appearance of MC and AD also was assessed.
Statistical Analysis—Numbers and percentages were used for categorical variables. Continuous variables were represented by mean and standard deviation. Categorical variables were compared using the χ2 test, and continuous variables between groups were compared using the Student t test. All statistical tests were 2-sided, with statistical significance defined as P≤.05. Statistical analysis was performed using SPSS software version 28 (IBM).
Results
Study Population—A total of 610 children were included in the study; 263 (43%) were female and 347 (57%) were male. The patients ranged in age from 4 months to 10 years, with a mean (SD) age of 4.87 (1.82) years. Five hundred fifty-six (91%) patients had AD, and 336 (55%) had MC. Within this cohort, 274 (45%) children had AD only, 54 (9%) had MC only, and 282 (46%) had both AD and MC. Regarding the temporal sequence, among the 282 children who had both AD and MC, AD preceded MC in 203 (72%) cases, both conditions were diagnosed concomitantly in 43 (15%) cases, and MC preceded AD in 36 (13%) cases. For cases in which the MC diagnosis followed the diagnosis of AD, the mean (SD) time between each diagnosis was 3.17 (1.5) years.
Comparison of Atopic and Nonatopic Children With MC—Although a higher proportion of males were diagnosed with MC (with or without concurrent AD), the differences in sex distribution between the 2 groups did not reach statistical significance. Among all children with MC, the majority (81.5% [274/336]) were aged 1 to 6 years at presentation. Patients with MC as their sole diagnosis had a similar mean age compared with those with concurrent AD. However, a detailed age subgroup analysis revealed a notable distinction: in the group with MC as the sole diagnosis, the majority (95% [51/54]) were younger than 7 years. In contrast, in the combined MC and AD group, MC manifested across a wider age range, with 21% (58/282) of patients being older than 7 years. In MC cases associated with AD, a notably higher lesion count and increased local inflammatory response were observed compared to those without AD. The time for complete resolution of all MC lesions was substantially prolonged in patients with comorbid AD. Specifically, 93% (50/54) of patients with MC without comorbid AD achieved full resolution within 1 year, whereas 52% (146/282) of patients with comorbid AD required more than 1 year for resolution (eTable 1).

Comparison of Atopic Children With and Without MC—Sex, age distribution, and disease duration showed no differences between atopic patients with and without MC. Atopic patients with MC exhibited greater body surface area involvement and higher validated Investigator Global Assessment Scale for Atopic Dermatitis scores compared to atopic patients without MC (eTable 2).

Comment
This study examined the relationship between MC and AD in pediatric patients, revealing a notable correlation and yielding valuable epidemiologic and clinical insights. Consistent with previous research, our study demonstrated a high prevalence of AD in children with MC.2,6,7,16-21 Previous studies indicated AD rates of 13% to 43% in pediatric patients with MC, whereas our study found a higher prevalence (84%), signifying a substantial majority of patients with MC in our cohort had AD. This discrepancy arises from factors such as demographic, genetic, and environmental differences, along with differences in access to medical care, referral practices, and diagnostic approaches across health care systems.14
Our temporal analysis of MC and AD diagnoses offers important insights. In the majority (72% [203/282]) of cases, the diagnosis of AD preceded MC, supporting previous research suggesting that the underlying pathophysiology of AD heightens susceptibility to MC.15,17-20 Less frequently, MC was diagnosed before or concurrently with AD, indicating that MC may occasionally trigger or exacerbate milder or undiagnosed AD, as previously proposed.20
A notable finding in our study was the expanded age range for MC onset in patients with AD, encompassing older age groups compared to patients with MC as their sole diagnosis, possibly due to persistent immune dysregulation. To the best of our knowledge, this specific observation has not been systematically reported or documented in prior cohort studies. Visible skin lesions of MC may have a psychological impact on patients, influencing self-consciousness and causing embarrassment and emotional distress. This may be more pronounced in older children, who are more aware of their appearance and social perceptions.23-25 These considerations should play a role in the management of MC.
Our study revealed that children with AD and MC displayed higher lesion counts, increased local inflammatory responses, and a more protracted resolution period compared to nonatopic children. In more than 50% of children with AD, MC took more than 1 year for resolution, whereas the majority of those without AD achieved resolution within 1 year. These findings may be attributed to AD-related immune dysregulation, influencing the natural course of MC. Consequently, it suggests that while nonatopic children with MC usually are managed through observation, atopic patients may benefit from an intervention-oriented approach.
Comparing atopic patients with and without MC showed a heightened occurrence of severe and extensive AD among those with concurrent MC. Several factors could contribute to this observation. On one hand, there could be a direct association between the extent and severity of AD, leading to an elevated susceptibility to MC. Conversely, MC might exacerbate immunologic dysregulation and intensify skin inflammation in atopic individuals.20 Additionally, itching related to both disorders may exacerbate inflammation and compromise the epidermal barrier, facilitating the spread of MC. This interplay suggests that each condition exacerbates the other in a self-reinforcing cycle. The importance of patient and caregiver education is underscored by recognizing these interactions. To manage both conditions effectively, health care providers should counsel patients and caregivers on maintaining proper skin care practices such as gentle cleansing with mild, fragrance-free products, regular moisturization, and avoidance of irritants, encourage them to avoid scratching, and recommend adopting an active treatment approach.
Our study had notable strengths. Firstly, a substantial sample size enhanced the statistical reliability of our findings. Additionally, valuable insights into the epidemiology and clinical aspects of AD and MC were obtained by utilizing real-world data from an outpatient dermatology practice. In our study, clinical evaluations covered body surface area involvement and disease severity for AD while also assessing lesion counts and the presence of inflammatory lesions for MC. This comprehensive approach facilitated a thorough analysis of both conditions. The extended data collection period not only allowed for observation of their clinical course and duration, but also enabled a detailed assessment of their interplay.
Our study also had several limitations. Primarily, its retrospective design relied on the accuracy and comprehensiveness of medical records, which may have introduced bias. The exclusion of some patients due to incomplete data further increased the potential for selection bias. Additionally, this study was conducted in a single outpatient dermatology practice in Israel, resulting in a study population composed predominantly of Jewish patients (94%), with a minority (6%) of Arab patients. Other ethnic groups, including Black, Asian, and Hispanic populations, were not represented. This reflects the country’s demographic composition rather than an intentional selection bias. However, the limited ethnic diversity reduces the generalizability of our findings. Differences in demographics, coding practices, health care utilization (eg, timeliness of seeking care, access to dermatology services), and treatment strategies also may impact the observed prevalence, clinical characteristics, and patient outcomes. Furthermore, while our study highlighted the potential advantages of a proactive treatment approach for atopic children with MC, it did not evaluate specific treatment protocols. Future research should aim to confirm the most efficacious therapeutic strategies for managing MC in atopic individuals and to include a more diverse population to better understand the applicability of findings across various ethnic groups.
Conclusion
Our study found a high prevalence of AD in children with MC and a strong bidirectional relationship between these conditions. Pediatric patients with AD display a broader age range for MC, greater lesion burden, increased local inflammatory responses, prolonged resolution times, and more extensive and severe AD.
Recognizing the interplay between MC and AD is crucial, highlighting the importance of health care providers educating patients and caregivers. Emphasizing skin hygiene, discouraging scratching, and implementing proactive treatment approaches can enhance the outcomes of both conditions. Further research into the underlying mechanisms of this association and effective therapeutic strategies for MC in atopic individuals is warranted.
Acknowledgments—The authors thank Zvi Segal, MD (Tel Hashomer, Israel) for his insightful contribution to the statistical analysis of the results. We would like to express our appreciation to the dedicated team of the dermatology practice in Netanya for the support throughout the performance of the study. Additionally, we thank all study participants and their parents for their participation and contribution to our research.
- Han H, Smythe C, Yousefian F, et al. Molluscum contagiosum virus evasion of immune surveillance: a review. J Drugs Dermatol. 2023;22182-189.
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54.
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305;E1;E2.
- Forbat E, Al-Niaimi F, Ali FR. Molluscum contagiosum: review and update on management. Pediatr Dermatol. 2017;34:504-515.
- Olsen JR, Gallacher J, Piguet V, et al. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31:130-136.
- Kakourou T, Zachariades A, Anastasiou T, et al. Molluscum contagiosum in Greek children: a case series. Int J Dermatol. 2005;44:221-223.
- Osio A, Deslandes E, Saada V, et al. Clinical characteristics of molluscum contagiosum in children in a private dermatology practice in the greater Paris area, France: a prospective study in 661 patients. Dermatology. 2011;222:314-320.
- Hebert AA, Bhatia N, Del Rosso JQ. Molluscum contagiosum: epidemiology, considerations, treatment options, and therapeutic gaps. J Clin Aesthet Dermatol. 2023;16(8 Suppl 1):S4-S11.
- Chao YC, Ko MJ, Tsai WC, et al. Comparative efficacy of treatments for molluscum contagiosum: a systematic review and network meta-analysis. J Dtsch Dermatol Ges. 2023;21:587-597.
- Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33:281-288.
- Hale G, Davies E, Grindlay DJC, et al. What’s new in atopic eczema? an analysis of systematic reviews published in 2017. part 2: epidemiology, etiology, and risk factors. Clin Exp Dermatol. 2019;44:868-873.
- Tracy A, Bhatti S, Eichenfield LF. Update on pediatric atopic dermatitis. Cutis. 2020;106:143-146.
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345-360.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Manti S, Amorini M, Cuppari C, et al. Filaggrin mutations and molluscum contagiosum skin infection in patients with atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119446-451.
- Seize M, Ianhez M, Cestari S. A study of the correlation between molluscum contagiosum and atopic dermatitis in children. An Bras Dermatol. 2011;86:663-668.
- Ren Z, Silverberg JI. Association of atopic dermatitis with bacterial, fungal, viral, and sexually transmitted skin infections. Dermatitis. 2020;31:157-164.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Han JH, Yoon JW, Yook HJ, et al. Evaluation of atopic dermatitis and cutaneous infectious disorders using sequential pattern mining: a nationwide population-based cohort study. J Clin Med. 2022;11:3422.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Hayashida S, Furusho N, Uchi H, et al. Are lifetime prevalence of impetigo, molluscum and herpes infection really increased in children having atopic dermatitis? J Dermatol Sci. 2010;60:173-178.
- Simpson E, Bissonnette R, Eichenfield LF, et al. The Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD): the development and reliability testing of a novel clinical outcome measurement instrument for the severity of atopic dermatitis. J Am Acad Dermatol. 2020;83:839-846.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195.
- Ðurovic´ MR, Jankovic´ J, Spiric´ VT, et al. Does age influence the quality of life in children with atopic dermatitis? PLoS One. 2019;14:E0224618.
- Chernyshov PV. Stigmatization and self-perception in children with atopic dermatitis. Clin Cosmet Investig Dermatol. 2016;9:159-166.
- Han H, Smythe C, Yousefian F, et al. Molluscum contagiosum virus evasion of immune surveillance: a review. J Drugs Dermatol. 2023;22182-189.
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54.
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305;E1;E2.
- Forbat E, Al-Niaimi F, Ali FR. Molluscum contagiosum: review and update on management. Pediatr Dermatol. 2017;34:504-515.
- Olsen JR, Gallacher J, Piguet V, et al. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31:130-136.
- Kakourou T, Zachariades A, Anastasiou T, et al. Molluscum contagiosum in Greek children: a case series. Int J Dermatol. 2005;44:221-223.
- Osio A, Deslandes E, Saada V, et al. Clinical characteristics of molluscum contagiosum in children in a private dermatology practice in the greater Paris area, France: a prospective study in 661 patients. Dermatology. 2011;222:314-320.
- Hebert AA, Bhatia N, Del Rosso JQ. Molluscum contagiosum: epidemiology, considerations, treatment options, and therapeutic gaps. J Clin Aesthet Dermatol. 2023;16(8 Suppl 1):S4-S11.
- Chao YC, Ko MJ, Tsai WC, et al. Comparative efficacy of treatments for molluscum contagiosum: a systematic review and network meta-analysis. J Dtsch Dermatol Ges. 2023;21:587-597.
- Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33:281-288.
- Hale G, Davies E, Grindlay DJC, et al. What’s new in atopic eczema? an analysis of systematic reviews published in 2017. part 2: epidemiology, etiology, and risk factors. Clin Exp Dermatol. 2019;44:868-873.
- Tracy A, Bhatti S, Eichenfield LF. Update on pediatric atopic dermatitis. Cutis. 2020;106:143-146.
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345-360.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Manti S, Amorini M, Cuppari C, et al. Filaggrin mutations and molluscum contagiosum skin infection in patients with atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119446-451.
- Seize M, Ianhez M, Cestari S. A study of the correlation between molluscum contagiosum and atopic dermatitis in children. An Bras Dermatol. 2011;86:663-668.
- Ren Z, Silverberg JI. Association of atopic dermatitis with bacterial, fungal, viral, and sexually transmitted skin infections. Dermatitis. 2020;31:157-164.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Han JH, Yoon JW, Yook HJ, et al. Evaluation of atopic dermatitis and cutaneous infectious disorders using sequential pattern mining: a nationwide population-based cohort study. J Clin Med. 2022;11:3422.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Hayashida S, Furusho N, Uchi H, et al. Are lifetime prevalence of impetigo, molluscum and herpes infection really increased in children having atopic dermatitis? J Dermatol Sci. 2010;60:173-178.
- Simpson E, Bissonnette R, Eichenfield LF, et al. The Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD): the development and reliability testing of a novel clinical outcome measurement instrument for the severity of atopic dermatitis. J Am Acad Dermatol. 2020;83:839-846.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195.
- Ðurovic´ MR, Jankovic´ J, Spiric´ VT, et al. Does age influence the quality of life in children with atopic dermatitis? PLoS One. 2019;14:E0224618.
- Chernyshov PV. Stigmatization and self-perception in children with atopic dermatitis. Clin Cosmet Investig Dermatol. 2016;9:159-166.
Epidemiologic and Clinical Evaluation of the Bidirectional Link Between Molluscum Contagiosum and Atopic Dermatitis in Children
Epidemiologic and Clinical Evaluation of the Bidirectional Link Between Molluscum Contagiosum and Atopic Dermatitis in Children
Practice Points
- There is a high prevalence of atopic dermatitis (AD) in children with molluscum contagiosum, with a strong bidirectional relationship between these conditions.
- Children with AD display a broader age range for molluscum contagiosum, greater lesion burden, increased local inflammatory responses, prolonged resolution time, and more extensive and severe disease.