User login
Endometriosis: Clinical Diagnosis and Empiric Treatment
Dr. Taylor: Endometriosis is a very common disease. Unfortunately, it's still widely under-recognized. It's estimated that perhaps up to 10% of reproductive age women have endometriosis, yet many are never diagnosed or diagnosed late. The average time that it takes for someone to be diagnosed is about 10 years; that is from the time they have classic symptoms of endometriosis until the time they get a definitive diagnosis and treatment.
I think it's very important that we recognize the clinical features of endometriosis. It's absolutely crucial if we want to shorten the time tom diagnosis. Often patients see multiple physicians before they get an accurate diagnosis. They're often dismissed by their early caregivers who are not very familiar with the disease.
For me, the most important feature is the pelvic pain. To identify endometriosis, I look specifically at the cyclic nature of the pelvic pain, and the progressive nature of the pelvic pain. Endometriosis is by far the most common reason that women have pelvic pain and initially it tends to be cyclic. It often starts as dysmenorrhea (painful periods), however it can progress to the point where pain occurs at times outside of menses. In fact, it can progress to the point where it's painful all the time. However, it almost always starts as dysmennorhea, and progresses.
Pelvic pain that someone had from their first menses on, i.e., from menarche, is less likely to be endometriosis than the pain that progresses and becomes worse or spreads to other times of the menstrual cycle. Endometriosis is a progressive disease, and that's one of the key distinctions in making me think somebody has endometriosis.
Most women with cyclic pelvic pain that gets worse over time, cyclic progressive pelvic pain, will have endometriosis. Those are the key features I look for.
It is also very important to recognize that endometriosis can have effects outside of the reproductive tract, can cause systemic inflammation, and impact other organ systems. Bowel and bladder dysfunction are very common. If that is cyclic and coincides with the pelvic pain, it’s very likely to be secondary to endometriosis. It's important not to get distracted or mislead into making other diagnoses.
What are some of the common symptoms and how does that impact your diagnosis evaluation?
Dr. Taylor: The most common symptom we see is that cyclic pelvic pain I just discussed. Pain, often starting as dysmenorrhea, can go on to include pain outside of the time of menses and become more than just dysmenorrhea- pain in other areas. The other common symptom is infertility. Many women with endometriosis do not have severe pain or may not have pain at all, but first present when they have trouble conceiving.
Endometriosis may be the cause of infertility. Sometimes we recognize that it's endometriosis when we do a physical exam or an ultrasound and find an endometrioma. Cyclic pelvic pain is the classic symptom we look for, and infertility is another key symptom that we shouldn't forget.
Can you talk a little bit about empiric treatment, primarily what that means and how prevalent it is in your current practice?
Dr. Taylor: I think one of the problems we have today with the treatment of endometriosis is that it doesn't get recognized and doesn't get treated right away. When we talk about emperic treatment, it's usually ruling out other potential etiologies of pain. We must ensure no one has have an infection, a tumor or other etiologies that are the cause of the pain, and not simply presume that any cyclic progressive pelvic pain is endometriosis – however most of the time it will be due to endometriosis.
Usually with a good history, physical exam, and sometimes with addition of a transvaginal ultrasound, you can rule out other etiologies of pain, and have a very good idea that this pain is related to endometriosis. Based on clinical presentation, and without needing surgery, we can make a clinical diagnosis of endometriosis.
Clinical diagnosis allows patients to have this disease recognized earlier. It allows them to get into treatment sooner and reverses this trend that we've seen of delayed recognition. This delay is especially difficult in younger women in formative years in their life, when they're in school, when they're in an early stage of their career. If they're held back because of this debilitating pain, these are critical times and opportunities they really can't completely make up.
It's very important that we recognize endometriosis early. If we require a laparoscopy to make the diagnosis, the threshold becomes very high and we don't make the diagnosis. We miss a lot of women with endometriosis and don't treat them early.
There was time when our medical options were limited, and we wanted to be sure of our diagnosis. But these days, I think we can make a clinical diagnosis knowing that we have several treatment options that are relatively easy and safe for patients. Our first line therapy-- the first thing I use when I make the clinical diagnosis of endometriosis-- the first emperic treatment I would use is an oral contraceptive.
I prefer giving the oral contraceptive in a continuous fashion, rather than in a cyclic fashion. If someone has dysmenorrhea, why have menses at all? Retrograde menstruation is the etiology of most endometriosis. If we can eliminate menstruation, potentially it may be reducing endometriosis in the long run.
A lot of women with endometriosis will not respond to progestins—a phenomenon called progestin resistance—therefore, not everyone who has endometriosis will respond to an oral contraceptive. Probably about a third of patients either will not initially respond or will develop a resistance to a progestin and fail to respond in the long-term. Still others have side effects due to progestin therapy-- breast tenderness, mood changes, or a feeling of bloating are very common progestin related side effects.
We now have agents like oral GnRH antagonists that we can use as a second line treatment for those that either don't respond to progestins or those who have side effects from a progestin, including a progestin based oral contraceptive. Additionally, in those with severe pain you may want to use something a little more aggressive.
GnRH antagonists are easy to use oral medications, with an immediate onset of action and are easily reversed. We have come a long way from when we had to use an injectable GnRH agonist as the second line therapy. We have much simpler, easy, second line medications that we can turn to that makes empiric treatment a lot easier.
In the past, when we made a surgical diagnosis it was often because we were afraid of committing them to a long course of Depo GnRH agonist treatment; we didn't use a lot of add back therapy and they had tremendous side effects and the risk of bone loss. Patients and their physicians were reluctant to use GnRH agonists.
Things have gotten a lot easier with oral contraceptives, that can be followed by GnRH antagonists, which are easy to use, simple medications that are very patient-friendly. I think we can make that clinical diagnosis. We can move to an empiric treatment, either first or second line with an oral contraceptive or a GnRH antagonist and easily treat these women without significant side effects. It is important that we advocate for women with cyclic pain, and recognize it more readily, clinically diagnosed it, and begin that empiric treatment. That paradigm really has a huge impact on women's lives.
What recent advancements have been made in diagnosing and treating endometriosis?
Dr. Taylor: I think one of the biggest things that I see improving is recognition. Hopefully, we're narrowing that long delay by closing that time gap from initial symptoms to recognition. As we see public awareness grow, patients are recognizing and looking for answers, and thinking to themselves that they may have endometriosis.
Years ago, people were embarrassed to talk about menstruation, painful menstruation, pain with intercourse, pain with bowel movements. Thankfully, we're a more open society now and we can talk about these things. Women are starting to realize that they may have a problem where it was just dismissed before or perhaps, they were embarrassed to talk about it. I think we have seen a huge advancement. Physicians, as well, are recognizing endometriosis even more than before.
I think we're also much more accepting of this clinical diagnosis paradigm and empiric treatment. A lot of that, as we just said, comes from having better, easier to use drugs available that are much more patient-friendly. The GnRH antagonist elagolix is currently available for treatment of endometriosis in the United States. There are two more GnRH antagonists in the pipeline-- relugolix, which we expect to be approved shortly, and linzagolix which is undergoing phase three clinical trials now. Hopefully, we will have several of these second line drugs, drugs which we can even use first line for severe endometriosis. Their availability is another huge advance.
I also think it is essential, that we don't put someone through surgery to recognize endometriosis. We must be better at taking a good history, doing an exam, and ultrasound when needed. You don't need a surgery to diagnose endometriosis.
However, we do still sometimes need surgery to treat endometriosis. Often, endometriosis will cause adhesions or scarring. These long-term sequelae of endometriosis can still require surgery. The medications we have available today are very good at stopping active disease. But the damage done from long-term endometriosis if we don’t treat may still require surgery. I'm hoping that the earlier we start treating people, the less damage will be done, and the less therapeutic surgeries needed. I think these changes are coming and are all very promising.
It would also be great if we had a non-invasive definitive diagnostic test. There are several of those under development, but nothing available yet. I suspect that we'll see those become available very shortly.
The other thing that we still need in the field is a treatment we can use for those women trying to get pregnant. We use in vitro fertilization, which works very well in the endometriosis population. But a medical therapy that can suppress endometriosis and allow people to try to conceive without needing IVF is something I hope for in the future. A specific endometriosis therapy that is not hormonal, that targets the specific pathophysiology of endometriosis, is something that I'd like to see developed and many of us are currently working on.
I think there is a lot coming, but we've already moved the needle a long way. The GnRH antagonists have given us much more confidence in moving forward with clinical diagnosis and empiric treatment of this disease. It's a huge boon for women's health, allowing early recognition and preventing long-term complications of endometriosis.
Dr. Taylor: Endometriosis is a very common disease. Unfortunately, it's still widely under-recognized. It's estimated that perhaps up to 10% of reproductive age women have endometriosis, yet many are never diagnosed or diagnosed late. The average time that it takes for someone to be diagnosed is about 10 years; that is from the time they have classic symptoms of endometriosis until the time they get a definitive diagnosis and treatment.
I think it's very important that we recognize the clinical features of endometriosis. It's absolutely crucial if we want to shorten the time tom diagnosis. Often patients see multiple physicians before they get an accurate diagnosis. They're often dismissed by their early caregivers who are not very familiar with the disease.
For me, the most important feature is the pelvic pain. To identify endometriosis, I look specifically at the cyclic nature of the pelvic pain, and the progressive nature of the pelvic pain. Endometriosis is by far the most common reason that women have pelvic pain and initially it tends to be cyclic. It often starts as dysmenorrhea (painful periods), however it can progress to the point where pain occurs at times outside of menses. In fact, it can progress to the point where it's painful all the time. However, it almost always starts as dysmennorhea, and progresses.
Pelvic pain that someone had from their first menses on, i.e., from menarche, is less likely to be endometriosis than the pain that progresses and becomes worse or spreads to other times of the menstrual cycle. Endometriosis is a progressive disease, and that's one of the key distinctions in making me think somebody has endometriosis.
Most women with cyclic pelvic pain that gets worse over time, cyclic progressive pelvic pain, will have endometriosis. Those are the key features I look for.
It is also very important to recognize that endometriosis can have effects outside of the reproductive tract, can cause systemic inflammation, and impact other organ systems. Bowel and bladder dysfunction are very common. If that is cyclic and coincides with the pelvic pain, it’s very likely to be secondary to endometriosis. It's important not to get distracted or mislead into making other diagnoses.
What are some of the common symptoms and how does that impact your diagnosis evaluation?
Dr. Taylor: The most common symptom we see is that cyclic pelvic pain I just discussed. Pain, often starting as dysmenorrhea, can go on to include pain outside of the time of menses and become more than just dysmenorrhea- pain in other areas. The other common symptom is infertility. Many women with endometriosis do not have severe pain or may not have pain at all, but first present when they have trouble conceiving.
Endometriosis may be the cause of infertility. Sometimes we recognize that it's endometriosis when we do a physical exam or an ultrasound and find an endometrioma. Cyclic pelvic pain is the classic symptom we look for, and infertility is another key symptom that we shouldn't forget.
Can you talk a little bit about empiric treatment, primarily what that means and how prevalent it is in your current practice?
Dr. Taylor: I think one of the problems we have today with the treatment of endometriosis is that it doesn't get recognized and doesn't get treated right away. When we talk about emperic treatment, it's usually ruling out other potential etiologies of pain. We must ensure no one has have an infection, a tumor or other etiologies that are the cause of the pain, and not simply presume that any cyclic progressive pelvic pain is endometriosis – however most of the time it will be due to endometriosis.
Usually with a good history, physical exam, and sometimes with addition of a transvaginal ultrasound, you can rule out other etiologies of pain, and have a very good idea that this pain is related to endometriosis. Based on clinical presentation, and without needing surgery, we can make a clinical diagnosis of endometriosis.
Clinical diagnosis allows patients to have this disease recognized earlier. It allows them to get into treatment sooner and reverses this trend that we've seen of delayed recognition. This delay is especially difficult in younger women in formative years in their life, when they're in school, when they're in an early stage of their career. If they're held back because of this debilitating pain, these are critical times and opportunities they really can't completely make up.
It's very important that we recognize endometriosis early. If we require a laparoscopy to make the diagnosis, the threshold becomes very high and we don't make the diagnosis. We miss a lot of women with endometriosis and don't treat them early.
There was time when our medical options were limited, and we wanted to be sure of our diagnosis. But these days, I think we can make a clinical diagnosis knowing that we have several treatment options that are relatively easy and safe for patients. Our first line therapy-- the first thing I use when I make the clinical diagnosis of endometriosis-- the first emperic treatment I would use is an oral contraceptive.
I prefer giving the oral contraceptive in a continuous fashion, rather than in a cyclic fashion. If someone has dysmenorrhea, why have menses at all? Retrograde menstruation is the etiology of most endometriosis. If we can eliminate menstruation, potentially it may be reducing endometriosis in the long run.
A lot of women with endometriosis will not respond to progestins—a phenomenon called progestin resistance—therefore, not everyone who has endometriosis will respond to an oral contraceptive. Probably about a third of patients either will not initially respond or will develop a resistance to a progestin and fail to respond in the long-term. Still others have side effects due to progestin therapy-- breast tenderness, mood changes, or a feeling of bloating are very common progestin related side effects.
We now have agents like oral GnRH antagonists that we can use as a second line treatment for those that either don't respond to progestins or those who have side effects from a progestin, including a progestin based oral contraceptive. Additionally, in those with severe pain you may want to use something a little more aggressive.
GnRH antagonists are easy to use oral medications, with an immediate onset of action and are easily reversed. We have come a long way from when we had to use an injectable GnRH agonist as the second line therapy. We have much simpler, easy, second line medications that we can turn to that makes empiric treatment a lot easier.
In the past, when we made a surgical diagnosis it was often because we were afraid of committing them to a long course of Depo GnRH agonist treatment; we didn't use a lot of add back therapy and they had tremendous side effects and the risk of bone loss. Patients and their physicians were reluctant to use GnRH agonists.
Things have gotten a lot easier with oral contraceptives, that can be followed by GnRH antagonists, which are easy to use, simple medications that are very patient-friendly. I think we can make that clinical diagnosis. We can move to an empiric treatment, either first or second line with an oral contraceptive or a GnRH antagonist and easily treat these women without significant side effects. It is important that we advocate for women with cyclic pain, and recognize it more readily, clinically diagnosed it, and begin that empiric treatment. That paradigm really has a huge impact on women's lives.
What recent advancements have been made in diagnosing and treating endometriosis?
Dr. Taylor: I think one of the biggest things that I see improving is recognition. Hopefully, we're narrowing that long delay by closing that time gap from initial symptoms to recognition. As we see public awareness grow, patients are recognizing and looking for answers, and thinking to themselves that they may have endometriosis.
Years ago, people were embarrassed to talk about menstruation, painful menstruation, pain with intercourse, pain with bowel movements. Thankfully, we're a more open society now and we can talk about these things. Women are starting to realize that they may have a problem where it was just dismissed before or perhaps, they were embarrassed to talk about it. I think we have seen a huge advancement. Physicians, as well, are recognizing endometriosis even more than before.
I think we're also much more accepting of this clinical diagnosis paradigm and empiric treatment. A lot of that, as we just said, comes from having better, easier to use drugs available that are much more patient-friendly. The GnRH antagonist elagolix is currently available for treatment of endometriosis in the United States. There are two more GnRH antagonists in the pipeline-- relugolix, which we expect to be approved shortly, and linzagolix which is undergoing phase three clinical trials now. Hopefully, we will have several of these second line drugs, drugs which we can even use first line for severe endometriosis. Their availability is another huge advance.
I also think it is essential, that we don't put someone through surgery to recognize endometriosis. We must be better at taking a good history, doing an exam, and ultrasound when needed. You don't need a surgery to diagnose endometriosis.
However, we do still sometimes need surgery to treat endometriosis. Often, endometriosis will cause adhesions or scarring. These long-term sequelae of endometriosis can still require surgery. The medications we have available today are very good at stopping active disease. But the damage done from long-term endometriosis if we don’t treat may still require surgery. I'm hoping that the earlier we start treating people, the less damage will be done, and the less therapeutic surgeries needed. I think these changes are coming and are all very promising.
It would also be great if we had a non-invasive definitive diagnostic test. There are several of those under development, but nothing available yet. I suspect that we'll see those become available very shortly.
The other thing that we still need in the field is a treatment we can use for those women trying to get pregnant. We use in vitro fertilization, which works very well in the endometriosis population. But a medical therapy that can suppress endometriosis and allow people to try to conceive without needing IVF is something I hope for in the future. A specific endometriosis therapy that is not hormonal, that targets the specific pathophysiology of endometriosis, is something that I'd like to see developed and many of us are currently working on.
I think there is a lot coming, but we've already moved the needle a long way. The GnRH antagonists have given us much more confidence in moving forward with clinical diagnosis and empiric treatment of this disease. It's a huge boon for women's health, allowing early recognition and preventing long-term complications of endometriosis.
Dr. Taylor: Endometriosis is a very common disease. Unfortunately, it's still widely under-recognized. It's estimated that perhaps up to 10% of reproductive age women have endometriosis, yet many are never diagnosed or diagnosed late. The average time that it takes for someone to be diagnosed is about 10 years; that is from the time they have classic symptoms of endometriosis until the time they get a definitive diagnosis and treatment.
I think it's very important that we recognize the clinical features of endometriosis. It's absolutely crucial if we want to shorten the time tom diagnosis. Often patients see multiple physicians before they get an accurate diagnosis. They're often dismissed by their early caregivers who are not very familiar with the disease.
For me, the most important feature is the pelvic pain. To identify endometriosis, I look specifically at the cyclic nature of the pelvic pain, and the progressive nature of the pelvic pain. Endometriosis is by far the most common reason that women have pelvic pain and initially it tends to be cyclic. It often starts as dysmenorrhea (painful periods), however it can progress to the point where pain occurs at times outside of menses. In fact, it can progress to the point where it's painful all the time. However, it almost always starts as dysmennorhea, and progresses.
Pelvic pain that someone had from their first menses on, i.e., from menarche, is less likely to be endometriosis than the pain that progresses and becomes worse or spreads to other times of the menstrual cycle. Endometriosis is a progressive disease, and that's one of the key distinctions in making me think somebody has endometriosis.
Most women with cyclic pelvic pain that gets worse over time, cyclic progressive pelvic pain, will have endometriosis. Those are the key features I look for.
It is also very important to recognize that endometriosis can have effects outside of the reproductive tract, can cause systemic inflammation, and impact other organ systems. Bowel and bladder dysfunction are very common. If that is cyclic and coincides with the pelvic pain, it’s very likely to be secondary to endometriosis. It's important not to get distracted or mislead into making other diagnoses.
What are some of the common symptoms and how does that impact your diagnosis evaluation?
Dr. Taylor: The most common symptom we see is that cyclic pelvic pain I just discussed. Pain, often starting as dysmenorrhea, can go on to include pain outside of the time of menses and become more than just dysmenorrhea- pain in other areas. The other common symptom is infertility. Many women with endometriosis do not have severe pain or may not have pain at all, but first present when they have trouble conceiving.
Endometriosis may be the cause of infertility. Sometimes we recognize that it's endometriosis when we do a physical exam or an ultrasound and find an endometrioma. Cyclic pelvic pain is the classic symptom we look for, and infertility is another key symptom that we shouldn't forget.
Can you talk a little bit about empiric treatment, primarily what that means and how prevalent it is in your current practice?
Dr. Taylor: I think one of the problems we have today with the treatment of endometriosis is that it doesn't get recognized and doesn't get treated right away. When we talk about emperic treatment, it's usually ruling out other potential etiologies of pain. We must ensure no one has have an infection, a tumor or other etiologies that are the cause of the pain, and not simply presume that any cyclic progressive pelvic pain is endometriosis – however most of the time it will be due to endometriosis.
Usually with a good history, physical exam, and sometimes with addition of a transvaginal ultrasound, you can rule out other etiologies of pain, and have a very good idea that this pain is related to endometriosis. Based on clinical presentation, and without needing surgery, we can make a clinical diagnosis of endometriosis.
Clinical diagnosis allows patients to have this disease recognized earlier. It allows them to get into treatment sooner and reverses this trend that we've seen of delayed recognition. This delay is especially difficult in younger women in formative years in their life, when they're in school, when they're in an early stage of their career. If they're held back because of this debilitating pain, these are critical times and opportunities they really can't completely make up.
It's very important that we recognize endometriosis early. If we require a laparoscopy to make the diagnosis, the threshold becomes very high and we don't make the diagnosis. We miss a lot of women with endometriosis and don't treat them early.
There was time when our medical options were limited, and we wanted to be sure of our diagnosis. But these days, I think we can make a clinical diagnosis knowing that we have several treatment options that are relatively easy and safe for patients. Our first line therapy-- the first thing I use when I make the clinical diagnosis of endometriosis-- the first emperic treatment I would use is an oral contraceptive.
I prefer giving the oral contraceptive in a continuous fashion, rather than in a cyclic fashion. If someone has dysmenorrhea, why have menses at all? Retrograde menstruation is the etiology of most endometriosis. If we can eliminate menstruation, potentially it may be reducing endometriosis in the long run.
A lot of women with endometriosis will not respond to progestins—a phenomenon called progestin resistance—therefore, not everyone who has endometriosis will respond to an oral contraceptive. Probably about a third of patients either will not initially respond or will develop a resistance to a progestin and fail to respond in the long-term. Still others have side effects due to progestin therapy-- breast tenderness, mood changes, or a feeling of bloating are very common progestin related side effects.
We now have agents like oral GnRH antagonists that we can use as a second line treatment for those that either don't respond to progestins or those who have side effects from a progestin, including a progestin based oral contraceptive. Additionally, in those with severe pain you may want to use something a little more aggressive.
GnRH antagonists are easy to use oral medications, with an immediate onset of action and are easily reversed. We have come a long way from when we had to use an injectable GnRH agonist as the second line therapy. We have much simpler, easy, second line medications that we can turn to that makes empiric treatment a lot easier.
In the past, when we made a surgical diagnosis it was often because we were afraid of committing them to a long course of Depo GnRH agonist treatment; we didn't use a lot of add back therapy and they had tremendous side effects and the risk of bone loss. Patients and their physicians were reluctant to use GnRH agonists.
Things have gotten a lot easier with oral contraceptives, that can be followed by GnRH antagonists, which are easy to use, simple medications that are very patient-friendly. I think we can make that clinical diagnosis. We can move to an empiric treatment, either first or second line with an oral contraceptive or a GnRH antagonist and easily treat these women without significant side effects. It is important that we advocate for women with cyclic pain, and recognize it more readily, clinically diagnosed it, and begin that empiric treatment. That paradigm really has a huge impact on women's lives.
What recent advancements have been made in diagnosing and treating endometriosis?
Dr. Taylor: I think one of the biggest things that I see improving is recognition. Hopefully, we're narrowing that long delay by closing that time gap from initial symptoms to recognition. As we see public awareness grow, patients are recognizing and looking for answers, and thinking to themselves that they may have endometriosis.
Years ago, people were embarrassed to talk about menstruation, painful menstruation, pain with intercourse, pain with bowel movements. Thankfully, we're a more open society now and we can talk about these things. Women are starting to realize that they may have a problem where it was just dismissed before or perhaps, they were embarrassed to talk about it. I think we have seen a huge advancement. Physicians, as well, are recognizing endometriosis even more than before.
I think we're also much more accepting of this clinical diagnosis paradigm and empiric treatment. A lot of that, as we just said, comes from having better, easier to use drugs available that are much more patient-friendly. The GnRH antagonist elagolix is currently available for treatment of endometriosis in the United States. There are two more GnRH antagonists in the pipeline-- relugolix, which we expect to be approved shortly, and linzagolix which is undergoing phase three clinical trials now. Hopefully, we will have several of these second line drugs, drugs which we can even use first line for severe endometriosis. Their availability is another huge advance.
I also think it is essential, that we don't put someone through surgery to recognize endometriosis. We must be better at taking a good history, doing an exam, and ultrasound when needed. You don't need a surgery to diagnose endometriosis.
However, we do still sometimes need surgery to treat endometriosis. Often, endometriosis will cause adhesions or scarring. These long-term sequelae of endometriosis can still require surgery. The medications we have available today are very good at stopping active disease. But the damage done from long-term endometriosis if we don’t treat may still require surgery. I'm hoping that the earlier we start treating people, the less damage will be done, and the less therapeutic surgeries needed. I think these changes are coming and are all very promising.
It would also be great if we had a non-invasive definitive diagnostic test. There are several of those under development, but nothing available yet. I suspect that we'll see those become available very shortly.
The other thing that we still need in the field is a treatment we can use for those women trying to get pregnant. We use in vitro fertilization, which works very well in the endometriosis population. But a medical therapy that can suppress endometriosis and allow people to try to conceive without needing IVF is something I hope for in the future. A specific endometriosis therapy that is not hormonal, that targets the specific pathophysiology of endometriosis, is something that I'd like to see developed and many of us are currently working on.
I think there is a lot coming, but we've already moved the needle a long way. The GnRH antagonists have given us much more confidence in moving forward with clinical diagnosis and empiric treatment of this disease. It's a huge boon for women's health, allowing early recognition and preventing long-term complications of endometriosis.
Exocrine Pancreatic Insufficiency: Clinical Presentation and Diagnosis
What is your approach to differentiating EPI from other pancreatic conditions when making a diagnosis?
Dr. Kothari: Exocrine pancreatic insufficiency, or EPI, is a condition largely defined by malabsorption as the result of inadequate digestive enzymes. The resulting symptoms from maldigestion include bloating, malodorous gas, abdominal pain, changes in bowel habits, and weight change. EPI can be caused by intrinsic pancreatic disorders (such as chronic pancreatitis, acute pancreatitis, cystic fibrosis or pancreatic cancer) or from extra-pancreatic diseases (including the result of gastrointestinal surgery). Thus, EPI should be considered a consequence of an already existing gastrointestinal disorder.
Can you a speak a little bit more about the signs and symptoms or characteristics that are most common in patients with EPI?
Dr. Kothari: The symptoms of EPI can range from bloating and abdominal pain with mild to overt steatorrhea with greasy and oily stools that are difficult to flush with malodorous flatulence, weight loss, and symptoms of vitamin and micronutrient deficiency. The pathophysiology of these symptoms results from inadequate enzymes which are needed for digestion. Particularly, lipase is the major enzyme needed for fat digestion and thus when not present leads to fat maldigestion resulting in symptoms. Furthermore, undigested fats result in alterations in gut motility which can further exacerbate symptoms to include nausea, vomiting, early satiety and inadequate stool evacuation.
Patients who have fat malabsorption, particularly for pancreatic insufficiency, can also have malnutrition as a result of inadequate absorption of nutrients and micronutrients. Particularly, we think about fat-soluble vitamins-- vitamin A, vitamin E, vitamin D, and vitamin K and in the initial evaluation of patients with established EPI, one could consider evaluation of comorbid bone disease.
How crucial is having the correct interpretation of the clinical presentation to pinpointing the diagnosis?
Dr. Kothari: This is a great question because, with exocrine pancreatic insufficiency as there is growing publicity for the disorder and because symptoms can be rather non-specific when mild, it is important to be informed on how best to make this diagnosis. Thus, it is important to review the predisposing conditions that may lead to the diagnosis of EPI. These conditions include cystic fibrosis, chronic pancreatitis, acute pancreatitis, previous pancreatic surgery, history of pancreatic cancer (or suspicion for new pancreatic cancer), history of diabetes, celiac disease, history of luminal surgeries (including bariatric surgery), and inflammatory bowel disease. Further, since EPI can be a result of intrinsic pancreatic pathologies, it is critical to consider the risk factors for chronic pancreatitis which include alcohol and tobacco ingestion, prior episodes of recurrent acute pancreatitis, genetic conditions that may predispose patients to chronic pancreatitis, including cystic fibrosis, and hereditary conditions that also result in pancreatitis. As clinicians, it is our role to obtain an accurate history to best gauge the risk factors for EPI.
After reviewing risk factors, we then must review the clinical presentation to know if the symptoms could be from EPI which include bloating, gas, abdominal pain, weight changes, changes in bowel habits and consequences of vitamin deficiencies. Since the symptoms of mild EPI can be similar to other GI conditions such as SIBO, celiac disease, and functional bowel disorders, gauging whether a patient has risk factors for EPI will help the clinician understand how likely a diagnosis of EPI may be and if and what testing would be appropriate.
In my practice, I consider diagnostic testing in patients who may be at risk for EPI and have mild symptoms such malodorous gas, bloating and mild steatorrhea. For patient with clear evidence of steatorrhea (weight loss and vitamin deficiencies) and have strong risk factors for EPI (i.e. heavy alcohol and tobacco and/or a history of recurrent or severe acute pancreatitis), I might consider imaging and/or empiric therapies as to expedite care.
How difficult is it to diagnose EPI and what steps do you take to ensure that you prescribe patients with the proper therapy?
Dr. Kothari: The diagnosis of pancreatic insufficiency, in my mind, needs to start with assessing the pre-test probability of the patient having EPI, since testing could lead to a false positive.
The test of choice in most scenarios for diagnosing pancreatic insufficiency is a stool test known as the fecal elastase. It is a measurement of pancreatic elastase in the stool. The test itself is a concentration. For any condition that results in a dilute stool sample, that'll result in a falsely low value that can give a patient a false positive test. Now, this can be corrected by the lab concentrating the stool sample before running the test, but that testing center needs to know how to do that.
The other assumption that we make with this stool test is that we assume that the elastase is a stable molecule that can traverse all the gut and be collected adequately. And for any reason, if that enzyme is degraded for any reason, it's also going to provide a low test, a low result, resulting in a false positive.
If they have risk factors for chronic pancreatitis or pancreatic disease and they're presenting with symptoms of EPI, then the usual test that I'll choose is dedicated pancreatic imaging such as an MRI or dedicated CT pancreatic imaging, or endoscopic ultrasound. If we clinch a diagnosis of chronic pancreatitis and they have symptoms of pancreatic insufficiency, I think that’s enough to presume a diagnosis of exocrine pancreatic insufficiency and start treatment.
On the other hand, in patients who do not have risk factors for pancreatic disease but there remains some clinical suspicion for exocrine pancreatic insufficiency, then it may be reasonable to check a fecal elastase to rule out pancreatic insufficiency. If the test results are low, then follow-up dedicated pancreas imaging would be the next step in delineating intrinsic pancreatic conditions form extra-pancreatic causes. If pancreas imaging effectively rules out pancreatic disease then I consider checking for celiac disease, ruling out small intestinal bacterial overgrowth and considering assessment of luminal motility (either with a capsule or small bowel follow through). Although functional neuroendocrine tumors have been previously considered a cause of EPI, these tumors tend to present with secretory diarrhea which typically present differently (and often more dramatically) than other causes of EPI. Thus, I do not routinely check vasoactive hormone levels.
I think the American Gastroenterological Association has great patient education documents for our patients. Thus, I would encourage our colleagues to use the AGA for their resources for our patients on EPI.
What is your approach to differentiating EPI from other pancreatic conditions when making a diagnosis?
Dr. Kothari: Exocrine pancreatic insufficiency, or EPI, is a condition largely defined by malabsorption as the result of inadequate digestive enzymes. The resulting symptoms from maldigestion include bloating, malodorous gas, abdominal pain, changes in bowel habits, and weight change. EPI can be caused by intrinsic pancreatic disorders (such as chronic pancreatitis, acute pancreatitis, cystic fibrosis or pancreatic cancer) or from extra-pancreatic diseases (including the result of gastrointestinal surgery). Thus, EPI should be considered a consequence of an already existing gastrointestinal disorder.
Can you a speak a little bit more about the signs and symptoms or characteristics that are most common in patients with EPI?
Dr. Kothari: The symptoms of EPI can range from bloating and abdominal pain with mild to overt steatorrhea with greasy and oily stools that are difficult to flush with malodorous flatulence, weight loss, and symptoms of vitamin and micronutrient deficiency. The pathophysiology of these symptoms results from inadequate enzymes which are needed for digestion. Particularly, lipase is the major enzyme needed for fat digestion and thus when not present leads to fat maldigestion resulting in symptoms. Furthermore, undigested fats result in alterations in gut motility which can further exacerbate symptoms to include nausea, vomiting, early satiety and inadequate stool evacuation.
Patients who have fat malabsorption, particularly for pancreatic insufficiency, can also have malnutrition as a result of inadequate absorption of nutrients and micronutrients. Particularly, we think about fat-soluble vitamins-- vitamin A, vitamin E, vitamin D, and vitamin K and in the initial evaluation of patients with established EPI, one could consider evaluation of comorbid bone disease.
How crucial is having the correct interpretation of the clinical presentation to pinpointing the diagnosis?
Dr. Kothari: This is a great question because, with exocrine pancreatic insufficiency as there is growing publicity for the disorder and because symptoms can be rather non-specific when mild, it is important to be informed on how best to make this diagnosis. Thus, it is important to review the predisposing conditions that may lead to the diagnosis of EPI. These conditions include cystic fibrosis, chronic pancreatitis, acute pancreatitis, previous pancreatic surgery, history of pancreatic cancer (or suspicion for new pancreatic cancer), history of diabetes, celiac disease, history of luminal surgeries (including bariatric surgery), and inflammatory bowel disease. Further, since EPI can be a result of intrinsic pancreatic pathologies, it is critical to consider the risk factors for chronic pancreatitis which include alcohol and tobacco ingestion, prior episodes of recurrent acute pancreatitis, genetic conditions that may predispose patients to chronic pancreatitis, including cystic fibrosis, and hereditary conditions that also result in pancreatitis. As clinicians, it is our role to obtain an accurate history to best gauge the risk factors for EPI.
After reviewing risk factors, we then must review the clinical presentation to know if the symptoms could be from EPI which include bloating, gas, abdominal pain, weight changes, changes in bowel habits and consequences of vitamin deficiencies. Since the symptoms of mild EPI can be similar to other GI conditions such as SIBO, celiac disease, and functional bowel disorders, gauging whether a patient has risk factors for EPI will help the clinician understand how likely a diagnosis of EPI may be and if and what testing would be appropriate.
In my practice, I consider diagnostic testing in patients who may be at risk for EPI and have mild symptoms such malodorous gas, bloating and mild steatorrhea. For patient with clear evidence of steatorrhea (weight loss and vitamin deficiencies) and have strong risk factors for EPI (i.e. heavy alcohol and tobacco and/or a history of recurrent or severe acute pancreatitis), I might consider imaging and/or empiric therapies as to expedite care.
How difficult is it to diagnose EPI and what steps do you take to ensure that you prescribe patients with the proper therapy?
Dr. Kothari: The diagnosis of pancreatic insufficiency, in my mind, needs to start with assessing the pre-test probability of the patient having EPI, since testing could lead to a false positive.
The test of choice in most scenarios for diagnosing pancreatic insufficiency is a stool test known as the fecal elastase. It is a measurement of pancreatic elastase in the stool. The test itself is a concentration. For any condition that results in a dilute stool sample, that'll result in a falsely low value that can give a patient a false positive test. Now, this can be corrected by the lab concentrating the stool sample before running the test, but that testing center needs to know how to do that.
The other assumption that we make with this stool test is that we assume that the elastase is a stable molecule that can traverse all the gut and be collected adequately. And for any reason, if that enzyme is degraded for any reason, it's also going to provide a low test, a low result, resulting in a false positive.
If they have risk factors for chronic pancreatitis or pancreatic disease and they're presenting with symptoms of EPI, then the usual test that I'll choose is dedicated pancreatic imaging such as an MRI or dedicated CT pancreatic imaging, or endoscopic ultrasound. If we clinch a diagnosis of chronic pancreatitis and they have symptoms of pancreatic insufficiency, I think that’s enough to presume a diagnosis of exocrine pancreatic insufficiency and start treatment.
On the other hand, in patients who do not have risk factors for pancreatic disease but there remains some clinical suspicion for exocrine pancreatic insufficiency, then it may be reasonable to check a fecal elastase to rule out pancreatic insufficiency. If the test results are low, then follow-up dedicated pancreas imaging would be the next step in delineating intrinsic pancreatic conditions form extra-pancreatic causes. If pancreas imaging effectively rules out pancreatic disease then I consider checking for celiac disease, ruling out small intestinal bacterial overgrowth and considering assessment of luminal motility (either with a capsule or small bowel follow through). Although functional neuroendocrine tumors have been previously considered a cause of EPI, these tumors tend to present with secretory diarrhea which typically present differently (and often more dramatically) than other causes of EPI. Thus, I do not routinely check vasoactive hormone levels.
I think the American Gastroenterological Association has great patient education documents for our patients. Thus, I would encourage our colleagues to use the AGA for their resources for our patients on EPI.
What is your approach to differentiating EPI from other pancreatic conditions when making a diagnosis?
Dr. Kothari: Exocrine pancreatic insufficiency, or EPI, is a condition largely defined by malabsorption as the result of inadequate digestive enzymes. The resulting symptoms from maldigestion include bloating, malodorous gas, abdominal pain, changes in bowel habits, and weight change. EPI can be caused by intrinsic pancreatic disorders (such as chronic pancreatitis, acute pancreatitis, cystic fibrosis or pancreatic cancer) or from extra-pancreatic diseases (including the result of gastrointestinal surgery). Thus, EPI should be considered a consequence of an already existing gastrointestinal disorder.
Can you a speak a little bit more about the signs and symptoms or characteristics that are most common in patients with EPI?
Dr. Kothari: The symptoms of EPI can range from bloating and abdominal pain with mild to overt steatorrhea with greasy and oily stools that are difficult to flush with malodorous flatulence, weight loss, and symptoms of vitamin and micronutrient deficiency. The pathophysiology of these symptoms results from inadequate enzymes which are needed for digestion. Particularly, lipase is the major enzyme needed for fat digestion and thus when not present leads to fat maldigestion resulting in symptoms. Furthermore, undigested fats result in alterations in gut motility which can further exacerbate symptoms to include nausea, vomiting, early satiety and inadequate stool evacuation.
Patients who have fat malabsorption, particularly for pancreatic insufficiency, can also have malnutrition as a result of inadequate absorption of nutrients and micronutrients. Particularly, we think about fat-soluble vitamins-- vitamin A, vitamin E, vitamin D, and vitamin K and in the initial evaluation of patients with established EPI, one could consider evaluation of comorbid bone disease.
How crucial is having the correct interpretation of the clinical presentation to pinpointing the diagnosis?
Dr. Kothari: This is a great question because, with exocrine pancreatic insufficiency as there is growing publicity for the disorder and because symptoms can be rather non-specific when mild, it is important to be informed on how best to make this diagnosis. Thus, it is important to review the predisposing conditions that may lead to the diagnosis of EPI. These conditions include cystic fibrosis, chronic pancreatitis, acute pancreatitis, previous pancreatic surgery, history of pancreatic cancer (or suspicion for new pancreatic cancer), history of diabetes, celiac disease, history of luminal surgeries (including bariatric surgery), and inflammatory bowel disease. Further, since EPI can be a result of intrinsic pancreatic pathologies, it is critical to consider the risk factors for chronic pancreatitis which include alcohol and tobacco ingestion, prior episodes of recurrent acute pancreatitis, genetic conditions that may predispose patients to chronic pancreatitis, including cystic fibrosis, and hereditary conditions that also result in pancreatitis. As clinicians, it is our role to obtain an accurate history to best gauge the risk factors for EPI.
After reviewing risk factors, we then must review the clinical presentation to know if the symptoms could be from EPI which include bloating, gas, abdominal pain, weight changes, changes in bowel habits and consequences of vitamin deficiencies. Since the symptoms of mild EPI can be similar to other GI conditions such as SIBO, celiac disease, and functional bowel disorders, gauging whether a patient has risk factors for EPI will help the clinician understand how likely a diagnosis of EPI may be and if and what testing would be appropriate.
In my practice, I consider diagnostic testing in patients who may be at risk for EPI and have mild symptoms such malodorous gas, bloating and mild steatorrhea. For patient with clear evidence of steatorrhea (weight loss and vitamin deficiencies) and have strong risk factors for EPI (i.e. heavy alcohol and tobacco and/or a history of recurrent or severe acute pancreatitis), I might consider imaging and/or empiric therapies as to expedite care.
How difficult is it to diagnose EPI and what steps do you take to ensure that you prescribe patients with the proper therapy?
Dr. Kothari: The diagnosis of pancreatic insufficiency, in my mind, needs to start with assessing the pre-test probability of the patient having EPI, since testing could lead to a false positive.
The test of choice in most scenarios for diagnosing pancreatic insufficiency is a stool test known as the fecal elastase. It is a measurement of pancreatic elastase in the stool. The test itself is a concentration. For any condition that results in a dilute stool sample, that'll result in a falsely low value that can give a patient a false positive test. Now, this can be corrected by the lab concentrating the stool sample before running the test, but that testing center needs to know how to do that.
The other assumption that we make with this stool test is that we assume that the elastase is a stable molecule that can traverse all the gut and be collected adequately. And for any reason, if that enzyme is degraded for any reason, it's also going to provide a low test, a low result, resulting in a false positive.
If they have risk factors for chronic pancreatitis or pancreatic disease and they're presenting with symptoms of EPI, then the usual test that I'll choose is dedicated pancreatic imaging such as an MRI or dedicated CT pancreatic imaging, or endoscopic ultrasound. If we clinch a diagnosis of chronic pancreatitis and they have symptoms of pancreatic insufficiency, I think that’s enough to presume a diagnosis of exocrine pancreatic insufficiency and start treatment.
On the other hand, in patients who do not have risk factors for pancreatic disease but there remains some clinical suspicion for exocrine pancreatic insufficiency, then it may be reasonable to check a fecal elastase to rule out pancreatic insufficiency. If the test results are low, then follow-up dedicated pancreas imaging would be the next step in delineating intrinsic pancreatic conditions form extra-pancreatic causes. If pancreas imaging effectively rules out pancreatic disease then I consider checking for celiac disease, ruling out small intestinal bacterial overgrowth and considering assessment of luminal motility (either with a capsule or small bowel follow through). Although functional neuroendocrine tumors have been previously considered a cause of EPI, these tumors tend to present with secretory diarrhea which typically present differently (and often more dramatically) than other causes of EPI. Thus, I do not routinely check vasoactive hormone levels.
I think the American Gastroenterological Association has great patient education documents for our patients. Thus, I would encourage our colleagues to use the AGA for their resources for our patients on EPI.
The Pharmacological Management of Constipation
Professor Satish Rao, MD is the J. Harold Harrison, MD, Distinguished University Chair in Gastroenterology. Dr. Rao is also founding Director of the Digestive Health Center and Clinical Research Center, and tenured Director and Professor of Medicine. His research interests in Neurogastroenterology/Motility have focused on gaining mechanistic insights, developing novel diagnostic tools and pioneering innovative treatments for constipation, dyssynergic defecation, fecal incontinence, IBS, food intolerance, gas and bloating and small intestinal bacterial and fungal overgrowth (SIBO/SIFO) and visceral pain. His latest invention translumbosacral neuromodulation therapy (TNT) is revolutionizing treatment for fecal incontinence.
As a gastroenterologist, with a focus on research that includes pathophysiological treatment of IBS and constipation as the primary objective, how prevalent is it in your current practice?
Dr. Rao: It is a pleasure to discuss a topic very close to my heart. It is a very important but often neglected topic, and very many times people go to pharmacies, over-the-counter, or their grandmothers, seeking treatment for constipation, whereas, with all the advances today, they should be coming to us, gastroenterologists, as the primary source for really managing this problem.
Constipation is very common. It all depends, to some extent, on how we define it. But if we define it based on some more popular criteria, such as those supported by the Rome Foundation criteria, the global prevalence, is between 10% to 15%. As you can see millions of Americans suffer with this problem, and almost all AGA members would have seen hundreds of these patients in the course of their practice every year. So, it is highly prevalent.
The term constipation is misunderstood by many people. Different people have different names, different people have different definitions and different criteria. For years, most physicians and most textbooks equated constipation to infrequent bowel movements. That logic has changed dramatically in the last 10 to 15 years, where we now recognize constipation as not only infrequent bowel movement but, more commonly, difficulty with bowel movement. This difficulty with bowel movement has been the missing link as we were all focused on infrequent bowel movement. We now recognize that constipation means one of six things.
What are those six things that tell us it’s constipation? One, there’s excessive straining to have a bowel movement; two after having had a bowel movement, you're left with a feeling of incomplete evacuation; three, the stools are hard and difficult to pass. We have a very famous scale, called the Bristol Stool Form Scale. If anybody takes the time to look at the scale, if your stool form happens to be 1, 2, or 3, then you're more likely to be constipated; four, a patient has to use digital maneuvers or some kind of support to try and evacuate stool; five, a patient reports a sensation of blockage at the time of bowel movement repeatedly, and at least with 25% of bowel movements; and six, stool frequency of less than three bowel movements per week.
In order to diagnose constipation, if a patient has any two of these symptoms, for 25% of bowel movements over a period of three to six months, then that individual should be considered as having chronic constipation.
When is pharmacological management of constipation appropriate? And what diagnostic approach do you usually take to determine treatment?
Dr. Rao: I usually take a very detailed history from these patients. One of the things we have recognized, recently, is how inadequate our history has become, not necessarily from a lack of asking questions, but it seems to be a multifactorial process. We tested this in a prospective study showing that only 30% of the time were patients history correct in letting us know why they came to the clinic. The same patient who answered a questionnaire about their symptoms, when they keep a diary for one week, there's only 30% of the time there is concordance; 70% of the time the story is different.
Hence, the first step really is to get an accurate story about your patient with constipation. Fortunately, there are some digital apps that are available, a constipation stool diary app, and there is a MyGiHealth app, et cetera. People can use these apps or they can keep a paper diary. The next step would be to determine what may be mechanistically going wrong. These two steps will guide your management approach.
We have some simple tests that we can do. One is called a colon transit study, where we measure the speed at which stool goes through the colon. And we have two tests that are commonly used. One is called a Sitz marker test. The patient swallows the capsule which has 24 plastic rings. And then they take it on day one. They get an X-ray on day six, which is 120 hours later, and you count how many of those markers are left behind with the X-ray. If there are more than five, it's abnormal. It says they have slow transit. In other words, lazy colon. On the other hand, if they pass most of the markers, then they don't have a lazy colon.
The second test we often do is a wireless motility capsule test, which is again a capsule they swallow. They wear a recorder for five days. And then that measures the speed at which the capsule goes through the colon. A very simple way of measuring colon speed as well. These tests tell us if you have a lazy colon.
Another test that we do is anorectal manometry. We do the test because 40%-50% of patients have pelvic floor dysfunction. And that gives us important insights mechanistically whether they have rectal hypersensitivity or whether they have a problem with evacuating stool. If they have rectal hypersensitivity and they're complaining of constipation, then it equates to irritable bowel syndrome with constipation. That's how we pick up that category of constipation.
The other category I mentioned earlier, lazy colon, is slow transit constipation. And the third category we often see is called dyssynergic defecation, where they are unable to evacuate stool in a timely, orderly fashion. And that is the third group of constipation. Use these tests to help you diagnose which kind of constipation a patient has. That will then guide towards appropriate management.
Is there a higher frequency in the need for pharmacological management of constipation? And is it in any particular patient, for instance, such as older adults, the elderly, or those that may be critically ill, or any other demographic?
Dr. Rao: In terms of managing the constipation itself, many times patients will go to the drugstore or talk to a pharmacist and start taking some over-the-counter preparations.
Until recently, there hasn’t been any good study to tell us which one works, which preparation works. But we recently published a systematic review of which I had the pleasure of serving as the first author, where we looked at the 30-year data on over-the-counter treatments. We found that there is very good evidence to support polyethylene glycol, PEG, as grade A, recommendation.
We also found good evidence for senna, and for magnesium. These three compounds had good evidence. Whereas, for other over-the-counter preparations, such as fiber supplements, there are some fruit-based supplements that are now available, lactulose and so on, the evidence was second grade.
With regards to prescriptions, a drug that is approved is linaclotide, which is approved at a dose of 145 micrograms a day in a capsule form for treatment of chronic constipation. It's also approved for IBS with constipation at a higher dose of 290 micrograms a day. We also have plecanatide 3mg tablets. Both linaclotide and plecanatide, are guanylate cyclase C receptor agonists. They activate secretion through the guanylate cyclase pathway in the gut. And then, by inducing secretion, they improve constipation. Both drugs are approved for IBS-C and chronic constipation.
Then we have lubiprostone, which is a chloride channel 2 activator drug that is approved at a dose of 24 micrograms twice a day with food for treatment of chronic constipation, and 8 micrograms twice a day for treatment of IBS with constipation.
The most recent drug that was approved is called prucalopride. And this is approved at 1 to 2 milligrams a day. And this is a serotonin compound, which speeds up the gut activity including the, stomach, small bowel, and the colon. And by speeding up gut activity, it improves constipation.
Regarding particular demographics, I find that older patients tend to be a little bit more sensitive to some drugs. Some older adults are more refractory to standard compounds, which makes their management a little bit more challenging. We have to really titrate the dose very carefully.
The critically ill group is a little bit more challenging. Often, it's acute constipation or they're in ICU settings and there the management becomes a little bit more complex. One component of that critically ill group-- and of course, you can also see them in outpatient practice-- is the opioid-induced constipation, which is a category in its own right and has been recognized by the FDA. Unfortunately, one of the major side effects of opioid is constipation. It takes a toll in the gut.
Fortunately, we have a new set of drugs, called PAMORAs, or peripherally acting mu-opioid receptor antagonists. These drugs, when you take them orally, will neutralize the effect of opioids in the gut, and thereby relieve constipation without affecting the analgesic effect of opioids. Examples of these class of drugs are methylnaltrexone, naloxegol, naldemedine, etc. These are all FDA approved now for treatment of opioid-induced constipation, which is part of your critical ill or hospitalized patients, and sometimes they are really outpatients as well.
Are there risk factors?
Dr. Rao: There are several risk factors for constipation. They include, for example, elderly, particularly people who are not very mobile for various reasons, people-- but I think the biggest risk factor that I would like to mention, emphasize, is drugs. There are many, many, many drugs that cause constipation. Opioids, as I just mentioned . Also a number of anti-hypertensive drugs. Calcium channel blockers, for example, are constipating.
Now, iron, heavy metals, and calcium, are very constipating. So are anticholinergics, and antidepressants. Many antidepressants, particularly the tricyclic class, are very constipating.
My first message to my colleagues is, when a patient is presenting to you in the clinic, the first thing I teach my students, residents, fellows, is to look at the drug list. Think about that drug as a mischief for constipation. If it is feasible and appropriate, remove the drug, or substitute the drug, as you are looking for other reasons for constipation.
Another important risk factor worth mentioning is acute constipation. Constipation in a 70-year-old person, suddenly over the last six weeks, is serious and may raise suspicion for cancer in the colon, and the need for investigation. If patients suddenly develop constipation like that, then, it is important to make sure that there is nothing blocking the colon, that is creating constipation.
One other important group is pregnancy. I did forget to mention that. Interestingly, between a quarter to a third of woman, otherwise healthy women, have never had any symptoms, however, during pregnancy they do become constipated. That's because of hormonal changes, particularly the rush of progesterone which is happening in their body. And so, they will have to be managed during their stage, and very many times they will come back to their normal lifestyle afterwards.
When it comes to the data on current and emerging treatments, what do you see in the future of pharmacological management of constipation?
Dr. Rao: I think one of the critical things in the pharmacologic management of constipation is to recognize that constipation is rarely a one-mechanism disorder. And it may be in a particular individual, but how do we know? For example, how do we know that their gut is not producing enough serotonin? And that is why they are serotonin depleted in the gut and we need to supplement them with a serotonin product, to just give you an example. We don't know that. We don't have their genetic makeup, and so on.
What I'm getting at is, constipation is a heterogenic problem, and there are multiple mechanisms that lead to it. Therefore, our current armamentarium has significantly improved in the last decade.
The first decade of the new Millennium saw significant new drugs that were introduced. The second decade has seen even more. But I think there are other compounds that are now coming up. There are sodium-hydrogen ion pump blocking drugs , and there are other mechanistic drugs . There's another drug which is available in Japan, not yet in the US, called elobixibat, which actually blocks bile acid. Normally, 95% of the bile is reabsorbed in the colon. But if you block bile acid reabsorption and allow some bile to spill into the colon, your own bile can become a laxative in the colon.
Another important approach has been through a capsule technology, called Vibrant capsule. . I'm part of an investigative group is investigating this drug. The phase III data is not yet available but has been submitted, You take a capsule once a day, and this gently agitates the colon, and thereby stimulating the colon muscles to move the stool, and then you evacuate. So, it is not a pharmacologic, but it is a form of a capsule device treatment .
These are some emerging treatments just around the corner. There is kind of a belt device that you can wear around the belly, which passes a small amount of electric current in a sequential manner, to stimulate peristalsis, called electrical interference therapy.
If pharmacological management is not an option, then what's next?
Dr. Rao: Pharmacologic management works for about 50% of patients. But it doesn't work in everybody. It's not because the drug itself is not working or has side effects, instead, the issue is that the problem is not likely to be fixed by pharmacologic management.
About 40% of patients have a pelvic floor dysfunction called dyssynergic defecation. These folks, unbeknownst to them, have learned a new process of pooping, where they are blocking their own pooping action. They're not doing it deliberately. They're totally unaware. A third of them have this problem right from childhood. 2/3 acquire this problem in adulthood. Needless to say, this problem affects 40% of patients.
So yes, you can give them medications, and that will temporarily help them. But because they cannot evacuate this stool, it will never help them permanently. These individuals are best helped by a behavioral treatment called biofeedback therapy. Hopefully in the future, home-based biofeedback tools can become available, and that can really make this treatment more widely available to the public.
Is there anything else you'd like to add before we conclude?
Dr. Rao: My most important message to my colleagues is that constipation is a very common problem. Please take time to spend with your patients. Please use an app or a diary to record the symptoms. If at all possible, perform a digital rectal examination on all your constipated patients to identify the pelvic floor dysfunction group of patients. Manage them appropriately with over the counter drugs or particularly the FDA-approved pharmacotherapies. And that will work in a majority.
But when drugs don’t work or patients have predominant symptoms of difficult defecation, put on your thinking hat. Don't give up on your patients. Instead of sending them to a surgeon, which many times we rush to do, try and see what other things you can do including manometric testing and biofeedback therapy.
Professor Satish Rao, MD is the J. Harold Harrison, MD, Distinguished University Chair in Gastroenterology. Dr. Rao is also founding Director of the Digestive Health Center and Clinical Research Center, and tenured Director and Professor of Medicine. His research interests in Neurogastroenterology/Motility have focused on gaining mechanistic insights, developing novel diagnostic tools and pioneering innovative treatments for constipation, dyssynergic defecation, fecal incontinence, IBS, food intolerance, gas and bloating and small intestinal bacterial and fungal overgrowth (SIBO/SIFO) and visceral pain. His latest invention translumbosacral neuromodulation therapy (TNT) is revolutionizing treatment for fecal incontinence.
As a gastroenterologist, with a focus on research that includes pathophysiological treatment of IBS and constipation as the primary objective, how prevalent is it in your current practice?
Dr. Rao: It is a pleasure to discuss a topic very close to my heart. It is a very important but often neglected topic, and very many times people go to pharmacies, over-the-counter, or their grandmothers, seeking treatment for constipation, whereas, with all the advances today, they should be coming to us, gastroenterologists, as the primary source for really managing this problem.
Constipation is very common. It all depends, to some extent, on how we define it. But if we define it based on some more popular criteria, such as those supported by the Rome Foundation criteria, the global prevalence, is between 10% to 15%. As you can see millions of Americans suffer with this problem, and almost all AGA members would have seen hundreds of these patients in the course of their practice every year. So, it is highly prevalent.
The term constipation is misunderstood by many people. Different people have different names, different people have different definitions and different criteria. For years, most physicians and most textbooks equated constipation to infrequent bowel movements. That logic has changed dramatically in the last 10 to 15 years, where we now recognize constipation as not only infrequent bowel movement but, more commonly, difficulty with bowel movement. This difficulty with bowel movement has been the missing link as we were all focused on infrequent bowel movement. We now recognize that constipation means one of six things.
What are those six things that tell us it’s constipation? One, there’s excessive straining to have a bowel movement; two after having had a bowel movement, you're left with a feeling of incomplete evacuation; three, the stools are hard and difficult to pass. We have a very famous scale, called the Bristol Stool Form Scale. If anybody takes the time to look at the scale, if your stool form happens to be 1, 2, or 3, then you're more likely to be constipated; four, a patient has to use digital maneuvers or some kind of support to try and evacuate stool; five, a patient reports a sensation of blockage at the time of bowel movement repeatedly, and at least with 25% of bowel movements; and six, stool frequency of less than three bowel movements per week.
In order to diagnose constipation, if a patient has any two of these symptoms, for 25% of bowel movements over a period of three to six months, then that individual should be considered as having chronic constipation.
When is pharmacological management of constipation appropriate? And what diagnostic approach do you usually take to determine treatment?
Dr. Rao: I usually take a very detailed history from these patients. One of the things we have recognized, recently, is how inadequate our history has become, not necessarily from a lack of asking questions, but it seems to be a multifactorial process. We tested this in a prospective study showing that only 30% of the time were patients history correct in letting us know why they came to the clinic. The same patient who answered a questionnaire about their symptoms, when they keep a diary for one week, there's only 30% of the time there is concordance; 70% of the time the story is different.
Hence, the first step really is to get an accurate story about your patient with constipation. Fortunately, there are some digital apps that are available, a constipation stool diary app, and there is a MyGiHealth app, et cetera. People can use these apps or they can keep a paper diary. The next step would be to determine what may be mechanistically going wrong. These two steps will guide your management approach.
We have some simple tests that we can do. One is called a colon transit study, where we measure the speed at which stool goes through the colon. And we have two tests that are commonly used. One is called a Sitz marker test. The patient swallows the capsule which has 24 plastic rings. And then they take it on day one. They get an X-ray on day six, which is 120 hours later, and you count how many of those markers are left behind with the X-ray. If there are more than five, it's abnormal. It says they have slow transit. In other words, lazy colon. On the other hand, if they pass most of the markers, then they don't have a lazy colon.
The second test we often do is a wireless motility capsule test, which is again a capsule they swallow. They wear a recorder for five days. And then that measures the speed at which the capsule goes through the colon. A very simple way of measuring colon speed as well. These tests tell us if you have a lazy colon.
Another test that we do is anorectal manometry. We do the test because 40%-50% of patients have pelvic floor dysfunction. And that gives us important insights mechanistically whether they have rectal hypersensitivity or whether they have a problem with evacuating stool. If they have rectal hypersensitivity and they're complaining of constipation, then it equates to irritable bowel syndrome with constipation. That's how we pick up that category of constipation.
The other category I mentioned earlier, lazy colon, is slow transit constipation. And the third category we often see is called dyssynergic defecation, where they are unable to evacuate stool in a timely, orderly fashion. And that is the third group of constipation. Use these tests to help you diagnose which kind of constipation a patient has. That will then guide towards appropriate management.
Is there a higher frequency in the need for pharmacological management of constipation? And is it in any particular patient, for instance, such as older adults, the elderly, or those that may be critically ill, or any other demographic?
Dr. Rao: In terms of managing the constipation itself, many times patients will go to the drugstore or talk to a pharmacist and start taking some over-the-counter preparations.
Until recently, there hasn’t been any good study to tell us which one works, which preparation works. But we recently published a systematic review of which I had the pleasure of serving as the first author, where we looked at the 30-year data on over-the-counter treatments. We found that there is very good evidence to support polyethylene glycol, PEG, as grade A, recommendation.
We also found good evidence for senna, and for magnesium. These three compounds had good evidence. Whereas, for other over-the-counter preparations, such as fiber supplements, there are some fruit-based supplements that are now available, lactulose and so on, the evidence was second grade.
With regards to prescriptions, a drug that is approved is linaclotide, which is approved at a dose of 145 micrograms a day in a capsule form for treatment of chronic constipation. It's also approved for IBS with constipation at a higher dose of 290 micrograms a day. We also have plecanatide 3mg tablets. Both linaclotide and plecanatide, are guanylate cyclase C receptor agonists. They activate secretion through the guanylate cyclase pathway in the gut. And then, by inducing secretion, they improve constipation. Both drugs are approved for IBS-C and chronic constipation.
Then we have lubiprostone, which is a chloride channel 2 activator drug that is approved at a dose of 24 micrograms twice a day with food for treatment of chronic constipation, and 8 micrograms twice a day for treatment of IBS with constipation.
The most recent drug that was approved is called prucalopride. And this is approved at 1 to 2 milligrams a day. And this is a serotonin compound, which speeds up the gut activity including the, stomach, small bowel, and the colon. And by speeding up gut activity, it improves constipation.
Regarding particular demographics, I find that older patients tend to be a little bit more sensitive to some drugs. Some older adults are more refractory to standard compounds, which makes their management a little bit more challenging. We have to really titrate the dose very carefully.
The critically ill group is a little bit more challenging. Often, it's acute constipation or they're in ICU settings and there the management becomes a little bit more complex. One component of that critically ill group-- and of course, you can also see them in outpatient practice-- is the opioid-induced constipation, which is a category in its own right and has been recognized by the FDA. Unfortunately, one of the major side effects of opioid is constipation. It takes a toll in the gut.
Fortunately, we have a new set of drugs, called PAMORAs, or peripherally acting mu-opioid receptor antagonists. These drugs, when you take them orally, will neutralize the effect of opioids in the gut, and thereby relieve constipation without affecting the analgesic effect of opioids. Examples of these class of drugs are methylnaltrexone, naloxegol, naldemedine, etc. These are all FDA approved now for treatment of opioid-induced constipation, which is part of your critical ill or hospitalized patients, and sometimes they are really outpatients as well.
Are there risk factors?
Dr. Rao: There are several risk factors for constipation. They include, for example, elderly, particularly people who are not very mobile for various reasons, people-- but I think the biggest risk factor that I would like to mention, emphasize, is drugs. There are many, many, many drugs that cause constipation. Opioids, as I just mentioned . Also a number of anti-hypertensive drugs. Calcium channel blockers, for example, are constipating.
Now, iron, heavy metals, and calcium, are very constipating. So are anticholinergics, and antidepressants. Many antidepressants, particularly the tricyclic class, are very constipating.
My first message to my colleagues is, when a patient is presenting to you in the clinic, the first thing I teach my students, residents, fellows, is to look at the drug list. Think about that drug as a mischief for constipation. If it is feasible and appropriate, remove the drug, or substitute the drug, as you are looking for other reasons for constipation.
Another important risk factor worth mentioning is acute constipation. Constipation in a 70-year-old person, suddenly over the last six weeks, is serious and may raise suspicion for cancer in the colon, and the need for investigation. If patients suddenly develop constipation like that, then, it is important to make sure that there is nothing blocking the colon, that is creating constipation.
One other important group is pregnancy. I did forget to mention that. Interestingly, between a quarter to a third of woman, otherwise healthy women, have never had any symptoms, however, during pregnancy they do become constipated. That's because of hormonal changes, particularly the rush of progesterone which is happening in their body. And so, they will have to be managed during their stage, and very many times they will come back to their normal lifestyle afterwards.
When it comes to the data on current and emerging treatments, what do you see in the future of pharmacological management of constipation?
Dr. Rao: I think one of the critical things in the pharmacologic management of constipation is to recognize that constipation is rarely a one-mechanism disorder. And it may be in a particular individual, but how do we know? For example, how do we know that their gut is not producing enough serotonin? And that is why they are serotonin depleted in the gut and we need to supplement them with a serotonin product, to just give you an example. We don't know that. We don't have their genetic makeup, and so on.
What I'm getting at is, constipation is a heterogenic problem, and there are multiple mechanisms that lead to it. Therefore, our current armamentarium has significantly improved in the last decade.
The first decade of the new Millennium saw significant new drugs that were introduced. The second decade has seen even more. But I think there are other compounds that are now coming up. There are sodium-hydrogen ion pump blocking drugs , and there are other mechanistic drugs . There's another drug which is available in Japan, not yet in the US, called elobixibat, which actually blocks bile acid. Normally, 95% of the bile is reabsorbed in the colon. But if you block bile acid reabsorption and allow some bile to spill into the colon, your own bile can become a laxative in the colon.
Another important approach has been through a capsule technology, called Vibrant capsule. . I'm part of an investigative group is investigating this drug. The phase III data is not yet available but has been submitted, You take a capsule once a day, and this gently agitates the colon, and thereby stimulating the colon muscles to move the stool, and then you evacuate. So, it is not a pharmacologic, but it is a form of a capsule device treatment .
These are some emerging treatments just around the corner. There is kind of a belt device that you can wear around the belly, which passes a small amount of electric current in a sequential manner, to stimulate peristalsis, called electrical interference therapy.
If pharmacological management is not an option, then what's next?
Dr. Rao: Pharmacologic management works for about 50% of patients. But it doesn't work in everybody. It's not because the drug itself is not working or has side effects, instead, the issue is that the problem is not likely to be fixed by pharmacologic management.
About 40% of patients have a pelvic floor dysfunction called dyssynergic defecation. These folks, unbeknownst to them, have learned a new process of pooping, where they are blocking their own pooping action. They're not doing it deliberately. They're totally unaware. A third of them have this problem right from childhood. 2/3 acquire this problem in adulthood. Needless to say, this problem affects 40% of patients.
So yes, you can give them medications, and that will temporarily help them. But because they cannot evacuate this stool, it will never help them permanently. These individuals are best helped by a behavioral treatment called biofeedback therapy. Hopefully in the future, home-based biofeedback tools can become available, and that can really make this treatment more widely available to the public.
Is there anything else you'd like to add before we conclude?
Dr. Rao: My most important message to my colleagues is that constipation is a very common problem. Please take time to spend with your patients. Please use an app or a diary to record the symptoms. If at all possible, perform a digital rectal examination on all your constipated patients to identify the pelvic floor dysfunction group of patients. Manage them appropriately with over the counter drugs or particularly the FDA-approved pharmacotherapies. And that will work in a majority.
But when drugs don’t work or patients have predominant symptoms of difficult defecation, put on your thinking hat. Don't give up on your patients. Instead of sending them to a surgeon, which many times we rush to do, try and see what other things you can do including manometric testing and biofeedback therapy.
Professor Satish Rao, MD is the J. Harold Harrison, MD, Distinguished University Chair in Gastroenterology. Dr. Rao is also founding Director of the Digestive Health Center and Clinical Research Center, and tenured Director and Professor of Medicine. His research interests in Neurogastroenterology/Motility have focused on gaining mechanistic insights, developing novel diagnostic tools and pioneering innovative treatments for constipation, dyssynergic defecation, fecal incontinence, IBS, food intolerance, gas and bloating and small intestinal bacterial and fungal overgrowth (SIBO/SIFO) and visceral pain. His latest invention translumbosacral neuromodulation therapy (TNT) is revolutionizing treatment for fecal incontinence.
As a gastroenterologist, with a focus on research that includes pathophysiological treatment of IBS and constipation as the primary objective, how prevalent is it in your current practice?
Dr. Rao: It is a pleasure to discuss a topic very close to my heart. It is a very important but often neglected topic, and very many times people go to pharmacies, over-the-counter, or their grandmothers, seeking treatment for constipation, whereas, with all the advances today, they should be coming to us, gastroenterologists, as the primary source for really managing this problem.
Constipation is very common. It all depends, to some extent, on how we define it. But if we define it based on some more popular criteria, such as those supported by the Rome Foundation criteria, the global prevalence, is between 10% to 15%. As you can see millions of Americans suffer with this problem, and almost all AGA members would have seen hundreds of these patients in the course of their practice every year. So, it is highly prevalent.
The term constipation is misunderstood by many people. Different people have different names, different people have different definitions and different criteria. For years, most physicians and most textbooks equated constipation to infrequent bowel movements. That logic has changed dramatically in the last 10 to 15 years, where we now recognize constipation as not only infrequent bowel movement but, more commonly, difficulty with bowel movement. This difficulty with bowel movement has been the missing link as we were all focused on infrequent bowel movement. We now recognize that constipation means one of six things.
What are those six things that tell us it’s constipation? One, there’s excessive straining to have a bowel movement; two after having had a bowel movement, you're left with a feeling of incomplete evacuation; three, the stools are hard and difficult to pass. We have a very famous scale, called the Bristol Stool Form Scale. If anybody takes the time to look at the scale, if your stool form happens to be 1, 2, or 3, then you're more likely to be constipated; four, a patient has to use digital maneuvers or some kind of support to try and evacuate stool; five, a patient reports a sensation of blockage at the time of bowel movement repeatedly, and at least with 25% of bowel movements; and six, stool frequency of less than three bowel movements per week.
In order to diagnose constipation, if a patient has any two of these symptoms, for 25% of bowel movements over a period of three to six months, then that individual should be considered as having chronic constipation.
When is pharmacological management of constipation appropriate? And what diagnostic approach do you usually take to determine treatment?
Dr. Rao: I usually take a very detailed history from these patients. One of the things we have recognized, recently, is how inadequate our history has become, not necessarily from a lack of asking questions, but it seems to be a multifactorial process. We tested this in a prospective study showing that only 30% of the time were patients history correct in letting us know why they came to the clinic. The same patient who answered a questionnaire about their symptoms, when they keep a diary for one week, there's only 30% of the time there is concordance; 70% of the time the story is different.
Hence, the first step really is to get an accurate story about your patient with constipation. Fortunately, there are some digital apps that are available, a constipation stool diary app, and there is a MyGiHealth app, et cetera. People can use these apps or they can keep a paper diary. The next step would be to determine what may be mechanistically going wrong. These two steps will guide your management approach.
We have some simple tests that we can do. One is called a colon transit study, where we measure the speed at which stool goes through the colon. And we have two tests that are commonly used. One is called a Sitz marker test. The patient swallows the capsule which has 24 plastic rings. And then they take it on day one. They get an X-ray on day six, which is 120 hours later, and you count how many of those markers are left behind with the X-ray. If there are more than five, it's abnormal. It says they have slow transit. In other words, lazy colon. On the other hand, if they pass most of the markers, then they don't have a lazy colon.
The second test we often do is a wireless motility capsule test, which is again a capsule they swallow. They wear a recorder for five days. And then that measures the speed at which the capsule goes through the colon. A very simple way of measuring colon speed as well. These tests tell us if you have a lazy colon.
Another test that we do is anorectal manometry. We do the test because 40%-50% of patients have pelvic floor dysfunction. And that gives us important insights mechanistically whether they have rectal hypersensitivity or whether they have a problem with evacuating stool. If they have rectal hypersensitivity and they're complaining of constipation, then it equates to irritable bowel syndrome with constipation. That's how we pick up that category of constipation.
The other category I mentioned earlier, lazy colon, is slow transit constipation. And the third category we often see is called dyssynergic defecation, where they are unable to evacuate stool in a timely, orderly fashion. And that is the third group of constipation. Use these tests to help you diagnose which kind of constipation a patient has. That will then guide towards appropriate management.
Is there a higher frequency in the need for pharmacological management of constipation? And is it in any particular patient, for instance, such as older adults, the elderly, or those that may be critically ill, or any other demographic?
Dr. Rao: In terms of managing the constipation itself, many times patients will go to the drugstore or talk to a pharmacist and start taking some over-the-counter preparations.
Until recently, there hasn’t been any good study to tell us which one works, which preparation works. But we recently published a systematic review of which I had the pleasure of serving as the first author, where we looked at the 30-year data on over-the-counter treatments. We found that there is very good evidence to support polyethylene glycol, PEG, as grade A, recommendation.
We also found good evidence for senna, and for magnesium. These three compounds had good evidence. Whereas, for other over-the-counter preparations, such as fiber supplements, there are some fruit-based supplements that are now available, lactulose and so on, the evidence was second grade.
With regards to prescriptions, a drug that is approved is linaclotide, which is approved at a dose of 145 micrograms a day in a capsule form for treatment of chronic constipation. It's also approved for IBS with constipation at a higher dose of 290 micrograms a day. We also have plecanatide 3mg tablets. Both linaclotide and plecanatide, are guanylate cyclase C receptor agonists. They activate secretion through the guanylate cyclase pathway in the gut. And then, by inducing secretion, they improve constipation. Both drugs are approved for IBS-C and chronic constipation.
Then we have lubiprostone, which is a chloride channel 2 activator drug that is approved at a dose of 24 micrograms twice a day with food for treatment of chronic constipation, and 8 micrograms twice a day for treatment of IBS with constipation.
The most recent drug that was approved is called prucalopride. And this is approved at 1 to 2 milligrams a day. And this is a serotonin compound, which speeds up the gut activity including the, stomach, small bowel, and the colon. And by speeding up gut activity, it improves constipation.
Regarding particular demographics, I find that older patients tend to be a little bit more sensitive to some drugs. Some older adults are more refractory to standard compounds, which makes their management a little bit more challenging. We have to really titrate the dose very carefully.
The critically ill group is a little bit more challenging. Often, it's acute constipation or they're in ICU settings and there the management becomes a little bit more complex. One component of that critically ill group-- and of course, you can also see them in outpatient practice-- is the opioid-induced constipation, which is a category in its own right and has been recognized by the FDA. Unfortunately, one of the major side effects of opioid is constipation. It takes a toll in the gut.
Fortunately, we have a new set of drugs, called PAMORAs, or peripherally acting mu-opioid receptor antagonists. These drugs, when you take them orally, will neutralize the effect of opioids in the gut, and thereby relieve constipation without affecting the analgesic effect of opioids. Examples of these class of drugs are methylnaltrexone, naloxegol, naldemedine, etc. These are all FDA approved now for treatment of opioid-induced constipation, which is part of your critical ill or hospitalized patients, and sometimes they are really outpatients as well.
Are there risk factors?
Dr. Rao: There are several risk factors for constipation. They include, for example, elderly, particularly people who are not very mobile for various reasons, people-- but I think the biggest risk factor that I would like to mention, emphasize, is drugs. There are many, many, many drugs that cause constipation. Opioids, as I just mentioned . Also a number of anti-hypertensive drugs. Calcium channel blockers, for example, are constipating.
Now, iron, heavy metals, and calcium, are very constipating. So are anticholinergics, and antidepressants. Many antidepressants, particularly the tricyclic class, are very constipating.
My first message to my colleagues is, when a patient is presenting to you in the clinic, the first thing I teach my students, residents, fellows, is to look at the drug list. Think about that drug as a mischief for constipation. If it is feasible and appropriate, remove the drug, or substitute the drug, as you are looking for other reasons for constipation.
Another important risk factor worth mentioning is acute constipation. Constipation in a 70-year-old person, suddenly over the last six weeks, is serious and may raise suspicion for cancer in the colon, and the need for investigation. If patients suddenly develop constipation like that, then, it is important to make sure that there is nothing blocking the colon, that is creating constipation.
One other important group is pregnancy. I did forget to mention that. Interestingly, between a quarter to a third of woman, otherwise healthy women, have never had any symptoms, however, during pregnancy they do become constipated. That's because of hormonal changes, particularly the rush of progesterone which is happening in their body. And so, they will have to be managed during their stage, and very many times they will come back to their normal lifestyle afterwards.
When it comes to the data on current and emerging treatments, what do you see in the future of pharmacological management of constipation?
Dr. Rao: I think one of the critical things in the pharmacologic management of constipation is to recognize that constipation is rarely a one-mechanism disorder. And it may be in a particular individual, but how do we know? For example, how do we know that their gut is not producing enough serotonin? And that is why they are serotonin depleted in the gut and we need to supplement them with a serotonin product, to just give you an example. We don't know that. We don't have their genetic makeup, and so on.
What I'm getting at is, constipation is a heterogenic problem, and there are multiple mechanisms that lead to it. Therefore, our current armamentarium has significantly improved in the last decade.
The first decade of the new Millennium saw significant new drugs that were introduced. The second decade has seen even more. But I think there are other compounds that are now coming up. There are sodium-hydrogen ion pump blocking drugs , and there are other mechanistic drugs . There's another drug which is available in Japan, not yet in the US, called elobixibat, which actually blocks bile acid. Normally, 95% of the bile is reabsorbed in the colon. But if you block bile acid reabsorption and allow some bile to spill into the colon, your own bile can become a laxative in the colon.
Another important approach has been through a capsule technology, called Vibrant capsule. . I'm part of an investigative group is investigating this drug. The phase III data is not yet available but has been submitted, You take a capsule once a day, and this gently agitates the colon, and thereby stimulating the colon muscles to move the stool, and then you evacuate. So, it is not a pharmacologic, but it is a form of a capsule device treatment .
These are some emerging treatments just around the corner. There is kind of a belt device that you can wear around the belly, which passes a small amount of electric current in a sequential manner, to stimulate peristalsis, called electrical interference therapy.
If pharmacological management is not an option, then what's next?
Dr. Rao: Pharmacologic management works for about 50% of patients. But it doesn't work in everybody. It's not because the drug itself is not working or has side effects, instead, the issue is that the problem is not likely to be fixed by pharmacologic management.
About 40% of patients have a pelvic floor dysfunction called dyssynergic defecation. These folks, unbeknownst to them, have learned a new process of pooping, where they are blocking their own pooping action. They're not doing it deliberately. They're totally unaware. A third of them have this problem right from childhood. 2/3 acquire this problem in adulthood. Needless to say, this problem affects 40% of patients.
So yes, you can give them medications, and that will temporarily help them. But because they cannot evacuate this stool, it will never help them permanently. These individuals are best helped by a behavioral treatment called biofeedback therapy. Hopefully in the future, home-based biofeedback tools can become available, and that can really make this treatment more widely available to the public.
Is there anything else you'd like to add before we conclude?
Dr. Rao: My most important message to my colleagues is that constipation is a very common problem. Please take time to spend with your patients. Please use an app or a diary to record the symptoms. If at all possible, perform a digital rectal examination on all your constipated patients to identify the pelvic floor dysfunction group of patients. Manage them appropriately with over the counter drugs or particularly the FDA-approved pharmacotherapies. And that will work in a majority.
But when drugs don’t work or patients have predominant symptoms of difficult defecation, put on your thinking hat. Don't give up on your patients. Instead of sending them to a surgeon, which many times we rush to do, try and see what other things you can do including manometric testing and biofeedback therapy.
2021 in Review: Key Trials in Type 2 Diabetes (T2D)
Ronald Goldenberg, MD, completed his residency in Internal Medicine in 1987 at the University of Toronto, and his fellowship in Endocrinology & Metabolism in 1989 at the University of Toronto. Dr. Goldenberg is a past chair of the Ontario Medical Association Section on Endocrinology & Metabolism and a previous President of the Toronto Diabetes Association. Dr. Goldenberg has been an investigator in a wide array of clinical trials in the areas of diabetes, hypertension, obesity, and dyslipidemia. His major areas of interest include clinical care of diabetes, obesity, dyslipidemia and thyroid disorders. He has been actively involved in Continuing Medical Education for the last 3 decades, with a strong focus on translating evidence-based medicine into practical patient care.
As a consultant endocrinologist with an area of interest that includes clinical care of diabetes, can you briefly tell us what the top 5 studies of 2021 were that are most likely to influence diabetes or obesity practice?
Dr. Goldenberg: 2021 was a banner year for clinicians managing diabetes and or obesity. There were many key trials that were published and or presented. In my mind, the most important ones that will really influence practice include the STEP program of semaglutide 2.4 mg once weekly in the management of overweight or obesity. There is the FIGARO-DKD and FIDELITY analysis of finerenone in patients with type 2 diabetes and chronic kidney disease. Other top studies include the SURPASS trials of a novel dual incretin agonist called tirzepatide, the EMPEROR-Preserved trial with empagliflozin and a pooled analysis of empagliflozin in both HFrEF and HFpEF trials, and the AMPLITUDE-O trial, which is a cardiovascular outcome trial with an exendin-based GLP-1 receptor agonist known as efpeglenatide.
2021 was definitely a landmark year in diabetes. Let's start with the STEP program with semaglutide 2.4. What were the important findings in these studies?
Dr. Goldenberg: STEP is the Phase III program for 2.4 milligrams once weekly in the management of overweight or obesity. The STEP program studies that have been published and/or presented in 2021 include four Phase IIIa trials STEP 1 through STEP 4, as well as three Phase IIIb trials, STEP 5, 6, and 8. They're all rather similar, as they each enrolled patients with overweight and/or obesity. Patients were up-titrated to semaglutide 2.4 milligrams once weekly, and the top-line summary across all of these trials is that patients randomized to semaglutide 2.4 mg once weekly lost 15% to 17% of their body weight amongst those that did not have diabetes, which is really a tremendous amount of weight loss for an anti-obesity drug. And even those with type 2 diabetes lost almost 10% of their body weight, which is pretty impressive given that patients with type 2 diabetes are often somewhat refractory to weight loss.
There was a high percentage of body weight loss across these trials, as roughly 86 to 90% of patients without diabetes achieved at least a 5% body weight loss and even in those with diabetes, almost 70% achieved a 5% loss in their body weight. As far as overall safety, the safety profile of semaglutide 2.4 mg once weekly was generally similar to the GLP-1 receptor agonist class. The most common side effects were gastrointestinal. Nausea occurred anywhere from 20% to 58% of patients, but it was generally transient. Very few people withdrew because of gastrointestinal side effects.
I think the key thing for clinicians to know about the STEP program is that it's the results of these trials that led to the FDA approving semaglutide 2.4 mg once weekly as a new agent for the management of overweight and/or obesity.
You mentioned FIGARO-DKD and FIDELITY with Finerenone. Can you talk more about the relevance of this data and summarize the key findings?
Dr. Goldenberg: Finerenone is a new selective non-steroidal mineralocorticoid receptor antagonist that interacts with the mineralocorticoid receptor in a different way compared to traditional steroidal mineralocorticoid receptor agonists. We know from pre-clinical data that this agent targets inflammation and fibrosis in both the kidney and the heart. The finerenone Phase III program focused on patients with type 2 diabetes and chronic kidney disease. In 2021, they published the FIGARO-DKD trial. This enrolled almost 7,500 patients with type 2 diabetes and an eGFR of 25 ml/min or more along with albuminuria.
The key result of this trial is the primary outcome of CV death non-fatal MI, non-fatal stroke, or hospitalization for heart failure was reduced by 13%. The number needed to treat after 3 ½ years was 47. The primary outcome was mainly driven by a reduction for hospitalization for heart failure. Key secondary outcomes included composite kidney outcomes, one of which was defined by a sustained eGFR reduction of 40% or more along with end-stage kidney disease or renal death. This did not quite reach statistical significance, but a more stringent outcome that included a reduction in eGFR of at least 57% or more was in fact reduced by 23%.
End-stage kidney disease was reduced by 36% in the FIGARO-DKD trial. Finerenone was well tolerated. Hyperkalemia occurred in 10.8% of patients on finerenone and 5.3% on placebo but it was quite unusual to have to stop finerenone because of hyperkalemia. Building on FIGARO-DKD in 2021 was a prespecified meta-analysis of two large Phase III trials, the FIGARO-DKD trial and also the previously published FIDELIO-DKD trial and in this pooled analysis the composite cardiovascular outcome was reduced by 14%. The benefit on cardiovascular events was independent of the baseline eGFR or urine albumin to creatinine ratio, as well as independent of the use of SGLT2 inhibitors or GLP-1 receptor agonists.
There was also a 23% reduction in a composite kidney outcome that used a sustained 57% reduction in eGFR as part of that outcome and essentially each component of the composite kidney outcome was reduced, including kidney failure, end-stage kidney disease, eGFR of less than 15 ml/min in addition to a ≥57% decrease in eGFR. And this kidney outcome showed a benefit irrespective of the use of SGLT2 inhibitor at baseline although the number of patients taking an SGLT2 inhibitor in this analysis was relatively small. So overall, the results of finerenone in 2021 support the use of this agent in patients with type 2 diabetes and chronic kidney disease to improve both cardiovascular and kidney outcomes.
Thank you for this insight. Regarding the SURPASS trials with tirzepatide, what is tirzepatide and what was its impact on glycemia and weight?
Dr. Goldenberg. My pleasure. Tirzepatide is a unique dual GIP/GLP-1 receptor agonist that has been formulated to be given as a once weekly injection. In 2021, we heard the first results from the Phase III program including SURPASS-1 through SURPASS-5. In these trials, patients were randomized to tirzepatide 5 mg, 10 mg, or 15 mg, and often compared to placebo or an active comparator. Across the SURPASS trials, the A1C reduction from baseline was between 1.9% to 2.6%. Up to 97% of patients on tirzepatide achieved a HbA1c of less than 7%, and up to 62% achieve a normal HbA1c of less than 5.7%.
In addition to these rather robust glycemic outcomes, there was excellent weight loss in the SURPASS program with the weight reduction ranging from 6 to 13 kg from baseline. Interestingly, in the SURPASS studies, tirzepatide showed superiority to semaglutide 1 mg and also superiority to titrated basal insulin. As far as safety, the side effect profile was similar to all GLP-1 receptor agonists with transient nausea being the most common side effect. Overall, tirzepatide will definitely add to our ability to treat our patients with type 2 diabetes with an incretin agent, and when this agent gets approved, hopefully, it will provide robust glycemic lowering and weight loss.
The fourth key study you mentioned is the EMPEROR-Preserved along with the EMPEROR-Pooled with the empagliflozin. What did they find in this analysis?
Dr. Goldenberg: The EMPEROR-Preserved was the first completed large randomized clinical trial of an SGLT2 inhibitor in patients with heart failure with preserved ejection fraction. They enrolled almost 6,000 patients with HFpEF with or without type 2 diabetes and they were randomized to empagliflozin 10 mg or placebo. The primary outcome of cardiovascular death or hospitalization for heart failure was reduced by 21% with empagliflozin and the number needed to treat was 31. This primary outcome was largely driven by a reduction in hospitalization for heart failure. The primary outcome showed consistent benefit across 15 prespecified subgroups, including those with or without type 2 diabetes, and including a spectrum of baseline left ventricular ejection fractions from 40% to 50% to greater than 60%.
There were also some key secondary endpoints: total hospitalization for heart failure was reduced by 27% and empagliflozin also slowed the decline of eGFR over time in the EMPEROR-Preserved trial. The agent was well tolerated. There was a slight signal for more hypotension and genital mycotic infections, but otherwise really no concerning adverse effects.
Building on the EMPEROR-Preserved trial was a prespecified pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved, the two large outcome trials with empagliflozin in heart failure patients. The prespecified primary outcome of this analysis was a major renal outcome which included a GFR reduction of ≥40%, renal replacement therapy or sustained eGFR <10-15 ml/min. While in EMPEROR-Reduced there was a significant 49% reduction in this composite renal outcome, in EMPEROR-Preserved there was no significant reduction. Because of the heterogeneity across these two trials, it was not statistically valid to pool these two results for the composite renal outcome. However, what they found in EMPEROR-Pooled is that if you use a more robust renal outcome including at least a 50% decline in eGFR, then there seems to be a trend that varies depending on baseline left ventricular ejection fraction, suggesting a benefit on the renal outcome if your baseline left ventricular function ranges from 40% to 60%, but lack of benefit with a baseline left ventricular ejection fraction of over 60%. The top line summary of this data is that for the first time we have robust evidence that an SGLT2 inhibitor, in this case empagliflozin 10 mg, can provide a cardiovascular benefit in patients with HFpEF, in addition to the known benefit in HFrEF patients.
Finally, there's the AMPLITUDE-O with efpeglenatide, an international randomized controlled trial conducted at approximately 344 sites in 28 countries. What are the key learnings and messages for this specific study?
Dr. Goldenberg: Efpeglenatide is an exendin-4-based GLP-1 receptor agonist that is given once weekly and the AMPLITUDE-O trial is the cardiovascular outcome trial with efpeglenatide done in patients with type 2 diabetes and either cardiovascular disease or chronic kidney disease plus at least one cardiovascular risk factor. It was an important trial because prior to this cardiovascular outcome trial studies of exendin-4-based GLP-1 receptor agonist have been neutral. However, the AMPLITUDE-O study showed for the first-time superiority with an exendin-4-based GLP-1 receptor agonist. In this case, efpeglenatide 4 or 6 milligrams versus placebo was associated with a 27% reduction in the primary outcome of CV death, non-fatal MI or non-fatal stroke.
Importantly, there was a consistent benefit with efpeglenatide across a spectrum of prespecified subgroups, the most important one being those that entered the trial on a background SGLT2 inhibitor, which represented about 15% of the patients. They derived the same overall benefit as those not taking an SGLT2 inhibitor. It is important to appreciate that this is probably the most robust data we have for showing a cardiovascular benefit of adding a GLP-1 receptor agonist to an SGLT2 inhibitor in high risk patients with type 2 diabetes. AMPLITUDE-O also adds to the already appreciated knowledge of the cardiovascular benefit of GLP-1 receptor agonists and builds on this story by showing that you can get a cardiovascular benefit with an exendin-4-based GLP-1 receptor agonist and you can get a benefit as an add on to SGLT2 inhibitors.
STEP Program
- Wilding et al. N Engl J Med 2021; doi:10.1056/NEJMoa2032183; 2. Davies et al. Lancet, 2021; doi.org/10.1016/S0140-6736(21)00213-0: 3. Wadden et al. JAMA. doi:10.1001/jama.2021.1831; 4. Rubino et al. JAMA. 2021 Apr 13;325(14):1414-1425. doi: 10.1001/jama.2021.3224. 5. Garvey et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021; 6. Kadowaki et al. Presented at the International Congress on Metabolic Syndrome hybrid meeting .September 2-4, 2021; 7. Rubino et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021.
FIGARO-DKD and FIDELITY
- Pitt et al. N Engl J Med 2021; 385:2252-2263.DOI: 10.1056/NEJMoa2110956; 2. Agarwal et al. European Heart Journal 2021).https://doi.org/10.1093/eurheartj/ehab777.
SURPASS trials
- Rosenstock J, et al. Lancet. 2021;398(10295):143-155; 2. Frias JP, et al. N Eng J Med. 2021;385(6):503-515; 3. Ludvik B, et al. Lancet. 2021;398(10300):583-598; 4. Del Prato S, et al. Lancet. 2021; 5. Dahl D, et al. Poster presented at: ADA 2021. Poster LB-20.
EMPEROR-Preserved and EMPEROR-Pooled
- Anker S et al. N Engl J Med 2021; 385:1451-1461. DOI: 10.1056/NEJMoa2107038; 2. Packer M et al. N Engl J Med 2021; 385:1531-1533DOI: 10.1056/NEJMc2112411.
AMPLITUDE-O
- Gerstein H et al. N Engl J Med 2021; 385:896-907. DOI: 10.1056/NEJMoa2108269.
Ronald Goldenberg, MD, completed his residency in Internal Medicine in 1987 at the University of Toronto, and his fellowship in Endocrinology & Metabolism in 1989 at the University of Toronto. Dr. Goldenberg is a past chair of the Ontario Medical Association Section on Endocrinology & Metabolism and a previous President of the Toronto Diabetes Association. Dr. Goldenberg has been an investigator in a wide array of clinical trials in the areas of diabetes, hypertension, obesity, and dyslipidemia. His major areas of interest include clinical care of diabetes, obesity, dyslipidemia and thyroid disorders. He has been actively involved in Continuing Medical Education for the last 3 decades, with a strong focus on translating evidence-based medicine into practical patient care.
As a consultant endocrinologist with an area of interest that includes clinical care of diabetes, can you briefly tell us what the top 5 studies of 2021 were that are most likely to influence diabetes or obesity practice?
Dr. Goldenberg: 2021 was a banner year for clinicians managing diabetes and or obesity. There were many key trials that were published and or presented. In my mind, the most important ones that will really influence practice include the STEP program of semaglutide 2.4 mg once weekly in the management of overweight or obesity. There is the FIGARO-DKD and FIDELITY analysis of finerenone in patients with type 2 diabetes and chronic kidney disease. Other top studies include the SURPASS trials of a novel dual incretin agonist called tirzepatide, the EMPEROR-Preserved trial with empagliflozin and a pooled analysis of empagliflozin in both HFrEF and HFpEF trials, and the AMPLITUDE-O trial, which is a cardiovascular outcome trial with an exendin-based GLP-1 receptor agonist known as efpeglenatide.
2021 was definitely a landmark year in diabetes. Let's start with the STEP program with semaglutide 2.4. What were the important findings in these studies?
Dr. Goldenberg: STEP is the Phase III program for 2.4 milligrams once weekly in the management of overweight or obesity. The STEP program studies that have been published and/or presented in 2021 include four Phase IIIa trials STEP 1 through STEP 4, as well as three Phase IIIb trials, STEP 5, 6, and 8. They're all rather similar, as they each enrolled patients with overweight and/or obesity. Patients were up-titrated to semaglutide 2.4 milligrams once weekly, and the top-line summary across all of these trials is that patients randomized to semaglutide 2.4 mg once weekly lost 15% to 17% of their body weight amongst those that did not have diabetes, which is really a tremendous amount of weight loss for an anti-obesity drug. And even those with type 2 diabetes lost almost 10% of their body weight, which is pretty impressive given that patients with type 2 diabetes are often somewhat refractory to weight loss.
There was a high percentage of body weight loss across these trials, as roughly 86 to 90% of patients without diabetes achieved at least a 5% body weight loss and even in those with diabetes, almost 70% achieved a 5% loss in their body weight. As far as overall safety, the safety profile of semaglutide 2.4 mg once weekly was generally similar to the GLP-1 receptor agonist class. The most common side effects were gastrointestinal. Nausea occurred anywhere from 20% to 58% of patients, but it was generally transient. Very few people withdrew because of gastrointestinal side effects.
I think the key thing for clinicians to know about the STEP program is that it's the results of these trials that led to the FDA approving semaglutide 2.4 mg once weekly as a new agent for the management of overweight and/or obesity.
You mentioned FIGARO-DKD and FIDELITY with Finerenone. Can you talk more about the relevance of this data and summarize the key findings?
Dr. Goldenberg: Finerenone is a new selective non-steroidal mineralocorticoid receptor antagonist that interacts with the mineralocorticoid receptor in a different way compared to traditional steroidal mineralocorticoid receptor agonists. We know from pre-clinical data that this agent targets inflammation and fibrosis in both the kidney and the heart. The finerenone Phase III program focused on patients with type 2 diabetes and chronic kidney disease. In 2021, they published the FIGARO-DKD trial. This enrolled almost 7,500 patients with type 2 diabetes and an eGFR of 25 ml/min or more along with albuminuria.
The key result of this trial is the primary outcome of CV death non-fatal MI, non-fatal stroke, or hospitalization for heart failure was reduced by 13%. The number needed to treat after 3 ½ years was 47. The primary outcome was mainly driven by a reduction for hospitalization for heart failure. Key secondary outcomes included composite kidney outcomes, one of which was defined by a sustained eGFR reduction of 40% or more along with end-stage kidney disease or renal death. This did not quite reach statistical significance, but a more stringent outcome that included a reduction in eGFR of at least 57% or more was in fact reduced by 23%.
End-stage kidney disease was reduced by 36% in the FIGARO-DKD trial. Finerenone was well tolerated. Hyperkalemia occurred in 10.8% of patients on finerenone and 5.3% on placebo but it was quite unusual to have to stop finerenone because of hyperkalemia. Building on FIGARO-DKD in 2021 was a prespecified meta-analysis of two large Phase III trials, the FIGARO-DKD trial and also the previously published FIDELIO-DKD trial and in this pooled analysis the composite cardiovascular outcome was reduced by 14%. The benefit on cardiovascular events was independent of the baseline eGFR or urine albumin to creatinine ratio, as well as independent of the use of SGLT2 inhibitors or GLP-1 receptor agonists.
There was also a 23% reduction in a composite kidney outcome that used a sustained 57% reduction in eGFR as part of that outcome and essentially each component of the composite kidney outcome was reduced, including kidney failure, end-stage kidney disease, eGFR of less than 15 ml/min in addition to a ≥57% decrease in eGFR. And this kidney outcome showed a benefit irrespective of the use of SGLT2 inhibitor at baseline although the number of patients taking an SGLT2 inhibitor in this analysis was relatively small. So overall, the results of finerenone in 2021 support the use of this agent in patients with type 2 diabetes and chronic kidney disease to improve both cardiovascular and kidney outcomes.
Thank you for this insight. Regarding the SURPASS trials with tirzepatide, what is tirzepatide and what was its impact on glycemia and weight?
Dr. Goldenberg. My pleasure. Tirzepatide is a unique dual GIP/GLP-1 receptor agonist that has been formulated to be given as a once weekly injection. In 2021, we heard the first results from the Phase III program including SURPASS-1 through SURPASS-5. In these trials, patients were randomized to tirzepatide 5 mg, 10 mg, or 15 mg, and often compared to placebo or an active comparator. Across the SURPASS trials, the A1C reduction from baseline was between 1.9% to 2.6%. Up to 97% of patients on tirzepatide achieved a HbA1c of less than 7%, and up to 62% achieve a normal HbA1c of less than 5.7%.
In addition to these rather robust glycemic outcomes, there was excellent weight loss in the SURPASS program with the weight reduction ranging from 6 to 13 kg from baseline. Interestingly, in the SURPASS studies, tirzepatide showed superiority to semaglutide 1 mg and also superiority to titrated basal insulin. As far as safety, the side effect profile was similar to all GLP-1 receptor agonists with transient nausea being the most common side effect. Overall, tirzepatide will definitely add to our ability to treat our patients with type 2 diabetes with an incretin agent, and when this agent gets approved, hopefully, it will provide robust glycemic lowering and weight loss.
The fourth key study you mentioned is the EMPEROR-Preserved along with the EMPEROR-Pooled with the empagliflozin. What did they find in this analysis?
Dr. Goldenberg: The EMPEROR-Preserved was the first completed large randomized clinical trial of an SGLT2 inhibitor in patients with heart failure with preserved ejection fraction. They enrolled almost 6,000 patients with HFpEF with or without type 2 diabetes and they were randomized to empagliflozin 10 mg or placebo. The primary outcome of cardiovascular death or hospitalization for heart failure was reduced by 21% with empagliflozin and the number needed to treat was 31. This primary outcome was largely driven by a reduction in hospitalization for heart failure. The primary outcome showed consistent benefit across 15 prespecified subgroups, including those with or without type 2 diabetes, and including a spectrum of baseline left ventricular ejection fractions from 40% to 50% to greater than 60%.
There were also some key secondary endpoints: total hospitalization for heart failure was reduced by 27% and empagliflozin also slowed the decline of eGFR over time in the EMPEROR-Preserved trial. The agent was well tolerated. There was a slight signal for more hypotension and genital mycotic infections, but otherwise really no concerning adverse effects.
Building on the EMPEROR-Preserved trial was a prespecified pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved, the two large outcome trials with empagliflozin in heart failure patients. The prespecified primary outcome of this analysis was a major renal outcome which included a GFR reduction of ≥40%, renal replacement therapy or sustained eGFR <10-15 ml/min. While in EMPEROR-Reduced there was a significant 49% reduction in this composite renal outcome, in EMPEROR-Preserved there was no significant reduction. Because of the heterogeneity across these two trials, it was not statistically valid to pool these two results for the composite renal outcome. However, what they found in EMPEROR-Pooled is that if you use a more robust renal outcome including at least a 50% decline in eGFR, then there seems to be a trend that varies depending on baseline left ventricular ejection fraction, suggesting a benefit on the renal outcome if your baseline left ventricular function ranges from 40% to 60%, but lack of benefit with a baseline left ventricular ejection fraction of over 60%. The top line summary of this data is that for the first time we have robust evidence that an SGLT2 inhibitor, in this case empagliflozin 10 mg, can provide a cardiovascular benefit in patients with HFpEF, in addition to the known benefit in HFrEF patients.
Finally, there's the AMPLITUDE-O with efpeglenatide, an international randomized controlled trial conducted at approximately 344 sites in 28 countries. What are the key learnings and messages for this specific study?
Dr. Goldenberg: Efpeglenatide is an exendin-4-based GLP-1 receptor agonist that is given once weekly and the AMPLITUDE-O trial is the cardiovascular outcome trial with efpeglenatide done in patients with type 2 diabetes and either cardiovascular disease or chronic kidney disease plus at least one cardiovascular risk factor. It was an important trial because prior to this cardiovascular outcome trial studies of exendin-4-based GLP-1 receptor agonist have been neutral. However, the AMPLITUDE-O study showed for the first-time superiority with an exendin-4-based GLP-1 receptor agonist. In this case, efpeglenatide 4 or 6 milligrams versus placebo was associated with a 27% reduction in the primary outcome of CV death, non-fatal MI or non-fatal stroke.
Importantly, there was a consistent benefit with efpeglenatide across a spectrum of prespecified subgroups, the most important one being those that entered the trial on a background SGLT2 inhibitor, which represented about 15% of the patients. They derived the same overall benefit as those not taking an SGLT2 inhibitor. It is important to appreciate that this is probably the most robust data we have for showing a cardiovascular benefit of adding a GLP-1 receptor agonist to an SGLT2 inhibitor in high risk patients with type 2 diabetes. AMPLITUDE-O also adds to the already appreciated knowledge of the cardiovascular benefit of GLP-1 receptor agonists and builds on this story by showing that you can get a cardiovascular benefit with an exendin-4-based GLP-1 receptor agonist and you can get a benefit as an add on to SGLT2 inhibitors.
Ronald Goldenberg, MD, completed his residency in Internal Medicine in 1987 at the University of Toronto, and his fellowship in Endocrinology & Metabolism in 1989 at the University of Toronto. Dr. Goldenberg is a past chair of the Ontario Medical Association Section on Endocrinology & Metabolism and a previous President of the Toronto Diabetes Association. Dr. Goldenberg has been an investigator in a wide array of clinical trials in the areas of diabetes, hypertension, obesity, and dyslipidemia. His major areas of interest include clinical care of diabetes, obesity, dyslipidemia and thyroid disorders. He has been actively involved in Continuing Medical Education for the last 3 decades, with a strong focus on translating evidence-based medicine into practical patient care.
As a consultant endocrinologist with an area of interest that includes clinical care of diabetes, can you briefly tell us what the top 5 studies of 2021 were that are most likely to influence diabetes or obesity practice?
Dr. Goldenberg: 2021 was a banner year for clinicians managing diabetes and or obesity. There were many key trials that were published and or presented. In my mind, the most important ones that will really influence practice include the STEP program of semaglutide 2.4 mg once weekly in the management of overweight or obesity. There is the FIGARO-DKD and FIDELITY analysis of finerenone in patients with type 2 diabetes and chronic kidney disease. Other top studies include the SURPASS trials of a novel dual incretin agonist called tirzepatide, the EMPEROR-Preserved trial with empagliflozin and a pooled analysis of empagliflozin in both HFrEF and HFpEF trials, and the AMPLITUDE-O trial, which is a cardiovascular outcome trial with an exendin-based GLP-1 receptor agonist known as efpeglenatide.
2021 was definitely a landmark year in diabetes. Let's start with the STEP program with semaglutide 2.4. What were the important findings in these studies?
Dr. Goldenberg: STEP is the Phase III program for 2.4 milligrams once weekly in the management of overweight or obesity. The STEP program studies that have been published and/or presented in 2021 include four Phase IIIa trials STEP 1 through STEP 4, as well as three Phase IIIb trials, STEP 5, 6, and 8. They're all rather similar, as they each enrolled patients with overweight and/or obesity. Patients were up-titrated to semaglutide 2.4 milligrams once weekly, and the top-line summary across all of these trials is that patients randomized to semaglutide 2.4 mg once weekly lost 15% to 17% of their body weight amongst those that did not have diabetes, which is really a tremendous amount of weight loss for an anti-obesity drug. And even those with type 2 diabetes lost almost 10% of their body weight, which is pretty impressive given that patients with type 2 diabetes are often somewhat refractory to weight loss.
There was a high percentage of body weight loss across these trials, as roughly 86 to 90% of patients without diabetes achieved at least a 5% body weight loss and even in those with diabetes, almost 70% achieved a 5% loss in their body weight. As far as overall safety, the safety profile of semaglutide 2.4 mg once weekly was generally similar to the GLP-1 receptor agonist class. The most common side effects were gastrointestinal. Nausea occurred anywhere from 20% to 58% of patients, but it was generally transient. Very few people withdrew because of gastrointestinal side effects.
I think the key thing for clinicians to know about the STEP program is that it's the results of these trials that led to the FDA approving semaglutide 2.4 mg once weekly as a new agent for the management of overweight and/or obesity.
You mentioned FIGARO-DKD and FIDELITY with Finerenone. Can you talk more about the relevance of this data and summarize the key findings?
Dr. Goldenberg: Finerenone is a new selective non-steroidal mineralocorticoid receptor antagonist that interacts with the mineralocorticoid receptor in a different way compared to traditional steroidal mineralocorticoid receptor agonists. We know from pre-clinical data that this agent targets inflammation and fibrosis in both the kidney and the heart. The finerenone Phase III program focused on patients with type 2 diabetes and chronic kidney disease. In 2021, they published the FIGARO-DKD trial. This enrolled almost 7,500 patients with type 2 diabetes and an eGFR of 25 ml/min or more along with albuminuria.
The key result of this trial is the primary outcome of CV death non-fatal MI, non-fatal stroke, or hospitalization for heart failure was reduced by 13%. The number needed to treat after 3 ½ years was 47. The primary outcome was mainly driven by a reduction for hospitalization for heart failure. Key secondary outcomes included composite kidney outcomes, one of which was defined by a sustained eGFR reduction of 40% or more along with end-stage kidney disease or renal death. This did not quite reach statistical significance, but a more stringent outcome that included a reduction in eGFR of at least 57% or more was in fact reduced by 23%.
End-stage kidney disease was reduced by 36% in the FIGARO-DKD trial. Finerenone was well tolerated. Hyperkalemia occurred in 10.8% of patients on finerenone and 5.3% on placebo but it was quite unusual to have to stop finerenone because of hyperkalemia. Building on FIGARO-DKD in 2021 was a prespecified meta-analysis of two large Phase III trials, the FIGARO-DKD trial and also the previously published FIDELIO-DKD trial and in this pooled analysis the composite cardiovascular outcome was reduced by 14%. The benefit on cardiovascular events was independent of the baseline eGFR or urine albumin to creatinine ratio, as well as independent of the use of SGLT2 inhibitors or GLP-1 receptor agonists.
There was also a 23% reduction in a composite kidney outcome that used a sustained 57% reduction in eGFR as part of that outcome and essentially each component of the composite kidney outcome was reduced, including kidney failure, end-stage kidney disease, eGFR of less than 15 ml/min in addition to a ≥57% decrease in eGFR. And this kidney outcome showed a benefit irrespective of the use of SGLT2 inhibitor at baseline although the number of patients taking an SGLT2 inhibitor in this analysis was relatively small. So overall, the results of finerenone in 2021 support the use of this agent in patients with type 2 diabetes and chronic kidney disease to improve both cardiovascular and kidney outcomes.
Thank you for this insight. Regarding the SURPASS trials with tirzepatide, what is tirzepatide and what was its impact on glycemia and weight?
Dr. Goldenberg. My pleasure. Tirzepatide is a unique dual GIP/GLP-1 receptor agonist that has been formulated to be given as a once weekly injection. In 2021, we heard the first results from the Phase III program including SURPASS-1 through SURPASS-5. In these trials, patients were randomized to tirzepatide 5 mg, 10 mg, or 15 mg, and often compared to placebo or an active comparator. Across the SURPASS trials, the A1C reduction from baseline was between 1.9% to 2.6%. Up to 97% of patients on tirzepatide achieved a HbA1c of less than 7%, and up to 62% achieve a normal HbA1c of less than 5.7%.
In addition to these rather robust glycemic outcomes, there was excellent weight loss in the SURPASS program with the weight reduction ranging from 6 to 13 kg from baseline. Interestingly, in the SURPASS studies, tirzepatide showed superiority to semaglutide 1 mg and also superiority to titrated basal insulin. As far as safety, the side effect profile was similar to all GLP-1 receptor agonists with transient nausea being the most common side effect. Overall, tirzepatide will definitely add to our ability to treat our patients with type 2 diabetes with an incretin agent, and when this agent gets approved, hopefully, it will provide robust glycemic lowering and weight loss.
The fourth key study you mentioned is the EMPEROR-Preserved along with the EMPEROR-Pooled with the empagliflozin. What did they find in this analysis?
Dr. Goldenberg: The EMPEROR-Preserved was the first completed large randomized clinical trial of an SGLT2 inhibitor in patients with heart failure with preserved ejection fraction. They enrolled almost 6,000 patients with HFpEF with or without type 2 diabetes and they were randomized to empagliflozin 10 mg or placebo. The primary outcome of cardiovascular death or hospitalization for heart failure was reduced by 21% with empagliflozin and the number needed to treat was 31. This primary outcome was largely driven by a reduction in hospitalization for heart failure. The primary outcome showed consistent benefit across 15 prespecified subgroups, including those with or without type 2 diabetes, and including a spectrum of baseline left ventricular ejection fractions from 40% to 50% to greater than 60%.
There were also some key secondary endpoints: total hospitalization for heart failure was reduced by 27% and empagliflozin also slowed the decline of eGFR over time in the EMPEROR-Preserved trial. The agent was well tolerated. There was a slight signal for more hypotension and genital mycotic infections, but otherwise really no concerning adverse effects.
Building on the EMPEROR-Preserved trial was a prespecified pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved, the two large outcome trials with empagliflozin in heart failure patients. The prespecified primary outcome of this analysis was a major renal outcome which included a GFR reduction of ≥40%, renal replacement therapy or sustained eGFR <10-15 ml/min. While in EMPEROR-Reduced there was a significant 49% reduction in this composite renal outcome, in EMPEROR-Preserved there was no significant reduction. Because of the heterogeneity across these two trials, it was not statistically valid to pool these two results for the composite renal outcome. However, what they found in EMPEROR-Pooled is that if you use a more robust renal outcome including at least a 50% decline in eGFR, then there seems to be a trend that varies depending on baseline left ventricular ejection fraction, suggesting a benefit on the renal outcome if your baseline left ventricular function ranges from 40% to 60%, but lack of benefit with a baseline left ventricular ejection fraction of over 60%. The top line summary of this data is that for the first time we have robust evidence that an SGLT2 inhibitor, in this case empagliflozin 10 mg, can provide a cardiovascular benefit in patients with HFpEF, in addition to the known benefit in HFrEF patients.
Finally, there's the AMPLITUDE-O with efpeglenatide, an international randomized controlled trial conducted at approximately 344 sites in 28 countries. What are the key learnings and messages for this specific study?
Dr. Goldenberg: Efpeglenatide is an exendin-4-based GLP-1 receptor agonist that is given once weekly and the AMPLITUDE-O trial is the cardiovascular outcome trial with efpeglenatide done in patients with type 2 diabetes and either cardiovascular disease or chronic kidney disease plus at least one cardiovascular risk factor. It was an important trial because prior to this cardiovascular outcome trial studies of exendin-4-based GLP-1 receptor agonist have been neutral. However, the AMPLITUDE-O study showed for the first-time superiority with an exendin-4-based GLP-1 receptor agonist. In this case, efpeglenatide 4 or 6 milligrams versus placebo was associated with a 27% reduction in the primary outcome of CV death, non-fatal MI or non-fatal stroke.
Importantly, there was a consistent benefit with efpeglenatide across a spectrum of prespecified subgroups, the most important one being those that entered the trial on a background SGLT2 inhibitor, which represented about 15% of the patients. They derived the same overall benefit as those not taking an SGLT2 inhibitor. It is important to appreciate that this is probably the most robust data we have for showing a cardiovascular benefit of adding a GLP-1 receptor agonist to an SGLT2 inhibitor in high risk patients with type 2 diabetes. AMPLITUDE-O also adds to the already appreciated knowledge of the cardiovascular benefit of GLP-1 receptor agonists and builds on this story by showing that you can get a cardiovascular benefit with an exendin-4-based GLP-1 receptor agonist and you can get a benefit as an add on to SGLT2 inhibitors.
STEP Program
- Wilding et al. N Engl J Med 2021; doi:10.1056/NEJMoa2032183; 2. Davies et al. Lancet, 2021; doi.org/10.1016/S0140-6736(21)00213-0: 3. Wadden et al. JAMA. doi:10.1001/jama.2021.1831; 4. Rubino et al. JAMA. 2021 Apr 13;325(14):1414-1425. doi: 10.1001/jama.2021.3224. 5. Garvey et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021; 6. Kadowaki et al. Presented at the International Congress on Metabolic Syndrome hybrid meeting .September 2-4, 2021; 7. Rubino et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021.
FIGARO-DKD and FIDELITY
- Pitt et al. N Engl J Med 2021; 385:2252-2263.DOI: 10.1056/NEJMoa2110956; 2. Agarwal et al. European Heart Journal 2021).https://doi.org/10.1093/eurheartj/ehab777.
SURPASS trials
- Rosenstock J, et al. Lancet. 2021;398(10295):143-155; 2. Frias JP, et al. N Eng J Med. 2021;385(6):503-515; 3. Ludvik B, et al. Lancet. 2021;398(10300):583-598; 4. Del Prato S, et al. Lancet. 2021; 5. Dahl D, et al. Poster presented at: ADA 2021. Poster LB-20.
EMPEROR-Preserved and EMPEROR-Pooled
- Anker S et al. N Engl J Med 2021; 385:1451-1461. DOI: 10.1056/NEJMoa2107038; 2. Packer M et al. N Engl J Med 2021; 385:1531-1533DOI: 10.1056/NEJMc2112411.
AMPLITUDE-O
- Gerstein H et al. N Engl J Med 2021; 385:896-907. DOI: 10.1056/NEJMoa2108269.
STEP Program
- Wilding et al. N Engl J Med 2021; doi:10.1056/NEJMoa2032183; 2. Davies et al. Lancet, 2021; doi.org/10.1016/S0140-6736(21)00213-0: 3. Wadden et al. JAMA. doi:10.1001/jama.2021.1831; 4. Rubino et al. JAMA. 2021 Apr 13;325(14):1414-1425. doi: 10.1001/jama.2021.3224. 5. Garvey et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021; 6. Kadowaki et al. Presented at the International Congress on Metabolic Syndrome hybrid meeting .September 2-4, 2021; 7. Rubino et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021.
FIGARO-DKD and FIDELITY
- Pitt et al. N Engl J Med 2021; 385:2252-2263.DOI: 10.1056/NEJMoa2110956; 2. Agarwal et al. European Heart Journal 2021).https://doi.org/10.1093/eurheartj/ehab777.
SURPASS trials
- Rosenstock J, et al. Lancet. 2021;398(10295):143-155; 2. Frias JP, et al. N Eng J Med. 2021;385(6):503-515; 3. Ludvik B, et al. Lancet. 2021;398(10300):583-598; 4. Del Prato S, et al. Lancet. 2021; 5. Dahl D, et al. Poster presented at: ADA 2021. Poster LB-20.
EMPEROR-Preserved and EMPEROR-Pooled
- Anker S et al. N Engl J Med 2021; 385:1451-1461. DOI: 10.1056/NEJMoa2107038; 2. Packer M et al. N Engl J Med 2021; 385:1531-1533DOI: 10.1056/NEJMc2112411.
AMPLITUDE-O
- Gerstein H et al. N Engl J Med 2021; 385:896-907. DOI: 10.1056/NEJMoa2108269.
Exploring the relationship of COVID-19 vaccines and fertility
Introduction
Amidst an aggressive vaccination campaign for COVID-19, misinformation has spread over the Internet, affecting public perception and making some people hesitant to participate in ongoing immunization campaigns. Of chief concern are issues pertaining to fertility or viability of sperm – information circulating on social networks posits that the coronavirus vaccine may influence infertility in men, which, according to physicians, is not grounded in reality. From the perspective of evidence-based medicine, there is a dearth of information suggesting an untoward effect of the vaccine on male fertility. The risk of adverse reactions arising from approved vaccines is negligible, with mild, albeit controllable, side effects demonstrated by patients in clinical trials. Therefore, there is no plausible reason for the general public to avoid vaccinations.1
Infertility following vaccination
The source of confusion can be traced back to a study conducted by researchers at the University of Miami Miller School of Medicine; the general public has conflated a side effect of the virus, namely, infertility and erectile dysfunction, with that of the vaccine.2 According to Ranjith Ramasamy, MD, director of the urology program at Miller, “We were the first to demonstrate that the COVID virus, itself, can affect male fertility and be a potential cause for erectile dysfunction. We are now the first to examine if there is any impact of the COVID vaccine on male fertility potential, which we did not find.”3
Coronavirus can indeed cause significant damage to the testicular tissue of infected men by means of mediating ACE2 expression on Leydig and Sertoli cells of the testis. It should be noted that COVID-19 may potentially attack any type of cell in the body that expresses the enzyme ACE2. However, it is particularly harmful to cells with high levels of expression of this enzyme, such as testicular cells. The spermatogenesis process can be affected, thereby posing a risk to male fertility.4
Expanding on the theme of fertility during the pandemic, a number of false claims5-7 about the vaccine and its overall effect on the placenta and fertility have also emerged as a contentious topic for debate on social media; doctors continue to explain why the theories are not reasonable or a cause for concern. The World Health Organization (WHO) provides recommendations on COVID-19 vaccinations for pregnant and/or lactating women and encourages a shared decision process involving risk/benefit assessment with the prescribing physician.5 Pregnant women, especially those with underlying comorbid conditions, are susceptible to developing severe symptom manifestations of COVID-19 with the disease also being associated with an increased likelihood of premature birth. As far as lactating women are concerned, the evidence thus far has indicated that the risk of side effects of the vaccine is very low, suggesting that these women could be vaccinated.5
The vaccine is the best option
While more studies are needed to ascertain the relationship between COVID-19 and male infertility, the vaccine is currently the best option for those who are concerned about their fertility from exposure to the coronavirus. Because of delayed wholesale acceptance of vaccines by the general population, clinicians should continue to emphasize the importance of preventive care with respect to disease exposure.6
In addition, those who are concerned with fertility can opt for ways to preserve their reproductive capacity, such as the removal of semen for freezing sperm, albeit with adherence to sperm-washing procedures to preclude cross-contamination from viruses.8,9 For the preservation of sperm, the noninvasive method is often performed, preferably collected in several samples. Then, the semen is cryopreserved.8 In some instances, the sperm can also be removed directly from the testicles with a simple needle or by means of a minor surgical procedure.
A wait and try approach is advocated by clinicians for individuals who have already experienced COVID-19 symptoms and are therefore concerned about the prospect of childbearing.10 If the couple is unable to conceive after a year of trying, it is recommended that they consult a reproductive specialist; the clinician can carry out a comprehensive evaluation and order a series of tests to identify the source of the problem, indicating whether there are alternative methods for helping the couple to start a family (addressing the underlying factors involved in infertility, or treating via assisted reproduction procedures, such as in vitro fertilization).11
Dr. Aman is faculty member at the biology department of City Colleges of Chicago, and a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF). She disclosed no relevant financial relationships. Dr. Islam is a medical writer for the IMCHF, Montreal, is based in New York, and disclosed no relevant financial relationships. Mr. Choudhry is a research assistant at the IMCHF and he has no disclosures. Dr. Zia Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He has no disclosures.
References
1. Berry SD et al. J Am Geriatr Soc. 2021 May;69(5):1140-6.
2. Achua JK et al. World J Men’s Health. 2021 Jan;39(1):65-74.
3. Broderick JM. Urology Times. 2021 June.
4. Huang C et al. Andrology. 2021 Jan;9(1):80-7.
5. Sajjadi NB et al. J Osteopath Med. 2021 Apr 12;121(6):583-7.
6. Sallam M et al. Vaccines. 2021 Jan;9(1):42.
7. Islam MS et al. PloS One. 2021 May 12;16(5):e0251605.
8. Tesarik J. J Fertil Preserv. 2021;2:art246111.
9. Adiga SK et al. Reprod BioMed Online. 2020 Dec;41(6):991-7.
10. FAQs related to COVID-19. Q: If I get sick or test positive for COVID-19, when is it safe to become pregnant? American Society for Reproductive Medicine.
11. Cross C. Wellness and Prevention: Why can’t I get pregnant? John Hopkins Medicine.
Introduction
Amidst an aggressive vaccination campaign for COVID-19, misinformation has spread over the Internet, affecting public perception and making some people hesitant to participate in ongoing immunization campaigns. Of chief concern are issues pertaining to fertility or viability of sperm – information circulating on social networks posits that the coronavirus vaccine may influence infertility in men, which, according to physicians, is not grounded in reality. From the perspective of evidence-based medicine, there is a dearth of information suggesting an untoward effect of the vaccine on male fertility. The risk of adverse reactions arising from approved vaccines is negligible, with mild, albeit controllable, side effects demonstrated by patients in clinical trials. Therefore, there is no plausible reason for the general public to avoid vaccinations.1
Infertility following vaccination
The source of confusion can be traced back to a study conducted by researchers at the University of Miami Miller School of Medicine; the general public has conflated a side effect of the virus, namely, infertility and erectile dysfunction, with that of the vaccine.2 According to Ranjith Ramasamy, MD, director of the urology program at Miller, “We were the first to demonstrate that the COVID virus, itself, can affect male fertility and be a potential cause for erectile dysfunction. We are now the first to examine if there is any impact of the COVID vaccine on male fertility potential, which we did not find.”3
Coronavirus can indeed cause significant damage to the testicular tissue of infected men by means of mediating ACE2 expression on Leydig and Sertoli cells of the testis. It should be noted that COVID-19 may potentially attack any type of cell in the body that expresses the enzyme ACE2. However, it is particularly harmful to cells with high levels of expression of this enzyme, such as testicular cells. The spermatogenesis process can be affected, thereby posing a risk to male fertility.4
Expanding on the theme of fertility during the pandemic, a number of false claims5-7 about the vaccine and its overall effect on the placenta and fertility have also emerged as a contentious topic for debate on social media; doctors continue to explain why the theories are not reasonable or a cause for concern. The World Health Organization (WHO) provides recommendations on COVID-19 vaccinations for pregnant and/or lactating women and encourages a shared decision process involving risk/benefit assessment with the prescribing physician.5 Pregnant women, especially those with underlying comorbid conditions, are susceptible to developing severe symptom manifestations of COVID-19 with the disease also being associated with an increased likelihood of premature birth. As far as lactating women are concerned, the evidence thus far has indicated that the risk of side effects of the vaccine is very low, suggesting that these women could be vaccinated.5
The vaccine is the best option
While more studies are needed to ascertain the relationship between COVID-19 and male infertility, the vaccine is currently the best option for those who are concerned about their fertility from exposure to the coronavirus. Because of delayed wholesale acceptance of vaccines by the general population, clinicians should continue to emphasize the importance of preventive care with respect to disease exposure.6
In addition, those who are concerned with fertility can opt for ways to preserve their reproductive capacity, such as the removal of semen for freezing sperm, albeit with adherence to sperm-washing procedures to preclude cross-contamination from viruses.8,9 For the preservation of sperm, the noninvasive method is often performed, preferably collected in several samples. Then, the semen is cryopreserved.8 In some instances, the sperm can also be removed directly from the testicles with a simple needle or by means of a minor surgical procedure.
A wait and try approach is advocated by clinicians for individuals who have already experienced COVID-19 symptoms and are therefore concerned about the prospect of childbearing.10 If the couple is unable to conceive after a year of trying, it is recommended that they consult a reproductive specialist; the clinician can carry out a comprehensive evaluation and order a series of tests to identify the source of the problem, indicating whether there are alternative methods for helping the couple to start a family (addressing the underlying factors involved in infertility, or treating via assisted reproduction procedures, such as in vitro fertilization).11
Dr. Aman is faculty member at the biology department of City Colleges of Chicago, and a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF). She disclosed no relevant financial relationships. Dr. Islam is a medical writer for the IMCHF, Montreal, is based in New York, and disclosed no relevant financial relationships. Mr. Choudhry is a research assistant at the IMCHF and he has no disclosures. Dr. Zia Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He has no disclosures.
References
1. Berry SD et al. J Am Geriatr Soc. 2021 May;69(5):1140-6.
2. Achua JK et al. World J Men’s Health. 2021 Jan;39(1):65-74.
3. Broderick JM. Urology Times. 2021 June.
4. Huang C et al. Andrology. 2021 Jan;9(1):80-7.
5. Sajjadi NB et al. J Osteopath Med. 2021 Apr 12;121(6):583-7.
6. Sallam M et al. Vaccines. 2021 Jan;9(1):42.
7. Islam MS et al. PloS One. 2021 May 12;16(5):e0251605.
8. Tesarik J. J Fertil Preserv. 2021;2:art246111.
9. Adiga SK et al. Reprod BioMed Online. 2020 Dec;41(6):991-7.
10. FAQs related to COVID-19. Q: If I get sick or test positive for COVID-19, when is it safe to become pregnant? American Society for Reproductive Medicine.
11. Cross C. Wellness and Prevention: Why can’t I get pregnant? John Hopkins Medicine.
Introduction
Amidst an aggressive vaccination campaign for COVID-19, misinformation has spread over the Internet, affecting public perception and making some people hesitant to participate in ongoing immunization campaigns. Of chief concern are issues pertaining to fertility or viability of sperm – information circulating on social networks posits that the coronavirus vaccine may influence infertility in men, which, according to physicians, is not grounded in reality. From the perspective of evidence-based medicine, there is a dearth of information suggesting an untoward effect of the vaccine on male fertility. The risk of adverse reactions arising from approved vaccines is negligible, with mild, albeit controllable, side effects demonstrated by patients in clinical trials. Therefore, there is no plausible reason for the general public to avoid vaccinations.1
Infertility following vaccination
The source of confusion can be traced back to a study conducted by researchers at the University of Miami Miller School of Medicine; the general public has conflated a side effect of the virus, namely, infertility and erectile dysfunction, with that of the vaccine.2 According to Ranjith Ramasamy, MD, director of the urology program at Miller, “We were the first to demonstrate that the COVID virus, itself, can affect male fertility and be a potential cause for erectile dysfunction. We are now the first to examine if there is any impact of the COVID vaccine on male fertility potential, which we did not find.”3
Coronavirus can indeed cause significant damage to the testicular tissue of infected men by means of mediating ACE2 expression on Leydig and Sertoli cells of the testis. It should be noted that COVID-19 may potentially attack any type of cell in the body that expresses the enzyme ACE2. However, it is particularly harmful to cells with high levels of expression of this enzyme, such as testicular cells. The spermatogenesis process can be affected, thereby posing a risk to male fertility.4
Expanding on the theme of fertility during the pandemic, a number of false claims5-7 about the vaccine and its overall effect on the placenta and fertility have also emerged as a contentious topic for debate on social media; doctors continue to explain why the theories are not reasonable or a cause for concern. The World Health Organization (WHO) provides recommendations on COVID-19 vaccinations for pregnant and/or lactating women and encourages a shared decision process involving risk/benefit assessment with the prescribing physician.5 Pregnant women, especially those with underlying comorbid conditions, are susceptible to developing severe symptom manifestations of COVID-19 with the disease also being associated with an increased likelihood of premature birth. As far as lactating women are concerned, the evidence thus far has indicated that the risk of side effects of the vaccine is very low, suggesting that these women could be vaccinated.5
The vaccine is the best option
While more studies are needed to ascertain the relationship between COVID-19 and male infertility, the vaccine is currently the best option for those who are concerned about their fertility from exposure to the coronavirus. Because of delayed wholesale acceptance of vaccines by the general population, clinicians should continue to emphasize the importance of preventive care with respect to disease exposure.6
In addition, those who are concerned with fertility can opt for ways to preserve their reproductive capacity, such as the removal of semen for freezing sperm, albeit with adherence to sperm-washing procedures to preclude cross-contamination from viruses.8,9 For the preservation of sperm, the noninvasive method is often performed, preferably collected in several samples. Then, the semen is cryopreserved.8 In some instances, the sperm can also be removed directly from the testicles with a simple needle or by means of a minor surgical procedure.
A wait and try approach is advocated by clinicians for individuals who have already experienced COVID-19 symptoms and are therefore concerned about the prospect of childbearing.10 If the couple is unable to conceive after a year of trying, it is recommended that they consult a reproductive specialist; the clinician can carry out a comprehensive evaluation and order a series of tests to identify the source of the problem, indicating whether there are alternative methods for helping the couple to start a family (addressing the underlying factors involved in infertility, or treating via assisted reproduction procedures, such as in vitro fertilization).11
Dr. Aman is faculty member at the biology department of City Colleges of Chicago, and a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF). She disclosed no relevant financial relationships. Dr. Islam is a medical writer for the IMCHF, Montreal, is based in New York, and disclosed no relevant financial relationships. Mr. Choudhry is a research assistant at the IMCHF and he has no disclosures. Dr. Zia Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He has no disclosures.
References
1. Berry SD et al. J Am Geriatr Soc. 2021 May;69(5):1140-6.
2. Achua JK et al. World J Men’s Health. 2021 Jan;39(1):65-74.
3. Broderick JM. Urology Times. 2021 June.
4. Huang C et al. Andrology. 2021 Jan;9(1):80-7.
5. Sajjadi NB et al. J Osteopath Med. 2021 Apr 12;121(6):583-7.
6. Sallam M et al. Vaccines. 2021 Jan;9(1):42.
7. Islam MS et al. PloS One. 2021 May 12;16(5):e0251605.
8. Tesarik J. J Fertil Preserv. 2021;2:art246111.
9. Adiga SK et al. Reprod BioMed Online. 2020 Dec;41(6):991-7.
10. FAQs related to COVID-19. Q: If I get sick or test positive for COVID-19, when is it safe to become pregnant? American Society for Reproductive Medicine.
11. Cross C. Wellness and Prevention: Why can’t I get pregnant? John Hopkins Medicine.
Assisting Surgeons with Management: Initial Presentation of Abnormal Bleeding and Diagnosing of Fibroids
As an Advanced Practice Provider, when and why might a patient with uterine fibroids be scheduled to visit with you?
Ms. Haibach: Typically, with the flow of how our practice runs, a patient would schedule with me as an initial visit to explore their abnormal or heavy bleeding. Oftentimes, a patient is unsure with what they have going on medically and will view APPs as a safe place to start. Other times, I will see a patient for a general wellness exam who will mention heavier menses over the years or just a change in their bleeding pattern-- longer flow, things like that.
It may stem from something that seems out of the ordinary for them or a symptom impacting their life. For example, if a patient says, “I have to run home and change my clothes,” or “I'm bleeding through my bed sheets.” Those statements prompt further evaluation. At times, patients who have already been diagnosed with fibroids, will come to see me if they have chosen medical management over surgical management of their fibroids. They continue to follow up with me to reevaluate the success of their treatment plan periodically. So, whether I start them on a plan, or a physician does, they can follow up with me to revisit their medical plan and ensure it remains appropriate.
You touched on this a bit, but can you dive deeper into exactly what you are looking for as part of that visit?
Ms. Haibach: Definitely. With an initial consult to me, the number one question that I would ask my patients first is, what is your most bothersome symptom? With this question, I'm looking to determine: is it pain that brought you to me? Is it heavy bleeding? Do you feel bulk and bloaty? Are you having issues getting pregnant? Do you have bowel or bladder issues?
The information I get from that one initial question, helps guide the remainder of my visit. If bleeding is the main concern, we would focus on getting that under control. So, we need to suppress the menses with medication options. If bulk and bloating is the main concern, for instance the patient feels like they have a pregnant-looking abdomen, typically surgical options are warranted. If the main complaint is infertility, we do have fertility specialists in our practice who remove fibroids to aid patients in achieving pregnancy.
The most important purpose of this visit is to really listen to the patient to find out how these symptoms are impacting their daily lives. From there, I can use that information to guide my treatment plan.
So, once it is determined that the patient is a good surgical candidate, what would be the next steps?
Ms. Haibach: If at the end of my visit, I determine that a patient is potentially a suitable surgical candidate, the first thing I would do is order appropriate imaging. For example, if the patient is interested in uterine preservation for future fertility, she is likely going to opt for a laparoscopic myomectomy, where fibroids would be removed, and her uterus would be left in place. In that case, she would require an MRI for fibroid mapping. If a woman has completed childbearing, then oftentimes a pelvic ultrasound would suffice, at least to start, since she'd likely elect hysterectomy if she has reached her fertility goals.
I would also perform an endometrial biopsy to rule out malignant process before going into surgery. To optimize a patient for our MIGS surgeons, I gather a thorough medical history to ensure their comorbidities are appropriately managed. For example, diabetes is under control, sleep apnea is being treated, no active infections. If there is anything else going on that needs to be addressed, I'd refer them to the appropriate provider first.
Once I have acceptable imaging, a negative endometrial biopsy and an adequate medical history, I would then assist the patient in scheduling with one of the surgeons on my team for a consult and physical exam to determine surgery planning. Once they see our physician, a surgery date is booked. The patient would come back to see me within 30 days of surgery, and we would do a preoperative education appointment. I see them again 2 weeks after surgery for a post-op visit. We’d perform the post-of visit virtually in our practice. We would see the patient sooner if there are any other concerns that arise post-operatively.
What if the patient is not a surgical candidate? How do you as an APP assist in ongoing medical management?
Ms. Haibach: The presence of fibroids alone, without symptoms, often does not require surgical intervention. There are occasions where a patient is, for example, seen in the emergency room for abdominal pain, whereas they’ll get a CT scan of the abdomen pelvis, and a fibroid is incidentally found. At that point, they are instructed to see gynecology for follow-up. If the patient was unaware of the fibroid, has no symptoms and there's no concerning imaging features, then management with ongoing surveillance (repeat imaging and office follow up) and instructions on when to return is usually appropriate.
Depending on the symptoms, medical management typically includes hormonal suppression of menses in the form of a birth control pill or an IUD. If bleeding is the main concern, it is my goal to at least slow her bleeding, if not try to stop it. Not all women are good candidates for hormone therapy, so there is a medication option that is non-hormonal. In my role, I would start a medication plan for a patient and initiate a new medication such as hormonal suppression in the form of birth control, IUD, non-hormonal medications etc.
Typically, when I do that, I'll have the patient follow up with me in about two to three months to reassess the medication’s effectiveness. The goal of the reassessment is to determine if it is working for her life, to be sure there are no major side-effects, and just to make sure she is amenable to the plan. As part of the medical management, sometimes it is necessary to monitor blood counts for anemia to be certain that medical management is still appropriate for her.
From your experience in practicing, are you more likely to be visited by one age bracket or ethnicity over another?
Ms. Haibach: Actually, data tells us that most fibroids occur in women of reproductive age. They are also diagnosed in African American women two to three times more frequently than in white women. Fibroids are infrequently seen in premenstrual women. A relief of symptoms of the fibroids often occurs at the time of menopause, when the menstrual cyclicity seizes and steroid hormone levels decrease. My demographic is consistent with the above statistics. I tend to see women within the ages of 20’s-50’s and more often African Americans.
Was there anything else that you'd like to mention?
Ms. Haibach: Abnormal bleeding can be very stressful for women. APPs are a great place to start an abnormal bleeding or fibroid work-up. Patients should rest assure that although we cannot perform surgery, APPs can help get them in the right direction for the best care possible.
US Department of Health and Human Services, Office on Women’s Health. Uterine fibroids. (https://www.womenshealth.gov/a-z-topics/uterine-fibroids) Accessed 1/26/2022.
The American College of Obstetricians and Gynecologists. Uterine Fibroids. (https://www.acog.org/patient-resources/faqs/gynecologic-problems/uterine-fibroids) Accessed 1/26/2022.
As an Advanced Practice Provider, when and why might a patient with uterine fibroids be scheduled to visit with you?
Ms. Haibach: Typically, with the flow of how our practice runs, a patient would schedule with me as an initial visit to explore their abnormal or heavy bleeding. Oftentimes, a patient is unsure with what they have going on medically and will view APPs as a safe place to start. Other times, I will see a patient for a general wellness exam who will mention heavier menses over the years or just a change in their bleeding pattern-- longer flow, things like that.
It may stem from something that seems out of the ordinary for them or a symptom impacting their life. For example, if a patient says, “I have to run home and change my clothes,” or “I'm bleeding through my bed sheets.” Those statements prompt further evaluation. At times, patients who have already been diagnosed with fibroids, will come to see me if they have chosen medical management over surgical management of their fibroids. They continue to follow up with me to reevaluate the success of their treatment plan periodically. So, whether I start them on a plan, or a physician does, they can follow up with me to revisit their medical plan and ensure it remains appropriate.
You touched on this a bit, but can you dive deeper into exactly what you are looking for as part of that visit?
Ms. Haibach: Definitely. With an initial consult to me, the number one question that I would ask my patients first is, what is your most bothersome symptom? With this question, I'm looking to determine: is it pain that brought you to me? Is it heavy bleeding? Do you feel bulk and bloaty? Are you having issues getting pregnant? Do you have bowel or bladder issues?
The information I get from that one initial question, helps guide the remainder of my visit. If bleeding is the main concern, we would focus on getting that under control. So, we need to suppress the menses with medication options. If bulk and bloating is the main concern, for instance the patient feels like they have a pregnant-looking abdomen, typically surgical options are warranted. If the main complaint is infertility, we do have fertility specialists in our practice who remove fibroids to aid patients in achieving pregnancy.
The most important purpose of this visit is to really listen to the patient to find out how these symptoms are impacting their daily lives. From there, I can use that information to guide my treatment plan.
So, once it is determined that the patient is a good surgical candidate, what would be the next steps?
Ms. Haibach: If at the end of my visit, I determine that a patient is potentially a suitable surgical candidate, the first thing I would do is order appropriate imaging. For example, if the patient is interested in uterine preservation for future fertility, she is likely going to opt for a laparoscopic myomectomy, where fibroids would be removed, and her uterus would be left in place. In that case, she would require an MRI for fibroid mapping. If a woman has completed childbearing, then oftentimes a pelvic ultrasound would suffice, at least to start, since she'd likely elect hysterectomy if she has reached her fertility goals.
I would also perform an endometrial biopsy to rule out malignant process before going into surgery. To optimize a patient for our MIGS surgeons, I gather a thorough medical history to ensure their comorbidities are appropriately managed. For example, diabetes is under control, sleep apnea is being treated, no active infections. If there is anything else going on that needs to be addressed, I'd refer them to the appropriate provider first.
Once I have acceptable imaging, a negative endometrial biopsy and an adequate medical history, I would then assist the patient in scheduling with one of the surgeons on my team for a consult and physical exam to determine surgery planning. Once they see our physician, a surgery date is booked. The patient would come back to see me within 30 days of surgery, and we would do a preoperative education appointment. I see them again 2 weeks after surgery for a post-op visit. We’d perform the post-of visit virtually in our practice. We would see the patient sooner if there are any other concerns that arise post-operatively.
What if the patient is not a surgical candidate? How do you as an APP assist in ongoing medical management?
Ms. Haibach: The presence of fibroids alone, without symptoms, often does not require surgical intervention. There are occasions where a patient is, for example, seen in the emergency room for abdominal pain, whereas they’ll get a CT scan of the abdomen pelvis, and a fibroid is incidentally found. At that point, they are instructed to see gynecology for follow-up. If the patient was unaware of the fibroid, has no symptoms and there's no concerning imaging features, then management with ongoing surveillance (repeat imaging and office follow up) and instructions on when to return is usually appropriate.
Depending on the symptoms, medical management typically includes hormonal suppression of menses in the form of a birth control pill or an IUD. If bleeding is the main concern, it is my goal to at least slow her bleeding, if not try to stop it. Not all women are good candidates for hormone therapy, so there is a medication option that is non-hormonal. In my role, I would start a medication plan for a patient and initiate a new medication such as hormonal suppression in the form of birth control, IUD, non-hormonal medications etc.
Typically, when I do that, I'll have the patient follow up with me in about two to three months to reassess the medication’s effectiveness. The goal of the reassessment is to determine if it is working for her life, to be sure there are no major side-effects, and just to make sure she is amenable to the plan. As part of the medical management, sometimes it is necessary to monitor blood counts for anemia to be certain that medical management is still appropriate for her.
From your experience in practicing, are you more likely to be visited by one age bracket or ethnicity over another?
Ms. Haibach: Actually, data tells us that most fibroids occur in women of reproductive age. They are also diagnosed in African American women two to three times more frequently than in white women. Fibroids are infrequently seen in premenstrual women. A relief of symptoms of the fibroids often occurs at the time of menopause, when the menstrual cyclicity seizes and steroid hormone levels decrease. My demographic is consistent with the above statistics. I tend to see women within the ages of 20’s-50’s and more often African Americans.
Was there anything else that you'd like to mention?
Ms. Haibach: Abnormal bleeding can be very stressful for women. APPs are a great place to start an abnormal bleeding or fibroid work-up. Patients should rest assure that although we cannot perform surgery, APPs can help get them in the right direction for the best care possible.
As an Advanced Practice Provider, when and why might a patient with uterine fibroids be scheduled to visit with you?
Ms. Haibach: Typically, with the flow of how our practice runs, a patient would schedule with me as an initial visit to explore their abnormal or heavy bleeding. Oftentimes, a patient is unsure with what they have going on medically and will view APPs as a safe place to start. Other times, I will see a patient for a general wellness exam who will mention heavier menses over the years or just a change in their bleeding pattern-- longer flow, things like that.
It may stem from something that seems out of the ordinary for them or a symptom impacting their life. For example, if a patient says, “I have to run home and change my clothes,” or “I'm bleeding through my bed sheets.” Those statements prompt further evaluation. At times, patients who have already been diagnosed with fibroids, will come to see me if they have chosen medical management over surgical management of their fibroids. They continue to follow up with me to reevaluate the success of their treatment plan periodically. So, whether I start them on a plan, or a physician does, they can follow up with me to revisit their medical plan and ensure it remains appropriate.
You touched on this a bit, but can you dive deeper into exactly what you are looking for as part of that visit?
Ms. Haibach: Definitely. With an initial consult to me, the number one question that I would ask my patients first is, what is your most bothersome symptom? With this question, I'm looking to determine: is it pain that brought you to me? Is it heavy bleeding? Do you feel bulk and bloaty? Are you having issues getting pregnant? Do you have bowel or bladder issues?
The information I get from that one initial question, helps guide the remainder of my visit. If bleeding is the main concern, we would focus on getting that under control. So, we need to suppress the menses with medication options. If bulk and bloating is the main concern, for instance the patient feels like they have a pregnant-looking abdomen, typically surgical options are warranted. If the main complaint is infertility, we do have fertility specialists in our practice who remove fibroids to aid patients in achieving pregnancy.
The most important purpose of this visit is to really listen to the patient to find out how these symptoms are impacting their daily lives. From there, I can use that information to guide my treatment plan.
So, once it is determined that the patient is a good surgical candidate, what would be the next steps?
Ms. Haibach: If at the end of my visit, I determine that a patient is potentially a suitable surgical candidate, the first thing I would do is order appropriate imaging. For example, if the patient is interested in uterine preservation for future fertility, she is likely going to opt for a laparoscopic myomectomy, where fibroids would be removed, and her uterus would be left in place. In that case, she would require an MRI for fibroid mapping. If a woman has completed childbearing, then oftentimes a pelvic ultrasound would suffice, at least to start, since she'd likely elect hysterectomy if she has reached her fertility goals.
I would also perform an endometrial biopsy to rule out malignant process before going into surgery. To optimize a patient for our MIGS surgeons, I gather a thorough medical history to ensure their comorbidities are appropriately managed. For example, diabetes is under control, sleep apnea is being treated, no active infections. If there is anything else going on that needs to be addressed, I'd refer them to the appropriate provider first.
Once I have acceptable imaging, a negative endometrial biopsy and an adequate medical history, I would then assist the patient in scheduling with one of the surgeons on my team for a consult and physical exam to determine surgery planning. Once they see our physician, a surgery date is booked. The patient would come back to see me within 30 days of surgery, and we would do a preoperative education appointment. I see them again 2 weeks after surgery for a post-op visit. We’d perform the post-of visit virtually in our practice. We would see the patient sooner if there are any other concerns that arise post-operatively.
What if the patient is not a surgical candidate? How do you as an APP assist in ongoing medical management?
Ms. Haibach: The presence of fibroids alone, without symptoms, often does not require surgical intervention. There are occasions where a patient is, for example, seen in the emergency room for abdominal pain, whereas they’ll get a CT scan of the abdomen pelvis, and a fibroid is incidentally found. At that point, they are instructed to see gynecology for follow-up. If the patient was unaware of the fibroid, has no symptoms and there's no concerning imaging features, then management with ongoing surveillance (repeat imaging and office follow up) and instructions on when to return is usually appropriate.
Depending on the symptoms, medical management typically includes hormonal suppression of menses in the form of a birth control pill or an IUD. If bleeding is the main concern, it is my goal to at least slow her bleeding, if not try to stop it. Not all women are good candidates for hormone therapy, so there is a medication option that is non-hormonal. In my role, I would start a medication plan for a patient and initiate a new medication such as hormonal suppression in the form of birth control, IUD, non-hormonal medications etc.
Typically, when I do that, I'll have the patient follow up with me in about two to three months to reassess the medication’s effectiveness. The goal of the reassessment is to determine if it is working for her life, to be sure there are no major side-effects, and just to make sure she is amenable to the plan. As part of the medical management, sometimes it is necessary to monitor blood counts for anemia to be certain that medical management is still appropriate for her.
From your experience in practicing, are you more likely to be visited by one age bracket or ethnicity over another?
Ms. Haibach: Actually, data tells us that most fibroids occur in women of reproductive age. They are also diagnosed in African American women two to three times more frequently than in white women. Fibroids are infrequently seen in premenstrual women. A relief of symptoms of the fibroids often occurs at the time of menopause, when the menstrual cyclicity seizes and steroid hormone levels decrease. My demographic is consistent with the above statistics. I tend to see women within the ages of 20’s-50’s and more often African Americans.
Was there anything else that you'd like to mention?
Ms. Haibach: Abnormal bleeding can be very stressful for women. APPs are a great place to start an abnormal bleeding or fibroid work-up. Patients should rest assure that although we cannot perform surgery, APPs can help get them in the right direction for the best care possible.
US Department of Health and Human Services, Office on Women’s Health. Uterine fibroids. (https://www.womenshealth.gov/a-z-topics/uterine-fibroids) Accessed 1/26/2022.
The American College of Obstetricians and Gynecologists. Uterine Fibroids. (https://www.acog.org/patient-resources/faqs/gynecologic-problems/uterine-fibroids) Accessed 1/26/2022.
US Department of Health and Human Services, Office on Women’s Health. Uterine fibroids. (https://www.womenshealth.gov/a-z-topics/uterine-fibroids) Accessed 1/26/2022.
The American College of Obstetricians and Gynecologists. Uterine Fibroids. (https://www.acog.org/patient-resources/faqs/gynecologic-problems/uterine-fibroids) Accessed 1/26/2022.
Multifactorial Effects of Endometriosis as a Chronic Systemic Disease
Can you talk about your research thus far and what your overall lab work has shown regarding endometriosis as a chronic systemic disease?
Dr. Flores: Endometriosis has traditionally been characterized by its pelvic manifestation however, it is important to understand that it is profoundly more than a pelvic disease—it is a chronic, systemic disease with multifactorial effects throughout the body.
We and other groups have found increased expression of several inflammatory cytokines in women with endometriosis. Our lab has found that compared to women without endometriosis, women with endometriosis not only have certain inflammatory cytokines elevated but also have altered expression of microRNAs. MicroRNAs are small noncoding RNAs that bind to and modulate translation of mRNA. To help determine whether these miRNAs were involved in mediating increased expression of inflammatory cytokines in women with endometriosis, we then transfected these miRNAs into a macrophage cell line, and again found altered inflammatory cytokine expression. We and others have also found a role for stem cells (from bone marrow and other sources) in the pathogenesis of endometriosis. In addition, we have found that in endometriosis, women have a low body-mass index and altered metabolism, which is related to induction of induction of hepatic (anorexigenic) gene expression and microRNA-mediated changes in adipocyte (metabolic) gene expression. Furthermore, we have found altered gene expression in regions of the brain associated with anxiety and depression and altered pain sensitization. Taken together, this work helps provide support for the systemic nature of endometriosis.
How can your findings in this space help us in diagnosing clinically and ultimately avoid diagnostic delay?
Dr. Flores: It’s about understanding that endometriosis is not just a pelvic disease and understanding that endometriosis is leading to inflammation and altered expression of miRNAs which allows endometriosis to have long-range effects. For example, women with endometriosis commonly have anxiety and depression and low BMI. As mentioned earlier, we have found that in a murine model of endometriosis, there is altered gene expression in regions of the brain associated with anxiety and depression and altered metabolism in a murine model of endometriosis. Other groups have also found changes in brain volume in these same areas in women with endometriosis, and we have seen low BMI in women with endometriosis. In fact, a common misconception was that being thin was a risk factor for endometriosis, however we have found that the endometriosis itself, is causing women alteration in genes associated with metabolism.
With respect to the endometrium, in addition to being a pelvic pain disorder, we also see that women with endometriosis have a higher likelihood of having infertility. And we think that's in part because one, just like the lesions can be resistant to progesterone, the endometrium of these women can also be resistant to progesterone. Progesterone is necessary for decidualization/implantation. We have also seen that stem cells can be recruited and ultimately incorrectly incorporated into the endometrium, which may also contribute to infertility in women with endometriosis.
If we can understand this multifactorial nature of endometriosis, I think this will help us not only shift toward diagnosing endometriosis clinically, but also avoid diagnostic delay. If we can understand that endometriosis is not just a pelvic disorder, but that It can also involve altered mood, bowel/bladder symptoms, inflammation, altered metabolism and/or cause infertility, I think that will ultimately help us to diagnosing earlier.
In addition, we can also utilize pelvic pain symptomatology to help with diagnosis as well. We can ask about cyclic pelvic pain that's been getting progressively worse over the years, not responding to non-steroidal anti-inflammatory medications. Also, in understanding that endometriosis can affect other organs, asking about cyclic pain/symptoms in other areas, such as cyclical bowel or bladder symptoms.
Thinking about the fact that if you do have a patient like that, you're seeing that they have altered mood symptoms, or alterations in inflammatory markers. Maybe that will help us shift from a disease that was typically only considered to be diagnosed by surgery, by switching to a clinical diagnosis for endometriosis. Doing that will hopefully help avoid diagnostic delay.
If we understand that while we typically describe endometriosis as causing cyclic pain symptoms, sometimes because of the existing diagnostic delay, ultimately women can present with chronic pelvic pain. Thus, it's also important to ask patients presenting with chronic pelvic pain what the symptoms were like beforehand (i.e., was the pain cyclic and progressively worsening over the years/before it became chronic) doing so will also help in terms of diagnosing sooner.
Lastly, circulating miRNAs have been considered promising biomarker candidates because they are stable in circulation and have highly specific expression profiles. We have found that the combination of several miRNAs reliably distinguished endometriosis patients from controls, and a prospective, blinded study showed that the combination of several miRNAs could be used to accurately identify patients with endometriosis, with an area under the receiver operating characteristic curve of 0.93.
Roughly 11%, or more than 6.5 million, women in the United States between the ages of 15–44 years, may have endometriosis. Is this disease more common in any particular age range or ethnicity?
Dr. Flores: We’re actually actively investigating that right now. And I think what makes it challenging, especially with respect to the age range, is now we're -- I think in part because of so much more awareness and more research is being done looking at this disease as a chronic systemic disease-- we're now starting to see/diagnose adolescents with endometriosis.
I think as we start gathering more information about these individuals, we'll be able to better say if there is a particular age range. Right now, we usually say it's in the reproductive years, however for some women it may be later if they were not diagnosed earlier. Conversely, some who are hopefully reading this, and also who conduct research on endometriosis, may be able to diagnose someone earlier that may have been missed until they were in their 30s or 40s, for example.
With respect to ethnicity, I'm the task force leader for diversity, equity, and inclusion in research and recruitment. This is something that I'm actively starting to work on, as are other groups. I don't have the answer for that yet, but as we continue to collect more data, we will have more information on this.
What are some of the existing hormonal therapies you rely upon as well as the biomarkers in predicting response to treatment, and are there any new research or treatments on the horizon?
Dr. Flores: I'll first start by telling you a bit about our existing treatment regimens, and then how I decide who would benefit from a given one. First line has always been progestin-based therapy, either in the form of a combined oral contraceptive pill or as progesterone only pills. However, up to 1/3 of women fail progestin-based therapy—this is termed progesterone resistance.
When progestin-based therapies fail, we then rely on other agents that are focused more on estrogen deprivation because, while we don't know the complete etiology of endometriosis, we do know that it is estrogen-dependent. There are two classes— gonadotropin releasing hormone (GnRH) agonists and GnRH antagonists. The agonist binds to the GnRH receptors, and initially can cause a flare effect due to its agonist properties, initially stimulate release of estradiol, and ultimately the GnRH receptor becomes downregulated and estradiol is decreased to the menopausal range. As a result we routinely provided add-back therapy with norethindrone to help prevent hot flashes and ensure bone protection.
Within the past three years, there has been a new oral GnRH receptor antagonist approved for treating endometriosis. The medication is available as a once a day or twice a day dosing regimen. As this is a GnRH antagonist, upon binding to the GnRH receptor, it blocks receptor activity, thus avoiding the flare affect; essentially, within 24 hours, there is a decrease in estradiol production.
As two doses are available, you can tailor how much you dial down estrogen for a given patient. The low dose lowers estradiol to a range of 40 picograms while the high (twice a day) dosing lowers your estrogen to about 6 picograms. Also, although it was not studied originally in terms of giving add-back therapy for the higher dose, given the safety (and effectiveness) of add-back therapy with GnRH agonists we are using the same norethindrone add-back therapy for women who are taking the GnRH receptor antagonist.
The next question is, how do we decide which medication a given patient receives? To answer that, I will tell you a bit about my precision-based medicine research. As mentioned before, while progestin-based therapy is first-line, failure rates are high, and unfortunately, we previously have not been able to identify who will or will not respond to first-line therapy. As such, I decided to assess progesterone receptor expression in endometriotic lesions from women who had undergone surgery for endometriosis, and determine whether progesterone receptor expression levels in lesions could be used to predict response to progestin-based therapy. I found that in women that had high levels of the progesterone receptor, they responded completely to progestin-based therapy-- there was a 100% response rate to progestin-based therapy. This is in sharp contrast to women who had low PR expression, where there was only a 6% response rate to progestin-based therapy.
While this is great with respect to being able to predict who will or will not respond to first line therapy, the one limitation is that would mean that women have to undergo surgery in order to determine progesterone receptor status/response to progestin-based therapy. However, given that within two to five years following surgery, up to 50% of women will have recurrence of pain symptoms, where I see my test coming into play is postoperatively. This is because many times , women who had pain, or who were failing a given agent, are placed back on that same medical therapy they were failing after surgery. Usually that was a progestin. Therefore, instead of putting them on that same therapy that they were failing, we can use my test to place them on an alternative therapy (such as a GnRH analogue) that more specifically targets estradiol production.
In terms of future directions with respect to treatment, there is a microRNA that has been found to be low in women with endometriosis—miRNALet-7b. In a murine model of endometriosis, we have found that if we supplement with Let-7, there is decreased inflammation and decreased lesion size of endometriosis. We have also found that supplementing miRNA Let-7b in human endometriotic lesions results in decreased inflammation in cell culture.
That would be future directions in terms of focusing on microRNAs and seeing how we can manipulate those to essentially block inflammation and lesion growth. Furthermore, such treatment would be non-hormonal, which would be a novel therapeutic approach.
As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006-1014.
Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296-1301
Cosar E, Mamillapalli R, Ersoy GS, Cho S, Seifer B, Taylor HS. Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array-based analysis. Fertil Steril. 2016;106(2):402-409.
Flores VA, Vanhie A, Dang T, Taylor HS. Progesterone Receptor Status Predicts Response to Progestin Therapy in Endometriosis. J Clin Endocrinol Metab. 2018 Dec 1;103(12):4561-4568
Goetz TG, Mamillapalli R, Taylor HS. Low Body Mass Index in Endometriosis Is Promoted by Hepatic Metabolic Gene Dysregulation in Mice. Biol Reprod. 2016;95(6):115.
Li T, et al. Endometriosis alters brain electrophysiology, gene expression and increases pain sensitization, anxiety, and depression in female mice. Biol Reprod. 2018;99(2):349-359.
Moustafa S, Burn M, Mamillapalli R, Nematian S, Flores V, Taylor HS. Accurate diagnosis of endometriosis using serum microRNAs. Am J Obstet Gynecol. 2020;223(4):557.e1-557.e11.
Nematian SE, et al. Systemic Inflammation Induced by microRNAs: Endometriosis-Derived Alterations in Circulating microRNA 125b-5p and Let-7b-5p Regulate Macrophage Cytokine Production. J Clin Endocrinol Metab. 2018;103(1):64-74.
Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
Rogers PA, D'Hooghe TM, Fazleabas A, et al. Defining future directions for endometriosis research: workshop report from the 2011 World Congress of Endometriosis In Montpellier, France. Reprod Sci. 2013;20(5):483-499.
Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021 Feb 27
Zolbin MM, et al. Adipocyte alterations in endometriosis: reduced numbers of stem cells and microRNA induced alterations in adipocyte metabolic gene expression. Reprod Biol Endocrinol. 2019;17(1):36.
Can you talk about your research thus far and what your overall lab work has shown regarding endometriosis as a chronic systemic disease?
Dr. Flores: Endometriosis has traditionally been characterized by its pelvic manifestation however, it is important to understand that it is profoundly more than a pelvic disease—it is a chronic, systemic disease with multifactorial effects throughout the body.
We and other groups have found increased expression of several inflammatory cytokines in women with endometriosis. Our lab has found that compared to women without endometriosis, women with endometriosis not only have certain inflammatory cytokines elevated but also have altered expression of microRNAs. MicroRNAs are small noncoding RNAs that bind to and modulate translation of mRNA. To help determine whether these miRNAs were involved in mediating increased expression of inflammatory cytokines in women with endometriosis, we then transfected these miRNAs into a macrophage cell line, and again found altered inflammatory cytokine expression. We and others have also found a role for stem cells (from bone marrow and other sources) in the pathogenesis of endometriosis. In addition, we have found that in endometriosis, women have a low body-mass index and altered metabolism, which is related to induction of induction of hepatic (anorexigenic) gene expression and microRNA-mediated changes in adipocyte (metabolic) gene expression. Furthermore, we have found altered gene expression in regions of the brain associated with anxiety and depression and altered pain sensitization. Taken together, this work helps provide support for the systemic nature of endometriosis.
How can your findings in this space help us in diagnosing clinically and ultimately avoid diagnostic delay?
Dr. Flores: It’s about understanding that endometriosis is not just a pelvic disease and understanding that endometriosis is leading to inflammation and altered expression of miRNAs which allows endometriosis to have long-range effects. For example, women with endometriosis commonly have anxiety and depression and low BMI. As mentioned earlier, we have found that in a murine model of endometriosis, there is altered gene expression in regions of the brain associated with anxiety and depression and altered metabolism in a murine model of endometriosis. Other groups have also found changes in brain volume in these same areas in women with endometriosis, and we have seen low BMI in women with endometriosis. In fact, a common misconception was that being thin was a risk factor for endometriosis, however we have found that the endometriosis itself, is causing women alteration in genes associated with metabolism.
With respect to the endometrium, in addition to being a pelvic pain disorder, we also see that women with endometriosis have a higher likelihood of having infertility. And we think that's in part because one, just like the lesions can be resistant to progesterone, the endometrium of these women can also be resistant to progesterone. Progesterone is necessary for decidualization/implantation. We have also seen that stem cells can be recruited and ultimately incorrectly incorporated into the endometrium, which may also contribute to infertility in women with endometriosis.
If we can understand this multifactorial nature of endometriosis, I think this will help us not only shift toward diagnosing endometriosis clinically, but also avoid diagnostic delay. If we can understand that endometriosis is not just a pelvic disorder, but that It can also involve altered mood, bowel/bladder symptoms, inflammation, altered metabolism and/or cause infertility, I think that will ultimately help us to diagnosing earlier.
In addition, we can also utilize pelvic pain symptomatology to help with diagnosis as well. We can ask about cyclic pelvic pain that's been getting progressively worse over the years, not responding to non-steroidal anti-inflammatory medications. Also, in understanding that endometriosis can affect other organs, asking about cyclic pain/symptoms in other areas, such as cyclical bowel or bladder symptoms.
Thinking about the fact that if you do have a patient like that, you're seeing that they have altered mood symptoms, or alterations in inflammatory markers. Maybe that will help us shift from a disease that was typically only considered to be diagnosed by surgery, by switching to a clinical diagnosis for endometriosis. Doing that will hopefully help avoid diagnostic delay.
If we understand that while we typically describe endometriosis as causing cyclic pain symptoms, sometimes because of the existing diagnostic delay, ultimately women can present with chronic pelvic pain. Thus, it's also important to ask patients presenting with chronic pelvic pain what the symptoms were like beforehand (i.e., was the pain cyclic and progressively worsening over the years/before it became chronic) doing so will also help in terms of diagnosing sooner.
Lastly, circulating miRNAs have been considered promising biomarker candidates because they are stable in circulation and have highly specific expression profiles. We have found that the combination of several miRNAs reliably distinguished endometriosis patients from controls, and a prospective, blinded study showed that the combination of several miRNAs could be used to accurately identify patients with endometriosis, with an area under the receiver operating characteristic curve of 0.93.
Roughly 11%, or more than 6.5 million, women in the United States between the ages of 15–44 years, may have endometriosis. Is this disease more common in any particular age range or ethnicity?
Dr. Flores: We’re actually actively investigating that right now. And I think what makes it challenging, especially with respect to the age range, is now we're -- I think in part because of so much more awareness and more research is being done looking at this disease as a chronic systemic disease-- we're now starting to see/diagnose adolescents with endometriosis.
I think as we start gathering more information about these individuals, we'll be able to better say if there is a particular age range. Right now, we usually say it's in the reproductive years, however for some women it may be later if they were not diagnosed earlier. Conversely, some who are hopefully reading this, and also who conduct research on endometriosis, may be able to diagnose someone earlier that may have been missed until they were in their 30s or 40s, for example.
With respect to ethnicity, I'm the task force leader for diversity, equity, and inclusion in research and recruitment. This is something that I'm actively starting to work on, as are other groups. I don't have the answer for that yet, but as we continue to collect more data, we will have more information on this.
What are some of the existing hormonal therapies you rely upon as well as the biomarkers in predicting response to treatment, and are there any new research or treatments on the horizon?
Dr. Flores: I'll first start by telling you a bit about our existing treatment regimens, and then how I decide who would benefit from a given one. First line has always been progestin-based therapy, either in the form of a combined oral contraceptive pill or as progesterone only pills. However, up to 1/3 of women fail progestin-based therapy—this is termed progesterone resistance.
When progestin-based therapies fail, we then rely on other agents that are focused more on estrogen deprivation because, while we don't know the complete etiology of endometriosis, we do know that it is estrogen-dependent. There are two classes— gonadotropin releasing hormone (GnRH) agonists and GnRH antagonists. The agonist binds to the GnRH receptors, and initially can cause a flare effect due to its agonist properties, initially stimulate release of estradiol, and ultimately the GnRH receptor becomes downregulated and estradiol is decreased to the menopausal range. As a result we routinely provided add-back therapy with norethindrone to help prevent hot flashes and ensure bone protection.
Within the past three years, there has been a new oral GnRH receptor antagonist approved for treating endometriosis. The medication is available as a once a day or twice a day dosing regimen. As this is a GnRH antagonist, upon binding to the GnRH receptor, it blocks receptor activity, thus avoiding the flare affect; essentially, within 24 hours, there is a decrease in estradiol production.
As two doses are available, you can tailor how much you dial down estrogen for a given patient. The low dose lowers estradiol to a range of 40 picograms while the high (twice a day) dosing lowers your estrogen to about 6 picograms. Also, although it was not studied originally in terms of giving add-back therapy for the higher dose, given the safety (and effectiveness) of add-back therapy with GnRH agonists we are using the same norethindrone add-back therapy for women who are taking the GnRH receptor antagonist.
The next question is, how do we decide which medication a given patient receives? To answer that, I will tell you a bit about my precision-based medicine research. As mentioned before, while progestin-based therapy is first-line, failure rates are high, and unfortunately, we previously have not been able to identify who will or will not respond to first-line therapy. As such, I decided to assess progesterone receptor expression in endometriotic lesions from women who had undergone surgery for endometriosis, and determine whether progesterone receptor expression levels in lesions could be used to predict response to progestin-based therapy. I found that in women that had high levels of the progesterone receptor, they responded completely to progestin-based therapy-- there was a 100% response rate to progestin-based therapy. This is in sharp contrast to women who had low PR expression, where there was only a 6% response rate to progestin-based therapy.
While this is great with respect to being able to predict who will or will not respond to first line therapy, the one limitation is that would mean that women have to undergo surgery in order to determine progesterone receptor status/response to progestin-based therapy. However, given that within two to five years following surgery, up to 50% of women will have recurrence of pain symptoms, where I see my test coming into play is postoperatively. This is because many times , women who had pain, or who were failing a given agent, are placed back on that same medical therapy they were failing after surgery. Usually that was a progestin. Therefore, instead of putting them on that same therapy that they were failing, we can use my test to place them on an alternative therapy (such as a GnRH analogue) that more specifically targets estradiol production.
In terms of future directions with respect to treatment, there is a microRNA that has been found to be low in women with endometriosis—miRNALet-7b. In a murine model of endometriosis, we have found that if we supplement with Let-7, there is decreased inflammation and decreased lesion size of endometriosis. We have also found that supplementing miRNA Let-7b in human endometriotic lesions results in decreased inflammation in cell culture.
That would be future directions in terms of focusing on microRNAs and seeing how we can manipulate those to essentially block inflammation and lesion growth. Furthermore, such treatment would be non-hormonal, which would be a novel therapeutic approach.
Can you talk about your research thus far and what your overall lab work has shown regarding endometriosis as a chronic systemic disease?
Dr. Flores: Endometriosis has traditionally been characterized by its pelvic manifestation however, it is important to understand that it is profoundly more than a pelvic disease—it is a chronic, systemic disease with multifactorial effects throughout the body.
We and other groups have found increased expression of several inflammatory cytokines in women with endometriosis. Our lab has found that compared to women without endometriosis, women with endometriosis not only have certain inflammatory cytokines elevated but also have altered expression of microRNAs. MicroRNAs are small noncoding RNAs that bind to and modulate translation of mRNA. To help determine whether these miRNAs were involved in mediating increased expression of inflammatory cytokines in women with endometriosis, we then transfected these miRNAs into a macrophage cell line, and again found altered inflammatory cytokine expression. We and others have also found a role for stem cells (from bone marrow and other sources) in the pathogenesis of endometriosis. In addition, we have found that in endometriosis, women have a low body-mass index and altered metabolism, which is related to induction of induction of hepatic (anorexigenic) gene expression and microRNA-mediated changes in adipocyte (metabolic) gene expression. Furthermore, we have found altered gene expression in regions of the brain associated with anxiety and depression and altered pain sensitization. Taken together, this work helps provide support for the systemic nature of endometriosis.
How can your findings in this space help us in diagnosing clinically and ultimately avoid diagnostic delay?
Dr. Flores: It’s about understanding that endometriosis is not just a pelvic disease and understanding that endometriosis is leading to inflammation and altered expression of miRNAs which allows endometriosis to have long-range effects. For example, women with endometriosis commonly have anxiety and depression and low BMI. As mentioned earlier, we have found that in a murine model of endometriosis, there is altered gene expression in regions of the brain associated with anxiety and depression and altered metabolism in a murine model of endometriosis. Other groups have also found changes in brain volume in these same areas in women with endometriosis, and we have seen low BMI in women with endometriosis. In fact, a common misconception was that being thin was a risk factor for endometriosis, however we have found that the endometriosis itself, is causing women alteration in genes associated with metabolism.
With respect to the endometrium, in addition to being a pelvic pain disorder, we also see that women with endometriosis have a higher likelihood of having infertility. And we think that's in part because one, just like the lesions can be resistant to progesterone, the endometrium of these women can also be resistant to progesterone. Progesterone is necessary for decidualization/implantation. We have also seen that stem cells can be recruited and ultimately incorrectly incorporated into the endometrium, which may also contribute to infertility in women with endometriosis.
If we can understand this multifactorial nature of endometriosis, I think this will help us not only shift toward diagnosing endometriosis clinically, but also avoid diagnostic delay. If we can understand that endometriosis is not just a pelvic disorder, but that It can also involve altered mood, bowel/bladder symptoms, inflammation, altered metabolism and/or cause infertility, I think that will ultimately help us to diagnosing earlier.
In addition, we can also utilize pelvic pain symptomatology to help with diagnosis as well. We can ask about cyclic pelvic pain that's been getting progressively worse over the years, not responding to non-steroidal anti-inflammatory medications. Also, in understanding that endometriosis can affect other organs, asking about cyclic pain/symptoms in other areas, such as cyclical bowel or bladder symptoms.
Thinking about the fact that if you do have a patient like that, you're seeing that they have altered mood symptoms, or alterations in inflammatory markers. Maybe that will help us shift from a disease that was typically only considered to be diagnosed by surgery, by switching to a clinical diagnosis for endometriosis. Doing that will hopefully help avoid diagnostic delay.
If we understand that while we typically describe endometriosis as causing cyclic pain symptoms, sometimes because of the existing diagnostic delay, ultimately women can present with chronic pelvic pain. Thus, it's also important to ask patients presenting with chronic pelvic pain what the symptoms were like beforehand (i.e., was the pain cyclic and progressively worsening over the years/before it became chronic) doing so will also help in terms of diagnosing sooner.
Lastly, circulating miRNAs have been considered promising biomarker candidates because they are stable in circulation and have highly specific expression profiles. We have found that the combination of several miRNAs reliably distinguished endometriosis patients from controls, and a prospective, blinded study showed that the combination of several miRNAs could be used to accurately identify patients with endometriosis, with an area under the receiver operating characteristic curve of 0.93.
Roughly 11%, or more than 6.5 million, women in the United States between the ages of 15–44 years, may have endometriosis. Is this disease more common in any particular age range or ethnicity?
Dr. Flores: We’re actually actively investigating that right now. And I think what makes it challenging, especially with respect to the age range, is now we're -- I think in part because of so much more awareness and more research is being done looking at this disease as a chronic systemic disease-- we're now starting to see/diagnose adolescents with endometriosis.
I think as we start gathering more information about these individuals, we'll be able to better say if there is a particular age range. Right now, we usually say it's in the reproductive years, however for some women it may be later if they were not diagnosed earlier. Conversely, some who are hopefully reading this, and also who conduct research on endometriosis, may be able to diagnose someone earlier that may have been missed until they were in their 30s or 40s, for example.
With respect to ethnicity, I'm the task force leader for diversity, equity, and inclusion in research and recruitment. This is something that I'm actively starting to work on, as are other groups. I don't have the answer for that yet, but as we continue to collect more data, we will have more information on this.
What are some of the existing hormonal therapies you rely upon as well as the biomarkers in predicting response to treatment, and are there any new research or treatments on the horizon?
Dr. Flores: I'll first start by telling you a bit about our existing treatment regimens, and then how I decide who would benefit from a given one. First line has always been progestin-based therapy, either in the form of a combined oral contraceptive pill or as progesterone only pills. However, up to 1/3 of women fail progestin-based therapy—this is termed progesterone resistance.
When progestin-based therapies fail, we then rely on other agents that are focused more on estrogen deprivation because, while we don't know the complete etiology of endometriosis, we do know that it is estrogen-dependent. There are two classes— gonadotropin releasing hormone (GnRH) agonists and GnRH antagonists. The agonist binds to the GnRH receptors, and initially can cause a flare effect due to its agonist properties, initially stimulate release of estradiol, and ultimately the GnRH receptor becomes downregulated and estradiol is decreased to the menopausal range. As a result we routinely provided add-back therapy with norethindrone to help prevent hot flashes and ensure bone protection.
Within the past three years, there has been a new oral GnRH receptor antagonist approved for treating endometriosis. The medication is available as a once a day or twice a day dosing regimen. As this is a GnRH antagonist, upon binding to the GnRH receptor, it blocks receptor activity, thus avoiding the flare affect; essentially, within 24 hours, there is a decrease in estradiol production.
As two doses are available, you can tailor how much you dial down estrogen for a given patient. The low dose lowers estradiol to a range of 40 picograms while the high (twice a day) dosing lowers your estrogen to about 6 picograms. Also, although it was not studied originally in terms of giving add-back therapy for the higher dose, given the safety (and effectiveness) of add-back therapy with GnRH agonists we are using the same norethindrone add-back therapy for women who are taking the GnRH receptor antagonist.
The next question is, how do we decide which medication a given patient receives? To answer that, I will tell you a bit about my precision-based medicine research. As mentioned before, while progestin-based therapy is first-line, failure rates are high, and unfortunately, we previously have not been able to identify who will or will not respond to first-line therapy. As such, I decided to assess progesterone receptor expression in endometriotic lesions from women who had undergone surgery for endometriosis, and determine whether progesterone receptor expression levels in lesions could be used to predict response to progestin-based therapy. I found that in women that had high levels of the progesterone receptor, they responded completely to progestin-based therapy-- there was a 100% response rate to progestin-based therapy. This is in sharp contrast to women who had low PR expression, where there was only a 6% response rate to progestin-based therapy.
While this is great with respect to being able to predict who will or will not respond to first line therapy, the one limitation is that would mean that women have to undergo surgery in order to determine progesterone receptor status/response to progestin-based therapy. However, given that within two to five years following surgery, up to 50% of women will have recurrence of pain symptoms, where I see my test coming into play is postoperatively. This is because many times , women who had pain, or who were failing a given agent, are placed back on that same medical therapy they were failing after surgery. Usually that was a progestin. Therefore, instead of putting them on that same therapy that they were failing, we can use my test to place them on an alternative therapy (such as a GnRH analogue) that more specifically targets estradiol production.
In terms of future directions with respect to treatment, there is a microRNA that has been found to be low in women with endometriosis—miRNALet-7b. In a murine model of endometriosis, we have found that if we supplement with Let-7, there is decreased inflammation and decreased lesion size of endometriosis. We have also found that supplementing miRNA Let-7b in human endometriotic lesions results in decreased inflammation in cell culture.
That would be future directions in terms of focusing on microRNAs and seeing how we can manipulate those to essentially block inflammation and lesion growth. Furthermore, such treatment would be non-hormonal, which would be a novel therapeutic approach.
As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006-1014.
Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296-1301
Cosar E, Mamillapalli R, Ersoy GS, Cho S, Seifer B, Taylor HS. Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array-based analysis. Fertil Steril. 2016;106(2):402-409.
Flores VA, Vanhie A, Dang T, Taylor HS. Progesterone Receptor Status Predicts Response to Progestin Therapy in Endometriosis. J Clin Endocrinol Metab. 2018 Dec 1;103(12):4561-4568
Goetz TG, Mamillapalli R, Taylor HS. Low Body Mass Index in Endometriosis Is Promoted by Hepatic Metabolic Gene Dysregulation in Mice. Biol Reprod. 2016;95(6):115.
Li T, et al. Endometriosis alters brain electrophysiology, gene expression and increases pain sensitization, anxiety, and depression in female mice. Biol Reprod. 2018;99(2):349-359.
Moustafa S, Burn M, Mamillapalli R, Nematian S, Flores V, Taylor HS. Accurate diagnosis of endometriosis using serum microRNAs. Am J Obstet Gynecol. 2020;223(4):557.e1-557.e11.
Nematian SE, et al. Systemic Inflammation Induced by microRNAs: Endometriosis-Derived Alterations in Circulating microRNA 125b-5p and Let-7b-5p Regulate Macrophage Cytokine Production. J Clin Endocrinol Metab. 2018;103(1):64-74.
Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
Rogers PA, D'Hooghe TM, Fazleabas A, et al. Defining future directions for endometriosis research: workshop report from the 2011 World Congress of Endometriosis In Montpellier, France. Reprod Sci. 2013;20(5):483-499.
Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021 Feb 27
Zolbin MM, et al. Adipocyte alterations in endometriosis: reduced numbers of stem cells and microRNA induced alterations in adipocyte metabolic gene expression. Reprod Biol Endocrinol. 2019;17(1):36.
As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006-1014.
Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296-1301
Cosar E, Mamillapalli R, Ersoy GS, Cho S, Seifer B, Taylor HS. Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array-based analysis. Fertil Steril. 2016;106(2):402-409.
Flores VA, Vanhie A, Dang T, Taylor HS. Progesterone Receptor Status Predicts Response to Progestin Therapy in Endometriosis. J Clin Endocrinol Metab. 2018 Dec 1;103(12):4561-4568
Goetz TG, Mamillapalli R, Taylor HS. Low Body Mass Index in Endometriosis Is Promoted by Hepatic Metabolic Gene Dysregulation in Mice. Biol Reprod. 2016;95(6):115.
Li T, et al. Endometriosis alters brain electrophysiology, gene expression and increases pain sensitization, anxiety, and depression in female mice. Biol Reprod. 2018;99(2):349-359.
Moustafa S, Burn M, Mamillapalli R, Nematian S, Flores V, Taylor HS. Accurate diagnosis of endometriosis using serum microRNAs. Am J Obstet Gynecol. 2020;223(4):557.e1-557.e11.
Nematian SE, et al. Systemic Inflammation Induced by microRNAs: Endometriosis-Derived Alterations in Circulating microRNA 125b-5p and Let-7b-5p Regulate Macrophage Cytokine Production. J Clin Endocrinol Metab. 2018;103(1):64-74.
Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
Rogers PA, D'Hooghe TM, Fazleabas A, et al. Defining future directions for endometriosis research: workshop report from the 2011 World Congress of Endometriosis In Montpellier, France. Reprod Sci. 2013;20(5):483-499.
Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021 Feb 27
Zolbin MM, et al. Adipocyte alterations in endometriosis: reduced numbers of stem cells and microRNA induced alterations in adipocyte metabolic gene expression. Reprod Biol Endocrinol. 2019;17(1):36.
The differences between IBS-C and CIC
Lin Chang, MD, serves as the Co-Director of the G. Oppenheimer Center for Neurobiology of Stress and Resilience at UCLA. She is also Program Director of the UCLA Gastroenterology Fellowship Program. Dr. Chang’s expertise is in disorders of gut-brain interaction (also known as functional gastrointestinal disorders), particularly irritable bowel syndrome (IBS). She has recently served as the Clinical Research Councilor of the AGA Governing Board. She previously served as President of the American Neurogastroenterology and Motility Society (ANMS) and is a member of the Rome Foundation Board of Directors.
As a gastroenterologist focused on the pathophysiology of IBS related to stress, sex differences, and neuroendocrine alterations, and the treatment of IBS, Dr. Chang, what exactly is IBS-C and how is CIC defined differently?
Dr. Chang: IBS-C is irritable bowel syndrome with predominantly constipation which is a type of IBS. IBS is a symptom-based diagnosis for a chronic or recurrent gastrointestinal condition where patients have abdominal pain that's associated with constipation, diarrhea, or both. IBS is subtyped by bowel habit predominance into IBS with constipation, IBS with diarrhea, and IBS with mixed bowel habits. With IBS-mixed, one of the subgroups of IBS, they have diarrhea as well as constipation.
Patients will present with abdominal pain for usually one day, a week or even more. Sometimes, a little less. But when they have pain, it's associated with a change in stool frequency, a change in stool form, and/or the pain is related to defecation, meaning that when a patient has a bowel movement, they'll either have more pain or they'll have some pain relief, which is more common.
Now, CIC is Chronic Idiopathic Constipation and that's the term used for chronic constipation where abdominal pain is not a predominant symptom. The main difference between IBS-C and CIC is that abdominal pain is not a predominant or frequent symptom.
Patients with CIC can occasionally get abdominal pain, particularly if they haven't had a bowel movement for a prolonged period of time. However, in patients with IBS-C, they can have some normalization of their bowel habits or their constipation with treatment, although they can still have abdominal pain and discomfort. So, these patients have an element of visceral hypersensitivity where the gut is more sensitive than usual.
Very interesting Dr. Chang, and are the causes of IBS-C and CIC different? And then if so, in what ways?
Dr. Chang: Well, IBS is a multifactorial disorder and is known as a functional GI disorder. It has been redefined as a disorder of gut-brain interaction, which is a term people are starting to use and hear more.
There's a lot of scientific evidence that has demonstrated that IBS and other similar conditions, including chronic constipation and functional dyspepsia, where there is no structural and biochemical abnormality that you can readily determine, but there's scientific evidence to support that there’s an alteration in the brain-gut communication associated with symptoms. Altered brain-gut interactions are manifested by one or more of the following, which is visceral hypersensitivity, immune function, gut microbiota, gut motility, and central nervous system processing of visceral information. So, this really is a true brain-gut disorder.
There are multiple risk factors when it comes to IBS. It could be infection, or it could be stressful life events, in childhood and/or as an adult. Evidence shows that there can be some familial or genetic predisposition. Food and stress are the main triggers of IBS. Whereas, CIC can be considered a brain-gut disorder, but there's been more focus on gut function, including abnormal motility and defecation. There are three main subtypes of chronic idiopathic constipation.
There are six signs or symptoms that are the diagnostic criteria for CIC and the patient, or the individual, must meet two out of the six criteria, which I ask patients who report having constipation.
The subtypes of CIC are slow transit constipation where the transit time of stool through the colon is slower than normal which can be measured. Then there's normal transit constipation where the transit time of stool through the colon is normal. This group has not been studied that well and it's not completely understood why these patients have constipation, but it could be that they have a greater perception of constipation even though the transit time of stool in the colon is not slow.
And then there's the third group--defecatory disorders. The transit time of stool through the colon of stool can be normal or slow, but coexisting with that, a patient can have a defecation disorder. A common one is called dyssynergic defecation where the pelvic floor and the anal sphincter muscles don't relax appropriately when trying to evacuate stool. In this case, the rectum cannot straighten as much, the pelvic floor doesn't relax and descend, and stool is not easily evacuated.
There are also other conditions such as a significant large rectocele and rectal prolapse. Those are examples of defecatory disorders. So, when you see a patient with CIC, you want to first rule out secondary constipation where another condition or medication is causing constipation, such as hypothyroidism, diabetes, or a neurodegenerative disorder, or medications like opioids or anticholinergics.
CIC means that there isn't another cause of constipation, that is it is not a secondary condition. It's a primary chronic idiopathic constipation.
Let’s talk about the symptoms you're looking for and how they present themselves differently for IBS-C and CIC, at different times, depending on the diagnosis.
Dr. Chang: Sure! I mentioned what the symptom criteria of IBS was, which is having pain of a certain frequency that is associated with altered bowel habits. To determine the bowel habit subtype of IBS, you must assess the predominant stool form. We use the Bristol Stool Form Scale which is a validated stool form scale that's well known. It's publicly available.
The investigators did a survey years ago and they looked at the general population and found that the description of stool really could be encompassed in seven types, and those seven types of stool form correlate with transit time through the bowel. There's type 1 to type 7. Type 1 and 2 are the constipation type stool form where there's harder, drier pellet-like stools and that's associated with slower transit time through the colon. Types 3, 4 and 5 are more within the normal range. Types 6 and 7 are the loose or watery stools are suggestive of faster stool transit and considered indicative of diarrhea.
In patients with IBS-C, at least 25% of their bowel movements are the type 1 or 2, which is the harder, drier stool and less than 25% of bowel movements are loose watery. For diarrhea, it's opposite. IBS-mixed bowel habits, at least 25% of bowel movements are type 1 or 2 and at least 25% are type 6 or 7.
Now, to meet the diagnostic criteria of CIC, you must meet two out of the six criteria. All but one of the criteria must be present with at least 25% of bowel movements. There’s a straining, sensation of incomplete evacuation, use of manual maneuvers to help facilitate stool evacuation, sensation of anorectal blockage, and a Bristol Stool Form Scale of type 1 or 2. The remaining criterion is less than three bowel movements per week. If a patient reports, or endorses, at least two of those six symptoms and signs, then their symptoms meet criteria.
Both IBS-C and CIC are chronic conditions. For the diagnosis of both IBS-C and CIC, symptoms are present for at least three months and started at least six months ago.
What's interesting is that if you ask health care providers and physicians what constipation is and what symptoms define constipation, most of them will say having less than three bowel movements per week or infrequent bowel movements. But it turns out that in chronic constipation patients, they'll report decreased bowel movement frequency. About only a third of them will report that. They'll report the other symptoms of a constipation.
They could have multiple symptoms, but straining is a very common symptom as is hard stools. Even after a bowel movement, they don't feel completely evacuated. That's called sensation of incomplete evacuation. In fact, patients will present with different types of symptoms.
Constipation is often considered a symptom and a diagnosis. And it's fine to use it as a diagnosis, but you really want to delve into what symptoms of constipation they’re experiencing. Are they experiencing straining? Hard stools? Are they not having a bowel movement frequently? That's really part of the history taking so you can determine what the patient perceives as constipation and which symptom are bothersome to them.
So once diagnosed, how different are the treatments for each of the diseases?
Dr. Chang: In both IBS-C and CIC, treatments can include diet, exercise or ambulating more. Often, I will make sure they're drinking plenty of fluids. Those are dietary recommendations such as increasing fiber with foods and/or fiber supplementation. When looking at the difference between IBS-C and CIC, the one thing I should say is that they really exist along a spectrum, so we shouldn't really think of them as two separate diagnoses.
This goes back to the idea we touched on earlier that patients can move back and forth between the different diagnoses. At one point, a patient could have frequent abdominal pain and constipation and the symptoms would meet the criteria for IBS-C. But in the future, the pain gets better or resolves, but there’s still constipation. Their symptoms are more indicative of CIC. So, these conditions really exist along a spectrum.
Because both patients will have constipation symptoms, medications or treatments that help improve constipation can be used for both IBS-C and CIC. The key difference with IBS-C is that in addition to having altered gut motility where they're not moving stool effectively through the bowel, they also have visceral hypersensitivity which manifests as abdominal pain, bloating, and discomfort. Although there may be a modest correlation with bowel habits and IBS, sometimes, they don't correlate that well.
There are some treatments that help pain and constipation and those are the treatments that you want to think about in those patients with IBS-C where they're reporting both pain and constipation.
Now, it's very reasonable to use similar treatments in patients with mild symptoms, whether it's IBS or CIC. But if someone's having more severe IBS-C and they're having a fair amount of pain associated with constipation, you really want to think about treatments that can help reduce pain and constipation and not just constipation.
Treatments can include fiber such as psyllium and osmotic laxatives like polyethylene glycol, which is called MiraLAX, and magnesium-based regimens. These help constipation symptoms, but they don't significantly relieve abdominal pain. If someone came to me with IBS-C and they said, well, I do have pain, but it is mild, maybe a 2 or 3 out of 10, I could probably give them any one of the treatments I just mentioned. But in patients who say that their pain is 8 out of 10 or that it is their predominant symptom, I wouldn't necessarily prescribe the same treatment and would more likely opt for a treatment that has been shown to effectively reduce abdominal pain and constipation.
When you’re looking at the data, are their studies that might show a focus on the treatments and how they might impact the patients differently for IBS-C compared to CIC?
Dr. Chang: Well, the primary study endpoints that are used to determine efficacy of treatment in clinical trials differ in studies of CIC and IBS-C. However, studies also assess individual gastrointestinal symptoms that can be similar in both studies.
So, I would say that treatments that have been shown to be efficacious both in IBS-C and CIC likely relieve constipation symptoms similarly in both groups assuming that the severity of symptoms is comparable. It's just that in the CIC patient population, abdominal pain is not evaluated as much as it is in IBS-C.
F. Mearin, B. E. Lacy, L. Chang, W. D. Chey, A. J. Lembo, M. Simren, et al. Gastroenterology 2016 Vol. 150 Pages 1393-1407. http://www.ncbi.nlm.nih.gov/pubmed/27144627
D. A. Drossman. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016 https://www.ncbi.nlm.nih.gov/pubmed/27144617
Chang L. How to Approach a Patient with Difficult-to-Treat IBS. Gastroenterology 2021 Accession Number: 34331916 DOI: S0016-5085(21)03285-6 [pii]
10.1053/j.gastro.2021.07.034 https://www.ncbi.nlm.nih.gov/pubmed/34331916
A. E. Bharucha and B. E. Lacy. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020 Vol. 158 Issue 5 Pages 1232-1249 e3
Accession Number: 31945360 PMCID: PMC7573977 DOI: S0016-5085(20)30080-9 [pii]10.1053/j.gastro.2019.12.034 https://www.ncbi.nlm.nih.gov/pubmed/31945360
Lin Chang, MD, serves as the Co-Director of the G. Oppenheimer Center for Neurobiology of Stress and Resilience at UCLA. She is also Program Director of the UCLA Gastroenterology Fellowship Program. Dr. Chang’s expertise is in disorders of gut-brain interaction (also known as functional gastrointestinal disorders), particularly irritable bowel syndrome (IBS). She has recently served as the Clinical Research Councilor of the AGA Governing Board. She previously served as President of the American Neurogastroenterology and Motility Society (ANMS) and is a member of the Rome Foundation Board of Directors.
As a gastroenterologist focused on the pathophysiology of IBS related to stress, sex differences, and neuroendocrine alterations, and the treatment of IBS, Dr. Chang, what exactly is IBS-C and how is CIC defined differently?
Dr. Chang: IBS-C is irritable bowel syndrome with predominantly constipation which is a type of IBS. IBS is a symptom-based diagnosis for a chronic or recurrent gastrointestinal condition where patients have abdominal pain that's associated with constipation, diarrhea, or both. IBS is subtyped by bowel habit predominance into IBS with constipation, IBS with diarrhea, and IBS with mixed bowel habits. With IBS-mixed, one of the subgroups of IBS, they have diarrhea as well as constipation.
Patients will present with abdominal pain for usually one day, a week or even more. Sometimes, a little less. But when they have pain, it's associated with a change in stool frequency, a change in stool form, and/or the pain is related to defecation, meaning that when a patient has a bowel movement, they'll either have more pain or they'll have some pain relief, which is more common.
Now, CIC is Chronic Idiopathic Constipation and that's the term used for chronic constipation where abdominal pain is not a predominant symptom. The main difference between IBS-C and CIC is that abdominal pain is not a predominant or frequent symptom.
Patients with CIC can occasionally get abdominal pain, particularly if they haven't had a bowel movement for a prolonged period of time. However, in patients with IBS-C, they can have some normalization of their bowel habits or their constipation with treatment, although they can still have abdominal pain and discomfort. So, these patients have an element of visceral hypersensitivity where the gut is more sensitive than usual.
Very interesting Dr. Chang, and are the causes of IBS-C and CIC different? And then if so, in what ways?
Dr. Chang: Well, IBS is a multifactorial disorder and is known as a functional GI disorder. It has been redefined as a disorder of gut-brain interaction, which is a term people are starting to use and hear more.
There's a lot of scientific evidence that has demonstrated that IBS and other similar conditions, including chronic constipation and functional dyspepsia, where there is no structural and biochemical abnormality that you can readily determine, but there's scientific evidence to support that there’s an alteration in the brain-gut communication associated with symptoms. Altered brain-gut interactions are manifested by one or more of the following, which is visceral hypersensitivity, immune function, gut microbiota, gut motility, and central nervous system processing of visceral information. So, this really is a true brain-gut disorder.
There are multiple risk factors when it comes to IBS. It could be infection, or it could be stressful life events, in childhood and/or as an adult. Evidence shows that there can be some familial or genetic predisposition. Food and stress are the main triggers of IBS. Whereas, CIC can be considered a brain-gut disorder, but there's been more focus on gut function, including abnormal motility and defecation. There are three main subtypes of chronic idiopathic constipation.
There are six signs or symptoms that are the diagnostic criteria for CIC and the patient, or the individual, must meet two out of the six criteria, which I ask patients who report having constipation.
The subtypes of CIC are slow transit constipation where the transit time of stool through the colon is slower than normal which can be measured. Then there's normal transit constipation where the transit time of stool through the colon is normal. This group has not been studied that well and it's not completely understood why these patients have constipation, but it could be that they have a greater perception of constipation even though the transit time of stool in the colon is not slow.
And then there's the third group--defecatory disorders. The transit time of stool through the colon of stool can be normal or slow, but coexisting with that, a patient can have a defecation disorder. A common one is called dyssynergic defecation where the pelvic floor and the anal sphincter muscles don't relax appropriately when trying to evacuate stool. In this case, the rectum cannot straighten as much, the pelvic floor doesn't relax and descend, and stool is not easily evacuated.
There are also other conditions such as a significant large rectocele and rectal prolapse. Those are examples of defecatory disorders. So, when you see a patient with CIC, you want to first rule out secondary constipation where another condition or medication is causing constipation, such as hypothyroidism, diabetes, or a neurodegenerative disorder, or medications like opioids or anticholinergics.
CIC means that there isn't another cause of constipation, that is it is not a secondary condition. It's a primary chronic idiopathic constipation.
Let’s talk about the symptoms you're looking for and how they present themselves differently for IBS-C and CIC, at different times, depending on the diagnosis.
Dr. Chang: Sure! I mentioned what the symptom criteria of IBS was, which is having pain of a certain frequency that is associated with altered bowel habits. To determine the bowel habit subtype of IBS, you must assess the predominant stool form. We use the Bristol Stool Form Scale which is a validated stool form scale that's well known. It's publicly available.
The investigators did a survey years ago and they looked at the general population and found that the description of stool really could be encompassed in seven types, and those seven types of stool form correlate with transit time through the bowel. There's type 1 to type 7. Type 1 and 2 are the constipation type stool form where there's harder, drier pellet-like stools and that's associated with slower transit time through the colon. Types 3, 4 and 5 are more within the normal range. Types 6 and 7 are the loose or watery stools are suggestive of faster stool transit and considered indicative of diarrhea.
In patients with IBS-C, at least 25% of their bowel movements are the type 1 or 2, which is the harder, drier stool and less than 25% of bowel movements are loose watery. For diarrhea, it's opposite. IBS-mixed bowel habits, at least 25% of bowel movements are type 1 or 2 and at least 25% are type 6 or 7.
Now, to meet the diagnostic criteria of CIC, you must meet two out of the six criteria. All but one of the criteria must be present with at least 25% of bowel movements. There’s a straining, sensation of incomplete evacuation, use of manual maneuvers to help facilitate stool evacuation, sensation of anorectal blockage, and a Bristol Stool Form Scale of type 1 or 2. The remaining criterion is less than three bowel movements per week. If a patient reports, or endorses, at least two of those six symptoms and signs, then their symptoms meet criteria.
Both IBS-C and CIC are chronic conditions. For the diagnosis of both IBS-C and CIC, symptoms are present for at least three months and started at least six months ago.
What's interesting is that if you ask health care providers and physicians what constipation is and what symptoms define constipation, most of them will say having less than three bowel movements per week or infrequent bowel movements. But it turns out that in chronic constipation patients, they'll report decreased bowel movement frequency. About only a third of them will report that. They'll report the other symptoms of a constipation.
They could have multiple symptoms, but straining is a very common symptom as is hard stools. Even after a bowel movement, they don't feel completely evacuated. That's called sensation of incomplete evacuation. In fact, patients will present with different types of symptoms.
Constipation is often considered a symptom and a diagnosis. And it's fine to use it as a diagnosis, but you really want to delve into what symptoms of constipation they’re experiencing. Are they experiencing straining? Hard stools? Are they not having a bowel movement frequently? That's really part of the history taking so you can determine what the patient perceives as constipation and which symptom are bothersome to them.
So once diagnosed, how different are the treatments for each of the diseases?
Dr. Chang: In both IBS-C and CIC, treatments can include diet, exercise or ambulating more. Often, I will make sure they're drinking plenty of fluids. Those are dietary recommendations such as increasing fiber with foods and/or fiber supplementation. When looking at the difference between IBS-C and CIC, the one thing I should say is that they really exist along a spectrum, so we shouldn't really think of them as two separate diagnoses.
This goes back to the idea we touched on earlier that patients can move back and forth between the different diagnoses. At one point, a patient could have frequent abdominal pain and constipation and the symptoms would meet the criteria for IBS-C. But in the future, the pain gets better or resolves, but there’s still constipation. Their symptoms are more indicative of CIC. So, these conditions really exist along a spectrum.
Because both patients will have constipation symptoms, medications or treatments that help improve constipation can be used for both IBS-C and CIC. The key difference with IBS-C is that in addition to having altered gut motility where they're not moving stool effectively through the bowel, they also have visceral hypersensitivity which manifests as abdominal pain, bloating, and discomfort. Although there may be a modest correlation with bowel habits and IBS, sometimes, they don't correlate that well.
There are some treatments that help pain and constipation and those are the treatments that you want to think about in those patients with IBS-C where they're reporting both pain and constipation.
Now, it's very reasonable to use similar treatments in patients with mild symptoms, whether it's IBS or CIC. But if someone's having more severe IBS-C and they're having a fair amount of pain associated with constipation, you really want to think about treatments that can help reduce pain and constipation and not just constipation.
Treatments can include fiber such as psyllium and osmotic laxatives like polyethylene glycol, which is called MiraLAX, and magnesium-based regimens. These help constipation symptoms, but they don't significantly relieve abdominal pain. If someone came to me with IBS-C and they said, well, I do have pain, but it is mild, maybe a 2 or 3 out of 10, I could probably give them any one of the treatments I just mentioned. But in patients who say that their pain is 8 out of 10 or that it is their predominant symptom, I wouldn't necessarily prescribe the same treatment and would more likely opt for a treatment that has been shown to effectively reduce abdominal pain and constipation.
When you’re looking at the data, are their studies that might show a focus on the treatments and how they might impact the patients differently for IBS-C compared to CIC?
Dr. Chang: Well, the primary study endpoints that are used to determine efficacy of treatment in clinical trials differ in studies of CIC and IBS-C. However, studies also assess individual gastrointestinal symptoms that can be similar in both studies.
So, I would say that treatments that have been shown to be efficacious both in IBS-C and CIC likely relieve constipation symptoms similarly in both groups assuming that the severity of symptoms is comparable. It's just that in the CIC patient population, abdominal pain is not evaluated as much as it is in IBS-C.
Lin Chang, MD, serves as the Co-Director of the G. Oppenheimer Center for Neurobiology of Stress and Resilience at UCLA. She is also Program Director of the UCLA Gastroenterology Fellowship Program. Dr. Chang’s expertise is in disorders of gut-brain interaction (also known as functional gastrointestinal disorders), particularly irritable bowel syndrome (IBS). She has recently served as the Clinical Research Councilor of the AGA Governing Board. She previously served as President of the American Neurogastroenterology and Motility Society (ANMS) and is a member of the Rome Foundation Board of Directors.
As a gastroenterologist focused on the pathophysiology of IBS related to stress, sex differences, and neuroendocrine alterations, and the treatment of IBS, Dr. Chang, what exactly is IBS-C and how is CIC defined differently?
Dr. Chang: IBS-C is irritable bowel syndrome with predominantly constipation which is a type of IBS. IBS is a symptom-based diagnosis for a chronic or recurrent gastrointestinal condition where patients have abdominal pain that's associated with constipation, diarrhea, or both. IBS is subtyped by bowel habit predominance into IBS with constipation, IBS with diarrhea, and IBS with mixed bowel habits. With IBS-mixed, one of the subgroups of IBS, they have diarrhea as well as constipation.
Patients will present with abdominal pain for usually one day, a week or even more. Sometimes, a little less. But when they have pain, it's associated with a change in stool frequency, a change in stool form, and/or the pain is related to defecation, meaning that when a patient has a bowel movement, they'll either have more pain or they'll have some pain relief, which is more common.
Now, CIC is Chronic Idiopathic Constipation and that's the term used for chronic constipation where abdominal pain is not a predominant symptom. The main difference between IBS-C and CIC is that abdominal pain is not a predominant or frequent symptom.
Patients with CIC can occasionally get abdominal pain, particularly if they haven't had a bowel movement for a prolonged period of time. However, in patients with IBS-C, they can have some normalization of their bowel habits or their constipation with treatment, although they can still have abdominal pain and discomfort. So, these patients have an element of visceral hypersensitivity where the gut is more sensitive than usual.
Very interesting Dr. Chang, and are the causes of IBS-C and CIC different? And then if so, in what ways?
Dr. Chang: Well, IBS is a multifactorial disorder and is known as a functional GI disorder. It has been redefined as a disorder of gut-brain interaction, which is a term people are starting to use and hear more.
There's a lot of scientific evidence that has demonstrated that IBS and other similar conditions, including chronic constipation and functional dyspepsia, where there is no structural and biochemical abnormality that you can readily determine, but there's scientific evidence to support that there’s an alteration in the brain-gut communication associated with symptoms. Altered brain-gut interactions are manifested by one or more of the following, which is visceral hypersensitivity, immune function, gut microbiota, gut motility, and central nervous system processing of visceral information. So, this really is a true brain-gut disorder.
There are multiple risk factors when it comes to IBS. It could be infection, or it could be stressful life events, in childhood and/or as an adult. Evidence shows that there can be some familial or genetic predisposition. Food and stress are the main triggers of IBS. Whereas, CIC can be considered a brain-gut disorder, but there's been more focus on gut function, including abnormal motility and defecation. There are three main subtypes of chronic idiopathic constipation.
There are six signs or symptoms that are the diagnostic criteria for CIC and the patient, or the individual, must meet two out of the six criteria, which I ask patients who report having constipation.
The subtypes of CIC are slow transit constipation where the transit time of stool through the colon is slower than normal which can be measured. Then there's normal transit constipation where the transit time of stool through the colon is normal. This group has not been studied that well and it's not completely understood why these patients have constipation, but it could be that they have a greater perception of constipation even though the transit time of stool in the colon is not slow.
And then there's the third group--defecatory disorders. The transit time of stool through the colon of stool can be normal or slow, but coexisting with that, a patient can have a defecation disorder. A common one is called dyssynergic defecation where the pelvic floor and the anal sphincter muscles don't relax appropriately when trying to evacuate stool. In this case, the rectum cannot straighten as much, the pelvic floor doesn't relax and descend, and stool is not easily evacuated.
There are also other conditions such as a significant large rectocele and rectal prolapse. Those are examples of defecatory disorders. So, when you see a patient with CIC, you want to first rule out secondary constipation where another condition or medication is causing constipation, such as hypothyroidism, diabetes, or a neurodegenerative disorder, or medications like opioids or anticholinergics.
CIC means that there isn't another cause of constipation, that is it is not a secondary condition. It's a primary chronic idiopathic constipation.
Let’s talk about the symptoms you're looking for and how they present themselves differently for IBS-C and CIC, at different times, depending on the diagnosis.
Dr. Chang: Sure! I mentioned what the symptom criteria of IBS was, which is having pain of a certain frequency that is associated with altered bowel habits. To determine the bowel habit subtype of IBS, you must assess the predominant stool form. We use the Bristol Stool Form Scale which is a validated stool form scale that's well known. It's publicly available.
The investigators did a survey years ago and they looked at the general population and found that the description of stool really could be encompassed in seven types, and those seven types of stool form correlate with transit time through the bowel. There's type 1 to type 7. Type 1 and 2 are the constipation type stool form where there's harder, drier pellet-like stools and that's associated with slower transit time through the colon. Types 3, 4 and 5 are more within the normal range. Types 6 and 7 are the loose or watery stools are suggestive of faster stool transit and considered indicative of diarrhea.
In patients with IBS-C, at least 25% of their bowel movements are the type 1 or 2, which is the harder, drier stool and less than 25% of bowel movements are loose watery. For diarrhea, it's opposite. IBS-mixed bowel habits, at least 25% of bowel movements are type 1 or 2 and at least 25% are type 6 or 7.
Now, to meet the diagnostic criteria of CIC, you must meet two out of the six criteria. All but one of the criteria must be present with at least 25% of bowel movements. There’s a straining, sensation of incomplete evacuation, use of manual maneuvers to help facilitate stool evacuation, sensation of anorectal blockage, and a Bristol Stool Form Scale of type 1 or 2. The remaining criterion is less than three bowel movements per week. If a patient reports, or endorses, at least two of those six symptoms and signs, then their symptoms meet criteria.
Both IBS-C and CIC are chronic conditions. For the diagnosis of both IBS-C and CIC, symptoms are present for at least three months and started at least six months ago.
What's interesting is that if you ask health care providers and physicians what constipation is and what symptoms define constipation, most of them will say having less than three bowel movements per week or infrequent bowel movements. But it turns out that in chronic constipation patients, they'll report decreased bowel movement frequency. About only a third of them will report that. They'll report the other symptoms of a constipation.
They could have multiple symptoms, but straining is a very common symptom as is hard stools. Even after a bowel movement, they don't feel completely evacuated. That's called sensation of incomplete evacuation. In fact, patients will present with different types of symptoms.
Constipation is often considered a symptom and a diagnosis. And it's fine to use it as a diagnosis, but you really want to delve into what symptoms of constipation they’re experiencing. Are they experiencing straining? Hard stools? Are they not having a bowel movement frequently? That's really part of the history taking so you can determine what the patient perceives as constipation and which symptom are bothersome to them.
So once diagnosed, how different are the treatments for each of the diseases?
Dr. Chang: In both IBS-C and CIC, treatments can include diet, exercise or ambulating more. Often, I will make sure they're drinking plenty of fluids. Those are dietary recommendations such as increasing fiber with foods and/or fiber supplementation. When looking at the difference between IBS-C and CIC, the one thing I should say is that they really exist along a spectrum, so we shouldn't really think of them as two separate diagnoses.
This goes back to the idea we touched on earlier that patients can move back and forth between the different diagnoses. At one point, a patient could have frequent abdominal pain and constipation and the symptoms would meet the criteria for IBS-C. But in the future, the pain gets better or resolves, but there’s still constipation. Their symptoms are more indicative of CIC. So, these conditions really exist along a spectrum.
Because both patients will have constipation symptoms, medications or treatments that help improve constipation can be used for both IBS-C and CIC. The key difference with IBS-C is that in addition to having altered gut motility where they're not moving stool effectively through the bowel, they also have visceral hypersensitivity which manifests as abdominal pain, bloating, and discomfort. Although there may be a modest correlation with bowel habits and IBS, sometimes, they don't correlate that well.
There are some treatments that help pain and constipation and those are the treatments that you want to think about in those patients with IBS-C where they're reporting both pain and constipation.
Now, it's very reasonable to use similar treatments in patients with mild symptoms, whether it's IBS or CIC. But if someone's having more severe IBS-C and they're having a fair amount of pain associated with constipation, you really want to think about treatments that can help reduce pain and constipation and not just constipation.
Treatments can include fiber such as psyllium and osmotic laxatives like polyethylene glycol, which is called MiraLAX, and magnesium-based regimens. These help constipation symptoms, but they don't significantly relieve abdominal pain. If someone came to me with IBS-C and they said, well, I do have pain, but it is mild, maybe a 2 or 3 out of 10, I could probably give them any one of the treatments I just mentioned. But in patients who say that their pain is 8 out of 10 or that it is their predominant symptom, I wouldn't necessarily prescribe the same treatment and would more likely opt for a treatment that has been shown to effectively reduce abdominal pain and constipation.
When you’re looking at the data, are their studies that might show a focus on the treatments and how they might impact the patients differently for IBS-C compared to CIC?
Dr. Chang: Well, the primary study endpoints that are used to determine efficacy of treatment in clinical trials differ in studies of CIC and IBS-C. However, studies also assess individual gastrointestinal symptoms that can be similar in both studies.
So, I would say that treatments that have been shown to be efficacious both in IBS-C and CIC likely relieve constipation symptoms similarly in both groups assuming that the severity of symptoms is comparable. It's just that in the CIC patient population, abdominal pain is not evaluated as much as it is in IBS-C.
F. Mearin, B. E. Lacy, L. Chang, W. D. Chey, A. J. Lembo, M. Simren, et al. Gastroenterology 2016 Vol. 150 Pages 1393-1407. http://www.ncbi.nlm.nih.gov/pubmed/27144627
D. A. Drossman. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016 https://www.ncbi.nlm.nih.gov/pubmed/27144617
Chang L. How to Approach a Patient with Difficult-to-Treat IBS. Gastroenterology 2021 Accession Number: 34331916 DOI: S0016-5085(21)03285-6 [pii]
10.1053/j.gastro.2021.07.034 https://www.ncbi.nlm.nih.gov/pubmed/34331916
A. E. Bharucha and B. E. Lacy. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020 Vol. 158 Issue 5 Pages 1232-1249 e3
Accession Number: 31945360 PMCID: PMC7573977 DOI: S0016-5085(20)30080-9 [pii]10.1053/j.gastro.2019.12.034 https://www.ncbi.nlm.nih.gov/pubmed/31945360
F. Mearin, B. E. Lacy, L. Chang, W. D. Chey, A. J. Lembo, M. Simren, et al. Gastroenterology 2016 Vol. 150 Pages 1393-1407. http://www.ncbi.nlm.nih.gov/pubmed/27144627
D. A. Drossman. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016 https://www.ncbi.nlm.nih.gov/pubmed/27144617
Chang L. How to Approach a Patient with Difficult-to-Treat IBS. Gastroenterology 2021 Accession Number: 34331916 DOI: S0016-5085(21)03285-6 [pii]
10.1053/j.gastro.2021.07.034 https://www.ncbi.nlm.nih.gov/pubmed/34331916
A. E. Bharucha and B. E. Lacy. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020 Vol. 158 Issue 5 Pages 1232-1249 e3
Accession Number: 31945360 PMCID: PMC7573977 DOI: S0016-5085(20)30080-9 [pii]10.1053/j.gastro.2019.12.034 https://www.ncbi.nlm.nih.gov/pubmed/31945360
When surgery is the next step in treating endometriosis—know your patient’s priorities and how to optimize long-term pain relief
Cara R. King, DO, MS, is a member of the Cleveland Clinic Section of Minimally Invasive Gynecologic Surgery (MIGS). She is the Director of Benign Gynecologic Surgery, and Associate Program Director of the MIGS Fellowship, and Director of Innovation for the Women’s Health Institute. She is a member of the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Surgeons (SGS), American College of Surgeons (ACS), and the American Congress of Obstetricians and Gynecologists (ACOG).
Q: How much of your surgical practice is dedicated to patients with endometriosis?
Dr. King: The majority of my practice is dedicated to treating women with endometriosis. I practice at the Cleveland Clinic in Cleveland, Ohio, which is a high-volume referral center, so many of my patients are coming to me for endometriosis or pelvic pain-type symptoms. For most of my patients, I serve as a consultant, which means it's not their initial visit for this issue. I'm often seeing patients who have not found relief through alternate medical or surgical treatments and typically, have more deeply infiltrating or complex endometriosis disease.
Q: How do you make the treatment decision with patients that surgery is the next or proper needed step?
Dr. King: This decision depends on the goals and priorities of each of my patients. I don't have a one-size-fits-all type approach as every patient's journey and unique experiences vary. Ultimately, deciding on the available options and order of treatment depends on the patient's symptoms and priorities. I always start with a thorough history, including a detailed physical exam. The pelvic exam includes evaluation of the bladder, bowel, pelvic floor muscles, nerves, as well as the gynecologic organs including vagina, uterus, cervix and adnexa. If I palpate a nodule on the uterosacral ligaments or behind the cervix, I will sometimes perform a rectovaginal exam to assess for deeply infiltrating bowel disease. Various imaging modalities, including a transvaginal ultrasound or an MRI, can be helpful to further characterize the disease. This allows us to create a treatment plan that best aligns with the patients’ priorities and goals. As a general rule, surgery is usually indicated if empiric options have failed or if they desire definitive diagnosis; meaning the patient is still having pain symptoms despite conservative options or if they have failed or are intolerant to medical options. Some patients are not candidates for medical therapy, such as those who desire pregnancy or who are trying to conceive, so medical options wouldn't be an option for these patients. For patients who prefer an immediate diagnosis, surgical intervention may also be the best option. When I see initial consults for patients who haven't previously seen an endometriosis specialist, if they're not trying to conceive and if they are candidates for medical therapy, I think that's a reasonable first step. We must understand that medications are not curative, they are merely suppressive for endometriosis, so when patients come to me that have been on medical therapy for more than 3 months without pain improvement, and they haven't been offered a surgical approach, diagnostic laparoscopy is often the next best step.
Q: Please detail the presurgical discussion, or the consent process, that would allow you to go beyond the agreed-to procedure, if necessary?
Dr. King: Endometriosis is extremely unique in that you sometimes cannot tell how deeply infiltrative the disease is until you start excising it. So, my consent process and discussions are substantial parts of all patient presurgical conversations. This is crucial for understanding how comfortable the patient is with more aggressive surgery and to fully understand each individual’s symptoms and priorities. I spend a significant amount of time talking to patients about their exact goals for surgery and I conduct a thorough workup before we get into the operating room so that when coupled with a proper physical exam and detailed imaging, the element for surprise, such as finding disease that is much more advanced than you had thought, is decreased. Understanding your patient's symptoms as well as how aggressive they want you to be with regards to surgery is of utmost importance. The more accurate the description that I have of the type of disease that we're working with allows me to talk about all possibilities that could occur before the patients get into the operating room so that we can ensure expectations are met, for the patient and for the surgeon.
Q: Do you have any protocols to share with the audience that relate to limiting reoperation for residual disease?
Dr. King: Conducting a thorough history and physical exam in addition to having detailed imaging is crucial to optimize success. That said, there are times when imaging may appear “normal” when endometriosis is actually present, which is why it is of utmost importance to listen to your patient’s history. With deeply infiltrating endometriosis, superficially, if you look at the peritoneum, it can sometimes appear as if the disease is not that invasive. Again, endometriosis is unique in that until you start excising it, sometimes you don’t know the extent of the infiltration. So, having detailed imaging is going to allow for better mapping of the endometriosis beforehand which will allow you to properly focus in on those areas and enhance preoperative counseling.
My second level of advice is to know your limits with regards to surgical complexity and your laparoscopic skills. For instance, if an endometrioma is present on imaging, you will most likely encounter peritoneal disease and fibrosis below that ovary on the pelvic side wall adjacent to the ureter. If you are not comfortable excising this disease, you should consider referring the patient to an advanced pelvic surgeon. When you see certain characteristics on imaging, understanding what the disease process will look like when you get in there and understanding your own skill level at which you can safely and efficiently perform that dissection is very important. And if you do not have that skill level or if you are still working on detailed knowledge of retroperitoneal anatomy, then the opportunity exists to build up your team; consider including another subspecialist within GYN or urology, colorectal surgery, or cardiothoracic surgery, if you are working with diaphragmatic endometriosis. Loading your boat will allow you to safely and efficiently remove as much of the disease as you can and decrease the risk of leaving any behind. You could also consider video based surgical coaching to further enhance your own laparoscopic skills and surgical performance when treating this complex disease.
Q: How do you approach postsurgical management to maximize the pain-free period for patients?
Dr. King: We know that the best intervention for pain relief is complete excision of endometriosis. By performing a complete excision, we know that this procedure will prolong the length of time for pain-free interval. So, getting as close as possible to a complete excision is going to be the first step. It is also important to treat alternate sources of pain that can be impacted by endometriosis such as spasm of the pelvic floor muscles or central sensitization. While it is difficult to say whether recurrent endometriosis pain is secondary to reactivation of residual disease as opposed to new disease, we do know that complete excision provides longer relief. Assuming surgery has relieved a majority of or, all of the endometriosis associated pain, then the main strategy that we can use to postoperatively maximize that pain-free period is to minimize ovulation. This is typically accomplished with hormonal suppression. It is worth nothing that this isn't indicated for all patients and it is not mandatory as we, again, must be mindful of the patient's goals and priorities. But a recent systematic review did find that when we start hormonal suppression within 6 weeks of our endometriosis surgery, there is a significant reduction in recurrent endometriosis pain scores for up to one year postoperative. Currently, there are no non-hormonal medications that we can offer, nor do we have any interventions to alter genetics or immune aspects of the disease, though it is hoped such could possibly become available in the near future. At the current point in time, hormonal suppressive options are typically the best route but again, I want to reiterate that medications are suppressive and are not curative. And with regards to details of medical options, pulling in patient preference, financial aspects, underlying comorbidities, and long-term reproductive plans, are factors that are important to consider when making weighing decision.
Cara R. King, DO, MS, is a member of the Cleveland Clinic Section of Minimally Invasive Gynecologic Surgery (MIGS). She is the Director of Benign Gynecologic Surgery, and Associate Program Director of the MIGS Fellowship, and Director of Innovation for the Women’s Health Institute. She is a member of the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Surgeons (SGS), American College of Surgeons (ACS), and the American Congress of Obstetricians and Gynecologists (ACOG).
Q: How much of your surgical practice is dedicated to patients with endometriosis?
Dr. King: The majority of my practice is dedicated to treating women with endometriosis. I practice at the Cleveland Clinic in Cleveland, Ohio, which is a high-volume referral center, so many of my patients are coming to me for endometriosis or pelvic pain-type symptoms. For most of my patients, I serve as a consultant, which means it's not their initial visit for this issue. I'm often seeing patients who have not found relief through alternate medical or surgical treatments and typically, have more deeply infiltrating or complex endometriosis disease.
Q: How do you make the treatment decision with patients that surgery is the next or proper needed step?
Dr. King: This decision depends on the goals and priorities of each of my patients. I don't have a one-size-fits-all type approach as every patient's journey and unique experiences vary. Ultimately, deciding on the available options and order of treatment depends on the patient's symptoms and priorities. I always start with a thorough history, including a detailed physical exam. The pelvic exam includes evaluation of the bladder, bowel, pelvic floor muscles, nerves, as well as the gynecologic organs including vagina, uterus, cervix and adnexa. If I palpate a nodule on the uterosacral ligaments or behind the cervix, I will sometimes perform a rectovaginal exam to assess for deeply infiltrating bowel disease. Various imaging modalities, including a transvaginal ultrasound or an MRI, can be helpful to further characterize the disease. This allows us to create a treatment plan that best aligns with the patients’ priorities and goals. As a general rule, surgery is usually indicated if empiric options have failed or if they desire definitive diagnosis; meaning the patient is still having pain symptoms despite conservative options or if they have failed or are intolerant to medical options. Some patients are not candidates for medical therapy, such as those who desire pregnancy or who are trying to conceive, so medical options wouldn't be an option for these patients. For patients who prefer an immediate diagnosis, surgical intervention may also be the best option. When I see initial consults for patients who haven't previously seen an endometriosis specialist, if they're not trying to conceive and if they are candidates for medical therapy, I think that's a reasonable first step. We must understand that medications are not curative, they are merely suppressive for endometriosis, so when patients come to me that have been on medical therapy for more than 3 months without pain improvement, and they haven't been offered a surgical approach, diagnostic laparoscopy is often the next best step.
Q: Please detail the presurgical discussion, or the consent process, that would allow you to go beyond the agreed-to procedure, if necessary?
Dr. King: Endometriosis is extremely unique in that you sometimes cannot tell how deeply infiltrative the disease is until you start excising it. So, my consent process and discussions are substantial parts of all patient presurgical conversations. This is crucial for understanding how comfortable the patient is with more aggressive surgery and to fully understand each individual’s symptoms and priorities. I spend a significant amount of time talking to patients about their exact goals for surgery and I conduct a thorough workup before we get into the operating room so that when coupled with a proper physical exam and detailed imaging, the element for surprise, such as finding disease that is much more advanced than you had thought, is decreased. Understanding your patient's symptoms as well as how aggressive they want you to be with regards to surgery is of utmost importance. The more accurate the description that I have of the type of disease that we're working with allows me to talk about all possibilities that could occur before the patients get into the operating room so that we can ensure expectations are met, for the patient and for the surgeon.
Q: Do you have any protocols to share with the audience that relate to limiting reoperation for residual disease?
Dr. King: Conducting a thorough history and physical exam in addition to having detailed imaging is crucial to optimize success. That said, there are times when imaging may appear “normal” when endometriosis is actually present, which is why it is of utmost importance to listen to your patient’s history. With deeply infiltrating endometriosis, superficially, if you look at the peritoneum, it can sometimes appear as if the disease is not that invasive. Again, endometriosis is unique in that until you start excising it, sometimes you don’t know the extent of the infiltration. So, having detailed imaging is going to allow for better mapping of the endometriosis beforehand which will allow you to properly focus in on those areas and enhance preoperative counseling.
My second level of advice is to know your limits with regards to surgical complexity and your laparoscopic skills. For instance, if an endometrioma is present on imaging, you will most likely encounter peritoneal disease and fibrosis below that ovary on the pelvic side wall adjacent to the ureter. If you are not comfortable excising this disease, you should consider referring the patient to an advanced pelvic surgeon. When you see certain characteristics on imaging, understanding what the disease process will look like when you get in there and understanding your own skill level at which you can safely and efficiently perform that dissection is very important. And if you do not have that skill level or if you are still working on detailed knowledge of retroperitoneal anatomy, then the opportunity exists to build up your team; consider including another subspecialist within GYN or urology, colorectal surgery, or cardiothoracic surgery, if you are working with diaphragmatic endometriosis. Loading your boat will allow you to safely and efficiently remove as much of the disease as you can and decrease the risk of leaving any behind. You could also consider video based surgical coaching to further enhance your own laparoscopic skills and surgical performance when treating this complex disease.
Q: How do you approach postsurgical management to maximize the pain-free period for patients?
Dr. King: We know that the best intervention for pain relief is complete excision of endometriosis. By performing a complete excision, we know that this procedure will prolong the length of time for pain-free interval. So, getting as close as possible to a complete excision is going to be the first step. It is also important to treat alternate sources of pain that can be impacted by endometriosis such as spasm of the pelvic floor muscles or central sensitization. While it is difficult to say whether recurrent endometriosis pain is secondary to reactivation of residual disease as opposed to new disease, we do know that complete excision provides longer relief. Assuming surgery has relieved a majority of or, all of the endometriosis associated pain, then the main strategy that we can use to postoperatively maximize that pain-free period is to minimize ovulation. This is typically accomplished with hormonal suppression. It is worth nothing that this isn't indicated for all patients and it is not mandatory as we, again, must be mindful of the patient's goals and priorities. But a recent systematic review did find that when we start hormonal suppression within 6 weeks of our endometriosis surgery, there is a significant reduction in recurrent endometriosis pain scores for up to one year postoperative. Currently, there are no non-hormonal medications that we can offer, nor do we have any interventions to alter genetics or immune aspects of the disease, though it is hoped such could possibly become available in the near future. At the current point in time, hormonal suppressive options are typically the best route but again, I want to reiterate that medications are suppressive and are not curative. And with regards to details of medical options, pulling in patient preference, financial aspects, underlying comorbidities, and long-term reproductive plans, are factors that are important to consider when making weighing decision.
Cara R. King, DO, MS, is a member of the Cleveland Clinic Section of Minimally Invasive Gynecologic Surgery (MIGS). She is the Director of Benign Gynecologic Surgery, and Associate Program Director of the MIGS Fellowship, and Director of Innovation for the Women’s Health Institute. She is a member of the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Surgeons (SGS), American College of Surgeons (ACS), and the American Congress of Obstetricians and Gynecologists (ACOG).
Q: How much of your surgical practice is dedicated to patients with endometriosis?
Dr. King: The majority of my practice is dedicated to treating women with endometriosis. I practice at the Cleveland Clinic in Cleveland, Ohio, which is a high-volume referral center, so many of my patients are coming to me for endometriosis or pelvic pain-type symptoms. For most of my patients, I serve as a consultant, which means it's not their initial visit for this issue. I'm often seeing patients who have not found relief through alternate medical or surgical treatments and typically, have more deeply infiltrating or complex endometriosis disease.
Q: How do you make the treatment decision with patients that surgery is the next or proper needed step?
Dr. King: This decision depends on the goals and priorities of each of my patients. I don't have a one-size-fits-all type approach as every patient's journey and unique experiences vary. Ultimately, deciding on the available options and order of treatment depends on the patient's symptoms and priorities. I always start with a thorough history, including a detailed physical exam. The pelvic exam includes evaluation of the bladder, bowel, pelvic floor muscles, nerves, as well as the gynecologic organs including vagina, uterus, cervix and adnexa. If I palpate a nodule on the uterosacral ligaments or behind the cervix, I will sometimes perform a rectovaginal exam to assess for deeply infiltrating bowel disease. Various imaging modalities, including a transvaginal ultrasound or an MRI, can be helpful to further characterize the disease. This allows us to create a treatment plan that best aligns with the patients’ priorities and goals. As a general rule, surgery is usually indicated if empiric options have failed or if they desire definitive diagnosis; meaning the patient is still having pain symptoms despite conservative options or if they have failed or are intolerant to medical options. Some patients are not candidates for medical therapy, such as those who desire pregnancy or who are trying to conceive, so medical options wouldn't be an option for these patients. For patients who prefer an immediate diagnosis, surgical intervention may also be the best option. When I see initial consults for patients who haven't previously seen an endometriosis specialist, if they're not trying to conceive and if they are candidates for medical therapy, I think that's a reasonable first step. We must understand that medications are not curative, they are merely suppressive for endometriosis, so when patients come to me that have been on medical therapy for more than 3 months without pain improvement, and they haven't been offered a surgical approach, diagnostic laparoscopy is often the next best step.
Q: Please detail the presurgical discussion, or the consent process, that would allow you to go beyond the agreed-to procedure, if necessary?
Dr. King: Endometriosis is extremely unique in that you sometimes cannot tell how deeply infiltrative the disease is until you start excising it. So, my consent process and discussions are substantial parts of all patient presurgical conversations. This is crucial for understanding how comfortable the patient is with more aggressive surgery and to fully understand each individual’s symptoms and priorities. I spend a significant amount of time talking to patients about their exact goals for surgery and I conduct a thorough workup before we get into the operating room so that when coupled with a proper physical exam and detailed imaging, the element for surprise, such as finding disease that is much more advanced than you had thought, is decreased. Understanding your patient's symptoms as well as how aggressive they want you to be with regards to surgery is of utmost importance. The more accurate the description that I have of the type of disease that we're working with allows me to talk about all possibilities that could occur before the patients get into the operating room so that we can ensure expectations are met, for the patient and for the surgeon.
Q: Do you have any protocols to share with the audience that relate to limiting reoperation for residual disease?
Dr. King: Conducting a thorough history and physical exam in addition to having detailed imaging is crucial to optimize success. That said, there are times when imaging may appear “normal” when endometriosis is actually present, which is why it is of utmost importance to listen to your patient’s history. With deeply infiltrating endometriosis, superficially, if you look at the peritoneum, it can sometimes appear as if the disease is not that invasive. Again, endometriosis is unique in that until you start excising it, sometimes you don’t know the extent of the infiltration. So, having detailed imaging is going to allow for better mapping of the endometriosis beforehand which will allow you to properly focus in on those areas and enhance preoperative counseling.
My second level of advice is to know your limits with regards to surgical complexity and your laparoscopic skills. For instance, if an endometrioma is present on imaging, you will most likely encounter peritoneal disease and fibrosis below that ovary on the pelvic side wall adjacent to the ureter. If you are not comfortable excising this disease, you should consider referring the patient to an advanced pelvic surgeon. When you see certain characteristics on imaging, understanding what the disease process will look like when you get in there and understanding your own skill level at which you can safely and efficiently perform that dissection is very important. And if you do not have that skill level or if you are still working on detailed knowledge of retroperitoneal anatomy, then the opportunity exists to build up your team; consider including another subspecialist within GYN or urology, colorectal surgery, or cardiothoracic surgery, if you are working with diaphragmatic endometriosis. Loading your boat will allow you to safely and efficiently remove as much of the disease as you can and decrease the risk of leaving any behind. You could also consider video based surgical coaching to further enhance your own laparoscopic skills and surgical performance when treating this complex disease.
Q: How do you approach postsurgical management to maximize the pain-free period for patients?
Dr. King: We know that the best intervention for pain relief is complete excision of endometriosis. By performing a complete excision, we know that this procedure will prolong the length of time for pain-free interval. So, getting as close as possible to a complete excision is going to be the first step. It is also important to treat alternate sources of pain that can be impacted by endometriosis such as spasm of the pelvic floor muscles or central sensitization. While it is difficult to say whether recurrent endometriosis pain is secondary to reactivation of residual disease as opposed to new disease, we do know that complete excision provides longer relief. Assuming surgery has relieved a majority of or, all of the endometriosis associated pain, then the main strategy that we can use to postoperatively maximize that pain-free period is to minimize ovulation. This is typically accomplished with hormonal suppression. It is worth nothing that this isn't indicated for all patients and it is not mandatory as we, again, must be mindful of the patient's goals and priorities. But a recent systematic review did find that when we start hormonal suppression within 6 weeks of our endometriosis surgery, there is a significant reduction in recurrent endometriosis pain scores for up to one year postoperative. Currently, there are no non-hormonal medications that we can offer, nor do we have any interventions to alter genetics or immune aspects of the disease, though it is hoped such could possibly become available in the near future. At the current point in time, hormonal suppressive options are typically the best route but again, I want to reiterate that medications are suppressive and are not curative. And with regards to details of medical options, pulling in patient preference, financial aspects, underlying comorbidities, and long-term reproductive plans, are factors that are important to consider when making weighing decision.
More tools for the COVID toolbox
I was recently asked to see a 16-year-old, unvaccinated (against COVID-19) adolescent with hypothyroidism and obesity (body mass index 37 kg/m2) seen in the pediatric emergency department with tachycardia, O2 saturation 96%, urinary tract infection, poor appetite, and nausea. Her chest x-ray had low lung volumes but no infiltrates. She was noted to be dehydrated. Testing for COVID-19 was PCR positive.1
She was observed overnight, tolerated oral rehydration, and was being readied for discharge. Pediatric Infectious Diseases was called about prescribing remdesivir.
Remdesivir was not indicated as its current use is limited to inpatients with oxygen desaturations less than 94%. Infectious Diseases Society of America guidelines do recommend the use of monoclonal antibodies against the SARS-CoV-2 spike protein for prevention of COVID disease progression in high-risk individuals. Specifically, the IDSA guidelines say, “Among ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease, bamlanivimab/etesevimab, casirivimab/imdevimab, or sotrovimab rather than no neutralizing antibody treatment.”
The Food and Drug Administration’s Emergency Use Authorization (EUA) allowed use of specific monoclonal antibodies (casirivimab/imdevimab in combination, bamlanivimab/etesevimab in combination, and sotrovimab alone) for individuals 12 years and above with a minimum weight of 40 kg with high-risk conditions, describing the evidence as moderate certainty.2
Several questions have arisen regarding their use. Which children qualify under the EUA? Are the available monoclonal antibodies effective for SARS-CoV-2 variants? What adverse events were observed? Are there implementation hurdles?
Unlike the EUA for prophylactic use, which targeted unvaccinated individuals and those unlikely to have a good antibody response to vaccine, use of monoclonal antibody for prevention of progression does not have such restrictions. Effectiveness may vary by local variant susceptibility and should be considered in the choice of the most appropriate monoclonal antibody therapy. Reductions in hospitalization and progression to critical disease status were reported from phase 3 studies; reductions were also observed in mortality in some, but not all, studies. Enhanced viral clearance on day 7 was observed with few subjects having persistent high viral load.
Which children qualify under the EUA? Adolescents 12 years and older and over 40 kg are eligible if a high risk condition is present. High-risk conditions include body mass index at the 85th percentile or higher, immunosuppressive disease, or receipt of immunosuppressive therapies, or baseline (pre-COVID infection) medical-related technological dependence such as tracheostomy or positive pressure ventilation. Additional high-risk conditions are neurodevelopmental disorders, sickle cell disease, congenital or acquired heart disease, asthma, or reactive airway or other chronic respiratory disease that requires daily medication for control, diabetes, chronic kidney disease, or pregnancy.3
Are the available monoclonal antibodies effective for SARS-CoV-2 variants? Of course, this is a critical question and relies on knowledge of the dominant variant in a specific geographic location. The CDC data on which variants are susceptible to which monoclonal therapies were updated as of Oct. 21 online (see Table 1). Local departments of public health often will have current data on the dominant variant in the community. Currently, the dominant variant in the United States is Delta and it is anticipated to be susceptible to the three monoclonal treatments authorized under the EUA based on in vitro neutralizing assays.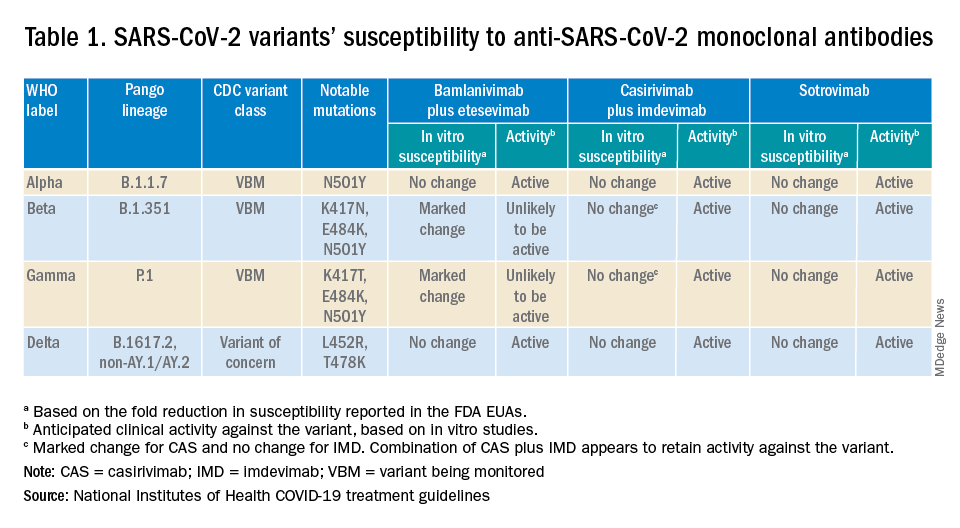
What adverse events were observed? Monoclonal antibody infusions are in general safe but anaphylaxis has been reported. Other infusion-related adverse events include urticaria, pruritis, flushing, pyrexia, shortness of breath, chest tightness, nausea, vomiting, and rash. Nearly all events were grade 1, mild, or grade 2, moderate. For nonsevere infusion-related reactions, consider slowing the infusion; if necessary, the infusion should be stopped.
Implementation challenges
The first challenge is finding a location to infuse the monoclonal antibodies. Although they can be given subcutaneously, the dose is large and little, if any, time is saved as the recommendation is for observation post administration for 1 hour. The challenge we and other centers may face is that the patients are COVID PCR+ and therefore our usual infusion program, which often is occupied by individuals already compromised and at high risk for severe COVID, is an undesirable location. We are planning to use the emergency department to accommodate such patients currently, but even that solution creates challenges for a busy, urban medical center.
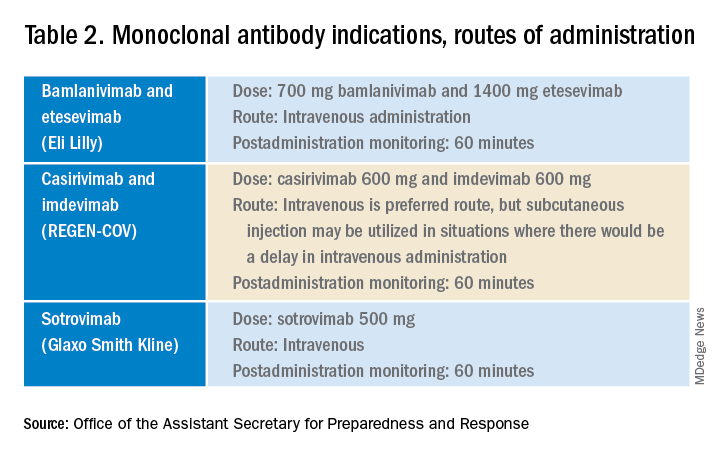
Summary
Anti–SARS-CoV-2 monoclonal antibodies are an important part of the therapeutic approach to minimizing disease severity. Clinicians should review high-risk conditions in adolescents who are PCR+ for SARS-CoV-2 and have mild to moderate symptoms. Medical care systems should implement programs to make monoclonal infusions available for such high-risk adolescents.4 Obesity and asthma reactive airways or requiring daily medication for control are the two most common conditions that place adolescents with COVID-19 at risk for progression to hospitalization and severe disease in addition to the more traditional immune-compromising conditions and medical fragility.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at pdnews@mdedge.com.
References
1. Federal Response to COVID-19: Monoclonal Antibody Clinical Implementation Guide. U.S. Department of Health and Human Services. 2021 Sep 2.
2. Bhimraj A et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Last updated 2021 Nov 9.
3. Anti-SARS-CoV-2 Monoclonal Antibodies. National Institutes of Health’s COVID 19 Treatment Guidelines. Last updated 2021 Oct 19.
4. Spreading the Word on the Benefits of Monoclonal Antibodies for COVID-19, by Hannah R. Buchdahl. CDC Foundation, 2021 Jul 2.
I was recently asked to see a 16-year-old, unvaccinated (against COVID-19) adolescent with hypothyroidism and obesity (body mass index 37 kg/m2) seen in the pediatric emergency department with tachycardia, O2 saturation 96%, urinary tract infection, poor appetite, and nausea. Her chest x-ray had low lung volumes but no infiltrates. She was noted to be dehydrated. Testing for COVID-19 was PCR positive.1
She was observed overnight, tolerated oral rehydration, and was being readied for discharge. Pediatric Infectious Diseases was called about prescribing remdesivir.
Remdesivir was not indicated as its current use is limited to inpatients with oxygen desaturations less than 94%. Infectious Diseases Society of America guidelines do recommend the use of monoclonal antibodies against the SARS-CoV-2 spike protein for prevention of COVID disease progression in high-risk individuals. Specifically, the IDSA guidelines say, “Among ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease, bamlanivimab/etesevimab, casirivimab/imdevimab, or sotrovimab rather than no neutralizing antibody treatment.”
The Food and Drug Administration’s Emergency Use Authorization (EUA) allowed use of specific monoclonal antibodies (casirivimab/imdevimab in combination, bamlanivimab/etesevimab in combination, and sotrovimab alone) for individuals 12 years and above with a minimum weight of 40 kg with high-risk conditions, describing the evidence as moderate certainty.2
Several questions have arisen regarding their use. Which children qualify under the EUA? Are the available monoclonal antibodies effective for SARS-CoV-2 variants? What adverse events were observed? Are there implementation hurdles?
Unlike the EUA for prophylactic use, which targeted unvaccinated individuals and those unlikely to have a good antibody response to vaccine, use of monoclonal antibody for prevention of progression does not have such restrictions. Effectiveness may vary by local variant susceptibility and should be considered in the choice of the most appropriate monoclonal antibody therapy. Reductions in hospitalization and progression to critical disease status were reported from phase 3 studies; reductions were also observed in mortality in some, but not all, studies. Enhanced viral clearance on day 7 was observed with few subjects having persistent high viral load.
Which children qualify under the EUA? Adolescents 12 years and older and over 40 kg are eligible if a high risk condition is present. High-risk conditions include body mass index at the 85th percentile or higher, immunosuppressive disease, or receipt of immunosuppressive therapies, or baseline (pre-COVID infection) medical-related technological dependence such as tracheostomy or positive pressure ventilation. Additional high-risk conditions are neurodevelopmental disorders, sickle cell disease, congenital or acquired heart disease, asthma, or reactive airway or other chronic respiratory disease that requires daily medication for control, diabetes, chronic kidney disease, or pregnancy.3
Are the available monoclonal antibodies effective for SARS-CoV-2 variants? Of course, this is a critical question and relies on knowledge of the dominant variant in a specific geographic location. The CDC data on which variants are susceptible to which monoclonal therapies were updated as of Oct. 21 online (see Table 1). Local departments of public health often will have current data on the dominant variant in the community. Currently, the dominant variant in the United States is Delta and it is anticipated to be susceptible to the three monoclonal treatments authorized under the EUA based on in vitro neutralizing assays.
What adverse events were observed? Monoclonal antibody infusions are in general safe but anaphylaxis has been reported. Other infusion-related adverse events include urticaria, pruritis, flushing, pyrexia, shortness of breath, chest tightness, nausea, vomiting, and rash. Nearly all events were grade 1, mild, or grade 2, moderate. For nonsevere infusion-related reactions, consider slowing the infusion; if necessary, the infusion should be stopped.
Implementation challenges
The first challenge is finding a location to infuse the monoclonal antibodies. Although they can be given subcutaneously, the dose is large and little, if any, time is saved as the recommendation is for observation post administration for 1 hour. The challenge we and other centers may face is that the patients are COVID PCR+ and therefore our usual infusion program, which often is occupied by individuals already compromised and at high risk for severe COVID, is an undesirable location. We are planning to use the emergency department to accommodate such patients currently, but even that solution creates challenges for a busy, urban medical center.

Summary
Anti–SARS-CoV-2 monoclonal antibodies are an important part of the therapeutic approach to minimizing disease severity. Clinicians should review high-risk conditions in adolescents who are PCR+ for SARS-CoV-2 and have mild to moderate symptoms. Medical care systems should implement programs to make monoclonal infusions available for such high-risk adolescents.4 Obesity and asthma reactive airways or requiring daily medication for control are the two most common conditions that place adolescents with COVID-19 at risk for progression to hospitalization and severe disease in addition to the more traditional immune-compromising conditions and medical fragility.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at pdnews@mdedge.com.
References
1. Federal Response to COVID-19: Monoclonal Antibody Clinical Implementation Guide. U.S. Department of Health and Human Services. 2021 Sep 2.
2. Bhimraj A et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Last updated 2021 Nov 9.
3. Anti-SARS-CoV-2 Monoclonal Antibodies. National Institutes of Health’s COVID 19 Treatment Guidelines. Last updated 2021 Oct 19.
4. Spreading the Word on the Benefits of Monoclonal Antibodies for COVID-19, by Hannah R. Buchdahl. CDC Foundation, 2021 Jul 2.
I was recently asked to see a 16-year-old, unvaccinated (against COVID-19) adolescent with hypothyroidism and obesity (body mass index 37 kg/m2) seen in the pediatric emergency department with tachycardia, O2 saturation 96%, urinary tract infection, poor appetite, and nausea. Her chest x-ray had low lung volumes but no infiltrates. She was noted to be dehydrated. Testing for COVID-19 was PCR positive.1
She was observed overnight, tolerated oral rehydration, and was being readied for discharge. Pediatric Infectious Diseases was called about prescribing remdesivir.
Remdesivir was not indicated as its current use is limited to inpatients with oxygen desaturations less than 94%. Infectious Diseases Society of America guidelines do recommend the use of monoclonal antibodies against the SARS-CoV-2 spike protein for prevention of COVID disease progression in high-risk individuals. Specifically, the IDSA guidelines say, “Among ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease, bamlanivimab/etesevimab, casirivimab/imdevimab, or sotrovimab rather than no neutralizing antibody treatment.”
The Food and Drug Administration’s Emergency Use Authorization (EUA) allowed use of specific monoclonal antibodies (casirivimab/imdevimab in combination, bamlanivimab/etesevimab in combination, and sotrovimab alone) for individuals 12 years and above with a minimum weight of 40 kg with high-risk conditions, describing the evidence as moderate certainty.2
Several questions have arisen regarding their use. Which children qualify under the EUA? Are the available monoclonal antibodies effective for SARS-CoV-2 variants? What adverse events were observed? Are there implementation hurdles?
Unlike the EUA for prophylactic use, which targeted unvaccinated individuals and those unlikely to have a good antibody response to vaccine, use of monoclonal antibody for prevention of progression does not have such restrictions. Effectiveness may vary by local variant susceptibility and should be considered in the choice of the most appropriate monoclonal antibody therapy. Reductions in hospitalization and progression to critical disease status were reported from phase 3 studies; reductions were also observed in mortality in some, but not all, studies. Enhanced viral clearance on day 7 was observed with few subjects having persistent high viral load.
Which children qualify under the EUA? Adolescents 12 years and older and over 40 kg are eligible if a high risk condition is present. High-risk conditions include body mass index at the 85th percentile or higher, immunosuppressive disease, or receipt of immunosuppressive therapies, or baseline (pre-COVID infection) medical-related technological dependence such as tracheostomy or positive pressure ventilation. Additional high-risk conditions are neurodevelopmental disorders, sickle cell disease, congenital or acquired heart disease, asthma, or reactive airway or other chronic respiratory disease that requires daily medication for control, diabetes, chronic kidney disease, or pregnancy.3
Are the available monoclonal antibodies effective for SARS-CoV-2 variants? Of course, this is a critical question and relies on knowledge of the dominant variant in a specific geographic location. The CDC data on which variants are susceptible to which monoclonal therapies were updated as of Oct. 21 online (see Table 1). Local departments of public health often will have current data on the dominant variant in the community. Currently, the dominant variant in the United States is Delta and it is anticipated to be susceptible to the three monoclonal treatments authorized under the EUA based on in vitro neutralizing assays.
What adverse events were observed? Monoclonal antibody infusions are in general safe but anaphylaxis has been reported. Other infusion-related adverse events include urticaria, pruritis, flushing, pyrexia, shortness of breath, chest tightness, nausea, vomiting, and rash. Nearly all events were grade 1, mild, or grade 2, moderate. For nonsevere infusion-related reactions, consider slowing the infusion; if necessary, the infusion should be stopped.
Implementation challenges
The first challenge is finding a location to infuse the monoclonal antibodies. Although they can be given subcutaneously, the dose is large and little, if any, time is saved as the recommendation is for observation post administration for 1 hour. The challenge we and other centers may face is that the patients are COVID PCR+ and therefore our usual infusion program, which often is occupied by individuals already compromised and at high risk for severe COVID, is an undesirable location. We are planning to use the emergency department to accommodate such patients currently, but even that solution creates challenges for a busy, urban medical center.

Summary
Anti–SARS-CoV-2 monoclonal antibodies are an important part of the therapeutic approach to minimizing disease severity. Clinicians should review high-risk conditions in adolescents who are PCR+ for SARS-CoV-2 and have mild to moderate symptoms. Medical care systems should implement programs to make monoclonal infusions available for such high-risk adolescents.4 Obesity and asthma reactive airways or requiring daily medication for control are the two most common conditions that place adolescents with COVID-19 at risk for progression to hospitalization and severe disease in addition to the more traditional immune-compromising conditions and medical fragility.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at pdnews@mdedge.com.
References
1. Federal Response to COVID-19: Monoclonal Antibody Clinical Implementation Guide. U.S. Department of Health and Human Services. 2021 Sep 2.
2. Bhimraj A et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Last updated 2021 Nov 9.
3. Anti-SARS-CoV-2 Monoclonal Antibodies. National Institutes of Health’s COVID 19 Treatment Guidelines. Last updated 2021 Oct 19.
4. Spreading the Word on the Benefits of Monoclonal Antibodies for COVID-19, by Hannah R. Buchdahl. CDC Foundation, 2021 Jul 2.









