User login
Confronting the epidemic of racism in ObGyn practice
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
Physician leadership: Racial disparities and racism. Where do we go from here?
The destructive toll COVID-19 has caused worldwide is devastating. In the United States, the disproportionate deaths of Black, Indigenous, and Latinx people due to structural racism, amplified by economic adversity, is unacceptable. Meanwhile, the continued murder of Black people by those sworn to protect the public is abhorrent and can no longer be ignored. Black lives matter. These crises have rightly gripped our attention, and should galvanize physicians individually and collectively to use our privileged voices and relative power for justice. We must strive for engaged, passionate, and innovative leadership deliberately aimed toward antiracism and equity.
The COVID-19 pandemic has illuminated the vast inequities in our country. It has highlighted the continued poor outcomes our health and health care systems create for Black, Indigenous, and Latinx communities. It also has demonstrated clearly that we are all connected—one large community, interdependent yet rife with differential power, privilege, and oppression. We must address these racial disparities—not only in the name of justice and good health for all but also because it is a moral and ethical imperative for us as physicians—and SARS-CoV-2 clearly shows us that it is in the best interest of everyone to do so.
First step: A deep dive look at systemic racism
What is first needed is an examination and acknowledgement by medicine and health care at large of the deeply entrenched roots of systemic and institutional racism in our profession and care systems, and their disproportionate and unjust impact on the health and livelihood of communities of color. The COVID-19 pandemic is only a recent example that highlights the perpetuation of a system that harms people of color. Racism, sexism, gender discrimination, economic and social injustice, religious persecution, and violence against women and children are age-old. We have yet to see health care institutions implement system-wide intersectional and antiracist practices to address them. Mandatory implicit bias training, policies for inclusion and diversity, and position statements are necessary first steps; however, they are not a panacea. They are insufficient to create the bold changes we need. The time for words has long passed. It is time to listen, to hear the cries of anguish and outrage, to examine our privileged position, to embrace change and discomfort, and most importantly to act, and to lead in dismantling the structures around us that perpetuate racial inequity.
How can we, as physicians and leaders, join in action and make an impact?
Dr. Camara Jones, past president of the American Public Health Association, describes 3 levels of racism:
- structural or systemic
- individual or personally mediated
- internalized.
Interventions at each level are important if we are to promote equity in health and health care. This framework can help us think about the following strategic initiatives.
Continue to: 1. Commit to becoming an antiracist and engage in independent study...
1. Commit to becoming antiracist and engage in independent study. This is an important first step as it will form the foundations for interventions—one cannot facilitate change without understanding the matter at hand. This step also may be the most personally challenging step forcing all of us to wrestle with discomfort, sadness, fear, guilt, and a host of other emotional responses. Remember that great change has never been born out of comfort, and the discomfort physicians may experience while unlearning racism and learning antiracism pales in comparison to what communities of color experience daily. We must actively work to unlearn the racist and anti-Black culture that is so deeply woven into every aspect of our existence.
Learn the history that was not given to us as kids in school. Read the brilliant literary works of Black, Indigenous, and Latinx artists and scholars on dismantling racism. Expand our vocabulary and knowledge of core concepts in racism, racial justice, and equity. Examine and reflect on our day-to-day practices. Be vocal in our commitment to antiracism—the time has passed for staying silent. If you are white, facilitate conversations about race with your white colleagues; the inherent power of racism relegates it to an issue that can never be on the table, but it is time to dismantle that power. Learn what acts of meaningful and intentional alliances are and when we need to give up power or privilege to a person of color. We also need to recognize that we as physicians, while leaders in many spaces, are not leaders in the powerful racial justice grassroots movements. We should learn from these movements, follow their lead, and use our privilege to uplift racial justice in our settings.
2. Embrace the current complexities with empathy and humility, finding ways to exercise our civic responsibility to the public with compassion. During the COVID-19 pandemic we have seen the devastation that social isolation, job loss, and illness can create. Suddenly those who could never have imagined themselves without food are waiting hours in their cars for food bank donations or are finding empty shelves in stores. Those who were not safe at home were suddenly imprisoned indefinitely in unsafe situations. Those who were comfortable, well-insured, and healthy are facing an invisible health threat, insecurity, fear, anxiety, and loss. Additionally, our civic institutions are failing. Those of us who always took our right to vote for granted are being forced to stand in hours’-long lines to exercise that right; while those who have been systematically disenfranchised are enduring even greater threats to their constitutional right to exercise their political power, disallowing them to speak for their families and communities and to vote for the justice they deserve. This may be an opportunity to stop blaming victims and recognize the toll that structural and systemic contributions to inequity have created over generations.
3. Meaningfully engage with and advocate for patients. In health and health care, we must begin to engage with the communities we serve and truly listen to their needs, desires, and barriers to care, and respond accordingly. Policies that try to address the social determinants of health without that engagement, and without the acknowledgement of the structural issues that cause them, however well-intentioned, are unlikely to accomplish their goals. We need to advocate as physicians and leaders in our settings for every policy, practice, and procedure to be scrutinized using an antiracist lens. To execute this, we need to:
- ask why clinic and hospital practices are built the way they are and how to make them more reflexive and responsive to individual patient’s needs
- examine what the disproportionate impacts might be on different groups of patients from a systems-level
- be ready to dismantle and/or rebuild something that is exacerbating disparate outcomes and experiences
- advocate for change that is built upon the narratives of patients and their communities.
We should include patients in the creation of hospital policies and guidelines in order to shift power toward them and to be transparent about how the system operates in order to facilitate trust and collaboration that centers patients and communities in the systems created to serve them.
Continue to: 4. Intentionally repair and build trust...
4. Intentionally repair and build trust. To create a safe environment, we must repair what we have broken and earn the trust of communities by uplifting their voices and redistributing our power to them in changing the systems and structures that have, for generations, kept Black, Indigenous, and Latinx people oppressed. Building trust requires first owning our histories of colonization, genocide, and slavery—now turned mass incarceration, debasement, and exploitation—that has existed for centuries. We as physicians need to do an honest examination of how we have eroded the trust of the very communities we care for since our profession’s creation. We need to acknowledge, as a white-dominant profession, the medical experimentation on and exploitation of Black and Brown bodies, and how this formed the foundation for a very valid deep distrust and fear of the medical establishment. We need to recognize how our inherent racial biases continue to feed this distrust, like when we don’t treat patients’ pain adequately or make them feel like we believe and listen to their needs and concerns. We must acknowledge our complicity in perpetuating the racial inequities in health, again highlighted by the COVID-19 pandemic.
5. Increase Black, Indigenous, and Latinx representation in physician and other health care professions’ workforce. Racism impacts not only patients but also our colleagues of color. The lack of racial diversity is a symptom of racism and a representation of the continued exclusion and devaluing of physicians of color. We must recognize this legacy of exclusion and facilitate intentional recruitment, retention, inclusion, and belonging of people of color into our workforce. Tokenism, the act of symbolically including one or few people from underrepresented groups, has been a weapon used by our workforce against physicians of color, resulting in isolation, “othering,” demoralization, and other deleterious impacts. We need to reverse this history and diversify our training programs and workforce to ensure justice in our own community.
6. Design multifaceted interventions. Multilevel problems require multilevel solutions. Interventions targeted solely at one level, while helpful, are unlikely to result in the larger scale changes our society needs to implement if we are to eradicate the impact of racism on health. We have long known that it is not just “preexisting conditions” or “poor” individual behaviors that lead to negative and disparate health outcomes—these are impacted by social and structural determinants much larger and more deleterious than that. It is critically important that we allocate and redistribute resources to create safe and affordable housing; childcare and preschool facilities; healthy, available, and affordable food; equitable and affordable educational opportunities; and a clean environment to support the health of all communities—not only those with the highest tax base. It is imperative that we strive to understand the lives of our fellow human beings who have been subjected to intergenerational social injustices and oppressions that have continued to place them at the margins of society. We need to center the lived experiences of communities of color in the design of multilevel interventions, especially Black and Indigenous communities. While we as physicians cannot individually impact education, economic, or food/environment systems, we can use our power to advocate for providing resources for the patients we care for and can create strategies within the health care system to address these needs in order to achieve optimal health. Robust and equitable social structures are the foundations for health, and ensuring equitable access to them is critical to reducing disparities.
Commit to lead
We must commit to unlearning our internalized racism, rebuilding relationships with communities of color, and engaging in antiracist practices. As a profession dedicated to healing, we have an obligation to be leaders in advocating for these changes, and dismantling the inequitable structure of our health care system.
Our challenge now is to articulate solutions. While antiracism should be informed by the lived experiences of communities of color, the work of antiracism is not their responsibility. In fact, it is the responsibility of our white-dominated systems and institutions to change.
There are some solutions that are easier to enumerate because they have easily measurable outcomes or activities, such as:
- collecting data transparently
- identifying inequities in access, treatment, and care
- conducting rigorous root cause analysis of those barriers to care
- increasing diverse racial and gender representation on decision-making bodies, from board rooms to committees, from leadership teams to research participants
- redistribute power by paving the way for underrepresented colleagues to participate in clinical, administrative, educational, executive, and health policy spaces
- mentoring new leaders who come from marginalized communities.
Every patient deserves our expertise and access to high-quality care. We should review our patient panels to ensure we are taking steps personally to be just and eliminate disparities, and we should monitor the results of those efforts.
Continue to: Be open to solutions that may make us “uncomfortable”...
Be open to solutions that may make us “uncomfortable”
There are other solutions, perhaps those that would be more effective on a larger scale, which may be harder to measure using our traditional ways of inquiry or measurement. Solutions that may create discomfort, anger, or fear for those who have held their power or positions for a long time. We need to begin to engage in developing, cultivating, and valuing innovative strategies that produce equally valid knowledge, evidence, and solutions without engaging in a randomized controlled trial. We need to reinvent the way inquiry, investigation, and implementation are done, and utilize novel, justice-informed strategies that include real-world evidence to produce results that are applicable to all (not just those willing to participate in sponsored trials). Only then will we be able to provide equitable health outcomes for all.
We also must accept responsibility for the past and humbly ask communities to work with us as we struggle to eliminate racism and dehumanization of Black lives by calling out our actions or inaction, recognizing the impact of our privileged status, and stepping down or stepping aside to allow others to lead. Sometimes it is as simple as turning off the Zoom camera so others can talk. By redistributing power and focusing this work upon the narratives of marginalized communities, we can improve our system for everyone. We must lead with action within our practices and systems; become advocates within our communities, institutions, and profession; strategize and organize interventions at both structural and individual levels to first recognize and name—then change—the systems; and unlearn behaviors that perpetuate racism.
Inaction is shirking our responsibility among the medical community
Benign inaction and unintentional acquiescence with “the way things are and have always been” abdicates our responsibility as physicians to improve the health of our patients and our communities. The modern Hippocratic Oath reminds us: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.” We have a professional and ethical responsibility to ensure health equity, and thus racial equity. As physicians, as healers, as leaders we must address racial inequities at all levels as we commit to improving the health of our nation. We can no longer stand silent in the face of the violence, brutality, and injustices our patients, friends, family, neighbors, communities, and society as a whole live through daily. It is unjust and inhumane to do so.
To be silent is to be complicit. As Gandhi said so long ago, we must “be the change we wish to see in the world.” And as Ijeoma Olua teaches us, “Anti-racism is the commitment to fight racism wherever you find it, including in yourself. And it’s the only way forward.”
- “So You Want to Talk about Race” Ijeoma Oluo
- “How to Be an Antiracist” Ibram X. Kendi
- “Between the World and Me” Ta-Nehisi Coates
- A conversation on race and privilege (Angela Davis and Jane Elliot) https://www.youtube.com/watch?reload=9&v=S0jf8D5WHoo
- Uncomfortable conversations with a Black man (Emmanuel Acho) https://www.youtube.com/watch?v=h8jUA7JBkF4
Antiracism – defined as the work of actively opposing racism by advocating for changes in political, economic, and social life. Antiracism tends to be an individualized approach, and set up in opposition to individual racist behaviors and impacts
Black Lives Matter – a political movement to address systemic and state violence against African Americans. Per the Black Lives Matter organizers: “In 2013, three radical Black organizers—Alicia Garza, Patrisse Cullors, and Opal Tometi—created a Black-centered political will and movement building project called BlackLivesMatter. It was in response to the acquittal of Trayvon Martin’s murderer, George Zimmerman. The project is now a member-led global network of more than 40 chapters. Members organize and build local power to intervene in violence inflicted on Black communities by the state and vigilantes. Black Lives Matter is an ideological and political intervention in a world where Black lives are systematically and intentionally targeted for demise. It is an affirmation of Black folks’ humanity, our contributions to this society, and our resilience in the face of deadly oppression.”
Implicit bias – also known as unconscious or hidden bias, implicit biases are negative associations that people unknowingly hold. They are expressed automatically, without conscious awareness. Many studies have indicated that implicit biases affect individuals’ attitudes and actions, thus creating real-world implications, even though individuals may not even be aware that those biases exist within themselves. Notably, implicit biases have been shown to trump individuals stated commitments to equality and fairness, thereby producing behavior that diverges from the explicit attitudes that many people profess.
Othering – view or treat (a person or group of people) as intrinsically different from and alien to oneself. (From https://lexico.com.)
For a full glossary of terms, visit RacialEquityTools.org (https://www.racialequitytools.org/glossary#anti-black)
The destructive toll COVID-19 has caused worldwide is devastating. In the United States, the disproportionate deaths of Black, Indigenous, and Latinx people due to structural racism, amplified by economic adversity, is unacceptable. Meanwhile, the continued murder of Black people by those sworn to protect the public is abhorrent and can no longer be ignored. Black lives matter. These crises have rightly gripped our attention, and should galvanize physicians individually and collectively to use our privileged voices and relative power for justice. We must strive for engaged, passionate, and innovative leadership deliberately aimed toward antiracism and equity.
The COVID-19 pandemic has illuminated the vast inequities in our country. It has highlighted the continued poor outcomes our health and health care systems create for Black, Indigenous, and Latinx communities. It also has demonstrated clearly that we are all connected—one large community, interdependent yet rife with differential power, privilege, and oppression. We must address these racial disparities—not only in the name of justice and good health for all but also because it is a moral and ethical imperative for us as physicians—and SARS-CoV-2 clearly shows us that it is in the best interest of everyone to do so.
First step: A deep dive look at systemic racism
What is first needed is an examination and acknowledgement by medicine and health care at large of the deeply entrenched roots of systemic and institutional racism in our profession and care systems, and their disproportionate and unjust impact on the health and livelihood of communities of color. The COVID-19 pandemic is only a recent example that highlights the perpetuation of a system that harms people of color. Racism, sexism, gender discrimination, economic and social injustice, religious persecution, and violence against women and children are age-old. We have yet to see health care institutions implement system-wide intersectional and antiracist practices to address them. Mandatory implicit bias training, policies for inclusion and diversity, and position statements are necessary first steps; however, they are not a panacea. They are insufficient to create the bold changes we need. The time for words has long passed. It is time to listen, to hear the cries of anguish and outrage, to examine our privileged position, to embrace change and discomfort, and most importantly to act, and to lead in dismantling the structures around us that perpetuate racial inequity.
How can we, as physicians and leaders, join in action and make an impact?
Dr. Camara Jones, past president of the American Public Health Association, describes 3 levels of racism:
- structural or systemic
- individual or personally mediated
- internalized.
Interventions at each level are important if we are to promote equity in health and health care. This framework can help us think about the following strategic initiatives.
Continue to: 1. Commit to becoming an antiracist and engage in independent study...
1. Commit to becoming antiracist and engage in independent study. This is an important first step as it will form the foundations for interventions—one cannot facilitate change without understanding the matter at hand. This step also may be the most personally challenging step forcing all of us to wrestle with discomfort, sadness, fear, guilt, and a host of other emotional responses. Remember that great change has never been born out of comfort, and the discomfort physicians may experience while unlearning racism and learning antiracism pales in comparison to what communities of color experience daily. We must actively work to unlearn the racist and anti-Black culture that is so deeply woven into every aspect of our existence.
Learn the history that was not given to us as kids in school. Read the brilliant literary works of Black, Indigenous, and Latinx artists and scholars on dismantling racism. Expand our vocabulary and knowledge of core concepts in racism, racial justice, and equity. Examine and reflect on our day-to-day practices. Be vocal in our commitment to antiracism—the time has passed for staying silent. If you are white, facilitate conversations about race with your white colleagues; the inherent power of racism relegates it to an issue that can never be on the table, but it is time to dismantle that power. Learn what acts of meaningful and intentional alliances are and when we need to give up power or privilege to a person of color. We also need to recognize that we as physicians, while leaders in many spaces, are not leaders in the powerful racial justice grassroots movements. We should learn from these movements, follow their lead, and use our privilege to uplift racial justice in our settings.
2. Embrace the current complexities with empathy and humility, finding ways to exercise our civic responsibility to the public with compassion. During the COVID-19 pandemic we have seen the devastation that social isolation, job loss, and illness can create. Suddenly those who could never have imagined themselves without food are waiting hours in their cars for food bank donations or are finding empty shelves in stores. Those who were not safe at home were suddenly imprisoned indefinitely in unsafe situations. Those who were comfortable, well-insured, and healthy are facing an invisible health threat, insecurity, fear, anxiety, and loss. Additionally, our civic institutions are failing. Those of us who always took our right to vote for granted are being forced to stand in hours’-long lines to exercise that right; while those who have been systematically disenfranchised are enduring even greater threats to their constitutional right to exercise their political power, disallowing them to speak for their families and communities and to vote for the justice they deserve. This may be an opportunity to stop blaming victims and recognize the toll that structural and systemic contributions to inequity have created over generations.
3. Meaningfully engage with and advocate for patients. In health and health care, we must begin to engage with the communities we serve and truly listen to their needs, desires, and barriers to care, and respond accordingly. Policies that try to address the social determinants of health without that engagement, and without the acknowledgement of the structural issues that cause them, however well-intentioned, are unlikely to accomplish their goals. We need to advocate as physicians and leaders in our settings for every policy, practice, and procedure to be scrutinized using an antiracist lens. To execute this, we need to:
- ask why clinic and hospital practices are built the way they are and how to make them more reflexive and responsive to individual patient’s needs
- examine what the disproportionate impacts might be on different groups of patients from a systems-level
- be ready to dismantle and/or rebuild something that is exacerbating disparate outcomes and experiences
- advocate for change that is built upon the narratives of patients and their communities.
We should include patients in the creation of hospital policies and guidelines in order to shift power toward them and to be transparent about how the system operates in order to facilitate trust and collaboration that centers patients and communities in the systems created to serve them.
Continue to: 4. Intentionally repair and build trust...
4. Intentionally repair and build trust. To create a safe environment, we must repair what we have broken and earn the trust of communities by uplifting their voices and redistributing our power to them in changing the systems and structures that have, for generations, kept Black, Indigenous, and Latinx people oppressed. Building trust requires first owning our histories of colonization, genocide, and slavery—now turned mass incarceration, debasement, and exploitation—that has existed for centuries. We as physicians need to do an honest examination of how we have eroded the trust of the very communities we care for since our profession’s creation. We need to acknowledge, as a white-dominant profession, the medical experimentation on and exploitation of Black and Brown bodies, and how this formed the foundation for a very valid deep distrust and fear of the medical establishment. We need to recognize how our inherent racial biases continue to feed this distrust, like when we don’t treat patients’ pain adequately or make them feel like we believe and listen to their needs and concerns. We must acknowledge our complicity in perpetuating the racial inequities in health, again highlighted by the COVID-19 pandemic.
5. Increase Black, Indigenous, and Latinx representation in physician and other health care professions’ workforce. Racism impacts not only patients but also our colleagues of color. The lack of racial diversity is a symptom of racism and a representation of the continued exclusion and devaluing of physicians of color. We must recognize this legacy of exclusion and facilitate intentional recruitment, retention, inclusion, and belonging of people of color into our workforce. Tokenism, the act of symbolically including one or few people from underrepresented groups, has been a weapon used by our workforce against physicians of color, resulting in isolation, “othering,” demoralization, and other deleterious impacts. We need to reverse this history and diversify our training programs and workforce to ensure justice in our own community.
6. Design multifaceted interventions. Multilevel problems require multilevel solutions. Interventions targeted solely at one level, while helpful, are unlikely to result in the larger scale changes our society needs to implement if we are to eradicate the impact of racism on health. We have long known that it is not just “preexisting conditions” or “poor” individual behaviors that lead to negative and disparate health outcomes—these are impacted by social and structural determinants much larger and more deleterious than that. It is critically important that we allocate and redistribute resources to create safe and affordable housing; childcare and preschool facilities; healthy, available, and affordable food; equitable and affordable educational opportunities; and a clean environment to support the health of all communities—not only those with the highest tax base. It is imperative that we strive to understand the lives of our fellow human beings who have been subjected to intergenerational social injustices and oppressions that have continued to place them at the margins of society. We need to center the lived experiences of communities of color in the design of multilevel interventions, especially Black and Indigenous communities. While we as physicians cannot individually impact education, economic, or food/environment systems, we can use our power to advocate for providing resources for the patients we care for and can create strategies within the health care system to address these needs in order to achieve optimal health. Robust and equitable social structures are the foundations for health, and ensuring equitable access to them is critical to reducing disparities.
Commit to lead
We must commit to unlearning our internalized racism, rebuilding relationships with communities of color, and engaging in antiracist practices. As a profession dedicated to healing, we have an obligation to be leaders in advocating for these changes, and dismantling the inequitable structure of our health care system.
Our challenge now is to articulate solutions. While antiracism should be informed by the lived experiences of communities of color, the work of antiracism is not their responsibility. In fact, it is the responsibility of our white-dominated systems and institutions to change.
There are some solutions that are easier to enumerate because they have easily measurable outcomes or activities, such as:
- collecting data transparently
- identifying inequities in access, treatment, and care
- conducting rigorous root cause analysis of those barriers to care
- increasing diverse racial and gender representation on decision-making bodies, from board rooms to committees, from leadership teams to research participants
- redistribute power by paving the way for underrepresented colleagues to participate in clinical, administrative, educational, executive, and health policy spaces
- mentoring new leaders who come from marginalized communities.
Every patient deserves our expertise and access to high-quality care. We should review our patient panels to ensure we are taking steps personally to be just and eliminate disparities, and we should monitor the results of those efforts.
Continue to: Be open to solutions that may make us “uncomfortable”...
Be open to solutions that may make us “uncomfortable”
There are other solutions, perhaps those that would be more effective on a larger scale, which may be harder to measure using our traditional ways of inquiry or measurement. Solutions that may create discomfort, anger, or fear for those who have held their power or positions for a long time. We need to begin to engage in developing, cultivating, and valuing innovative strategies that produce equally valid knowledge, evidence, and solutions without engaging in a randomized controlled trial. We need to reinvent the way inquiry, investigation, and implementation are done, and utilize novel, justice-informed strategies that include real-world evidence to produce results that are applicable to all (not just those willing to participate in sponsored trials). Only then will we be able to provide equitable health outcomes for all.
We also must accept responsibility for the past and humbly ask communities to work with us as we struggle to eliminate racism and dehumanization of Black lives by calling out our actions or inaction, recognizing the impact of our privileged status, and stepping down or stepping aside to allow others to lead. Sometimes it is as simple as turning off the Zoom camera so others can talk. By redistributing power and focusing this work upon the narratives of marginalized communities, we can improve our system for everyone. We must lead with action within our practices and systems; become advocates within our communities, institutions, and profession; strategize and organize interventions at both structural and individual levels to first recognize and name—then change—the systems; and unlearn behaviors that perpetuate racism.
Inaction is shirking our responsibility among the medical community
Benign inaction and unintentional acquiescence with “the way things are and have always been” abdicates our responsibility as physicians to improve the health of our patients and our communities. The modern Hippocratic Oath reminds us: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.” We have a professional and ethical responsibility to ensure health equity, and thus racial equity. As physicians, as healers, as leaders we must address racial inequities at all levels as we commit to improving the health of our nation. We can no longer stand silent in the face of the violence, brutality, and injustices our patients, friends, family, neighbors, communities, and society as a whole live through daily. It is unjust and inhumane to do so.
To be silent is to be complicit. As Gandhi said so long ago, we must “be the change we wish to see in the world.” And as Ijeoma Olua teaches us, “Anti-racism is the commitment to fight racism wherever you find it, including in yourself. And it’s the only way forward.”
- “So You Want to Talk about Race” Ijeoma Oluo
- “How to Be an Antiracist” Ibram X. Kendi
- “Between the World and Me” Ta-Nehisi Coates
- A conversation on race and privilege (Angela Davis and Jane Elliot) https://www.youtube.com/watch?reload=9&v=S0jf8D5WHoo
- Uncomfortable conversations with a Black man (Emmanuel Acho) https://www.youtube.com/watch?v=h8jUA7JBkF4
Antiracism – defined as the work of actively opposing racism by advocating for changes in political, economic, and social life. Antiracism tends to be an individualized approach, and set up in opposition to individual racist behaviors and impacts
Black Lives Matter – a political movement to address systemic and state violence against African Americans. Per the Black Lives Matter organizers: “In 2013, three radical Black organizers—Alicia Garza, Patrisse Cullors, and Opal Tometi—created a Black-centered political will and movement building project called BlackLivesMatter. It was in response to the acquittal of Trayvon Martin’s murderer, George Zimmerman. The project is now a member-led global network of more than 40 chapters. Members organize and build local power to intervene in violence inflicted on Black communities by the state and vigilantes. Black Lives Matter is an ideological and political intervention in a world where Black lives are systematically and intentionally targeted for demise. It is an affirmation of Black folks’ humanity, our contributions to this society, and our resilience in the face of deadly oppression.”
Implicit bias – also known as unconscious or hidden bias, implicit biases are negative associations that people unknowingly hold. They are expressed automatically, without conscious awareness. Many studies have indicated that implicit biases affect individuals’ attitudes and actions, thus creating real-world implications, even though individuals may not even be aware that those biases exist within themselves. Notably, implicit biases have been shown to trump individuals stated commitments to equality and fairness, thereby producing behavior that diverges from the explicit attitudes that many people profess.
Othering – view or treat (a person or group of people) as intrinsically different from and alien to oneself. (From https://lexico.com.)
For a full glossary of terms, visit RacialEquityTools.org (https://www.racialequitytools.org/glossary#anti-black)
The destructive toll COVID-19 has caused worldwide is devastating. In the United States, the disproportionate deaths of Black, Indigenous, and Latinx people due to structural racism, amplified by economic adversity, is unacceptable. Meanwhile, the continued murder of Black people by those sworn to protect the public is abhorrent and can no longer be ignored. Black lives matter. These crises have rightly gripped our attention, and should galvanize physicians individually and collectively to use our privileged voices and relative power for justice. We must strive for engaged, passionate, and innovative leadership deliberately aimed toward antiracism and equity.
The COVID-19 pandemic has illuminated the vast inequities in our country. It has highlighted the continued poor outcomes our health and health care systems create for Black, Indigenous, and Latinx communities. It also has demonstrated clearly that we are all connected—one large community, interdependent yet rife with differential power, privilege, and oppression. We must address these racial disparities—not only in the name of justice and good health for all but also because it is a moral and ethical imperative for us as physicians—and SARS-CoV-2 clearly shows us that it is in the best interest of everyone to do so.
First step: A deep dive look at systemic racism
What is first needed is an examination and acknowledgement by medicine and health care at large of the deeply entrenched roots of systemic and institutional racism in our profession and care systems, and their disproportionate and unjust impact on the health and livelihood of communities of color. The COVID-19 pandemic is only a recent example that highlights the perpetuation of a system that harms people of color. Racism, sexism, gender discrimination, economic and social injustice, religious persecution, and violence against women and children are age-old. We have yet to see health care institutions implement system-wide intersectional and antiracist practices to address them. Mandatory implicit bias training, policies for inclusion and diversity, and position statements are necessary first steps; however, they are not a panacea. They are insufficient to create the bold changes we need. The time for words has long passed. It is time to listen, to hear the cries of anguish and outrage, to examine our privileged position, to embrace change and discomfort, and most importantly to act, and to lead in dismantling the structures around us that perpetuate racial inequity.
How can we, as physicians and leaders, join in action and make an impact?
Dr. Camara Jones, past president of the American Public Health Association, describes 3 levels of racism:
- structural or systemic
- individual or personally mediated
- internalized.
Interventions at each level are important if we are to promote equity in health and health care. This framework can help us think about the following strategic initiatives.
Continue to: 1. Commit to becoming an antiracist and engage in independent study...
1. Commit to becoming antiracist and engage in independent study. This is an important first step as it will form the foundations for interventions—one cannot facilitate change without understanding the matter at hand. This step also may be the most personally challenging step forcing all of us to wrestle with discomfort, sadness, fear, guilt, and a host of other emotional responses. Remember that great change has never been born out of comfort, and the discomfort physicians may experience while unlearning racism and learning antiracism pales in comparison to what communities of color experience daily. We must actively work to unlearn the racist and anti-Black culture that is so deeply woven into every aspect of our existence.
Learn the history that was not given to us as kids in school. Read the brilliant literary works of Black, Indigenous, and Latinx artists and scholars on dismantling racism. Expand our vocabulary and knowledge of core concepts in racism, racial justice, and equity. Examine and reflect on our day-to-day practices. Be vocal in our commitment to antiracism—the time has passed for staying silent. If you are white, facilitate conversations about race with your white colleagues; the inherent power of racism relegates it to an issue that can never be on the table, but it is time to dismantle that power. Learn what acts of meaningful and intentional alliances are and when we need to give up power or privilege to a person of color. We also need to recognize that we as physicians, while leaders in many spaces, are not leaders in the powerful racial justice grassroots movements. We should learn from these movements, follow their lead, and use our privilege to uplift racial justice in our settings.
2. Embrace the current complexities with empathy and humility, finding ways to exercise our civic responsibility to the public with compassion. During the COVID-19 pandemic we have seen the devastation that social isolation, job loss, and illness can create. Suddenly those who could never have imagined themselves without food are waiting hours in their cars for food bank donations or are finding empty shelves in stores. Those who were not safe at home were suddenly imprisoned indefinitely in unsafe situations. Those who were comfortable, well-insured, and healthy are facing an invisible health threat, insecurity, fear, anxiety, and loss. Additionally, our civic institutions are failing. Those of us who always took our right to vote for granted are being forced to stand in hours’-long lines to exercise that right; while those who have been systematically disenfranchised are enduring even greater threats to their constitutional right to exercise their political power, disallowing them to speak for their families and communities and to vote for the justice they deserve. This may be an opportunity to stop blaming victims and recognize the toll that structural and systemic contributions to inequity have created over generations.
3. Meaningfully engage with and advocate for patients. In health and health care, we must begin to engage with the communities we serve and truly listen to their needs, desires, and barriers to care, and respond accordingly. Policies that try to address the social determinants of health without that engagement, and without the acknowledgement of the structural issues that cause them, however well-intentioned, are unlikely to accomplish their goals. We need to advocate as physicians and leaders in our settings for every policy, practice, and procedure to be scrutinized using an antiracist lens. To execute this, we need to:
- ask why clinic and hospital practices are built the way they are and how to make them more reflexive and responsive to individual patient’s needs
- examine what the disproportionate impacts might be on different groups of patients from a systems-level
- be ready to dismantle and/or rebuild something that is exacerbating disparate outcomes and experiences
- advocate for change that is built upon the narratives of patients and their communities.
We should include patients in the creation of hospital policies and guidelines in order to shift power toward them and to be transparent about how the system operates in order to facilitate trust and collaboration that centers patients and communities in the systems created to serve them.
Continue to: 4. Intentionally repair and build trust...
4. Intentionally repair and build trust. To create a safe environment, we must repair what we have broken and earn the trust of communities by uplifting their voices and redistributing our power to them in changing the systems and structures that have, for generations, kept Black, Indigenous, and Latinx people oppressed. Building trust requires first owning our histories of colonization, genocide, and slavery—now turned mass incarceration, debasement, and exploitation—that has existed for centuries. We as physicians need to do an honest examination of how we have eroded the trust of the very communities we care for since our profession’s creation. We need to acknowledge, as a white-dominant profession, the medical experimentation on and exploitation of Black and Brown bodies, and how this formed the foundation for a very valid deep distrust and fear of the medical establishment. We need to recognize how our inherent racial biases continue to feed this distrust, like when we don’t treat patients’ pain adequately or make them feel like we believe and listen to their needs and concerns. We must acknowledge our complicity in perpetuating the racial inequities in health, again highlighted by the COVID-19 pandemic.
5. Increase Black, Indigenous, and Latinx representation in physician and other health care professions’ workforce. Racism impacts not only patients but also our colleagues of color. The lack of racial diversity is a symptom of racism and a representation of the continued exclusion and devaluing of physicians of color. We must recognize this legacy of exclusion and facilitate intentional recruitment, retention, inclusion, and belonging of people of color into our workforce. Tokenism, the act of symbolically including one or few people from underrepresented groups, has been a weapon used by our workforce against physicians of color, resulting in isolation, “othering,” demoralization, and other deleterious impacts. We need to reverse this history and diversify our training programs and workforce to ensure justice in our own community.
6. Design multifaceted interventions. Multilevel problems require multilevel solutions. Interventions targeted solely at one level, while helpful, are unlikely to result in the larger scale changes our society needs to implement if we are to eradicate the impact of racism on health. We have long known that it is not just “preexisting conditions” or “poor” individual behaviors that lead to negative and disparate health outcomes—these are impacted by social and structural determinants much larger and more deleterious than that. It is critically important that we allocate and redistribute resources to create safe and affordable housing; childcare and preschool facilities; healthy, available, and affordable food; equitable and affordable educational opportunities; and a clean environment to support the health of all communities—not only those with the highest tax base. It is imperative that we strive to understand the lives of our fellow human beings who have been subjected to intergenerational social injustices and oppressions that have continued to place them at the margins of society. We need to center the lived experiences of communities of color in the design of multilevel interventions, especially Black and Indigenous communities. While we as physicians cannot individually impact education, economic, or food/environment systems, we can use our power to advocate for providing resources for the patients we care for and can create strategies within the health care system to address these needs in order to achieve optimal health. Robust and equitable social structures are the foundations for health, and ensuring equitable access to them is critical to reducing disparities.
Commit to lead
We must commit to unlearning our internalized racism, rebuilding relationships with communities of color, and engaging in antiracist practices. As a profession dedicated to healing, we have an obligation to be leaders in advocating for these changes, and dismantling the inequitable structure of our health care system.
Our challenge now is to articulate solutions. While antiracism should be informed by the lived experiences of communities of color, the work of antiracism is not their responsibility. In fact, it is the responsibility of our white-dominated systems and institutions to change.
There are some solutions that are easier to enumerate because they have easily measurable outcomes or activities, such as:
- collecting data transparently
- identifying inequities in access, treatment, and care
- conducting rigorous root cause analysis of those barriers to care
- increasing diverse racial and gender representation on decision-making bodies, from board rooms to committees, from leadership teams to research participants
- redistribute power by paving the way for underrepresented colleagues to participate in clinical, administrative, educational, executive, and health policy spaces
- mentoring new leaders who come from marginalized communities.
Every patient deserves our expertise and access to high-quality care. We should review our patient panels to ensure we are taking steps personally to be just and eliminate disparities, and we should monitor the results of those efforts.
Continue to: Be open to solutions that may make us “uncomfortable”...
Be open to solutions that may make us “uncomfortable”
There are other solutions, perhaps those that would be more effective on a larger scale, which may be harder to measure using our traditional ways of inquiry or measurement. Solutions that may create discomfort, anger, or fear for those who have held their power or positions for a long time. We need to begin to engage in developing, cultivating, and valuing innovative strategies that produce equally valid knowledge, evidence, and solutions without engaging in a randomized controlled trial. We need to reinvent the way inquiry, investigation, and implementation are done, and utilize novel, justice-informed strategies that include real-world evidence to produce results that are applicable to all (not just those willing to participate in sponsored trials). Only then will we be able to provide equitable health outcomes for all.
We also must accept responsibility for the past and humbly ask communities to work with us as we struggle to eliminate racism and dehumanization of Black lives by calling out our actions or inaction, recognizing the impact of our privileged status, and stepping down or stepping aside to allow others to lead. Sometimes it is as simple as turning off the Zoom camera so others can talk. By redistributing power and focusing this work upon the narratives of marginalized communities, we can improve our system for everyone. We must lead with action within our practices and systems; become advocates within our communities, institutions, and profession; strategize and organize interventions at both structural and individual levels to first recognize and name—then change—the systems; and unlearn behaviors that perpetuate racism.
Inaction is shirking our responsibility among the medical community
Benign inaction and unintentional acquiescence with “the way things are and have always been” abdicates our responsibility as physicians to improve the health of our patients and our communities. The modern Hippocratic Oath reminds us: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.” We have a professional and ethical responsibility to ensure health equity, and thus racial equity. As physicians, as healers, as leaders we must address racial inequities at all levels as we commit to improving the health of our nation. We can no longer stand silent in the face of the violence, brutality, and injustices our patients, friends, family, neighbors, communities, and society as a whole live through daily. It is unjust and inhumane to do so.
To be silent is to be complicit. As Gandhi said so long ago, we must “be the change we wish to see in the world.” And as Ijeoma Olua teaches us, “Anti-racism is the commitment to fight racism wherever you find it, including in yourself. And it’s the only way forward.”
- “So You Want to Talk about Race” Ijeoma Oluo
- “How to Be an Antiracist” Ibram X. Kendi
- “Between the World and Me” Ta-Nehisi Coates
- A conversation on race and privilege (Angela Davis and Jane Elliot) https://www.youtube.com/watch?reload=9&v=S0jf8D5WHoo
- Uncomfortable conversations with a Black man (Emmanuel Acho) https://www.youtube.com/watch?v=h8jUA7JBkF4
Antiracism – defined as the work of actively opposing racism by advocating for changes in political, economic, and social life. Antiracism tends to be an individualized approach, and set up in opposition to individual racist behaviors and impacts
Black Lives Matter – a political movement to address systemic and state violence against African Americans. Per the Black Lives Matter organizers: “In 2013, three radical Black organizers—Alicia Garza, Patrisse Cullors, and Opal Tometi—created a Black-centered political will and movement building project called BlackLivesMatter. It was in response to the acquittal of Trayvon Martin’s murderer, George Zimmerman. The project is now a member-led global network of more than 40 chapters. Members organize and build local power to intervene in violence inflicted on Black communities by the state and vigilantes. Black Lives Matter is an ideological and political intervention in a world where Black lives are systematically and intentionally targeted for demise. It is an affirmation of Black folks’ humanity, our contributions to this society, and our resilience in the face of deadly oppression.”
Implicit bias – also known as unconscious or hidden bias, implicit biases are negative associations that people unknowingly hold. They are expressed automatically, without conscious awareness. Many studies have indicated that implicit biases affect individuals’ attitudes and actions, thus creating real-world implications, even though individuals may not even be aware that those biases exist within themselves. Notably, implicit biases have been shown to trump individuals stated commitments to equality and fairness, thereby producing behavior that diverges from the explicit attitudes that many people profess.
Othering – view or treat (a person or group of people) as intrinsically different from and alien to oneself. (From https://lexico.com.)
For a full glossary of terms, visit RacialEquityTools.org (https://www.racialequitytools.org/glossary#anti-black)
The in-person postpartum blood pressure check: For whose benefit?
CASE Patient questions need for postpartum BP check
Ms. P presents at 28 weeks’ gestation with superimposed preeclampsia. She receives antenatal corticosteroids and titration of her nifedipine, but she is delivered at 29 weeks because of worsening fetal status. Her physician recommends a blood pressure (BP) visit in the office at 7 days postpartum.
She asks, “But can’t I just call you with the BP reading? And what do I do in the meantime?”
Hypertensive disorders of pregnancy and chronic hypertension remain among the leading causes of maternal morbidity and mortality in the United States and worldwide.1 The postpartum period remains a particularly high-risk time since up to 40% of maternal mortality can occur after delivery. To that end, the 2013 American College of Obstetricians and Gynecologists Hypertension in Pregnancy Task Force recommends postpartum follow-up 7 to 10 days after delivery in women with a hypertensive disorder of pregnancy.2
Why we need to find an alternative approach
Unfortunately, these guidelines are both cumbersome and insufficient. Up to one-third of patients do not attend their postpartum visit, particularly those who are young, uninsured, and nonwhite, a list uncomfortably similar to that for women most at risk for adverse outcomes after a high-risk pregnancy. In addition, the 7- to 10-day visit still represents only a single snapshot of the patient’s BP values rather than an ongoing assessment of symptoms or BP elevation over time. Moreover, studies also have shown that BP in both normotensive and hypertensive women often rises by the fifth day postpartum, suggesting that leaving this large window of time without surveillance may miss an opportunity to detect elevated BP in a more timely manner.3
It is time to break the habit of the in-office postpartum BP check and to evaluate the patient where she is and when she needs it. Research in the last 2 years shows that there are several solutions to our case patient’s question.

Solution 1: The provider-driven system
“Of course. Text us your numbers, and you will hear from the doctor if you need to do anything differently.”
One method that addresses both the communication and safety issues inherent in the 7- to 10-day routine in-office BP check is to have the patient send in her BP measurements for direct clinician review.
Researchers at the University of Pennsylvania developed a robust program using their Way to Health platform.4 Participating patients text their BP values twice daily, and they receive automated feedback for all values, with additional human feedback in real time from a clinician for severe-range values (>160 mm Hg systolic or >110 mm Hg diastolic). As an added safety measure, a physician reviews all inputted BPs daily and assesses the need for antihypertensive medication for BPs in the high mild range. Using this protocol, the researchers achieved a significant increase in adherence with the recommendation for reporting a BP value in the first 10 days after discharge (from 44% to 92%) as well as having fewer readmissions in the text-messaging arm (4% vs 0%).
Perhaps most impressive, though, is that the technology use eliminated pre-existing racial disparities in adherence. Black participants were as likely as nonblack participants to report a postpartum BP in the text-messaging system (93% vs 91%) despite being less than half as likely to keep a BP check visit (33% vs 70%).5
A similar solution is in place at the University of Pittsburgh, where a text message system on the Vivify platform is used to deliver patient BP measurements to a centralized monitoring team.6 This program is unique in that, rather than relying on a single physician, it is run through a nurse “call center” that allowed them to expand to 3 hospitals with the use of a single centralized monitoring team. To date, the program has enrolled more than 2,000 patients and achieved patient satisfaction rates greater than 94%.
A final program to consider was developed and piloted at the University of Wisconsin with an added technological advance: the use of a Bluetooth-enabled BP cuff that permits values to be automatically transmitted to a tablet that then uploads the information to a centralized database.7 This database was in turn monitored by trained nurses for safety and initiation or titration of antihypertensive medication as needed. Similar to the experience at the University of Pennsylvania, the researchers found improved adherence with monitoring and a notable reduction in readmissions (3.7% in controls vs 0.5% in the intervention arm). Of note, among those who did receive the ongoing monitoring, severe hypertension occurred in 56 (26.2%) of those patients and did so a mean of 6 days after discharge (that is, prior to when they typically would have seen a provider.)
The promise of such provider-driven systems is that they represent a true chronicle of a patient’s ongoing clinical course rather than a single snapshot of her BP in an artificial environment (and often after the highest risk time period!). In addition, direct monitoring by clinicians ensures an optimal safety profile.
Such systems, however, are also extremely resource intense in terms of both upfront information technology investment and ongoing provider surveillance. The systems above also relied on giving the patients a BP cuff, so it is unclear whether it was the technology support or this simple intervention that yielded the benefits. Nonetheless, the benefits were undeniable, and the financial costs saved by reducing even 1 hospital admission as well as the costs of outpatient surveillance may in the end justify these upfront expenditures.
Continue to: Solution 2: The algorithm-driven system...
Solution 2: The algorithm-driven system
“Sure. Plug your numbers into our system, and you’ll receive an automated response as to what to do next.”
One way to alleviate both the financial and opportunity cost of constant clinician surveillance would be to offload some tasks to algorithmic support. This approach—home BP monitoring accompanied by self-titration of antihypertensive medication—has been validated in outpatient primary care hypertension management in nonpregnant adults and more recently for postpartum patients as well.
In the SNAP-HT trial, investigators randomly assigned women to either usual care or algorithm-driven outpatient BP management.8 While both groups had serial visits (for safety monitoring), those in the experimental arm were advised only by the algorithm for any ongoing titration of medication. At 6 weeks, the investigators found that BPs were lower in the intervention group, and diastolic BPs remained lower at 6 months.
This methodology emphasizes the potential utility of true self-management of hypertension in the postpartum period. It relies, however, on having a highly developed system in place that can receive the data, respond with recommendations, and safely monitor for any aberrations in the feed. Still, this hybrid method may represent the sweet spot: a combination that ensures adequate surveillance while not overburdening the clinician with the simpler, initial steps in postpartum antihypertensive management.
Solution 3: The DIY system
“That’s a good point. I want to hear about your blood pressure readings in the meantime. Here’s what we can do.”
What about the 99% of practicing ObGyns who do not have an entire connected system for remote hypertension monitoring? A number of options can be put in place today with little cost and even less tech know-how (see “Do-it-yourself options for remote blood pressure monitoring,” below). Note that since many of these options would not be monitored in “real time” like the connected systems discussed above, the patient should be given strict parameters for contacting her clinician directly. These do-it-yourself, or DIY, methods are instead best for the purpose of chronic monitoring and medication titration but are still an improvement in communication over the single-serve BP check.
The bottom line
Pregnant women represent one of the most connected, Internet-savvy demographic groups of any patient population: More than three-quarters of pregnant women turn to the Internet for advice during their pregnancy.9,10 In addition, unlike most social determinants of health, such as housing, food access, and health care coverage, access to connected electronic devices differs little across racial lines, suggesting the potential for targeting health care inequities by implementing more—not less—technology into prenatal and postpartum care.
For this generation of new mothers, the in-office postpartum BP check is insufficient, artificial, and simply a waste of everyone’s time. While there is no one-size-fits-all approach, there are many options, and it is up to us as health care providers to facilitate the right care, in the right place, at the right time for our patients. ●
Acknowledgements: The authors would like to thank Haritha Pavuluri, Margaret Oliver, and Samantha Boniface for their assistance in the preparation of this manuscript.
Electronic health record (EHR) messaging
Most EHR systems have some form of patient messaging built in. Consider asking your patient to:
- message her blood pressure measurements every 1 to 2 days
- send a photo of handwritten blood pressure measurements
Vendor text messaging platforms
The year 2020 has seen the entire telehealth space grow tremendously, and platforms such as Doxy.me (https://doxy.me) and Updox (https://www.updox.com) allow secure text messaging with patients.
All-in-one connected vendor solutions
Third-party solutions are available that give the patient a connected blood pressure cuff, scale, and personalized app. For the clinician, these data then can be accessed either independently through a portal or can be integrated into the EHR. Examples of 2 companies include:
- Babyscripts (https://www.getbabyscripts.com)
- Wildflower Health (https://www.wildflowerhealth.com)
Telehealth visits
Scheduling weekly telephone or video visits (while not near the frequency of the above) would still yield greater engagement, and many payors currently reimburse for these visits at rates on par with in-person visits.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin summary, No. 222. Gestational hypertension and preeclampsia. Obstet Gynecol. 2020;135:1492-1495.
- American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122:1122-1131.
- Walters BN, Thompson ME, Lee A, et al. Blood pressure in the puerperium. Clin Sci. 1986;71:589-594.
- Hirshberg A, Downes K, Srinivas S. Comparing standard office-based follow-up with text-based remote monitoring in the management of postpartum hypertension: a randomised clinical trial. BMJ Qual Saf. 2018;27:871-877.
- Hirshberg A, Sammel MD, Srinivas SK. Text message remote monitoring reduced racial disparities in postpartum blood pressure ascertainment. Am J Obstet Gynecol. 2019;221:283-285.
- Hauspurg A, Lemon LS, Quinn BA, et al. A postpartum remote hypertension monitoring protocol implemented at the hospital level. Obstet Gynecol. 2019;134:685-691.
- Hoppe KK, Thomas N, Zernick M, et al. Telehealth with remote blood pressure monitoring compared to standard care for postpartum hypertension. Am J Obstet Gynecol. 2020;S0002-9378(20)30554-doi:10.1016/j.ajog.2020.05.027.
- Cairns AE, Tucker KL, Leeson P, et al. Self-management of postnatal hypertension. Hypertension. 2018;72:425-432.
- Pew Research Center. Mobile fact sheet, 2019. https://www.pewresearch.org/internet/fact-sheet/mobile/. Accessed June 16, 2020.
- Sayakhot P, Carolan-Olah M. Internet use by pregnant women seeking pregnancy-related information: a systematic review. BMC Pregnancy Childbirth. 2016;16:65
CASE Patient questions need for postpartum BP check
Ms. P presents at 28 weeks’ gestation with superimposed preeclampsia. She receives antenatal corticosteroids and titration of her nifedipine, but she is delivered at 29 weeks because of worsening fetal status. Her physician recommends a blood pressure (BP) visit in the office at 7 days postpartum.
She asks, “But can’t I just call you with the BP reading? And what do I do in the meantime?”
Hypertensive disorders of pregnancy and chronic hypertension remain among the leading causes of maternal morbidity and mortality in the United States and worldwide.1 The postpartum period remains a particularly high-risk time since up to 40% of maternal mortality can occur after delivery. To that end, the 2013 American College of Obstetricians and Gynecologists Hypertension in Pregnancy Task Force recommends postpartum follow-up 7 to 10 days after delivery in women with a hypertensive disorder of pregnancy.2
Why we need to find an alternative approach
Unfortunately, these guidelines are both cumbersome and insufficient. Up to one-third of patients do not attend their postpartum visit, particularly those who are young, uninsured, and nonwhite, a list uncomfortably similar to that for women most at risk for adverse outcomes after a high-risk pregnancy. In addition, the 7- to 10-day visit still represents only a single snapshot of the patient’s BP values rather than an ongoing assessment of symptoms or BP elevation over time. Moreover, studies also have shown that BP in both normotensive and hypertensive women often rises by the fifth day postpartum, suggesting that leaving this large window of time without surveillance may miss an opportunity to detect elevated BP in a more timely manner.3
It is time to break the habit of the in-office postpartum BP check and to evaluate the patient where she is and when she needs it. Research in the last 2 years shows that there are several solutions to our case patient’s question.

Solution 1: The provider-driven system
“Of course. Text us your numbers, and you will hear from the doctor if you need to do anything differently.”
One method that addresses both the communication and safety issues inherent in the 7- to 10-day routine in-office BP check is to have the patient send in her BP measurements for direct clinician review.
Researchers at the University of Pennsylvania developed a robust program using their Way to Health platform.4 Participating patients text their BP values twice daily, and they receive automated feedback for all values, with additional human feedback in real time from a clinician for severe-range values (>160 mm Hg systolic or >110 mm Hg diastolic). As an added safety measure, a physician reviews all inputted BPs daily and assesses the need for antihypertensive medication for BPs in the high mild range. Using this protocol, the researchers achieved a significant increase in adherence with the recommendation for reporting a BP value in the first 10 days after discharge (from 44% to 92%) as well as having fewer readmissions in the text-messaging arm (4% vs 0%).
Perhaps most impressive, though, is that the technology use eliminated pre-existing racial disparities in adherence. Black participants were as likely as nonblack participants to report a postpartum BP in the text-messaging system (93% vs 91%) despite being less than half as likely to keep a BP check visit (33% vs 70%).5
A similar solution is in place at the University of Pittsburgh, where a text message system on the Vivify platform is used to deliver patient BP measurements to a centralized monitoring team.6 This program is unique in that, rather than relying on a single physician, it is run through a nurse “call center” that allowed them to expand to 3 hospitals with the use of a single centralized monitoring team. To date, the program has enrolled more than 2,000 patients and achieved patient satisfaction rates greater than 94%.
A final program to consider was developed and piloted at the University of Wisconsin with an added technological advance: the use of a Bluetooth-enabled BP cuff that permits values to be automatically transmitted to a tablet that then uploads the information to a centralized database.7 This database was in turn monitored by trained nurses for safety and initiation or titration of antihypertensive medication as needed. Similar to the experience at the University of Pennsylvania, the researchers found improved adherence with monitoring and a notable reduction in readmissions (3.7% in controls vs 0.5% in the intervention arm). Of note, among those who did receive the ongoing monitoring, severe hypertension occurred in 56 (26.2%) of those patients and did so a mean of 6 days after discharge (that is, prior to when they typically would have seen a provider.)
The promise of such provider-driven systems is that they represent a true chronicle of a patient’s ongoing clinical course rather than a single snapshot of her BP in an artificial environment (and often after the highest risk time period!). In addition, direct monitoring by clinicians ensures an optimal safety profile.
Such systems, however, are also extremely resource intense in terms of both upfront information technology investment and ongoing provider surveillance. The systems above also relied on giving the patients a BP cuff, so it is unclear whether it was the technology support or this simple intervention that yielded the benefits. Nonetheless, the benefits were undeniable, and the financial costs saved by reducing even 1 hospital admission as well as the costs of outpatient surveillance may in the end justify these upfront expenditures.
Continue to: Solution 2: The algorithm-driven system...
Solution 2: The algorithm-driven system
“Sure. Plug your numbers into our system, and you’ll receive an automated response as to what to do next.”
One way to alleviate both the financial and opportunity cost of constant clinician surveillance would be to offload some tasks to algorithmic support. This approach—home BP monitoring accompanied by self-titration of antihypertensive medication—has been validated in outpatient primary care hypertension management in nonpregnant adults and more recently for postpartum patients as well.
In the SNAP-HT trial, investigators randomly assigned women to either usual care or algorithm-driven outpatient BP management.8 While both groups had serial visits (for safety monitoring), those in the experimental arm were advised only by the algorithm for any ongoing titration of medication. At 6 weeks, the investigators found that BPs were lower in the intervention group, and diastolic BPs remained lower at 6 months.
This methodology emphasizes the potential utility of true self-management of hypertension in the postpartum period. It relies, however, on having a highly developed system in place that can receive the data, respond with recommendations, and safely monitor for any aberrations in the feed. Still, this hybrid method may represent the sweet spot: a combination that ensures adequate surveillance while not overburdening the clinician with the simpler, initial steps in postpartum antihypertensive management.
Solution 3: The DIY system
“That’s a good point. I want to hear about your blood pressure readings in the meantime. Here’s what we can do.”
What about the 99% of practicing ObGyns who do not have an entire connected system for remote hypertension monitoring? A number of options can be put in place today with little cost and even less tech know-how (see “Do-it-yourself options for remote blood pressure monitoring,” below). Note that since many of these options would not be monitored in “real time” like the connected systems discussed above, the patient should be given strict parameters for contacting her clinician directly. These do-it-yourself, or DIY, methods are instead best for the purpose of chronic monitoring and medication titration but are still an improvement in communication over the single-serve BP check.
The bottom line
Pregnant women represent one of the most connected, Internet-savvy demographic groups of any patient population: More than three-quarters of pregnant women turn to the Internet for advice during their pregnancy.9,10 In addition, unlike most social determinants of health, such as housing, food access, and health care coverage, access to connected electronic devices differs little across racial lines, suggesting the potential for targeting health care inequities by implementing more—not less—technology into prenatal and postpartum care.
For this generation of new mothers, the in-office postpartum BP check is insufficient, artificial, and simply a waste of everyone’s time. While there is no one-size-fits-all approach, there are many options, and it is up to us as health care providers to facilitate the right care, in the right place, at the right time for our patients. ●
Acknowledgements: The authors would like to thank Haritha Pavuluri, Margaret Oliver, and Samantha Boniface for their assistance in the preparation of this manuscript.
Electronic health record (EHR) messaging
Most EHR systems have some form of patient messaging built in. Consider asking your patient to:
- message her blood pressure measurements every 1 to 2 days
- send a photo of handwritten blood pressure measurements
Vendor text messaging platforms
The year 2020 has seen the entire telehealth space grow tremendously, and platforms such as Doxy.me (https://doxy.me) and Updox (https://www.updox.com) allow secure text messaging with patients.
All-in-one connected vendor solutions
Third-party solutions are available that give the patient a connected blood pressure cuff, scale, and personalized app. For the clinician, these data then can be accessed either independently through a portal or can be integrated into the EHR. Examples of 2 companies include:
- Babyscripts (https://www.getbabyscripts.com)
- Wildflower Health (https://www.wildflowerhealth.com)
Telehealth visits
Scheduling weekly telephone or video visits (while not near the frequency of the above) would still yield greater engagement, and many payors currently reimburse for these visits at rates on par with in-person visits.
CASE Patient questions need for postpartum BP check
Ms. P presents at 28 weeks’ gestation with superimposed preeclampsia. She receives antenatal corticosteroids and titration of her nifedipine, but she is delivered at 29 weeks because of worsening fetal status. Her physician recommends a blood pressure (BP) visit in the office at 7 days postpartum.
She asks, “But can’t I just call you with the BP reading? And what do I do in the meantime?”
Hypertensive disorders of pregnancy and chronic hypertension remain among the leading causes of maternal morbidity and mortality in the United States and worldwide.1 The postpartum period remains a particularly high-risk time since up to 40% of maternal mortality can occur after delivery. To that end, the 2013 American College of Obstetricians and Gynecologists Hypertension in Pregnancy Task Force recommends postpartum follow-up 7 to 10 days after delivery in women with a hypertensive disorder of pregnancy.2
Why we need to find an alternative approach
Unfortunately, these guidelines are both cumbersome and insufficient. Up to one-third of patients do not attend their postpartum visit, particularly those who are young, uninsured, and nonwhite, a list uncomfortably similar to that for women most at risk for adverse outcomes after a high-risk pregnancy. In addition, the 7- to 10-day visit still represents only a single snapshot of the patient’s BP values rather than an ongoing assessment of symptoms or BP elevation over time. Moreover, studies also have shown that BP in both normotensive and hypertensive women often rises by the fifth day postpartum, suggesting that leaving this large window of time without surveillance may miss an opportunity to detect elevated BP in a more timely manner.3
It is time to break the habit of the in-office postpartum BP check and to evaluate the patient where she is and when she needs it. Research in the last 2 years shows that there are several solutions to our case patient’s question.

Solution 1: The provider-driven system
“Of course. Text us your numbers, and you will hear from the doctor if you need to do anything differently.”
One method that addresses both the communication and safety issues inherent in the 7- to 10-day routine in-office BP check is to have the patient send in her BP measurements for direct clinician review.
Researchers at the University of Pennsylvania developed a robust program using their Way to Health platform.4 Participating patients text their BP values twice daily, and they receive automated feedback for all values, with additional human feedback in real time from a clinician for severe-range values (>160 mm Hg systolic or >110 mm Hg diastolic). As an added safety measure, a physician reviews all inputted BPs daily and assesses the need for antihypertensive medication for BPs in the high mild range. Using this protocol, the researchers achieved a significant increase in adherence with the recommendation for reporting a BP value in the first 10 days after discharge (from 44% to 92%) as well as having fewer readmissions in the text-messaging arm (4% vs 0%).
Perhaps most impressive, though, is that the technology use eliminated pre-existing racial disparities in adherence. Black participants were as likely as nonblack participants to report a postpartum BP in the text-messaging system (93% vs 91%) despite being less than half as likely to keep a BP check visit (33% vs 70%).5
A similar solution is in place at the University of Pittsburgh, where a text message system on the Vivify platform is used to deliver patient BP measurements to a centralized monitoring team.6 This program is unique in that, rather than relying on a single physician, it is run through a nurse “call center” that allowed them to expand to 3 hospitals with the use of a single centralized monitoring team. To date, the program has enrolled more than 2,000 patients and achieved patient satisfaction rates greater than 94%.
A final program to consider was developed and piloted at the University of Wisconsin with an added technological advance: the use of a Bluetooth-enabled BP cuff that permits values to be automatically transmitted to a tablet that then uploads the information to a centralized database.7 This database was in turn monitored by trained nurses for safety and initiation or titration of antihypertensive medication as needed. Similar to the experience at the University of Pennsylvania, the researchers found improved adherence with monitoring and a notable reduction in readmissions (3.7% in controls vs 0.5% in the intervention arm). Of note, among those who did receive the ongoing monitoring, severe hypertension occurred in 56 (26.2%) of those patients and did so a mean of 6 days after discharge (that is, prior to when they typically would have seen a provider.)
The promise of such provider-driven systems is that they represent a true chronicle of a patient’s ongoing clinical course rather than a single snapshot of her BP in an artificial environment (and often after the highest risk time period!). In addition, direct monitoring by clinicians ensures an optimal safety profile.
Such systems, however, are also extremely resource intense in terms of both upfront information technology investment and ongoing provider surveillance. The systems above also relied on giving the patients a BP cuff, so it is unclear whether it was the technology support or this simple intervention that yielded the benefits. Nonetheless, the benefits were undeniable, and the financial costs saved by reducing even 1 hospital admission as well as the costs of outpatient surveillance may in the end justify these upfront expenditures.
Continue to: Solution 2: The algorithm-driven system...
Solution 2: The algorithm-driven system
“Sure. Plug your numbers into our system, and you’ll receive an automated response as to what to do next.”
One way to alleviate both the financial and opportunity cost of constant clinician surveillance would be to offload some tasks to algorithmic support. This approach—home BP monitoring accompanied by self-titration of antihypertensive medication—has been validated in outpatient primary care hypertension management in nonpregnant adults and more recently for postpartum patients as well.
In the SNAP-HT trial, investigators randomly assigned women to either usual care or algorithm-driven outpatient BP management.8 While both groups had serial visits (for safety monitoring), those in the experimental arm were advised only by the algorithm for any ongoing titration of medication. At 6 weeks, the investigators found that BPs were lower in the intervention group, and diastolic BPs remained lower at 6 months.
This methodology emphasizes the potential utility of true self-management of hypertension in the postpartum period. It relies, however, on having a highly developed system in place that can receive the data, respond with recommendations, and safely monitor for any aberrations in the feed. Still, this hybrid method may represent the sweet spot: a combination that ensures adequate surveillance while not overburdening the clinician with the simpler, initial steps in postpartum antihypertensive management.
Solution 3: The DIY system
“That’s a good point. I want to hear about your blood pressure readings in the meantime. Here’s what we can do.”
What about the 99% of practicing ObGyns who do not have an entire connected system for remote hypertension monitoring? A number of options can be put in place today with little cost and even less tech know-how (see “Do-it-yourself options for remote blood pressure monitoring,” below). Note that since many of these options would not be monitored in “real time” like the connected systems discussed above, the patient should be given strict parameters for contacting her clinician directly. These do-it-yourself, or DIY, methods are instead best for the purpose of chronic monitoring and medication titration but are still an improvement in communication over the single-serve BP check.
The bottom line
Pregnant women represent one of the most connected, Internet-savvy demographic groups of any patient population: More than three-quarters of pregnant women turn to the Internet for advice during their pregnancy.9,10 In addition, unlike most social determinants of health, such as housing, food access, and health care coverage, access to connected electronic devices differs little across racial lines, suggesting the potential for targeting health care inequities by implementing more—not less—technology into prenatal and postpartum care.
For this generation of new mothers, the in-office postpartum BP check is insufficient, artificial, and simply a waste of everyone’s time. While there is no one-size-fits-all approach, there are many options, and it is up to us as health care providers to facilitate the right care, in the right place, at the right time for our patients. ●
Acknowledgements: The authors would like to thank Haritha Pavuluri, Margaret Oliver, and Samantha Boniface for their assistance in the preparation of this manuscript.
Electronic health record (EHR) messaging
Most EHR systems have some form of patient messaging built in. Consider asking your patient to:
- message her blood pressure measurements every 1 to 2 days
- send a photo of handwritten blood pressure measurements
Vendor text messaging platforms
The year 2020 has seen the entire telehealth space grow tremendously, and platforms such as Doxy.me (https://doxy.me) and Updox (https://www.updox.com) allow secure text messaging with patients.
All-in-one connected vendor solutions
Third-party solutions are available that give the patient a connected blood pressure cuff, scale, and personalized app. For the clinician, these data then can be accessed either independently through a portal or can be integrated into the EHR. Examples of 2 companies include:
- Babyscripts (https://www.getbabyscripts.com)
- Wildflower Health (https://www.wildflowerhealth.com)
Telehealth visits
Scheduling weekly telephone or video visits (while not near the frequency of the above) would still yield greater engagement, and many payors currently reimburse for these visits at rates on par with in-person visits.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin summary, No. 222. Gestational hypertension and preeclampsia. Obstet Gynecol. 2020;135:1492-1495.
- American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122:1122-1131.
- Walters BN, Thompson ME, Lee A, et al. Blood pressure in the puerperium. Clin Sci. 1986;71:589-594.
- Hirshberg A, Downes K, Srinivas S. Comparing standard office-based follow-up with text-based remote monitoring in the management of postpartum hypertension: a randomised clinical trial. BMJ Qual Saf. 2018;27:871-877.
- Hirshberg A, Sammel MD, Srinivas SK. Text message remote monitoring reduced racial disparities in postpartum blood pressure ascertainment. Am J Obstet Gynecol. 2019;221:283-285.
- Hauspurg A, Lemon LS, Quinn BA, et al. A postpartum remote hypertension monitoring protocol implemented at the hospital level. Obstet Gynecol. 2019;134:685-691.
- Hoppe KK, Thomas N, Zernick M, et al. Telehealth with remote blood pressure monitoring compared to standard care for postpartum hypertension. Am J Obstet Gynecol. 2020;S0002-9378(20)30554-doi:10.1016/j.ajog.2020.05.027.
- Cairns AE, Tucker KL, Leeson P, et al. Self-management of postnatal hypertension. Hypertension. 2018;72:425-432.
- Pew Research Center. Mobile fact sheet, 2019. https://www.pewresearch.org/internet/fact-sheet/mobile/. Accessed June 16, 2020.
- Sayakhot P, Carolan-Olah M. Internet use by pregnant women seeking pregnancy-related information: a systematic review. BMC Pregnancy Childbirth. 2016;16:65
- American College of Obstetricians and Gynecologists. ACOG practice bulletin summary, No. 222. Gestational hypertension and preeclampsia. Obstet Gynecol. 2020;135:1492-1495.
- American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122:1122-1131.
- Walters BN, Thompson ME, Lee A, et al. Blood pressure in the puerperium. Clin Sci. 1986;71:589-594.
- Hirshberg A, Downes K, Srinivas S. Comparing standard office-based follow-up with text-based remote monitoring in the management of postpartum hypertension: a randomised clinical trial. BMJ Qual Saf. 2018;27:871-877.
- Hirshberg A, Sammel MD, Srinivas SK. Text message remote monitoring reduced racial disparities in postpartum blood pressure ascertainment. Am J Obstet Gynecol. 2019;221:283-285.
- Hauspurg A, Lemon LS, Quinn BA, et al. A postpartum remote hypertension monitoring protocol implemented at the hospital level. Obstet Gynecol. 2019;134:685-691.
- Hoppe KK, Thomas N, Zernick M, et al. Telehealth with remote blood pressure monitoring compared to standard care for postpartum hypertension. Am J Obstet Gynecol. 2020;S0002-9378(20)30554-doi:10.1016/j.ajog.2020.05.027.
- Cairns AE, Tucker KL, Leeson P, et al. Self-management of postnatal hypertension. Hypertension. 2018;72:425-432.
- Pew Research Center. Mobile fact sheet, 2019. https://www.pewresearch.org/internet/fact-sheet/mobile/. Accessed June 16, 2020.
- Sayakhot P, Carolan-Olah M. Internet use by pregnant women seeking pregnancy-related information: a systematic review. BMC Pregnancy Childbirth. 2016;16:65
Should all women with a history of OASI have a mediolateral episiotomy at their subsequent delivery?
Van Bavel J, Ravelli AC, Abu-Hanna A, et al. Risk factors for the recurrence of obstetrical anal sphincter injury and the role of a mediolateral episiotomy: an analysis of a national registry. BJOG. 2020;127:951-956.
EXPERT COMMENTARY
Women with a history of OASI are at increased risk for recurrence in a subsequent delivery. Higher rates of anal and fecal incontinence are reported in women with recurrent OASI (rOASI) compared with women who had an OASI only in their first delivery. Previous studies have reported recurrence rates of 5% to 7%,1 and some suggested that MLE may be protective, but standardized recommendations for mode of delivery and use of MLE currently are not available.
Recently, van Bavel and colleagues sought to determine the rate of rOASI in their population as well as the factors that increase and decrease the risk of this complication.
Details of the study
This cohort study used data from the Dutch Perinatal Registry (Perined) that included 268,607 women who had their first and second deliveries (singleton, term, vertex, < 43 weeks) vaginally in 2000–2009. The study’s primary objective was to determine the rate of rOASI in women who had OASI in their first delivery. The secondary objectives were to identify risk factors for rOASI and to assess the effect of MLE. For the purposes of this study, OASI was defined as subtotal and total rupture of the perineum, or grades 3A-4 as defined by the Royal College of Obstetricians and Gynaecologists.2
Within this cohort, 9,943 women had an OASI in their first delivery (4%), and the rate of rOASI was 5.8% (579 of 9,943). After multivariate analysis, the risk factors for rOASI were birth weight of 4,000 g or greater (odds ratio [OR], 2.1; 95% confidence interval [CI], 1.6–2.6) and duration of the second stage of labor of 30 minutes or longer (OR, 1.8; 95% CI, 1.4–2.3).
The MLE rate was 40.8% (4,054 of 9,943) and was associated with a lower rate of rOASI (OR, 0.3; 95% CI, 0.3–0.4). This association persisted when delivery type was separated into spontaneous and operative vaginal deliveries, with the number of MLEs needed to prevent one rOASI of 22 and 8, respectively. Birth weight of less than 3,000 g also was noted to be protective against rOASI (OR, 0.5; 95% CI, 0.3–0.9).
Based on these findings, as well as comparisons to previous studies, the authors concluded that MLE could be considered for routine use or at least discussed with all women with a prior OASI for prevention of rOASI.
Continue to: Study strengths and limitations...
Study strengths and limitations
A strength of this study was the large number of deliveries and the wide variation of practice included in the registry database, which promotes the generalizability of the results and reduces bias. This also provides an adequate base on which to determine an accurate rate of rOASI in the Dutch population.
One study limitation is that information is not available regarding how the episiotomies were performed (specifically, angle of incision), delivery techniques (“hands on” vs “hands off”), and indication for the episiotomy. Additional limitations suggested are that clinicians who perform an episiotomy may have an inherent bias regarding the protective nature of the procedure and may miss a rOASI due to inadequate examination postprocedure, overestimating its protective effect.
Finally, the relatively high rate of MLE and low rate of cesarean delivery (6.9%) in this study are specific to the Netherlands and do not reflect the obstetric practices used in many other countries. Generalizability of these results in the context of much lower MLE and higher cesarean delivery rates (as in the United States) would therefore be in question.●
Prevention of rOASI is important, as fecal incontinence is debilitating and difficult to treat. While this study provides evidence that MLE may protect against this complication, its results may not be generalizable to all patient or clinician populations. Differences in baseline rate of MLE and cesarean delivery, technique, indication, and comfort with repair—all not evaluated in this study—must be taken into account when counseling OASI patients about their options for delivery and the use of MLE in a subsequent pregnancy.
JAIMEY M. PAULI, MD
- Van Bavel J, Ravelli AC, Abu-Hanna A, et al. Risk factors for the recurrence of obstetrical anal sphincter injury and the role of a mediolateral episiotomy: an analysis of a national registry. BJOG. 2020;127:951-956.
- Royal College of Obstetricians and Gynaecologists. Green-top guideline No. 29: the management of third- and fourth-degree perineal tears. June 2014. https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-29.pdf. Accessed June 12, 2020.
Van Bavel J, Ravelli AC, Abu-Hanna A, et al. Risk factors for the recurrence of obstetrical anal sphincter injury and the role of a mediolateral episiotomy: an analysis of a national registry. BJOG. 2020;127:951-956.
EXPERT COMMENTARY
Women with a history of OASI are at increased risk for recurrence in a subsequent delivery. Higher rates of anal and fecal incontinence are reported in women with recurrent OASI (rOASI) compared with women who had an OASI only in their first delivery. Previous studies have reported recurrence rates of 5% to 7%,1 and some suggested that MLE may be protective, but standardized recommendations for mode of delivery and use of MLE currently are not available.
Recently, van Bavel and colleagues sought to determine the rate of rOASI in their population as well as the factors that increase and decrease the risk of this complication.
Details of the study
This cohort study used data from the Dutch Perinatal Registry (Perined) that included 268,607 women who had their first and second deliveries (singleton, term, vertex, < 43 weeks) vaginally in 2000–2009. The study’s primary objective was to determine the rate of rOASI in women who had OASI in their first delivery. The secondary objectives were to identify risk factors for rOASI and to assess the effect of MLE. For the purposes of this study, OASI was defined as subtotal and total rupture of the perineum, or grades 3A-4 as defined by the Royal College of Obstetricians and Gynaecologists.2
Within this cohort, 9,943 women had an OASI in their first delivery (4%), and the rate of rOASI was 5.8% (579 of 9,943). After multivariate analysis, the risk factors for rOASI were birth weight of 4,000 g or greater (odds ratio [OR], 2.1; 95% confidence interval [CI], 1.6–2.6) and duration of the second stage of labor of 30 minutes or longer (OR, 1.8; 95% CI, 1.4–2.3).
The MLE rate was 40.8% (4,054 of 9,943) and was associated with a lower rate of rOASI (OR, 0.3; 95% CI, 0.3–0.4). This association persisted when delivery type was separated into spontaneous and operative vaginal deliveries, with the number of MLEs needed to prevent one rOASI of 22 and 8, respectively. Birth weight of less than 3,000 g also was noted to be protective against rOASI (OR, 0.5; 95% CI, 0.3–0.9).
Based on these findings, as well as comparisons to previous studies, the authors concluded that MLE could be considered for routine use or at least discussed with all women with a prior OASI for prevention of rOASI.
Continue to: Study strengths and limitations...
Study strengths and limitations
A strength of this study was the large number of deliveries and the wide variation of practice included in the registry database, which promotes the generalizability of the results and reduces bias. This also provides an adequate base on which to determine an accurate rate of rOASI in the Dutch population.
One study limitation is that information is not available regarding how the episiotomies were performed (specifically, angle of incision), delivery techniques (“hands on” vs “hands off”), and indication for the episiotomy. Additional limitations suggested are that clinicians who perform an episiotomy may have an inherent bias regarding the protective nature of the procedure and may miss a rOASI due to inadequate examination postprocedure, overestimating its protective effect.
Finally, the relatively high rate of MLE and low rate of cesarean delivery (6.9%) in this study are specific to the Netherlands and do not reflect the obstetric practices used in many other countries. Generalizability of these results in the context of much lower MLE and higher cesarean delivery rates (as in the United States) would therefore be in question.●
Prevention of rOASI is important, as fecal incontinence is debilitating and difficult to treat. While this study provides evidence that MLE may protect against this complication, its results may not be generalizable to all patient or clinician populations. Differences in baseline rate of MLE and cesarean delivery, technique, indication, and comfort with repair—all not evaluated in this study—must be taken into account when counseling OASI patients about their options for delivery and the use of MLE in a subsequent pregnancy.
JAIMEY M. PAULI, MD
Van Bavel J, Ravelli AC, Abu-Hanna A, et al. Risk factors for the recurrence of obstetrical anal sphincter injury and the role of a mediolateral episiotomy: an analysis of a national registry. BJOG. 2020;127:951-956.
EXPERT COMMENTARY
Women with a history of OASI are at increased risk for recurrence in a subsequent delivery. Higher rates of anal and fecal incontinence are reported in women with recurrent OASI (rOASI) compared with women who had an OASI only in their first delivery. Previous studies have reported recurrence rates of 5% to 7%,1 and some suggested that MLE may be protective, but standardized recommendations for mode of delivery and use of MLE currently are not available.
Recently, van Bavel and colleagues sought to determine the rate of rOASI in their population as well as the factors that increase and decrease the risk of this complication.
Details of the study
This cohort study used data from the Dutch Perinatal Registry (Perined) that included 268,607 women who had their first and second deliveries (singleton, term, vertex, < 43 weeks) vaginally in 2000–2009. The study’s primary objective was to determine the rate of rOASI in women who had OASI in their first delivery. The secondary objectives were to identify risk factors for rOASI and to assess the effect of MLE. For the purposes of this study, OASI was defined as subtotal and total rupture of the perineum, or grades 3A-4 as defined by the Royal College of Obstetricians and Gynaecologists.2
Within this cohort, 9,943 women had an OASI in their first delivery (4%), and the rate of rOASI was 5.8% (579 of 9,943). After multivariate analysis, the risk factors for rOASI were birth weight of 4,000 g or greater (odds ratio [OR], 2.1; 95% confidence interval [CI], 1.6–2.6) and duration of the second stage of labor of 30 minutes or longer (OR, 1.8; 95% CI, 1.4–2.3).
The MLE rate was 40.8% (4,054 of 9,943) and was associated with a lower rate of rOASI (OR, 0.3; 95% CI, 0.3–0.4). This association persisted when delivery type was separated into spontaneous and operative vaginal deliveries, with the number of MLEs needed to prevent one rOASI of 22 and 8, respectively. Birth weight of less than 3,000 g also was noted to be protective against rOASI (OR, 0.5; 95% CI, 0.3–0.9).
Based on these findings, as well as comparisons to previous studies, the authors concluded that MLE could be considered for routine use or at least discussed with all women with a prior OASI for prevention of rOASI.
Continue to: Study strengths and limitations...
Study strengths and limitations
A strength of this study was the large number of deliveries and the wide variation of practice included in the registry database, which promotes the generalizability of the results and reduces bias. This also provides an adequate base on which to determine an accurate rate of rOASI in the Dutch population.
One study limitation is that information is not available regarding how the episiotomies were performed (specifically, angle of incision), delivery techniques (“hands on” vs “hands off”), and indication for the episiotomy. Additional limitations suggested are that clinicians who perform an episiotomy may have an inherent bias regarding the protective nature of the procedure and may miss a rOASI due to inadequate examination postprocedure, overestimating its protective effect.
Finally, the relatively high rate of MLE and low rate of cesarean delivery (6.9%) in this study are specific to the Netherlands and do not reflect the obstetric practices used in many other countries. Generalizability of these results in the context of much lower MLE and higher cesarean delivery rates (as in the United States) would therefore be in question.●
Prevention of rOASI is important, as fecal incontinence is debilitating and difficult to treat. While this study provides evidence that MLE may protect against this complication, its results may not be generalizable to all patient or clinician populations. Differences in baseline rate of MLE and cesarean delivery, technique, indication, and comfort with repair—all not evaluated in this study—must be taken into account when counseling OASI patients about their options for delivery and the use of MLE in a subsequent pregnancy.
JAIMEY M. PAULI, MD
- Van Bavel J, Ravelli AC, Abu-Hanna A, et al. Risk factors for the recurrence of obstetrical anal sphincter injury and the role of a mediolateral episiotomy: an analysis of a national registry. BJOG. 2020;127:951-956.
- Royal College of Obstetricians and Gynaecologists. Green-top guideline No. 29: the management of third- and fourth-degree perineal tears. June 2014. https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-29.pdf. Accessed June 12, 2020.
- Van Bavel J, Ravelli AC, Abu-Hanna A, et al. Risk factors for the recurrence of obstetrical anal sphincter injury and the role of a mediolateral episiotomy: an analysis of a national registry. BJOG. 2020;127:951-956.
- Royal College of Obstetricians and Gynaecologists. Green-top guideline No. 29: the management of third- and fourth-degree perineal tears. June 2014. https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-29.pdf. Accessed June 12, 2020.
How effective is elagolix treatment in women with fibroids and HMB?
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Expert Commentary
Uterine fibroids are common (occurring in up to 80% of reproductive-age women),1,2 and often associated with heavy menstrual bleeding (HMB). There are surgical and medical options, but typically medical options are used for short periods of time. Elagolix with hormonal add-back therapy was recently approved (May 29, 2020) by the US Food and Drug Administration (FDA) for treatment of HMB in women with uterine fibroids for up to 24 months.
Elagolix is an oral, nonpeptide gonadotropin-releasing hormone antagonist that results in a dose-dependent reduction of gonadotropins and ovarian sex hormones. There are now 2 approved products containing elagolix, with different indications:
- Orilissa. Elagolix was approved in 2018 by the FDA for moderate to severe pain associated with endometriosis. For that indication there are 2 dose options of elagolix (150 mg for up to 2 years and 200 mg for up to 6 months) and there is no hormonal add-back therapy.
- Oriahnn. Elagolix and hormonal add-back therapy was approved in 2020 for HMB associated with uterine fibroids for up to 24 months. The total daily dose of elagolix is 600 mg (elagolix 300 mg in the morning with estradiol 1 mg/norethindrone acetate 0.5 mg and then in the evening elagolix 300 mg and no hormonal add-back).
This new class of drug, GnRH antagonist, is an important one for women’s health, and emerging science will continue to expand its potential uses, such as in reproductive health, as well as long-term efficacy and safety. The difference in daily dose of elagolix for endometriosis (150 mg for 24 months) compared with HMB associated with fibroids (600 mg for 24 months) is why the hormonal add-back therapy is important and allows for protection of bone density.
This is an important manuscript because it highlights a medical option for women with HMB associated with fibroids, which can be used for a long period of time. Further, the improvement in bleeding is both impressive and maintained in the extension study. Approximately 90% of women show improvement in their menstrual bleeding associated with fibroids.
The question of what to do after 24 months of therapy with elagolix and hormonal add-back therapy is an important one, but providers should recognize that the limiting factor with this elagolix and hormonal add-back therapy is bone mineral density (BMD). We will only learn more and more moving forward if this is a clinically meaningful reason for stopping treatment at 24 months. The FDA takes a strict view of safety, and providers must weigh this with the benefit of therapy.
One other highlight between the 2 approved medications is that Orilissa does not have a black box warning, given that there is no hormonal add-back therapy. Oriahnn does have a warning, regarding thromboembolic disorders and vascular events:
- Estrogen and progestin combinations, including Oriahnn, increase the risk of thrombotic or thromboembolic disorders, especially in women at increased risk for these events.
- Oriahnn is contraindicated in women with current or a history of thrombotic or thromboembolic disorders and in women at increased risk for these events, including women over 35 years of age who smoke or women with uncontrolled hypertension.
Continue to: Details about the study...
Details about the study
The study by Simon et al is an extension study (UF-EXTEND), in that women could participate if they had completed 1 of the 2 pivotal studies on elagolix. The pivotal studies (Elaris UF1 and UF2) were both randomized, double-blinded, placebo-controlled studies with up to 6 months of therapy; for UF-EXTEND, however, participants were randomly assigned to either combined elagolix and hormone replacement therapy or elagolix alone for an additional 6 months of therapy. Although it was known that all participants would receive elagolix in UF-EXTEND, those who received hormonal add-back therapy were blinded. All women were then followed up for an additional 12 months after treatment ended.
The efficacy of elagolix was measured by the objective alkaline hematin method for menstrual blood loss with the a priori coprimary endpoints. The elagolix and hormonal add-back therapy group showed objective improvement in menstrual blood loss at 12 months in 87.9% of women in the extension study (89.4% in the elagolix alone group). This compares with 72.2% improvement at 6 months of treatment in the UF1 and UF2 studies for those taking elagolix and hormonal add-back therapy. These findings illustrate maintenance of the efficacy seen within the 6-month pivotal studies using elagolix over an extended amount of time.
The safety of elagolix also was demonstrated in UF-EXTEND. The 3 most common adverse events were similar to those found in Elaris UF1 and UF2 and included hot flushes, headache, and nausea. In the elagolix and hormonal add-back therapy group during the extension study, the percentage with hot flushes was 7%, headache 6%, and nausea 4%. These are small percentages, which is encouraging for providers and women with HMB associated with fibroids.
Effects on bone density
Bone density was evaluated at baseline in the UF1 and UF2 studies, through treatment, and then 12 months after the extended treatment was stopped. The hormonal add-back therapy of estradiol 1 mg/norethindrone acetate 0.5 mg significantly protected bone density. Some women did not have a decrease in bone density, but for those who did the average was less than 5% for the lumbar spine. The lumbar spine is considered the most reactive, so this illustrates the safety that combined therapy offers women with HMB and fibroids.
The lumbar spine is considered the most reactive, so this site is often used as the main focus with BMD studies. As Simon et al show, the lumbar spine mean BMD percent change from baseline for the elagolix with add-back therapy was -1.5% (95% confidence interval [CI], -1.9 to -1.0) in women who received up to 12 months of treatment at month 6 in the extension study. After stopping elagolix with add-back therapy, at 6 months the elagolix with add-back therapy had a Z-score of -0.6% (95% CI, -1.1 to -0.1). This shows a trend toward baseline, or a recovery within a short time from stopping medication.
Continue to: Study strengths and limitations...
Study strengths and limitations
Strengths of this study include its overall design; efficacy endpoints, which were all established a priori; the fact that measurement of menstrual blood loss was done with the objective alkaline hematin method; and the statistical analysis, which is thorough and well presented. This extension study allowed further evaluation of efficacy and safety for elagolix. Although the authors point out that there may be some selection bias in an extension study, the fact that so many women elected to continue into the extended study is a positive reflection of the treatment.
As providers learn of new therapies for management of HMB associated with fibroids, it is important to consider who will benefit the most. In my opinion, any woman with heavy periods associated with fibroids could be a candidate for elagolix with add-back therapy. This treatment is highly effective, well tolerated, and safe. My approach to management includes educating a woman on all potential therapies and this new option of elagolix and add-back therapy is an important one. The decision for an individual woman on how to manage heavy periods associated with fibroids should consider her contraceptive needs, medical issues, and the risk and benefit of individual therapies. ●
Elagolix and hormonal add-back therapy offer a long-term medical option for women with HMB associated with fibroids that is both effective and safe.
ANDREA S. LUKES, MD, MHSc
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Women’s Health. 2013;22:807-816.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Expert Commentary
Uterine fibroids are common (occurring in up to 80% of reproductive-age women),1,2 and often associated with heavy menstrual bleeding (HMB). There are surgical and medical options, but typically medical options are used for short periods of time. Elagolix with hormonal add-back therapy was recently approved (May 29, 2020) by the US Food and Drug Administration (FDA) for treatment of HMB in women with uterine fibroids for up to 24 months.
Elagolix is an oral, nonpeptide gonadotropin-releasing hormone antagonist that results in a dose-dependent reduction of gonadotropins and ovarian sex hormones. There are now 2 approved products containing elagolix, with different indications:
- Orilissa. Elagolix was approved in 2018 by the FDA for moderate to severe pain associated with endometriosis. For that indication there are 2 dose options of elagolix (150 mg for up to 2 years and 200 mg for up to 6 months) and there is no hormonal add-back therapy.
- Oriahnn. Elagolix and hormonal add-back therapy was approved in 2020 for HMB associated with uterine fibroids for up to 24 months. The total daily dose of elagolix is 600 mg (elagolix 300 mg in the morning with estradiol 1 mg/norethindrone acetate 0.5 mg and then in the evening elagolix 300 mg and no hormonal add-back).
This new class of drug, GnRH antagonist, is an important one for women’s health, and emerging science will continue to expand its potential uses, such as in reproductive health, as well as long-term efficacy and safety. The difference in daily dose of elagolix for endometriosis (150 mg for 24 months) compared with HMB associated with fibroids (600 mg for 24 months) is why the hormonal add-back therapy is important and allows for protection of bone density.
This is an important manuscript because it highlights a medical option for women with HMB associated with fibroids, which can be used for a long period of time. Further, the improvement in bleeding is both impressive and maintained in the extension study. Approximately 90% of women show improvement in their menstrual bleeding associated with fibroids.
The question of what to do after 24 months of therapy with elagolix and hormonal add-back therapy is an important one, but providers should recognize that the limiting factor with this elagolix and hormonal add-back therapy is bone mineral density (BMD). We will only learn more and more moving forward if this is a clinically meaningful reason for stopping treatment at 24 months. The FDA takes a strict view of safety, and providers must weigh this with the benefit of therapy.
One other highlight between the 2 approved medications is that Orilissa does not have a black box warning, given that there is no hormonal add-back therapy. Oriahnn does have a warning, regarding thromboembolic disorders and vascular events:
- Estrogen and progestin combinations, including Oriahnn, increase the risk of thrombotic or thromboembolic disorders, especially in women at increased risk for these events.
- Oriahnn is contraindicated in women with current or a history of thrombotic or thromboembolic disorders and in women at increased risk for these events, including women over 35 years of age who smoke or women with uncontrolled hypertension.
Continue to: Details about the study...
Details about the study
The study by Simon et al is an extension study (UF-EXTEND), in that women could participate if they had completed 1 of the 2 pivotal studies on elagolix. The pivotal studies (Elaris UF1 and UF2) were both randomized, double-blinded, placebo-controlled studies with up to 6 months of therapy; for UF-EXTEND, however, participants were randomly assigned to either combined elagolix and hormone replacement therapy or elagolix alone for an additional 6 months of therapy. Although it was known that all participants would receive elagolix in UF-EXTEND, those who received hormonal add-back therapy were blinded. All women were then followed up for an additional 12 months after treatment ended.
The efficacy of elagolix was measured by the objective alkaline hematin method for menstrual blood loss with the a priori coprimary endpoints. The elagolix and hormonal add-back therapy group showed objective improvement in menstrual blood loss at 12 months in 87.9% of women in the extension study (89.4% in the elagolix alone group). This compares with 72.2% improvement at 6 months of treatment in the UF1 and UF2 studies for those taking elagolix and hormonal add-back therapy. These findings illustrate maintenance of the efficacy seen within the 6-month pivotal studies using elagolix over an extended amount of time.
The safety of elagolix also was demonstrated in UF-EXTEND. The 3 most common adverse events were similar to those found in Elaris UF1 and UF2 and included hot flushes, headache, and nausea. In the elagolix and hormonal add-back therapy group during the extension study, the percentage with hot flushes was 7%, headache 6%, and nausea 4%. These are small percentages, which is encouraging for providers and women with HMB associated with fibroids.
Effects on bone density
Bone density was evaluated at baseline in the UF1 and UF2 studies, through treatment, and then 12 months after the extended treatment was stopped. The hormonal add-back therapy of estradiol 1 mg/norethindrone acetate 0.5 mg significantly protected bone density. Some women did not have a decrease in bone density, but for those who did the average was less than 5% for the lumbar spine. The lumbar spine is considered the most reactive, so this illustrates the safety that combined therapy offers women with HMB and fibroids.
The lumbar spine is considered the most reactive, so this site is often used as the main focus with BMD studies. As Simon et al show, the lumbar spine mean BMD percent change from baseline for the elagolix with add-back therapy was -1.5% (95% confidence interval [CI], -1.9 to -1.0) in women who received up to 12 months of treatment at month 6 in the extension study. After stopping elagolix with add-back therapy, at 6 months the elagolix with add-back therapy had a Z-score of -0.6% (95% CI, -1.1 to -0.1). This shows a trend toward baseline, or a recovery within a short time from stopping medication.
Continue to: Study strengths and limitations...
Study strengths and limitations
Strengths of this study include its overall design; efficacy endpoints, which were all established a priori; the fact that measurement of menstrual blood loss was done with the objective alkaline hematin method; and the statistical analysis, which is thorough and well presented. This extension study allowed further evaluation of efficacy and safety for elagolix. Although the authors point out that there may be some selection bias in an extension study, the fact that so many women elected to continue into the extended study is a positive reflection of the treatment.
As providers learn of new therapies for management of HMB associated with fibroids, it is important to consider who will benefit the most. In my opinion, any woman with heavy periods associated with fibroids could be a candidate for elagolix with add-back therapy. This treatment is highly effective, well tolerated, and safe. My approach to management includes educating a woman on all potential therapies and this new option of elagolix and add-back therapy is an important one. The decision for an individual woman on how to manage heavy periods associated with fibroids should consider her contraceptive needs, medical issues, and the risk and benefit of individual therapies. ●
Elagolix and hormonal add-back therapy offer a long-term medical option for women with HMB associated with fibroids that is both effective and safe.
ANDREA S. LUKES, MD, MHSc
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Expert Commentary
Uterine fibroids are common (occurring in up to 80% of reproductive-age women),1,2 and often associated with heavy menstrual bleeding (HMB). There are surgical and medical options, but typically medical options are used for short periods of time. Elagolix with hormonal add-back therapy was recently approved (May 29, 2020) by the US Food and Drug Administration (FDA) for treatment of HMB in women with uterine fibroids for up to 24 months.
Elagolix is an oral, nonpeptide gonadotropin-releasing hormone antagonist that results in a dose-dependent reduction of gonadotropins and ovarian sex hormones. There are now 2 approved products containing elagolix, with different indications:
- Orilissa. Elagolix was approved in 2018 by the FDA for moderate to severe pain associated with endometriosis. For that indication there are 2 dose options of elagolix (150 mg for up to 2 years and 200 mg for up to 6 months) and there is no hormonal add-back therapy.
- Oriahnn. Elagolix and hormonal add-back therapy was approved in 2020 for HMB associated with uterine fibroids for up to 24 months. The total daily dose of elagolix is 600 mg (elagolix 300 mg in the morning with estradiol 1 mg/norethindrone acetate 0.5 mg and then in the evening elagolix 300 mg and no hormonal add-back).
This new class of drug, GnRH antagonist, is an important one for women’s health, and emerging science will continue to expand its potential uses, such as in reproductive health, as well as long-term efficacy and safety. The difference in daily dose of elagolix for endometriosis (150 mg for 24 months) compared with HMB associated with fibroids (600 mg for 24 months) is why the hormonal add-back therapy is important and allows for protection of bone density.
This is an important manuscript because it highlights a medical option for women with HMB associated with fibroids, which can be used for a long period of time. Further, the improvement in bleeding is both impressive and maintained in the extension study. Approximately 90% of women show improvement in their menstrual bleeding associated with fibroids.
The question of what to do after 24 months of therapy with elagolix and hormonal add-back therapy is an important one, but providers should recognize that the limiting factor with this elagolix and hormonal add-back therapy is bone mineral density (BMD). We will only learn more and more moving forward if this is a clinically meaningful reason for stopping treatment at 24 months. The FDA takes a strict view of safety, and providers must weigh this with the benefit of therapy.
One other highlight between the 2 approved medications is that Orilissa does not have a black box warning, given that there is no hormonal add-back therapy. Oriahnn does have a warning, regarding thromboembolic disorders and vascular events:
- Estrogen and progestin combinations, including Oriahnn, increase the risk of thrombotic or thromboembolic disorders, especially in women at increased risk for these events.
- Oriahnn is contraindicated in women with current or a history of thrombotic or thromboembolic disorders and in women at increased risk for these events, including women over 35 years of age who smoke or women with uncontrolled hypertension.
Continue to: Details about the study...
Details about the study
The study by Simon et al is an extension study (UF-EXTEND), in that women could participate if they had completed 1 of the 2 pivotal studies on elagolix. The pivotal studies (Elaris UF1 and UF2) were both randomized, double-blinded, placebo-controlled studies with up to 6 months of therapy; for UF-EXTEND, however, participants were randomly assigned to either combined elagolix and hormone replacement therapy or elagolix alone for an additional 6 months of therapy. Although it was known that all participants would receive elagolix in UF-EXTEND, those who received hormonal add-back therapy were blinded. All women were then followed up for an additional 12 months after treatment ended.
The efficacy of elagolix was measured by the objective alkaline hematin method for menstrual blood loss with the a priori coprimary endpoints. The elagolix and hormonal add-back therapy group showed objective improvement in menstrual blood loss at 12 months in 87.9% of women in the extension study (89.4% in the elagolix alone group). This compares with 72.2% improvement at 6 months of treatment in the UF1 and UF2 studies for those taking elagolix and hormonal add-back therapy. These findings illustrate maintenance of the efficacy seen within the 6-month pivotal studies using elagolix over an extended amount of time.
The safety of elagolix also was demonstrated in UF-EXTEND. The 3 most common adverse events were similar to those found in Elaris UF1 and UF2 and included hot flushes, headache, and nausea. In the elagolix and hormonal add-back therapy group during the extension study, the percentage with hot flushes was 7%, headache 6%, and nausea 4%. These are small percentages, which is encouraging for providers and women with HMB associated with fibroids.
Effects on bone density
Bone density was evaluated at baseline in the UF1 and UF2 studies, through treatment, and then 12 months after the extended treatment was stopped. The hormonal add-back therapy of estradiol 1 mg/norethindrone acetate 0.5 mg significantly protected bone density. Some women did not have a decrease in bone density, but for those who did the average was less than 5% for the lumbar spine. The lumbar spine is considered the most reactive, so this illustrates the safety that combined therapy offers women with HMB and fibroids.
The lumbar spine is considered the most reactive, so this site is often used as the main focus with BMD studies. As Simon et al show, the lumbar spine mean BMD percent change from baseline for the elagolix with add-back therapy was -1.5% (95% confidence interval [CI], -1.9 to -1.0) in women who received up to 12 months of treatment at month 6 in the extension study. After stopping elagolix with add-back therapy, at 6 months the elagolix with add-back therapy had a Z-score of -0.6% (95% CI, -1.1 to -0.1). This shows a trend toward baseline, or a recovery within a short time from stopping medication.
Continue to: Study strengths and limitations...
Study strengths and limitations
Strengths of this study include its overall design; efficacy endpoints, which were all established a priori; the fact that measurement of menstrual blood loss was done with the objective alkaline hematin method; and the statistical analysis, which is thorough and well presented. This extension study allowed further evaluation of efficacy and safety for elagolix. Although the authors point out that there may be some selection bias in an extension study, the fact that so many women elected to continue into the extended study is a positive reflection of the treatment.
As providers learn of new therapies for management of HMB associated with fibroids, it is important to consider who will benefit the most. In my opinion, any woman with heavy periods associated with fibroids could be a candidate for elagolix with add-back therapy. This treatment is highly effective, well tolerated, and safe. My approach to management includes educating a woman on all potential therapies and this new option of elagolix and add-back therapy is an important one. The decision for an individual woman on how to manage heavy periods associated with fibroids should consider her contraceptive needs, medical issues, and the risk and benefit of individual therapies. ●
Elagolix and hormonal add-back therapy offer a long-term medical option for women with HMB associated with fibroids that is both effective and safe.
ANDREA S. LUKES, MD, MHSc
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Women’s Health. 2013;22:807-816.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Women’s Health. 2013;22:807-816.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
The Fetal Pillow: A new option for delivering the deeply impacted fetal head
Obstetricians know that a cesarean delivery (CD) for a woman with a prolonged second stage and a fetal head deeply impacted in the pelvis is challenging. In this situation, extensions of the uterine incision commonly occur, resulting in prolonged operative time and increased blood loss. Even more harrowing is the inability to deliver the fetal head, necessitating emergency assistance from other clinicians. In this situation, interventions that may be helpful include:
- extend or T the uterine incision
- enlist the aid of a clinician to push up on the fetal head with a vaginal hand (FIGURE 1)
- reverse breech extraction (FIGURE 2), and
- vaginal insertion of a Fetal Pillow prior to starting the delivery.
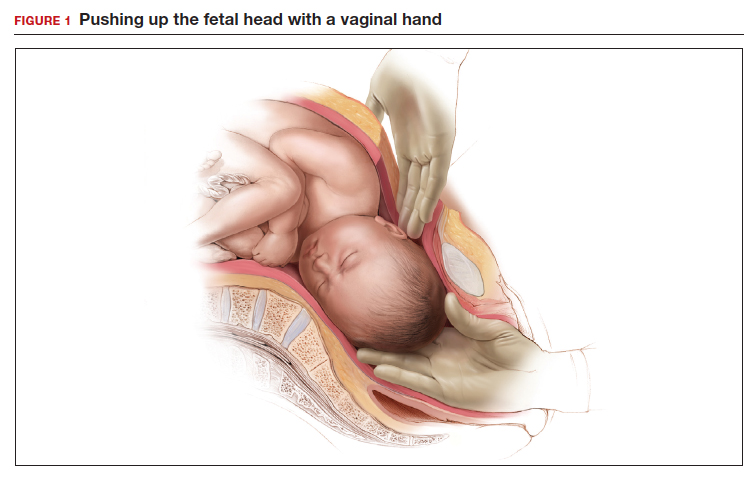
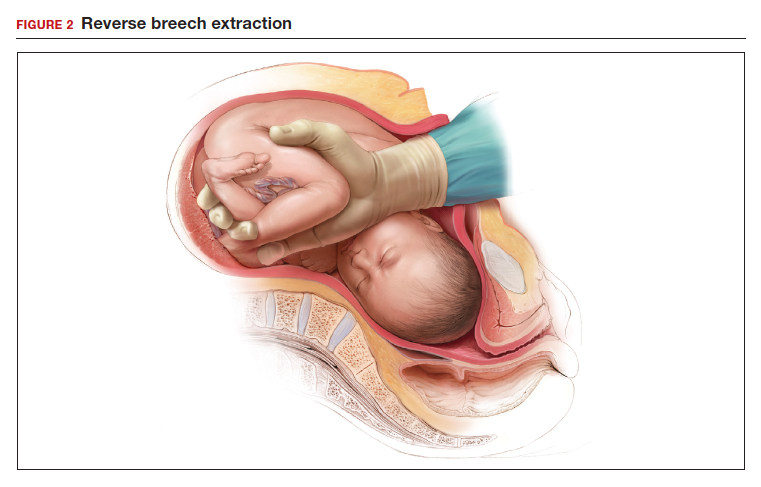
Evidence from clinical trials indicates that reverse breech extraction or insertion of a Fetal Pillow result in the best clinical outcomes.
Reverse breech extraction vs the push technique
Although the data are limited, most studies report that compared with pushing up with a vaginal hand (as shown in Figure 1), the reverse breech extraction technique (as shown in Figure 2) is associated with a reduction in extensions of the uterine incision, reduced blood loss, and reduced operative time.1 In a randomized trial, 108 women with obstructed labor undergoing CD in the second stage were randomly assigned to reverse breech extraction or pushing up with a vaginal hand.2 Following the uterine incision, the reverse breech extraction technique is performed by immediately reaching into the upper uterus and grasping the lower portion of the fetal leg and applying gentle traction on the leg until the second leg appeared. The lower legs are then pulled out of the uterus. Standard breech delivery maneuvers are used to deliver the shoulders and head. In the trial, compared with the push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (30% vs 11%; P<.05), less blood loss (899 mL vs 1,257 mL; P<.001), and shorter operative time (56 min vs 89 min, P<.001). Fetal injury was similar with the push and breech extraction techniques (6% and 7%).
In another randomized trial, 192 women undergoing CD for obstructed labor were randomly assigned to reverse breech extraction or pushing the head up with a hand in the vagina.3 Compared with the vaginal push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (19% vs 48%; P = .003), fewer cases of wound infection (2% vs 13%; P = .007), and fewer blood transfusions (2 vs 11; P = .012).
Additional options and adjuvants for facilitating delivery of a fetal head deeply impacted in the pelvis include: using a Coyne spoon, using nitroglycerine or terbutaline to relax the myometrium, breaking the vaginal suction on the fetal head before attempting delivery, keeping the wrist of the delivering hand as straight as possible to reduce uterine incision extensions, and incising the ring (if a Bandl’s ring is detected).
Continue to: The Fetal Pillow...
The Fetal Pillow
The Fetal Pillow (Safe Obstetric Systems, New York, New York) is a single-use fetal cephalic elevation device for managing the deeply impacted fetal head (FIGURE 3). The Fetal Pillow has a firm plastic base upon which is attached a soft silicon balloon. The Fetal Pillow is inserted into the vagina prior to initiating CD and the balloon is filled with 180 mL of saline, causing the fetal head to be pushed to a higher station (FIGURE 4). Use of the Fetal Pillow may be indicated prior to CD in the following situations:
- second stage labor with a deeply impacted head
- second stage labor and failed operative delivery
- occiput posterior position or deep transverse arrest
- absent progress in the first stage between 8 cm and 10 cm with a deeply impacted fetal head or excessive caput of the fetal head.
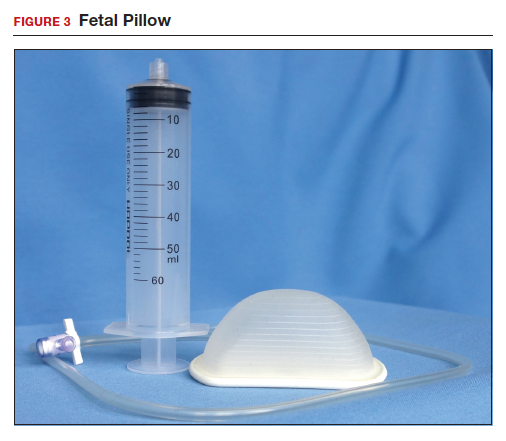
The Fetal Pillow is inserted after completing vaginal preparation for CD and before initiating skin preparation and abdominal draping. The steps for inserting the Fetal Pillow include:
- Use the 60 mL syringe to fully deflate the Fetal Pillow and leave the cock-stop open.
- Fold the Fetal Pillow by squeezing the firm plastic base, and with the patient’s legs in a frog-leg position, place the device in the vagina.
- Allow the firm plastic base to open to a flat position with the base against the posterior vaginal wall and the soft silicon balloon against the fetal head.
- Using pressure on the plastic base, gently push the Fetal Pillow posteriorly toward the sacrum of the mother.
- Use the 60 mL syringe to inflate the balloon with 180 mL of normal saline and close the valve.
- Straighten the patient’s legs and proceed with skin preparation and abdominal draping (FIGURE 4).
When the CD is completed, deflate the balloon by drawing out the saline with the 60 mL syringe and remove the device by hooking a finger around the firm plastic base. The Fetal Pillow is surprisingly easy to use.
Continue to: Effectiveness of the Fetal Pillow...
Effectiveness of the Fetal Pillow
In one randomized trial, 240 women undergoing CD were randomly allocated to a group in which the Fetal Pillow was placed in the vagina and inflated prior to the cesarean and a control group in which the Fetal Pillow was not used.
In another randomized trial, 60 nulliparous women undergoing CD in the second stage of labor had a Fetal Pillow inserted in the vagina and were randomly allocated to inflation of the pillow (Fetal Pillow group) or noninflation of the pillow (control group).5 In this study the mean length of the second stage was 4 hours. Compared with noninflation of the Fetal Pillow, use of the inflated Fetal Pillow was associated with a reduction in grade 3 extension of the uterine incision (extensions into the uterine artery, vagina, or bladder) (0% for inflation vs 13% for noninflation) and fewer difficult plus very difficult deliveries of the fetal head as reported by the surgeon (0% for inflation vs 37% for noninflation). There was no significant difference in blood loss between the two groups (800 mL vs 900 mL). These two randomized studies both reported that the use of the Fetal Pillow was associated with a reduction in grade 3 extensions of the uterine incision and a decrease in the difficulty of delivering the fetal head.
Consider trialing the Fetal Pillow
When a CD is performed after a prolonged second stage of labor, surgical complications are common, including extensions of the uterine incision and difficulty delivering the fetal head. When a grade 3 extension occurs—with tearing of a uterine artery, deep extension into the vagina, or damage to the bladder—the surgical repair can be extraordinarily challenging. Clinical trials report that both reverse breech extraction and the Fetal Pillow can facilitate CD in the setting of a prolonged second stage. For many obstetricians reverse breech extraction is a challenging obstetric maneuver. The insertion and inflation of a Fetal Pillow is a simple procedure. Obstetrician-gynecologists learn by doing. If you have never used the Fetal Pillow, I suggest you consider trialing it in your practice. ●
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123:337-345.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at cesarean section after prolonged obstructed labor: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22:375-378.
- Nooh AM, Abdeldayem HM, Ben-Affan O. Reverse breech extraction versus the standard approach of pushing the impacted fetal head up through the vagina in caesarean section for obstructed labour: a randomised controlled trial. J Obstet Gynaecol. 2017;37:459-463.
- Seal SL, Dey A, Barman SC, et al. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133:178-182.
- Lassey SC, Little SE, Saadeh M,et al. Cephalic elevation device for second-stage cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2020;135:879-884.
Obstetricians know that a cesarean delivery (CD) for a woman with a prolonged second stage and a fetal head deeply impacted in the pelvis is challenging. In this situation, extensions of the uterine incision commonly occur, resulting in prolonged operative time and increased blood loss. Even more harrowing is the inability to deliver the fetal head, necessitating emergency assistance from other clinicians. In this situation, interventions that may be helpful include:
- extend or T the uterine incision
- enlist the aid of a clinician to push up on the fetal head with a vaginal hand (FIGURE 1)
- reverse breech extraction (FIGURE 2), and
- vaginal insertion of a Fetal Pillow prior to starting the delivery.


Evidence from clinical trials indicates that reverse breech extraction or insertion of a Fetal Pillow result in the best clinical outcomes.
Reverse breech extraction vs the push technique
Although the data are limited, most studies report that compared with pushing up with a vaginal hand (as shown in Figure 1), the reverse breech extraction technique (as shown in Figure 2) is associated with a reduction in extensions of the uterine incision, reduced blood loss, and reduced operative time.1 In a randomized trial, 108 women with obstructed labor undergoing CD in the second stage were randomly assigned to reverse breech extraction or pushing up with a vaginal hand.2 Following the uterine incision, the reverse breech extraction technique is performed by immediately reaching into the upper uterus and grasping the lower portion of the fetal leg and applying gentle traction on the leg until the second leg appeared. The lower legs are then pulled out of the uterus. Standard breech delivery maneuvers are used to deliver the shoulders and head. In the trial, compared with the push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (30% vs 11%; P<.05), less blood loss (899 mL vs 1,257 mL; P<.001), and shorter operative time (56 min vs 89 min, P<.001). Fetal injury was similar with the push and breech extraction techniques (6% and 7%).
In another randomized trial, 192 women undergoing CD for obstructed labor were randomly assigned to reverse breech extraction or pushing the head up with a hand in the vagina.3 Compared with the vaginal push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (19% vs 48%; P = .003), fewer cases of wound infection (2% vs 13%; P = .007), and fewer blood transfusions (2 vs 11; P = .012).
Additional options and adjuvants for facilitating delivery of a fetal head deeply impacted in the pelvis include: using a Coyne spoon, using nitroglycerine or terbutaline to relax the myometrium, breaking the vaginal suction on the fetal head before attempting delivery, keeping the wrist of the delivering hand as straight as possible to reduce uterine incision extensions, and incising the ring (if a Bandl’s ring is detected).
Continue to: The Fetal Pillow...
The Fetal Pillow
The Fetal Pillow (Safe Obstetric Systems, New York, New York) is a single-use fetal cephalic elevation device for managing the deeply impacted fetal head (FIGURE 3). The Fetal Pillow has a firm plastic base upon which is attached a soft silicon balloon. The Fetal Pillow is inserted into the vagina prior to initiating CD and the balloon is filled with 180 mL of saline, causing the fetal head to be pushed to a higher station (FIGURE 4). Use of the Fetal Pillow may be indicated prior to CD in the following situations:
- second stage labor with a deeply impacted head
- second stage labor and failed operative delivery
- occiput posterior position or deep transverse arrest
- absent progress in the first stage between 8 cm and 10 cm with a deeply impacted fetal head or excessive caput of the fetal head.


The Fetal Pillow is inserted after completing vaginal preparation for CD and before initiating skin preparation and abdominal draping. The steps for inserting the Fetal Pillow include:
- Use the 60 mL syringe to fully deflate the Fetal Pillow and leave the cock-stop open.
- Fold the Fetal Pillow by squeezing the firm plastic base, and with the patient’s legs in a frog-leg position, place the device in the vagina.
- Allow the firm plastic base to open to a flat position with the base against the posterior vaginal wall and the soft silicon balloon against the fetal head.
- Using pressure on the plastic base, gently push the Fetal Pillow posteriorly toward the sacrum of the mother.
- Use the 60 mL syringe to inflate the balloon with 180 mL of normal saline and close the valve.
- Straighten the patient’s legs and proceed with skin preparation and abdominal draping (FIGURE 4).
When the CD is completed, deflate the balloon by drawing out the saline with the 60 mL syringe and remove the device by hooking a finger around the firm plastic base. The Fetal Pillow is surprisingly easy to use.
Continue to: Effectiveness of the Fetal Pillow...
Effectiveness of the Fetal Pillow
In one randomized trial, 240 women undergoing CD were randomly allocated to a group in which the Fetal Pillow was placed in the vagina and inflated prior to the cesarean and a control group in which the Fetal Pillow was not used.
In another randomized trial, 60 nulliparous women undergoing CD in the second stage of labor had a Fetal Pillow inserted in the vagina and were randomly allocated to inflation of the pillow (Fetal Pillow group) or noninflation of the pillow (control group).5 In this study the mean length of the second stage was 4 hours. Compared with noninflation of the Fetal Pillow, use of the inflated Fetal Pillow was associated with a reduction in grade 3 extension of the uterine incision (extensions into the uterine artery, vagina, or bladder) (0% for inflation vs 13% for noninflation) and fewer difficult plus very difficult deliveries of the fetal head as reported by the surgeon (0% for inflation vs 37% for noninflation). There was no significant difference in blood loss between the two groups (800 mL vs 900 mL). These two randomized studies both reported that the use of the Fetal Pillow was associated with a reduction in grade 3 extensions of the uterine incision and a decrease in the difficulty of delivering the fetal head.
Consider trialing the Fetal Pillow
When a CD is performed after a prolonged second stage of labor, surgical complications are common, including extensions of the uterine incision and difficulty delivering the fetal head. When a grade 3 extension occurs—with tearing of a uterine artery, deep extension into the vagina, or damage to the bladder—the surgical repair can be extraordinarily challenging. Clinical trials report that both reverse breech extraction and the Fetal Pillow can facilitate CD in the setting of a prolonged second stage. For many obstetricians reverse breech extraction is a challenging obstetric maneuver. The insertion and inflation of a Fetal Pillow is a simple procedure. Obstetrician-gynecologists learn by doing. If you have never used the Fetal Pillow, I suggest you consider trialing it in your practice. ●
Obstetricians know that a cesarean delivery (CD) for a woman with a prolonged second stage and a fetal head deeply impacted in the pelvis is challenging. In this situation, extensions of the uterine incision commonly occur, resulting in prolonged operative time and increased blood loss. Even more harrowing is the inability to deliver the fetal head, necessitating emergency assistance from other clinicians. In this situation, interventions that may be helpful include:
- extend or T the uterine incision
- enlist the aid of a clinician to push up on the fetal head with a vaginal hand (FIGURE 1)
- reverse breech extraction (FIGURE 2), and
- vaginal insertion of a Fetal Pillow prior to starting the delivery.


Evidence from clinical trials indicates that reverse breech extraction or insertion of a Fetal Pillow result in the best clinical outcomes.
Reverse breech extraction vs the push technique
Although the data are limited, most studies report that compared with pushing up with a vaginal hand (as shown in Figure 1), the reverse breech extraction technique (as shown in Figure 2) is associated with a reduction in extensions of the uterine incision, reduced blood loss, and reduced operative time.1 In a randomized trial, 108 women with obstructed labor undergoing CD in the second stage were randomly assigned to reverse breech extraction or pushing up with a vaginal hand.2 Following the uterine incision, the reverse breech extraction technique is performed by immediately reaching into the upper uterus and grasping the lower portion of the fetal leg and applying gentle traction on the leg until the second leg appeared. The lower legs are then pulled out of the uterus. Standard breech delivery maneuvers are used to deliver the shoulders and head. In the trial, compared with the push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (30% vs 11%; P<.05), less blood loss (899 mL vs 1,257 mL; P<.001), and shorter operative time (56 min vs 89 min, P<.001). Fetal injury was similar with the push and breech extraction techniques (6% and 7%).
In another randomized trial, 192 women undergoing CD for obstructed labor were randomly assigned to reverse breech extraction or pushing the head up with a hand in the vagina.3 Compared with the vaginal push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (19% vs 48%; P = .003), fewer cases of wound infection (2% vs 13%; P = .007), and fewer blood transfusions (2 vs 11; P = .012).
Additional options and adjuvants for facilitating delivery of a fetal head deeply impacted in the pelvis include: using a Coyne spoon, using nitroglycerine or terbutaline to relax the myometrium, breaking the vaginal suction on the fetal head before attempting delivery, keeping the wrist of the delivering hand as straight as possible to reduce uterine incision extensions, and incising the ring (if a Bandl’s ring is detected).
Continue to: The Fetal Pillow...
The Fetal Pillow
The Fetal Pillow (Safe Obstetric Systems, New York, New York) is a single-use fetal cephalic elevation device for managing the deeply impacted fetal head (FIGURE 3). The Fetal Pillow has a firm plastic base upon which is attached a soft silicon balloon. The Fetal Pillow is inserted into the vagina prior to initiating CD and the balloon is filled with 180 mL of saline, causing the fetal head to be pushed to a higher station (FIGURE 4). Use of the Fetal Pillow may be indicated prior to CD in the following situations:
- second stage labor with a deeply impacted head
- second stage labor and failed operative delivery
- occiput posterior position or deep transverse arrest
- absent progress in the first stage between 8 cm and 10 cm with a deeply impacted fetal head or excessive caput of the fetal head.


The Fetal Pillow is inserted after completing vaginal preparation for CD and before initiating skin preparation and abdominal draping. The steps for inserting the Fetal Pillow include:
- Use the 60 mL syringe to fully deflate the Fetal Pillow and leave the cock-stop open.
- Fold the Fetal Pillow by squeezing the firm plastic base, and with the patient’s legs in a frog-leg position, place the device in the vagina.
- Allow the firm plastic base to open to a flat position with the base against the posterior vaginal wall and the soft silicon balloon against the fetal head.
- Using pressure on the plastic base, gently push the Fetal Pillow posteriorly toward the sacrum of the mother.
- Use the 60 mL syringe to inflate the balloon with 180 mL of normal saline and close the valve.
- Straighten the patient’s legs and proceed with skin preparation and abdominal draping (FIGURE 4).
When the CD is completed, deflate the balloon by drawing out the saline with the 60 mL syringe and remove the device by hooking a finger around the firm plastic base. The Fetal Pillow is surprisingly easy to use.
Continue to: Effectiveness of the Fetal Pillow...
Effectiveness of the Fetal Pillow
In one randomized trial, 240 women undergoing CD were randomly allocated to a group in which the Fetal Pillow was placed in the vagina and inflated prior to the cesarean and a control group in which the Fetal Pillow was not used.
In another randomized trial, 60 nulliparous women undergoing CD in the second stage of labor had a Fetal Pillow inserted in the vagina and were randomly allocated to inflation of the pillow (Fetal Pillow group) or noninflation of the pillow (control group).5 In this study the mean length of the second stage was 4 hours. Compared with noninflation of the Fetal Pillow, use of the inflated Fetal Pillow was associated with a reduction in grade 3 extension of the uterine incision (extensions into the uterine artery, vagina, or bladder) (0% for inflation vs 13% for noninflation) and fewer difficult plus very difficult deliveries of the fetal head as reported by the surgeon (0% for inflation vs 37% for noninflation). There was no significant difference in blood loss between the two groups (800 mL vs 900 mL). These two randomized studies both reported that the use of the Fetal Pillow was associated with a reduction in grade 3 extensions of the uterine incision and a decrease in the difficulty of delivering the fetal head.
Consider trialing the Fetal Pillow
When a CD is performed after a prolonged second stage of labor, surgical complications are common, including extensions of the uterine incision and difficulty delivering the fetal head. When a grade 3 extension occurs—with tearing of a uterine artery, deep extension into the vagina, or damage to the bladder—the surgical repair can be extraordinarily challenging. Clinical trials report that both reverse breech extraction and the Fetal Pillow can facilitate CD in the setting of a prolonged second stage. For many obstetricians reverse breech extraction is a challenging obstetric maneuver. The insertion and inflation of a Fetal Pillow is a simple procedure. Obstetrician-gynecologists learn by doing. If you have never used the Fetal Pillow, I suggest you consider trialing it in your practice. ●
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123:337-345.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at cesarean section after prolonged obstructed labor: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22:375-378.
- Nooh AM, Abdeldayem HM, Ben-Affan O. Reverse breech extraction versus the standard approach of pushing the impacted fetal head up through the vagina in caesarean section for obstructed labour: a randomised controlled trial. J Obstet Gynaecol. 2017;37:459-463.
- Seal SL, Dey A, Barman SC, et al. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133:178-182.
- Lassey SC, Little SE, Saadeh M,et al. Cephalic elevation device for second-stage cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2020;135:879-884.
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123:337-345.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at cesarean section after prolonged obstructed labor: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22:375-378.
- Nooh AM, Abdeldayem HM, Ben-Affan O. Reverse breech extraction versus the standard approach of pushing the impacted fetal head up through the vagina in caesarean section for obstructed labour: a randomised controlled trial. J Obstet Gynaecol. 2017;37:459-463.
- Seal SL, Dey A, Barman SC, et al. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133:178-182.
- Lassey SC, Little SE, Saadeh M,et al. Cephalic elevation device for second-stage cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2020;135:879-884.
ASCCP guidelines for managing abnormal cervical cancer tests: What’s new?
The 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors Consensus Guidelines, which represent a consensus of nearly 20 professional organizations and patient advocates, are a culmination of almost 10 years of research.1 With the last version issued in 2012,2 these latest guidelines offer the most recent recommendations regarding safely triaging women with abnormal cervical cancer screening results.
According to the consensus, research has shown that risk-based management allows clinicians to better discriminate women who will likely develop precancer from those who can safely continue with routine screening. As you will hear from guidelines coauthor Dr. Warner Huh, one of the most important differences between these guidelines and the 2012 version is a new emphasis on the principle of “equal management for equal risk.” Essentially, this insures that all women who have the same amount of risk for progression to precancer or cancer are managed the same.
The guidelines were once again published in the Journal of Lower Genital Tract Disease, and the tables they reference are publicly available. Additionally, ASCCP is developing a new management guidelines app to facilitate the use of the guidelines on smartphones and computers. With the publicly available risk tables, and the ASCCP navigation app, the guidelines will more easily accommodate updates as new information and technology become available.
OBG Management : The latest ASCCP guidelines, published in April, represent a “paradigm shift” from results to risk-based guidelines. Can you explain what this means and why the shift was undertaken?
Warner K. Huh, MD: Yes, the shift occurred because we needed to focus less on algorithms and more on risk. We started promulgating a concept of “equal management for equal risk” back in 2012. What this means is that if we have a method to look up a risk score based on relevant test results and other pieces of information, then all patients with that score should be managed in the same manner. We wanted that to be the underlying principle.
Focusing on risk tables also makes it easier to incorporate any future technologies used for risk estimation without having to rebuild algorithms from scratch. ASCCP is developing a new management guidelines app to streamline navigation of the guidelines. This app makes them easier for clinicians to use; they simply plug in certain variables from the patient’s history and receive 1 of 5 outputs: treatment, colposcopy, or surveillance at 1, 3, or 5 years.
The only drawbacks, if you view them as such, are that the clinician must plug in all the variables, and then must sit back and trust in what we have done. Clinicians have to trust that the system works and will simplify the clinical decision making.
We spent a lot of time determining what the risk thresholds should be. Some may argue they are arbitrary, but the decisions were data-driven, and carefully, thoughtfully vetted; we deliberated about whether the cut points actually made sense clinically to a practicing clinician base. The clinical action thresholds refer to a specific percentage below which a woman falls into one bucket and above which she falls into another bucket.
The other element that is unique about the guidelines is that instead of looking at the patient’s current screening result in isolation, the user sees it along with the prior one because prior history dictates subsequent risk.
It’s important that clinicians understand why this system is so markedly different from what we have done previously, and why risk-based guidelines make infinitely more sense than algorithmic ones. It’s because: 1) they can be easier to use; 2) they incorporate new data more efficiently and effectively than algorithm-based guidelines; and 3) they can incorporate future technologies seamlessly rather than having to create yet another algorithm.
Continue to: OBG Management : What do clinicians need in order to execute the guidelines?...
OBG Management : What do clinicians need in order to execute the guidelines?
Dr. Huh: Nothing. All of the information needed—the guidelines article and risk tables—are publicly available. However, to make navigation of the guidelines easier, the plan is for the app that I mentioned. I have the app on my phone and am actively beta testing it now. We’re planning on creating a web-based application as well, that will allow users to access the Internet and their electronic health record system so that they can plug in information directly from patient charts. The web-based app will be similar to the web-based Breast Cancer Surveillance Consortium’s Risk Calculator (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm). You will pull it up, plug in the requested information, including the patient’s age; their Pap smear and genotyping results; and their previous screening history.
OBG Management : When will the app be available for users?
Dr. Huh: It will be available for release on June 8.
OBG Management : Were HPV vaccination levels incorporated into the new guidelines?
OBG Management : Have recommendations regarding colposcopy changed?
Dr. Huh: Not really. About 3 years ago, we created basic colposcopy guidelines—the ASCCP Colposcopy Standards—so everything about colposcopy references back to those guidelines. Those colposcopy standards covered terminology and risk-based colposcopy, which actually aligns beautifully with these guidelines.
OBG Management : To narrow in on some changes from the prior guidelines, can colposcopy be deferred in certain patients?
Dr. Huh: Yes. Not everyone who has an abnormal screening test needs to come back for colposcopy.
OBG Management : How has guidance for expedited treatment or treatment without colposcopic biopsy changed?
Dr. Huh: This was heavily debated within not only the treatment group that I co-chaired with Richard Guido, MD, but also within the entire steering committee. The recommendation is that if the patient has an immediate risk of CIN 3 that is >60%, the patient should go straight to treatment without a colposcopic biopsy. The main reason for this is that you do not want to biopsy a patient and then lose them to follow-up.
When a woman has >60% immediate risk of CIN 3, we are fairly certain that colposcopy is not going to change management ultimately, so we recommend that patients receive treatment right away. We have already been doing this for 15 to 20 years, so this is not a new concept. It is just more formally codified here by assigning a percentage to the risk. Those who have between 25% and 60% immediate risk of CIN 3 should receive immediate colposcopy. We realize that not all clinicians have the ability to do this, so if clinicians can’t treat immediately, we recommend they do whatever they can to prevent losing the patient to follow-up.
OBG Management : How should a positive primary HPV screening test be managed?
Dr. Huh: If a woman has a positive primary HPV screening test, genotyping should be performed. If genotyping reveals HPV 16 or 18, then the patient should proceed to colposcopy. If genotyping reveals other forms of HPV, reflex cytology or a Pap smear should follow. ●
- Perkins RB, Guido RS, Castle PE, et al, for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020;24(2):102-131.
- Massad LS, Einstein MH, Huh WK, et al, for the 2012 ASCCP Consensus Guidelines Conference. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2013;17(5):S1-S27.
The 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors Consensus Guidelines, which represent a consensus of nearly 20 professional organizations and patient advocates, are a culmination of almost 10 years of research.1 With the last version issued in 2012,2 these latest guidelines offer the most recent recommendations regarding safely triaging women with abnormal cervical cancer screening results.
According to the consensus, research has shown that risk-based management allows clinicians to better discriminate women who will likely develop precancer from those who can safely continue with routine screening. As you will hear from guidelines coauthor Dr. Warner Huh, one of the most important differences between these guidelines and the 2012 version is a new emphasis on the principle of “equal management for equal risk.” Essentially, this insures that all women who have the same amount of risk for progression to precancer or cancer are managed the same.
The guidelines were once again published in the Journal of Lower Genital Tract Disease, and the tables they reference are publicly available. Additionally, ASCCP is developing a new management guidelines app to facilitate the use of the guidelines on smartphones and computers. With the publicly available risk tables, and the ASCCP navigation app, the guidelines will more easily accommodate updates as new information and technology become available.
OBG Management : The latest ASCCP guidelines, published in April, represent a “paradigm shift” from results to risk-based guidelines. Can you explain what this means and why the shift was undertaken?
Warner K. Huh, MD: Yes, the shift occurred because we needed to focus less on algorithms and more on risk. We started promulgating a concept of “equal management for equal risk” back in 2012. What this means is that if we have a method to look up a risk score based on relevant test results and other pieces of information, then all patients with that score should be managed in the same manner. We wanted that to be the underlying principle.
Focusing on risk tables also makes it easier to incorporate any future technologies used for risk estimation without having to rebuild algorithms from scratch. ASCCP is developing a new management guidelines app to streamline navigation of the guidelines. This app makes them easier for clinicians to use; they simply plug in certain variables from the patient’s history and receive 1 of 5 outputs: treatment, colposcopy, or surveillance at 1, 3, or 5 years.
The only drawbacks, if you view them as such, are that the clinician must plug in all the variables, and then must sit back and trust in what we have done. Clinicians have to trust that the system works and will simplify the clinical decision making.
We spent a lot of time determining what the risk thresholds should be. Some may argue they are arbitrary, but the decisions were data-driven, and carefully, thoughtfully vetted; we deliberated about whether the cut points actually made sense clinically to a practicing clinician base. The clinical action thresholds refer to a specific percentage below which a woman falls into one bucket and above which she falls into another bucket.
The other element that is unique about the guidelines is that instead of looking at the patient’s current screening result in isolation, the user sees it along with the prior one because prior history dictates subsequent risk.
It’s important that clinicians understand why this system is so markedly different from what we have done previously, and why risk-based guidelines make infinitely more sense than algorithmic ones. It’s because: 1) they can be easier to use; 2) they incorporate new data more efficiently and effectively than algorithm-based guidelines; and 3) they can incorporate future technologies seamlessly rather than having to create yet another algorithm.
Continue to: OBG Management : What do clinicians need in order to execute the guidelines?...
OBG Management : What do clinicians need in order to execute the guidelines?
Dr. Huh: Nothing. All of the information needed—the guidelines article and risk tables—are publicly available. However, to make navigation of the guidelines easier, the plan is for the app that I mentioned. I have the app on my phone and am actively beta testing it now. We’re planning on creating a web-based application as well, that will allow users to access the Internet and their electronic health record system so that they can plug in information directly from patient charts. The web-based app will be similar to the web-based Breast Cancer Surveillance Consortium’s Risk Calculator (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm). You will pull it up, plug in the requested information, including the patient’s age; their Pap smear and genotyping results; and their previous screening history.
OBG Management : When will the app be available for users?
Dr. Huh: It will be available for release on June 8.
OBG Management : Were HPV vaccination levels incorporated into the new guidelines?
OBG Management : Have recommendations regarding colposcopy changed?
Dr. Huh: Not really. About 3 years ago, we created basic colposcopy guidelines—the ASCCP Colposcopy Standards—so everything about colposcopy references back to those guidelines. Those colposcopy standards covered terminology and risk-based colposcopy, which actually aligns beautifully with these guidelines.
OBG Management : To narrow in on some changes from the prior guidelines, can colposcopy be deferred in certain patients?
Dr. Huh: Yes. Not everyone who has an abnormal screening test needs to come back for colposcopy.
OBG Management : How has guidance for expedited treatment or treatment without colposcopic biopsy changed?
Dr. Huh: This was heavily debated within not only the treatment group that I co-chaired with Richard Guido, MD, but also within the entire steering committee. The recommendation is that if the patient has an immediate risk of CIN 3 that is >60%, the patient should go straight to treatment without a colposcopic biopsy. The main reason for this is that you do not want to biopsy a patient and then lose them to follow-up.
When a woman has >60% immediate risk of CIN 3, we are fairly certain that colposcopy is not going to change management ultimately, so we recommend that patients receive treatment right away. We have already been doing this for 15 to 20 years, so this is not a new concept. It is just more formally codified here by assigning a percentage to the risk. Those who have between 25% and 60% immediate risk of CIN 3 should receive immediate colposcopy. We realize that not all clinicians have the ability to do this, so if clinicians can’t treat immediately, we recommend they do whatever they can to prevent losing the patient to follow-up.
OBG Management : How should a positive primary HPV screening test be managed?
Dr. Huh: If a woman has a positive primary HPV screening test, genotyping should be performed. If genotyping reveals HPV 16 or 18, then the patient should proceed to colposcopy. If genotyping reveals other forms of HPV, reflex cytology or a Pap smear should follow. ●
The 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors Consensus Guidelines, which represent a consensus of nearly 20 professional organizations and patient advocates, are a culmination of almost 10 years of research.1 With the last version issued in 2012,2 these latest guidelines offer the most recent recommendations regarding safely triaging women with abnormal cervical cancer screening results.
According to the consensus, research has shown that risk-based management allows clinicians to better discriminate women who will likely develop precancer from those who can safely continue with routine screening. As you will hear from guidelines coauthor Dr. Warner Huh, one of the most important differences between these guidelines and the 2012 version is a new emphasis on the principle of “equal management for equal risk.” Essentially, this insures that all women who have the same amount of risk for progression to precancer or cancer are managed the same.
The guidelines were once again published in the Journal of Lower Genital Tract Disease, and the tables they reference are publicly available. Additionally, ASCCP is developing a new management guidelines app to facilitate the use of the guidelines on smartphones and computers. With the publicly available risk tables, and the ASCCP navigation app, the guidelines will more easily accommodate updates as new information and technology become available.
OBG Management : The latest ASCCP guidelines, published in April, represent a “paradigm shift” from results to risk-based guidelines. Can you explain what this means and why the shift was undertaken?
Warner K. Huh, MD: Yes, the shift occurred because we needed to focus less on algorithms and more on risk. We started promulgating a concept of “equal management for equal risk” back in 2012. What this means is that if we have a method to look up a risk score based on relevant test results and other pieces of information, then all patients with that score should be managed in the same manner. We wanted that to be the underlying principle.
Focusing on risk tables also makes it easier to incorporate any future technologies used for risk estimation without having to rebuild algorithms from scratch. ASCCP is developing a new management guidelines app to streamline navigation of the guidelines. This app makes them easier for clinicians to use; they simply plug in certain variables from the patient’s history and receive 1 of 5 outputs: treatment, colposcopy, or surveillance at 1, 3, or 5 years.
The only drawbacks, if you view them as such, are that the clinician must plug in all the variables, and then must sit back and trust in what we have done. Clinicians have to trust that the system works and will simplify the clinical decision making.
We spent a lot of time determining what the risk thresholds should be. Some may argue they are arbitrary, but the decisions were data-driven, and carefully, thoughtfully vetted; we deliberated about whether the cut points actually made sense clinically to a practicing clinician base. The clinical action thresholds refer to a specific percentage below which a woman falls into one bucket and above which she falls into another bucket.
The other element that is unique about the guidelines is that instead of looking at the patient’s current screening result in isolation, the user sees it along with the prior one because prior history dictates subsequent risk.
It’s important that clinicians understand why this system is so markedly different from what we have done previously, and why risk-based guidelines make infinitely more sense than algorithmic ones. It’s because: 1) they can be easier to use; 2) they incorporate new data more efficiently and effectively than algorithm-based guidelines; and 3) they can incorporate future technologies seamlessly rather than having to create yet another algorithm.
Continue to: OBG Management : What do clinicians need in order to execute the guidelines?...
OBG Management : What do clinicians need in order to execute the guidelines?
Dr. Huh: Nothing. All of the information needed—the guidelines article and risk tables—are publicly available. However, to make navigation of the guidelines easier, the plan is for the app that I mentioned. I have the app on my phone and am actively beta testing it now. We’re planning on creating a web-based application as well, that will allow users to access the Internet and their electronic health record system so that they can plug in information directly from patient charts. The web-based app will be similar to the web-based Breast Cancer Surveillance Consortium’s Risk Calculator (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm). You will pull it up, plug in the requested information, including the patient’s age; their Pap smear and genotyping results; and their previous screening history.
OBG Management : When will the app be available for users?
Dr. Huh: It will be available for release on June 8.
OBG Management : Were HPV vaccination levels incorporated into the new guidelines?
OBG Management : Have recommendations regarding colposcopy changed?
Dr. Huh: Not really. About 3 years ago, we created basic colposcopy guidelines—the ASCCP Colposcopy Standards—so everything about colposcopy references back to those guidelines. Those colposcopy standards covered terminology and risk-based colposcopy, which actually aligns beautifully with these guidelines.
OBG Management : To narrow in on some changes from the prior guidelines, can colposcopy be deferred in certain patients?
Dr. Huh: Yes. Not everyone who has an abnormal screening test needs to come back for colposcopy.
OBG Management : How has guidance for expedited treatment or treatment without colposcopic biopsy changed?
Dr. Huh: This was heavily debated within not only the treatment group that I co-chaired with Richard Guido, MD, but also within the entire steering committee. The recommendation is that if the patient has an immediate risk of CIN 3 that is >60%, the patient should go straight to treatment without a colposcopic biopsy. The main reason for this is that you do not want to biopsy a patient and then lose them to follow-up.
When a woman has >60% immediate risk of CIN 3, we are fairly certain that colposcopy is not going to change management ultimately, so we recommend that patients receive treatment right away. We have already been doing this for 15 to 20 years, so this is not a new concept. It is just more formally codified here by assigning a percentage to the risk. Those who have between 25% and 60% immediate risk of CIN 3 should receive immediate colposcopy. We realize that not all clinicians have the ability to do this, so if clinicians can’t treat immediately, we recommend they do whatever they can to prevent losing the patient to follow-up.
OBG Management : How should a positive primary HPV screening test be managed?
Dr. Huh: If a woman has a positive primary HPV screening test, genotyping should be performed. If genotyping reveals HPV 16 or 18, then the patient should proceed to colposcopy. If genotyping reveals other forms of HPV, reflex cytology or a Pap smear should follow. ●
- Perkins RB, Guido RS, Castle PE, et al, for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020;24(2):102-131.
- Massad LS, Einstein MH, Huh WK, et al, for the 2012 ASCCP Consensus Guidelines Conference. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2013;17(5):S1-S27.
- Perkins RB, Guido RS, Castle PE, et al, for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020;24(2):102-131.
- Massad LS, Einstein MH, Huh WK, et al, for the 2012 ASCCP Consensus Guidelines Conference. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2013;17(5):S1-S27.
Respiratory particles generated by speech can remain airborne for up to 14 minutes
Stadnytskyi and colleagues explored the size of droplets created by speech using a highly sensitive laser system. They reported in PNAS that speaking resulted in the generation of a high number of medium-sized droplets (10- to 100-µm in diameter). Under the conditions of their experiment (27% humidity and 23° C) they reported that speech probably generates droplets that originate at a size of 12 to 21 µm in diameter and quickly dehydrate to an estimated diameter of 4 µm. The 4 µm-sized particles had a falling rate of only 0.06 cm·s−1 and remained airborne for 8 to 14 minutes.1
As reported by Hamner and colleagues, on March 10, 2020, 61 persons attended a 2.5-hour choir practice. One choir member had symptoms of an upper respiratory infection that began on March 7. Eventually that choir member tested positive for SARS-CoV-2. Of the 60 remaining persons, 52 (86.7%) eventually developed an upper respiratory illness. In total, 33 cases of SARS-CoV-2 were confirmed by nucleic acid testing and 20 probable cases were diagnosed (these individuals declined testing). The choir attendees developed symptoms at a median of 3 days following the practice, with a range of 1 to 12 days. Three of the 53 ill people were hospitalized, and two died.2
The Stadnytskyi study suggests that speech generates large respiratory droplets that dehydrate into very small droplets that may remain in the air for an extended period of time. If the SARS-CoV-2 virus were in the original large droplet, the rapid dehydration of the droplet would result in prolonged airborne presence of the virus and enhance its infectivity.
The Hamner study highlights the importance of vocalization and respiratory particles in transmitting the SARS-CoV-2 virus. For clinicians and patients, both studies support many recommendations to reduce viral transmission, including:
- all clinicians and patients need to wear face masks
- all clinicians and patients should avoid face-to-face contact if alternative approaches to communication are possible
- all clinicians and patients should avoid gathering in large groups or crowded public spaces and need to maintain physical distancing.
The COVID pandemic has dramatically changed how we practice medicine and socialize.
- Stadnytskyi V, Bax CE, Bax A, et al. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. PNAS. May 13, 2020. https://doi.org/10.1073/pnas.2006874117.
- Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at choir practice—Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606-610. Early release, May 12, 2020.
Stadnytskyi and colleagues explored the size of droplets created by speech using a highly sensitive laser system. They reported in PNAS that speaking resulted in the generation of a high number of medium-sized droplets (10- to 100-µm in diameter). Under the conditions of their experiment (27% humidity and 23° C) they reported that speech probably generates droplets that originate at a size of 12 to 21 µm in diameter and quickly dehydrate to an estimated diameter of 4 µm. The 4 µm-sized particles had a falling rate of only 0.06 cm·s−1 and remained airborne for 8 to 14 minutes.1
As reported by Hamner and colleagues, on March 10, 2020, 61 persons attended a 2.5-hour choir practice. One choir member had symptoms of an upper respiratory infection that began on March 7. Eventually that choir member tested positive for SARS-CoV-2. Of the 60 remaining persons, 52 (86.7%) eventually developed an upper respiratory illness. In total, 33 cases of SARS-CoV-2 were confirmed by nucleic acid testing and 20 probable cases were diagnosed (these individuals declined testing). The choir attendees developed symptoms at a median of 3 days following the practice, with a range of 1 to 12 days. Three of the 53 ill people were hospitalized, and two died.2
The Stadnytskyi study suggests that speech generates large respiratory droplets that dehydrate into very small droplets that may remain in the air for an extended period of time. If the SARS-CoV-2 virus were in the original large droplet, the rapid dehydration of the droplet would result in prolonged airborne presence of the virus and enhance its infectivity.
The Hamner study highlights the importance of vocalization and respiratory particles in transmitting the SARS-CoV-2 virus. For clinicians and patients, both studies support many recommendations to reduce viral transmission, including:
- all clinicians and patients need to wear face masks
- all clinicians and patients should avoid face-to-face contact if alternative approaches to communication are possible
- all clinicians and patients should avoid gathering in large groups or crowded public spaces and need to maintain physical distancing.
The COVID pandemic has dramatically changed how we practice medicine and socialize.
Stadnytskyi and colleagues explored the size of droplets created by speech using a highly sensitive laser system. They reported in PNAS that speaking resulted in the generation of a high number of medium-sized droplets (10- to 100-µm in diameter). Under the conditions of their experiment (27% humidity and 23° C) they reported that speech probably generates droplets that originate at a size of 12 to 21 µm in diameter and quickly dehydrate to an estimated diameter of 4 µm. The 4 µm-sized particles had a falling rate of only 0.06 cm·s−1 and remained airborne for 8 to 14 minutes.1
As reported by Hamner and colleagues, on March 10, 2020, 61 persons attended a 2.5-hour choir practice. One choir member had symptoms of an upper respiratory infection that began on March 7. Eventually that choir member tested positive for SARS-CoV-2. Of the 60 remaining persons, 52 (86.7%) eventually developed an upper respiratory illness. In total, 33 cases of SARS-CoV-2 were confirmed by nucleic acid testing and 20 probable cases were diagnosed (these individuals declined testing). The choir attendees developed symptoms at a median of 3 days following the practice, with a range of 1 to 12 days. Three of the 53 ill people were hospitalized, and two died.2
The Stadnytskyi study suggests that speech generates large respiratory droplets that dehydrate into very small droplets that may remain in the air for an extended period of time. If the SARS-CoV-2 virus were in the original large droplet, the rapid dehydration of the droplet would result in prolonged airborne presence of the virus and enhance its infectivity.
The Hamner study highlights the importance of vocalization and respiratory particles in transmitting the SARS-CoV-2 virus. For clinicians and patients, both studies support many recommendations to reduce viral transmission, including:
- all clinicians and patients need to wear face masks
- all clinicians and patients should avoid face-to-face contact if alternative approaches to communication are possible
- all clinicians and patients should avoid gathering in large groups or crowded public spaces and need to maintain physical distancing.
The COVID pandemic has dramatically changed how we practice medicine and socialize.
- Stadnytskyi V, Bax CE, Bax A, et al. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. PNAS. May 13, 2020. https://doi.org/10.1073/pnas.2006874117.
- Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at choir practice—Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606-610. Early release, May 12, 2020.
- Stadnytskyi V, Bax CE, Bax A, et al. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. PNAS. May 13, 2020. https://doi.org/10.1073/pnas.2006874117.
- Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at choir practice—Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606-610. Early release, May 12, 2020.