User login
The joys and rewards of an asymmetric life
The benefits of living a balanced life is a very popular concept. But I beg to differ. Balance in one’s life is overrated. Allocating equal time to the various components of one’s life may sound admirable, but it is a recipe for an ordinary life, with no major achievements or a memorable legacy. Scoring a “moonshot” achievement while living a balanced life is highly unlikely.
The benefits of deliberately leading an “asymmetric life” is an epiphany I acquired as a young boy addicted to watching stellar Olympic athletes win gold medals. I dreamed about being the best in the world in a sport, or in something else. As I read about the lives of my Olympic idols, my mind was opened to the fact that each of them led an unbalanced life in the pursuit of their cherished goal to be the best in the world: a gold medalist. I found out that for several years before the Olympic games, these athletes spent a disproportionate amount of their waking time (≥10 hours a day) practicing their sport, strengthening their muscles, building up their stamina, and honing their physical skills and mental toughness. Those sacrifices were necessary—in fact, indispensable—to set themselves apart from us mere mortals. Their social life was quite restricted, and even their educational pursuits had to be reduced or deferred.
I realized at a young age that to be the world’s best athlete, one must lead a purpose-driven life and channel a tremendous amount of time and energy to achieve the cherished goal of an Olympic gold medal. I understood the sacrifices necessary to excel in sports, and concluded the same was also true outside of sports, such as for Nobel Laureates, world-class pianists, prodigious authors, ballet dancers, opera divas, or self-employed entrepreneurs.
As I grew up, I repeatedly heard people praise “the balanced life,” but in my heart, I knew that was a fallacy. I had already decided in high school that I wanted to become a psychiatric physician. I was a premed major in college and very aware that our medical school enrolled only 44 students into the Med 1 class. There were >350 other premed undergraduates. Thus, without hesitation, and with gusto, I deliberately led an unbalanced life, studying countless hours each day to achieve an A grade in all required and elective courses to earn a spot on the Dean’s list. I already had confidence in my academic skills because of my excellent performance in high school, but I was not going to take any chances because I recalled a quote commonly attributed to Thomas Edison: “Genius is 1% inspiration and 99% perspiration.” This is obviously antithetical to living a balanced life.
I matriculated in medical school, and my unbalanced lifestyle continued unabated. Most readers of this journal are fellow physicians who know well the heavy demands of medical school on our lives, in both the preclinical and clinical years. Trying to lead a balanced life during the 4 years of medical school can have disastrous consequences. We all led an “asymmetric existence” with 75% (or more) of our waking hours invested in our careers and 25% (or less) directed to our social lives (and fortunately, our families and friends generally understood). That is what it takes to earn the coveted MD, the equivalent of an Olympic medal for intellectual athletes.
Then came 4 more years of psychiatric residency training, and the long hours of work continued, along with many nights and weekends on call. As a resident, I treasured the modest but precious amount of time I had outside work. I was lucky to have a very supportive and competent wife (a psychologist), who spared me from having to wake up at night to feed our first baby or do various household chores, so I could read the many articles and books on my desk and catch up on my sleep after my frequent night and weekend call shifts.
My unbalanced life continued when I pursued a postresidency fellowship at the National Institutes of Health, where I conducted numerous clinical research trials, brain imaging studies, and postmortem research on a large collection of brains from deceased patients with schizophrenia or bipolar disorder. I worked 12 to 15 hours a day to write up the data I had collected, submit it to scientific journals, and revise it as needed. I knew from the strategic plan I had set for my life that the neuroscience fellowship would launch my academic career, and indeed it did.
Continue to: Reaping the benefits
Reaping the benefits
Fast forward 30 years and you will still find me leading an unbalanced but joyful and fulfilling life. People often ask me how I was able to achieve so much (authoring several hundred scientific publications; publishing 13 books; receiving dozens of grants; editing 3 scientific journals; founding an international schizophrenia society; assuming many leadership positions, including becoming a department chair at 2 universities and being elected to the presidency of several associations; lecturing around the world and making hundreds of scientific presentations at national and international conferences; seeing thousands of patients; teaching, supervising, and mentoring countless medical students, psychiatric residents, and young faculty members; and creating a nonprofit foundation [CURESZ.org] with a former patient who recovered completely after 5 years of home classes and treatment-refractory command hallucinations who then graduated from college with honors in molecular biology after I prescribed clozapine to “cure” her from what was deemed a hopeless and irreversible mental disability1). In all, thanks to my unbalanced life, I have achieved 12 moonshots and each is a major achievement of which I am proud.
My answer to those who ask me how I did all that is simple: I have strategically led an unbalanced life, enjoying every minute of it, and reaping the fruits of my labor. I do not waste an inordinate amount of time watching TV or participating in social media like many others might. And more importantly, despite this unbalanced life, I have been married to my college sweetheart for several decades and have a son and a daughter who are very high achievers and make me proud. I do budget time to regularly take my children and grandchildren on family vacations to exotic locations. I have dinner with my family every night. I am very happy with this so-called unbalanced life. I have received numerous awards and recognitions for my accomplishments, including the Distinguished Scholar Award (the highest academic recognition at The Ohio State University), the coveted Stanley Dean Award for research into schizophrenia from the American College of Psychiatrists, 4 Golden Apple Teaching Awards, and the Daniel Drake Medal, the highest honor that the University of Cincinnati College of Medicine bestows on a faculty member. (Dr. Drake founded the University of Cincinnati College of Medicine 200 years ago, a major moonshot, and among his many accomplishments, he also established the first psychiatric hospital in Ohio, another consequential moonshot. I am sure he led a very productive, unbalanced life, and that is why he is still remembered and revered 200 years later.)
It is said that at the height of his prominence 90 years ago, Sigmund Freud was asked, “What is life all about?” He responded with 2 words: “Liebe und arbeit” (love and work). Importantly, he did not specify which proportions those 2 major functions should occupy in one’s life. It was left up to each individual to make that choice. In the constitution of our country, that freedom of choice is the secret sauce of “the pursuit of happiness.”
1. The CURESZ Foundation. Who we are. Accessed April 11, 2023. https://curesz.org/about/who-we-are/
The benefits of living a balanced life is a very popular concept. But I beg to differ. Balance in one’s life is overrated. Allocating equal time to the various components of one’s life may sound admirable, but it is a recipe for an ordinary life, with no major achievements or a memorable legacy. Scoring a “moonshot” achievement while living a balanced life is highly unlikely.
The benefits of deliberately leading an “asymmetric life” is an epiphany I acquired as a young boy addicted to watching stellar Olympic athletes win gold medals. I dreamed about being the best in the world in a sport, or in something else. As I read about the lives of my Olympic idols, my mind was opened to the fact that each of them led an unbalanced life in the pursuit of their cherished goal to be the best in the world: a gold medalist. I found out that for several years before the Olympic games, these athletes spent a disproportionate amount of their waking time (≥10 hours a day) practicing their sport, strengthening their muscles, building up their stamina, and honing their physical skills and mental toughness. Those sacrifices were necessary—in fact, indispensable—to set themselves apart from us mere mortals. Their social life was quite restricted, and even their educational pursuits had to be reduced or deferred.
I realized at a young age that to be the world’s best athlete, one must lead a purpose-driven life and channel a tremendous amount of time and energy to achieve the cherished goal of an Olympic gold medal. I understood the sacrifices necessary to excel in sports, and concluded the same was also true outside of sports, such as for Nobel Laureates, world-class pianists, prodigious authors, ballet dancers, opera divas, or self-employed entrepreneurs.
As I grew up, I repeatedly heard people praise “the balanced life,” but in my heart, I knew that was a fallacy. I had already decided in high school that I wanted to become a psychiatric physician. I was a premed major in college and very aware that our medical school enrolled only 44 students into the Med 1 class. There were >350 other premed undergraduates. Thus, without hesitation, and with gusto, I deliberately led an unbalanced life, studying countless hours each day to achieve an A grade in all required and elective courses to earn a spot on the Dean’s list. I already had confidence in my academic skills because of my excellent performance in high school, but I was not going to take any chances because I recalled a quote commonly attributed to Thomas Edison: “Genius is 1% inspiration and 99% perspiration.” This is obviously antithetical to living a balanced life.
I matriculated in medical school, and my unbalanced lifestyle continued unabated. Most readers of this journal are fellow physicians who know well the heavy demands of medical school on our lives, in both the preclinical and clinical years. Trying to lead a balanced life during the 4 years of medical school can have disastrous consequences. We all led an “asymmetric existence” with 75% (or more) of our waking hours invested in our careers and 25% (or less) directed to our social lives (and fortunately, our families and friends generally understood). That is what it takes to earn the coveted MD, the equivalent of an Olympic medal for intellectual athletes.
Then came 4 more years of psychiatric residency training, and the long hours of work continued, along with many nights and weekends on call. As a resident, I treasured the modest but precious amount of time I had outside work. I was lucky to have a very supportive and competent wife (a psychologist), who spared me from having to wake up at night to feed our first baby or do various household chores, so I could read the many articles and books on my desk and catch up on my sleep after my frequent night and weekend call shifts.
My unbalanced life continued when I pursued a postresidency fellowship at the National Institutes of Health, where I conducted numerous clinical research trials, brain imaging studies, and postmortem research on a large collection of brains from deceased patients with schizophrenia or bipolar disorder. I worked 12 to 15 hours a day to write up the data I had collected, submit it to scientific journals, and revise it as needed. I knew from the strategic plan I had set for my life that the neuroscience fellowship would launch my academic career, and indeed it did.
Continue to: Reaping the benefits
Reaping the benefits
Fast forward 30 years and you will still find me leading an unbalanced but joyful and fulfilling life. People often ask me how I was able to achieve so much (authoring several hundred scientific publications; publishing 13 books; receiving dozens of grants; editing 3 scientific journals; founding an international schizophrenia society; assuming many leadership positions, including becoming a department chair at 2 universities and being elected to the presidency of several associations; lecturing around the world and making hundreds of scientific presentations at national and international conferences; seeing thousands of patients; teaching, supervising, and mentoring countless medical students, psychiatric residents, and young faculty members; and creating a nonprofit foundation [CURESZ.org] with a former patient who recovered completely after 5 years of home classes and treatment-refractory command hallucinations who then graduated from college with honors in molecular biology after I prescribed clozapine to “cure” her from what was deemed a hopeless and irreversible mental disability1). In all, thanks to my unbalanced life, I have achieved 12 moonshots and each is a major achievement of which I am proud.
My answer to those who ask me how I did all that is simple: I have strategically led an unbalanced life, enjoying every minute of it, and reaping the fruits of my labor. I do not waste an inordinate amount of time watching TV or participating in social media like many others might. And more importantly, despite this unbalanced life, I have been married to my college sweetheart for several decades and have a son and a daughter who are very high achievers and make me proud. I do budget time to regularly take my children and grandchildren on family vacations to exotic locations. I have dinner with my family every night. I am very happy with this so-called unbalanced life. I have received numerous awards and recognitions for my accomplishments, including the Distinguished Scholar Award (the highest academic recognition at The Ohio State University), the coveted Stanley Dean Award for research into schizophrenia from the American College of Psychiatrists, 4 Golden Apple Teaching Awards, and the Daniel Drake Medal, the highest honor that the University of Cincinnati College of Medicine bestows on a faculty member. (Dr. Drake founded the University of Cincinnati College of Medicine 200 years ago, a major moonshot, and among his many accomplishments, he also established the first psychiatric hospital in Ohio, another consequential moonshot. I am sure he led a very productive, unbalanced life, and that is why he is still remembered and revered 200 years later.)
It is said that at the height of his prominence 90 years ago, Sigmund Freud was asked, “What is life all about?” He responded with 2 words: “Liebe und arbeit” (love and work). Importantly, he did not specify which proportions those 2 major functions should occupy in one’s life. It was left up to each individual to make that choice. In the constitution of our country, that freedom of choice is the secret sauce of “the pursuit of happiness.”
The benefits of living a balanced life is a very popular concept. But I beg to differ. Balance in one’s life is overrated. Allocating equal time to the various components of one’s life may sound admirable, but it is a recipe for an ordinary life, with no major achievements or a memorable legacy. Scoring a “moonshot” achievement while living a balanced life is highly unlikely.
The benefits of deliberately leading an “asymmetric life” is an epiphany I acquired as a young boy addicted to watching stellar Olympic athletes win gold medals. I dreamed about being the best in the world in a sport, or in something else. As I read about the lives of my Olympic idols, my mind was opened to the fact that each of them led an unbalanced life in the pursuit of their cherished goal to be the best in the world: a gold medalist. I found out that for several years before the Olympic games, these athletes spent a disproportionate amount of their waking time (≥10 hours a day) practicing their sport, strengthening their muscles, building up their stamina, and honing their physical skills and mental toughness. Those sacrifices were necessary—in fact, indispensable—to set themselves apart from us mere mortals. Their social life was quite restricted, and even their educational pursuits had to be reduced or deferred.
I realized at a young age that to be the world’s best athlete, one must lead a purpose-driven life and channel a tremendous amount of time and energy to achieve the cherished goal of an Olympic gold medal. I understood the sacrifices necessary to excel in sports, and concluded the same was also true outside of sports, such as for Nobel Laureates, world-class pianists, prodigious authors, ballet dancers, opera divas, or self-employed entrepreneurs.
As I grew up, I repeatedly heard people praise “the balanced life,” but in my heart, I knew that was a fallacy. I had already decided in high school that I wanted to become a psychiatric physician. I was a premed major in college and very aware that our medical school enrolled only 44 students into the Med 1 class. There were >350 other premed undergraduates. Thus, without hesitation, and with gusto, I deliberately led an unbalanced life, studying countless hours each day to achieve an A grade in all required and elective courses to earn a spot on the Dean’s list. I already had confidence in my academic skills because of my excellent performance in high school, but I was not going to take any chances because I recalled a quote commonly attributed to Thomas Edison: “Genius is 1% inspiration and 99% perspiration.” This is obviously antithetical to living a balanced life.
I matriculated in medical school, and my unbalanced lifestyle continued unabated. Most readers of this journal are fellow physicians who know well the heavy demands of medical school on our lives, in both the preclinical and clinical years. Trying to lead a balanced life during the 4 years of medical school can have disastrous consequences. We all led an “asymmetric existence” with 75% (or more) of our waking hours invested in our careers and 25% (or less) directed to our social lives (and fortunately, our families and friends generally understood). That is what it takes to earn the coveted MD, the equivalent of an Olympic medal for intellectual athletes.
Then came 4 more years of psychiatric residency training, and the long hours of work continued, along with many nights and weekends on call. As a resident, I treasured the modest but precious amount of time I had outside work. I was lucky to have a very supportive and competent wife (a psychologist), who spared me from having to wake up at night to feed our first baby or do various household chores, so I could read the many articles and books on my desk and catch up on my sleep after my frequent night and weekend call shifts.
My unbalanced life continued when I pursued a postresidency fellowship at the National Institutes of Health, where I conducted numerous clinical research trials, brain imaging studies, and postmortem research on a large collection of brains from deceased patients with schizophrenia or bipolar disorder. I worked 12 to 15 hours a day to write up the data I had collected, submit it to scientific journals, and revise it as needed. I knew from the strategic plan I had set for my life that the neuroscience fellowship would launch my academic career, and indeed it did.
Continue to: Reaping the benefits
Reaping the benefits
Fast forward 30 years and you will still find me leading an unbalanced but joyful and fulfilling life. People often ask me how I was able to achieve so much (authoring several hundred scientific publications; publishing 13 books; receiving dozens of grants; editing 3 scientific journals; founding an international schizophrenia society; assuming many leadership positions, including becoming a department chair at 2 universities and being elected to the presidency of several associations; lecturing around the world and making hundreds of scientific presentations at national and international conferences; seeing thousands of patients; teaching, supervising, and mentoring countless medical students, psychiatric residents, and young faculty members; and creating a nonprofit foundation [CURESZ.org] with a former patient who recovered completely after 5 years of home classes and treatment-refractory command hallucinations who then graduated from college with honors in molecular biology after I prescribed clozapine to “cure” her from what was deemed a hopeless and irreversible mental disability1). In all, thanks to my unbalanced life, I have achieved 12 moonshots and each is a major achievement of which I am proud.
My answer to those who ask me how I did all that is simple: I have strategically led an unbalanced life, enjoying every minute of it, and reaping the fruits of my labor. I do not waste an inordinate amount of time watching TV or participating in social media like many others might. And more importantly, despite this unbalanced life, I have been married to my college sweetheart for several decades and have a son and a daughter who are very high achievers and make me proud. I do budget time to regularly take my children and grandchildren on family vacations to exotic locations. I have dinner with my family every night. I am very happy with this so-called unbalanced life. I have received numerous awards and recognitions for my accomplishments, including the Distinguished Scholar Award (the highest academic recognition at The Ohio State University), the coveted Stanley Dean Award for research into schizophrenia from the American College of Psychiatrists, 4 Golden Apple Teaching Awards, and the Daniel Drake Medal, the highest honor that the University of Cincinnati College of Medicine bestows on a faculty member. (Dr. Drake founded the University of Cincinnati College of Medicine 200 years ago, a major moonshot, and among his many accomplishments, he also established the first psychiatric hospital in Ohio, another consequential moonshot. I am sure he led a very productive, unbalanced life, and that is why he is still remembered and revered 200 years later.)
It is said that at the height of his prominence 90 years ago, Sigmund Freud was asked, “What is life all about?” He responded with 2 words: “Liebe und arbeit” (love and work). Importantly, he did not specify which proportions those 2 major functions should occupy in one’s life. It was left up to each individual to make that choice. In the constitution of our country, that freedom of choice is the secret sauce of “the pursuit of happiness.”
1. The CURESZ Foundation. Who we are. Accessed April 11, 2023. https://curesz.org/about/who-we-are/
1. The CURESZ Foundation. Who we are. Accessed April 11, 2023. https://curesz.org/about/who-we-are/
What is the most effective management of first trimester miscarriage?
First trimester miscarriage, the presence of a nonviable intrauterine pregnancy before 13 weeks’ gestation, is a common complication occurring in approximately 15% of clinical pregnancies.1,2 The goals for the holistic management of first-trimester miscarriage are to 1) reduce the risk of complications such as excessive bleeding and infection, 2) ensure that the patient is supported during a time of great distress, and 3) optimally counsel the patient about treatment options and elicit the patient’s preferences for care.3 To resolve a miscarriage, the intrauterine pregnancy tissue must be expelled, restoring normal reproductive function.
The options for the management of a nonviable intrauterine pregnancy include expectant management, medication treatment with mifepristone plus misoprostol or misoprostol-alone, or uterine aspiration. In the absence of uterine hemorrhage, infection, or another severe complication of miscarriage, the patient’s preferences should guide the choice of treatment. Many patients with miscarriage prioritize avoiding medical interventions and may prefer expectant management. A patient who prefers rapid and reliable completion of the pregnancy loss process may prefer uterine aspiration. If the patient prefers to avoid uterine aspiration but desires control over the time and location of the expulsion process, medication treatment may be optimal. Many other factors influence a patient’s choice of miscarriage treatment, including balancing work and childcare issues and the ease of scheduling a uterine aspiration. In counseling patients about the options for miscarriage treatment it is helpful to know the success rate of each treatment option.4 This editorial reviews miscarriage treatment outcomes as summarized in a recent Cochrane network meta-analysis.5
Uterine aspiration versus mifepristone-misoprostol
In 2 clinical trials that included 899 patients with miscarriage, successful treatment with uterine aspira-tion versus mifepristone-misoprostolwas reported in 95% and 66% of cases, respectively.6,7
In the largest clinical trial comparing uterine aspiration to mifepristone-misoprostol, 801 patients with first-trimester miscarriage were randomly assigned to uterine aspiration or mifepristone-misoprostol.6 Uterine aspiration and mifepristone-misoprostol were associated with successful miscarriage treatment in 95% and 64% of cases, respectively. In the uterine aspiration group, a second uterine aspiration occurred in 5% of patients. Two patients in the uterine aspiration group needed a third uterine aspiration to resolve the miscarriage. In the mifepristone-misoprostol group, 36% of patients had a uterine aspiration. It should be noted that the trial protocol guided patients having a medication abortion to uterine aspiration if expulsion of miscarriage tissue had not occurred within 8 hours of receiving misoprostol. If the trial protocol permitted 1 to 4 weeks of monitoring after mifepristone-misoprostol treatment, the success rate with medication treatment would be greater. Six to 8 weeks following miscarriage treatment, patient-reported anxiety and depression symptoms were similar in both groups.6
Uterine aspiration versus misoprostol
Among 3 clinical trials that limited enrollment to patients with missed miscarriage, involving 308 patients, the success rates for uterine aspiration and misoprostol treatment was 95% and 62%, respectively.5
In a study sponsored by the National Institutes of Health, 652 patients with missed miscarriage or incomplete miscarriage were randomly assigned in a 1:3 ratioto uterine aspiration or misoprostol treatment (800 µg vaginally). After 8 days of follow-up, successful treatment rates among the patients treated with uterine evacuation or misoprostol was 97% and 84%, respectively.8 Of note, with misoprostol treatment the success rate increased from day 3 to day 8 of follow-up—from 71% to 84%.8
Continue to: Mifepristone-misoprostol versus misoprostol...
Mifepristone-misoprostol versus misoprostol
The combined results of 7 clinical trials of medication management of missed miscarriage that included 1,812 patients showed that successful treatment with mifepristone-misoprostol or misoprostol alone occurred in 80% and 70% of cases, respectively.5
Schreiber and colleagues9 reported a study of 300 patients with an anembryonic gestation or embryonic demise that were between 5 and 12 completed weeks of gestation and randomly assigned to treatment with mifepristone (200 mg) plus vaginal misoprostol (800 µg) administered 24 to 48 hours after mifepristone or vaginal misoprostol (800 µg) alone. Ultrasonography was performed 1 to 4 days after misoprostol administration. Successful treatment was defined as expulsion of the gestational sac plus no additional surgical or medical intervention within 30 days after treatment. In this study, the dual-medication regimen of mifepristone-misoprostol was more successful than misoprostol alone in resolving the miscarriage, 84% and 67%, respectively (relative risk [RR], 1.25; 95% CI, 1.09–1.43). Surgical evacuation of the uterus occurred less often with mifepristone-misoprostol treatment (9%) than with misoprostol monotherapy (24%) (RR, 0.37; 95% CI, 0.21 ̶ 0.68). Pelvic infection occurred in 2 patients (1.3%) in each group. Uterine bleeding managed with blood transfusion occurred in 3 patients who received mifepristone-misoprostol and 1 patient who received misoprostol alone. In this study, clinical factors, including active bleeding, parity, and gestational age did not influence treatment success with the mifepristone-misoprostol regimen.10 The mifepristone-misoprostol regimen was reported to be more cost-effective than misoprostol alone.11Chu and colleagues12 reporteda study of medication treatmentof missed miscarriage that included more than 700 patients randomly assigned to treatment with mifepristone-misoprostol or placebo-misoprostol. Missed miscarriage was diagnosed by an ultrasound demonstrating a gestational sac and a nonviable pregnancy. The doses of mifepristone and misoprostol were 200 mg and 800 µg, respectively. In this study, the misoprostol was administered 48 hours following mifepristone or placebo using a vaginal, oral, or buccal route; 90% of patients used the vaginal route. Treatment was considered successful if the patient passed the gestational sac as determined by an ultrasound performed 7 days after entry into the study. If the gestational sac was passed, the patients were asked to do a urine pregnancy test 3 weeks after entering the study to conclude their care episode. If patients did not pass the gestational sac, they were offered a second dose of misoprostol or surgical evacuation. At 7 days of follow-up, the success rates in the mifepristone-misoprostol and misoprostol-alone groups were 83% and 76%, respectively. Surgical intervention was performed in 25% of patients treated with placebo-misoprostol and 17% of patients treated with mifepristone-misoprostol (RR, 0.73; 95% CI, 0.53 ̶ 0.95; P=.021).12 A cost-effectiveness analysis of the trial results reported that the combination of mifepristone-misoprostol was less costly than misoprostolalone for the management of missed miscarriages.13
 Photo: Getty Images
Photo: Getty Images

Expectant management versus uterine aspiration
The combined results of 7 clinical trials that included a total of 1,693 patients showed that successful treatment of miscarriage with expectant management or uterine aspiration occurred in 68% and 93% of cases, respectively.5 In one study, 700 patients with miscarriage were randomly assigned to expectant management or uterine aspiration. Treatment was successful for 56% and 95% of patients in the expectant management and uterine aspiration groups, respectively.6
The Cochrane network meta-analysis concluded that cervical preparation followed by uterine aspiration may be more effective than expectant management, with a reported risk ratio (RR) of 2.12 (95% CI, 1.41–3.20) with low-certainty evidence.5 In addition, uterine aspiration compared with expectant management may reduce the risk of serious complications (RR, 0.55; 95% CI, 0.23–1.32), with a wide range of treatment effects in reported trials and low-certainty evidence.5
In the treatment of miscarriage, the efficacy of expectant management may vary by the type of miscarriage. In one study, following the identification of a miscarriage, the percent of patients who have completed the expulsion of pregnancy tissue by 14 days was reported to be 84% for incomplete miscarriage, 59% for pregnancy loss with no expulsion of tissue, and 52% with ultrasound detection of a nonviable pregnancy with a gestational sac.14
Expectant management versus mifepristone-misoprostol
Aggregated data from 3 clinical trials that included a total of 910 patients showed that successful treatment with expectant management or mifepristone-misoprostol was reported in 48% and 68% of cases, respectively.5 The Cochrane network meta-analysis concluded that mifepristone-misoprostol may be more effective than expectant management, with a risk ratio of 1.42 (95% CI, 1.22–1.66) with low-certainty evidence. In addition, mifepristone-misoprostol compared with expectant management may reduce the risk for serious complications (RR, 0.76; 95% CI, 0.31–1.84) with wide range of treatment effects and low-certainty evidence.5
Continue to: Expectant management versus misoprostol...
Expectant management versus misoprostol
The combined results of 10 clinical trials that included a total of 838 patients with miscarriage, showed that successful treatment with expectant management or misoprostol-alone occurred in 44% and 75% of cases, respectively.5 Among 3 studies limiting enrollment to patients with missed miscarriage, successful treatment with expectant management or misoprostol-alone occurred in 32% and 70%, respectively.5
The Cochrane analysis concluded that misoprostol-alone may be more effective than expectant management, with a reported risk ratio of 1.30 (95% CI, 1.16–1.46) with low-certainty evidence. In addition, misoprostol-alone compared with expectant management may reduce the risk of serious complications (RR, 0.50; 95% CI, 0.22–1.15) with a wide range of treatment effects and low-certainty evidence.5
Patient experience of miscarriage care
Pregnancy loss is often a distressing experience, which is associated with grief, anxiety, depression, and guilt, lasting up to 2 years for some patients.15,16 Patient dissatisfaction with miscarriage care often focuses on 4 issues: a perceived lack of emotional support, failure to elicit patient preferences for treatment, insufficient provision of information, and inconsistent posttreatment follow-up.17-19 When caring for patients with miscarriage, key goals are to communicate medical information with empathy and to provide emotional support. In the setting of a miscarriage, it is easy for patients to perceive that the clinician is insensitive and cold.15 Expressions of sympathy, compassion, and condolence help build an emotional connection and improve trust with the patient. Communications that may be helpful include: “I am sorry for your loss,” “I wish the outcome could be different,” “Our clinical team wants to provide you the best care possible,” and “May I ask how you are feeling?” Many patients report that they would like to have been offered mental health services as part of their miscarriage care.15
The Cochrane network meta-analysis of miscarriage concluded that uterine aspiration, misoprostol-mifepristone, and misoprostol-alone were likely more effective in resolving a miscarriage than expectant management.5 The strength of the conclusion was limited because of significant heterogeneity among studies, including different inclusion criteria, definition of success, and length of follow-up. Clinical trials with follow-up intervals more than 7 days generally reported greater success rates with expectant14 and medication management8 than studies with short follow-up intervals. Generally, expectant or medication management treatment is more likely to be successful in cases of incomplete abortion than in cases of missed miscarriage.5
In a rank analysis of treatment efficacy, uterine aspiration was top-ranked, followed by medication management. Expectant management had the greatest probability of being associated with unplanned uterine aspiration. Based on my analysis of available miscarriage studies, I estimate that the treatment success rates are approximately:
- uterine aspiration (93% to 99%)
- misoprostol-mifepristone (66% to 84%)
- misoprostol-alone (62% to 76%)
- expectant management (32% to 68%).
Although there may be significant differences in efficacy among the treatment options, offering patients all available approaches to treatment, providing information about the relative success of each approach, and eliciting the patient preference for care ensures an optimal patient experience during a major life event. ●
- Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. Br Med J. 1997;315:32-34.
- Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189-194.
- Wallace R, DiLaura A, Dehlendorf C. “Every person’s just different”: women’s experiences with counseling for early pregnancy loss management. Womens Health Issues. 2017;27:456-462.
- Early pregnancy loss. ACOG Practice Bulletin No. 200. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132: E197-E207.
- Ghosh J, Papadopoulou A, Devall AJ, et al. Methods for managing miscarriage: a network meta-analysis. Cochrane Database Syst Rev. 2021;CD012602.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical or surgical? Br Med J. 2006;332:1235-1240.
- Niinimaki M, Jouppila P, Martikainen H, et al. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367-372.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:21612170.
- Sonalkar S, Koelper N, Creinin MD, et al. Management of early pregnancy loss with mifepristone and misoprostol: clinical predictors of treatment success from a randomized trial. Am J Obstet Gynecol. 2020;223:551.e1-7.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:E201594.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Okeke-Ogwulu CB, Williams EV, Chu JJ, et al. Cost-effectiveness of mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage: an economic evaluation based on the MifeMiso trial. BJOG. 2021;128:1534-1545.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. Br Med J. 2002;324:873-875.
- Smith LF, Frost J, Levitas R, et al. Women’s experience of three early miscarriage options. Br J Gen Pract. 2006;56:198-205.
- Leppert PC, Pahlka BS. Grieving characteristics after spontaneous abortion: a management approach. Obstet Gynecol. 1984;64:119-122.
- Ho AL, Hernandez A, Robb JM, et al. Spontaneous miscarriage management experience: a systematic review. Cureus. 2022;14:E24269. 1
- Geller PA, Psaros C, Levine Kornfield S. Satisfaction with pregnancy loss aftercare: are women getting what they want? Arch Women’s Ment Health. 2010;13:111-124.
- Miller CA, Roe AH, McAllister A, et al. Patient experiences with miscarriage management in the emergency and ambulatory settings. Obstet Gynecol. 2019;134:1285-1292.
First trimester miscarriage, the presence of a nonviable intrauterine pregnancy before 13 weeks’ gestation, is a common complication occurring in approximately 15% of clinical pregnancies.1,2 The goals for the holistic management of first-trimester miscarriage are to 1) reduce the risk of complications such as excessive bleeding and infection, 2) ensure that the patient is supported during a time of great distress, and 3) optimally counsel the patient about treatment options and elicit the patient’s preferences for care.3 To resolve a miscarriage, the intrauterine pregnancy tissue must be expelled, restoring normal reproductive function.
The options for the management of a nonviable intrauterine pregnancy include expectant management, medication treatment with mifepristone plus misoprostol or misoprostol-alone, or uterine aspiration. In the absence of uterine hemorrhage, infection, or another severe complication of miscarriage, the patient’s preferences should guide the choice of treatment. Many patients with miscarriage prioritize avoiding medical interventions and may prefer expectant management. A patient who prefers rapid and reliable completion of the pregnancy loss process may prefer uterine aspiration. If the patient prefers to avoid uterine aspiration but desires control over the time and location of the expulsion process, medication treatment may be optimal. Many other factors influence a patient’s choice of miscarriage treatment, including balancing work and childcare issues and the ease of scheduling a uterine aspiration. In counseling patients about the options for miscarriage treatment it is helpful to know the success rate of each treatment option.4 This editorial reviews miscarriage treatment outcomes as summarized in a recent Cochrane network meta-analysis.5
Uterine aspiration versus mifepristone-misoprostol
In 2 clinical trials that included 899 patients with miscarriage, successful treatment with uterine aspira-tion versus mifepristone-misoprostolwas reported in 95% and 66% of cases, respectively.6,7
In the largest clinical trial comparing uterine aspiration to mifepristone-misoprostol, 801 patients with first-trimester miscarriage were randomly assigned to uterine aspiration or mifepristone-misoprostol.6 Uterine aspiration and mifepristone-misoprostol were associated with successful miscarriage treatment in 95% and 64% of cases, respectively. In the uterine aspiration group, a second uterine aspiration occurred in 5% of patients. Two patients in the uterine aspiration group needed a third uterine aspiration to resolve the miscarriage. In the mifepristone-misoprostol group, 36% of patients had a uterine aspiration. It should be noted that the trial protocol guided patients having a medication abortion to uterine aspiration if expulsion of miscarriage tissue had not occurred within 8 hours of receiving misoprostol. If the trial protocol permitted 1 to 4 weeks of monitoring after mifepristone-misoprostol treatment, the success rate with medication treatment would be greater. Six to 8 weeks following miscarriage treatment, patient-reported anxiety and depression symptoms were similar in both groups.6
Uterine aspiration versus misoprostol
Among 3 clinical trials that limited enrollment to patients with missed miscarriage, involving 308 patients, the success rates for uterine aspiration and misoprostol treatment was 95% and 62%, respectively.5
In a study sponsored by the National Institutes of Health, 652 patients with missed miscarriage or incomplete miscarriage were randomly assigned in a 1:3 ratioto uterine aspiration or misoprostol treatment (800 µg vaginally). After 8 days of follow-up, successful treatment rates among the patients treated with uterine evacuation or misoprostol was 97% and 84%, respectively.8 Of note, with misoprostol treatment the success rate increased from day 3 to day 8 of follow-up—from 71% to 84%.8
Continue to: Mifepristone-misoprostol versus misoprostol...
Mifepristone-misoprostol versus misoprostol
The combined results of 7 clinical trials of medication management of missed miscarriage that included 1,812 patients showed that successful treatment with mifepristone-misoprostol or misoprostol alone occurred in 80% and 70% of cases, respectively.5
Schreiber and colleagues9 reported a study of 300 patients with an anembryonic gestation or embryonic demise that were between 5 and 12 completed weeks of gestation and randomly assigned to treatment with mifepristone (200 mg) plus vaginal misoprostol (800 µg) administered 24 to 48 hours after mifepristone or vaginal misoprostol (800 µg) alone. Ultrasonography was performed 1 to 4 days after misoprostol administration. Successful treatment was defined as expulsion of the gestational sac plus no additional surgical or medical intervention within 30 days after treatment. In this study, the dual-medication regimen of mifepristone-misoprostol was more successful than misoprostol alone in resolving the miscarriage, 84% and 67%, respectively (relative risk [RR], 1.25; 95% CI, 1.09–1.43). Surgical evacuation of the uterus occurred less often with mifepristone-misoprostol treatment (9%) than with misoprostol monotherapy (24%) (RR, 0.37; 95% CI, 0.21 ̶ 0.68). Pelvic infection occurred in 2 patients (1.3%) in each group. Uterine bleeding managed with blood transfusion occurred in 3 patients who received mifepristone-misoprostol and 1 patient who received misoprostol alone. In this study, clinical factors, including active bleeding, parity, and gestational age did not influence treatment success with the mifepristone-misoprostol regimen.10 The mifepristone-misoprostol regimen was reported to be more cost-effective than misoprostol alone.11Chu and colleagues12 reporteda study of medication treatmentof missed miscarriage that included more than 700 patients randomly assigned to treatment with mifepristone-misoprostol or placebo-misoprostol. Missed miscarriage was diagnosed by an ultrasound demonstrating a gestational sac and a nonviable pregnancy. The doses of mifepristone and misoprostol were 200 mg and 800 µg, respectively. In this study, the misoprostol was administered 48 hours following mifepristone or placebo using a vaginal, oral, or buccal route; 90% of patients used the vaginal route. Treatment was considered successful if the patient passed the gestational sac as determined by an ultrasound performed 7 days after entry into the study. If the gestational sac was passed, the patients were asked to do a urine pregnancy test 3 weeks after entering the study to conclude their care episode. If patients did not pass the gestational sac, they were offered a second dose of misoprostol or surgical evacuation. At 7 days of follow-up, the success rates in the mifepristone-misoprostol and misoprostol-alone groups were 83% and 76%, respectively. Surgical intervention was performed in 25% of patients treated with placebo-misoprostol and 17% of patients treated with mifepristone-misoprostol (RR, 0.73; 95% CI, 0.53 ̶ 0.95; P=.021).12 A cost-effectiveness analysis of the trial results reported that the combination of mifepristone-misoprostol was less costly than misoprostolalone for the management of missed miscarriages.13
 Photo: Getty Images
Photo: Getty Images

Expectant management versus uterine aspiration
The combined results of 7 clinical trials that included a total of 1,693 patients showed that successful treatment of miscarriage with expectant management or uterine aspiration occurred in 68% and 93% of cases, respectively.5 In one study, 700 patients with miscarriage were randomly assigned to expectant management or uterine aspiration. Treatment was successful for 56% and 95% of patients in the expectant management and uterine aspiration groups, respectively.6
The Cochrane network meta-analysis concluded that cervical preparation followed by uterine aspiration may be more effective than expectant management, with a reported risk ratio (RR) of 2.12 (95% CI, 1.41–3.20) with low-certainty evidence.5 In addition, uterine aspiration compared with expectant management may reduce the risk of serious complications (RR, 0.55; 95% CI, 0.23–1.32), with a wide range of treatment effects in reported trials and low-certainty evidence.5
In the treatment of miscarriage, the efficacy of expectant management may vary by the type of miscarriage. In one study, following the identification of a miscarriage, the percent of patients who have completed the expulsion of pregnancy tissue by 14 days was reported to be 84% for incomplete miscarriage, 59% for pregnancy loss with no expulsion of tissue, and 52% with ultrasound detection of a nonviable pregnancy with a gestational sac.14
Expectant management versus mifepristone-misoprostol
Aggregated data from 3 clinical trials that included a total of 910 patients showed that successful treatment with expectant management or mifepristone-misoprostol was reported in 48% and 68% of cases, respectively.5 The Cochrane network meta-analysis concluded that mifepristone-misoprostol may be more effective than expectant management, with a risk ratio of 1.42 (95% CI, 1.22–1.66) with low-certainty evidence. In addition, mifepristone-misoprostol compared with expectant management may reduce the risk for serious complications (RR, 0.76; 95% CI, 0.31–1.84) with wide range of treatment effects and low-certainty evidence.5
Continue to: Expectant management versus misoprostol...
Expectant management versus misoprostol
The combined results of 10 clinical trials that included a total of 838 patients with miscarriage, showed that successful treatment with expectant management or misoprostol-alone occurred in 44% and 75% of cases, respectively.5 Among 3 studies limiting enrollment to patients with missed miscarriage, successful treatment with expectant management or misoprostol-alone occurred in 32% and 70%, respectively.5
The Cochrane analysis concluded that misoprostol-alone may be more effective than expectant management, with a reported risk ratio of 1.30 (95% CI, 1.16–1.46) with low-certainty evidence. In addition, misoprostol-alone compared with expectant management may reduce the risk of serious complications (RR, 0.50; 95% CI, 0.22–1.15) with a wide range of treatment effects and low-certainty evidence.5
Patient experience of miscarriage care
Pregnancy loss is often a distressing experience, which is associated with grief, anxiety, depression, and guilt, lasting up to 2 years for some patients.15,16 Patient dissatisfaction with miscarriage care often focuses on 4 issues: a perceived lack of emotional support, failure to elicit patient preferences for treatment, insufficient provision of information, and inconsistent posttreatment follow-up.17-19 When caring for patients with miscarriage, key goals are to communicate medical information with empathy and to provide emotional support. In the setting of a miscarriage, it is easy for patients to perceive that the clinician is insensitive and cold.15 Expressions of sympathy, compassion, and condolence help build an emotional connection and improve trust with the patient. Communications that may be helpful include: “I am sorry for your loss,” “I wish the outcome could be different,” “Our clinical team wants to provide you the best care possible,” and “May I ask how you are feeling?” Many patients report that they would like to have been offered mental health services as part of their miscarriage care.15
The Cochrane network meta-analysis of miscarriage concluded that uterine aspiration, misoprostol-mifepristone, and misoprostol-alone were likely more effective in resolving a miscarriage than expectant management.5 The strength of the conclusion was limited because of significant heterogeneity among studies, including different inclusion criteria, definition of success, and length of follow-up. Clinical trials with follow-up intervals more than 7 days generally reported greater success rates with expectant14 and medication management8 than studies with short follow-up intervals. Generally, expectant or medication management treatment is more likely to be successful in cases of incomplete abortion than in cases of missed miscarriage.5
In a rank analysis of treatment efficacy, uterine aspiration was top-ranked, followed by medication management. Expectant management had the greatest probability of being associated with unplanned uterine aspiration. Based on my analysis of available miscarriage studies, I estimate that the treatment success rates are approximately:
- uterine aspiration (93% to 99%)
- misoprostol-mifepristone (66% to 84%)
- misoprostol-alone (62% to 76%)
- expectant management (32% to 68%).
Although there may be significant differences in efficacy among the treatment options, offering patients all available approaches to treatment, providing information about the relative success of each approach, and eliciting the patient preference for care ensures an optimal patient experience during a major life event. ●
First trimester miscarriage, the presence of a nonviable intrauterine pregnancy before 13 weeks’ gestation, is a common complication occurring in approximately 15% of clinical pregnancies.1,2 The goals for the holistic management of first-trimester miscarriage are to 1) reduce the risk of complications such as excessive bleeding and infection, 2) ensure that the patient is supported during a time of great distress, and 3) optimally counsel the patient about treatment options and elicit the patient’s preferences for care.3 To resolve a miscarriage, the intrauterine pregnancy tissue must be expelled, restoring normal reproductive function.
The options for the management of a nonviable intrauterine pregnancy include expectant management, medication treatment with mifepristone plus misoprostol or misoprostol-alone, or uterine aspiration. In the absence of uterine hemorrhage, infection, or another severe complication of miscarriage, the patient’s preferences should guide the choice of treatment. Many patients with miscarriage prioritize avoiding medical interventions and may prefer expectant management. A patient who prefers rapid and reliable completion of the pregnancy loss process may prefer uterine aspiration. If the patient prefers to avoid uterine aspiration but desires control over the time and location of the expulsion process, medication treatment may be optimal. Many other factors influence a patient’s choice of miscarriage treatment, including balancing work and childcare issues and the ease of scheduling a uterine aspiration. In counseling patients about the options for miscarriage treatment it is helpful to know the success rate of each treatment option.4 This editorial reviews miscarriage treatment outcomes as summarized in a recent Cochrane network meta-analysis.5
Uterine aspiration versus mifepristone-misoprostol
In 2 clinical trials that included 899 patients with miscarriage, successful treatment with uterine aspira-tion versus mifepristone-misoprostolwas reported in 95% and 66% of cases, respectively.6,7
In the largest clinical trial comparing uterine aspiration to mifepristone-misoprostol, 801 patients with first-trimester miscarriage were randomly assigned to uterine aspiration or mifepristone-misoprostol.6 Uterine aspiration and mifepristone-misoprostol were associated with successful miscarriage treatment in 95% and 64% of cases, respectively. In the uterine aspiration group, a second uterine aspiration occurred in 5% of patients. Two patients in the uterine aspiration group needed a third uterine aspiration to resolve the miscarriage. In the mifepristone-misoprostol group, 36% of patients had a uterine aspiration. It should be noted that the trial protocol guided patients having a medication abortion to uterine aspiration if expulsion of miscarriage tissue had not occurred within 8 hours of receiving misoprostol. If the trial protocol permitted 1 to 4 weeks of monitoring after mifepristone-misoprostol treatment, the success rate with medication treatment would be greater. Six to 8 weeks following miscarriage treatment, patient-reported anxiety and depression symptoms were similar in both groups.6
Uterine aspiration versus misoprostol
Among 3 clinical trials that limited enrollment to patients with missed miscarriage, involving 308 patients, the success rates for uterine aspiration and misoprostol treatment was 95% and 62%, respectively.5
In a study sponsored by the National Institutes of Health, 652 patients with missed miscarriage or incomplete miscarriage were randomly assigned in a 1:3 ratioto uterine aspiration or misoprostol treatment (800 µg vaginally). After 8 days of follow-up, successful treatment rates among the patients treated with uterine evacuation or misoprostol was 97% and 84%, respectively.8 Of note, with misoprostol treatment the success rate increased from day 3 to day 8 of follow-up—from 71% to 84%.8
Continue to: Mifepristone-misoprostol versus misoprostol...
Mifepristone-misoprostol versus misoprostol
The combined results of 7 clinical trials of medication management of missed miscarriage that included 1,812 patients showed that successful treatment with mifepristone-misoprostol or misoprostol alone occurred in 80% and 70% of cases, respectively.5
Schreiber and colleagues9 reported a study of 300 patients with an anembryonic gestation or embryonic demise that were between 5 and 12 completed weeks of gestation and randomly assigned to treatment with mifepristone (200 mg) plus vaginal misoprostol (800 µg) administered 24 to 48 hours after mifepristone or vaginal misoprostol (800 µg) alone. Ultrasonography was performed 1 to 4 days after misoprostol administration. Successful treatment was defined as expulsion of the gestational sac plus no additional surgical or medical intervention within 30 days after treatment. In this study, the dual-medication regimen of mifepristone-misoprostol was more successful than misoprostol alone in resolving the miscarriage, 84% and 67%, respectively (relative risk [RR], 1.25; 95% CI, 1.09–1.43). Surgical evacuation of the uterus occurred less often with mifepristone-misoprostol treatment (9%) than with misoprostol monotherapy (24%) (RR, 0.37; 95% CI, 0.21 ̶ 0.68). Pelvic infection occurred in 2 patients (1.3%) in each group. Uterine bleeding managed with blood transfusion occurred in 3 patients who received mifepristone-misoprostol and 1 patient who received misoprostol alone. In this study, clinical factors, including active bleeding, parity, and gestational age did not influence treatment success with the mifepristone-misoprostol regimen.10 The mifepristone-misoprostol regimen was reported to be more cost-effective than misoprostol alone.11Chu and colleagues12 reporteda study of medication treatmentof missed miscarriage that included more than 700 patients randomly assigned to treatment with mifepristone-misoprostol or placebo-misoprostol. Missed miscarriage was diagnosed by an ultrasound demonstrating a gestational sac and a nonviable pregnancy. The doses of mifepristone and misoprostol were 200 mg and 800 µg, respectively. In this study, the misoprostol was administered 48 hours following mifepristone or placebo using a vaginal, oral, or buccal route; 90% of patients used the vaginal route. Treatment was considered successful if the patient passed the gestational sac as determined by an ultrasound performed 7 days after entry into the study. If the gestational sac was passed, the patients were asked to do a urine pregnancy test 3 weeks after entering the study to conclude their care episode. If patients did not pass the gestational sac, they were offered a second dose of misoprostol or surgical evacuation. At 7 days of follow-up, the success rates in the mifepristone-misoprostol and misoprostol-alone groups were 83% and 76%, respectively. Surgical intervention was performed in 25% of patients treated with placebo-misoprostol and 17% of patients treated with mifepristone-misoprostol (RR, 0.73; 95% CI, 0.53 ̶ 0.95; P=.021).12 A cost-effectiveness analysis of the trial results reported that the combination of mifepristone-misoprostol was less costly than misoprostolalone for the management of missed miscarriages.13
 Photo: Getty Images
Photo: Getty Images

Expectant management versus uterine aspiration
The combined results of 7 clinical trials that included a total of 1,693 patients showed that successful treatment of miscarriage with expectant management or uterine aspiration occurred in 68% and 93% of cases, respectively.5 In one study, 700 patients with miscarriage were randomly assigned to expectant management or uterine aspiration. Treatment was successful for 56% and 95% of patients in the expectant management and uterine aspiration groups, respectively.6
The Cochrane network meta-analysis concluded that cervical preparation followed by uterine aspiration may be more effective than expectant management, with a reported risk ratio (RR) of 2.12 (95% CI, 1.41–3.20) with low-certainty evidence.5 In addition, uterine aspiration compared with expectant management may reduce the risk of serious complications (RR, 0.55; 95% CI, 0.23–1.32), with a wide range of treatment effects in reported trials and low-certainty evidence.5
In the treatment of miscarriage, the efficacy of expectant management may vary by the type of miscarriage. In one study, following the identification of a miscarriage, the percent of patients who have completed the expulsion of pregnancy tissue by 14 days was reported to be 84% for incomplete miscarriage, 59% for pregnancy loss with no expulsion of tissue, and 52% with ultrasound detection of a nonviable pregnancy with a gestational sac.14
Expectant management versus mifepristone-misoprostol
Aggregated data from 3 clinical trials that included a total of 910 patients showed that successful treatment with expectant management or mifepristone-misoprostol was reported in 48% and 68% of cases, respectively.5 The Cochrane network meta-analysis concluded that mifepristone-misoprostol may be more effective than expectant management, with a risk ratio of 1.42 (95% CI, 1.22–1.66) with low-certainty evidence. In addition, mifepristone-misoprostol compared with expectant management may reduce the risk for serious complications (RR, 0.76; 95% CI, 0.31–1.84) with wide range of treatment effects and low-certainty evidence.5
Continue to: Expectant management versus misoprostol...
Expectant management versus misoprostol
The combined results of 10 clinical trials that included a total of 838 patients with miscarriage, showed that successful treatment with expectant management or misoprostol-alone occurred in 44% and 75% of cases, respectively.5 Among 3 studies limiting enrollment to patients with missed miscarriage, successful treatment with expectant management or misoprostol-alone occurred in 32% and 70%, respectively.5
The Cochrane analysis concluded that misoprostol-alone may be more effective than expectant management, with a reported risk ratio of 1.30 (95% CI, 1.16–1.46) with low-certainty evidence. In addition, misoprostol-alone compared with expectant management may reduce the risk of serious complications (RR, 0.50; 95% CI, 0.22–1.15) with a wide range of treatment effects and low-certainty evidence.5
Patient experience of miscarriage care
Pregnancy loss is often a distressing experience, which is associated with grief, anxiety, depression, and guilt, lasting up to 2 years for some patients.15,16 Patient dissatisfaction with miscarriage care often focuses on 4 issues: a perceived lack of emotional support, failure to elicit patient preferences for treatment, insufficient provision of information, and inconsistent posttreatment follow-up.17-19 When caring for patients with miscarriage, key goals are to communicate medical information with empathy and to provide emotional support. In the setting of a miscarriage, it is easy for patients to perceive that the clinician is insensitive and cold.15 Expressions of sympathy, compassion, and condolence help build an emotional connection and improve trust with the patient. Communications that may be helpful include: “I am sorry for your loss,” “I wish the outcome could be different,” “Our clinical team wants to provide you the best care possible,” and “May I ask how you are feeling?” Many patients report that they would like to have been offered mental health services as part of their miscarriage care.15
The Cochrane network meta-analysis of miscarriage concluded that uterine aspiration, misoprostol-mifepristone, and misoprostol-alone were likely more effective in resolving a miscarriage than expectant management.5 The strength of the conclusion was limited because of significant heterogeneity among studies, including different inclusion criteria, definition of success, and length of follow-up. Clinical trials with follow-up intervals more than 7 days generally reported greater success rates with expectant14 and medication management8 than studies with short follow-up intervals. Generally, expectant or medication management treatment is more likely to be successful in cases of incomplete abortion than in cases of missed miscarriage.5
In a rank analysis of treatment efficacy, uterine aspiration was top-ranked, followed by medication management. Expectant management had the greatest probability of being associated with unplanned uterine aspiration. Based on my analysis of available miscarriage studies, I estimate that the treatment success rates are approximately:
- uterine aspiration (93% to 99%)
- misoprostol-mifepristone (66% to 84%)
- misoprostol-alone (62% to 76%)
- expectant management (32% to 68%).
Although there may be significant differences in efficacy among the treatment options, offering patients all available approaches to treatment, providing information about the relative success of each approach, and eliciting the patient preference for care ensures an optimal patient experience during a major life event. ●
- Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. Br Med J. 1997;315:32-34.
- Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189-194.
- Wallace R, DiLaura A, Dehlendorf C. “Every person’s just different”: women’s experiences with counseling for early pregnancy loss management. Womens Health Issues. 2017;27:456-462.
- Early pregnancy loss. ACOG Practice Bulletin No. 200. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132: E197-E207.
- Ghosh J, Papadopoulou A, Devall AJ, et al. Methods for managing miscarriage: a network meta-analysis. Cochrane Database Syst Rev. 2021;CD012602.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical or surgical? Br Med J. 2006;332:1235-1240.
- Niinimaki M, Jouppila P, Martikainen H, et al. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367-372.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:21612170.
- Sonalkar S, Koelper N, Creinin MD, et al. Management of early pregnancy loss with mifepristone and misoprostol: clinical predictors of treatment success from a randomized trial. Am J Obstet Gynecol. 2020;223:551.e1-7.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:E201594.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Okeke-Ogwulu CB, Williams EV, Chu JJ, et al. Cost-effectiveness of mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage: an economic evaluation based on the MifeMiso trial. BJOG. 2021;128:1534-1545.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. Br Med J. 2002;324:873-875.
- Smith LF, Frost J, Levitas R, et al. Women’s experience of three early miscarriage options. Br J Gen Pract. 2006;56:198-205.
- Leppert PC, Pahlka BS. Grieving characteristics after spontaneous abortion: a management approach. Obstet Gynecol. 1984;64:119-122.
- Ho AL, Hernandez A, Robb JM, et al. Spontaneous miscarriage management experience: a systematic review. Cureus. 2022;14:E24269. 1
- Geller PA, Psaros C, Levine Kornfield S. Satisfaction with pregnancy loss aftercare: are women getting what they want? Arch Women’s Ment Health. 2010;13:111-124.
- Miller CA, Roe AH, McAllister A, et al. Patient experiences with miscarriage management in the emergency and ambulatory settings. Obstet Gynecol. 2019;134:1285-1292.
- Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. Br Med J. 1997;315:32-34.
- Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189-194.
- Wallace R, DiLaura A, Dehlendorf C. “Every person’s just different”: women’s experiences with counseling for early pregnancy loss management. Womens Health Issues. 2017;27:456-462.
- Early pregnancy loss. ACOG Practice Bulletin No. 200. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132: E197-E207.
- Ghosh J, Papadopoulou A, Devall AJ, et al. Methods for managing miscarriage: a network meta-analysis. Cochrane Database Syst Rev. 2021;CD012602.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical or surgical? Br Med J. 2006;332:1235-1240.
- Niinimaki M, Jouppila P, Martikainen H, et al. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367-372.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:21612170.
- Sonalkar S, Koelper N, Creinin MD, et al. Management of early pregnancy loss with mifepristone and misoprostol: clinical predictors of treatment success from a randomized trial. Am J Obstet Gynecol. 2020;223:551.e1-7.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:E201594.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Okeke-Ogwulu CB, Williams EV, Chu JJ, et al. Cost-effectiveness of mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage: an economic evaluation based on the MifeMiso trial. BJOG. 2021;128:1534-1545.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. Br Med J. 2002;324:873-875.
- Smith LF, Frost J, Levitas R, et al. Women’s experience of three early miscarriage options. Br J Gen Pract. 2006;56:198-205.
- Leppert PC, Pahlka BS. Grieving characteristics after spontaneous abortion: a management approach. Obstet Gynecol. 1984;64:119-122.
- Ho AL, Hernandez A, Robb JM, et al. Spontaneous miscarriage management experience: a systematic review. Cureus. 2022;14:E24269. 1
- Geller PA, Psaros C, Levine Kornfield S. Satisfaction with pregnancy loss aftercare: are women getting what they want? Arch Women’s Ment Health. 2010;13:111-124.
- Miller CA, Roe AH, McAllister A, et al. Patient experiences with miscarriage management in the emergency and ambulatory settings. Obstet Gynecol. 2019;134:1285-1292.
A ‘guest editorial’ … generated by ChatGPT?
The artificial intelligence (AI) program ChatGPT (generative pre-trained transformer) was introduced to the public by Open AI on November 30, 2022. It has been described as a game-changer for society, culture, literature, arts, and science, with a potential impact that rivals that of the internet and social media.
ChatGPT can produce human-like essays in response to queries. Some researchers used ChatGPT to generate abstracts based on the titles of articles published in 5 high-impact journals. These abstracts were so “real” that they escaped detection by expert peer reviewers in 32% of cases.1 In addition, several medical/science articles were published that included ChatGPT as a bylined author.2
Like other journals,3-7
- Nonhuman AI technologies do not qualify for named authorship.
- Authors should be transparent about the use of AI technologies to aid in writing content. Authors should disclose which tool was used and how it was applied in the article submitted to the journal.
- Human authors are responsible for the accuracy of work compiled by an AI tool, including the absence of plagiarism. Proper referencing of statements is necessary.
- Images created by AI tools are not permitted for publication.
Because the overwhelming majority of articles published in
Can AI generate an acceptable ‘guest editorial?’
In addition to creating formulaic writing, some of the concerns about using AI in scientific publishing is that the results might be outdated, incorrect, or lacking appropriate references, and that it can produce “plausible-sounding but incorrect or nonsensical” answers to questions.3 To test this, I asked ChatGPT to write an article on the same topic as 4 of my articles published in
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
1. Psychiatry is not a real medical specialty: This is a false myth. Psychiatry is a recognized medical specialty, and psychiatrists are licensed medical doctors who have undergone extensive training in the diagnosis and treatment of mental illness.
2. Mental illness is not a real illness: Mental illness is a real and biologically based medical condition, just like physical illness. Research has shown that mental illness is caused by a combination of genetic, environmental, and lifestyle factors.
3. Antidepressants are not effective: Antidepressants have been shown to be effective in treating depression and other mental illnesses. While they are not a cure, they can provide significant symptom relief and improve quality of life for many people.
4. Psychiatric drugs are dangerous and have serious side effects: While all drugs have potential side effects, psychiatric medications have been proven to be safe and effective for many people. The risks and benefits of taking any medication should be carefully considered and discussed with a healthcare professional.
5. Therapy is not effective: Therapy, also known as psychotherapy, has been shown to be effective in treating a wide range of mental illnesses, including depression, anxiety, and post-traumatic stress disorder (PTSD).
6. People with mental illness are violent: People with mental illness are no more likely to be violent than the general population. In fact, people with mental illness are more likely to be victims of violence than perpetrators.
It is important to dispel these myths and misconceptions about psychiatry, as they can prevent people from seeking the help they need and can also contribute to stigma and discrimination towards those with mental illness.
In conclusion, there are many myths and misconceptions surrounding psychiatry. Dispelling these myths and increasing public understanding about mental illness and its treatment can help reduce stigma and encourage more people to seek the help they need.
What do you think? I encourage you to send me your take on this AI-generated “guest editorial,” and whether you deem its quality to be similar to that of an article authored by a human psychiatrist.
1. Else H. Abstracts written by ChatGPT fool scientists. Nature. 2023;613(7944):423. doi: 10.1038/d41586-023-00056-7
2. Stokel-Walker C. ChatGPT listed as author on research papers: many scientists disapprove. Nature. 2023;613(7945):620-621. doi:10.1038/d41586-023-00107-z
3. Flanagin A, Bibbins-Domingo K, Berkwits M, et al. Nonhuman “authors” and implications for the integrity of scientific publication and medical knowledge. JAMA. 2023;329(8):637-639. doi:10.1001/jama.2023.1344
4. Tools such as ChatGPT threaten transparent science; here are our ground rules for their use. Nature. 2023;613(7945):612. doi:10.1038/d41586-023-00191-1
5. Thorp HH. ChatGPT is fun, but not an author. Science. 2023;379(6630):313. doi:10.1126/science.adg7879
6. PNAS. The PNAS journals outline their policies for ChatGPT and generative AI. February 21, 2023. Accessed March 9, 2023. https://www.pnas.org/post/update/pnas-policy-for-chatgpt-generative-ai
7. Marušic’ A. JoGH policy on the use of artificial intelligence in scholarly manuscripts. J Glob Health. 2023;13:01002. doi:10.7189/jogh.13.01002
The artificial intelligence (AI) program ChatGPT (generative pre-trained transformer) was introduced to the public by Open AI on November 30, 2022. It has been described as a game-changer for society, culture, literature, arts, and science, with a potential impact that rivals that of the internet and social media.
ChatGPT can produce human-like essays in response to queries. Some researchers used ChatGPT to generate abstracts based on the titles of articles published in 5 high-impact journals. These abstracts were so “real” that they escaped detection by expert peer reviewers in 32% of cases.1 In addition, several medical/science articles were published that included ChatGPT as a bylined author.2
Like other journals,3-7
- Nonhuman AI technologies do not qualify for named authorship.
- Authors should be transparent about the use of AI technologies to aid in writing content. Authors should disclose which tool was used and how it was applied in the article submitted to the journal.
- Human authors are responsible for the accuracy of work compiled by an AI tool, including the absence of plagiarism. Proper referencing of statements is necessary.
- Images created by AI tools are not permitted for publication.
Because the overwhelming majority of articles published in
Can AI generate an acceptable ‘guest editorial?’
In addition to creating formulaic writing, some of the concerns about using AI in scientific publishing is that the results might be outdated, incorrect, or lacking appropriate references, and that it can produce “plausible-sounding but incorrect or nonsensical” answers to questions.3 To test this, I asked ChatGPT to write an article on the same topic as 4 of my articles published in
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
1. Psychiatry is not a real medical specialty: This is a false myth. Psychiatry is a recognized medical specialty, and psychiatrists are licensed medical doctors who have undergone extensive training in the diagnosis and treatment of mental illness.
2. Mental illness is not a real illness: Mental illness is a real and biologically based medical condition, just like physical illness. Research has shown that mental illness is caused by a combination of genetic, environmental, and lifestyle factors.
3. Antidepressants are not effective: Antidepressants have been shown to be effective in treating depression and other mental illnesses. While they are not a cure, they can provide significant symptom relief and improve quality of life for many people.
4. Psychiatric drugs are dangerous and have serious side effects: While all drugs have potential side effects, psychiatric medications have been proven to be safe and effective for many people. The risks and benefits of taking any medication should be carefully considered and discussed with a healthcare professional.
5. Therapy is not effective: Therapy, also known as psychotherapy, has been shown to be effective in treating a wide range of mental illnesses, including depression, anxiety, and post-traumatic stress disorder (PTSD).
6. People with mental illness are violent: People with mental illness are no more likely to be violent than the general population. In fact, people with mental illness are more likely to be victims of violence than perpetrators.
It is important to dispel these myths and misconceptions about psychiatry, as they can prevent people from seeking the help they need and can also contribute to stigma and discrimination towards those with mental illness.
In conclusion, there are many myths and misconceptions surrounding psychiatry. Dispelling these myths and increasing public understanding about mental illness and its treatment can help reduce stigma and encourage more people to seek the help they need.
What do you think? I encourage you to send me your take on this AI-generated “guest editorial,” and whether you deem its quality to be similar to that of an article authored by a human psychiatrist.
The artificial intelligence (AI) program ChatGPT (generative pre-trained transformer) was introduced to the public by Open AI on November 30, 2022. It has been described as a game-changer for society, culture, literature, arts, and science, with a potential impact that rivals that of the internet and social media.
ChatGPT can produce human-like essays in response to queries. Some researchers used ChatGPT to generate abstracts based on the titles of articles published in 5 high-impact journals. These abstracts were so “real” that they escaped detection by expert peer reviewers in 32% of cases.1 In addition, several medical/science articles were published that included ChatGPT as a bylined author.2
Like other journals,3-7
- Nonhuman AI technologies do not qualify for named authorship.
- Authors should be transparent about the use of AI technologies to aid in writing content. Authors should disclose which tool was used and how it was applied in the article submitted to the journal.
- Human authors are responsible for the accuracy of work compiled by an AI tool, including the absence of plagiarism. Proper referencing of statements is necessary.
- Images created by AI tools are not permitted for publication.
Because the overwhelming majority of articles published in
Can AI generate an acceptable ‘guest editorial?’
In addition to creating formulaic writing, some of the concerns about using AI in scientific publishing is that the results might be outdated, incorrect, or lacking appropriate references, and that it can produce “plausible-sounding but incorrect or nonsensical” answers to questions.3 To test this, I asked ChatGPT to write an article on the same topic as 4 of my articles published in
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
1. Psychiatry is not a real medical specialty: This is a false myth. Psychiatry is a recognized medical specialty, and psychiatrists are licensed medical doctors who have undergone extensive training in the diagnosis and treatment of mental illness.
2. Mental illness is not a real illness: Mental illness is a real and biologically based medical condition, just like physical illness. Research has shown that mental illness is caused by a combination of genetic, environmental, and lifestyle factors.
3. Antidepressants are not effective: Antidepressants have been shown to be effective in treating depression and other mental illnesses. While they are not a cure, they can provide significant symptom relief and improve quality of life for many people.
4. Psychiatric drugs are dangerous and have serious side effects: While all drugs have potential side effects, psychiatric medications have been proven to be safe and effective for many people. The risks and benefits of taking any medication should be carefully considered and discussed with a healthcare professional.
5. Therapy is not effective: Therapy, also known as psychotherapy, has been shown to be effective in treating a wide range of mental illnesses, including depression, anxiety, and post-traumatic stress disorder (PTSD).
6. People with mental illness are violent: People with mental illness are no more likely to be violent than the general population. In fact, people with mental illness are more likely to be victims of violence than perpetrators.
It is important to dispel these myths and misconceptions about psychiatry, as they can prevent people from seeking the help they need and can also contribute to stigma and discrimination towards those with mental illness.
In conclusion, there are many myths and misconceptions surrounding psychiatry. Dispelling these myths and increasing public understanding about mental illness and its treatment can help reduce stigma and encourage more people to seek the help they need.
What do you think? I encourage you to send me your take on this AI-generated “guest editorial,” and whether you deem its quality to be similar to that of an article authored by a human psychiatrist.
1. Else H. Abstracts written by ChatGPT fool scientists. Nature. 2023;613(7944):423. doi: 10.1038/d41586-023-00056-7
2. Stokel-Walker C. ChatGPT listed as author on research papers: many scientists disapprove. Nature. 2023;613(7945):620-621. doi:10.1038/d41586-023-00107-z
3. Flanagin A, Bibbins-Domingo K, Berkwits M, et al. Nonhuman “authors” and implications for the integrity of scientific publication and medical knowledge. JAMA. 2023;329(8):637-639. doi:10.1001/jama.2023.1344
4. Tools such as ChatGPT threaten transparent science; here are our ground rules for their use. Nature. 2023;613(7945):612. doi:10.1038/d41586-023-00191-1
5. Thorp HH. ChatGPT is fun, but not an author. Science. 2023;379(6630):313. doi:10.1126/science.adg7879
6. PNAS. The PNAS journals outline their policies for ChatGPT and generative AI. February 21, 2023. Accessed March 9, 2023. https://www.pnas.org/post/update/pnas-policy-for-chatgpt-generative-ai
7. Marušic’ A. JoGH policy on the use of artificial intelligence in scholarly manuscripts. J Glob Health. 2023;13:01002. doi:10.7189/jogh.13.01002
1. Else H. Abstracts written by ChatGPT fool scientists. Nature. 2023;613(7944):423. doi: 10.1038/d41586-023-00056-7
2. Stokel-Walker C. ChatGPT listed as author on research papers: many scientists disapprove. Nature. 2023;613(7945):620-621. doi:10.1038/d41586-023-00107-z
3. Flanagin A, Bibbins-Domingo K, Berkwits M, et al. Nonhuman “authors” and implications for the integrity of scientific publication and medical knowledge. JAMA. 2023;329(8):637-639. doi:10.1001/jama.2023.1344
4. Tools such as ChatGPT threaten transparent science; here are our ground rules for their use. Nature. 2023;613(7945):612. doi:10.1038/d41586-023-00191-1
5. Thorp HH. ChatGPT is fun, but not an author. Science. 2023;379(6630):313. doi:10.1126/science.adg7879
6. PNAS. The PNAS journals outline their policies for ChatGPT and generative AI. February 21, 2023. Accessed March 9, 2023. https://www.pnas.org/post/update/pnas-policy-for-chatgpt-generative-ai
7. Marušic’ A. JoGH policy on the use of artificial intelligence in scholarly manuscripts. J Glob Health. 2023;13:01002. doi:10.7189/jogh.13.01002
Advances in the treatment of fetal demise in the second and third trimester
Clinical care for fetal demise is complex and multidimensional, including empathic emotional support for the patient and family members who are experiencing a tragedy, investigation of the cause of the demise, and a plan for emptying the uterus. This editorial narrowly focuses on the options for treatment of fetal demise with the goal of emptying the uterus while minimizing complications.
When planning treatment of fetal demise, focus on fetal size and gestational age
Most guidelines for the treatment of fetal demise use gestational age to guide selection of a treatment.1,2 I believe that fetal size is as important as gestational age for selecting a treatment plan. When considering treatment, there are 2 reasons why fetal size is as important as gestational age:
- The physiologic processes that caused fetal demise may have caused fetal growth restriction, resulting in a fetal size that is 2 or more weeks below expected fetal size for gestational age.
- Fetal demise may have occurred weeks before the diagnosis was made, resulting in gestational age being greater than fetal size. This editorial will use ultrasonography estimate of fetal size in gestational weeks to guide treatment recommendations. When discussing fetal size, we will use the convention of weeks-days (w-d). Twenty-five weeks and zero days gestation is represented as 25w0d.
Treatment in the second and third trimester is a 2-step process
Step 1: Cervical preparation
In most cases of first trimester fetal demise, no cervical preparation is necessary. Cervical dilation with metal dilators followed by uterine evacuation with an appropriately sized vacuum catheter is a highly successful treatment.3 However for second and third trimester fetal demise, it is best to use a 2-step process, beginning with cervical preparation followed by emptying the uterus. For example, at a fetal size of 13w0d to 16w0d, cervical preparation can be achieved by administering a single buccal dose of misoprostol 400 µg 3 to 4 hours prior to uterine evacuation or by inserting a Dilapan-S (Medicem Inc) osmotic cervical dilator 3 to 6 hours prior to uterine evacuation.4-7 At a fetal size of 16w0d to 19w6d, cervical preparation can be achieved by placing osmotic cervical dilators 4 to 6 hours before surgical evacuation and administering buccal misoprostol 400 µg 3 hours before surgical evacuation.8
Alternatively, from 16w0d to 25w0d osmotic cervical dilators can be placed on day 1 of a 2-day process, and the patient can return on day 2 to have the cervical dilators removed followed by surgical evacuation of the uterus. Mifepristone 200 mg oral dose can be administered on day 1 to facilitate cervical preparation. In my practice, I use mifepristone 200 mg on day 1 when the fetal size is ≥20w0d gestation. Options for cervical preparation include use of osmotic dilators, cervical balloons, misoprostol, and/or mifepristone. These options are discussed below. With fetal demise, natural physiologic processes often have caused sufficient cervical softening and dilation that no cervical preparation is necessary and immediate uterine surgical evacuation or induction of labor can be initiated.
Step 2: Emptying the uterus
In the second and third trimesters, the approach to uterine evacuation is based on fetal size. At fetal sizes <25w0d, options for emptying the uterus include surgical evacuation with a vacuum catheter and grasping forceps or induction of labor with misoprostol followed by vaginal birth and expulsion of the placenta. At fetal sizes ˃25w0d gestation, following completion of cervical preparation, the most common approaches to uterine evacuation are induction of labor with misoprostol or oxytocin. Rarely, with a stillbirth at term, some clinicians will select hysterotomy to empty the uterus, avoiding uterine rupture during labor induction for patients at the highest risk, including those with a prior classical cesarean birth or more than 2 prior cesarean births with a low-transverse uterine incision.
Osmotic cervical dilators
The 2 most used cervical dilators are Dilapan-S, a polyacrylate-based hydrogel rod, and laminaria, dried compressed seaweed stipe (stalk) from Laminaria japonica or Laminaria digitata. Dilapan-S rods are available in diameters of 3 mm and 4 mm and rod lengths of 55 mm and 65 mm. Laminaria dilators are available in diameters of 2, 3, 4, 5, 6, 8 and 10 mm and rod length of 60 and 70 mm. Dilapan-S dilators reach near-maximal dilation in approximately 4 to 6 hours but continue to expand over the following 18 hours to achieve a maximum dilation of 3.3 to 3.6 times their dry diameter.9 Laminaria dilators expand to 2.7 to 2.9 times their dry diameter over 24 hours.9
A general rule is that as many dilators as possible should be placed until significant resistance to the placement of additional dilators is encountered.10 In my practice, for fetal size ≥20 weeks’ gestation, I place 2 Dilapan-S rods, 4 mm in diameter, 55 mm in length, and then encircle the Dilapan-S with laminaria rods that are 4 mm in diameter and 60 mm in length. Once cervical resistance to the placement of the 4 mm laminaria rods is observed, I encircle those laminaria with laminaria 2 mm in diameter, filling in the interstices between the 4 mm laminaria. The next day, cervical dilation is routinely ≥3 cm.
In a retrospective study of 491 patients undergoing pregnancy termination after 14 weeks’ gestation, with a mean gestational age of 24 weeks, compared with no osmotic cervical dilators, inserting osmotic cervical dilators the day before initiating misoprostol for induction of labor resulted in a decrease in time to delivery (428 min vs 640 min; P<.001) and a decrease in total misoprostol dose (990 µg vs 1,449 µg; P<.0001).11
Cervical balloons
All clinicians know that a Foley catheter or a Cook cervical ripening balloon can be used for cervical preparation in the third trimester.12,13 The Foley catheter also has been reported to be useful for cervical preparation in the second trimester. In one study of 43 patients 17 to 24 weeks’ gestation scheduled for a second-trimester dilation and evacuation, an intracervical Foley catheter was placed the evening before evacuation, and the balloon was inflated with 30 mL to 50 mL of saline. At the same time, mifepristone 200 mg was administered to the patients.14 The following day, dilation and evacuation was performed. In 72% of cases no additional cervical dilation was required on the day of evacuation. The investigators concluded that if osmotic cervical dilators are not available, the placement of an intracervical Foley catheter plus administration of mifepristone facilitates performance of an evacuation on the following day. If the patient prefers a 1-day procedure, the Foley can be inserted in the morning to facilitate cervical preparation, and the uterus can be evacuated in the afternoon.
Continue to: Misoprostol...
Misoprostol
Misoprostol, a derivative of prostaglandin E1, is useful for both cervical preparation and induction of labor. The dose of misoprostol and the route of administration are major determinants of uterine response.15-19 When administered by an oral route, misoprostol has fast onset and offset of action and often does not cause sustained uterine contractions. Hence, oral misoprostol, at a low dose is useful for cervical ripening, but not as useful for stimulation of sustained uterine contractions for induction of labor. When administered by a buccal or vaginal route, misoprostol has prolonged activity and often results in sustained uterine contractions. At any given dose of misoprostol, buccal and vaginal misoprostol administration are more effective than oral administration in inducing sustained uterine contractions sufficient to empty the uterus.15-19
Mifepristone
Mifepristone, an anti-progestin, is useful for cervical preparation and sensitizing myocytes to the action of uterotonics. Progesterone reduces cell-to-cell communication among uterine myocytes, facilitating uterine quiescence by suppressing connexin 43 and other proteins. Mifepristone blocks the effect of progesterone, inducing the production of myocyte connexin 43, enhancing efficient cell-to-cell communication, permitting uterine myoctes to contract in unison, creating the potential for powerful and sustained contractions.20-23 Randomized clinical trials report that administration of mifepristone 200 mg prior to misoprostol induced labor results in more rapid emptying of the uterus.24-27
It takes time for mifepristone to have its full effect on uterine myocytes. Hence, most protocols recommend waiting 24 hours following mifepristone administration before initiating treatment with an agent to stimulate uterine contractions such as misoprostol or oxytocin. However, preliminary data suggest that partial benefit of mifepristone can be obtained when initiating misoprostol 3 to 5 hours after mifepristone administration.28 In a study of 481 patients undergoing induction of labor in the second or third trimester, the time from initiation of misoprostol to vaginal birth was 15 hours with no mifepristone pretreatment, 13.2 hours if mifepristone was administered 3 to 5 hours before initiating misoprostol, 9.3 hours if mifepristone was administered 24 hours before initiating misoprostol, and 10.5 hours if mifepristone was administered 48 hours before initiating misoprostol.28
Fetal size <25w0d gestation: Cervical preparation and surgical evacuation
For fetal demise at a fetal size less than 25w0d, if clinical experts are available, the best treatment option is cervical preparation followed by surgical evacuation of the uterus using a vacuum catheter and grasping forceps to empty the uterus.29,30 A disadvantage of surgical evacuation of the uterus is that an intact fetus is not available for the patient to hold and mourn, and pathologic examination of an intact fetus is not possible. An alternative approach is cervical preparation followed by induction of labor using misoprostol with the goal of delivering an intact fetus. Although no prospective clinical trials are available comparing these 2 options, retrospective studies have reported that, at fetal size <25w0d gestation, compared with induction of labor, surgical evacuation of the uterus results in fewer complications,30 including fewer cases of retained placenta requiring an unplanned procedure and fewer presumed uterine infections.29
For surgical evacuation of fetal demise with a fetal size of <25w0d gestation, the first step on day 1 is placement of osmotic cervical dilators. In addition to osmotic cervical dilators, if the gestational age or fetal size is ≥19 weeks’ gestation an oral dose of mifepristone 200 mg to facilitate cervical preparation may be considered. On day 2, the osmotic dilators are removed and surgical evacuation is performed. In one randomized study, for pregnancies at 19 to 24 weeks’ gestation, compared with osmotic dilators alone, administration of mifepristone 200 mg at the time of placement of osmotic dilators resulted in fewer procedures that were difficult to complete.31 In some cases, 2 consecutive days of cervical preparation with osmotic dilators may be needed to properly prepare the cervix for uterine evacuation. For example, the cervix of a nulliparous teenage patient may require 2 days of cervical preparation with osmotic dilators to facilitate uterine evacuation. In some cases of fetal demise, the cervix is already dilated to ≥3 cm and surgical evacuation of the uterus or induction of labor can be initiated without the need for cervical preparation.
Continue to: Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor...
Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor
Treatment of fetal demise at 14w0d to 28w6d gestation with the goal of the vaginal birth of an intact fetus is optimized by the administration of mifepristone for cervical preparation followed by induction of labor with misoprostol.26,27
In one clinical trial, 66 patients with fetal demise between 14w0d and 28w6d gestation were randomly assigned to receive mifepristone 200 mg or placebo followed 24 to 48 hours later with initiation of misoprostol induction of labor.26 Among the patients from 14w0d to 24 weeks’ gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24w0d to 28 weeks’ gestation, the misoprostol dose was 200 µg vaginally every 4 hours. At 24 hours, a consultant obstetrician determined if additional misoprostol should be given. The median time from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups was 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P= .002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage, 4 in the placebo group and 1 in the mifepristone group.26
In a second clinical trial, 105 patients with fetal demise after 20 weeks of gestation were randomly assigned to receive mifepristone 200 mg or placebo.27 In this study, 86% of the patients were ≥26w0d gestation, with a mean gestational age of approximately 32w2d. Thirty-six to 48 hours later, misoprostol induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostoldose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients from ≥26 weeks’ gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours, respectively (P=.001). Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg; P<.001). Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.27
Fetal size ≥29w0d gestation
At a fetal size ≥29w0d gestation, if the cervix is ripe with a Bishop score of ≥7, oxytocin induction of labor is often used as a first-line treatment. If the cervix is not ripe, misoprostol induction of labor may be considered at doses less than those used in the second trimester of pregnancy.32 TABLES 1,1, 26, 33–36 2,37 and 337 summarize regimens proposed for fetal size ≥29w0d. One regimen begins with an initial misoprostol dose of 50 µg. If adequate uterine contractions occur, the 50 µg dose is repeated every 4 hours up to 6 total doses. If contractions are inadequate, the dose can be increased to 100 µg every 4 hours for 5 additional doses.
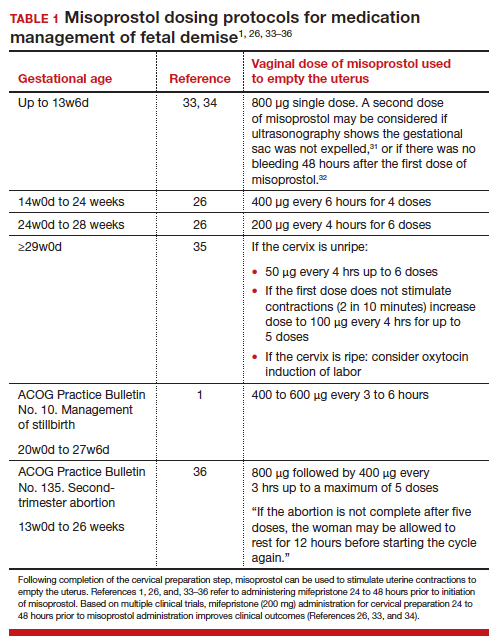
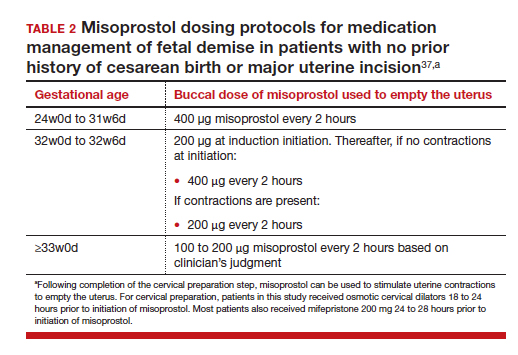

For fetal demise after 28w0d gestation, the
A multidisciplinary approach can optimize compassionate care
There are many gaps in the holistic care of patients and partners experiencing fetal demise. Patients with fetal demise often report that they did not receive sufficient information about the cause of the demise and wanted more opportunity to be involved in decision making about their care.39 The patient’s partner often reports feeling unacknowledged as a grieving parent.40 Fetal demise is experienced by many patients as a tragedy, triggering feelings of grief, anger, denial, anxiety and depression, sometimes resulting in isolation and substance misuse.
Using a 5-round Delphi process, experts identified 8 core goals in the care of patients with fetal demise:
- reduce stigma
- provide respectful care
- involve patients in care planning
- attempt to provide an explanation for the demise1
- acknowledge the depth of the grief response and provide emotional support
- offer information about ongoing psychological support
- provide information about future pregnancy planning
- provide opportunities for specialized training and support for care providers.41
Management of stillbirth is optimized by a multidisciplinary approach that includes the expert care of obstetrician-gynecologists, obstetric nurses, anesthesiologists, and expert consultation from social work, chaplaincy, and pathology. A heart-to-heart connection between clinician and patient is a key component of stillbirth care. ●
- American College of Obstetricians and Gynecologists. Management of stillbirth. ACOG Obstetric Care Consensus. No. 10. Obstet Gynecol. 2020;135:e110-132.
- Tsakiridis I, Giouleka S, Mamopoulos A, et al. Investigation and management of stillbirths: a descriptive review of major guidelines. J Perinat Med. 2022;50:796-813.
- Spingler T, Sonek J, Hoopman M, et al. Complication rate after termination of pregnancy due to fetal defects. Ultrasound Obstet Gynecol. 2023;Epub January 7.
- Goldberg AB, Drey EA, Whitaker AK, et al. Misoprostol compared with laminaria before early second-trimester surgical abortion: a randomized trial. Obstet Gynecol. 2005;106:234-241.
- Meirik O, My Huong NT, Piaggio G, et al. WHOR-GoP-MoF Regulation. Complications of first trimester abortion by vacuum aspiration after cervical preparation with and without misoprostol: a multicentre randomised trial. Lancet. 2012;379(9829):1817-1824.
- Bartz D, Maurer R, Allen RH, et al. Buccal misoprostol compared with synthetic osmotic cervical dilator before surgical abortion: a randomized controlled trial. Obstet Gynecol. 2013;122:57-63.
- Ngo LL, Mokashi M, Janiak E, et al. Acute complications with same-day versus overnight cervical preparation before dilation and evacuation at 14 to 16 weeks. Contraception. 2023;117:61-66.
- Kim CS, Dragoman M, Prosch L, et al. Same-day compared with overnight cervical preparation before dilation and evacuation between 16 and 19 6/7 weeks of gestation: a randomized controlled trial. Obstet Gynecol. 2022;139:1141-1144.
- Drunecky T, Reidingerova M, Plisova M, et al. Experimental comparison of properties of natural and synthetic osmotic dilators. Arch Gynecol Obstet. 2015;292:349-354.
- Hern WM. Laminaria versus Dilapan osmotic cervical dilators for outpatient dilation and evacuation abortion: randomized cohort comparison of 1001 patients. Am J Obstet Gynecol. 1994;171:1324-1328.
- Berthold C, Gomes David M, Gabriel P, et al. Effect of the addition of osmotic dilators to medical induction of labor abortion: a before-and-after study. Eur J Obstet Gynecol. 2020;244:185-189.
- Kemper JI, Li W, Goni S, et al. Foley catheter vs oral misoprostol for induction of labor: individual participant data meta-analysis. Ultrasound Obstet Gynecol. 2021;57:215-223.
- Attalli E, Kern Guy, Fouks Y, et al. Labor induction in third trimester non-viable fetus. J Matern Fetal Neonatal Med. 2022;Epub October 1.
- Fessehaye Sium A, Prager S, Wolderufael M, et al. Foley catheter for cervical preparation prior to second trimester dilation and evacuation: a supply-based alternative for surgical abortion: a case series. Contracept X. 2022;4:100085.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590.
- Barbieri RL. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022;34:8-10, 12.
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290.
- Barbieri RL. Mifepristone for the treatment of miscarriage and fetal demise. OBG Manag. 2022;34:811, 15.
- Kapp N, Borgatta L, Stubblefield P, et al. Mifepristone in second-trimester medical abortion. Obstet Gynecol. 2007;110:1304-1310.
- Ngoc NTN, Shochet T, Raghavan S, et al. Mifepristone and misoprostol compared with misoprostol alone for second trimester abortion: a randomized controlled trial. Obstet Gynecol. 2011;118:601608.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890.
- Prodan N, Breisch J, Hoopman M, et al. Dosing interval between mifepristone and misoprostol in second and third trimester termination. Arch Gynecol Obstet. 2019;299:675-679.
- Edlow AG, Hou MY, Maurer R, et al. Uterine evacuation for second trimester fetal death and maternal morbidity. Obstet Gynecol. 2011;117:1-10.
- Bryan AG, Grimes DA, Garrett JM, et al. Second-trimester abortion for fetal anomalies or fetal death. Obstet Gynecol. 2011;117:788-792.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol or mifepristone compared with overnight osmotic dilators alone. Obstet Gynecol. 2015;126:599-609.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- American College of Obstetricians and Gynecologists. Second-trimester abortion. Practice Bulletin No. 135. Obstet Gynecol. 2013;121:1394-1406.
- Wingo E, Raifman S, Landau C, et al. Mifepristone-misoprostol versus misoprostol-alone regimen for medication abortion at ≥ 24 weeks gestation. Contraception. Appendix 1. 2020;102:99-103.
- American College of Obstetricians and Gynecologists. Vaginal birth after cesarean delivery. ACOG Practice Bulletin No. 205. Obstet Gynecol. 2019;133:e110-e127.
- Atkins B, Blencowe H, Boyle FM, et al. Is care of stillborn babies and their parents respectful? Results from an international online survey. BJOG. 2022;129:1731-1739.
- Haezell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387(10018):604-616.
- Shakespeare C, Merriel A, Bakhbakhi D, et al. The RESPECT Study for consensus on global bereavement care after stillbirth. Int J Gynaecol Obstet. 2020;149:137-147.
Clinical care for fetal demise is complex and multidimensional, including empathic emotional support for the patient and family members who are experiencing a tragedy, investigation of the cause of the demise, and a plan for emptying the uterus. This editorial narrowly focuses on the options for treatment of fetal demise with the goal of emptying the uterus while minimizing complications.
When planning treatment of fetal demise, focus on fetal size and gestational age
Most guidelines for the treatment of fetal demise use gestational age to guide selection of a treatment.1,2 I believe that fetal size is as important as gestational age for selecting a treatment plan. When considering treatment, there are 2 reasons why fetal size is as important as gestational age:
- The physiologic processes that caused fetal demise may have caused fetal growth restriction, resulting in a fetal size that is 2 or more weeks below expected fetal size for gestational age.
- Fetal demise may have occurred weeks before the diagnosis was made, resulting in gestational age being greater than fetal size. This editorial will use ultrasonography estimate of fetal size in gestational weeks to guide treatment recommendations. When discussing fetal size, we will use the convention of weeks-days (w-d). Twenty-five weeks and zero days gestation is represented as 25w0d.
Treatment in the second and third trimester is a 2-step process
Step 1: Cervical preparation
In most cases of first trimester fetal demise, no cervical preparation is necessary. Cervical dilation with metal dilators followed by uterine evacuation with an appropriately sized vacuum catheter is a highly successful treatment.3 However for second and third trimester fetal demise, it is best to use a 2-step process, beginning with cervical preparation followed by emptying the uterus. For example, at a fetal size of 13w0d to 16w0d, cervical preparation can be achieved by administering a single buccal dose of misoprostol 400 µg 3 to 4 hours prior to uterine evacuation or by inserting a Dilapan-S (Medicem Inc) osmotic cervical dilator 3 to 6 hours prior to uterine evacuation.4-7 At a fetal size of 16w0d to 19w6d, cervical preparation can be achieved by placing osmotic cervical dilators 4 to 6 hours before surgical evacuation and administering buccal misoprostol 400 µg 3 hours before surgical evacuation.8
Alternatively, from 16w0d to 25w0d osmotic cervical dilators can be placed on day 1 of a 2-day process, and the patient can return on day 2 to have the cervical dilators removed followed by surgical evacuation of the uterus. Mifepristone 200 mg oral dose can be administered on day 1 to facilitate cervical preparation. In my practice, I use mifepristone 200 mg on day 1 when the fetal size is ≥20w0d gestation. Options for cervical preparation include use of osmotic dilators, cervical balloons, misoprostol, and/or mifepristone. These options are discussed below. With fetal demise, natural physiologic processes often have caused sufficient cervical softening and dilation that no cervical preparation is necessary and immediate uterine surgical evacuation or induction of labor can be initiated.
Step 2: Emptying the uterus
In the second and third trimesters, the approach to uterine evacuation is based on fetal size. At fetal sizes <25w0d, options for emptying the uterus include surgical evacuation with a vacuum catheter and grasping forceps or induction of labor with misoprostol followed by vaginal birth and expulsion of the placenta. At fetal sizes ˃25w0d gestation, following completion of cervical preparation, the most common approaches to uterine evacuation are induction of labor with misoprostol or oxytocin. Rarely, with a stillbirth at term, some clinicians will select hysterotomy to empty the uterus, avoiding uterine rupture during labor induction for patients at the highest risk, including those with a prior classical cesarean birth or more than 2 prior cesarean births with a low-transverse uterine incision.
Osmotic cervical dilators
The 2 most used cervical dilators are Dilapan-S, a polyacrylate-based hydrogel rod, and laminaria, dried compressed seaweed stipe (stalk) from Laminaria japonica or Laminaria digitata. Dilapan-S rods are available in diameters of 3 mm and 4 mm and rod lengths of 55 mm and 65 mm. Laminaria dilators are available in diameters of 2, 3, 4, 5, 6, 8 and 10 mm and rod length of 60 and 70 mm. Dilapan-S dilators reach near-maximal dilation in approximately 4 to 6 hours but continue to expand over the following 18 hours to achieve a maximum dilation of 3.3 to 3.6 times their dry diameter.9 Laminaria dilators expand to 2.7 to 2.9 times their dry diameter over 24 hours.9
A general rule is that as many dilators as possible should be placed until significant resistance to the placement of additional dilators is encountered.10 In my practice, for fetal size ≥20 weeks’ gestation, I place 2 Dilapan-S rods, 4 mm in diameter, 55 mm in length, and then encircle the Dilapan-S with laminaria rods that are 4 mm in diameter and 60 mm in length. Once cervical resistance to the placement of the 4 mm laminaria rods is observed, I encircle those laminaria with laminaria 2 mm in diameter, filling in the interstices between the 4 mm laminaria. The next day, cervical dilation is routinely ≥3 cm.
In a retrospective study of 491 patients undergoing pregnancy termination after 14 weeks’ gestation, with a mean gestational age of 24 weeks, compared with no osmotic cervical dilators, inserting osmotic cervical dilators the day before initiating misoprostol for induction of labor resulted in a decrease in time to delivery (428 min vs 640 min; P<.001) and a decrease in total misoprostol dose (990 µg vs 1,449 µg; P<.0001).11
Cervical balloons
All clinicians know that a Foley catheter or a Cook cervical ripening balloon can be used for cervical preparation in the third trimester.12,13 The Foley catheter also has been reported to be useful for cervical preparation in the second trimester. In one study of 43 patients 17 to 24 weeks’ gestation scheduled for a second-trimester dilation and evacuation, an intracervical Foley catheter was placed the evening before evacuation, and the balloon was inflated with 30 mL to 50 mL of saline. At the same time, mifepristone 200 mg was administered to the patients.14 The following day, dilation and evacuation was performed. In 72% of cases no additional cervical dilation was required on the day of evacuation. The investigators concluded that if osmotic cervical dilators are not available, the placement of an intracervical Foley catheter plus administration of mifepristone facilitates performance of an evacuation on the following day. If the patient prefers a 1-day procedure, the Foley can be inserted in the morning to facilitate cervical preparation, and the uterus can be evacuated in the afternoon.
Continue to: Misoprostol...
Misoprostol
Misoprostol, a derivative of prostaglandin E1, is useful for both cervical preparation and induction of labor. The dose of misoprostol and the route of administration are major determinants of uterine response.15-19 When administered by an oral route, misoprostol has fast onset and offset of action and often does not cause sustained uterine contractions. Hence, oral misoprostol, at a low dose is useful for cervical ripening, but not as useful for stimulation of sustained uterine contractions for induction of labor. When administered by a buccal or vaginal route, misoprostol has prolonged activity and often results in sustained uterine contractions. At any given dose of misoprostol, buccal and vaginal misoprostol administration are more effective than oral administration in inducing sustained uterine contractions sufficient to empty the uterus.15-19
Mifepristone
Mifepristone, an anti-progestin, is useful for cervical preparation and sensitizing myocytes to the action of uterotonics. Progesterone reduces cell-to-cell communication among uterine myocytes, facilitating uterine quiescence by suppressing connexin 43 and other proteins. Mifepristone blocks the effect of progesterone, inducing the production of myocyte connexin 43, enhancing efficient cell-to-cell communication, permitting uterine myoctes to contract in unison, creating the potential for powerful and sustained contractions.20-23 Randomized clinical trials report that administration of mifepristone 200 mg prior to misoprostol induced labor results in more rapid emptying of the uterus.24-27
It takes time for mifepristone to have its full effect on uterine myocytes. Hence, most protocols recommend waiting 24 hours following mifepristone administration before initiating treatment with an agent to stimulate uterine contractions such as misoprostol or oxytocin. However, preliminary data suggest that partial benefit of mifepristone can be obtained when initiating misoprostol 3 to 5 hours after mifepristone administration.28 In a study of 481 patients undergoing induction of labor in the second or third trimester, the time from initiation of misoprostol to vaginal birth was 15 hours with no mifepristone pretreatment, 13.2 hours if mifepristone was administered 3 to 5 hours before initiating misoprostol, 9.3 hours if mifepristone was administered 24 hours before initiating misoprostol, and 10.5 hours if mifepristone was administered 48 hours before initiating misoprostol.28
Fetal size <25w0d gestation: Cervical preparation and surgical evacuation
For fetal demise at a fetal size less than 25w0d, if clinical experts are available, the best treatment option is cervical preparation followed by surgical evacuation of the uterus using a vacuum catheter and grasping forceps to empty the uterus.29,30 A disadvantage of surgical evacuation of the uterus is that an intact fetus is not available for the patient to hold and mourn, and pathologic examination of an intact fetus is not possible. An alternative approach is cervical preparation followed by induction of labor using misoprostol with the goal of delivering an intact fetus. Although no prospective clinical trials are available comparing these 2 options, retrospective studies have reported that, at fetal size <25w0d gestation, compared with induction of labor, surgical evacuation of the uterus results in fewer complications,30 including fewer cases of retained placenta requiring an unplanned procedure and fewer presumed uterine infections.29
For surgical evacuation of fetal demise with a fetal size of <25w0d gestation, the first step on day 1 is placement of osmotic cervical dilators. In addition to osmotic cervical dilators, if the gestational age or fetal size is ≥19 weeks’ gestation an oral dose of mifepristone 200 mg to facilitate cervical preparation may be considered. On day 2, the osmotic dilators are removed and surgical evacuation is performed. In one randomized study, for pregnancies at 19 to 24 weeks’ gestation, compared with osmotic dilators alone, administration of mifepristone 200 mg at the time of placement of osmotic dilators resulted in fewer procedures that were difficult to complete.31 In some cases, 2 consecutive days of cervical preparation with osmotic dilators may be needed to properly prepare the cervix for uterine evacuation. For example, the cervix of a nulliparous teenage patient may require 2 days of cervical preparation with osmotic dilators to facilitate uterine evacuation. In some cases of fetal demise, the cervix is already dilated to ≥3 cm and surgical evacuation of the uterus or induction of labor can be initiated without the need for cervical preparation.
Continue to: Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor...
Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor
Treatment of fetal demise at 14w0d to 28w6d gestation with the goal of the vaginal birth of an intact fetus is optimized by the administration of mifepristone for cervical preparation followed by induction of labor with misoprostol.26,27
In one clinical trial, 66 patients with fetal demise between 14w0d and 28w6d gestation were randomly assigned to receive mifepristone 200 mg or placebo followed 24 to 48 hours later with initiation of misoprostol induction of labor.26 Among the patients from 14w0d to 24 weeks’ gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24w0d to 28 weeks’ gestation, the misoprostol dose was 200 µg vaginally every 4 hours. At 24 hours, a consultant obstetrician determined if additional misoprostol should be given. The median time from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups was 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P= .002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage, 4 in the placebo group and 1 in the mifepristone group.26
In a second clinical trial, 105 patients with fetal demise after 20 weeks of gestation were randomly assigned to receive mifepristone 200 mg or placebo.27 In this study, 86% of the patients were ≥26w0d gestation, with a mean gestational age of approximately 32w2d. Thirty-six to 48 hours later, misoprostol induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostoldose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients from ≥26 weeks’ gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours, respectively (P=.001). Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg; P<.001). Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.27
Fetal size ≥29w0d gestation
At a fetal size ≥29w0d gestation, if the cervix is ripe with a Bishop score of ≥7, oxytocin induction of labor is often used as a first-line treatment. If the cervix is not ripe, misoprostol induction of labor may be considered at doses less than those used in the second trimester of pregnancy.32 TABLES 1,1, 26, 33–36 2,37 and 337 summarize regimens proposed for fetal size ≥29w0d. One regimen begins with an initial misoprostol dose of 50 µg. If adequate uterine contractions occur, the 50 µg dose is repeated every 4 hours up to 6 total doses. If contractions are inadequate, the dose can be increased to 100 µg every 4 hours for 5 additional doses.



For fetal demise after 28w0d gestation, the
A multidisciplinary approach can optimize compassionate care
There are many gaps in the holistic care of patients and partners experiencing fetal demise. Patients with fetal demise often report that they did not receive sufficient information about the cause of the demise and wanted more opportunity to be involved in decision making about their care.39 The patient’s partner often reports feeling unacknowledged as a grieving parent.40 Fetal demise is experienced by many patients as a tragedy, triggering feelings of grief, anger, denial, anxiety and depression, sometimes resulting in isolation and substance misuse.
Using a 5-round Delphi process, experts identified 8 core goals in the care of patients with fetal demise:
- reduce stigma
- provide respectful care
- involve patients in care planning
- attempt to provide an explanation for the demise1
- acknowledge the depth of the grief response and provide emotional support
- offer information about ongoing psychological support
- provide information about future pregnancy planning
- provide opportunities for specialized training and support for care providers.41
Management of stillbirth is optimized by a multidisciplinary approach that includes the expert care of obstetrician-gynecologists, obstetric nurses, anesthesiologists, and expert consultation from social work, chaplaincy, and pathology. A heart-to-heart connection between clinician and patient is a key component of stillbirth care. ●
Clinical care for fetal demise is complex and multidimensional, including empathic emotional support for the patient and family members who are experiencing a tragedy, investigation of the cause of the demise, and a plan for emptying the uterus. This editorial narrowly focuses on the options for treatment of fetal demise with the goal of emptying the uterus while minimizing complications.
When planning treatment of fetal demise, focus on fetal size and gestational age
Most guidelines for the treatment of fetal demise use gestational age to guide selection of a treatment.1,2 I believe that fetal size is as important as gestational age for selecting a treatment plan. When considering treatment, there are 2 reasons why fetal size is as important as gestational age:
- The physiologic processes that caused fetal demise may have caused fetal growth restriction, resulting in a fetal size that is 2 or more weeks below expected fetal size for gestational age.
- Fetal demise may have occurred weeks before the diagnosis was made, resulting in gestational age being greater than fetal size. This editorial will use ultrasonography estimate of fetal size in gestational weeks to guide treatment recommendations. When discussing fetal size, we will use the convention of weeks-days (w-d). Twenty-five weeks and zero days gestation is represented as 25w0d.
Treatment in the second and third trimester is a 2-step process
Step 1: Cervical preparation
In most cases of first trimester fetal demise, no cervical preparation is necessary. Cervical dilation with metal dilators followed by uterine evacuation with an appropriately sized vacuum catheter is a highly successful treatment.3 However for second and third trimester fetal demise, it is best to use a 2-step process, beginning with cervical preparation followed by emptying the uterus. For example, at a fetal size of 13w0d to 16w0d, cervical preparation can be achieved by administering a single buccal dose of misoprostol 400 µg 3 to 4 hours prior to uterine evacuation or by inserting a Dilapan-S (Medicem Inc) osmotic cervical dilator 3 to 6 hours prior to uterine evacuation.4-7 At a fetal size of 16w0d to 19w6d, cervical preparation can be achieved by placing osmotic cervical dilators 4 to 6 hours before surgical evacuation and administering buccal misoprostol 400 µg 3 hours before surgical evacuation.8
Alternatively, from 16w0d to 25w0d osmotic cervical dilators can be placed on day 1 of a 2-day process, and the patient can return on day 2 to have the cervical dilators removed followed by surgical evacuation of the uterus. Mifepristone 200 mg oral dose can be administered on day 1 to facilitate cervical preparation. In my practice, I use mifepristone 200 mg on day 1 when the fetal size is ≥20w0d gestation. Options for cervical preparation include use of osmotic dilators, cervical balloons, misoprostol, and/or mifepristone. These options are discussed below. With fetal demise, natural physiologic processes often have caused sufficient cervical softening and dilation that no cervical preparation is necessary and immediate uterine surgical evacuation or induction of labor can be initiated.
Step 2: Emptying the uterus
In the second and third trimesters, the approach to uterine evacuation is based on fetal size. At fetal sizes <25w0d, options for emptying the uterus include surgical evacuation with a vacuum catheter and grasping forceps or induction of labor with misoprostol followed by vaginal birth and expulsion of the placenta. At fetal sizes ˃25w0d gestation, following completion of cervical preparation, the most common approaches to uterine evacuation are induction of labor with misoprostol or oxytocin. Rarely, with a stillbirth at term, some clinicians will select hysterotomy to empty the uterus, avoiding uterine rupture during labor induction for patients at the highest risk, including those with a prior classical cesarean birth or more than 2 prior cesarean births with a low-transverse uterine incision.
Osmotic cervical dilators
The 2 most used cervical dilators are Dilapan-S, a polyacrylate-based hydrogel rod, and laminaria, dried compressed seaweed stipe (stalk) from Laminaria japonica or Laminaria digitata. Dilapan-S rods are available in diameters of 3 mm and 4 mm and rod lengths of 55 mm and 65 mm. Laminaria dilators are available in diameters of 2, 3, 4, 5, 6, 8 and 10 mm and rod length of 60 and 70 mm. Dilapan-S dilators reach near-maximal dilation in approximately 4 to 6 hours but continue to expand over the following 18 hours to achieve a maximum dilation of 3.3 to 3.6 times their dry diameter.9 Laminaria dilators expand to 2.7 to 2.9 times their dry diameter over 24 hours.9
A general rule is that as many dilators as possible should be placed until significant resistance to the placement of additional dilators is encountered.10 In my practice, for fetal size ≥20 weeks’ gestation, I place 2 Dilapan-S rods, 4 mm in diameter, 55 mm in length, and then encircle the Dilapan-S with laminaria rods that are 4 mm in diameter and 60 mm in length. Once cervical resistance to the placement of the 4 mm laminaria rods is observed, I encircle those laminaria with laminaria 2 mm in diameter, filling in the interstices between the 4 mm laminaria. The next day, cervical dilation is routinely ≥3 cm.
In a retrospective study of 491 patients undergoing pregnancy termination after 14 weeks’ gestation, with a mean gestational age of 24 weeks, compared with no osmotic cervical dilators, inserting osmotic cervical dilators the day before initiating misoprostol for induction of labor resulted in a decrease in time to delivery (428 min vs 640 min; P<.001) and a decrease in total misoprostol dose (990 µg vs 1,449 µg; P<.0001).11
Cervical balloons
All clinicians know that a Foley catheter or a Cook cervical ripening balloon can be used for cervical preparation in the third trimester.12,13 The Foley catheter also has been reported to be useful for cervical preparation in the second trimester. In one study of 43 patients 17 to 24 weeks’ gestation scheduled for a second-trimester dilation and evacuation, an intracervical Foley catheter was placed the evening before evacuation, and the balloon was inflated with 30 mL to 50 mL of saline. At the same time, mifepristone 200 mg was administered to the patients.14 The following day, dilation and evacuation was performed. In 72% of cases no additional cervical dilation was required on the day of evacuation. The investigators concluded that if osmotic cervical dilators are not available, the placement of an intracervical Foley catheter plus administration of mifepristone facilitates performance of an evacuation on the following day. If the patient prefers a 1-day procedure, the Foley can be inserted in the morning to facilitate cervical preparation, and the uterus can be evacuated in the afternoon.
Continue to: Misoprostol...
Misoprostol
Misoprostol, a derivative of prostaglandin E1, is useful for both cervical preparation and induction of labor. The dose of misoprostol and the route of administration are major determinants of uterine response.15-19 When administered by an oral route, misoprostol has fast onset and offset of action and often does not cause sustained uterine contractions. Hence, oral misoprostol, at a low dose is useful for cervical ripening, but not as useful for stimulation of sustained uterine contractions for induction of labor. When administered by a buccal or vaginal route, misoprostol has prolonged activity and often results in sustained uterine contractions. At any given dose of misoprostol, buccal and vaginal misoprostol administration are more effective than oral administration in inducing sustained uterine contractions sufficient to empty the uterus.15-19
Mifepristone
Mifepristone, an anti-progestin, is useful for cervical preparation and sensitizing myocytes to the action of uterotonics. Progesterone reduces cell-to-cell communication among uterine myocytes, facilitating uterine quiescence by suppressing connexin 43 and other proteins. Mifepristone blocks the effect of progesterone, inducing the production of myocyte connexin 43, enhancing efficient cell-to-cell communication, permitting uterine myoctes to contract in unison, creating the potential for powerful and sustained contractions.20-23 Randomized clinical trials report that administration of mifepristone 200 mg prior to misoprostol induced labor results in more rapid emptying of the uterus.24-27
It takes time for mifepristone to have its full effect on uterine myocytes. Hence, most protocols recommend waiting 24 hours following mifepristone administration before initiating treatment with an agent to stimulate uterine contractions such as misoprostol or oxytocin. However, preliminary data suggest that partial benefit of mifepristone can be obtained when initiating misoprostol 3 to 5 hours after mifepristone administration.28 In a study of 481 patients undergoing induction of labor in the second or third trimester, the time from initiation of misoprostol to vaginal birth was 15 hours with no mifepristone pretreatment, 13.2 hours if mifepristone was administered 3 to 5 hours before initiating misoprostol, 9.3 hours if mifepristone was administered 24 hours before initiating misoprostol, and 10.5 hours if mifepristone was administered 48 hours before initiating misoprostol.28
Fetal size <25w0d gestation: Cervical preparation and surgical evacuation
For fetal demise at a fetal size less than 25w0d, if clinical experts are available, the best treatment option is cervical preparation followed by surgical evacuation of the uterus using a vacuum catheter and grasping forceps to empty the uterus.29,30 A disadvantage of surgical evacuation of the uterus is that an intact fetus is not available for the patient to hold and mourn, and pathologic examination of an intact fetus is not possible. An alternative approach is cervical preparation followed by induction of labor using misoprostol with the goal of delivering an intact fetus. Although no prospective clinical trials are available comparing these 2 options, retrospective studies have reported that, at fetal size <25w0d gestation, compared with induction of labor, surgical evacuation of the uterus results in fewer complications,30 including fewer cases of retained placenta requiring an unplanned procedure and fewer presumed uterine infections.29
For surgical evacuation of fetal demise with a fetal size of <25w0d gestation, the first step on day 1 is placement of osmotic cervical dilators. In addition to osmotic cervical dilators, if the gestational age or fetal size is ≥19 weeks’ gestation an oral dose of mifepristone 200 mg to facilitate cervical preparation may be considered. On day 2, the osmotic dilators are removed and surgical evacuation is performed. In one randomized study, for pregnancies at 19 to 24 weeks’ gestation, compared with osmotic dilators alone, administration of mifepristone 200 mg at the time of placement of osmotic dilators resulted in fewer procedures that were difficult to complete.31 In some cases, 2 consecutive days of cervical preparation with osmotic dilators may be needed to properly prepare the cervix for uterine evacuation. For example, the cervix of a nulliparous teenage patient may require 2 days of cervical preparation with osmotic dilators to facilitate uterine evacuation. In some cases of fetal demise, the cervix is already dilated to ≥3 cm and surgical evacuation of the uterus or induction of labor can be initiated without the need for cervical preparation.
Continue to: Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor...
Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor
Treatment of fetal demise at 14w0d to 28w6d gestation with the goal of the vaginal birth of an intact fetus is optimized by the administration of mifepristone for cervical preparation followed by induction of labor with misoprostol.26,27
In one clinical trial, 66 patients with fetal demise between 14w0d and 28w6d gestation were randomly assigned to receive mifepristone 200 mg or placebo followed 24 to 48 hours later with initiation of misoprostol induction of labor.26 Among the patients from 14w0d to 24 weeks’ gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24w0d to 28 weeks’ gestation, the misoprostol dose was 200 µg vaginally every 4 hours. At 24 hours, a consultant obstetrician determined if additional misoprostol should be given. The median time from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups was 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P= .002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage, 4 in the placebo group and 1 in the mifepristone group.26
In a second clinical trial, 105 patients with fetal demise after 20 weeks of gestation were randomly assigned to receive mifepristone 200 mg or placebo.27 In this study, 86% of the patients were ≥26w0d gestation, with a mean gestational age of approximately 32w2d. Thirty-six to 48 hours later, misoprostol induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostoldose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients from ≥26 weeks’ gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours, respectively (P=.001). Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg; P<.001). Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.27
Fetal size ≥29w0d gestation
At a fetal size ≥29w0d gestation, if the cervix is ripe with a Bishop score of ≥7, oxytocin induction of labor is often used as a first-line treatment. If the cervix is not ripe, misoprostol induction of labor may be considered at doses less than those used in the second trimester of pregnancy.32 TABLES 1,1, 26, 33–36 2,37 and 337 summarize regimens proposed for fetal size ≥29w0d. One regimen begins with an initial misoprostol dose of 50 µg. If adequate uterine contractions occur, the 50 µg dose is repeated every 4 hours up to 6 total doses. If contractions are inadequate, the dose can be increased to 100 µg every 4 hours for 5 additional doses.



For fetal demise after 28w0d gestation, the
A multidisciplinary approach can optimize compassionate care
There are many gaps in the holistic care of patients and partners experiencing fetal demise. Patients with fetal demise often report that they did not receive sufficient information about the cause of the demise and wanted more opportunity to be involved in decision making about their care.39 The patient’s partner often reports feeling unacknowledged as a grieving parent.40 Fetal demise is experienced by many patients as a tragedy, triggering feelings of grief, anger, denial, anxiety and depression, sometimes resulting in isolation and substance misuse.
Using a 5-round Delphi process, experts identified 8 core goals in the care of patients with fetal demise:
- reduce stigma
- provide respectful care
- involve patients in care planning
- attempt to provide an explanation for the demise1
- acknowledge the depth of the grief response and provide emotional support
- offer information about ongoing psychological support
- provide information about future pregnancy planning
- provide opportunities for specialized training and support for care providers.41
Management of stillbirth is optimized by a multidisciplinary approach that includes the expert care of obstetrician-gynecologists, obstetric nurses, anesthesiologists, and expert consultation from social work, chaplaincy, and pathology. A heart-to-heart connection between clinician and patient is a key component of stillbirth care. ●
- American College of Obstetricians and Gynecologists. Management of stillbirth. ACOG Obstetric Care Consensus. No. 10. Obstet Gynecol. 2020;135:e110-132.
- Tsakiridis I, Giouleka S, Mamopoulos A, et al. Investigation and management of stillbirths: a descriptive review of major guidelines. J Perinat Med. 2022;50:796-813.
- Spingler T, Sonek J, Hoopman M, et al. Complication rate after termination of pregnancy due to fetal defects. Ultrasound Obstet Gynecol. 2023;Epub January 7.
- Goldberg AB, Drey EA, Whitaker AK, et al. Misoprostol compared with laminaria before early second-trimester surgical abortion: a randomized trial. Obstet Gynecol. 2005;106:234-241.
- Meirik O, My Huong NT, Piaggio G, et al. WHOR-GoP-MoF Regulation. Complications of first trimester abortion by vacuum aspiration after cervical preparation with and without misoprostol: a multicentre randomised trial. Lancet. 2012;379(9829):1817-1824.
- Bartz D, Maurer R, Allen RH, et al. Buccal misoprostol compared with synthetic osmotic cervical dilator before surgical abortion: a randomized controlled trial. Obstet Gynecol. 2013;122:57-63.
- Ngo LL, Mokashi M, Janiak E, et al. Acute complications with same-day versus overnight cervical preparation before dilation and evacuation at 14 to 16 weeks. Contraception. 2023;117:61-66.
- Kim CS, Dragoman M, Prosch L, et al. Same-day compared with overnight cervical preparation before dilation and evacuation between 16 and 19 6/7 weeks of gestation: a randomized controlled trial. Obstet Gynecol. 2022;139:1141-1144.
- Drunecky T, Reidingerova M, Plisova M, et al. Experimental comparison of properties of natural and synthetic osmotic dilators. Arch Gynecol Obstet. 2015;292:349-354.
- Hern WM. Laminaria versus Dilapan osmotic cervical dilators for outpatient dilation and evacuation abortion: randomized cohort comparison of 1001 patients. Am J Obstet Gynecol. 1994;171:1324-1328.
- Berthold C, Gomes David M, Gabriel P, et al. Effect of the addition of osmotic dilators to medical induction of labor abortion: a before-and-after study. Eur J Obstet Gynecol. 2020;244:185-189.
- Kemper JI, Li W, Goni S, et al. Foley catheter vs oral misoprostol for induction of labor: individual participant data meta-analysis. Ultrasound Obstet Gynecol. 2021;57:215-223.
- Attalli E, Kern Guy, Fouks Y, et al. Labor induction in third trimester non-viable fetus. J Matern Fetal Neonatal Med. 2022;Epub October 1.
- Fessehaye Sium A, Prager S, Wolderufael M, et al. Foley catheter for cervical preparation prior to second trimester dilation and evacuation: a supply-based alternative for surgical abortion: a case series. Contracept X. 2022;4:100085.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590.
- Barbieri RL. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022;34:8-10, 12.
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290.
- Barbieri RL. Mifepristone for the treatment of miscarriage and fetal demise. OBG Manag. 2022;34:811, 15.
- Kapp N, Borgatta L, Stubblefield P, et al. Mifepristone in second-trimester medical abortion. Obstet Gynecol. 2007;110:1304-1310.
- Ngoc NTN, Shochet T, Raghavan S, et al. Mifepristone and misoprostol compared with misoprostol alone for second trimester abortion: a randomized controlled trial. Obstet Gynecol. 2011;118:601608.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890.
- Prodan N, Breisch J, Hoopman M, et al. Dosing interval between mifepristone and misoprostol in second and third trimester termination. Arch Gynecol Obstet. 2019;299:675-679.
- Edlow AG, Hou MY, Maurer R, et al. Uterine evacuation for second trimester fetal death and maternal morbidity. Obstet Gynecol. 2011;117:1-10.
- Bryan AG, Grimes DA, Garrett JM, et al. Second-trimester abortion for fetal anomalies or fetal death. Obstet Gynecol. 2011;117:788-792.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol or mifepristone compared with overnight osmotic dilators alone. Obstet Gynecol. 2015;126:599-609.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- American College of Obstetricians and Gynecologists. Second-trimester abortion. Practice Bulletin No. 135. Obstet Gynecol. 2013;121:1394-1406.
- Wingo E, Raifman S, Landau C, et al. Mifepristone-misoprostol versus misoprostol-alone regimen for medication abortion at ≥ 24 weeks gestation. Contraception. Appendix 1. 2020;102:99-103.
- American College of Obstetricians and Gynecologists. Vaginal birth after cesarean delivery. ACOG Practice Bulletin No. 205. Obstet Gynecol. 2019;133:e110-e127.
- Atkins B, Blencowe H, Boyle FM, et al. Is care of stillborn babies and their parents respectful? Results from an international online survey. BJOG. 2022;129:1731-1739.
- Haezell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387(10018):604-616.
- Shakespeare C, Merriel A, Bakhbakhi D, et al. The RESPECT Study for consensus on global bereavement care after stillbirth. Int J Gynaecol Obstet. 2020;149:137-147.
- American College of Obstetricians and Gynecologists. Management of stillbirth. ACOG Obstetric Care Consensus. No. 10. Obstet Gynecol. 2020;135:e110-132.
- Tsakiridis I, Giouleka S, Mamopoulos A, et al. Investigation and management of stillbirths: a descriptive review of major guidelines. J Perinat Med. 2022;50:796-813.
- Spingler T, Sonek J, Hoopman M, et al. Complication rate after termination of pregnancy due to fetal defects. Ultrasound Obstet Gynecol. 2023;Epub January 7.
- Goldberg AB, Drey EA, Whitaker AK, et al. Misoprostol compared with laminaria before early second-trimester surgical abortion: a randomized trial. Obstet Gynecol. 2005;106:234-241.
- Meirik O, My Huong NT, Piaggio G, et al. WHOR-GoP-MoF Regulation. Complications of first trimester abortion by vacuum aspiration after cervical preparation with and without misoprostol: a multicentre randomised trial. Lancet. 2012;379(9829):1817-1824.
- Bartz D, Maurer R, Allen RH, et al. Buccal misoprostol compared with synthetic osmotic cervical dilator before surgical abortion: a randomized controlled trial. Obstet Gynecol. 2013;122:57-63.
- Ngo LL, Mokashi M, Janiak E, et al. Acute complications with same-day versus overnight cervical preparation before dilation and evacuation at 14 to 16 weeks. Contraception. 2023;117:61-66.
- Kim CS, Dragoman M, Prosch L, et al. Same-day compared with overnight cervical preparation before dilation and evacuation between 16 and 19 6/7 weeks of gestation: a randomized controlled trial. Obstet Gynecol. 2022;139:1141-1144.
- Drunecky T, Reidingerova M, Plisova M, et al. Experimental comparison of properties of natural and synthetic osmotic dilators. Arch Gynecol Obstet. 2015;292:349-354.
- Hern WM. Laminaria versus Dilapan osmotic cervical dilators for outpatient dilation and evacuation abortion: randomized cohort comparison of 1001 patients. Am J Obstet Gynecol. 1994;171:1324-1328.
- Berthold C, Gomes David M, Gabriel P, et al. Effect of the addition of osmotic dilators to medical induction of labor abortion: a before-and-after study. Eur J Obstet Gynecol. 2020;244:185-189.
- Kemper JI, Li W, Goni S, et al. Foley catheter vs oral misoprostol for induction of labor: individual participant data meta-analysis. Ultrasound Obstet Gynecol. 2021;57:215-223.
- Attalli E, Kern Guy, Fouks Y, et al. Labor induction in third trimester non-viable fetus. J Matern Fetal Neonatal Med. 2022;Epub October 1.
- Fessehaye Sium A, Prager S, Wolderufael M, et al. Foley catheter for cervical preparation prior to second trimester dilation and evacuation: a supply-based alternative for surgical abortion: a case series. Contracept X. 2022;4:100085.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590.
- Barbieri RL. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022;34:8-10, 12.
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290.
- Barbieri RL. Mifepristone for the treatment of miscarriage and fetal demise. OBG Manag. 2022;34:811, 15.
- Kapp N, Borgatta L, Stubblefield P, et al. Mifepristone in second-trimester medical abortion. Obstet Gynecol. 2007;110:1304-1310.
- Ngoc NTN, Shochet T, Raghavan S, et al. Mifepristone and misoprostol compared with misoprostol alone for second trimester abortion: a randomized controlled trial. Obstet Gynecol. 2011;118:601608.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890.
- Prodan N, Breisch J, Hoopman M, et al. Dosing interval between mifepristone and misoprostol in second and third trimester termination. Arch Gynecol Obstet. 2019;299:675-679.
- Edlow AG, Hou MY, Maurer R, et al. Uterine evacuation for second trimester fetal death and maternal morbidity. Obstet Gynecol. 2011;117:1-10.
- Bryan AG, Grimes DA, Garrett JM, et al. Second-trimester abortion for fetal anomalies or fetal death. Obstet Gynecol. 2011;117:788-792.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol or mifepristone compared with overnight osmotic dilators alone. Obstet Gynecol. 2015;126:599-609.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- American College of Obstetricians and Gynecologists. Second-trimester abortion. Practice Bulletin No. 135. Obstet Gynecol. 2013;121:1394-1406.
- Wingo E, Raifman S, Landau C, et al. Mifepristone-misoprostol versus misoprostol-alone regimen for medication abortion at ≥ 24 weeks gestation. Contraception. Appendix 1. 2020;102:99-103.
- American College of Obstetricians and Gynecologists. Vaginal birth after cesarean delivery. ACOG Practice Bulletin No. 205. Obstet Gynecol. 2019;133:e110-e127.
- Atkins B, Blencowe H, Boyle FM, et al. Is care of stillborn babies and their parents respectful? Results from an international online survey. BJOG. 2022;129:1731-1739.
- Haezell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387(10018):604-616.
- Shakespeare C, Merriel A, Bakhbakhi D, et al. The RESPECT Study for consensus on global bereavement care after stillbirth. Int J Gynaecol Obstet. 2020;149:137-147.
Financial navigators saved about $2,500 per cancer patient
Cancer patients in the United States face complex financial issues in navigating with medical insurance companies to cover their care. This “financial toxicity” has come to be regarded as a side effect of cancer treatment.
Patients with hematologic malignancies may be particularly vulnerable to financial toxicity, owing to the nature of their treatment, which often includes bone marrow transplantation, lengthy hospital stays, and prolonged intensive follow-up, as well as potential treatment-related complications, such as graft vs. host disease.
The results from this small study suggest that using an oncology financial navigator could be helpful. But not all cancer patients have access to such a person, explained lead author Jean S. Edward, PhD, RN, associate professor in the college of nursing at the University of Kentucky, Lexington.
“Unfortunately, it’s not as common as we would like, especially in underserved areas with patient and caregiver populations that need it the most,” she said. Dr. Edward is hopeful that the results from this study, even though it is small, might help to boost use of this intervention. “OFN [oncology financial navigation] is not necessarily a cutting-edge program or ‘novel’ intervention, but the lack of programs and limitations in implementing in cancer centers does make it a gap in practice,” Dr. Edward told this news organization.
“There are gaps in evidence on how to incorporate an oncology financial navigator in current workflows and sustainability of positions, but as our study has shown, the return on investment to the health care system and/or financial benefits to patients/caregivers could help cover the cost of implementing such programs,” she said.
The study was published in JCO Oncology Practice.
The intervention used in this study, Coverage and Cost-of-Care Links (CC Links), was designed specifically to address financial toxicity among patients with hematologic cancers.
The study’s primary outcomes were defined as improvements in financial distress as well as in physical and mental quality of life.
A total of 54 patients and 32 caregivers completed the intervention and pre-/postintervention surveys. More than half of participants were women. The average age was 63 years. Less than a quarter of the patients were employed (23%), about one-third had income that was below the federal poverty level, and almost all had insurance. About 59% of the caregivers were employed.
The navigators’ functions included screening for financial toxicity using FACIT-Comprehensive Score for Financial Toxicity (COST) and the National Comprehensive Cancer Network’s Distress Thermometer and Problem List. They also helped patients to estimate cost of care, assessed health insurance coverage, and connected patients/caregivers with disease-specific resources and other external assistance programs, among other things.
Participants had an average of three in-person meetings and five telephone interactions with the financial navigator. The most common concern was in regard to high out-of-pocket costs. The most frequently provided services from the navigator were helping with financial assistance programs and grant applications. Overall, the navigator was able to obtain $124,600 in financial benefits for 48 participants, as well as money for travel ($24,000), urgent needs ($16,000), patient financial assistance ($9,100), and copay assistance grants ($75,500).
With regard to scores on the screening tools, the only significant change from pre- to postintervention was in the psychological response score, or COST. It decreased by an average of 2.30 points (P = .019; Hedges’ g = 0.33). For caregivers, there was a significant improvement in COST (average decrease, 2.97 points; P = .021; g = 0.43), material condition scores (average decrease, 0.63 points; P = .031; g = 0.39), and total financial toxicity scores (average decrease, 0.13 points; P = .041; g = 0.37).
Most of the participants gave the intervention high ratings for acceptability (89%) and appropriateness (88%).
“Standardized screening for financial toxicity in cancer care settings is essential to support early identification of financial needs that serve as barriers to care,” the authors conclude. “Close collaboration and coordination with existing services and workflows are essential for the seamless integration of OFN interventions within health systems and to help facilitate contact and communication with participants.”
The study was supported by the National Cancer Institute; the University of Kentucky’s Markey Cancer Center; the Research Communications Office of the Patient Oriented and Population Science Shared Resource Facilities; Joan Scales, LCSW, and the Psych-Oncology Program at the University of Kentucky Markey Cancer Center; and UK HealthCare’s Patient Financial Services. Dr. Edward has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer patients in the United States face complex financial issues in navigating with medical insurance companies to cover their care. This “financial toxicity” has come to be regarded as a side effect of cancer treatment.
Patients with hematologic malignancies may be particularly vulnerable to financial toxicity, owing to the nature of their treatment, which often includes bone marrow transplantation, lengthy hospital stays, and prolonged intensive follow-up, as well as potential treatment-related complications, such as graft vs. host disease.
The results from this small study suggest that using an oncology financial navigator could be helpful. But not all cancer patients have access to such a person, explained lead author Jean S. Edward, PhD, RN, associate professor in the college of nursing at the University of Kentucky, Lexington.
“Unfortunately, it’s not as common as we would like, especially in underserved areas with patient and caregiver populations that need it the most,” she said. Dr. Edward is hopeful that the results from this study, even though it is small, might help to boost use of this intervention. “OFN [oncology financial navigation] is not necessarily a cutting-edge program or ‘novel’ intervention, but the lack of programs and limitations in implementing in cancer centers does make it a gap in practice,” Dr. Edward told this news organization.
“There are gaps in evidence on how to incorporate an oncology financial navigator in current workflows and sustainability of positions, but as our study has shown, the return on investment to the health care system and/or financial benefits to patients/caregivers could help cover the cost of implementing such programs,” she said.
The study was published in JCO Oncology Practice.
The intervention used in this study, Coverage and Cost-of-Care Links (CC Links), was designed specifically to address financial toxicity among patients with hematologic cancers.
The study’s primary outcomes were defined as improvements in financial distress as well as in physical and mental quality of life.
A total of 54 patients and 32 caregivers completed the intervention and pre-/postintervention surveys. More than half of participants were women. The average age was 63 years. Less than a quarter of the patients were employed (23%), about one-third had income that was below the federal poverty level, and almost all had insurance. About 59% of the caregivers were employed.
The navigators’ functions included screening for financial toxicity using FACIT-Comprehensive Score for Financial Toxicity (COST) and the National Comprehensive Cancer Network’s Distress Thermometer and Problem List. They also helped patients to estimate cost of care, assessed health insurance coverage, and connected patients/caregivers with disease-specific resources and other external assistance programs, among other things.
Participants had an average of three in-person meetings and five telephone interactions with the financial navigator. The most common concern was in regard to high out-of-pocket costs. The most frequently provided services from the navigator were helping with financial assistance programs and grant applications. Overall, the navigator was able to obtain $124,600 in financial benefits for 48 participants, as well as money for travel ($24,000), urgent needs ($16,000), patient financial assistance ($9,100), and copay assistance grants ($75,500).
With regard to scores on the screening tools, the only significant change from pre- to postintervention was in the psychological response score, or COST. It decreased by an average of 2.30 points (P = .019; Hedges’ g = 0.33). For caregivers, there was a significant improvement in COST (average decrease, 2.97 points; P = .021; g = 0.43), material condition scores (average decrease, 0.63 points; P = .031; g = 0.39), and total financial toxicity scores (average decrease, 0.13 points; P = .041; g = 0.37).
Most of the participants gave the intervention high ratings for acceptability (89%) and appropriateness (88%).
“Standardized screening for financial toxicity in cancer care settings is essential to support early identification of financial needs that serve as barriers to care,” the authors conclude. “Close collaboration and coordination with existing services and workflows are essential for the seamless integration of OFN interventions within health systems and to help facilitate contact and communication with participants.”
The study was supported by the National Cancer Institute; the University of Kentucky’s Markey Cancer Center; the Research Communications Office of the Patient Oriented and Population Science Shared Resource Facilities; Joan Scales, LCSW, and the Psych-Oncology Program at the University of Kentucky Markey Cancer Center; and UK HealthCare’s Patient Financial Services. Dr. Edward has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer patients in the United States face complex financial issues in navigating with medical insurance companies to cover their care. This “financial toxicity” has come to be regarded as a side effect of cancer treatment.
Patients with hematologic malignancies may be particularly vulnerable to financial toxicity, owing to the nature of their treatment, which often includes bone marrow transplantation, lengthy hospital stays, and prolonged intensive follow-up, as well as potential treatment-related complications, such as graft vs. host disease.
The results from this small study suggest that using an oncology financial navigator could be helpful. But not all cancer patients have access to such a person, explained lead author Jean S. Edward, PhD, RN, associate professor in the college of nursing at the University of Kentucky, Lexington.
“Unfortunately, it’s not as common as we would like, especially in underserved areas with patient and caregiver populations that need it the most,” she said. Dr. Edward is hopeful that the results from this study, even though it is small, might help to boost use of this intervention. “OFN [oncology financial navigation] is not necessarily a cutting-edge program or ‘novel’ intervention, but the lack of programs and limitations in implementing in cancer centers does make it a gap in practice,” Dr. Edward told this news organization.
“There are gaps in evidence on how to incorporate an oncology financial navigator in current workflows and sustainability of positions, but as our study has shown, the return on investment to the health care system and/or financial benefits to patients/caregivers could help cover the cost of implementing such programs,” she said.
The study was published in JCO Oncology Practice.
The intervention used in this study, Coverage and Cost-of-Care Links (CC Links), was designed specifically to address financial toxicity among patients with hematologic cancers.
The study’s primary outcomes were defined as improvements in financial distress as well as in physical and mental quality of life.
A total of 54 patients and 32 caregivers completed the intervention and pre-/postintervention surveys. More than half of participants were women. The average age was 63 years. Less than a quarter of the patients were employed (23%), about one-third had income that was below the federal poverty level, and almost all had insurance. About 59% of the caregivers were employed.
The navigators’ functions included screening for financial toxicity using FACIT-Comprehensive Score for Financial Toxicity (COST) and the National Comprehensive Cancer Network’s Distress Thermometer and Problem List. They also helped patients to estimate cost of care, assessed health insurance coverage, and connected patients/caregivers with disease-specific resources and other external assistance programs, among other things.
Participants had an average of three in-person meetings and five telephone interactions with the financial navigator. The most common concern was in regard to high out-of-pocket costs. The most frequently provided services from the navigator were helping with financial assistance programs and grant applications. Overall, the navigator was able to obtain $124,600 in financial benefits for 48 participants, as well as money for travel ($24,000), urgent needs ($16,000), patient financial assistance ($9,100), and copay assistance grants ($75,500).
With regard to scores on the screening tools, the only significant change from pre- to postintervention was in the psychological response score, or COST. It decreased by an average of 2.30 points (P = .019; Hedges’ g = 0.33). For caregivers, there was a significant improvement in COST (average decrease, 2.97 points; P = .021; g = 0.43), material condition scores (average decrease, 0.63 points; P = .031; g = 0.39), and total financial toxicity scores (average decrease, 0.13 points; P = .041; g = 0.37).
Most of the participants gave the intervention high ratings for acceptability (89%) and appropriateness (88%).
“Standardized screening for financial toxicity in cancer care settings is essential to support early identification of financial needs that serve as barriers to care,” the authors conclude. “Close collaboration and coordination with existing services and workflows are essential for the seamless integration of OFN interventions within health systems and to help facilitate contact and communication with participants.”
The study was supported by the National Cancer Institute; the University of Kentucky’s Markey Cancer Center; the Research Communications Office of the Patient Oriented and Population Science Shared Resource Facilities; Joan Scales, LCSW, and the Psych-Oncology Program at the University of Kentucky Markey Cancer Center; and UK HealthCare’s Patient Financial Services. Dr. Edward has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JCO ONCOLOGY PRACTICE
Reimagining psychiatric assessment and interventions as procedures
Many psychiatric physicians lament the dearth of procedures in psychiatry compared to other medical specialties such as surgery, cardiology, gastroenterology, or radiology. The few procedures in psychiatry include electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation, and vagus nerve stimulation, which are restricted to a small number of sites and not available for most psychiatric practitioners. This lack of tangible/physical procedures should not be surprising because psychiatry deals with disorders of the mind, which are invisible.
However, when one closely examines what psychiatrists do in daily practice to heal our patients, most of what we do actually qualifies as “procedures” although no hardware, machines, or gadgets are involved. Treating psychiatric brain disorders (aka mental illness) requires exquisite skills and expertise, just like medical specialties that use machines to measure or treat various body organs.
It’s time to relabel psychiatric interventions as procedures designed to improve anomalous thoughts, affect, emotions, cognition, and behavior. After giving it some thought (and with a bit of tongue in cheek), I came up with the following list of “psychiatric procedures”:
- Psychosocial exploratory laparotomy: The comprehensive psychiatric assessment and mental status exam.
- Chemotherapy: Oral or injective pharmacotherapeutic intervention.
- Psychoplastic repair: Neuroplasticity, including neurogenesis, synaptogenesis, and dendritic spine regeneration, have been shown to be associated with both psychotherapy and psychotropic medications.1,2
- Suicidectomy: Extracting the lethal urge to die by suicide.
- Anger debridement: Removing the irritability and destructive anger outbursts frequently associated with various psychopathologies.
- Anxiety ablation: Eliminating the noxious emotional state of anxiety and frightening panic attacks.
- Empathy infusion: Enabling patients to become more understanding of other people and bolstering their impaired “theory of mind.”
- Personality transplant: Replacing a maladaptive personality with a healthier one (eg, using dialectical behavior therapy for borderline personality disorder).
- Cognitive LASIK: To improve insight, analogous to how ophthalmologic LASIK improves sight.
- Mental embolectomy: Removing a blockage to repair rigid attitudes and develop “open-mindedness.”
- Behavioral dilation and curettage (D&C): To rid patients of negative attributes such as impulsivity or reckless behavior.
- Psychotherapeutic anesthesia: Numbing emotional pain or severe grief reaction.
- Social anastomosis: Helping patients who are schizoid or isolative via group therapy, an effective interpersonal and social procedure.
- Psychotherapeutic stent: To open the vessels of narrow-mindedness.
- Cortico-psychological resuscitation (CPR): For patients experiencing stress-induced behavioral arrhythmias or emotional infarction.
- Immunotherapy: Using various neuroprotective psychotropic medications with anti-inflammatory properties or employing evidence-based psychotherapy such as cognitive-behavior therapy (aka neuropsychotherapy), which have been shown to reduce inflammatory biomarkers such as C-reactive protein and cytokines.3
- Psychotherapy: A neuromodulation procedure for a variety of psychiatric disorders.4
- Neurobiological facelift: It is well established that neurogenesis, synaptogenesis, and dendritic spine sprouting are significantly increased with both neuroprotective psychotropic medications (antidepressants, lithium, valproate, and second-generationantipsychotics5) as well as with psychotherapy. There is growing evidence of “premature brain aging” in schizophrenia, bipolar disorder, and depression, with shrinkage in the volume of the cortex and subcortical regions, especially the hippocampus. Psychiatric biopsychosocial intervention rebuilds those brain regions by stimulating and replenishing the neuropil and neurogenic regions (dentate gyrus and subventricular zone). This is like performing virtual plastic surgery on a wrinkled brain and its sagging mind. MRI scans before and after ECT show a remarkable ≥10% increase in the volume of the hippocampus and amygdala, which translates to billions of new neurons, glia, and synapses.6
Reinventing psychiatric therapies as procedures may elicit sarcasm from skeptics, but when you think about it, it is justified. Excising depression is like excising a tumor, not with a scalpel, but virtually. Stabilizing the broken brain and mind after a psychotic episode (aka brain attack) is like stabilizing the heart after a myocardial infarction (aka heart attack). Just because the mind is virtual doesn’t mean it is not “real and tangible.” A desktop computer is visible, but the software that brings it to life is invisible. Healing the human mind requires multiple medical interventions by psychiatrists in hospitals and clinics, just like surgeons and endoscopists or cardiologists. Mental health care is as much procedural as other medical and surgical specialties.
One more thing: the validated clinical rating scales for various psychiatric brain disorders (eg, the Positive and Negative Syndrome Scale for schizophrenia, Montgomery-Åsberg Depression Rating Scale for depression, Young Mania Rating Scale for bipolar mania, Hamilton Anxiety Rating Scale for anxiety, Yale-Brown Obsessive Compulsive Scale for obsessive-compulsive disorder) are actual measurement procedures for the severity of the illness, just as a sphygmomanometer measures blood pressure and its improvement with treatment. There are also multiple cognitive test batteries to measure cognitive impairment.7
Finally, unlike psychiatric reimbursement, which is tethered to time, procedures are compensated more generously, irrespective of the time involved. The complexities of diagnosing and treating psychiatric brain disorders that dangerously disrupt thoughts, feelings, behavior, and cognition are just as intricate and demanding as the diagnosis and treatment of general medical and surgical conditions. They should all be equally appreciated as vital life-saving procedures for the human body, brain, and mind.
1. Nasrallah HA, Hopkins T, Pixley SK. Differential effects of antipsychotic and antidepressant drugs on neurogenic regions in rats. Brain Res. 2010;1354:23-29.
2. Tomasino B, Fabbro F. Increases in the right dorsolateral prefrontal cortex and decreases the rostral prefrontal cortex activation after-8 weeks of focused attention based mindfulness meditation. Brain Cogn. 2016;102:46-54.
3. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
4. Nasrallah HA. Optimal psychiatric treatment: Target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
5. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
6. Gryglewski G, Lanzenberger R, Silberbauer LR, et al. Meta-analysis of brain structural changes after electroconvulsive therapy in depression. Brain Stimul. 2021;14(4):927-937.
7. Nasrallah HA. The Cognition Self-Assessment Rating Scale for patients with schizophrenia. Current Psychiatry. 2023;22(3):30-34.
Many psychiatric physicians lament the dearth of procedures in psychiatry compared to other medical specialties such as surgery, cardiology, gastroenterology, or radiology. The few procedures in psychiatry include electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation, and vagus nerve stimulation, which are restricted to a small number of sites and not available for most psychiatric practitioners. This lack of tangible/physical procedures should not be surprising because psychiatry deals with disorders of the mind, which are invisible.
However, when one closely examines what psychiatrists do in daily practice to heal our patients, most of what we do actually qualifies as “procedures” although no hardware, machines, or gadgets are involved. Treating psychiatric brain disorders (aka mental illness) requires exquisite skills and expertise, just like medical specialties that use machines to measure or treat various body organs.
It’s time to relabel psychiatric interventions as procedures designed to improve anomalous thoughts, affect, emotions, cognition, and behavior. After giving it some thought (and with a bit of tongue in cheek), I came up with the following list of “psychiatric procedures”:
- Psychosocial exploratory laparotomy: The comprehensive psychiatric assessment and mental status exam.
- Chemotherapy: Oral or injective pharmacotherapeutic intervention.
- Psychoplastic repair: Neuroplasticity, including neurogenesis, synaptogenesis, and dendritic spine regeneration, have been shown to be associated with both psychotherapy and psychotropic medications.1,2
- Suicidectomy: Extracting the lethal urge to die by suicide.
- Anger debridement: Removing the irritability and destructive anger outbursts frequently associated with various psychopathologies.
- Anxiety ablation: Eliminating the noxious emotional state of anxiety and frightening panic attacks.
- Empathy infusion: Enabling patients to become more understanding of other people and bolstering their impaired “theory of mind.”
- Personality transplant: Replacing a maladaptive personality with a healthier one (eg, using dialectical behavior therapy for borderline personality disorder).
- Cognitive LASIK: To improve insight, analogous to how ophthalmologic LASIK improves sight.
- Mental embolectomy: Removing a blockage to repair rigid attitudes and develop “open-mindedness.”
- Behavioral dilation and curettage (D&C): To rid patients of negative attributes such as impulsivity or reckless behavior.
- Psychotherapeutic anesthesia: Numbing emotional pain or severe grief reaction.
- Social anastomosis: Helping patients who are schizoid or isolative via group therapy, an effective interpersonal and social procedure.
- Psychotherapeutic stent: To open the vessels of narrow-mindedness.
- Cortico-psychological resuscitation (CPR): For patients experiencing stress-induced behavioral arrhythmias or emotional infarction.
- Immunotherapy: Using various neuroprotective psychotropic medications with anti-inflammatory properties or employing evidence-based psychotherapy such as cognitive-behavior therapy (aka neuropsychotherapy), which have been shown to reduce inflammatory biomarkers such as C-reactive protein and cytokines.3
- Psychotherapy: A neuromodulation procedure for a variety of psychiatric disorders.4
- Neurobiological facelift: It is well established that neurogenesis, synaptogenesis, and dendritic spine sprouting are significantly increased with both neuroprotective psychotropic medications (antidepressants, lithium, valproate, and second-generationantipsychotics5) as well as with psychotherapy. There is growing evidence of “premature brain aging” in schizophrenia, bipolar disorder, and depression, with shrinkage in the volume of the cortex and subcortical regions, especially the hippocampus. Psychiatric biopsychosocial intervention rebuilds those brain regions by stimulating and replenishing the neuropil and neurogenic regions (dentate gyrus and subventricular zone). This is like performing virtual plastic surgery on a wrinkled brain and its sagging mind. MRI scans before and after ECT show a remarkable ≥10% increase in the volume of the hippocampus and amygdala, which translates to billions of new neurons, glia, and synapses.6
Reinventing psychiatric therapies as procedures may elicit sarcasm from skeptics, but when you think about it, it is justified. Excising depression is like excising a tumor, not with a scalpel, but virtually. Stabilizing the broken brain and mind after a psychotic episode (aka brain attack) is like stabilizing the heart after a myocardial infarction (aka heart attack). Just because the mind is virtual doesn’t mean it is not “real and tangible.” A desktop computer is visible, but the software that brings it to life is invisible. Healing the human mind requires multiple medical interventions by psychiatrists in hospitals and clinics, just like surgeons and endoscopists or cardiologists. Mental health care is as much procedural as other medical and surgical specialties.
One more thing: the validated clinical rating scales for various psychiatric brain disorders (eg, the Positive and Negative Syndrome Scale for schizophrenia, Montgomery-Åsberg Depression Rating Scale for depression, Young Mania Rating Scale for bipolar mania, Hamilton Anxiety Rating Scale for anxiety, Yale-Brown Obsessive Compulsive Scale for obsessive-compulsive disorder) are actual measurement procedures for the severity of the illness, just as a sphygmomanometer measures blood pressure and its improvement with treatment. There are also multiple cognitive test batteries to measure cognitive impairment.7
Finally, unlike psychiatric reimbursement, which is tethered to time, procedures are compensated more generously, irrespective of the time involved. The complexities of diagnosing and treating psychiatric brain disorders that dangerously disrupt thoughts, feelings, behavior, and cognition are just as intricate and demanding as the diagnosis and treatment of general medical and surgical conditions. They should all be equally appreciated as vital life-saving procedures for the human body, brain, and mind.
Many psychiatric physicians lament the dearth of procedures in psychiatry compared to other medical specialties such as surgery, cardiology, gastroenterology, or radiology. The few procedures in psychiatry include electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation, and vagus nerve stimulation, which are restricted to a small number of sites and not available for most psychiatric practitioners. This lack of tangible/physical procedures should not be surprising because psychiatry deals with disorders of the mind, which are invisible.
However, when one closely examines what psychiatrists do in daily practice to heal our patients, most of what we do actually qualifies as “procedures” although no hardware, machines, or gadgets are involved. Treating psychiatric brain disorders (aka mental illness) requires exquisite skills and expertise, just like medical specialties that use machines to measure or treat various body organs.
It’s time to relabel psychiatric interventions as procedures designed to improve anomalous thoughts, affect, emotions, cognition, and behavior. After giving it some thought (and with a bit of tongue in cheek), I came up with the following list of “psychiatric procedures”:
- Psychosocial exploratory laparotomy: The comprehensive psychiatric assessment and mental status exam.
- Chemotherapy: Oral or injective pharmacotherapeutic intervention.
- Psychoplastic repair: Neuroplasticity, including neurogenesis, synaptogenesis, and dendritic spine regeneration, have been shown to be associated with both psychotherapy and psychotropic medications.1,2
- Suicidectomy: Extracting the lethal urge to die by suicide.
- Anger debridement: Removing the irritability and destructive anger outbursts frequently associated with various psychopathologies.
- Anxiety ablation: Eliminating the noxious emotional state of anxiety and frightening panic attacks.
- Empathy infusion: Enabling patients to become more understanding of other people and bolstering their impaired “theory of mind.”
- Personality transplant: Replacing a maladaptive personality with a healthier one (eg, using dialectical behavior therapy for borderline personality disorder).
- Cognitive LASIK: To improve insight, analogous to how ophthalmologic LASIK improves sight.
- Mental embolectomy: Removing a blockage to repair rigid attitudes and develop “open-mindedness.”
- Behavioral dilation and curettage (D&C): To rid patients of negative attributes such as impulsivity or reckless behavior.
- Psychotherapeutic anesthesia: Numbing emotional pain or severe grief reaction.
- Social anastomosis: Helping patients who are schizoid or isolative via group therapy, an effective interpersonal and social procedure.
- Psychotherapeutic stent: To open the vessels of narrow-mindedness.
- Cortico-psychological resuscitation (CPR): For patients experiencing stress-induced behavioral arrhythmias or emotional infarction.
- Immunotherapy: Using various neuroprotective psychotropic medications with anti-inflammatory properties or employing evidence-based psychotherapy such as cognitive-behavior therapy (aka neuropsychotherapy), which have been shown to reduce inflammatory biomarkers such as C-reactive protein and cytokines.3
- Psychotherapy: A neuromodulation procedure for a variety of psychiatric disorders.4
- Neurobiological facelift: It is well established that neurogenesis, synaptogenesis, and dendritic spine sprouting are significantly increased with both neuroprotective psychotropic medications (antidepressants, lithium, valproate, and second-generationantipsychotics5) as well as with psychotherapy. There is growing evidence of “premature brain aging” in schizophrenia, bipolar disorder, and depression, with shrinkage in the volume of the cortex and subcortical regions, especially the hippocampus. Psychiatric biopsychosocial intervention rebuilds those brain regions by stimulating and replenishing the neuropil and neurogenic regions (dentate gyrus and subventricular zone). This is like performing virtual plastic surgery on a wrinkled brain and its sagging mind. MRI scans before and after ECT show a remarkable ≥10% increase in the volume of the hippocampus and amygdala, which translates to billions of new neurons, glia, and synapses.6
Reinventing psychiatric therapies as procedures may elicit sarcasm from skeptics, but when you think about it, it is justified. Excising depression is like excising a tumor, not with a scalpel, but virtually. Stabilizing the broken brain and mind after a psychotic episode (aka brain attack) is like stabilizing the heart after a myocardial infarction (aka heart attack). Just because the mind is virtual doesn’t mean it is not “real and tangible.” A desktop computer is visible, but the software that brings it to life is invisible. Healing the human mind requires multiple medical interventions by psychiatrists in hospitals and clinics, just like surgeons and endoscopists or cardiologists. Mental health care is as much procedural as other medical and surgical specialties.
One more thing: the validated clinical rating scales for various psychiatric brain disorders (eg, the Positive and Negative Syndrome Scale for schizophrenia, Montgomery-Åsberg Depression Rating Scale for depression, Young Mania Rating Scale for bipolar mania, Hamilton Anxiety Rating Scale for anxiety, Yale-Brown Obsessive Compulsive Scale for obsessive-compulsive disorder) are actual measurement procedures for the severity of the illness, just as a sphygmomanometer measures blood pressure and its improvement with treatment. There are also multiple cognitive test batteries to measure cognitive impairment.7
Finally, unlike psychiatric reimbursement, which is tethered to time, procedures are compensated more generously, irrespective of the time involved. The complexities of diagnosing and treating psychiatric brain disorders that dangerously disrupt thoughts, feelings, behavior, and cognition are just as intricate and demanding as the diagnosis and treatment of general medical and surgical conditions. They should all be equally appreciated as vital life-saving procedures for the human body, brain, and mind.
1. Nasrallah HA, Hopkins T, Pixley SK. Differential effects of antipsychotic and antidepressant drugs on neurogenic regions in rats. Brain Res. 2010;1354:23-29.
2. Tomasino B, Fabbro F. Increases in the right dorsolateral prefrontal cortex and decreases the rostral prefrontal cortex activation after-8 weeks of focused attention based mindfulness meditation. Brain Cogn. 2016;102:46-54.
3. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
4. Nasrallah HA. Optimal psychiatric treatment: Target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
5. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
6. Gryglewski G, Lanzenberger R, Silberbauer LR, et al. Meta-analysis of brain structural changes after electroconvulsive therapy in depression. Brain Stimul. 2021;14(4):927-937.
7. Nasrallah HA. The Cognition Self-Assessment Rating Scale for patients with schizophrenia. Current Psychiatry. 2023;22(3):30-34.
1. Nasrallah HA, Hopkins T, Pixley SK. Differential effects of antipsychotic and antidepressant drugs on neurogenic regions in rats. Brain Res. 2010;1354:23-29.
2. Tomasino B, Fabbro F. Increases in the right dorsolateral prefrontal cortex and decreases the rostral prefrontal cortex activation after-8 weeks of focused attention based mindfulness meditation. Brain Cogn. 2016;102:46-54.
3. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
4. Nasrallah HA. Optimal psychiatric treatment: Target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
5. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
6. Gryglewski G, Lanzenberger R, Silberbauer LR, et al. Meta-analysis of brain structural changes after electroconvulsive therapy in depression. Brain Stimul. 2021;14(4):927-937.
7. Nasrallah HA. The Cognition Self-Assessment Rating Scale for patients with schizophrenia. Current Psychiatry. 2023;22(3):30-34.



