User login
Can Sea Lions Expand Our Knowledge of Epilepsy?
In 2022, the Journal of Neurosurgery took the unusual step of publishing a case report of a nonhuman subject, in this case an 8-year-old male sea lion named Cronutt, who had severe epilepsy that in many ways mirrored the common form seen in humans.
A 2020 xenotransplantation of interneuron progenitor cells in the sea lion’s hippocampus may have cured him. “He’s doing fantastic,” said Scott Baraban, PhD, a professor of neurological surgery at the University of California San Francisco Weill Institute for Neurosciences. The procedure offered a confirmation of success stories in mice and could serve as a bridge to human studies, although the science remains murky.
Cronutt’s story began the first time he was found stranded along the California coast in San Luis Obispo County, in November 2017, lethargic and disoriented. Nevertheless, he improved quickly under the care of the Marine Mammal Rescue Center in Sausalito, California, and they released him back into the wild 2 weeks later. Unfortunately, his troubles weren’t over. In the next 2 months, he came to the attention of the rehabilitation center two more times, and the resulting habituation to humans meant that he could not be re-released to the wild.
Things only got worse from there. He experienced a convulsive seizure and loss of consciousness in early 2018 and another seizure a year later. Despite anti-seizure medication, the episodes continued, and eventually, he stopped eating for long periods and lost a quarter of his body weight. It looked like euthanasia was the only alternative.
Neurosurgeons Take Notice
Baraban has devoted his career to animal models of epilepsy, focusing mainly on mice. He developed a method to transplant GABAergic interneurons from pigs, which can pick up the inhibitory role of endogenous neurons. In epileptic mice, mouse-derived progenitor cells mature into inhibitory synapses and dampen seizures, and even reverse behavioral problems.
Baraban was intrigued by the potential of the procedure to help sea lions and potentially bridge the gap between murine and human studies, and he was eager to try the approach after he learned of Cronutt’s case through his colleague Paul Buckmaster at Stanford University, California, who had collaborated with the Marine Mammal Rescue Center to demonstrate that sea lions suffer hippocampal damage that is very similar to human temporal lobe epilepsy.
It turns out that surgery on a sea lion is more complicated than on a mouse. The surgeon needs to conduct an MRI scan to locate the lesion and guide the placement of transplanted cells, and the big animals need to be sedated and intubated. Their caretakers are not enthusiastic about invasive procedures, but Cronutt had already been imaged as part of an earlier study of the effects of exposure to domoic acid on the sea lion brain, giving neurosurgeons at UCSF the information they needed.
In 2020, they transplanted GABAergic interneurons into Cronutt’s hippocampus in a last-ditch effort to save his life. He improved rapidly, and his caregivers eventually tapered off his anti-seizure medications. Today, Cronutt is living seizure-free at the Six Flags Discovery Kingdom in Vallejo, California. He even began participating in shows at Six Flags. “He is able to learn things and do things cognitively that he couldn’t do before,” said Baraban.
The researchers have not followed up with more imaging. “We just haven’t had any reason to bother him. It’s very invasive to get him to an MRI center,” said Baraban.
There haven’t been any more procedures on sea lions, in part because sea lions at rescue centers must be returned to the wild within 6-8 months, and animals with pig-based progenitor cells can’t be released. Any sea lion that would undergo such a procedure would have to live permanently in captivity, potentially for 20-30 years. “It’s a huge undertaking and expense,” said Baraban. There are also logistical challenges with the need to have MRI and other facilities close at hand to where the sea lion is kept.
Human Connections
As a veterinarian interested in human epilepsy, Buckmaster sought to find additional animal models for human temporal lobe epilepsy. He later met a veterinarian who worked with the Marine Mammal Center, who told him about temporal lobe epilepsy in sea lions.
Cronutt was just one of hundreds of California sea lions and other mammals rendered helpless by epileptic seizures induced by exposure to domoic acid, a chemical released by toxic algal blooms. Sea otters, whales, and even birds can likewise be affected. Humans, too, though it is rare: A domoic acid poisoning event in mussels from Prince Edward Island led to convulsions and an eventual diagnosis of temporal lobe epilepsy in an 84-year-old Canadian man, and three others died.
Most of Buckmaster’s work had been done in rats, but seizures are different than what are seen in humans. The damage isn’t unilateral, as in human epilepsy. The damage is unilateral in sea lions, and they suffer damage to similar neurons to those injured in humans. “In rats, you don’t see much loss of granule cells, which are the main neuron in the dentate gyrus, a region that’s really affected in temporal lobe epilepsy. In people, there’s a lot of variation, but [on average] you lose about half your granule cells, and that’s similar to sea lions. In rats, they just don’t lose them,” said Buckmaster.
That makes sea lions a useful model, but they hardly crack the case of human epilepsy. “To be humble about it, we don’t really know what’s causing the seizures. We have an idea of what region of the brain the seizures are coming from, but in terms of the cellular mechanisms, it’s still not clear,” said Buckmaster.
He also noted that, unlike sea lions, the vast majority of patients with human epilepsy have no exposure to domoic acid. That said, the case of sea lions and other marine animals makes researchers wonder. “This is speculative, but a lot of people with temporal epilepsy will have a history of some kind of precipitating event. Typically, it’s earlier in life, and a common one is prolonged febrile seizures. That could be a link to the domoic acid exposure in sea lions because the domoic acid exposure in sea lions frequently causes prolonged seizures. If the seizures are severe enough and long enough, they cause permanent brain damage, and that can cause temporal lobe epilepsy. That’s what we strongly believe is causing the temporal lobe epilepsy in sea lions. Prolonged, severe seizures can be caused by many things other than domoic acid, and that could be what causes it in many cases of human temporal lobe epilepsy,” said Buckmaster.
The approach taken with Cronutt makes sense to Buckmaster, who was not directly involved in the procedure. “The ideal treatment would be something that you would administer once, and it would cause cessation of seizures without any side effects. The transplantation of inhibitory cells is a treatment that would last and hopefully wouldn’t have any side effects and hopefully would provide complete control of seizures. It’s unlikely to reach all those goals, but it’s a step in the right direction, hopefully,” said Buckmaster.
He noted that Cronutt is only a single case and not proof that the procedure will work more generally. It’s possible the sea lion would have improved on his own without treatment, and the procedure itself could have influenced the brain. “That’s been the case with other neurological conditions that have attempted to be treated with cell transplantation,” said Buckmaster.
A phase 1/2 clinical trial, sponsored by Neurona Therapeutics, is currently recruiting patients to test transplant of human interneurons created from pluripotent stem cells into both temporal lobes of patients with drug-resistant bilateral mesial temporal lobe epilepsy.
Cronutt’s Course
Although he has improved dramatically, it’s possible that Cronutt continues to experience nonconvulsive seizures, which would not be easy to detect in an animal. “You don’t know if Cronutt’s seizures are controlled or not unless you can look at the electrical activity in Cronutt’s brain, and to my knowledge that has not been done,” said Buckmaster.
Previous studies in rats did repeatedly demonstrate the potential of the treatment approach. Despite the feel-good story of Cronutt, “that’s probably the most compelling evidence in support of that arm of treatment,” said Buckmaster.
Buckmaster and Barbaran had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
In 2022, the Journal of Neurosurgery took the unusual step of publishing a case report of a nonhuman subject, in this case an 8-year-old male sea lion named Cronutt, who had severe epilepsy that in many ways mirrored the common form seen in humans.
A 2020 xenotransplantation of interneuron progenitor cells in the sea lion’s hippocampus may have cured him. “He’s doing fantastic,” said Scott Baraban, PhD, a professor of neurological surgery at the University of California San Francisco Weill Institute for Neurosciences. The procedure offered a confirmation of success stories in mice and could serve as a bridge to human studies, although the science remains murky.
Cronutt’s story began the first time he was found stranded along the California coast in San Luis Obispo County, in November 2017, lethargic and disoriented. Nevertheless, he improved quickly under the care of the Marine Mammal Rescue Center in Sausalito, California, and they released him back into the wild 2 weeks later. Unfortunately, his troubles weren’t over. In the next 2 months, he came to the attention of the rehabilitation center two more times, and the resulting habituation to humans meant that he could not be re-released to the wild.
Things only got worse from there. He experienced a convulsive seizure and loss of consciousness in early 2018 and another seizure a year later. Despite anti-seizure medication, the episodes continued, and eventually, he stopped eating for long periods and lost a quarter of his body weight. It looked like euthanasia was the only alternative.
Neurosurgeons Take Notice
Baraban has devoted his career to animal models of epilepsy, focusing mainly on mice. He developed a method to transplant GABAergic interneurons from pigs, which can pick up the inhibitory role of endogenous neurons. In epileptic mice, mouse-derived progenitor cells mature into inhibitory synapses and dampen seizures, and even reverse behavioral problems.
Baraban was intrigued by the potential of the procedure to help sea lions and potentially bridge the gap between murine and human studies, and he was eager to try the approach after he learned of Cronutt’s case through his colleague Paul Buckmaster at Stanford University, California, who had collaborated with the Marine Mammal Rescue Center to demonstrate that sea lions suffer hippocampal damage that is very similar to human temporal lobe epilepsy.
It turns out that surgery on a sea lion is more complicated than on a mouse. The surgeon needs to conduct an MRI scan to locate the lesion and guide the placement of transplanted cells, and the big animals need to be sedated and intubated. Their caretakers are not enthusiastic about invasive procedures, but Cronutt had already been imaged as part of an earlier study of the effects of exposure to domoic acid on the sea lion brain, giving neurosurgeons at UCSF the information they needed.
In 2020, they transplanted GABAergic interneurons into Cronutt’s hippocampus in a last-ditch effort to save his life. He improved rapidly, and his caregivers eventually tapered off his anti-seizure medications. Today, Cronutt is living seizure-free at the Six Flags Discovery Kingdom in Vallejo, California. He even began participating in shows at Six Flags. “He is able to learn things and do things cognitively that he couldn’t do before,” said Baraban.
The researchers have not followed up with more imaging. “We just haven’t had any reason to bother him. It’s very invasive to get him to an MRI center,” said Baraban.
There haven’t been any more procedures on sea lions, in part because sea lions at rescue centers must be returned to the wild within 6-8 months, and animals with pig-based progenitor cells can’t be released. Any sea lion that would undergo such a procedure would have to live permanently in captivity, potentially for 20-30 years. “It’s a huge undertaking and expense,” said Baraban. There are also logistical challenges with the need to have MRI and other facilities close at hand to where the sea lion is kept.
Human Connections
As a veterinarian interested in human epilepsy, Buckmaster sought to find additional animal models for human temporal lobe epilepsy. He later met a veterinarian who worked with the Marine Mammal Center, who told him about temporal lobe epilepsy in sea lions.
Cronutt was just one of hundreds of California sea lions and other mammals rendered helpless by epileptic seizures induced by exposure to domoic acid, a chemical released by toxic algal blooms. Sea otters, whales, and even birds can likewise be affected. Humans, too, though it is rare: A domoic acid poisoning event in mussels from Prince Edward Island led to convulsions and an eventual diagnosis of temporal lobe epilepsy in an 84-year-old Canadian man, and three others died.
Most of Buckmaster’s work had been done in rats, but seizures are different than what are seen in humans. The damage isn’t unilateral, as in human epilepsy. The damage is unilateral in sea lions, and they suffer damage to similar neurons to those injured in humans. “In rats, you don’t see much loss of granule cells, which are the main neuron in the dentate gyrus, a region that’s really affected in temporal lobe epilepsy. In people, there’s a lot of variation, but [on average] you lose about half your granule cells, and that’s similar to sea lions. In rats, they just don’t lose them,” said Buckmaster.
That makes sea lions a useful model, but they hardly crack the case of human epilepsy. “To be humble about it, we don’t really know what’s causing the seizures. We have an idea of what region of the brain the seizures are coming from, but in terms of the cellular mechanisms, it’s still not clear,” said Buckmaster.
He also noted that, unlike sea lions, the vast majority of patients with human epilepsy have no exposure to domoic acid. That said, the case of sea lions and other marine animals makes researchers wonder. “This is speculative, but a lot of people with temporal epilepsy will have a history of some kind of precipitating event. Typically, it’s earlier in life, and a common one is prolonged febrile seizures. That could be a link to the domoic acid exposure in sea lions because the domoic acid exposure in sea lions frequently causes prolonged seizures. If the seizures are severe enough and long enough, they cause permanent brain damage, and that can cause temporal lobe epilepsy. That’s what we strongly believe is causing the temporal lobe epilepsy in sea lions. Prolonged, severe seizures can be caused by many things other than domoic acid, and that could be what causes it in many cases of human temporal lobe epilepsy,” said Buckmaster.
The approach taken with Cronutt makes sense to Buckmaster, who was not directly involved in the procedure. “The ideal treatment would be something that you would administer once, and it would cause cessation of seizures without any side effects. The transplantation of inhibitory cells is a treatment that would last and hopefully wouldn’t have any side effects and hopefully would provide complete control of seizures. It’s unlikely to reach all those goals, but it’s a step in the right direction, hopefully,” said Buckmaster.
He noted that Cronutt is only a single case and not proof that the procedure will work more generally. It’s possible the sea lion would have improved on his own without treatment, and the procedure itself could have influenced the brain. “That’s been the case with other neurological conditions that have attempted to be treated with cell transplantation,” said Buckmaster.
A phase 1/2 clinical trial, sponsored by Neurona Therapeutics, is currently recruiting patients to test transplant of human interneurons created from pluripotent stem cells into both temporal lobes of patients with drug-resistant bilateral mesial temporal lobe epilepsy.
Cronutt’s Course
Although he has improved dramatically, it’s possible that Cronutt continues to experience nonconvulsive seizures, which would not be easy to detect in an animal. “You don’t know if Cronutt’s seizures are controlled or not unless you can look at the electrical activity in Cronutt’s brain, and to my knowledge that has not been done,” said Buckmaster.
Previous studies in rats did repeatedly demonstrate the potential of the treatment approach. Despite the feel-good story of Cronutt, “that’s probably the most compelling evidence in support of that arm of treatment,” said Buckmaster.
Buckmaster and Barbaran had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
In 2022, the Journal of Neurosurgery took the unusual step of publishing a case report of a nonhuman subject, in this case an 8-year-old male sea lion named Cronutt, who had severe epilepsy that in many ways mirrored the common form seen in humans.
A 2020 xenotransplantation of interneuron progenitor cells in the sea lion’s hippocampus may have cured him. “He’s doing fantastic,” said Scott Baraban, PhD, a professor of neurological surgery at the University of California San Francisco Weill Institute for Neurosciences. The procedure offered a confirmation of success stories in mice and could serve as a bridge to human studies, although the science remains murky.
Cronutt’s story began the first time he was found stranded along the California coast in San Luis Obispo County, in November 2017, lethargic and disoriented. Nevertheless, he improved quickly under the care of the Marine Mammal Rescue Center in Sausalito, California, and they released him back into the wild 2 weeks later. Unfortunately, his troubles weren’t over. In the next 2 months, he came to the attention of the rehabilitation center two more times, and the resulting habituation to humans meant that he could not be re-released to the wild.
Things only got worse from there. He experienced a convulsive seizure and loss of consciousness in early 2018 and another seizure a year later. Despite anti-seizure medication, the episodes continued, and eventually, he stopped eating for long periods and lost a quarter of his body weight. It looked like euthanasia was the only alternative.
Neurosurgeons Take Notice
Baraban has devoted his career to animal models of epilepsy, focusing mainly on mice. He developed a method to transplant GABAergic interneurons from pigs, which can pick up the inhibitory role of endogenous neurons. In epileptic mice, mouse-derived progenitor cells mature into inhibitory synapses and dampen seizures, and even reverse behavioral problems.
Baraban was intrigued by the potential of the procedure to help sea lions and potentially bridge the gap between murine and human studies, and he was eager to try the approach after he learned of Cronutt’s case through his colleague Paul Buckmaster at Stanford University, California, who had collaborated with the Marine Mammal Rescue Center to demonstrate that sea lions suffer hippocampal damage that is very similar to human temporal lobe epilepsy.
It turns out that surgery on a sea lion is more complicated than on a mouse. The surgeon needs to conduct an MRI scan to locate the lesion and guide the placement of transplanted cells, and the big animals need to be sedated and intubated. Their caretakers are not enthusiastic about invasive procedures, but Cronutt had already been imaged as part of an earlier study of the effects of exposure to domoic acid on the sea lion brain, giving neurosurgeons at UCSF the information they needed.
In 2020, they transplanted GABAergic interneurons into Cronutt’s hippocampus in a last-ditch effort to save his life. He improved rapidly, and his caregivers eventually tapered off his anti-seizure medications. Today, Cronutt is living seizure-free at the Six Flags Discovery Kingdom in Vallejo, California. He even began participating in shows at Six Flags. “He is able to learn things and do things cognitively that he couldn’t do before,” said Baraban.
The researchers have not followed up with more imaging. “We just haven’t had any reason to bother him. It’s very invasive to get him to an MRI center,” said Baraban.
There haven’t been any more procedures on sea lions, in part because sea lions at rescue centers must be returned to the wild within 6-8 months, and animals with pig-based progenitor cells can’t be released. Any sea lion that would undergo such a procedure would have to live permanently in captivity, potentially for 20-30 years. “It’s a huge undertaking and expense,” said Baraban. There are also logistical challenges with the need to have MRI and other facilities close at hand to where the sea lion is kept.
Human Connections
As a veterinarian interested in human epilepsy, Buckmaster sought to find additional animal models for human temporal lobe epilepsy. He later met a veterinarian who worked with the Marine Mammal Center, who told him about temporal lobe epilepsy in sea lions.
Cronutt was just one of hundreds of California sea lions and other mammals rendered helpless by epileptic seizures induced by exposure to domoic acid, a chemical released by toxic algal blooms. Sea otters, whales, and even birds can likewise be affected. Humans, too, though it is rare: A domoic acid poisoning event in mussels from Prince Edward Island led to convulsions and an eventual diagnosis of temporal lobe epilepsy in an 84-year-old Canadian man, and three others died.
Most of Buckmaster’s work had been done in rats, but seizures are different than what are seen in humans. The damage isn’t unilateral, as in human epilepsy. The damage is unilateral in sea lions, and they suffer damage to similar neurons to those injured in humans. “In rats, you don’t see much loss of granule cells, which are the main neuron in the dentate gyrus, a region that’s really affected in temporal lobe epilepsy. In people, there’s a lot of variation, but [on average] you lose about half your granule cells, and that’s similar to sea lions. In rats, they just don’t lose them,” said Buckmaster.
That makes sea lions a useful model, but they hardly crack the case of human epilepsy. “To be humble about it, we don’t really know what’s causing the seizures. We have an idea of what region of the brain the seizures are coming from, but in terms of the cellular mechanisms, it’s still not clear,” said Buckmaster.
He also noted that, unlike sea lions, the vast majority of patients with human epilepsy have no exposure to domoic acid. That said, the case of sea lions and other marine animals makes researchers wonder. “This is speculative, but a lot of people with temporal epilepsy will have a history of some kind of precipitating event. Typically, it’s earlier in life, and a common one is prolonged febrile seizures. That could be a link to the domoic acid exposure in sea lions because the domoic acid exposure in sea lions frequently causes prolonged seizures. If the seizures are severe enough and long enough, they cause permanent brain damage, and that can cause temporal lobe epilepsy. That’s what we strongly believe is causing the temporal lobe epilepsy in sea lions. Prolonged, severe seizures can be caused by many things other than domoic acid, and that could be what causes it in many cases of human temporal lobe epilepsy,” said Buckmaster.
The approach taken with Cronutt makes sense to Buckmaster, who was not directly involved in the procedure. “The ideal treatment would be something that you would administer once, and it would cause cessation of seizures without any side effects. The transplantation of inhibitory cells is a treatment that would last and hopefully wouldn’t have any side effects and hopefully would provide complete control of seizures. It’s unlikely to reach all those goals, but it’s a step in the right direction, hopefully,” said Buckmaster.
He noted that Cronutt is only a single case and not proof that the procedure will work more generally. It’s possible the sea lion would have improved on his own without treatment, and the procedure itself could have influenced the brain. “That’s been the case with other neurological conditions that have attempted to be treated with cell transplantation,” said Buckmaster.
A phase 1/2 clinical trial, sponsored by Neurona Therapeutics, is currently recruiting patients to test transplant of human interneurons created from pluripotent stem cells into both temporal lobes of patients with drug-resistant bilateral mesial temporal lobe epilepsy.
Cronutt’s Course
Although he has improved dramatically, it’s possible that Cronutt continues to experience nonconvulsive seizures, which would not be easy to detect in an animal. “You don’t know if Cronutt’s seizures are controlled or not unless you can look at the electrical activity in Cronutt’s brain, and to my knowledge that has not been done,” said Buckmaster.
Previous studies in rats did repeatedly demonstrate the potential of the treatment approach. Despite the feel-good story of Cronutt, “that’s probably the most compelling evidence in support of that arm of treatment,” said Buckmaster.
Buckmaster and Barbaran had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
COVID-19 Takes a Greater Toll on Kidneys Than Pneumonia
TOPLINE:
This decline in kidney function, measured by the estimated glomerular filtration rate (eGFR), is particularly steep among individuals who require hospitalization for COVID-19.
METHODOLOGY:
- SARS-CoV-2, the virus that causes COVID-19, has been associated with acute kidney injury, but its potential impact on long-term kidney function remains unclear.
- Researchers investigated the decline in kidney function after COVID-19 vs pneumonia by including all hospitalized and nonhospitalized adults from the Stockholm Creatinine Measurements Project who had at least one eGFR measurement in the 2 years before a positive COVID-19 test result or pneumonia diagnosis.
- Overall, 134,565 individuals (median age, 51 years; 55.6% women) who had their first SARS-CoV-2 infection between February 2020 and January 2022 were included, of whom 13.3% required hospitalization within 28 days of their first positive COVID-19 test result.
- They were compared with 35,987 patients (median age, 71 years; 53.8% women) who were diagnosed with pneumonia between February 2018 and January 2020; 46.5% of them required hospitalization.
- The primary outcome measure focused on the mean annual change in eGFR slopes before and after each infection; the secondary outcome assessed was the annual change in postinfection eGFR slopes between COVID-19 and pneumonia cases.
TAKEAWAY:
- Before COVID-19, eGFR changes were minimal, but after the infection, the average decline increased to 4.1 (95% CI, 3.8-4.4) mL/min/1.73 m2; however, in the pneumonia cohort, a decline in eGFR was noted both before and after the infection.
- After COVID-19, the mean annual decline in eGFR was 3.4% (95% CI, 3.2%-3.5%), increasing to 5.4% (95% CI, 5.2%-5.6%) for those who were hospitalized.
- In contrast, the pneumonia group experienced an average annual decline of 2.3% (95% CI, 2.1%-2.5%) after the infection, which remained unchanged when analyzing only patients who were hospitalized.
- The risk for a 25% reduction in eGFR was higher in patients with COVID-19 than in those with pneumonia (hazard ratio [HR], 1.19; 95% CI, 1.07-1.34), with the risk being even higher among those who required hospitalization (HR, 1.42; 95% CI, 1.22-1.64).
IN PRACTICE:
“These findings help inform decisions regarding the need to monitor kidney function in survivors of COVID-19 and could have implications for policymakers regarding future healthcare planning and kidney service provision,” the authors wrote.
SOURCE:
This study was led by Viyaasan Mahalingasivam, MPhil, London School of Hygiene & Tropical Medicine, London, England. It was published online in JAMA Network Open.
LIMITATIONS:
This study lacked information on important confounders such as ethnicity and body mass index. The follow-up period was not long enough to fully evaluate the long-term association of COVID-19 with kidney function. Some individuals may have been misclassified as nonhospitalized if their first infection was mild and a subsequent infection required hospitalization.
DISCLOSURES:
This study was supported by grants from the National Institute for Health and Care Research, Njurfonden, Stig and Gunborg Westman Foundation, and the Swedish Research Council. One author reported receiving a Career Development Award from the National Institute for Health and Care Research, and another author reported receiving grants from Njurfonden, Stig and Gunborg Westman Foundation, Swedish Research Council, Swedish Heart Lung Foundation, and Region Stockholm during the conduct of the study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
This decline in kidney function, measured by the estimated glomerular filtration rate (eGFR), is particularly steep among individuals who require hospitalization for COVID-19.
METHODOLOGY:
- SARS-CoV-2, the virus that causes COVID-19, has been associated with acute kidney injury, but its potential impact on long-term kidney function remains unclear.
- Researchers investigated the decline in kidney function after COVID-19 vs pneumonia by including all hospitalized and nonhospitalized adults from the Stockholm Creatinine Measurements Project who had at least one eGFR measurement in the 2 years before a positive COVID-19 test result or pneumonia diagnosis.
- Overall, 134,565 individuals (median age, 51 years; 55.6% women) who had their first SARS-CoV-2 infection between February 2020 and January 2022 were included, of whom 13.3% required hospitalization within 28 days of their first positive COVID-19 test result.
- They were compared with 35,987 patients (median age, 71 years; 53.8% women) who were diagnosed with pneumonia between February 2018 and January 2020; 46.5% of them required hospitalization.
- The primary outcome measure focused on the mean annual change in eGFR slopes before and after each infection; the secondary outcome assessed was the annual change in postinfection eGFR slopes between COVID-19 and pneumonia cases.
TAKEAWAY:
- Before COVID-19, eGFR changes were minimal, but after the infection, the average decline increased to 4.1 (95% CI, 3.8-4.4) mL/min/1.73 m2; however, in the pneumonia cohort, a decline in eGFR was noted both before and after the infection.
- After COVID-19, the mean annual decline in eGFR was 3.4% (95% CI, 3.2%-3.5%), increasing to 5.4% (95% CI, 5.2%-5.6%) for those who were hospitalized.
- In contrast, the pneumonia group experienced an average annual decline of 2.3% (95% CI, 2.1%-2.5%) after the infection, which remained unchanged when analyzing only patients who were hospitalized.
- The risk for a 25% reduction in eGFR was higher in patients with COVID-19 than in those with pneumonia (hazard ratio [HR], 1.19; 95% CI, 1.07-1.34), with the risk being even higher among those who required hospitalization (HR, 1.42; 95% CI, 1.22-1.64).
IN PRACTICE:
“These findings help inform decisions regarding the need to monitor kidney function in survivors of COVID-19 and could have implications for policymakers regarding future healthcare planning and kidney service provision,” the authors wrote.
SOURCE:
This study was led by Viyaasan Mahalingasivam, MPhil, London School of Hygiene & Tropical Medicine, London, England. It was published online in JAMA Network Open.
LIMITATIONS:
This study lacked information on important confounders such as ethnicity and body mass index. The follow-up period was not long enough to fully evaluate the long-term association of COVID-19 with kidney function. Some individuals may have been misclassified as nonhospitalized if their first infection was mild and a subsequent infection required hospitalization.
DISCLOSURES:
This study was supported by grants from the National Institute for Health and Care Research, Njurfonden, Stig and Gunborg Westman Foundation, and the Swedish Research Council. One author reported receiving a Career Development Award from the National Institute for Health and Care Research, and another author reported receiving grants from Njurfonden, Stig and Gunborg Westman Foundation, Swedish Research Council, Swedish Heart Lung Foundation, and Region Stockholm during the conduct of the study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
This decline in kidney function, measured by the estimated glomerular filtration rate (eGFR), is particularly steep among individuals who require hospitalization for COVID-19.
METHODOLOGY:
- SARS-CoV-2, the virus that causes COVID-19, has been associated with acute kidney injury, but its potential impact on long-term kidney function remains unclear.
- Researchers investigated the decline in kidney function after COVID-19 vs pneumonia by including all hospitalized and nonhospitalized adults from the Stockholm Creatinine Measurements Project who had at least one eGFR measurement in the 2 years before a positive COVID-19 test result or pneumonia diagnosis.
- Overall, 134,565 individuals (median age, 51 years; 55.6% women) who had their first SARS-CoV-2 infection between February 2020 and January 2022 were included, of whom 13.3% required hospitalization within 28 days of their first positive COVID-19 test result.
- They were compared with 35,987 patients (median age, 71 years; 53.8% women) who were diagnosed with pneumonia between February 2018 and January 2020; 46.5% of them required hospitalization.
- The primary outcome measure focused on the mean annual change in eGFR slopes before and after each infection; the secondary outcome assessed was the annual change in postinfection eGFR slopes between COVID-19 and pneumonia cases.
TAKEAWAY:
- Before COVID-19, eGFR changes were minimal, but after the infection, the average decline increased to 4.1 (95% CI, 3.8-4.4) mL/min/1.73 m2; however, in the pneumonia cohort, a decline in eGFR was noted both before and after the infection.
- After COVID-19, the mean annual decline in eGFR was 3.4% (95% CI, 3.2%-3.5%), increasing to 5.4% (95% CI, 5.2%-5.6%) for those who were hospitalized.
- In contrast, the pneumonia group experienced an average annual decline of 2.3% (95% CI, 2.1%-2.5%) after the infection, which remained unchanged when analyzing only patients who were hospitalized.
- The risk for a 25% reduction in eGFR was higher in patients with COVID-19 than in those with pneumonia (hazard ratio [HR], 1.19; 95% CI, 1.07-1.34), with the risk being even higher among those who required hospitalization (HR, 1.42; 95% CI, 1.22-1.64).
IN PRACTICE:
“These findings help inform decisions regarding the need to monitor kidney function in survivors of COVID-19 and could have implications for policymakers regarding future healthcare planning and kidney service provision,” the authors wrote.
SOURCE:
This study was led by Viyaasan Mahalingasivam, MPhil, London School of Hygiene & Tropical Medicine, London, England. It was published online in JAMA Network Open.
LIMITATIONS:
This study lacked information on important confounders such as ethnicity and body mass index. The follow-up period was not long enough to fully evaluate the long-term association of COVID-19 with kidney function. Some individuals may have been misclassified as nonhospitalized if their first infection was mild and a subsequent infection required hospitalization.
DISCLOSURES:
This study was supported by grants from the National Institute for Health and Care Research, Njurfonden, Stig and Gunborg Westman Foundation, and the Swedish Research Council. One author reported receiving a Career Development Award from the National Institute for Health and Care Research, and another author reported receiving grants from Njurfonden, Stig and Gunborg Westman Foundation, Swedish Research Council, Swedish Heart Lung Foundation, and Region Stockholm during the conduct of the study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Younger People and Long COVID: Underreported, Undertreated
John Bolecek, 41, of Richmond, Virginia, was diagnosed with long COVID in May of 2022. While his acute infection was mild, once everyone else in his family had recovered, the heavy fatigue he experienced from the start has never lifted.
“When I wake up in the morning, I feel like I haven’t gone to sleep at all,” Bolecek said. “It’s this super fatigue that’s just never gone away.”
The urban planner who once rode his bike to work daily and spent weekends cycling had to quit working and now can barely get through a light walk before long COVID symptoms of post-exertional malaise, an intense fatigue after previously tolerated physical or mental activity, set in. His unrefreshing sleep, fatigue, and dysautonomia — a disruption of the autonomic nervous system that causes dizziness, heart rate changes, and nausea — have made it nearly impossible to share household duties with his wife. She has to do most of the cooking, cleaning, and tending to their two sons, ages 6 and 8 years.
It’s an increasingly familiar story for those hit with long COVID in their prime, a period of life when young and middle-aged adults are the most productive and the busiest, often in the thick of parenting while also taking care of their aging parents. And it’s a group that is among the hardest hit by long COVID both because of the sheer number of patients with the condition and the mental and financial strain that it’s putting on this age group. According to the Centers for Disease Control and Prevention (CDC), 6.9% of adults aged 18-34 years and 8.9% of adults aged 35-49 years have the disorder compared with 4.1% of older adults aged > 65 years who are the least likely to have long COVID.
In a study published recently in Scientific Reports, researchers found that in a population of California residents with long COVID, older individuals (who were sicker to start) had more severe symptoms associated with the condition. But researchers also found that younger people (aged 18-49 years) were more likely to experience symptoms that reduced their productivity and quality of life. They suggested this is both because they have more to do in a given day and because they have a longer life ahead of them living with a chronic condition.
“Much of California’s population falls within the 18-49 age group, [so] we would expect to see the highest overall burden coming from these individuals,” said lead study author Sophie Zhu, a researcher in the Division of Communicable Disease Control at the California Department of Public Health.
The Impact on Work and Life Productivity
Adults and especially those in middle age tend to have a lot of competing stressors during this period of life, said Nisha Viswanathan, MD, director of the UCLA Health Long COVID program. “Patients may need to decrease some of the pressures of life for their health and that can be impossible to do because they have so many other people who are depending on them.”
It’s a different set of circumstances compared with older individuals who may have more severe symptoms because they have underlying conditions. But older Americans are also more likely to be retired and don’t have children who are financially dependent on them. Previous research has shown the burden that long COVID is having on the workforce. A study published in the August 2023 edition of The Lancet Regional Health found that 5.8% of participating patients with long COVID reported occupational changes like moving to part time or remote work, including 1.6% who had completely dropped out of the workforce.
Middle age is also a time of life when patients may not have time to seek the care they need. The chronic nature of long COVID means that treatment can be time consuming and expensive, all of which drains resources from patients who are often supporting spouses, children, and sometimes older parents. A study published in Disability and Health Journal found that patients with long COVID have significantly higher rates of housing instability and financial concerns, such as worries about paying rent or a mortgage, than those without the condition.
The Financial Strain of Long COVID
For those who can’t work, the process of applying for long-term disability can also be complicated. That’s especially true for people whose illness keeps them from doing even basic tasks like filling out paperwork and dealing with disability insurance claims. It requires those applying as a result of their long COVID symptoms to show all records connected to long COVID as well as a medical history, the beginning of their symptoms, and their current treatments.
Even then, many patients complain of having their claims rejected, which can be financially disastrous to families already struggling to get by. Still, experts contend that it’s important to understand that as of July 2021, long COVID is considered a disability under the Americans with Disabilities Act (ADA).
“Long COVID is recognized as a disability under Section 504 of the ADA, and yet day after day, we see violations of the ADA for people with long COVID not getting the accommodations that they need in order to work,” said David Putrino, PhD, the Nash Family director of the Cohen Center for Recovery from Complex Chronic Illness at Mount Sinai in New York City and a renowned expert in long COVID.
He added that short- and long-term disability claims are sometimes denied because of a lack of diagnostic testing to prove a patient has the condition. “This is nonsensical and absurd because the CDC does not require a blood test for the diagnosis of long COVID. It’s at your physician’s discretion,” Putrino said.
Viswanathan agreed. She said that for many of her patients, getting long-term disability has been particularly challenging because there’s no blood test for long COVID to prove patients have the condition. “As a result, for many of our patients, especially when they’re young, they may have to return to work in one form or another,” Viswanathan said.
The Impact of Long COVID on Quality of Life
What’s worse, the full impact is yet unknown because this is likely an underestimated cohort as many of these patients had mild cases of acute COVID-19 and fewer underlying conditions. For others, their long COVID is undiagnosed.
“Much of the impact on productivity and quality of life for this group remains hidden,” said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St. Louis Health Care System in Missouri.
Unfortunately, the impact on Bolecek’s life isn’t so hidden. He can’t work, which has been a financial stressor on the family. He spends much of the day in bed so that he can help with a few things when his wife gets home from work. He can’t cycle anymore and, as a result, has lost many of the friends associated with his favorite hobby.
But he remains hopeful, and more than anything else, he’s thankful for his family. His wife and kids have given him the strength to push on even when the days are hard. “I just don’t know where I’d be without them,” Bolecek said.
A version of this article first appeared on Medscape.com.
John Bolecek, 41, of Richmond, Virginia, was diagnosed with long COVID in May of 2022. While his acute infection was mild, once everyone else in his family had recovered, the heavy fatigue he experienced from the start has never lifted.
“When I wake up in the morning, I feel like I haven’t gone to sleep at all,” Bolecek said. “It’s this super fatigue that’s just never gone away.”
The urban planner who once rode his bike to work daily and spent weekends cycling had to quit working and now can barely get through a light walk before long COVID symptoms of post-exertional malaise, an intense fatigue after previously tolerated physical or mental activity, set in. His unrefreshing sleep, fatigue, and dysautonomia — a disruption of the autonomic nervous system that causes dizziness, heart rate changes, and nausea — have made it nearly impossible to share household duties with his wife. She has to do most of the cooking, cleaning, and tending to their two sons, ages 6 and 8 years.
It’s an increasingly familiar story for those hit with long COVID in their prime, a period of life when young and middle-aged adults are the most productive and the busiest, often in the thick of parenting while also taking care of their aging parents. And it’s a group that is among the hardest hit by long COVID both because of the sheer number of patients with the condition and the mental and financial strain that it’s putting on this age group. According to the Centers for Disease Control and Prevention (CDC), 6.9% of adults aged 18-34 years and 8.9% of adults aged 35-49 years have the disorder compared with 4.1% of older adults aged > 65 years who are the least likely to have long COVID.
In a study published recently in Scientific Reports, researchers found that in a population of California residents with long COVID, older individuals (who were sicker to start) had more severe symptoms associated with the condition. But researchers also found that younger people (aged 18-49 years) were more likely to experience symptoms that reduced their productivity and quality of life. They suggested this is both because they have more to do in a given day and because they have a longer life ahead of them living with a chronic condition.
“Much of California’s population falls within the 18-49 age group, [so] we would expect to see the highest overall burden coming from these individuals,” said lead study author Sophie Zhu, a researcher in the Division of Communicable Disease Control at the California Department of Public Health.
The Impact on Work and Life Productivity
Adults and especially those in middle age tend to have a lot of competing stressors during this period of life, said Nisha Viswanathan, MD, director of the UCLA Health Long COVID program. “Patients may need to decrease some of the pressures of life for their health and that can be impossible to do because they have so many other people who are depending on them.”
It’s a different set of circumstances compared with older individuals who may have more severe symptoms because they have underlying conditions. But older Americans are also more likely to be retired and don’t have children who are financially dependent on them. Previous research has shown the burden that long COVID is having on the workforce. A study published in the August 2023 edition of The Lancet Regional Health found that 5.8% of participating patients with long COVID reported occupational changes like moving to part time or remote work, including 1.6% who had completely dropped out of the workforce.
Middle age is also a time of life when patients may not have time to seek the care they need. The chronic nature of long COVID means that treatment can be time consuming and expensive, all of which drains resources from patients who are often supporting spouses, children, and sometimes older parents. A study published in Disability and Health Journal found that patients with long COVID have significantly higher rates of housing instability and financial concerns, such as worries about paying rent or a mortgage, than those without the condition.
The Financial Strain of Long COVID
For those who can’t work, the process of applying for long-term disability can also be complicated. That’s especially true for people whose illness keeps them from doing even basic tasks like filling out paperwork and dealing with disability insurance claims. It requires those applying as a result of their long COVID symptoms to show all records connected to long COVID as well as a medical history, the beginning of their symptoms, and their current treatments.
Even then, many patients complain of having their claims rejected, which can be financially disastrous to families already struggling to get by. Still, experts contend that it’s important to understand that as of July 2021, long COVID is considered a disability under the Americans with Disabilities Act (ADA).
“Long COVID is recognized as a disability under Section 504 of the ADA, and yet day after day, we see violations of the ADA for people with long COVID not getting the accommodations that they need in order to work,” said David Putrino, PhD, the Nash Family director of the Cohen Center for Recovery from Complex Chronic Illness at Mount Sinai in New York City and a renowned expert in long COVID.
He added that short- and long-term disability claims are sometimes denied because of a lack of diagnostic testing to prove a patient has the condition. “This is nonsensical and absurd because the CDC does not require a blood test for the diagnosis of long COVID. It’s at your physician’s discretion,” Putrino said.
Viswanathan agreed. She said that for many of her patients, getting long-term disability has been particularly challenging because there’s no blood test for long COVID to prove patients have the condition. “As a result, for many of our patients, especially when they’re young, they may have to return to work in one form or another,” Viswanathan said.
The Impact of Long COVID on Quality of Life
What’s worse, the full impact is yet unknown because this is likely an underestimated cohort as many of these patients had mild cases of acute COVID-19 and fewer underlying conditions. For others, their long COVID is undiagnosed.
“Much of the impact on productivity and quality of life for this group remains hidden,” said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St. Louis Health Care System in Missouri.
Unfortunately, the impact on Bolecek’s life isn’t so hidden. He can’t work, which has been a financial stressor on the family. He spends much of the day in bed so that he can help with a few things when his wife gets home from work. He can’t cycle anymore and, as a result, has lost many of the friends associated with his favorite hobby.
But he remains hopeful, and more than anything else, he’s thankful for his family. His wife and kids have given him the strength to push on even when the days are hard. “I just don’t know where I’d be without them,” Bolecek said.
A version of this article first appeared on Medscape.com.
John Bolecek, 41, of Richmond, Virginia, was diagnosed with long COVID in May of 2022. While his acute infection was mild, once everyone else in his family had recovered, the heavy fatigue he experienced from the start has never lifted.
“When I wake up in the morning, I feel like I haven’t gone to sleep at all,” Bolecek said. “It’s this super fatigue that’s just never gone away.”
The urban planner who once rode his bike to work daily and spent weekends cycling had to quit working and now can barely get through a light walk before long COVID symptoms of post-exertional malaise, an intense fatigue after previously tolerated physical or mental activity, set in. His unrefreshing sleep, fatigue, and dysautonomia — a disruption of the autonomic nervous system that causes dizziness, heart rate changes, and nausea — have made it nearly impossible to share household duties with his wife. She has to do most of the cooking, cleaning, and tending to their two sons, ages 6 and 8 years.
It’s an increasingly familiar story for those hit with long COVID in their prime, a period of life when young and middle-aged adults are the most productive and the busiest, often in the thick of parenting while also taking care of their aging parents. And it’s a group that is among the hardest hit by long COVID both because of the sheer number of patients with the condition and the mental and financial strain that it’s putting on this age group. According to the Centers for Disease Control and Prevention (CDC), 6.9% of adults aged 18-34 years and 8.9% of adults aged 35-49 years have the disorder compared with 4.1% of older adults aged > 65 years who are the least likely to have long COVID.
In a study published recently in Scientific Reports, researchers found that in a population of California residents with long COVID, older individuals (who were sicker to start) had more severe symptoms associated with the condition. But researchers also found that younger people (aged 18-49 years) were more likely to experience symptoms that reduced their productivity and quality of life. They suggested this is both because they have more to do in a given day and because they have a longer life ahead of them living with a chronic condition.
“Much of California’s population falls within the 18-49 age group, [so] we would expect to see the highest overall burden coming from these individuals,” said lead study author Sophie Zhu, a researcher in the Division of Communicable Disease Control at the California Department of Public Health.
The Impact on Work and Life Productivity
Adults and especially those in middle age tend to have a lot of competing stressors during this period of life, said Nisha Viswanathan, MD, director of the UCLA Health Long COVID program. “Patients may need to decrease some of the pressures of life for their health and that can be impossible to do because they have so many other people who are depending on them.”
It’s a different set of circumstances compared with older individuals who may have more severe symptoms because they have underlying conditions. But older Americans are also more likely to be retired and don’t have children who are financially dependent on them. Previous research has shown the burden that long COVID is having on the workforce. A study published in the August 2023 edition of The Lancet Regional Health found that 5.8% of participating patients with long COVID reported occupational changes like moving to part time or remote work, including 1.6% who had completely dropped out of the workforce.
Middle age is also a time of life when patients may not have time to seek the care they need. The chronic nature of long COVID means that treatment can be time consuming and expensive, all of which drains resources from patients who are often supporting spouses, children, and sometimes older parents. A study published in Disability and Health Journal found that patients with long COVID have significantly higher rates of housing instability and financial concerns, such as worries about paying rent or a mortgage, than those without the condition.
The Financial Strain of Long COVID
For those who can’t work, the process of applying for long-term disability can also be complicated. That’s especially true for people whose illness keeps them from doing even basic tasks like filling out paperwork and dealing with disability insurance claims. It requires those applying as a result of their long COVID symptoms to show all records connected to long COVID as well as a medical history, the beginning of their symptoms, and their current treatments.
Even then, many patients complain of having their claims rejected, which can be financially disastrous to families already struggling to get by. Still, experts contend that it’s important to understand that as of July 2021, long COVID is considered a disability under the Americans with Disabilities Act (ADA).
“Long COVID is recognized as a disability under Section 504 of the ADA, and yet day after day, we see violations of the ADA for people with long COVID not getting the accommodations that they need in order to work,” said David Putrino, PhD, the Nash Family director of the Cohen Center for Recovery from Complex Chronic Illness at Mount Sinai in New York City and a renowned expert in long COVID.
He added that short- and long-term disability claims are sometimes denied because of a lack of diagnostic testing to prove a patient has the condition. “This is nonsensical and absurd because the CDC does not require a blood test for the diagnosis of long COVID. It’s at your physician’s discretion,” Putrino said.
Viswanathan agreed. She said that for many of her patients, getting long-term disability has been particularly challenging because there’s no blood test for long COVID to prove patients have the condition. “As a result, for many of our patients, especially when they’re young, they may have to return to work in one form or another,” Viswanathan said.
The Impact of Long COVID on Quality of Life
What’s worse, the full impact is yet unknown because this is likely an underestimated cohort as many of these patients had mild cases of acute COVID-19 and fewer underlying conditions. For others, their long COVID is undiagnosed.
“Much of the impact on productivity and quality of life for this group remains hidden,” said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St. Louis Health Care System in Missouri.
Unfortunately, the impact on Bolecek’s life isn’t so hidden. He can’t work, which has been a financial stressor on the family. He spends much of the day in bed so that he can help with a few things when his wife gets home from work. He can’t cycle anymore and, as a result, has lost many of the friends associated with his favorite hobby.
But he remains hopeful, and more than anything else, he’s thankful for his family. His wife and kids have given him the strength to push on even when the days are hard. “I just don’t know where I’d be without them,” Bolecek said.
A version of this article first appeared on Medscape.com.
Early Oseltamivir Benefits Hospitalized Influenza Patients
TOPLINE:
Early treatment with oseltamivir on the same day as hospital admission was associated with fewer severe clinical outcomes, such as worsening pulmonary disease, need for invasive ventilation, organ failure, and in-hospital death in adults hospitalized with influenza.
METHODOLOGY:
- The 2018 guidelines from the Infectious Disease Society of America recommend prompt administration of oseltamivir to hospitalized patients with suspected or confirmed influenza, regardless of the time of symptom onset; however, variations in treatment practices and circulating virus strains may affect the effectiveness of this practice guideline.
- Researchers conducted a multicenter observational study across 24 hospitals in the United States during the 2022-2023 flu season to assess the benefits of initiating oseltamivir treatment on the same day as hospital admission for adults with acute influenza, compared with late or no treatment.
- They included 840 adults (age, ≥ 18 years) with laboratory-confirmed influenza, of which 415 patients initiated oseltamivir on the same day as hospital admission (early treatment).
- Among the 425 patients in the late/no treatment group, most (78%) received oseltamivir 1 day after admission, while 124 did not receive oseltamivir at all.
- The primary outcome was the peak pulmonary disease severity level that patients experienced during hospitalization, and secondary outcomes included hospital length of stay, ICU admission, initiation of extrapulmonary organ support using vasopressors or kidney replacement therapy, and in-hospital death.
TAKEAWAY:
- Patients in the early treatment group were less likely to experience progression and severe progression of pulmonary disease after the day of hospital admission, compared with those in the late or no treatment group (P < .001 and P = .027, respectively).
- Patients who received early oseltamivir treatment had 40% lower peak pulmonary disease severity than those who received late or no treatment (proportional adjusted odds ratio [paOR], 0.60; 95% CI, 0.49-0.72).
- They also showed lower odds of ICU admission (aOR, 0.25; 95% CI, 0.13-0.49) and use of acute kidney replacement therapy or vasopressors (aOR, 0.40; 95% CI, 0.22-0.67).
- Those in the early treatment group also had a shorter hospital length of stay (median, 4 days vs 4 days) and faced a 64% lower risk for in-hospital mortality (aOR, 0.36; 95% CI, 0.19-0.69) compared with those in the late or no treatment group.
IN PRACTICE:
“These findings support current recommendations, such as the IDSA [Infectious Disease Society of America] Influenza Clinical Practice Guidelines and CDC [Centers for Disease Control and Prevention] guidance, to initiate oseltamivir treatment as soon as possible for adult patients hospitalized with influenza,” the authors wrote.
SOURCE:
The study was led by Nathaniel M. Lewis, PhD, Influenza Division, CDC, Atlanta, Georgia, and was published online in Clinical Infectious Diseases.
LIMITATIONS:
This study may not be generalizable to seasons when influenza A(H1N1)pdm09 or B viruses are predominant as it was conducted during an influenza A(H3N2) virus–predominant season. The study lacked sufficient power to examine various oseltamivir treatment initiation timepoints or identify a potential maximum time-to-treatment threshold for effectiveness. Moreover, variables such as outpatient antiviral treatment before hospital admission and other treatments using macrolides, statins, corticosteroids, or immunomodulators before or during hospitalization were not collected, which may have influenced the study findings.
DISCLOSURES:
The study received funding from the CDC and the National Center for Immunization and Respiratory Diseases. Some authors reported receiving research support, consulting fees, funding, grants, or fees for participation in an advisory board and having other ties with certain institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Early treatment with oseltamivir on the same day as hospital admission was associated with fewer severe clinical outcomes, such as worsening pulmonary disease, need for invasive ventilation, organ failure, and in-hospital death in adults hospitalized with influenza.
METHODOLOGY:
- The 2018 guidelines from the Infectious Disease Society of America recommend prompt administration of oseltamivir to hospitalized patients with suspected or confirmed influenza, regardless of the time of symptom onset; however, variations in treatment practices and circulating virus strains may affect the effectiveness of this practice guideline.
- Researchers conducted a multicenter observational study across 24 hospitals in the United States during the 2022-2023 flu season to assess the benefits of initiating oseltamivir treatment on the same day as hospital admission for adults with acute influenza, compared with late or no treatment.
- They included 840 adults (age, ≥ 18 years) with laboratory-confirmed influenza, of which 415 patients initiated oseltamivir on the same day as hospital admission (early treatment).
- Among the 425 patients in the late/no treatment group, most (78%) received oseltamivir 1 day after admission, while 124 did not receive oseltamivir at all.
- The primary outcome was the peak pulmonary disease severity level that patients experienced during hospitalization, and secondary outcomes included hospital length of stay, ICU admission, initiation of extrapulmonary organ support using vasopressors or kidney replacement therapy, and in-hospital death.
TAKEAWAY:
- Patients in the early treatment group were less likely to experience progression and severe progression of pulmonary disease after the day of hospital admission, compared with those in the late or no treatment group (P < .001 and P = .027, respectively).
- Patients who received early oseltamivir treatment had 40% lower peak pulmonary disease severity than those who received late or no treatment (proportional adjusted odds ratio [paOR], 0.60; 95% CI, 0.49-0.72).
- They also showed lower odds of ICU admission (aOR, 0.25; 95% CI, 0.13-0.49) and use of acute kidney replacement therapy or vasopressors (aOR, 0.40; 95% CI, 0.22-0.67).
- Those in the early treatment group also had a shorter hospital length of stay (median, 4 days vs 4 days) and faced a 64% lower risk for in-hospital mortality (aOR, 0.36; 95% CI, 0.19-0.69) compared with those in the late or no treatment group.
IN PRACTICE:
“These findings support current recommendations, such as the IDSA [Infectious Disease Society of America] Influenza Clinical Practice Guidelines and CDC [Centers for Disease Control and Prevention] guidance, to initiate oseltamivir treatment as soon as possible for adult patients hospitalized with influenza,” the authors wrote.
SOURCE:
The study was led by Nathaniel M. Lewis, PhD, Influenza Division, CDC, Atlanta, Georgia, and was published online in Clinical Infectious Diseases.
LIMITATIONS:
This study may not be generalizable to seasons when influenza A(H1N1)pdm09 or B viruses are predominant as it was conducted during an influenza A(H3N2) virus–predominant season. The study lacked sufficient power to examine various oseltamivir treatment initiation timepoints or identify a potential maximum time-to-treatment threshold for effectiveness. Moreover, variables such as outpatient antiviral treatment before hospital admission and other treatments using macrolides, statins, corticosteroids, or immunomodulators before or during hospitalization were not collected, which may have influenced the study findings.
DISCLOSURES:
The study received funding from the CDC and the National Center for Immunization and Respiratory Diseases. Some authors reported receiving research support, consulting fees, funding, grants, or fees for participation in an advisory board and having other ties with certain institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Early treatment with oseltamivir on the same day as hospital admission was associated with fewer severe clinical outcomes, such as worsening pulmonary disease, need for invasive ventilation, organ failure, and in-hospital death in adults hospitalized with influenza.
METHODOLOGY:
- The 2018 guidelines from the Infectious Disease Society of America recommend prompt administration of oseltamivir to hospitalized patients with suspected or confirmed influenza, regardless of the time of symptom onset; however, variations in treatment practices and circulating virus strains may affect the effectiveness of this practice guideline.
- Researchers conducted a multicenter observational study across 24 hospitals in the United States during the 2022-2023 flu season to assess the benefits of initiating oseltamivir treatment on the same day as hospital admission for adults with acute influenza, compared with late or no treatment.
- They included 840 adults (age, ≥ 18 years) with laboratory-confirmed influenza, of which 415 patients initiated oseltamivir on the same day as hospital admission (early treatment).
- Among the 425 patients in the late/no treatment group, most (78%) received oseltamivir 1 day after admission, while 124 did not receive oseltamivir at all.
- The primary outcome was the peak pulmonary disease severity level that patients experienced during hospitalization, and secondary outcomes included hospital length of stay, ICU admission, initiation of extrapulmonary organ support using vasopressors or kidney replacement therapy, and in-hospital death.
TAKEAWAY:
- Patients in the early treatment group were less likely to experience progression and severe progression of pulmonary disease after the day of hospital admission, compared with those in the late or no treatment group (P < .001 and P = .027, respectively).
- Patients who received early oseltamivir treatment had 40% lower peak pulmonary disease severity than those who received late or no treatment (proportional adjusted odds ratio [paOR], 0.60; 95% CI, 0.49-0.72).
- They also showed lower odds of ICU admission (aOR, 0.25; 95% CI, 0.13-0.49) and use of acute kidney replacement therapy or vasopressors (aOR, 0.40; 95% CI, 0.22-0.67).
- Those in the early treatment group also had a shorter hospital length of stay (median, 4 days vs 4 days) and faced a 64% lower risk for in-hospital mortality (aOR, 0.36; 95% CI, 0.19-0.69) compared with those in the late or no treatment group.
IN PRACTICE:
“These findings support current recommendations, such as the IDSA [Infectious Disease Society of America] Influenza Clinical Practice Guidelines and CDC [Centers for Disease Control and Prevention] guidance, to initiate oseltamivir treatment as soon as possible for adult patients hospitalized with influenza,” the authors wrote.
SOURCE:
The study was led by Nathaniel M. Lewis, PhD, Influenza Division, CDC, Atlanta, Georgia, and was published online in Clinical Infectious Diseases.
LIMITATIONS:
This study may not be generalizable to seasons when influenza A(H1N1)pdm09 or B viruses are predominant as it was conducted during an influenza A(H3N2) virus–predominant season. The study lacked sufficient power to examine various oseltamivir treatment initiation timepoints or identify a potential maximum time-to-treatment threshold for effectiveness. Moreover, variables such as outpatient antiviral treatment before hospital admission and other treatments using macrolides, statins, corticosteroids, or immunomodulators before or during hospitalization were not collected, which may have influenced the study findings.
DISCLOSURES:
The study received funding from the CDC and the National Center for Immunization and Respiratory Diseases. Some authors reported receiving research support, consulting fees, funding, grants, or fees for participation in an advisory board and having other ties with certain institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Your Guide to COVID Vaccines for 2024-2025
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
How Much Does Long COVID Cost Society? New Data Shed Light
Long COVID, a major public health crisis, is also becoming a significant economic crisis. A new study in Nature reports that the global annual economic impact of long COVID has hit $1 trillion — or about 1% of the global economy.
Long COVID is estimated to affect 6%-7% of adults. Those afflicted are often unable to work for extended periods, and some simply stop working altogether.
Besides damaging individual lives, long COVID is having wide-ranging impacts on health systems and economies worldwide, as those who suffer from it have large absences from work, leading to lower productivity. Even those who return to work after weeks, months, or even up to a year out of work may come back with worse productivity and some functional impairment — as a few of the condition’s common symptoms include fatigue and brain fog.
Experts say more is needed not only in terms of scientific research into new treatments for long COVID but also from a public policy perspective.
Long COVID’s impact on the labor force is already having ripple effects throughout the economy of the United States and other countries. Earlier this year, the US Government Accountability Office stated long COVID potentially affects up to 23 million Americans, with as many as a million people out of work. The healthcare industry is particularly hard hit.
The latest survey from the National Center for Health Statistics estimated 17.3%-18.6% of adults have experienced long COVID. This isn’t the same as those who have it now, only a broad indicator of people who’ve ever experienced symptoms.
Public health experts, economists, researchers, and physicians say they are only beginning to focus on ways to reduce long COVID’s impact.
They suggest a range of potential solutions to address the public health crisis and the economic impacts — including implementing a more thorough surveillance system to track long COVID cases, building better ventilation systems in hospitals and buildings to reduce the spread of the virus, increasing vaccination efforts as new viral strains continuously emerge, and more funding for long COVID research to better quantify and qualify the disease’s impact.
Shaky Statistics, Inconsistent Surveillance
David Smith, MD, an infectious disease specialist at the University of California, San Diego, said more needs to be done to survey, quantify, and qualify the impacts of long COVID on the economy before practical solutions can be identified.
“Our surveillance system sucks,” Smith said. “I can see how many people test positive for COVID, but how many of those people have long COVID?”
Long COVID also doesn’t have a true definition or standard diagnosis, which complicates surveillance efforts. It includes a spectrum of symptoms such as shortness of breath, chronic fatigue, and brain fog that linger for 2-3 months after an acute infection. But there’s no “concrete case definition,” Smith said. “And not everybody’s long COVID is exactly the same as everybody else’s.”
As a result, epidemiologists can’t effectively characterize the disease, and health economists can’t measure its exact economic impact.
Few countries have established comprehensive surveillance systems to estimate the burden of long COVID at the population level.
The United States currently tracks new cases by measuring wastewater levels, which isn’t as comprehensive as the tracking that was done during the pandemic. But positive wastewater samples can’t tell us who is infected in an area, nor can it distinguish whether a visitor/tourist or resident is mostly contributing to the wastewater analysis — an important distinction in public health studies.
Wastewater surveillance is an excellent complement to traditional disease surveillance with advantages and disadvantages, but it shouldn’t be the sole way to measure disease.
What Research Best Informs the Debate?
A study by Economist Impact — a think tank that partners with corporations, foundations, NGOs, and governments to help drive policy — estimated between a 0.5% and 2.3% gross domestic product (GDP) loss across eight separate countries in 2024. The study included the United Kingdom and United States.
Meanwhile, Australian researchers recently detailed how long COVID-related reductions in labor supply affected its productivity and GDP from 2022 to 2024. The study found that long COVID could be costing the Australian economy about 0.5% of its GDP, which researchers deemed a conservative estimate.
Public health researchers in New Zealand used the estimate of GDP loss in Australia to measure their own potential losses and advocated for strengthening occupational support across all sectors to protect health.
But these studies can’t quite compare with what would have to be done for the United States economy.
“New Zealand is small ... and has an excellent public health system with good delivery of vaccines and treatments…so how do we compare that to us?” Smith said. “They do better in all of their public health metrics than we do.”
Measuring the Economic Impact
Gopi Shah Goda, PhD, a health economist and senior fellow in economic studies at the Brookings Institution, co-authored a 2023 study that found COVID-19 reduced the US labor force by about 500,000 people.
Plus, workers who missed a full week due to COVID-19 absences became 7% less likely to return to the labor force a year later compared with workers who didn’t miss work for health reasons. That amounts to 0.2% of the labor force, a significant number.
“Even a small percent of the labor force is a big number…it’s like an extra year of populating aging,” Goda said.
“Some people who get long COVID might have dropped out of the labor force anyway,” Goda added.
The study concluded that average individual earnings lost from long COVID were $9000, and the total lost labor supply amounted to $62 billion annually — about half the estimated productivity losses from cancer or diabetes.
But research into long COVID research continues to be underfunded compared with other health conditions, experts noted.
Cancer and diabetes both receive billions of research dollars annually from the National Institutes of Health. Long COVID research gets only a few million, according to Goda.
Informing Public Health Policy
When it comes to caring for patients with long COVID, the big issue facing every nation’s public policy leaders is how best to allocate limited health resources.
“Public health never has enough money ... Do they buy more vaccines? Do they do educational programs? Who do they target the most?” Smith said.
Though Smith thinks the best preventative measure is increased vaccination, vaccination rates remain low in the United States.
“Unfortunately, as last fall demonstrated, there’s a lot of vaccine indifference and skepticism,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University School of Medicine, Nashville, Tennessee.
Over the past year, only 14% of eligible children and 22% of adults received the 2023-2024 COVID vaccine boosters.
Schaffner said public health experts wrestle with ways to assure the public vaccines are safe and effective.
“They’re trying to provide a level of comfort that [getting vaccinated] is the socially appropriate thing to do,” which remains a significant challenge, Schaffner said.
Some people don’t have access to vaccines and comprehensive medical services because they lack insurance, Medicaid, and Medicare. And the United States still doesn’t distribute vaccines as well as other countries, Schaffner added.
“In other countries, every doctor’s office gets vaccines for free ... here, we have a large commercial enterprise that basically runs it…there are still populations who aren’t reached,” he said.
Long COVID clinics that have opened around the country have offered help to some patients with long COVID. A year and a half ago, Yale University, New Haven, Connecticut, established its Long COVID Care Center. Stanford University, Stanford, California, opened its Long COVID Clinic back in 2021. Vanderbilt University now has its own, as well — the Adult Post-COVID Clinic.
But these clinics have faced declining federal resources, forcing some to close and others to face questions about whether they will be able to continue to operate without more aggressive federal direction and policy planning.
“With some central direction, we could provide better supportive care for the many patients with long COVID out there,” Schaffner said.
For countries with universal healthcare systems, services such as occupational health, extended sick leave, extended time for disability, and workers’ compensation benefits are readily available.
But in the United States, it’s often left to the physicians and their patients to figure out a plan.
“I think we could make physicians more aware of options for their patients…for example, regularly check eligibility for workers compensation,” Schaffner said.
A version of this article first appeared on Medscape.com.
Long COVID, a major public health crisis, is also becoming a significant economic crisis. A new study in Nature reports that the global annual economic impact of long COVID has hit $1 trillion — or about 1% of the global economy.
Long COVID is estimated to affect 6%-7% of adults. Those afflicted are often unable to work for extended periods, and some simply stop working altogether.
Besides damaging individual lives, long COVID is having wide-ranging impacts on health systems and economies worldwide, as those who suffer from it have large absences from work, leading to lower productivity. Even those who return to work after weeks, months, or even up to a year out of work may come back with worse productivity and some functional impairment — as a few of the condition’s common symptoms include fatigue and brain fog.
Experts say more is needed not only in terms of scientific research into new treatments for long COVID but also from a public policy perspective.
Long COVID’s impact on the labor force is already having ripple effects throughout the economy of the United States and other countries. Earlier this year, the US Government Accountability Office stated long COVID potentially affects up to 23 million Americans, with as many as a million people out of work. The healthcare industry is particularly hard hit.
The latest survey from the National Center for Health Statistics estimated 17.3%-18.6% of adults have experienced long COVID. This isn’t the same as those who have it now, only a broad indicator of people who’ve ever experienced symptoms.
Public health experts, economists, researchers, and physicians say they are only beginning to focus on ways to reduce long COVID’s impact.
They suggest a range of potential solutions to address the public health crisis and the economic impacts — including implementing a more thorough surveillance system to track long COVID cases, building better ventilation systems in hospitals and buildings to reduce the spread of the virus, increasing vaccination efforts as new viral strains continuously emerge, and more funding for long COVID research to better quantify and qualify the disease’s impact.
Shaky Statistics, Inconsistent Surveillance
David Smith, MD, an infectious disease specialist at the University of California, San Diego, said more needs to be done to survey, quantify, and qualify the impacts of long COVID on the economy before practical solutions can be identified.
“Our surveillance system sucks,” Smith said. “I can see how many people test positive for COVID, but how many of those people have long COVID?”
Long COVID also doesn’t have a true definition or standard diagnosis, which complicates surveillance efforts. It includes a spectrum of symptoms such as shortness of breath, chronic fatigue, and brain fog that linger for 2-3 months after an acute infection. But there’s no “concrete case definition,” Smith said. “And not everybody’s long COVID is exactly the same as everybody else’s.”
As a result, epidemiologists can’t effectively characterize the disease, and health economists can’t measure its exact economic impact.
Few countries have established comprehensive surveillance systems to estimate the burden of long COVID at the population level.
The United States currently tracks new cases by measuring wastewater levels, which isn’t as comprehensive as the tracking that was done during the pandemic. But positive wastewater samples can’t tell us who is infected in an area, nor can it distinguish whether a visitor/tourist or resident is mostly contributing to the wastewater analysis — an important distinction in public health studies.
Wastewater surveillance is an excellent complement to traditional disease surveillance with advantages and disadvantages, but it shouldn’t be the sole way to measure disease.
What Research Best Informs the Debate?
A study by Economist Impact — a think tank that partners with corporations, foundations, NGOs, and governments to help drive policy — estimated between a 0.5% and 2.3% gross domestic product (GDP) loss across eight separate countries in 2024. The study included the United Kingdom and United States.
Meanwhile, Australian researchers recently detailed how long COVID-related reductions in labor supply affected its productivity and GDP from 2022 to 2024. The study found that long COVID could be costing the Australian economy about 0.5% of its GDP, which researchers deemed a conservative estimate.
Public health researchers in New Zealand used the estimate of GDP loss in Australia to measure their own potential losses and advocated for strengthening occupational support across all sectors to protect health.
But these studies can’t quite compare with what would have to be done for the United States economy.
“New Zealand is small ... and has an excellent public health system with good delivery of vaccines and treatments…so how do we compare that to us?” Smith said. “They do better in all of their public health metrics than we do.”
Measuring the Economic Impact
Gopi Shah Goda, PhD, a health economist and senior fellow in economic studies at the Brookings Institution, co-authored a 2023 study that found COVID-19 reduced the US labor force by about 500,000 people.
Plus, workers who missed a full week due to COVID-19 absences became 7% less likely to return to the labor force a year later compared with workers who didn’t miss work for health reasons. That amounts to 0.2% of the labor force, a significant number.
“Even a small percent of the labor force is a big number…it’s like an extra year of populating aging,” Goda said.
“Some people who get long COVID might have dropped out of the labor force anyway,” Goda added.
The study concluded that average individual earnings lost from long COVID were $9000, and the total lost labor supply amounted to $62 billion annually — about half the estimated productivity losses from cancer or diabetes.
But research into long COVID research continues to be underfunded compared with other health conditions, experts noted.
Cancer and diabetes both receive billions of research dollars annually from the National Institutes of Health. Long COVID research gets only a few million, according to Goda.
Informing Public Health Policy
When it comes to caring for patients with long COVID, the big issue facing every nation’s public policy leaders is how best to allocate limited health resources.
“Public health never has enough money ... Do they buy more vaccines? Do they do educational programs? Who do they target the most?” Smith said.
Though Smith thinks the best preventative measure is increased vaccination, vaccination rates remain low in the United States.
“Unfortunately, as last fall demonstrated, there’s a lot of vaccine indifference and skepticism,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University School of Medicine, Nashville, Tennessee.
Over the past year, only 14% of eligible children and 22% of adults received the 2023-2024 COVID vaccine boosters.
Schaffner said public health experts wrestle with ways to assure the public vaccines are safe and effective.
“They’re trying to provide a level of comfort that [getting vaccinated] is the socially appropriate thing to do,” which remains a significant challenge, Schaffner said.
Some people don’t have access to vaccines and comprehensive medical services because they lack insurance, Medicaid, and Medicare. And the United States still doesn’t distribute vaccines as well as other countries, Schaffner added.
“In other countries, every doctor’s office gets vaccines for free ... here, we have a large commercial enterprise that basically runs it…there are still populations who aren’t reached,” he said.
Long COVID clinics that have opened around the country have offered help to some patients with long COVID. A year and a half ago, Yale University, New Haven, Connecticut, established its Long COVID Care Center. Stanford University, Stanford, California, opened its Long COVID Clinic back in 2021. Vanderbilt University now has its own, as well — the Adult Post-COVID Clinic.
But these clinics have faced declining federal resources, forcing some to close and others to face questions about whether they will be able to continue to operate without more aggressive federal direction and policy planning.
“With some central direction, we could provide better supportive care for the many patients with long COVID out there,” Schaffner said.
For countries with universal healthcare systems, services such as occupational health, extended sick leave, extended time for disability, and workers’ compensation benefits are readily available.
But in the United States, it’s often left to the physicians and their patients to figure out a plan.
“I think we could make physicians more aware of options for their patients…for example, regularly check eligibility for workers compensation,” Schaffner said.
A version of this article first appeared on Medscape.com.
Long COVID, a major public health crisis, is also becoming a significant economic crisis. A new study in Nature reports that the global annual economic impact of long COVID has hit $1 trillion — or about 1% of the global economy.
Long COVID is estimated to affect 6%-7% of adults. Those afflicted are often unable to work for extended periods, and some simply stop working altogether.
Besides damaging individual lives, long COVID is having wide-ranging impacts on health systems and economies worldwide, as those who suffer from it have large absences from work, leading to lower productivity. Even those who return to work after weeks, months, or even up to a year out of work may come back with worse productivity and some functional impairment — as a few of the condition’s common symptoms include fatigue and brain fog.
Experts say more is needed not only in terms of scientific research into new treatments for long COVID but also from a public policy perspective.
Long COVID’s impact on the labor force is already having ripple effects throughout the economy of the United States and other countries. Earlier this year, the US Government Accountability Office stated long COVID potentially affects up to 23 million Americans, with as many as a million people out of work. The healthcare industry is particularly hard hit.
The latest survey from the National Center for Health Statistics estimated 17.3%-18.6% of adults have experienced long COVID. This isn’t the same as those who have it now, only a broad indicator of people who’ve ever experienced symptoms.
Public health experts, economists, researchers, and physicians say they are only beginning to focus on ways to reduce long COVID’s impact.
They suggest a range of potential solutions to address the public health crisis and the economic impacts — including implementing a more thorough surveillance system to track long COVID cases, building better ventilation systems in hospitals and buildings to reduce the spread of the virus, increasing vaccination efforts as new viral strains continuously emerge, and more funding for long COVID research to better quantify and qualify the disease’s impact.
Shaky Statistics, Inconsistent Surveillance
David Smith, MD, an infectious disease specialist at the University of California, San Diego, said more needs to be done to survey, quantify, and qualify the impacts of long COVID on the economy before practical solutions can be identified.
“Our surveillance system sucks,” Smith said. “I can see how many people test positive for COVID, but how many of those people have long COVID?”
Long COVID also doesn’t have a true definition or standard diagnosis, which complicates surveillance efforts. It includes a spectrum of symptoms such as shortness of breath, chronic fatigue, and brain fog that linger for 2-3 months after an acute infection. But there’s no “concrete case definition,” Smith said. “And not everybody’s long COVID is exactly the same as everybody else’s.”
As a result, epidemiologists can’t effectively characterize the disease, and health economists can’t measure its exact economic impact.
Few countries have established comprehensive surveillance systems to estimate the burden of long COVID at the population level.
The United States currently tracks new cases by measuring wastewater levels, which isn’t as comprehensive as the tracking that was done during the pandemic. But positive wastewater samples can’t tell us who is infected in an area, nor can it distinguish whether a visitor/tourist or resident is mostly contributing to the wastewater analysis — an important distinction in public health studies.
Wastewater surveillance is an excellent complement to traditional disease surveillance with advantages and disadvantages, but it shouldn’t be the sole way to measure disease.
What Research Best Informs the Debate?
A study by Economist Impact — a think tank that partners with corporations, foundations, NGOs, and governments to help drive policy — estimated between a 0.5% and 2.3% gross domestic product (GDP) loss across eight separate countries in 2024. The study included the United Kingdom and United States.
Meanwhile, Australian researchers recently detailed how long COVID-related reductions in labor supply affected its productivity and GDP from 2022 to 2024. The study found that long COVID could be costing the Australian economy about 0.5% of its GDP, which researchers deemed a conservative estimate.
Public health researchers in New Zealand used the estimate of GDP loss in Australia to measure their own potential losses and advocated for strengthening occupational support across all sectors to protect health.
But these studies can’t quite compare with what would have to be done for the United States economy.
“New Zealand is small ... and has an excellent public health system with good delivery of vaccines and treatments…so how do we compare that to us?” Smith said. “They do better in all of their public health metrics than we do.”
Measuring the Economic Impact
Gopi Shah Goda, PhD, a health economist and senior fellow in economic studies at the Brookings Institution, co-authored a 2023 study that found COVID-19 reduced the US labor force by about 500,000 people.
Plus, workers who missed a full week due to COVID-19 absences became 7% less likely to return to the labor force a year later compared with workers who didn’t miss work for health reasons. That amounts to 0.2% of the labor force, a significant number.
“Even a small percent of the labor force is a big number…it’s like an extra year of populating aging,” Goda said.
“Some people who get long COVID might have dropped out of the labor force anyway,” Goda added.
The study concluded that average individual earnings lost from long COVID were $9000, and the total lost labor supply amounted to $62 billion annually — about half the estimated productivity losses from cancer or diabetes.
But research into long COVID research continues to be underfunded compared with other health conditions, experts noted.
Cancer and diabetes both receive billions of research dollars annually from the National Institutes of Health. Long COVID research gets only a few million, according to Goda.
Informing Public Health Policy
When it comes to caring for patients with long COVID, the big issue facing every nation’s public policy leaders is how best to allocate limited health resources.
“Public health never has enough money ... Do they buy more vaccines? Do they do educational programs? Who do they target the most?” Smith said.
Though Smith thinks the best preventative measure is increased vaccination, vaccination rates remain low in the United States.
“Unfortunately, as last fall demonstrated, there’s a lot of vaccine indifference and skepticism,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University School of Medicine, Nashville, Tennessee.
Over the past year, only 14% of eligible children and 22% of adults received the 2023-2024 COVID vaccine boosters.
Schaffner said public health experts wrestle with ways to assure the public vaccines are safe and effective.
“They’re trying to provide a level of comfort that [getting vaccinated] is the socially appropriate thing to do,” which remains a significant challenge, Schaffner said.
Some people don’t have access to vaccines and comprehensive medical services because they lack insurance, Medicaid, and Medicare. And the United States still doesn’t distribute vaccines as well as other countries, Schaffner added.
“In other countries, every doctor’s office gets vaccines for free ... here, we have a large commercial enterprise that basically runs it…there are still populations who aren’t reached,” he said.
Long COVID clinics that have opened around the country have offered help to some patients with long COVID. A year and a half ago, Yale University, New Haven, Connecticut, established its Long COVID Care Center. Stanford University, Stanford, California, opened its Long COVID Clinic back in 2021. Vanderbilt University now has its own, as well — the Adult Post-COVID Clinic.
But these clinics have faced declining federal resources, forcing some to close and others to face questions about whether they will be able to continue to operate without more aggressive federal direction and policy planning.
“With some central direction, we could provide better supportive care for the many patients with long COVID out there,” Schaffner said.
For countries with universal healthcare systems, services such as occupational health, extended sick leave, extended time for disability, and workers’ compensation benefits are readily available.
But in the United States, it’s often left to the physicians and their patients to figure out a plan.
“I think we could make physicians more aware of options for their patients…for example, regularly check eligibility for workers compensation,” Schaffner said.
A version of this article first appeared on Medscape.com.
FROM NATURE
Sperm Appear to Have a Nonreproductive Function
Brazilian researchers have identified a previously unrecognized function of sperm that is unrelated to reproduction. A study of 13 patients admitted to the Hospital das Clínicas da Universidade de São Paulo with moderate to severe COVID-19 showed that male gametes released extracellular traps (in a process called ETosis) in response to the infection. This immune response, which is common to macrophages and neutrophils, had never been observed in mammalian reproductive cells.
“It opens up a new line of research,” said Jorge Hallak, a professor at the University of São Paulo School of Medicine, São Paulo, Brazil, and first author of the article published in Andrology. “This may be an innovative mechanism, or it may have always existed, and no one knew.”
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in cells more than 3 months after infection in 11 participants, although polymerase chain reaction tests were negative. These findings suggest the potential for drafting a protocol or guidance on when to attempt a pregnancy. “My concern is with assisted reproduction, in which, in general, only one basic spermogram is done, without diagnostic investigation or serology for coronavirus,” said Hallak.
Symptomatic infections hinder the reproductive process because symptoms such as high fever impair cell function by triggering increased DNA fragmentation, reduced mitochondrial activity, decreased acrosome reaction, and cell death, thus affecting sperm count and gamete mobility.
The new findings indicate that the impact of SARS-CoV-2 infection can continue for as long as 90 days after symptoms and signs disappear and affect sperm count and gamete quality for even longer. “With the sperm selection technique, you are at risk of taking a cell with viruses and injecting it into the egg. It is not known what changes this may cause to the embryo,” said Hallak.
The expert emphasized that the finding contributes to the understanding of reproductive difficulties that previously had no plausible explanation. It serves as a warning against negligence in the evaluation of men in assisted reproductive treatments.
Daniel Zylberstein, urologist and member of the Brazilian Association of Assisted Reproduction, who did not participate in the research, noted that the result comes from a small study that should be expanded to try to develop guidance for doctors.
“There is still no protocol for these cases. The ideal approach would be to wait for complete spermatogenesis, which takes about 3 months, before putting patients on treatment. This often does not happen, and treatment begins shortly after clinical recovery. In the case of moderate to severe COVID-19, this period should be longer than 90 days,” he said.
The study suggests establishing a quarantine period for reproduction until the sperm are free of the virus, said Zylberstein. “With infected sperm, it makes no sense to start reproductive treatment. This sperm is spending energy to fight the pathogen. Assisted reproduction is expensive and exhaustive and may not have the expected outcome because of SARS-CoV-2 infectivity.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Brazilian researchers have identified a previously unrecognized function of sperm that is unrelated to reproduction. A study of 13 patients admitted to the Hospital das Clínicas da Universidade de São Paulo with moderate to severe COVID-19 showed that male gametes released extracellular traps (in a process called ETosis) in response to the infection. This immune response, which is common to macrophages and neutrophils, had never been observed in mammalian reproductive cells.
“It opens up a new line of research,” said Jorge Hallak, a professor at the University of São Paulo School of Medicine, São Paulo, Brazil, and first author of the article published in Andrology. “This may be an innovative mechanism, or it may have always existed, and no one knew.”
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in cells more than 3 months after infection in 11 participants, although polymerase chain reaction tests were negative. These findings suggest the potential for drafting a protocol or guidance on when to attempt a pregnancy. “My concern is with assisted reproduction, in which, in general, only one basic spermogram is done, without diagnostic investigation or serology for coronavirus,” said Hallak.
Symptomatic infections hinder the reproductive process because symptoms such as high fever impair cell function by triggering increased DNA fragmentation, reduced mitochondrial activity, decreased acrosome reaction, and cell death, thus affecting sperm count and gamete mobility.
The new findings indicate that the impact of SARS-CoV-2 infection can continue for as long as 90 days after symptoms and signs disappear and affect sperm count and gamete quality for even longer. “With the sperm selection technique, you are at risk of taking a cell with viruses and injecting it into the egg. It is not known what changes this may cause to the embryo,” said Hallak.
The expert emphasized that the finding contributes to the understanding of reproductive difficulties that previously had no plausible explanation. It serves as a warning against negligence in the evaluation of men in assisted reproductive treatments.
Daniel Zylberstein, urologist and member of the Brazilian Association of Assisted Reproduction, who did not participate in the research, noted that the result comes from a small study that should be expanded to try to develop guidance for doctors.
“There is still no protocol for these cases. The ideal approach would be to wait for complete spermatogenesis, which takes about 3 months, before putting patients on treatment. This often does not happen, and treatment begins shortly after clinical recovery. In the case of moderate to severe COVID-19, this period should be longer than 90 days,” he said.
The study suggests establishing a quarantine period for reproduction until the sperm are free of the virus, said Zylberstein. “With infected sperm, it makes no sense to start reproductive treatment. This sperm is spending energy to fight the pathogen. Assisted reproduction is expensive and exhaustive and may not have the expected outcome because of SARS-CoV-2 infectivity.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Brazilian researchers have identified a previously unrecognized function of sperm that is unrelated to reproduction. A study of 13 patients admitted to the Hospital das Clínicas da Universidade de São Paulo with moderate to severe COVID-19 showed that male gametes released extracellular traps (in a process called ETosis) in response to the infection. This immune response, which is common to macrophages and neutrophils, had never been observed in mammalian reproductive cells.
“It opens up a new line of research,” said Jorge Hallak, a professor at the University of São Paulo School of Medicine, São Paulo, Brazil, and first author of the article published in Andrology. “This may be an innovative mechanism, or it may have always existed, and no one knew.”
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in cells more than 3 months after infection in 11 participants, although polymerase chain reaction tests were negative. These findings suggest the potential for drafting a protocol or guidance on when to attempt a pregnancy. “My concern is with assisted reproduction, in which, in general, only one basic spermogram is done, without diagnostic investigation or serology for coronavirus,” said Hallak.
Symptomatic infections hinder the reproductive process because symptoms such as high fever impair cell function by triggering increased DNA fragmentation, reduced mitochondrial activity, decreased acrosome reaction, and cell death, thus affecting sperm count and gamete mobility.
The new findings indicate that the impact of SARS-CoV-2 infection can continue for as long as 90 days after symptoms and signs disappear and affect sperm count and gamete quality for even longer. “With the sperm selection technique, you are at risk of taking a cell with viruses and injecting it into the egg. It is not known what changes this may cause to the embryo,” said Hallak.
The expert emphasized that the finding contributes to the understanding of reproductive difficulties that previously had no plausible explanation. It serves as a warning against negligence in the evaluation of men in assisted reproductive treatments.
Daniel Zylberstein, urologist and member of the Brazilian Association of Assisted Reproduction, who did not participate in the research, noted that the result comes from a small study that should be expanded to try to develop guidance for doctors.
“There is still no protocol for these cases. The ideal approach would be to wait for complete spermatogenesis, which takes about 3 months, before putting patients on treatment. This often does not happen, and treatment begins shortly after clinical recovery. In the case of moderate to severe COVID-19, this period should be longer than 90 days,” he said.
The study suggests establishing a quarantine period for reproduction until the sperm are free of the virus, said Zylberstein. “With infected sperm, it makes no sense to start reproductive treatment. This sperm is spending energy to fight the pathogen. Assisted reproduction is expensive and exhaustive and may not have the expected outcome because of SARS-CoV-2 infectivity.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
FROM ANDROLOGY
FDA Okays Subcutaneous Ocrelizumab for MS
The subcutaneous (SC) injection can be administered by a healthcare professional in approximately 10 minutes and is the first and only twice-a-year SC injection approved for both RMS and PPMS, according to a company news release.
The FDA approval is based on pivotal data from the phase 3 OCARINA II trial, which showed no clinically significant difference in blood levels of ocrelizumab when administered subcutaneously and an efficacy profile consistent with the intravenous (IV) formulation.
“The trial met its primary and secondary endpoints, demonstrating SC injection was noninferior to IV infusion based on [ocrelizumab] levels in the blood, and consistent control of clinical (relapses) and radiological (MRI lesions) disease activity,” the company said in the release.
The safety profile of SC ocrelizumab was consistent with the safety profile of IV ocrelizumab, with the exception of injection site reactions, the most common adverse event.
Injection reactions were more often reported with the first injection, with 49% of trial participants experiencing an injection reaction after the first injection. All injection reactions were mild or moderate, and none led to treatment withdrawal.
Ocrevus Zunovo “may offer greater flexibility for healthcare providers and people living with multiple sclerosis, based on their individual treatment needs,” Levi Garraway, MD, PhD, chief medical officer for Genentech, said in the press release. “We are pleased that with a new method of delivery, there is now an additional option for those who need flexibility in the route of administration or treatment time,” Natalie Blake, executive director of the MS Foundation, said in the release.
The SC formulation of ocrelizumab was approved by the European Commission in June.
Complete prescribing information is available online.
A version of this article appeared on Medscape.com.
The subcutaneous (SC) injection can be administered by a healthcare professional in approximately 10 minutes and is the first and only twice-a-year SC injection approved for both RMS and PPMS, according to a company news release.
The FDA approval is based on pivotal data from the phase 3 OCARINA II trial, which showed no clinically significant difference in blood levels of ocrelizumab when administered subcutaneously and an efficacy profile consistent with the intravenous (IV) formulation.
“The trial met its primary and secondary endpoints, demonstrating SC injection was noninferior to IV infusion based on [ocrelizumab] levels in the blood, and consistent control of clinical (relapses) and radiological (MRI lesions) disease activity,” the company said in the release.
The safety profile of SC ocrelizumab was consistent with the safety profile of IV ocrelizumab, with the exception of injection site reactions, the most common adverse event.
Injection reactions were more often reported with the first injection, with 49% of trial participants experiencing an injection reaction after the first injection. All injection reactions were mild or moderate, and none led to treatment withdrawal.
Ocrevus Zunovo “may offer greater flexibility for healthcare providers and people living with multiple sclerosis, based on their individual treatment needs,” Levi Garraway, MD, PhD, chief medical officer for Genentech, said in the press release. “We are pleased that with a new method of delivery, there is now an additional option for those who need flexibility in the route of administration or treatment time,” Natalie Blake, executive director of the MS Foundation, said in the release.
The SC formulation of ocrelizumab was approved by the European Commission in June.
Complete prescribing information is available online.
A version of this article appeared on Medscape.com.
The subcutaneous (SC) injection can be administered by a healthcare professional in approximately 10 minutes and is the first and only twice-a-year SC injection approved for both RMS and PPMS, according to a company news release.
The FDA approval is based on pivotal data from the phase 3 OCARINA II trial, which showed no clinically significant difference in blood levels of ocrelizumab when administered subcutaneously and an efficacy profile consistent with the intravenous (IV) formulation.
“The trial met its primary and secondary endpoints, demonstrating SC injection was noninferior to IV infusion based on [ocrelizumab] levels in the blood, and consistent control of clinical (relapses) and radiological (MRI lesions) disease activity,” the company said in the release.
The safety profile of SC ocrelizumab was consistent with the safety profile of IV ocrelizumab, with the exception of injection site reactions, the most common adverse event.
Injection reactions were more often reported with the first injection, with 49% of trial participants experiencing an injection reaction after the first injection. All injection reactions were mild or moderate, and none led to treatment withdrawal.
Ocrevus Zunovo “may offer greater flexibility for healthcare providers and people living with multiple sclerosis, based on their individual treatment needs,” Levi Garraway, MD, PhD, chief medical officer for Genentech, said in the press release. “We are pleased that with a new method of delivery, there is now an additional option for those who need flexibility in the route of administration or treatment time,” Natalie Blake, executive director of the MS Foundation, said in the release.
The SC formulation of ocrelizumab was approved by the European Commission in June.
Complete prescribing information is available online.
A version of this article appeared on Medscape.com.
How Safe is Anti–IL-6 Therapy During Pregnancy?
TOPLINE:
The maternal and neonatal outcomes in pregnant women treated with anti–interleukin (IL)-6 therapy for COVID-19 are largely favorable, with transient neonatal cytopenia observed in around one third of the babies being the only possible adverse outcome that could be related to anti–IL-6 therapy.
METHODOLOGY:
- Despite guidance, very few pregnant women with COVID-19 are offered evidence-based therapies such as anti–IL-6 due to concerns regarding fetal safety in later pregnancy.
- In this retrospective study, researchers evaluated maternal and neonatal outcomes in 25 pregnant women with COVID-19 (mean age at admission, 33 years) treated with anti–IL-6 (tocilizumab or sarilumab) at two tertiary hospitals in London.
- Most women (n = 16) received anti–IL-6 in the third trimester of pregnancy, whereas nine received it during the second trimester.
- Maternal and neonatal outcomes were assessed through medical record reviews and maternal medicine networks, with follow-up for 12 months.
- The women included in the study constituted a high-risk population with severe COVID-19; 24 required level two or three critical care. All women were receiving at least three concomitant medications due to their critical illness.
TAKEAWAY:
- Overall, 24 of 25 women treated with IL-6 receptor antibodies survived until hospital discharge.
- The sole death occurred in a woman with severe COVID-19 pneumonitis who later developed myocarditis and cardiac arrest. The physicians believed that these complications were more likely due to severe COVID-19 rather than anti–IL-6 therapy.
- All pregnancies resulted in live births; however, 16 babies had to be delivered preterm due to COVID-19 complications.
- Transient cytopenia was observed in 6 of 19 babies in whom a full blood count was performed. All the six babies were premature, with cytopenia resolving within 7 days in four babies; one baby died from complications associated with extreme prematurity.
IN PRACTICE:
“Although the authors found mild, transitory cytopenia in some (6 of 19) exposed infants, most had been delivered prematurely due to progressive COVID-19–related morbidity, and distinguishing drug effects from similar prematurity-related effects is difficult,” wrote Steven L. Clark, MD, from the Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, in an accompanying editorial.
SOURCE:
The study was led by Melanie Nana, MRCP, from the Department of Obstetric Medicine, St Thomas’ Hospital, London, England. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study was retrospective in design, which may have introduced bias. The small sample size of 25 women may have limited the generalizability of the findings. Additionally, the study did not include a control group, which made it difficult to attribute outcomes solely to anti–IL-6 therapy. The lack of long-term follow-up data on the neonates also limited the understanding of potential long-term effects.
DISCLOSURES:
This study did not receive any funding. Some authors, including the lead author, received speaker fees, grants, or consultancy fees from academic institutions or pharmaceutical companies or had other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The maternal and neonatal outcomes in pregnant women treated with anti–interleukin (IL)-6 therapy for COVID-19 are largely favorable, with transient neonatal cytopenia observed in around one third of the babies being the only possible adverse outcome that could be related to anti–IL-6 therapy.
METHODOLOGY:
- Despite guidance, very few pregnant women with COVID-19 are offered evidence-based therapies such as anti–IL-6 due to concerns regarding fetal safety in later pregnancy.
- In this retrospective study, researchers evaluated maternal and neonatal outcomes in 25 pregnant women with COVID-19 (mean age at admission, 33 years) treated with anti–IL-6 (tocilizumab or sarilumab) at two tertiary hospitals in London.
- Most women (n = 16) received anti–IL-6 in the third trimester of pregnancy, whereas nine received it during the second trimester.
- Maternal and neonatal outcomes were assessed through medical record reviews and maternal medicine networks, with follow-up for 12 months.
- The women included in the study constituted a high-risk population with severe COVID-19; 24 required level two or three critical care. All women were receiving at least three concomitant medications due to their critical illness.
TAKEAWAY:
- Overall, 24 of 25 women treated with IL-6 receptor antibodies survived until hospital discharge.
- The sole death occurred in a woman with severe COVID-19 pneumonitis who later developed myocarditis and cardiac arrest. The physicians believed that these complications were more likely due to severe COVID-19 rather than anti–IL-6 therapy.
- All pregnancies resulted in live births; however, 16 babies had to be delivered preterm due to COVID-19 complications.
- Transient cytopenia was observed in 6 of 19 babies in whom a full blood count was performed. All the six babies were premature, with cytopenia resolving within 7 days in four babies; one baby died from complications associated with extreme prematurity.
IN PRACTICE:
“Although the authors found mild, transitory cytopenia in some (6 of 19) exposed infants, most had been delivered prematurely due to progressive COVID-19–related morbidity, and distinguishing drug effects from similar prematurity-related effects is difficult,” wrote Steven L. Clark, MD, from the Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, in an accompanying editorial.
SOURCE:
The study was led by Melanie Nana, MRCP, from the Department of Obstetric Medicine, St Thomas’ Hospital, London, England. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study was retrospective in design, which may have introduced bias. The small sample size of 25 women may have limited the generalizability of the findings. Additionally, the study did not include a control group, which made it difficult to attribute outcomes solely to anti–IL-6 therapy. The lack of long-term follow-up data on the neonates also limited the understanding of potential long-term effects.
DISCLOSURES:
This study did not receive any funding. Some authors, including the lead author, received speaker fees, grants, or consultancy fees from academic institutions or pharmaceutical companies or had other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The maternal and neonatal outcomes in pregnant women treated with anti–interleukin (IL)-6 therapy for COVID-19 are largely favorable, with transient neonatal cytopenia observed in around one third of the babies being the only possible adverse outcome that could be related to anti–IL-6 therapy.
METHODOLOGY:
- Despite guidance, very few pregnant women with COVID-19 are offered evidence-based therapies such as anti–IL-6 due to concerns regarding fetal safety in later pregnancy.
- In this retrospective study, researchers evaluated maternal and neonatal outcomes in 25 pregnant women with COVID-19 (mean age at admission, 33 years) treated with anti–IL-6 (tocilizumab or sarilumab) at two tertiary hospitals in London.
- Most women (n = 16) received anti–IL-6 in the third trimester of pregnancy, whereas nine received it during the second trimester.
- Maternal and neonatal outcomes were assessed through medical record reviews and maternal medicine networks, with follow-up for 12 months.
- The women included in the study constituted a high-risk population with severe COVID-19; 24 required level two or three critical care. All women were receiving at least three concomitant medications due to their critical illness.
TAKEAWAY:
- Overall, 24 of 25 women treated with IL-6 receptor antibodies survived until hospital discharge.
- The sole death occurred in a woman with severe COVID-19 pneumonitis who later developed myocarditis and cardiac arrest. The physicians believed that these complications were more likely due to severe COVID-19 rather than anti–IL-6 therapy.
- All pregnancies resulted in live births; however, 16 babies had to be delivered preterm due to COVID-19 complications.
- Transient cytopenia was observed in 6 of 19 babies in whom a full blood count was performed. All the six babies were premature, with cytopenia resolving within 7 days in four babies; one baby died from complications associated with extreme prematurity.
IN PRACTICE:
“Although the authors found mild, transitory cytopenia in some (6 of 19) exposed infants, most had been delivered prematurely due to progressive COVID-19–related morbidity, and distinguishing drug effects from similar prematurity-related effects is difficult,” wrote Steven L. Clark, MD, from the Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, in an accompanying editorial.
SOURCE:
The study was led by Melanie Nana, MRCP, from the Department of Obstetric Medicine, St Thomas’ Hospital, London, England. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study was retrospective in design, which may have introduced bias. The small sample size of 25 women may have limited the generalizability of the findings. Additionally, the study did not include a control group, which made it difficult to attribute outcomes solely to anti–IL-6 therapy. The lack of long-term follow-up data on the neonates also limited the understanding of potential long-term effects.
DISCLOSURES:
This study did not receive any funding. Some authors, including the lead author, received speaker fees, grants, or consultancy fees from academic institutions or pharmaceutical companies or had other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
New First-Line Therapies for Migraine Prevention
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 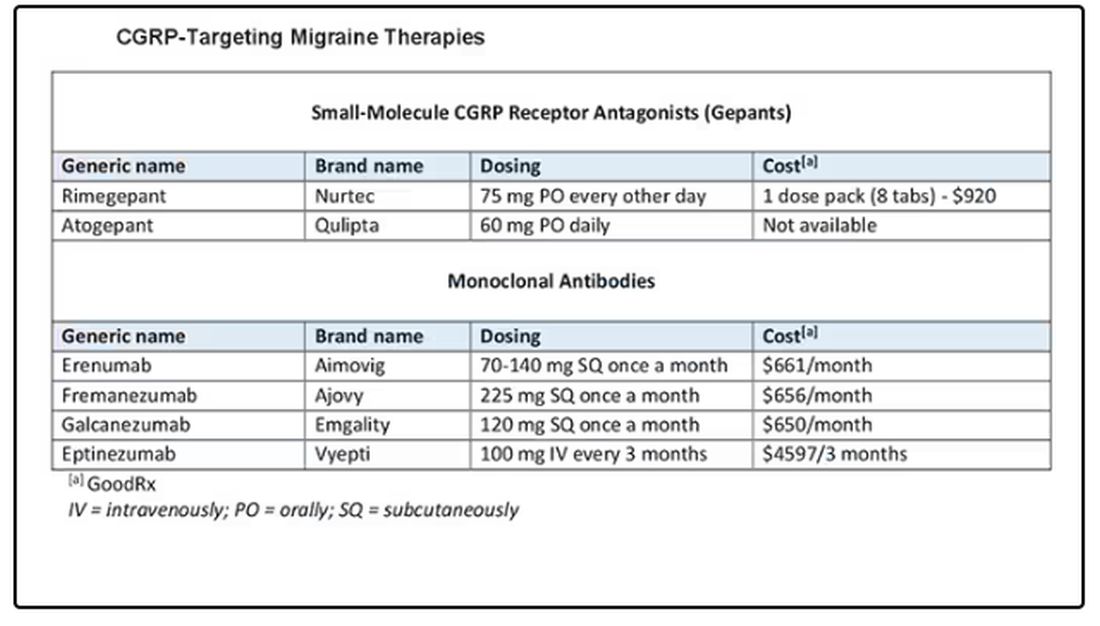
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.