User login
Things We Do For No Reason: Echocardiogram in Unselected Patients with Syncope
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

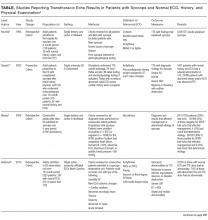
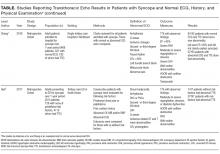
Syncope is a common cause of emergency department (ED) visits and hospitalizations. Echocardiogram is frequently used as a diagnostic tool in the evaluation of syncope, performed in 39%-91% of patients.
CLINICAL SCENARIO
A 57-year-old woman presented to the ED after a syncopal episode. She had just eaten dinner when she slumped over and became unresponsive. Her husband estimated that she regained consciousness 30 seconds later and quickly returned to baseline mental status. She denied chest pain, shortness of breath, or palpitations. Her medical history included hypertension and hypothyroidism. Her medication regimen was unchanged.
Vital signs, including orthostatic blood pressures, were within normal ranges. A physical examination revealed regular heart sounds without murmur, rub, or gallop. ECG showed normal sinus rhythm, normal axis, and normal intervals. Chest radiograph, complete blood count, chemistry, pro-brain natriuretic peptide (pro-BNP), and troponin were within normal ranges.
BACKGROUND
Syncope, defined as “abrupt, transient, complete loss of consciousness, associated with inability to maintain postural tone, with rapid and spontaneous recovery,”1 is a common clinical problem, accounting for 1% of ED visits in the United States.2 As syncope has been shown to be associated with increased mortality,3 the primary goal of syncope evaluation is to identify modifiable underlying causes, particularly cardiac causes. Current guidelines recommend a complete history and physical, orthostatic blood pressure measurement, and ECG as the initial evaluation for syncope.1 Echocardiogram is a frequent additional test, performed in 39%-91% of patients.4-8
WHY YOU MAY THINK ECHOCARDIOGRAM IS HELPFUL
Echocardiogram may identify depressed ejection fraction, a risk factor for ventricular arrhythmias, along with structural causes of syncope, including aortic stenosis, pulmonary hypertension, and hypertrophic cardiomyopathy.9 Structural heart disease is the underlying etiology in about 3% of patients with syncope.10
Prior guidelines stated that “an echocardiogram is a helpful screening test if the history, physical examination, and ECG do not provide a diagnosis or if underlying heart disease is suspected.”11 A separate guideline for the appropriate use of echocardiogram assigned a score of appropriateness on a 1-9 scale based on increasing indication.12 Echocardiogram for syncope was scored a 7 in patients with “no other symptoms or signs of cardiovascular disease.”12 Only 25%-40% of patients with syncope will have a cause identified after the history, physical examination, and ECG,13,14 creating diagnostic uncertainty that often leads to further testing.
WHY ECHOCARDIOGRAM IS NOT NECESSARY IN ALL PATIENTS
Mendu et al.5 performed a single-center, retrospective study of the diagnostic yield of testing for syncope in 2106 consecutive patients older than 65 admitted over the course of 5 years. They retrospectively applied the San Francisco Syncope Rule (SFSR), which patients met if they had congestive heart failure, hematocrit <30%, abnormal ECG, shortness of breath, or systolic blood pressure <90 mm Hg. There were 821 patients (39%) who underwent echocardiogram. Among the 488 with no SFSR criteria, 10 patients (2%) had echocardiogram results that affected management, and 4 patients (1%) had results that helped determine the etiology of syncope.
Anderson et al. studied 323 syncope patients in a single ED observation unit over 18 months.6 Patients with high-risk features, including unstable vital signs, abnormal cardiac biomarkers, or ischemic ECG changes, were excluded from the unit. The initial ECG was considered abnormal if it contained arrhythmia, premature atrial or ventricular contractions, pacing, second- or third-degree heart block, or left bundle branch block. Of the 235 patients with a normal ECG who underwent echocardiogram, none had an abnormal study.
Chang et al.7 performed a retrospective review of 468 patients admitted with syncope at a single hospital. Charts were reviewed for ECG and echocardiogram results. Abnormal ECGs were defined as those containing arrhythmias, Q waves, ischemic changes, second- and third-degree heart block, paced rhythm, corrected QT interval (QTc) >500 ms, left bundle branch or bifasicular block, Brugada pattern, or abnormal axis. Among 321 patients with normal ECGs, echocardiograms were performed in 192. Eleven of those echocardiograms were abnormal: 3 demonstrated aortic stenosis in patients who already carried the diagnosis, and the other 8 abnormal echocardiograms revealed unexpected left ventricular ejection fractions <45% or other nonaortic valvular pathology. None of the findings were felt to be the cause of syncope.
Han et al.8 performed a retrospective cohort study of all syncope patients presenting to a single ED over the course of 1 year. Patients were stratified as high risk if they had chest pain, palpitations, a history of cardiac disease (defined as prior arrhythmia, heart failure, coronary artery disease, or structural heart disease), abnormal cardiac biomarkers, or an abnormal ECG (defined as sinus bradycardia, arrhythmia, premature beats, second- or third-degree heart block, ventricular hypertrophy, ischemic Q or ST changes, or abnormal QT interval). Patients with none of those symptoms or findings were considered low risk. Of those categorized as low risk (n = 115), 47 underwent echocardiogram, only 1 of which was abnormal.
Across studies, the percentage of patients with a normal cardiac history, examination, and ECG with new, significant abnormalities on echocardiogram was 0% in 3 studies (n = 340),4,6,15 2% in 1 study (10/488 patients),5 2.1% in 1 study (1/47 patients),8 and 4.2% in 1 study (8/192 patients).7 The 11 echocardiograms with significant findings in the studies by Mendu et al.5 and Han et al.8 were not further described. The 8 patients with abnormal echocardiograms reported by Chang et al.7 had depressed left ventricular ejection fraction or nonaortic valvular disease that did not represent a definitive etiology of their syncope. Given the cost of $1,000 to $2,220 per study,16 routine echocardiograms in patients with a normal history, examination, and ECG would thus require $60,000 to $132,000 in spending to find 1 new significant abnormality, which may be unrelated to the actual cause of syncope.
SITUATIONS IN WHICH ECHOCARDIOGRAM MAY BE HELPFUL
The diagnostic yield of echocardiogram is higher in patients with a positive cardiac history or abnormal ECG. In the prospective study by Sarasin et al.15 a total of 27% of patients with a positive cardiac history or abnormal ECG were found to have an ejection fraction less than or equal to 40%. Other studies reporting percentages of abnormal echocardiograms in patients with abnormal history, ECG, or examination found rates of 8% (26/333),5 20% (7/35),6 28% (27/97),8 and 29% (27/93).7 It should be noted that not all of these abnormalities were felt to be the cause of syncope. For example, Sarasin et al.15 reported that only half of the patients with newly identified depressed ejection fraction were diagnosed with arrhythmia-related syncope. Chang et al7 reported that 6 of the 27 patients (22%) with abnormal ECG and echocardiogram had the cause of syncope established by echocardiogram.
Finally, some syncope patients will have cardiac biomarkers sent in the ED. Han et al.8 found that among patients with syncope, those with abnormal versus normal echocardiogram were more likely to have elevated BNP (70% vs 23%) and troponin (36% vs 12.4%). Thus, obtaining an echocardiogram in patients with syncope and abnormal cardiac biomarkers may be reasonable. It should be noted, however, that while some studies have suggested a role for biomarkers in differentiating cardiac from noncardiac syncope,17-20 current guidelines state that the usefulness of these tests is uncertain.1
WHAT YOU SHOULD DO INSTEAD OF ECHOCARDIOGRAM FOR ALL PATIENTS
Clinicians should carefully screen patients with syncope for abnormal findings suggesting cardiac disease on history, physical examination, and ECG. Relevant cardiac history includes known coronary artery disease, valvular heart disease, arrhythmia, congestive heart failure, and risk factors for cardiac syncope (supplemental Appendix). The definition of abnormal ECG varies among studies, but abnormalities that should prompt an echocardiogram include arrhythmia, premature atrial or ventricular contractions, second- or third-degree heart block, sinus bradycardia, bundle branch or fascicular blocks, left ventricular hypertrophy, ischemic ST or T wave changes, Q waves, or a prolonged QTc interval. New guidelines from the American College of Cardiology state, “Routine cardiac imaging is not useful in the evaluation of patients with syncope unless cardiac etiology is suspected on the basis of an initial evaluation, including history, physical examination, or ECG.”1
RECOMMENDATIONS
- All patients with syncope should receive a complete history, physical examination, orthostatic vital signs, and ECG.
- Perform echocardiogram on patients with syncope and a history of cardiac disease, examination suggestive of structural heart disease or congestive heart failure, or abnormal ECG.
- Echocardiogram may be reasonable in patients with syncope and abnormal cardiac biomarkers.
CONCLUSIONS
While commonly performed as part of syncope evaluations, echocardiogram has a very low diagnostic yield in patients with a normal history, physical, and ECG. The patient described in the initial case scenario would have an extremely low likelihood of having important diagnostic information found on echocardiogram.
Disclosure
The authors have no conflicts of interest relevant to this article.
1. Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70(5):620-633. PubMed
2. Sun BC, Emond JA, Camargo CA Jr. Characteristics and admission patterns of patients presenting with syncope to U.S. emergency departments, 1992-2000. Acad Emerg Med. 2004;11(10):1029-1034. PubMed
3. Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347(12):878-885. PubMed
4. Recchia D, Barzilai B. Echocardiography in the evaluation of patients with syncope. J Gen Intern Med. 1995;10(12):649-655. PubMed
5. Mendu ML, McAvay G, Lampert R, Stoehr J, Tinetti ME. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169(14):1299-1305. PubMed
6. Anderson KL, Limkakeng A, Damuth E, Chandra A. Cardiac evaluation for structural abnormalities may not be required in patients presenting with syncope and a normal ECG result in an observation unit setting. Ann Emerg Med. 2012;60(4):478-484.e1. PubMed
7. Chang NL, Shah P, Bajaj S, Virk H, Bikkina M, Shamoon F. Diagnostic Yield of Echocardiography in Syncope Patients with Normal ECG. Cardiol Res Pract. 2016;2016:1251637. PubMed
8. Han SK, Yeom SR, Lee SH, et al. Transthoracic echocardiogram in syncope patients with normal initial evaluation. Am J Emerg Med. 2017;35(2):281-284. PubMed
9. Task Force for the Diagnosis and Management of Syncope, European Society of Cardiology, European Heart Rhythm Association, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30(21):2631-2671.
10. Alboni P, Brignole M, Menozzi C, et al. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol. 2001;37(7):1921-1928. PubMed
11. Strickberger SA, Benson DW, Biaggioni I, et al. AHA/ACCF Scientific Statement on the evaluation of syncope: from the American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke, and the Quality of Care and Outcomes Research Interdisciplinary Working Group; and the American College of Cardiology Foundation: in collaboration with the Heart Rhythm Society: endorsed by the American Autonomic Society. Circulation. 2006;113(2):316-327. PubMed
12. American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance Endorsed by the American College of Chest Physicians. J Am Coll Cardiol. 2011;57(9):1126-1166. PubMed
13. Crane SD. Risk stratification of patients with syncope in an accident and emergency department. Emerg Med J. 2002;19(1):23-27. PubMed
14. Croci F, Brignole M, Alboni P, et al. The application of a standardized strategy of evaluation in patients with syncope referred to three syncope units. Europace. 2002;4(4):351-355. PubMed
15. Sarasin FP, Junod AF, Carballo D, Slama S, Unger PF, Louis-Simonet M. Role of echocardiography in the evaluation of syncope: a prospective study. Heart. 2002;88(4):363-367. PubMed
16. Echocardiogram Cost. http://health.costhelper.com/echocardiograms.html. 2017. Accessed January 26, 2017.
17. Thiruganasambandamoorthy V, Ramaekers R, Rahman MO, et al. Prognostic value of cardiac biomarkers in the risk stratification of syncope: a systematic review. Intern Emerg Med. 2015;10(8):1003-1014. PubMed
18. Pfister R, Diedrichs H, Larbig R, Erdmann E, Schneider CA. NT-pro-BNP for differential diagnosis in patients with syncope. Int J Cardiol. 2009;133(1):51-54. PubMed
19. Reed MJ, Mills NL, Weir CJ. Sensitive troponin assay predicts outcome in syncope. Emerg Med J. 2012;29(12):1001-1003. PubMed
20. Tanimoto K, Yukiiri K, Mizushige K, et al. Usefulness of brain natriuretic peptide as a marker for separating cardiac and noncardiac causes of syncope. Am J Cardiol. 2004;93(2):228-230. PubMed
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Syncope is a common cause of emergency department (ED) visits and hospitalizations. Echocardiogram is frequently used as a diagnostic tool in the evaluation of syncope, performed in 39%-91% of patients.
CLINICAL SCENARIO
A 57-year-old woman presented to the ED after a syncopal episode. She had just eaten dinner when she slumped over and became unresponsive. Her husband estimated that she regained consciousness 30 seconds later and quickly returned to baseline mental status. She denied chest pain, shortness of breath, or palpitations. Her medical history included hypertension and hypothyroidism. Her medication regimen was unchanged.
Vital signs, including orthostatic blood pressures, were within normal ranges. A physical examination revealed regular heart sounds without murmur, rub, or gallop. ECG showed normal sinus rhythm, normal axis, and normal intervals. Chest radiograph, complete blood count, chemistry, pro-brain natriuretic peptide (pro-BNP), and troponin were within normal ranges.
BACKGROUND
Syncope, defined as “abrupt, transient, complete loss of consciousness, associated with inability to maintain postural tone, with rapid and spontaneous recovery,”1 is a common clinical problem, accounting for 1% of ED visits in the United States.2 As syncope has been shown to be associated with increased mortality,3 the primary goal of syncope evaluation is to identify modifiable underlying causes, particularly cardiac causes. Current guidelines recommend a complete history and physical, orthostatic blood pressure measurement, and ECG as the initial evaluation for syncope.1 Echocardiogram is a frequent additional test, performed in 39%-91% of patients.4-8
WHY YOU MAY THINK ECHOCARDIOGRAM IS HELPFUL
Echocardiogram may identify depressed ejection fraction, a risk factor for ventricular arrhythmias, along with structural causes of syncope, including aortic stenosis, pulmonary hypertension, and hypertrophic cardiomyopathy.9 Structural heart disease is the underlying etiology in about 3% of patients with syncope.10
Prior guidelines stated that “an echocardiogram is a helpful screening test if the history, physical examination, and ECG do not provide a diagnosis or if underlying heart disease is suspected.”11 A separate guideline for the appropriate use of echocardiogram assigned a score of appropriateness on a 1-9 scale based on increasing indication.12 Echocardiogram for syncope was scored a 7 in patients with “no other symptoms or signs of cardiovascular disease.”12 Only 25%-40% of patients with syncope will have a cause identified after the history, physical examination, and ECG,13,14 creating diagnostic uncertainty that often leads to further testing.
WHY ECHOCARDIOGRAM IS NOT NECESSARY IN ALL PATIENTS
Mendu et al.5 performed a single-center, retrospective study of the diagnostic yield of testing for syncope in 2106 consecutive patients older than 65 admitted over the course of 5 years. They retrospectively applied the San Francisco Syncope Rule (SFSR), which patients met if they had congestive heart failure, hematocrit <30%, abnormal ECG, shortness of breath, or systolic blood pressure <90 mm Hg. There were 821 patients (39%) who underwent echocardiogram. Among the 488 with no SFSR criteria, 10 patients (2%) had echocardiogram results that affected management, and 4 patients (1%) had results that helped determine the etiology of syncope.
Anderson et al. studied 323 syncope patients in a single ED observation unit over 18 months.6 Patients with high-risk features, including unstable vital signs, abnormal cardiac biomarkers, or ischemic ECG changes, were excluded from the unit. The initial ECG was considered abnormal if it contained arrhythmia, premature atrial or ventricular contractions, pacing, second- or third-degree heart block, or left bundle branch block. Of the 235 patients with a normal ECG who underwent echocardiogram, none had an abnormal study.
Chang et al.7 performed a retrospective review of 468 patients admitted with syncope at a single hospital. Charts were reviewed for ECG and echocardiogram results. Abnormal ECGs were defined as those containing arrhythmias, Q waves, ischemic changes, second- and third-degree heart block, paced rhythm, corrected QT interval (QTc) >500 ms, left bundle branch or bifasicular block, Brugada pattern, or abnormal axis. Among 321 patients with normal ECGs, echocardiograms were performed in 192. Eleven of those echocardiograms were abnormal: 3 demonstrated aortic stenosis in patients who already carried the diagnosis, and the other 8 abnormal echocardiograms revealed unexpected left ventricular ejection fractions <45% or other nonaortic valvular pathology. None of the findings were felt to be the cause of syncope.
Han et al.8 performed a retrospective cohort study of all syncope patients presenting to a single ED over the course of 1 year. Patients were stratified as high risk if they had chest pain, palpitations, a history of cardiac disease (defined as prior arrhythmia, heart failure, coronary artery disease, or structural heart disease), abnormal cardiac biomarkers, or an abnormal ECG (defined as sinus bradycardia, arrhythmia, premature beats, second- or third-degree heart block, ventricular hypertrophy, ischemic Q or ST changes, or abnormal QT interval). Patients with none of those symptoms or findings were considered low risk. Of those categorized as low risk (n = 115), 47 underwent echocardiogram, only 1 of which was abnormal.
Across studies, the percentage of patients with a normal cardiac history, examination, and ECG with new, significant abnormalities on echocardiogram was 0% in 3 studies (n = 340),4,6,15 2% in 1 study (10/488 patients),5 2.1% in 1 study (1/47 patients),8 and 4.2% in 1 study (8/192 patients).7 The 11 echocardiograms with significant findings in the studies by Mendu et al.5 and Han et al.8 were not further described. The 8 patients with abnormal echocardiograms reported by Chang et al.7 had depressed left ventricular ejection fraction or nonaortic valvular disease that did not represent a definitive etiology of their syncope. Given the cost of $1,000 to $2,220 per study,16 routine echocardiograms in patients with a normal history, examination, and ECG would thus require $60,000 to $132,000 in spending to find 1 new significant abnormality, which may be unrelated to the actual cause of syncope.
SITUATIONS IN WHICH ECHOCARDIOGRAM MAY BE HELPFUL
The diagnostic yield of echocardiogram is higher in patients with a positive cardiac history or abnormal ECG. In the prospective study by Sarasin et al.15 a total of 27% of patients with a positive cardiac history or abnormal ECG were found to have an ejection fraction less than or equal to 40%. Other studies reporting percentages of abnormal echocardiograms in patients with abnormal history, ECG, or examination found rates of 8% (26/333),5 20% (7/35),6 28% (27/97),8 and 29% (27/93).7 It should be noted that not all of these abnormalities were felt to be the cause of syncope. For example, Sarasin et al.15 reported that only half of the patients with newly identified depressed ejection fraction were diagnosed with arrhythmia-related syncope. Chang et al7 reported that 6 of the 27 patients (22%) with abnormal ECG and echocardiogram had the cause of syncope established by echocardiogram.
Finally, some syncope patients will have cardiac biomarkers sent in the ED. Han et al.8 found that among patients with syncope, those with abnormal versus normal echocardiogram were more likely to have elevated BNP (70% vs 23%) and troponin (36% vs 12.4%). Thus, obtaining an echocardiogram in patients with syncope and abnormal cardiac biomarkers may be reasonable. It should be noted, however, that while some studies have suggested a role for biomarkers in differentiating cardiac from noncardiac syncope,17-20 current guidelines state that the usefulness of these tests is uncertain.1
WHAT YOU SHOULD DO INSTEAD OF ECHOCARDIOGRAM FOR ALL PATIENTS
Clinicians should carefully screen patients with syncope for abnormal findings suggesting cardiac disease on history, physical examination, and ECG. Relevant cardiac history includes known coronary artery disease, valvular heart disease, arrhythmia, congestive heart failure, and risk factors for cardiac syncope (supplemental Appendix). The definition of abnormal ECG varies among studies, but abnormalities that should prompt an echocardiogram include arrhythmia, premature atrial or ventricular contractions, second- or third-degree heart block, sinus bradycardia, bundle branch or fascicular blocks, left ventricular hypertrophy, ischemic ST or T wave changes, Q waves, or a prolonged QTc interval. New guidelines from the American College of Cardiology state, “Routine cardiac imaging is not useful in the evaluation of patients with syncope unless cardiac etiology is suspected on the basis of an initial evaluation, including history, physical examination, or ECG.”1
RECOMMENDATIONS
- All patients with syncope should receive a complete history, physical examination, orthostatic vital signs, and ECG.
- Perform echocardiogram on patients with syncope and a history of cardiac disease, examination suggestive of structural heart disease or congestive heart failure, or abnormal ECG.
- Echocardiogram may be reasonable in patients with syncope and abnormal cardiac biomarkers.
CONCLUSIONS
While commonly performed as part of syncope evaluations, echocardiogram has a very low diagnostic yield in patients with a normal history, physical, and ECG. The patient described in the initial case scenario would have an extremely low likelihood of having important diagnostic information found on echocardiogram.
Disclosure
The authors have no conflicts of interest relevant to this article.
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Syncope is a common cause of emergency department (ED) visits and hospitalizations. Echocardiogram is frequently used as a diagnostic tool in the evaluation of syncope, performed in 39%-91% of patients.
CLINICAL SCENARIO
A 57-year-old woman presented to the ED after a syncopal episode. She had just eaten dinner when she slumped over and became unresponsive. Her husband estimated that she regained consciousness 30 seconds later and quickly returned to baseline mental status. She denied chest pain, shortness of breath, or palpitations. Her medical history included hypertension and hypothyroidism. Her medication regimen was unchanged.
Vital signs, including orthostatic blood pressures, were within normal ranges. A physical examination revealed regular heart sounds without murmur, rub, or gallop. ECG showed normal sinus rhythm, normal axis, and normal intervals. Chest radiograph, complete blood count, chemistry, pro-brain natriuretic peptide (pro-BNP), and troponin were within normal ranges.
BACKGROUND
Syncope, defined as “abrupt, transient, complete loss of consciousness, associated with inability to maintain postural tone, with rapid and spontaneous recovery,”1 is a common clinical problem, accounting for 1% of ED visits in the United States.2 As syncope has been shown to be associated with increased mortality,3 the primary goal of syncope evaluation is to identify modifiable underlying causes, particularly cardiac causes. Current guidelines recommend a complete history and physical, orthostatic blood pressure measurement, and ECG as the initial evaluation for syncope.1 Echocardiogram is a frequent additional test, performed in 39%-91% of patients.4-8
WHY YOU MAY THINK ECHOCARDIOGRAM IS HELPFUL
Echocardiogram may identify depressed ejection fraction, a risk factor for ventricular arrhythmias, along with structural causes of syncope, including aortic stenosis, pulmonary hypertension, and hypertrophic cardiomyopathy.9 Structural heart disease is the underlying etiology in about 3% of patients with syncope.10
Prior guidelines stated that “an echocardiogram is a helpful screening test if the history, physical examination, and ECG do not provide a diagnosis or if underlying heart disease is suspected.”11 A separate guideline for the appropriate use of echocardiogram assigned a score of appropriateness on a 1-9 scale based on increasing indication.12 Echocardiogram for syncope was scored a 7 in patients with “no other symptoms or signs of cardiovascular disease.”12 Only 25%-40% of patients with syncope will have a cause identified after the history, physical examination, and ECG,13,14 creating diagnostic uncertainty that often leads to further testing.
WHY ECHOCARDIOGRAM IS NOT NECESSARY IN ALL PATIENTS
Mendu et al.5 performed a single-center, retrospective study of the diagnostic yield of testing for syncope in 2106 consecutive patients older than 65 admitted over the course of 5 years. They retrospectively applied the San Francisco Syncope Rule (SFSR), which patients met if they had congestive heart failure, hematocrit <30%, abnormal ECG, shortness of breath, or systolic blood pressure <90 mm Hg. There were 821 patients (39%) who underwent echocardiogram. Among the 488 with no SFSR criteria, 10 patients (2%) had echocardiogram results that affected management, and 4 patients (1%) had results that helped determine the etiology of syncope.
Anderson et al. studied 323 syncope patients in a single ED observation unit over 18 months.6 Patients with high-risk features, including unstable vital signs, abnormal cardiac biomarkers, or ischemic ECG changes, were excluded from the unit. The initial ECG was considered abnormal if it contained arrhythmia, premature atrial or ventricular contractions, pacing, second- or third-degree heart block, or left bundle branch block. Of the 235 patients with a normal ECG who underwent echocardiogram, none had an abnormal study.
Chang et al.7 performed a retrospective review of 468 patients admitted with syncope at a single hospital. Charts were reviewed for ECG and echocardiogram results. Abnormal ECGs were defined as those containing arrhythmias, Q waves, ischemic changes, second- and third-degree heart block, paced rhythm, corrected QT interval (QTc) >500 ms, left bundle branch or bifasicular block, Brugada pattern, or abnormal axis. Among 321 patients with normal ECGs, echocardiograms were performed in 192. Eleven of those echocardiograms were abnormal: 3 demonstrated aortic stenosis in patients who already carried the diagnosis, and the other 8 abnormal echocardiograms revealed unexpected left ventricular ejection fractions <45% or other nonaortic valvular pathology. None of the findings were felt to be the cause of syncope.
Han et al.8 performed a retrospective cohort study of all syncope patients presenting to a single ED over the course of 1 year. Patients were stratified as high risk if they had chest pain, palpitations, a history of cardiac disease (defined as prior arrhythmia, heart failure, coronary artery disease, or structural heart disease), abnormal cardiac biomarkers, or an abnormal ECG (defined as sinus bradycardia, arrhythmia, premature beats, second- or third-degree heart block, ventricular hypertrophy, ischemic Q or ST changes, or abnormal QT interval). Patients with none of those symptoms or findings were considered low risk. Of those categorized as low risk (n = 115), 47 underwent echocardiogram, only 1 of which was abnormal.
Across studies, the percentage of patients with a normal cardiac history, examination, and ECG with new, significant abnormalities on echocardiogram was 0% in 3 studies (n = 340),4,6,15 2% in 1 study (10/488 patients),5 2.1% in 1 study (1/47 patients),8 and 4.2% in 1 study (8/192 patients).7 The 11 echocardiograms with significant findings in the studies by Mendu et al.5 and Han et al.8 were not further described. The 8 patients with abnormal echocardiograms reported by Chang et al.7 had depressed left ventricular ejection fraction or nonaortic valvular disease that did not represent a definitive etiology of their syncope. Given the cost of $1,000 to $2,220 per study,16 routine echocardiograms in patients with a normal history, examination, and ECG would thus require $60,000 to $132,000 in spending to find 1 new significant abnormality, which may be unrelated to the actual cause of syncope.
SITUATIONS IN WHICH ECHOCARDIOGRAM MAY BE HELPFUL
The diagnostic yield of echocardiogram is higher in patients with a positive cardiac history or abnormal ECG. In the prospective study by Sarasin et al.15 a total of 27% of patients with a positive cardiac history or abnormal ECG were found to have an ejection fraction less than or equal to 40%. Other studies reporting percentages of abnormal echocardiograms in patients with abnormal history, ECG, or examination found rates of 8% (26/333),5 20% (7/35),6 28% (27/97),8 and 29% (27/93).7 It should be noted that not all of these abnormalities were felt to be the cause of syncope. For example, Sarasin et al.15 reported that only half of the patients with newly identified depressed ejection fraction were diagnosed with arrhythmia-related syncope. Chang et al7 reported that 6 of the 27 patients (22%) with abnormal ECG and echocardiogram had the cause of syncope established by echocardiogram.
Finally, some syncope patients will have cardiac biomarkers sent in the ED. Han et al.8 found that among patients with syncope, those with abnormal versus normal echocardiogram were more likely to have elevated BNP (70% vs 23%) and troponin (36% vs 12.4%). Thus, obtaining an echocardiogram in patients with syncope and abnormal cardiac biomarkers may be reasonable. It should be noted, however, that while some studies have suggested a role for biomarkers in differentiating cardiac from noncardiac syncope,17-20 current guidelines state that the usefulness of these tests is uncertain.1
WHAT YOU SHOULD DO INSTEAD OF ECHOCARDIOGRAM FOR ALL PATIENTS
Clinicians should carefully screen patients with syncope for abnormal findings suggesting cardiac disease on history, physical examination, and ECG. Relevant cardiac history includes known coronary artery disease, valvular heart disease, arrhythmia, congestive heart failure, and risk factors for cardiac syncope (supplemental Appendix). The definition of abnormal ECG varies among studies, but abnormalities that should prompt an echocardiogram include arrhythmia, premature atrial or ventricular contractions, second- or third-degree heart block, sinus bradycardia, bundle branch or fascicular blocks, left ventricular hypertrophy, ischemic ST or T wave changes, Q waves, or a prolonged QTc interval. New guidelines from the American College of Cardiology state, “Routine cardiac imaging is not useful in the evaluation of patients with syncope unless cardiac etiology is suspected on the basis of an initial evaluation, including history, physical examination, or ECG.”1
RECOMMENDATIONS
- All patients with syncope should receive a complete history, physical examination, orthostatic vital signs, and ECG.
- Perform echocardiogram on patients with syncope and a history of cardiac disease, examination suggestive of structural heart disease or congestive heart failure, or abnormal ECG.
- Echocardiogram may be reasonable in patients with syncope and abnormal cardiac biomarkers.
CONCLUSIONS
While commonly performed as part of syncope evaluations, echocardiogram has a very low diagnostic yield in patients with a normal history, physical, and ECG. The patient described in the initial case scenario would have an extremely low likelihood of having important diagnostic information found on echocardiogram.
Disclosure
The authors have no conflicts of interest relevant to this article.
1. Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70(5):620-633. PubMed
2. Sun BC, Emond JA, Camargo CA Jr. Characteristics and admission patterns of patients presenting with syncope to U.S. emergency departments, 1992-2000. Acad Emerg Med. 2004;11(10):1029-1034. PubMed
3. Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347(12):878-885. PubMed
4. Recchia D, Barzilai B. Echocardiography in the evaluation of patients with syncope. J Gen Intern Med. 1995;10(12):649-655. PubMed
5. Mendu ML, McAvay G, Lampert R, Stoehr J, Tinetti ME. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169(14):1299-1305. PubMed
6. Anderson KL, Limkakeng A, Damuth E, Chandra A. Cardiac evaluation for structural abnormalities may not be required in patients presenting with syncope and a normal ECG result in an observation unit setting. Ann Emerg Med. 2012;60(4):478-484.e1. PubMed
7. Chang NL, Shah P, Bajaj S, Virk H, Bikkina M, Shamoon F. Diagnostic Yield of Echocardiography in Syncope Patients with Normal ECG. Cardiol Res Pract. 2016;2016:1251637. PubMed
8. Han SK, Yeom SR, Lee SH, et al. Transthoracic echocardiogram in syncope patients with normal initial evaluation. Am J Emerg Med. 2017;35(2):281-284. PubMed
9. Task Force for the Diagnosis and Management of Syncope, European Society of Cardiology, European Heart Rhythm Association, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30(21):2631-2671.
10. Alboni P, Brignole M, Menozzi C, et al. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol. 2001;37(7):1921-1928. PubMed
11. Strickberger SA, Benson DW, Biaggioni I, et al. AHA/ACCF Scientific Statement on the evaluation of syncope: from the American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke, and the Quality of Care and Outcomes Research Interdisciplinary Working Group; and the American College of Cardiology Foundation: in collaboration with the Heart Rhythm Society: endorsed by the American Autonomic Society. Circulation. 2006;113(2):316-327. PubMed
12. American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance Endorsed by the American College of Chest Physicians. J Am Coll Cardiol. 2011;57(9):1126-1166. PubMed
13. Crane SD. Risk stratification of patients with syncope in an accident and emergency department. Emerg Med J. 2002;19(1):23-27. PubMed
14. Croci F, Brignole M, Alboni P, et al. The application of a standardized strategy of evaluation in patients with syncope referred to three syncope units. Europace. 2002;4(4):351-355. PubMed
15. Sarasin FP, Junod AF, Carballo D, Slama S, Unger PF, Louis-Simonet M. Role of echocardiography in the evaluation of syncope: a prospective study. Heart. 2002;88(4):363-367. PubMed
16. Echocardiogram Cost. http://health.costhelper.com/echocardiograms.html. 2017. Accessed January 26, 2017.
17. Thiruganasambandamoorthy V, Ramaekers R, Rahman MO, et al. Prognostic value of cardiac biomarkers in the risk stratification of syncope: a systematic review. Intern Emerg Med. 2015;10(8):1003-1014. PubMed
18. Pfister R, Diedrichs H, Larbig R, Erdmann E, Schneider CA. NT-pro-BNP for differential diagnosis in patients with syncope. Int J Cardiol. 2009;133(1):51-54. PubMed
19. Reed MJ, Mills NL, Weir CJ. Sensitive troponin assay predicts outcome in syncope. Emerg Med J. 2012;29(12):1001-1003. PubMed
20. Tanimoto K, Yukiiri K, Mizushige K, et al. Usefulness of brain natriuretic peptide as a marker for separating cardiac and noncardiac causes of syncope. Am J Cardiol. 2004;93(2):228-230. PubMed
1. Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70(5):620-633. PubMed
2. Sun BC, Emond JA, Camargo CA Jr. Characteristics and admission patterns of patients presenting with syncope to U.S. emergency departments, 1992-2000. Acad Emerg Med. 2004;11(10):1029-1034. PubMed
3. Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347(12):878-885. PubMed
4. Recchia D, Barzilai B. Echocardiography in the evaluation of patients with syncope. J Gen Intern Med. 1995;10(12):649-655. PubMed
5. Mendu ML, McAvay G, Lampert R, Stoehr J, Tinetti ME. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169(14):1299-1305. PubMed
6. Anderson KL, Limkakeng A, Damuth E, Chandra A. Cardiac evaluation for structural abnormalities may not be required in patients presenting with syncope and a normal ECG result in an observation unit setting. Ann Emerg Med. 2012;60(4):478-484.e1. PubMed
7. Chang NL, Shah P, Bajaj S, Virk H, Bikkina M, Shamoon F. Diagnostic Yield of Echocardiography in Syncope Patients with Normal ECG. Cardiol Res Pract. 2016;2016:1251637. PubMed
8. Han SK, Yeom SR, Lee SH, et al. Transthoracic echocardiogram in syncope patients with normal initial evaluation. Am J Emerg Med. 2017;35(2):281-284. PubMed
9. Task Force for the Diagnosis and Management of Syncope, European Society of Cardiology, European Heart Rhythm Association, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30(21):2631-2671.
10. Alboni P, Brignole M, Menozzi C, et al. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol. 2001;37(7):1921-1928. PubMed
11. Strickberger SA, Benson DW, Biaggioni I, et al. AHA/ACCF Scientific Statement on the evaluation of syncope: from the American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke, and the Quality of Care and Outcomes Research Interdisciplinary Working Group; and the American College of Cardiology Foundation: in collaboration with the Heart Rhythm Society: endorsed by the American Autonomic Society. Circulation. 2006;113(2):316-327. PubMed
12. American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance Endorsed by the American College of Chest Physicians. J Am Coll Cardiol. 2011;57(9):1126-1166. PubMed
13. Crane SD. Risk stratification of patients with syncope in an accident and emergency department. Emerg Med J. 2002;19(1):23-27. PubMed
14. Croci F, Brignole M, Alboni P, et al. The application of a standardized strategy of evaluation in patients with syncope referred to three syncope units. Europace. 2002;4(4):351-355. PubMed
15. Sarasin FP, Junod AF, Carballo D, Slama S, Unger PF, Louis-Simonet M. Role of echocardiography in the evaluation of syncope: a prospective study. Heart. 2002;88(4):363-367. PubMed
16. Echocardiogram Cost. http://health.costhelper.com/echocardiograms.html. 2017. Accessed January 26, 2017.
17. Thiruganasambandamoorthy V, Ramaekers R, Rahman MO, et al. Prognostic value of cardiac biomarkers in the risk stratification of syncope: a systematic review. Intern Emerg Med. 2015;10(8):1003-1014. PubMed
18. Pfister R, Diedrichs H, Larbig R, Erdmann E, Schneider CA. NT-pro-BNP for differential diagnosis in patients with syncope. Int J Cardiol. 2009;133(1):51-54. PubMed
19. Reed MJ, Mills NL, Weir CJ. Sensitive troponin assay predicts outcome in syncope. Emerg Med J. 2012;29(12):1001-1003. PubMed
20. Tanimoto K, Yukiiri K, Mizushige K, et al. Usefulness of brain natriuretic peptide as a marker for separating cardiac and noncardiac causes of syncope. Am J Cardiol. 2004;93(2):228-230. PubMed
© 2017 Society of Hospital Medicine
Things We Do For No Reason: Against Medical Advice Discharges
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Against medical advice (AMA) discharges, which account for up to 2% of all inpatient discharges, are associated with worse health and health services outcomes and disproportionately affect vulnerable patient populations. This paper will review the background data on AMA discharges as well as the reasons physicians may choose to discharge patients AMA. From a healthcare quality perspective, the designation of a discharge as AMA is low-value care in that it is a routine hospital practice without demonstrated benefit and is not supported by a strong evidence base. We argue that designating discharges as AMA has never been shown to advance patient care and that it has the potential to harm patients by reducing access to care and promoting stigma. We believe that greater attention to both shared decision-making as well as harm reduction principles in discharge planning can serve as effective, patient-centered alternatives when patients choose not to follow a healthcare professional’s recommended advice.
CASE PRESENTATION
A 54-year-old man with active intravenous (IV) drug use and hepatitis C was admitted with lower extremity cellulitis. On hospital day 2, the patient insisted that he wanted to go home. The treatment team informed the patient that an additional 2-3 days of IV antibiotics would produce a more reliable cure and reduce the risk of readmission. Should the team inform the patient that he will be discharged against medical advice (AMA) if he chooses to leave the hospital prematurely?
BACKGROUND
In the United States, patients are discharged AMA approximately 500,000 times per year (1%-2% of all discharges).1 These discharges represent a wide array of clinical scenarios that all culminate in the formal recognition and documentation of a competent patient’s choice to decline further inpatient medical care and leave the hospital prior to a recommended clinical endpoint. Compared with standard discharges, AMA discharges are associated with an increased adjusted relative risk of 30-day mortality as high as 10% and 30-day readmission rates that are 20%-40% higher than readmission rates following standard discharges.2 AMA discharges are more likely among patients with substance use disorders, psychiatric illness, and HIV.3
WHY YOU MIGHT THINK AMA DISCHARGES ARE HELPFUL
Although there are little empirical data to inform how and why physicians choose to designate a discharge as AMA when patients decline recommended care, the existing evidence suggests that fears of legal liability are strongly driving the practice.4 Physicians may believe that they must discharge patients AMA in order to fulfill their legal and ethical responsibilities, or to demonstrate in writing the physician’s concern and the significant risk of leaving.5,6 Clinicians may have been acculturated during training to believe that an AMA discharge may also be seen as a way of formally distancing themselves from the patient’s request for a nonstandard or unsafe discharge plan, thus deflecting any potential blame for worse patient outcomes.
Finally, clinicians and administrators may also believe that an AMA discharge is the appropriate designation for a hospital stay that ended because the patient chose to prematurely discontinue the treatment relationship or to decline the postdischarge placement recommendations. This reasoning may explain why the hospital penalties authorized by Medicare’s Hospital Readmission Reduction Program generally exclude initial admissions ending in an AMA discharge7 and may provide the rationale (and perhaps a financial incentive) to discharge patients AMA in order to limit CMS readmission penalties.
WHY AMA DISCHARGES ADD NO VALUE TO A PATIENT’S FULLY INFORMED DECLINATION OF CARE
The AMA discharge is a routine hospital practice without demonstrated patient benefit and which disproportionately affects vulnerable populations. There is also a growing literature that demonstrates that AMA discharges stigmatize patients, reduce their access to care, and can reduce the quality of informed consent discussions in discharge planning.8-10 Although there are no conclusive data that AMA discharges are more likely among underrepresented racial minorities, the disproportionate burden of AMA discharges and their worse health outcomes are borne by the homeless, those with substance use disorders, and the uninsured.3,11
Compared to patients discharged conventionally from an emergency department, 25% of patients discharged AMA reported not wanting to return for follow-up care.8 This reluctance to return for care is in part mediated by provider-generated stigma and blame9,12 and may be exacerbated when patients believe that their decision to leave AMA was based upon extenuating circumstance or competing necessity (eg, limited care options for their dependents, poor quality hospital care, etc.).
To persuade patients to remain hospitalized, 85% of trainees and 67% of attending physicians in one study incorrectly informed their patients that insurance will not reimburse a hospitalization if they leave AMA.13 Because this study demonstrated that there is no empirical evidence that payment after AMA discharges is denied by private or government payers, physicians sharing this misinformation can breed distrust and coercively undermine patients’ ability to make a voluntary choice.
When clinicians assert they are bound by duty to discharge a patient AMA, they may be conflating a presumed legal obligation to formally designate the discharge as AMA in the medical record with their actual obligation to obtain the patient’s informed consent for the discharge. In other words, there is no identifiable medico-legal requirement to specifically designate a discharge as AMA.
Although clinicians may presume that the AMA designation provides protection from liability, the claim is not supported by the available literature.14,15 In these studies, which reviewed relevant case law, defendants prevailed not because of the physician’s AMA designation, but because the plaintiff was not able to prove negligence. The proper execution of the discharge process, not the specific designation of AMA, is what conferred liability protection.5 Indeed, malpractice claims, which are associated with patient perceptions of feeling deserted or devalued,16 might be more likely with AMA discharges when they result from flawed and stigmatizing communication processes.17
Finally, there are no clinical, regulatory, or professional standards that specify the designation of an AMA discharge. Neither the Joint Commission nor any other professional organization specify under what conditions a clinician should discharge a patient AMA, thus promoting wide variability in its use and further limiting it as a valid and reliable healthcare metric.
WHAT SHOULD PHYSICIANS DO INSTEAD: AVOID THE AMA DESIGNATION AND PROMOTE SHARED DECISION-MAKING AND HARM REDUCTION
Because all competent patients have the right to decline recommended inpatient treatment, the ethical and legal standard is that the physician obtain the patient’s informed consent to leave by communicating the risks, benefits, and alternatives to leaving and fully documenting the conversation in the medical record.2 The additional steps of formalizing the discharge as AMA and providing AMA forms for the patient to sign have never been demonstrated to improve quality (and add needless clerical work). When declining any treatment, even life-sustaining treatment, the request for a patient signature to decline such treatment has not been demonstrated to improve risk communication and is not considered a best practice for informed consent.18 When the physician’s motives for this behavior are punitive or directed primarily at reducing liability, it may distract the physician from their fiduciary duty to put patients first.
The solution to improve quality is straightforward—avoid designating discharges as AMA. Instead, clinicians should maintain a single discharge process with clear, objective documentation including providing appropriate prescriptions and follow-up appointments regardless of whether the patient’s choice is consistent with a physician’s recommendation. In its place, the physician should use shared decision-making (SDM) and harm reduction principles to enhance the patient’s well-being within the identified constraints. SDM involves physicians and patients making healthcare decisions together by combining the patients’ values and preferences for care with the physicians’ expertise and knowledge of medical evidence. Harm reduction practices seek to reduce the adverse health consequences that may come from unhealthy behaviors while assuming that patients will likely continue such behaviors. Evidence-based and widely accepted examples of harm reduction strategies include nicotine replacement therapy and needle exchange programs.19
SDM in discharge planning provides a range of discharge and transitional care options that are within prevailing medical standards, not simply a single recommendation that prioritizes health promotion to the exclusion of other identified patient goals. Quality discharge planning should provide the “right care for the right patient at the right time”20 that moves beyond the false choice of either remaining in the hospital under the conditions specified by the physician or leaving AMA. Although physicians are understandably concerned about patients making choices that do not prioritize their health, physicians can consider the evidence for harm reduction programs’ effectiveness in improving health outcomes21 and accommodate patients by providing harm-reducing discharge options that, while suboptimal, may not be substandard.22
Physicians who wish to promote stronger patient-centered discharge practices may find that avoiding or limiting AMA discharges may conflict with their institution’s policy. In those cases, physicians should work closely with their leadership and legal counsel to ensure that any proposed practice changes are legally compliant but also improve SDM and reduce stigma for this population.
Although ending the clinical practice of designating discharges as AMA is unlikely to completely ameliorate the morbidity and costs associated with patients declining episodes of inpatient care, there is reasonable face validity to conclude that replacing the AMA practice with greater attention to harm reduction and SDM can reduce some of the preventable harms like stigmatization and reduced access to care. Together, these practices demonstrate the profession’s continued commitment to the public to practice patient-centered care.
RECOMMENDATIONS
- Treat all discharges similarly. Avoid designating an inpatient discharge as AMA.
- Ensure there is objective documentation of the patient’s informed choice to leave the hospital.
- When patients wish to leave the hospital prior to a physician-recommended clinical endpoint, engage in SDM with a focus on providing all medically reasonable treatment options that promote harm reduction.
- If you choose to designate a discharge as AMA, approach the discharge planning process consistently and with patient-centered principles by optimizing SDM and harm reduction.
CONCLUSION
The physician informed the patient of the risks, benefits, and alternatives to leaving the hospital prior to the completion of IV antibiotics and confirmed the patient’s decision-making capacity. Next, the physician elicited the patient’s preferences for care and identified competing priorities. The patient wanted treatment for his cellulitis, but he was experiencing pain and opioid withdrawal. The physician then expanded the range of potential treatment options, including evaluation for medication-assisted treatment for the patient’s opioid use disorder (OUD) and harm reduction measures such as safer injection practices, needle exchange, housing assistance, and overdose prevention and treatment education.23 An alternative harm-reducing option included discharge with oral antibiotics and follow-up with his primary physician in 48-72 hours. After the patient indicated that he wanted to leave because he was not yet ready for OUD treatment, he was discharged with the standard discharge paperwork and antibiotics, and the physician documented the informed consent discussion.
Disclosure
The authors report no conflicts of interest, financial or otherwise. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs, the VA National Center for Ethics in Health Care or the US Government.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org
1. Ibrahim SA, Kwoh CK, Krishnan E. Factors associated with patients who leave acute-care hospitals against medical advice. Am J Public Health. 2007;97(12):2204-2208. PubMed
2. Alfandre DJ. “I’m going home”: discharges against medical advice. Mayo Clin Proc. 2009;84(3):255-260. PubMed
3. Kraut A, Fransoo R, Olafson K, Ramsey CD, Yogendran M, Garland A. A population-based analysis of leaving the hospital against medical advice: incidence and associated variables. BMC Health Serv Res. 2013;13:415. PubMed
4. Green P, Watts D, Poole S, Dhopesh V. Why patients sign out against medical advice (AMA): factors motivating patients to sign out AMA. Am J Drug Alcohol Abuse. 2004;30(2):489-493. PubMed
5. Levy F, Mareiniss DP, Iacovelli C. The Importance of a Proper Against-Medical-Advice (AMA) Discharge: How Signing Out AMA May Create Significant Liability Protection for Providers. J Emerg Med. 2012;43(3):516-520. PubMed
6. Brenner J, Joslin J, Goulette A, Grant WD, Wojcik SM. Against Medical Advice: A Survey of ED Clinicians’ Rationale for Use. J Emerg Nurs. 2016;42(5):408-411. PubMed
7. Hospital-Wide (All-Condition) 30-Day Risk-Standardized Readmission Measure. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/downloads/MMSHospital-WideAll-ConditionReadmissionRate.pdf. Accessed on July 22, 2016.
8. Jerrard DA, Chasm RM. Patients leaving against medical advice (AMA) from the emergency department--disease prevalence and willingness to return. J Emerg Med. 2011;41(4):412-417. PubMed
9. Haywood C, Jr, Lanzkron S, Hughes MT, et al. A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med. 2011;26(5):518-523. PubMed
10. Wigder HN, Propp DA, Leslie K, Mathew A. Insurance companies refusing payment for patients who leave the emergency department against medical advice is a myth. Ann Emerg Med. 2010;55(4):393. PubMed
11. Saab D, Nisenbaum R, Dhalla I, Hwang SW. Hospital Readmissions in a Community-based Sample of Homeless Adults: a Matched-cohort Study. J Gen Intern Med. 2016;31(9):1011-1018. PubMed
12. Lekas HM, Alfandre D, Gordon P, Harwood K, Yin MT. The role of patient-provider interactions: Using an accounts framework to explain hospital discharges against medical advice. Soc Sci Med. 2016;156:106-113. PubMed
13. Schaefer GR, Matus H, Schumann JH, et al. Financial Responsibility of Hospitalized Patients Who Left Against Medical Advice: Medical Urban Legend? J Gen Intern Med. 2012;27(7):825-830. PubMed
14. Devitt PJ, Devitt AC, Dewan M. Does identifying a discharge as “against medical advice” confer legal protection? J Fam Pract. 2000;49(3):224-227. PubMed
15. Devitt PJ, Devitt AC, Dewan M. An examination of whether discharging patients against medical advice protects physicians from malpractice charges. Psychiatr Serv. 2000;51(7):899-902. PubMed
16. Beckman HB, Markakis KM, Suchman AL, Frankel RM. The doctor-patient relationship and malpractice. Lessons from plaintiff depositions. Arch Intern Med. 1994;154(12):1365-1370. PubMed
17. Windish DM, Ratanawongsa N. Providers’ perceptions of relationships and professional roles when caring for patients who leave the hospital against medical advice. J Gen Intern Med. 2008;23(10):1698-1707. PubMed
18. Sulmasy DP, Sood JR, Texiera K, McAuley RL, McGugins J, Ury WA. A prospective trial of a new policy eliminating signed consent for do not resuscitate orders. J Gen Intern Med. 2006;21(12):1261-1268. PubMed
19. Stratton K, Shetty P, Wallace R, Bondurant S. Clearing the smoke: the science base for tobacco harm reduction--executive summary. Tob Control. 2001;10(2):189-195. PubMed
20. What is Health Care Quality and Who Decides?. March 2009. Agency for Healthcare Research and Quality, Rockville, MD. https://archive.ahrq.gov/news/speech/test031809.html
21. Hobden KL, Cunningham JA. Barriers to the dissemination of four harm reduction strategies: a survey of addiction treatment providers in Ontario. Harm Reduct J. 2006;3:35. PubMed
22. Alfandre D. Clinical Recommendations in Medical Practice: A Proposed Framework to Reduce Bias and Improve the Quality of Medical Decisions. J Clin Ethics. 2016;27(1):21-27. PubMed
23. Fanucchi L, Lofwall MR. Putting Parity into Practice - Integrating Opioid-Use Disorder Treatment into the Hospital Setting. N Engl J Med. 2016;375(9):811-813. PubMed
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Against medical advice (AMA) discharges, which account for up to 2% of all inpatient discharges, are associated with worse health and health services outcomes and disproportionately affect vulnerable patient populations. This paper will review the background data on AMA discharges as well as the reasons physicians may choose to discharge patients AMA. From a healthcare quality perspective, the designation of a discharge as AMA is low-value care in that it is a routine hospital practice without demonstrated benefit and is not supported by a strong evidence base. We argue that designating discharges as AMA has never been shown to advance patient care and that it has the potential to harm patients by reducing access to care and promoting stigma. We believe that greater attention to both shared decision-making as well as harm reduction principles in discharge planning can serve as effective, patient-centered alternatives when patients choose not to follow a healthcare professional’s recommended advice.
CASE PRESENTATION
A 54-year-old man with active intravenous (IV) drug use and hepatitis C was admitted with lower extremity cellulitis. On hospital day 2, the patient insisted that he wanted to go home. The treatment team informed the patient that an additional 2-3 days of IV antibiotics would produce a more reliable cure and reduce the risk of readmission. Should the team inform the patient that he will be discharged against medical advice (AMA) if he chooses to leave the hospital prematurely?
BACKGROUND
In the United States, patients are discharged AMA approximately 500,000 times per year (1%-2% of all discharges).1 These discharges represent a wide array of clinical scenarios that all culminate in the formal recognition and documentation of a competent patient’s choice to decline further inpatient medical care and leave the hospital prior to a recommended clinical endpoint. Compared with standard discharges, AMA discharges are associated with an increased adjusted relative risk of 30-day mortality as high as 10% and 30-day readmission rates that are 20%-40% higher than readmission rates following standard discharges.2 AMA discharges are more likely among patients with substance use disorders, psychiatric illness, and HIV.3
WHY YOU MIGHT THINK AMA DISCHARGES ARE HELPFUL
Although there are little empirical data to inform how and why physicians choose to designate a discharge as AMA when patients decline recommended care, the existing evidence suggests that fears of legal liability are strongly driving the practice.4 Physicians may believe that they must discharge patients AMA in order to fulfill their legal and ethical responsibilities, or to demonstrate in writing the physician’s concern and the significant risk of leaving.5,6 Clinicians may have been acculturated during training to believe that an AMA discharge may also be seen as a way of formally distancing themselves from the patient’s request for a nonstandard or unsafe discharge plan, thus deflecting any potential blame for worse patient outcomes.
Finally, clinicians and administrators may also believe that an AMA discharge is the appropriate designation for a hospital stay that ended because the patient chose to prematurely discontinue the treatment relationship or to decline the postdischarge placement recommendations. This reasoning may explain why the hospital penalties authorized by Medicare’s Hospital Readmission Reduction Program generally exclude initial admissions ending in an AMA discharge7 and may provide the rationale (and perhaps a financial incentive) to discharge patients AMA in order to limit CMS readmission penalties.
WHY AMA DISCHARGES ADD NO VALUE TO A PATIENT’S FULLY INFORMED DECLINATION OF CARE
The AMA discharge is a routine hospital practice without demonstrated patient benefit and which disproportionately affects vulnerable populations. There is also a growing literature that demonstrates that AMA discharges stigmatize patients, reduce their access to care, and can reduce the quality of informed consent discussions in discharge planning.8-10 Although there are no conclusive data that AMA discharges are more likely among underrepresented racial minorities, the disproportionate burden of AMA discharges and their worse health outcomes are borne by the homeless, those with substance use disorders, and the uninsured.3,11
Compared to patients discharged conventionally from an emergency department, 25% of patients discharged AMA reported not wanting to return for follow-up care.8 This reluctance to return for care is in part mediated by provider-generated stigma and blame9,12 and may be exacerbated when patients believe that their decision to leave AMA was based upon extenuating circumstance or competing necessity (eg, limited care options for their dependents, poor quality hospital care, etc.).
To persuade patients to remain hospitalized, 85% of trainees and 67% of attending physicians in one study incorrectly informed their patients that insurance will not reimburse a hospitalization if they leave AMA.13 Because this study demonstrated that there is no empirical evidence that payment after AMA discharges is denied by private or government payers, physicians sharing this misinformation can breed distrust and coercively undermine patients’ ability to make a voluntary choice.
When clinicians assert they are bound by duty to discharge a patient AMA, they may be conflating a presumed legal obligation to formally designate the discharge as AMA in the medical record with their actual obligation to obtain the patient’s informed consent for the discharge. In other words, there is no identifiable medico-legal requirement to specifically designate a discharge as AMA.
Although clinicians may presume that the AMA designation provides protection from liability, the claim is not supported by the available literature.14,15 In these studies, which reviewed relevant case law, defendants prevailed not because of the physician’s AMA designation, but because the plaintiff was not able to prove negligence. The proper execution of the discharge process, not the specific designation of AMA, is what conferred liability protection.5 Indeed, malpractice claims, which are associated with patient perceptions of feeling deserted or devalued,16 might be more likely with AMA discharges when they result from flawed and stigmatizing communication processes.17
Finally, there are no clinical, regulatory, or professional standards that specify the designation of an AMA discharge. Neither the Joint Commission nor any other professional organization specify under what conditions a clinician should discharge a patient AMA, thus promoting wide variability in its use and further limiting it as a valid and reliable healthcare metric.
WHAT SHOULD PHYSICIANS DO INSTEAD: AVOID THE AMA DESIGNATION AND PROMOTE SHARED DECISION-MAKING AND HARM REDUCTION
Because all competent patients have the right to decline recommended inpatient treatment, the ethical and legal standard is that the physician obtain the patient’s informed consent to leave by communicating the risks, benefits, and alternatives to leaving and fully documenting the conversation in the medical record.2 The additional steps of formalizing the discharge as AMA and providing AMA forms for the patient to sign have never been demonstrated to improve quality (and add needless clerical work). When declining any treatment, even life-sustaining treatment, the request for a patient signature to decline such treatment has not been demonstrated to improve risk communication and is not considered a best practice for informed consent.18 When the physician’s motives for this behavior are punitive or directed primarily at reducing liability, it may distract the physician from their fiduciary duty to put patients first.
The solution to improve quality is straightforward—avoid designating discharges as AMA. Instead, clinicians should maintain a single discharge process with clear, objective documentation including providing appropriate prescriptions and follow-up appointments regardless of whether the patient’s choice is consistent with a physician’s recommendation. In its place, the physician should use shared decision-making (SDM) and harm reduction principles to enhance the patient’s well-being within the identified constraints. SDM involves physicians and patients making healthcare decisions together by combining the patients’ values and preferences for care with the physicians’ expertise and knowledge of medical evidence. Harm reduction practices seek to reduce the adverse health consequences that may come from unhealthy behaviors while assuming that patients will likely continue such behaviors. Evidence-based and widely accepted examples of harm reduction strategies include nicotine replacement therapy and needle exchange programs.19
SDM in discharge planning provides a range of discharge and transitional care options that are within prevailing medical standards, not simply a single recommendation that prioritizes health promotion to the exclusion of other identified patient goals. Quality discharge planning should provide the “right care for the right patient at the right time”20 that moves beyond the false choice of either remaining in the hospital under the conditions specified by the physician or leaving AMA. Although physicians are understandably concerned about patients making choices that do not prioritize their health, physicians can consider the evidence for harm reduction programs’ effectiveness in improving health outcomes21 and accommodate patients by providing harm-reducing discharge options that, while suboptimal, may not be substandard.22
Physicians who wish to promote stronger patient-centered discharge practices may find that avoiding or limiting AMA discharges may conflict with their institution’s policy. In those cases, physicians should work closely with their leadership and legal counsel to ensure that any proposed practice changes are legally compliant but also improve SDM and reduce stigma for this population.
Although ending the clinical practice of designating discharges as AMA is unlikely to completely ameliorate the morbidity and costs associated with patients declining episodes of inpatient care, there is reasonable face validity to conclude that replacing the AMA practice with greater attention to harm reduction and SDM can reduce some of the preventable harms like stigmatization and reduced access to care. Together, these practices demonstrate the profession’s continued commitment to the public to practice patient-centered care.
RECOMMENDATIONS
- Treat all discharges similarly. Avoid designating an inpatient discharge as AMA.
- Ensure there is objective documentation of the patient’s informed choice to leave the hospital.
- When patients wish to leave the hospital prior to a physician-recommended clinical endpoint, engage in SDM with a focus on providing all medically reasonable treatment options that promote harm reduction.
- If you choose to designate a discharge as AMA, approach the discharge planning process consistently and with patient-centered principles by optimizing SDM and harm reduction.
CONCLUSION
The physician informed the patient of the risks, benefits, and alternatives to leaving the hospital prior to the completion of IV antibiotics and confirmed the patient’s decision-making capacity. Next, the physician elicited the patient’s preferences for care and identified competing priorities. The patient wanted treatment for his cellulitis, but he was experiencing pain and opioid withdrawal. The physician then expanded the range of potential treatment options, including evaluation for medication-assisted treatment for the patient’s opioid use disorder (OUD) and harm reduction measures such as safer injection practices, needle exchange, housing assistance, and overdose prevention and treatment education.23 An alternative harm-reducing option included discharge with oral antibiotics and follow-up with his primary physician in 48-72 hours. After the patient indicated that he wanted to leave because he was not yet ready for OUD treatment, he was discharged with the standard discharge paperwork and antibiotics, and the physician documented the informed consent discussion.
Disclosure
The authors report no conflicts of interest, financial or otherwise. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs, the VA National Center for Ethics in Health Care or the US Government.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Against medical advice (AMA) discharges, which account for up to 2% of all inpatient discharges, are associated with worse health and health services outcomes and disproportionately affect vulnerable patient populations. This paper will review the background data on AMA discharges as well as the reasons physicians may choose to discharge patients AMA. From a healthcare quality perspective, the designation of a discharge as AMA is low-value care in that it is a routine hospital practice without demonstrated benefit and is not supported by a strong evidence base. We argue that designating discharges as AMA has never been shown to advance patient care and that it has the potential to harm patients by reducing access to care and promoting stigma. We believe that greater attention to both shared decision-making as well as harm reduction principles in discharge planning can serve as effective, patient-centered alternatives when patients choose not to follow a healthcare professional’s recommended advice.
CASE PRESENTATION
A 54-year-old man with active intravenous (IV) drug use and hepatitis C was admitted with lower extremity cellulitis. On hospital day 2, the patient insisted that he wanted to go home. The treatment team informed the patient that an additional 2-3 days of IV antibiotics would produce a more reliable cure and reduce the risk of readmission. Should the team inform the patient that he will be discharged against medical advice (AMA) if he chooses to leave the hospital prematurely?
BACKGROUND
In the United States, patients are discharged AMA approximately 500,000 times per year (1%-2% of all discharges).1 These discharges represent a wide array of clinical scenarios that all culminate in the formal recognition and documentation of a competent patient’s choice to decline further inpatient medical care and leave the hospital prior to a recommended clinical endpoint. Compared with standard discharges, AMA discharges are associated with an increased adjusted relative risk of 30-day mortality as high as 10% and 30-day readmission rates that are 20%-40% higher than readmission rates following standard discharges.2 AMA discharges are more likely among patients with substance use disorders, psychiatric illness, and HIV.3
WHY YOU MIGHT THINK AMA DISCHARGES ARE HELPFUL
Although there are little empirical data to inform how and why physicians choose to designate a discharge as AMA when patients decline recommended care, the existing evidence suggests that fears of legal liability are strongly driving the practice.4 Physicians may believe that they must discharge patients AMA in order to fulfill their legal and ethical responsibilities, or to demonstrate in writing the physician’s concern and the significant risk of leaving.5,6 Clinicians may have been acculturated during training to believe that an AMA discharge may also be seen as a way of formally distancing themselves from the patient’s request for a nonstandard or unsafe discharge plan, thus deflecting any potential blame for worse patient outcomes.
Finally, clinicians and administrators may also believe that an AMA discharge is the appropriate designation for a hospital stay that ended because the patient chose to prematurely discontinue the treatment relationship or to decline the postdischarge placement recommendations. This reasoning may explain why the hospital penalties authorized by Medicare’s Hospital Readmission Reduction Program generally exclude initial admissions ending in an AMA discharge7 and may provide the rationale (and perhaps a financial incentive) to discharge patients AMA in order to limit CMS readmission penalties.
WHY AMA DISCHARGES ADD NO VALUE TO A PATIENT’S FULLY INFORMED DECLINATION OF CARE
The AMA discharge is a routine hospital practice without demonstrated patient benefit and which disproportionately affects vulnerable populations. There is also a growing literature that demonstrates that AMA discharges stigmatize patients, reduce their access to care, and can reduce the quality of informed consent discussions in discharge planning.8-10 Although there are no conclusive data that AMA discharges are more likely among underrepresented racial minorities, the disproportionate burden of AMA discharges and their worse health outcomes are borne by the homeless, those with substance use disorders, and the uninsured.3,11
Compared to patients discharged conventionally from an emergency department, 25% of patients discharged AMA reported not wanting to return for follow-up care.8 This reluctance to return for care is in part mediated by provider-generated stigma and blame9,12 and may be exacerbated when patients believe that their decision to leave AMA was based upon extenuating circumstance or competing necessity (eg, limited care options for their dependents, poor quality hospital care, etc.).
To persuade patients to remain hospitalized, 85% of trainees and 67% of attending physicians in one study incorrectly informed their patients that insurance will not reimburse a hospitalization if they leave AMA.13 Because this study demonstrated that there is no empirical evidence that payment after AMA discharges is denied by private or government payers, physicians sharing this misinformation can breed distrust and coercively undermine patients’ ability to make a voluntary choice.
When clinicians assert they are bound by duty to discharge a patient AMA, they may be conflating a presumed legal obligation to formally designate the discharge as AMA in the medical record with their actual obligation to obtain the patient’s informed consent for the discharge. In other words, there is no identifiable medico-legal requirement to specifically designate a discharge as AMA.
Although clinicians may presume that the AMA designation provides protection from liability, the claim is not supported by the available literature.14,15 In these studies, which reviewed relevant case law, defendants prevailed not because of the physician’s AMA designation, but because the plaintiff was not able to prove negligence. The proper execution of the discharge process, not the specific designation of AMA, is what conferred liability protection.5 Indeed, malpractice claims, which are associated with patient perceptions of feeling deserted or devalued,16 might be more likely with AMA discharges when they result from flawed and stigmatizing communication processes.17
Finally, there are no clinical, regulatory, or professional standards that specify the designation of an AMA discharge. Neither the Joint Commission nor any other professional organization specify under what conditions a clinician should discharge a patient AMA, thus promoting wide variability in its use and further limiting it as a valid and reliable healthcare metric.
WHAT SHOULD PHYSICIANS DO INSTEAD: AVOID THE AMA DESIGNATION AND PROMOTE SHARED DECISION-MAKING AND HARM REDUCTION
Because all competent patients have the right to decline recommended inpatient treatment, the ethical and legal standard is that the physician obtain the patient’s informed consent to leave by communicating the risks, benefits, and alternatives to leaving and fully documenting the conversation in the medical record.2 The additional steps of formalizing the discharge as AMA and providing AMA forms for the patient to sign have never been demonstrated to improve quality (and add needless clerical work). When declining any treatment, even life-sustaining treatment, the request for a patient signature to decline such treatment has not been demonstrated to improve risk communication and is not considered a best practice for informed consent.18 When the physician’s motives for this behavior are punitive or directed primarily at reducing liability, it may distract the physician from their fiduciary duty to put patients first.
The solution to improve quality is straightforward—avoid designating discharges as AMA. Instead, clinicians should maintain a single discharge process with clear, objective documentation including providing appropriate prescriptions and follow-up appointments regardless of whether the patient’s choice is consistent with a physician’s recommendation. In its place, the physician should use shared decision-making (SDM) and harm reduction principles to enhance the patient’s well-being within the identified constraints. SDM involves physicians and patients making healthcare decisions together by combining the patients’ values and preferences for care with the physicians’ expertise and knowledge of medical evidence. Harm reduction practices seek to reduce the adverse health consequences that may come from unhealthy behaviors while assuming that patients will likely continue such behaviors. Evidence-based and widely accepted examples of harm reduction strategies include nicotine replacement therapy and needle exchange programs.19
SDM in discharge planning provides a range of discharge and transitional care options that are within prevailing medical standards, not simply a single recommendation that prioritizes health promotion to the exclusion of other identified patient goals. Quality discharge planning should provide the “right care for the right patient at the right time”20 that moves beyond the false choice of either remaining in the hospital under the conditions specified by the physician or leaving AMA. Although physicians are understandably concerned about patients making choices that do not prioritize their health, physicians can consider the evidence for harm reduction programs’ effectiveness in improving health outcomes21 and accommodate patients by providing harm-reducing discharge options that, while suboptimal, may not be substandard.22
Physicians who wish to promote stronger patient-centered discharge practices may find that avoiding or limiting AMA discharges may conflict with their institution’s policy. In those cases, physicians should work closely with their leadership and legal counsel to ensure that any proposed practice changes are legally compliant but also improve SDM and reduce stigma for this population.
Although ending the clinical practice of designating discharges as AMA is unlikely to completely ameliorate the morbidity and costs associated with patients declining episodes of inpatient care, there is reasonable face validity to conclude that replacing the AMA practice with greater attention to harm reduction and SDM can reduce some of the preventable harms like stigmatization and reduced access to care. Together, these practices demonstrate the profession’s continued commitment to the public to practice patient-centered care.
RECOMMENDATIONS
- Treat all discharges similarly. Avoid designating an inpatient discharge as AMA.
- Ensure there is objective documentation of the patient’s informed choice to leave the hospital.
- When patients wish to leave the hospital prior to a physician-recommended clinical endpoint, engage in SDM with a focus on providing all medically reasonable treatment options that promote harm reduction.
- If you choose to designate a discharge as AMA, approach the discharge planning process consistently and with patient-centered principles by optimizing SDM and harm reduction.
CONCLUSION
The physician informed the patient of the risks, benefits, and alternatives to leaving the hospital prior to the completion of IV antibiotics and confirmed the patient’s decision-making capacity. Next, the physician elicited the patient’s preferences for care and identified competing priorities. The patient wanted treatment for his cellulitis, but he was experiencing pain and opioid withdrawal. The physician then expanded the range of potential treatment options, including evaluation for medication-assisted treatment for the patient’s opioid use disorder (OUD) and harm reduction measures such as safer injection practices, needle exchange, housing assistance, and overdose prevention and treatment education.23 An alternative harm-reducing option included discharge with oral antibiotics and follow-up with his primary physician in 48-72 hours. After the patient indicated that he wanted to leave because he was not yet ready for OUD treatment, he was discharged with the standard discharge paperwork and antibiotics, and the physician documented the informed consent discussion.
Disclosure
The authors report no conflicts of interest, financial or otherwise. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs, the VA National Center for Ethics in Health Care or the US Government.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org
1. Ibrahim SA, Kwoh CK, Krishnan E. Factors associated with patients who leave acute-care hospitals against medical advice. Am J Public Health. 2007;97(12):2204-2208. PubMed
2. Alfandre DJ. “I’m going home”: discharges against medical advice. Mayo Clin Proc. 2009;84(3):255-260. PubMed
3. Kraut A, Fransoo R, Olafson K, Ramsey CD, Yogendran M, Garland A. A population-based analysis of leaving the hospital against medical advice: incidence and associated variables. BMC Health Serv Res. 2013;13:415. PubMed
4. Green P, Watts D, Poole S, Dhopesh V. Why patients sign out against medical advice (AMA): factors motivating patients to sign out AMA. Am J Drug Alcohol Abuse. 2004;30(2):489-493. PubMed
5. Levy F, Mareiniss DP, Iacovelli C. The Importance of a Proper Against-Medical-Advice (AMA) Discharge: How Signing Out AMA May Create Significant Liability Protection for Providers. J Emerg Med. 2012;43(3):516-520. PubMed
6. Brenner J, Joslin J, Goulette A, Grant WD, Wojcik SM. Against Medical Advice: A Survey of ED Clinicians’ Rationale for Use. J Emerg Nurs. 2016;42(5):408-411. PubMed
7. Hospital-Wide (All-Condition) 30-Day Risk-Standardized Readmission Measure. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/downloads/MMSHospital-WideAll-ConditionReadmissionRate.pdf. Accessed on July 22, 2016.
8. Jerrard DA, Chasm RM. Patients leaving against medical advice (AMA) from the emergency department--disease prevalence and willingness to return. J Emerg Med. 2011;41(4):412-417. PubMed
9. Haywood C, Jr, Lanzkron S, Hughes MT, et al. A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med. 2011;26(5):518-523. PubMed
10. Wigder HN, Propp DA, Leslie K, Mathew A. Insurance companies refusing payment for patients who leave the emergency department against medical advice is a myth. Ann Emerg Med. 2010;55(4):393. PubMed
11. Saab D, Nisenbaum R, Dhalla I, Hwang SW. Hospital Readmissions in a Community-based Sample of Homeless Adults: a Matched-cohort Study. J Gen Intern Med. 2016;31(9):1011-1018. PubMed
12. Lekas HM, Alfandre D, Gordon P, Harwood K, Yin MT. The role of patient-provider interactions: Using an accounts framework to explain hospital discharges against medical advice. Soc Sci Med. 2016;156:106-113. PubMed
13. Schaefer GR, Matus H, Schumann JH, et al. Financial Responsibility of Hospitalized Patients Who Left Against Medical Advice: Medical Urban Legend? J Gen Intern Med. 2012;27(7):825-830. PubMed
14. Devitt PJ, Devitt AC, Dewan M. Does identifying a discharge as “against medical advice” confer legal protection? J Fam Pract. 2000;49(3):224-227. PubMed
15. Devitt PJ, Devitt AC, Dewan M. An examination of whether discharging patients against medical advice protects physicians from malpractice charges. Psychiatr Serv. 2000;51(7):899-902. PubMed
16. Beckman HB, Markakis KM, Suchman AL, Frankel RM. The doctor-patient relationship and malpractice. Lessons from plaintiff depositions. Arch Intern Med. 1994;154(12):1365-1370. PubMed
17. Windish DM, Ratanawongsa N. Providers’ perceptions of relationships and professional roles when caring for patients who leave the hospital against medical advice. J Gen Intern Med. 2008;23(10):1698-1707. PubMed
18. Sulmasy DP, Sood JR, Texiera K, McAuley RL, McGugins J, Ury WA. A prospective trial of a new policy eliminating signed consent for do not resuscitate orders. J Gen Intern Med. 2006;21(12):1261-1268. PubMed
19. Stratton K, Shetty P, Wallace R, Bondurant S. Clearing the smoke: the science base for tobacco harm reduction--executive summary. Tob Control. 2001;10(2):189-195. PubMed
20. What is Health Care Quality and Who Decides?. March 2009. Agency for Healthcare Research and Quality, Rockville, MD. https://archive.ahrq.gov/news/speech/test031809.html
21. Hobden KL, Cunningham JA. Barriers to the dissemination of four harm reduction strategies: a survey of addiction treatment providers in Ontario. Harm Reduct J. 2006;3:35. PubMed
22. Alfandre D. Clinical Recommendations in Medical Practice: A Proposed Framework to Reduce Bias and Improve the Quality of Medical Decisions. J Clin Ethics. 2016;27(1):21-27. PubMed
23. Fanucchi L, Lofwall MR. Putting Parity into Practice - Integrating Opioid-Use Disorder Treatment into the Hospital Setting. N Engl J Med. 2016;375(9):811-813. PubMed
1. Ibrahim SA, Kwoh CK, Krishnan E. Factors associated with patients who leave acute-care hospitals against medical advice. Am J Public Health. 2007;97(12):2204-2208. PubMed
2. Alfandre DJ. “I’m going home”: discharges against medical advice. Mayo Clin Proc. 2009;84(3):255-260. PubMed
3. Kraut A, Fransoo R, Olafson K, Ramsey CD, Yogendran M, Garland A. A population-based analysis of leaving the hospital against medical advice: incidence and associated variables. BMC Health Serv Res. 2013;13:415. PubMed
4. Green P, Watts D, Poole S, Dhopesh V. Why patients sign out against medical advice (AMA): factors motivating patients to sign out AMA. Am J Drug Alcohol Abuse. 2004;30(2):489-493. PubMed
5. Levy F, Mareiniss DP, Iacovelli C. The Importance of a Proper Against-Medical-Advice (AMA) Discharge: How Signing Out AMA May Create Significant Liability Protection for Providers. J Emerg Med. 2012;43(3):516-520. PubMed
6. Brenner J, Joslin J, Goulette A, Grant WD, Wojcik SM. Against Medical Advice: A Survey of ED Clinicians’ Rationale for Use. J Emerg Nurs. 2016;42(5):408-411. PubMed
7. Hospital-Wide (All-Condition) 30-Day Risk-Standardized Readmission Measure. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/downloads/MMSHospital-WideAll-ConditionReadmissionRate.pdf. Accessed on July 22, 2016.
8. Jerrard DA, Chasm RM. Patients leaving against medical advice (AMA) from the emergency department--disease prevalence and willingness to return. J Emerg Med. 2011;41(4):412-417. PubMed
9. Haywood C, Jr, Lanzkron S, Hughes MT, et al. A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med. 2011;26(5):518-523. PubMed
10. Wigder HN, Propp DA, Leslie K, Mathew A. Insurance companies refusing payment for patients who leave the emergency department against medical advice is a myth. Ann Emerg Med. 2010;55(4):393. PubMed
11. Saab D, Nisenbaum R, Dhalla I, Hwang SW. Hospital Readmissions in a Community-based Sample of Homeless Adults: a Matched-cohort Study. J Gen Intern Med. 2016;31(9):1011-1018. PubMed
12. Lekas HM, Alfandre D, Gordon P, Harwood K, Yin MT. The role of patient-provider interactions: Using an accounts framework to explain hospital discharges against medical advice. Soc Sci Med. 2016;156:106-113. PubMed
13. Schaefer GR, Matus H, Schumann JH, et al. Financial Responsibility of Hospitalized Patients Who Left Against Medical Advice: Medical Urban Legend? J Gen Intern Med. 2012;27(7):825-830. PubMed
14. Devitt PJ, Devitt AC, Dewan M. Does identifying a discharge as “against medical advice” confer legal protection? J Fam Pract. 2000;49(3):224-227. PubMed
15. Devitt PJ, Devitt AC, Dewan M. An examination of whether discharging patients against medical advice protects physicians from malpractice charges. Psychiatr Serv. 2000;51(7):899-902. PubMed
16. Beckman HB, Markakis KM, Suchman AL, Frankel RM. The doctor-patient relationship and malpractice. Lessons from plaintiff depositions. Arch Intern Med. 1994;154(12):1365-1370. PubMed
17. Windish DM, Ratanawongsa N. Providers’ perceptions of relationships and professional roles when caring for patients who leave the hospital against medical advice. J Gen Intern Med. 2008;23(10):1698-1707. PubMed
18. Sulmasy DP, Sood JR, Texiera K, McAuley RL, McGugins J, Ury WA. A prospective trial of a new policy eliminating signed consent for do not resuscitate orders. J Gen Intern Med. 2006;21(12):1261-1268. PubMed
19. Stratton K, Shetty P, Wallace R, Bondurant S. Clearing the smoke: the science base for tobacco harm reduction--executive summary. Tob Control. 2001;10(2):189-195. PubMed
20. What is Health Care Quality and Who Decides?. March 2009. Agency for Healthcare Research and Quality, Rockville, MD. https://archive.ahrq.gov/news/speech/test031809.html
21. Hobden KL, Cunningham JA. Barriers to the dissemination of four harm reduction strategies: a survey of addiction treatment providers in Ontario. Harm Reduct J. 2006;3:35. PubMed
22. Alfandre D. Clinical Recommendations in Medical Practice: A Proposed Framework to Reduce Bias and Improve the Quality of Medical Decisions. J Clin Ethics. 2016;27(1):21-27. PubMed
23. Fanucchi L, Lofwall MR. Putting Parity into Practice - Integrating Opioid-Use Disorder Treatment into the Hospital Setting. N Engl J Med. 2016;375(9):811-813. PubMed
©2017 Society of Hospital Medicine
Things We Do For No Reason: Two-Unit Red Cell Transfusions in Stable Anemic Patients
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

INTRODUCTION
Blood transfusion is not only the most common procedure performed in US hospitals but is also widely overused, according to The Joint Commission. Unnecessary transfusions can increase risks and costs, and now, multiple landmark trials support using restrictive transfusion strategies. This manuscript discusses the importance and potential impacts of giving single-unit red blood cell (RBC) transfusions in anemic patients who are not actively bleeding and are hemodynamically stable. The “thing we do for no reason” is giving 2-unit RBC transfusions when 1 unit would suffice. We call this the “Why give 2 when 1 will do?” campaign for RBC transfusion.
CASE PRESENTATION
A 74-year-old, 70-kg male with a known history of myelodysplastic syndrome is admitted for dizziness and shortness of breath. His hemoglobin (Hb) concentration is 6.2 g/dL (baseline Hb of 8 g/dL). The patient denies any hematuria, hematemesis, and melena. Physical examination is remarkable only for tachycardia—heart rate of 110. The admitting hospitalist ponders whether to order a 2-unit red blood cell (RBC) transfusion.
WHY YOU MIGHT THINK DOUBLE UNIT RED BLOOD CELL TRANSFUSIONS ARE HELPFUL
RBC transfusion is the most common procedure performed in US hospitals, with about 12 million RBC units given to patients in the United States each year.1 Based on an opinion paper published in 1942 by Adams and Lundy2 the “10/30 rule” set the standard that the ideal transfusion thresholds were an Hb of 10 g/dL or a hematocrit of 30%. Until human immunodeficiency virus (HIV) became a threat to the nation’s blood supply in the early 1980s, few questioned the 10/30 rule. There is no doubt that blood transfusions can be lifesaving in the presence of active bleeding or hemorrhagic shock; in fact, many hospitals have blood donation campaigns reminding us to “give blood—save a life.” To some, these messages may suggest that more blood is better. Prior to the 1990s, clinicians were taught that if the patient needed an RBC transfusion, 2 units was the optimal dose for adult patients. In fact, single-unit transfusions were strongly discouraged, and authorities on the risks of transfusion wrote that single-unit transfusions were acknowledged to be unnecessary.3
WHY THERE IS “NO REASON” TO ROUTINELY ORDER DOUBLE UNIT TRANSFUSIONS
According to a recent Joint Commission Overuse Summit, transfusion was identified as 1 of the top 5 overused medical procedures.4 Blood transfusions can cause complications such as transfusion-related acute lung injury and transfusion-associated circulatory overload, the number 1 and 2 causes of transfusion-related deaths, respectively,5 as well as other transfusion reactions (eg, allergic and hemolytic) and alloimmunization. Transfusion-related morbidity and mortality have been shown to be dose dependent,6 suggesting that the lowest effective number of units should be transfused. Although, with modern-day testing, the risks of HIV and viral hepatitis are exceedingly low, emerging infectious diseases such as the Zika virus and Babesiosis represent new threats to the nation’s blood supply, with potential transfusion-related transmission and severe consequences, especially for the immunosuppressed. As quality-improvement, patient safety, and cost-saving initiatives, many hospitals have implemented strategies to reduce unnecessary transfusions and decrease overall blood utilization.
The above-mentioned studies support the concept that oftentimes less is more for transfusions, which includes giving the lowest effective amount of transfused blood. These trials have enrolled multiple patient populations, such as critically ill patients in the intensive care unit,11,13 elderly orthopedic surgery patients,14 cardiac surgery patients,12 and patients with gastrointestinal hemorrhage,15 traumatic brain injury,17 and septicemia.16 Outcomes in the trials included mortality, serious infections, thrombotic and ischemic events, neurologic deficits, multiple-organ dysfunction, and inability to ambulate (Table). The findings in these studies suggest that we increase risks and cost without improving outcomes only by giving more blood than is necessary. Since most of these trials were published in the last decade, some very recently, clinicians have not fully adopted these newer, restrictive transfusion strategies.19
ARE THERE REASONS TO ORDER 2-UNIT TRANSFUSIONS IN CERTAIN CIRCUMSTANCES?
Perhaps the most common indication for ordering multiunit RBC transfusions is active bleeding, as it is clear that whatever Hb threshold is chosen, transfusion should be given in sufficient amounts to stay ahead of the bleeding.20 It is important to remember that we treat patients and their symptoms, not just their laboratory values. Good medical care adapts and/or modifies treatment protocols and guidelines according to the clinical situation. Intravascular volume is also important to consider because what really matters for oxygen content and delivery is the total red cell mass (ie, the Hb concentration times the blood volume). If a patient is hypovolemic and/or actively bleeding, the Hb transfusion trigger, as well as the dose of blood, may need to be adjusted upward, creating clinical scenarios in which 2-unit RBC transfusions may be appropriate. Other clinical settings for which multiunit RBC transfusions may be indicated include patients with severe anemia, for whom both the pretransfusion Hb (the trigger) and the posttransfusion Hb (the target) should be considered. Patients with hemoglobinopathies (eg, sickle cell or thalassemia) sometimes require multiunit transfusions or even exchange transfusions to improve oxygen delivery. Other patients who may benefit from higher Hb levels achieved by multiunit transfusions include those with acute coronary syndromes; however, the ideal Hb transfusion threshold in this setting has yet to be determined.21
WHAT YOU SHOULD DO INSTEAD
For hemodynamically stable patients and in the absence of active bleeding, single-unit RBC transfusions, followed by reassessment, should be the standard for most patients. The reassessment should include measuring the posttransfusion Hb level and checking for improvement in vital sign abnormalities and signs or symptoms of anemia or end-organ ischemia. A recent publication on our hospital-wide campaign called “Why give 2 when 1 will do?” showed a significant (35%) reduction in 2-unit transfusion orders along with an 18% overall decrease in RBC utilization and substantial cost savings (≈$600,000 per year).10 These findings demonstrate that there is a large opportunity to reduce transfusion overuse by encouraging single-unit transfusions.
RECOMMENDATIONS
- For nonbleeding, hemodynamically stable patients who require a transfusion, transfuse a single RBC unit and then reassess the Hb level before transfusing a second unit.
- The decision to transfuse RBCs should take into account the patient’s overall condition, including their symptoms, intravascular volume, and the occurrence and rate of active bleeding, not just the Hb value alone.
CONCLUSIONS
In stable patients, a single unit of RBCs often is adequate to raise the Hb to an acceptable level and relieve the signs and symptoms of anemia. Additional units should be prescribed only after reassessment of the patient and the Hb level. For our patient with symptomatic anemia, it is reasonable to transfuse 1 RBC unit, and then measure the Hb level, and reassess his symptoms before giving additional RBC units.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Acknowledgments
This publication is dedicated to our beloved colleague, Dr. Rajiv N. Thakkar, who recently and unexpectedly suffered a fatal cardiac event. We will miss him dearly.
Disclosure
S.M.F. has been on advisory boards for the Haemonetics Corporation (Braintree, MA), Medtronic Inc. (Minneapolis, MN), and Zimmer Biomet (Warsaw, IN). All other authors declare no competing interests.
1. Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion. 2016;56:2173-2183. PubMed
2. Adams C, Lundy JS. Anesthesia in cases of poor surgical risk – Some suggestions for decreasing the risk. Surg Gynec Obstet. 1942;74:1011-1019.
3. Morton JH. An evaluation of blood-transfusion practices on a surgical service. N Engl J Med. 1960;263:1285-1287. PubMed
4. Pfunter A, Wier LM, Stocks C. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb165.pdf. Accessed January 7, 2017.
5. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406-3417. PubMed
6. Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608-1616. PubMed
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025-2035. PubMed
8. Ferraris VA, Brown JR, Despotis GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944-982. PubMed
9. Callum JL, Waters JH, Shaz BH, et al. The AABB recommendations for the Choosing Wisely campaign of the American Board of Internal Medicine. Transfusion. 2014;54:2344-2352. PubMed
10. Podlasek SJ, Thakkar RN, Rotello LC, et al. Implementing a “Why give 2 when 1 will do?” Choosing Wisely campaign. Transfusion. 2016;56:2164. PubMed
11. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-417. PubMed
12. Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: The TRACS randomized controlled trial. JAMA. 2010;304:1559-1567. PubMed
13. Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609-1619. PubMed
14. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453-2462. PubMed
15. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. New Engl J Med. 2013;368:11-21. PubMed
16. Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381-1391. PubMed
17. Robertson CS, Hannay HJ, Yamal JM, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: A randomized clinical trial. JAMA. 2014;312:36-47. PubMed
18. Murphy GJ, Pike K, Rogers CA, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015;372:997-1008.
19. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev. 2017;31:62-71. PubMed
20. Frank SM, Resar LM, Rothschild JA, et al. A novel method of data analysis for utilization of red blood cell transfusion. Transfusion. 2013;53:3052-9. PubMed
21. Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964.el-971.e1. PubMed
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

INTRODUCTION
Blood transfusion is not only the most common procedure performed in US hospitals but is also widely overused, according to The Joint Commission. Unnecessary transfusions can increase risks and costs, and now, multiple landmark trials support using restrictive transfusion strategies. This manuscript discusses the importance and potential impacts of giving single-unit red blood cell (RBC) transfusions in anemic patients who are not actively bleeding and are hemodynamically stable. The “thing we do for no reason” is giving 2-unit RBC transfusions when 1 unit would suffice. We call this the “Why give 2 when 1 will do?” campaign for RBC transfusion.
CASE PRESENTATION
A 74-year-old, 70-kg male with a known history of myelodysplastic syndrome is admitted for dizziness and shortness of breath. His hemoglobin (Hb) concentration is 6.2 g/dL (baseline Hb of 8 g/dL). The patient denies any hematuria, hematemesis, and melena. Physical examination is remarkable only for tachycardia—heart rate of 110. The admitting hospitalist ponders whether to order a 2-unit red blood cell (RBC) transfusion.
WHY YOU MIGHT THINK DOUBLE UNIT RED BLOOD CELL TRANSFUSIONS ARE HELPFUL
RBC transfusion is the most common procedure performed in US hospitals, with about 12 million RBC units given to patients in the United States each year.1 Based on an opinion paper published in 1942 by Adams and Lundy2 the “10/30 rule” set the standard that the ideal transfusion thresholds were an Hb of 10 g/dL or a hematocrit of 30%. Until human immunodeficiency virus (HIV) became a threat to the nation’s blood supply in the early 1980s, few questioned the 10/30 rule. There is no doubt that blood transfusions can be lifesaving in the presence of active bleeding or hemorrhagic shock; in fact, many hospitals have blood donation campaigns reminding us to “give blood—save a life.” To some, these messages may suggest that more blood is better. Prior to the 1990s, clinicians were taught that if the patient needed an RBC transfusion, 2 units was the optimal dose for adult patients. In fact, single-unit transfusions were strongly discouraged, and authorities on the risks of transfusion wrote that single-unit transfusions were acknowledged to be unnecessary.3
WHY THERE IS “NO REASON” TO ROUTINELY ORDER DOUBLE UNIT TRANSFUSIONS
According to a recent Joint Commission Overuse Summit, transfusion was identified as 1 of the top 5 overused medical procedures.4 Blood transfusions can cause complications such as transfusion-related acute lung injury and transfusion-associated circulatory overload, the number 1 and 2 causes of transfusion-related deaths, respectively,5 as well as other transfusion reactions (eg, allergic and hemolytic) and alloimmunization. Transfusion-related morbidity and mortality have been shown to be dose dependent,6 suggesting that the lowest effective number of units should be transfused. Although, with modern-day testing, the risks of HIV and viral hepatitis are exceedingly low, emerging infectious diseases such as the Zika virus and Babesiosis represent new threats to the nation’s blood supply, with potential transfusion-related transmission and severe consequences, especially for the immunosuppressed. As quality-improvement, patient safety, and cost-saving initiatives, many hospitals have implemented strategies to reduce unnecessary transfusions and decrease overall blood utilization.
The above-mentioned studies support the concept that oftentimes less is more for transfusions, which includes giving the lowest effective amount of transfused blood. These trials have enrolled multiple patient populations, such as critically ill patients in the intensive care unit,11,13 elderly orthopedic surgery patients,14 cardiac surgery patients,12 and patients with gastrointestinal hemorrhage,15 traumatic brain injury,17 and septicemia.16 Outcomes in the trials included mortality, serious infections, thrombotic and ischemic events, neurologic deficits, multiple-organ dysfunction, and inability to ambulate (Table). The findings in these studies suggest that we increase risks and cost without improving outcomes only by giving more blood than is necessary. Since most of these trials were published in the last decade, some very recently, clinicians have not fully adopted these newer, restrictive transfusion strategies.19
ARE THERE REASONS TO ORDER 2-UNIT TRANSFUSIONS IN CERTAIN CIRCUMSTANCES?
Perhaps the most common indication for ordering multiunit RBC transfusions is active bleeding, as it is clear that whatever Hb threshold is chosen, transfusion should be given in sufficient amounts to stay ahead of the bleeding.20 It is important to remember that we treat patients and their symptoms, not just their laboratory values. Good medical care adapts and/or modifies treatment protocols and guidelines according to the clinical situation. Intravascular volume is also important to consider because what really matters for oxygen content and delivery is the total red cell mass (ie, the Hb concentration times the blood volume). If a patient is hypovolemic and/or actively bleeding, the Hb transfusion trigger, as well as the dose of blood, may need to be adjusted upward, creating clinical scenarios in which 2-unit RBC transfusions may be appropriate. Other clinical settings for which multiunit RBC transfusions may be indicated include patients with severe anemia, for whom both the pretransfusion Hb (the trigger) and the posttransfusion Hb (the target) should be considered. Patients with hemoglobinopathies (eg, sickle cell or thalassemia) sometimes require multiunit transfusions or even exchange transfusions to improve oxygen delivery. Other patients who may benefit from higher Hb levels achieved by multiunit transfusions include those with acute coronary syndromes; however, the ideal Hb transfusion threshold in this setting has yet to be determined.21
WHAT YOU SHOULD DO INSTEAD
For hemodynamically stable patients and in the absence of active bleeding, single-unit RBC transfusions, followed by reassessment, should be the standard for most patients. The reassessment should include measuring the posttransfusion Hb level and checking for improvement in vital sign abnormalities and signs or symptoms of anemia or end-organ ischemia. A recent publication on our hospital-wide campaign called “Why give 2 when 1 will do?” showed a significant (35%) reduction in 2-unit transfusion orders along with an 18% overall decrease in RBC utilization and substantial cost savings (≈$600,000 per year).10 These findings demonstrate that there is a large opportunity to reduce transfusion overuse by encouraging single-unit transfusions.
RECOMMENDATIONS
- For nonbleeding, hemodynamically stable patients who require a transfusion, transfuse a single RBC unit and then reassess the Hb level before transfusing a second unit.
- The decision to transfuse RBCs should take into account the patient’s overall condition, including their symptoms, intravascular volume, and the occurrence and rate of active bleeding, not just the Hb value alone.
CONCLUSIONS
In stable patients, a single unit of RBCs often is adequate to raise the Hb to an acceptable level and relieve the signs and symptoms of anemia. Additional units should be prescribed only after reassessment of the patient and the Hb level. For our patient with symptomatic anemia, it is reasonable to transfuse 1 RBC unit, and then measure the Hb level, and reassess his symptoms before giving additional RBC units.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Acknowledgments
This publication is dedicated to our beloved colleague, Dr. Rajiv N. Thakkar, who recently and unexpectedly suffered a fatal cardiac event. We will miss him dearly.
Disclosure
S.M.F. has been on advisory boards for the Haemonetics Corporation (Braintree, MA), Medtronic Inc. (Minneapolis, MN), and Zimmer Biomet (Warsaw, IN). All other authors declare no competing interests.
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

INTRODUCTION
Blood transfusion is not only the most common procedure performed in US hospitals but is also widely overused, according to The Joint Commission. Unnecessary transfusions can increase risks and costs, and now, multiple landmark trials support using restrictive transfusion strategies. This manuscript discusses the importance and potential impacts of giving single-unit red blood cell (RBC) transfusions in anemic patients who are not actively bleeding and are hemodynamically stable. The “thing we do for no reason” is giving 2-unit RBC transfusions when 1 unit would suffice. We call this the “Why give 2 when 1 will do?” campaign for RBC transfusion.
CASE PRESENTATION
A 74-year-old, 70-kg male with a known history of myelodysplastic syndrome is admitted for dizziness and shortness of breath. His hemoglobin (Hb) concentration is 6.2 g/dL (baseline Hb of 8 g/dL). The patient denies any hematuria, hematemesis, and melena. Physical examination is remarkable only for tachycardia—heart rate of 110. The admitting hospitalist ponders whether to order a 2-unit red blood cell (RBC) transfusion.
WHY YOU MIGHT THINK DOUBLE UNIT RED BLOOD CELL TRANSFUSIONS ARE HELPFUL
RBC transfusion is the most common procedure performed in US hospitals, with about 12 million RBC units given to patients in the United States each year.1 Based on an opinion paper published in 1942 by Adams and Lundy2 the “10/30 rule” set the standard that the ideal transfusion thresholds were an Hb of 10 g/dL or a hematocrit of 30%. Until human immunodeficiency virus (HIV) became a threat to the nation’s blood supply in the early 1980s, few questioned the 10/30 rule. There is no doubt that blood transfusions can be lifesaving in the presence of active bleeding or hemorrhagic shock; in fact, many hospitals have blood donation campaigns reminding us to “give blood—save a life.” To some, these messages may suggest that more blood is better. Prior to the 1990s, clinicians were taught that if the patient needed an RBC transfusion, 2 units was the optimal dose for adult patients. In fact, single-unit transfusions were strongly discouraged, and authorities on the risks of transfusion wrote that single-unit transfusions were acknowledged to be unnecessary.3
WHY THERE IS “NO REASON” TO ROUTINELY ORDER DOUBLE UNIT TRANSFUSIONS
According to a recent Joint Commission Overuse Summit, transfusion was identified as 1 of the top 5 overused medical procedures.4 Blood transfusions can cause complications such as transfusion-related acute lung injury and transfusion-associated circulatory overload, the number 1 and 2 causes of transfusion-related deaths, respectively,5 as well as other transfusion reactions (eg, allergic and hemolytic) and alloimmunization. Transfusion-related morbidity and mortality have been shown to be dose dependent,6 suggesting that the lowest effective number of units should be transfused. Although, with modern-day testing, the risks of HIV and viral hepatitis are exceedingly low, emerging infectious diseases such as the Zika virus and Babesiosis represent new threats to the nation’s blood supply, with potential transfusion-related transmission and severe consequences, especially for the immunosuppressed. As quality-improvement, patient safety, and cost-saving initiatives, many hospitals have implemented strategies to reduce unnecessary transfusions and decrease overall blood utilization.
The above-mentioned studies support the concept that oftentimes less is more for transfusions, which includes giving the lowest effective amount of transfused blood. These trials have enrolled multiple patient populations, such as critically ill patients in the intensive care unit,11,13 elderly orthopedic surgery patients,14 cardiac surgery patients,12 and patients with gastrointestinal hemorrhage,15 traumatic brain injury,17 and septicemia.16 Outcomes in the trials included mortality, serious infections, thrombotic and ischemic events, neurologic deficits, multiple-organ dysfunction, and inability to ambulate (Table). The findings in these studies suggest that we increase risks and cost without improving outcomes only by giving more blood than is necessary. Since most of these trials were published in the last decade, some very recently, clinicians have not fully adopted these newer, restrictive transfusion strategies.19
ARE THERE REASONS TO ORDER 2-UNIT TRANSFUSIONS IN CERTAIN CIRCUMSTANCES?
Perhaps the most common indication for ordering multiunit RBC transfusions is active bleeding, as it is clear that whatever Hb threshold is chosen, transfusion should be given in sufficient amounts to stay ahead of the bleeding.20 It is important to remember that we treat patients and their symptoms, not just their laboratory values. Good medical care adapts and/or modifies treatment protocols and guidelines according to the clinical situation. Intravascular volume is also important to consider because what really matters for oxygen content and delivery is the total red cell mass (ie, the Hb concentration times the blood volume). If a patient is hypovolemic and/or actively bleeding, the Hb transfusion trigger, as well as the dose of blood, may need to be adjusted upward, creating clinical scenarios in which 2-unit RBC transfusions may be appropriate. Other clinical settings for which multiunit RBC transfusions may be indicated include patients with severe anemia, for whom both the pretransfusion Hb (the trigger) and the posttransfusion Hb (the target) should be considered. Patients with hemoglobinopathies (eg, sickle cell or thalassemia) sometimes require multiunit transfusions or even exchange transfusions to improve oxygen delivery. Other patients who may benefit from higher Hb levels achieved by multiunit transfusions include those with acute coronary syndromes; however, the ideal Hb transfusion threshold in this setting has yet to be determined.21
WHAT YOU SHOULD DO INSTEAD
For hemodynamically stable patients and in the absence of active bleeding, single-unit RBC transfusions, followed by reassessment, should be the standard for most patients. The reassessment should include measuring the posttransfusion Hb level and checking for improvement in vital sign abnormalities and signs or symptoms of anemia or end-organ ischemia. A recent publication on our hospital-wide campaign called “Why give 2 when 1 will do?” showed a significant (35%) reduction in 2-unit transfusion orders along with an 18% overall decrease in RBC utilization and substantial cost savings (≈$600,000 per year).10 These findings demonstrate that there is a large opportunity to reduce transfusion overuse by encouraging single-unit transfusions.
RECOMMENDATIONS
- For nonbleeding, hemodynamically stable patients who require a transfusion, transfuse a single RBC unit and then reassess the Hb level before transfusing a second unit.
- The decision to transfuse RBCs should take into account the patient’s overall condition, including their symptoms, intravascular volume, and the occurrence and rate of active bleeding, not just the Hb value alone.
CONCLUSIONS
In stable patients, a single unit of RBCs often is adequate to raise the Hb to an acceptable level and relieve the signs and symptoms of anemia. Additional units should be prescribed only after reassessment of the patient and the Hb level. For our patient with symptomatic anemia, it is reasonable to transfuse 1 RBC unit, and then measure the Hb level, and reassess his symptoms before giving additional RBC units.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Acknowledgments
This publication is dedicated to our beloved colleague, Dr. Rajiv N. Thakkar, who recently and unexpectedly suffered a fatal cardiac event. We will miss him dearly.
Disclosure
S.M.F. has been on advisory boards for the Haemonetics Corporation (Braintree, MA), Medtronic Inc. (Minneapolis, MN), and Zimmer Biomet (Warsaw, IN). All other authors declare no competing interests.
1. Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion. 2016;56:2173-2183. PubMed
2. Adams C, Lundy JS. Anesthesia in cases of poor surgical risk – Some suggestions for decreasing the risk. Surg Gynec Obstet. 1942;74:1011-1019.
3. Morton JH. An evaluation of blood-transfusion practices on a surgical service. N Engl J Med. 1960;263:1285-1287. PubMed
4. Pfunter A, Wier LM, Stocks C. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb165.pdf. Accessed January 7, 2017.
5. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406-3417. PubMed
6. Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608-1616. PubMed
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025-2035. PubMed
8. Ferraris VA, Brown JR, Despotis GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944-982. PubMed
9. Callum JL, Waters JH, Shaz BH, et al. The AABB recommendations for the Choosing Wisely campaign of the American Board of Internal Medicine. Transfusion. 2014;54:2344-2352. PubMed
10. Podlasek SJ, Thakkar RN, Rotello LC, et al. Implementing a “Why give 2 when 1 will do?” Choosing Wisely campaign. Transfusion. 2016;56:2164. PubMed
11. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-417. PubMed
12. Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: The TRACS randomized controlled trial. JAMA. 2010;304:1559-1567. PubMed
13. Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609-1619. PubMed
14. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453-2462. PubMed
15. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. New Engl J Med. 2013;368:11-21. PubMed
16. Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381-1391. PubMed
17. Robertson CS, Hannay HJ, Yamal JM, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: A randomized clinical trial. JAMA. 2014;312:36-47. PubMed
18. Murphy GJ, Pike K, Rogers CA, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015;372:997-1008.
19. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev. 2017;31:62-71. PubMed
20. Frank SM, Resar LM, Rothschild JA, et al. A novel method of data analysis for utilization of red blood cell transfusion. Transfusion. 2013;53:3052-9. PubMed
21. Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964.el-971.e1. PubMed
1. Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion. 2016;56:2173-2183. PubMed
2. Adams C, Lundy JS. Anesthesia in cases of poor surgical risk – Some suggestions for decreasing the risk. Surg Gynec Obstet. 1942;74:1011-1019.
3. Morton JH. An evaluation of blood-transfusion practices on a surgical service. N Engl J Med. 1960;263:1285-1287. PubMed
4. Pfunter A, Wier LM, Stocks C. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb165.pdf. Accessed January 7, 2017.
5. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406-3417. PubMed
6. Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608-1616. PubMed
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025-2035. PubMed
8. Ferraris VA, Brown JR, Despotis GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944-982. PubMed
9. Callum JL, Waters JH, Shaz BH, et al. The AABB recommendations for the Choosing Wisely campaign of the American Board of Internal Medicine. Transfusion. 2014;54:2344-2352. PubMed
10. Podlasek SJ, Thakkar RN, Rotello LC, et al. Implementing a “Why give 2 when 1 will do?” Choosing Wisely campaign. Transfusion. 2016;56:2164. PubMed
11. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-417. PubMed
12. Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: The TRACS randomized controlled trial. JAMA. 2010;304:1559-1567. PubMed
13. Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609-1619. PubMed
14. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453-2462. PubMed
15. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. New Engl J Med. 2013;368:11-21. PubMed
16. Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381-1391. PubMed
17. Robertson CS, Hannay HJ, Yamal JM, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: A randomized clinical trial. JAMA. 2014;312:36-47. PubMed
18. Murphy GJ, Pike K, Rogers CA, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015;372:997-1008.
19. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev. 2017;31:62-71. PubMed
20. Frank SM, Resar LM, Rothschild JA, et al. A novel method of data analysis for utilization of red blood cell transfusion. Transfusion. 2013;53:3052-9. PubMed
21. Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964.el-971.e1. PubMed
© 2017 Society of Hospital Medicine
Ammonia Levels and Hepatic Encephalopathy in Patients with Known Chronic Liver Disease
© 2017 Society of Hospital Medicine
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Ammonia is predominantly generated in the gut by intestinal bacteria and enzymes and detoxified primarily in the liver. Since the 1930s, ammonia has been identified as the principal culprit in hepatic encephalopathy (HE). Many physicians utilize serum ammonia to diagnose, assess severity, and determine the resolution of HE in patients with chronic liver disease (CLD) despite research showing that ammonia levels are unhelpful in all of these clinical circumstances. HE in patients with CLD is a clinical diagnosis of exclusion that should not be based on ammonia levels.
CASE PRESENTATION
A 62-year-old man diagnosed with cirrhosis due to Hepatitis C and alcoholism was brought to the emergency department for alteration in mentation. He had scant melenic stools 5 days preceding his admission and did not exhibit overt signs or symptoms of infection. His systemic examination was normal except for somnolence, disorientation to space and time, asterixis, and ascites. His lab parameters were within normal limits except for an elevated blood urea nitrogen and thrombocytopenia. His blood cultures did not grow any organisms, and paracentesis ruled out spontaneous bacterial peritonitis. During his hospital stay, he underwent esophageal variceal banding and was effectively managed with lactulose and rifaximin. The patient was alert, fully oriented, and without asterixis at the time of discharge 6 days later. Would an elevated venous ammonia level at admission alter management? If the ammonia level was elevated, would serial ammonia measurements affect management?
BACKGROUND
The colonic microbiome produces ammonia from dietary nitrogen. In health, approximately 85% of it is detoxified by the liver and excreted as urea in urine, while muscle and brain tissue metabolize the remaining 15%. The process of transamination and the urea cycle prevents this metabolic product from accumulating in the body. The elevated levels of nitrogenous toxins, including ammonia, in the systemic circulation of patients with CLD occur due to hepatocellular dysfunction and/or portosystemic shunting. This hyperammonemia is compounded by reduced peripheral metabolism of ammonia by muscle as a consequence of cachexia and muscle atrophy. Astrocytes synthesize glutamine excessively in the setting of hyperammonemia, resulting in astrocyte swelling and the generation of reactive oxygen species. Astrocyte swelling, free radical generation, and increased inhibitory function of gamma-Aminobutyric Acid result in cerebral dysfunction.1,2 HE manifests as a broad spectrum of neurological or psychiatric abnormalities ranging from subclinical alterations to coma and was commonly graded on the West Haven Criteria (WHC) of 0 to 4 (Table).3 The Grade 0 from the previous WHC, referenced in many trials included in this article, has been replaced with minimal HE in the newly updated WHC by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver.4,5
WHY YOU MIGHT THINK AMMONIA LEVELS HELP TO GUIDE TREATMENT OF HE IN PATIENTS WITH CLD
The ammonia hypothesis posits that ammonia is key in the pathogenesis of HE.6-10 Some of the common precipitants of HE—gastrointestinal bleeding, infection, and renal failure—promote hyperammonemia.11 HE is treated with nonabsorbable disaccharides (lactulose and lactitol) and rifaximin, which reduce the serum concentration of ammonia. Given these associations between HE and ammonia, physicians have for decades tested serum ammonia levels to diagnose HE and chart its resolution. In a study conducted by the Bavarian Society of Gastroenterology,12 60% of the respondents to an anonymous questionnaire regularly performed ammonia analysis in all their patients with liver cirrhosis, believing that it efficiently diagnosed HE.
WHY SERUM AMMONIA LEVELS DO NOT HELP IN THE DIAGNOSIS OR MANAGEMENT OF HE IN CLD PATIENTS
Accuracy of Serum Ammonia
Multiple factors affect the accuracy of ammonia levels. First, fist clenching or the use of a tourniquet during the process of phlebotomy can falsely increase ammonia levels.13 Second, some authors have argued that the source of the ammonia sample matters. Kramer et al.14 reported that partial pressure of ammonia correlated closely with the degree of clinical and electrophysiological abnormalities of HE. However, Nicolao et al.15 and Ong et al.16 showed that the blood ammonia levels, whether measured by total venous, total arterial, or partial pressure methods, were equivalent. Third, ammonia levels are dependent on the time to processing of the specimen. Inaccurate results may occur if the blood sample is not immediately placed on ice after collection or if it is not centrifuged within 15 minutes of collection.17,18
Ammonia Levels and Diagnosis of HE
Even with proper collection and processing, ammonia levels in patients with CLD do not reliably diagnose HE. Gundling et al.19 determined the sensitivity and specificity of venous ammonia levels ≥ 55 µmol/L to diagnose HE to be 47.2% and 78.3%, respectively, by using a gold standard of the WHC and the critical flicker frequency test (a psychophysiologic test). The positive predictive and negative predictive values of ammonia were 77.3% and 48.6%, with an overall diagnostic accuracy of 59.3%. Approximately 60% of the patients with Grade 3 WHC HE had a normal ammonia level in this study. Ong et al16 found that only 31% of patients with CLD and no evidence of HE had a normal ammonia level.In other words, CLD patients with normal ammonia levels can have HE, and patients with elevated ammonia levels may have normal cognitive functioning.
Furthermore, ammonia levels are not a valid tool to diagnose HE even with an oral glutamine challenge.20 Most importantly, HE is a clinical diagnosis reached following the exclusion of other likely causes of cerebral dysfunction, independent of the ammonia level.
Ammonia Levels and Staging HE
The grading of HE was introduced to assess the response to an intervention in patients with HE enrolled in clinical trials.21 Tools like the WHC (Table) categorize the severity of HE. Nicolao et al.15 noted significant overlap in the levels of ammonia between patients with HE Grades 1 and 2 when compared with patients with Grades 3 and 4. This considerable overlap in levels of ammonia was more evident among patients with Grades 0 to 2 per Ong’s study.16 Most importantly, hospitalists do not need ammonia levels to determine that a patient has HE Grade 3 or HE Grade 4 symptoms, as the stage is graded on clinical grounds only. Once other causes for cerebral dysfunction have been ruled out, the ammonia level does not add to the clinical picture.
Serial Ammonia Levels and Resolution of HE
If the ammonia hypothesis is the sole explanation for the pathogenesis of HE, then the resolution of HE symptoms should be associated with normalization of ammonia levels. Physicians have commonly followed ammonia levels serially throughout a hospital stay. Nicolao et al.15 evaluated the association of ammonia with HE. They noted that some of the CLD patients had unchanged or increasing levels of ammonia despite overt neurological improvement from their HE.15 Some have argued that the normalization of ammonia levels lag behind the clinical improvement by 48 hours after resolution of symptoms. In the Nicolao et al.15 study, ammonia levels for almost all of the patients did not normalize 48 hours after resolution of neurologic symptoms. Moreover, 29% of the patients were noted to have higher venous ammonia levels 48 hours after the resolution of neurologic symptoms.15 These data underscore why serial measurements of ammonia in patients with CLD are not useful. For patients with overt symptoms, clinicians can determine improvement based on serial exams.
RECOMMENDATIONS
- HE is a diagnosis of exclusion and is made on clinical grounds.
- Do not check serum ammonia levels in patients with CLD to diagnose HE, to assess the severity of HE, or to determine whether HE is resolving.
- Use your clinical evaluation to determine the severity and course of HE.
- Treatment should be tailored according to clinical findings, not ammonia levels.
CONCLUSION
The attraction of the ammonia theory to explain HE continues to lead physicians to check and follow blood ammonia levels in patients with CLD and suspected HE. However, ammonia measurement, as in the clinical vignette, should be replaced by a thorough clinical evaluation to rule out other causes for altered mental status. Serial exams of the patient should guide management, not ammonia levels.
Disclosure
The authors report no conflicts of interest.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook, and don’t forget to “Like It” on Facebook or retweet it on Twitter.
1. Tapper EB, Jiang ZG, Patwardhan VR. Refining the ammonia hypothesis: A physiology-driven approach to the treatment of hepatic encephalopathy. Mayo Clin Proc. 2015;90:646-658. PubMed
2. Parekh PJ, Balart LA. Ammonia and Its Role in the Pathogenesis of Hepatic Encephalopathy. Clin Liver Dis. 2015;19:529-537. PubMed
3. Blei AT, Córdoba J. Hepatic Encephalopathy. Am J Gastroenterol. 2001;96:1968-1976. PubMed
4. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study Of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. PubMed
5. Bajaj JS, Cordoba J, Mullen KD, et al. Review Article: the design of clinical trials in Hepatic Encephalopathy - an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33:739-747. PubMed
6. Ahboucha S, Butterworth RF. Pathophysiology of hepatic encephalopathy: A new look at GABA from the molecular standpoint. Metab Brain Dis. 2004;19:331-343. PubMed
7. Butterworth RF. Pathophysiology of Hepatic Encephalopathy: A New Look at Ammonia. 2003;17:1-7. PubMed
8. Schafer DF, Fowler JM, Munson PJ, Thakur AK, Waggoner JG, Jones EA. Gamma-aminobutyric acid and benzodiazepine receptors in an animal model of fulminant hepatic failure. J Lab Clin Med. 1983;102:870-880. PubMed
9. Michalak A, Rose C, Butterworth J, Butterworth RF. Neuroactive amino acids and glutamate (NMDA) receptors in frontal cortex of rats with experimental acute liver failure. Hepatology. 1996;24:908-13. PubMed
10. Bassett ML, Mullen KD, Scholz B, Fenstermacher JD, Jones EA. Increased brain uptake of gamma-aminobutyric acid in a rabbit model of hepatic encephalopathy. Gastroenterology. 1990;98:747-757. PubMed
11. Clay AS, Hainline BE. Hyperammonemia in the ICU. Chest. 2007;132:1368-1378. PubMed
12. Gundling F, Seidl H, Schmidt T, Schepp W. Blood ammonia level in liver cirrhosis: a conditio sine qua non to confirm hepatic encephalopathy? Eur J Gastroenterol Hepatol. 2008;20:246-247. PubMed
13. Stahl J. Studies of the Blood Ammonia in Liver Disease: Its Diagnostic, Prognostic and Therapeutic Significance. Ann Intern Med. 1963;58:1–24. PubMed
14. Kramer L, Tribl B, Gendo A, et al. Partial pressure of ammonia versus ammonia in hepatic encephalopathy. Hepatology. 2000;31:30-34. PubMed
15. Nicolao F, Masini A, Manuela M, Attili AF, Riggio O. Role of determination of partial pressure of ammonia in cirrhotic patients with or without hepatic encephalopathy. J Hepatol. 2003;38:441-446. PubMed
16. Ong JP, Aggarwal A, Krieger D, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114:188-193. PubMed
17. Da Fonseca-Wollheim F. Preanalytical increase of ammonia in blood specimens from healthy subjects. Clin Chem. 1990;36:1483-1487. PubMed
18. Howanitz JH, Howanitz PJ, Skrodzki CA, Iwanski JA. Influences of specimen processing and storage conditions on results for plasma ammonia. Clin Chem. 1984;30:906-908. PubMed
19. Gundling F, Zelihic E, Seidl H, et al. How to diagnose hepatic encephalopathy in the emergency department. Ann Hepatol. 2013;12:108-114. PubMed
20. Ditisheim S, Giostra E, Burkhard PR, et al. A capillary blood ammonia bedside test following glutamine load to improve the diagnosis of hepatic encephalopathy in cirrhosis. BMC Gastroenterol. 2011;11:134. PubMed
21. Conn HO, Leevy CM, Vlahcevic ZR, et al. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology. 1977;72:573-583. PubMed
© 2017 Society of Hospital Medicine
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Ammonia is predominantly generated in the gut by intestinal bacteria and enzymes and detoxified primarily in the liver. Since the 1930s, ammonia has been identified as the principal culprit in hepatic encephalopathy (HE). Many physicians utilize serum ammonia to diagnose, assess severity, and determine the resolution of HE in patients with chronic liver disease (CLD) despite research showing that ammonia levels are unhelpful in all of these clinical circumstances. HE in patients with CLD is a clinical diagnosis of exclusion that should not be based on ammonia levels.
CASE PRESENTATION
A 62-year-old man diagnosed with cirrhosis due to Hepatitis C and alcoholism was brought to the emergency department for alteration in mentation. He had scant melenic stools 5 days preceding his admission and did not exhibit overt signs or symptoms of infection. His systemic examination was normal except for somnolence, disorientation to space and time, asterixis, and ascites. His lab parameters were within normal limits except for an elevated blood urea nitrogen and thrombocytopenia. His blood cultures did not grow any organisms, and paracentesis ruled out spontaneous bacterial peritonitis. During his hospital stay, he underwent esophageal variceal banding and was effectively managed with lactulose and rifaximin. The patient was alert, fully oriented, and without asterixis at the time of discharge 6 days later. Would an elevated venous ammonia level at admission alter management? If the ammonia level was elevated, would serial ammonia measurements affect management?
BACKGROUND
The colonic microbiome produces ammonia from dietary nitrogen. In health, approximately 85% of it is detoxified by the liver and excreted as urea in urine, while muscle and brain tissue metabolize the remaining 15%. The process of transamination and the urea cycle prevents this metabolic product from accumulating in the body. The elevated levels of nitrogenous toxins, including ammonia, in the systemic circulation of patients with CLD occur due to hepatocellular dysfunction and/or portosystemic shunting. This hyperammonemia is compounded by reduced peripheral metabolism of ammonia by muscle as a consequence of cachexia and muscle atrophy. Astrocytes synthesize glutamine excessively in the setting of hyperammonemia, resulting in astrocyte swelling and the generation of reactive oxygen species. Astrocyte swelling, free radical generation, and increased inhibitory function of gamma-Aminobutyric Acid result in cerebral dysfunction.1,2 HE manifests as a broad spectrum of neurological or psychiatric abnormalities ranging from subclinical alterations to coma and was commonly graded on the West Haven Criteria (WHC) of 0 to 4 (Table).3 The Grade 0 from the previous WHC, referenced in many trials included in this article, has been replaced with minimal HE in the newly updated WHC by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver.4,5
WHY YOU MIGHT THINK AMMONIA LEVELS HELP TO GUIDE TREATMENT OF HE IN PATIENTS WITH CLD
The ammonia hypothesis posits that ammonia is key in the pathogenesis of HE.6-10 Some of the common precipitants of HE—gastrointestinal bleeding, infection, and renal failure—promote hyperammonemia.11 HE is treated with nonabsorbable disaccharides (lactulose and lactitol) and rifaximin, which reduce the serum concentration of ammonia. Given these associations between HE and ammonia, physicians have for decades tested serum ammonia levels to diagnose HE and chart its resolution. In a study conducted by the Bavarian Society of Gastroenterology,12 60% of the respondents to an anonymous questionnaire regularly performed ammonia analysis in all their patients with liver cirrhosis, believing that it efficiently diagnosed HE.
WHY SERUM AMMONIA LEVELS DO NOT HELP IN THE DIAGNOSIS OR MANAGEMENT OF HE IN CLD PATIENTS
Accuracy of Serum Ammonia
Multiple factors affect the accuracy of ammonia levels. First, fist clenching or the use of a tourniquet during the process of phlebotomy can falsely increase ammonia levels.13 Second, some authors have argued that the source of the ammonia sample matters. Kramer et al.14 reported that partial pressure of ammonia correlated closely with the degree of clinical and electrophysiological abnormalities of HE. However, Nicolao et al.15 and Ong et al.16 showed that the blood ammonia levels, whether measured by total venous, total arterial, or partial pressure methods, were equivalent. Third, ammonia levels are dependent on the time to processing of the specimen. Inaccurate results may occur if the blood sample is not immediately placed on ice after collection or if it is not centrifuged within 15 minutes of collection.17,18
Ammonia Levels and Diagnosis of HE
Even with proper collection and processing, ammonia levels in patients with CLD do not reliably diagnose HE. Gundling et al.19 determined the sensitivity and specificity of venous ammonia levels ≥ 55 µmol/L to diagnose HE to be 47.2% and 78.3%, respectively, by using a gold standard of the WHC and the critical flicker frequency test (a psychophysiologic test). The positive predictive and negative predictive values of ammonia were 77.3% and 48.6%, with an overall diagnostic accuracy of 59.3%. Approximately 60% of the patients with Grade 3 WHC HE had a normal ammonia level in this study. Ong et al16 found that only 31% of patients with CLD and no evidence of HE had a normal ammonia level.In other words, CLD patients with normal ammonia levels can have HE, and patients with elevated ammonia levels may have normal cognitive functioning.
Furthermore, ammonia levels are not a valid tool to diagnose HE even with an oral glutamine challenge.20 Most importantly, HE is a clinical diagnosis reached following the exclusion of other likely causes of cerebral dysfunction, independent of the ammonia level.
Ammonia Levels and Staging HE
The grading of HE was introduced to assess the response to an intervention in patients with HE enrolled in clinical trials.21 Tools like the WHC (Table) categorize the severity of HE. Nicolao et al.15 noted significant overlap in the levels of ammonia between patients with HE Grades 1 and 2 when compared with patients with Grades 3 and 4. This considerable overlap in levels of ammonia was more evident among patients with Grades 0 to 2 per Ong’s study.16 Most importantly, hospitalists do not need ammonia levels to determine that a patient has HE Grade 3 or HE Grade 4 symptoms, as the stage is graded on clinical grounds only. Once other causes for cerebral dysfunction have been ruled out, the ammonia level does not add to the clinical picture.
Serial Ammonia Levels and Resolution of HE
If the ammonia hypothesis is the sole explanation for the pathogenesis of HE, then the resolution of HE symptoms should be associated with normalization of ammonia levels. Physicians have commonly followed ammonia levels serially throughout a hospital stay. Nicolao et al.15 evaluated the association of ammonia with HE. They noted that some of the CLD patients had unchanged or increasing levels of ammonia despite overt neurological improvement from their HE.15 Some have argued that the normalization of ammonia levels lag behind the clinical improvement by 48 hours after resolution of symptoms. In the Nicolao et al.15 study, ammonia levels for almost all of the patients did not normalize 48 hours after resolution of neurologic symptoms. Moreover, 29% of the patients were noted to have higher venous ammonia levels 48 hours after the resolution of neurologic symptoms.15 These data underscore why serial measurements of ammonia in patients with CLD are not useful. For patients with overt symptoms, clinicians can determine improvement based on serial exams.
RECOMMENDATIONS
- HE is a diagnosis of exclusion and is made on clinical grounds.
- Do not check serum ammonia levels in patients with CLD to diagnose HE, to assess the severity of HE, or to determine whether HE is resolving.
- Use your clinical evaluation to determine the severity and course of HE.
- Treatment should be tailored according to clinical findings, not ammonia levels.
CONCLUSION
The attraction of the ammonia theory to explain HE continues to lead physicians to check and follow blood ammonia levels in patients with CLD and suspected HE. However, ammonia measurement, as in the clinical vignette, should be replaced by a thorough clinical evaluation to rule out other causes for altered mental status. Serial exams of the patient should guide management, not ammonia levels.
Disclosure
The authors report no conflicts of interest.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook, and don’t forget to “Like It” on Facebook or retweet it on Twitter.
© 2017 Society of Hospital Medicine
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Ammonia is predominantly generated in the gut by intestinal bacteria and enzymes and detoxified primarily in the liver. Since the 1930s, ammonia has been identified as the principal culprit in hepatic encephalopathy (HE). Many physicians utilize serum ammonia to diagnose, assess severity, and determine the resolution of HE in patients with chronic liver disease (CLD) despite research showing that ammonia levels are unhelpful in all of these clinical circumstances. HE in patients with CLD is a clinical diagnosis of exclusion that should not be based on ammonia levels.
CASE PRESENTATION
A 62-year-old man diagnosed with cirrhosis due to Hepatitis C and alcoholism was brought to the emergency department for alteration in mentation. He had scant melenic stools 5 days preceding his admission and did not exhibit overt signs or symptoms of infection. His systemic examination was normal except for somnolence, disorientation to space and time, asterixis, and ascites. His lab parameters were within normal limits except for an elevated blood urea nitrogen and thrombocytopenia. His blood cultures did not grow any organisms, and paracentesis ruled out spontaneous bacterial peritonitis. During his hospital stay, he underwent esophageal variceal banding and was effectively managed with lactulose and rifaximin. The patient was alert, fully oriented, and without asterixis at the time of discharge 6 days later. Would an elevated venous ammonia level at admission alter management? If the ammonia level was elevated, would serial ammonia measurements affect management?
BACKGROUND
The colonic microbiome produces ammonia from dietary nitrogen. In health, approximately 85% of it is detoxified by the liver and excreted as urea in urine, while muscle and brain tissue metabolize the remaining 15%. The process of transamination and the urea cycle prevents this metabolic product from accumulating in the body. The elevated levels of nitrogenous toxins, including ammonia, in the systemic circulation of patients with CLD occur due to hepatocellular dysfunction and/or portosystemic shunting. This hyperammonemia is compounded by reduced peripheral metabolism of ammonia by muscle as a consequence of cachexia and muscle atrophy. Astrocytes synthesize glutamine excessively in the setting of hyperammonemia, resulting in astrocyte swelling and the generation of reactive oxygen species. Astrocyte swelling, free radical generation, and increased inhibitory function of gamma-Aminobutyric Acid result in cerebral dysfunction.1,2 HE manifests as a broad spectrum of neurological or psychiatric abnormalities ranging from subclinical alterations to coma and was commonly graded on the West Haven Criteria (WHC) of 0 to 4 (Table).3 The Grade 0 from the previous WHC, referenced in many trials included in this article, has been replaced with minimal HE in the newly updated WHC by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver.4,5
WHY YOU MIGHT THINK AMMONIA LEVELS HELP TO GUIDE TREATMENT OF HE IN PATIENTS WITH CLD
The ammonia hypothesis posits that ammonia is key in the pathogenesis of HE.6-10 Some of the common precipitants of HE—gastrointestinal bleeding, infection, and renal failure—promote hyperammonemia.11 HE is treated with nonabsorbable disaccharides (lactulose and lactitol) and rifaximin, which reduce the serum concentration of ammonia. Given these associations between HE and ammonia, physicians have for decades tested serum ammonia levels to diagnose HE and chart its resolution. In a study conducted by the Bavarian Society of Gastroenterology,12 60% of the respondents to an anonymous questionnaire regularly performed ammonia analysis in all their patients with liver cirrhosis, believing that it efficiently diagnosed HE.
WHY SERUM AMMONIA LEVELS DO NOT HELP IN THE DIAGNOSIS OR MANAGEMENT OF HE IN CLD PATIENTS
Accuracy of Serum Ammonia
Multiple factors affect the accuracy of ammonia levels. First, fist clenching or the use of a tourniquet during the process of phlebotomy can falsely increase ammonia levels.13 Second, some authors have argued that the source of the ammonia sample matters. Kramer et al.14 reported that partial pressure of ammonia correlated closely with the degree of clinical and electrophysiological abnormalities of HE. However, Nicolao et al.15 and Ong et al.16 showed that the blood ammonia levels, whether measured by total venous, total arterial, or partial pressure methods, were equivalent. Third, ammonia levels are dependent on the time to processing of the specimen. Inaccurate results may occur if the blood sample is not immediately placed on ice after collection or if it is not centrifuged within 15 minutes of collection.17,18
Ammonia Levels and Diagnosis of HE
Even with proper collection and processing, ammonia levels in patients with CLD do not reliably diagnose HE. Gundling et al.19 determined the sensitivity and specificity of venous ammonia levels ≥ 55 µmol/L to diagnose HE to be 47.2% and 78.3%, respectively, by using a gold standard of the WHC and the critical flicker frequency test (a psychophysiologic test). The positive predictive and negative predictive values of ammonia were 77.3% and 48.6%, with an overall diagnostic accuracy of 59.3%. Approximately 60% of the patients with Grade 3 WHC HE had a normal ammonia level in this study. Ong et al16 found that only 31% of patients with CLD and no evidence of HE had a normal ammonia level.In other words, CLD patients with normal ammonia levels can have HE, and patients with elevated ammonia levels may have normal cognitive functioning.
Furthermore, ammonia levels are not a valid tool to diagnose HE even with an oral glutamine challenge.20 Most importantly, HE is a clinical diagnosis reached following the exclusion of other likely causes of cerebral dysfunction, independent of the ammonia level.
Ammonia Levels and Staging HE
The grading of HE was introduced to assess the response to an intervention in patients with HE enrolled in clinical trials.21 Tools like the WHC (Table) categorize the severity of HE. Nicolao et al.15 noted significant overlap in the levels of ammonia between patients with HE Grades 1 and 2 when compared with patients with Grades 3 and 4. This considerable overlap in levels of ammonia was more evident among patients with Grades 0 to 2 per Ong’s study.16 Most importantly, hospitalists do not need ammonia levels to determine that a patient has HE Grade 3 or HE Grade 4 symptoms, as the stage is graded on clinical grounds only. Once other causes for cerebral dysfunction have been ruled out, the ammonia level does not add to the clinical picture.
Serial Ammonia Levels and Resolution of HE
If the ammonia hypothesis is the sole explanation for the pathogenesis of HE, then the resolution of HE symptoms should be associated with normalization of ammonia levels. Physicians have commonly followed ammonia levels serially throughout a hospital stay. Nicolao et al.15 evaluated the association of ammonia with HE. They noted that some of the CLD patients had unchanged or increasing levels of ammonia despite overt neurological improvement from their HE.15 Some have argued that the normalization of ammonia levels lag behind the clinical improvement by 48 hours after resolution of symptoms. In the Nicolao et al.15 study, ammonia levels for almost all of the patients did not normalize 48 hours after resolution of neurologic symptoms. Moreover, 29% of the patients were noted to have higher venous ammonia levels 48 hours after the resolution of neurologic symptoms.15 These data underscore why serial measurements of ammonia in patients with CLD are not useful. For patients with overt symptoms, clinicians can determine improvement based on serial exams.
RECOMMENDATIONS
- HE is a diagnosis of exclusion and is made on clinical grounds.
- Do not check serum ammonia levels in patients with CLD to diagnose HE, to assess the severity of HE, or to determine whether HE is resolving.
- Use your clinical evaluation to determine the severity and course of HE.
- Treatment should be tailored according to clinical findings, not ammonia levels.
CONCLUSION
The attraction of the ammonia theory to explain HE continues to lead physicians to check and follow blood ammonia levels in patients with CLD and suspected HE. However, ammonia measurement, as in the clinical vignette, should be replaced by a thorough clinical evaluation to rule out other causes for altered mental status. Serial exams of the patient should guide management, not ammonia levels.
Disclosure
The authors report no conflicts of interest.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook, and don’t forget to “Like It” on Facebook or retweet it on Twitter.
1. Tapper EB, Jiang ZG, Patwardhan VR. Refining the ammonia hypothesis: A physiology-driven approach to the treatment of hepatic encephalopathy. Mayo Clin Proc. 2015;90:646-658. PubMed
2. Parekh PJ, Balart LA. Ammonia and Its Role in the Pathogenesis of Hepatic Encephalopathy. Clin Liver Dis. 2015;19:529-537. PubMed
3. Blei AT, Córdoba J. Hepatic Encephalopathy. Am J Gastroenterol. 2001;96:1968-1976. PubMed
4. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study Of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. PubMed
5. Bajaj JS, Cordoba J, Mullen KD, et al. Review Article: the design of clinical trials in Hepatic Encephalopathy - an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33:739-747. PubMed
6. Ahboucha S, Butterworth RF. Pathophysiology of hepatic encephalopathy: A new look at GABA from the molecular standpoint. Metab Brain Dis. 2004;19:331-343. PubMed
7. Butterworth RF. Pathophysiology of Hepatic Encephalopathy: A New Look at Ammonia. 2003;17:1-7. PubMed
8. Schafer DF, Fowler JM, Munson PJ, Thakur AK, Waggoner JG, Jones EA. Gamma-aminobutyric acid and benzodiazepine receptors in an animal model of fulminant hepatic failure. J Lab Clin Med. 1983;102:870-880. PubMed
9. Michalak A, Rose C, Butterworth J, Butterworth RF. Neuroactive amino acids and glutamate (NMDA) receptors in frontal cortex of rats with experimental acute liver failure. Hepatology. 1996;24:908-13. PubMed
10. Bassett ML, Mullen KD, Scholz B, Fenstermacher JD, Jones EA. Increased brain uptake of gamma-aminobutyric acid in a rabbit model of hepatic encephalopathy. Gastroenterology. 1990;98:747-757. PubMed
11. Clay AS, Hainline BE. Hyperammonemia in the ICU. Chest. 2007;132:1368-1378. PubMed
12. Gundling F, Seidl H, Schmidt T, Schepp W. Blood ammonia level in liver cirrhosis: a conditio sine qua non to confirm hepatic encephalopathy? Eur J Gastroenterol Hepatol. 2008;20:246-247. PubMed
13. Stahl J. Studies of the Blood Ammonia in Liver Disease: Its Diagnostic, Prognostic and Therapeutic Significance. Ann Intern Med. 1963;58:1–24. PubMed
14. Kramer L, Tribl B, Gendo A, et al. Partial pressure of ammonia versus ammonia in hepatic encephalopathy. Hepatology. 2000;31:30-34. PubMed
15. Nicolao F, Masini A, Manuela M, Attili AF, Riggio O. Role of determination of partial pressure of ammonia in cirrhotic patients with or without hepatic encephalopathy. J Hepatol. 2003;38:441-446. PubMed
16. Ong JP, Aggarwal A, Krieger D, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114:188-193. PubMed
17. Da Fonseca-Wollheim F. Preanalytical increase of ammonia in blood specimens from healthy subjects. Clin Chem. 1990;36:1483-1487. PubMed
18. Howanitz JH, Howanitz PJ, Skrodzki CA, Iwanski JA. Influences of specimen processing and storage conditions on results for plasma ammonia. Clin Chem. 1984;30:906-908. PubMed
19. Gundling F, Zelihic E, Seidl H, et al. How to diagnose hepatic encephalopathy in the emergency department. Ann Hepatol. 2013;12:108-114. PubMed
20. Ditisheim S, Giostra E, Burkhard PR, et al. A capillary blood ammonia bedside test following glutamine load to improve the diagnosis of hepatic encephalopathy in cirrhosis. BMC Gastroenterol. 2011;11:134. PubMed
21. Conn HO, Leevy CM, Vlahcevic ZR, et al. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology. 1977;72:573-583. PubMed
1. Tapper EB, Jiang ZG, Patwardhan VR. Refining the ammonia hypothesis: A physiology-driven approach to the treatment of hepatic encephalopathy. Mayo Clin Proc. 2015;90:646-658. PubMed
2. Parekh PJ, Balart LA. Ammonia and Its Role in the Pathogenesis of Hepatic Encephalopathy. Clin Liver Dis. 2015;19:529-537. PubMed
3. Blei AT, Córdoba J. Hepatic Encephalopathy. Am J Gastroenterol. 2001;96:1968-1976. PubMed
4. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study Of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. PubMed
5. Bajaj JS, Cordoba J, Mullen KD, et al. Review Article: the design of clinical trials in Hepatic Encephalopathy - an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33:739-747. PubMed
6. Ahboucha S, Butterworth RF. Pathophysiology of hepatic encephalopathy: A new look at GABA from the molecular standpoint. Metab Brain Dis. 2004;19:331-343. PubMed
7. Butterworth RF. Pathophysiology of Hepatic Encephalopathy: A New Look at Ammonia. 2003;17:1-7. PubMed
8. Schafer DF, Fowler JM, Munson PJ, Thakur AK, Waggoner JG, Jones EA. Gamma-aminobutyric acid and benzodiazepine receptors in an animal model of fulminant hepatic failure. J Lab Clin Med. 1983;102:870-880. PubMed
9. Michalak A, Rose C, Butterworth J, Butterworth RF. Neuroactive amino acids and glutamate (NMDA) receptors in frontal cortex of rats with experimental acute liver failure. Hepatology. 1996;24:908-13. PubMed
10. Bassett ML, Mullen KD, Scholz B, Fenstermacher JD, Jones EA. Increased brain uptake of gamma-aminobutyric acid in a rabbit model of hepatic encephalopathy. Gastroenterology. 1990;98:747-757. PubMed
11. Clay AS, Hainline BE. Hyperammonemia in the ICU. Chest. 2007;132:1368-1378. PubMed
12. Gundling F, Seidl H, Schmidt T, Schepp W. Blood ammonia level in liver cirrhosis: a conditio sine qua non to confirm hepatic encephalopathy? Eur J Gastroenterol Hepatol. 2008;20:246-247. PubMed
13. Stahl J. Studies of the Blood Ammonia in Liver Disease: Its Diagnostic, Prognostic and Therapeutic Significance. Ann Intern Med. 1963;58:1–24. PubMed
14. Kramer L, Tribl B, Gendo A, et al. Partial pressure of ammonia versus ammonia in hepatic encephalopathy. Hepatology. 2000;31:30-34. PubMed
15. Nicolao F, Masini A, Manuela M, Attili AF, Riggio O. Role of determination of partial pressure of ammonia in cirrhotic patients with or without hepatic encephalopathy. J Hepatol. 2003;38:441-446. PubMed
16. Ong JP, Aggarwal A, Krieger D, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114:188-193. PubMed
17. Da Fonseca-Wollheim F. Preanalytical increase of ammonia in blood specimens from healthy subjects. Clin Chem. 1990;36:1483-1487. PubMed
18. Howanitz JH, Howanitz PJ, Skrodzki CA, Iwanski JA. Influences of specimen processing and storage conditions on results for plasma ammonia. Clin Chem. 1984;30:906-908. PubMed
19. Gundling F, Zelihic E, Seidl H, et al. How to diagnose hepatic encephalopathy in the emergency department. Ann Hepatol. 2013;12:108-114. PubMed
20. Ditisheim S, Giostra E, Burkhard PR, et al. A capillary blood ammonia bedside test following glutamine load to improve the diagnosis of hepatic encephalopathy in cirrhosis. BMC Gastroenterol. 2011;11:134. PubMed
21. Conn HO, Leevy CM, Vlahcevic ZR, et al. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology. 1977;72:573-583. PubMed
Fecal occult blood testing in hospitalized patients with upper gastrointestinal bleeding
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 47-year-old man with a history of alcohol abuse, cirrhosis, and grade II esophageal varices is admitted for treatment of alcohol withdrawal. He reports having some dark-colored stools a week prior to admission, but his stools since then have been normal in color. A repeat hemoglobin is stable, but a fecal occult blood test is positive. What should be done next?
BACKGROUND
The US Preventive Services Task Force and the American College of Gastroenterology recommend fecal occult blood testing (FOBT) as one method for colorectal cancer (CRC) screening in average risk populations.1,2 FOBTs can be divided into guaiac-based tests (gFOBTs), which measure heme, and fecal immunochemical tests (FITs), which measure the globin portion of human hemoglobin (Hb). In gFOBTs, heme present in the sample reacts with a hydrogen peroxide developer to oxidize guaiac, producing a blue color.3 Screening gFOBT was shown to decrease mortality from CRC in several landmark studies in the 1990s, but its sensitivity is poor, ranging from 30% to 57%.4 Because the guaiac-induced color change is determined visually, interpretation of gFOBT results are subject to error. In a survey of 173 medical providers, 12% did not accurately interpret gFOBT results.5 In light of these limitations, recent guidelines support the use of newer FITs for CRC screening. FITs utilize antibodies directed against the human globin moiety and demonstrate an increased sensitivity when compared with gFOBTs (by 32% to 62%) for detecting neoplasm.6 While evidence supports the use of FOBTs in CRC screening, providers use these tests for nonvalidated purposes, including the evaluation of suspected acute upper gastrointestinal bleeding (UGIB).
WHY YOU MIGHT THINK FOBT is HELPFUL FOR EVALUATION OF INPATIENTS WITH SUSPECTED ACUTE UGIB
Given the incidence (up to 100 per 100,000 persons per year) and high mortality of UGIB (up to 20,000 deaths annually in the United States),7 there would ideally be a noninvasive test available to help guide management. In evaluating a patient with possible acute UGIB, FOBT affords several theoretical benefits. FOBT is quick, inexpensive, and can be performed by any health professional. In contrast, the primary diagnostic procedure for UGIB, esophagogastroduodenoscopy (EGD), carries procedural and sedation-related risks, can be costly and time-consuming, and requires consultation from subspecialty providers.
WHY FOBT is NOT HELPFUL FOR EVALUATION OF INPATIENTS WITH SUSPECTED ACUTE UGIB
While FOBTs are valuable as screening tests for CRC in the outpatient setting, their use has been extended to diagnose gastrointestinal (GI) bleeding in the inpatient setting without supporting data. As is true for many screening tests, FOBT is associated with a high incidence of false-positive results, or type I errors.8,9 False-positive FOBT results can occur from ingested blood via extra-intestinal sources (eg, epistaxis, gingival bleeding, pharyngitis, hemoptysis), or in medical conditions with intestinal mucosal inflammation (eg, esophagitis, gastritis, inflammatory bowel disease). False-positive results can also be due to clinically insignificant GI blood loss induced by medications (eg, aspirin, nonsteroidal anti-inflammatory drugs), alcohol,10 or by ingestion of meats, fruits, or vegetables containing peroxidase (eg, broccoli, cauliflower).11
Outpatients using FOBTs for cancer screening are advised to hold medications and avoid foods that may lead to false-positive results. Despite institution of these restrictions, false-positive rates are still high, as 37% to 53% of CRC screening patients with a positive FOBT have a subsequent negative colonoscopy, and only 11% to 21% of these patients have a source of bleeding identified on subsequent EGD.12 False-positive results might be even higher in the inpatient setting, where patients typically do not adhere to these restrictions. A review of FOBTs performed in 3 acute care hospitals revealed that 65% of patients tested were on at least one medication that impacted the validity of gFOBT results, and 98% had no evidence of dietary restriction prior to testing.13
The use of FOBTs (particularly FITs) is also subject to false-negative results, or type II errors. While FITs have increased specificity for lower GI bleeding, their ability to detect UGIB is limited, because most Hb is digested in the small intestine and not present in rectal stool.14 In a study of more than 2,700 patients, FIT results were not correlated with the presence of upper GI pathology.15 False-negative results are less common with gFOBTs, although these may occur with low volume, slow or intermittent bleeding,16 or with ingestion of substances that inhibit oxidation, such as vitamin C.17
Beyond these test limitations, studies suggest that the majority of inpatient FOBT results do not impact immediate medical decision-making or management. In one study, only 34% of hospitalized patients with a positive FOBT underwent further GI studies, with the majority of those patients (60%) receiving endoscopy before the results of the FOBT were known.18 In another study of 201 FOBTs performed on hospitalized patients, those with negative results underwent further GI evaluation at a higher rate than those with positive results (41% vs 38%).8 This aligns with a study that revealed the majority of patients suspected of having a GI bleed underwent endoscopic evaluation regardless of the FOBT result.9
WHEN MIGHT FOBT BE HELPFUL?
FOBT currently has a role in CRC screening and
WHAT WE SHOULD DO INSTEAD
A careful history, physical examination, and visual inspection of the stool remain the foundation of establishing UGIB as the etiology of anemia. Observed melena (either by passed stool or a rectal examination) has a likelihood ratio (LR) of 25 for UGIB; a patient’s self-report of stools that sounds melenic (black or tarry) has an LR of 5-6.19 An upper GI source may be further supported by an elevated blood urea nitrogen (BUN) to creatinine ratio, as blood is absorbed through the small bowel and patients may have concomitant decreased renal perfusion. A BUN to creatinine ratio of >30 is associated with a positive LR (LR+) of 7.5 for UGIB.19 Recall that the higher the LR+, and the lower the negative LR (LR-), the better the test is at ruling in and out the diagnosis, respectively. LR+ of 2–10 and LR– of 0.1–0.5 represent a modestly helpful diagnostic test, whereas LR+ >10 and LR- <0.1 are considered robust. These are generalizations only, as value of LR+/LR- depends on pretest probability.
Although Gastroccult23 may be considered for the detection of occult blood in gastric juice, its package insert states: “As with any occult blood test, results with the Gastroccult test cannot be considered conclusive evidence of the presence or absence of upper gastrointestinal bleeding or pathology.” As with any diagnostic evaluation, we would only recommend this test if it would change management.
- FOBT should not be performed to diagnose UGIB.
- When there is clinical suspicion of acute GI bleeding, the best diagnostic tools are a good history, physical examination, and visual inspection of the stool by the clinician to determine the presence of hematochezia or melena.
- Deferring FOBT to the ambulatory setting may improve test performance characteristics.
CONCLUSION
FOBT is validated as an outpatient colon cancer screening tool in asymptomatic patients, not for inpatient evaluation of acute GIB. Given the poor positive predictive value for a positive FOBT in an acute GIB scenario, the potential risk for unnecessary treatments or procedures is real. Conversely, a negative FOBT (particularly FIT) does not rule out GI bleeding and risks a false sense of security that may result in under-treatment. In most scenarios in which FOBT is performed, clinicians can make decisions based on a composite of history, physical exam, visual inspection of the stool, and laboratory investigation. Until further research substantiates the utility of FOBT for this purpose, we would recommend against the routine use of FOBT for evaluating UGIB in hospitalized patients.
Acknowledgment
Disclosure: The authors do not have any relevant financial disclosures to report. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailingTWDFNR@hospitalmedicine.org.
1. U.S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137:129-131. PubMed
2. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. PubMed
3. Carroll MRR, Seaman HE, Halloran HP. Tests and investigations for colorectal cancer screening. Clinical Biochemistry. 2014;47:921-939. PubMed
4. Tinmouth J, Lansdorp-Vogelaar I, Allison JE. Faecal immunochemical tests versus guaiac faecal occult blood tests: what clinicians and colorectal cancer screening programme organisers need to know. Gut. 2015;64(8):1327-1337. PubMed
5. Selinger RR, et al. Failure of health care professionals to interpret fecal occult blood tests accurately. Am J Med. 2003;114(1):64-67. PubMed
6. Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008. Am J Gastroenterol. 2009;104(3):739-750. PubMed
7. El-Tawil AM. Trends on gastrointestinal bleeding and mortality: Where are we standing? World J Gastroenterol. 2012;18(11):1154. PubMed
8. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269. PubMed
9. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426. PubMed
10. Fleming, JL, Ahlquist DA, McGill DB, Zinsmeister AR, Ellefson RD, Schwartz S. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163. PubMed
11. Macrae FA, St John DJB. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82:891-898. PubMed
12. Allard J, et al. Gastroscopy following a positive fecal occult blood test and negative colonoscopy: systematic review and guideline. Can J Gastroenterol. 2010;24(2):113-120. PubMed
13. Friedman A, Chan A, Chin LC, Deen A, Hammerschlag G, Lee M, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111. PubMed
14. Allison JE, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst. 2007;99(19):1462-1470. PubMed
15. Chiang TH, Lee YC, Tu CH, Chiu HM, Wu MS. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481. PubMed
16. Bassett ML, Goulston KJ. False positive and negative hemoccult reactions on a normal diet and effect of diet restriction. Aust N Z J Med. 1980;10(1):1-4. PubMed
17. Jaffe, RM, Kasten B, Young DS, MacLowry JD. False-negative stool occult blood tests caused by ingestion of ascorbic acid (vitamin C). Ann Intern Med. 1975;83(6):824-826. PubMed
18. Ip S, Sokoro AAH, Kaita L, Ruiz C, McIntyre E, Singh H. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494. PubMed
19. Srygley FD, Gerardo CJ, Trun T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072-1079. PubMed
20. Logue KA. Data Request - FOBT. June 2016. Regions Hospital, HealthPartners Laboratory, Saint Paul, Minnesota.
21. Population Clock. http://www.census.gov/popclock/. Accessed July 8, 2016.
22. Mosadeghi S, Ren H, Yen I, Bhuket T. Evaluation of fecal occult blood testing in the acute hospital setting. Gastrointestinal Endoscopy. 2015;81(5).
23. Gastroccult [package insert]. Beckman Coulter, Brea, CA. https://www.beckmancoulter.com/wsrportal/wsr/diagnostics/clinical-products/rapid-diagnostics/gas troccult/index.htm. Accessed March 18, 2008.
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 47-year-old man with a history of alcohol abuse, cirrhosis, and grade II esophageal varices is admitted for treatment of alcohol withdrawal. He reports having some dark-colored stools a week prior to admission, but his stools since then have been normal in color. A repeat hemoglobin is stable, but a fecal occult blood test is positive. What should be done next?
BACKGROUND
The US Preventive Services Task Force and the American College of Gastroenterology recommend fecal occult blood testing (FOBT) as one method for colorectal cancer (CRC) screening in average risk populations.1,2 FOBTs can be divided into guaiac-based tests (gFOBTs), which measure heme, and fecal immunochemical tests (FITs), which measure the globin portion of human hemoglobin (Hb). In gFOBTs, heme present in the sample reacts with a hydrogen peroxide developer to oxidize guaiac, producing a blue color.3 Screening gFOBT was shown to decrease mortality from CRC in several landmark studies in the 1990s, but its sensitivity is poor, ranging from 30% to 57%.4 Because the guaiac-induced color change is determined visually, interpretation of gFOBT results are subject to error. In a survey of 173 medical providers, 12% did not accurately interpret gFOBT results.5 In light of these limitations, recent guidelines support the use of newer FITs for CRC screening. FITs utilize antibodies directed against the human globin moiety and demonstrate an increased sensitivity when compared with gFOBTs (by 32% to 62%) for detecting neoplasm.6 While evidence supports the use of FOBTs in CRC screening, providers use these tests for nonvalidated purposes, including the evaluation of suspected acute upper gastrointestinal bleeding (UGIB).
WHY YOU MIGHT THINK FOBT is HELPFUL FOR EVALUATION OF INPATIENTS WITH SUSPECTED ACUTE UGIB
Given the incidence (up to 100 per 100,000 persons per year) and high mortality of UGIB (up to 20,000 deaths annually in the United States),7 there would ideally be a noninvasive test available to help guide management. In evaluating a patient with possible acute UGIB, FOBT affords several theoretical benefits. FOBT is quick, inexpensive, and can be performed by any health professional. In contrast, the primary diagnostic procedure for UGIB, esophagogastroduodenoscopy (EGD), carries procedural and sedation-related risks, can be costly and time-consuming, and requires consultation from subspecialty providers.
WHY FOBT is NOT HELPFUL FOR EVALUATION OF INPATIENTS WITH SUSPECTED ACUTE UGIB
While FOBTs are valuable as screening tests for CRC in the outpatient setting, their use has been extended to diagnose gastrointestinal (GI) bleeding in the inpatient setting without supporting data. As is true for many screening tests, FOBT is associated with a high incidence of false-positive results, or type I errors.8,9 False-positive FOBT results can occur from ingested blood via extra-intestinal sources (eg, epistaxis, gingival bleeding, pharyngitis, hemoptysis), or in medical conditions with intestinal mucosal inflammation (eg, esophagitis, gastritis, inflammatory bowel disease). False-positive results can also be due to clinically insignificant GI blood loss induced by medications (eg, aspirin, nonsteroidal anti-inflammatory drugs), alcohol,10 or by ingestion of meats, fruits, or vegetables containing peroxidase (eg, broccoli, cauliflower).11
Outpatients using FOBTs for cancer screening are advised to hold medications and avoid foods that may lead to false-positive results. Despite institution of these restrictions, false-positive rates are still high, as 37% to 53% of CRC screening patients with a positive FOBT have a subsequent negative colonoscopy, and only 11% to 21% of these patients have a source of bleeding identified on subsequent EGD.12 False-positive results might be even higher in the inpatient setting, where patients typically do not adhere to these restrictions. A review of FOBTs performed in 3 acute care hospitals revealed that 65% of patients tested were on at least one medication that impacted the validity of gFOBT results, and 98% had no evidence of dietary restriction prior to testing.13
The use of FOBTs (particularly FITs) is also subject to false-negative results, or type II errors. While FITs have increased specificity for lower GI bleeding, their ability to detect UGIB is limited, because most Hb is digested in the small intestine and not present in rectal stool.14 In a study of more than 2,700 patients, FIT results were not correlated with the presence of upper GI pathology.15 False-negative results are less common with gFOBTs, although these may occur with low volume, slow or intermittent bleeding,16 or with ingestion of substances that inhibit oxidation, such as vitamin C.17
Beyond these test limitations, studies suggest that the majority of inpatient FOBT results do not impact immediate medical decision-making or management. In one study, only 34% of hospitalized patients with a positive FOBT underwent further GI studies, with the majority of those patients (60%) receiving endoscopy before the results of the FOBT were known.18 In another study of 201 FOBTs performed on hospitalized patients, those with negative results underwent further GI evaluation at a higher rate than those with positive results (41% vs 38%).8 This aligns with a study that revealed the majority of patients suspected of having a GI bleed underwent endoscopic evaluation regardless of the FOBT result.9
WHEN MIGHT FOBT BE HELPFUL?
FOBT currently has a role in CRC screening and
WHAT WE SHOULD DO INSTEAD
A careful history, physical examination, and visual inspection of the stool remain the foundation of establishing UGIB as the etiology of anemia. Observed melena (either by passed stool or a rectal examination) has a likelihood ratio (LR) of 25 for UGIB; a patient’s self-report of stools that sounds melenic (black or tarry) has an LR of 5-6.19 An upper GI source may be further supported by an elevated blood urea nitrogen (BUN) to creatinine ratio, as blood is absorbed through the small bowel and patients may have concomitant decreased renal perfusion. A BUN to creatinine ratio of >30 is associated with a positive LR (LR+) of 7.5 for UGIB.19 Recall that the higher the LR+, and the lower the negative LR (LR-), the better the test is at ruling in and out the diagnosis, respectively. LR+ of 2–10 and LR– of 0.1–0.5 represent a modestly helpful diagnostic test, whereas LR+ >10 and LR- <0.1 are considered robust. These are generalizations only, as value of LR+/LR- depends on pretest probability.
Although Gastroccult23 may be considered for the detection of occult blood in gastric juice, its package insert states: “As with any occult blood test, results with the Gastroccult test cannot be considered conclusive evidence of the presence or absence of upper gastrointestinal bleeding or pathology.” As with any diagnostic evaluation, we would only recommend this test if it would change management.
- FOBT should not be performed to diagnose UGIB.
- When there is clinical suspicion of acute GI bleeding, the best diagnostic tools are a good history, physical examination, and visual inspection of the stool by the clinician to determine the presence of hematochezia or melena.
- Deferring FOBT to the ambulatory setting may improve test performance characteristics.
CONCLUSION
FOBT is validated as an outpatient colon cancer screening tool in asymptomatic patients, not for inpatient evaluation of acute GIB. Given the poor positive predictive value for a positive FOBT in an acute GIB scenario, the potential risk for unnecessary treatments or procedures is real. Conversely, a negative FOBT (particularly FIT) does not rule out GI bleeding and risks a false sense of security that may result in under-treatment. In most scenarios in which FOBT is performed, clinicians can make decisions based on a composite of history, physical exam, visual inspection of the stool, and laboratory investigation. Until further research substantiates the utility of FOBT for this purpose, we would recommend against the routine use of FOBT for evaluating UGIB in hospitalized patients.
Acknowledgment
Disclosure: The authors do not have any relevant financial disclosures to report. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailingTWDFNR@hospitalmedicine.org.
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 47-year-old man with a history of alcohol abuse, cirrhosis, and grade II esophageal varices is admitted for treatment of alcohol withdrawal. He reports having some dark-colored stools a week prior to admission, but his stools since then have been normal in color. A repeat hemoglobin is stable, but a fecal occult blood test is positive. What should be done next?
BACKGROUND
The US Preventive Services Task Force and the American College of Gastroenterology recommend fecal occult blood testing (FOBT) as one method for colorectal cancer (CRC) screening in average risk populations.1,2 FOBTs can be divided into guaiac-based tests (gFOBTs), which measure heme, and fecal immunochemical tests (FITs), which measure the globin portion of human hemoglobin (Hb). In gFOBTs, heme present in the sample reacts with a hydrogen peroxide developer to oxidize guaiac, producing a blue color.3 Screening gFOBT was shown to decrease mortality from CRC in several landmark studies in the 1990s, but its sensitivity is poor, ranging from 30% to 57%.4 Because the guaiac-induced color change is determined visually, interpretation of gFOBT results are subject to error. In a survey of 173 medical providers, 12% did not accurately interpret gFOBT results.5 In light of these limitations, recent guidelines support the use of newer FITs for CRC screening. FITs utilize antibodies directed against the human globin moiety and demonstrate an increased sensitivity when compared with gFOBTs (by 32% to 62%) for detecting neoplasm.6 While evidence supports the use of FOBTs in CRC screening, providers use these tests for nonvalidated purposes, including the evaluation of suspected acute upper gastrointestinal bleeding (UGIB).
WHY YOU MIGHT THINK FOBT is HELPFUL FOR EVALUATION OF INPATIENTS WITH SUSPECTED ACUTE UGIB
Given the incidence (up to 100 per 100,000 persons per year) and high mortality of UGIB (up to 20,000 deaths annually in the United States),7 there would ideally be a noninvasive test available to help guide management. In evaluating a patient with possible acute UGIB, FOBT affords several theoretical benefits. FOBT is quick, inexpensive, and can be performed by any health professional. In contrast, the primary diagnostic procedure for UGIB, esophagogastroduodenoscopy (EGD), carries procedural and sedation-related risks, can be costly and time-consuming, and requires consultation from subspecialty providers.
WHY FOBT is NOT HELPFUL FOR EVALUATION OF INPATIENTS WITH SUSPECTED ACUTE UGIB
While FOBTs are valuable as screening tests for CRC in the outpatient setting, their use has been extended to diagnose gastrointestinal (GI) bleeding in the inpatient setting without supporting data. As is true for many screening tests, FOBT is associated with a high incidence of false-positive results, or type I errors.8,9 False-positive FOBT results can occur from ingested blood via extra-intestinal sources (eg, epistaxis, gingival bleeding, pharyngitis, hemoptysis), or in medical conditions with intestinal mucosal inflammation (eg, esophagitis, gastritis, inflammatory bowel disease). False-positive results can also be due to clinically insignificant GI blood loss induced by medications (eg, aspirin, nonsteroidal anti-inflammatory drugs), alcohol,10 or by ingestion of meats, fruits, or vegetables containing peroxidase (eg, broccoli, cauliflower).11
Outpatients using FOBTs for cancer screening are advised to hold medications and avoid foods that may lead to false-positive results. Despite institution of these restrictions, false-positive rates are still high, as 37% to 53% of CRC screening patients with a positive FOBT have a subsequent negative colonoscopy, and only 11% to 21% of these patients have a source of bleeding identified on subsequent EGD.12 False-positive results might be even higher in the inpatient setting, where patients typically do not adhere to these restrictions. A review of FOBTs performed in 3 acute care hospitals revealed that 65% of patients tested were on at least one medication that impacted the validity of gFOBT results, and 98% had no evidence of dietary restriction prior to testing.13
The use of FOBTs (particularly FITs) is also subject to false-negative results, or type II errors. While FITs have increased specificity for lower GI bleeding, their ability to detect UGIB is limited, because most Hb is digested in the small intestine and not present in rectal stool.14 In a study of more than 2,700 patients, FIT results were not correlated with the presence of upper GI pathology.15 False-negative results are less common with gFOBTs, although these may occur with low volume, slow or intermittent bleeding,16 or with ingestion of substances that inhibit oxidation, such as vitamin C.17
Beyond these test limitations, studies suggest that the majority of inpatient FOBT results do not impact immediate medical decision-making or management. In one study, only 34% of hospitalized patients with a positive FOBT underwent further GI studies, with the majority of those patients (60%) receiving endoscopy before the results of the FOBT were known.18 In another study of 201 FOBTs performed on hospitalized patients, those with negative results underwent further GI evaluation at a higher rate than those with positive results (41% vs 38%).8 This aligns with a study that revealed the majority of patients suspected of having a GI bleed underwent endoscopic evaluation regardless of the FOBT result.9
WHEN MIGHT FOBT BE HELPFUL?
FOBT currently has a role in CRC screening and
WHAT WE SHOULD DO INSTEAD
A careful history, physical examination, and visual inspection of the stool remain the foundation of establishing UGIB as the etiology of anemia. Observed melena (either by passed stool or a rectal examination) has a likelihood ratio (LR) of 25 for UGIB; a patient’s self-report of stools that sounds melenic (black or tarry) has an LR of 5-6.19 An upper GI source may be further supported by an elevated blood urea nitrogen (BUN) to creatinine ratio, as blood is absorbed through the small bowel and patients may have concomitant decreased renal perfusion. A BUN to creatinine ratio of >30 is associated with a positive LR (LR+) of 7.5 for UGIB.19 Recall that the higher the LR+, and the lower the negative LR (LR-), the better the test is at ruling in and out the diagnosis, respectively. LR+ of 2–10 and LR– of 0.1–0.5 represent a modestly helpful diagnostic test, whereas LR+ >10 and LR- <0.1 are considered robust. These are generalizations only, as value of LR+/LR- depends on pretest probability.
Although Gastroccult23 may be considered for the detection of occult blood in gastric juice, its package insert states: “As with any occult blood test, results with the Gastroccult test cannot be considered conclusive evidence of the presence or absence of upper gastrointestinal bleeding or pathology.” As with any diagnostic evaluation, we would only recommend this test if it would change management.
- FOBT should not be performed to diagnose UGIB.
- When there is clinical suspicion of acute GI bleeding, the best diagnostic tools are a good history, physical examination, and visual inspection of the stool by the clinician to determine the presence of hematochezia or melena.
- Deferring FOBT to the ambulatory setting may improve test performance characteristics.
CONCLUSION
FOBT is validated as an outpatient colon cancer screening tool in asymptomatic patients, not for inpatient evaluation of acute GIB. Given the poor positive predictive value for a positive FOBT in an acute GIB scenario, the potential risk for unnecessary treatments or procedures is real. Conversely, a negative FOBT (particularly FIT) does not rule out GI bleeding and risks a false sense of security that may result in under-treatment. In most scenarios in which FOBT is performed, clinicians can make decisions based on a composite of history, physical exam, visual inspection of the stool, and laboratory investigation. Until further research substantiates the utility of FOBT for this purpose, we would recommend against the routine use of FOBT for evaluating UGIB in hospitalized patients.
Acknowledgment
Disclosure: The authors do not have any relevant financial disclosures to report. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailingTWDFNR@hospitalmedicine.org.
1. U.S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137:129-131. PubMed
2. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. PubMed
3. Carroll MRR, Seaman HE, Halloran HP. Tests and investigations for colorectal cancer screening. Clinical Biochemistry. 2014;47:921-939. PubMed
4. Tinmouth J, Lansdorp-Vogelaar I, Allison JE. Faecal immunochemical tests versus guaiac faecal occult blood tests: what clinicians and colorectal cancer screening programme organisers need to know. Gut. 2015;64(8):1327-1337. PubMed
5. Selinger RR, et al. Failure of health care professionals to interpret fecal occult blood tests accurately. Am J Med. 2003;114(1):64-67. PubMed
6. Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008. Am J Gastroenterol. 2009;104(3):739-750. PubMed
7. El-Tawil AM. Trends on gastrointestinal bleeding and mortality: Where are we standing? World J Gastroenterol. 2012;18(11):1154. PubMed
8. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269. PubMed
9. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426. PubMed
10. Fleming, JL, Ahlquist DA, McGill DB, Zinsmeister AR, Ellefson RD, Schwartz S. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163. PubMed
11. Macrae FA, St John DJB. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82:891-898. PubMed
12. Allard J, et al. Gastroscopy following a positive fecal occult blood test and negative colonoscopy: systematic review and guideline. Can J Gastroenterol. 2010;24(2):113-120. PubMed
13. Friedman A, Chan A, Chin LC, Deen A, Hammerschlag G, Lee M, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111. PubMed
14. Allison JE, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst. 2007;99(19):1462-1470. PubMed
15. Chiang TH, Lee YC, Tu CH, Chiu HM, Wu MS. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481. PubMed
16. Bassett ML, Goulston KJ. False positive and negative hemoccult reactions on a normal diet and effect of diet restriction. Aust N Z J Med. 1980;10(1):1-4. PubMed
17. Jaffe, RM, Kasten B, Young DS, MacLowry JD. False-negative stool occult blood tests caused by ingestion of ascorbic acid (vitamin C). Ann Intern Med. 1975;83(6):824-826. PubMed
18. Ip S, Sokoro AAH, Kaita L, Ruiz C, McIntyre E, Singh H. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494. PubMed
19. Srygley FD, Gerardo CJ, Trun T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072-1079. PubMed
20. Logue KA. Data Request - FOBT. June 2016. Regions Hospital, HealthPartners Laboratory, Saint Paul, Minnesota.
21. Population Clock. http://www.census.gov/popclock/. Accessed July 8, 2016.
22. Mosadeghi S, Ren H, Yen I, Bhuket T. Evaluation of fecal occult blood testing in the acute hospital setting. Gastrointestinal Endoscopy. 2015;81(5).
23. Gastroccult [package insert]. Beckman Coulter, Brea, CA. https://www.beckmancoulter.com/wsrportal/wsr/diagnostics/clinical-products/rapid-diagnostics/gas troccult/index.htm. Accessed March 18, 2008.
1. U.S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137:129-131. PubMed
2. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. PubMed
3. Carroll MRR, Seaman HE, Halloran HP. Tests and investigations for colorectal cancer screening. Clinical Biochemistry. 2014;47:921-939. PubMed
4. Tinmouth J, Lansdorp-Vogelaar I, Allison JE. Faecal immunochemical tests versus guaiac faecal occult blood tests: what clinicians and colorectal cancer screening programme organisers need to know. Gut. 2015;64(8):1327-1337. PubMed
5. Selinger RR, et al. Failure of health care professionals to interpret fecal occult blood tests accurately. Am J Med. 2003;114(1):64-67. PubMed
6. Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008. Am J Gastroenterol. 2009;104(3):739-750. PubMed
7. El-Tawil AM. Trends on gastrointestinal bleeding and mortality: Where are we standing? World J Gastroenterol. 2012;18(11):1154. PubMed
8. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269. PubMed
9. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426. PubMed
10. Fleming, JL, Ahlquist DA, McGill DB, Zinsmeister AR, Ellefson RD, Schwartz S. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163. PubMed
11. Macrae FA, St John DJB. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82:891-898. PubMed
12. Allard J, et al. Gastroscopy following a positive fecal occult blood test and negative colonoscopy: systematic review and guideline. Can J Gastroenterol. 2010;24(2):113-120. PubMed
13. Friedman A, Chan A, Chin LC, Deen A, Hammerschlag G, Lee M, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111. PubMed
14. Allison JE, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst. 2007;99(19):1462-1470. PubMed
15. Chiang TH, Lee YC, Tu CH, Chiu HM, Wu MS. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481. PubMed
16. Bassett ML, Goulston KJ. False positive and negative hemoccult reactions on a normal diet and effect of diet restriction. Aust N Z J Med. 1980;10(1):1-4. PubMed
17. Jaffe, RM, Kasten B, Young DS, MacLowry JD. False-negative stool occult blood tests caused by ingestion of ascorbic acid (vitamin C). Ann Intern Med. 1975;83(6):824-826. PubMed
18. Ip S, Sokoro AAH, Kaita L, Ruiz C, McIntyre E, Singh H. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494. PubMed
19. Srygley FD, Gerardo CJ, Trun T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072-1079. PubMed
20. Logue KA. Data Request - FOBT. June 2016. Regions Hospital, HealthPartners Laboratory, Saint Paul, Minnesota.
21. Population Clock. http://www.census.gov/popclock/. Accessed July 8, 2016.
22. Mosadeghi S, Ren H, Yen I, Bhuket T. Evaluation of fecal occult blood testing in the acute hospital setting. Gastrointestinal Endoscopy. 2015;81(5).
23. Gastroccult [package insert]. Beckman Coulter, Brea, CA. https://www.beckmancoulter.com/wsrportal/wsr/diagnostics/clinical-products/rapid-diagnostics/gas troccult/index.htm. Accessed March 18, 2008.
© 2017 Society of Hospital Medicine
Empiric <i>Listeria monocytogenes</i> antibiotic coverage for febrile infants (age, 0-90 days)
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Evaluation and treatment of the febrile infant 0 to 90 days of age are common clinical issues in pediatrics, family medicine, emergency medicine, and pediatric hospital medicine. Traditional teaching has been that Listeria monocytogenes is 1 of the 3 most common pathogens causing neonatal sepsis. Many practitioners routinely use antibiotic regimens, including ampicillin, to specifically target Listeria. However, a large body of evidence, including a meta-analysis and several multicenter studies, has shown that listeriosis is extremely rare in the United States. The practice of empiric ampicillin thus exposes the patient to harms and costs with little if any potential benefit, while increasing pressure on the bacterial flora in the community to generate antibiotic resistance. Empiric ampicillin for all infants admitted for sepsis evaluation is a tradition-based practice no longer founded on the best available evidence.
CASE REPORT
A 32-day-old, full-term, previously healthy girl presented with fever of 1 day’s duration. Her parents reported she had appeared well until the evening before admission, when she became a bit less active and spent less time breastfeeding. The morning of admission, she was fussier than usual. Rectal temperature, taken by her parents, was 101°F. There were no other symptoms and no sick contacts.
On examination, the patient’s rectal temperature was 101.5°F. Her other vitals and the physical examination findings were unremarkable. Laboratory test results included a normal urinalysis and a peripheral white blood cell (WBC) count of 21,300 cells/µL. Cerebrospinal fluid (CSF) analysis revealed normal protein and glucose levels with 3 WBCs/µL and a negative gram stain. Due to stratifying at higher risk for serious bacterial infection (SBI), the child was admitted and started on ampicillin and cefotaxime while awaiting culture results.
BACKGROUND
Evaluation and treatment of febrile infants are common clinical issues in pediatrics, emergency medicine, and general practice. Practice guidelines for evaluation of febrile infants recommend hospitalization and parenteral antibiotics for children younger than 28 days and children 29 to 90 days old if stratified at high risk for SBI.1,2 Recommendations for empiric antibiotic regimens include ampicillin in addition to either gentamicin or cefotaxime.1,2
WHY YOU MIGHT THINK AMPICILLIN IS HELPFUL
Generations of pediatrics students have been taught that the 3 pathogens most likely to cause bacterial sepsis in infants are group B Streptococcus (GBS), Escherichia coli, and Listeria monocytogenes. This teaching is still espoused in the latest editions of pediatrics textbooks.3 Ampicillin is specifically recommended for covering Listeria, and studies have found that 62% to 78% of practitioners choose empiric ampicillin-containing antibiotic regimens for the treatment of febrile infants.4-6
WHY EMPIRIC AMPICILLIN IS UNNECESSARY
In the past, Listeria was a potential though still uncommon infant pathogen. Over the past few decades, however, the epidemiology of infant sepsis has changed significantly. Estimates of the rate of infection with Listeria now range from extremely rare to nonexistent across multiple studies4,7-15 (Table). In a 4-year retrospective case series at a single urban academic center in Washington, DC, Sadow et al.4 reported no instances of Listeria among 121 positive bacterial cultures in infants younger than 60 days seen in the emergency department (ED). Byington et al.7 examined all positive cultures for infants 0 to 90 days old at a large academic referral center in Utah over a 3-year period and reported no cases of Listeria (1298 patients, 105 SBI cases). A study at a North Carolina academic center found 1 case of Listeria meningitis among 72 SBIs (668 febrile infants) without a localizing source.8 At a large group-practice in northern California, Greenhow et al.9 examined all blood cultures (N = 4255) performed over 4 years for otherwise healthy infants 1 week to 3 months old and found no cases of Listeria. In a follow-up study, the same authors examined all blood (n = 5396), urine (n = 4599), and CSF (n = 1796) cultures in the same population and found no Listeria cases.10 Hassoun et al.11 studied SBI rates among infants younger than 28 days with any blood, urine, or CSF culture performed over 4 years at two Michigan EDs. One (0.08%) of the 1192 infants evaluated had bacteremia caused by Listeria.
Multicenter studies have reported similar results. In a study of 6 hospital systems in geographically diverse areas of the United States, Biondi et al.12 examined all positive blood cultures (N = 181) for febrile infants younger than 90 days admitted to a general pediatric ward, and found no listeriosis. Mischler et al.13 examined all positive blood cultures (N = 392) for otherwise healthy febrile infants 0 to 90 days old admitted to a hospital in 1 of 17 geographically diverse healthcare systems and found no cases of Listeria. A recent meta-analysis of studies that reported SBI rates for febrile infants 0 to 90 days old found the weighted prevalence of Listeria bacteremia to be 0.03% (2/20,703) and that of meningitis to be 0.02% (3/13,375).14 Veesenmeyer and Edmonson15 used a national inpatient database to identify all Listeria cases among infants over a 6-year period and estimated listeriosis rates for the US population. Over the 6 years, there were 212 total cases, which were extrapolated to 344 in the United States during that period, yielding a pooled annual incidence rate of 1.41 in 100,000 births.
Ampicillin offers no significant improvement in coverage for GBS or E coli beyond other β-lactam antibiotics, such as cefotaxime. Therefore, though the cost and potential harms of 24 to 48 hours of intravenous ampicillin are low for the individual patient, there is almost no potential benefit. Using the weighted prevalence of 0.03% for Listeria bacteremia reported in the recent meta-analysis,14 the number needed to treat to cover 1 case of Listeria bacteremia would be 3333. In addition, the increasing incidence of ampicillin resistance, particularly among gram-negative organisms,4,7,9 argues strongly for better antibiotic stewardship on a national level. A number of expert authors have advocated dropping empiric Listeria coverage as part of the treatment of febrile infants, particularly infants 29 to 90 days old.16,17 Some authors continue to advocate empiric Listeria coverage.6 It is interesting to note, however, that the incidence of Staph aureus bacteremia in recent case series is much higher than that reported for Listeria, accounting for 6-9% of bacteremia cases.9,11,13 Yet few if any authors advocate for empiric S. aureus coverage.
WHEN EMPIRIC AMPICILLIN COVERAGE MAY BE REASONABLE
The rate of listeriosis remains low across age groups in recent studies, but the rate is slightly higher in very young infants. In the recent national database study of listeriosis cases over a 6-year period, almost half involved infants younger than 7 days, and most of these infants showed no evidence of meningitis.15 Therefore, it may be reasonable to include empiric Listeria coverage in febrile infants younger than 7 days, though the study authors estimated 22.5 annual cases of Listeria in this age range in the United States. Eighty percent of the Listeria cases were in infants younger than 28 days, but more than 85% of infants 7 to 28 days old had meningitis. Therefore, broad antimicrobial coverage for infants with CSF pleocytosis and/or a high bacterial meningitis score is reasonable, especially for infants younger than 28 days.
Other potential indications for ampicillin are enterococcal infections. Though enteroccocal SBI rates in febrile infants are also quite low,7-9,11,12 if Enterococcus were highly suspected, such as in an infant with pyuria and gram positive organisms on gram stain, ampicillin offers good additional coverage. In the case of a local outbreak of listeriosis, or a specific exposure to Listeria-contaminated products on a patient history, antibiotics with efficacy against Listeria should be used. Last, in cases in which gentamicin is used as empiric coverage for gram-negative organisms, ampicillin offers important additional coverage for GBS.
Some practitioners advocate ampicillin and gentamicin over cefotaxime regimens on the basis of an often cited study that found a survival benefit for febrile neonates in the intensive care setting.18 There are a number of reasons that this study should not influence care for typical infants admitted with possible sepsis. First, the study was retrospective and limited by its use of administrative data. The authors acknowledged that a potential explanation for their results is unmeasured confounding. Second, the patients included in the study were dramatically different from the group of well infants admitted with possible sepsis; the study included neonatal critical care unit patients treated with antibiotics within the first 3 days of life. Third, the study’s results have not been replicated in otherwise healthy febrile infants.
WHAT YOU SHOULD USE INSTEAD OF AMPICILLIN FOR EMPIRIC LISTERIA COVERAGE
For febrile children 0 to 90 days old, empiric antibiotic coverage should be aimed at covering the current predominant pathogens, which include E coli and GBS. Therefore, for most children and US regions, a third-generation cephalosporin (eg, cefotaxime) is sufficient.
RECOMMENDATIONS
- Empiric antibiotics for treatment of febrile children 0-90 days should target E. coli and GBS; a third generation cephalosporin, (e.g. cefotaxime) alone is a reasonable choice for most patients.
- Prescribing ampicillin to specifically cover Listeria is unnecessary for the vast majority of febrile infants
- Prescribing ampicillin is reasonable in certain subgroups of febrile infants: those less than seven days of age, those with evidence of bacterial meningitis (especially if also <28 days of age), those in whom enterococcal infection is strongly suspected, and those with specific Listeria exposures related to local outbreaks.
CONCLUSION
The 32-day-old infant described in the clinical scenario was at extremely low risk for listeriosis. Antibiotic coverage with a third-generation cephalosporin is sufficient for the most likely pathogens. The common practice of empirically covering Listeria in otherwise healthy febrile infants considered to be at higher risk for SBI is no longer based on best available evidence and represents overtreatment with at least theoretical harms. Avoidance of the risks associated with the side effects of antibiotics, costs saved by forgoing multiple antibiotics, a decrease in medication dosing frequency, and improved antibiotic stewardship for the general population all argue forcefully for making empiric Listeria coverage a thing of the past.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med. 1993;22(7):1198-1210. PubMed
2. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42(4):530-545. PubMed
3. Nield L, Kamat D. Fever without a focus. In: Kliegman R, Stanton B, eds. Nelson’s Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016.
4. Sadow KB, Derr R, Teach SJ. Bacterial infections in infants 60 days and younger: epidemiology, resistance, and implications for treatment. Arch Pediatr Adolesc Med. 1999;153(6):611-614. PubMed
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. PubMed
6. Cantey JB, Lopez-Medina E, Nguyen S, Doern C, Garcia C. Empiric antibiotics for serious bacterial infection in young infants: opportunities for stewardship. Pediatr Emerg Care. 2015;31(8):568-571. PubMed
7. Byington CL, Rittichier KK, Bassett KE, et al. Serious bacterial infections in febrile infants younger than 90 days of age: the importance of ampicillin-resistant pathogens. Pediatrics. 2003;111(5 pt 1):964-968. PubMed
8. Watt K, Waddle E, Jhaveri R. Changing epidemiology of serious bacterial infections in febrile infants without localizing signs. PLoS One. 2010;5(8):e12448. PubMed
9. Greenhow TL, Hung YY, Herz AM. Changing epidemiology of bacteremia in infants aged 1 week to 3 months. Pediatrics. 2012;129(3):e590-e596. PubMed
10. Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. PubMed
11. Hassoun A, Stankovic C, Rogers A, et al. Listeria and enterococcal infections in neonates 28 days of age and younger: is empiric parenteral ampicillin still indicated? Pediatr Emerg Care. 2014;30(4):240-243. PubMed
12. Biondi E, Evans R, Mischler M, et al. Epidemiology of bacteremia in febrile infants in the United States. Pediatrics. 2013;132(6):990-996. PubMed
13. Mischler M, Ryan MS, Leyenaar JK, et al. Epidemiology of bacteremia in previously healthy febrile infants: a follow-up study. Hosp Pediatr. 2015;5(6):293-300. PubMed
14. Leazer R, Perkins AM, Shomaker K, Fine B. A meta-analysis of the rates of Listeria monocytogenes and Enterococcus in febrile infants. Hosp Pediatr. 2016;6(4):187-195. PubMed
15. Veesenmeyer AF, Edmonson MB. Trends in US hospital stays for listeriosis in infants. Hosp Pediatr. 2016;6(4):196-203. PubMed
16. Schroeder AR, Roberts KB. Is tradition trumping evidence in the treatment of young, febrile infants? Hosp Pediatr. 2016;6(4):252-253. PubMed
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. PubMed
18. Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117(1):67-74. PubMed
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Evaluation and treatment of the febrile infant 0 to 90 days of age are common clinical issues in pediatrics, family medicine, emergency medicine, and pediatric hospital medicine. Traditional teaching has been that Listeria monocytogenes is 1 of the 3 most common pathogens causing neonatal sepsis. Many practitioners routinely use antibiotic regimens, including ampicillin, to specifically target Listeria. However, a large body of evidence, including a meta-analysis and several multicenter studies, has shown that listeriosis is extremely rare in the United States. The practice of empiric ampicillin thus exposes the patient to harms and costs with little if any potential benefit, while increasing pressure on the bacterial flora in the community to generate antibiotic resistance. Empiric ampicillin for all infants admitted for sepsis evaluation is a tradition-based practice no longer founded on the best available evidence.
CASE REPORT
A 32-day-old, full-term, previously healthy girl presented with fever of 1 day’s duration. Her parents reported she had appeared well until the evening before admission, when she became a bit less active and spent less time breastfeeding. The morning of admission, she was fussier than usual. Rectal temperature, taken by her parents, was 101°F. There were no other symptoms and no sick contacts.
On examination, the patient’s rectal temperature was 101.5°F. Her other vitals and the physical examination findings were unremarkable. Laboratory test results included a normal urinalysis and a peripheral white blood cell (WBC) count of 21,300 cells/µL. Cerebrospinal fluid (CSF) analysis revealed normal protein and glucose levels with 3 WBCs/µL and a negative gram stain. Due to stratifying at higher risk for serious bacterial infection (SBI), the child was admitted and started on ampicillin and cefotaxime while awaiting culture results.
BACKGROUND
Evaluation and treatment of febrile infants are common clinical issues in pediatrics, emergency medicine, and general practice. Practice guidelines for evaluation of febrile infants recommend hospitalization and parenteral antibiotics for children younger than 28 days and children 29 to 90 days old if stratified at high risk for SBI.1,2 Recommendations for empiric antibiotic regimens include ampicillin in addition to either gentamicin or cefotaxime.1,2
WHY YOU MIGHT THINK AMPICILLIN IS HELPFUL
Generations of pediatrics students have been taught that the 3 pathogens most likely to cause bacterial sepsis in infants are group B Streptococcus (GBS), Escherichia coli, and Listeria monocytogenes. This teaching is still espoused in the latest editions of pediatrics textbooks.3 Ampicillin is specifically recommended for covering Listeria, and studies have found that 62% to 78% of practitioners choose empiric ampicillin-containing antibiotic regimens for the treatment of febrile infants.4-6
WHY EMPIRIC AMPICILLIN IS UNNECESSARY
In the past, Listeria was a potential though still uncommon infant pathogen. Over the past few decades, however, the epidemiology of infant sepsis has changed significantly. Estimates of the rate of infection with Listeria now range from extremely rare to nonexistent across multiple studies4,7-15 (Table). In a 4-year retrospective case series at a single urban academic center in Washington, DC, Sadow et al.4 reported no instances of Listeria among 121 positive bacterial cultures in infants younger than 60 days seen in the emergency department (ED). Byington et al.7 examined all positive cultures for infants 0 to 90 days old at a large academic referral center in Utah over a 3-year period and reported no cases of Listeria (1298 patients, 105 SBI cases). A study at a North Carolina academic center found 1 case of Listeria meningitis among 72 SBIs (668 febrile infants) without a localizing source.8 At a large group-practice in northern California, Greenhow et al.9 examined all blood cultures (N = 4255) performed over 4 years for otherwise healthy infants 1 week to 3 months old and found no cases of Listeria. In a follow-up study, the same authors examined all blood (n = 5396), urine (n = 4599), and CSF (n = 1796) cultures in the same population and found no Listeria cases.10 Hassoun et al.11 studied SBI rates among infants younger than 28 days with any blood, urine, or CSF culture performed over 4 years at two Michigan EDs. One (0.08%) of the 1192 infants evaluated had bacteremia caused by Listeria.
Multicenter studies have reported similar results. In a study of 6 hospital systems in geographically diverse areas of the United States, Biondi et al.12 examined all positive blood cultures (N = 181) for febrile infants younger than 90 days admitted to a general pediatric ward, and found no listeriosis. Mischler et al.13 examined all positive blood cultures (N = 392) for otherwise healthy febrile infants 0 to 90 days old admitted to a hospital in 1 of 17 geographically diverse healthcare systems and found no cases of Listeria. A recent meta-analysis of studies that reported SBI rates for febrile infants 0 to 90 days old found the weighted prevalence of Listeria bacteremia to be 0.03% (2/20,703) and that of meningitis to be 0.02% (3/13,375).14 Veesenmeyer and Edmonson15 used a national inpatient database to identify all Listeria cases among infants over a 6-year period and estimated listeriosis rates for the US population. Over the 6 years, there were 212 total cases, which were extrapolated to 344 in the United States during that period, yielding a pooled annual incidence rate of 1.41 in 100,000 births.
Ampicillin offers no significant improvement in coverage for GBS or E coli beyond other β-lactam antibiotics, such as cefotaxime. Therefore, though the cost and potential harms of 24 to 48 hours of intravenous ampicillin are low for the individual patient, there is almost no potential benefit. Using the weighted prevalence of 0.03% for Listeria bacteremia reported in the recent meta-analysis,14 the number needed to treat to cover 1 case of Listeria bacteremia would be 3333. In addition, the increasing incidence of ampicillin resistance, particularly among gram-negative organisms,4,7,9 argues strongly for better antibiotic stewardship on a national level. A number of expert authors have advocated dropping empiric Listeria coverage as part of the treatment of febrile infants, particularly infants 29 to 90 days old.16,17 Some authors continue to advocate empiric Listeria coverage.6 It is interesting to note, however, that the incidence of Staph aureus bacteremia in recent case series is much higher than that reported for Listeria, accounting for 6-9% of bacteremia cases.9,11,13 Yet few if any authors advocate for empiric S. aureus coverage.
WHEN EMPIRIC AMPICILLIN COVERAGE MAY BE REASONABLE
The rate of listeriosis remains low across age groups in recent studies, but the rate is slightly higher in very young infants. In the recent national database study of listeriosis cases over a 6-year period, almost half involved infants younger than 7 days, and most of these infants showed no evidence of meningitis.15 Therefore, it may be reasonable to include empiric Listeria coverage in febrile infants younger than 7 days, though the study authors estimated 22.5 annual cases of Listeria in this age range in the United States. Eighty percent of the Listeria cases were in infants younger than 28 days, but more than 85% of infants 7 to 28 days old had meningitis. Therefore, broad antimicrobial coverage for infants with CSF pleocytosis and/or a high bacterial meningitis score is reasonable, especially for infants younger than 28 days.
Other potential indications for ampicillin are enterococcal infections. Though enteroccocal SBI rates in febrile infants are also quite low,7-9,11,12 if Enterococcus were highly suspected, such as in an infant with pyuria and gram positive organisms on gram stain, ampicillin offers good additional coverage. In the case of a local outbreak of listeriosis, or a specific exposure to Listeria-contaminated products on a patient history, antibiotics with efficacy against Listeria should be used. Last, in cases in which gentamicin is used as empiric coverage for gram-negative organisms, ampicillin offers important additional coverage for GBS.
Some practitioners advocate ampicillin and gentamicin over cefotaxime regimens on the basis of an often cited study that found a survival benefit for febrile neonates in the intensive care setting.18 There are a number of reasons that this study should not influence care for typical infants admitted with possible sepsis. First, the study was retrospective and limited by its use of administrative data. The authors acknowledged that a potential explanation for their results is unmeasured confounding. Second, the patients included in the study were dramatically different from the group of well infants admitted with possible sepsis; the study included neonatal critical care unit patients treated with antibiotics within the first 3 days of life. Third, the study’s results have not been replicated in otherwise healthy febrile infants.
WHAT YOU SHOULD USE INSTEAD OF AMPICILLIN FOR EMPIRIC LISTERIA COVERAGE
For febrile children 0 to 90 days old, empiric antibiotic coverage should be aimed at covering the current predominant pathogens, which include E coli and GBS. Therefore, for most children and US regions, a third-generation cephalosporin (eg, cefotaxime) is sufficient.
RECOMMENDATIONS
- Empiric antibiotics for treatment of febrile children 0-90 days should target E. coli and GBS; a third generation cephalosporin, (e.g. cefotaxime) alone is a reasonable choice for most patients.
- Prescribing ampicillin to specifically cover Listeria is unnecessary for the vast majority of febrile infants
- Prescribing ampicillin is reasonable in certain subgroups of febrile infants: those less than seven days of age, those with evidence of bacterial meningitis (especially if also <28 days of age), those in whom enterococcal infection is strongly suspected, and those with specific Listeria exposures related to local outbreaks.
CONCLUSION
The 32-day-old infant described in the clinical scenario was at extremely low risk for listeriosis. Antibiotic coverage with a third-generation cephalosporin is sufficient for the most likely pathogens. The common practice of empirically covering Listeria in otherwise healthy febrile infants considered to be at higher risk for SBI is no longer based on best available evidence and represents overtreatment with at least theoretical harms. Avoidance of the risks associated with the side effects of antibiotics, costs saved by forgoing multiple antibiotics, a decrease in medication dosing frequency, and improved antibiotic stewardship for the general population all argue forcefully for making empiric Listeria coverage a thing of the past.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Evaluation and treatment of the febrile infant 0 to 90 days of age are common clinical issues in pediatrics, family medicine, emergency medicine, and pediatric hospital medicine. Traditional teaching has been that Listeria monocytogenes is 1 of the 3 most common pathogens causing neonatal sepsis. Many practitioners routinely use antibiotic regimens, including ampicillin, to specifically target Listeria. However, a large body of evidence, including a meta-analysis and several multicenter studies, has shown that listeriosis is extremely rare in the United States. The practice of empiric ampicillin thus exposes the patient to harms and costs with little if any potential benefit, while increasing pressure on the bacterial flora in the community to generate antibiotic resistance. Empiric ampicillin for all infants admitted for sepsis evaluation is a tradition-based practice no longer founded on the best available evidence.
CASE REPORT
A 32-day-old, full-term, previously healthy girl presented with fever of 1 day’s duration. Her parents reported she had appeared well until the evening before admission, when she became a bit less active and spent less time breastfeeding. The morning of admission, she was fussier than usual. Rectal temperature, taken by her parents, was 101°F. There were no other symptoms and no sick contacts.
On examination, the patient’s rectal temperature was 101.5°F. Her other vitals and the physical examination findings were unremarkable. Laboratory test results included a normal urinalysis and a peripheral white blood cell (WBC) count of 21,300 cells/µL. Cerebrospinal fluid (CSF) analysis revealed normal protein and glucose levels with 3 WBCs/µL and a negative gram stain. Due to stratifying at higher risk for serious bacterial infection (SBI), the child was admitted and started on ampicillin and cefotaxime while awaiting culture results.
BACKGROUND
Evaluation and treatment of febrile infants are common clinical issues in pediatrics, emergency medicine, and general practice. Practice guidelines for evaluation of febrile infants recommend hospitalization and parenteral antibiotics for children younger than 28 days and children 29 to 90 days old if stratified at high risk for SBI.1,2 Recommendations for empiric antibiotic regimens include ampicillin in addition to either gentamicin or cefotaxime.1,2
WHY YOU MIGHT THINK AMPICILLIN IS HELPFUL
Generations of pediatrics students have been taught that the 3 pathogens most likely to cause bacterial sepsis in infants are group B Streptococcus (GBS), Escherichia coli, and Listeria monocytogenes. This teaching is still espoused in the latest editions of pediatrics textbooks.3 Ampicillin is specifically recommended for covering Listeria, and studies have found that 62% to 78% of practitioners choose empiric ampicillin-containing antibiotic regimens for the treatment of febrile infants.4-6
WHY EMPIRIC AMPICILLIN IS UNNECESSARY
In the past, Listeria was a potential though still uncommon infant pathogen. Over the past few decades, however, the epidemiology of infant sepsis has changed significantly. Estimates of the rate of infection with Listeria now range from extremely rare to nonexistent across multiple studies4,7-15 (Table). In a 4-year retrospective case series at a single urban academic center in Washington, DC, Sadow et al.4 reported no instances of Listeria among 121 positive bacterial cultures in infants younger than 60 days seen in the emergency department (ED). Byington et al.7 examined all positive cultures for infants 0 to 90 days old at a large academic referral center in Utah over a 3-year period and reported no cases of Listeria (1298 patients, 105 SBI cases). A study at a North Carolina academic center found 1 case of Listeria meningitis among 72 SBIs (668 febrile infants) without a localizing source.8 At a large group-practice in northern California, Greenhow et al.9 examined all blood cultures (N = 4255) performed over 4 years for otherwise healthy infants 1 week to 3 months old and found no cases of Listeria. In a follow-up study, the same authors examined all blood (n = 5396), urine (n = 4599), and CSF (n = 1796) cultures in the same population and found no Listeria cases.10 Hassoun et al.11 studied SBI rates among infants younger than 28 days with any blood, urine, or CSF culture performed over 4 years at two Michigan EDs. One (0.08%) of the 1192 infants evaluated had bacteremia caused by Listeria.
Multicenter studies have reported similar results. In a study of 6 hospital systems in geographically diverse areas of the United States, Biondi et al.12 examined all positive blood cultures (N = 181) for febrile infants younger than 90 days admitted to a general pediatric ward, and found no listeriosis. Mischler et al.13 examined all positive blood cultures (N = 392) for otherwise healthy febrile infants 0 to 90 days old admitted to a hospital in 1 of 17 geographically diverse healthcare systems and found no cases of Listeria. A recent meta-analysis of studies that reported SBI rates for febrile infants 0 to 90 days old found the weighted prevalence of Listeria bacteremia to be 0.03% (2/20,703) and that of meningitis to be 0.02% (3/13,375).14 Veesenmeyer and Edmonson15 used a national inpatient database to identify all Listeria cases among infants over a 6-year period and estimated listeriosis rates for the US population. Over the 6 years, there were 212 total cases, which were extrapolated to 344 in the United States during that period, yielding a pooled annual incidence rate of 1.41 in 100,000 births.
Ampicillin offers no significant improvement in coverage for GBS or E coli beyond other β-lactam antibiotics, such as cefotaxime. Therefore, though the cost and potential harms of 24 to 48 hours of intravenous ampicillin are low for the individual patient, there is almost no potential benefit. Using the weighted prevalence of 0.03% for Listeria bacteremia reported in the recent meta-analysis,14 the number needed to treat to cover 1 case of Listeria bacteremia would be 3333. In addition, the increasing incidence of ampicillin resistance, particularly among gram-negative organisms,4,7,9 argues strongly for better antibiotic stewardship on a national level. A number of expert authors have advocated dropping empiric Listeria coverage as part of the treatment of febrile infants, particularly infants 29 to 90 days old.16,17 Some authors continue to advocate empiric Listeria coverage.6 It is interesting to note, however, that the incidence of Staph aureus bacteremia in recent case series is much higher than that reported for Listeria, accounting for 6-9% of bacteremia cases.9,11,13 Yet few if any authors advocate for empiric S. aureus coverage.
WHEN EMPIRIC AMPICILLIN COVERAGE MAY BE REASONABLE
The rate of listeriosis remains low across age groups in recent studies, but the rate is slightly higher in very young infants. In the recent national database study of listeriosis cases over a 6-year period, almost half involved infants younger than 7 days, and most of these infants showed no evidence of meningitis.15 Therefore, it may be reasonable to include empiric Listeria coverage in febrile infants younger than 7 days, though the study authors estimated 22.5 annual cases of Listeria in this age range in the United States. Eighty percent of the Listeria cases were in infants younger than 28 days, but more than 85% of infants 7 to 28 days old had meningitis. Therefore, broad antimicrobial coverage for infants with CSF pleocytosis and/or a high bacterial meningitis score is reasonable, especially for infants younger than 28 days.
Other potential indications for ampicillin are enterococcal infections. Though enteroccocal SBI rates in febrile infants are also quite low,7-9,11,12 if Enterococcus were highly suspected, such as in an infant with pyuria and gram positive organisms on gram stain, ampicillin offers good additional coverage. In the case of a local outbreak of listeriosis, or a specific exposure to Listeria-contaminated products on a patient history, antibiotics with efficacy against Listeria should be used. Last, in cases in which gentamicin is used as empiric coverage for gram-negative organisms, ampicillin offers important additional coverage for GBS.
Some practitioners advocate ampicillin and gentamicin over cefotaxime regimens on the basis of an often cited study that found a survival benefit for febrile neonates in the intensive care setting.18 There are a number of reasons that this study should not influence care for typical infants admitted with possible sepsis. First, the study was retrospective and limited by its use of administrative data. The authors acknowledged that a potential explanation for their results is unmeasured confounding. Second, the patients included in the study were dramatically different from the group of well infants admitted with possible sepsis; the study included neonatal critical care unit patients treated with antibiotics within the first 3 days of life. Third, the study’s results have not been replicated in otherwise healthy febrile infants.
WHAT YOU SHOULD USE INSTEAD OF AMPICILLIN FOR EMPIRIC LISTERIA COVERAGE
For febrile children 0 to 90 days old, empiric antibiotic coverage should be aimed at covering the current predominant pathogens, which include E coli and GBS. Therefore, for most children and US regions, a third-generation cephalosporin (eg, cefotaxime) is sufficient.
RECOMMENDATIONS
- Empiric antibiotics for treatment of febrile children 0-90 days should target E. coli and GBS; a third generation cephalosporin, (e.g. cefotaxime) alone is a reasonable choice for most patients.
- Prescribing ampicillin to specifically cover Listeria is unnecessary for the vast majority of febrile infants
- Prescribing ampicillin is reasonable in certain subgroups of febrile infants: those less than seven days of age, those with evidence of bacterial meningitis (especially if also <28 days of age), those in whom enterococcal infection is strongly suspected, and those with specific Listeria exposures related to local outbreaks.
CONCLUSION
The 32-day-old infant described in the clinical scenario was at extremely low risk for listeriosis. Antibiotic coverage with a third-generation cephalosporin is sufficient for the most likely pathogens. The common practice of empirically covering Listeria in otherwise healthy febrile infants considered to be at higher risk for SBI is no longer based on best available evidence and represents overtreatment with at least theoretical harms. Avoidance of the risks associated with the side effects of antibiotics, costs saved by forgoing multiple antibiotics, a decrease in medication dosing frequency, and improved antibiotic stewardship for the general population all argue forcefully for making empiric Listeria coverage a thing of the past.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med. 1993;22(7):1198-1210. PubMed
2. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42(4):530-545. PubMed
3. Nield L, Kamat D. Fever without a focus. In: Kliegman R, Stanton B, eds. Nelson’s Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016.
4. Sadow KB, Derr R, Teach SJ. Bacterial infections in infants 60 days and younger: epidemiology, resistance, and implications for treatment. Arch Pediatr Adolesc Med. 1999;153(6):611-614. PubMed
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. PubMed
6. Cantey JB, Lopez-Medina E, Nguyen S, Doern C, Garcia C. Empiric antibiotics for serious bacterial infection in young infants: opportunities for stewardship. Pediatr Emerg Care. 2015;31(8):568-571. PubMed
7. Byington CL, Rittichier KK, Bassett KE, et al. Serious bacterial infections in febrile infants younger than 90 days of age: the importance of ampicillin-resistant pathogens. Pediatrics. 2003;111(5 pt 1):964-968. PubMed
8. Watt K, Waddle E, Jhaveri R. Changing epidemiology of serious bacterial infections in febrile infants without localizing signs. PLoS One. 2010;5(8):e12448. PubMed
9. Greenhow TL, Hung YY, Herz AM. Changing epidemiology of bacteremia in infants aged 1 week to 3 months. Pediatrics. 2012;129(3):e590-e596. PubMed
10. Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. PubMed
11. Hassoun A, Stankovic C, Rogers A, et al. Listeria and enterococcal infections in neonates 28 days of age and younger: is empiric parenteral ampicillin still indicated? Pediatr Emerg Care. 2014;30(4):240-243. PubMed
12. Biondi E, Evans R, Mischler M, et al. Epidemiology of bacteremia in febrile infants in the United States. Pediatrics. 2013;132(6):990-996. PubMed
13. Mischler M, Ryan MS, Leyenaar JK, et al. Epidemiology of bacteremia in previously healthy febrile infants: a follow-up study. Hosp Pediatr. 2015;5(6):293-300. PubMed
14. Leazer R, Perkins AM, Shomaker K, Fine B. A meta-analysis of the rates of Listeria monocytogenes and Enterococcus in febrile infants. Hosp Pediatr. 2016;6(4):187-195. PubMed
15. Veesenmeyer AF, Edmonson MB. Trends in US hospital stays for listeriosis in infants. Hosp Pediatr. 2016;6(4):196-203. PubMed
16. Schroeder AR, Roberts KB. Is tradition trumping evidence in the treatment of young, febrile infants? Hosp Pediatr. 2016;6(4):252-253. PubMed
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. PubMed
18. Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117(1):67-74. PubMed
1. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med. 1993;22(7):1198-1210. PubMed
2. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42(4):530-545. PubMed
3. Nield L, Kamat D. Fever without a focus. In: Kliegman R, Stanton B, eds. Nelson’s Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016.
4. Sadow KB, Derr R, Teach SJ. Bacterial infections in infants 60 days and younger: epidemiology, resistance, and implications for treatment. Arch Pediatr Adolesc Med. 1999;153(6):611-614. PubMed
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. PubMed
6. Cantey JB, Lopez-Medina E, Nguyen S, Doern C, Garcia C. Empiric antibiotics for serious bacterial infection in young infants: opportunities for stewardship. Pediatr Emerg Care. 2015;31(8):568-571. PubMed
7. Byington CL, Rittichier KK, Bassett KE, et al. Serious bacterial infections in febrile infants younger than 90 days of age: the importance of ampicillin-resistant pathogens. Pediatrics. 2003;111(5 pt 1):964-968. PubMed
8. Watt K, Waddle E, Jhaveri R. Changing epidemiology of serious bacterial infections in febrile infants without localizing signs. PLoS One. 2010;5(8):e12448. PubMed
9. Greenhow TL, Hung YY, Herz AM. Changing epidemiology of bacteremia in infants aged 1 week to 3 months. Pediatrics. 2012;129(3):e590-e596. PubMed
10. Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. PubMed
11. Hassoun A, Stankovic C, Rogers A, et al. Listeria and enterococcal infections in neonates 28 days of age and younger: is empiric parenteral ampicillin still indicated? Pediatr Emerg Care. 2014;30(4):240-243. PubMed
12. Biondi E, Evans R, Mischler M, et al. Epidemiology of bacteremia in febrile infants in the United States. Pediatrics. 2013;132(6):990-996. PubMed
13. Mischler M, Ryan MS, Leyenaar JK, et al. Epidemiology of bacteremia in previously healthy febrile infants: a follow-up study. Hosp Pediatr. 2015;5(6):293-300. PubMed
14. Leazer R, Perkins AM, Shomaker K, Fine B. A meta-analysis of the rates of Listeria monocytogenes and Enterococcus in febrile infants. Hosp Pediatr. 2016;6(4):187-195. PubMed
15. Veesenmeyer AF, Edmonson MB. Trends in US hospital stays for listeriosis in infants. Hosp Pediatr. 2016;6(4):196-203. PubMed
16. Schroeder AR, Roberts KB. Is tradition trumping evidence in the treatment of young, febrile infants? Hosp Pediatr. 2016;6(4):252-253. PubMed
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. PubMed
18. Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117(1):67-74. PubMed
© 2017 Society of Hospital Medicine
Urine eosinophils for acute interstitial nephritis
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Acute interstitial nephritis (AIN) is an important cause of acute kidney injury (AKI) in the hospital setting. However, the diagnosis of AIN is challenging because of its nonspecific clinical manifestations and the invasiveness of kidney biopsy, the gold standard for diagnosis. Urine eosinophils (UEs) emerged several decades ago as a noninvasive alternative for diagnosing AIN. Initial studies found UEs had a significant diagnostic value, but these studies had small sample sizes, and the diagnosis of AIN was made on clinical grounds only, without biopsy confirmation. In this article, we review the literature on the diagnostic value of UEs in the diagnosis of AIN.
CASE REPORT
A 62-year-old woman with type 2 diabetes mellitus, systemic hypertension, coronary artery disease, and obesity is admitted for AKI found on routine laboratory testing. She has been taking amoxicillin and doxycycline for left leg cellulitis the past 5 days, but improvement has been minimal. On admission, blood pressure is 120/74 mm Hg, and heart rate is 89 beats per minute. Serum creatinine level is increased, from 0.7 mg/dL at baseline to 3.6 mg/dL on admission. Complete urinalysis reveals 1+ protein and presence of white blood cells and isormorphic red blood cells. No casts or crystals are seen. Given the possibility of AIN, UE testing is ordered. UEs are positive at 25%. Does this result significantly increase the patient’s posttest probability of having AIN?
WHY YOU MIGHT THINK ORDERING URINE EOSINOPHILS IN THE EVALUATION OF AIN IS HELPFUL
AKI occurs in more than 1 in 5 hospitalizations and is associated with a more than 4-fold increased likelihood of in-hospital mortality at 21 days.1 AIN is an important cause of AKI and has been found in 6% to 30% of AKI patients who had biopsies performed.2-4 AIN is characterized by infiltration of inflammatory cells in the kidney interstitium and is more commonly caused by drugs, especially beta-lactam antibiotics, and less commonly by autoimmune or systemic diseases and infections. As the signs and symptoms of AIN are nonspecific, and the gold-standard test is renal biopsy, diagnosticians have sought a noninvasive test, such as UEs.
In 1978, Galpin et al.5 found that UEs comprised 10% to 60% of urine white blood cells in 9 of 9 patients with methicillin-induced interstitial nephritis; 6 of the 9 had biopsy-proven AIN. In 1980, Linton et al.6 found UEs in 6 of 9 patients with drug-induced AIN; 8 of the 9 had biopsy-proven AIN. In 1986, Nolan et al.7 reported that, compared with Wright stain, Hansel stain was more sensitive in visualizing UEs; they did not use biopsy for confirmation. Wright-stain detection of UEs is limited by the variable staining characteristics of “eosinophilic” granules in body fluids other than blood. With Hansel stain, UEs are readily identified by their brilliant red-pink granules. These 3 small studies helped make UEs the go-to noninvasive test for assessing for AIN.8
WHY THERE IS LITTLE REASON TO ORDER URINE EOSINOPHILS IN PATIENTS WITH SUSPICION FOR AIN
While initial studies indicated UEs might be diagnostically helpful, subsequent studies did not. In 1985, Corwin et al.9 used Wright stain and found UEs in 65 of 470 adults with AKI. Only 9 (14%) of the 65 had a diagnosis of AIN, which was made mostly on clinical grounds. These findings showed that UEs were produced by other renal or urinary tract abnormalities, such as urinary tract infections, acute tubular necrosis, and glomerulonephritis. In a second study, Corwin et al.10 found that Hansel stain (vs Wright stain) improved the sensitivity of UEs for AIN diagnosis, from 25% to 62.5%. Sensitivity was improved at the expense of specificity, as Hansel stain was positive in other diagnoses as well. The AIN diagnosis was not confirmed by kidney biopsy in the large majority of patients in this study. Lack of confirmation by biopsy, the gold-standard diagnostic test, was a methodologic flaw of this study and others.
Sutton11 reviewed data from 10 studies and found AIN could not be reliably excluded in the absence of UEs (only 19 of 32 biopsy-confirmed AIN cases had UEs present). In addition, Ruffing et al.12 used Hansel stain and concluded that the positive predictive value of UEs was inadequate in diagnosing AIN. Only 6 of their 15 patients with AIN had positive UEs. Urine eosinophils were also present in patients with other diagnoses (glomerulonephritis, chronic kidney disease, acute pyelonephritis, prerenal azotemia). Like many other investigators, Ruffing et al. made the AIN diagnosis on clinical grounds in the large majority of cases.
Muriithi et al.13 reported similarly negative results in their retrospective AKI study involving 566 Mayo Clinic patients and spanning almost 2 decades. The study included patients who underwent both Hansel-stain UE testing and kidney biopsy within a week of each other. Only 28 (30%) of 91 biopsy-proven AIN cases were positive for UEs. Using the 1% cutoff for a positive UE test yielded only 30.8% sensitivity and 68.2% specificity. Using the 5% cutoff increased specificity to 91.2%, at the expense of sensitivity (19.2%); positive predictive value improved to only 30%, and negative predictive value remained relatively unchanged, at 85.6%. In short, Muriithi et al. found that UE testing had no utility in AIN diagnosis.
In summary, initial studies, such as those by Corwin et al,9,10 supported the conclusion that UEs are useful in AIN diagnosis but had questionable validity owing to methodologic issues, including small sample size and lack of biopsy confirmation of AIN. On the other hand, more recent studies, such as the one conducted by Muriithi et al.,13 had larger sample sizes and biopsy-proven diagnoses and confirmed the poor diagnostic value of UEs in AIN.
The poor sensitivity and specificity of UE tests can have important consequences. A false positive test may cause the clinician to incorrectly diagnose the patient with AIN and prompt the clinician to remove medications that may be vitally important. The clinician may also consider treating the patient with steroids empirically. A false negative test may inappropriately reassure the clinician that the patient does not have AIN and does not need cessation of the culprit drug. This may also lead the clinician to forego a necessary kidney biopsy.
WHAT YOU SHOULD DO INSTEAD
A history of recent exposure to a classic offending drug (eg, beta-lactam, proton pump inhibitor, nonsteroidal anti-inflammatory drug) in combination with the classic triad of fever, rash, and peripheral eosinophilia suggests an AIN diagnosis. However, less than 5% to 10% of patients present with this triad.14,15 Regardless of the triad’s presence, if other causes of AKI have been excluded, stopping a potential offending agent and monitoring for improvement are recommended. If a culprit drug cannot be safely discontinued, renal biopsy may be necessary for confirmation of the diagnosis. Moreover, if kidney function continues to deteriorate, a nephrology consultation may be warranted for guidance on the risks and benefits of performing a kidney biopsy to confirm the diagnosis and/or the use of corticosteroids.
RECOMMENDATIONS
- Urine eosinophils should not be used in the diagnosis of AIN.
- The clinical diagnosis of drug-associated AIN should be based on excluding other possible likely etiologies of AKI and confirming the history of drug exposure. This is reinforced when kidney function improves upon discontinuation of offending agent.
- Kidney biopsy is the gold standard for AIN and should be performed if the clinical picture is unclear or the renal function is not improving upon discontinuation of offending agent.
CONCLUSION
Since the mid-1980s, studies have found that UEs are too insensitive and nonspecific to confirm or exclude the diagnosis of AIN in patients with AKI (Table). UEs are seen in other AKI etiologies, such as pyelonephritis, acute tubular necrosis, atheroembolic renal disease, and glomerulonephritis. Current evidence-based medicine does not support use of UEs as a biomarker for AIN. False-positive and false-negative results confuse the overall picture and result either in discontinuation of important medications and unnecessary steroid treatment or in delayed removal of a culprit medication.16
Our case’s positive UE test does not affect the posttest probability that our patient has AIN. Presence of a culprit drug and absence of clinical data suggesting an alternative diagnosis would lead most clinicians to change antibiotic therapy and observe for improvement in renal function.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35(4):349-355.
2. Farrington K, Levison DA, Greenwood RN, Cattell WR, Baker LR. Renal biopsy in patients with unexplained renal impairment and normal kidney size. Q J Med. 1989;70(263):221-233.
3. Michel DM, Kelly CJ. Acute interstitial nephritis. J Am Soc Nephrol. 1998;9(3):506-515.
4. Neilson EG. Pathogenesis and therapy of interstitial nephritis. Kidney Int. 1989;35(5):1257-1270.
5. Galpin JE, Shinaberger JH, Stanley TM, et al. Acute interstitial nephritis due to methicillin. Am J Med. 1978;65(5):756-765.
6. Linton AL, Clark WF, Driedger AA, Turnbull DI, Lindsay RM. Acute interstitial nephritis due to drugs: review of the literature with a report of nine cases. Ann Intern Med. 1980;93(5):735-741.
7. Nolan CR 3rd, Anger MS, Kelleher SP. Eosinophiluria—a new method of detection and definition of the clinical spectrum. N Engl J Med. 1986;315(24):1516-1519.
8. Perazella MA, Bomback AS. Urinary eosinophils in AIN: farewell to an old biomarker? Clin J Am Soc Nephrol. 2013;8(11):1841-1843.
9. Corwin HL, Korbet SM, Schwartz MM. Clinical correlates of eosinophiluria. Arch Intern Med. 1985;145(6):1097-1099.
10. Corwin HL, Bray RA, Haber MH. The detection and interpretation of urinary eosinophils. Arch Pathol Lab Med. 1989;113(11):1256-1258.
11. Sutton JM. Urinary eosinophils. Arch Intern Med. 1986;146(11):2243-2244.
12. Ruffing KA, Hoppes P, Blend D, Cugino A, Jarjoura D, Whittier FC. Eosinophils in urine revisited. Clin Nephrol. 1994;41(3):163-166.
13. Muriithi AK, Nasr SH, Leung N. Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol. 2013;8(11):1857-1862.
14. Clarkson MR, Giblin L, O’Connell FP, et al. Acute interstitial nephritis: clinical features and response to corticosteroid therapy. Nephrol Dial Transplant. 2004;19(11):2778-2783.
15. Rossert J. Drug-induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817.
16. Fletcher A. Eosinophiluria and acute interstitial nephritis. N Engl J Med. 2008;358(16):1760-1761.
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Acute interstitial nephritis (AIN) is an important cause of acute kidney injury (AKI) in the hospital setting. However, the diagnosis of AIN is challenging because of its nonspecific clinical manifestations and the invasiveness of kidney biopsy, the gold standard for diagnosis. Urine eosinophils (UEs) emerged several decades ago as a noninvasive alternative for diagnosing AIN. Initial studies found UEs had a significant diagnostic value, but these studies had small sample sizes, and the diagnosis of AIN was made on clinical grounds only, without biopsy confirmation. In this article, we review the literature on the diagnostic value of UEs in the diagnosis of AIN.
CASE REPORT
A 62-year-old woman with type 2 diabetes mellitus, systemic hypertension, coronary artery disease, and obesity is admitted for AKI found on routine laboratory testing. She has been taking amoxicillin and doxycycline for left leg cellulitis the past 5 days, but improvement has been minimal. On admission, blood pressure is 120/74 mm Hg, and heart rate is 89 beats per minute. Serum creatinine level is increased, from 0.7 mg/dL at baseline to 3.6 mg/dL on admission. Complete urinalysis reveals 1+ protein and presence of white blood cells and isormorphic red blood cells. No casts or crystals are seen. Given the possibility of AIN, UE testing is ordered. UEs are positive at 25%. Does this result significantly increase the patient’s posttest probability of having AIN?
WHY YOU MIGHT THINK ORDERING URINE EOSINOPHILS IN THE EVALUATION OF AIN IS HELPFUL
AKI occurs in more than 1 in 5 hospitalizations and is associated with a more than 4-fold increased likelihood of in-hospital mortality at 21 days.1 AIN is an important cause of AKI and has been found in 6% to 30% of AKI patients who had biopsies performed.2-4 AIN is characterized by infiltration of inflammatory cells in the kidney interstitium and is more commonly caused by drugs, especially beta-lactam antibiotics, and less commonly by autoimmune or systemic diseases and infections. As the signs and symptoms of AIN are nonspecific, and the gold-standard test is renal biopsy, diagnosticians have sought a noninvasive test, such as UEs.
In 1978, Galpin et al.5 found that UEs comprised 10% to 60% of urine white blood cells in 9 of 9 patients with methicillin-induced interstitial nephritis; 6 of the 9 had biopsy-proven AIN. In 1980, Linton et al.6 found UEs in 6 of 9 patients with drug-induced AIN; 8 of the 9 had biopsy-proven AIN. In 1986, Nolan et al.7 reported that, compared with Wright stain, Hansel stain was more sensitive in visualizing UEs; they did not use biopsy for confirmation. Wright-stain detection of UEs is limited by the variable staining characteristics of “eosinophilic” granules in body fluids other than blood. With Hansel stain, UEs are readily identified by their brilliant red-pink granules. These 3 small studies helped make UEs the go-to noninvasive test for assessing for AIN.8
WHY THERE IS LITTLE REASON TO ORDER URINE EOSINOPHILS IN PATIENTS WITH SUSPICION FOR AIN
While initial studies indicated UEs might be diagnostically helpful, subsequent studies did not. In 1985, Corwin et al.9 used Wright stain and found UEs in 65 of 470 adults with AKI. Only 9 (14%) of the 65 had a diagnosis of AIN, which was made mostly on clinical grounds. These findings showed that UEs were produced by other renal or urinary tract abnormalities, such as urinary tract infections, acute tubular necrosis, and glomerulonephritis. In a second study, Corwin et al.10 found that Hansel stain (vs Wright stain) improved the sensitivity of UEs for AIN diagnosis, from 25% to 62.5%. Sensitivity was improved at the expense of specificity, as Hansel stain was positive in other diagnoses as well. The AIN diagnosis was not confirmed by kidney biopsy in the large majority of patients in this study. Lack of confirmation by biopsy, the gold-standard diagnostic test, was a methodologic flaw of this study and others.
Sutton11 reviewed data from 10 studies and found AIN could not be reliably excluded in the absence of UEs (only 19 of 32 biopsy-confirmed AIN cases had UEs present). In addition, Ruffing et al.12 used Hansel stain and concluded that the positive predictive value of UEs was inadequate in diagnosing AIN. Only 6 of their 15 patients with AIN had positive UEs. Urine eosinophils were also present in patients with other diagnoses (glomerulonephritis, chronic kidney disease, acute pyelonephritis, prerenal azotemia). Like many other investigators, Ruffing et al. made the AIN diagnosis on clinical grounds in the large majority of cases.
Muriithi et al.13 reported similarly negative results in their retrospective AKI study involving 566 Mayo Clinic patients and spanning almost 2 decades. The study included patients who underwent both Hansel-stain UE testing and kidney biopsy within a week of each other. Only 28 (30%) of 91 biopsy-proven AIN cases were positive for UEs. Using the 1% cutoff for a positive UE test yielded only 30.8% sensitivity and 68.2% specificity. Using the 5% cutoff increased specificity to 91.2%, at the expense of sensitivity (19.2%); positive predictive value improved to only 30%, and negative predictive value remained relatively unchanged, at 85.6%. In short, Muriithi et al. found that UE testing had no utility in AIN diagnosis.
In summary, initial studies, such as those by Corwin et al,9,10 supported the conclusion that UEs are useful in AIN diagnosis but had questionable validity owing to methodologic issues, including small sample size and lack of biopsy confirmation of AIN. On the other hand, more recent studies, such as the one conducted by Muriithi et al.,13 had larger sample sizes and biopsy-proven diagnoses and confirmed the poor diagnostic value of UEs in AIN.
The poor sensitivity and specificity of UE tests can have important consequences. A false positive test may cause the clinician to incorrectly diagnose the patient with AIN and prompt the clinician to remove medications that may be vitally important. The clinician may also consider treating the patient with steroids empirically. A false negative test may inappropriately reassure the clinician that the patient does not have AIN and does not need cessation of the culprit drug. This may also lead the clinician to forego a necessary kidney biopsy.
WHAT YOU SHOULD DO INSTEAD
A history of recent exposure to a classic offending drug (eg, beta-lactam, proton pump inhibitor, nonsteroidal anti-inflammatory drug) in combination with the classic triad of fever, rash, and peripheral eosinophilia suggests an AIN diagnosis. However, less than 5% to 10% of patients present with this triad.14,15 Regardless of the triad’s presence, if other causes of AKI have been excluded, stopping a potential offending agent and monitoring for improvement are recommended. If a culprit drug cannot be safely discontinued, renal biopsy may be necessary for confirmation of the diagnosis. Moreover, if kidney function continues to deteriorate, a nephrology consultation may be warranted for guidance on the risks and benefits of performing a kidney biopsy to confirm the diagnosis and/or the use of corticosteroids.
RECOMMENDATIONS
- Urine eosinophils should not be used in the diagnosis of AIN.
- The clinical diagnosis of drug-associated AIN should be based on excluding other possible likely etiologies of AKI and confirming the history of drug exposure. This is reinforced when kidney function improves upon discontinuation of offending agent.
- Kidney biopsy is the gold standard for AIN and should be performed if the clinical picture is unclear or the renal function is not improving upon discontinuation of offending agent.
CONCLUSION
Since the mid-1980s, studies have found that UEs are too insensitive and nonspecific to confirm or exclude the diagnosis of AIN in patients with AKI (Table). UEs are seen in other AKI etiologies, such as pyelonephritis, acute tubular necrosis, atheroembolic renal disease, and glomerulonephritis. Current evidence-based medicine does not support use of UEs as a biomarker for AIN. False-positive and false-negative results confuse the overall picture and result either in discontinuation of important medications and unnecessary steroid treatment or in delayed removal of a culprit medication.16
Our case’s positive UE test does not affect the posttest probability that our patient has AIN. Presence of a culprit drug and absence of clinical data suggesting an alternative diagnosis would lead most clinicians to change antibiotic therapy and observe for improvement in renal function.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Acute interstitial nephritis (AIN) is an important cause of acute kidney injury (AKI) in the hospital setting. However, the diagnosis of AIN is challenging because of its nonspecific clinical manifestations and the invasiveness of kidney biopsy, the gold standard for diagnosis. Urine eosinophils (UEs) emerged several decades ago as a noninvasive alternative for diagnosing AIN. Initial studies found UEs had a significant diagnostic value, but these studies had small sample sizes, and the diagnosis of AIN was made on clinical grounds only, without biopsy confirmation. In this article, we review the literature on the diagnostic value of UEs in the diagnosis of AIN.
CASE REPORT
A 62-year-old woman with type 2 diabetes mellitus, systemic hypertension, coronary artery disease, and obesity is admitted for AKI found on routine laboratory testing. She has been taking amoxicillin and doxycycline for left leg cellulitis the past 5 days, but improvement has been minimal. On admission, blood pressure is 120/74 mm Hg, and heart rate is 89 beats per minute. Serum creatinine level is increased, from 0.7 mg/dL at baseline to 3.6 mg/dL on admission. Complete urinalysis reveals 1+ protein and presence of white blood cells and isormorphic red blood cells. No casts or crystals are seen. Given the possibility of AIN, UE testing is ordered. UEs are positive at 25%. Does this result significantly increase the patient’s posttest probability of having AIN?
WHY YOU MIGHT THINK ORDERING URINE EOSINOPHILS IN THE EVALUATION OF AIN IS HELPFUL
AKI occurs in more than 1 in 5 hospitalizations and is associated with a more than 4-fold increased likelihood of in-hospital mortality at 21 days.1 AIN is an important cause of AKI and has been found in 6% to 30% of AKI patients who had biopsies performed.2-4 AIN is characterized by infiltration of inflammatory cells in the kidney interstitium and is more commonly caused by drugs, especially beta-lactam antibiotics, and less commonly by autoimmune or systemic diseases and infections. As the signs and symptoms of AIN are nonspecific, and the gold-standard test is renal biopsy, diagnosticians have sought a noninvasive test, such as UEs.
In 1978, Galpin et al.5 found that UEs comprised 10% to 60% of urine white blood cells in 9 of 9 patients with methicillin-induced interstitial nephritis; 6 of the 9 had biopsy-proven AIN. In 1980, Linton et al.6 found UEs in 6 of 9 patients with drug-induced AIN; 8 of the 9 had biopsy-proven AIN. In 1986, Nolan et al.7 reported that, compared with Wright stain, Hansel stain was more sensitive in visualizing UEs; they did not use biopsy for confirmation. Wright-stain detection of UEs is limited by the variable staining characteristics of “eosinophilic” granules in body fluids other than blood. With Hansel stain, UEs are readily identified by their brilliant red-pink granules. These 3 small studies helped make UEs the go-to noninvasive test for assessing for AIN.8
WHY THERE IS LITTLE REASON TO ORDER URINE EOSINOPHILS IN PATIENTS WITH SUSPICION FOR AIN
While initial studies indicated UEs might be diagnostically helpful, subsequent studies did not. In 1985, Corwin et al.9 used Wright stain and found UEs in 65 of 470 adults with AKI. Only 9 (14%) of the 65 had a diagnosis of AIN, which was made mostly on clinical grounds. These findings showed that UEs were produced by other renal or urinary tract abnormalities, such as urinary tract infections, acute tubular necrosis, and glomerulonephritis. In a second study, Corwin et al.10 found that Hansel stain (vs Wright stain) improved the sensitivity of UEs for AIN diagnosis, from 25% to 62.5%. Sensitivity was improved at the expense of specificity, as Hansel stain was positive in other diagnoses as well. The AIN diagnosis was not confirmed by kidney biopsy in the large majority of patients in this study. Lack of confirmation by biopsy, the gold-standard diagnostic test, was a methodologic flaw of this study and others.
Sutton11 reviewed data from 10 studies and found AIN could not be reliably excluded in the absence of UEs (only 19 of 32 biopsy-confirmed AIN cases had UEs present). In addition, Ruffing et al.12 used Hansel stain and concluded that the positive predictive value of UEs was inadequate in diagnosing AIN. Only 6 of their 15 patients with AIN had positive UEs. Urine eosinophils were also present in patients with other diagnoses (glomerulonephritis, chronic kidney disease, acute pyelonephritis, prerenal azotemia). Like many other investigators, Ruffing et al. made the AIN diagnosis on clinical grounds in the large majority of cases.
Muriithi et al.13 reported similarly negative results in their retrospective AKI study involving 566 Mayo Clinic patients and spanning almost 2 decades. The study included patients who underwent both Hansel-stain UE testing and kidney biopsy within a week of each other. Only 28 (30%) of 91 biopsy-proven AIN cases were positive for UEs. Using the 1% cutoff for a positive UE test yielded only 30.8% sensitivity and 68.2% specificity. Using the 5% cutoff increased specificity to 91.2%, at the expense of sensitivity (19.2%); positive predictive value improved to only 30%, and negative predictive value remained relatively unchanged, at 85.6%. In short, Muriithi et al. found that UE testing had no utility in AIN diagnosis.
In summary, initial studies, such as those by Corwin et al,9,10 supported the conclusion that UEs are useful in AIN diagnosis but had questionable validity owing to methodologic issues, including small sample size and lack of biopsy confirmation of AIN. On the other hand, more recent studies, such as the one conducted by Muriithi et al.,13 had larger sample sizes and biopsy-proven diagnoses and confirmed the poor diagnostic value of UEs in AIN.
The poor sensitivity and specificity of UE tests can have important consequences. A false positive test may cause the clinician to incorrectly diagnose the patient with AIN and prompt the clinician to remove medications that may be vitally important. The clinician may also consider treating the patient with steroids empirically. A false negative test may inappropriately reassure the clinician that the patient does not have AIN and does not need cessation of the culprit drug. This may also lead the clinician to forego a necessary kidney biopsy.
WHAT YOU SHOULD DO INSTEAD
A history of recent exposure to a classic offending drug (eg, beta-lactam, proton pump inhibitor, nonsteroidal anti-inflammatory drug) in combination with the classic triad of fever, rash, and peripheral eosinophilia suggests an AIN diagnosis. However, less than 5% to 10% of patients present with this triad.14,15 Regardless of the triad’s presence, if other causes of AKI have been excluded, stopping a potential offending agent and monitoring for improvement are recommended. If a culprit drug cannot be safely discontinued, renal biopsy may be necessary for confirmation of the diagnosis. Moreover, if kidney function continues to deteriorate, a nephrology consultation may be warranted for guidance on the risks and benefits of performing a kidney biopsy to confirm the diagnosis and/or the use of corticosteroids.
RECOMMENDATIONS
- Urine eosinophils should not be used in the diagnosis of AIN.
- The clinical diagnosis of drug-associated AIN should be based on excluding other possible likely etiologies of AKI and confirming the history of drug exposure. This is reinforced when kidney function improves upon discontinuation of offending agent.
- Kidney biopsy is the gold standard for AIN and should be performed if the clinical picture is unclear or the renal function is not improving upon discontinuation of offending agent.
CONCLUSION
Since the mid-1980s, studies have found that UEs are too insensitive and nonspecific to confirm or exclude the diagnosis of AIN in patients with AKI (Table). UEs are seen in other AKI etiologies, such as pyelonephritis, acute tubular necrosis, atheroembolic renal disease, and glomerulonephritis. Current evidence-based medicine does not support use of UEs as a biomarker for AIN. False-positive and false-negative results confuse the overall picture and result either in discontinuation of important medications and unnecessary steroid treatment or in delayed removal of a culprit medication.16
Our case’s positive UE test does not affect the posttest probability that our patient has AIN. Presence of a culprit drug and absence of clinical data suggesting an alternative diagnosis would lead most clinicians to change antibiotic therapy and observe for improvement in renal function.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35(4):349-355.
2. Farrington K, Levison DA, Greenwood RN, Cattell WR, Baker LR. Renal biopsy in patients with unexplained renal impairment and normal kidney size. Q J Med. 1989;70(263):221-233.
3. Michel DM, Kelly CJ. Acute interstitial nephritis. J Am Soc Nephrol. 1998;9(3):506-515.
4. Neilson EG. Pathogenesis and therapy of interstitial nephritis. Kidney Int. 1989;35(5):1257-1270.
5. Galpin JE, Shinaberger JH, Stanley TM, et al. Acute interstitial nephritis due to methicillin. Am J Med. 1978;65(5):756-765.
6. Linton AL, Clark WF, Driedger AA, Turnbull DI, Lindsay RM. Acute interstitial nephritis due to drugs: review of the literature with a report of nine cases. Ann Intern Med. 1980;93(5):735-741.
7. Nolan CR 3rd, Anger MS, Kelleher SP. Eosinophiluria—a new method of detection and definition of the clinical spectrum. N Engl J Med. 1986;315(24):1516-1519.
8. Perazella MA, Bomback AS. Urinary eosinophils in AIN: farewell to an old biomarker? Clin J Am Soc Nephrol. 2013;8(11):1841-1843.
9. Corwin HL, Korbet SM, Schwartz MM. Clinical correlates of eosinophiluria. Arch Intern Med. 1985;145(6):1097-1099.
10. Corwin HL, Bray RA, Haber MH. The detection and interpretation of urinary eosinophils. Arch Pathol Lab Med. 1989;113(11):1256-1258.
11. Sutton JM. Urinary eosinophils. Arch Intern Med. 1986;146(11):2243-2244.
12. Ruffing KA, Hoppes P, Blend D, Cugino A, Jarjoura D, Whittier FC. Eosinophils in urine revisited. Clin Nephrol. 1994;41(3):163-166.
13. Muriithi AK, Nasr SH, Leung N. Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol. 2013;8(11):1857-1862.
14. Clarkson MR, Giblin L, O’Connell FP, et al. Acute interstitial nephritis: clinical features and response to corticosteroid therapy. Nephrol Dial Transplant. 2004;19(11):2778-2783.
15. Rossert J. Drug-induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817.
16. Fletcher A. Eosinophiluria and acute interstitial nephritis. N Engl J Med. 2008;358(16):1760-1761.
1. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35(4):349-355.
2. Farrington K, Levison DA, Greenwood RN, Cattell WR, Baker LR. Renal biopsy in patients with unexplained renal impairment and normal kidney size. Q J Med. 1989;70(263):221-233.
3. Michel DM, Kelly CJ. Acute interstitial nephritis. J Am Soc Nephrol. 1998;9(3):506-515.
4. Neilson EG. Pathogenesis and therapy of interstitial nephritis. Kidney Int. 1989;35(5):1257-1270.
5. Galpin JE, Shinaberger JH, Stanley TM, et al. Acute interstitial nephritis due to methicillin. Am J Med. 1978;65(5):756-765.
6. Linton AL, Clark WF, Driedger AA, Turnbull DI, Lindsay RM. Acute interstitial nephritis due to drugs: review of the literature with a report of nine cases. Ann Intern Med. 1980;93(5):735-741.
7. Nolan CR 3rd, Anger MS, Kelleher SP. Eosinophiluria—a new method of detection and definition of the clinical spectrum. N Engl J Med. 1986;315(24):1516-1519.
8. Perazella MA, Bomback AS. Urinary eosinophils in AIN: farewell to an old biomarker? Clin J Am Soc Nephrol. 2013;8(11):1841-1843.
9. Corwin HL, Korbet SM, Schwartz MM. Clinical correlates of eosinophiluria. Arch Intern Med. 1985;145(6):1097-1099.
10. Corwin HL, Bray RA, Haber MH. The detection and interpretation of urinary eosinophils. Arch Pathol Lab Med. 1989;113(11):1256-1258.
11. Sutton JM. Urinary eosinophils. Arch Intern Med. 1986;146(11):2243-2244.
12. Ruffing KA, Hoppes P, Blend D, Cugino A, Jarjoura D, Whittier FC. Eosinophils in urine revisited. Clin Nephrol. 1994;41(3):163-166.
13. Muriithi AK, Nasr SH, Leung N. Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol. 2013;8(11):1857-1862.
14. Clarkson MR, Giblin L, O’Connell FP, et al. Acute interstitial nephritis: clinical features and response to corticosteroid therapy. Nephrol Dial Transplant. 2004;19(11):2778-2783.
15. Rossert J. Drug-induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817.
16. Fletcher A. Eosinophiluria and acute interstitial nephritis. N Engl J Med. 2008;358(16):1760-1761.
© 2017 Society of Hospital Medicine
The value of using ultrasound to rule out deep vein thrombosis in cases of cellulitis
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Because of overlapping clinical manifestations, clinicians often order ultrasound to rule out deep vein thrombosis (DVT) in cases of cellulitis. Ultrasound testing is performed for 16% to 73% of patients diagnosed with cellulitis. Although testing is common, the pooled incidence of DVT is low (3.1%). Few data elucidate which patients with cellulitis are more likely to have concurrent DVT and require further testing. The Wells clinical prediction rule with
CASE REPORT
A 50-year-old man presented to the emergency department with a 3-day-old cut on his anterior right shin. Associated redness, warmth, pain, and swelling had progressed. The patient had no history of prior DVT or pulmonary embolism (PE). His temperature was 38.5°C, and his white blood cell count of 18,000. On review of systems, he denied shortness of breath and chest pain. He was diagnosed with cellulitis and administered intravenous fluids and cefazolin. The clinician wondered whether to perform lower extremity ultrasound to rule out concurrent DVT.
WHY YOU MIGHT THINK ULTRASOUND IS HELPFUL IN RULING OUT DVT IN CELLULITIS
Lower extremity cellulitis, a common infection of the skin and subcutaneous tissues, is characterized by unilateral erythema, pain, warmth, and swelling. The infection usually follows a skin breach that allows bacteria to enter. DVT may present similarly, and symptoms can include mild leukocytosis and elevated temperature. Because of the clinical similarities, clinicians often order compression ultrasound of the extremity to rule out concurrent DVT in cellulitis. Further impetus for testing stems from fear of the potential complications of untreated DVT, including post-thrombotic syndrome, chronic venous insufficiency, and venous ulceration. A subsequent PE can be fatal, or can cause significant morbidity, including chronic VTE with associated pulmonary hypertension. An estimated quarter of all PEs present as sudden death.1
WHY ULTRASOUND IS NOT HELPFUL IN THIS SETTING
Studies have shown that ultrasound is ordered for 16% to 73% of patients with a cellulitis diagnosis.2,3 Although testing is commonly performed, a meta-analysis of 9 studies of cellulitis patients who underwent ultrasound testing for concurrent DVT revealed a low pooled incidence of total DVT (3.1%) and proximal DVT (2.1%).4 Maze et al.2 retrospectively reviewed 1515 cellulitis cases (identified by International Classification of Diseases, Ninth Revision codes) at a single center in New Zealand over 3 years. Of the 1515 patients, 240 (16%) had ultrasound performed, and only 3 (1.3%) were found to have DVT. Two of the 3 had active malignancy, and the third had injected battery acid into the area. In a 5-year retrospective cohort study at a Veterans Administration hospital in Connecticut, Gunderson and Chang3 reviewed the cases of 183 patients with cellulitis and found ultrasound testing commonly performed (73% of cases) to assess for DVT. Only 1 patient (<1%) was diagnosed with new DVT in the ipsilateral leg, and acute DVT was diagnosed in the contralateral leg of 2 other patients. Overall, these studies indicate the incidence of concurrent DVT in cellulitis is low, regardless of the frequency of ultrasound testing.
Although the cost of a single ultrasound test is not prohibitive, annual total costs hospital-wide and nationally are large. In the United States, the charge for a unilateral duplex ultrasound of the extremity ranges from $260 to $1300, and there is an additional charge for interpretation by a radiologist.5 In a retrospective study spanning 3.5 years and involving 2 community hospitals in Michigan, an estimated $290,000 was spent on ultrasound tests defined as unnecessary for patients with cellulitis.6 A limitation of the study was defining a test as unnecessary based on its result being negative.
DOES WELLS SCORE WITH D-DIMER HELP DEFINE A LOW-RISK POPULATION?
The Wells clinical prediction rule is commonly used to assess the pretest probability of DVT in patients presenting with unilateral leg symptoms. The Wells score is often combined with
WHEN MIGHT ULTRASOUND
Investigators have described possible DVT risk factors in patients with cellulitis, but definitive associations are lacking because of the insufficient number of patients studied.8,9 The most consistently identified DVT risk factor is history of previous thromboembolism. In a retrospective analysis of patients with cellulitis, Afzal et al.6 found that, of the 66.8% who underwent ultrasound testing, 5.5% were identified as having concurrent DVT. The authors performed univariate analyses of 15 potential risk factors, including active malignancy, oral contraceptive pill use, recent hospitalization, and surgery. A higher incidence of DVT was found for patients with history of VTE (odds ratio [OR], 5.7; 95% confidence interval [CI], 2.3-13.7), calf swelling (OR, 4.5; 95% CI, 1.3-15.8), CVA (OR, 3.5; 95% CI, 1.2-10.1), or hypertension (OR, 3.5; 95% CI, 0.98-12.2). Given the wide confidence intervals, paucity of studies, and lack of definitive data in the setting of cellulitis, clinicians may want to consider the risk factors established in larger trials in other settings, including known immobility (OR, <2); thrombophilia, CHF, and CVA with hemiparesis (OR, 2-9); and trauma and recent surgery (OR, >10).10
WHAT YOU SHOULD DO INSTEAD
As the incidence of concurrent VTE in patients with cellulitis is low, the essential step is to make a clear diagnosis of cellulitis based on its established signs and symptoms. A 2-center trial of 145 patients found that cellulitis was diagnosed accurately by general medicine and emergency medicine physicians 72% of the time, with evaluation by dermatologists and infectious disease specialists used as the gold standard. Only 5% of the misdiagnosed patients were diagnosed with DVT; stasis dermatitis was the most common alternative diagnosis. Taking a thorough history may elicit risk factors consistent with cellulitis, such as a recent injury with a break in the skin. On examination, cellulitis should be suspected for patients with fever and localized pain, redness, swelling, and warmth—the cardinal signs of dolor, rubor, tumor, and calor. An injury or entry site and leukocytosis also support the diagnosis of cellulitis. Distinct margins of erythema on the skin are highly suspicious for erysipelas.11 Other physical findings (eg, laceration, purulent drainage, lymphangitic spread, fluctuating mass) also are consistent with a diagnosis of cellulitis.
The patient’s history is also essential in determining whether any DVT risk factors are present. Past medical history of VTE or CVA, or recent history of surgery, immobility, or trauma, should alert the clinician to the possibility of DVT. Family history of VTE increases the likelihood of DVT. Acute shortness of breath or chest pain in the setting of concerning lower extremity findings for DVT should raise concern for DVT and concurrent PE.
If the classic features of cellulitis are present, empiric antibiotics should be initiated. Routine ultrasound testing for all patients with cellulitis is of low value. However, as the incidence of DVT in this population is not negligible, those with VTE risk factors should be targeted for testing. Studies in the setting of cellulitis provide little guidance regarding specific risk factors that can be used to determine who should undergo further testing. Given this limitation, we suggest that clinicians incorporate into their decision making the well-established VTE risk factors identified for large populations studied in other settings, such as the postoperative period. Specifically, clinicians should consider ultrasound testing for patients with cellulitis and prior history of VTE; immobility; thrombophilia, CHF, and CVA with hemiparesis; or trauma and recent surgery.10-12 Ultrasound should also be considered for patients with cellulitis that does not improve and for patients whose localized symptoms worsen despite use of antibiotics.
RECOMMENDATIONS
Do not routinely perform ultrasound to rule out concurrent DVT in cases of cellulitis.
Consider compression ultrasound if there is a history of VTE; immobility; thrombophilia, CHF, and CVA with hemiparesis; or trauma and recent surgery. Also consider it for patients who do not respond to antibiotics.
- In cases of cellulitis, avoid use of the Wells score alone or with
D -dimer testing, as it likely overestimates the DVT risk.
CONCLUSION
The current evidence shows that, for most patients with cellulitis, routine ultrasound testing for DVT is unnecessary. Ultrasound should be considered for patients with potent VTE risk factors. If symptoms do not improve, or if they worsen despite use of antibiotics, clinicians should be alert to potential anchoring bias and consider DVT. The Wells clinical prediction rule overestimates the incidence of DVT in cellulitis and has little value in this setting.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Heit JA. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J Thromb Thrombolysis. 2006;21(1):23-29. PubMed
2. Maze MJ, Pithie A, Dawes T, Chambers ST. An audit of venous duplex ultrasonography in patients with lower limb cellulitis. N Z Med J. 2011;124(1329):53-56. PubMed
3. Gunderson CG, Chang JJ. Overuse of compression ultrasound for patients with lower extremity cellulitis. Thromb Res. 2014;134(4):846-850. PubMed
4. Gunderson CG, Chang JJ. Risk of deep vein thrombosis in patients with cellulitis and erysipelas: a systematic review and meta-analysis. Thromb Res. 2013;132(3):336-340. PubMed
5. Extremity ultrasound (nonvascular) cost and procedure information. http://www.newchoicehealth.com/procedures/extremity-ultrasound-nonvascular. Accessed February 15, 2016.
6. Afzal MZ, Saleh MM, Razvi S, Hashmi H, Lampen R. Utility of lower extremity Doppler in patients with lower extremity cellulitis: a need to change the practice? South Med J. 2015;108(7):439-444. PubMed
7. Goodacre S, Sutton AJ, Sampson FC. Meta-analysis: the value of clinical assessment in the diagnosis of deep venous thrombosis. Ann Intern Med. 2005;143(2):129-139. PubMed
8. Maze MJ, Skea S, Pithie A, Metcalf S, Pearson JF, Chambers ST. Prevalence of concurrent deep vein thrombosis in patients with lower limb cellulitis: a prospective cohort study. BMC Infect Dis. 2013;13:141. PubMed
9. Bersier D, Bounameaux H. Cellulitis and deep vein thrombosis: a controversial association. J Thromb Haemost. 2003;1(4):867-868. PubMed
10. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23 suppl 1):I9-I16. PubMed
11. Rabuka CE, Azoulay LY, Kahn SR. Predictors of a positive duplex scan in patients with a clinical presentation compatible with deep vein thrombosis or cellulitis. Can J Infect Dis. 2003;14(4):210-214. PubMed
12. Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius Study. Arch Intern Med. 2000;160(22):3415-3420. PubMed
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Because of overlapping clinical manifestations, clinicians often order ultrasound to rule out deep vein thrombosis (DVT) in cases of cellulitis. Ultrasound testing is performed for 16% to 73% of patients diagnosed with cellulitis. Although testing is common, the pooled incidence of DVT is low (3.1%). Few data elucidate which patients with cellulitis are more likely to have concurrent DVT and require further testing. The Wells clinical prediction rule with
CASE REPORT
A 50-year-old man presented to the emergency department with a 3-day-old cut on his anterior right shin. Associated redness, warmth, pain, and swelling had progressed. The patient had no history of prior DVT or pulmonary embolism (PE). His temperature was 38.5°C, and his white blood cell count of 18,000. On review of systems, he denied shortness of breath and chest pain. He was diagnosed with cellulitis and administered intravenous fluids and cefazolin. The clinician wondered whether to perform lower extremity ultrasound to rule out concurrent DVT.
WHY YOU MIGHT THINK ULTRASOUND IS HELPFUL IN RULING OUT DVT IN CELLULITIS
Lower extremity cellulitis, a common infection of the skin and subcutaneous tissues, is characterized by unilateral erythema, pain, warmth, and swelling. The infection usually follows a skin breach that allows bacteria to enter. DVT may present similarly, and symptoms can include mild leukocytosis and elevated temperature. Because of the clinical similarities, clinicians often order compression ultrasound of the extremity to rule out concurrent DVT in cellulitis. Further impetus for testing stems from fear of the potential complications of untreated DVT, including post-thrombotic syndrome, chronic venous insufficiency, and venous ulceration. A subsequent PE can be fatal, or can cause significant morbidity, including chronic VTE with associated pulmonary hypertension. An estimated quarter of all PEs present as sudden death.1
WHY ULTRASOUND IS NOT HELPFUL IN THIS SETTING
Studies have shown that ultrasound is ordered for 16% to 73% of patients with a cellulitis diagnosis.2,3 Although testing is commonly performed, a meta-analysis of 9 studies of cellulitis patients who underwent ultrasound testing for concurrent DVT revealed a low pooled incidence of total DVT (3.1%) and proximal DVT (2.1%).4 Maze et al.2 retrospectively reviewed 1515 cellulitis cases (identified by International Classification of Diseases, Ninth Revision codes) at a single center in New Zealand over 3 years. Of the 1515 patients, 240 (16%) had ultrasound performed, and only 3 (1.3%) were found to have DVT. Two of the 3 had active malignancy, and the third had injected battery acid into the area. In a 5-year retrospective cohort study at a Veterans Administration hospital in Connecticut, Gunderson and Chang3 reviewed the cases of 183 patients with cellulitis and found ultrasound testing commonly performed (73% of cases) to assess for DVT. Only 1 patient (<1%) was diagnosed with new DVT in the ipsilateral leg, and acute DVT was diagnosed in the contralateral leg of 2 other patients. Overall, these studies indicate the incidence of concurrent DVT in cellulitis is low, regardless of the frequency of ultrasound testing.
Although the cost of a single ultrasound test is not prohibitive, annual total costs hospital-wide and nationally are large. In the United States, the charge for a unilateral duplex ultrasound of the extremity ranges from $260 to $1300, and there is an additional charge for interpretation by a radiologist.5 In a retrospective study spanning 3.5 years and involving 2 community hospitals in Michigan, an estimated $290,000 was spent on ultrasound tests defined as unnecessary for patients with cellulitis.6 A limitation of the study was defining a test as unnecessary based on its result being negative.
DOES WELLS SCORE WITH D-DIMER HELP DEFINE A LOW-RISK POPULATION?
The Wells clinical prediction rule is commonly used to assess the pretest probability of DVT in patients presenting with unilateral leg symptoms. The Wells score is often combined with
WHEN MIGHT ULTRASOUND
Investigators have described possible DVT risk factors in patients with cellulitis, but definitive associations are lacking because of the insufficient number of patients studied.8,9 The most consistently identified DVT risk factor is history of previous thromboembolism. In a retrospective analysis of patients with cellulitis, Afzal et al.6 found that, of the 66.8% who underwent ultrasound testing, 5.5% were identified as having concurrent DVT. The authors performed univariate analyses of 15 potential risk factors, including active malignancy, oral contraceptive pill use, recent hospitalization, and surgery. A higher incidence of DVT was found for patients with history of VTE (odds ratio [OR], 5.7; 95% confidence interval [CI], 2.3-13.7), calf swelling (OR, 4.5; 95% CI, 1.3-15.8), CVA (OR, 3.5; 95% CI, 1.2-10.1), or hypertension (OR, 3.5; 95% CI, 0.98-12.2). Given the wide confidence intervals, paucity of studies, and lack of definitive data in the setting of cellulitis, clinicians may want to consider the risk factors established in larger trials in other settings, including known immobility (OR, <2); thrombophilia, CHF, and CVA with hemiparesis (OR, 2-9); and trauma and recent surgery (OR, >10).10
WHAT YOU SHOULD DO INSTEAD
As the incidence of concurrent VTE in patients with cellulitis is low, the essential step is to make a clear diagnosis of cellulitis based on its established signs and symptoms. A 2-center trial of 145 patients found that cellulitis was diagnosed accurately by general medicine and emergency medicine physicians 72% of the time, with evaluation by dermatologists and infectious disease specialists used as the gold standard. Only 5% of the misdiagnosed patients were diagnosed with DVT; stasis dermatitis was the most common alternative diagnosis. Taking a thorough history may elicit risk factors consistent with cellulitis, such as a recent injury with a break in the skin. On examination, cellulitis should be suspected for patients with fever and localized pain, redness, swelling, and warmth—the cardinal signs of dolor, rubor, tumor, and calor. An injury or entry site and leukocytosis also support the diagnosis of cellulitis. Distinct margins of erythema on the skin are highly suspicious for erysipelas.11 Other physical findings (eg, laceration, purulent drainage, lymphangitic spread, fluctuating mass) also are consistent with a diagnosis of cellulitis.
The patient’s history is also essential in determining whether any DVT risk factors are present. Past medical history of VTE or CVA, or recent history of surgery, immobility, or trauma, should alert the clinician to the possibility of DVT. Family history of VTE increases the likelihood of DVT. Acute shortness of breath or chest pain in the setting of concerning lower extremity findings for DVT should raise concern for DVT and concurrent PE.
If the classic features of cellulitis are present, empiric antibiotics should be initiated. Routine ultrasound testing for all patients with cellulitis is of low value. However, as the incidence of DVT in this population is not negligible, those with VTE risk factors should be targeted for testing. Studies in the setting of cellulitis provide little guidance regarding specific risk factors that can be used to determine who should undergo further testing. Given this limitation, we suggest that clinicians incorporate into their decision making the well-established VTE risk factors identified for large populations studied in other settings, such as the postoperative period. Specifically, clinicians should consider ultrasound testing for patients with cellulitis and prior history of VTE; immobility; thrombophilia, CHF, and CVA with hemiparesis; or trauma and recent surgery.10-12 Ultrasound should also be considered for patients with cellulitis that does not improve and for patients whose localized symptoms worsen despite use of antibiotics.
RECOMMENDATIONS
Do not routinely perform ultrasound to rule out concurrent DVT in cases of cellulitis.
Consider compression ultrasound if there is a history of VTE; immobility; thrombophilia, CHF, and CVA with hemiparesis; or trauma and recent surgery. Also consider it for patients who do not respond to antibiotics.
- In cases of cellulitis, avoid use of the Wells score alone or with
D -dimer testing, as it likely overestimates the DVT risk.
CONCLUSION
The current evidence shows that, for most patients with cellulitis, routine ultrasound testing for DVT is unnecessary. Ultrasound should be considered for patients with potent VTE risk factors. If symptoms do not improve, or if they worsen despite use of antibiotics, clinicians should be alert to potential anchoring bias and consider DVT. The Wells clinical prediction rule overestimates the incidence of DVT in cellulitis and has little value in this setting.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Because of overlapping clinical manifestations, clinicians often order ultrasound to rule out deep vein thrombosis (DVT) in cases of cellulitis. Ultrasound testing is performed for 16% to 73% of patients diagnosed with cellulitis. Although testing is common, the pooled incidence of DVT is low (3.1%). Few data elucidate which patients with cellulitis are more likely to have concurrent DVT and require further testing. The Wells clinical prediction rule with
CASE REPORT
A 50-year-old man presented to the emergency department with a 3-day-old cut on his anterior right shin. Associated redness, warmth, pain, and swelling had progressed. The patient had no history of prior DVT or pulmonary embolism (PE). His temperature was 38.5°C, and his white blood cell count of 18,000. On review of systems, he denied shortness of breath and chest pain. He was diagnosed with cellulitis and administered intravenous fluids and cefazolin. The clinician wondered whether to perform lower extremity ultrasound to rule out concurrent DVT.
WHY YOU MIGHT THINK ULTRASOUND IS HELPFUL IN RULING OUT DVT IN CELLULITIS
Lower extremity cellulitis, a common infection of the skin and subcutaneous tissues, is characterized by unilateral erythema, pain, warmth, and swelling. The infection usually follows a skin breach that allows bacteria to enter. DVT may present similarly, and symptoms can include mild leukocytosis and elevated temperature. Because of the clinical similarities, clinicians often order compression ultrasound of the extremity to rule out concurrent DVT in cellulitis. Further impetus for testing stems from fear of the potential complications of untreated DVT, including post-thrombotic syndrome, chronic venous insufficiency, and venous ulceration. A subsequent PE can be fatal, or can cause significant morbidity, including chronic VTE with associated pulmonary hypertension. An estimated quarter of all PEs present as sudden death.1
WHY ULTRASOUND IS NOT HELPFUL IN THIS SETTING
Studies have shown that ultrasound is ordered for 16% to 73% of patients with a cellulitis diagnosis.2,3 Although testing is commonly performed, a meta-analysis of 9 studies of cellulitis patients who underwent ultrasound testing for concurrent DVT revealed a low pooled incidence of total DVT (3.1%) and proximal DVT (2.1%).4 Maze et al.2 retrospectively reviewed 1515 cellulitis cases (identified by International Classification of Diseases, Ninth Revision codes) at a single center in New Zealand over 3 years. Of the 1515 patients, 240 (16%) had ultrasound performed, and only 3 (1.3%) were found to have DVT. Two of the 3 had active malignancy, and the third had injected battery acid into the area. In a 5-year retrospective cohort study at a Veterans Administration hospital in Connecticut, Gunderson and Chang3 reviewed the cases of 183 patients with cellulitis and found ultrasound testing commonly performed (73% of cases) to assess for DVT. Only 1 patient (<1%) was diagnosed with new DVT in the ipsilateral leg, and acute DVT was diagnosed in the contralateral leg of 2 other patients. Overall, these studies indicate the incidence of concurrent DVT in cellulitis is low, regardless of the frequency of ultrasound testing.
Although the cost of a single ultrasound test is not prohibitive, annual total costs hospital-wide and nationally are large. In the United States, the charge for a unilateral duplex ultrasound of the extremity ranges from $260 to $1300, and there is an additional charge for interpretation by a radiologist.5 In a retrospective study spanning 3.5 years and involving 2 community hospitals in Michigan, an estimated $290,000 was spent on ultrasound tests defined as unnecessary for patients with cellulitis.6 A limitation of the study was defining a test as unnecessary based on its result being negative.
DOES WELLS SCORE WITH D-DIMER HELP DEFINE A LOW-RISK POPULATION?
The Wells clinical prediction rule is commonly used to assess the pretest probability of DVT in patients presenting with unilateral leg symptoms. The Wells score is often combined with
WHEN MIGHT ULTRASOUND
Investigators have described possible DVT risk factors in patients with cellulitis, but definitive associations are lacking because of the insufficient number of patients studied.8,9 The most consistently identified DVT risk factor is history of previous thromboembolism. In a retrospective analysis of patients with cellulitis, Afzal et al.6 found that, of the 66.8% who underwent ultrasound testing, 5.5% were identified as having concurrent DVT. The authors performed univariate analyses of 15 potential risk factors, including active malignancy, oral contraceptive pill use, recent hospitalization, and surgery. A higher incidence of DVT was found for patients with history of VTE (odds ratio [OR], 5.7; 95% confidence interval [CI], 2.3-13.7), calf swelling (OR, 4.5; 95% CI, 1.3-15.8), CVA (OR, 3.5; 95% CI, 1.2-10.1), or hypertension (OR, 3.5; 95% CI, 0.98-12.2). Given the wide confidence intervals, paucity of studies, and lack of definitive data in the setting of cellulitis, clinicians may want to consider the risk factors established in larger trials in other settings, including known immobility (OR, <2); thrombophilia, CHF, and CVA with hemiparesis (OR, 2-9); and trauma and recent surgery (OR, >10).10
WHAT YOU SHOULD DO INSTEAD
As the incidence of concurrent VTE in patients with cellulitis is low, the essential step is to make a clear diagnosis of cellulitis based on its established signs and symptoms. A 2-center trial of 145 patients found that cellulitis was diagnosed accurately by general medicine and emergency medicine physicians 72% of the time, with evaluation by dermatologists and infectious disease specialists used as the gold standard. Only 5% of the misdiagnosed patients were diagnosed with DVT; stasis dermatitis was the most common alternative diagnosis. Taking a thorough history may elicit risk factors consistent with cellulitis, such as a recent injury with a break in the skin. On examination, cellulitis should be suspected for patients with fever and localized pain, redness, swelling, and warmth—the cardinal signs of dolor, rubor, tumor, and calor. An injury or entry site and leukocytosis also support the diagnosis of cellulitis. Distinct margins of erythema on the skin are highly suspicious for erysipelas.11 Other physical findings (eg, laceration, purulent drainage, lymphangitic spread, fluctuating mass) also are consistent with a diagnosis of cellulitis.
The patient’s history is also essential in determining whether any DVT risk factors are present. Past medical history of VTE or CVA, or recent history of surgery, immobility, or trauma, should alert the clinician to the possibility of DVT. Family history of VTE increases the likelihood of DVT. Acute shortness of breath or chest pain in the setting of concerning lower extremity findings for DVT should raise concern for DVT and concurrent PE.
If the classic features of cellulitis are present, empiric antibiotics should be initiated. Routine ultrasound testing for all patients with cellulitis is of low value. However, as the incidence of DVT in this population is not negligible, those with VTE risk factors should be targeted for testing. Studies in the setting of cellulitis provide little guidance regarding specific risk factors that can be used to determine who should undergo further testing. Given this limitation, we suggest that clinicians incorporate into their decision making the well-established VTE risk factors identified for large populations studied in other settings, such as the postoperative period. Specifically, clinicians should consider ultrasound testing for patients with cellulitis and prior history of VTE; immobility; thrombophilia, CHF, and CVA with hemiparesis; or trauma and recent surgery.10-12 Ultrasound should also be considered for patients with cellulitis that does not improve and for patients whose localized symptoms worsen despite use of antibiotics.
RECOMMENDATIONS
Do not routinely perform ultrasound to rule out concurrent DVT in cases of cellulitis.
Consider compression ultrasound if there is a history of VTE; immobility; thrombophilia, CHF, and CVA with hemiparesis; or trauma and recent surgery. Also consider it for patients who do not respond to antibiotics.
- In cases of cellulitis, avoid use of the Wells score alone or with
D -dimer testing, as it likely overestimates the DVT risk.
CONCLUSION
The current evidence shows that, for most patients with cellulitis, routine ultrasound testing for DVT is unnecessary. Ultrasound should be considered for patients with potent VTE risk factors. If symptoms do not improve, or if they worsen despite use of antibiotics, clinicians should be alert to potential anchoring bias and consider DVT. The Wells clinical prediction rule overestimates the incidence of DVT in cellulitis and has little value in this setting.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Heit JA. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J Thromb Thrombolysis. 2006;21(1):23-29. PubMed
2. Maze MJ, Pithie A, Dawes T, Chambers ST. An audit of venous duplex ultrasonography in patients with lower limb cellulitis. N Z Med J. 2011;124(1329):53-56. PubMed
3. Gunderson CG, Chang JJ. Overuse of compression ultrasound for patients with lower extremity cellulitis. Thromb Res. 2014;134(4):846-850. PubMed
4. Gunderson CG, Chang JJ. Risk of deep vein thrombosis in patients with cellulitis and erysipelas: a systematic review and meta-analysis. Thromb Res. 2013;132(3):336-340. PubMed
5. Extremity ultrasound (nonvascular) cost and procedure information. http://www.newchoicehealth.com/procedures/extremity-ultrasound-nonvascular. Accessed February 15, 2016.
6. Afzal MZ, Saleh MM, Razvi S, Hashmi H, Lampen R. Utility of lower extremity Doppler in patients with lower extremity cellulitis: a need to change the practice? South Med J. 2015;108(7):439-444. PubMed
7. Goodacre S, Sutton AJ, Sampson FC. Meta-analysis: the value of clinical assessment in the diagnosis of deep venous thrombosis. Ann Intern Med. 2005;143(2):129-139. PubMed
8. Maze MJ, Skea S, Pithie A, Metcalf S, Pearson JF, Chambers ST. Prevalence of concurrent deep vein thrombosis in patients with lower limb cellulitis: a prospective cohort study. BMC Infect Dis. 2013;13:141. PubMed
9. Bersier D, Bounameaux H. Cellulitis and deep vein thrombosis: a controversial association. J Thromb Haemost. 2003;1(4):867-868. PubMed
10. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23 suppl 1):I9-I16. PubMed
11. Rabuka CE, Azoulay LY, Kahn SR. Predictors of a positive duplex scan in patients with a clinical presentation compatible with deep vein thrombosis or cellulitis. Can J Infect Dis. 2003;14(4):210-214. PubMed
12. Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius Study. Arch Intern Med. 2000;160(22):3415-3420. PubMed
1. Heit JA. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J Thromb Thrombolysis. 2006;21(1):23-29. PubMed
2. Maze MJ, Pithie A, Dawes T, Chambers ST. An audit of venous duplex ultrasonography in patients with lower limb cellulitis. N Z Med J. 2011;124(1329):53-56. PubMed
3. Gunderson CG, Chang JJ. Overuse of compression ultrasound for patients with lower extremity cellulitis. Thromb Res. 2014;134(4):846-850. PubMed
4. Gunderson CG, Chang JJ. Risk of deep vein thrombosis in patients with cellulitis and erysipelas: a systematic review and meta-analysis. Thromb Res. 2013;132(3):336-340. PubMed
5. Extremity ultrasound (nonvascular) cost and procedure information. http://www.newchoicehealth.com/procedures/extremity-ultrasound-nonvascular. Accessed February 15, 2016.
6. Afzal MZ, Saleh MM, Razvi S, Hashmi H, Lampen R. Utility of lower extremity Doppler in patients with lower extremity cellulitis: a need to change the practice? South Med J. 2015;108(7):439-444. PubMed
7. Goodacre S, Sutton AJ, Sampson FC. Meta-analysis: the value of clinical assessment in the diagnosis of deep venous thrombosis. Ann Intern Med. 2005;143(2):129-139. PubMed
8. Maze MJ, Skea S, Pithie A, Metcalf S, Pearson JF, Chambers ST. Prevalence of concurrent deep vein thrombosis in patients with lower limb cellulitis: a prospective cohort study. BMC Infect Dis. 2013;13:141. PubMed
9. Bersier D, Bounameaux H. Cellulitis and deep vein thrombosis: a controversial association. J Thromb Haemost. 2003;1(4):867-868. PubMed
10. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23 suppl 1):I9-I16. PubMed
11. Rabuka CE, Azoulay LY, Kahn SR. Predictors of a positive duplex scan in patients with a clinical presentation compatible with deep vein thrombosis or cellulitis. Can J Infect Dis. 2003;14(4):210-214. PubMed
12. Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius Study. Arch Intern Med. 2000;160(22):3415-3420. PubMed
© 2017 Society of Hospital Medicine
Nondirected testing for inpatients with severe liver injury
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 68-year-old woman with ischemic cardiomyopathy was admitted with abdominal cramping, diarrhea, and nausea, which had left her unable to keep food and liquids down for 2 days. She had been taking diuretics and had a remote history of intravenous drug use. On admission, she was afebrile and had blood pressure of 100/60 mm Hg and a heart rate of 100 bpm. Her extremities were cool and clammy. Blood test results showed an alanine aminotransferase (ALT) level of 1510 IU/L and an aspartate aminotransferase (AST) level of 1643 IU/L. The patient’s clinician did not know her baseline ALT and AST levels and thought the best approach was to identify the cause of the transaminase elevation.
Severe acute liver injury (liver enzymes, >10 × upper limit of normal [ULN], usually 40 IU/L) is a common presentation among hospitalized patients. Between 1997 and 2015, 1.5% of patients admitted to our hospital had severe liver injury. In another large cohort of hospitalized patients,1 0.6% had an ALT level higher than 1000 IU/L (~20 × ULN). A precise diagnosis is often needed to direct appropriate therapy, and serologic tests are available for many conditions, both common and rare (Table). Given the relative ease of bundled blood testing, nondirected testing has emerged as a popular, if reflexive, strategy.2-5 In this approach, clinicians evaluate each patient for the set of testable diseases all at once—in contrast to taking a directed, stepwise testing approach guided by the patient’s history.
Use of nondirected testing is common in patients with severe acute liver injury. Of the 5795 such patients treated at our hospital between 2000 and 2015, within the same day of service 53% were tested for hepatitis C virus antibody, 38% for hemochromatosis (ferritin test), 28% for autoimmune hepatitis (antinuclear antibody test), and 15% for primary biliary cholangitis (antimitochondrial antibody test) by our clinical laboratory. Of the 5023 patients who had send-out tests performed for Wilson disease (ceruloplasmin), 81% were queried for hepatitis B virus infection, 76% for hepatitis C virus infection, 75% for autoimmune hepatitis, and 73.1% for hemochromatosis.2 Similar trends were found for patients with severe liver injury tested for α1-antitrypsin (AAT) deficiency.3 In sum, these data showed that each patient with severe liver injury was tested out of concern about diseases with markedly different epidemiology and clinical presentations (Table).
WHY YOU MIGHT THINK NONDIRECTED TESTING IS HELPFUL
Use of nondirected testing may reflect perceived urgency, convenience, and thoroughness.2-6 Alternatively, it may simply involve following a consultant’s recommendations.4 As severe acute liver injury is often associated with tremendous morbidity, clinicians seeking answers may perceive directed, stepwise testing as inappropriately slow given the urgency of the presentation; they may think that nondirected testing can reduce hospital length of stay.
WHY NONDIRECTED TESTING IS NOT HELPFUL
Nondirected testing is a problem for at least 4 reasons: limited benefit of reflexive testing for rare diseases, no meaningful impact on outcomes, false positives, and financial cost.
First, immediately testing for rare causes of liver disease is unlikely to benefit patients with severe liver injury. The underlying etiologies of severe liver injury are relatively well circumscribed (Table). Overall, 42% of patients with severe liver injury and 57% of those with an ALT level higher than 1000 IU/L have ischemic hepatitis.7 Accounting for a significant percentage of severe liver injury cases are acute biliary obstruction (24%), drug-induced injury (10%-13%), and viral hepatitis (4%-7%).1,8 Of the small subset of patients with severe liver injury that progresses to acute liver failure (ALF; encephalopathy, coagulopathy), 0.5% have autoimmune hepatitis and 0.1% have Wilson disease.9 Furthermore, many patients are tested for AAT deficiency, hemochromatosis, and primary biliary cholangitis, but these are never causes of severe acute liver injury (Table).
Second, diagnosing a rarer cause of acute liver injury modestly earlier has no meaningful impact on outcome. Work-up for more common etiologies can usually be completed within 2 or 3 days. This is true even for patients with ALF. Specific therapies generally are lacking for ALF, save for use of N-acetylcysteine for acetaminophen overdose and antiviral therapy for hepatitis B virus infection.9,10 Furthermore, although effective therapies are available for both autoimmune hepatitis and Wilson disease, the potential benefit stems from altering the longer term course of disease. Initial management, even for these rare conditions, is no different from that for other etiologies. Conversely, acute liver injury caused by ischemic hepatitis, biliary disease, or drug-induced liver injury requires swift corrective action. Even if normotensive, patients with ischemic hepatitis are often in cardiogenic shock and benefit from careful monitoring and critical care.7 Patients with acute biliary obstruction may need therapeutic endoscopy. Last, patients with drug-induced liver injury benefit from immediate discontinuation of the offending drug.
Third, in the testing of patients with low pretest probabilities, false positives are common. For example, at our institution and at an institution in Austria, severe liver injury patients with a low ceruloplasmin level have a 95.1% to 98.1% chance of a false-positive result (they have a low ceruloplasmin level but do not have Wilson disease).3,4 Furthermore, 91% of positive tests are never confirmed,3 indicating either that clinicians never valued the initial test or that other diagnoses were much more likely. Even worse, as was the case in 65% of patients with low AAT levels,2,3 genetic diagnoses were based on unconfirmed, potentially false-positive serologic tests.
Fourth, although the financial cost for each individual test is small, at the population level the cost of nondirected testing is significant. For example, although each reflects testing for conditions that do not cause acute liver injury, the costs of ferritin, AAT, and antimitochondrial antibody tests are $13, $16, and $37, respectively (Medicare/Medicaid reimbursements in 2016 $US).11 About 1.5% of admitted patients are found to have severe liver injury. If this proportion holds true for the roughly 40 million discharges from US hospitals each year, then there would be an annual cost of about $40 million if all 3 tests were performed for each patient with severe liver injury. In addition, although nondirected testing may seem clinically expedient, there are no data suggesting it reduces length of stay. In fact, ceruloplasmin, AAT, and many other tests are sent to external laboratories and are unlikely to be returned before discharge. If clinicians delay discharge for results, then nondirected testing would increase rather than decrease length of stay.
WHAT YOU SHOULD DO INSTEAD
In this era of increasing cost-consciousness, nondirected testing has escaped relatively unscathed. Indeed, nondirected testing is prevalent, yet has pitfalls similar to those of serologic testing (eg, vasculitis or arthritis,6 acute renal injury, infectious disease12). The alternative is deliberate, empirical, patient-centered testing that is attentive to the patient’s presentation and the harms of false positives. The idea is to select tests for each patient with acute liver injury according to presentation and the most likely corresponding diagnoses (Table, Figure).
The “one-stop shopping” in providers’ electronic order entry systems makes it too easy to over-order tests. Fortunately, these systems’ simple and effective decision supports can force pauses in the ordering process, create barriers to waste, and provide education about test characteristics and costs.4,5,13 Our medical center’s volume of ceruloplasmin orders decreased by 80% after a change was made to its ordering system; the ordering of a ceruloplasmin test is now interrupted by a pop-up screen that displays test characteristics and an option to continue or cancel the order.4,5 Hospitals should consider implementing clinical decision supports in this area. Successful interventions provide electronic rather than paper-based support as part of the clinical workflow, during the ordering process, and recommendations rather than assessments.13
RECOMMENDATIONS
- For each patient with severe acute liver injury, select tests on the basis of the presentation (Figure). Testing for rare diseases should be performed only after common diseases have been excluded.
- Avoid testing for hemochromatosis (iron indices, genetic tests), AAT deficiency (AAT levels or phenotypes), and primary biliary cholangitis (antimitochondrial antibodies) in patients with severe acute liver injury.
- Consider implementing decision supports that can curb nondirected testing in areas in which it is common.
CONCLUSION
Nondirected testing is associated with false positives and increased costs in the evaluation and management of severe acute liver injury. The alternative is deliberate, epidemiologically and clinically driven directed testing. Electronic ordering system decision supports can be useful in curtailing nondirected testing.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
1. Johnson RD, O’Connor ML, Kerr RM. Extreme serum elevations of aspartate aminotransferase. Am J Gastroenterol. 1995;90(8):1244-1245. PubMed
2. Tapper EB, Patwardhan VR, Curry M. Low yield and utilization of confirmatory testing in a cohort of patients with liver disease assessed for alpha-1 antitrypsin deficiency. Dig Dis Sci. 2015;60(6):1589-1594. PubMed
3. Tapper EB, Rahni DO, Arnaout R, Lai M. The overuse of serum ceruloplasmin measurement. Am J Med. 2013;126(10):926.e1-e5. PubMed
4. Tapper EB, Sengupta N, Lai M, Horowitz G. Understanding and reducing ceruloplasmin overuse with a decision support intervention for liver disease evaluation. Am J Med. 2016;129(1):115.e17-e22. PubMed
5. Tapper EB, Sengupta N, Lai M, Horowitz G. A decision support tool to reduce overtesting for ceruloplasmin and improve adherence with clinical guidelines. JAMA Intern Med. 2015;175(9):1561-1562. PubMed
6. Lichtenstein MJ, Pincus T. How useful are combinations of blood tests in “rheumatic panels” in diagnosis of rheumatic diseases? J Gen Intern Med. 1988;3(5):435-442. PubMed
7. Tapper EB, Sengupta N, Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med. 2015;128(12):1314-1321. PubMed
8. Whitehead MW, Hawkes ND, Hainsworth I, Kingham JG. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999;45(1):129-133. PubMed
9. Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92(4):761-794. PubMed
10. Lee WM, Larson AM, Stravitz RT. AASLD Position Paper: The Management of Acute Liver Failure: Update 2011. American Association for the Study of Liver Diseases website. https://www.aasld.org/sites/default/files/guideline_documents/alfenhanced.pdf. Published 2011. Accessed January 26, 2017.
11. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367-1384. PubMed
12. Aesif SW, Parenti DM, Lesky L, Keiser JF. A cost-effective interdisciplinary approach to microbiologic send-out test use. Arch Pathol Lab Med. 2015;139(2):194-198. PubMed
13. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
14. Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6(3):635-647. PubMed
15. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(1):328-343. PubMed
16. Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181-1188. PubMed
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 68-year-old woman with ischemic cardiomyopathy was admitted with abdominal cramping, diarrhea, and nausea, which had left her unable to keep food and liquids down for 2 days. She had been taking diuretics and had a remote history of intravenous drug use. On admission, she was afebrile and had blood pressure of 100/60 mm Hg and a heart rate of 100 bpm. Her extremities were cool and clammy. Blood test results showed an alanine aminotransferase (ALT) level of 1510 IU/L and an aspartate aminotransferase (AST) level of 1643 IU/L. The patient’s clinician did not know her baseline ALT and AST levels and thought the best approach was to identify the cause of the transaminase elevation.
Severe acute liver injury (liver enzymes, >10 × upper limit of normal [ULN], usually 40 IU/L) is a common presentation among hospitalized patients. Between 1997 and 2015, 1.5% of patients admitted to our hospital had severe liver injury. In another large cohort of hospitalized patients,1 0.6% had an ALT level higher than 1000 IU/L (~20 × ULN). A precise diagnosis is often needed to direct appropriate therapy, and serologic tests are available for many conditions, both common and rare (Table). Given the relative ease of bundled blood testing, nondirected testing has emerged as a popular, if reflexive, strategy.2-5 In this approach, clinicians evaluate each patient for the set of testable diseases all at once—in contrast to taking a directed, stepwise testing approach guided by the patient’s history.
Use of nondirected testing is common in patients with severe acute liver injury. Of the 5795 such patients treated at our hospital between 2000 and 2015, within the same day of service 53% were tested for hepatitis C virus antibody, 38% for hemochromatosis (ferritin test), 28% for autoimmune hepatitis (antinuclear antibody test), and 15% for primary biliary cholangitis (antimitochondrial antibody test) by our clinical laboratory. Of the 5023 patients who had send-out tests performed for Wilson disease (ceruloplasmin), 81% were queried for hepatitis B virus infection, 76% for hepatitis C virus infection, 75% for autoimmune hepatitis, and 73.1% for hemochromatosis.2 Similar trends were found for patients with severe liver injury tested for α1-antitrypsin (AAT) deficiency.3 In sum, these data showed that each patient with severe liver injury was tested out of concern about diseases with markedly different epidemiology and clinical presentations (Table).
WHY YOU MIGHT THINK NONDIRECTED TESTING IS HELPFUL
Use of nondirected testing may reflect perceived urgency, convenience, and thoroughness.2-6 Alternatively, it may simply involve following a consultant’s recommendations.4 As severe acute liver injury is often associated with tremendous morbidity, clinicians seeking answers may perceive directed, stepwise testing as inappropriately slow given the urgency of the presentation; they may think that nondirected testing can reduce hospital length of stay.
WHY NONDIRECTED TESTING IS NOT HELPFUL
Nondirected testing is a problem for at least 4 reasons: limited benefit of reflexive testing for rare diseases, no meaningful impact on outcomes, false positives, and financial cost.
First, immediately testing for rare causes of liver disease is unlikely to benefit patients with severe liver injury. The underlying etiologies of severe liver injury are relatively well circumscribed (Table). Overall, 42% of patients with severe liver injury and 57% of those with an ALT level higher than 1000 IU/L have ischemic hepatitis.7 Accounting for a significant percentage of severe liver injury cases are acute biliary obstruction (24%), drug-induced injury (10%-13%), and viral hepatitis (4%-7%).1,8 Of the small subset of patients with severe liver injury that progresses to acute liver failure (ALF; encephalopathy, coagulopathy), 0.5% have autoimmune hepatitis and 0.1% have Wilson disease.9 Furthermore, many patients are tested for AAT deficiency, hemochromatosis, and primary biliary cholangitis, but these are never causes of severe acute liver injury (Table).
Second, diagnosing a rarer cause of acute liver injury modestly earlier has no meaningful impact on outcome. Work-up for more common etiologies can usually be completed within 2 or 3 days. This is true even for patients with ALF. Specific therapies generally are lacking for ALF, save for use of N-acetylcysteine for acetaminophen overdose and antiviral therapy for hepatitis B virus infection.9,10 Furthermore, although effective therapies are available for both autoimmune hepatitis and Wilson disease, the potential benefit stems from altering the longer term course of disease. Initial management, even for these rare conditions, is no different from that for other etiologies. Conversely, acute liver injury caused by ischemic hepatitis, biliary disease, or drug-induced liver injury requires swift corrective action. Even if normotensive, patients with ischemic hepatitis are often in cardiogenic shock and benefit from careful monitoring and critical care.7 Patients with acute biliary obstruction may need therapeutic endoscopy. Last, patients with drug-induced liver injury benefit from immediate discontinuation of the offending drug.
Third, in the testing of patients with low pretest probabilities, false positives are common. For example, at our institution and at an institution in Austria, severe liver injury patients with a low ceruloplasmin level have a 95.1% to 98.1% chance of a false-positive result (they have a low ceruloplasmin level but do not have Wilson disease).3,4 Furthermore, 91% of positive tests are never confirmed,3 indicating either that clinicians never valued the initial test or that other diagnoses were much more likely. Even worse, as was the case in 65% of patients with low AAT levels,2,3 genetic diagnoses were based on unconfirmed, potentially false-positive serologic tests.
Fourth, although the financial cost for each individual test is small, at the population level the cost of nondirected testing is significant. For example, although each reflects testing for conditions that do not cause acute liver injury, the costs of ferritin, AAT, and antimitochondrial antibody tests are $13, $16, and $37, respectively (Medicare/Medicaid reimbursements in 2016 $US).11 About 1.5% of admitted patients are found to have severe liver injury. If this proportion holds true for the roughly 40 million discharges from US hospitals each year, then there would be an annual cost of about $40 million if all 3 tests were performed for each patient with severe liver injury. In addition, although nondirected testing may seem clinically expedient, there are no data suggesting it reduces length of stay. In fact, ceruloplasmin, AAT, and many other tests are sent to external laboratories and are unlikely to be returned before discharge. If clinicians delay discharge for results, then nondirected testing would increase rather than decrease length of stay.
WHAT YOU SHOULD DO INSTEAD
In this era of increasing cost-consciousness, nondirected testing has escaped relatively unscathed. Indeed, nondirected testing is prevalent, yet has pitfalls similar to those of serologic testing (eg, vasculitis or arthritis,6 acute renal injury, infectious disease12). The alternative is deliberate, empirical, patient-centered testing that is attentive to the patient’s presentation and the harms of false positives. The idea is to select tests for each patient with acute liver injury according to presentation and the most likely corresponding diagnoses (Table, Figure).
The “one-stop shopping” in providers’ electronic order entry systems makes it too easy to over-order tests. Fortunately, these systems’ simple and effective decision supports can force pauses in the ordering process, create barriers to waste, and provide education about test characteristics and costs.4,5,13 Our medical center’s volume of ceruloplasmin orders decreased by 80% after a change was made to its ordering system; the ordering of a ceruloplasmin test is now interrupted by a pop-up screen that displays test characteristics and an option to continue or cancel the order.4,5 Hospitals should consider implementing clinical decision supports in this area. Successful interventions provide electronic rather than paper-based support as part of the clinical workflow, during the ordering process, and recommendations rather than assessments.13
RECOMMENDATIONS
- For each patient with severe acute liver injury, select tests on the basis of the presentation (Figure). Testing for rare diseases should be performed only after common diseases have been excluded.
- Avoid testing for hemochromatosis (iron indices, genetic tests), AAT deficiency (AAT levels or phenotypes), and primary biliary cholangitis (antimitochondrial antibodies) in patients with severe acute liver injury.
- Consider implementing decision supports that can curb nondirected testing in areas in which it is common.
CONCLUSION
Nondirected testing is associated with false positives and increased costs in the evaluation and management of severe acute liver injury. The alternative is deliberate, epidemiologically and clinically driven directed testing. Electronic ordering system decision supports can be useful in curtailing nondirected testing.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 68-year-old woman with ischemic cardiomyopathy was admitted with abdominal cramping, diarrhea, and nausea, which had left her unable to keep food and liquids down for 2 days. She had been taking diuretics and had a remote history of intravenous drug use. On admission, she was afebrile and had blood pressure of 100/60 mm Hg and a heart rate of 100 bpm. Her extremities were cool and clammy. Blood test results showed an alanine aminotransferase (ALT) level of 1510 IU/L and an aspartate aminotransferase (AST) level of 1643 IU/L. The patient’s clinician did not know her baseline ALT and AST levels and thought the best approach was to identify the cause of the transaminase elevation.
Severe acute liver injury (liver enzymes, >10 × upper limit of normal [ULN], usually 40 IU/L) is a common presentation among hospitalized patients. Between 1997 and 2015, 1.5% of patients admitted to our hospital had severe liver injury. In another large cohort of hospitalized patients,1 0.6% had an ALT level higher than 1000 IU/L (~20 × ULN). A precise diagnosis is often needed to direct appropriate therapy, and serologic tests are available for many conditions, both common and rare (Table). Given the relative ease of bundled blood testing, nondirected testing has emerged as a popular, if reflexive, strategy.2-5 In this approach, clinicians evaluate each patient for the set of testable diseases all at once—in contrast to taking a directed, stepwise testing approach guided by the patient’s history.
Use of nondirected testing is common in patients with severe acute liver injury. Of the 5795 such patients treated at our hospital between 2000 and 2015, within the same day of service 53% were tested for hepatitis C virus antibody, 38% for hemochromatosis (ferritin test), 28% for autoimmune hepatitis (antinuclear antibody test), and 15% for primary biliary cholangitis (antimitochondrial antibody test) by our clinical laboratory. Of the 5023 patients who had send-out tests performed for Wilson disease (ceruloplasmin), 81% were queried for hepatitis B virus infection, 76% for hepatitis C virus infection, 75% for autoimmune hepatitis, and 73.1% for hemochromatosis.2 Similar trends were found for patients with severe liver injury tested for α1-antitrypsin (AAT) deficiency.3 In sum, these data showed that each patient with severe liver injury was tested out of concern about diseases with markedly different epidemiology and clinical presentations (Table).
WHY YOU MIGHT THINK NONDIRECTED TESTING IS HELPFUL
Use of nondirected testing may reflect perceived urgency, convenience, and thoroughness.2-6 Alternatively, it may simply involve following a consultant’s recommendations.4 As severe acute liver injury is often associated with tremendous morbidity, clinicians seeking answers may perceive directed, stepwise testing as inappropriately slow given the urgency of the presentation; they may think that nondirected testing can reduce hospital length of stay.
WHY NONDIRECTED TESTING IS NOT HELPFUL
Nondirected testing is a problem for at least 4 reasons: limited benefit of reflexive testing for rare diseases, no meaningful impact on outcomes, false positives, and financial cost.
First, immediately testing for rare causes of liver disease is unlikely to benefit patients with severe liver injury. The underlying etiologies of severe liver injury are relatively well circumscribed (Table). Overall, 42% of patients with severe liver injury and 57% of those with an ALT level higher than 1000 IU/L have ischemic hepatitis.7 Accounting for a significant percentage of severe liver injury cases are acute biliary obstruction (24%), drug-induced injury (10%-13%), and viral hepatitis (4%-7%).1,8 Of the small subset of patients with severe liver injury that progresses to acute liver failure (ALF; encephalopathy, coagulopathy), 0.5% have autoimmune hepatitis and 0.1% have Wilson disease.9 Furthermore, many patients are tested for AAT deficiency, hemochromatosis, and primary biliary cholangitis, but these are never causes of severe acute liver injury (Table).
Second, diagnosing a rarer cause of acute liver injury modestly earlier has no meaningful impact on outcome. Work-up for more common etiologies can usually be completed within 2 or 3 days. This is true even for patients with ALF. Specific therapies generally are lacking for ALF, save for use of N-acetylcysteine for acetaminophen overdose and antiviral therapy for hepatitis B virus infection.9,10 Furthermore, although effective therapies are available for both autoimmune hepatitis and Wilson disease, the potential benefit stems from altering the longer term course of disease. Initial management, even for these rare conditions, is no different from that for other etiologies. Conversely, acute liver injury caused by ischemic hepatitis, biliary disease, or drug-induced liver injury requires swift corrective action. Even if normotensive, patients with ischemic hepatitis are often in cardiogenic shock and benefit from careful monitoring and critical care.7 Patients with acute biliary obstruction may need therapeutic endoscopy. Last, patients with drug-induced liver injury benefit from immediate discontinuation of the offending drug.
Third, in the testing of patients with low pretest probabilities, false positives are common. For example, at our institution and at an institution in Austria, severe liver injury patients with a low ceruloplasmin level have a 95.1% to 98.1% chance of a false-positive result (they have a low ceruloplasmin level but do not have Wilson disease).3,4 Furthermore, 91% of positive tests are never confirmed,3 indicating either that clinicians never valued the initial test or that other diagnoses were much more likely. Even worse, as was the case in 65% of patients with low AAT levels,2,3 genetic diagnoses were based on unconfirmed, potentially false-positive serologic tests.
Fourth, although the financial cost for each individual test is small, at the population level the cost of nondirected testing is significant. For example, although each reflects testing for conditions that do not cause acute liver injury, the costs of ferritin, AAT, and antimitochondrial antibody tests are $13, $16, and $37, respectively (Medicare/Medicaid reimbursements in 2016 $US).11 About 1.5% of admitted patients are found to have severe liver injury. If this proportion holds true for the roughly 40 million discharges from US hospitals each year, then there would be an annual cost of about $40 million if all 3 tests were performed for each patient with severe liver injury. In addition, although nondirected testing may seem clinically expedient, there are no data suggesting it reduces length of stay. In fact, ceruloplasmin, AAT, and many other tests are sent to external laboratories and are unlikely to be returned before discharge. If clinicians delay discharge for results, then nondirected testing would increase rather than decrease length of stay.
WHAT YOU SHOULD DO INSTEAD
In this era of increasing cost-consciousness, nondirected testing has escaped relatively unscathed. Indeed, nondirected testing is prevalent, yet has pitfalls similar to those of serologic testing (eg, vasculitis or arthritis,6 acute renal injury, infectious disease12). The alternative is deliberate, empirical, patient-centered testing that is attentive to the patient’s presentation and the harms of false positives. The idea is to select tests for each patient with acute liver injury according to presentation and the most likely corresponding diagnoses (Table, Figure).
The “one-stop shopping” in providers’ electronic order entry systems makes it too easy to over-order tests. Fortunately, these systems’ simple and effective decision supports can force pauses in the ordering process, create barriers to waste, and provide education about test characteristics and costs.4,5,13 Our medical center’s volume of ceruloplasmin orders decreased by 80% after a change was made to its ordering system; the ordering of a ceruloplasmin test is now interrupted by a pop-up screen that displays test characteristics and an option to continue or cancel the order.4,5 Hospitals should consider implementing clinical decision supports in this area. Successful interventions provide electronic rather than paper-based support as part of the clinical workflow, during the ordering process, and recommendations rather than assessments.13
RECOMMENDATIONS
- For each patient with severe acute liver injury, select tests on the basis of the presentation (Figure). Testing for rare diseases should be performed only after common diseases have been excluded.
- Avoid testing for hemochromatosis (iron indices, genetic tests), AAT deficiency (AAT levels or phenotypes), and primary biliary cholangitis (antimitochondrial antibodies) in patients with severe acute liver injury.
- Consider implementing decision supports that can curb nondirected testing in areas in which it is common.
CONCLUSION
Nondirected testing is associated with false positives and increased costs in the evaluation and management of severe acute liver injury. The alternative is deliberate, epidemiologically and clinically driven directed testing. Electronic ordering system decision supports can be useful in curtailing nondirected testing.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
1. Johnson RD, O’Connor ML, Kerr RM. Extreme serum elevations of aspartate aminotransferase. Am J Gastroenterol. 1995;90(8):1244-1245. PubMed
2. Tapper EB, Patwardhan VR, Curry M. Low yield and utilization of confirmatory testing in a cohort of patients with liver disease assessed for alpha-1 antitrypsin deficiency. Dig Dis Sci. 2015;60(6):1589-1594. PubMed
3. Tapper EB, Rahni DO, Arnaout R, Lai M. The overuse of serum ceruloplasmin measurement. Am J Med. 2013;126(10):926.e1-e5. PubMed
4. Tapper EB, Sengupta N, Lai M, Horowitz G. Understanding and reducing ceruloplasmin overuse with a decision support intervention for liver disease evaluation. Am J Med. 2016;129(1):115.e17-e22. PubMed
5. Tapper EB, Sengupta N, Lai M, Horowitz G. A decision support tool to reduce overtesting for ceruloplasmin and improve adherence with clinical guidelines. JAMA Intern Med. 2015;175(9):1561-1562. PubMed
6. Lichtenstein MJ, Pincus T. How useful are combinations of blood tests in “rheumatic panels” in diagnosis of rheumatic diseases? J Gen Intern Med. 1988;3(5):435-442. PubMed
7. Tapper EB, Sengupta N, Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med. 2015;128(12):1314-1321. PubMed
8. Whitehead MW, Hawkes ND, Hainsworth I, Kingham JG. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999;45(1):129-133. PubMed
9. Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92(4):761-794. PubMed
10. Lee WM, Larson AM, Stravitz RT. AASLD Position Paper: The Management of Acute Liver Failure: Update 2011. American Association for the Study of Liver Diseases website. https://www.aasld.org/sites/default/files/guideline_documents/alfenhanced.pdf. Published 2011. Accessed January 26, 2017.
11. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367-1384. PubMed
12. Aesif SW, Parenti DM, Lesky L, Keiser JF. A cost-effective interdisciplinary approach to microbiologic send-out test use. Arch Pathol Lab Med. 2015;139(2):194-198. PubMed
13. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
14. Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6(3):635-647. PubMed
15. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(1):328-343. PubMed
16. Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181-1188. PubMed
1. Johnson RD, O’Connor ML, Kerr RM. Extreme serum elevations of aspartate aminotransferase. Am J Gastroenterol. 1995;90(8):1244-1245. PubMed
2. Tapper EB, Patwardhan VR, Curry M. Low yield and utilization of confirmatory testing in a cohort of patients with liver disease assessed for alpha-1 antitrypsin deficiency. Dig Dis Sci. 2015;60(6):1589-1594. PubMed
3. Tapper EB, Rahni DO, Arnaout R, Lai M. The overuse of serum ceruloplasmin measurement. Am J Med. 2013;126(10):926.e1-e5. PubMed
4. Tapper EB, Sengupta N, Lai M, Horowitz G. Understanding and reducing ceruloplasmin overuse with a decision support intervention for liver disease evaluation. Am J Med. 2016;129(1):115.e17-e22. PubMed
5. Tapper EB, Sengupta N, Lai M, Horowitz G. A decision support tool to reduce overtesting for ceruloplasmin and improve adherence with clinical guidelines. JAMA Intern Med. 2015;175(9):1561-1562. PubMed
6. Lichtenstein MJ, Pincus T. How useful are combinations of blood tests in “rheumatic panels” in diagnosis of rheumatic diseases? J Gen Intern Med. 1988;3(5):435-442. PubMed
7. Tapper EB, Sengupta N, Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med. 2015;128(12):1314-1321. PubMed
8. Whitehead MW, Hawkes ND, Hainsworth I, Kingham JG. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999;45(1):129-133. PubMed
9. Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92(4):761-794. PubMed
10. Lee WM, Larson AM, Stravitz RT. AASLD Position Paper: The Management of Acute Liver Failure: Update 2011. American Association for the Study of Liver Diseases website. https://www.aasld.org/sites/default/files/guideline_documents/alfenhanced.pdf. Published 2011. Accessed January 26, 2017.
11. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367-1384. PubMed
12. Aesif SW, Parenti DM, Lesky L, Keiser JF. A cost-effective interdisciplinary approach to microbiologic send-out test use. Arch Pathol Lab Med. 2015;139(2):194-198. PubMed
13. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
14. Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6(3):635-647. PubMed
15. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(1):328-343. PubMed
16. Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181-1188. PubMed
© 2017 Society of Hospital Medicine