User login
Worsening agitation and hallucinations: Could it be PTSD?
CASE Confusion, hallucinations
Mr. G, age 57, is brought to the emergency department (ED) from a hospice care facility for worsening agitation and psychosis over 2 days. His wife, who accompanies him, describes a 2-month onset of “confusion” with occasional visual hallucinations. She says that at baseline Mr. G was alert and oriented and able to engage appropriately in conversations. The hospice facility administered emergency medications, including unknown dosages of haloperidol and chlorpromazine, the morning before transfer to the ED.
Mr. G has a history of posttraumatic stress disorder (PTSD), anxiety, and depression that has been managed for 6 years with several trials of antidepressant monotherapy, including fluoxetine, citalopram, mirtazapine, bupropion, and augmentation using aripiprazole, risperidone, topiramate, and zolpidem. At the time of this hospital presentation, his symptoms are controlled on clonazepam, 2 mg/d, and trazodone, 50 mg/d. For his pain attributed to non-small cell lung cancer (NSCLC), he receives methadone, 25 mg, 6 times a day, and hydromorphone, 8 mg, every 4 hours as needed, for breakthrough pain. Mr. G underwent a right upper lobectomy 5 years ago and neurosurgery with a right suboccipital craniectomy for right-sided cerebellar metastatic tumor measuring 2 × 1 × 0.6 cm, along with chemotherapy and radiation for metastasis in the brain 1 year ago. His last chemotherapy session was 3 months ago.
In the ED, Mr. G is sedated and oriented only to person and his wife. He is observed mumbling incoherently. Abnormal vital signs and laboratory findings are elevated pulse, 97 beats per minute; mild anemia, 13.5 g/dL hemoglobin and 40.8% hematocrit; an elevated glucose of 136 mg/dL; and small amounts of blood, trace ketones, and hyaline casts in urinalysis. Vital signs, laboratory resu
In addition to psychotropic and pain medication, Mr. G is taking cyclobenzaprine, 5 mg, every 6 hours as needed, for muscle spasms; docusate, 200 mg/d; enoxaparin, 100 mg/1mL, every 12 hours; folic acid, 1 mg/d; gabapentin, 600 mg, 3 times daily; lidocaine ointment, twice daily as needed, for pain; omeprazole, 80 mg/d; ondansetron, 4 mg, every 8 hours as needed, for nausea; and tamsulosin, 0.4 mg/d.
What is your differential diagnosis for Mr. G?
a) brain metastases
b) infection
c) PTSD
d) polypharmacy
e) benzodiazepine withdrawal
The authors’ observations
Altered mental status (AMS), or acute confusional state, describes an individual who fails to interact with environmental stimuli in an appropriate, anticipated manner. The disturbance usually is acute and transient.1 Often providers struggle to obtain relevant facts about a patient’s history of illness and must use laboratory and diagnostic data to determine the underlying cause of the patient’s disorientation.
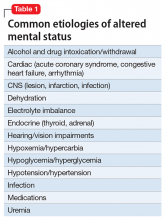
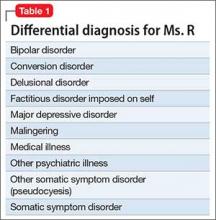
Mental status includes 2 components: arousal and awareness. Arousal refers to a person’s wakeful state and how an individual responds to his (her) surroundings. Impairment in arousal can result in variable states including lethargy, drowsiness, and even coma. Awareness, on the other hand, is an individual’s perception of his environment, including orientation to surroundings, executive functioning, and memory. Although arousal level is controlled by the reticular activating system of the brainstem, awareness of consciousness is mediated at the cortical level. Mr. G experienced increased arousal and AMS with a clear change in behavior from his baseline. With increasing frequency of hallucinations and agitated behaviors, several tests must be ordered to determine the etiology of his altered mentation (Table 1).
Which test would you order next?
a) urine drug screen (UDS)
b) chest CT with pulmonary embolism protocol
c) CT of the head
d) blood cultures
e) chest radiography
EVALUATION Awake, still confused
The ED physician orders a UDS, non-contrasted CT of the head, and chest radiography for preliminary workup investigating the cause of Mr. G’s AMS. UDS is negative for illicit substances. The non-contrasted CT of the head shows a stable, right cerebellar hemisphere lesion from a prior lung metastasis. Mr. G’s chest radiography reading describes an ill-defined opacity at the left lung base.
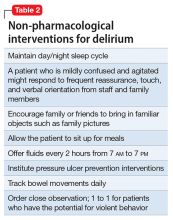
Mr. G is admitted to the medical service and is started on dexamethasone, 8 mg/d, for his NSCLC with brain metastasis. Clonazepam is continued to prevent benzodiazepine withdrawal. The psychiatry and palliative care teams are consulted to determine if Mr. G’s PTSD symptoms and/or opioids are contributing to his AMS and psychosis. After evaluation, the psychiatry team recommends decreasing clonazepam to 0.5 mg, twice daily, starting olanzapine, 5 mg, every 12 hours, for agitation and psychosis involving auditory and visual hallucinations as well as paranoid themes related to food contamination, and using non-pharmacologic interventions for delirium treatment (Table 2). In a prospective, randomized controlled trial of olanzapine vs haloperidol, clinical improvement in delirious states was seen in individuals who received either antipsychotic medication; however, haloperidol was associated with extrapyramidal side effects. Therefore, olanzapine is a safe alternative to haloperidol in delirious patients.2
The psychiatry consult service suspects delirium due to polypharmacy or Mr. G’s metastatic brain lesion. However, other collaborating treatment teams feel that Mr. G’s presentation was precipitated by an exacerbation of PTSD symptoms because of the observed psychotic themes, in addition to metabolic encephalopathy. Acute stress disorder can present with emotional numbing, depersonalization, reduced awareness of surroundings, or dissociative amnesia. However, Mr. G has not experienced PTSD symptoms involving mental status changes with fluctuating orientation in the past nor has he displayed persistent dissociation during outpatient psychiatric care. Therefore, it is unlikely that PTSD is the primary cause of his hospital admission.
The palliative care team recommends switching Mr. G’s pain medications to methadone, 20 mg, every 6 hours, to reduce possibility that opioids are contributing to his delirious state. Mr. G’s medical providers report that the chest radiography is suspicious for pneumonia and start him on levofloxacin, 500 mg/d.
The authors’ observations
DSM-5 criteria for delirium has 4 components:
- disturbance in attention and awareness
- change in cognition
- the disturbance develops over a short period of time
- there is evidence that the disturbance is a direct consequence of a medical condition, medication, or substance, or more than 1 cause.3
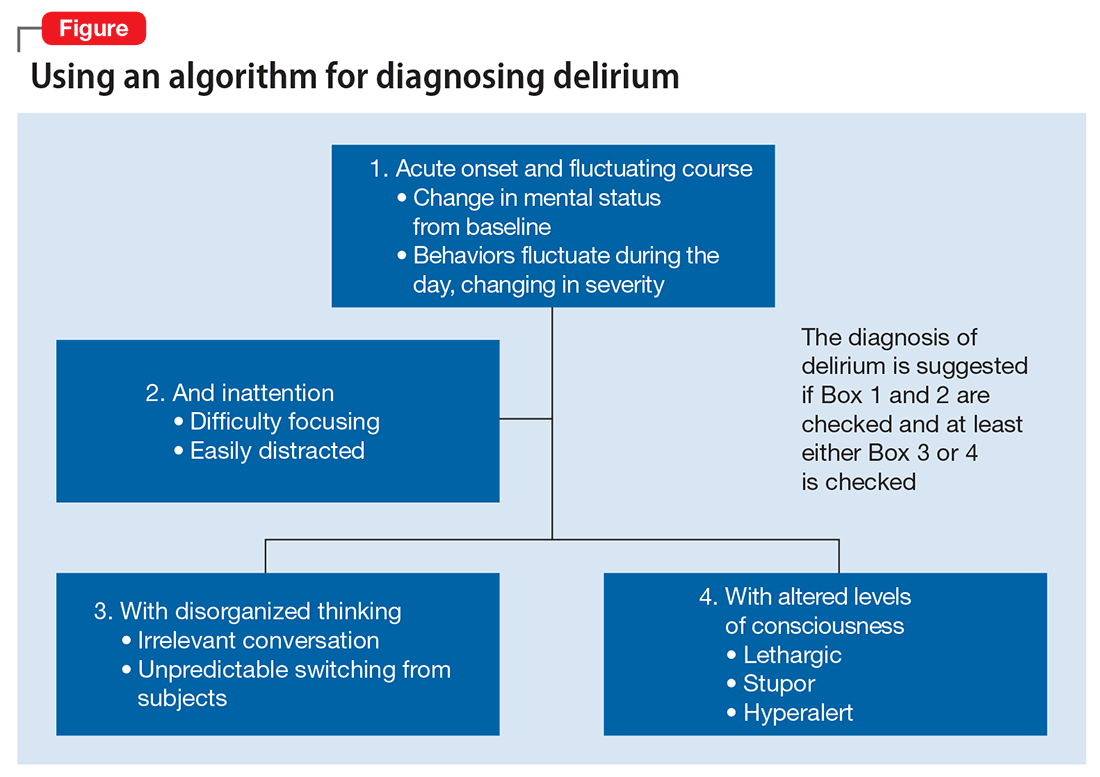
Mr. G presented with multi-factorial delirium, and as a result, all underlying contributions, including infection, polypharmacy, brain metastasis, and steroids needed to be considered. Treating delirium requires investigating the underlying cause and keeping the patient safe in the process (Figure). Mr. G was agitated at presentation; therefore, low-dosage olanzapine was initiated to address the imbalance between the cholinergic and dopaminergic systems in the CNS, which are thought to be the mechanism behind delirious presentations.
In Mr. G’s case, methadone was lowered, with continual monitoring and evaluation for his comfort. Infections, specifically urinary tract infections and pneumonia, can cause delirium states and must be treated with appropriate antibiotics. Metastatic tumors have been known to precipitate changes in mental status and can be ruled out via imaging. In Mr. G’s case, his metastatic lesion remained stable from prior radiographic studies.
TREATMENT Delirium resolves
Mr. G slowly responds to multi-modal treatment including decreased opioids and benzodiazepines and the use of low-dosage antipsychotics. He begins to return to baseline with antibiotic administration. By hospital day 5, Mr. G is alert and oriented. He notes resolution of his auditory and visual hallucinations and denies any persistent paranoia or delusions. The medical team observes Mr. G is having difficulty swallowing with meals, and orders a speech therapy evaluation. After assessment, the team suspects that aspiration pneumonia could have precipitated Mr. G’s initial decline and recommends a mechanic diet with thin liquids to reduce the risk of future aspiration.
Mr. G is discharged home in his wife’s care with home hospice to continue end-of-life care. His medication regimen includes olanzapine, 10 mg/d, to continue until his next outpatient appointment, trazodone, 50 mg/d, for depression and PTSD symptoms, and clonazepam is decreased to 0.5 mg, at bedtime, for anxiety.
The authors’ observations
Mr. G’s case highlights the importance of fully evaluating all common underlying causes of delirium. The etiology of delirium is more likely to be missed in medically complex patients or in patients with a history of psychiatric illness. Palliative care patients have several risk factors for delirium, such as benzodiazepine or opioid treatment, dementia, and organic diseases such as brain metastasis.6 A recent study assessed the frequency of delirium in cancer patients admitted to an inpatient palliative unit and found that 71% of individuals had a diagnosis of delirium at admission and 26% developed delirium afterward.7 Despite the increased likelihood of developing delirium, more than one-half of palliative patients have delirium that is missed by their primary providers.8 Similarly, patients with documented psychiatric illness were approximately 2.5 times more likely to have overlooked delirium compared with patients without psychiatric illness.9
Risk and prevention
Patients with risk factors for delirium—which includes sedative and narcotic usage, advanced cancer, older age, prolonged hospital stays, surgical procedures, and/or cognitive impairment—should receive interventions to prevent delirium. However, if symptoms of AMS are present, providers should perform a complete workup for underlying causes of delirium. Remembering that individuals with delirium have an impaired ability to voice symptoms, such as dyspnea, dysuria, and headache, clinicians should have a high index of suspicion for delirium in patients at heightened risk.10
Perhaps most important, teams treating patients at high risk for delirium should employ preventive measures to reduce the development of delirium. Although more studies are needed to clarify the role of drug therapies for preventing delirium, there is strong evidence for several non-pharmacotherapeutic interventions including:
- frequent orientation activities
- early mobilization
- maintaining healthy sleep–wake cycles
- minimizing the use of psychoactive drugs and frequently reviewing the medication regimen
- allowing use of eyeglasses and hearing aids
- treating volume depletion.10
These preventive measures are important when treating delirium, such as minimizing Mr. G’s use of benzodiazepine and opioids—medications known to contribute to iatrogenic delirium.
A delirium diagnosis portends grave adverse outcomes. Research has shown significant associations with morbidity and mortality, financial and emotional burden, and prolonged hospitalizations. Often, symptoms of delirium persist for months and patients do not recover completely. However, studies have found that when underlying causes are treated effectively, delirium is more likely to be reversible.11
The prompt diagnosis of delirium with good interdisciplinary communication can reduce the risk of these adverse outcomes.12 Consultation-liaison psychiatrists are well positioned to facilitate the diagnoses of delirium and play a role in educating other health care providers of the importance of prevention, early symptom recognition, full workup, and effective treatment of its underlying causes.
1. Posner JB, Saper CB, Schiff ND, et al. Plum and Posner’s diagnosis of stupor and coma. New York, NY: Oxford University Press; 2007.
2. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haldoperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30(3):444-449.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Lonergan E, Luxenberg J, Areosa Sastre A, et al. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(1):CD006379. doi: 10.1002/14651858.CD006379.pub2.
5. Vella-Brincat J, Macleod AD. Adverse effects of opioids on the central nervous system of palliative care patients. J Pain Palliat Care Pharmacother. 2007;21(1):15-25.
6. Grassi L, Caraceni A, Mitchell AJ, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(3):550.
7. de la Cruz M, Ransing V, Yennu S, et al. The frequency, characteristics, and outcomes among cancer patients with delirium admitted to an acute palliative care unit. Oncologist. 2015;20(12):1425-1431.
8. de la Cruz, M, Fan J, Yennu S, et al. The frequency of missed delirium in patients referred to palliative care in a comprehensive cancer center. Support Care Cancer. 2015;23(8):2427-2433.
9. Swigart SE, Kishi Y, Thurber S, et al. Misdiagnosed delirium in patient referrals to a university-based hospital psychiatry department. Psychosomatics. 2008;49(2):104-108.
10. Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676.
11. Dasgupta M, Hillier LM. Factors associated with prolonged delirium: a systematic review. Int Psychogeriatr. 2010;22(3):373-394.
12. Detweiler MB, Kenneth A, Bader G, et al. Can improved intra- and inter-team communication reduce missed delirium? Psychiatr Q. 2014;85(2):211-224.
CASE Confusion, hallucinations
Mr. G, age 57, is brought to the emergency department (ED) from a hospice care facility for worsening agitation and psychosis over 2 days. His wife, who accompanies him, describes a 2-month onset of “confusion” with occasional visual hallucinations. She says that at baseline Mr. G was alert and oriented and able to engage appropriately in conversations. The hospice facility administered emergency medications, including unknown dosages of haloperidol and chlorpromazine, the morning before transfer to the ED.
Mr. G has a history of posttraumatic stress disorder (PTSD), anxiety, and depression that has been managed for 6 years with several trials of antidepressant monotherapy, including fluoxetine, citalopram, mirtazapine, bupropion, and augmentation using aripiprazole, risperidone, topiramate, and zolpidem. At the time of this hospital presentation, his symptoms are controlled on clonazepam, 2 mg/d, and trazodone, 50 mg/d. For his pain attributed to non-small cell lung cancer (NSCLC), he receives methadone, 25 mg, 6 times a day, and hydromorphone, 8 mg, every 4 hours as needed, for breakthrough pain. Mr. G underwent a right upper lobectomy 5 years ago and neurosurgery with a right suboccipital craniectomy for right-sided cerebellar metastatic tumor measuring 2 × 1 × 0.6 cm, along with chemotherapy and radiation for metastasis in the brain 1 year ago. His last chemotherapy session was 3 months ago.
In the ED, Mr. G is sedated and oriented only to person and his wife. He is observed mumbling incoherently. Abnormal vital signs and laboratory findings are elevated pulse, 97 beats per minute; mild anemia, 13.5 g/dL hemoglobin and 40.8% hematocrit; an elevated glucose of 136 mg/dL; and small amounts of blood, trace ketones, and hyaline casts in urinalysis. Vital signs, laboratory resu
In addition to psychotropic and pain medication, Mr. G is taking cyclobenzaprine, 5 mg, every 6 hours as needed, for muscle spasms; docusate, 200 mg/d; enoxaparin, 100 mg/1mL, every 12 hours; folic acid, 1 mg/d; gabapentin, 600 mg, 3 times daily; lidocaine ointment, twice daily as needed, for pain; omeprazole, 80 mg/d; ondansetron, 4 mg, every 8 hours as needed, for nausea; and tamsulosin, 0.4 mg/d.
What is your differential diagnosis for Mr. G?
a) brain metastases
b) infection
c) PTSD
d) polypharmacy
e) benzodiazepine withdrawal
The authors’ observations
Altered mental status (AMS), or acute confusional state, describes an individual who fails to interact with environmental stimuli in an appropriate, anticipated manner. The disturbance usually is acute and transient.1 Often providers struggle to obtain relevant facts about a patient’s history of illness and must use laboratory and diagnostic data to determine the underlying cause of the patient’s disorientation.
Mental status includes 2 components: arousal and awareness. Arousal refers to a person’s wakeful state and how an individual responds to his (her) surroundings. Impairment in arousal can result in variable states including lethargy, drowsiness, and even coma. Awareness, on the other hand, is an individual’s perception of his environment, including orientation to surroundings, executive functioning, and memory. Although arousal level is controlled by the reticular activating system of the brainstem, awareness of consciousness is mediated at the cortical level. Mr. G experienced increased arousal and AMS with a clear change in behavior from his baseline. With increasing frequency of hallucinations and agitated behaviors, several tests must be ordered to determine the etiology of his altered mentation (Table 1).
Which test would you order next?
a) urine drug screen (UDS)
b) chest CT with pulmonary embolism protocol
c) CT of the head
d) blood cultures
e) chest radiography
EVALUATION Awake, still confused
The ED physician orders a UDS, non-contrasted CT of the head, and chest radiography for preliminary workup investigating the cause of Mr. G’s AMS. UDS is negative for illicit substances. The non-contrasted CT of the head shows a stable, right cerebellar hemisphere lesion from a prior lung metastasis. Mr. G’s chest radiography reading describes an ill-defined opacity at the left lung base.
Mr. G is admitted to the medical service and is started on dexamethasone, 8 mg/d, for his NSCLC with brain metastasis. Clonazepam is continued to prevent benzodiazepine withdrawal. The psychiatry and palliative care teams are consulted to determine if Mr. G’s PTSD symptoms and/or opioids are contributing to his AMS and psychosis. After evaluation, the psychiatry team recommends decreasing clonazepam to 0.5 mg, twice daily, starting olanzapine, 5 mg, every 12 hours, for agitation and psychosis involving auditory and visual hallucinations as well as paranoid themes related to food contamination, and using non-pharmacologic interventions for delirium treatment (Table 2). In a prospective, randomized controlled trial of olanzapine vs haloperidol, clinical improvement in delirious states was seen in individuals who received either antipsychotic medication; however, haloperidol was associated with extrapyramidal side effects. Therefore, olanzapine is a safe alternative to haloperidol in delirious patients.2
The psychiatry consult service suspects delirium due to polypharmacy or Mr. G’s metastatic brain lesion. However, other collaborating treatment teams feel that Mr. G’s presentation was precipitated by an exacerbation of PTSD symptoms because of the observed psychotic themes, in addition to metabolic encephalopathy. Acute stress disorder can present with emotional numbing, depersonalization, reduced awareness of surroundings, or dissociative amnesia. However, Mr. G has not experienced PTSD symptoms involving mental status changes with fluctuating orientation in the past nor has he displayed persistent dissociation during outpatient psychiatric care. Therefore, it is unlikely that PTSD is the primary cause of his hospital admission.
The palliative care team recommends switching Mr. G’s pain medications to methadone, 20 mg, every 6 hours, to reduce possibility that opioids are contributing to his delirious state. Mr. G’s medical providers report that the chest radiography is suspicious for pneumonia and start him on levofloxacin, 500 mg/d.
The authors’ observations
DSM-5 criteria for delirium has 4 components:
- disturbance in attention and awareness
- change in cognition
- the disturbance develops over a short period of time
- there is evidence that the disturbance is a direct consequence of a medical condition, medication, or substance, or more than 1 cause.3
Mr. G presented with multi-factorial delirium, and as a result, all underlying contributions, including infection, polypharmacy, brain metastasis, and steroids needed to be considered. Treating delirium requires investigating the underlying cause and keeping the patient safe in the process (Figure). Mr. G was agitated at presentation; therefore, low-dosage olanzapine was initiated to address the imbalance between the cholinergic and dopaminergic systems in the CNS, which are thought to be the mechanism behind delirious presentations.
In Mr. G’s case, methadone was lowered, with continual monitoring and evaluation for his comfort. Infections, specifically urinary tract infections and pneumonia, can cause delirium states and must be treated with appropriate antibiotics. Metastatic tumors have been known to precipitate changes in mental status and can be ruled out via imaging. In Mr. G’s case, his metastatic lesion remained stable from prior radiographic studies.
TREATMENT Delirium resolves
Mr. G slowly responds to multi-modal treatment including decreased opioids and benzodiazepines and the use of low-dosage antipsychotics. He begins to return to baseline with antibiotic administration. By hospital day 5, Mr. G is alert and oriented. He notes resolution of his auditory and visual hallucinations and denies any persistent paranoia or delusions. The medical team observes Mr. G is having difficulty swallowing with meals, and orders a speech therapy evaluation. After assessment, the team suspects that aspiration pneumonia could have precipitated Mr. G’s initial decline and recommends a mechanic diet with thin liquids to reduce the risk of future aspiration.
Mr. G is discharged home in his wife’s care with home hospice to continue end-of-life care. His medication regimen includes olanzapine, 10 mg/d, to continue until his next outpatient appointment, trazodone, 50 mg/d, for depression and PTSD symptoms, and clonazepam is decreased to 0.5 mg, at bedtime, for anxiety.
The authors’ observations
Mr. G’s case highlights the importance of fully evaluating all common underlying causes of delirium. The etiology of delirium is more likely to be missed in medically complex patients or in patients with a history of psychiatric illness. Palliative care patients have several risk factors for delirium, such as benzodiazepine or opioid treatment, dementia, and organic diseases such as brain metastasis.6 A recent study assessed the frequency of delirium in cancer patients admitted to an inpatient palliative unit and found that 71% of individuals had a diagnosis of delirium at admission and 26% developed delirium afterward.7 Despite the increased likelihood of developing delirium, more than one-half of palliative patients have delirium that is missed by their primary providers.8 Similarly, patients with documented psychiatric illness were approximately 2.5 times more likely to have overlooked delirium compared with patients without psychiatric illness.9
Risk and prevention
Patients with risk factors for delirium—which includes sedative and narcotic usage, advanced cancer, older age, prolonged hospital stays, surgical procedures, and/or cognitive impairment—should receive interventions to prevent delirium. However, if symptoms of AMS are present, providers should perform a complete workup for underlying causes of delirium. Remembering that individuals with delirium have an impaired ability to voice symptoms, such as dyspnea, dysuria, and headache, clinicians should have a high index of suspicion for delirium in patients at heightened risk.10
Perhaps most important, teams treating patients at high risk for delirium should employ preventive measures to reduce the development of delirium. Although more studies are needed to clarify the role of drug therapies for preventing delirium, there is strong evidence for several non-pharmacotherapeutic interventions including:
- frequent orientation activities
- early mobilization
- maintaining healthy sleep–wake cycles
- minimizing the use of psychoactive drugs and frequently reviewing the medication regimen
- allowing use of eyeglasses and hearing aids
- treating volume depletion.10
These preventive measures are important when treating delirium, such as minimizing Mr. G’s use of benzodiazepine and opioids—medications known to contribute to iatrogenic delirium.
A delirium diagnosis portends grave adverse outcomes. Research has shown significant associations with morbidity and mortality, financial and emotional burden, and prolonged hospitalizations. Often, symptoms of delirium persist for months and patients do not recover completely. However, studies have found that when underlying causes are treated effectively, delirium is more likely to be reversible.11
The prompt diagnosis of delirium with good interdisciplinary communication can reduce the risk of these adverse outcomes.12 Consultation-liaison psychiatrists are well positioned to facilitate the diagnoses of delirium and play a role in educating other health care providers of the importance of prevention, early symptom recognition, full workup, and effective treatment of its underlying causes.
CASE Confusion, hallucinations
Mr. G, age 57, is brought to the emergency department (ED) from a hospice care facility for worsening agitation and psychosis over 2 days. His wife, who accompanies him, describes a 2-month onset of “confusion” with occasional visual hallucinations. She says that at baseline Mr. G was alert and oriented and able to engage appropriately in conversations. The hospice facility administered emergency medications, including unknown dosages of haloperidol and chlorpromazine, the morning before transfer to the ED.
Mr. G has a history of posttraumatic stress disorder (PTSD), anxiety, and depression that has been managed for 6 years with several trials of antidepressant monotherapy, including fluoxetine, citalopram, mirtazapine, bupropion, and augmentation using aripiprazole, risperidone, topiramate, and zolpidem. At the time of this hospital presentation, his symptoms are controlled on clonazepam, 2 mg/d, and trazodone, 50 mg/d. For his pain attributed to non-small cell lung cancer (NSCLC), he receives methadone, 25 mg, 6 times a day, and hydromorphone, 8 mg, every 4 hours as needed, for breakthrough pain. Mr. G underwent a right upper lobectomy 5 years ago and neurosurgery with a right suboccipital craniectomy for right-sided cerebellar metastatic tumor measuring 2 × 1 × 0.6 cm, along with chemotherapy and radiation for metastasis in the brain 1 year ago. His last chemotherapy session was 3 months ago.
In the ED, Mr. G is sedated and oriented only to person and his wife. He is observed mumbling incoherently. Abnormal vital signs and laboratory findings are elevated pulse, 97 beats per minute; mild anemia, 13.5 g/dL hemoglobin and 40.8% hematocrit; an elevated glucose of 136 mg/dL; and small amounts of blood, trace ketones, and hyaline casts in urinalysis. Vital signs, laboratory resu
In addition to psychotropic and pain medication, Mr. G is taking cyclobenzaprine, 5 mg, every 6 hours as needed, for muscle spasms; docusate, 200 mg/d; enoxaparin, 100 mg/1mL, every 12 hours; folic acid, 1 mg/d; gabapentin, 600 mg, 3 times daily; lidocaine ointment, twice daily as needed, for pain; omeprazole, 80 mg/d; ondansetron, 4 mg, every 8 hours as needed, for nausea; and tamsulosin, 0.4 mg/d.
What is your differential diagnosis for Mr. G?
a) brain metastases
b) infection
c) PTSD
d) polypharmacy
e) benzodiazepine withdrawal
The authors’ observations
Altered mental status (AMS), or acute confusional state, describes an individual who fails to interact with environmental stimuli in an appropriate, anticipated manner. The disturbance usually is acute and transient.1 Often providers struggle to obtain relevant facts about a patient’s history of illness and must use laboratory and diagnostic data to determine the underlying cause of the patient’s disorientation.
Mental status includes 2 components: arousal and awareness. Arousal refers to a person’s wakeful state and how an individual responds to his (her) surroundings. Impairment in arousal can result in variable states including lethargy, drowsiness, and even coma. Awareness, on the other hand, is an individual’s perception of his environment, including orientation to surroundings, executive functioning, and memory. Although arousal level is controlled by the reticular activating system of the brainstem, awareness of consciousness is mediated at the cortical level. Mr. G experienced increased arousal and AMS with a clear change in behavior from his baseline. With increasing frequency of hallucinations and agitated behaviors, several tests must be ordered to determine the etiology of his altered mentation (Table 1).
Which test would you order next?
a) urine drug screen (UDS)
b) chest CT with pulmonary embolism protocol
c) CT of the head
d) blood cultures
e) chest radiography
EVALUATION Awake, still confused
The ED physician orders a UDS, non-contrasted CT of the head, and chest radiography for preliminary workup investigating the cause of Mr. G’s AMS. UDS is negative for illicit substances. The non-contrasted CT of the head shows a stable, right cerebellar hemisphere lesion from a prior lung metastasis. Mr. G’s chest radiography reading describes an ill-defined opacity at the left lung base.
Mr. G is admitted to the medical service and is started on dexamethasone, 8 mg/d, for his NSCLC with brain metastasis. Clonazepam is continued to prevent benzodiazepine withdrawal. The psychiatry and palliative care teams are consulted to determine if Mr. G’s PTSD symptoms and/or opioids are contributing to his AMS and psychosis. After evaluation, the psychiatry team recommends decreasing clonazepam to 0.5 mg, twice daily, starting olanzapine, 5 mg, every 12 hours, for agitation and psychosis involving auditory and visual hallucinations as well as paranoid themes related to food contamination, and using non-pharmacologic interventions for delirium treatment (Table 2). In a prospective, randomized controlled trial of olanzapine vs haloperidol, clinical improvement in delirious states was seen in individuals who received either antipsychotic medication; however, haloperidol was associated with extrapyramidal side effects. Therefore, olanzapine is a safe alternative to haloperidol in delirious patients.2
The psychiatry consult service suspects delirium due to polypharmacy or Mr. G’s metastatic brain lesion. However, other collaborating treatment teams feel that Mr. G’s presentation was precipitated by an exacerbation of PTSD symptoms because of the observed psychotic themes, in addition to metabolic encephalopathy. Acute stress disorder can present with emotional numbing, depersonalization, reduced awareness of surroundings, or dissociative amnesia. However, Mr. G has not experienced PTSD symptoms involving mental status changes with fluctuating orientation in the past nor has he displayed persistent dissociation during outpatient psychiatric care. Therefore, it is unlikely that PTSD is the primary cause of his hospital admission.
The palliative care team recommends switching Mr. G’s pain medications to methadone, 20 mg, every 6 hours, to reduce possibility that opioids are contributing to his delirious state. Mr. G’s medical providers report that the chest radiography is suspicious for pneumonia and start him on levofloxacin, 500 mg/d.
The authors’ observations
DSM-5 criteria for delirium has 4 components:
- disturbance in attention and awareness
- change in cognition
- the disturbance develops over a short period of time
- there is evidence that the disturbance is a direct consequence of a medical condition, medication, or substance, or more than 1 cause.3
Mr. G presented with multi-factorial delirium, and as a result, all underlying contributions, including infection, polypharmacy, brain metastasis, and steroids needed to be considered. Treating delirium requires investigating the underlying cause and keeping the patient safe in the process (Figure). Mr. G was agitated at presentation; therefore, low-dosage olanzapine was initiated to address the imbalance between the cholinergic and dopaminergic systems in the CNS, which are thought to be the mechanism behind delirious presentations.
In Mr. G’s case, methadone was lowered, with continual monitoring and evaluation for his comfort. Infections, specifically urinary tract infections and pneumonia, can cause delirium states and must be treated with appropriate antibiotics. Metastatic tumors have been known to precipitate changes in mental status and can be ruled out via imaging. In Mr. G’s case, his metastatic lesion remained stable from prior radiographic studies.
TREATMENT Delirium resolves
Mr. G slowly responds to multi-modal treatment including decreased opioids and benzodiazepines and the use of low-dosage antipsychotics. He begins to return to baseline with antibiotic administration. By hospital day 5, Mr. G is alert and oriented. He notes resolution of his auditory and visual hallucinations and denies any persistent paranoia or delusions. The medical team observes Mr. G is having difficulty swallowing with meals, and orders a speech therapy evaluation. After assessment, the team suspects that aspiration pneumonia could have precipitated Mr. G’s initial decline and recommends a mechanic diet with thin liquids to reduce the risk of future aspiration.
Mr. G is discharged home in his wife’s care with home hospice to continue end-of-life care. His medication regimen includes olanzapine, 10 mg/d, to continue until his next outpatient appointment, trazodone, 50 mg/d, for depression and PTSD symptoms, and clonazepam is decreased to 0.5 mg, at bedtime, for anxiety.
The authors’ observations
Mr. G’s case highlights the importance of fully evaluating all common underlying causes of delirium. The etiology of delirium is more likely to be missed in medically complex patients or in patients with a history of psychiatric illness. Palliative care patients have several risk factors for delirium, such as benzodiazepine or opioid treatment, dementia, and organic diseases such as brain metastasis.6 A recent study assessed the frequency of delirium in cancer patients admitted to an inpatient palliative unit and found that 71% of individuals had a diagnosis of delirium at admission and 26% developed delirium afterward.7 Despite the increased likelihood of developing delirium, more than one-half of palliative patients have delirium that is missed by their primary providers.8 Similarly, patients with documented psychiatric illness were approximately 2.5 times more likely to have overlooked delirium compared with patients without psychiatric illness.9
Risk and prevention
Patients with risk factors for delirium—which includes sedative and narcotic usage, advanced cancer, older age, prolonged hospital stays, surgical procedures, and/or cognitive impairment—should receive interventions to prevent delirium. However, if symptoms of AMS are present, providers should perform a complete workup for underlying causes of delirium. Remembering that individuals with delirium have an impaired ability to voice symptoms, such as dyspnea, dysuria, and headache, clinicians should have a high index of suspicion for delirium in patients at heightened risk.10
Perhaps most important, teams treating patients at high risk for delirium should employ preventive measures to reduce the development of delirium. Although more studies are needed to clarify the role of drug therapies for preventing delirium, there is strong evidence for several non-pharmacotherapeutic interventions including:
- frequent orientation activities
- early mobilization
- maintaining healthy sleep–wake cycles
- minimizing the use of psychoactive drugs and frequently reviewing the medication regimen
- allowing use of eyeglasses and hearing aids
- treating volume depletion.10
These preventive measures are important when treating delirium, such as minimizing Mr. G’s use of benzodiazepine and opioids—medications known to contribute to iatrogenic delirium.
A delirium diagnosis portends grave adverse outcomes. Research has shown significant associations with morbidity and mortality, financial and emotional burden, and prolonged hospitalizations. Often, symptoms of delirium persist for months and patients do not recover completely. However, studies have found that when underlying causes are treated effectively, delirium is more likely to be reversible.11
The prompt diagnosis of delirium with good interdisciplinary communication can reduce the risk of these adverse outcomes.12 Consultation-liaison psychiatrists are well positioned to facilitate the diagnoses of delirium and play a role in educating other health care providers of the importance of prevention, early symptom recognition, full workup, and effective treatment of its underlying causes.
1. Posner JB, Saper CB, Schiff ND, et al. Plum and Posner’s diagnosis of stupor and coma. New York, NY: Oxford University Press; 2007.
2. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haldoperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30(3):444-449.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Lonergan E, Luxenberg J, Areosa Sastre A, et al. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(1):CD006379. doi: 10.1002/14651858.CD006379.pub2.
5. Vella-Brincat J, Macleod AD. Adverse effects of opioids on the central nervous system of palliative care patients. J Pain Palliat Care Pharmacother. 2007;21(1):15-25.
6. Grassi L, Caraceni A, Mitchell AJ, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(3):550.
7. de la Cruz M, Ransing V, Yennu S, et al. The frequency, characteristics, and outcomes among cancer patients with delirium admitted to an acute palliative care unit. Oncologist. 2015;20(12):1425-1431.
8. de la Cruz, M, Fan J, Yennu S, et al. The frequency of missed delirium in patients referred to palliative care in a comprehensive cancer center. Support Care Cancer. 2015;23(8):2427-2433.
9. Swigart SE, Kishi Y, Thurber S, et al. Misdiagnosed delirium in patient referrals to a university-based hospital psychiatry department. Psychosomatics. 2008;49(2):104-108.
10. Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676.
11. Dasgupta M, Hillier LM. Factors associated with prolonged delirium: a systematic review. Int Psychogeriatr. 2010;22(3):373-394.
12. Detweiler MB, Kenneth A, Bader G, et al. Can improved intra- and inter-team communication reduce missed delirium? Psychiatr Q. 2014;85(2):211-224.
1. Posner JB, Saper CB, Schiff ND, et al. Plum and Posner’s diagnosis of stupor and coma. New York, NY: Oxford University Press; 2007.
2. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haldoperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30(3):444-449.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Lonergan E, Luxenberg J, Areosa Sastre A, et al. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(1):CD006379. doi: 10.1002/14651858.CD006379.pub2.
5. Vella-Brincat J, Macleod AD. Adverse effects of opioids on the central nervous system of palliative care patients. J Pain Palliat Care Pharmacother. 2007;21(1):15-25.
6. Grassi L, Caraceni A, Mitchell AJ, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(3):550.
7. de la Cruz M, Ransing V, Yennu S, et al. The frequency, characteristics, and outcomes among cancer patients with delirium admitted to an acute palliative care unit. Oncologist. 2015;20(12):1425-1431.
8. de la Cruz, M, Fan J, Yennu S, et al. The frequency of missed delirium in patients referred to palliative care in a comprehensive cancer center. Support Care Cancer. 2015;23(8):2427-2433.
9. Swigart SE, Kishi Y, Thurber S, et al. Misdiagnosed delirium in patient referrals to a university-based hospital psychiatry department. Psychosomatics. 2008;49(2):104-108.
10. Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676.
11. Dasgupta M, Hillier LM. Factors associated with prolonged delirium: a systematic review. Int Psychogeriatr. 2010;22(3):373-394.
12. Detweiler MB, Kenneth A, Bader G, et al. Can improved intra- and inter-team communication reduce missed delirium? Psychiatr Q. 2014;85(2):211-224.
Suicidal and asking for money for food
CASE Suicidal and hungry
Mr. L, age 59, attempts suicide by taking approximately 20 acetaminophen tablets of unknown dosage. He immediately comes to the emergency department where blood work reveals a 4-hour acetaminophen level of 94.8 μg/mL (therapeutic range, 10 to 30 μg/mL; toxic range, >150 μg/mL); administration of N-acetylcysteine is unnecessary. Mr. L is admitted to general medical services for monitoring and is transferred to our unit for psychiatric evaluation and management.
During our initial interview, Mr. L, who has a developmental disability, is grossly oriented and generally cooperative, reporting depressed mood with an irritable affect. He is preoccupied with having limited funds and repeatedly states he is worried that he can’t buy food, but says that the hospital could help by providing for him. Mr. L states that his depressed mood is directly related to his financial situation and, that if he had more money, he would not be suicidal. He cites worsening visual impairment that requires surgery as an additional stressor.
On several occasions, Mr. L states that the only way to help him is to give him $600 so that he can buy food and pay for medical treatment. Mr. L says he does not feel supported by his family, despite having a sister who lives nearby.
What would you include in the differential diagnosis for Mr. L?
a) major depressive disorder (MDD)
b) depression secondary to a medical condition
c) neurocognitive disorder
d) adjustment disorder with depressive features
e) factitious disorder
The authors’ observations
Our differential diagnosis included MDD, adjustment disorder, neurocognitive disorder, and factitious disorder. He did not meet criteria for MDD because he did not have excessive guilt, loss of interest, change in sleep or appetite, psychomotor dysregulation, or change in energy level. Although suicidal behavior could indicate MDD, the fact that he immediately walked to the hospital after taking an excessive amount of acetaminophen suggests that he did not want to die. Further, he attributed his suicidal thoughts to environmental stressors. Similarly, we ruled out adjustment disorder because he had no reported or observed changes in mood or anxiety. Although financial difficulties might have overwhelmed his limited coping abilities, he took too much acetaminophen to ensure that he was hospitalized. His motivation for seeking hospitalization ruled out factitious disorder. Mr. L has a developmental disability, but information obtained from collateral sources ruled out an acute change to cognitive functioning.
HISTORY Repeated admissions
Mr. L has a history of a psychiatric hospitalization 3 weeks prior to this admission. He presented to an emergency department stating that his blood glucose was low. Mr. L was noted to be confused and anxious and said he was convinced he was going to die. At that time, his thought content was hyper-religious and he claimed he could hear the devil. Mr. L was hospitalized and started on low-dosage risperidone. At discharge, he declined referral for outpatient mental health treatment because he denied having a mental illness. However, he was amenable to follow up at a wellness clinic.
Mr. L has worked at a local supermarket for 19 years and has lived independently throughout his adult life. After he returned to the community, he was repeatedly absent from work, which further exacerbated his financial strain. He attended a follow-up outpatient appointment but reported, “They didn’t help me,” although it was unclear what he meant.
Between admissions to our hospital, Mr. L had 2 visits to an emergency department, the first time saying he felt depressed and the second reporting he attempted suicide by taking 5 acetaminophen tablets. On both occasions he requested placement in a residential facility but was discharged home after an initial assessment. Emergency room records indicated that Mr. L stated, “If you cannot give me money for food, then there is no use and I would rather die.”
What is the most likely DSM-5 diagnosis for Mr. L?
a) schizophrenia
b) malingering
c) brief psychotic disorder
d) dependent personality disorder
The authors’ observations
Malingering in DSM-5 is defined as the “intentional production of false or grossly exaggerated physical or psychological symptoms, motivated by external incentives.”1 These external incentives include financial compensation, avoiding military duties, evading criminal charges, and avoiding work, and are collectively considered as secondary gain. Although not considered a diagnosis in the strictest sense, clinicians must differentiate malingering from other psychiatric disorders. In the literature, case reports describe patients who feigned an array of symptoms including those of posttraumatic stress disorder, paraphilias, cognitive dysfunction, depression, anxiety, and psychosis.2-5
In Mr. L’s case, malingering presented as suicidal behavior with an inadvertently high fatality risk. Notably, Mr. L came to an emergency room a few days before this admission after swallowing 5 acetaminophen tablets in a suicide attempt, which did not lead to a medical or psychiatric hospitalization. In an attempt to ensure admission, Mr. L then took a potentially lethal dose of 20 acetaminophen tablets. In our assessment and according to his statements, the primary motivation for the suicide attempt was to obtain reliable food and housing. Mr. L’s developmental disability might have contributed to a relative lack of understanding of the consequences of his actions. In addition, poor overall communication and coping skills led to an exaggerated response to psychosocial stressors.
Malingering and suicide attempts
Few studies have investigated malingering in regards to suicide and other psychiatric emergencies. In a study of 227 consecutive psychiatric emergencies assessed for evidence of malingering, 13% were thought to be feigned or exaggerated.6 Interestingly, the most commonly reported secondary gain was food and shelter, similar to Mr. L. This study did not report the types of psychiatric emergencies, therefore suicidal actions associated with malingering could not be evaluated.
In another study, 40 patients hospitalized for suicidal ideation (n = 29, 72%) or suicidal gestures (n = 11, 28%) in a large, urban tertiary care center were evaluated for malingering by anonymous report of feigned or exaggerated symptoms.7 Most of these patients were diagnosed with a mood disorder (28%) and/or an adjustment disorder (53%). Four (10%) admitted to malingering. Among the malingerers, reasons for feigning illness included:
- wanting to be hospitalized
- wanting to make someone angry or feel sorry
- gaining access to detoxification programs
- getting treatment for emotional problems.
Interestingly, an analysis of demographic factors associated with malingering reveals an association with suicide attempts but not persistent suicidal ideations. This could be because of selection bias; patients who reported a suicide attempt might be more likely to be hospitalized.
A follow-up study8 evaluated 50 additional consecutive psychiatric inpatients admitted to the same tertiary care hospital for suicide risk. Unlike the previous study, a larger proportion of these patients had made a suicide attempt (n = 21, 42%) and a greater number had made a previous suicide attempt (n = 33, 66%). Primary mood disorders comprised most of the psychiatric diagnoses (n = 28, 56%). In this study, the exact nature of the suicide gestures was not documented, leaving open the question of lethality of the attempts. These studies do not suggest that those who malinger are not at risk for suicide, only that these patients tend to exaggerate the severity of their ideations or behaviors.
OUTCOME Reluctantly discharged
We contact Mr. L’s siblings, who offer to provide temporary housing and financial support and assist him with medical needs. This abated Mr. L’s suicidal ideation; however, he wishes to remain in the hospital with the goals of obtaining eyeglasses and dentures. We explain that psychiatric hospitalization is no longer indicated and he is discharged.
Which of the following is the most effective management strategy for malingering?
a) direct confrontation of the malingering patient
b) immediate discharge once malingering is identified
c) evaluation for possible comorbid psychiatric conditions
d) neuropsychiatric consultation
The authors’ observations
The challenges of treating patients who malinger include clinician uncertainty in making the diagnosis and high variability in occurrence across settings (Table 1). Current estimates indicate that 4% to 8% of medical and psychiatric cases not involved in litigation or compensation have an element of feigned symptoms.3,9 The rate could be higher in specific circumstances such as medicolegal disputes and criminal cases.10
The societal impact of malingering is significant. Therefore, identifying these patients is an important clinical intervention that can have a wide impact.11 However, it is also important to acknowledge that genuine psychiatric illness could be comorbid with malingering. Although differentiating a patient’s true from feigned symptoms can be difficult, it is critical to carefully evaluate the patient in order to provide the best treatment.
It seems that physicians can detect malingering, but documentation often is not provided. In the Rissmiller et al study,7 all 4 cases of malingering were identified retrospectively by study psychiatrists; however, none of their medical records included documentation of malingering, a finding also reported in the Yates et al study.6 Also concerning, the clinicians suspected malingering in some patients who were not feigning symptoms, suggesting that a relatively high threshold is necessary for making the diagnosis.
How to help patients who malinger
Identifying malingering in patients with obvious secondary gain is important to prevent exposure to potential adverse effects of medication and unnecessary use of medical resources. In addition, obtaining collateral information, records from previous admissions or outpatient treatment, and psychological testing adds to the body of evidence suggesting malingering. We also recommend a comprehensive psychosocial evaluation to identify the presence of secondary gain.
Management of malingering (Table 2) includes building a strong therapeutic alliance, exploring reasons for feigning symptoms, open discussion of inciting external factors such as interpersonal conflict or difficulties at work, and/or confrontation.10 In addition, supportive psychotherapy might help strengthen coping mechanisms and problem solving strategies, thereby removing the need for secondary gain.12 Additionally, face-saving mechanisms that allow the patient to discard their feigned symptoms, or enable the person to alter his (her) history, could be to his benefit. Lastly, and importantly, clinicians should focus efforts on ruling out or effectively treating comorbid psychiatric conditions.
From a risk management standpoint, include all available data to support the malingering diagnosis in your progress notes and discharge summaries. A clinician seeking to discharge a patient suspected of malingering who is still endorsing suicidal or homicidal intent will benefit from administrative review, including legal counsel to mitigate risk, and be more confident discharging somebody assessed to be malingering.
We recognize that certain patients could trigger countertransference reactions that impel clinicians to take on a significant caretaking role. Patients skillful at deception could manifest a desire to rescue or save them. In these instances, clinicians should examine why and how these feelings have come about, particularly if there is evidence that the individual could be attempting to use the interaction to achieve secondary gain. Awareness of these feelings could help with the diagnostic formulation. Moreover, a clinician who has such strong feelings might be tempted to abet a patient in achieving the secondary gain, or protect him (her) from the natural consequences of individual’s deception (eg, not discharging a hospitalized patient). This is counter-therapeutic and reinforces maladaptive behaviors and coping processes.13
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington DC: American Psychiatric Association; 2013.
2. Fedoroff JP, Hanson A, McGuire M, et al. Simulated paraphilias: a preliminary study of patients who imitate or exaggerate paraphilic symptoms and behaviors. J Forensic Sci. 1992;37(3):902-911.
3. Mittenberg W, Patton C, Canyock EM, et al. Base rates of malingering and symptom exaggeration. J Clin Exp Neuropsychol. 2002;24(8):1094-1102.
4. Waite S, Geddes A. Malingered psychosis leading to involuntary psychiatric hospitalization. Australas Psychiatry. 2006;14(4):419-421.
5. Hall RC, Hall RC. Malingering of PTSD: forensic and diagnostic consideration, characteristics of malingerers and clinical presentations. Gen Hosp Psychiatry. 2006;28(6):525-535.
6. Yates BD, Nordquist CR, Shultz-Ross RA. Feigned psychiatric symptoms in the emergency room. Psychiatr Serv. 1996;47(9):998-1000.
7. Rissmiller DJ, Wayslow A, Madison H, et al. Prevalence of malingering in inpatient suicidal ideators and attempters. Crisis. 1998;19(2):62-66.
8. Rissmiller D, Steer RA, Friedman M, et al. Prevalence of malingering in suicidal psychiatric inpatients: a replication. Psychol Rep. 1999;84(3 pt 1):726-730.
9. Sullivan K, Lange RT, Dawes S. Methods of detecting malingering and estimated symptom exaggeration base rates in Australia. Journal of Forensic Neuropsychology. 2007;4(4):49-70.
10. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
11. Chafetz M, Underhill J. Estimated costs of malingered disability. Arch Clin Neuropsychol. 2013;28(7):633-639.
12. Peebles R, Sabel
13. Malone RD, Lange CL. A clinical approach to the malingering patient. J Am Acad Psychoanal Dyn Psychiatry. 2007;35(1):13-21.
CASE Suicidal and hungry
Mr. L, age 59, attempts suicide by taking approximately 20 acetaminophen tablets of unknown dosage. He immediately comes to the emergency department where blood work reveals a 4-hour acetaminophen level of 94.8 μg/mL (therapeutic range, 10 to 30 μg/mL; toxic range, >150 μg/mL); administration of N-acetylcysteine is unnecessary. Mr. L is admitted to general medical services for monitoring and is transferred to our unit for psychiatric evaluation and management.
During our initial interview, Mr. L, who has a developmental disability, is grossly oriented and generally cooperative, reporting depressed mood with an irritable affect. He is preoccupied with having limited funds and repeatedly states he is worried that he can’t buy food, but says that the hospital could help by providing for him. Mr. L states that his depressed mood is directly related to his financial situation and, that if he had more money, he would not be suicidal. He cites worsening visual impairment that requires surgery as an additional stressor.
On several occasions, Mr. L states that the only way to help him is to give him $600 so that he can buy food and pay for medical treatment. Mr. L says he does not feel supported by his family, despite having a sister who lives nearby.
What would you include in the differential diagnosis for Mr. L?
a) major depressive disorder (MDD)
b) depression secondary to a medical condition
c) neurocognitive disorder
d) adjustment disorder with depressive features
e) factitious disorder
The authors’ observations
Our differential diagnosis included MDD, adjustment disorder, neurocognitive disorder, and factitious disorder. He did not meet criteria for MDD because he did not have excessive guilt, loss of interest, change in sleep or appetite, psychomotor dysregulation, or change in energy level. Although suicidal behavior could indicate MDD, the fact that he immediately walked to the hospital after taking an excessive amount of acetaminophen suggests that he did not want to die. Further, he attributed his suicidal thoughts to environmental stressors. Similarly, we ruled out adjustment disorder because he had no reported or observed changes in mood or anxiety. Although financial difficulties might have overwhelmed his limited coping abilities, he took too much acetaminophen to ensure that he was hospitalized. His motivation for seeking hospitalization ruled out factitious disorder. Mr. L has a developmental disability, but information obtained from collateral sources ruled out an acute change to cognitive functioning.
HISTORY Repeated admissions
Mr. L has a history of a psychiatric hospitalization 3 weeks prior to this admission. He presented to an emergency department stating that his blood glucose was low. Mr. L was noted to be confused and anxious and said he was convinced he was going to die. At that time, his thought content was hyper-religious and he claimed he could hear the devil. Mr. L was hospitalized and started on low-dosage risperidone. At discharge, he declined referral for outpatient mental health treatment because he denied having a mental illness. However, he was amenable to follow up at a wellness clinic.
Mr. L has worked at a local supermarket for 19 years and has lived independently throughout his adult life. After he returned to the community, he was repeatedly absent from work, which further exacerbated his financial strain. He attended a follow-up outpatient appointment but reported, “They didn’t help me,” although it was unclear what he meant.
Between admissions to our hospital, Mr. L had 2 visits to an emergency department, the first time saying he felt depressed and the second reporting he attempted suicide by taking 5 acetaminophen tablets. On both occasions he requested placement in a residential facility but was discharged home after an initial assessment. Emergency room records indicated that Mr. L stated, “If you cannot give me money for food, then there is no use and I would rather die.”
What is the most likely DSM-5 diagnosis for Mr. L?
a) schizophrenia
b) malingering
c) brief psychotic disorder
d) dependent personality disorder
The authors’ observations
Malingering in DSM-5 is defined as the “intentional production of false or grossly exaggerated physical or psychological symptoms, motivated by external incentives.”1 These external incentives include financial compensation, avoiding military duties, evading criminal charges, and avoiding work, and are collectively considered as secondary gain. Although not considered a diagnosis in the strictest sense, clinicians must differentiate malingering from other psychiatric disorders. In the literature, case reports describe patients who feigned an array of symptoms including those of posttraumatic stress disorder, paraphilias, cognitive dysfunction, depression, anxiety, and psychosis.2-5
In Mr. L’s case, malingering presented as suicidal behavior with an inadvertently high fatality risk. Notably, Mr. L came to an emergency room a few days before this admission after swallowing 5 acetaminophen tablets in a suicide attempt, which did not lead to a medical or psychiatric hospitalization. In an attempt to ensure admission, Mr. L then took a potentially lethal dose of 20 acetaminophen tablets. In our assessment and according to his statements, the primary motivation for the suicide attempt was to obtain reliable food and housing. Mr. L’s developmental disability might have contributed to a relative lack of understanding of the consequences of his actions. In addition, poor overall communication and coping skills led to an exaggerated response to psychosocial stressors.
Malingering and suicide attempts
Few studies have investigated malingering in regards to suicide and other psychiatric emergencies. In a study of 227 consecutive psychiatric emergencies assessed for evidence of malingering, 13% were thought to be feigned or exaggerated.6 Interestingly, the most commonly reported secondary gain was food and shelter, similar to Mr. L. This study did not report the types of psychiatric emergencies, therefore suicidal actions associated with malingering could not be evaluated.
In another study, 40 patients hospitalized for suicidal ideation (n = 29, 72%) or suicidal gestures (n = 11, 28%) in a large, urban tertiary care center were evaluated for malingering by anonymous report of feigned or exaggerated symptoms.7 Most of these patients were diagnosed with a mood disorder (28%) and/or an adjustment disorder (53%). Four (10%) admitted to malingering. Among the malingerers, reasons for feigning illness included:
- wanting to be hospitalized
- wanting to make someone angry or feel sorry
- gaining access to detoxification programs
- getting treatment for emotional problems.
Interestingly, an analysis of demographic factors associated with malingering reveals an association with suicide attempts but not persistent suicidal ideations. This could be because of selection bias; patients who reported a suicide attempt might be more likely to be hospitalized.
A follow-up study8 evaluated 50 additional consecutive psychiatric inpatients admitted to the same tertiary care hospital for suicide risk. Unlike the previous study, a larger proportion of these patients had made a suicide attempt (n = 21, 42%) and a greater number had made a previous suicide attempt (n = 33, 66%). Primary mood disorders comprised most of the psychiatric diagnoses (n = 28, 56%). In this study, the exact nature of the suicide gestures was not documented, leaving open the question of lethality of the attempts. These studies do not suggest that those who malinger are not at risk for suicide, only that these patients tend to exaggerate the severity of their ideations or behaviors.
OUTCOME Reluctantly discharged
We contact Mr. L’s siblings, who offer to provide temporary housing and financial support and assist him with medical needs. This abated Mr. L’s suicidal ideation; however, he wishes to remain in the hospital with the goals of obtaining eyeglasses and dentures. We explain that psychiatric hospitalization is no longer indicated and he is discharged.
Which of the following is the most effective management strategy for malingering?
a) direct confrontation of the malingering patient
b) immediate discharge once malingering is identified
c) evaluation for possible comorbid psychiatric conditions
d) neuropsychiatric consultation
The authors’ observations
The challenges of treating patients who malinger include clinician uncertainty in making the diagnosis and high variability in occurrence across settings (Table 1). Current estimates indicate that 4% to 8% of medical and psychiatric cases not involved in litigation or compensation have an element of feigned symptoms.3,9 The rate could be higher in specific circumstances such as medicolegal disputes and criminal cases.10
The societal impact of malingering is significant. Therefore, identifying these patients is an important clinical intervention that can have a wide impact.11 However, it is also important to acknowledge that genuine psychiatric illness could be comorbid with malingering. Although differentiating a patient’s true from feigned symptoms can be difficult, it is critical to carefully evaluate the patient in order to provide the best treatment.
It seems that physicians can detect malingering, but documentation often is not provided. In the Rissmiller et al study,7 all 4 cases of malingering were identified retrospectively by study psychiatrists; however, none of their medical records included documentation of malingering, a finding also reported in the Yates et al study.6 Also concerning, the clinicians suspected malingering in some patients who were not feigning symptoms, suggesting that a relatively high threshold is necessary for making the diagnosis.
How to help patients who malinger
Identifying malingering in patients with obvious secondary gain is important to prevent exposure to potential adverse effects of medication and unnecessary use of medical resources. In addition, obtaining collateral information, records from previous admissions or outpatient treatment, and psychological testing adds to the body of evidence suggesting malingering. We also recommend a comprehensive psychosocial evaluation to identify the presence of secondary gain.
Management of malingering (Table 2) includes building a strong therapeutic alliance, exploring reasons for feigning symptoms, open discussion of inciting external factors such as interpersonal conflict or difficulties at work, and/or confrontation.10 In addition, supportive psychotherapy might help strengthen coping mechanisms and problem solving strategies, thereby removing the need for secondary gain.12 Additionally, face-saving mechanisms that allow the patient to discard their feigned symptoms, or enable the person to alter his (her) history, could be to his benefit. Lastly, and importantly, clinicians should focus efforts on ruling out or effectively treating comorbid psychiatric conditions.
From a risk management standpoint, include all available data to support the malingering diagnosis in your progress notes and discharge summaries. A clinician seeking to discharge a patient suspected of malingering who is still endorsing suicidal or homicidal intent will benefit from administrative review, including legal counsel to mitigate risk, and be more confident discharging somebody assessed to be malingering.
We recognize that certain patients could trigger countertransference reactions that impel clinicians to take on a significant caretaking role. Patients skillful at deception could manifest a desire to rescue or save them. In these instances, clinicians should examine why and how these feelings have come about, particularly if there is evidence that the individual could be attempting to use the interaction to achieve secondary gain. Awareness of these feelings could help with the diagnostic formulation. Moreover, a clinician who has such strong feelings might be tempted to abet a patient in achieving the secondary gain, or protect him (her) from the natural consequences of individual’s deception (eg, not discharging a hospitalized patient). This is counter-therapeutic and reinforces maladaptive behaviors and coping processes.13
CASE Suicidal and hungry
Mr. L, age 59, attempts suicide by taking approximately 20 acetaminophen tablets of unknown dosage. He immediately comes to the emergency department where blood work reveals a 4-hour acetaminophen level of 94.8 μg/mL (therapeutic range, 10 to 30 μg/mL; toxic range, >150 μg/mL); administration of N-acetylcysteine is unnecessary. Mr. L is admitted to general medical services for monitoring and is transferred to our unit for psychiatric evaluation and management.
During our initial interview, Mr. L, who has a developmental disability, is grossly oriented and generally cooperative, reporting depressed mood with an irritable affect. He is preoccupied with having limited funds and repeatedly states he is worried that he can’t buy food, but says that the hospital could help by providing for him. Mr. L states that his depressed mood is directly related to his financial situation and, that if he had more money, he would not be suicidal. He cites worsening visual impairment that requires surgery as an additional stressor.
On several occasions, Mr. L states that the only way to help him is to give him $600 so that he can buy food and pay for medical treatment. Mr. L says he does not feel supported by his family, despite having a sister who lives nearby.
What would you include in the differential diagnosis for Mr. L?
a) major depressive disorder (MDD)
b) depression secondary to a medical condition
c) neurocognitive disorder
d) adjustment disorder with depressive features
e) factitious disorder
The authors’ observations
Our differential diagnosis included MDD, adjustment disorder, neurocognitive disorder, and factitious disorder. He did not meet criteria for MDD because he did not have excessive guilt, loss of interest, change in sleep or appetite, psychomotor dysregulation, or change in energy level. Although suicidal behavior could indicate MDD, the fact that he immediately walked to the hospital after taking an excessive amount of acetaminophen suggests that he did not want to die. Further, he attributed his suicidal thoughts to environmental stressors. Similarly, we ruled out adjustment disorder because he had no reported or observed changes in mood or anxiety. Although financial difficulties might have overwhelmed his limited coping abilities, he took too much acetaminophen to ensure that he was hospitalized. His motivation for seeking hospitalization ruled out factitious disorder. Mr. L has a developmental disability, but information obtained from collateral sources ruled out an acute change to cognitive functioning.
HISTORY Repeated admissions
Mr. L has a history of a psychiatric hospitalization 3 weeks prior to this admission. He presented to an emergency department stating that his blood glucose was low. Mr. L was noted to be confused and anxious and said he was convinced he was going to die. At that time, his thought content was hyper-religious and he claimed he could hear the devil. Mr. L was hospitalized and started on low-dosage risperidone. At discharge, he declined referral for outpatient mental health treatment because he denied having a mental illness. However, he was amenable to follow up at a wellness clinic.
Mr. L has worked at a local supermarket for 19 years and has lived independently throughout his adult life. After he returned to the community, he was repeatedly absent from work, which further exacerbated his financial strain. He attended a follow-up outpatient appointment but reported, “They didn’t help me,” although it was unclear what he meant.
Between admissions to our hospital, Mr. L had 2 visits to an emergency department, the first time saying he felt depressed and the second reporting he attempted suicide by taking 5 acetaminophen tablets. On both occasions he requested placement in a residential facility but was discharged home after an initial assessment. Emergency room records indicated that Mr. L stated, “If you cannot give me money for food, then there is no use and I would rather die.”
What is the most likely DSM-5 diagnosis for Mr. L?
a) schizophrenia
b) malingering
c) brief psychotic disorder
d) dependent personality disorder
The authors’ observations
Malingering in DSM-5 is defined as the “intentional production of false or grossly exaggerated physical or psychological symptoms, motivated by external incentives.”1 These external incentives include financial compensation, avoiding military duties, evading criminal charges, and avoiding work, and are collectively considered as secondary gain. Although not considered a diagnosis in the strictest sense, clinicians must differentiate malingering from other psychiatric disorders. In the literature, case reports describe patients who feigned an array of symptoms including those of posttraumatic stress disorder, paraphilias, cognitive dysfunction, depression, anxiety, and psychosis.2-5
In Mr. L’s case, malingering presented as suicidal behavior with an inadvertently high fatality risk. Notably, Mr. L came to an emergency room a few days before this admission after swallowing 5 acetaminophen tablets in a suicide attempt, which did not lead to a medical or psychiatric hospitalization. In an attempt to ensure admission, Mr. L then took a potentially lethal dose of 20 acetaminophen tablets. In our assessment and according to his statements, the primary motivation for the suicide attempt was to obtain reliable food and housing. Mr. L’s developmental disability might have contributed to a relative lack of understanding of the consequences of his actions. In addition, poor overall communication and coping skills led to an exaggerated response to psychosocial stressors.
Malingering and suicide attempts
Few studies have investigated malingering in regards to suicide and other psychiatric emergencies. In a study of 227 consecutive psychiatric emergencies assessed for evidence of malingering, 13% were thought to be feigned or exaggerated.6 Interestingly, the most commonly reported secondary gain was food and shelter, similar to Mr. L. This study did not report the types of psychiatric emergencies, therefore suicidal actions associated with malingering could not be evaluated.
In another study, 40 patients hospitalized for suicidal ideation (n = 29, 72%) or suicidal gestures (n = 11, 28%) in a large, urban tertiary care center were evaluated for malingering by anonymous report of feigned or exaggerated symptoms.7 Most of these patients were diagnosed with a mood disorder (28%) and/or an adjustment disorder (53%). Four (10%) admitted to malingering. Among the malingerers, reasons for feigning illness included:
- wanting to be hospitalized
- wanting to make someone angry or feel sorry
- gaining access to detoxification programs
- getting treatment for emotional problems.
Interestingly, an analysis of demographic factors associated with malingering reveals an association with suicide attempts but not persistent suicidal ideations. This could be because of selection bias; patients who reported a suicide attempt might be more likely to be hospitalized.
A follow-up study8 evaluated 50 additional consecutive psychiatric inpatients admitted to the same tertiary care hospital for suicide risk. Unlike the previous study, a larger proportion of these patients had made a suicide attempt (n = 21, 42%) and a greater number had made a previous suicide attempt (n = 33, 66%). Primary mood disorders comprised most of the psychiatric diagnoses (n = 28, 56%). In this study, the exact nature of the suicide gestures was not documented, leaving open the question of lethality of the attempts. These studies do not suggest that those who malinger are not at risk for suicide, only that these patients tend to exaggerate the severity of their ideations or behaviors.
OUTCOME Reluctantly discharged
We contact Mr. L’s siblings, who offer to provide temporary housing and financial support and assist him with medical needs. This abated Mr. L’s suicidal ideation; however, he wishes to remain in the hospital with the goals of obtaining eyeglasses and dentures. We explain that psychiatric hospitalization is no longer indicated and he is discharged.
Which of the following is the most effective management strategy for malingering?
a) direct confrontation of the malingering patient
b) immediate discharge once malingering is identified
c) evaluation for possible comorbid psychiatric conditions
d) neuropsychiatric consultation
The authors’ observations
The challenges of treating patients who malinger include clinician uncertainty in making the diagnosis and high variability in occurrence across settings (Table 1). Current estimates indicate that 4% to 8% of medical and psychiatric cases not involved in litigation or compensation have an element of feigned symptoms.3,9 The rate could be higher in specific circumstances such as medicolegal disputes and criminal cases.10
The societal impact of malingering is significant. Therefore, identifying these patients is an important clinical intervention that can have a wide impact.11 However, it is also important to acknowledge that genuine psychiatric illness could be comorbid with malingering. Although differentiating a patient’s true from feigned symptoms can be difficult, it is critical to carefully evaluate the patient in order to provide the best treatment.
It seems that physicians can detect malingering, but documentation often is not provided. In the Rissmiller et al study,7 all 4 cases of malingering were identified retrospectively by study psychiatrists; however, none of their medical records included documentation of malingering, a finding also reported in the Yates et al study.6 Also concerning, the clinicians suspected malingering in some patients who were not feigning symptoms, suggesting that a relatively high threshold is necessary for making the diagnosis.
How to help patients who malinger
Identifying malingering in patients with obvious secondary gain is important to prevent exposure to potential adverse effects of medication and unnecessary use of medical resources. In addition, obtaining collateral information, records from previous admissions or outpatient treatment, and psychological testing adds to the body of evidence suggesting malingering. We also recommend a comprehensive psychosocial evaluation to identify the presence of secondary gain.
Management of malingering (Table 2) includes building a strong therapeutic alliance, exploring reasons for feigning symptoms, open discussion of inciting external factors such as interpersonal conflict or difficulties at work, and/or confrontation.10 In addition, supportive psychotherapy might help strengthen coping mechanisms and problem solving strategies, thereby removing the need for secondary gain.12 Additionally, face-saving mechanisms that allow the patient to discard their feigned symptoms, or enable the person to alter his (her) history, could be to his benefit. Lastly, and importantly, clinicians should focus efforts on ruling out or effectively treating comorbid psychiatric conditions.
From a risk management standpoint, include all available data to support the malingering diagnosis in your progress notes and discharge summaries. A clinician seeking to discharge a patient suspected of malingering who is still endorsing suicidal or homicidal intent will benefit from administrative review, including legal counsel to mitigate risk, and be more confident discharging somebody assessed to be malingering.
We recognize that certain patients could trigger countertransference reactions that impel clinicians to take on a significant caretaking role. Patients skillful at deception could manifest a desire to rescue or save them. In these instances, clinicians should examine why and how these feelings have come about, particularly if there is evidence that the individual could be attempting to use the interaction to achieve secondary gain. Awareness of these feelings could help with the diagnostic formulation. Moreover, a clinician who has such strong feelings might be tempted to abet a patient in achieving the secondary gain, or protect him (her) from the natural consequences of individual’s deception (eg, not discharging a hospitalized patient). This is counter-therapeutic and reinforces maladaptive behaviors and coping processes.13
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington DC: American Psychiatric Association; 2013.
2. Fedoroff JP, Hanson A, McGuire M, et al. Simulated paraphilias: a preliminary study of patients who imitate or exaggerate paraphilic symptoms and behaviors. J Forensic Sci. 1992;37(3):902-911.
3. Mittenberg W, Patton C, Canyock EM, et al. Base rates of malingering and symptom exaggeration. J Clin Exp Neuropsychol. 2002;24(8):1094-1102.
4. Waite S, Geddes A. Malingered psychosis leading to involuntary psychiatric hospitalization. Australas Psychiatry. 2006;14(4):419-421.
5. Hall RC, Hall RC. Malingering of PTSD: forensic and diagnostic consideration, characteristics of malingerers and clinical presentations. Gen Hosp Psychiatry. 2006;28(6):525-535.
6. Yates BD, Nordquist CR, Shultz-Ross RA. Feigned psychiatric symptoms in the emergency room. Psychiatr Serv. 1996;47(9):998-1000.
7. Rissmiller DJ, Wayslow A, Madison H, et al. Prevalence of malingering in inpatient suicidal ideators and attempters. Crisis. 1998;19(2):62-66.
8. Rissmiller D, Steer RA, Friedman M, et al. Prevalence of malingering in suicidal psychiatric inpatients: a replication. Psychol Rep. 1999;84(3 pt 1):726-730.
9. Sullivan K, Lange RT, Dawes S. Methods of detecting malingering and estimated symptom exaggeration base rates in Australia. Journal of Forensic Neuropsychology. 2007;4(4):49-70.
10. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
11. Chafetz M, Underhill J. Estimated costs of malingered disability. Arch Clin Neuropsychol. 2013;28(7):633-639.
12. Peebles R, Sabel
13. Malone RD, Lange CL. A clinical approach to the malingering patient. J Am Acad Psychoanal Dyn Psychiatry. 2007;35(1):13-21.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington DC: American Psychiatric Association; 2013.
2. Fedoroff JP, Hanson A, McGuire M, et al. Simulated paraphilias: a preliminary study of patients who imitate or exaggerate paraphilic symptoms and behaviors. J Forensic Sci. 1992;37(3):902-911.
3. Mittenberg W, Patton C, Canyock EM, et al. Base rates of malingering and symptom exaggeration. J Clin Exp Neuropsychol. 2002;24(8):1094-1102.
4. Waite S, Geddes A. Malingered psychosis leading to involuntary psychiatric hospitalization. Australas Psychiatry. 2006;14(4):419-421.
5. Hall RC, Hall RC. Malingering of PTSD: forensic and diagnostic consideration, characteristics of malingerers and clinical presentations. Gen Hosp Psychiatry. 2006;28(6):525-535.
6. Yates BD, Nordquist CR, Shultz-Ross RA. Feigned psychiatric symptoms in the emergency room. Psychiatr Serv. 1996;47(9):998-1000.
7. Rissmiller DJ, Wayslow A, Madison H, et al. Prevalence of malingering in inpatient suicidal ideators and attempters. Crisis. 1998;19(2):62-66.
8. Rissmiller D, Steer RA, Friedman M, et al. Prevalence of malingering in suicidal psychiatric inpatients: a replication. Psychol Rep. 1999;84(3 pt 1):726-730.
9. Sullivan K, Lange RT, Dawes S. Methods of detecting malingering and estimated symptom exaggeration base rates in Australia. Journal of Forensic Neuropsychology. 2007;4(4):49-70.
10. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
11. Chafetz M, Underhill J. Estimated costs of malingered disability. Arch Clin Neuropsychol. 2013;28(7):633-639.
12. Peebles R, Sabel
13. Malone RD, Lange CL. A clinical approach to the malingering patient. J Am Acad Psychoanal Dyn Psychiatry. 2007;35(1):13-21.
A girl repeatedly jabs her finger up her nose: Compulsion or self-injury?
CASE Anxious and self-injurious
A, age 6, is forcibly inserting her finger into her nose repeatedly until she bleeds profusely, as many as 20 times per day. She is not nose-picking but is jabbing her finger into her nose as far as possible in a repetitive ramming motion. Less frequently, she inserts her finger into her vagina, resulting in chronic urinary tract infections (UTIs). She has bedtime checking rituals; worries that her parents will die; has a fear of vomiting to the point where she stopped eating normally and lost 5 lb in 6 months; intense fear of storms; refusal to use public bathrooms; and involuntary throat clearing, facial grimacing, and lip twitches.
A’s symptoms began at age 3. There is no history of physical or sexual abuse. She does well in school, but these behaviors have had a significant impact on her social functioning. She is not taking any medications and has been in weekly cognitive-behavioral therapy (CBT) for the last year. A has had several UTIs but otherwise is physically healthy.
Which diagnosis best describes A’s condition?
a) non-suicidal self-injury (NSSI)
b) generalized anxiety disorder (GAD)
c) obsessive-compulsive disorder (OCD)
d) Tourette’s disorder (TD)
The authors’ observations
A is causing herself to bleed and says she wants to stop this behavior. Onset of NSSI typically is age 12 to 14 and could be accompanied by traits of cluster B personality disorders.1 In A’s case, her age and absence of any stated desire to relieve stress or intense negative affective states rules out NSSI.
Because A has multiple and frequent fears, worries, and anxieties that have been present for years and have caused significant functional impairment, a diagnosis of GAD is warranted. Because she has had both motor and vocal tics for more than 1 year, she also meets diagnostic criteria for TD (Table 1).
In young children, OCD manifests primarily with compulsive behavior, such as excessive hand washing, counting, and ordering, that interferes with functioning. Although A has bedtime checking rituals, she has no significant functional impairment from these rituals alone. A’s finger-insertion behavior could be interpreted as a complex motor tic or as a compulsion, in which case impairment was significant enough to justify a diagnosis of OCD.
Many individuals with OCD report the need to engage in compulsive behavior to decrease anxiety or until they experience a “just right” feeling.2 However, neither A nor her mother reported the need for the “just right” feeling. The child recognized the urge to put her finger in her nose and did experience relief of anxiety after drawing blood. Although A said that she was unable to control her hands, she was observed frequently touching the side of her nose in an attempt to avoid inserting her finger in her nose.
Compulsive behavior that results in self-injury typically is not seen in OCD except in children with severe neurologic complications, low intellectual functioning, psychosis, or autism.3
It often is difficult to determine if complex motor or vocal tics are compulsions (Table 2). Indeed, the same biologic mechanisms are thought to be implicated in TD and OCD.4 A significant percentage of children with OCD have tics, and patients often report that they are unable to distinguish between compulsions and complex tics.5 Therefore, we thought that a reasonable differential included both TD and OCD, but more careful assessment over time was required.
Treatment options
A has been receiving CBT for more than 1 year but her symptoms were worsening, which prompted her parents to seek evaluation in our clinic. Because of the level of interference with daily functioning and significant distress, our priority was developing a treatment plan that has the best chance of quickly reducing symptom severity and frequency. The results of the large-scale Pediatric OCD Treatment Study (POTS), which evaluated children age 7 to 17, and the Child/Adolescent Multimodal Anxiety Study, which evaluated children age <12, indicated that the combination of CBT with a selective serotonin reuptake inhibitor (SSRI) reduced OCD symptoms more than either modality alone.6,7 Considerations for using SSRIs in this age group include:
- the risk of behavioral activation
- poor tolerability
- lack of an evidence base for dosage optimization.
The American Academy of Child and Adolescent Psychiatry’s Preschool Psychopharmacology Working Group’s guidelines for treating anxiety in preschoolers state that pharmacotherapeutic intervention can be considered when symptoms are intolerable and adequate psychotherapy interventions have been tried.8 In A’s case, she had been receiving CBT for a year without improvement in symptoms; therefore, initiating medication was indicated, as well as an examination of therapeutic modalities being used.
Treatment Next steps
A is started on liquid fluoxetine, 20 mg/5 mL, 1 mL (4 mg) daily, because of her inability to swallow pills and her young age. According to her mother, a week later A is sleeping better and seems happier. The entire family seems less stressed. During the third week, A successfully goes on a camping trip with her family and is starting to eat better. Her finger-in-nose insertions still are occurring but, according to her mother, she is not putting her finger in her vagina. In session, she is not observed putting her finger in her nose or touching her nose, which she had done frequently during the initial evaluation. Fluoxetine seems to be well tolerated and the dosage is increased to 2 mL (8 mg) per day.
Although A has weekly scheduled appointments, she is not brought in again until a month later. At that time her mother reports an approximately 40% improvement in overall symptoms, including less frequent nose-insertion behaviors.
What type of psychotherapy would you employ for A?
a) CBT
b) behavioral therapy
c) habit reversal training (HRT)
d) pharmacotherapy alone
The authors’ observations
The treatment team planned to begin psychotherapy after A showed a decrease in anxiety and frequency of problem behaviors to a point where she could benefit. Evidence-based treatment for compulsions and tics is CBT and/or HRT.9 However, clinicians frequently encounter special challenges in helping young children (age 5 to 8) who have OCD. Factors such as family functioning, parental accommodation to the child’s symptoms, and the child’s ability to understand symptoms, exposure and response prevention, and willingness to tolerate discomfort should be considered if treatment is to be effective.
Research has shown that including parents when treating anxious children—especially young children—can facilitate gains and hasten positive outcomes.10,11 The POTS Jr study showed the relative efficacy of a family-based CBT model for young children with OCD that emphasizes consistent involvement of parents in all phases of treatment.12 In this case, A and her mother were seen together for psychotherapy, with an initial focus on learning more about the antecedents and consequences of the child’s behaviors.
OUTCOME Inconsistencies
Treatment was initiated during the summer. With the upcoming start of the school year, A begins to complain of daily headache, stomachache, and anxiety related to the start of school. Fluoxetine is increased to 3 mL/d (12 mg/d). After school starts, her mother stops going to work and begins attending school daily with A to relieve both her and the child’s anxiety.
The following week, the mother pages the psychiatrist, hysterical and crying because she thought the child was “pulling her hair out so much she looks like a cancer survivor.” Both parents blame the increase in fluoxetine for the heightened anxiety. At the next visit, the treatment team does not notice any evidence of unusual hair loss on the child. A has not attended school for several weeks, and her mother has not returned to work. Her parents report that the finger-to-nose behavior has increased, although it is not observed during the session, and fluoxetine is tapered as her parents requested.
At the next session, her mother notes a significant increase in finger-to-nose behavior and requests that the child be put back on fluoxetine, saying, “I would give anything to have the child I had on Prozac back.”
How would you proceed?
a) confront the mother’s inconsistencies
b) restart fluoxetine and continue psychotherapy
c) refer A to another clinic or therapist
d) refer A to inpatient care
The authors’ observations
The treatment team identified several barriers to successful treatment in our clinic. The level of functional interference caused by A’s symptoms indicated sessions more often than once a week, but the parents felt that the distance from our clinic to their home made this too difficult. The mother’s anxiety and obvious distress over her daughter’s symptoms precluded working closely with child. Parental anxiety is correlated with the child’s anxiety and can moderate treatment outcome.11 In response to the suffering of their anxious children, especially young ones, parents often will become anxious and accommodate to the child’s symptoms, which we strongly suspected was happening with A’s mother.
Parents’ concerns about A’s symptoms and response to treatment were addressed during a family meeting. Recognizing that the level of care needed by this family was higher than could be provided in our clinic, we recommended referral to a specialty clinic. A was brought to another clinic, and treatment at our facility was terminated.
1. Klonsky ED. The functions of deliberate self-injury: a review of the evidence. Clin Psychol Rev. 2007;27(2):226-239.
2. Miguel EC, do Rosário-Campos MC, Prado HS, et al. Sensory phenomena in obsessive-compulsive disorder and Tourette’s disorder. J Clin Psychiatry. 2000;61(2):150-156.
3. Nock MK, Favazza A. Non-suicidal self-injury: definition and classification. In: Nock MK, ed. Understanding nonsuicidal self-injury: origins, assessment, and treatment. Washington, DC: American Psychological Association; 2009:9-18.
4. Goodman WK, Storch EA, Geffken GR, et al. Obsessive-compulsive disorder in Tourette syndrome. J Child Neurol. 2006;21(8):704-714.
5. Garcia AM, Freeman JB, Himle MB, et al. Phenomenology of early childhood onset obsessive-compulsive disorder. J Psychopathol Behav Assess. 2009;31(2):104-111.
6. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969-1976.
7. Piacentini JC, Bennett S, Compton SN, et al. 24- and 36-week outcomes for the Child/Adolescent Anxiety Multimodal Study (CAMS). J Am Acad Child Adolesc Psychiatry. 2014;53(3):297-310.
8. Gleason MM, Egger HL, Emslie GJ, et al. Psychopharmacological treatment for very young children: contexts and guidelines. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1532-1572.
9. Abramowitz JS, Whiteside SP, Deacon BJ. The effectiveness of treatment for pediatric obsessive-compulsive disorder: a meta-analysis. Behavior Therapy. 2005;36(1):55-63.
10. Barmish AJ, Kendall PC. Should parents be co-clients in cognitive-behavioral therapy for anxious youth. J Clin Child Adolesc Psychol. 2005;34(3):569-581.
11. Drake KL, Ginsburg GS. Family factors in the development, treatment, and prevention of childhood anxiety disorders. Clin Child Fam Psychol Rev. 2012;15(2):144-162.
12. Freeman J, Sapyta J, Garcia A, et al. Family-based treatment of early childhood obsessive-compulsive disorder: the Pediatric Obsessive-Compulsive Disorder Treatment Study for Young Children (POTS Jr)—a randomized clinical trial. JAMA Psychiatry. 2014;71(6):689-698.
CASE Anxious and self-injurious
A, age 6, is forcibly inserting her finger into her nose repeatedly until she bleeds profusely, as many as 20 times per day. She is not nose-picking but is jabbing her finger into her nose as far as possible in a repetitive ramming motion. Less frequently, she inserts her finger into her vagina, resulting in chronic urinary tract infections (UTIs). She has bedtime checking rituals; worries that her parents will die; has a fear of vomiting to the point where she stopped eating normally and lost 5 lb in 6 months; intense fear of storms; refusal to use public bathrooms; and involuntary throat clearing, facial grimacing, and lip twitches.
A’s symptoms began at age 3. There is no history of physical or sexual abuse. She does well in school, but these behaviors have had a significant impact on her social functioning. She is not taking any medications and has been in weekly cognitive-behavioral therapy (CBT) for the last year. A has had several UTIs but otherwise is physically healthy.
Which diagnosis best describes A’s condition?
a) non-suicidal self-injury (NSSI)
b) generalized anxiety disorder (GAD)
c) obsessive-compulsive disorder (OCD)
d) Tourette’s disorder (TD)
The authors’ observations
A is causing herself to bleed and says she wants to stop this behavior. Onset of NSSI typically is age 12 to 14 and could be accompanied by traits of cluster B personality disorders.1 In A’s case, her age and absence of any stated desire to relieve stress or intense negative affective states rules out NSSI.
Because A has multiple and frequent fears, worries, and anxieties that have been present for years and have caused significant functional impairment, a diagnosis of GAD is warranted. Because she has had both motor and vocal tics for more than 1 year, she also meets diagnostic criteria for TD (Table 1).
In young children, OCD manifests primarily with compulsive behavior, such as excessive hand washing, counting, and ordering, that interferes with functioning. Although A has bedtime checking rituals, she has no significant functional impairment from these rituals alone. A’s finger-insertion behavior could be interpreted as a complex motor tic or as a compulsion, in which case impairment was significant enough to justify a diagnosis of OCD.
Many individuals with OCD report the need to engage in compulsive behavior to decrease anxiety or until they experience a “just right” feeling.2 However, neither A nor her mother reported the need for the “just right” feeling. The child recognized the urge to put her finger in her nose and did experience relief of anxiety after drawing blood. Although A said that she was unable to control her hands, she was observed frequently touching the side of her nose in an attempt to avoid inserting her finger in her nose.
Compulsive behavior that results in self-injury typically is not seen in OCD except in children with severe neurologic complications, low intellectual functioning, psychosis, or autism.3
It often is difficult to determine if complex motor or vocal tics are compulsions (Table 2). Indeed, the same biologic mechanisms are thought to be implicated in TD and OCD.4 A significant percentage of children with OCD have tics, and patients often report that they are unable to distinguish between compulsions and complex tics.5 Therefore, we thought that a reasonable differential included both TD and OCD, but more careful assessment over time was required.
Treatment options
A has been receiving CBT for more than 1 year but her symptoms were worsening, which prompted her parents to seek evaluation in our clinic. Because of the level of interference with daily functioning and significant distress, our priority was developing a treatment plan that has the best chance of quickly reducing symptom severity and frequency. The results of the large-scale Pediatric OCD Treatment Study (POTS), which evaluated children age 7 to 17, and the Child/Adolescent Multimodal Anxiety Study, which evaluated children age <12, indicated that the combination of CBT with a selective serotonin reuptake inhibitor (SSRI) reduced OCD symptoms more than either modality alone.6,7 Considerations for using SSRIs in this age group include:
- the risk of behavioral activation
- poor tolerability
- lack of an evidence base for dosage optimization.
The American Academy of Child and Adolescent Psychiatry’s Preschool Psychopharmacology Working Group’s guidelines for treating anxiety in preschoolers state that pharmacotherapeutic intervention can be considered when symptoms are intolerable and adequate psychotherapy interventions have been tried.8 In A’s case, she had been receiving CBT for a year without improvement in symptoms; therefore, initiating medication was indicated, as well as an examination of therapeutic modalities being used.
Treatment Next steps
A is started on liquid fluoxetine, 20 mg/5 mL, 1 mL (4 mg) daily, because of her inability to swallow pills and her young age. According to her mother, a week later A is sleeping better and seems happier. The entire family seems less stressed. During the third week, A successfully goes on a camping trip with her family and is starting to eat better. Her finger-in-nose insertions still are occurring but, according to her mother, she is not putting her finger in her vagina. In session, she is not observed putting her finger in her nose or touching her nose, which she had done frequently during the initial evaluation. Fluoxetine seems to be well tolerated and the dosage is increased to 2 mL (8 mg) per day.
Although A has weekly scheduled appointments, she is not brought in again until a month later. At that time her mother reports an approximately 40% improvement in overall symptoms, including less frequent nose-insertion behaviors.
What type of psychotherapy would you employ for A?
a) CBT
b) behavioral therapy
c) habit reversal training (HRT)
d) pharmacotherapy alone
The authors’ observations
The treatment team planned to begin psychotherapy after A showed a decrease in anxiety and frequency of problem behaviors to a point where she could benefit. Evidence-based treatment for compulsions and tics is CBT and/or HRT.9 However, clinicians frequently encounter special challenges in helping young children (age 5 to 8) who have OCD. Factors such as family functioning, parental accommodation to the child’s symptoms, and the child’s ability to understand symptoms, exposure and response prevention, and willingness to tolerate discomfort should be considered if treatment is to be effective.
Research has shown that including parents when treating anxious children—especially young children—can facilitate gains and hasten positive outcomes.10,11 The POTS Jr study showed the relative efficacy of a family-based CBT model for young children with OCD that emphasizes consistent involvement of parents in all phases of treatment.12 In this case, A and her mother were seen together for psychotherapy, with an initial focus on learning more about the antecedents and consequences of the child’s behaviors.
OUTCOME Inconsistencies
Treatment was initiated during the summer. With the upcoming start of the school year, A begins to complain of daily headache, stomachache, and anxiety related to the start of school. Fluoxetine is increased to 3 mL/d (12 mg/d). After school starts, her mother stops going to work and begins attending school daily with A to relieve both her and the child’s anxiety.
The following week, the mother pages the psychiatrist, hysterical and crying because she thought the child was “pulling her hair out so much she looks like a cancer survivor.” Both parents blame the increase in fluoxetine for the heightened anxiety. At the next visit, the treatment team does not notice any evidence of unusual hair loss on the child. A has not attended school for several weeks, and her mother has not returned to work. Her parents report that the finger-to-nose behavior has increased, although it is not observed during the session, and fluoxetine is tapered as her parents requested.
At the next session, her mother notes a significant increase in finger-to-nose behavior and requests that the child be put back on fluoxetine, saying, “I would give anything to have the child I had on Prozac back.”
How would you proceed?
a) confront the mother’s inconsistencies
b) restart fluoxetine and continue psychotherapy
c) refer A to another clinic or therapist
d) refer A to inpatient care
The authors’ observations
The treatment team identified several barriers to successful treatment in our clinic. The level of functional interference caused by A’s symptoms indicated sessions more often than once a week, but the parents felt that the distance from our clinic to their home made this too difficult. The mother’s anxiety and obvious distress over her daughter’s symptoms precluded working closely with child. Parental anxiety is correlated with the child’s anxiety and can moderate treatment outcome.11 In response to the suffering of their anxious children, especially young ones, parents often will become anxious and accommodate to the child’s symptoms, which we strongly suspected was happening with A’s mother.
Parents’ concerns about A’s symptoms and response to treatment were addressed during a family meeting. Recognizing that the level of care needed by this family was higher than could be provided in our clinic, we recommended referral to a specialty clinic. A was brought to another clinic, and treatment at our facility was terminated.
CASE Anxious and self-injurious
A, age 6, is forcibly inserting her finger into her nose repeatedly until she bleeds profusely, as many as 20 times per day. She is not nose-picking but is jabbing her finger into her nose as far as possible in a repetitive ramming motion. Less frequently, she inserts her finger into her vagina, resulting in chronic urinary tract infections (UTIs). She has bedtime checking rituals; worries that her parents will die; has a fear of vomiting to the point where she stopped eating normally and lost 5 lb in 6 months; intense fear of storms; refusal to use public bathrooms; and involuntary throat clearing, facial grimacing, and lip twitches.
A’s symptoms began at age 3. There is no history of physical or sexual abuse. She does well in school, but these behaviors have had a significant impact on her social functioning. She is not taking any medications and has been in weekly cognitive-behavioral therapy (CBT) for the last year. A has had several UTIs but otherwise is physically healthy.
Which diagnosis best describes A’s condition?
a) non-suicidal self-injury (NSSI)
b) generalized anxiety disorder (GAD)
c) obsessive-compulsive disorder (OCD)
d) Tourette’s disorder (TD)
The authors’ observations
A is causing herself to bleed and says she wants to stop this behavior. Onset of NSSI typically is age 12 to 14 and could be accompanied by traits of cluster B personality disorders.1 In A’s case, her age and absence of any stated desire to relieve stress or intense negative affective states rules out NSSI.
Because A has multiple and frequent fears, worries, and anxieties that have been present for years and have caused significant functional impairment, a diagnosis of GAD is warranted. Because she has had both motor and vocal tics for more than 1 year, she also meets diagnostic criteria for TD (Table 1).
In young children, OCD manifests primarily with compulsive behavior, such as excessive hand washing, counting, and ordering, that interferes with functioning. Although A has bedtime checking rituals, she has no significant functional impairment from these rituals alone. A’s finger-insertion behavior could be interpreted as a complex motor tic or as a compulsion, in which case impairment was significant enough to justify a diagnosis of OCD.
Many individuals with OCD report the need to engage in compulsive behavior to decrease anxiety or until they experience a “just right” feeling.2 However, neither A nor her mother reported the need for the “just right” feeling. The child recognized the urge to put her finger in her nose and did experience relief of anxiety after drawing blood. Although A said that she was unable to control her hands, she was observed frequently touching the side of her nose in an attempt to avoid inserting her finger in her nose.
Compulsive behavior that results in self-injury typically is not seen in OCD except in children with severe neurologic complications, low intellectual functioning, psychosis, or autism.3
It often is difficult to determine if complex motor or vocal tics are compulsions (Table 2). Indeed, the same biologic mechanisms are thought to be implicated in TD and OCD.4 A significant percentage of children with OCD have tics, and patients often report that they are unable to distinguish between compulsions and complex tics.5 Therefore, we thought that a reasonable differential included both TD and OCD, but more careful assessment over time was required.
Treatment options
A has been receiving CBT for more than 1 year but her symptoms were worsening, which prompted her parents to seek evaluation in our clinic. Because of the level of interference with daily functioning and significant distress, our priority was developing a treatment plan that has the best chance of quickly reducing symptom severity and frequency. The results of the large-scale Pediatric OCD Treatment Study (POTS), which evaluated children age 7 to 17, and the Child/Adolescent Multimodal Anxiety Study, which evaluated children age <12, indicated that the combination of CBT with a selective serotonin reuptake inhibitor (SSRI) reduced OCD symptoms more than either modality alone.6,7 Considerations for using SSRIs in this age group include:
- the risk of behavioral activation
- poor tolerability
- lack of an evidence base for dosage optimization.
The American Academy of Child and Adolescent Psychiatry’s Preschool Psychopharmacology Working Group’s guidelines for treating anxiety in preschoolers state that pharmacotherapeutic intervention can be considered when symptoms are intolerable and adequate psychotherapy interventions have been tried.8 In A’s case, she had been receiving CBT for a year without improvement in symptoms; therefore, initiating medication was indicated, as well as an examination of therapeutic modalities being used.
Treatment Next steps
A is started on liquid fluoxetine, 20 mg/5 mL, 1 mL (4 mg) daily, because of her inability to swallow pills and her young age. According to her mother, a week later A is sleeping better and seems happier. The entire family seems less stressed. During the third week, A successfully goes on a camping trip with her family and is starting to eat better. Her finger-in-nose insertions still are occurring but, according to her mother, she is not putting her finger in her vagina. In session, she is not observed putting her finger in her nose or touching her nose, which she had done frequently during the initial evaluation. Fluoxetine seems to be well tolerated and the dosage is increased to 2 mL (8 mg) per day.
Although A has weekly scheduled appointments, she is not brought in again until a month later. At that time her mother reports an approximately 40% improvement in overall symptoms, including less frequent nose-insertion behaviors.
What type of psychotherapy would you employ for A?
a) CBT
b) behavioral therapy
c) habit reversal training (HRT)
d) pharmacotherapy alone
The authors’ observations
The treatment team planned to begin psychotherapy after A showed a decrease in anxiety and frequency of problem behaviors to a point where she could benefit. Evidence-based treatment for compulsions and tics is CBT and/or HRT.9 However, clinicians frequently encounter special challenges in helping young children (age 5 to 8) who have OCD. Factors such as family functioning, parental accommodation to the child’s symptoms, and the child’s ability to understand symptoms, exposure and response prevention, and willingness to tolerate discomfort should be considered if treatment is to be effective.
Research has shown that including parents when treating anxious children—especially young children—can facilitate gains and hasten positive outcomes.10,11 The POTS Jr study showed the relative efficacy of a family-based CBT model for young children with OCD that emphasizes consistent involvement of parents in all phases of treatment.12 In this case, A and her mother were seen together for psychotherapy, with an initial focus on learning more about the antecedents and consequences of the child’s behaviors.
OUTCOME Inconsistencies
Treatment was initiated during the summer. With the upcoming start of the school year, A begins to complain of daily headache, stomachache, and anxiety related to the start of school. Fluoxetine is increased to 3 mL/d (12 mg/d). After school starts, her mother stops going to work and begins attending school daily with A to relieve both her and the child’s anxiety.
The following week, the mother pages the psychiatrist, hysterical and crying because she thought the child was “pulling her hair out so much she looks like a cancer survivor.” Both parents blame the increase in fluoxetine for the heightened anxiety. At the next visit, the treatment team does not notice any evidence of unusual hair loss on the child. A has not attended school for several weeks, and her mother has not returned to work. Her parents report that the finger-to-nose behavior has increased, although it is not observed during the session, and fluoxetine is tapered as her parents requested.
At the next session, her mother notes a significant increase in finger-to-nose behavior and requests that the child be put back on fluoxetine, saying, “I would give anything to have the child I had on Prozac back.”
How would you proceed?
a) confront the mother’s inconsistencies
b) restart fluoxetine and continue psychotherapy
c) refer A to another clinic or therapist
d) refer A to inpatient care
The authors’ observations
The treatment team identified several barriers to successful treatment in our clinic. The level of functional interference caused by A’s symptoms indicated sessions more often than once a week, but the parents felt that the distance from our clinic to their home made this too difficult. The mother’s anxiety and obvious distress over her daughter’s symptoms precluded working closely with child. Parental anxiety is correlated with the child’s anxiety and can moderate treatment outcome.11 In response to the suffering of their anxious children, especially young ones, parents often will become anxious and accommodate to the child’s symptoms, which we strongly suspected was happening with A’s mother.
Parents’ concerns about A’s symptoms and response to treatment were addressed during a family meeting. Recognizing that the level of care needed by this family was higher than could be provided in our clinic, we recommended referral to a specialty clinic. A was brought to another clinic, and treatment at our facility was terminated.
1. Klonsky ED. The functions of deliberate self-injury: a review of the evidence. Clin Psychol Rev. 2007;27(2):226-239.
2. Miguel EC, do Rosário-Campos MC, Prado HS, et al. Sensory phenomena in obsessive-compulsive disorder and Tourette’s disorder. J Clin Psychiatry. 2000;61(2):150-156.
3. Nock MK, Favazza A. Non-suicidal self-injury: definition and classification. In: Nock MK, ed. Understanding nonsuicidal self-injury: origins, assessment, and treatment. Washington, DC: American Psychological Association; 2009:9-18.
4. Goodman WK, Storch EA, Geffken GR, et al. Obsessive-compulsive disorder in Tourette syndrome. J Child Neurol. 2006;21(8):704-714.
5. Garcia AM, Freeman JB, Himle MB, et al. Phenomenology of early childhood onset obsessive-compulsive disorder. J Psychopathol Behav Assess. 2009;31(2):104-111.
6. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969-1976.
7. Piacentini JC, Bennett S, Compton SN, et al. 24- and 36-week outcomes for the Child/Adolescent Anxiety Multimodal Study (CAMS). J Am Acad Child Adolesc Psychiatry. 2014;53(3):297-310.
8. Gleason MM, Egger HL, Emslie GJ, et al. Psychopharmacological treatment for very young children: contexts and guidelines. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1532-1572.
9. Abramowitz JS, Whiteside SP, Deacon BJ. The effectiveness of treatment for pediatric obsessive-compulsive disorder: a meta-analysis. Behavior Therapy. 2005;36(1):55-63.
10. Barmish AJ, Kendall PC. Should parents be co-clients in cognitive-behavioral therapy for anxious youth. J Clin Child Adolesc Psychol. 2005;34(3):569-581.
11. Drake KL, Ginsburg GS. Family factors in the development, treatment, and prevention of childhood anxiety disorders. Clin Child Fam Psychol Rev. 2012;15(2):144-162.
12. Freeman J, Sapyta J, Garcia A, et al. Family-based treatment of early childhood obsessive-compulsive disorder: the Pediatric Obsessive-Compulsive Disorder Treatment Study for Young Children (POTS Jr)—a randomized clinical trial. JAMA Psychiatry. 2014;71(6):689-698.
1. Klonsky ED. The functions of deliberate self-injury: a review of the evidence. Clin Psychol Rev. 2007;27(2):226-239.
2. Miguel EC, do Rosário-Campos MC, Prado HS, et al. Sensory phenomena in obsessive-compulsive disorder and Tourette’s disorder. J Clin Psychiatry. 2000;61(2):150-156.
3. Nock MK, Favazza A. Non-suicidal self-injury: definition and classification. In: Nock MK, ed. Understanding nonsuicidal self-injury: origins, assessment, and treatment. Washington, DC: American Psychological Association; 2009:9-18.
4. Goodman WK, Storch EA, Geffken GR, et al. Obsessive-compulsive disorder in Tourette syndrome. J Child Neurol. 2006;21(8):704-714.
5. Garcia AM, Freeman JB, Himle MB, et al. Phenomenology of early childhood onset obsessive-compulsive disorder. J Psychopathol Behav Assess. 2009;31(2):104-111.
6. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969-1976.
7. Piacentini JC, Bennett S, Compton SN, et al. 24- and 36-week outcomes for the Child/Adolescent Anxiety Multimodal Study (CAMS). J Am Acad Child Adolesc Psychiatry. 2014;53(3):297-310.
8. Gleason MM, Egger HL, Emslie GJ, et al. Psychopharmacological treatment for very young children: contexts and guidelines. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1532-1572.
9. Abramowitz JS, Whiteside SP, Deacon BJ. The effectiveness of treatment for pediatric obsessive-compulsive disorder: a meta-analysis. Behavior Therapy. 2005;36(1):55-63.
10. Barmish AJ, Kendall PC. Should parents be co-clients in cognitive-behavioral therapy for anxious youth. J Clin Child Adolesc Psychol. 2005;34(3):569-581.
11. Drake KL, Ginsburg GS. Family factors in the development, treatment, and prevention of childhood anxiety disorders. Clin Child Fam Psychol Rev. 2012;15(2):144-162.
12. Freeman J, Sapyta J, Garcia A, et al. Family-based treatment of early childhood obsessive-compulsive disorder: the Pediatric Obsessive-Compulsive Disorder Treatment Study for Young Children (POTS Jr)—a randomized clinical trial. JAMA Psychiatry. 2014;71(6):689-698.
Stabilized schizoaffective disorder; later confusion and depression appears
CASE
Disoriented and confused
Mr. D, age 42, presents to our emergency department (ED) accompanied by his family with recent onset of disorientation, confusion, depressive mood with labile affect, sleep disturbances, purposeless movements, and grossly reduced kinetics/verbal output. He has a history of schizoaffective disorder, bipolar type, and recurrent admissions for psychotic mood instability.
A few months earlier, Mr. D was treated at our facility for acute exacerbation of his schizoaffective disorder. He was stabilized and discharged with aripiprazole, 30 mg/d, and mirtazapine, 15 mg/d—he had been taking both medications for some time—and newly started extended-release divalproex, 500 mg in the morning/1000 mg nightly (13.2 mg/kg). His trough valproic acid serum level was 70 µg/mL at discharge. He continued on this medication regimen until he returns to our ED with his family.
Mr. D has several medical problems, such as type 2 diabetes mellitus and hypertension, for which he has been receiving metformin, 1,000 mg/d, lisinopril, 10 mg/d, and simvastatin, 20 mg/d. He has no history of alcohol or substance abuse and does not smoke.
Serum and urine analyses are unremarkable and include finger-stick blood glucose, complete blood count, urinalysis, urine drug screen, comprehensive metabolic panel, magnesium, γ-glutamyl transpeptidase (GGTP), amylase, thyroid-stimulating hormone, and blood alcohol level. Random valproic acid serum level taken in the ED is 64 µg/mL. Non-contrast head CT is interpreted as non-acute. There are no documented abnormal findings during the physical exam.
What could be causing Mr. D’s altered mental status?
a) symptoms of a medical illness
b) medication, undetected substance intoxication, or withdrawal-related symptoms
c) acute exacerbation of schizoaffective disorder
d) delirium
e) catatonia of undetected and/or multiple causes
The authors’ observations
The differential diagnosis was broad at the time of Mr. D’s presentation to the ED because his symptoms overlapped across clinical considerations. The initial medical evaluation was negative, which suggested an active primary mental illness. However, Mr. D’s presenting symptoms warranted continued vigilance for concurrent or emergent delirium or catatonia, especially because of the potential morbidity if these conditions are not detected and managed.
EVALUATION
Fluctuating status
Mr. D is admitted to the mental health unit for treatment of presumptive bipolar depression with catatonic features. The initial admitting team continues aripiprazole, increased divalproex extended release to 1,000 mg in the morning/1,500 mg at night, held mirtazapine, and started lorazepam, 2 mg, 3 times daily, for catatonia. Metformin, lisinopril, and simvastatin are continued. Mr. D’s mental status and behavior fluctuates over the next 48 hours prompting the treatment team to consider an emergent delirious process.
On day 3, the primary team assumes care and observes fluctuations in level of arousal with disorientation, inattention, labile affect, disorganized speech and behavior, and responsiveness to internal (visual) stimuli. Finger-stick blood glucose level remains stable. Review of physical symptoms is notable for nausea and examination reveals unsteady gait and asterixis. His family denies that Mr. D used alcohol or drugs before admission. Collateral information from the family and review of Mr. D’s outpatient records is consistent with an acutely fluctuating confusional state that began 10 days before admission.
At this point, what is your differential diagnosis for Mr. D’s altered mental status?
a) symptoms of a medical illness
b) medication, undetected substance intoxication, or withdrawal-related symptoms
c) acute exacerbation of schizoaffective disorder
d) delirium
e) catatonia of undetected or multiple causes
TREATMENT
Valproate stopped
Mr. D’s ammonia level is 119 µg/dL (reference range, 15 to 45 μg/dL) on hospital day 3. Divalproex and lorazepam are discontinued, and standing lactulose is started because it is evident that he has active valproate-related hyperammonemic encephalopathy (VHE), also known as delirium due to valproate-related hyperammonemia.
Awake and drowsy EEG within 24 hours reveals “diffuse irregular slow activity” without epileptogenic features. HIV, syphilis, and vitamin B12 and red blood cell folate screening are negative. We confirm that Mr. D is not a vegetarian (dietary carnitine deficiency is a risk factor for VHE). He is not screened for a urea cycle disorder.
The authors’ observations
Divalproex is a commonly used FDA-approved treatment for a variety of neurologic and psychiatric conditions including acute bipolar mania.1-3 It also is used for off-label control of various psychiatric symptoms. It is a stable coordination compound composed of sodium valproate and valproic acid that dissipates into the valproate ion in the gastrointestinal tract.1 (In this article, references to valproate [VPA] include valproic acid and divalproex.) The drug is relatively well-tolerated; however, use may carry teratogenic risk and can adversely impact a variety of body systems, especially hematopoietic, gastrointestinal, and neurologic systems.1-3 Adverse effects can be idiosyncratic or in part related to VPA serum levels.1,4 VPA toxicity increases the likelihood of some adverse health outcomes, such as nausea, diarrhea, and tremors.1
Identifying and treating VHE
Asymptomatic elevations in ammonia without evidence of hepatic injury are common, might be related to valproic serum levels, and may occur in up to one-half of psychiatric patients receiving VPA.2-4 In contrast, VHE is a rare and potentially lethal idiosyncratic event unrelated to duration of VPA treatment, dosage, or valproic serum level.2-4 In addition, prior safe use might not protect against future VHE.3,4
VHE presents as delirium with characteristic acute changes in mental status, including alterations in cognition or level of consciousness ranging from lethargy to coma, along with possible focal neurological findings or vomiting.1,3,4 Although more common among patients with a seizure disorder, VHE also might be associated with new seizure activity in patients who do not have a seizure disorder.5
Although symptomatically acute in onset, emergence is unpredictable and can occur within days or up to years of use with therapeutic VPA dosing and valproic serum levels.2,4 Complicating identification, laboratory transaminase or ammonia elevations may or may not be present2-4; however, VHE typically occurs in the setting of hyperammonemia and normal transaminase levels.2 Reversible EEG findings are nonspecific2 and could show generalized slowing with occasional bursts of frontal intermittent rhythmic delta activity and triphasic waves.2,4
Pathophysiological descriptions of emergent VHE have been hypothesized,2-4 but the definitive causal mechanism remains unclear.6 Published VHE risk factors2-6 include:
- polypharmacy (especially anti-convulsants)
- inherited or dietary-based carnitine deficiency
- urea cycle disorders
- mental retardation.
How would you treat VHE?
a) cholinesterase inhibitors
b) antipsychotic therapy
c) supportive care
d) ammonia-reducing agents such as lactulose, carnitine, and neomycin
e) discontinue valproate
Outcome Normalized ammonia
Four days after discontinuing divalproex and starting lactulose, Mr. D’s fluctuating level of arousal, orientation, attention, and perceptual disturbances resolve along with restoration of environmental relatedness in setting of normalized ammonia level to 39 µg/dL. He is euthymic, non-psychotic, and without cognitive impairment at time of discharge. An “allergy” to divalproex is entered in his electronic medical record in an effort to discourage future retrial.
The authors’ observations
Once identified, management of VHE invariably includes consideration for discontinuation of valproate1,2,4,19; other adjunctive, expediting, ammonia-reducing strategies, including lactulose and carnitine, have also been described.2,4,5,20 Although lactulose is more commonly used, carnitine supplementation might be associated with a preferable dosing schedule and drug interaction and side-effect profile.20 Rapidly deteriorating clinical status could indicate hemodialysis.4
Of critical importance, these management strategies rely on awareness of and prompt identification of the condition, which includes an ability to distinguish emergent VHE from the mental illness VPA is used to treat.
Stopping the offending agent generally results in complete recovery in VHE patients with psychiatric illness.4 Most (>90%, n = 31) psychiatric patients in our and prior5 case series reviews recovered within 2 weeks of intervention.5 Cautious resumption of divalproex could be considered if there is a compelling clinical indication and you suspect that a putative polypharmacy agent such as topiramate has been removed; otherwise future retrial of VPA should be avoided.14
Mr. D’s case was consistent with a valproate-related hyperammonemic delirious event. He had preadmission acute onset, intra-daily fluctuating confusion, and visual perceptual disturbances with nausea, asterixis, gait disturbance, elevated ammonia, and a supportive EEG months after starting divalproex. Similar to our case, some challenging aspects of identifying emergent VHE include:
- earlier safe use of divalproex over extended periods
- lack of elevated VPA serum level
- lack of transaminase elevation
- lack of apparent risk factors
- presence of background serious mental illness, which can distract from VHE detection via misattribution to uncontrolled primary mental illness.
This last point is critical because it can delay VHE identification and treatment or worse, result in misdiagnosis with accompanying continuation or escalation of VPA dosing as has initially occurred in Mr. D’s case. Similar concerns have been raised2,5 and occurred,5,19 which is not surprising given the frequency of VPA use for psychiatric conditions and symptoms.
Providers should have a low threshold for checking an ammonia level in clinical scenarios that involve any alteration in mental status that may resemble delirium in psychiatric patients treated with valproate. From a preventative perspective, it may be prudent to avoid valproate in psychiatric patients with known VHE risk factors. Either way, promotion of VHE awareness and detection across medical disciplines is paramount.
1. Depakote [package insert]. Chicago, IL: AbbVie; 2016.
2. Lewis C, Deshpande A, Tesar G, et al. Valproate-induced hyperammonemic encephalopathy: a brief review. Curr Med Res Opin. 2012;28(6):1039-1042.
3. Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013;46(15):1323-1338.
4. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
5. Carr RB, Shrewsbury K. Hyperammonemia due to valproic acid in the psychiatric setting. Am J Psychiatry. 2007;164(7):1020-1027.
6. Hung C, Li T, Wei I, et al. The real mechanism of VPA-induced hyperammonemia remains unknown. Gen Hosp Psychiatry. 2011;33(1):84.e3-84.e4.
7. Starer J, Chang G. Hyperammonemic encephalopathy, valproic acid, and benzodiazepine withdrawal: a case series. Am J Drug Alcohol Abuse. 2010;36(2):98-101.
8. Eubanks AL, Aguirre B, Bourgeois JA. Severe acute hyperammonemia after brief exposure to valproate. Psychosomatics. 2008;49(1):82-83.
9. Fan CC, Huang MC, Liu HC. Lamotrigine might potentiate valproic acid-induced hyperammonemic encephalopathy. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(7):1747-1748.
10. Deutsch SI, Burket JA, Rosse RB. Valproate-induced hyperammonemic encephalopathy and normal liver functions: possible synergism with topiramate. Clin Neuropharmacol. 2009;32(6):350-352.
11. Rodrigues-Silva N, Venâncio Ä, Bouça J. Risperidone, a risk for valproate induced encephalopathy? Gen Hosp Psychiatry. 2013;35(4):452.e5-452.e6.
12. Sunkavalli KK, Iqbal FM, Singh B, et al. Valproate-induced hyperammonemic encephalopathy: a case report and brief review of the literature. Am J Ther. 2013;20(5):569-571.
13. Abreu LN, Issler C, Lafer B. Valproate-induced reversible pseudoatrophy of the brain and hyperammonemic encephalopathy in a bipolar patient. Aust N Z J Psychiatry. 2009;43(5):484-485.
14. Hong L, Schutz J, Nance M. A case of valproate-induced encephalopathy. Aust N Z J Psychiatry. 2012;46(12):1200-1201.
15. Kimmel RJ, Irwin SA, Meyer JM. Valproic acid-associated hyperammonemic encephalopathy: a case report from the psychiatric setting. Int Clin Psychopharmacol. 2005;20(1):57-58.
16. Elgudin L, Hall Y, Schubert D. Ammonia induced encephalopathy from valproic acid in a bipolar patient: case report. Int J Psychiatry Med. 2003;33(1):91-96.
17. Stewart JT. A case of hyperammonemic encephalopathy after 11 years of valproate therapy. J Clin Psychopharmacol. 2008;28(3):361-362.
18. Wadzinski J, Franks R, Roane D, et al. Valproate-associated hyperammonemic encephalopathy. J Am Board Fam Med. 2007;20(5):499-502.
19. Chang M, Tang X, Wen S, et al. Valproate (VPA)-associated hyperammonemic encephalopathy independent of elevated serum VPA levels: 21 cases in China from May 2000 to May 2012. Compr Psychiatry. 2013;54(5):562-567.
20. Sonik P, Hilty DM, Rossaro L, et al. Carnitine supplementation for valproate-related hyperammonemia to maintain therapeutic valproate level. J Clin Psychopharmacol. 2011;31(5):680-682.
CASE
Disoriented and confused
Mr. D, age 42, presents to our emergency department (ED) accompanied by his family with recent onset of disorientation, confusion, depressive mood with labile affect, sleep disturbances, purposeless movements, and grossly reduced kinetics/verbal output. He has a history of schizoaffective disorder, bipolar type, and recurrent admissions for psychotic mood instability.
A few months earlier, Mr. D was treated at our facility for acute exacerbation of his schizoaffective disorder. He was stabilized and discharged with aripiprazole, 30 mg/d, and mirtazapine, 15 mg/d—he had been taking both medications for some time—and newly started extended-release divalproex, 500 mg in the morning/1000 mg nightly (13.2 mg/kg). His trough valproic acid serum level was 70 µg/mL at discharge. He continued on this medication regimen until he returns to our ED with his family.
Mr. D has several medical problems, such as type 2 diabetes mellitus and hypertension, for which he has been receiving metformin, 1,000 mg/d, lisinopril, 10 mg/d, and simvastatin, 20 mg/d. He has no history of alcohol or substance abuse and does not smoke.
Serum and urine analyses are unremarkable and include finger-stick blood glucose, complete blood count, urinalysis, urine drug screen, comprehensive metabolic panel, magnesium, γ-glutamyl transpeptidase (GGTP), amylase, thyroid-stimulating hormone, and blood alcohol level. Random valproic acid serum level taken in the ED is 64 µg/mL. Non-contrast head CT is interpreted as non-acute. There are no documented abnormal findings during the physical exam.
What could be causing Mr. D’s altered mental status?
a) symptoms of a medical illness
b) medication, undetected substance intoxication, or withdrawal-related symptoms
c) acute exacerbation of schizoaffective disorder
d) delirium
e) catatonia of undetected and/or multiple causes
The authors’ observations
The differential diagnosis was broad at the time of Mr. D’s presentation to the ED because his symptoms overlapped across clinical considerations. The initial medical evaluation was negative, which suggested an active primary mental illness. However, Mr. D’s presenting symptoms warranted continued vigilance for concurrent or emergent delirium or catatonia, especially because of the potential morbidity if these conditions are not detected and managed.
EVALUATION
Fluctuating status
Mr. D is admitted to the mental health unit for treatment of presumptive bipolar depression with catatonic features. The initial admitting team continues aripiprazole, increased divalproex extended release to 1,000 mg in the morning/1,500 mg at night, held mirtazapine, and started lorazepam, 2 mg, 3 times daily, for catatonia. Metformin, lisinopril, and simvastatin are continued. Mr. D’s mental status and behavior fluctuates over the next 48 hours prompting the treatment team to consider an emergent delirious process.
On day 3, the primary team assumes care and observes fluctuations in level of arousal with disorientation, inattention, labile affect, disorganized speech and behavior, and responsiveness to internal (visual) stimuli. Finger-stick blood glucose level remains stable. Review of physical symptoms is notable for nausea and examination reveals unsteady gait and asterixis. His family denies that Mr. D used alcohol or drugs before admission. Collateral information from the family and review of Mr. D’s outpatient records is consistent with an acutely fluctuating confusional state that began 10 days before admission.
At this point, what is your differential diagnosis for Mr. D’s altered mental status?
a) symptoms of a medical illness
b) medication, undetected substance intoxication, or withdrawal-related symptoms
c) acute exacerbation of schizoaffective disorder
d) delirium
e) catatonia of undetected or multiple causes
TREATMENT
Valproate stopped
Mr. D’s ammonia level is 119 µg/dL (reference range, 15 to 45 μg/dL) on hospital day 3. Divalproex and lorazepam are discontinued, and standing lactulose is started because it is evident that he has active valproate-related hyperammonemic encephalopathy (VHE), also known as delirium due to valproate-related hyperammonemia.
Awake and drowsy EEG within 24 hours reveals “diffuse irregular slow activity” without epileptogenic features. HIV, syphilis, and vitamin B12 and red blood cell folate screening are negative. We confirm that Mr. D is not a vegetarian (dietary carnitine deficiency is a risk factor for VHE). He is not screened for a urea cycle disorder.
The authors’ observations
Divalproex is a commonly used FDA-approved treatment for a variety of neurologic and psychiatric conditions including acute bipolar mania.1-3 It also is used for off-label control of various psychiatric symptoms. It is a stable coordination compound composed of sodium valproate and valproic acid that dissipates into the valproate ion in the gastrointestinal tract.1 (In this article, references to valproate [VPA] include valproic acid and divalproex.) The drug is relatively well-tolerated; however, use may carry teratogenic risk and can adversely impact a variety of body systems, especially hematopoietic, gastrointestinal, and neurologic systems.1-3 Adverse effects can be idiosyncratic or in part related to VPA serum levels.1,4 VPA toxicity increases the likelihood of some adverse health outcomes, such as nausea, diarrhea, and tremors.1
Identifying and treating VHE
Asymptomatic elevations in ammonia without evidence of hepatic injury are common, might be related to valproic serum levels, and may occur in up to one-half of psychiatric patients receiving VPA.2-4 In contrast, VHE is a rare and potentially lethal idiosyncratic event unrelated to duration of VPA treatment, dosage, or valproic serum level.2-4 In addition, prior safe use might not protect against future VHE.3,4
VHE presents as delirium with characteristic acute changes in mental status, including alterations in cognition or level of consciousness ranging from lethargy to coma, along with possible focal neurological findings or vomiting.1,3,4 Although more common among patients with a seizure disorder, VHE also might be associated with new seizure activity in patients who do not have a seizure disorder.5
Although symptomatically acute in onset, emergence is unpredictable and can occur within days or up to years of use with therapeutic VPA dosing and valproic serum levels.2,4 Complicating identification, laboratory transaminase or ammonia elevations may or may not be present2-4; however, VHE typically occurs in the setting of hyperammonemia and normal transaminase levels.2 Reversible EEG findings are nonspecific2 and could show generalized slowing with occasional bursts of frontal intermittent rhythmic delta activity and triphasic waves.2,4
Pathophysiological descriptions of emergent VHE have been hypothesized,2-4 but the definitive causal mechanism remains unclear.6 Published VHE risk factors2-6 include:
- polypharmacy (especially anti-convulsants)
- inherited or dietary-based carnitine deficiency
- urea cycle disorders
- mental retardation.
How would you treat VHE?
a) cholinesterase inhibitors
b) antipsychotic therapy
c) supportive care
d) ammonia-reducing agents such as lactulose, carnitine, and neomycin
e) discontinue valproate
Outcome Normalized ammonia
Four days after discontinuing divalproex and starting lactulose, Mr. D’s fluctuating level of arousal, orientation, attention, and perceptual disturbances resolve along with restoration of environmental relatedness in setting of normalized ammonia level to 39 µg/dL. He is euthymic, non-psychotic, and without cognitive impairment at time of discharge. An “allergy” to divalproex is entered in his electronic medical record in an effort to discourage future retrial.
The authors’ observations
Once identified, management of VHE invariably includes consideration for discontinuation of valproate1,2,4,19; other adjunctive, expediting, ammonia-reducing strategies, including lactulose and carnitine, have also been described.2,4,5,20 Although lactulose is more commonly used, carnitine supplementation might be associated with a preferable dosing schedule and drug interaction and side-effect profile.20 Rapidly deteriorating clinical status could indicate hemodialysis.4
Of critical importance, these management strategies rely on awareness of and prompt identification of the condition, which includes an ability to distinguish emergent VHE from the mental illness VPA is used to treat.
Stopping the offending agent generally results in complete recovery in VHE patients with psychiatric illness.4 Most (>90%, n = 31) psychiatric patients in our and prior5 case series reviews recovered within 2 weeks of intervention.5 Cautious resumption of divalproex could be considered if there is a compelling clinical indication and you suspect that a putative polypharmacy agent such as topiramate has been removed; otherwise future retrial of VPA should be avoided.14
Mr. D’s case was consistent with a valproate-related hyperammonemic delirious event. He had preadmission acute onset, intra-daily fluctuating confusion, and visual perceptual disturbances with nausea, asterixis, gait disturbance, elevated ammonia, and a supportive EEG months after starting divalproex. Similar to our case, some challenging aspects of identifying emergent VHE include:
- earlier safe use of divalproex over extended periods
- lack of elevated VPA serum level
- lack of transaminase elevation
- lack of apparent risk factors
- presence of background serious mental illness, which can distract from VHE detection via misattribution to uncontrolled primary mental illness.
This last point is critical because it can delay VHE identification and treatment or worse, result in misdiagnosis with accompanying continuation or escalation of VPA dosing as has initially occurred in Mr. D’s case. Similar concerns have been raised2,5 and occurred,5,19 which is not surprising given the frequency of VPA use for psychiatric conditions and symptoms.
Providers should have a low threshold for checking an ammonia level in clinical scenarios that involve any alteration in mental status that may resemble delirium in psychiatric patients treated with valproate. From a preventative perspective, it may be prudent to avoid valproate in psychiatric patients with known VHE risk factors. Either way, promotion of VHE awareness and detection across medical disciplines is paramount.
CASE
Disoriented and confused
Mr. D, age 42, presents to our emergency department (ED) accompanied by his family with recent onset of disorientation, confusion, depressive mood with labile affect, sleep disturbances, purposeless movements, and grossly reduced kinetics/verbal output. He has a history of schizoaffective disorder, bipolar type, and recurrent admissions for psychotic mood instability.
A few months earlier, Mr. D was treated at our facility for acute exacerbation of his schizoaffective disorder. He was stabilized and discharged with aripiprazole, 30 mg/d, and mirtazapine, 15 mg/d—he had been taking both medications for some time—and newly started extended-release divalproex, 500 mg in the morning/1000 mg nightly (13.2 mg/kg). His trough valproic acid serum level was 70 µg/mL at discharge. He continued on this medication regimen until he returns to our ED with his family.
Mr. D has several medical problems, such as type 2 diabetes mellitus and hypertension, for which he has been receiving metformin, 1,000 mg/d, lisinopril, 10 mg/d, and simvastatin, 20 mg/d. He has no history of alcohol or substance abuse and does not smoke.
Serum and urine analyses are unremarkable and include finger-stick blood glucose, complete blood count, urinalysis, urine drug screen, comprehensive metabolic panel, magnesium, γ-glutamyl transpeptidase (GGTP), amylase, thyroid-stimulating hormone, and blood alcohol level. Random valproic acid serum level taken in the ED is 64 µg/mL. Non-contrast head CT is interpreted as non-acute. There are no documented abnormal findings during the physical exam.
What could be causing Mr. D’s altered mental status?
a) symptoms of a medical illness
b) medication, undetected substance intoxication, or withdrawal-related symptoms
c) acute exacerbation of schizoaffective disorder
d) delirium
e) catatonia of undetected and/or multiple causes
The authors’ observations
The differential diagnosis was broad at the time of Mr. D’s presentation to the ED because his symptoms overlapped across clinical considerations. The initial medical evaluation was negative, which suggested an active primary mental illness. However, Mr. D’s presenting symptoms warranted continued vigilance for concurrent or emergent delirium or catatonia, especially because of the potential morbidity if these conditions are not detected and managed.
EVALUATION
Fluctuating status
Mr. D is admitted to the mental health unit for treatment of presumptive bipolar depression with catatonic features. The initial admitting team continues aripiprazole, increased divalproex extended release to 1,000 mg in the morning/1,500 mg at night, held mirtazapine, and started lorazepam, 2 mg, 3 times daily, for catatonia. Metformin, lisinopril, and simvastatin are continued. Mr. D’s mental status and behavior fluctuates over the next 48 hours prompting the treatment team to consider an emergent delirious process.
On day 3, the primary team assumes care and observes fluctuations in level of arousal with disorientation, inattention, labile affect, disorganized speech and behavior, and responsiveness to internal (visual) stimuli. Finger-stick blood glucose level remains stable. Review of physical symptoms is notable for nausea and examination reveals unsteady gait and asterixis. His family denies that Mr. D used alcohol or drugs before admission. Collateral information from the family and review of Mr. D’s outpatient records is consistent with an acutely fluctuating confusional state that began 10 days before admission.
At this point, what is your differential diagnosis for Mr. D’s altered mental status?
a) symptoms of a medical illness
b) medication, undetected substance intoxication, or withdrawal-related symptoms
c) acute exacerbation of schizoaffective disorder
d) delirium
e) catatonia of undetected or multiple causes
TREATMENT
Valproate stopped
Mr. D’s ammonia level is 119 µg/dL (reference range, 15 to 45 μg/dL) on hospital day 3. Divalproex and lorazepam are discontinued, and standing lactulose is started because it is evident that he has active valproate-related hyperammonemic encephalopathy (VHE), also known as delirium due to valproate-related hyperammonemia.
Awake and drowsy EEG within 24 hours reveals “diffuse irregular slow activity” without epileptogenic features. HIV, syphilis, and vitamin B12 and red blood cell folate screening are negative. We confirm that Mr. D is not a vegetarian (dietary carnitine deficiency is a risk factor for VHE). He is not screened for a urea cycle disorder.
The authors’ observations
Divalproex is a commonly used FDA-approved treatment for a variety of neurologic and psychiatric conditions including acute bipolar mania.1-3 It also is used for off-label control of various psychiatric symptoms. It is a stable coordination compound composed of sodium valproate and valproic acid that dissipates into the valproate ion in the gastrointestinal tract.1 (In this article, references to valproate [VPA] include valproic acid and divalproex.) The drug is relatively well-tolerated; however, use may carry teratogenic risk and can adversely impact a variety of body systems, especially hematopoietic, gastrointestinal, and neurologic systems.1-3 Adverse effects can be idiosyncratic or in part related to VPA serum levels.1,4 VPA toxicity increases the likelihood of some adverse health outcomes, such as nausea, diarrhea, and tremors.1
Identifying and treating VHE
Asymptomatic elevations in ammonia without evidence of hepatic injury are common, might be related to valproic serum levels, and may occur in up to one-half of psychiatric patients receiving VPA.2-4 In contrast, VHE is a rare and potentially lethal idiosyncratic event unrelated to duration of VPA treatment, dosage, or valproic serum level.2-4 In addition, prior safe use might not protect against future VHE.3,4
VHE presents as delirium with characteristic acute changes in mental status, including alterations in cognition or level of consciousness ranging from lethargy to coma, along with possible focal neurological findings or vomiting.1,3,4 Although more common among patients with a seizure disorder, VHE also might be associated with new seizure activity in patients who do not have a seizure disorder.5
Although symptomatically acute in onset, emergence is unpredictable and can occur within days or up to years of use with therapeutic VPA dosing and valproic serum levels.2,4 Complicating identification, laboratory transaminase or ammonia elevations may or may not be present2-4; however, VHE typically occurs in the setting of hyperammonemia and normal transaminase levels.2 Reversible EEG findings are nonspecific2 and could show generalized slowing with occasional bursts of frontal intermittent rhythmic delta activity and triphasic waves.2,4
Pathophysiological descriptions of emergent VHE have been hypothesized,2-4 but the definitive causal mechanism remains unclear.6 Published VHE risk factors2-6 include:
- polypharmacy (especially anti-convulsants)
- inherited or dietary-based carnitine deficiency
- urea cycle disorders
- mental retardation.
How would you treat VHE?
a) cholinesterase inhibitors
b) antipsychotic therapy
c) supportive care
d) ammonia-reducing agents such as lactulose, carnitine, and neomycin
e) discontinue valproate
Outcome Normalized ammonia
Four days after discontinuing divalproex and starting lactulose, Mr. D’s fluctuating level of arousal, orientation, attention, and perceptual disturbances resolve along with restoration of environmental relatedness in setting of normalized ammonia level to 39 µg/dL. He is euthymic, non-psychotic, and without cognitive impairment at time of discharge. An “allergy” to divalproex is entered in his electronic medical record in an effort to discourage future retrial.
The authors’ observations
Once identified, management of VHE invariably includes consideration for discontinuation of valproate1,2,4,19; other adjunctive, expediting, ammonia-reducing strategies, including lactulose and carnitine, have also been described.2,4,5,20 Although lactulose is more commonly used, carnitine supplementation might be associated with a preferable dosing schedule and drug interaction and side-effect profile.20 Rapidly deteriorating clinical status could indicate hemodialysis.4
Of critical importance, these management strategies rely on awareness of and prompt identification of the condition, which includes an ability to distinguish emergent VHE from the mental illness VPA is used to treat.
Stopping the offending agent generally results in complete recovery in VHE patients with psychiatric illness.4 Most (>90%, n = 31) psychiatric patients in our and prior5 case series reviews recovered within 2 weeks of intervention.5 Cautious resumption of divalproex could be considered if there is a compelling clinical indication and you suspect that a putative polypharmacy agent such as topiramate has been removed; otherwise future retrial of VPA should be avoided.14
Mr. D’s case was consistent with a valproate-related hyperammonemic delirious event. He had preadmission acute onset, intra-daily fluctuating confusion, and visual perceptual disturbances with nausea, asterixis, gait disturbance, elevated ammonia, and a supportive EEG months after starting divalproex. Similar to our case, some challenging aspects of identifying emergent VHE include:
- earlier safe use of divalproex over extended periods
- lack of elevated VPA serum level
- lack of transaminase elevation
- lack of apparent risk factors
- presence of background serious mental illness, which can distract from VHE detection via misattribution to uncontrolled primary mental illness.
This last point is critical because it can delay VHE identification and treatment or worse, result in misdiagnosis with accompanying continuation or escalation of VPA dosing as has initially occurred in Mr. D’s case. Similar concerns have been raised2,5 and occurred,5,19 which is not surprising given the frequency of VPA use for psychiatric conditions and symptoms.
Providers should have a low threshold for checking an ammonia level in clinical scenarios that involve any alteration in mental status that may resemble delirium in psychiatric patients treated with valproate. From a preventative perspective, it may be prudent to avoid valproate in psychiatric patients with known VHE risk factors. Either way, promotion of VHE awareness and detection across medical disciplines is paramount.
1. Depakote [package insert]. Chicago, IL: AbbVie; 2016.
2. Lewis C, Deshpande A, Tesar G, et al. Valproate-induced hyperammonemic encephalopathy: a brief review. Curr Med Res Opin. 2012;28(6):1039-1042.
3. Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013;46(15):1323-1338.
4. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
5. Carr RB, Shrewsbury K. Hyperammonemia due to valproic acid in the psychiatric setting. Am J Psychiatry. 2007;164(7):1020-1027.
6. Hung C, Li T, Wei I, et al. The real mechanism of VPA-induced hyperammonemia remains unknown. Gen Hosp Psychiatry. 2011;33(1):84.e3-84.e4.
7. Starer J, Chang G. Hyperammonemic encephalopathy, valproic acid, and benzodiazepine withdrawal: a case series. Am J Drug Alcohol Abuse. 2010;36(2):98-101.
8. Eubanks AL, Aguirre B, Bourgeois JA. Severe acute hyperammonemia after brief exposure to valproate. Psychosomatics. 2008;49(1):82-83.
9. Fan CC, Huang MC, Liu HC. Lamotrigine might potentiate valproic acid-induced hyperammonemic encephalopathy. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(7):1747-1748.
10. Deutsch SI, Burket JA, Rosse RB. Valproate-induced hyperammonemic encephalopathy and normal liver functions: possible synergism with topiramate. Clin Neuropharmacol. 2009;32(6):350-352.
11. Rodrigues-Silva N, Venâncio Ä, Bouça J. Risperidone, a risk for valproate induced encephalopathy? Gen Hosp Psychiatry. 2013;35(4):452.e5-452.e6.
12. Sunkavalli KK, Iqbal FM, Singh B, et al. Valproate-induced hyperammonemic encephalopathy: a case report and brief review of the literature. Am J Ther. 2013;20(5):569-571.
13. Abreu LN, Issler C, Lafer B. Valproate-induced reversible pseudoatrophy of the brain and hyperammonemic encephalopathy in a bipolar patient. Aust N Z J Psychiatry. 2009;43(5):484-485.
14. Hong L, Schutz J, Nance M. A case of valproate-induced encephalopathy. Aust N Z J Psychiatry. 2012;46(12):1200-1201.
15. Kimmel RJ, Irwin SA, Meyer JM. Valproic acid-associated hyperammonemic encephalopathy: a case report from the psychiatric setting. Int Clin Psychopharmacol. 2005;20(1):57-58.
16. Elgudin L, Hall Y, Schubert D. Ammonia induced encephalopathy from valproic acid in a bipolar patient: case report. Int J Psychiatry Med. 2003;33(1):91-96.
17. Stewart JT. A case of hyperammonemic encephalopathy after 11 years of valproate therapy. J Clin Psychopharmacol. 2008;28(3):361-362.
18. Wadzinski J, Franks R, Roane D, et al. Valproate-associated hyperammonemic encephalopathy. J Am Board Fam Med. 2007;20(5):499-502.
19. Chang M, Tang X, Wen S, et al. Valproate (VPA)-associated hyperammonemic encephalopathy independent of elevated serum VPA levels: 21 cases in China from May 2000 to May 2012. Compr Psychiatry. 2013;54(5):562-567.
20. Sonik P, Hilty DM, Rossaro L, et al. Carnitine supplementation for valproate-related hyperammonemia to maintain therapeutic valproate level. J Clin Psychopharmacol. 2011;31(5):680-682.
1. Depakote [package insert]. Chicago, IL: AbbVie; 2016.
2. Lewis C, Deshpande A, Tesar G, et al. Valproate-induced hyperammonemic encephalopathy: a brief review. Curr Med Res Opin. 2012;28(6):1039-1042.
3. Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013;46(15):1323-1338.
4. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
5. Carr RB, Shrewsbury K. Hyperammonemia due to valproic acid in the psychiatric setting. Am J Psychiatry. 2007;164(7):1020-1027.
6. Hung C, Li T, Wei I, et al. The real mechanism of VPA-induced hyperammonemia remains unknown. Gen Hosp Psychiatry. 2011;33(1):84.e3-84.e4.
7. Starer J, Chang G. Hyperammonemic encephalopathy, valproic acid, and benzodiazepine withdrawal: a case series. Am J Drug Alcohol Abuse. 2010;36(2):98-101.
8. Eubanks AL, Aguirre B, Bourgeois JA. Severe acute hyperammonemia after brief exposure to valproate. Psychosomatics. 2008;49(1):82-83.
9. Fan CC, Huang MC, Liu HC. Lamotrigine might potentiate valproic acid-induced hyperammonemic encephalopathy. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(7):1747-1748.
10. Deutsch SI, Burket JA, Rosse RB. Valproate-induced hyperammonemic encephalopathy and normal liver functions: possible synergism with topiramate. Clin Neuropharmacol. 2009;32(6):350-352.
11. Rodrigues-Silva N, Venâncio Ä, Bouça J. Risperidone, a risk for valproate induced encephalopathy? Gen Hosp Psychiatry. 2013;35(4):452.e5-452.e6.
12. Sunkavalli KK, Iqbal FM, Singh B, et al. Valproate-induced hyperammonemic encephalopathy: a case report and brief review of the literature. Am J Ther. 2013;20(5):569-571.
13. Abreu LN, Issler C, Lafer B. Valproate-induced reversible pseudoatrophy of the brain and hyperammonemic encephalopathy in a bipolar patient. Aust N Z J Psychiatry. 2009;43(5):484-485.
14. Hong L, Schutz J, Nance M. A case of valproate-induced encephalopathy. Aust N Z J Psychiatry. 2012;46(12):1200-1201.
15. Kimmel RJ, Irwin SA, Meyer JM. Valproic acid-associated hyperammonemic encephalopathy: a case report from the psychiatric setting. Int Clin Psychopharmacol. 2005;20(1):57-58.
16. Elgudin L, Hall Y, Schubert D. Ammonia induced encephalopathy from valproic acid in a bipolar patient: case report. Int J Psychiatry Med. 2003;33(1):91-96.
17. Stewart JT. A case of hyperammonemic encephalopathy after 11 years of valproate therapy. J Clin Psychopharmacol. 2008;28(3):361-362.
18. Wadzinski J, Franks R, Roane D, et al. Valproate-associated hyperammonemic encephalopathy. J Am Board Fam Med. 2007;20(5):499-502.
19. Chang M, Tang X, Wen S, et al. Valproate (VPA)-associated hyperammonemic encephalopathy independent of elevated serum VPA levels: 21 cases in China from May 2000 to May 2012. Compr Psychiatry. 2013;54(5):562-567.
20. Sonik P, Hilty DM, Rossaro L, et al. Carnitine supplementation for valproate-related hyperammonemia to maintain therapeutic valproate level. J Clin Psychopharmacol. 2011;31(5):680-682.
ADHD symptoms are stable, then a sudden relapse
CASE
Sudden deterioration
R, age 11, has attention-deficit/hyperactivity disorder (ADHD), combined type, and oppositional defiant disorder, which has been stable for more than a year on extended-release (ER) methylphenidate (brand name: Concerta), 54 mg/d (1.2 mg/kg). With combined pharmacotherapy and behavioral management, his symptoms of hyperactivity, inattention, and impulsivity improved at school and at home. He shows some academic gains as evidenced by improved achievement at school.
Over 2 months, R experiences a substantial deterioration in behavioral and academic performance. Along with core symptoms of ADHD, he begins to exhibit physical and verbal aggression. A report from school states that R has been using obscene language and destroying property, and has had episodes of provoked aggression toward his peers. His grades drop and he receives 2 school suspensions because of aggressive behavior.
What could be causing R’s ADHD symptoms to reemerge?
a) nonadherence to treatment
b) substance abuse
c) medication change
d) all of the above
The authors’ observations
Worsening of psychiatric symptoms in a stable patient is relatively common. Many factors can contribute to patient destabilization. Treatment nonadherence is a leading cause, along with psychosocial stressors and substance use (Table).
EVALUATION
Adherence confirmed
R is hyperactive and distracted during his visit, a clear deterioration from his baseline status. R is oppositional and defiant toward his mother during the session, but shows good social skills when communicating with the physician.
R’s mother reports that her son seldom forgets to take his medication, and she ensures that he is swallowing the pill, rather than chewing it. Data from the prescription drug-monitoring program show that the family is filling the prescriptions regularly. The ER methylphenidate dosage is raised to 72 mg/d. The clinicians provide psychoeducation about adherence to a medication regimen to R and his family. Also, his parents and teachers receive Vanderbilt Assessment Scales for ADHD to assess the symptoms in different settings.
At a follow-up visit a week later, R’s mother reports that her son continues to have problems in school and at home. The Vanderbilt scales reveal that R is having clinically significant problems with attention, hyperactivity, impulse control, and oppositional behavior.
A urine drug screen is ordered to rule out the possibility of a sudden deterioration of ADHD symptoms secondary to substance use disorder. To ensure compliance, we recommend that R take his medication at the school nurse’s office in the morning.
A week later
Although R takes his medication at school, he continues to show core symptoms of ADHD without improvement. The urine drug screen is negative. A physical examination does not reveal any medical illness. The treatment team calls the pharmacist to obtain a complete list of medications R is taking, who confirms that he is only receiving ER methylphenidate, 72 mg/d. The pharmacist also notes that R’s medication was switched from the brand-name drug to a generic 3 months ago because of a change in insurance coverage. This change coincided with the reemergence of his ADHD symptoms.
R’s mother reports that the new pills do not look like the old ones even before the dosage was raised. A new brand-necessary prescription is sent to the pharmacy. With the brand-name medication, R’s symptoms quickly improve, and remain improved when the dosage is decreased to the previous dosage of 54 mg/d.
With osmotic-controlled release oral delivery system (OROS) and outer coating of ER methylphenidate, how much drug is released immediately vs slow release?
a) 22% immediate release and 78% slow release
b) 78% immediate release and 22% slow release
c) 50% immediate release and 50% slow release
The authors’ observations
Generic substitution of a brand medication can result in worsening of symptoms and increased adverse effects. Possible bioequivalence issues can lead to failure of drug therapy.1
In 2013, the FDA determined that 2 specific generic formulations of ER methylphenidate do not have therapeutic equivalency to the brand-name medication, Concerta. The FDA stated, “Based on an analysis of data, FDA has concerns about whether or not two approved generic versions of Concerta tablets (methylphenidate hydrochloride extended-release tablets), used to treat attention-deficit hyperactivity disorder in adults and children, are therapeutically equiv
In an apparent confirmation of the FDA’s concerns, a case series of children and adolescents with ADHD observed that almost all of the patients showed symptom improvement when they switched from a non-OROS formulation to an OROS preparation at the same dosage.3
The OROS preparation is thought to provide more predictable medication delivery over an extended period of time (Figure). A patient taking an ER formulation without OROS might lose this benefit, which could lead to symptom destabilization, even if the patient is taking the medication as instructed.
Brand vs generic
Under FDA regulations, companies seeking approval for generic formulations of approved drugs must demonstrate that their products are the same as the brand-name drug in terms of:
- active ingredients
- strength
- dosage form
- route of administration
- packaging label.
In addition, the pharmaceutical company must demonstrate that the generic form is absorbed and distributed to the part of the body at which it has its effect at acceptably similar levels to the brand-name drug. All medications—new or generic, in clinical trials or approved, prescription or over-the-counter—must be manufactured under controlled conditions that assure product quality.
However, some studies have disputed this equivalency. In 1 study, patients with schizophrenia receiving generic olanzapine had lower serum concentration than patients with schizophrenia taking equivalent dosages of brand-name olanzapine.4 Similarly, studies comparing generic and brand-name venlafaxine showed significant differences in peak plasma concentration (Cmax)between generic and brand-name compounds.5
The FDA has considered upgrading the manufacturers’ warnings about the risk of generic medications, but has delayed the decision to 2017.6
FDA’s approval process for generic drugs
To receive approval of a generic formulation in the United States, the FDA requires that the generic drug should be compared with the corresponding brand-name drug in small crossover trials involving at least 24 to 36 healthy volunteers.
Bioequivalence is then established based on assessments of the rate of absorption (Cmax and area under the plasma concentration-time curve [AUC]). The FDA’s criteria are designed to achieve 90% confidence that the ratios of the test-to-reference log-transformed mean values for AUC and Cmax are within the interval of 80% to 125%. The FDA accepts −20% to 25% variation in Cmax and AUC in products that are considered bioequivalent. This is much less stringent than its −5% to 5% standard used for brand-name products. The FDA publishes a list of generic drugs that have been certified as bioequivalent, known as the “Orange Book.”5
Considerations when substituting generic medication
Because of the growing number of generic formulations of the same medication, generic–generic switches are becoming more commonplace. Theoretically, any 2 generic versions of the same medication can have a variation of up to 40% in AUC and Cmax. Generic medications are tested in healthy human controls through single-dose studies, which raises concerns about their applicability to the entire patient population.
Bioequivalence. It is a matter of debate whether bioequivalence translates to therapeutic equivalency. For medications with a narrow therapeutic index, the FDA has accepted that these 2 phenomena are not necessarily linked. With the exception of a few medications, including lithium and some anticonvulsants such as divalproex sodium and carbamazepine, serum level of the medications usually does not predict clinical response.
Inert ingredients. Generic medications can include inert ingredients (excipients) that are different from those in their branded counterparts. Some of these inactive ingredients can cause adverse effects. A study comparing paroxetine mesylate and paroxetine hydrochloride showed differences in bioequivalence and clinical efficacy.7
In some cases, brand-to-generic substitution can thwart clinical progress in a stable patient. This small change in the medication could destabilize the patient’s condition, which, in turn, may lead to unnecessary and significant social and financial burdens on the patient’s family, school, community, and the health care system.
Recommendations
In the event of a change in clinical response, clinicians first should evaluate adherence and explore other factors, such as biological, psychological, medical, and social issues. Adherence can be adversely affected by a change in the physical characteristics of the pill. Prescribers should remain cognizant of brand–generic and generic–generic switches. It may be reasonable to adjust the dosage of the new generic medication to address changes in clinical effectiveness.
If these strategies are ineffective, consider switching to a brand-name medication. Write “Dispense As Written” on the prescription to ensure delivery of the branded medication or a specific generic version of the medication.
An insurance company might require prior authorization to approve payment for the brand medication. To save time, use electronic forms or fax for communicating with the insurance company. Adding references to FDA statements and research papers, along with the patient’s history and presentations, would be prudent to demonstrate doubts about efficacy of the generic medication.
1. Atif M, Azeem M, Sarwar MR. Potential problems and recommendations regarding substitution of generic antiepileptic drugs: a systematic review of literature. Springerplus. 2016;5:182. doi: 10.1186/s40064-016-1824-2.
2. U.S. Food and Drug Administration. Methylphenidate hydrochloride extended release tablets (generic Concerta) made by Mallinckrodt and Kudco. http://www.fda.gov/Drugs/DrugSafety/ucm422568.htm. Updated November 13, 2014. Accessed August 29, 2016.
3. Lally MD, Kral MC, Boan AD. Not all generic Concerta is created equal: comparison of OROS versus non-OROS for the treatment of ADHD [published online October 14, 2015]. Clin Pediatr (Phila). doi:10.1177/0009922815611647.
4. Italiano DD, Bruno A, Santoro V, et al. Generic olanzapine substitution in patients with schizophrenia: assessment of serum concentrations and therapeutic response after switching. Ther Drug Monit. 2015;37(6):827-830.
5. Borgheini GG. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25(6):1578-1592.
6. Thomas K. F.D.A. delays rule on generic drug labels. http://www.nytimes.com/2016/05/20/business/fda-delays-rule-on-generic-drug-labels.html. Published May 19, 2016. Accessed August 29, 2016.
7. Pae CU, Misra A, Ham BJ, et al. Paroxetine mesylate: comparable to paroxetine hydrochloride? Expert Opin Pharmacother. 2010;11(2):185-193
CASE
Sudden deterioration
R, age 11, has attention-deficit/hyperactivity disorder (ADHD), combined type, and oppositional defiant disorder, which has been stable for more than a year on extended-release (ER) methylphenidate (brand name: Concerta), 54 mg/d (1.2 mg/kg). With combined pharmacotherapy and behavioral management, his symptoms of hyperactivity, inattention, and impulsivity improved at school and at home. He shows some academic gains as evidenced by improved achievement at school.
Over 2 months, R experiences a substantial deterioration in behavioral and academic performance. Along with core symptoms of ADHD, he begins to exhibit physical and verbal aggression. A report from school states that R has been using obscene language and destroying property, and has had episodes of provoked aggression toward his peers. His grades drop and he receives 2 school suspensions because of aggressive behavior.
What could be causing R’s ADHD symptoms to reemerge?
a) nonadherence to treatment
b) substance abuse
c) medication change
d) all of the above
The authors’ observations
Worsening of psychiatric symptoms in a stable patient is relatively common. Many factors can contribute to patient destabilization. Treatment nonadherence is a leading cause, along with psychosocial stressors and substance use (Table).
EVALUATION
Adherence confirmed
R is hyperactive and distracted during his visit, a clear deterioration from his baseline status. R is oppositional and defiant toward his mother during the session, but shows good social skills when communicating with the physician.
R’s mother reports that her son seldom forgets to take his medication, and she ensures that he is swallowing the pill, rather than chewing it. Data from the prescription drug-monitoring program show that the family is filling the prescriptions regularly. The ER methylphenidate dosage is raised to 72 mg/d. The clinicians provide psychoeducation about adherence to a medication regimen to R and his family. Also, his parents and teachers receive Vanderbilt Assessment Scales for ADHD to assess the symptoms in different settings.
At a follow-up visit a week later, R’s mother reports that her son continues to have problems in school and at home. The Vanderbilt scales reveal that R is having clinically significant problems with attention, hyperactivity, impulse control, and oppositional behavior.
A urine drug screen is ordered to rule out the possibility of a sudden deterioration of ADHD symptoms secondary to substance use disorder. To ensure compliance, we recommend that R take his medication at the school nurse’s office in the morning.
A week later
Although R takes his medication at school, he continues to show core symptoms of ADHD without improvement. The urine drug screen is negative. A physical examination does not reveal any medical illness. The treatment team calls the pharmacist to obtain a complete list of medications R is taking, who confirms that he is only receiving ER methylphenidate, 72 mg/d. The pharmacist also notes that R’s medication was switched from the brand-name drug to a generic 3 months ago because of a change in insurance coverage. This change coincided with the reemergence of his ADHD symptoms.
R’s mother reports that the new pills do not look like the old ones even before the dosage was raised. A new brand-necessary prescription is sent to the pharmacy. With the brand-name medication, R’s symptoms quickly improve, and remain improved when the dosage is decreased to the previous dosage of 54 mg/d.
With osmotic-controlled release oral delivery system (OROS) and outer coating of ER methylphenidate, how much drug is released immediately vs slow release?
a) 22% immediate release and 78% slow release
b) 78% immediate release and 22% slow release
c) 50% immediate release and 50% slow release
The authors’ observations
Generic substitution of a brand medication can result in worsening of symptoms and increased adverse effects. Possible bioequivalence issues can lead to failure of drug therapy.1
In 2013, the FDA determined that 2 specific generic formulations of ER methylphenidate do not have therapeutic equivalency to the brand-name medication, Concerta. The FDA stated, “Based on an analysis of data, FDA has concerns about whether or not two approved generic versions of Concerta tablets (methylphenidate hydrochloride extended-release tablets), used to treat attention-deficit hyperactivity disorder in adults and children, are therapeutically equiv
In an apparent confirmation of the FDA’s concerns, a case series of children and adolescents with ADHD observed that almost all of the patients showed symptom improvement when they switched from a non-OROS formulation to an OROS preparation at the same dosage.3
The OROS preparation is thought to provide more predictable medication delivery over an extended period of time (Figure). A patient taking an ER formulation without OROS might lose this benefit, which could lead to symptom destabilization, even if the patient is taking the medication as instructed.
Brand vs generic
Under FDA regulations, companies seeking approval for generic formulations of approved drugs must demonstrate that their products are the same as the brand-name drug in terms of:
- active ingredients
- strength
- dosage form
- route of administration
- packaging label.
In addition, the pharmaceutical company must demonstrate that the generic form is absorbed and distributed to the part of the body at which it has its effect at acceptably similar levels to the brand-name drug. All medications—new or generic, in clinical trials or approved, prescription or over-the-counter—must be manufactured under controlled conditions that assure product quality.
However, some studies have disputed this equivalency. In 1 study, patients with schizophrenia receiving generic olanzapine had lower serum concentration than patients with schizophrenia taking equivalent dosages of brand-name olanzapine.4 Similarly, studies comparing generic and brand-name venlafaxine showed significant differences in peak plasma concentration (Cmax)between generic and brand-name compounds.5
The FDA has considered upgrading the manufacturers’ warnings about the risk of generic medications, but has delayed the decision to 2017.6
FDA’s approval process for generic drugs
To receive approval of a generic formulation in the United States, the FDA requires that the generic drug should be compared with the corresponding brand-name drug in small crossover trials involving at least 24 to 36 healthy volunteers.
Bioequivalence is then established based on assessments of the rate of absorption (Cmax and area under the plasma concentration-time curve [AUC]). The FDA’s criteria are designed to achieve 90% confidence that the ratios of the test-to-reference log-transformed mean values for AUC and Cmax are within the interval of 80% to 125%. The FDA accepts −20% to 25% variation in Cmax and AUC in products that are considered bioequivalent. This is much less stringent than its −5% to 5% standard used for brand-name products. The FDA publishes a list of generic drugs that have been certified as bioequivalent, known as the “Orange Book.”5
Considerations when substituting generic medication
Because of the growing number of generic formulations of the same medication, generic–generic switches are becoming more commonplace. Theoretically, any 2 generic versions of the same medication can have a variation of up to 40% in AUC and Cmax. Generic medications are tested in healthy human controls through single-dose studies, which raises concerns about their applicability to the entire patient population.
Bioequivalence. It is a matter of debate whether bioequivalence translates to therapeutic equivalency. For medications with a narrow therapeutic index, the FDA has accepted that these 2 phenomena are not necessarily linked. With the exception of a few medications, including lithium and some anticonvulsants such as divalproex sodium and carbamazepine, serum level of the medications usually does not predict clinical response.
Inert ingredients. Generic medications can include inert ingredients (excipients) that are different from those in their branded counterparts. Some of these inactive ingredients can cause adverse effects. A study comparing paroxetine mesylate and paroxetine hydrochloride showed differences in bioequivalence and clinical efficacy.7
In some cases, brand-to-generic substitution can thwart clinical progress in a stable patient. This small change in the medication could destabilize the patient’s condition, which, in turn, may lead to unnecessary and significant social and financial burdens on the patient’s family, school, community, and the health care system.
Recommendations
In the event of a change in clinical response, clinicians first should evaluate adherence and explore other factors, such as biological, psychological, medical, and social issues. Adherence can be adversely affected by a change in the physical characteristics of the pill. Prescribers should remain cognizant of brand–generic and generic–generic switches. It may be reasonable to adjust the dosage of the new generic medication to address changes in clinical effectiveness.
If these strategies are ineffective, consider switching to a brand-name medication. Write “Dispense As Written” on the prescription to ensure delivery of the branded medication or a specific generic version of the medication.
An insurance company might require prior authorization to approve payment for the brand medication. To save time, use electronic forms or fax for communicating with the insurance company. Adding references to FDA statements and research papers, along with the patient’s history and presentations, would be prudent to demonstrate doubts about efficacy of the generic medication.
CASE
Sudden deterioration
R, age 11, has attention-deficit/hyperactivity disorder (ADHD), combined type, and oppositional defiant disorder, which has been stable for more than a year on extended-release (ER) methylphenidate (brand name: Concerta), 54 mg/d (1.2 mg/kg). With combined pharmacotherapy and behavioral management, his symptoms of hyperactivity, inattention, and impulsivity improved at school and at home. He shows some academic gains as evidenced by improved achievement at school.
Over 2 months, R experiences a substantial deterioration in behavioral and academic performance. Along with core symptoms of ADHD, he begins to exhibit physical and verbal aggression. A report from school states that R has been using obscene language and destroying property, and has had episodes of provoked aggression toward his peers. His grades drop and he receives 2 school suspensions because of aggressive behavior.
What could be causing R’s ADHD symptoms to reemerge?
a) nonadherence to treatment
b) substance abuse
c) medication change
d) all of the above
The authors’ observations
Worsening of psychiatric symptoms in a stable patient is relatively common. Many factors can contribute to patient destabilization. Treatment nonadherence is a leading cause, along with psychosocial stressors and substance use (Table).
EVALUATION
Adherence confirmed
R is hyperactive and distracted during his visit, a clear deterioration from his baseline status. R is oppositional and defiant toward his mother during the session, but shows good social skills when communicating with the physician.
R’s mother reports that her son seldom forgets to take his medication, and she ensures that he is swallowing the pill, rather than chewing it. Data from the prescription drug-monitoring program show that the family is filling the prescriptions regularly. The ER methylphenidate dosage is raised to 72 mg/d. The clinicians provide psychoeducation about adherence to a medication regimen to R and his family. Also, his parents and teachers receive Vanderbilt Assessment Scales for ADHD to assess the symptoms in different settings.
At a follow-up visit a week later, R’s mother reports that her son continues to have problems in school and at home. The Vanderbilt scales reveal that R is having clinically significant problems with attention, hyperactivity, impulse control, and oppositional behavior.
A urine drug screen is ordered to rule out the possibility of a sudden deterioration of ADHD symptoms secondary to substance use disorder. To ensure compliance, we recommend that R take his medication at the school nurse’s office in the morning.
A week later
Although R takes his medication at school, he continues to show core symptoms of ADHD without improvement. The urine drug screen is negative. A physical examination does not reveal any medical illness. The treatment team calls the pharmacist to obtain a complete list of medications R is taking, who confirms that he is only receiving ER methylphenidate, 72 mg/d. The pharmacist also notes that R’s medication was switched from the brand-name drug to a generic 3 months ago because of a change in insurance coverage. This change coincided with the reemergence of his ADHD symptoms.
R’s mother reports that the new pills do not look like the old ones even before the dosage was raised. A new brand-necessary prescription is sent to the pharmacy. With the brand-name medication, R’s symptoms quickly improve, and remain improved when the dosage is decreased to the previous dosage of 54 mg/d.
With osmotic-controlled release oral delivery system (OROS) and outer coating of ER methylphenidate, how much drug is released immediately vs slow release?
a) 22% immediate release and 78% slow release
b) 78% immediate release and 22% slow release
c) 50% immediate release and 50% slow release
The authors’ observations
Generic substitution of a brand medication can result in worsening of symptoms and increased adverse effects. Possible bioequivalence issues can lead to failure of drug therapy.1
In 2013, the FDA determined that 2 specific generic formulations of ER methylphenidate do not have therapeutic equivalency to the brand-name medication, Concerta. The FDA stated, “Based on an analysis of data, FDA has concerns about whether or not two approved generic versions of Concerta tablets (methylphenidate hydrochloride extended-release tablets), used to treat attention-deficit hyperactivity disorder in adults and children, are therapeutically equiv
In an apparent confirmation of the FDA’s concerns, a case series of children and adolescents with ADHD observed that almost all of the patients showed symptom improvement when they switched from a non-OROS formulation to an OROS preparation at the same dosage.3
The OROS preparation is thought to provide more predictable medication delivery over an extended period of time (Figure). A patient taking an ER formulation without OROS might lose this benefit, which could lead to symptom destabilization, even if the patient is taking the medication as instructed.
Brand vs generic
Under FDA regulations, companies seeking approval for generic formulations of approved drugs must demonstrate that their products are the same as the brand-name drug in terms of:
- active ingredients
- strength
- dosage form
- route of administration
- packaging label.
In addition, the pharmaceutical company must demonstrate that the generic form is absorbed and distributed to the part of the body at which it has its effect at acceptably similar levels to the brand-name drug. All medications—new or generic, in clinical trials or approved, prescription or over-the-counter—must be manufactured under controlled conditions that assure product quality.
However, some studies have disputed this equivalency. In 1 study, patients with schizophrenia receiving generic olanzapine had lower serum concentration than patients with schizophrenia taking equivalent dosages of brand-name olanzapine.4 Similarly, studies comparing generic and brand-name venlafaxine showed significant differences in peak plasma concentration (Cmax)between generic and brand-name compounds.5
The FDA has considered upgrading the manufacturers’ warnings about the risk of generic medications, but has delayed the decision to 2017.6
FDA’s approval process for generic drugs
To receive approval of a generic formulation in the United States, the FDA requires that the generic drug should be compared with the corresponding brand-name drug in small crossover trials involving at least 24 to 36 healthy volunteers.
Bioequivalence is then established based on assessments of the rate of absorption (Cmax and area under the plasma concentration-time curve [AUC]). The FDA’s criteria are designed to achieve 90% confidence that the ratios of the test-to-reference log-transformed mean values for AUC and Cmax are within the interval of 80% to 125%. The FDA accepts −20% to 25% variation in Cmax and AUC in products that are considered bioequivalent. This is much less stringent than its −5% to 5% standard used for brand-name products. The FDA publishes a list of generic drugs that have been certified as bioequivalent, known as the “Orange Book.”5
Considerations when substituting generic medication
Because of the growing number of generic formulations of the same medication, generic–generic switches are becoming more commonplace. Theoretically, any 2 generic versions of the same medication can have a variation of up to 40% in AUC and Cmax. Generic medications are tested in healthy human controls through single-dose studies, which raises concerns about their applicability to the entire patient population.
Bioequivalence. It is a matter of debate whether bioequivalence translates to therapeutic equivalency. For medications with a narrow therapeutic index, the FDA has accepted that these 2 phenomena are not necessarily linked. With the exception of a few medications, including lithium and some anticonvulsants such as divalproex sodium and carbamazepine, serum level of the medications usually does not predict clinical response.
Inert ingredients. Generic medications can include inert ingredients (excipients) that are different from those in their branded counterparts. Some of these inactive ingredients can cause adverse effects. A study comparing paroxetine mesylate and paroxetine hydrochloride showed differences in bioequivalence and clinical efficacy.7
In some cases, brand-to-generic substitution can thwart clinical progress in a stable patient. This small change in the medication could destabilize the patient’s condition, which, in turn, may lead to unnecessary and significant social and financial burdens on the patient’s family, school, community, and the health care system.
Recommendations
In the event of a change in clinical response, clinicians first should evaluate adherence and explore other factors, such as biological, psychological, medical, and social issues. Adherence can be adversely affected by a change in the physical characteristics of the pill. Prescribers should remain cognizant of brand–generic and generic–generic switches. It may be reasonable to adjust the dosage of the new generic medication to address changes in clinical effectiveness.
If these strategies are ineffective, consider switching to a brand-name medication. Write “Dispense As Written” on the prescription to ensure delivery of the branded medication or a specific generic version of the medication.
An insurance company might require prior authorization to approve payment for the brand medication. To save time, use electronic forms or fax for communicating with the insurance company. Adding references to FDA statements and research papers, along with the patient’s history and presentations, would be prudent to demonstrate doubts about efficacy of the generic medication.
1. Atif M, Azeem M, Sarwar MR. Potential problems and recommendations regarding substitution of generic antiepileptic drugs: a systematic review of literature. Springerplus. 2016;5:182. doi: 10.1186/s40064-016-1824-2.
2. U.S. Food and Drug Administration. Methylphenidate hydrochloride extended release tablets (generic Concerta) made by Mallinckrodt and Kudco. http://www.fda.gov/Drugs/DrugSafety/ucm422568.htm. Updated November 13, 2014. Accessed August 29, 2016.
3. Lally MD, Kral MC, Boan AD. Not all generic Concerta is created equal: comparison of OROS versus non-OROS for the treatment of ADHD [published online October 14, 2015]. Clin Pediatr (Phila). doi:10.1177/0009922815611647.
4. Italiano DD, Bruno A, Santoro V, et al. Generic olanzapine substitution in patients with schizophrenia: assessment of serum concentrations and therapeutic response after switching. Ther Drug Monit. 2015;37(6):827-830.
5. Borgheini GG. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25(6):1578-1592.
6. Thomas K. F.D.A. delays rule on generic drug labels. http://www.nytimes.com/2016/05/20/business/fda-delays-rule-on-generic-drug-labels.html. Published May 19, 2016. Accessed August 29, 2016.
7. Pae CU, Misra A, Ham BJ, et al. Paroxetine mesylate: comparable to paroxetine hydrochloride? Expert Opin Pharmacother. 2010;11(2):185-193
1. Atif M, Azeem M, Sarwar MR. Potential problems and recommendations regarding substitution of generic antiepileptic drugs: a systematic review of literature. Springerplus. 2016;5:182. doi: 10.1186/s40064-016-1824-2.
2. U.S. Food and Drug Administration. Methylphenidate hydrochloride extended release tablets (generic Concerta) made by Mallinckrodt and Kudco. http://www.fda.gov/Drugs/DrugSafety/ucm422568.htm. Updated November 13, 2014. Accessed August 29, 2016.
3. Lally MD, Kral MC, Boan AD. Not all generic Concerta is created equal: comparison of OROS versus non-OROS for the treatment of ADHD [published online October 14, 2015]. Clin Pediatr (Phila). doi:10.1177/0009922815611647.
4. Italiano DD, Bruno A, Santoro V, et al. Generic olanzapine substitution in patients with schizophrenia: assessment of serum concentrations and therapeutic response after switching. Ther Drug Monit. 2015;37(6):827-830.
5. Borgheini GG. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25(6):1578-1592.
6. Thomas K. F.D.A. delays rule on generic drug labels. http://www.nytimes.com/2016/05/20/business/fda-delays-rule-on-generic-drug-labels.html. Published May 19, 2016. Accessed August 29, 2016.
7. Pae CU, Misra A, Ham BJ, et al. Paroxetine mesylate: comparable to paroxetine hydrochloride? Expert Opin Pharmacother. 2010;11(2):185-193
Pregnant nearly a year? The patient has symptoms but evidence is lacking
Mrs. X, age 43, gravida 4 para 1, is a married woman of sub-Saharan African heritage with a history of idiopathic hypertension, uterine leiomyomas, and multiple spontaneous miscarriages. She has no psychiatric history and had never been evaluated by a mental health professional. Mrs. X is well known to the hospital’s emergency room and obstetrics and gynecology services for several presentations claiming to be pregnant, continuously, over the last 11 months, despite evidence—several negative serum beta human chorionic gonadotropin (ß-hCG) tests and transvaginal sonograms—to the contrary.
Mrs. X reports that after feeling ill for “a few days,” she began to believe that she was “losing [her] mucous plug” and needed urgent evaluation in preparation for the delivery of her “child.” She again is given a ß-hCG test, which is negative, as well as a negative transvaginal sonogram.
Mrs. X’s blood pressure is 220/113 mm Hg, and she emergently receives captopril, 25 mg sublingually, which lowers her systolic blood pressure to 194 mm Hg. The internal medicine team learns that Mrs. X stopped taking her blood pressure medications, lisinopril and hydrochlorothiazide, approximately 2 weeks earlier because she “didn’t want it [the antihypertensive agents] to hurt [her] baby.”
What explains Mrs. X’s belief that she is pregnant?
a) polycystic ovary syndrome (PCOS)
b) delusional disorder
c) bipolar I disorder
d) somatic symptom disorder
The authors’ observations
Pseudocyesis is a psychosomatic condition with an estimated incidence of 1 in 160 maternity admissions in many African countries and 1 in 22,000 in the United States.1 According to DSM-5, pseudocyesis is a false belief of being pregnant along with signs and symptoms of pregnancy.2
Pseudocyesis is more common in:
- developing countries
- areas of low socioeconomic status with minimal education
- societies that place great importance on childbirth
- areas with low access to care.3
The primary presenting symptoms are changes in menses, enlarging abdomen, awareness of fetal movement, enlarged and tender breasts, galactorrhea, and weight gain.4
The exact pathophysiology of the disorder has not been determined, but we believe the psychosomatic hypothesis offers the most compelling explanation. According to this hypothesis, intense social pressures, such as an overwhelming desire to become pregnant because of cultural considerations, personal reasons, or both, could alter the normal function of the hypothalamic-pituitary-ovarian axis,5 which could result in physical manifestations of pregnancy. Tarín et al1 found that rodents with chronic psychosocial stress had decreased brain norepinephrine and dopamine activity and elevated plasma levels of norepinephrine. This can translate to human models, in which a deficit or dysfunction of catecholaminergic activity in the brain could lead to increased pulsatile gonadotropin-releasing hormone, luteinizing hormone (LH), prolactin, and an elevated LH:follicle-stimulating hormone ratio.1 These endocrine changes could induce traits found in most women with pseudocyesis, such as hypomenorrhea or amenorrhea, diurnal or nocturnal hyperprolactinemia (or both), and galactorrhea.1
How would you approach Mrs. X’s care?
a) confront her with the negative pregnancy tests
b) admit her to the inpatient psychiatric unit
c) begin antipsychotic therapy
d) discharge her with outpatient follow-up
EVALUATION A curse on her
Although Mrs. X initially refused to see the psychiatry team, she is more receptive on hospital Day 3. Mrs. X reports that she and her husband had been trying to have a child since they were married 17 years earlier. She had a child with another man before she met her husband, causing her in-laws in Africa to become suspicious that she is intentionally not producing a child for her husband. She had 3 spontaneous abortions since her marriage; these added stress to the relationship because the couple would feel elated when learning of a pregnancy and increasingly devastated with each miscarriage.
Mrs. X reports that she and her husband have been seeing a number of reproductive endocrinologists for 7 years to try to become pregnant. She reports feeling that these physicians are not listening to her or giving her adequate treatment, which is why she has not been able to become pregnant. At the time of the evaluation, she reports that she is pregnant, and the tests have been negative because her mother-in-law placed a “curse” on her. This “curse” caused the baby to be invisible to the laboratory tests and sonograms.
During the psychiatric evaluation, Mrs. X displays her protuberant abdomen and says that she feels the fetus kicking. In addition, she also reports amenorrhea and breast tenderness and engorgement.
During her hospital stay, Mrs. X’s mental status exam does not demonstrate signs or symptoms of a mood disorder, bipolar disorder, or psychosis. Nonetheless, she remains delusional and holds to her fixed false belief of being pregnant. She refuses to be swayed by evidence that she is not pregnant. Despite this, clinicians build enough rapport that Mrs. X agrees to follow up with psychiatry in the outpatient clinic after discharge.
The internal medicine team is apprehensive that Mrs. X will continue to refuse antihypertensive medications out of concern that the medications would harm her pregnancy, as she had in the hospital. She remains hypertensive, with average systolic blood pressure in the 180 to 200 mm Hg range; however, after much discussion with her and her family members, she agrees to try amlodipine, 5 mg/d, a category C drug. She says that she will adhere to the medication if she does not experience any side effects.
Mrs. X is discharged on hospital Day 4 to outpatient follow-up.
The authors’ observations
When considering a diagnosis of pseudocyesis, the condition should be distinguished from others with similar presentations. Before beginning a psychiatric evaluation, a normal pregnancy must be ruled out. This is easily done with a positive urine or serum ß-hCG and an abdominal or transvaginal ultrasound. Pseudocyesis can be differentiated from:
- delusion of pregnancy (sometimes referred to as psychotic pregnancy)—a delusional disorder often seen in psychotic illness without any physical manifestations of pregnancy
- pseudopregnancy (sometimes referred to as erroneous pseudocyesis), another rare condition in which signs and symptoms of pregnancy are manifested1,6,7 but the patient does not have a delusion of pregnancy.
Pseudocyesis, in contrast, comprises the delusion of pregnancy and physical manifestations.2 These distinctions could be difficult to make clinically; for example, an increase in abdominal girth could be a result of pseudocyesis or obesity. In the setting of physical manifestations of pregnancy, a diagnosis of pseudocyesis is more likely (Table1).
Patients with pseudocyesis exhibit subjective and objective findings of pregnancy, such as abdominal distension, enlarged breasts, enhanced pigmentation, lordotic posture, cessation of menses, morning sickness, and weight gain.8,9 Furthermore, approximately 1% of pseudocyesis patients have false labor, as Mrs. X did.10 Typically, the duration of these symptoms range from a few weeks to 9 months. In some cases, symptoms can last longer11; at admission, Mrs. X reported that she was 11 months pregnant. She saw nothing wrong with this assertion, despite knowing that human gestation lasts 9 months.
In delusion of pregnancy, a patient might exhibit abdominal distension and cessation of menses but have no other objective findings of pregnancy.7 Rather than being a somatoform disorder such as pseudocyesis, a delusion of pregnancy is a symptom of psychosis or, rarely, dementia.12
Pseudopregnancy is a somatic state resembling pregnancy that can arise from a variety of medical conditions. A full medical workup and intensive mental status and cognitive evaluation are necessary for diagnostic clarity. Although the pathology and workup of delusional pregnancy is beyond the scope of this article, we suggest Seeman13 for a review and Chatterjee et al14 and Tarín et al1 for guidance on making the diagnosis.
Theories about pathophysiology
As with many psychosomatic conditions, the pathological process of pseudocyesis originally was thought of in a psychodynamic context. Several psychodynamic theories have been proposed, including instances in which the internal desire to be pregnant is strong enough to induce a series of physiological changes akin to the state of pregnancy.6
Other examiners of pseudocyesis have noted its development from fears and societal pressure, including the loss of companionship or “womanhood.”6,9 Last, the tenuous interplay of desire for a child and substantial fear of pregnancy appears to play a role in many cases.9-11 Rosenberg et al15 reported on a teenager with pseudocyesis who desired to be pregnant to appease her husband and family, but feared pregnancy and the implications of having a child at such a young age. As this team wrote, “this pregnancy sans child fulfilled the needs of the entire family, at least temporarily.”15
Prevailing modern theories behind the somatic presentations of these patients hinge on an imbalance of the hypothalamic-pituitary-adrenal axis.9 Although this remains the area of ongoing research, most literature has not shown a consistent change or trend in laboratory levels of hormones associated with pseudocyesis.16 Tarín et al,1 however, did show a similar hormonal profile between patients with pseudocyesis and those with PCOS. Although urine or serum pregnancy testing and ultrasonography are indicated to rule out pseudopregnancy, we see no benefit in obtaining other lab work in most cases beyond that of a general medical workup, because such evaluations are not helpful in diagnosis or treatment.
Mrs. X’s abdomen was protuberant and she displayed the typical linea nigra of pregnancy. Many authors have theorized the physiological mechanism behind the abdominal enlargement to include contraction of the diaphragm, which reduces the abdominal cavity and forces the bowel outwards. As abdominal fat increases, the patient becomes constipated, and the bowel becomes distended.10,16 Although the cause of our patient’s abdominal enlargement was not pursued, we note that the literature reported that the abdominal enlargement disappears when the patient is under general anesthesia.10,16,17
Characteristics of pseudocyesis
Bivin and Klinger’s 1937 compilation of >400 cases of pseudocyesis over nearly 200 years remains a landmark in the study of this condition.18 In their analysis, patients range in age from 20 to 44; >75% were married. The authors noted that many of the women they studied had borne children previously. Further social and psychological studies came from this breakthrough article, which shed light on the dynamics of pseudocyesis in many patients with the condition.
According to Koic,11 pseudocyesis is a form of conversion disorder with underlying depression. This theory is based on literature reports of patients displaying similar personal, cultural, and social factors. These similarities, although not comprehensive, are paramount in both the diagnosis and treatment of this condition.
Often, pseudocyesis presents in patients with lower education and socioeconomic status.1,3,11 This is particularly true in developing nations in sub-Saharan Africa and the Indian subcontinent. Case reports, cross-sectional, and longitudinal studies from these developing nations in particular note the extremely high stress placed on women to produce children for their husbands and family in male-dominated society; it is common for a woman to be rejected by her husband and family if she is unable to reproduce.3
The effect of a lower level of education on development of pseudocyesis appears to be multifactorial:
- Lack of understanding of the human body and reproductive health can lead to misperception of signs of pregnancy and bodily changes
- Low education correlates with poor earnings and worse prenatal care; delayed or no prenatal care also has been associated with an increased incidence of pseudocyesis.3
In Ouj’s study of pseudocyesis in Nigeria, the author postulated that an educated woman does not endure the same stress of fertility as an uneducated woman; she is already respected in her society and will not be rejected if she does not have children.3
Mrs. X’s ethnic background and continued close ties with sub-Saharan Africa are notable: Her background is one that is typically associated with pseudocyesis. She is from an developing country, did not complete higher education, was ostracized by her mother-in-law because of her inability to conceive, and was told several times, during her visits to Ghana, that she was indeed pregnant.
Mrs. X noted a strong desire to conceive for her husband and family and carried with her perhaps an even stronger fear of loss of marriage and female identity—which has been bolstered by the importance placed on the woman’s raison d’être in the family by her cultural upbringing.3,6,9-11,15 What Mrs. X never made clear, however, was whether she wanted another child at her age and in the setting of having many friends and rewarding full-time employment.
Epidemiology of pseudocyesis worldwide has been evaluated in a handful of studies. As compiled by Cohen,8 the prevalence of pseudocyesis in Boston, Massachusetts, was 1/22,000 births, whereas it was dramatically higher in Sudan (1/160 women who had previously been managed for reproductive failure).1 This discrepancy in prevalance is consistent with current theories on patient characteristics that lead to increased incidence of pseudocyesis in underdeveloped nations. A 1951 study at an academic hospital in Philadelphia, Pennsylvania, noted 27 cases of pseudocyesis in maternity admissions during the study period—an incidence of 1 in 250.19 Of note, 85% of cases were of African American heritage; in 89% of cases, the woman had been trying to conceive for as long as 17 years.
Avoiding confrontation
Initially, Mrs. X was resistant to talking with a psychiatrist; this is consistent with studies showing that a patient can be suspicious and even hostile when a clinician attempts to engage her in mental health treatment.10,16 The patient interprets the physical sensations she experiences during pseudocyesis, for example, as a real pregnancy, a perception that is contradicted by medical testing.
It is important to understand this conflict and to avoid confronting the patient directly about false beliefs; confrontation has been shown to be detrimental to patient recovery. Instead, offer the patient alternatives to her symptoms (ie, sensations of abdominal movement also can be caused by indigestion), while not directly discounting her experiences.6,9 Indeed, from early on in the study of pseudocyesis, there have been many reports of resolution of symptoms when the physician helped the patient understand that she is not pregnant.20,21
OUTCOME Supportive therapy
Mrs. X is seen for outpatient psychiatry follow-up several weeks after hospitalization. She acknowledges that, although she still thought pregnancy is possible, she is willing to entertain the idea that there could be another medical explanation for her symptoms.
Mrs. X is provided with supportive therapy techniques, and her marital and societal stressors are discussed. Psychotropic medications are considered, but eventually deemed unnecessary; the treatment team is concerned that Mrs. X, who remains wary of mental health providers, would view the offer of medication as offensive.
Mrs. X is seen in the gynecology clinic approximately 2 weeks later; there, a diagnosis of secondary anovulation is made and a workup for PCOS initiated.
Subsequent review of the medical record states that, during further follow-up with gynecology, Mrs. X no longer believes that she is pregnant.
1. Tarín JJ, Hermenegildo C, García-Pérez MA, et al. Endocrinology and physiology of pseudocyesis. Reprod Biol Endocrinol. 2013;11:39.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Ouj U. Pseudocyesis in a rural southeast Nigerian community. J Obstet Gynaecol Res. 2009;35(4):660-665.
4. Signer SF, Weinstein RP, Munoz RA, et al. Pseudocyesis in organic mood disorders. Six cases. Psychosomatics. 1992;33(3):316-323.
5. Omer H, Elizur Y, Barnea T, et al. Psychological variables and premature labour: a possible solution for some methodological problems. J Psychosom Res. 1986;30(5):559-565.
6. Starkman MN, Marshall JC, La Ferla J, et al. Pseudocyesis: psychologic and neuroendocrine interrelationships. Psychosom Med. 1985;47(1):46-57.
7. Yadav T, Balhara YP, Kataria DK. Pseudocyesis versus delusion of pregnancy: differential diagnoses to be kept in mind. Indian J Psychol Med. 2012;34(1):82-84.
8. Cohen LM. A current perspective of pseudocyesis. Am J Psychiatry. 1982;139(9):1140-1144.
9. Brown E, Barglow P. Pseudocyesis. A paradigm for psychophysiological interactions. Arch Gen Psychiatry. 1971;24(3):221-229.
10. Small GW. Pseudocyesis: an overview. Can J Psychiatry. 1986;31(5):452-457.
11. Koi´c E, Mu´zin´c L, Đordevic V, et al. Pseudocyesis and couvade syndrome. Drustvena Istrazivanja. 2002;11:1031-1047.
12. Bhattacharyya S, Chaturvedi SK. Metamorphosis of delusion of pregnancy. Can J Psychiatry. 2001;46(6):561-562.
13. Seeman MV. Pseudocyesis, delusional pregnancy, and psychosis: the birth of a delusion. World J Clin Cases. 2014;2(8):338-344.
14. Chatterjee SS, Nath N, Dasgupta G, et al. Delusion of pregnancy and other pregnancy-mimicking conditions: dissecting through differential diagnosis. Medical Journal of Dr. D.Y. Patil University. 2014;7(3):369-372.
15. Rosenberg HK, Coleman BG, Croop J, et al. Pseudocyesis in an adolescent patient. Clin Pediatr (Phila). 1983;22(10):708-712.
16. O’Grady JP, Rosenthal M. Pseudocyesis: a modern perspective on an old disorder. Obstet Gynecol Surv. 1989;44(7):500-511.
17. Whelan CI, Stewart DE. Pseudocyesis–a review and report of six cases. Int J Psychiatry Med. 1990;20(1):97-108.
18. Bivin GD, Klinger MP. Pseudocyesis. Bloomington, IN: Principia Press; 1937.
19. Fried PH, Rakoff AE, Schopbach RR, et al. Pseudocyesis; a psychosomatic study in gynecology. J Am Med Assoc. 1951;145(17):1329-1335.
20. Dunbar F. Emotions and bodily changes. 3rd ed. New York, NY: Columbia University Press; 1947.
21. Steinberg A, Pastor N, Winheld EB, et al. Psychoendocrine relationship in pseudocyesis. Psychosom Med. 1946;8(3):176-179.
Mrs. X, age 43, gravida 4 para 1, is a married woman of sub-Saharan African heritage with a history of idiopathic hypertension, uterine leiomyomas, and multiple spontaneous miscarriages. She has no psychiatric history and had never been evaluated by a mental health professional. Mrs. X is well known to the hospital’s emergency room and obstetrics and gynecology services for several presentations claiming to be pregnant, continuously, over the last 11 months, despite evidence—several negative serum beta human chorionic gonadotropin (ß-hCG) tests and transvaginal sonograms—to the contrary.
Mrs. X reports that after feeling ill for “a few days,” she began to believe that she was “losing [her] mucous plug” and needed urgent evaluation in preparation for the delivery of her “child.” She again is given a ß-hCG test, which is negative, as well as a negative transvaginal sonogram.
Mrs. X’s blood pressure is 220/113 mm Hg, and she emergently receives captopril, 25 mg sublingually, which lowers her systolic blood pressure to 194 mm Hg. The internal medicine team learns that Mrs. X stopped taking her blood pressure medications, lisinopril and hydrochlorothiazide, approximately 2 weeks earlier because she “didn’t want it [the antihypertensive agents] to hurt [her] baby.”
What explains Mrs. X’s belief that she is pregnant?
a) polycystic ovary syndrome (PCOS)
b) delusional disorder
c) bipolar I disorder
d) somatic symptom disorder
The authors’ observations
Pseudocyesis is a psychosomatic condition with an estimated incidence of 1 in 160 maternity admissions in many African countries and 1 in 22,000 in the United States.1 According to DSM-5, pseudocyesis is a false belief of being pregnant along with signs and symptoms of pregnancy.2
Pseudocyesis is more common in:
- developing countries
- areas of low socioeconomic status with minimal education
- societies that place great importance on childbirth
- areas with low access to care.3
The primary presenting symptoms are changes in menses, enlarging abdomen, awareness of fetal movement, enlarged and tender breasts, galactorrhea, and weight gain.4
The exact pathophysiology of the disorder has not been determined, but we believe the psychosomatic hypothesis offers the most compelling explanation. According to this hypothesis, intense social pressures, such as an overwhelming desire to become pregnant because of cultural considerations, personal reasons, or both, could alter the normal function of the hypothalamic-pituitary-ovarian axis,5 which could result in physical manifestations of pregnancy. Tarín et al1 found that rodents with chronic psychosocial stress had decreased brain norepinephrine and dopamine activity and elevated plasma levels of norepinephrine. This can translate to human models, in which a deficit or dysfunction of catecholaminergic activity in the brain could lead to increased pulsatile gonadotropin-releasing hormone, luteinizing hormone (LH), prolactin, and an elevated LH:follicle-stimulating hormone ratio.1 These endocrine changes could induce traits found in most women with pseudocyesis, such as hypomenorrhea or amenorrhea, diurnal or nocturnal hyperprolactinemia (or both), and galactorrhea.1
How would you approach Mrs. X’s care?
a) confront her with the negative pregnancy tests
b) admit her to the inpatient psychiatric unit
c) begin antipsychotic therapy
d) discharge her with outpatient follow-up
EVALUATION A curse on her
Although Mrs. X initially refused to see the psychiatry team, she is more receptive on hospital Day 3. Mrs. X reports that she and her husband had been trying to have a child since they were married 17 years earlier. She had a child with another man before she met her husband, causing her in-laws in Africa to become suspicious that she is intentionally not producing a child for her husband. She had 3 spontaneous abortions since her marriage; these added stress to the relationship because the couple would feel elated when learning of a pregnancy and increasingly devastated with each miscarriage.
Mrs. X reports that she and her husband have been seeing a number of reproductive endocrinologists for 7 years to try to become pregnant. She reports feeling that these physicians are not listening to her or giving her adequate treatment, which is why she has not been able to become pregnant. At the time of the evaluation, she reports that she is pregnant, and the tests have been negative because her mother-in-law placed a “curse” on her. This “curse” caused the baby to be invisible to the laboratory tests and sonograms.
During the psychiatric evaluation, Mrs. X displays her protuberant abdomen and says that she feels the fetus kicking. In addition, she also reports amenorrhea and breast tenderness and engorgement.
During her hospital stay, Mrs. X’s mental status exam does not demonstrate signs or symptoms of a mood disorder, bipolar disorder, or psychosis. Nonetheless, she remains delusional and holds to her fixed false belief of being pregnant. She refuses to be swayed by evidence that she is not pregnant. Despite this, clinicians build enough rapport that Mrs. X agrees to follow up with psychiatry in the outpatient clinic after discharge.
The internal medicine team is apprehensive that Mrs. X will continue to refuse antihypertensive medications out of concern that the medications would harm her pregnancy, as she had in the hospital. She remains hypertensive, with average systolic blood pressure in the 180 to 200 mm Hg range; however, after much discussion with her and her family members, she agrees to try amlodipine, 5 mg/d, a category C drug. She says that she will adhere to the medication if she does not experience any side effects.
Mrs. X is discharged on hospital Day 4 to outpatient follow-up.
The authors’ observations
When considering a diagnosis of pseudocyesis, the condition should be distinguished from others with similar presentations. Before beginning a psychiatric evaluation, a normal pregnancy must be ruled out. This is easily done with a positive urine or serum ß-hCG and an abdominal or transvaginal ultrasound. Pseudocyesis can be differentiated from:
- delusion of pregnancy (sometimes referred to as psychotic pregnancy)—a delusional disorder often seen in psychotic illness without any physical manifestations of pregnancy
- pseudopregnancy (sometimes referred to as erroneous pseudocyesis), another rare condition in which signs and symptoms of pregnancy are manifested1,6,7 but the patient does not have a delusion of pregnancy.
Pseudocyesis, in contrast, comprises the delusion of pregnancy and physical manifestations.2 These distinctions could be difficult to make clinically; for example, an increase in abdominal girth could be a result of pseudocyesis or obesity. In the setting of physical manifestations of pregnancy, a diagnosis of pseudocyesis is more likely (Table1).
Patients with pseudocyesis exhibit subjective and objective findings of pregnancy, such as abdominal distension, enlarged breasts, enhanced pigmentation, lordotic posture, cessation of menses, morning sickness, and weight gain.8,9 Furthermore, approximately 1% of pseudocyesis patients have false labor, as Mrs. X did.10 Typically, the duration of these symptoms range from a few weeks to 9 months. In some cases, symptoms can last longer11; at admission, Mrs. X reported that she was 11 months pregnant. She saw nothing wrong with this assertion, despite knowing that human gestation lasts 9 months.
In delusion of pregnancy, a patient might exhibit abdominal distension and cessation of menses but have no other objective findings of pregnancy.7 Rather than being a somatoform disorder such as pseudocyesis, a delusion of pregnancy is a symptom of psychosis or, rarely, dementia.12
Pseudopregnancy is a somatic state resembling pregnancy that can arise from a variety of medical conditions. A full medical workup and intensive mental status and cognitive evaluation are necessary for diagnostic clarity. Although the pathology and workup of delusional pregnancy is beyond the scope of this article, we suggest Seeman13 for a review and Chatterjee et al14 and Tarín et al1 for guidance on making the diagnosis.
Theories about pathophysiology
As with many psychosomatic conditions, the pathological process of pseudocyesis originally was thought of in a psychodynamic context. Several psychodynamic theories have been proposed, including instances in which the internal desire to be pregnant is strong enough to induce a series of physiological changes akin to the state of pregnancy.6
Other examiners of pseudocyesis have noted its development from fears and societal pressure, including the loss of companionship or “womanhood.”6,9 Last, the tenuous interplay of desire for a child and substantial fear of pregnancy appears to play a role in many cases.9-11 Rosenberg et al15 reported on a teenager with pseudocyesis who desired to be pregnant to appease her husband and family, but feared pregnancy and the implications of having a child at such a young age. As this team wrote, “this pregnancy sans child fulfilled the needs of the entire family, at least temporarily.”15
Prevailing modern theories behind the somatic presentations of these patients hinge on an imbalance of the hypothalamic-pituitary-adrenal axis.9 Although this remains the area of ongoing research, most literature has not shown a consistent change or trend in laboratory levels of hormones associated with pseudocyesis.16 Tarín et al,1 however, did show a similar hormonal profile between patients with pseudocyesis and those with PCOS. Although urine or serum pregnancy testing and ultrasonography are indicated to rule out pseudopregnancy, we see no benefit in obtaining other lab work in most cases beyond that of a general medical workup, because such evaluations are not helpful in diagnosis or treatment.
Mrs. X’s abdomen was protuberant and she displayed the typical linea nigra of pregnancy. Many authors have theorized the physiological mechanism behind the abdominal enlargement to include contraction of the diaphragm, which reduces the abdominal cavity and forces the bowel outwards. As abdominal fat increases, the patient becomes constipated, and the bowel becomes distended.10,16 Although the cause of our patient’s abdominal enlargement was not pursued, we note that the literature reported that the abdominal enlargement disappears when the patient is under general anesthesia.10,16,17
Characteristics of pseudocyesis
Bivin and Klinger’s 1937 compilation of >400 cases of pseudocyesis over nearly 200 years remains a landmark in the study of this condition.18 In their analysis, patients range in age from 20 to 44; >75% were married. The authors noted that many of the women they studied had borne children previously. Further social and psychological studies came from this breakthrough article, which shed light on the dynamics of pseudocyesis in many patients with the condition.
According to Koic,11 pseudocyesis is a form of conversion disorder with underlying depression. This theory is based on literature reports of patients displaying similar personal, cultural, and social factors. These similarities, although not comprehensive, are paramount in both the diagnosis and treatment of this condition.
Often, pseudocyesis presents in patients with lower education and socioeconomic status.1,3,11 This is particularly true in developing nations in sub-Saharan Africa and the Indian subcontinent. Case reports, cross-sectional, and longitudinal studies from these developing nations in particular note the extremely high stress placed on women to produce children for their husbands and family in male-dominated society; it is common for a woman to be rejected by her husband and family if she is unable to reproduce.3
The effect of a lower level of education on development of pseudocyesis appears to be multifactorial:
- Lack of understanding of the human body and reproductive health can lead to misperception of signs of pregnancy and bodily changes
- Low education correlates with poor earnings and worse prenatal care; delayed or no prenatal care also has been associated with an increased incidence of pseudocyesis.3
In Ouj’s study of pseudocyesis in Nigeria, the author postulated that an educated woman does not endure the same stress of fertility as an uneducated woman; she is already respected in her society and will not be rejected if she does not have children.3
Mrs. X’s ethnic background and continued close ties with sub-Saharan Africa are notable: Her background is one that is typically associated with pseudocyesis. She is from an developing country, did not complete higher education, was ostracized by her mother-in-law because of her inability to conceive, and was told several times, during her visits to Ghana, that she was indeed pregnant.
Mrs. X noted a strong desire to conceive for her husband and family and carried with her perhaps an even stronger fear of loss of marriage and female identity—which has been bolstered by the importance placed on the woman’s raison d’être in the family by her cultural upbringing.3,6,9-11,15 What Mrs. X never made clear, however, was whether she wanted another child at her age and in the setting of having many friends and rewarding full-time employment.
Epidemiology of pseudocyesis worldwide has been evaluated in a handful of studies. As compiled by Cohen,8 the prevalence of pseudocyesis in Boston, Massachusetts, was 1/22,000 births, whereas it was dramatically higher in Sudan (1/160 women who had previously been managed for reproductive failure).1 This discrepancy in prevalance is consistent with current theories on patient characteristics that lead to increased incidence of pseudocyesis in underdeveloped nations. A 1951 study at an academic hospital in Philadelphia, Pennsylvania, noted 27 cases of pseudocyesis in maternity admissions during the study period—an incidence of 1 in 250.19 Of note, 85% of cases were of African American heritage; in 89% of cases, the woman had been trying to conceive for as long as 17 years.
Avoiding confrontation
Initially, Mrs. X was resistant to talking with a psychiatrist; this is consistent with studies showing that a patient can be suspicious and even hostile when a clinician attempts to engage her in mental health treatment.10,16 The patient interprets the physical sensations she experiences during pseudocyesis, for example, as a real pregnancy, a perception that is contradicted by medical testing.
It is important to understand this conflict and to avoid confronting the patient directly about false beliefs; confrontation has been shown to be detrimental to patient recovery. Instead, offer the patient alternatives to her symptoms (ie, sensations of abdominal movement also can be caused by indigestion), while not directly discounting her experiences.6,9 Indeed, from early on in the study of pseudocyesis, there have been many reports of resolution of symptoms when the physician helped the patient understand that she is not pregnant.20,21
OUTCOME Supportive therapy
Mrs. X is seen for outpatient psychiatry follow-up several weeks after hospitalization. She acknowledges that, although she still thought pregnancy is possible, she is willing to entertain the idea that there could be another medical explanation for her symptoms.
Mrs. X is provided with supportive therapy techniques, and her marital and societal stressors are discussed. Psychotropic medications are considered, but eventually deemed unnecessary; the treatment team is concerned that Mrs. X, who remains wary of mental health providers, would view the offer of medication as offensive.
Mrs. X is seen in the gynecology clinic approximately 2 weeks later; there, a diagnosis of secondary anovulation is made and a workup for PCOS initiated.
Subsequent review of the medical record states that, during further follow-up with gynecology, Mrs. X no longer believes that she is pregnant.
Mrs. X, age 43, gravida 4 para 1, is a married woman of sub-Saharan African heritage with a history of idiopathic hypertension, uterine leiomyomas, and multiple spontaneous miscarriages. She has no psychiatric history and had never been evaluated by a mental health professional. Mrs. X is well known to the hospital’s emergency room and obstetrics and gynecology services for several presentations claiming to be pregnant, continuously, over the last 11 months, despite evidence—several negative serum beta human chorionic gonadotropin (ß-hCG) tests and transvaginal sonograms—to the contrary.
Mrs. X reports that after feeling ill for “a few days,” she began to believe that she was “losing [her] mucous plug” and needed urgent evaluation in preparation for the delivery of her “child.” She again is given a ß-hCG test, which is negative, as well as a negative transvaginal sonogram.
Mrs. X’s blood pressure is 220/113 mm Hg, and she emergently receives captopril, 25 mg sublingually, which lowers her systolic blood pressure to 194 mm Hg. The internal medicine team learns that Mrs. X stopped taking her blood pressure medications, lisinopril and hydrochlorothiazide, approximately 2 weeks earlier because she “didn’t want it [the antihypertensive agents] to hurt [her] baby.”
What explains Mrs. X’s belief that she is pregnant?
a) polycystic ovary syndrome (PCOS)
b) delusional disorder
c) bipolar I disorder
d) somatic symptom disorder
The authors’ observations
Pseudocyesis is a psychosomatic condition with an estimated incidence of 1 in 160 maternity admissions in many African countries and 1 in 22,000 in the United States.1 According to DSM-5, pseudocyesis is a false belief of being pregnant along with signs and symptoms of pregnancy.2
Pseudocyesis is more common in:
- developing countries
- areas of low socioeconomic status with minimal education
- societies that place great importance on childbirth
- areas with low access to care.3
The primary presenting symptoms are changes in menses, enlarging abdomen, awareness of fetal movement, enlarged and tender breasts, galactorrhea, and weight gain.4
The exact pathophysiology of the disorder has not been determined, but we believe the psychosomatic hypothesis offers the most compelling explanation. According to this hypothesis, intense social pressures, such as an overwhelming desire to become pregnant because of cultural considerations, personal reasons, or both, could alter the normal function of the hypothalamic-pituitary-ovarian axis,5 which could result in physical manifestations of pregnancy. Tarín et al1 found that rodents with chronic psychosocial stress had decreased brain norepinephrine and dopamine activity and elevated plasma levels of norepinephrine. This can translate to human models, in which a deficit or dysfunction of catecholaminergic activity in the brain could lead to increased pulsatile gonadotropin-releasing hormone, luteinizing hormone (LH), prolactin, and an elevated LH:follicle-stimulating hormone ratio.1 These endocrine changes could induce traits found in most women with pseudocyesis, such as hypomenorrhea or amenorrhea, diurnal or nocturnal hyperprolactinemia (or both), and galactorrhea.1
How would you approach Mrs. X’s care?
a) confront her with the negative pregnancy tests
b) admit her to the inpatient psychiatric unit
c) begin antipsychotic therapy
d) discharge her with outpatient follow-up
EVALUATION A curse on her
Although Mrs. X initially refused to see the psychiatry team, she is more receptive on hospital Day 3. Mrs. X reports that she and her husband had been trying to have a child since they were married 17 years earlier. She had a child with another man before she met her husband, causing her in-laws in Africa to become suspicious that she is intentionally not producing a child for her husband. She had 3 spontaneous abortions since her marriage; these added stress to the relationship because the couple would feel elated when learning of a pregnancy and increasingly devastated with each miscarriage.
Mrs. X reports that she and her husband have been seeing a number of reproductive endocrinologists for 7 years to try to become pregnant. She reports feeling that these physicians are not listening to her or giving her adequate treatment, which is why she has not been able to become pregnant. At the time of the evaluation, she reports that she is pregnant, and the tests have been negative because her mother-in-law placed a “curse” on her. This “curse” caused the baby to be invisible to the laboratory tests and sonograms.
During the psychiatric evaluation, Mrs. X displays her protuberant abdomen and says that she feels the fetus kicking. In addition, she also reports amenorrhea and breast tenderness and engorgement.
During her hospital stay, Mrs. X’s mental status exam does not demonstrate signs or symptoms of a mood disorder, bipolar disorder, or psychosis. Nonetheless, she remains delusional and holds to her fixed false belief of being pregnant. She refuses to be swayed by evidence that she is not pregnant. Despite this, clinicians build enough rapport that Mrs. X agrees to follow up with psychiatry in the outpatient clinic after discharge.
The internal medicine team is apprehensive that Mrs. X will continue to refuse antihypertensive medications out of concern that the medications would harm her pregnancy, as she had in the hospital. She remains hypertensive, with average systolic blood pressure in the 180 to 200 mm Hg range; however, after much discussion with her and her family members, she agrees to try amlodipine, 5 mg/d, a category C drug. She says that she will adhere to the medication if she does not experience any side effects.
Mrs. X is discharged on hospital Day 4 to outpatient follow-up.
The authors’ observations
When considering a diagnosis of pseudocyesis, the condition should be distinguished from others with similar presentations. Before beginning a psychiatric evaluation, a normal pregnancy must be ruled out. This is easily done with a positive urine or serum ß-hCG and an abdominal or transvaginal ultrasound. Pseudocyesis can be differentiated from:
- delusion of pregnancy (sometimes referred to as psychotic pregnancy)—a delusional disorder often seen in psychotic illness without any physical manifestations of pregnancy
- pseudopregnancy (sometimes referred to as erroneous pseudocyesis), another rare condition in which signs and symptoms of pregnancy are manifested1,6,7 but the patient does not have a delusion of pregnancy.
Pseudocyesis, in contrast, comprises the delusion of pregnancy and physical manifestations.2 These distinctions could be difficult to make clinically; for example, an increase in abdominal girth could be a result of pseudocyesis or obesity. In the setting of physical manifestations of pregnancy, a diagnosis of pseudocyesis is more likely (Table1).
Patients with pseudocyesis exhibit subjective and objective findings of pregnancy, such as abdominal distension, enlarged breasts, enhanced pigmentation, lordotic posture, cessation of menses, morning sickness, and weight gain.8,9 Furthermore, approximately 1% of pseudocyesis patients have false labor, as Mrs. X did.10 Typically, the duration of these symptoms range from a few weeks to 9 months. In some cases, symptoms can last longer11; at admission, Mrs. X reported that she was 11 months pregnant. She saw nothing wrong with this assertion, despite knowing that human gestation lasts 9 months.
In delusion of pregnancy, a patient might exhibit abdominal distension and cessation of menses but have no other objective findings of pregnancy.7 Rather than being a somatoform disorder such as pseudocyesis, a delusion of pregnancy is a symptom of psychosis or, rarely, dementia.12
Pseudopregnancy is a somatic state resembling pregnancy that can arise from a variety of medical conditions. A full medical workup and intensive mental status and cognitive evaluation are necessary for diagnostic clarity. Although the pathology and workup of delusional pregnancy is beyond the scope of this article, we suggest Seeman13 for a review and Chatterjee et al14 and Tarín et al1 for guidance on making the diagnosis.
Theories about pathophysiology
As with many psychosomatic conditions, the pathological process of pseudocyesis originally was thought of in a psychodynamic context. Several psychodynamic theories have been proposed, including instances in which the internal desire to be pregnant is strong enough to induce a series of physiological changes akin to the state of pregnancy.6
Other examiners of pseudocyesis have noted its development from fears and societal pressure, including the loss of companionship or “womanhood.”6,9 Last, the tenuous interplay of desire for a child and substantial fear of pregnancy appears to play a role in many cases.9-11 Rosenberg et al15 reported on a teenager with pseudocyesis who desired to be pregnant to appease her husband and family, but feared pregnancy and the implications of having a child at such a young age. As this team wrote, “this pregnancy sans child fulfilled the needs of the entire family, at least temporarily.”15
Prevailing modern theories behind the somatic presentations of these patients hinge on an imbalance of the hypothalamic-pituitary-adrenal axis.9 Although this remains the area of ongoing research, most literature has not shown a consistent change or trend in laboratory levels of hormones associated with pseudocyesis.16 Tarín et al,1 however, did show a similar hormonal profile between patients with pseudocyesis and those with PCOS. Although urine or serum pregnancy testing and ultrasonography are indicated to rule out pseudopregnancy, we see no benefit in obtaining other lab work in most cases beyond that of a general medical workup, because such evaluations are not helpful in diagnosis or treatment.
Mrs. X’s abdomen was protuberant and she displayed the typical linea nigra of pregnancy. Many authors have theorized the physiological mechanism behind the abdominal enlargement to include contraction of the diaphragm, which reduces the abdominal cavity and forces the bowel outwards. As abdominal fat increases, the patient becomes constipated, and the bowel becomes distended.10,16 Although the cause of our patient’s abdominal enlargement was not pursued, we note that the literature reported that the abdominal enlargement disappears when the patient is under general anesthesia.10,16,17
Characteristics of pseudocyesis
Bivin and Klinger’s 1937 compilation of >400 cases of pseudocyesis over nearly 200 years remains a landmark in the study of this condition.18 In their analysis, patients range in age from 20 to 44; >75% were married. The authors noted that many of the women they studied had borne children previously. Further social and psychological studies came from this breakthrough article, which shed light on the dynamics of pseudocyesis in many patients with the condition.
According to Koic,11 pseudocyesis is a form of conversion disorder with underlying depression. This theory is based on literature reports of patients displaying similar personal, cultural, and social factors. These similarities, although not comprehensive, are paramount in both the diagnosis and treatment of this condition.
Often, pseudocyesis presents in patients with lower education and socioeconomic status.1,3,11 This is particularly true in developing nations in sub-Saharan Africa and the Indian subcontinent. Case reports, cross-sectional, and longitudinal studies from these developing nations in particular note the extremely high stress placed on women to produce children for their husbands and family in male-dominated society; it is common for a woman to be rejected by her husband and family if she is unable to reproduce.3
The effect of a lower level of education on development of pseudocyesis appears to be multifactorial:
- Lack of understanding of the human body and reproductive health can lead to misperception of signs of pregnancy and bodily changes
- Low education correlates with poor earnings and worse prenatal care; delayed or no prenatal care also has been associated with an increased incidence of pseudocyesis.3
In Ouj’s study of pseudocyesis in Nigeria, the author postulated that an educated woman does not endure the same stress of fertility as an uneducated woman; she is already respected in her society and will not be rejected if she does not have children.3
Mrs. X’s ethnic background and continued close ties with sub-Saharan Africa are notable: Her background is one that is typically associated with pseudocyesis. She is from an developing country, did not complete higher education, was ostracized by her mother-in-law because of her inability to conceive, and was told several times, during her visits to Ghana, that she was indeed pregnant.
Mrs. X noted a strong desire to conceive for her husband and family and carried with her perhaps an even stronger fear of loss of marriage and female identity—which has been bolstered by the importance placed on the woman’s raison d’être in the family by her cultural upbringing.3,6,9-11,15 What Mrs. X never made clear, however, was whether she wanted another child at her age and in the setting of having many friends and rewarding full-time employment.
Epidemiology of pseudocyesis worldwide has been evaluated in a handful of studies. As compiled by Cohen,8 the prevalence of pseudocyesis in Boston, Massachusetts, was 1/22,000 births, whereas it was dramatically higher in Sudan (1/160 women who had previously been managed for reproductive failure).1 This discrepancy in prevalance is consistent with current theories on patient characteristics that lead to increased incidence of pseudocyesis in underdeveloped nations. A 1951 study at an academic hospital in Philadelphia, Pennsylvania, noted 27 cases of pseudocyesis in maternity admissions during the study period—an incidence of 1 in 250.19 Of note, 85% of cases were of African American heritage; in 89% of cases, the woman had been trying to conceive for as long as 17 years.
Avoiding confrontation
Initially, Mrs. X was resistant to talking with a psychiatrist; this is consistent with studies showing that a patient can be suspicious and even hostile when a clinician attempts to engage her in mental health treatment.10,16 The patient interprets the physical sensations she experiences during pseudocyesis, for example, as a real pregnancy, a perception that is contradicted by medical testing.
It is important to understand this conflict and to avoid confronting the patient directly about false beliefs; confrontation has been shown to be detrimental to patient recovery. Instead, offer the patient alternatives to her symptoms (ie, sensations of abdominal movement also can be caused by indigestion), while not directly discounting her experiences.6,9 Indeed, from early on in the study of pseudocyesis, there have been many reports of resolution of symptoms when the physician helped the patient understand that she is not pregnant.20,21
OUTCOME Supportive therapy
Mrs. X is seen for outpatient psychiatry follow-up several weeks after hospitalization. She acknowledges that, although she still thought pregnancy is possible, she is willing to entertain the idea that there could be another medical explanation for her symptoms.
Mrs. X is provided with supportive therapy techniques, and her marital and societal stressors are discussed. Psychotropic medications are considered, but eventually deemed unnecessary; the treatment team is concerned that Mrs. X, who remains wary of mental health providers, would view the offer of medication as offensive.
Mrs. X is seen in the gynecology clinic approximately 2 weeks later; there, a diagnosis of secondary anovulation is made and a workup for PCOS initiated.
Subsequent review of the medical record states that, during further follow-up with gynecology, Mrs. X no longer believes that she is pregnant.
1. Tarín JJ, Hermenegildo C, García-Pérez MA, et al. Endocrinology and physiology of pseudocyesis. Reprod Biol Endocrinol. 2013;11:39.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Ouj U. Pseudocyesis in a rural southeast Nigerian community. J Obstet Gynaecol Res. 2009;35(4):660-665.
4. Signer SF, Weinstein RP, Munoz RA, et al. Pseudocyesis in organic mood disorders. Six cases. Psychosomatics. 1992;33(3):316-323.
5. Omer H, Elizur Y, Barnea T, et al. Psychological variables and premature labour: a possible solution for some methodological problems. J Psychosom Res. 1986;30(5):559-565.
6. Starkman MN, Marshall JC, La Ferla J, et al. Pseudocyesis: psychologic and neuroendocrine interrelationships. Psychosom Med. 1985;47(1):46-57.
7. Yadav T, Balhara YP, Kataria DK. Pseudocyesis versus delusion of pregnancy: differential diagnoses to be kept in mind. Indian J Psychol Med. 2012;34(1):82-84.
8. Cohen LM. A current perspective of pseudocyesis. Am J Psychiatry. 1982;139(9):1140-1144.
9. Brown E, Barglow P. Pseudocyesis. A paradigm for psychophysiological interactions. Arch Gen Psychiatry. 1971;24(3):221-229.
10. Small GW. Pseudocyesis: an overview. Can J Psychiatry. 1986;31(5):452-457.
11. Koi´c E, Mu´zin´c L, Đordevic V, et al. Pseudocyesis and couvade syndrome. Drustvena Istrazivanja. 2002;11:1031-1047.
12. Bhattacharyya S, Chaturvedi SK. Metamorphosis of delusion of pregnancy. Can J Psychiatry. 2001;46(6):561-562.
13. Seeman MV. Pseudocyesis, delusional pregnancy, and psychosis: the birth of a delusion. World J Clin Cases. 2014;2(8):338-344.
14. Chatterjee SS, Nath N, Dasgupta G, et al. Delusion of pregnancy and other pregnancy-mimicking conditions: dissecting through differential diagnosis. Medical Journal of Dr. D.Y. Patil University. 2014;7(3):369-372.
15. Rosenberg HK, Coleman BG, Croop J, et al. Pseudocyesis in an adolescent patient. Clin Pediatr (Phila). 1983;22(10):708-712.
16. O’Grady JP, Rosenthal M. Pseudocyesis: a modern perspective on an old disorder. Obstet Gynecol Surv. 1989;44(7):500-511.
17. Whelan CI, Stewart DE. Pseudocyesis–a review and report of six cases. Int J Psychiatry Med. 1990;20(1):97-108.
18. Bivin GD, Klinger MP. Pseudocyesis. Bloomington, IN: Principia Press; 1937.
19. Fried PH, Rakoff AE, Schopbach RR, et al. Pseudocyesis; a psychosomatic study in gynecology. J Am Med Assoc. 1951;145(17):1329-1335.
20. Dunbar F. Emotions and bodily changes. 3rd ed. New York, NY: Columbia University Press; 1947.
21. Steinberg A, Pastor N, Winheld EB, et al. Psychoendocrine relationship in pseudocyesis. Psychosom Med. 1946;8(3):176-179.
1. Tarín JJ, Hermenegildo C, García-Pérez MA, et al. Endocrinology and physiology of pseudocyesis. Reprod Biol Endocrinol. 2013;11:39.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Ouj U. Pseudocyesis in a rural southeast Nigerian community. J Obstet Gynaecol Res. 2009;35(4):660-665.
4. Signer SF, Weinstein RP, Munoz RA, et al. Pseudocyesis in organic mood disorders. Six cases. Psychosomatics. 1992;33(3):316-323.
5. Omer H, Elizur Y, Barnea T, et al. Psychological variables and premature labour: a possible solution for some methodological problems. J Psychosom Res. 1986;30(5):559-565.
6. Starkman MN, Marshall JC, La Ferla J, et al. Pseudocyesis: psychologic and neuroendocrine interrelationships. Psychosom Med. 1985;47(1):46-57.
7. Yadav T, Balhara YP, Kataria DK. Pseudocyesis versus delusion of pregnancy: differential diagnoses to be kept in mind. Indian J Psychol Med. 2012;34(1):82-84.
8. Cohen LM. A current perspective of pseudocyesis. Am J Psychiatry. 1982;139(9):1140-1144.
9. Brown E, Barglow P. Pseudocyesis. A paradigm for psychophysiological interactions. Arch Gen Psychiatry. 1971;24(3):221-229.
10. Small GW. Pseudocyesis: an overview. Can J Psychiatry. 1986;31(5):452-457.
11. Koi´c E, Mu´zin´c L, Đordevic V, et al. Pseudocyesis and couvade syndrome. Drustvena Istrazivanja. 2002;11:1031-1047.
12. Bhattacharyya S, Chaturvedi SK. Metamorphosis of delusion of pregnancy. Can J Psychiatry. 2001;46(6):561-562.
13. Seeman MV. Pseudocyesis, delusional pregnancy, and psychosis: the birth of a delusion. World J Clin Cases. 2014;2(8):338-344.
14. Chatterjee SS, Nath N, Dasgupta G, et al. Delusion of pregnancy and other pregnancy-mimicking conditions: dissecting through differential diagnosis. Medical Journal of Dr. D.Y. Patil University. 2014;7(3):369-372.
15. Rosenberg HK, Coleman BG, Croop J, et al. Pseudocyesis in an adolescent patient. Clin Pediatr (Phila). 1983;22(10):708-712.
16. O’Grady JP, Rosenthal M. Pseudocyesis: a modern perspective on an old disorder. Obstet Gynecol Surv. 1989;44(7):500-511.
17. Whelan CI, Stewart DE. Pseudocyesis–a review and report of six cases. Int J Psychiatry Med. 1990;20(1):97-108.
18. Bivin GD, Klinger MP. Pseudocyesis. Bloomington, IN: Principia Press; 1937.
19. Fried PH, Rakoff AE, Schopbach RR, et al. Pseudocyesis; a psychosomatic study in gynecology. J Am Med Assoc. 1951;145(17):1329-1335.
20. Dunbar F. Emotions and bodily changes. 3rd ed. New York, NY: Columbia University Press; 1947.
21. Steinberg A, Pastor N, Winheld EB, et al. Psychoendocrine relationship in pseudocyesis. Psychosom Med. 1946;8(3):176-179.
Psychosis in treated neurosyphilis: Is now the time to stop his antipsychotic?
CASE Hallucinations, impaired memory
Mr. C is a 61-year-old African American man who visits the outpatient clinic for management of antipsychotic therapy for psychosis and depression. His most recent inpatient psychiatric hospitalization for auditory and visual hallucinations, paranoia, and agitation was more than 10 years ago. He has been taking chlorpromazine, 100 mg/d, for 11 years. Mr. C reports that he has had no psychotic symptoms in the past 3 years; he continues taking chlorpromazine, he says, because it helps him sleep.
How would you proceed with Mr. C’s care?
a) continue chlorpromazine because he has been symptom free
b) consider tapering and discontinuing chlorpromazine
c) obtain a more detailed history from Mr. C and perform additional tests
HISTORY Validation of diagnosis
Mr. C reports that, at age 48, he started hearing babies crying and started seeing dead infants crawling out of the incinerator at the hospital where he worked. He denies any psychiatric symptoms before that time. He stopped working 10 years ago because of his psychiatric symptoms and decline in cognition.
Subsequently, Mr. C had 3 inpatient psychiatric hospitalizations for auditory hallucinations; chlorpromazine, 100 mg/d, was prescribed for psychosis. Later efforts to discontinue chlorpromazine resulted in relapse of psychotic symptoms. Mr. C has no family history of psychiatric illness.
Mr. C’s medical history is significant for aortic regurgitation, congestive cardiac failure, hypertension, and left-sided sensorineural hearing loss. He has a history of cocaine abuse from age 21 to 45, but denies using any other substances, including alcohol and nicotine.
Urine toxicology and routine blood tests are within normal limits. The QTc is slightly prolonged over the past 2 years, recording 512, 520, and 505 milliseconds on serial electrocardiograms.
Mr. C is able to perform simple abstractions. He has a goal-directed thought process, devoid of any preoccupation, paranoia, and perceptual abnormalities. Cognitive screening reveals significant impairment of memory, registration, calculation, attention, and visuospatial skills.
Careful review of Mr. C’s history and medical records reveals a diagnosis of syphilis at age 48 after unprotected sexual intercourse. He recalls that he had a solitary genital lesion, which resolved over a few weeks. He then developed a slightly itchy, non-tender macular rash over his upper back, which he did not report to a physician. After a few months, he developed unsteady gait, blurry vision, and weakness of limbs, and had to crawl to the hospital. There, he was given a diagnosis of neurosyphilis. He also developed left-sided hearing loss during that time.
Mr. C was treated with aqueous penicillin G benzathine, 4 million units IV for 2 weeks. No follow-up cerebrospinal fluid (CSF) examination was documented after antibiotic treatment. He developed auditory and visual hallucinations and paranoia a few months after completing penicillin treatment. During the following year, he had 3 inpatient psychiatric hospitalizations for psychosis, agitation, and depressed mood.
How would you treat a patient with a history of neurosyphilis who presents with psychosis years after diagnosis?
a) repeat antibiotic treatment and stop the antipsychotic
b) repeat antibiotic treatment and continue the antipsychotic
c) attempt to discontinue the antipsychotic
d) continue the antipsychotic
The authors’ observations
Mr. C’s psychotic symptoms seem to be temporally related to his diagnosis of neurosyphilis at age 48. He and his family members deny that Mr. C had any history of psychosis or depression before the neurosyphilis diagnosis. All inpatient psychiatric hospitalizations were within 1 year of the neurosyphilis diagnosis.
Mr. C has been on a low dosage of chlorpromazine, which has significant antihistaminic action. Chlorpromazine also is known to cause QTc prolongation, especially in patients with heart disease.
TREATMENT Medication change
A serum rapid plasma reagin test is non-reactive, but Treponema pallidum particle agglutination is positive. MRI shows moderate atrophy suggestive of diffuse small-vessel disease.
Mr. C’s psychotic symptoms are considered to be sequelae of neurosyphilis, based on (1) the presence of positive antibody tests, (2) residual neurologic deficits, (3) other suggestive sequelae (aortic regurgitation, sensorineural deafness), and (4) age-inappropriate gradual cognitive decline in the absence of other psychiatric history.
Because we are concerned about the prolonged QTc, chlorpromazine is discontinued. Haloperidol, 5 mg at bedtime, is started. The neurology team does not recommend antibiotic treatment because symptoms have been stable for years. Mr. C refuses a lumbar puncture.
Mr. C returns to the outpatient clinic monthly. He is psychiatrically stable without any worsening of psychosis. Cognitive impairment remains stable over the next 6 months. Haloperidol is tapered to 2 mg at bedtime 6 months after initial evaluation. Mr. C remains psychiatrically stable on subsequent follow-up visits.
The authors’ observations
Mr. C’s psychotic symptoms persisted after standard antibiotic treatment of neurosyphilis and lapsed when he stopped taking antipsychotic medication 10 years after the initial treatment of neurosyphilis. He carried a diagnosis of schizophrenia for many years, even though his psychotic symptoms were atypical for the presentation of schizophrenia.
It is important to understand the natural course of syphilis, its implication on psychiatric symptom production, and long-term psychiatric prognosis.
Syphilis is a sexually transmitted infectious disease caused by T pallidum, a spirochete, that has varied clinical presentations. Osler called syphilis the “great imitator” for its array of system involvement, ranging from asymptomatic infection and afferent pupillary defect to depression, psychosis, and dementia. With wide use of penicillin, the rate of neurosyphilis declined steadily during the mid 1990s. By 1997, the overall rate reached its lowest point in the United States; in 1999 the Centers for Disease Control and Prevention released a national plan to eliminate syphilis.1 By 2004, however, prevalence had increased to 4.7/100,000. It is thought that this increase is mainly associated with substance use (especially crack cocaine) and HIV co-infection. Most cases were distributed in economically depressed geographical areas.
Psychiatric patients are at higher risk of acquiring the infection because of substance use, lack of education on safer sex practices, and impulsive behavior.
Stages of syphilis
Syphilis does not follow a step-wise progression. One-third of cases progress to the tertiary stage, even many years after initial infection, without adequate treatment.2
Almost 10% syphilis cases present with neurologic symptoms,3 and neurologic involvement can occur at any stage of disease progression. The most common symptoms of syphilis are presented in Table 1.
A range of psychiatric symptoms have been reported among patients with syphilis, including anhedonia, suicidality, mania, grandiosity, persecutory delusions, auditory and visual hallucinations, paranoia, and cognitive impairment. The incidence of psychiatric symptoms is not clearly described in literature.
Diagnosis and treatment
Neurosyphilis, at any disease stage, should be suspected if a patient:
- exhibits suggestive symptoms
- does not respond to antibiotic treatment
- has late latent syphilis
- is immunocompromised.
Lumbar puncture and examination of CSF is the most useful diagnostic test. Dark field microscopy to reveal T pallidum is definitive, but only is applicable during the primary stage. The role of dark field microscopy of the CSF sample to diagnose neurologic involvement has not been established. Tests and treatment protocol are described in Table 2.2-5
Treatment of psychiatric symptoms of neurosyphilis
There are inconsistent and limited data about the prevalence of psychiatric symptoms in neurosyphilis. A retrospective study6 of 161 patients with neurosyphilis in South Africa reported that 50.9% exhibited a complex spectrum of symptoms that included delirium and dementia. Of treated patients, 17% continued to have residual symptoms during follow-up.
A review of the literature did not reveal any widely accepted guideline for screening for neurosyphilis in general psychiatry practice or a treatment protocol for psychiatric symptoms. This lack of guidance could be attributed to the rarity of the disease, cost-benefit analyses, and low specificity of antibody tests. In the literature, syphilis screening is recommended as a routine protocol when evaluating and treating dementia.7
In most studies, a diagnosis of neurosyphilis was confirmed by CSF examination; however, many of these studies did not report a specific follow-up CSF examination protocol. Most of these patients were treated with an antipsychotic with partial improvement in symptoms, even after standard antibiotic protocol.8
First- and second-generation antipsychotics and mood stabilizers have been shown to be useful in the acute treatment of psychosis and agitation.8 In few instances, the psychotropic medication was continued beyond several months and the patient was placed in a long-term care facility. Psychiatric symptoms persisted for many years with or without residual neurosyphilis symptoms, possibly because of permanent neuronal loss.
Clinical considerations
It often is difficult to distinguish a preexisting psychiatric disorder made worse by neurosyphilis from a secondary psychiatric disorder caused by neurosyphilis. The 2 might coexist, or psychiatric symptoms could be wrongly attributed to schizophrenia because of a lack of careful clinical evaluation.
Often, the follow-up diagnostic protocol for neurosyphilis is not followed; as a result, the need for re-treatment remains unclear. Rarity of the disease makes it difficult to perform a prospective, randomized study to determine the duration and effect of long-term psychiatric treatment.
Close follow-up and consideration of the risk vs benefit of psychotropic medication is key. Because there are no proven guidelines for the length of treatment with antipsychotics, it is prudent to minimize their use until psychiatrically indicated. Side effects, such as (in Mr. C’s case) changes in the QTc interval, should warrant consideration of discontinuing psychotropic medication. Interdisciplinary collaboration with neurology and infectious disease will improve the overall outcome of a complex clinical presentation.
1. Centers for Disease Control and Prevention. National plan to eliminate syphilis from the United States. http://www.cdc.gov/stopsyphilis/plan.htm. Updated December 7, 2007. Accessed July 7, 2016.
2. Friedrich F, Aigner M, Fearns N, et al. Psychosis in neurosyphilis—clinical aspects and implications. Psychopathology. 2014;47(1):3-9.
3. Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68(2):283-290.
4. Romanowski B, Sutherland R, Fick GH, et al. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114(12):1005-1009.
5. Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines. Syphilis. http://www.cdc.gov/std/tg2015/syphilis.htm. Updated June 4, 2015. Accessed July 13, 2016.
6. Timmermans M, Carr J. Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry. 2004;75(12):1727-1730.
7. Scott KR, Barrett AM. Dementia syndrome: evaluation and treatment. Expert Rev Neurother. 2007;7(4):407-422.
8. Sanchez FM, Zisselman MH. Treatment of psychiatric symptoms associated with neurosyphilis. Psychosomatics. 2007;48(5):440-445.
CASE Hallucinations, impaired memory
Mr. C is a 61-year-old African American man who visits the outpatient clinic for management of antipsychotic therapy for psychosis and depression. His most recent inpatient psychiatric hospitalization for auditory and visual hallucinations, paranoia, and agitation was more than 10 years ago. He has been taking chlorpromazine, 100 mg/d, for 11 years. Mr. C reports that he has had no psychotic symptoms in the past 3 years; he continues taking chlorpromazine, he says, because it helps him sleep.
How would you proceed with Mr. C’s care?
a) continue chlorpromazine because he has been symptom free
b) consider tapering and discontinuing chlorpromazine
c) obtain a more detailed history from Mr. C and perform additional tests
HISTORY Validation of diagnosis
Mr. C reports that, at age 48, he started hearing babies crying and started seeing dead infants crawling out of the incinerator at the hospital where he worked. He denies any psychiatric symptoms before that time. He stopped working 10 years ago because of his psychiatric symptoms and decline in cognition.
Subsequently, Mr. C had 3 inpatient psychiatric hospitalizations for auditory hallucinations; chlorpromazine, 100 mg/d, was prescribed for psychosis. Later efforts to discontinue chlorpromazine resulted in relapse of psychotic symptoms. Mr. C has no family history of psychiatric illness.
Mr. C’s medical history is significant for aortic regurgitation, congestive cardiac failure, hypertension, and left-sided sensorineural hearing loss. He has a history of cocaine abuse from age 21 to 45, but denies using any other substances, including alcohol and nicotine.
Urine toxicology and routine blood tests are within normal limits. The QTc is slightly prolonged over the past 2 years, recording 512, 520, and 505 milliseconds on serial electrocardiograms.
Mr. C is able to perform simple abstractions. He has a goal-directed thought process, devoid of any preoccupation, paranoia, and perceptual abnormalities. Cognitive screening reveals significant impairment of memory, registration, calculation, attention, and visuospatial skills.
Careful review of Mr. C’s history and medical records reveals a diagnosis of syphilis at age 48 after unprotected sexual intercourse. He recalls that he had a solitary genital lesion, which resolved over a few weeks. He then developed a slightly itchy, non-tender macular rash over his upper back, which he did not report to a physician. After a few months, he developed unsteady gait, blurry vision, and weakness of limbs, and had to crawl to the hospital. There, he was given a diagnosis of neurosyphilis. He also developed left-sided hearing loss during that time.
Mr. C was treated with aqueous penicillin G benzathine, 4 million units IV for 2 weeks. No follow-up cerebrospinal fluid (CSF) examination was documented after antibiotic treatment. He developed auditory and visual hallucinations and paranoia a few months after completing penicillin treatment. During the following year, he had 3 inpatient psychiatric hospitalizations for psychosis, agitation, and depressed mood.
How would you treat a patient with a history of neurosyphilis who presents with psychosis years after diagnosis?
a) repeat antibiotic treatment and stop the antipsychotic
b) repeat antibiotic treatment and continue the antipsychotic
c) attempt to discontinue the antipsychotic
d) continue the antipsychotic
The authors’ observations
Mr. C’s psychotic symptoms seem to be temporally related to his diagnosis of neurosyphilis at age 48. He and his family members deny that Mr. C had any history of psychosis or depression before the neurosyphilis diagnosis. All inpatient psychiatric hospitalizations were within 1 year of the neurosyphilis diagnosis.
Mr. C has been on a low dosage of chlorpromazine, which has significant antihistaminic action. Chlorpromazine also is known to cause QTc prolongation, especially in patients with heart disease.
TREATMENT Medication change
A serum rapid plasma reagin test is non-reactive, but Treponema pallidum particle agglutination is positive. MRI shows moderate atrophy suggestive of diffuse small-vessel disease.
Mr. C’s psychotic symptoms are considered to be sequelae of neurosyphilis, based on (1) the presence of positive antibody tests, (2) residual neurologic deficits, (3) other suggestive sequelae (aortic regurgitation, sensorineural deafness), and (4) age-inappropriate gradual cognitive decline in the absence of other psychiatric history.
Because we are concerned about the prolonged QTc, chlorpromazine is discontinued. Haloperidol, 5 mg at bedtime, is started. The neurology team does not recommend antibiotic treatment because symptoms have been stable for years. Mr. C refuses a lumbar puncture.
Mr. C returns to the outpatient clinic monthly. He is psychiatrically stable without any worsening of psychosis. Cognitive impairment remains stable over the next 6 months. Haloperidol is tapered to 2 mg at bedtime 6 months after initial evaluation. Mr. C remains psychiatrically stable on subsequent follow-up visits.
The authors’ observations
Mr. C’s psychotic symptoms persisted after standard antibiotic treatment of neurosyphilis and lapsed when he stopped taking antipsychotic medication 10 years after the initial treatment of neurosyphilis. He carried a diagnosis of schizophrenia for many years, even though his psychotic symptoms were atypical for the presentation of schizophrenia.
It is important to understand the natural course of syphilis, its implication on psychiatric symptom production, and long-term psychiatric prognosis.
Syphilis is a sexually transmitted infectious disease caused by T pallidum, a spirochete, that has varied clinical presentations. Osler called syphilis the “great imitator” for its array of system involvement, ranging from asymptomatic infection and afferent pupillary defect to depression, psychosis, and dementia. With wide use of penicillin, the rate of neurosyphilis declined steadily during the mid 1990s. By 1997, the overall rate reached its lowest point in the United States; in 1999 the Centers for Disease Control and Prevention released a national plan to eliminate syphilis.1 By 2004, however, prevalence had increased to 4.7/100,000. It is thought that this increase is mainly associated with substance use (especially crack cocaine) and HIV co-infection. Most cases were distributed in economically depressed geographical areas.
Psychiatric patients are at higher risk of acquiring the infection because of substance use, lack of education on safer sex practices, and impulsive behavior.
Stages of syphilis
Syphilis does not follow a step-wise progression. One-third of cases progress to the tertiary stage, even many years after initial infection, without adequate treatment.2
Almost 10% syphilis cases present with neurologic symptoms,3 and neurologic involvement can occur at any stage of disease progression. The most common symptoms of syphilis are presented in Table 1.
A range of psychiatric symptoms have been reported among patients with syphilis, including anhedonia, suicidality, mania, grandiosity, persecutory delusions, auditory and visual hallucinations, paranoia, and cognitive impairment. The incidence of psychiatric symptoms is not clearly described in literature.
Diagnosis and treatment
Neurosyphilis, at any disease stage, should be suspected if a patient:
- exhibits suggestive symptoms
- does not respond to antibiotic treatment
- has late latent syphilis
- is immunocompromised.
Lumbar puncture and examination of CSF is the most useful diagnostic test. Dark field microscopy to reveal T pallidum is definitive, but only is applicable during the primary stage. The role of dark field microscopy of the CSF sample to diagnose neurologic involvement has not been established. Tests and treatment protocol are described in Table 2.2-5
Treatment of psychiatric symptoms of neurosyphilis
There are inconsistent and limited data about the prevalence of psychiatric symptoms in neurosyphilis. A retrospective study6 of 161 patients with neurosyphilis in South Africa reported that 50.9% exhibited a complex spectrum of symptoms that included delirium and dementia. Of treated patients, 17% continued to have residual symptoms during follow-up.
A review of the literature did not reveal any widely accepted guideline for screening for neurosyphilis in general psychiatry practice or a treatment protocol for psychiatric symptoms. This lack of guidance could be attributed to the rarity of the disease, cost-benefit analyses, and low specificity of antibody tests. In the literature, syphilis screening is recommended as a routine protocol when evaluating and treating dementia.7
In most studies, a diagnosis of neurosyphilis was confirmed by CSF examination; however, many of these studies did not report a specific follow-up CSF examination protocol. Most of these patients were treated with an antipsychotic with partial improvement in symptoms, even after standard antibiotic protocol.8
First- and second-generation antipsychotics and mood stabilizers have been shown to be useful in the acute treatment of psychosis and agitation.8 In few instances, the psychotropic medication was continued beyond several months and the patient was placed in a long-term care facility. Psychiatric symptoms persisted for many years with or without residual neurosyphilis symptoms, possibly because of permanent neuronal loss.
Clinical considerations
It often is difficult to distinguish a preexisting psychiatric disorder made worse by neurosyphilis from a secondary psychiatric disorder caused by neurosyphilis. The 2 might coexist, or psychiatric symptoms could be wrongly attributed to schizophrenia because of a lack of careful clinical evaluation.
Often, the follow-up diagnostic protocol for neurosyphilis is not followed; as a result, the need for re-treatment remains unclear. Rarity of the disease makes it difficult to perform a prospective, randomized study to determine the duration and effect of long-term psychiatric treatment.
Close follow-up and consideration of the risk vs benefit of psychotropic medication is key. Because there are no proven guidelines for the length of treatment with antipsychotics, it is prudent to minimize their use until psychiatrically indicated. Side effects, such as (in Mr. C’s case) changes in the QTc interval, should warrant consideration of discontinuing psychotropic medication. Interdisciplinary collaboration with neurology and infectious disease will improve the overall outcome of a complex clinical presentation.
CASE Hallucinations, impaired memory
Mr. C is a 61-year-old African American man who visits the outpatient clinic for management of antipsychotic therapy for psychosis and depression. His most recent inpatient psychiatric hospitalization for auditory and visual hallucinations, paranoia, and agitation was more than 10 years ago. He has been taking chlorpromazine, 100 mg/d, for 11 years. Mr. C reports that he has had no psychotic symptoms in the past 3 years; he continues taking chlorpromazine, he says, because it helps him sleep.
How would you proceed with Mr. C’s care?
a) continue chlorpromazine because he has been symptom free
b) consider tapering and discontinuing chlorpromazine
c) obtain a more detailed history from Mr. C and perform additional tests
HISTORY Validation of diagnosis
Mr. C reports that, at age 48, he started hearing babies crying and started seeing dead infants crawling out of the incinerator at the hospital where he worked. He denies any psychiatric symptoms before that time. He stopped working 10 years ago because of his psychiatric symptoms and decline in cognition.
Subsequently, Mr. C had 3 inpatient psychiatric hospitalizations for auditory hallucinations; chlorpromazine, 100 mg/d, was prescribed for psychosis. Later efforts to discontinue chlorpromazine resulted in relapse of psychotic symptoms. Mr. C has no family history of psychiatric illness.
Mr. C’s medical history is significant for aortic regurgitation, congestive cardiac failure, hypertension, and left-sided sensorineural hearing loss. He has a history of cocaine abuse from age 21 to 45, but denies using any other substances, including alcohol and nicotine.
Urine toxicology and routine blood tests are within normal limits. The QTc is slightly prolonged over the past 2 years, recording 512, 520, and 505 milliseconds on serial electrocardiograms.
Mr. C is able to perform simple abstractions. He has a goal-directed thought process, devoid of any preoccupation, paranoia, and perceptual abnormalities. Cognitive screening reveals significant impairment of memory, registration, calculation, attention, and visuospatial skills.
Careful review of Mr. C’s history and medical records reveals a diagnosis of syphilis at age 48 after unprotected sexual intercourse. He recalls that he had a solitary genital lesion, which resolved over a few weeks. He then developed a slightly itchy, non-tender macular rash over his upper back, which he did not report to a physician. After a few months, he developed unsteady gait, blurry vision, and weakness of limbs, and had to crawl to the hospital. There, he was given a diagnosis of neurosyphilis. He also developed left-sided hearing loss during that time.
Mr. C was treated with aqueous penicillin G benzathine, 4 million units IV for 2 weeks. No follow-up cerebrospinal fluid (CSF) examination was documented after antibiotic treatment. He developed auditory and visual hallucinations and paranoia a few months after completing penicillin treatment. During the following year, he had 3 inpatient psychiatric hospitalizations for psychosis, agitation, and depressed mood.
How would you treat a patient with a history of neurosyphilis who presents with psychosis years after diagnosis?
a) repeat antibiotic treatment and stop the antipsychotic
b) repeat antibiotic treatment and continue the antipsychotic
c) attempt to discontinue the antipsychotic
d) continue the antipsychotic
The authors’ observations
Mr. C’s psychotic symptoms seem to be temporally related to his diagnosis of neurosyphilis at age 48. He and his family members deny that Mr. C had any history of psychosis or depression before the neurosyphilis diagnosis. All inpatient psychiatric hospitalizations were within 1 year of the neurosyphilis diagnosis.
Mr. C has been on a low dosage of chlorpromazine, which has significant antihistaminic action. Chlorpromazine also is known to cause QTc prolongation, especially in patients with heart disease.
TREATMENT Medication change
A serum rapid plasma reagin test is non-reactive, but Treponema pallidum particle agglutination is positive. MRI shows moderate atrophy suggestive of diffuse small-vessel disease.
Mr. C’s psychotic symptoms are considered to be sequelae of neurosyphilis, based on (1) the presence of positive antibody tests, (2) residual neurologic deficits, (3) other suggestive sequelae (aortic regurgitation, sensorineural deafness), and (4) age-inappropriate gradual cognitive decline in the absence of other psychiatric history.
Because we are concerned about the prolonged QTc, chlorpromazine is discontinued. Haloperidol, 5 mg at bedtime, is started. The neurology team does not recommend antibiotic treatment because symptoms have been stable for years. Mr. C refuses a lumbar puncture.
Mr. C returns to the outpatient clinic monthly. He is psychiatrically stable without any worsening of psychosis. Cognitive impairment remains stable over the next 6 months. Haloperidol is tapered to 2 mg at bedtime 6 months after initial evaluation. Mr. C remains psychiatrically stable on subsequent follow-up visits.
The authors’ observations
Mr. C’s psychotic symptoms persisted after standard antibiotic treatment of neurosyphilis and lapsed when he stopped taking antipsychotic medication 10 years after the initial treatment of neurosyphilis. He carried a diagnosis of schizophrenia for many years, even though his psychotic symptoms were atypical for the presentation of schizophrenia.
It is important to understand the natural course of syphilis, its implication on psychiatric symptom production, and long-term psychiatric prognosis.
Syphilis is a sexually transmitted infectious disease caused by T pallidum, a spirochete, that has varied clinical presentations. Osler called syphilis the “great imitator” for its array of system involvement, ranging from asymptomatic infection and afferent pupillary defect to depression, psychosis, and dementia. With wide use of penicillin, the rate of neurosyphilis declined steadily during the mid 1990s. By 1997, the overall rate reached its lowest point in the United States; in 1999 the Centers for Disease Control and Prevention released a national plan to eliminate syphilis.1 By 2004, however, prevalence had increased to 4.7/100,000. It is thought that this increase is mainly associated with substance use (especially crack cocaine) and HIV co-infection. Most cases were distributed in economically depressed geographical areas.
Psychiatric patients are at higher risk of acquiring the infection because of substance use, lack of education on safer sex practices, and impulsive behavior.
Stages of syphilis
Syphilis does not follow a step-wise progression. One-third of cases progress to the tertiary stage, even many years after initial infection, without adequate treatment.2
Almost 10% syphilis cases present with neurologic symptoms,3 and neurologic involvement can occur at any stage of disease progression. The most common symptoms of syphilis are presented in Table 1.
A range of psychiatric symptoms have been reported among patients with syphilis, including anhedonia, suicidality, mania, grandiosity, persecutory delusions, auditory and visual hallucinations, paranoia, and cognitive impairment. The incidence of psychiatric symptoms is not clearly described in literature.
Diagnosis and treatment
Neurosyphilis, at any disease stage, should be suspected if a patient:
- exhibits suggestive symptoms
- does not respond to antibiotic treatment
- has late latent syphilis
- is immunocompromised.
Lumbar puncture and examination of CSF is the most useful diagnostic test. Dark field microscopy to reveal T pallidum is definitive, but only is applicable during the primary stage. The role of dark field microscopy of the CSF sample to diagnose neurologic involvement has not been established. Tests and treatment protocol are described in Table 2.2-5
Treatment of psychiatric symptoms of neurosyphilis
There are inconsistent and limited data about the prevalence of psychiatric symptoms in neurosyphilis. A retrospective study6 of 161 patients with neurosyphilis in South Africa reported that 50.9% exhibited a complex spectrum of symptoms that included delirium and dementia. Of treated patients, 17% continued to have residual symptoms during follow-up.
A review of the literature did not reveal any widely accepted guideline for screening for neurosyphilis in general psychiatry practice or a treatment protocol for psychiatric symptoms. This lack of guidance could be attributed to the rarity of the disease, cost-benefit analyses, and low specificity of antibody tests. In the literature, syphilis screening is recommended as a routine protocol when evaluating and treating dementia.7
In most studies, a diagnosis of neurosyphilis was confirmed by CSF examination; however, many of these studies did not report a specific follow-up CSF examination protocol. Most of these patients were treated with an antipsychotic with partial improvement in symptoms, even after standard antibiotic protocol.8
First- and second-generation antipsychotics and mood stabilizers have been shown to be useful in the acute treatment of psychosis and agitation.8 In few instances, the psychotropic medication was continued beyond several months and the patient was placed in a long-term care facility. Psychiatric symptoms persisted for many years with or without residual neurosyphilis symptoms, possibly because of permanent neuronal loss.
Clinical considerations
It often is difficult to distinguish a preexisting psychiatric disorder made worse by neurosyphilis from a secondary psychiatric disorder caused by neurosyphilis. The 2 might coexist, or psychiatric symptoms could be wrongly attributed to schizophrenia because of a lack of careful clinical evaluation.
Often, the follow-up diagnostic protocol for neurosyphilis is not followed; as a result, the need for re-treatment remains unclear. Rarity of the disease makes it difficult to perform a prospective, randomized study to determine the duration and effect of long-term psychiatric treatment.
Close follow-up and consideration of the risk vs benefit of psychotropic medication is key. Because there are no proven guidelines for the length of treatment with antipsychotics, it is prudent to minimize their use until psychiatrically indicated. Side effects, such as (in Mr. C’s case) changes in the QTc interval, should warrant consideration of discontinuing psychotropic medication. Interdisciplinary collaboration with neurology and infectious disease will improve the overall outcome of a complex clinical presentation.
1. Centers for Disease Control and Prevention. National plan to eliminate syphilis from the United States. http://www.cdc.gov/stopsyphilis/plan.htm. Updated December 7, 2007. Accessed July 7, 2016.
2. Friedrich F, Aigner M, Fearns N, et al. Psychosis in neurosyphilis—clinical aspects and implications. Psychopathology. 2014;47(1):3-9.
3. Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68(2):283-290.
4. Romanowski B, Sutherland R, Fick GH, et al. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114(12):1005-1009.
5. Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines. Syphilis. http://www.cdc.gov/std/tg2015/syphilis.htm. Updated June 4, 2015. Accessed July 13, 2016.
6. Timmermans M, Carr J. Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry. 2004;75(12):1727-1730.
7. Scott KR, Barrett AM. Dementia syndrome: evaluation and treatment. Expert Rev Neurother. 2007;7(4):407-422.
8. Sanchez FM, Zisselman MH. Treatment of psychiatric symptoms associated with neurosyphilis. Psychosomatics. 2007;48(5):440-445.
1. Centers for Disease Control and Prevention. National plan to eliminate syphilis from the United States. http://www.cdc.gov/stopsyphilis/plan.htm. Updated December 7, 2007. Accessed July 7, 2016.
2. Friedrich F, Aigner M, Fearns N, et al. Psychosis in neurosyphilis—clinical aspects and implications. Psychopathology. 2014;47(1):3-9.
3. Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68(2):283-290.
4. Romanowski B, Sutherland R, Fick GH, et al. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114(12):1005-1009.
5. Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines. Syphilis. http://www.cdc.gov/std/tg2015/syphilis.htm. Updated June 4, 2015. Accessed July 13, 2016.
6. Timmermans M, Carr J. Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry. 2004;75(12):1727-1730.
7. Scott KR, Barrett AM. Dementia syndrome: evaluation and treatment. Expert Rev Neurother. 2007;7(4):407-422.
8. Sanchez FM, Zisselman MH. Treatment of psychiatric symptoms associated with neurosyphilis. Psychosomatics. 2007;48(5):440-445.
No evidence of pregnancy, but she is suicidal and depressed after ‘my baby died’
CASE Depressed after she says her baby died
Ms. R, age 50, is an African-American woman who presents to a psychiatric hospital under an involuntary commitment executed by local law enforcement. Her sister called the authorities because Ms. R reportedly told her that she is “very depressed” and wants to “end [her] life” by taking an overdose of medications after the death of her newborn 1 week earlier.
Ms. R states that she delivered a child at “full term” in the emergency department of an outside community hospital, and that her current psychiatric symptoms began after the child died from “SIDS” [sudden infant death syndrome] shortly after birth.
Ms. R describes depressive symptoms including depressed mood, anhedonia, decreased energy, feelings of guilt, decreased concentration, poor sleep, and suicidal ideation. She denies substance use or a medical condition that could have induced these symptoms, and denies symptoms of mania, anxiety, or psychosis at admission or during the previous year.
Ms. R reports a history of manic episodes that includes periods of elevated mood or irritability, impulsivity, increased energy, excessive spending despite negative consequences, lack of need for sleep, rapid thoughts, and rapid speech that impaired her social and occupational functioning. Her most recent manic episode was approximately 3 years before this admission. She reports a previous suicide attempt and a history of physical abuse from a former intimate partner.
Neither the findings of a physical examination nor the results of a screening test for serum β-human chorionic gonadotropin (βHCG) are consistent with pregnancy. Ms. R’s medical record reveals that she was hospitalized for a “cardiac workup” a week earlier and requested investigation of possible pregnancy, which was negative. Records also reveal that she had a hysterectomy 10 years earlier.
Although Ms. R’s sister and boyfriend support her claim of pregnancy, the patient’s young adult son refutes it and states that she “does stuff like this for attention.” Her son also reports receiving a forged sonogram picture that his mother found online 1 month earlier. Ms. R presents an obituary from a local newspaper for the child but, on further investigation, the photograph of the infant was discovered to be of another child, also obtained online. Ms. R’s family denies knowledge of potential external reward Ms. R could gain by claiming to be pregnant.
Which of the following diagnoses can be considered after Ms. R’s initial presentation?
a) somatic symptom disorder
b) major depressive disorder
c) bipolar I disorder
d) delusional disorder
The authors’ observations
Ms. R reported the recent death of a newborn that was incompatible with her medical history. Her family members revealed that Ms. R made an active effort to deceive them about the reported pregnancy. She also exhibited symptoms of a major depressive episode in the context of previous manic episodes and expressed suicidal ideation.
The first step in the diagnostic pathway was to rule out possible medical explanations, including pregnancy, which could account for the patient’s symptoms. Although the serum βHCG level usually returns to non-pregnant levels 2 to 4 weeks after delivery, it can take even longer in some women.1 The absence of βHCG along with the recorded history of hysterectomy indicated that Ms. R was not pregnant at the time of testing or within the preceding few weeks. Once medical anomalies and substance use were ruled out, further classification of the psychiatric condition was undertaken.
One aspect of establishing a diagnosis for Ms. R is determining the presence of psychosis (eg, delusional thinking) (Table 1). Ms. R deliberately fabricated evidence of her pregnancy and manipulated family members, which indicated a low likelihood of delusions and supported a diagnostic alternative to psychosis.
Ms. R has a well-described history of manic episodes with current symptoms of a major depressive episode. The treatment team makes a diagnosis of bipolar I disorder, most recent episode depressed. The depressive symptoms Ms. R described were consistent with bipolar depression but did not explain her report of a pregnancy that is inconsistent with reality.
As is the case with Ms. R, diagnostic clarity often requires observation and evaluation over time. Building a strong therapeutic relationship with Ms. R in the context of an appropriate treatment plan allows the treatment team to explore the origin, motivations, and evolution of her thought content while managing her illness.
Confronting a patient about her false claims is likely to result in which of the following?
a) spontaneous resolution of symptoms
b) improved therapeutic alliance
c) degradation of the patient’s coping mechanism
d) violent outbursts by the patient
EVALUATION Confrontation
At admission, Ms. R remains resolute that she was pregnant and is suffering immense psychological distress secondary to the death of her child. Early in the treatment course, she is confronted with evidence indicating that her pregnancy was impossible. Shortly after this interaction, nursing staff alerts the treating physician that Ms. R experienced a “seizure-like spell” characterized by gross non-stereotyped jerking of the upper extremities, intact orientation, retention of bowel and bladder function, and coherent speech consistent with a diagnosis of pseudoseizure.2
Ms. R is transferred to a tertiary care facility for neurologic evaluation and observation. Ms. R repeatedly presents a photograph that she claims to be of her deceased child and implores the allied treatment team to advocate for discharge. Evaluation of Ms. R’s neurologic symptoms revealed no medical explanation for the “seizure-like spell” and she is transferred to the inpatient psychiatric hospital.
Upon return to the inpatient psychiatric unit, Ms. R receives intensive psychological exploration of her symptoms, thought content, and the foundation of her pregnancy claim. Within days, she acknowledges that the pregnancy was “not real” and that she was conscious of this fact in the months prior to hospitalization. She cites turmoil in her romantic relationship as the primary stimulus for her actions.
The authors’ observations
Ms. R’s reported pregnancy was not a delusion, but rather a deceitful exposition constructed with appropriate reality testing and a conscious awareness of the manipulation. This eliminated delusions as the explanation of her pregnancy claim. Although Ms. R initially rejected evidence refuting her belief of pregnancy, she recognized and accepted reality with appropriate intervention.
Factitious disorder vs malingering
Factitious disorder and malingering can present with intentional induction or report of symptoms or signs of a physical abnormality:
Factitious disorder imposed on the self is a willful misrepresentation or fabrication of signs or symptoms of an illness by a person in the absence of obvious personal gain that cannot be explained by a separate physical or mental illness (Table 2).3,4
Malingering is the intentional production or exaggeration of physical or psychological signs or symptoms with obvious secondary gain.
Malingering can be excluded in Ms. R’s case: She did not gain external reward by falsely reporting pregnancy. Although DSM-IV-TR (Table 2) assumes that the motivation for the patient with factitious disorder is to assume the sick role, DSM-5 merely states that the she (he) should present themselves as ill, impaired, or injured.3,4
Ms. R’s treatment team diagnosed factitious disorder imposed on self after careful exclusion of other causes for her symptoms. Bipolar I disorder, most recent episode depressed, also was diagnosed after considering Ms. R’s previous history of manic episodes and depressive symptoms at presentation.
Factitious disorder and other psychiatric conditions often are comorbid. Bipolar disorder, as in Ms. R’s case, as well as major depressive disorder commonly are comorbid with factitious disorder. It is also important to note that factitious disorder often occurs in the context of a personality disorder.5
Which of the following medications are FDA-approved for treating factitious disorder?
a) olanzapine-fluoxetine combination
b) lurasidone
c) valproic acid
d) all of the above
e) no medications are approved for treating factitious disorder
TREATMENT Support, drug therapy
Treatment of Ms. R’s factitious disorder consists of psychological interventions via psychotherapy and strengthening of social support. She participates in daily individual therapy sessions as well as several group therapy activities. Ms. R engages with her social worker to facilitate a successful transition to an appropriate support network and access community resources to aid her wellness.
The treatment team feels that her diagnosis of bipolar I disorder, most recent episode depressed, warrants pharmacologic intervention. Ms. R agrees to begin a mood stabilizer, valproic acid, instead of medications FDA-approved to treat bipolar depression, such as lurasidone or quetiapine, because she reports good efficacy and tolerability when she took it during a major depressive episode approximately 4 years earlier.
Valproic acid is started at 250 mg/d and increased to 1,000 mg/d. Ms. R tolerates the medication without observed or reported adverse effects.
The authors’ observations
Managing factitious disorder can be challenging; patients can evoke strong feelings of countertransference during treatment.3,6,7 Providers might feel that the patient does not need to be treated, or that the patient is “not really sick.” This may induce anger and animosity toward the patient (therapeutic nihilism).8 These negative emotions are likely to disrupt the patient–provider relationship and exacerbate the patient’s symptoms.
It is generally accepted that the patient should be made aware of the treatment plan, in an indirect and tactful way, so that the patient does not feel “outed.” Unmasking the patient—the process of instilling insight—is a delicate step and can be a stressful time for the patient.9 A confrontational approach often places the patient’s sick role in doubt and does not address the pathological aspect of the disorder.
It is rare for a patient to admit to fabricating symptoms; confronted, the patient is likely to double their efforts to maintain the rouse of a fictional disease.10,11 It is important for the treatment team to be aware that patients frequently leave the treatment facility against medical advice, seek a different provider, or even pursue legal action for defamation against the treating physician.
Treating comorbid medical and psychiatric conditions is important for successful management of a patient with factitious disorder. Initiating valproic acid to address Ms. R’s bipolar depression contributed to her overall psychiatric stability. Initial treatment with a medication that is FDA-approved for treating bipolar depression, such as lurasidone, quetiapine, or olanzapine-fluoxetine combination, should be considered as an alternative. We chose valproic acid for Ms. R because of its previous efficacy, good tolerability, and the patient’s high level of comfort with the medication.
Which of the following are risk factors for factitious disorder?
a) lengthy medical treatments or hospitalizations as a child
b) female sex
c) experience as a health care worker
d) all of the above
OUTCOME Stabilization
Successful treatment during Ms. R’s inpatient psychiatric admission results in improved insight, remission of suicidal ideation, and stabilization of mood lability. She is discharged to the care of her family with a plan to follow up with a psychotherapist and psychiatrist. Continued administration of valproic acid continues to be effective after discharge.
Ms. R engages in frequent follow-up with outpatient psychiatric services. She remains engaged in psychotherapy and psychiatric care 1 year after discharge. Ms. R has made no report of pregnancy or required hospitalization during this time. She expresses trust in the mental health care system and acknowledges the role treatment played in her improvement.
1. Reyes FI, Winter JS, Faiman C. Postpartum disappearance of chorionic gonadotropin from the maternal and neonatal circulations. Am J Obstet Gynecol. 1985;153(5):486-489.
2. Avbersek A, Sisodiya S. Does the primary literature provide support for clinical signs used to distinguish psychogenic nonepileptic seizures from epileptic seizures? J Neurol Neurosurg Psychiatry. 2010;81(7):719-725.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Kapfhammer HP, Rothenhausler HM, Dietrich E, et al. Artifactual disorders—between deception and self-mutilation. Experiences in consultation psychiatry at a university clinic [in German]. Nervenarzt. 1998;69(5):401-409.
6. Feldman MD, Feldman JM. Tangled in the web: countertransference in the therapy of factitious disorders. Int J Psychiatry Med. 1995;25(4):389-399.
7. Wedel KR. A therapeutic confrontation approach to treating patients with factitious illness. Soc Work. 1971;16(2):69-73.
8. Feldman MD, Hamilton JC, Deemer HN. Factitious disorder. In: Phillips KA, ed. Somatoform and factitious disorder. Washington, DC: American Psychiatric Press; 2001:129-159.
9. Scher LM, Knudsen P, Leamon M. Somatic symptom and related disorders. In: Hales RE, Yudofsky SC, Weiss Roberts L, eds. The American Publishing Psychiatric Publishing textbook of psychiatry. Arlington, VA: American Psychiatric Publishing; 2014:531-556.
10. Lipsitt DR. Introduction. In: Feldman MD, Eisendrath SJ, eds. The spectrum of factitious disorders. Washington, DC: American Psychiatric Press; 1996:xix-xxviii.
11. van der Feltz-Cornelis CM. Confronting patients about a factitious disorder [in Dutch]. Ned Tidjschr Geneeskd. 2000;144(12):545-548.
CASE Depressed after she says her baby died
Ms. R, age 50, is an African-American woman who presents to a psychiatric hospital under an involuntary commitment executed by local law enforcement. Her sister called the authorities because Ms. R reportedly told her that she is “very depressed” and wants to “end [her] life” by taking an overdose of medications after the death of her newborn 1 week earlier.
Ms. R states that she delivered a child at “full term” in the emergency department of an outside community hospital, and that her current psychiatric symptoms began after the child died from “SIDS” [sudden infant death syndrome] shortly after birth.
Ms. R describes depressive symptoms including depressed mood, anhedonia, decreased energy, feelings of guilt, decreased concentration, poor sleep, and suicidal ideation. She denies substance use or a medical condition that could have induced these symptoms, and denies symptoms of mania, anxiety, or psychosis at admission or during the previous year.
Ms. R reports a history of manic episodes that includes periods of elevated mood or irritability, impulsivity, increased energy, excessive spending despite negative consequences, lack of need for sleep, rapid thoughts, and rapid speech that impaired her social and occupational functioning. Her most recent manic episode was approximately 3 years before this admission. She reports a previous suicide attempt and a history of physical abuse from a former intimate partner.
Neither the findings of a physical examination nor the results of a screening test for serum β-human chorionic gonadotropin (βHCG) are consistent with pregnancy. Ms. R’s medical record reveals that she was hospitalized for a “cardiac workup” a week earlier and requested investigation of possible pregnancy, which was negative. Records also reveal that she had a hysterectomy 10 years earlier.
Although Ms. R’s sister and boyfriend support her claim of pregnancy, the patient’s young adult son refutes it and states that she “does stuff like this for attention.” Her son also reports receiving a forged sonogram picture that his mother found online 1 month earlier. Ms. R presents an obituary from a local newspaper for the child but, on further investigation, the photograph of the infant was discovered to be of another child, also obtained online. Ms. R’s family denies knowledge of potential external reward Ms. R could gain by claiming to be pregnant.
Which of the following diagnoses can be considered after Ms. R’s initial presentation?
a) somatic symptom disorder
b) major depressive disorder
c) bipolar I disorder
d) delusional disorder
The authors’ observations
Ms. R reported the recent death of a newborn that was incompatible with her medical history. Her family members revealed that Ms. R made an active effort to deceive them about the reported pregnancy. She also exhibited symptoms of a major depressive episode in the context of previous manic episodes and expressed suicidal ideation.
The first step in the diagnostic pathway was to rule out possible medical explanations, including pregnancy, which could account for the patient’s symptoms. Although the serum βHCG level usually returns to non-pregnant levels 2 to 4 weeks after delivery, it can take even longer in some women.1 The absence of βHCG along with the recorded history of hysterectomy indicated that Ms. R was not pregnant at the time of testing or within the preceding few weeks. Once medical anomalies and substance use were ruled out, further classification of the psychiatric condition was undertaken.
One aspect of establishing a diagnosis for Ms. R is determining the presence of psychosis (eg, delusional thinking) (Table 1). Ms. R deliberately fabricated evidence of her pregnancy and manipulated family members, which indicated a low likelihood of delusions and supported a diagnostic alternative to psychosis.
Ms. R has a well-described history of manic episodes with current symptoms of a major depressive episode. The treatment team makes a diagnosis of bipolar I disorder, most recent episode depressed. The depressive symptoms Ms. R described were consistent with bipolar depression but did not explain her report of a pregnancy that is inconsistent with reality.
As is the case with Ms. R, diagnostic clarity often requires observation and evaluation over time. Building a strong therapeutic relationship with Ms. R in the context of an appropriate treatment plan allows the treatment team to explore the origin, motivations, and evolution of her thought content while managing her illness.
Confronting a patient about her false claims is likely to result in which of the following?
a) spontaneous resolution of symptoms
b) improved therapeutic alliance
c) degradation of the patient’s coping mechanism
d) violent outbursts by the patient
EVALUATION Confrontation
At admission, Ms. R remains resolute that she was pregnant and is suffering immense psychological distress secondary to the death of her child. Early in the treatment course, she is confronted with evidence indicating that her pregnancy was impossible. Shortly after this interaction, nursing staff alerts the treating physician that Ms. R experienced a “seizure-like spell” characterized by gross non-stereotyped jerking of the upper extremities, intact orientation, retention of bowel and bladder function, and coherent speech consistent with a diagnosis of pseudoseizure.2
Ms. R is transferred to a tertiary care facility for neurologic evaluation and observation. Ms. R repeatedly presents a photograph that she claims to be of her deceased child and implores the allied treatment team to advocate for discharge. Evaluation of Ms. R’s neurologic symptoms revealed no medical explanation for the “seizure-like spell” and she is transferred to the inpatient psychiatric hospital.
Upon return to the inpatient psychiatric unit, Ms. R receives intensive psychological exploration of her symptoms, thought content, and the foundation of her pregnancy claim. Within days, she acknowledges that the pregnancy was “not real” and that she was conscious of this fact in the months prior to hospitalization. She cites turmoil in her romantic relationship as the primary stimulus for her actions.
The authors’ observations
Ms. R’s reported pregnancy was not a delusion, but rather a deceitful exposition constructed with appropriate reality testing and a conscious awareness of the manipulation. This eliminated delusions as the explanation of her pregnancy claim. Although Ms. R initially rejected evidence refuting her belief of pregnancy, she recognized and accepted reality with appropriate intervention.
Factitious disorder vs malingering
Factitious disorder and malingering can present with intentional induction or report of symptoms or signs of a physical abnormality:
Factitious disorder imposed on the self is a willful misrepresentation or fabrication of signs or symptoms of an illness by a person in the absence of obvious personal gain that cannot be explained by a separate physical or mental illness (Table 2).3,4
Malingering is the intentional production or exaggeration of physical or psychological signs or symptoms with obvious secondary gain.
Malingering can be excluded in Ms. R’s case: She did not gain external reward by falsely reporting pregnancy. Although DSM-IV-TR (Table 2) assumes that the motivation for the patient with factitious disorder is to assume the sick role, DSM-5 merely states that the she (he) should present themselves as ill, impaired, or injured.3,4
Ms. R’s treatment team diagnosed factitious disorder imposed on self after careful exclusion of other causes for her symptoms. Bipolar I disorder, most recent episode depressed, also was diagnosed after considering Ms. R’s previous history of manic episodes and depressive symptoms at presentation.
Factitious disorder and other psychiatric conditions often are comorbid. Bipolar disorder, as in Ms. R’s case, as well as major depressive disorder commonly are comorbid with factitious disorder. It is also important to note that factitious disorder often occurs in the context of a personality disorder.5
Which of the following medications are FDA-approved for treating factitious disorder?
a) olanzapine-fluoxetine combination
b) lurasidone
c) valproic acid
d) all of the above
e) no medications are approved for treating factitious disorder
TREATMENT Support, drug therapy
Treatment of Ms. R’s factitious disorder consists of psychological interventions via psychotherapy and strengthening of social support. She participates in daily individual therapy sessions as well as several group therapy activities. Ms. R engages with her social worker to facilitate a successful transition to an appropriate support network and access community resources to aid her wellness.
The treatment team feels that her diagnosis of bipolar I disorder, most recent episode depressed, warrants pharmacologic intervention. Ms. R agrees to begin a mood stabilizer, valproic acid, instead of medications FDA-approved to treat bipolar depression, such as lurasidone or quetiapine, because she reports good efficacy and tolerability when she took it during a major depressive episode approximately 4 years earlier.
Valproic acid is started at 250 mg/d and increased to 1,000 mg/d. Ms. R tolerates the medication without observed or reported adverse effects.
The authors’ observations
Managing factitious disorder can be challenging; patients can evoke strong feelings of countertransference during treatment.3,6,7 Providers might feel that the patient does not need to be treated, or that the patient is “not really sick.” This may induce anger and animosity toward the patient (therapeutic nihilism).8 These negative emotions are likely to disrupt the patient–provider relationship and exacerbate the patient’s symptoms.
It is generally accepted that the patient should be made aware of the treatment plan, in an indirect and tactful way, so that the patient does not feel “outed.” Unmasking the patient—the process of instilling insight—is a delicate step and can be a stressful time for the patient.9 A confrontational approach often places the patient’s sick role in doubt and does not address the pathological aspect of the disorder.
It is rare for a patient to admit to fabricating symptoms; confronted, the patient is likely to double their efforts to maintain the rouse of a fictional disease.10,11 It is important for the treatment team to be aware that patients frequently leave the treatment facility against medical advice, seek a different provider, or even pursue legal action for defamation against the treating physician.
Treating comorbid medical and psychiatric conditions is important for successful management of a patient with factitious disorder. Initiating valproic acid to address Ms. R’s bipolar depression contributed to her overall psychiatric stability. Initial treatment with a medication that is FDA-approved for treating bipolar depression, such as lurasidone, quetiapine, or olanzapine-fluoxetine combination, should be considered as an alternative. We chose valproic acid for Ms. R because of its previous efficacy, good tolerability, and the patient’s high level of comfort with the medication.
Which of the following are risk factors for factitious disorder?
a) lengthy medical treatments or hospitalizations as a child
b) female sex
c) experience as a health care worker
d) all of the above
OUTCOME Stabilization
Successful treatment during Ms. R’s inpatient psychiatric admission results in improved insight, remission of suicidal ideation, and stabilization of mood lability. She is discharged to the care of her family with a plan to follow up with a psychotherapist and psychiatrist. Continued administration of valproic acid continues to be effective after discharge.
Ms. R engages in frequent follow-up with outpatient psychiatric services. She remains engaged in psychotherapy and psychiatric care 1 year after discharge. Ms. R has made no report of pregnancy or required hospitalization during this time. She expresses trust in the mental health care system and acknowledges the role treatment played in her improvement.
CASE Depressed after she says her baby died
Ms. R, age 50, is an African-American woman who presents to a psychiatric hospital under an involuntary commitment executed by local law enforcement. Her sister called the authorities because Ms. R reportedly told her that she is “very depressed” and wants to “end [her] life” by taking an overdose of medications after the death of her newborn 1 week earlier.
Ms. R states that she delivered a child at “full term” in the emergency department of an outside community hospital, and that her current psychiatric symptoms began after the child died from “SIDS” [sudden infant death syndrome] shortly after birth.
Ms. R describes depressive symptoms including depressed mood, anhedonia, decreased energy, feelings of guilt, decreased concentration, poor sleep, and suicidal ideation. She denies substance use or a medical condition that could have induced these symptoms, and denies symptoms of mania, anxiety, or psychosis at admission or during the previous year.
Ms. R reports a history of manic episodes that includes periods of elevated mood or irritability, impulsivity, increased energy, excessive spending despite negative consequences, lack of need for sleep, rapid thoughts, and rapid speech that impaired her social and occupational functioning. Her most recent manic episode was approximately 3 years before this admission. She reports a previous suicide attempt and a history of physical abuse from a former intimate partner.
Neither the findings of a physical examination nor the results of a screening test for serum β-human chorionic gonadotropin (βHCG) are consistent with pregnancy. Ms. R’s medical record reveals that she was hospitalized for a “cardiac workup” a week earlier and requested investigation of possible pregnancy, which was negative. Records also reveal that she had a hysterectomy 10 years earlier.
Although Ms. R’s sister and boyfriend support her claim of pregnancy, the patient’s young adult son refutes it and states that she “does stuff like this for attention.” Her son also reports receiving a forged sonogram picture that his mother found online 1 month earlier. Ms. R presents an obituary from a local newspaper for the child but, on further investigation, the photograph of the infant was discovered to be of another child, also obtained online. Ms. R’s family denies knowledge of potential external reward Ms. R could gain by claiming to be pregnant.
Which of the following diagnoses can be considered after Ms. R’s initial presentation?
a) somatic symptom disorder
b) major depressive disorder
c) bipolar I disorder
d) delusional disorder
The authors’ observations
Ms. R reported the recent death of a newborn that was incompatible with her medical history. Her family members revealed that Ms. R made an active effort to deceive them about the reported pregnancy. She also exhibited symptoms of a major depressive episode in the context of previous manic episodes and expressed suicidal ideation.
The first step in the diagnostic pathway was to rule out possible medical explanations, including pregnancy, which could account for the patient’s symptoms. Although the serum βHCG level usually returns to non-pregnant levels 2 to 4 weeks after delivery, it can take even longer in some women.1 The absence of βHCG along with the recorded history of hysterectomy indicated that Ms. R was not pregnant at the time of testing or within the preceding few weeks. Once medical anomalies and substance use were ruled out, further classification of the psychiatric condition was undertaken.
One aspect of establishing a diagnosis for Ms. R is determining the presence of psychosis (eg, delusional thinking) (Table 1). Ms. R deliberately fabricated evidence of her pregnancy and manipulated family members, which indicated a low likelihood of delusions and supported a diagnostic alternative to psychosis.
Ms. R has a well-described history of manic episodes with current symptoms of a major depressive episode. The treatment team makes a diagnosis of bipolar I disorder, most recent episode depressed. The depressive symptoms Ms. R described were consistent with bipolar depression but did not explain her report of a pregnancy that is inconsistent with reality.
As is the case with Ms. R, diagnostic clarity often requires observation and evaluation over time. Building a strong therapeutic relationship with Ms. R in the context of an appropriate treatment plan allows the treatment team to explore the origin, motivations, and evolution of her thought content while managing her illness.
Confronting a patient about her false claims is likely to result in which of the following?
a) spontaneous resolution of symptoms
b) improved therapeutic alliance
c) degradation of the patient’s coping mechanism
d) violent outbursts by the patient
EVALUATION Confrontation
At admission, Ms. R remains resolute that she was pregnant and is suffering immense psychological distress secondary to the death of her child. Early in the treatment course, she is confronted with evidence indicating that her pregnancy was impossible. Shortly after this interaction, nursing staff alerts the treating physician that Ms. R experienced a “seizure-like spell” characterized by gross non-stereotyped jerking of the upper extremities, intact orientation, retention of bowel and bladder function, and coherent speech consistent with a diagnosis of pseudoseizure.2
Ms. R is transferred to a tertiary care facility for neurologic evaluation and observation. Ms. R repeatedly presents a photograph that she claims to be of her deceased child and implores the allied treatment team to advocate for discharge. Evaluation of Ms. R’s neurologic symptoms revealed no medical explanation for the “seizure-like spell” and she is transferred to the inpatient psychiatric hospital.
Upon return to the inpatient psychiatric unit, Ms. R receives intensive psychological exploration of her symptoms, thought content, and the foundation of her pregnancy claim. Within days, she acknowledges that the pregnancy was “not real” and that she was conscious of this fact in the months prior to hospitalization. She cites turmoil in her romantic relationship as the primary stimulus for her actions.
The authors’ observations
Ms. R’s reported pregnancy was not a delusion, but rather a deceitful exposition constructed with appropriate reality testing and a conscious awareness of the manipulation. This eliminated delusions as the explanation of her pregnancy claim. Although Ms. R initially rejected evidence refuting her belief of pregnancy, she recognized and accepted reality with appropriate intervention.
Factitious disorder vs malingering
Factitious disorder and malingering can present with intentional induction or report of symptoms or signs of a physical abnormality:
Factitious disorder imposed on the self is a willful misrepresentation or fabrication of signs or symptoms of an illness by a person in the absence of obvious personal gain that cannot be explained by a separate physical or mental illness (Table 2).3,4
Malingering is the intentional production or exaggeration of physical or psychological signs or symptoms with obvious secondary gain.
Malingering can be excluded in Ms. R’s case: She did not gain external reward by falsely reporting pregnancy. Although DSM-IV-TR (Table 2) assumes that the motivation for the patient with factitious disorder is to assume the sick role, DSM-5 merely states that the she (he) should present themselves as ill, impaired, or injured.3,4
Ms. R’s treatment team diagnosed factitious disorder imposed on self after careful exclusion of other causes for her symptoms. Bipolar I disorder, most recent episode depressed, also was diagnosed after considering Ms. R’s previous history of manic episodes and depressive symptoms at presentation.
Factitious disorder and other psychiatric conditions often are comorbid. Bipolar disorder, as in Ms. R’s case, as well as major depressive disorder commonly are comorbid with factitious disorder. It is also important to note that factitious disorder often occurs in the context of a personality disorder.5
Which of the following medications are FDA-approved for treating factitious disorder?
a) olanzapine-fluoxetine combination
b) lurasidone
c) valproic acid
d) all of the above
e) no medications are approved for treating factitious disorder
TREATMENT Support, drug therapy
Treatment of Ms. R’s factitious disorder consists of psychological interventions via psychotherapy and strengthening of social support. She participates in daily individual therapy sessions as well as several group therapy activities. Ms. R engages with her social worker to facilitate a successful transition to an appropriate support network and access community resources to aid her wellness.
The treatment team feels that her diagnosis of bipolar I disorder, most recent episode depressed, warrants pharmacologic intervention. Ms. R agrees to begin a mood stabilizer, valproic acid, instead of medications FDA-approved to treat bipolar depression, such as lurasidone or quetiapine, because she reports good efficacy and tolerability when she took it during a major depressive episode approximately 4 years earlier.
Valproic acid is started at 250 mg/d and increased to 1,000 mg/d. Ms. R tolerates the medication without observed or reported adverse effects.
The authors’ observations
Managing factitious disorder can be challenging; patients can evoke strong feelings of countertransference during treatment.3,6,7 Providers might feel that the patient does not need to be treated, or that the patient is “not really sick.” This may induce anger and animosity toward the patient (therapeutic nihilism).8 These negative emotions are likely to disrupt the patient–provider relationship and exacerbate the patient’s symptoms.
It is generally accepted that the patient should be made aware of the treatment plan, in an indirect and tactful way, so that the patient does not feel “outed.” Unmasking the patient—the process of instilling insight—is a delicate step and can be a stressful time for the patient.9 A confrontational approach often places the patient’s sick role in doubt and does not address the pathological aspect of the disorder.
It is rare for a patient to admit to fabricating symptoms; confronted, the patient is likely to double their efforts to maintain the rouse of a fictional disease.10,11 It is important for the treatment team to be aware that patients frequently leave the treatment facility against medical advice, seek a different provider, or even pursue legal action for defamation against the treating physician.
Treating comorbid medical and psychiatric conditions is important for successful management of a patient with factitious disorder. Initiating valproic acid to address Ms. R’s bipolar depression contributed to her overall psychiatric stability. Initial treatment with a medication that is FDA-approved for treating bipolar depression, such as lurasidone, quetiapine, or olanzapine-fluoxetine combination, should be considered as an alternative. We chose valproic acid for Ms. R because of its previous efficacy, good tolerability, and the patient’s high level of comfort with the medication.
Which of the following are risk factors for factitious disorder?
a) lengthy medical treatments or hospitalizations as a child
b) female sex
c) experience as a health care worker
d) all of the above
OUTCOME Stabilization
Successful treatment during Ms. R’s inpatient psychiatric admission results in improved insight, remission of suicidal ideation, and stabilization of mood lability. She is discharged to the care of her family with a plan to follow up with a psychotherapist and psychiatrist. Continued administration of valproic acid continues to be effective after discharge.
Ms. R engages in frequent follow-up with outpatient psychiatric services. She remains engaged in psychotherapy and psychiatric care 1 year after discharge. Ms. R has made no report of pregnancy or required hospitalization during this time. She expresses trust in the mental health care system and acknowledges the role treatment played in her improvement.
1. Reyes FI, Winter JS, Faiman C. Postpartum disappearance of chorionic gonadotropin from the maternal and neonatal circulations. Am J Obstet Gynecol. 1985;153(5):486-489.
2. Avbersek A, Sisodiya S. Does the primary literature provide support for clinical signs used to distinguish psychogenic nonepileptic seizures from epileptic seizures? J Neurol Neurosurg Psychiatry. 2010;81(7):719-725.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Kapfhammer HP, Rothenhausler HM, Dietrich E, et al. Artifactual disorders—between deception and self-mutilation. Experiences in consultation psychiatry at a university clinic [in German]. Nervenarzt. 1998;69(5):401-409.
6. Feldman MD, Feldman JM. Tangled in the web: countertransference in the therapy of factitious disorders. Int J Psychiatry Med. 1995;25(4):389-399.
7. Wedel KR. A therapeutic confrontation approach to treating patients with factitious illness. Soc Work. 1971;16(2):69-73.
8. Feldman MD, Hamilton JC, Deemer HN. Factitious disorder. In: Phillips KA, ed. Somatoform and factitious disorder. Washington, DC: American Psychiatric Press; 2001:129-159.
9. Scher LM, Knudsen P, Leamon M. Somatic symptom and related disorders. In: Hales RE, Yudofsky SC, Weiss Roberts L, eds. The American Publishing Psychiatric Publishing textbook of psychiatry. Arlington, VA: American Psychiatric Publishing; 2014:531-556.
10. Lipsitt DR. Introduction. In: Feldman MD, Eisendrath SJ, eds. The spectrum of factitious disorders. Washington, DC: American Psychiatric Press; 1996:xix-xxviii.
11. van der Feltz-Cornelis CM. Confronting patients about a factitious disorder [in Dutch]. Ned Tidjschr Geneeskd. 2000;144(12):545-548.
1. Reyes FI, Winter JS, Faiman C. Postpartum disappearance of chorionic gonadotropin from the maternal and neonatal circulations. Am J Obstet Gynecol. 1985;153(5):486-489.
2. Avbersek A, Sisodiya S. Does the primary literature provide support for clinical signs used to distinguish psychogenic nonepileptic seizures from epileptic seizures? J Neurol Neurosurg Psychiatry. 2010;81(7):719-725.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Kapfhammer HP, Rothenhausler HM, Dietrich E, et al. Artifactual disorders—between deception and self-mutilation. Experiences in consultation psychiatry at a university clinic [in German]. Nervenarzt. 1998;69(5):401-409.
6. Feldman MD, Feldman JM. Tangled in the web: countertransference in the therapy of factitious disorders. Int J Psychiatry Med. 1995;25(4):389-399.
7. Wedel KR. A therapeutic confrontation approach to treating patients with factitious illness. Soc Work. 1971;16(2):69-73.
8. Feldman MD, Hamilton JC, Deemer HN. Factitious disorder. In: Phillips KA, ed. Somatoform and factitious disorder. Washington, DC: American Psychiatric Press; 2001:129-159.
9. Scher LM, Knudsen P, Leamon M. Somatic symptom and related disorders. In: Hales RE, Yudofsky SC, Weiss Roberts L, eds. The American Publishing Psychiatric Publishing textbook of psychiatry. Arlington, VA: American Psychiatric Publishing; 2014:531-556.
10. Lipsitt DR. Introduction. In: Feldman MD, Eisendrath SJ, eds. The spectrum of factitious disorders. Washington, DC: American Psychiatric Press; 1996:xix-xxviii.
11. van der Feltz-Cornelis CM. Confronting patients about a factitious disorder [in Dutch]. Ned Tidjschr Geneeskd. 2000;144(12):545-548.
Treated with a mood stabilizer, he becomes incontinent and walks oddly
CASE Rapid decline
Mr. X, age 67, is a businessman who had a diagnosis of bipolar depression 8 years ago, and who is being evaluated now for new-onset cognitive impairment, gait disturbance that resembles child-like steps, dyskinesia, and urinary incontinence of approximately 2 months’ duration. He has been treated for bipolar depression with valproic acid, 1,000 mg/d, and venlafaxine, 150 mg/d, without complaint until now, since the diagnosis was made 8 years ago. The serum valproic acid level, tested every month, is within the therapeutic range; liver function tests, ordered every 6 months, also are within the normal range.
Mr. X has become confined to his bedroom and needs assistance to walk. He has to be lifted to a standing position by 2 attendants, who bear his weight and instruct him to take one step at a time. He wears a diaper and needs assistance shaving, showering, and getting dressed. When the treatment team asks him about his condition, Mr. X turns to his wife to respond on his behalf. He is slow to speak and struggles to remember the details about his condition or the duration of his disability.
Mr. X is referred to a neurologist, based on cognitive impairment and gait disturbance, who orders an MRI scan of the brain that shows enlarged ventricles and some cortical atrophy (Figure 1). A neurosurgeon removes approximately 25 mL of CSF as a diagnostic and therapeutic intervention.
Videography of his ambulation, recorded before and after the CSF tap, shows slight improvement in gait. Mr. X is seen by a neurosurgery team, who recommends that he receive a ventriculoperitoneal shunt for hydrocephalus.
While awaiting surgical treatment, Mr. X’s psychotropic medications are withheld, and he is closely monitored for reemergence of psychiatric symptoms. Mr. X shows gradual but significant improvement in his gait within 8 to 10 weeks. His dyskinesia improves significantly, as does his cognitive function.
What additional testing is recommended beyond MRI?
a) complete blood count with differential
b) blood ammonia level
c) neuropsychological evaluation
d) APOE-e4 genetic testing
e) all the above
The authors’ observations
Normal pressure hydrocephalus (NPH) is characterized by gait disturbance, dementia, or urinary incontinence that is associated with dilation of the brain’s ventricular system with normal opening CSF pressure (Table 1). Several studies have reported that patients with NPH might exhibit neuropsychiatric symptoms,1-4 possibly related to alterations in central neurotransmitter activity.5 NPH patients could present with symptoms reflecting frontal dominance (Table 2,6-9). In a study of 35 patients with idiopathic NPH in a tertiary hospital in Brazil,10 psychiatric symptoms were established by formal psychiatric evaluation in 71%, notably anxiety, depression, and psychotic syndromes.
Mechanism responsible for gait disturbance
Gait disturbance typically is the first and most prominent symptom of the NPH triad. Gait disturbance in NPH can be progressive because of expansion of the ventricular system, mainly the lateral ventricles, leading to pressure on the corticospinal motor fibers descending to the lumbosacral spinal cord. Although there is no one type of gait disturbance indicative of NPH, it often is described as shuffling, magnetic, and wide-based.11 Slowness of gait and gait imbalance or disequilibrium are common and more likely to respond to shunting.12
Drug-induced gait disturbance is likely to result in parkinsonian symptoms.13 A possible mechanism involves inhibition of neurite outgrowth. Qian et al14 found that therapeutic plasma levels of valproic acid reduced cell proliferation and neurite outgrowth, using SY5Y neuroblastoma cells as a neuronal model. Researchers also reported that valproic acid reduced mRNA and protein levels of neurofilament 160; a possible mechanistic explanation involves inhibition of neurite outgrowth that leads to gait disturbance. These effects reversed 2 days after stopping valproic acid.
Another possible mechanism is related to γ-aminobutyric acid (GABA) pathway disturbance leading to dopamine inhibition. This postulates that valproic acid or a metabolite of valproic acid, such as Δ-2-valproate, which may be a more potent inhibitor of the GABA-degrading enzyme than valproic acid, could cause a transient inhibitory effect on dopaminergic pathways.15
Mechanism of mood stabilizer action
Valproic acid is incorporated into neuronal membranes in a saturable manner and appears to displace naturally occurring branched-chain phospholipids.16 Chronic valproic acid use reduces protein kinase C (PKC) activity in patients with mania.17 Elevated PKC activity has been observed in patients with mania and in animal models of mania.18 Valproic acid has antioxidant effects and has reversed early DNA damage caused by amphetamine in an animal model of mania.19 Valproic acid and lithium both reduce inositol biosynthesis; the mechanism of action for valproic acid is unique, however, resulting from decreased myo-inositol-1-phosphate synthase inhibition.20
There is not a strong correlation between serum valproic acid levels and antimanic effects, but levels in the range of 50 to 150 μg/mL generally are required for therapeutic effect.
Neuropsychiatric adverse effects of valproic acid
With most antiepileptic drugs, adverse effects mainly are dose-related and include sedation, drowsiness, incoordination, nausea, and fatigue. Careful dose titration can reduce the risk of these adverse effects. Research on mothers with epilepsy has shown an association between valproic acid exposure in utero and lower IQ and a higher prevalence of autism spectrum disorder in children.21
Adverse effects on cognitive functioning are infrequent; valproic acid improves cognition in select patients.22 In a 20-week randomized, observer-blinded, parallel-group trial, adding valproic acid to carbamazepine resulted in improvement in short-term verbal memory.23 In a group of geriatric patients (mean age 77 years), no adverse cognitive effects were observed with valproic acid use.24
Masmoudi et al25 evaluated dementia and extrapyramidal symptoms associated with long-term valproic acid use. Among the side effects attributed to valproic acid, parkinsonian syndromes and cognitive impairment were not commonly reported. In a prospective study, Armon et al26 found several abnormal symptoms and signs related to motor and cognitive function impairment in patients on long-term valproic acid therapy. These side effects might be related to a disturbance in the GABAergic pathways in the basal ganglia system. Note that Δ2-valproic acid, a metabolite of valproic acid, preferentially accumulates in select areas of the brain: the substantia nigra, superior and inferior colliculus, hippocampus, and medulla.
What is the next best step in management?
a) surgically implant a shunt
b) adjust the dosage of valproic acid
c) switch to monotherapy
d) switch to an alternative psychotropic medication
e) provide observation and follow-up
The authors’ observations
Unusual appearances of NPH symptoms could hinder early diagnosis and proper treatment. Mr. X was taking valproic acid and venlafaxine for bipolar depression, without any complaints, and was asymptomatic for 8 years—until he developed symptoms of NPH.
In patients who have what can be considered classic symptoms of NPH and are taking valproic acid, consider discontinuing the drug on a trial basis before resorting to a more invasive procedure. This strategy could significantly reduce the cost of health care and contribute to the overall well-being of the patient.
NPH associated with chronic valproic acid use is rare, supported by only 1 case report13 in our literature review. Based on the severity of symptoms and chance for misdiagnosis, it is essential to identify such cases and differentiate them from others with underlying neuropathology or a secondary cause, such as age-related dementia or Parkinson’s disease, to avoid the burden of unnecessary diagnostic testing on the patient and physician.
Family history also is important in cases presenting with sensorineural hearing loss,13 which follows a pattern of maternal inheritance. Consider genetic testing in such cases.
Earlier diagnosis of valproic acid-induced NPH enables specific interventions and treatment. Treatment of NPH includes one of several forms of shunting and appropriate neuroleptic therapy for behavioral symptoms. Although there is a significant risk (40% to 50%) of psychiatric and behavioral symptoms as a shunt-related complication, as many as 60% of operated patients showed objective improvement. This makes the diagnosis of NPH, and referral for appropriate surgical treatment of NPH, an important challenge to the psychiatrist.27
OUTCOME No reemergence
Findings on a repeat MRI 2.5 months after the CSF tap remain unchanged. Surgery is cancelled and medications are discontinued. Mr. X is advised to continue outpatient follow-up for monitoring of re-emerging symptoms of bipolar depression.
At a follow-up visit, Mr. X’s condition has returned to baseline. He ambulates spontaneously and responds to questions without evidence of cognitive deficit. He no longer is incontinent.
Follow-up MRI is performed and indicated normal results.
Neuropsychological testing is deemed unnecessary because Mr. X has fully recovered from cognitive clouding (and there would be no baseline results against which to compare current findings). Based on the medication history, the team concludes that prolonged use of valproic acid may have led to development of signs and symptoms of an NPH-like syndrome.
The authors’ observations
Awareness of an association of NPH with neuropsychiatric changes is important for clinical psychiatrists because early assessment and appropriate intervention can prevent associated long-term complications. Valproic acid is considered a relatively safe medication with few neurologic side effects, but the association of an NPH-like syndrome with chronic valproic acid use, documented in this case report, emphasizes the importance of studying long-term consequences of using valproic acid in geriatric patients. More such case reports need to be evaluated to study the association of neuropsychiatric complications with chronic valproic use in the geriatric population.
Mr. X apparently had cerebral atrophy with enlarged ventricles that was consistently evident for 10 years (Figure 2), although he has been maintained on valproic acid for 8 years. What is intriguing in this case is that discontinuing valproic acid relieved the triad of incontinence, imbalance, and memory deficits indicative of NPH. Mr. X remains free of these symptoms.
1. Pinner G, Johnson H, Bouman WP, et al. Psychiatric manifestations of normal-pressure hydrocephalus: a short review and unusual case. Int Psychogeriatr. 1997;9(4):465-470.
2. Alao AO, Naprawa SA. Psychiatric complications of hydrocephalus. Int J Psychiatry Med. 2001;31(3):337-340.
3. Lindqvist G, Andersson H, Bilting M, et al. Normal pressure hydrocephalus: psychiatric findings before and after shunt operation classified in a new diagnostic system for organic psychiatry. Acta Psychiatr Scand Suppl. 1993;373:18-32.
4. Kito Y, Kazui H, Kubo Y, et al. Neuropsychiatric symptoms in patients with idiopathic normal pressure hydrocephalus. Behav Neurol. 2009;21(3):165-174.
5. Markianos M, Lafazanos S, Koutsis G, et al. CSF neurotransmitter metabolites and neuropsychiatric symptomatology in patients with normal pressure hydrocephalus. Clin Neurol Neurosurg. 2009;111(3):231-234.
6. McIntyre AW, Emsley RA. Shoplifting associated with normal-pressure hydrocephalus: report of a case. J Geriatr Psychiatry Neurol. 1990;3(4):229-230.
7. Kwentus JA, Hart RP. Normal pressure hydrocephalus presenting as mania. J Nerv Ment Dis. 1987;175(8):500-502.
8. Bloom KK, Kraft WA. Paranoia—an unusual presentation of hydrocephalus. Am J Phys Med Rehabil. 1998;77(2):157-159.
9. Yusim A, Anbarasan D, Bernstein C, et al. Normal pressure hydrocephalus presenting as Othello syndrome: case presentation and review of the literature. Am J Psychiatry. 2008;165(9):1119-1125.
10. Oliveira MF, Oliveira JR, Rotta JM, et al. Psychiatric symptoms are present in most of the patients with idiopathic normal pressure hydrocephalus. Arq Neuropsiquiatr. 2014;72(6):435-438.
11. Marmarou A, Young HF, Aygok GA, et al. Diagnosis and management of idiopathic normal-pressure hydrocephalus: a prospective study in 151 patients. J Neurosurg. 2005;102(6):987-997.
12. Bugalho P, Guimarães J. Gait disturbance in normal pressure hydrocephalus: a clinical study. Parkinsonism Relat Disord. 2007;13(7):434-437.
13. Evans MD, Shinar R, Yaari R. Reversible dementia and gait disturbance after prolonged use of valproic acid. Seizure. 2011;20(6):509-511.
14. Qian Y, Zheng Y, Tiffany-Castiglioni E. Valproate reversibly reduces neurite outgrowth by human SY5Y neuroblastoma cells. Brain Res. 2009;1302:21-33.
15. Löscher W. Pharmacological, toxicological and neurochemical effects of delta 2(E)-valproate in animals. Pharm Weekbl Sci. 1992;14(3A):139-143.
16. Siafaka-Kapadai A, Patiris M, Bowden C, et al. Incorporation of [3H]-valproic acid into lipids in GT1-7 neurons. Biochem Pharmacol. 1998;56(2):207-212.
17. Hahn CG, Umapathy, Wagn HY, et al. Lithium and valproic acid treatments reduce PKC activation and receptor-G-protein coupling in platelets of bipolar manic patients. J Psychiatr Res. 2005;39(4):35-63.
18. Einat H, Manji HK. Cellular plasticity cascades: genes-to-behavior pathways in animal models of bipolar disorder. Biol Psychiatry. 2006;59(12):1160-1171.
19. Andreazza AC, Frey BN, Stertz L, et al. Effects of lithium and valproate on DNA damage and oxidative stress markers in an animal model of mania [abstract P10]. Bipolar Disord. 2007;9(suppl 1):16.
20. Galit S, Shirley M, Ora K, et al. Effect of valproate derivatives on human brain myo-inositol-1-phosphate (MIP) synthase activity and amphetamine-induced rearing. Pharmacol Rep. 2007;59(4):402-407.
21. Kennedy GM, Lhatoo SD. CNS adverse events associated with antiepileptic drugs. CNS Drugs. 2008;22(9):739-760.
22. Prevey ML, Delaney RC, Cramer JA, et al. Effect of valproate on cognitive functioning. Comparison with carbamazepine. The Department of Veteran Affairs Epilepsy Cooperative Study 264 Group. Arch Neurol. 1996;53(10):1008-1016.
23. Aldenkamp AP, Baker G, Mulder OG, et al. A multicenter randomized clinical study to evaluate the effect on cognitive function of topiramate compared with valproate as add-on therapy to carbamazepine in patients with partial-onset seizures. Epilepsia. 2000;41(9):1167-1178.
24. Craig I, Tallis R. Impact of valproate and phenytoin on cognitive function in elderly patients: results of a single-blind randomized comparative study. Epilepsia. 1994;35(2):381-390.
25. Masmoudi K, Gras-Champel V, Bonnet I, et al. Dementia and extrapyramidal problems caused by long-term valproic acid [in French]. Therapie. 2000;55(5):629-634.
26. Armon C, Shin C, Miller P, et al. Reversible parkinsonism and cognitive impairment with chronic valproate use. Neurology. 1996;47(3):626-635.
27. Price TR, Tucker GJ. Psychiatric and behavioral manifestations of normal pressure hydrocephalus. A case report and brief review. J Nerv Ment Dis. 1977;164(1):51-55.
CASE Rapid decline
Mr. X, age 67, is a businessman who had a diagnosis of bipolar depression 8 years ago, and who is being evaluated now for new-onset cognitive impairment, gait disturbance that resembles child-like steps, dyskinesia, and urinary incontinence of approximately 2 months’ duration. He has been treated for bipolar depression with valproic acid, 1,000 mg/d, and venlafaxine, 150 mg/d, without complaint until now, since the diagnosis was made 8 years ago. The serum valproic acid level, tested every month, is within the therapeutic range; liver function tests, ordered every 6 months, also are within the normal range.
Mr. X has become confined to his bedroom and needs assistance to walk. He has to be lifted to a standing position by 2 attendants, who bear his weight and instruct him to take one step at a time. He wears a diaper and needs assistance shaving, showering, and getting dressed. When the treatment team asks him about his condition, Mr. X turns to his wife to respond on his behalf. He is slow to speak and struggles to remember the details about his condition or the duration of his disability.
Mr. X is referred to a neurologist, based on cognitive impairment and gait disturbance, who orders an MRI scan of the brain that shows enlarged ventricles and some cortical atrophy (Figure 1). A neurosurgeon removes approximately 25 mL of CSF as a diagnostic and therapeutic intervention.
Videography of his ambulation, recorded before and after the CSF tap, shows slight improvement in gait. Mr. X is seen by a neurosurgery team, who recommends that he receive a ventriculoperitoneal shunt for hydrocephalus.
While awaiting surgical treatment, Mr. X’s psychotropic medications are withheld, and he is closely monitored for reemergence of psychiatric symptoms. Mr. X shows gradual but significant improvement in his gait within 8 to 10 weeks. His dyskinesia improves significantly, as does his cognitive function.
What additional testing is recommended beyond MRI?
a) complete blood count with differential
b) blood ammonia level
c) neuropsychological evaluation
d) APOE-e4 genetic testing
e) all the above
The authors’ observations
Normal pressure hydrocephalus (NPH) is characterized by gait disturbance, dementia, or urinary incontinence that is associated with dilation of the brain’s ventricular system with normal opening CSF pressure (Table 1). Several studies have reported that patients with NPH might exhibit neuropsychiatric symptoms,1-4 possibly related to alterations in central neurotransmitter activity.5 NPH patients could present with symptoms reflecting frontal dominance (Table 2,6-9). In a study of 35 patients with idiopathic NPH in a tertiary hospital in Brazil,10 psychiatric symptoms were established by formal psychiatric evaluation in 71%, notably anxiety, depression, and psychotic syndromes.
Mechanism responsible for gait disturbance
Gait disturbance typically is the first and most prominent symptom of the NPH triad. Gait disturbance in NPH can be progressive because of expansion of the ventricular system, mainly the lateral ventricles, leading to pressure on the corticospinal motor fibers descending to the lumbosacral spinal cord. Although there is no one type of gait disturbance indicative of NPH, it often is described as shuffling, magnetic, and wide-based.11 Slowness of gait and gait imbalance or disequilibrium are common and more likely to respond to shunting.12
Drug-induced gait disturbance is likely to result in parkinsonian symptoms.13 A possible mechanism involves inhibition of neurite outgrowth. Qian et al14 found that therapeutic plasma levels of valproic acid reduced cell proliferation and neurite outgrowth, using SY5Y neuroblastoma cells as a neuronal model. Researchers also reported that valproic acid reduced mRNA and protein levels of neurofilament 160; a possible mechanistic explanation involves inhibition of neurite outgrowth that leads to gait disturbance. These effects reversed 2 days after stopping valproic acid.
Another possible mechanism is related to γ-aminobutyric acid (GABA) pathway disturbance leading to dopamine inhibition. This postulates that valproic acid or a metabolite of valproic acid, such as Δ-2-valproate, which may be a more potent inhibitor of the GABA-degrading enzyme than valproic acid, could cause a transient inhibitory effect on dopaminergic pathways.15
Mechanism of mood stabilizer action
Valproic acid is incorporated into neuronal membranes in a saturable manner and appears to displace naturally occurring branched-chain phospholipids.16 Chronic valproic acid use reduces protein kinase C (PKC) activity in patients with mania.17 Elevated PKC activity has been observed in patients with mania and in animal models of mania.18 Valproic acid has antioxidant effects and has reversed early DNA damage caused by amphetamine in an animal model of mania.19 Valproic acid and lithium both reduce inositol biosynthesis; the mechanism of action for valproic acid is unique, however, resulting from decreased myo-inositol-1-phosphate synthase inhibition.20
There is not a strong correlation between serum valproic acid levels and antimanic effects, but levels in the range of 50 to 150 μg/mL generally are required for therapeutic effect.
Neuropsychiatric adverse effects of valproic acid
With most antiepileptic drugs, adverse effects mainly are dose-related and include sedation, drowsiness, incoordination, nausea, and fatigue. Careful dose titration can reduce the risk of these adverse effects. Research on mothers with epilepsy has shown an association between valproic acid exposure in utero and lower IQ and a higher prevalence of autism spectrum disorder in children.21
Adverse effects on cognitive functioning are infrequent; valproic acid improves cognition in select patients.22 In a 20-week randomized, observer-blinded, parallel-group trial, adding valproic acid to carbamazepine resulted in improvement in short-term verbal memory.23 In a group of geriatric patients (mean age 77 years), no adverse cognitive effects were observed with valproic acid use.24
Masmoudi et al25 evaluated dementia and extrapyramidal symptoms associated with long-term valproic acid use. Among the side effects attributed to valproic acid, parkinsonian syndromes and cognitive impairment were not commonly reported. In a prospective study, Armon et al26 found several abnormal symptoms and signs related to motor and cognitive function impairment in patients on long-term valproic acid therapy. These side effects might be related to a disturbance in the GABAergic pathways in the basal ganglia system. Note that Δ2-valproic acid, a metabolite of valproic acid, preferentially accumulates in select areas of the brain: the substantia nigra, superior and inferior colliculus, hippocampus, and medulla.
What is the next best step in management?
a) surgically implant a shunt
b) adjust the dosage of valproic acid
c) switch to monotherapy
d) switch to an alternative psychotropic medication
e) provide observation and follow-up
The authors’ observations
Unusual appearances of NPH symptoms could hinder early diagnosis and proper treatment. Mr. X was taking valproic acid and venlafaxine for bipolar depression, without any complaints, and was asymptomatic for 8 years—until he developed symptoms of NPH.
In patients who have what can be considered classic symptoms of NPH and are taking valproic acid, consider discontinuing the drug on a trial basis before resorting to a more invasive procedure. This strategy could significantly reduce the cost of health care and contribute to the overall well-being of the patient.
NPH associated with chronic valproic acid use is rare, supported by only 1 case report13 in our literature review. Based on the severity of symptoms and chance for misdiagnosis, it is essential to identify such cases and differentiate them from others with underlying neuropathology or a secondary cause, such as age-related dementia or Parkinson’s disease, to avoid the burden of unnecessary diagnostic testing on the patient and physician.
Family history also is important in cases presenting with sensorineural hearing loss,13 which follows a pattern of maternal inheritance. Consider genetic testing in such cases.
Earlier diagnosis of valproic acid-induced NPH enables specific interventions and treatment. Treatment of NPH includes one of several forms of shunting and appropriate neuroleptic therapy for behavioral symptoms. Although there is a significant risk (40% to 50%) of psychiatric and behavioral symptoms as a shunt-related complication, as many as 60% of operated patients showed objective improvement. This makes the diagnosis of NPH, and referral for appropriate surgical treatment of NPH, an important challenge to the psychiatrist.27
OUTCOME No reemergence
Findings on a repeat MRI 2.5 months after the CSF tap remain unchanged. Surgery is cancelled and medications are discontinued. Mr. X is advised to continue outpatient follow-up for monitoring of re-emerging symptoms of bipolar depression.
At a follow-up visit, Mr. X’s condition has returned to baseline. He ambulates spontaneously and responds to questions without evidence of cognitive deficit. He no longer is incontinent.
Follow-up MRI is performed and indicated normal results.
Neuropsychological testing is deemed unnecessary because Mr. X has fully recovered from cognitive clouding (and there would be no baseline results against which to compare current findings). Based on the medication history, the team concludes that prolonged use of valproic acid may have led to development of signs and symptoms of an NPH-like syndrome.
The authors’ observations
Awareness of an association of NPH with neuropsychiatric changes is important for clinical psychiatrists because early assessment and appropriate intervention can prevent associated long-term complications. Valproic acid is considered a relatively safe medication with few neurologic side effects, but the association of an NPH-like syndrome with chronic valproic acid use, documented in this case report, emphasizes the importance of studying long-term consequences of using valproic acid in geriatric patients. More such case reports need to be evaluated to study the association of neuropsychiatric complications with chronic valproic use in the geriatric population.
Mr. X apparently had cerebral atrophy with enlarged ventricles that was consistently evident for 10 years (Figure 2), although he has been maintained on valproic acid for 8 years. What is intriguing in this case is that discontinuing valproic acid relieved the triad of incontinence, imbalance, and memory deficits indicative of NPH. Mr. X remains free of these symptoms.
CASE Rapid decline
Mr. X, age 67, is a businessman who had a diagnosis of bipolar depression 8 years ago, and who is being evaluated now for new-onset cognitive impairment, gait disturbance that resembles child-like steps, dyskinesia, and urinary incontinence of approximately 2 months’ duration. He has been treated for bipolar depression with valproic acid, 1,000 mg/d, and venlafaxine, 150 mg/d, without complaint until now, since the diagnosis was made 8 years ago. The serum valproic acid level, tested every month, is within the therapeutic range; liver function tests, ordered every 6 months, also are within the normal range.
Mr. X has become confined to his bedroom and needs assistance to walk. He has to be lifted to a standing position by 2 attendants, who bear his weight and instruct him to take one step at a time. He wears a diaper and needs assistance shaving, showering, and getting dressed. When the treatment team asks him about his condition, Mr. X turns to his wife to respond on his behalf. He is slow to speak and struggles to remember the details about his condition or the duration of his disability.
Mr. X is referred to a neurologist, based on cognitive impairment and gait disturbance, who orders an MRI scan of the brain that shows enlarged ventricles and some cortical atrophy (Figure 1). A neurosurgeon removes approximately 25 mL of CSF as a diagnostic and therapeutic intervention.
Videography of his ambulation, recorded before and after the CSF tap, shows slight improvement in gait. Mr. X is seen by a neurosurgery team, who recommends that he receive a ventriculoperitoneal shunt for hydrocephalus.
While awaiting surgical treatment, Mr. X’s psychotropic medications are withheld, and he is closely monitored for reemergence of psychiatric symptoms. Mr. X shows gradual but significant improvement in his gait within 8 to 10 weeks. His dyskinesia improves significantly, as does his cognitive function.
What additional testing is recommended beyond MRI?
a) complete blood count with differential
b) blood ammonia level
c) neuropsychological evaluation
d) APOE-e4 genetic testing
e) all the above
The authors’ observations
Normal pressure hydrocephalus (NPH) is characterized by gait disturbance, dementia, or urinary incontinence that is associated with dilation of the brain’s ventricular system with normal opening CSF pressure (Table 1). Several studies have reported that patients with NPH might exhibit neuropsychiatric symptoms,1-4 possibly related to alterations in central neurotransmitter activity.5 NPH patients could present with symptoms reflecting frontal dominance (Table 2,6-9). In a study of 35 patients with idiopathic NPH in a tertiary hospital in Brazil,10 psychiatric symptoms were established by formal psychiatric evaluation in 71%, notably anxiety, depression, and psychotic syndromes.
Mechanism responsible for gait disturbance
Gait disturbance typically is the first and most prominent symptom of the NPH triad. Gait disturbance in NPH can be progressive because of expansion of the ventricular system, mainly the lateral ventricles, leading to pressure on the corticospinal motor fibers descending to the lumbosacral spinal cord. Although there is no one type of gait disturbance indicative of NPH, it often is described as shuffling, magnetic, and wide-based.11 Slowness of gait and gait imbalance or disequilibrium are common and more likely to respond to shunting.12
Drug-induced gait disturbance is likely to result in parkinsonian symptoms.13 A possible mechanism involves inhibition of neurite outgrowth. Qian et al14 found that therapeutic plasma levels of valproic acid reduced cell proliferation and neurite outgrowth, using SY5Y neuroblastoma cells as a neuronal model. Researchers also reported that valproic acid reduced mRNA and protein levels of neurofilament 160; a possible mechanistic explanation involves inhibition of neurite outgrowth that leads to gait disturbance. These effects reversed 2 days after stopping valproic acid.
Another possible mechanism is related to γ-aminobutyric acid (GABA) pathway disturbance leading to dopamine inhibition. This postulates that valproic acid or a metabolite of valproic acid, such as Δ-2-valproate, which may be a more potent inhibitor of the GABA-degrading enzyme than valproic acid, could cause a transient inhibitory effect on dopaminergic pathways.15
Mechanism of mood stabilizer action
Valproic acid is incorporated into neuronal membranes in a saturable manner and appears to displace naturally occurring branched-chain phospholipids.16 Chronic valproic acid use reduces protein kinase C (PKC) activity in patients with mania.17 Elevated PKC activity has been observed in patients with mania and in animal models of mania.18 Valproic acid has antioxidant effects and has reversed early DNA damage caused by amphetamine in an animal model of mania.19 Valproic acid and lithium both reduce inositol biosynthesis; the mechanism of action for valproic acid is unique, however, resulting from decreased myo-inositol-1-phosphate synthase inhibition.20
There is not a strong correlation between serum valproic acid levels and antimanic effects, but levels in the range of 50 to 150 μg/mL generally are required for therapeutic effect.
Neuropsychiatric adverse effects of valproic acid
With most antiepileptic drugs, adverse effects mainly are dose-related and include sedation, drowsiness, incoordination, nausea, and fatigue. Careful dose titration can reduce the risk of these adverse effects. Research on mothers with epilepsy has shown an association between valproic acid exposure in utero and lower IQ and a higher prevalence of autism spectrum disorder in children.21
Adverse effects on cognitive functioning are infrequent; valproic acid improves cognition in select patients.22 In a 20-week randomized, observer-blinded, parallel-group trial, adding valproic acid to carbamazepine resulted in improvement in short-term verbal memory.23 In a group of geriatric patients (mean age 77 years), no adverse cognitive effects were observed with valproic acid use.24
Masmoudi et al25 evaluated dementia and extrapyramidal symptoms associated with long-term valproic acid use. Among the side effects attributed to valproic acid, parkinsonian syndromes and cognitive impairment were not commonly reported. In a prospective study, Armon et al26 found several abnormal symptoms and signs related to motor and cognitive function impairment in patients on long-term valproic acid therapy. These side effects might be related to a disturbance in the GABAergic pathways in the basal ganglia system. Note that Δ2-valproic acid, a metabolite of valproic acid, preferentially accumulates in select areas of the brain: the substantia nigra, superior and inferior colliculus, hippocampus, and medulla.
What is the next best step in management?
a) surgically implant a shunt
b) adjust the dosage of valproic acid
c) switch to monotherapy
d) switch to an alternative psychotropic medication
e) provide observation and follow-up
The authors’ observations
Unusual appearances of NPH symptoms could hinder early diagnosis and proper treatment. Mr. X was taking valproic acid and venlafaxine for bipolar depression, without any complaints, and was asymptomatic for 8 years—until he developed symptoms of NPH.
In patients who have what can be considered classic symptoms of NPH and are taking valproic acid, consider discontinuing the drug on a trial basis before resorting to a more invasive procedure. This strategy could significantly reduce the cost of health care and contribute to the overall well-being of the patient.
NPH associated with chronic valproic acid use is rare, supported by only 1 case report13 in our literature review. Based on the severity of symptoms and chance for misdiagnosis, it is essential to identify such cases and differentiate them from others with underlying neuropathology or a secondary cause, such as age-related dementia or Parkinson’s disease, to avoid the burden of unnecessary diagnostic testing on the patient and physician.
Family history also is important in cases presenting with sensorineural hearing loss,13 which follows a pattern of maternal inheritance. Consider genetic testing in such cases.
Earlier diagnosis of valproic acid-induced NPH enables specific interventions and treatment. Treatment of NPH includes one of several forms of shunting and appropriate neuroleptic therapy for behavioral symptoms. Although there is a significant risk (40% to 50%) of psychiatric and behavioral symptoms as a shunt-related complication, as many as 60% of operated patients showed objective improvement. This makes the diagnosis of NPH, and referral for appropriate surgical treatment of NPH, an important challenge to the psychiatrist.27
OUTCOME No reemergence
Findings on a repeat MRI 2.5 months after the CSF tap remain unchanged. Surgery is cancelled and medications are discontinued. Mr. X is advised to continue outpatient follow-up for monitoring of re-emerging symptoms of bipolar depression.
At a follow-up visit, Mr. X’s condition has returned to baseline. He ambulates spontaneously and responds to questions without evidence of cognitive deficit. He no longer is incontinent.
Follow-up MRI is performed and indicated normal results.
Neuropsychological testing is deemed unnecessary because Mr. X has fully recovered from cognitive clouding (and there would be no baseline results against which to compare current findings). Based on the medication history, the team concludes that prolonged use of valproic acid may have led to development of signs and symptoms of an NPH-like syndrome.
The authors’ observations
Awareness of an association of NPH with neuropsychiatric changes is important for clinical psychiatrists because early assessment and appropriate intervention can prevent associated long-term complications. Valproic acid is considered a relatively safe medication with few neurologic side effects, but the association of an NPH-like syndrome with chronic valproic acid use, documented in this case report, emphasizes the importance of studying long-term consequences of using valproic acid in geriatric patients. More such case reports need to be evaluated to study the association of neuropsychiatric complications with chronic valproic use in the geriatric population.
Mr. X apparently had cerebral atrophy with enlarged ventricles that was consistently evident for 10 years (Figure 2), although he has been maintained on valproic acid for 8 years. What is intriguing in this case is that discontinuing valproic acid relieved the triad of incontinence, imbalance, and memory deficits indicative of NPH. Mr. X remains free of these symptoms.
1. Pinner G, Johnson H, Bouman WP, et al. Psychiatric manifestations of normal-pressure hydrocephalus: a short review and unusual case. Int Psychogeriatr. 1997;9(4):465-470.
2. Alao AO, Naprawa SA. Psychiatric complications of hydrocephalus. Int J Psychiatry Med. 2001;31(3):337-340.
3. Lindqvist G, Andersson H, Bilting M, et al. Normal pressure hydrocephalus: psychiatric findings before and after shunt operation classified in a new diagnostic system for organic psychiatry. Acta Psychiatr Scand Suppl. 1993;373:18-32.
4. Kito Y, Kazui H, Kubo Y, et al. Neuropsychiatric symptoms in patients with idiopathic normal pressure hydrocephalus. Behav Neurol. 2009;21(3):165-174.
5. Markianos M, Lafazanos S, Koutsis G, et al. CSF neurotransmitter metabolites and neuropsychiatric symptomatology in patients with normal pressure hydrocephalus. Clin Neurol Neurosurg. 2009;111(3):231-234.
6. McIntyre AW, Emsley RA. Shoplifting associated with normal-pressure hydrocephalus: report of a case. J Geriatr Psychiatry Neurol. 1990;3(4):229-230.
7. Kwentus JA, Hart RP. Normal pressure hydrocephalus presenting as mania. J Nerv Ment Dis. 1987;175(8):500-502.
8. Bloom KK, Kraft WA. Paranoia—an unusual presentation of hydrocephalus. Am J Phys Med Rehabil. 1998;77(2):157-159.
9. Yusim A, Anbarasan D, Bernstein C, et al. Normal pressure hydrocephalus presenting as Othello syndrome: case presentation and review of the literature. Am J Psychiatry. 2008;165(9):1119-1125.
10. Oliveira MF, Oliveira JR, Rotta JM, et al. Psychiatric symptoms are present in most of the patients with idiopathic normal pressure hydrocephalus. Arq Neuropsiquiatr. 2014;72(6):435-438.
11. Marmarou A, Young HF, Aygok GA, et al. Diagnosis and management of idiopathic normal-pressure hydrocephalus: a prospective study in 151 patients. J Neurosurg. 2005;102(6):987-997.
12. Bugalho P, Guimarães J. Gait disturbance in normal pressure hydrocephalus: a clinical study. Parkinsonism Relat Disord. 2007;13(7):434-437.
13. Evans MD, Shinar R, Yaari R. Reversible dementia and gait disturbance after prolonged use of valproic acid. Seizure. 2011;20(6):509-511.
14. Qian Y, Zheng Y, Tiffany-Castiglioni E. Valproate reversibly reduces neurite outgrowth by human SY5Y neuroblastoma cells. Brain Res. 2009;1302:21-33.
15. Löscher W. Pharmacological, toxicological and neurochemical effects of delta 2(E)-valproate in animals. Pharm Weekbl Sci. 1992;14(3A):139-143.
16. Siafaka-Kapadai A, Patiris M, Bowden C, et al. Incorporation of [3H]-valproic acid into lipids in GT1-7 neurons. Biochem Pharmacol. 1998;56(2):207-212.
17. Hahn CG, Umapathy, Wagn HY, et al. Lithium and valproic acid treatments reduce PKC activation and receptor-G-protein coupling in platelets of bipolar manic patients. J Psychiatr Res. 2005;39(4):35-63.
18. Einat H, Manji HK. Cellular plasticity cascades: genes-to-behavior pathways in animal models of bipolar disorder. Biol Psychiatry. 2006;59(12):1160-1171.
19. Andreazza AC, Frey BN, Stertz L, et al. Effects of lithium and valproate on DNA damage and oxidative stress markers in an animal model of mania [abstract P10]. Bipolar Disord. 2007;9(suppl 1):16.
20. Galit S, Shirley M, Ora K, et al. Effect of valproate derivatives on human brain myo-inositol-1-phosphate (MIP) synthase activity and amphetamine-induced rearing. Pharmacol Rep. 2007;59(4):402-407.
21. Kennedy GM, Lhatoo SD. CNS adverse events associated with antiepileptic drugs. CNS Drugs. 2008;22(9):739-760.
22. Prevey ML, Delaney RC, Cramer JA, et al. Effect of valproate on cognitive functioning. Comparison with carbamazepine. The Department of Veteran Affairs Epilepsy Cooperative Study 264 Group. Arch Neurol. 1996;53(10):1008-1016.
23. Aldenkamp AP, Baker G, Mulder OG, et al. A multicenter randomized clinical study to evaluate the effect on cognitive function of topiramate compared with valproate as add-on therapy to carbamazepine in patients with partial-onset seizures. Epilepsia. 2000;41(9):1167-1178.
24. Craig I, Tallis R. Impact of valproate and phenytoin on cognitive function in elderly patients: results of a single-blind randomized comparative study. Epilepsia. 1994;35(2):381-390.
25. Masmoudi K, Gras-Champel V, Bonnet I, et al. Dementia and extrapyramidal problems caused by long-term valproic acid [in French]. Therapie. 2000;55(5):629-634.
26. Armon C, Shin C, Miller P, et al. Reversible parkinsonism and cognitive impairment with chronic valproate use. Neurology. 1996;47(3):626-635.
27. Price TR, Tucker GJ. Psychiatric and behavioral manifestations of normal pressure hydrocephalus. A case report and brief review. J Nerv Ment Dis. 1977;164(1):51-55.
1. Pinner G, Johnson H, Bouman WP, et al. Psychiatric manifestations of normal-pressure hydrocephalus: a short review and unusual case. Int Psychogeriatr. 1997;9(4):465-470.
2. Alao AO, Naprawa SA. Psychiatric complications of hydrocephalus. Int J Psychiatry Med. 2001;31(3):337-340.
3. Lindqvist G, Andersson H, Bilting M, et al. Normal pressure hydrocephalus: psychiatric findings before and after shunt operation classified in a new diagnostic system for organic psychiatry. Acta Psychiatr Scand Suppl. 1993;373:18-32.
4. Kito Y, Kazui H, Kubo Y, et al. Neuropsychiatric symptoms in patients with idiopathic normal pressure hydrocephalus. Behav Neurol. 2009;21(3):165-174.
5. Markianos M, Lafazanos S, Koutsis G, et al. CSF neurotransmitter metabolites and neuropsychiatric symptomatology in patients with normal pressure hydrocephalus. Clin Neurol Neurosurg. 2009;111(3):231-234.
6. McIntyre AW, Emsley RA. Shoplifting associated with normal-pressure hydrocephalus: report of a case. J Geriatr Psychiatry Neurol. 1990;3(4):229-230.
7. Kwentus JA, Hart RP. Normal pressure hydrocephalus presenting as mania. J Nerv Ment Dis. 1987;175(8):500-502.
8. Bloom KK, Kraft WA. Paranoia—an unusual presentation of hydrocephalus. Am J Phys Med Rehabil. 1998;77(2):157-159.
9. Yusim A, Anbarasan D, Bernstein C, et al. Normal pressure hydrocephalus presenting as Othello syndrome: case presentation and review of the literature. Am J Psychiatry. 2008;165(9):1119-1125.
10. Oliveira MF, Oliveira JR, Rotta JM, et al. Psychiatric symptoms are present in most of the patients with idiopathic normal pressure hydrocephalus. Arq Neuropsiquiatr. 2014;72(6):435-438.
11. Marmarou A, Young HF, Aygok GA, et al. Diagnosis and management of idiopathic normal-pressure hydrocephalus: a prospective study in 151 patients. J Neurosurg. 2005;102(6):987-997.
12. Bugalho P, Guimarães J. Gait disturbance in normal pressure hydrocephalus: a clinical study. Parkinsonism Relat Disord. 2007;13(7):434-437.
13. Evans MD, Shinar R, Yaari R. Reversible dementia and gait disturbance after prolonged use of valproic acid. Seizure. 2011;20(6):509-511.
14. Qian Y, Zheng Y, Tiffany-Castiglioni E. Valproate reversibly reduces neurite outgrowth by human SY5Y neuroblastoma cells. Brain Res. 2009;1302:21-33.
15. Löscher W. Pharmacological, toxicological and neurochemical effects of delta 2(E)-valproate in animals. Pharm Weekbl Sci. 1992;14(3A):139-143.
16. Siafaka-Kapadai A, Patiris M, Bowden C, et al. Incorporation of [3H]-valproic acid into lipids in GT1-7 neurons. Biochem Pharmacol. 1998;56(2):207-212.
17. Hahn CG, Umapathy, Wagn HY, et al. Lithium and valproic acid treatments reduce PKC activation and receptor-G-protein coupling in platelets of bipolar manic patients. J Psychiatr Res. 2005;39(4):35-63.
18. Einat H, Manji HK. Cellular plasticity cascades: genes-to-behavior pathways in animal models of bipolar disorder. Biol Psychiatry. 2006;59(12):1160-1171.
19. Andreazza AC, Frey BN, Stertz L, et al. Effects of lithium and valproate on DNA damage and oxidative stress markers in an animal model of mania [abstract P10]. Bipolar Disord. 2007;9(suppl 1):16.
20. Galit S, Shirley M, Ora K, et al. Effect of valproate derivatives on human brain myo-inositol-1-phosphate (MIP) synthase activity and amphetamine-induced rearing. Pharmacol Rep. 2007;59(4):402-407.
21. Kennedy GM, Lhatoo SD. CNS adverse events associated with antiepileptic drugs. CNS Drugs. 2008;22(9):739-760.
22. Prevey ML, Delaney RC, Cramer JA, et al. Effect of valproate on cognitive functioning. Comparison with carbamazepine. The Department of Veteran Affairs Epilepsy Cooperative Study 264 Group. Arch Neurol. 1996;53(10):1008-1016.
23. Aldenkamp AP, Baker G, Mulder OG, et al. A multicenter randomized clinical study to evaluate the effect on cognitive function of topiramate compared with valproate as add-on therapy to carbamazepine in patients with partial-onset seizures. Epilepsia. 2000;41(9):1167-1178.
24. Craig I, Tallis R. Impact of valproate and phenytoin on cognitive function in elderly patients: results of a single-blind randomized comparative study. Epilepsia. 1994;35(2):381-390.
25. Masmoudi K, Gras-Champel V, Bonnet I, et al. Dementia and extrapyramidal problems caused by long-term valproic acid [in French]. Therapie. 2000;55(5):629-634.
26. Armon C, Shin C, Miller P, et al. Reversible parkinsonism and cognitive impairment with chronic valproate use. Neurology. 1996;47(3):626-635.
27. Price TR, Tucker GJ. Psychiatric and behavioral manifestations of normal pressure hydrocephalus. A case report and brief review. J Nerv Ment Dis. 1977;164(1):51-55.