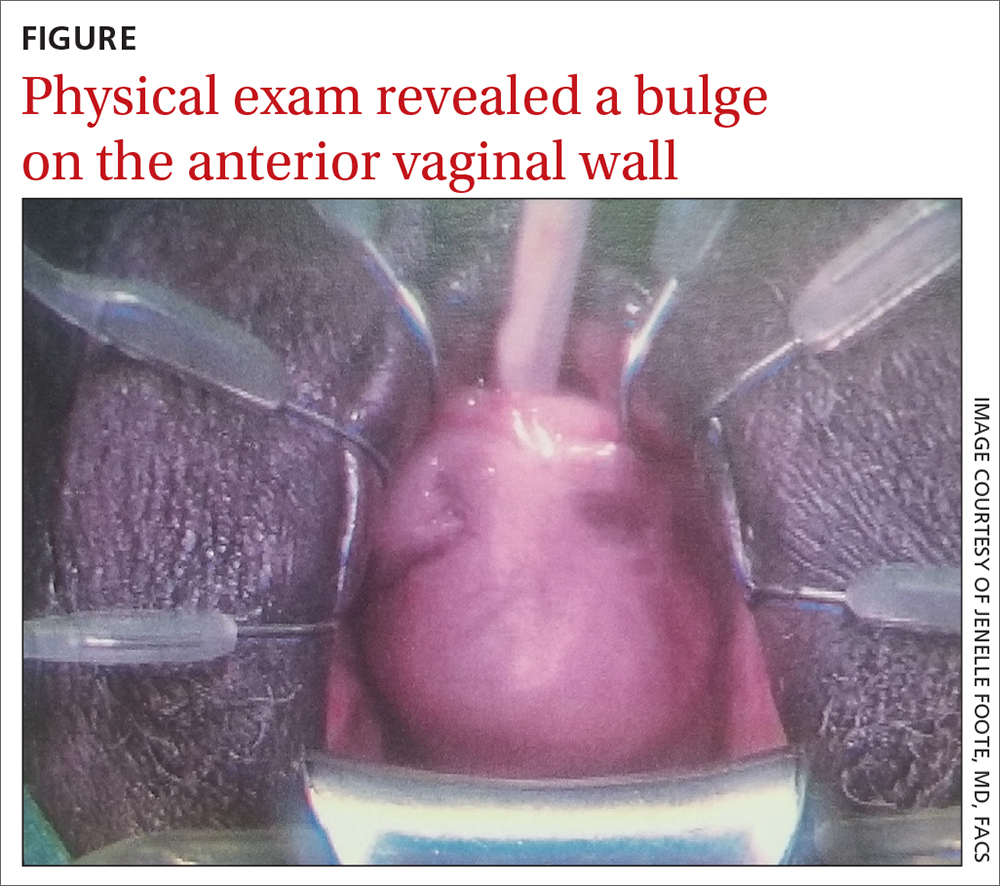
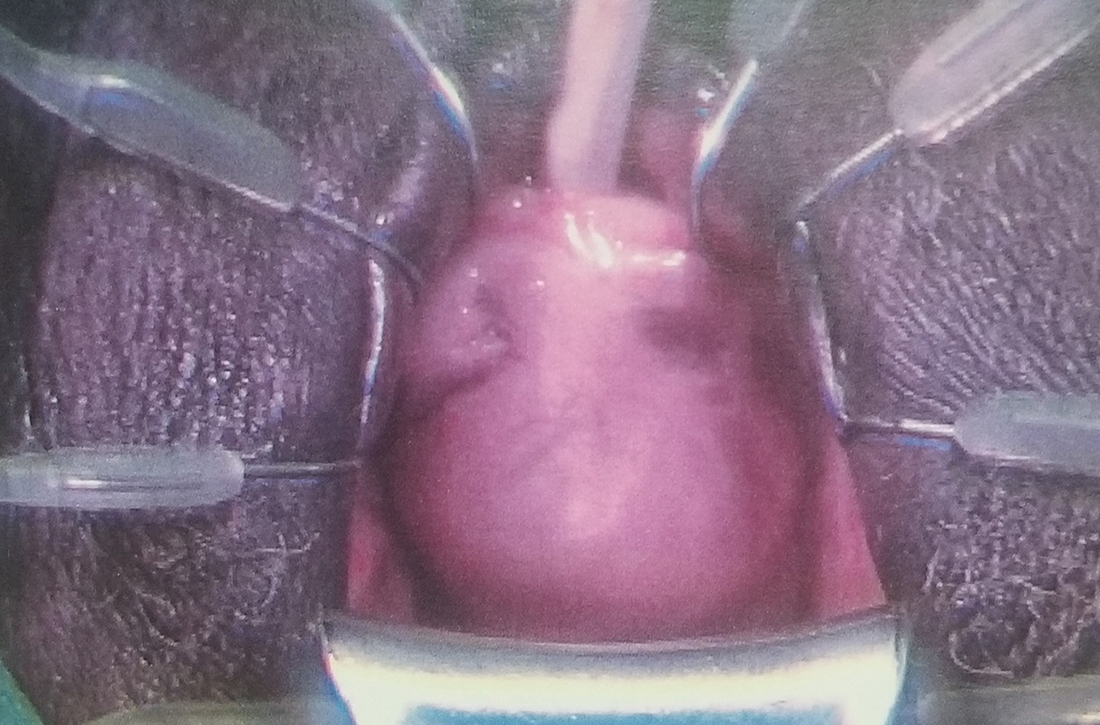
User login
Approach to Pancytopenia in a Deployed Service Member
Pancytopenia is a condition in which all 3 hematologic cell lines are lower than expected in the blood, often representing either an increase in cellular destruction or decrease in bone marrow production. Destruction often occurs in the setting of autoimmune conditions (eg, systemic lupus erythematosus, rheumatoid arthritis) or splenic sequestration, often affecting erythrocytes and platelets more than leukocytes. Decreased production represents central etiologies, which are often due to nutritional deficiencies, infections, drug toxicities, or malabsorption.1 Pancytopenia secondary to vitamin B12 deficiency is rare, accounting for about 5% of the hematologic manifestations of symptomatic vitamin B12 deficient patients.2
Pernicious anemia, named for a once lethal disease, is a form of vitamin B12 (cobalamin) deficiency that results from an autoimmune (type II hypersensitivity) reaction to gastric parietal cells or intrinsic factor. Antibodies bind to gastric parietal cells and reduce gastric acid production, leading to atrophic gastritis, or they bind intrinsic factor and block the binding and absorption of vitamin B12 in the gastrointestinal tract. While first described in the 1820s, it was not until a century later when scientists were studying hematopoiesis in response to the heavy casualty burden from battlefield exsanguination in World War I that dogs fed raw liver were noted to have significantly better blood regeneration response than those fed cooked liver. This discovery led physicians Minot and Murphy to use raw liver to treat pernicious anemia and found that jaundice improved, reticulocyte counts increased, and hemoglobin (Hb) concentration improved, resulting in the duo becoming the first American recipients of the Nobel Prize in physiology or medicine.3 It was ultimately determined in 1948 by chemists Folkers and Todd that the active ingredient in raw liver responsible for this phenomenon was vitamin B12.4
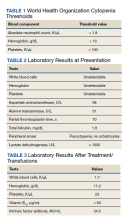
Patients with pernicious anemia typically present with macrocytic anemia, low reticulocyte count, hypersegmented neutrophils, as well as mild leukopenia and/or thrombocytopenia, distinguishable from folate deficiency by an elevated serum methylmalonic acid level. World Health Organization cytopenia thresholds are listed in Table 1.5 Treatment consists of lifelong vitamin B12 supplementation, and endoscopic screening is often recommended after diagnosis due to increased risk of gastrointestinal malignancy.6 Pernicious anemia can be difficult to distinguish from thrombotic thrombocytopenia purpura (TTP), a microangiopathic hemolytic anemia that can cause rapid end-organ failure and death if treatment is delayed.7 While pernicious anemia is not typically hemolytic, case reports of hemolysis in severe deficiency have been reported.7 Adequate bone marrow response to hemolysis in TTP results in an elevated reticulocyte count, which can be useful in differentiating from pernicious anemia where there is typically an inadequate bone marrow response and low reticulocyte count.8,9
The approach to working up pancytopenia begins with a detailed history inquiring about medications, exposures (benzenes, pesticides), alcohol use, and infection history. A thorough physical examination may help point the health care practitioner (HCP) toward a certain etiology, as the differential for pancytopenia is broad. In the deployed soldier downrange, resources are often limited, and the history/physical are crucial in preventing an expensive and unnecessary workup.
Case Presentation
A 24-year-old active-duty female patient presented in late December 2020 to a theater hospital in Djibouti after a witnessed syncopal episode. She had a history of Hashimoto thyroiditis and was taking levothyroxine sodium 75 mcg daily. The patient reported gluten intolerance, which was never formally evaluated. The syncopal episode lasted a few seconds and was not associated with any prodromal or postictal symptoms. No seizure activity was observed, and she had no history of syncopal episodes. She reported that she had been feeling ill 24 to 48 hours prior, with nausea, fatigue, decreased oral intake, decreased urine output, and 2 episodes of nonbilious, nonbloody emesis.
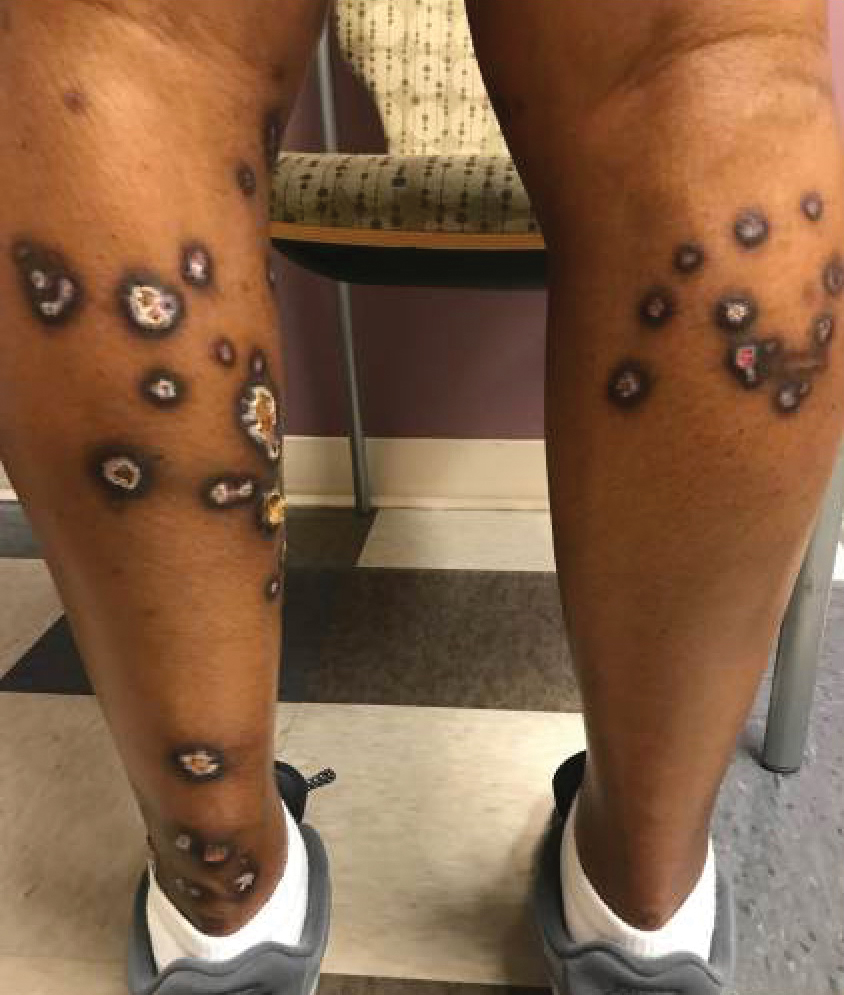
When the patient arrived, she was tachycardic with heart rate in the 130s beats per minute (baseline, 100-110 beats per minute), febrile (103 °F), and had systolic blood pressure (SBP) in the low 100s (baseline, SBP 120s-130s). An electrocardiogram and chest radiographs were unremarkable. Her complete blood count (CBC) could not be processed due to Hb and platelet levels too low to detect on assay (Table 2). Lactate dehydrogenase (LDH) was elevated at > 1000 U/L with mild elevation in liver enzymes (aspartate aminotransferase, 98 U/L; alanine aminotransferase, 51 U/L) and prolonged partial thromboplastin time 70 seconds. She did not report any increased bleeding or bruising. The peripheral blood smear demonstrated pancytopenia, without any schistocytes, and she was started on broad-spectrum antibiotics for presumed sepsis from urinary source and possible TTP.
The patient received 5 units of packed red blood cells, transfusion of platelets, and 2 doses of vitamin B12 in Djibouti with clinical improvement and resolution of orthostasis, hypotension, tachycardia, and fever. Her final posttransfusion CBC showed a Hb level of 11.2 g/dL, white blood cell (WBC) count of 1.7 K/µL, and platelet count of 23 K/µL (Table 3). Two days later her Hb level was 9.0 g/dL, WBC count 1.8 K/µL, and platelet count was 12 K/µL. She was evacuated via air to Landstuhl Regional Medical Center (LRMC) in Germany within 48 hours of presentation, given limited testing capabilities and persistent anemia and thrombocytopenia, refractory to transfusion, concerning for aplastic anemia or acute leukemia.
On arrival at LRMC, she was transfused 1 unit of platelets and given 3 doses of intramuscular vitamin B12 for undetectable levels (< 50 pg/mL) at presentation. An extensive infectious workup was obtained, which did not reveal any viral, bacterial, or parasitic causes. The patient also had a bone marrow biopsy performed at a civilian site, which revealed hypocellular bone marrow. She was transferred to Walter Reed National Military Medical Center (WRNMMC) for further workup and evaluation, given the infectious workup, which was negative. Concern for hematologic malignancy remained. At the time of her arrival, the laboratory values had drastically improved with vitamin supplementation. The patient’s absolute reticulocyte count indicated adequate bone marrow response and because of her improvement, a repeat bone marrow biopsy was not performed.
Intrinsic factor antibodies were elevated (34.5 AU/mL; reference range, 0.0-1.1), which confirmed that this patient’s underlying etiology was secondary to pernicious anemia. The patient continued to improve and repeat vitamin B12 and folate levels revealed that she was responding to therapy. At discharge, intramuscular vitamin B12 injections were planned to continue monthly, indefinitely per guidelines. Oral supplementation is typically avoided due to poor absorption.
Of note, during her inpatient admission at WRNMMC, further evaluation of reported gluten intolerance was performed, which revealed a negative celiac disease panel (IgG/IgA tissue transglutaminase antibodies). On discharge, she was to establish care with gastroenterology for further evaluation, likely including endoscopic evaluation, at her next duty station. She was able to resume full travel and duty functions on discharge from WRNMMC.
Discussion
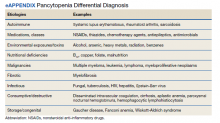
We highlight a complex case of pancytopenia secondary to pernicious anemia in a deployed service member. With limited resources downrange, the workup of pancytopenia can be resource intensive, expensive, and time sensitive, which can have detrimental impacts on medical readiness. Additionally, undiagnosed coagulopathies can have lethal consequences in a deployed service member where bleeding risk may be elevated depending on the mission. The differential for pancytopenia is vast, and given its relative rarity in pernicious anemia, the HCP must use key components of the history and laboratory results to narrow the differential (eAppendix).10
Pernicious anemia commonly presents as an isolated anemia. In a study looking at the hematologic manifestations of 201 cohort patients with well-documented vitamin B12 deficiency, 5% had symptomatic pancytopenia and 1.5% had a hemolytic anemia.2 The majority (> 67%) of hematologic abnormalities were correctable with cobalamin replacement.2 In our case, the solider presented with symptomatic anemia, manifesting as syncope, and was found to have transfusion-resistant pancytopenia.She had a hemolytic anemia with an LDH > 1000 U/L, haptoglobin < 3 mg/dL, and mild transaminitis with hyperbilirubinemia (1.8 mg/dL). No schistocytes were observed on peripheral smear, suggesting intramedullary hemolysis, which is believed to be due to the destruction of megaloblastic cells by macrophages in bone marrow.11 A French study found high LDH levels and low reticulocyte counts to be strongly suggestive of vitamin B12 deficiency and helpful in differentiating pernicious anemia from TTP, given that bone marrow response to anemia in TTP is preserved.8
While vitamin B12 deficiency is not often associated with hemolytic anemia, multiple cases have been reported in the literature.6 Screening for vitamin B12 deficiency may have shortened this patient’s clinical course and limited the need for air evacuation to a stateside quaternary medical center. However, testing for cobalamin levels in overseas deployed environments is difficult, timely, and costly. New technologies, such as optical sensors, can detect vitamin B12 levels in the blood in < 1 minute and offer portable, low-cost options that may be useful in the deployed military setting.12
Diet plays a key role in this case, since the patient had a reported history of gluten intolerance, although it was never documented or evaluated prior to this presentation. Prior to deployment, the patient ate mostly rice, potatoes, and vegetables. While deployed in an austere environment, food options were limited. These conditions forced her to intermittently consume gluten products, which led to gastrointestinal issues, exacerbating her nutritional deficiencies. In the 2 months before her first syncopal episode, she reported worsening fatigue that impacted her ability to exercise. Vitamin B12 stores often take years to deplete, suggesting that she had a chronic nutritional deficiency before deployment. Another possibility was that she developed an autoimmune gastritis that acutely worsened in the setting of poor nutritional intake. Her history of Hashimoto thyroiditis is also important, as up to one-third of patients with autoimmune thyroid disease have been associated with pernicious anemia (range, 3%-32%) with certain shared human leukocyte antigen alleles implicated in autoimmune gastritis.13,14
Conclusions
This rare case of pernicious anemia presenting as pancytopenia illustrates the challenge in working up pancytopenia, especially in austere military environments with limited testing capabilities. Screening for chronic dietary and nutritional deficiency is important in a service member, raising the question of what role predeployment screening may have and what dietary accommodations may be available during overseas deployments, which can potentially dampen inflammation of the gastrointestinal tract, especially for those with preexisting autoimmune gastrointestinal conditions. Also, newer technology allows portable, low-cost testing of cobalamin and may aid in its diagnosis. In patients who are anemic with low vitamin B12, HCPs can begin vitamin B12 supplementation while continuing the workup (eg, antibody testing, endoscopy). If the patient responds appropriately, further workup becomes less urgent, therefore, decreasing resource use and increasing military readiness. When hemolysis is present, a low reticulocyte count can be beneficial to help differentiate this condition from TTP, a life-threatening condition that must also be ruled out or treated. Pernicious anemia should be on the differential in any patients with autoimmune conditions presenting with cytopenias, especially in those with a history of autoimmune thyroid disorders.
1. Takeshima M, Ishikawa H, Kitadate A, et al. Anorexia nervosa-associated pancytopenia mimicking idiopathic aplastic anemia: a case report. BMC Psychiatry. 2018;18(1):150. doi:10.1186/s12888-018-1743-6
2. Andrès E, Affenberger S, Zimmer J, et al. Current hematological findings in cobalamin deficiency. A study of 201 consecutive patients with documented cobalamin deficiency. Clin Lab Haematol. 2006;28(1):50-56. doi:10.1111/j.1365-2257.2006.00755.x
3. Sinclair L. Recognizing, treating and understanding pernicious anaemia. J R Soc Med. 2008;101(5):262-264. doi:10.1258/jrsm.2008.081006
4. Shampo MA, Kyle RA, Steensma DP. William Murphy—Nobel Prize for the treatment of pernicious anemia. Mayo Clin Proc. 2006;81(6):726. doi:10.4065/81.6.726
5. Hong M, He G. The 2016 revision to the World Health Organization classification of myelodysplastic syndromes. J Transl Int Med. 2017;5(3):139-143. doi:10.1515/jtim-2017-0002
6. Tunio NA, Sheriff MZ, Cooper G. Prevalence of gastric cancer in patients with pernicious anemia: a population-based study. Am J Gastroenterol. 2020;115:S665. doi:10.14309/01.ajg.0000707332.16739.72
7. Bailey M, Maestas T, Betancourt R, Mikhael D, Babiker HM. A rare cause of thrombotic thrombocytopenic purpura- (TTP-) like syndrome, vitamin B12 deficiency: interpretation of significant pathological findings. Case Rep Hematol. 2019;2019:1529306. doi:10.1155/2019/1529306
8. Stanley M, Michalski JM. Thrombotic Thrombocytopenic Purpura. StatPearls Publishing LLC; 2021.
9. Noël N, Maigné G, Tertian G, et al. Hemolysis and schistocytosis in the emergency department: consider pseudothrombotic microangiopathy related to vitamin B12 deficiency. QJM. 2013;106(11):1017-1022. doi:10.1093/qjmed/hct142
10. Chiravuri S, De Jesus O. Pancytopenia. StatPearls Publishing LLC; 2021.
11. Gladstone E. Pernicious anemia presenting with pancytopenia and hemolysis: a case report. February 8, 2019. Accessed June 9, 2022. https://www.journalmc.org/index.php/JMC/article/view/3269/2563
12. ScienceDaily. Developing a sensor for vitamin B12 deficiency. October 17, 2016. Accessed June 9, 2022. https://www.sciencedaily.com/releases/2016/10/161017103221.htm
13. Rodriguez NM, Shackelford K. Pernicious Anemia. StatPearls Publishing LLC; 2021.
14. Fernando MM, Stevens CR, Walsh EC, et al. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet. 2008;4(4):e1000024. doi:10.1371/journal.pgen.1000024
Pancytopenia is a condition in which all 3 hematologic cell lines are lower than expected in the blood, often representing either an increase in cellular destruction or decrease in bone marrow production. Destruction often occurs in the setting of autoimmune conditions (eg, systemic lupus erythematosus, rheumatoid arthritis) or splenic sequestration, often affecting erythrocytes and platelets more than leukocytes. Decreased production represents central etiologies, which are often due to nutritional deficiencies, infections, drug toxicities, or malabsorption.1 Pancytopenia secondary to vitamin B12 deficiency is rare, accounting for about 5% of the hematologic manifestations of symptomatic vitamin B12 deficient patients.2
Pernicious anemia, named for a once lethal disease, is a form of vitamin B12 (cobalamin) deficiency that results from an autoimmune (type II hypersensitivity) reaction to gastric parietal cells or intrinsic factor. Antibodies bind to gastric parietal cells and reduce gastric acid production, leading to atrophic gastritis, or they bind intrinsic factor and block the binding and absorption of vitamin B12 in the gastrointestinal tract. While first described in the 1820s, it was not until a century later when scientists were studying hematopoiesis in response to the heavy casualty burden from battlefield exsanguination in World War I that dogs fed raw liver were noted to have significantly better blood regeneration response than those fed cooked liver. This discovery led physicians Minot and Murphy to use raw liver to treat pernicious anemia and found that jaundice improved, reticulocyte counts increased, and hemoglobin (Hb) concentration improved, resulting in the duo becoming the first American recipients of the Nobel Prize in physiology or medicine.3 It was ultimately determined in 1948 by chemists Folkers and Todd that the active ingredient in raw liver responsible for this phenomenon was vitamin B12.4
Patients with pernicious anemia typically present with macrocytic anemia, low reticulocyte count, hypersegmented neutrophils, as well as mild leukopenia and/or thrombocytopenia, distinguishable from folate deficiency by an elevated serum methylmalonic acid level. World Health Organization cytopenia thresholds are listed in Table 1.5 Treatment consists of lifelong vitamin B12 supplementation, and endoscopic screening is often recommended after diagnosis due to increased risk of gastrointestinal malignancy.6 Pernicious anemia can be difficult to distinguish from thrombotic thrombocytopenia purpura (TTP), a microangiopathic hemolytic anemia that can cause rapid end-organ failure and death if treatment is delayed.7 While pernicious anemia is not typically hemolytic, case reports of hemolysis in severe deficiency have been reported.7 Adequate bone marrow response to hemolysis in TTP results in an elevated reticulocyte count, which can be useful in differentiating from pernicious anemia where there is typically an inadequate bone marrow response and low reticulocyte count.8,9
The approach to working up pancytopenia begins with a detailed history inquiring about medications, exposures (benzenes, pesticides), alcohol use, and infection history. A thorough physical examination may help point the health care practitioner (HCP) toward a certain etiology, as the differential for pancytopenia is broad. In the deployed soldier downrange, resources are often limited, and the history/physical are crucial in preventing an expensive and unnecessary workup.
Case Presentation
A 24-year-old active-duty female patient presented in late December 2020 to a theater hospital in Djibouti after a witnessed syncopal episode. She had a history of Hashimoto thyroiditis and was taking levothyroxine sodium 75 mcg daily. The patient reported gluten intolerance, which was never formally evaluated. The syncopal episode lasted a few seconds and was not associated with any prodromal or postictal symptoms. No seizure activity was observed, and she had no history of syncopal episodes. She reported that she had been feeling ill 24 to 48 hours prior, with nausea, fatigue, decreased oral intake, decreased urine output, and 2 episodes of nonbilious, nonbloody emesis.
When the patient arrived, she was tachycardic with heart rate in the 130s beats per minute (baseline, 100-110 beats per minute), febrile (103 °F), and had systolic blood pressure (SBP) in the low 100s (baseline, SBP 120s-130s). An electrocardiogram and chest radiographs were unremarkable. Her complete blood count (CBC) could not be processed due to Hb and platelet levels too low to detect on assay (Table 2). Lactate dehydrogenase (LDH) was elevated at > 1000 U/L with mild elevation in liver enzymes (aspartate aminotransferase, 98 U/L; alanine aminotransferase, 51 U/L) and prolonged partial thromboplastin time 70 seconds. She did not report any increased bleeding or bruising. The peripheral blood smear demonstrated pancytopenia, without any schistocytes, and she was started on broad-spectrum antibiotics for presumed sepsis from urinary source and possible TTP.
The patient received 5 units of packed red blood cells, transfusion of platelets, and 2 doses of vitamin B12 in Djibouti with clinical improvement and resolution of orthostasis, hypotension, tachycardia, and fever. Her final posttransfusion CBC showed a Hb level of 11.2 g/dL, white blood cell (WBC) count of 1.7 K/µL, and platelet count of 23 K/µL (Table 3). Two days later her Hb level was 9.0 g/dL, WBC count 1.8 K/µL, and platelet count was 12 K/µL. She was evacuated via air to Landstuhl Regional Medical Center (LRMC) in Germany within 48 hours of presentation, given limited testing capabilities and persistent anemia and thrombocytopenia, refractory to transfusion, concerning for aplastic anemia or acute leukemia.
On arrival at LRMC, she was transfused 1 unit of platelets and given 3 doses of intramuscular vitamin B12 for undetectable levels (< 50 pg/mL) at presentation. An extensive infectious workup was obtained, which did not reveal any viral, bacterial, or parasitic causes. The patient also had a bone marrow biopsy performed at a civilian site, which revealed hypocellular bone marrow. She was transferred to Walter Reed National Military Medical Center (WRNMMC) for further workup and evaluation, given the infectious workup, which was negative. Concern for hematologic malignancy remained. At the time of her arrival, the laboratory values had drastically improved with vitamin supplementation. The patient’s absolute reticulocyte count indicated adequate bone marrow response and because of her improvement, a repeat bone marrow biopsy was not performed.
Intrinsic factor antibodies were elevated (34.5 AU/mL; reference range, 0.0-1.1), which confirmed that this patient’s underlying etiology was secondary to pernicious anemia. The patient continued to improve and repeat vitamin B12 and folate levels revealed that she was responding to therapy. At discharge, intramuscular vitamin B12 injections were planned to continue monthly, indefinitely per guidelines. Oral supplementation is typically avoided due to poor absorption.
Of note, during her inpatient admission at WRNMMC, further evaluation of reported gluten intolerance was performed, which revealed a negative celiac disease panel (IgG/IgA tissue transglutaminase antibodies). On discharge, she was to establish care with gastroenterology for further evaluation, likely including endoscopic evaluation, at her next duty station. She was able to resume full travel and duty functions on discharge from WRNMMC.
Discussion
We highlight a complex case of pancytopenia secondary to pernicious anemia in a deployed service member. With limited resources downrange, the workup of pancytopenia can be resource intensive, expensive, and time sensitive, which can have detrimental impacts on medical readiness. Additionally, undiagnosed coagulopathies can have lethal consequences in a deployed service member where bleeding risk may be elevated depending on the mission. The differential for pancytopenia is vast, and given its relative rarity in pernicious anemia, the HCP must use key components of the history and laboratory results to narrow the differential (eAppendix).10
Pernicious anemia commonly presents as an isolated anemia. In a study looking at the hematologic manifestations of 201 cohort patients with well-documented vitamin B12 deficiency, 5% had symptomatic pancytopenia and 1.5% had a hemolytic anemia.2 The majority (> 67%) of hematologic abnormalities were correctable with cobalamin replacement.2 In our case, the solider presented with symptomatic anemia, manifesting as syncope, and was found to have transfusion-resistant pancytopenia.She had a hemolytic anemia with an LDH > 1000 U/L, haptoglobin < 3 mg/dL, and mild transaminitis with hyperbilirubinemia (1.8 mg/dL). No schistocytes were observed on peripheral smear, suggesting intramedullary hemolysis, which is believed to be due to the destruction of megaloblastic cells by macrophages in bone marrow.11 A French study found high LDH levels and low reticulocyte counts to be strongly suggestive of vitamin B12 deficiency and helpful in differentiating pernicious anemia from TTP, given that bone marrow response to anemia in TTP is preserved.8
While vitamin B12 deficiency is not often associated with hemolytic anemia, multiple cases have been reported in the literature.6 Screening for vitamin B12 deficiency may have shortened this patient’s clinical course and limited the need for air evacuation to a stateside quaternary medical center. However, testing for cobalamin levels in overseas deployed environments is difficult, timely, and costly. New technologies, such as optical sensors, can detect vitamin B12 levels in the blood in < 1 minute and offer portable, low-cost options that may be useful in the deployed military setting.12
Diet plays a key role in this case, since the patient had a reported history of gluten intolerance, although it was never documented or evaluated prior to this presentation. Prior to deployment, the patient ate mostly rice, potatoes, and vegetables. While deployed in an austere environment, food options were limited. These conditions forced her to intermittently consume gluten products, which led to gastrointestinal issues, exacerbating her nutritional deficiencies. In the 2 months before her first syncopal episode, she reported worsening fatigue that impacted her ability to exercise. Vitamin B12 stores often take years to deplete, suggesting that she had a chronic nutritional deficiency before deployment. Another possibility was that she developed an autoimmune gastritis that acutely worsened in the setting of poor nutritional intake. Her history of Hashimoto thyroiditis is also important, as up to one-third of patients with autoimmune thyroid disease have been associated with pernicious anemia (range, 3%-32%) with certain shared human leukocyte antigen alleles implicated in autoimmune gastritis.13,14
Conclusions
This rare case of pernicious anemia presenting as pancytopenia illustrates the challenge in working up pancytopenia, especially in austere military environments with limited testing capabilities. Screening for chronic dietary and nutritional deficiency is important in a service member, raising the question of what role predeployment screening may have and what dietary accommodations may be available during overseas deployments, which can potentially dampen inflammation of the gastrointestinal tract, especially for those with preexisting autoimmune gastrointestinal conditions. Also, newer technology allows portable, low-cost testing of cobalamin and may aid in its diagnosis. In patients who are anemic with low vitamin B12, HCPs can begin vitamin B12 supplementation while continuing the workup (eg, antibody testing, endoscopy). If the patient responds appropriately, further workup becomes less urgent, therefore, decreasing resource use and increasing military readiness. When hemolysis is present, a low reticulocyte count can be beneficial to help differentiate this condition from TTP, a life-threatening condition that must also be ruled out or treated. Pernicious anemia should be on the differential in any patients with autoimmune conditions presenting with cytopenias, especially in those with a history of autoimmune thyroid disorders.
Pancytopenia is a condition in which all 3 hematologic cell lines are lower than expected in the blood, often representing either an increase in cellular destruction or decrease in bone marrow production. Destruction often occurs in the setting of autoimmune conditions (eg, systemic lupus erythematosus, rheumatoid arthritis) or splenic sequestration, often affecting erythrocytes and platelets more than leukocytes. Decreased production represents central etiologies, which are often due to nutritional deficiencies, infections, drug toxicities, or malabsorption.1 Pancytopenia secondary to vitamin B12 deficiency is rare, accounting for about 5% of the hematologic manifestations of symptomatic vitamin B12 deficient patients.2
Pernicious anemia, named for a once lethal disease, is a form of vitamin B12 (cobalamin) deficiency that results from an autoimmune (type II hypersensitivity) reaction to gastric parietal cells or intrinsic factor. Antibodies bind to gastric parietal cells and reduce gastric acid production, leading to atrophic gastritis, or they bind intrinsic factor and block the binding and absorption of vitamin B12 in the gastrointestinal tract. While first described in the 1820s, it was not until a century later when scientists were studying hematopoiesis in response to the heavy casualty burden from battlefield exsanguination in World War I that dogs fed raw liver were noted to have significantly better blood regeneration response than those fed cooked liver. This discovery led physicians Minot and Murphy to use raw liver to treat pernicious anemia and found that jaundice improved, reticulocyte counts increased, and hemoglobin (Hb) concentration improved, resulting in the duo becoming the first American recipients of the Nobel Prize in physiology or medicine.3 It was ultimately determined in 1948 by chemists Folkers and Todd that the active ingredient in raw liver responsible for this phenomenon was vitamin B12.4
Patients with pernicious anemia typically present with macrocytic anemia, low reticulocyte count, hypersegmented neutrophils, as well as mild leukopenia and/or thrombocytopenia, distinguishable from folate deficiency by an elevated serum methylmalonic acid level. World Health Organization cytopenia thresholds are listed in Table 1.5 Treatment consists of lifelong vitamin B12 supplementation, and endoscopic screening is often recommended after diagnosis due to increased risk of gastrointestinal malignancy.6 Pernicious anemia can be difficult to distinguish from thrombotic thrombocytopenia purpura (TTP), a microangiopathic hemolytic anemia that can cause rapid end-organ failure and death if treatment is delayed.7 While pernicious anemia is not typically hemolytic, case reports of hemolysis in severe deficiency have been reported.7 Adequate bone marrow response to hemolysis in TTP results in an elevated reticulocyte count, which can be useful in differentiating from pernicious anemia where there is typically an inadequate bone marrow response and low reticulocyte count.8,9
The approach to working up pancytopenia begins with a detailed history inquiring about medications, exposures (benzenes, pesticides), alcohol use, and infection history. A thorough physical examination may help point the health care practitioner (HCP) toward a certain etiology, as the differential for pancytopenia is broad. In the deployed soldier downrange, resources are often limited, and the history/physical are crucial in preventing an expensive and unnecessary workup.
Case Presentation
A 24-year-old active-duty female patient presented in late December 2020 to a theater hospital in Djibouti after a witnessed syncopal episode. She had a history of Hashimoto thyroiditis and was taking levothyroxine sodium 75 mcg daily. The patient reported gluten intolerance, which was never formally evaluated. The syncopal episode lasted a few seconds and was not associated with any prodromal or postictal symptoms. No seizure activity was observed, and she had no history of syncopal episodes. She reported that she had been feeling ill 24 to 48 hours prior, with nausea, fatigue, decreased oral intake, decreased urine output, and 2 episodes of nonbilious, nonbloody emesis.
When the patient arrived, she was tachycardic with heart rate in the 130s beats per minute (baseline, 100-110 beats per minute), febrile (103 °F), and had systolic blood pressure (SBP) in the low 100s (baseline, SBP 120s-130s). An electrocardiogram and chest radiographs were unremarkable. Her complete blood count (CBC) could not be processed due to Hb and platelet levels too low to detect on assay (Table 2). Lactate dehydrogenase (LDH) was elevated at > 1000 U/L with mild elevation in liver enzymes (aspartate aminotransferase, 98 U/L; alanine aminotransferase, 51 U/L) and prolonged partial thromboplastin time 70 seconds. She did not report any increased bleeding or bruising. The peripheral blood smear demonstrated pancytopenia, without any schistocytes, and she was started on broad-spectrum antibiotics for presumed sepsis from urinary source and possible TTP.
The patient received 5 units of packed red blood cells, transfusion of platelets, and 2 doses of vitamin B12 in Djibouti with clinical improvement and resolution of orthostasis, hypotension, tachycardia, and fever. Her final posttransfusion CBC showed a Hb level of 11.2 g/dL, white blood cell (WBC) count of 1.7 K/µL, and platelet count of 23 K/µL (Table 3). Two days later her Hb level was 9.0 g/dL, WBC count 1.8 K/µL, and platelet count was 12 K/µL. She was evacuated via air to Landstuhl Regional Medical Center (LRMC) in Germany within 48 hours of presentation, given limited testing capabilities and persistent anemia and thrombocytopenia, refractory to transfusion, concerning for aplastic anemia or acute leukemia.
On arrival at LRMC, she was transfused 1 unit of platelets and given 3 doses of intramuscular vitamin B12 for undetectable levels (< 50 pg/mL) at presentation. An extensive infectious workup was obtained, which did not reveal any viral, bacterial, or parasitic causes. The patient also had a bone marrow biopsy performed at a civilian site, which revealed hypocellular bone marrow. She was transferred to Walter Reed National Military Medical Center (WRNMMC) for further workup and evaluation, given the infectious workup, which was negative. Concern for hematologic malignancy remained. At the time of her arrival, the laboratory values had drastically improved with vitamin supplementation. The patient’s absolute reticulocyte count indicated adequate bone marrow response and because of her improvement, a repeat bone marrow biopsy was not performed.
Intrinsic factor antibodies were elevated (34.5 AU/mL; reference range, 0.0-1.1), which confirmed that this patient’s underlying etiology was secondary to pernicious anemia. The patient continued to improve and repeat vitamin B12 and folate levels revealed that she was responding to therapy. At discharge, intramuscular vitamin B12 injections were planned to continue monthly, indefinitely per guidelines. Oral supplementation is typically avoided due to poor absorption.
Of note, during her inpatient admission at WRNMMC, further evaluation of reported gluten intolerance was performed, which revealed a negative celiac disease panel (IgG/IgA tissue transglutaminase antibodies). On discharge, she was to establish care with gastroenterology for further evaluation, likely including endoscopic evaluation, at her next duty station. She was able to resume full travel and duty functions on discharge from WRNMMC.
Discussion
We highlight a complex case of pancytopenia secondary to pernicious anemia in a deployed service member. With limited resources downrange, the workup of pancytopenia can be resource intensive, expensive, and time sensitive, which can have detrimental impacts on medical readiness. Additionally, undiagnosed coagulopathies can have lethal consequences in a deployed service member where bleeding risk may be elevated depending on the mission. The differential for pancytopenia is vast, and given its relative rarity in pernicious anemia, the HCP must use key components of the history and laboratory results to narrow the differential (eAppendix).10
Pernicious anemia commonly presents as an isolated anemia. In a study looking at the hematologic manifestations of 201 cohort patients with well-documented vitamin B12 deficiency, 5% had symptomatic pancytopenia and 1.5% had a hemolytic anemia.2 The majority (> 67%) of hematologic abnormalities were correctable with cobalamin replacement.2 In our case, the solider presented with symptomatic anemia, manifesting as syncope, and was found to have transfusion-resistant pancytopenia.She had a hemolytic anemia with an LDH > 1000 U/L, haptoglobin < 3 mg/dL, and mild transaminitis with hyperbilirubinemia (1.8 mg/dL). No schistocytes were observed on peripheral smear, suggesting intramedullary hemolysis, which is believed to be due to the destruction of megaloblastic cells by macrophages in bone marrow.11 A French study found high LDH levels and low reticulocyte counts to be strongly suggestive of vitamin B12 deficiency and helpful in differentiating pernicious anemia from TTP, given that bone marrow response to anemia in TTP is preserved.8
While vitamin B12 deficiency is not often associated with hemolytic anemia, multiple cases have been reported in the literature.6 Screening for vitamin B12 deficiency may have shortened this patient’s clinical course and limited the need for air evacuation to a stateside quaternary medical center. However, testing for cobalamin levels in overseas deployed environments is difficult, timely, and costly. New technologies, such as optical sensors, can detect vitamin B12 levels in the blood in < 1 minute and offer portable, low-cost options that may be useful in the deployed military setting.12
Diet plays a key role in this case, since the patient had a reported history of gluten intolerance, although it was never documented or evaluated prior to this presentation. Prior to deployment, the patient ate mostly rice, potatoes, and vegetables. While deployed in an austere environment, food options were limited. These conditions forced her to intermittently consume gluten products, which led to gastrointestinal issues, exacerbating her nutritional deficiencies. In the 2 months before her first syncopal episode, she reported worsening fatigue that impacted her ability to exercise. Vitamin B12 stores often take years to deplete, suggesting that she had a chronic nutritional deficiency before deployment. Another possibility was that she developed an autoimmune gastritis that acutely worsened in the setting of poor nutritional intake. Her history of Hashimoto thyroiditis is also important, as up to one-third of patients with autoimmune thyroid disease have been associated with pernicious anemia (range, 3%-32%) with certain shared human leukocyte antigen alleles implicated in autoimmune gastritis.13,14
Conclusions
This rare case of pernicious anemia presenting as pancytopenia illustrates the challenge in working up pancytopenia, especially in austere military environments with limited testing capabilities. Screening for chronic dietary and nutritional deficiency is important in a service member, raising the question of what role predeployment screening may have and what dietary accommodations may be available during overseas deployments, which can potentially dampen inflammation of the gastrointestinal tract, especially for those with preexisting autoimmune gastrointestinal conditions. Also, newer technology allows portable, low-cost testing of cobalamin and may aid in its diagnosis. In patients who are anemic with low vitamin B12, HCPs can begin vitamin B12 supplementation while continuing the workup (eg, antibody testing, endoscopy). If the patient responds appropriately, further workup becomes less urgent, therefore, decreasing resource use and increasing military readiness. When hemolysis is present, a low reticulocyte count can be beneficial to help differentiate this condition from TTP, a life-threatening condition that must also be ruled out or treated. Pernicious anemia should be on the differential in any patients with autoimmune conditions presenting with cytopenias, especially in those with a history of autoimmune thyroid disorders.
1. Takeshima M, Ishikawa H, Kitadate A, et al. Anorexia nervosa-associated pancytopenia mimicking idiopathic aplastic anemia: a case report. BMC Psychiatry. 2018;18(1):150. doi:10.1186/s12888-018-1743-6
2. Andrès E, Affenberger S, Zimmer J, et al. Current hematological findings in cobalamin deficiency. A study of 201 consecutive patients with documented cobalamin deficiency. Clin Lab Haematol. 2006;28(1):50-56. doi:10.1111/j.1365-2257.2006.00755.x
3. Sinclair L. Recognizing, treating and understanding pernicious anaemia. J R Soc Med. 2008;101(5):262-264. doi:10.1258/jrsm.2008.081006
4. Shampo MA, Kyle RA, Steensma DP. William Murphy—Nobel Prize for the treatment of pernicious anemia. Mayo Clin Proc. 2006;81(6):726. doi:10.4065/81.6.726
5. Hong M, He G. The 2016 revision to the World Health Organization classification of myelodysplastic syndromes. J Transl Int Med. 2017;5(3):139-143. doi:10.1515/jtim-2017-0002
6. Tunio NA, Sheriff MZ, Cooper G. Prevalence of gastric cancer in patients with pernicious anemia: a population-based study. Am J Gastroenterol. 2020;115:S665. doi:10.14309/01.ajg.0000707332.16739.72
7. Bailey M, Maestas T, Betancourt R, Mikhael D, Babiker HM. A rare cause of thrombotic thrombocytopenic purpura- (TTP-) like syndrome, vitamin B12 deficiency: interpretation of significant pathological findings. Case Rep Hematol. 2019;2019:1529306. doi:10.1155/2019/1529306
8. Stanley M, Michalski JM. Thrombotic Thrombocytopenic Purpura. StatPearls Publishing LLC; 2021.
9. Noël N, Maigné G, Tertian G, et al. Hemolysis and schistocytosis in the emergency department: consider pseudothrombotic microangiopathy related to vitamin B12 deficiency. QJM. 2013;106(11):1017-1022. doi:10.1093/qjmed/hct142
10. Chiravuri S, De Jesus O. Pancytopenia. StatPearls Publishing LLC; 2021.
11. Gladstone E. Pernicious anemia presenting with pancytopenia and hemolysis: a case report. February 8, 2019. Accessed June 9, 2022. https://www.journalmc.org/index.php/JMC/article/view/3269/2563
12. ScienceDaily. Developing a sensor for vitamin B12 deficiency. October 17, 2016. Accessed June 9, 2022. https://www.sciencedaily.com/releases/2016/10/161017103221.htm
13. Rodriguez NM, Shackelford K. Pernicious Anemia. StatPearls Publishing LLC; 2021.
14. Fernando MM, Stevens CR, Walsh EC, et al. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet. 2008;4(4):e1000024. doi:10.1371/journal.pgen.1000024
1. Takeshima M, Ishikawa H, Kitadate A, et al. Anorexia nervosa-associated pancytopenia mimicking idiopathic aplastic anemia: a case report. BMC Psychiatry. 2018;18(1):150. doi:10.1186/s12888-018-1743-6
2. Andrès E, Affenberger S, Zimmer J, et al. Current hematological findings in cobalamin deficiency. A study of 201 consecutive patients with documented cobalamin deficiency. Clin Lab Haematol. 2006;28(1):50-56. doi:10.1111/j.1365-2257.2006.00755.x
3. Sinclair L. Recognizing, treating and understanding pernicious anaemia. J R Soc Med. 2008;101(5):262-264. doi:10.1258/jrsm.2008.081006
4. Shampo MA, Kyle RA, Steensma DP. William Murphy—Nobel Prize for the treatment of pernicious anemia. Mayo Clin Proc. 2006;81(6):726. doi:10.4065/81.6.726
5. Hong M, He G. The 2016 revision to the World Health Organization classification of myelodysplastic syndromes. J Transl Int Med. 2017;5(3):139-143. doi:10.1515/jtim-2017-0002
6. Tunio NA, Sheriff MZ, Cooper G. Prevalence of gastric cancer in patients with pernicious anemia: a population-based study. Am J Gastroenterol. 2020;115:S665. doi:10.14309/01.ajg.0000707332.16739.72
7. Bailey M, Maestas T, Betancourt R, Mikhael D, Babiker HM. A rare cause of thrombotic thrombocytopenic purpura- (TTP-) like syndrome, vitamin B12 deficiency: interpretation of significant pathological findings. Case Rep Hematol. 2019;2019:1529306. doi:10.1155/2019/1529306
8. Stanley M, Michalski JM. Thrombotic Thrombocytopenic Purpura. StatPearls Publishing LLC; 2021.
9. Noël N, Maigné G, Tertian G, et al. Hemolysis and schistocytosis in the emergency department: consider pseudothrombotic microangiopathy related to vitamin B12 deficiency. QJM. 2013;106(11):1017-1022. doi:10.1093/qjmed/hct142
10. Chiravuri S, De Jesus O. Pancytopenia. StatPearls Publishing LLC; 2021.
11. Gladstone E. Pernicious anemia presenting with pancytopenia and hemolysis: a case report. February 8, 2019. Accessed June 9, 2022. https://www.journalmc.org/index.php/JMC/article/view/3269/2563
12. ScienceDaily. Developing a sensor for vitamin B12 deficiency. October 17, 2016. Accessed June 9, 2022. https://www.sciencedaily.com/releases/2016/10/161017103221.htm
13. Rodriguez NM, Shackelford K. Pernicious Anemia. StatPearls Publishing LLC; 2021.
14. Fernando MM, Stevens CR, Walsh EC, et al. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet. 2008;4(4):e1000024. doi:10.1371/journal.pgen.1000024
Simultaneous Cases of Carfilzomib-Induced Thrombotic Microangiopathy in 2 Patients With Multiple Myeloma
As a class of drugs, proteasome inhibitors are known to rarely cause drug-induced thrombotic microangiopathy (DITMA). In particular, carfilzomib is a second-generation, irreversible proteasome inhibitor approved for the treatment of relapsed, refractory multiple myeloma (MM) in combination with other therapeutic agents.1 Although generally well tolerated, carfilzomib has been associated with serious adverse events such as cardiovascular toxicity and DITMA.2-4 Thrombotic microangiopathy (TMA) is a life-threatening disorder characterized by thrombocytopenia, microangiopathic hemolytic anemia, and end-organ damage.5 Its occurrence secondary to carfilzomib has been reported only rarely in clinical trials of MM, and the most effective management of the disorder as well as the concurrent risk factors that contribute to its development remain incompletely understood.6,7 As a result, given both the expanding use of carfilzomib in practice and the morbidity of TMA, descriptions of carfilzomib-induced TMA from the real-world setting continue to provide important contributions to our understanding of the disorder.
At our US Department of Veterans Affairs (VA) medical center, 2 patients developed severe carfilzomib-induced TMA within days of one another. The presentation of simultaneous cases was highly unexpected and offered the unique opportunity to compare clinical features in real time. Here, we describe our 2 cases in detail, review their presentations and management in the context of the prior literature, and discuss potential insights gained into the disease.
Case Presentation
Case 1
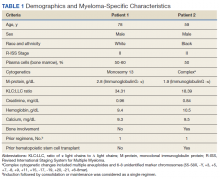
A 78-year-old male patient was diagnosed with monoclonal gammopathy of undetermined significance in 2012 that progressed to Revised International Staging System stage II IgG-κ MM in 2016 due to worsening anemia with a hemoglobin level < 10 g/dL (Table 1). He was treated initially with 8 cycles of first-line bortezomib, lenalidomide, and dexamethasone, to which he achieved a partial response with > 50% reduction in serum M-protein. He then received 3 cycles of maintenance bortezomib until relapse, at which time he was switched to second-line therapy consisting of carfilzomib 20 mg/m2 on days 1 and 2 and 56 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 56 mg/m2 on days 1, 2, 8, 9, 15, and 16 for subsequent cycles plus dexamethasone 20 mg twice weekly every 28 days.
After the patient received cycle 3, day 1 of carfilzomib, he developed subjective fevers, chills, and diarrhea. He missed his day 2 infusion and instead presented to the VA emergency department, where his vital signs were stable and laboratory tests were notable for the following levels: leukocytosis of20.3 K/µL (91.7% neutrophils), hemoglobin 12.4 g/dL (prior 13.5 g/dL), platelet count 171 K/µL, and creatinine 1.39 mg/dL (prior 1.13 g/dL). A chest X-ray demonstrated diffuse bilateral opacities concerning for edema vs infection, and he was started empirically on vancomycin, piperacillin-tazobactam, and azithromycin. His outpatient medications, which included acyclovir, aspirin, finasteride, oxybutynin, ranitidine, omega-3 fatty acids, fish oil, vitamin D, and senna, were continued as indicated.
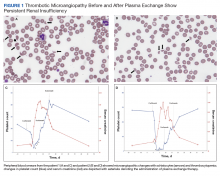
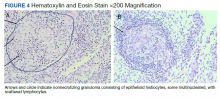
On hospital day 2, the patient’s platelet count dropped to 81 K/µL and creatinine level rose to 1.78 mg/dL. He developed dark urine (urinalysis [UA] 3+ blood, 6-11 red blood cells per high power field [RBC/HPF]) and had laboratory tests suggestive of hemolysis, including lactic dehydrogenase (LDH) > 1,200 IU/L (reference range, 60-250 IU/L), haptoglobin < 30 mg/dL (reference range, 44-215 mg/dL), total bilirubin 3.2 mg/dL (reference range, 0.2-1.3 mg/dL; indirect bilirubin, 2.6 mg/dL), and a peripheral blood smear demonstrating moderate microangiopathy (Figure 1).
Workup for alternative causes of thrombocytopenia included a negative heparin-induced thrombocytopenia panel and a disseminated intravascular coagulation (DIC) panel showing elevated fibrinogen (515 mg/dL; reference range, 200-400 mg/dL) and mildly elevated international normalized ratio (INR) (1.3). Blood cultures were negative, and a 22-pathogen gastrointestinal polymerase chain reaction (PCR) panel failed to identify viral or bacterial pathogens, including Escherichia coli O157:H7. C3 (81 mg/dL; reference range, 90-180 mg/dL) and C4 (16 mg/dL; reference range, 16-47 mg/dL) complement levels were borderline to mildly reduced.
Based on this constellation of findings, a diagnosis of TMA was made, and the patient was started empirically on plasma exchange and pulse-dosed steroids. After 4 cycles of plasma exchange, the platelet count had normalized from its nadir of 29 K/µL. ADAMTS13 activity (98% enzyme activity) ruled out thrombotic thrombocytopenic purpura (TTP), and the patient continued to have anuric renal failure (creatinine, 8.62 mg/dL) necessitating the initiation of hemodialysis. Given persistent renal insufficiency, a diagnosis of atypical hemolytic uremic syndrome (HUS) was considered, and eculizumab 900 mg was administered on days 8 and 15 with stabilization of renal function. By the time of discharge on day 18, the patient’s creatinine level had decreased to 3.89 mg/dL, and platelet count was 403 K/µL. Creatinine normalized to 1.07 mg/dL by day 46.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for mutations in the following genes associated with atypical HUS: CFH, CFI, MCP (CD46), THBD, CFB, C3, DGKE, ADAMTS13, C4BPA, C4BPB, LMNA, CFHR1, CFHR3, CFHR4, and CFHR5. The patient subsequently remained off all antimyeloma therapy for > 1 year until eventually starting third-line pomalidomide plus dexamethasone without reinitiation of proteasome inhibitor therapy.
Case 2
A 59-year-old male patient, diagnosed in 2013 with ISS stage I IgG-κ MM after presenting with compression fractures, completed 8 cycles of cyclophosphamide, bortezomib, and dexamethasone before undergoing autologous hematopoietic stem cell transplantation with complete response (Table 1). He subsequently received single-agent maintenance bortezomib until relapse nearly 2 years later, at which time he started second-line carfilzomib 20 mg/m2 on days 1 and 2 and 27 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 27 mg/m2 on days 8, 9, 15, and 16 for cycles 2 to 8, lenalidomide 25 mg on days 1 to 21, and dexamethasone 40 mg weekly every 28 days. Serum free light chain levels normalized after 9 cycles, and he subsequently began maintenance carfilzomib 70 mg/m2 on days 1 and 15 plus lenalidomide 10 mg on days 1 to 21 every 28 days.
On the morning before admission, the patient received C6D17 of maintenance carfilzomib, which had been delayed from day 15 because of the holiday. Later that evening, he developed nausea, vomiting, and fever of 101.3 °F. He presented to the VA emergency department and was tachycardic (108 beats per minute) and hypotensive (86/55 mm Hg). Laboratory tests were notable for hemoglobin level 9.9 g/dL (prior 11.6 g/dL), platelet count 270 K/µL, and creatinine level 1.86 mg/dL (prior 1.12 mg/dL). A respiratory viral panel was positive for influenza A, and antimicrobial agents were eventually broadened to piperacillin-tazobactam, azithromycin, and oseltamivir. His outpatient medications, which included acyclovir, zoledronic acid, sulfamethoxazole/trimethoprim, aspirin, amlodipine, atorvastatin, omeprazole, zolpidem, calcium, vitamin D, loratadine, ascorbic acid, and prochlorperazine, were continued as indicated.
On hospital day 2, the patient’s platelet count declined from 211 to 57 K/µL. He developed tea-colored urine (UA 2+ blood, 0-2 RBC/HPF) and had laboratory tests suggestive of hemolysis, including LDH 910 IU/L (reference range, 60-250 IU/L), total bilirubin 3.3 mg/dL (reference range, 0.2-1.3 mg/dL; no direct or indirect available), and a peripheral blood smear demonstrating moderate microangiopathy. Although haptoglobin level was normal at this time (206 mg/dL; reference range, 44-215 mg/dL), it decreased to 42 mg/dL by the following day. Additional workup included a negative direct Coombs and a DIC panel showing elevated fibrinogen (596 mg/dL; reference range, 200-400 mg/dL) and mildly elevated INR (1.16). Blood cultures remained negative, and a 22-pathogen GI PCR panel identified no viral or bacterial pathogens, including E coli O157:H7. C3 (114 mg/dL; reference range, 90-180 mg/dL) and C4 (40 mg/dL; reference range, 16-47 mg/dL) complement levels were both normal.
Based on these findings, empiric treatment was started with plasma exchange and pulse-dosed steroids. The patient received 3 cycles of plasma exchange until the results of the ADAMTS13 activity ruled out TTP (63% enzyme activity). Over the next 6 days, his platelet count reached a nadir of 6 K/µL and creatinine level peaked at 10.36 mg/dL, necessitating the initiation of hemodialysis. Given severe renal insufficiency, a diagnosis of atypical HUS was again considered, and eculizumab 900 mg was administered on days 9 and 16 with stabilization of renal function. By the time of discharge on day 17, the patient’s creatinine level had decreased to 4.17 mg/dL and platelet count was 164 K/µL. Creatinine level normalized to 1.02 mg/dL by day 72.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for gene mutations associated with atypical HUS. Approximately 1 month after discharge, the patient resumed maintenance lenalidomide alone without reinitiation of proteasome inhibitor therapy.
Discussion
In this case series, we describe the uncommon drug-related adverse event of TMA occurring in 2 patients with MM after receiving carfilzomib. Although the incidence of TMA disorders is low, reaching up to 2.8% in patients receiving carfilzomib plus cyclophosphamide and dexamethasone in the phase 2 CARDAMON trial, our experience suggests that a high index of suspicion for carfilzomib-induced TMA is warranted in the real-world setting.8 TMA syndromes, including TTP, HUS, and DITMA, are characterized by microvascular endothelial injury and thrombosis leading to thrombocytopenia and microangiopathic hemolytic anemia.5,9 Several drug culprits of DITMA are recognized, including quinine, gemcitabine, tacrolimus, and proteasome inhibitors (bortezomib, carfilzomib, ixazomib).10-12 In a real-world series of patients receiving proteasome inhibitor therapy, either carfilzomib (n=8) or bortezomib (n=3), common clinical features of DITMA included thrombocytopenia, microangiopathic hemolytic anemia, gastrointestinal symptoms, and renal insufficiency with or without a need for hemodialysis.2 Although DITMA has been described primarily as an early event, its occurrence after 12 months of proteasome inhibitor therapy has also been reported, both in this series and elsewhere, thereby suggesting an ongoing risk for DITMA throughout the duration of carfilzomib treatment.2,13
The diagnosis of DITMA can be challenging given its nonspecific symptoms that overlap with other TMA syndromes. Previous studies have proposed that for a drug to be associated with DITMA, there should be: (1) evidence of clinical and/or pathologic findings of TMA; (2) exclusion of alternative causes of TMA; (3) no other new drug exposures other than the suspected culprit medication; and (4) a lack of recurrence of TMA in absence of the drug.10 In the case of patients with MM, other causes of TMA have also been described, including the underlying plasma cell disorder itself and stem cell transplantation.14 In the 2 cases we have described, these alternative causes were considered unlikely given that only 1 patient underwent transplantation remotely and neither had a previous history of TMA secondary to their disease. With respect to other TMA syndromes, ADAMTS13 levels > 10% and negative stool studies for E coli O157:H7 suggested against TTP or typical HUS, respectively. No other drug culprits were identified, and the close timing between the receipt of carfilzomib and symptom onset supported a causal relationship.
Because specific therapies are lacking, management of DITMA has traditionally included drug discontinuation and supportive care for end-organ injury.5 The terminal complement inhibitor, eculizumab, improves hematologic abnormalities and renal function in patients with atypical HUS but its use for treating patients with DITMA is not standard.15 Therefore, the decision to administer eculizumab to our 2 patients was driven by their severe renal insufficiency without improvement after plasma exchange, which suggested a phenotype similar to atypical HUS. After administration of eculizumab, renal function stabilized and then gradually improved over weeks to months, a time course similar to that described in cases of patients with DITMA secondary to other anticancer therapies treated with eculizumab.16 Although these results suggest a potential role for eculizumab in proteasome inhibitor–induced TMA, distinguishing the benefit of eculizumab over drug discontinuation alone remains challenging, and well-designed prospective investigations are needed.
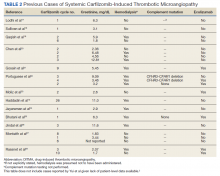
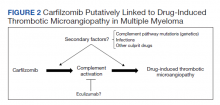
The clustered occurrence of our 2 cases is unique from previous reports that describe carfilzomib-induced TMA as a sporadic event (Table 2).13,17-28 Both immune-mediated and direct toxic effects have been proposed as mechanisms of DITMA, and while our cases do not differentiate between these mechanisms, we considered whether a combined model of initiation, whereby patient or environmental risk factors modulate occurrence of the disease in conjunction with the inciting drug, could explain the clustered occurrence of cases. In this series, drug manufacturing was not a shared risk factor as each patient received carfilzomib from different lot numbers. Furthermore, other patients at our center received carfilzomib from the same batches without developing DITMA. We also considered the role of infection given that 1 patient was diagnosed with influenza A and both presented with nonspecific, viral-like symptoms during the winter season. Interestingly, concurrent viral infections have been reported in other cases of carfilzomib-induced DITMA as well and have also been discussed as a trigger of atypical HUS.20,29 Finally, genetic testing was negative for complement pathway mutations that might predispose to complement dysregulation.
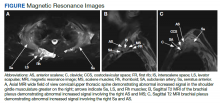
The absence of complement mutations in our 2 patients differs from a recent series describing heterozygous CFHR3-CHFR1 deletions in association with carfilzomib-induced TMA.22 In that report, the authors hypothesized that carfilzomib decreases expression of complement factor H (CFH), a negative regulator of complement activation, thereby leading to complement dysregulation in patients who are genetically predisposed. In a second series, plasma from patients with DITMA secondary to carfilzomib induced the deposition of the complement complex, C5b-9, on endothelial cells in culture, suggesting activation of the complement pathway.30 The effective use of eculizumab would also point to a role for complement activation, and ongoing investigations should aim to identify the triggers and mechanisms of complement dysregulation in this setting, especially for patients like ours in whom genetic testing for complement pathway mutations is negative (Figure 2).
Conclusions
DITMA is a known risk of proteasome inhibitors and is listed as a safety warning in the prescribing information for bortezomib, carfilzomib, and ixazomib.12 Given the overall rarity of this adverse event, the simultaneous presentation of our 2 cases was unexpected and underscores the need for heightened awareness in clinical practice. In addition, while no underlying complement mutations were identified, eculizumab was used in both cases to successfully stabilize renal function. Further research investigating the efficacy of eculizumab and the role of complement activation in proteasome inhibitor–induced TMA will be valuable.
Acknowledgments
The authors would like to thank the patients whose histories are reported in this manuscript as well as the physicians and staff who provided care during the hospitalizations and beyond. We also thank Oscar Silva, MD, PhD, for his assistance in reviewing and formatting the peripheral blood smear images.
1. McBride A, Klaus JO, Stockeri-Goldstein K. Carfilzomib: a second-generation proteasome inhibitor for the treatment of multiple myeloma. Am J Health Syst Pharm. 2015;72(5):353-360. doi:10.2146/ajhp130281
2. Yui JC, Van Keer J, Weiss BM, et al. Proteasome inhibitor associated thrombotic microangiopathy. Am J Hematol. 2016;91(9):E348-E352. doi:10.1002/ajh.24447
3. Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv. 2017;1(7):449-454. doi:10.1182/bloodadvances.2016003269
4. Chari A, Stewart AK, Russell SD, et al. Analysis of carfilzomib cardiovascular safety profile across relapsed and/or refractory multiple myeloma clinical trials. Blood Adv. 2018;2(13):1633-1644. doi:10.1182/bloodadvances.2017015545
5. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654-666. doi:10.1056/NEJMra1312353
6. Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27-38. doi:10.1016/S1470-2045(15)00464-7
7. Dimopoulos M, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396(10245):186-197. doi:10.1016/S0140-6736(20)30734-0
8. Camilleri M, Cuadrado M, Phillips E, et al. Thrombotic microangiopathy in untreated myeloma patients receiving carfilzomib, cyclophosphamide and dexamethasone on the CARDAMON study. Br J Haematol. 2021;193(4):750-760. doi:10.1111/bjh.17377
9. Masias C, Vasu S, Cataland SR. None of the above: thrombotic microangiopathy beyond TTP and HUS. Blood. 2017;129(21):2857-2863. doi:10.1182/blood-2016-11-743104
10. Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN. Drug-induced thrombotic microangiopathy: a systemic review of published reports. Blood. 2015;125(4):616-618. doi:10.1182/blood-2014-11-611335
11. Saleem R, Reese JA, George JN. Drug-induced thrombotic-microangiopathy: an updated systematic review, 2014-2018. Am J Hematol. 2018;93(9):E241-E243. doi:10.1002/ajh.25208
12 Nguyen MN, Nayernama A, Jones SC, Kanapuru B, Gormley N, Waldron PE. Proteasome inhibitor-associated thrombotic microangiopathy: a review of cases reported to the FDA adverse event reporting system and published in the literature. Am J Hematol. 2020;95(9):E218-E222. doi:10.1002/ajh.25832
13. Haddadin M, Al-Sadawi M, Madanat S, et al. Late presentation of carfilzomib associated thrombotic microangiopathy. Am J Med Case Rep. 2019;7(10):240-243. doi:10.12691/ajmcr-7-10-5
14 Portuguese AJ, Gleber C, Passero Jr FC, Lipe B. A review of thrombotic microangiopathies in multiple myeloma. Leuk Res. 2019;85:106195. doi:10.1016/j.leukres.2019.106195
15. Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368(23):2169-2181. doi:10.1056/NEJMoa1208981
16. Olson SR, Lu E, Sulpizio E, Shatzel JJ, Rueda JF, DeLoughery TG. When to stop eculizumab in complement-mediated thrombotic microangiopathies. Am J Nephrol. 2018;48(2):96-107. doi:10.1159/000492033
17. Lodhi A, Kumar A, Saqlain MU, Suneja M. Thrombotic microangiopathy associated with proteasome inhibitors. Clin Kidney J. 2015;8(5):632-636. doi:10.1093/ckj/sfv059
18. Sullivan MR, Danilov AV, Lansigan F, Dunbar NM. Carfilzomib associated thrombotic microangiopathy initially treated with therapeutic plasma exchange. J Clin Apher., 2015;30(5):308-310. doi:10.1002/jca.21371
19. Qaqish I, Schlam IM, Chakkera HA, Fonseca R, Adamski J. Carfilzomib: a cause of drug associated thrombotic microangiopathy. Transfus Apher Sci. 2016;54(3):401-404. doi:10.1016/j.transci.2016.03.002
20. Chen Y, Ooi M, Lim SF, et al. Thrombotic microangiopathy during carfilzomib use: case series in Singapore. Blood Cancer J. 2016;6(7):e450. doi:10.1038/bcj.2016.62
21. Gosain R, Gill A, Fuqua J, et al. Gemcitabine and carfilzomib induced thrombotic microangiopathy: eculizumab as a life-saving treatment. Clin Case Rep. 2017;5(12):1926-1930. doi:10.1002/ccr3.1214
22. Portuguese AJ, Lipe B. Carfilzomib-induced aHUS responds to early eculizumab and may be associated with heterozygrous CFHR3-CFHR1 deletion. Blood Adv. 2018;2(23):3443-3446. doi:10.1182/bloodadvances.2018027532
23. Moliz C, Gutiérrez E, Cavero T, Redondo B, Praga M. Eculizumab as a treatment for atypical hemolytic syndrome secondary to carfilzomib. Nefrologia (Engl Ed). 2019;39(1):86-88. doi:10.1016/j.nefro.2018.02.005
24. Jeyaraman P, Borah P, Singh A, et al., Thrombotic microangiopathy after carfilzomib in a very young myeloma patient. Blood Cells Mol Dis. 2020;81:102400. doi:10.1016/j.bcmd.2019.102400
25. Bhutani D, Assal A, Mapara MY, Prinzing S, Lentzsch S. Case report: carfilzomib-induced thrombotic microangiopathy with complement activation treated successfully with eculizumab. Clin Lymphoma Myeloma Leuk. 2020;20(4):e155-e157. doi:10.1016/j.clml.2020.01.016
26. Jindal N, Jandial A, Jain A, et al. Carfilzomib-induced thrombotic microangiopathy: a case based review. Hematol Oncol Stem Cell Ther. 2020;S1658-3876(20)30118-7. doi:10.1016/j.hemonc.2020.07.001
27. Monteith BE, Venner CP, Reece DE, et al. Drug-induced thrombotic microangiopathy with concurrent proteasome inhibitor use in the treatment of multiple myeloma: a case series and review of the literature. Clin Lymphoma Myeloma Leuk. 2020;20(11):e791-e780. doi:10.1016/j.clml.2020.04.014
28. Rassner M, Baur R, Wäsch R, et al. Two cases of carfilzomib-induced thrombotic microangiopathy successfully treated with eculizumab in multiple myeloma. BMC Nephrol. 2021;22(1):32. doi:10.1186/s12882-020-02226-5
29. Kavanagh D, Goodship THJ. Atypical hemolytic uremic syndrome, genetic basis, and clinical manifestations. Hematology Am Soc Hematol Educ Program. 2011;2011:15-20. doi:10.1182/asheducation-2011.1.15
30. Blasco M, Martínez-Roca A, Rodríguez-Lobato LG, et al. Complement as the enabler of carfilzomib-induced thrombotic microangiopathy. Br J Haematol. 2021;193(1):181-187. doi:10.1111/bjh.16796
As a class of drugs, proteasome inhibitors are known to rarely cause drug-induced thrombotic microangiopathy (DITMA). In particular, carfilzomib is a second-generation, irreversible proteasome inhibitor approved for the treatment of relapsed, refractory multiple myeloma (MM) in combination with other therapeutic agents.1 Although generally well tolerated, carfilzomib has been associated with serious adverse events such as cardiovascular toxicity and DITMA.2-4 Thrombotic microangiopathy (TMA) is a life-threatening disorder characterized by thrombocytopenia, microangiopathic hemolytic anemia, and end-organ damage.5 Its occurrence secondary to carfilzomib has been reported only rarely in clinical trials of MM, and the most effective management of the disorder as well as the concurrent risk factors that contribute to its development remain incompletely understood.6,7 As a result, given both the expanding use of carfilzomib in practice and the morbidity of TMA, descriptions of carfilzomib-induced TMA from the real-world setting continue to provide important contributions to our understanding of the disorder.
At our US Department of Veterans Affairs (VA) medical center, 2 patients developed severe carfilzomib-induced TMA within days of one another. The presentation of simultaneous cases was highly unexpected and offered the unique opportunity to compare clinical features in real time. Here, we describe our 2 cases in detail, review their presentations and management in the context of the prior literature, and discuss potential insights gained into the disease.
Case Presentation
Case 1
A 78-year-old male patient was diagnosed with monoclonal gammopathy of undetermined significance in 2012 that progressed to Revised International Staging System stage II IgG-κ MM in 2016 due to worsening anemia with a hemoglobin level < 10 g/dL (Table 1). He was treated initially with 8 cycles of first-line bortezomib, lenalidomide, and dexamethasone, to which he achieved a partial response with > 50% reduction in serum M-protein. He then received 3 cycles of maintenance bortezomib until relapse, at which time he was switched to second-line therapy consisting of carfilzomib 20 mg/m2 on days 1 and 2 and 56 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 56 mg/m2 on days 1, 2, 8, 9, 15, and 16 for subsequent cycles plus dexamethasone 20 mg twice weekly every 28 days.
After the patient received cycle 3, day 1 of carfilzomib, he developed subjective fevers, chills, and diarrhea. He missed his day 2 infusion and instead presented to the VA emergency department, where his vital signs were stable and laboratory tests were notable for the following levels: leukocytosis of20.3 K/µL (91.7% neutrophils), hemoglobin 12.4 g/dL (prior 13.5 g/dL), platelet count 171 K/µL, and creatinine 1.39 mg/dL (prior 1.13 g/dL). A chest X-ray demonstrated diffuse bilateral opacities concerning for edema vs infection, and he was started empirically on vancomycin, piperacillin-tazobactam, and azithromycin. His outpatient medications, which included acyclovir, aspirin, finasteride, oxybutynin, ranitidine, omega-3 fatty acids, fish oil, vitamin D, and senna, were continued as indicated.
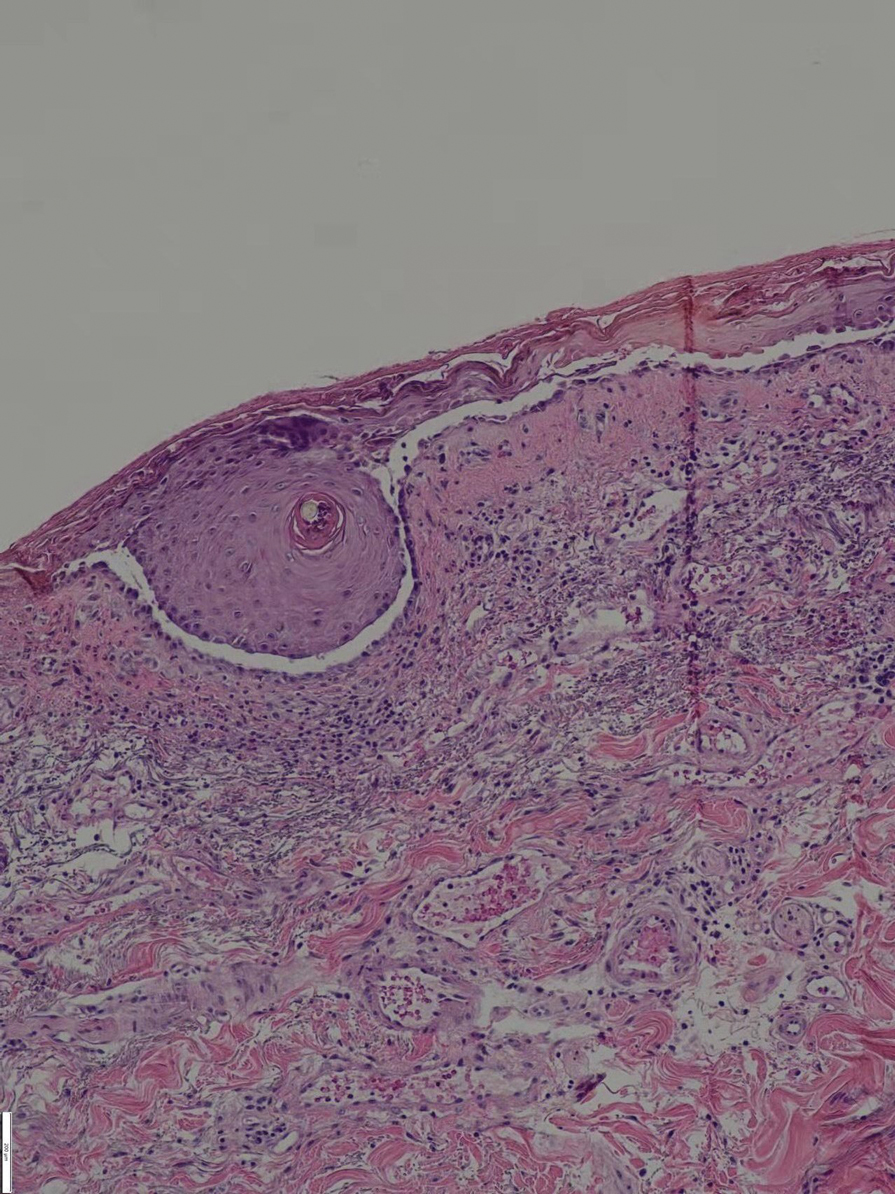
On hospital day 2, the patient’s platelet count dropped to 81 K/µL and creatinine level rose to 1.78 mg/dL. He developed dark urine (urinalysis [UA] 3+ blood, 6-11 red blood cells per high power field [RBC/HPF]) and had laboratory tests suggestive of hemolysis, including lactic dehydrogenase (LDH) > 1,200 IU/L (reference range, 60-250 IU/L), haptoglobin < 30 mg/dL (reference range, 44-215 mg/dL), total bilirubin 3.2 mg/dL (reference range, 0.2-1.3 mg/dL; indirect bilirubin, 2.6 mg/dL), and a peripheral blood smear demonstrating moderate microangiopathy (Figure 1).
Workup for alternative causes of thrombocytopenia included a negative heparin-induced thrombocytopenia panel and a disseminated intravascular coagulation (DIC) panel showing elevated fibrinogen (515 mg/dL; reference range, 200-400 mg/dL) and mildly elevated international normalized ratio (INR) (1.3). Blood cultures were negative, and a 22-pathogen gastrointestinal polymerase chain reaction (PCR) panel failed to identify viral or bacterial pathogens, including Escherichia coli O157:H7. C3 (81 mg/dL; reference range, 90-180 mg/dL) and C4 (16 mg/dL; reference range, 16-47 mg/dL) complement levels were borderline to mildly reduced.
Based on this constellation of findings, a diagnosis of TMA was made, and the patient was started empirically on plasma exchange and pulse-dosed steroids. After 4 cycles of plasma exchange, the platelet count had normalized from its nadir of 29 K/µL. ADAMTS13 activity (98% enzyme activity) ruled out thrombotic thrombocytopenic purpura (TTP), and the patient continued to have anuric renal failure (creatinine, 8.62 mg/dL) necessitating the initiation of hemodialysis. Given persistent renal insufficiency, a diagnosis of atypical hemolytic uremic syndrome (HUS) was considered, and eculizumab 900 mg was administered on days 8 and 15 with stabilization of renal function. By the time of discharge on day 18, the patient’s creatinine level had decreased to 3.89 mg/dL, and platelet count was 403 K/µL. Creatinine normalized to 1.07 mg/dL by day 46.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for mutations in the following genes associated with atypical HUS: CFH, CFI, MCP (CD46), THBD, CFB, C3, DGKE, ADAMTS13, C4BPA, C4BPB, LMNA, CFHR1, CFHR3, CFHR4, and CFHR5. The patient subsequently remained off all antimyeloma therapy for > 1 year until eventually starting third-line pomalidomide plus dexamethasone without reinitiation of proteasome inhibitor therapy.
Case 2
A 59-year-old male patient, diagnosed in 2013 with ISS stage I IgG-κ MM after presenting with compression fractures, completed 8 cycles of cyclophosphamide, bortezomib, and dexamethasone before undergoing autologous hematopoietic stem cell transplantation with complete response (Table 1). He subsequently received single-agent maintenance bortezomib until relapse nearly 2 years later, at which time he started second-line carfilzomib 20 mg/m2 on days 1 and 2 and 27 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 27 mg/m2 on days 8, 9, 15, and 16 for cycles 2 to 8, lenalidomide 25 mg on days 1 to 21, and dexamethasone 40 mg weekly every 28 days. Serum free light chain levels normalized after 9 cycles, and he subsequently began maintenance carfilzomib 70 mg/m2 on days 1 and 15 plus lenalidomide 10 mg on days 1 to 21 every 28 days.
On the morning before admission, the patient received C6D17 of maintenance carfilzomib, which had been delayed from day 15 because of the holiday. Later that evening, he developed nausea, vomiting, and fever of 101.3 °F. He presented to the VA emergency department and was tachycardic (108 beats per minute) and hypotensive (86/55 mm Hg). Laboratory tests were notable for hemoglobin level 9.9 g/dL (prior 11.6 g/dL), platelet count 270 K/µL, and creatinine level 1.86 mg/dL (prior 1.12 mg/dL). A respiratory viral panel was positive for influenza A, and antimicrobial agents were eventually broadened to piperacillin-tazobactam, azithromycin, and oseltamivir. His outpatient medications, which included acyclovir, zoledronic acid, sulfamethoxazole/trimethoprim, aspirin, amlodipine, atorvastatin, omeprazole, zolpidem, calcium, vitamin D, loratadine, ascorbic acid, and prochlorperazine, were continued as indicated.
On hospital day 2, the patient’s platelet count declined from 211 to 57 K/µL. He developed tea-colored urine (UA 2+ blood, 0-2 RBC/HPF) and had laboratory tests suggestive of hemolysis, including LDH 910 IU/L (reference range, 60-250 IU/L), total bilirubin 3.3 mg/dL (reference range, 0.2-1.3 mg/dL; no direct or indirect available), and a peripheral blood smear demonstrating moderate microangiopathy. Although haptoglobin level was normal at this time (206 mg/dL; reference range, 44-215 mg/dL), it decreased to 42 mg/dL by the following day. Additional workup included a negative direct Coombs and a DIC panel showing elevated fibrinogen (596 mg/dL; reference range, 200-400 mg/dL) and mildly elevated INR (1.16). Blood cultures remained negative, and a 22-pathogen GI PCR panel identified no viral or bacterial pathogens, including E coli O157:H7. C3 (114 mg/dL; reference range, 90-180 mg/dL) and C4 (40 mg/dL; reference range, 16-47 mg/dL) complement levels were both normal.
Based on these findings, empiric treatment was started with plasma exchange and pulse-dosed steroids. The patient received 3 cycles of plasma exchange until the results of the ADAMTS13 activity ruled out TTP (63% enzyme activity). Over the next 6 days, his platelet count reached a nadir of 6 K/µL and creatinine level peaked at 10.36 mg/dL, necessitating the initiation of hemodialysis. Given severe renal insufficiency, a diagnosis of atypical HUS was again considered, and eculizumab 900 mg was administered on days 9 and 16 with stabilization of renal function. By the time of discharge on day 17, the patient’s creatinine level had decreased to 4.17 mg/dL and platelet count was 164 K/µL. Creatinine level normalized to 1.02 mg/dL by day 72.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for gene mutations associated with atypical HUS. Approximately 1 month after discharge, the patient resumed maintenance lenalidomide alone without reinitiation of proteasome inhibitor therapy.
Discussion
In this case series, we describe the uncommon drug-related adverse event of TMA occurring in 2 patients with MM after receiving carfilzomib. Although the incidence of TMA disorders is low, reaching up to 2.8% in patients receiving carfilzomib plus cyclophosphamide and dexamethasone in the phase 2 CARDAMON trial, our experience suggests that a high index of suspicion for carfilzomib-induced TMA is warranted in the real-world setting.8 TMA syndromes, including TTP, HUS, and DITMA, are characterized by microvascular endothelial injury and thrombosis leading to thrombocytopenia and microangiopathic hemolytic anemia.5,9 Several drug culprits of DITMA are recognized, including quinine, gemcitabine, tacrolimus, and proteasome inhibitors (bortezomib, carfilzomib, ixazomib).10-12 In a real-world series of patients receiving proteasome inhibitor therapy, either carfilzomib (n=8) or bortezomib (n=3), common clinical features of DITMA included thrombocytopenia, microangiopathic hemolytic anemia, gastrointestinal symptoms, and renal insufficiency with or without a need for hemodialysis.2 Although DITMA has been described primarily as an early event, its occurrence after 12 months of proteasome inhibitor therapy has also been reported, both in this series and elsewhere, thereby suggesting an ongoing risk for DITMA throughout the duration of carfilzomib treatment.2,13
The diagnosis of DITMA can be challenging given its nonspecific symptoms that overlap with other TMA syndromes. Previous studies have proposed that for a drug to be associated with DITMA, there should be: (1) evidence of clinical and/or pathologic findings of TMA; (2) exclusion of alternative causes of TMA; (3) no other new drug exposures other than the suspected culprit medication; and (4) a lack of recurrence of TMA in absence of the drug.10 In the case of patients with MM, other causes of TMA have also been described, including the underlying plasma cell disorder itself and stem cell transplantation.14 In the 2 cases we have described, these alternative causes were considered unlikely given that only 1 patient underwent transplantation remotely and neither had a previous history of TMA secondary to their disease. With respect to other TMA syndromes, ADAMTS13 levels > 10% and negative stool studies for E coli O157:H7 suggested against TTP or typical HUS, respectively. No other drug culprits were identified, and the close timing between the receipt of carfilzomib and symptom onset supported a causal relationship.
Because specific therapies are lacking, management of DITMA has traditionally included drug discontinuation and supportive care for end-organ injury.5 The terminal complement inhibitor, eculizumab, improves hematologic abnormalities and renal function in patients with atypical HUS but its use for treating patients with DITMA is not standard.15 Therefore, the decision to administer eculizumab to our 2 patients was driven by their severe renal insufficiency without improvement after plasma exchange, which suggested a phenotype similar to atypical HUS. After administration of eculizumab, renal function stabilized and then gradually improved over weeks to months, a time course similar to that described in cases of patients with DITMA secondary to other anticancer therapies treated with eculizumab.16 Although these results suggest a potential role for eculizumab in proteasome inhibitor–induced TMA, distinguishing the benefit of eculizumab over drug discontinuation alone remains challenging, and well-designed prospective investigations are needed.
The clustered occurrence of our 2 cases is unique from previous reports that describe carfilzomib-induced TMA as a sporadic event (Table 2).13,17-28 Both immune-mediated and direct toxic effects have been proposed as mechanisms of DITMA, and while our cases do not differentiate between these mechanisms, we considered whether a combined model of initiation, whereby patient or environmental risk factors modulate occurrence of the disease in conjunction with the inciting drug, could explain the clustered occurrence of cases. In this series, drug manufacturing was not a shared risk factor as each patient received carfilzomib from different lot numbers. Furthermore, other patients at our center received carfilzomib from the same batches without developing DITMA. We also considered the role of infection given that 1 patient was diagnosed with influenza A and both presented with nonspecific, viral-like symptoms during the winter season. Interestingly, concurrent viral infections have been reported in other cases of carfilzomib-induced DITMA as well and have also been discussed as a trigger of atypical HUS.20,29 Finally, genetic testing was negative for complement pathway mutations that might predispose to complement dysregulation.
The absence of complement mutations in our 2 patients differs from a recent series describing heterozygous CFHR3-CHFR1 deletions in association with carfilzomib-induced TMA.22 In that report, the authors hypothesized that carfilzomib decreases expression of complement factor H (CFH), a negative regulator of complement activation, thereby leading to complement dysregulation in patients who are genetically predisposed. In a second series, plasma from patients with DITMA secondary to carfilzomib induced the deposition of the complement complex, C5b-9, on endothelial cells in culture, suggesting activation of the complement pathway.30 The effective use of eculizumab would also point to a role for complement activation, and ongoing investigations should aim to identify the triggers and mechanisms of complement dysregulation in this setting, especially for patients like ours in whom genetic testing for complement pathway mutations is negative (Figure 2).
Conclusions
DITMA is a known risk of proteasome inhibitors and is listed as a safety warning in the prescribing information for bortezomib, carfilzomib, and ixazomib.12 Given the overall rarity of this adverse event, the simultaneous presentation of our 2 cases was unexpected and underscores the need for heightened awareness in clinical practice. In addition, while no underlying complement mutations were identified, eculizumab was used in both cases to successfully stabilize renal function. Further research investigating the efficacy of eculizumab and the role of complement activation in proteasome inhibitor–induced TMA will be valuable.
Acknowledgments
The authors would like to thank the patients whose histories are reported in this manuscript as well as the physicians and staff who provided care during the hospitalizations and beyond. We also thank Oscar Silva, MD, PhD, for his assistance in reviewing and formatting the peripheral blood smear images.
As a class of drugs, proteasome inhibitors are known to rarely cause drug-induced thrombotic microangiopathy (DITMA). In particular, carfilzomib is a second-generation, irreversible proteasome inhibitor approved for the treatment of relapsed, refractory multiple myeloma (MM) in combination with other therapeutic agents.1 Although generally well tolerated, carfilzomib has been associated with serious adverse events such as cardiovascular toxicity and DITMA.2-4 Thrombotic microangiopathy (TMA) is a life-threatening disorder characterized by thrombocytopenia, microangiopathic hemolytic anemia, and end-organ damage.5 Its occurrence secondary to carfilzomib has been reported only rarely in clinical trials of MM, and the most effective management of the disorder as well as the concurrent risk factors that contribute to its development remain incompletely understood.6,7 As a result, given both the expanding use of carfilzomib in practice and the morbidity of TMA, descriptions of carfilzomib-induced TMA from the real-world setting continue to provide important contributions to our understanding of the disorder.
At our US Department of Veterans Affairs (VA) medical center, 2 patients developed severe carfilzomib-induced TMA within days of one another. The presentation of simultaneous cases was highly unexpected and offered the unique opportunity to compare clinical features in real time. Here, we describe our 2 cases in detail, review their presentations and management in the context of the prior literature, and discuss potential insights gained into the disease.
Case Presentation
Case 1
A 78-year-old male patient was diagnosed with monoclonal gammopathy of undetermined significance in 2012 that progressed to Revised International Staging System stage II IgG-κ MM in 2016 due to worsening anemia with a hemoglobin level < 10 g/dL (Table 1). He was treated initially with 8 cycles of first-line bortezomib, lenalidomide, and dexamethasone, to which he achieved a partial response with > 50% reduction in serum M-protein. He then received 3 cycles of maintenance bortezomib until relapse, at which time he was switched to second-line therapy consisting of carfilzomib 20 mg/m2 on days 1 and 2 and 56 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 56 mg/m2 on days 1, 2, 8, 9, 15, and 16 for subsequent cycles plus dexamethasone 20 mg twice weekly every 28 days.
After the patient received cycle 3, day 1 of carfilzomib, he developed subjective fevers, chills, and diarrhea. He missed his day 2 infusion and instead presented to the VA emergency department, where his vital signs were stable and laboratory tests were notable for the following levels: leukocytosis of20.3 K/µL (91.7% neutrophils), hemoglobin 12.4 g/dL (prior 13.5 g/dL), platelet count 171 K/µL, and creatinine 1.39 mg/dL (prior 1.13 g/dL). A chest X-ray demonstrated diffuse bilateral opacities concerning for edema vs infection, and he was started empirically on vancomycin, piperacillin-tazobactam, and azithromycin. His outpatient medications, which included acyclovir, aspirin, finasteride, oxybutynin, ranitidine, omega-3 fatty acids, fish oil, vitamin D, and senna, were continued as indicated.
On hospital day 2, the patient’s platelet count dropped to 81 K/µL and creatinine level rose to 1.78 mg/dL. He developed dark urine (urinalysis [UA] 3+ blood, 6-11 red blood cells per high power field [RBC/HPF]) and had laboratory tests suggestive of hemolysis, including lactic dehydrogenase (LDH) > 1,200 IU/L (reference range, 60-250 IU/L), haptoglobin < 30 mg/dL (reference range, 44-215 mg/dL), total bilirubin 3.2 mg/dL (reference range, 0.2-1.3 mg/dL; indirect bilirubin, 2.6 mg/dL), and a peripheral blood smear demonstrating moderate microangiopathy (Figure 1).
Workup for alternative causes of thrombocytopenia included a negative heparin-induced thrombocytopenia panel and a disseminated intravascular coagulation (DIC) panel showing elevated fibrinogen (515 mg/dL; reference range, 200-400 mg/dL) and mildly elevated international normalized ratio (INR) (1.3). Blood cultures were negative, and a 22-pathogen gastrointestinal polymerase chain reaction (PCR) panel failed to identify viral or bacterial pathogens, including Escherichia coli O157:H7. C3 (81 mg/dL; reference range, 90-180 mg/dL) and C4 (16 mg/dL; reference range, 16-47 mg/dL) complement levels were borderline to mildly reduced.
Based on this constellation of findings, a diagnosis of TMA was made, and the patient was started empirically on plasma exchange and pulse-dosed steroids. After 4 cycles of plasma exchange, the platelet count had normalized from its nadir of 29 K/µL. ADAMTS13 activity (98% enzyme activity) ruled out thrombotic thrombocytopenic purpura (TTP), and the patient continued to have anuric renal failure (creatinine, 8.62 mg/dL) necessitating the initiation of hemodialysis. Given persistent renal insufficiency, a diagnosis of atypical hemolytic uremic syndrome (HUS) was considered, and eculizumab 900 mg was administered on days 8 and 15 with stabilization of renal function. By the time of discharge on day 18, the patient’s creatinine level had decreased to 3.89 mg/dL, and platelet count was 403 K/µL. Creatinine normalized to 1.07 mg/dL by day 46.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for mutations in the following genes associated with atypical HUS: CFH, CFI, MCP (CD46), THBD, CFB, C3, DGKE, ADAMTS13, C4BPA, C4BPB, LMNA, CFHR1, CFHR3, CFHR4, and CFHR5. The patient subsequently remained off all antimyeloma therapy for > 1 year until eventually starting third-line pomalidomide plus dexamethasone without reinitiation of proteasome inhibitor therapy.
Case 2
A 59-year-old male patient, diagnosed in 2013 with ISS stage I IgG-κ MM after presenting with compression fractures, completed 8 cycles of cyclophosphamide, bortezomib, and dexamethasone before undergoing autologous hematopoietic stem cell transplantation with complete response (Table 1). He subsequently received single-agent maintenance bortezomib until relapse nearly 2 years later, at which time he started second-line carfilzomib 20 mg/m2 on days 1 and 2 and 27 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 27 mg/m2 on days 8, 9, 15, and 16 for cycles 2 to 8, lenalidomide 25 mg on days 1 to 21, and dexamethasone 40 mg weekly every 28 days. Serum free light chain levels normalized after 9 cycles, and he subsequently began maintenance carfilzomib 70 mg/m2 on days 1 and 15 plus lenalidomide 10 mg on days 1 to 21 every 28 days.
On the morning before admission, the patient received C6D17 of maintenance carfilzomib, which had been delayed from day 15 because of the holiday. Later that evening, he developed nausea, vomiting, and fever of 101.3 °F. He presented to the VA emergency department and was tachycardic (108 beats per minute) and hypotensive (86/55 mm Hg). Laboratory tests were notable for hemoglobin level 9.9 g/dL (prior 11.6 g/dL), platelet count 270 K/µL, and creatinine level 1.86 mg/dL (prior 1.12 mg/dL). A respiratory viral panel was positive for influenza A, and antimicrobial agents were eventually broadened to piperacillin-tazobactam, azithromycin, and oseltamivir. His outpatient medications, which included acyclovir, zoledronic acid, sulfamethoxazole/trimethoprim, aspirin, amlodipine, atorvastatin, omeprazole, zolpidem, calcium, vitamin D, loratadine, ascorbic acid, and prochlorperazine, were continued as indicated.
On hospital day 2, the patient’s platelet count declined from 211 to 57 K/µL. He developed tea-colored urine (UA 2+ blood, 0-2 RBC/HPF) and had laboratory tests suggestive of hemolysis, including LDH 910 IU/L (reference range, 60-250 IU/L), total bilirubin 3.3 mg/dL (reference range, 0.2-1.3 mg/dL; no direct or indirect available), and a peripheral blood smear demonstrating moderate microangiopathy. Although haptoglobin level was normal at this time (206 mg/dL; reference range, 44-215 mg/dL), it decreased to 42 mg/dL by the following day. Additional workup included a negative direct Coombs and a DIC panel showing elevated fibrinogen (596 mg/dL; reference range, 200-400 mg/dL) and mildly elevated INR (1.16). Blood cultures remained negative, and a 22-pathogen GI PCR panel identified no viral or bacterial pathogens, including E coli O157:H7. C3 (114 mg/dL; reference range, 90-180 mg/dL) and C4 (40 mg/dL; reference range, 16-47 mg/dL) complement levels were both normal.
Based on these findings, empiric treatment was started with plasma exchange and pulse-dosed steroids. The patient received 3 cycles of plasma exchange until the results of the ADAMTS13 activity ruled out TTP (63% enzyme activity). Over the next 6 days, his platelet count reached a nadir of 6 K/µL and creatinine level peaked at 10.36 mg/dL, necessitating the initiation of hemodialysis. Given severe renal insufficiency, a diagnosis of atypical HUS was again considered, and eculizumab 900 mg was administered on days 9 and 16 with stabilization of renal function. By the time of discharge on day 17, the patient’s creatinine level had decreased to 4.17 mg/dL and platelet count was 164 K/µL. Creatinine level normalized to 1.02 mg/dL by day 72.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for gene mutations associated with atypical HUS. Approximately 1 month after discharge, the patient resumed maintenance lenalidomide alone without reinitiation of proteasome inhibitor therapy.
Discussion
In this case series, we describe the uncommon drug-related adverse event of TMA occurring in 2 patients with MM after receiving carfilzomib. Although the incidence of TMA disorders is low, reaching up to 2.8% in patients receiving carfilzomib plus cyclophosphamide and dexamethasone in the phase 2 CARDAMON trial, our experience suggests that a high index of suspicion for carfilzomib-induced TMA is warranted in the real-world setting.8 TMA syndromes, including TTP, HUS, and DITMA, are characterized by microvascular endothelial injury and thrombosis leading to thrombocytopenia and microangiopathic hemolytic anemia.5,9 Several drug culprits of DITMA are recognized, including quinine, gemcitabine, tacrolimus, and proteasome inhibitors (bortezomib, carfilzomib, ixazomib).10-12 In a real-world series of patients receiving proteasome inhibitor therapy, either carfilzomib (n=8) or bortezomib (n=3), common clinical features of DITMA included thrombocytopenia, microangiopathic hemolytic anemia, gastrointestinal symptoms, and renal insufficiency with or without a need for hemodialysis.2 Although DITMA has been described primarily as an early event, its occurrence after 12 months of proteasome inhibitor therapy has also been reported, both in this series and elsewhere, thereby suggesting an ongoing risk for DITMA throughout the duration of carfilzomib treatment.2,13
The diagnosis of DITMA can be challenging given its nonspecific symptoms that overlap with other TMA syndromes. Previous studies have proposed that for a drug to be associated with DITMA, there should be: (1) evidence of clinical and/or pathologic findings of TMA; (2) exclusion of alternative causes of TMA; (3) no other new drug exposures other than the suspected culprit medication; and (4) a lack of recurrence of TMA in absence of the drug.10 In the case of patients with MM, other causes of TMA have also been described, including the underlying plasma cell disorder itself and stem cell transplantation.14 In the 2 cases we have described, these alternative causes were considered unlikely given that only 1 patient underwent transplantation remotely and neither had a previous history of TMA secondary to their disease. With respect to other TMA syndromes, ADAMTS13 levels > 10% and negative stool studies for E coli O157:H7 suggested against TTP or typical HUS, respectively. No other drug culprits were identified, and the close timing between the receipt of carfilzomib and symptom onset supported a causal relationship.
Because specific therapies are lacking, management of DITMA has traditionally included drug discontinuation and supportive care for end-organ injury.5 The terminal complement inhibitor, eculizumab, improves hematologic abnormalities and renal function in patients with atypical HUS but its use for treating patients with DITMA is not standard.15 Therefore, the decision to administer eculizumab to our 2 patients was driven by their severe renal insufficiency without improvement after plasma exchange, which suggested a phenotype similar to atypical HUS. After administration of eculizumab, renal function stabilized and then gradually improved over weeks to months, a time course similar to that described in cases of patients with DITMA secondary to other anticancer therapies treated with eculizumab.16 Although these results suggest a potential role for eculizumab in proteasome inhibitor–induced TMA, distinguishing the benefit of eculizumab over drug discontinuation alone remains challenging, and well-designed prospective investigations are needed.
The clustered occurrence of our 2 cases is unique from previous reports that describe carfilzomib-induced TMA as a sporadic event (Table 2).13,17-28 Both immune-mediated and direct toxic effects have been proposed as mechanisms of DITMA, and while our cases do not differentiate between these mechanisms, we considered whether a combined model of initiation, whereby patient or environmental risk factors modulate occurrence of the disease in conjunction with the inciting drug, could explain the clustered occurrence of cases. In this series, drug manufacturing was not a shared risk factor as each patient received carfilzomib from different lot numbers. Furthermore, other patients at our center received carfilzomib from the same batches without developing DITMA. We also considered the role of infection given that 1 patient was diagnosed with influenza A and both presented with nonspecific, viral-like symptoms during the winter season. Interestingly, concurrent viral infections have been reported in other cases of carfilzomib-induced DITMA as well and have also been discussed as a trigger of atypical HUS.20,29 Finally, genetic testing was negative for complement pathway mutations that might predispose to complement dysregulation.
The absence of complement mutations in our 2 patients differs from a recent series describing heterozygous CFHR3-CHFR1 deletions in association with carfilzomib-induced TMA.22 In that report, the authors hypothesized that carfilzomib decreases expression of complement factor H (CFH), a negative regulator of complement activation, thereby leading to complement dysregulation in patients who are genetically predisposed. In a second series, plasma from patients with DITMA secondary to carfilzomib induced the deposition of the complement complex, C5b-9, on endothelial cells in culture, suggesting activation of the complement pathway.30 The effective use of eculizumab would also point to a role for complement activation, and ongoing investigations should aim to identify the triggers and mechanisms of complement dysregulation in this setting, especially for patients like ours in whom genetic testing for complement pathway mutations is negative (Figure 2).
Conclusions
DITMA is a known risk of proteasome inhibitors and is listed as a safety warning in the prescribing information for bortezomib, carfilzomib, and ixazomib.12 Given the overall rarity of this adverse event, the simultaneous presentation of our 2 cases was unexpected and underscores the need for heightened awareness in clinical practice. In addition, while no underlying complement mutations were identified, eculizumab was used in both cases to successfully stabilize renal function. Further research investigating the efficacy of eculizumab and the role of complement activation in proteasome inhibitor–induced TMA will be valuable.
Acknowledgments
The authors would like to thank the patients whose histories are reported in this manuscript as well as the physicians and staff who provided care during the hospitalizations and beyond. We also thank Oscar Silva, MD, PhD, for his assistance in reviewing and formatting the peripheral blood smear images.
1. McBride A, Klaus JO, Stockeri-Goldstein K. Carfilzomib: a second-generation proteasome inhibitor for the treatment of multiple myeloma. Am J Health Syst Pharm. 2015;72(5):353-360. doi:10.2146/ajhp130281
2. Yui JC, Van Keer J, Weiss BM, et al. Proteasome inhibitor associated thrombotic microangiopathy. Am J Hematol. 2016;91(9):E348-E352. doi:10.1002/ajh.24447
3. Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv. 2017;1(7):449-454. doi:10.1182/bloodadvances.2016003269
4. Chari A, Stewart AK, Russell SD, et al. Analysis of carfilzomib cardiovascular safety profile across relapsed and/or refractory multiple myeloma clinical trials. Blood Adv. 2018;2(13):1633-1644. doi:10.1182/bloodadvances.2017015545
5. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654-666. doi:10.1056/NEJMra1312353
6. Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27-38. doi:10.1016/S1470-2045(15)00464-7
7. Dimopoulos M, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396(10245):186-197. doi:10.1016/S0140-6736(20)30734-0
8. Camilleri M, Cuadrado M, Phillips E, et al. Thrombotic microangiopathy in untreated myeloma patients receiving carfilzomib, cyclophosphamide and dexamethasone on the CARDAMON study. Br J Haematol. 2021;193(4):750-760. doi:10.1111/bjh.17377
9. Masias C, Vasu S, Cataland SR. None of the above: thrombotic microangiopathy beyond TTP and HUS. Blood. 2017;129(21):2857-2863. doi:10.1182/blood-2016-11-743104
10. Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN. Drug-induced thrombotic microangiopathy: a systemic review of published reports. Blood. 2015;125(4):616-618. doi:10.1182/blood-2014-11-611335
11. Saleem R, Reese JA, George JN. Drug-induced thrombotic-microangiopathy: an updated systematic review, 2014-2018. Am J Hematol. 2018;93(9):E241-E243. doi:10.1002/ajh.25208
12 Nguyen MN, Nayernama A, Jones SC, Kanapuru B, Gormley N, Waldron PE. Proteasome inhibitor-associated thrombotic microangiopathy: a review of cases reported to the FDA adverse event reporting system and published in the literature. Am J Hematol. 2020;95(9):E218-E222. doi:10.1002/ajh.25832
13. Haddadin M, Al-Sadawi M, Madanat S, et al. Late presentation of carfilzomib associated thrombotic microangiopathy. Am J Med Case Rep. 2019;7(10):240-243. doi:10.12691/ajmcr-7-10-5
14 Portuguese AJ, Gleber C, Passero Jr FC, Lipe B. A review of thrombotic microangiopathies in multiple myeloma. Leuk Res. 2019;85:106195. doi:10.1016/j.leukres.2019.106195
15. Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368(23):2169-2181. doi:10.1056/NEJMoa1208981
16. Olson SR, Lu E, Sulpizio E, Shatzel JJ, Rueda JF, DeLoughery TG. When to stop eculizumab in complement-mediated thrombotic microangiopathies. Am J Nephrol. 2018;48(2):96-107. doi:10.1159/000492033
17. Lodhi A, Kumar A, Saqlain MU, Suneja M. Thrombotic microangiopathy associated with proteasome inhibitors. Clin Kidney J. 2015;8(5):632-636. doi:10.1093/ckj/sfv059
18. Sullivan MR, Danilov AV, Lansigan F, Dunbar NM. Carfilzomib associated thrombotic microangiopathy initially treated with therapeutic plasma exchange. J Clin Apher., 2015;30(5):308-310. doi:10.1002/jca.21371
19. Qaqish I, Schlam IM, Chakkera HA, Fonseca R, Adamski J. Carfilzomib: a cause of drug associated thrombotic microangiopathy. Transfus Apher Sci. 2016;54(3):401-404. doi:10.1016/j.transci.2016.03.002
20. Chen Y, Ooi M, Lim SF, et al. Thrombotic microangiopathy during carfilzomib use: case series in Singapore. Blood Cancer J. 2016;6(7):e450. doi:10.1038/bcj.2016.62
21. Gosain R, Gill A, Fuqua J, et al. Gemcitabine and carfilzomib induced thrombotic microangiopathy: eculizumab as a life-saving treatment. Clin Case Rep. 2017;5(12):1926-1930. doi:10.1002/ccr3.1214
22. Portuguese AJ, Lipe B. Carfilzomib-induced aHUS responds to early eculizumab and may be associated with heterozygrous CFHR3-CFHR1 deletion. Blood Adv. 2018;2(23):3443-3446. doi:10.1182/bloodadvances.2018027532
23. Moliz C, Gutiérrez E, Cavero T, Redondo B, Praga M. Eculizumab as a treatment for atypical hemolytic syndrome secondary to carfilzomib. Nefrologia (Engl Ed). 2019;39(1):86-88. doi:10.1016/j.nefro.2018.02.005
24. Jeyaraman P, Borah P, Singh A, et al., Thrombotic microangiopathy after carfilzomib in a very young myeloma patient. Blood Cells Mol Dis. 2020;81:102400. doi:10.1016/j.bcmd.2019.102400
25. Bhutani D, Assal A, Mapara MY, Prinzing S, Lentzsch S. Case report: carfilzomib-induced thrombotic microangiopathy with complement activation treated successfully with eculizumab. Clin Lymphoma Myeloma Leuk. 2020;20(4):e155-e157. doi:10.1016/j.clml.2020.01.016
26. Jindal N, Jandial A, Jain A, et al. Carfilzomib-induced thrombotic microangiopathy: a case based review. Hematol Oncol Stem Cell Ther. 2020;S1658-3876(20)30118-7. doi:10.1016/j.hemonc.2020.07.001
27. Monteith BE, Venner CP, Reece DE, et al. Drug-induced thrombotic microangiopathy with concurrent proteasome inhibitor use in the treatment of multiple myeloma: a case series and review of the literature. Clin Lymphoma Myeloma Leuk. 2020;20(11):e791-e780. doi:10.1016/j.clml.2020.04.014
28. Rassner M, Baur R, Wäsch R, et al. Two cases of carfilzomib-induced thrombotic microangiopathy successfully treated with eculizumab in multiple myeloma. BMC Nephrol. 2021;22(1):32. doi:10.1186/s12882-020-02226-5
29. Kavanagh D, Goodship THJ. Atypical hemolytic uremic syndrome, genetic basis, and clinical manifestations. Hematology Am Soc Hematol Educ Program. 2011;2011:15-20. doi:10.1182/asheducation-2011.1.15
30. Blasco M, Martínez-Roca A, Rodríguez-Lobato LG, et al. Complement as the enabler of carfilzomib-induced thrombotic microangiopathy. Br J Haematol. 2021;193(1):181-187. doi:10.1111/bjh.16796
1. McBride A, Klaus JO, Stockeri-Goldstein K. Carfilzomib: a second-generation proteasome inhibitor for the treatment of multiple myeloma. Am J Health Syst Pharm. 2015;72(5):353-360. doi:10.2146/ajhp130281
2. Yui JC, Van Keer J, Weiss BM, et al. Proteasome inhibitor associated thrombotic microangiopathy. Am J Hematol. 2016;91(9):E348-E352. doi:10.1002/ajh.24447
3. Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv. 2017;1(7):449-454. doi:10.1182/bloodadvances.2016003269
4. Chari A, Stewart AK, Russell SD, et al. Analysis of carfilzomib cardiovascular safety profile across relapsed and/or refractory multiple myeloma clinical trials. Blood Adv. 2018;2(13):1633-1644. doi:10.1182/bloodadvances.2017015545
5. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654-666. doi:10.1056/NEJMra1312353
6. Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27-38. doi:10.1016/S1470-2045(15)00464-7
7. Dimopoulos M, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396(10245):186-197. doi:10.1016/S0140-6736(20)30734-0
8. Camilleri M, Cuadrado M, Phillips E, et al. Thrombotic microangiopathy in untreated myeloma patients receiving carfilzomib, cyclophosphamide and dexamethasone on the CARDAMON study. Br J Haematol. 2021;193(4):750-760. doi:10.1111/bjh.17377
9. Masias C, Vasu S, Cataland SR. None of the above: thrombotic microangiopathy beyond TTP and HUS. Blood. 2017;129(21):2857-2863. doi:10.1182/blood-2016-11-743104
10. Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN. Drug-induced thrombotic microangiopathy: a systemic review of published reports. Blood. 2015;125(4):616-618. doi:10.1182/blood-2014-11-611335
11. Saleem R, Reese JA, George JN. Drug-induced thrombotic-microangiopathy: an updated systematic review, 2014-2018. Am J Hematol. 2018;93(9):E241-E243. doi:10.1002/ajh.25208
12 Nguyen MN, Nayernama A, Jones SC, Kanapuru B, Gormley N, Waldron PE. Proteasome inhibitor-associated thrombotic microangiopathy: a review of cases reported to the FDA adverse event reporting system and published in the literature. Am J Hematol. 2020;95(9):E218-E222. doi:10.1002/ajh.25832
13. Haddadin M, Al-Sadawi M, Madanat S, et al. Late presentation of carfilzomib associated thrombotic microangiopathy. Am J Med Case Rep. 2019;7(10):240-243. doi:10.12691/ajmcr-7-10-5
14 Portuguese AJ, Gleber C, Passero Jr FC, Lipe B. A review of thrombotic microangiopathies in multiple myeloma. Leuk Res. 2019;85:106195. doi:10.1016/j.leukres.2019.106195
15. Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368(23):2169-2181. doi:10.1056/NEJMoa1208981
16. Olson SR, Lu E, Sulpizio E, Shatzel JJ, Rueda JF, DeLoughery TG. When to stop eculizumab in complement-mediated thrombotic microangiopathies. Am J Nephrol. 2018;48(2):96-107. doi:10.1159/000492033
17. Lodhi A, Kumar A, Saqlain MU, Suneja M. Thrombotic microangiopathy associated with proteasome inhibitors. Clin Kidney J. 2015;8(5):632-636. doi:10.1093/ckj/sfv059
18. Sullivan MR, Danilov AV, Lansigan F, Dunbar NM. Carfilzomib associated thrombotic microangiopathy initially treated with therapeutic plasma exchange. J Clin Apher., 2015;30(5):308-310. doi:10.1002/jca.21371
19. Qaqish I, Schlam IM, Chakkera HA, Fonseca R, Adamski J. Carfilzomib: a cause of drug associated thrombotic microangiopathy. Transfus Apher Sci. 2016;54(3):401-404. doi:10.1016/j.transci.2016.03.002
20. Chen Y, Ooi M, Lim SF, et al. Thrombotic microangiopathy during carfilzomib use: case series in Singapore. Blood Cancer J. 2016;6(7):e450. doi:10.1038/bcj.2016.62
21. Gosain R, Gill A, Fuqua J, et al. Gemcitabine and carfilzomib induced thrombotic microangiopathy: eculizumab as a life-saving treatment. Clin Case Rep. 2017;5(12):1926-1930. doi:10.1002/ccr3.1214
22. Portuguese AJ, Lipe B. Carfilzomib-induced aHUS responds to early eculizumab and may be associated with heterozygrous CFHR3-CFHR1 deletion. Blood Adv. 2018;2(23):3443-3446. doi:10.1182/bloodadvances.2018027532
23. Moliz C, Gutiérrez E, Cavero T, Redondo B, Praga M. Eculizumab as a treatment for atypical hemolytic syndrome secondary to carfilzomib. Nefrologia (Engl Ed). 2019;39(1):86-88. doi:10.1016/j.nefro.2018.02.005
24. Jeyaraman P, Borah P, Singh A, et al., Thrombotic microangiopathy after carfilzomib in a very young myeloma patient. Blood Cells Mol Dis. 2020;81:102400. doi:10.1016/j.bcmd.2019.102400
25. Bhutani D, Assal A, Mapara MY, Prinzing S, Lentzsch S. Case report: carfilzomib-induced thrombotic microangiopathy with complement activation treated successfully with eculizumab. Clin Lymphoma Myeloma Leuk. 2020;20(4):e155-e157. doi:10.1016/j.clml.2020.01.016
26. Jindal N, Jandial A, Jain A, et al. Carfilzomib-induced thrombotic microangiopathy: a case based review. Hematol Oncol Stem Cell Ther. 2020;S1658-3876(20)30118-7. doi:10.1016/j.hemonc.2020.07.001
27. Monteith BE, Venner CP, Reece DE, et al. Drug-induced thrombotic microangiopathy with concurrent proteasome inhibitor use in the treatment of multiple myeloma: a case series and review of the literature. Clin Lymphoma Myeloma Leuk. 2020;20(11):e791-e780. doi:10.1016/j.clml.2020.04.014
28. Rassner M, Baur R, Wäsch R, et al. Two cases of carfilzomib-induced thrombotic microangiopathy successfully treated with eculizumab in multiple myeloma. BMC Nephrol. 2021;22(1):32. doi:10.1186/s12882-020-02226-5
29. Kavanagh D, Goodship THJ. Atypical hemolytic uremic syndrome, genetic basis, and clinical manifestations. Hematology Am Soc Hematol Educ Program. 2011;2011:15-20. doi:10.1182/asheducation-2011.1.15
30. Blasco M, Martínez-Roca A, Rodríguez-Lobato LG, et al. Complement as the enabler of carfilzomib-induced thrombotic microangiopathy. Br J Haematol. 2021;193(1):181-187. doi:10.1111/bjh.16796
Rheumatologic Perspective on Persistent Right-Hand Tenosynovitis Secondary to Mycobacterium marinum Infection
Rheumatologic conditions and infections may imitate each other, often making diagnosis challenging. Therefore, it is imperative to obtain adequate histories and have a keen eye for these potentially confounding differential diagnoses. Immunosuppressants used in managing rheumatologic etiologies have detrimental consequences in undiagnosed underlying infections. Consequently, worsening symptoms with standard therapy should raise awareness to a different diagnosis.
Nontuberculous mycobacteria (NTM) are slow-growing organisms difficult to yield in culture. Initial negative synovial fluid stains and cultures when suspecting NTM infectious arthritis or tenosynovitis should not exclude the diagnosis if there is a strong clinical scenario. The identification of Mycobacterium marinum (M marinum) infection in the hand is of utmost importance given that delayed treatment may cause significant and even permanent disability.
We present the case of a 73-year-old male patient with progressively worsening right-hand tenosynovitis who was evaluated for crystal-induced and sarcoid arthropathies in the setting of negative synovial biopsy cultures but was subsequently diagnosed with M marinum infectious tenosynovitis after a second surgical debridement.
Case Presentation
A 73-year-old male patient with history of type 2 diabetes mellitus, hypertension, hyperlipidemia, hypothyroidism, bilateral knee osteoarthritis, obstructive sleep apnea, and posttraumatic stress disorder presented to the emergency department (ED) with right wrist swelling and pain for 4 days. The patient reported that he was working in his garden when symptoms started. He did not recall any skin abrasions or wounds, insect bites, thorn punctures, trauma, or exposure to swimming pools or fish tanks. Patient was afebrile, and vital signs were within normal range. On physical examination, there was erythema, swelling, and tenderness in the dorsum of the right hand and over the dorsal aspect of the fourth metacarpophalangeal joint (Figure 1). The skin was intact.
Symptoms had not responded to 7 days of cefalexin nor to a short course of oral steroids. Leukocytosis of 14.35 × 109/L (reference range, 3.90-9.90 × 109/L) with neutrophilia at 11.10 × 109/L (reference range, 1.73-6.37 × 109/L) was noted. Sedimentation rate and C-reactive protein levels were normal. Right-hand X-ray was remarkable for chondrocalcinosis in the triangular fibrocartilage. Right upper extremity magnetic resonance imaging (MRI) revealed diffuse inflammation in the right wrist and hand (Figure 2). There was no evidence of septic arthritis or osteomyelitis. Consequently, orthopedic service recommended no surgical intervention. Additionally, the patient had preserved range of motion that further indicated tenosynovitis, which could be medically managed with antibiotics, rather than a septic joint.
One dose of IV piperacillin/tazobactam was given at the ED, and he was admitted to the internal medicine ward with right hand and wrist cellulitis and indolent suppurative tenosynovitis. Empiric IV ceftriaxone and vancomycin were started as per infectious disease (ID) service with adequate response defined as a reduction of the swelling, erythema, and tenderness of the right hand and wrist. Differential diagnosis included sporotrichosis, nocardia vs NTM infection.
Interventional radiology was consulted for right wrist drainage. However, only 1 mL of fluid was obtained. Synovial fluid was sent for cell count and differential, crystal analysis, bacterial cultures, fungal cultures, and acid-fast bacilli (AFB) stains and culture. Neutrophils were 43% and lymphocytes were 57%. Crystal analysis was negative. Bacterial culture and mycology were negative. AFB stain and culture results were negative after 6 weeks. Based on gardening history and risk of thorn exposure and low suspicion for common bacterial pathogens, ID service switched antibiotics to moxifloxacin, minocycline, and linezolid for broad coverage to complete 3 weeks as outpatient. The patient reported significantly improved pain and handgrip with notable decrease in swelling. Nonetheless, 3 weeks after completing antibiotics, the right-hand pain recurred, raising concern for complex regional syndrome vs crystalline arthropathy.
The patient was referred to rheumatology service for evaluation of crystal-induced arthropathy given chondrocalcinosis. Physical examination revealed right third proximal interphalangeal joint swelling and tenderness with overimposed tophilike nodule. No erythema or palpable effusions were appreciated. Range of motion was preserved. Laboratory workup showed resolved leukocytosis and neutrophilia, and normal sedimentation rate or C-reactive protein levels. Antinuclear antibody panel, rheumatoid factor, and anti–cyclic citrullinated peptide levels were normal. Serum uric acid levels were 5.9 mg/dL. Chlamydia, gonorrhea, and HIV tests were negative. Short course of low-dose oral prednisone starting at 15 mg daily with tapering by 5 mg every 3 days was given for presumptive calcium pyrophosphate deposition vs gout. Nevertheless, right-hand swelling and pain worsened after steroids. Repeat right upper extremity MRI showed persistent soft tissue edema and inflammation along the dorsum of the hand extending to the digits, tenosynovitis, and fluid in the third metacarpophalangeal that could represent a superficial abscess. The patient was hospitalized given concerns of infection.
The relapse of tenosynovitis raised concerns for a persistent infection secondary to a fastidious organism, such as NTM. Thus, inquiries specifically pertaining to any contact with bodies of water were entertained. The patient remembered that he had gone scuba diving in the ocean weeks before symptom onset. This meant scuba diving could then be the inciting event rather than gardening, which placed NTM higher in the differential. ID service did not recommend antibiotics until new cultures were available. Orthopedic service was consulted for surgical debridement. The right dorsal hand, wrist, and distal forearm tendon sheaths were surgically opened to obtain a synovial biopsy.
Synovial fluid was sent for fungal, bacterial, and AFB cultures, and synovial biopsy for AFB stains, PCR amplification/sequencing assay, and cultures. Results showed nonnecrotizing granulomas and all cultures were negative (Figures 3, 4). Rheumatology was again consulted for evaluation for sarcoidosis given negative cultures and noncaseating granulomas. Review of systems was completely negative for sarcoidosis. Computed tomography (CT) of the thorax did not show any pulmonary abnormalities, lymphadenopathy, and hilar adenopathy. Serum calcium and angiotensin-converting enzyme levels were normal. ID service recommended against empiric antibiotics given negative culture. Given persistent pain, and reported cases of isolated sarcoid tenosynovitis, low-dose oral prednisone 20 mg daily was given after clearance by ID service. Nonetheless, the right wrist and hand swelling, erythema, and tenderness relapsed with 1 dose of prednisone, leading to a repeat right upper extremity synovial biopsy due to high suspicion for persistent infection with a fastidious organism. New synovial tissue biopsy revealed fibro-adipose tissue with prominent vessels and fibrosis, nonnecrotizing, sarcoidlike granuloma with giant cell granulomatous reaction. The AFB and Grocott methenamine silver stains were negative. PCR was negative for AFB. No crystals were reported. After 5 weeks, the synovial biopsy culture was positive for M marinum. Patient was started on oral azithromycin 500 mg daily, rifabutin 300 mg daily, and ethambutol 15 mg/kg daily. At the time of this report, the patient was still completing antibiotic therapy with adequate response and undergoing occupational therapy rehabilitation (Figure 5).
Discussion
M marinum is an NTM found in bodies of water and marine settings. Infection arises after direct contact of lacerated skin with contaminated water. In a review article of 5 cases of M marinum tenosynovitis, they found that all individuals had wounds with exposure to fish or shrimp while in the water or while handling seafood.1 The incidence of this infection is infrequent, estimated to be 0.04 cases per 100,000, with only about 25% of these cases presenting as tenosynovitis.2 The incubation period ranges from 2 to 4 weeks.3 Late identification of this organism is common because of its slow development. For example, presentation from first exposure to symptom onset may take as long as 32 days.1 In addition, in the same review, surgical intervention occurred in 63 days.1 It has been reported that AFB stains are positive in just 9% of cases, which confounds diagnosis even more.4 After synovial tissue culture is obtained, it takes approximately 6 weeks for the organism to grow. Moreover, diagnosis may take longer if it is not suspected.5
Four types of M marinum infections have been described.5 The status of the immune system plays a role in how the manifestations present. The first type is limited, which is seen in immunocompetent persons, characterized by skin involvement, such as erythematous nodular lesions, that may improve on their own in months or years.4 Conversely, in immunosuppressed patients, the second type of infection may cause sporotrichoid spreading described as following lymphangitic pattern. The third type presents with musculoskeletal findings, such as arthritis, tenosynovitis, bursitis, or osteomyelitis, as seen in our patient. The fourth type consists of systemic manifestations.5 Medications that lower the immune system, such as corticosteroids, chemotherapy, and biologic disease modifying agents, may increase the risk for developing this entity.4 Specifically, antitumor necrosis factor inhibitors have been historically associated with mycobacterium infections.6
Patients are frequently diagnosed with soft tissue infection, such as abscesses or cellulitis, as in our case. They may at times be found to have other musculoskeletal conditions such as trigger finger.1 Other similar presenting entities are psoriatic arthritis, rheumatoid arthritis, and remitting seronegative arthritis.4 These clinical resemblances complicate the scenario, especially when initial cultures are negative, as the treatment for these rheumatic diseases is immunosuppression, which adversely impact the fastidious infection. In our case, the improved swelling and range of motion after the 3-week course of empiric antibiotics for suppurative tenosynovitis was initially reassuring that the previous infection had been successfully treated. Subsequently, the presence of chondrocalcinosis in the triangular fibrocartilage in the right-hand X-rays, persistent pain, and the tophi-like appearance of the right third proximal interphalangeal nodule raised concerns for crystalline arthropathies, such as calcium pyrophosphate deposition vs gout. Nonetheless, given the lack of response to low-dose steroids, an ongoing infectious process was strongly considered.
Sarcoidosis was a concern after the first synovial biopsy revealed noncaseating granulomas and negative stains and cultures. Sarcoid tenosynovitis is rare with only 22 cases described as per a 2015 report.7 Musculoskeletal involvement in sarcoidosis has been reported in 1 to 13% of sarcoid patients.7 Once again, unresponsiveness to steroids led to another synovial biopsy for culture due to potential infection. Akin to other cases, more than one surgical debridement was required to diagnose our patient.
Conclusions
Our case reinforces the vital role of history gathering in establishing diagnoses. It underscores the value of clinical suspicion especially in patients unresponsive to standard treatment for inflammatory arthritis, namely corticosteroids. Tissue biopsy with culture for AFB is crucial for accurate diagnosis in NTM infection, which may imitate rheumatic inflammatory arthritis. Clinicians should be keenly aware of this fastidious, indolent organism in the setting of persistent localized tenosynovitis.
1. Pang HN, Lee JY, Puhaindran ME, Tan SH, Tan AB, Yong FC. Mycobacterium marinum as a cause of chronic granulomatous tenosynovitis in the hand. J Infect. 2007;54(6):584-588. doi:10.1016/j.jinf.2006.11.014
2. Wongworawat MD, Holtom P, Learch TJ, Fedenko A, Stevanovic MV. A prolonged case of Mycobacterium marinum flexor tenosynovitis: radiographic and histological correlation, and review of the literature. Skeletal Radiol. 2003;32(9):542-545. doi:10.1007/s00256-003-0636-y
3. Schubert N, Schill T, Plüß M, Korsten P. Flare or foe? - Mycobacterium marinum infection mimicking rheumatoid arthritis tenosynovitis: case report and literature review. BMC Rheumatol. 2020;4:11. Published 2020 Mar 16. doi:10.1186/s41927-020-0114-3
4. Lam A, Toma W, Schlesinger N. Mycobacterium marinum arthritis mimicking rheumatoid arthritis. J Rheumatol. 2006;33(4):817-819.
5. Hashish E, Merwad A, Elgaml S, et al. Mycobacterium marinum infection in fish and man: epidemiology, pathophysiology and management; a review. Vet Q. 2018;38(1):35-46. doi:10.1080/01652176.2018.1447171
6. Thanou-Stavraki A, Sawalha AH, Crowson AN, Harley JB. Noodling and Mycobacterium marinum infection mimicking seronegative rheumatoid arthritis complicated by anti-tumor necrosis factor α therapy. Arthritis Care Res (Hoboken). 2011;63(1):160-164. doi:10.1002/acr.20303
7. Al-Ani Z, Oh TC, Macphie E, Woodruff MJ. Sarcoid tenosynovitis, rare presentation of a common disease. Case report and literature review. J Radiol Case Rep. 2015;9(8):16-23. Published 2015 Aug 31. doi:10.3941/jrcr.v9i8.2311
Rheumatologic conditions and infections may imitate each other, often making diagnosis challenging. Therefore, it is imperative to obtain adequate histories and have a keen eye for these potentially confounding differential diagnoses. Immunosuppressants used in managing rheumatologic etiologies have detrimental consequences in undiagnosed underlying infections. Consequently, worsening symptoms with standard therapy should raise awareness to a different diagnosis.
Nontuberculous mycobacteria (NTM) are slow-growing organisms difficult to yield in culture. Initial negative synovial fluid stains and cultures when suspecting NTM infectious arthritis or tenosynovitis should not exclude the diagnosis if there is a strong clinical scenario. The identification of Mycobacterium marinum (M marinum) infection in the hand is of utmost importance given that delayed treatment may cause significant and even permanent disability.
We present the case of a 73-year-old male patient with progressively worsening right-hand tenosynovitis who was evaluated for crystal-induced and sarcoid arthropathies in the setting of negative synovial biopsy cultures but was subsequently diagnosed with M marinum infectious tenosynovitis after a second surgical debridement.
Case Presentation
A 73-year-old male patient with history of type 2 diabetes mellitus, hypertension, hyperlipidemia, hypothyroidism, bilateral knee osteoarthritis, obstructive sleep apnea, and posttraumatic stress disorder presented to the emergency department (ED) with right wrist swelling and pain for 4 days. The patient reported that he was working in his garden when symptoms started. He did not recall any skin abrasions or wounds, insect bites, thorn punctures, trauma, or exposure to swimming pools or fish tanks. Patient was afebrile, and vital signs were within normal range. On physical examination, there was erythema, swelling, and tenderness in the dorsum of the right hand and over the dorsal aspect of the fourth metacarpophalangeal joint (Figure 1). The skin was intact.
Symptoms had not responded to 7 days of cefalexin nor to a short course of oral steroids. Leukocytosis of 14.35 × 109/L (reference range, 3.90-9.90 × 109/L) with neutrophilia at 11.10 × 109/L (reference range, 1.73-6.37 × 109/L) was noted. Sedimentation rate and C-reactive protein levels were normal. Right-hand X-ray was remarkable for chondrocalcinosis in the triangular fibrocartilage. Right upper extremity magnetic resonance imaging (MRI) revealed diffuse inflammation in the right wrist and hand (Figure 2). There was no evidence of septic arthritis or osteomyelitis. Consequently, orthopedic service recommended no surgical intervention. Additionally, the patient had preserved range of motion that further indicated tenosynovitis, which could be medically managed with antibiotics, rather than a septic joint.
One dose of IV piperacillin/tazobactam was given at the ED, and he was admitted to the internal medicine ward with right hand and wrist cellulitis and indolent suppurative tenosynovitis. Empiric IV ceftriaxone and vancomycin were started as per infectious disease (ID) service with adequate response defined as a reduction of the swelling, erythema, and tenderness of the right hand and wrist. Differential diagnosis included sporotrichosis, nocardia vs NTM infection.
Interventional radiology was consulted for right wrist drainage. However, only 1 mL of fluid was obtained. Synovial fluid was sent for cell count and differential, crystal analysis, bacterial cultures, fungal cultures, and acid-fast bacilli (AFB) stains and culture. Neutrophils were 43% and lymphocytes were 57%. Crystal analysis was negative. Bacterial culture and mycology were negative. AFB stain and culture results were negative after 6 weeks. Based on gardening history and risk of thorn exposure and low suspicion for common bacterial pathogens, ID service switched antibiotics to moxifloxacin, minocycline, and linezolid for broad coverage to complete 3 weeks as outpatient. The patient reported significantly improved pain and handgrip with notable decrease in swelling. Nonetheless, 3 weeks after completing antibiotics, the right-hand pain recurred, raising concern for complex regional syndrome vs crystalline arthropathy.
The patient was referred to rheumatology service for evaluation of crystal-induced arthropathy given chondrocalcinosis. Physical examination revealed right third proximal interphalangeal joint swelling and tenderness with overimposed tophilike nodule. No erythema or palpable effusions were appreciated. Range of motion was preserved. Laboratory workup showed resolved leukocytosis and neutrophilia, and normal sedimentation rate or C-reactive protein levels. Antinuclear antibody panel, rheumatoid factor, and anti–cyclic citrullinated peptide levels were normal. Serum uric acid levels were 5.9 mg/dL. Chlamydia, gonorrhea, and HIV tests were negative. Short course of low-dose oral prednisone starting at 15 mg daily with tapering by 5 mg every 3 days was given for presumptive calcium pyrophosphate deposition vs gout. Nevertheless, right-hand swelling and pain worsened after steroids. Repeat right upper extremity MRI showed persistent soft tissue edema and inflammation along the dorsum of the hand extending to the digits, tenosynovitis, and fluid in the third metacarpophalangeal that could represent a superficial abscess. The patient was hospitalized given concerns of infection.
The relapse of tenosynovitis raised concerns for a persistent infection secondary to a fastidious organism, such as NTM. Thus, inquiries specifically pertaining to any contact with bodies of water were entertained. The patient remembered that he had gone scuba diving in the ocean weeks before symptom onset. This meant scuba diving could then be the inciting event rather than gardening, which placed NTM higher in the differential. ID service did not recommend antibiotics until new cultures were available. Orthopedic service was consulted for surgical debridement. The right dorsal hand, wrist, and distal forearm tendon sheaths were surgically opened to obtain a synovial biopsy.
Synovial fluid was sent for fungal, bacterial, and AFB cultures, and synovial biopsy for AFB stains, PCR amplification/sequencing assay, and cultures. Results showed nonnecrotizing granulomas and all cultures were negative (Figures 3, 4). Rheumatology was again consulted for evaluation for sarcoidosis given negative cultures and noncaseating granulomas. Review of systems was completely negative for sarcoidosis. Computed tomography (CT) of the thorax did not show any pulmonary abnormalities, lymphadenopathy, and hilar adenopathy. Serum calcium and angiotensin-converting enzyme levels were normal. ID service recommended against empiric antibiotics given negative culture. Given persistent pain, and reported cases of isolated sarcoid tenosynovitis, low-dose oral prednisone 20 mg daily was given after clearance by ID service. Nonetheless, the right wrist and hand swelling, erythema, and tenderness relapsed with 1 dose of prednisone, leading to a repeat right upper extremity synovial biopsy due to high suspicion for persistent infection with a fastidious organism. New synovial tissue biopsy revealed fibro-adipose tissue with prominent vessels and fibrosis, nonnecrotizing, sarcoidlike granuloma with giant cell granulomatous reaction. The AFB and Grocott methenamine silver stains were negative. PCR was negative for AFB. No crystals were reported. After 5 weeks, the synovial biopsy culture was positive for M marinum. Patient was started on oral azithromycin 500 mg daily, rifabutin 300 mg daily, and ethambutol 15 mg/kg daily. At the time of this report, the patient was still completing antibiotic therapy with adequate response and undergoing occupational therapy rehabilitation (Figure 5).
Discussion
M marinum is an NTM found in bodies of water and marine settings. Infection arises after direct contact of lacerated skin with contaminated water. In a review article of 5 cases of M marinum tenosynovitis, they found that all individuals had wounds with exposure to fish or shrimp while in the water or while handling seafood.1 The incidence of this infection is infrequent, estimated to be 0.04 cases per 100,000, with only about 25% of these cases presenting as tenosynovitis.2 The incubation period ranges from 2 to 4 weeks.3 Late identification of this organism is common because of its slow development. For example, presentation from first exposure to symptom onset may take as long as 32 days.1 In addition, in the same review, surgical intervention occurred in 63 days.1 It has been reported that AFB stains are positive in just 9% of cases, which confounds diagnosis even more.4 After synovial tissue culture is obtained, it takes approximately 6 weeks for the organism to grow. Moreover, diagnosis may take longer if it is not suspected.5
Four types of M marinum infections have been described.5 The status of the immune system plays a role in how the manifestations present. The first type is limited, which is seen in immunocompetent persons, characterized by skin involvement, such as erythematous nodular lesions, that may improve on their own in months or years.4 Conversely, in immunosuppressed patients, the second type of infection may cause sporotrichoid spreading described as following lymphangitic pattern. The third type presents with musculoskeletal findings, such as arthritis, tenosynovitis, bursitis, or osteomyelitis, as seen in our patient. The fourth type consists of systemic manifestations.5 Medications that lower the immune system, such as corticosteroids, chemotherapy, and biologic disease modifying agents, may increase the risk for developing this entity.4 Specifically, antitumor necrosis factor inhibitors have been historically associated with mycobacterium infections.6
Patients are frequently diagnosed with soft tissue infection, such as abscesses or cellulitis, as in our case. They may at times be found to have other musculoskeletal conditions such as trigger finger.1 Other similar presenting entities are psoriatic arthritis, rheumatoid arthritis, and remitting seronegative arthritis.4 These clinical resemblances complicate the scenario, especially when initial cultures are negative, as the treatment for these rheumatic diseases is immunosuppression, which adversely impact the fastidious infection. In our case, the improved swelling and range of motion after the 3-week course of empiric antibiotics for suppurative tenosynovitis was initially reassuring that the previous infection had been successfully treated. Subsequently, the presence of chondrocalcinosis in the triangular fibrocartilage in the right-hand X-rays, persistent pain, and the tophi-like appearance of the right third proximal interphalangeal nodule raised concerns for crystalline arthropathies, such as calcium pyrophosphate deposition vs gout. Nonetheless, given the lack of response to low-dose steroids, an ongoing infectious process was strongly considered.
Sarcoidosis was a concern after the first synovial biopsy revealed noncaseating granulomas and negative stains and cultures. Sarcoid tenosynovitis is rare with only 22 cases described as per a 2015 report.7 Musculoskeletal involvement in sarcoidosis has been reported in 1 to 13% of sarcoid patients.7 Once again, unresponsiveness to steroids led to another synovial biopsy for culture due to potential infection. Akin to other cases, more than one surgical debridement was required to diagnose our patient.
Conclusions
Our case reinforces the vital role of history gathering in establishing diagnoses. It underscores the value of clinical suspicion especially in patients unresponsive to standard treatment for inflammatory arthritis, namely corticosteroids. Tissue biopsy with culture for AFB is crucial for accurate diagnosis in NTM infection, which may imitate rheumatic inflammatory arthritis. Clinicians should be keenly aware of this fastidious, indolent organism in the setting of persistent localized tenosynovitis.
Rheumatologic conditions and infections may imitate each other, often making diagnosis challenging. Therefore, it is imperative to obtain adequate histories and have a keen eye for these potentially confounding differential diagnoses. Immunosuppressants used in managing rheumatologic etiologies have detrimental consequences in undiagnosed underlying infections. Consequently, worsening symptoms with standard therapy should raise awareness to a different diagnosis.
Nontuberculous mycobacteria (NTM) are slow-growing organisms difficult to yield in culture. Initial negative synovial fluid stains and cultures when suspecting NTM infectious arthritis or tenosynovitis should not exclude the diagnosis if there is a strong clinical scenario. The identification of Mycobacterium marinum (M marinum) infection in the hand is of utmost importance given that delayed treatment may cause significant and even permanent disability.
We present the case of a 73-year-old male patient with progressively worsening right-hand tenosynovitis who was evaluated for crystal-induced and sarcoid arthropathies in the setting of negative synovial biopsy cultures but was subsequently diagnosed with M marinum infectious tenosynovitis after a second surgical debridement.
Case Presentation
A 73-year-old male patient with history of type 2 diabetes mellitus, hypertension, hyperlipidemia, hypothyroidism, bilateral knee osteoarthritis, obstructive sleep apnea, and posttraumatic stress disorder presented to the emergency department (ED) with right wrist swelling and pain for 4 days. The patient reported that he was working in his garden when symptoms started. He did not recall any skin abrasions or wounds, insect bites, thorn punctures, trauma, or exposure to swimming pools or fish tanks. Patient was afebrile, and vital signs were within normal range. On physical examination, there was erythema, swelling, and tenderness in the dorsum of the right hand and over the dorsal aspect of the fourth metacarpophalangeal joint (Figure 1). The skin was intact.
Symptoms had not responded to 7 days of cefalexin nor to a short course of oral steroids. Leukocytosis of 14.35 × 109/L (reference range, 3.90-9.90 × 109/L) with neutrophilia at 11.10 × 109/L (reference range, 1.73-6.37 × 109/L) was noted. Sedimentation rate and C-reactive protein levels were normal. Right-hand X-ray was remarkable for chondrocalcinosis in the triangular fibrocartilage. Right upper extremity magnetic resonance imaging (MRI) revealed diffuse inflammation in the right wrist and hand (Figure 2). There was no evidence of septic arthritis or osteomyelitis. Consequently, orthopedic service recommended no surgical intervention. Additionally, the patient had preserved range of motion that further indicated tenosynovitis, which could be medically managed with antibiotics, rather than a septic joint.
One dose of IV piperacillin/tazobactam was given at the ED, and he was admitted to the internal medicine ward with right hand and wrist cellulitis and indolent suppurative tenosynovitis. Empiric IV ceftriaxone and vancomycin were started as per infectious disease (ID) service with adequate response defined as a reduction of the swelling, erythema, and tenderness of the right hand and wrist. Differential diagnosis included sporotrichosis, nocardia vs NTM infection.
Interventional radiology was consulted for right wrist drainage. However, only 1 mL of fluid was obtained. Synovial fluid was sent for cell count and differential, crystal analysis, bacterial cultures, fungal cultures, and acid-fast bacilli (AFB) stains and culture. Neutrophils were 43% and lymphocytes were 57%. Crystal analysis was negative. Bacterial culture and mycology were negative. AFB stain and culture results were negative after 6 weeks. Based on gardening history and risk of thorn exposure and low suspicion for common bacterial pathogens, ID service switched antibiotics to moxifloxacin, minocycline, and linezolid for broad coverage to complete 3 weeks as outpatient. The patient reported significantly improved pain and handgrip with notable decrease in swelling. Nonetheless, 3 weeks after completing antibiotics, the right-hand pain recurred, raising concern for complex regional syndrome vs crystalline arthropathy.
The patient was referred to rheumatology service for evaluation of crystal-induced arthropathy given chondrocalcinosis. Physical examination revealed right third proximal interphalangeal joint swelling and tenderness with overimposed tophilike nodule. No erythema or palpable effusions were appreciated. Range of motion was preserved. Laboratory workup showed resolved leukocytosis and neutrophilia, and normal sedimentation rate or C-reactive protein levels. Antinuclear antibody panel, rheumatoid factor, and anti–cyclic citrullinated peptide levels were normal. Serum uric acid levels were 5.9 mg/dL. Chlamydia, gonorrhea, and HIV tests were negative. Short course of low-dose oral prednisone starting at 15 mg daily with tapering by 5 mg every 3 days was given for presumptive calcium pyrophosphate deposition vs gout. Nevertheless, right-hand swelling and pain worsened after steroids. Repeat right upper extremity MRI showed persistent soft tissue edema and inflammation along the dorsum of the hand extending to the digits, tenosynovitis, and fluid in the third metacarpophalangeal that could represent a superficial abscess. The patient was hospitalized given concerns of infection.
The relapse of tenosynovitis raised concerns for a persistent infection secondary to a fastidious organism, such as NTM. Thus, inquiries specifically pertaining to any contact with bodies of water were entertained. The patient remembered that he had gone scuba diving in the ocean weeks before symptom onset. This meant scuba diving could then be the inciting event rather than gardening, which placed NTM higher in the differential. ID service did not recommend antibiotics until new cultures were available. Orthopedic service was consulted for surgical debridement. The right dorsal hand, wrist, and distal forearm tendon sheaths were surgically opened to obtain a synovial biopsy.
Synovial fluid was sent for fungal, bacterial, and AFB cultures, and synovial biopsy for AFB stains, PCR amplification/sequencing assay, and cultures. Results showed nonnecrotizing granulomas and all cultures were negative (Figures 3, 4). Rheumatology was again consulted for evaluation for sarcoidosis given negative cultures and noncaseating granulomas. Review of systems was completely negative for sarcoidosis. Computed tomography (CT) of the thorax did not show any pulmonary abnormalities, lymphadenopathy, and hilar adenopathy. Serum calcium and angiotensin-converting enzyme levels were normal. ID service recommended against empiric antibiotics given negative culture. Given persistent pain, and reported cases of isolated sarcoid tenosynovitis, low-dose oral prednisone 20 mg daily was given after clearance by ID service. Nonetheless, the right wrist and hand swelling, erythema, and tenderness relapsed with 1 dose of prednisone, leading to a repeat right upper extremity synovial biopsy due to high suspicion for persistent infection with a fastidious organism. New synovial tissue biopsy revealed fibro-adipose tissue with prominent vessels and fibrosis, nonnecrotizing, sarcoidlike granuloma with giant cell granulomatous reaction. The AFB and Grocott methenamine silver stains were negative. PCR was negative for AFB. No crystals were reported. After 5 weeks, the synovial biopsy culture was positive for M marinum. Patient was started on oral azithromycin 500 mg daily, rifabutin 300 mg daily, and ethambutol 15 mg/kg daily. At the time of this report, the patient was still completing antibiotic therapy with adequate response and undergoing occupational therapy rehabilitation (Figure 5).
Discussion
M marinum is an NTM found in bodies of water and marine settings. Infection arises after direct contact of lacerated skin with contaminated water. In a review article of 5 cases of M marinum tenosynovitis, they found that all individuals had wounds with exposure to fish or shrimp while in the water or while handling seafood.1 The incidence of this infection is infrequent, estimated to be 0.04 cases per 100,000, with only about 25% of these cases presenting as tenosynovitis.2 The incubation period ranges from 2 to 4 weeks.3 Late identification of this organism is common because of its slow development. For example, presentation from first exposure to symptom onset may take as long as 32 days.1 In addition, in the same review, surgical intervention occurred in 63 days.1 It has been reported that AFB stains are positive in just 9% of cases, which confounds diagnosis even more.4 After synovial tissue culture is obtained, it takes approximately 6 weeks for the organism to grow. Moreover, diagnosis may take longer if it is not suspected.5
Four types of M marinum infections have been described.5 The status of the immune system plays a role in how the manifestations present. The first type is limited, which is seen in immunocompetent persons, characterized by skin involvement, such as erythematous nodular lesions, that may improve on their own in months or years.4 Conversely, in immunosuppressed patients, the second type of infection may cause sporotrichoid spreading described as following lymphangitic pattern. The third type presents with musculoskeletal findings, such as arthritis, tenosynovitis, bursitis, or osteomyelitis, as seen in our patient. The fourth type consists of systemic manifestations.5 Medications that lower the immune system, such as corticosteroids, chemotherapy, and biologic disease modifying agents, may increase the risk for developing this entity.4 Specifically, antitumor necrosis factor inhibitors have been historically associated with mycobacterium infections.6
Patients are frequently diagnosed with soft tissue infection, such as abscesses or cellulitis, as in our case. They may at times be found to have other musculoskeletal conditions such as trigger finger.1 Other similar presenting entities are psoriatic arthritis, rheumatoid arthritis, and remitting seronegative arthritis.4 These clinical resemblances complicate the scenario, especially when initial cultures are negative, as the treatment for these rheumatic diseases is immunosuppression, which adversely impact the fastidious infection. In our case, the improved swelling and range of motion after the 3-week course of empiric antibiotics for suppurative tenosynovitis was initially reassuring that the previous infection had been successfully treated. Subsequently, the presence of chondrocalcinosis in the triangular fibrocartilage in the right-hand X-rays, persistent pain, and the tophi-like appearance of the right third proximal interphalangeal nodule raised concerns for crystalline arthropathies, such as calcium pyrophosphate deposition vs gout. Nonetheless, given the lack of response to low-dose steroids, an ongoing infectious process was strongly considered.
Sarcoidosis was a concern after the first synovial biopsy revealed noncaseating granulomas and negative stains and cultures. Sarcoid tenosynovitis is rare with only 22 cases described as per a 2015 report.7 Musculoskeletal involvement in sarcoidosis has been reported in 1 to 13% of sarcoid patients.7 Once again, unresponsiveness to steroids led to another synovial biopsy for culture due to potential infection. Akin to other cases, more than one surgical debridement was required to diagnose our patient.
Conclusions
Our case reinforces the vital role of history gathering in establishing diagnoses. It underscores the value of clinical suspicion especially in patients unresponsive to standard treatment for inflammatory arthritis, namely corticosteroids. Tissue biopsy with culture for AFB is crucial for accurate diagnosis in NTM infection, which may imitate rheumatic inflammatory arthritis. Clinicians should be keenly aware of this fastidious, indolent organism in the setting of persistent localized tenosynovitis.
1. Pang HN, Lee JY, Puhaindran ME, Tan SH, Tan AB, Yong FC. Mycobacterium marinum as a cause of chronic granulomatous tenosynovitis in the hand. J Infect. 2007;54(6):584-588. doi:10.1016/j.jinf.2006.11.014
2. Wongworawat MD, Holtom P, Learch TJ, Fedenko A, Stevanovic MV. A prolonged case of Mycobacterium marinum flexor tenosynovitis: radiographic and histological correlation, and review of the literature. Skeletal Radiol. 2003;32(9):542-545. doi:10.1007/s00256-003-0636-y
3. Schubert N, Schill T, Plüß M, Korsten P. Flare or foe? - Mycobacterium marinum infection mimicking rheumatoid arthritis tenosynovitis: case report and literature review. BMC Rheumatol. 2020;4:11. Published 2020 Mar 16. doi:10.1186/s41927-020-0114-3
4. Lam A, Toma W, Schlesinger N. Mycobacterium marinum arthritis mimicking rheumatoid arthritis. J Rheumatol. 2006;33(4):817-819.
5. Hashish E, Merwad A, Elgaml S, et al. Mycobacterium marinum infection in fish and man: epidemiology, pathophysiology and management; a review. Vet Q. 2018;38(1):35-46. doi:10.1080/01652176.2018.1447171
6. Thanou-Stavraki A, Sawalha AH, Crowson AN, Harley JB. Noodling and Mycobacterium marinum infection mimicking seronegative rheumatoid arthritis complicated by anti-tumor necrosis factor α therapy. Arthritis Care Res (Hoboken). 2011;63(1):160-164. doi:10.1002/acr.20303
7. Al-Ani Z, Oh TC, Macphie E, Woodruff MJ. Sarcoid tenosynovitis, rare presentation of a common disease. Case report and literature review. J Radiol Case Rep. 2015;9(8):16-23. Published 2015 Aug 31. doi:10.3941/jrcr.v9i8.2311
1. Pang HN, Lee JY, Puhaindran ME, Tan SH, Tan AB, Yong FC. Mycobacterium marinum as a cause of chronic granulomatous tenosynovitis in the hand. J Infect. 2007;54(6):584-588. doi:10.1016/j.jinf.2006.11.014
2. Wongworawat MD, Holtom P, Learch TJ, Fedenko A, Stevanovic MV. A prolonged case of Mycobacterium marinum flexor tenosynovitis: radiographic and histological correlation, and review of the literature. Skeletal Radiol. 2003;32(9):542-545. doi:10.1007/s00256-003-0636-y
3. Schubert N, Schill T, Plüß M, Korsten P. Flare or foe? - Mycobacterium marinum infection mimicking rheumatoid arthritis tenosynovitis: case report and literature review. BMC Rheumatol. 2020;4:11. Published 2020 Mar 16. doi:10.1186/s41927-020-0114-3
4. Lam A, Toma W, Schlesinger N. Mycobacterium marinum arthritis mimicking rheumatoid arthritis. J Rheumatol. 2006;33(4):817-819.
5. Hashish E, Merwad A, Elgaml S, et al. Mycobacterium marinum infection in fish and man: epidemiology, pathophysiology and management; a review. Vet Q. 2018;38(1):35-46. doi:10.1080/01652176.2018.1447171
6. Thanou-Stavraki A, Sawalha AH, Crowson AN, Harley JB. Noodling and Mycobacterium marinum infection mimicking seronegative rheumatoid arthritis complicated by anti-tumor necrosis factor α therapy. Arthritis Care Res (Hoboken). 2011;63(1):160-164. doi:10.1002/acr.20303
7. Al-Ani Z, Oh TC, Macphie E, Woodruff MJ. Sarcoid tenosynovitis, rare presentation of a common disease. Case report and literature review. J Radiol Case Rep. 2015;9(8):16-23. Published 2015 Aug 31. doi:10.3941/jrcr.v9i8.2311
Rhabdomyolysis Occurring After Use of Cocaine Contaminated With Fentanyl Causing Bilateral Brachial Plexopathy
The brachial plexus is a group of interwoven nerves arising from the cervical spinal cord and coursing through the neck, shoulder, and axilla with terminal branches extending to the distal arm.1 Disorders of the brachial plexus are more rare than other isolated peripheral nerve disorders, trauma being the most common etiology.1 Traction, neoplasms, radiation exposure, external compression, and inflammatory processes, such as Parsonage-Turner syndrome, have also been described as less common etiologies.2
Rhabdomyolysis, a condition in which muscle breakdown occurs, is an uncommon and perhaps underrecognized cause of brachial plexopathy. Rhabdomyolysis is often caused by muscle overuse, trauma, prolonged immobilization, drugs, or toxins. Substances indicated as precipitating factors include alcohol, opioids, cocaine, and amphetamines.3,4 As rhabdomyolysis progresses, swelling and edema can compress surrounding structures. Therefore, in cases of rhabdomyolysis involving the muscles of the neck and shoulder girdle, external compression of the brachial plexus can potentially cause brachial plexopathy. Rare cases of this phenomenon occurring as a sequela of substance use have been described.1,5-9 Few cases have been reported in the literature.
The following case report describes a patient who
Case Presentation
A 68-year-old male patient with a history of polysubstance use disorder presented to the emergency department with complete loss of sensory and motor function of both arms. He had fallen asleep on his couch the previous evening with his arms crossed over his chest in the prone position.
On admission, the patient presented with an agitated mental status. The patient presented with 0/5 strength bilaterally in the upper extremities (UEs) accompanied by numbness and tingling. Radial pulses were palpable in both arms. All UE reflexes were absent, but patellar reflex was intact bilaterally. On hospital day 2, the patient was awake, alert, and oriented to person, place, and time and could provide a full history. The patient’s cranial nerves were intact with shoulder shrug testing mildly weak at 4/5 strength.
Serum electrolytes and glucose levels were normal. The creatine phosphokinase (CPK) level was elevated at 21,292 IU/L. Creatinine and blood urea nitrogen levels were elevated at 1.7 mg/dL and 32 mg/dL, respectively. Serum B12, thyroid-stimulating hormone, and hemoglobin A1c levels were normal.
Due to the absence of evidence of spinal cord injury, presence of normal motor and sensory function of the lower extremities, an elevated CPK level, signal hyperintensities of the muscles of the shoulder girdle, and the patient’s history, the leading diagnosis at this time was brachial plexopathy secondary to focal rhabdomyolysis.
Over the next week, the patient regained some motor function of the left hand and some sensory function bilaterally. At 8 weeks postadmission, a nerve conduction study showed prolonged latencies in the median and ulnar nerves bilaterally. The following week, the patient reported pain in both shoulders (left greater than the right) as well as weakness of shoulder movement on the left greater than the right. There was pain in the right arm throughout. On examination, there was improved function of the arms distal to the elbow, which was better on the right side despite the associated pain (Table). There was atrophy of the left scapular muscles, hypothenar eminence, and deltoid muscle. There was weakness of the left triceps, with slight fourth and fifth finger flexion. The patient was unable to elevate or abduct the left shoulder but could elevate the right shoulder up to 45°. Sensation was decreased over the right outer arm and left posterior upper arm, with hypersensitivity in the right medial upper and lower arm. Deep tendon reflexes were absent in the upper arm aside from the biceps reflex (1+). All reflexes of the lower extremities were normal. It is interesting to note the relative greater improvement on the right despite the edema found on initial imaging being more prominent on the right.
Discussion
Rhabdomyolysis is a condition defined by myocyte necrosis that results in release of cellular contents and local edema. Inciting events may be traumatic, metabolic, ischemic, or substance induced. Common substances indicated include cocaine, amphetamines, acetaminophen, opioids, and alcohol.10 It classically presents with muscle pain and a marked elevation in serum CPK level, but other metabolic disturbances, acute kidney injury, or toxic hepatitis may also occur. A more uncommon sequela of rhabdomyolysis is plexopathy caused by edematous swelling and compression of the surrounding structures.
Rare cases of brachial plexopathy caused by rhabdomyolysis following substance use have been described. In many of these cases, rhabdomyolysis occurred after alcohol use with or without concurrent use of prescription opioids or heroin.7-9 One case following use of 3,4-methylenedioxy-N-methylamptamine (MDMA) and marijuana use was reported.1 Another case of concurrent brachial plexopathy and Horner syndrome in a 29-year-old male patient following ingestion of alcohol and opioids has also been described.5 The rate of occurrence of this phenomenon in the general population is unknown.
The pathophysiology of rhabdomyolysis caused by substance use has not been definitively identified, but it is hypothesized that the cause is 2-fold. The first insult is the direct toxicity of the substances to myocytes.8,9 The second factor is prolonged immobilization in a position that compresses the affected musculature and blood supply, causing both mechanical stress and ischemia to the muscles and brachial plexus. This prolonged immobilization can frequently follow use of substances, such as alcohol or opioids.9 Cases have been reported wherein rhabdomyolysis causing brachial plexopathy occurred despite relatively normal positioning of the arms and shoulders during sleep.9 In our case, the patient had fallen asleep with his arms crossed over his chest in the prone position with his head turned, though he could not recall to which side. Although he stated that he had slept in this position regularly, the effects of fentanyl may have prevented the patient from waking to adjust his posture. This position had potential to compress the musculature of the neck and shoulders and restrict blood flow, resulting in the focal rhabdomyolysis seen in this patient. In theory, the position could also cause a stretch injury of the brachial plexus, although a pure stretch injury would more likely present unilaterally and without evidence of rhabdomyolysis.
Chronic ethanol use may have been a major contributor by both sensitizing the muscles to toxicity of other substances and induction of CYP450 enzymes that are normally responsible for metabolizing other drugs.8 Alcohol also inhibits gluconeogenesis and leads to hyperpolarization of myocytes, further contributing to their susceptibility to damage.9 Our patient had a prior history of alcohol use years before this event, but not at the time of this event.
Our patient had other known risk factors for rhabdomyolysis, including his long-term statin therapy, but it is unclear whether these were contributing factors in his case.10 Of the medications that are known to cause rhabdomyolysis, statins are among the most commonly described, although the mechanism through which this process occurs is not clear. A case of rhabdomyolysis following use of cocaine and heroin in a patient on long-standing statin therapy has been described.13 Our review of the literature found no cases of statin-induced rhabdomyolysis associated with brachial plexopathy. It is possible that concurrent statin therapy has an additive effect to other substances in inducing rhabdomyolysis.
Parsonage-Turner syndrome, also known as neuralgic amyotrophy, should also be included in the differential diagnosis. While there have been multiple etiologies proposed for Parsonage-Turner syndrome, it is generally thought to begin as a primary inflammatory process targeting the brachial plexus. One case report describes Parsonage-Turner syndrome progressing to secondary rhabdomyolysis.6 In this case, no primary etiology was identified, so the Parsonage-Turner syndrome diagnosis was made with secondary rhabdomyolysis.6 We believe it is possible that this case and others may have been misdiagnosed as Parsonage-Turner syndrome.
Aside from physical rehabilitation programs, cases of plexopathy secondary to rhabdomyolysis similar to our patient have largely been treated with supportive therapy and symptom management. Pain management was the primary goal in this patient, which was achieved with moderate success using a combination of muscle relaxants, antiepileptics, tramadol, and serotonin-norepinephrine reuptake inhibitors. Some surgical approaches have been reported in the literature. One case of rhabdomyolysis of the shoulder girdle causing a similar process benefitted from fasciotomy and surgical decompression.7 This patient had a complete recovery of all motor functions aside from shoulder abduction at 8 weeks postoperation, but neuropathic pain persisted in both arms. It is possible our patient may have benefitted from a similar treatment. Further research is necessary to determine the utility of this type of procedure when treating such cases.
Conclusions
This case report adds to the literature describing focal rhabdomyolysis causing secondary bilateral brachial plexopathy after substance use. Further research is needed to establish a definitive pathophysiology as well as treatment guidelines. Evidence-based treatment could mean better outcomes and quicker recoveries for future patients with this condition.
1. Eker Büyüks¸ireci D, Polat M, Zinnurog˘lu M, Cengiz B, Kaymak Karatas¸ GK. Bilateral pan-plexus lesion after substance use: A case report. Turk J Phys Med Rehabil. 2019;65(4):411-414. doi:10.5606/tftrd.2019.3157
2. Rubin DI. Brachial and lumbosacral plexopathies: a review. Clin Neurophysiol Pract. 2020;5:173-193. doi:10.1016/j.cnp.2020.07.005
3. Oshima Y. Characteristics of drug-associated rhabdomyolysis: analysis of 8,610 cases reported to the US Food and Drug Administration. Intern Med. 2011;50(8):845-853. doi:10.2169/internalmedicine.50.4484
4. Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity-a euro-den study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal.pone.0246297
5. Lee SC, Geannette C, Wolfe SW, Feinberg JH, Sneag DB. Rhabdomyolysis resulting in concurrent Horner’s syndrome and brachial plexopathy: a case report. Skeletal Radiology. 2017;46(8):1131-1136. doi:10.1007/s00256-017-2634-5
6. Goetsch MR, Shen J, Jones JA, Memon A, Chatham W. Neuralgic amyotrophy presenting with multifocal myonecrosis and rhabdomyolysis. Cureus. 2020;12(3):e7382. doi:10.7759/cureus.7382
7. Tonetti DA, Tarkin IS, Bandi K, Moossy JJ. Complete bilateral brachial plexus injury from rhabdomyolysis and compartment syndrome: surgical case report. Oper Neurosurg (Hagerstown). 2019;17(2):E68-e72. doi:10.1093/ons/opy289
8. Riggs JE, Schochet SS Jr, Hogg JP. Focal rhabdomyolysis and brachial plexopathy: an association with heroin and chronic ethanol use. Mil Med. 1999;164(3):228-229.
9. Maddison P. Acute rhabdomyolysis and brachial plexopathy following alcohol ingestion. Muscle Nerve. 2002;25(2):283-285. doi:10.1002/mus.10021.abs
10. Giannoglou GD, Chatzizisis YS, Misirli G. The syndrome of rhabdomyolysis: pathophysiology and diagnosis. Eur J Intern Med. 2007;18(2):90-100. doi:10.1016/j.ejim.2006.09.020
11. Meacham MC, Lynch KL, Coffin PO, Wade A, Wheeler E, Riley ED. Addressing overdose risk among unstably housed women in San Francisco, California: an examination of potential fentanyl contamination of multiple substances. Harm Reduct J. 2020;17(1). doi:10.1186/s12954-020-00361-8
12. Klar SA, Brodkin E, Gibson E, et al. Notes from the field: furanyl-fentanyl overdose events caused by smoking contaminated crack cocaine - British Columbia, Canada, July 15-18, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(37):1015-1016. doi:10.15585/mmwr.mm6537a6
13. Mitaritonno M, Lupo M, Greco I, Mazza A, Cervellin G. Severe rhabdomyolysis induced by co-administration of cocaine and heroin in a 45 years old man treated with rosuvastatin: a case report. Acta Biomed. 2021;92(S1):e2021089. doi:10.23750/abm.v92iS1.8858
The brachial plexus is a group of interwoven nerves arising from the cervical spinal cord and coursing through the neck, shoulder, and axilla with terminal branches extending to the distal arm.1 Disorders of the brachial plexus are more rare than other isolated peripheral nerve disorders, trauma being the most common etiology.1 Traction, neoplasms, radiation exposure, external compression, and inflammatory processes, such as Parsonage-Turner syndrome, have also been described as less common etiologies.2
Rhabdomyolysis, a condition in which muscle breakdown occurs, is an uncommon and perhaps underrecognized cause of brachial plexopathy. Rhabdomyolysis is often caused by muscle overuse, trauma, prolonged immobilization, drugs, or toxins. Substances indicated as precipitating factors include alcohol, opioids, cocaine, and amphetamines.3,4 As rhabdomyolysis progresses, swelling and edema can compress surrounding structures. Therefore, in cases of rhabdomyolysis involving the muscles of the neck and shoulder girdle, external compression of the brachial plexus can potentially cause brachial plexopathy. Rare cases of this phenomenon occurring as a sequela of substance use have been described.1,5-9 Few cases have been reported in the literature.
The following case report describes a patient who
Case Presentation
A 68-year-old male patient with a history of polysubstance use disorder presented to the emergency department with complete loss of sensory and motor function of both arms. He had fallen asleep on his couch the previous evening with his arms crossed over his chest in the prone position.
On admission, the patient presented with an agitated mental status. The patient presented with 0/5 strength bilaterally in the upper extremities (UEs) accompanied by numbness and tingling. Radial pulses were palpable in both arms. All UE reflexes were absent, but patellar reflex was intact bilaterally. On hospital day 2, the patient was awake, alert, and oriented to person, place, and time and could provide a full history. The patient’s cranial nerves were intact with shoulder shrug testing mildly weak at 4/5 strength.
Serum electrolytes and glucose levels were normal. The creatine phosphokinase (CPK) level was elevated at 21,292 IU/L. Creatinine and blood urea nitrogen levels were elevated at 1.7 mg/dL and 32 mg/dL, respectively. Serum B12, thyroid-stimulating hormone, and hemoglobin A1c levels were normal.
Due to the absence of evidence of spinal cord injury, presence of normal motor and sensory function of the lower extremities, an elevated CPK level, signal hyperintensities of the muscles of the shoulder girdle, and the patient’s history, the leading diagnosis at this time was brachial plexopathy secondary to focal rhabdomyolysis.
Over the next week, the patient regained some motor function of the left hand and some sensory function bilaterally. At 8 weeks postadmission, a nerve conduction study showed prolonged latencies in the median and ulnar nerves bilaterally. The following week, the patient reported pain in both shoulders (left greater than the right) as well as weakness of shoulder movement on the left greater than the right. There was pain in the right arm throughout. On examination, there was improved function of the arms distal to the elbow, which was better on the right side despite the associated pain (Table). There was atrophy of the left scapular muscles, hypothenar eminence, and deltoid muscle. There was weakness of the left triceps, with slight fourth and fifth finger flexion. The patient was unable to elevate or abduct the left shoulder but could elevate the right shoulder up to 45°. Sensation was decreased over the right outer arm and left posterior upper arm, with hypersensitivity in the right medial upper and lower arm. Deep tendon reflexes were absent in the upper arm aside from the biceps reflex (1+). All reflexes of the lower extremities were normal. It is interesting to note the relative greater improvement on the right despite the edema found on initial imaging being more prominent on the right.
Discussion
Rhabdomyolysis is a condition defined by myocyte necrosis that results in release of cellular contents and local edema. Inciting events may be traumatic, metabolic, ischemic, or substance induced. Common substances indicated include cocaine, amphetamines, acetaminophen, opioids, and alcohol.10 It classically presents with muscle pain and a marked elevation in serum CPK level, but other metabolic disturbances, acute kidney injury, or toxic hepatitis may also occur. A more uncommon sequela of rhabdomyolysis is plexopathy caused by edematous swelling and compression of the surrounding structures.
Rare cases of brachial plexopathy caused by rhabdomyolysis following substance use have been described. In many of these cases, rhabdomyolysis occurred after alcohol use with or without concurrent use of prescription opioids or heroin.7-9 One case following use of 3,4-methylenedioxy-N-methylamptamine (MDMA) and marijuana use was reported.1 Another case of concurrent brachial plexopathy and Horner syndrome in a 29-year-old male patient following ingestion of alcohol and opioids has also been described.5 The rate of occurrence of this phenomenon in the general population is unknown.
The pathophysiology of rhabdomyolysis caused by substance use has not been definitively identified, but it is hypothesized that the cause is 2-fold. The first insult is the direct toxicity of the substances to myocytes.8,9 The second factor is prolonged immobilization in a position that compresses the affected musculature and blood supply, causing both mechanical stress and ischemia to the muscles and brachial plexus. This prolonged immobilization can frequently follow use of substances, such as alcohol or opioids.9 Cases have been reported wherein rhabdomyolysis causing brachial plexopathy occurred despite relatively normal positioning of the arms and shoulders during sleep.9 In our case, the patient had fallen asleep with his arms crossed over his chest in the prone position with his head turned, though he could not recall to which side. Although he stated that he had slept in this position regularly, the effects of fentanyl may have prevented the patient from waking to adjust his posture. This position had potential to compress the musculature of the neck and shoulders and restrict blood flow, resulting in the focal rhabdomyolysis seen in this patient. In theory, the position could also cause a stretch injury of the brachial plexus, although a pure stretch injury would more likely present unilaterally and without evidence of rhabdomyolysis.
Chronic ethanol use may have been a major contributor by both sensitizing the muscles to toxicity of other substances and induction of CYP450 enzymes that are normally responsible for metabolizing other drugs.8 Alcohol also inhibits gluconeogenesis and leads to hyperpolarization of myocytes, further contributing to their susceptibility to damage.9 Our patient had a prior history of alcohol use years before this event, but not at the time of this event.
Our patient had other known risk factors for rhabdomyolysis, including his long-term statin therapy, but it is unclear whether these were contributing factors in his case.10 Of the medications that are known to cause rhabdomyolysis, statins are among the most commonly described, although the mechanism through which this process occurs is not clear. A case of rhabdomyolysis following use of cocaine and heroin in a patient on long-standing statin therapy has been described.13 Our review of the literature found no cases of statin-induced rhabdomyolysis associated with brachial plexopathy. It is possible that concurrent statin therapy has an additive effect to other substances in inducing rhabdomyolysis.
Parsonage-Turner syndrome, also known as neuralgic amyotrophy, should also be included in the differential diagnosis. While there have been multiple etiologies proposed for Parsonage-Turner syndrome, it is generally thought to begin as a primary inflammatory process targeting the brachial plexus. One case report describes Parsonage-Turner syndrome progressing to secondary rhabdomyolysis.6 In this case, no primary etiology was identified, so the Parsonage-Turner syndrome diagnosis was made with secondary rhabdomyolysis.6 We believe it is possible that this case and others may have been misdiagnosed as Parsonage-Turner syndrome.
Aside from physical rehabilitation programs, cases of plexopathy secondary to rhabdomyolysis similar to our patient have largely been treated with supportive therapy and symptom management. Pain management was the primary goal in this patient, which was achieved with moderate success using a combination of muscle relaxants, antiepileptics, tramadol, and serotonin-norepinephrine reuptake inhibitors. Some surgical approaches have been reported in the literature. One case of rhabdomyolysis of the shoulder girdle causing a similar process benefitted from fasciotomy and surgical decompression.7 This patient had a complete recovery of all motor functions aside from shoulder abduction at 8 weeks postoperation, but neuropathic pain persisted in both arms. It is possible our patient may have benefitted from a similar treatment. Further research is necessary to determine the utility of this type of procedure when treating such cases.
Conclusions
This case report adds to the literature describing focal rhabdomyolysis causing secondary bilateral brachial plexopathy after substance use. Further research is needed to establish a definitive pathophysiology as well as treatment guidelines. Evidence-based treatment could mean better outcomes and quicker recoveries for future patients with this condition.
The brachial plexus is a group of interwoven nerves arising from the cervical spinal cord and coursing through the neck, shoulder, and axilla with terminal branches extending to the distal arm.1 Disorders of the brachial plexus are more rare than other isolated peripheral nerve disorders, trauma being the most common etiology.1 Traction, neoplasms, radiation exposure, external compression, and inflammatory processes, such as Parsonage-Turner syndrome, have also been described as less common etiologies.2
Rhabdomyolysis, a condition in which muscle breakdown occurs, is an uncommon and perhaps underrecognized cause of brachial plexopathy. Rhabdomyolysis is often caused by muscle overuse, trauma, prolonged immobilization, drugs, or toxins. Substances indicated as precipitating factors include alcohol, opioids, cocaine, and amphetamines.3,4 As rhabdomyolysis progresses, swelling and edema can compress surrounding structures. Therefore, in cases of rhabdomyolysis involving the muscles of the neck and shoulder girdle, external compression of the brachial plexus can potentially cause brachial plexopathy. Rare cases of this phenomenon occurring as a sequela of substance use have been described.1,5-9 Few cases have been reported in the literature.
The following case report describes a patient who
Case Presentation
A 68-year-old male patient with a history of polysubstance use disorder presented to the emergency department with complete loss of sensory and motor function of both arms. He had fallen asleep on his couch the previous evening with his arms crossed over his chest in the prone position.
On admission, the patient presented with an agitated mental status. The patient presented with 0/5 strength bilaterally in the upper extremities (UEs) accompanied by numbness and tingling. Radial pulses were palpable in both arms. All UE reflexes were absent, but patellar reflex was intact bilaterally. On hospital day 2, the patient was awake, alert, and oriented to person, place, and time and could provide a full history. The patient’s cranial nerves were intact with shoulder shrug testing mildly weak at 4/5 strength.
Serum electrolytes and glucose levels were normal. The creatine phosphokinase (CPK) level was elevated at 21,292 IU/L. Creatinine and blood urea nitrogen levels were elevated at 1.7 mg/dL and 32 mg/dL, respectively. Serum B12, thyroid-stimulating hormone, and hemoglobin A1c levels were normal.
Due to the absence of evidence of spinal cord injury, presence of normal motor and sensory function of the lower extremities, an elevated CPK level, signal hyperintensities of the muscles of the shoulder girdle, and the patient’s history, the leading diagnosis at this time was brachial plexopathy secondary to focal rhabdomyolysis.
Over the next week, the patient regained some motor function of the left hand and some sensory function bilaterally. At 8 weeks postadmission, a nerve conduction study showed prolonged latencies in the median and ulnar nerves bilaterally. The following week, the patient reported pain in both shoulders (left greater than the right) as well as weakness of shoulder movement on the left greater than the right. There was pain in the right arm throughout. On examination, there was improved function of the arms distal to the elbow, which was better on the right side despite the associated pain (Table). There was atrophy of the left scapular muscles, hypothenar eminence, and deltoid muscle. There was weakness of the left triceps, with slight fourth and fifth finger flexion. The patient was unable to elevate or abduct the left shoulder but could elevate the right shoulder up to 45°. Sensation was decreased over the right outer arm and left posterior upper arm, with hypersensitivity in the right medial upper and lower arm. Deep tendon reflexes were absent in the upper arm aside from the biceps reflex (1+). All reflexes of the lower extremities were normal. It is interesting to note the relative greater improvement on the right despite the edema found on initial imaging being more prominent on the right.
Discussion
Rhabdomyolysis is a condition defined by myocyte necrosis that results in release of cellular contents and local edema. Inciting events may be traumatic, metabolic, ischemic, or substance induced. Common substances indicated include cocaine, amphetamines, acetaminophen, opioids, and alcohol.10 It classically presents with muscle pain and a marked elevation in serum CPK level, but other metabolic disturbances, acute kidney injury, or toxic hepatitis may also occur. A more uncommon sequela of rhabdomyolysis is plexopathy caused by edematous swelling and compression of the surrounding structures.
Rare cases of brachial plexopathy caused by rhabdomyolysis following substance use have been described. In many of these cases, rhabdomyolysis occurred after alcohol use with or without concurrent use of prescription opioids or heroin.7-9 One case following use of 3,4-methylenedioxy-N-methylamptamine (MDMA) and marijuana use was reported.1 Another case of concurrent brachial plexopathy and Horner syndrome in a 29-year-old male patient following ingestion of alcohol and opioids has also been described.5 The rate of occurrence of this phenomenon in the general population is unknown.
The pathophysiology of rhabdomyolysis caused by substance use has not been definitively identified, but it is hypothesized that the cause is 2-fold. The first insult is the direct toxicity of the substances to myocytes.8,9 The second factor is prolonged immobilization in a position that compresses the affected musculature and blood supply, causing both mechanical stress and ischemia to the muscles and brachial plexus. This prolonged immobilization can frequently follow use of substances, such as alcohol or opioids.9 Cases have been reported wherein rhabdomyolysis causing brachial plexopathy occurred despite relatively normal positioning of the arms and shoulders during sleep.9 In our case, the patient had fallen asleep with his arms crossed over his chest in the prone position with his head turned, though he could not recall to which side. Although he stated that he had slept in this position regularly, the effects of fentanyl may have prevented the patient from waking to adjust his posture. This position had potential to compress the musculature of the neck and shoulders and restrict blood flow, resulting in the focal rhabdomyolysis seen in this patient. In theory, the position could also cause a stretch injury of the brachial plexus, although a pure stretch injury would more likely present unilaterally and without evidence of rhabdomyolysis.
Chronic ethanol use may have been a major contributor by both sensitizing the muscles to toxicity of other substances and induction of CYP450 enzymes that are normally responsible for metabolizing other drugs.8 Alcohol also inhibits gluconeogenesis and leads to hyperpolarization of myocytes, further contributing to their susceptibility to damage.9 Our patient had a prior history of alcohol use years before this event, but not at the time of this event.
Our patient had other known risk factors for rhabdomyolysis, including his long-term statin therapy, but it is unclear whether these were contributing factors in his case.10 Of the medications that are known to cause rhabdomyolysis, statins are among the most commonly described, although the mechanism through which this process occurs is not clear. A case of rhabdomyolysis following use of cocaine and heroin in a patient on long-standing statin therapy has been described.13 Our review of the literature found no cases of statin-induced rhabdomyolysis associated with brachial plexopathy. It is possible that concurrent statin therapy has an additive effect to other substances in inducing rhabdomyolysis.
Parsonage-Turner syndrome, also known as neuralgic amyotrophy, should also be included in the differential diagnosis. While there have been multiple etiologies proposed for Parsonage-Turner syndrome, it is generally thought to begin as a primary inflammatory process targeting the brachial plexus. One case report describes Parsonage-Turner syndrome progressing to secondary rhabdomyolysis.6 In this case, no primary etiology was identified, so the Parsonage-Turner syndrome diagnosis was made with secondary rhabdomyolysis.6 We believe it is possible that this case and others may have been misdiagnosed as Parsonage-Turner syndrome.
Aside from physical rehabilitation programs, cases of plexopathy secondary to rhabdomyolysis similar to our patient have largely been treated with supportive therapy and symptom management. Pain management was the primary goal in this patient, which was achieved with moderate success using a combination of muscle relaxants, antiepileptics, tramadol, and serotonin-norepinephrine reuptake inhibitors. Some surgical approaches have been reported in the literature. One case of rhabdomyolysis of the shoulder girdle causing a similar process benefitted from fasciotomy and surgical decompression.7 This patient had a complete recovery of all motor functions aside from shoulder abduction at 8 weeks postoperation, but neuropathic pain persisted in both arms. It is possible our patient may have benefitted from a similar treatment. Further research is necessary to determine the utility of this type of procedure when treating such cases.
Conclusions
This case report adds to the literature describing focal rhabdomyolysis causing secondary bilateral brachial plexopathy after substance use. Further research is needed to establish a definitive pathophysiology as well as treatment guidelines. Evidence-based treatment could mean better outcomes and quicker recoveries for future patients with this condition.
1. Eker Büyüks¸ireci D, Polat M, Zinnurog˘lu M, Cengiz B, Kaymak Karatas¸ GK. Bilateral pan-plexus lesion after substance use: A case report. Turk J Phys Med Rehabil. 2019;65(4):411-414. doi:10.5606/tftrd.2019.3157
2. Rubin DI. Brachial and lumbosacral plexopathies: a review. Clin Neurophysiol Pract. 2020;5:173-193. doi:10.1016/j.cnp.2020.07.005
3. Oshima Y. Characteristics of drug-associated rhabdomyolysis: analysis of 8,610 cases reported to the US Food and Drug Administration. Intern Med. 2011;50(8):845-853. doi:10.2169/internalmedicine.50.4484
4. Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity-a euro-den study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal.pone.0246297
5. Lee SC, Geannette C, Wolfe SW, Feinberg JH, Sneag DB. Rhabdomyolysis resulting in concurrent Horner’s syndrome and brachial plexopathy: a case report. Skeletal Radiology. 2017;46(8):1131-1136. doi:10.1007/s00256-017-2634-5
6. Goetsch MR, Shen J, Jones JA, Memon A, Chatham W. Neuralgic amyotrophy presenting with multifocal myonecrosis and rhabdomyolysis. Cureus. 2020;12(3):e7382. doi:10.7759/cureus.7382
7. Tonetti DA, Tarkin IS, Bandi K, Moossy JJ. Complete bilateral brachial plexus injury from rhabdomyolysis and compartment syndrome: surgical case report. Oper Neurosurg (Hagerstown). 2019;17(2):E68-e72. doi:10.1093/ons/opy289
8. Riggs JE, Schochet SS Jr, Hogg JP. Focal rhabdomyolysis and brachial plexopathy: an association with heroin and chronic ethanol use. Mil Med. 1999;164(3):228-229.
9. Maddison P. Acute rhabdomyolysis and brachial plexopathy following alcohol ingestion. Muscle Nerve. 2002;25(2):283-285. doi:10.1002/mus.10021.abs
10. Giannoglou GD, Chatzizisis YS, Misirli G. The syndrome of rhabdomyolysis: pathophysiology and diagnosis. Eur J Intern Med. 2007;18(2):90-100. doi:10.1016/j.ejim.2006.09.020
11. Meacham MC, Lynch KL, Coffin PO, Wade A, Wheeler E, Riley ED. Addressing overdose risk among unstably housed women in San Francisco, California: an examination of potential fentanyl contamination of multiple substances. Harm Reduct J. 2020;17(1). doi:10.1186/s12954-020-00361-8
12. Klar SA, Brodkin E, Gibson E, et al. Notes from the field: furanyl-fentanyl overdose events caused by smoking contaminated crack cocaine - British Columbia, Canada, July 15-18, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(37):1015-1016. doi:10.15585/mmwr.mm6537a6
13. Mitaritonno M, Lupo M, Greco I, Mazza A, Cervellin G. Severe rhabdomyolysis induced by co-administration of cocaine and heroin in a 45 years old man treated with rosuvastatin: a case report. Acta Biomed. 2021;92(S1):e2021089. doi:10.23750/abm.v92iS1.8858
1. Eker Büyüks¸ireci D, Polat M, Zinnurog˘lu M, Cengiz B, Kaymak Karatas¸ GK. Bilateral pan-plexus lesion after substance use: A case report. Turk J Phys Med Rehabil. 2019;65(4):411-414. doi:10.5606/tftrd.2019.3157
2. Rubin DI. Brachial and lumbosacral plexopathies: a review. Clin Neurophysiol Pract. 2020;5:173-193. doi:10.1016/j.cnp.2020.07.005
3. Oshima Y. Characteristics of drug-associated rhabdomyolysis: analysis of 8,610 cases reported to the US Food and Drug Administration. Intern Med. 2011;50(8):845-853. doi:10.2169/internalmedicine.50.4484
4. Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity-a euro-den study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal.pone.0246297
5. Lee SC, Geannette C, Wolfe SW, Feinberg JH, Sneag DB. Rhabdomyolysis resulting in concurrent Horner’s syndrome and brachial plexopathy: a case report. Skeletal Radiology. 2017;46(8):1131-1136. doi:10.1007/s00256-017-2634-5
6. Goetsch MR, Shen J, Jones JA, Memon A, Chatham W. Neuralgic amyotrophy presenting with multifocal myonecrosis and rhabdomyolysis. Cureus. 2020;12(3):e7382. doi:10.7759/cureus.7382
7. Tonetti DA, Tarkin IS, Bandi K, Moossy JJ. Complete bilateral brachial plexus injury from rhabdomyolysis and compartment syndrome: surgical case report. Oper Neurosurg (Hagerstown). 2019;17(2):E68-e72. doi:10.1093/ons/opy289
8. Riggs JE, Schochet SS Jr, Hogg JP. Focal rhabdomyolysis and brachial plexopathy: an association with heroin and chronic ethanol use. Mil Med. 1999;164(3):228-229.
9. Maddison P. Acute rhabdomyolysis and brachial plexopathy following alcohol ingestion. Muscle Nerve. 2002;25(2):283-285. doi:10.1002/mus.10021.abs
10. Giannoglou GD, Chatzizisis YS, Misirli G. The syndrome of rhabdomyolysis: pathophysiology and diagnosis. Eur J Intern Med. 2007;18(2):90-100. doi:10.1016/j.ejim.2006.09.020
11. Meacham MC, Lynch KL, Coffin PO, Wade A, Wheeler E, Riley ED. Addressing overdose risk among unstably housed women in San Francisco, California: an examination of potential fentanyl contamination of multiple substances. Harm Reduct J. 2020;17(1). doi:10.1186/s12954-020-00361-8
12. Klar SA, Brodkin E, Gibson E, et al. Notes from the field: furanyl-fentanyl overdose events caused by smoking contaminated crack cocaine - British Columbia, Canada, July 15-18, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(37):1015-1016. doi:10.15585/mmwr.mm6537a6
13. Mitaritonno M, Lupo M, Greco I, Mazza A, Cervellin G. Severe rhabdomyolysis induced by co-administration of cocaine and heroin in a 45 years old man treated with rosuvastatin: a case report. Acta Biomed. 2021;92(S1):e2021089. doi:10.23750/abm.v92iS1.8858
Benign Pneumatosis Intestinalis: A Case Report and Review of the Literature
Pneumatosis intestinalis (PI) is the finding of gas within the walls of the intestine on imaging. It is most commonly detected via radiograph or computed tomography (CT). The diseases leading to the accumulation of gas within the submucosal space of the gastrointestinal (GI) tract are heterogenous, and the finding of PI itself has a wide range of clinical implications from impending clinical deterioration to an incidental finding of minimal consequence.
We present the case of a veteran who had sustained a remote anoxic brain injury resulting in chronic dependence on a gastrostomy tube for enteral nutrition, found incidentally to have PI without signs of intra-abdominal catastrophe. An exclusion of other, more lifethreatening causes of PI led to a diagnosis of benign PI secondary to the presence of his gastrostomy tube. This case highlights the importance of interpreting the finding of PI in the clinical context of the specific patient and how conservative management may be appropriate in some cases.
Case Presentation
A 61-year-old male patient was admitted for fever. The patient had a remote history of cardiac arrest complicated by anoxic brain injury requiring tracheostomy, gastrostomy tube, and a suprapubic catheter with recurrent catheter-associated urinary tract infections (CAUTI), secondary seizure disorder, atrial fibrillation off anticoagulation due to recurrent GI bleeding, and treatment naive chronic hepatitis C virus. His ability to provide a clinical history was limited by his nonverbal status. He had no prior surgical history but had presented a month earlier for a high-grade small bowel obstruction (SBO) with pneumobilia that was managed conservatively as the surgical team deemed him a poor candidate for surgical intervention with his extensive comorbidities. A bioethics consultation at the time supported minimizing potential surgical risk in favor of conservative medical management; this was discussed with the patient’s surrogate decision maker, who also wished to avoid surgery. The SBO resolved with conservative management. He had been residing in a nursing home and doing well until 24 hours prior to admission when he developed fevers.
Vital signs on admission showed a temperature of 100.8 °F, heart rate 100 beats per minute, blood pressure 116/85, respiratory rate 22 per minute, and oxygen saturation of 100% on 6 L of oxygen via tracheostomy collar. His initial examination was notable for clear lung sounds, a nondistended nonrigid abdomen with an indwelling percutaneous gastrostomy tube, and absence of areas of skin breakdown or erythema. Notable laboratory studies showed a leukocytosis and urinalysis suggestive of CAUTI (Table). His urinary catheter was exchanged, he was fluid resuscitated and started on empiric vancomycin and piperacillin-tazobactam for management of sepsis due to CAUTI.
For the first 3 days of his hospitalization, he demonstrated clinical improvement on vancomycin and piperacillin-tazobactam while awaiting results from his urine bacterial culture. On hospital day 3, hedeveloped recurrent nonbloody, nonbilious emesis despite no change in the rate or formulation of his enteral nutrition. He also had 3 watery brown bowel movements. His vital signs remained within normal limits. His abdominal examination at this point showed mild distention and was hypertympanic to percussion, but there was no rigidity or involuntary guarding. On hospital day 4, he continued to have emesis with an unchanged abdominal examination. The differential diagnosis included recurrence of prior SBO, ileus, intestinal ischemia, enteral nutrition intolerance, Clostridioides difficile (C difficile) colitis, and GI dysmotility because of his anoxic brain injury.
Testing for C difficile was negative. An abdominal radiograph was obtained and revealed no bowel obstruction but, alarmingly, showed extensive intramural bowel gas, suggestive of PI (Figure 1). His leukocyte count, serum bicarbonate, and serum lactate levels remained within normal limits. A CT with contrast of the abdomen and pelvis demonstrated no vascular obstruction but confirmed the presence of diffuse intramural gas in his stomach and proximal small bowel, as well as the presence of mesenteric and portal venous gas (Figures 2 and 3). Although his abdominal examination had not changed and did not suggest peritonitis, general surgery was consulted to discuss the need for surgical intervention. Given his overall clinical stability and high surgical risk due to his many comorbidities, surgery recommended a conservative approach.
Through the following hospital days, his enteral nutrition was held and serial abdominal examinations were performed without change. Serial laboratory studies, including serum lactate and leukocyte count, remained reassuringly within normal limits. His urine culture eventually revealed multidrugresistant Pseudomonas aeruginosa. Antimicrobial therapy was narrowed to piperacillintazobactam for a complete course. Enteral nutrition was gradually reintroduced at a low rate, ultimately reaching goal rate with return of bowel function by hospital day 9. Despite extensive workup, the etiology of his transient enteral nutrition intolerance remained uncertain, though an adverse effect of antibiotic therapy was thought possible. Follow-up abdominal radiographs demonstrated interval improvement of PI. He was discharged back to his skilled nursing facility on hospital day 11 without incident.
Discussion
PI is an incompletely understood condition seen in multiple diseases. Patients may present with highly variable symptoms, often more attributable to the underlying disease causing the PI than the presence of PI, as patients may be entirely asymptomatic. When symptoms are attributed to PI, those most reported are abdominal pain, bloody stools, and diarrhea.1 It is often detected on abdominal plain films. Alternative methods of diagnosis include ultrasonography, barium enema, and endoscopy although the last method has been known to occasionally lead to bowel perforation.2-6 The most sensitive method of detection is CT, which also provides additional information about abdominal pathology and may identify the underlying process responsible for the PI.7
While not fully understood, much information about PI and its pathogenesis is known. Understanding the mechanisms of PI is vital to direct the clinician’s evaluation of the patient for reversible conditions that may cause PI. Early descriptions of PI in the literature documented an association with pyloric stenosis, leading to the theory that gas from the intestinal lumen is driven into the submucosal space during episodes of forceful vomiting with increased intraluminal pressure.8 As PI was subsequently described in multiple other disease states not typically associated with increased intraluminal pressure such as inflammatory bowel disease, GI malignancy, cryptosporidiosis and CMV infection, additional theories about the pathogenesis of PI have arisen.9-24 There is now experimental data to support multiple mechanisms of intramural gas accumulation. It has become accepted that PI represents a common pathway shared across various pathologic states and results from multifactorial mechanisms of gas entry into the intestinal wall.25-29
Factors leading to the development of PI include bacterial production of gas, intraluminal GI gas compositions, increased intraluminal pressure, pulmonary gas tracking through vessels communicating with the thorax, and mucosal disruption. PI has been linked to bacterial infections of the GI tract in humans including C difficile, Klebsiella, and Whipple disease.14-18 In animal models, C difficile within the walls of rat intestine results in the appearance of pneumocysts, or discrete collections of submucosal gas, which are the hallmark feature of PI.30 It is thought that direct invasion of bacteria into intramural spaces can cause PI in humans, although bacteria have yet to be directly isolated from the pneumocysts. Translocation of luminal gas into pneumocysts found in PI is theorized to be driven by differences in partial pressures.31 The concentration of hydrogen within the intestinal lumen is high due to bacterial production. Hydrogen, diffusing along its partial pressure gradient between the lumen and blood, accumulates within the intestinal wall and causes the formation of pneumocysts. This phenomenon has been hypothesized to explain the tendency for pneumocysts to form around the mesenteric vasculature.
Gas from the lumen can also be forced into the intestinal wall during an abrupt increase in intra-abdominal pressure, such as that seen with forceful vomiting. The final possible origin of the gas is the lungs, as PI has been associated with lung disease. It was previously thought that gas from ruptured alveoli tracks along mediastinal vessels, below the diaphragm, and into the mesentery.30 Newer theories argue that increased intra-abdominal pressure, typical of patients with obstructive lung disease and frequent coughing, is the driver of PI by the mechanism previously described.32-34 Additionally, mucosal disruption leads to increased permeability and allows accumulation of gas within the intestinal walls. Mucosal abnormalities have been described in histopathologic studies of patients with PI and associated with conditions known to compromise mucosal integrity, such as immunodeficiencies, inflammatory bowel disease, and the receipt of cytotoxic chemotherapy.10,12,19-23
Our patient likely had mucosal disruption due to his gastrostomy tube as well as increased intraluminal pressure from recurrent vomiting, contributing to translocation of otherwise normal intraluminal gas. The presence of portal venous gas, as seen in this case, has historically portended a worse prognosis, with 37% mortality in one series.7,35,36 However, portal venous gas as well as pneumoperitoneum occur in benign etiologies of PI as well. It is thought that this occurs due to rupture of the submucosal pneumocysts through the wall opposite the intestinal lumen and thus does not result in a direct communication between the intestinal lumen and the peritoneal cavity.12
PI is not a diagnosis but a manifestation of an underlying disease. As such, the treatment of PI is targeted toward the underlying condition. Of note, the pattern and extent of PI seen on imaging has not been shown to correlate with the severity of the underlying pathologic process.35,37 Instead, assessment of the patient and their clinical trajectory should determine the appropriate treatment. The decision facing the clinician when PI is discovered is whether urgent surgery is indicated, as is the case in mesenteric ischemia, bowel necrosis, or intestinal perforation, conditions known to be associated with PI. Otherwise, there is no definitive treatment for PI. Bowel rest is almost universally pursued. There are reports of treating with supranormal levels of supplemental oxygen, maintaining arterial partial pressure of oxygen above 300 mm Hg, with a face mask and 8 L/min flow rate.38,39 The proposed mechanisms of benefit include establishing a favorable diffusion gradient for intramural gas to exit the pneumocysts as well as creating an inhospitable, aerobic environment for hydrogenproducing anaerobic enteric bacteria. A prudent approach for most cases of PI is conservative management with bowel rest and supplemental oxygen unless there is a definitive indication for urgent surgical intervention, such as peritonitis, abdominal sepsis, or perforation.40,41 Management recommendations suggest that up to 50% of cases can be successfully managed nonoperatively.42
Conclusions
PI is the radiographic finding of gas within the walls of the intestinal tract and has variable clinical significance. It can represent a benign incidental finding or a sequela of intraabdominal emergencies such as mesenteric ischemia or bowel necrosis. Because PI is seen in a variety of disorders, several proposed mechanisms are supported in the medical literature. These include bacterial production of gas, gas pressure gradients between the intestinal lumen and the blood, increased intraluminal pressure, pulmonary gas tracking from intrathoracic vessels, and mucosal disruption. The evaluation of a patient with PI must begin with an assessment for the need for urgent surgical intervention. Additional management measures include bowel rest, IV hydration, and supplemental oxygen administration. Because of its wide variety of etiologies of varying clinical urgency, placing the finding of PI in the context of the patient is paramount to selecting an appropriate management strategy.
1. Jamart J. Pneumatosis cystoides intestinalis. A statistical study of 919 cases. Acta Hepatogastroenterol (Stuttg). 1979;26(5):419-422.
2. Lafortune M, Trinh BC, Burns PN, et al. Air in the portal vein: sonographic and Doppler manifestations. Radiology. 1991;180(3):667-670. doi:10.1148/radiology.180.3.1871276
3. Kriegshauser JS, Reading CC, King BF, Welch TJ. Combined systemic and portal venous gas: sonographic and CT detection in two cases. AJR Am J Roentgenol. 1990;154(6):1219-1221. doi:10.2214/ajr.154.6.2110731
4. Goske MJ, Goldblum JR, Applegate KE, Mitchell CS, Bardo D. The “circle sign”: a new sonographic sign of pneumatosis intestinalis - clinical, pathologic and experimental findings. Pediatr Radiol. 1999;29(7):530-535. doi:10.1007/s002470050638
5. Marshak RH, Lindner AE, Maklansky D. Pneumatosis cystoides coli. Gastrointest Radiol. 1977;2(2):85-89. doi:10.1007/BF02256475
6. Jensen R, Gutnik SH. Pneumatosis cystoides intestinalis: a complication of colonoscopic polypectomy. S D J Med. 1991;44(7):177-179.
7. Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990;212(2):160-165. doi:10.1097/00000658-199008000-00008
8. Koss LG. Abdominal gas cysts (Pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol. 1952;53(6):523-549.
9. Jona JZ. Benign pneumatosis intestinalis coli after blunt trauma to the abdomen in a child. J Pediatr Surg. 2000;35(7):1109-1111. doi:10.1053/jpsu.2000.7837
10. Gagliardi G, Thompson IW, Hershman MJ, Forbes A, Hawley PR, Talbot IC. Pneumatosis coli: a proposed pathogenesis based on study of 25 cases and review of the literature. Int J Colorectal Dis. 1996;11(3):111-118. doi:10.1007/s003840050031
11. Seto T, Koide N, Taniuchi N, Yamada T, Hamaguchi M, Goto S. Pneumatosis cystoides intestinalis complicating carcinoma of the small intestine. Am J Surg. 2001;182(3):287-288. doi:10.1016/S0002-9610(01)00710-3
12. Galandiuk S, Fazio VW, Petras RE. Pneumatosis cystoides intestinalis in Crohn’s disease. Report of two cases. Dis Colon Rectum. 1985;28(12):951-956. doi:10.1007/BF02554315
13. Parra JA, Acinas O, Bueno J, Madrazo C, Fariñas C. An unusual form of pneumatosis intestinalis associated with appendicitis. Br J Radiol. 1998;71(843):326-328. doi:10.1259/bjr.71.843.9616245
14. Schenk P, Madl C, Kramer L, et al. Pneumatosis intestinalis with Clostridium difficile colitis as a cause of acute abdomen after lung transplantation. Dig Dis Sci. 1998;43(11):2455-2458. doi:10.1023/a:1026682131847
15. Kreiss C, Forohar F, Smithline AE, Brandt LJ. Pneumatosis intestinalis complicating C. difficile pseudomembranous colitis. Am J Gastroenterol. 1999;94(9):2560-2561. doi:10.1111/j.1572-0241.1999.01397.x
16. Day DL, Ramsay NK, Letourneau JG. Pneumatosis intestinalis after bone marrow transplantation. AJR Am J Roentgenol. 1988;151(1):85-87. doi:10.2214/ajr.151.1.85
17. Tahara S, Sakai Y, Katsuno H, Urano M, Kuroda M, Tsukamoto T. Pneumatosis intestinalis and hepatic portal venous gas associated with gas-forming bacterial translocation due to postoperative paralytic ileus: A case report. Medicine (Baltimore). 2019;98(2):e14079. doi:10.1097/MD.0000000000014079
18. Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of whipple’s disease in a patient mistakenly on anti-tnf therapy. ACG Case Rep J. 2013;1(1):25- 28. Published 2013 Oct 8. doi:10.14309/crj.2013.11
19. Burton EM, Mercado-Deane MG, Patel K. Pneumatosis intestinalis in a child with AIDS and pseudomembranous colitis. Pediatr Radiol. 1994;24(8):609-610. doi:10.1007/BF02012750
20. Berk RN, Wall SD, McArdle CB, et al. Cryptosporidiosis of the stomach and small intestine in patients with AIDS. AJR Am J Roentgenol. 1984;143(3):549-554. doi:10.2214/ajr.143.3.549
21. Samson VE, Brown WR. Pneumatosis cystoides intestinalis in AIDS-associated cryptosporidiosis. More than an incidental finding? J Clin Gastroenterol. 1996;22(4):311-312.doi:10.1097/00004836-199606000-00015
22. Tjon A Tham RT, Vlasveld LT, Willemze R. Gastrointestinal complications of cytosine-arabinoside chemotherapy: findings on plain abdominal radiographs. AJR Am J Roentgenol. 1990;154(1):95-98. doi:10.2214/ajr.154.1.2104733
23. Hashimoto S, Saitoh H, Wada K, et al. Pneumatosis cystoides intestinalis after chemotherapy for hematological malignancies: report of 4 cases. Intern Med. 1995;34(3):212-215. doi:10.2169/internalmedicine.34.212
24. Gelman SF, Brandt LJ. Pneumatosis intestinalis and AIDS: a case report and review of the literature. Am J Gastroenterol. 1998;93(4):646-650. doi:10.1111/j.1572-0241.1998.183_b.x
25. Gillon J, Tadesse K, Logan RF, Holt S, Sircus W. Breath hydrogen in pneumatosis cystoides intestinalis. Gut. 1979;20(11):1008-1011. doi:10.1136/gut.20.11.1008
26. Hughes DT, Gordon KC, Swann JC, Bolt GL. Pneumatosis cystoides intestinalis. Gut. 1966;7(5):553-557. doi:10.1136/gut.7.5.553
27. Read NW, Al-Janabi MN, Cann PA. Is raised breath hydrogen related to the pathogenesis of pneumatosis coli? Gut. 1984;25(8):839-845. doi:10.1136/gut.25.8.839
28. van der Linden W, Marsell R. Pneumatosis cystoides coli associated with high H2 excretion. Treatment with an elemental diet. Scand J Gastroenterol. 1979;14(2):173-174. doi:10.3109/00365527909179864
29. Christl SU, Gibson GR, Murgatroyd PR, Scheppach W, Cummings JH. Impaired hydrogen metabolism in pneumatosis cystoides intestinalis. Gastroenterology. 1993;104(2):392-397. doi:10.1016/0016-5085(93)90406-3
30. Keyting WS, Mccarver RR, Kovarik JL, Daywitt AL. Pneumatosis intestinalis: a new concept. Radiology. 1961;76:733-741. doi:10.1148/76.5.733
31. Florin TH, Hills BA. Does counterperfusion supersaturation cause gas cysts in pneumatosis cystoides coli, and can breathing heliox reduce them? Lancet. 1995;345(8959):1220-1222. doi:10.1016/S0140-6736(95)91996-1
32. Grieve DA, Unsworth IP. Pneumatosis cystoides intestinalis: an experience with hyperbaric oxygen treatment. Aust N Z J Surg. 1991;61(6):423-426.
33. Micklefield GH, Kuntz HD, May B. Pneumatosis cystoides intestinalis: case reports and review of the literature. Mater Med Pol. 1990;22(2):70-72.
34. Yale CE, Balish E, Wu JP. The bacterial etiology of pneumatosis cystoides intestinalis. Arch Surg. 1974;109(1):89- 94. doi:10.1001/archsurg.1974.01360010067017
35. Fenton LZ, Buonomo C. Benign pneumatosis in children. Pediatr Radiol. 2000;30(11):786-793. doi:10.1007/s002470000303
36. Tobias R, Coleman S, Helman CA. Pneumatosis coli simulating hepatomegaly. Am J Gastroenterol. 1985;80(2):146-149.
37. Feczko PJ, Mezwa DG, Farah MC, White BD. Clinical significance of pneumatosis of the bowel w a l l . Radiographics. 1992;12(6):1069-1078. doi:10.1148/radiographics.12.6.1439012
38. Masterson JS, Fratkin LB, Osler TR, Trapp WG. Treatment of pneumatosis cystoides intestinalis with hyperbaric oxygen. Ann Surg. 1978;187(3):245-247. doi:10.1097/00000658-197803000-00005
39. Höflin F, Linden W van der. Pneumatosis cystoides intestinalis treated by oxygen breathing. Scandinavian J Gastroenterol . 1974;9(5) :427-430. doi:10.1080/00365521.1974.12096852
40. St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138(1):68-75. doi:10.1001/archsurg.138.1.68
41. Ling F, Guo D, Zhu L. Pneumatosis cystoides intestinalis: a case report and literature review. BMC Gastroenterol. 2019;19(1):176. Published 2019 Nov 6. doi:10.1186/s12876-019-1087-9
42. Morris MS, Gee AC, Cho SD, et al. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195(5):679-682. doi:10.1016/j.amjsurg.2008.01.011
Pneumatosis intestinalis (PI) is the finding of gas within the walls of the intestine on imaging. It is most commonly detected via radiograph or computed tomography (CT). The diseases leading to the accumulation of gas within the submucosal space of the gastrointestinal (GI) tract are heterogenous, and the finding of PI itself has a wide range of clinical implications from impending clinical deterioration to an incidental finding of minimal consequence.
We present the case of a veteran who had sustained a remote anoxic brain injury resulting in chronic dependence on a gastrostomy tube for enteral nutrition, found incidentally to have PI without signs of intra-abdominal catastrophe. An exclusion of other, more lifethreatening causes of PI led to a diagnosis of benign PI secondary to the presence of his gastrostomy tube. This case highlights the importance of interpreting the finding of PI in the clinical context of the specific patient and how conservative management may be appropriate in some cases.
Case Presentation
A 61-year-old male patient was admitted for fever. The patient had a remote history of cardiac arrest complicated by anoxic brain injury requiring tracheostomy, gastrostomy tube, and a suprapubic catheter with recurrent catheter-associated urinary tract infections (CAUTI), secondary seizure disorder, atrial fibrillation off anticoagulation due to recurrent GI bleeding, and treatment naive chronic hepatitis C virus. His ability to provide a clinical history was limited by his nonverbal status. He had no prior surgical history but had presented a month earlier for a high-grade small bowel obstruction (SBO) with pneumobilia that was managed conservatively as the surgical team deemed him a poor candidate for surgical intervention with his extensive comorbidities. A bioethics consultation at the time supported minimizing potential surgical risk in favor of conservative medical management; this was discussed with the patient’s surrogate decision maker, who also wished to avoid surgery. The SBO resolved with conservative management. He had been residing in a nursing home and doing well until 24 hours prior to admission when he developed fevers.
Vital signs on admission showed a temperature of 100.8 °F, heart rate 100 beats per minute, blood pressure 116/85, respiratory rate 22 per minute, and oxygen saturation of 100% on 6 L of oxygen via tracheostomy collar. His initial examination was notable for clear lung sounds, a nondistended nonrigid abdomen with an indwelling percutaneous gastrostomy tube, and absence of areas of skin breakdown or erythema. Notable laboratory studies showed a leukocytosis and urinalysis suggestive of CAUTI (Table). His urinary catheter was exchanged, he was fluid resuscitated and started on empiric vancomycin and piperacillin-tazobactam for management of sepsis due to CAUTI.
For the first 3 days of his hospitalization, he demonstrated clinical improvement on vancomycin and piperacillin-tazobactam while awaiting results from his urine bacterial culture. On hospital day 3, hedeveloped recurrent nonbloody, nonbilious emesis despite no change in the rate or formulation of his enteral nutrition. He also had 3 watery brown bowel movements. His vital signs remained within normal limits. His abdominal examination at this point showed mild distention and was hypertympanic to percussion, but there was no rigidity or involuntary guarding. On hospital day 4, he continued to have emesis with an unchanged abdominal examination. The differential diagnosis included recurrence of prior SBO, ileus, intestinal ischemia, enteral nutrition intolerance, Clostridioides difficile (C difficile) colitis, and GI dysmotility because of his anoxic brain injury.
Testing for C difficile was negative. An abdominal radiograph was obtained and revealed no bowel obstruction but, alarmingly, showed extensive intramural bowel gas, suggestive of PI (Figure 1). His leukocyte count, serum bicarbonate, and serum lactate levels remained within normal limits. A CT with contrast of the abdomen and pelvis demonstrated no vascular obstruction but confirmed the presence of diffuse intramural gas in his stomach and proximal small bowel, as well as the presence of mesenteric and portal venous gas (Figures 2 and 3). Although his abdominal examination had not changed and did not suggest peritonitis, general surgery was consulted to discuss the need for surgical intervention. Given his overall clinical stability and high surgical risk due to his many comorbidities, surgery recommended a conservative approach.
Through the following hospital days, his enteral nutrition was held and serial abdominal examinations were performed without change. Serial laboratory studies, including serum lactate and leukocyte count, remained reassuringly within normal limits. His urine culture eventually revealed multidrugresistant Pseudomonas aeruginosa. Antimicrobial therapy was narrowed to piperacillintazobactam for a complete course. Enteral nutrition was gradually reintroduced at a low rate, ultimately reaching goal rate with return of bowel function by hospital day 9. Despite extensive workup, the etiology of his transient enteral nutrition intolerance remained uncertain, though an adverse effect of antibiotic therapy was thought possible. Follow-up abdominal radiographs demonstrated interval improvement of PI. He was discharged back to his skilled nursing facility on hospital day 11 without incident.
Discussion
PI is an incompletely understood condition seen in multiple diseases. Patients may present with highly variable symptoms, often more attributable to the underlying disease causing the PI than the presence of PI, as patients may be entirely asymptomatic. When symptoms are attributed to PI, those most reported are abdominal pain, bloody stools, and diarrhea.1 It is often detected on abdominal plain films. Alternative methods of diagnosis include ultrasonography, barium enema, and endoscopy although the last method has been known to occasionally lead to bowel perforation.2-6 The most sensitive method of detection is CT, which also provides additional information about abdominal pathology and may identify the underlying process responsible for the PI.7
While not fully understood, much information about PI and its pathogenesis is known. Understanding the mechanisms of PI is vital to direct the clinician’s evaluation of the patient for reversible conditions that may cause PI. Early descriptions of PI in the literature documented an association with pyloric stenosis, leading to the theory that gas from the intestinal lumen is driven into the submucosal space during episodes of forceful vomiting with increased intraluminal pressure.8 As PI was subsequently described in multiple other disease states not typically associated with increased intraluminal pressure such as inflammatory bowel disease, GI malignancy, cryptosporidiosis and CMV infection, additional theories about the pathogenesis of PI have arisen.9-24 There is now experimental data to support multiple mechanisms of intramural gas accumulation. It has become accepted that PI represents a common pathway shared across various pathologic states and results from multifactorial mechanisms of gas entry into the intestinal wall.25-29
Factors leading to the development of PI include bacterial production of gas, intraluminal GI gas compositions, increased intraluminal pressure, pulmonary gas tracking through vessels communicating with the thorax, and mucosal disruption. PI has been linked to bacterial infections of the GI tract in humans including C difficile, Klebsiella, and Whipple disease.14-18 In animal models, C difficile within the walls of rat intestine results in the appearance of pneumocysts, or discrete collections of submucosal gas, which are the hallmark feature of PI.30 It is thought that direct invasion of bacteria into intramural spaces can cause PI in humans, although bacteria have yet to be directly isolated from the pneumocysts. Translocation of luminal gas into pneumocysts found in PI is theorized to be driven by differences in partial pressures.31 The concentration of hydrogen within the intestinal lumen is high due to bacterial production. Hydrogen, diffusing along its partial pressure gradient between the lumen and blood, accumulates within the intestinal wall and causes the formation of pneumocysts. This phenomenon has been hypothesized to explain the tendency for pneumocysts to form around the mesenteric vasculature.
Gas from the lumen can also be forced into the intestinal wall during an abrupt increase in intra-abdominal pressure, such as that seen with forceful vomiting. The final possible origin of the gas is the lungs, as PI has been associated with lung disease. It was previously thought that gas from ruptured alveoli tracks along mediastinal vessels, below the diaphragm, and into the mesentery.30 Newer theories argue that increased intra-abdominal pressure, typical of patients with obstructive lung disease and frequent coughing, is the driver of PI by the mechanism previously described.32-34 Additionally, mucosal disruption leads to increased permeability and allows accumulation of gas within the intestinal walls. Mucosal abnormalities have been described in histopathologic studies of patients with PI and associated with conditions known to compromise mucosal integrity, such as immunodeficiencies, inflammatory bowel disease, and the receipt of cytotoxic chemotherapy.10,12,19-23
Our patient likely had mucosal disruption due to his gastrostomy tube as well as increased intraluminal pressure from recurrent vomiting, contributing to translocation of otherwise normal intraluminal gas. The presence of portal venous gas, as seen in this case, has historically portended a worse prognosis, with 37% mortality in one series.7,35,36 However, portal venous gas as well as pneumoperitoneum occur in benign etiologies of PI as well. It is thought that this occurs due to rupture of the submucosal pneumocysts through the wall opposite the intestinal lumen and thus does not result in a direct communication between the intestinal lumen and the peritoneal cavity.12
PI is not a diagnosis but a manifestation of an underlying disease. As such, the treatment of PI is targeted toward the underlying condition. Of note, the pattern and extent of PI seen on imaging has not been shown to correlate with the severity of the underlying pathologic process.35,37 Instead, assessment of the patient and their clinical trajectory should determine the appropriate treatment. The decision facing the clinician when PI is discovered is whether urgent surgery is indicated, as is the case in mesenteric ischemia, bowel necrosis, or intestinal perforation, conditions known to be associated with PI. Otherwise, there is no definitive treatment for PI. Bowel rest is almost universally pursued. There are reports of treating with supranormal levels of supplemental oxygen, maintaining arterial partial pressure of oxygen above 300 mm Hg, with a face mask and 8 L/min flow rate.38,39 The proposed mechanisms of benefit include establishing a favorable diffusion gradient for intramural gas to exit the pneumocysts as well as creating an inhospitable, aerobic environment for hydrogenproducing anaerobic enteric bacteria. A prudent approach for most cases of PI is conservative management with bowel rest and supplemental oxygen unless there is a definitive indication for urgent surgical intervention, such as peritonitis, abdominal sepsis, or perforation.40,41 Management recommendations suggest that up to 50% of cases can be successfully managed nonoperatively.42
Conclusions
PI is the radiographic finding of gas within the walls of the intestinal tract and has variable clinical significance. It can represent a benign incidental finding or a sequela of intraabdominal emergencies such as mesenteric ischemia or bowel necrosis. Because PI is seen in a variety of disorders, several proposed mechanisms are supported in the medical literature. These include bacterial production of gas, gas pressure gradients between the intestinal lumen and the blood, increased intraluminal pressure, pulmonary gas tracking from intrathoracic vessels, and mucosal disruption. The evaluation of a patient with PI must begin with an assessment for the need for urgent surgical intervention. Additional management measures include bowel rest, IV hydration, and supplemental oxygen administration. Because of its wide variety of etiologies of varying clinical urgency, placing the finding of PI in the context of the patient is paramount to selecting an appropriate management strategy.
Pneumatosis intestinalis (PI) is the finding of gas within the walls of the intestine on imaging. It is most commonly detected via radiograph or computed tomography (CT). The diseases leading to the accumulation of gas within the submucosal space of the gastrointestinal (GI) tract are heterogenous, and the finding of PI itself has a wide range of clinical implications from impending clinical deterioration to an incidental finding of minimal consequence.
We present the case of a veteran who had sustained a remote anoxic brain injury resulting in chronic dependence on a gastrostomy tube for enteral nutrition, found incidentally to have PI without signs of intra-abdominal catastrophe. An exclusion of other, more lifethreatening causes of PI led to a diagnosis of benign PI secondary to the presence of his gastrostomy tube. This case highlights the importance of interpreting the finding of PI in the clinical context of the specific patient and how conservative management may be appropriate in some cases.
Case Presentation
A 61-year-old male patient was admitted for fever. The patient had a remote history of cardiac arrest complicated by anoxic brain injury requiring tracheostomy, gastrostomy tube, and a suprapubic catheter with recurrent catheter-associated urinary tract infections (CAUTI), secondary seizure disorder, atrial fibrillation off anticoagulation due to recurrent GI bleeding, and treatment naive chronic hepatitis C virus. His ability to provide a clinical history was limited by his nonverbal status. He had no prior surgical history but had presented a month earlier for a high-grade small bowel obstruction (SBO) with pneumobilia that was managed conservatively as the surgical team deemed him a poor candidate for surgical intervention with his extensive comorbidities. A bioethics consultation at the time supported minimizing potential surgical risk in favor of conservative medical management; this was discussed with the patient’s surrogate decision maker, who also wished to avoid surgery. The SBO resolved with conservative management. He had been residing in a nursing home and doing well until 24 hours prior to admission when he developed fevers.
Vital signs on admission showed a temperature of 100.8 °F, heart rate 100 beats per minute, blood pressure 116/85, respiratory rate 22 per minute, and oxygen saturation of 100% on 6 L of oxygen via tracheostomy collar. His initial examination was notable for clear lung sounds, a nondistended nonrigid abdomen with an indwelling percutaneous gastrostomy tube, and absence of areas of skin breakdown or erythema. Notable laboratory studies showed a leukocytosis and urinalysis suggestive of CAUTI (Table). His urinary catheter was exchanged, he was fluid resuscitated and started on empiric vancomycin and piperacillin-tazobactam for management of sepsis due to CAUTI.
For the first 3 days of his hospitalization, he demonstrated clinical improvement on vancomycin and piperacillin-tazobactam while awaiting results from his urine bacterial culture. On hospital day 3, hedeveloped recurrent nonbloody, nonbilious emesis despite no change in the rate or formulation of his enteral nutrition. He also had 3 watery brown bowel movements. His vital signs remained within normal limits. His abdominal examination at this point showed mild distention and was hypertympanic to percussion, but there was no rigidity or involuntary guarding. On hospital day 4, he continued to have emesis with an unchanged abdominal examination. The differential diagnosis included recurrence of prior SBO, ileus, intestinal ischemia, enteral nutrition intolerance, Clostridioides difficile (C difficile) colitis, and GI dysmotility because of his anoxic brain injury.
Testing for C difficile was negative. An abdominal radiograph was obtained and revealed no bowel obstruction but, alarmingly, showed extensive intramural bowel gas, suggestive of PI (Figure 1). His leukocyte count, serum bicarbonate, and serum lactate levels remained within normal limits. A CT with contrast of the abdomen and pelvis demonstrated no vascular obstruction but confirmed the presence of diffuse intramural gas in his stomach and proximal small bowel, as well as the presence of mesenteric and portal venous gas (Figures 2 and 3). Although his abdominal examination had not changed and did not suggest peritonitis, general surgery was consulted to discuss the need for surgical intervention. Given his overall clinical stability and high surgical risk due to his many comorbidities, surgery recommended a conservative approach.
Through the following hospital days, his enteral nutrition was held and serial abdominal examinations were performed without change. Serial laboratory studies, including serum lactate and leukocyte count, remained reassuringly within normal limits. His urine culture eventually revealed multidrugresistant Pseudomonas aeruginosa. Antimicrobial therapy was narrowed to piperacillintazobactam for a complete course. Enteral nutrition was gradually reintroduced at a low rate, ultimately reaching goal rate with return of bowel function by hospital day 9. Despite extensive workup, the etiology of his transient enteral nutrition intolerance remained uncertain, though an adverse effect of antibiotic therapy was thought possible. Follow-up abdominal radiographs demonstrated interval improvement of PI. He was discharged back to his skilled nursing facility on hospital day 11 without incident.
Discussion
PI is an incompletely understood condition seen in multiple diseases. Patients may present with highly variable symptoms, often more attributable to the underlying disease causing the PI than the presence of PI, as patients may be entirely asymptomatic. When symptoms are attributed to PI, those most reported are abdominal pain, bloody stools, and diarrhea.1 It is often detected on abdominal plain films. Alternative methods of diagnosis include ultrasonography, barium enema, and endoscopy although the last method has been known to occasionally lead to bowel perforation.2-6 The most sensitive method of detection is CT, which also provides additional information about abdominal pathology and may identify the underlying process responsible for the PI.7
While not fully understood, much information about PI and its pathogenesis is known. Understanding the mechanisms of PI is vital to direct the clinician’s evaluation of the patient for reversible conditions that may cause PI. Early descriptions of PI in the literature documented an association with pyloric stenosis, leading to the theory that gas from the intestinal lumen is driven into the submucosal space during episodes of forceful vomiting with increased intraluminal pressure.8 As PI was subsequently described in multiple other disease states not typically associated with increased intraluminal pressure such as inflammatory bowel disease, GI malignancy, cryptosporidiosis and CMV infection, additional theories about the pathogenesis of PI have arisen.9-24 There is now experimental data to support multiple mechanisms of intramural gas accumulation. It has become accepted that PI represents a common pathway shared across various pathologic states and results from multifactorial mechanisms of gas entry into the intestinal wall.25-29
Factors leading to the development of PI include bacterial production of gas, intraluminal GI gas compositions, increased intraluminal pressure, pulmonary gas tracking through vessels communicating with the thorax, and mucosal disruption. PI has been linked to bacterial infections of the GI tract in humans including C difficile, Klebsiella, and Whipple disease.14-18 In animal models, C difficile within the walls of rat intestine results in the appearance of pneumocysts, or discrete collections of submucosal gas, which are the hallmark feature of PI.30 It is thought that direct invasion of bacteria into intramural spaces can cause PI in humans, although bacteria have yet to be directly isolated from the pneumocysts. Translocation of luminal gas into pneumocysts found in PI is theorized to be driven by differences in partial pressures.31 The concentration of hydrogen within the intestinal lumen is high due to bacterial production. Hydrogen, diffusing along its partial pressure gradient between the lumen and blood, accumulates within the intestinal wall and causes the formation of pneumocysts. This phenomenon has been hypothesized to explain the tendency for pneumocysts to form around the mesenteric vasculature.
Gas from the lumen can also be forced into the intestinal wall during an abrupt increase in intra-abdominal pressure, such as that seen with forceful vomiting. The final possible origin of the gas is the lungs, as PI has been associated with lung disease. It was previously thought that gas from ruptured alveoli tracks along mediastinal vessels, below the diaphragm, and into the mesentery.30 Newer theories argue that increased intra-abdominal pressure, typical of patients with obstructive lung disease and frequent coughing, is the driver of PI by the mechanism previously described.32-34 Additionally, mucosal disruption leads to increased permeability and allows accumulation of gas within the intestinal walls. Mucosal abnormalities have been described in histopathologic studies of patients with PI and associated with conditions known to compromise mucosal integrity, such as immunodeficiencies, inflammatory bowel disease, and the receipt of cytotoxic chemotherapy.10,12,19-23
Our patient likely had mucosal disruption due to his gastrostomy tube as well as increased intraluminal pressure from recurrent vomiting, contributing to translocation of otherwise normal intraluminal gas. The presence of portal venous gas, as seen in this case, has historically portended a worse prognosis, with 37% mortality in one series.7,35,36 However, portal venous gas as well as pneumoperitoneum occur in benign etiologies of PI as well. It is thought that this occurs due to rupture of the submucosal pneumocysts through the wall opposite the intestinal lumen and thus does not result in a direct communication between the intestinal lumen and the peritoneal cavity.12
PI is not a diagnosis but a manifestation of an underlying disease. As such, the treatment of PI is targeted toward the underlying condition. Of note, the pattern and extent of PI seen on imaging has not been shown to correlate with the severity of the underlying pathologic process.35,37 Instead, assessment of the patient and their clinical trajectory should determine the appropriate treatment. The decision facing the clinician when PI is discovered is whether urgent surgery is indicated, as is the case in mesenteric ischemia, bowel necrosis, or intestinal perforation, conditions known to be associated with PI. Otherwise, there is no definitive treatment for PI. Bowel rest is almost universally pursued. There are reports of treating with supranormal levels of supplemental oxygen, maintaining arterial partial pressure of oxygen above 300 mm Hg, with a face mask and 8 L/min flow rate.38,39 The proposed mechanisms of benefit include establishing a favorable diffusion gradient for intramural gas to exit the pneumocysts as well as creating an inhospitable, aerobic environment for hydrogenproducing anaerobic enteric bacteria. A prudent approach for most cases of PI is conservative management with bowel rest and supplemental oxygen unless there is a definitive indication for urgent surgical intervention, such as peritonitis, abdominal sepsis, or perforation.40,41 Management recommendations suggest that up to 50% of cases can be successfully managed nonoperatively.42
Conclusions
PI is the radiographic finding of gas within the walls of the intestinal tract and has variable clinical significance. It can represent a benign incidental finding or a sequela of intraabdominal emergencies such as mesenteric ischemia or bowel necrosis. Because PI is seen in a variety of disorders, several proposed mechanisms are supported in the medical literature. These include bacterial production of gas, gas pressure gradients between the intestinal lumen and the blood, increased intraluminal pressure, pulmonary gas tracking from intrathoracic vessels, and mucosal disruption. The evaluation of a patient with PI must begin with an assessment for the need for urgent surgical intervention. Additional management measures include bowel rest, IV hydration, and supplemental oxygen administration. Because of its wide variety of etiologies of varying clinical urgency, placing the finding of PI in the context of the patient is paramount to selecting an appropriate management strategy.
1. Jamart J. Pneumatosis cystoides intestinalis. A statistical study of 919 cases. Acta Hepatogastroenterol (Stuttg). 1979;26(5):419-422.
2. Lafortune M, Trinh BC, Burns PN, et al. Air in the portal vein: sonographic and Doppler manifestations. Radiology. 1991;180(3):667-670. doi:10.1148/radiology.180.3.1871276
3. Kriegshauser JS, Reading CC, King BF, Welch TJ. Combined systemic and portal venous gas: sonographic and CT detection in two cases. AJR Am J Roentgenol. 1990;154(6):1219-1221. doi:10.2214/ajr.154.6.2110731
4. Goske MJ, Goldblum JR, Applegate KE, Mitchell CS, Bardo D. The “circle sign”: a new sonographic sign of pneumatosis intestinalis - clinical, pathologic and experimental findings. Pediatr Radiol. 1999;29(7):530-535. doi:10.1007/s002470050638
5. Marshak RH, Lindner AE, Maklansky D. Pneumatosis cystoides coli. Gastrointest Radiol. 1977;2(2):85-89. doi:10.1007/BF02256475
6. Jensen R, Gutnik SH. Pneumatosis cystoides intestinalis: a complication of colonoscopic polypectomy. S D J Med. 1991;44(7):177-179.
7. Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990;212(2):160-165. doi:10.1097/00000658-199008000-00008
8. Koss LG. Abdominal gas cysts (Pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol. 1952;53(6):523-549.
9. Jona JZ. Benign pneumatosis intestinalis coli after blunt trauma to the abdomen in a child. J Pediatr Surg. 2000;35(7):1109-1111. doi:10.1053/jpsu.2000.7837
10. Gagliardi G, Thompson IW, Hershman MJ, Forbes A, Hawley PR, Talbot IC. Pneumatosis coli: a proposed pathogenesis based on study of 25 cases and review of the literature. Int J Colorectal Dis. 1996;11(3):111-118. doi:10.1007/s003840050031
11. Seto T, Koide N, Taniuchi N, Yamada T, Hamaguchi M, Goto S. Pneumatosis cystoides intestinalis complicating carcinoma of the small intestine. Am J Surg. 2001;182(3):287-288. doi:10.1016/S0002-9610(01)00710-3
12. Galandiuk S, Fazio VW, Petras RE. Pneumatosis cystoides intestinalis in Crohn’s disease. Report of two cases. Dis Colon Rectum. 1985;28(12):951-956. doi:10.1007/BF02554315
13. Parra JA, Acinas O, Bueno J, Madrazo C, Fariñas C. An unusual form of pneumatosis intestinalis associated with appendicitis. Br J Radiol. 1998;71(843):326-328. doi:10.1259/bjr.71.843.9616245
14. Schenk P, Madl C, Kramer L, et al. Pneumatosis intestinalis with Clostridium difficile colitis as a cause of acute abdomen after lung transplantation. Dig Dis Sci. 1998;43(11):2455-2458. doi:10.1023/a:1026682131847
15. Kreiss C, Forohar F, Smithline AE, Brandt LJ. Pneumatosis intestinalis complicating C. difficile pseudomembranous colitis. Am J Gastroenterol. 1999;94(9):2560-2561. doi:10.1111/j.1572-0241.1999.01397.x
16. Day DL, Ramsay NK, Letourneau JG. Pneumatosis intestinalis after bone marrow transplantation. AJR Am J Roentgenol. 1988;151(1):85-87. doi:10.2214/ajr.151.1.85
17. Tahara S, Sakai Y, Katsuno H, Urano M, Kuroda M, Tsukamoto T. Pneumatosis intestinalis and hepatic portal venous gas associated with gas-forming bacterial translocation due to postoperative paralytic ileus: A case report. Medicine (Baltimore). 2019;98(2):e14079. doi:10.1097/MD.0000000000014079
18. Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of whipple’s disease in a patient mistakenly on anti-tnf therapy. ACG Case Rep J. 2013;1(1):25- 28. Published 2013 Oct 8. doi:10.14309/crj.2013.11
19. Burton EM, Mercado-Deane MG, Patel K. Pneumatosis intestinalis in a child with AIDS and pseudomembranous colitis. Pediatr Radiol. 1994;24(8):609-610. doi:10.1007/BF02012750
20. Berk RN, Wall SD, McArdle CB, et al. Cryptosporidiosis of the stomach and small intestine in patients with AIDS. AJR Am J Roentgenol. 1984;143(3):549-554. doi:10.2214/ajr.143.3.549
21. Samson VE, Brown WR. Pneumatosis cystoides intestinalis in AIDS-associated cryptosporidiosis. More than an incidental finding? J Clin Gastroenterol. 1996;22(4):311-312.doi:10.1097/00004836-199606000-00015
22. Tjon A Tham RT, Vlasveld LT, Willemze R. Gastrointestinal complications of cytosine-arabinoside chemotherapy: findings on plain abdominal radiographs. AJR Am J Roentgenol. 1990;154(1):95-98. doi:10.2214/ajr.154.1.2104733
23. Hashimoto S, Saitoh H, Wada K, et al. Pneumatosis cystoides intestinalis after chemotherapy for hematological malignancies: report of 4 cases. Intern Med. 1995;34(3):212-215. doi:10.2169/internalmedicine.34.212
24. Gelman SF, Brandt LJ. Pneumatosis intestinalis and AIDS: a case report and review of the literature. Am J Gastroenterol. 1998;93(4):646-650. doi:10.1111/j.1572-0241.1998.183_b.x
25. Gillon J, Tadesse K, Logan RF, Holt S, Sircus W. Breath hydrogen in pneumatosis cystoides intestinalis. Gut. 1979;20(11):1008-1011. doi:10.1136/gut.20.11.1008
26. Hughes DT, Gordon KC, Swann JC, Bolt GL. Pneumatosis cystoides intestinalis. Gut. 1966;7(5):553-557. doi:10.1136/gut.7.5.553
27. Read NW, Al-Janabi MN, Cann PA. Is raised breath hydrogen related to the pathogenesis of pneumatosis coli? Gut. 1984;25(8):839-845. doi:10.1136/gut.25.8.839
28. van der Linden W, Marsell R. Pneumatosis cystoides coli associated with high H2 excretion. Treatment with an elemental diet. Scand J Gastroenterol. 1979;14(2):173-174. doi:10.3109/00365527909179864
29. Christl SU, Gibson GR, Murgatroyd PR, Scheppach W, Cummings JH. Impaired hydrogen metabolism in pneumatosis cystoides intestinalis. Gastroenterology. 1993;104(2):392-397. doi:10.1016/0016-5085(93)90406-3
30. Keyting WS, Mccarver RR, Kovarik JL, Daywitt AL. Pneumatosis intestinalis: a new concept. Radiology. 1961;76:733-741. doi:10.1148/76.5.733
31. Florin TH, Hills BA. Does counterperfusion supersaturation cause gas cysts in pneumatosis cystoides coli, and can breathing heliox reduce them? Lancet. 1995;345(8959):1220-1222. doi:10.1016/S0140-6736(95)91996-1
32. Grieve DA, Unsworth IP. Pneumatosis cystoides intestinalis: an experience with hyperbaric oxygen treatment. Aust N Z J Surg. 1991;61(6):423-426.
33. Micklefield GH, Kuntz HD, May B. Pneumatosis cystoides intestinalis: case reports and review of the literature. Mater Med Pol. 1990;22(2):70-72.
34. Yale CE, Balish E, Wu JP. The bacterial etiology of pneumatosis cystoides intestinalis. Arch Surg. 1974;109(1):89- 94. doi:10.1001/archsurg.1974.01360010067017
35. Fenton LZ, Buonomo C. Benign pneumatosis in children. Pediatr Radiol. 2000;30(11):786-793. doi:10.1007/s002470000303
36. Tobias R, Coleman S, Helman CA. Pneumatosis coli simulating hepatomegaly. Am J Gastroenterol. 1985;80(2):146-149.
37. Feczko PJ, Mezwa DG, Farah MC, White BD. Clinical significance of pneumatosis of the bowel w a l l . Radiographics. 1992;12(6):1069-1078. doi:10.1148/radiographics.12.6.1439012
38. Masterson JS, Fratkin LB, Osler TR, Trapp WG. Treatment of pneumatosis cystoides intestinalis with hyperbaric oxygen. Ann Surg. 1978;187(3):245-247. doi:10.1097/00000658-197803000-00005
39. Höflin F, Linden W van der. Pneumatosis cystoides intestinalis treated by oxygen breathing. Scandinavian J Gastroenterol . 1974;9(5) :427-430. doi:10.1080/00365521.1974.12096852
40. St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138(1):68-75. doi:10.1001/archsurg.138.1.68
41. Ling F, Guo D, Zhu L. Pneumatosis cystoides intestinalis: a case report and literature review. BMC Gastroenterol. 2019;19(1):176. Published 2019 Nov 6. doi:10.1186/s12876-019-1087-9
42. Morris MS, Gee AC, Cho SD, et al. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195(5):679-682. doi:10.1016/j.amjsurg.2008.01.011
1. Jamart J. Pneumatosis cystoides intestinalis. A statistical study of 919 cases. Acta Hepatogastroenterol (Stuttg). 1979;26(5):419-422.
2. Lafortune M, Trinh BC, Burns PN, et al. Air in the portal vein: sonographic and Doppler manifestations. Radiology. 1991;180(3):667-670. doi:10.1148/radiology.180.3.1871276
3. Kriegshauser JS, Reading CC, King BF, Welch TJ. Combined systemic and portal venous gas: sonographic and CT detection in two cases. AJR Am J Roentgenol. 1990;154(6):1219-1221. doi:10.2214/ajr.154.6.2110731
4. Goske MJ, Goldblum JR, Applegate KE, Mitchell CS, Bardo D. The “circle sign”: a new sonographic sign of pneumatosis intestinalis - clinical, pathologic and experimental findings. Pediatr Radiol. 1999;29(7):530-535. doi:10.1007/s002470050638
5. Marshak RH, Lindner AE, Maklansky D. Pneumatosis cystoides coli. Gastrointest Radiol. 1977;2(2):85-89. doi:10.1007/BF02256475
6. Jensen R, Gutnik SH. Pneumatosis cystoides intestinalis: a complication of colonoscopic polypectomy. S D J Med. 1991;44(7):177-179.
7. Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990;212(2):160-165. doi:10.1097/00000658-199008000-00008
8. Koss LG. Abdominal gas cysts (Pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol. 1952;53(6):523-549.
9. Jona JZ. Benign pneumatosis intestinalis coli after blunt trauma to the abdomen in a child. J Pediatr Surg. 2000;35(7):1109-1111. doi:10.1053/jpsu.2000.7837
10. Gagliardi G, Thompson IW, Hershman MJ, Forbes A, Hawley PR, Talbot IC. Pneumatosis coli: a proposed pathogenesis based on study of 25 cases and review of the literature. Int J Colorectal Dis. 1996;11(3):111-118. doi:10.1007/s003840050031
11. Seto T, Koide N, Taniuchi N, Yamada T, Hamaguchi M, Goto S. Pneumatosis cystoides intestinalis complicating carcinoma of the small intestine. Am J Surg. 2001;182(3):287-288. doi:10.1016/S0002-9610(01)00710-3
12. Galandiuk S, Fazio VW, Petras RE. Pneumatosis cystoides intestinalis in Crohn’s disease. Report of two cases. Dis Colon Rectum. 1985;28(12):951-956. doi:10.1007/BF02554315
13. Parra JA, Acinas O, Bueno J, Madrazo C, Fariñas C. An unusual form of pneumatosis intestinalis associated with appendicitis. Br J Radiol. 1998;71(843):326-328. doi:10.1259/bjr.71.843.9616245
14. Schenk P, Madl C, Kramer L, et al. Pneumatosis intestinalis with Clostridium difficile colitis as a cause of acute abdomen after lung transplantation. Dig Dis Sci. 1998;43(11):2455-2458. doi:10.1023/a:1026682131847
15. Kreiss C, Forohar F, Smithline AE, Brandt LJ. Pneumatosis intestinalis complicating C. difficile pseudomembranous colitis. Am J Gastroenterol. 1999;94(9):2560-2561. doi:10.1111/j.1572-0241.1999.01397.x
16. Day DL, Ramsay NK, Letourneau JG. Pneumatosis intestinalis after bone marrow transplantation. AJR Am J Roentgenol. 1988;151(1):85-87. doi:10.2214/ajr.151.1.85
17. Tahara S, Sakai Y, Katsuno H, Urano M, Kuroda M, Tsukamoto T. Pneumatosis intestinalis and hepatic portal venous gas associated with gas-forming bacterial translocation due to postoperative paralytic ileus: A case report. Medicine (Baltimore). 2019;98(2):e14079. doi:10.1097/MD.0000000000014079
18. Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of whipple’s disease in a patient mistakenly on anti-tnf therapy. ACG Case Rep J. 2013;1(1):25- 28. Published 2013 Oct 8. doi:10.14309/crj.2013.11
19. Burton EM, Mercado-Deane MG, Patel K. Pneumatosis intestinalis in a child with AIDS and pseudomembranous colitis. Pediatr Radiol. 1994;24(8):609-610. doi:10.1007/BF02012750
20. Berk RN, Wall SD, McArdle CB, et al. Cryptosporidiosis of the stomach and small intestine in patients with AIDS. AJR Am J Roentgenol. 1984;143(3):549-554. doi:10.2214/ajr.143.3.549
21. Samson VE, Brown WR. Pneumatosis cystoides intestinalis in AIDS-associated cryptosporidiosis. More than an incidental finding? J Clin Gastroenterol. 1996;22(4):311-312.doi:10.1097/00004836-199606000-00015
22. Tjon A Tham RT, Vlasveld LT, Willemze R. Gastrointestinal complications of cytosine-arabinoside chemotherapy: findings on plain abdominal radiographs. AJR Am J Roentgenol. 1990;154(1):95-98. doi:10.2214/ajr.154.1.2104733
23. Hashimoto S, Saitoh H, Wada K, et al. Pneumatosis cystoides intestinalis after chemotherapy for hematological malignancies: report of 4 cases. Intern Med. 1995;34(3):212-215. doi:10.2169/internalmedicine.34.212
24. Gelman SF, Brandt LJ. Pneumatosis intestinalis and AIDS: a case report and review of the literature. Am J Gastroenterol. 1998;93(4):646-650. doi:10.1111/j.1572-0241.1998.183_b.x
25. Gillon J, Tadesse K, Logan RF, Holt S, Sircus W. Breath hydrogen in pneumatosis cystoides intestinalis. Gut. 1979;20(11):1008-1011. doi:10.1136/gut.20.11.1008
26. Hughes DT, Gordon KC, Swann JC, Bolt GL. Pneumatosis cystoides intestinalis. Gut. 1966;7(5):553-557. doi:10.1136/gut.7.5.553
27. Read NW, Al-Janabi MN, Cann PA. Is raised breath hydrogen related to the pathogenesis of pneumatosis coli? Gut. 1984;25(8):839-845. doi:10.1136/gut.25.8.839
28. van der Linden W, Marsell R. Pneumatosis cystoides coli associated with high H2 excretion. Treatment with an elemental diet. Scand J Gastroenterol. 1979;14(2):173-174. doi:10.3109/00365527909179864
29. Christl SU, Gibson GR, Murgatroyd PR, Scheppach W, Cummings JH. Impaired hydrogen metabolism in pneumatosis cystoides intestinalis. Gastroenterology. 1993;104(2):392-397. doi:10.1016/0016-5085(93)90406-3
30. Keyting WS, Mccarver RR, Kovarik JL, Daywitt AL. Pneumatosis intestinalis: a new concept. Radiology. 1961;76:733-741. doi:10.1148/76.5.733
31. Florin TH, Hills BA. Does counterperfusion supersaturation cause gas cysts in pneumatosis cystoides coli, and can breathing heliox reduce them? Lancet. 1995;345(8959):1220-1222. doi:10.1016/S0140-6736(95)91996-1
32. Grieve DA, Unsworth IP. Pneumatosis cystoides intestinalis: an experience with hyperbaric oxygen treatment. Aust N Z J Surg. 1991;61(6):423-426.
33. Micklefield GH, Kuntz HD, May B. Pneumatosis cystoides intestinalis: case reports and review of the literature. Mater Med Pol. 1990;22(2):70-72.
34. Yale CE, Balish E, Wu JP. The bacterial etiology of pneumatosis cystoides intestinalis. Arch Surg. 1974;109(1):89- 94. doi:10.1001/archsurg.1974.01360010067017
35. Fenton LZ, Buonomo C. Benign pneumatosis in children. Pediatr Radiol. 2000;30(11):786-793. doi:10.1007/s002470000303
36. Tobias R, Coleman S, Helman CA. Pneumatosis coli simulating hepatomegaly. Am J Gastroenterol. 1985;80(2):146-149.
37. Feczko PJ, Mezwa DG, Farah MC, White BD. Clinical significance of pneumatosis of the bowel w a l l . Radiographics. 1992;12(6):1069-1078. doi:10.1148/radiographics.12.6.1439012
38. Masterson JS, Fratkin LB, Osler TR, Trapp WG. Treatment of pneumatosis cystoides intestinalis with hyperbaric oxygen. Ann Surg. 1978;187(3):245-247. doi:10.1097/00000658-197803000-00005
39. Höflin F, Linden W van der. Pneumatosis cystoides intestinalis treated by oxygen breathing. Scandinavian J Gastroenterol . 1974;9(5) :427-430. doi:10.1080/00365521.1974.12096852
40. St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138(1):68-75. doi:10.1001/archsurg.138.1.68
41. Ling F, Guo D, Zhu L. Pneumatosis cystoides intestinalis: a case report and literature review. BMC Gastroenterol. 2019;19(1):176. Published 2019 Nov 6. doi:10.1186/s12876-019-1087-9
42. Morris MS, Gee AC, Cho SD, et al. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195(5):679-682. doi:10.1016/j.amjsurg.2008.01.011
62-year-old woman • dysuria • dyspareunia • urinary incontinence • Dx?
THE CASE
A 62-year-old postmenopausal woman presented to the clinic as a new patient for her annual physical examination. She reported a 9-year history of symptoms including dysuria, post-void dribbling, dyspareunia, and urinary incontinence on review of systems. Her physical examination revealed an anterior vaginal wall bulge (FIGURE). Results of a urinalysis were negative. The patient was referred to Urology for further evaluation.
THE DIAGNOSIS
A pelvic magnetic resonance imaging (MRI) scan revealed a large periurethral diverticulum with a horseshoe shape.
DISCUSSION
Women are more likely than men to develop urethral diverticulum, and it can manifest at any age, usually in the third through seventh decade.4,5 It was once thought to be more common in Black women, although the literature does not support this.6 Black women are 3 times more likely to be operated on than White women to treat urethral diverticula.7
Unknown origin. Most cases of urethral diverticulum are acquired; the etiology is uncertain.8,9 The assumption is that urethral diverticulum occurs as a result of repeated infection of the periurethral glands with subsequent obstruction, abscess formation, and chronic inflammation.1,2,4 Childbirth trauma, iatrogenic causes, and urethral instrumentation have also been implicated.3,4 In rare cases of congenital urethral diverticula, the diverticula are thought to be remnants of Gartner duct cysts, and yet, incidence in the pediatric population is low.8
Diagnosis is confirmed through physical exam and imaging
The urethral diverticulum manifests anteriorly and palpation of the anterior vaginal wall may reveal a painful mass.10 A split-speculum is used for careful inspection and palpation of the anterior vaginal wall.9 If the diverticulum is found to be firm on palpation, or there is bloody urethral drainage, malignancy (although rare) must be ruled out.4,5 Refer such patients to a urologist or urogynecologist.
Radiologic imaging (eg, ultrasound,
Continue to: Nonspecific symptoms may lead to misdiagnosis
Nonspecific symptoms may lead to misdiagnosis. The symptoms associated with urethral diverticulum are diverse and linked to several differential diagnoses (TABLE).3,4,12 The most common signs and symptoms are pelvic pain, urethral mass, dyspareunia, dysuria, urinary incontinence, and post-void dribbling—all of which are considered nonspecific.3,10,11 These nonspecific symptoms (or even an absence of symptoms), along with a physician’s lack of familiarity with urethral diverticulum, can result in a misdiagnosis or even a delayed diagnosis (up to 5.2 years).3,10

Managing symptoms vs preventing recurrence
Conservative management with antibiotics, anticholinergics, and/or observation is acceptable for patients with mild symptoms and those who are pregnant or who have a current infection or serious comorbidities that preclude surgery.3,9 Complete excision of the urethral diverticulum with reconstruction is considered the most effective surgical management for symptom relief and recurrence prevention.3,4,11,14
Our patient underwent a successful transvaginal suburethral diverticulectomy.
THE TAKEAWAY
The diagnosis of female urethral diverticulum is often delayed or misdiagnosed because symptoms are diverse and nonspecific. One should have a high degree of suspicion for urethral diverticulum in patients with dysuria, dyspareunia, pelvic pain, urinary incontinence, and irritative voiding symptoms who are not responding to conservative management. Ultrasound is an appropriate first-line imaging modality. However, a pelvic MRI is the most sensitive and specific in diagnosing urethral diverticulum.12
CORRESPONDENCE
Folashade Omole, MD, FAAFP, 720 Westview Drive, Atlanta, GA 30310; fomole@msm.edu
1. Billow M, James R, Resnick K, et al. An unusual presentation of a urethral diverticulum as a vaginal wall mass: a case report. J Med Case Rep. 2013;7:171. doi: 10.1186/1752-1947-7-171
2. El-Nashar SA, Bacon MM, Kim-Fine S, et al. Incidence of female urethral diverticulum: a population-based analysis and literature review. Int Urogynecol J. 2014;25:73-79. doi: 10.1007/s00192-013-2155-2
3. Cameron AP. Urethral diverticulum in the female: a meta-analysis of modern series. Minerva Ginecol. 2016;68:186-210.
4. Greiman AK, Rolef J, Rovner ES. Urethral diverticulum: a systematic review. Arab J Urol. 2019;17:49-57. doi: 10.1080/2090598X.2019.1589748
5. Allen D, Mishra V, Pepper W, et al. A single-center experience of symptomatic male urethral diverticula. Urology. 2007;70:650-653. doi: 10.1016/j.urology.2007.06.1111
6. O’Connor E, Iatropoulou D, Hashimoto S, et al. Urethral diverticulum carcinoma in females—a case series and review of the English and Japanese literature. Transl Androl Urol. 2018;7:703-729. doi: 10.21037/tau.2018.07.08
7. Burrows LJ, Howden NL, Meyn L, et al. Surgical procedures for urethral diverticula in women in the United States, 1979-1997. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:158-161. doi: 10.1007/s00192-004-1145-9
8. Riyach O, Ahsaini M, Tazi MF, et al. Female urethral diverticulum: cases report and literature. Ann Surg Innov Res. 2014;8:1. doi: 10.1186/1750-1164-8-1
9. Antosh DD, Gutman RE. Diagnosis and management of female urethral diverticulum. Female Pelvic Med Reconstr Surg. 2011;17:264-271. doi: 10.1097/SPV.0b013e318234a242
10. Romanzi LJ, Groutz A, Blaivas JG. Urethral diverticulum in women: diverse presentations resulting in diagnostic delay and mismanagement. J Urol. 2000;164:428-433.
11. Reeves FA, Inman RD, Chapple CR. Management of symptomatic urethral diverticula in women: a single-centre experience. Eur Urol. 2014;66:164-172. doi: 10.1016/j.eururo.2014.02.041
12. Dwarkasing RS, Dinkelaar W, Hop WCJ, et al. MRI evaluation of urethral diverticula and differential diagnosis in symptomatic women. AJR Am J Roentgenol. 2011;197:676-682. doi: 10.2214/AJR.10.6144
13. Porten S, Kielb S. Diagnosis of female diverticula using magnetic resonance imaging. Adv Urol. 2008;2008:213516. doi: 10.1155/2008/213516
14. Ockrim JL, Allen DJ, Shah PJ, et al. A tertiary experience of urethral diverticulectomy: diagnosis, imaging and surgical outcomes. BJU Int. 2009;103:1550-1554. doi: 10.1111/j.1464-410X.2009.08348.x
THE CASE
A 62-year-old postmenopausal woman presented to the clinic as a new patient for her annual physical examination. She reported a 9-year history of symptoms including dysuria, post-void dribbling, dyspareunia, and urinary incontinence on review of systems. Her physical examination revealed an anterior vaginal wall bulge (FIGURE). Results of a urinalysis were negative. The patient was referred to Urology for further evaluation.
THE DIAGNOSIS
A pelvic magnetic resonance imaging (MRI) scan revealed a large periurethral diverticulum with a horseshoe shape.
DISCUSSION
Women are more likely than men to develop urethral diverticulum, and it can manifest at any age, usually in the third through seventh decade.4,5 It was once thought to be more common in Black women, although the literature does not support this.6 Black women are 3 times more likely to be operated on than White women to treat urethral diverticula.7
Unknown origin. Most cases of urethral diverticulum are acquired; the etiology is uncertain.8,9 The assumption is that urethral diverticulum occurs as a result of repeated infection of the periurethral glands with subsequent obstruction, abscess formation, and chronic inflammation.1,2,4 Childbirth trauma, iatrogenic causes, and urethral instrumentation have also been implicated.3,4 In rare cases of congenital urethral diverticula, the diverticula are thought to be remnants of Gartner duct cysts, and yet, incidence in the pediatric population is low.8
Diagnosis is confirmed through physical exam and imaging
The urethral diverticulum manifests anteriorly and palpation of the anterior vaginal wall may reveal a painful mass.10 A split-speculum is used for careful inspection and palpation of the anterior vaginal wall.9 If the diverticulum is found to be firm on palpation, or there is bloody urethral drainage, malignancy (although rare) must be ruled out.4,5 Refer such patients to a urologist or urogynecologist.
Radiologic imaging (eg, ultrasound,
Continue to: Nonspecific symptoms may lead to misdiagnosis
Nonspecific symptoms may lead to misdiagnosis. The symptoms associated with urethral diverticulum are diverse and linked to several differential diagnoses (TABLE).3,4,12 The most common signs and symptoms are pelvic pain, urethral mass, dyspareunia, dysuria, urinary incontinence, and post-void dribbling—all of which are considered nonspecific.3,10,11 These nonspecific symptoms (or even an absence of symptoms), along with a physician’s lack of familiarity with urethral diverticulum, can result in a misdiagnosis or even a delayed diagnosis (up to 5.2 years).3,10

Managing symptoms vs preventing recurrence
Conservative management with antibiotics, anticholinergics, and/or observation is acceptable for patients with mild symptoms and those who are pregnant or who have a current infection or serious comorbidities that preclude surgery.3,9 Complete excision of the urethral diverticulum with reconstruction is considered the most effective surgical management for symptom relief and recurrence prevention.3,4,11,14
Our patient underwent a successful transvaginal suburethral diverticulectomy.
THE TAKEAWAY
The diagnosis of female urethral diverticulum is often delayed or misdiagnosed because symptoms are diverse and nonspecific. One should have a high degree of suspicion for urethral diverticulum in patients with dysuria, dyspareunia, pelvic pain, urinary incontinence, and irritative voiding symptoms who are not responding to conservative management. Ultrasound is an appropriate first-line imaging modality. However, a pelvic MRI is the most sensitive and specific in diagnosing urethral diverticulum.12
CORRESPONDENCE
Folashade Omole, MD, FAAFP, 720 Westview Drive, Atlanta, GA 30310; fomole@msm.edu
THE CASE
A 62-year-old postmenopausal woman presented to the clinic as a new patient for her annual physical examination. She reported a 9-year history of symptoms including dysuria, post-void dribbling, dyspareunia, and urinary incontinence on review of systems. Her physical examination revealed an anterior vaginal wall bulge (FIGURE). Results of a urinalysis were negative. The patient was referred to Urology for further evaluation.
THE DIAGNOSIS
A pelvic magnetic resonance imaging (MRI) scan revealed a large periurethral diverticulum with a horseshoe shape.
DISCUSSION
Women are more likely than men to develop urethral diverticulum, and it can manifest at any age, usually in the third through seventh decade.4,5 It was once thought to be more common in Black women, although the literature does not support this.6 Black women are 3 times more likely to be operated on than White women to treat urethral diverticula.7
Unknown origin. Most cases of urethral diverticulum are acquired; the etiology is uncertain.8,9 The assumption is that urethral diverticulum occurs as a result of repeated infection of the periurethral glands with subsequent obstruction, abscess formation, and chronic inflammation.1,2,4 Childbirth trauma, iatrogenic causes, and urethral instrumentation have also been implicated.3,4 In rare cases of congenital urethral diverticula, the diverticula are thought to be remnants of Gartner duct cysts, and yet, incidence in the pediatric population is low.8
Diagnosis is confirmed through physical exam and imaging
The urethral diverticulum manifests anteriorly and palpation of the anterior vaginal wall may reveal a painful mass.10 A split-speculum is used for careful inspection and palpation of the anterior vaginal wall.9 If the diverticulum is found to be firm on palpation, or there is bloody urethral drainage, malignancy (although rare) must be ruled out.4,5 Refer such patients to a urologist or urogynecologist.
Radiologic imaging (eg, ultrasound,
Continue to: Nonspecific symptoms may lead to misdiagnosis
Nonspecific symptoms may lead to misdiagnosis. The symptoms associated with urethral diverticulum are diverse and linked to several differential diagnoses (TABLE).3,4,12 The most common signs and symptoms are pelvic pain, urethral mass, dyspareunia, dysuria, urinary incontinence, and post-void dribbling—all of which are considered nonspecific.3,10,11 These nonspecific symptoms (or even an absence of symptoms), along with a physician’s lack of familiarity with urethral diverticulum, can result in a misdiagnosis or even a delayed diagnosis (up to 5.2 years).3,10

Managing symptoms vs preventing recurrence
Conservative management with antibiotics, anticholinergics, and/or observation is acceptable for patients with mild symptoms and those who are pregnant or who have a current infection or serious comorbidities that preclude surgery.3,9 Complete excision of the urethral diverticulum with reconstruction is considered the most effective surgical management for symptom relief and recurrence prevention.3,4,11,14
Our patient underwent a successful transvaginal suburethral diverticulectomy.
THE TAKEAWAY
The diagnosis of female urethral diverticulum is often delayed or misdiagnosed because symptoms are diverse and nonspecific. One should have a high degree of suspicion for urethral diverticulum in patients with dysuria, dyspareunia, pelvic pain, urinary incontinence, and irritative voiding symptoms who are not responding to conservative management. Ultrasound is an appropriate first-line imaging modality. However, a pelvic MRI is the most sensitive and specific in diagnosing urethral diverticulum.12
CORRESPONDENCE
Folashade Omole, MD, FAAFP, 720 Westview Drive, Atlanta, GA 30310; fomole@msm.edu
1. Billow M, James R, Resnick K, et al. An unusual presentation of a urethral diverticulum as a vaginal wall mass: a case report. J Med Case Rep. 2013;7:171. doi: 10.1186/1752-1947-7-171
2. El-Nashar SA, Bacon MM, Kim-Fine S, et al. Incidence of female urethral diverticulum: a population-based analysis and literature review. Int Urogynecol J. 2014;25:73-79. doi: 10.1007/s00192-013-2155-2
3. Cameron AP. Urethral diverticulum in the female: a meta-analysis of modern series. Minerva Ginecol. 2016;68:186-210.
4. Greiman AK, Rolef J, Rovner ES. Urethral diverticulum: a systematic review. Arab J Urol. 2019;17:49-57. doi: 10.1080/2090598X.2019.1589748
5. Allen D, Mishra V, Pepper W, et al. A single-center experience of symptomatic male urethral diverticula. Urology. 2007;70:650-653. doi: 10.1016/j.urology.2007.06.1111
6. O’Connor E, Iatropoulou D, Hashimoto S, et al. Urethral diverticulum carcinoma in females—a case series and review of the English and Japanese literature. Transl Androl Urol. 2018;7:703-729. doi: 10.21037/tau.2018.07.08
7. Burrows LJ, Howden NL, Meyn L, et al. Surgical procedures for urethral diverticula in women in the United States, 1979-1997. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:158-161. doi: 10.1007/s00192-004-1145-9
8. Riyach O, Ahsaini M, Tazi MF, et al. Female urethral diverticulum: cases report and literature. Ann Surg Innov Res. 2014;8:1. doi: 10.1186/1750-1164-8-1
9. Antosh DD, Gutman RE. Diagnosis and management of female urethral diverticulum. Female Pelvic Med Reconstr Surg. 2011;17:264-271. doi: 10.1097/SPV.0b013e318234a242
10. Romanzi LJ, Groutz A, Blaivas JG. Urethral diverticulum in women: diverse presentations resulting in diagnostic delay and mismanagement. J Urol. 2000;164:428-433.
11. Reeves FA, Inman RD, Chapple CR. Management of symptomatic urethral diverticula in women: a single-centre experience. Eur Urol. 2014;66:164-172. doi: 10.1016/j.eururo.2014.02.041
12. Dwarkasing RS, Dinkelaar W, Hop WCJ, et al. MRI evaluation of urethral diverticula and differential diagnosis in symptomatic women. AJR Am J Roentgenol. 2011;197:676-682. doi: 10.2214/AJR.10.6144
13. Porten S, Kielb S. Diagnosis of female diverticula using magnetic resonance imaging. Adv Urol. 2008;2008:213516. doi: 10.1155/2008/213516
14. Ockrim JL, Allen DJ, Shah PJ, et al. A tertiary experience of urethral diverticulectomy: diagnosis, imaging and surgical outcomes. BJU Int. 2009;103:1550-1554. doi: 10.1111/j.1464-410X.2009.08348.x
1. Billow M, James R, Resnick K, et al. An unusual presentation of a urethral diverticulum as a vaginal wall mass: a case report. J Med Case Rep. 2013;7:171. doi: 10.1186/1752-1947-7-171
2. El-Nashar SA, Bacon MM, Kim-Fine S, et al. Incidence of female urethral diverticulum: a population-based analysis and literature review. Int Urogynecol J. 2014;25:73-79. doi: 10.1007/s00192-013-2155-2
3. Cameron AP. Urethral diverticulum in the female: a meta-analysis of modern series. Minerva Ginecol. 2016;68:186-210.
4. Greiman AK, Rolef J, Rovner ES. Urethral diverticulum: a systematic review. Arab J Urol. 2019;17:49-57. doi: 10.1080/2090598X.2019.1589748
5. Allen D, Mishra V, Pepper W, et al. A single-center experience of symptomatic male urethral diverticula. Urology. 2007;70:650-653. doi: 10.1016/j.urology.2007.06.1111
6. O’Connor E, Iatropoulou D, Hashimoto S, et al. Urethral diverticulum carcinoma in females—a case series and review of the English and Japanese literature. Transl Androl Urol. 2018;7:703-729. doi: 10.21037/tau.2018.07.08
7. Burrows LJ, Howden NL, Meyn L, et al. Surgical procedures for urethral diverticula in women in the United States, 1979-1997. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:158-161. doi: 10.1007/s00192-004-1145-9
8. Riyach O, Ahsaini M, Tazi MF, et al. Female urethral diverticulum: cases report and literature. Ann Surg Innov Res. 2014;8:1. doi: 10.1186/1750-1164-8-1
9. Antosh DD, Gutman RE. Diagnosis and management of female urethral diverticulum. Female Pelvic Med Reconstr Surg. 2011;17:264-271. doi: 10.1097/SPV.0b013e318234a242
10. Romanzi LJ, Groutz A, Blaivas JG. Urethral diverticulum in women: diverse presentations resulting in diagnostic delay and mismanagement. J Urol. 2000;164:428-433.
11. Reeves FA, Inman RD, Chapple CR. Management of symptomatic urethral diverticula in women: a single-centre experience. Eur Urol. 2014;66:164-172. doi: 10.1016/j.eururo.2014.02.041
12. Dwarkasing RS, Dinkelaar W, Hop WCJ, et al. MRI evaluation of urethral diverticula and differential diagnosis in symptomatic women. AJR Am J Roentgenol. 2011;197:676-682. doi: 10.2214/AJR.10.6144
13. Porten S, Kielb S. Diagnosis of female diverticula using magnetic resonance imaging. Adv Urol. 2008;2008:213516. doi: 10.1155/2008/213516
14. Ockrim JL, Allen DJ, Shah PJ, et al. A tertiary experience of urethral diverticulectomy: diagnosis, imaging and surgical outcomes. BJU Int. 2009;103:1550-1554. doi: 10.1111/j.1464-410X.2009.08348.x
Deployed Airbag Causes Bullous Reaction Following a Motor Vehicle Accident
Airbags are lifesaving during motor vehicle accidents (MVAs), but their deployment has been associated with skin issues such as irritant dermatitis1; lacerations2; abrasions3; and thermal, friction, and chemical burns.4-6 Ocular issues such as alkaline chemical keratitis7 and ocular alkali injuries8 also have been described.
Airbag deployment is triggered by rapid deceleration and impact, which ignite a sodium azide cartridge, causing the woven nylon bag to inflate with hydrocarbon gases.8 This leads to release of sodium hydroxide, sodium bicarbonate, and metallic oxides in an aerosolized form. If a tear in the meshwork of the airbag occurs, exposure to an even larger amount of powder containing caustic alkali chemicals can occur.8
We describe a patient who developed a bullous reaction to airbag contents after he was involved in an MVA in which the airbag deployed.
Case Report
A 35-year-old man with a history of type 2 diabetes mellitus and chronic hepatitis B presented to the dermatology clinic for an evaluation of new-onset blisters. The rash occurred 1 day after the patient was involved in an MVA in which he was exposed to the airbag’s contents after it burst. He had been evaluated twice in the emergency department for the skin eruption before being referred to dermatology. He noted the lesions were pruritic and painful. Prior treatments included silver sulfadiazine cream 1% and clobetasol cream 0.05%, though he discontinued using the latter because of burning with application. Physical examination revealed tense vesicles and bullae on an erythematous base on the right lower flank, forearms, and legs, with the exception of the lower right leg where a cast had been from a prior injury (Figure 1).
Two punch biopsies of the left arm were performed and sent for hematoxylin and eosin staining and direct immunofluorescence to rule out bullous diseases, such as bullous pemphigoid, linear IgA, and bullous lupus. Hematoxylin and eosin staining revealed extensive spongiosis with blister formation and a dense perivascular infiltrate in the superficial and mid dermis composed of lymphocytes with numerous scattered eosinophils (Figures 2 and 3). Direct immunofluorescence studies were negative. Treatment with oral prednisone and oral antihistamines was initiated.
At 10-day follow-up, the patient had a few residual bullae; most lesions were demonstrating various stages of healing (Figure 4). The patient’s cast had been removed, and there were no lesions in this previously covered area. At 6-week follow-up he had continued healing of the bullae and erosions as well as postinflammatory hyperpigmentation (Figure 5).
Comment
With the advent of airbags for safety purposes, these potentially lifesaving devices also have been known to cause injury.9 In 1998, the most commonly reported airbag injuries were ocular injuries.10 Cutaneous manifestations of airbag injury are less well known.11
Two cases of airbag deployment with skin blistering have been reported in the literature based on a PubMed search of articles indexed for MEDLINE using the terms airbag blistering or airbag bullae12,13; however, the blistering was described in the context of a burn. One case of the effects of airbag deployment residue highlights a patient arriving to the emergency department with erythema and blisters on the hands within 48 hours of airbag deployment in an MVA, and the treatment was standard burn therapy.12 Another case report described a patient with a second-degree burn with a 12-cm blister occurring on the radial side of the hand and distal forearm following an MVA and airbag deployment, which was treated conservatively.13 Cases of thermal burns, chemical burns, and irritant contact dermatitis after airbag deployment have been described in the literature.4-6,11,12,14,15 Our patient’s distal right lower leg was covered with a cast for osteomyelitis, and no blisters had developed in this area. It is likely that the transfer of airbag contents occurred during the process of unbuckling his seatbelt, which could explain the bullae that developed on the right flank. Per the Centers for Disease Control and Prevention, individuals should quickly remove clothing and wash their body with large amounts of soap and water following exposure to sodium azide.16
In 1989, the Federal Motor Vehicle Safety Standard No. 208 (occupant crash protection) became effective, stating all cars must have vehicle crash protection.12 Prior to 1993, it was reported that there had been no associated chemical injuries with airbag deployment. Subsequently, a 6-month retrospective study in 1993 showed that dermal injuries were found in connection with the presence of sodium hydroxide in automobile airbags.12 By 2004, it was known that airbags could cause chemical and thermal burns in addition to traumatic injuries from deployment.1 Since 2007, all motor vehicles have been required to have advanced airbags, which are engineered to sense the presence of passengers and determine if the airbag will deploy, and if so, how much to deploy to minimize airbag-related injury.3
The brand of car that our patient drove during the MVA is one with known airbag recalls due to safety defects; however, the year and actual model of the vehicle are not known, so specific information about the airbag in question is not available. It has been noted that some defective airbag inflators that were exposed to excess moisture during the manufacturing process could explode during deployment, causing shrapnel and airbag rupture, which has been linked to nearly 300 injuries worldwide.17
Conclusion
It is evident that the use of airbag devices reduces morbidity and mortality due to MVAs.9 It also had been reported that up to 96% of airbag-related injuries are relatively minor, which many would argue justifies their use.18 Furthermore, it has been reported that 99.8% of skin injuries following airbag deployment are minor.19 In the United States, it is mandated that every vehicle have functional airbags installed.8
This case highlights the potential for substantial airbag-induced skin reactions, specifically a bullous reaction, following airbag deployment. The persistent pruritus and lasting postinflammatory hyperpigmentation seen in this case were certainly worrisome for our patient. We also present this case to remind dermatology providers of possible treatment approaches to these skin reactions. Immediate cleansing of the affected areas of skin may help avoid such reactions.
- Corazza M, Trincone S, Zampino MR, et al. Air bags and the skin. Skinmed. 2004;3:256-258.
- Corazza M, Trincone S, Virgili A. Effects of airbag deployment: lesions, epidemiology, and management. Am J Clin Dermatol. 2004;5:295-300.
- Kuska TC. Air bag safety: an update. J Emerg Nurs. 2016;42:438-441.
- Ulrich D, Noah EM, Fuchs P, et al. Burn injuries caused by air bag deployment. Burns. 2001;27:196-199.
- Erpenbeck SP, Roy E, Ziembicki JA, et al. A systematic review on airbag-induced burns. J Burn Care Res. 2021;42:481-487.
- Skibba KEH, Cleveland CN, Bell DE. Airbag burns: an unfortunate consequence of motor vehicle safety. J Burn Care Res. 2021;42:71-73.
- Smally AJ, Binzer A, Dolin S, et al. Alkaline chemical keratitis: eye injury from airbags. Ann Emerg Med. 1992;21:1400-1402.
- Barnes SS, Wong W Jr, Affeldt JC. A case of severe airbag related ocular alkali injury. Hawaii J Med Public Health. 2012;71:229-231.
- Wallis LA, Greaves I. Injuries associated with airbag deployment. Emerg Med J. 2002;19:490-493.
- Mohamed AA, Banerjee A. Patterns of injury associated with automobile airbag use. Postgrad Med J. 1998;74:455-458.
- Foley E, Helm TN. Air bag injury and the dermatologist. Cutis. 2000;66:251-252.
- Swanson-Biearman B, Mrvos R, Dean BS, et al. Air bags: lifesaving with toxic potential? Am J Emerg Med. 1993;11:38-39.
- Roth T, Meredith P. Traumatic lesions caused by the “air-bag” system [in French]. Z Unfallchir Versicherungsmed. 1993;86:189-193.
- Wu JJ, Sanchez-Palacios C, Brieva J, et al. A case of air bag dermatitis. Arch Dermatol. 2002;138:1383-1384.
- Vitello W, Kim M, Johnson RM, et al. Full-thickness burn to the hand from an automobile airbag. J Burn Care Rehabil. 1999;20:212-215.
- Centers for Disease Control and Prevention. Facts about sodium azide. Updated April 4, 2018. Accessed May 15, 2022. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp
- Shepardson D. Honda to recall 1.2 million vehicles in North America to replace Takata airbags. March 12, 2019. Accessed March 22, 2022. https://www.reuters.com/article/us-honda-takata-recall/honda-to-recall-1-2-million-vehicles-in-north-america-to-replace-takata-airbags-idUSKBN1QT1C9
- Gabauer DJ, Gabler HC. The effects of airbags and seatbelts on occupant injury in longitudinal barrier crashes. J Safety Res. 2010;41:9-15.
- Rath AL, Jernigan MV, Stitzel JD, et al. The effects of depowered airbags on skin injuries in frontal automobile crashes. Plast Reconstr Surg. 2005;115:428-435.
Airbags are lifesaving during motor vehicle accidents (MVAs), but their deployment has been associated with skin issues such as irritant dermatitis1; lacerations2; abrasions3; and thermal, friction, and chemical burns.4-6 Ocular issues such as alkaline chemical keratitis7 and ocular alkali injuries8 also have been described.
Airbag deployment is triggered by rapid deceleration and impact, which ignite a sodium azide cartridge, causing the woven nylon bag to inflate with hydrocarbon gases.8 This leads to release of sodium hydroxide, sodium bicarbonate, and metallic oxides in an aerosolized form. If a tear in the meshwork of the airbag occurs, exposure to an even larger amount of powder containing caustic alkali chemicals can occur.8
We describe a patient who developed a bullous reaction to airbag contents after he was involved in an MVA in which the airbag deployed.
Case Report
A 35-year-old man with a history of type 2 diabetes mellitus and chronic hepatitis B presented to the dermatology clinic for an evaluation of new-onset blisters. The rash occurred 1 day after the patient was involved in an MVA in which he was exposed to the airbag’s contents after it burst. He had been evaluated twice in the emergency department for the skin eruption before being referred to dermatology. He noted the lesions were pruritic and painful. Prior treatments included silver sulfadiazine cream 1% and clobetasol cream 0.05%, though he discontinued using the latter because of burning with application. Physical examination revealed tense vesicles and bullae on an erythematous base on the right lower flank, forearms, and legs, with the exception of the lower right leg where a cast had been from a prior injury (Figure 1).
Two punch biopsies of the left arm were performed and sent for hematoxylin and eosin staining and direct immunofluorescence to rule out bullous diseases, such as bullous pemphigoid, linear IgA, and bullous lupus. Hematoxylin and eosin staining revealed extensive spongiosis with blister formation and a dense perivascular infiltrate in the superficial and mid dermis composed of lymphocytes with numerous scattered eosinophils (Figures 2 and 3). Direct immunofluorescence studies were negative. Treatment with oral prednisone and oral antihistamines was initiated.
At 10-day follow-up, the patient had a few residual bullae; most lesions were demonstrating various stages of healing (Figure 4). The patient’s cast had been removed, and there were no lesions in this previously covered area. At 6-week follow-up he had continued healing of the bullae and erosions as well as postinflammatory hyperpigmentation (Figure 5).
Comment
With the advent of airbags for safety purposes, these potentially lifesaving devices also have been known to cause injury.9 In 1998, the most commonly reported airbag injuries were ocular injuries.10 Cutaneous manifestations of airbag injury are less well known.11
Two cases of airbag deployment with skin blistering have been reported in the literature based on a PubMed search of articles indexed for MEDLINE using the terms airbag blistering or airbag bullae12,13; however, the blistering was described in the context of a burn. One case of the effects of airbag deployment residue highlights a patient arriving to the emergency department with erythema and blisters on the hands within 48 hours of airbag deployment in an MVA, and the treatment was standard burn therapy.12 Another case report described a patient with a second-degree burn with a 12-cm blister occurring on the radial side of the hand and distal forearm following an MVA and airbag deployment, which was treated conservatively.13 Cases of thermal burns, chemical burns, and irritant contact dermatitis after airbag deployment have been described in the literature.4-6,11,12,14,15 Our patient’s distal right lower leg was covered with a cast for osteomyelitis, and no blisters had developed in this area. It is likely that the transfer of airbag contents occurred during the process of unbuckling his seatbelt, which could explain the bullae that developed on the right flank. Per the Centers for Disease Control and Prevention, individuals should quickly remove clothing and wash their body with large amounts of soap and water following exposure to sodium azide.16
In 1989, the Federal Motor Vehicle Safety Standard No. 208 (occupant crash protection) became effective, stating all cars must have vehicle crash protection.12 Prior to 1993, it was reported that there had been no associated chemical injuries with airbag deployment. Subsequently, a 6-month retrospective study in 1993 showed that dermal injuries were found in connection with the presence of sodium hydroxide in automobile airbags.12 By 2004, it was known that airbags could cause chemical and thermal burns in addition to traumatic injuries from deployment.1 Since 2007, all motor vehicles have been required to have advanced airbags, which are engineered to sense the presence of passengers and determine if the airbag will deploy, and if so, how much to deploy to minimize airbag-related injury.3
The brand of car that our patient drove during the MVA is one with known airbag recalls due to safety defects; however, the year and actual model of the vehicle are not known, so specific information about the airbag in question is not available. It has been noted that some defective airbag inflators that were exposed to excess moisture during the manufacturing process could explode during deployment, causing shrapnel and airbag rupture, which has been linked to nearly 300 injuries worldwide.17
Conclusion
It is evident that the use of airbag devices reduces morbidity and mortality due to MVAs.9 It also had been reported that up to 96% of airbag-related injuries are relatively minor, which many would argue justifies their use.18 Furthermore, it has been reported that 99.8% of skin injuries following airbag deployment are minor.19 In the United States, it is mandated that every vehicle have functional airbags installed.8
This case highlights the potential for substantial airbag-induced skin reactions, specifically a bullous reaction, following airbag deployment. The persistent pruritus and lasting postinflammatory hyperpigmentation seen in this case were certainly worrisome for our patient. We also present this case to remind dermatology providers of possible treatment approaches to these skin reactions. Immediate cleansing of the affected areas of skin may help avoid such reactions.
Airbags are lifesaving during motor vehicle accidents (MVAs), but their deployment has been associated with skin issues such as irritant dermatitis1; lacerations2; abrasions3; and thermal, friction, and chemical burns.4-6 Ocular issues such as alkaline chemical keratitis7 and ocular alkali injuries8 also have been described.
Airbag deployment is triggered by rapid deceleration and impact, which ignite a sodium azide cartridge, causing the woven nylon bag to inflate with hydrocarbon gases.8 This leads to release of sodium hydroxide, sodium bicarbonate, and metallic oxides in an aerosolized form. If a tear in the meshwork of the airbag occurs, exposure to an even larger amount of powder containing caustic alkali chemicals can occur.8
We describe a patient who developed a bullous reaction to airbag contents after he was involved in an MVA in which the airbag deployed.
Case Report
A 35-year-old man with a history of type 2 diabetes mellitus and chronic hepatitis B presented to the dermatology clinic for an evaluation of new-onset blisters. The rash occurred 1 day after the patient was involved in an MVA in which he was exposed to the airbag’s contents after it burst. He had been evaluated twice in the emergency department for the skin eruption before being referred to dermatology. He noted the lesions were pruritic and painful. Prior treatments included silver sulfadiazine cream 1% and clobetasol cream 0.05%, though he discontinued using the latter because of burning with application. Physical examination revealed tense vesicles and bullae on an erythematous base on the right lower flank, forearms, and legs, with the exception of the lower right leg where a cast had been from a prior injury (Figure 1).
Two punch biopsies of the left arm were performed and sent for hematoxylin and eosin staining and direct immunofluorescence to rule out bullous diseases, such as bullous pemphigoid, linear IgA, and bullous lupus. Hematoxylin and eosin staining revealed extensive spongiosis with blister formation and a dense perivascular infiltrate in the superficial and mid dermis composed of lymphocytes with numerous scattered eosinophils (Figures 2 and 3). Direct immunofluorescence studies were negative. Treatment with oral prednisone and oral antihistamines was initiated.
At 10-day follow-up, the patient had a few residual bullae; most lesions were demonstrating various stages of healing (Figure 4). The patient’s cast had been removed, and there were no lesions in this previously covered area. At 6-week follow-up he had continued healing of the bullae and erosions as well as postinflammatory hyperpigmentation (Figure 5).
Comment
With the advent of airbags for safety purposes, these potentially lifesaving devices also have been known to cause injury.9 In 1998, the most commonly reported airbag injuries were ocular injuries.10 Cutaneous manifestations of airbag injury are less well known.11
Two cases of airbag deployment with skin blistering have been reported in the literature based on a PubMed search of articles indexed for MEDLINE using the terms airbag blistering or airbag bullae12,13; however, the blistering was described in the context of a burn. One case of the effects of airbag deployment residue highlights a patient arriving to the emergency department with erythema and blisters on the hands within 48 hours of airbag deployment in an MVA, and the treatment was standard burn therapy.12 Another case report described a patient with a second-degree burn with a 12-cm blister occurring on the radial side of the hand and distal forearm following an MVA and airbag deployment, which was treated conservatively.13 Cases of thermal burns, chemical burns, and irritant contact dermatitis after airbag deployment have been described in the literature.4-6,11,12,14,15 Our patient’s distal right lower leg was covered with a cast for osteomyelitis, and no blisters had developed in this area. It is likely that the transfer of airbag contents occurred during the process of unbuckling his seatbelt, which could explain the bullae that developed on the right flank. Per the Centers for Disease Control and Prevention, individuals should quickly remove clothing and wash their body with large amounts of soap and water following exposure to sodium azide.16
In 1989, the Federal Motor Vehicle Safety Standard No. 208 (occupant crash protection) became effective, stating all cars must have vehicle crash protection.12 Prior to 1993, it was reported that there had been no associated chemical injuries with airbag deployment. Subsequently, a 6-month retrospective study in 1993 showed that dermal injuries were found in connection with the presence of sodium hydroxide in automobile airbags.12 By 2004, it was known that airbags could cause chemical and thermal burns in addition to traumatic injuries from deployment.1 Since 2007, all motor vehicles have been required to have advanced airbags, which are engineered to sense the presence of passengers and determine if the airbag will deploy, and if so, how much to deploy to minimize airbag-related injury.3
The brand of car that our patient drove during the MVA is one with known airbag recalls due to safety defects; however, the year and actual model of the vehicle are not known, so specific information about the airbag in question is not available. It has been noted that some defective airbag inflators that were exposed to excess moisture during the manufacturing process could explode during deployment, causing shrapnel and airbag rupture, which has been linked to nearly 300 injuries worldwide.17
Conclusion
It is evident that the use of airbag devices reduces morbidity and mortality due to MVAs.9 It also had been reported that up to 96% of airbag-related injuries are relatively minor, which many would argue justifies their use.18 Furthermore, it has been reported that 99.8% of skin injuries following airbag deployment are minor.19 In the United States, it is mandated that every vehicle have functional airbags installed.8
This case highlights the potential for substantial airbag-induced skin reactions, specifically a bullous reaction, following airbag deployment. The persistent pruritus and lasting postinflammatory hyperpigmentation seen in this case were certainly worrisome for our patient. We also present this case to remind dermatology providers of possible treatment approaches to these skin reactions. Immediate cleansing of the affected areas of skin may help avoid such reactions.
- Corazza M, Trincone S, Zampino MR, et al. Air bags and the skin. Skinmed. 2004;3:256-258.
- Corazza M, Trincone S, Virgili A. Effects of airbag deployment: lesions, epidemiology, and management. Am J Clin Dermatol. 2004;5:295-300.
- Kuska TC. Air bag safety: an update. J Emerg Nurs. 2016;42:438-441.
- Ulrich D, Noah EM, Fuchs P, et al. Burn injuries caused by air bag deployment. Burns. 2001;27:196-199.
- Erpenbeck SP, Roy E, Ziembicki JA, et al. A systematic review on airbag-induced burns. J Burn Care Res. 2021;42:481-487.
- Skibba KEH, Cleveland CN, Bell DE. Airbag burns: an unfortunate consequence of motor vehicle safety. J Burn Care Res. 2021;42:71-73.
- Smally AJ, Binzer A, Dolin S, et al. Alkaline chemical keratitis: eye injury from airbags. Ann Emerg Med. 1992;21:1400-1402.
- Barnes SS, Wong W Jr, Affeldt JC. A case of severe airbag related ocular alkali injury. Hawaii J Med Public Health. 2012;71:229-231.
- Wallis LA, Greaves I. Injuries associated with airbag deployment. Emerg Med J. 2002;19:490-493.
- Mohamed AA, Banerjee A. Patterns of injury associated with automobile airbag use. Postgrad Med J. 1998;74:455-458.
- Foley E, Helm TN. Air bag injury and the dermatologist. Cutis. 2000;66:251-252.
- Swanson-Biearman B, Mrvos R, Dean BS, et al. Air bags: lifesaving with toxic potential? Am J Emerg Med. 1993;11:38-39.
- Roth T, Meredith P. Traumatic lesions caused by the “air-bag” system [in French]. Z Unfallchir Versicherungsmed. 1993;86:189-193.
- Wu JJ, Sanchez-Palacios C, Brieva J, et al. A case of air bag dermatitis. Arch Dermatol. 2002;138:1383-1384.
- Vitello W, Kim M, Johnson RM, et al. Full-thickness burn to the hand from an automobile airbag. J Burn Care Rehabil. 1999;20:212-215.
- Centers for Disease Control and Prevention. Facts about sodium azide. Updated April 4, 2018. Accessed May 15, 2022. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp
- Shepardson D. Honda to recall 1.2 million vehicles in North America to replace Takata airbags. March 12, 2019. Accessed March 22, 2022. https://www.reuters.com/article/us-honda-takata-recall/honda-to-recall-1-2-million-vehicles-in-north-america-to-replace-takata-airbags-idUSKBN1QT1C9
- Gabauer DJ, Gabler HC. The effects of airbags and seatbelts on occupant injury in longitudinal barrier crashes. J Safety Res. 2010;41:9-15.
- Rath AL, Jernigan MV, Stitzel JD, et al. The effects of depowered airbags on skin injuries in frontal automobile crashes. Plast Reconstr Surg. 2005;115:428-435.
- Corazza M, Trincone S, Zampino MR, et al. Air bags and the skin. Skinmed. 2004;3:256-258.
- Corazza M, Trincone S, Virgili A. Effects of airbag deployment: lesions, epidemiology, and management. Am J Clin Dermatol. 2004;5:295-300.
- Kuska TC. Air bag safety: an update. J Emerg Nurs. 2016;42:438-441.
- Ulrich D, Noah EM, Fuchs P, et al. Burn injuries caused by air bag deployment. Burns. 2001;27:196-199.
- Erpenbeck SP, Roy E, Ziembicki JA, et al. A systematic review on airbag-induced burns. J Burn Care Res. 2021;42:481-487.
- Skibba KEH, Cleveland CN, Bell DE. Airbag burns: an unfortunate consequence of motor vehicle safety. J Burn Care Res. 2021;42:71-73.
- Smally AJ, Binzer A, Dolin S, et al. Alkaline chemical keratitis: eye injury from airbags. Ann Emerg Med. 1992;21:1400-1402.
- Barnes SS, Wong W Jr, Affeldt JC. A case of severe airbag related ocular alkali injury. Hawaii J Med Public Health. 2012;71:229-231.
- Wallis LA, Greaves I. Injuries associated with airbag deployment. Emerg Med J. 2002;19:490-493.
- Mohamed AA, Banerjee A. Patterns of injury associated with automobile airbag use. Postgrad Med J. 1998;74:455-458.
- Foley E, Helm TN. Air bag injury and the dermatologist. Cutis. 2000;66:251-252.
- Swanson-Biearman B, Mrvos R, Dean BS, et al. Air bags: lifesaving with toxic potential? Am J Emerg Med. 1993;11:38-39.
- Roth T, Meredith P. Traumatic lesions caused by the “air-bag” system [in French]. Z Unfallchir Versicherungsmed. 1993;86:189-193.
- Wu JJ, Sanchez-Palacios C, Brieva J, et al. A case of air bag dermatitis. Arch Dermatol. 2002;138:1383-1384.
- Vitello W, Kim M, Johnson RM, et al. Full-thickness burn to the hand from an automobile airbag. J Burn Care Rehabil. 1999;20:212-215.
- Centers for Disease Control and Prevention. Facts about sodium azide. Updated April 4, 2018. Accessed May 15, 2022. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp
- Shepardson D. Honda to recall 1.2 million vehicles in North America to replace Takata airbags. March 12, 2019. Accessed March 22, 2022. https://www.reuters.com/article/us-honda-takata-recall/honda-to-recall-1-2-million-vehicles-in-north-america-to-replace-takata-airbags-idUSKBN1QT1C9
- Gabauer DJ, Gabler HC. The effects of airbags and seatbelts on occupant injury in longitudinal barrier crashes. J Safety Res. 2010;41:9-15.
- Rath AL, Jernigan MV, Stitzel JD, et al. The effects of depowered airbags on skin injuries in frontal automobile crashes. Plast Reconstr Surg. 2005;115:428-435.
Practice Points
- This case highlights the potential for a bullous reaction following airbag deployment.
- After airbag deployment, it is important to immediately cleanse the affected areas of skin with soap and water.
Pemphigus Vulgaris Aggravated: Rifampicin Found at the Scene of the Crime
Case Report
A 60-year-old man presented with eroded areas in the mouth and blistering eruptions on the scalp, face, trunk, arms, and legs. He initially presented to an outside hospital 4 years prior and was treated with oral prednisone 50 mg daily, to which the eruptions responded rapidly; however, following a nearly 5-mg reduction of the dose per week by the patient and irregular oral administration, he experienced several episodes of recurrence, but he could not remember the exact dosage of prednisone he had taken during that period. Subsequently, he was admitted to our hospital because of large areas of erythema and erosions on the scalp, trunk, arms, and legs.
Since starting the prednisone regimen 4 years prior, the patient had experienced onset of hypertension, diabetes, glaucoma, cataracts, optic nerve atrophy, aseptic necrosis of the femoral head, and osteoporosis. Biopsy of a new skin lesion
The patient initially was started again prednisone 50 mg daily, to which the skin eruptions responded, and 2 weeks later, the disease was considered controlled. The prednisone dosage was tapered to 20 mg daily 3 months later with no new blister formation. However, 2 weeks later, the patient was diagnosed by a tuberculosis specialist with pulmonary tuberculosis, and a daily regimen of isoniazid, rifampicin, ethambutol, and levofloxacin was instituted.
Ten days after starting antituberculosis therapy, the patient developed new erythematous blisters that could not be controlled and self-adjusted the prednisone dose to 50 mg daily. Two months later, blister formation continued.
Six months after the initial presentation, the patient returned to our hospital because of uncontrollable rashes (Figure 2). On admission, he had a Pemphigus Disease Area Index (PDAI) score of 32 with disease involving 30% of the body surface area. Laboratory testing showed a desmoglein 1 level of 233 U/mL and desmoglein 3 level of 228 U/mL. A tuberculosis specialist from an outside hospital was consulted to evaluate the patient’s condition and assist in treatment. Based on findings from a pulmonary computed tomography scan, which showed the inflammation was considerably absorbed, treatment was adjusted to stop using ethambutol and levofloxacin and continue rifampicin and isoniazid. For the PV, prednisone was titrated upward to 75 mg daily, mycophenolate mofetil (MMF) 1 g twice daily was added, and IVIG 400 mg/kg daily was administered for 7 days. After 3 weeks, the rash still expanded.
In considering possible interactions between the drugs, we consulted the literature and found reports1-3 that rifampicin accelerated glucocorticoid metabolism, of which the tuberculosis specialist that we consulted was not aware. Therefore, rifampicin was stopped, and the antituberculosis therapy was adjusted to levofloxacin and isoniazid. Meanwhile, the steroid was changed to methylprednisolone 120 mg daily for 3 days, then to 80 mg daily for 2 days.
After 5 days, the rash was controlled with no new development and the patient was discharged. He continued on prednisone 80 mg daily and MMF 1 g twice daily.
At 2-month follow-up, no new rash had developed. The patient had already self-discontinued the MMF for 1 month because it was difficult to obtain at local hospitals. The prednisone was reduced to 40 mg daily. Pulmonary computed tomography showed no signs of reactivation of tuberculosis.
Comment
Drugs that depend on these enzymes for their metabolism are prone to
Rifampicin causes a marked reduction in dose-corrected mycophenolic acid exposure when administered simultaneously with MMF through induction of glucuronidation activity and inhibition of enterohepatic recirculation.5,10In in vitro studies, rifampin and other cytochrome P450 inducers have been identified as potentially useful for increasing the rate of cyclophosphamide and ifosfamide (an isomeric analogue of cyclophosphamide) 4-hydroxylation in the human liver in a manner that could have a favorable impact on the clinical pharmacokinetics of these anticancer prodrugs.11 However, clinical analysis of 16 patients indicated that co-administration of ifosfamide with rifampin did not result in changes in the pharmacokinetics of the parent drug or its metabolites.12
The steroids and
Conclusion
In our patient, the use of rifapentine resulted in a recurrence of previously controlled PV and resistance to treatment. The patient’s disease was quickly controlled after discontinuation of rifampicin and with a short-term course of high-dose methylprednisolone and remained stable when the dosages of MMF and prednisone were reduced.
- Miyagawa S, Yamashina Y, Okuchi T, et al. Exacerbation of pemphigus by rifampicin. Br J Dermatol. 1986;114:729-732. doi:10.1111/j.1365-2133.1986.tb04882.x
- Gange RW, Rhodes EL, Edwards CO, et al. Pemphigus induced by rifampicin. Br J Dermatol. 1976;95:445-448. doi:10.1111/j.1365-2133.1976.tb00849.x
- Bergrem H, Refvem OK. Altered prednisolone pharmacokinetics in patients treated with rifampicin. Acta Med Scand. 1983;213:339-343. doi:10.1111/j.0954-6820.1983.tb03748.x
- McAllister WA, Thompson PJ, Al-Habet SM, et al. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed). 1983;286:923-925. doi:10.1136/bmj.286.6369.923
- Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310:95-106. doi:10.1007/s00403-017-1790-8
- Barman H, Dass R, Duwarah SG. Use of high-dose prednisolone to overcome rifampicin-induced corticosteroid non-responsiveness in childhood nephrotic syndrome. Saudi J Kidney Dis Transpl. 2016;27:157-160. doi:10.4103/1319-2442.174198
- Okey AB, Roberts EA, Harper PA, et al. Induction of drug-metabolizing enzymes: mechanisms and consequences. Clin Biochem. 1986;19:132-141. doi:10.1016/s0009-9120(86)80060-1
- Venkatesan K. Pharmacokinetic interactions with rifampicin. Clin Pharmacokinet. 1992;22:47-65. doi:10.2165/00003088-199222010-00005
- Naesens M, Kuypers DRJ, Streit F, et al. Rifampin induces alterations in mycophenolic acid glucuronidation and elimination: implications for drug exposure in renal allograft recipients. Clin Pharmacol Ther. 2006;80:509-521. doi:10.1016/j.clpt.2006.08.002
- Kuypers DRJ, Verleden G, Naesens M, et al. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate–glucuronosyltransferase. Clin Pharmacol Ther. 2005;78:81-88. doi:10.1016/j.clpt.2005.03.004
- Chenhsu RY, Loong CC, Chou MH, et al. Renal allograft dysfunction associated with rifampin–tacrolimus interaction. Ann Pharmacother. 2000;34:27-31. doi:10.1345/aph.19069
- Douglas JG, McLeod MJ. Pharmacokinetic factors in the modern drug treatment of tuberculosis. Clin Pharmacokinet. 1999;37:127-146. doi:10.2165/00003088-199937020-00003
Case Report
A 60-year-old man presented with eroded areas in the mouth and blistering eruptions on the scalp, face, trunk, arms, and legs. He initially presented to an outside hospital 4 years prior and was treated with oral prednisone 50 mg daily, to which the eruptions responded rapidly; however, following a nearly 5-mg reduction of the dose per week by the patient and irregular oral administration, he experienced several episodes of recurrence, but he could not remember the exact dosage of prednisone he had taken during that period. Subsequently, he was admitted to our hospital because of large areas of erythema and erosions on the scalp, trunk, arms, and legs.
Since starting the prednisone regimen 4 years prior, the patient had experienced onset of hypertension, diabetes, glaucoma, cataracts, optic nerve atrophy, aseptic necrosis of the femoral head, and osteoporosis. Biopsy of a new skin lesion
The patient initially was started again prednisone 50 mg daily, to which the skin eruptions responded, and 2 weeks later, the disease was considered controlled. The prednisone dosage was tapered to 20 mg daily 3 months later with no new blister formation. However, 2 weeks later, the patient was diagnosed by a tuberculosis specialist with pulmonary tuberculosis, and a daily regimen of isoniazid, rifampicin, ethambutol, and levofloxacin was instituted.
Ten days after starting antituberculosis therapy, the patient developed new erythematous blisters that could not be controlled and self-adjusted the prednisone dose to 50 mg daily. Two months later, blister formation continued.
Six months after the initial presentation, the patient returned to our hospital because of uncontrollable rashes (Figure 2). On admission, he had a Pemphigus Disease Area Index (PDAI) score of 32 with disease involving 30% of the body surface area. Laboratory testing showed a desmoglein 1 level of 233 U/mL and desmoglein 3 level of 228 U/mL. A tuberculosis specialist from an outside hospital was consulted to evaluate the patient’s condition and assist in treatment. Based on findings from a pulmonary computed tomography scan, which showed the inflammation was considerably absorbed, treatment was adjusted to stop using ethambutol and levofloxacin and continue rifampicin and isoniazid. For the PV, prednisone was titrated upward to 75 mg daily, mycophenolate mofetil (MMF) 1 g twice daily was added, and IVIG 400 mg/kg daily was administered for 7 days. After 3 weeks, the rash still expanded.
In considering possible interactions between the drugs, we consulted the literature and found reports1-3 that rifampicin accelerated glucocorticoid metabolism, of which the tuberculosis specialist that we consulted was not aware. Therefore, rifampicin was stopped, and the antituberculosis therapy was adjusted to levofloxacin and isoniazid. Meanwhile, the steroid was changed to methylprednisolone 120 mg daily for 3 days, then to 80 mg daily for 2 days.
After 5 days, the rash was controlled with no new development and the patient was discharged. He continued on prednisone 80 mg daily and MMF 1 g twice daily.
At 2-month follow-up, no new rash had developed. The patient had already self-discontinued the MMF for 1 month because it was difficult to obtain at local hospitals. The prednisone was reduced to 40 mg daily. Pulmonary computed tomography showed no signs of reactivation of tuberculosis.
Comment
Drugs that depend on these enzymes for their metabolism are prone to
Rifampicin causes a marked reduction in dose-corrected mycophenolic acid exposure when administered simultaneously with MMF through induction of glucuronidation activity and inhibition of enterohepatic recirculation.5,10In in vitro studies, rifampin and other cytochrome P450 inducers have been identified as potentially useful for increasing the rate of cyclophosphamide and ifosfamide (an isomeric analogue of cyclophosphamide) 4-hydroxylation in the human liver in a manner that could have a favorable impact on the clinical pharmacokinetics of these anticancer prodrugs.11 However, clinical analysis of 16 patients indicated that co-administration of ifosfamide with rifampin did not result in changes in the pharmacokinetics of the parent drug or its metabolites.12
The steroids and
Conclusion
In our patient, the use of rifapentine resulted in a recurrence of previously controlled PV and resistance to treatment. The patient’s disease was quickly controlled after discontinuation of rifampicin and with a short-term course of high-dose methylprednisolone and remained stable when the dosages of MMF and prednisone were reduced.
Case Report
A 60-year-old man presented with eroded areas in the mouth and blistering eruptions on the scalp, face, trunk, arms, and legs. He initially presented to an outside hospital 4 years prior and was treated with oral prednisone 50 mg daily, to which the eruptions responded rapidly; however, following a nearly 5-mg reduction of the dose per week by the patient and irregular oral administration, he experienced several episodes of recurrence, but he could not remember the exact dosage of prednisone he had taken during that period. Subsequently, he was admitted to our hospital because of large areas of erythema and erosions on the scalp, trunk, arms, and legs.
Since starting the prednisone regimen 4 years prior, the patient had experienced onset of hypertension, diabetes, glaucoma, cataracts, optic nerve atrophy, aseptic necrosis of the femoral head, and osteoporosis. Biopsy of a new skin lesion
The patient initially was started again prednisone 50 mg daily, to which the skin eruptions responded, and 2 weeks later, the disease was considered controlled. The prednisone dosage was tapered to 20 mg daily 3 months later with no new blister formation. However, 2 weeks later, the patient was diagnosed by a tuberculosis specialist with pulmonary tuberculosis, and a daily regimen of isoniazid, rifampicin, ethambutol, and levofloxacin was instituted.
Ten days after starting antituberculosis therapy, the patient developed new erythematous blisters that could not be controlled and self-adjusted the prednisone dose to 50 mg daily. Two months later, blister formation continued.
Six months after the initial presentation, the patient returned to our hospital because of uncontrollable rashes (Figure 2). On admission, he had a Pemphigus Disease Area Index (PDAI) score of 32 with disease involving 30% of the body surface area. Laboratory testing showed a desmoglein 1 level of 233 U/mL and desmoglein 3 level of 228 U/mL. A tuberculosis specialist from an outside hospital was consulted to evaluate the patient’s condition and assist in treatment. Based on findings from a pulmonary computed tomography scan, which showed the inflammation was considerably absorbed, treatment was adjusted to stop using ethambutol and levofloxacin and continue rifampicin and isoniazid. For the PV, prednisone was titrated upward to 75 mg daily, mycophenolate mofetil (MMF) 1 g twice daily was added, and IVIG 400 mg/kg daily was administered for 7 days. After 3 weeks, the rash still expanded.
In considering possible interactions between the drugs, we consulted the literature and found reports1-3 that rifampicin accelerated glucocorticoid metabolism, of which the tuberculosis specialist that we consulted was not aware. Therefore, rifampicin was stopped, and the antituberculosis therapy was adjusted to levofloxacin and isoniazid. Meanwhile, the steroid was changed to methylprednisolone 120 mg daily for 3 days, then to 80 mg daily for 2 days.
After 5 days, the rash was controlled with no new development and the patient was discharged. He continued on prednisone 80 mg daily and MMF 1 g twice daily.
At 2-month follow-up, no new rash had developed. The patient had already self-discontinued the MMF for 1 month because it was difficult to obtain at local hospitals. The prednisone was reduced to 40 mg daily. Pulmonary computed tomography showed no signs of reactivation of tuberculosis.
Comment
Drugs that depend on these enzymes for their metabolism are prone to
Rifampicin causes a marked reduction in dose-corrected mycophenolic acid exposure when administered simultaneously with MMF through induction of glucuronidation activity and inhibition of enterohepatic recirculation.5,10In in vitro studies, rifampin and other cytochrome P450 inducers have been identified as potentially useful for increasing the rate of cyclophosphamide and ifosfamide (an isomeric analogue of cyclophosphamide) 4-hydroxylation in the human liver in a manner that could have a favorable impact on the clinical pharmacokinetics of these anticancer prodrugs.11 However, clinical analysis of 16 patients indicated that co-administration of ifosfamide with rifampin did not result in changes in the pharmacokinetics of the parent drug or its metabolites.12
The steroids and
Conclusion
In our patient, the use of rifapentine resulted in a recurrence of previously controlled PV and resistance to treatment. The patient’s disease was quickly controlled after discontinuation of rifampicin and with a short-term course of high-dose methylprednisolone and remained stable when the dosages of MMF and prednisone were reduced.
- Miyagawa S, Yamashina Y, Okuchi T, et al. Exacerbation of pemphigus by rifampicin. Br J Dermatol. 1986;114:729-732. doi:10.1111/j.1365-2133.1986.tb04882.x
- Gange RW, Rhodes EL, Edwards CO, et al. Pemphigus induced by rifampicin. Br J Dermatol. 1976;95:445-448. doi:10.1111/j.1365-2133.1976.tb00849.x
- Bergrem H, Refvem OK. Altered prednisolone pharmacokinetics in patients treated with rifampicin. Acta Med Scand. 1983;213:339-343. doi:10.1111/j.0954-6820.1983.tb03748.x
- McAllister WA, Thompson PJ, Al-Habet SM, et al. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed). 1983;286:923-925. doi:10.1136/bmj.286.6369.923
- Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310:95-106. doi:10.1007/s00403-017-1790-8
- Barman H, Dass R, Duwarah SG. Use of high-dose prednisolone to overcome rifampicin-induced corticosteroid non-responsiveness in childhood nephrotic syndrome. Saudi J Kidney Dis Transpl. 2016;27:157-160. doi:10.4103/1319-2442.174198
- Okey AB, Roberts EA, Harper PA, et al. Induction of drug-metabolizing enzymes: mechanisms and consequences. Clin Biochem. 1986;19:132-141. doi:10.1016/s0009-9120(86)80060-1
- Venkatesan K. Pharmacokinetic interactions with rifampicin. Clin Pharmacokinet. 1992;22:47-65. doi:10.2165/00003088-199222010-00005
- Naesens M, Kuypers DRJ, Streit F, et al. Rifampin induces alterations in mycophenolic acid glucuronidation and elimination: implications for drug exposure in renal allograft recipients. Clin Pharmacol Ther. 2006;80:509-521. doi:10.1016/j.clpt.2006.08.002
- Kuypers DRJ, Verleden G, Naesens M, et al. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate–glucuronosyltransferase. Clin Pharmacol Ther. 2005;78:81-88. doi:10.1016/j.clpt.2005.03.004
- Chenhsu RY, Loong CC, Chou MH, et al. Renal allograft dysfunction associated with rifampin–tacrolimus interaction. Ann Pharmacother. 2000;34:27-31. doi:10.1345/aph.19069
- Douglas JG, McLeod MJ. Pharmacokinetic factors in the modern drug treatment of tuberculosis. Clin Pharmacokinet. 1999;37:127-146. doi:10.2165/00003088-199937020-00003
- Miyagawa S, Yamashina Y, Okuchi T, et al. Exacerbation of pemphigus by rifampicin. Br J Dermatol. 1986;114:729-732. doi:10.1111/j.1365-2133.1986.tb04882.x
- Gange RW, Rhodes EL, Edwards CO, et al. Pemphigus induced by rifampicin. Br J Dermatol. 1976;95:445-448. doi:10.1111/j.1365-2133.1976.tb00849.x
- Bergrem H, Refvem OK. Altered prednisolone pharmacokinetics in patients treated with rifampicin. Acta Med Scand. 1983;213:339-343. doi:10.1111/j.0954-6820.1983.tb03748.x
- McAllister WA, Thompson PJ, Al-Habet SM, et al. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed). 1983;286:923-925. doi:10.1136/bmj.286.6369.923
- Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310:95-106. doi:10.1007/s00403-017-1790-8
- Barman H, Dass R, Duwarah SG. Use of high-dose prednisolone to overcome rifampicin-induced corticosteroid non-responsiveness in childhood nephrotic syndrome. Saudi J Kidney Dis Transpl. 2016;27:157-160. doi:10.4103/1319-2442.174198
- Okey AB, Roberts EA, Harper PA, et al. Induction of drug-metabolizing enzymes: mechanisms and consequences. Clin Biochem. 1986;19:132-141. doi:10.1016/s0009-9120(86)80060-1
- Venkatesan K. Pharmacokinetic interactions with rifampicin. Clin Pharmacokinet. 1992;22:47-65. doi:10.2165/00003088-199222010-00005
- Naesens M, Kuypers DRJ, Streit F, et al. Rifampin induces alterations in mycophenolic acid glucuronidation and elimination: implications for drug exposure in renal allograft recipients. Clin Pharmacol Ther. 2006;80:509-521. doi:10.1016/j.clpt.2006.08.002
- Kuypers DRJ, Verleden G, Naesens M, et al. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate–glucuronosyltransferase. Clin Pharmacol Ther. 2005;78:81-88. doi:10.1016/j.clpt.2005.03.004
- Chenhsu RY, Loong CC, Chou MH, et al. Renal allograft dysfunction associated with rifampin–tacrolimus interaction. Ann Pharmacother. 2000;34:27-31. doi:10.1345/aph.19069
- Douglas JG, McLeod MJ. Pharmacokinetic factors in the modern drug treatment of tuberculosis. Clin Pharmacokinet. 1999;37:127-146. doi:10.2165/00003088-199937020-00003
Practice Points
- Long-term use of immunosuppressants requires constant attention for infections, especially latent infections in the body.
- Clinicians should carefully inquire with patients about concomitant diseases and medications used, and be vigilant about drug interactions.
Leiomyosarcoma of the Penis: A Case Report and Re-Appraisal
Penile cancer is rare with a worldwide incidence of 0.8 cases per 100,000 men.1 The most common type is squamous cell carcinoma (SCC) followed by soft tissue sarcoma (STS) and Kaposi sarcoma.2 Leiomyosarcoma (LMS) is the second most common STS subtype at this location.3 Approximately 50 cases of penile LMS have been reported in the English literature, most as isolated case reports while Fetsch and colleagues reported 14 cases from a single institute.4 We present a case of penile LMS with a review of 31 cases. We also describe presentation, treatment options, and recurrence pattern of this rare malignancy.
Case Presentation
A patient aged 70 years presented to the urology clinic with 1-year history of a slowly enlarging penile mass associated with phimosis. He reported no pain, dysuria, or hesitancy. On examination a 2 × 2-cm smooth, mobile, nonulcerating mass was seen on the tip of his left glans without inguinal lymphadenopathy. He underwent circumcision and excision biopsy that revealed an encapsulated tan-white mass measuring 3 × 2.2 × 1.5 cm under the surface of the foreskin. Histology showed a spindle cell tumor with areas of increased cellularity, prominent atypia, and pleomorphism, focal necrosis, and scattered mitoses, including atypical forms. The tumor stained positive for smooth muscle actin and desmin. Ki-67 staining showed foci with a very high proliferation index (Figure). Resection margins were negative. Final Fédération Nationale des Centres de Lutte Contre Le Cancer score was grade 2 (differentiation, 1; mitotic, 3; necrosis, 1). Computed tomography of the chest, abdomen, and pelvis did not show evidence of metastasis. The tumor was classified as superficial, stage IIA (pT1cN0cM0). Local excision with negative margins was deemed adequate treatment.
Discussion
Penile LMS is rare and arises from smooth muscles, which in the penis can be from dartos fascia, erector pili in the skin covering the shaft, or from tunica media of the superficial vessels and cavernosa.5 It commonly presents as a nodule or ulcer that might be accompanied by paraphimosis, phimosis, erectile dysfunction, and lower urinary tract symptoms depending on the extent of local tissue involvement. In our review of 31 cases, the age at presentation ranged from 38 to 85 years, with 1 case report of LMS in a 6-year-old. The highest incidence was in the 6th decade. Tumor behavior can be indolent or aggressive. Most patients in our review had asymptomatic, slow-growing lesions for 6 to 24 months before presentation—including our patient—while others had an aggressive tumor with symptoms for a few weeks followed by rapid metastatic spread.6,7
Histology and Staging
Diagnosis requires biopsy followed by histologic examination and immunohistochemistry of the lesion. Typically, LMS shows fascicles of spindle cells with varying degrees of nuclear atypia, pleomorphisms, and necrotic regions. Mitotic rate is variable and usually > 5 per high power field. Cells stain positive for smooth muscle actin, desmin, and h-caldesmon.8 TNM (tumor, nodes, metastasis) stage is determined by the American Joint Committee on Cancer guidelines for STS.
Pratt and colleagues were the first to categorize penile LMS as superficial or deep.9 The former includes all lesions superficial to tunica albuginea while the latter run deep to this layer. Anatomical distinction is an important factor in tumor behavior, treatment selection, and prognosis. In our review, we found 14 cases of superficial and 17 cases of deep LMS.
Treatment
There are no established guidelines on optimum treatment of penile LMS. However, we can extrapolate principles from current guidelines on penile cancer, cutaneous leiomyosarcoma, and limb sarcomas. At present, the first-line treatment for superficial penile LMS is wide local excision to achieve negative margins. Circumcision alone might be sufficient for tumors of the distal prepuce, as in our case.10 Radical resection generally is not required for these early-stage tumors. In our review, no patient in this category developed recurrence or metastasis regardless of initial surgery type (Table 1).6,11,12
For deep lesions, partial—if functional penile stump and negative margins can be achieved—or total penectomy is required.10 In our review, more conservative approaches to deep tumors were associated with local recurrences.7,13,14 Lymphatic spread is rare for LMS. Additionally, involvement of local lymph nodes usually coincides with distant spread. Inguinal lymph node dissection is not indicated if initial negative surgical margins are achieved.
For STS at other sites in the body, radiation therapy is recommended postoperatively for high-grade lesions, which can be extrapolated to penile LMS as well. The benefit of preoperative radiation therapy is less certain. In limb sarcomas, radiation is associated with better local control for large-sized tumors and is used for patients with initial unresectable tumors.15 Similar recommendation could be extended to penile LMS with local spread to inguinal lymph nodes, scrotum, or abdominal wall. In our review, postoperative radiation therapy was used in 3 patients with deep tumors.16-18 Of these, short-term relapse occurred in 1 patient.
Chemotherapy for LMS remains controversial. The tumor generally is resistant to chemotherapy and systemic therapy, if employed, is for palliative purpose. The most promising results for adjuvant chemotherapy for resectable STS is seen in limb and uterine sarcomas with high-grade, metastatic, or relapsed tumors but improvement in overall survival has been marginal.19,20Single and multidrug regimens based on doxorubicin, ifosfamide, and gemcitabine have been studied with results showing no efficacy or a slight benefit.8,21 Immunotherapy and targeted therapy for penile STS have not been studied. In our review, postoperative chemotherapy was used for 2 patients with deep tumors and 1 patient with a superficial tumor while preoperative chemotherapy was used for 1 patient.16,18,22 Short-term relapse was seen in 2 of 4 of these patients (Table 2).
Metastatic Disease
LMS tends to metastasize hematogenously and lymphatic spread is uncommon. In our review, 7 patients developed metastasis. These patients had deep tumors at presentation with tumor size > 3 cm. Five of 7 patients had involvement of corpora cavernosa at presentation. The lung was the most common site of metastasis, followed by local extension to lower abdominal wall and scrotum. Of the 7 patients, 3 were treated with initial limited excision or partial penectomy and then experienced local recurrence or distant metastasis.7,13,14,23 This supports the use of radical surgery in large, deep tumors. In an additional 4 cases, metastasis occurred despite initial treatment with total penectomy and use of adjuvant chemoradiation therapy.
In most cases penile LMS is a de novo tumor, however, on occasion it could be accompanied by another epithelial malignancy. Similarly, penile LMS might be a site of recurrence for a primary LMS at another site, as seen in 3 of the reviewed cases. In the first, a patient presented with a nodule on the glans suspicious for SCC, second with synchronous SCC and LMS, and a third case where a patient presented with penile LMS 9 years after being treated for similar tumor in the epididymis.17,24,25
Prognosis
Penile LMS prognosis is difficult to ascertain because reported cases are rare. In our review, the longest documented disease-free survival was 3.5 years for a patient with superficial LMS treated with local excision.26 In cases of distant metastasis, average survival was 4.6 months, while the longest survival since initial presentation and last documented local recurrence was 16 years.14 Five-year survival has not been reported.
Conclusions
LMS of the penis is a rare and potentially aggressive malignancy. It can be classified as superficial or deep based on tumor relation to the tunica albuginea. Deep tumors, those > 3 cm, high-grade lesions, and tumors with involvement of corpora cavernosa, tend to spread locally, metastasize to distant areas, and require more radical surgery with or without postoperative radiation therapy. In comparison, superficial lesions can be treated with local excision only. Both superficial and deep tumors require close follow-up.
1. Montes Cardona CE, García-Perdomo HA. Incidence of penile cancer worldwide: systematic review and meta-analysis. Rev Panam Salud Publica. 2017;41:e117. Published 2017 Nov 30. doi:10.26633/RPSP.2017.117
2. Volker HU, Zettl A, Haralambieva E, et al. Leiomyosarcoma of the larynx as a local relapse of squamous cell carcinoma—report of an unusual case. Head Neck. 2010;32(5):679-683. doi:10.1002/hed.21127
3. Wollina U, Steinbach F, Verma S, et al. Penile tumours: a review. J Eur Acad Dermatol Venereol. 2014;28(10):1267-1276. doi:10.1111/jdv.12491
4. Fetsch JF, Davis CJ Jr, Miettinen M, Sesterhenn IA. Leiomyosarcoma of the penis: a clinicopathologic study of 14 cases with review of the literature and discussion of the differential diagnosis. Am J Surg Pathol. 2004;28(1):115-125. doi:10.1097/00000478-200401000-00014
5. Sundersingh S, Majhi U, Narayanaswamy K, Balasubramanian S. Primary leiomyosarcoma of the penis. Indian J Pathol Microbiol. 2009;52(3):447-448. doi:10.4103/0377-4929.55028
6. Mendis D, Bott SR, Davies JH. Subcutaneous leiomyosarcoma of the frenulum. Scientific World J. 2005;5:571-575. doi:10.1100/tsw.2005.76
7. Elem B, Nieslanik J. Leiomyosarcoma of the penis. Br J Urol. 1979;51(1):46. doi:10.1111/j.1464-410x.1979.tb04244.x
8. Serrano C, George S. Leiomyosarcoma. Hematol Oncol Clin North Am. 2013;27(5):957-974. doi:10.1016/j.hoc.2013.07.002
9. Pratt RM, Ross RT. Leiomyosarcoma of the penis. A report of a case. Br J Surg. 1969;56(11):870-872. doi:10.1002/bjs.1800561122
10. National Comprehensive Cancer Network. Penile cancer. NCCN evidence blocks. Version 2.2022 Updated January 26, 2022. Accessed March 16, 2022. https://www.nccn.org/professionals/physician_gls/pdf/penile_blocks.pdf
11. Ashley DJ, Edwards EC. Sarcoma of the penis; leiomyosarcoma of the penis: report of a case with a review of the literature on sarcoma of the penis. Br J Surg. 1957;45(190):170-179. doi:10.1002/bjs.18004519011
12. Pow-Sang MR, Orihuela E. Leiomyosarcoma of the penis. J Urol. 1994;151(6):1643-1645. doi:10.1016/s0022-5347(17)35328-413. Isa SS, Almaraz R, Magovern J. Leiomyosarcoma of the penis. Case report and review of the literature. Cancer. 1984;54(5):939-942. doi:10.1002/1097-0142(19840901)54:5<939::aid-cncr2820540533>3.0.co;2-y
14. Hutcheson JB, Wittaker WW, Fronstin MH. Leiomyosarcoma of the penis: case report and review of literature. J Urol. 1969;101(6):874-875. doi:10.1016/s0022-5347(17)62446-7
15. Grimer R, Judson I, Peake D, et al. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010:506182. doi:10.1155/2010/506182
16. McDonald MW, O’Connell JR, Manning JT, Benjamin RS. Leiomyosarcoma of the penis. J Urol. 1983;130(4):788-789. doi:10.1016/s0022-5347(17)51464-0
17. Planz B, Brunner K, Kalem T, Schlick RW, Kind M. Primary leiomyosarcoma of the epididymis and late recurrence on the penis. J Urol. 1998;159(2):508. doi:10.1016/s0022-5347(01)63966-1
18. Smart RH. Leiomyosarcoma of the penis. J Urol. 1984;132(2):356-357. doi:10.1016/s0022-5347(17)49624-8
19. Patrikidou A, Domont J, Cioffi A, Le Cesne A. Treating soft tissue sarcomas with adjuvant chemotherapy. Curr Treat Options Oncol. 2011;12(1):21-31. doi:10.1007/s11864-011-0145-5
20. Italiano A, Delva F, Mathoulin-Pelissier S, et al. Effect of adjuvant chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: a multivariate analysis of the French Sarcoma Group Database. Ann Oncol. 2010;21(12):2436-2441. doi:10.1093/annonc/mdq238
21. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113(3):573-581. doi:10.1002/cncr.23592
22. Lacarrière E, Galliot I, Gobet F, Sibert L. Leiomyosarcoma of the corpus cavernosum mimicking a Peyronie’s plaque. Urology. 2012;79(4):e53-e54. doi:10.1016/j.urology.2011.07.1410
23. Hamal PB. Leiomyosarcoma of penis—case report and review of the literature. Br J Urol. 1975;47(3):319-324. doi:10.1111/j.1464-410x.1975.tb03974.x
24. Greenwood N, Fox H, Edwards EC. Leiomyosarcoma of the penis. Cancer. 1972;29(2):481-483. doi:10.1002/1097-0142(197202)29:2<481::aid -cncr2820290237>3.0.co;2-q
25. Koizumi H, Nagano K, Kosaka S. A case of penile tumor: combination of leiomyosarcoma and squamous cell carcinoma. Hinyokika Kiyo. 1987;33(9):1489-1491.
26. Romero Gonzalez EJ, Marenco Jimenez JL, Mayorga Pineda MP, Martínez Morán A, Castiñeiras Fernández J. Leiomyosarcoma of the penis, an exceptional entity. Urol Case Rep. 2015;3(3):63-64. doi:10.1016/j.eucr.2014.12.007
Penile cancer is rare with a worldwide incidence of 0.8 cases per 100,000 men.1 The most common type is squamous cell carcinoma (SCC) followed by soft tissue sarcoma (STS) and Kaposi sarcoma.2 Leiomyosarcoma (LMS) is the second most common STS subtype at this location.3 Approximately 50 cases of penile LMS have been reported in the English literature, most as isolated case reports while Fetsch and colleagues reported 14 cases from a single institute.4 We present a case of penile LMS with a review of 31 cases. We also describe presentation, treatment options, and recurrence pattern of this rare malignancy.
Case Presentation
A patient aged 70 years presented to the urology clinic with 1-year history of a slowly enlarging penile mass associated with phimosis. He reported no pain, dysuria, or hesitancy. On examination a 2 × 2-cm smooth, mobile, nonulcerating mass was seen on the tip of his left glans without inguinal lymphadenopathy. He underwent circumcision and excision biopsy that revealed an encapsulated tan-white mass measuring 3 × 2.2 × 1.5 cm under the surface of the foreskin. Histology showed a spindle cell tumor with areas of increased cellularity, prominent atypia, and pleomorphism, focal necrosis, and scattered mitoses, including atypical forms. The tumor stained positive for smooth muscle actin and desmin. Ki-67 staining showed foci with a very high proliferation index (Figure). Resection margins were negative. Final Fédération Nationale des Centres de Lutte Contre Le Cancer score was grade 2 (differentiation, 1; mitotic, 3; necrosis, 1). Computed tomography of the chest, abdomen, and pelvis did not show evidence of metastasis. The tumor was classified as superficial, stage IIA (pT1cN0cM0). Local excision with negative margins was deemed adequate treatment.
Discussion
Penile LMS is rare and arises from smooth muscles, which in the penis can be from dartos fascia, erector pili in the skin covering the shaft, or from tunica media of the superficial vessels and cavernosa.5 It commonly presents as a nodule or ulcer that might be accompanied by paraphimosis, phimosis, erectile dysfunction, and lower urinary tract symptoms depending on the extent of local tissue involvement. In our review of 31 cases, the age at presentation ranged from 38 to 85 years, with 1 case report of LMS in a 6-year-old. The highest incidence was in the 6th decade. Tumor behavior can be indolent or aggressive. Most patients in our review had asymptomatic, slow-growing lesions for 6 to 24 months before presentation—including our patient—while others had an aggressive tumor with symptoms for a few weeks followed by rapid metastatic spread.6,7
Histology and Staging
Diagnosis requires biopsy followed by histologic examination and immunohistochemistry of the lesion. Typically, LMS shows fascicles of spindle cells with varying degrees of nuclear atypia, pleomorphisms, and necrotic regions. Mitotic rate is variable and usually > 5 per high power field. Cells stain positive for smooth muscle actin, desmin, and h-caldesmon.8 TNM (tumor, nodes, metastasis) stage is determined by the American Joint Committee on Cancer guidelines for STS.
Pratt and colleagues were the first to categorize penile LMS as superficial or deep.9 The former includes all lesions superficial to tunica albuginea while the latter run deep to this layer. Anatomical distinction is an important factor in tumor behavior, treatment selection, and prognosis. In our review, we found 14 cases of superficial and 17 cases of deep LMS.
Treatment
There are no established guidelines on optimum treatment of penile LMS. However, we can extrapolate principles from current guidelines on penile cancer, cutaneous leiomyosarcoma, and limb sarcomas. At present, the first-line treatment for superficial penile LMS is wide local excision to achieve negative margins. Circumcision alone might be sufficient for tumors of the distal prepuce, as in our case.10 Radical resection generally is not required for these early-stage tumors. In our review, no patient in this category developed recurrence or metastasis regardless of initial surgery type (Table 1).6,11,12
For deep lesions, partial—if functional penile stump and negative margins can be achieved—or total penectomy is required.10 In our review, more conservative approaches to deep tumors were associated with local recurrences.7,13,14 Lymphatic spread is rare for LMS. Additionally, involvement of local lymph nodes usually coincides with distant spread. Inguinal lymph node dissection is not indicated if initial negative surgical margins are achieved.
For STS at other sites in the body, radiation therapy is recommended postoperatively for high-grade lesions, which can be extrapolated to penile LMS as well. The benefit of preoperative radiation therapy is less certain. In limb sarcomas, radiation is associated with better local control for large-sized tumors and is used for patients with initial unresectable tumors.15 Similar recommendation could be extended to penile LMS with local spread to inguinal lymph nodes, scrotum, or abdominal wall. In our review, postoperative radiation therapy was used in 3 patients with deep tumors.16-18 Of these, short-term relapse occurred in 1 patient.
Chemotherapy for LMS remains controversial. The tumor generally is resistant to chemotherapy and systemic therapy, if employed, is for palliative purpose. The most promising results for adjuvant chemotherapy for resectable STS is seen in limb and uterine sarcomas with high-grade, metastatic, or relapsed tumors but improvement in overall survival has been marginal.19,20Single and multidrug regimens based on doxorubicin, ifosfamide, and gemcitabine have been studied with results showing no efficacy or a slight benefit.8,21 Immunotherapy and targeted therapy for penile STS have not been studied. In our review, postoperative chemotherapy was used for 2 patients with deep tumors and 1 patient with a superficial tumor while preoperative chemotherapy was used for 1 patient.16,18,22 Short-term relapse was seen in 2 of 4 of these patients (Table 2).
Metastatic Disease
LMS tends to metastasize hematogenously and lymphatic spread is uncommon. In our review, 7 patients developed metastasis. These patients had deep tumors at presentation with tumor size > 3 cm. Five of 7 patients had involvement of corpora cavernosa at presentation. The lung was the most common site of metastasis, followed by local extension to lower abdominal wall and scrotum. Of the 7 patients, 3 were treated with initial limited excision or partial penectomy and then experienced local recurrence or distant metastasis.7,13,14,23 This supports the use of radical surgery in large, deep tumors. In an additional 4 cases, metastasis occurred despite initial treatment with total penectomy and use of adjuvant chemoradiation therapy.
In most cases penile LMS is a de novo tumor, however, on occasion it could be accompanied by another epithelial malignancy. Similarly, penile LMS might be a site of recurrence for a primary LMS at another site, as seen in 3 of the reviewed cases. In the first, a patient presented with a nodule on the glans suspicious for SCC, second with synchronous SCC and LMS, and a third case where a patient presented with penile LMS 9 years after being treated for similar tumor in the epididymis.17,24,25
Prognosis
Penile LMS prognosis is difficult to ascertain because reported cases are rare. In our review, the longest documented disease-free survival was 3.5 years for a patient with superficial LMS treated with local excision.26 In cases of distant metastasis, average survival was 4.6 months, while the longest survival since initial presentation and last documented local recurrence was 16 years.14 Five-year survival has not been reported.
Conclusions
LMS of the penis is a rare and potentially aggressive malignancy. It can be classified as superficial or deep based on tumor relation to the tunica albuginea. Deep tumors, those > 3 cm, high-grade lesions, and tumors with involvement of corpora cavernosa, tend to spread locally, metastasize to distant areas, and require more radical surgery with or without postoperative radiation therapy. In comparison, superficial lesions can be treated with local excision only. Both superficial and deep tumors require close follow-up.
Penile cancer is rare with a worldwide incidence of 0.8 cases per 100,000 men.1 The most common type is squamous cell carcinoma (SCC) followed by soft tissue sarcoma (STS) and Kaposi sarcoma.2 Leiomyosarcoma (LMS) is the second most common STS subtype at this location.3 Approximately 50 cases of penile LMS have been reported in the English literature, most as isolated case reports while Fetsch and colleagues reported 14 cases from a single institute.4 We present a case of penile LMS with a review of 31 cases. We also describe presentation, treatment options, and recurrence pattern of this rare malignancy.
Case Presentation
A patient aged 70 years presented to the urology clinic with 1-year history of a slowly enlarging penile mass associated with phimosis. He reported no pain, dysuria, or hesitancy. On examination a 2 × 2-cm smooth, mobile, nonulcerating mass was seen on the tip of his left glans without inguinal lymphadenopathy. He underwent circumcision and excision biopsy that revealed an encapsulated tan-white mass measuring 3 × 2.2 × 1.5 cm under the surface of the foreskin. Histology showed a spindle cell tumor with areas of increased cellularity, prominent atypia, and pleomorphism, focal necrosis, and scattered mitoses, including atypical forms. The tumor stained positive for smooth muscle actin and desmin. Ki-67 staining showed foci with a very high proliferation index (Figure). Resection margins were negative. Final Fédération Nationale des Centres de Lutte Contre Le Cancer score was grade 2 (differentiation, 1; mitotic, 3; necrosis, 1). Computed tomography of the chest, abdomen, and pelvis did not show evidence of metastasis. The tumor was classified as superficial, stage IIA (pT1cN0cM0). Local excision with negative margins was deemed adequate treatment.
Discussion
Penile LMS is rare and arises from smooth muscles, which in the penis can be from dartos fascia, erector pili in the skin covering the shaft, or from tunica media of the superficial vessels and cavernosa.5 It commonly presents as a nodule or ulcer that might be accompanied by paraphimosis, phimosis, erectile dysfunction, and lower urinary tract symptoms depending on the extent of local tissue involvement. In our review of 31 cases, the age at presentation ranged from 38 to 85 years, with 1 case report of LMS in a 6-year-old. The highest incidence was in the 6th decade. Tumor behavior can be indolent or aggressive. Most patients in our review had asymptomatic, slow-growing lesions for 6 to 24 months before presentation—including our patient—while others had an aggressive tumor with symptoms for a few weeks followed by rapid metastatic spread.6,7
Histology and Staging
Diagnosis requires biopsy followed by histologic examination and immunohistochemistry of the lesion. Typically, LMS shows fascicles of spindle cells with varying degrees of nuclear atypia, pleomorphisms, and necrotic regions. Mitotic rate is variable and usually > 5 per high power field. Cells stain positive for smooth muscle actin, desmin, and h-caldesmon.8 TNM (tumor, nodes, metastasis) stage is determined by the American Joint Committee on Cancer guidelines for STS.
Pratt and colleagues were the first to categorize penile LMS as superficial or deep.9 The former includes all lesions superficial to tunica albuginea while the latter run deep to this layer. Anatomical distinction is an important factor in tumor behavior, treatment selection, and prognosis. In our review, we found 14 cases of superficial and 17 cases of deep LMS.
Treatment
There are no established guidelines on optimum treatment of penile LMS. However, we can extrapolate principles from current guidelines on penile cancer, cutaneous leiomyosarcoma, and limb sarcomas. At present, the first-line treatment for superficial penile LMS is wide local excision to achieve negative margins. Circumcision alone might be sufficient for tumors of the distal prepuce, as in our case.10 Radical resection generally is not required for these early-stage tumors. In our review, no patient in this category developed recurrence or metastasis regardless of initial surgery type (Table 1).6,11,12
For deep lesions, partial—if functional penile stump and negative margins can be achieved—or total penectomy is required.10 In our review, more conservative approaches to deep tumors were associated with local recurrences.7,13,14 Lymphatic spread is rare for LMS. Additionally, involvement of local lymph nodes usually coincides with distant spread. Inguinal lymph node dissection is not indicated if initial negative surgical margins are achieved.
For STS at other sites in the body, radiation therapy is recommended postoperatively for high-grade lesions, which can be extrapolated to penile LMS as well. The benefit of preoperative radiation therapy is less certain. In limb sarcomas, radiation is associated with better local control for large-sized tumors and is used for patients with initial unresectable tumors.15 Similar recommendation could be extended to penile LMS with local spread to inguinal lymph nodes, scrotum, or abdominal wall. In our review, postoperative radiation therapy was used in 3 patients with deep tumors.16-18 Of these, short-term relapse occurred in 1 patient.
Chemotherapy for LMS remains controversial. The tumor generally is resistant to chemotherapy and systemic therapy, if employed, is for palliative purpose. The most promising results for adjuvant chemotherapy for resectable STS is seen in limb and uterine sarcomas with high-grade, metastatic, or relapsed tumors but improvement in overall survival has been marginal.19,20Single and multidrug regimens based on doxorubicin, ifosfamide, and gemcitabine have been studied with results showing no efficacy or a slight benefit.8,21 Immunotherapy and targeted therapy for penile STS have not been studied. In our review, postoperative chemotherapy was used for 2 patients with deep tumors and 1 patient with a superficial tumor while preoperative chemotherapy was used for 1 patient.16,18,22 Short-term relapse was seen in 2 of 4 of these patients (Table 2).
Metastatic Disease
LMS tends to metastasize hematogenously and lymphatic spread is uncommon. In our review, 7 patients developed metastasis. These patients had deep tumors at presentation with tumor size > 3 cm. Five of 7 patients had involvement of corpora cavernosa at presentation. The lung was the most common site of metastasis, followed by local extension to lower abdominal wall and scrotum. Of the 7 patients, 3 were treated with initial limited excision or partial penectomy and then experienced local recurrence or distant metastasis.7,13,14,23 This supports the use of radical surgery in large, deep tumors. In an additional 4 cases, metastasis occurred despite initial treatment with total penectomy and use of adjuvant chemoradiation therapy.
In most cases penile LMS is a de novo tumor, however, on occasion it could be accompanied by another epithelial malignancy. Similarly, penile LMS might be a site of recurrence for a primary LMS at another site, as seen in 3 of the reviewed cases. In the first, a patient presented with a nodule on the glans suspicious for SCC, second with synchronous SCC and LMS, and a third case where a patient presented with penile LMS 9 years after being treated for similar tumor in the epididymis.17,24,25
Prognosis
Penile LMS prognosis is difficult to ascertain because reported cases are rare. In our review, the longest documented disease-free survival was 3.5 years for a patient with superficial LMS treated with local excision.26 In cases of distant metastasis, average survival was 4.6 months, while the longest survival since initial presentation and last documented local recurrence was 16 years.14 Five-year survival has not been reported.
Conclusions
LMS of the penis is a rare and potentially aggressive malignancy. It can be classified as superficial or deep based on tumor relation to the tunica albuginea. Deep tumors, those > 3 cm, high-grade lesions, and tumors with involvement of corpora cavernosa, tend to spread locally, metastasize to distant areas, and require more radical surgery with or without postoperative radiation therapy. In comparison, superficial lesions can be treated with local excision only. Both superficial and deep tumors require close follow-up.
1. Montes Cardona CE, García-Perdomo HA. Incidence of penile cancer worldwide: systematic review and meta-analysis. Rev Panam Salud Publica. 2017;41:e117. Published 2017 Nov 30. doi:10.26633/RPSP.2017.117
2. Volker HU, Zettl A, Haralambieva E, et al. Leiomyosarcoma of the larynx as a local relapse of squamous cell carcinoma—report of an unusual case. Head Neck. 2010;32(5):679-683. doi:10.1002/hed.21127
3. Wollina U, Steinbach F, Verma S, et al. Penile tumours: a review. J Eur Acad Dermatol Venereol. 2014;28(10):1267-1276. doi:10.1111/jdv.12491
4. Fetsch JF, Davis CJ Jr, Miettinen M, Sesterhenn IA. Leiomyosarcoma of the penis: a clinicopathologic study of 14 cases with review of the literature and discussion of the differential diagnosis. Am J Surg Pathol. 2004;28(1):115-125. doi:10.1097/00000478-200401000-00014
5. Sundersingh S, Majhi U, Narayanaswamy K, Balasubramanian S. Primary leiomyosarcoma of the penis. Indian J Pathol Microbiol. 2009;52(3):447-448. doi:10.4103/0377-4929.55028
6. Mendis D, Bott SR, Davies JH. Subcutaneous leiomyosarcoma of the frenulum. Scientific World J. 2005;5:571-575. doi:10.1100/tsw.2005.76
7. Elem B, Nieslanik J. Leiomyosarcoma of the penis. Br J Urol. 1979;51(1):46. doi:10.1111/j.1464-410x.1979.tb04244.x
8. Serrano C, George S. Leiomyosarcoma. Hematol Oncol Clin North Am. 2013;27(5):957-974. doi:10.1016/j.hoc.2013.07.002
9. Pratt RM, Ross RT. Leiomyosarcoma of the penis. A report of a case. Br J Surg. 1969;56(11):870-872. doi:10.1002/bjs.1800561122
10. National Comprehensive Cancer Network. Penile cancer. NCCN evidence blocks. Version 2.2022 Updated January 26, 2022. Accessed March 16, 2022. https://www.nccn.org/professionals/physician_gls/pdf/penile_blocks.pdf
11. Ashley DJ, Edwards EC. Sarcoma of the penis; leiomyosarcoma of the penis: report of a case with a review of the literature on sarcoma of the penis. Br J Surg. 1957;45(190):170-179. doi:10.1002/bjs.18004519011
12. Pow-Sang MR, Orihuela E. Leiomyosarcoma of the penis. J Urol. 1994;151(6):1643-1645. doi:10.1016/s0022-5347(17)35328-413. Isa SS, Almaraz R, Magovern J. Leiomyosarcoma of the penis. Case report and review of the literature. Cancer. 1984;54(5):939-942. doi:10.1002/1097-0142(19840901)54:5<939::aid-cncr2820540533>3.0.co;2-y
14. Hutcheson JB, Wittaker WW, Fronstin MH. Leiomyosarcoma of the penis: case report and review of literature. J Urol. 1969;101(6):874-875. doi:10.1016/s0022-5347(17)62446-7
15. Grimer R, Judson I, Peake D, et al. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010:506182. doi:10.1155/2010/506182
16. McDonald MW, O’Connell JR, Manning JT, Benjamin RS. Leiomyosarcoma of the penis. J Urol. 1983;130(4):788-789. doi:10.1016/s0022-5347(17)51464-0
17. Planz B, Brunner K, Kalem T, Schlick RW, Kind M. Primary leiomyosarcoma of the epididymis and late recurrence on the penis. J Urol. 1998;159(2):508. doi:10.1016/s0022-5347(01)63966-1
18. Smart RH. Leiomyosarcoma of the penis. J Urol. 1984;132(2):356-357. doi:10.1016/s0022-5347(17)49624-8
19. Patrikidou A, Domont J, Cioffi A, Le Cesne A. Treating soft tissue sarcomas with adjuvant chemotherapy. Curr Treat Options Oncol. 2011;12(1):21-31. doi:10.1007/s11864-011-0145-5
20. Italiano A, Delva F, Mathoulin-Pelissier S, et al. Effect of adjuvant chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: a multivariate analysis of the French Sarcoma Group Database. Ann Oncol. 2010;21(12):2436-2441. doi:10.1093/annonc/mdq238
21. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113(3):573-581. doi:10.1002/cncr.23592
22. Lacarrière E, Galliot I, Gobet F, Sibert L. Leiomyosarcoma of the corpus cavernosum mimicking a Peyronie’s plaque. Urology. 2012;79(4):e53-e54. doi:10.1016/j.urology.2011.07.1410
23. Hamal PB. Leiomyosarcoma of penis—case report and review of the literature. Br J Urol. 1975;47(3):319-324. doi:10.1111/j.1464-410x.1975.tb03974.x
24. Greenwood N, Fox H, Edwards EC. Leiomyosarcoma of the penis. Cancer. 1972;29(2):481-483. doi:10.1002/1097-0142(197202)29:2<481::aid -cncr2820290237>3.0.co;2-q
25. Koizumi H, Nagano K, Kosaka S. A case of penile tumor: combination of leiomyosarcoma and squamous cell carcinoma. Hinyokika Kiyo. 1987;33(9):1489-1491.
26. Romero Gonzalez EJ, Marenco Jimenez JL, Mayorga Pineda MP, Martínez Morán A, Castiñeiras Fernández J. Leiomyosarcoma of the penis, an exceptional entity. Urol Case Rep. 2015;3(3):63-64. doi:10.1016/j.eucr.2014.12.007
1. Montes Cardona CE, García-Perdomo HA. Incidence of penile cancer worldwide: systematic review and meta-analysis. Rev Panam Salud Publica. 2017;41:e117. Published 2017 Nov 30. doi:10.26633/RPSP.2017.117
2. Volker HU, Zettl A, Haralambieva E, et al. Leiomyosarcoma of the larynx as a local relapse of squamous cell carcinoma—report of an unusual case. Head Neck. 2010;32(5):679-683. doi:10.1002/hed.21127
3. Wollina U, Steinbach F, Verma S, et al. Penile tumours: a review. J Eur Acad Dermatol Venereol. 2014;28(10):1267-1276. doi:10.1111/jdv.12491
4. Fetsch JF, Davis CJ Jr, Miettinen M, Sesterhenn IA. Leiomyosarcoma of the penis: a clinicopathologic study of 14 cases with review of the literature and discussion of the differential diagnosis. Am J Surg Pathol. 2004;28(1):115-125. doi:10.1097/00000478-200401000-00014
5. Sundersingh S, Majhi U, Narayanaswamy K, Balasubramanian S. Primary leiomyosarcoma of the penis. Indian J Pathol Microbiol. 2009;52(3):447-448. doi:10.4103/0377-4929.55028
6. Mendis D, Bott SR, Davies JH. Subcutaneous leiomyosarcoma of the frenulum. Scientific World J. 2005;5:571-575. doi:10.1100/tsw.2005.76
7. Elem B, Nieslanik J. Leiomyosarcoma of the penis. Br J Urol. 1979;51(1):46. doi:10.1111/j.1464-410x.1979.tb04244.x
8. Serrano C, George S. Leiomyosarcoma. Hematol Oncol Clin North Am. 2013;27(5):957-974. doi:10.1016/j.hoc.2013.07.002
9. Pratt RM, Ross RT. Leiomyosarcoma of the penis. A report of a case. Br J Surg. 1969;56(11):870-872. doi:10.1002/bjs.1800561122
10. National Comprehensive Cancer Network. Penile cancer. NCCN evidence blocks. Version 2.2022 Updated January 26, 2022. Accessed March 16, 2022. https://www.nccn.org/professionals/physician_gls/pdf/penile_blocks.pdf
11. Ashley DJ, Edwards EC. Sarcoma of the penis; leiomyosarcoma of the penis: report of a case with a review of the literature on sarcoma of the penis. Br J Surg. 1957;45(190):170-179. doi:10.1002/bjs.18004519011
12. Pow-Sang MR, Orihuela E. Leiomyosarcoma of the penis. J Urol. 1994;151(6):1643-1645. doi:10.1016/s0022-5347(17)35328-413. Isa SS, Almaraz R, Magovern J. Leiomyosarcoma of the penis. Case report and review of the literature. Cancer. 1984;54(5):939-942. doi:10.1002/1097-0142(19840901)54:5<939::aid-cncr2820540533>3.0.co;2-y
14. Hutcheson JB, Wittaker WW, Fronstin MH. Leiomyosarcoma of the penis: case report and review of literature. J Urol. 1969;101(6):874-875. doi:10.1016/s0022-5347(17)62446-7
15. Grimer R, Judson I, Peake D, et al. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010:506182. doi:10.1155/2010/506182
16. McDonald MW, O’Connell JR, Manning JT, Benjamin RS. Leiomyosarcoma of the penis. J Urol. 1983;130(4):788-789. doi:10.1016/s0022-5347(17)51464-0
17. Planz B, Brunner K, Kalem T, Schlick RW, Kind M. Primary leiomyosarcoma of the epididymis and late recurrence on the penis. J Urol. 1998;159(2):508. doi:10.1016/s0022-5347(01)63966-1
18. Smart RH. Leiomyosarcoma of the penis. J Urol. 1984;132(2):356-357. doi:10.1016/s0022-5347(17)49624-8
19. Patrikidou A, Domont J, Cioffi A, Le Cesne A. Treating soft tissue sarcomas with adjuvant chemotherapy. Curr Treat Options Oncol. 2011;12(1):21-31. doi:10.1007/s11864-011-0145-5
20. Italiano A, Delva F, Mathoulin-Pelissier S, et al. Effect of adjuvant chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: a multivariate analysis of the French Sarcoma Group Database. Ann Oncol. 2010;21(12):2436-2441. doi:10.1093/annonc/mdq238
21. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113(3):573-581. doi:10.1002/cncr.23592
22. Lacarrière E, Galliot I, Gobet F, Sibert L. Leiomyosarcoma of the corpus cavernosum mimicking a Peyronie’s plaque. Urology. 2012;79(4):e53-e54. doi:10.1016/j.urology.2011.07.1410
23. Hamal PB. Leiomyosarcoma of penis—case report and review of the literature. Br J Urol. 1975;47(3):319-324. doi:10.1111/j.1464-410x.1975.tb03974.x
24. Greenwood N, Fox H, Edwards EC. Leiomyosarcoma of the penis. Cancer. 1972;29(2):481-483. doi:10.1002/1097-0142(197202)29:2<481::aid -cncr2820290237>3.0.co;2-q
25. Koizumi H, Nagano K, Kosaka S. A case of penile tumor: combination of leiomyosarcoma and squamous cell carcinoma. Hinyokika Kiyo. 1987;33(9):1489-1491.
26. Romero Gonzalez EJ, Marenco Jimenez JL, Mayorga Pineda MP, Martínez Morán A, Castiñeiras Fernández J. Leiomyosarcoma of the penis, an exceptional entity. Urol Case Rep. 2015;3(3):63-64. doi:10.1016/j.eucr.2014.12.007