User login
Juvenile Dermatomyositis–Associated Panniculitis
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.
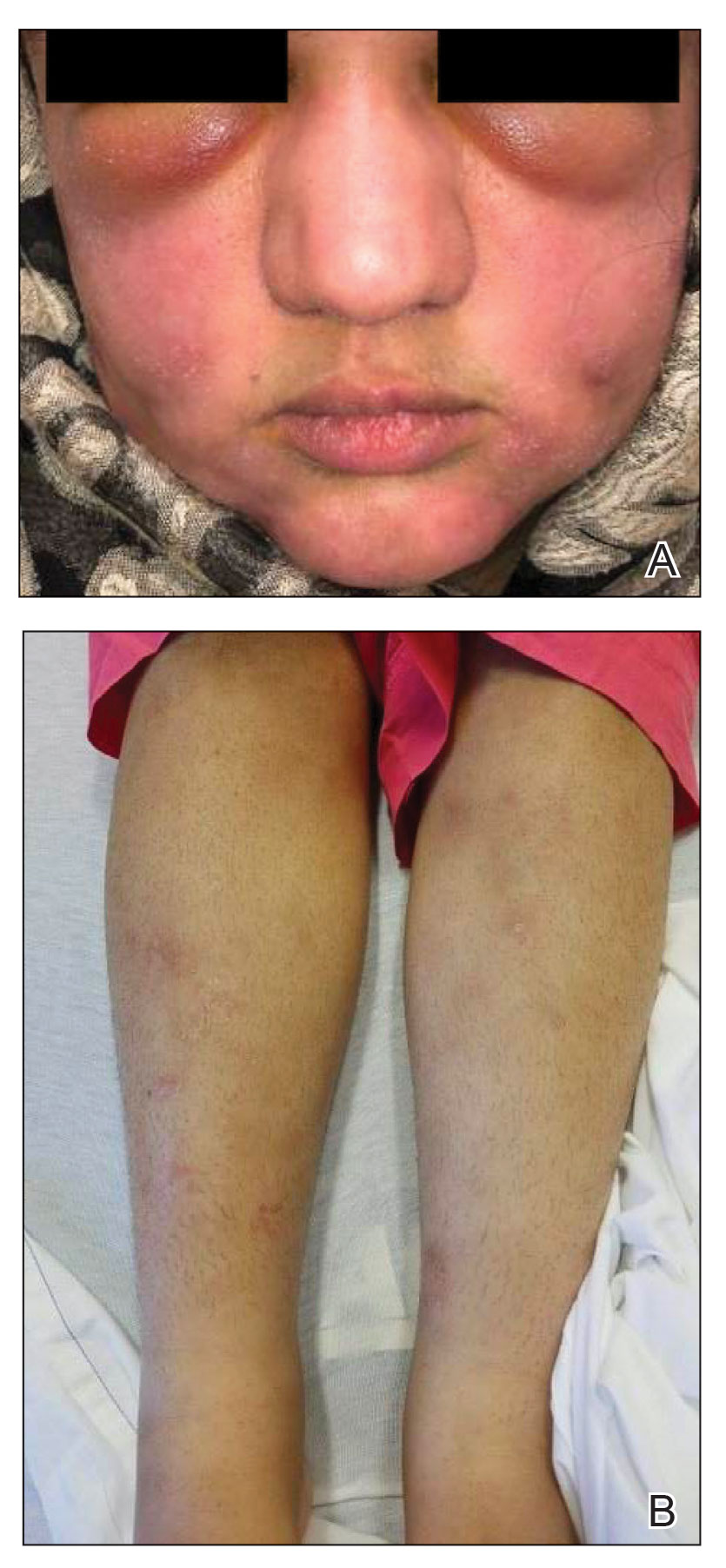
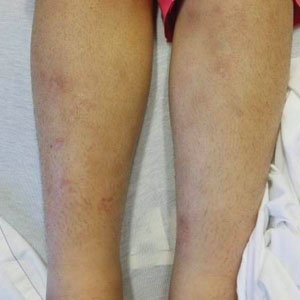
A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.
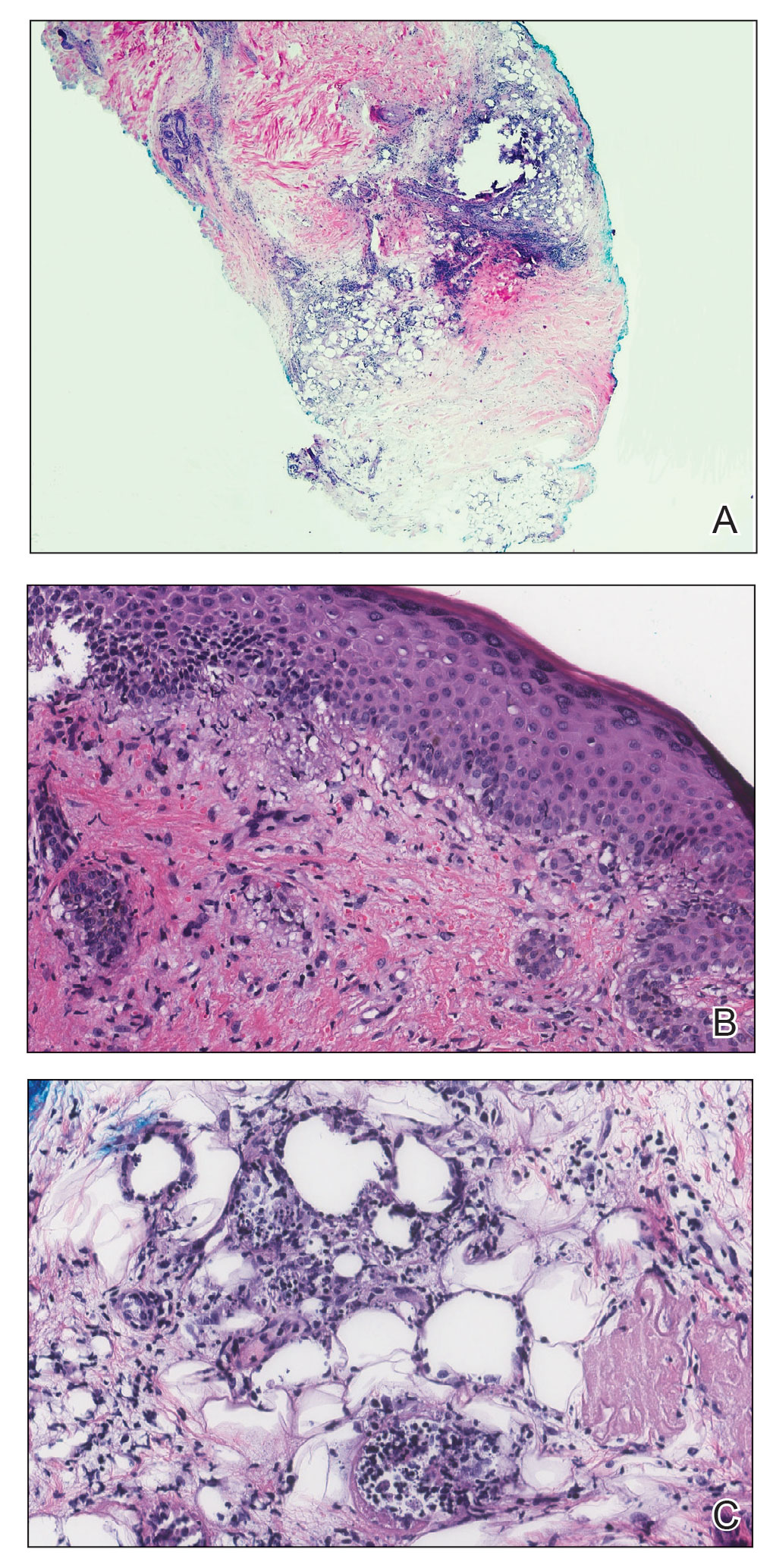
Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.

A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.

Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.

A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.

Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
Practice Points
- Juvenile dermatomyositis is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin.
- Juvenile dermatomyositis has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant, dermatomyositis (DM).
- Panniculitis is a rare but severe complication of DM, and this subset of DM may be challenging to treat, requiring aggressive therapy.
Skin Manifestations of Complex Regional Pain Syndrome
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
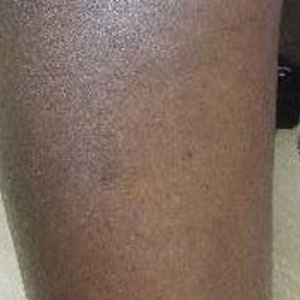
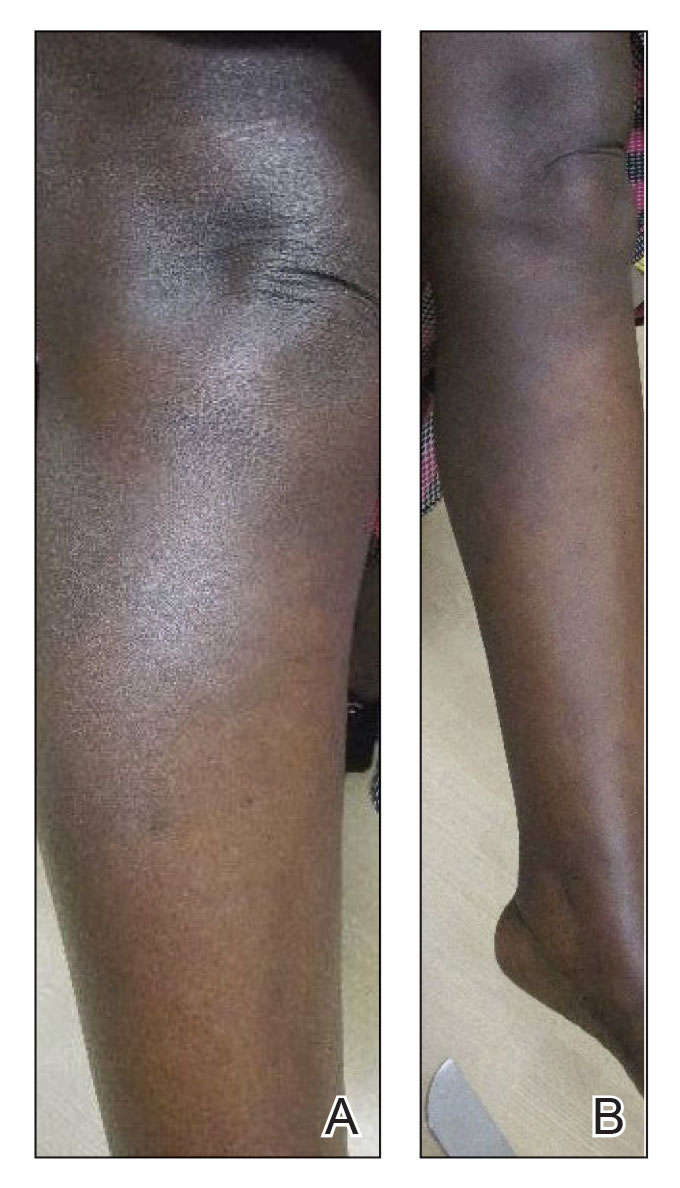
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.
To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.

To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.

To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
PRACTICE POINTS
- Common dermatologic manifestations of complex regional pain syndrome (CRPS), which often are nonspecific and often the presenting symptoms of the syndrome, include allodynia, edema, erythema, hypopigmentation or hyperpigmentation, and petechiae.
- Diagnosis and management of CRPS are the most important steps in treating dermatologic manifestations of the syndrome.
Primary Malignant Melanoma of the Middle Ear
To the Editor:
An 82-year-old man presented to our dermatology clinic for a total-body skin examination due to a recently diagnosed primary melanoma of the left middle ear. He reported pain of the left ear and water behind the left eardrum of 1 year’s duration. An otorhinolaryngologist performed surgery due to the severe mastoiditis. A biopsy of the contents of the left middle ear revealed malignant melanoma. Positron emission tomography–computed tomography revealed the mass was mainly located in the anterior aspect of the left middle ear with suspicion of tumor extension into the bony portion of the eustachian tube. No other disease was present. Prior to presentation to dermatology, gross excision of the left middle ear with removal of additional melanoma was confirmed by biopsy, and further analysis revealed v-Raf murine sarcoma viral oncogene (BRAF) was not detected while cellular proto-oncogene receptor kinase (KIT) mutation was detected on exon 13p (K642E).
The patient had no family history of melanoma. He never smoked and did not have contact with hazardous material. Initial examination at our clinic revealed no other suspicious pigmented lesions. After additional negative workup by the oncologist, the patient was presented to the tumor board, and postoperative radiotherapy was recommended to improve local control. Eight months after the patient’s initial diagnosis of the primary middle ear melanoma, a computed tomography–guided right lung biopsy showed metastatic melanoma. After various treatment modalities were discussed with the patient and his family, he was started on pembrolizumab. After 6 months on pembrolizumab, the patient developed autoimmune pneumonitis and pembrolizumab was discontinued. The patient elected to discontinue treatment and died 6 months later.
Malignant melanoma with primary involvement of the middle ear and mastoid mucosa rarely has been reported.1-3 Primary malignant melanoma of the middle ear mucosa is difficult to diagnose clinically. Difficulty and delay in diagnosis occur because of the location and frequent lack of pathognomonic symptoms of the disease.2 A comprehensive literature review by Maxwell et al3 in 2018 of the 10 reported primary middle ear mucosal melanomas found that patients most commonly presented with otorrhea, aural fullness, and hearing loss. Less common symptoms included otalgia, tinnitus, and facial weakness. Clinical examination revealed patients presented with serous otitis and/or a visible mass within the middle ear or external auditory canal. These melanomas demonstrated particularly poor outcomes, with 70% mortality, 20% local recurrence, and 50% distant metastasis. Distant metastases that occurred with primary middle ear mucosal melanoma include lung, liver, intraparotid, abdomen, and cutaneous metastasis.3
The specific pathophysiologic factors underlying the development of primary malignant melanoma of the middle ear mucosa are not known.2 The middle ear and its components develop from the first and second pharyngeal arches.4 Melanocyte precursors from the neural crest migrate during the seventh or eighth week of embryogenesis. These precursors migrate to the epidermis, various mucosal epithelial, hair follicles, dermis, retina, uveal tract, leptomeninges, inner ear, and other tissues.5 The ossicles of the middle ear develop from the neural crest6 and remain in the mesenchyme until the eighth month, when the surrounding tissue dissolves.4 Cutaneous melanomas arise from the malignant transformation of melanocytes in the skin of neural crest lineage. Noncutaneous melanomas are hypothesized to arise from melanoblasts migrating to noncutaneous organs after neural crest cells undergo an epithelial-mesenchymal translation.7
Melanoma 5-year survival rates vary based on the melanoma disease stage: 98% for stage 1, 90% for stage 2, 70% for stage 3, and 10% for stage 4. Although early-stage disease mainly is treated with surgery, advanced and unresectable disease is managed with different therapeutic options, including BRAF inhibitors such as vemurafenib, dabrafenib mesylate, and encorafenib; immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab; and oncolytic virus such as talimogene laherparepvec.8,9
Ninety percent of melanomas are of cutaneous origin. Extracutaneous melanomas may be derived from the uvea, leptomeninges, mucous membranes, and gastrointestinal tract.10 Mucosal melanomas are rare and represent only approximately 1% of all melanomas.11 In order of frequency, primary mucosal melanomas include the head and neck, anorectal region, vulvovaginal region, and urinary tract. UV radiation exposure is an important risk factor for cutaneous melanoma but has not been associated with the development of mucosal melanoma.7 In 2019, Altieri et al11 analyzed 1824 cases of mucosal melanoma and found that anatomic site influences survival because mucosal melanomas in the most occult anatomic sites—spinal/central nervous system, lung and pleura, liver, and pancreas—have the worst prognosis, likely because they have already metastasized by the time they are diagnosed. Due to their occult anatomic location and lack of early presenting signs and symptoms, mucosal melanomas are difficult to diagnose at an early stage, resulting in a poorer prognosis compared with cutaneous melanomas. The most important prognostic indicator for cutaneous melanomas of tumor thickness (ie, Breslow depth) provides less prognostic value for patients with mucosal melanoma. Limitations also include the lack of a standardized staging system for mucosal melanoma, but Altieri et al11 found that poorer survival in patients with mucosal melanoma was observed in relation to stage based on the clinical and pathologic tumor-node-metastasis staging system of the Surveillance, Epidemiology, and End Results program. An aggregate 5-year survival estimate of patients diagnosed with mucosal melanoma is 28%, underscoring that mucosal melanoma is an aggressive melanoma that carries a poor prognosis and warrants a more aggressive treatment approach at the time of diagnosis.11
Common treatment of primary middle ear mucosal melanoma involves a multimodality therapy including surgical oncological resection for most patients. Currently, radiation is in use for adjuvant treatment and definitive therapy in unresectable tumors or patients who are poor surgical candidates. Malignant melanoma traditionally was considered radioresistant, yet considerable variability in responsiveness has been observed both within and between tumors. Although there are no defined indications for adjuvant therapy, it is often administered in advanced or recurrent cases and those with positive or close margins. Chemotherapy generally is reserved for patients with systemic disease. The chemotherapeutic agents that have been used in the treatment of patients with melanoma of the middle ear include the alkylating agents dacarbazine, cisplatin, nimustine, paclitaxel, and temozolomide. Also, chemotherapeutic agents that have been reported in the treatment of melanoma of the middle ear include tamoxifen, the selective estrogen receptor inhibitor, and interferon. Most recently, programed cell death protein 1 inhibitors pembrolizumab and nivolumab have been used in the treatment of middle ear melanoma. Outcomes remain poor with a high rate of mortality. Novel immunotherapeutic agents combined with adjuvant radiotherapy have been proposed to improve disease control and survival rates.3
Data on systemic therapies for mucosal melanomas are limited due to the rarity of the disease. Even with the development of novel therapies, outcomes remain poor for mucosal melanomas, and additional treatment strategies are needed. Although proto-oncogene BRAF mutations occur in 50% to 70% of cutaneous melanomas, these mutations are rare in mucosal melanomas.3 In mucosal melanomas, activating mutations of the cell receptor KIT are identified more frequently.7 Alterations in proto-oncogene KIT have been found in acral, mucosal, and cutaneous melanoma. KIT mutations were found on exons 11 and 13.12 Variability in the biology of KIT is suggested. Treatment of melanomas with the KIT mutations with tyrosine inhibitors imatinib and nilotinib have shown variable benefits.10 In a 2019 study of 44 patients with mucosal melanoma, Moya-Plana et al13 found that in cases of unresectable and/or metastatic disease, immunotherapy with pembrolizumab had a better benefit-risk ratio than immune treatment with ipilimumab, a cytotoxic T-cell lymphocyte-associated protein 4 inhibitor.
Primary malignant melanoma of the middle ear is unusual and difficult to diagnose clinically. These melanomas have a poor prognosis and can have distant metastasis including cutaneous metastasis. We present this case to emphasize the need to be aware that melanoma can arise in the middle ear.
- Ozturk O, Baglam T, Uneri C, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Kulak Burun Bogaz Ihtis Derg. 2006;16:83-86.
- Idris IA, Daud KM, Yusof Z, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Egypt J ENT Allied Sci. 2017;18:307-309.
- Maxwell AK, Takeda H, Gubbels SP. Primary middle ear mucosal melanoma: case report and comprehensive literature review of 21 cases of primary middle ear and eustachian tube melanoma. Ann Otol Rhinol Laryngol. 2018;127:856-863.
- Sadler TW. Ear. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:324-325.
- Jakubovic HR, Akerman AB. Structure and function of skin: development, morphology and physiology. In: Moschella SL, Hurley HJ, eds. Dermatology. Vol 1. WB Saunders Co; 1985:22-23.
- Sadler TW. The axial skeleton. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:133-137.
- Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366-375.
- Abdutaali R, Alkhattib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22-28.
- Skudalski L, Waldeman R, Kerr PE, et al. Melanoma: an update on systemic therapies. J Am Acad Dermatol. 2022;86:515-524.
- Heymann WR. A step toward demystifying melanomas of unknown primary sites. J Am Acad Dermatol. 2018;79:208-209.
- Altieri L, Eguchi M, Peng DH, et al. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol. 2019;81:136-142.
- Volpe VO, Klufas DM, Hegde U, et al. The new paradigm of systemic therapies for metastatic melanoma. J Am Acad Dermatol. 2017;77:356-368.
- Moya-Plana A, Herrera Gomez RG, Rossoni C, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68:1171-1178.
To the Editor:
An 82-year-old man presented to our dermatology clinic for a total-body skin examination due to a recently diagnosed primary melanoma of the left middle ear. He reported pain of the left ear and water behind the left eardrum of 1 year’s duration. An otorhinolaryngologist performed surgery due to the severe mastoiditis. A biopsy of the contents of the left middle ear revealed malignant melanoma. Positron emission tomography–computed tomography revealed the mass was mainly located in the anterior aspect of the left middle ear with suspicion of tumor extension into the bony portion of the eustachian tube. No other disease was present. Prior to presentation to dermatology, gross excision of the left middle ear with removal of additional melanoma was confirmed by biopsy, and further analysis revealed v-Raf murine sarcoma viral oncogene (BRAF) was not detected while cellular proto-oncogene receptor kinase (KIT) mutation was detected on exon 13p (K642E).
The patient had no family history of melanoma. He never smoked and did not have contact with hazardous material. Initial examination at our clinic revealed no other suspicious pigmented lesions. After additional negative workup by the oncologist, the patient was presented to the tumor board, and postoperative radiotherapy was recommended to improve local control. Eight months after the patient’s initial diagnosis of the primary middle ear melanoma, a computed tomography–guided right lung biopsy showed metastatic melanoma. After various treatment modalities were discussed with the patient and his family, he was started on pembrolizumab. After 6 months on pembrolizumab, the patient developed autoimmune pneumonitis and pembrolizumab was discontinued. The patient elected to discontinue treatment and died 6 months later.
Malignant melanoma with primary involvement of the middle ear and mastoid mucosa rarely has been reported.1-3 Primary malignant melanoma of the middle ear mucosa is difficult to diagnose clinically. Difficulty and delay in diagnosis occur because of the location and frequent lack of pathognomonic symptoms of the disease.2 A comprehensive literature review by Maxwell et al3 in 2018 of the 10 reported primary middle ear mucosal melanomas found that patients most commonly presented with otorrhea, aural fullness, and hearing loss. Less common symptoms included otalgia, tinnitus, and facial weakness. Clinical examination revealed patients presented with serous otitis and/or a visible mass within the middle ear or external auditory canal. These melanomas demonstrated particularly poor outcomes, with 70% mortality, 20% local recurrence, and 50% distant metastasis. Distant metastases that occurred with primary middle ear mucosal melanoma include lung, liver, intraparotid, abdomen, and cutaneous metastasis.3
The specific pathophysiologic factors underlying the development of primary malignant melanoma of the middle ear mucosa are not known.2 The middle ear and its components develop from the first and second pharyngeal arches.4 Melanocyte precursors from the neural crest migrate during the seventh or eighth week of embryogenesis. These precursors migrate to the epidermis, various mucosal epithelial, hair follicles, dermis, retina, uveal tract, leptomeninges, inner ear, and other tissues.5 The ossicles of the middle ear develop from the neural crest6 and remain in the mesenchyme until the eighth month, when the surrounding tissue dissolves.4 Cutaneous melanomas arise from the malignant transformation of melanocytes in the skin of neural crest lineage. Noncutaneous melanomas are hypothesized to arise from melanoblasts migrating to noncutaneous organs after neural crest cells undergo an epithelial-mesenchymal translation.7
Melanoma 5-year survival rates vary based on the melanoma disease stage: 98% for stage 1, 90% for stage 2, 70% for stage 3, and 10% for stage 4. Although early-stage disease mainly is treated with surgery, advanced and unresectable disease is managed with different therapeutic options, including BRAF inhibitors such as vemurafenib, dabrafenib mesylate, and encorafenib; immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab; and oncolytic virus such as talimogene laherparepvec.8,9
Ninety percent of melanomas are of cutaneous origin. Extracutaneous melanomas may be derived from the uvea, leptomeninges, mucous membranes, and gastrointestinal tract.10 Mucosal melanomas are rare and represent only approximately 1% of all melanomas.11 In order of frequency, primary mucosal melanomas include the head and neck, anorectal region, vulvovaginal region, and urinary tract. UV radiation exposure is an important risk factor for cutaneous melanoma but has not been associated with the development of mucosal melanoma.7 In 2019, Altieri et al11 analyzed 1824 cases of mucosal melanoma and found that anatomic site influences survival because mucosal melanomas in the most occult anatomic sites—spinal/central nervous system, lung and pleura, liver, and pancreas—have the worst prognosis, likely because they have already metastasized by the time they are diagnosed. Due to their occult anatomic location and lack of early presenting signs and symptoms, mucosal melanomas are difficult to diagnose at an early stage, resulting in a poorer prognosis compared with cutaneous melanomas. The most important prognostic indicator for cutaneous melanomas of tumor thickness (ie, Breslow depth) provides less prognostic value for patients with mucosal melanoma. Limitations also include the lack of a standardized staging system for mucosal melanoma, but Altieri et al11 found that poorer survival in patients with mucosal melanoma was observed in relation to stage based on the clinical and pathologic tumor-node-metastasis staging system of the Surveillance, Epidemiology, and End Results program. An aggregate 5-year survival estimate of patients diagnosed with mucosal melanoma is 28%, underscoring that mucosal melanoma is an aggressive melanoma that carries a poor prognosis and warrants a more aggressive treatment approach at the time of diagnosis.11
Common treatment of primary middle ear mucosal melanoma involves a multimodality therapy including surgical oncological resection for most patients. Currently, radiation is in use for adjuvant treatment and definitive therapy in unresectable tumors or patients who are poor surgical candidates. Malignant melanoma traditionally was considered radioresistant, yet considerable variability in responsiveness has been observed both within and between tumors. Although there are no defined indications for adjuvant therapy, it is often administered in advanced or recurrent cases and those with positive or close margins. Chemotherapy generally is reserved for patients with systemic disease. The chemotherapeutic agents that have been used in the treatment of patients with melanoma of the middle ear include the alkylating agents dacarbazine, cisplatin, nimustine, paclitaxel, and temozolomide. Also, chemotherapeutic agents that have been reported in the treatment of melanoma of the middle ear include tamoxifen, the selective estrogen receptor inhibitor, and interferon. Most recently, programed cell death protein 1 inhibitors pembrolizumab and nivolumab have been used in the treatment of middle ear melanoma. Outcomes remain poor with a high rate of mortality. Novel immunotherapeutic agents combined with adjuvant radiotherapy have been proposed to improve disease control and survival rates.3
Data on systemic therapies for mucosal melanomas are limited due to the rarity of the disease. Even with the development of novel therapies, outcomes remain poor for mucosal melanomas, and additional treatment strategies are needed. Although proto-oncogene BRAF mutations occur in 50% to 70% of cutaneous melanomas, these mutations are rare in mucosal melanomas.3 In mucosal melanomas, activating mutations of the cell receptor KIT are identified more frequently.7 Alterations in proto-oncogene KIT have been found in acral, mucosal, and cutaneous melanoma. KIT mutations were found on exons 11 and 13.12 Variability in the biology of KIT is suggested. Treatment of melanomas with the KIT mutations with tyrosine inhibitors imatinib and nilotinib have shown variable benefits.10 In a 2019 study of 44 patients with mucosal melanoma, Moya-Plana et al13 found that in cases of unresectable and/or metastatic disease, immunotherapy with pembrolizumab had a better benefit-risk ratio than immune treatment with ipilimumab, a cytotoxic T-cell lymphocyte-associated protein 4 inhibitor.
Primary malignant melanoma of the middle ear is unusual and difficult to diagnose clinically. These melanomas have a poor prognosis and can have distant metastasis including cutaneous metastasis. We present this case to emphasize the need to be aware that melanoma can arise in the middle ear.
To the Editor:
An 82-year-old man presented to our dermatology clinic for a total-body skin examination due to a recently diagnosed primary melanoma of the left middle ear. He reported pain of the left ear and water behind the left eardrum of 1 year’s duration. An otorhinolaryngologist performed surgery due to the severe mastoiditis. A biopsy of the contents of the left middle ear revealed malignant melanoma. Positron emission tomography–computed tomography revealed the mass was mainly located in the anterior aspect of the left middle ear with suspicion of tumor extension into the bony portion of the eustachian tube. No other disease was present. Prior to presentation to dermatology, gross excision of the left middle ear with removal of additional melanoma was confirmed by biopsy, and further analysis revealed v-Raf murine sarcoma viral oncogene (BRAF) was not detected while cellular proto-oncogene receptor kinase (KIT) mutation was detected on exon 13p (K642E).
The patient had no family history of melanoma. He never smoked and did not have contact with hazardous material. Initial examination at our clinic revealed no other suspicious pigmented lesions. After additional negative workup by the oncologist, the patient was presented to the tumor board, and postoperative radiotherapy was recommended to improve local control. Eight months after the patient’s initial diagnosis of the primary middle ear melanoma, a computed tomography–guided right lung biopsy showed metastatic melanoma. After various treatment modalities were discussed with the patient and his family, he was started on pembrolizumab. After 6 months on pembrolizumab, the patient developed autoimmune pneumonitis and pembrolizumab was discontinued. The patient elected to discontinue treatment and died 6 months later.
Malignant melanoma with primary involvement of the middle ear and mastoid mucosa rarely has been reported.1-3 Primary malignant melanoma of the middle ear mucosa is difficult to diagnose clinically. Difficulty and delay in diagnosis occur because of the location and frequent lack of pathognomonic symptoms of the disease.2 A comprehensive literature review by Maxwell et al3 in 2018 of the 10 reported primary middle ear mucosal melanomas found that patients most commonly presented with otorrhea, aural fullness, and hearing loss. Less common symptoms included otalgia, tinnitus, and facial weakness. Clinical examination revealed patients presented with serous otitis and/or a visible mass within the middle ear or external auditory canal. These melanomas demonstrated particularly poor outcomes, with 70% mortality, 20% local recurrence, and 50% distant metastasis. Distant metastases that occurred with primary middle ear mucosal melanoma include lung, liver, intraparotid, abdomen, and cutaneous metastasis.3
The specific pathophysiologic factors underlying the development of primary malignant melanoma of the middle ear mucosa are not known.2 The middle ear and its components develop from the first and second pharyngeal arches.4 Melanocyte precursors from the neural crest migrate during the seventh or eighth week of embryogenesis. These precursors migrate to the epidermis, various mucosal epithelial, hair follicles, dermis, retina, uveal tract, leptomeninges, inner ear, and other tissues.5 The ossicles of the middle ear develop from the neural crest6 and remain in the mesenchyme until the eighth month, when the surrounding tissue dissolves.4 Cutaneous melanomas arise from the malignant transformation of melanocytes in the skin of neural crest lineage. Noncutaneous melanomas are hypothesized to arise from melanoblasts migrating to noncutaneous organs after neural crest cells undergo an epithelial-mesenchymal translation.7
Melanoma 5-year survival rates vary based on the melanoma disease stage: 98% for stage 1, 90% for stage 2, 70% for stage 3, and 10% for stage 4. Although early-stage disease mainly is treated with surgery, advanced and unresectable disease is managed with different therapeutic options, including BRAF inhibitors such as vemurafenib, dabrafenib mesylate, and encorafenib; immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab; and oncolytic virus such as talimogene laherparepvec.8,9
Ninety percent of melanomas are of cutaneous origin. Extracutaneous melanomas may be derived from the uvea, leptomeninges, mucous membranes, and gastrointestinal tract.10 Mucosal melanomas are rare and represent only approximately 1% of all melanomas.11 In order of frequency, primary mucosal melanomas include the head and neck, anorectal region, vulvovaginal region, and urinary tract. UV radiation exposure is an important risk factor for cutaneous melanoma but has not been associated with the development of mucosal melanoma.7 In 2019, Altieri et al11 analyzed 1824 cases of mucosal melanoma and found that anatomic site influences survival because mucosal melanomas in the most occult anatomic sites—spinal/central nervous system, lung and pleura, liver, and pancreas—have the worst prognosis, likely because they have already metastasized by the time they are diagnosed. Due to their occult anatomic location and lack of early presenting signs and symptoms, mucosal melanomas are difficult to diagnose at an early stage, resulting in a poorer prognosis compared with cutaneous melanomas. The most important prognostic indicator for cutaneous melanomas of tumor thickness (ie, Breslow depth) provides less prognostic value for patients with mucosal melanoma. Limitations also include the lack of a standardized staging system for mucosal melanoma, but Altieri et al11 found that poorer survival in patients with mucosal melanoma was observed in relation to stage based on the clinical and pathologic tumor-node-metastasis staging system of the Surveillance, Epidemiology, and End Results program. An aggregate 5-year survival estimate of patients diagnosed with mucosal melanoma is 28%, underscoring that mucosal melanoma is an aggressive melanoma that carries a poor prognosis and warrants a more aggressive treatment approach at the time of diagnosis.11
Common treatment of primary middle ear mucosal melanoma involves a multimodality therapy including surgical oncological resection for most patients. Currently, radiation is in use for adjuvant treatment and definitive therapy in unresectable tumors or patients who are poor surgical candidates. Malignant melanoma traditionally was considered radioresistant, yet considerable variability in responsiveness has been observed both within and between tumors. Although there are no defined indications for adjuvant therapy, it is often administered in advanced or recurrent cases and those with positive or close margins. Chemotherapy generally is reserved for patients with systemic disease. The chemotherapeutic agents that have been used in the treatment of patients with melanoma of the middle ear include the alkylating agents dacarbazine, cisplatin, nimustine, paclitaxel, and temozolomide. Also, chemotherapeutic agents that have been reported in the treatment of melanoma of the middle ear include tamoxifen, the selective estrogen receptor inhibitor, and interferon. Most recently, programed cell death protein 1 inhibitors pembrolizumab and nivolumab have been used in the treatment of middle ear melanoma. Outcomes remain poor with a high rate of mortality. Novel immunotherapeutic agents combined with adjuvant radiotherapy have been proposed to improve disease control and survival rates.3
Data on systemic therapies for mucosal melanomas are limited due to the rarity of the disease. Even with the development of novel therapies, outcomes remain poor for mucosal melanomas, and additional treatment strategies are needed. Although proto-oncogene BRAF mutations occur in 50% to 70% of cutaneous melanomas, these mutations are rare in mucosal melanomas.3 In mucosal melanomas, activating mutations of the cell receptor KIT are identified more frequently.7 Alterations in proto-oncogene KIT have been found in acral, mucosal, and cutaneous melanoma. KIT mutations were found on exons 11 and 13.12 Variability in the biology of KIT is suggested. Treatment of melanomas with the KIT mutations with tyrosine inhibitors imatinib and nilotinib have shown variable benefits.10 In a 2019 study of 44 patients with mucosal melanoma, Moya-Plana et al13 found that in cases of unresectable and/or metastatic disease, immunotherapy with pembrolizumab had a better benefit-risk ratio than immune treatment with ipilimumab, a cytotoxic T-cell lymphocyte-associated protein 4 inhibitor.
Primary malignant melanoma of the middle ear is unusual and difficult to diagnose clinically. These melanomas have a poor prognosis and can have distant metastasis including cutaneous metastasis. We present this case to emphasize the need to be aware that melanoma can arise in the middle ear.
- Ozturk O, Baglam T, Uneri C, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Kulak Burun Bogaz Ihtis Derg. 2006;16:83-86.
- Idris IA, Daud KM, Yusof Z, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Egypt J ENT Allied Sci. 2017;18:307-309.
- Maxwell AK, Takeda H, Gubbels SP. Primary middle ear mucosal melanoma: case report and comprehensive literature review of 21 cases of primary middle ear and eustachian tube melanoma. Ann Otol Rhinol Laryngol. 2018;127:856-863.
- Sadler TW. Ear. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:324-325.
- Jakubovic HR, Akerman AB. Structure and function of skin: development, morphology and physiology. In: Moschella SL, Hurley HJ, eds. Dermatology. Vol 1. WB Saunders Co; 1985:22-23.
- Sadler TW. The axial skeleton. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:133-137.
- Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366-375.
- Abdutaali R, Alkhattib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22-28.
- Skudalski L, Waldeman R, Kerr PE, et al. Melanoma: an update on systemic therapies. J Am Acad Dermatol. 2022;86:515-524.
- Heymann WR. A step toward demystifying melanomas of unknown primary sites. J Am Acad Dermatol. 2018;79:208-209.
- Altieri L, Eguchi M, Peng DH, et al. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol. 2019;81:136-142.
- Volpe VO, Klufas DM, Hegde U, et al. The new paradigm of systemic therapies for metastatic melanoma. J Am Acad Dermatol. 2017;77:356-368.
- Moya-Plana A, Herrera Gomez RG, Rossoni C, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68:1171-1178.
- Ozturk O, Baglam T, Uneri C, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Kulak Burun Bogaz Ihtis Derg. 2006;16:83-86.
- Idris IA, Daud KM, Yusof Z, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Egypt J ENT Allied Sci. 2017;18:307-309.
- Maxwell AK, Takeda H, Gubbels SP. Primary middle ear mucosal melanoma: case report and comprehensive literature review of 21 cases of primary middle ear and eustachian tube melanoma. Ann Otol Rhinol Laryngol. 2018;127:856-863.
- Sadler TW. Ear. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:324-325.
- Jakubovic HR, Akerman AB. Structure and function of skin: development, morphology and physiology. In: Moschella SL, Hurley HJ, eds. Dermatology. Vol 1. WB Saunders Co; 1985:22-23.
- Sadler TW. The axial skeleton. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:133-137.
- Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366-375.
- Abdutaali R, Alkhattib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22-28.
- Skudalski L, Waldeman R, Kerr PE, et al. Melanoma: an update on systemic therapies. J Am Acad Dermatol. 2022;86:515-524.
- Heymann WR. A step toward demystifying melanomas of unknown primary sites. J Am Acad Dermatol. 2018;79:208-209.
- Altieri L, Eguchi M, Peng DH, et al. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol. 2019;81:136-142.
- Volpe VO, Klufas DM, Hegde U, et al. The new paradigm of systemic therapies for metastatic melanoma. J Am Acad Dermatol. 2017;77:356-368.
- Moya-Plana A, Herrera Gomez RG, Rossoni C, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68:1171-1178.
PRACTICE POINTS
- Primary malignant melanoma of the middle ear is rare and has poor prognosis.
- Distant metastasis, including cutaneous metastasis, results from primary middle ear melanoma.
A Trauma-Induced Fatty Mass: The Facts About Posttraumatic Pseudolipomas
To the Editor:
The posttraumatic pseudolipoma (PTL) is a painless localized mass comprised of unencapsulated adipose tissue that develops at the site of acute or prolonged blunt soft tissue trauma. It may be round or fusiform in shape and has areas of saponification leading to fat necrosis.1 Posttraumatic pseudolipomas are 12 times more likely to occur in females, which may be attributed to sex-determined adipose tissue distribution or cosmetic concerns.2 Most PTLs are found in areas of the body with high adiposity, including the hip, thigh, and gluteal regions.3 A patient history of a traumatic event resulting in a hematoma and a subsequent latent period of several months to years before the pseudolipoma formation occurs is common.1,2,4-6
A 27-year-old woman presented to the family medicine clinic for examination of a deformity on the right buttock. She noticed a soft protruding mass months after landing on the buttocks and on top of a stick during routine physical training. Prior ultrasonography of the deformity proved unhelpful in determining the etiology. Physical examination revealed a protruding, 2-cm, flesh-colored mass on the right buttock intergluteal fold that was soft, compressible, and nontender (Figure 1). There was no capsule, nodule, loculation, or sinus tract. The patient underwent excisional resection with findings of benign-appearing unencapsulated adipose tissue (Figure 2). The wound was closed without difficulty. After several weeks, she had a well-healing scar without contour deficits of the buttocks. Two to 3 months after the initial repair, the patient presented to the family medicine clinic with recurrence of the fatty protrusion. She was referred for consultation and definitive management to a plastic surgeon but was lost to follow up.
In a systematic review of the literature to research pathogenesis theories, a PubMed search of articles indexed for MEDLINE using the terms trauma and pseudolipoma, lipoma, fat, or adipose yielded 45 citations, with only 10 publications addressing the pathology specific to pseudolipomas. Two leading theories of the pathogenesis of PTLs include the adipose herniation pathway and the inflammatory proliferation pathway.4,5
Adipose tissue comprises fat lobules that are organized underneath the supportive elastic fascial layers. Injury from forces exceeding the fascial strength is the basis for the oldest pathogenesis theory. The adipose herniation theory suggests that fat lobules are displaced through the damaged septae, allowing for the development of an epidermal pseudolipoma at the site of blunt trauma.7 This theory has been supported by many case reports; however, more recent reports have identified a larger number of PTL cases that showed no identifiable disruptions in the fascia.1,4,8
In 1997, the inflammatory proliferation theory began to gain attention. The theory describes how local tissue trauma leads to the release of inflammatory cytokines, which successively signals the development of preadipocytes or adipose tissue–derived stem cells (ASCs) into mature adipocytes.4 Most patients report a history of a hematoma in the area of pseudolipoma development, which strongly supports this newer theory. Studies exploring hematomas have found elevated levels of growth factors and inflammatory markers.2,9 In particular, tumor necrosis factor α, peroxisome proliferator–activated receptor γ, vascular endothelial growth factor, and IL-6 and IL-8 may foster an environment in which adipogenic cells are both chemotaxed to the area of trauma and differentiated to white adipose tissue.2,10
Despite addressing the role of the preadipocyte, the available research fails to address the general development of mesenchymal cells into the preadipocyte. White adipose tissue develops at sites of neovascularization and frequently has been observed spreading into the nearby tissue toward other blood vessels. Furthermore, these white adipose tissue expansions remain reliant on multiple growth factors and cell-signaling molecules.10 Numerous investigations into stem cell grafting have found that implantation of ASCs in vivo within animal models does not result in the proliferation and differentiation of ASCs unless specific conditions have been met such as prior tissue injury or immunodeficiency.10-12 These investigations support and expand on the inflammatory proliferation pathway. Thus, most of the true PTLs in the available research appear as de novo tumors and are more congruent with the inflammatory proliferation model.1,2,4-6,8
Typical treatment of a PTL is surgical excision or liposuction depending on the pathology and size of the pseudolipoma. Biopsy examination prior to liposuction is critical for evaluation of liposarcoma and may help identify damage to Scarpa fascia. Recurrence of a PTL is rare regardless of treatment method; however, in a study of 31 PTL cases, only 6 were pathologically identified as PTLs without fibrous material.1
Our patient experienced a blunt trauma to the buttocks and subsequently developed a PTL that was surgically excised and recurred within 3 months. Research surrounding the pathogenesis of the PTL has evolved from the theory of physical herniation of adipose tissue to an inflammatory differentiation of preadipocytes, but there is still much to learn about how and why it occurs and the mesenchymal differentiation following tissue injury.
- Aust MC, Spies M, Kall S, et al. Lipomas after blunt soft tissue trauma: are they real? analysis of 31 cases. Br J Dermatol. 2007;157:92-99. doi:10.1111/j.1365-2133.2007.07970.x
- Galea LA, Penington AJ, Morrison WA. Post-traumatic pseudolipomas—a review and postulated mechanisms of their development. J Plast Reconstr Aesthet Surg. 2009;62:737-741. doi:10.1016/j.bjps.2008.12.021
- Zajac JC, Mandelbaum M, Economides JM, et al. Immediate massive posttraumatic pseudolipoma of the buttocks: a case of a heterotopic “love handle.” Plast Reconstr Surg Glob Open. 2018;6:E1887. doi:10.1097/GOX.0000000000001887
- Signorini M, Campiglio GL. Posttraumatic lipomas: where do they really come from? Plast Reconstr Surg. 1998;101:699-705. doi:10.1097/00006534-199803000-00017
- Khadilkar AS, Goyal A, Gauba K. The enigma of “traumatic pseudolipoma” and “traumatic herniation of buccal fat pad”: a systematic review and new classification system of post-traumatic craniofacial fatty masses. J Oral Maxillofac Surg. 2018;76:1267-1278. doi:10.1016/j.joms.2017.01.024
- Copcu E, Sivrioglu NS. Posttraumatic lipoma: analysis of 10 cases and explanation of possible mechanisms. Dermatol Surg. 2003;29:215-220. doi:10.1046/j.1524-4725.2003.29052.x
- Penoff JH. Traumatic lipomas/pseudolipomas. J Trauma. 1982;22:63-65. doi:10.1097/00005373-198201000-00013
- Theumann N, Abdelmoumene A, Wintermark M, et al. Posttraumatic pseudolipoma: MRI appearances. Eur Radiol. 2005;15:1876-1880. doi:10.1007/s00330-005-2757-2
- David LR, DeFranzo A, Marks M, et al. Posttraumatic pseudolipoma. J Trauma. 1996;40:396-400. doi:10.1097/00005373-199603000-00012
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227-246. doi:10.1194/jlr.R021089
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153-163. doi:10.1038/ncb2015
- Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. doi:10.1161/01.Cir.0000135466.16823.D0
To the Editor:
The posttraumatic pseudolipoma (PTL) is a painless localized mass comprised of unencapsulated adipose tissue that develops at the site of acute or prolonged blunt soft tissue trauma. It may be round or fusiform in shape and has areas of saponification leading to fat necrosis.1 Posttraumatic pseudolipomas are 12 times more likely to occur in females, which may be attributed to sex-determined adipose tissue distribution or cosmetic concerns.2 Most PTLs are found in areas of the body with high adiposity, including the hip, thigh, and gluteal regions.3 A patient history of a traumatic event resulting in a hematoma and a subsequent latent period of several months to years before the pseudolipoma formation occurs is common.1,2,4-6
A 27-year-old woman presented to the family medicine clinic for examination of a deformity on the right buttock. She noticed a soft protruding mass months after landing on the buttocks and on top of a stick during routine physical training. Prior ultrasonography of the deformity proved unhelpful in determining the etiology. Physical examination revealed a protruding, 2-cm, flesh-colored mass on the right buttock intergluteal fold that was soft, compressible, and nontender (Figure 1). There was no capsule, nodule, loculation, or sinus tract. The patient underwent excisional resection with findings of benign-appearing unencapsulated adipose tissue (Figure 2). The wound was closed without difficulty. After several weeks, she had a well-healing scar without contour deficits of the buttocks. Two to 3 months after the initial repair, the patient presented to the family medicine clinic with recurrence of the fatty protrusion. She was referred for consultation and definitive management to a plastic surgeon but was lost to follow up.

In a systematic review of the literature to research pathogenesis theories, a PubMed search of articles indexed for MEDLINE using the terms trauma and pseudolipoma, lipoma, fat, or adipose yielded 45 citations, with only 10 publications addressing the pathology specific to pseudolipomas. Two leading theories of the pathogenesis of PTLs include the adipose herniation pathway and the inflammatory proliferation pathway.4,5

Adipose tissue comprises fat lobules that are organized underneath the supportive elastic fascial layers. Injury from forces exceeding the fascial strength is the basis for the oldest pathogenesis theory. The adipose herniation theory suggests that fat lobules are displaced through the damaged septae, allowing for the development of an epidermal pseudolipoma at the site of blunt trauma.7 This theory has been supported by many case reports; however, more recent reports have identified a larger number of PTL cases that showed no identifiable disruptions in the fascia.1,4,8
In 1997, the inflammatory proliferation theory began to gain attention. The theory describes how local tissue trauma leads to the release of inflammatory cytokines, which successively signals the development of preadipocytes or adipose tissue–derived stem cells (ASCs) into mature adipocytes.4 Most patients report a history of a hematoma in the area of pseudolipoma development, which strongly supports this newer theory. Studies exploring hematomas have found elevated levels of growth factors and inflammatory markers.2,9 In particular, tumor necrosis factor α, peroxisome proliferator–activated receptor γ, vascular endothelial growth factor, and IL-6 and IL-8 may foster an environment in which adipogenic cells are both chemotaxed to the area of trauma and differentiated to white adipose tissue.2,10
Despite addressing the role of the preadipocyte, the available research fails to address the general development of mesenchymal cells into the preadipocyte. White adipose tissue develops at sites of neovascularization and frequently has been observed spreading into the nearby tissue toward other blood vessels. Furthermore, these white adipose tissue expansions remain reliant on multiple growth factors and cell-signaling molecules.10 Numerous investigations into stem cell grafting have found that implantation of ASCs in vivo within animal models does not result in the proliferation and differentiation of ASCs unless specific conditions have been met such as prior tissue injury or immunodeficiency.10-12 These investigations support and expand on the inflammatory proliferation pathway. Thus, most of the true PTLs in the available research appear as de novo tumors and are more congruent with the inflammatory proliferation model.1,2,4-6,8
Typical treatment of a PTL is surgical excision or liposuction depending on the pathology and size of the pseudolipoma. Biopsy examination prior to liposuction is critical for evaluation of liposarcoma and may help identify damage to Scarpa fascia. Recurrence of a PTL is rare regardless of treatment method; however, in a study of 31 PTL cases, only 6 were pathologically identified as PTLs without fibrous material.1
Our patient experienced a blunt trauma to the buttocks and subsequently developed a PTL that was surgically excised and recurred within 3 months. Research surrounding the pathogenesis of the PTL has evolved from the theory of physical herniation of adipose tissue to an inflammatory differentiation of preadipocytes, but there is still much to learn about how and why it occurs and the mesenchymal differentiation following tissue injury.
To the Editor:
The posttraumatic pseudolipoma (PTL) is a painless localized mass comprised of unencapsulated adipose tissue that develops at the site of acute or prolonged blunt soft tissue trauma. It may be round or fusiform in shape and has areas of saponification leading to fat necrosis.1 Posttraumatic pseudolipomas are 12 times more likely to occur in females, which may be attributed to sex-determined adipose tissue distribution or cosmetic concerns.2 Most PTLs are found in areas of the body with high adiposity, including the hip, thigh, and gluteal regions.3 A patient history of a traumatic event resulting in a hematoma and a subsequent latent period of several months to years before the pseudolipoma formation occurs is common.1,2,4-6
A 27-year-old woman presented to the family medicine clinic for examination of a deformity on the right buttock. She noticed a soft protruding mass months after landing on the buttocks and on top of a stick during routine physical training. Prior ultrasonography of the deformity proved unhelpful in determining the etiology. Physical examination revealed a protruding, 2-cm, flesh-colored mass on the right buttock intergluteal fold that was soft, compressible, and nontender (Figure 1). There was no capsule, nodule, loculation, or sinus tract. The patient underwent excisional resection with findings of benign-appearing unencapsulated adipose tissue (Figure 2). The wound was closed without difficulty. After several weeks, she had a well-healing scar without contour deficits of the buttocks. Two to 3 months after the initial repair, the patient presented to the family medicine clinic with recurrence of the fatty protrusion. She was referred for consultation and definitive management to a plastic surgeon but was lost to follow up.

In a systematic review of the literature to research pathogenesis theories, a PubMed search of articles indexed for MEDLINE using the terms trauma and pseudolipoma, lipoma, fat, or adipose yielded 45 citations, with only 10 publications addressing the pathology specific to pseudolipomas. Two leading theories of the pathogenesis of PTLs include the adipose herniation pathway and the inflammatory proliferation pathway.4,5

Adipose tissue comprises fat lobules that are organized underneath the supportive elastic fascial layers. Injury from forces exceeding the fascial strength is the basis for the oldest pathogenesis theory. The adipose herniation theory suggests that fat lobules are displaced through the damaged septae, allowing for the development of an epidermal pseudolipoma at the site of blunt trauma.7 This theory has been supported by many case reports; however, more recent reports have identified a larger number of PTL cases that showed no identifiable disruptions in the fascia.1,4,8
In 1997, the inflammatory proliferation theory began to gain attention. The theory describes how local tissue trauma leads to the release of inflammatory cytokines, which successively signals the development of preadipocytes or adipose tissue–derived stem cells (ASCs) into mature adipocytes.4 Most patients report a history of a hematoma in the area of pseudolipoma development, which strongly supports this newer theory. Studies exploring hematomas have found elevated levels of growth factors and inflammatory markers.2,9 In particular, tumor necrosis factor α, peroxisome proliferator–activated receptor γ, vascular endothelial growth factor, and IL-6 and IL-8 may foster an environment in which adipogenic cells are both chemotaxed to the area of trauma and differentiated to white adipose tissue.2,10
Despite addressing the role of the preadipocyte, the available research fails to address the general development of mesenchymal cells into the preadipocyte. White adipose tissue develops at sites of neovascularization and frequently has been observed spreading into the nearby tissue toward other blood vessels. Furthermore, these white adipose tissue expansions remain reliant on multiple growth factors and cell-signaling molecules.10 Numerous investigations into stem cell grafting have found that implantation of ASCs in vivo within animal models does not result in the proliferation and differentiation of ASCs unless specific conditions have been met such as prior tissue injury or immunodeficiency.10-12 These investigations support and expand on the inflammatory proliferation pathway. Thus, most of the true PTLs in the available research appear as de novo tumors and are more congruent with the inflammatory proliferation model.1,2,4-6,8
Typical treatment of a PTL is surgical excision or liposuction depending on the pathology and size of the pseudolipoma. Biopsy examination prior to liposuction is critical for evaluation of liposarcoma and may help identify damage to Scarpa fascia. Recurrence of a PTL is rare regardless of treatment method; however, in a study of 31 PTL cases, only 6 were pathologically identified as PTLs without fibrous material.1
Our patient experienced a blunt trauma to the buttocks and subsequently developed a PTL that was surgically excised and recurred within 3 months. Research surrounding the pathogenesis of the PTL has evolved from the theory of physical herniation of adipose tissue to an inflammatory differentiation of preadipocytes, but there is still much to learn about how and why it occurs and the mesenchymal differentiation following tissue injury.
- Aust MC, Spies M, Kall S, et al. Lipomas after blunt soft tissue trauma: are they real? analysis of 31 cases. Br J Dermatol. 2007;157:92-99. doi:10.1111/j.1365-2133.2007.07970.x
- Galea LA, Penington AJ, Morrison WA. Post-traumatic pseudolipomas—a review and postulated mechanisms of their development. J Plast Reconstr Aesthet Surg. 2009;62:737-741. doi:10.1016/j.bjps.2008.12.021
- Zajac JC, Mandelbaum M, Economides JM, et al. Immediate massive posttraumatic pseudolipoma of the buttocks: a case of a heterotopic “love handle.” Plast Reconstr Surg Glob Open. 2018;6:E1887. doi:10.1097/GOX.0000000000001887
- Signorini M, Campiglio GL. Posttraumatic lipomas: where do they really come from? Plast Reconstr Surg. 1998;101:699-705. doi:10.1097/00006534-199803000-00017
- Khadilkar AS, Goyal A, Gauba K. The enigma of “traumatic pseudolipoma” and “traumatic herniation of buccal fat pad”: a systematic review and new classification system of post-traumatic craniofacial fatty masses. J Oral Maxillofac Surg. 2018;76:1267-1278. doi:10.1016/j.joms.2017.01.024
- Copcu E, Sivrioglu NS. Posttraumatic lipoma: analysis of 10 cases and explanation of possible mechanisms. Dermatol Surg. 2003;29:215-220. doi:10.1046/j.1524-4725.2003.29052.x
- Penoff JH. Traumatic lipomas/pseudolipomas. J Trauma. 1982;22:63-65. doi:10.1097/00005373-198201000-00013
- Theumann N, Abdelmoumene A, Wintermark M, et al. Posttraumatic pseudolipoma: MRI appearances. Eur Radiol. 2005;15:1876-1880. doi:10.1007/s00330-005-2757-2
- David LR, DeFranzo A, Marks M, et al. Posttraumatic pseudolipoma. J Trauma. 1996;40:396-400. doi:10.1097/00005373-199603000-00012
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227-246. doi:10.1194/jlr.R021089
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153-163. doi:10.1038/ncb2015
- Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. doi:10.1161/01.Cir.0000135466.16823.D0
- Aust MC, Spies M, Kall S, et al. Lipomas after blunt soft tissue trauma: are they real? analysis of 31 cases. Br J Dermatol. 2007;157:92-99. doi:10.1111/j.1365-2133.2007.07970.x
- Galea LA, Penington AJ, Morrison WA. Post-traumatic pseudolipomas—a review and postulated mechanisms of their development. J Plast Reconstr Aesthet Surg. 2009;62:737-741. doi:10.1016/j.bjps.2008.12.021
- Zajac JC, Mandelbaum M, Economides JM, et al. Immediate massive posttraumatic pseudolipoma of the buttocks: a case of a heterotopic “love handle.” Plast Reconstr Surg Glob Open. 2018;6:E1887. doi:10.1097/GOX.0000000000001887
- Signorini M, Campiglio GL. Posttraumatic lipomas: where do they really come from? Plast Reconstr Surg. 1998;101:699-705. doi:10.1097/00006534-199803000-00017
- Khadilkar AS, Goyal A, Gauba K. The enigma of “traumatic pseudolipoma” and “traumatic herniation of buccal fat pad”: a systematic review and new classification system of post-traumatic craniofacial fatty masses. J Oral Maxillofac Surg. 2018;76:1267-1278. doi:10.1016/j.joms.2017.01.024
- Copcu E, Sivrioglu NS. Posttraumatic lipoma: analysis of 10 cases and explanation of possible mechanisms. Dermatol Surg. 2003;29:215-220. doi:10.1046/j.1524-4725.2003.29052.x
- Penoff JH. Traumatic lipomas/pseudolipomas. J Trauma. 1982;22:63-65. doi:10.1097/00005373-198201000-00013
- Theumann N, Abdelmoumene A, Wintermark M, et al. Posttraumatic pseudolipoma: MRI appearances. Eur Radiol. 2005;15:1876-1880. doi:10.1007/s00330-005-2757-2
- David LR, DeFranzo A, Marks M, et al. Posttraumatic pseudolipoma. J Trauma. 1996;40:396-400. doi:10.1097/00005373-199603000-00012
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227-246. doi:10.1194/jlr.R021089
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153-163. doi:10.1038/ncb2015
- Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. doi:10.1161/01.Cir.0000135466.16823.D0
Practice Points
- Physicians should include pseudolipoma in the differential diagnosis when evaluating masses that develop in patients at sites of blunt or prolonged trauma.
- A pseudolipoma is an unencapsulated, round, or fusiform fatty mass that differs from a traditional lipoma by the absence of a capsule.
- Further research may elucidate the pathogenesis of these adiposities.
Dupilumab as a Therapeutic Approach in Alopecia Universalis
To the Editor:
Atopic diseases, specifically atopic dermatitis (AD) and alopecia areata (AA), are at the forefront of a new era in dermatology involving molecular-directed therapy. Dupilumab is one specific example, having received US Food and Drug Administration approval in March 2017 for the treatment of adults with moderate to severe AD.1 It currently is being investigated for use in pediatric AD. The most commonly reported side effects associated with the use of dupilumab include headaches, conjunctivitis, keratitis, blepharitis, nasopharyngitis, and injection-site reactions.2 We discuss a case of hair regrowth in a patient who was previously diagnosed with AA after treatment with dupilumab for refractory AD.
A 65-year-old White man presented with a history of AD since childhood. Additional medical history included hyperlipidemia; herpes simplex virus infection; asthma; and a diagnosis of AA 6 years prior, which eventually progressed to alopecia universalis. Physical examination demonstrated scattered erythematous lichenified plaques with excoriations involving the arms, legs, and trunk. The patient’s face and scalp were spared of lesions. Complete loss of body hair including the eyelashes and eyebrows also was noted, which was consistent with alopecia universalis.
The patient was started on dupilumab for refractory AD after multiple courses of topical and systemic steroids failed. Prior treatment for AD did not include immunosuppressive or light therapy. The standard dosage of dupilumab was administered, which consisted of a 600-mg subcutaneous loading dose, followed by 300 mg every 2 weeks. There was no concurrent topical corticosteroid or topical calcineurin inhibitor prescribed. After 1 month of treatment with dupilumab, near-complete resolution of the patient’s AD was noted, and after 10 months of treatment, the patient experienced regrowth of the eyelashes, terminal hairs of the beard area (Figure), and vellus hairs of the eyebrows. This hair regrowth persists today with continued dupilumab treatment, and the patient has experienced no additional side effects.

Multiple retrospective and meta-analysis studies have demonstrated a high occurrence of AD comorbid with AA, which strongly suggests a common pathogenesis.3,4 Atopic dermatitis is an inflammatory skin disease mediated by IL-4, IL-5, and IL-13 of the helper T-cell type 2 (TH2) pathway.1 Dupilumab is a human monoclonal antibody that binds to IL-4Rα, which also is found in IL-13 receptors. Dupilumab prevents TH2 pathway-related downstream signaling effects of both cytokines. Although this effect was originally utilized to suppress the TH2-mediated signaling in AD, our patient and others have demonstrated successful hair regrowth with dupilumab, which likely stems from a similar TH2-related antagonism in AA.5,6
The cause of AA is unknown, but IL-4 and IL-13 of the TH2 pathway have been implicated, which renders support for the therapeutic effect of dupilumab in the treatment of AA. Scalp samples of patients with AA have demonstrated upregulation of TH2, helper T-cell type 1 (TH1), and IL-23 cytokines, suggesting efficacy with the use of anti-TH2, anti-TH1, and anti–IL-23 therapies.7 Polymerase chain reaction testing performed on serum samples in patients with AA displayed marked elevation of TH2 cytokines, notably IL-13, which were reduced following intralesional corticosteroid treatment.8 It also has been demonstrated that multiple TH2-related genes contribute to the genetic susceptibility of developing AA, specifically IL-4 and IL-13.9,10
Prior case reports have shown contradicting effects (dupilumab-induced AA), which are speculated to be caused by a stronger TH1 response from TH2 suppression.11,12 In one report, dupilumab was initiated for AD refractory to multiple topical and oral interventions. New-onset hair loss to the scalp was noted after 18 weeks of therapy. Twenty-six weeks into therapy with dupilumab, full hair regrowth was then reported.11 Despite this report, our patient’s hair regrowth after the use of dupilumab for refractory AD further strengthens support for the use of dupilumab as a potential therapy for alopecia universalis and other lymphocyte-mediated hair loss conditions. However, a large disparity in response time and an overall slower progression of hair regrowth reported in our case separate it from other reports of rapid voluminous hair regrowth.5,6 Our findings support the potential use of dupilumab in the treatment of patients with AA.
- Shirly M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Ou Z, Chen C, Chen A, et al. Adverse events of dupilumab in adults with moderate-to-severe atopic dermatitis: a meta-analysis. Int Immunopharmacol. 2018;54:303-310.
- Andersen YMF, Egeberg A, Gislason GH, et al. Autoimmune diseases in adults with atopic dermatitis. J Am Acad Dermatol. 2017;76:274-280.e1.
- Mohan GC, Silverberg JI. Association of vitiligo and alopecia areata with atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol. 2015;15:522-528.
- Penzi LR, Yasuda M, Manatis-Lornell A, et al. Hair regrowth in a patient with long-standing alopecia totalis and atopic dermatitis treated with dupilumab. JAMA Dermatol. 2018;154:1358-1360.
- Alniemi DT, McGevna L. Dupilumab treatment for atopic dermatitis leading to unexpected treatment for alopecia universalis. JAAD Case Rep. 2019;5:111-112.
- Suárez-Fariñas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136:1277-1287.
- Fuentes-Duculan J, Gulati N, Bonifacio KM, et al. Biomarkers of alopecia areata disease activity and response to corticosteroid treatment. Exp Dermatol. 2016;25:282-286.
- Jagielska D, Redler S, Brockschmidt FF, et al. Follow-up study of the first genome-wide association scan in alopecia areata: IL13 and KIAA0350 as susceptibility loci supported with genome-wide significance. J Invest Dermatol. 2012;132:2192-2197.
- Kalkan G, Karakus N, Bas¸ Y, et al. The association between interleukin (IL)-4 gene intron 3 VNTR polymorphism and alopecia areata (AA) in Turkish population. Gene. 2013;527:565-569.
- Flanagan K, Sperling L, Lin J. Drug-induced alopecia after dupilumab therapy. JAAD Case Rep. 2019;5:54-56.
- Mitchell K, Levitt J. Alopecia areata after dupilumab for atopic dermatitis. JAAD Case Rep. 2018;4:143-144.
To the Editor:
Atopic diseases, specifically atopic dermatitis (AD) and alopecia areata (AA), are at the forefront of a new era in dermatology involving molecular-directed therapy. Dupilumab is one specific example, having received US Food and Drug Administration approval in March 2017 for the treatment of adults with moderate to severe AD.1 It currently is being investigated for use in pediatric AD. The most commonly reported side effects associated with the use of dupilumab include headaches, conjunctivitis, keratitis, blepharitis, nasopharyngitis, and injection-site reactions.2 We discuss a case of hair regrowth in a patient who was previously diagnosed with AA after treatment with dupilumab for refractory AD.
A 65-year-old White man presented with a history of AD since childhood. Additional medical history included hyperlipidemia; herpes simplex virus infection; asthma; and a diagnosis of AA 6 years prior, which eventually progressed to alopecia universalis. Physical examination demonstrated scattered erythematous lichenified plaques with excoriations involving the arms, legs, and trunk. The patient’s face and scalp were spared of lesions. Complete loss of body hair including the eyelashes and eyebrows also was noted, which was consistent with alopecia universalis.
The patient was started on dupilumab for refractory AD after multiple courses of topical and systemic steroids failed. Prior treatment for AD did not include immunosuppressive or light therapy. The standard dosage of dupilumab was administered, which consisted of a 600-mg subcutaneous loading dose, followed by 300 mg every 2 weeks. There was no concurrent topical corticosteroid or topical calcineurin inhibitor prescribed. After 1 month of treatment with dupilumab, near-complete resolution of the patient’s AD was noted, and after 10 months of treatment, the patient experienced regrowth of the eyelashes, terminal hairs of the beard area (Figure), and vellus hairs of the eyebrows. This hair regrowth persists today with continued dupilumab treatment, and the patient has experienced no additional side effects.

Multiple retrospective and meta-analysis studies have demonstrated a high occurrence of AD comorbid with AA, which strongly suggests a common pathogenesis.3,4 Atopic dermatitis is an inflammatory skin disease mediated by IL-4, IL-5, and IL-13 of the helper T-cell type 2 (TH2) pathway.1 Dupilumab is a human monoclonal antibody that binds to IL-4Rα, which also is found in IL-13 receptors. Dupilumab prevents TH2 pathway-related downstream signaling effects of both cytokines. Although this effect was originally utilized to suppress the TH2-mediated signaling in AD, our patient and others have demonstrated successful hair regrowth with dupilumab, which likely stems from a similar TH2-related antagonism in AA.5,6
The cause of AA is unknown, but IL-4 and IL-13 of the TH2 pathway have been implicated, which renders support for the therapeutic effect of dupilumab in the treatment of AA. Scalp samples of patients with AA have demonstrated upregulation of TH2, helper T-cell type 1 (TH1), and IL-23 cytokines, suggesting efficacy with the use of anti-TH2, anti-TH1, and anti–IL-23 therapies.7 Polymerase chain reaction testing performed on serum samples in patients with AA displayed marked elevation of TH2 cytokines, notably IL-13, which were reduced following intralesional corticosteroid treatment.8 It also has been demonstrated that multiple TH2-related genes contribute to the genetic susceptibility of developing AA, specifically IL-4 and IL-13.9,10
Prior case reports have shown contradicting effects (dupilumab-induced AA), which are speculated to be caused by a stronger TH1 response from TH2 suppression.11,12 In one report, dupilumab was initiated for AD refractory to multiple topical and oral interventions. New-onset hair loss to the scalp was noted after 18 weeks of therapy. Twenty-six weeks into therapy with dupilumab, full hair regrowth was then reported.11 Despite this report, our patient’s hair regrowth after the use of dupilumab for refractory AD further strengthens support for the use of dupilumab as a potential therapy for alopecia universalis and other lymphocyte-mediated hair loss conditions. However, a large disparity in response time and an overall slower progression of hair regrowth reported in our case separate it from other reports of rapid voluminous hair regrowth.5,6 Our findings support the potential use of dupilumab in the treatment of patients with AA.
To the Editor:
Atopic diseases, specifically atopic dermatitis (AD) and alopecia areata (AA), are at the forefront of a new era in dermatology involving molecular-directed therapy. Dupilumab is one specific example, having received US Food and Drug Administration approval in March 2017 for the treatment of adults with moderate to severe AD.1 It currently is being investigated for use in pediatric AD. The most commonly reported side effects associated with the use of dupilumab include headaches, conjunctivitis, keratitis, blepharitis, nasopharyngitis, and injection-site reactions.2 We discuss a case of hair regrowth in a patient who was previously diagnosed with AA after treatment with dupilumab for refractory AD.
A 65-year-old White man presented with a history of AD since childhood. Additional medical history included hyperlipidemia; herpes simplex virus infection; asthma; and a diagnosis of AA 6 years prior, which eventually progressed to alopecia universalis. Physical examination demonstrated scattered erythematous lichenified plaques with excoriations involving the arms, legs, and trunk. The patient’s face and scalp were spared of lesions. Complete loss of body hair including the eyelashes and eyebrows also was noted, which was consistent with alopecia universalis.
The patient was started on dupilumab for refractory AD after multiple courses of topical and systemic steroids failed. Prior treatment for AD did not include immunosuppressive or light therapy. The standard dosage of dupilumab was administered, which consisted of a 600-mg subcutaneous loading dose, followed by 300 mg every 2 weeks. There was no concurrent topical corticosteroid or topical calcineurin inhibitor prescribed. After 1 month of treatment with dupilumab, near-complete resolution of the patient’s AD was noted, and after 10 months of treatment, the patient experienced regrowth of the eyelashes, terminal hairs of the beard area (Figure), and vellus hairs of the eyebrows. This hair regrowth persists today with continued dupilumab treatment, and the patient has experienced no additional side effects.

Multiple retrospective and meta-analysis studies have demonstrated a high occurrence of AD comorbid with AA, which strongly suggests a common pathogenesis.3,4 Atopic dermatitis is an inflammatory skin disease mediated by IL-4, IL-5, and IL-13 of the helper T-cell type 2 (TH2) pathway.1 Dupilumab is a human monoclonal antibody that binds to IL-4Rα, which also is found in IL-13 receptors. Dupilumab prevents TH2 pathway-related downstream signaling effects of both cytokines. Although this effect was originally utilized to suppress the TH2-mediated signaling in AD, our patient and others have demonstrated successful hair regrowth with dupilumab, which likely stems from a similar TH2-related antagonism in AA.5,6
The cause of AA is unknown, but IL-4 and IL-13 of the TH2 pathway have been implicated, which renders support for the therapeutic effect of dupilumab in the treatment of AA. Scalp samples of patients with AA have demonstrated upregulation of TH2, helper T-cell type 1 (TH1), and IL-23 cytokines, suggesting efficacy with the use of anti-TH2, anti-TH1, and anti–IL-23 therapies.7 Polymerase chain reaction testing performed on serum samples in patients with AA displayed marked elevation of TH2 cytokines, notably IL-13, which were reduced following intralesional corticosteroid treatment.8 It also has been demonstrated that multiple TH2-related genes contribute to the genetic susceptibility of developing AA, specifically IL-4 and IL-13.9,10
Prior case reports have shown contradicting effects (dupilumab-induced AA), which are speculated to be caused by a stronger TH1 response from TH2 suppression.11,12 In one report, dupilumab was initiated for AD refractory to multiple topical and oral interventions. New-onset hair loss to the scalp was noted after 18 weeks of therapy. Twenty-six weeks into therapy with dupilumab, full hair regrowth was then reported.11 Despite this report, our patient’s hair regrowth after the use of dupilumab for refractory AD further strengthens support for the use of dupilumab as a potential therapy for alopecia universalis and other lymphocyte-mediated hair loss conditions. However, a large disparity in response time and an overall slower progression of hair regrowth reported in our case separate it from other reports of rapid voluminous hair regrowth.5,6 Our findings support the potential use of dupilumab in the treatment of patients with AA.
- Shirly M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Ou Z, Chen C, Chen A, et al. Adverse events of dupilumab in adults with moderate-to-severe atopic dermatitis: a meta-analysis. Int Immunopharmacol. 2018;54:303-310.
- Andersen YMF, Egeberg A, Gislason GH, et al. Autoimmune diseases in adults with atopic dermatitis. J Am Acad Dermatol. 2017;76:274-280.e1.
- Mohan GC, Silverberg JI. Association of vitiligo and alopecia areata with atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol. 2015;15:522-528.
- Penzi LR, Yasuda M, Manatis-Lornell A, et al. Hair regrowth in a patient with long-standing alopecia totalis and atopic dermatitis treated with dupilumab. JAMA Dermatol. 2018;154:1358-1360.
- Alniemi DT, McGevna L. Dupilumab treatment for atopic dermatitis leading to unexpected treatment for alopecia universalis. JAAD Case Rep. 2019;5:111-112.
- Suárez-Fariñas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136:1277-1287.
- Fuentes-Duculan J, Gulati N, Bonifacio KM, et al. Biomarkers of alopecia areata disease activity and response to corticosteroid treatment. Exp Dermatol. 2016;25:282-286.
- Jagielska D, Redler S, Brockschmidt FF, et al. Follow-up study of the first genome-wide association scan in alopecia areata: IL13 and KIAA0350 as susceptibility loci supported with genome-wide significance. J Invest Dermatol. 2012;132:2192-2197.
- Kalkan G, Karakus N, Bas¸ Y, et al. The association between interleukin (IL)-4 gene intron 3 VNTR polymorphism and alopecia areata (AA) in Turkish population. Gene. 2013;527:565-569.
- Flanagan K, Sperling L, Lin J. Drug-induced alopecia after dupilumab therapy. JAAD Case Rep. 2019;5:54-56.
- Mitchell K, Levitt J. Alopecia areata after dupilumab for atopic dermatitis. JAAD Case Rep. 2018;4:143-144.
- Shirly M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Ou Z, Chen C, Chen A, et al. Adverse events of dupilumab in adults with moderate-to-severe atopic dermatitis: a meta-analysis. Int Immunopharmacol. 2018;54:303-310.
- Andersen YMF, Egeberg A, Gislason GH, et al. Autoimmune diseases in adults with atopic dermatitis. J Am Acad Dermatol. 2017;76:274-280.e1.
- Mohan GC, Silverberg JI. Association of vitiligo and alopecia areata with atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol. 2015;15:522-528.
- Penzi LR, Yasuda M, Manatis-Lornell A, et al. Hair regrowth in a patient with long-standing alopecia totalis and atopic dermatitis treated with dupilumab. JAMA Dermatol. 2018;154:1358-1360.
- Alniemi DT, McGevna L. Dupilumab treatment for atopic dermatitis leading to unexpected treatment for alopecia universalis. JAAD Case Rep. 2019;5:111-112.
- Suárez-Fariñas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136:1277-1287.
- Fuentes-Duculan J, Gulati N, Bonifacio KM, et al. Biomarkers of alopecia areata disease activity and response to corticosteroid treatment. Exp Dermatol. 2016;25:282-286.
- Jagielska D, Redler S, Brockschmidt FF, et al. Follow-up study of the first genome-wide association scan in alopecia areata: IL13 and KIAA0350 as susceptibility loci supported with genome-wide significance. J Invest Dermatol. 2012;132:2192-2197.
- Kalkan G, Karakus N, Bas¸ Y, et al. The association between interleukin (IL)-4 gene intron 3 VNTR polymorphism and alopecia areata (AA) in Turkish population. Gene. 2013;527:565-569.
- Flanagan K, Sperling L, Lin J. Drug-induced alopecia after dupilumab therapy. JAAD Case Rep. 2019;5:54-56.
- Mitchell K, Levitt J. Alopecia areata after dupilumab for atopic dermatitis. JAAD Case Rep. 2018;4:143-144.
Practice Points
- Practicing dermatologists should be aware of the shared pathophysiology of alopecia areata and atopic dermatitis and the relief patients with these conditions can experience when treated with dupilumab.
- As molecular-directed biologic therapies emerge in the marketplace, their potential for targeting one atopic disease may offer notable benefits for use in the treatment of other atopic diseases.
Psoriasiform Dermatitis Associated With the Moderna COVID-19 Messenger RNA Vaccine
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.

A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.

Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).

The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.

A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.

Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).

The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.

A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.

Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).

The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
PRACTICE POINTS
- The differential diagnosis for a new-onset psoriasiform rash in an elderly patient should include a vaccine-related rash.
- A rash following vaccination that necessitates systemic corticosteroid therapy can decrease vaccine efficacy.
Hyaluronidase for Skin Necrosis Induced by Amiodarone
To the Editor:
Amiodarone is an oral or intravenous (IV) drug commonly used to treat supraventricular and ventricular arrhythmia as well as atrial fibrillation.1 Adverse drug reactions associated with the use of amiodarone include pulmonary, gastrointestinal, thyroid, ocular, neurologic, and cutaneous reactions.1 Long-term use of amiodarone—typically more than 4 months—can lead to slate-gray skin discoloration and photosensitivity, both of which can be reversed with drug withdrawal.2,3 Phlebitis also has been described in less than 3% of patients who receive peripheral IV administration of amiodarone.4
Amiodarone-induced skin necrosis due to extravasation is a rare complication of this antiarrhythmic medication, with only 3 reported cases in the literature according to a PubMed search of articles indexed for MEDLINE using the search terms amiodarone and skin and (necrosis or ischemia or extravasation or reaction).5–7 Although hyaluronidase is a known therapy for extravasation of fluids, including parenteral nutrition and chemotherapy, its use for the treatment of extravasation from amiodarone is not well documented.6 We report a case of skin necrosis of the left dorsal forearm and the left dorsal and ventral hand following infusion of amiodarone through a peripheral IV line, which was treated with injections of hyaluronidase.
A 77-year-old man was admitted to the emergency department for sepsis secondary to cholangitis in the setting of an obstructive gallbladder stone. His medical history was notable for multivessel coronary artery disease and atrial flutter treated with ablation. One day after admission, endoscopic retrograde cholangiopancreatography was attempted and aborted due to atrial fibrillation with rapid ventricular response. A second endoscopic retrograde cholangiopancreatography attempt was made 4 days later, during which the patient underwent cardiac arrest. During this event, amiodarone was administered in a 200-mL solution (1.8 mg/mL) in 5% dextrose through a peripheral IV line in the left forearm. The patient was stabilized and transferred to the intensive care unit.
Twenty-four hours after amiodarone administration, erythema was noted on the left dorsal forearm. Within hours, the digits of the hand became a dark, dusky color, which spread to involve the forearm. Surgical debridement was not deemed necessary; the left arm was elevated, and warm compresses were applied regularly. Within the next week, the skin of the left hand and dorsal forearm had progressively worsened and took on a well-demarcated, dusky blue hue surrounded by an erythematous border involving the proximal forearm and upper arm (Figure 1A). The skin was fragile and had overlying bullae (Figure 1B).
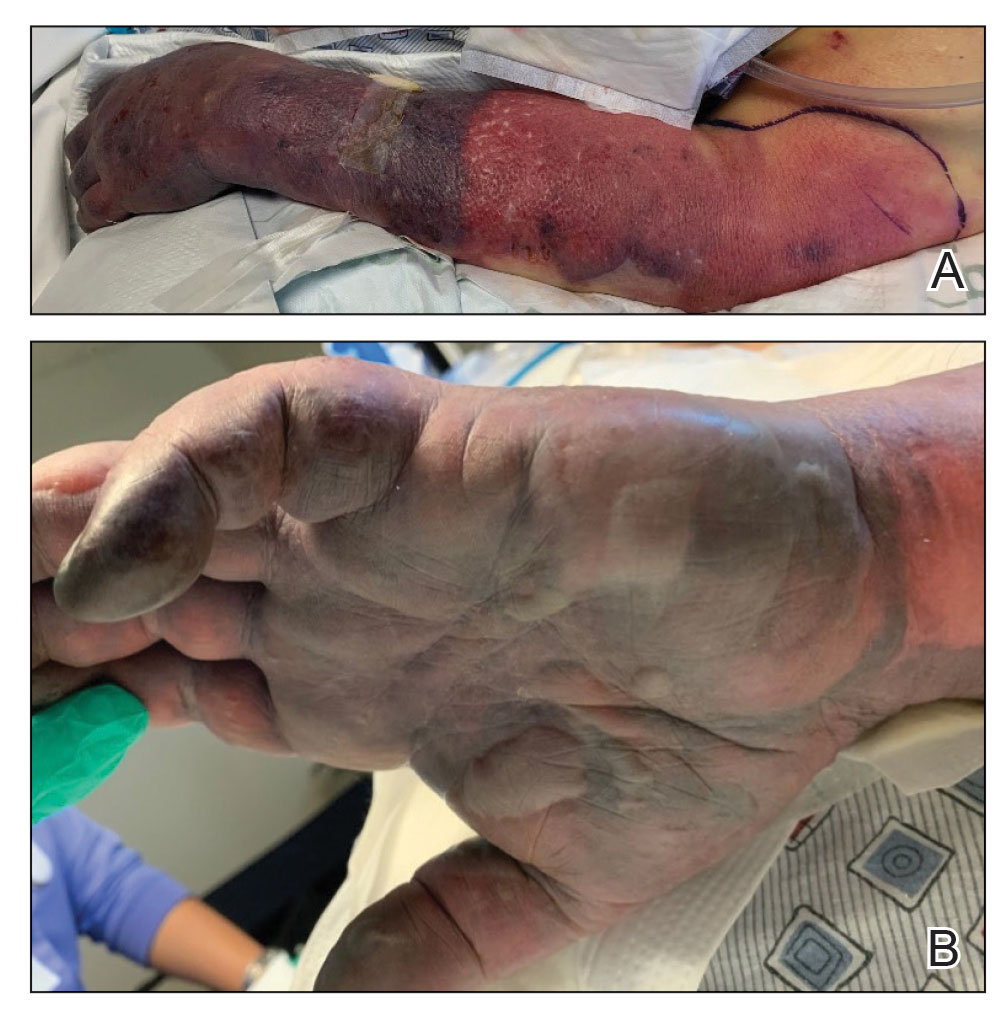
Hyaluronidase (1000 U) was injected into the surrounding areas of erythema, which resolved from the left proximal forearm to the elbow within 2 days after injection (Figure 2). The dusky violaceous patches were persistent, and the necrotic bullae were unchanged. Hyaluronidase (1000 U) was injected into necrotic skin of the left dorsal forearm and dorsal and ventral hand. No improvement was noted on subsequent evaluations of this area. While still an inpatient, he received wound care and twice-daily Doppler ultrasounds in the areas of necrosis. The patient lost sensation in the left hand with increased soft tissue necrosis and developed an eschar on the left dorsal forearm. Due to the progressive loss of function and necrosis, a partial forearm amputation was performed that healed well, and the patient experienced improvement in range of motion of the left upper extremity.
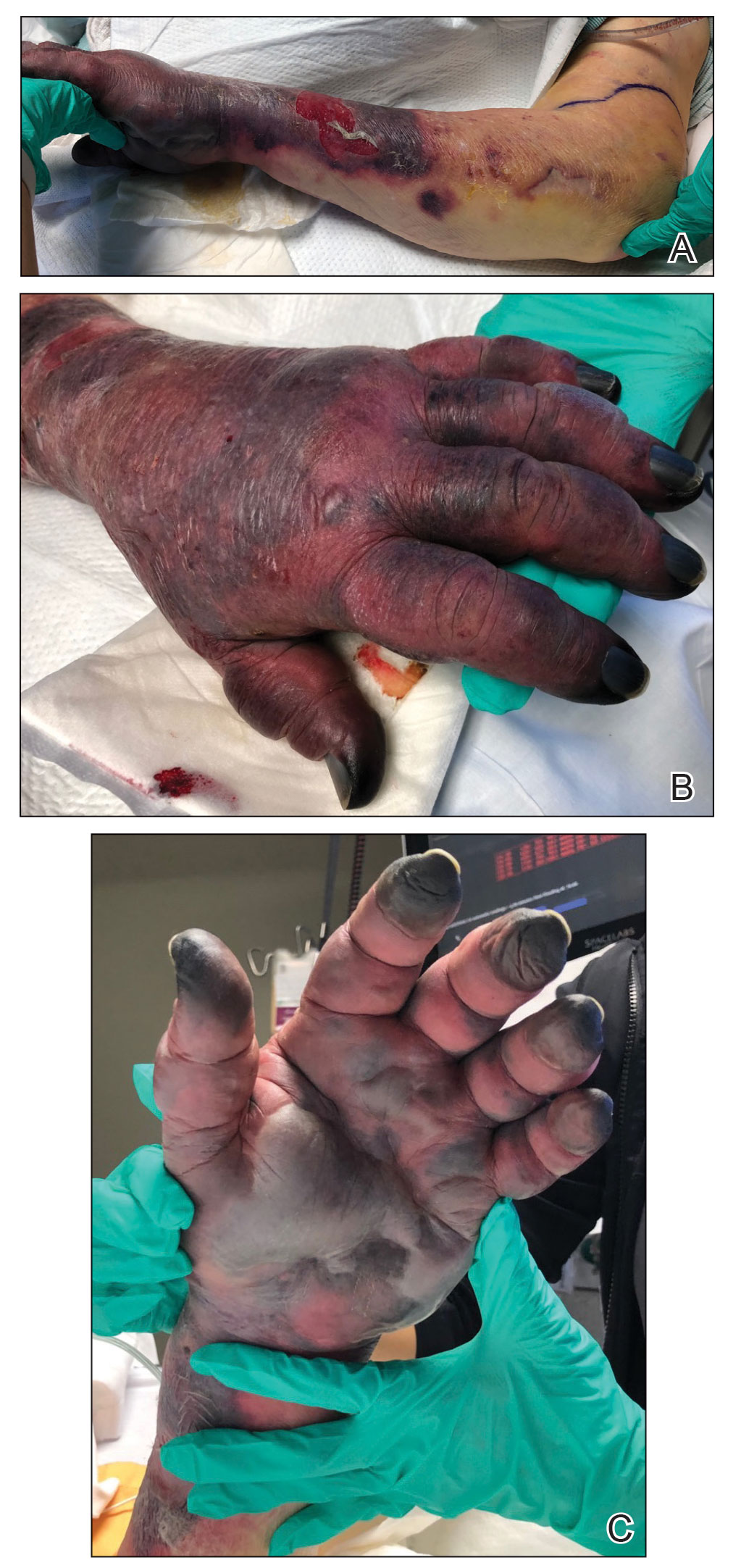
Well-known adverse reactions of amiodarone treatment include pulmonary fibrosis, hepatic dysfunction, hypothyroidism and hyperthyroidism, peripheral neuropathy, and corneal deposits.1 Cutaneous adverse reactions include photosensitivity (phototoxic and photoallergic reactions), hyperpigmentation, pseudoporphyria, and linear IgA bullous dermatosis. Less commonly, it also can cause urticaria, pruritus, erythema nodosum, purpura, and toxic epidermal necrolysis.3 Amiodarone-induced skin necrosis is rare, first described by Russell and Saltissi5 in 2006 in a 60-year-old man who developed dark discoloration and edema of the forearm 24 hours after initiation of an amiodarone peripheral IV. The patient was treated with hot or cold packs and steroid cream per the pharmaceutical company’s recommendations; however, patient outcomes were not discussed.5 A 77-year-old man who received subcutaneous amiodarone due to misplaced vascular access developed edema and bullae of the forearm followed by tissue necrosis, resulting in notably reduced mobility.6 Fox et al7 described a 60-year-old man who developed atrial fibrillation after emergent spinal fusion and laminectomy. He received intradermal hyaluronidase administration within 24 hours of developing severe pain from extravasation induced by amiodarone with no adverse outcomes and full recovery.7
There are numerous properties of amiodarone that may have resulted in the skin necrosis seen in these cases. The acidic pH (3.5–4.5) of amiodarone can contribute to coagulative necrosis, cellular desiccation, eschar formation, and edema.8 It also can contain additives such as polysorbate and benzyl alcohol, which may contribute to the drug’s vesicant properties.9
Current recommendations for IV administration of amiodarone include delivery through a central vein with high concentrations (>2 mg/mL) because peripheral infusion is slower and may cause phlebitis.4 In-line filters also may be a potential method of preventing phlebitis with peripheral IV administration of amiodarone.10 Extravasation of amiodarone can be treated nonpharmacologically with limb elevation and warm compresses, as these methods may promote vasodilation and enhance drug removal.5-7 However, when extravasation leads to progressive erythema and skin necrosis or is refractory to these therapies, intradermal injection of hyaluronidase should be considered. Hyaluronidase mediates the degradation of hyaluronic acid in the extracellular matrix, allowing for increased permeability of injected fluids into tissues and diluting the concentration of toxins at the site of exposure.9,11 It has been used to treat extravasation of fluids such as parenteral nutrition, electrolyte infusion, antibiotics, aminophylline, mannitol, and chemotherapy.11 Although hyaluronidase has been recognized as therapeutic for extravasation, there is no established consistent dosing or proper technique. In the setting of infiltration of chemotherapy, doses of hyaluronidase ranging from 150 to 1500 U/mL can be subcutaneously or intradermally injected into the site within 1 hour of extravasation. Side effects of using hyaluronidase are rare, including local pruritus, allergic reactions, urticaria, and angioedema.12
The patient described by Fox et al7 who fully recovered from amiodarone extravasation after hyaluronidase injections likely benefited from quick intervention, as he received amiodarone within 24 hours of the care team identifying initial erythema. Although our patient did have improvement of the areas of erythema on the forearm, evidence of skin and subcutaneous tissue necrosis on the left hand and proximal forearm was already apparent and not reversible, most likely caused by late intervention of intradermal hyaluronidase almost a week after the extravasation event. It is important to identify amiodarone as the source of extravasation and administer intradermal hyaluronidase in a timely fashion for extravasation refractory to conventional measurements to prevent progression to severe tissue damage.
Our case draws attention to the risk for skin necrosis with peripheral IV administration of amiodarone. Interventions include limb elevation, warm compresses, and consideration of intradermal hyaluronidase within 24 hours of extravasation, as this may reduce the severity of subsequent tissue damage with minimal side effects.
- Epstein AE, Olshansky B, Naccarelli GV, et al. Practical management guide for clinicians who treat patients with amiodarone. Am J Med. 2016;129:468-475. doi:10.1016/j.amjmed.2015.08.039
- Harris L, McKenna WJ, Rowland E, et al. Side effects of long-term amiodarone therapy. Circulation. 1983;67:45-51. doi:10.1161/01.cir.67.1.45
- Jaworski K, Walecka I, Rudnicka L, et al. Cutaneous adverse reactions of amiodarone. Med Sci Monit. 2014;20:2369-2372. doi:10.12659/MSM.890881
- Kowey Peter R, Marinchak Roger A, Rials Seth J, et al. Intravenous amiodarone. J Am Coll Cardiol. 1997;29:1190-1198. doi:10.1016/S0735-1097(97)00069-7
- Russell SJ, Saltissi S. Amiodarone induced skin necrosis. Heart. 2006;92:1395. doi:10.1136/hrt.2005.086157
- Grove EL. Skin necrosis and consequences of accidental subcutaneous administration of amiodarone. Ugeskr Laeger. 2015;177:V66928.
- Fox AN, Villanueva R, Miller JL. Management of amiodarone extravasation with intradermal hyaluronidase. Am J Health Syst Pharm. 2017;74:1545-1548. doi:10.2146/ajhp160737
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632. doi:https://doi.org/10.1002/phar.1396
- Le A, Patel S. Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014;48:870-886. doi:10.1177/1060028014527820
- Slim AM, Roth JE, Duffy B, et al. The incidence of phlebitis with intravenous amiodarone at guideline dose recommendations. Mil Med. 2007;172:1279-1283.
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921-1943. doi:10.1016/j.lfs.2007.02.037
- Jung H. Hyaluronidase: an overview of its properties, applications, and side effects. Arch Plast Surg. 2020;47:297-300. doi:10.5999/aps.2020.00752
To the Editor:
Amiodarone is an oral or intravenous (IV) drug commonly used to treat supraventricular and ventricular arrhythmia as well as atrial fibrillation.1 Adverse drug reactions associated with the use of amiodarone include pulmonary, gastrointestinal, thyroid, ocular, neurologic, and cutaneous reactions.1 Long-term use of amiodarone—typically more than 4 months—can lead to slate-gray skin discoloration and photosensitivity, both of which can be reversed with drug withdrawal.2,3 Phlebitis also has been described in less than 3% of patients who receive peripheral IV administration of amiodarone.4
Amiodarone-induced skin necrosis due to extravasation is a rare complication of this antiarrhythmic medication, with only 3 reported cases in the literature according to a PubMed search of articles indexed for MEDLINE using the search terms amiodarone and skin and (necrosis or ischemia or extravasation or reaction).5–7 Although hyaluronidase is a known therapy for extravasation of fluids, including parenteral nutrition and chemotherapy, its use for the treatment of extravasation from amiodarone is not well documented.6 We report a case of skin necrosis of the left dorsal forearm and the left dorsal and ventral hand following infusion of amiodarone through a peripheral IV line, which was treated with injections of hyaluronidase.
A 77-year-old man was admitted to the emergency department for sepsis secondary to cholangitis in the setting of an obstructive gallbladder stone. His medical history was notable for multivessel coronary artery disease and atrial flutter treated with ablation. One day after admission, endoscopic retrograde cholangiopancreatography was attempted and aborted due to atrial fibrillation with rapid ventricular response. A second endoscopic retrograde cholangiopancreatography attempt was made 4 days later, during which the patient underwent cardiac arrest. During this event, amiodarone was administered in a 200-mL solution (1.8 mg/mL) in 5% dextrose through a peripheral IV line in the left forearm. The patient was stabilized and transferred to the intensive care unit.
Twenty-four hours after amiodarone administration, erythema was noted on the left dorsal forearm. Within hours, the digits of the hand became a dark, dusky color, which spread to involve the forearm. Surgical debridement was not deemed necessary; the left arm was elevated, and warm compresses were applied regularly. Within the next week, the skin of the left hand and dorsal forearm had progressively worsened and took on a well-demarcated, dusky blue hue surrounded by an erythematous border involving the proximal forearm and upper arm (Figure 1A). The skin was fragile and had overlying bullae (Figure 1B).

Hyaluronidase (1000 U) was injected into the surrounding areas of erythema, which resolved from the left proximal forearm to the elbow within 2 days after injection (Figure 2). The dusky violaceous patches were persistent, and the necrotic bullae were unchanged. Hyaluronidase (1000 U) was injected into necrotic skin of the left dorsal forearm and dorsal and ventral hand. No improvement was noted on subsequent evaluations of this area. While still an inpatient, he received wound care and twice-daily Doppler ultrasounds in the areas of necrosis. The patient lost sensation in the left hand with increased soft tissue necrosis and developed an eschar on the left dorsal forearm. Due to the progressive loss of function and necrosis, a partial forearm amputation was performed that healed well, and the patient experienced improvement in range of motion of the left upper extremity.

Well-known adverse reactions of amiodarone treatment include pulmonary fibrosis, hepatic dysfunction, hypothyroidism and hyperthyroidism, peripheral neuropathy, and corneal deposits.1 Cutaneous adverse reactions include photosensitivity (phototoxic and photoallergic reactions), hyperpigmentation, pseudoporphyria, and linear IgA bullous dermatosis. Less commonly, it also can cause urticaria, pruritus, erythema nodosum, purpura, and toxic epidermal necrolysis.3 Amiodarone-induced skin necrosis is rare, first described by Russell and Saltissi5 in 2006 in a 60-year-old man who developed dark discoloration and edema of the forearm 24 hours after initiation of an amiodarone peripheral IV. The patient was treated with hot or cold packs and steroid cream per the pharmaceutical company’s recommendations; however, patient outcomes were not discussed.5 A 77-year-old man who received subcutaneous amiodarone due to misplaced vascular access developed edema and bullae of the forearm followed by tissue necrosis, resulting in notably reduced mobility.6 Fox et al7 described a 60-year-old man who developed atrial fibrillation after emergent spinal fusion and laminectomy. He received intradermal hyaluronidase administration within 24 hours of developing severe pain from extravasation induced by amiodarone with no adverse outcomes and full recovery.7
There are numerous properties of amiodarone that may have resulted in the skin necrosis seen in these cases. The acidic pH (3.5–4.5) of amiodarone can contribute to coagulative necrosis, cellular desiccation, eschar formation, and edema.8 It also can contain additives such as polysorbate and benzyl alcohol, which may contribute to the drug’s vesicant properties.9
Current recommendations for IV administration of amiodarone include delivery through a central vein with high concentrations (>2 mg/mL) because peripheral infusion is slower and may cause phlebitis.4 In-line filters also may be a potential method of preventing phlebitis with peripheral IV administration of amiodarone.10 Extravasation of amiodarone can be treated nonpharmacologically with limb elevation and warm compresses, as these methods may promote vasodilation and enhance drug removal.5-7 However, when extravasation leads to progressive erythema and skin necrosis or is refractory to these therapies, intradermal injection of hyaluronidase should be considered. Hyaluronidase mediates the degradation of hyaluronic acid in the extracellular matrix, allowing for increased permeability of injected fluids into tissues and diluting the concentration of toxins at the site of exposure.9,11 It has been used to treat extravasation of fluids such as parenteral nutrition, electrolyte infusion, antibiotics, aminophylline, mannitol, and chemotherapy.11 Although hyaluronidase has been recognized as therapeutic for extravasation, there is no established consistent dosing or proper technique. In the setting of infiltration of chemotherapy, doses of hyaluronidase ranging from 150 to 1500 U/mL can be subcutaneously or intradermally injected into the site within 1 hour of extravasation. Side effects of using hyaluronidase are rare, including local pruritus, allergic reactions, urticaria, and angioedema.12
The patient described by Fox et al7 who fully recovered from amiodarone extravasation after hyaluronidase injections likely benefited from quick intervention, as he received amiodarone within 24 hours of the care team identifying initial erythema. Although our patient did have improvement of the areas of erythema on the forearm, evidence of skin and subcutaneous tissue necrosis on the left hand and proximal forearm was already apparent and not reversible, most likely caused by late intervention of intradermal hyaluronidase almost a week after the extravasation event. It is important to identify amiodarone as the source of extravasation and administer intradermal hyaluronidase in a timely fashion for extravasation refractory to conventional measurements to prevent progression to severe tissue damage.
Our case draws attention to the risk for skin necrosis with peripheral IV administration of amiodarone. Interventions include limb elevation, warm compresses, and consideration of intradermal hyaluronidase within 24 hours of extravasation, as this may reduce the severity of subsequent tissue damage with minimal side effects.
To the Editor:
Amiodarone is an oral or intravenous (IV) drug commonly used to treat supraventricular and ventricular arrhythmia as well as atrial fibrillation.1 Adverse drug reactions associated with the use of amiodarone include pulmonary, gastrointestinal, thyroid, ocular, neurologic, and cutaneous reactions.1 Long-term use of amiodarone—typically more than 4 months—can lead to slate-gray skin discoloration and photosensitivity, both of which can be reversed with drug withdrawal.2,3 Phlebitis also has been described in less than 3% of patients who receive peripheral IV administration of amiodarone.4
Amiodarone-induced skin necrosis due to extravasation is a rare complication of this antiarrhythmic medication, with only 3 reported cases in the literature according to a PubMed search of articles indexed for MEDLINE using the search terms amiodarone and skin and (necrosis or ischemia or extravasation or reaction).5–7 Although hyaluronidase is a known therapy for extravasation of fluids, including parenteral nutrition and chemotherapy, its use for the treatment of extravasation from amiodarone is not well documented.6 We report a case of skin necrosis of the left dorsal forearm and the left dorsal and ventral hand following infusion of amiodarone through a peripheral IV line, which was treated with injections of hyaluronidase.
A 77-year-old man was admitted to the emergency department for sepsis secondary to cholangitis in the setting of an obstructive gallbladder stone. His medical history was notable for multivessel coronary artery disease and atrial flutter treated with ablation. One day after admission, endoscopic retrograde cholangiopancreatography was attempted and aborted due to atrial fibrillation with rapid ventricular response. A second endoscopic retrograde cholangiopancreatography attempt was made 4 days later, during which the patient underwent cardiac arrest. During this event, amiodarone was administered in a 200-mL solution (1.8 mg/mL) in 5% dextrose through a peripheral IV line in the left forearm. The patient was stabilized and transferred to the intensive care unit.
Twenty-four hours after amiodarone administration, erythema was noted on the left dorsal forearm. Within hours, the digits of the hand became a dark, dusky color, which spread to involve the forearm. Surgical debridement was not deemed necessary; the left arm was elevated, and warm compresses were applied regularly. Within the next week, the skin of the left hand and dorsal forearm had progressively worsened and took on a well-demarcated, dusky blue hue surrounded by an erythematous border involving the proximal forearm and upper arm (Figure 1A). The skin was fragile and had overlying bullae (Figure 1B).

Hyaluronidase (1000 U) was injected into the surrounding areas of erythema, which resolved from the left proximal forearm to the elbow within 2 days after injection (Figure 2). The dusky violaceous patches were persistent, and the necrotic bullae were unchanged. Hyaluronidase (1000 U) was injected into necrotic skin of the left dorsal forearm and dorsal and ventral hand. No improvement was noted on subsequent evaluations of this area. While still an inpatient, he received wound care and twice-daily Doppler ultrasounds in the areas of necrosis. The patient lost sensation in the left hand with increased soft tissue necrosis and developed an eschar on the left dorsal forearm. Due to the progressive loss of function and necrosis, a partial forearm amputation was performed that healed well, and the patient experienced improvement in range of motion of the left upper extremity.

Well-known adverse reactions of amiodarone treatment include pulmonary fibrosis, hepatic dysfunction, hypothyroidism and hyperthyroidism, peripheral neuropathy, and corneal deposits.1 Cutaneous adverse reactions include photosensitivity (phototoxic and photoallergic reactions), hyperpigmentation, pseudoporphyria, and linear IgA bullous dermatosis. Less commonly, it also can cause urticaria, pruritus, erythema nodosum, purpura, and toxic epidermal necrolysis.3 Amiodarone-induced skin necrosis is rare, first described by Russell and Saltissi5 in 2006 in a 60-year-old man who developed dark discoloration and edema of the forearm 24 hours after initiation of an amiodarone peripheral IV. The patient was treated with hot or cold packs and steroid cream per the pharmaceutical company’s recommendations; however, patient outcomes were not discussed.5 A 77-year-old man who received subcutaneous amiodarone due to misplaced vascular access developed edema and bullae of the forearm followed by tissue necrosis, resulting in notably reduced mobility.6 Fox et al7 described a 60-year-old man who developed atrial fibrillation after emergent spinal fusion and laminectomy. He received intradermal hyaluronidase administration within 24 hours of developing severe pain from extravasation induced by amiodarone with no adverse outcomes and full recovery.7
There are numerous properties of amiodarone that may have resulted in the skin necrosis seen in these cases. The acidic pH (3.5–4.5) of amiodarone can contribute to coagulative necrosis, cellular desiccation, eschar formation, and edema.8 It also can contain additives such as polysorbate and benzyl alcohol, which may contribute to the drug’s vesicant properties.9
Current recommendations for IV administration of amiodarone include delivery through a central vein with high concentrations (>2 mg/mL) because peripheral infusion is slower and may cause phlebitis.4 In-line filters also may be a potential method of preventing phlebitis with peripheral IV administration of amiodarone.10 Extravasation of amiodarone can be treated nonpharmacologically with limb elevation and warm compresses, as these methods may promote vasodilation and enhance drug removal.5-7 However, when extravasation leads to progressive erythema and skin necrosis or is refractory to these therapies, intradermal injection of hyaluronidase should be considered. Hyaluronidase mediates the degradation of hyaluronic acid in the extracellular matrix, allowing for increased permeability of injected fluids into tissues and diluting the concentration of toxins at the site of exposure.9,11 It has been used to treat extravasation of fluids such as parenteral nutrition, electrolyte infusion, antibiotics, aminophylline, mannitol, and chemotherapy.11 Although hyaluronidase has been recognized as therapeutic for extravasation, there is no established consistent dosing or proper technique. In the setting of infiltration of chemotherapy, doses of hyaluronidase ranging from 150 to 1500 U/mL can be subcutaneously or intradermally injected into the site within 1 hour of extravasation. Side effects of using hyaluronidase are rare, including local pruritus, allergic reactions, urticaria, and angioedema.12
The patient described by Fox et al7 who fully recovered from amiodarone extravasation after hyaluronidase injections likely benefited from quick intervention, as he received amiodarone within 24 hours of the care team identifying initial erythema. Although our patient did have improvement of the areas of erythema on the forearm, evidence of skin and subcutaneous tissue necrosis on the left hand and proximal forearm was already apparent and not reversible, most likely caused by late intervention of intradermal hyaluronidase almost a week after the extravasation event. It is important to identify amiodarone as the source of extravasation and administer intradermal hyaluronidase in a timely fashion for extravasation refractory to conventional measurements to prevent progression to severe tissue damage.
Our case draws attention to the risk for skin necrosis with peripheral IV administration of amiodarone. Interventions include limb elevation, warm compresses, and consideration of intradermal hyaluronidase within 24 hours of extravasation, as this may reduce the severity of subsequent tissue damage with minimal side effects.
- Epstein AE, Olshansky B, Naccarelli GV, et al. Practical management guide for clinicians who treat patients with amiodarone. Am J Med. 2016;129:468-475. doi:10.1016/j.amjmed.2015.08.039
- Harris L, McKenna WJ, Rowland E, et al. Side effects of long-term amiodarone therapy. Circulation. 1983;67:45-51. doi:10.1161/01.cir.67.1.45
- Jaworski K, Walecka I, Rudnicka L, et al. Cutaneous adverse reactions of amiodarone. Med Sci Monit. 2014;20:2369-2372. doi:10.12659/MSM.890881
- Kowey Peter R, Marinchak Roger A, Rials Seth J, et al. Intravenous amiodarone. J Am Coll Cardiol. 1997;29:1190-1198. doi:10.1016/S0735-1097(97)00069-7
- Russell SJ, Saltissi S. Amiodarone induced skin necrosis. Heart. 2006;92:1395. doi:10.1136/hrt.2005.086157
- Grove EL. Skin necrosis and consequences of accidental subcutaneous administration of amiodarone. Ugeskr Laeger. 2015;177:V66928.
- Fox AN, Villanueva R, Miller JL. Management of amiodarone extravasation with intradermal hyaluronidase. Am J Health Syst Pharm. 2017;74:1545-1548. doi:10.2146/ajhp160737
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632. doi:https://doi.org/10.1002/phar.1396
- Le A, Patel S. Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014;48:870-886. doi:10.1177/1060028014527820
- Slim AM, Roth JE, Duffy B, et al. The incidence of phlebitis with intravenous amiodarone at guideline dose recommendations. Mil Med. 2007;172:1279-1283.
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921-1943. doi:10.1016/j.lfs.2007.02.037
- Jung H. Hyaluronidase: an overview of its properties, applications, and side effects. Arch Plast Surg. 2020;47:297-300. doi:10.5999/aps.2020.00752
- Epstein AE, Olshansky B, Naccarelli GV, et al. Practical management guide for clinicians who treat patients with amiodarone. Am J Med. 2016;129:468-475. doi:10.1016/j.amjmed.2015.08.039
- Harris L, McKenna WJ, Rowland E, et al. Side effects of long-term amiodarone therapy. Circulation. 1983;67:45-51. doi:10.1161/01.cir.67.1.45
- Jaworski K, Walecka I, Rudnicka L, et al. Cutaneous adverse reactions of amiodarone. Med Sci Monit. 2014;20:2369-2372. doi:10.12659/MSM.890881
- Kowey Peter R, Marinchak Roger A, Rials Seth J, et al. Intravenous amiodarone. J Am Coll Cardiol. 1997;29:1190-1198. doi:10.1016/S0735-1097(97)00069-7
- Russell SJ, Saltissi S. Amiodarone induced skin necrosis. Heart. 2006;92:1395. doi:10.1136/hrt.2005.086157
- Grove EL. Skin necrosis and consequences of accidental subcutaneous administration of amiodarone. Ugeskr Laeger. 2015;177:V66928.
- Fox AN, Villanueva R, Miller JL. Management of amiodarone extravasation with intradermal hyaluronidase. Am J Health Syst Pharm. 2017;74:1545-1548. doi:10.2146/ajhp160737
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632. doi:https://doi.org/10.1002/phar.1396
- Le A, Patel S. Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014;48:870-886. doi:10.1177/1060028014527820
- Slim AM, Roth JE, Duffy B, et al. The incidence of phlebitis with intravenous amiodarone at guideline dose recommendations. Mil Med. 2007;172:1279-1283.
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921-1943. doi:10.1016/j.lfs.2007.02.037
- Jung H. Hyaluronidase: an overview of its properties, applications, and side effects. Arch Plast Surg. 2020;47:297-300. doi:10.5999/aps.2020.00752
Practice Points
- Intravenous amiodarone administered peripherally can induce skin extravasation, leading to necrosis.
- Dermatologists should be aware that early intervention with intradermal hyaluronidase may reduce the severity of tissue damage caused by amiodarone-induced skin necrosis.
Genital Lentiginosis: A Benign Pigmentary Abnormality Often Raising Concern for Melanoma
To the Editor:
Genital lentiginosis (also known as mucosal melanotic macules, vulvar melanosis, penile melanosis, and penile lentigines) occurs in men and women.1 Lesions present in adult life as multifocal, asymmetrical, pigmented patches that can have a mottled appearance or exhibit skip areas. The irregular appearance of the pigmented areas often raises concern for melanoma. Biopsy reveals increased pigmentation along the basal layer of the epidermis; the irregular distribution of single melanocytes and pagetoid spread typical of melanoma in situ is not identified.
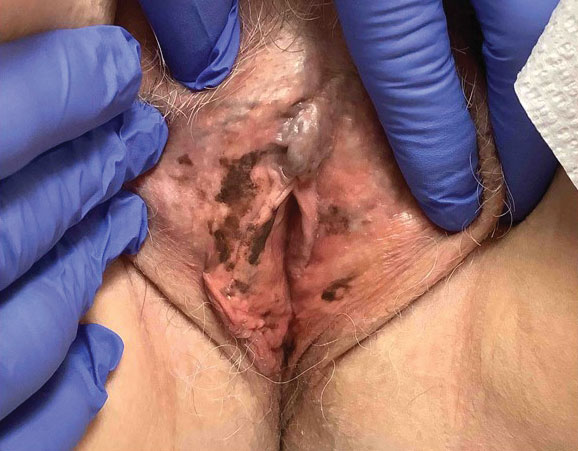
Genital lentiginosis usually occurs as an isolated finding; however, the condition can be a manifestation of Laugier-Hunziker syndrome, Carney complex, and Bannayan-Riley-Ruvalcaba syndrome.1-3 When it occurs as an isolated finding, the patient can be reassured and treatment is unnecessary. Because genital lentiginosis may mimic the appearance of melanoma, it is important for physicians to differentiate the two and make a correct diagnosis. We present a case of genital lentiginosis that mimicked vulvar melanoma.
A 64-year-old woman was referred by her gynecologist to dermatology to rule out vulvar melanoma. The patient had a history of hypothyroidism and hypercholesterolemia but was otherwise in good health. Genital examination revealed asymptomatic pigmented macules and patches of unknown duration (Figure 1). Specimens were taken from 3 areas by punch biopsy to clarify the diagnosis. All 3 specimens showed identical features including basilar pigmentation, occasional melanophages in the papillary dermis, and no evidence of nests or pagetoid spread of atypical melanocytes (Figures 2 and 3). Histologic findings were diagnostic for genital lentiginosis. The patient was reassured, and no treatment was provided. At 6-month follow-up there was no change in clinical appearance.
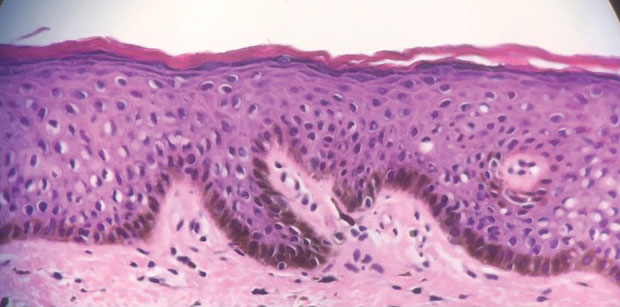
Genital lentiginosis is characterized by brown lesions that can have a mottled appearance and often are associated with skip areas.1 Lesions can be strikingly irregular and darkly pigmented.
Although the lesions of genital lentiginosis most often are isolated findings, they can be a clue to several uncommon syndromes such as autosomal-dominant Bannayan-Riley-Ruvalcaba syndrome, which is associated with genital lentiginosis, intestinal polyposis, and macrocephaly.3 Vascular malformations, lipomatosis, verrucal keratoses, and acrochordons can occur. Bannayan-Riley-Ruvalcaba syndrome and Cowden syndrome may share genetic linkage; mutations in the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) has been implicated in both syndromes.4 Underlying Carney complex should be excluded when genital lentiginosis is encountered.
Genital lentiginosis is idiopathic in most instances, but reports of lesions occurring after annular lichen planus suggest a possible mechanism.5 The disappearance of lentigines after imatinib therapy suggests a role for c-kit, a receptor tyrosine kinase that is involved in intracellular signaling, in some cases.6 At times, lesions can simulate trichrome vitiligo or have a reticulate pattern.7
Men and women present at different points in the course of disease. Men often present with penile lesions 14 years after onset, on average; they notice a gradual increase in the size of lesions. Because women can have greater difficulty self-examining the genital region, they tend to present much later in the course but often within a few months after initial inspection.1,8
Genital lentiginosis can mimic melanoma with nonhomogeneous pigmentation, asymmetry, and unilateral distribution, which makes dermoscopic assessment of colors helpful in narrowing the differential diagnosis. Melanoma is associated with combinations of gray, red, blue, and white, which are not found in genital lentiginosis.9
Biopsy of a genital lentigo is diagnostic, distinguishing the lesion from melanoma—failing to reveal the atypical melanocytes and pagetoid spread characteristic of melanoma in situ. Histologic findings can cause diagnostic difficulties when concurrent lichen sclerosus is associated with genital lentigines or nevi.10
Lentigines on sun-damaged skin or in the setting of xeroderma pigmentosum have been associated with melanoma,11-13 but genital lentigines are not considered a form of precancerous melanosis. In women, early diagnosis is important when there is concern for melanoma because the prognosis for vulvar melanoma is improved in thin lesions.14
Other entities in the differential include secondary syphilis, which commonly presents as macules and scaly papules and can be found on mucosal surfaces such as the oral cavity,15 as well as Kaposi sarcoma, which is characterized by purplish, brown, or black macules, plaques, and nodules, more commonly in immunosuppressed patients.16
To avoid unwarranted concern and unnecessary surgery, dermatologists should be aware of genital lentigines and their characteristic presentation in adults.
- Hwang L, Wilson H, Orengo I. Off-center fold: irregular, pigmented genital macules. Arch Dermatol. 2000;136:1559-1564. doi:10.1001/archderm.136.12.1559-b
- Rhodes AR, Silverman RA, Harrist TJ, et al. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the “LAMB” syndrome. J Am Acad Dermatol. 1984;10:72-82. doi:10.1016/s0190-9622(84)80047-x
- Erkek E, Hizel S, Sanl C, et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639-643. doi:10.1016/j.jaad.2005.06.022
- Blum RR, Rahimizadeh A, Kardon N, et al. Genital lentigines in a 6-year-old boy with a family history of Cowden’s disease: clinical and genetic evidence of the linkage between Bannayan-Riley-Ruvalcaba syndrome and Cowden’s disease. J Cutan Med Surg. 2001;5:228-230. doi:10.1177/120347540100500307
- Isbary G, Dyall-Smith D, Coras-Stepanek B, et al. Penile lentigo (genital mucosal macule) following annular lichen planus: a possible association? Australas J Dermatol. 2014;55:159-161. doi:10.1111/ajd.12169
- Campbell T, Felsten L, Moore J. Disappearance of lentigines in a patient receiving imatinib treatment for familial gastrointestinal stromal tumor syndrome. Arch Dermatol. 2009;145:1313-1316. doi:10.1001/archdermatol.2009.263
- Romero- A, R, , et al. Reticulate genital pigmentation associated with localized vitiligo. Arch Dermatol. 2010; 146:574-575. doi:10.1001/archdermatol.2010.69
- Barnhill RL, Albert LS, Shama SK, et al. Genital lentiginosis: a clinical and histopathologic study. J Am Acad Dermatol. 1990;22:453-460. doi:10.1016/0190-9622(90)70064-o
- De Giorgi V, Gori A, Salvati L, et al. Clinical and dermoscopic features of vulvar melanosis over the last 20 years. JAMA Dermatol. 2020;156:1185–1191. doi:10.1001/jamadermatol.2020.2528
- El Shabrawi-Caelen L, Soyer HP, Schaeppi H, et al. Genital lentigines and melanocytic nevi with superimposed lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol. 2004;50:690-694. doi:10.1016/j.jaad.2003.09.034
- Shatkin M, Helm MF, Muhlbauer A, et al. Solar lentigo evolving into fatal metastatic melanoma in a patient who initially refused surgery. N A J Med Sci. 2020;1:28-31. doi:10.7156/najms.2020.1301028
- Stern JB, Peck GL, Haupt HM, et al. Malignant melanoma in xeroderma pigmentosum: search for a precursor lesion. J Am Acad Dermatol. 1993;28:591-594. doi:10.1016/0190-9622(93)70079-9
- Byrom L, Barksdale S, Weedon D, et al. Unstable solar lentigo: a defined separate entity. Australas J Dermatol. 2016;57:229-234. doi:10.1111/ajd.12447
- Panizzon RG. Vulvar melanoma. Semin Dermatol. 1996;15:67-70. doi:10.1016/s1085-5629(96)80021-6
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164. doi:10.1097/00007435-198010000-00002
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151. doi:10.1002/jso.20090
To the Editor:
Genital lentiginosis (also known as mucosal melanotic macules, vulvar melanosis, penile melanosis, and penile lentigines) occurs in men and women.1 Lesions present in adult life as multifocal, asymmetrical, pigmented patches that can have a mottled appearance or exhibit skip areas. The irregular appearance of the pigmented areas often raises concern for melanoma. Biopsy reveals increased pigmentation along the basal layer of the epidermis; the irregular distribution of single melanocytes and pagetoid spread typical of melanoma in situ is not identified.

Genital lentiginosis usually occurs as an isolated finding; however, the condition can be a manifestation of Laugier-Hunziker syndrome, Carney complex, and Bannayan-Riley-Ruvalcaba syndrome.1-3 When it occurs as an isolated finding, the patient can be reassured and treatment is unnecessary. Because genital lentiginosis may mimic the appearance of melanoma, it is important for physicians to differentiate the two and make a correct diagnosis. We present a case of genital lentiginosis that mimicked vulvar melanoma.

A 64-year-old woman was referred by her gynecologist to dermatology to rule out vulvar melanoma. The patient had a history of hypothyroidism and hypercholesterolemia but was otherwise in good health. Genital examination revealed asymptomatic pigmented macules and patches of unknown duration (Figure 1). Specimens were taken from 3 areas by punch biopsy to clarify the diagnosis. All 3 specimens showed identical features including basilar pigmentation, occasional melanophages in the papillary dermis, and no evidence of nests or pagetoid spread of atypical melanocytes (Figures 2 and 3). Histologic findings were diagnostic for genital lentiginosis. The patient was reassured, and no treatment was provided. At 6-month follow-up there was no change in clinical appearance.

Genital lentiginosis is characterized by brown lesions that can have a mottled appearance and often are associated with skip areas.1 Lesions can be strikingly irregular and darkly pigmented.
Although the lesions of genital lentiginosis most often are isolated findings, they can be a clue to several uncommon syndromes such as autosomal-dominant Bannayan-Riley-Ruvalcaba syndrome, which is associated with genital lentiginosis, intestinal polyposis, and macrocephaly.3 Vascular malformations, lipomatosis, verrucal keratoses, and acrochordons can occur. Bannayan-Riley-Ruvalcaba syndrome and Cowden syndrome may share genetic linkage; mutations in the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) has been implicated in both syndromes.4 Underlying Carney complex should be excluded when genital lentiginosis is encountered.
Genital lentiginosis is idiopathic in most instances, but reports of lesions occurring after annular lichen planus suggest a possible mechanism.5 The disappearance of lentigines after imatinib therapy suggests a role for c-kit, a receptor tyrosine kinase that is involved in intracellular signaling, in some cases.6 At times, lesions can simulate trichrome vitiligo or have a reticulate pattern.7
Men and women present at different points in the course of disease. Men often present with penile lesions 14 years after onset, on average; they notice a gradual increase in the size of lesions. Because women can have greater difficulty self-examining the genital region, they tend to present much later in the course but often within a few months after initial inspection.1,8
Genital lentiginosis can mimic melanoma with nonhomogeneous pigmentation, asymmetry, and unilateral distribution, which makes dermoscopic assessment of colors helpful in narrowing the differential diagnosis. Melanoma is associated with combinations of gray, red, blue, and white, which are not found in genital lentiginosis.9
Biopsy of a genital lentigo is diagnostic, distinguishing the lesion from melanoma—failing to reveal the atypical melanocytes and pagetoid spread characteristic of melanoma in situ. Histologic findings can cause diagnostic difficulties when concurrent lichen sclerosus is associated with genital lentigines or nevi.10
Lentigines on sun-damaged skin or in the setting of xeroderma pigmentosum have been associated with melanoma,11-13 but genital lentigines are not considered a form of precancerous melanosis. In women, early diagnosis is important when there is concern for melanoma because the prognosis for vulvar melanoma is improved in thin lesions.14
Other entities in the differential include secondary syphilis, which commonly presents as macules and scaly papules and can be found on mucosal surfaces such as the oral cavity,15 as well as Kaposi sarcoma, which is characterized by purplish, brown, or black macules, plaques, and nodules, more commonly in immunosuppressed patients.16
To avoid unwarranted concern and unnecessary surgery, dermatologists should be aware of genital lentigines and their characteristic presentation in adults.
To the Editor:
Genital lentiginosis (also known as mucosal melanotic macules, vulvar melanosis, penile melanosis, and penile lentigines) occurs in men and women.1 Lesions present in adult life as multifocal, asymmetrical, pigmented patches that can have a mottled appearance or exhibit skip areas. The irregular appearance of the pigmented areas often raises concern for melanoma. Biopsy reveals increased pigmentation along the basal layer of the epidermis; the irregular distribution of single melanocytes and pagetoid spread typical of melanoma in situ is not identified.

Genital lentiginosis usually occurs as an isolated finding; however, the condition can be a manifestation of Laugier-Hunziker syndrome, Carney complex, and Bannayan-Riley-Ruvalcaba syndrome.1-3 When it occurs as an isolated finding, the patient can be reassured and treatment is unnecessary. Because genital lentiginosis may mimic the appearance of melanoma, it is important for physicians to differentiate the two and make a correct diagnosis. We present a case of genital lentiginosis that mimicked vulvar melanoma.

A 64-year-old woman was referred by her gynecologist to dermatology to rule out vulvar melanoma. The patient had a history of hypothyroidism and hypercholesterolemia but was otherwise in good health. Genital examination revealed asymptomatic pigmented macules and patches of unknown duration (Figure 1). Specimens were taken from 3 areas by punch biopsy to clarify the diagnosis. All 3 specimens showed identical features including basilar pigmentation, occasional melanophages in the papillary dermis, and no evidence of nests or pagetoid spread of atypical melanocytes (Figures 2 and 3). Histologic findings were diagnostic for genital lentiginosis. The patient was reassured, and no treatment was provided. At 6-month follow-up there was no change in clinical appearance.

Genital lentiginosis is characterized by brown lesions that can have a mottled appearance and often are associated with skip areas.1 Lesions can be strikingly irregular and darkly pigmented.
Although the lesions of genital lentiginosis most often are isolated findings, they can be a clue to several uncommon syndromes such as autosomal-dominant Bannayan-Riley-Ruvalcaba syndrome, which is associated with genital lentiginosis, intestinal polyposis, and macrocephaly.3 Vascular malformations, lipomatosis, verrucal keratoses, and acrochordons can occur. Bannayan-Riley-Ruvalcaba syndrome and Cowden syndrome may share genetic linkage; mutations in the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) has been implicated in both syndromes.4 Underlying Carney complex should be excluded when genital lentiginosis is encountered.
Genital lentiginosis is idiopathic in most instances, but reports of lesions occurring after annular lichen planus suggest a possible mechanism.5 The disappearance of lentigines after imatinib therapy suggests a role for c-kit, a receptor tyrosine kinase that is involved in intracellular signaling, in some cases.6 At times, lesions can simulate trichrome vitiligo or have a reticulate pattern.7
Men and women present at different points in the course of disease. Men often present with penile lesions 14 years after onset, on average; they notice a gradual increase in the size of lesions. Because women can have greater difficulty self-examining the genital region, they tend to present much later in the course but often within a few months after initial inspection.1,8
Genital lentiginosis can mimic melanoma with nonhomogeneous pigmentation, asymmetry, and unilateral distribution, which makes dermoscopic assessment of colors helpful in narrowing the differential diagnosis. Melanoma is associated with combinations of gray, red, blue, and white, which are not found in genital lentiginosis.9
Biopsy of a genital lentigo is diagnostic, distinguishing the lesion from melanoma—failing to reveal the atypical melanocytes and pagetoid spread characteristic of melanoma in situ. Histologic findings can cause diagnostic difficulties when concurrent lichen sclerosus is associated with genital lentigines or nevi.10
Lentigines on sun-damaged skin or in the setting of xeroderma pigmentosum have been associated with melanoma,11-13 but genital lentigines are not considered a form of precancerous melanosis. In women, early diagnosis is important when there is concern for melanoma because the prognosis for vulvar melanoma is improved in thin lesions.14
Other entities in the differential include secondary syphilis, which commonly presents as macules and scaly papules and can be found on mucosal surfaces such as the oral cavity,15 as well as Kaposi sarcoma, which is characterized by purplish, brown, or black macules, plaques, and nodules, more commonly in immunosuppressed patients.16
To avoid unwarranted concern and unnecessary surgery, dermatologists should be aware of genital lentigines and their characteristic presentation in adults.
- Hwang L, Wilson H, Orengo I. Off-center fold: irregular, pigmented genital macules. Arch Dermatol. 2000;136:1559-1564. doi:10.1001/archderm.136.12.1559-b
- Rhodes AR, Silverman RA, Harrist TJ, et al. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the “LAMB” syndrome. J Am Acad Dermatol. 1984;10:72-82. doi:10.1016/s0190-9622(84)80047-x
- Erkek E, Hizel S, Sanl C, et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639-643. doi:10.1016/j.jaad.2005.06.022
- Blum RR, Rahimizadeh A, Kardon N, et al. Genital lentigines in a 6-year-old boy with a family history of Cowden’s disease: clinical and genetic evidence of the linkage between Bannayan-Riley-Ruvalcaba syndrome and Cowden’s disease. J Cutan Med Surg. 2001;5:228-230. doi:10.1177/120347540100500307
- Isbary G, Dyall-Smith D, Coras-Stepanek B, et al. Penile lentigo (genital mucosal macule) following annular lichen planus: a possible association? Australas J Dermatol. 2014;55:159-161. doi:10.1111/ajd.12169
- Campbell T, Felsten L, Moore J. Disappearance of lentigines in a patient receiving imatinib treatment for familial gastrointestinal stromal tumor syndrome. Arch Dermatol. 2009;145:1313-1316. doi:10.1001/archdermatol.2009.263
- Romero- A, R, , et al. Reticulate genital pigmentation associated with localized vitiligo. Arch Dermatol. 2010; 146:574-575. doi:10.1001/archdermatol.2010.69
- Barnhill RL, Albert LS, Shama SK, et al. Genital lentiginosis: a clinical and histopathologic study. J Am Acad Dermatol. 1990;22:453-460. doi:10.1016/0190-9622(90)70064-o
- De Giorgi V, Gori A, Salvati L, et al. Clinical and dermoscopic features of vulvar melanosis over the last 20 years. JAMA Dermatol. 2020;156:1185–1191. doi:10.1001/jamadermatol.2020.2528
- El Shabrawi-Caelen L, Soyer HP, Schaeppi H, et al. Genital lentigines and melanocytic nevi with superimposed lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol. 2004;50:690-694. doi:10.1016/j.jaad.2003.09.034
- Shatkin M, Helm MF, Muhlbauer A, et al. Solar lentigo evolving into fatal metastatic melanoma in a patient who initially refused surgery. N A J Med Sci. 2020;1:28-31. doi:10.7156/najms.2020.1301028
- Stern JB, Peck GL, Haupt HM, et al. Malignant melanoma in xeroderma pigmentosum: search for a precursor lesion. J Am Acad Dermatol. 1993;28:591-594. doi:10.1016/0190-9622(93)70079-9
- Byrom L, Barksdale S, Weedon D, et al. Unstable solar lentigo: a defined separate entity. Australas J Dermatol. 2016;57:229-234. doi:10.1111/ajd.12447
- Panizzon RG. Vulvar melanoma. Semin Dermatol. 1996;15:67-70. doi:10.1016/s1085-5629(96)80021-6
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164. doi:10.1097/00007435-198010000-00002
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151. doi:10.1002/jso.20090
- Hwang L, Wilson H, Orengo I. Off-center fold: irregular, pigmented genital macules. Arch Dermatol. 2000;136:1559-1564. doi:10.1001/archderm.136.12.1559-b
- Rhodes AR, Silverman RA, Harrist TJ, et al. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the “LAMB” syndrome. J Am Acad Dermatol. 1984;10:72-82. doi:10.1016/s0190-9622(84)80047-x
- Erkek E, Hizel S, Sanl C, et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639-643. doi:10.1016/j.jaad.2005.06.022
- Blum RR, Rahimizadeh A, Kardon N, et al. Genital lentigines in a 6-year-old boy with a family history of Cowden’s disease: clinical and genetic evidence of the linkage between Bannayan-Riley-Ruvalcaba syndrome and Cowden’s disease. J Cutan Med Surg. 2001;5:228-230. doi:10.1177/120347540100500307
- Isbary G, Dyall-Smith D, Coras-Stepanek B, et al. Penile lentigo (genital mucosal macule) following annular lichen planus: a possible association? Australas J Dermatol. 2014;55:159-161. doi:10.1111/ajd.12169
- Campbell T, Felsten L, Moore J. Disappearance of lentigines in a patient receiving imatinib treatment for familial gastrointestinal stromal tumor syndrome. Arch Dermatol. 2009;145:1313-1316. doi:10.1001/archdermatol.2009.263
- Romero- A, R, , et al. Reticulate genital pigmentation associated with localized vitiligo. Arch Dermatol. 2010; 146:574-575. doi:10.1001/archdermatol.2010.69
- Barnhill RL, Albert LS, Shama SK, et al. Genital lentiginosis: a clinical and histopathologic study. J Am Acad Dermatol. 1990;22:453-460. doi:10.1016/0190-9622(90)70064-o
- De Giorgi V, Gori A, Salvati L, et al. Clinical and dermoscopic features of vulvar melanosis over the last 20 years. JAMA Dermatol. 2020;156:1185–1191. doi:10.1001/jamadermatol.2020.2528
- El Shabrawi-Caelen L, Soyer HP, Schaeppi H, et al. Genital lentigines and melanocytic nevi with superimposed lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol. 2004;50:690-694. doi:10.1016/j.jaad.2003.09.034
- Shatkin M, Helm MF, Muhlbauer A, et al. Solar lentigo evolving into fatal metastatic melanoma in a patient who initially refused surgery. N A J Med Sci. 2020;1:28-31. doi:10.7156/najms.2020.1301028
- Stern JB, Peck GL, Haupt HM, et al. Malignant melanoma in xeroderma pigmentosum: search for a precursor lesion. J Am Acad Dermatol. 1993;28:591-594. doi:10.1016/0190-9622(93)70079-9
- Byrom L, Barksdale S, Weedon D, et al. Unstable solar lentigo: a defined separate entity. Australas J Dermatol. 2016;57:229-234. doi:10.1111/ajd.12447
- Panizzon RG. Vulvar melanoma. Semin Dermatol. 1996;15:67-70. doi:10.1016/s1085-5629(96)80021-6
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164. doi:10.1097/00007435-198010000-00002
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151. doi:10.1002/jso.20090
Practice Points
- The irregular appearance of genital lentiginosis—multifocal, asymmetric, irregular, and darkly pigmented patches—often raises concern for melanoma but is benign.
- Certain genetic conditions can present with genital lentiginosis.
- Dermoscopic assessment of the lesion color is highly helpful in narrowing the differential diagnosis; seeing no gray, red, blue, or white makes melanoma less likely.
- Be aware of genital lentigines and their characteristic presentation in adulthood to avoid unwarranted concern and unneeded surgery.
Atypical Localized Scleroderma Development During Nivolumab Therapy for Metastatic Lung Adenocarcinoma
To the Editor:
Immune checkpoint inhibitors such as anti–programmed cell death protein 1 (anti–PD-1) and anticytotoxic T lymphocyte–associated protein 4 therapies are a promising class of cancer therapeutics. However, they are associated with a variety of immune-related adverse events (irAEs), including cutaneous toxicity.1 The PD-1/programmed death ligand 1 (PD-L1) pathway is important for the maintenance of immune tolerance, and a blockade has been shown to lead to development of various autoimmune diseases.2 We present the case of a patient who developed new-onset localized scleroderma during treatment with the PD-1 inhibitor nivolumab.
A 65-year-old woman presented with a rash on the left thigh that was associated with pruritus, pain, and a pulling sensation. She had a history of stage IV lung adenocarcinoma, with a mass in the right upper lobe with metastatic foci to the left femur, right humerus, right hilar, and pretracheal lymph nodes. She received palliative radiation to the left femur and was started on carboplatin and pemetrexed. Metastasis to the liver was noted after completion of 6 cycles of therapy, and the patient’s treatment was changed to nivolumab. After 17 months on nivolumab therapy (2 years after initial diagnosis and 20 months after radiation therapy), she presented to our dermatology clinic with a cutaneous eruption on the buttocks that spread to the left thigh. The rash failed to improve after 1 month of treatment with emollients and triamcinolone cream 0.1%.
At the current presentation, which was 2 months after she initially presented to our clinic, dermatologic examination revealed erythematous and sclerotic plaques on the left lateral thigh (Figure 1A). Betamethasone cream 0.05% was prescribed, and nivolumab was discontinued due to progression of cutaneous symptoms. A punch biopsy from the left thigh demonstrated superficial dermal sclerosis that was suggestive of chronic radiation dermatitis; direct immunofluorescence testing was negative. The patient was started on prednisone 50 mg daily, which resulted in mild improvement in symptoms.
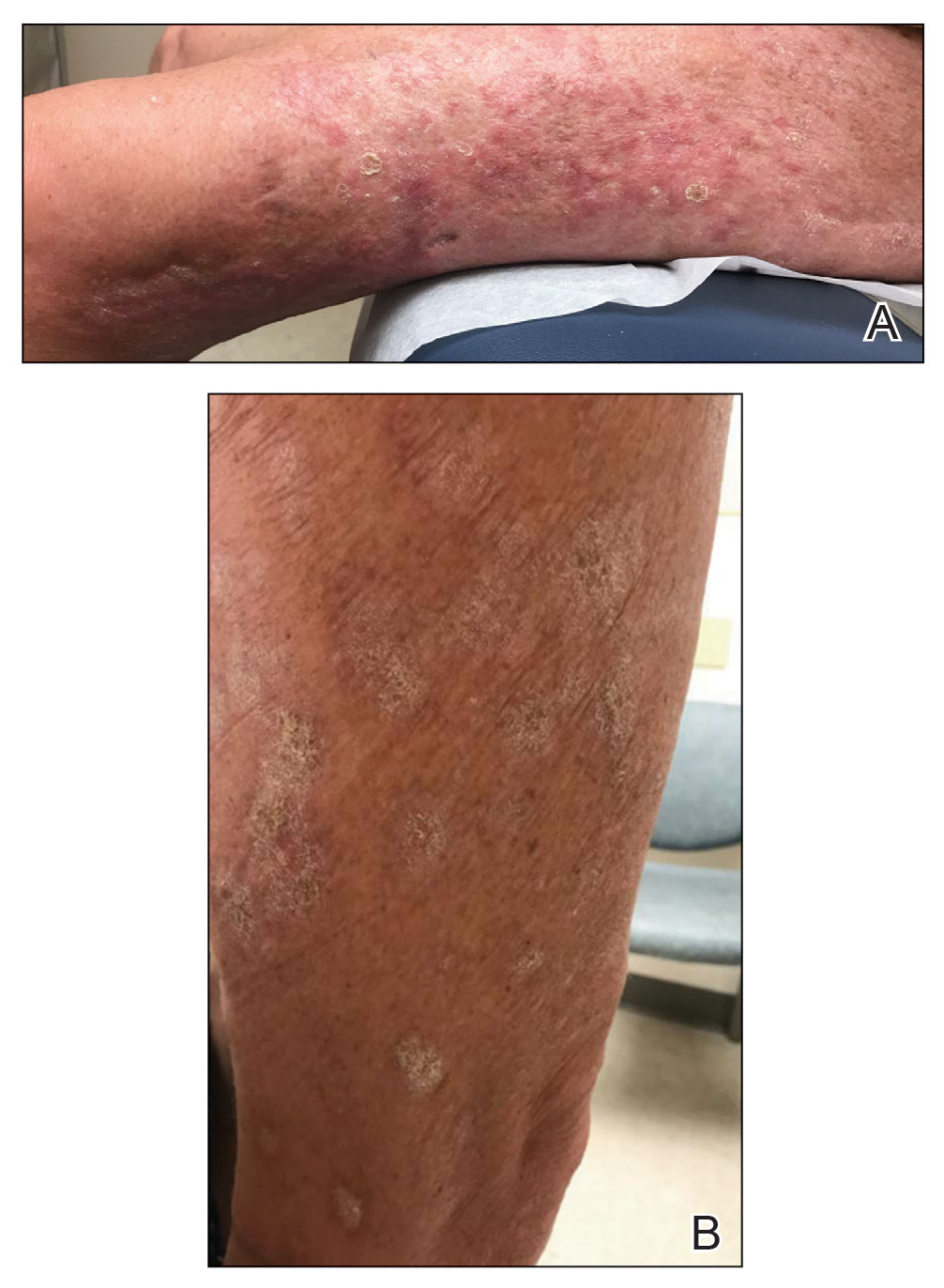
Within 6 months, new sclerotic plaques developed on the patient’s back and right thigh (Figure 1B). Because the lesions were located outside the radiation field of the left femur, a second biopsy was obtained from the right thigh. Histopathology revealed extensive dermal sclerosis and a perivascular lymphoplasmacytic infiltrate (Figure 2). An antinuclear antibody test was weakly positive (1:40, nucleolar pattern) with a negative extractable nuclear antigen panel result. Anti–double-stranded DNA, anti–topoisomerase 1, anti-Smith, antiribonucleoprotein, anti–Sjögren syndrome type A, anti–Sjögren syndrome type B, and anticentromere serology test results were negative. The patient denied decreased oral aperture, difficulty swallowing, or Raynaud phenomenon. Due to the atypical clinical presentation in the setting of PD-1 inhibitor therapy, the etiology of the eruption was potentially attributable to nivolumab. She was started on treatment with methotrexate 20 mg weekly and clobetasol cream 0.05% twice daily; she continued taking prednisone 5 mg daily. The cutaneous manifestations on the patient’s back completely resolved, and the legs continued to gradually improve on this regimen. Immunotherapy continued to be held due to skin toxicity.
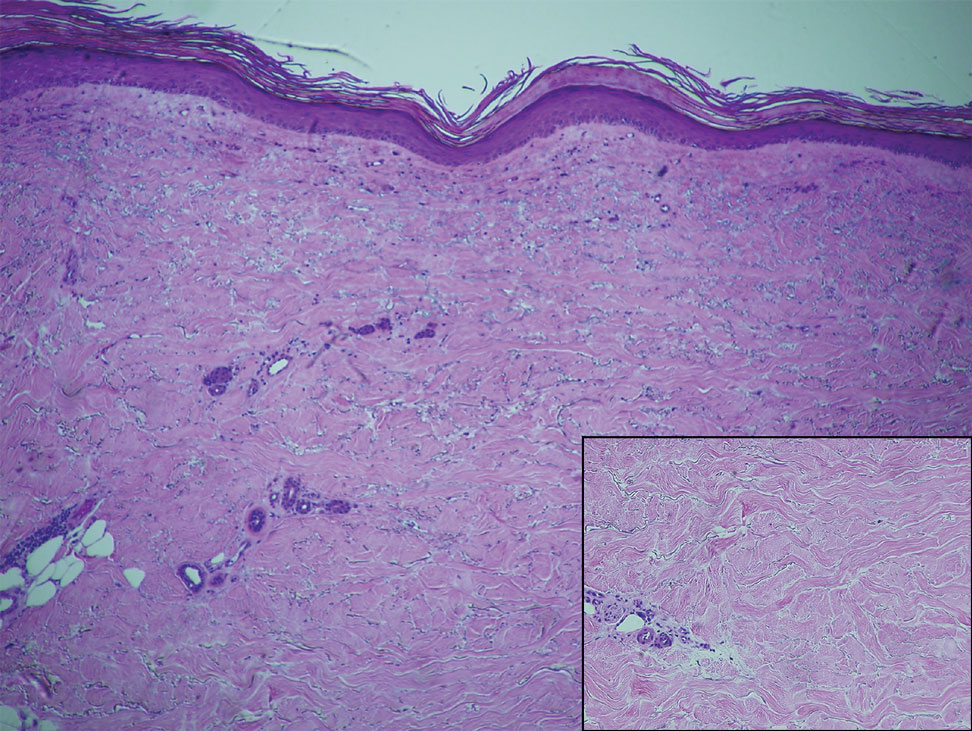
Localized scleroderma is an autoimmune disorder characterized by inflammation and skin thickening. Overactive fibroblasts produce excess collagen, leading to the clinical symptoms of skin thickening, hardening, and discoloration.3 Lesions frequently develop on the arms, face, or legs and can present as patches or linear bands. Unlike systemic sclerosis, the internal organs typically are uninvolved; however, sclerotic lesions can be disfiguring and cause notable disability if they impede joint movement.
The PD-1/PD-L1 pathway is a negative regulator of the immune response that inactivates T cells and helps maintain self-tolerance. Modulation of the PD-1/PD-L1 pathway and overexpression of PD-L1 are seen in various cancers as a mechanism to help malignant cells avoid immune destruction.4 Conversely, inhibition of this pathway can be used to stimulate an antitumor immune response. This checkpoint inhibition strategy has been highly successful for the treatment of various cancers including melanoma and non–small cell lung carcinoma. There are several checkpoint inhibitors approved in the United States that are used for cancer therapy and target the PD-1/PD-L1 pathway, such as nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.4 A downside of checkpoint inhibitor treatment is that uncontrolled T-cell activation can lead to irAEs, including cutaneous eruptions, pruritus, diarrhea, colitis, hepatitis, endocrinopathies, pneumonitis, and renal insufficiency.5 These toxicities are reversible if treated appropriately but can cause notable morbidity and mortality if left unrecognized. Cutaneous eruption is one of the most common irAEs associated with anti–PD-1 and anti–PD-L1 therapies and can limit therapeutic efficacy, as the drug may need to be held or discontinued due to the severity of the eruption.6 Mid-potency to high-potency topical corticosteroids and systemic antihistamines are first-line treatments of grades 1 and 2 skin toxicities associated with PD-1 inhibitor therapy. For eruptions classified as grades 3 or 4 or refractory grade 2, discontinuation of the drug and systemic corticosteroids is recommended.7
The cutaneous eruption in immunotherapy-mediated dermatitis is thought to be largely mediated by activated T cells infiltrating the dermis.8 In localized scleroderma, increased tumor necrosis factor α, IFN-γ, IFN-γ–induced protein 10, and granulocyte macrophage colony stimulating factor activity have been shown to correlate with disease activity.9,10 Interestingly, increased tumor necrosis factor α and IFN-γ correlate with better response and increased overall survival in PD-1 inhibition therapy, suggesting a correlation between PD-1 inhibition and T helper activation as noted by the etiology of sclerosis in our patient.11 Additionally, history of radiation was a confounding factor in the diagnosis of our patient, as both sclerodermoid reactions and chronic radiation dermatitis can present with dermal sclerosis. However, the progression of disease outside of the radiation field excluded this etiology. Although new-onset sclerodermoid reactions have been reported with PD-1 inhibitors, they have been described secondary to sclerodermoid reactions from treatment with pembrolizumab.12,13 One case series reported a case of diffuse sclerodermoid reaction and a limited reaction in response to pembrolizumab treatment, while another case report described a relapse of generalized morphea in response to pembrolizumab treatment.12,13 One case of relapsing morphea in response to nivolumab treatment for stage IV lung adenocarcinoma also has been reported.14
Cutaneous toxicities are one of the most common irAEs associated with checkpoint inhibitors and are seen in more than one-third of treated patients. Most frequently, these irAEs manifest as spongiotic dermatitis on histopathology, but a broad spectrum of cutaneous reactions have been observed.15 Although sclerodermoid reactions have been reported with PD-1 inhibitors, most are described secondary to sclerodermoid reactions with pembrolizumab and involve relapse of previously diagnosed morphea rather than new-onset disease.12-14
Our case highlights new-onset localized scleroderma in the setting of nivolumab therapy that showed clinical improvement with methotrexate and topical and systemic steroids. This reaction pattern should be considered in all patients who develop cutaneous eruptions when treated with a PD-1 inhibitor. There should be a high index of suspicion for the potential occurrence of irAEs to ensure early recognition and treatment to minimize morbidity and maximize adherence to therapy for the underlying malignancy.
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793.
- Dai S, Jia R, Zhang X, et al. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290:72-79.
- Badea I, Taylor M, Rosenberg A, et al. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology (Oxford). 2009;48:213-221.
- Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84-106.
- Villadolid J, Asim A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- O’Kane GM, Labbé C, Doherty MK, et al. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist. 2017;22:70-80.
- Shi VJ, Rodic N, Gettinger S, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed celldeath 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152:1128-1136.
- Torok KS, Kurzinski K, Kelsey C, et al. Peripheral blood cytokine and chemokine profiles in juvenile localized scleroderma: T-helper cell-associated cytokine profiles. Semin Arthritis Rheum. 2015;45:284-293.
- Guo X, Higgs BW, Bay-Jensen AC, et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 mAb, anifrolumab, in adult patients with systemic sclerosis. J Invest Dermatol. 2015;135:2402-2409.
- Boutsikou E, Domvri K, Hardavella G, et al. Tumor necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol. 2018;10:1758835918768238.
- Barbosa NS, Wetter DA, Wieland CN, et al. Scleroderma induced by pembrolizumab: a case series. Mayo Clin Proc. 2017;92:1158-1163.
- Cheng MW, Hisaw LD, Bernet L. Generalized morphea in the setting of pembrolizumab. Int J Dermatol. 2019;58:736-738.
- Alegre-Sánchez A, Fonda-Pascual P, Saceda-Corralo D, et al. Relapse of morphea during nivolumab therapy for lung adenocarcinoma. Actas Dermosifiliogr. 2017;108:69-70.
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19:345-361.
To the Editor:
Immune checkpoint inhibitors such as anti–programmed cell death protein 1 (anti–PD-1) and anticytotoxic T lymphocyte–associated protein 4 therapies are a promising class of cancer therapeutics. However, they are associated with a variety of immune-related adverse events (irAEs), including cutaneous toxicity.1 The PD-1/programmed death ligand 1 (PD-L1) pathway is important for the maintenance of immune tolerance, and a blockade has been shown to lead to development of various autoimmune diseases.2 We present the case of a patient who developed new-onset localized scleroderma during treatment with the PD-1 inhibitor nivolumab.
A 65-year-old woman presented with a rash on the left thigh that was associated with pruritus, pain, and a pulling sensation. She had a history of stage IV lung adenocarcinoma, with a mass in the right upper lobe with metastatic foci to the left femur, right humerus, right hilar, and pretracheal lymph nodes. She received palliative radiation to the left femur and was started on carboplatin and pemetrexed. Metastasis to the liver was noted after completion of 6 cycles of therapy, and the patient’s treatment was changed to nivolumab. After 17 months on nivolumab therapy (2 years after initial diagnosis and 20 months after radiation therapy), she presented to our dermatology clinic with a cutaneous eruption on the buttocks that spread to the left thigh. The rash failed to improve after 1 month of treatment with emollients and triamcinolone cream 0.1%.
At the current presentation, which was 2 months after she initially presented to our clinic, dermatologic examination revealed erythematous and sclerotic plaques on the left lateral thigh (Figure 1A). Betamethasone cream 0.05% was prescribed, and nivolumab was discontinued due to progression of cutaneous symptoms. A punch biopsy from the left thigh demonstrated superficial dermal sclerosis that was suggestive of chronic radiation dermatitis; direct immunofluorescence testing was negative. The patient was started on prednisone 50 mg daily, which resulted in mild improvement in symptoms.

Within 6 months, new sclerotic plaques developed on the patient’s back and right thigh (Figure 1B). Because the lesions were located outside the radiation field of the left femur, a second biopsy was obtained from the right thigh. Histopathology revealed extensive dermal sclerosis and a perivascular lymphoplasmacytic infiltrate (Figure 2). An antinuclear antibody test was weakly positive (1:40, nucleolar pattern) with a negative extractable nuclear antigen panel result. Anti–double-stranded DNA, anti–topoisomerase 1, anti-Smith, antiribonucleoprotein, anti–Sjögren syndrome type A, anti–Sjögren syndrome type B, and anticentromere serology test results were negative. The patient denied decreased oral aperture, difficulty swallowing, or Raynaud phenomenon. Due to the atypical clinical presentation in the setting of PD-1 inhibitor therapy, the etiology of the eruption was potentially attributable to nivolumab. She was started on treatment with methotrexate 20 mg weekly and clobetasol cream 0.05% twice daily; she continued taking prednisone 5 mg daily. The cutaneous manifestations on the patient’s back completely resolved, and the legs continued to gradually improve on this regimen. Immunotherapy continued to be held due to skin toxicity.

Localized scleroderma is an autoimmune disorder characterized by inflammation and skin thickening. Overactive fibroblasts produce excess collagen, leading to the clinical symptoms of skin thickening, hardening, and discoloration.3 Lesions frequently develop on the arms, face, or legs and can present as patches or linear bands. Unlike systemic sclerosis, the internal organs typically are uninvolved; however, sclerotic lesions can be disfiguring and cause notable disability if they impede joint movement.
The PD-1/PD-L1 pathway is a negative regulator of the immune response that inactivates T cells and helps maintain self-tolerance. Modulation of the PD-1/PD-L1 pathway and overexpression of PD-L1 are seen in various cancers as a mechanism to help malignant cells avoid immune destruction.4 Conversely, inhibition of this pathway can be used to stimulate an antitumor immune response. This checkpoint inhibition strategy has been highly successful for the treatment of various cancers including melanoma and non–small cell lung carcinoma. There are several checkpoint inhibitors approved in the United States that are used for cancer therapy and target the PD-1/PD-L1 pathway, such as nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.4 A downside of checkpoint inhibitor treatment is that uncontrolled T-cell activation can lead to irAEs, including cutaneous eruptions, pruritus, diarrhea, colitis, hepatitis, endocrinopathies, pneumonitis, and renal insufficiency.5 These toxicities are reversible if treated appropriately but can cause notable morbidity and mortality if left unrecognized. Cutaneous eruption is one of the most common irAEs associated with anti–PD-1 and anti–PD-L1 therapies and can limit therapeutic efficacy, as the drug may need to be held or discontinued due to the severity of the eruption.6 Mid-potency to high-potency topical corticosteroids and systemic antihistamines are first-line treatments of grades 1 and 2 skin toxicities associated with PD-1 inhibitor therapy. For eruptions classified as grades 3 or 4 or refractory grade 2, discontinuation of the drug and systemic corticosteroids is recommended.7
The cutaneous eruption in immunotherapy-mediated dermatitis is thought to be largely mediated by activated T cells infiltrating the dermis.8 In localized scleroderma, increased tumor necrosis factor α, IFN-γ, IFN-γ–induced protein 10, and granulocyte macrophage colony stimulating factor activity have been shown to correlate with disease activity.9,10 Interestingly, increased tumor necrosis factor α and IFN-γ correlate with better response and increased overall survival in PD-1 inhibition therapy, suggesting a correlation between PD-1 inhibition and T helper activation as noted by the etiology of sclerosis in our patient.11 Additionally, history of radiation was a confounding factor in the diagnosis of our patient, as both sclerodermoid reactions and chronic radiation dermatitis can present with dermal sclerosis. However, the progression of disease outside of the radiation field excluded this etiology. Although new-onset sclerodermoid reactions have been reported with PD-1 inhibitors, they have been described secondary to sclerodermoid reactions from treatment with pembrolizumab.12,13 One case series reported a case of diffuse sclerodermoid reaction and a limited reaction in response to pembrolizumab treatment, while another case report described a relapse of generalized morphea in response to pembrolizumab treatment.12,13 One case of relapsing morphea in response to nivolumab treatment for stage IV lung adenocarcinoma also has been reported.14
Cutaneous toxicities are one of the most common irAEs associated with checkpoint inhibitors and are seen in more than one-third of treated patients. Most frequently, these irAEs manifest as spongiotic dermatitis on histopathology, but a broad spectrum of cutaneous reactions have been observed.15 Although sclerodermoid reactions have been reported with PD-1 inhibitors, most are described secondary to sclerodermoid reactions with pembrolizumab and involve relapse of previously diagnosed morphea rather than new-onset disease.12-14
Our case highlights new-onset localized scleroderma in the setting of nivolumab therapy that showed clinical improvement with methotrexate and topical and systemic steroids. This reaction pattern should be considered in all patients who develop cutaneous eruptions when treated with a PD-1 inhibitor. There should be a high index of suspicion for the potential occurrence of irAEs to ensure early recognition and treatment to minimize morbidity and maximize adherence to therapy for the underlying malignancy.
To the Editor:
Immune checkpoint inhibitors such as anti–programmed cell death protein 1 (anti–PD-1) and anticytotoxic T lymphocyte–associated protein 4 therapies are a promising class of cancer therapeutics. However, they are associated with a variety of immune-related adverse events (irAEs), including cutaneous toxicity.1 The PD-1/programmed death ligand 1 (PD-L1) pathway is important for the maintenance of immune tolerance, and a blockade has been shown to lead to development of various autoimmune diseases.2 We present the case of a patient who developed new-onset localized scleroderma during treatment with the PD-1 inhibitor nivolumab.
A 65-year-old woman presented with a rash on the left thigh that was associated with pruritus, pain, and a pulling sensation. She had a history of stage IV lung adenocarcinoma, with a mass in the right upper lobe with metastatic foci to the left femur, right humerus, right hilar, and pretracheal lymph nodes. She received palliative radiation to the left femur and was started on carboplatin and pemetrexed. Metastasis to the liver was noted after completion of 6 cycles of therapy, and the patient’s treatment was changed to nivolumab. After 17 months on nivolumab therapy (2 years after initial diagnosis and 20 months after radiation therapy), she presented to our dermatology clinic with a cutaneous eruption on the buttocks that spread to the left thigh. The rash failed to improve after 1 month of treatment with emollients and triamcinolone cream 0.1%.
At the current presentation, which was 2 months after she initially presented to our clinic, dermatologic examination revealed erythematous and sclerotic plaques on the left lateral thigh (Figure 1A). Betamethasone cream 0.05% was prescribed, and nivolumab was discontinued due to progression of cutaneous symptoms. A punch biopsy from the left thigh demonstrated superficial dermal sclerosis that was suggestive of chronic radiation dermatitis; direct immunofluorescence testing was negative. The patient was started on prednisone 50 mg daily, which resulted in mild improvement in symptoms.

Within 6 months, new sclerotic plaques developed on the patient’s back and right thigh (Figure 1B). Because the lesions were located outside the radiation field of the left femur, a second biopsy was obtained from the right thigh. Histopathology revealed extensive dermal sclerosis and a perivascular lymphoplasmacytic infiltrate (Figure 2). An antinuclear antibody test was weakly positive (1:40, nucleolar pattern) with a negative extractable nuclear antigen panel result. Anti–double-stranded DNA, anti–topoisomerase 1, anti-Smith, antiribonucleoprotein, anti–Sjögren syndrome type A, anti–Sjögren syndrome type B, and anticentromere serology test results were negative. The patient denied decreased oral aperture, difficulty swallowing, or Raynaud phenomenon. Due to the atypical clinical presentation in the setting of PD-1 inhibitor therapy, the etiology of the eruption was potentially attributable to nivolumab. She was started on treatment with methotrexate 20 mg weekly and clobetasol cream 0.05% twice daily; she continued taking prednisone 5 mg daily. The cutaneous manifestations on the patient’s back completely resolved, and the legs continued to gradually improve on this regimen. Immunotherapy continued to be held due to skin toxicity.

Localized scleroderma is an autoimmune disorder characterized by inflammation and skin thickening. Overactive fibroblasts produce excess collagen, leading to the clinical symptoms of skin thickening, hardening, and discoloration.3 Lesions frequently develop on the arms, face, or legs and can present as patches or linear bands. Unlike systemic sclerosis, the internal organs typically are uninvolved; however, sclerotic lesions can be disfiguring and cause notable disability if they impede joint movement.
The PD-1/PD-L1 pathway is a negative regulator of the immune response that inactivates T cells and helps maintain self-tolerance. Modulation of the PD-1/PD-L1 pathway and overexpression of PD-L1 are seen in various cancers as a mechanism to help malignant cells avoid immune destruction.4 Conversely, inhibition of this pathway can be used to stimulate an antitumor immune response. This checkpoint inhibition strategy has been highly successful for the treatment of various cancers including melanoma and non–small cell lung carcinoma. There are several checkpoint inhibitors approved in the United States that are used for cancer therapy and target the PD-1/PD-L1 pathway, such as nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.4 A downside of checkpoint inhibitor treatment is that uncontrolled T-cell activation can lead to irAEs, including cutaneous eruptions, pruritus, diarrhea, colitis, hepatitis, endocrinopathies, pneumonitis, and renal insufficiency.5 These toxicities are reversible if treated appropriately but can cause notable morbidity and mortality if left unrecognized. Cutaneous eruption is one of the most common irAEs associated with anti–PD-1 and anti–PD-L1 therapies and can limit therapeutic efficacy, as the drug may need to be held or discontinued due to the severity of the eruption.6 Mid-potency to high-potency topical corticosteroids and systemic antihistamines are first-line treatments of grades 1 and 2 skin toxicities associated with PD-1 inhibitor therapy. For eruptions classified as grades 3 or 4 or refractory grade 2, discontinuation of the drug and systemic corticosteroids is recommended.7
The cutaneous eruption in immunotherapy-mediated dermatitis is thought to be largely mediated by activated T cells infiltrating the dermis.8 In localized scleroderma, increased tumor necrosis factor α, IFN-γ, IFN-γ–induced protein 10, and granulocyte macrophage colony stimulating factor activity have been shown to correlate with disease activity.9,10 Interestingly, increased tumor necrosis factor α and IFN-γ correlate with better response and increased overall survival in PD-1 inhibition therapy, suggesting a correlation between PD-1 inhibition and T helper activation as noted by the etiology of sclerosis in our patient.11 Additionally, history of radiation was a confounding factor in the diagnosis of our patient, as both sclerodermoid reactions and chronic radiation dermatitis can present with dermal sclerosis. However, the progression of disease outside of the radiation field excluded this etiology. Although new-onset sclerodermoid reactions have been reported with PD-1 inhibitors, they have been described secondary to sclerodermoid reactions from treatment with pembrolizumab.12,13 One case series reported a case of diffuse sclerodermoid reaction and a limited reaction in response to pembrolizumab treatment, while another case report described a relapse of generalized morphea in response to pembrolizumab treatment.12,13 One case of relapsing morphea in response to nivolumab treatment for stage IV lung adenocarcinoma also has been reported.14
Cutaneous toxicities are one of the most common irAEs associated with checkpoint inhibitors and are seen in more than one-third of treated patients. Most frequently, these irAEs manifest as spongiotic dermatitis on histopathology, but a broad spectrum of cutaneous reactions have been observed.15 Although sclerodermoid reactions have been reported with PD-1 inhibitors, most are described secondary to sclerodermoid reactions with pembrolizumab and involve relapse of previously diagnosed morphea rather than new-onset disease.12-14
Our case highlights new-onset localized scleroderma in the setting of nivolumab therapy that showed clinical improvement with methotrexate and topical and systemic steroids. This reaction pattern should be considered in all patients who develop cutaneous eruptions when treated with a PD-1 inhibitor. There should be a high index of suspicion for the potential occurrence of irAEs to ensure early recognition and treatment to minimize morbidity and maximize adherence to therapy for the underlying malignancy.
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793.
- Dai S, Jia R, Zhang X, et al. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290:72-79.
- Badea I, Taylor M, Rosenberg A, et al. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology (Oxford). 2009;48:213-221.
- Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84-106.
- Villadolid J, Asim A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- O’Kane GM, Labbé C, Doherty MK, et al. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist. 2017;22:70-80.
- Shi VJ, Rodic N, Gettinger S, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed celldeath 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152:1128-1136.
- Torok KS, Kurzinski K, Kelsey C, et al. Peripheral blood cytokine and chemokine profiles in juvenile localized scleroderma: T-helper cell-associated cytokine profiles. Semin Arthritis Rheum. 2015;45:284-293.
- Guo X, Higgs BW, Bay-Jensen AC, et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 mAb, anifrolumab, in adult patients with systemic sclerosis. J Invest Dermatol. 2015;135:2402-2409.
- Boutsikou E, Domvri K, Hardavella G, et al. Tumor necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol. 2018;10:1758835918768238.
- Barbosa NS, Wetter DA, Wieland CN, et al. Scleroderma induced by pembrolizumab: a case series. Mayo Clin Proc. 2017;92:1158-1163.
- Cheng MW, Hisaw LD, Bernet L. Generalized morphea in the setting of pembrolizumab. Int J Dermatol. 2019;58:736-738.
- Alegre-Sánchez A, Fonda-Pascual P, Saceda-Corralo D, et al. Relapse of morphea during nivolumab therapy for lung adenocarcinoma. Actas Dermosifiliogr. 2017;108:69-70.
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19:345-361.
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793.
- Dai S, Jia R, Zhang X, et al. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290:72-79.
- Badea I, Taylor M, Rosenberg A, et al. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology (Oxford). 2009;48:213-221.
- Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84-106.
- Villadolid J, Asim A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- O’Kane GM, Labbé C, Doherty MK, et al. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist. 2017;22:70-80.
- Shi VJ, Rodic N, Gettinger S, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed celldeath 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152:1128-1136.
- Torok KS, Kurzinski K, Kelsey C, et al. Peripheral blood cytokine and chemokine profiles in juvenile localized scleroderma: T-helper cell-associated cytokine profiles. Semin Arthritis Rheum. 2015;45:284-293.
- Guo X, Higgs BW, Bay-Jensen AC, et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 mAb, anifrolumab, in adult patients with systemic sclerosis. J Invest Dermatol. 2015;135:2402-2409.
- Boutsikou E, Domvri K, Hardavella G, et al. Tumor necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol. 2018;10:1758835918768238.
- Barbosa NS, Wetter DA, Wieland CN, et al. Scleroderma induced by pembrolizumab: a case series. Mayo Clin Proc. 2017;92:1158-1163.
- Cheng MW, Hisaw LD, Bernet L. Generalized morphea in the setting of pembrolizumab. Int J Dermatol. 2019;58:736-738.
- Alegre-Sánchez A, Fonda-Pascual P, Saceda-Corralo D, et al. Relapse of morphea during nivolumab therapy for lung adenocarcinoma. Actas Dermosifiliogr. 2017;108:69-70.
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19:345-361.
Practice Points
- Immune checkpoint inhibitors such as nivolumab, a programmed cell death protein 1 (PD-1) inhibitor, are associated with immune-related adverse events (irAEs) such as skin toxicity.
- Scleroderma should be considered in the differential diagnosis of patients who develop cutaneous eruptions during treatment with PD-1 inhibitors.
- To ensure prompt recognition and treatment, health care providers should maintain a high index of suspicion for development of cutaneous irAEs in patients using checkpoint inhibitors.
Ossification and Migration of a Nodule Following Calcium Hydroxylapatite Injection
To the Editor:
Calcium hydroxylapatite is an injectable filler approved by the US Food and Drug Administration for moderate to severe rhytides of the face and the treatment of facial lipodystrophy in patients with HIV.1 This long-lasting filler generally is well tolerated with minimal side effects; however, there have been reports of nodules or granulomatous formation following injection.2 We present a case of a migrating nodule following injection of a calcium hydroxylapatite filler that appeared ossified on radiographic imaging. We highlight this rarely reported phenomenon to increase awareness of this complication.
A 72-year-old woman presented to our clinic with a mass on the left cheek. The patient had a history of treatment with facial fillers but no notable medical conditions. She initially received hyaluronic acid injectable gel dermal filler twice—3 years apart—before switching to calcium hydroxylapatite injections twice—4 months apart—from an outside provider. One month after the second treatment, she noticed a mass on the left cheek and promptly returned to the provider who performed the calcium hydroxylapatite injections. The provider, who had originally injected in the infraorbital area, stated it was unlikely that the filler would have migrated to the mid cheek and referred the patient to a general dentist who suspected salivary gland pathology. The patient was referred to an oral and maxillofacial surgeon who suspected the mass was related to the parotid gland. Maxillofacial computed tomography (CT) revealed heterotopic ossification vs myositis ossificans, possibly related to the recent injection. The patient was eventually referred to the Division of Plastic Surgery, Department of Surgery, at the University of Texas Medical Branch (Galveston, Texas) for further evaluation. Physical examination revealed a 2×1-cm firm, mobile, nontender mass in the left cheek in the area of the buccinator muscles. The mass did not express any fluid and was most easily palpable from the oral cavity. Radiography findings showed that the calcium hydroxylapatite filler had migrated to this location and formed a nodule (Figure). Because calcium hydroxylapatite fillers generally last 12 to 18 months, we opted to observe the lesion for spontaneous resolution. Four months later, the patient presented to our clinic for follow-up and the mass had reduced in size and appeared to be spontaneously resolving.
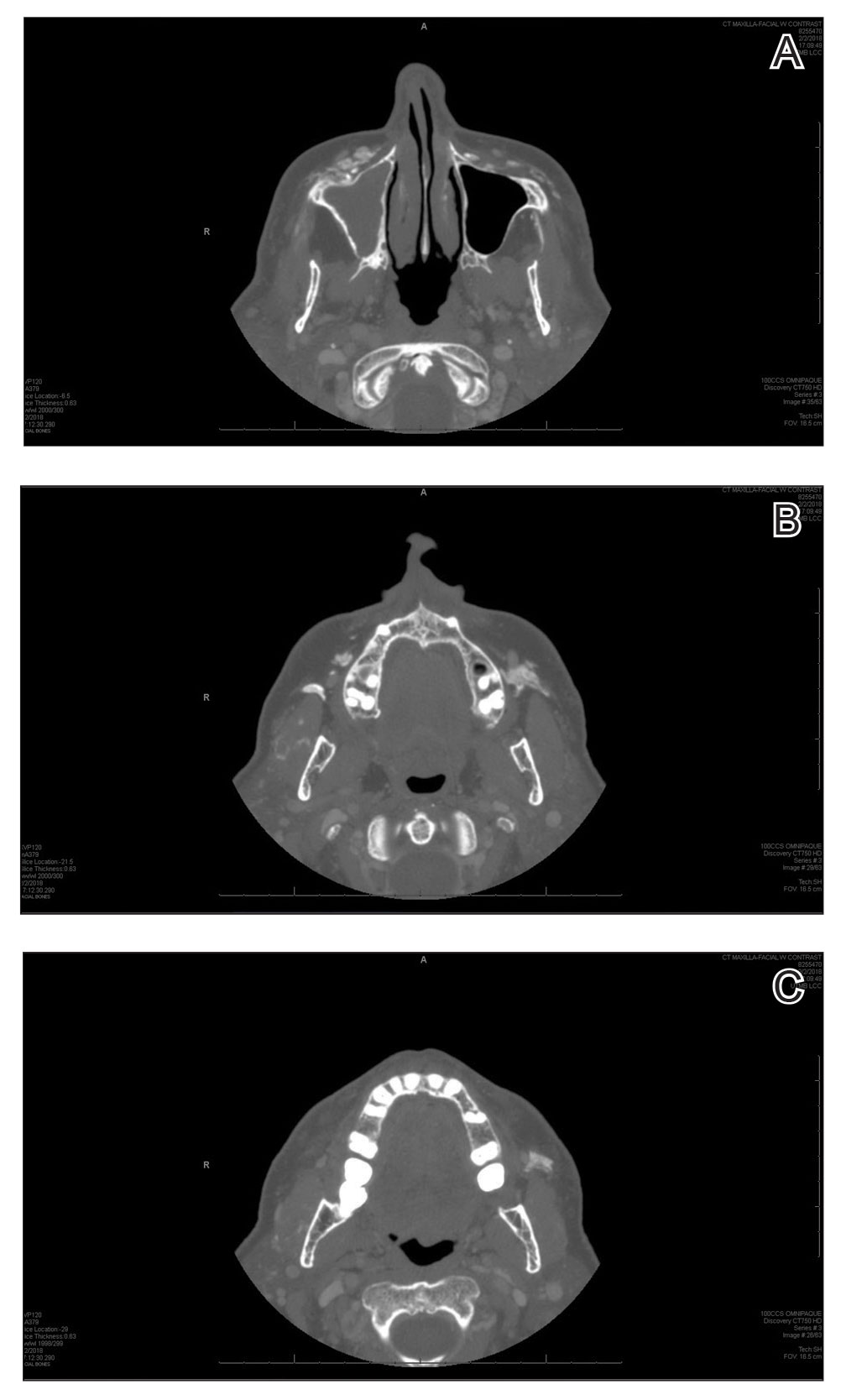
We present a unique case of a migrating nodule that occurred after injection with calcium hydroxylapatite, which led to concern for neoplastic tumor formation. This complication is rare, and it is important for practitioners who inject calcium hydroxylapatite as well as those who these patients may be referred to for evaluation to be aware that migrating nodules can occur. This awareness can help reduce unnecessary referrals, medical procedures, and anxiety.
Calcium hydroxylapatite filler is composed of 30% calcium hydroxylapatite microspheres suspended in a 70% sodium carboxymethylcellulose gel. The water-soluble gel rapidly becomes absorbed upon injection; however, the microspheres form a scaffold for the production of newly synthesized collagen. The filling effect generally lasts 12 to 18 months.1
Calcium hydroxylapatite, similar to most fillers, generally is well tolerated with a low complication rate of 3%.1 Although nodule formation with calcium hydroxylapatite is rare, it is the most common adverse event and encompasses 96% of complications. The remaining 4% of complications include persistent inflammation, swelling, erythema, and technical mistakes leading to overcorrection.1 Migrating nodules are rare; however, Beer3 reported a similar case.
Treatment of calcium hydroxylapatite nodules depends on differentiating a cause based on the time of onset. Early nodules that occur within 1 to 2 weeks of the injection usually represent incorrect positioning of the filler and can be treated by massaging the nodule. Other more invasive techniques involve aspiration or injection of sterile water. Late-onset nodules have shown response to corticosteroid injections. For inflammatory nodules of infectious origin, antibiotics can be useful. Surgical excision of the nodule rarely is required, as most nodules will resolve spontaneously, even without intervention.1,2
Radiologic findings of calcium hydroxylapatite appear as high-attenuation linear streaks or masses on CT (280–700 HU) and as low to intermediate signal intensity on T1- or T2-weighted sequences on magnetic resonance imaging. Oftentimes, calcium hydroxylapatite has a similar radiographic appearance to bone and can persist for 2 years or more on radiographic imaging, longer than they are clinically visible.4 The nodule formation from injection with calcium hydroxylapatite can mimic pathologic conditions such as miliary osteomas, myositis ossificans, heterotrophic/dystrophic calcifications, and foreign bodies on CT. Our patient’s CT findings of high attenuation linear streaks and nodules of similar signal intensity to bone were consistent with those previously described in the radiographic literature.
Calcium hydroxylapatite fillers have a good safety profile, but it is important to recognize that nodule formation is a common adverse event and that migration of nodules can occur. Practitioners should recognize this possibility in patients presenting with new masses after filler injection before advocating for potentially invasive and costly procedures and diagnostic modalities.
- Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol. 2017;16:152-161.
- Moulinets I, Arnaud E, Bui P, et al. Foreign body reaction to Radiesse: 2 cases. Am J Dermatopathol. 2013;35:e37-40.
- Beer KR. Radiesse nodule of the lips from a distant injection site: report of a case and consideration of etiology and management. J Drugs Dermatol. 2007;6:846-847.
- Ginat DT, Schatz CJ. Imaging features of midface injectable fillers and associated complications. AJNR Am J Neuroradiol. 2013;34:1488-1495.
To the Editor:
Calcium hydroxylapatite is an injectable filler approved by the US Food and Drug Administration for moderate to severe rhytides of the face and the treatment of facial lipodystrophy in patients with HIV.1 This long-lasting filler generally is well tolerated with minimal side effects; however, there have been reports of nodules or granulomatous formation following injection.2 We present a case of a migrating nodule following injection of a calcium hydroxylapatite filler that appeared ossified on radiographic imaging. We highlight this rarely reported phenomenon to increase awareness of this complication.
A 72-year-old woman presented to our clinic with a mass on the left cheek. The patient had a history of treatment with facial fillers but no notable medical conditions. She initially received hyaluronic acid injectable gel dermal filler twice—3 years apart—before switching to calcium hydroxylapatite injections twice—4 months apart—from an outside provider. One month after the second treatment, she noticed a mass on the left cheek and promptly returned to the provider who performed the calcium hydroxylapatite injections. The provider, who had originally injected in the infraorbital area, stated it was unlikely that the filler would have migrated to the mid cheek and referred the patient to a general dentist who suspected salivary gland pathology. The patient was referred to an oral and maxillofacial surgeon who suspected the mass was related to the parotid gland. Maxillofacial computed tomography (CT) revealed heterotopic ossification vs myositis ossificans, possibly related to the recent injection. The patient was eventually referred to the Division of Plastic Surgery, Department of Surgery, at the University of Texas Medical Branch (Galveston, Texas) for further evaluation. Physical examination revealed a 2×1-cm firm, mobile, nontender mass in the left cheek in the area of the buccinator muscles. The mass did not express any fluid and was most easily palpable from the oral cavity. Radiography findings showed that the calcium hydroxylapatite filler had migrated to this location and formed a nodule (Figure). Because calcium hydroxylapatite fillers generally last 12 to 18 months, we opted to observe the lesion for spontaneous resolution. Four months later, the patient presented to our clinic for follow-up and the mass had reduced in size and appeared to be spontaneously resolving.

We present a unique case of a migrating nodule that occurred after injection with calcium hydroxylapatite, which led to concern for neoplastic tumor formation. This complication is rare, and it is important for practitioners who inject calcium hydroxylapatite as well as those who these patients may be referred to for evaluation to be aware that migrating nodules can occur. This awareness can help reduce unnecessary referrals, medical procedures, and anxiety.
Calcium hydroxylapatite filler is composed of 30% calcium hydroxylapatite microspheres suspended in a 70% sodium carboxymethylcellulose gel. The water-soluble gel rapidly becomes absorbed upon injection; however, the microspheres form a scaffold for the production of newly synthesized collagen. The filling effect generally lasts 12 to 18 months.1
Calcium hydroxylapatite, similar to most fillers, generally is well tolerated with a low complication rate of 3%.1 Although nodule formation with calcium hydroxylapatite is rare, it is the most common adverse event and encompasses 96% of complications. The remaining 4% of complications include persistent inflammation, swelling, erythema, and technical mistakes leading to overcorrection.1 Migrating nodules are rare; however, Beer3 reported a similar case.
Treatment of calcium hydroxylapatite nodules depends on differentiating a cause based on the time of onset. Early nodules that occur within 1 to 2 weeks of the injection usually represent incorrect positioning of the filler and can be treated by massaging the nodule. Other more invasive techniques involve aspiration or injection of sterile water. Late-onset nodules have shown response to corticosteroid injections. For inflammatory nodules of infectious origin, antibiotics can be useful. Surgical excision of the nodule rarely is required, as most nodules will resolve spontaneously, even without intervention.1,2
Radiologic findings of calcium hydroxylapatite appear as high-attenuation linear streaks or masses on CT (280–700 HU) and as low to intermediate signal intensity on T1- or T2-weighted sequences on magnetic resonance imaging. Oftentimes, calcium hydroxylapatite has a similar radiographic appearance to bone and can persist for 2 years or more on radiographic imaging, longer than they are clinically visible.4 The nodule formation from injection with calcium hydroxylapatite can mimic pathologic conditions such as miliary osteomas, myositis ossificans, heterotrophic/dystrophic calcifications, and foreign bodies on CT. Our patient’s CT findings of high attenuation linear streaks and nodules of similar signal intensity to bone were consistent with those previously described in the radiographic literature.
Calcium hydroxylapatite fillers have a good safety profile, but it is important to recognize that nodule formation is a common adverse event and that migration of nodules can occur. Practitioners should recognize this possibility in patients presenting with new masses after filler injection before advocating for potentially invasive and costly procedures and diagnostic modalities.
To the Editor:
Calcium hydroxylapatite is an injectable filler approved by the US Food and Drug Administration for moderate to severe rhytides of the face and the treatment of facial lipodystrophy in patients with HIV.1 This long-lasting filler generally is well tolerated with minimal side effects; however, there have been reports of nodules or granulomatous formation following injection.2 We present a case of a migrating nodule following injection of a calcium hydroxylapatite filler that appeared ossified on radiographic imaging. We highlight this rarely reported phenomenon to increase awareness of this complication.
A 72-year-old woman presented to our clinic with a mass on the left cheek. The patient had a history of treatment with facial fillers but no notable medical conditions. She initially received hyaluronic acid injectable gel dermal filler twice—3 years apart—before switching to calcium hydroxylapatite injections twice—4 months apart—from an outside provider. One month after the second treatment, she noticed a mass on the left cheek and promptly returned to the provider who performed the calcium hydroxylapatite injections. The provider, who had originally injected in the infraorbital area, stated it was unlikely that the filler would have migrated to the mid cheek and referred the patient to a general dentist who suspected salivary gland pathology. The patient was referred to an oral and maxillofacial surgeon who suspected the mass was related to the parotid gland. Maxillofacial computed tomography (CT) revealed heterotopic ossification vs myositis ossificans, possibly related to the recent injection. The patient was eventually referred to the Division of Plastic Surgery, Department of Surgery, at the University of Texas Medical Branch (Galveston, Texas) for further evaluation. Physical examination revealed a 2×1-cm firm, mobile, nontender mass in the left cheek in the area of the buccinator muscles. The mass did not express any fluid and was most easily palpable from the oral cavity. Radiography findings showed that the calcium hydroxylapatite filler had migrated to this location and formed a nodule (Figure). Because calcium hydroxylapatite fillers generally last 12 to 18 months, we opted to observe the lesion for spontaneous resolution. Four months later, the patient presented to our clinic for follow-up and the mass had reduced in size and appeared to be spontaneously resolving.

We present a unique case of a migrating nodule that occurred after injection with calcium hydroxylapatite, which led to concern for neoplastic tumor formation. This complication is rare, and it is important for practitioners who inject calcium hydroxylapatite as well as those who these patients may be referred to for evaluation to be aware that migrating nodules can occur. This awareness can help reduce unnecessary referrals, medical procedures, and anxiety.
Calcium hydroxylapatite filler is composed of 30% calcium hydroxylapatite microspheres suspended in a 70% sodium carboxymethylcellulose gel. The water-soluble gel rapidly becomes absorbed upon injection; however, the microspheres form a scaffold for the production of newly synthesized collagen. The filling effect generally lasts 12 to 18 months.1
Calcium hydroxylapatite, similar to most fillers, generally is well tolerated with a low complication rate of 3%.1 Although nodule formation with calcium hydroxylapatite is rare, it is the most common adverse event and encompasses 96% of complications. The remaining 4% of complications include persistent inflammation, swelling, erythema, and technical mistakes leading to overcorrection.1 Migrating nodules are rare; however, Beer3 reported a similar case.
Treatment of calcium hydroxylapatite nodules depends on differentiating a cause based on the time of onset. Early nodules that occur within 1 to 2 weeks of the injection usually represent incorrect positioning of the filler and can be treated by massaging the nodule. Other more invasive techniques involve aspiration or injection of sterile water. Late-onset nodules have shown response to corticosteroid injections. For inflammatory nodules of infectious origin, antibiotics can be useful. Surgical excision of the nodule rarely is required, as most nodules will resolve spontaneously, even without intervention.1,2
Radiologic findings of calcium hydroxylapatite appear as high-attenuation linear streaks or masses on CT (280–700 HU) and as low to intermediate signal intensity on T1- or T2-weighted sequences on magnetic resonance imaging. Oftentimes, calcium hydroxylapatite has a similar radiographic appearance to bone and can persist for 2 years or more on radiographic imaging, longer than they are clinically visible.4 The nodule formation from injection with calcium hydroxylapatite can mimic pathologic conditions such as miliary osteomas, myositis ossificans, heterotrophic/dystrophic calcifications, and foreign bodies on CT. Our patient’s CT findings of high attenuation linear streaks and nodules of similar signal intensity to bone were consistent with those previously described in the radiographic literature.
Calcium hydroxylapatite fillers have a good safety profile, but it is important to recognize that nodule formation is a common adverse event and that migration of nodules can occur. Practitioners should recognize this possibility in patients presenting with new masses after filler injection before advocating for potentially invasive and costly procedures and diagnostic modalities.
- Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol. 2017;16:152-161.
- Moulinets I, Arnaud E, Bui P, et al. Foreign body reaction to Radiesse: 2 cases. Am J Dermatopathol. 2013;35:e37-40.
- Beer KR. Radiesse nodule of the lips from a distant injection site: report of a case and consideration of etiology and management. J Drugs Dermatol. 2007;6:846-847.
- Ginat DT, Schatz CJ. Imaging features of midface injectable fillers and associated complications. AJNR Am J Neuroradiol. 2013;34:1488-1495.
- Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol. 2017;16:152-161.
- Moulinets I, Arnaud E, Bui P, et al. Foreign body reaction to Radiesse: 2 cases. Am J Dermatopathol. 2013;35:e37-40.
- Beer KR. Radiesse nodule of the lips from a distant injection site: report of a case and consideration of etiology and management. J Drugs Dermatol. 2007;6:846-847.
- Ginat DT, Schatz CJ. Imaging features of midface injectable fillers and associated complications. AJNR Am J Neuroradiol. 2013;34:1488-1495.
Practice Points
- Calcium hydroxylapatite filler can migrate and form nodules in distant locations from the original injection site.
- Practitioners of calcium hydroxylapatite fillers should be aware of the potential for nodule migration to avoid costly, time-consuming, and invasive referrals and procedures.