User login
Using measurement-based care to improve outcomes for patients with depression
Ms. H, age 42, is being treated by her family physician for her second episode of major depressive disorder (MDD). When she was 35, Ms. H experienced her first episode of MDD, which was successfully treated with
At the 8-week follow-up appointment, the physician notes how much better Ms. H seems to be doing. He says that because she has had such a good response, she should continue the fluoxetine and come back in 3 months. Later that evening, Ms. H reflects on her visit. Although she feels better, she still does not feel normal. In fact, she is not sure that she has really felt normal since before her first depressive episode. Ms. H decides to see a psychiatrist.
At her first appointment, the psychiatrist asks Ms. H to complete the Quick Inventory of Depressive Symptoms–Self Rated (QIDS-SR) scale. Her QIDS-SR score is 6, which is consistent with mild residual symptoms of depression.1 The psychiatrist increases the fluoxetine dosage to 40 mg/d and recommends that she complete a course of cognitive-behavioral therapy (CBT).
Although psychiatry currently does not have tests that provide continuous data such as blood pressure or HbA1c, well-validated rating scales can help clinicians in getting their patients to achieve symptom remission. Measurement-based care is the “systematic use of measurement tools to monitor progress and guide treatment choices.”1 Originally, psychometric rating scales were designed for research; typically, they were administered by the clinician, and were too long to be used in routine outpatient clinical practice. Subsequently, it was determined that patients without psychotic symptoms or cognitive deficits can accurately assess their own symptoms, and this led to the development of short self-assessment scales that have a high level of reliability when compared with longer, clinician-administered instruments. Despite the availability of several validated, brief rating scales, it is estimated that only approximately 18% of psychiatrists use them in clinical practice.2
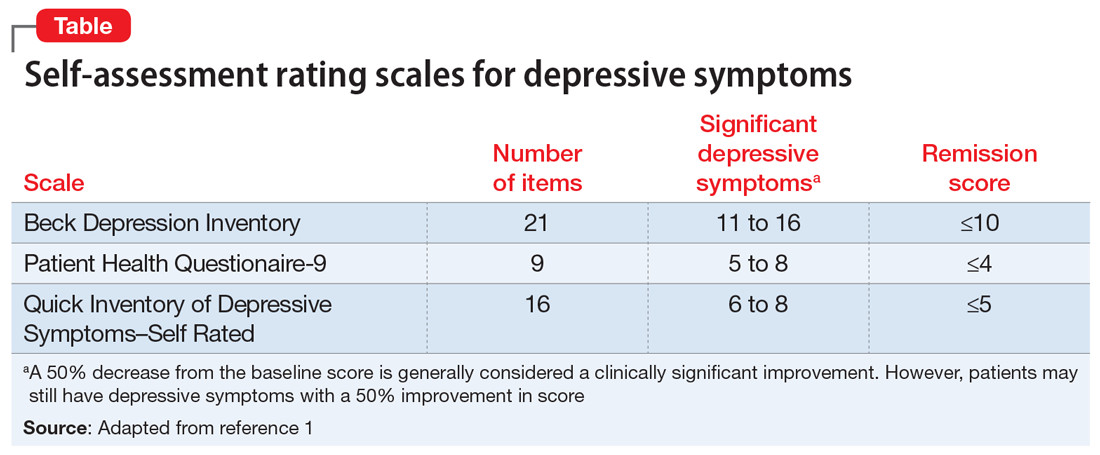
Self-rated scales for depression have been shown to be as valid as clinician-rated scales. For depression, the Patient Health Questionaire-9 (PHQ-9), based on the 9 symptom criteria associated with a diagnosis of MDD, is likely the most commonly used self-assessment scale.1 However, the QIDS-SR and the Beck Depression Inventory are both well-validated.1 In particular, QIDS-SR scores and score changes have been shown to be comparable with those on the QIDS-Clinician Rating (QIDS-C) scale.3 A 50% decrease in score typically is defined as a clinical response. Remission of symptoms is often defined as a score ≤4 on the PHQ-9 or ≤5 on the QIDS-SR (Table1). Similar to laboratory tests, rating scales are not diagnostic, but are a piece of information for the clinician to use in making diagnostic and treatment decisions.
The use of brief rating scales can help identify symptoms that may not come up in discussion with the patient, and it provides a systematic method of reviewing symptoms. Patients may be encouraged when they see a decrease in their scores after beginning treatment.2 Patients with depression need to complete rating scales frequently, just as a patient with hypertension would need their blood pressure frequently monitored.2 Frequent measurement with rating scales may help identify residual depressive symptoms that indicate the need for additional intervention. Residual depressive symptoms are the best predictor of the recurrence of depression, and treatment to remission is essential in preventing recurrence. In fact, recurrence is 2 to 3 times more likely in patients who do not achieve remission.1
Continue to: Optimizing the use of self-rating scales...
Optimizing the use of self-rating scales
To save time, patients can complete a rating scale before seeing the clinician, and the use of computerized applications can automatically sum scores and plot response graphs.4 Some researchers have suggested that some patients may be more honest in completing a self-assessment than in their verbal responses to the clinician.4 It is important to discuss the rating scale results with the patient.2 With a newly diagnosed patient, goals for treatment and the treatment plan can be outlined. During follow-up visits, clinicians should note areas of improvement and provide encouragement. If the patient’s symptoms are not improving appropriately, the clinician should discuss treatment options and offer the patient hope. This may improve the patient’s engagement in care and their understanding of how symptoms are associated with their illness.2 Studies have suggested that the use of validated rating tools (along with other interventions) can result in faster improvement in symptoms and higher response rates, and can assist in achieving remission.1,2,5
CASE CONTINUED
After 6 weeks of CBT and the increased fluoxetine dose, Ms. H returns to her psychiatrist for a follow-up visit. Her QIDS-SR score is 4, which is down from her initial score of 6. Ms. H is elated when she sees that her symptoms score has decreased since the previous visit. To confirm this finding, the psychiatrist completes the QIDS-C, and records a score of 3. The psychiatrist discusses the appropriate continuation of fluoxetine and CBT.
In this case, the use of a brief clinical rating scale helped Ms. H’s psychiatrist identify residual depressive symptoms and modify treatment so that she achieved remission. Using patient-reported outcomes also helps facilitate meaningful conversations between the patient and clinician and helps identify symptoms suggestive of relapse.2 Although this case focused on the use of measurement-based care in depression, brief symptom rating scales for most major psychiatric disorders—many of them self-assessments—also are available, as are brief rating scales to assess medication adverse effects and adherence.5
Just as clinicians in other areas of medicine use assessments such as laboratory tests and blood pressure monitoring for initial assessment and in following response to treatment, measurement-based care allows for a quasi-objective evaluation of patients with psychiatric disorders. Improved response rates, time to response, and patient engagement are all positive results of measurement-based care
Related Resources
- Martin-Cook K, Palmer L, Thornton L, et al. Setting measurement-based care in motion: practical lessons in the implementation and integration of measurement-based care in psychiatry clinical practice. Neuropsychiatric Disease & Treatment. 2021;17:1621-1631.
- Aboraya A, Nasrallah HA, Elswick DE, et al. Measurementbased care in psychiatry-past, present, and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
Drug Brand Names
Fluoxetine • Prozac
- Self-rated scales are believed to be as reliable as clinician-rated scales in assessing symptoms in patients who are not cognitively impaired.
- The use of rating scales can enhance engagement of the patient with the clinician.
- Utilizing computer- or smartphone appbased rating scales allows for automatic scoring and graphing.
- The use of rating scales in the pharmacotherapy of depression has been associated with more rapid symptoms improvement, greater response rates, and a greater likelihood of achieving remission.
- Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry 2009;70(suppl 6):26-31. doi:10.4088/ JCP.8133su1c.04
- Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76(3):324-335.
- Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
- Trivedi MH, Papakostas GI, Jackson WC, et al. Implementing measurement-based care to determine and treat inadequate response. J Clin Psychiatry 2020;81(3):OT19037BR1. doi: 10.4088/JCP.OT19037BR1
- Morris DW, Trivedi MH. Measurement-based care for unipolar depression. Curr Psychiatry Rep. 2011;13(6):446-458.
Ms. H, age 42, is being treated by her family physician for her second episode of major depressive disorder (MDD). When she was 35, Ms. H experienced her first episode of MDD, which was successfully treated with
At the 8-week follow-up appointment, the physician notes how much better Ms. H seems to be doing. He says that because she has had such a good response, she should continue the fluoxetine and come back in 3 months. Later that evening, Ms. H reflects on her visit. Although she feels better, she still does not feel normal. In fact, she is not sure that she has really felt normal since before her first depressive episode. Ms. H decides to see a psychiatrist.
At her first appointment, the psychiatrist asks Ms. H to complete the Quick Inventory of Depressive Symptoms–Self Rated (QIDS-SR) scale. Her QIDS-SR score is 6, which is consistent with mild residual symptoms of depression.1 The psychiatrist increases the fluoxetine dosage to 40 mg/d and recommends that she complete a course of cognitive-behavioral therapy (CBT).
Although psychiatry currently does not have tests that provide continuous data such as blood pressure or HbA1c, well-validated rating scales can help clinicians in getting their patients to achieve symptom remission. Measurement-based care is the “systematic use of measurement tools to monitor progress and guide treatment choices.”1 Originally, psychometric rating scales were designed for research; typically, they were administered by the clinician, and were too long to be used in routine outpatient clinical practice. Subsequently, it was determined that patients without psychotic symptoms or cognitive deficits can accurately assess their own symptoms, and this led to the development of short self-assessment scales that have a high level of reliability when compared with longer, clinician-administered instruments. Despite the availability of several validated, brief rating scales, it is estimated that only approximately 18% of psychiatrists use them in clinical practice.2
Self-rated scales for depression have been shown to be as valid as clinician-rated scales. For depression, the Patient Health Questionaire-9 (PHQ-9), based on the 9 symptom criteria associated with a diagnosis of MDD, is likely the most commonly used self-assessment scale.1 However, the QIDS-SR and the Beck Depression Inventory are both well-validated.1 In particular, QIDS-SR scores and score changes have been shown to be comparable with those on the QIDS-Clinician Rating (QIDS-C) scale.3 A 50% decrease in score typically is defined as a clinical response. Remission of symptoms is often defined as a score ≤4 on the PHQ-9 or ≤5 on the QIDS-SR (Table1). Similar to laboratory tests, rating scales are not diagnostic, but are a piece of information for the clinician to use in making diagnostic and treatment decisions.

The use of brief rating scales can help identify symptoms that may not come up in discussion with the patient, and it provides a systematic method of reviewing symptoms. Patients may be encouraged when they see a decrease in their scores after beginning treatment.2 Patients with depression need to complete rating scales frequently, just as a patient with hypertension would need their blood pressure frequently monitored.2 Frequent measurement with rating scales may help identify residual depressive symptoms that indicate the need for additional intervention. Residual depressive symptoms are the best predictor of the recurrence of depression, and treatment to remission is essential in preventing recurrence. In fact, recurrence is 2 to 3 times more likely in patients who do not achieve remission.1
Continue to: Optimizing the use of self-rating scales...
Optimizing the use of self-rating scales
To save time, patients can complete a rating scale before seeing the clinician, and the use of computerized applications can automatically sum scores and plot response graphs.4 Some researchers have suggested that some patients may be more honest in completing a self-assessment than in their verbal responses to the clinician.4 It is important to discuss the rating scale results with the patient.2 With a newly diagnosed patient, goals for treatment and the treatment plan can be outlined. During follow-up visits, clinicians should note areas of improvement and provide encouragement. If the patient’s symptoms are not improving appropriately, the clinician should discuss treatment options and offer the patient hope. This may improve the patient’s engagement in care and their understanding of how symptoms are associated with their illness.2 Studies have suggested that the use of validated rating tools (along with other interventions) can result in faster improvement in symptoms and higher response rates, and can assist in achieving remission.1,2,5
CASE CONTINUED
After 6 weeks of CBT and the increased fluoxetine dose, Ms. H returns to her psychiatrist for a follow-up visit. Her QIDS-SR score is 4, which is down from her initial score of 6. Ms. H is elated when she sees that her symptoms score has decreased since the previous visit. To confirm this finding, the psychiatrist completes the QIDS-C, and records a score of 3. The psychiatrist discusses the appropriate continuation of fluoxetine and CBT.
In this case, the use of a brief clinical rating scale helped Ms. H’s psychiatrist identify residual depressive symptoms and modify treatment so that she achieved remission. Using patient-reported outcomes also helps facilitate meaningful conversations between the patient and clinician and helps identify symptoms suggestive of relapse.2 Although this case focused on the use of measurement-based care in depression, brief symptom rating scales for most major psychiatric disorders—many of them self-assessments—also are available, as are brief rating scales to assess medication adverse effects and adherence.5
Just as clinicians in other areas of medicine use assessments such as laboratory tests and blood pressure monitoring for initial assessment and in following response to treatment, measurement-based care allows for a quasi-objective evaluation of patients with psychiatric disorders. Improved response rates, time to response, and patient engagement are all positive results of measurement-based care
Related Resources
- Martin-Cook K, Palmer L, Thornton L, et al. Setting measurement-based care in motion: practical lessons in the implementation and integration of measurement-based care in psychiatry clinical practice. Neuropsychiatric Disease & Treatment. 2021;17:1621-1631.
- Aboraya A, Nasrallah HA, Elswick DE, et al. Measurementbased care in psychiatry-past, present, and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
Drug Brand Names
Fluoxetine • Prozac
- Self-rated scales are believed to be as reliable as clinician-rated scales in assessing symptoms in patients who are not cognitively impaired.
- The use of rating scales can enhance engagement of the patient with the clinician.
- Utilizing computer- or smartphone appbased rating scales allows for automatic scoring and graphing.
- The use of rating scales in the pharmacotherapy of depression has been associated with more rapid symptoms improvement, greater response rates, and a greater likelihood of achieving remission.
Ms. H, age 42, is being treated by her family physician for her second episode of major depressive disorder (MDD). When she was 35, Ms. H experienced her first episode of MDD, which was successfully treated with
At the 8-week follow-up appointment, the physician notes how much better Ms. H seems to be doing. He says that because she has had such a good response, she should continue the fluoxetine and come back in 3 months. Later that evening, Ms. H reflects on her visit. Although she feels better, she still does not feel normal. In fact, she is not sure that she has really felt normal since before her first depressive episode. Ms. H decides to see a psychiatrist.
At her first appointment, the psychiatrist asks Ms. H to complete the Quick Inventory of Depressive Symptoms–Self Rated (QIDS-SR) scale. Her QIDS-SR score is 6, which is consistent with mild residual symptoms of depression.1 The psychiatrist increases the fluoxetine dosage to 40 mg/d and recommends that she complete a course of cognitive-behavioral therapy (CBT).
Although psychiatry currently does not have tests that provide continuous data such as blood pressure or HbA1c, well-validated rating scales can help clinicians in getting their patients to achieve symptom remission. Measurement-based care is the “systematic use of measurement tools to monitor progress and guide treatment choices.”1 Originally, psychometric rating scales were designed for research; typically, they were administered by the clinician, and were too long to be used in routine outpatient clinical practice. Subsequently, it was determined that patients without psychotic symptoms or cognitive deficits can accurately assess their own symptoms, and this led to the development of short self-assessment scales that have a high level of reliability when compared with longer, clinician-administered instruments. Despite the availability of several validated, brief rating scales, it is estimated that only approximately 18% of psychiatrists use them in clinical practice.2
Self-rated scales for depression have been shown to be as valid as clinician-rated scales. For depression, the Patient Health Questionaire-9 (PHQ-9), based on the 9 symptom criteria associated with a diagnosis of MDD, is likely the most commonly used self-assessment scale.1 However, the QIDS-SR and the Beck Depression Inventory are both well-validated.1 In particular, QIDS-SR scores and score changes have been shown to be comparable with those on the QIDS-Clinician Rating (QIDS-C) scale.3 A 50% decrease in score typically is defined as a clinical response. Remission of symptoms is often defined as a score ≤4 on the PHQ-9 or ≤5 on the QIDS-SR (Table1). Similar to laboratory tests, rating scales are not diagnostic, but are a piece of information for the clinician to use in making diagnostic and treatment decisions.

The use of brief rating scales can help identify symptoms that may not come up in discussion with the patient, and it provides a systematic method of reviewing symptoms. Patients may be encouraged when they see a decrease in their scores after beginning treatment.2 Patients with depression need to complete rating scales frequently, just as a patient with hypertension would need their blood pressure frequently monitored.2 Frequent measurement with rating scales may help identify residual depressive symptoms that indicate the need for additional intervention. Residual depressive symptoms are the best predictor of the recurrence of depression, and treatment to remission is essential in preventing recurrence. In fact, recurrence is 2 to 3 times more likely in patients who do not achieve remission.1
Continue to: Optimizing the use of self-rating scales...
Optimizing the use of self-rating scales
To save time, patients can complete a rating scale before seeing the clinician, and the use of computerized applications can automatically sum scores and plot response graphs.4 Some researchers have suggested that some patients may be more honest in completing a self-assessment than in their verbal responses to the clinician.4 It is important to discuss the rating scale results with the patient.2 With a newly diagnosed patient, goals for treatment and the treatment plan can be outlined. During follow-up visits, clinicians should note areas of improvement and provide encouragement. If the patient’s symptoms are not improving appropriately, the clinician should discuss treatment options and offer the patient hope. This may improve the patient’s engagement in care and their understanding of how symptoms are associated with their illness.2 Studies have suggested that the use of validated rating tools (along with other interventions) can result in faster improvement in symptoms and higher response rates, and can assist in achieving remission.1,2,5
CASE CONTINUED
After 6 weeks of CBT and the increased fluoxetine dose, Ms. H returns to her psychiatrist for a follow-up visit. Her QIDS-SR score is 4, which is down from her initial score of 6. Ms. H is elated when she sees that her symptoms score has decreased since the previous visit. To confirm this finding, the psychiatrist completes the QIDS-C, and records a score of 3. The psychiatrist discusses the appropriate continuation of fluoxetine and CBT.
In this case, the use of a brief clinical rating scale helped Ms. H’s psychiatrist identify residual depressive symptoms and modify treatment so that she achieved remission. Using patient-reported outcomes also helps facilitate meaningful conversations between the patient and clinician and helps identify symptoms suggestive of relapse.2 Although this case focused on the use of measurement-based care in depression, brief symptom rating scales for most major psychiatric disorders—many of them self-assessments—also are available, as are brief rating scales to assess medication adverse effects and adherence.5
Just as clinicians in other areas of medicine use assessments such as laboratory tests and blood pressure monitoring for initial assessment and in following response to treatment, measurement-based care allows for a quasi-objective evaluation of patients with psychiatric disorders. Improved response rates, time to response, and patient engagement are all positive results of measurement-based care
Related Resources
- Martin-Cook K, Palmer L, Thornton L, et al. Setting measurement-based care in motion: practical lessons in the implementation and integration of measurement-based care in psychiatry clinical practice. Neuropsychiatric Disease & Treatment. 2021;17:1621-1631.
- Aboraya A, Nasrallah HA, Elswick DE, et al. Measurementbased care in psychiatry-past, present, and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
Drug Brand Names
Fluoxetine • Prozac
- Self-rated scales are believed to be as reliable as clinician-rated scales in assessing symptoms in patients who are not cognitively impaired.
- The use of rating scales can enhance engagement of the patient with the clinician.
- Utilizing computer- or smartphone appbased rating scales allows for automatic scoring and graphing.
- The use of rating scales in the pharmacotherapy of depression has been associated with more rapid symptoms improvement, greater response rates, and a greater likelihood of achieving remission.
- Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry 2009;70(suppl 6):26-31. doi:10.4088/ JCP.8133su1c.04
- Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76(3):324-335.
- Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
- Trivedi MH, Papakostas GI, Jackson WC, et al. Implementing measurement-based care to determine and treat inadequate response. J Clin Psychiatry 2020;81(3):OT19037BR1. doi: 10.4088/JCP.OT19037BR1
- Morris DW, Trivedi MH. Measurement-based care for unipolar depression. Curr Psychiatry Rep. 2011;13(6):446-458.
- Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry 2009;70(suppl 6):26-31. doi:10.4088/ JCP.8133su1c.04
- Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76(3):324-335.
- Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
- Trivedi MH, Papakostas GI, Jackson WC, et al. Implementing measurement-based care to determine and treat inadequate response. J Clin Psychiatry 2020;81(3):OT19037BR1. doi: 10.4088/JCP.OT19037BR1
- Morris DW, Trivedi MH. Measurement-based care for unipolar depression. Curr Psychiatry Rep. 2011;13(6):446-458.
Calcineurin inhibitor–induced psychosis
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.
Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.

Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.

Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
Persistent altered mental status
CASE Sluggish, weak, and incoherent
Mr. O, age 24, who has a history of schizophrenia and obesity, presents to the emergency department (ED) for altered mental status (AMS). His mother reports that he has been sluggish, weak, incoherent, had no appetite, and that on the day before admission, he was drinking excessive amounts of water and urinating every 10 minutes.
HISTORY Multiple ineffective antipsychotics
Mr. O was diagnosed with schizophrenia at age 21 and struggled with medication adherence, which resulted in multiple hospitalizations for stabilization. Trials of haloperidol, risperidone, paliperidone palmitate, and valproic acid had been ineffective. At the time of admission, his psychotropic medication regimen is fluphenazine decanoate, 25 mg injection every 2 weeks; clozapine, 50 mg/d; lithium carbonate, 300 mg twice a day; benztropine, 2 mg every night; and trazodone, 50 mg every night.
EVALUATION Fever, tachycardia, and diabetic ketoacidosis
Upon arrival to the ED, Mr. O is obtunded, unable to follow commands, and does not respond to painful stimuli. On physical exam, he has a fever of 38.4°C (reference range 35.1°C to 37.9°C); tachycardia with a heart rate of 142 beats per minute (bpm) (reference range 60 to 100); tachypnea with a respiratory rate of 35 breaths per minute (reference range 12 to 20); a blood pressure of 116/76 mmHg (reference range 90/60 to 130/80); and hypoxemia with an oxygen saturation of 90% on room air (reference range 94% to 100%).
Mr. O is admitted to the hospital and his laboratory workup indicates diabetic ketoacidosis (DKA), with a glucose of 1,700 mg/dL; anion gap of 30 (reference range 4 to 12 mmol/L); pH 7.04 (reference range 7.32 to 7.42); serum bicarbonate 6 (reference range 20 to 24 mEq/L); beta-hydroxybutyrate 11.04 (reference range 0 to 0.27 mmol/L); urine ketones, serum osmolality 407 (reference range 280 to 300 mOsm/kg); and an elevated white blood cell count of 18.4 (reference range 4.5 to 11.0 × 109/L). A CT scan of the head is negative for acute pathology.
Initially, all psychotropic medications are held. On Day 3 of hospitalization, psychiatry is consulted and clozapine, 50 mg/d; lithium, 300 mg/d; and benztropine, 1 mg at night, are restarted; however, fluphenazine decanoate and trazodone are held. The team recommends IV haloperidol, 2 mg as needed for agitation; however, it is never administered.
Imaging rules out deep vein thrombosis, cardiac dysfunction, and stroke, but a CT chest scan is notable for bilateral lung infiltrates, which suggests aspiration pneumonia.
Mr. O is diagnosed with diabetes, complicated by DKA, and is treated in the intensive care unit (ICU). Despite resolution of the DKA, he remains altered with fever and tachycardia.
Continue to: On Day 6 of hospitalization...
On Day 6 of hospitalization, Mr. O continues to be tachycardic and obtunded with nuchal rigidity. The team decides to transfer Mr. O to another hospital for a higher level of care and continued workup of his persistent AMS.
Immediately upon arrival at the second hospital, infectious disease and neurology teams are consulted for further evaluation. Mr. O’s AMS continues despite no clear signs of infection or other neurologic insults.
[polldaddy:10930631]
The authors’ observations
Based on Mr. O’s psychiatric history and laboratory results, the first medical team concluded his initial AMS was likely secondary to DKA; however, the AMS continued after the DKA resolved. At the second hospital, Mr. O’s treatment team continued to dig for answers.
EVALUATION Exploring the differential diagnosis
At the second hospital, Mr. O is admitted to the ICU with fever (37.8°C), tachycardia (120 bpm), tachypnea, withdrawal from painful stimuli, decreased reflexes, and muscle rigidity, including clenched jaw. The differential diagnoses include meningitis, sepsis from aspiration pneumonia, severe metabolic encephalopathy with prolonged recovery, central pontine myelinolysis, anoxic brain injury, and subclinical seizures.
Empiric vancomycin, 1.75 g every 12 hours; ceftriaxone, 2 g/d; and acyclovir, 900 mg every 8 hours are started for meningoencephalitis, and all psychotropic medications are discontinued. Case reports have documented a relationship between hyperglycemic hyperosmolar syndrome (HHS) and malignant hyperthermia in rare cases1; however, HHS is ruled out based on Mr. O’s laboratory results.A lumbar puncture and imaging rules out CNS infection. Antibiotic treatment is narrowed to ampicillin-sulbactam due to Mr. O’s prior CT chest showing concern for aspiration pneumonia. An MRI of the brain rules out central pontine myelinolysis, acute stroke, and anoxic brain injury, and an EEG shows nonspecific encephalopathy. On Day 10 of hospitalization, a neurologic exam shows flaccid paralysis and bilateral clonus, and Mr. O is mute. On Day 14 of hospitalization, his fever resolves, and his blood cultures are negative. On Day 15 of hospitalization, Mr. O’s creatine kinase (CK) level is elevated at 1,308 U/L (reference range 26 to 192 U/L), suggesting rhabdomyolysis.
Continue to: Given the neurologic exam findings...
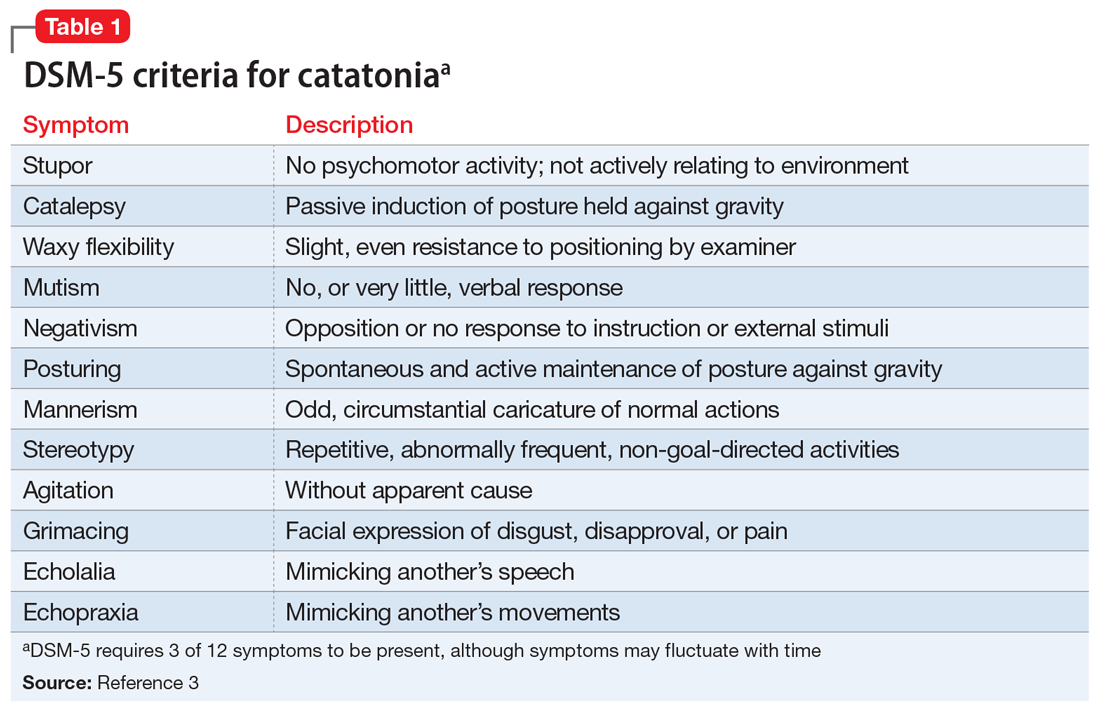
Given the neurologic exam findings, and the limited evidence of infection, the differential diagnosis for Mr. O’s AMS is broadened to include catatonia, neuroleptic malignant syndrome (NMS), serotonin syndrome, and autoimmune encephalitis. The psychiatry team evaluates Mr. O for catatonia. He scores 14 on the Bush-Francis Catatonia Rating Scale, with findings of immobility/stupor, mutism, staring, autonomic instability, and withdrawal indicating the presence of catatonia.2
The authors’ observations
When Mr. O was transferred to the second hospital, the primary concern was to rule out meningitis due to his unstable vitals, obtunded mental state, and nuchal rigidity. A comprehensive infectious workup, including lumbar puncture, was imperative because infection can not only lead to AMS, but also precipitate episodes of DKA. Mr. O’s persistently abnormal vital signs indicated an underlying process may have been missed by focusing on treating DKA.
TREATMENT Finally, the diagnosis is established
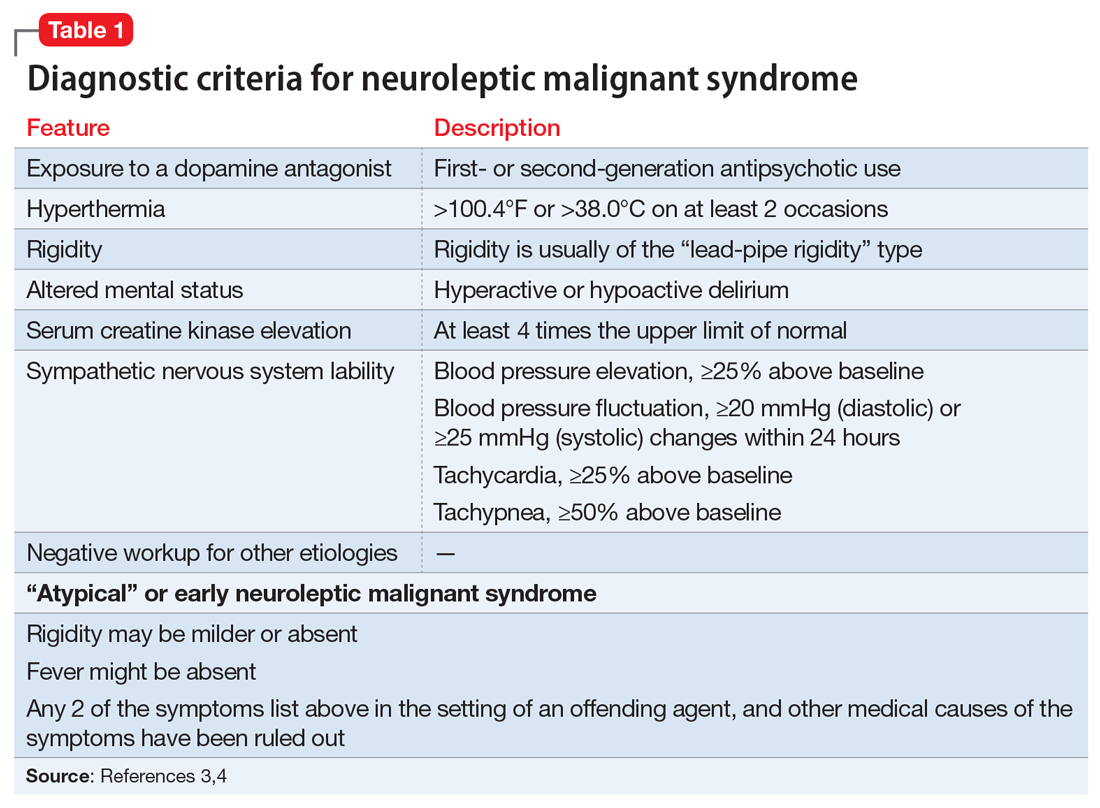
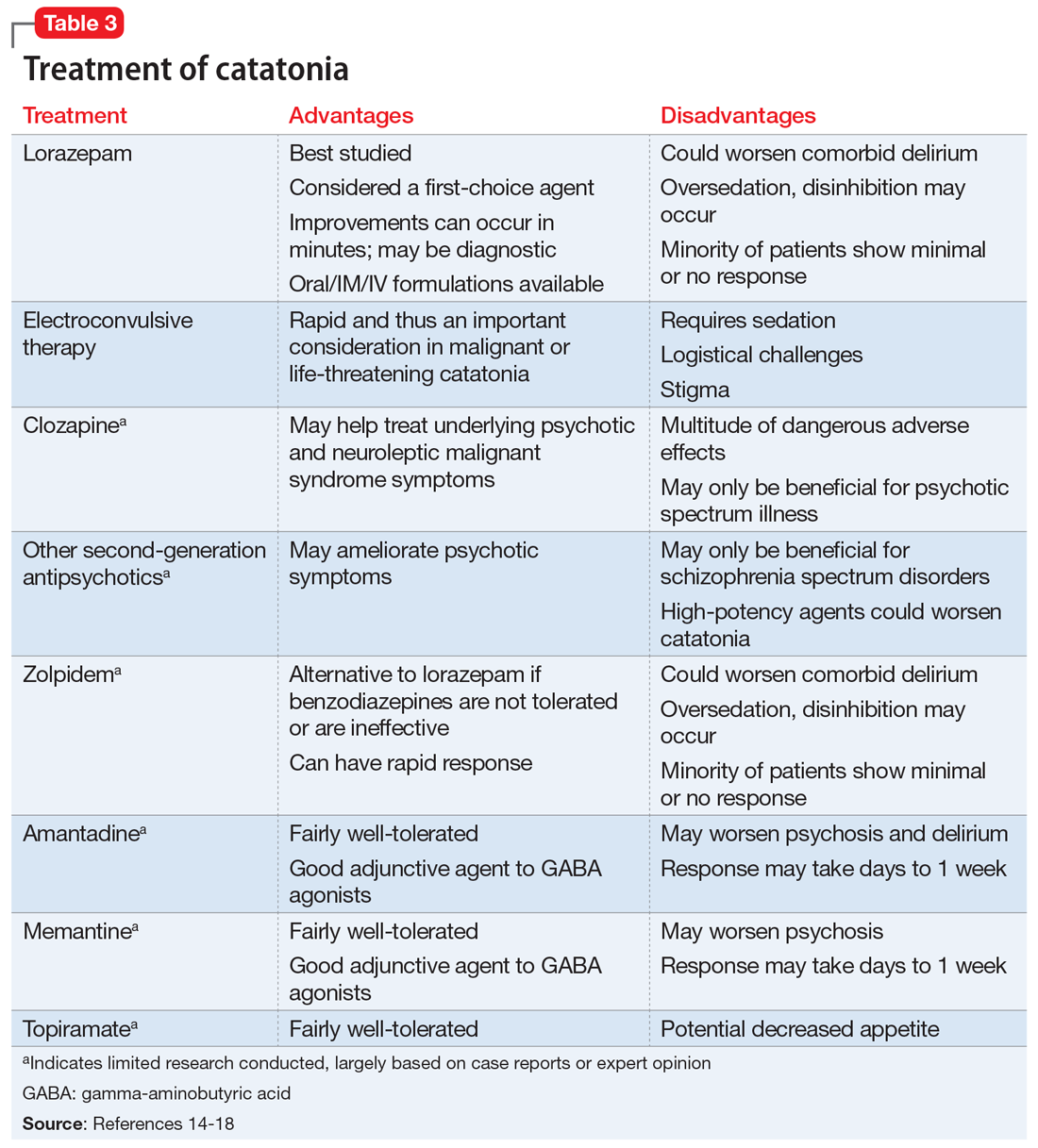
A lorazepam challenge is performed, and Mr. O receives 4 mg of lorazepam over 24 hours with little change in his catatonia symptoms. Given his persistent fever, tachycardia, and an elevated CK levels in the context of recent exposure to antipsychotic medications, Mr. O is diagnosed with NMS (Table 13,4 ) and is started on bromocriptine, 5 mg 3 times daily.
[polldaddy:10930632]
The authors’ observations
Mr. O’s complicated medical state—starting with DKA, halting the use of antipsychotic medications, and the suspicion of catatonia due to his history of schizophrenia—all distracted from the ultimate diagnosis of NMS as the cause of his enduring AMS and autonomic instability. Catatonia and NMS have overlapping symptomatology, including rigidity, autonomic instability, and stupor, which make the diagnosis of either condition complicated. A positive lorazepam test to diagnose catatonia is defined as a marked reduction in catatonia symptoms (typically a 50% reduction) as measured on a standardized rating scale.5 However, a negative lorazepam challenge does not definitely rule out catatonia because some cases are resistant to benzodiazepines.6
NMS risk factors relevant in this case include male sex, young age, acute medical illness, dehydration, and exposure to multiple psychotropic medications, including 2 antipsychotics, clozapine and fluphenazine.7 DKA is especially pertinent due to its acute onset and cause of significant dehydration. NMS can occur at any point of antipsychotic exposure, although the risk is highest during the initial weeks of treatment and during dosage changes. Unfortunately, Mr. O’s treatment team was unable to determine whether his medication had been recently changed, so it is not known what role this may have played in the development of NMS. Although first-generation antipsychotics are considered more likely to cause NMS, second-generation antipsychotics (SGAs) dominate the treatment of schizophrenia and bipolar disorder, and these medications also can cause NMS.8 As occurred in this case, long-acting injectable antipsychotics can be easily forgotten when not administered in the hospital, and their presence in the body persists for weeks. For example, the half-life of fluphenazine decanoate is approximately 10 days, and the half-life of haloperidol decanoate is 21 days.9
Continue to: OUTCOME Improvement with bromocriptine
OUTCOME Improvement with bromocriptine
After 4 days of bromocriptine, 5 mg 3 times daily, Mr. O is more alert, able to say “hello,” and can follow 1-step commands. By Day 26 of hospitalization, his CK levels decrease to 296 U/L, his CSF autoimmune panel is negative, and he is able to participate in physical therapy. After failing multiple swallow tests, Mr. O requires a percutaneous endoscopic gastrostomy (PEG) tube. He is discharged from the hospital to a long-term acute care facility with the plan to taper bromocriptine and restart a psychotropic regimen with his outpatient psychiatrist. At the time of discharge, he is able to sit at the edge of the bed independently, state his name, and respond to questions with multiple-word answers.
[polldaddy:10930633]
The authors’ observations
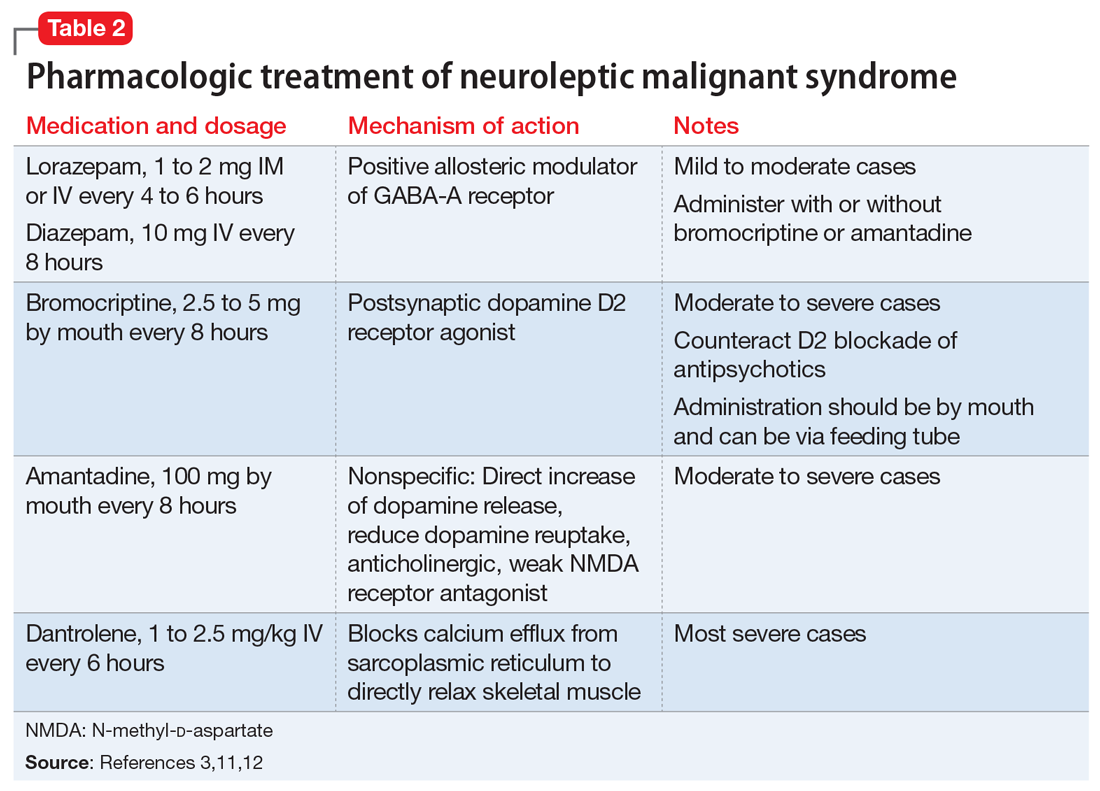
The most common pharmacologic treatments for NMS are dantrolene, bromocriptine, benzodiazepines (lorazepam or diazepam), and amantadine.3 Mild cases of NMS should be treated with discontinuation of all antipsychotics, supportive care, and benzodiazepines.3 Bromocriptine or amantadine are more appropriate for moderate cases and dantrolene for severe cases of NMS.3 All antipsychotics should be discontinued while a patient is experiencing an episode of NMS; however, once the NMS has resolved, clinicians must thoroughly evaluate the risks and benefits of restarting antipsychotic medication. After a patient has experienced an episode of NMS, clinicians generally should avoid prescribing the agent(s) that caused NMS and long-acting injections, and slowly titrate a low-potency SGA such as quetiapine.10Table 23,11,12 outlines the pharmacologic treatment of NMS.
Bottom Line
Neuroleptic malignant syndrome (NMS) should always be part of the differential diagnosis in patients with mental illness and altered mental status. The risk of NMS is especially high in patients with acute medical illness and exposure to antipsychotic medications.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Acyclovir • Zovirax
Amantadine • Gocovri
Ampicillin-sulbactam • Unasyn
Aripiprazole • Abilify Maintena
Benztropine • Cogentin
Bromocriptine • Cycloset, Parlodel
Ceftriaxone • Rocephin
Clozapine • Clozaril
Dantrolene • Dantrium
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Valproate sodium • Depakote
Trazodone • Oleptro
Vancomycin • Vancocin
1. Zeitler P, Haqq A, Rosenbloom A, et al. Hyperglycemic hyperosmolar syndrome in children: pathophysiological considerations and suggested guidelines for treatment. J Pediatr. 2011;158(1):9-14.e1-2. doi: 10.1016/j.jpeds.2010.09.048
2. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185. doi: 10.1007/s11920-010-0113-y
3. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981. doi:10.1177/1060028016657553
4. Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011;72(9):1222-1228. doi:10.4088/JCP.10m06438
5. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181. doi:10.3389/fpsyt.2014.00181
6. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380. doi:10.1176/jnp.2009.21.4.371
7. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin. 2004;22(2):389-411. doi:10.1016/j.ncl.2003.12.006
8. Tse L, Barr AM, Scarapicchia V, et al. Neuroleptic malignant syndrome: a review from a clinically oriented perspective. Curr Neuropharmacol. 2015;13(3):395-406. doi:10.2174/1570159x13999150424113345
9. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59. doi:10.1007/s40263-020-00779-5
10. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876. doi:10.1176/ajp.2007.164.6.870
11. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
12. Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care. 2007;11(1):R4. doi:10.1186/cc5148
CASE Sluggish, weak, and incoherent
Mr. O, age 24, who has a history of schizophrenia and obesity, presents to the emergency department (ED) for altered mental status (AMS). His mother reports that he has been sluggish, weak, incoherent, had no appetite, and that on the day before admission, he was drinking excessive amounts of water and urinating every 10 minutes.
HISTORY Multiple ineffective antipsychotics
Mr. O was diagnosed with schizophrenia at age 21 and struggled with medication adherence, which resulted in multiple hospitalizations for stabilization. Trials of haloperidol, risperidone, paliperidone palmitate, and valproic acid had been ineffective. At the time of admission, his psychotropic medication regimen is fluphenazine decanoate, 25 mg injection every 2 weeks; clozapine, 50 mg/d; lithium carbonate, 300 mg twice a day; benztropine, 2 mg every night; and trazodone, 50 mg every night.
EVALUATION Fever, tachycardia, and diabetic ketoacidosis
Upon arrival to the ED, Mr. O is obtunded, unable to follow commands, and does not respond to painful stimuli. On physical exam, he has a fever of 38.4°C (reference range 35.1°C to 37.9°C); tachycardia with a heart rate of 142 beats per minute (bpm) (reference range 60 to 100); tachypnea with a respiratory rate of 35 breaths per minute (reference range 12 to 20); a blood pressure of 116/76 mmHg (reference range 90/60 to 130/80); and hypoxemia with an oxygen saturation of 90% on room air (reference range 94% to 100%).
Mr. O is admitted to the hospital and his laboratory workup indicates diabetic ketoacidosis (DKA), with a glucose of 1,700 mg/dL; anion gap of 30 (reference range 4 to 12 mmol/L); pH 7.04 (reference range 7.32 to 7.42); serum bicarbonate 6 (reference range 20 to 24 mEq/L); beta-hydroxybutyrate 11.04 (reference range 0 to 0.27 mmol/L); urine ketones, serum osmolality 407 (reference range 280 to 300 mOsm/kg); and an elevated white blood cell count of 18.4 (reference range 4.5 to 11.0 × 109/L). A CT scan of the head is negative for acute pathology.
Initially, all psychotropic medications are held. On Day 3 of hospitalization, psychiatry is consulted and clozapine, 50 mg/d; lithium, 300 mg/d; and benztropine, 1 mg at night, are restarted; however, fluphenazine decanoate and trazodone are held. The team recommends IV haloperidol, 2 mg as needed for agitation; however, it is never administered.
Imaging rules out deep vein thrombosis, cardiac dysfunction, and stroke, but a CT chest scan is notable for bilateral lung infiltrates, which suggests aspiration pneumonia.
Mr. O is diagnosed with diabetes, complicated by DKA, and is treated in the intensive care unit (ICU). Despite resolution of the DKA, he remains altered with fever and tachycardia.
Continue to: On Day 6 of hospitalization...
On Day 6 of hospitalization, Mr. O continues to be tachycardic and obtunded with nuchal rigidity. The team decides to transfer Mr. O to another hospital for a higher level of care and continued workup of his persistent AMS.
Immediately upon arrival at the second hospital, infectious disease and neurology teams are consulted for further evaluation. Mr. O’s AMS continues despite no clear signs of infection or other neurologic insults.
[polldaddy:10930631]
The authors’ observations
Based on Mr. O’s psychiatric history and laboratory results, the first medical team concluded his initial AMS was likely secondary to DKA; however, the AMS continued after the DKA resolved. At the second hospital, Mr. O’s treatment team continued to dig for answers.
EVALUATION Exploring the differential diagnosis
At the second hospital, Mr. O is admitted to the ICU with fever (37.8°C), tachycardia (120 bpm), tachypnea, withdrawal from painful stimuli, decreased reflexes, and muscle rigidity, including clenched jaw. The differential diagnoses include meningitis, sepsis from aspiration pneumonia, severe metabolic encephalopathy with prolonged recovery, central pontine myelinolysis, anoxic brain injury, and subclinical seizures.
Empiric vancomycin, 1.75 g every 12 hours; ceftriaxone, 2 g/d; and acyclovir, 900 mg every 8 hours are started for meningoencephalitis, and all psychotropic medications are discontinued. Case reports have documented a relationship between hyperglycemic hyperosmolar syndrome (HHS) and malignant hyperthermia in rare cases1; however, HHS is ruled out based on Mr. O’s laboratory results.A lumbar puncture and imaging rules out CNS infection. Antibiotic treatment is narrowed to ampicillin-sulbactam due to Mr. O’s prior CT chest showing concern for aspiration pneumonia. An MRI of the brain rules out central pontine myelinolysis, acute stroke, and anoxic brain injury, and an EEG shows nonspecific encephalopathy. On Day 10 of hospitalization, a neurologic exam shows flaccid paralysis and bilateral clonus, and Mr. O is mute. On Day 14 of hospitalization, his fever resolves, and his blood cultures are negative. On Day 15 of hospitalization, Mr. O’s creatine kinase (CK) level is elevated at 1,308 U/L (reference range 26 to 192 U/L), suggesting rhabdomyolysis.
Continue to: Given the neurologic exam findings...
Given the neurologic exam findings, and the limited evidence of infection, the differential diagnosis for Mr. O’s AMS is broadened to include catatonia, neuroleptic malignant syndrome (NMS), serotonin syndrome, and autoimmune encephalitis. The psychiatry team evaluates Mr. O for catatonia. He scores 14 on the Bush-Francis Catatonia Rating Scale, with findings of immobility/stupor, mutism, staring, autonomic instability, and withdrawal indicating the presence of catatonia.2
The authors’ observations
When Mr. O was transferred to the second hospital, the primary concern was to rule out meningitis due to his unstable vitals, obtunded mental state, and nuchal rigidity. A comprehensive infectious workup, including lumbar puncture, was imperative because infection can not only lead to AMS, but also precipitate episodes of DKA. Mr. O’s persistently abnormal vital signs indicated an underlying process may have been missed by focusing on treating DKA.
TREATMENT Finally, the diagnosis is established
A lorazepam challenge is performed, and Mr. O receives 4 mg of lorazepam over 24 hours with little change in his catatonia symptoms. Given his persistent fever, tachycardia, and an elevated CK levels in the context of recent exposure to antipsychotic medications, Mr. O is diagnosed with NMS (Table 13,4 ) and is started on bromocriptine, 5 mg 3 times daily.
[polldaddy:10930632]
The authors’ observations
Mr. O’s complicated medical state—starting with DKA, halting the use of antipsychotic medications, and the suspicion of catatonia due to his history of schizophrenia—all distracted from the ultimate diagnosis of NMS as the cause of his enduring AMS and autonomic instability. Catatonia and NMS have overlapping symptomatology, including rigidity, autonomic instability, and stupor, which make the diagnosis of either condition complicated. A positive lorazepam test to diagnose catatonia is defined as a marked reduction in catatonia symptoms (typically a 50% reduction) as measured on a standardized rating scale.5 However, a negative lorazepam challenge does not definitely rule out catatonia because some cases are resistant to benzodiazepines.6
NMS risk factors relevant in this case include male sex, young age, acute medical illness, dehydration, and exposure to multiple psychotropic medications, including 2 antipsychotics, clozapine and fluphenazine.7 DKA is especially pertinent due to its acute onset and cause of significant dehydration. NMS can occur at any point of antipsychotic exposure, although the risk is highest during the initial weeks of treatment and during dosage changes. Unfortunately, Mr. O’s treatment team was unable to determine whether his medication had been recently changed, so it is not known what role this may have played in the development of NMS. Although first-generation antipsychotics are considered more likely to cause NMS, second-generation antipsychotics (SGAs) dominate the treatment of schizophrenia and bipolar disorder, and these medications also can cause NMS.8 As occurred in this case, long-acting injectable antipsychotics can be easily forgotten when not administered in the hospital, and their presence in the body persists for weeks. For example, the half-life of fluphenazine decanoate is approximately 10 days, and the half-life of haloperidol decanoate is 21 days.9
Continue to: OUTCOME Improvement with bromocriptine
OUTCOME Improvement with bromocriptine
After 4 days of bromocriptine, 5 mg 3 times daily, Mr. O is more alert, able to say “hello,” and can follow 1-step commands. By Day 26 of hospitalization, his CK levels decrease to 296 U/L, his CSF autoimmune panel is negative, and he is able to participate in physical therapy. After failing multiple swallow tests, Mr. O requires a percutaneous endoscopic gastrostomy (PEG) tube. He is discharged from the hospital to a long-term acute care facility with the plan to taper bromocriptine and restart a psychotropic regimen with his outpatient psychiatrist. At the time of discharge, he is able to sit at the edge of the bed independently, state his name, and respond to questions with multiple-word answers.
[polldaddy:10930633]
The authors’ observations
The most common pharmacologic treatments for NMS are dantrolene, bromocriptine, benzodiazepines (lorazepam or diazepam), and amantadine.3 Mild cases of NMS should be treated with discontinuation of all antipsychotics, supportive care, and benzodiazepines.3 Bromocriptine or amantadine are more appropriate for moderate cases and dantrolene for severe cases of NMS.3 All antipsychotics should be discontinued while a patient is experiencing an episode of NMS; however, once the NMS has resolved, clinicians must thoroughly evaluate the risks and benefits of restarting antipsychotic medication. After a patient has experienced an episode of NMS, clinicians generally should avoid prescribing the agent(s) that caused NMS and long-acting injections, and slowly titrate a low-potency SGA such as quetiapine.10Table 23,11,12 outlines the pharmacologic treatment of NMS.
Bottom Line
Neuroleptic malignant syndrome (NMS) should always be part of the differential diagnosis in patients with mental illness and altered mental status. The risk of NMS is especially high in patients with acute medical illness and exposure to antipsychotic medications.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Acyclovir • Zovirax
Amantadine • Gocovri
Ampicillin-sulbactam • Unasyn
Aripiprazole • Abilify Maintena
Benztropine • Cogentin
Bromocriptine • Cycloset, Parlodel
Ceftriaxone • Rocephin
Clozapine • Clozaril
Dantrolene • Dantrium
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Valproate sodium • Depakote
Trazodone • Oleptro
Vancomycin • Vancocin
CASE Sluggish, weak, and incoherent
Mr. O, age 24, who has a history of schizophrenia and obesity, presents to the emergency department (ED) for altered mental status (AMS). His mother reports that he has been sluggish, weak, incoherent, had no appetite, and that on the day before admission, he was drinking excessive amounts of water and urinating every 10 minutes.
HISTORY Multiple ineffective antipsychotics
Mr. O was diagnosed with schizophrenia at age 21 and struggled with medication adherence, which resulted in multiple hospitalizations for stabilization. Trials of haloperidol, risperidone, paliperidone palmitate, and valproic acid had been ineffective. At the time of admission, his psychotropic medication regimen is fluphenazine decanoate, 25 mg injection every 2 weeks; clozapine, 50 mg/d; lithium carbonate, 300 mg twice a day; benztropine, 2 mg every night; and trazodone, 50 mg every night.
EVALUATION Fever, tachycardia, and diabetic ketoacidosis
Upon arrival to the ED, Mr. O is obtunded, unable to follow commands, and does not respond to painful stimuli. On physical exam, he has a fever of 38.4°C (reference range 35.1°C to 37.9°C); tachycardia with a heart rate of 142 beats per minute (bpm) (reference range 60 to 100); tachypnea with a respiratory rate of 35 breaths per minute (reference range 12 to 20); a blood pressure of 116/76 mmHg (reference range 90/60 to 130/80); and hypoxemia with an oxygen saturation of 90% on room air (reference range 94% to 100%).
Mr. O is admitted to the hospital and his laboratory workup indicates diabetic ketoacidosis (DKA), with a glucose of 1,700 mg/dL; anion gap of 30 (reference range 4 to 12 mmol/L); pH 7.04 (reference range 7.32 to 7.42); serum bicarbonate 6 (reference range 20 to 24 mEq/L); beta-hydroxybutyrate 11.04 (reference range 0 to 0.27 mmol/L); urine ketones, serum osmolality 407 (reference range 280 to 300 mOsm/kg); and an elevated white blood cell count of 18.4 (reference range 4.5 to 11.0 × 109/L). A CT scan of the head is negative for acute pathology.
Initially, all psychotropic medications are held. On Day 3 of hospitalization, psychiatry is consulted and clozapine, 50 mg/d; lithium, 300 mg/d; and benztropine, 1 mg at night, are restarted; however, fluphenazine decanoate and trazodone are held. The team recommends IV haloperidol, 2 mg as needed for agitation; however, it is never administered.
Imaging rules out deep vein thrombosis, cardiac dysfunction, and stroke, but a CT chest scan is notable for bilateral lung infiltrates, which suggests aspiration pneumonia.
Mr. O is diagnosed with diabetes, complicated by DKA, and is treated in the intensive care unit (ICU). Despite resolution of the DKA, he remains altered with fever and tachycardia.
Continue to: On Day 6 of hospitalization...
On Day 6 of hospitalization, Mr. O continues to be tachycardic and obtunded with nuchal rigidity. The team decides to transfer Mr. O to another hospital for a higher level of care and continued workup of his persistent AMS.
Immediately upon arrival at the second hospital, infectious disease and neurology teams are consulted for further evaluation. Mr. O’s AMS continues despite no clear signs of infection or other neurologic insults.
[polldaddy:10930631]
The authors’ observations
Based on Mr. O’s psychiatric history and laboratory results, the first medical team concluded his initial AMS was likely secondary to DKA; however, the AMS continued after the DKA resolved. At the second hospital, Mr. O’s treatment team continued to dig for answers.
EVALUATION Exploring the differential diagnosis
At the second hospital, Mr. O is admitted to the ICU with fever (37.8°C), tachycardia (120 bpm), tachypnea, withdrawal from painful stimuli, decreased reflexes, and muscle rigidity, including clenched jaw. The differential diagnoses include meningitis, sepsis from aspiration pneumonia, severe metabolic encephalopathy with prolonged recovery, central pontine myelinolysis, anoxic brain injury, and subclinical seizures.
Empiric vancomycin, 1.75 g every 12 hours; ceftriaxone, 2 g/d; and acyclovir, 900 mg every 8 hours are started for meningoencephalitis, and all psychotropic medications are discontinued. Case reports have documented a relationship between hyperglycemic hyperosmolar syndrome (HHS) and malignant hyperthermia in rare cases1; however, HHS is ruled out based on Mr. O’s laboratory results.A lumbar puncture and imaging rules out CNS infection. Antibiotic treatment is narrowed to ampicillin-sulbactam due to Mr. O’s prior CT chest showing concern for aspiration pneumonia. An MRI of the brain rules out central pontine myelinolysis, acute stroke, and anoxic brain injury, and an EEG shows nonspecific encephalopathy. On Day 10 of hospitalization, a neurologic exam shows flaccid paralysis and bilateral clonus, and Mr. O is mute. On Day 14 of hospitalization, his fever resolves, and his blood cultures are negative. On Day 15 of hospitalization, Mr. O’s creatine kinase (CK) level is elevated at 1,308 U/L (reference range 26 to 192 U/L), suggesting rhabdomyolysis.
Continue to: Given the neurologic exam findings...
Given the neurologic exam findings, and the limited evidence of infection, the differential diagnosis for Mr. O’s AMS is broadened to include catatonia, neuroleptic malignant syndrome (NMS), serotonin syndrome, and autoimmune encephalitis. The psychiatry team evaluates Mr. O for catatonia. He scores 14 on the Bush-Francis Catatonia Rating Scale, with findings of immobility/stupor, mutism, staring, autonomic instability, and withdrawal indicating the presence of catatonia.2
The authors’ observations
When Mr. O was transferred to the second hospital, the primary concern was to rule out meningitis due to his unstable vitals, obtunded mental state, and nuchal rigidity. A comprehensive infectious workup, including lumbar puncture, was imperative because infection can not only lead to AMS, but also precipitate episodes of DKA. Mr. O’s persistently abnormal vital signs indicated an underlying process may have been missed by focusing on treating DKA.
TREATMENT Finally, the diagnosis is established
A lorazepam challenge is performed, and Mr. O receives 4 mg of lorazepam over 24 hours with little change in his catatonia symptoms. Given his persistent fever, tachycardia, and an elevated CK levels in the context of recent exposure to antipsychotic medications, Mr. O is diagnosed with NMS (Table 13,4 ) and is started on bromocriptine, 5 mg 3 times daily.
[polldaddy:10930632]
The authors’ observations
Mr. O’s complicated medical state—starting with DKA, halting the use of antipsychotic medications, and the suspicion of catatonia due to his history of schizophrenia—all distracted from the ultimate diagnosis of NMS as the cause of his enduring AMS and autonomic instability. Catatonia and NMS have overlapping symptomatology, including rigidity, autonomic instability, and stupor, which make the diagnosis of either condition complicated. A positive lorazepam test to diagnose catatonia is defined as a marked reduction in catatonia symptoms (typically a 50% reduction) as measured on a standardized rating scale.5 However, a negative lorazepam challenge does not definitely rule out catatonia because some cases are resistant to benzodiazepines.6
NMS risk factors relevant in this case include male sex, young age, acute medical illness, dehydration, and exposure to multiple psychotropic medications, including 2 antipsychotics, clozapine and fluphenazine.7 DKA is especially pertinent due to its acute onset and cause of significant dehydration. NMS can occur at any point of antipsychotic exposure, although the risk is highest during the initial weeks of treatment and during dosage changes. Unfortunately, Mr. O’s treatment team was unable to determine whether his medication had been recently changed, so it is not known what role this may have played in the development of NMS. Although first-generation antipsychotics are considered more likely to cause NMS, second-generation antipsychotics (SGAs) dominate the treatment of schizophrenia and bipolar disorder, and these medications also can cause NMS.8 As occurred in this case, long-acting injectable antipsychotics can be easily forgotten when not administered in the hospital, and their presence in the body persists for weeks. For example, the half-life of fluphenazine decanoate is approximately 10 days, and the half-life of haloperidol decanoate is 21 days.9
Continue to: OUTCOME Improvement with bromocriptine
OUTCOME Improvement with bromocriptine
After 4 days of bromocriptine, 5 mg 3 times daily, Mr. O is more alert, able to say “hello,” and can follow 1-step commands. By Day 26 of hospitalization, his CK levels decrease to 296 U/L, his CSF autoimmune panel is negative, and he is able to participate in physical therapy. After failing multiple swallow tests, Mr. O requires a percutaneous endoscopic gastrostomy (PEG) tube. He is discharged from the hospital to a long-term acute care facility with the plan to taper bromocriptine and restart a psychotropic regimen with his outpatient psychiatrist. At the time of discharge, he is able to sit at the edge of the bed independently, state his name, and respond to questions with multiple-word answers.
[polldaddy:10930633]
The authors’ observations
The most common pharmacologic treatments for NMS are dantrolene, bromocriptine, benzodiazepines (lorazepam or diazepam), and amantadine.3 Mild cases of NMS should be treated with discontinuation of all antipsychotics, supportive care, and benzodiazepines.3 Bromocriptine or amantadine are more appropriate for moderate cases and dantrolene for severe cases of NMS.3 All antipsychotics should be discontinued while a patient is experiencing an episode of NMS; however, once the NMS has resolved, clinicians must thoroughly evaluate the risks and benefits of restarting antipsychotic medication. After a patient has experienced an episode of NMS, clinicians generally should avoid prescribing the agent(s) that caused NMS and long-acting injections, and slowly titrate a low-potency SGA such as quetiapine.10Table 23,11,12 outlines the pharmacologic treatment of NMS.
Bottom Line
Neuroleptic malignant syndrome (NMS) should always be part of the differential diagnosis in patients with mental illness and altered mental status. The risk of NMS is especially high in patients with acute medical illness and exposure to antipsychotic medications.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Acyclovir • Zovirax
Amantadine • Gocovri
Ampicillin-sulbactam • Unasyn
Aripiprazole • Abilify Maintena
Benztropine • Cogentin
Bromocriptine • Cycloset, Parlodel
Ceftriaxone • Rocephin
Clozapine • Clozaril
Dantrolene • Dantrium
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Valproate sodium • Depakote
Trazodone • Oleptro
Vancomycin • Vancocin
1. Zeitler P, Haqq A, Rosenbloom A, et al. Hyperglycemic hyperosmolar syndrome in children: pathophysiological considerations and suggested guidelines for treatment. J Pediatr. 2011;158(1):9-14.e1-2. doi: 10.1016/j.jpeds.2010.09.048
2. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185. doi: 10.1007/s11920-010-0113-y
3. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981. doi:10.1177/1060028016657553
4. Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011;72(9):1222-1228. doi:10.4088/JCP.10m06438
5. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181. doi:10.3389/fpsyt.2014.00181
6. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380. doi:10.1176/jnp.2009.21.4.371
7. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin. 2004;22(2):389-411. doi:10.1016/j.ncl.2003.12.006
8. Tse L, Barr AM, Scarapicchia V, et al. Neuroleptic malignant syndrome: a review from a clinically oriented perspective. Curr Neuropharmacol. 2015;13(3):395-406. doi:10.2174/1570159x13999150424113345
9. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59. doi:10.1007/s40263-020-00779-5
10. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876. doi:10.1176/ajp.2007.164.6.870
11. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
12. Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care. 2007;11(1):R4. doi:10.1186/cc5148
1. Zeitler P, Haqq A, Rosenbloom A, et al. Hyperglycemic hyperosmolar syndrome in children: pathophysiological considerations and suggested guidelines for treatment. J Pediatr. 2011;158(1):9-14.e1-2. doi: 10.1016/j.jpeds.2010.09.048
2. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185. doi: 10.1007/s11920-010-0113-y
3. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981. doi:10.1177/1060028016657553
4. Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011;72(9):1222-1228. doi:10.4088/JCP.10m06438
5. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181. doi:10.3389/fpsyt.2014.00181
6. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380. doi:10.1176/jnp.2009.21.4.371
7. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin. 2004;22(2):389-411. doi:10.1016/j.ncl.2003.12.006
8. Tse L, Barr AM, Scarapicchia V, et al. Neuroleptic malignant syndrome: a review from a clinically oriented perspective. Curr Neuropharmacol. 2015;13(3):395-406. doi:10.2174/1570159x13999150424113345
9. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59. doi:10.1007/s40263-020-00779-5
10. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876. doi:10.1176/ajp.2007.164.6.870
11. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
12. Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care. 2007;11(1):R4. doi:10.1186/cc5148
Serotonergic antidepressants’ effects on bone health
Mrs. D, age 67, has a history of major depressive disorder. She has had adequate treatment trials with duloxetine, mirtazapine, and sertraline; each failed to produce remission. She is currently prescribed paroxetine, 40 mg/d, and aripiprazole, 10 mg/d, with good efficacy. She also has a history of hypertension and seasonal allergies, for which she receives amlodipine, 10 mg/d, and loratadine, 10 mg/d, respectively.
Mrs. D’s depressive symptoms were well controlled until 2 months ago, when she fell and fractured her hip. With encouragement from her prescriber, she enrolled in a partial hospitalization program for more intensive psychotherapy. During a medication education session, she is surprised to learn that antidepressants may affect bone health.
During a medication management meeting with her prescriber, Mrs. D asks about the risk of osteoporosis, and whether her antidepressant could have contributed to her hip fracture.
Bone is a dynamic tissue that undergoes a continuous process of remodeling. Osteoblasts are responsible for bone formation, whereas osteoclasts are responsible for bone resorption. Osteocytes—the predominant cell type in bone—along with cytokines, hormones, and growth factors help to orchestrate these actions.1 Serotonin is increasingly recognized as a factor in bone homeostasis. Bone synthesizes serotonin, expresses serotonin transporters, and contains a variety of serotonin receptors.2
Serotonin serves many physiologic functions outside of the CNS, and it appears to have opposing actions on bone metabolism (Table 11,3). Peripheral (gut-derived) serotonin inhibits bone formation through its effects on osteoblasts, whereas the actions of serotonin in the CNS promote bone growth through inhibitory effects on sympathetic output.2 Selective serotonin reuptake inhibitor (SSRI) enhancement of peripheral serotonin and its negative effect on bone may outweigh the benefits caused by SSRI enhancement of central serotonin neurotransmission.1 In vitro data suggest SSRIs inhibit osteoblast and osteoclast function, theoretically decreasing bone turnover and increasing fracture risk.4 Other data indicate SSRI treatment may decrease procollagen type 1 N-terminal propeptide, a peripheral marker of bone formation.5 Both SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) have been associated with lower cortical bone mineral density (BMD).6Table 27,8 details the relative affinity of select antidepressants for the serotonin transporter.
Both serotonergic antidepressants and depression have been associated with decreased BMD and increased fracture risk.1,9 Behavioral aspects of depression, such as inadequate nutrition or physical inactivity, overlap with risk factors for poor bone health. In addition, elevated levels of circulating cortisol and proinflammatory cytokines in patients with depressive symptoms may contribute to decreased bone mass.10,11 Modifiable risk factors for osteoporosis and fractures include low calcium and vitamin D intake, low body weight, and a sedentary lifestyle. Nonmodifiable risk factors include advancing age, female sex, Asian or White ethnicity, malabsorptive conditions, and chronic corticosteroid use.12
What the evidence says
Evidence for the correlation between fractures and serotonergic antidepressant use is mixed. One meta-analysis found a significant association between SSRIs and fractures, suggesting a 1.62-fold increased risk.13 Another meta-analysis investigated SSRIs and SNRIs and the risk of fracture.14 The SSRIs had a 1.67-fold increased risk; however, a lack of studies prohibited making conclusions about SNRIs. The number needed to harm was calculated at 85, 46, and 19 with 1, 2, and 5 years of SSRI exposure, respectively. A third meta-analysis found increased fracture risk related to depression and reported a hazard ratio of 1.26 after adjusting for confounders.9 This analysis suggests depression affects fracture risk and may limit the interpretation of causation from SSRI use. Studies included in these meta-analyses had significant heterogeneity.
Continue to: The effect of SSRIs...
The effect of SSRIs vs non-SSRIs on BMD also has been studied. The SSRIs were associated with significantly reduced BMD of the lumbar spine but not the total hip or femoral neck as compared to non-SSRIs; however, this BMD loss was not examined in relation to the presence of fractures. Older patients had more pronounced bone loss.15 Conversely, another meta-analysis examined BMD in women receiving SSRIs or tricyclic antidepressants.10 Neither medication class was associated with lower BMD at measured locations, including lumbar spine, femoral neck, and total hip. This analysis was limited by the lack of available trials; only 4 were included.
Other recent research has continued to explore the relationship between antidepressants and fracture in various patient populations. In a study of patients receiving maintenance dialysis treatment, short- and long-term SSRI use increased hip fracture risk. The authors speculated that short-term risk may be mediated by adverse effects that increase fall risk (eg, hyponatremia, orthostasis), whereas long-term risk may be influenced by changes in bone homeostasis.16 In two 6-month analyses of fluoxetine treatment in patients following an acute stroke, fluoxetine increased the risk of bone fractures.17,18 Finally, in women with osteoporosis receiving risedronate or teriparatide, in both groups a higher fracture risk was observed for patients who were also receiving an SSRI or SNRI.19
Monitor BMD and educate patients about bone health
Available literature has not identified any clear risk factors for fracture with SSRI use. Guidelines suggest monitoring BMD in patients with risk factors for osteoporosis, if clinically indicated, as well as monitoring BMD in those receiving long-term antidepressant treatment.20-22 Educate patients on strategies that promote optimal bone health, such as consuming a balanced diet that meets the recommended dietary allowance of calcium, vitamin D, and limits soda consumption. Teach patients to avoid tobacco and excessive alcohol use because both adversely impact BMD. Maintaining a healthy weight, physical activity, and adequate sleep also support bone health.11 Instruct patients receiving antidepressants to report unexplained bone pain, tenderness, swelling, or bruising because these symptoms may be indicative of fracture.
CASE CONTINUED
Mrs. D’s age, sex, and depression place her at higher risk of fracture. Paroxetine is the only SSRI that has bone fracture listed as a precaution in its labeling.23 In addition, it is the most anticholinergic SSRI and may have contributed to her fall. Switching to bupropion by cross titration may benefit Mrs. D because bupropion is not serotonergic. Little data exist regarding the effects of bupropion on bone. Her prescriber monitors Mrs. D’s BMD periodically, and educates her on dietary considerations. He also recommends calcium, 1,200 mg/d, and vitamin D, 800 IU/d, to help prevent fractures,24 and that she continue physical therapy exercises and increase physical activity as tolerated.
Related Resources
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
- Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
- Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
- US National Library of Medicine. DailyMed. https://dailymed.nlm.nih.gov/dailymed
Drug Brand Names
Amitriptyline • Elavil
Amlodipine • Norvasc
Aripiprazole • Abilify
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Doxepin • Silenor, Sinequan
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Levomilnacipran • Fetzima
Loratadine • Claritin
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Risedronate • Actonel
Sertraline • Zoloft
Teriparatide • Forteo
Trazodone • Desyrel
Venlafaxine • Effexor
Vortioxetine • Trintellix
1. Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
2. Lavoie B, Lian JB, Mawe GM. Regulation of bone metabolism by serotonin. Adv Exp Med Biol. 2017;1033:35-46.
3. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355-366.
4. Hodge JM, Wang Y, Berk M, et al. Selective serotonin reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. Biol Psychiatry. 2013;74(1):32-39.
5. Kumar M, Jiloha RC, Kataria D, et al. Effect of selective serotonin reuptake inhibitors on markers of bone loss. Psychiatry Res. 2019;276:39-44.
6. Agarwal S, Germosen C, Kil N, et al. Current anti-depressant use is associated with cortical bone deficits and reduced physical function in elderly women. Bone. 2020;140:115552.
7. DeBattista C. Antidepressant agents. In: Katzung BG, ed. Basic and clinical pharmacology. 14th ed. McGraw-Hill; 2018.
8. Kasper S, Pail G. Milnacipran: a unique antidepressant? Neuropsychiatr Dis Treat. 2010;6(Suppl 1):23-31.
9. Wu Q, Liu B, Tonmoy S. Depression and risk of fracture and bone loss: an updated meta-analysis of prospective studies. Osteoporos Int. 2018;29(6):1303-1312.
10. Schweiger JU, Schweiger U, Hüppe M, et al. The use of antidepressant agents and bone mineral density in women: a meta-analysis. Int J Environ Res Public Health. 2018;15(7):1373.
11. Rizzoli R, Cooper C, Reginster JY, et al. Antidepressant medications and osteoporosis. Bone. 2012;51(3):606-613.
12. Rice JN, Gillett CB, Malas NM. The impact of psychotropic medications on bone health in youth. Curr Psychiatry Rep. 2018;20(11):104.
13. Kumar M, Bajpai R, Shaik AR, et al. Alliance between selective serotonin reuptake inhibitors and fracture risk: an updated systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76(10):1373-1392.
14. Khanassov V, Hu J, Reeves D, et al. Selective serotonin reuptake inhibitor and selective serotonin and norepinephrine reuptake inhibitor use and risk of fractures in adults: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2018;33(12):1688-1708.
15. Zhou C, Fang L, Chen Y, et al. Effect of selective serotonin reuptake inhibitors on bone mineral density: a systematic review and meta-analysis. Osteoporos Int. 2018;29(6):1243-1251.
16. Vangala C, Niu J, Montez-Rath ME, et al. Selective serotonin reuptake inhibitor use and hip fracture risk among patients on hemodialysis. Am J Kidney Dis. 2020;75(3):351-360.
17. Hankey GJ, Hackett ML, Almeida OP, et al. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):651-660.
18. Lundström E, Isaksson E, Näsman P, et al. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):661-669.
19. Kendler DL, Marin F, Geusens P, et al. Psychotropic medications and proton pump inhibitors and the risk of fractures in the teriparatide versus risedronate VERO clinical trial. Bone. 2020;130:115113.
20. Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
21. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. Published October 2010. Accessed February 8, 2021. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
22. Agacayak KS, Guler R, Ilyasov B. Evaluation of the effect of long-term use of antidepressants in the SSRI group on bone density with dental volumetric tomography. Drug Des Devel Ther. 2019;13:3477-3484.
23. US National Library of Medicine. DailyMed. Accessed February 8, 2021. https://dailymed.nlm.nih.gov/dailymed
24. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
Mrs. D, age 67, has a history of major depressive disorder. She has had adequate treatment trials with duloxetine, mirtazapine, and sertraline; each failed to produce remission. She is currently prescribed paroxetine, 40 mg/d, and aripiprazole, 10 mg/d, with good efficacy. She also has a history of hypertension and seasonal allergies, for which she receives amlodipine, 10 mg/d, and loratadine, 10 mg/d, respectively.
Mrs. D’s depressive symptoms were well controlled until 2 months ago, when she fell and fractured her hip. With encouragement from her prescriber, she enrolled in a partial hospitalization program for more intensive psychotherapy. During a medication education session, she is surprised to learn that antidepressants may affect bone health.
During a medication management meeting with her prescriber, Mrs. D asks about the risk of osteoporosis, and whether her antidepressant could have contributed to her hip fracture.
Bone is a dynamic tissue that undergoes a continuous process of remodeling. Osteoblasts are responsible for bone formation, whereas osteoclasts are responsible for bone resorption. Osteocytes—the predominant cell type in bone—along with cytokines, hormones, and growth factors help to orchestrate these actions.1 Serotonin is increasingly recognized as a factor in bone homeostasis. Bone synthesizes serotonin, expresses serotonin transporters, and contains a variety of serotonin receptors.2
Serotonin serves many physiologic functions outside of the CNS, and it appears to have opposing actions on bone metabolism (Table 11,3). Peripheral (gut-derived) serotonin inhibits bone formation through its effects on osteoblasts, whereas the actions of serotonin in the CNS promote bone growth through inhibitory effects on sympathetic output.2 Selective serotonin reuptake inhibitor (SSRI) enhancement of peripheral serotonin and its negative effect on bone may outweigh the benefits caused by SSRI enhancement of central serotonin neurotransmission.1 In vitro data suggest SSRIs inhibit osteoblast and osteoclast function, theoretically decreasing bone turnover and increasing fracture risk.4 Other data indicate SSRI treatment may decrease procollagen type 1 N-terminal propeptide, a peripheral marker of bone formation.5 Both SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) have been associated with lower cortical bone mineral density (BMD).6Table 27,8 details the relative affinity of select antidepressants for the serotonin transporter.
Both serotonergic antidepressants and depression have been associated with decreased BMD and increased fracture risk.1,9 Behavioral aspects of depression, such as inadequate nutrition or physical inactivity, overlap with risk factors for poor bone health. In addition, elevated levels of circulating cortisol and proinflammatory cytokines in patients with depressive symptoms may contribute to decreased bone mass.10,11 Modifiable risk factors for osteoporosis and fractures include low calcium and vitamin D intake, low body weight, and a sedentary lifestyle. Nonmodifiable risk factors include advancing age, female sex, Asian or White ethnicity, malabsorptive conditions, and chronic corticosteroid use.12
What the evidence says
Evidence for the correlation between fractures and serotonergic antidepressant use is mixed. One meta-analysis found a significant association between SSRIs and fractures, suggesting a 1.62-fold increased risk.13 Another meta-analysis investigated SSRIs and SNRIs and the risk of fracture.14 The SSRIs had a 1.67-fold increased risk; however, a lack of studies prohibited making conclusions about SNRIs. The number needed to harm was calculated at 85, 46, and 19 with 1, 2, and 5 years of SSRI exposure, respectively. A third meta-analysis found increased fracture risk related to depression and reported a hazard ratio of 1.26 after adjusting for confounders.9 This analysis suggests depression affects fracture risk and may limit the interpretation of causation from SSRI use. Studies included in these meta-analyses had significant heterogeneity.
Continue to: The effect of SSRIs...
The effect of SSRIs vs non-SSRIs on BMD also has been studied. The SSRIs were associated with significantly reduced BMD of the lumbar spine but not the total hip or femoral neck as compared to non-SSRIs; however, this BMD loss was not examined in relation to the presence of fractures. Older patients had more pronounced bone loss.15 Conversely, another meta-analysis examined BMD in women receiving SSRIs or tricyclic antidepressants.10 Neither medication class was associated with lower BMD at measured locations, including lumbar spine, femoral neck, and total hip. This analysis was limited by the lack of available trials; only 4 were included.
Other recent research has continued to explore the relationship between antidepressants and fracture in various patient populations. In a study of patients receiving maintenance dialysis treatment, short- and long-term SSRI use increased hip fracture risk. The authors speculated that short-term risk may be mediated by adverse effects that increase fall risk (eg, hyponatremia, orthostasis), whereas long-term risk may be influenced by changes in bone homeostasis.16 In two 6-month analyses of fluoxetine treatment in patients following an acute stroke, fluoxetine increased the risk of bone fractures.17,18 Finally, in women with osteoporosis receiving risedronate or teriparatide, in both groups a higher fracture risk was observed for patients who were also receiving an SSRI or SNRI.19
Monitor BMD and educate patients about bone health
Available literature has not identified any clear risk factors for fracture with SSRI use. Guidelines suggest monitoring BMD in patients with risk factors for osteoporosis, if clinically indicated, as well as monitoring BMD in those receiving long-term antidepressant treatment.20-22 Educate patients on strategies that promote optimal bone health, such as consuming a balanced diet that meets the recommended dietary allowance of calcium, vitamin D, and limits soda consumption. Teach patients to avoid tobacco and excessive alcohol use because both adversely impact BMD. Maintaining a healthy weight, physical activity, and adequate sleep also support bone health.11 Instruct patients receiving antidepressants to report unexplained bone pain, tenderness, swelling, or bruising because these symptoms may be indicative of fracture.
CASE CONTINUED
Mrs. D’s age, sex, and depression place her at higher risk of fracture. Paroxetine is the only SSRI that has bone fracture listed as a precaution in its labeling.23 In addition, it is the most anticholinergic SSRI and may have contributed to her fall. Switching to bupropion by cross titration may benefit Mrs. D because bupropion is not serotonergic. Little data exist regarding the effects of bupropion on bone. Her prescriber monitors Mrs. D’s BMD periodically, and educates her on dietary considerations. He also recommends calcium, 1,200 mg/d, and vitamin D, 800 IU/d, to help prevent fractures,24 and that she continue physical therapy exercises and increase physical activity as tolerated.
Related Resources
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
- Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
- Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
- US National Library of Medicine. DailyMed. https://dailymed.nlm.nih.gov/dailymed
Drug Brand Names
Amitriptyline • Elavil
Amlodipine • Norvasc
Aripiprazole • Abilify
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Doxepin • Silenor, Sinequan
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Levomilnacipran • Fetzima
Loratadine • Claritin
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Risedronate • Actonel
Sertraline • Zoloft
Teriparatide • Forteo
Trazodone • Desyrel
Venlafaxine • Effexor
Vortioxetine • Trintellix
Mrs. D, age 67, has a history of major depressive disorder. She has had adequate treatment trials with duloxetine, mirtazapine, and sertraline; each failed to produce remission. She is currently prescribed paroxetine, 40 mg/d, and aripiprazole, 10 mg/d, with good efficacy. She also has a history of hypertension and seasonal allergies, for which she receives amlodipine, 10 mg/d, and loratadine, 10 mg/d, respectively.
Mrs. D’s depressive symptoms were well controlled until 2 months ago, when she fell and fractured her hip. With encouragement from her prescriber, she enrolled in a partial hospitalization program for more intensive psychotherapy. During a medication education session, she is surprised to learn that antidepressants may affect bone health.
During a medication management meeting with her prescriber, Mrs. D asks about the risk of osteoporosis, and whether her antidepressant could have contributed to her hip fracture.
Bone is a dynamic tissue that undergoes a continuous process of remodeling. Osteoblasts are responsible for bone formation, whereas osteoclasts are responsible for bone resorption. Osteocytes—the predominant cell type in bone—along with cytokines, hormones, and growth factors help to orchestrate these actions.1 Serotonin is increasingly recognized as a factor in bone homeostasis. Bone synthesizes serotonin, expresses serotonin transporters, and contains a variety of serotonin receptors.2
Serotonin serves many physiologic functions outside of the CNS, and it appears to have opposing actions on bone metabolism (Table 11,3). Peripheral (gut-derived) serotonin inhibits bone formation through its effects on osteoblasts, whereas the actions of serotonin in the CNS promote bone growth through inhibitory effects on sympathetic output.2 Selective serotonin reuptake inhibitor (SSRI) enhancement of peripheral serotonin and its negative effect on bone may outweigh the benefits caused by SSRI enhancement of central serotonin neurotransmission.1 In vitro data suggest SSRIs inhibit osteoblast and osteoclast function, theoretically decreasing bone turnover and increasing fracture risk.4 Other data indicate SSRI treatment may decrease procollagen type 1 N-terminal propeptide, a peripheral marker of bone formation.5 Both SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) have been associated with lower cortical bone mineral density (BMD).6Table 27,8 details the relative affinity of select antidepressants for the serotonin transporter.
Both serotonergic antidepressants and depression have been associated with decreased BMD and increased fracture risk.1,9 Behavioral aspects of depression, such as inadequate nutrition or physical inactivity, overlap with risk factors for poor bone health. In addition, elevated levels of circulating cortisol and proinflammatory cytokines in patients with depressive symptoms may contribute to decreased bone mass.10,11 Modifiable risk factors for osteoporosis and fractures include low calcium and vitamin D intake, low body weight, and a sedentary lifestyle. Nonmodifiable risk factors include advancing age, female sex, Asian or White ethnicity, malabsorptive conditions, and chronic corticosteroid use.12
What the evidence says
Evidence for the correlation between fractures and serotonergic antidepressant use is mixed. One meta-analysis found a significant association between SSRIs and fractures, suggesting a 1.62-fold increased risk.13 Another meta-analysis investigated SSRIs and SNRIs and the risk of fracture.14 The SSRIs had a 1.67-fold increased risk; however, a lack of studies prohibited making conclusions about SNRIs. The number needed to harm was calculated at 85, 46, and 19 with 1, 2, and 5 years of SSRI exposure, respectively. A third meta-analysis found increased fracture risk related to depression and reported a hazard ratio of 1.26 after adjusting for confounders.9 This analysis suggests depression affects fracture risk and may limit the interpretation of causation from SSRI use. Studies included in these meta-analyses had significant heterogeneity.
Continue to: The effect of SSRIs...
The effect of SSRIs vs non-SSRIs on BMD also has been studied. The SSRIs were associated with significantly reduced BMD of the lumbar spine but not the total hip or femoral neck as compared to non-SSRIs; however, this BMD loss was not examined in relation to the presence of fractures. Older patients had more pronounced bone loss.15 Conversely, another meta-analysis examined BMD in women receiving SSRIs or tricyclic antidepressants.10 Neither medication class was associated with lower BMD at measured locations, including lumbar spine, femoral neck, and total hip. This analysis was limited by the lack of available trials; only 4 were included.
Other recent research has continued to explore the relationship between antidepressants and fracture in various patient populations. In a study of patients receiving maintenance dialysis treatment, short- and long-term SSRI use increased hip fracture risk. The authors speculated that short-term risk may be mediated by adverse effects that increase fall risk (eg, hyponatremia, orthostasis), whereas long-term risk may be influenced by changes in bone homeostasis.16 In two 6-month analyses of fluoxetine treatment in patients following an acute stroke, fluoxetine increased the risk of bone fractures.17,18 Finally, in women with osteoporosis receiving risedronate or teriparatide, in both groups a higher fracture risk was observed for patients who were also receiving an SSRI or SNRI.19
Monitor BMD and educate patients about bone health
Available literature has not identified any clear risk factors for fracture with SSRI use. Guidelines suggest monitoring BMD in patients with risk factors for osteoporosis, if clinically indicated, as well as monitoring BMD in those receiving long-term antidepressant treatment.20-22 Educate patients on strategies that promote optimal bone health, such as consuming a balanced diet that meets the recommended dietary allowance of calcium, vitamin D, and limits soda consumption. Teach patients to avoid tobacco and excessive alcohol use because both adversely impact BMD. Maintaining a healthy weight, physical activity, and adequate sleep also support bone health.11 Instruct patients receiving antidepressants to report unexplained bone pain, tenderness, swelling, or bruising because these symptoms may be indicative of fracture.
CASE CONTINUED
Mrs. D’s age, sex, and depression place her at higher risk of fracture. Paroxetine is the only SSRI that has bone fracture listed as a precaution in its labeling.23 In addition, it is the most anticholinergic SSRI and may have contributed to her fall. Switching to bupropion by cross titration may benefit Mrs. D because bupropion is not serotonergic. Little data exist regarding the effects of bupropion on bone. Her prescriber monitors Mrs. D’s BMD periodically, and educates her on dietary considerations. He also recommends calcium, 1,200 mg/d, and vitamin D, 800 IU/d, to help prevent fractures,24 and that she continue physical therapy exercises and increase physical activity as tolerated.
Related Resources
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
- Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
- Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
- US National Library of Medicine. DailyMed. https://dailymed.nlm.nih.gov/dailymed
Drug Brand Names
Amitriptyline • Elavil
Amlodipine • Norvasc
Aripiprazole • Abilify
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Doxepin • Silenor, Sinequan
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Levomilnacipran • Fetzima
Loratadine • Claritin
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Risedronate • Actonel
Sertraline • Zoloft
Teriparatide • Forteo
Trazodone • Desyrel
Venlafaxine • Effexor
Vortioxetine • Trintellix
1. Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
2. Lavoie B, Lian JB, Mawe GM. Regulation of bone metabolism by serotonin. Adv Exp Med Biol. 2017;1033:35-46.
3. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355-366.
4. Hodge JM, Wang Y, Berk M, et al. Selective serotonin reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. Biol Psychiatry. 2013;74(1):32-39.
5. Kumar M, Jiloha RC, Kataria D, et al. Effect of selective serotonin reuptake inhibitors on markers of bone loss. Psychiatry Res. 2019;276:39-44.
6. Agarwal S, Germosen C, Kil N, et al. Current anti-depressant use is associated with cortical bone deficits and reduced physical function in elderly women. Bone. 2020;140:115552.
7. DeBattista C. Antidepressant agents. In: Katzung BG, ed. Basic and clinical pharmacology. 14th ed. McGraw-Hill; 2018.
8. Kasper S, Pail G. Milnacipran: a unique antidepressant? Neuropsychiatr Dis Treat. 2010;6(Suppl 1):23-31.
9. Wu Q, Liu B, Tonmoy S. Depression and risk of fracture and bone loss: an updated meta-analysis of prospective studies. Osteoporos Int. 2018;29(6):1303-1312.
10. Schweiger JU, Schweiger U, Hüppe M, et al. The use of antidepressant agents and bone mineral density in women: a meta-analysis. Int J Environ Res Public Health. 2018;15(7):1373.
11. Rizzoli R, Cooper C, Reginster JY, et al. Antidepressant medications and osteoporosis. Bone. 2012;51(3):606-613.
12. Rice JN, Gillett CB, Malas NM. The impact of psychotropic medications on bone health in youth. Curr Psychiatry Rep. 2018;20(11):104.
13. Kumar M, Bajpai R, Shaik AR, et al. Alliance between selective serotonin reuptake inhibitors and fracture risk: an updated systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76(10):1373-1392.
14. Khanassov V, Hu J, Reeves D, et al. Selective serotonin reuptake inhibitor and selective serotonin and norepinephrine reuptake inhibitor use and risk of fractures in adults: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2018;33(12):1688-1708.
15. Zhou C, Fang L, Chen Y, et al. Effect of selective serotonin reuptake inhibitors on bone mineral density: a systematic review and meta-analysis. Osteoporos Int. 2018;29(6):1243-1251.
16. Vangala C, Niu J, Montez-Rath ME, et al. Selective serotonin reuptake inhibitor use and hip fracture risk among patients on hemodialysis. Am J Kidney Dis. 2020;75(3):351-360.
17. Hankey GJ, Hackett ML, Almeida OP, et al. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):651-660.
18. Lundström E, Isaksson E, Näsman P, et al. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):661-669.
19. Kendler DL, Marin F, Geusens P, et al. Psychotropic medications and proton pump inhibitors and the risk of fractures in the teriparatide versus risedronate VERO clinical trial. Bone. 2020;130:115113.
20. Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
21. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. Published October 2010. Accessed February 8, 2021. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
22. Agacayak KS, Guler R, Ilyasov B. Evaluation of the effect of long-term use of antidepressants in the SSRI group on bone density with dental volumetric tomography. Drug Des Devel Ther. 2019;13:3477-3484.
23. US National Library of Medicine. DailyMed. Accessed February 8, 2021. https://dailymed.nlm.nih.gov/dailymed
24. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
1. Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
2. Lavoie B, Lian JB, Mawe GM. Regulation of bone metabolism by serotonin. Adv Exp Med Biol. 2017;1033:35-46.
3. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355-366.
4. Hodge JM, Wang Y, Berk M, et al. Selective serotonin reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. Biol Psychiatry. 2013;74(1):32-39.
5. Kumar M, Jiloha RC, Kataria D, et al. Effect of selective serotonin reuptake inhibitors on markers of bone loss. Psychiatry Res. 2019;276:39-44.
6. Agarwal S, Germosen C, Kil N, et al. Current anti-depressant use is associated with cortical bone deficits and reduced physical function in elderly women. Bone. 2020;140:115552.
7. DeBattista C. Antidepressant agents. In: Katzung BG, ed. Basic and clinical pharmacology. 14th ed. McGraw-Hill; 2018.
8. Kasper S, Pail G. Milnacipran: a unique antidepressant? Neuropsychiatr Dis Treat. 2010;6(Suppl 1):23-31.
9. Wu Q, Liu B, Tonmoy S. Depression and risk of fracture and bone loss: an updated meta-analysis of prospective studies. Osteoporos Int. 2018;29(6):1303-1312.
10. Schweiger JU, Schweiger U, Hüppe M, et al. The use of antidepressant agents and bone mineral density in women: a meta-analysis. Int J Environ Res Public Health. 2018;15(7):1373.
11. Rizzoli R, Cooper C, Reginster JY, et al. Antidepressant medications and osteoporosis. Bone. 2012;51(3):606-613.
12. Rice JN, Gillett CB, Malas NM. The impact of psychotropic medications on bone health in youth. Curr Psychiatry Rep. 2018;20(11):104.
13. Kumar M, Bajpai R, Shaik AR, et al. Alliance between selective serotonin reuptake inhibitors and fracture risk: an updated systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76(10):1373-1392.
14. Khanassov V, Hu J, Reeves D, et al. Selective serotonin reuptake inhibitor and selective serotonin and norepinephrine reuptake inhibitor use and risk of fractures in adults: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2018;33(12):1688-1708.
15. Zhou C, Fang L, Chen Y, et al. Effect of selective serotonin reuptake inhibitors on bone mineral density: a systematic review and meta-analysis. Osteoporos Int. 2018;29(6):1243-1251.
16. Vangala C, Niu J, Montez-Rath ME, et al. Selective serotonin reuptake inhibitor use and hip fracture risk among patients on hemodialysis. Am J Kidney Dis. 2020;75(3):351-360.
17. Hankey GJ, Hackett ML, Almeida OP, et al. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):651-660.
18. Lundström E, Isaksson E, Näsman P, et al. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):661-669.
19. Kendler DL, Marin F, Geusens P, et al. Psychotropic medications and proton pump inhibitors and the risk of fractures in the teriparatide versus risedronate VERO clinical trial. Bone. 2020;130:115113.
20. Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
21. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. Published October 2010. Accessed February 8, 2021. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
22. Agacayak KS, Guler R, Ilyasov B. Evaluation of the effect of long-term use of antidepressants in the SSRI group on bone density with dental volumetric tomography. Drug Des Devel Ther. 2019;13:3477-3484.
23. US National Library of Medicine. DailyMed. Accessed February 8, 2021. https://dailymed.nlm.nih.gov/dailymed
24. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
An unquenchable thirst
CASE Unresponsive after a presumed seizure
Mr. F, age 44, has schizophrenia. He is brought to the hospital by ambulance after he is found on the ground outside of his mother’s house following a presumed seizure and fall. On arrival to the emergency department, he is unresponsive. His laboratory values are significant for a sodium level of 110 mEq/L (reference range: 135 to 145 mEq/L), indicating hyponatremia.
HISTORY Fixated on purity
Mr. F’s mother reports that Mr. F had an unremarkable childhood. He was raised in a household with both parents and a younger sister. Mr. F did well academically and studied engineering and physics in college. There was no reported history of trauma or substance use.
During his senior year of college, Mr. F began experiencing paranoia, auditory hallucinations, and religious delusions. He required hospitalization and was diagnosed with schizophrenia. Following multiple hospitalizations over 5 years, he moved in with his mother, who was granted guardianship.
His mother said Mr. F’s religious delusions were of purity and cleansing the soul. He spent hours memorizing the Bible and would go for days without eating but would drink large amounts of water. She said she thought this was due to his desire to flush out imperfections.
In the past 3 years, Mr. F has been hospitalized several times for severe hyponatremia. At home, his mother attempted to restrict his water intake. However, Mr. F would still drink out of sinks and hoses. Mr. F’s mother reports that over the past month he had become more isolated. He would spend entire days reading the Bible, and his water intake had further increased.
Prior medication trials for Mr. F included haloperidol, up to 10 mg twice per day; aripiprazole, up to 20 mg/d; and risperidone, up to 6 mg nightly. These had been effective, but Mr. F had difficulty with adherence. He did not receive a long-acting injectable (LAI) antipsychotic initially due to lack of access at the rural clinic where he was treated, and later due to his mother’s preference for her son to receive oral medications. Prior to his current presentation, Mr. F’s medication regimen was olanzapine, 10 mg twice a day; perphenazine, 8 mg twice a day; and long-acting propranolol, 60 mg/d. Mr. F had no other chronic medical problems.
EVALUATION Hyponatremia, but why?
Mr. F is intubated and admitted to the surgical service for stabilization due to injuries from his fall. He has fractures of his right sinus and bilateral nasal bones, which are managed nonoperatively. He is delirious, with waxing and waning attention, memory disturbances, and disorientation. His psychotropic medications are held.
Continue to: Imaging of his head...
Imaging of his head does not reveal acute abnormalities suggesting a malignant or paraneoplastic process, and there are no concerns for ongoing seizures. An infection workup is negative. His urine toxicology is negative and blood alcohol level is 0. His sodium normalizes after 3 days of IV fluids and fluid restriction. Therefore, further tests to differentiate the causes of hyponatremia, such as urine electrolytes and urine osmolality, are not pursued.
[polldaddy:10910406]
The authors’ observations
The differential diagnosis for hyponatremia is broad in the setting of psychiatric illness. Low sodium levels could be due to psychotropic medications, psychiatrically-driven behaviors, or an underlying medical problem. Our differential diagnosis for Mr. F included iatrogenic syndrome of inappropriate antidiuretic hormone (SIADH), diabetes insipidus, or psychogenic polydipsia, a form of primary polydipsia. Other causes of primary polydipsia are related to substances, such as heavy beer intakeor use of 3,4-methylenedioxymethamphetamine (MDMA, also known as “ecstasy”), or brain lesions,1 but these causes were less likely given Mr. F’s negative urine toxicology and head imaging.
While psychogenic polydipsia is due to increased water consumption, both SIADH and diabetes insipidus are due to alterations in fluid homeostasis.2,3 Table 12-4 outlines distinguishing characteristics of SIADH, diabetes insipidus, and psychogenic polydipsia. Urine studies were not pursued because Mr. F’s sodium resolved and acute concerns, such as malignancy or infection, were ruled out. Mr. F’s hyponatremia was presumed to be due to psychogenic polydipsia because of his increased fluid intake and normalization of sodium with hypertonic fluids and subsequent fluid restriction. During this time, he was managed on the surgical service; the plan was to pursue urine studies and possibly a fluid challenge if his hyponatremia persisted.
EVALUATION Delirium resolves, delusions persist
While Mr. F is on the surgical service, the treatment team focuses on stabilizing his sodium level and assessing for causes of altered mental status that led to his fall. Psychiatry is consulted for management of his agitation. Following the gradual correction of his sodium level and extubation, his sensorium improves. By hospital Day 5, Mr. F’s delirium resolves.
During this time, Mr. F’s disorganization and religious delusions become apparent. He spends much of his time reading his Bible. He has poor hygiene and limited engagement in activities of daily living. Due to his psychosis and inability to care for himself, Mr. F is transferred to the psychiatric unit with consent from his mother.
Continue to: TREATMENT Olanzapine and fluid restriction
TREATMENT Olanzapine and fluid restriction
In the psychiatric unit, Mr. F is restarted on olanzapine, but not on perphenazine due to anticholinergic effects and not on propranolol due to continued orthostatic hypotension. Five days later, he is at his baseline level of functioning with residual psychosis. His fluid intake is restricted to <1.5 L per day and he is easily compliant.
Mr. F’s mother is comfortable with his discharge home on a regimen of olanzapine, 25 mg/d, and the team discusses the fluid restrictions with her. The treatment team suggests initiating an LAI before Mr. F is discharged, but this is not pursued because his mother thinks he is doing well with the oral medication. She wants to monitor him with the medication changes in the clinic before pursuing an LAI; however, she is open to it in the future.
The authors’ observations
Approximately 20% of patients with schizophrenia may experience psychogenic polydipsia.4,5 The cause of psychogenic polydipsia in patients with serious mental illness is multifactorial. It may stem from malfunction of the hypothalamic-pituitary axis, which leads to alterations in antidiuretic hormone secretion and function.4-6
Mr. F’s case highlights several challenges associated with treating psychogenic polydipsia in patients with serious mental illness. Antipsychotics with high dopamine affinity, such as risperidone and haloperidol, may increase the risk of psychogenic polydipsia, while antipsychotics with lower dopamine affinity, such as clozapine, may decrease the occurrence.5 Antipsychotics block postsynaptic dopamine receptors, which can induce supersensitivity by increasing presynaptic dopamine release in the hypothalamic areas, where thirst regulation occurs. This increase in dopamine leads to increased thirst drive and fluid intake.3
Quetiapine or clozapine may have been a better antipsychotic choice because these agents have lower D2 receptor affinity, whereas olanzapine has intermediate binding to D2 receptors.6,7 However, quetiapine and clozapine are more strongly associated with orthostasis, which was a concern during Mr. F’s hospitalization. The weekly laboratory testing required with clozapine use would have been an unfeasible burden for Mr. F because he lived in a rural environment. Perphenazine was not continued due to higher D2 affinity and anticholinergic effects, which can increase thirst.6
Continue to: In addition to switching...
In addition to switching to an antipsychotic with looser D2 binding, other medications for treating polydipsia have been studied. It is hypothesized that the alpha-2 adrenergic system may play a role in thirst regulation. For example, mianserin, an alpha-2 antagonist, may decrease water intake. However, studies have been small and inconsistent.8,9 Propranolol,10 a beta adrenergic receptor blocker; irbesartan,11 an angiotensin-II receptor blocker; demeclocycline,12 a tetracycline that inhibits antidiuretic hormone action; and naltrexone,9 a mu opioid antagonist, have been studied with inconclusive results and a variety of adverse effects5,7,13 (Table 28-13).
Behavioral interventions for patients with psychogenic polydipsia include fluid restriction, twice-daily weight checks, cognitive-behavioral therapy, and reinforcement schedules, which may be useful but less realistic due to need for increased supervision.11,12 Patient and family education on the signs of hyponatremia are important to prevent serious complications, such as those Mr. F experienced.
OUTCOME Repeated hospitalizations
Mr. F is discharged with follow-up in our psychiatry clinic and attends 1 appointment. At that time, his mother reports that Mr. F is compliant with his medication and has limited fluid intake. However, over the next 2 months, he is admitted to our psychiatric unit twice with similar presentations. Each time, the treatment team has extensive discussions with Mr. F’s mother about strategies to limit his water intake and the possibility of residential placement due to his need for a higher level of care. Although she acknowledges that nursing home placement may be needed in the future, she is not ready to take this step.
Three months later, Mr. F returns to our hospital with severe abdominal pain and is found to have a perforated bowel obstruction. His sodium is within normal limits on presentation, and the psychiatry team is not involved during this hospitalization. Mr. F is treated for sepsis and undergoes 3 exploratory laparotomies with continued decline in his health. He dies during this hospitalization. The cause of Mr. F’s perforated bowel obstruction is not determined, and his family does not pursue an autopsy.
The authors’ observations
At Mr. F’s final hospital presentation, his sodium was normal. It is possible Mr. F and his mother had found an acceptable fluid restriction routine, and he may have been doing better from a psychiatric perspective, but this will remain unknown.
Continue to: This case highlights...
This case highlights the clinical and ethical complexity of treating patients with psychogenic polydipsia. Because Mr. F no longer had autonomy, we had to determine if his mother was acting in his best interest as his guardian. Guardianship requirements and expectations vary by state. In our state of Missouri, a guardian is appointed by the court to act in the best interest of the ward, and may be a family member (preferred) or state-appointed. The guardian is responsible for providing the ward’s care and is in charge of financial and medical decisions. In Missouri, the guardian must assure the ward resides in the “least restrictive setting reasonably available,” which is the minimum necessary to provide the ward safe care and housing.14 Full guardianship, as in Mr. F’s case, is different from limited guardianship, which is an option in states such as Missouri. In limited guardianship, the court decides the extent of the guardian’s role in decisions for the ward.14,15
Mr. F’s mother believed she was acting in her son’s best interest by having him home with his family. She believed by living at home, he would derive more enjoyment from life than living in a nursing home. By the time Mr. F presented to our hospital, he had been living with decompensated schizophrenia for years, so some level of psychosis was likely to persist, even with treatment. Given his increasingly frequent hospitalizations for hyponatremia due to increased water intake, more intense supervision may have been needed to maintain his safety, in line with nonmaleficence. The treatment team considered Mr. F’s best interest when discussing placement and worked to understand his mother’s preferences.
His mother continued to acknowledge the need for changes and adjustments at home. She was receptive to the need for fluid restriction and increased structure at home. Therefore, we felt she continued to be acting in his best interest, and his home would be the least restrictive setting for his care. If Mr. F had continued to require repeated hospitalizations and had not passed away, we would have pursued an ethics consult to discuss the need for nursing home placement and how to best approach this with Mr. F’s mother.
Bottom Line
Patients with serious mental illness who present with hyponatremia should be evaluated for psychogenic polydipsia by assessing their dietary and fluid intakes, along with collateral from family. The use of antipsychotics with high dopamine affinity may increase the risk of psychogenic polydipsia. Behavioral interventions include fluid restriction, weight checks, cognitive-behavioral therapy, and reinforcement schedules.
Related Resources
- Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/ 978-3-319-58260-3_21
- Sailer C, Winzeler B, Christ-Crain M. Primary polydipsia in the medical and psychiatric patient: characteristics, complications and therapy. Swiss Med Wkly. 2017;147:w14514. doi:10.4414/ smw.2017.14514
Drug Brand Names
Amiloride • Midamor
Aripiprazole • Abilify
Clonidine • Catapres
Clozapine • Clozaril
Demeclocycline • Declomycin
Desmopressin • DDAVP
Haloperidol • Haldol
Irbesartan • Avapro
Lithium • Eskalith, Lithobid
Losartan • Cozaar
Mianserin • Tolvon
Naloxone • Narcan
Naltrexone • Revia
Olanzapine • Zyprexa
Perphenazine • Trilafon
Propranolol • Inderal LA
Quetiapine • Seroquel
Risperidone • Risperda
1. Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/978-3-319-58260-3_21
2. Gross P. Clinical management of SIADH. Ther Adv Endocrinol Metab. 2012;3(2):61-73. doi:10.1177/2042018812437561
3. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primer. 2019;5(1):54. doi:10.1038/s41572-019-0103-2
4. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi:10.1016/j.beem.2020.101469
5. Kirino S, Sakuma M, Misawa F, et al. Relationship between polydipsia and antipsychotics: a systematic review of clinical studies and case reports. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109756. doi:10.1016/j.pnpbp.2019.109756
6. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: from receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223. doi:10.2174/1570159X15666170630163616
7. Seeman P, Tallerico T. Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry. 1998;3(2):123-134. doi:10.1038/sj.mp.4000336
8. Hayashi T, Nishikawa T, Koga I, et al. Involvement of the α 2 -adrenergic system in polydipsia in schizophrenic patients: a pilot study. Psychopharmacology (Berl). 1997;130(4):382-386. doi:10.1007/s002130050254
9. Rizvi S, Gold J, Khan AM. Role of naltrexone in improving compulsive drinking in psychogenic polydipsia. Cureus. 2019;11(8):e5320. doi:10.7759/cureus.5320
10. Kishi Y, Kurosawa H, Endo S. Is propranolol effective in primary polydipsia? Int J Psychiatry Med. 1998;28(3):315-325. doi:10.2190/QPWL-14H7-HPGG-A29D
11. Kruse D, Pantelis C, Rudd R, et al. Treatment of psychogenic polydipsia: comparison of risperidone and olanzapine, and the effects of an adjunctive angiotensin-II receptor blocking drug (irbesartan). Aust N Z J Psychiatry. 2001;35(1):65-68. doi:10.1046/j.1440-1614.2001.00847.x
12. Alexander RC, Karp BI, Thompson S, et al. A double blind, placebo-controlled trial of demeclocycline treatment of polydipsia-hyponatremia in chronically psychotic patients. Biol Psychiatry. 1991;30(4):417-420. doi:10.1016/0006-3223(91)90300-B
13. Valente S, Fisher D. Recognizing and managing psychogenic polydipsia in mental health. J Nurse Pract. 2010;6(7):546-550. doi:10.1016/j.nurpra.2010.03.004
14. Barton R, Esq SL, Lockett LL. The use of conservatorships and adult guardianships and other options in the care of the mentally ill in the United States. World Guard Congr. Published May 29, 2014. Accessed June 18, 2021. http://www.guardianship.org/IRL/Resources/Handouts/Family%20Members%20as%20Guardians_Handout.pdf
15. ABA Commission on Law & Aging. Adult Guardianship Statutory Table of Authorities. ABA. Published January 2021. Accessed June 17, 2021. https://www.americanbar.org/content/dam/aba/administrative/law_aging/2019-adult-guardianship-statutory-table-of-authorities.pdf
CASE Unresponsive after a presumed seizure
Mr. F, age 44, has schizophrenia. He is brought to the hospital by ambulance after he is found on the ground outside of his mother’s house following a presumed seizure and fall. On arrival to the emergency department, he is unresponsive. His laboratory values are significant for a sodium level of 110 mEq/L (reference range: 135 to 145 mEq/L), indicating hyponatremia.
HISTORY Fixated on purity
Mr. F’s mother reports that Mr. F had an unremarkable childhood. He was raised in a household with both parents and a younger sister. Mr. F did well academically and studied engineering and physics in college. There was no reported history of trauma or substance use.
During his senior year of college, Mr. F began experiencing paranoia, auditory hallucinations, and religious delusions. He required hospitalization and was diagnosed with schizophrenia. Following multiple hospitalizations over 5 years, he moved in with his mother, who was granted guardianship.
His mother said Mr. F’s religious delusions were of purity and cleansing the soul. He spent hours memorizing the Bible and would go for days without eating but would drink large amounts of water. She said she thought this was due to his desire to flush out imperfections.
In the past 3 years, Mr. F has been hospitalized several times for severe hyponatremia. At home, his mother attempted to restrict his water intake. However, Mr. F would still drink out of sinks and hoses. Mr. F’s mother reports that over the past month he had become more isolated. He would spend entire days reading the Bible, and his water intake had further increased.
Prior medication trials for Mr. F included haloperidol, up to 10 mg twice per day; aripiprazole, up to 20 mg/d; and risperidone, up to 6 mg nightly. These had been effective, but Mr. F had difficulty with adherence. He did not receive a long-acting injectable (LAI) antipsychotic initially due to lack of access at the rural clinic where he was treated, and later due to his mother’s preference for her son to receive oral medications. Prior to his current presentation, Mr. F’s medication regimen was olanzapine, 10 mg twice a day; perphenazine, 8 mg twice a day; and long-acting propranolol, 60 mg/d. Mr. F had no other chronic medical problems.
EVALUATION Hyponatremia, but why?
Mr. F is intubated and admitted to the surgical service for stabilization due to injuries from his fall. He has fractures of his right sinus and bilateral nasal bones, which are managed nonoperatively. He is delirious, with waxing and waning attention, memory disturbances, and disorientation. His psychotropic medications are held.
Continue to: Imaging of his head...
Imaging of his head does not reveal acute abnormalities suggesting a malignant or paraneoplastic process, and there are no concerns for ongoing seizures. An infection workup is negative. His urine toxicology is negative and blood alcohol level is 0. His sodium normalizes after 3 days of IV fluids and fluid restriction. Therefore, further tests to differentiate the causes of hyponatremia, such as urine electrolytes and urine osmolality, are not pursued.
[polldaddy:10910406]
The authors’ observations
The differential diagnosis for hyponatremia is broad in the setting of psychiatric illness. Low sodium levels could be due to psychotropic medications, psychiatrically-driven behaviors, or an underlying medical problem. Our differential diagnosis for Mr. F included iatrogenic syndrome of inappropriate antidiuretic hormone (SIADH), diabetes insipidus, or psychogenic polydipsia, a form of primary polydipsia. Other causes of primary polydipsia are related to substances, such as heavy beer intakeor use of 3,4-methylenedioxymethamphetamine (MDMA, also known as “ecstasy”), or brain lesions,1 but these causes were less likely given Mr. F’s negative urine toxicology and head imaging.
While psychogenic polydipsia is due to increased water consumption, both SIADH and diabetes insipidus are due to alterations in fluid homeostasis.2,3 Table 12-4 outlines distinguishing characteristics of SIADH, diabetes insipidus, and psychogenic polydipsia. Urine studies were not pursued because Mr. F’s sodium resolved and acute concerns, such as malignancy or infection, were ruled out. Mr. F’s hyponatremia was presumed to be due to psychogenic polydipsia because of his increased fluid intake and normalization of sodium with hypertonic fluids and subsequent fluid restriction. During this time, he was managed on the surgical service; the plan was to pursue urine studies and possibly a fluid challenge if his hyponatremia persisted.
EVALUATION Delirium resolves, delusions persist
While Mr. F is on the surgical service, the treatment team focuses on stabilizing his sodium level and assessing for causes of altered mental status that led to his fall. Psychiatry is consulted for management of his agitation. Following the gradual correction of his sodium level and extubation, his sensorium improves. By hospital Day 5, Mr. F’s delirium resolves.
During this time, Mr. F’s disorganization and religious delusions become apparent. He spends much of his time reading his Bible. He has poor hygiene and limited engagement in activities of daily living. Due to his psychosis and inability to care for himself, Mr. F is transferred to the psychiatric unit with consent from his mother.
Continue to: TREATMENT Olanzapine and fluid restriction
TREATMENT Olanzapine and fluid restriction
In the psychiatric unit, Mr. F is restarted on olanzapine, but not on perphenazine due to anticholinergic effects and not on propranolol due to continued orthostatic hypotension. Five days later, he is at his baseline level of functioning with residual psychosis. His fluid intake is restricted to <1.5 L per day and he is easily compliant.
Mr. F’s mother is comfortable with his discharge home on a regimen of olanzapine, 25 mg/d, and the team discusses the fluid restrictions with her. The treatment team suggests initiating an LAI before Mr. F is discharged, but this is not pursued because his mother thinks he is doing well with the oral medication. She wants to monitor him with the medication changes in the clinic before pursuing an LAI; however, she is open to it in the future.
The authors’ observations
Approximately 20% of patients with schizophrenia may experience psychogenic polydipsia.4,5 The cause of psychogenic polydipsia in patients with serious mental illness is multifactorial. It may stem from malfunction of the hypothalamic-pituitary axis, which leads to alterations in antidiuretic hormone secretion and function.4-6
Mr. F’s case highlights several challenges associated with treating psychogenic polydipsia in patients with serious mental illness. Antipsychotics with high dopamine affinity, such as risperidone and haloperidol, may increase the risk of psychogenic polydipsia, while antipsychotics with lower dopamine affinity, such as clozapine, may decrease the occurrence.5 Antipsychotics block postsynaptic dopamine receptors, which can induce supersensitivity by increasing presynaptic dopamine release in the hypothalamic areas, where thirst regulation occurs. This increase in dopamine leads to increased thirst drive and fluid intake.3
Quetiapine or clozapine may have been a better antipsychotic choice because these agents have lower D2 receptor affinity, whereas olanzapine has intermediate binding to D2 receptors.6,7 However, quetiapine and clozapine are more strongly associated with orthostasis, which was a concern during Mr. F’s hospitalization. The weekly laboratory testing required with clozapine use would have been an unfeasible burden for Mr. F because he lived in a rural environment. Perphenazine was not continued due to higher D2 affinity and anticholinergic effects, which can increase thirst.6
Continue to: In addition to switching...
In addition to switching to an antipsychotic with looser D2 binding, other medications for treating polydipsia have been studied. It is hypothesized that the alpha-2 adrenergic system may play a role in thirst regulation. For example, mianserin, an alpha-2 antagonist, may decrease water intake. However, studies have been small and inconsistent.8,9 Propranolol,10 a beta adrenergic receptor blocker; irbesartan,11 an angiotensin-II receptor blocker; demeclocycline,12 a tetracycline that inhibits antidiuretic hormone action; and naltrexone,9 a mu opioid antagonist, have been studied with inconclusive results and a variety of adverse effects5,7,13 (Table 28-13).
Behavioral interventions for patients with psychogenic polydipsia include fluid restriction, twice-daily weight checks, cognitive-behavioral therapy, and reinforcement schedules, which may be useful but less realistic due to need for increased supervision.11,12 Patient and family education on the signs of hyponatremia are important to prevent serious complications, such as those Mr. F experienced.
OUTCOME Repeated hospitalizations
Mr. F is discharged with follow-up in our psychiatry clinic and attends 1 appointment. At that time, his mother reports that Mr. F is compliant with his medication and has limited fluid intake. However, over the next 2 months, he is admitted to our psychiatric unit twice with similar presentations. Each time, the treatment team has extensive discussions with Mr. F’s mother about strategies to limit his water intake and the possibility of residential placement due to his need for a higher level of care. Although she acknowledges that nursing home placement may be needed in the future, she is not ready to take this step.
Three months later, Mr. F returns to our hospital with severe abdominal pain and is found to have a perforated bowel obstruction. His sodium is within normal limits on presentation, and the psychiatry team is not involved during this hospitalization. Mr. F is treated for sepsis and undergoes 3 exploratory laparotomies with continued decline in his health. He dies during this hospitalization. The cause of Mr. F’s perforated bowel obstruction is not determined, and his family does not pursue an autopsy.
The authors’ observations
At Mr. F’s final hospital presentation, his sodium was normal. It is possible Mr. F and his mother had found an acceptable fluid restriction routine, and he may have been doing better from a psychiatric perspective, but this will remain unknown.
Continue to: This case highlights...
This case highlights the clinical and ethical complexity of treating patients with psychogenic polydipsia. Because Mr. F no longer had autonomy, we had to determine if his mother was acting in his best interest as his guardian. Guardianship requirements and expectations vary by state. In our state of Missouri, a guardian is appointed by the court to act in the best interest of the ward, and may be a family member (preferred) or state-appointed. The guardian is responsible for providing the ward’s care and is in charge of financial and medical decisions. In Missouri, the guardian must assure the ward resides in the “least restrictive setting reasonably available,” which is the minimum necessary to provide the ward safe care and housing.14 Full guardianship, as in Mr. F’s case, is different from limited guardianship, which is an option in states such as Missouri. In limited guardianship, the court decides the extent of the guardian’s role in decisions for the ward.14,15
Mr. F’s mother believed she was acting in her son’s best interest by having him home with his family. She believed by living at home, he would derive more enjoyment from life than living in a nursing home. By the time Mr. F presented to our hospital, he had been living with decompensated schizophrenia for years, so some level of psychosis was likely to persist, even with treatment. Given his increasingly frequent hospitalizations for hyponatremia due to increased water intake, more intense supervision may have been needed to maintain his safety, in line with nonmaleficence. The treatment team considered Mr. F’s best interest when discussing placement and worked to understand his mother’s preferences.
His mother continued to acknowledge the need for changes and adjustments at home. She was receptive to the need for fluid restriction and increased structure at home. Therefore, we felt she continued to be acting in his best interest, and his home would be the least restrictive setting for his care. If Mr. F had continued to require repeated hospitalizations and had not passed away, we would have pursued an ethics consult to discuss the need for nursing home placement and how to best approach this with Mr. F’s mother.
Bottom Line
Patients with serious mental illness who present with hyponatremia should be evaluated for psychogenic polydipsia by assessing their dietary and fluid intakes, along with collateral from family. The use of antipsychotics with high dopamine affinity may increase the risk of psychogenic polydipsia. Behavioral interventions include fluid restriction, weight checks, cognitive-behavioral therapy, and reinforcement schedules.
Related Resources
- Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/ 978-3-319-58260-3_21
- Sailer C, Winzeler B, Christ-Crain M. Primary polydipsia in the medical and psychiatric patient: characteristics, complications and therapy. Swiss Med Wkly. 2017;147:w14514. doi:10.4414/ smw.2017.14514
Drug Brand Names
Amiloride • Midamor
Aripiprazole • Abilify
Clonidine • Catapres
Clozapine • Clozaril
Demeclocycline • Declomycin
Desmopressin • DDAVP
Haloperidol • Haldol
Irbesartan • Avapro
Lithium • Eskalith, Lithobid
Losartan • Cozaar
Mianserin • Tolvon
Naloxone • Narcan
Naltrexone • Revia
Olanzapine • Zyprexa
Perphenazine • Trilafon
Propranolol • Inderal LA
Quetiapine • Seroquel
Risperidone • Risperda
CASE Unresponsive after a presumed seizure
Mr. F, age 44, has schizophrenia. He is brought to the hospital by ambulance after he is found on the ground outside of his mother’s house following a presumed seizure and fall. On arrival to the emergency department, he is unresponsive. His laboratory values are significant for a sodium level of 110 mEq/L (reference range: 135 to 145 mEq/L), indicating hyponatremia.
HISTORY Fixated on purity
Mr. F’s mother reports that Mr. F had an unremarkable childhood. He was raised in a household with both parents and a younger sister. Mr. F did well academically and studied engineering and physics in college. There was no reported history of trauma or substance use.
During his senior year of college, Mr. F began experiencing paranoia, auditory hallucinations, and religious delusions. He required hospitalization and was diagnosed with schizophrenia. Following multiple hospitalizations over 5 years, he moved in with his mother, who was granted guardianship.
His mother said Mr. F’s religious delusions were of purity and cleansing the soul. He spent hours memorizing the Bible and would go for days without eating but would drink large amounts of water. She said she thought this was due to his desire to flush out imperfections.
In the past 3 years, Mr. F has been hospitalized several times for severe hyponatremia. At home, his mother attempted to restrict his water intake. However, Mr. F would still drink out of sinks and hoses. Mr. F’s mother reports that over the past month he had become more isolated. He would spend entire days reading the Bible, and his water intake had further increased.
Prior medication trials for Mr. F included haloperidol, up to 10 mg twice per day; aripiprazole, up to 20 mg/d; and risperidone, up to 6 mg nightly. These had been effective, but Mr. F had difficulty with adherence. He did not receive a long-acting injectable (LAI) antipsychotic initially due to lack of access at the rural clinic where he was treated, and later due to his mother’s preference for her son to receive oral medications. Prior to his current presentation, Mr. F’s medication regimen was olanzapine, 10 mg twice a day; perphenazine, 8 mg twice a day; and long-acting propranolol, 60 mg/d. Mr. F had no other chronic medical problems.
EVALUATION Hyponatremia, but why?
Mr. F is intubated and admitted to the surgical service for stabilization due to injuries from his fall. He has fractures of his right sinus and bilateral nasal bones, which are managed nonoperatively. He is delirious, with waxing and waning attention, memory disturbances, and disorientation. His psychotropic medications are held.
Continue to: Imaging of his head...
Imaging of his head does not reveal acute abnormalities suggesting a malignant or paraneoplastic process, and there are no concerns for ongoing seizures. An infection workup is negative. His urine toxicology is negative and blood alcohol level is 0. His sodium normalizes after 3 days of IV fluids and fluid restriction. Therefore, further tests to differentiate the causes of hyponatremia, such as urine electrolytes and urine osmolality, are not pursued.
[polldaddy:10910406]
The authors’ observations
The differential diagnosis for hyponatremia is broad in the setting of psychiatric illness. Low sodium levels could be due to psychotropic medications, psychiatrically-driven behaviors, or an underlying medical problem. Our differential diagnosis for Mr. F included iatrogenic syndrome of inappropriate antidiuretic hormone (SIADH), diabetes insipidus, or psychogenic polydipsia, a form of primary polydipsia. Other causes of primary polydipsia are related to substances, such as heavy beer intakeor use of 3,4-methylenedioxymethamphetamine (MDMA, also known as “ecstasy”), or brain lesions,1 but these causes were less likely given Mr. F’s negative urine toxicology and head imaging.
While psychogenic polydipsia is due to increased water consumption, both SIADH and diabetes insipidus are due to alterations in fluid homeostasis.2,3 Table 12-4 outlines distinguishing characteristics of SIADH, diabetes insipidus, and psychogenic polydipsia. Urine studies were not pursued because Mr. F’s sodium resolved and acute concerns, such as malignancy or infection, were ruled out. Mr. F’s hyponatremia was presumed to be due to psychogenic polydipsia because of his increased fluid intake and normalization of sodium with hypertonic fluids and subsequent fluid restriction. During this time, he was managed on the surgical service; the plan was to pursue urine studies and possibly a fluid challenge if his hyponatremia persisted.
EVALUATION Delirium resolves, delusions persist
While Mr. F is on the surgical service, the treatment team focuses on stabilizing his sodium level and assessing for causes of altered mental status that led to his fall. Psychiatry is consulted for management of his agitation. Following the gradual correction of his sodium level and extubation, his sensorium improves. By hospital Day 5, Mr. F’s delirium resolves.
During this time, Mr. F’s disorganization and religious delusions become apparent. He spends much of his time reading his Bible. He has poor hygiene and limited engagement in activities of daily living. Due to his psychosis and inability to care for himself, Mr. F is transferred to the psychiatric unit with consent from his mother.
Continue to: TREATMENT Olanzapine and fluid restriction
TREATMENT Olanzapine and fluid restriction
In the psychiatric unit, Mr. F is restarted on olanzapine, but not on perphenazine due to anticholinergic effects and not on propranolol due to continued orthostatic hypotension. Five days later, he is at his baseline level of functioning with residual psychosis. His fluid intake is restricted to <1.5 L per day and he is easily compliant.
Mr. F’s mother is comfortable with his discharge home on a regimen of olanzapine, 25 mg/d, and the team discusses the fluid restrictions with her. The treatment team suggests initiating an LAI before Mr. F is discharged, but this is not pursued because his mother thinks he is doing well with the oral medication. She wants to monitor him with the medication changes in the clinic before pursuing an LAI; however, she is open to it in the future.
The authors’ observations
Approximately 20% of patients with schizophrenia may experience psychogenic polydipsia.4,5 The cause of psychogenic polydipsia in patients with serious mental illness is multifactorial. It may stem from malfunction of the hypothalamic-pituitary axis, which leads to alterations in antidiuretic hormone secretion and function.4-6
Mr. F’s case highlights several challenges associated with treating psychogenic polydipsia in patients with serious mental illness. Antipsychotics with high dopamine affinity, such as risperidone and haloperidol, may increase the risk of psychogenic polydipsia, while antipsychotics with lower dopamine affinity, such as clozapine, may decrease the occurrence.5 Antipsychotics block postsynaptic dopamine receptors, which can induce supersensitivity by increasing presynaptic dopamine release in the hypothalamic areas, where thirst regulation occurs. This increase in dopamine leads to increased thirst drive and fluid intake.3
Quetiapine or clozapine may have been a better antipsychotic choice because these agents have lower D2 receptor affinity, whereas olanzapine has intermediate binding to D2 receptors.6,7 However, quetiapine and clozapine are more strongly associated with orthostasis, which was a concern during Mr. F’s hospitalization. The weekly laboratory testing required with clozapine use would have been an unfeasible burden for Mr. F because he lived in a rural environment. Perphenazine was not continued due to higher D2 affinity and anticholinergic effects, which can increase thirst.6
Continue to: In addition to switching...
In addition to switching to an antipsychotic with looser D2 binding, other medications for treating polydipsia have been studied. It is hypothesized that the alpha-2 adrenergic system may play a role in thirst regulation. For example, mianserin, an alpha-2 antagonist, may decrease water intake. However, studies have been small and inconsistent.8,9 Propranolol,10 a beta adrenergic receptor blocker; irbesartan,11 an angiotensin-II receptor blocker; demeclocycline,12 a tetracycline that inhibits antidiuretic hormone action; and naltrexone,9 a mu opioid antagonist, have been studied with inconclusive results and a variety of adverse effects5,7,13 (Table 28-13).
Behavioral interventions for patients with psychogenic polydipsia include fluid restriction, twice-daily weight checks, cognitive-behavioral therapy, and reinforcement schedules, which may be useful but less realistic due to need for increased supervision.11,12 Patient and family education on the signs of hyponatremia are important to prevent serious complications, such as those Mr. F experienced.
OUTCOME Repeated hospitalizations
Mr. F is discharged with follow-up in our psychiatry clinic and attends 1 appointment. At that time, his mother reports that Mr. F is compliant with his medication and has limited fluid intake. However, over the next 2 months, he is admitted to our psychiatric unit twice with similar presentations. Each time, the treatment team has extensive discussions with Mr. F’s mother about strategies to limit his water intake and the possibility of residential placement due to his need for a higher level of care. Although she acknowledges that nursing home placement may be needed in the future, she is not ready to take this step.
Three months later, Mr. F returns to our hospital with severe abdominal pain and is found to have a perforated bowel obstruction. His sodium is within normal limits on presentation, and the psychiatry team is not involved during this hospitalization. Mr. F is treated for sepsis and undergoes 3 exploratory laparotomies with continued decline in his health. He dies during this hospitalization. The cause of Mr. F’s perforated bowel obstruction is not determined, and his family does not pursue an autopsy.
The authors’ observations
At Mr. F’s final hospital presentation, his sodium was normal. It is possible Mr. F and his mother had found an acceptable fluid restriction routine, and he may have been doing better from a psychiatric perspective, but this will remain unknown.
Continue to: This case highlights...
This case highlights the clinical and ethical complexity of treating patients with psychogenic polydipsia. Because Mr. F no longer had autonomy, we had to determine if his mother was acting in his best interest as his guardian. Guardianship requirements and expectations vary by state. In our state of Missouri, a guardian is appointed by the court to act in the best interest of the ward, and may be a family member (preferred) or state-appointed. The guardian is responsible for providing the ward’s care and is in charge of financial and medical decisions. In Missouri, the guardian must assure the ward resides in the “least restrictive setting reasonably available,” which is the minimum necessary to provide the ward safe care and housing.14 Full guardianship, as in Mr. F’s case, is different from limited guardianship, which is an option in states such as Missouri. In limited guardianship, the court decides the extent of the guardian’s role in decisions for the ward.14,15
Mr. F’s mother believed she was acting in her son’s best interest by having him home with his family. She believed by living at home, he would derive more enjoyment from life than living in a nursing home. By the time Mr. F presented to our hospital, he had been living with decompensated schizophrenia for years, so some level of psychosis was likely to persist, even with treatment. Given his increasingly frequent hospitalizations for hyponatremia due to increased water intake, more intense supervision may have been needed to maintain his safety, in line with nonmaleficence. The treatment team considered Mr. F’s best interest when discussing placement and worked to understand his mother’s preferences.
His mother continued to acknowledge the need for changes and adjustments at home. She was receptive to the need for fluid restriction and increased structure at home. Therefore, we felt she continued to be acting in his best interest, and his home would be the least restrictive setting for his care. If Mr. F had continued to require repeated hospitalizations and had not passed away, we would have pursued an ethics consult to discuss the need for nursing home placement and how to best approach this with Mr. F’s mother.
Bottom Line
Patients with serious mental illness who present with hyponatremia should be evaluated for psychogenic polydipsia by assessing their dietary and fluid intakes, along with collateral from family. The use of antipsychotics with high dopamine affinity may increase the risk of psychogenic polydipsia. Behavioral interventions include fluid restriction, weight checks, cognitive-behavioral therapy, and reinforcement schedules.
Related Resources
- Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/ 978-3-319-58260-3_21
- Sailer C, Winzeler B, Christ-Crain M. Primary polydipsia in the medical and psychiatric patient: characteristics, complications and therapy. Swiss Med Wkly. 2017;147:w14514. doi:10.4414/ smw.2017.14514
Drug Brand Names
Amiloride • Midamor
Aripiprazole • Abilify
Clonidine • Catapres
Clozapine • Clozaril
Demeclocycline • Declomycin
Desmopressin • DDAVP
Haloperidol • Haldol
Irbesartan • Avapro
Lithium • Eskalith, Lithobid
Losartan • Cozaar
Mianserin • Tolvon
Naloxone • Narcan
Naltrexone • Revia
Olanzapine • Zyprexa
Perphenazine • Trilafon
Propranolol • Inderal LA
Quetiapine • Seroquel
Risperidone • Risperda
1. Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/978-3-319-58260-3_21
2. Gross P. Clinical management of SIADH. Ther Adv Endocrinol Metab. 2012;3(2):61-73. doi:10.1177/2042018812437561
3. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primer. 2019;5(1):54. doi:10.1038/s41572-019-0103-2
4. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi:10.1016/j.beem.2020.101469
5. Kirino S, Sakuma M, Misawa F, et al. Relationship between polydipsia and antipsychotics: a systematic review of clinical studies and case reports. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109756. doi:10.1016/j.pnpbp.2019.109756
6. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: from receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223. doi:10.2174/1570159X15666170630163616
7. Seeman P, Tallerico T. Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry. 1998;3(2):123-134. doi:10.1038/sj.mp.4000336
8. Hayashi T, Nishikawa T, Koga I, et al. Involvement of the α 2 -adrenergic system in polydipsia in schizophrenic patients: a pilot study. Psychopharmacology (Berl). 1997;130(4):382-386. doi:10.1007/s002130050254
9. Rizvi S, Gold J, Khan AM. Role of naltrexone in improving compulsive drinking in psychogenic polydipsia. Cureus. 2019;11(8):e5320. doi:10.7759/cureus.5320
10. Kishi Y, Kurosawa H, Endo S. Is propranolol effective in primary polydipsia? Int J Psychiatry Med. 1998;28(3):315-325. doi:10.2190/QPWL-14H7-HPGG-A29D
11. Kruse D, Pantelis C, Rudd R, et al. Treatment of psychogenic polydipsia: comparison of risperidone and olanzapine, and the effects of an adjunctive angiotensin-II receptor blocking drug (irbesartan). Aust N Z J Psychiatry. 2001;35(1):65-68. doi:10.1046/j.1440-1614.2001.00847.x
12. Alexander RC, Karp BI, Thompson S, et al. A double blind, placebo-controlled trial of demeclocycline treatment of polydipsia-hyponatremia in chronically psychotic patients. Biol Psychiatry. 1991;30(4):417-420. doi:10.1016/0006-3223(91)90300-B
13. Valente S, Fisher D. Recognizing and managing psychogenic polydipsia in mental health. J Nurse Pract. 2010;6(7):546-550. doi:10.1016/j.nurpra.2010.03.004
14. Barton R, Esq SL, Lockett LL. The use of conservatorships and adult guardianships and other options in the care of the mentally ill in the United States. World Guard Congr. Published May 29, 2014. Accessed June 18, 2021. http://www.guardianship.org/IRL/Resources/Handouts/Family%20Members%20as%20Guardians_Handout.pdf
15. ABA Commission on Law & Aging. Adult Guardianship Statutory Table of Authorities. ABA. Published January 2021. Accessed June 17, 2021. https://www.americanbar.org/content/dam/aba/administrative/law_aging/2019-adult-guardianship-statutory-table-of-authorities.pdf
1. Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/978-3-319-58260-3_21
2. Gross P. Clinical management of SIADH. Ther Adv Endocrinol Metab. 2012;3(2):61-73. doi:10.1177/2042018812437561
3. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primer. 2019;5(1):54. doi:10.1038/s41572-019-0103-2
4. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi:10.1016/j.beem.2020.101469
5. Kirino S, Sakuma M, Misawa F, et al. Relationship between polydipsia and antipsychotics: a systematic review of clinical studies and case reports. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109756. doi:10.1016/j.pnpbp.2019.109756
6. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: from receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223. doi:10.2174/1570159X15666170630163616
7. Seeman P, Tallerico T. Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry. 1998;3(2):123-134. doi:10.1038/sj.mp.4000336
8. Hayashi T, Nishikawa T, Koga I, et al. Involvement of the α 2 -adrenergic system in polydipsia in schizophrenic patients: a pilot study. Psychopharmacology (Berl). 1997;130(4):382-386. doi:10.1007/s002130050254
9. Rizvi S, Gold J, Khan AM. Role of naltrexone in improving compulsive drinking in psychogenic polydipsia. Cureus. 2019;11(8):e5320. doi:10.7759/cureus.5320
10. Kishi Y, Kurosawa H, Endo S. Is propranolol effective in primary polydipsia? Int J Psychiatry Med. 1998;28(3):315-325. doi:10.2190/QPWL-14H7-HPGG-A29D
11. Kruse D, Pantelis C, Rudd R, et al. Treatment of psychogenic polydipsia: comparison of risperidone and olanzapine, and the effects of an adjunctive angiotensin-II receptor blocking drug (irbesartan). Aust N Z J Psychiatry. 2001;35(1):65-68. doi:10.1046/j.1440-1614.2001.00847.x
12. Alexander RC, Karp BI, Thompson S, et al. A double blind, placebo-controlled trial of demeclocycline treatment of polydipsia-hyponatremia in chronically psychotic patients. Biol Psychiatry. 1991;30(4):417-420. doi:10.1016/0006-3223(91)90300-B
13. Valente S, Fisher D. Recognizing and managing psychogenic polydipsia in mental health. J Nurse Pract. 2010;6(7):546-550. doi:10.1016/j.nurpra.2010.03.004
14. Barton R, Esq SL, Lockett LL. The use of conservatorships and adult guardianships and other options in the care of the mentally ill in the United States. World Guard Congr. Published May 29, 2014. Accessed June 18, 2021. http://www.guardianship.org/IRL/Resources/Handouts/Family%20Members%20as%20Guardians_Handout.pdf
15. ABA Commission on Law & Aging. Adult Guardianship Statutory Table of Authorities. ABA. Published January 2021. Accessed June 17, 2021. https://www.americanbar.org/content/dam/aba/administrative/law_aging/2019-adult-guardianship-statutory-table-of-authorities.pdf
Late-onset, treatment-resistant anxiety and depression
CASE Anxious and can’t sleep
Mr. A, age 41, presents to his primary care physician (PCP) with anxiety and insomnia. He describes having generalized anxiety with initial and middle insomnia, and says he is sleeping an average of 2 hours per night. He denies any other psychiatric symptoms. Mr. A has no significant psychiatric or medical history.
Mr. A is initiated on zolpidem tartrate, 12.5 mg every night at bedtime, and paroxetine, 20 mg every night at bedtime, for anxiety and insomnia, but these medications result in little to no improvement.
During a 4-month period, he is treated with trials of alprazolam, 0.5 mg every 8 hours as needed; diazepam 5 mg twice a day as needed; diphenhydramine, 50 mg at bedtime; and eszopiclone, 3 mg at bedtime. Despite these treatments, he experiences increased anxiety and insomnia, and develops depressive symptoms, including depressed mood, poor concentration, general malaise, extreme fatigue, a 15-pound unintentional weight loss, erectile dysfunction, and decreased libido. Mr. A denies having suicidal or homicidal ideations. Additionally, he typically goes to the gym approximately 3 times per week, and has noticed that the amount of weight he is able to lift has decreased, which is distressing. Previously, he had been able to lift 300 pounds, but now he can only lift 200 pounds.
[polldaddy:10891920]
The authors’ observations
Insomnia, anxiety, and depression are common chief complaints in medical settings. However, some psychiatric presentations may have an underlying medical etiology.
DSM-5 requires that medical conditions be ruled out in order for a patient to meet criteria for a psychiatric diagnosis.1 Medical differential diagnoses for patients with psychiatric symptoms can include autoimmune, drug/toxin, metabolic, infectious, neoplastic, neurologic, and nutritional etiologies (Table 12). To rule out the possibility of an underlying medical etiology, general screening guidelines include complete blood count, complete metabolic panel, urinalysis, and urine drug screen with alcohol. Human immunodeficiency virus testing and thyroid hormone testing are also commonly ordered.3 Further laboratory testing and imaging is typically not warranted in the absence of historical or physical findings because they are not advocated as cost-effective, so health care professionals must use their clinical judgment to determine appropriate further evaluation. The onset of anxiety most commonly occurs in late adolescence early and adulthood, but Mr. A experienced his first symptoms of anxiety at age 41.2 Mr. A’s age, lack of psychiatric or family history of mental illness, acute onset of symptoms, and failure of symptoms to abate with standard psychiatric treatments warrant a more extensive workup.
EVALUATION Imaging reveals an important finding
Because Mr. A’s symptoms do not improve with standard psychiatric treatments, his PCP orders standard laboratory bloodwork to investigate a possible medical etiology; however, his results are all within normal range.
After the PCP’s niece is coincidentally diagnosed with a pituitary macroadenoma, the PCP orders brain imaging for Mr. A. Results of an MRI show that Mr. A has a 1.6-cm macroadenoma of the pituitary. He is referred to an endocrinologist, who orders additional laboratory tests that show an elevated 24-hour free urine cortisol level of 73 μg/24 h (normal range: 3.5 to 45 μg/24 h), suggesting that Mr. A’s anxiety may be due to Cushing’s disease or that his anxiety caused falsely elevated urinary cortisol levels. Four weeks later, bloodwork is repeated and shows an abnormal dexamethasone suppression test, and 2 more elevated 24-hour free urine cortisol levels of 76 μg/24 h and 150 μg/24 h. A repeat MRI shows a 1.8-cm, mostly cystic sellar mass, indicating the need for surgical intervention. Although the tumor is large and shows optic nerve compression, Mr. A does not complain of headaches or changes in vision.
Continue to: Two months later...
Two months later, Mr. A undergoes a transsphenoidal tumor resection of the pituitary adenoma, and biopsy results confirm an adrenocorticotropic hormone (ACTH)-secreting pituitary macroadenoma, which is consistent with Cushing’s disease. Following surgery, steroid treatment with dexamethasone is discontinued due to a persistently elevated
[polldaddy:10891923]
The authors’ observations
Chronic excess glucocorticoid production is the underlying pathophysiology of Cushing’s disease, which is most commonly caused by an ACTH-producing adenoma.4,5 When these hormones become dysregulated, the result can be over- or underproduction of cortisol, which can lead to physical and psychiatric manifestations.6
Cushing’s disease most commonly manifests with the physical symptoms of centripetal fat deposition, abdominal striae, facial plethora, muscle atrophy, bone density loss, immunosuppression, and cardiovascular complications.5
Hypercortisolism can precipitate anxiety (12% to 79%), mood disorders (50% to 70%), and (less commonly) psychotic disorders; however, in a clinical setting, if a patient presented with one of these as a chief complaint, they would likely first be treated psychiatrically rather than worked up medically for a rare medical condition.5,7-13
Mr. A’s initial bloodwork was unremarkable, but cortisol levels were not obtained at that time because testing for cortisol levels to rule out an underlying medical condition is not routine in patients with depression and anxiety. In Mr. A’s case, a neuroendocrine workup was only ordered once his PCP’s niece coincidentally was diagnosed with a pituitary adenoma.
Continue to: For Mr. A...
For Mr. A, Cushing’s disease presented as a psychiatric disorder with anxiety and insomnia that were resistant to numerous psychiatric medications during an 8-month period. If Mr. A’s PCP had not ordered a brain MRI, he may have continued to receive ineffective psychiatric treatment for some time. Many of Mr. A’s physical symptoms were consistent with Cushing’s disease and mental illness, including erectile dysfunction, fatigue, and muscle weakness; however, his 15-pound weight loss pointed more toward psychiatric illness and further disguised his underlying medical diagnosis, because sudden weight gain is commonly seen in Cushing’s disease (Table 24,5,7,9).
TREATMENT Persistent psychiatric symptoms, then finally relief
Four weeks after surgery, Mr. A’s psychiatric symptoms gradually intensify, which prompts him to see a psychiatrist. A mental status examination (MSE) shows that he is well-nourished, with normal activity, appropriate behavior, and coherent thought process, but depressed mood and flat affect. He denies suicidal or homicidal ideation. He reports that despite being advised to have realistic expectations, he had high hopes that the surgery would lead to remission of all his symptoms, and expresses disappointment that he does not feel “back to normal.”
Six days later, Mr. A’s wife takes him to the hospital. His MSE shows that he has a tense appearance, fidgety activity, depressed and anxious mood, restricted affect, circumstantial thought process, and paranoid delusions that his wife was plotting against him. He says he still is experiencing insomnia. He also discloses having suicidal ideations with a plan and intent to overdose on medication, as well as homicidal ideations about killing his wife and children. Mr. A provides reasons for why he would want to hurt his family, and does not appear to be bothered by these thoughts.
Mr. A is admitted to the inpatient psychiatric unit and is prescribed quetiapine, 100 mg every night at bedtime. During the next 2 days, quetiapine is titrated to 300 mg every night at bedtime. On hospital Day 3, Mr. A says he is feeling worse than the previous days. He is still having vague suicidal thoughts and feels agitated, guilty, and depressed. To treat these persistent symptoms, quetiapine is further increased to 400 mg every night at bedtime, and he is initiated on bupropion XL, 150 mg, to treat persistent symptoms.
After 1 week of hospitalization, the treatment team meets with Mr. A and his wife, who has been supportive throughout her husband’s hospitalization. During the meeting, they both agree that Mr. A has experienced some improvement because he is no longer having suicidal or homicidal thoughts, but he is still feeling depressed and frustrated by his continued insomnia. Following the meeting, Mr. A’s quetiapine is further increased to 450 mg every night at bedtime to address continued insomnia, and bupropion XL is increased to 300 mg/d to address continued depressive symptoms. During the next few days, his affective symptoms improve; however, his initial insomnia continues, and quetiapine is further increased to 500 mg every night at bedtime.
Continue to: On hospital Day 20...
On hospital Day 20, Mr. A is discharged back to his outpatient psychiatrist and receives quetiapine, 500 mg every night at bedtime, and bupropion XL, 300 mg/d. Although Mr. A’s depression and anxiety continue to be well controlled, his insomnia persists. Sleep hygiene is addressed, and alprazolam, 0.5 mg every night at bedtime, is added to his regimen, which proves to be effective.
OUTCOME A slow remission
After a year of treatment, Mr. A is slowly tapered off of all medications. Two years later, he is in complete remission of all psychiatric symptoms and no longer requires any psychotropic medications.
The authors’ observations
Treatment for hypercortisolism in patients with psychiatric symptoms triggered by glucocorticoid imbalance has typically resulted in a decrease in the severity of their psychiatric symptoms.9,11 A prospective longitudinal study examining 33 patients found that correction of hypercortisolism in patients with Cushing’s syndrome often led to resolution of their psychiatric symptoms, with 87.9% of patients back to baseline within 1 year.14 However, to our knowledge, few reports have described the management of patients whose symptoms are resistant to treatment of hypercortisolism.
In our case, after transsphenoidal resection of an adenoma, Mr. A became suicidal and paranoid, and his anxiety and insomnia also persisted. A possible explanation for the worsening of Mr. A’s symptoms after surgery could be the slow recovery of the hypothalamic-pituitary-adrenal (HPA) axis and therefore a temporary deficiency in glucocorticoid, which caused an increase in catecholamines, leading to an increase in stress.14 This concept of a “slow recovery” is supported by the fact that Mr. A was successfully weaned off all medication after 1 year of treatment, and achieved complete remission of psychiatric symptoms for >2 years. Furthermore, the severity of Mr. A’s symptoms appeared to correlate with his 24-hour urine cortisol and
Future research should evaluate the utility of screening all patients with treatment-resistant anxiety and/or insomnia for hypercortisolism. Even without other clues to endocrinopathies, serum cortisol levels can be used as a screening tool for diagnosing underlying medical causes in patients with anxiety and depression.2 A greater understanding of the relationship between medical and psychiatric manifestations will allow clinicians to better care for patients. Further research is needed to elucidate the quantitative relationship between cortisol levels and anxiety to evaluate severity, guide treatment planning, and follow treatment response for patients with anxiety. It may be useful to determine the threshold between elevated cortisol levels due to anxiety vs elevated cortisol due to an underlying medical pathology such as Cushing’s disease. Additionally, little research has been conducted to compare how psychiatric symptoms respond to pituitary macroadenoma resection alone, pharmaceutical intervention alone, or a combination of these approaches. It would be beneficial to evaluate these treatment strategies to elucidate the most effective method to reduce psychiatric symptoms in patients with hypercortisolism, and perhaps to reduce the incidence of post-resection worsening of psychiatric symptoms.
Continue to: This case was challenging...
This case was challenging because Mr. A did not initially respond to psychiatric intervention, his psychiatric symptoms worsened after transsphenoidal resection of the pituitary adenoma, and his symptoms were alleviated only after psychiatric medications were re-initiated following surgery. This case highlights the importance of considering an underlying medically diagnosable and treatable cause of psychiatric illness, and illustrates the complex ongoing management that may be necessary to help a patient with this condition achieve their baseline. Further, Mr. A’s case shows that the absence of response to standard psychiatric therapies should warrant earlier laboratory and/or imaging evaluation prior to or in conjunction with psychiatric referral. Additionally, testing for cortisol levels is not typically done for a patient with treatment-resistant anxiety, and this case highlights the importance of considering hypercortisolism in such circumstances.
Bottom Line
Consider testing cortisol levels in patients with treatment-resistant anxiety and insomnia, because cortisol plays a role in Cushing’s disease and anxiety. The severity of psychiatric manifestations of Cushing’s disease may correlate with cortisol levels. Treatment should focus on symptomatic management and underlying etiology.
Related Resources
- Roberts LW, Hales RE, Yudofsky SC, ed. The American Psychiatric Association Publishing Textbook of Psychiatry. 7th ed. American Psychiatric Association Publishing; 2019.
- Rotham J. Cushing’s syndrome: a tale of frequent misdiagnosis. National Center for Health Research. 2020. www.center4research.org/cushings-syndrome-frequent-misdiagnosis/
- Middleman D. Psychiatric issues of Cushing’s patients: coping with Cushing’s. Cushing’s Support and Research Foundation. www.csrf.net/coping-with-cushings/psychiatric-issues-of-cushings-patients/
Drug Brand Names
Alprazolam • Xanax
Bupropion • Wellbutrin
Dexamethasone • Decadron
Diazepam • Valium
Eszopiclone • Lunesta
Paroxetine • Paxil
Quetiapine • Seroquel
Zolpidem tartrate • Ambien CR
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Sadock BJ, Sadock VA, Ruiz P, et al. Neural sciences. In: Sadock BJ, Sadock VA, Ruiz P, et al. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 11th ed. Wolters Kluwer; 2015.
3. Anfinson TJ, Kathol RG. Screening laboratory evaluation in psychiatric patients: a review. Gen Hosp Psychiatry. 1992;14(4):248-257.
4. Fehm HL, Voigt KH. Pathophysiology of Cushing’s disease. Pathobiol Annu. 1979;9:225-255.
5. Fujii Y, Mizoguchi Y, Masuoka J, et al. Cushing’s syndrome and psychosis: a case report and literature review. Prim Care Companion CNS Disord. 2018;20(5):18.
6. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2011;4(2):739-769.
7. Santos A, Resimini E, Pascual JC, et al. Psychiatric symptoms in patients with Cushing’s syndrome: prevalence diagnosis, and management. Drugs. 2017;77(8):829-842.
8. Arnaldi G, Angeli A, Atkinson B, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593-5602.
9. Sonino N, Fava GA. Psychosomatic aspects of Cushing’s disease. Psychother Psychosom. 1998;67(3):140-146.
10. Loosen PT, Chambliss B, DeBold CR, et al. Psychiatric phenomenology in Cushing’s disease. Pharmacopsychiatry. 1992;25(4):192-198.
11. Kelly WF, Kelly MJ, Faragher B. A prospective study of psychiatric and psychological aspects of Cushing’s syndrome. Clin Endocrinol. 1996;45(6):715-720.
12. Katho RG, Delahunt JW, Hannah L. Transition from bipolar affective disorder to intermittent Cushing’s syndrome: case report. J Clin Psychiatry. 1985;46(5):194-196.
13. Hirsh D, Orr G, Kantarovich V, et al. Cushing’s syndrome presenting as a schizophrenia-like psychotic state. Isr J Psychiatry Relat Sci. 2000;37(1):46-50.
14. Dorn LD, Burgess ES, Friedman TC, et al. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82(3):912-919.
15. Starkman MN, Schteingart DE, Schork MA. Cushing’s syndrome after treatment: changes in cortisol and ACTH levels, and amelioration of the depressive syndrome. Psychiatry Res. 1986;19(3):177-178.
CASE Anxious and can’t sleep
Mr. A, age 41, presents to his primary care physician (PCP) with anxiety and insomnia. He describes having generalized anxiety with initial and middle insomnia, and says he is sleeping an average of 2 hours per night. He denies any other psychiatric symptoms. Mr. A has no significant psychiatric or medical history.
Mr. A is initiated on zolpidem tartrate, 12.5 mg every night at bedtime, and paroxetine, 20 mg every night at bedtime, for anxiety and insomnia, but these medications result in little to no improvement.
During a 4-month period, he is treated with trials of alprazolam, 0.5 mg every 8 hours as needed; diazepam 5 mg twice a day as needed; diphenhydramine, 50 mg at bedtime; and eszopiclone, 3 mg at bedtime. Despite these treatments, he experiences increased anxiety and insomnia, and develops depressive symptoms, including depressed mood, poor concentration, general malaise, extreme fatigue, a 15-pound unintentional weight loss, erectile dysfunction, and decreased libido. Mr. A denies having suicidal or homicidal ideations. Additionally, he typically goes to the gym approximately 3 times per week, and has noticed that the amount of weight he is able to lift has decreased, which is distressing. Previously, he had been able to lift 300 pounds, but now he can only lift 200 pounds.
[polldaddy:10891920]
The authors’ observations
Insomnia, anxiety, and depression are common chief complaints in medical settings. However, some psychiatric presentations may have an underlying medical etiology.
DSM-5 requires that medical conditions be ruled out in order for a patient to meet criteria for a psychiatric diagnosis.1 Medical differential diagnoses for patients with psychiatric symptoms can include autoimmune, drug/toxin, metabolic, infectious, neoplastic, neurologic, and nutritional etiologies (Table 12). To rule out the possibility of an underlying medical etiology, general screening guidelines include complete blood count, complete metabolic panel, urinalysis, and urine drug screen with alcohol. Human immunodeficiency virus testing and thyroid hormone testing are also commonly ordered.3 Further laboratory testing and imaging is typically not warranted in the absence of historical or physical findings because they are not advocated as cost-effective, so health care professionals must use their clinical judgment to determine appropriate further evaluation. The onset of anxiety most commonly occurs in late adolescence early and adulthood, but Mr. A experienced his first symptoms of anxiety at age 41.2 Mr. A’s age, lack of psychiatric or family history of mental illness, acute onset of symptoms, and failure of symptoms to abate with standard psychiatric treatments warrant a more extensive workup.
EVALUATION Imaging reveals an important finding
Because Mr. A’s symptoms do not improve with standard psychiatric treatments, his PCP orders standard laboratory bloodwork to investigate a possible medical etiology; however, his results are all within normal range.
After the PCP’s niece is coincidentally diagnosed with a pituitary macroadenoma, the PCP orders brain imaging for Mr. A. Results of an MRI show that Mr. A has a 1.6-cm macroadenoma of the pituitary. He is referred to an endocrinologist, who orders additional laboratory tests that show an elevated 24-hour free urine cortisol level of 73 μg/24 h (normal range: 3.5 to 45 μg/24 h), suggesting that Mr. A’s anxiety may be due to Cushing’s disease or that his anxiety caused falsely elevated urinary cortisol levels. Four weeks later, bloodwork is repeated and shows an abnormal dexamethasone suppression test, and 2 more elevated 24-hour free urine cortisol levels of 76 μg/24 h and 150 μg/24 h. A repeat MRI shows a 1.8-cm, mostly cystic sellar mass, indicating the need for surgical intervention. Although the tumor is large and shows optic nerve compression, Mr. A does not complain of headaches or changes in vision.
Continue to: Two months later...
Two months later, Mr. A undergoes a transsphenoidal tumor resection of the pituitary adenoma, and biopsy results confirm an adrenocorticotropic hormone (ACTH)-secreting pituitary macroadenoma, which is consistent with Cushing’s disease. Following surgery, steroid treatment with dexamethasone is discontinued due to a persistently elevated
[polldaddy:10891923]
The authors’ observations
Chronic excess glucocorticoid production is the underlying pathophysiology of Cushing’s disease, which is most commonly caused by an ACTH-producing adenoma.4,5 When these hormones become dysregulated, the result can be over- or underproduction of cortisol, which can lead to physical and psychiatric manifestations.6
Cushing’s disease most commonly manifests with the physical symptoms of centripetal fat deposition, abdominal striae, facial plethora, muscle atrophy, bone density loss, immunosuppression, and cardiovascular complications.5
Hypercortisolism can precipitate anxiety (12% to 79%), mood disorders (50% to 70%), and (less commonly) psychotic disorders; however, in a clinical setting, if a patient presented with one of these as a chief complaint, they would likely first be treated psychiatrically rather than worked up medically for a rare medical condition.5,7-13
Mr. A’s initial bloodwork was unremarkable, but cortisol levels were not obtained at that time because testing for cortisol levels to rule out an underlying medical condition is not routine in patients with depression and anxiety. In Mr. A’s case, a neuroendocrine workup was only ordered once his PCP’s niece coincidentally was diagnosed with a pituitary adenoma.
Continue to: For Mr. A...
For Mr. A, Cushing’s disease presented as a psychiatric disorder with anxiety and insomnia that were resistant to numerous psychiatric medications during an 8-month period. If Mr. A’s PCP had not ordered a brain MRI, he may have continued to receive ineffective psychiatric treatment for some time. Many of Mr. A’s physical symptoms were consistent with Cushing’s disease and mental illness, including erectile dysfunction, fatigue, and muscle weakness; however, his 15-pound weight loss pointed more toward psychiatric illness and further disguised his underlying medical diagnosis, because sudden weight gain is commonly seen in Cushing’s disease (Table 24,5,7,9).
TREATMENT Persistent psychiatric symptoms, then finally relief
Four weeks after surgery, Mr. A’s psychiatric symptoms gradually intensify, which prompts him to see a psychiatrist. A mental status examination (MSE) shows that he is well-nourished, with normal activity, appropriate behavior, and coherent thought process, but depressed mood and flat affect. He denies suicidal or homicidal ideation. He reports that despite being advised to have realistic expectations, he had high hopes that the surgery would lead to remission of all his symptoms, and expresses disappointment that he does not feel “back to normal.”
Six days later, Mr. A’s wife takes him to the hospital. His MSE shows that he has a tense appearance, fidgety activity, depressed and anxious mood, restricted affect, circumstantial thought process, and paranoid delusions that his wife was plotting against him. He says he still is experiencing insomnia. He also discloses having suicidal ideations with a plan and intent to overdose on medication, as well as homicidal ideations about killing his wife and children. Mr. A provides reasons for why he would want to hurt his family, and does not appear to be bothered by these thoughts.
Mr. A is admitted to the inpatient psychiatric unit and is prescribed quetiapine, 100 mg every night at bedtime. During the next 2 days, quetiapine is titrated to 300 mg every night at bedtime. On hospital Day 3, Mr. A says he is feeling worse than the previous days. He is still having vague suicidal thoughts and feels agitated, guilty, and depressed. To treat these persistent symptoms, quetiapine is further increased to 400 mg every night at bedtime, and he is initiated on bupropion XL, 150 mg, to treat persistent symptoms.
After 1 week of hospitalization, the treatment team meets with Mr. A and his wife, who has been supportive throughout her husband’s hospitalization. During the meeting, they both agree that Mr. A has experienced some improvement because he is no longer having suicidal or homicidal thoughts, but he is still feeling depressed and frustrated by his continued insomnia. Following the meeting, Mr. A’s quetiapine is further increased to 450 mg every night at bedtime to address continued insomnia, and bupropion XL is increased to 300 mg/d to address continued depressive symptoms. During the next few days, his affective symptoms improve; however, his initial insomnia continues, and quetiapine is further increased to 500 mg every night at bedtime.
Continue to: On hospital Day 20...
On hospital Day 20, Mr. A is discharged back to his outpatient psychiatrist and receives quetiapine, 500 mg every night at bedtime, and bupropion XL, 300 mg/d. Although Mr. A’s depression and anxiety continue to be well controlled, his insomnia persists. Sleep hygiene is addressed, and alprazolam, 0.5 mg every night at bedtime, is added to his regimen, which proves to be effective.
OUTCOME A slow remission
After a year of treatment, Mr. A is slowly tapered off of all medications. Two years later, he is in complete remission of all psychiatric symptoms and no longer requires any psychotropic medications.
The authors’ observations
Treatment for hypercortisolism in patients with psychiatric symptoms triggered by glucocorticoid imbalance has typically resulted in a decrease in the severity of their psychiatric symptoms.9,11 A prospective longitudinal study examining 33 patients found that correction of hypercortisolism in patients with Cushing’s syndrome often led to resolution of their psychiatric symptoms, with 87.9% of patients back to baseline within 1 year.14 However, to our knowledge, few reports have described the management of patients whose symptoms are resistant to treatment of hypercortisolism.
In our case, after transsphenoidal resection of an adenoma, Mr. A became suicidal and paranoid, and his anxiety and insomnia also persisted. A possible explanation for the worsening of Mr. A’s symptoms after surgery could be the slow recovery of the hypothalamic-pituitary-adrenal (HPA) axis and therefore a temporary deficiency in glucocorticoid, which caused an increase in catecholamines, leading to an increase in stress.14 This concept of a “slow recovery” is supported by the fact that Mr. A was successfully weaned off all medication after 1 year of treatment, and achieved complete remission of psychiatric symptoms for >2 years. Furthermore, the severity of Mr. A’s symptoms appeared to correlate with his 24-hour urine cortisol and
Future research should evaluate the utility of screening all patients with treatment-resistant anxiety and/or insomnia for hypercortisolism. Even without other clues to endocrinopathies, serum cortisol levels can be used as a screening tool for diagnosing underlying medical causes in patients with anxiety and depression.2 A greater understanding of the relationship between medical and psychiatric manifestations will allow clinicians to better care for patients. Further research is needed to elucidate the quantitative relationship between cortisol levels and anxiety to evaluate severity, guide treatment planning, and follow treatment response for patients with anxiety. It may be useful to determine the threshold between elevated cortisol levels due to anxiety vs elevated cortisol due to an underlying medical pathology such as Cushing’s disease. Additionally, little research has been conducted to compare how psychiatric symptoms respond to pituitary macroadenoma resection alone, pharmaceutical intervention alone, or a combination of these approaches. It would be beneficial to evaluate these treatment strategies to elucidate the most effective method to reduce psychiatric symptoms in patients with hypercortisolism, and perhaps to reduce the incidence of post-resection worsening of psychiatric symptoms.
Continue to: This case was challenging...
This case was challenging because Mr. A did not initially respond to psychiatric intervention, his psychiatric symptoms worsened after transsphenoidal resection of the pituitary adenoma, and his symptoms were alleviated only after psychiatric medications were re-initiated following surgery. This case highlights the importance of considering an underlying medically diagnosable and treatable cause of psychiatric illness, and illustrates the complex ongoing management that may be necessary to help a patient with this condition achieve their baseline. Further, Mr. A’s case shows that the absence of response to standard psychiatric therapies should warrant earlier laboratory and/or imaging evaluation prior to or in conjunction with psychiatric referral. Additionally, testing for cortisol levels is not typically done for a patient with treatment-resistant anxiety, and this case highlights the importance of considering hypercortisolism in such circumstances.
Bottom Line
Consider testing cortisol levels in patients with treatment-resistant anxiety and insomnia, because cortisol plays a role in Cushing’s disease and anxiety. The severity of psychiatric manifestations of Cushing’s disease may correlate with cortisol levels. Treatment should focus on symptomatic management and underlying etiology.
Related Resources
- Roberts LW, Hales RE, Yudofsky SC, ed. The American Psychiatric Association Publishing Textbook of Psychiatry. 7th ed. American Psychiatric Association Publishing; 2019.
- Rotham J. Cushing’s syndrome: a tale of frequent misdiagnosis. National Center for Health Research. 2020. www.center4research.org/cushings-syndrome-frequent-misdiagnosis/
- Middleman D. Psychiatric issues of Cushing’s patients: coping with Cushing’s. Cushing’s Support and Research Foundation. www.csrf.net/coping-with-cushings/psychiatric-issues-of-cushings-patients/
Drug Brand Names
Alprazolam • Xanax
Bupropion • Wellbutrin
Dexamethasone • Decadron
Diazepam • Valium
Eszopiclone • Lunesta
Paroxetine • Paxil
Quetiapine • Seroquel
Zolpidem tartrate • Ambien CR
CASE Anxious and can’t sleep
Mr. A, age 41, presents to his primary care physician (PCP) with anxiety and insomnia. He describes having generalized anxiety with initial and middle insomnia, and says he is sleeping an average of 2 hours per night. He denies any other psychiatric symptoms. Mr. A has no significant psychiatric or medical history.
Mr. A is initiated on zolpidem tartrate, 12.5 mg every night at bedtime, and paroxetine, 20 mg every night at bedtime, for anxiety and insomnia, but these medications result in little to no improvement.
During a 4-month period, he is treated with trials of alprazolam, 0.5 mg every 8 hours as needed; diazepam 5 mg twice a day as needed; diphenhydramine, 50 mg at bedtime; and eszopiclone, 3 mg at bedtime. Despite these treatments, he experiences increased anxiety and insomnia, and develops depressive symptoms, including depressed mood, poor concentration, general malaise, extreme fatigue, a 15-pound unintentional weight loss, erectile dysfunction, and decreased libido. Mr. A denies having suicidal or homicidal ideations. Additionally, he typically goes to the gym approximately 3 times per week, and has noticed that the amount of weight he is able to lift has decreased, which is distressing. Previously, he had been able to lift 300 pounds, but now he can only lift 200 pounds.
[polldaddy:10891920]
The authors’ observations
Insomnia, anxiety, and depression are common chief complaints in medical settings. However, some psychiatric presentations may have an underlying medical etiology.
DSM-5 requires that medical conditions be ruled out in order for a patient to meet criteria for a psychiatric diagnosis.1 Medical differential diagnoses for patients with psychiatric symptoms can include autoimmune, drug/toxin, metabolic, infectious, neoplastic, neurologic, and nutritional etiologies (Table 12). To rule out the possibility of an underlying medical etiology, general screening guidelines include complete blood count, complete metabolic panel, urinalysis, and urine drug screen with alcohol. Human immunodeficiency virus testing and thyroid hormone testing are also commonly ordered.3 Further laboratory testing and imaging is typically not warranted in the absence of historical or physical findings because they are not advocated as cost-effective, so health care professionals must use their clinical judgment to determine appropriate further evaluation. The onset of anxiety most commonly occurs in late adolescence early and adulthood, but Mr. A experienced his first symptoms of anxiety at age 41.2 Mr. A’s age, lack of psychiatric or family history of mental illness, acute onset of symptoms, and failure of symptoms to abate with standard psychiatric treatments warrant a more extensive workup.
EVALUATION Imaging reveals an important finding
Because Mr. A’s symptoms do not improve with standard psychiatric treatments, his PCP orders standard laboratory bloodwork to investigate a possible medical etiology; however, his results are all within normal range.
After the PCP’s niece is coincidentally diagnosed with a pituitary macroadenoma, the PCP orders brain imaging for Mr. A. Results of an MRI show that Mr. A has a 1.6-cm macroadenoma of the pituitary. He is referred to an endocrinologist, who orders additional laboratory tests that show an elevated 24-hour free urine cortisol level of 73 μg/24 h (normal range: 3.5 to 45 μg/24 h), suggesting that Mr. A’s anxiety may be due to Cushing’s disease or that his anxiety caused falsely elevated urinary cortisol levels. Four weeks later, bloodwork is repeated and shows an abnormal dexamethasone suppression test, and 2 more elevated 24-hour free urine cortisol levels of 76 μg/24 h and 150 μg/24 h. A repeat MRI shows a 1.8-cm, mostly cystic sellar mass, indicating the need for surgical intervention. Although the tumor is large and shows optic nerve compression, Mr. A does not complain of headaches or changes in vision.
Continue to: Two months later...
Two months later, Mr. A undergoes a transsphenoidal tumor resection of the pituitary adenoma, and biopsy results confirm an adrenocorticotropic hormone (ACTH)-secreting pituitary macroadenoma, which is consistent with Cushing’s disease. Following surgery, steroid treatment with dexamethasone is discontinued due to a persistently elevated
[polldaddy:10891923]
The authors’ observations
Chronic excess glucocorticoid production is the underlying pathophysiology of Cushing’s disease, which is most commonly caused by an ACTH-producing adenoma.4,5 When these hormones become dysregulated, the result can be over- or underproduction of cortisol, which can lead to physical and psychiatric manifestations.6
Cushing’s disease most commonly manifests with the physical symptoms of centripetal fat deposition, abdominal striae, facial plethora, muscle atrophy, bone density loss, immunosuppression, and cardiovascular complications.5
Hypercortisolism can precipitate anxiety (12% to 79%), mood disorders (50% to 70%), and (less commonly) psychotic disorders; however, in a clinical setting, if a patient presented with one of these as a chief complaint, they would likely first be treated psychiatrically rather than worked up medically for a rare medical condition.5,7-13
Mr. A’s initial bloodwork was unremarkable, but cortisol levels were not obtained at that time because testing for cortisol levels to rule out an underlying medical condition is not routine in patients with depression and anxiety. In Mr. A’s case, a neuroendocrine workup was only ordered once his PCP’s niece coincidentally was diagnosed with a pituitary adenoma.
Continue to: For Mr. A...
For Mr. A, Cushing’s disease presented as a psychiatric disorder with anxiety and insomnia that were resistant to numerous psychiatric medications during an 8-month period. If Mr. A’s PCP had not ordered a brain MRI, he may have continued to receive ineffective psychiatric treatment for some time. Many of Mr. A’s physical symptoms were consistent with Cushing’s disease and mental illness, including erectile dysfunction, fatigue, and muscle weakness; however, his 15-pound weight loss pointed more toward psychiatric illness and further disguised his underlying medical diagnosis, because sudden weight gain is commonly seen in Cushing’s disease (Table 24,5,7,9).
TREATMENT Persistent psychiatric symptoms, then finally relief
Four weeks after surgery, Mr. A’s psychiatric symptoms gradually intensify, which prompts him to see a psychiatrist. A mental status examination (MSE) shows that he is well-nourished, with normal activity, appropriate behavior, and coherent thought process, but depressed mood and flat affect. He denies suicidal or homicidal ideation. He reports that despite being advised to have realistic expectations, he had high hopes that the surgery would lead to remission of all his symptoms, and expresses disappointment that he does not feel “back to normal.”
Six days later, Mr. A’s wife takes him to the hospital. His MSE shows that he has a tense appearance, fidgety activity, depressed and anxious mood, restricted affect, circumstantial thought process, and paranoid delusions that his wife was plotting against him. He says he still is experiencing insomnia. He also discloses having suicidal ideations with a plan and intent to overdose on medication, as well as homicidal ideations about killing his wife and children. Mr. A provides reasons for why he would want to hurt his family, and does not appear to be bothered by these thoughts.
Mr. A is admitted to the inpatient psychiatric unit and is prescribed quetiapine, 100 mg every night at bedtime. During the next 2 days, quetiapine is titrated to 300 mg every night at bedtime. On hospital Day 3, Mr. A says he is feeling worse than the previous days. He is still having vague suicidal thoughts and feels agitated, guilty, and depressed. To treat these persistent symptoms, quetiapine is further increased to 400 mg every night at bedtime, and he is initiated on bupropion XL, 150 mg, to treat persistent symptoms.
After 1 week of hospitalization, the treatment team meets with Mr. A and his wife, who has been supportive throughout her husband’s hospitalization. During the meeting, they both agree that Mr. A has experienced some improvement because he is no longer having suicidal or homicidal thoughts, but he is still feeling depressed and frustrated by his continued insomnia. Following the meeting, Mr. A’s quetiapine is further increased to 450 mg every night at bedtime to address continued insomnia, and bupropion XL is increased to 300 mg/d to address continued depressive symptoms. During the next few days, his affective symptoms improve; however, his initial insomnia continues, and quetiapine is further increased to 500 mg every night at bedtime.
Continue to: On hospital Day 20...
On hospital Day 20, Mr. A is discharged back to his outpatient psychiatrist and receives quetiapine, 500 mg every night at bedtime, and bupropion XL, 300 mg/d. Although Mr. A’s depression and anxiety continue to be well controlled, his insomnia persists. Sleep hygiene is addressed, and alprazolam, 0.5 mg every night at bedtime, is added to his regimen, which proves to be effective.
OUTCOME A slow remission
After a year of treatment, Mr. A is slowly tapered off of all medications. Two years later, he is in complete remission of all psychiatric symptoms and no longer requires any psychotropic medications.
The authors’ observations
Treatment for hypercortisolism in patients with psychiatric symptoms triggered by glucocorticoid imbalance has typically resulted in a decrease in the severity of their psychiatric symptoms.9,11 A prospective longitudinal study examining 33 patients found that correction of hypercortisolism in patients with Cushing’s syndrome often led to resolution of their psychiatric symptoms, with 87.9% of patients back to baseline within 1 year.14 However, to our knowledge, few reports have described the management of patients whose symptoms are resistant to treatment of hypercortisolism.
In our case, after transsphenoidal resection of an adenoma, Mr. A became suicidal and paranoid, and his anxiety and insomnia also persisted. A possible explanation for the worsening of Mr. A’s symptoms after surgery could be the slow recovery of the hypothalamic-pituitary-adrenal (HPA) axis and therefore a temporary deficiency in glucocorticoid, which caused an increase in catecholamines, leading to an increase in stress.14 This concept of a “slow recovery” is supported by the fact that Mr. A was successfully weaned off all medication after 1 year of treatment, and achieved complete remission of psychiatric symptoms for >2 years. Furthermore, the severity of Mr. A’s symptoms appeared to correlate with his 24-hour urine cortisol and
Future research should evaluate the utility of screening all patients with treatment-resistant anxiety and/or insomnia for hypercortisolism. Even without other clues to endocrinopathies, serum cortisol levels can be used as a screening tool for diagnosing underlying medical causes in patients with anxiety and depression.2 A greater understanding of the relationship between medical and psychiatric manifestations will allow clinicians to better care for patients. Further research is needed to elucidate the quantitative relationship between cortisol levels and anxiety to evaluate severity, guide treatment planning, and follow treatment response for patients with anxiety. It may be useful to determine the threshold between elevated cortisol levels due to anxiety vs elevated cortisol due to an underlying medical pathology such as Cushing’s disease. Additionally, little research has been conducted to compare how psychiatric symptoms respond to pituitary macroadenoma resection alone, pharmaceutical intervention alone, or a combination of these approaches. It would be beneficial to evaluate these treatment strategies to elucidate the most effective method to reduce psychiatric symptoms in patients with hypercortisolism, and perhaps to reduce the incidence of post-resection worsening of psychiatric symptoms.
Continue to: This case was challenging...
This case was challenging because Mr. A did not initially respond to psychiatric intervention, his psychiatric symptoms worsened after transsphenoidal resection of the pituitary adenoma, and his symptoms were alleviated only after psychiatric medications were re-initiated following surgery. This case highlights the importance of considering an underlying medically diagnosable and treatable cause of psychiatric illness, and illustrates the complex ongoing management that may be necessary to help a patient with this condition achieve their baseline. Further, Mr. A’s case shows that the absence of response to standard psychiatric therapies should warrant earlier laboratory and/or imaging evaluation prior to or in conjunction with psychiatric referral. Additionally, testing for cortisol levels is not typically done for a patient with treatment-resistant anxiety, and this case highlights the importance of considering hypercortisolism in such circumstances.
Bottom Line
Consider testing cortisol levels in patients with treatment-resistant anxiety and insomnia, because cortisol plays a role in Cushing’s disease and anxiety. The severity of psychiatric manifestations of Cushing’s disease may correlate with cortisol levels. Treatment should focus on symptomatic management and underlying etiology.
Related Resources
- Roberts LW, Hales RE, Yudofsky SC, ed. The American Psychiatric Association Publishing Textbook of Psychiatry. 7th ed. American Psychiatric Association Publishing; 2019.
- Rotham J. Cushing’s syndrome: a tale of frequent misdiagnosis. National Center for Health Research. 2020. www.center4research.org/cushings-syndrome-frequent-misdiagnosis/
- Middleman D. Psychiatric issues of Cushing’s patients: coping with Cushing’s. Cushing’s Support and Research Foundation. www.csrf.net/coping-with-cushings/psychiatric-issues-of-cushings-patients/
Drug Brand Names
Alprazolam • Xanax
Bupropion • Wellbutrin
Dexamethasone • Decadron
Diazepam • Valium
Eszopiclone • Lunesta
Paroxetine • Paxil
Quetiapine • Seroquel
Zolpidem tartrate • Ambien CR
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Sadock BJ, Sadock VA, Ruiz P, et al. Neural sciences. In: Sadock BJ, Sadock VA, Ruiz P, et al. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 11th ed. Wolters Kluwer; 2015.
3. Anfinson TJ, Kathol RG. Screening laboratory evaluation in psychiatric patients: a review. Gen Hosp Psychiatry. 1992;14(4):248-257.
4. Fehm HL, Voigt KH. Pathophysiology of Cushing’s disease. Pathobiol Annu. 1979;9:225-255.
5. Fujii Y, Mizoguchi Y, Masuoka J, et al. Cushing’s syndrome and psychosis: a case report and literature review. Prim Care Companion CNS Disord. 2018;20(5):18.
6. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2011;4(2):739-769.
7. Santos A, Resimini E, Pascual JC, et al. Psychiatric symptoms in patients with Cushing’s syndrome: prevalence diagnosis, and management. Drugs. 2017;77(8):829-842.
8. Arnaldi G, Angeli A, Atkinson B, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593-5602.
9. Sonino N, Fava GA. Psychosomatic aspects of Cushing’s disease. Psychother Psychosom. 1998;67(3):140-146.
10. Loosen PT, Chambliss B, DeBold CR, et al. Psychiatric phenomenology in Cushing’s disease. Pharmacopsychiatry. 1992;25(4):192-198.
11. Kelly WF, Kelly MJ, Faragher B. A prospective study of psychiatric and psychological aspects of Cushing’s syndrome. Clin Endocrinol. 1996;45(6):715-720.
12. Katho RG, Delahunt JW, Hannah L. Transition from bipolar affective disorder to intermittent Cushing’s syndrome: case report. J Clin Psychiatry. 1985;46(5):194-196.
13. Hirsh D, Orr G, Kantarovich V, et al. Cushing’s syndrome presenting as a schizophrenia-like psychotic state. Isr J Psychiatry Relat Sci. 2000;37(1):46-50.
14. Dorn LD, Burgess ES, Friedman TC, et al. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82(3):912-919.
15. Starkman MN, Schteingart DE, Schork MA. Cushing’s syndrome after treatment: changes in cortisol and ACTH levels, and amelioration of the depressive syndrome. Psychiatry Res. 1986;19(3):177-178.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Sadock BJ, Sadock VA, Ruiz P, et al. Neural sciences. In: Sadock BJ, Sadock VA, Ruiz P, et al. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 11th ed. Wolters Kluwer; 2015.
3. Anfinson TJ, Kathol RG. Screening laboratory evaluation in psychiatric patients: a review. Gen Hosp Psychiatry. 1992;14(4):248-257.
4. Fehm HL, Voigt KH. Pathophysiology of Cushing’s disease. Pathobiol Annu. 1979;9:225-255.
5. Fujii Y, Mizoguchi Y, Masuoka J, et al. Cushing’s syndrome and psychosis: a case report and literature review. Prim Care Companion CNS Disord. 2018;20(5):18.
6. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2011;4(2):739-769.
7. Santos A, Resimini E, Pascual JC, et al. Psychiatric symptoms in patients with Cushing’s syndrome: prevalence diagnosis, and management. Drugs. 2017;77(8):829-842.
8. Arnaldi G, Angeli A, Atkinson B, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593-5602.
9. Sonino N, Fava GA. Psychosomatic aspects of Cushing’s disease. Psychother Psychosom. 1998;67(3):140-146.
10. Loosen PT, Chambliss B, DeBold CR, et al. Psychiatric phenomenology in Cushing’s disease. Pharmacopsychiatry. 1992;25(4):192-198.
11. Kelly WF, Kelly MJ, Faragher B. A prospective study of psychiatric and psychological aspects of Cushing’s syndrome. Clin Endocrinol. 1996;45(6):715-720.
12. Katho RG, Delahunt JW, Hannah L. Transition from bipolar affective disorder to intermittent Cushing’s syndrome: case report. J Clin Psychiatry. 1985;46(5):194-196.
13. Hirsh D, Orr G, Kantarovich V, et al. Cushing’s syndrome presenting as a schizophrenia-like psychotic state. Isr J Psychiatry Relat Sci. 2000;37(1):46-50.
14. Dorn LD, Burgess ES, Friedman TC, et al. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82(3):912-919.
15. Starkman MN, Schteingart DE, Schork MA. Cushing’s syndrome after treatment: changes in cortisol and ACTH levels, and amelioration of the depressive syndrome. Psychiatry Res. 1986;19(3):177-178.
Mood stabilizers: Balancing tolerability, serum levels, and dosage
Mr. B, age 32, was diagnosed with bipolar disorder 10 years ago after experiencing a manic episode that resulted in his first psychiatric hospitalization. He was prescribed quetiapine, 400 mg/d, and remained stable for the next several years. Unfortunately, Mr. B developed significant metabolic adverse effects, including diabetes and a 30-pound weight gain, so he was switched from quetiapine to lithium. Mr. B was unable to tolerate the sedation and cognitive effects of lithium, and the dose could not be titrated to within the therapeutic window. As a result, Mr. B experienced a moderate depressive episode. His current clinician would like to initiate lamotrigine at a starting dose of 25 mg/d. Mr. B has not had a manic episode since the index hospitalization, and this is his first depressive episode.
The term “mood stabilizer” has come to refer to medications that treat a depressive and/or manic episode without inducing the other. In conventional terms, it refers to non-antipsychotic medications such as lithium, divalproex, and lamotrigine. Except for lithium, mood stabilizers are also antiepileptic drugs (AEDs). The role of AEDs for treating psychiatric conditions was discovered after they were originally FDA-approved for treating seizures. Following this discovery, the recommended doses and therapeutic ranges for these agents when applied to psychiatric treatment fell into a gray area.
Every patient is different and requires an individualized treatment plan, but this often leaves the clinician wondering, “How high is too high for this mood stabilizer?” or “My patient is responding well, but could a higher dose be even more effective?” In the case of Mr. B, who has trialed 2 medications with poor tolerability, how high can the lamotrigine dose be titrated to achieve a therapeutic response without adverse effects? The literature on this topic does not provide an exact answer, but does shed some light on key considerations for such decisions.
Which mood stabilizers are recommended?
One of the most recently updated guidelines for the treatment of bipolar disorder was released in 2018 by the Canadian Network for Mood and Anxiety Treatments (CANMAT).1 Lithium, divalproex, and lamotrigine were each recommended as a first-line option for treating bipolar disorder. For lithium and divalproex, the CANMAT guidelines recommend serum level monitoring for efficacy and tolerability; however, they do not recommend serum level monitoring for lamotrigine. Lithium and divalproex each have safety and tolerability concerns, particularly when selected for maintenance therapy, whereas lamotrigine is typically much better tolerated.1 Divalproex and lithium can cause weight gain, gastrointestinal adverse effects (nausea, vomiting, diarrhea), and tremor. Additional tolerability concerns with lithium include renal toxicity, electrocardiogram abnormalities, hypothyroidism, cognitive impairment, and dermatologic reactions. Divalproex can produce greater levels of sedation and may impact reproductive function (oligomenorrhea or hyperandrogenism). One of the most common adverse effects of lamotrigine is a non-serious rash; however, slow dose titration is necessary to decrease the risk of a serious, life-threatening rash such as Stevens-Johnson syndrome.
Lithium
Lithium continues to be regarded as a gold-standard therapy for bipolar disorder. The exact serum levels corresponding to efficacy and tolerability vary. The Lithiumeter: Version 2.0 is a schematic that incorporates the various levels recommended by different clinical guidelines.2 The recommended serum levels range from 0.6 to 1.0 mEq/L for mania and 0.4 to 0.8 mEq/L for depression.2 One of the main issues with lithium dosing is balancing a therapeutic level with tolerability and toxicity. Toxicity may begin when lithium levels exceed 1.2 mEq/L, and levels >2.0 mEq/L can be lethal. Signs of acute toxicity include tremor, headache, arrhythmia, nausea, vomiting, diarrhea, polyuria, and polydipsia. Conversely, chronic lithium use may lead to chronic toxicity as patients age and their physical health changes. Signs of chronic toxicity include ataxia, confusion, renal dysfunction, and tremor. There is no “one size fits all” when it comes to lithium dosing. Individualized dosing is necessary to balance efficacy and tolerability.
Divalproex
Divalproex was initially studied for use as an AED, and its therapeutic levels as an AED are not the same as those indicated for bipolar disorder. Generally, patients with bipolar disorder require a divalproex serum level >50 µg/mL. Ranges closer to 100 µg/mL have been found to be most effective for treating acute mania.3 A loading dose of 20 to 30 mg/kg/d can be administered to help achieve mood stabilization. Again, efficacy must be balanced against toxicity. The maximum dose of divalproex is 60 mg/kg/d, which is rarely seen in psychiatric practice. Early studies of divalproex found adverse effects greatest in individuals with plasma levels >100 µg/mL. Reported adverse effects included alopecia, weight gain, tremor, and mental status changes.4
Lamotrigine
Unlike lithium and divalproex, lamotrigine therapeutic drug monitoring is not common. The accepted therapeutic reference range (TRR) for lamotrigine as an AED is 3,000 to 14,000 ng/mL. Unholzer et al5 evaluated the dose and TRR for individuals with bipolar disorder treated with lamotrigine. No statistically significant difference in lamotrigine serum levels was found in responders vs nonresponders.5 Most patients were prescribed ≤200 mg/d; however, some were prescribed higher doses. The maximum dose recommended when lamotrigine is used as an AED is 400 mg/d; however, this study furthered the evidence that lower doses tend to be effective in bipolar disorder.
Continue to: CASE
CASE CONTINUED
It has been 3 months since Mr. B was initiated on lamotrigine, and he has since been titrated to his current, stable dose of 100 mg/d. Mr. B is no longer experiencing the sedation he had with lithium and has the energy to commit to an exercise routine. This has allowed him to lose 15 pounds so far and greatly improve control of his diabetes.
Dosage summary
Most available evidence supports dosing lithium and divalproex to effect, typically seen between 0.6 to 1.0 mEq/L and 50 to 125 µg/mL, respectively. Higher plasma levels tend to correspond to more adverse effects and toxicity. Lamotrigine does not have such a narrow therapeutic window. Lamotrigine for psychiatric treatment yields greatest efficacy at approximately 200 mg/d, but doses can be increased if warranted, which could be the case in Mr. B.
Table 11-5 outlines dosing strategies and therapeutic serum levels for lithium, divalproex, and lamotrigine. Table 22 lists signs and symptoms of lithium toxicity, and Table 31,2 describes strategies for managing adverse effects of lithium and divalproex.
1. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
2. Malhi GS, Gershon S, Outhred T. Lithiumeter: version 2.0. Bipolar Disord. 2016;18(8):631-641.
3. Allen MH, Hirschfeld RM, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry. 2006;163(2):272-275.
4. Turnbull DM, Rawlins MD, Weightman D, et al. Plasma concentrations of sodium valproate: their clinical value. Ann Neurol. 1983;14(1):38-42.
5. Unholzer S, Haen E. Retrospective analysis of therapeutic drug monitoring data for treatment of bipolar disorder with lamotrigine. Pharmacopsychiatry. 2015;48(7):296.
Mr. B, age 32, was diagnosed with bipolar disorder 10 years ago after experiencing a manic episode that resulted in his first psychiatric hospitalization. He was prescribed quetiapine, 400 mg/d, and remained stable for the next several years. Unfortunately, Mr. B developed significant metabolic adverse effects, including diabetes and a 30-pound weight gain, so he was switched from quetiapine to lithium. Mr. B was unable to tolerate the sedation and cognitive effects of lithium, and the dose could not be titrated to within the therapeutic window. As a result, Mr. B experienced a moderate depressive episode. His current clinician would like to initiate lamotrigine at a starting dose of 25 mg/d. Mr. B has not had a manic episode since the index hospitalization, and this is his first depressive episode.
The term “mood stabilizer” has come to refer to medications that treat a depressive and/or manic episode without inducing the other. In conventional terms, it refers to non-antipsychotic medications such as lithium, divalproex, and lamotrigine. Except for lithium, mood stabilizers are also antiepileptic drugs (AEDs). The role of AEDs for treating psychiatric conditions was discovered after they were originally FDA-approved for treating seizures. Following this discovery, the recommended doses and therapeutic ranges for these agents when applied to psychiatric treatment fell into a gray area.
Every patient is different and requires an individualized treatment plan, but this often leaves the clinician wondering, “How high is too high for this mood stabilizer?” or “My patient is responding well, but could a higher dose be even more effective?” In the case of Mr. B, who has trialed 2 medications with poor tolerability, how high can the lamotrigine dose be titrated to achieve a therapeutic response without adverse effects? The literature on this topic does not provide an exact answer, but does shed some light on key considerations for such decisions.
Which mood stabilizers are recommended?
One of the most recently updated guidelines for the treatment of bipolar disorder was released in 2018 by the Canadian Network for Mood and Anxiety Treatments (CANMAT).1 Lithium, divalproex, and lamotrigine were each recommended as a first-line option for treating bipolar disorder. For lithium and divalproex, the CANMAT guidelines recommend serum level monitoring for efficacy and tolerability; however, they do not recommend serum level monitoring for lamotrigine. Lithium and divalproex each have safety and tolerability concerns, particularly when selected for maintenance therapy, whereas lamotrigine is typically much better tolerated.1 Divalproex and lithium can cause weight gain, gastrointestinal adverse effects (nausea, vomiting, diarrhea), and tremor. Additional tolerability concerns with lithium include renal toxicity, electrocardiogram abnormalities, hypothyroidism, cognitive impairment, and dermatologic reactions. Divalproex can produce greater levels of sedation and may impact reproductive function (oligomenorrhea or hyperandrogenism). One of the most common adverse effects of lamotrigine is a non-serious rash; however, slow dose titration is necessary to decrease the risk of a serious, life-threatening rash such as Stevens-Johnson syndrome.
Lithium
Lithium continues to be regarded as a gold-standard therapy for bipolar disorder. The exact serum levels corresponding to efficacy and tolerability vary. The Lithiumeter: Version 2.0 is a schematic that incorporates the various levels recommended by different clinical guidelines.2 The recommended serum levels range from 0.6 to 1.0 mEq/L for mania and 0.4 to 0.8 mEq/L for depression.2 One of the main issues with lithium dosing is balancing a therapeutic level with tolerability and toxicity. Toxicity may begin when lithium levels exceed 1.2 mEq/L, and levels >2.0 mEq/L can be lethal. Signs of acute toxicity include tremor, headache, arrhythmia, nausea, vomiting, diarrhea, polyuria, and polydipsia. Conversely, chronic lithium use may lead to chronic toxicity as patients age and their physical health changes. Signs of chronic toxicity include ataxia, confusion, renal dysfunction, and tremor. There is no “one size fits all” when it comes to lithium dosing. Individualized dosing is necessary to balance efficacy and tolerability.
Divalproex
Divalproex was initially studied for use as an AED, and its therapeutic levels as an AED are not the same as those indicated for bipolar disorder. Generally, patients with bipolar disorder require a divalproex serum level >50 µg/mL. Ranges closer to 100 µg/mL have been found to be most effective for treating acute mania.3 A loading dose of 20 to 30 mg/kg/d can be administered to help achieve mood stabilization. Again, efficacy must be balanced against toxicity. The maximum dose of divalproex is 60 mg/kg/d, which is rarely seen in psychiatric practice. Early studies of divalproex found adverse effects greatest in individuals with plasma levels >100 µg/mL. Reported adverse effects included alopecia, weight gain, tremor, and mental status changes.4
Lamotrigine
Unlike lithium and divalproex, lamotrigine therapeutic drug monitoring is not common. The accepted therapeutic reference range (TRR) for lamotrigine as an AED is 3,000 to 14,000 ng/mL. Unholzer et al5 evaluated the dose and TRR for individuals with bipolar disorder treated with lamotrigine. No statistically significant difference in lamotrigine serum levels was found in responders vs nonresponders.5 Most patients were prescribed ≤200 mg/d; however, some were prescribed higher doses. The maximum dose recommended when lamotrigine is used as an AED is 400 mg/d; however, this study furthered the evidence that lower doses tend to be effective in bipolar disorder.
Continue to: CASE
CASE CONTINUED
It has been 3 months since Mr. B was initiated on lamotrigine, and he has since been titrated to his current, stable dose of 100 mg/d. Mr. B is no longer experiencing the sedation he had with lithium and has the energy to commit to an exercise routine. This has allowed him to lose 15 pounds so far and greatly improve control of his diabetes.
Dosage summary
Most available evidence supports dosing lithium and divalproex to effect, typically seen between 0.6 to 1.0 mEq/L and 50 to 125 µg/mL, respectively. Higher plasma levels tend to correspond to more adverse effects and toxicity. Lamotrigine does not have such a narrow therapeutic window. Lamotrigine for psychiatric treatment yields greatest efficacy at approximately 200 mg/d, but doses can be increased if warranted, which could be the case in Mr. B.
Table 11-5 outlines dosing strategies and therapeutic serum levels for lithium, divalproex, and lamotrigine. Table 22 lists signs and symptoms of lithium toxicity, and Table 31,2 describes strategies for managing adverse effects of lithium and divalproex.
Mr. B, age 32, was diagnosed with bipolar disorder 10 years ago after experiencing a manic episode that resulted in his first psychiatric hospitalization. He was prescribed quetiapine, 400 mg/d, and remained stable for the next several years. Unfortunately, Mr. B developed significant metabolic adverse effects, including diabetes and a 30-pound weight gain, so he was switched from quetiapine to lithium. Mr. B was unable to tolerate the sedation and cognitive effects of lithium, and the dose could not be titrated to within the therapeutic window. As a result, Mr. B experienced a moderate depressive episode. His current clinician would like to initiate lamotrigine at a starting dose of 25 mg/d. Mr. B has not had a manic episode since the index hospitalization, and this is his first depressive episode.
The term “mood stabilizer” has come to refer to medications that treat a depressive and/or manic episode without inducing the other. In conventional terms, it refers to non-antipsychotic medications such as lithium, divalproex, and lamotrigine. Except for lithium, mood stabilizers are also antiepileptic drugs (AEDs). The role of AEDs for treating psychiatric conditions was discovered after they were originally FDA-approved for treating seizures. Following this discovery, the recommended doses and therapeutic ranges for these agents when applied to psychiatric treatment fell into a gray area.
Every patient is different and requires an individualized treatment plan, but this often leaves the clinician wondering, “How high is too high for this mood stabilizer?” or “My patient is responding well, but could a higher dose be even more effective?” In the case of Mr. B, who has trialed 2 medications with poor tolerability, how high can the lamotrigine dose be titrated to achieve a therapeutic response without adverse effects? The literature on this topic does not provide an exact answer, but does shed some light on key considerations for such decisions.
Which mood stabilizers are recommended?
One of the most recently updated guidelines for the treatment of bipolar disorder was released in 2018 by the Canadian Network for Mood and Anxiety Treatments (CANMAT).1 Lithium, divalproex, and lamotrigine were each recommended as a first-line option for treating bipolar disorder. For lithium and divalproex, the CANMAT guidelines recommend serum level monitoring for efficacy and tolerability; however, they do not recommend serum level monitoring for lamotrigine. Lithium and divalproex each have safety and tolerability concerns, particularly when selected for maintenance therapy, whereas lamotrigine is typically much better tolerated.1 Divalproex and lithium can cause weight gain, gastrointestinal adverse effects (nausea, vomiting, diarrhea), and tremor. Additional tolerability concerns with lithium include renal toxicity, electrocardiogram abnormalities, hypothyroidism, cognitive impairment, and dermatologic reactions. Divalproex can produce greater levels of sedation and may impact reproductive function (oligomenorrhea or hyperandrogenism). One of the most common adverse effects of lamotrigine is a non-serious rash; however, slow dose titration is necessary to decrease the risk of a serious, life-threatening rash such as Stevens-Johnson syndrome.
Lithium
Lithium continues to be regarded as a gold-standard therapy for bipolar disorder. The exact serum levels corresponding to efficacy and tolerability vary. The Lithiumeter: Version 2.0 is a schematic that incorporates the various levels recommended by different clinical guidelines.2 The recommended serum levels range from 0.6 to 1.0 mEq/L for mania and 0.4 to 0.8 mEq/L for depression.2 One of the main issues with lithium dosing is balancing a therapeutic level with tolerability and toxicity. Toxicity may begin when lithium levels exceed 1.2 mEq/L, and levels >2.0 mEq/L can be lethal. Signs of acute toxicity include tremor, headache, arrhythmia, nausea, vomiting, diarrhea, polyuria, and polydipsia. Conversely, chronic lithium use may lead to chronic toxicity as patients age and their physical health changes. Signs of chronic toxicity include ataxia, confusion, renal dysfunction, and tremor. There is no “one size fits all” when it comes to lithium dosing. Individualized dosing is necessary to balance efficacy and tolerability.
Divalproex
Divalproex was initially studied for use as an AED, and its therapeutic levels as an AED are not the same as those indicated for bipolar disorder. Generally, patients with bipolar disorder require a divalproex serum level >50 µg/mL. Ranges closer to 100 µg/mL have been found to be most effective for treating acute mania.3 A loading dose of 20 to 30 mg/kg/d can be administered to help achieve mood stabilization. Again, efficacy must be balanced against toxicity. The maximum dose of divalproex is 60 mg/kg/d, which is rarely seen in psychiatric practice. Early studies of divalproex found adverse effects greatest in individuals with plasma levels >100 µg/mL. Reported adverse effects included alopecia, weight gain, tremor, and mental status changes.4
Lamotrigine
Unlike lithium and divalproex, lamotrigine therapeutic drug monitoring is not common. The accepted therapeutic reference range (TRR) for lamotrigine as an AED is 3,000 to 14,000 ng/mL. Unholzer et al5 evaluated the dose and TRR for individuals with bipolar disorder treated with lamotrigine. No statistically significant difference in lamotrigine serum levels was found in responders vs nonresponders.5 Most patients were prescribed ≤200 mg/d; however, some were prescribed higher doses. The maximum dose recommended when lamotrigine is used as an AED is 400 mg/d; however, this study furthered the evidence that lower doses tend to be effective in bipolar disorder.
Continue to: CASE
CASE CONTINUED
It has been 3 months since Mr. B was initiated on lamotrigine, and he has since been titrated to his current, stable dose of 100 mg/d. Mr. B is no longer experiencing the sedation he had with lithium and has the energy to commit to an exercise routine. This has allowed him to lose 15 pounds so far and greatly improve control of his diabetes.
Dosage summary
Most available evidence supports dosing lithium and divalproex to effect, typically seen between 0.6 to 1.0 mEq/L and 50 to 125 µg/mL, respectively. Higher plasma levels tend to correspond to more adverse effects and toxicity. Lamotrigine does not have such a narrow therapeutic window. Lamotrigine for psychiatric treatment yields greatest efficacy at approximately 200 mg/d, but doses can be increased if warranted, which could be the case in Mr. B.
Table 11-5 outlines dosing strategies and therapeutic serum levels for lithium, divalproex, and lamotrigine. Table 22 lists signs and symptoms of lithium toxicity, and Table 31,2 describes strategies for managing adverse effects of lithium and divalproex.
1. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
2. Malhi GS, Gershon S, Outhred T. Lithiumeter: version 2.0. Bipolar Disord. 2016;18(8):631-641.
3. Allen MH, Hirschfeld RM, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry. 2006;163(2):272-275.
4. Turnbull DM, Rawlins MD, Weightman D, et al. Plasma concentrations of sodium valproate: their clinical value. Ann Neurol. 1983;14(1):38-42.
5. Unholzer S, Haen E. Retrospective analysis of therapeutic drug monitoring data for treatment of bipolar disorder with lamotrigine. Pharmacopsychiatry. 2015;48(7):296.
1. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
2. Malhi GS, Gershon S, Outhred T. Lithiumeter: version 2.0. Bipolar Disord. 2016;18(8):631-641.
3. Allen MH, Hirschfeld RM, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry. 2006;163(2):272-275.
4. Turnbull DM, Rawlins MD, Weightman D, et al. Plasma concentrations of sodium valproate: their clinical value. Ann Neurol. 1983;14(1):38-42.
5. Unholzer S, Haen E. Retrospective analysis of therapeutic drug monitoring data for treatment of bipolar disorder with lamotrigine. Pharmacopsychiatry. 2015;48(7):296.
Stuck in a rut with the wrong diagnosis
CASE Aggressive behaviors, psychosis
Ms. N, age 58, has a long history of bipolar disorder with psychotic features. She presents to our emergency department (ED) after an acute fall and frequent violent behaviors at her nursing home, where she had resided since being diagnosed with an unspecified neurocognitive disorder. For several weeks before her fall, she was physically aggressive, throwing objects at nursing home staff, and was unable to have her behavior redirected.
While in the ED, Ms. N rambles and appears to be responding to internal stimuli. Suddenly, she stops responding and begins to stare.
HISTORY Severe, chronic psychosis and hospitalization
Ms. N is well-known at our inpatient psychiatry and electroconvulsive therapy (ECT) services. During the last 10 years, she has had worsening manic, psychotic, and catatonic (both excited and stuporous subtype) episodes. Three years ago, she had experienced a period of severe, chronic psychosis and excited catatonia that required extended inpatient treatment. While hospitalized, Ms. N had marginal responses to clozapine and benzodiazepines, but improved dramatically with ECT. After Ms. N left the hospital, she went to live with her boyfriend. She remained stable on monthly maintenance ECT treatments (bifrontal) before she was lost to follow-up 14 months prior to the current presentation. Ms. N’s family reports that she needed a cardiac clearance before continuing ECT treatment; however, she was hospitalized at another hospital with pneumonia and subsequent complications that interrupted the maintenance ECT treatments.
Approximately 3 months after medical issues requiring hospitalization began, Ms. N received a diagnosis of neurocognitive disorder due to difficulty with activities of daily living and cognitive decline. She was transferred to a nursing home by the outside hospital. When Ms. N’s symptoms of psychosis returned and she required inpatient psychiatric care, she was transferred to a nearby facility that did not have ECT available or knowledge of her history of catatonia resistant to pharmacologic management. Ms. N had a documented history of catatonia that spanned 10 years. During the last 4 years, Ms. N often required ECT treatment. Her current medication regimen prescribed by an outpatient psychiatrist includes clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily, both for bipolar disorder.
EVALUATION An unusual mix of symptoms
In the ED, Ms. N undergoes a CT of the head, which is found to be nonacute. Laboratory results show that her white blood cell count is 14.3 K/µL, which is mildly elevated. Results from a urinalysis and electrocardiogram (ECG) are unremarkable.
After Ms. N punches a radiology technician, she is administered IV lorazepam, 2 mg once, for her agitation. Twenty minutes after receiving IV lorazepam, she is calm and cooperative. However, approximately 4 hours later, Ms. N is yelling, tearful, and expressing delusions of grandeur—she believes she is God.
After she is admitted to the medical floor, Ms. N is seen by our consultation and liaison psychiatry service. She exhibits several signs of catatonia, including grasp reflex, gegenhalten (oppositional paratonia), waxy flexibility, and echolalia. Ms. N also has an episode of urinary incontinence. At some parts of the day, she is alert and oriented to self and location; at other times, she is somnolent and disoriented. The treatment team continues Ms. N’s previous medication regimen of clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily. Unfortunately, at times Ms. N spits out and hides her administered oral medications, which leads to the decision to discontinue clozapine. Once medically cleared, Ms. N is transferred to the psychiatric floor.
[polldaddy:10869949]
Continue to: TREATMENT
TREATMENT Bifrontal ECT initiated
On hospital Day 3 Ms. N is administered a trial of IM lorazepam, titrated up to 6 mg/d (maximum tolerated dose) while the treatment team initiates the legal process to conduct ECT because she is unable to give consent. Once Ms. N begins tolerating oral medications, amantadine, 100 mg twice daily, is added to treat her catatonia. As in prior hospitalizations, Ms. N is unresponsive to pharmacotherapy alone for her catatonic symptoms. On hospital Day 8, forced ECT is granted, which is 5 days after the process of filing paperwork was started. Bifrontal ECT is utilized with the following settings: frequency 70 Hz, pulse width 1.5 ms, 100% energy dose, 504 mC. Ms. N does not experience a significant improvement until she receives 10 ECT treatments as part of a 3-times-per-week acute series protocol. The Bush-Francis Catatonia Rating Scale (BFCRS) and the KANNER scale are used to monitor her progress. Her initial BFCRS score is 17 and initial KANNER scale, part 2 score is 26.
Ms. N spends a total of 61 days in the hospital, which is significantly longer than her previous hospital admissions on our psychiatric unit; these previous admissions were for treatment of both stuporous and excited subtypes of catatonia. This increased length of stay coincides with a significantly longer duration of untreated catatonia. Knowledge of her history of both the stuporous and excited subtypes of catatonia would have allowed for faster diagnosis and treatment.1
The authors’ observations
Originally conceptualized as a separate syndrome by Karl Kahlbaum, catatonia was considered only as a specifier for neuropsychiatric conditions (primarily schizophrenia) as recently as DSM-IV-TR.2 DSM-5 describes catatonia as a marked psychomotor disturbance and acknowledges its connection to schizophrenia by keeping it in the same chapter.3 DSM-5 includes separate diagnoses for catatonia, catatonia due to a general medical condition, and unspecified catatonia (for catatonia without a known underlying disorder).3 A recent meta-analysis found the prevalence of catatonia is higher in patients with medical/neurologic illness, bipolar disorder, and autism than in those with schizophrenia.4
Table 13 highlights the DSM-5 criteria for catatonia. DSM-5 requires 3 of 12 symptoms to be present, although symptoms may fluctuate with time.3 If a clinician is not specifically looking for catatonia, it can be a difficult syndrome to diagnose. Does rigidity indicate catatonia, or excessive dopamine blockade from an antipsychotic? How can seemingly contradictory symptoms be part of the same syndrome? Many clinicians associate catatonia with the stuporous subtype (immobility, posturing, catalepsy), which is more prevalent, but the excited subtype, which may involve severe agitation, autonomic dysfunction, and impaired consciousness, can be lethal.2 The diversity in presentation of catatonia is not unlike the challenging variety of symptoms of heart attacks.
A retrospective study of all adults admitted to a hospital found that only 41% of patients who met criteria for catatonia received this diagnosis.5 Further complicating the diagnosis, delirium and catatonia can co-exist; one study found this was the case in 1 of 3 critically ill patients.6 DSM-5 criteria for catatonia due to another medical condition exclude the diagnosis if delirium is present, but this study and others suggest this needs to be reconsidered.3
Continue to: A standardized evaluation is key
A standardized evaluation is key
Just as a patient who presents with chest pain requires a standardized evaluation, including a pertinent history, laboratory workup, and ECG, psychiatrists may also use standardized diagnostic instruments to aid in the diagnosis of catatonia. One study of hospitalized patients with schizophrenia found that using a standardized diagnostic procedure for catatonia resulted in a 7-fold increase in the diagnosis.7 The BFCRS is the most common standardized instrument for catatonia, likely due to its high inter-rater reliability.8 Other scales include the KANNER scale and Northoff Catatonia Scale, which emphasize different aspects of the disease or for certain clinical populations (eg, the KANNER scale adjusts for patients who are nonverbal at baseline). One study suggested that BFCRS has lower reliability for less-severe illness.9 These differences emphasize that psychiatry does not have a thorough understanding of the intricacies of catatonia. However, using validated screening tools can lead to more consistent diagnoses and continue important research on this often-misunderstood illness.
Dangers of untreated catatonia
Rapid treatment of catatonia is necessary to prevent mortality. A study of patients in Kentucky’s state psychiatric hospitals found that untreated catatonia with resultant death from pulmonary embolism was the leading cause of preventable death.10 A 17-year retrospective study of patients with schizophrenia admitted to 1 hospital found that those with catatonia were >4 times as likely to die during hospitalization than those without catatonia.11 The significant morbidity and mortality from untreated catatonia are typically attributed to the consequences of poorly controlled movements, immobility, autonomic instability, and poor/no oral intake. Reduced oral intake can result in malnutrition, dehydration, arrhythmias, and increased risk of infections. Furthermore, chronic catatonic episodes are more difficult to treat.12 In addition to the aggressive management of neuropsychiatric symptoms, it is vital to evaluate relevant medical etiologies that may be contributing to the syndrome (Table 213). Tracking vital signs and laboratory values, such as creatine kinase, electrolytes, and complete blood count, is required to ensure the medical condition does not become life-threatening.
Treatment options
Studies and expert opinion suggest that benzodiazepines (specifically lorazepam, because it is the most studied agent) are the first-line treatment for catatonia. A lorazepam challenge test—providing 1 or 2 mg of IV lorazepam—is considered diagnostic and therapeutic given the high rate of response within 10 minutes.14 Patients with limited response to lorazepam or who are medically compromised should undergo ECT. Electroconvulsive therapy is considered the gold-standard treatment for catatonia; estimated response rates range from 59% to 100%, even in patients who fail to respond to pharmacotherapy.15 Although highly effective, ECT is often hindered by the time required to initiate treatment, stigma, lack of access, and other logistical challenges.
Table 314-18 highlights the advantages and disadvantages of treatment options for catatonia. Some researchers have suggested a zolpidem challenge test could augment lorazepam because some patients respond only to zolpidem.14 The efficacy of these medications along with some evidence of anti-N-methyl-
Ms. N was ultimately diagnosed with bipolar disorder, current episode mixed, with psychotic and catatonic features. Ms. N had symptoms of mania including grandiosity, periods of lack of sleep, delusions as well as depressive symptoms of tearfulness and low mood. The treatment team had considered that Ms. N had delirious mania because she had fluctuating sensorium, which included varying degrees of orientation and ability to answer questioning. However, the literature supporting the differentiation between delirious mania and excited catatonia is unclear, and both conditions may respond to ECT.18 A diagnosis of catatonia allowed the team to use rating scales to track Ms. N’s progress by monitoring for specific signs, such as grasp reflex and waxy flexibility.
Continue to: OUTCOME
OUTCOME Return to baseline
Before discharge, Ms. N’s BFCRS score decreases from the initial score of 17 to 0, and her KANNER scale score decreases from the initial score of 26 to 4, which correlates with vast improvement in clinical presentation. Once Ms. N completes the acute ECT treatment, she returns to her baseline level of functioning, and is discharged to live with her boyfriend. She is advised to continue weekly ECT for the first several months to ensure clinical stability. This regimen is later transitioned to biweekly and then monthly. Electroconvulsive therapy protocols from previous research were utilized in Ms. N’s case, but ultimately the lowest number of ECT treatments needed to maintain stability is determined clinically over many years.19 Ms. N is discharged on aripiprazole, 15 mg/d; bupropion ER, 300 mg/d (added after depressive symptoms emerge while catatonia symptoms improve midway through her lengthy hospitalization); and memantine, 10 mg/d. Ideally, clozapine would have been continued; however, due to her history of nonadherence and frequent restarting of the medication at a low dose, clozapine was discontinued and aripiprazole initiated.
More than 1 year later, Ms. N remains stable and continues to receive monthly ECT maintenance treatments.
Bottom Line
Catatonia should always be considered in a patient who presents with acute neuropsychiatric symptoms. Rapid diagnosis with standardized screening instruments and aggressive treatment are vital to prevent morbidity and mortality.
Related Resource
- Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing textbook of psychosomatic medicine and consultation-liaison psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Baclofen • Ozobax
Bupropion ER • Wellbutrin XL
Clonazepam • Klonopin
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Metoclopramide • Reglan
Memantine • Namenda
Topiramate • Topamax
Zolpidem • Ambien
1. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums. 2000;5(7):26-33.
2. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391‐398.
3. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013. 119-121.
4. Solmi M, Pigato GG, Roiter B, et al. Prevalence of catatonia and its moderators in clinical samples: results from a meta-analysis and meta-regression analysis. Schizophrenia Bulletin. 2017;44(5):1133-1150.
5. Llesuy JR, Medina M, Jacobson KC, et al. Catatonia under-diagnosis in the general hospital. J Neuropsychiatry Clin Neurosci. 2018;30(2):145-151.
6. Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients. Crit Care Med. 2017;45(11):1837-1844.
7. Heijden FVD, Tuinier S, Arts N, et al. Catatonia: disappeared or under-diagnosed? Psychopathology. 2005;38(1):3-8.
8. Sarkar S, Sakey S, Mathan K, et al. Assessing catatonia using four different instruments: inter-rater reliability and prevalence in inpatient clinical population. Asian J Psychiatr. 2016;23:27-31.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
10. Puentes R, Brenzel A, Leon JD. Pulmonary embolism during stuporous episodes of catatonia was found to be the most frequent cause of preventable death according to a state mortality review: 6 deaths in 15 years. Clin Schizophr Relat Psychoses. 2017; doi:10.3371/csrp.rpab.071317
11. Funayama M, Takata T, Koreki A, et al. Catatonic stupor in schizophrenic disorders and subsequent medical complications and mortality. Psychosomatic Medicine. 2018:80(4):370-376.
12. Perugi G, Medda P, Toni C, et al. The role of electroconvulsive therapy (ECT) in bipolar disorder: effectiveness in 522 patients with bipolar depression, mixed-state, mania and catatonic features. Curr Neuropharmacol. 2017;15(3):359-371.
13. Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
14. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
15. Pelzer A, Heijden FVD, Boer ED. Systematic review of catatonia treatment. Neuropsychiatr Dis Treat. 2018;14:317-326.
16. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry and Clin Neurosci. 2007;19(4):406-412.
17. Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatr Scand Suppl. 2013;(441):1-47.
18. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge University Press; 2006.
19. Petrides G, Tobias KG, Kellner CH, et al. Continuation and maintenance electroconvulsive therapy for mood disorders: review of the literature. Neuropsychobiology. 2011;64(3):129-140.
CASE Aggressive behaviors, psychosis
Ms. N, age 58, has a long history of bipolar disorder with psychotic features. She presents to our emergency department (ED) after an acute fall and frequent violent behaviors at her nursing home, where she had resided since being diagnosed with an unspecified neurocognitive disorder. For several weeks before her fall, she was physically aggressive, throwing objects at nursing home staff, and was unable to have her behavior redirected.
While in the ED, Ms. N rambles and appears to be responding to internal stimuli. Suddenly, she stops responding and begins to stare.
HISTORY Severe, chronic psychosis and hospitalization
Ms. N is well-known at our inpatient psychiatry and electroconvulsive therapy (ECT) services. During the last 10 years, she has had worsening manic, psychotic, and catatonic (both excited and stuporous subtype) episodes. Three years ago, she had experienced a period of severe, chronic psychosis and excited catatonia that required extended inpatient treatment. While hospitalized, Ms. N had marginal responses to clozapine and benzodiazepines, but improved dramatically with ECT. After Ms. N left the hospital, she went to live with her boyfriend. She remained stable on monthly maintenance ECT treatments (bifrontal) before she was lost to follow-up 14 months prior to the current presentation. Ms. N’s family reports that she needed a cardiac clearance before continuing ECT treatment; however, she was hospitalized at another hospital with pneumonia and subsequent complications that interrupted the maintenance ECT treatments.
Approximately 3 months after medical issues requiring hospitalization began, Ms. N received a diagnosis of neurocognitive disorder due to difficulty with activities of daily living and cognitive decline. She was transferred to a nursing home by the outside hospital. When Ms. N’s symptoms of psychosis returned and she required inpatient psychiatric care, she was transferred to a nearby facility that did not have ECT available or knowledge of her history of catatonia resistant to pharmacologic management. Ms. N had a documented history of catatonia that spanned 10 years. During the last 4 years, Ms. N often required ECT treatment. Her current medication regimen prescribed by an outpatient psychiatrist includes clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily, both for bipolar disorder.
EVALUATION An unusual mix of symptoms
In the ED, Ms. N undergoes a CT of the head, which is found to be nonacute. Laboratory results show that her white blood cell count is 14.3 K/µL, which is mildly elevated. Results from a urinalysis and electrocardiogram (ECG) are unremarkable.
After Ms. N punches a radiology technician, she is administered IV lorazepam, 2 mg once, for her agitation. Twenty minutes after receiving IV lorazepam, she is calm and cooperative. However, approximately 4 hours later, Ms. N is yelling, tearful, and expressing delusions of grandeur—she believes she is God.
After she is admitted to the medical floor, Ms. N is seen by our consultation and liaison psychiatry service. She exhibits several signs of catatonia, including grasp reflex, gegenhalten (oppositional paratonia), waxy flexibility, and echolalia. Ms. N also has an episode of urinary incontinence. At some parts of the day, she is alert and oriented to self and location; at other times, she is somnolent and disoriented. The treatment team continues Ms. N’s previous medication regimen of clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily. Unfortunately, at times Ms. N spits out and hides her administered oral medications, which leads to the decision to discontinue clozapine. Once medically cleared, Ms. N is transferred to the psychiatric floor.
[polldaddy:10869949]
Continue to: TREATMENT
TREATMENT Bifrontal ECT initiated
On hospital Day 3 Ms. N is administered a trial of IM lorazepam, titrated up to 6 mg/d (maximum tolerated dose) while the treatment team initiates the legal process to conduct ECT because she is unable to give consent. Once Ms. N begins tolerating oral medications, amantadine, 100 mg twice daily, is added to treat her catatonia. As in prior hospitalizations, Ms. N is unresponsive to pharmacotherapy alone for her catatonic symptoms. On hospital Day 8, forced ECT is granted, which is 5 days after the process of filing paperwork was started. Bifrontal ECT is utilized with the following settings: frequency 70 Hz, pulse width 1.5 ms, 100% energy dose, 504 mC. Ms. N does not experience a significant improvement until she receives 10 ECT treatments as part of a 3-times-per-week acute series protocol. The Bush-Francis Catatonia Rating Scale (BFCRS) and the KANNER scale are used to monitor her progress. Her initial BFCRS score is 17 and initial KANNER scale, part 2 score is 26.
Ms. N spends a total of 61 days in the hospital, which is significantly longer than her previous hospital admissions on our psychiatric unit; these previous admissions were for treatment of both stuporous and excited subtypes of catatonia. This increased length of stay coincides with a significantly longer duration of untreated catatonia. Knowledge of her history of both the stuporous and excited subtypes of catatonia would have allowed for faster diagnosis and treatment.1
The authors’ observations
Originally conceptualized as a separate syndrome by Karl Kahlbaum, catatonia was considered only as a specifier for neuropsychiatric conditions (primarily schizophrenia) as recently as DSM-IV-TR.2 DSM-5 describes catatonia as a marked psychomotor disturbance and acknowledges its connection to schizophrenia by keeping it in the same chapter.3 DSM-5 includes separate diagnoses for catatonia, catatonia due to a general medical condition, and unspecified catatonia (for catatonia without a known underlying disorder).3 A recent meta-analysis found the prevalence of catatonia is higher in patients with medical/neurologic illness, bipolar disorder, and autism than in those with schizophrenia.4
Table 13 highlights the DSM-5 criteria for catatonia. DSM-5 requires 3 of 12 symptoms to be present, although symptoms may fluctuate with time.3 If a clinician is not specifically looking for catatonia, it can be a difficult syndrome to diagnose. Does rigidity indicate catatonia, or excessive dopamine blockade from an antipsychotic? How can seemingly contradictory symptoms be part of the same syndrome? Many clinicians associate catatonia with the stuporous subtype (immobility, posturing, catalepsy), which is more prevalent, but the excited subtype, which may involve severe agitation, autonomic dysfunction, and impaired consciousness, can be lethal.2 The diversity in presentation of catatonia is not unlike the challenging variety of symptoms of heart attacks.
A retrospective study of all adults admitted to a hospital found that only 41% of patients who met criteria for catatonia received this diagnosis.5 Further complicating the diagnosis, delirium and catatonia can co-exist; one study found this was the case in 1 of 3 critically ill patients.6 DSM-5 criteria for catatonia due to another medical condition exclude the diagnosis if delirium is present, but this study and others suggest this needs to be reconsidered.3
Continue to: A standardized evaluation is key
A standardized evaluation is key
Just as a patient who presents with chest pain requires a standardized evaluation, including a pertinent history, laboratory workup, and ECG, psychiatrists may also use standardized diagnostic instruments to aid in the diagnosis of catatonia. One study of hospitalized patients with schizophrenia found that using a standardized diagnostic procedure for catatonia resulted in a 7-fold increase in the diagnosis.7 The BFCRS is the most common standardized instrument for catatonia, likely due to its high inter-rater reliability.8 Other scales include the KANNER scale and Northoff Catatonia Scale, which emphasize different aspects of the disease or for certain clinical populations (eg, the KANNER scale adjusts for patients who are nonverbal at baseline). One study suggested that BFCRS has lower reliability for less-severe illness.9 These differences emphasize that psychiatry does not have a thorough understanding of the intricacies of catatonia. However, using validated screening tools can lead to more consistent diagnoses and continue important research on this often-misunderstood illness.
Dangers of untreated catatonia
Rapid treatment of catatonia is necessary to prevent mortality. A study of patients in Kentucky’s state psychiatric hospitals found that untreated catatonia with resultant death from pulmonary embolism was the leading cause of preventable death.10 A 17-year retrospective study of patients with schizophrenia admitted to 1 hospital found that those with catatonia were >4 times as likely to die during hospitalization than those without catatonia.11 The significant morbidity and mortality from untreated catatonia are typically attributed to the consequences of poorly controlled movements, immobility, autonomic instability, and poor/no oral intake. Reduced oral intake can result in malnutrition, dehydration, arrhythmias, and increased risk of infections. Furthermore, chronic catatonic episodes are more difficult to treat.12 In addition to the aggressive management of neuropsychiatric symptoms, it is vital to evaluate relevant medical etiologies that may be contributing to the syndrome (Table 213). Tracking vital signs and laboratory values, such as creatine kinase, electrolytes, and complete blood count, is required to ensure the medical condition does not become life-threatening.
Treatment options
Studies and expert opinion suggest that benzodiazepines (specifically lorazepam, because it is the most studied agent) are the first-line treatment for catatonia. A lorazepam challenge test—providing 1 or 2 mg of IV lorazepam—is considered diagnostic and therapeutic given the high rate of response within 10 minutes.14 Patients with limited response to lorazepam or who are medically compromised should undergo ECT. Electroconvulsive therapy is considered the gold-standard treatment for catatonia; estimated response rates range from 59% to 100%, even in patients who fail to respond to pharmacotherapy.15 Although highly effective, ECT is often hindered by the time required to initiate treatment, stigma, lack of access, and other logistical challenges.
Table 314-18 highlights the advantages and disadvantages of treatment options for catatonia. Some researchers have suggested a zolpidem challenge test could augment lorazepam because some patients respond only to zolpidem.14 The efficacy of these medications along with some evidence of anti-N-methyl-
Ms. N was ultimately diagnosed with bipolar disorder, current episode mixed, with psychotic and catatonic features. Ms. N had symptoms of mania including grandiosity, periods of lack of sleep, delusions as well as depressive symptoms of tearfulness and low mood. The treatment team had considered that Ms. N had delirious mania because she had fluctuating sensorium, which included varying degrees of orientation and ability to answer questioning. However, the literature supporting the differentiation between delirious mania and excited catatonia is unclear, and both conditions may respond to ECT.18 A diagnosis of catatonia allowed the team to use rating scales to track Ms. N’s progress by monitoring for specific signs, such as grasp reflex and waxy flexibility.
Continue to: OUTCOME
OUTCOME Return to baseline
Before discharge, Ms. N’s BFCRS score decreases from the initial score of 17 to 0, and her KANNER scale score decreases from the initial score of 26 to 4, which correlates with vast improvement in clinical presentation. Once Ms. N completes the acute ECT treatment, she returns to her baseline level of functioning, and is discharged to live with her boyfriend. She is advised to continue weekly ECT for the first several months to ensure clinical stability. This regimen is later transitioned to biweekly and then monthly. Electroconvulsive therapy protocols from previous research were utilized in Ms. N’s case, but ultimately the lowest number of ECT treatments needed to maintain stability is determined clinically over many years.19 Ms. N is discharged on aripiprazole, 15 mg/d; bupropion ER, 300 mg/d (added after depressive symptoms emerge while catatonia symptoms improve midway through her lengthy hospitalization); and memantine, 10 mg/d. Ideally, clozapine would have been continued; however, due to her history of nonadherence and frequent restarting of the medication at a low dose, clozapine was discontinued and aripiprazole initiated.
More than 1 year later, Ms. N remains stable and continues to receive monthly ECT maintenance treatments.
Bottom Line
Catatonia should always be considered in a patient who presents with acute neuropsychiatric symptoms. Rapid diagnosis with standardized screening instruments and aggressive treatment are vital to prevent morbidity and mortality.
Related Resource
- Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing textbook of psychosomatic medicine and consultation-liaison psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Baclofen • Ozobax
Bupropion ER • Wellbutrin XL
Clonazepam • Klonopin
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Metoclopramide • Reglan
Memantine • Namenda
Topiramate • Topamax
Zolpidem • Ambien
CASE Aggressive behaviors, psychosis
Ms. N, age 58, has a long history of bipolar disorder with psychotic features. She presents to our emergency department (ED) after an acute fall and frequent violent behaviors at her nursing home, where she had resided since being diagnosed with an unspecified neurocognitive disorder. For several weeks before her fall, she was physically aggressive, throwing objects at nursing home staff, and was unable to have her behavior redirected.
While in the ED, Ms. N rambles and appears to be responding to internal stimuli. Suddenly, she stops responding and begins to stare.
HISTORY Severe, chronic psychosis and hospitalization
Ms. N is well-known at our inpatient psychiatry and electroconvulsive therapy (ECT) services. During the last 10 years, she has had worsening manic, psychotic, and catatonic (both excited and stuporous subtype) episodes. Three years ago, she had experienced a period of severe, chronic psychosis and excited catatonia that required extended inpatient treatment. While hospitalized, Ms. N had marginal responses to clozapine and benzodiazepines, but improved dramatically with ECT. After Ms. N left the hospital, she went to live with her boyfriend. She remained stable on monthly maintenance ECT treatments (bifrontal) before she was lost to follow-up 14 months prior to the current presentation. Ms. N’s family reports that she needed a cardiac clearance before continuing ECT treatment; however, she was hospitalized at another hospital with pneumonia and subsequent complications that interrupted the maintenance ECT treatments.
Approximately 3 months after medical issues requiring hospitalization began, Ms. N received a diagnosis of neurocognitive disorder due to difficulty with activities of daily living and cognitive decline. She was transferred to a nursing home by the outside hospital. When Ms. N’s symptoms of psychosis returned and she required inpatient psychiatric care, she was transferred to a nearby facility that did not have ECT available or knowledge of her history of catatonia resistant to pharmacologic management. Ms. N had a documented history of catatonia that spanned 10 years. During the last 4 years, Ms. N often required ECT treatment. Her current medication regimen prescribed by an outpatient psychiatrist includes clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily, both for bipolar disorder.
EVALUATION An unusual mix of symptoms
In the ED, Ms. N undergoes a CT of the head, which is found to be nonacute. Laboratory results show that her white blood cell count is 14.3 K/µL, which is mildly elevated. Results from a urinalysis and electrocardiogram (ECG) are unremarkable.
After Ms. N punches a radiology technician, she is administered IV lorazepam, 2 mg once, for her agitation. Twenty minutes after receiving IV lorazepam, she is calm and cooperative. However, approximately 4 hours later, Ms. N is yelling, tearful, and expressing delusions of grandeur—she believes she is God.
After she is admitted to the medical floor, Ms. N is seen by our consultation and liaison psychiatry service. She exhibits several signs of catatonia, including grasp reflex, gegenhalten (oppositional paratonia), waxy flexibility, and echolalia. Ms. N also has an episode of urinary incontinence. At some parts of the day, she is alert and oriented to self and location; at other times, she is somnolent and disoriented. The treatment team continues Ms. N’s previous medication regimen of clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily. Unfortunately, at times Ms. N spits out and hides her administered oral medications, which leads to the decision to discontinue clozapine. Once medically cleared, Ms. N is transferred to the psychiatric floor.
[polldaddy:10869949]
Continue to: TREATMENT
TREATMENT Bifrontal ECT initiated
On hospital Day 3 Ms. N is administered a trial of IM lorazepam, titrated up to 6 mg/d (maximum tolerated dose) while the treatment team initiates the legal process to conduct ECT because she is unable to give consent. Once Ms. N begins tolerating oral medications, amantadine, 100 mg twice daily, is added to treat her catatonia. As in prior hospitalizations, Ms. N is unresponsive to pharmacotherapy alone for her catatonic symptoms. On hospital Day 8, forced ECT is granted, which is 5 days after the process of filing paperwork was started. Bifrontal ECT is utilized with the following settings: frequency 70 Hz, pulse width 1.5 ms, 100% energy dose, 504 mC. Ms. N does not experience a significant improvement until she receives 10 ECT treatments as part of a 3-times-per-week acute series protocol. The Bush-Francis Catatonia Rating Scale (BFCRS) and the KANNER scale are used to monitor her progress. Her initial BFCRS score is 17 and initial KANNER scale, part 2 score is 26.
Ms. N spends a total of 61 days in the hospital, which is significantly longer than her previous hospital admissions on our psychiatric unit; these previous admissions were for treatment of both stuporous and excited subtypes of catatonia. This increased length of stay coincides with a significantly longer duration of untreated catatonia. Knowledge of her history of both the stuporous and excited subtypes of catatonia would have allowed for faster diagnosis and treatment.1
The authors’ observations
Originally conceptualized as a separate syndrome by Karl Kahlbaum, catatonia was considered only as a specifier for neuropsychiatric conditions (primarily schizophrenia) as recently as DSM-IV-TR.2 DSM-5 describes catatonia as a marked psychomotor disturbance and acknowledges its connection to schizophrenia by keeping it in the same chapter.3 DSM-5 includes separate diagnoses for catatonia, catatonia due to a general medical condition, and unspecified catatonia (for catatonia without a known underlying disorder).3 A recent meta-analysis found the prevalence of catatonia is higher in patients with medical/neurologic illness, bipolar disorder, and autism than in those with schizophrenia.4
Table 13 highlights the DSM-5 criteria for catatonia. DSM-5 requires 3 of 12 symptoms to be present, although symptoms may fluctuate with time.3 If a clinician is not specifically looking for catatonia, it can be a difficult syndrome to diagnose. Does rigidity indicate catatonia, or excessive dopamine blockade from an antipsychotic? How can seemingly contradictory symptoms be part of the same syndrome? Many clinicians associate catatonia with the stuporous subtype (immobility, posturing, catalepsy), which is more prevalent, but the excited subtype, which may involve severe agitation, autonomic dysfunction, and impaired consciousness, can be lethal.2 The diversity in presentation of catatonia is not unlike the challenging variety of symptoms of heart attacks.
A retrospective study of all adults admitted to a hospital found that only 41% of patients who met criteria for catatonia received this diagnosis.5 Further complicating the diagnosis, delirium and catatonia can co-exist; one study found this was the case in 1 of 3 critically ill patients.6 DSM-5 criteria for catatonia due to another medical condition exclude the diagnosis if delirium is present, but this study and others suggest this needs to be reconsidered.3
Continue to: A standardized evaluation is key
A standardized evaluation is key
Just as a patient who presents with chest pain requires a standardized evaluation, including a pertinent history, laboratory workup, and ECG, psychiatrists may also use standardized diagnostic instruments to aid in the diagnosis of catatonia. One study of hospitalized patients with schizophrenia found that using a standardized diagnostic procedure for catatonia resulted in a 7-fold increase in the diagnosis.7 The BFCRS is the most common standardized instrument for catatonia, likely due to its high inter-rater reliability.8 Other scales include the KANNER scale and Northoff Catatonia Scale, which emphasize different aspects of the disease or for certain clinical populations (eg, the KANNER scale adjusts for patients who are nonverbal at baseline). One study suggested that BFCRS has lower reliability for less-severe illness.9 These differences emphasize that psychiatry does not have a thorough understanding of the intricacies of catatonia. However, using validated screening tools can lead to more consistent diagnoses and continue important research on this often-misunderstood illness.
Dangers of untreated catatonia
Rapid treatment of catatonia is necessary to prevent mortality. A study of patients in Kentucky’s state psychiatric hospitals found that untreated catatonia with resultant death from pulmonary embolism was the leading cause of preventable death.10 A 17-year retrospective study of patients with schizophrenia admitted to 1 hospital found that those with catatonia were >4 times as likely to die during hospitalization than those without catatonia.11 The significant morbidity and mortality from untreated catatonia are typically attributed to the consequences of poorly controlled movements, immobility, autonomic instability, and poor/no oral intake. Reduced oral intake can result in malnutrition, dehydration, arrhythmias, and increased risk of infections. Furthermore, chronic catatonic episodes are more difficult to treat.12 In addition to the aggressive management of neuropsychiatric symptoms, it is vital to evaluate relevant medical etiologies that may be contributing to the syndrome (Table 213). Tracking vital signs and laboratory values, such as creatine kinase, electrolytes, and complete blood count, is required to ensure the medical condition does not become life-threatening.
Treatment options
Studies and expert opinion suggest that benzodiazepines (specifically lorazepam, because it is the most studied agent) are the first-line treatment for catatonia. A lorazepam challenge test—providing 1 or 2 mg of IV lorazepam—is considered diagnostic and therapeutic given the high rate of response within 10 minutes.14 Patients with limited response to lorazepam or who are medically compromised should undergo ECT. Electroconvulsive therapy is considered the gold-standard treatment for catatonia; estimated response rates range from 59% to 100%, even in patients who fail to respond to pharmacotherapy.15 Although highly effective, ECT is often hindered by the time required to initiate treatment, stigma, lack of access, and other logistical challenges.
Table 314-18 highlights the advantages and disadvantages of treatment options for catatonia. Some researchers have suggested a zolpidem challenge test could augment lorazepam because some patients respond only to zolpidem.14 The efficacy of these medications along with some evidence of anti-N-methyl-
Ms. N was ultimately diagnosed with bipolar disorder, current episode mixed, with psychotic and catatonic features. Ms. N had symptoms of mania including grandiosity, periods of lack of sleep, delusions as well as depressive symptoms of tearfulness and low mood. The treatment team had considered that Ms. N had delirious mania because she had fluctuating sensorium, which included varying degrees of orientation and ability to answer questioning. However, the literature supporting the differentiation between delirious mania and excited catatonia is unclear, and both conditions may respond to ECT.18 A diagnosis of catatonia allowed the team to use rating scales to track Ms. N’s progress by monitoring for specific signs, such as grasp reflex and waxy flexibility.
Continue to: OUTCOME
OUTCOME Return to baseline
Before discharge, Ms. N’s BFCRS score decreases from the initial score of 17 to 0, and her KANNER scale score decreases from the initial score of 26 to 4, which correlates with vast improvement in clinical presentation. Once Ms. N completes the acute ECT treatment, she returns to her baseline level of functioning, and is discharged to live with her boyfriend. She is advised to continue weekly ECT for the first several months to ensure clinical stability. This regimen is later transitioned to biweekly and then monthly. Electroconvulsive therapy protocols from previous research were utilized in Ms. N’s case, but ultimately the lowest number of ECT treatments needed to maintain stability is determined clinically over many years.19 Ms. N is discharged on aripiprazole, 15 mg/d; bupropion ER, 300 mg/d (added after depressive symptoms emerge while catatonia symptoms improve midway through her lengthy hospitalization); and memantine, 10 mg/d. Ideally, clozapine would have been continued; however, due to her history of nonadherence and frequent restarting of the medication at a low dose, clozapine was discontinued and aripiprazole initiated.
More than 1 year later, Ms. N remains stable and continues to receive monthly ECT maintenance treatments.
Bottom Line
Catatonia should always be considered in a patient who presents with acute neuropsychiatric symptoms. Rapid diagnosis with standardized screening instruments and aggressive treatment are vital to prevent morbidity and mortality.
Related Resource
- Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing textbook of psychosomatic medicine and consultation-liaison psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Baclofen • Ozobax
Bupropion ER • Wellbutrin XL
Clonazepam • Klonopin
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Metoclopramide • Reglan
Memantine • Namenda
Topiramate • Topamax
Zolpidem • Ambien
1. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums. 2000;5(7):26-33.
2. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391‐398.
3. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013. 119-121.
4. Solmi M, Pigato GG, Roiter B, et al. Prevalence of catatonia and its moderators in clinical samples: results from a meta-analysis and meta-regression analysis. Schizophrenia Bulletin. 2017;44(5):1133-1150.
5. Llesuy JR, Medina M, Jacobson KC, et al. Catatonia under-diagnosis in the general hospital. J Neuropsychiatry Clin Neurosci. 2018;30(2):145-151.
6. Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients. Crit Care Med. 2017;45(11):1837-1844.
7. Heijden FVD, Tuinier S, Arts N, et al. Catatonia: disappeared or under-diagnosed? Psychopathology. 2005;38(1):3-8.
8. Sarkar S, Sakey S, Mathan K, et al. Assessing catatonia using four different instruments: inter-rater reliability and prevalence in inpatient clinical population. Asian J Psychiatr. 2016;23:27-31.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
10. Puentes R, Brenzel A, Leon JD. Pulmonary embolism during stuporous episodes of catatonia was found to be the most frequent cause of preventable death according to a state mortality review: 6 deaths in 15 years. Clin Schizophr Relat Psychoses. 2017; doi:10.3371/csrp.rpab.071317
11. Funayama M, Takata T, Koreki A, et al. Catatonic stupor in schizophrenic disorders and subsequent medical complications and mortality. Psychosomatic Medicine. 2018:80(4):370-376.
12. Perugi G, Medda P, Toni C, et al. The role of electroconvulsive therapy (ECT) in bipolar disorder: effectiveness in 522 patients with bipolar depression, mixed-state, mania and catatonic features. Curr Neuropharmacol. 2017;15(3):359-371.
13. Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
14. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
15. Pelzer A, Heijden FVD, Boer ED. Systematic review of catatonia treatment. Neuropsychiatr Dis Treat. 2018;14:317-326.
16. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry and Clin Neurosci. 2007;19(4):406-412.
17. Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatr Scand Suppl. 2013;(441):1-47.
18. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge University Press; 2006.
19. Petrides G, Tobias KG, Kellner CH, et al. Continuation and maintenance electroconvulsive therapy for mood disorders: review of the literature. Neuropsychobiology. 2011;64(3):129-140.
1. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums. 2000;5(7):26-33.
2. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391‐398.
3. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013. 119-121.
4. Solmi M, Pigato GG, Roiter B, et al. Prevalence of catatonia and its moderators in clinical samples: results from a meta-analysis and meta-regression analysis. Schizophrenia Bulletin. 2017;44(5):1133-1150.
5. Llesuy JR, Medina M, Jacobson KC, et al. Catatonia under-diagnosis in the general hospital. J Neuropsychiatry Clin Neurosci. 2018;30(2):145-151.
6. Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients. Crit Care Med. 2017;45(11):1837-1844.
7. Heijden FVD, Tuinier S, Arts N, et al. Catatonia: disappeared or under-diagnosed? Psychopathology. 2005;38(1):3-8.
8. Sarkar S, Sakey S, Mathan K, et al. Assessing catatonia using four different instruments: inter-rater reliability and prevalence in inpatient clinical population. Asian J Psychiatr. 2016;23:27-31.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
10. Puentes R, Brenzel A, Leon JD. Pulmonary embolism during stuporous episodes of catatonia was found to be the most frequent cause of preventable death according to a state mortality review: 6 deaths in 15 years. Clin Schizophr Relat Psychoses. 2017; doi:10.3371/csrp.rpab.071317
11. Funayama M, Takata T, Koreki A, et al. Catatonic stupor in schizophrenic disorders and subsequent medical complications and mortality. Psychosomatic Medicine. 2018:80(4):370-376.
12. Perugi G, Medda P, Toni C, et al. The role of electroconvulsive therapy (ECT) in bipolar disorder: effectiveness in 522 patients with bipolar depression, mixed-state, mania and catatonic features. Curr Neuropharmacol. 2017;15(3):359-371.
13. Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
14. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
15. Pelzer A, Heijden FVD, Boer ED. Systematic review of catatonia treatment. Neuropsychiatr Dis Treat. 2018;14:317-326.
16. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry and Clin Neurosci. 2007;19(4):406-412.
17. Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatr Scand Suppl. 2013;(441):1-47.
18. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge University Press; 2006.
19. Petrides G, Tobias KG, Kellner CH, et al. Continuation and maintenance electroconvulsive therapy for mood disorders: review of the literature. Neuropsychobiology. 2011;64(3):129-140.
Efficacy and safety of high-dose antipsychotic therapy
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).
Steps to take before increasing the dose
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.
SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).
Steps to take before increasing the dose
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.
SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).
Steps to take before increasing the dose
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.
SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
Nothing up his sleeve: Decompensation after bariatric surgery
CASE Sudden-onset low mood
Mr. G, age 64, is obese (body mass index [BMI] 37 kg/m2) and has a history of schizoaffective disorder. He is recovering from a sleeve gastrectomy, a surgical weight-loss procedure in which a large portion of the stomach is removed. Seven weeks after his surgery, he experiences a sudden onset of “low mood” and fears that he will become suicidal; he has a history of suicide attempts. Mr. G calls his long-term outpatient clinic and is advised to go to the emergency department (ED).
For years, Mr. G had been stable in a group home setting, and had always been adherent to treatment and forthcoming about his medications with both his bariatric surgeon and psychiatrist. Within the last month, he had been seen at the clinic, had no psychiatric symptoms, and was recovering well from the sleeve gastrectomy.
HISTORY A stable regimen
Mr. G’s psychiatric symptoms initially developed when he was in his 20s, during a time in which he reported using “a lot of drugs.” He had multiple suicide attempts, and multiple inpatient and outpatient treatments. He was diagnosed with schizoaffective disorder.
Mr. G has been stable on medications for the last 2 years. His outpatient psychotropic regimen is divalproex sodium extended-release (ER), 2,500 mg every night at bedtime; iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime.
In the group home, Mr. G spends his days socializing, studying philosophy, and writing essays. He hopes to find a job in the craftsman industry.
Mr. G’s medical history includes obesity (BMI: 37 kg/m2). Since the surgery, he has been receiving omeprazole, 40 mg/d, a proton pump inhibitor (PPI), to decrease the amount of acid in his stomach. Three weeks after surgery, he had an unremarkable postoperative outpatient psychiatry visit. Divalproex sodium ER was maintained at the pre-surgical dose of 2,500 mg/d.
EVALUATION Depressed and frightened
In the ED, Mr. G’s vitals are normal, but his serum valproic acid (VPA) level is 33.68 µg/mL (therapeutic range: 50 to 125 µg/mL), despite being compliant with treatment. Mr. G is discharged from the ED and told to follow up with his outpatient psychiatrist the next day.
Continue to: At his outpatient psychiatry appointment...
At his outpatient psychiatry appointment, Mr. G’s vital signs are normal, but he reports increasing depression and worsened mood. On mental status examination, Mr. G’s appearance is well groomed, and no agitation nor fidgeting are observed. His behavior is cooperative but somewhat disorganized. He is perseverative on “feeling so low.” He has poor eye contact, which is unusual for him. Mr. G’s speech is loud compared with his baseline. Affect is congruent to mood, which he describes as “depressed and frightened.” He is also noted to be irritable. His thought process is abstract and tangential, which is within his baseline. Mr. G’s thought content is fearful and negativistic, despite his usual positivity and optimism. He denies hallucinations and is oriented to time, place, and person. His judgment, attention, and memory are all within normal limits.
[polldaddy:10790537]
The authors’ observations
The psychiatrist rules out malingering/nonadherence due to Mr. G’s long history of treatment compliance, as evidenced by his past symptom control and therapeutic serum VPA levels. Mr. G was compliant with his postoperative appointments and has been healing well. Therefore, the treatment team believed that Mr. G’s intense and acute decompensation had to be related to a recent change. The notable changes in Mr. G’s case included his sleeve gastrectomy, and the addition of omeprazole to his medication regimen.
The treatment team observed that Mr. G had a long history of compliance with his medications and his symptoms were consistent with a low serum VPA level, which led to the conclusion that the low serum VPA level measured while he was in the ED was likely accurate. This prompted the team to consider Mr. G’s recent surgery. It is well documented that some bariatric surgeries can cause poor absorption of certain vitamins, minerals, and medications. However, Mr. G had a sleeve gastrectomy, which preserves absorption. At this point, the team considered if the patient’s recent medication change was the source of his low VPA level.
The psychiatrist concluded that the issue must have been with the addition of omeprazole because Mr. G’s sleeve gastrectomy was noneventful, he was healing well and being closely monitored by his bariatric surgeon, and this type of surgery preserves absorption. Fortunately, Mr. G was a good historian and had informed his psychiatrist about the addition of omeprazole after his sleeve gastrectomy. The psychiatrist knew acidity was important for the absorption of some medications. Although she was unsure as to whether the problem was a lack of absorption or lack of delivery, the psychiatrist knew a medication change was necessary to raise Mr. G’s serum VPA levels.
TREATMENT A change in divalproex formulation
The psychiatrist switches Mr. G’s formulation of divalproex sodium ER, 2,500 mg/d, to valproic acid immediate-release (IR) liquid capsules. He receives a total daily dose of 2,500 mg, but the dosage is split into 3 times a day. The omeprazole is continued to maintain the postoperative healing process, and he receives his other medications as well (iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime).
[polldaddy:10790540]
Continue to: The authors' observations
The authors’ observations
The key component to creating a treatment plan for Mr. G centered on understanding drug metabolism and delivery. Acidity plays a role in dissolution of many medications, which led the team to surmise that the PPI, omeprazole, was the culprit. Through research, they understood that the divalproex sodium ER formulation needed a more acidic environment to dissolve, and therefore, an IR formulation was needed.
Different formulations, different characteristics
Medications can be produced in different formulations such as IR, delayed-release (DR), and ER formulations. Different formulations may contain the same medication at identical strengths; however, they may not be bioequivalent and should be titrated based on both the properties of the medication and the release type.1
Immediate-release formulations are developed to dissolve without delaying or prolonging absorption of the medication. These formulations typically include “superdisintegrants” containing croscarmellose sodium2 so that they disintegrate, de-aggregate, and/or dissolve when they come into contact with water or the gastrointestinal tract.3-7
Delayed-release formulations rely on the gastrointestinal pH to release the medication after a certain amount of time has elapsed due to the enteric coating surrounding the tablet. This enteric coating prevents gastric mucosa/gastric juices from inactivating an acid-labile medication.8
Extended-release formulations, such as the divalproex sodium ER that was originally prescribed to Mr. G, are designed to release the medication in a controlled manner over an extended period of time, and at a predetermined rate and location following administration.8-9 The advantage of this type of formulation is that it can be used to reduce dose frequency and improve adherence.10 Extended-release formulations are designed to minimize fluctuations in serum drug concentration between doses,11 thereby reducing the risk of adverse effects.12,13 A list of some common extended-release psychiatric medications is shown in the Table.
Continue to: The 5 oral formulations...
The 5 oral formulations of medications that contain valproic acid include:
- syrup
- capsule
- sprinkle
- enteric-coated delayed-release and extended-release
A parenteral form via IV is available for patients who are unable to swallow.
Absorption vs delivery
Any gastric bypass surgery can have postoperative complications, one of which can include absorption deficiencies of vitamins and minerals. Sleeve gastrectomy has the least amount of absorption-related nutritional deficiencies.14 Additionally, this procedure preserves the stomach’s ability to produce gastric acid. Therefore, regardless of formulation, there should be no initial postsurgical need to change psychotropic medication formulations. However, because VPA is related to B-vitamin deficiency, supplementation can be considered.
Omeprazole is a PPI that increases pH in the stomach and is often prescribed to promote healing of gastric surgery. However, in Mr. G’s case, omeprazole created a non-acidic environment in his stomach, which prevented the divalproex sodium ER formulation from being dissolved and the medication from being delivered. Mr. G’s absorption ability was preserved, which was confirmed by his rapid recovery and increased serum VPA levels once he was switched to the IR formulation. There is no literature supporting a recommended length of time a patient can receive omeprazole therapy for sleeve gastrectomy; this is at the surgeon’s discretion. Mr. G’s prescription for omeprazole was for 3 months.
Proper valproate dosing
In Mr. G’s case, it could be hypothesized that the VPA dosing was incorrect. For mood disorders, oral VPA dosing is 25 mg/kg/d. Mr. G lost 40 pounds, which would translate to a 450-mg reduction in dose. Despite maintaining his original dose, his serum VPA levels decreased by almost 50% and could not be attributed to trough measurement. In this case, Mr. G was prescribed a higher dose than needed given his weight loss.
Continue to: Divalproex sodium ER...
Divalproex sodium ER is a hydrophilic matrix tablet that requires a low pH to dissolve. Switching to an IR formulation bypassed the need for a low pH and the VPA was delivered and absorbed. Mr. G was always able to absorb the medication, but only when delivered. The Table lists other psychiatric medications that clinicians should be aware of that utilize similar hydrophilic matrix technology to slowly release medications through the gastrointestinal tract and also require low pH to release the medication from the tablet.
OUTCOME Stable once again
Two and a half weeks after his medication formulation is changed from divalproex sodium ER to IR, Mr. G shows improvement in his symptoms. His serum VPA level is 52 µg/mL, which is within therapeutic limits. He continues receiving his previous medications as well. He reports “feeling much better” and denies having any depressive symptoms nor anxiety. Mr. G is able to maintain eye contact, and has positive thought content, improved organization of thinking, and retained abstraction.
Bottom Line
All medication changes should be identified at each visit. Many extended-release psychiatric medications require lower pH to release the medication from the tablet. When evaluating nonresponse to psychotropic medications, anything that affects pH in the stomach should be considered.
Related Resources
- Monte SV, Russo KM, Mustafa E. Impact of sleeve gastrectomy on psychiatric medication use and symptoms. J Obes. 2018; 2018:8532602. doi: 10.1155/2018/8532602
- Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
- ObesityHelp, Inc. https://www.obesityhelp.com/medications-after-bariatric-surgery-wls/
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Clonidine ER • Kapvay
Divalproex sodium extended- release tablets • Depakote ER
Escitalopram • Lexapro
Iloperidone • Fanapt
Methylphenidate ER tablet • Concerta
Methylphenidate ER capsule • Metadate, Jornay
Methylphenidate LA capsule • Ritalin LA
Mirtazapine • Remeron
Omeprazole • Prilosec, Zegerid
Paroxetine • Paxil
Valproic acid immediate- release capsules and solution • Depakene
Valproate sodium IV • Depacon
Venlafaxine • Effexor
1. Wheless JW, Phelps SJ. A clinician’s guide to oral extended-release drug delivery systems in epilepsy. J Pediatr Pharmacol Ther. 2018;23(4):277-292.
2. Jaimini M, Ranga S, Kumar A, et al. A review on immediate release drug delivery system by using design of experiment. J Drug Discov Therap. 2013;1(12):21-27.
3. Bhandari N, Kumar A, Choudhary A, et al. A review on immediate release drug delivery system. Int Res J Pharm App Sci. 2014;49(1):78-87.
4. Eatock J, Baker GA. Managing patient adherence and quality of life in epilepsy. Neuropsychiatr Dis Treat. 2007;3(1):117-131.
5. Manjunath R, Davis KL, Candrilli SD, et al. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14(2):372-378.
6. Samsonsen C, Reimers A, Bråthen G, et al. Nonadherence to treatment causing acute hospitalizations in people with epilepsy: an observational, prospective study. Epilepsia. 2014;55(11):e125-e128. doi: 10.1111/epi.12801
7. Mangal M, Thakral S, Goswami M, et al. Superdisintegrants: an updated review. Int Pharmacy Pharmaceut Sci Res. 2012;2(2):26-35.
8. Tablets. United States Pharmacopeia. Accessed January 21, 2021. http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1151s87.html
9. Holquist C, Fava W. FDA safety page: delayed- vs. extended-release Rxs. Drug Topics. Published July 23, 2007. Accessed January 21, 2021. https://www.drugtopics.com/view/fda-safety-page-delayed-release-vs-extended-release-rxs
10. Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
11. Perucca E. Extended-release formulations of antiepileptic drugs: rationale and comparative value. Epilepsy Curr. 2009;9(6):153-157.
12. Bialer M. Extended-release formulations for the treatment of epilepsy. CNS Drugs. 2007;21(9):765-774.
13. Pellock JM, Smith MC, Cloyd JC, et al. Extended-release formulations: simplifying strategies in the management of antiepileptic drug therapy. Epilepsy Behav. 2004;5(3):301-307.
14. Sarkhosh K, Birch DW, Sharma A, et al. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon’s guide. Can J Surg 2013;56(5):347-352.
CASE Sudden-onset low mood
Mr. G, age 64, is obese (body mass index [BMI] 37 kg/m2) and has a history of schizoaffective disorder. He is recovering from a sleeve gastrectomy, a surgical weight-loss procedure in which a large portion of the stomach is removed. Seven weeks after his surgery, he experiences a sudden onset of “low mood” and fears that he will become suicidal; he has a history of suicide attempts. Mr. G calls his long-term outpatient clinic and is advised to go to the emergency department (ED).
For years, Mr. G had been stable in a group home setting, and had always been adherent to treatment and forthcoming about his medications with both his bariatric surgeon and psychiatrist. Within the last month, he had been seen at the clinic, had no psychiatric symptoms, and was recovering well from the sleeve gastrectomy.
HISTORY A stable regimen
Mr. G’s psychiatric symptoms initially developed when he was in his 20s, during a time in which he reported using “a lot of drugs.” He had multiple suicide attempts, and multiple inpatient and outpatient treatments. He was diagnosed with schizoaffective disorder.
Mr. G has been stable on medications for the last 2 years. His outpatient psychotropic regimen is divalproex sodium extended-release (ER), 2,500 mg every night at bedtime; iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime.
In the group home, Mr. G spends his days socializing, studying philosophy, and writing essays. He hopes to find a job in the craftsman industry.
Mr. G’s medical history includes obesity (BMI: 37 kg/m2). Since the surgery, he has been receiving omeprazole, 40 mg/d, a proton pump inhibitor (PPI), to decrease the amount of acid in his stomach. Three weeks after surgery, he had an unremarkable postoperative outpatient psychiatry visit. Divalproex sodium ER was maintained at the pre-surgical dose of 2,500 mg/d.
EVALUATION Depressed and frightened
In the ED, Mr. G’s vitals are normal, but his serum valproic acid (VPA) level is 33.68 µg/mL (therapeutic range: 50 to 125 µg/mL), despite being compliant with treatment. Mr. G is discharged from the ED and told to follow up with his outpatient psychiatrist the next day.
Continue to: At his outpatient psychiatry appointment...
At his outpatient psychiatry appointment, Mr. G’s vital signs are normal, but he reports increasing depression and worsened mood. On mental status examination, Mr. G’s appearance is well groomed, and no agitation nor fidgeting are observed. His behavior is cooperative but somewhat disorganized. He is perseverative on “feeling so low.” He has poor eye contact, which is unusual for him. Mr. G’s speech is loud compared with his baseline. Affect is congruent to mood, which he describes as “depressed and frightened.” He is also noted to be irritable. His thought process is abstract and tangential, which is within his baseline. Mr. G’s thought content is fearful and negativistic, despite his usual positivity and optimism. He denies hallucinations and is oriented to time, place, and person. His judgment, attention, and memory are all within normal limits.
[polldaddy:10790537]
The authors’ observations
The psychiatrist rules out malingering/nonadherence due to Mr. G’s long history of treatment compliance, as evidenced by his past symptom control and therapeutic serum VPA levels. Mr. G was compliant with his postoperative appointments and has been healing well. Therefore, the treatment team believed that Mr. G’s intense and acute decompensation had to be related to a recent change. The notable changes in Mr. G’s case included his sleeve gastrectomy, and the addition of omeprazole to his medication regimen.
The treatment team observed that Mr. G had a long history of compliance with his medications and his symptoms were consistent with a low serum VPA level, which led to the conclusion that the low serum VPA level measured while he was in the ED was likely accurate. This prompted the team to consider Mr. G’s recent surgery. It is well documented that some bariatric surgeries can cause poor absorption of certain vitamins, minerals, and medications. However, Mr. G had a sleeve gastrectomy, which preserves absorption. At this point, the team considered if the patient’s recent medication change was the source of his low VPA level.
The psychiatrist concluded that the issue must have been with the addition of omeprazole because Mr. G’s sleeve gastrectomy was noneventful, he was healing well and being closely monitored by his bariatric surgeon, and this type of surgery preserves absorption. Fortunately, Mr. G was a good historian and had informed his psychiatrist about the addition of omeprazole after his sleeve gastrectomy. The psychiatrist knew acidity was important for the absorption of some medications. Although she was unsure as to whether the problem was a lack of absorption or lack of delivery, the psychiatrist knew a medication change was necessary to raise Mr. G’s serum VPA levels.
TREATMENT A change in divalproex formulation
The psychiatrist switches Mr. G’s formulation of divalproex sodium ER, 2,500 mg/d, to valproic acid immediate-release (IR) liquid capsules. He receives a total daily dose of 2,500 mg, but the dosage is split into 3 times a day. The omeprazole is continued to maintain the postoperative healing process, and he receives his other medications as well (iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime).
[polldaddy:10790540]
Continue to: The authors' observations
The authors’ observations
The key component to creating a treatment plan for Mr. G centered on understanding drug metabolism and delivery. Acidity plays a role in dissolution of many medications, which led the team to surmise that the PPI, omeprazole, was the culprit. Through research, they understood that the divalproex sodium ER formulation needed a more acidic environment to dissolve, and therefore, an IR formulation was needed.
Different formulations, different characteristics
Medications can be produced in different formulations such as IR, delayed-release (DR), and ER formulations. Different formulations may contain the same medication at identical strengths; however, they may not be bioequivalent and should be titrated based on both the properties of the medication and the release type.1
Immediate-release formulations are developed to dissolve without delaying or prolonging absorption of the medication. These formulations typically include “superdisintegrants” containing croscarmellose sodium2 so that they disintegrate, de-aggregate, and/or dissolve when they come into contact with water or the gastrointestinal tract.3-7
Delayed-release formulations rely on the gastrointestinal pH to release the medication after a certain amount of time has elapsed due to the enteric coating surrounding the tablet. This enteric coating prevents gastric mucosa/gastric juices from inactivating an acid-labile medication.8
Extended-release formulations, such as the divalproex sodium ER that was originally prescribed to Mr. G, are designed to release the medication in a controlled manner over an extended period of time, and at a predetermined rate and location following administration.8-9 The advantage of this type of formulation is that it can be used to reduce dose frequency and improve adherence.10 Extended-release formulations are designed to minimize fluctuations in serum drug concentration between doses,11 thereby reducing the risk of adverse effects.12,13 A list of some common extended-release psychiatric medications is shown in the Table.
Continue to: The 5 oral formulations...
The 5 oral formulations of medications that contain valproic acid include:
- syrup
- capsule
- sprinkle
- enteric-coated delayed-release and extended-release
A parenteral form via IV is available for patients who are unable to swallow.
Absorption vs delivery
Any gastric bypass surgery can have postoperative complications, one of which can include absorption deficiencies of vitamins and minerals. Sleeve gastrectomy has the least amount of absorption-related nutritional deficiencies.14 Additionally, this procedure preserves the stomach’s ability to produce gastric acid. Therefore, regardless of formulation, there should be no initial postsurgical need to change psychotropic medication formulations. However, because VPA is related to B-vitamin deficiency, supplementation can be considered.
Omeprazole is a PPI that increases pH in the stomach and is often prescribed to promote healing of gastric surgery. However, in Mr. G’s case, omeprazole created a non-acidic environment in his stomach, which prevented the divalproex sodium ER formulation from being dissolved and the medication from being delivered. Mr. G’s absorption ability was preserved, which was confirmed by his rapid recovery and increased serum VPA levels once he was switched to the IR formulation. There is no literature supporting a recommended length of time a patient can receive omeprazole therapy for sleeve gastrectomy; this is at the surgeon’s discretion. Mr. G’s prescription for omeprazole was for 3 months.
Proper valproate dosing
In Mr. G’s case, it could be hypothesized that the VPA dosing was incorrect. For mood disorders, oral VPA dosing is 25 mg/kg/d. Mr. G lost 40 pounds, which would translate to a 450-mg reduction in dose. Despite maintaining his original dose, his serum VPA levels decreased by almost 50% and could not be attributed to trough measurement. In this case, Mr. G was prescribed a higher dose than needed given his weight loss.
Continue to: Divalproex sodium ER...
Divalproex sodium ER is a hydrophilic matrix tablet that requires a low pH to dissolve. Switching to an IR formulation bypassed the need for a low pH and the VPA was delivered and absorbed. Mr. G was always able to absorb the medication, but only when delivered. The Table lists other psychiatric medications that clinicians should be aware of that utilize similar hydrophilic matrix technology to slowly release medications through the gastrointestinal tract and also require low pH to release the medication from the tablet.
OUTCOME Stable once again
Two and a half weeks after his medication formulation is changed from divalproex sodium ER to IR, Mr. G shows improvement in his symptoms. His serum VPA level is 52 µg/mL, which is within therapeutic limits. He continues receiving his previous medications as well. He reports “feeling much better” and denies having any depressive symptoms nor anxiety. Mr. G is able to maintain eye contact, and has positive thought content, improved organization of thinking, and retained abstraction.
Bottom Line
All medication changes should be identified at each visit. Many extended-release psychiatric medications require lower pH to release the medication from the tablet. When evaluating nonresponse to psychotropic medications, anything that affects pH in the stomach should be considered.
Related Resources
- Monte SV, Russo KM, Mustafa E. Impact of sleeve gastrectomy on psychiatric medication use and symptoms. J Obes. 2018; 2018:8532602. doi: 10.1155/2018/8532602
- Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
- ObesityHelp, Inc. https://www.obesityhelp.com/medications-after-bariatric-surgery-wls/
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Clonidine ER • Kapvay
Divalproex sodium extended- release tablets • Depakote ER
Escitalopram • Lexapro
Iloperidone • Fanapt
Methylphenidate ER tablet • Concerta
Methylphenidate ER capsule • Metadate, Jornay
Methylphenidate LA capsule • Ritalin LA
Mirtazapine • Remeron
Omeprazole • Prilosec, Zegerid
Paroxetine • Paxil
Valproic acid immediate- release capsules and solution • Depakene
Valproate sodium IV • Depacon
Venlafaxine • Effexor
CASE Sudden-onset low mood
Mr. G, age 64, is obese (body mass index [BMI] 37 kg/m2) and has a history of schizoaffective disorder. He is recovering from a sleeve gastrectomy, a surgical weight-loss procedure in which a large portion of the stomach is removed. Seven weeks after his surgery, he experiences a sudden onset of “low mood” and fears that he will become suicidal; he has a history of suicide attempts. Mr. G calls his long-term outpatient clinic and is advised to go to the emergency department (ED).
For years, Mr. G had been stable in a group home setting, and had always been adherent to treatment and forthcoming about his medications with both his bariatric surgeon and psychiatrist. Within the last month, he had been seen at the clinic, had no psychiatric symptoms, and was recovering well from the sleeve gastrectomy.
HISTORY A stable regimen
Mr. G’s psychiatric symptoms initially developed when he was in his 20s, during a time in which he reported using “a lot of drugs.” He had multiple suicide attempts, and multiple inpatient and outpatient treatments. He was diagnosed with schizoaffective disorder.
Mr. G has been stable on medications for the last 2 years. His outpatient psychotropic regimen is divalproex sodium extended-release (ER), 2,500 mg every night at bedtime; iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime.
In the group home, Mr. G spends his days socializing, studying philosophy, and writing essays. He hopes to find a job in the craftsman industry.
Mr. G’s medical history includes obesity (BMI: 37 kg/m2). Since the surgery, he has been receiving omeprazole, 40 mg/d, a proton pump inhibitor (PPI), to decrease the amount of acid in his stomach. Three weeks after surgery, he had an unremarkable postoperative outpatient psychiatry visit. Divalproex sodium ER was maintained at the pre-surgical dose of 2,500 mg/d.
EVALUATION Depressed and frightened
In the ED, Mr. G’s vitals are normal, but his serum valproic acid (VPA) level is 33.68 µg/mL (therapeutic range: 50 to 125 µg/mL), despite being compliant with treatment. Mr. G is discharged from the ED and told to follow up with his outpatient psychiatrist the next day.
Continue to: At his outpatient psychiatry appointment...
At his outpatient psychiatry appointment, Mr. G’s vital signs are normal, but he reports increasing depression and worsened mood. On mental status examination, Mr. G’s appearance is well groomed, and no agitation nor fidgeting are observed. His behavior is cooperative but somewhat disorganized. He is perseverative on “feeling so low.” He has poor eye contact, which is unusual for him. Mr. G’s speech is loud compared with his baseline. Affect is congruent to mood, which he describes as “depressed and frightened.” He is also noted to be irritable. His thought process is abstract and tangential, which is within his baseline. Mr. G’s thought content is fearful and negativistic, despite his usual positivity and optimism. He denies hallucinations and is oriented to time, place, and person. His judgment, attention, and memory are all within normal limits.
[polldaddy:10790537]
The authors’ observations
The psychiatrist rules out malingering/nonadherence due to Mr. G’s long history of treatment compliance, as evidenced by his past symptom control and therapeutic serum VPA levels. Mr. G was compliant with his postoperative appointments and has been healing well. Therefore, the treatment team believed that Mr. G’s intense and acute decompensation had to be related to a recent change. The notable changes in Mr. G’s case included his sleeve gastrectomy, and the addition of omeprazole to his medication regimen.
The treatment team observed that Mr. G had a long history of compliance with his medications and his symptoms were consistent with a low serum VPA level, which led to the conclusion that the low serum VPA level measured while he was in the ED was likely accurate. This prompted the team to consider Mr. G’s recent surgery. It is well documented that some bariatric surgeries can cause poor absorption of certain vitamins, minerals, and medications. However, Mr. G had a sleeve gastrectomy, which preserves absorption. At this point, the team considered if the patient’s recent medication change was the source of his low VPA level.
The psychiatrist concluded that the issue must have been with the addition of omeprazole because Mr. G’s sleeve gastrectomy was noneventful, he was healing well and being closely monitored by his bariatric surgeon, and this type of surgery preserves absorption. Fortunately, Mr. G was a good historian and had informed his psychiatrist about the addition of omeprazole after his sleeve gastrectomy. The psychiatrist knew acidity was important for the absorption of some medications. Although she was unsure as to whether the problem was a lack of absorption or lack of delivery, the psychiatrist knew a medication change was necessary to raise Mr. G’s serum VPA levels.
TREATMENT A change in divalproex formulation
The psychiatrist switches Mr. G’s formulation of divalproex sodium ER, 2,500 mg/d, to valproic acid immediate-release (IR) liquid capsules. He receives a total daily dose of 2,500 mg, but the dosage is split into 3 times a day. The omeprazole is continued to maintain the postoperative healing process, and he receives his other medications as well (iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime).
[polldaddy:10790540]
Continue to: The authors' observations
The authors’ observations
The key component to creating a treatment plan for Mr. G centered on understanding drug metabolism and delivery. Acidity plays a role in dissolution of many medications, which led the team to surmise that the PPI, omeprazole, was the culprit. Through research, they understood that the divalproex sodium ER formulation needed a more acidic environment to dissolve, and therefore, an IR formulation was needed.
Different formulations, different characteristics
Medications can be produced in different formulations such as IR, delayed-release (DR), and ER formulations. Different formulations may contain the same medication at identical strengths; however, they may not be bioequivalent and should be titrated based on both the properties of the medication and the release type.1
Immediate-release formulations are developed to dissolve without delaying or prolonging absorption of the medication. These formulations typically include “superdisintegrants” containing croscarmellose sodium2 so that they disintegrate, de-aggregate, and/or dissolve when they come into contact with water or the gastrointestinal tract.3-7
Delayed-release formulations rely on the gastrointestinal pH to release the medication after a certain amount of time has elapsed due to the enteric coating surrounding the tablet. This enteric coating prevents gastric mucosa/gastric juices from inactivating an acid-labile medication.8
Extended-release formulations, such as the divalproex sodium ER that was originally prescribed to Mr. G, are designed to release the medication in a controlled manner over an extended period of time, and at a predetermined rate and location following administration.8-9 The advantage of this type of formulation is that it can be used to reduce dose frequency and improve adherence.10 Extended-release formulations are designed to minimize fluctuations in serum drug concentration between doses,11 thereby reducing the risk of adverse effects.12,13 A list of some common extended-release psychiatric medications is shown in the Table.
Continue to: The 5 oral formulations...
The 5 oral formulations of medications that contain valproic acid include:
- syrup
- capsule
- sprinkle
- enteric-coated delayed-release and extended-release
A parenteral form via IV is available for patients who are unable to swallow.
Absorption vs delivery
Any gastric bypass surgery can have postoperative complications, one of which can include absorption deficiencies of vitamins and minerals. Sleeve gastrectomy has the least amount of absorption-related nutritional deficiencies.14 Additionally, this procedure preserves the stomach’s ability to produce gastric acid. Therefore, regardless of formulation, there should be no initial postsurgical need to change psychotropic medication formulations. However, because VPA is related to B-vitamin deficiency, supplementation can be considered.
Omeprazole is a PPI that increases pH in the stomach and is often prescribed to promote healing of gastric surgery. However, in Mr. G’s case, omeprazole created a non-acidic environment in his stomach, which prevented the divalproex sodium ER formulation from being dissolved and the medication from being delivered. Mr. G’s absorption ability was preserved, which was confirmed by his rapid recovery and increased serum VPA levels once he was switched to the IR formulation. There is no literature supporting a recommended length of time a patient can receive omeprazole therapy for sleeve gastrectomy; this is at the surgeon’s discretion. Mr. G’s prescription for omeprazole was for 3 months.
Proper valproate dosing
In Mr. G’s case, it could be hypothesized that the VPA dosing was incorrect. For mood disorders, oral VPA dosing is 25 mg/kg/d. Mr. G lost 40 pounds, which would translate to a 450-mg reduction in dose. Despite maintaining his original dose, his serum VPA levels decreased by almost 50% and could not be attributed to trough measurement. In this case, Mr. G was prescribed a higher dose than needed given his weight loss.
Continue to: Divalproex sodium ER...
Divalproex sodium ER is a hydrophilic matrix tablet that requires a low pH to dissolve. Switching to an IR formulation bypassed the need for a low pH and the VPA was delivered and absorbed. Mr. G was always able to absorb the medication, but only when delivered. The Table lists other psychiatric medications that clinicians should be aware of that utilize similar hydrophilic matrix technology to slowly release medications through the gastrointestinal tract and also require low pH to release the medication from the tablet.
OUTCOME Stable once again
Two and a half weeks after his medication formulation is changed from divalproex sodium ER to IR, Mr. G shows improvement in his symptoms. His serum VPA level is 52 µg/mL, which is within therapeutic limits. He continues receiving his previous medications as well. He reports “feeling much better” and denies having any depressive symptoms nor anxiety. Mr. G is able to maintain eye contact, and has positive thought content, improved organization of thinking, and retained abstraction.
Bottom Line
All medication changes should be identified at each visit. Many extended-release psychiatric medications require lower pH to release the medication from the tablet. When evaluating nonresponse to psychotropic medications, anything that affects pH in the stomach should be considered.
Related Resources
- Monte SV, Russo KM, Mustafa E. Impact of sleeve gastrectomy on psychiatric medication use and symptoms. J Obes. 2018; 2018:8532602. doi: 10.1155/2018/8532602
- Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
- ObesityHelp, Inc. https://www.obesityhelp.com/medications-after-bariatric-surgery-wls/
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Clonidine ER • Kapvay
Divalproex sodium extended- release tablets • Depakote ER
Escitalopram • Lexapro
Iloperidone • Fanapt
Methylphenidate ER tablet • Concerta
Methylphenidate ER capsule • Metadate, Jornay
Methylphenidate LA capsule • Ritalin LA
Mirtazapine • Remeron
Omeprazole • Prilosec, Zegerid
Paroxetine • Paxil
Valproic acid immediate- release capsules and solution • Depakene
Valproate sodium IV • Depacon
Venlafaxine • Effexor
1. Wheless JW, Phelps SJ. A clinician’s guide to oral extended-release drug delivery systems in epilepsy. J Pediatr Pharmacol Ther. 2018;23(4):277-292.
2. Jaimini M, Ranga S, Kumar A, et al. A review on immediate release drug delivery system by using design of experiment. J Drug Discov Therap. 2013;1(12):21-27.
3. Bhandari N, Kumar A, Choudhary A, et al. A review on immediate release drug delivery system. Int Res J Pharm App Sci. 2014;49(1):78-87.
4. Eatock J, Baker GA. Managing patient adherence and quality of life in epilepsy. Neuropsychiatr Dis Treat. 2007;3(1):117-131.
5. Manjunath R, Davis KL, Candrilli SD, et al. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14(2):372-378.
6. Samsonsen C, Reimers A, Bråthen G, et al. Nonadherence to treatment causing acute hospitalizations in people with epilepsy: an observational, prospective study. Epilepsia. 2014;55(11):e125-e128. doi: 10.1111/epi.12801
7. Mangal M, Thakral S, Goswami M, et al. Superdisintegrants: an updated review. Int Pharmacy Pharmaceut Sci Res. 2012;2(2):26-35.
8. Tablets. United States Pharmacopeia. Accessed January 21, 2021. http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1151s87.html
9. Holquist C, Fava W. FDA safety page: delayed- vs. extended-release Rxs. Drug Topics. Published July 23, 2007. Accessed January 21, 2021. https://www.drugtopics.com/view/fda-safety-page-delayed-release-vs-extended-release-rxs
10. Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
11. Perucca E. Extended-release formulations of antiepileptic drugs: rationale and comparative value. Epilepsy Curr. 2009;9(6):153-157.
12. Bialer M. Extended-release formulations for the treatment of epilepsy. CNS Drugs. 2007;21(9):765-774.
13. Pellock JM, Smith MC, Cloyd JC, et al. Extended-release formulations: simplifying strategies in the management of antiepileptic drug therapy. Epilepsy Behav. 2004;5(3):301-307.
14. Sarkhosh K, Birch DW, Sharma A, et al. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon’s guide. Can J Surg 2013;56(5):347-352.
1. Wheless JW, Phelps SJ. A clinician’s guide to oral extended-release drug delivery systems in epilepsy. J Pediatr Pharmacol Ther. 2018;23(4):277-292.
2. Jaimini M, Ranga S, Kumar A, et al. A review on immediate release drug delivery system by using design of experiment. J Drug Discov Therap. 2013;1(12):21-27.
3. Bhandari N, Kumar A, Choudhary A, et al. A review on immediate release drug delivery system. Int Res J Pharm App Sci. 2014;49(1):78-87.
4. Eatock J, Baker GA. Managing patient adherence and quality of life in epilepsy. Neuropsychiatr Dis Treat. 2007;3(1):117-131.
5. Manjunath R, Davis KL, Candrilli SD, et al. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14(2):372-378.
6. Samsonsen C, Reimers A, Bråthen G, et al. Nonadherence to treatment causing acute hospitalizations in people with epilepsy: an observational, prospective study. Epilepsia. 2014;55(11):e125-e128. doi: 10.1111/epi.12801
7. Mangal M, Thakral S, Goswami M, et al. Superdisintegrants: an updated review. Int Pharmacy Pharmaceut Sci Res. 2012;2(2):26-35.
8. Tablets. United States Pharmacopeia. Accessed January 21, 2021. http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1151s87.html
9. Holquist C, Fava W. FDA safety page: delayed- vs. extended-release Rxs. Drug Topics. Published July 23, 2007. Accessed January 21, 2021. https://www.drugtopics.com/view/fda-safety-page-delayed-release-vs-extended-release-rxs
10. Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
11. Perucca E. Extended-release formulations of antiepileptic drugs: rationale and comparative value. Epilepsy Curr. 2009;9(6):153-157.
12. Bialer M. Extended-release formulations for the treatment of epilepsy. CNS Drugs. 2007;21(9):765-774.
13. Pellock JM, Smith MC, Cloyd JC, et al. Extended-release formulations: simplifying strategies in the management of antiepileptic drug therapy. Epilepsy Behav. 2004;5(3):301-307.
14. Sarkhosh K, Birch DW, Sharma A, et al. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon’s guide. Can J Surg 2013;56(5):347-352.