User login
Calcinosis universalis
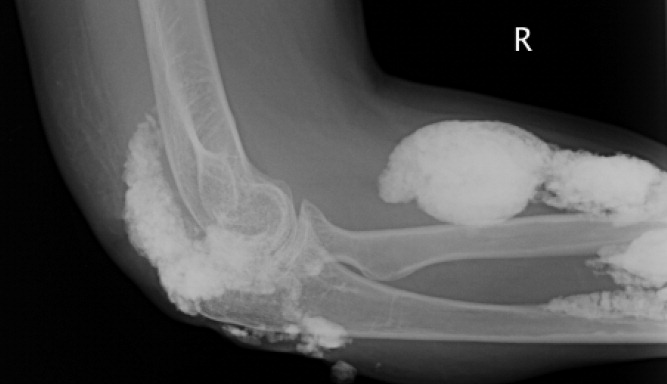
A 38‐year‐old woman with juvenile dermatomyositis (JDM) and calcinosis universalis presented with 3 days of drainage from a lesion on her right elbow. An examination of the elbow revealed diffuse and firm subcutaneous nodules with overlying erythema. X‐rays illustrated soft‐tissue calcifications in the forearm and elbow without evidence of osteomyelitis (Figure 1). Wound cultures grew Staphylococcus aureus, and the patient was started on intravenous antibiotics for abscess treatment.
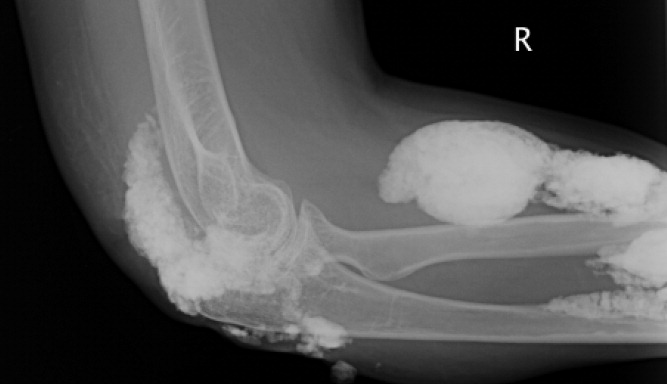
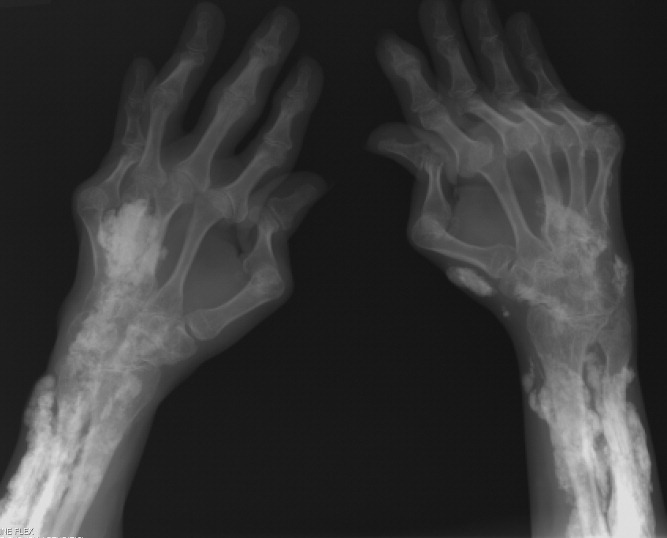
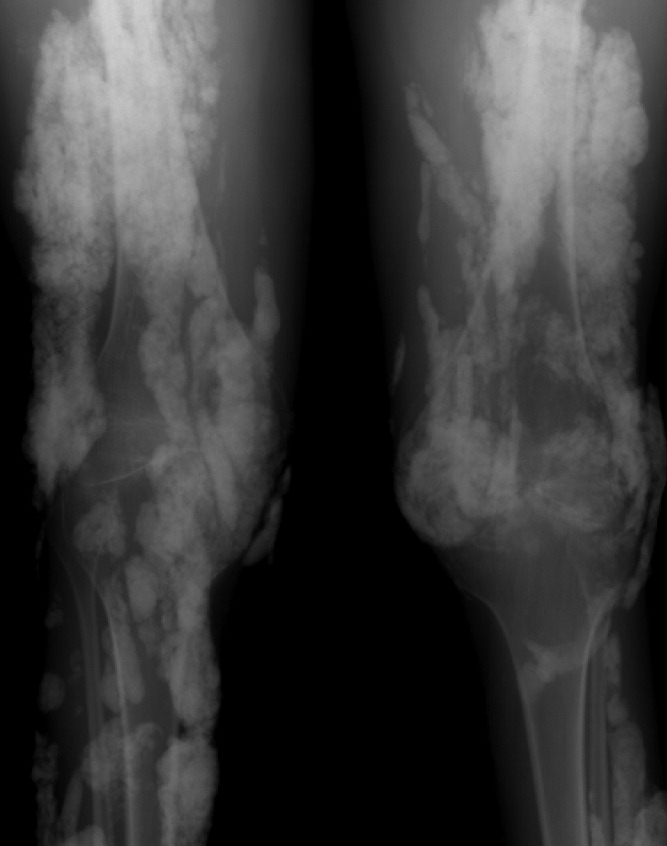
Calcinosis universalis is soft‐tissue calcification presenting as a complication of JDM. It is often detected in childhood in 30% to 70% of patients. It is hypothesized that calcinosis is due to chronic tissue inflammation, as seen in JDM, leading to muscle damage, releasing calcium, and inducing mineralization. Calcinosis universalis often presents as calcified nodules and plaques in areas of repeated trauma, such as joints, extremities, and buttocks (Figures 13). Calcification is localized in subcutaneous tissue, fascial planes, tendons, or intramuscular areas. It can cause debilitating secondary complications such as skin ulcerations expressing calcified material, superimposed infections of skin lesions, joint contractures with severe arthralgias, and muscle atrophy. Calcinosis has been correlated with severity of JDM with presence of cardiac involvement and use of more than one immunosuppression medication.1 It has also been associated with the degree of vasculopathy and delay in initiation of therapy for controlling inflammation in JDM.2
Soft‐tissue calcification can be classified into 5 categories:
-
Dystrophic calcification occurs in injured tissues with normal calcium, phosphorus, and parathyroid hormone levels, as seen in this patient. Calcified nodules or plaques occur in the extremities and buttocks. This is most often seen in JDM, scleroderma, and systemic lupus erythematosus.
-
Metastatic calcification affects normal tissues with abnormal levels of calcium and phosphorus. It is seen in large joints as well as arteries and visceral organs. It is associated with hyperparathyroidism, hypervitaminosis D, and malignancies.
-
Calciphylaxis with abnormal calcium and phosphorus metabolism causes small‐vessel calcification in patients with chronic renal failure.
-
Tumoral calcification is a familial condition with normal calcium levels but elevated phosphorus levels. Large subcutaneous calcifications are seen near high‐pressure areas and joints.
-
Idiopathic calcification is seen in healthy children and young adults with normal calcium metabolism and appears as multiple subcutaneous calcifications.2
Although multiple therapeutic options have been tried for the management or prevention of calcinosis, there is currently no accepted standard of treatment. In patients with calcinosis, warfarin, probenecid, colchicine, bisphosphonates, minocycline, diltiazem, aluminum hydroxide, corticosteroids, and salicylate have been attempted with variable results. Other therapeutic options include carbon dioxide laser treatments and surgical excision of large plaques. Decreasing muscle inflammation with aggressive treatment of JDM may improve outcomes and decrease the incidence of calcification.3 Unfortunately, once calcinosis has occurred, it is highly refractory to medical therapy.
Calcinosis universalis can lead to severe functional impairment. It can be distinguished from other types of calcinosis by diffuse involvement of muscle and fascia in connective tissue disease with normal calcium and phosphorus levels. New management modalities such as cyclosporine, intravenous immunoglobulin, and tumor necrosis factor alpha inhibitors are currently being evaluated.
- ,,, et al.Risk factors associated with calcinosis of juvenile dermatomyositis.JPediatr (Rio J).2008;84(1):68–74.
- ,,,.Calcinosis in rheumatic diseases.Semin Arthritis Rheum.2005;34(6):805–812.
- ,,, et al.Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis.J Am Acad Dermatol.2002;47(4):505–511.
A 38‐year‐old woman with juvenile dermatomyositis (JDM) and calcinosis universalis presented with 3 days of drainage from a lesion on her right elbow. An examination of the elbow revealed diffuse and firm subcutaneous nodules with overlying erythema. X‐rays illustrated soft‐tissue calcifications in the forearm and elbow without evidence of osteomyelitis (Figure 1). Wound cultures grew Staphylococcus aureus, and the patient was started on intravenous antibiotics for abscess treatment.

Calcinosis universalis is soft‐tissue calcification presenting as a complication of JDM. It is often detected in childhood in 30% to 70% of patients. It is hypothesized that calcinosis is due to chronic tissue inflammation, as seen in JDM, leading to muscle damage, releasing calcium, and inducing mineralization. Calcinosis universalis often presents as calcified nodules and plaques in areas of repeated trauma, such as joints, extremities, and buttocks (Figures 13). Calcification is localized in subcutaneous tissue, fascial planes, tendons, or intramuscular areas. It can cause debilitating secondary complications such as skin ulcerations expressing calcified material, superimposed infections of skin lesions, joint contractures with severe arthralgias, and muscle atrophy. Calcinosis has been correlated with severity of JDM with presence of cardiac involvement and use of more than one immunosuppression medication.1 It has also been associated with the degree of vasculopathy and delay in initiation of therapy for controlling inflammation in JDM.2
Soft‐tissue calcification can be classified into 5 categories:
-
Dystrophic calcification occurs in injured tissues with normal calcium, phosphorus, and parathyroid hormone levels, as seen in this patient. Calcified nodules or plaques occur in the extremities and buttocks. This is most often seen in JDM, scleroderma, and systemic lupus erythematosus.
-
Metastatic calcification affects normal tissues with abnormal levels of calcium and phosphorus. It is seen in large joints as well as arteries and visceral organs. It is associated with hyperparathyroidism, hypervitaminosis D, and malignancies.
-
Calciphylaxis with abnormal calcium and phosphorus metabolism causes small‐vessel calcification in patients with chronic renal failure.
-
Tumoral calcification is a familial condition with normal calcium levels but elevated phosphorus levels. Large subcutaneous calcifications are seen near high‐pressure areas and joints.
-
Idiopathic calcification is seen in healthy children and young adults with normal calcium metabolism and appears as multiple subcutaneous calcifications.2


Although multiple therapeutic options have been tried for the management or prevention of calcinosis, there is currently no accepted standard of treatment. In patients with calcinosis, warfarin, probenecid, colchicine, bisphosphonates, minocycline, diltiazem, aluminum hydroxide, corticosteroids, and salicylate have been attempted with variable results. Other therapeutic options include carbon dioxide laser treatments and surgical excision of large plaques. Decreasing muscle inflammation with aggressive treatment of JDM may improve outcomes and decrease the incidence of calcification.3 Unfortunately, once calcinosis has occurred, it is highly refractory to medical therapy.
Calcinosis universalis can lead to severe functional impairment. It can be distinguished from other types of calcinosis by diffuse involvement of muscle and fascia in connective tissue disease with normal calcium and phosphorus levels. New management modalities such as cyclosporine, intravenous immunoglobulin, and tumor necrosis factor alpha inhibitors are currently being evaluated.
A 38‐year‐old woman with juvenile dermatomyositis (JDM) and calcinosis universalis presented with 3 days of drainage from a lesion on her right elbow. An examination of the elbow revealed diffuse and firm subcutaneous nodules with overlying erythema. X‐rays illustrated soft‐tissue calcifications in the forearm and elbow without evidence of osteomyelitis (Figure 1). Wound cultures grew Staphylococcus aureus, and the patient was started on intravenous antibiotics for abscess treatment.

Calcinosis universalis is soft‐tissue calcification presenting as a complication of JDM. It is often detected in childhood in 30% to 70% of patients. It is hypothesized that calcinosis is due to chronic tissue inflammation, as seen in JDM, leading to muscle damage, releasing calcium, and inducing mineralization. Calcinosis universalis often presents as calcified nodules and plaques in areas of repeated trauma, such as joints, extremities, and buttocks (Figures 13). Calcification is localized in subcutaneous tissue, fascial planes, tendons, or intramuscular areas. It can cause debilitating secondary complications such as skin ulcerations expressing calcified material, superimposed infections of skin lesions, joint contractures with severe arthralgias, and muscle atrophy. Calcinosis has been correlated with severity of JDM with presence of cardiac involvement and use of more than one immunosuppression medication.1 It has also been associated with the degree of vasculopathy and delay in initiation of therapy for controlling inflammation in JDM.2
Soft‐tissue calcification can be classified into 5 categories:
-
Dystrophic calcification occurs in injured tissues with normal calcium, phosphorus, and parathyroid hormone levels, as seen in this patient. Calcified nodules or plaques occur in the extremities and buttocks. This is most often seen in JDM, scleroderma, and systemic lupus erythematosus.
-
Metastatic calcification affects normal tissues with abnormal levels of calcium and phosphorus. It is seen in large joints as well as arteries and visceral organs. It is associated with hyperparathyroidism, hypervitaminosis D, and malignancies.
-
Calciphylaxis with abnormal calcium and phosphorus metabolism causes small‐vessel calcification in patients with chronic renal failure.
-
Tumoral calcification is a familial condition with normal calcium levels but elevated phosphorus levels. Large subcutaneous calcifications are seen near high‐pressure areas and joints.
-
Idiopathic calcification is seen in healthy children and young adults with normal calcium metabolism and appears as multiple subcutaneous calcifications.2


Although multiple therapeutic options have been tried for the management or prevention of calcinosis, there is currently no accepted standard of treatment. In patients with calcinosis, warfarin, probenecid, colchicine, bisphosphonates, minocycline, diltiazem, aluminum hydroxide, corticosteroids, and salicylate have been attempted with variable results. Other therapeutic options include carbon dioxide laser treatments and surgical excision of large plaques. Decreasing muscle inflammation with aggressive treatment of JDM may improve outcomes and decrease the incidence of calcification.3 Unfortunately, once calcinosis has occurred, it is highly refractory to medical therapy.
Calcinosis universalis can lead to severe functional impairment. It can be distinguished from other types of calcinosis by diffuse involvement of muscle and fascia in connective tissue disease with normal calcium and phosphorus levels. New management modalities such as cyclosporine, intravenous immunoglobulin, and tumor necrosis factor alpha inhibitors are currently being evaluated.
- ,,, et al.Risk factors associated with calcinosis of juvenile dermatomyositis.JPediatr (Rio J).2008;84(1):68–74.
- ,,,.Calcinosis in rheumatic diseases.Semin Arthritis Rheum.2005;34(6):805–812.
- ,,, et al.Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis.J Am Acad Dermatol.2002;47(4):505–511.
- ,,, et al.Risk factors associated with calcinosis of juvenile dermatomyositis.JPediatr (Rio J).2008;84(1):68–74.
- ,,,.Calcinosis in rheumatic diseases.Semin Arthritis Rheum.2005;34(6):805–812.
- ,,, et al.Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis.J Am Acad Dermatol.2002;47(4):505–511.
Evaluation of glycemic control following discontinuation of an intensive insulin protocol
Hyperglycemia and insulin resistance are common occurrences in critically ill patients, even those without a past medical history of diabetes.1, 2 This hyperglycemic state is associated with adverse outcomes, including severe infections, polyneuropathy, multiple‐organ failure, and death.3 Several studies have shown benefit in keeping patients' blood glucose (BG) tightly controlled.37 In a randomized controlled study, strict BG control (80‐110 mg/dL) with an insulin drip significantly reduced morbidity and mortality in critically ill patients.3 A recent meta‐analysis concluded that avoiding BG levels >150 mg/dL appeared to be crucial to reducing mortality in a mixed medical and surgical intensive care unit (ICU) population.7
The Diabetes Mellitus Insulin‐Glucose Infusion in Acute Myocardial Infarction study addressed the issue of tight glycemic control both acutely and chronically in 620 diabetic patients postmyocardial infarction. Patients were randomized to tight glycemic control (126‐180 mg/dL) followed by a transition to maintenance insulin or to standard care. This intervention demonstrated a sustained mortality reduction of 7.5% at 1 year.8 In contrast, the CREATE‐ECLA study showed a neutral mortality benefit of a short‐term (24‐hour) insulin infusion in postmyocardial infarction patients.9 These data demonstrate the need for clinicians to consider insulin requirements throughout the hospital stay and after discharge. To date, there are no published studies evaluating glycemic control following discontinuation of an intensive insulin protocol (IIP). Therefore, the current study was conducted to compare BG control during the use of an IIP and for the 5 days following intensive insulin therapy.
METHODS
Patient Population
This retrospective chart review was conducted at Methodist University Hospital (MUH), Memphis, TN. MUH is a 500‐bed, university‐affiliated tertiary referral hospital. The study was approved by the hospital institutional review board. From January 2006 to January 2007, a computer‐generated pharmacy report was used to identify all patients receiving the hospital‐approved IIP. Patients were included if they were 18 years old and received the IIP for 24 hours. Patients were excluded from the study if they met any of the following criteria: (1) complete BG measurements were not retrievable while the patient received the IIP or for the 5 days following discontinuation of the IIP, (2) the patient died while receiving the IIP, and (3) an endocrinologist was involved in the care of the patient.
IIP
The hospital‐approved IIP is a paper‐based, physician‐initiated, nurse‐managed protocol. Criteria required before initiating the IIP include (1) ICU admission, (2) 2 BG measurements >150 mg/dL, (3) administration of continuous exogenous glucose, and (4) absence of diabetic ketoacidosis. The goal range of the IIP is 80 to 150 mg/dL. Hourly BG measurements are initially required, but as control is achieved, measurements may be extended to every 2 hours and then every 4 hours. In general, the criteria used for transitioning off the IIP include stability during the last 12 hours. Patients were considered to be stable on the IIP if they had >70% of their glucose measurements within the goal range during the last 12 hours.
Data Collection
When inclusion criteria were met, patients' medical records were reviewed. Data collection included basic demographic information, concurrent medications, duration of IIP, amount of insulin administered during the last 12 hours of the IIP, insulin regimen post‐IIP, and BG measurements during the last 12 hours on the IIP and for a total of 5 days after the IIP was stopped (follow‐up period). For this study, hyperglycemia was defined as a BG value >150 mg/dL, significant hyperglycemia was defined as >200 mg/dL, and severe hyperglycemia was defined as >300 mg/dL. Hypoglycemia was defined as a BG value <60 mg/dL. The values of <60 mg/dL, >150 mg/dL, and >200 mg/dL were chosen on the basis of the criteria used in the MUH IIP and standard sliding‐scale protocols. A value of >300 mg/dL was used to better describe patients with hyperglycemia. Poor glycemic control following the IIP was defined as a >30% change in mean BG during the last 12 hours on the drip and on the first day after discontinuation of the drip.
Statistical Analysis
The primary objective of this study was to compare BG control during the last 12 hours of an IIP and for the 5 days following its discontinuation. Secondary objectives were to evaluate the incidence of hyperglycemia and hypoglycemia during the transition period and to identify patients at risk of poor glycemic control following discontinuation of the IIP. Continuous data are appropriately reported as the mean standard deviation or median (interquartile range), depending on the distribution. Continuous variables were compared with the Student t test or Wilcoxon rank sum test. Discrete variables were compared with chi‐square analysis and Bonferroni Correction where appropriate. For comparisons of BG during the IIP and on days 1 to 5 of the follow‐up period, repeated‐measures analysis of variance on ranks was conducted because of the distribution. These statistical analyses were performed with SigmaStat version 2.03 (Systat Software, Inc., Richmond, VA). A P value of less than 0.05 was considered significant. However, when the Bonferroni correction was used, a value of less than 0.01 was considered significant. Multivariable logistic regression was used to determine independent predictors of a greater than 30% change in the mean BG value between the last 12 hours of the IIP and the first day off the insulin drip. Potential independent variables included in the analysis were stability on protocol, requiring less than 20 units of insulin in the last 12 hours on the IIP, use of antibiotics, use of steroids, history of diabetes, and type of insulin to which the patient was transitioned (none, sliding scale, and scheduled and sliding scale). The model was built in a backwards, stepwise fashion with SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
A total of 171 patients received the IIP during the study period. Ninety‐seven patients did not meet inclusion criteria because they received the IIP for less than 24 hours. Of the 74 patients meeting inclusion criteria, 9 were excluded (5 had insufficient glucose data, 3 were cared for by an endocrinologist, and 1 died while receiving the IIP). Thus, 65 patients were included in the study.
Table 1 lists the baseline demographics for all patients and those with and without a history of diabetes mellitus (DM). The majority of the patients (n = 49) underwent a surgical procedure, with the most common procedure being coronary artery bypass graft (n = 38). Patients undergoing coronary artery bypass graft had the IIP included in their standard postoperative order set. The majority of patients were considered stable during the 12 hours prior to discontinuation of the IIP, including 23 patients with a history of DM. Of the 65 patients who were included in the study, 25 (38.5%) received a scheduled insulin order following discontinuation of the IIP, whereas 38 (58.5%) received some form of sliding‐scale insulin (SSI). Additionally, 2 (3%) patients did not receive any form of insulin order after stopping the IIP. Of those receiving scheduled insulin, 15 (60%) received neutral protamine Hagedorn, 5 (20%) received glargine, 5 (20%) received 70/30, and 1 (4%) received regular insulin. Of those receiving SSI only, the prescribed frequency was as follows: every 4 hours for 17 (45%), before meals and at bedtime for 15 (39%), every 6 hours for 5 (13%), and every 2 hours for 1 (3%).
| All Patients (n = 65) | PMH of DM (n = 36) | No PMH of DM (n = 29) | |
|---|---|---|---|
| |||
| Age, mean years SD | 62 11 | 61 10 | 64 12 |
| Male gender, n (%) | 38 (58) | 22 (61) | 16 (55.2) |
| BMI SD | 30 7.2 | 31 7 | 30 6.5 |
| Surgery, n (%) | 49 (74.2) | 27 (75) | 21 (72.4) |
| CABG, n | 38 | 22 | 16 |
| Liver transplant, n | 6 | 1 | 4 |
| Other, n | 5 | 4 | 1 |
| Last 24 hours on IIP, n (%) | |||
| Ventilator | 37 (56.9) | 22 (61.1) | 15 (51.7) |
| Antibiotics | 37 (56.9) | 20 (55.6) | 17 (58.6) |
| Vasopressors | 11 (16.9) | 5 (13.9) | 6 (20.7) |
| Hemodialysis | 8 (12.3) | 5 (13.9) | 3 (10.3) |
| Steroids | 16 (24.6) | 9 (25) | 7 (24.1) |
| Duration of IIP, mean hours SD | 72 65 | 80 78 | 62 45 |
| Insulin during last 12 hours of IIP, mean units SD | 47 37 | 51 30 | 46 45 |
| Type of insulin received following IIP, n (%) | |||
| Scheduled + sliding scale | 25 (38.5) | 19 (52.8) | 6 (20.7) |
| Sliding scale only | 38 (58.5) | 16 (44.4) | 22 (75.9) |
| None | 2 (3) | 1 (2.8) | 1 (3.4) |
| Total daily insulin following IIP, mean units SD | 28 41 | 38 49 | 17 24 |
| Patients stable on IIP | 44 (67.7) | 23 (64.8) | 21 (72.4) |
| Hospital LOS, mean days SD | 24 18 | 24 17 | 23 19 |
| Mortality, n (%) | 15 (23.1) | 5 (13.8) | 10 (34.5) |
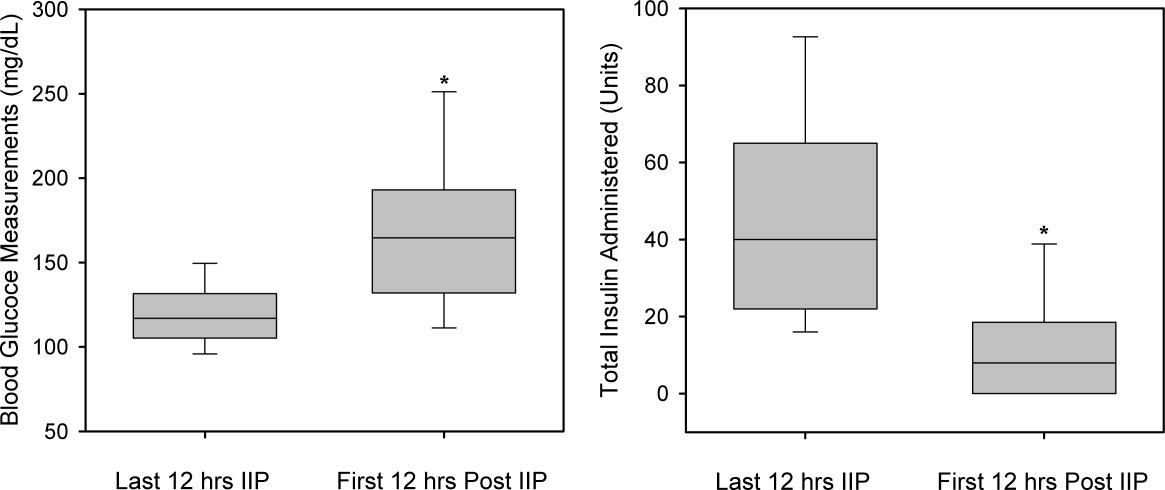
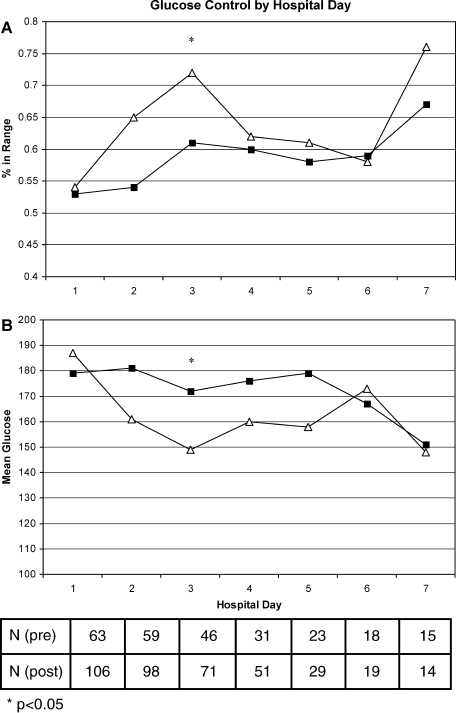
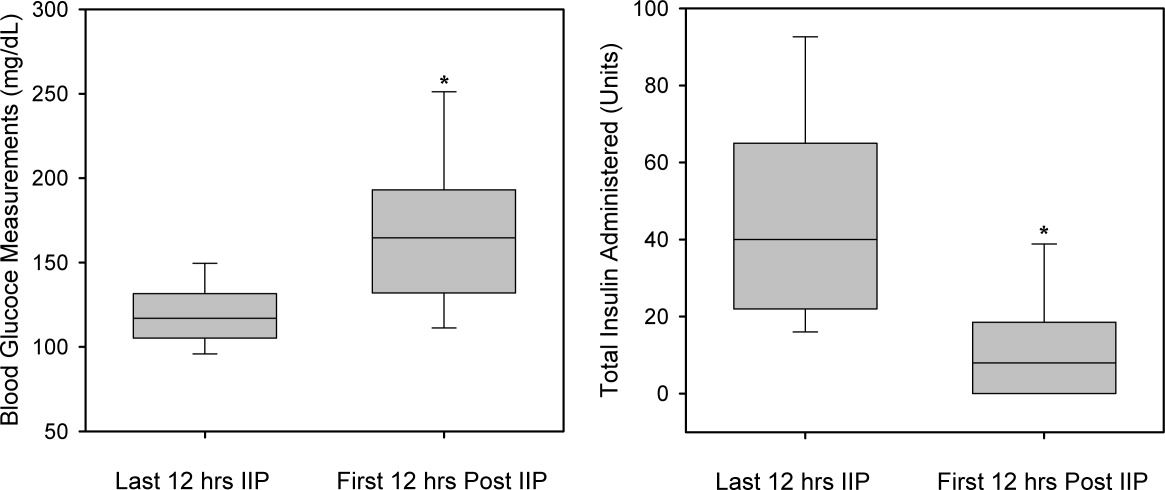
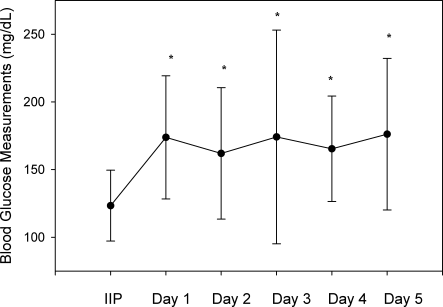
A total of 562 glucose measurements were collected during the last 12 hours on the IIP, whereas 201 were collected during the first 12 hours immediately following the IIP. Patients demonstrated a significant increase in BG (mean standard deviation) during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP (168 50 mg/dL versus 123 26 mg/dL, P < 0.001). This corresponded to a significant decrease in the median (interquartile range) insulin administered during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP [8 (0‐18) units versus 40 (22‐65) units, P < 0.001; Figure 1]. A total of 1914 BG measurements were collected during the follow‐up period. Figure 2 shows mean BG values for all patients on the IIP compared to mean BG values for each day of the follow‐up period. There was a significant increase in mean BG measurements when the IIP was compared to each day of the follow‐up period, but there was no difference between days of the follow‐up period. Table 2 shows the proportion of patients experiencing at least 1 episode of hyperglycemia (BG > 150 mg/dL), significant hyperglycemia (BG > 200 mg/dL), severe hyperglycemia (BG > 300 mg/dL), or hypoglycemia (BG < 60 mg/dL) while receiving the IIP and during the follow‐up period. When comparing the IIP to the follow‐up period, we found a significant increase in the proportion of patients with at least 1 BG > 150 mg/dL. This was also true for patients with a BG of > 200 mg/dL.
| IIP (n = 65) | Day 1 (n = 65) | Day 2 (n = 65) | Day 3 (n = 64) | Day 4 (n = 62) | Day 5 (n = 59) | |
|---|---|---|---|---|---|---|
| ||||||
| Patients with >150 mg/dL, n (%) | 33 (51) | 54 (83)* | 54 (83)* | 52 (81)* | 51 (82)* | 48 (81)* |
| Patients with >200 mg/dL, n (%) | 11 (17) | 37 (57)* | 31 (48)* | 26 (41)* | 33 (53)* | 34 (58)* |
| Patients with >300 mg/dL, n (%) | 2 (3) | 11 (17) | 7 (11) | 8 (12) | 5 (8) | 10 (17) |
| Patients with <60 mg/dL, n (%) | 6 (9) | 5 (8) | 2 (3) | 2 (3) | 2 (3) | 0 (0) |
The only independent predictor of a greater than 30% change in mean BG was the requirement for more than 20 units of insulin (>1.7 units/hour) during the last 12 hours on the IIP. The odds of a greater than 30% change was 4.62 times higher (95% confidence interval: 1.1718.17) in patients requiring more than 20 units during the last 12 hours on IIP after adjustments for stability on the protocol and past medical history of diabetes. Stability on the protocol was not identified as an independent predictor, with an adjusted odds ratio of 2.40 (95% confidence interval: 0.797.32).
DISCUSSION
This is the first study to describe glycemic control following the transition from an IIP to subcutaneous insulin. We observed that during the 5 days following discontinuation of an IIP, patients had significantly elevated mean BG values. These data are highlighted by the fact that patients received significantly less insulin during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP. Additionally, a larger than expected proportion of patients exhibited at least 1 episode of hyperglycemia during the follow‐up period. We also found that an increased insulin requirement of >1.7 units/hour during the last 12 hours of the IIP was an independent risk factor for a greater than 30% increase in mean BG on day 1 of the follow‐up period.
Increasing evidence demonstrates that the development of hyperglycemia in the hospital setting is a marker of poor clinical outcome and mortality. In fact, hyperglycemia has been associated with prolonged hospital stay, infection, disability after discharge, and death in patients on general surgical and medical wards.1012 This makes the increase in mean BG found in our study following discontinuation of the IIP a concern.
SSI with subcutaneous short‐acting insulin has been used for inpatients as the standard of care for many years. However, evidence supporting the effectiveness of SSI alone is lacking, and it is not recommended by the American Diabetes Association.13 Queale et al.14 showed that SSI regimens when used alone were associated with suboptimal glycemic control and a 3‐fold higher risk of hyperglycemic episodes.1 Two retrospective studies have also demonstrated that SSI is less effective and widely variable in comparison with proactive preventative therapy.15, 16 In the current study, 58.5% of patients received SSI alone during the follow‐up period. As indicated in Figure 1, there was a significant increase in mean BG during this time interval. The choice of an inappropriate insulin regimen might be a contributing factor to poor glycemic control.
Because only 38.5% of patients were transitioned to scheduled insulin in our study, one possible strategy to help improve glycemic control would be to transition patients to a scheduled insulin regimen. Umpierrez et al.12 conducted a prospective, multicenter randomized trial to compare the efficacy and safety of a basal‐bolus insulin regimen with that of SSI in hospitalized type 2 diabetics. These authors found that patients treated with insulin glargine and glulisine had greater improvement in glycemic control than those treated with SSI (P < 0.01).12 Interestingly, the basal‐bolus method provides a maintenance insulin regimen that is aggressively titrated upward as well as an adjustable SSI based on insulin sensitivity. Patients in the current study may have benefited from a similar approach as many did not have their scheduled insulin adjusted despite persistent hyperglycemia.
With the increasing evidence for tight glycemic control in the ICU, a standardized transition from an intensive insulin infusion to a subcutaneous basal‐bolus regimen or other scheduled regimen is needed. To date, the current study is the first to describe this transition. Based on these data, recommendations for transitioning patients off an IIP provided by Furnary and Braithwaite17 should be considered by clinicians. In fact, one of their proposed predictors for unsuccessful transition was an insulin requirement of 2 units/hour. Indeed, the only independent risk factor for poor glycemic control identified in the current study was a requirement of >20 units (>1.7 units/hour) during the last 12 hours of the IIP. Further research is required to verify the other predictors suggested by Furnary and Braithwaite. They recommended using a standardized conversion protocol to transition patients off an IIP.
More recently, Kitabchi et al.18 recommended that a BG target of less than 180 mg/dL be maintained for the hospitalized patient.18 Although our study showed a mean BG less than 180 mg/dL during the follow‐up period, the variability in these values raises concerns for individual patients.
The current study is limited by its size and retrospective nature. As with all retrospective studies, the inability to control the implementation and discontinuation of the IIP may confound the results. However, this study demonstrates a real world experience with an IIP and illustrates the difficulties with transitioning patients to a subcutaneous regimen. BG values and administered insulin were collected only for the last 12 hours on the IIP. This duration is considered appropriate as this time period is used clinically at MUH, and previous recommendations for transitioning patients suggest using a time period of 6 to 8 hours to guide the transition insulin regimen.17 In addition, data regarding the severity of illness and new onset of infections were not collected for patients in the study. Both could affect glucose control. All patients had to be in an ICU to receive the IIP, but their location during the follow‐up period varied. Although these data were not available, control of BG is a problem that should be addressed whether the patient is in the ICU or not. Another possible limitation of the study was the identification of patients with or without a past medical history of DM. The inability to identify new‐onset or previously undiagnosed DM may have affected analyses based on this variable.
CONCLUSIONS
This study demonstrated a significant increase in mean BG following discontinuation of an IIP; this corresponded to a significant decrease in the amount of insulin administered. This increase was sustained for a period of at least 5 days. Additionally, an independent risk factor for poor glycemic control immediately following discontinuation of an IIP was an insulin requirement of >1.7 units/hour during the previous 12 hours. Further study into transitioning off an IIP is warranted.
- ,,.Stress‐induced hyperglycemia.Crit Care Clin.2001;17:107–124.
- .Alterations in carbohydrate metabolism during stress: a review of the literature.Am J Med.1995;98:75–84.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79(8):992–1000.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2) effects on mortality and morbidity.Eur Heart J.2005;26:651–661.
- ,,, et al.Intensive insulin therapy in mixed medical/surgical intensive care units.Diabetes.2006;55:3151–3159.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:56–65.
- ,,, et al.Effect of glucose‐insulin‐potassium infusion on mortality in patients with acute ST‐segment elevation myocardial infarction: the CREATE‐ECLA randomized controlled trial.JAMA.2005;293(4):437–446.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
- ,,, et al.,for the American Diabetes Association Professional Practice Committee. Diagnosis and classification of diabetes mellitus.Diabetes Care.2006;29(suppl 1):43–48.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157(5):545–552.
- ,,,,.Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding‐scale insulin therapy.Pharmacotherapy.2006;26(10):1421–1432.
- ,,,,.Efficacy of sliding‐scale insulin therapy: a comparison with prospective regimens.Fam Pract Res J.1994;14(4):313–322.
- ,.Effects of outcome on in‐hospital transition from intravenous insulin infusion to subcutaneous therapy.Am J Cardiol.2006;98:557–564.
- ,,.Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients.Metabolism.2008;57:116–120.
Hyperglycemia and insulin resistance are common occurrences in critically ill patients, even those without a past medical history of diabetes.1, 2 This hyperglycemic state is associated with adverse outcomes, including severe infections, polyneuropathy, multiple‐organ failure, and death.3 Several studies have shown benefit in keeping patients' blood glucose (BG) tightly controlled.37 In a randomized controlled study, strict BG control (80‐110 mg/dL) with an insulin drip significantly reduced morbidity and mortality in critically ill patients.3 A recent meta‐analysis concluded that avoiding BG levels >150 mg/dL appeared to be crucial to reducing mortality in a mixed medical and surgical intensive care unit (ICU) population.7
The Diabetes Mellitus Insulin‐Glucose Infusion in Acute Myocardial Infarction study addressed the issue of tight glycemic control both acutely and chronically in 620 diabetic patients postmyocardial infarction. Patients were randomized to tight glycemic control (126‐180 mg/dL) followed by a transition to maintenance insulin or to standard care. This intervention demonstrated a sustained mortality reduction of 7.5% at 1 year.8 In contrast, the CREATE‐ECLA study showed a neutral mortality benefit of a short‐term (24‐hour) insulin infusion in postmyocardial infarction patients.9 These data demonstrate the need for clinicians to consider insulin requirements throughout the hospital stay and after discharge. To date, there are no published studies evaluating glycemic control following discontinuation of an intensive insulin protocol (IIP). Therefore, the current study was conducted to compare BG control during the use of an IIP and for the 5 days following intensive insulin therapy.
METHODS
Patient Population
This retrospective chart review was conducted at Methodist University Hospital (MUH), Memphis, TN. MUH is a 500‐bed, university‐affiliated tertiary referral hospital. The study was approved by the hospital institutional review board. From January 2006 to January 2007, a computer‐generated pharmacy report was used to identify all patients receiving the hospital‐approved IIP. Patients were included if they were 18 years old and received the IIP for 24 hours. Patients were excluded from the study if they met any of the following criteria: (1) complete BG measurements were not retrievable while the patient received the IIP or for the 5 days following discontinuation of the IIP, (2) the patient died while receiving the IIP, and (3) an endocrinologist was involved in the care of the patient.
IIP
The hospital‐approved IIP is a paper‐based, physician‐initiated, nurse‐managed protocol. Criteria required before initiating the IIP include (1) ICU admission, (2) 2 BG measurements >150 mg/dL, (3) administration of continuous exogenous glucose, and (4) absence of diabetic ketoacidosis. The goal range of the IIP is 80 to 150 mg/dL. Hourly BG measurements are initially required, but as control is achieved, measurements may be extended to every 2 hours and then every 4 hours. In general, the criteria used for transitioning off the IIP include stability during the last 12 hours. Patients were considered to be stable on the IIP if they had >70% of their glucose measurements within the goal range during the last 12 hours.
Data Collection
When inclusion criteria were met, patients' medical records were reviewed. Data collection included basic demographic information, concurrent medications, duration of IIP, amount of insulin administered during the last 12 hours of the IIP, insulin regimen post‐IIP, and BG measurements during the last 12 hours on the IIP and for a total of 5 days after the IIP was stopped (follow‐up period). For this study, hyperglycemia was defined as a BG value >150 mg/dL, significant hyperglycemia was defined as >200 mg/dL, and severe hyperglycemia was defined as >300 mg/dL. Hypoglycemia was defined as a BG value <60 mg/dL. The values of <60 mg/dL, >150 mg/dL, and >200 mg/dL were chosen on the basis of the criteria used in the MUH IIP and standard sliding‐scale protocols. A value of >300 mg/dL was used to better describe patients with hyperglycemia. Poor glycemic control following the IIP was defined as a >30% change in mean BG during the last 12 hours on the drip and on the first day after discontinuation of the drip.
Statistical Analysis
The primary objective of this study was to compare BG control during the last 12 hours of an IIP and for the 5 days following its discontinuation. Secondary objectives were to evaluate the incidence of hyperglycemia and hypoglycemia during the transition period and to identify patients at risk of poor glycemic control following discontinuation of the IIP. Continuous data are appropriately reported as the mean standard deviation or median (interquartile range), depending on the distribution. Continuous variables were compared with the Student t test or Wilcoxon rank sum test. Discrete variables were compared with chi‐square analysis and Bonferroni Correction where appropriate. For comparisons of BG during the IIP and on days 1 to 5 of the follow‐up period, repeated‐measures analysis of variance on ranks was conducted because of the distribution. These statistical analyses were performed with SigmaStat version 2.03 (Systat Software, Inc., Richmond, VA). A P value of less than 0.05 was considered significant. However, when the Bonferroni correction was used, a value of less than 0.01 was considered significant. Multivariable logistic regression was used to determine independent predictors of a greater than 30% change in the mean BG value between the last 12 hours of the IIP and the first day off the insulin drip. Potential independent variables included in the analysis were stability on protocol, requiring less than 20 units of insulin in the last 12 hours on the IIP, use of antibiotics, use of steroids, history of diabetes, and type of insulin to which the patient was transitioned (none, sliding scale, and scheduled and sliding scale). The model was built in a backwards, stepwise fashion with SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
A total of 171 patients received the IIP during the study period. Ninety‐seven patients did not meet inclusion criteria because they received the IIP for less than 24 hours. Of the 74 patients meeting inclusion criteria, 9 were excluded (5 had insufficient glucose data, 3 were cared for by an endocrinologist, and 1 died while receiving the IIP). Thus, 65 patients were included in the study.
Table 1 lists the baseline demographics for all patients and those with and without a history of diabetes mellitus (DM). The majority of the patients (n = 49) underwent a surgical procedure, with the most common procedure being coronary artery bypass graft (n = 38). Patients undergoing coronary artery bypass graft had the IIP included in their standard postoperative order set. The majority of patients were considered stable during the 12 hours prior to discontinuation of the IIP, including 23 patients with a history of DM. Of the 65 patients who were included in the study, 25 (38.5%) received a scheduled insulin order following discontinuation of the IIP, whereas 38 (58.5%) received some form of sliding‐scale insulin (SSI). Additionally, 2 (3%) patients did not receive any form of insulin order after stopping the IIP. Of those receiving scheduled insulin, 15 (60%) received neutral protamine Hagedorn, 5 (20%) received glargine, 5 (20%) received 70/30, and 1 (4%) received regular insulin. Of those receiving SSI only, the prescribed frequency was as follows: every 4 hours for 17 (45%), before meals and at bedtime for 15 (39%), every 6 hours for 5 (13%), and every 2 hours for 1 (3%).
| All Patients (n = 65) | PMH of DM (n = 36) | No PMH of DM (n = 29) | |
|---|---|---|---|
| |||
| Age, mean years SD | 62 11 | 61 10 | 64 12 |
| Male gender, n (%) | 38 (58) | 22 (61) | 16 (55.2) |
| BMI SD | 30 7.2 | 31 7 | 30 6.5 |
| Surgery, n (%) | 49 (74.2) | 27 (75) | 21 (72.4) |
| CABG, n | 38 | 22 | 16 |
| Liver transplant, n | 6 | 1 | 4 |
| Other, n | 5 | 4 | 1 |
| Last 24 hours on IIP, n (%) | |||
| Ventilator | 37 (56.9) | 22 (61.1) | 15 (51.7) |
| Antibiotics | 37 (56.9) | 20 (55.6) | 17 (58.6) |
| Vasopressors | 11 (16.9) | 5 (13.9) | 6 (20.7) |
| Hemodialysis | 8 (12.3) | 5 (13.9) | 3 (10.3) |
| Steroids | 16 (24.6) | 9 (25) | 7 (24.1) |
| Duration of IIP, mean hours SD | 72 65 | 80 78 | 62 45 |
| Insulin during last 12 hours of IIP, mean units SD | 47 37 | 51 30 | 46 45 |
| Type of insulin received following IIP, n (%) | |||
| Scheduled + sliding scale | 25 (38.5) | 19 (52.8) | 6 (20.7) |
| Sliding scale only | 38 (58.5) | 16 (44.4) | 22 (75.9) |
| None | 2 (3) | 1 (2.8) | 1 (3.4) |
| Total daily insulin following IIP, mean units SD | 28 41 | 38 49 | 17 24 |
| Patients stable on IIP | 44 (67.7) | 23 (64.8) | 21 (72.4) |
| Hospital LOS, mean days SD | 24 18 | 24 17 | 23 19 |
| Mortality, n (%) | 15 (23.1) | 5 (13.8) | 10 (34.5) |
A total of 562 glucose measurements were collected during the last 12 hours on the IIP, whereas 201 were collected during the first 12 hours immediately following the IIP. Patients demonstrated a significant increase in BG (mean standard deviation) during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP (168 50 mg/dL versus 123 26 mg/dL, P < 0.001). This corresponded to a significant decrease in the median (interquartile range) insulin administered during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP [8 (0‐18) units versus 40 (22‐65) units, P < 0.001; Figure 1]. A total of 1914 BG measurements were collected during the follow‐up period. Figure 2 shows mean BG values for all patients on the IIP compared to mean BG values for each day of the follow‐up period. There was a significant increase in mean BG measurements when the IIP was compared to each day of the follow‐up period, but there was no difference between days of the follow‐up period. Table 2 shows the proportion of patients experiencing at least 1 episode of hyperglycemia (BG > 150 mg/dL), significant hyperglycemia (BG > 200 mg/dL), severe hyperglycemia (BG > 300 mg/dL), or hypoglycemia (BG < 60 mg/dL) while receiving the IIP and during the follow‐up period. When comparing the IIP to the follow‐up period, we found a significant increase in the proportion of patients with at least 1 BG > 150 mg/dL. This was also true for patients with a BG of > 200 mg/dL.


| IIP (n = 65) | Day 1 (n = 65) | Day 2 (n = 65) | Day 3 (n = 64) | Day 4 (n = 62) | Day 5 (n = 59) | |
|---|---|---|---|---|---|---|
| ||||||
| Patients with >150 mg/dL, n (%) | 33 (51) | 54 (83)* | 54 (83)* | 52 (81)* | 51 (82)* | 48 (81)* |
| Patients with >200 mg/dL, n (%) | 11 (17) | 37 (57)* | 31 (48)* | 26 (41)* | 33 (53)* | 34 (58)* |
| Patients with >300 mg/dL, n (%) | 2 (3) | 11 (17) | 7 (11) | 8 (12) | 5 (8) | 10 (17) |
| Patients with <60 mg/dL, n (%) | 6 (9) | 5 (8) | 2 (3) | 2 (3) | 2 (3) | 0 (0) |
The only independent predictor of a greater than 30% change in mean BG was the requirement for more than 20 units of insulin (>1.7 units/hour) during the last 12 hours on the IIP. The odds of a greater than 30% change was 4.62 times higher (95% confidence interval: 1.1718.17) in patients requiring more than 20 units during the last 12 hours on IIP after adjustments for stability on the protocol and past medical history of diabetes. Stability on the protocol was not identified as an independent predictor, with an adjusted odds ratio of 2.40 (95% confidence interval: 0.797.32).
DISCUSSION
This is the first study to describe glycemic control following the transition from an IIP to subcutaneous insulin. We observed that during the 5 days following discontinuation of an IIP, patients had significantly elevated mean BG values. These data are highlighted by the fact that patients received significantly less insulin during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP. Additionally, a larger than expected proportion of patients exhibited at least 1 episode of hyperglycemia during the follow‐up period. We also found that an increased insulin requirement of >1.7 units/hour during the last 12 hours of the IIP was an independent risk factor for a greater than 30% increase in mean BG on day 1 of the follow‐up period.
Increasing evidence demonstrates that the development of hyperglycemia in the hospital setting is a marker of poor clinical outcome and mortality. In fact, hyperglycemia has been associated with prolonged hospital stay, infection, disability after discharge, and death in patients on general surgical and medical wards.1012 This makes the increase in mean BG found in our study following discontinuation of the IIP a concern.
SSI with subcutaneous short‐acting insulin has been used for inpatients as the standard of care for many years. However, evidence supporting the effectiveness of SSI alone is lacking, and it is not recommended by the American Diabetes Association.13 Queale et al.14 showed that SSI regimens when used alone were associated with suboptimal glycemic control and a 3‐fold higher risk of hyperglycemic episodes.1 Two retrospective studies have also demonstrated that SSI is less effective and widely variable in comparison with proactive preventative therapy.15, 16 In the current study, 58.5% of patients received SSI alone during the follow‐up period. As indicated in Figure 1, there was a significant increase in mean BG during this time interval. The choice of an inappropriate insulin regimen might be a contributing factor to poor glycemic control.
Because only 38.5% of patients were transitioned to scheduled insulin in our study, one possible strategy to help improve glycemic control would be to transition patients to a scheduled insulin regimen. Umpierrez et al.12 conducted a prospective, multicenter randomized trial to compare the efficacy and safety of a basal‐bolus insulin regimen with that of SSI in hospitalized type 2 diabetics. These authors found that patients treated with insulin glargine and glulisine had greater improvement in glycemic control than those treated with SSI (P < 0.01).12 Interestingly, the basal‐bolus method provides a maintenance insulin regimen that is aggressively titrated upward as well as an adjustable SSI based on insulin sensitivity. Patients in the current study may have benefited from a similar approach as many did not have their scheduled insulin adjusted despite persistent hyperglycemia.
With the increasing evidence for tight glycemic control in the ICU, a standardized transition from an intensive insulin infusion to a subcutaneous basal‐bolus regimen or other scheduled regimen is needed. To date, the current study is the first to describe this transition. Based on these data, recommendations for transitioning patients off an IIP provided by Furnary and Braithwaite17 should be considered by clinicians. In fact, one of their proposed predictors for unsuccessful transition was an insulin requirement of 2 units/hour. Indeed, the only independent risk factor for poor glycemic control identified in the current study was a requirement of >20 units (>1.7 units/hour) during the last 12 hours of the IIP. Further research is required to verify the other predictors suggested by Furnary and Braithwaite. They recommended using a standardized conversion protocol to transition patients off an IIP.
More recently, Kitabchi et al.18 recommended that a BG target of less than 180 mg/dL be maintained for the hospitalized patient.18 Although our study showed a mean BG less than 180 mg/dL during the follow‐up period, the variability in these values raises concerns for individual patients.
The current study is limited by its size and retrospective nature. As with all retrospective studies, the inability to control the implementation and discontinuation of the IIP may confound the results. However, this study demonstrates a real world experience with an IIP and illustrates the difficulties with transitioning patients to a subcutaneous regimen. BG values and administered insulin were collected only for the last 12 hours on the IIP. This duration is considered appropriate as this time period is used clinically at MUH, and previous recommendations for transitioning patients suggest using a time period of 6 to 8 hours to guide the transition insulin regimen.17 In addition, data regarding the severity of illness and new onset of infections were not collected for patients in the study. Both could affect glucose control. All patients had to be in an ICU to receive the IIP, but their location during the follow‐up period varied. Although these data were not available, control of BG is a problem that should be addressed whether the patient is in the ICU or not. Another possible limitation of the study was the identification of patients with or without a past medical history of DM. The inability to identify new‐onset or previously undiagnosed DM may have affected analyses based on this variable.
CONCLUSIONS
This study demonstrated a significant increase in mean BG following discontinuation of an IIP; this corresponded to a significant decrease in the amount of insulin administered. This increase was sustained for a period of at least 5 days. Additionally, an independent risk factor for poor glycemic control immediately following discontinuation of an IIP was an insulin requirement of >1.7 units/hour during the previous 12 hours. Further study into transitioning off an IIP is warranted.
Hyperglycemia and insulin resistance are common occurrences in critically ill patients, even those without a past medical history of diabetes.1, 2 This hyperglycemic state is associated with adverse outcomes, including severe infections, polyneuropathy, multiple‐organ failure, and death.3 Several studies have shown benefit in keeping patients' blood glucose (BG) tightly controlled.37 In a randomized controlled study, strict BG control (80‐110 mg/dL) with an insulin drip significantly reduced morbidity and mortality in critically ill patients.3 A recent meta‐analysis concluded that avoiding BG levels >150 mg/dL appeared to be crucial to reducing mortality in a mixed medical and surgical intensive care unit (ICU) population.7
The Diabetes Mellitus Insulin‐Glucose Infusion in Acute Myocardial Infarction study addressed the issue of tight glycemic control both acutely and chronically in 620 diabetic patients postmyocardial infarction. Patients were randomized to tight glycemic control (126‐180 mg/dL) followed by a transition to maintenance insulin or to standard care. This intervention demonstrated a sustained mortality reduction of 7.5% at 1 year.8 In contrast, the CREATE‐ECLA study showed a neutral mortality benefit of a short‐term (24‐hour) insulin infusion in postmyocardial infarction patients.9 These data demonstrate the need for clinicians to consider insulin requirements throughout the hospital stay and after discharge. To date, there are no published studies evaluating glycemic control following discontinuation of an intensive insulin protocol (IIP). Therefore, the current study was conducted to compare BG control during the use of an IIP and for the 5 days following intensive insulin therapy.
METHODS
Patient Population
This retrospective chart review was conducted at Methodist University Hospital (MUH), Memphis, TN. MUH is a 500‐bed, university‐affiliated tertiary referral hospital. The study was approved by the hospital institutional review board. From January 2006 to January 2007, a computer‐generated pharmacy report was used to identify all patients receiving the hospital‐approved IIP. Patients were included if they were 18 years old and received the IIP for 24 hours. Patients were excluded from the study if they met any of the following criteria: (1) complete BG measurements were not retrievable while the patient received the IIP or for the 5 days following discontinuation of the IIP, (2) the patient died while receiving the IIP, and (3) an endocrinologist was involved in the care of the patient.
IIP
The hospital‐approved IIP is a paper‐based, physician‐initiated, nurse‐managed protocol. Criteria required before initiating the IIP include (1) ICU admission, (2) 2 BG measurements >150 mg/dL, (3) administration of continuous exogenous glucose, and (4) absence of diabetic ketoacidosis. The goal range of the IIP is 80 to 150 mg/dL. Hourly BG measurements are initially required, but as control is achieved, measurements may be extended to every 2 hours and then every 4 hours. In general, the criteria used for transitioning off the IIP include stability during the last 12 hours. Patients were considered to be stable on the IIP if they had >70% of their glucose measurements within the goal range during the last 12 hours.
Data Collection
When inclusion criteria were met, patients' medical records were reviewed. Data collection included basic demographic information, concurrent medications, duration of IIP, amount of insulin administered during the last 12 hours of the IIP, insulin regimen post‐IIP, and BG measurements during the last 12 hours on the IIP and for a total of 5 days after the IIP was stopped (follow‐up period). For this study, hyperglycemia was defined as a BG value >150 mg/dL, significant hyperglycemia was defined as >200 mg/dL, and severe hyperglycemia was defined as >300 mg/dL. Hypoglycemia was defined as a BG value <60 mg/dL. The values of <60 mg/dL, >150 mg/dL, and >200 mg/dL were chosen on the basis of the criteria used in the MUH IIP and standard sliding‐scale protocols. A value of >300 mg/dL was used to better describe patients with hyperglycemia. Poor glycemic control following the IIP was defined as a >30% change in mean BG during the last 12 hours on the drip and on the first day after discontinuation of the drip.
Statistical Analysis
The primary objective of this study was to compare BG control during the last 12 hours of an IIP and for the 5 days following its discontinuation. Secondary objectives were to evaluate the incidence of hyperglycemia and hypoglycemia during the transition period and to identify patients at risk of poor glycemic control following discontinuation of the IIP. Continuous data are appropriately reported as the mean standard deviation or median (interquartile range), depending on the distribution. Continuous variables were compared with the Student t test or Wilcoxon rank sum test. Discrete variables were compared with chi‐square analysis and Bonferroni Correction where appropriate. For comparisons of BG during the IIP and on days 1 to 5 of the follow‐up period, repeated‐measures analysis of variance on ranks was conducted because of the distribution. These statistical analyses were performed with SigmaStat version 2.03 (Systat Software, Inc., Richmond, VA). A P value of less than 0.05 was considered significant. However, when the Bonferroni correction was used, a value of less than 0.01 was considered significant. Multivariable logistic regression was used to determine independent predictors of a greater than 30% change in the mean BG value between the last 12 hours of the IIP and the first day off the insulin drip. Potential independent variables included in the analysis were stability on protocol, requiring less than 20 units of insulin in the last 12 hours on the IIP, use of antibiotics, use of steroids, history of diabetes, and type of insulin to which the patient was transitioned (none, sliding scale, and scheduled and sliding scale). The model was built in a backwards, stepwise fashion with SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
A total of 171 patients received the IIP during the study period. Ninety‐seven patients did not meet inclusion criteria because they received the IIP for less than 24 hours. Of the 74 patients meeting inclusion criteria, 9 were excluded (5 had insufficient glucose data, 3 were cared for by an endocrinologist, and 1 died while receiving the IIP). Thus, 65 patients were included in the study.
Table 1 lists the baseline demographics for all patients and those with and without a history of diabetes mellitus (DM). The majority of the patients (n = 49) underwent a surgical procedure, with the most common procedure being coronary artery bypass graft (n = 38). Patients undergoing coronary artery bypass graft had the IIP included in their standard postoperative order set. The majority of patients were considered stable during the 12 hours prior to discontinuation of the IIP, including 23 patients with a history of DM. Of the 65 patients who were included in the study, 25 (38.5%) received a scheduled insulin order following discontinuation of the IIP, whereas 38 (58.5%) received some form of sliding‐scale insulin (SSI). Additionally, 2 (3%) patients did not receive any form of insulin order after stopping the IIP. Of those receiving scheduled insulin, 15 (60%) received neutral protamine Hagedorn, 5 (20%) received glargine, 5 (20%) received 70/30, and 1 (4%) received regular insulin. Of those receiving SSI only, the prescribed frequency was as follows: every 4 hours for 17 (45%), before meals and at bedtime for 15 (39%), every 6 hours for 5 (13%), and every 2 hours for 1 (3%).
| All Patients (n = 65) | PMH of DM (n = 36) | No PMH of DM (n = 29) | |
|---|---|---|---|
| |||
| Age, mean years SD | 62 11 | 61 10 | 64 12 |
| Male gender, n (%) | 38 (58) | 22 (61) | 16 (55.2) |
| BMI SD | 30 7.2 | 31 7 | 30 6.5 |
| Surgery, n (%) | 49 (74.2) | 27 (75) | 21 (72.4) |
| CABG, n | 38 | 22 | 16 |
| Liver transplant, n | 6 | 1 | 4 |
| Other, n | 5 | 4 | 1 |
| Last 24 hours on IIP, n (%) | |||
| Ventilator | 37 (56.9) | 22 (61.1) | 15 (51.7) |
| Antibiotics | 37 (56.9) | 20 (55.6) | 17 (58.6) |
| Vasopressors | 11 (16.9) | 5 (13.9) | 6 (20.7) |
| Hemodialysis | 8 (12.3) | 5 (13.9) | 3 (10.3) |
| Steroids | 16 (24.6) | 9 (25) | 7 (24.1) |
| Duration of IIP, mean hours SD | 72 65 | 80 78 | 62 45 |
| Insulin during last 12 hours of IIP, mean units SD | 47 37 | 51 30 | 46 45 |
| Type of insulin received following IIP, n (%) | |||
| Scheduled + sliding scale | 25 (38.5) | 19 (52.8) | 6 (20.7) |
| Sliding scale only | 38 (58.5) | 16 (44.4) | 22 (75.9) |
| None | 2 (3) | 1 (2.8) | 1 (3.4) |
| Total daily insulin following IIP, mean units SD | 28 41 | 38 49 | 17 24 |
| Patients stable on IIP | 44 (67.7) | 23 (64.8) | 21 (72.4) |
| Hospital LOS, mean days SD | 24 18 | 24 17 | 23 19 |
| Mortality, n (%) | 15 (23.1) | 5 (13.8) | 10 (34.5) |
A total of 562 glucose measurements were collected during the last 12 hours on the IIP, whereas 201 were collected during the first 12 hours immediately following the IIP. Patients demonstrated a significant increase in BG (mean standard deviation) during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP (168 50 mg/dL versus 123 26 mg/dL, P < 0.001). This corresponded to a significant decrease in the median (interquartile range) insulin administered during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP [8 (0‐18) units versus 40 (22‐65) units, P < 0.001; Figure 1]. A total of 1914 BG measurements were collected during the follow‐up period. Figure 2 shows mean BG values for all patients on the IIP compared to mean BG values for each day of the follow‐up period. There was a significant increase in mean BG measurements when the IIP was compared to each day of the follow‐up period, but there was no difference between days of the follow‐up period. Table 2 shows the proportion of patients experiencing at least 1 episode of hyperglycemia (BG > 150 mg/dL), significant hyperglycemia (BG > 200 mg/dL), severe hyperglycemia (BG > 300 mg/dL), or hypoglycemia (BG < 60 mg/dL) while receiving the IIP and during the follow‐up period. When comparing the IIP to the follow‐up period, we found a significant increase in the proportion of patients with at least 1 BG > 150 mg/dL. This was also true for patients with a BG of > 200 mg/dL.


| IIP (n = 65) | Day 1 (n = 65) | Day 2 (n = 65) | Day 3 (n = 64) | Day 4 (n = 62) | Day 5 (n = 59) | |
|---|---|---|---|---|---|---|
| ||||||
| Patients with >150 mg/dL, n (%) | 33 (51) | 54 (83)* | 54 (83)* | 52 (81)* | 51 (82)* | 48 (81)* |
| Patients with >200 mg/dL, n (%) | 11 (17) | 37 (57)* | 31 (48)* | 26 (41)* | 33 (53)* | 34 (58)* |
| Patients with >300 mg/dL, n (%) | 2 (3) | 11 (17) | 7 (11) | 8 (12) | 5 (8) | 10 (17) |
| Patients with <60 mg/dL, n (%) | 6 (9) | 5 (8) | 2 (3) | 2 (3) | 2 (3) | 0 (0) |
The only independent predictor of a greater than 30% change in mean BG was the requirement for more than 20 units of insulin (>1.7 units/hour) during the last 12 hours on the IIP. The odds of a greater than 30% change was 4.62 times higher (95% confidence interval: 1.1718.17) in patients requiring more than 20 units during the last 12 hours on IIP after adjustments for stability on the protocol and past medical history of diabetes. Stability on the protocol was not identified as an independent predictor, with an adjusted odds ratio of 2.40 (95% confidence interval: 0.797.32).
DISCUSSION
This is the first study to describe glycemic control following the transition from an IIP to subcutaneous insulin. We observed that during the 5 days following discontinuation of an IIP, patients had significantly elevated mean BG values. These data are highlighted by the fact that patients received significantly less insulin during the first 12 hours of the follow‐up period versus the last 12 hours of the IIP. Additionally, a larger than expected proportion of patients exhibited at least 1 episode of hyperglycemia during the follow‐up period. We also found that an increased insulin requirement of >1.7 units/hour during the last 12 hours of the IIP was an independent risk factor for a greater than 30% increase in mean BG on day 1 of the follow‐up period.
Increasing evidence demonstrates that the development of hyperglycemia in the hospital setting is a marker of poor clinical outcome and mortality. In fact, hyperglycemia has been associated with prolonged hospital stay, infection, disability after discharge, and death in patients on general surgical and medical wards.1012 This makes the increase in mean BG found in our study following discontinuation of the IIP a concern.
SSI with subcutaneous short‐acting insulin has been used for inpatients as the standard of care for many years. However, evidence supporting the effectiveness of SSI alone is lacking, and it is not recommended by the American Diabetes Association.13 Queale et al.14 showed that SSI regimens when used alone were associated with suboptimal glycemic control and a 3‐fold higher risk of hyperglycemic episodes.1 Two retrospective studies have also demonstrated that SSI is less effective and widely variable in comparison with proactive preventative therapy.15, 16 In the current study, 58.5% of patients received SSI alone during the follow‐up period. As indicated in Figure 1, there was a significant increase in mean BG during this time interval. The choice of an inappropriate insulin regimen might be a contributing factor to poor glycemic control.
Because only 38.5% of patients were transitioned to scheduled insulin in our study, one possible strategy to help improve glycemic control would be to transition patients to a scheduled insulin regimen. Umpierrez et al.12 conducted a prospective, multicenter randomized trial to compare the efficacy and safety of a basal‐bolus insulin regimen with that of SSI in hospitalized type 2 diabetics. These authors found that patients treated with insulin glargine and glulisine had greater improvement in glycemic control than those treated with SSI (P < 0.01).12 Interestingly, the basal‐bolus method provides a maintenance insulin regimen that is aggressively titrated upward as well as an adjustable SSI based on insulin sensitivity. Patients in the current study may have benefited from a similar approach as many did not have their scheduled insulin adjusted despite persistent hyperglycemia.
With the increasing evidence for tight glycemic control in the ICU, a standardized transition from an intensive insulin infusion to a subcutaneous basal‐bolus regimen or other scheduled regimen is needed. To date, the current study is the first to describe this transition. Based on these data, recommendations for transitioning patients off an IIP provided by Furnary and Braithwaite17 should be considered by clinicians. In fact, one of their proposed predictors for unsuccessful transition was an insulin requirement of 2 units/hour. Indeed, the only independent risk factor for poor glycemic control identified in the current study was a requirement of >20 units (>1.7 units/hour) during the last 12 hours of the IIP. Further research is required to verify the other predictors suggested by Furnary and Braithwaite. They recommended using a standardized conversion protocol to transition patients off an IIP.
More recently, Kitabchi et al.18 recommended that a BG target of less than 180 mg/dL be maintained for the hospitalized patient.18 Although our study showed a mean BG less than 180 mg/dL during the follow‐up period, the variability in these values raises concerns for individual patients.
The current study is limited by its size and retrospective nature. As with all retrospective studies, the inability to control the implementation and discontinuation of the IIP may confound the results. However, this study demonstrates a real world experience with an IIP and illustrates the difficulties with transitioning patients to a subcutaneous regimen. BG values and administered insulin were collected only for the last 12 hours on the IIP. This duration is considered appropriate as this time period is used clinically at MUH, and previous recommendations for transitioning patients suggest using a time period of 6 to 8 hours to guide the transition insulin regimen.17 In addition, data regarding the severity of illness and new onset of infections were not collected for patients in the study. Both could affect glucose control. All patients had to be in an ICU to receive the IIP, but their location during the follow‐up period varied. Although these data were not available, control of BG is a problem that should be addressed whether the patient is in the ICU or not. Another possible limitation of the study was the identification of patients with or without a past medical history of DM. The inability to identify new‐onset or previously undiagnosed DM may have affected analyses based on this variable.
CONCLUSIONS
This study demonstrated a significant increase in mean BG following discontinuation of an IIP; this corresponded to a significant decrease in the amount of insulin administered. This increase was sustained for a period of at least 5 days. Additionally, an independent risk factor for poor glycemic control immediately following discontinuation of an IIP was an insulin requirement of >1.7 units/hour during the previous 12 hours. Further study into transitioning off an IIP is warranted.
- ,,.Stress‐induced hyperglycemia.Crit Care Clin.2001;17:107–124.
- .Alterations in carbohydrate metabolism during stress: a review of the literature.Am J Med.1995;98:75–84.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79(8):992–1000.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2) effects on mortality and morbidity.Eur Heart J.2005;26:651–661.
- ,,, et al.Intensive insulin therapy in mixed medical/surgical intensive care units.Diabetes.2006;55:3151–3159.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:56–65.
- ,,, et al.Effect of glucose‐insulin‐potassium infusion on mortality in patients with acute ST‐segment elevation myocardial infarction: the CREATE‐ECLA randomized controlled trial.JAMA.2005;293(4):437–446.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
- ,,, et al.,for the American Diabetes Association Professional Practice Committee. Diagnosis and classification of diabetes mellitus.Diabetes Care.2006;29(suppl 1):43–48.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157(5):545–552.
- ,,,,.Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding‐scale insulin therapy.Pharmacotherapy.2006;26(10):1421–1432.
- ,,,,.Efficacy of sliding‐scale insulin therapy: a comparison with prospective regimens.Fam Pract Res J.1994;14(4):313–322.
- ,.Effects of outcome on in‐hospital transition from intravenous insulin infusion to subcutaneous therapy.Am J Cardiol.2006;98:557–564.
- ,,.Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients.Metabolism.2008;57:116–120.
- ,,.Stress‐induced hyperglycemia.Crit Care Clin.2001;17:107–124.
- .Alterations in carbohydrate metabolism during stress: a review of the literature.Am J Med.1995;98:75–84.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79(8):992–1000.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2) effects on mortality and morbidity.Eur Heart J.2005;26:651–661.
- ,,, et al.Intensive insulin therapy in mixed medical/surgical intensive care units.Diabetes.2006;55:3151–3159.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:56–65.
- ,,, et al.Effect of glucose‐insulin‐potassium infusion on mortality in patients with acute ST‐segment elevation myocardial infarction: the CREATE‐ECLA randomized controlled trial.JAMA.2005;293(4):437–446.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
- ,,, et al.,for the American Diabetes Association Professional Practice Committee. Diagnosis and classification of diabetes mellitus.Diabetes Care.2006;29(suppl 1):43–48.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157(5):545–552.
- ,,,,.Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding‐scale insulin therapy.Pharmacotherapy.2006;26(10):1421–1432.
- ,,,,.Efficacy of sliding‐scale insulin therapy: a comparison with prospective regimens.Fam Pract Res J.1994;14(4):313–322.
- ,.Effects of outcome on in‐hospital transition from intravenous insulin infusion to subcutaneous therapy.Am J Cardiol.2006;98:557–564.
- ,,.Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients.Metabolism.2008;57:116–120.
Copyright © 2009 Society of Hospital Medicine
Pericardial Effusion
Eight days prior to admission to our hospital, a 50‐year‐old morbidly obese patient presented to his primary care clinic, complaining of weakness, dizziness, and symptoms consistent with paroxysmal nocturnal dyspnea. He had a history of diabetes mellitus type 2, hypertension, chronic renal insufficiency with baseline creatinine in the range of 2.0 mg/dL, and atrial fibrillation on warfarin (10 mg/day). He was given diuretics and sent home. Two days later, the patient presented to a rural emergency department, complaining of leg pain and swelling. He was given cephalexin for cellulitis and discharged home. The evening prior to admission to our hospital, the patient developed sharp left‐sided chest pain, orthopnea, and worsening dyspnea. He again presented to the rural emergency department and was found to have a blood pressure of 60/40 mm Hg. He was admitted and given boluses of intravenous fluids and a dose of ceftriaxone. Laboratory studies at that time demonstrated the following: sodium, 130 mEq/L; potassium, 6.5 mEq/L; CO2, 21 mEq/L; blood urea nitrogen (BUN), 105 mg/dL; creatinine, 5.5 mg/dL; alkaline phosphatase, 330 IU/L; aspartate aminotransferase (AST), 507 IU/L; and alanine aminotransferase (ALT), 145 IU/L. The following morning, he was oliguric, and his liver transaminases had increased to an AST level of 1862 IU/L and an ALT level of 1055 IU/L. At this point, he was transferred to our hospital for hemodialysis with a diagnosis of acute oliguric renal failure.
On admission to our hospital, the patient was afebrile, had a blood pressure of 112/80 mm Hg, and was hypoxic with an O2 saturation of 93% on 4 L of oxygen by nasal cannula. His weight was 201 kg. He was alert and cooperative. Heart sounds were distant but had a regular rate and rhythm without murmurs, rubs, or gallops. No jugular venous pulsations were appreciated. He had decreased lung sounds throughout both lung fields. His abdomen was morbidly obese and nontender. Extremities demonstrated chronic venous stasis changes with multiple superficial ulcers and bilateral pitting edema.
In light of the elevated liver function tests reported by the referring hospital, these studies were repeated on arrival, revealing an AST level of 4780 IU/L and an ALT level of 1876 IU/L. Other initial laboratory studies demonstrated a BUN level of 116 mg/dL, a creatinine level of 6 mg/dL, a potassium level of 7.2 mEq/L, negative cardiac enzymes, and an international normalized ratio greater than 9.0. A urinalysis could not be performed because the patient was anuric. An electrocardiogram demonstrated a normal sinus rhythm with a right bundle branch block and nonspecific ST segment and T wave changes. There was no prior electrocardiogram available for comparison. Blood acetaminophen level and infectious hepatitis panels were negative.
Within 1 hour of admission, the patient became hypotensive, with his systolic blood pressure decreasing into the 70s. Over the next 24 hours, the patient was treated with intravenous fluids and pressors (neosynephrine) but remained hypotensive, with his systolic blood pressure in the range of 64 to 80 mm Hg.
At 14 hours after admission, liver function tests were repeated and revealed further elevation of his transaminases (AST, 5135 IU/L; ALT, 2468 IU/L). The diagnosis of shock liver was entertained. A portable chest X‐ray (CXR) demonstrated an enlarged cardiac silhouette suggestive of cardiomegaly or pericardial effusion. No prior CXR was available for comparison. An echocardiogram showed probable effusion but was not diagnostic secondary to his body habitus and poor windows. A computed tomography (CT) scan confirmed a large pericardial effusion. This finding, in combination with diminished heart sounds and hypotension, presented a clinical picture consistent with cardiac tamponade. The patient underwent a subxiphoid pericardial window, and 1.5 L of bloody effusion was removed. Intraoperatively, his systolic blood pressure immediately improved from 95 to 135 mm Hg. Urine output increased from 0 to 80 mL/hour in the first hour. Five days later, his creatinine returned to his baseline of 1.9 mg/dL, and other laboratory values had decreased: AST, 306 IU/L; ALT, 520 IU/L; alkaline phosphatase, 122 IU/L; and BUN, 86 mg/dL.
DISCUSSION
Acute renal failure (ARF) is a common condition, with an incidence of 1% on admission to the hospital; 70% of these cases are due to prerenal causes.1 Among patients already in the hospital, prerenal azotemia is responsible for 21% to 39% of cases of ARF.2, 3 Prerenal ARF is most commonly caused by hypotension, which may be cardiogenic, hypovolemic, septic, or due to vasodilatation. Adrenal insufficiency and other etiologies should also be considered. In our patient, chronic renal failure and superimposed hypotension contributed to acute oliguric renal failure. In searching for the etiology of his hypotension, we concentrated on cardiogenic causes in view of his enlarged cardiac silhouette.
In considering the cardiogenic causes of hypotension, we did not initially focus on tamponade. Although oliguria with renal failure is recognized as a complication of cardiac tamponade, relatively little has been written about ARF as the presenting problem in tamponade. The literature is limited to a few case reports, such as those described by Queffeulou et al.4 and Saklayen et al.5 In our patient, the diagnosis of tamponade was made more difficult by the patient's large size, which made the interpretation of physical findings such as heart sounds more difficult and compromised the quality of essential imaging studies.
His rising transaminases suggested shock liver and eventually provided the key to the patient's diagnosis. In light of his worsening liver function tests, chest pain, shortness of breath, and cardiomegaly on CXR, we focused on a cardiac etiology. An echocardiogram, which often can be used to identify pericardial effusion and hence tamponade, was nondiagnostic because of the patient's obesity. Despite the concerns regarding the weight limits of the examination table, the diagnosis was finally established with a CT scan.
In our patient, no definite cause for his bloody pericardial effusion was found. Cardiac enzymes were negative, and this helped rule out ischemia. Nothing indicative of a neoplasm was seen on the CT of his chest. Blood cultures were negative. Viral studies were not performed, and the effusion was not sent for special studies. He may have had an underlying pericarditis secondary to his chronic kidney disease or a viral syndrome that, combined with his anticoagulation treatment, resulted in a bloody effusion.
In addition to illustrating the difficulties in properly diagnosing acutely ill morbidly obese patients, this report also demonstrates that in patients with shock liver and ARF, pericardial tamponade may be the culprit. If preliminary studies including CXR and echocardiogram are equivocal and sufficient suspicion remains, then a CT is warranted.
- ,,,.Community‐acquired acute renal failure.Am J Kidney Dis.1991;17:191–198.
- ,.Epidemiology of acute renal failure: a prospective, multicenter, community‐based study. Madrid Acute Renal Failure Study Group.Kidney Int.1996;50(3):811–818.
- ,,.Hospital‐acquired renal insufficiency.Am J Kidney Dis.2002;39:930–936.
- ,,.Acute renal and hepatic failure: do not miss pericardial tamponade!Nephrol Dial Transplant.1999;14(9):2260.
- ,,.Pericardial effusion leading to acute renal failure: two case reports and discussion of pathophysiology.Am J Kidney Dis.2002;40(4):837–841.
Eight days prior to admission to our hospital, a 50‐year‐old morbidly obese patient presented to his primary care clinic, complaining of weakness, dizziness, and symptoms consistent with paroxysmal nocturnal dyspnea. He had a history of diabetes mellitus type 2, hypertension, chronic renal insufficiency with baseline creatinine in the range of 2.0 mg/dL, and atrial fibrillation on warfarin (10 mg/day). He was given diuretics and sent home. Two days later, the patient presented to a rural emergency department, complaining of leg pain and swelling. He was given cephalexin for cellulitis and discharged home. The evening prior to admission to our hospital, the patient developed sharp left‐sided chest pain, orthopnea, and worsening dyspnea. He again presented to the rural emergency department and was found to have a blood pressure of 60/40 mm Hg. He was admitted and given boluses of intravenous fluids and a dose of ceftriaxone. Laboratory studies at that time demonstrated the following: sodium, 130 mEq/L; potassium, 6.5 mEq/L; CO2, 21 mEq/L; blood urea nitrogen (BUN), 105 mg/dL; creatinine, 5.5 mg/dL; alkaline phosphatase, 330 IU/L; aspartate aminotransferase (AST), 507 IU/L; and alanine aminotransferase (ALT), 145 IU/L. The following morning, he was oliguric, and his liver transaminases had increased to an AST level of 1862 IU/L and an ALT level of 1055 IU/L. At this point, he was transferred to our hospital for hemodialysis with a diagnosis of acute oliguric renal failure.
On admission to our hospital, the patient was afebrile, had a blood pressure of 112/80 mm Hg, and was hypoxic with an O2 saturation of 93% on 4 L of oxygen by nasal cannula. His weight was 201 kg. He was alert and cooperative. Heart sounds were distant but had a regular rate and rhythm without murmurs, rubs, or gallops. No jugular venous pulsations were appreciated. He had decreased lung sounds throughout both lung fields. His abdomen was morbidly obese and nontender. Extremities demonstrated chronic venous stasis changes with multiple superficial ulcers and bilateral pitting edema.
In light of the elevated liver function tests reported by the referring hospital, these studies were repeated on arrival, revealing an AST level of 4780 IU/L and an ALT level of 1876 IU/L. Other initial laboratory studies demonstrated a BUN level of 116 mg/dL, a creatinine level of 6 mg/dL, a potassium level of 7.2 mEq/L, negative cardiac enzymes, and an international normalized ratio greater than 9.0. A urinalysis could not be performed because the patient was anuric. An electrocardiogram demonstrated a normal sinus rhythm with a right bundle branch block and nonspecific ST segment and T wave changes. There was no prior electrocardiogram available for comparison. Blood acetaminophen level and infectious hepatitis panels were negative.
Within 1 hour of admission, the patient became hypotensive, with his systolic blood pressure decreasing into the 70s. Over the next 24 hours, the patient was treated with intravenous fluids and pressors (neosynephrine) but remained hypotensive, with his systolic blood pressure in the range of 64 to 80 mm Hg.
At 14 hours after admission, liver function tests were repeated and revealed further elevation of his transaminases (AST, 5135 IU/L; ALT, 2468 IU/L). The diagnosis of shock liver was entertained. A portable chest X‐ray (CXR) demonstrated an enlarged cardiac silhouette suggestive of cardiomegaly or pericardial effusion. No prior CXR was available for comparison. An echocardiogram showed probable effusion but was not diagnostic secondary to his body habitus and poor windows. A computed tomography (CT) scan confirmed a large pericardial effusion. This finding, in combination with diminished heart sounds and hypotension, presented a clinical picture consistent with cardiac tamponade. The patient underwent a subxiphoid pericardial window, and 1.5 L of bloody effusion was removed. Intraoperatively, his systolic blood pressure immediately improved from 95 to 135 mm Hg. Urine output increased from 0 to 80 mL/hour in the first hour. Five days later, his creatinine returned to his baseline of 1.9 mg/dL, and other laboratory values had decreased: AST, 306 IU/L; ALT, 520 IU/L; alkaline phosphatase, 122 IU/L; and BUN, 86 mg/dL.
DISCUSSION
Acute renal failure (ARF) is a common condition, with an incidence of 1% on admission to the hospital; 70% of these cases are due to prerenal causes.1 Among patients already in the hospital, prerenal azotemia is responsible for 21% to 39% of cases of ARF.2, 3 Prerenal ARF is most commonly caused by hypotension, which may be cardiogenic, hypovolemic, septic, or due to vasodilatation. Adrenal insufficiency and other etiologies should also be considered. In our patient, chronic renal failure and superimposed hypotension contributed to acute oliguric renal failure. In searching for the etiology of his hypotension, we concentrated on cardiogenic causes in view of his enlarged cardiac silhouette.
In considering the cardiogenic causes of hypotension, we did not initially focus on tamponade. Although oliguria with renal failure is recognized as a complication of cardiac tamponade, relatively little has been written about ARF as the presenting problem in tamponade. The literature is limited to a few case reports, such as those described by Queffeulou et al.4 and Saklayen et al.5 In our patient, the diagnosis of tamponade was made more difficult by the patient's large size, which made the interpretation of physical findings such as heart sounds more difficult and compromised the quality of essential imaging studies.
His rising transaminases suggested shock liver and eventually provided the key to the patient's diagnosis. In light of his worsening liver function tests, chest pain, shortness of breath, and cardiomegaly on CXR, we focused on a cardiac etiology. An echocardiogram, which often can be used to identify pericardial effusion and hence tamponade, was nondiagnostic because of the patient's obesity. Despite the concerns regarding the weight limits of the examination table, the diagnosis was finally established with a CT scan.
In our patient, no definite cause for his bloody pericardial effusion was found. Cardiac enzymes were negative, and this helped rule out ischemia. Nothing indicative of a neoplasm was seen on the CT of his chest. Blood cultures were negative. Viral studies were not performed, and the effusion was not sent for special studies. He may have had an underlying pericarditis secondary to his chronic kidney disease or a viral syndrome that, combined with his anticoagulation treatment, resulted in a bloody effusion.
In addition to illustrating the difficulties in properly diagnosing acutely ill morbidly obese patients, this report also demonstrates that in patients with shock liver and ARF, pericardial tamponade may be the culprit. If preliminary studies including CXR and echocardiogram are equivocal and sufficient suspicion remains, then a CT is warranted.
Eight days prior to admission to our hospital, a 50‐year‐old morbidly obese patient presented to his primary care clinic, complaining of weakness, dizziness, and symptoms consistent with paroxysmal nocturnal dyspnea. He had a history of diabetes mellitus type 2, hypertension, chronic renal insufficiency with baseline creatinine in the range of 2.0 mg/dL, and atrial fibrillation on warfarin (10 mg/day). He was given diuretics and sent home. Two days later, the patient presented to a rural emergency department, complaining of leg pain and swelling. He was given cephalexin for cellulitis and discharged home. The evening prior to admission to our hospital, the patient developed sharp left‐sided chest pain, orthopnea, and worsening dyspnea. He again presented to the rural emergency department and was found to have a blood pressure of 60/40 mm Hg. He was admitted and given boluses of intravenous fluids and a dose of ceftriaxone. Laboratory studies at that time demonstrated the following: sodium, 130 mEq/L; potassium, 6.5 mEq/L; CO2, 21 mEq/L; blood urea nitrogen (BUN), 105 mg/dL; creatinine, 5.5 mg/dL; alkaline phosphatase, 330 IU/L; aspartate aminotransferase (AST), 507 IU/L; and alanine aminotransferase (ALT), 145 IU/L. The following morning, he was oliguric, and his liver transaminases had increased to an AST level of 1862 IU/L and an ALT level of 1055 IU/L. At this point, he was transferred to our hospital for hemodialysis with a diagnosis of acute oliguric renal failure.
On admission to our hospital, the patient was afebrile, had a blood pressure of 112/80 mm Hg, and was hypoxic with an O2 saturation of 93% on 4 L of oxygen by nasal cannula. His weight was 201 kg. He was alert and cooperative. Heart sounds were distant but had a regular rate and rhythm without murmurs, rubs, or gallops. No jugular venous pulsations were appreciated. He had decreased lung sounds throughout both lung fields. His abdomen was morbidly obese and nontender. Extremities demonstrated chronic venous stasis changes with multiple superficial ulcers and bilateral pitting edema.
In light of the elevated liver function tests reported by the referring hospital, these studies were repeated on arrival, revealing an AST level of 4780 IU/L and an ALT level of 1876 IU/L. Other initial laboratory studies demonstrated a BUN level of 116 mg/dL, a creatinine level of 6 mg/dL, a potassium level of 7.2 mEq/L, negative cardiac enzymes, and an international normalized ratio greater than 9.0. A urinalysis could not be performed because the patient was anuric. An electrocardiogram demonstrated a normal sinus rhythm with a right bundle branch block and nonspecific ST segment and T wave changes. There was no prior electrocardiogram available for comparison. Blood acetaminophen level and infectious hepatitis panels were negative.
Within 1 hour of admission, the patient became hypotensive, with his systolic blood pressure decreasing into the 70s. Over the next 24 hours, the patient was treated with intravenous fluids and pressors (neosynephrine) but remained hypotensive, with his systolic blood pressure in the range of 64 to 80 mm Hg.
At 14 hours after admission, liver function tests were repeated and revealed further elevation of his transaminases (AST, 5135 IU/L; ALT, 2468 IU/L). The diagnosis of shock liver was entertained. A portable chest X‐ray (CXR) demonstrated an enlarged cardiac silhouette suggestive of cardiomegaly or pericardial effusion. No prior CXR was available for comparison. An echocardiogram showed probable effusion but was not diagnostic secondary to his body habitus and poor windows. A computed tomography (CT) scan confirmed a large pericardial effusion. This finding, in combination with diminished heart sounds and hypotension, presented a clinical picture consistent with cardiac tamponade. The patient underwent a subxiphoid pericardial window, and 1.5 L of bloody effusion was removed. Intraoperatively, his systolic blood pressure immediately improved from 95 to 135 mm Hg. Urine output increased from 0 to 80 mL/hour in the first hour. Five days later, his creatinine returned to his baseline of 1.9 mg/dL, and other laboratory values had decreased: AST, 306 IU/L; ALT, 520 IU/L; alkaline phosphatase, 122 IU/L; and BUN, 86 mg/dL.
DISCUSSION
Acute renal failure (ARF) is a common condition, with an incidence of 1% on admission to the hospital; 70% of these cases are due to prerenal causes.1 Among patients already in the hospital, prerenal azotemia is responsible for 21% to 39% of cases of ARF.2, 3 Prerenal ARF is most commonly caused by hypotension, which may be cardiogenic, hypovolemic, septic, or due to vasodilatation. Adrenal insufficiency and other etiologies should also be considered. In our patient, chronic renal failure and superimposed hypotension contributed to acute oliguric renal failure. In searching for the etiology of his hypotension, we concentrated on cardiogenic causes in view of his enlarged cardiac silhouette.
In considering the cardiogenic causes of hypotension, we did not initially focus on tamponade. Although oliguria with renal failure is recognized as a complication of cardiac tamponade, relatively little has been written about ARF as the presenting problem in tamponade. The literature is limited to a few case reports, such as those described by Queffeulou et al.4 and Saklayen et al.5 In our patient, the diagnosis of tamponade was made more difficult by the patient's large size, which made the interpretation of physical findings such as heart sounds more difficult and compromised the quality of essential imaging studies.
His rising transaminases suggested shock liver and eventually provided the key to the patient's diagnosis. In light of his worsening liver function tests, chest pain, shortness of breath, and cardiomegaly on CXR, we focused on a cardiac etiology. An echocardiogram, which often can be used to identify pericardial effusion and hence tamponade, was nondiagnostic because of the patient's obesity. Despite the concerns regarding the weight limits of the examination table, the diagnosis was finally established with a CT scan.
In our patient, no definite cause for his bloody pericardial effusion was found. Cardiac enzymes were negative, and this helped rule out ischemia. Nothing indicative of a neoplasm was seen on the CT of his chest. Blood cultures were negative. Viral studies were not performed, and the effusion was not sent for special studies. He may have had an underlying pericarditis secondary to his chronic kidney disease or a viral syndrome that, combined with his anticoagulation treatment, resulted in a bloody effusion.
In addition to illustrating the difficulties in properly diagnosing acutely ill morbidly obese patients, this report also demonstrates that in patients with shock liver and ARF, pericardial tamponade may be the culprit. If preliminary studies including CXR and echocardiogram are equivocal and sufficient suspicion remains, then a CT is warranted.
- ,,,.Community‐acquired acute renal failure.Am J Kidney Dis.1991;17:191–198.
- ,.Epidemiology of acute renal failure: a prospective, multicenter, community‐based study. Madrid Acute Renal Failure Study Group.Kidney Int.1996;50(3):811–818.
- ,,.Hospital‐acquired renal insufficiency.Am J Kidney Dis.2002;39:930–936.
- ,,.Acute renal and hepatic failure: do not miss pericardial tamponade!Nephrol Dial Transplant.1999;14(9):2260.
- ,,.Pericardial effusion leading to acute renal failure: two case reports and discussion of pathophysiology.Am J Kidney Dis.2002;40(4):837–841.
- ,,,.Community‐acquired acute renal failure.Am J Kidney Dis.1991;17:191–198.
- ,.Epidemiology of acute renal failure: a prospective, multicenter, community‐based study. Madrid Acute Renal Failure Study Group.Kidney Int.1996;50(3):811–818.
- ,,.Hospital‐acquired renal insufficiency.Am J Kidney Dis.2002;39:930–936.
- ,,.Acute renal and hepatic failure: do not miss pericardial tamponade!Nephrol Dial Transplant.1999;14(9):2260.
- ,,.Pericardial effusion leading to acute renal failure: two case reports and discussion of pathophysiology.Am J Kidney Dis.2002;40(4):837–841.
Improving Inpatient Glycemic Control
Diabetes mellitus and/or inpatient hyperglycemia are common comorbid conditions in hospitalized patients. Recent surveys show that over 90% of hospitalized diabetic patients experience hyperglycemia (>200 mg/dL), and in nearly 1 in 5 of these patients hyperglycemia persists for 3 days or more.1 Hyperglycemia among inpatients without a previous history of diabetes mellitus is also very common.2 Observational studies have shown that hyperglycemia in hospitalized patients is associated with adverse outcomes including infectious complications, increased length of stay, and increased mortality.27 Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units.8, 9
Based on the available data, the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90 to 130 mg/dL and peak postprandial glucose levels <180 mg/dL in hospitalized nonintensive care unit (ICU) patients.10 To reach these targets, the ADA and American College of Endocrinology (ACE) suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.11 Protocols should promote the use of continuous intravenous insulin infusions or scheduled basal‐bolus subcutaneous insulin regimens. Subcutaneous insulin protocols should include target glucose levels, basal, nutritional, and supplemental insulin, and daily dose adjustments.6 A recent randomized controlled trial of non‐ICU inpatients demonstrated that such a basal‐bolus insulin regimen results in improved glucose control compared with a sliding scale only regimen.12
To date, few published studies have investigated the best ways to implement such management protocols; those that have are often resource‐intensive, for example involving daily involvement of nurse practitioners or diabetologists.13, 14 It is therefore not known how best to implement an inpatient diabetes management program that is effective, efficient, and self‐perpetuating. At Brigham and Women's Hospital (BWH), we have been refining a subcutaneous insulin protocol, focused provider education, and more recently a computerized order set to overcome barriers related to fear of hypoglycemia, delays in insulin prescribing, and unfamiliarity with inpatient glucose management.15 The aims of this current trial were to evaluate the effects of these interventions on a geographically localized general medical service previously naive to these interventions to evaluate their effects on glycemic control, patient safety, and processes of care. We hypothesized that these interventions would improve glycemic control and increase use of basal‐bolus insulin orders without increasing the rate of hypoglycemia.
METHODS
Setting and Participants
This prospective, before‐after trial was conducted at BWH from July 15, 2005 through June 22, 2006. Eligible subjects were patients scheduled for admission to the BWH Physician Assistant/Clinician Educator (PACE) Service with either a known diagnosis of type 2 diabetes mellitus or inpatient hyperglycemia (at least 1 random laboratory glucose >180 mg/dL). The PACE service is a geographically‐localized general medicine service of up to 15 beds where patients are cared for by a single cadre of nurses, 2 physician's assistants (PAs), and 1 hospitalist attending. A moonlighter covers the service at night. The PACE service does not accept patients transferred from other acute care hospitals or from ICUs, but does not otherwise have triage guidelines related to diagnosis, complexity, or acuity. Patients were excluded if they had type 1 diabetes, presented with hyperosmolar hyperglycemic state (HHS) or diabetic ketoacidosis (DKA), received total parenteral nutrition (TPN), or were receiving palliative care. This study was approved by the BWH Institutional Review Board; patient consent was deemed not to be necessary for this study given the relatively nonsensitive nature of the data, noninvasive means of data collection, and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
The study intervention consisted of three components, initiated in January 2006:
Glycemic management protocol: a multidisciplinary team of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (Jennifer Trujillo) developed a subcutaneous insulin protocol based on ADA guidelines (Table 1; see the appendix for complete protocol). The protocol was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee and refined through 6 months of pilot testing on other general medical services.15 The protocol consisted of a set of specific treatment recommendations, including: (1) bedside glucose monitoring; (2) stopping oral diabetes agents in most patients; (3) estimating total daily insulin requirements; (4) prescribing basal, nutritional, and supplemental insulin based on the patient's total insulin requirements, preadmission medication regimen, and nutritional status; (5) adjusting insulin on a daily basis as needed; (6) managing hypoglycemia; (7) suggestions for discharge orders; and (8) indications for an endocrinology consultation. The protocol was printed as a pocket guide, distributed to all members of the PACE service, and used to guide all other interventions.
Diabetes education: all PAs received 2 one‐hour educational sessions: a lecture by a diabetologist (M.L.P.) reviewing the rationale for tight glycemic control and general principles of management, and a workshop by a hospitalist (J.L.S.) in which specific cases were reviewed to illustrate how the protocol could be used in practice (eg, when oral agents could be safely continued, how to prescribe insulin on admission, and how to make subsequent adjustments in dose). All hospitalist attendings received a 1‐hour lecture summarizing the above material. All nurses on the service received a lecture that focused on issues unique to nursing care, such as insulin administration, glucose testing, managing patients with unpredictable oral (PO) intake, and patient education. (All materials are available from the authors upon request).
Order Set: an order set, built into BWH's proprietary computer provider order entry (CPOE) system, was created to parallel the glycemic management protocol and facilitate insulin orders for patients eating discrete meals, receiving continuous liquid enteral nutrition (tube feeds), or receiving nothing by mouth (NPO). Other components of the order set facilitated glucose monitoring and other laboratory tests and ordering consultation when appropriate.
| Oral Agents | Stop Oral Agents in Most Patients |
|---|---|
| |
| Glucose testing | Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if NPO |
| Insulin | |
| 1. Estimate total daily insulin dose | 0.5 to 0.7 units/kg/day, depending on patient's age, size, renal function, insulin sensitivity, history of hypoglycemia, and steroid use |
| 2. Start basal insulin | Patient's home dose or 50% of calculated total daily dose; NPH qAM/qHS or insulin glargine qHS; If NPO, use one‐half the home dose unless hyperglycemic |
| 3. Start nutritional insulin if not NPO | Patient's home dose or 50% of calculated total daily dose, less if poor or unknown intake; discrete meals: insulin aspart split over 3 meals, 0 to 15 minutes prior to eating; continuous tube feeds or IV dextrose: regular insulin every 6 hours |
| 4. Start correctional insulin | 1 of 3 scales provided based on total daily dose of insulin; same type as nutritional insulin; regular insulin if NPO |
| 5. Daily adjustment | Calculate total administered dose from prior day, adjust for degree of hyperglycemia or hypoglycemia, renal function, PO intake, steroid use, and degree of illness, and redistribute as 50% basal, 50% nutritional, or 100% basal if NPO |
| Hypoglycemia orders | Juice, IV dextrose, or IM glucagon depending on ability to take oral nutrition and IV access |
| Discharge orders | Based on A1C: either home regimen, titration of home regimen, or new insulin regimen (if latter, simple regimen with aggressive patient education and prompt follow‐up) |
| Indications for endocrine consultation | Labile blood sugars, poor control, prolonged NPO period, question of type 1 or type 2 diabetes |
Study Protocol and Data Collection
A research assistant prospectively identified eligible patients each weekday by screening all patients scheduled for admission to the PACE service using the daily computerized sign‐out system used on all general medical teams. Specifically, laboratory random glucose levels, inpatient medications, and medical histories were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were then confirmed by medical record review and adjudicated by one study author (J.L.S.) if necessary. Further medical record review was performed to identify specific patient populations (eg, diet‐controlled, steroid‐induced, or previously undiagnosed diabetes), determine preadmission diabetes medications, and determine the patient's weight. Hospital computerized clinical and administrative records were abstracted to obtain patient demographics (age, sex, race, insurance status), laboratory data (glucose level on admission, A1C level [taken during or within 6 months prior to admission]), clinical data (length of stay, billing‐based Charlson comorbidity score,16 and diagnosis‐related group [DRG] case mix index), all inpatient insulin and oral diabetes medication orders, frequency of bedside glucose testing, and diet orders. Electronic medication administration record (eMAR) data were used to determine all doses and times of insulin administration.
Outcomes
The primary outcome was the mean percent of glucose readings between 60 and 180 mg/dL per patient (ie, calculated for each patient and averaged across all eligible patients in each study arm). Only bedside glucose readings were used given the lack of additional useful information typically provided by laboratory (venous plasma) glucose readings.17 Readings drawn within 1 hour of a previous reading were excluded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Only readings while on the study service were used. Readings on hospital day 1 were excluded because our intervention was expected to have little impact on the first day's glucose control; for patients with undiagnosed diabetes, data collection began the day following the first elevated glucose reading. Readings beyond hospital day 14 were also excluded to avoid biased data from patients with exceptionally long lengths of stay.
Secondary outcomes included the following:
Glycemic control:
Patient‐day weighted mean glucose (ie, mean glucose for each patient‐day, averaged across all patient days);
Mean glucose per patient for each hospital day (days 17).
Patient safety:
Proportion of patient‐days with any glucose reading <60 mg/dL (hypoglycemia) and <40 mg/dL (severe hypoglycemia).
Processes of care:
Use of any NPH insulin or insulin glargine (basal) insulin during the hospitalization if 2 or more glucose readings were >180 mg/dL.
Adequacy of basal dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission basal dose or 0.20 to 0.42 units/kg if not known or not taken prior to admission. If not eating, half the above calculations.
Use of any scheduled nutritional insulin during the hospitalization if ever prescribed a diet and 2 or more glucose readings were greater than 180 mg/dL.
Adequacy of nutritional dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission nutritional dose or 0.20 to 0.42 units/kg/day if not known or not taken prior to admission. Patients on clear liquid diets, enteral feeds, or receiving glucocorticoids were excluded from this analysis.
Correct type of nutritional insulin: if eating discrete meals, insulin aspart (the rapid‐acting insulin on formulary at BWH); if prescribed tube feeds, regular insulin.
Use of supplemental insulin by itself (without scheduled basal or nutritional insulin), a marker of poor care.
A1C testing within 1 month prior to or during hospitalization.
Clinical inertia: if at least two glucose readings <60 mg/dL or >180 mg/dL on a patient‐day, lack of any change to any insulin order the following day if still on the study service.
Healthcare utilization:
Hospital length of stay in hours, calculated from the exact time of admission until the exact time of discharge, using hospital administrative data.
Analyses
Study results were compared prior to the intervention (July 15 through December 12, 2005) with those during the intervention (January 18 through June 20, 2006). Patient data and clinical outcomes were analyzed descriptively using proportions, means with standard deviations (SDs), or medians with interquartile ranges (IQRs) as appropriate. Comparisons between groups were calculated using Fisher's exact test for dichotomous and categorical variables, and Student t test or Wilcoxon rank sum test for continuous variables as appropriate. The primary outcome was first analyzed using linear regression with study group as the independent variable and percent of glucose readings within range per patient as the dependent variable. We then adjusted for potential confounders by putting each covariate into the model, one at a time. All significant predictors of the outcome at a P value <0.10 were retained in the final model. We used general estimating equations to adjust for clustering of results by each PA. Similar analyses were performed for hospital length of stay per patient using a negative binomial model, so chosen because it fit the data distribution much better than the typically used Poisson model. With a planned sample size of 115 patients and 1250 glucose readings per arm, an intraclass correlation coefficient of 0.10, and an alpha of 0.05, the study had 90% power to detect an increase in percent of glucose readings in range from 67% to 75%. All analyses were based on the intention‐to‐treat principle. Except as above, 2‐sided P values <0.05 were considered significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 248 potential patients for the study. We subsequently excluded 79 patients for the following reasons: no glucose readings beyond hospital day 1 while on PACE service (34 patients); never admitted to PACE service (15 patients); no diabetes or inpatient hyperglycemia (9 patients, mostly patients prescribed an insulin sliding scale prophylactically to avoid steroid‐induced hyperglycemia); type 1 diabetes (13 patients); TPN, DKA, or HHS (5 patients); and palliative care (3 patients). The remaining 169 patients included 63 from the preintervention period(out of 489 total admissions to the PACE service; 13%) and 106 patients in the postintervention period (out of 565 admissions; 19%). These patients had 2447 glucose readings, or an average of 3.6 glucose readings per monitored patient‐day in the preintervention period and 3.3 glucose readings per patient‐day in the postintervention period. Even including the 34 patients who were excluded for lack of glucose readings, glucose data were still available for 717 out of a potential 775 patient‐days (93%). Characteristics for all included patients are shown in Table 2. The mean admission glucose was 197 mg/dL, mean A1C was 8.4%, 54% of the patients were prescribed insulin prior to admission, and 7% had no prior diagnosis of diabetes. There were no significant differences in baseline characteristics between the 2 patient groups except for Charlson score, which was higher in the preintervention group (87% versus 74% with score 2 or higher; Table 2). The top diagnosis‐related groups for the entire cohort included: heart failure and shock (12 patients); kidney and urinary tract infections (12 patients); esophagitis, gastroenteritis, and miscellaneous digestive disorders (11 patients); chronic obstructive pulmonary disease (10 patients); renal failure (10 patients); simple pneumonia and pleurisy (7 patients); disorders of the pancreas except malignancy (6 patients); chest pain (5 patients); and cellulitis (5 patients).
| Preintervention (n = 63) | Postintervention (n = 106) | P Value | |
|---|---|---|---|
| |||
| Mean age, year (SD) | 63.0 (15.7) | 64.7 (14.3) | 0.52 |
| Male, n (%) | 25 (40) | 52 (49) | 0.27 |
| Race, n (%) | 0.33 | ||
| White | 29 (46) | 42 (40) | |
| Black | 21 (33) | 28 (26) | |
| Hispanic | 11 (17) | 30 (28) | |
| Unknown | 2 (3) | 6 (6) | |
| Admission glucose, mg/dL (SD) | 188 (90.9) | 203 (96.1) | 0.33 |
| A1C, % (SD) | 8.5 (2.4) | 8.3 (2.4) | 0.85 |
| Insulin use prior to admission, n (%) | 38 (60) | 54 (51) | 0.48 |
| Case mix index, median (IQR) | 0.89 (0.781.11) | 0.91 (0.841.22) | 0.33 |
| Charlson index, n (%) | 0.03 | ||
| 01 | 8 (13) | 28 (26) | |
| 23 | 29 (46) | 27 (26) | |
| 45 | 15 (24) | 29 (27) | |
| >5 | 11 (17) | 22 (21) | |
| Known history of diabetes, n (%) | 62 (98) | 96 (91) | 0.06 |
With respect to insulin ordering practices, there was no significant difference in the use of basal insulin in hyperglycemic patients between the preintervention period and postintervention period (81% versus 91%; P = 0.17), nor in the dose of basal insulin prescribed (results not shown), but there was an increase in the use of scheduled nutritional insulin for those patients with hyperglycemia receiving nutrition: 40% versus 75%, P < 0.001 (Table 3). The percent of patients receiving supplemental (sliding scale) insulin by itself (ie, without ever receiving basal or nutritional insulin) was lower during the postintervention period (29% versus 8%, P < 0.001). Nonsignificant differences were seen in the rates of prescribing an appropriate dose and type of nutritional insulin. Notably, there was no difference at all in the proportion of patient‐days in which insulin adjustments were made when 2 or more episodes of hyperglycemia or hypoglycemia were present during the previous day (56% of patient‐days in both groups; P = 0.90).
| Preintervention (n = 63) | Postintervention (n = 106) | Unadjusted Effect Size (95% CI) | Adjusted Effect Size (95% CI) | |
|---|---|---|---|---|
| ||||
| Mean percent glucose readings 60180 mg/dL per patient (SD) | 59.1 (0.28) | 64.7 (0.27) | +5.6 (3.0 to +14.3) | +9.7 (+0.6 to +18.8)*, |
| Patient‐day weighted mean glucose, mg/dL (SD) | 174.7 (60.0) | 164.6 (54.2) | 10.1 (1.6 to 18.5) | 15.6 (6.4 to 24.9), |
| Percent patient‐days with any glucose <60 mg/dL | 16/293 (5.5%) | 26/424 (6.1%) | 1.1 (0.6 to 2.1) | 1.1 (0.6 to 2.1) |
| Percent patient‐days with any glucose <40 mg/dL | 3/293 (1.0%) | 5/424 (1.2%) | 1.3 (0.3 to 5.9) | 1.1 (0.3 to 5.1) |
| Hospital length of stay, hours, mean (SD) | 112.2 (63.3) | 86.0 (89.6) | 30% (5% to 51%) | 25% (6% to 44%),∥ |
| Basal insulin if inpatient hyperglycemia (2 or more readings >180 mg/dL) | 39/48 (81%) | 67/74 (91%) | 2.2 (0.8 to 6.4) | |
| Nutritional insulin if inpatient hyperglycemia and PO intake | 19/48 (40%) | 53/71 (75%) | 4.5 (2.0 to 9.9), | |
| Adequate initial dose of nutritional insulin (home dose or 0.200.42 units/kg/day)# | 2/9 (22%) | 22/49 (45%) | 2.9 (0.5 to 15.1) | |
| Supplemental insulin alone (without basal or nutritional insulin) | 16/56 (29%) | 7/92 (8%) | 0.2 (0.08 to 0.5), | |
| Insulin changed if previous day's glucose out of range (2 or more values <60 or >180 mg/dL) | 70/126 (56%) | 76/135 (56%) | 1.0 (0.6 to 1.6) | |
| A1C tested during hospitalization if not available within 30 days prior | 38/63 (60%) | 74/106 (70%) | 1.5 (0.8 to 2.9) | |
The primary outcome, the mean percent of glucose readings between 60 and 180 mg/dL per patient, was 59.1% in the preintervention period and 64.7% in the postintervention (P = 0.13 in unadjusted analysis; Table 3). When adjusted for A1C, admission glucose, and insulin use prior to admission, the adjusted absolute difference in the percent of glucose readings within range was 9.7% (95% confidence interval [CI], 0.6%‐18.8%; P = 0.04; Table 3). Regarding other measures of glucose control, the patient‐day weighted mean glucose was 174.7 mg/dL in the preintervention period and 164.6 mg/dL postintervention (P = 0.02), and there was no significant difference in the percent of patient‐days with any hypoglycemia (glucose <60 mg/dL) or severe hypoglycemia (glucose <40 mg/dL; Table 3). There were also no significant differences in the mean number of hypoglycemic events per patient‐day (6.8 versus 6.6 per 100 patient‐days; relative risk, 0.95; 95% CI, 0.541.67; P = 0.87) or severe hypoglycemic events per patient‐day (1.0 versus 1.4 per 100 patient‐days; relative risk, 1.38; 95% CI, 0.355.53; P = 0.65).
We also compared hospital length of stay in hours between the study groups (Table 3). Length of stay (LOS) was shorter in the postintervention arm in unadjusted analyses (112 versus 86 hours; P < 0.001), and this difference persisted when adjusted for patient insurance, race, gender, and Charlson comorbidity score (25% shorter; 95% CI, 6%‐44%). A comparison of LOS among nonstudy patients on the PACE service during these 2 time periods revealed no difference (105 versus 101 hours). When the length of stay analysis was limited to study patients with a known diagnosis of diabetes, the adjusted effect size was a 31% relative decrease in length of stay.
Figure 1A shows the percent glucose readings within range per patient by hospital day. The greatest differences between groups can be seen on hospital days 2 and 3 (11% absolute differences on both days). Similarly, Figure 1B shows the mean glucose per patient by hospital day. Again, the biggest differences are seen on hospital days 2 and 3 (20 and 23 mg/dL difference between groups, respectively). In both cases, only the day 3 comparisons were significantly different between study groups.
DISCUSSION
In this before‐after study, we found that a multifaceted intervention consisting of a subcutaneous insulin protocol, focused education, and an order set built into the hospital's CPOE system was associated with a significantly higher percentage of glucose readings within range per patient in analyses adjusted for patient demographics and severity of diabetes. We also found a significant decrease in patient‐day weighted mean glucose, a marked increase in appropriate use of scheduled nutritional insulin, and a concomitant decrease in sliding scale insulin only regimens during the postintervention period. Moreover, we found a shorter length of stay during the postintervention period that persisted after adjustment for several clinical factors. Importantly, the interventions described in this study require very few resources to continue indefinitely: printing costs for the management protocol, 4 hours of education delivered per year, and routine upkeep of an electronic order set.
Because this was a before‐after study, we cannot exclude the possibility that these improvements in process and outcome were due to cointerventions and/or temporal trends. However, we know of no other interventions aimed at improving diabetes care in this self‐contained service of nurses, PAs, and hospitalists. Moreover, the process improvements, especially the increase in scheduled nutritional insulin, were rather marked, unlikely to be due to temporal trends alone, and likely capable of producing the corresponding improvements in glucose control. That glucose control stopped improving after hospital day 3 may be due to the fact that subsequent adjustment to insulin orders occurred infrequently and no more often than prior to the intervention. That we did not see greater improvements in glycemic control overall may also reflect the fact that 81% of study patients with inpatient hyperglycemia received basal insulin prior to the intervention.
The reduction in patient LOS was somewhat surprising given the relatively small sample size. However, the results are consistent with those of other studies linking hyperglycemia to LOS18, 19 and we found no evidence for a temporal trend toward lower LOS on the PACE service as a whole during the same time period. While a greater proportion of patients on the PACE service were in the study in the post‐intervention period compared with the preintervention period, we found no evidence that the difference in length of stay was due to increased surveillance for nondiabetics, especially because eligibility criteria depended on phlebotomy glucose values, which were uniformly tested in all inpatients. Also, effects on length of stay were actually stronger when limited to patients with known diabetes. Finally, we controlled for several predictors of length of stay, although we still cannot exclude the possibility of unmeasured confounding between groups.
Since ADA and ACE issued guidelines for inpatient management of diabetes and hyperglycemia, many institutions have developed subcutaneous insulin algorithms, educational curricula, and/or order sets to increase compliance with these guidelines and improve glycemic control. Some of these efforts have been studied and some have been successful in their efforts.13, 14, 2023 Unfortunately, most of these programs have not rigorously assessed their impact on process and outcomes, and the most effective studies published to date have involved interventions much more intensive than those described here. For example, Rush University's intervention was associated with a 50 mg/dL decrease in mean blood glucose but involved an endocrinologist rounding twice daily with house officers for 2 weeks at a time.13 At Northwestern University, a diabetes management service run by nurse practitioners was established, and the focus was on the conversion from intravenous to subcutaneous insulin regimens.14 The RABBIT 2 study that demonstrated the benefits of a basal‐bolus insulin regimen used daily rounding with an endocrinologist.12 More modestly, a program in Pitt County Memorial Hospital in Greenville, NC, relied mostly on diabetes nurse case managers, a strategy which reduced hospital‐wide mean glucose levels as well as LOS, although the greatest improvements in glycemic control were seen in the ICU.19 Our findings are much more consistent with those from University of California San Diego, as yet unpublished, which also used an algorithm, computerized order set, education, as well as continuous quality improvement methods to achieve its aims.22
Our study has several limitations, including being conducted on 1 general medicine service at 1 academic medical center. Moreover, this service, using a physician assistant/hospitalist model, a closed geographic unit, and fairly generous staffing ratio, is likely different from those in many settings and may limit the generalizability of our findings. However, this model allowed us to conduct the study in a laboratory relatively untouched by other cointerventions. Furthermore, the use of PAs in this way may become more common as both academic and community hospitals rely more on mid‐level providers. Our study had a relatively low percentage of patients without a known diagnosis of diabetes compared with other studies, again potentially but not necessarily limiting generalizability. This finding has been shown in other studies at our institution24 and may be due to the high rate of screening for diabetes in the community. Another limitation is that this was a nonrandomized, before‐after trial. However, all subjects were prospectively enrolled to improve comparability, and we performed rigorous adjustment for multiple potential confounding factors. Also, this study had limited statistical power to detect differences in hypoglycemia rates. The preintervention arm was smaller than planned due to fewer diabetic patients than expected on the service and a higher number of exclusions; we prolonged the postintervention period to achieve the desired sample size for that arm of the study.
Our study also has several strengths, including electronic capture of many processes of care and a methodology to operationalize them into measures of protocol adherence. Our metrics of glycemic control were rigorously designed and based on a national task force on inpatient glycemic control sponsored by the Society of Hospital Medicine, with representation from the ADA and AACE.25
Potential future improvements to this intervention include modifications to the daily adjustment algorithm to improve its usability and ability to improve glucose control. Another is the use of high‐reliability methods to improve order set use and daily insulin adjustment, including alerts within the CPOE system and nurse empowerment to contact medical teams if glucose levels are out of range (eg, if greater than 180 mg/dL, not just if greater than 350 or 400 mg/dL). Future research directions include multicenter, randomized controlled trials of these types of interventions and an analysis of more distal patient outcomes including total healthcare utilization, infection rates, end‐organ damage, and mortality.
In conclusion, we found a relationship between a relatively low‐cost quality improvement intervention and improved glycemic control in the non‐ICU general medical setting. Such a finding suggests the benefits of the algorithm itself to improve glucose control and of our implementation strategy. Other institutions may find this intervention a useful starting point for their own quality improvement efforts. Both the algorithm and implementation strategy are deserving of further improvements and future study.
Acknowledgements
We thank Paul Szumita, Karen Fiumara, Jennifer Trujillo, and the other members of the BWH Diabetes Pharmacy and Therapeutics Subcommittee for their help designing and implementing the intervention; Aubre McClendon, Nicole Auclair, Emily Dattwyler, Mariya Fiman, and Alison Pietras for valuable research assistance; Deborah Williams for data analysis; Amy Bloom for project support; and Stuart Lipsitz for biostatistical expertise.
APPENDIX
INPATIENT DIABETES MANAGEMENT PROTOCOL
Management of Diabetes and Hyperglycemia in Hospitalized Non‐ICU Patients
Rationale
Increasing data show a strong association between hyperglycemia and adverse inpatient outcomes. The American Diabetes Association and the American College of Clinical Endocrinology recommend all glucose levels be below 180 mg/dL in non‐ICU patients. Because hospitalizations are unstable situations, even patients who are well controlled on oral agents as outpatients are usually best managed with insulin.
Insulin may be safely administered even to patients without previously diagnosed diabetes. As long as the prescribed doses are below what is normally produced by the pancreas, the patient will not become hypoglycemic. If the glucose level drops, endogenous insulin secretion will reduce to compensate.
Total insulin requirements in insulin‐sensitive patients (eg, type 1 diabetes mellitus) is 0.50.7/units/kg/day. Insulin requirements in insulin‐resistant type 2 diabetic patients may vary greatly, and can exceed 12 units/kg/day. A conservative estimate for initial insulin therapy in any patient with diabetes is to start with the type 1 diabetes mellitus dose, 0.50.7 units/kg/day.
Overview
Effective inpatient insulin regimens typically include 3 components:
Basal insulin (eg, scheduled NPH or insulin glargine [Lantus]), which is used to manage fasting and premeal hyperglycemia.
Nutritional or prandial insulin (eg, scheduled regular insulin, insulin lispro [Humalog] or insulin aspart [Novolog]) which controls hyperglycemia from nutritional (eg, discrete meals, TPN, IV dextrose) sources.
Supplemental or correctional insulin (eg, regular insulin, insulin lispro, or insulin aspart), which is used in addition to scheduled insulin to meet unexpected basal hyperglycemia that is not covered by the scheduled insulin.
Sample Orders (Not for Patients with Uncontrolled Type 1 Diabetes, DKA, Hyperglycemic Hyperosmolar State, or Other Absolute Need for IV Insulin)
Check (fingerstick) capillary blood glucose qAC, qHS.
NPH insulin subcutaneously (SC) ___ units qAM, ___ units qHS.
Insulin aspart SC ___ units pre‐breakfast, ___ units pre‐lunch, ___ units pre‐dinner, hold if NPO or premeal BS <60 mg/dL; give 015 minutes before meals.
Insulin aspart SC sliding scale (see Table 6) qAC, in addition to standing nutritional insulin, 015 minutes before meals.
For BS <60 mg/dL:
If patient can take PO
Give 15 g of fast acting carbohydrate (4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets).
Repeat finger capillary glucose every 15 (q15) minutes and repeat above (5.a.i.) if BG <60 mg/dL.
When BG >60 mg/dL, give snack or meal in a half‐hour.
If patient cannot take PO
Give 25 mL of 50% dextrose (D50) as an IV push.;
Repeat finger capillary glucose q15 minutes and repeat above (5.b.i.) if BG <80 mg/dL.
Guidelines
Stop oral diabetes agents in most patients (see Table 7 for list of contraindications and precautions).
Check bedside blood glucose (BBG or fingerstick) qAC and qHS (or at 0600 hours, 1200 hours, 1800 hours, and 2400 hours if no discrete meals).
Estimate total daily insulin requirement:
For most patients, conservative estimate is 0.50.7 units/kg/day, but may be much higher.
Reasons for lower end of the range: renal insufficiency, small size, insulin sensitive (eg, type 1), recent hypoglycemia, decreasing doses of steroids, older age.
Reasons for higher end of the range: obese, initiation or increasing doses of steroids, marked hyperglycemia.
Start basal insulin if any premeal BG >140 mg/dL and no recent glucose <60 mg/dL off insulin (Table 5).
Start nutritional or prandial insulinhold if nutrition is stopped/held or premeal BS <60 (Table 5).
Start supplemental/correctional insulin in addition to nutritional (prandial) insulin (Table 6):
Discrete meals: Insulin aspart qAC (with nutritional insulin). 0
No discrete meals: Regular insulin q6h.
On a daily basis, adjust scheduled insulin based on previous days' blood sugars:
Add up total insulin given the previous day, including scheduled and supplemental insulin, to determine new total daily insulin requirement.
Adjust total daily insulin requirement based on clinical considerations (eg, give more if marked hyperglycemia, eating more, improving renal function, increasing steroids; give less if eating less, worsening renal function, tapering steroids, recovering from severe illness).
Give 50% of requirement as basal and 50% as nutritional, as above (may need proportionately less nutritional insulin if appetite poor or unknown).
Adjust sliding scale if needed based on total scheduled insulin dose (see step 6, above).
For BG <60 mg/dL:
If patient can take PO, give 15 g of fast acting carbohydrate.
(4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets; not juice plus sugar).
Repeat finger capillary glucose q15 minutes and repeat above if BG <60.
When BG >60, give snack or meal in half an hour.
If patient cannot take PO, give 25 mL of D50 as IV push.
Check finger capillary glucose q15 minutes and repeat above if BG <80.
Discharge orders:
Patient should be discharged home on a medication regimen that was similar to the admission regimen (ie, the regimen prescribed by their PCP). Exceptions include
The patient has a contraindication to an admission medication.
There is evidence of severe hyperglycemia (eg, very high A1C) or hypoglycemia on admission regimen.
If a patient is admitted with no insulin, and requires insulin to be continued as an outpatient (eg, newly‐diagnosed type 1 diabetes, A1C very high, and contraindication to or on maximum oral regimen), limit discharge insulin regimen to no more than 1 injection per day (eg, hs NPH; an exception to this is for type 1 diabetic patients, who are optimally treated with 34 injections/day). Make sure the patient has prompt follow‐up with their primary care provider (PCP).
Avoid discharging home on sliding scale.
If a patient is going to require insulin injections and self‐monitoring blood glucose as an outpatient, make sure they are instructed about how to perform these.
Indications for calling an endocrine consult:
Labile blood sugars.
Prolonged periods of NPO, eg, for procedures, especially in patients with type 1 diabetes
Marked hyperglycemia despite following this guideline.
Question of type 1 versus type 2 versus other type of diabetes. 0
Basil Insulin Guidelines Home Insulin Regimen Starting Dose of Basal Insulin Considerations NOTE: Patients with T1DM require basal insulin at all times! Basal never should be held!
Abbreviations: NPO, nothing by mouth.
On basal (eg, NPH or glargine) insulin at home Patient's home dose of NPH or glargine If NPO, consider starting half of NPH or glargine home dose, unless hyperglycemic at home. Not on basal (eg, NPH or glargine) insulin at home NPH 50% of total daily insulin requirement, given qHS or split qAM/qHS (maximum starting dose 20 units/day) Same dose if patient has previously diagnosed or undiagnosed diabetes Nutritional Insulin Guidelines Type of Nutrition Common Nutritional Regimens Sample Starting Doses Abbreviation: qAM, every morning; qHS, at bed time.
If receiving cycled tube feeds at night, give nutritional NPH qHS only.
Discrete meals Aspart given 015 minutes before meals Home dose, if known or 50% of total insulin requirement, split over 3 meals, may need less if poor or unknown appetite Continuous tube feeding,* IV dextrose NPH qHS or qAM/qHS 50% of total insulin requirement (in addition to basal dose), may need less if not at goal caloric intake Glargine given every day (qd), anytime Regular every 6 hours (q6h) Sample Supplemental/Correctional Insulin Scales Blood Glucose Scheduled Insulin < 40 Units/Day Scheduled Insulin of 4080 Units/Day Scheduled Insulin > 80 Units/Day Individualized NOTE: Avoid supplemental insulin qHS unless patient is very hyperglycemic and obese.
150199 1 unit 1 unit 2 units ____ units 200249 2 units 3 units 4 units ____ units 250299 3 units 5 units 7 units ____ units 300349 4 units 7 units 10 units ____ units >349 5 units + call HO 8 units + call HO 12 units + call HO ___ units + call HO Notes on Oral Agents Agents Considerations Metabolism Sulfonylureas/secretagogues: glyburide, glipizide, glimeperide (Amaryl); repaglinide (Prandin); nateglinide (Starlix) Risk for hypoglycemia Metabolized in liver; Glyburide metabolized to active metabolites; 50% renally eliminated Metformin Contraindicated in heart failure and renal dysfunction (creatinine [Cr] >1.5 mg/dL in men and 1.4 mg/dL in women) Eliminated renally Should be held at time of iodinated contrast studies. (May be restarted after normal postcontrast renal function is confirmed) Adverse effects include diarrhea, nausea, and anorexia Thiazolidinediones: pioglitazone (Actos), rosiglitazone (Avandia) Contraindicated in class III and IV heart failure Metabolized in liver Use with caution in patients with edema Adverse effects include increased intravascular volume Slow onset of action Avoid in hepatic dysfunction Glucosidease inhibitors: acarbose (Precose), miglitol (Glycet) Gastrointestinal intolerance Acarbose eliminated in gut and renally
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax.2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- Standards of medical care in diabetes, 2007.Diabetes Care.2007;30(Suppl 1):S4–S41.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3:55–63.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,,.Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation.Diabetes Care.2007;30:993–994.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23:757–765.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,,.Effect of a standardized insulin order set and an insulin management algorithm on inpatient glycemic control and hypoglycemia. Society of Hospital Medicine Annual Meeting, 2007; Dallas, TX;2007.
- ,,, et al.Trends in bed occupancy for inpatients with diabetes before and after the introduction of a diabetes inpatient specialist nurse service.Diabet Med.2006;23:1008–1015.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al. Improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes, 2006. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/GC_Imp_Guide.cfm. Accessed October 2008.
Diabetes mellitus and/or inpatient hyperglycemia are common comorbid conditions in hospitalized patients. Recent surveys show that over 90% of hospitalized diabetic patients experience hyperglycemia (>200 mg/dL), and in nearly 1 in 5 of these patients hyperglycemia persists for 3 days or more.1 Hyperglycemia among inpatients without a previous history of diabetes mellitus is also very common.2 Observational studies have shown that hyperglycemia in hospitalized patients is associated with adverse outcomes including infectious complications, increased length of stay, and increased mortality.27 Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units.8, 9
Based on the available data, the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90 to 130 mg/dL and peak postprandial glucose levels <180 mg/dL in hospitalized nonintensive care unit (ICU) patients.10 To reach these targets, the ADA and American College of Endocrinology (ACE) suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.11 Protocols should promote the use of continuous intravenous insulin infusions or scheduled basal‐bolus subcutaneous insulin regimens. Subcutaneous insulin protocols should include target glucose levels, basal, nutritional, and supplemental insulin, and daily dose adjustments.6 A recent randomized controlled trial of non‐ICU inpatients demonstrated that such a basal‐bolus insulin regimen results in improved glucose control compared with a sliding scale only regimen.12
To date, few published studies have investigated the best ways to implement such management protocols; those that have are often resource‐intensive, for example involving daily involvement of nurse practitioners or diabetologists.13, 14 It is therefore not known how best to implement an inpatient diabetes management program that is effective, efficient, and self‐perpetuating. At Brigham and Women's Hospital (BWH), we have been refining a subcutaneous insulin protocol, focused provider education, and more recently a computerized order set to overcome barriers related to fear of hypoglycemia, delays in insulin prescribing, and unfamiliarity with inpatient glucose management.15 The aims of this current trial were to evaluate the effects of these interventions on a geographically localized general medical service previously naive to these interventions to evaluate their effects on glycemic control, patient safety, and processes of care. We hypothesized that these interventions would improve glycemic control and increase use of basal‐bolus insulin orders without increasing the rate of hypoglycemia.
METHODS
Setting and Participants
This prospective, before‐after trial was conducted at BWH from July 15, 2005 through June 22, 2006. Eligible subjects were patients scheduled for admission to the BWH Physician Assistant/Clinician Educator (PACE) Service with either a known diagnosis of type 2 diabetes mellitus or inpatient hyperglycemia (at least 1 random laboratory glucose >180 mg/dL). The PACE service is a geographically‐localized general medicine service of up to 15 beds where patients are cared for by a single cadre of nurses, 2 physician's assistants (PAs), and 1 hospitalist attending. A moonlighter covers the service at night. The PACE service does not accept patients transferred from other acute care hospitals or from ICUs, but does not otherwise have triage guidelines related to diagnosis, complexity, or acuity. Patients were excluded if they had type 1 diabetes, presented with hyperosmolar hyperglycemic state (HHS) or diabetic ketoacidosis (DKA), received total parenteral nutrition (TPN), or were receiving palliative care. This study was approved by the BWH Institutional Review Board; patient consent was deemed not to be necessary for this study given the relatively nonsensitive nature of the data, noninvasive means of data collection, and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
The study intervention consisted of three components, initiated in January 2006:
Glycemic management protocol: a multidisciplinary team of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (Jennifer Trujillo) developed a subcutaneous insulin protocol based on ADA guidelines (Table 1; see the appendix for complete protocol). The protocol was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee and refined through 6 months of pilot testing on other general medical services.15 The protocol consisted of a set of specific treatment recommendations, including: (1) bedside glucose monitoring; (2) stopping oral diabetes agents in most patients; (3) estimating total daily insulin requirements; (4) prescribing basal, nutritional, and supplemental insulin based on the patient's total insulin requirements, preadmission medication regimen, and nutritional status; (5) adjusting insulin on a daily basis as needed; (6) managing hypoglycemia; (7) suggestions for discharge orders; and (8) indications for an endocrinology consultation. The protocol was printed as a pocket guide, distributed to all members of the PACE service, and used to guide all other interventions.
Diabetes education: all PAs received 2 one‐hour educational sessions: a lecture by a diabetologist (M.L.P.) reviewing the rationale for tight glycemic control and general principles of management, and a workshop by a hospitalist (J.L.S.) in which specific cases were reviewed to illustrate how the protocol could be used in practice (eg, when oral agents could be safely continued, how to prescribe insulin on admission, and how to make subsequent adjustments in dose). All hospitalist attendings received a 1‐hour lecture summarizing the above material. All nurses on the service received a lecture that focused on issues unique to nursing care, such as insulin administration, glucose testing, managing patients with unpredictable oral (PO) intake, and patient education. (All materials are available from the authors upon request).
Order Set: an order set, built into BWH's proprietary computer provider order entry (CPOE) system, was created to parallel the glycemic management protocol and facilitate insulin orders for patients eating discrete meals, receiving continuous liquid enteral nutrition (tube feeds), or receiving nothing by mouth (NPO). Other components of the order set facilitated glucose monitoring and other laboratory tests and ordering consultation when appropriate.
| Oral Agents | Stop Oral Agents in Most Patients |
|---|---|
| |
| Glucose testing | Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if NPO |
| Insulin | |
| 1. Estimate total daily insulin dose | 0.5 to 0.7 units/kg/day, depending on patient's age, size, renal function, insulin sensitivity, history of hypoglycemia, and steroid use |
| 2. Start basal insulin | Patient's home dose or 50% of calculated total daily dose; NPH qAM/qHS or insulin glargine qHS; If NPO, use one‐half the home dose unless hyperglycemic |
| 3. Start nutritional insulin if not NPO | Patient's home dose or 50% of calculated total daily dose, less if poor or unknown intake; discrete meals: insulin aspart split over 3 meals, 0 to 15 minutes prior to eating; continuous tube feeds or IV dextrose: regular insulin every 6 hours |
| 4. Start correctional insulin | 1 of 3 scales provided based on total daily dose of insulin; same type as nutritional insulin; regular insulin if NPO |
| 5. Daily adjustment | Calculate total administered dose from prior day, adjust for degree of hyperglycemia or hypoglycemia, renal function, PO intake, steroid use, and degree of illness, and redistribute as 50% basal, 50% nutritional, or 100% basal if NPO |
| Hypoglycemia orders | Juice, IV dextrose, or IM glucagon depending on ability to take oral nutrition and IV access |
| Discharge orders | Based on A1C: either home regimen, titration of home regimen, or new insulin regimen (if latter, simple regimen with aggressive patient education and prompt follow‐up) |
| Indications for endocrine consultation | Labile blood sugars, poor control, prolonged NPO period, question of type 1 or type 2 diabetes |
Study Protocol and Data Collection
A research assistant prospectively identified eligible patients each weekday by screening all patients scheduled for admission to the PACE service using the daily computerized sign‐out system used on all general medical teams. Specifically, laboratory random glucose levels, inpatient medications, and medical histories were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were then confirmed by medical record review and adjudicated by one study author (J.L.S.) if necessary. Further medical record review was performed to identify specific patient populations (eg, diet‐controlled, steroid‐induced, or previously undiagnosed diabetes), determine preadmission diabetes medications, and determine the patient's weight. Hospital computerized clinical and administrative records were abstracted to obtain patient demographics (age, sex, race, insurance status), laboratory data (glucose level on admission, A1C level [taken during or within 6 months prior to admission]), clinical data (length of stay, billing‐based Charlson comorbidity score,16 and diagnosis‐related group [DRG] case mix index), all inpatient insulin and oral diabetes medication orders, frequency of bedside glucose testing, and diet orders. Electronic medication administration record (eMAR) data were used to determine all doses and times of insulin administration.
Outcomes
The primary outcome was the mean percent of glucose readings between 60 and 180 mg/dL per patient (ie, calculated for each patient and averaged across all eligible patients in each study arm). Only bedside glucose readings were used given the lack of additional useful information typically provided by laboratory (venous plasma) glucose readings.17 Readings drawn within 1 hour of a previous reading were excluded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Only readings while on the study service were used. Readings on hospital day 1 were excluded because our intervention was expected to have little impact on the first day's glucose control; for patients with undiagnosed diabetes, data collection began the day following the first elevated glucose reading. Readings beyond hospital day 14 were also excluded to avoid biased data from patients with exceptionally long lengths of stay.
Secondary outcomes included the following:
Glycemic control:
Patient‐day weighted mean glucose (ie, mean glucose for each patient‐day, averaged across all patient days);
Mean glucose per patient for each hospital day (days 17).
Patient safety:
Proportion of patient‐days with any glucose reading <60 mg/dL (hypoglycemia) and <40 mg/dL (severe hypoglycemia).
Processes of care:
Use of any NPH insulin or insulin glargine (basal) insulin during the hospitalization if 2 or more glucose readings were >180 mg/dL.
Adequacy of basal dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission basal dose or 0.20 to 0.42 units/kg if not known or not taken prior to admission. If not eating, half the above calculations.
Use of any scheduled nutritional insulin during the hospitalization if ever prescribed a diet and 2 or more glucose readings were greater than 180 mg/dL.
Adequacy of nutritional dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission nutritional dose or 0.20 to 0.42 units/kg/day if not known or not taken prior to admission. Patients on clear liquid diets, enteral feeds, or receiving glucocorticoids were excluded from this analysis.
Correct type of nutritional insulin: if eating discrete meals, insulin aspart (the rapid‐acting insulin on formulary at BWH); if prescribed tube feeds, regular insulin.
Use of supplemental insulin by itself (without scheduled basal or nutritional insulin), a marker of poor care.
A1C testing within 1 month prior to or during hospitalization.
Clinical inertia: if at least two glucose readings <60 mg/dL or >180 mg/dL on a patient‐day, lack of any change to any insulin order the following day if still on the study service.
Healthcare utilization:
Hospital length of stay in hours, calculated from the exact time of admission until the exact time of discharge, using hospital administrative data.
Analyses
Study results were compared prior to the intervention (July 15 through December 12, 2005) with those during the intervention (January 18 through June 20, 2006). Patient data and clinical outcomes were analyzed descriptively using proportions, means with standard deviations (SDs), or medians with interquartile ranges (IQRs) as appropriate. Comparisons between groups were calculated using Fisher's exact test for dichotomous and categorical variables, and Student t test or Wilcoxon rank sum test for continuous variables as appropriate. The primary outcome was first analyzed using linear regression with study group as the independent variable and percent of glucose readings within range per patient as the dependent variable. We then adjusted for potential confounders by putting each covariate into the model, one at a time. All significant predictors of the outcome at a P value <0.10 were retained in the final model. We used general estimating equations to adjust for clustering of results by each PA. Similar analyses were performed for hospital length of stay per patient using a negative binomial model, so chosen because it fit the data distribution much better than the typically used Poisson model. With a planned sample size of 115 patients and 1250 glucose readings per arm, an intraclass correlation coefficient of 0.10, and an alpha of 0.05, the study had 90% power to detect an increase in percent of glucose readings in range from 67% to 75%. All analyses were based on the intention‐to‐treat principle. Except as above, 2‐sided P values <0.05 were considered significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 248 potential patients for the study. We subsequently excluded 79 patients for the following reasons: no glucose readings beyond hospital day 1 while on PACE service (34 patients); never admitted to PACE service (15 patients); no diabetes or inpatient hyperglycemia (9 patients, mostly patients prescribed an insulin sliding scale prophylactically to avoid steroid‐induced hyperglycemia); type 1 diabetes (13 patients); TPN, DKA, or HHS (5 patients); and palliative care (3 patients). The remaining 169 patients included 63 from the preintervention period(out of 489 total admissions to the PACE service; 13%) and 106 patients in the postintervention period (out of 565 admissions; 19%). These patients had 2447 glucose readings, or an average of 3.6 glucose readings per monitored patient‐day in the preintervention period and 3.3 glucose readings per patient‐day in the postintervention period. Even including the 34 patients who were excluded for lack of glucose readings, glucose data were still available for 717 out of a potential 775 patient‐days (93%). Characteristics for all included patients are shown in Table 2. The mean admission glucose was 197 mg/dL, mean A1C was 8.4%, 54% of the patients were prescribed insulin prior to admission, and 7% had no prior diagnosis of diabetes. There were no significant differences in baseline characteristics between the 2 patient groups except for Charlson score, which was higher in the preintervention group (87% versus 74% with score 2 or higher; Table 2). The top diagnosis‐related groups for the entire cohort included: heart failure and shock (12 patients); kidney and urinary tract infections (12 patients); esophagitis, gastroenteritis, and miscellaneous digestive disorders (11 patients); chronic obstructive pulmonary disease (10 patients); renal failure (10 patients); simple pneumonia and pleurisy (7 patients); disorders of the pancreas except malignancy (6 patients); chest pain (5 patients); and cellulitis (5 patients).
| Preintervention (n = 63) | Postintervention (n = 106) | P Value | |
|---|---|---|---|
| |||
| Mean age, year (SD) | 63.0 (15.7) | 64.7 (14.3) | 0.52 |
| Male, n (%) | 25 (40) | 52 (49) | 0.27 |
| Race, n (%) | 0.33 | ||
| White | 29 (46) | 42 (40) | |
| Black | 21 (33) | 28 (26) | |
| Hispanic | 11 (17) | 30 (28) | |
| Unknown | 2 (3) | 6 (6) | |
| Admission glucose, mg/dL (SD) | 188 (90.9) | 203 (96.1) | 0.33 |
| A1C, % (SD) | 8.5 (2.4) | 8.3 (2.4) | 0.85 |
| Insulin use prior to admission, n (%) | 38 (60) | 54 (51) | 0.48 |
| Case mix index, median (IQR) | 0.89 (0.781.11) | 0.91 (0.841.22) | 0.33 |
| Charlson index, n (%) | 0.03 | ||
| 01 | 8 (13) | 28 (26) | |
| 23 | 29 (46) | 27 (26) | |
| 45 | 15 (24) | 29 (27) | |
| >5 | 11 (17) | 22 (21) | |
| Known history of diabetes, n (%) | 62 (98) | 96 (91) | 0.06 |
With respect to insulin ordering practices, there was no significant difference in the use of basal insulin in hyperglycemic patients between the preintervention period and postintervention period (81% versus 91%; P = 0.17), nor in the dose of basal insulin prescribed (results not shown), but there was an increase in the use of scheduled nutritional insulin for those patients with hyperglycemia receiving nutrition: 40% versus 75%, P < 0.001 (Table 3). The percent of patients receiving supplemental (sliding scale) insulin by itself (ie, without ever receiving basal or nutritional insulin) was lower during the postintervention period (29% versus 8%, P < 0.001). Nonsignificant differences were seen in the rates of prescribing an appropriate dose and type of nutritional insulin. Notably, there was no difference at all in the proportion of patient‐days in which insulin adjustments were made when 2 or more episodes of hyperglycemia or hypoglycemia were present during the previous day (56% of patient‐days in both groups; P = 0.90).
| Preintervention (n = 63) | Postintervention (n = 106) | Unadjusted Effect Size (95% CI) | Adjusted Effect Size (95% CI) | |
|---|---|---|---|---|
| ||||
| Mean percent glucose readings 60180 mg/dL per patient (SD) | 59.1 (0.28) | 64.7 (0.27) | +5.6 (3.0 to +14.3) | +9.7 (+0.6 to +18.8)*, |
| Patient‐day weighted mean glucose, mg/dL (SD) | 174.7 (60.0) | 164.6 (54.2) | 10.1 (1.6 to 18.5) | 15.6 (6.4 to 24.9), |
| Percent patient‐days with any glucose <60 mg/dL | 16/293 (5.5%) | 26/424 (6.1%) | 1.1 (0.6 to 2.1) | 1.1 (0.6 to 2.1) |
| Percent patient‐days with any glucose <40 mg/dL | 3/293 (1.0%) | 5/424 (1.2%) | 1.3 (0.3 to 5.9) | 1.1 (0.3 to 5.1) |
| Hospital length of stay, hours, mean (SD) | 112.2 (63.3) | 86.0 (89.6) | 30% (5% to 51%) | 25% (6% to 44%),∥ |
| Basal insulin if inpatient hyperglycemia (2 or more readings >180 mg/dL) | 39/48 (81%) | 67/74 (91%) | 2.2 (0.8 to 6.4) | |
| Nutritional insulin if inpatient hyperglycemia and PO intake | 19/48 (40%) | 53/71 (75%) | 4.5 (2.0 to 9.9), | |
| Adequate initial dose of nutritional insulin (home dose or 0.200.42 units/kg/day)# | 2/9 (22%) | 22/49 (45%) | 2.9 (0.5 to 15.1) | |
| Supplemental insulin alone (without basal or nutritional insulin) | 16/56 (29%) | 7/92 (8%) | 0.2 (0.08 to 0.5), | |
| Insulin changed if previous day's glucose out of range (2 or more values <60 or >180 mg/dL) | 70/126 (56%) | 76/135 (56%) | 1.0 (0.6 to 1.6) | |
| A1C tested during hospitalization if not available within 30 days prior | 38/63 (60%) | 74/106 (70%) | 1.5 (0.8 to 2.9) | |
The primary outcome, the mean percent of glucose readings between 60 and 180 mg/dL per patient, was 59.1% in the preintervention period and 64.7% in the postintervention (P = 0.13 in unadjusted analysis; Table 3). When adjusted for A1C, admission glucose, and insulin use prior to admission, the adjusted absolute difference in the percent of glucose readings within range was 9.7% (95% confidence interval [CI], 0.6%‐18.8%; P = 0.04; Table 3). Regarding other measures of glucose control, the patient‐day weighted mean glucose was 174.7 mg/dL in the preintervention period and 164.6 mg/dL postintervention (P = 0.02), and there was no significant difference in the percent of patient‐days with any hypoglycemia (glucose <60 mg/dL) or severe hypoglycemia (glucose <40 mg/dL; Table 3). There were also no significant differences in the mean number of hypoglycemic events per patient‐day (6.8 versus 6.6 per 100 patient‐days; relative risk, 0.95; 95% CI, 0.541.67; P = 0.87) or severe hypoglycemic events per patient‐day (1.0 versus 1.4 per 100 patient‐days; relative risk, 1.38; 95% CI, 0.355.53; P = 0.65).
We also compared hospital length of stay in hours between the study groups (Table 3). Length of stay (LOS) was shorter in the postintervention arm in unadjusted analyses (112 versus 86 hours; P < 0.001), and this difference persisted when adjusted for patient insurance, race, gender, and Charlson comorbidity score (25% shorter; 95% CI, 6%‐44%). A comparison of LOS among nonstudy patients on the PACE service during these 2 time periods revealed no difference (105 versus 101 hours). When the length of stay analysis was limited to study patients with a known diagnosis of diabetes, the adjusted effect size was a 31% relative decrease in length of stay.
Figure 1A shows the percent glucose readings within range per patient by hospital day. The greatest differences between groups can be seen on hospital days 2 and 3 (11% absolute differences on both days). Similarly, Figure 1B shows the mean glucose per patient by hospital day. Again, the biggest differences are seen on hospital days 2 and 3 (20 and 23 mg/dL difference between groups, respectively). In both cases, only the day 3 comparisons were significantly different between study groups.

DISCUSSION
In this before‐after study, we found that a multifaceted intervention consisting of a subcutaneous insulin protocol, focused education, and an order set built into the hospital's CPOE system was associated with a significantly higher percentage of glucose readings within range per patient in analyses adjusted for patient demographics and severity of diabetes. We also found a significant decrease in patient‐day weighted mean glucose, a marked increase in appropriate use of scheduled nutritional insulin, and a concomitant decrease in sliding scale insulin only regimens during the postintervention period. Moreover, we found a shorter length of stay during the postintervention period that persisted after adjustment for several clinical factors. Importantly, the interventions described in this study require very few resources to continue indefinitely: printing costs for the management protocol, 4 hours of education delivered per year, and routine upkeep of an electronic order set.
Because this was a before‐after study, we cannot exclude the possibility that these improvements in process and outcome were due to cointerventions and/or temporal trends. However, we know of no other interventions aimed at improving diabetes care in this self‐contained service of nurses, PAs, and hospitalists. Moreover, the process improvements, especially the increase in scheduled nutritional insulin, were rather marked, unlikely to be due to temporal trends alone, and likely capable of producing the corresponding improvements in glucose control. That glucose control stopped improving after hospital day 3 may be due to the fact that subsequent adjustment to insulin orders occurred infrequently and no more often than prior to the intervention. That we did not see greater improvements in glycemic control overall may also reflect the fact that 81% of study patients with inpatient hyperglycemia received basal insulin prior to the intervention.
The reduction in patient LOS was somewhat surprising given the relatively small sample size. However, the results are consistent with those of other studies linking hyperglycemia to LOS18, 19 and we found no evidence for a temporal trend toward lower LOS on the PACE service as a whole during the same time period. While a greater proportion of patients on the PACE service were in the study in the post‐intervention period compared with the preintervention period, we found no evidence that the difference in length of stay was due to increased surveillance for nondiabetics, especially because eligibility criteria depended on phlebotomy glucose values, which were uniformly tested in all inpatients. Also, effects on length of stay were actually stronger when limited to patients with known diabetes. Finally, we controlled for several predictors of length of stay, although we still cannot exclude the possibility of unmeasured confounding between groups.
Since ADA and ACE issued guidelines for inpatient management of diabetes and hyperglycemia, many institutions have developed subcutaneous insulin algorithms, educational curricula, and/or order sets to increase compliance with these guidelines and improve glycemic control. Some of these efforts have been studied and some have been successful in their efforts.13, 14, 2023 Unfortunately, most of these programs have not rigorously assessed their impact on process and outcomes, and the most effective studies published to date have involved interventions much more intensive than those described here. For example, Rush University's intervention was associated with a 50 mg/dL decrease in mean blood glucose but involved an endocrinologist rounding twice daily with house officers for 2 weeks at a time.13 At Northwestern University, a diabetes management service run by nurse practitioners was established, and the focus was on the conversion from intravenous to subcutaneous insulin regimens.14 The RABBIT 2 study that demonstrated the benefits of a basal‐bolus insulin regimen used daily rounding with an endocrinologist.12 More modestly, a program in Pitt County Memorial Hospital in Greenville, NC, relied mostly on diabetes nurse case managers, a strategy which reduced hospital‐wide mean glucose levels as well as LOS, although the greatest improvements in glycemic control were seen in the ICU.19 Our findings are much more consistent with those from University of California San Diego, as yet unpublished, which also used an algorithm, computerized order set, education, as well as continuous quality improvement methods to achieve its aims.22
Our study has several limitations, including being conducted on 1 general medicine service at 1 academic medical center. Moreover, this service, using a physician assistant/hospitalist model, a closed geographic unit, and fairly generous staffing ratio, is likely different from those in many settings and may limit the generalizability of our findings. However, this model allowed us to conduct the study in a laboratory relatively untouched by other cointerventions. Furthermore, the use of PAs in this way may become more common as both academic and community hospitals rely more on mid‐level providers. Our study had a relatively low percentage of patients without a known diagnosis of diabetes compared with other studies, again potentially but not necessarily limiting generalizability. This finding has been shown in other studies at our institution24 and may be due to the high rate of screening for diabetes in the community. Another limitation is that this was a nonrandomized, before‐after trial. However, all subjects were prospectively enrolled to improve comparability, and we performed rigorous adjustment for multiple potential confounding factors. Also, this study had limited statistical power to detect differences in hypoglycemia rates. The preintervention arm was smaller than planned due to fewer diabetic patients than expected on the service and a higher number of exclusions; we prolonged the postintervention period to achieve the desired sample size for that arm of the study.
Our study also has several strengths, including electronic capture of many processes of care and a methodology to operationalize them into measures of protocol adherence. Our metrics of glycemic control were rigorously designed and based on a national task force on inpatient glycemic control sponsored by the Society of Hospital Medicine, with representation from the ADA and AACE.25
Potential future improvements to this intervention include modifications to the daily adjustment algorithm to improve its usability and ability to improve glucose control. Another is the use of high‐reliability methods to improve order set use and daily insulin adjustment, including alerts within the CPOE system and nurse empowerment to contact medical teams if glucose levels are out of range (eg, if greater than 180 mg/dL, not just if greater than 350 or 400 mg/dL). Future research directions include multicenter, randomized controlled trials of these types of interventions and an analysis of more distal patient outcomes including total healthcare utilization, infection rates, end‐organ damage, and mortality.
In conclusion, we found a relationship between a relatively low‐cost quality improvement intervention and improved glycemic control in the non‐ICU general medical setting. Such a finding suggests the benefits of the algorithm itself to improve glucose control and of our implementation strategy. Other institutions may find this intervention a useful starting point for their own quality improvement efforts. Both the algorithm and implementation strategy are deserving of further improvements and future study.
Acknowledgements
We thank Paul Szumita, Karen Fiumara, Jennifer Trujillo, and the other members of the BWH Diabetes Pharmacy and Therapeutics Subcommittee for their help designing and implementing the intervention; Aubre McClendon, Nicole Auclair, Emily Dattwyler, Mariya Fiman, and Alison Pietras for valuable research assistance; Deborah Williams for data analysis; Amy Bloom for project support; and Stuart Lipsitz for biostatistical expertise.
APPENDIX
INPATIENT DIABETES MANAGEMENT PROTOCOL
Management of Diabetes and Hyperglycemia in Hospitalized Non‐ICU Patients
Rationale
Increasing data show a strong association between hyperglycemia and adverse inpatient outcomes. The American Diabetes Association and the American College of Clinical Endocrinology recommend all glucose levels be below 180 mg/dL in non‐ICU patients. Because hospitalizations are unstable situations, even patients who are well controlled on oral agents as outpatients are usually best managed with insulin.
Insulin may be safely administered even to patients without previously diagnosed diabetes. As long as the prescribed doses are below what is normally produced by the pancreas, the patient will not become hypoglycemic. If the glucose level drops, endogenous insulin secretion will reduce to compensate.
Total insulin requirements in insulin‐sensitive patients (eg, type 1 diabetes mellitus) is 0.50.7/units/kg/day. Insulin requirements in insulin‐resistant type 2 diabetic patients may vary greatly, and can exceed 12 units/kg/day. A conservative estimate for initial insulin therapy in any patient with diabetes is to start with the type 1 diabetes mellitus dose, 0.50.7 units/kg/day.
Overview
Effective inpatient insulin regimens typically include 3 components:
Basal insulin (eg, scheduled NPH or insulin glargine [Lantus]), which is used to manage fasting and premeal hyperglycemia.
Nutritional or prandial insulin (eg, scheduled regular insulin, insulin lispro [Humalog] or insulin aspart [Novolog]) which controls hyperglycemia from nutritional (eg, discrete meals, TPN, IV dextrose) sources.
Supplemental or correctional insulin (eg, regular insulin, insulin lispro, or insulin aspart), which is used in addition to scheduled insulin to meet unexpected basal hyperglycemia that is not covered by the scheduled insulin.
Sample Orders (Not for Patients with Uncontrolled Type 1 Diabetes, DKA, Hyperglycemic Hyperosmolar State, or Other Absolute Need for IV Insulin)
Check (fingerstick) capillary blood glucose qAC, qHS.
NPH insulin subcutaneously (SC) ___ units qAM, ___ units qHS.
Insulin aspart SC ___ units pre‐breakfast, ___ units pre‐lunch, ___ units pre‐dinner, hold if NPO or premeal BS <60 mg/dL; give 015 minutes before meals.
Insulin aspart SC sliding scale (see Table 6) qAC, in addition to standing nutritional insulin, 015 minutes before meals.
For BS <60 mg/dL:
If patient can take PO
Give 15 g of fast acting carbohydrate (4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets).
Repeat finger capillary glucose every 15 (q15) minutes and repeat above (5.a.i.) if BG <60 mg/dL.
When BG >60 mg/dL, give snack or meal in a half‐hour.
If patient cannot take PO
Give 25 mL of 50% dextrose (D50) as an IV push.;
Repeat finger capillary glucose q15 minutes and repeat above (5.b.i.) if BG <80 mg/dL.
Guidelines
Stop oral diabetes agents in most patients (see Table 7 for list of contraindications and precautions).
Check bedside blood glucose (BBG or fingerstick) qAC and qHS (or at 0600 hours, 1200 hours, 1800 hours, and 2400 hours if no discrete meals).
Estimate total daily insulin requirement:
For most patients, conservative estimate is 0.50.7 units/kg/day, but may be much higher.
Reasons for lower end of the range: renal insufficiency, small size, insulin sensitive (eg, type 1), recent hypoglycemia, decreasing doses of steroids, older age.
Reasons for higher end of the range: obese, initiation or increasing doses of steroids, marked hyperglycemia.
Start basal insulin if any premeal BG >140 mg/dL and no recent glucose <60 mg/dL off insulin (Table 5).
Start nutritional or prandial insulinhold if nutrition is stopped/held or premeal BS <60 (Table 5).
Start supplemental/correctional insulin in addition to nutritional (prandial) insulin (Table 6):
Discrete meals: Insulin aspart qAC (with nutritional insulin). 0
No discrete meals: Regular insulin q6h.
On a daily basis, adjust scheduled insulin based on previous days' blood sugars:
Add up total insulin given the previous day, including scheduled and supplemental insulin, to determine new total daily insulin requirement.
Adjust total daily insulin requirement based on clinical considerations (eg, give more if marked hyperglycemia, eating more, improving renal function, increasing steroids; give less if eating less, worsening renal function, tapering steroids, recovering from severe illness).
Give 50% of requirement as basal and 50% as nutritional, as above (may need proportionately less nutritional insulin if appetite poor or unknown).
Adjust sliding scale if needed based on total scheduled insulin dose (see step 6, above).
For BG <60 mg/dL:
If patient can take PO, give 15 g of fast acting carbohydrate.
(4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets; not juice plus sugar).
Repeat finger capillary glucose q15 minutes and repeat above if BG <60.
When BG >60, give snack or meal in half an hour.
If patient cannot take PO, give 25 mL of D50 as IV push.
Check finger capillary glucose q15 minutes and repeat above if BG <80.
Discharge orders:
Patient should be discharged home on a medication regimen that was similar to the admission regimen (ie, the regimen prescribed by their PCP). Exceptions include
The patient has a contraindication to an admission medication.
There is evidence of severe hyperglycemia (eg, very high A1C) or hypoglycemia on admission regimen.
If a patient is admitted with no insulin, and requires insulin to be continued as an outpatient (eg, newly‐diagnosed type 1 diabetes, A1C very high, and contraindication to or on maximum oral regimen), limit discharge insulin regimen to no more than 1 injection per day (eg, hs NPH; an exception to this is for type 1 diabetic patients, who are optimally treated with 34 injections/day). Make sure the patient has prompt follow‐up with their primary care provider (PCP).
Avoid discharging home on sliding scale.
If a patient is going to require insulin injections and self‐monitoring blood glucose as an outpatient, make sure they are instructed about how to perform these.
Indications for calling an endocrine consult:
Labile blood sugars.
Prolonged periods of NPO, eg, for procedures, especially in patients with type 1 diabetes
Marked hyperglycemia despite following this guideline.
Question of type 1 versus type 2 versus other type of diabetes. 0
Basil Insulin Guidelines Home Insulin Regimen Starting Dose of Basal Insulin Considerations NOTE: Patients with T1DM require basal insulin at all times! Basal never should be held!
Abbreviations: NPO, nothing by mouth.
On basal (eg, NPH or glargine) insulin at home Patient's home dose of NPH or glargine If NPO, consider starting half of NPH or glargine home dose, unless hyperglycemic at home. Not on basal (eg, NPH or glargine) insulin at home NPH 50% of total daily insulin requirement, given qHS or split qAM/qHS (maximum starting dose 20 units/day) Same dose if patient has previously diagnosed or undiagnosed diabetes Nutritional Insulin Guidelines Type of Nutrition Common Nutritional Regimens Sample Starting Doses Abbreviation: qAM, every morning; qHS, at bed time.
If receiving cycled tube feeds at night, give nutritional NPH qHS only.
Discrete meals Aspart given 015 minutes before meals Home dose, if known or 50% of total insulin requirement, split over 3 meals, may need less if poor or unknown appetite Continuous tube feeding,* IV dextrose NPH qHS or qAM/qHS 50% of total insulin requirement (in addition to basal dose), may need less if not at goal caloric intake Glargine given every day (qd), anytime Regular every 6 hours (q6h) Sample Supplemental/Correctional Insulin Scales Blood Glucose Scheduled Insulin < 40 Units/Day Scheduled Insulin of 4080 Units/Day Scheduled Insulin > 80 Units/Day Individualized NOTE: Avoid supplemental insulin qHS unless patient is very hyperglycemic and obese.
150199 1 unit 1 unit 2 units ____ units 200249 2 units 3 units 4 units ____ units 250299 3 units 5 units 7 units ____ units 300349 4 units 7 units 10 units ____ units >349 5 units + call HO 8 units + call HO 12 units + call HO ___ units + call HO Notes on Oral Agents Agents Considerations Metabolism Sulfonylureas/secretagogues: glyburide, glipizide, glimeperide (Amaryl); repaglinide (Prandin); nateglinide (Starlix) Risk for hypoglycemia Metabolized in liver; Glyburide metabolized to active metabolites; 50% renally eliminated Metformin Contraindicated in heart failure and renal dysfunction (creatinine [Cr] >1.5 mg/dL in men and 1.4 mg/dL in women) Eliminated renally Should be held at time of iodinated contrast studies. (May be restarted after normal postcontrast renal function is confirmed) Adverse effects include diarrhea, nausea, and anorexia Thiazolidinediones: pioglitazone (Actos), rosiglitazone (Avandia) Contraindicated in class III and IV heart failure Metabolized in liver Use with caution in patients with edema Adverse effects include increased intravascular volume Slow onset of action Avoid in hepatic dysfunction Glucosidease inhibitors: acarbose (Precose), miglitol (Glycet) Gastrointestinal intolerance Acarbose eliminated in gut and renally
Diabetes mellitus and/or inpatient hyperglycemia are common comorbid conditions in hospitalized patients. Recent surveys show that over 90% of hospitalized diabetic patients experience hyperglycemia (>200 mg/dL), and in nearly 1 in 5 of these patients hyperglycemia persists for 3 days or more.1 Hyperglycemia among inpatients without a previous history of diabetes mellitus is also very common.2 Observational studies have shown that hyperglycemia in hospitalized patients is associated with adverse outcomes including infectious complications, increased length of stay, and increased mortality.27 Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units.8, 9
Based on the available data, the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90 to 130 mg/dL and peak postprandial glucose levels <180 mg/dL in hospitalized nonintensive care unit (ICU) patients.10 To reach these targets, the ADA and American College of Endocrinology (ACE) suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.11 Protocols should promote the use of continuous intravenous insulin infusions or scheduled basal‐bolus subcutaneous insulin regimens. Subcutaneous insulin protocols should include target glucose levels, basal, nutritional, and supplemental insulin, and daily dose adjustments.6 A recent randomized controlled trial of non‐ICU inpatients demonstrated that such a basal‐bolus insulin regimen results in improved glucose control compared with a sliding scale only regimen.12
To date, few published studies have investigated the best ways to implement such management protocols; those that have are often resource‐intensive, for example involving daily involvement of nurse practitioners or diabetologists.13, 14 It is therefore not known how best to implement an inpatient diabetes management program that is effective, efficient, and self‐perpetuating. At Brigham and Women's Hospital (BWH), we have been refining a subcutaneous insulin protocol, focused provider education, and more recently a computerized order set to overcome barriers related to fear of hypoglycemia, delays in insulin prescribing, and unfamiliarity with inpatient glucose management.15 The aims of this current trial were to evaluate the effects of these interventions on a geographically localized general medical service previously naive to these interventions to evaluate their effects on glycemic control, patient safety, and processes of care. We hypothesized that these interventions would improve glycemic control and increase use of basal‐bolus insulin orders without increasing the rate of hypoglycemia.
METHODS
Setting and Participants
This prospective, before‐after trial was conducted at BWH from July 15, 2005 through June 22, 2006. Eligible subjects were patients scheduled for admission to the BWH Physician Assistant/Clinician Educator (PACE) Service with either a known diagnosis of type 2 diabetes mellitus or inpatient hyperglycemia (at least 1 random laboratory glucose >180 mg/dL). The PACE service is a geographically‐localized general medicine service of up to 15 beds where patients are cared for by a single cadre of nurses, 2 physician's assistants (PAs), and 1 hospitalist attending. A moonlighter covers the service at night. The PACE service does not accept patients transferred from other acute care hospitals or from ICUs, but does not otherwise have triage guidelines related to diagnosis, complexity, or acuity. Patients were excluded if they had type 1 diabetes, presented with hyperosmolar hyperglycemic state (HHS) or diabetic ketoacidosis (DKA), received total parenteral nutrition (TPN), or were receiving palliative care. This study was approved by the BWH Institutional Review Board; patient consent was deemed not to be necessary for this study given the relatively nonsensitive nature of the data, noninvasive means of data collection, and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
The study intervention consisted of three components, initiated in January 2006:
Glycemic management protocol: a multidisciplinary team of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (Jennifer Trujillo) developed a subcutaneous insulin protocol based on ADA guidelines (Table 1; see the appendix for complete protocol). The protocol was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee and refined through 6 months of pilot testing on other general medical services.15 The protocol consisted of a set of specific treatment recommendations, including: (1) bedside glucose monitoring; (2) stopping oral diabetes agents in most patients; (3) estimating total daily insulin requirements; (4) prescribing basal, nutritional, and supplemental insulin based on the patient's total insulin requirements, preadmission medication regimen, and nutritional status; (5) adjusting insulin on a daily basis as needed; (6) managing hypoglycemia; (7) suggestions for discharge orders; and (8) indications for an endocrinology consultation. The protocol was printed as a pocket guide, distributed to all members of the PACE service, and used to guide all other interventions.
Diabetes education: all PAs received 2 one‐hour educational sessions: a lecture by a diabetologist (M.L.P.) reviewing the rationale for tight glycemic control and general principles of management, and a workshop by a hospitalist (J.L.S.) in which specific cases were reviewed to illustrate how the protocol could be used in practice (eg, when oral agents could be safely continued, how to prescribe insulin on admission, and how to make subsequent adjustments in dose). All hospitalist attendings received a 1‐hour lecture summarizing the above material. All nurses on the service received a lecture that focused on issues unique to nursing care, such as insulin administration, glucose testing, managing patients with unpredictable oral (PO) intake, and patient education. (All materials are available from the authors upon request).
Order Set: an order set, built into BWH's proprietary computer provider order entry (CPOE) system, was created to parallel the glycemic management protocol and facilitate insulin orders for patients eating discrete meals, receiving continuous liquid enteral nutrition (tube feeds), or receiving nothing by mouth (NPO). Other components of the order set facilitated glucose monitoring and other laboratory tests and ordering consultation when appropriate.
| Oral Agents | Stop Oral Agents in Most Patients |
|---|---|
| |
| Glucose testing | Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if NPO |
| Insulin | |
| 1. Estimate total daily insulin dose | 0.5 to 0.7 units/kg/day, depending on patient's age, size, renal function, insulin sensitivity, history of hypoglycemia, and steroid use |
| 2. Start basal insulin | Patient's home dose or 50% of calculated total daily dose; NPH qAM/qHS or insulin glargine qHS; If NPO, use one‐half the home dose unless hyperglycemic |
| 3. Start nutritional insulin if not NPO | Patient's home dose or 50% of calculated total daily dose, less if poor or unknown intake; discrete meals: insulin aspart split over 3 meals, 0 to 15 minutes prior to eating; continuous tube feeds or IV dextrose: regular insulin every 6 hours |
| 4. Start correctional insulin | 1 of 3 scales provided based on total daily dose of insulin; same type as nutritional insulin; regular insulin if NPO |
| 5. Daily adjustment | Calculate total administered dose from prior day, adjust for degree of hyperglycemia or hypoglycemia, renal function, PO intake, steroid use, and degree of illness, and redistribute as 50% basal, 50% nutritional, or 100% basal if NPO |
| Hypoglycemia orders | Juice, IV dextrose, or IM glucagon depending on ability to take oral nutrition and IV access |
| Discharge orders | Based on A1C: either home regimen, titration of home regimen, or new insulin regimen (if latter, simple regimen with aggressive patient education and prompt follow‐up) |
| Indications for endocrine consultation | Labile blood sugars, poor control, prolonged NPO period, question of type 1 or type 2 diabetes |
Study Protocol and Data Collection
A research assistant prospectively identified eligible patients each weekday by screening all patients scheduled for admission to the PACE service using the daily computerized sign‐out system used on all general medical teams. Specifically, laboratory random glucose levels, inpatient medications, and medical histories were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were then confirmed by medical record review and adjudicated by one study author (J.L.S.) if necessary. Further medical record review was performed to identify specific patient populations (eg, diet‐controlled, steroid‐induced, or previously undiagnosed diabetes), determine preadmission diabetes medications, and determine the patient's weight. Hospital computerized clinical and administrative records were abstracted to obtain patient demographics (age, sex, race, insurance status), laboratory data (glucose level on admission, A1C level [taken during or within 6 months prior to admission]), clinical data (length of stay, billing‐based Charlson comorbidity score,16 and diagnosis‐related group [DRG] case mix index), all inpatient insulin and oral diabetes medication orders, frequency of bedside glucose testing, and diet orders. Electronic medication administration record (eMAR) data were used to determine all doses and times of insulin administration.
Outcomes
The primary outcome was the mean percent of glucose readings between 60 and 180 mg/dL per patient (ie, calculated for each patient and averaged across all eligible patients in each study arm). Only bedside glucose readings were used given the lack of additional useful information typically provided by laboratory (venous plasma) glucose readings.17 Readings drawn within 1 hour of a previous reading were excluded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Only readings while on the study service were used. Readings on hospital day 1 were excluded because our intervention was expected to have little impact on the first day's glucose control; for patients with undiagnosed diabetes, data collection began the day following the first elevated glucose reading. Readings beyond hospital day 14 were also excluded to avoid biased data from patients with exceptionally long lengths of stay.
Secondary outcomes included the following:
Glycemic control:
Patient‐day weighted mean glucose (ie, mean glucose for each patient‐day, averaged across all patient days);
Mean glucose per patient for each hospital day (days 17).
Patient safety:
Proportion of patient‐days with any glucose reading <60 mg/dL (hypoglycemia) and <40 mg/dL (severe hypoglycemia).
Processes of care:
Use of any NPH insulin or insulin glargine (basal) insulin during the hospitalization if 2 or more glucose readings were >180 mg/dL.
Adequacy of basal dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission basal dose or 0.20 to 0.42 units/kg if not known or not taken prior to admission. If not eating, half the above calculations.
Use of any scheduled nutritional insulin during the hospitalization if ever prescribed a diet and 2 or more glucose readings were greater than 180 mg/dL.
Adequacy of nutritional dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission nutritional dose or 0.20 to 0.42 units/kg/day if not known or not taken prior to admission. Patients on clear liquid diets, enteral feeds, or receiving glucocorticoids were excluded from this analysis.
Correct type of nutritional insulin: if eating discrete meals, insulin aspart (the rapid‐acting insulin on formulary at BWH); if prescribed tube feeds, regular insulin.
Use of supplemental insulin by itself (without scheduled basal or nutritional insulin), a marker of poor care.
A1C testing within 1 month prior to or during hospitalization.
Clinical inertia: if at least two glucose readings <60 mg/dL or >180 mg/dL on a patient‐day, lack of any change to any insulin order the following day if still on the study service.
Healthcare utilization:
Hospital length of stay in hours, calculated from the exact time of admission until the exact time of discharge, using hospital administrative data.
Analyses
Study results were compared prior to the intervention (July 15 through December 12, 2005) with those during the intervention (January 18 through June 20, 2006). Patient data and clinical outcomes were analyzed descriptively using proportions, means with standard deviations (SDs), or medians with interquartile ranges (IQRs) as appropriate. Comparisons between groups were calculated using Fisher's exact test for dichotomous and categorical variables, and Student t test or Wilcoxon rank sum test for continuous variables as appropriate. The primary outcome was first analyzed using linear regression with study group as the independent variable and percent of glucose readings within range per patient as the dependent variable. We then adjusted for potential confounders by putting each covariate into the model, one at a time. All significant predictors of the outcome at a P value <0.10 were retained in the final model. We used general estimating equations to adjust for clustering of results by each PA. Similar analyses were performed for hospital length of stay per patient using a negative binomial model, so chosen because it fit the data distribution much better than the typically used Poisson model. With a planned sample size of 115 patients and 1250 glucose readings per arm, an intraclass correlation coefficient of 0.10, and an alpha of 0.05, the study had 90% power to detect an increase in percent of glucose readings in range from 67% to 75%. All analyses were based on the intention‐to‐treat principle. Except as above, 2‐sided P values <0.05 were considered significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 248 potential patients for the study. We subsequently excluded 79 patients for the following reasons: no glucose readings beyond hospital day 1 while on PACE service (34 patients); never admitted to PACE service (15 patients); no diabetes or inpatient hyperglycemia (9 patients, mostly patients prescribed an insulin sliding scale prophylactically to avoid steroid‐induced hyperglycemia); type 1 diabetes (13 patients); TPN, DKA, or HHS (5 patients); and palliative care (3 patients). The remaining 169 patients included 63 from the preintervention period(out of 489 total admissions to the PACE service; 13%) and 106 patients in the postintervention period (out of 565 admissions; 19%). These patients had 2447 glucose readings, or an average of 3.6 glucose readings per monitored patient‐day in the preintervention period and 3.3 glucose readings per patient‐day in the postintervention period. Even including the 34 patients who were excluded for lack of glucose readings, glucose data were still available for 717 out of a potential 775 patient‐days (93%). Characteristics for all included patients are shown in Table 2. The mean admission glucose was 197 mg/dL, mean A1C was 8.4%, 54% of the patients were prescribed insulin prior to admission, and 7% had no prior diagnosis of diabetes. There were no significant differences in baseline characteristics between the 2 patient groups except for Charlson score, which was higher in the preintervention group (87% versus 74% with score 2 or higher; Table 2). The top diagnosis‐related groups for the entire cohort included: heart failure and shock (12 patients); kidney and urinary tract infections (12 patients); esophagitis, gastroenteritis, and miscellaneous digestive disorders (11 patients); chronic obstructive pulmonary disease (10 patients); renal failure (10 patients); simple pneumonia and pleurisy (7 patients); disorders of the pancreas except malignancy (6 patients); chest pain (5 patients); and cellulitis (5 patients).
| Preintervention (n = 63) | Postintervention (n = 106) | P Value | |
|---|---|---|---|
| |||
| Mean age, year (SD) | 63.0 (15.7) | 64.7 (14.3) | 0.52 |
| Male, n (%) | 25 (40) | 52 (49) | 0.27 |
| Race, n (%) | 0.33 | ||
| White | 29 (46) | 42 (40) | |
| Black | 21 (33) | 28 (26) | |
| Hispanic | 11 (17) | 30 (28) | |
| Unknown | 2 (3) | 6 (6) | |
| Admission glucose, mg/dL (SD) | 188 (90.9) | 203 (96.1) | 0.33 |
| A1C, % (SD) | 8.5 (2.4) | 8.3 (2.4) | 0.85 |
| Insulin use prior to admission, n (%) | 38 (60) | 54 (51) | 0.48 |
| Case mix index, median (IQR) | 0.89 (0.781.11) | 0.91 (0.841.22) | 0.33 |
| Charlson index, n (%) | 0.03 | ||
| 01 | 8 (13) | 28 (26) | |
| 23 | 29 (46) | 27 (26) | |
| 45 | 15 (24) | 29 (27) | |
| >5 | 11 (17) | 22 (21) | |
| Known history of diabetes, n (%) | 62 (98) | 96 (91) | 0.06 |
With respect to insulin ordering practices, there was no significant difference in the use of basal insulin in hyperglycemic patients between the preintervention period and postintervention period (81% versus 91%; P = 0.17), nor in the dose of basal insulin prescribed (results not shown), but there was an increase in the use of scheduled nutritional insulin for those patients with hyperglycemia receiving nutrition: 40% versus 75%, P < 0.001 (Table 3). The percent of patients receiving supplemental (sliding scale) insulin by itself (ie, without ever receiving basal or nutritional insulin) was lower during the postintervention period (29% versus 8%, P < 0.001). Nonsignificant differences were seen in the rates of prescribing an appropriate dose and type of nutritional insulin. Notably, there was no difference at all in the proportion of patient‐days in which insulin adjustments were made when 2 or more episodes of hyperglycemia or hypoglycemia were present during the previous day (56% of patient‐days in both groups; P = 0.90).
| Preintervention (n = 63) | Postintervention (n = 106) | Unadjusted Effect Size (95% CI) | Adjusted Effect Size (95% CI) | |
|---|---|---|---|---|
| ||||
| Mean percent glucose readings 60180 mg/dL per patient (SD) | 59.1 (0.28) | 64.7 (0.27) | +5.6 (3.0 to +14.3) | +9.7 (+0.6 to +18.8)*, |
| Patient‐day weighted mean glucose, mg/dL (SD) | 174.7 (60.0) | 164.6 (54.2) | 10.1 (1.6 to 18.5) | 15.6 (6.4 to 24.9), |
| Percent patient‐days with any glucose <60 mg/dL | 16/293 (5.5%) | 26/424 (6.1%) | 1.1 (0.6 to 2.1) | 1.1 (0.6 to 2.1) |
| Percent patient‐days with any glucose <40 mg/dL | 3/293 (1.0%) | 5/424 (1.2%) | 1.3 (0.3 to 5.9) | 1.1 (0.3 to 5.1) |
| Hospital length of stay, hours, mean (SD) | 112.2 (63.3) | 86.0 (89.6) | 30% (5% to 51%) | 25% (6% to 44%),∥ |
| Basal insulin if inpatient hyperglycemia (2 or more readings >180 mg/dL) | 39/48 (81%) | 67/74 (91%) | 2.2 (0.8 to 6.4) | |
| Nutritional insulin if inpatient hyperglycemia and PO intake | 19/48 (40%) | 53/71 (75%) | 4.5 (2.0 to 9.9), | |
| Adequate initial dose of nutritional insulin (home dose or 0.200.42 units/kg/day)# | 2/9 (22%) | 22/49 (45%) | 2.9 (0.5 to 15.1) | |
| Supplemental insulin alone (without basal or nutritional insulin) | 16/56 (29%) | 7/92 (8%) | 0.2 (0.08 to 0.5), | |
| Insulin changed if previous day's glucose out of range (2 or more values <60 or >180 mg/dL) | 70/126 (56%) | 76/135 (56%) | 1.0 (0.6 to 1.6) | |
| A1C tested during hospitalization if not available within 30 days prior | 38/63 (60%) | 74/106 (70%) | 1.5 (0.8 to 2.9) | |
The primary outcome, the mean percent of glucose readings between 60 and 180 mg/dL per patient, was 59.1% in the preintervention period and 64.7% in the postintervention (P = 0.13 in unadjusted analysis; Table 3). When adjusted for A1C, admission glucose, and insulin use prior to admission, the adjusted absolute difference in the percent of glucose readings within range was 9.7% (95% confidence interval [CI], 0.6%‐18.8%; P = 0.04; Table 3). Regarding other measures of glucose control, the patient‐day weighted mean glucose was 174.7 mg/dL in the preintervention period and 164.6 mg/dL postintervention (P = 0.02), and there was no significant difference in the percent of patient‐days with any hypoglycemia (glucose <60 mg/dL) or severe hypoglycemia (glucose <40 mg/dL; Table 3). There were also no significant differences in the mean number of hypoglycemic events per patient‐day (6.8 versus 6.6 per 100 patient‐days; relative risk, 0.95; 95% CI, 0.541.67; P = 0.87) or severe hypoglycemic events per patient‐day (1.0 versus 1.4 per 100 patient‐days; relative risk, 1.38; 95% CI, 0.355.53; P = 0.65).
We also compared hospital length of stay in hours between the study groups (Table 3). Length of stay (LOS) was shorter in the postintervention arm in unadjusted analyses (112 versus 86 hours; P < 0.001), and this difference persisted when adjusted for patient insurance, race, gender, and Charlson comorbidity score (25% shorter; 95% CI, 6%‐44%). A comparison of LOS among nonstudy patients on the PACE service during these 2 time periods revealed no difference (105 versus 101 hours). When the length of stay analysis was limited to study patients with a known diagnosis of diabetes, the adjusted effect size was a 31% relative decrease in length of stay.
Figure 1A shows the percent glucose readings within range per patient by hospital day. The greatest differences between groups can be seen on hospital days 2 and 3 (11% absolute differences on both days). Similarly, Figure 1B shows the mean glucose per patient by hospital day. Again, the biggest differences are seen on hospital days 2 and 3 (20 and 23 mg/dL difference between groups, respectively). In both cases, only the day 3 comparisons were significantly different between study groups.

DISCUSSION
In this before‐after study, we found that a multifaceted intervention consisting of a subcutaneous insulin protocol, focused education, and an order set built into the hospital's CPOE system was associated with a significantly higher percentage of glucose readings within range per patient in analyses adjusted for patient demographics and severity of diabetes. We also found a significant decrease in patient‐day weighted mean glucose, a marked increase in appropriate use of scheduled nutritional insulin, and a concomitant decrease in sliding scale insulin only regimens during the postintervention period. Moreover, we found a shorter length of stay during the postintervention period that persisted after adjustment for several clinical factors. Importantly, the interventions described in this study require very few resources to continue indefinitely: printing costs for the management protocol, 4 hours of education delivered per year, and routine upkeep of an electronic order set.
Because this was a before‐after study, we cannot exclude the possibility that these improvements in process and outcome were due to cointerventions and/or temporal trends. However, we know of no other interventions aimed at improving diabetes care in this self‐contained service of nurses, PAs, and hospitalists. Moreover, the process improvements, especially the increase in scheduled nutritional insulin, were rather marked, unlikely to be due to temporal trends alone, and likely capable of producing the corresponding improvements in glucose control. That glucose control stopped improving after hospital day 3 may be due to the fact that subsequent adjustment to insulin orders occurred infrequently and no more often than prior to the intervention. That we did not see greater improvements in glycemic control overall may also reflect the fact that 81% of study patients with inpatient hyperglycemia received basal insulin prior to the intervention.
The reduction in patient LOS was somewhat surprising given the relatively small sample size. However, the results are consistent with those of other studies linking hyperglycemia to LOS18, 19 and we found no evidence for a temporal trend toward lower LOS on the PACE service as a whole during the same time period. While a greater proportion of patients on the PACE service were in the study in the post‐intervention period compared with the preintervention period, we found no evidence that the difference in length of stay was due to increased surveillance for nondiabetics, especially because eligibility criteria depended on phlebotomy glucose values, which were uniformly tested in all inpatients. Also, effects on length of stay were actually stronger when limited to patients with known diabetes. Finally, we controlled for several predictors of length of stay, although we still cannot exclude the possibility of unmeasured confounding between groups.
Since ADA and ACE issued guidelines for inpatient management of diabetes and hyperglycemia, many institutions have developed subcutaneous insulin algorithms, educational curricula, and/or order sets to increase compliance with these guidelines and improve glycemic control. Some of these efforts have been studied and some have been successful in their efforts.13, 14, 2023 Unfortunately, most of these programs have not rigorously assessed their impact on process and outcomes, and the most effective studies published to date have involved interventions much more intensive than those described here. For example, Rush University's intervention was associated with a 50 mg/dL decrease in mean blood glucose but involved an endocrinologist rounding twice daily with house officers for 2 weeks at a time.13 At Northwestern University, a diabetes management service run by nurse practitioners was established, and the focus was on the conversion from intravenous to subcutaneous insulin regimens.14 The RABBIT 2 study that demonstrated the benefits of a basal‐bolus insulin regimen used daily rounding with an endocrinologist.12 More modestly, a program in Pitt County Memorial Hospital in Greenville, NC, relied mostly on diabetes nurse case managers, a strategy which reduced hospital‐wide mean glucose levels as well as LOS, although the greatest improvements in glycemic control were seen in the ICU.19 Our findings are much more consistent with those from University of California San Diego, as yet unpublished, which also used an algorithm, computerized order set, education, as well as continuous quality improvement methods to achieve its aims.22
Our study has several limitations, including being conducted on 1 general medicine service at 1 academic medical center. Moreover, this service, using a physician assistant/hospitalist model, a closed geographic unit, and fairly generous staffing ratio, is likely different from those in many settings and may limit the generalizability of our findings. However, this model allowed us to conduct the study in a laboratory relatively untouched by other cointerventions. Furthermore, the use of PAs in this way may become more common as both academic and community hospitals rely more on mid‐level providers. Our study had a relatively low percentage of patients without a known diagnosis of diabetes compared with other studies, again potentially but not necessarily limiting generalizability. This finding has been shown in other studies at our institution24 and may be due to the high rate of screening for diabetes in the community. Another limitation is that this was a nonrandomized, before‐after trial. However, all subjects were prospectively enrolled to improve comparability, and we performed rigorous adjustment for multiple potential confounding factors. Also, this study had limited statistical power to detect differences in hypoglycemia rates. The preintervention arm was smaller than planned due to fewer diabetic patients than expected on the service and a higher number of exclusions; we prolonged the postintervention period to achieve the desired sample size for that arm of the study.
Our study also has several strengths, including electronic capture of many processes of care and a methodology to operationalize them into measures of protocol adherence. Our metrics of glycemic control were rigorously designed and based on a national task force on inpatient glycemic control sponsored by the Society of Hospital Medicine, with representation from the ADA and AACE.25
Potential future improvements to this intervention include modifications to the daily adjustment algorithm to improve its usability and ability to improve glucose control. Another is the use of high‐reliability methods to improve order set use and daily insulin adjustment, including alerts within the CPOE system and nurse empowerment to contact medical teams if glucose levels are out of range (eg, if greater than 180 mg/dL, not just if greater than 350 or 400 mg/dL). Future research directions include multicenter, randomized controlled trials of these types of interventions and an analysis of more distal patient outcomes including total healthcare utilization, infection rates, end‐organ damage, and mortality.
In conclusion, we found a relationship between a relatively low‐cost quality improvement intervention and improved glycemic control in the non‐ICU general medical setting. Such a finding suggests the benefits of the algorithm itself to improve glucose control and of our implementation strategy. Other institutions may find this intervention a useful starting point for their own quality improvement efforts. Both the algorithm and implementation strategy are deserving of further improvements and future study.
Acknowledgements
We thank Paul Szumita, Karen Fiumara, Jennifer Trujillo, and the other members of the BWH Diabetes Pharmacy and Therapeutics Subcommittee for their help designing and implementing the intervention; Aubre McClendon, Nicole Auclair, Emily Dattwyler, Mariya Fiman, and Alison Pietras for valuable research assistance; Deborah Williams for data analysis; Amy Bloom for project support; and Stuart Lipsitz for biostatistical expertise.
APPENDIX
INPATIENT DIABETES MANAGEMENT PROTOCOL
Management of Diabetes and Hyperglycemia in Hospitalized Non‐ICU Patients
Rationale
Increasing data show a strong association between hyperglycemia and adverse inpatient outcomes. The American Diabetes Association and the American College of Clinical Endocrinology recommend all glucose levels be below 180 mg/dL in non‐ICU patients. Because hospitalizations are unstable situations, even patients who are well controlled on oral agents as outpatients are usually best managed with insulin.
Insulin may be safely administered even to patients without previously diagnosed diabetes. As long as the prescribed doses are below what is normally produced by the pancreas, the patient will not become hypoglycemic. If the glucose level drops, endogenous insulin secretion will reduce to compensate.
Total insulin requirements in insulin‐sensitive patients (eg, type 1 diabetes mellitus) is 0.50.7/units/kg/day. Insulin requirements in insulin‐resistant type 2 diabetic patients may vary greatly, and can exceed 12 units/kg/day. A conservative estimate for initial insulin therapy in any patient with diabetes is to start with the type 1 diabetes mellitus dose, 0.50.7 units/kg/day.
Overview
Effective inpatient insulin regimens typically include 3 components:
Basal insulin (eg, scheduled NPH or insulin glargine [Lantus]), which is used to manage fasting and premeal hyperglycemia.
Nutritional or prandial insulin (eg, scheduled regular insulin, insulin lispro [Humalog] or insulin aspart [Novolog]) which controls hyperglycemia from nutritional (eg, discrete meals, TPN, IV dextrose) sources.
Supplemental or correctional insulin (eg, regular insulin, insulin lispro, or insulin aspart), which is used in addition to scheduled insulin to meet unexpected basal hyperglycemia that is not covered by the scheduled insulin.
Sample Orders (Not for Patients with Uncontrolled Type 1 Diabetes, DKA, Hyperglycemic Hyperosmolar State, or Other Absolute Need for IV Insulin)
Check (fingerstick) capillary blood glucose qAC, qHS.
NPH insulin subcutaneously (SC) ___ units qAM, ___ units qHS.
Insulin aspart SC ___ units pre‐breakfast, ___ units pre‐lunch, ___ units pre‐dinner, hold if NPO or premeal BS <60 mg/dL; give 015 minutes before meals.
Insulin aspart SC sliding scale (see Table 6) qAC, in addition to standing nutritional insulin, 015 minutes before meals.
For BS <60 mg/dL:
If patient can take PO
Give 15 g of fast acting carbohydrate (4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets).
Repeat finger capillary glucose every 15 (q15) minutes and repeat above (5.a.i.) if BG <60 mg/dL.
When BG >60 mg/dL, give snack or meal in a half‐hour.
If patient cannot take PO
Give 25 mL of 50% dextrose (D50) as an IV push.;
Repeat finger capillary glucose q15 minutes and repeat above (5.b.i.) if BG <80 mg/dL.
Guidelines
Stop oral diabetes agents in most patients (see Table 7 for list of contraindications and precautions).
Check bedside blood glucose (BBG or fingerstick) qAC and qHS (or at 0600 hours, 1200 hours, 1800 hours, and 2400 hours if no discrete meals).
Estimate total daily insulin requirement:
For most patients, conservative estimate is 0.50.7 units/kg/day, but may be much higher.
Reasons for lower end of the range: renal insufficiency, small size, insulin sensitive (eg, type 1), recent hypoglycemia, decreasing doses of steroids, older age.
Reasons for higher end of the range: obese, initiation or increasing doses of steroids, marked hyperglycemia.
Start basal insulin if any premeal BG >140 mg/dL and no recent glucose <60 mg/dL off insulin (Table 5).
Start nutritional or prandial insulinhold if nutrition is stopped/held or premeal BS <60 (Table 5).
Start supplemental/correctional insulin in addition to nutritional (prandial) insulin (Table 6):
Discrete meals: Insulin aspart qAC (with nutritional insulin). 0
No discrete meals: Regular insulin q6h.
On a daily basis, adjust scheduled insulin based on previous days' blood sugars:
Add up total insulin given the previous day, including scheduled and supplemental insulin, to determine new total daily insulin requirement.
Adjust total daily insulin requirement based on clinical considerations (eg, give more if marked hyperglycemia, eating more, improving renal function, increasing steroids; give less if eating less, worsening renal function, tapering steroids, recovering from severe illness).
Give 50% of requirement as basal and 50% as nutritional, as above (may need proportionately less nutritional insulin if appetite poor or unknown).
Adjust sliding scale if needed based on total scheduled insulin dose (see step 6, above).
For BG <60 mg/dL:
If patient can take PO, give 15 g of fast acting carbohydrate.
(4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets; not juice plus sugar).
Repeat finger capillary glucose q15 minutes and repeat above if BG <60.
When BG >60, give snack or meal in half an hour.
If patient cannot take PO, give 25 mL of D50 as IV push.
Check finger capillary glucose q15 minutes and repeat above if BG <80.
Discharge orders:
Patient should be discharged home on a medication regimen that was similar to the admission regimen (ie, the regimen prescribed by their PCP). Exceptions include
The patient has a contraindication to an admission medication.
There is evidence of severe hyperglycemia (eg, very high A1C) or hypoglycemia on admission regimen.
If a patient is admitted with no insulin, and requires insulin to be continued as an outpatient (eg, newly‐diagnosed type 1 diabetes, A1C very high, and contraindication to or on maximum oral regimen), limit discharge insulin regimen to no more than 1 injection per day (eg, hs NPH; an exception to this is for type 1 diabetic patients, who are optimally treated with 34 injections/day). Make sure the patient has prompt follow‐up with their primary care provider (PCP).
Avoid discharging home on sliding scale.
If a patient is going to require insulin injections and self‐monitoring blood glucose as an outpatient, make sure they are instructed about how to perform these.
Indications for calling an endocrine consult:
Labile blood sugars.
Prolonged periods of NPO, eg, for procedures, especially in patients with type 1 diabetes
Marked hyperglycemia despite following this guideline.
Question of type 1 versus type 2 versus other type of diabetes. 0
Basil Insulin Guidelines Home Insulin Regimen Starting Dose of Basal Insulin Considerations NOTE: Patients with T1DM require basal insulin at all times! Basal never should be held!
Abbreviations: NPO, nothing by mouth.
On basal (eg, NPH or glargine) insulin at home Patient's home dose of NPH or glargine If NPO, consider starting half of NPH or glargine home dose, unless hyperglycemic at home. Not on basal (eg, NPH or glargine) insulin at home NPH 50% of total daily insulin requirement, given qHS or split qAM/qHS (maximum starting dose 20 units/day) Same dose if patient has previously diagnosed or undiagnosed diabetes Nutritional Insulin Guidelines Type of Nutrition Common Nutritional Regimens Sample Starting Doses Abbreviation: qAM, every morning; qHS, at bed time.
If receiving cycled tube feeds at night, give nutritional NPH qHS only.
Discrete meals Aspart given 015 minutes before meals Home dose, if known or 50% of total insulin requirement, split over 3 meals, may need less if poor or unknown appetite Continuous tube feeding,* IV dextrose NPH qHS or qAM/qHS 50% of total insulin requirement (in addition to basal dose), may need less if not at goal caloric intake Glargine given every day (qd), anytime Regular every 6 hours (q6h) Sample Supplemental/Correctional Insulin Scales Blood Glucose Scheduled Insulin < 40 Units/Day Scheduled Insulin of 4080 Units/Day Scheduled Insulin > 80 Units/Day Individualized NOTE: Avoid supplemental insulin qHS unless patient is very hyperglycemic and obese.
150199 1 unit 1 unit 2 units ____ units 200249 2 units 3 units 4 units ____ units 250299 3 units 5 units 7 units ____ units 300349 4 units 7 units 10 units ____ units >349 5 units + call HO 8 units + call HO 12 units + call HO ___ units + call HO Notes on Oral Agents Agents Considerations Metabolism Sulfonylureas/secretagogues: glyburide, glipizide, glimeperide (Amaryl); repaglinide (Prandin); nateglinide (Starlix) Risk for hypoglycemia Metabolized in liver; Glyburide metabolized to active metabolites; 50% renally eliminated Metformin Contraindicated in heart failure and renal dysfunction (creatinine [Cr] >1.5 mg/dL in men and 1.4 mg/dL in women) Eliminated renally Should be held at time of iodinated contrast studies. (May be restarted after normal postcontrast renal function is confirmed) Adverse effects include diarrhea, nausea, and anorexia Thiazolidinediones: pioglitazone (Actos), rosiglitazone (Avandia) Contraindicated in class III and IV heart failure Metabolized in liver Use with caution in patients with edema Adverse effects include increased intravascular volume Slow onset of action Avoid in hepatic dysfunction Glucosidease inhibitors: acarbose (Precose), miglitol (Glycet) Gastrointestinal intolerance Acarbose eliminated in gut and renally
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax.2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- Standards of medical care in diabetes, 2007.Diabetes Care.2007;30(Suppl 1):S4–S41.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3:55–63.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,,.Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation.Diabetes Care.2007;30:993–994.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23:757–765.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,,.Effect of a standardized insulin order set and an insulin management algorithm on inpatient glycemic control and hypoglycemia. Society of Hospital Medicine Annual Meeting, 2007; Dallas, TX;2007.
- ,,, et al.Trends in bed occupancy for inpatients with diabetes before and after the introduction of a diabetes inpatient specialist nurse service.Diabet Med.2006;23:1008–1015.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al. Improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes, 2006. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/GC_Imp_Guide.cfm. Accessed October 2008.
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax.2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- Standards of medical care in diabetes, 2007.Diabetes Care.2007;30(Suppl 1):S4–S41.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3:55–63.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,,.Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation.Diabetes Care.2007;30:993–994.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23:757–765.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,,.Effect of a standardized insulin order set and an insulin management algorithm on inpatient glycemic control and hypoglycemia. Society of Hospital Medicine Annual Meeting, 2007; Dallas, TX;2007.
- ,,, et al.Trends in bed occupancy for inpatients with diabetes before and after the introduction of a diabetes inpatient specialist nurse service.Diabet Med.2006;23:1008–1015.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al. Improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes, 2006. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/GC_Imp_Guide.cfm. Accessed October 2008.
Copyright © 2009 Society of Hospital Medicine
Welcome to My World
Is hospital medicine a bona fide specialty? Do something long enough, and as Justice Potter Stewart said when defining a certain taboo carnal subject many years ago, I know it when I see it. Although working groups may struggle to conceive a master set of core competencies for hospitalists, I will tell you this: no texts are needed, and you know that you are on to something when 2 hospitalists practicing 3000 miles apart shoot each other a knowing glance and, without words, just understand what the other is thinking. After 10 years of practice in several hospitals, I have had enough mind melds to last a lifetime. Who needs science after all? I mean, how many of us can keep a straight face when asked if we have ever heard this line: Ah, yes, Dr. Flansbaum, umm, I am Dr. Smith from the surgical ICU, and we have a patient hospital day 34 status post Whipple that is no longer surgically active. See, you are smiling already. Do I have to finish the sentence for you?
What follows is a collective experience of things that I call the grind: things so small, so inconsequential, that no one will ever cite them individually as the deal breakers of the day. Collectively, they are the fabric of who we are and that little sore on the inside of our cheek that we just have to touch every few minutes in order to remind ourselves of why dermatologists always look so happy. Any accompanying sage lessons are also free of charge.
-
Why at the end of the day, always on a weekend after you have found a comfortable seat as far away from the nurse's station as possible, do you open up the chart and see 1 line of open space left on the last page of the progress notepaper? Even better, why is the note above it a follow‐up from vascular surgery, written in font size 24, in a form of Sanskrit that not even Steven Hawkings would recognize?
-
Okay, how about this: For patients with loooong lengths of stay, how many creative ways can you write Awaiting placement, afebrile, no complaints (in compliance with billing rules of course)? The correct answer is between 16 and 23. A thesaurus helps for word number 1try stable to startand change your pen from fine point to medium point on odd days. Then add tolerated breakfast on Monday, lunch on Tuesday, and dinner on Wednesday. Voila! Who said this was tough?
-
Okay, this one boggles my mind. You wish to auscultate a set of lungs. The patient is sitting on his gown. You attempt to lift the gown, and instead of raising his tush, the patient continues to sit while you tug away. Is this just me? It happens every week.
-
The medical students are great. They are ambitious and make teaching fun. Why though, at 9:59 AM, with an upcoming meeting with your chief at 10, are there no PC terminals at the station available except for the MSIII on MySpace.com with the chart you could not find (for 5 minutes) underneath his clipboard? Yes, Virginia, make me remember those days.
-
Clearly, this is one of the more helpful lines you can get from a consult: Patient needs to be medically optimized, and consider head MRI. Consider? Is not that why I called the consult in the first place? Let us consider not to use the word consider any more. Consider that. I feel better.
-
While we are on the topic of consultants, a dollar goes to you if this has never happened during your time on the wards: (1) the consultant visits, (2) the consultant evaluates, (3) you speak with the consultant, (4) all of you agree that the patient can go home, and (5) you then read the consult after the patient is dressed and his IV is out. Umm, a head CT before discharge and please have the neurosurgery clear the patient before discharge? Am I working in a parallel universe? Too much caffeine? Lord, give me strength.
-
Your beeper goes off at 2:57 PM. At 2:57 PM plus 5 seconds, you call the number you were paged from and no one answers. Does the word ponderous come to mind? This invariably happens every day, of course, typically when I am in the midst of multitasking 4 conversations. However, I extract some form of perverse cosmic revenge when I need to make a call and pick up an open extension from a ringing multiline phone. Invariably, I click the button to engage a line and, oops, good bye caller. I am only kidding; that never happens (is my nose really growing?). Just think, I could have been the one screaming, Is Laverne here?
-
You get an admission, a patient whom you have never met, and his room is listed as 428. You walk in, and the patient nearest to you is a well‐groomed middle‐age individual with a welcoming smile. The patient next to him is breathing fire, screaming at an imaginary executioner, and claiming that you are the guilty party and need to die. Which patient do you think is yours?
-
Those of you who work with housestaff will appreciate this one (file it under systems issue: fix next week): You have a discussion with a patient regarding his PM discharge at 11 AM. You arrange the follow‐up, you review the new medications, you discuss who will pick him up after dinner, and so forth. You get that warm and fuzzy feeling that you have done your job and all is right in the universe. Naturally, you also tell the resident that the patient can go home. Lo and behold, you look at your census the following morning, and the name of the aforementioned patient radiates like a beacon from the screen. You then poke your head into the room, feeling assured that it is merely an error, and the aforementioned patient is lying in bed, smiling and happy to see you. You ask, What happened? The reply, I don't know. Didn't your son come last night to pick you up? The response is yes. After the penetrating ulcer in your stomach bores a little deeper, you discover the official discharge order did not occur, and the patient was content eating chipped beef and sleeping on said contoured mattress 1 more night. Serenity now, serenity now.
-
The fifth vital sign? Is that the new black? Heck, number 5, I think we are up to 11 or 12 these days. Need a new metric installed? You guessed it: add it to the list!
-
Do you ever get LOS fatigue with a particular patient that is so severe you go to bed the previous night and have problems falling asleep? Really, what do you say to a person when his hospital stay exceeds, say, 5 months? Yes, I actually think of topics and issues that I can incorporate into the conversation which will spice up the relationship. New bed sheets, a fresh coat of paint? It would make a good Seinfeld episode, no?
-
Is it me, or is having 2 patients in the same room like being a flight attendant wheeling around the beverage cart? Get one the peanuts, and then the other wants the pretzels. For sure, add 10 minutes to your time in room 728 tomorrow.
-
If a patient is unable to leave the hospital for reasons unrelated to discharge planning (locked out of his house, the child is out of town until the next morning, etc.), why do I feel so naughty when I get off the phone with the MCO medical director after offering explanations? I do not get it. You think that the hospital employs a battery of runners to padlock homes and steal patient's clothing. Who wrote this playbook?
-
I love my consultants. Really, I do. I am not picking on them today. The high points of my day are the exchanges that I have with my subspecialty colleagues. However, the myopia that pervades some sign off notes give me pause. For example, a patient admitted for gastrointestinal bleed, s/p EGD, and stable at 72 hours post arrival receives a consultant note as follows: if patient eating and ambulating, can be discharged home as per PMD. Surely, when the level of transferred oversight shifts to the level of caloric ingestion and sneaker use, well, let us just say that I am all for some new E&M codes. They did not tell me about this in hospitalist school.
-
Don't you love the feeling of your beeper going off, and 30 seconds after the first page, boom, it goes off againboth to the same extension? I mean really, I am a nice guy, but do you really want to rile me up this early in the morning?
-
The nurse pages you from 1 floor away10 seconds from where you are standing. You recognize that number, you knew that it was coming, and for sure, waiting on the other end is that family member who hails from a foreboding place. How quickly does your brain do the computationdo I use the phone and let my fingers do the talking, or make that stroll and have that face‐to‐face summit? No sarcastic comment is needed. I see us now, hands joined, joyfully singing Kumbaya in a loving embrace.
-
Gee, it is not busy today. Say that on the wards and you get a leering glance. However, say that in the emergency room and you will meet your death. There is something about that phrase and the emergency department. The nurses there do not forget, although a Starbucks cappuccino does put a nice salve on the wound.
-
On your day off, do you ever notice that your beeper vibrates on your belt and you are not wearing it? I am not kidding.
-
An irony of life: I have developed an immunity to cigarette smoke in hospital bathrooms. Why is that? It is like peanut butter and jelly. They just seem so happy together.
-
Do you want to transfer that patient to psychiatry? No, no, no, you silly hospitalistdid you not notice that abnormal BUN and atypical lymphocyte on the peripheral smear? Hey, Dr. Freud, can you write me for some of that Prozac too!
-
We need to consult a rulebook on chair ownership. Did you ever notice (a tinge of Andy Rooney here) that case managers own their seats? I know that the world is not quite right when a case manager shoots me that dastardly glare, as if to say, Flee, you silly physician, I live at this station, you are merely my guest! As far as that chair is concerned, perhaps if a small plaque is added to the backrest with a suitable donation, my legs will finally get their deserved daily rest.
-
Finally, do you want to become invisible? Go to the reading board and stand behind a radiologist at 10:30 AM. Do it long enough, and after a few days, you will be saying I'm Good Enough, I'm Smart Enough, and Doggone It, People Like Me! Do you want to disappear completely? Try it on a Friday.
Okay, okay, I will stop there. It is funny, though; this stuff really happens. Despite the aggravation, I see these commonalities as the glue that binds us, assists in building the esprit de corps in our profession, and adds a little levity to the work place. Outside the hospital (not inside, of course), I can confidently state that these routines are part of who I am. After all, it is all about the knowing glance that I mentioned previously. The humorous part is that we are certainly on someone else's list. Probably a nurse, an emergency room doctor, or maybe a physician's assistant is scribing away at this minute, and we are number 7: Those hospitalists really tick me off.
EPILOGUE
Note to selflook in the mirror occasionally; you might learn something.
I apologize to all my nonhospitalist colleagues if you sneered. I love all of you. Today.
Is hospital medicine a bona fide specialty? Do something long enough, and as Justice Potter Stewart said when defining a certain taboo carnal subject many years ago, I know it when I see it. Although working groups may struggle to conceive a master set of core competencies for hospitalists, I will tell you this: no texts are needed, and you know that you are on to something when 2 hospitalists practicing 3000 miles apart shoot each other a knowing glance and, without words, just understand what the other is thinking. After 10 years of practice in several hospitals, I have had enough mind melds to last a lifetime. Who needs science after all? I mean, how many of us can keep a straight face when asked if we have ever heard this line: Ah, yes, Dr. Flansbaum, umm, I am Dr. Smith from the surgical ICU, and we have a patient hospital day 34 status post Whipple that is no longer surgically active. See, you are smiling already. Do I have to finish the sentence for you?
What follows is a collective experience of things that I call the grind: things so small, so inconsequential, that no one will ever cite them individually as the deal breakers of the day. Collectively, they are the fabric of who we are and that little sore on the inside of our cheek that we just have to touch every few minutes in order to remind ourselves of why dermatologists always look so happy. Any accompanying sage lessons are also free of charge.
-
Why at the end of the day, always on a weekend after you have found a comfortable seat as far away from the nurse's station as possible, do you open up the chart and see 1 line of open space left on the last page of the progress notepaper? Even better, why is the note above it a follow‐up from vascular surgery, written in font size 24, in a form of Sanskrit that not even Steven Hawkings would recognize?
-
Okay, how about this: For patients with loooong lengths of stay, how many creative ways can you write Awaiting placement, afebrile, no complaints (in compliance with billing rules of course)? The correct answer is between 16 and 23. A thesaurus helps for word number 1try stable to startand change your pen from fine point to medium point on odd days. Then add tolerated breakfast on Monday, lunch on Tuesday, and dinner on Wednesday. Voila! Who said this was tough?
-
Okay, this one boggles my mind. You wish to auscultate a set of lungs. The patient is sitting on his gown. You attempt to lift the gown, and instead of raising his tush, the patient continues to sit while you tug away. Is this just me? It happens every week.
-
The medical students are great. They are ambitious and make teaching fun. Why though, at 9:59 AM, with an upcoming meeting with your chief at 10, are there no PC terminals at the station available except for the MSIII on MySpace.com with the chart you could not find (for 5 minutes) underneath his clipboard? Yes, Virginia, make me remember those days.
-
Clearly, this is one of the more helpful lines you can get from a consult: Patient needs to be medically optimized, and consider head MRI. Consider? Is not that why I called the consult in the first place? Let us consider not to use the word consider any more. Consider that. I feel better.
-
While we are on the topic of consultants, a dollar goes to you if this has never happened during your time on the wards: (1) the consultant visits, (2) the consultant evaluates, (3) you speak with the consultant, (4) all of you agree that the patient can go home, and (5) you then read the consult after the patient is dressed and his IV is out. Umm, a head CT before discharge and please have the neurosurgery clear the patient before discharge? Am I working in a parallel universe? Too much caffeine? Lord, give me strength.
-
Your beeper goes off at 2:57 PM. At 2:57 PM plus 5 seconds, you call the number you were paged from and no one answers. Does the word ponderous come to mind? This invariably happens every day, of course, typically when I am in the midst of multitasking 4 conversations. However, I extract some form of perverse cosmic revenge when I need to make a call and pick up an open extension from a ringing multiline phone. Invariably, I click the button to engage a line and, oops, good bye caller. I am only kidding; that never happens (is my nose really growing?). Just think, I could have been the one screaming, Is Laverne here?
-
You get an admission, a patient whom you have never met, and his room is listed as 428. You walk in, and the patient nearest to you is a well‐groomed middle‐age individual with a welcoming smile. The patient next to him is breathing fire, screaming at an imaginary executioner, and claiming that you are the guilty party and need to die. Which patient do you think is yours?
-
Those of you who work with housestaff will appreciate this one (file it under systems issue: fix next week): You have a discussion with a patient regarding his PM discharge at 11 AM. You arrange the follow‐up, you review the new medications, you discuss who will pick him up after dinner, and so forth. You get that warm and fuzzy feeling that you have done your job and all is right in the universe. Naturally, you also tell the resident that the patient can go home. Lo and behold, you look at your census the following morning, and the name of the aforementioned patient radiates like a beacon from the screen. You then poke your head into the room, feeling assured that it is merely an error, and the aforementioned patient is lying in bed, smiling and happy to see you. You ask, What happened? The reply, I don't know. Didn't your son come last night to pick you up? The response is yes. After the penetrating ulcer in your stomach bores a little deeper, you discover the official discharge order did not occur, and the patient was content eating chipped beef and sleeping on said contoured mattress 1 more night. Serenity now, serenity now.
-
The fifth vital sign? Is that the new black? Heck, number 5, I think we are up to 11 or 12 these days. Need a new metric installed? You guessed it: add it to the list!
-
Do you ever get LOS fatigue with a particular patient that is so severe you go to bed the previous night and have problems falling asleep? Really, what do you say to a person when his hospital stay exceeds, say, 5 months? Yes, I actually think of topics and issues that I can incorporate into the conversation which will spice up the relationship. New bed sheets, a fresh coat of paint? It would make a good Seinfeld episode, no?
-
Is it me, or is having 2 patients in the same room like being a flight attendant wheeling around the beverage cart? Get one the peanuts, and then the other wants the pretzels. For sure, add 10 minutes to your time in room 728 tomorrow.
-
If a patient is unable to leave the hospital for reasons unrelated to discharge planning (locked out of his house, the child is out of town until the next morning, etc.), why do I feel so naughty when I get off the phone with the MCO medical director after offering explanations? I do not get it. You think that the hospital employs a battery of runners to padlock homes and steal patient's clothing. Who wrote this playbook?
-
I love my consultants. Really, I do. I am not picking on them today. The high points of my day are the exchanges that I have with my subspecialty colleagues. However, the myopia that pervades some sign off notes give me pause. For example, a patient admitted for gastrointestinal bleed, s/p EGD, and stable at 72 hours post arrival receives a consultant note as follows: if patient eating and ambulating, can be discharged home as per PMD. Surely, when the level of transferred oversight shifts to the level of caloric ingestion and sneaker use, well, let us just say that I am all for some new E&M codes. They did not tell me about this in hospitalist school.
-
Don't you love the feeling of your beeper going off, and 30 seconds after the first page, boom, it goes off againboth to the same extension? I mean really, I am a nice guy, but do you really want to rile me up this early in the morning?
-
The nurse pages you from 1 floor away10 seconds from where you are standing. You recognize that number, you knew that it was coming, and for sure, waiting on the other end is that family member who hails from a foreboding place. How quickly does your brain do the computationdo I use the phone and let my fingers do the talking, or make that stroll and have that face‐to‐face summit? No sarcastic comment is needed. I see us now, hands joined, joyfully singing Kumbaya in a loving embrace.
-
Gee, it is not busy today. Say that on the wards and you get a leering glance. However, say that in the emergency room and you will meet your death. There is something about that phrase and the emergency department. The nurses there do not forget, although a Starbucks cappuccino does put a nice salve on the wound.
-
On your day off, do you ever notice that your beeper vibrates on your belt and you are not wearing it? I am not kidding.
-
An irony of life: I have developed an immunity to cigarette smoke in hospital bathrooms. Why is that? It is like peanut butter and jelly. They just seem so happy together.
-
Do you want to transfer that patient to psychiatry? No, no, no, you silly hospitalistdid you not notice that abnormal BUN and atypical lymphocyte on the peripheral smear? Hey, Dr. Freud, can you write me for some of that Prozac too!
-
We need to consult a rulebook on chair ownership. Did you ever notice (a tinge of Andy Rooney here) that case managers own their seats? I know that the world is not quite right when a case manager shoots me that dastardly glare, as if to say, Flee, you silly physician, I live at this station, you are merely my guest! As far as that chair is concerned, perhaps if a small plaque is added to the backrest with a suitable donation, my legs will finally get their deserved daily rest.
-
Finally, do you want to become invisible? Go to the reading board and stand behind a radiologist at 10:30 AM. Do it long enough, and after a few days, you will be saying I'm Good Enough, I'm Smart Enough, and Doggone It, People Like Me! Do you want to disappear completely? Try it on a Friday.
Okay, okay, I will stop there. It is funny, though; this stuff really happens. Despite the aggravation, I see these commonalities as the glue that binds us, assists in building the esprit de corps in our profession, and adds a little levity to the work place. Outside the hospital (not inside, of course), I can confidently state that these routines are part of who I am. After all, it is all about the knowing glance that I mentioned previously. The humorous part is that we are certainly on someone else's list. Probably a nurse, an emergency room doctor, or maybe a physician's assistant is scribing away at this minute, and we are number 7: Those hospitalists really tick me off.
EPILOGUE
Note to selflook in the mirror occasionally; you might learn something.
I apologize to all my nonhospitalist colleagues if you sneered. I love all of you. Today.
Is hospital medicine a bona fide specialty? Do something long enough, and as Justice Potter Stewart said when defining a certain taboo carnal subject many years ago, I know it when I see it. Although working groups may struggle to conceive a master set of core competencies for hospitalists, I will tell you this: no texts are needed, and you know that you are on to something when 2 hospitalists practicing 3000 miles apart shoot each other a knowing glance and, without words, just understand what the other is thinking. After 10 years of practice in several hospitals, I have had enough mind melds to last a lifetime. Who needs science after all? I mean, how many of us can keep a straight face when asked if we have ever heard this line: Ah, yes, Dr. Flansbaum, umm, I am Dr. Smith from the surgical ICU, and we have a patient hospital day 34 status post Whipple that is no longer surgically active. See, you are smiling already. Do I have to finish the sentence for you?
What follows is a collective experience of things that I call the grind: things so small, so inconsequential, that no one will ever cite them individually as the deal breakers of the day. Collectively, they are the fabric of who we are and that little sore on the inside of our cheek that we just have to touch every few minutes in order to remind ourselves of why dermatologists always look so happy. Any accompanying sage lessons are also free of charge.
-
Why at the end of the day, always on a weekend after you have found a comfortable seat as far away from the nurse's station as possible, do you open up the chart and see 1 line of open space left on the last page of the progress notepaper? Even better, why is the note above it a follow‐up from vascular surgery, written in font size 24, in a form of Sanskrit that not even Steven Hawkings would recognize?
-
Okay, how about this: For patients with loooong lengths of stay, how many creative ways can you write Awaiting placement, afebrile, no complaints (in compliance with billing rules of course)? The correct answer is between 16 and 23. A thesaurus helps for word number 1try stable to startand change your pen from fine point to medium point on odd days. Then add tolerated breakfast on Monday, lunch on Tuesday, and dinner on Wednesday. Voila! Who said this was tough?
-
Okay, this one boggles my mind. You wish to auscultate a set of lungs. The patient is sitting on his gown. You attempt to lift the gown, and instead of raising his tush, the patient continues to sit while you tug away. Is this just me? It happens every week.
-
The medical students are great. They are ambitious and make teaching fun. Why though, at 9:59 AM, with an upcoming meeting with your chief at 10, are there no PC terminals at the station available except for the MSIII on MySpace.com with the chart you could not find (for 5 minutes) underneath his clipboard? Yes, Virginia, make me remember those days.
-
Clearly, this is one of the more helpful lines you can get from a consult: Patient needs to be medically optimized, and consider head MRI. Consider? Is not that why I called the consult in the first place? Let us consider not to use the word consider any more. Consider that. I feel better.
-
While we are on the topic of consultants, a dollar goes to you if this has never happened during your time on the wards: (1) the consultant visits, (2) the consultant evaluates, (3) you speak with the consultant, (4) all of you agree that the patient can go home, and (5) you then read the consult after the patient is dressed and his IV is out. Umm, a head CT before discharge and please have the neurosurgery clear the patient before discharge? Am I working in a parallel universe? Too much caffeine? Lord, give me strength.
-
Your beeper goes off at 2:57 PM. At 2:57 PM plus 5 seconds, you call the number you were paged from and no one answers. Does the word ponderous come to mind? This invariably happens every day, of course, typically when I am in the midst of multitasking 4 conversations. However, I extract some form of perverse cosmic revenge when I need to make a call and pick up an open extension from a ringing multiline phone. Invariably, I click the button to engage a line and, oops, good bye caller. I am only kidding; that never happens (is my nose really growing?). Just think, I could have been the one screaming, Is Laverne here?
-
You get an admission, a patient whom you have never met, and his room is listed as 428. You walk in, and the patient nearest to you is a well‐groomed middle‐age individual with a welcoming smile. The patient next to him is breathing fire, screaming at an imaginary executioner, and claiming that you are the guilty party and need to die. Which patient do you think is yours?
-
Those of you who work with housestaff will appreciate this one (file it under systems issue: fix next week): You have a discussion with a patient regarding his PM discharge at 11 AM. You arrange the follow‐up, you review the new medications, you discuss who will pick him up after dinner, and so forth. You get that warm and fuzzy feeling that you have done your job and all is right in the universe. Naturally, you also tell the resident that the patient can go home. Lo and behold, you look at your census the following morning, and the name of the aforementioned patient radiates like a beacon from the screen. You then poke your head into the room, feeling assured that it is merely an error, and the aforementioned patient is lying in bed, smiling and happy to see you. You ask, What happened? The reply, I don't know. Didn't your son come last night to pick you up? The response is yes. After the penetrating ulcer in your stomach bores a little deeper, you discover the official discharge order did not occur, and the patient was content eating chipped beef and sleeping on said contoured mattress 1 more night. Serenity now, serenity now.
-
The fifth vital sign? Is that the new black? Heck, number 5, I think we are up to 11 or 12 these days. Need a new metric installed? You guessed it: add it to the list!
-
Do you ever get LOS fatigue with a particular patient that is so severe you go to bed the previous night and have problems falling asleep? Really, what do you say to a person when his hospital stay exceeds, say, 5 months? Yes, I actually think of topics and issues that I can incorporate into the conversation which will spice up the relationship. New bed sheets, a fresh coat of paint? It would make a good Seinfeld episode, no?
-
Is it me, or is having 2 patients in the same room like being a flight attendant wheeling around the beverage cart? Get one the peanuts, and then the other wants the pretzels. For sure, add 10 minutes to your time in room 728 tomorrow.
-
If a patient is unable to leave the hospital for reasons unrelated to discharge planning (locked out of his house, the child is out of town until the next morning, etc.), why do I feel so naughty when I get off the phone with the MCO medical director after offering explanations? I do not get it. You think that the hospital employs a battery of runners to padlock homes and steal patient's clothing. Who wrote this playbook?
-
I love my consultants. Really, I do. I am not picking on them today. The high points of my day are the exchanges that I have with my subspecialty colleagues. However, the myopia that pervades some sign off notes give me pause. For example, a patient admitted for gastrointestinal bleed, s/p EGD, and stable at 72 hours post arrival receives a consultant note as follows: if patient eating and ambulating, can be discharged home as per PMD. Surely, when the level of transferred oversight shifts to the level of caloric ingestion and sneaker use, well, let us just say that I am all for some new E&M codes. They did not tell me about this in hospitalist school.
-
Don't you love the feeling of your beeper going off, and 30 seconds after the first page, boom, it goes off againboth to the same extension? I mean really, I am a nice guy, but do you really want to rile me up this early in the morning?
-
The nurse pages you from 1 floor away10 seconds from where you are standing. You recognize that number, you knew that it was coming, and for sure, waiting on the other end is that family member who hails from a foreboding place. How quickly does your brain do the computationdo I use the phone and let my fingers do the talking, or make that stroll and have that face‐to‐face summit? No sarcastic comment is needed. I see us now, hands joined, joyfully singing Kumbaya in a loving embrace.
-
Gee, it is not busy today. Say that on the wards and you get a leering glance. However, say that in the emergency room and you will meet your death. There is something about that phrase and the emergency department. The nurses there do not forget, although a Starbucks cappuccino does put a nice salve on the wound.
-
On your day off, do you ever notice that your beeper vibrates on your belt and you are not wearing it? I am not kidding.
-
An irony of life: I have developed an immunity to cigarette smoke in hospital bathrooms. Why is that? It is like peanut butter and jelly. They just seem so happy together.
-
Do you want to transfer that patient to psychiatry? No, no, no, you silly hospitalistdid you not notice that abnormal BUN and atypical lymphocyte on the peripheral smear? Hey, Dr. Freud, can you write me for some of that Prozac too!
-
We need to consult a rulebook on chair ownership. Did you ever notice (a tinge of Andy Rooney here) that case managers own their seats? I know that the world is not quite right when a case manager shoots me that dastardly glare, as if to say, Flee, you silly physician, I live at this station, you are merely my guest! As far as that chair is concerned, perhaps if a small plaque is added to the backrest with a suitable donation, my legs will finally get their deserved daily rest.
-
Finally, do you want to become invisible? Go to the reading board and stand behind a radiologist at 10:30 AM. Do it long enough, and after a few days, you will be saying I'm Good Enough, I'm Smart Enough, and Doggone It, People Like Me! Do you want to disappear completely? Try it on a Friday.
Okay, okay, I will stop there. It is funny, though; this stuff really happens. Despite the aggravation, I see these commonalities as the glue that binds us, assists in building the esprit de corps in our profession, and adds a little levity to the work place. Outside the hospital (not inside, of course), I can confidently state that these routines are part of who I am. After all, it is all about the knowing glance that I mentioned previously. The humorous part is that we are certainly on someone else's list. Probably a nurse, an emergency room doctor, or maybe a physician's assistant is scribing away at this minute, and we are number 7: Those hospitalists really tick me off.
EPILOGUE
Note to selflook in the mirror occasionally; you might learn something.
I apologize to all my nonhospitalist colleagues if you sneered. I love all of you. Today.
Improved Glycemic Control and Hypoglycemia
Diabetes has reached epidemic proportions in the United States, affecting over 20 million individuals,1 and further rises are expected. A disproportionate increase in diabetes has occurred in the inpatient setting.2 Furthermore, for every 2 patients in the hospital with known diabetes, there may be an additional 1 with newly observed hyperglycemia. Both are common. In 1 report, for example, 24% of inpatients with hyperglycemia had a prior diagnosis of diabetes, whereas another 12% had hyperglycemia without a prior diagnosis of diabetes.3
Although there is a paucity of high quality randomized controlled trials to support tight glycemic control in non‐critical care inpatient settings, poor glycemic control in hospitalized patients is strongly associated with undesirable outcomes for a variety of conditions, including pneumonia,4 cancer chemotherapy,5 renal transplant,6 and postsurgical wound infections.7, 8 Hyperglycemia also induces dehydration, fluid and electrolyte imbalance, gastric motility problems, and venous thromboembolism formation.9
Structured subcutaneous insulin order sets and insulin management protocols have been widely advocated as a method to encourage basal bolus insulin regimens and enhance glycemic control,2, 9, 10 but the effect of these interventions on glycemic control, hypoglycemia, and insulin use patterns in the real world setting has not been well reported. Fear of inducing hypoglycemia is often the main barrier for initiating basal insulin containing regimens and pursuing glycemic targets.2 The evidence would suggest, however, that sliding scale regimens, as opposed to more physiologic basal bolus regimens, may actually increase both hypoglycemic and hyperglycemic excursions.11 A convincing demonstration of the efficacy (improved insulin use patterns and reduced hyperglycemia) and safety (reduced hypoglycemia) of structured insulin order sets and insulin management protocols would foster a more rapid adoption of these strategies.
PATIENTS AND METHODS
In our 400‐bed university hospital, we formed a hospitalist‐led multidisciplinary team in early 2003, with the focus of improving the care delivered to non‐critical care patients with diabetes or hyperglycemia. We used a Plan‐Do‐Study‐Act (PDSA) performance improvement framework, and conducted institutional review board (IRB)‐approved prospective observational research in parallel with the performance improvement efforts, with a waiver for individual informed consent. The study population consisted of all adult inpatients on non‐critical care units with electronically reported point of care (POC) glucose testing from November 2002 through December 2005. We excluded patients who did not have either a discharge diagnosis of Diabetes (ICD 9 codes 250‐251.XX) or demonstrated hyperglycemia (fasting POC glucose >130 mg/dL 2, or a random value of >180 mg/dL) from analysis of glycemic control and hypoglycemia. Women admitted to Obstetrics were excluded. Monthly and quarterly summaries on glycemic control, hypoglycemia, and insulin use patterns (metrics described below) were reported to the improvement team and other groups on a regular basis throughout the intervention period. POC glucose data, demographics, markers of severity of illness, and diagnosis codes were retrieved from the electronic health record.
Interventions
We introduced several interventions and educational efforts throughout the course of our improvement. The 2 key interventions were as follows:
Structured subcutaneous insulin order sets (November, 2003).
An insulin management algorithm, described below (May 2005).
Key Intervention #1: Structured Subcutaneous Insulin Order Set Implementation
In November 2003, we introduced a paper‐based structured subcutaneous insulin order set. This order set encouraged the use of scheduled basal and nutritional insulin, provided guidance for monitoring glucose levels, and for insulin dosing. A hypoglycemia protocol and a standardized correction insulin table were embedded in the order set. This set was similar to examples of structured insulin ordering subsequently presented in the literature.9 In a parallel effort, the University of California, San Diego Medical Center (UCSDMC) was developing a computer physician order entry (CPOE) module for our comprehensive clinical information system, Invision (Siemens Medical Systems, Malvern, PA), that heretofore had primarily focused on result review, patient schedule management, and nursing documentation. In anticipation of CPOE and for the purpose of standardization, we removed outdated sliding scale insulin regimens from a variety preexisting order sets and inserted references to the standardized subcutaneous insulin order set in their stead. The medication administration record (MAR) was changed to reflect the basal/nutritional/correction insulin terminology. It became more difficult to order a stand‐alone insulin sliding scale even before CPOE versions became available. The standardized order set was the only preprinted correction scale insulin order available, and ordering physicians have to specifically opt out of basal and nutritional insulin choices to order sliding scale only regimens. Verbal orders for correction dose scales were deemed unacceptable by medical staff committees. Correctional insulin doses could be ordered as a 1‐time order, but the pharmacy rejected ongoing insulin orders that were not entered on the structured form.
We introduced our first standardized CPOE subcutaneous insulin order set in January 2004 at the smaller of our 2 campuses, and subsequently completed full deployment across both campuses in all adult medical‐surgical care areas by September 2004.
The CPOE version, like the paper version that immediately preceded it, encouraged the use of basal/bolus insulin regimens, promoted the terms basal, nutritional or premeal, and adjustment dose insulin in the order sets and the medication administration record, and was mandatory for providers wishing to order anything but a 1‐time order of insulin. Figure 1 depicts a screen shot of the CPOE version. Similar to the paper version, the ordering physician had to specifically opt out of ordering scheduled premeal and basal insulin to order a sliding scale only regimen. The first screen also ensured that appropriate POC glucose monitoring was ordered and endorsed a standing hypoglycemia protocol order. The CPOE version had only a few additional features not possible on paper. Obvious benefits included elimination of unapproved abbreviations and handwriting errors. Nutritional and correction insulin types were forced to be identical. Fundamentally, however, both the paper and online structured ordering experiences had the same degree of control over provider ordering patterns, and there was no increment in guidance for choosing insulin regimens, hence their combined analysis as structured orders.
Key Intervention #2: Insulin Management Algorithm
The structured insulin order set had many advantages, but also had many limitations. Guidance for preferred insulin regimens for patients in different nutritional situations was not inherent in the order set, and all basal and nutritional insulin options were offered as equally acceptable choices. The order set gave very general guidance for insulin dosing, but did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components, and guidance for setting a glycemic target or adjusting insulin was lacking.
Recognizing these limitations, we devised an insulin management algorithm to provide guidance incremental to that offered in the order set. In April 2005, 3 hospitalists piloted a paper‐based insulin management algorithm (Figure 2, front; Figure 3, reverse) on their teaching services. This 1‐page algorithm provided guidance on insulin dosing and monitoring, and provided institutionally preferred insulin regimens for patients in different nutritional situations. As an example, of the several acceptable subcutaneous insulin regimens that an eating patient might use in the inpatient setting, we advocated the use of 1 preferred regimen (a relatively peakless, long‐acting basal insulin once a day, along with a rapid acting analog nutritional insulin with each meal). We introduced the concept of a ward glycemic target, provided prompts for diabetes education, and generally recommended discontinuation of oral hypoglycemic agents in the inpatient setting. The hospitalists were introduced to the concepts and the algorithm via 1 of the authors (G.M.) in a 1‐hour session. The algorithm was introduced on each teaching team during routine teaching rounds with a slide set (approximately 15 slides) that outlined the basic principles of insulin dosing, and gave example cases which modeled the proper use of the algorithm. The principles were reinforced on daily patient work rounds as they were applied on inpatients with hyperglycemia. The pilot results on 25 patients, compared to 250 historical control patients, were very promising, with markedly improved glycemic control and no increase in hypoglycemia. We therefore sought to spread the use of the algorithm. In May 2005 the insulin management algorithm and teaching slide set were promoted on all 7 hospitalist‐run services, and the results of the pilot and concepts of the algorithm were shared with a variety of house staff and service leaders in approximately a dozen sessions: educational grand rounds, assorted noon lectures, and subsequently, at new intern orientations. Easy access to the algorithm was assured by providing a link to the file within the CPOE insulin order set.
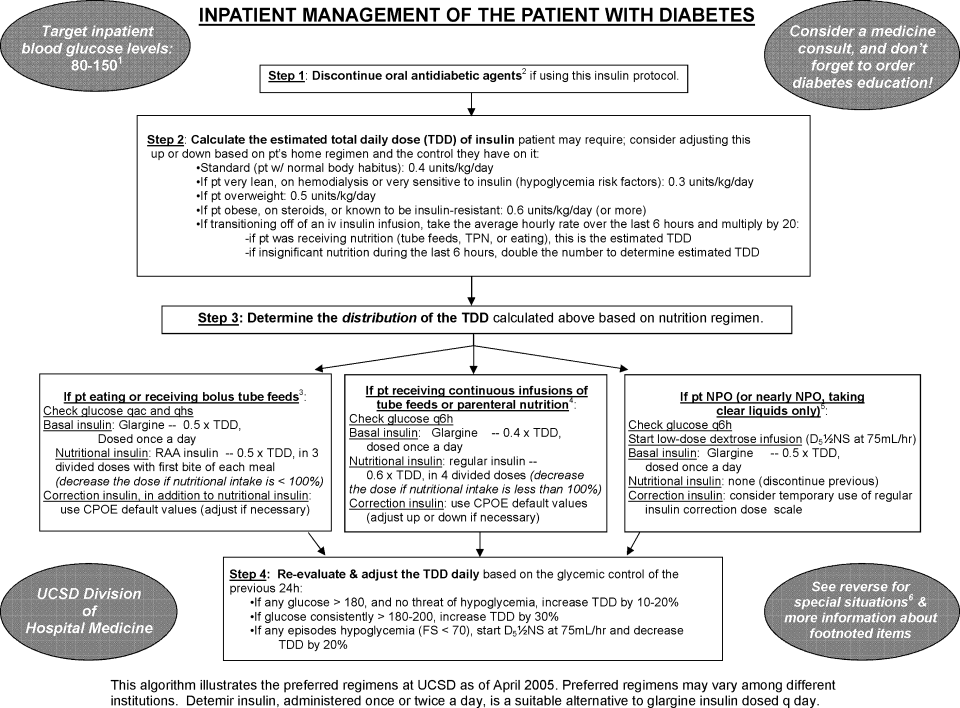

Other Attempts to Improve Care
Several other issues were addressed in the context of the larger performance improvement effort by the team. In many cases, hard data were not gathered to assess the effectiveness of the interventions, or the interventions were ongoing and could be considered the background milieu for the key interventions listed above.
During each intervention, education sessions were given throughout the hospital to staff, including physicians, residents, and nurses, using departmental grand rounds, nursing rounds, and in‐services to describe the process and goals. Patient education programs were also redesigned and implemented, using preprinted brochure. Front‐line nursing staff teaching skills were bolstered via Clinical Nurse Specialist educational sessions, and the use of a template for patient teaching. The educational template assessed patient readiness to learn, home environment, current knowledge, and other factors. Approximately 6 conferences directed at various physician staff per year became part of the regular curriculum.
We recognized that there was often poor coordination between glucose monitoring, nutrition delivery, and insulin administration. The traditional nursing practice of the 6:00 AM finger stick and insulin administration was changed to match a formalized nutrition delivery schedule. Nutrition services and nursing were engaged to address timeliness of nutrition delivery, insulin administration, and POC glucose documentation in the electronic health record.
Feedback to individual medicine resident teams on reaching glycemic targets, with movie ticket/coffee coupon rewards to high performing teams, was tried from April 2004 to September 2004.
Measures and Analyses
Assessing Insulin Use Patterns
A convenience sample gathering all subcutaneous insulin orders from 4 to 5 selected days per month yielded 70 to 90 subcutaneous insulin orders for review each month. Sampling was originally performed each month, followed by less frequent sampling once stability in insulin use patterns was reached. Regimens were categorized by pharmacy and hospitalist review as to whether basal insulin was part of the insulin regimen or not. The percentage of insulin regimens incorporating basal insulin was calculated for each sampled month and followed in run charts, and comparisons between preorder set and postorder set time periods were made using Pearson's chi square statistic.
Assessing Glycemic Control
Glycemic control and hypoglycemia parameters were monitored for the entire 38‐month observation period.
Routinely monitored POC glucose values were used to assess glycemic control. During the initial data examination, it was found after 14 days of the hospital stay, there was a notable stabilization and improvement in glucose control and fewer hypoglycemic events, therefore we examined only the first 14 days of hospitalization, thereby eliminating a potential source of bias from length of stay outliers.
A mean glucose value was recorded for each patient‐day with 1 or more recorded values. Glycemic control for each patient‐stay was calculated by averaging the patient‐day mean values, which we will refer to as the day‐weighted mean. Hypoglycemic values (60 mg/dL) were excluded from calculation of the mean glucose, to avoid equating frequent hypoglycemia with optimal glycemic control. An uncontrolled patient‐day was defined as a monitored patient‐day with a mean glucose 180 mg/dL. An uncontrolled patient‐stay is defined as a patient‐stay with a day‐weighted mean glucose value 180 mg/dL.
We theorized that the greatest impact of the interventions would be realized in patients with longer monitoring periods, and that those with only a few POC glucose values could potentially misrepresent the impact of our interventions: therefore we performed a second analysis restricted to patients with 8 POC glucose values.
Assessing Hypoglycemia
Hypoglycemia was defined as a glucose 60 mg/dL, and severe hypoglycemia was defined as a glucose 40 mg/dL. These parameters were characterized by 2 methods. First, we calculated the percentage of monitored patients suffering from 1 or more hypoglycemic events or severe hypoglycemic events over the course of their entire admission. A second method tracked the percentage of monitored patient‐days with hypoglycemia and severe hypoglycemia, thereby correcting for potential misinterpretation from clustered repeated measures or variable length of stay. As with the glycemic control analysis, we repeated the hypoglycemia analysis in the subset of patients with 8 POC glucose values.
Summary Analysis of Glycemic Control and Hypoglycemia
Pearson chi square values, with relative risks (RRs) and 95% confidence intervals (CIs) were calculated to compare glycemic control and hypoglycemia in the 2 key interventions and baseline. The interventions and data reporting were grouped as follows:
Baseline: November 2002 to October 2003) = Time Period 1 (TP1)
Structured Order Set: November 2003 to April 2005) = Time Period 2 (TP2)
Algorithm plus Structured Order Set: May 2005 to December 2005) = Time Period 3 (TP3)
A P value of less than 0.05 was determined as significant and data were analyzed using STATA, Version 8 (STATA Corp., College Station, TX).
We assigned the RR of uncontrolled hyperglycemia and the RR of hypoglycemia during the baseline time (TP1) with values of 1.0, and calculated the RR and CIs for the same parameters during TP2 and TP3.
RESULTS
Just over 11,000 patients were identified for POC glucose testing over the 38 month observation period. Of these, 9314 patients had either a diagnosis of diabetes or documented hyperglycemia. The characteristics of this study population are depicted in Table 1. There were no differences between the groups and the demographics of age, gender, or length of stay (P > 0.05 for all parameters). There was a slight increase in the percent of patients with any intensive care unit days over the 3 time periods and a similar increase in the case mix index.
| Patients Meeting Criteria of Diabetes Mellitus Diagnosis or Hyperglycemia (n = 9,314 patients) | Baseline | TP2 | TP3 |
|---|---|---|---|
| |||
| Time period (TP) | November 2002 to October 2003 | November 2003 to April 2005 | May 2005 to December 2005 |
| Monitored patient days (44,232) | 11,571 | 21,126 | 11,535 |
| Number of patients (9,314) | 2,504 | 4,515 | 2,295 |
| Males (%) | 55 | 54 | 56 |
| Average age standard deviation | 56 17 | 56 17 | 56 16 |
| Length of stay (excluding highest 1% of outliers) | 4.6 5.9 | 4.6 5.7 | 4.8 5.8 |
| % With any intensive care unit days* | 18 | 20 | 22 |
| Case mix index score (mean SD) | 1.8 2.1 | 2.0 2.3 | 2.1 2.1 |
| Case mix index (median score) | 1.1 | 1.3 | 1.3 |
Of the 9314 study patients, 5530 had 8 or more POC glucose values, and were included in a secondary analysis of glycemic control and hypoglycemia.
Insulin Use Patterns
Figure 4 demonstrates the dramatic improvement that took place with the introduction of the structured order set. In the 6 months preceding the introduction of the structured insulin order set (May‐October 2003) 72% of 477 sampled patients with insulin orders were on sliding scale‐only insulin regimens (with no basal insulin), compared to just 26% of 499 patients sampled in the March to August 2004 time period subsequent to order set implementation (P < .0001, chi square statistic). Intermittent monthly checks on insulin use patterns reveal this change has been sustained.
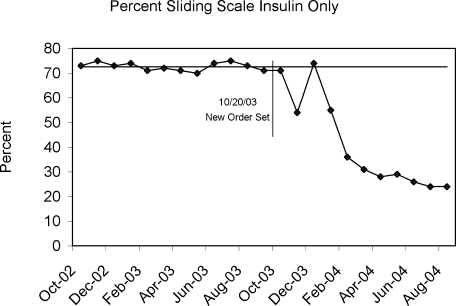
Glycemic Control
A total of 9314 patients with 44,232 monitored patient‐days and over 120,000 POC glucose values were analyzed to assess glycemic control, which was improved with structured insulin orders and improved incrementally with the introduction of the insulin management algorithm.
The percent of patient‐days that were uncontrolled, defined as a monitored day with a mean glucose of 180 mg/dL, was reduced over the 3 time periods (37.8% versus 33.9% versus 30.1%, P < 0.005, Pearson chi square statistic), representing a 21% RR reduction of uncontrolled patient‐days from TP1 versus TP3. Table 2 shows the summary results for glycemic control, including the RR and CIs between the 3 time periods.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 179 66 | 170 65 | 165 58 | |
| Median | 160 | 155 | 151 | |
| Uncontrolled patient‐days* | 4,372 | 7,162 | 3,465 | |
| Monitored patient‐days | 11,555 | 21,135 | 11,531 | |
| % Uncontrolled patient‐days | 37.8 | 33.9 | 30.1 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.89 (0.87‐0.92) | 0.79 (0.77‐0.82) | 0.89 (0.86‐0.92) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 177 57 | 174 54 | 170 50 | |
| Day‐weighted median | 167 | 162 | 158 | |
| Uncontrolled patient‐stay (%) | 1,038 | 1,696 | 784 | |
| Monitored patient‐stay | 2,504 | 4,515 | 2,295 | |
| % Uncontrolled patient‐stays | 41.5 | 37.6 | 34.2 | |
| RR: uncontrolled patient‐stay (95% confidence interval) | 0.91 (0.85‐0.96) | 0.84 (0.77‐0.89) | 0.91 (0.85‐0.97) | |
In a similar fashion, the percent of patients with uncontrolled patient‐stays (day‐weighted mean glucose 180 mg/dL) was also reduced over the 3 time periods (41.5% versus 37.6% versus 34.2%, P < 0.05, Pearson chi square statistic, with an RR reduction of 16% for TP3:TP1). Figure 5 depicts a statistical process control chart of the percent of patients experiencing uncontrolled patient‐stays over time, and is more effective in displaying the temporal relationship of the interventions with the improved results.
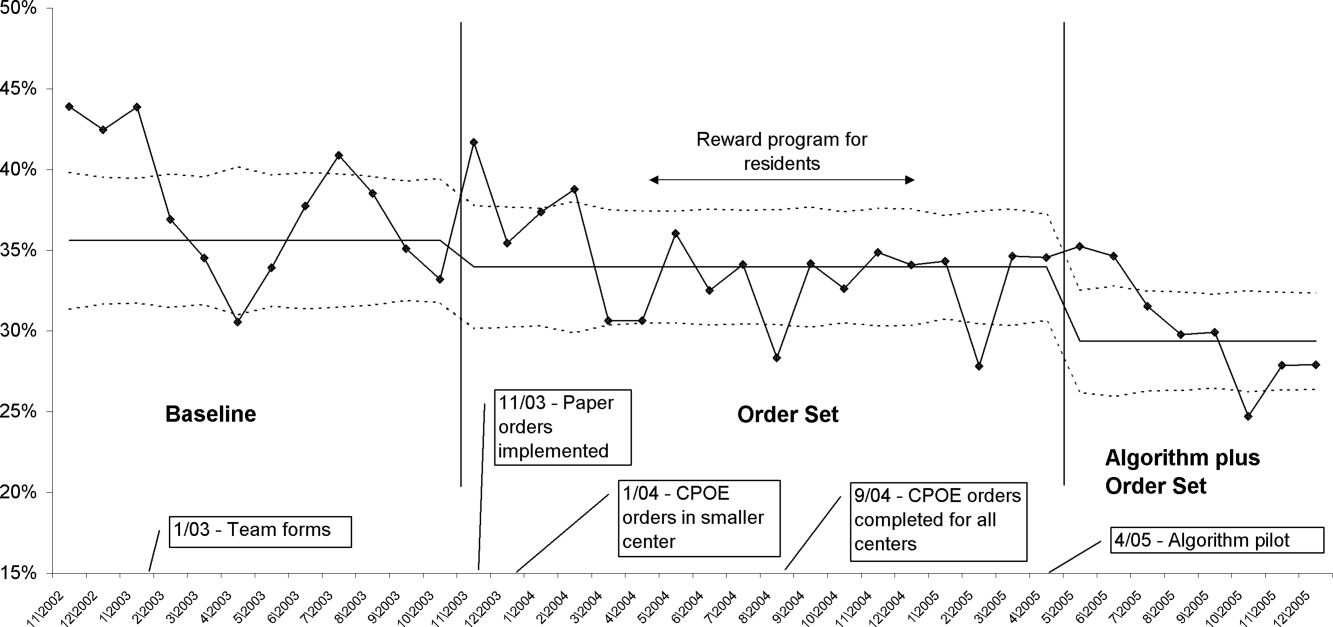
Uncontrolled hyperglycemic days and stays were reduced incrementally from TP3 versus TP2, reflecting the added benefit of the insulin management algorithm, compared to the benefit enjoyed with the structured order set alone.
When the analyses were repeated after excluding patients with fewer than 8 POC glucose readings (Table 3), the findings were similar, but as predicted, the effect was slightly more pronounced, with a 23% relative reduction in uncontrolled patient‐days and a 27% reduction in uncontrolled patient‐stays of TP3 versus TP1.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 172 65 | 169 64 | 163 57 | |
| Median | 159 | 154 | 149 | |
| Uncontrolled patient‐days* | 3,469 | 5,639 | 2,766 | |
| Monitored patient‐days | 9,304 | 17,278 | 9,671 | |
| % Uncontrolled patient‐days | 37.3 | 32.6 | 28.6 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.87 (0.85‐0.90) | 0.77 (0.74‐0.80) | 0.88 (0.84‐0.91) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 175 51 | 169 47 | 166 45 | |
| Day‐weighted median | 167 | 158 | 155 | |
| Uncontrolled patient‐stay (%) | 588 | 908 | 425 | |
| Monitored patient‐stay | 1,439 | 2,659 | 1,426 | |
| % Uncontrolled patient‐stays | 40.1 | 34.1 | 29.8 | |
| RR: Uncontrolled patient‐stay (95% confidence interval) | 0.84 (0.77‐0.91) | 0.73 (0.66‐0.81) | 0.87 (0.79‐0.96) | |
Hypoglycemia
Table 4 summarizes the results for hypoglycemia and severe hypoglycemia in the study population, and Table 5 summarizes the secondary analyses of hypoglycemia in the subset with at least 8 POC glucose readings.
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 2504 | 4515 | 2295 | |
| Stays with hypoglycemia (%) | 296 (11.8) | 437 (9.7) | 210 (9.2) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.82 (0.72‐0.94) | 0.77 (0.65‐0.92) | 0.95 (0.81‐1.10) |
| Stays with severe hypoglycemia (%) | 73 (2.9) | 96 (2.1) | 55 (2.4) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.73 (0.54‐0.98) | 0.82 (0.58‐1.16) | 1.13 (0.81‐1.56) |
| Monitored patient‐days | 11,584 | 21,158 | 11,548 | |
| Days with hypoglycemia (%) | 441 (3.8) | 623 (2.9) | 300 (2.6) | |
| RR hypoglycemic day (CI) | 1.0 | 0.77 (0.69‐0.87) | 0.68 (0.59‐0.78) | 0.88 (0.77‐1.01) |
| Days with severe hypoglycemia (%) | 86 (0.74) | 109 (0.52) | 66 (0.57) | |
| RR Severe hypoglycemic day (CI) | 1.0 | 0.69 (0.52‐0.92) | 0.77 (0.56‐1.06) | 1.10 (0.82‐1.5) |
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 1440 | 2664 | 1426 | |
| Stays with hypoglycemia (%) | 237 (16.5) | 384 (14.4) | 180 (12.6) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.88 (0.76‐1.02) | 0.77 (0.64‐0.92) | 0.88 (0.75‐1.03) |
| Stays with severe hypoglycemia (%) | 58 (4.0) | 93 (3.5) | 47 (3.3) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.87 (0.63‐1.2) | 0.82 (0.56‐1.19) | 0.94 (0.67‐1.33) |
| Monitored patient‐days | 9,317 | 17,310 | 9,684 | |
| Days with hypoglycemia (%) | 379 (4.1) | 569 (3.3) | 269 (2.7) | |
| RR hypoglycemic day (CI) | 1.0 | 0.81 (0.71‐0.92) | 0.68 (0.59‐0.80) | 0.85 (0.73‐0.98) |
| Days with severe hypoglycemia (%) | 71 (0.76) | 106 (0.61) | 58 (0.60) | |
| RR severe hypoglycemic day (CI) | 1.0 | 0.80 (0.60‐1.08) | 0.79 (0.56‐1.11) | 0.98 (0.71‐1.34) |
Analysis by Patient‐Stay
The percent of patients that suffered 1 or more hypoglycemic event over the course of their inpatient stay was 11.8% in TP1, 9.7% in TP2, and 9.2% in TP3. The RR of a patient suffering from a hypoglycemic event was significantly improved in the intervention time periods compared to baseline, with the RR of TP3:TP1 = 0.77 (CI, 0.65‐0.92). There was a strong trend for incremental improvement in hypoglycemic patient‐stays for TP3 versus TP2, but the trend just missed statistical significance (P < 0.07). Similar trends in improvement were found for severe hypoglycemia by patient‐stay, but these trends were only statistically significant for TP2 versus TP1. The findings were similar in the subset of patients with at least 8 POC glucose readings (Table 5).
Analysis by Patient‐Day
Of monitored patient days in the baseline TP1, 3.8% contained a hypoglycemic value of 60 mg/dL. With the introduction of structured insulin orders in TP2, this was reduced to 2.9%, and in TP3 it was 2.6%. The RR of a hypoglycemic patient‐day of TP2 compared to TP1 was 0.77 (CI, 0.69‐0.87), whereas the cumulative impact of the structured order set and algorithm (TP3:TP1) was 0.68 (CI, 0.59‐0.78), representing a 32% reduction of the baseline risk of suffering from a hypoglycemic day. Similar reductions were seen for the risk of a severe hypoglycemic patient‐day.
The secondary analysis of hypoglycemic and severe hypoglycemic patient‐days showed very similar results, except that the TP3:TP2 RR for hypoglycemia of 0.85 (CI, 0.73‐0.98) reached statistical significance, again demonstrating the incrementally beneficial effect of the insulin management algorithm.
DISCUSSION
Our study convincingly demonstrates that significant improvement in glycemic control can be achieved with implementation of structured subcutaneous insulin orders and a simple insulin management protocol. Perhaps more importantly, these gains in glycemic control are not gained at the expense of increased iatrogenic hypoglycemia, and in fact, we observed a 32% decline in the percent of patient‐days with hypoglycemia. This is extremely important because fear of hypoglycemia is the most significant barrier to glycemic control efforts.
Strengths and Limitations
Our study has several strengths. The study is large and incorporates all patients with diabetes or hyperglycemia captured by POC glucose testing, and the observation period is long enough that bias from merely being observed is not a factor. We used metrics for glycemic control, hypoglycemia, and insulin use patterns that are of high quality and are generally in line with the Society of Hospital Medicine (SHM) Glycemic Control Task force recommendations,12, 13 and examined data by both patient‐stay and patient‐day.
The increased use of anticipatory physiologic subcutaneous insulin regimens, and the subsequent decline in the use of sliding scale insulin, is the most likely mechanism for improvement. The improvements seen are fairly dramatic for an institution in absolute terms, because inpatient hyperglycemia and hypoglycemia are so common. For example, on an annualized basis for our 400‐bed medical center, these interventions prevent 124 patients from experiencing 208 hypoglycemic days.
Other institutions should be able to replicate our results. We received administrative support to create a multidisciplinary steering committee, but we did not have incremental resources to create a dedicated team for insulin management, mandated endocrinology comanagement or consultations, or manual data collection. In fact, we had only 1 diabetes educator for 400 adult beds at 2 sites, and were relatively underresourced in this area by community standards. There was some time and expense in creating the glycemic control reports, but all of the glucose data collected were part of normal care, and the data retrieval became automated.
The main limitation of this study lies in the observational study design. There were multiple interventions in addition to structured insulin orders and the insulin management algorithm, and these educational and organizational changes undoubtedly also contributed to the overall success of our program. Since we did not perform a randomized controlled trial, the reader might reasonably question if the structured order sets and insulin management algorithm were actually the cause of the improvement seen, as opposed to these ancillary efforts or secular change. However, there are several factors that make this unlikely. First, the study population was well‐defined, having diabetes or documented hyperglycemia in all 3 time periods. Second, the demographics remained constant or actually worked against improvement trends, since the markers of patient acuity suggest increased patient acuity over the observation period. Third, the temporal relationship of the improvement to the introduction of our key interventions, as viewed on statistical process control charts shown in Figure 5, strongly suggest a causal relationship. This temporal relationship was consistently observed no matter how we chose to define uncontrolled hyperglycemia, and was also seen on hypoglycemia control charts. We view the ancillary interventions (such as educational efforts) as necessary, but not sufficient, in and of themselves, to effect major improvement.
We did not analyze the impact of the improved glycemic control on patient outcomes. In the absence of a randomized controlled trial design, controlling for the various confounders is a challenging task. Also, it is likely that not all hypoglycemic events were attributable to inpatient glycemic control regimens, though the secondary analysis probably eliminated many hypoglycemia admissions.
Lessons Learned: Implications from our study
We agree with the American Association of Clinical Endocrinologists (AACE)/American Diabetes Association (ADA)2 and the SHM Glycemic Control Task Force12 about the essential elements needed for successful implementation of inpatient glycemic control programs:
An appropriate level of administrative support.
Formation of a multidisciplinary steering committee to drive the development of initiatives, empowered to enact changes.
Assessment of current processes, quality of care, and barriers to practice change.
Development and implementation of interventions, including standardized order sets, protocols, policies, and algorithms with associated educational programs.
Metrics for evaluation of glycemic control, hypoglycemia, insulin use patterns, and other aspects of care.
Metrics to follow hypoglycemia are extremely important. The voluntary reporting on insulin‐induced hypoglycemia fluctuated widely over the course of our project. These fluctuations did not correlate well with the more objective and accurate measures we followed, and this objective data was very helpful in reducing the fear of hypoglycemia, and spreading the wider use of basal bolus insulin regimens. We strongly recommend that improvement teams formulate and follow measures of glycemic control, hypoglycemia, and insulin use, similar to those outlined in the SHM Glycemic Control Improvement Guide12 and the SHM Glycemic Control Task Force summary on glucometrics.13
Although we introduced our structured insulin order set first, with a long lag time until we introduced the insulin management algorithm, we advocate a different approach for institutions grappling with these issues. This approach is well‐described by the SHM Glycemic Control Task Force.14 An insulin management algorithm should be crafted first, integrating guidance for insulin dosing, preferred insulin regimens for different nutritional situations, a glycemic target, insulin dosing adjustment, glucose monitoring, and prompts for ordering a glycosylated hemoglobin (A1c) level. Next, the order set and the supporting educational programs should integrate this guidance as much as possible, making the key guidance available at the point of patient care.
This guidance was available in our algorithm but was not inherent in the structured insulin orders described in this report, and all basal and nutritional insulin options were offered as equally acceptable choices. This version did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components. Only a single adjustment dose scale was offered, leaving appropriate modifications up to the end user, and from a usability standpoint, our CPOE insulin orders lacked dynamic flexibility (revising a single insulin required discontinuing all prior orders and reentering all orders). These limitations have subsequently been addressed with Version 2 of our CPOE insulin orders, and the details will soon be available in the literature.15
We are now exploring further improvement with concurrent identification and intervention of hyperglycemic patients that are not on physiologic insulin regimens or not meeting glycemic targets, and implementing protocols addressing the transition from infusion insulin.
CONCLUSION
We significantly improved glycemic control and simultaneously reduced hypoglycemia across all major medical and surgical services at our medical center, thereby addressing the number 1 barrier to improved inpatient glycemic control. We achieved this via systems changes with the introduction of structured subcutaneous insulin orders and the insulin management algorithm, along with education, but did not otherwise mandate or monitor adherence to our algorithm.
Implementing an institutional insulin management algorithm and structured insulin orders should now be viewed as a potent safety intervention as well as an intervention to enhance quality, and we have demonstrated that non‐critical care glycemic control efforts can clearly be a win‐win situation.
- Centers for Disease Control and Prevention.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2002.Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention;2003. Available at: www.cdc.gov/diabetes/pubs/factsheet.htm. Accessed January 21, 2006.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955‐1962.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978‐982.
- ,,, et al.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810‐815.
- ,,, et al.Cancer.2004;100:1179‐1185.
- ,,, et al.Early perioperative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321‐1324.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22:77‐81.
- ,,, et al.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356‐361.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553‐591.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77‐82.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity? [Editorial].J Hosp Med.2006;1:141‐144.
- Society of Hospital Medicine Glycemic Control Task Force: Optimizing Glycemic Control and Reducing Hypoglycemia at Your Medical Center. Society of Hospital Medicine, Glycemic Control Quality Improvement Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed October2008.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(S5):66–75.
- ,,,;for the SHM Glycemic Control Task Force.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(S5):29–41.
- ,,,.Indication‐based ordering: a new paradigm for glycemic control in hospitalized inpatients.J Diabetes Sci Tech.2008;2(3):349‐356.
Diabetes has reached epidemic proportions in the United States, affecting over 20 million individuals,1 and further rises are expected. A disproportionate increase in diabetes has occurred in the inpatient setting.2 Furthermore, for every 2 patients in the hospital with known diabetes, there may be an additional 1 with newly observed hyperglycemia. Both are common. In 1 report, for example, 24% of inpatients with hyperglycemia had a prior diagnosis of diabetes, whereas another 12% had hyperglycemia without a prior diagnosis of diabetes.3
Although there is a paucity of high quality randomized controlled trials to support tight glycemic control in non‐critical care inpatient settings, poor glycemic control in hospitalized patients is strongly associated with undesirable outcomes for a variety of conditions, including pneumonia,4 cancer chemotherapy,5 renal transplant,6 and postsurgical wound infections.7, 8 Hyperglycemia also induces dehydration, fluid and electrolyte imbalance, gastric motility problems, and venous thromboembolism formation.9
Structured subcutaneous insulin order sets and insulin management protocols have been widely advocated as a method to encourage basal bolus insulin regimens and enhance glycemic control,2, 9, 10 but the effect of these interventions on glycemic control, hypoglycemia, and insulin use patterns in the real world setting has not been well reported. Fear of inducing hypoglycemia is often the main barrier for initiating basal insulin containing regimens and pursuing glycemic targets.2 The evidence would suggest, however, that sliding scale regimens, as opposed to more physiologic basal bolus regimens, may actually increase both hypoglycemic and hyperglycemic excursions.11 A convincing demonstration of the efficacy (improved insulin use patterns and reduced hyperglycemia) and safety (reduced hypoglycemia) of structured insulin order sets and insulin management protocols would foster a more rapid adoption of these strategies.
PATIENTS AND METHODS
In our 400‐bed university hospital, we formed a hospitalist‐led multidisciplinary team in early 2003, with the focus of improving the care delivered to non‐critical care patients with diabetes or hyperglycemia. We used a Plan‐Do‐Study‐Act (PDSA) performance improvement framework, and conducted institutional review board (IRB)‐approved prospective observational research in parallel with the performance improvement efforts, with a waiver for individual informed consent. The study population consisted of all adult inpatients on non‐critical care units with electronically reported point of care (POC) glucose testing from November 2002 through December 2005. We excluded patients who did not have either a discharge diagnosis of Diabetes (ICD 9 codes 250‐251.XX) or demonstrated hyperglycemia (fasting POC glucose >130 mg/dL 2, or a random value of >180 mg/dL) from analysis of glycemic control and hypoglycemia. Women admitted to Obstetrics were excluded. Monthly and quarterly summaries on glycemic control, hypoglycemia, and insulin use patterns (metrics described below) were reported to the improvement team and other groups on a regular basis throughout the intervention period. POC glucose data, demographics, markers of severity of illness, and diagnosis codes were retrieved from the electronic health record.
Interventions
We introduced several interventions and educational efforts throughout the course of our improvement. The 2 key interventions were as follows:
Structured subcutaneous insulin order sets (November, 2003).
An insulin management algorithm, described below (May 2005).
Key Intervention #1: Structured Subcutaneous Insulin Order Set Implementation
In November 2003, we introduced a paper‐based structured subcutaneous insulin order set. This order set encouraged the use of scheduled basal and nutritional insulin, provided guidance for monitoring glucose levels, and for insulin dosing. A hypoglycemia protocol and a standardized correction insulin table were embedded in the order set. This set was similar to examples of structured insulin ordering subsequently presented in the literature.9 In a parallel effort, the University of California, San Diego Medical Center (UCSDMC) was developing a computer physician order entry (CPOE) module for our comprehensive clinical information system, Invision (Siemens Medical Systems, Malvern, PA), that heretofore had primarily focused on result review, patient schedule management, and nursing documentation. In anticipation of CPOE and for the purpose of standardization, we removed outdated sliding scale insulin regimens from a variety preexisting order sets and inserted references to the standardized subcutaneous insulin order set in their stead. The medication administration record (MAR) was changed to reflect the basal/nutritional/correction insulin terminology. It became more difficult to order a stand‐alone insulin sliding scale even before CPOE versions became available. The standardized order set was the only preprinted correction scale insulin order available, and ordering physicians have to specifically opt out of basal and nutritional insulin choices to order sliding scale only regimens. Verbal orders for correction dose scales were deemed unacceptable by medical staff committees. Correctional insulin doses could be ordered as a 1‐time order, but the pharmacy rejected ongoing insulin orders that were not entered on the structured form.
We introduced our first standardized CPOE subcutaneous insulin order set in January 2004 at the smaller of our 2 campuses, and subsequently completed full deployment across both campuses in all adult medical‐surgical care areas by September 2004.
The CPOE version, like the paper version that immediately preceded it, encouraged the use of basal/bolus insulin regimens, promoted the terms basal, nutritional or premeal, and adjustment dose insulin in the order sets and the medication administration record, and was mandatory for providers wishing to order anything but a 1‐time order of insulin. Figure 1 depicts a screen shot of the CPOE version. Similar to the paper version, the ordering physician had to specifically opt out of ordering scheduled premeal and basal insulin to order a sliding scale only regimen. The first screen also ensured that appropriate POC glucose monitoring was ordered and endorsed a standing hypoglycemia protocol order. The CPOE version had only a few additional features not possible on paper. Obvious benefits included elimination of unapproved abbreviations and handwriting errors. Nutritional and correction insulin types were forced to be identical. Fundamentally, however, both the paper and online structured ordering experiences had the same degree of control over provider ordering patterns, and there was no increment in guidance for choosing insulin regimens, hence their combined analysis as structured orders.

Key Intervention #2: Insulin Management Algorithm
The structured insulin order set had many advantages, but also had many limitations. Guidance for preferred insulin regimens for patients in different nutritional situations was not inherent in the order set, and all basal and nutritional insulin options were offered as equally acceptable choices. The order set gave very general guidance for insulin dosing, but did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components, and guidance for setting a glycemic target or adjusting insulin was lacking.
Recognizing these limitations, we devised an insulin management algorithm to provide guidance incremental to that offered in the order set. In April 2005, 3 hospitalists piloted a paper‐based insulin management algorithm (Figure 2, front; Figure 3, reverse) on their teaching services. This 1‐page algorithm provided guidance on insulin dosing and monitoring, and provided institutionally preferred insulin regimens for patients in different nutritional situations. As an example, of the several acceptable subcutaneous insulin regimens that an eating patient might use in the inpatient setting, we advocated the use of 1 preferred regimen (a relatively peakless, long‐acting basal insulin once a day, along with a rapid acting analog nutritional insulin with each meal). We introduced the concept of a ward glycemic target, provided prompts for diabetes education, and generally recommended discontinuation of oral hypoglycemic agents in the inpatient setting. The hospitalists were introduced to the concepts and the algorithm via 1 of the authors (G.M.) in a 1‐hour session. The algorithm was introduced on each teaching team during routine teaching rounds with a slide set (approximately 15 slides) that outlined the basic principles of insulin dosing, and gave example cases which modeled the proper use of the algorithm. The principles were reinforced on daily patient work rounds as they were applied on inpatients with hyperglycemia. The pilot results on 25 patients, compared to 250 historical control patients, were very promising, with markedly improved glycemic control and no increase in hypoglycemia. We therefore sought to spread the use of the algorithm. In May 2005 the insulin management algorithm and teaching slide set were promoted on all 7 hospitalist‐run services, and the results of the pilot and concepts of the algorithm were shared with a variety of house staff and service leaders in approximately a dozen sessions: educational grand rounds, assorted noon lectures, and subsequently, at new intern orientations. Easy access to the algorithm was assured by providing a link to the file within the CPOE insulin order set.


Other Attempts to Improve Care
Several other issues were addressed in the context of the larger performance improvement effort by the team. In many cases, hard data were not gathered to assess the effectiveness of the interventions, or the interventions were ongoing and could be considered the background milieu for the key interventions listed above.
During each intervention, education sessions were given throughout the hospital to staff, including physicians, residents, and nurses, using departmental grand rounds, nursing rounds, and in‐services to describe the process and goals. Patient education programs were also redesigned and implemented, using preprinted brochure. Front‐line nursing staff teaching skills were bolstered via Clinical Nurse Specialist educational sessions, and the use of a template for patient teaching. The educational template assessed patient readiness to learn, home environment, current knowledge, and other factors. Approximately 6 conferences directed at various physician staff per year became part of the regular curriculum.
We recognized that there was often poor coordination between glucose monitoring, nutrition delivery, and insulin administration. The traditional nursing practice of the 6:00 AM finger stick and insulin administration was changed to match a formalized nutrition delivery schedule. Nutrition services and nursing were engaged to address timeliness of nutrition delivery, insulin administration, and POC glucose documentation in the electronic health record.
Feedback to individual medicine resident teams on reaching glycemic targets, with movie ticket/coffee coupon rewards to high performing teams, was tried from April 2004 to September 2004.
Measures and Analyses
Assessing Insulin Use Patterns
A convenience sample gathering all subcutaneous insulin orders from 4 to 5 selected days per month yielded 70 to 90 subcutaneous insulin orders for review each month. Sampling was originally performed each month, followed by less frequent sampling once stability in insulin use patterns was reached. Regimens were categorized by pharmacy and hospitalist review as to whether basal insulin was part of the insulin regimen or not. The percentage of insulin regimens incorporating basal insulin was calculated for each sampled month and followed in run charts, and comparisons between preorder set and postorder set time periods were made using Pearson's chi square statistic.
Assessing Glycemic Control
Glycemic control and hypoglycemia parameters were monitored for the entire 38‐month observation period.
Routinely monitored POC glucose values were used to assess glycemic control. During the initial data examination, it was found after 14 days of the hospital stay, there was a notable stabilization and improvement in glucose control and fewer hypoglycemic events, therefore we examined only the first 14 days of hospitalization, thereby eliminating a potential source of bias from length of stay outliers.
A mean glucose value was recorded for each patient‐day with 1 or more recorded values. Glycemic control for each patient‐stay was calculated by averaging the patient‐day mean values, which we will refer to as the day‐weighted mean. Hypoglycemic values (60 mg/dL) were excluded from calculation of the mean glucose, to avoid equating frequent hypoglycemia with optimal glycemic control. An uncontrolled patient‐day was defined as a monitored patient‐day with a mean glucose 180 mg/dL. An uncontrolled patient‐stay is defined as a patient‐stay with a day‐weighted mean glucose value 180 mg/dL.
We theorized that the greatest impact of the interventions would be realized in patients with longer monitoring periods, and that those with only a few POC glucose values could potentially misrepresent the impact of our interventions: therefore we performed a second analysis restricted to patients with 8 POC glucose values.
Assessing Hypoglycemia
Hypoglycemia was defined as a glucose 60 mg/dL, and severe hypoglycemia was defined as a glucose 40 mg/dL. These parameters were characterized by 2 methods. First, we calculated the percentage of monitored patients suffering from 1 or more hypoglycemic events or severe hypoglycemic events over the course of their entire admission. A second method tracked the percentage of monitored patient‐days with hypoglycemia and severe hypoglycemia, thereby correcting for potential misinterpretation from clustered repeated measures or variable length of stay. As with the glycemic control analysis, we repeated the hypoglycemia analysis in the subset of patients with 8 POC glucose values.
Summary Analysis of Glycemic Control and Hypoglycemia
Pearson chi square values, with relative risks (RRs) and 95% confidence intervals (CIs) were calculated to compare glycemic control and hypoglycemia in the 2 key interventions and baseline. The interventions and data reporting were grouped as follows:
Baseline: November 2002 to October 2003) = Time Period 1 (TP1)
Structured Order Set: November 2003 to April 2005) = Time Period 2 (TP2)
Algorithm plus Structured Order Set: May 2005 to December 2005) = Time Period 3 (TP3)
A P value of less than 0.05 was determined as significant and data were analyzed using STATA, Version 8 (STATA Corp., College Station, TX).
We assigned the RR of uncontrolled hyperglycemia and the RR of hypoglycemia during the baseline time (TP1) with values of 1.0, and calculated the RR and CIs for the same parameters during TP2 and TP3.
RESULTS
Just over 11,000 patients were identified for POC glucose testing over the 38 month observation period. Of these, 9314 patients had either a diagnosis of diabetes or documented hyperglycemia. The characteristics of this study population are depicted in Table 1. There were no differences between the groups and the demographics of age, gender, or length of stay (P > 0.05 for all parameters). There was a slight increase in the percent of patients with any intensive care unit days over the 3 time periods and a similar increase in the case mix index.
| Patients Meeting Criteria of Diabetes Mellitus Diagnosis or Hyperglycemia (n = 9,314 patients) | Baseline | TP2 | TP3 |
|---|---|---|---|
| |||
| Time period (TP) | November 2002 to October 2003 | November 2003 to April 2005 | May 2005 to December 2005 |
| Monitored patient days (44,232) | 11,571 | 21,126 | 11,535 |
| Number of patients (9,314) | 2,504 | 4,515 | 2,295 |
| Males (%) | 55 | 54 | 56 |
| Average age standard deviation | 56 17 | 56 17 | 56 16 |
| Length of stay (excluding highest 1% of outliers) | 4.6 5.9 | 4.6 5.7 | 4.8 5.8 |
| % With any intensive care unit days* | 18 | 20 | 22 |
| Case mix index score (mean SD) | 1.8 2.1 | 2.0 2.3 | 2.1 2.1 |
| Case mix index (median score) | 1.1 | 1.3 | 1.3 |
Of the 9314 study patients, 5530 had 8 or more POC glucose values, and were included in a secondary analysis of glycemic control and hypoglycemia.
Insulin Use Patterns
Figure 4 demonstrates the dramatic improvement that took place with the introduction of the structured order set. In the 6 months preceding the introduction of the structured insulin order set (May‐October 2003) 72% of 477 sampled patients with insulin orders were on sliding scale‐only insulin regimens (with no basal insulin), compared to just 26% of 499 patients sampled in the March to August 2004 time period subsequent to order set implementation (P < .0001, chi square statistic). Intermittent monthly checks on insulin use patterns reveal this change has been sustained.

Glycemic Control
A total of 9314 patients with 44,232 monitored patient‐days and over 120,000 POC glucose values were analyzed to assess glycemic control, which was improved with structured insulin orders and improved incrementally with the introduction of the insulin management algorithm.
The percent of patient‐days that were uncontrolled, defined as a monitored day with a mean glucose of 180 mg/dL, was reduced over the 3 time periods (37.8% versus 33.9% versus 30.1%, P < 0.005, Pearson chi square statistic), representing a 21% RR reduction of uncontrolled patient‐days from TP1 versus TP3. Table 2 shows the summary results for glycemic control, including the RR and CIs between the 3 time periods.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 179 66 | 170 65 | 165 58 | |
| Median | 160 | 155 | 151 | |
| Uncontrolled patient‐days* | 4,372 | 7,162 | 3,465 | |
| Monitored patient‐days | 11,555 | 21,135 | 11,531 | |
| % Uncontrolled patient‐days | 37.8 | 33.9 | 30.1 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.89 (0.87‐0.92) | 0.79 (0.77‐0.82) | 0.89 (0.86‐0.92) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 177 57 | 174 54 | 170 50 | |
| Day‐weighted median | 167 | 162 | 158 | |
| Uncontrolled patient‐stay (%) | 1,038 | 1,696 | 784 | |
| Monitored patient‐stay | 2,504 | 4,515 | 2,295 | |
| % Uncontrolled patient‐stays | 41.5 | 37.6 | 34.2 | |
| RR: uncontrolled patient‐stay (95% confidence interval) | 0.91 (0.85‐0.96) | 0.84 (0.77‐0.89) | 0.91 (0.85‐0.97) | |
In a similar fashion, the percent of patients with uncontrolled patient‐stays (day‐weighted mean glucose 180 mg/dL) was also reduced over the 3 time periods (41.5% versus 37.6% versus 34.2%, P < 0.05, Pearson chi square statistic, with an RR reduction of 16% for TP3:TP1). Figure 5 depicts a statistical process control chart of the percent of patients experiencing uncontrolled patient‐stays over time, and is more effective in displaying the temporal relationship of the interventions with the improved results.

Uncontrolled hyperglycemic days and stays were reduced incrementally from TP3 versus TP2, reflecting the added benefit of the insulin management algorithm, compared to the benefit enjoyed with the structured order set alone.
When the analyses were repeated after excluding patients with fewer than 8 POC glucose readings (Table 3), the findings were similar, but as predicted, the effect was slightly more pronounced, with a 23% relative reduction in uncontrolled patient‐days and a 27% reduction in uncontrolled patient‐stays of TP3 versus TP1.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 172 65 | 169 64 | 163 57 | |
| Median | 159 | 154 | 149 | |
| Uncontrolled patient‐days* | 3,469 | 5,639 | 2,766 | |
| Monitored patient‐days | 9,304 | 17,278 | 9,671 | |
| % Uncontrolled patient‐days | 37.3 | 32.6 | 28.6 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.87 (0.85‐0.90) | 0.77 (0.74‐0.80) | 0.88 (0.84‐0.91) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 175 51 | 169 47 | 166 45 | |
| Day‐weighted median | 167 | 158 | 155 | |
| Uncontrolled patient‐stay (%) | 588 | 908 | 425 | |
| Monitored patient‐stay | 1,439 | 2,659 | 1,426 | |
| % Uncontrolled patient‐stays | 40.1 | 34.1 | 29.8 | |
| RR: Uncontrolled patient‐stay (95% confidence interval) | 0.84 (0.77‐0.91) | 0.73 (0.66‐0.81) | 0.87 (0.79‐0.96) | |
Hypoglycemia
Table 4 summarizes the results for hypoglycemia and severe hypoglycemia in the study population, and Table 5 summarizes the secondary analyses of hypoglycemia in the subset with at least 8 POC glucose readings.
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 2504 | 4515 | 2295 | |
| Stays with hypoglycemia (%) | 296 (11.8) | 437 (9.7) | 210 (9.2) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.82 (0.72‐0.94) | 0.77 (0.65‐0.92) | 0.95 (0.81‐1.10) |
| Stays with severe hypoglycemia (%) | 73 (2.9) | 96 (2.1) | 55 (2.4) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.73 (0.54‐0.98) | 0.82 (0.58‐1.16) | 1.13 (0.81‐1.56) |
| Monitored patient‐days | 11,584 | 21,158 | 11,548 | |
| Days with hypoglycemia (%) | 441 (3.8) | 623 (2.9) | 300 (2.6) | |
| RR hypoglycemic day (CI) | 1.0 | 0.77 (0.69‐0.87) | 0.68 (0.59‐0.78) | 0.88 (0.77‐1.01) |
| Days with severe hypoglycemia (%) | 86 (0.74) | 109 (0.52) | 66 (0.57) | |
| RR Severe hypoglycemic day (CI) | 1.0 | 0.69 (0.52‐0.92) | 0.77 (0.56‐1.06) | 1.10 (0.82‐1.5) |
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 1440 | 2664 | 1426 | |
| Stays with hypoglycemia (%) | 237 (16.5) | 384 (14.4) | 180 (12.6) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.88 (0.76‐1.02) | 0.77 (0.64‐0.92) | 0.88 (0.75‐1.03) |
| Stays with severe hypoglycemia (%) | 58 (4.0) | 93 (3.5) | 47 (3.3) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.87 (0.63‐1.2) | 0.82 (0.56‐1.19) | 0.94 (0.67‐1.33) |
| Monitored patient‐days | 9,317 | 17,310 | 9,684 | |
| Days with hypoglycemia (%) | 379 (4.1) | 569 (3.3) | 269 (2.7) | |
| RR hypoglycemic day (CI) | 1.0 | 0.81 (0.71‐0.92) | 0.68 (0.59‐0.80) | 0.85 (0.73‐0.98) |
| Days with severe hypoglycemia (%) | 71 (0.76) | 106 (0.61) | 58 (0.60) | |
| RR severe hypoglycemic day (CI) | 1.0 | 0.80 (0.60‐1.08) | 0.79 (0.56‐1.11) | 0.98 (0.71‐1.34) |
Analysis by Patient‐Stay
The percent of patients that suffered 1 or more hypoglycemic event over the course of their inpatient stay was 11.8% in TP1, 9.7% in TP2, and 9.2% in TP3. The RR of a patient suffering from a hypoglycemic event was significantly improved in the intervention time periods compared to baseline, with the RR of TP3:TP1 = 0.77 (CI, 0.65‐0.92). There was a strong trend for incremental improvement in hypoglycemic patient‐stays for TP3 versus TP2, but the trend just missed statistical significance (P < 0.07). Similar trends in improvement were found for severe hypoglycemia by patient‐stay, but these trends were only statistically significant for TP2 versus TP1. The findings were similar in the subset of patients with at least 8 POC glucose readings (Table 5).
Analysis by Patient‐Day
Of monitored patient days in the baseline TP1, 3.8% contained a hypoglycemic value of 60 mg/dL. With the introduction of structured insulin orders in TP2, this was reduced to 2.9%, and in TP3 it was 2.6%. The RR of a hypoglycemic patient‐day of TP2 compared to TP1 was 0.77 (CI, 0.69‐0.87), whereas the cumulative impact of the structured order set and algorithm (TP3:TP1) was 0.68 (CI, 0.59‐0.78), representing a 32% reduction of the baseline risk of suffering from a hypoglycemic day. Similar reductions were seen for the risk of a severe hypoglycemic patient‐day.
The secondary analysis of hypoglycemic and severe hypoglycemic patient‐days showed very similar results, except that the TP3:TP2 RR for hypoglycemia of 0.85 (CI, 0.73‐0.98) reached statistical significance, again demonstrating the incrementally beneficial effect of the insulin management algorithm.
DISCUSSION
Our study convincingly demonstrates that significant improvement in glycemic control can be achieved with implementation of structured subcutaneous insulin orders and a simple insulin management protocol. Perhaps more importantly, these gains in glycemic control are not gained at the expense of increased iatrogenic hypoglycemia, and in fact, we observed a 32% decline in the percent of patient‐days with hypoglycemia. This is extremely important because fear of hypoglycemia is the most significant barrier to glycemic control efforts.
Strengths and Limitations
Our study has several strengths. The study is large and incorporates all patients with diabetes or hyperglycemia captured by POC glucose testing, and the observation period is long enough that bias from merely being observed is not a factor. We used metrics for glycemic control, hypoglycemia, and insulin use patterns that are of high quality and are generally in line with the Society of Hospital Medicine (SHM) Glycemic Control Task force recommendations,12, 13 and examined data by both patient‐stay and patient‐day.
The increased use of anticipatory physiologic subcutaneous insulin regimens, and the subsequent decline in the use of sliding scale insulin, is the most likely mechanism for improvement. The improvements seen are fairly dramatic for an institution in absolute terms, because inpatient hyperglycemia and hypoglycemia are so common. For example, on an annualized basis for our 400‐bed medical center, these interventions prevent 124 patients from experiencing 208 hypoglycemic days.
Other institutions should be able to replicate our results. We received administrative support to create a multidisciplinary steering committee, but we did not have incremental resources to create a dedicated team for insulin management, mandated endocrinology comanagement or consultations, or manual data collection. In fact, we had only 1 diabetes educator for 400 adult beds at 2 sites, and were relatively underresourced in this area by community standards. There was some time and expense in creating the glycemic control reports, but all of the glucose data collected were part of normal care, and the data retrieval became automated.
The main limitation of this study lies in the observational study design. There were multiple interventions in addition to structured insulin orders and the insulin management algorithm, and these educational and organizational changes undoubtedly also contributed to the overall success of our program. Since we did not perform a randomized controlled trial, the reader might reasonably question if the structured order sets and insulin management algorithm were actually the cause of the improvement seen, as opposed to these ancillary efforts or secular change. However, there are several factors that make this unlikely. First, the study population was well‐defined, having diabetes or documented hyperglycemia in all 3 time periods. Second, the demographics remained constant or actually worked against improvement trends, since the markers of patient acuity suggest increased patient acuity over the observation period. Third, the temporal relationship of the improvement to the introduction of our key interventions, as viewed on statistical process control charts shown in Figure 5, strongly suggest a causal relationship. This temporal relationship was consistently observed no matter how we chose to define uncontrolled hyperglycemia, and was also seen on hypoglycemia control charts. We view the ancillary interventions (such as educational efforts) as necessary, but not sufficient, in and of themselves, to effect major improvement.
We did not analyze the impact of the improved glycemic control on patient outcomes. In the absence of a randomized controlled trial design, controlling for the various confounders is a challenging task. Also, it is likely that not all hypoglycemic events were attributable to inpatient glycemic control regimens, though the secondary analysis probably eliminated many hypoglycemia admissions.
Lessons Learned: Implications from our study
We agree with the American Association of Clinical Endocrinologists (AACE)/American Diabetes Association (ADA)2 and the SHM Glycemic Control Task Force12 about the essential elements needed for successful implementation of inpatient glycemic control programs:
An appropriate level of administrative support.
Formation of a multidisciplinary steering committee to drive the development of initiatives, empowered to enact changes.
Assessment of current processes, quality of care, and barriers to practice change.
Development and implementation of interventions, including standardized order sets, protocols, policies, and algorithms with associated educational programs.
Metrics for evaluation of glycemic control, hypoglycemia, insulin use patterns, and other aspects of care.
Metrics to follow hypoglycemia are extremely important. The voluntary reporting on insulin‐induced hypoglycemia fluctuated widely over the course of our project. These fluctuations did not correlate well with the more objective and accurate measures we followed, and this objective data was very helpful in reducing the fear of hypoglycemia, and spreading the wider use of basal bolus insulin regimens. We strongly recommend that improvement teams formulate and follow measures of glycemic control, hypoglycemia, and insulin use, similar to those outlined in the SHM Glycemic Control Improvement Guide12 and the SHM Glycemic Control Task Force summary on glucometrics.13
Although we introduced our structured insulin order set first, with a long lag time until we introduced the insulin management algorithm, we advocate a different approach for institutions grappling with these issues. This approach is well‐described by the SHM Glycemic Control Task Force.14 An insulin management algorithm should be crafted first, integrating guidance for insulin dosing, preferred insulin regimens for different nutritional situations, a glycemic target, insulin dosing adjustment, glucose monitoring, and prompts for ordering a glycosylated hemoglobin (A1c) level. Next, the order set and the supporting educational programs should integrate this guidance as much as possible, making the key guidance available at the point of patient care.
This guidance was available in our algorithm but was not inherent in the structured insulin orders described in this report, and all basal and nutritional insulin options were offered as equally acceptable choices. This version did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components. Only a single adjustment dose scale was offered, leaving appropriate modifications up to the end user, and from a usability standpoint, our CPOE insulin orders lacked dynamic flexibility (revising a single insulin required discontinuing all prior orders and reentering all orders). These limitations have subsequently been addressed with Version 2 of our CPOE insulin orders, and the details will soon be available in the literature.15
We are now exploring further improvement with concurrent identification and intervention of hyperglycemic patients that are not on physiologic insulin regimens or not meeting glycemic targets, and implementing protocols addressing the transition from infusion insulin.
CONCLUSION
We significantly improved glycemic control and simultaneously reduced hypoglycemia across all major medical and surgical services at our medical center, thereby addressing the number 1 barrier to improved inpatient glycemic control. We achieved this via systems changes with the introduction of structured subcutaneous insulin orders and the insulin management algorithm, along with education, but did not otherwise mandate or monitor adherence to our algorithm.
Implementing an institutional insulin management algorithm and structured insulin orders should now be viewed as a potent safety intervention as well as an intervention to enhance quality, and we have demonstrated that non‐critical care glycemic control efforts can clearly be a win‐win situation.
Diabetes has reached epidemic proportions in the United States, affecting over 20 million individuals,1 and further rises are expected. A disproportionate increase in diabetes has occurred in the inpatient setting.2 Furthermore, for every 2 patients in the hospital with known diabetes, there may be an additional 1 with newly observed hyperglycemia. Both are common. In 1 report, for example, 24% of inpatients with hyperglycemia had a prior diagnosis of diabetes, whereas another 12% had hyperglycemia without a prior diagnosis of diabetes.3
Although there is a paucity of high quality randomized controlled trials to support tight glycemic control in non‐critical care inpatient settings, poor glycemic control in hospitalized patients is strongly associated with undesirable outcomes for a variety of conditions, including pneumonia,4 cancer chemotherapy,5 renal transplant,6 and postsurgical wound infections.7, 8 Hyperglycemia also induces dehydration, fluid and electrolyte imbalance, gastric motility problems, and venous thromboembolism formation.9
Structured subcutaneous insulin order sets and insulin management protocols have been widely advocated as a method to encourage basal bolus insulin regimens and enhance glycemic control,2, 9, 10 but the effect of these interventions on glycemic control, hypoglycemia, and insulin use patterns in the real world setting has not been well reported. Fear of inducing hypoglycemia is often the main barrier for initiating basal insulin containing regimens and pursuing glycemic targets.2 The evidence would suggest, however, that sliding scale regimens, as opposed to more physiologic basal bolus regimens, may actually increase both hypoglycemic and hyperglycemic excursions.11 A convincing demonstration of the efficacy (improved insulin use patterns and reduced hyperglycemia) and safety (reduced hypoglycemia) of structured insulin order sets and insulin management protocols would foster a more rapid adoption of these strategies.
PATIENTS AND METHODS
In our 400‐bed university hospital, we formed a hospitalist‐led multidisciplinary team in early 2003, with the focus of improving the care delivered to non‐critical care patients with diabetes or hyperglycemia. We used a Plan‐Do‐Study‐Act (PDSA) performance improvement framework, and conducted institutional review board (IRB)‐approved prospective observational research in parallel with the performance improvement efforts, with a waiver for individual informed consent. The study population consisted of all adult inpatients on non‐critical care units with electronically reported point of care (POC) glucose testing from November 2002 through December 2005. We excluded patients who did not have either a discharge diagnosis of Diabetes (ICD 9 codes 250‐251.XX) or demonstrated hyperglycemia (fasting POC glucose >130 mg/dL 2, or a random value of >180 mg/dL) from analysis of glycemic control and hypoglycemia. Women admitted to Obstetrics were excluded. Monthly and quarterly summaries on glycemic control, hypoglycemia, and insulin use patterns (metrics described below) were reported to the improvement team and other groups on a regular basis throughout the intervention period. POC glucose data, demographics, markers of severity of illness, and diagnosis codes were retrieved from the electronic health record.
Interventions
We introduced several interventions and educational efforts throughout the course of our improvement. The 2 key interventions were as follows:
Structured subcutaneous insulin order sets (November, 2003).
An insulin management algorithm, described below (May 2005).
Key Intervention #1: Structured Subcutaneous Insulin Order Set Implementation
In November 2003, we introduced a paper‐based structured subcutaneous insulin order set. This order set encouraged the use of scheduled basal and nutritional insulin, provided guidance for monitoring glucose levels, and for insulin dosing. A hypoglycemia protocol and a standardized correction insulin table were embedded in the order set. This set was similar to examples of structured insulin ordering subsequently presented in the literature.9 In a parallel effort, the University of California, San Diego Medical Center (UCSDMC) was developing a computer physician order entry (CPOE) module for our comprehensive clinical information system, Invision (Siemens Medical Systems, Malvern, PA), that heretofore had primarily focused on result review, patient schedule management, and nursing documentation. In anticipation of CPOE and for the purpose of standardization, we removed outdated sliding scale insulin regimens from a variety preexisting order sets and inserted references to the standardized subcutaneous insulin order set in their stead. The medication administration record (MAR) was changed to reflect the basal/nutritional/correction insulin terminology. It became more difficult to order a stand‐alone insulin sliding scale even before CPOE versions became available. The standardized order set was the only preprinted correction scale insulin order available, and ordering physicians have to specifically opt out of basal and nutritional insulin choices to order sliding scale only regimens. Verbal orders for correction dose scales were deemed unacceptable by medical staff committees. Correctional insulin doses could be ordered as a 1‐time order, but the pharmacy rejected ongoing insulin orders that were not entered on the structured form.
We introduced our first standardized CPOE subcutaneous insulin order set in January 2004 at the smaller of our 2 campuses, and subsequently completed full deployment across both campuses in all adult medical‐surgical care areas by September 2004.
The CPOE version, like the paper version that immediately preceded it, encouraged the use of basal/bolus insulin regimens, promoted the terms basal, nutritional or premeal, and adjustment dose insulin in the order sets and the medication administration record, and was mandatory for providers wishing to order anything but a 1‐time order of insulin. Figure 1 depicts a screen shot of the CPOE version. Similar to the paper version, the ordering physician had to specifically opt out of ordering scheduled premeal and basal insulin to order a sliding scale only regimen. The first screen also ensured that appropriate POC glucose monitoring was ordered and endorsed a standing hypoglycemia protocol order. The CPOE version had only a few additional features not possible on paper. Obvious benefits included elimination of unapproved abbreviations and handwriting errors. Nutritional and correction insulin types were forced to be identical. Fundamentally, however, both the paper and online structured ordering experiences had the same degree of control over provider ordering patterns, and there was no increment in guidance for choosing insulin regimens, hence their combined analysis as structured orders.

Key Intervention #2: Insulin Management Algorithm
The structured insulin order set had many advantages, but also had many limitations. Guidance for preferred insulin regimens for patients in different nutritional situations was not inherent in the order set, and all basal and nutritional insulin options were offered as equally acceptable choices. The order set gave very general guidance for insulin dosing, but did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components, and guidance for setting a glycemic target or adjusting insulin was lacking.
Recognizing these limitations, we devised an insulin management algorithm to provide guidance incremental to that offered in the order set. In April 2005, 3 hospitalists piloted a paper‐based insulin management algorithm (Figure 2, front; Figure 3, reverse) on their teaching services. This 1‐page algorithm provided guidance on insulin dosing and monitoring, and provided institutionally preferred insulin regimens for patients in different nutritional situations. As an example, of the several acceptable subcutaneous insulin regimens that an eating patient might use in the inpatient setting, we advocated the use of 1 preferred regimen (a relatively peakless, long‐acting basal insulin once a day, along with a rapid acting analog nutritional insulin with each meal). We introduced the concept of a ward glycemic target, provided prompts for diabetes education, and generally recommended discontinuation of oral hypoglycemic agents in the inpatient setting. The hospitalists were introduced to the concepts and the algorithm via 1 of the authors (G.M.) in a 1‐hour session. The algorithm was introduced on each teaching team during routine teaching rounds with a slide set (approximately 15 slides) that outlined the basic principles of insulin dosing, and gave example cases which modeled the proper use of the algorithm. The principles were reinforced on daily patient work rounds as they were applied on inpatients with hyperglycemia. The pilot results on 25 patients, compared to 250 historical control patients, were very promising, with markedly improved glycemic control and no increase in hypoglycemia. We therefore sought to spread the use of the algorithm. In May 2005 the insulin management algorithm and teaching slide set were promoted on all 7 hospitalist‐run services, and the results of the pilot and concepts of the algorithm were shared with a variety of house staff and service leaders in approximately a dozen sessions: educational grand rounds, assorted noon lectures, and subsequently, at new intern orientations. Easy access to the algorithm was assured by providing a link to the file within the CPOE insulin order set.


Other Attempts to Improve Care
Several other issues were addressed in the context of the larger performance improvement effort by the team. In many cases, hard data were not gathered to assess the effectiveness of the interventions, or the interventions were ongoing and could be considered the background milieu for the key interventions listed above.
During each intervention, education sessions were given throughout the hospital to staff, including physicians, residents, and nurses, using departmental grand rounds, nursing rounds, and in‐services to describe the process and goals. Patient education programs were also redesigned and implemented, using preprinted brochure. Front‐line nursing staff teaching skills were bolstered via Clinical Nurse Specialist educational sessions, and the use of a template for patient teaching. The educational template assessed patient readiness to learn, home environment, current knowledge, and other factors. Approximately 6 conferences directed at various physician staff per year became part of the regular curriculum.
We recognized that there was often poor coordination between glucose monitoring, nutrition delivery, and insulin administration. The traditional nursing practice of the 6:00 AM finger stick and insulin administration was changed to match a formalized nutrition delivery schedule. Nutrition services and nursing were engaged to address timeliness of nutrition delivery, insulin administration, and POC glucose documentation in the electronic health record.
Feedback to individual medicine resident teams on reaching glycemic targets, with movie ticket/coffee coupon rewards to high performing teams, was tried from April 2004 to September 2004.
Measures and Analyses
Assessing Insulin Use Patterns
A convenience sample gathering all subcutaneous insulin orders from 4 to 5 selected days per month yielded 70 to 90 subcutaneous insulin orders for review each month. Sampling was originally performed each month, followed by less frequent sampling once stability in insulin use patterns was reached. Regimens were categorized by pharmacy and hospitalist review as to whether basal insulin was part of the insulin regimen or not. The percentage of insulin regimens incorporating basal insulin was calculated for each sampled month and followed in run charts, and comparisons between preorder set and postorder set time periods were made using Pearson's chi square statistic.
Assessing Glycemic Control
Glycemic control and hypoglycemia parameters were monitored for the entire 38‐month observation period.
Routinely monitored POC glucose values were used to assess glycemic control. During the initial data examination, it was found after 14 days of the hospital stay, there was a notable stabilization and improvement in glucose control and fewer hypoglycemic events, therefore we examined only the first 14 days of hospitalization, thereby eliminating a potential source of bias from length of stay outliers.
A mean glucose value was recorded for each patient‐day with 1 or more recorded values. Glycemic control for each patient‐stay was calculated by averaging the patient‐day mean values, which we will refer to as the day‐weighted mean. Hypoglycemic values (60 mg/dL) were excluded from calculation of the mean glucose, to avoid equating frequent hypoglycemia with optimal glycemic control. An uncontrolled patient‐day was defined as a monitored patient‐day with a mean glucose 180 mg/dL. An uncontrolled patient‐stay is defined as a patient‐stay with a day‐weighted mean glucose value 180 mg/dL.
We theorized that the greatest impact of the interventions would be realized in patients with longer monitoring periods, and that those with only a few POC glucose values could potentially misrepresent the impact of our interventions: therefore we performed a second analysis restricted to patients with 8 POC glucose values.
Assessing Hypoglycemia
Hypoglycemia was defined as a glucose 60 mg/dL, and severe hypoglycemia was defined as a glucose 40 mg/dL. These parameters were characterized by 2 methods. First, we calculated the percentage of monitored patients suffering from 1 or more hypoglycemic events or severe hypoglycemic events over the course of their entire admission. A second method tracked the percentage of monitored patient‐days with hypoglycemia and severe hypoglycemia, thereby correcting for potential misinterpretation from clustered repeated measures or variable length of stay. As with the glycemic control analysis, we repeated the hypoglycemia analysis in the subset of patients with 8 POC glucose values.
Summary Analysis of Glycemic Control and Hypoglycemia
Pearson chi square values, with relative risks (RRs) and 95% confidence intervals (CIs) were calculated to compare glycemic control and hypoglycemia in the 2 key interventions and baseline. The interventions and data reporting were grouped as follows:
Baseline: November 2002 to October 2003) = Time Period 1 (TP1)
Structured Order Set: November 2003 to April 2005) = Time Period 2 (TP2)
Algorithm plus Structured Order Set: May 2005 to December 2005) = Time Period 3 (TP3)
A P value of less than 0.05 was determined as significant and data were analyzed using STATA, Version 8 (STATA Corp., College Station, TX).
We assigned the RR of uncontrolled hyperglycemia and the RR of hypoglycemia during the baseline time (TP1) with values of 1.0, and calculated the RR and CIs for the same parameters during TP2 and TP3.
RESULTS
Just over 11,000 patients were identified for POC glucose testing over the 38 month observation period. Of these, 9314 patients had either a diagnosis of diabetes or documented hyperglycemia. The characteristics of this study population are depicted in Table 1. There were no differences between the groups and the demographics of age, gender, or length of stay (P > 0.05 for all parameters). There was a slight increase in the percent of patients with any intensive care unit days over the 3 time periods and a similar increase in the case mix index.
| Patients Meeting Criteria of Diabetes Mellitus Diagnosis or Hyperglycemia (n = 9,314 patients) | Baseline | TP2 | TP3 |
|---|---|---|---|
| |||
| Time period (TP) | November 2002 to October 2003 | November 2003 to April 2005 | May 2005 to December 2005 |
| Monitored patient days (44,232) | 11,571 | 21,126 | 11,535 |
| Number of patients (9,314) | 2,504 | 4,515 | 2,295 |
| Males (%) | 55 | 54 | 56 |
| Average age standard deviation | 56 17 | 56 17 | 56 16 |
| Length of stay (excluding highest 1% of outliers) | 4.6 5.9 | 4.6 5.7 | 4.8 5.8 |
| % With any intensive care unit days* | 18 | 20 | 22 |
| Case mix index score (mean SD) | 1.8 2.1 | 2.0 2.3 | 2.1 2.1 |
| Case mix index (median score) | 1.1 | 1.3 | 1.3 |
Of the 9314 study patients, 5530 had 8 or more POC glucose values, and were included in a secondary analysis of glycemic control and hypoglycemia.
Insulin Use Patterns
Figure 4 demonstrates the dramatic improvement that took place with the introduction of the structured order set. In the 6 months preceding the introduction of the structured insulin order set (May‐October 2003) 72% of 477 sampled patients with insulin orders were on sliding scale‐only insulin regimens (with no basal insulin), compared to just 26% of 499 patients sampled in the March to August 2004 time period subsequent to order set implementation (P < .0001, chi square statistic). Intermittent monthly checks on insulin use patterns reveal this change has been sustained.

Glycemic Control
A total of 9314 patients with 44,232 monitored patient‐days and over 120,000 POC glucose values were analyzed to assess glycemic control, which was improved with structured insulin orders and improved incrementally with the introduction of the insulin management algorithm.
The percent of patient‐days that were uncontrolled, defined as a monitored day with a mean glucose of 180 mg/dL, was reduced over the 3 time periods (37.8% versus 33.9% versus 30.1%, P < 0.005, Pearson chi square statistic), representing a 21% RR reduction of uncontrolled patient‐days from TP1 versus TP3. Table 2 shows the summary results for glycemic control, including the RR and CIs between the 3 time periods.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 179 66 | 170 65 | 165 58 | |
| Median | 160 | 155 | 151 | |
| Uncontrolled patient‐days* | 4,372 | 7,162 | 3,465 | |
| Monitored patient‐days | 11,555 | 21,135 | 11,531 | |
| % Uncontrolled patient‐days | 37.8 | 33.9 | 30.1 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.89 (0.87‐0.92) | 0.79 (0.77‐0.82) | 0.89 (0.86‐0.92) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 177 57 | 174 54 | 170 50 | |
| Day‐weighted median | 167 | 162 | 158 | |
| Uncontrolled patient‐stay (%) | 1,038 | 1,696 | 784 | |
| Monitored patient‐stay | 2,504 | 4,515 | 2,295 | |
| % Uncontrolled patient‐stays | 41.5 | 37.6 | 34.2 | |
| RR: uncontrolled patient‐stay (95% confidence interval) | 0.91 (0.85‐0.96) | 0.84 (0.77‐0.89) | 0.91 (0.85‐0.97) | |
In a similar fashion, the percent of patients with uncontrolled patient‐stays (day‐weighted mean glucose 180 mg/dL) was also reduced over the 3 time periods (41.5% versus 37.6% versus 34.2%, P < 0.05, Pearson chi square statistic, with an RR reduction of 16% for TP3:TP1). Figure 5 depicts a statistical process control chart of the percent of patients experiencing uncontrolled patient‐stays over time, and is more effective in displaying the temporal relationship of the interventions with the improved results.

Uncontrolled hyperglycemic days and stays were reduced incrementally from TP3 versus TP2, reflecting the added benefit of the insulin management algorithm, compared to the benefit enjoyed with the structured order set alone.
When the analyses were repeated after excluding patients with fewer than 8 POC glucose readings (Table 3), the findings were similar, but as predicted, the effect was slightly more pronounced, with a 23% relative reduction in uncontrolled patient‐days and a 27% reduction in uncontrolled patient‐stays of TP3 versus TP1.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 172 65 | 169 64 | 163 57 | |
| Median | 159 | 154 | 149 | |
| Uncontrolled patient‐days* | 3,469 | 5,639 | 2,766 | |
| Monitored patient‐days | 9,304 | 17,278 | 9,671 | |
| % Uncontrolled patient‐days | 37.3 | 32.6 | 28.6 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.87 (0.85‐0.90) | 0.77 (0.74‐0.80) | 0.88 (0.84‐0.91) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 175 51 | 169 47 | 166 45 | |
| Day‐weighted median | 167 | 158 | 155 | |
| Uncontrolled patient‐stay (%) | 588 | 908 | 425 | |
| Monitored patient‐stay | 1,439 | 2,659 | 1,426 | |
| % Uncontrolled patient‐stays | 40.1 | 34.1 | 29.8 | |
| RR: Uncontrolled patient‐stay (95% confidence interval) | 0.84 (0.77‐0.91) | 0.73 (0.66‐0.81) | 0.87 (0.79‐0.96) | |
Hypoglycemia
Table 4 summarizes the results for hypoglycemia and severe hypoglycemia in the study population, and Table 5 summarizes the secondary analyses of hypoglycemia in the subset with at least 8 POC glucose readings.
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 2504 | 4515 | 2295 | |
| Stays with hypoglycemia (%) | 296 (11.8) | 437 (9.7) | 210 (9.2) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.82 (0.72‐0.94) | 0.77 (0.65‐0.92) | 0.95 (0.81‐1.10) |
| Stays with severe hypoglycemia (%) | 73 (2.9) | 96 (2.1) | 55 (2.4) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.73 (0.54‐0.98) | 0.82 (0.58‐1.16) | 1.13 (0.81‐1.56) |
| Monitored patient‐days | 11,584 | 21,158 | 11,548 | |
| Days with hypoglycemia (%) | 441 (3.8) | 623 (2.9) | 300 (2.6) | |
| RR hypoglycemic day (CI) | 1.0 | 0.77 (0.69‐0.87) | 0.68 (0.59‐0.78) | 0.88 (0.77‐1.01) |
| Days with severe hypoglycemia (%) | 86 (0.74) | 109 (0.52) | 66 (0.57) | |
| RR Severe hypoglycemic day (CI) | 1.0 | 0.69 (0.52‐0.92) | 0.77 (0.56‐1.06) | 1.10 (0.82‐1.5) |
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 1440 | 2664 | 1426 | |
| Stays with hypoglycemia (%) | 237 (16.5) | 384 (14.4) | 180 (12.6) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.88 (0.76‐1.02) | 0.77 (0.64‐0.92) | 0.88 (0.75‐1.03) |
| Stays with severe hypoglycemia (%) | 58 (4.0) | 93 (3.5) | 47 (3.3) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.87 (0.63‐1.2) | 0.82 (0.56‐1.19) | 0.94 (0.67‐1.33) |
| Monitored patient‐days | 9,317 | 17,310 | 9,684 | |
| Days with hypoglycemia (%) | 379 (4.1) | 569 (3.3) | 269 (2.7) | |
| RR hypoglycemic day (CI) | 1.0 | 0.81 (0.71‐0.92) | 0.68 (0.59‐0.80) | 0.85 (0.73‐0.98) |
| Days with severe hypoglycemia (%) | 71 (0.76) | 106 (0.61) | 58 (0.60) | |
| RR severe hypoglycemic day (CI) | 1.0 | 0.80 (0.60‐1.08) | 0.79 (0.56‐1.11) | 0.98 (0.71‐1.34) |
Analysis by Patient‐Stay
The percent of patients that suffered 1 or more hypoglycemic event over the course of their inpatient stay was 11.8% in TP1, 9.7% in TP2, and 9.2% in TP3. The RR of a patient suffering from a hypoglycemic event was significantly improved in the intervention time periods compared to baseline, with the RR of TP3:TP1 = 0.77 (CI, 0.65‐0.92). There was a strong trend for incremental improvement in hypoglycemic patient‐stays for TP3 versus TP2, but the trend just missed statistical significance (P < 0.07). Similar trends in improvement were found for severe hypoglycemia by patient‐stay, but these trends were only statistically significant for TP2 versus TP1. The findings were similar in the subset of patients with at least 8 POC glucose readings (Table 5).
Analysis by Patient‐Day
Of monitored patient days in the baseline TP1, 3.8% contained a hypoglycemic value of 60 mg/dL. With the introduction of structured insulin orders in TP2, this was reduced to 2.9%, and in TP3 it was 2.6%. The RR of a hypoglycemic patient‐day of TP2 compared to TP1 was 0.77 (CI, 0.69‐0.87), whereas the cumulative impact of the structured order set and algorithm (TP3:TP1) was 0.68 (CI, 0.59‐0.78), representing a 32% reduction of the baseline risk of suffering from a hypoglycemic day. Similar reductions were seen for the risk of a severe hypoglycemic patient‐day.
The secondary analysis of hypoglycemic and severe hypoglycemic patient‐days showed very similar results, except that the TP3:TP2 RR for hypoglycemia of 0.85 (CI, 0.73‐0.98) reached statistical significance, again demonstrating the incrementally beneficial effect of the insulin management algorithm.
DISCUSSION
Our study convincingly demonstrates that significant improvement in glycemic control can be achieved with implementation of structured subcutaneous insulin orders and a simple insulin management protocol. Perhaps more importantly, these gains in glycemic control are not gained at the expense of increased iatrogenic hypoglycemia, and in fact, we observed a 32% decline in the percent of patient‐days with hypoglycemia. This is extremely important because fear of hypoglycemia is the most significant barrier to glycemic control efforts.
Strengths and Limitations
Our study has several strengths. The study is large and incorporates all patients with diabetes or hyperglycemia captured by POC glucose testing, and the observation period is long enough that bias from merely being observed is not a factor. We used metrics for glycemic control, hypoglycemia, and insulin use patterns that are of high quality and are generally in line with the Society of Hospital Medicine (SHM) Glycemic Control Task force recommendations,12, 13 and examined data by both patient‐stay and patient‐day.
The increased use of anticipatory physiologic subcutaneous insulin regimens, and the subsequent decline in the use of sliding scale insulin, is the most likely mechanism for improvement. The improvements seen are fairly dramatic for an institution in absolute terms, because inpatient hyperglycemia and hypoglycemia are so common. For example, on an annualized basis for our 400‐bed medical center, these interventions prevent 124 patients from experiencing 208 hypoglycemic days.
Other institutions should be able to replicate our results. We received administrative support to create a multidisciplinary steering committee, but we did not have incremental resources to create a dedicated team for insulin management, mandated endocrinology comanagement or consultations, or manual data collection. In fact, we had only 1 diabetes educator for 400 adult beds at 2 sites, and were relatively underresourced in this area by community standards. There was some time and expense in creating the glycemic control reports, but all of the glucose data collected were part of normal care, and the data retrieval became automated.
The main limitation of this study lies in the observational study design. There were multiple interventions in addition to structured insulin orders and the insulin management algorithm, and these educational and organizational changes undoubtedly also contributed to the overall success of our program. Since we did not perform a randomized controlled trial, the reader might reasonably question if the structured order sets and insulin management algorithm were actually the cause of the improvement seen, as opposed to these ancillary efforts or secular change. However, there are several factors that make this unlikely. First, the study population was well‐defined, having diabetes or documented hyperglycemia in all 3 time periods. Second, the demographics remained constant or actually worked against improvement trends, since the markers of patient acuity suggest increased patient acuity over the observation period. Third, the temporal relationship of the improvement to the introduction of our key interventions, as viewed on statistical process control charts shown in Figure 5, strongly suggest a causal relationship. This temporal relationship was consistently observed no matter how we chose to define uncontrolled hyperglycemia, and was also seen on hypoglycemia control charts. We view the ancillary interventions (such as educational efforts) as necessary, but not sufficient, in and of themselves, to effect major improvement.
We did not analyze the impact of the improved glycemic control on patient outcomes. In the absence of a randomized controlled trial design, controlling for the various confounders is a challenging task. Also, it is likely that not all hypoglycemic events were attributable to inpatient glycemic control regimens, though the secondary analysis probably eliminated many hypoglycemia admissions.
Lessons Learned: Implications from our study
We agree with the American Association of Clinical Endocrinologists (AACE)/American Diabetes Association (ADA)2 and the SHM Glycemic Control Task Force12 about the essential elements needed for successful implementation of inpatient glycemic control programs:
An appropriate level of administrative support.
Formation of a multidisciplinary steering committee to drive the development of initiatives, empowered to enact changes.
Assessment of current processes, quality of care, and barriers to practice change.
Development and implementation of interventions, including standardized order sets, protocols, policies, and algorithms with associated educational programs.
Metrics for evaluation of glycemic control, hypoglycemia, insulin use patterns, and other aspects of care.
Metrics to follow hypoglycemia are extremely important. The voluntary reporting on insulin‐induced hypoglycemia fluctuated widely over the course of our project. These fluctuations did not correlate well with the more objective and accurate measures we followed, and this objective data was very helpful in reducing the fear of hypoglycemia, and spreading the wider use of basal bolus insulin regimens. We strongly recommend that improvement teams formulate and follow measures of glycemic control, hypoglycemia, and insulin use, similar to those outlined in the SHM Glycemic Control Improvement Guide12 and the SHM Glycemic Control Task Force summary on glucometrics.13
Although we introduced our structured insulin order set first, with a long lag time until we introduced the insulin management algorithm, we advocate a different approach for institutions grappling with these issues. This approach is well‐described by the SHM Glycemic Control Task Force.14 An insulin management algorithm should be crafted first, integrating guidance for insulin dosing, preferred insulin regimens for different nutritional situations, a glycemic target, insulin dosing adjustment, glucose monitoring, and prompts for ordering a glycosylated hemoglobin (A1c) level. Next, the order set and the supporting educational programs should integrate this guidance as much as possible, making the key guidance available at the point of patient care.
This guidance was available in our algorithm but was not inherent in the structured insulin orders described in this report, and all basal and nutritional insulin options were offered as equally acceptable choices. This version did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components. Only a single adjustment dose scale was offered, leaving appropriate modifications up to the end user, and from a usability standpoint, our CPOE insulin orders lacked dynamic flexibility (revising a single insulin required discontinuing all prior orders and reentering all orders). These limitations have subsequently been addressed with Version 2 of our CPOE insulin orders, and the details will soon be available in the literature.15
We are now exploring further improvement with concurrent identification and intervention of hyperglycemic patients that are not on physiologic insulin regimens or not meeting glycemic targets, and implementing protocols addressing the transition from infusion insulin.
CONCLUSION
We significantly improved glycemic control and simultaneously reduced hypoglycemia across all major medical and surgical services at our medical center, thereby addressing the number 1 barrier to improved inpatient glycemic control. We achieved this via systems changes with the introduction of structured subcutaneous insulin orders and the insulin management algorithm, along with education, but did not otherwise mandate or monitor adherence to our algorithm.
Implementing an institutional insulin management algorithm and structured insulin orders should now be viewed as a potent safety intervention as well as an intervention to enhance quality, and we have demonstrated that non‐critical care glycemic control efforts can clearly be a win‐win situation.
- Centers for Disease Control and Prevention.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2002.Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention;2003. Available at: www.cdc.gov/diabetes/pubs/factsheet.htm. Accessed January 21, 2006.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955‐1962.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978‐982.
- ,,, et al.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810‐815.
- ,,, et al.Cancer.2004;100:1179‐1185.
- ,,, et al.Early perioperative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321‐1324.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22:77‐81.
- ,,, et al.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356‐361.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553‐591.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77‐82.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity? [Editorial].J Hosp Med.2006;1:141‐144.
- Society of Hospital Medicine Glycemic Control Task Force: Optimizing Glycemic Control and Reducing Hypoglycemia at Your Medical Center. Society of Hospital Medicine, Glycemic Control Quality Improvement Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed October2008.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(S5):66–75.
- ,,,;for the SHM Glycemic Control Task Force.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(S5):29–41.
- ,,,.Indication‐based ordering: a new paradigm for glycemic control in hospitalized inpatients.J Diabetes Sci Tech.2008;2(3):349‐356.
- Centers for Disease Control and Prevention.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2002.Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention;2003. Available at: www.cdc.gov/diabetes/pubs/factsheet.htm. Accessed January 21, 2006.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955‐1962.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978‐982.
- ,,, et al.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810‐815.
- ,,, et al.Cancer.2004;100:1179‐1185.
- ,,, et al.Early perioperative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321‐1324.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22:77‐81.
- ,,, et al.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356‐361.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553‐591.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77‐82.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity? [Editorial].J Hosp Med.2006;1:141‐144.
- Society of Hospital Medicine Glycemic Control Task Force: Optimizing Glycemic Control and Reducing Hypoglycemia at Your Medical Center. Society of Hospital Medicine, Glycemic Control Quality Improvement Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed October2008.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(S5):66–75.
- ,,,;for the SHM Glycemic Control Task Force.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(S5):29–41.
- ,,,.Indication‐based ordering: a new paradigm for glycemic control in hospitalized inpatients.J Diabetes Sci Tech.2008;2(3):349‐356.
Copyright © 2009 Society of Hospital Medicine
Sleep in hospitalized medical patients, Part 2: Behavioral and pharmacological management of sleep disturbances
In Part 1, we reviewed normal sleep architecture, and discussed the numerous factors that often disrupt the sleep of hospitalized medical patients. Effective management of sleep complaints among acutely ill patients includes a thorough assessment of medical and psychiatric conditions, medications and other psychosocial factors that may be directly or indirectly impairing sleep. In Part 2, we review and introduce an algorithm for assessing and managing sleep complaints in acutely ill hospitalized patients.
ASSESSMENT AND EVALUATION OF SLEEP COMPLAINTS
Assessment and evaluation of a sleep complaint begins with (Figure 1) an initial review of the medical record for documentation of the signs and symptoms of an underlying primary sleep disorder, which may be exacerbated during an acute medical illness. Common sleep disorders that are often overlooked include obstructive sleep apnea (OSA), restless leg syndrome (RLS), and periodic limb movement disorder (PLMD). Predisposing factors, characteristic clinical features, and differential diagnoses of these disorders are described in Table 1.
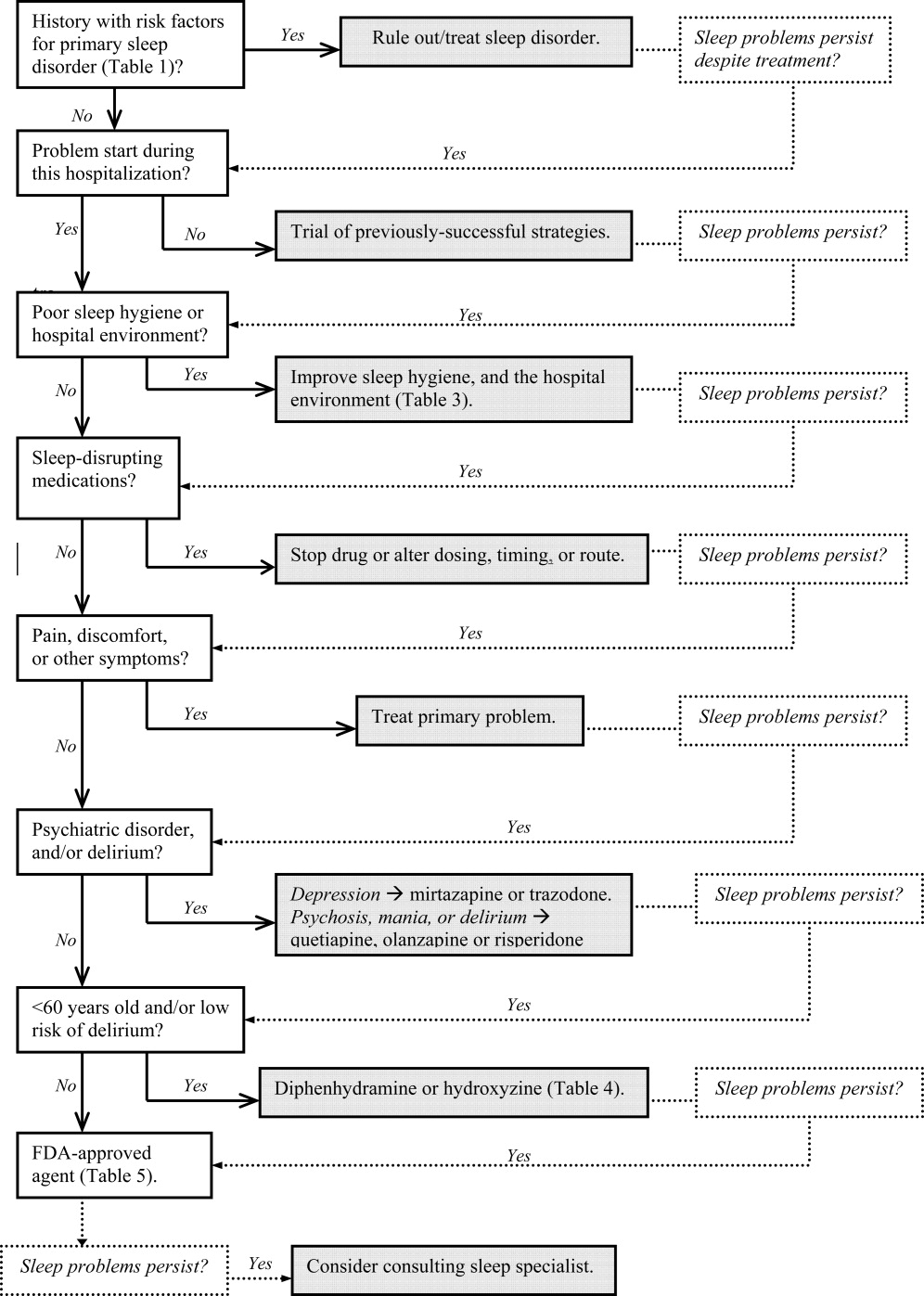
| Sleep Disorder | Predisposing Factors | Clinical Features | Differential Diagnosis |
|---|---|---|---|
| |||
| Obstructive sleep apnea (OSA) | Nasopharyngeal abnormalities, craniofacial abnormalities, obesity, >40 years old, men > women (2:1), neurologic disorder (eg, recent stroke) | Repetitive episodes of upper airway obstruction that occur during sleep, usually associated with oxygen desaturation. Episodes include loud snoring or gasps lasting 2030 seconds. Associated with morning headaches and dry mouth. | Sleep‐related laryngospasm, nocturnal gastroesophageal reflux, narcolepsy, hypersomnia, PLMD, central alveolar hypoventilation, paroxysmal nocturnal dyspnea, primary snoring, Cheyne‐Stokes ventilation, nocturnal asthma |
| Periodic limb movement disorder (PLMD) | OSA. RLS, or narcolepsy; aging; chronic uremia; TCAs or MAOIs; withdrawal from antiepileptic agents, or other sedating agents | Periodic episodes of repetitive and stereotyped limb movements: extension of the big toe with partial flexion of the ankles, knees, or hips. Muscle contractions last 0.5 to 5 seconds, with 20‐second to 40‐second intervals between them. | Sleep starts (occur just prior to, not during, sleep, and do not have a regular periodicity like PLMD), nocturnal epileptic seizures, myoclonic epilepsy |
| Restless leg syndrome (RLS) | Pregnancy (>20 weeks gestation), uremia, anemia, rheumatoid arthritis, peak onset is middle age | Uncomfortable leg sensations that occur prior to sleep onset that leads to an irresistible urge to move the legs. Described as achy, crawling, pulling, prickling, or tingling, and disrupts sleep onset. | Chronic myelopathy, peripheral neuropathy, akathisia, fasciculation syndromes, anemia |
| Sleep starts | Can worsen with anxiety, caffeine or other stimulants, daytime physical exertion | Sudden, brief contraction of the legs that occurs at sleep onset. Usually benign, but may worsen during hospitalization, and interfere with sleep. | PLMD, RLS, hyperekplexia syndrome, in which generalized myoclonus is readily elicited by stimuli |
Obtain a focused history by using questions listed in Table 2 to characterize the onset, duration, frequency, and specific characteristics of the patient's current sleep patterns. Next, establish whether the onset of the patient's sleep complaint began with the time of hospitalization. Subsequent questions can then focus on factors that may be impairing sleep such as the hospital environment and sleep hygiene behaviors by comparing the patient's home sleep habits with those during hospitalization. Inquire about the use or abuse of substances such as sedatives, antidepressants, sedatives, antiepileptic drugs (AEDs), and opioids. Ask questions about the presence of pain syndromes and other comorbidities that often impact sleep.
| Focus | Examples of Questions |
|---|---|
| |
| Sleep pattern | Do you have problems falling asleep or staying asleep? How often do you wake up during the night? How long does it take you to fall back asleep? When did the problem start? What can we do to help you sleep? What time do you try to go to sleep, and what time do you wake up? |
| Behavioral factors | Compare your bedtime routine at home, and in the hospital. |
| Environment | Does the lighting or noise level in the hospital disrupt your sleep? How so? Are you awoken from sleep for laboratory work, monitoring, bathing, or other nursing/medical procedures? |
| Patient comfort | Is your pain adequately controlled at night? If not, are you on a scheduled analgesic regimen, or do you have to ask for pain medications? Do you have breathing problems, gastroesophageal reflux, or other type of discomfort that keeps you from sleeping well? |
| Substances | Do you drink alcohol? How much, and how often? When was your last alcoholic beverage? Inquire about cocaine, methamphetamine, marijuana, and medically‐unsupervised use of opioids. |
| Psychosocial | How was your mood just prior to being hospitalized? How has your mood been since you were admitted? Have you experienced any emotionally or physically traumatic event prior to, or during, this hospitalization that continues to bother you (eg, intubation, resuscitation, surgery, blood draws, MRI scanning)? |
MANAGEMENT OF SLEEP COMPLAINTS
Management of sleep disturbance is multifactorial and consists of nonpharmacologic as well as pharmacologic therapies. A stepwise approach is suggested and begins with nonpharmacologic strategies.
Nonpharmacologic Interventions
Before using sedative/hypnotic agents, address sleep hygiene and other factors that disrupt sleep during a hospitalization such as those listed in Table 3.
| Barriers to Sleep | Strategies To Optimize Sleep in the Hospital |
|---|---|
| |
| Noise | Limit the volume level of television sets, and do not allow patients or visitors to increase the volume. |
| Promptly respond to alarm monitors, and consider liberalizing the monitor alarm setting, if appropriate. | |
| Keep patients' doors closed, if possible. | |
| Post signs to remind staff and visitors to minimize conversations at or near the bedside. | |
| Adhere strictly to visiting hours. | |
| Encourage staff to switch their beepers and other electronic devices to vibrate at night. | |
| Limit the number of visitors at a time and/or if appropriate, have the patient meet with visitors in another location (eg, conference room, cafeteria). | |
| Offer earplugs. | |
| Ask patients to turn their phone ringers off when visiting hours are over. | |
| Anxiety | Encourage visitors to minimize discussing emotionally difficult topics with patients near bedtime. |
| Lighting | Offer eye masks. |
| Encourage exposure to brighter light during the day (turn on the lights, open the curtains), and turn off the lights by 9 PM. | |
| Poor sleep hygiene | Encourage regular nocturnal sleep time, and discourage lengthy naps during the day. |
| Medications and substances | Minimize BzRAs for sleep. Try to wean patients off BzRAs prior to discharge. At discharge, provide the minimum number of pills until they are scheduled to see their primary care clinician posthospitalization, and do not provide refills. |
|
Avoid starting multiple medications at one time. Minimize use of sleep‐disrupting medications (see Part 1, Table 3). |
|
| Change medication regimens to promote sleep; eg, avoid night‐time diuretics if possible. | |
| No caffeine or cigarette smoking after 6 PM. | |
| Effects of treatments | Minimize bathing, dressing changes, room switches, and other activities at night. |
| Regularly review nighttime orders to see if you could decrease the frequency of overnight monitoring (eg, fingersticks, labdraws, checking vitals). | |
| Delirium | Provide an updated calendar to facilitate cognitive orientation. |
| Discontinue nonessential medications. Minimize use of BzRAs, barbiturates, opiates, antihistamines, and anticholinergic agents. | |
| Regularly provide verbal and other cues to orient patients to the date, time, location, and circumstances. | |
| Nocturnal discomfort | Optimize nighttime glycemic control, and maximize pain management. |
| For patients with reflux: No oral intake after 8 PM, and keep head of bed elevated 30 degrees. | |
| Provide nocturnal O2, CPAP, and/or other medications, as appropriate. If patient is on CPAP, assess the mask's fit and comfort. | |
Pharmacologic (Sedative/Hypnotic) Interventions
Pharmacologic therapy may be necessary to treat disordered sleep. The ideal sleep aid would reduce sleep latency or time to fall asleep, increase total sleep time (TST), not cause next‐day sedation, improve daytime functioning, and minimize the development of tolerance. Unfortunately, no single agent meets all these independent criteria. In the past 10 years, newer benzodiazepines (BzRAs) with shorter half‐lives have been shown to be efficacious in reducing sleep latency, but the problem of sleep maintenance without next‐day sedation persists.1 To choose an appropriate sleep agent, evaluate the drug's efficacy, mechanism of action, and side‐effect profile. Then, match these characteristics with the patient's clinical condition(s). In patients with comorbid sleep and psychiatric problems, consider using a sedating psychotropic at bedtime to promote sleep.
Non‐Food and Drug AdministrationApproved (Off‐Label) Sleep Aids: Psychotropic Medications
Limited data exist on the efficacy of non‐Food and Drug Administration (FDA)approved medications for insomnia,2 such as antidepressants and atypical antipsychotics (AAPs), and antihistamines; examples of which are listed in Table 4. The administration of antihistamines, barbiturates, chloral hydrate, and alternative/herbal therapies has been discouraged, because the benefits rarely outweigh the risks associated with their use. Currently, trazodone is the most commonly prescribed antidepressant for the treatment of insomnia, despite the relative lack of data regarding its use for insomnia.3 Prescription data suggest that trazodoneat hypnotic doses, which are lower than the full antidepressant doseis more commonly prescribed for insomnia rather than for its FDA‐approved use for depression.4 In general, sleep specialists refrain from recommending sedating antidepressants for primary insomnia due to insufficient data regarding efficacy and safety. In addition, trazodone has been associated with arrhythmias in patients with preexisting cardiac conduction system disease. Curry et al.3 speculated that trazodone is popular among prescribers because, unlike most BzRAs, trazodone does not have a recommended limited duration of use and is perceived as being safer than BzRAs. Walsh et al.5 conducted a randomized double‐blind, placebo‐controlled trial (n = 589) that compared the hypnotic efficacy and other sleep‐associated variables of trazodone (50 mg) and zolpidem (10 mg). During the first week of treatment, the subjects on trazodone or zolpidem decreased their time to fall asleep, or sleep latency, by 22% and 35%, respectively, compared to placebo. Sleep latency was significantly shorter on zolpidem (57.75 2.7 minutes) than for trazodone (57.7 + 4.0 minutes). By the second week, subjects on zolpidem continued to have a reduction in the time to fall asleep, but there was no significant difference between subjects on trazodone and placebo.5 Trazodone may be an acceptable short‐term alternative to BzRAs for patients with hypercapnia or hypoxemia, and in those with a history of drug abuse or dependence. At doses of 150 to 450 mg, trazodone may be an appropriate medication in patients with major depressive disorder and problems with sleep maintenance.6 Tolerance to trazodone's sedating property tends to develop after 2 weeks of treatment, however, so other treatments may need to be considered if sleep problems persist. The available data address relatively short‐term use of trazodone, so questions of safety and efficacy for chronic insomnia remain unanswered.
| Drug | Pertinent Side Effects | Comments |
|---|---|---|
| ||
| Antidepressants | ||
| Mirtazapine (Remeron) | Somnolence, appetite, weight, dry mouth | May be beneficial for comorbid depression and insomnia. Lower doses (15 mg) increase sedation. |
| Trazodone | Residual daytime sedation, headache, orthostatic hypotension, priapism, cardiac arrhythmias | May be beneficial for comorbid depression and insomnia. Not recommended as first‐line agent for insomnia.3 May be an alternative if BzRAs are contraindicated (severe hypercapnia or hypoxemia or history of substance abuse). Tolerance usually develops within 2 weeks. Lower doses (50100 mg) than when used for depression (400 mg). |
| TCAs | Delirium, cognition, seizure threshold, orthostatic hypotension, tachycardia, acquired prolonged QT syndrome, heart block, acute hepatitis | Avoid in hospitalized patients due to their anticholinergic, antihistaminic, and cardiovascular side effects. May be beneficial for comorbid depression and insomnia. |
| Antihistamines | ||
| Diphenhydramine (Benadryl) | Residual daytime sedation, delirium, orthostatic hypotension, psychomotor function, prolonged QT syndrome, blurred vision, urinary retention | Better than placebo to treat insomnia,12 but data is lacking to definitively endorse diphenhydramine for insomnia.13 Tolerance to antihistamines develops within a few days. Avoid in patients >60 years old.18 |
| Hydroxyzine | Drowsiness, dry mouth, dizziness, agitation, cognitive function | Efficacy as anxiolytic for >4 months use not established. Not FDA‐approved for insomnia. Avoid in patients >60 years old, closed‐angle glaucoma, prostatic hypertrophy, severe asthma, and COPD. |
| Antipsychotics | ||
| Quetiapine (Seroquel) | Sedation, orthostatic hypotension, hyperglycemia, appetite, weight, hyperlipidemia | The most sedating of the atypical antipsychotics, it is frequently used as a sleep aid. Not recommended for insomnia or other sleep problems unless there is a comorbid psychiatric disorder. Dosed lower (25100 mg) when used for insomnia versus for FDA‐approved indications (600 mg). |
| Olanzapine (Zyprexa) | Sedation, hyperglycemia, appetite, weight, hyperlipidemia | Of atypical antipsychotics, olanzapine is the most likely to cause metabolic complications. Should not be used solely for insomnia. |
| Barbiturate | ||
| Chloral hydrate | Oversedation, respiratory depression, nausea, vomiting, diarrhea, drowsiness, cognitive function, psychotic symptoms (paranoia, hallucinations), vertigo, dizziness, headache | Chloral hydrate has been used for the short‐term (2 weeks) treatment of insomnia, but is currently not FDA‐approved for that indication. Additive CNS depression may occur if given with other sedative‐hypnotics. Caution in patients with severe cardiac disease. Contraindicated in marked hepatic or renal impairment. Highly lethal in overdose, and should be avoided in patients with risk of suicide. |
Mirtazapine (Remeron), which promotes both sleep and appetite, may be particularly helpful for patients with cancer, acquired immunodeficiency syndrome (AIDS), and other conditions in which the triad of poor sleep, anorexia, and depression are common. Mirtazapine is a noradrenergic and specific serotonergic agent that causes inverse, dose‐dependent sedation (doses 15 mg are less sedating).7 To target sleeplessness, start with a dose between 7.5 and 15 mg. If ineffective at this dose, it is unlikely that increasing the dose will be of benefit for sleep. A small randomized, double‐blind, placebo‐controlled trial found that low‐dose mirtazapine reduced the apnea‐hypopnea index (API) by half in newly‐diagnosed subjects with OSA (n = 12).8 The results were promising in terms of the use of mixed‐profile serotonergic drugs in treating OSA. However, as pointed out by the researchers, mirtazapine's tendency to cause weight gain, is problematic in this patient population.
Although sedating, tricyclic antidepressants (TCAs) should not be used to promote sleep in hospitalized patients. TCAs increase the risk of cardiac conduction abnormalities, decrease seizure threshold, and have significant anticholinergic and anti‐alpha‐adrenergic effects. In dementia patients, the anticholinergic effect of TCAs may precipitate delirium.
AAPs should not be used routinely as first‐line agents for insomnia, except in patients who are in the midst of acute manic or psychotic episodes.9 With chronic use of AAPs, the risks of hyperglycemia, hyperlipidemia, and weight gain outweigh the potential sleep benefits of these agents. AAPs, especially risperidone, may cause extrapyramidal syndrome (EPS). Risperidone, ziprasidone and quetiapine have been associated with prolonged QTc interval, but the relatively low doses of AAPs that are used purely for sedative purposes makes this risk relatively low. If a patient has a history of Parkinsonism or other EPS, risperidone should generally be avoided. If a patient treated with risperidone develops EPS, another AAP should be considered. A reasonable precaution is to obtain a pretreatment 12‐lead electrocardiogram. If the QTc is greater than 450 msec, consider using olanzapine rather than ziprasidone, risperidone, or quetiapine. Sedating AAPs include risperidone (Risperdal), olanzapine (Zyprexa), and quetiapine (Seroquel), with the latter 2 being especially sedating. Quetiapine may also cause orthostatic hypotension. The recent practice of using AAPs for delirium has not been reported to be associated with significant safety risks, probably because delirium treatment is typically of short duration under a period of close clinical observation. These agents should not be used indefinitely for insomnia without close monitoring of metabolic, psychiatric, and neurologic status. However, recent data suggest that the risk of serious adverse effects of AAPs may outweigh the potential benefits for the treatment of aggression or agitation in patients with Alzheimer's disease.10
A meta‐analysis of randomized placebo‐controlled trials of AAP use among dementia patients showed that overall, the use of AAP drugs for periods of less than 8 to 12 weeks was associated with a small increased risk for death compared with placebo.11 Data indicated that most patients' behaviors improved substantially during the first 1 to 4 weeks of treatment. In a double‐blind, placebo‐controlled trial, 421 patients with Alzheimer's disease and psychosis, aggression or agitation were randomly assigned to receive olanzapine (mean dose, 5.5 mg per day), quetiapine (mean dose, 56.5 mg per day), risperidone (mean dose, 1.0 mg per day), or placebo. Improvement was observed in 32% of patients assigned to olanzapine, 26% of patients assigned to quetiapine, 29% of patients assigned to risperidone, and 21% of patients assigned to placebo. A lower, but significant, proportion of the patients (24%, 16%, 18%, and 5%, respectively) discontinued these medications due to intolerable side effects. Thus, if minimal improvement is observed even after 8 weeks of treatment, prescribers should consider discontinuing the AAP. The management of agitation in dementia, particularly in the elderly, calls for an integrative and creative psychopharmacological approach, including the use of antidepressants, nonbenzodiazepine anxiolytics such as buspirone, and mood stabilizers such as divalproex sodium (Depakote) before exposing patients to the risks of AAPs.
Antihistamines are the most commonly used over‐the‐counter agents for chronic insomnia.1 Diphenhydramine (Benadryl) has been shown to be better than placebo to treat insomnia,12 but data is lacking to definitively endorse its use to promote sleep.13 Diphenhydramine is also limited by the development of tolerance within a few days of daily use. The anticholinergic action of antihistamines may lead to orthostatic hypotension, urinary retention, and may induce delirium in vulnerable patients. Therefore, diphenhydramine should be avoided in hospitalized patients.
Recent data suggest that hydroxyzine, an antihistamine, may be an appropriate sleep aid for patients with hepatic encephalopathy in whom BzRAs are contraindicated.14 Subjective improvement in sleep was observed in 40% of hydroxyzine‐treated patients with hepatic encephalopathy compared to placebo.
Chloral hydrate is one of the Western world's oldest known sedative‐hypnotics and was commonly used as a sleep aid through the 1970s.15 Chloral hydrate was eventually supplanted by BzRAs,16 and fell out of favor as a sleep aid due to its relatively high tolerance rate, drug‐drug interaction profile, and the high risk of death in an overdose. Doses of 500 to 1000 mg sufficed to promote sleep in most of the hospitalized subjects. More recent data regarding its use for treating insomnia are not available, but chloral hydrate may be an alternative short‐term treatment for insomnia in selected hospitalized patients. Because of its high‐risk profile, chloral hydrate would be used as a last‐resort medication, preferably with input from critical care and/or sleep medicine specialists.
FDA‐Approved Sleep Aids
As shown in Table 5, the FDA has approved 3 classes of medications for the treatment of insomnia: benzodiazepine gamma‐aminobutyric acid (GABA)A receptor agonists (BzRAs), nonbenzodiazepine GABAA receptor agonists (non‐BzRAs), and melatonin‐receptor agonists.17 BzRAs include estazolam (ProSom), flurazepam (Dalmane), quazepam (Doral), temazepam (Restoril), and triazolam (Halcion). Though BzRAs decrease sleep latency, increase TST, and decrease slow wave or deep sleep, they also have adverse side effects such as daytime sedation, anterograde amnesia, cognitive impairment, motor incoordination, dependence, tolerance, and rebound insomnia.18 Because of these side effects, BzRAs should be limited to generally healthy, young (ie, 45 years old) patients who are expected to have brief hospital stays.
| Drugs | Adult Dose (mg) | Half‐Life (hours)* | Onset (minutes) | Peak Effect (hours) | Major Effects/Clinical Comments |
|---|---|---|---|---|---|
| |||||
| BzRAs | Caution in elderly patients. Tolerance to BzRAs develop to the sedative, hypnotic, and anticonvulsant effects. | ||||
| Estazolam (ProSom) | 12 | 1024 | 60 | 0.51.5 | Short‐term (710 days) treatment for frequent arousals, early morning awakening. Not as useful for sleep onset. Avoid in patients with OSA. Caution in elderly patients, liver disease. High doses can cause respiratory depression. |
| Flurazepam (Dalmane) | 1530 | 47100 | 1520 | 36 | In general, avoid in hospitalized medical patients, especially elderly patients. |
| Quazepam (Doral) | 7.515 | 25114 | 1.5 | In general, avoid in hospitalized medical patients, especially elderly patients. | |
| Temazepam (Restoril) | 1530 | 616 | 23 | Short‐term (710 days) treatment for sleep onset and maintenance. Doses 30 mg/day: morning grogginess, nausea, headache, and vivid dreaming. | |
| Triazolam (Halcion) | 0.1250.25 | 1.55.5 | 1530 | 1.75 | Maximum dose is 0.5 mg. Short‐term (710 days) treatment. Rapid onset; should be in bed when taking medication. Contraindicated with atazanavir, ketoconazole, itraconazole, nefazodone, ritonavir. |
| Non‐BzRAs | |||||
| Eszopiclone (Lunesta) | 23 | 69 | 1 | In elderly: difficulty falling asleep, then initial: 1 mg; maximum 2 mg. Difficulty staying asleep: 2 mg. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with high‐fat foods. No tolerance after 6 months. | |
| Zaleplon (Sonata) | 520 | 1 | Rapid | 1 | Short‐term (710 days) treatment for falling asleep and/or next‐day wakefulness is crucial (eg, shift workers). |
| Zopiclone (Imovane) | 515 | 3.86.5 (510 in elderly) | 30 | 2 | Transient and short‐term (710 days) treatment. Contraindicated in severe respiratory impairment. Caution in liver disease and depression; elderly prone to side effects. Anticholinergic agents may plasma level. |
| Zolpidem (Ambien) | 520 | 1.44.5 | 30 | 2 | Short‐term (710 days) treatment for sleep onset and maintenance. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with food. No tolerance after 50 weeks. |
| Melatonin agonist | |||||
| Ramelton (Rozerem) | 8 | 12 | 30 | 11.5 | For sleep onset. For faster sleep onset, do not ingest with high‐fat foods. No tolerance. Contraindicated with fluvoxamine. |
Efficacy and safety studies have generally been limited to healthy, younger individuals without a history of primary sleep disorder. Potential adverse effects of BzRAs may become even more pronounced in hospitalized medical patients due to older age, acute illness, cointeraction drugs, and multidrug regimens. Although BzRAs are FDA‐approved for the treatment of insomnia, flurazepam and quazepam should generally be avoided in hospitalized patients. These agents' long half‐lives increase the risk of drug‐drug interactions and adverse events such as respiratory depression, cognitive decline, and delirium in acutely ill patients. For similar reasons, other long‐acting BzRAs such as clonazepam (Klonopin) and diazepam (Valium) should also not be used to treat insomnia in hospitalized patients. An exception to this is a patient with RLS, in which clonazepam is an approved treatment. However, now that ropinirole HCl (Requip) is FDA‐approved for RLS, BzARs may be able to be avoided. Lorazepam (Ativan), due to its relatively short half‐life and its anxiolytic property, is frequently used to treat insomnia in hospitalized medical patients.18 Start with the lowest dose possible (eg, 0.5 mg) as a one‐time‐only order, or on a as needed basis for 3 days. Alprazolam (Xanax), a potent, fast‐acting BzRA with a relatively short half‐life, has developed a reputation as being notoriously addictive, and experts feel alprazolam has similar potential for withdrawal and rebound.19, 20
The use of BzRAs should be minimized in all patients, and avoided in the elderly or those with a particularly high risk for delirium (eg, traumatic brain injury, stroke, multiple new medications). All BzRAs should be avoided in patients with a prior history of sedative‐hypnotic and/or alcohol dependence unless medically indicated, such as in alcohol withdrawal. Refrain from ordering nightly scheduled BzRAs without a specific time limit to ensure that sedative‐hypnotic use is closely monitored.
For the past 2 decades, physicians have been advised against using long‐acting BzRAs in the elderly (>65 years old) due to the increased risks of hip fractures, falls, motor vehicle accidents, daytime sedation, and adverse cognitive events such as delirium.2124 A large 5‐year prospective study in Quebec found that the risk of injury varied by the BzRA, and was independent of half‐life.25 Importantly, the risk of injury was dose‐dependent: the higher the dose of oxazepam, flurazepam, or chlordiazepoxide, the higher the risk of injury in the elderly.
Non‐BzRAs seem to have a superior side‐effect profile when compared to BzRAs, but should also be used with caution in the elderly. Non‐BzRAs include eszopiclone (Lunesta), zaleplon (Sonata), zolpidem (Ambien), and zolpidem extended‐release. The number of comparison studies is limited, but the available data reveal that: (1) zolpidem (Ambien) may be better than temazepam (Restoril) in terms of sleep latency and quality; and (2) zaleplon (Sonata) may lead to a shorter sleep latency than zolpidem (Ambien), but the latter is associated with longer sleep duration.26 Non‐BzRAs have less next‐day sedation, psychomotor dysfunction, tolerance/withdrawal, and rapid‐eye‐movement (REM) sleep rebound; and lower abuse potential than BzRAs.27
The most commonly prescribed hypnotic, zolpidem has a short half‐life, and seems to reduce sleep latency with minimal residual side effects when compared to BzRAs. The results of a recent multicenter, randomized, double‐blind, placebo‐controlled trial indicated that zolpidem extended‐release may be efficacious for up to 6 months in outpatients with chronic insomnia.28
The sole melatonin‐receptor agonist, ramelteon (Rozerem), also reduces time to fall asleep without next‐day psychomotor and memory effects.29 Ramelteon is believed to target receptors melatonin 1 and 2 receptors located in the brain's suprachiasmatic nucleus to stabilize circadian rhythms and stabilize the sleep‐wake cycle.30
CONCLUSION
Hospitalization is often associated with disrupted sleep, which can affect recovery from illness. Understanding the major factors that impair sleep during hospitalization allows clinicians to systemically evaluate and treat sleep problems. More than just prescribing a sedative/hypnotic, the treatment for sleep disruption includes addressing sleep hygiene and hospital environment issues, identifying medications that could disrupt sleep, and treating specific syndromes that impair sleep. We suggest a practical algorithm to guide clinical assessment, treatment options, and selection of appropriate sleeping medications. Critical to optimizing recovery from illness, sleep may be considered as the sixth vital sign, and should be part of the routine evaluation of every hospitalized patient.
- .Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies.Ann Clin Psychiatry.2006;18(1):49–56.
- ,.Treatment of insomnia.Prim Psychiatry.2005;12(8):47–56.
- ,,.Pharmacologic management of insomnia: past, present, and future.Psychiatr Clin North Am.2006;29:871–893.
- ,.“Hypnotic” prescription patterns in a large managed‐care population.Sleep Med.2004;5(5):463–466.
- ,,, et al.Subjective hypnotic efficacy of trazodone and zolpidem in DSM III‐R primary insomnia.Hum Psychopharmacol.1998;13:191–198.
- ,,, et al.Mirtazapine is more effective than trazodone: a double‐blind controlled study in hospitalized patients with major depression.Int Clin Psychopharmacol.1995;10:3–9.
- ,,.Mirtazapine: an antidepressant with noradrenergic and specific serotonergic effects.Pharmacotherapy.1997;17:10–21.
- ,,,.Efficacy of mirtazapine in obstructive sleep apnea syndrome.Sleep.2007;30(1):35–41.
- ,.Atypical antipsychotics in bipolar disorder: systematic review of randomised trials.BMC Psychiatry.2007;7:40:1–17.
- ,,, et al.Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's Disease.N Engl J Med.2006;355:1525–1538.
- ,,.Risk of death with atypical antipsychotic drug treatment for dementia: meta‐analysis of randomized placebo‐controlled trials.JAMA.2005;294(15):1934–1943.
- ,.Clinical evaluation of diphenhydramine hydrochloride for the treatment of insomnia in psychiatric patients: a double‐blind study.J Clin Pharmacol.1983;23:234–242.
- .Diagnosis and treatment of chronic insomnia: a review.Psychiatr Serv.2005;56:332–343.
- ,,,,.Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial.Am J Gastroenterol.2007;102:744–753.
- ,.Clinical effects of chloral hydrate in hospitalized medical patients.J Clin Pharmacol.1979;19(10):669–674.
- Miller RD, editor.Miller's Anesthesia.6th ed.Philadelphia, PA:Elsevier;2005.
- .State‐of‐the‐art sleep management. Awakening insomnia management. Proceedings from a satellite symposium at SLEEP 2006: 20th Anniversary Meeting of the Associated Professional Sleep Societies, Salt Lake City, UT.2006:6–12.
- ,,.Use of a computer‐based reminder to improve sedative‐hypnotic prescribing in older hospitalized patients.J Am Geriatr Soc.2007;55:43–47.
- ,,,.Topiramate use in alprazolam addiction.World J Biol Psychiatry.2006;7(4):265–267.
- ,,,.Trends in recommendations for the pharmacotherapy of anxiety disorders by an international expert panel, 1992–1997.Eur Neuropsychopharmacol.1999;9(Suppl 6):S393–S398.
- ,,,,.Benzodiazepine use and the risk of motor vehicle crash in the elderly.JAMA.1997;278:27–31.
- ,,,,.Psychotropic drug use and the risk of hip fracture.NEngl J Med.1987;316:363–369.
- ,,,,.Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits.BMJ.2005;331:1169–1175.
- ,,,,,.Delirium in hospitalized older persons: outcomes and predictors.J Am Geriatr Soc.1994;42:809–815.
- ,,,,.A 5‐year prospective assessment of the risk associated with individual benzodiazdepines and doses in new elderly users.J Am Geriatr Soc.2005;53:233–241.
- ,,, et al.Newer hypnotic drugs for the short‐term management of insomnia: a systematic review and economic evaluation.Health Technol Assess.2004;19:305–322.
- .Medications and their effect on sleep.Prim Care Clin Off Pract.2005;32:401–509.
- ,,,,.Long‐term efficacy and safety of zolpidem extended‐release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6‐month, randomized, double‐blind, placebo‐controlled, parallel‐group, multicenter study.Sleep.2008;31(1):79–90.
- ,,,.An efficacy, safety, and dose‐response study of Ramelteon in patients with chronic primary insomnia.Sleep Med.2006;7(1):17–24.
- ,.Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists.Sleep Med.2004;5(6):523–532.
In Part 1, we reviewed normal sleep architecture, and discussed the numerous factors that often disrupt the sleep of hospitalized medical patients. Effective management of sleep complaints among acutely ill patients includes a thorough assessment of medical and psychiatric conditions, medications and other psychosocial factors that may be directly or indirectly impairing sleep. In Part 2, we review and introduce an algorithm for assessing and managing sleep complaints in acutely ill hospitalized patients.
ASSESSMENT AND EVALUATION OF SLEEP COMPLAINTS
Assessment and evaluation of a sleep complaint begins with (Figure 1) an initial review of the medical record for documentation of the signs and symptoms of an underlying primary sleep disorder, which may be exacerbated during an acute medical illness. Common sleep disorders that are often overlooked include obstructive sleep apnea (OSA), restless leg syndrome (RLS), and periodic limb movement disorder (PLMD). Predisposing factors, characteristic clinical features, and differential diagnoses of these disorders are described in Table 1.

| Sleep Disorder | Predisposing Factors | Clinical Features | Differential Diagnosis |
|---|---|---|---|
| |||
| Obstructive sleep apnea (OSA) | Nasopharyngeal abnormalities, craniofacial abnormalities, obesity, >40 years old, men > women (2:1), neurologic disorder (eg, recent stroke) | Repetitive episodes of upper airway obstruction that occur during sleep, usually associated with oxygen desaturation. Episodes include loud snoring or gasps lasting 2030 seconds. Associated with morning headaches and dry mouth. | Sleep‐related laryngospasm, nocturnal gastroesophageal reflux, narcolepsy, hypersomnia, PLMD, central alveolar hypoventilation, paroxysmal nocturnal dyspnea, primary snoring, Cheyne‐Stokes ventilation, nocturnal asthma |
| Periodic limb movement disorder (PLMD) | OSA. RLS, or narcolepsy; aging; chronic uremia; TCAs or MAOIs; withdrawal from antiepileptic agents, or other sedating agents | Periodic episodes of repetitive and stereotyped limb movements: extension of the big toe with partial flexion of the ankles, knees, or hips. Muscle contractions last 0.5 to 5 seconds, with 20‐second to 40‐second intervals between them. | Sleep starts (occur just prior to, not during, sleep, and do not have a regular periodicity like PLMD), nocturnal epileptic seizures, myoclonic epilepsy |
| Restless leg syndrome (RLS) | Pregnancy (>20 weeks gestation), uremia, anemia, rheumatoid arthritis, peak onset is middle age | Uncomfortable leg sensations that occur prior to sleep onset that leads to an irresistible urge to move the legs. Described as achy, crawling, pulling, prickling, or tingling, and disrupts sleep onset. | Chronic myelopathy, peripheral neuropathy, akathisia, fasciculation syndromes, anemia |
| Sleep starts | Can worsen with anxiety, caffeine or other stimulants, daytime physical exertion | Sudden, brief contraction of the legs that occurs at sleep onset. Usually benign, but may worsen during hospitalization, and interfere with sleep. | PLMD, RLS, hyperekplexia syndrome, in which generalized myoclonus is readily elicited by stimuli |
Obtain a focused history by using questions listed in Table 2 to characterize the onset, duration, frequency, and specific characteristics of the patient's current sleep patterns. Next, establish whether the onset of the patient's sleep complaint began with the time of hospitalization. Subsequent questions can then focus on factors that may be impairing sleep such as the hospital environment and sleep hygiene behaviors by comparing the patient's home sleep habits with those during hospitalization. Inquire about the use or abuse of substances such as sedatives, antidepressants, sedatives, antiepileptic drugs (AEDs), and opioids. Ask questions about the presence of pain syndromes and other comorbidities that often impact sleep.
| Focus | Examples of Questions |
|---|---|
| |
| Sleep pattern | Do you have problems falling asleep or staying asleep? How often do you wake up during the night? How long does it take you to fall back asleep? When did the problem start? What can we do to help you sleep? What time do you try to go to sleep, and what time do you wake up? |
| Behavioral factors | Compare your bedtime routine at home, and in the hospital. |
| Environment | Does the lighting or noise level in the hospital disrupt your sleep? How so? Are you awoken from sleep for laboratory work, monitoring, bathing, or other nursing/medical procedures? |
| Patient comfort | Is your pain adequately controlled at night? If not, are you on a scheduled analgesic regimen, or do you have to ask for pain medications? Do you have breathing problems, gastroesophageal reflux, or other type of discomfort that keeps you from sleeping well? |
| Substances | Do you drink alcohol? How much, and how often? When was your last alcoholic beverage? Inquire about cocaine, methamphetamine, marijuana, and medically‐unsupervised use of opioids. |
| Psychosocial | How was your mood just prior to being hospitalized? How has your mood been since you were admitted? Have you experienced any emotionally or physically traumatic event prior to, or during, this hospitalization that continues to bother you (eg, intubation, resuscitation, surgery, blood draws, MRI scanning)? |
MANAGEMENT OF SLEEP COMPLAINTS
Management of sleep disturbance is multifactorial and consists of nonpharmacologic as well as pharmacologic therapies. A stepwise approach is suggested and begins with nonpharmacologic strategies.
Nonpharmacologic Interventions
Before using sedative/hypnotic agents, address sleep hygiene and other factors that disrupt sleep during a hospitalization such as those listed in Table 3.
| Barriers to Sleep | Strategies To Optimize Sleep in the Hospital |
|---|---|
| |
| Noise | Limit the volume level of television sets, and do not allow patients or visitors to increase the volume. |
| Promptly respond to alarm monitors, and consider liberalizing the monitor alarm setting, if appropriate. | |
| Keep patients' doors closed, if possible. | |
| Post signs to remind staff and visitors to minimize conversations at or near the bedside. | |
| Adhere strictly to visiting hours. | |
| Encourage staff to switch their beepers and other electronic devices to vibrate at night. | |
| Limit the number of visitors at a time and/or if appropriate, have the patient meet with visitors in another location (eg, conference room, cafeteria). | |
| Offer earplugs. | |
| Ask patients to turn their phone ringers off when visiting hours are over. | |
| Anxiety | Encourage visitors to minimize discussing emotionally difficult topics with patients near bedtime. |
| Lighting | Offer eye masks. |
| Encourage exposure to brighter light during the day (turn on the lights, open the curtains), and turn off the lights by 9 PM. | |
| Poor sleep hygiene | Encourage regular nocturnal sleep time, and discourage lengthy naps during the day. |
| Medications and substances | Minimize BzRAs for sleep. Try to wean patients off BzRAs prior to discharge. At discharge, provide the minimum number of pills until they are scheduled to see their primary care clinician posthospitalization, and do not provide refills. |
|
Avoid starting multiple medications at one time. Minimize use of sleep‐disrupting medications (see Part 1, Table 3). |
|
| Change medication regimens to promote sleep; eg, avoid night‐time diuretics if possible. | |
| No caffeine or cigarette smoking after 6 PM. | |
| Effects of treatments | Minimize bathing, dressing changes, room switches, and other activities at night. |
| Regularly review nighttime orders to see if you could decrease the frequency of overnight monitoring (eg, fingersticks, labdraws, checking vitals). | |
| Delirium | Provide an updated calendar to facilitate cognitive orientation. |
| Discontinue nonessential medications. Minimize use of BzRAs, barbiturates, opiates, antihistamines, and anticholinergic agents. | |
| Regularly provide verbal and other cues to orient patients to the date, time, location, and circumstances. | |
| Nocturnal discomfort | Optimize nighttime glycemic control, and maximize pain management. |
| For patients with reflux: No oral intake after 8 PM, and keep head of bed elevated 30 degrees. | |
| Provide nocturnal O2, CPAP, and/or other medications, as appropriate. If patient is on CPAP, assess the mask's fit and comfort. | |
Pharmacologic (Sedative/Hypnotic) Interventions
Pharmacologic therapy may be necessary to treat disordered sleep. The ideal sleep aid would reduce sleep latency or time to fall asleep, increase total sleep time (TST), not cause next‐day sedation, improve daytime functioning, and minimize the development of tolerance. Unfortunately, no single agent meets all these independent criteria. In the past 10 years, newer benzodiazepines (BzRAs) with shorter half‐lives have been shown to be efficacious in reducing sleep latency, but the problem of sleep maintenance without next‐day sedation persists.1 To choose an appropriate sleep agent, evaluate the drug's efficacy, mechanism of action, and side‐effect profile. Then, match these characteristics with the patient's clinical condition(s). In patients with comorbid sleep and psychiatric problems, consider using a sedating psychotropic at bedtime to promote sleep.
Non‐Food and Drug AdministrationApproved (Off‐Label) Sleep Aids: Psychotropic Medications
Limited data exist on the efficacy of non‐Food and Drug Administration (FDA)approved medications for insomnia,2 such as antidepressants and atypical antipsychotics (AAPs), and antihistamines; examples of which are listed in Table 4. The administration of antihistamines, barbiturates, chloral hydrate, and alternative/herbal therapies has been discouraged, because the benefits rarely outweigh the risks associated with their use. Currently, trazodone is the most commonly prescribed antidepressant for the treatment of insomnia, despite the relative lack of data regarding its use for insomnia.3 Prescription data suggest that trazodoneat hypnotic doses, which are lower than the full antidepressant doseis more commonly prescribed for insomnia rather than for its FDA‐approved use for depression.4 In general, sleep specialists refrain from recommending sedating antidepressants for primary insomnia due to insufficient data regarding efficacy and safety. In addition, trazodone has been associated with arrhythmias in patients with preexisting cardiac conduction system disease. Curry et al.3 speculated that trazodone is popular among prescribers because, unlike most BzRAs, trazodone does not have a recommended limited duration of use and is perceived as being safer than BzRAs. Walsh et al.5 conducted a randomized double‐blind, placebo‐controlled trial (n = 589) that compared the hypnotic efficacy and other sleep‐associated variables of trazodone (50 mg) and zolpidem (10 mg). During the first week of treatment, the subjects on trazodone or zolpidem decreased their time to fall asleep, or sleep latency, by 22% and 35%, respectively, compared to placebo. Sleep latency was significantly shorter on zolpidem (57.75 2.7 minutes) than for trazodone (57.7 + 4.0 minutes). By the second week, subjects on zolpidem continued to have a reduction in the time to fall asleep, but there was no significant difference between subjects on trazodone and placebo.5 Trazodone may be an acceptable short‐term alternative to BzRAs for patients with hypercapnia or hypoxemia, and in those with a history of drug abuse or dependence. At doses of 150 to 450 mg, trazodone may be an appropriate medication in patients with major depressive disorder and problems with sleep maintenance.6 Tolerance to trazodone's sedating property tends to develop after 2 weeks of treatment, however, so other treatments may need to be considered if sleep problems persist. The available data address relatively short‐term use of trazodone, so questions of safety and efficacy for chronic insomnia remain unanswered.
| Drug | Pertinent Side Effects | Comments |
|---|---|---|
| ||
| Antidepressants | ||
| Mirtazapine (Remeron) | Somnolence, appetite, weight, dry mouth | May be beneficial for comorbid depression and insomnia. Lower doses (15 mg) increase sedation. |
| Trazodone | Residual daytime sedation, headache, orthostatic hypotension, priapism, cardiac arrhythmias | May be beneficial for comorbid depression and insomnia. Not recommended as first‐line agent for insomnia.3 May be an alternative if BzRAs are contraindicated (severe hypercapnia or hypoxemia or history of substance abuse). Tolerance usually develops within 2 weeks. Lower doses (50100 mg) than when used for depression (400 mg). |
| TCAs | Delirium, cognition, seizure threshold, orthostatic hypotension, tachycardia, acquired prolonged QT syndrome, heart block, acute hepatitis | Avoid in hospitalized patients due to their anticholinergic, antihistaminic, and cardiovascular side effects. May be beneficial for comorbid depression and insomnia. |
| Antihistamines | ||
| Diphenhydramine (Benadryl) | Residual daytime sedation, delirium, orthostatic hypotension, psychomotor function, prolonged QT syndrome, blurred vision, urinary retention | Better than placebo to treat insomnia,12 but data is lacking to definitively endorse diphenhydramine for insomnia.13 Tolerance to antihistamines develops within a few days. Avoid in patients >60 years old.18 |
| Hydroxyzine | Drowsiness, dry mouth, dizziness, agitation, cognitive function | Efficacy as anxiolytic for >4 months use not established. Not FDA‐approved for insomnia. Avoid in patients >60 years old, closed‐angle glaucoma, prostatic hypertrophy, severe asthma, and COPD. |
| Antipsychotics | ||
| Quetiapine (Seroquel) | Sedation, orthostatic hypotension, hyperglycemia, appetite, weight, hyperlipidemia | The most sedating of the atypical antipsychotics, it is frequently used as a sleep aid. Not recommended for insomnia or other sleep problems unless there is a comorbid psychiatric disorder. Dosed lower (25100 mg) when used for insomnia versus for FDA‐approved indications (600 mg). |
| Olanzapine (Zyprexa) | Sedation, hyperglycemia, appetite, weight, hyperlipidemia | Of atypical antipsychotics, olanzapine is the most likely to cause metabolic complications. Should not be used solely for insomnia. |
| Barbiturate | ||
| Chloral hydrate | Oversedation, respiratory depression, nausea, vomiting, diarrhea, drowsiness, cognitive function, psychotic symptoms (paranoia, hallucinations), vertigo, dizziness, headache | Chloral hydrate has been used for the short‐term (2 weeks) treatment of insomnia, but is currently not FDA‐approved for that indication. Additive CNS depression may occur if given with other sedative‐hypnotics. Caution in patients with severe cardiac disease. Contraindicated in marked hepatic or renal impairment. Highly lethal in overdose, and should be avoided in patients with risk of suicide. |
Mirtazapine (Remeron), which promotes both sleep and appetite, may be particularly helpful for patients with cancer, acquired immunodeficiency syndrome (AIDS), and other conditions in which the triad of poor sleep, anorexia, and depression are common. Mirtazapine is a noradrenergic and specific serotonergic agent that causes inverse, dose‐dependent sedation (doses 15 mg are less sedating).7 To target sleeplessness, start with a dose between 7.5 and 15 mg. If ineffective at this dose, it is unlikely that increasing the dose will be of benefit for sleep. A small randomized, double‐blind, placebo‐controlled trial found that low‐dose mirtazapine reduced the apnea‐hypopnea index (API) by half in newly‐diagnosed subjects with OSA (n = 12).8 The results were promising in terms of the use of mixed‐profile serotonergic drugs in treating OSA. However, as pointed out by the researchers, mirtazapine's tendency to cause weight gain, is problematic in this patient population.
Although sedating, tricyclic antidepressants (TCAs) should not be used to promote sleep in hospitalized patients. TCAs increase the risk of cardiac conduction abnormalities, decrease seizure threshold, and have significant anticholinergic and anti‐alpha‐adrenergic effects. In dementia patients, the anticholinergic effect of TCAs may precipitate delirium.
AAPs should not be used routinely as first‐line agents for insomnia, except in patients who are in the midst of acute manic or psychotic episodes.9 With chronic use of AAPs, the risks of hyperglycemia, hyperlipidemia, and weight gain outweigh the potential sleep benefits of these agents. AAPs, especially risperidone, may cause extrapyramidal syndrome (EPS). Risperidone, ziprasidone and quetiapine have been associated with prolonged QTc interval, but the relatively low doses of AAPs that are used purely for sedative purposes makes this risk relatively low. If a patient has a history of Parkinsonism or other EPS, risperidone should generally be avoided. If a patient treated with risperidone develops EPS, another AAP should be considered. A reasonable precaution is to obtain a pretreatment 12‐lead electrocardiogram. If the QTc is greater than 450 msec, consider using olanzapine rather than ziprasidone, risperidone, or quetiapine. Sedating AAPs include risperidone (Risperdal), olanzapine (Zyprexa), and quetiapine (Seroquel), with the latter 2 being especially sedating. Quetiapine may also cause orthostatic hypotension. The recent practice of using AAPs for delirium has not been reported to be associated with significant safety risks, probably because delirium treatment is typically of short duration under a period of close clinical observation. These agents should not be used indefinitely for insomnia without close monitoring of metabolic, psychiatric, and neurologic status. However, recent data suggest that the risk of serious adverse effects of AAPs may outweigh the potential benefits for the treatment of aggression or agitation in patients with Alzheimer's disease.10
A meta‐analysis of randomized placebo‐controlled trials of AAP use among dementia patients showed that overall, the use of AAP drugs for periods of less than 8 to 12 weeks was associated with a small increased risk for death compared with placebo.11 Data indicated that most patients' behaviors improved substantially during the first 1 to 4 weeks of treatment. In a double‐blind, placebo‐controlled trial, 421 patients with Alzheimer's disease and psychosis, aggression or agitation were randomly assigned to receive olanzapine (mean dose, 5.5 mg per day), quetiapine (mean dose, 56.5 mg per day), risperidone (mean dose, 1.0 mg per day), or placebo. Improvement was observed in 32% of patients assigned to olanzapine, 26% of patients assigned to quetiapine, 29% of patients assigned to risperidone, and 21% of patients assigned to placebo. A lower, but significant, proportion of the patients (24%, 16%, 18%, and 5%, respectively) discontinued these medications due to intolerable side effects. Thus, if minimal improvement is observed even after 8 weeks of treatment, prescribers should consider discontinuing the AAP. The management of agitation in dementia, particularly in the elderly, calls for an integrative and creative psychopharmacological approach, including the use of antidepressants, nonbenzodiazepine anxiolytics such as buspirone, and mood stabilizers such as divalproex sodium (Depakote) before exposing patients to the risks of AAPs.
Antihistamines are the most commonly used over‐the‐counter agents for chronic insomnia.1 Diphenhydramine (Benadryl) has been shown to be better than placebo to treat insomnia,12 but data is lacking to definitively endorse its use to promote sleep.13 Diphenhydramine is also limited by the development of tolerance within a few days of daily use. The anticholinergic action of antihistamines may lead to orthostatic hypotension, urinary retention, and may induce delirium in vulnerable patients. Therefore, diphenhydramine should be avoided in hospitalized patients.
Recent data suggest that hydroxyzine, an antihistamine, may be an appropriate sleep aid for patients with hepatic encephalopathy in whom BzRAs are contraindicated.14 Subjective improvement in sleep was observed in 40% of hydroxyzine‐treated patients with hepatic encephalopathy compared to placebo.
Chloral hydrate is one of the Western world's oldest known sedative‐hypnotics and was commonly used as a sleep aid through the 1970s.15 Chloral hydrate was eventually supplanted by BzRAs,16 and fell out of favor as a sleep aid due to its relatively high tolerance rate, drug‐drug interaction profile, and the high risk of death in an overdose. Doses of 500 to 1000 mg sufficed to promote sleep in most of the hospitalized subjects. More recent data regarding its use for treating insomnia are not available, but chloral hydrate may be an alternative short‐term treatment for insomnia in selected hospitalized patients. Because of its high‐risk profile, chloral hydrate would be used as a last‐resort medication, preferably with input from critical care and/or sleep medicine specialists.
FDA‐Approved Sleep Aids
As shown in Table 5, the FDA has approved 3 classes of medications for the treatment of insomnia: benzodiazepine gamma‐aminobutyric acid (GABA)A receptor agonists (BzRAs), nonbenzodiazepine GABAA receptor agonists (non‐BzRAs), and melatonin‐receptor agonists.17 BzRAs include estazolam (ProSom), flurazepam (Dalmane), quazepam (Doral), temazepam (Restoril), and triazolam (Halcion). Though BzRAs decrease sleep latency, increase TST, and decrease slow wave or deep sleep, they also have adverse side effects such as daytime sedation, anterograde amnesia, cognitive impairment, motor incoordination, dependence, tolerance, and rebound insomnia.18 Because of these side effects, BzRAs should be limited to generally healthy, young (ie, 45 years old) patients who are expected to have brief hospital stays.
| Drugs | Adult Dose (mg) | Half‐Life (hours)* | Onset (minutes) | Peak Effect (hours) | Major Effects/Clinical Comments |
|---|---|---|---|---|---|
| |||||
| BzRAs | Caution in elderly patients. Tolerance to BzRAs develop to the sedative, hypnotic, and anticonvulsant effects. | ||||
| Estazolam (ProSom) | 12 | 1024 | 60 | 0.51.5 | Short‐term (710 days) treatment for frequent arousals, early morning awakening. Not as useful for sleep onset. Avoid in patients with OSA. Caution in elderly patients, liver disease. High doses can cause respiratory depression. |
| Flurazepam (Dalmane) | 1530 | 47100 | 1520 | 36 | In general, avoid in hospitalized medical patients, especially elderly patients. |
| Quazepam (Doral) | 7.515 | 25114 | 1.5 | In general, avoid in hospitalized medical patients, especially elderly patients. | |
| Temazepam (Restoril) | 1530 | 616 | 23 | Short‐term (710 days) treatment for sleep onset and maintenance. Doses 30 mg/day: morning grogginess, nausea, headache, and vivid dreaming. | |
| Triazolam (Halcion) | 0.1250.25 | 1.55.5 | 1530 | 1.75 | Maximum dose is 0.5 mg. Short‐term (710 days) treatment. Rapid onset; should be in bed when taking medication. Contraindicated with atazanavir, ketoconazole, itraconazole, nefazodone, ritonavir. |
| Non‐BzRAs | |||||
| Eszopiclone (Lunesta) | 23 | 69 | 1 | In elderly: difficulty falling asleep, then initial: 1 mg; maximum 2 mg. Difficulty staying asleep: 2 mg. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with high‐fat foods. No tolerance after 6 months. | |
| Zaleplon (Sonata) | 520 | 1 | Rapid | 1 | Short‐term (710 days) treatment for falling asleep and/or next‐day wakefulness is crucial (eg, shift workers). |
| Zopiclone (Imovane) | 515 | 3.86.5 (510 in elderly) | 30 | 2 | Transient and short‐term (710 days) treatment. Contraindicated in severe respiratory impairment. Caution in liver disease and depression; elderly prone to side effects. Anticholinergic agents may plasma level. |
| Zolpidem (Ambien) | 520 | 1.44.5 | 30 | 2 | Short‐term (710 days) treatment for sleep onset and maintenance. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with food. No tolerance after 50 weeks. |
| Melatonin agonist | |||||
| Ramelton (Rozerem) | 8 | 12 | 30 | 11.5 | For sleep onset. For faster sleep onset, do not ingest with high‐fat foods. No tolerance. Contraindicated with fluvoxamine. |
Efficacy and safety studies have generally been limited to healthy, younger individuals without a history of primary sleep disorder. Potential adverse effects of BzRAs may become even more pronounced in hospitalized medical patients due to older age, acute illness, cointeraction drugs, and multidrug regimens. Although BzRAs are FDA‐approved for the treatment of insomnia, flurazepam and quazepam should generally be avoided in hospitalized patients. These agents' long half‐lives increase the risk of drug‐drug interactions and adverse events such as respiratory depression, cognitive decline, and delirium in acutely ill patients. For similar reasons, other long‐acting BzRAs such as clonazepam (Klonopin) and diazepam (Valium) should also not be used to treat insomnia in hospitalized patients. An exception to this is a patient with RLS, in which clonazepam is an approved treatment. However, now that ropinirole HCl (Requip) is FDA‐approved for RLS, BzARs may be able to be avoided. Lorazepam (Ativan), due to its relatively short half‐life and its anxiolytic property, is frequently used to treat insomnia in hospitalized medical patients.18 Start with the lowest dose possible (eg, 0.5 mg) as a one‐time‐only order, or on a as needed basis for 3 days. Alprazolam (Xanax), a potent, fast‐acting BzRA with a relatively short half‐life, has developed a reputation as being notoriously addictive, and experts feel alprazolam has similar potential for withdrawal and rebound.19, 20
The use of BzRAs should be minimized in all patients, and avoided in the elderly or those with a particularly high risk for delirium (eg, traumatic brain injury, stroke, multiple new medications). All BzRAs should be avoided in patients with a prior history of sedative‐hypnotic and/or alcohol dependence unless medically indicated, such as in alcohol withdrawal. Refrain from ordering nightly scheduled BzRAs without a specific time limit to ensure that sedative‐hypnotic use is closely monitored.
For the past 2 decades, physicians have been advised against using long‐acting BzRAs in the elderly (>65 years old) due to the increased risks of hip fractures, falls, motor vehicle accidents, daytime sedation, and adverse cognitive events such as delirium.2124 A large 5‐year prospective study in Quebec found that the risk of injury varied by the BzRA, and was independent of half‐life.25 Importantly, the risk of injury was dose‐dependent: the higher the dose of oxazepam, flurazepam, or chlordiazepoxide, the higher the risk of injury in the elderly.
Non‐BzRAs seem to have a superior side‐effect profile when compared to BzRAs, but should also be used with caution in the elderly. Non‐BzRAs include eszopiclone (Lunesta), zaleplon (Sonata), zolpidem (Ambien), and zolpidem extended‐release. The number of comparison studies is limited, but the available data reveal that: (1) zolpidem (Ambien) may be better than temazepam (Restoril) in terms of sleep latency and quality; and (2) zaleplon (Sonata) may lead to a shorter sleep latency than zolpidem (Ambien), but the latter is associated with longer sleep duration.26 Non‐BzRAs have less next‐day sedation, psychomotor dysfunction, tolerance/withdrawal, and rapid‐eye‐movement (REM) sleep rebound; and lower abuse potential than BzRAs.27
The most commonly prescribed hypnotic, zolpidem has a short half‐life, and seems to reduce sleep latency with minimal residual side effects when compared to BzRAs. The results of a recent multicenter, randomized, double‐blind, placebo‐controlled trial indicated that zolpidem extended‐release may be efficacious for up to 6 months in outpatients with chronic insomnia.28
The sole melatonin‐receptor agonist, ramelteon (Rozerem), also reduces time to fall asleep without next‐day psychomotor and memory effects.29 Ramelteon is believed to target receptors melatonin 1 and 2 receptors located in the brain's suprachiasmatic nucleus to stabilize circadian rhythms and stabilize the sleep‐wake cycle.30
CONCLUSION
Hospitalization is often associated with disrupted sleep, which can affect recovery from illness. Understanding the major factors that impair sleep during hospitalization allows clinicians to systemically evaluate and treat sleep problems. More than just prescribing a sedative/hypnotic, the treatment for sleep disruption includes addressing sleep hygiene and hospital environment issues, identifying medications that could disrupt sleep, and treating specific syndromes that impair sleep. We suggest a practical algorithm to guide clinical assessment, treatment options, and selection of appropriate sleeping medications. Critical to optimizing recovery from illness, sleep may be considered as the sixth vital sign, and should be part of the routine evaluation of every hospitalized patient.
In Part 1, we reviewed normal sleep architecture, and discussed the numerous factors that often disrupt the sleep of hospitalized medical patients. Effective management of sleep complaints among acutely ill patients includes a thorough assessment of medical and psychiatric conditions, medications and other psychosocial factors that may be directly or indirectly impairing sleep. In Part 2, we review and introduce an algorithm for assessing and managing sleep complaints in acutely ill hospitalized patients.
ASSESSMENT AND EVALUATION OF SLEEP COMPLAINTS
Assessment and evaluation of a sleep complaint begins with (Figure 1) an initial review of the medical record for documentation of the signs and symptoms of an underlying primary sleep disorder, which may be exacerbated during an acute medical illness. Common sleep disorders that are often overlooked include obstructive sleep apnea (OSA), restless leg syndrome (RLS), and periodic limb movement disorder (PLMD). Predisposing factors, characteristic clinical features, and differential diagnoses of these disorders are described in Table 1.

| Sleep Disorder | Predisposing Factors | Clinical Features | Differential Diagnosis |
|---|---|---|---|
| |||
| Obstructive sleep apnea (OSA) | Nasopharyngeal abnormalities, craniofacial abnormalities, obesity, >40 years old, men > women (2:1), neurologic disorder (eg, recent stroke) | Repetitive episodes of upper airway obstruction that occur during sleep, usually associated with oxygen desaturation. Episodes include loud snoring or gasps lasting 2030 seconds. Associated with morning headaches and dry mouth. | Sleep‐related laryngospasm, nocturnal gastroesophageal reflux, narcolepsy, hypersomnia, PLMD, central alveolar hypoventilation, paroxysmal nocturnal dyspnea, primary snoring, Cheyne‐Stokes ventilation, nocturnal asthma |
| Periodic limb movement disorder (PLMD) | OSA. RLS, or narcolepsy; aging; chronic uremia; TCAs or MAOIs; withdrawal from antiepileptic agents, or other sedating agents | Periodic episodes of repetitive and stereotyped limb movements: extension of the big toe with partial flexion of the ankles, knees, or hips. Muscle contractions last 0.5 to 5 seconds, with 20‐second to 40‐second intervals between them. | Sleep starts (occur just prior to, not during, sleep, and do not have a regular periodicity like PLMD), nocturnal epileptic seizures, myoclonic epilepsy |
| Restless leg syndrome (RLS) | Pregnancy (>20 weeks gestation), uremia, anemia, rheumatoid arthritis, peak onset is middle age | Uncomfortable leg sensations that occur prior to sleep onset that leads to an irresistible urge to move the legs. Described as achy, crawling, pulling, prickling, or tingling, and disrupts sleep onset. | Chronic myelopathy, peripheral neuropathy, akathisia, fasciculation syndromes, anemia |
| Sleep starts | Can worsen with anxiety, caffeine or other stimulants, daytime physical exertion | Sudden, brief contraction of the legs that occurs at sleep onset. Usually benign, but may worsen during hospitalization, and interfere with sleep. | PLMD, RLS, hyperekplexia syndrome, in which generalized myoclonus is readily elicited by stimuli |
Obtain a focused history by using questions listed in Table 2 to characterize the onset, duration, frequency, and specific characteristics of the patient's current sleep patterns. Next, establish whether the onset of the patient's sleep complaint began with the time of hospitalization. Subsequent questions can then focus on factors that may be impairing sleep such as the hospital environment and sleep hygiene behaviors by comparing the patient's home sleep habits with those during hospitalization. Inquire about the use or abuse of substances such as sedatives, antidepressants, sedatives, antiepileptic drugs (AEDs), and opioids. Ask questions about the presence of pain syndromes and other comorbidities that often impact sleep.
| Focus | Examples of Questions |
|---|---|
| |
| Sleep pattern | Do you have problems falling asleep or staying asleep? How often do you wake up during the night? How long does it take you to fall back asleep? When did the problem start? What can we do to help you sleep? What time do you try to go to sleep, and what time do you wake up? |
| Behavioral factors | Compare your bedtime routine at home, and in the hospital. |
| Environment | Does the lighting or noise level in the hospital disrupt your sleep? How so? Are you awoken from sleep for laboratory work, monitoring, bathing, or other nursing/medical procedures? |
| Patient comfort | Is your pain adequately controlled at night? If not, are you on a scheduled analgesic regimen, or do you have to ask for pain medications? Do you have breathing problems, gastroesophageal reflux, or other type of discomfort that keeps you from sleeping well? |
| Substances | Do you drink alcohol? How much, and how often? When was your last alcoholic beverage? Inquire about cocaine, methamphetamine, marijuana, and medically‐unsupervised use of opioids. |
| Psychosocial | How was your mood just prior to being hospitalized? How has your mood been since you were admitted? Have you experienced any emotionally or physically traumatic event prior to, or during, this hospitalization that continues to bother you (eg, intubation, resuscitation, surgery, blood draws, MRI scanning)? |
MANAGEMENT OF SLEEP COMPLAINTS
Management of sleep disturbance is multifactorial and consists of nonpharmacologic as well as pharmacologic therapies. A stepwise approach is suggested and begins with nonpharmacologic strategies.
Nonpharmacologic Interventions
Before using sedative/hypnotic agents, address sleep hygiene and other factors that disrupt sleep during a hospitalization such as those listed in Table 3.
| Barriers to Sleep | Strategies To Optimize Sleep in the Hospital |
|---|---|
| |
| Noise | Limit the volume level of television sets, and do not allow patients or visitors to increase the volume. |
| Promptly respond to alarm monitors, and consider liberalizing the monitor alarm setting, if appropriate. | |
| Keep patients' doors closed, if possible. | |
| Post signs to remind staff and visitors to minimize conversations at or near the bedside. | |
| Adhere strictly to visiting hours. | |
| Encourage staff to switch their beepers and other electronic devices to vibrate at night. | |
| Limit the number of visitors at a time and/or if appropriate, have the patient meet with visitors in another location (eg, conference room, cafeteria). | |
| Offer earplugs. | |
| Ask patients to turn their phone ringers off when visiting hours are over. | |
| Anxiety | Encourage visitors to minimize discussing emotionally difficult topics with patients near bedtime. |
| Lighting | Offer eye masks. |
| Encourage exposure to brighter light during the day (turn on the lights, open the curtains), and turn off the lights by 9 PM. | |
| Poor sleep hygiene | Encourage regular nocturnal sleep time, and discourage lengthy naps during the day. |
| Medications and substances | Minimize BzRAs for sleep. Try to wean patients off BzRAs prior to discharge. At discharge, provide the minimum number of pills until they are scheduled to see their primary care clinician posthospitalization, and do not provide refills. |
|
Avoid starting multiple medications at one time. Minimize use of sleep‐disrupting medications (see Part 1, Table 3). |
|
| Change medication regimens to promote sleep; eg, avoid night‐time diuretics if possible. | |
| No caffeine or cigarette smoking after 6 PM. | |
| Effects of treatments | Minimize bathing, dressing changes, room switches, and other activities at night. |
| Regularly review nighttime orders to see if you could decrease the frequency of overnight monitoring (eg, fingersticks, labdraws, checking vitals). | |
| Delirium | Provide an updated calendar to facilitate cognitive orientation. |
| Discontinue nonessential medications. Minimize use of BzRAs, barbiturates, opiates, antihistamines, and anticholinergic agents. | |
| Regularly provide verbal and other cues to orient patients to the date, time, location, and circumstances. | |
| Nocturnal discomfort | Optimize nighttime glycemic control, and maximize pain management. |
| For patients with reflux: No oral intake after 8 PM, and keep head of bed elevated 30 degrees. | |
| Provide nocturnal O2, CPAP, and/or other medications, as appropriate. If patient is on CPAP, assess the mask's fit and comfort. | |
Pharmacologic (Sedative/Hypnotic) Interventions
Pharmacologic therapy may be necessary to treat disordered sleep. The ideal sleep aid would reduce sleep latency or time to fall asleep, increase total sleep time (TST), not cause next‐day sedation, improve daytime functioning, and minimize the development of tolerance. Unfortunately, no single agent meets all these independent criteria. In the past 10 years, newer benzodiazepines (BzRAs) with shorter half‐lives have been shown to be efficacious in reducing sleep latency, but the problem of sleep maintenance without next‐day sedation persists.1 To choose an appropriate sleep agent, evaluate the drug's efficacy, mechanism of action, and side‐effect profile. Then, match these characteristics with the patient's clinical condition(s). In patients with comorbid sleep and psychiatric problems, consider using a sedating psychotropic at bedtime to promote sleep.
Non‐Food and Drug AdministrationApproved (Off‐Label) Sleep Aids: Psychotropic Medications
Limited data exist on the efficacy of non‐Food and Drug Administration (FDA)approved medications for insomnia,2 such as antidepressants and atypical antipsychotics (AAPs), and antihistamines; examples of which are listed in Table 4. The administration of antihistamines, barbiturates, chloral hydrate, and alternative/herbal therapies has been discouraged, because the benefits rarely outweigh the risks associated with their use. Currently, trazodone is the most commonly prescribed antidepressant for the treatment of insomnia, despite the relative lack of data regarding its use for insomnia.3 Prescription data suggest that trazodoneat hypnotic doses, which are lower than the full antidepressant doseis more commonly prescribed for insomnia rather than for its FDA‐approved use for depression.4 In general, sleep specialists refrain from recommending sedating antidepressants for primary insomnia due to insufficient data regarding efficacy and safety. In addition, trazodone has been associated with arrhythmias in patients with preexisting cardiac conduction system disease. Curry et al.3 speculated that trazodone is popular among prescribers because, unlike most BzRAs, trazodone does not have a recommended limited duration of use and is perceived as being safer than BzRAs. Walsh et al.5 conducted a randomized double‐blind, placebo‐controlled trial (n = 589) that compared the hypnotic efficacy and other sleep‐associated variables of trazodone (50 mg) and zolpidem (10 mg). During the first week of treatment, the subjects on trazodone or zolpidem decreased their time to fall asleep, or sleep latency, by 22% and 35%, respectively, compared to placebo. Sleep latency was significantly shorter on zolpidem (57.75 2.7 minutes) than for trazodone (57.7 + 4.0 minutes). By the second week, subjects on zolpidem continued to have a reduction in the time to fall asleep, but there was no significant difference between subjects on trazodone and placebo.5 Trazodone may be an acceptable short‐term alternative to BzRAs for patients with hypercapnia or hypoxemia, and in those with a history of drug abuse or dependence. At doses of 150 to 450 mg, trazodone may be an appropriate medication in patients with major depressive disorder and problems with sleep maintenance.6 Tolerance to trazodone's sedating property tends to develop after 2 weeks of treatment, however, so other treatments may need to be considered if sleep problems persist. The available data address relatively short‐term use of trazodone, so questions of safety and efficacy for chronic insomnia remain unanswered.
| Drug | Pertinent Side Effects | Comments |
|---|---|---|
| ||
| Antidepressants | ||
| Mirtazapine (Remeron) | Somnolence, appetite, weight, dry mouth | May be beneficial for comorbid depression and insomnia. Lower doses (15 mg) increase sedation. |
| Trazodone | Residual daytime sedation, headache, orthostatic hypotension, priapism, cardiac arrhythmias | May be beneficial for comorbid depression and insomnia. Not recommended as first‐line agent for insomnia.3 May be an alternative if BzRAs are contraindicated (severe hypercapnia or hypoxemia or history of substance abuse). Tolerance usually develops within 2 weeks. Lower doses (50100 mg) than when used for depression (400 mg). |
| TCAs | Delirium, cognition, seizure threshold, orthostatic hypotension, tachycardia, acquired prolonged QT syndrome, heart block, acute hepatitis | Avoid in hospitalized patients due to their anticholinergic, antihistaminic, and cardiovascular side effects. May be beneficial for comorbid depression and insomnia. |
| Antihistamines | ||
| Diphenhydramine (Benadryl) | Residual daytime sedation, delirium, orthostatic hypotension, psychomotor function, prolonged QT syndrome, blurred vision, urinary retention | Better than placebo to treat insomnia,12 but data is lacking to definitively endorse diphenhydramine for insomnia.13 Tolerance to antihistamines develops within a few days. Avoid in patients >60 years old.18 |
| Hydroxyzine | Drowsiness, dry mouth, dizziness, agitation, cognitive function | Efficacy as anxiolytic for >4 months use not established. Not FDA‐approved for insomnia. Avoid in patients >60 years old, closed‐angle glaucoma, prostatic hypertrophy, severe asthma, and COPD. |
| Antipsychotics | ||
| Quetiapine (Seroquel) | Sedation, orthostatic hypotension, hyperglycemia, appetite, weight, hyperlipidemia | The most sedating of the atypical antipsychotics, it is frequently used as a sleep aid. Not recommended for insomnia or other sleep problems unless there is a comorbid psychiatric disorder. Dosed lower (25100 mg) when used for insomnia versus for FDA‐approved indications (600 mg). |
| Olanzapine (Zyprexa) | Sedation, hyperglycemia, appetite, weight, hyperlipidemia | Of atypical antipsychotics, olanzapine is the most likely to cause metabolic complications. Should not be used solely for insomnia. |
| Barbiturate | ||
| Chloral hydrate | Oversedation, respiratory depression, nausea, vomiting, diarrhea, drowsiness, cognitive function, psychotic symptoms (paranoia, hallucinations), vertigo, dizziness, headache | Chloral hydrate has been used for the short‐term (2 weeks) treatment of insomnia, but is currently not FDA‐approved for that indication. Additive CNS depression may occur if given with other sedative‐hypnotics. Caution in patients with severe cardiac disease. Contraindicated in marked hepatic or renal impairment. Highly lethal in overdose, and should be avoided in patients with risk of suicide. |
Mirtazapine (Remeron), which promotes both sleep and appetite, may be particularly helpful for patients with cancer, acquired immunodeficiency syndrome (AIDS), and other conditions in which the triad of poor sleep, anorexia, and depression are common. Mirtazapine is a noradrenergic and specific serotonergic agent that causes inverse, dose‐dependent sedation (doses 15 mg are less sedating).7 To target sleeplessness, start with a dose between 7.5 and 15 mg. If ineffective at this dose, it is unlikely that increasing the dose will be of benefit for sleep. A small randomized, double‐blind, placebo‐controlled trial found that low‐dose mirtazapine reduced the apnea‐hypopnea index (API) by half in newly‐diagnosed subjects with OSA (n = 12).8 The results were promising in terms of the use of mixed‐profile serotonergic drugs in treating OSA. However, as pointed out by the researchers, mirtazapine's tendency to cause weight gain, is problematic in this patient population.
Although sedating, tricyclic antidepressants (TCAs) should not be used to promote sleep in hospitalized patients. TCAs increase the risk of cardiac conduction abnormalities, decrease seizure threshold, and have significant anticholinergic and anti‐alpha‐adrenergic effects. In dementia patients, the anticholinergic effect of TCAs may precipitate delirium.
AAPs should not be used routinely as first‐line agents for insomnia, except in patients who are in the midst of acute manic or psychotic episodes.9 With chronic use of AAPs, the risks of hyperglycemia, hyperlipidemia, and weight gain outweigh the potential sleep benefits of these agents. AAPs, especially risperidone, may cause extrapyramidal syndrome (EPS). Risperidone, ziprasidone and quetiapine have been associated with prolonged QTc interval, but the relatively low doses of AAPs that are used purely for sedative purposes makes this risk relatively low. If a patient has a history of Parkinsonism or other EPS, risperidone should generally be avoided. If a patient treated with risperidone develops EPS, another AAP should be considered. A reasonable precaution is to obtain a pretreatment 12‐lead electrocardiogram. If the QTc is greater than 450 msec, consider using olanzapine rather than ziprasidone, risperidone, or quetiapine. Sedating AAPs include risperidone (Risperdal), olanzapine (Zyprexa), and quetiapine (Seroquel), with the latter 2 being especially sedating. Quetiapine may also cause orthostatic hypotension. The recent practice of using AAPs for delirium has not been reported to be associated with significant safety risks, probably because delirium treatment is typically of short duration under a period of close clinical observation. These agents should not be used indefinitely for insomnia without close monitoring of metabolic, psychiatric, and neurologic status. However, recent data suggest that the risk of serious adverse effects of AAPs may outweigh the potential benefits for the treatment of aggression or agitation in patients with Alzheimer's disease.10
A meta‐analysis of randomized placebo‐controlled trials of AAP use among dementia patients showed that overall, the use of AAP drugs for periods of less than 8 to 12 weeks was associated with a small increased risk for death compared with placebo.11 Data indicated that most patients' behaviors improved substantially during the first 1 to 4 weeks of treatment. In a double‐blind, placebo‐controlled trial, 421 patients with Alzheimer's disease and psychosis, aggression or agitation were randomly assigned to receive olanzapine (mean dose, 5.5 mg per day), quetiapine (mean dose, 56.5 mg per day), risperidone (mean dose, 1.0 mg per day), or placebo. Improvement was observed in 32% of patients assigned to olanzapine, 26% of patients assigned to quetiapine, 29% of patients assigned to risperidone, and 21% of patients assigned to placebo. A lower, but significant, proportion of the patients (24%, 16%, 18%, and 5%, respectively) discontinued these medications due to intolerable side effects. Thus, if minimal improvement is observed even after 8 weeks of treatment, prescribers should consider discontinuing the AAP. The management of agitation in dementia, particularly in the elderly, calls for an integrative and creative psychopharmacological approach, including the use of antidepressants, nonbenzodiazepine anxiolytics such as buspirone, and mood stabilizers such as divalproex sodium (Depakote) before exposing patients to the risks of AAPs.
Antihistamines are the most commonly used over‐the‐counter agents for chronic insomnia.1 Diphenhydramine (Benadryl) has been shown to be better than placebo to treat insomnia,12 but data is lacking to definitively endorse its use to promote sleep.13 Diphenhydramine is also limited by the development of tolerance within a few days of daily use. The anticholinergic action of antihistamines may lead to orthostatic hypotension, urinary retention, and may induce delirium in vulnerable patients. Therefore, diphenhydramine should be avoided in hospitalized patients.
Recent data suggest that hydroxyzine, an antihistamine, may be an appropriate sleep aid for patients with hepatic encephalopathy in whom BzRAs are contraindicated.14 Subjective improvement in sleep was observed in 40% of hydroxyzine‐treated patients with hepatic encephalopathy compared to placebo.
Chloral hydrate is one of the Western world's oldest known sedative‐hypnotics and was commonly used as a sleep aid through the 1970s.15 Chloral hydrate was eventually supplanted by BzRAs,16 and fell out of favor as a sleep aid due to its relatively high tolerance rate, drug‐drug interaction profile, and the high risk of death in an overdose. Doses of 500 to 1000 mg sufficed to promote sleep in most of the hospitalized subjects. More recent data regarding its use for treating insomnia are not available, but chloral hydrate may be an alternative short‐term treatment for insomnia in selected hospitalized patients. Because of its high‐risk profile, chloral hydrate would be used as a last‐resort medication, preferably with input from critical care and/or sleep medicine specialists.
FDA‐Approved Sleep Aids
As shown in Table 5, the FDA has approved 3 classes of medications for the treatment of insomnia: benzodiazepine gamma‐aminobutyric acid (GABA)A receptor agonists (BzRAs), nonbenzodiazepine GABAA receptor agonists (non‐BzRAs), and melatonin‐receptor agonists.17 BzRAs include estazolam (ProSom), flurazepam (Dalmane), quazepam (Doral), temazepam (Restoril), and triazolam (Halcion). Though BzRAs decrease sleep latency, increase TST, and decrease slow wave or deep sleep, they also have adverse side effects such as daytime sedation, anterograde amnesia, cognitive impairment, motor incoordination, dependence, tolerance, and rebound insomnia.18 Because of these side effects, BzRAs should be limited to generally healthy, young (ie, 45 years old) patients who are expected to have brief hospital stays.
| Drugs | Adult Dose (mg) | Half‐Life (hours)* | Onset (minutes) | Peak Effect (hours) | Major Effects/Clinical Comments |
|---|---|---|---|---|---|
| |||||
| BzRAs | Caution in elderly patients. Tolerance to BzRAs develop to the sedative, hypnotic, and anticonvulsant effects. | ||||
| Estazolam (ProSom) | 12 | 1024 | 60 | 0.51.5 | Short‐term (710 days) treatment for frequent arousals, early morning awakening. Not as useful for sleep onset. Avoid in patients with OSA. Caution in elderly patients, liver disease. High doses can cause respiratory depression. |
| Flurazepam (Dalmane) | 1530 | 47100 | 1520 | 36 | In general, avoid in hospitalized medical patients, especially elderly patients. |
| Quazepam (Doral) | 7.515 | 25114 | 1.5 | In general, avoid in hospitalized medical patients, especially elderly patients. | |
| Temazepam (Restoril) | 1530 | 616 | 23 | Short‐term (710 days) treatment for sleep onset and maintenance. Doses 30 mg/day: morning grogginess, nausea, headache, and vivid dreaming. | |
| Triazolam (Halcion) | 0.1250.25 | 1.55.5 | 1530 | 1.75 | Maximum dose is 0.5 mg. Short‐term (710 days) treatment. Rapid onset; should be in bed when taking medication. Contraindicated with atazanavir, ketoconazole, itraconazole, nefazodone, ritonavir. |
| Non‐BzRAs | |||||
| Eszopiclone (Lunesta) | 23 | 69 | 1 | In elderly: difficulty falling asleep, then initial: 1 mg; maximum 2 mg. Difficulty staying asleep: 2 mg. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with high‐fat foods. No tolerance after 6 months. | |
| Zaleplon (Sonata) | 520 | 1 | Rapid | 1 | Short‐term (710 days) treatment for falling asleep and/or next‐day wakefulness is crucial (eg, shift workers). |
| Zopiclone (Imovane) | 515 | 3.86.5 (510 in elderly) | 30 | 2 | Transient and short‐term (710 days) treatment. Contraindicated in severe respiratory impairment. Caution in liver disease and depression; elderly prone to side effects. Anticholinergic agents may plasma level. |
| Zolpidem (Ambien) | 520 | 1.44.5 | 30 | 2 | Short‐term (710 days) treatment for sleep onset and maintenance. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with food. No tolerance after 50 weeks. |
| Melatonin agonist | |||||
| Ramelton (Rozerem) | 8 | 12 | 30 | 11.5 | For sleep onset. For faster sleep onset, do not ingest with high‐fat foods. No tolerance. Contraindicated with fluvoxamine. |
Efficacy and safety studies have generally been limited to healthy, younger individuals without a history of primary sleep disorder. Potential adverse effects of BzRAs may become even more pronounced in hospitalized medical patients due to older age, acute illness, cointeraction drugs, and multidrug regimens. Although BzRAs are FDA‐approved for the treatment of insomnia, flurazepam and quazepam should generally be avoided in hospitalized patients. These agents' long half‐lives increase the risk of drug‐drug interactions and adverse events such as respiratory depression, cognitive decline, and delirium in acutely ill patients. For similar reasons, other long‐acting BzRAs such as clonazepam (Klonopin) and diazepam (Valium) should also not be used to treat insomnia in hospitalized patients. An exception to this is a patient with RLS, in which clonazepam is an approved treatment. However, now that ropinirole HCl (Requip) is FDA‐approved for RLS, BzARs may be able to be avoided. Lorazepam (Ativan), due to its relatively short half‐life and its anxiolytic property, is frequently used to treat insomnia in hospitalized medical patients.18 Start with the lowest dose possible (eg, 0.5 mg) as a one‐time‐only order, or on a as needed basis for 3 days. Alprazolam (Xanax), a potent, fast‐acting BzRA with a relatively short half‐life, has developed a reputation as being notoriously addictive, and experts feel alprazolam has similar potential for withdrawal and rebound.19, 20
The use of BzRAs should be minimized in all patients, and avoided in the elderly or those with a particularly high risk for delirium (eg, traumatic brain injury, stroke, multiple new medications). All BzRAs should be avoided in patients with a prior history of sedative‐hypnotic and/or alcohol dependence unless medically indicated, such as in alcohol withdrawal. Refrain from ordering nightly scheduled BzRAs without a specific time limit to ensure that sedative‐hypnotic use is closely monitored.
For the past 2 decades, physicians have been advised against using long‐acting BzRAs in the elderly (>65 years old) due to the increased risks of hip fractures, falls, motor vehicle accidents, daytime sedation, and adverse cognitive events such as delirium.2124 A large 5‐year prospective study in Quebec found that the risk of injury varied by the BzRA, and was independent of half‐life.25 Importantly, the risk of injury was dose‐dependent: the higher the dose of oxazepam, flurazepam, or chlordiazepoxide, the higher the risk of injury in the elderly.
Non‐BzRAs seem to have a superior side‐effect profile when compared to BzRAs, but should also be used with caution in the elderly. Non‐BzRAs include eszopiclone (Lunesta), zaleplon (Sonata), zolpidem (Ambien), and zolpidem extended‐release. The number of comparison studies is limited, but the available data reveal that: (1) zolpidem (Ambien) may be better than temazepam (Restoril) in terms of sleep latency and quality; and (2) zaleplon (Sonata) may lead to a shorter sleep latency than zolpidem (Ambien), but the latter is associated with longer sleep duration.26 Non‐BzRAs have less next‐day sedation, psychomotor dysfunction, tolerance/withdrawal, and rapid‐eye‐movement (REM) sleep rebound; and lower abuse potential than BzRAs.27
The most commonly prescribed hypnotic, zolpidem has a short half‐life, and seems to reduce sleep latency with minimal residual side effects when compared to BzRAs. The results of a recent multicenter, randomized, double‐blind, placebo‐controlled trial indicated that zolpidem extended‐release may be efficacious for up to 6 months in outpatients with chronic insomnia.28
The sole melatonin‐receptor agonist, ramelteon (Rozerem), also reduces time to fall asleep without next‐day psychomotor and memory effects.29 Ramelteon is believed to target receptors melatonin 1 and 2 receptors located in the brain's suprachiasmatic nucleus to stabilize circadian rhythms and stabilize the sleep‐wake cycle.30
CONCLUSION
Hospitalization is often associated with disrupted sleep, which can affect recovery from illness. Understanding the major factors that impair sleep during hospitalization allows clinicians to systemically evaluate and treat sleep problems. More than just prescribing a sedative/hypnotic, the treatment for sleep disruption includes addressing sleep hygiene and hospital environment issues, identifying medications that could disrupt sleep, and treating specific syndromes that impair sleep. We suggest a practical algorithm to guide clinical assessment, treatment options, and selection of appropriate sleeping medications. Critical to optimizing recovery from illness, sleep may be considered as the sixth vital sign, and should be part of the routine evaluation of every hospitalized patient.
- .Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies.Ann Clin Psychiatry.2006;18(1):49–56.
- ,.Treatment of insomnia.Prim Psychiatry.2005;12(8):47–56.
- ,,.Pharmacologic management of insomnia: past, present, and future.Psychiatr Clin North Am.2006;29:871–893.
- ,.“Hypnotic” prescription patterns in a large managed‐care population.Sleep Med.2004;5(5):463–466.
- ,,, et al.Subjective hypnotic efficacy of trazodone and zolpidem in DSM III‐R primary insomnia.Hum Psychopharmacol.1998;13:191–198.
- ,,, et al.Mirtazapine is more effective than trazodone: a double‐blind controlled study in hospitalized patients with major depression.Int Clin Psychopharmacol.1995;10:3–9.
- ,,.Mirtazapine: an antidepressant with noradrenergic and specific serotonergic effects.Pharmacotherapy.1997;17:10–21.
- ,,,.Efficacy of mirtazapine in obstructive sleep apnea syndrome.Sleep.2007;30(1):35–41.
- ,.Atypical antipsychotics in bipolar disorder: systematic review of randomised trials.BMC Psychiatry.2007;7:40:1–17.
- ,,, et al.Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's Disease.N Engl J Med.2006;355:1525–1538.
- ,,.Risk of death with atypical antipsychotic drug treatment for dementia: meta‐analysis of randomized placebo‐controlled trials.JAMA.2005;294(15):1934–1943.
- ,.Clinical evaluation of diphenhydramine hydrochloride for the treatment of insomnia in psychiatric patients: a double‐blind study.J Clin Pharmacol.1983;23:234–242.
- .Diagnosis and treatment of chronic insomnia: a review.Psychiatr Serv.2005;56:332–343.
- ,,,,.Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial.Am J Gastroenterol.2007;102:744–753.
- ,.Clinical effects of chloral hydrate in hospitalized medical patients.J Clin Pharmacol.1979;19(10):669–674.
- Miller RD, editor.Miller's Anesthesia.6th ed.Philadelphia, PA:Elsevier;2005.
- .State‐of‐the‐art sleep management. Awakening insomnia management. Proceedings from a satellite symposium at SLEEP 2006: 20th Anniversary Meeting of the Associated Professional Sleep Societies, Salt Lake City, UT.2006:6–12.
- ,,.Use of a computer‐based reminder to improve sedative‐hypnotic prescribing in older hospitalized patients.J Am Geriatr Soc.2007;55:43–47.
- ,,,.Topiramate use in alprazolam addiction.World J Biol Psychiatry.2006;7(4):265–267.
- ,,,.Trends in recommendations for the pharmacotherapy of anxiety disorders by an international expert panel, 1992–1997.Eur Neuropsychopharmacol.1999;9(Suppl 6):S393–S398.
- ,,,,.Benzodiazepine use and the risk of motor vehicle crash in the elderly.JAMA.1997;278:27–31.
- ,,,,.Psychotropic drug use and the risk of hip fracture.NEngl J Med.1987;316:363–369.
- ,,,,.Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits.BMJ.2005;331:1169–1175.
- ,,,,,.Delirium in hospitalized older persons: outcomes and predictors.J Am Geriatr Soc.1994;42:809–815.
- ,,,,.A 5‐year prospective assessment of the risk associated with individual benzodiazdepines and doses in new elderly users.J Am Geriatr Soc.2005;53:233–241.
- ,,, et al.Newer hypnotic drugs for the short‐term management of insomnia: a systematic review and economic evaluation.Health Technol Assess.2004;19:305–322.
- .Medications and their effect on sleep.Prim Care Clin Off Pract.2005;32:401–509.
- ,,,,.Long‐term efficacy and safety of zolpidem extended‐release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6‐month, randomized, double‐blind, placebo‐controlled, parallel‐group, multicenter study.Sleep.2008;31(1):79–90.
- ,,,.An efficacy, safety, and dose‐response study of Ramelteon in patients with chronic primary insomnia.Sleep Med.2006;7(1):17–24.
- ,.Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists.Sleep Med.2004;5(6):523–532.
- .Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies.Ann Clin Psychiatry.2006;18(1):49–56.
- ,.Treatment of insomnia.Prim Psychiatry.2005;12(8):47–56.
- ,,.Pharmacologic management of insomnia: past, present, and future.Psychiatr Clin North Am.2006;29:871–893.
- ,.“Hypnotic” prescription patterns in a large managed‐care population.Sleep Med.2004;5(5):463–466.
- ,,, et al.Subjective hypnotic efficacy of trazodone and zolpidem in DSM III‐R primary insomnia.Hum Psychopharmacol.1998;13:191–198.
- ,,, et al.Mirtazapine is more effective than trazodone: a double‐blind controlled study in hospitalized patients with major depression.Int Clin Psychopharmacol.1995;10:3–9.
- ,,.Mirtazapine: an antidepressant with noradrenergic and specific serotonergic effects.Pharmacotherapy.1997;17:10–21.
- ,,,.Efficacy of mirtazapine in obstructive sleep apnea syndrome.Sleep.2007;30(1):35–41.
- ,.Atypical antipsychotics in bipolar disorder: systematic review of randomised trials.BMC Psychiatry.2007;7:40:1–17.
- ,,, et al.Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's Disease.N Engl J Med.2006;355:1525–1538.
- ,,.Risk of death with atypical antipsychotic drug treatment for dementia: meta‐analysis of randomized placebo‐controlled trials.JAMA.2005;294(15):1934–1943.
- ,.Clinical evaluation of diphenhydramine hydrochloride for the treatment of insomnia in psychiatric patients: a double‐blind study.J Clin Pharmacol.1983;23:234–242.
- .Diagnosis and treatment of chronic insomnia: a review.Psychiatr Serv.2005;56:332–343.
- ,,,,.Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial.Am J Gastroenterol.2007;102:744–753.
- ,.Clinical effects of chloral hydrate in hospitalized medical patients.J Clin Pharmacol.1979;19(10):669–674.
- Miller RD, editor.Miller's Anesthesia.6th ed.Philadelphia, PA:Elsevier;2005.
- .State‐of‐the‐art sleep management. Awakening insomnia management. Proceedings from a satellite symposium at SLEEP 2006: 20th Anniversary Meeting of the Associated Professional Sleep Societies, Salt Lake City, UT.2006:6–12.
- ,,.Use of a computer‐based reminder to improve sedative‐hypnotic prescribing in older hospitalized patients.J Am Geriatr Soc.2007;55:43–47.
- ,,,.Topiramate use in alprazolam addiction.World J Biol Psychiatry.2006;7(4):265–267.
- ,,,.Trends in recommendations for the pharmacotherapy of anxiety disorders by an international expert panel, 1992–1997.Eur Neuropsychopharmacol.1999;9(Suppl 6):S393–S398.
- ,,,,.Benzodiazepine use and the risk of motor vehicle crash in the elderly.JAMA.1997;278:27–31.
- ,,,,.Psychotropic drug use and the risk of hip fracture.NEngl J Med.1987;316:363–369.
- ,,,,.Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits.BMJ.2005;331:1169–1175.
- ,,,,,.Delirium in hospitalized older persons: outcomes and predictors.J Am Geriatr Soc.1994;42:809–815.
- ,,,,.A 5‐year prospective assessment of the risk associated with individual benzodiazdepines and doses in new elderly users.J Am Geriatr Soc.2005;53:233–241.
- ,,, et al.Newer hypnotic drugs for the short‐term management of insomnia: a systematic review and economic evaluation.Health Technol Assess.2004;19:305–322.
- .Medications and their effect on sleep.Prim Care Clin Off Pract.2005;32:401–509.
- ,,,,.Long‐term efficacy and safety of zolpidem extended‐release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6‐month, randomized, double‐blind, placebo‐controlled, parallel‐group, multicenter study.Sleep.2008;31(1):79–90.
- ,,,.An efficacy, safety, and dose‐response study of Ramelteon in patients with chronic primary insomnia.Sleep Med.2006;7(1):17–24.
- ,.Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists.Sleep Med.2004;5(6):523–532.
Limited communication and management of emergency department hyperglycemia in hospitalized patients
While increasing evidence suggests that hyperglycemia during illness is associated with poor clinical outcome,1, 2 hyperglycemia in the hospital setting is often overlooked and unaddressed.3, 4 Early and intensive management of hyperglycemia may improve outcomes in hospitalized patients.57 Emergency Department (ED) glucose values may present an early opportunity to identify hyperglycemic patients as having unrecognized glucose intolerance and improve early glycemic control for hospitalized patients. Serum glucose values are available for 18% of 110 million annual ED visits in the United States, and many others undergo capillary glucose measurements.8 Although stressors and lack of fasting may contribute to ED hyperglycemia, communication and management should be similar.5 In this study, we hypothesized that in less than 20% of patients ED hyperglycemia would be recognized, communicated to patients, or they would receive ED treatment.
PATIENTS AND METHODS
Study Design
This was a retrospective cohort study using a structured medical record review of consecutive ED patients presenting between September 1, 2004 and August 31, 2005. We obtained our Institutional Review Board's approval with waiver of informed consent.
Study Setting and Population
The site of data collection was an urban, academic institution with approximately 50,000 annual ED visits. Care of hospitalized patients on the medical service is provided or supervised by staff hospitalists. Using the hospital's electronic records, we identified all patients with serum glucose ordered from the ED during the study time period. When there were multiple glucose results, we included only the first glucose values. Based on conservative thresholds for association of random glucose with poor clinical outcomes in hospitalized patients and with undiagnosed diabetes,5, 9 we considered glucose 140 mg/dL (7.8 mmol/L) as normal and categorized the remaining values into 2 groups: 140‐199 mg/dL (7.8‐11.0 mmol/L) and 200 mg/dL (11.1 mmol/L).
Study Protocol
We selected 200 patients from each glucose group using a random number generator, and 2 investigators (D.J.S., A.A.G.) performed a detailed chart review using a standardized data abstraction form. The research team met frequently to maintain consistency in data collection and to resolve disputes.
We recorded demographic data, presence of a primary care provider, relevant past medical history, current medications, ED treatment (insulin, oral hypoglycemic agents, and intravenous fluids), disposition (admission or discharge), and final diagnoses. Additionally, we evaluated capillary blood glucose values during the ED stay and serum glucose values during the ED and hospital stay to evaluate for hypoglycemia (defined as glucose 65 mg/dL). We also evaluate diagnosis codes to identify concurrent infection, sepsis, or trauma that may have been associated with the hyperglycemia, based on previously reported methodology.10, 11 Finally, we examined the inpatient or ED written discharge instructions to evaluate newly started antidiabetic medications, communication of hyperglycemia, and recommendation of repeat glucose/diabetes testing.
Data Analysis
We performed statistical analyses using Stata 9.0 (Stata Corp., College Station, TX) and summarized data using basic descriptive statistics with 95% confidence intervals (95%CIs). We measured interrater agreement for chart abstraction by calculating the kappa statistic for a 5% sample of charts abstracted by both investigators. We considered kappa >0.80 as high interrater agreement. We evaluated differences between subgroups of interest using chi square test. All P values are 2‐tailed, with P 0.05 considered statistically significant.
RESULTS
During the data collection period, 27,688 (58%) ED visits had at least 1 serum glucose result. After excluding multiple glucose results for the same visit, the median glucose value was 106 mg/dL (range, 7‐2280 mg/dL); 3517 (13%) values were 140‐199 mg/dL, and 2304 (8%) values were 200 mg/dL. We located 385 of the 400 (96%) randomly selected charts. Interrater agreement for chart review was high (kappa = 0.91‐0.98).
Table 1 shows demographic characteristics and Table 2 shows clinical data of the sample, stratified by glucose group and charted diagnosis of diabetes. Overall, 55% of patients with glucose values 140‐199 mg/dL and 16% of patients with glucose 200 mg/dL had no prior diabetes diagnosis. Hyperglycemia was associated with sepsis for 22% of patients, infection without sepsis for 13% of patients, and traumatic injury for 19% of patients.
| Glucose 140199 mg/dL % (95%CI) or Median (IQR) | Glucose 200 mg/dL % (95%CI) or Median (IQR) | ||||
|---|---|---|---|---|---|
| Variable | Diabetes (n = 87) | No Diabetes (n = 107) | Diabetes (n = 160) | No Diabetes (n = 31) | Total n (%) or Median (IQR) (n = 385) |
| |||||
| Demographics | |||||
| Age | 66 (5475) | 68 (5083) | 63 (5275) | 58 (3376) | 64 (5176) |
| Female sex | 39% (2950) | 58% (4867) | 55% (4763) | 26% (1245) | 50% (4555) |
| Race/ethnicity | |||||
| White | 67% (5676) | 75% (6583) | 61% (5369) | 71% (5286) | 258 (67%) |
| Black | 22% (1432) | 9% (517) | 21% (1528) | 10% (226) | 65 (17%) |
| Hispanic | 2% (08) | 4% (19) | 6% (310) | 3% (017) | 26 (4%) |
| Other | 9% (417) | 12% (720) | 12% (819) | 16% (534) | 46 (12%) |
| Insurance | |||||
| Private | 32% (2343) | 41% (3251) | 32% (2540) | 45% (2764) | 137 (36%) |
| Medicare | 61% (5071) | 47%(3757) | 49% (4157) | 32% (1751) | 192 (50%) |
| Medicaid | 6% (213) | 7% (314) | 16% (1022) | 6% (121) | 40 (10%) |
| None | 1% (06) | 5% (211) | 3% (17) | 16% (534) | 16 (4%) |
| Assigned PCP | 95% (8999) | 84% (7690) | 86% (8091) | 71% (5286) | 86% (8390) |
| Past medical history | |||||
| Hypertension | 61% (5071) | 45% (3555) | 58% (5066) | 39% (2156) | 206 (54%) |
| Hyperlipidemia | 28% (1938) | 21% (1329) | 25% (1932) | 10% (226) | 90 (23%) |
| Coronary artery disease | 41% (3152) | 29% (2138) | 26% (2034) | 13% (430) | 113 (29%) |
| Current medications | |||||
| Insulin | 36% (2647) | 0 | 54% (4662) | 0 | 117 (30%) |
| Sulfonylurea | 25% (1736) | 0 | 26% (1933) | 0 | 63 (16%) |
| Other oral hypoglycemic | 39% (2950) | 0 | 24% (1832) | 0 | 73 (19%) |
| Systemic corticosteroids | 5% (111) | 10% (517) | 4% (18) | 6% (121) | 23 (6%) |
| Glucose 140199 mg/dL % (95%CI) or Median (IQR) | Glucose 200 mg/dL % (95%CI) or Median (IQR) | ||||
|---|---|---|---|---|---|
| Variable | Diabetes | No Diabetes | Diabetes | No Diabetes | Total n (%) or Median (IQR) |
| |||||
| ED clinical data | (n = 87) | (n = 107) | (n = 160) | (n = 31) | (n = 385) |
| Glucose value, mg/dL | 167 (163170) | 160 (157163) | 308 (285330) | 272 (242300) | 231 (220244) |
| Insulin | 6% (213) | 1% (03) | 31% (2439) | 19% (737) | 61 (16%) |
| IVF without dextrose* | 44% (3355) | 54% (4464) | 51% (4358) | 68% (4983) | 198 (51%) |
| Hyperglycemia charted as diagnosis | 3% (110) | 0 | 18% (1225) | 16% (534) | 36 (9%) |
| Hospital admission | 76% (6584) | 79% (7187) | 73% (6680) | 84% (6695) | 293 (76%) |
| Discharge data | (n = 84) | (n = 98) | (n = 156) | (n = 25) | (n = 363) |
| New insulin Rx | 8% (316) | 5% (212) | 6% (310) | 16% (536) | 26 (7%) |
| New sulfonylurea Rx | 2% (08) | 1% (06) | 4% (18) | 0 | 10 (3%) |
| New other oral hypoglycemic Rx | 1% (06) | 1% (06) | 3% (17) | 8% (126) | 9 (2%) |
| Any new diabetes Rx | 12% (621) | 7% (314) | 12% (718) | 24% (945) | 42 (12%) |
| Hyperglycemia noted in written instructions | 4% (110) | 3% (19) | 15% (1021) | 24% (945) | 36 (10%) |
| Repeat glucose/diabetes testing charted | 5% (112) | 1% (06) | 9% (515) | 16% (536) | 23 (6%) |
No patient received intravenous fluids with dextrose prior to initial serum glucose determination, and there was no difference in home corticosteroid use between groups (P = 0.23). Patients with known diabetes were more likely to receive insulin in the ED (P 0.01). Only 1 patient received an oral hypoglycemic agent in the ED. Three patients had documented hypoglycemia on capillary blood glucose during the ED stay, and no patients had hypoglycemia based on serum glucose during the ED or hospital stay. Among hospitalized patients, 61% had inpatient orders for diabetic‐consistent/carbohydrate‐consistent diet, 65% for capillary glucose tests daily, and 63% for sliding scale insulin.
We also present written discharge instructions data for 363 visits (253 inpatient and 110 ED) in Table 2; discharge instructions were not available for 22 visits (12 deaths during hospitalization, 10 missing instructions). New antidiabetic medications were prescribed for 42 (12%) patients, all from the inpatient setting. There was no difference between inpatient and ED communication of hyperglycemia (10% [95%CI, 7%‐14%] versus 9% [95%CI, 4%‐15%]) and recommendation for further outpatient testing (8% [95%CI, 4%‐11%] versus 4% [95%CI, 0%‐7%]) in written discharge instructions (P = 0.73 and 0.16, respectively). Compared to those with glucose 140‐199 mg/dL, patients with glucose 200 mg/dL were more likely to receive written communication of hyperglycemia (17% [95%CI, 11%‐22%] versus 3% [95%CI, 0%‐6%]) and recommendation for further outpatient testing (10% [95%CI, 6%‐14%] versus 3% [95%CI, 0%‐5%] (both, P 0.01).
DISCUSSION
Although noncritical ED glucose values may be overlooked, values sufficient to motivate inpatient and long‐term management are sometimes uncovered, and when unrecognized may be missed opportunities. Indeed, admission hyperglycemia has been linked to poor clinical outcomes in hospitalized patients for a variety of conditions, particularly for myocardial infarction, stroke, and critical illness.1215
In this study, we evaluated recognition, communication, and management of ED glucose values above a relatively conservative threshold of 140 mg/dL, occurring in 21% of ED glucose results. Diabetes screening thresholds for casual glucose values as low as 120 mg/dL,9 and intensive glycemic control in critically ill patients to a target as low as 110 mg/dL have been suggested.5 Nevertheless, only 16% of our sample received insulin in the ED for hyperglycemia, and hyperglycemia was charted as a diagnosis in only 9% of cases.
This is especially important because 77% of ED visits without hyperglycemia charted as a diagnosis resulted in hospitalization, and early glycemic control was infrequently initiated. Limited ED management of hyperglycemia may be driven by the presence of more critical management issues (eg, 54% of patients had concomitant infection or trauma), lack of familiarity with guidelines, which suggest treatment to glucose 140 mg/dL in critically ill patients and 180 mg/dL in all hospitalized patients,16 or fear of adverse events, such as hypoglycemia. Additionally, ED crowding has been shown to effect decreased quality and timeliness of treatment for pneumonia, and may have similar effects for hyperglycemia.17 Inpatient recognition of hyperglycemia, based on orders for diet, glucose checks, and insulin, appeared significantly better, but this did not translate to improved communication in written discharge instructions. Additionally, many hospitalized patients may spend many hours, or even days, in the ED waiting for beds, which currently is a missed opportunity to initiate early therapy.
Written discharge instructions informed less than 10% of patients of their hyperglycemia or outlined a plan for further evaluation and management. Our prior work suggests that nearly all (95%) ED patients want to be informed of elevated blood glucose and are willing to follow‐up, if instructed.18 The current data suggests that hyperglycemia in ED and hospitalized patients is frequently unrecognized and undertreated, and opportunities to institute an outpatient plan to address hyperglycemia are frequently missed.
This study has several potential limitations. This study was performed at a single academic center, which limits generalizability to other geographic areas and hospital types. Accuracy of abstracted data depended on chart review, which is limited by the possibility of missing, incomplete, or unreliable information. Standardized definitions and abstraction forms limited potential for bias, and high interrater agreement demonstrated internal reliability of the chart review. We considered only initial glucose values and were unable to determine nutritional status; it is possible that subsequent measurements were within an acceptable range. Conversely, hospitalized patients may have developed hyperglycemia subsequent to the initial glucose result, which would underestimate the scope of inpatient hyperglycemia. Also, because there are limited data for interpretation of ED hyperglycemia, we were unable to determine optimal glucose thresholds. Finally, we were unable to evaluate the content of verbal instructions or letters to outpatient providers, which limited our ability to fully describe communication of abnormal findings. However, patients do not often retain information in verbal instructions, in the context of new diagnoses and complex medical regimens.
In summary, recognition, management, and communication of ED hyperglycemia were suboptimal in our patient population and represent a missed opportunity. Enhanced recognition, management, and referral for hyperglycemia observed during usual ED care may provide an unobtrusive method to improve identification of undiagnosed diabetes/prediabetes and initiation of intensive glycemic control for hospitalized patients.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,,,.Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.JAm Coll Cardiol.1995;26:57–65.
- ,,.National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary.Adv Data.2007;386:1–32.
- ,,, et al.Performance of recommended screening tests for undiagnosed diabetes and dysglycemia.Diabetes Care.2001;24:1899–1903.
- ,,, et al.The epidemiology of sepsis in the United States from 1979 through 2000.N Engl J Med.2003;348:1546–1554.
- ,,,,,.Completeness and accuracy of International Classification of Disease (ICD) external cause of injury codes in emergency department electronic data.Inj Prev.2007;13:422–425.
- ,,,.Stress hyperglycemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,, et al.Admission blood glucose level as a risk indicator of death after myocardial infarction in patients with and without diabetes mellitus.Arch Intern Med.2004;164:982–989.
- ,,, et al.Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke.Neurology.2002;59:67–71.
- .Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients.Mayo Clin Proc.2003;78:1471–1478.
- American Diabetes Association.Standards of Medical Care in Diabetes—2008.Diabetes Care.2008;31;S12–S54.
- ,,,.Effect of emergency department crowding on time to antibiotics in patients admitted with community‐acquired pneumonia.Ann Emerg Med.2007;50:501–509.
- ,,,,.Estimated risk for undiagnosed diabetes in the emergency department: a multicenter survey.Acad Emerg Med.2007;14:492–495.
While increasing evidence suggests that hyperglycemia during illness is associated with poor clinical outcome,1, 2 hyperglycemia in the hospital setting is often overlooked and unaddressed.3, 4 Early and intensive management of hyperglycemia may improve outcomes in hospitalized patients.57 Emergency Department (ED) glucose values may present an early opportunity to identify hyperglycemic patients as having unrecognized glucose intolerance and improve early glycemic control for hospitalized patients. Serum glucose values are available for 18% of 110 million annual ED visits in the United States, and many others undergo capillary glucose measurements.8 Although stressors and lack of fasting may contribute to ED hyperglycemia, communication and management should be similar.5 In this study, we hypothesized that in less than 20% of patients ED hyperglycemia would be recognized, communicated to patients, or they would receive ED treatment.
PATIENTS AND METHODS
Study Design
This was a retrospective cohort study using a structured medical record review of consecutive ED patients presenting between September 1, 2004 and August 31, 2005. We obtained our Institutional Review Board's approval with waiver of informed consent.
Study Setting and Population
The site of data collection was an urban, academic institution with approximately 50,000 annual ED visits. Care of hospitalized patients on the medical service is provided or supervised by staff hospitalists. Using the hospital's electronic records, we identified all patients with serum glucose ordered from the ED during the study time period. When there were multiple glucose results, we included only the first glucose values. Based on conservative thresholds for association of random glucose with poor clinical outcomes in hospitalized patients and with undiagnosed diabetes,5, 9 we considered glucose 140 mg/dL (7.8 mmol/L) as normal and categorized the remaining values into 2 groups: 140‐199 mg/dL (7.8‐11.0 mmol/L) and 200 mg/dL (11.1 mmol/L).
Study Protocol
We selected 200 patients from each glucose group using a random number generator, and 2 investigators (D.J.S., A.A.G.) performed a detailed chart review using a standardized data abstraction form. The research team met frequently to maintain consistency in data collection and to resolve disputes.
We recorded demographic data, presence of a primary care provider, relevant past medical history, current medications, ED treatment (insulin, oral hypoglycemic agents, and intravenous fluids), disposition (admission or discharge), and final diagnoses. Additionally, we evaluated capillary blood glucose values during the ED stay and serum glucose values during the ED and hospital stay to evaluate for hypoglycemia (defined as glucose 65 mg/dL). We also evaluate diagnosis codes to identify concurrent infection, sepsis, or trauma that may have been associated with the hyperglycemia, based on previously reported methodology.10, 11 Finally, we examined the inpatient or ED written discharge instructions to evaluate newly started antidiabetic medications, communication of hyperglycemia, and recommendation of repeat glucose/diabetes testing.
Data Analysis
We performed statistical analyses using Stata 9.0 (Stata Corp., College Station, TX) and summarized data using basic descriptive statistics with 95% confidence intervals (95%CIs). We measured interrater agreement for chart abstraction by calculating the kappa statistic for a 5% sample of charts abstracted by both investigators. We considered kappa >0.80 as high interrater agreement. We evaluated differences between subgroups of interest using chi square test. All P values are 2‐tailed, with P 0.05 considered statistically significant.
RESULTS
During the data collection period, 27,688 (58%) ED visits had at least 1 serum glucose result. After excluding multiple glucose results for the same visit, the median glucose value was 106 mg/dL (range, 7‐2280 mg/dL); 3517 (13%) values were 140‐199 mg/dL, and 2304 (8%) values were 200 mg/dL. We located 385 of the 400 (96%) randomly selected charts. Interrater agreement for chart review was high (kappa = 0.91‐0.98).
Table 1 shows demographic characteristics and Table 2 shows clinical data of the sample, stratified by glucose group and charted diagnosis of diabetes. Overall, 55% of patients with glucose values 140‐199 mg/dL and 16% of patients with glucose 200 mg/dL had no prior diabetes diagnosis. Hyperglycemia was associated with sepsis for 22% of patients, infection without sepsis for 13% of patients, and traumatic injury for 19% of patients.
| Glucose 140199 mg/dL % (95%CI) or Median (IQR) | Glucose 200 mg/dL % (95%CI) or Median (IQR) | ||||
|---|---|---|---|---|---|
| Variable | Diabetes (n = 87) | No Diabetes (n = 107) | Diabetes (n = 160) | No Diabetes (n = 31) | Total n (%) or Median (IQR) (n = 385) |
| |||||
| Demographics | |||||
| Age | 66 (5475) | 68 (5083) | 63 (5275) | 58 (3376) | 64 (5176) |
| Female sex | 39% (2950) | 58% (4867) | 55% (4763) | 26% (1245) | 50% (4555) |
| Race/ethnicity | |||||
| White | 67% (5676) | 75% (6583) | 61% (5369) | 71% (5286) | 258 (67%) |
| Black | 22% (1432) | 9% (517) | 21% (1528) | 10% (226) | 65 (17%) |
| Hispanic | 2% (08) | 4% (19) | 6% (310) | 3% (017) | 26 (4%) |
| Other | 9% (417) | 12% (720) | 12% (819) | 16% (534) | 46 (12%) |
| Insurance | |||||
| Private | 32% (2343) | 41% (3251) | 32% (2540) | 45% (2764) | 137 (36%) |
| Medicare | 61% (5071) | 47%(3757) | 49% (4157) | 32% (1751) | 192 (50%) |
| Medicaid | 6% (213) | 7% (314) | 16% (1022) | 6% (121) | 40 (10%) |
| None | 1% (06) | 5% (211) | 3% (17) | 16% (534) | 16 (4%) |
| Assigned PCP | 95% (8999) | 84% (7690) | 86% (8091) | 71% (5286) | 86% (8390) |
| Past medical history | |||||
| Hypertension | 61% (5071) | 45% (3555) | 58% (5066) | 39% (2156) | 206 (54%) |
| Hyperlipidemia | 28% (1938) | 21% (1329) | 25% (1932) | 10% (226) | 90 (23%) |
| Coronary artery disease | 41% (3152) | 29% (2138) | 26% (2034) | 13% (430) | 113 (29%) |
| Current medications | |||||
| Insulin | 36% (2647) | 0 | 54% (4662) | 0 | 117 (30%) |
| Sulfonylurea | 25% (1736) | 0 | 26% (1933) | 0 | 63 (16%) |
| Other oral hypoglycemic | 39% (2950) | 0 | 24% (1832) | 0 | 73 (19%) |
| Systemic corticosteroids | 5% (111) | 10% (517) | 4% (18) | 6% (121) | 23 (6%) |
| Glucose 140199 mg/dL % (95%CI) or Median (IQR) | Glucose 200 mg/dL % (95%CI) or Median (IQR) | ||||
|---|---|---|---|---|---|
| Variable | Diabetes | No Diabetes | Diabetes | No Diabetes | Total n (%) or Median (IQR) |
| |||||
| ED clinical data | (n = 87) | (n = 107) | (n = 160) | (n = 31) | (n = 385) |
| Glucose value, mg/dL | 167 (163170) | 160 (157163) | 308 (285330) | 272 (242300) | 231 (220244) |
| Insulin | 6% (213) | 1% (03) | 31% (2439) | 19% (737) | 61 (16%) |
| IVF without dextrose* | 44% (3355) | 54% (4464) | 51% (4358) | 68% (4983) | 198 (51%) |
| Hyperglycemia charted as diagnosis | 3% (110) | 0 | 18% (1225) | 16% (534) | 36 (9%) |
| Hospital admission | 76% (6584) | 79% (7187) | 73% (6680) | 84% (6695) | 293 (76%) |
| Discharge data | (n = 84) | (n = 98) | (n = 156) | (n = 25) | (n = 363) |
| New insulin Rx | 8% (316) | 5% (212) | 6% (310) | 16% (536) | 26 (7%) |
| New sulfonylurea Rx | 2% (08) | 1% (06) | 4% (18) | 0 | 10 (3%) |
| New other oral hypoglycemic Rx | 1% (06) | 1% (06) | 3% (17) | 8% (126) | 9 (2%) |
| Any new diabetes Rx | 12% (621) | 7% (314) | 12% (718) | 24% (945) | 42 (12%) |
| Hyperglycemia noted in written instructions | 4% (110) | 3% (19) | 15% (1021) | 24% (945) | 36 (10%) |
| Repeat glucose/diabetes testing charted | 5% (112) | 1% (06) | 9% (515) | 16% (536) | 23 (6%) |
No patient received intravenous fluids with dextrose prior to initial serum glucose determination, and there was no difference in home corticosteroid use between groups (P = 0.23). Patients with known diabetes were more likely to receive insulin in the ED (P 0.01). Only 1 patient received an oral hypoglycemic agent in the ED. Three patients had documented hypoglycemia on capillary blood glucose during the ED stay, and no patients had hypoglycemia based on serum glucose during the ED or hospital stay. Among hospitalized patients, 61% had inpatient orders for diabetic‐consistent/carbohydrate‐consistent diet, 65% for capillary glucose tests daily, and 63% for sliding scale insulin.
We also present written discharge instructions data for 363 visits (253 inpatient and 110 ED) in Table 2; discharge instructions were not available for 22 visits (12 deaths during hospitalization, 10 missing instructions). New antidiabetic medications were prescribed for 42 (12%) patients, all from the inpatient setting. There was no difference between inpatient and ED communication of hyperglycemia (10% [95%CI, 7%‐14%] versus 9% [95%CI, 4%‐15%]) and recommendation for further outpatient testing (8% [95%CI, 4%‐11%] versus 4% [95%CI, 0%‐7%]) in written discharge instructions (P = 0.73 and 0.16, respectively). Compared to those with glucose 140‐199 mg/dL, patients with glucose 200 mg/dL were more likely to receive written communication of hyperglycemia (17% [95%CI, 11%‐22%] versus 3% [95%CI, 0%‐6%]) and recommendation for further outpatient testing (10% [95%CI, 6%‐14%] versus 3% [95%CI, 0%‐5%] (both, P 0.01).
DISCUSSION
Although noncritical ED glucose values may be overlooked, values sufficient to motivate inpatient and long‐term management are sometimes uncovered, and when unrecognized may be missed opportunities. Indeed, admission hyperglycemia has been linked to poor clinical outcomes in hospitalized patients for a variety of conditions, particularly for myocardial infarction, stroke, and critical illness.1215
In this study, we evaluated recognition, communication, and management of ED glucose values above a relatively conservative threshold of 140 mg/dL, occurring in 21% of ED glucose results. Diabetes screening thresholds for casual glucose values as low as 120 mg/dL,9 and intensive glycemic control in critically ill patients to a target as low as 110 mg/dL have been suggested.5 Nevertheless, only 16% of our sample received insulin in the ED for hyperglycemia, and hyperglycemia was charted as a diagnosis in only 9% of cases.
This is especially important because 77% of ED visits without hyperglycemia charted as a diagnosis resulted in hospitalization, and early glycemic control was infrequently initiated. Limited ED management of hyperglycemia may be driven by the presence of more critical management issues (eg, 54% of patients had concomitant infection or trauma), lack of familiarity with guidelines, which suggest treatment to glucose 140 mg/dL in critically ill patients and 180 mg/dL in all hospitalized patients,16 or fear of adverse events, such as hypoglycemia. Additionally, ED crowding has been shown to effect decreased quality and timeliness of treatment for pneumonia, and may have similar effects for hyperglycemia.17 Inpatient recognition of hyperglycemia, based on orders for diet, glucose checks, and insulin, appeared significantly better, but this did not translate to improved communication in written discharge instructions. Additionally, many hospitalized patients may spend many hours, or even days, in the ED waiting for beds, which currently is a missed opportunity to initiate early therapy.
Written discharge instructions informed less than 10% of patients of their hyperglycemia or outlined a plan for further evaluation and management. Our prior work suggests that nearly all (95%) ED patients want to be informed of elevated blood glucose and are willing to follow‐up, if instructed.18 The current data suggests that hyperglycemia in ED and hospitalized patients is frequently unrecognized and undertreated, and opportunities to institute an outpatient plan to address hyperglycemia are frequently missed.
This study has several potential limitations. This study was performed at a single academic center, which limits generalizability to other geographic areas and hospital types. Accuracy of abstracted data depended on chart review, which is limited by the possibility of missing, incomplete, or unreliable information. Standardized definitions and abstraction forms limited potential for bias, and high interrater agreement demonstrated internal reliability of the chart review. We considered only initial glucose values and were unable to determine nutritional status; it is possible that subsequent measurements were within an acceptable range. Conversely, hospitalized patients may have developed hyperglycemia subsequent to the initial glucose result, which would underestimate the scope of inpatient hyperglycemia. Also, because there are limited data for interpretation of ED hyperglycemia, we were unable to determine optimal glucose thresholds. Finally, we were unable to evaluate the content of verbal instructions or letters to outpatient providers, which limited our ability to fully describe communication of abnormal findings. However, patients do not often retain information in verbal instructions, in the context of new diagnoses and complex medical regimens.
In summary, recognition, management, and communication of ED hyperglycemia were suboptimal in our patient population and represent a missed opportunity. Enhanced recognition, management, and referral for hyperglycemia observed during usual ED care may provide an unobtrusive method to improve identification of undiagnosed diabetes/prediabetes and initiation of intensive glycemic control for hospitalized patients.
While increasing evidence suggests that hyperglycemia during illness is associated with poor clinical outcome,1, 2 hyperglycemia in the hospital setting is often overlooked and unaddressed.3, 4 Early and intensive management of hyperglycemia may improve outcomes in hospitalized patients.57 Emergency Department (ED) glucose values may present an early opportunity to identify hyperglycemic patients as having unrecognized glucose intolerance and improve early glycemic control for hospitalized patients. Serum glucose values are available for 18% of 110 million annual ED visits in the United States, and many others undergo capillary glucose measurements.8 Although stressors and lack of fasting may contribute to ED hyperglycemia, communication and management should be similar.5 In this study, we hypothesized that in less than 20% of patients ED hyperglycemia would be recognized, communicated to patients, or they would receive ED treatment.
PATIENTS AND METHODS
Study Design
This was a retrospective cohort study using a structured medical record review of consecutive ED patients presenting between September 1, 2004 and August 31, 2005. We obtained our Institutional Review Board's approval with waiver of informed consent.
Study Setting and Population
The site of data collection was an urban, academic institution with approximately 50,000 annual ED visits. Care of hospitalized patients on the medical service is provided or supervised by staff hospitalists. Using the hospital's electronic records, we identified all patients with serum glucose ordered from the ED during the study time period. When there were multiple glucose results, we included only the first glucose values. Based on conservative thresholds for association of random glucose with poor clinical outcomes in hospitalized patients and with undiagnosed diabetes,5, 9 we considered glucose 140 mg/dL (7.8 mmol/L) as normal and categorized the remaining values into 2 groups: 140‐199 mg/dL (7.8‐11.0 mmol/L) and 200 mg/dL (11.1 mmol/L).
Study Protocol
We selected 200 patients from each glucose group using a random number generator, and 2 investigators (D.J.S., A.A.G.) performed a detailed chart review using a standardized data abstraction form. The research team met frequently to maintain consistency in data collection and to resolve disputes.
We recorded demographic data, presence of a primary care provider, relevant past medical history, current medications, ED treatment (insulin, oral hypoglycemic agents, and intravenous fluids), disposition (admission or discharge), and final diagnoses. Additionally, we evaluated capillary blood glucose values during the ED stay and serum glucose values during the ED and hospital stay to evaluate for hypoglycemia (defined as glucose 65 mg/dL). We also evaluate diagnosis codes to identify concurrent infection, sepsis, or trauma that may have been associated with the hyperglycemia, based on previously reported methodology.10, 11 Finally, we examined the inpatient or ED written discharge instructions to evaluate newly started antidiabetic medications, communication of hyperglycemia, and recommendation of repeat glucose/diabetes testing.
Data Analysis
We performed statistical analyses using Stata 9.0 (Stata Corp., College Station, TX) and summarized data using basic descriptive statistics with 95% confidence intervals (95%CIs). We measured interrater agreement for chart abstraction by calculating the kappa statistic for a 5% sample of charts abstracted by both investigators. We considered kappa >0.80 as high interrater agreement. We evaluated differences between subgroups of interest using chi square test. All P values are 2‐tailed, with P 0.05 considered statistically significant.
RESULTS
During the data collection period, 27,688 (58%) ED visits had at least 1 serum glucose result. After excluding multiple glucose results for the same visit, the median glucose value was 106 mg/dL (range, 7‐2280 mg/dL); 3517 (13%) values were 140‐199 mg/dL, and 2304 (8%) values were 200 mg/dL. We located 385 of the 400 (96%) randomly selected charts. Interrater agreement for chart review was high (kappa = 0.91‐0.98).
Table 1 shows demographic characteristics and Table 2 shows clinical data of the sample, stratified by glucose group and charted diagnosis of diabetes. Overall, 55% of patients with glucose values 140‐199 mg/dL and 16% of patients with glucose 200 mg/dL had no prior diabetes diagnosis. Hyperglycemia was associated with sepsis for 22% of patients, infection without sepsis for 13% of patients, and traumatic injury for 19% of patients.
| Glucose 140199 mg/dL % (95%CI) or Median (IQR) | Glucose 200 mg/dL % (95%CI) or Median (IQR) | ||||
|---|---|---|---|---|---|
| Variable | Diabetes (n = 87) | No Diabetes (n = 107) | Diabetes (n = 160) | No Diabetes (n = 31) | Total n (%) or Median (IQR) (n = 385) |
| |||||
| Demographics | |||||
| Age | 66 (5475) | 68 (5083) | 63 (5275) | 58 (3376) | 64 (5176) |
| Female sex | 39% (2950) | 58% (4867) | 55% (4763) | 26% (1245) | 50% (4555) |
| Race/ethnicity | |||||
| White | 67% (5676) | 75% (6583) | 61% (5369) | 71% (5286) | 258 (67%) |
| Black | 22% (1432) | 9% (517) | 21% (1528) | 10% (226) | 65 (17%) |
| Hispanic | 2% (08) | 4% (19) | 6% (310) | 3% (017) | 26 (4%) |
| Other | 9% (417) | 12% (720) | 12% (819) | 16% (534) | 46 (12%) |
| Insurance | |||||
| Private | 32% (2343) | 41% (3251) | 32% (2540) | 45% (2764) | 137 (36%) |
| Medicare | 61% (5071) | 47%(3757) | 49% (4157) | 32% (1751) | 192 (50%) |
| Medicaid | 6% (213) | 7% (314) | 16% (1022) | 6% (121) | 40 (10%) |
| None | 1% (06) | 5% (211) | 3% (17) | 16% (534) | 16 (4%) |
| Assigned PCP | 95% (8999) | 84% (7690) | 86% (8091) | 71% (5286) | 86% (8390) |
| Past medical history | |||||
| Hypertension | 61% (5071) | 45% (3555) | 58% (5066) | 39% (2156) | 206 (54%) |
| Hyperlipidemia | 28% (1938) | 21% (1329) | 25% (1932) | 10% (226) | 90 (23%) |
| Coronary artery disease | 41% (3152) | 29% (2138) | 26% (2034) | 13% (430) | 113 (29%) |
| Current medications | |||||
| Insulin | 36% (2647) | 0 | 54% (4662) | 0 | 117 (30%) |
| Sulfonylurea | 25% (1736) | 0 | 26% (1933) | 0 | 63 (16%) |
| Other oral hypoglycemic | 39% (2950) | 0 | 24% (1832) | 0 | 73 (19%) |
| Systemic corticosteroids | 5% (111) | 10% (517) | 4% (18) | 6% (121) | 23 (6%) |
| Glucose 140199 mg/dL % (95%CI) or Median (IQR) | Glucose 200 mg/dL % (95%CI) or Median (IQR) | ||||
|---|---|---|---|---|---|
| Variable | Diabetes | No Diabetes | Diabetes | No Diabetes | Total n (%) or Median (IQR) |
| |||||
| ED clinical data | (n = 87) | (n = 107) | (n = 160) | (n = 31) | (n = 385) |
| Glucose value, mg/dL | 167 (163170) | 160 (157163) | 308 (285330) | 272 (242300) | 231 (220244) |
| Insulin | 6% (213) | 1% (03) | 31% (2439) | 19% (737) | 61 (16%) |
| IVF without dextrose* | 44% (3355) | 54% (4464) | 51% (4358) | 68% (4983) | 198 (51%) |
| Hyperglycemia charted as diagnosis | 3% (110) | 0 | 18% (1225) | 16% (534) | 36 (9%) |
| Hospital admission | 76% (6584) | 79% (7187) | 73% (6680) | 84% (6695) | 293 (76%) |
| Discharge data | (n = 84) | (n = 98) | (n = 156) | (n = 25) | (n = 363) |
| New insulin Rx | 8% (316) | 5% (212) | 6% (310) | 16% (536) | 26 (7%) |
| New sulfonylurea Rx | 2% (08) | 1% (06) | 4% (18) | 0 | 10 (3%) |
| New other oral hypoglycemic Rx | 1% (06) | 1% (06) | 3% (17) | 8% (126) | 9 (2%) |
| Any new diabetes Rx | 12% (621) | 7% (314) | 12% (718) | 24% (945) | 42 (12%) |
| Hyperglycemia noted in written instructions | 4% (110) | 3% (19) | 15% (1021) | 24% (945) | 36 (10%) |
| Repeat glucose/diabetes testing charted | 5% (112) | 1% (06) | 9% (515) | 16% (536) | 23 (6%) |
No patient received intravenous fluids with dextrose prior to initial serum glucose determination, and there was no difference in home corticosteroid use between groups (P = 0.23). Patients with known diabetes were more likely to receive insulin in the ED (P 0.01). Only 1 patient received an oral hypoglycemic agent in the ED. Three patients had documented hypoglycemia on capillary blood glucose during the ED stay, and no patients had hypoglycemia based on serum glucose during the ED or hospital stay. Among hospitalized patients, 61% had inpatient orders for diabetic‐consistent/carbohydrate‐consistent diet, 65% for capillary glucose tests daily, and 63% for sliding scale insulin.
We also present written discharge instructions data for 363 visits (253 inpatient and 110 ED) in Table 2; discharge instructions were not available for 22 visits (12 deaths during hospitalization, 10 missing instructions). New antidiabetic medications were prescribed for 42 (12%) patients, all from the inpatient setting. There was no difference between inpatient and ED communication of hyperglycemia (10% [95%CI, 7%‐14%] versus 9% [95%CI, 4%‐15%]) and recommendation for further outpatient testing (8% [95%CI, 4%‐11%] versus 4% [95%CI, 0%‐7%]) in written discharge instructions (P = 0.73 and 0.16, respectively). Compared to those with glucose 140‐199 mg/dL, patients with glucose 200 mg/dL were more likely to receive written communication of hyperglycemia (17% [95%CI, 11%‐22%] versus 3% [95%CI, 0%‐6%]) and recommendation for further outpatient testing (10% [95%CI, 6%‐14%] versus 3% [95%CI, 0%‐5%] (both, P 0.01).
DISCUSSION
Although noncritical ED glucose values may be overlooked, values sufficient to motivate inpatient and long‐term management are sometimes uncovered, and when unrecognized may be missed opportunities. Indeed, admission hyperglycemia has been linked to poor clinical outcomes in hospitalized patients for a variety of conditions, particularly for myocardial infarction, stroke, and critical illness.1215
In this study, we evaluated recognition, communication, and management of ED glucose values above a relatively conservative threshold of 140 mg/dL, occurring in 21% of ED glucose results. Diabetes screening thresholds for casual glucose values as low as 120 mg/dL,9 and intensive glycemic control in critically ill patients to a target as low as 110 mg/dL have been suggested.5 Nevertheless, only 16% of our sample received insulin in the ED for hyperglycemia, and hyperglycemia was charted as a diagnosis in only 9% of cases.
This is especially important because 77% of ED visits without hyperglycemia charted as a diagnosis resulted in hospitalization, and early glycemic control was infrequently initiated. Limited ED management of hyperglycemia may be driven by the presence of more critical management issues (eg, 54% of patients had concomitant infection or trauma), lack of familiarity with guidelines, which suggest treatment to glucose 140 mg/dL in critically ill patients and 180 mg/dL in all hospitalized patients,16 or fear of adverse events, such as hypoglycemia. Additionally, ED crowding has been shown to effect decreased quality and timeliness of treatment for pneumonia, and may have similar effects for hyperglycemia.17 Inpatient recognition of hyperglycemia, based on orders for diet, glucose checks, and insulin, appeared significantly better, but this did not translate to improved communication in written discharge instructions. Additionally, many hospitalized patients may spend many hours, or even days, in the ED waiting for beds, which currently is a missed opportunity to initiate early therapy.
Written discharge instructions informed less than 10% of patients of their hyperglycemia or outlined a plan for further evaluation and management. Our prior work suggests that nearly all (95%) ED patients want to be informed of elevated blood glucose and are willing to follow‐up, if instructed.18 The current data suggests that hyperglycemia in ED and hospitalized patients is frequently unrecognized and undertreated, and opportunities to institute an outpatient plan to address hyperglycemia are frequently missed.
This study has several potential limitations. This study was performed at a single academic center, which limits generalizability to other geographic areas and hospital types. Accuracy of abstracted data depended on chart review, which is limited by the possibility of missing, incomplete, or unreliable information. Standardized definitions and abstraction forms limited potential for bias, and high interrater agreement demonstrated internal reliability of the chart review. We considered only initial glucose values and were unable to determine nutritional status; it is possible that subsequent measurements were within an acceptable range. Conversely, hospitalized patients may have developed hyperglycemia subsequent to the initial glucose result, which would underestimate the scope of inpatient hyperglycemia. Also, because there are limited data for interpretation of ED hyperglycemia, we were unable to determine optimal glucose thresholds. Finally, we were unable to evaluate the content of verbal instructions or letters to outpatient providers, which limited our ability to fully describe communication of abnormal findings. However, patients do not often retain information in verbal instructions, in the context of new diagnoses and complex medical regimens.
In summary, recognition, management, and communication of ED hyperglycemia were suboptimal in our patient population and represent a missed opportunity. Enhanced recognition, management, and referral for hyperglycemia observed during usual ED care may provide an unobtrusive method to improve identification of undiagnosed diabetes/prediabetes and initiation of intensive glycemic control for hospitalized patients.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,,,.Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.JAm Coll Cardiol.1995;26:57–65.
- ,,.National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary.Adv Data.2007;386:1–32.
- ,,, et al.Performance of recommended screening tests for undiagnosed diabetes and dysglycemia.Diabetes Care.2001;24:1899–1903.
- ,,, et al.The epidemiology of sepsis in the United States from 1979 through 2000.N Engl J Med.2003;348:1546–1554.
- ,,,,,.Completeness and accuracy of International Classification of Disease (ICD) external cause of injury codes in emergency department electronic data.Inj Prev.2007;13:422–425.
- ,,,.Stress hyperglycemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,, et al.Admission blood glucose level as a risk indicator of death after myocardial infarction in patients with and without diabetes mellitus.Arch Intern Med.2004;164:982–989.
- ,,, et al.Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke.Neurology.2002;59:67–71.
- .Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients.Mayo Clin Proc.2003;78:1471–1478.
- American Diabetes Association.Standards of Medical Care in Diabetes—2008.Diabetes Care.2008;31;S12–S54.
- ,,,.Effect of emergency department crowding on time to antibiotics in patients admitted with community‐acquired pneumonia.Ann Emerg Med.2007;50:501–509.
- ,,,,.Estimated risk for undiagnosed diabetes in the emergency department: a multicenter survey.Acad Emerg Med.2007;14:492–495.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,,,.Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.JAm Coll Cardiol.1995;26:57–65.
- ,,.National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary.Adv Data.2007;386:1–32.
- ,,, et al.Performance of recommended screening tests for undiagnosed diabetes and dysglycemia.Diabetes Care.2001;24:1899–1903.
- ,,, et al.The epidemiology of sepsis in the United States from 1979 through 2000.N Engl J Med.2003;348:1546–1554.
- ,,,,,.Completeness and accuracy of International Classification of Disease (ICD) external cause of injury codes in emergency department electronic data.Inj Prev.2007;13:422–425.
- ,,,.Stress hyperglycemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,, et al.Admission blood glucose level as a risk indicator of death after myocardial infarction in patients with and without diabetes mellitus.Arch Intern Med.2004;164:982–989.
- ,,, et al.Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke.Neurology.2002;59:67–71.
- .Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients.Mayo Clin Proc.2003;78:1471–1478.
- American Diabetes Association.Standards of Medical Care in Diabetes—2008.Diabetes Care.2008;31;S12–S54.
- ,,,.Effect of emergency department crowding on time to antibiotics in patients admitted with community‐acquired pneumonia.Ann Emerg Med.2007;50:501–509.
- ,,,,.Estimated risk for undiagnosed diabetes in the emergency department: a multicenter survey.Acad Emerg Med.2007;14:492–495.
Glycemic Control in Academic Hospitals
Hyperglycemia is a common occurrence in hospitalized patients, with and without a prior diagnosis of diabetes mellitus.13 Estimates of prevalence of diabetes mellitus in hospitalized adult patients range from 12% to 25%.4 Hyperglycemia is a strong predictor of adverse clinical outcome in a range of diseases such as acute stroke, congestive heart failure, community‐acquired pneumonia, and acute myocardial infarction.58 Hyperglycemia is also a risk factor for surgical infection in patients undergoing cardiac surgery.9, 10 A landmark prospective randomized controlled clinical trial by van den Berghe et al.11 demonstrated that tight glucose control (target blood glucose level 80110 mg/dL) with intravenous insulin in critically ill surgical patients led to dramatic reductions in acute renal failure, critical illness polyneuropathy, hospital mortality, and bloodstream infection. Other clinical studies have demonstrated that glycemic control with intravenous insulin improves clinical outcomes and reduces length of stay in patients with diabetes undergoing cardiac surgery.12, 13
Based upon these findings, the American College of Endocrinology (ACE) published recommendations in 2004 for hospital diabetes and metabolic control.14 Similar recommendations for hospital glycemic control have been included in the American Diabetes Association (ADA) guidelines since 2005.15 There is now emerging consensus that use of continuous insulin infusion given through a standardized protocol is the standard of care to control hyperglycemia in critically ill patients.1618 Likewise, use of specific hospital insulin regimens that include basal and short‐acting insulin with appropriate bedside glucose monitoring and avoiding use of sliding scale short‐acting insulin alone has become recognized as the most effective approach for glucose management in hospitalized patients not requiring intravenous insulin.4, 1921
The University HealthSystem Consortium (UHC) is an alliance of 97 academic health centers and 153 of their associated hospitals that conducts benchmarking studies on clinical and operational topics with member academic medical centers and develops new programs to improve quality of care, patient safety, and operational, clinical, and financial performance. In late 2004, UHC launched the Glycemic Control Benchmarking Project to determine the current status of glycemic control in adult patients admitted to academic medical centers, types of treatment employed to control glucose, and operational measures and practices of care for glycemic control in the hospital setting. The goal of the project was to describe contemporary glucose management for the purpose of identifying best practices. The information was later shared with each participating medical center to allow them to better align care delivery with ADA and ACE guidelines. Thirty‐seven academic medical centers agreed to participate and submit patient level data as well as an operational survey of current policies and practices for hospital glycemic control. This report summarizes the key findings from retrospective analyses of hospital and patient‐level data and describes contemporary management of hyperglycemia in academic medical centers.
PATIENTS AND METHODS
To be eligible for the study, hospital patients at each participating medical center had to be 18 years of age, have a 72‐hour or longer length of stay, and be admitted with 1 or more of the following Diagnostic‐related group (DRG) codes: 89 (simple pneumonia/ pleurisy), 109 (coronary artery bypass grafting without catheterization), 127 (heart failure and shock), 143 (chest pain), 209 (joint/limb procedure), 316 (renal failure), 478 (other vascular procedures), or 527 (percutaneous intervention with drug eluting stent without acute myocardial infarction). The DRG codes were selected from analysis of the UHC Clinical Data Base because they were the most common adult medical and surgical admission codes that included diabetes as a secondary diagnosis for academic medical centers and were believed to best represent the majority of hospital admissions. Each participating medical center received a secure electronic listing of their eligible patients discharged between July 1, 2004 and September 30, 2004 from the UHC Clinical Data Base. Each center identified data extractors who were trained via teleconference and received technical and content support by UHC staff. The data were collected by chart review and submitted electronically to UHC from February to April 2005.
For each medical center, patients were screened in reverse chronological order proceeding back in time until the minimum number of 50 eligible cases was obtained or until all potential cases were screened. Although 50 cases was the recommended minimum sample size per site, each medical center was encouraged to submit as many eligible cases as possible. The median number of cases submitted by site was 50 (interquartile range [IQR], 4251). Cases were entered into the study if they met the eligibility criteria and at least one of the following inclusion criteria: (1) two consecutive blood glucose readings >180 mg/dL within a 24hour period, or (2) insulin treatment at any time during the hospitalization. Exclusion criteria included history of pancreatic transplant, pregnancy at time of admission, hospice or palliative care during hospital admission, and patients who received insulin for a reason other than blood glucose control (ie, hyperkalemia). Early in the data collection, DRG 209 was dropped from potential screening due to the low yield of meeting screening criteria for blood glucose readings. Of the 315 cases screened for DRG 209 only 44 met all inclusion criteria and remain in the study population.
A maximum of 3 consecutive days of blood glucose (BG) readings were collected for each patient, referred to as measurement day 1, measurement day 2, and measurement day 3. Measurement day 1 is defined as the day the first of 2 consecutive blood glucose levels >180 mg/dL occurred during the hospitalization or as the first day insulin was administered during the hospitalization, whichever came first; 40.6% of patients had the day of admission as their first measurement day. Glucose measurements were recorded by hour for each measurement day as available, and if more than 1 glucose value was available within a particular hour, only the first result was recorded. Both bedside and laboratory serum glucose values were utilized, and glycosylated hemoglobin (A1C) values were included if they were recorded during the hospitalization or within 30 days prior to admission;22 95.7% of patients had BG results reported for all 3 measurement days. We defined estimated 6 AM glucose for each subject as: the 6 AM glucose if it was available; otherwise the average of the 5 AM and 7 AM glucose values if at least 1 of them was available; otherwise the average of the 4 AM and 8 AM glucose values if at least 1 of them was available. Relevant demographics, medical history, hospitalization details, type and route of insulin administration, and discharge data were also collected. For subcutaneous insulin administration, use of regular, lispro, or aspart insulin was classified as short‐acting insulin; use of neutral protamine Hagedorn (NPH), ultralente, or glargine insulin was classified as long‐acting insulin. For analysis of glycemic control measures, patient‐days in which location or glucose data were not recorded were excluded from analysis. For the analysis comparing subcutaneous versus intravenous insulin treatment on glucose control, patients who received a combination of therapy with subcutaneous and intravenous insulin on the same measurement day were excluded from the analysis (44 patients on day 1, 96 on day 2, and 47 on day 3). For this retrospective analysis, UHC provided a deidentified data set to the authors. The study protocol was reviewed by the Vanderbilt University Institutional Review Board and deemed to be nonhuman subject research since the data set contained no personal or institutional identifiers. Therefore, no informed consent of subjects was required.
Measures of glucose control (median glucose and estimated 6 AM glucose) were analyzed by patient‐day,23 and were compared by a Wilcoxon rank sum test or an analysis of variance, as indicated. P values <0.05 were considered significant. To compare effects of intravenous (IV) insulin, subcutaneous long‐acting short‐acting insulin, and subcutaneous short‐acting insulin use alone on glycemic control, mixed effects linear regression modeling for median glucose and mixed effects logistic regression modeling for hyperglycemia and hypoglycemia were used to adjust for fixed effects of age, gender, diabetes status, all patient refined diagnosis related groups (APR‐DRG) severity of illness score, outpatient diabetes treatment, patient location, admission diagnosis, and random effect of hospital site. Separate regression models were performed for measurement days 2 and 3. Statistical analyses were performed with Stata version 8 (Stata Corporation, College Station, TX), R version 2.1.0 (R Foundation for Statistical Computing, Vienna, Austria;
RESULTS
Thirty‐seven US academic medical centers from 24 states contributed to the analysis. A total of 4,367 cases meeting age, length of stay, and DRG criteria were screened for inclusion in the study; 2,649 (60.7%) screened cases were excluded due to failure to meet inclusion criteria (51%) or presence of exclusionary conditions (9.7%); 1,718 (39.3%) screened cases met all criteria and were included in this analysis. Patient characteristics are summarized in Table 1. A majority of patients (79%) had a documented history of diabetes, and most of these were classified as type 2 diabetes in the hospital record. Of the patients who were classified as having diabetes on admission, 50.8% were on some form of outpatient insulin therapy with or without oral diabetes agents. Patients with a diagnosis of diabetes had a median admission glucose of 158 mg/dL (IQR, 118221), which was significantly higher than the median admission glucose of 119 mg/dL (IQR, 100160) for patients without diabetes (P < 0.001, rank‐sum test).
| |
| n | 1718 |
| Age (years), median (IQR) | 65 (5674) |
| Male | 928 (54) |
| Female | 790 (46) |
| Admission glucose (mg/dL) | 149 (111207) |
| Race/Ethnicity | |
| White | 1048 (61.0) |
| Black | 480 (27.9) |
| Hispanic | 67 (3.9) |
| Other | 123 (7.2) |
| Diabetes history | 1358 (79.0) |
| Type 2 diabetes mellitus | 996 (58.0) |
| Type 1 diabetes mellitus | 128 (7.5) |
| Unspecified/other diabetes mellitus | 234 (13.6) |
| No history of diabetes mellitus | 360 (21.0) |
| Outpatient diabetes treatment | |
| Insulin only | 522 (30.4) |
| Oral agents only | 505 (29.4) |
| Insulin and oral agents | 168 (9.8) |
| No drug therapy | 137 (8.0) |
| Not documented | 26 (1.5) |
| Hospitalization DRG | |
| 127 Heart failure | 443 (25.8) |
| 109 Coronary artery bypass grafting | 389 (22.6) |
| 316 Renal failure | 251 (14.6) |
| 478 Other vascular procedure | 195 (11.4) |
| 89 Pneumonia | 186 (10.8) |
| 527 Percutaneous intervention with stent | 136 (7.9) |
| 143 Chest pain | 74 (4.3) |
| 209 Joint/limb procedure | 44 (2.6) |
| Primary insurer | |
| Medicare | 961 (56.0) |
| Private/commercial | 392 (22.8) |
| Medicaid | 200 (11.6) |
| Government | 88 (5.1) |
| Self‐pay | 67 (3.9) |
| Other/unknown | 10 (0.6) |
To determine overall glycemic control for the cohort, median glucose was calculated for each patient, stratified by diabetes status and location for each measurement day (Table 2). Patient‐days with a location of emergency department (96 patients on day 1, 6 on day 2, and 2 on day 3) and two patients whose location was not defined were excluded from the analysis. Overall, median glucose declined from measurement day 1 to day 3. For patients with diabetes, median glucose was significantly lower in the intensive care unit (ICU) compared to the general ward or intermediate care for measurement days 1 and 2, but not day 3. This difference was more pronounced in patients without diabetes, with median glucose significantly lower in the ICU for all 3 measurement days compared to other locations. As expected, median glucose was lower for patients without diabetes compared to patients with diabetes for all measurement days and locations. Hyperglycemia was common; 867 of 1,718 (50%) patients had at least 1 glucose measurement 180 mg/dL on both days 2 and 3; 18% of all patients had a median glucose 180 mg/dL on all 3 measurement days. Daily 6 AM glucose was the summary glycemic control measure in the clinical trial by van den Berghe et al.,11 with goal glucose of 80 to 110 mg/dL in the intensive treatment group. Since the glycemic target of the American College of Endocrinology Position Statement is <110 mg/dL (based largely on van den Berghe et al.11) we also calculated estimated 6 AM glucose for ICU patient‐days to determine the proportion of patients attaining this target.14 Estimated 6 AM glucose was lower in ICU patients without diabetes compared to those with diabetes. For patients with diabetes, only 20% of patients in the ICU had an estimated 6 AM glucose 110 mg/dL on measurement day 2, and only 24% on day 3. For patients without diabetes, 27% and 25% had an estimated 6 AM glucose 110 mg/dL on days 2 and 3, respectively.
| Measurement by Location | |||
|---|---|---|---|
| Day 1 | Day 2 | Day 3 | |
| |||
| Patients with diabetes | |||
| Estimated 6 AM glucose (mg/dL) | |||
| Intensive care unit | 153.0 (119.0204.0) | 148.0 (118.0183.0) | 144.0 (113.0191.0) |
| n | 167 | 231 | 161 |
| Median glucose (mg/dL) | |||
| General floor | 186.0 (151.0229.0) | 163.0 (131.0210.0) | 161.0 (127.0203.4) |
| n | 681 | 757 | 758 |
| Intermediate care | 193.0 (155.3233.8) | 170.0 (137.0215.5) | 169.0 (137.9215.6) |
| n | 291 | 333 | 348 |
| Intensive care unit | 177.5 (149.6213.6) | 152.5 (128.3187.0) | 156.5 (124.5194.3) |
| n | 294 | 247 | 175 |
| P value* | 0.038 | <0.001 | 0.068 |
| Patients without diabetes | |||
| Estimated 6 AM glucose (mg/dL) | |||
| Intensive care unit | 133.0 (104.5174.0) | 134.0 (109.0169.0) | 128.0 (111.5151.3) |
| n | 98 | 157 | 80 |
| Median glucose (mg/dL) | |||
| General floor | 179.0 (149.5209.5) | 161.3 (131.4188.3) | 143.5 (122.0170.0) |
| n | 91 | 96 | 133 |
| Intermediate care | 168.3 (138.1193.8) | 137.0 (119.8161.5) | 129.3 (116.3145.5) |
| n | 46 | 71 | 86 |
| Intensive care unit | 153.8 (132.9188.8) | 136.5 (120.0157.0) | 129.0 (116.0143.8) |
| n | 218 | 186 | 106 |
| P value* | <0.001 | <0.001 | <0.001 |
For the overall cohort, insulin was the most common treatment for hyperglycemia, with 84.6% of all patients receiving some form of insulin therapy on the second measurement day. On the second day, 30.8% received short‐acting subcutaneous insulin only, 8.2% received intravenous insulin infusion, 22.5% received both short‐acting and long‐acting subcutaneous insulin, 3.9% received oral agents, 23% received some combination of insulin therapies and/or oral agents, and 11.9% received no treatment. To determine the effect of intravenous versus subcutaneous insulin treatment on glycemic control, we compared patients by insulin treatment and location for each measurement day (Table 3). Intravenous insulin was used predominantly in the ICU, and was associated with significantly lower median glucose compared to subcutaneous insulin in both locations for all 3 measurement days. As expected, the average number of glucose measures per patient was significantly higher for those receiving intravenous insulin. Intravenous insulin use in the ICU was associated with a significantly lower number of patients with hyperglycemia, defined as the number who had 1 or more glucose values 180 mg/dL during a given measurement day. Of note, intravenous insulin use in the ICU was associated with a significantly higher proportion of patients who had hypoglycemia (defined as the number of patients who had one or more glucose values <70 mg/dL) compared to subcutaneous insulin only on measurement day 1 (8.1% versus 2.9%; P = 0.021), but not on days 2 (12.7% versus 8.0%; P > 0.05) or 3 (12.7% versus 7.8%; P > 0.05). Severe hypoglycemia, defined as a blood glucose recording <50 mg/dL,24 was rare, and occurred in only 2.8% of all patient days. On measurement day 1, 34 patients had a total of 49 severe hypoglycemic events; on day 2, 54 patients had 68 severe hypoglycemic events; on day 3, 54 patients had 68 severe hypoglycemic events. Only 3 patients had severe hypoglycemic events on all 3 measurement days. Analysis of severe hypoglycemia events stratified by intravenous versus subcutaneous insulin did not show any significant differences for any of the 3 measurement days (data not shown).
| Location/Day | Outcome | Intravenous Insulin | Subcutaneous Insulin | P Value* |
|---|---|---|---|---|
| ||||
| Intensive Care Unit, Day 1 | Patient's glucose, median (mg/dL) | 148.0 | 183.0 | <0.001 |
| Interquartile range | 128.0178.0 | 154.8211.0 | ||
| Hypoglycemic patients, n (%) | 16 (8.1) | 6 (2.9) | 0.021 | |
| Hyperglycemic patients, n (%) | 130 (66.0) | 175 (85.0) | <0.001 | |
| Average glucose measures/patient | 8.4 | 4.8 | <0.001 | |
| Patients, n | 197 | 206 | ||
| Intermediate/General Ward, Day 1 | Patient's glucose, median (mg/dL) | 152.0 | 186.5 | <0.001 |
| Interquartile range | 131.0164.5 | 150.0230.0 | ||
| Hypoglycemic patients, n (%) | 1 (4.1) | 71 (7.4) | ns | |
| Hyperglycemic patients, n (%) | 18 (78.3) | 808 (83.9) | ns | |
| Average glucose measures/patient | 9.7 | 3.8 | <0.001 | |
| Patients, n | 23 | 962 | ||
| Intensive Care Unit, Day 2 | Patient's glucose, median (mg/dL) | 124.8 | 159.8 | <0.001 |
| Interquartile range | 110.4140.5 | 138.6197.4 | ||
| Hypoglycemic patients, n (%) | 15 (12.7) | 14 (8.0) | ns | |
| Hyperglycemic patients, n (%) | 53 (44.9) | 135 (76.7) | <0.001 | |
| Average glucose measures/patient | 12.5 | 5.3 | <0.001 | |
| Patients, n | 118 | 176 | ||
| Intermediate/General Ward, Day 2 | Patient's glucose, median (mg/dL) | 136.0 | 168.8 | <0.001 |
| Interquartile range | 116.0168.0 | 136.1215.5 | ||
| Hypoglycemic patients, n (%) | 2 (6.7) | 113 (11.3) | ns | |
| Hyperglycemic patients, n (%) | 18 (60.0) | 784 (78.6) | 0.015 | |
| Average glucose measures/patient | 11.0 | 4.6 | <0.001 | |
| Patients, n | 30 | 996 | ||
| Intensive Care Unit, Day 3 | Patient's glucose, median (mg/dL) | 123.5 | 171.0 | <0.001 |
| Interquartile range | 110.0137.1 | 137.3198.5 | ||
| Hypoglycemic patients, n (%) | 7 (12.7) | 11 (7.8) | ns | |
| Hyperglycemic patients, n (%) | 24 (43.6) | 101 (71.1) | <0.001 | |
| Average glucose measures/patient | 11.4 | 4.8 | <0.001 | |
| Patients, n | 54 | 141 | ||
| Intermediate/General Ward, Day 3 | Patient's glucose, median (mg/dL) | 129.8 | 166.0 | <0.001 |
| Interquartile range | 120.5142.3 | 131.5208.0 | ||
| Hypoglycemic patients, n (%) | 3 (13.6) | 104 (9.8) | ns | |
| Hyperglycemic patients, n (%) | 13 (59.1) | 773 (72.7) | ns | |
| Average glucose measures/patient | 10.3 | 4.3 | <0.001 | |
| Patients, n | 22 | 1,055 | ||
We hypothesized that use of subcutaneous long‐acting (basal) insulin (with or without short‐acting insulin) would be associated with superior glucose control compared to use of subcutaneous short‐acting insulin (sliding scale and/or scheduled prandial insulin) alone. We performed an exploratory multivariate regression analysis to compare the effect of IV insulin, long acting subcutaneous insulin short acting insulin, or short acting subcutaneous insulin alone on median glucose, hyperglycemic events (glucose 180 mg/dL), and hypoglycemic events (glucose <70 mg/dL) for days 2 and 3 (Table 4). Compared to short‐acting subcutaneous insulin alone, use of IV insulin but not long‐acting subcutaneous insulin was predictive of lower median glucose for days 2 and 3. Use of long‐acting subcutaneous insulin was not associated with significantly lower odds of hyperglycemic events for days 2 and 3, but was associated with higher odds of hypoglycemic events on day 2 (odds ratio [OR], 1.8; P = 0.01) when compared to short‐acting subcutaneous insulin alone.
| Glucose Control Measure | Intravenous Insulin Infusion | Long‐Acting Subcutaneous Insulin |
|---|---|---|
| ||
| Median glucose | ||
| Day 2, n = 1,297 | 32.0 (45.4 to 18.5); P < 0.001* | 5.1 (13.8 to 3.6); P = 0.25* |
| Day 3, n = 1,251 | 33.0 (48.9 to 17); P < 0.001* | 3.4 (5.2 to 11.9); P = 0.44* |
| Patient has 1 hyperglycemic event | ||
| Day 2, n = 1,298 | 0.4 (0.20.6); P < 0.001 | 0.7 (0.51.1); P = 0.11 |
| Day 3, n = 1,261 | 0.6 (0.31.1); P = 0.11 | 0.8 (0.61.1); P = 0.24 |
| Patient has 1 hypoglycemic event | ||
| Day 2, n = 1,298 | 2.1 (1.04.7); P = 0.07 | 1.8 (1.22.9); P = 0.010 |
| Day 3, n = 1,261 | 4.0 (1.69.8); P = 0.003 | 1.4 (0.92.3); P = 0.13 |
We measured the performance of recommended hospital diabetes care practices (A1C assessment, documentation of diabetes history in the hospital record, admission laboratory glucose assessment, bedside glucose monitoring, recommended insulin therapy)14, 15 for all study patients, and also stratified performance by hospital (Table 5); 98.6% of all patients with a diagnosis of diabetes had physician documentation of their diabetes status recorded in the hospital record, and there was consistently high performance of this by hospital (Table 5); 77% of all patients with a history of diabetes had a laboratory blood glucose result recorded within 8 hours of hospital admission, and 81.3% of patients with a history of diabetes had blood glucose monitored at least 4 times on measurement day 2. Performance by hospital (Table 5) varied widely for glucose monitoring (range, 56.5%95.5% of patients by hospital) and admission laboratory glucose assessment (range, 39.0%97.1% of patients by hospital).
| Diabetes Care Measure | Mean Hospital Performance (%) | Standard Deviation (%) | Range (%) |
|---|---|---|---|
| |||
| Physician documentation of diabetes history in medical record | 98.8 | 2.1 | 91.5100 |
| A1C assessment documented for diabetes patients (measured during hospitalization or within 30 days prior to admission) | 33.7 | 15.4 | 3.162.9 |
| Laboratory glucose assessment within 8 hours of hospital presentation for diabetes patients | 77.0 | 13.4 | 39.097.1 |
| Blood glucose monitoring at least 4 times on second measurement day for diabetes patients | 81.6 | 10.8 | 56.595.5 |
| Percentage of patients receiving insulin therapy who were given short and long‐acting insulin OR IV insulin infusion OR insulin pump therapy on second measurement day | 44.9 | 14.3 | 12.176.5 |
Of all patients, 31% had A1C measurement recorded during their hospitalization or within 30 days prior to admission. There was wide variation in hospital performance of A1C assessment in patients with diabetes (Table 5). Patients with a diagnosis of diabetes had a median A1C of 7.4% (IQR, 6.4%8.9%; n = 473), and those without a diagnosis of diabetes had a median A1C of 5.9% (IQR, 5.6%6.4%; n = 70). Of the patients with a history of diabetes who had A1C recorded, 59% had a value >7%. Of the patients without a history of diabetes who had A1C recorded, 43% had a value >6.0%, suggesting previously undiagnosed diabetes.25
We found wide variation among hospitals (range, 12.1%76.5%) in use of recommended regimens of insulin therapy, defined as short‐acting and long‐acting subcutaneous insulin or IV insulin infusion or insulin pump therapy on second measurement day. Endocrine/diabetes consultation was infrequent, only 9% of all patients were evaluated by an endocrinologist or diabetologist at any time during the hospitalization.
DISCUSSION
In this retrospective analysis of hospitalized patients who had 2 consecutive blood glucose values 180 mg/dL and/or received insulin therapy, hyperglycemia was common and hypoglycemia was infrequent. Use of intravenous insulin was associated with better glucose control, and did not increase the frequency of severe hypoglycemic events (glucose <50 mg/dL). The majority of patients with a history of diabetes had physician documentation in the hospital chart, laboratory serum glucose obtained within 8 hours of hospital admission, and at least 4 blood glucose determinations on the second measurement day.
Only 35% of patients with diabetes had an A1C measurement and of these almost 60% had an A1C level >7%. Though the A1C may not greatly affect acute glucose management in the hospital setting, it does identify patients that may require intensification of diabetes therapy at hospital discharge and coordination of outpatient follow‐up. A report of a UHC clinical benchmarking project of ambulatory diabetes care in academic medical centers demonstrated high rates of diagnostic testing, but only 34% of patients were at the A1C goal, and only 40% of patients above the A1C goal had adjustment of their diabetes regimen at their last clinic visit.26 In a retrospective study of patients with diabetes mellitus admitted to an academic teaching hospital, only 20% of discharges indicated a plan for diabetes follow‐up.27 Thus, intensification of antihyperglycemic therapy and formulation of a diabetes follow‐up plan on hospital discharge in those patients with A1C >7% represents an opportunity to improve glycemic control in the ambulatory setting. Also, measurement of A1C can be used for diabetes case‐finding in hospitalized patients with hyperglycemia.25 Previously unrecognized diabetes is a common finding in patients admitted with cardiovascular disease. In a study of patients admitted with myocardial infarction, 25% were found to have previously undiagnosed diabetes.28 Hospital patients with hyperglycemia but without a prior diagnosis of diabetes who have an elevation of A1C >6.0% can be identified as at‐risk for diabetes and postdischarge glucose evaluation can be arranged.
The target of maintaining all glucose values 180 mg/dL recommended in the 20052007 American Diabetes Association guidelines for hospital diabetes management was not commonly achieved, with over 70% of patients who received subcutaneous insulin therapy having 1 or more glucose values >180 on all 3 measurement days, regardless of patient location.15 The target of maintaining critically ill patients as close to 110 mg/dL as possible was also difficult to achieve, with only 25% of ICU patients having an estimated 6 AM glucose <110 mg/dL on measurement day 3. A prospective cohort study of 107 inpatients with diabetes at Brigham and Women's Hospital showed a 76% prevalence of patients with at least one BG >180 mg/dL.29 In that study, 90% of patients had a sliding‐scale order, 36% received an oral diabetes agent, and 43% received basal insulin at some time during hospitalization. A recently published analysis by Wexler et al.30 compiled data of hospitalized patients with diabetes from an earlier 2003 UHC Diabetes Benchmarking Project (n = 274) and patients from 15 not‐for‐profit member hospitals of VHA, Incorporated (n = 725) to examine the prevalence of hyperglycemia and hypoglycemia. Hyperglycemia (defined as a single BG value >200 mg/dL) was common, occurring in 77% of patients in the UHC cohort and 76% in the VHA, Inc. cohort. This was comparable to our findings that 76.7% of ICU patients and 78.6% of ward patients treated with subcutaneous insulin had 1 or more BG values 180 mg/dL on measurement day 2. Wexler et al.30 also determined that use of basal insulin was associated with a higher prevalence of hyperglycemia and hypoglycemia in their study. Our regression analysis finding that long‐acting (basal) insulin use was not associated with improvement in glycemic control is consistent with the findings of the aforementioned study. There are a number of potential explanations for this: (1) underdosing of basal insulin or lack of adequate prandial insulin coverage for nutritional intake; (2) lack of effective titration in response to hyperglycemia; and (3) variation in the ordering and administration of basal insulin at different hospital sites.
Use of both manual and computerized IV insulin protocols has been shown to provide effective glucose control in critically ill patients.1618 Though intravenous insulin use was associated with better overall glucose control in our study; only about 50% of ICU patients received it on measurement day 1. A recent prospective randomized clinical trial demonstrated superior glycemic control in noncritically ill hospitalized patients with type 2 diabetes with basal/bolus insulin therapy compared to sliding scale insulin alone.31 Use of basal/bolus insulin regimens as part of a comprehensive hospital diabetes management program has been shown to improve glycemic control in an academic medical center.20 Therefore, we do not believe that our regression analysis findings invalidate the concept of basal/bolus insulin for inpatients with hyperglycemia, but rather indicate the need for more research into subcutaneous insulin regimens and hospital care practices that lead to improved glucose control. We found wide variation in hospital use of basal/bolus insulin regimens. Overall only 22.5% of all patients on the second measurement day received both short‐acting and long‐acting subcutaneous insulin, compared to 30.8% who received short‐acting subcutaneous insulin only. A recent consensus statement on inpatient glycemic control by the American College of Endocrinology and American Diabetes Association highlighted the systematic barriers to improved glycemic control in hospitals, such as inadequate knowledge of diabetes management techniques, fear of hypoglycemia, and skepticism about benefits of tighter glucose control.32
There are some important limitations to this study. The data are retrospective and only a limited number of hospital days and clinical variables could be assessed for each patient. As indicated in Table 3, there were significant differences in the frequency of glucose measurement depending on treatment, which can potentially bias estimated prevalence of hyperglycemia and hypoglycemia. We did not have a practical method to assess nutritional status or the adequacy of insulin dosing over time for each patient. We also could not assess the association of glycemic control on clinical outcomes such as hospital mortality or infection rates. Since this study was exclusively in academic medical centers, the generalization of findings to community‐based medical centers may be limited. The risk‐benefit of tight glycemic control in medical ICU patients based on clinical trial evidence has been unclear, and there is not broad agreement among clinicians on the recommended target for glycemic control in this group.3335 When we analyzed glycemic control in ICU patients we did not have a practical method to control for type of ICU and variations in individual ICU glycemic control targets. We recognize that the 2004 American College of Endocrinology recommendation of maintaining glucose 110 mg/dL may not be appropriate for all critically ill patients.14 Finally, clinical trial data are lacking on the effect of tight glucose control on major clinical outcomes for noncritically ill hospital patients. This has led to significant controversy regarding glycemic targets for different subgroups of hospitalized patients.34, 36
In summary, we found a high prevalence of persistent hyperglycemia in this large cohort of hospitalized patients, and hypoglycemia was infrequent. Use of IV insulin was associated with improvement in glycemic control, but was used in less than half of ICU patients. There was wide variation in hospital performance of recommended diabetes care measures. Opportunities to improve care in academic medical centers include expanded use of intravenous and subcutaneous basal/bolus insulin protocols and increased frequency of A1C testing.
- ,,, et al.Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke.Neurology.2002;59:67–71.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,,.Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,.Admission plasma glucose. Independent risk factor for long‐term prognosis after myocardial infarction even in nondiabetic patients.Diabetes Care.1999;22:1827–1831.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Modifiable risk factors associated with deep sternal site infection after coronary artery bypass grafting.J Thorac Cardiovasc Surg.2000;119:108–114.
- ,,,,.The association of diabetes and glucose control with surgical‐site infections among cardiothoracic surgery patients.Infect Control Hosp Epidemiol.2001;22:607–612.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109:1497–1502.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(Suppl 2):21–33.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10(Suppl 2):4–9.
- American Diabetes Association.Standards of medical care in diabetes.Diabetes Care.2005;28(Suppl 1):S4–S36.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27:461–467.
- ,,,,.Use of a computerized guideline for glucose regulation in the intensive care unit improved both guideline adherence and glucose regulation.J Am Med Inform Assoc.2005;12:172–180.
- ,,, et al.Computer‐based insulin infusion protocol improves glycemia control over manual protocol.J Am Med Inform Assoc.2007;14:278–287.
- ,,,,.Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding‐scale insulin therapy.Pharmacotherapy.2006;26:1421–1432.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,.70/30 insulin algorithm versus sliding scale insulin.Ann Pharmacother.2005;39:1606–1610.
- ,,,.Glucose measurement: confounding issues in setting targets for inpatient management.Diabetes Care.2007;30:403–409.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- American Diabetes Association.Hospital admission guidelines for diabetes.Diabetes Care.2004;27(Suppl 1):S103.
- ,,,,,,.Utility of HbA(1c) levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,.Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change.Diabetes Care.2005;28:337–442.
- ,,, et al.Diabetes care in the hospital: is there clinical inertia?J Hosp Med.2006;1:151–160.
- ,,, et al.Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study.Lancet.2002;359:2140–2144.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- The ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,.Tight blood glucose control with insulin in the ICU: facts and controversies.Chest.2007;132:268–278.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.NEngl J Med.2008;358:125–139.
- ,.Counterpoint: Inpatient glucose management: a premature call to arms?Diabetes Care.2005;28:976–979.
Hyperglycemia is a common occurrence in hospitalized patients, with and without a prior diagnosis of diabetes mellitus.13 Estimates of prevalence of diabetes mellitus in hospitalized adult patients range from 12% to 25%.4 Hyperglycemia is a strong predictor of adverse clinical outcome in a range of diseases such as acute stroke, congestive heart failure, community‐acquired pneumonia, and acute myocardial infarction.58 Hyperglycemia is also a risk factor for surgical infection in patients undergoing cardiac surgery.9, 10 A landmark prospective randomized controlled clinical trial by van den Berghe et al.11 demonstrated that tight glucose control (target blood glucose level 80110 mg/dL) with intravenous insulin in critically ill surgical patients led to dramatic reductions in acute renal failure, critical illness polyneuropathy, hospital mortality, and bloodstream infection. Other clinical studies have demonstrated that glycemic control with intravenous insulin improves clinical outcomes and reduces length of stay in patients with diabetes undergoing cardiac surgery.12, 13
Based upon these findings, the American College of Endocrinology (ACE) published recommendations in 2004 for hospital diabetes and metabolic control.14 Similar recommendations for hospital glycemic control have been included in the American Diabetes Association (ADA) guidelines since 2005.15 There is now emerging consensus that use of continuous insulin infusion given through a standardized protocol is the standard of care to control hyperglycemia in critically ill patients.1618 Likewise, use of specific hospital insulin regimens that include basal and short‐acting insulin with appropriate bedside glucose monitoring and avoiding use of sliding scale short‐acting insulin alone has become recognized as the most effective approach for glucose management in hospitalized patients not requiring intravenous insulin.4, 1921
The University HealthSystem Consortium (UHC) is an alliance of 97 academic health centers and 153 of their associated hospitals that conducts benchmarking studies on clinical and operational topics with member academic medical centers and develops new programs to improve quality of care, patient safety, and operational, clinical, and financial performance. In late 2004, UHC launched the Glycemic Control Benchmarking Project to determine the current status of glycemic control in adult patients admitted to academic medical centers, types of treatment employed to control glucose, and operational measures and practices of care for glycemic control in the hospital setting. The goal of the project was to describe contemporary glucose management for the purpose of identifying best practices. The information was later shared with each participating medical center to allow them to better align care delivery with ADA and ACE guidelines. Thirty‐seven academic medical centers agreed to participate and submit patient level data as well as an operational survey of current policies and practices for hospital glycemic control. This report summarizes the key findings from retrospective analyses of hospital and patient‐level data and describes contemporary management of hyperglycemia in academic medical centers.
PATIENTS AND METHODS
To be eligible for the study, hospital patients at each participating medical center had to be 18 years of age, have a 72‐hour or longer length of stay, and be admitted with 1 or more of the following Diagnostic‐related group (DRG) codes: 89 (simple pneumonia/ pleurisy), 109 (coronary artery bypass grafting without catheterization), 127 (heart failure and shock), 143 (chest pain), 209 (joint/limb procedure), 316 (renal failure), 478 (other vascular procedures), or 527 (percutaneous intervention with drug eluting stent without acute myocardial infarction). The DRG codes were selected from analysis of the UHC Clinical Data Base because they were the most common adult medical and surgical admission codes that included diabetes as a secondary diagnosis for academic medical centers and were believed to best represent the majority of hospital admissions. Each participating medical center received a secure electronic listing of their eligible patients discharged between July 1, 2004 and September 30, 2004 from the UHC Clinical Data Base. Each center identified data extractors who were trained via teleconference and received technical and content support by UHC staff. The data were collected by chart review and submitted electronically to UHC from February to April 2005.
For each medical center, patients were screened in reverse chronological order proceeding back in time until the minimum number of 50 eligible cases was obtained or until all potential cases were screened. Although 50 cases was the recommended minimum sample size per site, each medical center was encouraged to submit as many eligible cases as possible. The median number of cases submitted by site was 50 (interquartile range [IQR], 4251). Cases were entered into the study if they met the eligibility criteria and at least one of the following inclusion criteria: (1) two consecutive blood glucose readings >180 mg/dL within a 24hour period, or (2) insulin treatment at any time during the hospitalization. Exclusion criteria included history of pancreatic transplant, pregnancy at time of admission, hospice or palliative care during hospital admission, and patients who received insulin for a reason other than blood glucose control (ie, hyperkalemia). Early in the data collection, DRG 209 was dropped from potential screening due to the low yield of meeting screening criteria for blood glucose readings. Of the 315 cases screened for DRG 209 only 44 met all inclusion criteria and remain in the study population.
A maximum of 3 consecutive days of blood glucose (BG) readings were collected for each patient, referred to as measurement day 1, measurement day 2, and measurement day 3. Measurement day 1 is defined as the day the first of 2 consecutive blood glucose levels >180 mg/dL occurred during the hospitalization or as the first day insulin was administered during the hospitalization, whichever came first; 40.6% of patients had the day of admission as their first measurement day. Glucose measurements were recorded by hour for each measurement day as available, and if more than 1 glucose value was available within a particular hour, only the first result was recorded. Both bedside and laboratory serum glucose values were utilized, and glycosylated hemoglobin (A1C) values were included if they were recorded during the hospitalization or within 30 days prior to admission;22 95.7% of patients had BG results reported for all 3 measurement days. We defined estimated 6 AM glucose for each subject as: the 6 AM glucose if it was available; otherwise the average of the 5 AM and 7 AM glucose values if at least 1 of them was available; otherwise the average of the 4 AM and 8 AM glucose values if at least 1 of them was available. Relevant demographics, medical history, hospitalization details, type and route of insulin administration, and discharge data were also collected. For subcutaneous insulin administration, use of regular, lispro, or aspart insulin was classified as short‐acting insulin; use of neutral protamine Hagedorn (NPH), ultralente, or glargine insulin was classified as long‐acting insulin. For analysis of glycemic control measures, patient‐days in which location or glucose data were not recorded were excluded from analysis. For the analysis comparing subcutaneous versus intravenous insulin treatment on glucose control, patients who received a combination of therapy with subcutaneous and intravenous insulin on the same measurement day were excluded from the analysis (44 patients on day 1, 96 on day 2, and 47 on day 3). For this retrospective analysis, UHC provided a deidentified data set to the authors. The study protocol was reviewed by the Vanderbilt University Institutional Review Board and deemed to be nonhuman subject research since the data set contained no personal or institutional identifiers. Therefore, no informed consent of subjects was required.
Measures of glucose control (median glucose and estimated 6 AM glucose) were analyzed by patient‐day,23 and were compared by a Wilcoxon rank sum test or an analysis of variance, as indicated. P values <0.05 were considered significant. To compare effects of intravenous (IV) insulin, subcutaneous long‐acting short‐acting insulin, and subcutaneous short‐acting insulin use alone on glycemic control, mixed effects linear regression modeling for median glucose and mixed effects logistic regression modeling for hyperglycemia and hypoglycemia were used to adjust for fixed effects of age, gender, diabetes status, all patient refined diagnosis related groups (APR‐DRG) severity of illness score, outpatient diabetes treatment, patient location, admission diagnosis, and random effect of hospital site. Separate regression models were performed for measurement days 2 and 3. Statistical analyses were performed with Stata version 8 (Stata Corporation, College Station, TX), R version 2.1.0 (R Foundation for Statistical Computing, Vienna, Austria;
RESULTS
Thirty‐seven US academic medical centers from 24 states contributed to the analysis. A total of 4,367 cases meeting age, length of stay, and DRG criteria were screened for inclusion in the study; 2,649 (60.7%) screened cases were excluded due to failure to meet inclusion criteria (51%) or presence of exclusionary conditions (9.7%); 1,718 (39.3%) screened cases met all criteria and were included in this analysis. Patient characteristics are summarized in Table 1. A majority of patients (79%) had a documented history of diabetes, and most of these were classified as type 2 diabetes in the hospital record. Of the patients who were classified as having diabetes on admission, 50.8% were on some form of outpatient insulin therapy with or without oral diabetes agents. Patients with a diagnosis of diabetes had a median admission glucose of 158 mg/dL (IQR, 118221), which was significantly higher than the median admission glucose of 119 mg/dL (IQR, 100160) for patients without diabetes (P < 0.001, rank‐sum test).
| |
| n | 1718 |
| Age (years), median (IQR) | 65 (5674) |
| Male | 928 (54) |
| Female | 790 (46) |
| Admission glucose (mg/dL) | 149 (111207) |
| Race/Ethnicity | |
| White | 1048 (61.0) |
| Black | 480 (27.9) |
| Hispanic | 67 (3.9) |
| Other | 123 (7.2) |
| Diabetes history | 1358 (79.0) |
| Type 2 diabetes mellitus | 996 (58.0) |
| Type 1 diabetes mellitus | 128 (7.5) |
| Unspecified/other diabetes mellitus | 234 (13.6) |
| No history of diabetes mellitus | 360 (21.0) |
| Outpatient diabetes treatment | |
| Insulin only | 522 (30.4) |
| Oral agents only | 505 (29.4) |
| Insulin and oral agents | 168 (9.8) |
| No drug therapy | 137 (8.0) |
| Not documented | 26 (1.5) |
| Hospitalization DRG | |
| 127 Heart failure | 443 (25.8) |
| 109 Coronary artery bypass grafting | 389 (22.6) |
| 316 Renal failure | 251 (14.6) |
| 478 Other vascular procedure | 195 (11.4) |
| 89 Pneumonia | 186 (10.8) |
| 527 Percutaneous intervention with stent | 136 (7.9) |
| 143 Chest pain | 74 (4.3) |
| 209 Joint/limb procedure | 44 (2.6) |
| Primary insurer | |
| Medicare | 961 (56.0) |
| Private/commercial | 392 (22.8) |
| Medicaid | 200 (11.6) |
| Government | 88 (5.1) |
| Self‐pay | 67 (3.9) |
| Other/unknown | 10 (0.6) |
To determine overall glycemic control for the cohort, median glucose was calculated for each patient, stratified by diabetes status and location for each measurement day (Table 2). Patient‐days with a location of emergency department (96 patients on day 1, 6 on day 2, and 2 on day 3) and two patients whose location was not defined were excluded from the analysis. Overall, median glucose declined from measurement day 1 to day 3. For patients with diabetes, median glucose was significantly lower in the intensive care unit (ICU) compared to the general ward or intermediate care for measurement days 1 and 2, but not day 3. This difference was more pronounced in patients without diabetes, with median glucose significantly lower in the ICU for all 3 measurement days compared to other locations. As expected, median glucose was lower for patients without diabetes compared to patients with diabetes for all measurement days and locations. Hyperglycemia was common; 867 of 1,718 (50%) patients had at least 1 glucose measurement 180 mg/dL on both days 2 and 3; 18% of all patients had a median glucose 180 mg/dL on all 3 measurement days. Daily 6 AM glucose was the summary glycemic control measure in the clinical trial by van den Berghe et al.,11 with goal glucose of 80 to 110 mg/dL in the intensive treatment group. Since the glycemic target of the American College of Endocrinology Position Statement is <110 mg/dL (based largely on van den Berghe et al.11) we also calculated estimated 6 AM glucose for ICU patient‐days to determine the proportion of patients attaining this target.14 Estimated 6 AM glucose was lower in ICU patients without diabetes compared to those with diabetes. For patients with diabetes, only 20% of patients in the ICU had an estimated 6 AM glucose 110 mg/dL on measurement day 2, and only 24% on day 3. For patients without diabetes, 27% and 25% had an estimated 6 AM glucose 110 mg/dL on days 2 and 3, respectively.
| Measurement by Location | |||
|---|---|---|---|
| Day 1 | Day 2 | Day 3 | |
| |||
| Patients with diabetes | |||
| Estimated 6 AM glucose (mg/dL) | |||
| Intensive care unit | 153.0 (119.0204.0) | 148.0 (118.0183.0) | 144.0 (113.0191.0) |
| n | 167 | 231 | 161 |
| Median glucose (mg/dL) | |||
| General floor | 186.0 (151.0229.0) | 163.0 (131.0210.0) | 161.0 (127.0203.4) |
| n | 681 | 757 | 758 |
| Intermediate care | 193.0 (155.3233.8) | 170.0 (137.0215.5) | 169.0 (137.9215.6) |
| n | 291 | 333 | 348 |
| Intensive care unit | 177.5 (149.6213.6) | 152.5 (128.3187.0) | 156.5 (124.5194.3) |
| n | 294 | 247 | 175 |
| P value* | 0.038 | <0.001 | 0.068 |
| Patients without diabetes | |||
| Estimated 6 AM glucose (mg/dL) | |||
| Intensive care unit | 133.0 (104.5174.0) | 134.0 (109.0169.0) | 128.0 (111.5151.3) |
| n | 98 | 157 | 80 |
| Median glucose (mg/dL) | |||
| General floor | 179.0 (149.5209.5) | 161.3 (131.4188.3) | 143.5 (122.0170.0) |
| n | 91 | 96 | 133 |
| Intermediate care | 168.3 (138.1193.8) | 137.0 (119.8161.5) | 129.3 (116.3145.5) |
| n | 46 | 71 | 86 |
| Intensive care unit | 153.8 (132.9188.8) | 136.5 (120.0157.0) | 129.0 (116.0143.8) |
| n | 218 | 186 | 106 |
| P value* | <0.001 | <0.001 | <0.001 |
For the overall cohort, insulin was the most common treatment for hyperglycemia, with 84.6% of all patients receiving some form of insulin therapy on the second measurement day. On the second day, 30.8% received short‐acting subcutaneous insulin only, 8.2% received intravenous insulin infusion, 22.5% received both short‐acting and long‐acting subcutaneous insulin, 3.9% received oral agents, 23% received some combination of insulin therapies and/or oral agents, and 11.9% received no treatment. To determine the effect of intravenous versus subcutaneous insulin treatment on glycemic control, we compared patients by insulin treatment and location for each measurement day (Table 3). Intravenous insulin was used predominantly in the ICU, and was associated with significantly lower median glucose compared to subcutaneous insulin in both locations for all 3 measurement days. As expected, the average number of glucose measures per patient was significantly higher for those receiving intravenous insulin. Intravenous insulin use in the ICU was associated with a significantly lower number of patients with hyperglycemia, defined as the number who had 1 or more glucose values 180 mg/dL during a given measurement day. Of note, intravenous insulin use in the ICU was associated with a significantly higher proportion of patients who had hypoglycemia (defined as the number of patients who had one or more glucose values <70 mg/dL) compared to subcutaneous insulin only on measurement day 1 (8.1% versus 2.9%; P = 0.021), but not on days 2 (12.7% versus 8.0%; P > 0.05) or 3 (12.7% versus 7.8%; P > 0.05). Severe hypoglycemia, defined as a blood glucose recording <50 mg/dL,24 was rare, and occurred in only 2.8% of all patient days. On measurement day 1, 34 patients had a total of 49 severe hypoglycemic events; on day 2, 54 patients had 68 severe hypoglycemic events; on day 3, 54 patients had 68 severe hypoglycemic events. Only 3 patients had severe hypoglycemic events on all 3 measurement days. Analysis of severe hypoglycemia events stratified by intravenous versus subcutaneous insulin did not show any significant differences for any of the 3 measurement days (data not shown).
| Location/Day | Outcome | Intravenous Insulin | Subcutaneous Insulin | P Value* |
|---|---|---|---|---|
| ||||
| Intensive Care Unit, Day 1 | Patient's glucose, median (mg/dL) | 148.0 | 183.0 | <0.001 |
| Interquartile range | 128.0178.0 | 154.8211.0 | ||
| Hypoglycemic patients, n (%) | 16 (8.1) | 6 (2.9) | 0.021 | |
| Hyperglycemic patients, n (%) | 130 (66.0) | 175 (85.0) | <0.001 | |
| Average glucose measures/patient | 8.4 | 4.8 | <0.001 | |
| Patients, n | 197 | 206 | ||
| Intermediate/General Ward, Day 1 | Patient's glucose, median (mg/dL) | 152.0 | 186.5 | <0.001 |
| Interquartile range | 131.0164.5 | 150.0230.0 | ||
| Hypoglycemic patients, n (%) | 1 (4.1) | 71 (7.4) | ns | |
| Hyperglycemic patients, n (%) | 18 (78.3) | 808 (83.9) | ns | |
| Average glucose measures/patient | 9.7 | 3.8 | <0.001 | |
| Patients, n | 23 | 962 | ||
| Intensive Care Unit, Day 2 | Patient's glucose, median (mg/dL) | 124.8 | 159.8 | <0.001 |
| Interquartile range | 110.4140.5 | 138.6197.4 | ||
| Hypoglycemic patients, n (%) | 15 (12.7) | 14 (8.0) | ns | |
| Hyperglycemic patients, n (%) | 53 (44.9) | 135 (76.7) | <0.001 | |
| Average glucose measures/patient | 12.5 | 5.3 | <0.001 | |
| Patients, n | 118 | 176 | ||
| Intermediate/General Ward, Day 2 | Patient's glucose, median (mg/dL) | 136.0 | 168.8 | <0.001 |
| Interquartile range | 116.0168.0 | 136.1215.5 | ||
| Hypoglycemic patients, n (%) | 2 (6.7) | 113 (11.3) | ns | |
| Hyperglycemic patients, n (%) | 18 (60.0) | 784 (78.6) | 0.015 | |
| Average glucose measures/patient | 11.0 | 4.6 | <0.001 | |
| Patients, n | 30 | 996 | ||
| Intensive Care Unit, Day 3 | Patient's glucose, median (mg/dL) | 123.5 | 171.0 | <0.001 |
| Interquartile range | 110.0137.1 | 137.3198.5 | ||
| Hypoglycemic patients, n (%) | 7 (12.7) | 11 (7.8) | ns | |
| Hyperglycemic patients, n (%) | 24 (43.6) | 101 (71.1) | <0.001 | |
| Average glucose measures/patient | 11.4 | 4.8 | <0.001 | |
| Patients, n | 54 | 141 | ||
| Intermediate/General Ward, Day 3 | Patient's glucose, median (mg/dL) | 129.8 | 166.0 | <0.001 |
| Interquartile range | 120.5142.3 | 131.5208.0 | ||
| Hypoglycemic patients, n (%) | 3 (13.6) | 104 (9.8) | ns | |
| Hyperglycemic patients, n (%) | 13 (59.1) | 773 (72.7) | ns | |
| Average glucose measures/patient | 10.3 | 4.3 | <0.001 | |
| Patients, n | 22 | 1,055 | ||
We hypothesized that use of subcutaneous long‐acting (basal) insulin (with or without short‐acting insulin) would be associated with superior glucose control compared to use of subcutaneous short‐acting insulin (sliding scale and/or scheduled prandial insulin) alone. We performed an exploratory multivariate regression analysis to compare the effect of IV insulin, long acting subcutaneous insulin short acting insulin, or short acting subcutaneous insulin alone on median glucose, hyperglycemic events (glucose 180 mg/dL), and hypoglycemic events (glucose <70 mg/dL) for days 2 and 3 (Table 4). Compared to short‐acting subcutaneous insulin alone, use of IV insulin but not long‐acting subcutaneous insulin was predictive of lower median glucose for days 2 and 3. Use of long‐acting subcutaneous insulin was not associated with significantly lower odds of hyperglycemic events for days 2 and 3, but was associated with higher odds of hypoglycemic events on day 2 (odds ratio [OR], 1.8; P = 0.01) when compared to short‐acting subcutaneous insulin alone.
| Glucose Control Measure | Intravenous Insulin Infusion | Long‐Acting Subcutaneous Insulin |
|---|---|---|
| ||
| Median glucose | ||
| Day 2, n = 1,297 | 32.0 (45.4 to 18.5); P < 0.001* | 5.1 (13.8 to 3.6); P = 0.25* |
| Day 3, n = 1,251 | 33.0 (48.9 to 17); P < 0.001* | 3.4 (5.2 to 11.9); P = 0.44* |
| Patient has 1 hyperglycemic event | ||
| Day 2, n = 1,298 | 0.4 (0.20.6); P < 0.001 | 0.7 (0.51.1); P = 0.11 |
| Day 3, n = 1,261 | 0.6 (0.31.1); P = 0.11 | 0.8 (0.61.1); P = 0.24 |
| Patient has 1 hypoglycemic event | ||
| Day 2, n = 1,298 | 2.1 (1.04.7); P = 0.07 | 1.8 (1.22.9); P = 0.010 |
| Day 3, n = 1,261 | 4.0 (1.69.8); P = 0.003 | 1.4 (0.92.3); P = 0.13 |
We measured the performance of recommended hospital diabetes care practices (A1C assessment, documentation of diabetes history in the hospital record, admission laboratory glucose assessment, bedside glucose monitoring, recommended insulin therapy)14, 15 for all study patients, and also stratified performance by hospital (Table 5); 98.6% of all patients with a diagnosis of diabetes had physician documentation of their diabetes status recorded in the hospital record, and there was consistently high performance of this by hospital (Table 5); 77% of all patients with a history of diabetes had a laboratory blood glucose result recorded within 8 hours of hospital admission, and 81.3% of patients with a history of diabetes had blood glucose monitored at least 4 times on measurement day 2. Performance by hospital (Table 5) varied widely for glucose monitoring (range, 56.5%95.5% of patients by hospital) and admission laboratory glucose assessment (range, 39.0%97.1% of patients by hospital).
| Diabetes Care Measure | Mean Hospital Performance (%) | Standard Deviation (%) | Range (%) |
|---|---|---|---|
| |||
| Physician documentation of diabetes history in medical record | 98.8 | 2.1 | 91.5100 |
| A1C assessment documented for diabetes patients (measured during hospitalization or within 30 days prior to admission) | 33.7 | 15.4 | 3.162.9 |
| Laboratory glucose assessment within 8 hours of hospital presentation for diabetes patients | 77.0 | 13.4 | 39.097.1 |
| Blood glucose monitoring at least 4 times on second measurement day for diabetes patients | 81.6 | 10.8 | 56.595.5 |
| Percentage of patients receiving insulin therapy who were given short and long‐acting insulin OR IV insulin infusion OR insulin pump therapy on second measurement day | 44.9 | 14.3 | 12.176.5 |
Of all patients, 31% had A1C measurement recorded during their hospitalization or within 30 days prior to admission. There was wide variation in hospital performance of A1C assessment in patients with diabetes (Table 5). Patients with a diagnosis of diabetes had a median A1C of 7.4% (IQR, 6.4%8.9%; n = 473), and those without a diagnosis of diabetes had a median A1C of 5.9% (IQR, 5.6%6.4%; n = 70). Of the patients with a history of diabetes who had A1C recorded, 59% had a value >7%. Of the patients without a history of diabetes who had A1C recorded, 43% had a value >6.0%, suggesting previously undiagnosed diabetes.25
We found wide variation among hospitals (range, 12.1%76.5%) in use of recommended regimens of insulin therapy, defined as short‐acting and long‐acting subcutaneous insulin or IV insulin infusion or insulin pump therapy on second measurement day. Endocrine/diabetes consultation was infrequent, only 9% of all patients were evaluated by an endocrinologist or diabetologist at any time during the hospitalization.
DISCUSSION
In this retrospective analysis of hospitalized patients who had 2 consecutive blood glucose values 180 mg/dL and/or received insulin therapy, hyperglycemia was common and hypoglycemia was infrequent. Use of intravenous insulin was associated with better glucose control, and did not increase the frequency of severe hypoglycemic events (glucose <50 mg/dL). The majority of patients with a history of diabetes had physician documentation in the hospital chart, laboratory serum glucose obtained within 8 hours of hospital admission, and at least 4 blood glucose determinations on the second measurement day.
Only 35% of patients with diabetes had an A1C measurement and of these almost 60% had an A1C level >7%. Though the A1C may not greatly affect acute glucose management in the hospital setting, it does identify patients that may require intensification of diabetes therapy at hospital discharge and coordination of outpatient follow‐up. A report of a UHC clinical benchmarking project of ambulatory diabetes care in academic medical centers demonstrated high rates of diagnostic testing, but only 34% of patients were at the A1C goal, and only 40% of patients above the A1C goal had adjustment of their diabetes regimen at their last clinic visit.26 In a retrospective study of patients with diabetes mellitus admitted to an academic teaching hospital, only 20% of discharges indicated a plan for diabetes follow‐up.27 Thus, intensification of antihyperglycemic therapy and formulation of a diabetes follow‐up plan on hospital discharge in those patients with A1C >7% represents an opportunity to improve glycemic control in the ambulatory setting. Also, measurement of A1C can be used for diabetes case‐finding in hospitalized patients with hyperglycemia.25 Previously unrecognized diabetes is a common finding in patients admitted with cardiovascular disease. In a study of patients admitted with myocardial infarction, 25% were found to have previously undiagnosed diabetes.28 Hospital patients with hyperglycemia but without a prior diagnosis of diabetes who have an elevation of A1C >6.0% can be identified as at‐risk for diabetes and postdischarge glucose evaluation can be arranged.
The target of maintaining all glucose values 180 mg/dL recommended in the 20052007 American Diabetes Association guidelines for hospital diabetes management was not commonly achieved, with over 70% of patients who received subcutaneous insulin therapy having 1 or more glucose values >180 on all 3 measurement days, regardless of patient location.15 The target of maintaining critically ill patients as close to 110 mg/dL as possible was also difficult to achieve, with only 25% of ICU patients having an estimated 6 AM glucose <110 mg/dL on measurement day 3. A prospective cohort study of 107 inpatients with diabetes at Brigham and Women's Hospital showed a 76% prevalence of patients with at least one BG >180 mg/dL.29 In that study, 90% of patients had a sliding‐scale order, 36% received an oral diabetes agent, and 43% received basal insulin at some time during hospitalization. A recently published analysis by Wexler et al.30 compiled data of hospitalized patients with diabetes from an earlier 2003 UHC Diabetes Benchmarking Project (n = 274) and patients from 15 not‐for‐profit member hospitals of VHA, Incorporated (n = 725) to examine the prevalence of hyperglycemia and hypoglycemia. Hyperglycemia (defined as a single BG value >200 mg/dL) was common, occurring in 77% of patients in the UHC cohort and 76% in the VHA, Inc. cohort. This was comparable to our findings that 76.7% of ICU patients and 78.6% of ward patients treated with subcutaneous insulin had 1 or more BG values 180 mg/dL on measurement day 2. Wexler et al.30 also determined that use of basal insulin was associated with a higher prevalence of hyperglycemia and hypoglycemia in their study. Our regression analysis finding that long‐acting (basal) insulin use was not associated with improvement in glycemic control is consistent with the findings of the aforementioned study. There are a number of potential explanations for this: (1) underdosing of basal insulin or lack of adequate prandial insulin coverage for nutritional intake; (2) lack of effective titration in response to hyperglycemia; and (3) variation in the ordering and administration of basal insulin at different hospital sites.
Use of both manual and computerized IV insulin protocols has been shown to provide effective glucose control in critically ill patients.1618 Though intravenous insulin use was associated with better overall glucose control in our study; only about 50% of ICU patients received it on measurement day 1. A recent prospective randomized clinical trial demonstrated superior glycemic control in noncritically ill hospitalized patients with type 2 diabetes with basal/bolus insulin therapy compared to sliding scale insulin alone.31 Use of basal/bolus insulin regimens as part of a comprehensive hospital diabetes management program has been shown to improve glycemic control in an academic medical center.20 Therefore, we do not believe that our regression analysis findings invalidate the concept of basal/bolus insulin for inpatients with hyperglycemia, but rather indicate the need for more research into subcutaneous insulin regimens and hospital care practices that lead to improved glucose control. We found wide variation in hospital use of basal/bolus insulin regimens. Overall only 22.5% of all patients on the second measurement day received both short‐acting and long‐acting subcutaneous insulin, compared to 30.8% who received short‐acting subcutaneous insulin only. A recent consensus statement on inpatient glycemic control by the American College of Endocrinology and American Diabetes Association highlighted the systematic barriers to improved glycemic control in hospitals, such as inadequate knowledge of diabetes management techniques, fear of hypoglycemia, and skepticism about benefits of tighter glucose control.32
There are some important limitations to this study. The data are retrospective and only a limited number of hospital days and clinical variables could be assessed for each patient. As indicated in Table 3, there were significant differences in the frequency of glucose measurement depending on treatment, which can potentially bias estimated prevalence of hyperglycemia and hypoglycemia. We did not have a practical method to assess nutritional status or the adequacy of insulin dosing over time for each patient. We also could not assess the association of glycemic control on clinical outcomes such as hospital mortality or infection rates. Since this study was exclusively in academic medical centers, the generalization of findings to community‐based medical centers may be limited. The risk‐benefit of tight glycemic control in medical ICU patients based on clinical trial evidence has been unclear, and there is not broad agreement among clinicians on the recommended target for glycemic control in this group.3335 When we analyzed glycemic control in ICU patients we did not have a practical method to control for type of ICU and variations in individual ICU glycemic control targets. We recognize that the 2004 American College of Endocrinology recommendation of maintaining glucose 110 mg/dL may not be appropriate for all critically ill patients.14 Finally, clinical trial data are lacking on the effect of tight glucose control on major clinical outcomes for noncritically ill hospital patients. This has led to significant controversy regarding glycemic targets for different subgroups of hospitalized patients.34, 36
In summary, we found a high prevalence of persistent hyperglycemia in this large cohort of hospitalized patients, and hypoglycemia was infrequent. Use of IV insulin was associated with improvement in glycemic control, but was used in less than half of ICU patients. There was wide variation in hospital performance of recommended diabetes care measures. Opportunities to improve care in academic medical centers include expanded use of intravenous and subcutaneous basal/bolus insulin protocols and increased frequency of A1C testing.
Hyperglycemia is a common occurrence in hospitalized patients, with and without a prior diagnosis of diabetes mellitus.13 Estimates of prevalence of diabetes mellitus in hospitalized adult patients range from 12% to 25%.4 Hyperglycemia is a strong predictor of adverse clinical outcome in a range of diseases such as acute stroke, congestive heart failure, community‐acquired pneumonia, and acute myocardial infarction.58 Hyperglycemia is also a risk factor for surgical infection in patients undergoing cardiac surgery.9, 10 A landmark prospective randomized controlled clinical trial by van den Berghe et al.11 demonstrated that tight glucose control (target blood glucose level 80110 mg/dL) with intravenous insulin in critically ill surgical patients led to dramatic reductions in acute renal failure, critical illness polyneuropathy, hospital mortality, and bloodstream infection. Other clinical studies have demonstrated that glycemic control with intravenous insulin improves clinical outcomes and reduces length of stay in patients with diabetes undergoing cardiac surgery.12, 13
Based upon these findings, the American College of Endocrinology (ACE) published recommendations in 2004 for hospital diabetes and metabolic control.14 Similar recommendations for hospital glycemic control have been included in the American Diabetes Association (ADA) guidelines since 2005.15 There is now emerging consensus that use of continuous insulin infusion given through a standardized protocol is the standard of care to control hyperglycemia in critically ill patients.1618 Likewise, use of specific hospital insulin regimens that include basal and short‐acting insulin with appropriate bedside glucose monitoring and avoiding use of sliding scale short‐acting insulin alone has become recognized as the most effective approach for glucose management in hospitalized patients not requiring intravenous insulin.4, 1921
The University HealthSystem Consortium (UHC) is an alliance of 97 academic health centers and 153 of their associated hospitals that conducts benchmarking studies on clinical and operational topics with member academic medical centers and develops new programs to improve quality of care, patient safety, and operational, clinical, and financial performance. In late 2004, UHC launched the Glycemic Control Benchmarking Project to determine the current status of glycemic control in adult patients admitted to academic medical centers, types of treatment employed to control glucose, and operational measures and practices of care for glycemic control in the hospital setting. The goal of the project was to describe contemporary glucose management for the purpose of identifying best practices. The information was later shared with each participating medical center to allow them to better align care delivery with ADA and ACE guidelines. Thirty‐seven academic medical centers agreed to participate and submit patient level data as well as an operational survey of current policies and practices for hospital glycemic control. This report summarizes the key findings from retrospective analyses of hospital and patient‐level data and describes contemporary management of hyperglycemia in academic medical centers.
PATIENTS AND METHODS
To be eligible for the study, hospital patients at each participating medical center had to be 18 years of age, have a 72‐hour or longer length of stay, and be admitted with 1 or more of the following Diagnostic‐related group (DRG) codes: 89 (simple pneumonia/ pleurisy), 109 (coronary artery bypass grafting without catheterization), 127 (heart failure and shock), 143 (chest pain), 209 (joint/limb procedure), 316 (renal failure), 478 (other vascular procedures), or 527 (percutaneous intervention with drug eluting stent without acute myocardial infarction). The DRG codes were selected from analysis of the UHC Clinical Data Base because they were the most common adult medical and surgical admission codes that included diabetes as a secondary diagnosis for academic medical centers and were believed to best represent the majority of hospital admissions. Each participating medical center received a secure electronic listing of their eligible patients discharged between July 1, 2004 and September 30, 2004 from the UHC Clinical Data Base. Each center identified data extractors who were trained via teleconference and received technical and content support by UHC staff. The data were collected by chart review and submitted electronically to UHC from February to April 2005.
For each medical center, patients were screened in reverse chronological order proceeding back in time until the minimum number of 50 eligible cases was obtained or until all potential cases were screened. Although 50 cases was the recommended minimum sample size per site, each medical center was encouraged to submit as many eligible cases as possible. The median number of cases submitted by site was 50 (interquartile range [IQR], 4251). Cases were entered into the study if they met the eligibility criteria and at least one of the following inclusion criteria: (1) two consecutive blood glucose readings >180 mg/dL within a 24hour period, or (2) insulin treatment at any time during the hospitalization. Exclusion criteria included history of pancreatic transplant, pregnancy at time of admission, hospice or palliative care during hospital admission, and patients who received insulin for a reason other than blood glucose control (ie, hyperkalemia). Early in the data collection, DRG 209 was dropped from potential screening due to the low yield of meeting screening criteria for blood glucose readings. Of the 315 cases screened for DRG 209 only 44 met all inclusion criteria and remain in the study population.
A maximum of 3 consecutive days of blood glucose (BG) readings were collected for each patient, referred to as measurement day 1, measurement day 2, and measurement day 3. Measurement day 1 is defined as the day the first of 2 consecutive blood glucose levels >180 mg/dL occurred during the hospitalization or as the first day insulin was administered during the hospitalization, whichever came first; 40.6% of patients had the day of admission as their first measurement day. Glucose measurements were recorded by hour for each measurement day as available, and if more than 1 glucose value was available within a particular hour, only the first result was recorded. Both bedside and laboratory serum glucose values were utilized, and glycosylated hemoglobin (A1C) values were included if they were recorded during the hospitalization or within 30 days prior to admission;22 95.7% of patients had BG results reported for all 3 measurement days. We defined estimated 6 AM glucose for each subject as: the 6 AM glucose if it was available; otherwise the average of the 5 AM and 7 AM glucose values if at least 1 of them was available; otherwise the average of the 4 AM and 8 AM glucose values if at least 1 of them was available. Relevant demographics, medical history, hospitalization details, type and route of insulin administration, and discharge data were also collected. For subcutaneous insulin administration, use of regular, lispro, or aspart insulin was classified as short‐acting insulin; use of neutral protamine Hagedorn (NPH), ultralente, or glargine insulin was classified as long‐acting insulin. For analysis of glycemic control measures, patient‐days in which location or glucose data were not recorded were excluded from analysis. For the analysis comparing subcutaneous versus intravenous insulin treatment on glucose control, patients who received a combination of therapy with subcutaneous and intravenous insulin on the same measurement day were excluded from the analysis (44 patients on day 1, 96 on day 2, and 47 on day 3). For this retrospective analysis, UHC provided a deidentified data set to the authors. The study protocol was reviewed by the Vanderbilt University Institutional Review Board and deemed to be nonhuman subject research since the data set contained no personal or institutional identifiers. Therefore, no informed consent of subjects was required.
Measures of glucose control (median glucose and estimated 6 AM glucose) were analyzed by patient‐day,23 and were compared by a Wilcoxon rank sum test or an analysis of variance, as indicated. P values <0.05 were considered significant. To compare effects of intravenous (IV) insulin, subcutaneous long‐acting short‐acting insulin, and subcutaneous short‐acting insulin use alone on glycemic control, mixed effects linear regression modeling for median glucose and mixed effects logistic regression modeling for hyperglycemia and hypoglycemia were used to adjust for fixed effects of age, gender, diabetes status, all patient refined diagnosis related groups (APR‐DRG) severity of illness score, outpatient diabetes treatment, patient location, admission diagnosis, and random effect of hospital site. Separate regression models were performed for measurement days 2 and 3. Statistical analyses were performed with Stata version 8 (Stata Corporation, College Station, TX), R version 2.1.0 (R Foundation for Statistical Computing, Vienna, Austria;
RESULTS
Thirty‐seven US academic medical centers from 24 states contributed to the analysis. A total of 4,367 cases meeting age, length of stay, and DRG criteria were screened for inclusion in the study; 2,649 (60.7%) screened cases were excluded due to failure to meet inclusion criteria (51%) or presence of exclusionary conditions (9.7%); 1,718 (39.3%) screened cases met all criteria and were included in this analysis. Patient characteristics are summarized in Table 1. A majority of patients (79%) had a documented history of diabetes, and most of these were classified as type 2 diabetes in the hospital record. Of the patients who were classified as having diabetes on admission, 50.8% were on some form of outpatient insulin therapy with or without oral diabetes agents. Patients with a diagnosis of diabetes had a median admission glucose of 158 mg/dL (IQR, 118221), which was significantly higher than the median admission glucose of 119 mg/dL (IQR, 100160) for patients without diabetes (P < 0.001, rank‐sum test).
| |
| n | 1718 |
| Age (years), median (IQR) | 65 (5674) |
| Male | 928 (54) |
| Female | 790 (46) |
| Admission glucose (mg/dL) | 149 (111207) |
| Race/Ethnicity | |
| White | 1048 (61.0) |
| Black | 480 (27.9) |
| Hispanic | 67 (3.9) |
| Other | 123 (7.2) |
| Diabetes history | 1358 (79.0) |
| Type 2 diabetes mellitus | 996 (58.0) |
| Type 1 diabetes mellitus | 128 (7.5) |
| Unspecified/other diabetes mellitus | 234 (13.6) |
| No history of diabetes mellitus | 360 (21.0) |
| Outpatient diabetes treatment | |
| Insulin only | 522 (30.4) |
| Oral agents only | 505 (29.4) |
| Insulin and oral agents | 168 (9.8) |
| No drug therapy | 137 (8.0) |
| Not documented | 26 (1.5) |
| Hospitalization DRG | |
| 127 Heart failure | 443 (25.8) |
| 109 Coronary artery bypass grafting | 389 (22.6) |
| 316 Renal failure | 251 (14.6) |
| 478 Other vascular procedure | 195 (11.4) |
| 89 Pneumonia | 186 (10.8) |
| 527 Percutaneous intervention with stent | 136 (7.9) |
| 143 Chest pain | 74 (4.3) |
| 209 Joint/limb procedure | 44 (2.6) |
| Primary insurer | |
| Medicare | 961 (56.0) |
| Private/commercial | 392 (22.8) |
| Medicaid | 200 (11.6) |
| Government | 88 (5.1) |
| Self‐pay | 67 (3.9) |
| Other/unknown | 10 (0.6) |
To determine overall glycemic control for the cohort, median glucose was calculated for each patient, stratified by diabetes status and location for each measurement day (Table 2). Patient‐days with a location of emergency department (96 patients on day 1, 6 on day 2, and 2 on day 3) and two patients whose location was not defined were excluded from the analysis. Overall, median glucose declined from measurement day 1 to day 3. For patients with diabetes, median glucose was significantly lower in the intensive care unit (ICU) compared to the general ward or intermediate care for measurement days 1 and 2, but not day 3. This difference was more pronounced in patients without diabetes, with median glucose significantly lower in the ICU for all 3 measurement days compared to other locations. As expected, median glucose was lower for patients without diabetes compared to patients with diabetes for all measurement days and locations. Hyperglycemia was common; 867 of 1,718 (50%) patients had at least 1 glucose measurement 180 mg/dL on both days 2 and 3; 18% of all patients had a median glucose 180 mg/dL on all 3 measurement days. Daily 6 AM glucose was the summary glycemic control measure in the clinical trial by van den Berghe et al.,11 with goal glucose of 80 to 110 mg/dL in the intensive treatment group. Since the glycemic target of the American College of Endocrinology Position Statement is <110 mg/dL (based largely on van den Berghe et al.11) we also calculated estimated 6 AM glucose for ICU patient‐days to determine the proportion of patients attaining this target.14 Estimated 6 AM glucose was lower in ICU patients without diabetes compared to those with diabetes. For patients with diabetes, only 20% of patients in the ICU had an estimated 6 AM glucose 110 mg/dL on measurement day 2, and only 24% on day 3. For patients without diabetes, 27% and 25% had an estimated 6 AM glucose 110 mg/dL on days 2 and 3, respectively.
| Measurement by Location | |||
|---|---|---|---|
| Day 1 | Day 2 | Day 3 | |
| |||
| Patients with diabetes | |||
| Estimated 6 AM glucose (mg/dL) | |||
| Intensive care unit | 153.0 (119.0204.0) | 148.0 (118.0183.0) | 144.0 (113.0191.0) |
| n | 167 | 231 | 161 |
| Median glucose (mg/dL) | |||
| General floor | 186.0 (151.0229.0) | 163.0 (131.0210.0) | 161.0 (127.0203.4) |
| n | 681 | 757 | 758 |
| Intermediate care | 193.0 (155.3233.8) | 170.0 (137.0215.5) | 169.0 (137.9215.6) |
| n | 291 | 333 | 348 |
| Intensive care unit | 177.5 (149.6213.6) | 152.5 (128.3187.0) | 156.5 (124.5194.3) |
| n | 294 | 247 | 175 |
| P value* | 0.038 | <0.001 | 0.068 |
| Patients without diabetes | |||
| Estimated 6 AM glucose (mg/dL) | |||
| Intensive care unit | 133.0 (104.5174.0) | 134.0 (109.0169.0) | 128.0 (111.5151.3) |
| n | 98 | 157 | 80 |
| Median glucose (mg/dL) | |||
| General floor | 179.0 (149.5209.5) | 161.3 (131.4188.3) | 143.5 (122.0170.0) |
| n | 91 | 96 | 133 |
| Intermediate care | 168.3 (138.1193.8) | 137.0 (119.8161.5) | 129.3 (116.3145.5) |
| n | 46 | 71 | 86 |
| Intensive care unit | 153.8 (132.9188.8) | 136.5 (120.0157.0) | 129.0 (116.0143.8) |
| n | 218 | 186 | 106 |
| P value* | <0.001 | <0.001 | <0.001 |
For the overall cohort, insulin was the most common treatment for hyperglycemia, with 84.6% of all patients receiving some form of insulin therapy on the second measurement day. On the second day, 30.8% received short‐acting subcutaneous insulin only, 8.2% received intravenous insulin infusion, 22.5% received both short‐acting and long‐acting subcutaneous insulin, 3.9% received oral agents, 23% received some combination of insulin therapies and/or oral agents, and 11.9% received no treatment. To determine the effect of intravenous versus subcutaneous insulin treatment on glycemic control, we compared patients by insulin treatment and location for each measurement day (Table 3). Intravenous insulin was used predominantly in the ICU, and was associated with significantly lower median glucose compared to subcutaneous insulin in both locations for all 3 measurement days. As expected, the average number of glucose measures per patient was significantly higher for those receiving intravenous insulin. Intravenous insulin use in the ICU was associated with a significantly lower number of patients with hyperglycemia, defined as the number who had 1 or more glucose values 180 mg/dL during a given measurement day. Of note, intravenous insulin use in the ICU was associated with a significantly higher proportion of patients who had hypoglycemia (defined as the number of patients who had one or more glucose values <70 mg/dL) compared to subcutaneous insulin only on measurement day 1 (8.1% versus 2.9%; P = 0.021), but not on days 2 (12.7% versus 8.0%; P > 0.05) or 3 (12.7% versus 7.8%; P > 0.05). Severe hypoglycemia, defined as a blood glucose recording <50 mg/dL,24 was rare, and occurred in only 2.8% of all patient days. On measurement day 1, 34 patients had a total of 49 severe hypoglycemic events; on day 2, 54 patients had 68 severe hypoglycemic events; on day 3, 54 patients had 68 severe hypoglycemic events. Only 3 patients had severe hypoglycemic events on all 3 measurement days. Analysis of severe hypoglycemia events stratified by intravenous versus subcutaneous insulin did not show any significant differences for any of the 3 measurement days (data not shown).
| Location/Day | Outcome | Intravenous Insulin | Subcutaneous Insulin | P Value* |
|---|---|---|---|---|
| ||||
| Intensive Care Unit, Day 1 | Patient's glucose, median (mg/dL) | 148.0 | 183.0 | <0.001 |
| Interquartile range | 128.0178.0 | 154.8211.0 | ||
| Hypoglycemic patients, n (%) | 16 (8.1) | 6 (2.9) | 0.021 | |
| Hyperglycemic patients, n (%) | 130 (66.0) | 175 (85.0) | <0.001 | |
| Average glucose measures/patient | 8.4 | 4.8 | <0.001 | |
| Patients, n | 197 | 206 | ||
| Intermediate/General Ward, Day 1 | Patient's glucose, median (mg/dL) | 152.0 | 186.5 | <0.001 |
| Interquartile range | 131.0164.5 | 150.0230.0 | ||
| Hypoglycemic patients, n (%) | 1 (4.1) | 71 (7.4) | ns | |
| Hyperglycemic patients, n (%) | 18 (78.3) | 808 (83.9) | ns | |
| Average glucose measures/patient | 9.7 | 3.8 | <0.001 | |
| Patients, n | 23 | 962 | ||
| Intensive Care Unit, Day 2 | Patient's glucose, median (mg/dL) | 124.8 | 159.8 | <0.001 |
| Interquartile range | 110.4140.5 | 138.6197.4 | ||
| Hypoglycemic patients, n (%) | 15 (12.7) | 14 (8.0) | ns | |
| Hyperglycemic patients, n (%) | 53 (44.9) | 135 (76.7) | <0.001 | |
| Average glucose measures/patient | 12.5 | 5.3 | <0.001 | |
| Patients, n | 118 | 176 | ||
| Intermediate/General Ward, Day 2 | Patient's glucose, median (mg/dL) | 136.0 | 168.8 | <0.001 |
| Interquartile range | 116.0168.0 | 136.1215.5 | ||
| Hypoglycemic patients, n (%) | 2 (6.7) | 113 (11.3) | ns | |
| Hyperglycemic patients, n (%) | 18 (60.0) | 784 (78.6) | 0.015 | |
| Average glucose measures/patient | 11.0 | 4.6 | <0.001 | |
| Patients, n | 30 | 996 | ||
| Intensive Care Unit, Day 3 | Patient's glucose, median (mg/dL) | 123.5 | 171.0 | <0.001 |
| Interquartile range | 110.0137.1 | 137.3198.5 | ||
| Hypoglycemic patients, n (%) | 7 (12.7) | 11 (7.8) | ns | |
| Hyperglycemic patients, n (%) | 24 (43.6) | 101 (71.1) | <0.001 | |
| Average glucose measures/patient | 11.4 | 4.8 | <0.001 | |
| Patients, n | 54 | 141 | ||
| Intermediate/General Ward, Day 3 | Patient's glucose, median (mg/dL) | 129.8 | 166.0 | <0.001 |
| Interquartile range | 120.5142.3 | 131.5208.0 | ||
| Hypoglycemic patients, n (%) | 3 (13.6) | 104 (9.8) | ns | |
| Hyperglycemic patients, n (%) | 13 (59.1) | 773 (72.7) | ns | |
| Average glucose measures/patient | 10.3 | 4.3 | <0.001 | |
| Patients, n | 22 | 1,055 | ||
We hypothesized that use of subcutaneous long‐acting (basal) insulin (with or without short‐acting insulin) would be associated with superior glucose control compared to use of subcutaneous short‐acting insulin (sliding scale and/or scheduled prandial insulin) alone. We performed an exploratory multivariate regression analysis to compare the effect of IV insulin, long acting subcutaneous insulin short acting insulin, or short acting subcutaneous insulin alone on median glucose, hyperglycemic events (glucose 180 mg/dL), and hypoglycemic events (glucose <70 mg/dL) for days 2 and 3 (Table 4). Compared to short‐acting subcutaneous insulin alone, use of IV insulin but not long‐acting subcutaneous insulin was predictive of lower median glucose for days 2 and 3. Use of long‐acting subcutaneous insulin was not associated with significantly lower odds of hyperglycemic events for days 2 and 3, but was associated with higher odds of hypoglycemic events on day 2 (odds ratio [OR], 1.8; P = 0.01) when compared to short‐acting subcutaneous insulin alone.
| Glucose Control Measure | Intravenous Insulin Infusion | Long‐Acting Subcutaneous Insulin |
|---|---|---|
| ||
| Median glucose | ||
| Day 2, n = 1,297 | 32.0 (45.4 to 18.5); P < 0.001* | 5.1 (13.8 to 3.6); P = 0.25* |
| Day 3, n = 1,251 | 33.0 (48.9 to 17); P < 0.001* | 3.4 (5.2 to 11.9); P = 0.44* |
| Patient has 1 hyperglycemic event | ||
| Day 2, n = 1,298 | 0.4 (0.20.6); P < 0.001 | 0.7 (0.51.1); P = 0.11 |
| Day 3, n = 1,261 | 0.6 (0.31.1); P = 0.11 | 0.8 (0.61.1); P = 0.24 |
| Patient has 1 hypoglycemic event | ||
| Day 2, n = 1,298 | 2.1 (1.04.7); P = 0.07 | 1.8 (1.22.9); P = 0.010 |
| Day 3, n = 1,261 | 4.0 (1.69.8); P = 0.003 | 1.4 (0.92.3); P = 0.13 |
We measured the performance of recommended hospital diabetes care practices (A1C assessment, documentation of diabetes history in the hospital record, admission laboratory glucose assessment, bedside glucose monitoring, recommended insulin therapy)14, 15 for all study patients, and also stratified performance by hospital (Table 5); 98.6% of all patients with a diagnosis of diabetes had physician documentation of their diabetes status recorded in the hospital record, and there was consistently high performance of this by hospital (Table 5); 77% of all patients with a history of diabetes had a laboratory blood glucose result recorded within 8 hours of hospital admission, and 81.3% of patients with a history of diabetes had blood glucose monitored at least 4 times on measurement day 2. Performance by hospital (Table 5) varied widely for glucose monitoring (range, 56.5%95.5% of patients by hospital) and admission laboratory glucose assessment (range, 39.0%97.1% of patients by hospital).
| Diabetes Care Measure | Mean Hospital Performance (%) | Standard Deviation (%) | Range (%) |
|---|---|---|---|
| |||
| Physician documentation of diabetes history in medical record | 98.8 | 2.1 | 91.5100 |
| A1C assessment documented for diabetes patients (measured during hospitalization or within 30 days prior to admission) | 33.7 | 15.4 | 3.162.9 |
| Laboratory glucose assessment within 8 hours of hospital presentation for diabetes patients | 77.0 | 13.4 | 39.097.1 |
| Blood glucose monitoring at least 4 times on second measurement day for diabetes patients | 81.6 | 10.8 | 56.595.5 |
| Percentage of patients receiving insulin therapy who were given short and long‐acting insulin OR IV insulin infusion OR insulin pump therapy on second measurement day | 44.9 | 14.3 | 12.176.5 |
Of all patients, 31% had A1C measurement recorded during their hospitalization or within 30 days prior to admission. There was wide variation in hospital performance of A1C assessment in patients with diabetes (Table 5). Patients with a diagnosis of diabetes had a median A1C of 7.4% (IQR, 6.4%8.9%; n = 473), and those without a diagnosis of diabetes had a median A1C of 5.9% (IQR, 5.6%6.4%; n = 70). Of the patients with a history of diabetes who had A1C recorded, 59% had a value >7%. Of the patients without a history of diabetes who had A1C recorded, 43% had a value >6.0%, suggesting previously undiagnosed diabetes.25
We found wide variation among hospitals (range, 12.1%76.5%) in use of recommended regimens of insulin therapy, defined as short‐acting and long‐acting subcutaneous insulin or IV insulin infusion or insulin pump therapy on second measurement day. Endocrine/diabetes consultation was infrequent, only 9% of all patients were evaluated by an endocrinologist or diabetologist at any time during the hospitalization.
DISCUSSION
In this retrospective analysis of hospitalized patients who had 2 consecutive blood glucose values 180 mg/dL and/or received insulin therapy, hyperglycemia was common and hypoglycemia was infrequent. Use of intravenous insulin was associated with better glucose control, and did not increase the frequency of severe hypoglycemic events (glucose <50 mg/dL). The majority of patients with a history of diabetes had physician documentation in the hospital chart, laboratory serum glucose obtained within 8 hours of hospital admission, and at least 4 blood glucose determinations on the second measurement day.
Only 35% of patients with diabetes had an A1C measurement and of these almost 60% had an A1C level >7%. Though the A1C may not greatly affect acute glucose management in the hospital setting, it does identify patients that may require intensification of diabetes therapy at hospital discharge and coordination of outpatient follow‐up. A report of a UHC clinical benchmarking project of ambulatory diabetes care in academic medical centers demonstrated high rates of diagnostic testing, but only 34% of patients were at the A1C goal, and only 40% of patients above the A1C goal had adjustment of their diabetes regimen at their last clinic visit.26 In a retrospective study of patients with diabetes mellitus admitted to an academic teaching hospital, only 20% of discharges indicated a plan for diabetes follow‐up.27 Thus, intensification of antihyperglycemic therapy and formulation of a diabetes follow‐up plan on hospital discharge in those patients with A1C >7% represents an opportunity to improve glycemic control in the ambulatory setting. Also, measurement of A1C can be used for diabetes case‐finding in hospitalized patients with hyperglycemia.25 Previously unrecognized diabetes is a common finding in patients admitted with cardiovascular disease. In a study of patients admitted with myocardial infarction, 25% were found to have previously undiagnosed diabetes.28 Hospital patients with hyperglycemia but without a prior diagnosis of diabetes who have an elevation of A1C >6.0% can be identified as at‐risk for diabetes and postdischarge glucose evaluation can be arranged.
The target of maintaining all glucose values 180 mg/dL recommended in the 20052007 American Diabetes Association guidelines for hospital diabetes management was not commonly achieved, with over 70% of patients who received subcutaneous insulin therapy having 1 or more glucose values >180 on all 3 measurement days, regardless of patient location.15 The target of maintaining critically ill patients as close to 110 mg/dL as possible was also difficult to achieve, with only 25% of ICU patients having an estimated 6 AM glucose <110 mg/dL on measurement day 3. A prospective cohort study of 107 inpatients with diabetes at Brigham and Women's Hospital showed a 76% prevalence of patients with at least one BG >180 mg/dL.29 In that study, 90% of patients had a sliding‐scale order, 36% received an oral diabetes agent, and 43% received basal insulin at some time during hospitalization. A recently published analysis by Wexler et al.30 compiled data of hospitalized patients with diabetes from an earlier 2003 UHC Diabetes Benchmarking Project (n = 274) and patients from 15 not‐for‐profit member hospitals of VHA, Incorporated (n = 725) to examine the prevalence of hyperglycemia and hypoglycemia. Hyperglycemia (defined as a single BG value >200 mg/dL) was common, occurring in 77% of patients in the UHC cohort and 76% in the VHA, Inc. cohort. This was comparable to our findings that 76.7% of ICU patients and 78.6% of ward patients treated with subcutaneous insulin had 1 or more BG values 180 mg/dL on measurement day 2. Wexler et al.30 also determined that use of basal insulin was associated with a higher prevalence of hyperglycemia and hypoglycemia in their study. Our regression analysis finding that long‐acting (basal) insulin use was not associated with improvement in glycemic control is consistent with the findings of the aforementioned study. There are a number of potential explanations for this: (1) underdosing of basal insulin or lack of adequate prandial insulin coverage for nutritional intake; (2) lack of effective titration in response to hyperglycemia; and (3) variation in the ordering and administration of basal insulin at different hospital sites.
Use of both manual and computerized IV insulin protocols has been shown to provide effective glucose control in critically ill patients.1618 Though intravenous insulin use was associated with better overall glucose control in our study; only about 50% of ICU patients received it on measurement day 1. A recent prospective randomized clinical trial demonstrated superior glycemic control in noncritically ill hospitalized patients with type 2 diabetes with basal/bolus insulin therapy compared to sliding scale insulin alone.31 Use of basal/bolus insulin regimens as part of a comprehensive hospital diabetes management program has been shown to improve glycemic control in an academic medical center.20 Therefore, we do not believe that our regression analysis findings invalidate the concept of basal/bolus insulin for inpatients with hyperglycemia, but rather indicate the need for more research into subcutaneous insulin regimens and hospital care practices that lead to improved glucose control. We found wide variation in hospital use of basal/bolus insulin regimens. Overall only 22.5% of all patients on the second measurement day received both short‐acting and long‐acting subcutaneous insulin, compared to 30.8% who received short‐acting subcutaneous insulin only. A recent consensus statement on inpatient glycemic control by the American College of Endocrinology and American Diabetes Association highlighted the systematic barriers to improved glycemic control in hospitals, such as inadequate knowledge of diabetes management techniques, fear of hypoglycemia, and skepticism about benefits of tighter glucose control.32
There are some important limitations to this study. The data are retrospective and only a limited number of hospital days and clinical variables could be assessed for each patient. As indicated in Table 3, there were significant differences in the frequency of glucose measurement depending on treatment, which can potentially bias estimated prevalence of hyperglycemia and hypoglycemia. We did not have a practical method to assess nutritional status or the adequacy of insulin dosing over time for each patient. We also could not assess the association of glycemic control on clinical outcomes such as hospital mortality or infection rates. Since this study was exclusively in academic medical centers, the generalization of findings to community‐based medical centers may be limited. The risk‐benefit of tight glycemic control in medical ICU patients based on clinical trial evidence has been unclear, and there is not broad agreement among clinicians on the recommended target for glycemic control in this group.3335 When we analyzed glycemic control in ICU patients we did not have a practical method to control for type of ICU and variations in individual ICU glycemic control targets. We recognize that the 2004 American College of Endocrinology recommendation of maintaining glucose 110 mg/dL may not be appropriate for all critically ill patients.14 Finally, clinical trial data are lacking on the effect of tight glucose control on major clinical outcomes for noncritically ill hospital patients. This has led to significant controversy regarding glycemic targets for different subgroups of hospitalized patients.34, 36
In summary, we found a high prevalence of persistent hyperglycemia in this large cohort of hospitalized patients, and hypoglycemia was infrequent. Use of IV insulin was associated with improvement in glycemic control, but was used in less than half of ICU patients. There was wide variation in hospital performance of recommended diabetes care measures. Opportunities to improve care in academic medical centers include expanded use of intravenous and subcutaneous basal/bolus insulin protocols and increased frequency of A1C testing.
- ,,, et al.Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke.Neurology.2002;59:67–71.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,,.Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,.Admission plasma glucose. Independent risk factor for long‐term prognosis after myocardial infarction even in nondiabetic patients.Diabetes Care.1999;22:1827–1831.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Modifiable risk factors associated with deep sternal site infection after coronary artery bypass grafting.J Thorac Cardiovasc Surg.2000;119:108–114.
- ,,,,.The association of diabetes and glucose control with surgical‐site infections among cardiothoracic surgery patients.Infect Control Hosp Epidemiol.2001;22:607–612.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109:1497–1502.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(Suppl 2):21–33.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10(Suppl 2):4–9.
- American Diabetes Association.Standards of medical care in diabetes.Diabetes Care.2005;28(Suppl 1):S4–S36.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27:461–467.
- ,,,,.Use of a computerized guideline for glucose regulation in the intensive care unit improved both guideline adherence and glucose regulation.J Am Med Inform Assoc.2005;12:172–180.
- ,,, et al.Computer‐based insulin infusion protocol improves glycemia control over manual protocol.J Am Med Inform Assoc.2007;14:278–287.
- ,,,,.Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding‐scale insulin therapy.Pharmacotherapy.2006;26:1421–1432.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,.70/30 insulin algorithm versus sliding scale insulin.Ann Pharmacother.2005;39:1606–1610.
- ,,,.Glucose measurement: confounding issues in setting targets for inpatient management.Diabetes Care.2007;30:403–409.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- American Diabetes Association.Hospital admission guidelines for diabetes.Diabetes Care.2004;27(Suppl 1):S103.
- ,,,,,,.Utility of HbA(1c) levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,.Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change.Diabetes Care.2005;28:337–442.
- ,,, et al.Diabetes care in the hospital: is there clinical inertia?J Hosp Med.2006;1:151–160.
- ,,, et al.Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study.Lancet.2002;359:2140–2144.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- The ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,.Tight blood glucose control with insulin in the ICU: facts and controversies.Chest.2007;132:268–278.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.NEngl J Med.2008;358:125–139.
- ,.Counterpoint: Inpatient glucose management: a premature call to arms?Diabetes Care.2005;28:976–979.
- ,,, et al.Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke.Neurology.2002;59:67–71.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,,.Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,.Admission plasma glucose. Independent risk factor for long‐term prognosis after myocardial infarction even in nondiabetic patients.Diabetes Care.1999;22:1827–1831.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Modifiable risk factors associated with deep sternal site infection after coronary artery bypass grafting.J Thorac Cardiovasc Surg.2000;119:108–114.
- ,,,,.The association of diabetes and glucose control with surgical‐site infections among cardiothoracic surgery patients.Infect Control Hosp Epidemiol.2001;22:607–612.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109:1497–1502.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(Suppl 2):21–33.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10(Suppl 2):4–9.
- American Diabetes Association.Standards of medical care in diabetes.Diabetes Care.2005;28(Suppl 1):S4–S36.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27:461–467.
- ,,,,.Use of a computerized guideline for glucose regulation in the intensive care unit improved both guideline adherence and glucose regulation.J Am Med Inform Assoc.2005;12:172–180.
- ,,, et al.Computer‐based insulin infusion protocol improves glycemia control over manual protocol.J Am Med Inform Assoc.2007;14:278–287.
- ,,,,.Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding‐scale insulin therapy.Pharmacotherapy.2006;26:1421–1432.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,.70/30 insulin algorithm versus sliding scale insulin.Ann Pharmacother.2005;39:1606–1610.
- ,,,.Glucose measurement: confounding issues in setting targets for inpatient management.Diabetes Care.2007;30:403–409.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- American Diabetes Association.Hospital admission guidelines for diabetes.Diabetes Care.2004;27(Suppl 1):S103.
- ,,,,,,.Utility of HbA(1c) levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,.Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change.Diabetes Care.2005;28:337–442.
- ,,, et al.Diabetes care in the hospital: is there clinical inertia?J Hosp Med.2006;1:151–160.
- ,,, et al.Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study.Lancet.2002;359:2140–2144.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- The ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,.Tight blood glucose control with insulin in the ICU: facts and controversies.Chest.2007;132:268–278.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.NEngl J Med.2008;358:125–139.
- ,.Counterpoint: Inpatient glucose management: a premature call to arms?Diabetes Care.2005;28:976–979.
Copyright © 2009 Society of Hospital Medicine
Paging goldilocks: How much glycemic control is just right?
There is no doubt that hyperglycemia among hospitalized patients correlates with worse prognosis. Further, there are well‐documented mechanisms by which poor glycemic control may directly impact outcomes. For example, hyperglycemia and insulin deficiency can impair neutrophil function, exacerbate inflammation, and impair endothelium‐mediated dilatation,1, 2 whereas hypoglycemia increases sympathetic tone. And both severe hyperglycemia and hypoglycemia, of course, can precipitate altered mental status. But certainly not all of the morbid outcomes associated with poor glycemic control in the hospitalincluding infection, cardiac events and deathare caused by poor glycemic control in the hospital. Elevated glucose levels in the hospital are often seen in sicker patients with raging stress hormones and in brittle diabetics with a present‐on‐admission condition that has been ravaging their vasculature for years. This means that virtually all observational studies demonstrating worse outcomes in the setting of poor glucose control in the hospital will be severely confounded by comorbid illness, and much confounding will remain even after multivariate adjustment.3
Nonetheless, high‐quality randomized controlled trials that have focused on critically ill patients,4, 5 rather than general medical patients, have generated intense interest and fostered the belief that controlling the glucose level of all hospitalized patients is probably a good idea. (Although, more recently, even the data supporting glycemic control in the critically ill have been challenged.)6 Enthusiasm for implementing aggressive glycemic control protocols outside of the intensive care unit (ICU) appears widespread, as is evident in this issue of JHM.711 In this issue, two articles detail the challenges of implementing glycemia control protocols.7, 8 The research teams employed different protocols and used different metrics, but there are common themes: (1) The process was iterative. Interventions were piloted, then rolled out, and substantial effort was needed to foster continued attention to the interventions. (2) The process was multidisciplinary. Buy‐in and input were needed not only from physicians, but also from nurses, pharmacists, dieticians, clinical data system experts, and probably patients. (3) Impacting process measures was easier than impacting surrogate outcome measures. Specifically, despite dramatic changes in the use of carefully vetted order sets and protocols, the impact on glycemia was modest and sometimes inconsistent.
These studies illustrate that implementing protocols to control glycemia is neither easy, nor consistently associated with improved glycemic controllet alone improved major clinical outcomes. Three complementary observational studies911 further illustrate how hard it is to optimize glycemic control in the hospital setting. Together, the observational and interventional studies demonstrate how difficult it is to measure success. Should we focus on the mean glucose value achieved or the frequency of extreme glucose values (which are, by definition, more dangerous)? Should we look at glycemic control in every patient who is placed on a protocol, even those who barely need any insulin at all, or should we focus our interventions and analyses on those patients with more severe dysglycemia at baseline? This latter issue is fundamentally important, since the rollout of any systemwide glycemia protocol that results in higher catchment rates will appear more effective than it really is by enriching the postintervention data with healthier patients.
Before embarking on time‐intensive efforts to improve care, maybe we should be sure that the evidence supports our efforts.12 Recent recommendations from the American Diabetes Association state that for non‐critically ill patients: there is no clear evidence for specific blood glucose goals.13 (This recommendation, based on expert consensus or clinical experience, further states that because cohort data suggest that outcomes are better in hospitalized patients with fasting glucose 126 mg/dL and all random glucose values 180 to 200 mg/dL, these goals are reasonable if they can be safely achieved.) But given the challenges associated with implementing glycemia protocols, one might argue that hospitalists should invest their quality improvement efforts elsewhere.
So where does this leave us? What target glucose is not too high, not too low, but just right? Given the ever‐increasing number of quality improvement measures and interventions that are expected in the hospital, what amount of time, effort, and money devoted to improving inpatient glycemic control is just right? And what do our patients think? Should we be feeding our patients low glycemic load diets, or letting them indulge in one of the few creature comforts remaining in a semiprivate room?
What is clear from the results of the research published in this issue of JHM (regardless of whether we think that an inpatient pre‐meal glucose of 160 mg/dL is good, bad, or neither), is that we need to continue to develop systems, strategies, and teams to rapidly disseminate quality improvement interventions locally. We need multidisciplinary inputfrom physicians, nurses, dieticians, pharmacists, and patientsto do it right. So, even if the pendulum swings away from tight glycemic control in the hospital, the lessons we learned from these authors' valiant efforts to tame inpatient glycemia may provide us with the tools and knowledge required to successfully tackle other clinical issues such as delirium prevention, pain control, medication reconciliation, and handoffs. The striking obstacles (both in implementation and analysis) faced and overcome by the authors of the articles in this issue of JHM will hopefully embolden them to take on other quality improvement interventions that are perhaps more likely to help hospitalized patients.
- ,,,,.Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose‐binding lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,,.In search of fewer independent risk factors.Arch Intern Med.2005;165:138–145.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,.Benefits and risks of tight glucose control in critically ill adults: a meta‐analysis.JAMA.2008;300:933–944.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,,; for the SHM Glycemic Control Task Force.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(S5):29–41.
- , ,, et al.Computer‐based insulin infusion protocol improves glycemia control over manual protocol.J Am Med Inform Assoc.2007;14:278–287.
- ,,,,.Estimated risk for undiagnosed diabetes in the emergency department: a multicenter survey.Acad Emerg Med.2007;14:492–495.
- ,,,,,.Evaluation of glycemic control following discontinuation of an intensive insulin protocol.J Hosp Med.2009;28–34.
- ,,.The tension between needing to improve care and knowing how to do it.N Engl J Med.2007;357:608–613.
- American Diabetes Association. Standards of medical care in diabetes—2008.Diabetes Care.2008;31(Suppl 1):S12–S54.
There is no doubt that hyperglycemia among hospitalized patients correlates with worse prognosis. Further, there are well‐documented mechanisms by which poor glycemic control may directly impact outcomes. For example, hyperglycemia and insulin deficiency can impair neutrophil function, exacerbate inflammation, and impair endothelium‐mediated dilatation,1, 2 whereas hypoglycemia increases sympathetic tone. And both severe hyperglycemia and hypoglycemia, of course, can precipitate altered mental status. But certainly not all of the morbid outcomes associated with poor glycemic control in the hospitalincluding infection, cardiac events and deathare caused by poor glycemic control in the hospital. Elevated glucose levels in the hospital are often seen in sicker patients with raging stress hormones and in brittle diabetics with a present‐on‐admission condition that has been ravaging their vasculature for years. This means that virtually all observational studies demonstrating worse outcomes in the setting of poor glucose control in the hospital will be severely confounded by comorbid illness, and much confounding will remain even after multivariate adjustment.3
Nonetheless, high‐quality randomized controlled trials that have focused on critically ill patients,4, 5 rather than general medical patients, have generated intense interest and fostered the belief that controlling the glucose level of all hospitalized patients is probably a good idea. (Although, more recently, even the data supporting glycemic control in the critically ill have been challenged.)6 Enthusiasm for implementing aggressive glycemic control protocols outside of the intensive care unit (ICU) appears widespread, as is evident in this issue of JHM.711 In this issue, two articles detail the challenges of implementing glycemia control protocols.7, 8 The research teams employed different protocols and used different metrics, but there are common themes: (1) The process was iterative. Interventions were piloted, then rolled out, and substantial effort was needed to foster continued attention to the interventions. (2) The process was multidisciplinary. Buy‐in and input were needed not only from physicians, but also from nurses, pharmacists, dieticians, clinical data system experts, and probably patients. (3) Impacting process measures was easier than impacting surrogate outcome measures. Specifically, despite dramatic changes in the use of carefully vetted order sets and protocols, the impact on glycemia was modest and sometimes inconsistent.
These studies illustrate that implementing protocols to control glycemia is neither easy, nor consistently associated with improved glycemic controllet alone improved major clinical outcomes. Three complementary observational studies911 further illustrate how hard it is to optimize glycemic control in the hospital setting. Together, the observational and interventional studies demonstrate how difficult it is to measure success. Should we focus on the mean glucose value achieved or the frequency of extreme glucose values (which are, by definition, more dangerous)? Should we look at glycemic control in every patient who is placed on a protocol, even those who barely need any insulin at all, or should we focus our interventions and analyses on those patients with more severe dysglycemia at baseline? This latter issue is fundamentally important, since the rollout of any systemwide glycemia protocol that results in higher catchment rates will appear more effective than it really is by enriching the postintervention data with healthier patients.
Before embarking on time‐intensive efforts to improve care, maybe we should be sure that the evidence supports our efforts.12 Recent recommendations from the American Diabetes Association state that for non‐critically ill patients: there is no clear evidence for specific blood glucose goals.13 (This recommendation, based on expert consensus or clinical experience, further states that because cohort data suggest that outcomes are better in hospitalized patients with fasting glucose 126 mg/dL and all random glucose values 180 to 200 mg/dL, these goals are reasonable if they can be safely achieved.) But given the challenges associated with implementing glycemia protocols, one might argue that hospitalists should invest their quality improvement efforts elsewhere.
So where does this leave us? What target glucose is not too high, not too low, but just right? Given the ever‐increasing number of quality improvement measures and interventions that are expected in the hospital, what amount of time, effort, and money devoted to improving inpatient glycemic control is just right? And what do our patients think? Should we be feeding our patients low glycemic load diets, or letting them indulge in one of the few creature comforts remaining in a semiprivate room?
What is clear from the results of the research published in this issue of JHM (regardless of whether we think that an inpatient pre‐meal glucose of 160 mg/dL is good, bad, or neither), is that we need to continue to develop systems, strategies, and teams to rapidly disseminate quality improvement interventions locally. We need multidisciplinary inputfrom physicians, nurses, dieticians, pharmacists, and patientsto do it right. So, even if the pendulum swings away from tight glycemic control in the hospital, the lessons we learned from these authors' valiant efforts to tame inpatient glycemia may provide us with the tools and knowledge required to successfully tackle other clinical issues such as delirium prevention, pain control, medication reconciliation, and handoffs. The striking obstacles (both in implementation and analysis) faced and overcome by the authors of the articles in this issue of JHM will hopefully embolden them to take on other quality improvement interventions that are perhaps more likely to help hospitalized patients.
There is no doubt that hyperglycemia among hospitalized patients correlates with worse prognosis. Further, there are well‐documented mechanisms by which poor glycemic control may directly impact outcomes. For example, hyperglycemia and insulin deficiency can impair neutrophil function, exacerbate inflammation, and impair endothelium‐mediated dilatation,1, 2 whereas hypoglycemia increases sympathetic tone. And both severe hyperglycemia and hypoglycemia, of course, can precipitate altered mental status. But certainly not all of the morbid outcomes associated with poor glycemic control in the hospitalincluding infection, cardiac events and deathare caused by poor glycemic control in the hospital. Elevated glucose levels in the hospital are often seen in sicker patients with raging stress hormones and in brittle diabetics with a present‐on‐admission condition that has been ravaging their vasculature for years. This means that virtually all observational studies demonstrating worse outcomes in the setting of poor glucose control in the hospital will be severely confounded by comorbid illness, and much confounding will remain even after multivariate adjustment.3
Nonetheless, high‐quality randomized controlled trials that have focused on critically ill patients,4, 5 rather than general medical patients, have generated intense interest and fostered the belief that controlling the glucose level of all hospitalized patients is probably a good idea. (Although, more recently, even the data supporting glycemic control in the critically ill have been challenged.)6 Enthusiasm for implementing aggressive glycemic control protocols outside of the intensive care unit (ICU) appears widespread, as is evident in this issue of JHM.711 In this issue, two articles detail the challenges of implementing glycemia control protocols.7, 8 The research teams employed different protocols and used different metrics, but there are common themes: (1) The process was iterative. Interventions were piloted, then rolled out, and substantial effort was needed to foster continued attention to the interventions. (2) The process was multidisciplinary. Buy‐in and input were needed not only from physicians, but also from nurses, pharmacists, dieticians, clinical data system experts, and probably patients. (3) Impacting process measures was easier than impacting surrogate outcome measures. Specifically, despite dramatic changes in the use of carefully vetted order sets and protocols, the impact on glycemia was modest and sometimes inconsistent.
These studies illustrate that implementing protocols to control glycemia is neither easy, nor consistently associated with improved glycemic controllet alone improved major clinical outcomes. Three complementary observational studies911 further illustrate how hard it is to optimize glycemic control in the hospital setting. Together, the observational and interventional studies demonstrate how difficult it is to measure success. Should we focus on the mean glucose value achieved or the frequency of extreme glucose values (which are, by definition, more dangerous)? Should we look at glycemic control in every patient who is placed on a protocol, even those who barely need any insulin at all, or should we focus our interventions and analyses on those patients with more severe dysglycemia at baseline? This latter issue is fundamentally important, since the rollout of any systemwide glycemia protocol that results in higher catchment rates will appear more effective than it really is by enriching the postintervention data with healthier patients.
Before embarking on time‐intensive efforts to improve care, maybe we should be sure that the evidence supports our efforts.12 Recent recommendations from the American Diabetes Association state that for non‐critically ill patients: there is no clear evidence for specific blood glucose goals.13 (This recommendation, based on expert consensus or clinical experience, further states that because cohort data suggest that outcomes are better in hospitalized patients with fasting glucose 126 mg/dL and all random glucose values 180 to 200 mg/dL, these goals are reasonable if they can be safely achieved.) But given the challenges associated with implementing glycemia protocols, one might argue that hospitalists should invest their quality improvement efforts elsewhere.
So where does this leave us? What target glucose is not too high, not too low, but just right? Given the ever‐increasing number of quality improvement measures and interventions that are expected in the hospital, what amount of time, effort, and money devoted to improving inpatient glycemic control is just right? And what do our patients think? Should we be feeding our patients low glycemic load diets, or letting them indulge in one of the few creature comforts remaining in a semiprivate room?
What is clear from the results of the research published in this issue of JHM (regardless of whether we think that an inpatient pre‐meal glucose of 160 mg/dL is good, bad, or neither), is that we need to continue to develop systems, strategies, and teams to rapidly disseminate quality improvement interventions locally. We need multidisciplinary inputfrom physicians, nurses, dieticians, pharmacists, and patientsto do it right. So, even if the pendulum swings away from tight glycemic control in the hospital, the lessons we learned from these authors' valiant efforts to tame inpatient glycemia may provide us with the tools and knowledge required to successfully tackle other clinical issues such as delirium prevention, pain control, medication reconciliation, and handoffs. The striking obstacles (both in implementation and analysis) faced and overcome by the authors of the articles in this issue of JHM will hopefully embolden them to take on other quality improvement interventions that are perhaps more likely to help hospitalized patients.
- ,,,,.Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose‐binding lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,,.In search of fewer independent risk factors.Arch Intern Med.2005;165:138–145.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,.Benefits and risks of tight glucose control in critically ill adults: a meta‐analysis.JAMA.2008;300:933–944.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,,; for the SHM Glycemic Control Task Force.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(S5):29–41.
- , ,, et al.Computer‐based insulin infusion protocol improves glycemia control over manual protocol.J Am Med Inform Assoc.2007;14:278–287.
- ,,,,.Estimated risk for undiagnosed diabetes in the emergency department: a multicenter survey.Acad Emerg Med.2007;14:492–495.
- ,,,,,.Evaluation of glycemic control following discontinuation of an intensive insulin protocol.J Hosp Med.2009;28–34.
- ,,.The tension between needing to improve care and knowing how to do it.N Engl J Med.2007;357:608–613.
- American Diabetes Association. Standards of medical care in diabetes—2008.Diabetes Care.2008;31(Suppl 1):S12–S54.
- ,,,,.Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose‐binding lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,,.In search of fewer independent risk factors.Arch Intern Med.2005;165:138–145.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,.Benefits and risks of tight glucose control in critically ill adults: a meta‐analysis.JAMA.2008;300:933–944.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,,; for the SHM Glycemic Control Task Force.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(S5):29–41.
- , ,, et al.Computer‐based insulin infusion protocol improves glycemia control over manual protocol.J Am Med Inform Assoc.2007;14:278–287.
- ,,,,.Estimated risk for undiagnosed diabetes in the emergency department: a multicenter survey.Acad Emerg Med.2007;14:492–495.
- ,,,,,.Evaluation of glycemic control following discontinuation of an intensive insulin protocol.J Hosp Med.2009;28–34.
- ,,.The tension between needing to improve care and knowing how to do it.N Engl J Med.2007;357:608–613.
- American Diabetes Association. Standards of medical care in diabetes—2008.Diabetes Care.2008;31(Suppl 1):S12–S54.