User login
Prevention of Hospital‐Acquired VTE
Pulmonary embolism (PE) and deep vein thrombosis (DVT), collectively referred to as venous thromboembolism (VTE), represent a major public health problem, affecting hundreds of thousands of Americans each year.1 The best estimates are that at least 100,000 deaths are attributable to VTE each year in the United States alone.1 VTE is primarily a problem of hospitalized and recently‐hospitalized patients.2 Although a recent meta‐analysis did not prove mortality benefit of prophylaxis in the medical population,3 PE is frequently estimated to be the most common preventable cause of hospital death.46
Pharmacologic methods to prevent VTE are safe, effective, cost‐effective, and advocated by authoritative guidelines.7 Even though the majority of medical and surgical inpatients have multiple risk factors for VTE, large prospective studies continue to demonstrate that these preventive methods are significantly underutilized, often with only 30% to 50% eligible patients receiving prophylaxis.812
The reasons for this underutilization include lack of physician familiarity or agreement with guidelines, underestimation of VTE risk, concern over risk of bleeding, and the perception that the guidelines are resource‐intensive or difficult to implement in a practical fashion.13 While many VTE risk‐assessment models are available in the literature,1418 a lack of prospectively validated models and issues regarding ease of use have further hampered widespread integration of VTE risk assessments into order sets and inpatient practice.
We sought to optimize prevention of hospital‐acquired (HA) VTE in our 350‐bed tertiary‐care academic center using a VTE prevention protocol and a multifaceted approach that could be replicated across a wide variety of medical centers.
Patients and Methods
Study Design
We developed, implemented, and refined a VTE prevention protocol and examined the impact of our efforts. We observed adult inpatients on a longitudinal basis for the prevalence of adequate VTE prophylaxis and for the incidence of HA VTE throughout a 36‐month period from calendar year 2005 through 2007, and performed a retrospective analysis for any potential adverse effects of increased VTE prophylaxis. The project adhered to the HIPAA requirements for privacy involving health‐related data from human research participants. The study was approved by the Institutional Review Board of the University of California, San Diego, which waived the requirement for individual patient informed consent.
We included all hospitalized adult patients (medical and surgical services) at our medical center in our observations and interventions, including patients of all ethnic groups, geriatric patients, prisoners, and the socially and economically disadvantaged in our population. Exclusion criteria were age under 14 years, and hospitalization on Psychiatry or Obstetrics/Gynecology services.
Development of a VTE Risk‐assessment Model and VTE Prevention Protocol
A core multidisciplinary team with hospitalists, pulmonary critical care VTE experts, pharmacists, nurses, and information specialists was formed. After gaining administrative support for standardization, we worked with medical staff leaders to gain consensus on a VTE prevention protocol for all medical and surgical areas from mid‐2005 through mid‐2006. The VTE prevention protocol included the elements of VTE risk stratification, definitions of adequate VTE prevention measures linked to the level of VTE risk, and definitions for contraindications to pharmacologic prophylactic measures. We piloted risk‐assessment model (RAM) drafts for ease of use and clarity, using rapid cycle feedback from pharmacy residents, house staff, and medical staff attending physicians. Models often cited in the literature15, 18 that include point‐based scoring of VTE risk factors (with prophylaxis choices hinging on the additive sum of scoring) were rejected based on the pilot experience.
We adopted a simple model with 3 levels of VTE risk that could be completed by the physician in seconds, and then proceeded to integrate this RAM into standardized data collection instruments and eventually (April 2006) into a computerized provider order entry (CPOE) order set (Siemmens Invision v26). Each level of VTE risk was firmly linked to a menu of acceptable prophylaxis options (Table 1). Simple text cues were used to define risk assessment, with more exhaustive listings of risk factors being relegated to accessible reference tables.
| Low | Moderate | High |
|---|---|---|
| ||
| Ambulatory patient without VTE risk factors; observation patient with expected LOS 2 days; same day surgery or minor surgery | All other patients (not in low‐risk or high‐risk category); most medical/surgical patients; respiratory insufficiency, heart failure, acute infectious, or inflammatory disease | Lower extremity arthroplasty; hip, pelvic, or severe lower extremity fractures; acute SCI with paresis; multiple major trauma; abdominal or pelvic surgery for cancer |
| Early ambulation | UFH 5000 units SC q 8 hours; OR LMWH q day; OR UFH 5000 units SC q 12 hours (if weight < 50 kg or age > 75 years); AND suggest adding IPC | LMWH (UFH if ESRD); OR fondaparinux 2.5 mg SC daily; OR warfarin, INR 2‐3; AND IPC (unless not feasible) |
Intermittent pneumatic compression devices were endorsed as an adjunct in all patients in the highest risk level, and as the primary method in patients with contraindications to pharmacologic prophylaxis. Aspirin was deemed an inappropriate choice for VTE prophylaxis. Subcutaneous unfractionated or low‐molecular‐weight heparin were endorsed as the primary method of prophylaxis for the majority of patients without contraindications.
Integration of the VTE Protocol into Order Sets
An essential strategy for the success of the VTE protocol included integrating guidance for the physician into the flow of patient care, via standardized order sets. The CPOE VTE prevention order set was modular by design, as opposed to a stand alone design. After conferring with appropriate stakeholders, preexisting and nonstandardized prompts for VTE prophylaxis were removed from commonly used order sets, and the standardized module was inserted in its place. This allowed for integration of the standardized VTE prevention module into all admission and transfer order sets, essentially insuring that all patients admitted or transferred within the medical center would be exposed to the protocol. Physicians using a variety of admission and transfer order sets were prompted to select each patient's risk for VTE, and declare the presence or absence of contraindications to pharmacologic prophylaxis. Only the VTE prevention options most appropriate for the patient's VTE and anticoagulation risk profile were presented as the default choice for VTE prophylaxis. Explicit designation of VTE risk level and a prophylaxis choice were presented in a hard stop mechanism, and utilization of these orders was therefore mandatory, not optional. Proper use (such as the proper classification of VTE risk by the ordering physician) was actively monitored on an auditing basis, and order sets were modified occasionally on the basis of subjective and objective feedback.
Assessment of VTE Risk Assessment Interobserver Agreement
Data from 150 randomly selected patients from the audit pool (from late 2005 through mid‐2006) were abstracted by the nurse practitioner in a detailed manner. Five independent reviewers assessed each patient for VTE risk level, and for a determination of whether or not they were on adequate VTE prophylaxis on the day of the audit per protocol. Interobserver agreement was calculated for these parameters using kappa scores.
Prospective Monitoring of Adequate VTE Prophylaxis
A daily medical center inpatient census report of eligible patients in the medical center for >48 hours was downloaded into an Microsoft Excel spreadsheet, with each patient assigned a consecutive number. The Excel random number generator plug‐in function was used to generate a randomly sequenced list of the patients. The research nurse practitioner targeted serial patients on the list for further study, until she accomplished the requisite number of audits each day. The mean number of audits per month declined over the study years as the trends stabilized and as grant funding expired, but remained robust throughout (2005: 107 audits per month; 2006: 80 audits per month; and 2007: 57 audits per month).
The data collected on each patient randomly selected for audit included age, gender, location, service, date and time of review, and date of admission. The audit VTE RAM (identical to the VTE RAM incorporated into the order set), was used to classify each patient's VTE risk as low, moderate, or high. For each audit, we determined if the patient was on an adequate VTE prevention regimen consistent with our protocol, given their VTE risk level, demographics, and absence or presence of contraindications to pharmacologic prophylaxis. All questionable cases were reviewed by at least 2 physicians at weekly meetings with a final consensus determination. Adequacy of the VTE regimen was judged by orders entered on the day of the audit, but we also noted whether or not ordered intermittent compression devices were in place and functioning at the time of the audit.
Prospective (Concurrent) Discovery and Analysis of VTE Cases
The team nurse practitioner used the PACS radiology reporting and archival system (IMPAX version 4.5; AGFA Healthcare Informatics, Greenville, SC) to identify all new diagnoses of VTE, in the process described below.
Procedure codes for following studies were entered into the IMPAX search engine to locate all such exams performed in the previous 1 to 3 days:
Ultrasound exams of the neck, upper extremities, and lower extremities;
Computed tomography (CT) angiograms of the chest;
Ventilation/perfusion nuclear medicine scans; and
Pulmonary angiograms.
Negative studies and studies that revealed unchanged chronic thromboses were excluded, while clots with a chronic appearance but no evidence of prior diagnosis were included. Iliofemoral, popliteal, calf vein, subclavian, internal and external jugular vein, and axillary vein thromboses were therefore included, as were all PEs. Less common locations, such as renal vein and cavernous sinus thromboses, were excluded. The improvement/research team exerted no influence over decisions about whether or not testing was done.
Each new case of VTE was then classified as HA VTE or community‐acquired VTE. A new VTE was classified as HA if the diagnosis was first suspected and made in the hospital. A newly diagnosed VTE was also classified as HA if the VTE was suspected in the ambulatory setting, but the patient had been hospitalized within the arbitrary window of the preceding 30 days.
Each new diagnosis of HA VTE was reviewed by core members of the multidisciplinary support team. This investigation included a determination of whether the patient was on an adequate VTE prophylaxis regimen at the time of the HA VTE, using the RAM and linked prophylaxis menu described above. The VTE prevention regimen ordered at the time the inpatient developed the HA VTE was classified as adherent or nonadherent to the University of California, San Diego (UCSD) protocol: patients who developed VTE when on suboptimal prophylaxis per protocol were classified as having a potentially preventable case. Potentially iatrogenic precipitants of VTE (such as the presence of a central venous catheter or restraints) were also noted. All data were entered into a Microsoft Access database for ease of retrieval and reporting.
All tests for VTE were performed based on clinical signs and symptoms, rather than routine screening, except for the Trauma and Burn services, which also screen for VTE in high‐risk patients per their established screening protocols.
Statistical Analysis of VTE Prophylaxis and HA VTE Cases
Gender differences between cases of VTE and randomly sampled and audited inpatients were examined by chi‐square analysis, and analysis of variance (ANOVA) was used to examine any age or body mass index (BMI) differences between audits and cases.
The unadjusted risk ratio (RR) for adequate prophylaxis was compared by year, with year 2005 being the baseline (comparison) year, by chi‐square analysis.
The unadjusted RR of HA VTE was calculated by dividing the number of cases found in the calendar year by the hospital census of adult inpatients at risk. For each case, a classification for the type of VTE (PE vs. DVT vs. combinations) was recorded. Cases not receiving adequate prophylaxis were categorized as preventable DVT. Unadjusted RRs were calculated for each year by chi‐square analysis, compared to the baseline (2005) year.
All data were analyzed using Stata (version 10; Stata Corp., College Station, TX). Results for the different analysis were considered significant at P < 0.05.
Retrospective Study of Unintentional Adverse Effects
The increase in anticoagulant use accompanying the introduction of the VTE prophylaxis order set warranted an evaluation of any subsequent rise in related adverse events. A study was done to determine the rates of bleeding and heparin‐induced thrombocytopenia (HIT) before and after the implementation of the VTE prophylaxis order set.
A retrospective analysis was conducted to evaluate outcomes in our inpatients from December 2004 through November 2006, with April to November, 2006 representing the post‐order set implementation time period. Any patient with a discharge diagnosis code of e934.2 (anticoagulant‐related adverse event) was selected for study to identify possible bleeding attributable to pharmacologic VTE prophylaxis. Major or minor bleeding attributable to pharmacologic VTE prophylaxis was defined as a bleed occurring 72 hours after receiving pharmacologic VTE prophylaxis. Major bleeding was defined as cerebrovascular, gastrointestinal, retroperitoneal, or overt bleeding with a decrease in hemoglobin 2 mg/dL with clinical symptoms such as hypotension or hypoxia (not associated with hemodialysis) or transfusion of 2 units of packed red blood cells. Minor bleeding was defined as ecchymosis, epistaxis, hematoma, hematuria, hemoptysis, petechiae, or bleeding without a decrease in hemoglobin 2 g/dL.
Possible cases of HIT were identified by screening for a concomitant secondary thrombocytopenia code (287.4). Chart review was then conducted to determine a causal relationship between the use of pharmacologic VTE prophylaxis and adverse events during the hospital stay. HIT attributable to pharmacologic VTE prophylaxis was determined by assessing if patients developed any of the following clinical criteria after receiving pharmacologic VTE prophylaxis: platelet count <150 109/L or 50% decrease from baseline, with or without an associated venous or arterial thrombosis or other sequelae (skin lesions at injection site, acute systemic reaction) and/or a positive heparin‐induced platelet activation (HIPA) test. In order to receive a diagnosis of HIT, thrombocytopenia must have occurred between days 5 to 15 of heparin therapy, unless existing evidence suggested that the patient developed rapid‐onset HIT or delayed‐onset HIT. Rapid‐onset HIT was defined as an abrupt drop in platelet count upon receiving a heparin product, due to heparin exposure within the previous 100 days. Delayed‐onset HIT was defined as HIT that developed several days after discontinuation of heparin. Other evident causes of thrombocytopenia were ruled out.
Statistical Analysis of Retrospective Study of Unintentional Adverse Effects
Regression analysis with chi‐square and ANOVA were used in the analysis of the demographic data. RRs were calculated for the number of cases coded with an anticoagulant‐related adverse event secondary thrombocytopenia before and after the order set implementation.
Educational Efforts and Feedback
Members of the multidisciplinary team presented information on HA VTE and the VTE prevention protocol at Medical and Surgical grand rounds, teaching rounds, and noon conference, averaging 1 educational session per quarter. Feedback and education was provided to physicians and nursing staff when audits revealed that a patient had inadequate prophylaxis with reference to the protocol standard. In addition, these conversations provided on opportunity to explore reasons for nonadherence with the protocol, confusion regarding the VTE RAM, and other barriers to effective prophylaxis, thereby providing guidance for further protocol revision and educational efforts. We adjusted the order set based on active monitoring of order set use and the audit process.
Results
There were 30,850 adult medical/surgical inpatients admitted to the medical center with a length of stay of 48 hours or more in 2005 to 2007, representing 186,397 patient‐days of observation. A total of 2,924 of these patients were randomly sampled during the VTE prophylaxis audit process (mean 81 audits per month). Table 2 shows the characteristics of randomly sampled audit patients and of the patients diagnosed with HA VTE. The demographics of the 30,850‐inpatient population (mean age = 50 years; 60.7% male; 52% Surgical Services) mirrored the demographics of the randomly sampled inpatients that underwent audits, validating the random sampling methods.
| Number (n = 3285) | % of Study Population* | Cases (n = 361) [n (%)] | Audits (n = 2924) [n (%)] | OR (95% CI) | |
|---|---|---|---|---|---|
| |||||
| Age (years) mean SD | 51 16 (range 15‐100) | 53 17 | 50 17 | 1.01 (1.003‐1.016) | |
| Gender, males | 1993 | 61 | 213 (59) | 1782 (61) | 0.93 (0.744‐1.16) |
| Major service: | |||||
| Surgery | 1714 | 52 | 200 (55) | 1516 (52) | |
| Medicine | 1566 | 48 | 161 (45) | 1408 (48) | |
| Service, detail | |||||
| Hospitalist | 1041 | 32 | 83 (23) | 958 (33) | |
| General surgery | 831 | 25 | 75 (21) | 756 (26) | |
| Trauma | 419 | 13 | 77 (22) | 342 (12) | |
| Cardiology | 313 | 10 | 45 (13) | 268 (9) | |
| Orthopedics | 244 | 7 | 15 (4) | 229 (8) | |
| Burn unit | 205 | 6 | 29 (8) | 176 (6) | |
| Other | 222 | 7 | 30 (8) | 192 (7) | |
The majority of inpatients sampled in the audits were in the moderate VTE risk category (84%), 12% were in the high‐risk category, and 4% were in the low‐risk category. The distribution of VTE risk did not change significantly over this time period.
Interobserver Agreement
The VTE RAM interobserver agreement was assessed on 150 patients with 5 observers as described above. The kappa score for the VTE risk level was 0.81. The kappa score for the judgment of whether the patient was on adequate prophylaxis or not was 0.90.
Impact on Percent of Patients with Adequate Prophylaxis (Longitudinal Audits)
Audits of randomly sampled inpatients occurred longitudinally throughout the study period as described above. Based on the intervention, the percent of patients on adequate prophylaxis improved significantly (P < 0.001) by each calendar year (see Table 3), from a baseline of 58% in 2005 to 78% in 2006 (unadjusted relative benefit = 1.35; 95% confidence interval [CI] = 1.28‐1.43), and 93% in 2007 (unadjusted relative benefit = 1.61; 95% CI = 1.52, 1.69). The improvement seen was more marked in the moderate VTE risk patients when compared to the high VTE risk patients. The percent of audited VTE prophylaxis improved from 53% in calendar year (CY) 2005 to 93% in 2007 (unadjusted relative benefit = 1.75; 95% CI = 1.70‐1.81) in the moderate VTE risk group, while the high VTE risk group improved from 83% to 92% in the same time period (unadjusted relative benefit = 1.11; 95% CI = 0.95‐1.25).
| 2005 | 2006 | 2007 | |
|---|---|---|---|
| |||
| All audits | 1279 | 960 | 679 |
| Prophylaxis adequate, n (%) | 740 (58) | 751 (78) | 631 (93) |
| Relative benefit (95% CI) | 1 | 1.35* (1.28‐1.43) | 1.61* (1.52‐1.69) |
Overall, adequate VTE prophylaxis was present in over 98% of audited patients in the last 6 months of 2007, and this high rate has been sustained throughout 2008. Age, ethnicity, and gender were not associated with differential rates of adequate VTE prophylaxis.
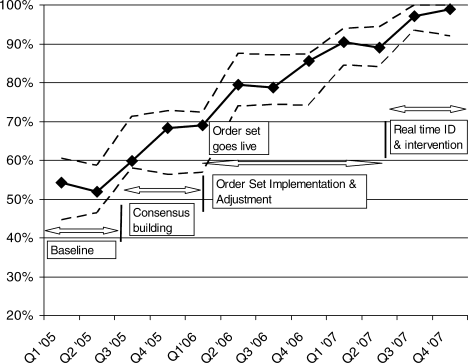
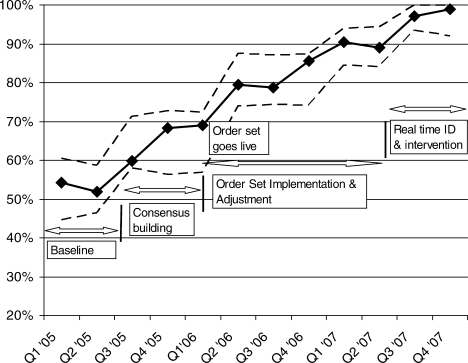
Figure 1 is a timeline of interventions and the impact on the prevalence of adequate VTE prophylaxis. The first 7 to 8 months represent the baseline rate 50% to 55% of VTE prophylaxis. In this baseline period, the improvement team was meeting, but had not yet begun meeting with the large variety of medical and surgical service leaders. Consensus‐building sessions with these leaders in the latter part of 2005 through mid‐2006 correlated with improvement in adequate VTE prophylaxis rates to near 70%. The consensus‐building sessions also prepared these varied services for a go live date of the modular order set that was incorporated into all admit and transfer order sets, often replacing preexisting orders referring to VTE prevention measures. The order set resulted in an improvement to 80% adequate prophylaxis, with the incremental improvement occurring virtually overnight with the go live date at the onset of quarter 2 (Q2) of 2006. Monitoring of the order set use confirmed that it was easy and efficient to use, but also revealed that physicians were at times classifying patients as low VTE risk inaccurately, when they possessed qualities that actually qualified them for moderate risk status by our protocol. We therefore inserted a secondary CPOE screen when patients were categorized as low VTE risk, asking the physician to deny or confirm that the patient had no risk factors that qualified them for moderate risk status. This confirmation screen essentially acted as a reminder to the physician to ask Are you sure this patient does not need VTE prophylaxis? This minor modification of the CPOE order set improved adequate VTE prophylaxis rates to 90%. Finally, we asked nurses to evaluate patients who were not on therapeutic or prophylactic doses of anticoagulants. Patients with VTE risk factors but no obvious contraindications generated a note from the nurse to the doctor, prompting the doctor to reassess VTE risk and potential contraindications. This simple intervention raised the percent of audited patients on adequate VTE prophylaxis to 98% in the last 6 months of 2007.
Description of Prospectively Identified VTE
We identified 748 cases of VTE among patients admitted to the medical center over the 36‐month study period; 387 (52%) were community‐acquired VTE. There were 361 HA cases (48% of total cases) over the same time period. There was no difference in age, gender, or BMI between the community‐acquired and hospital‐related VTE.
Of the 361 HA cases, 199 (55%) occurred on Surgical Services and 162 (45%) occurred on Medical Services; 58 (16%) unique patients had pulmonary emboli, while 303 (84%) patients experienced only DVT. Remarkably, almost one‐third of the DVT occurred in the upper extremities (108 upper extremities, 240 lower extremities), and most (80%) of the upper‐extremity DVT were associated with central venous catheters.
Of 361 HA VTE cases, 292 (81%) occurred in those in the moderate VTE risk category, 69 HA VTE cases occurred in high‐risk category patients (19%), and no VTE occurred in patients in the low‐risk category.
Improvement in HA VTE
HA VTE were identified and each case analyzed on an ongoing basis over the entire 3 year study period, as described above. Table 4 depicts a comparison of HA VTE on a year‐to‐year basis and the impact of the VTE prevention protocol on the incidence of HA VTE. In 2007 (the first full CY after the implementation of the order set) there was a 39% relative risk reduction (RRR) in the risk of experiencing an HA VTE. The reduction in the risk of preventable HA VTE was even more marked (RRR = 86%; 7 preventable VTE in 2007, compared to 44 in baseline year of 2005; RR = 0.14; 95% CI = 0.06‐0.31).
| HA VTE by Year | |||
|---|---|---|---|
| 2005 | 2006 | 2007 | |
| |||
| Patients at Risk | 9720 | 9923 | 11,207 |
| Cases with any HA VTE | 131 | 138 | 92 |
| Risk for HA VTE | 1 in 76 | 1 in 73 | 1 in 122 |
| Unadjusted relative risk (95% CI) | 1.0 | 1.03 (0.81‐1.31) | 0.61* (0.47‐0.79) |
| Cases with PE | 21 | 22 | 15 |
| Risk for PE | 1 in 463 | 1 in 451 | 1 in 747 |
| Unadjusted relative risk (95% CI) | 1.0 | 1.03 (0.56‐1.86) | 0.62 (0.32‐1.20) |
| Cases with DVT (and no PE) | 110 | 116 | 77 |
| Risk for DVT | 1 in 88 | 1 in 85 | 1 in 146 |
| Unadjusted relative risk (95% CI) | 1.0 | 1.03 (0.80‐1.33) | 0.61* (0.45‐0.81) |
| Cases with preventable VTE | 44 | 21 | 7 |
| Risk for preventable VTE | 1 in 221 | 1 in 473 | 1 in 1601 |
| Unadjusted relative risk (95% CI) | 1.0 | 0.47 (0.28‐0.79) | 0.14* (0.06‐0.31) |
Retrospective Analysis of Impact on HIT and Bleeding
There were no statistically significant differences in the number of cases coded for an anticoagulant‐related bleed or secondary thrombocytopenia (Table 5). Chart review revealed there were 2 cases of minor bleeding attributable to pharmacologic VTE prophylaxis before the order set implementation. There were no cases after implementation. No cases of HIT attributable to pharmacologic VTE prophylaxis were identified in either study period, with all cases being attributed to therapeutic anticoagulation.
| Pre‐order Set | Post‐order Set | Post‐order Set RR (CI) | |
|---|---|---|---|
| |||
| Bleeding events | 74 | 28 | 0.70 (0.46‐1.08) |
| Due to prophylaxis | 2 (minor) | 0 | |
| HIT events | 9 | 7 | 1.44 (0.54‐3.85) |
| Due to prophylaxis | 0 | 0 | |
| Patient admissions | 32117 | 17294 | |
Discussion
We demonstrated that implementation of a standardized VTE prevention protocol and order set could result in a dramatic and sustained increase in adequate VTE prophylaxis across an entire adult inpatient population. This achievement is more remarkable given the rigorous criteria defining adequate prophylaxis. Mechanical compression devices were not accepted as primary prophylaxis in moderate‐risk or high‐risk patients unless there was a documented contraindication to pharmacologic prophylaxis, and high VTE risk patients required both mechanical and pharmacologic prophylaxis to be considered adequately protected, for example. The relegation of mechanical prophylaxis to an ancillary role was endorsed by our direct observations, in that we were only able to verify that ordered mechanical prophylaxis was in place 60% of the time.
The passive dissemination of guidelines is ineffective in securing VTE prophylaxis.19 Improvement in VTE prophylaxis has been suboptimal when options for VTE prophylaxis are offered without providing guidance for VTE risk stratification and all options (pharmacologic, mechanical, or no prophylaxis) are presented as equally acceptable choices.20, 21 Our multifaceted strategy using multiple interventions is an approach endorsed by a recent systematic review19 and others in the literature.22, 23 The interventions we enacted included a method to prompt clinicians to assess patients for VTE risk, and then to assist in the selection of appropriate prophylaxis from standardized options. Decision support and clinical reminders have been shown to be more effective when integrated into the workflow19, 24; therefore, a key strategy of our study involved embedding the VTE risk assessment tool and guidance toward appropriate prophylactic regimens into commonly used admission/transfer order sets. We addressed the barriers of physician unfamiliarity or disagreement with guidelines10 with education and consensus‐building sessions with clinical leadership. Clinical feedback from audits, peer review, and nursing‐led interventions rounded out the layered multifaceted interventional approach.
We designed and prospectively validated a VTE RAM during the course of our improvement efforts, and to our knowledge our simple 3‐category (or 3‐level) VTE risk assessment model is the only validated model. The VTE risk assessment/prevention protocol was validated by several important parameters. First, it proved to be practical and easy to use, taking only seconds to complete, and it was readily adopted by all adult medical and surgical services. Second, the VTE RAM demonstrated excellent interobserver agreement for VTE risk level and decisions about adequacy of VTE prophylaxis with 5 physician reviewers. Third, the VTE RAM predicted risk for VTE. All patients suffering from HA VTE were in the moderate‐risk to high‐risk categories, and HA VTE occurred disproportionately in those meeting criteria for high risk. Fourth, implementation of the VTE RAM/protocol resulted in very high, sustained levels of VTE prophylaxis without any detectable safety concerns. Finally and perhaps most importantly, high rates of adherence to the VTE protocol resulted in a 40% decline in the incidence of HA VTE in our institution.
The improved prevalence of adequate VTE prophylaxis reduced, but did not eliminate, HA VTE. The reduction observed is consistent with the 40% to 50% efficacy of prophylaxis reported in the literature.7 Our experience highlights the recent controversy over proposals by the Centers for Medicare & Medicaid Services (CMS) to add HA VTE to the list of do not pay conditions later this year,25 as it is clear from our data that even near‐perfect adherence with accepted VTE prevention measures will not eliminate HA VTE. After vigorous pushback about the fairness of this measure, the HA VTE do not pay scope was narrowed to include only certain major orthopedic procedure patients.
Services with a preponderance of moderate‐risk patients had the largest reduction in HA VTE. Efforts that are focused only on high‐risk orthopedic, trauma, and critical care patients will miss the larger opportunities for maximal reduction in HA VTE for multiple reasons. First, moderate VTE risk patients are far more prevalent than high VTE risk patients (84% vs. 12% of inpatients at our institution). Second, high‐risk patients are already at a baseline relatively high rate of VTE prophylaxis compared to their moderate VTE risk counterparts (83% vs. 53% at our institution). Third, a large portion of patients at high risk for VTE (such as trauma patients) also have the largest prevalence of absolute or relative contraindications to pharmacologic prophylaxis, limiting the effect size of prevention efforts.
Major strengths of this study included ongoing rigorous concurrent measurement of both processes (percent of patients on adequate prophylaxis) and outcomes (HA VTE diagnosed via imaging studies) over a prolonged time period. The robust random sampling of inpatients insured that changes in VTE prophylaxis rates were not due to changes in the distribution of VTE risk or bias potentially introduced from convenience samples. The longitudinal monitoring of imaging study results for VTE cases is vastly superior to using administrative data that is reliant on coding. The recent University Healthsystem Consortium (UHC) benchmarking data on venous thromboembolism were sobering but instructive.26 UHC used administrative discharge codes for VTE in a secondary position to identify patients with HA VTE, which is a common strategy to follow the incidence of HA VTE. The accuracy of identifying surgical patients with an HA VTE was only 60%. Proper use of the present on admission (POA) designation would have improved this to 83%, but 17% of cases either did not occur or had history only with a labor‐intensive manual chart review. Performance was even worse in medical patients, with only a 30% accuracy rate, potentially improved to 79% if accurate POA designation had been used, and 21% of cases identified by administrative methods either did not occur or had history only. In essence, unless an improvement team uses chart review of each case potentially identified as a HA VTE case, the administrative data are not reliable. Concurrent discovery of VTE cases allows for a more accurate and timely chart review, and allows for near real‐time feedback to the responsible treatment team.
The major limitation of this study is inherent in the observational design and the lack of a control population. Other factors besides our VTE‐specific improvement efforts could affect process and outcomes, and reductions in HA VTE could conceivably occur because of changes in the make‐up of the admitted inpatient population. These limitations are mitigated to some degree by several observations. The VTE risk distribution in the randomly sampled inpatient population did not vary significantly from year to year. The number of HA VTE was reduced in 2007 even though the number of patients and patient days at risk for developing VTE went up. The incidence of community‐acquired VTE remained constant over the same time period, highlighting the consistency of our measurement techniques and the VTE risk in the community we serve. Last, the improvements in VTE prophylaxis rates increased at times that correlated well with the introduction of layered interventions, as depicted in Figure 1.
There were several limitations to the internal study on adverse effects of VTE protocol implementation. First, this was a retrospective study, so much of the data collection was dependent upon physician progress notes and discharge summaries. Lack of documentation could have precluded the appropriate diagnosis codes from being assigned. Next, the study population was generated from coding data, so subjectivity could have been introduced during the coding process. Also, a majority of the patients did not fit the study criteria due to discharge with the e934.2 code, because they were found to have an elevated international normalized ratio (INR) after being admitted on warfarin. Finally, chart‐reviewer bias could have affected the results, as the chart reviewer became more proficient at reviewing charts over time. Despite these limitations, the study methodology allowed for screening of a large population for rare events. Bleeding may be a frequent concern with primary thromboprophylaxis, but data from clinical trials and this study help to demonstrate that rates of adverse events from pharmacologic VTE prophylaxis are very rare.
Another potential limitation is raised by the question of whether our methods can be generalized to other sites. Our site is an academic medical center and we have CPOE, which is present in only a small minority of centers. Furthermore, one could question how feasible it is to get institution‐wide consensus for a VTE prevention protocol in settings with heterogenous medical staffs. To address these issues, we used a proven performance improvement framework calling for administrative support, a multidisciplinary improvement team, reliable measures, and a multifaceted approach to interventions. This framework and our experiences have been incorporated into improvement guides27, 28 that have been the centerpiece of the Society of Hospital Medicine VTE Prevention Collaborative improvement efforts in a wide variety of medical environments. The collaborative leadership has observed that success is the rule when this model is followed, in institutions large and small, academic or community, and in both paper and CPOE environments. Not all of these sites use a VTE RAM identical to ours, and there are local nuances to preferred choices of prophylaxis. However, they all incorporated simple VTE risk stratification with only a few levels of risk. Reinforcing the expectation that pharmacologic prophylaxis is indicated for the majority of inpatients is likely more important than the nuances of choices for each risk level.
We demonstrated that dramatic improvement in VTE prophylaxis is achievable, safe, and effective in reducing the incidence of HA VTE. We used scalable, portable methods to make a large and convincing impact on the incidence of HA VTE, while also developing and prospectively validating a VTE RAM. A wide variety of institutions are achieving significant improvement using similar strategies. Future research and improvement efforts should focus on how to accelerate integration of this model across networks of hospitals, leveraging networks with common order sets or information systems. Widespread success in improving VTE prophylaxis would likely have a far‐reaching benefit on morbidity and PE‐related mortality.
- U.S. Department of Health and Human Services. Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism.2008 Clean-up Rule No. CU01 invoked here. . Available at: http://www.surgeongeneral.gov/topics/deepvein. Accessed June 2009.
- ,,, et al.Incidence of venous thromboembolism in hospitalized patients vs. community residents.Mayo Clin Proc.2001;76:1102–1110.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism.Arch Intern Med.2002;162:1245–1248.
- ,,, et al.Antithrombotic therapy practices in US hospitals in an era of practice guidelines.Arch Intern Med.2005;165:1458–1464.
- ,,, et al.Prevention of venous thromboembolism.Chest.1995;108:312–334.
- ,,, et al.Prevention of venous thromboembolism: ACCP Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6 Suppl):381S–453S.
- ,.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259–262.
- ,,, et al.The outcome after treatment of venous thromboembolism is different in surgical and acutely ill medical patients. Findings from the RIETE registry.J Thromb Haemost.2004;2:1892–1898.
- ,,, et al.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the international medical prevention registry on venous thromboembolism.Chest.2007;132(3):936–945.
- ,,, et al.Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada.Thromb Res.2007;119(2):145–155.
- ,,, et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- ,,.Compliance with recommended prophylaxis for venous thromboembolism: improving the use and rate of uptake of clinical practice guidelines.J Thromb Haemost.2004;2:221–227.
- ,.Risk factors for venous thromboembolism.Circulation.2003;107:I‐9–I‐16.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 suppl 5):12–19.
- ,,,,.Identification of candidates for prevention of venous thromboembolism.Semin Thromb Hemost.1997;23(1):55–67.
- .Venous thromboembolic risk and its prevention in hospitalized medical patients.Semin Thromb Hemost.2002;28(6);577–583.
- ,,, et al.A guide to venous thromboembolism risk factor assessment.J Thromb Thrombolysis.2000;9:253–262.
- ,,, et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241:397–415.
- ,,,.Medical admission order sets to improve deep vein thrombosis prophylaxis rates and other outcomes.J Hosp Med.2009;4(2):81–89.
- .Medical admission order sets to improve deep vein thrombosis prevention: a model for others or a prescription for mediocrity? [Editorial].J Hosp Med.2009;4(2):77–80.
- ,,,.No magic bullets: a systematic review of 102 trials of interventions to improve professional practice.CMAJ.1995;153:1423–1431.
- ,,.Innovative approaches to increase deep vein thrombosis prophylaxis rate resulting in a decrease in hospital‐acquired deep vein thrombosis at a tertiary‐care teaching hospital.J Hosp Med.2008;3(2):148–155.
- ,,,.Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies.Rockville, MD:Agency for Healthcare Research and Quality;2004.
- CMS Office of Public Affairs. Fact Sheet: CMS Proposes Additions to List of Hospital‐Acquired Conditions for Fiscal Year 2009. Available at: http://www.cms.hhs.gov/apps/media/press/factsheet.asp?Counter=3042. Accessed June2009.
- The DVT/PE 2007 Knowledge Transfer Meeting. Proceedings of November 30, 2007 meeting. Available at: http://www.uhc.edu/21801.htm. Accessed June2009.
- ,. Preventing Hospital‐Acquired Venous Thromboembolism. A Guide for Effective Quality Improvement. Society of Hospital Medicine, VTE Quality Improvement Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_VTE/VTE_Home.cfm. Accessed June 2009.
- ,.Preventing Hospital‐Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement. Prepared by the Society of Hospital Medicine. AHRQ Publication No. 08–0075.Rockville, MD:Agency for Healthcare Research and Quality. September2008. Available at: http://www.ahrq.gov/qual/vtguide. Accessed June 2009.
Pulmonary embolism (PE) and deep vein thrombosis (DVT), collectively referred to as venous thromboembolism (VTE), represent a major public health problem, affecting hundreds of thousands of Americans each year.1 The best estimates are that at least 100,000 deaths are attributable to VTE each year in the United States alone.1 VTE is primarily a problem of hospitalized and recently‐hospitalized patients.2 Although a recent meta‐analysis did not prove mortality benefit of prophylaxis in the medical population,3 PE is frequently estimated to be the most common preventable cause of hospital death.46
Pharmacologic methods to prevent VTE are safe, effective, cost‐effective, and advocated by authoritative guidelines.7 Even though the majority of medical and surgical inpatients have multiple risk factors for VTE, large prospective studies continue to demonstrate that these preventive methods are significantly underutilized, often with only 30% to 50% eligible patients receiving prophylaxis.812
The reasons for this underutilization include lack of physician familiarity or agreement with guidelines, underestimation of VTE risk, concern over risk of bleeding, and the perception that the guidelines are resource‐intensive or difficult to implement in a practical fashion.13 While many VTE risk‐assessment models are available in the literature,1418 a lack of prospectively validated models and issues regarding ease of use have further hampered widespread integration of VTE risk assessments into order sets and inpatient practice.
We sought to optimize prevention of hospital‐acquired (HA) VTE in our 350‐bed tertiary‐care academic center using a VTE prevention protocol and a multifaceted approach that could be replicated across a wide variety of medical centers.
Patients and Methods
Study Design
We developed, implemented, and refined a VTE prevention protocol and examined the impact of our efforts. We observed adult inpatients on a longitudinal basis for the prevalence of adequate VTE prophylaxis and for the incidence of HA VTE throughout a 36‐month period from calendar year 2005 through 2007, and performed a retrospective analysis for any potential adverse effects of increased VTE prophylaxis. The project adhered to the HIPAA requirements for privacy involving health‐related data from human research participants. The study was approved by the Institutional Review Board of the University of California, San Diego, which waived the requirement for individual patient informed consent.
We included all hospitalized adult patients (medical and surgical services) at our medical center in our observations and interventions, including patients of all ethnic groups, geriatric patients, prisoners, and the socially and economically disadvantaged in our population. Exclusion criteria were age under 14 years, and hospitalization on Psychiatry or Obstetrics/Gynecology services.
Development of a VTE Risk‐assessment Model and VTE Prevention Protocol
A core multidisciplinary team with hospitalists, pulmonary critical care VTE experts, pharmacists, nurses, and information specialists was formed. After gaining administrative support for standardization, we worked with medical staff leaders to gain consensus on a VTE prevention protocol for all medical and surgical areas from mid‐2005 through mid‐2006. The VTE prevention protocol included the elements of VTE risk stratification, definitions of adequate VTE prevention measures linked to the level of VTE risk, and definitions for contraindications to pharmacologic prophylactic measures. We piloted risk‐assessment model (RAM) drafts for ease of use and clarity, using rapid cycle feedback from pharmacy residents, house staff, and medical staff attending physicians. Models often cited in the literature15, 18 that include point‐based scoring of VTE risk factors (with prophylaxis choices hinging on the additive sum of scoring) were rejected based on the pilot experience.
We adopted a simple model with 3 levels of VTE risk that could be completed by the physician in seconds, and then proceeded to integrate this RAM into standardized data collection instruments and eventually (April 2006) into a computerized provider order entry (CPOE) order set (Siemmens Invision v26). Each level of VTE risk was firmly linked to a menu of acceptable prophylaxis options (Table 1). Simple text cues were used to define risk assessment, with more exhaustive listings of risk factors being relegated to accessible reference tables.
| Low | Moderate | High |
|---|---|---|
| ||
| Ambulatory patient without VTE risk factors; observation patient with expected LOS 2 days; same day surgery or minor surgery | All other patients (not in low‐risk or high‐risk category); most medical/surgical patients; respiratory insufficiency, heart failure, acute infectious, or inflammatory disease | Lower extremity arthroplasty; hip, pelvic, or severe lower extremity fractures; acute SCI with paresis; multiple major trauma; abdominal or pelvic surgery for cancer |
| Early ambulation | UFH 5000 units SC q 8 hours; OR LMWH q day; OR UFH 5000 units SC q 12 hours (if weight < 50 kg or age > 75 years); AND suggest adding IPC | LMWH (UFH if ESRD); OR fondaparinux 2.5 mg SC daily; OR warfarin, INR 2‐3; AND IPC (unless not feasible) |
Intermittent pneumatic compression devices were endorsed as an adjunct in all patients in the highest risk level, and as the primary method in patients with contraindications to pharmacologic prophylaxis. Aspirin was deemed an inappropriate choice for VTE prophylaxis. Subcutaneous unfractionated or low‐molecular‐weight heparin were endorsed as the primary method of prophylaxis for the majority of patients without contraindications.
Integration of the VTE Protocol into Order Sets
An essential strategy for the success of the VTE protocol included integrating guidance for the physician into the flow of patient care, via standardized order sets. The CPOE VTE prevention order set was modular by design, as opposed to a stand alone design. After conferring with appropriate stakeholders, preexisting and nonstandardized prompts for VTE prophylaxis were removed from commonly used order sets, and the standardized module was inserted in its place. This allowed for integration of the standardized VTE prevention module into all admission and transfer order sets, essentially insuring that all patients admitted or transferred within the medical center would be exposed to the protocol. Physicians using a variety of admission and transfer order sets were prompted to select each patient's risk for VTE, and declare the presence or absence of contraindications to pharmacologic prophylaxis. Only the VTE prevention options most appropriate for the patient's VTE and anticoagulation risk profile were presented as the default choice for VTE prophylaxis. Explicit designation of VTE risk level and a prophylaxis choice were presented in a hard stop mechanism, and utilization of these orders was therefore mandatory, not optional. Proper use (such as the proper classification of VTE risk by the ordering physician) was actively monitored on an auditing basis, and order sets were modified occasionally on the basis of subjective and objective feedback.
Assessment of VTE Risk Assessment Interobserver Agreement
Data from 150 randomly selected patients from the audit pool (from late 2005 through mid‐2006) were abstracted by the nurse practitioner in a detailed manner. Five independent reviewers assessed each patient for VTE risk level, and for a determination of whether or not they were on adequate VTE prophylaxis on the day of the audit per protocol. Interobserver agreement was calculated for these parameters using kappa scores.
Prospective Monitoring of Adequate VTE Prophylaxis
A daily medical center inpatient census report of eligible patients in the medical center for >48 hours was downloaded into an Microsoft Excel spreadsheet, with each patient assigned a consecutive number. The Excel random number generator plug‐in function was used to generate a randomly sequenced list of the patients. The research nurse practitioner targeted serial patients on the list for further study, until she accomplished the requisite number of audits each day. The mean number of audits per month declined over the study years as the trends stabilized and as grant funding expired, but remained robust throughout (2005: 107 audits per month; 2006: 80 audits per month; and 2007: 57 audits per month).
The data collected on each patient randomly selected for audit included age, gender, location, service, date and time of review, and date of admission. The audit VTE RAM (identical to the VTE RAM incorporated into the order set), was used to classify each patient's VTE risk as low, moderate, or high. For each audit, we determined if the patient was on an adequate VTE prevention regimen consistent with our protocol, given their VTE risk level, demographics, and absence or presence of contraindications to pharmacologic prophylaxis. All questionable cases were reviewed by at least 2 physicians at weekly meetings with a final consensus determination. Adequacy of the VTE regimen was judged by orders entered on the day of the audit, but we also noted whether or not ordered intermittent compression devices were in place and functioning at the time of the audit.
Prospective (Concurrent) Discovery and Analysis of VTE Cases
The team nurse practitioner used the PACS radiology reporting and archival system (IMPAX version 4.5; AGFA Healthcare Informatics, Greenville, SC) to identify all new diagnoses of VTE, in the process described below.
Procedure codes for following studies were entered into the IMPAX search engine to locate all such exams performed in the previous 1 to 3 days:
Ultrasound exams of the neck, upper extremities, and lower extremities;
Computed tomography (CT) angiograms of the chest;
Ventilation/perfusion nuclear medicine scans; and
Pulmonary angiograms.
Negative studies and studies that revealed unchanged chronic thromboses were excluded, while clots with a chronic appearance but no evidence of prior diagnosis were included. Iliofemoral, popliteal, calf vein, subclavian, internal and external jugular vein, and axillary vein thromboses were therefore included, as were all PEs. Less common locations, such as renal vein and cavernous sinus thromboses, were excluded. The improvement/research team exerted no influence over decisions about whether or not testing was done.
Each new case of VTE was then classified as HA VTE or community‐acquired VTE. A new VTE was classified as HA if the diagnosis was first suspected and made in the hospital. A newly diagnosed VTE was also classified as HA if the VTE was suspected in the ambulatory setting, but the patient had been hospitalized within the arbitrary window of the preceding 30 days.
Each new diagnosis of HA VTE was reviewed by core members of the multidisciplinary support team. This investigation included a determination of whether the patient was on an adequate VTE prophylaxis regimen at the time of the HA VTE, using the RAM and linked prophylaxis menu described above. The VTE prevention regimen ordered at the time the inpatient developed the HA VTE was classified as adherent or nonadherent to the University of California, San Diego (UCSD) protocol: patients who developed VTE when on suboptimal prophylaxis per protocol were classified as having a potentially preventable case. Potentially iatrogenic precipitants of VTE (such as the presence of a central venous catheter or restraints) were also noted. All data were entered into a Microsoft Access database for ease of retrieval and reporting.
All tests for VTE were performed based on clinical signs and symptoms, rather than routine screening, except for the Trauma and Burn services, which also screen for VTE in high‐risk patients per their established screening protocols.
Statistical Analysis of VTE Prophylaxis and HA VTE Cases
Gender differences between cases of VTE and randomly sampled and audited inpatients were examined by chi‐square analysis, and analysis of variance (ANOVA) was used to examine any age or body mass index (BMI) differences between audits and cases.
The unadjusted risk ratio (RR) for adequate prophylaxis was compared by year, with year 2005 being the baseline (comparison) year, by chi‐square analysis.
The unadjusted RR of HA VTE was calculated by dividing the number of cases found in the calendar year by the hospital census of adult inpatients at risk. For each case, a classification for the type of VTE (PE vs. DVT vs. combinations) was recorded. Cases not receiving adequate prophylaxis were categorized as preventable DVT. Unadjusted RRs were calculated for each year by chi‐square analysis, compared to the baseline (2005) year.
All data were analyzed using Stata (version 10; Stata Corp., College Station, TX). Results for the different analysis were considered significant at P < 0.05.
Retrospective Study of Unintentional Adverse Effects
The increase in anticoagulant use accompanying the introduction of the VTE prophylaxis order set warranted an evaluation of any subsequent rise in related adverse events. A study was done to determine the rates of bleeding and heparin‐induced thrombocytopenia (HIT) before and after the implementation of the VTE prophylaxis order set.
A retrospective analysis was conducted to evaluate outcomes in our inpatients from December 2004 through November 2006, with April to November, 2006 representing the post‐order set implementation time period. Any patient with a discharge diagnosis code of e934.2 (anticoagulant‐related adverse event) was selected for study to identify possible bleeding attributable to pharmacologic VTE prophylaxis. Major or minor bleeding attributable to pharmacologic VTE prophylaxis was defined as a bleed occurring 72 hours after receiving pharmacologic VTE prophylaxis. Major bleeding was defined as cerebrovascular, gastrointestinal, retroperitoneal, or overt bleeding with a decrease in hemoglobin 2 mg/dL with clinical symptoms such as hypotension or hypoxia (not associated with hemodialysis) or transfusion of 2 units of packed red blood cells. Minor bleeding was defined as ecchymosis, epistaxis, hematoma, hematuria, hemoptysis, petechiae, or bleeding without a decrease in hemoglobin 2 g/dL.
Possible cases of HIT were identified by screening for a concomitant secondary thrombocytopenia code (287.4). Chart review was then conducted to determine a causal relationship between the use of pharmacologic VTE prophylaxis and adverse events during the hospital stay. HIT attributable to pharmacologic VTE prophylaxis was determined by assessing if patients developed any of the following clinical criteria after receiving pharmacologic VTE prophylaxis: platelet count <150 109/L or 50% decrease from baseline, with or without an associated venous or arterial thrombosis or other sequelae (skin lesions at injection site, acute systemic reaction) and/or a positive heparin‐induced platelet activation (HIPA) test. In order to receive a diagnosis of HIT, thrombocytopenia must have occurred between days 5 to 15 of heparin therapy, unless existing evidence suggested that the patient developed rapid‐onset HIT or delayed‐onset HIT. Rapid‐onset HIT was defined as an abrupt drop in platelet count upon receiving a heparin product, due to heparin exposure within the previous 100 days. Delayed‐onset HIT was defined as HIT that developed several days after discontinuation of heparin. Other evident causes of thrombocytopenia were ruled out.
Statistical Analysis of Retrospective Study of Unintentional Adverse Effects
Regression analysis with chi‐square and ANOVA were used in the analysis of the demographic data. RRs were calculated for the number of cases coded with an anticoagulant‐related adverse event secondary thrombocytopenia before and after the order set implementation.
Educational Efforts and Feedback
Members of the multidisciplinary team presented information on HA VTE and the VTE prevention protocol at Medical and Surgical grand rounds, teaching rounds, and noon conference, averaging 1 educational session per quarter. Feedback and education was provided to physicians and nursing staff when audits revealed that a patient had inadequate prophylaxis with reference to the protocol standard. In addition, these conversations provided on opportunity to explore reasons for nonadherence with the protocol, confusion regarding the VTE RAM, and other barriers to effective prophylaxis, thereby providing guidance for further protocol revision and educational efforts. We adjusted the order set based on active monitoring of order set use and the audit process.
Results
There were 30,850 adult medical/surgical inpatients admitted to the medical center with a length of stay of 48 hours or more in 2005 to 2007, representing 186,397 patient‐days of observation. A total of 2,924 of these patients were randomly sampled during the VTE prophylaxis audit process (mean 81 audits per month). Table 2 shows the characteristics of randomly sampled audit patients and of the patients diagnosed with HA VTE. The demographics of the 30,850‐inpatient population (mean age = 50 years; 60.7% male; 52% Surgical Services) mirrored the demographics of the randomly sampled inpatients that underwent audits, validating the random sampling methods.
| Number (n = 3285) | % of Study Population* | Cases (n = 361) [n (%)] | Audits (n = 2924) [n (%)] | OR (95% CI) | |
|---|---|---|---|---|---|
| |||||
| Age (years) mean SD | 51 16 (range 15‐100) | 53 17 | 50 17 | 1.01 (1.003‐1.016) | |
| Gender, males | 1993 | 61 | 213 (59) | 1782 (61) | 0.93 (0.744‐1.16) |
| Major service: | |||||
| Surgery | 1714 | 52 | 200 (55) | 1516 (52) | |
| Medicine | 1566 | 48 | 161 (45) | 1408 (48) | |
| Service, detail | |||||
| Hospitalist | 1041 | 32 | 83 (23) | 958 (33) | |
| General surgery | 831 | 25 | 75 (21) | 756 (26) | |
| Trauma | 419 | 13 | 77 (22) | 342 (12) | |
| Cardiology | 313 | 10 | 45 (13) | 268 (9) | |
| Orthopedics | 244 | 7 | 15 (4) | 229 (8) | |
| Burn unit | 205 | 6 | 29 (8) | 176 (6) | |
| Other | 222 | 7 | 30 (8) | 192 (7) | |
The majority of inpatients sampled in the audits were in the moderate VTE risk category (84%), 12% were in the high‐risk category, and 4% were in the low‐risk category. The distribution of VTE risk did not change significantly over this time period.
Interobserver Agreement
The VTE RAM interobserver agreement was assessed on 150 patients with 5 observers as described above. The kappa score for the VTE risk level was 0.81. The kappa score for the judgment of whether the patient was on adequate prophylaxis or not was 0.90.
Impact on Percent of Patients with Adequate Prophylaxis (Longitudinal Audits)
Audits of randomly sampled inpatients occurred longitudinally throughout the study period as described above. Based on the intervention, the percent of patients on adequate prophylaxis improved significantly (P < 0.001) by each calendar year (see Table 3), from a baseline of 58% in 2005 to 78% in 2006 (unadjusted relative benefit = 1.35; 95% confidence interval [CI] = 1.28‐1.43), and 93% in 2007 (unadjusted relative benefit = 1.61; 95% CI = 1.52, 1.69). The improvement seen was more marked in the moderate VTE risk patients when compared to the high VTE risk patients. The percent of audited VTE prophylaxis improved from 53% in calendar year (CY) 2005 to 93% in 2007 (unadjusted relative benefit = 1.75; 95% CI = 1.70‐1.81) in the moderate VTE risk group, while the high VTE risk group improved from 83% to 92% in the same time period (unadjusted relative benefit = 1.11; 95% CI = 0.95‐1.25).
| 2005 | 2006 | 2007 | |
|---|---|---|---|
| |||
| All audits | 1279 | 960 | 679 |
| Prophylaxis adequate, n (%) | 740 (58) | 751 (78) | 631 (93) |
| Relative benefit (95% CI) | 1 | 1.35* (1.28‐1.43) | 1.61* (1.52‐1.69) |
Overall, adequate VTE prophylaxis was present in over 98% of audited patients in the last 6 months of 2007, and this high rate has been sustained throughout 2008. Age, ethnicity, and gender were not associated with differential rates of adequate VTE prophylaxis.
Figure 1 is a timeline of interventions and the impact on the prevalence of adequate VTE prophylaxis. The first 7 to 8 months represent the baseline rate 50% to 55% of VTE prophylaxis. In this baseline period, the improvement team was meeting, but had not yet begun meeting with the large variety of medical and surgical service leaders. Consensus‐building sessions with these leaders in the latter part of 2005 through mid‐2006 correlated with improvement in adequate VTE prophylaxis rates to near 70%. The consensus‐building sessions also prepared these varied services for a go live date of the modular order set that was incorporated into all admit and transfer order sets, often replacing preexisting orders referring to VTE prevention measures. The order set resulted in an improvement to 80% adequate prophylaxis, with the incremental improvement occurring virtually overnight with the go live date at the onset of quarter 2 (Q2) of 2006. Monitoring of the order set use confirmed that it was easy and efficient to use, but also revealed that physicians were at times classifying patients as low VTE risk inaccurately, when they possessed qualities that actually qualified them for moderate risk status by our protocol. We therefore inserted a secondary CPOE screen when patients were categorized as low VTE risk, asking the physician to deny or confirm that the patient had no risk factors that qualified them for moderate risk status. This confirmation screen essentially acted as a reminder to the physician to ask Are you sure this patient does not need VTE prophylaxis? This minor modification of the CPOE order set improved adequate VTE prophylaxis rates to 90%. Finally, we asked nurses to evaluate patients who were not on therapeutic or prophylactic doses of anticoagulants. Patients with VTE risk factors but no obvious contraindications generated a note from the nurse to the doctor, prompting the doctor to reassess VTE risk and potential contraindications. This simple intervention raised the percent of audited patients on adequate VTE prophylaxis to 98% in the last 6 months of 2007.

Description of Prospectively Identified VTE
We identified 748 cases of VTE among patients admitted to the medical center over the 36‐month study period; 387 (52%) were community‐acquired VTE. There were 361 HA cases (48% of total cases) over the same time period. There was no difference in age, gender, or BMI between the community‐acquired and hospital‐related VTE.
Of the 361 HA cases, 199 (55%) occurred on Surgical Services and 162 (45%) occurred on Medical Services; 58 (16%) unique patients had pulmonary emboli, while 303 (84%) patients experienced only DVT. Remarkably, almost one‐third of the DVT occurred in the upper extremities (108 upper extremities, 240 lower extremities), and most (80%) of the upper‐extremity DVT were associated with central venous catheters.
Of 361 HA VTE cases, 292 (81%) occurred in those in the moderate VTE risk category, 69 HA VTE cases occurred in high‐risk category patients (19%), and no VTE occurred in patients in the low‐risk category.
Improvement in HA VTE
HA VTE were identified and each case analyzed on an ongoing basis over the entire 3 year study period, as described above. Table 4 depicts a comparison of HA VTE on a year‐to‐year basis and the impact of the VTE prevention protocol on the incidence of HA VTE. In 2007 (the first full CY after the implementation of the order set) there was a 39% relative risk reduction (RRR) in the risk of experiencing an HA VTE. The reduction in the risk of preventable HA VTE was even more marked (RRR = 86%; 7 preventable VTE in 2007, compared to 44 in baseline year of 2005; RR = 0.14; 95% CI = 0.06‐0.31).
| HA VTE by Year | |||
|---|---|---|---|
| 2005 | 2006 | 2007 | |
| |||
| Patients at Risk | 9720 | 9923 | 11,207 |
| Cases with any HA VTE | 131 | 138 | 92 |
| Risk for HA VTE | 1 in 76 | 1 in 73 | 1 in 122 |
| Unadjusted relative risk (95% CI) | 1.0 | 1.03 (0.81‐1.31) | 0.61* (0.47‐0.79) |
| Cases with PE | 21 | 22 | 15 |
| Risk for PE | 1 in 463 | 1 in 451 | 1 in 747 |
| Unadjusted relative risk (95% CI) | 1.0 | 1.03 (0.56‐1.86) | 0.62 (0.32‐1.20) |
| Cases with DVT (and no PE) | 110 | 116 | 77 |
| Risk for DVT | 1 in 88 | 1 in 85 | 1 in 146 |
| Unadjusted relative risk (95% CI) | 1.0 | 1.03 (0.80‐1.33) | 0.61* (0.45‐0.81) |
| Cases with preventable VTE | 44 | 21 | 7 |
| Risk for preventable VTE | 1 in 221 | 1 in 473 | 1 in 1601 |
| Unadjusted relative risk (95% CI) | 1.0 | 0.47 (0.28‐0.79) | 0.14* (0.06‐0.31) |
Retrospective Analysis of Impact on HIT and Bleeding
There were no statistically significant differences in the number of cases coded for an anticoagulant‐related bleed or secondary thrombocytopenia (Table 5). Chart review revealed there were 2 cases of minor bleeding attributable to pharmacologic VTE prophylaxis before the order set implementation. There were no cases after implementation. No cases of HIT attributable to pharmacologic VTE prophylaxis were identified in either study period, with all cases being attributed to therapeutic anticoagulation.
| Pre‐order Set | Post‐order Set | Post‐order Set RR (CI) | |
|---|---|---|---|
| |||
| Bleeding events | 74 | 28 | 0.70 (0.46‐1.08) |
| Due to prophylaxis | 2 (minor) | 0 | |
| HIT events | 9 | 7 | 1.44 (0.54‐3.85) |
| Due to prophylaxis | 0 | 0 | |
| Patient admissions | 32117 | 17294 | |
Discussion
We demonstrated that implementation of a standardized VTE prevention protocol and order set could result in a dramatic and sustained increase in adequate VTE prophylaxis across an entire adult inpatient population. This achievement is more remarkable given the rigorous criteria defining adequate prophylaxis. Mechanical compression devices were not accepted as primary prophylaxis in moderate‐risk or high‐risk patients unless there was a documented contraindication to pharmacologic prophylaxis, and high VTE risk patients required both mechanical and pharmacologic prophylaxis to be considered adequately protected, for example. The relegation of mechanical prophylaxis to an ancillary role was endorsed by our direct observations, in that we were only able to verify that ordered mechanical prophylaxis was in place 60% of the time.
The passive dissemination of guidelines is ineffective in securing VTE prophylaxis.19 Improvement in VTE prophylaxis has been suboptimal when options for VTE prophylaxis are offered without providing guidance for VTE risk stratification and all options (pharmacologic, mechanical, or no prophylaxis) are presented as equally acceptable choices.20, 21 Our multifaceted strategy using multiple interventions is an approach endorsed by a recent systematic review19 and others in the literature.22, 23 The interventions we enacted included a method to prompt clinicians to assess patients for VTE risk, and then to assist in the selection of appropriate prophylaxis from standardized options. Decision support and clinical reminders have been shown to be more effective when integrated into the workflow19, 24; therefore, a key strategy of our study involved embedding the VTE risk assessment tool and guidance toward appropriate prophylactic regimens into commonly used admission/transfer order sets. We addressed the barriers of physician unfamiliarity or disagreement with guidelines10 with education and consensus‐building sessions with clinical leadership. Clinical feedback from audits, peer review, and nursing‐led interventions rounded out the layered multifaceted interventional approach.
We designed and prospectively validated a VTE RAM during the course of our improvement efforts, and to our knowledge our simple 3‐category (or 3‐level) VTE risk assessment model is the only validated model. The VTE risk assessment/prevention protocol was validated by several important parameters. First, it proved to be practical and easy to use, taking only seconds to complete, and it was readily adopted by all adult medical and surgical services. Second, the VTE RAM demonstrated excellent interobserver agreement for VTE risk level and decisions about adequacy of VTE prophylaxis with 5 physician reviewers. Third, the VTE RAM predicted risk for VTE. All patients suffering from HA VTE were in the moderate‐risk to high‐risk categories, and HA VTE occurred disproportionately in those meeting criteria for high risk. Fourth, implementation of the VTE RAM/protocol resulted in very high, sustained levels of VTE prophylaxis without any detectable safety concerns. Finally and perhaps most importantly, high rates of adherence to the VTE protocol resulted in a 40% decline in the incidence of HA VTE in our institution.
The improved prevalence of adequate VTE prophylaxis reduced, but did not eliminate, HA VTE. The reduction observed is consistent with the 40% to 50% efficacy of prophylaxis reported in the literature.7 Our experience highlights the recent controversy over proposals by the Centers for Medicare & Medicaid Services (CMS) to add HA VTE to the list of do not pay conditions later this year,25 as it is clear from our data that even near‐perfect adherence with accepted VTE prevention measures will not eliminate HA VTE. After vigorous pushback about the fairness of this measure, the HA VTE do not pay scope was narrowed to include only certain major orthopedic procedure patients.
Services with a preponderance of moderate‐risk patients had the largest reduction in HA VTE. Efforts that are focused only on high‐risk orthopedic, trauma, and critical care patients will miss the larger opportunities for maximal reduction in HA VTE for multiple reasons. First, moderate VTE risk patients are far more prevalent than high VTE risk patients (84% vs. 12% of inpatients at our institution). Second, high‐risk patients are already at a baseline relatively high rate of VTE prophylaxis compared to their moderate VTE risk counterparts (83% vs. 53% at our institution). Third, a large portion of patients at high risk for VTE (such as trauma patients) also have the largest prevalence of absolute or relative contraindications to pharmacologic prophylaxis, limiting the effect size of prevention efforts.
Major strengths of this study included ongoing rigorous concurrent measurement of both processes (percent of patients on adequate prophylaxis) and outcomes (HA VTE diagnosed via imaging studies) over a prolonged time period. The robust random sampling of inpatients insured that changes in VTE prophylaxis rates were not due to changes in the distribution of VTE risk or bias potentially introduced from convenience samples. The longitudinal monitoring of imaging study results for VTE cases is vastly superior to using administrative data that is reliant on coding. The recent University Healthsystem Consortium (UHC) benchmarking data on venous thromboembolism were sobering but instructive.26 UHC used administrative discharge codes for VTE in a secondary position to identify patients with HA VTE, which is a common strategy to follow the incidence of HA VTE. The accuracy of identifying surgical patients with an HA VTE was only 60%. Proper use of the present on admission (POA) designation would have improved this to 83%, but 17% of cases either did not occur or had history only with a labor‐intensive manual chart review. Performance was even worse in medical patients, with only a 30% accuracy rate, potentially improved to 79% if accurate POA designation had been used, and 21% of cases identified by administrative methods either did not occur or had history only. In essence, unless an improvement team uses chart review of each case potentially identified as a HA VTE case, the administrative data are not reliable. Concurrent discovery of VTE cases allows for a more accurate and timely chart review, and allows for near real‐time feedback to the responsible treatment team.
The major limitation of this study is inherent in the observational design and the lack of a control population. Other factors besides our VTE‐specific improvement efforts could affect process and outcomes, and reductions in HA VTE could conceivably occur because of changes in the make‐up of the admitted inpatient population. These limitations are mitigated to some degree by several observations. The VTE risk distribution in the randomly sampled inpatient population did not vary significantly from year to year. The number of HA VTE was reduced in 2007 even though the number of patients and patient days at risk for developing VTE went up. The incidence of community‐acquired VTE remained constant over the same time period, highlighting the consistency of our measurement techniques and the VTE risk in the community we serve. Last, the improvements in VTE prophylaxis rates increased at times that correlated well with the introduction of layered interventions, as depicted in Figure 1.
There were several limitations to the internal study on adverse effects of VTE protocol implementation. First, this was a retrospective study, so much of the data collection was dependent upon physician progress notes and discharge summaries. Lack of documentation could have precluded the appropriate diagnosis codes from being assigned. Next, the study population was generated from coding data, so subjectivity could have been introduced during the coding process. Also, a majority of the patients did not fit the study criteria due to discharge with the e934.2 code, because they were found to have an elevated international normalized ratio (INR) after being admitted on warfarin. Finally, chart‐reviewer bias could have affected the results, as the chart reviewer became more proficient at reviewing charts over time. Despite these limitations, the study methodology allowed for screening of a large population for rare events. Bleeding may be a frequent concern with primary thromboprophylaxis, but data from clinical trials and this study help to demonstrate that rates of adverse events from pharmacologic VTE prophylaxis are very rare.
Another potential limitation is raised by the question of whether our methods can be generalized to other sites. Our site is an academic medical center and we have CPOE, which is present in only a small minority of centers. Furthermore, one could question how feasible it is to get institution‐wide consensus for a VTE prevention protocol in settings with heterogenous medical staffs. To address these issues, we used a proven performance improvement framework calling for administrative support, a multidisciplinary improvement team, reliable measures, and a multifaceted approach to interventions. This framework and our experiences have been incorporated into improvement guides27, 28 that have been the centerpiece of the Society of Hospital Medicine VTE Prevention Collaborative improvement efforts in a wide variety of medical environments. The collaborative leadership has observed that success is the rule when this model is followed, in institutions large and small, academic or community, and in both paper and CPOE environments. Not all of these sites use a VTE RAM identical to ours, and there are local nuances to preferred choices of prophylaxis. However, they all incorporated simple VTE risk stratification with only a few levels of risk. Reinforcing the expectation that pharmacologic prophylaxis is indicated for the majority of inpatients is likely more important than the nuances of choices for each risk level.
We demonstrated that dramatic improvement in VTE prophylaxis is achievable, safe, and effective in reducing the incidence of HA VTE. We used scalable, portable methods to make a large and convincing impact on the incidence of HA VTE, while also developing and prospectively validating a VTE RAM. A wide variety of institutions are achieving significant improvement using similar strategies. Future research and improvement efforts should focus on how to accelerate integration of this model across networks of hospitals, leveraging networks with common order sets or information systems. Widespread success in improving VTE prophylaxis would likely have a far‐reaching benefit on morbidity and PE‐related mortality.
Pulmonary embolism (PE) and deep vein thrombosis (DVT), collectively referred to as venous thromboembolism (VTE), represent a major public health problem, affecting hundreds of thousands of Americans each year.1 The best estimates are that at least 100,000 deaths are attributable to VTE each year in the United States alone.1 VTE is primarily a problem of hospitalized and recently‐hospitalized patients.2 Although a recent meta‐analysis did not prove mortality benefit of prophylaxis in the medical population,3 PE is frequently estimated to be the most common preventable cause of hospital death.46
Pharmacologic methods to prevent VTE are safe, effective, cost‐effective, and advocated by authoritative guidelines.7 Even though the majority of medical and surgical inpatients have multiple risk factors for VTE, large prospective studies continue to demonstrate that these preventive methods are significantly underutilized, often with only 30% to 50% eligible patients receiving prophylaxis.812
The reasons for this underutilization include lack of physician familiarity or agreement with guidelines, underestimation of VTE risk, concern over risk of bleeding, and the perception that the guidelines are resource‐intensive or difficult to implement in a practical fashion.13 While many VTE risk‐assessment models are available in the literature,1418 a lack of prospectively validated models and issues regarding ease of use have further hampered widespread integration of VTE risk assessments into order sets and inpatient practice.
We sought to optimize prevention of hospital‐acquired (HA) VTE in our 350‐bed tertiary‐care academic center using a VTE prevention protocol and a multifaceted approach that could be replicated across a wide variety of medical centers.
Patients and Methods
Study Design
We developed, implemented, and refined a VTE prevention protocol and examined the impact of our efforts. We observed adult inpatients on a longitudinal basis for the prevalence of adequate VTE prophylaxis and for the incidence of HA VTE throughout a 36‐month period from calendar year 2005 through 2007, and performed a retrospective analysis for any potential adverse effects of increased VTE prophylaxis. The project adhered to the HIPAA requirements for privacy involving health‐related data from human research participants. The study was approved by the Institutional Review Board of the University of California, San Diego, which waived the requirement for individual patient informed consent.
We included all hospitalized adult patients (medical and surgical services) at our medical center in our observations and interventions, including patients of all ethnic groups, geriatric patients, prisoners, and the socially and economically disadvantaged in our population. Exclusion criteria were age under 14 years, and hospitalization on Psychiatry or Obstetrics/Gynecology services.
Development of a VTE Risk‐assessment Model and VTE Prevention Protocol
A core multidisciplinary team with hospitalists, pulmonary critical care VTE experts, pharmacists, nurses, and information specialists was formed. After gaining administrative support for standardization, we worked with medical staff leaders to gain consensus on a VTE prevention protocol for all medical and surgical areas from mid‐2005 through mid‐2006. The VTE prevention protocol included the elements of VTE risk stratification, definitions of adequate VTE prevention measures linked to the level of VTE risk, and definitions for contraindications to pharmacologic prophylactic measures. We piloted risk‐assessment model (RAM) drafts for ease of use and clarity, using rapid cycle feedback from pharmacy residents, house staff, and medical staff attending physicians. Models often cited in the literature15, 18 that include point‐based scoring of VTE risk factors (with prophylaxis choices hinging on the additive sum of scoring) were rejected based on the pilot experience.
We adopted a simple model with 3 levels of VTE risk that could be completed by the physician in seconds, and then proceeded to integrate this RAM into standardized data collection instruments and eventually (April 2006) into a computerized provider order entry (CPOE) order set (Siemmens Invision v26). Each level of VTE risk was firmly linked to a menu of acceptable prophylaxis options (Table 1). Simple text cues were used to define risk assessment, with more exhaustive listings of risk factors being relegated to accessible reference tables.
| Low | Moderate | High |
|---|---|---|
| ||
| Ambulatory patient without VTE risk factors; observation patient with expected LOS 2 days; same day surgery or minor surgery | All other patients (not in low‐risk or high‐risk category); most medical/surgical patients; respiratory insufficiency, heart failure, acute infectious, or inflammatory disease | Lower extremity arthroplasty; hip, pelvic, or severe lower extremity fractures; acute SCI with paresis; multiple major trauma; abdominal or pelvic surgery for cancer |
| Early ambulation | UFH 5000 units SC q 8 hours; OR LMWH q day; OR UFH 5000 units SC q 12 hours (if weight < 50 kg or age > 75 years); AND suggest adding IPC | LMWH (UFH if ESRD); OR fondaparinux 2.5 mg SC daily; OR warfarin, INR 2‐3; AND IPC (unless not feasible) |
Intermittent pneumatic compression devices were endorsed as an adjunct in all patients in the highest risk level, and as the primary method in patients with contraindications to pharmacologic prophylaxis. Aspirin was deemed an inappropriate choice for VTE prophylaxis. Subcutaneous unfractionated or low‐molecular‐weight heparin were endorsed as the primary method of prophylaxis for the majority of patients without contraindications.
Integration of the VTE Protocol into Order Sets
An essential strategy for the success of the VTE protocol included integrating guidance for the physician into the flow of patient care, via standardized order sets. The CPOE VTE prevention order set was modular by design, as opposed to a stand alone design. After conferring with appropriate stakeholders, preexisting and nonstandardized prompts for VTE prophylaxis were removed from commonly used order sets, and the standardized module was inserted in its place. This allowed for integration of the standardized VTE prevention module into all admission and transfer order sets, essentially insuring that all patients admitted or transferred within the medical center would be exposed to the protocol. Physicians using a variety of admission and transfer order sets were prompted to select each patient's risk for VTE, and declare the presence or absence of contraindications to pharmacologic prophylaxis. Only the VTE prevention options most appropriate for the patient's VTE and anticoagulation risk profile were presented as the default choice for VTE prophylaxis. Explicit designation of VTE risk level and a prophylaxis choice were presented in a hard stop mechanism, and utilization of these orders was therefore mandatory, not optional. Proper use (such as the proper classification of VTE risk by the ordering physician) was actively monitored on an auditing basis, and order sets were modified occasionally on the basis of subjective and objective feedback.
Assessment of VTE Risk Assessment Interobserver Agreement
Data from 150 randomly selected patients from the audit pool (from late 2005 through mid‐2006) were abstracted by the nurse practitioner in a detailed manner. Five independent reviewers assessed each patient for VTE risk level, and for a determination of whether or not they were on adequate VTE prophylaxis on the day of the audit per protocol. Interobserver agreement was calculated for these parameters using kappa scores.
Prospective Monitoring of Adequate VTE Prophylaxis
A daily medical center inpatient census report of eligible patients in the medical center for >48 hours was downloaded into an Microsoft Excel spreadsheet, with each patient assigned a consecutive number. The Excel random number generator plug‐in function was used to generate a randomly sequenced list of the patients. The research nurse practitioner targeted serial patients on the list for further study, until she accomplished the requisite number of audits each day. The mean number of audits per month declined over the study years as the trends stabilized and as grant funding expired, but remained robust throughout (2005: 107 audits per month; 2006: 80 audits per month; and 2007: 57 audits per month).
The data collected on each patient randomly selected for audit included age, gender, location, service, date and time of review, and date of admission. The audit VTE RAM (identical to the VTE RAM incorporated into the order set), was used to classify each patient's VTE risk as low, moderate, or high. For each audit, we determined if the patient was on an adequate VTE prevention regimen consistent with our protocol, given their VTE risk level, demographics, and absence or presence of contraindications to pharmacologic prophylaxis. All questionable cases were reviewed by at least 2 physicians at weekly meetings with a final consensus determination. Adequacy of the VTE regimen was judged by orders entered on the day of the audit, but we also noted whether or not ordered intermittent compression devices were in place and functioning at the time of the audit.
Prospective (Concurrent) Discovery and Analysis of VTE Cases
The team nurse practitioner used the PACS radiology reporting and archival system (IMPAX version 4.5; AGFA Healthcare Informatics, Greenville, SC) to identify all new diagnoses of VTE, in the process described below.
Procedure codes for following studies were entered into the IMPAX search engine to locate all such exams performed in the previous 1 to 3 days:
Ultrasound exams of the neck, upper extremities, and lower extremities;
Computed tomography (CT) angiograms of the chest;
Ventilation/perfusion nuclear medicine scans; and
Pulmonary angiograms.
Negative studies and studies that revealed unchanged chronic thromboses were excluded, while clots with a chronic appearance but no evidence of prior diagnosis were included. Iliofemoral, popliteal, calf vein, subclavian, internal and external jugular vein, and axillary vein thromboses were therefore included, as were all PEs. Less common locations, such as renal vein and cavernous sinus thromboses, were excluded. The improvement/research team exerted no influence over decisions about whether or not testing was done.
Each new case of VTE was then classified as HA VTE or community‐acquired VTE. A new VTE was classified as HA if the diagnosis was first suspected and made in the hospital. A newly diagnosed VTE was also classified as HA if the VTE was suspected in the ambulatory setting, but the patient had been hospitalized within the arbitrary window of the preceding 30 days.
Each new diagnosis of HA VTE was reviewed by core members of the multidisciplinary support team. This investigation included a determination of whether the patient was on an adequate VTE prophylaxis regimen at the time of the HA VTE, using the RAM and linked prophylaxis menu described above. The VTE prevention regimen ordered at the time the inpatient developed the HA VTE was classified as adherent or nonadherent to the University of California, San Diego (UCSD) protocol: patients who developed VTE when on suboptimal prophylaxis per protocol were classified as having a potentially preventable case. Potentially iatrogenic precipitants of VTE (such as the presence of a central venous catheter or restraints) were also noted. All data were entered into a Microsoft Access database for ease of retrieval and reporting.
All tests for VTE were performed based on clinical signs and symptoms, rather than routine screening, except for the Trauma and Burn services, which also screen for VTE in high‐risk patients per their established screening protocols.
Statistical Analysis of VTE Prophylaxis and HA VTE Cases
Gender differences between cases of VTE and randomly sampled and audited inpatients were examined by chi‐square analysis, and analysis of variance (ANOVA) was used to examine any age or body mass index (BMI) differences between audits and cases.
The unadjusted risk ratio (RR) for adequate prophylaxis was compared by year, with year 2005 being the baseline (comparison) year, by chi‐square analysis.
The unadjusted RR of HA VTE was calculated by dividing the number of cases found in the calendar year by the hospital census of adult inpatients at risk. For each case, a classification for the type of VTE (PE vs. DVT vs. combinations) was recorded. Cases not receiving adequate prophylaxis were categorized as preventable DVT. Unadjusted RRs were calculated for each year by chi‐square analysis, compared to the baseline (2005) year.
All data were analyzed using Stata (version 10; Stata Corp., College Station, TX). Results for the different analysis were considered significant at P < 0.05.
Retrospective Study of Unintentional Adverse Effects
The increase in anticoagulant use accompanying the introduction of the VTE prophylaxis order set warranted an evaluation of any subsequent rise in related adverse events. A study was done to determine the rates of bleeding and heparin‐induced thrombocytopenia (HIT) before and after the implementation of the VTE prophylaxis order set.
A retrospective analysis was conducted to evaluate outcomes in our inpatients from December 2004 through November 2006, with April to November, 2006 representing the post‐order set implementation time period. Any patient with a discharge diagnosis code of e934.2 (anticoagulant‐related adverse event) was selected for study to identify possible bleeding attributable to pharmacologic VTE prophylaxis. Major or minor bleeding attributable to pharmacologic VTE prophylaxis was defined as a bleed occurring 72 hours after receiving pharmacologic VTE prophylaxis. Major bleeding was defined as cerebrovascular, gastrointestinal, retroperitoneal, or overt bleeding with a decrease in hemoglobin 2 mg/dL with clinical symptoms such as hypotension or hypoxia (not associated with hemodialysis) or transfusion of 2 units of packed red blood cells. Minor bleeding was defined as ecchymosis, epistaxis, hematoma, hematuria, hemoptysis, petechiae, or bleeding without a decrease in hemoglobin 2 g/dL.
Possible cases of HIT were identified by screening for a concomitant secondary thrombocytopenia code (287.4). Chart review was then conducted to determine a causal relationship between the use of pharmacologic VTE prophylaxis and adverse events during the hospital stay. HIT attributable to pharmacologic VTE prophylaxis was determined by assessing if patients developed any of the following clinical criteria after receiving pharmacologic VTE prophylaxis: platelet count <150 109/L or 50% decrease from baseline, with or without an associated venous or arterial thrombosis or other sequelae (skin lesions at injection site, acute systemic reaction) and/or a positive heparin‐induced platelet activation (HIPA) test. In order to receive a diagnosis of HIT, thrombocytopenia must have occurred between days 5 to 15 of heparin therapy, unless existing evidence suggested that the patient developed rapid‐onset HIT or delayed‐onset HIT. Rapid‐onset HIT was defined as an abrupt drop in platelet count upon receiving a heparin product, due to heparin exposure within the previous 100 days. Delayed‐onset HIT was defined as HIT that developed several days after discontinuation of heparin. Other evident causes of thrombocytopenia were ruled out.
Statistical Analysis of Retrospective Study of Unintentional Adverse Effects
Regression analysis with chi‐square and ANOVA were used in the analysis of the demographic data. RRs were calculated for the number of cases coded with an anticoagulant‐related adverse event secondary thrombocytopenia before and after the order set implementation.
Educational Efforts and Feedback
Members of the multidisciplinary team presented information on HA VTE and the VTE prevention protocol at Medical and Surgical grand rounds, teaching rounds, and noon conference, averaging 1 educational session per quarter. Feedback and education was provided to physicians and nursing staff when audits revealed that a patient had inadequate prophylaxis with reference to the protocol standard. In addition, these conversations provided on opportunity to explore reasons for nonadherence with the protocol, confusion regarding the VTE RAM, and other barriers to effective prophylaxis, thereby providing guidance for further protocol revision and educational efforts. We adjusted the order set based on active monitoring of order set use and the audit process.
Results
There were 30,850 adult medical/surgical inpatients admitted to the medical center with a length of stay of 48 hours or more in 2005 to 2007, representing 186,397 patient‐days of observation. A total of 2,924 of these patients were randomly sampled during the VTE prophylaxis audit process (mean 81 audits per month). Table 2 shows the characteristics of randomly sampled audit patients and of the patients diagnosed with HA VTE. The demographics of the 30,850‐inpatient population (mean age = 50 years; 60.7% male; 52% Surgical Services) mirrored the demographics of the randomly sampled inpatients that underwent audits, validating the random sampling methods.
| Number (n = 3285) | % of Study Population* | Cases (n = 361) [n (%)] | Audits (n = 2924) [n (%)] | OR (95% CI) | |
|---|---|---|---|---|---|
| |||||
| Age (years) mean SD | 51 16 (range 15‐100) | 53 17 | 50 17 | 1.01 (1.003‐1.016) | |
| Gender, males | 1993 | 61 | 213 (59) | 1782 (61) | 0.93 (0.744‐1.16) |
| Major service: | |||||
| Surgery | 1714 | 52 | 200 (55) | 1516 (52) | |
| Medicine | 1566 | 48 | 161 (45) | 1408 (48) | |
| Service, detail | |||||
| Hospitalist | 1041 | 32 | 83 (23) | 958 (33) | |
| General surgery | 831 | 25 | 75 (21) | 756 (26) | |
| Trauma | 419 | 13 | 77 (22) | 342 (12) | |
| Cardiology | 313 | 10 | 45 (13) | 268 (9) | |
| Orthopedics | 244 | 7 | 15 (4) | 229 (8) | |
| Burn unit | 205 | 6 | 29 (8) | 176 (6) | |
| Other | 222 | 7 | 30 (8) | 192 (7) | |
The majority of inpatients sampled in the audits were in the moderate VTE risk category (84%), 12% were in the high‐risk category, and 4% were in the low‐risk category. The distribution of VTE risk did not change significantly over this time period.
Interobserver Agreement
The VTE RAM interobserver agreement was assessed on 150 patients with 5 observers as described above. The kappa score for the VTE risk level was 0.81. The kappa score for the judgment of whether the patient was on adequate prophylaxis or not was 0.90.
Impact on Percent of Patients with Adequate Prophylaxis (Longitudinal Audits)
Audits of randomly sampled inpatients occurred longitudinally throughout the study period as described above. Based on the intervention, the percent of patients on adequate prophylaxis improved significantly (P < 0.001) by each calendar year (see Table 3), from a baseline of 58% in 2005 to 78% in 2006 (unadjusted relative benefit = 1.35; 95% confidence interval [CI] = 1.28‐1.43), and 93% in 2007 (unadjusted relative benefit = 1.61; 95% CI = 1.52, 1.69). The improvement seen was more marked in the moderate VTE risk patients when compared to the high VTE risk patients. The percent of audited VTE prophylaxis improved from 53% in calendar year (CY) 2005 to 93% in 2007 (unadjusted relative benefit = 1.75; 95% CI = 1.70‐1.81) in the moderate VTE risk group, while the high VTE risk group improved from 83% to 92% in the same time period (unadjusted relative benefit = 1.11; 95% CI = 0.95‐1.25).
| 2005 | 2006 | 2007 | |
|---|---|---|---|
| |||
| All audits | 1279 | 960 | 679 |
| Prophylaxis adequate, n (%) | 740 (58) | 751 (78) | 631 (93) |
| Relative benefit (95% CI) | 1 | 1.35* (1.28‐1.43) | 1.61* (1.52‐1.69) |
Overall, adequate VTE prophylaxis was present in over 98% of audited patients in the last 6 months of 2007, and this high rate has been sustained throughout 2008. Age, ethnicity, and gender were not associated with differential rates of adequate VTE prophylaxis.
Figure 1 is a timeline of interventions and the impact on the prevalence of adequate VTE prophylaxis. The first 7 to 8 months represent the baseline rate 50% to 55% of VTE prophylaxis. In this baseline period, the improvement team was meeting, but had not yet begun meeting with the large variety of medical and surgical service leaders. Consensus‐building sessions with these leaders in the latter part of 2005 through mid‐2006 correlated with improvement in adequate VTE prophylaxis rates to near 70%. The consensus‐building sessions also prepared these varied services for a go live date of the modular order set that was incorporated into all admit and transfer order sets, often replacing preexisting orders referring to VTE prevention measures. The order set resulted in an improvement to 80% adequate prophylaxis, with the incremental improvement occurring virtually overnight with the go live date at the onset of quarter 2 (Q2) of 2006. Monitoring of the order set use confirmed that it was easy and efficient to use, but also revealed that physicians were at times classifying patients as low VTE risk inaccurately, when they possessed qualities that actually qualified them for moderate risk status by our protocol. We therefore inserted a secondary CPOE screen when patients were categorized as low VTE risk, asking the physician to deny or confirm that the patient had no risk factors that qualified them for moderate risk status. This confirmation screen essentially acted as a reminder to the physician to ask Are you sure this patient does not need VTE prophylaxis? This minor modification of the CPOE order set improved adequate VTE prophylaxis rates to 90%. Finally, we asked nurses to evaluate patients who were not on therapeutic or prophylactic doses of anticoagulants. Patients with VTE risk factors but no obvious contraindications generated a note from the nurse to the doctor, prompting the doctor to reassess VTE risk and potential contraindications. This simple intervention raised the percent of audited patients on adequate VTE prophylaxis to 98% in the last 6 months of 2007.

Description of Prospectively Identified VTE
We identified 748 cases of VTE among patients admitted to the medical center over the 36‐month study period; 387 (52%) were community‐acquired VTE. There were 361 HA cases (48% of total cases) over the same time period. There was no difference in age, gender, or BMI between the community‐acquired and hospital‐related VTE.
Of the 361 HA cases, 199 (55%) occurred on Surgical Services and 162 (45%) occurred on Medical Services; 58 (16%) unique patients had pulmonary emboli, while 303 (84%) patients experienced only DVT. Remarkably, almost one‐third of the DVT occurred in the upper extremities (108 upper extremities, 240 lower extremities), and most (80%) of the upper‐extremity DVT were associated with central venous catheters.
Of 361 HA VTE cases, 292 (81%) occurred in those in the moderate VTE risk category, 69 HA VTE cases occurred in high‐risk category patients (19%), and no VTE occurred in patients in the low‐risk category.
Improvement in HA VTE
HA VTE were identified and each case analyzed on an ongoing basis over the entire 3 year study period, as described above. Table 4 depicts a comparison of HA VTE on a year‐to‐year basis and the impact of the VTE prevention protocol on the incidence of HA VTE. In 2007 (the first full CY after the implementation of the order set) there was a 39% relative risk reduction (RRR) in the risk of experiencing an HA VTE. The reduction in the risk of preventable HA VTE was even more marked (RRR = 86%; 7 preventable VTE in 2007, compared to 44 in baseline year of 2005; RR = 0.14; 95% CI = 0.06‐0.31).
| HA VTE by Year | |||
|---|---|---|---|
| 2005 | 2006 | 2007 | |
| |||
| Patients at Risk | 9720 | 9923 | 11,207 |
| Cases with any HA VTE | 131 | 138 | 92 |
| Risk for HA VTE | 1 in 76 | 1 in 73 | 1 in 122 |
| Unadjusted relative risk (95% CI) | 1.0 | 1.03 (0.81‐1.31) | 0.61* (0.47‐0.79) |
| Cases with PE | 21 | 22 | 15 |
| Risk for PE | 1 in 463 | 1 in 451 | 1 in 747 |
| Unadjusted relative risk (95% CI) | 1.0 | 1.03 (0.56‐1.86) | 0.62 (0.32‐1.20) |
| Cases with DVT (and no PE) | 110 | 116 | 77 |
| Risk for DVT | 1 in 88 | 1 in 85 | 1 in 146 |
| Unadjusted relative risk (95% CI) | 1.0 | 1.03 (0.80‐1.33) | 0.61* (0.45‐0.81) |
| Cases with preventable VTE | 44 | 21 | 7 |
| Risk for preventable VTE | 1 in 221 | 1 in 473 | 1 in 1601 |
| Unadjusted relative risk (95% CI) | 1.0 | 0.47 (0.28‐0.79) | 0.14* (0.06‐0.31) |
Retrospective Analysis of Impact on HIT and Bleeding
There were no statistically significant differences in the number of cases coded for an anticoagulant‐related bleed or secondary thrombocytopenia (Table 5). Chart review revealed there were 2 cases of minor bleeding attributable to pharmacologic VTE prophylaxis before the order set implementation. There were no cases after implementation. No cases of HIT attributable to pharmacologic VTE prophylaxis were identified in either study period, with all cases being attributed to therapeutic anticoagulation.
| Pre‐order Set | Post‐order Set | Post‐order Set RR (CI) | |
|---|---|---|---|
| |||
| Bleeding events | 74 | 28 | 0.70 (0.46‐1.08) |
| Due to prophylaxis | 2 (minor) | 0 | |
| HIT events | 9 | 7 | 1.44 (0.54‐3.85) |
| Due to prophylaxis | 0 | 0 | |
| Patient admissions | 32117 | 17294 | |
Discussion
We demonstrated that implementation of a standardized VTE prevention protocol and order set could result in a dramatic and sustained increase in adequate VTE prophylaxis across an entire adult inpatient population. This achievement is more remarkable given the rigorous criteria defining adequate prophylaxis. Mechanical compression devices were not accepted as primary prophylaxis in moderate‐risk or high‐risk patients unless there was a documented contraindication to pharmacologic prophylaxis, and high VTE risk patients required both mechanical and pharmacologic prophylaxis to be considered adequately protected, for example. The relegation of mechanical prophylaxis to an ancillary role was endorsed by our direct observations, in that we were only able to verify that ordered mechanical prophylaxis was in place 60% of the time.
The passive dissemination of guidelines is ineffective in securing VTE prophylaxis.19 Improvement in VTE prophylaxis has been suboptimal when options for VTE prophylaxis are offered without providing guidance for VTE risk stratification and all options (pharmacologic, mechanical, or no prophylaxis) are presented as equally acceptable choices.20, 21 Our multifaceted strategy using multiple interventions is an approach endorsed by a recent systematic review19 and others in the literature.22, 23 The interventions we enacted included a method to prompt clinicians to assess patients for VTE risk, and then to assist in the selection of appropriate prophylaxis from standardized options. Decision support and clinical reminders have been shown to be more effective when integrated into the workflow19, 24; therefore, a key strategy of our study involved embedding the VTE risk assessment tool and guidance toward appropriate prophylactic regimens into commonly used admission/transfer order sets. We addressed the barriers of physician unfamiliarity or disagreement with guidelines10 with education and consensus‐building sessions with clinical leadership. Clinical feedback from audits, peer review, and nursing‐led interventions rounded out the layered multifaceted interventional approach.
We designed and prospectively validated a VTE RAM during the course of our improvement efforts, and to our knowledge our simple 3‐category (or 3‐level) VTE risk assessment model is the only validated model. The VTE risk assessment/prevention protocol was validated by several important parameters. First, it proved to be practical and easy to use, taking only seconds to complete, and it was readily adopted by all adult medical and surgical services. Second, the VTE RAM demonstrated excellent interobserver agreement for VTE risk level and decisions about adequacy of VTE prophylaxis with 5 physician reviewers. Third, the VTE RAM predicted risk for VTE. All patients suffering from HA VTE were in the moderate‐risk to high‐risk categories, and HA VTE occurred disproportionately in those meeting criteria for high risk. Fourth, implementation of the VTE RAM/protocol resulted in very high, sustained levels of VTE prophylaxis without any detectable safety concerns. Finally and perhaps most importantly, high rates of adherence to the VTE protocol resulted in a 40% decline in the incidence of HA VTE in our institution.
The improved prevalence of adequate VTE prophylaxis reduced, but did not eliminate, HA VTE. The reduction observed is consistent with the 40% to 50% efficacy of prophylaxis reported in the literature.7 Our experience highlights the recent controversy over proposals by the Centers for Medicare & Medicaid Services (CMS) to add HA VTE to the list of do not pay conditions later this year,25 as it is clear from our data that even near‐perfect adherence with accepted VTE prevention measures will not eliminate HA VTE. After vigorous pushback about the fairness of this measure, the HA VTE do not pay scope was narrowed to include only certain major orthopedic procedure patients.
Services with a preponderance of moderate‐risk patients had the largest reduction in HA VTE. Efforts that are focused only on high‐risk orthopedic, trauma, and critical care patients will miss the larger opportunities for maximal reduction in HA VTE for multiple reasons. First, moderate VTE risk patients are far more prevalent than high VTE risk patients (84% vs. 12% of inpatients at our institution). Second, high‐risk patients are already at a baseline relatively high rate of VTE prophylaxis compared to their moderate VTE risk counterparts (83% vs. 53% at our institution). Third, a large portion of patients at high risk for VTE (such as trauma patients) also have the largest prevalence of absolute or relative contraindications to pharmacologic prophylaxis, limiting the effect size of prevention efforts.
Major strengths of this study included ongoing rigorous concurrent measurement of both processes (percent of patients on adequate prophylaxis) and outcomes (HA VTE diagnosed via imaging studies) over a prolonged time period. The robust random sampling of inpatients insured that changes in VTE prophylaxis rates were not due to changes in the distribution of VTE risk or bias potentially introduced from convenience samples. The longitudinal monitoring of imaging study results for VTE cases is vastly superior to using administrative data that is reliant on coding. The recent University Healthsystem Consortium (UHC) benchmarking data on venous thromboembolism were sobering but instructive.26 UHC used administrative discharge codes for VTE in a secondary position to identify patients with HA VTE, which is a common strategy to follow the incidence of HA VTE. The accuracy of identifying surgical patients with an HA VTE was only 60%. Proper use of the present on admission (POA) designation would have improved this to 83%, but 17% of cases either did not occur or had history only with a labor‐intensive manual chart review. Performance was even worse in medical patients, with only a 30% accuracy rate, potentially improved to 79% if accurate POA designation had been used, and 21% of cases identified by administrative methods either did not occur or had history only. In essence, unless an improvement team uses chart review of each case potentially identified as a HA VTE case, the administrative data are not reliable. Concurrent discovery of VTE cases allows for a more accurate and timely chart review, and allows for near real‐time feedback to the responsible treatment team.
The major limitation of this study is inherent in the observational design and the lack of a control population. Other factors besides our VTE‐specific improvement efforts could affect process and outcomes, and reductions in HA VTE could conceivably occur because of changes in the make‐up of the admitted inpatient population. These limitations are mitigated to some degree by several observations. The VTE risk distribution in the randomly sampled inpatient population did not vary significantly from year to year. The number of HA VTE was reduced in 2007 even though the number of patients and patient days at risk for developing VTE went up. The incidence of community‐acquired VTE remained constant over the same time period, highlighting the consistency of our measurement techniques and the VTE risk in the community we serve. Last, the improvements in VTE prophylaxis rates increased at times that correlated well with the introduction of layered interventions, as depicted in Figure 1.
There were several limitations to the internal study on adverse effects of VTE protocol implementation. First, this was a retrospective study, so much of the data collection was dependent upon physician progress notes and discharge summaries. Lack of documentation could have precluded the appropriate diagnosis codes from being assigned. Next, the study population was generated from coding data, so subjectivity could have been introduced during the coding process. Also, a majority of the patients did not fit the study criteria due to discharge with the e934.2 code, because they were found to have an elevated international normalized ratio (INR) after being admitted on warfarin. Finally, chart‐reviewer bias could have affected the results, as the chart reviewer became more proficient at reviewing charts over time. Despite these limitations, the study methodology allowed for screening of a large population for rare events. Bleeding may be a frequent concern with primary thromboprophylaxis, but data from clinical trials and this study help to demonstrate that rates of adverse events from pharmacologic VTE prophylaxis are very rare.
Another potential limitation is raised by the question of whether our methods can be generalized to other sites. Our site is an academic medical center and we have CPOE, which is present in only a small minority of centers. Furthermore, one could question how feasible it is to get institution‐wide consensus for a VTE prevention protocol in settings with heterogenous medical staffs. To address these issues, we used a proven performance improvement framework calling for administrative support, a multidisciplinary improvement team, reliable measures, and a multifaceted approach to interventions. This framework and our experiences have been incorporated into improvement guides27, 28 that have been the centerpiece of the Society of Hospital Medicine VTE Prevention Collaborative improvement efforts in a wide variety of medical environments. The collaborative leadership has observed that success is the rule when this model is followed, in institutions large and small, academic or community, and in both paper and CPOE environments. Not all of these sites use a VTE RAM identical to ours, and there are local nuances to preferred choices of prophylaxis. However, they all incorporated simple VTE risk stratification with only a few levels of risk. Reinforcing the expectation that pharmacologic prophylaxis is indicated for the majority of inpatients is likely more important than the nuances of choices for each risk level.
We demonstrated that dramatic improvement in VTE prophylaxis is achievable, safe, and effective in reducing the incidence of HA VTE. We used scalable, portable methods to make a large and convincing impact on the incidence of HA VTE, while also developing and prospectively validating a VTE RAM. A wide variety of institutions are achieving significant improvement using similar strategies. Future research and improvement efforts should focus on how to accelerate integration of this model across networks of hospitals, leveraging networks with common order sets or information systems. Widespread success in improving VTE prophylaxis would likely have a far‐reaching benefit on morbidity and PE‐related mortality.
- U.S. Department of Health and Human Services. Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism.2008 Clean-up Rule No. CU01 invoked here. . Available at: http://www.surgeongeneral.gov/topics/deepvein. Accessed June 2009.
- ,,, et al.Incidence of venous thromboembolism in hospitalized patients vs. community residents.Mayo Clin Proc.2001;76:1102–1110.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism.Arch Intern Med.2002;162:1245–1248.
- ,,, et al.Antithrombotic therapy practices in US hospitals in an era of practice guidelines.Arch Intern Med.2005;165:1458–1464.
- ,,, et al.Prevention of venous thromboembolism.Chest.1995;108:312–334.
- ,,, et al.Prevention of venous thromboembolism: ACCP Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6 Suppl):381S–453S.
- ,.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259–262.
- ,,, et al.The outcome after treatment of venous thromboembolism is different in surgical and acutely ill medical patients. Findings from the RIETE registry.J Thromb Haemost.2004;2:1892–1898.
- ,,, et al.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the international medical prevention registry on venous thromboembolism.Chest.2007;132(3):936–945.
- ,,, et al.Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada.Thromb Res.2007;119(2):145–155.
- ,,, et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- ,,.Compliance with recommended prophylaxis for venous thromboembolism: improving the use and rate of uptake of clinical practice guidelines.J Thromb Haemost.2004;2:221–227.
- ,.Risk factors for venous thromboembolism.Circulation.2003;107:I‐9–I‐16.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 suppl 5):12–19.
- ,,,,.Identification of candidates for prevention of venous thromboembolism.Semin Thromb Hemost.1997;23(1):55–67.
- .Venous thromboembolic risk and its prevention in hospitalized medical patients.Semin Thromb Hemost.2002;28(6);577–583.
- ,,, et al.A guide to venous thromboembolism risk factor assessment.J Thromb Thrombolysis.2000;9:253–262.
- ,,, et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241:397–415.
- ,,,.Medical admission order sets to improve deep vein thrombosis prophylaxis rates and other outcomes.J Hosp Med.2009;4(2):81–89.
- .Medical admission order sets to improve deep vein thrombosis prevention: a model for others or a prescription for mediocrity? [Editorial].J Hosp Med.2009;4(2):77–80.
- ,,,.No magic bullets: a systematic review of 102 trials of interventions to improve professional practice.CMAJ.1995;153:1423–1431.
- ,,.Innovative approaches to increase deep vein thrombosis prophylaxis rate resulting in a decrease in hospital‐acquired deep vein thrombosis at a tertiary‐care teaching hospital.J Hosp Med.2008;3(2):148–155.
- ,,,.Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies.Rockville, MD:Agency for Healthcare Research and Quality;2004.
- CMS Office of Public Affairs. Fact Sheet: CMS Proposes Additions to List of Hospital‐Acquired Conditions for Fiscal Year 2009. Available at: http://www.cms.hhs.gov/apps/media/press/factsheet.asp?Counter=3042. Accessed June2009.
- The DVT/PE 2007 Knowledge Transfer Meeting. Proceedings of November 30, 2007 meeting. Available at: http://www.uhc.edu/21801.htm. Accessed June2009.
- ,. Preventing Hospital‐Acquired Venous Thromboembolism. A Guide for Effective Quality Improvement. Society of Hospital Medicine, VTE Quality Improvement Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_VTE/VTE_Home.cfm. Accessed June 2009.
- ,.Preventing Hospital‐Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement. Prepared by the Society of Hospital Medicine. AHRQ Publication No. 08–0075.Rockville, MD:Agency for Healthcare Research and Quality. September2008. Available at: http://www.ahrq.gov/qual/vtguide. Accessed June 2009.
- U.S. Department of Health and Human Services. Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism.2008 Clean-up Rule No. CU01 invoked here. . Available at: http://www.surgeongeneral.gov/topics/deepvein. Accessed June 2009.
- ,,, et al.Incidence of venous thromboembolism in hospitalized patients vs. community residents.Mayo Clin Proc.2001;76:1102–1110.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism.Arch Intern Med.2002;162:1245–1248.
- ,,, et al.Antithrombotic therapy practices in US hospitals in an era of practice guidelines.Arch Intern Med.2005;165:1458–1464.
- ,,, et al.Prevention of venous thromboembolism.Chest.1995;108:312–334.
- ,,, et al.Prevention of venous thromboembolism: ACCP Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6 Suppl):381S–453S.
- ,.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259–262.
- ,,, et al.The outcome after treatment of venous thromboembolism is different in surgical and acutely ill medical patients. Findings from the RIETE registry.J Thromb Haemost.2004;2:1892–1898.
- ,,, et al.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the international medical prevention registry on venous thromboembolism.Chest.2007;132(3):936–945.
- ,,, et al.Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada.Thromb Res.2007;119(2):145–155.
- ,,, et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- ,,.Compliance with recommended prophylaxis for venous thromboembolism: improving the use and rate of uptake of clinical practice guidelines.J Thromb Haemost.2004;2:221–227.
- ,.Risk factors for venous thromboembolism.Circulation.2003;107:I‐9–I‐16.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 suppl 5):12–19.
- ,,,,.Identification of candidates for prevention of venous thromboembolism.Semin Thromb Hemost.1997;23(1):55–67.
- .Venous thromboembolic risk and its prevention in hospitalized medical patients.Semin Thromb Hemost.2002;28(6);577–583.
- ,,, et al.A guide to venous thromboembolism risk factor assessment.J Thromb Thrombolysis.2000;9:253–262.
- ,,, et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241:397–415.
- ,,,.Medical admission order sets to improve deep vein thrombosis prophylaxis rates and other outcomes.J Hosp Med.2009;4(2):81–89.
- .Medical admission order sets to improve deep vein thrombosis prevention: a model for others or a prescription for mediocrity? [Editorial].J Hosp Med.2009;4(2):77–80.
- ,,,.No magic bullets: a systematic review of 102 trials of interventions to improve professional practice.CMAJ.1995;153:1423–1431.
- ,,.Innovative approaches to increase deep vein thrombosis prophylaxis rate resulting in a decrease in hospital‐acquired deep vein thrombosis at a tertiary‐care teaching hospital.J Hosp Med.2008;3(2):148–155.
- ,,,.Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies.Rockville, MD:Agency for Healthcare Research and Quality;2004.
- CMS Office of Public Affairs. Fact Sheet: CMS Proposes Additions to List of Hospital‐Acquired Conditions for Fiscal Year 2009. Available at: http://www.cms.hhs.gov/apps/media/press/factsheet.asp?Counter=3042. Accessed June2009.
- The DVT/PE 2007 Knowledge Transfer Meeting. Proceedings of November 30, 2007 meeting. Available at: http://www.uhc.edu/21801.htm. Accessed June2009.
- ,. Preventing Hospital‐Acquired Venous Thromboembolism. A Guide for Effective Quality Improvement. Society of Hospital Medicine, VTE Quality Improvement Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_VTE/VTE_Home.cfm. Accessed June 2009.
- ,.Preventing Hospital‐Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement. Prepared by the Society of Hospital Medicine. AHRQ Publication No. 08–0075.Rockville, MD:Agency for Healthcare Research and Quality. September2008. Available at: http://www.ahrq.gov/qual/vtguide. Accessed June 2009.
Copyright © 2010 Society of Hospital Medicine
Money Man
Founded in 1974, the Congressional Budget Office (CBO) assists Congress by preparing objective, nonpartisan analyses to aid in budgetary decisions. To do this, the CBO turns to its panel of health and economic advisers to examine frontier research in healthcare policy and other issues facing the nation.
David Meltzer, MD, PhD, FHM, a hospitalist and associate professor in the Department of Medicine and the Graduate School of Public Policy Studies at the University of Chicago, was recently appointed to a two-year term on the CBO’s health advisory panel. We spoke with Dr. Meltzer to learn more about his appointment.
Question: Can you explain the purpose of your role as a health adviser for the Congressional Budget Office?
Answer: The general purpose of having health advisors to the CBO is to provide thorough advice on issues relevant to public policies that are being considered. It is a tool to help clarify CBO’s thinking before they make public statements.
Q: Why is your role as a hospitalist beneficial to the advisory board?
A: Obviously, a lot of policy takes place in the hospital setting—both in terms of costs and interventions, which affect people's outcomes. My training as a hospitalist shapes how I think about that. Being a hospitalist makes me aware of some of the challenges in coordinating this care. This is something that I bring to the CBO.
Q: What topics do you typically discuss during your meetings with the CBO?
A: While I can’t disclose what we specifically discuss in the CBO because of confidentiality reasons, I can say that the advice we give is mostly general in nature, but occasionally it can be about more specific issues at hand.
Q: What role does hospital medicine play within the CBO’s analysis of healthcare issues?
A: In general, a lot of the issues facing the healthcare system are about how to control healthcare costs while maintaining and controlling quality. Hospital medicine has been very involved in measuring and improving quality of care and the coordination of care in the inpatient and outpatient setting—a broad issue for the whole U.S. healthcare system.
Founded in 1974, the Congressional Budget Office (CBO) assists Congress by preparing objective, nonpartisan analyses to aid in budgetary decisions. To do this, the CBO turns to its panel of health and economic advisers to examine frontier research in healthcare policy and other issues facing the nation.
David Meltzer, MD, PhD, FHM, a hospitalist and associate professor in the Department of Medicine and the Graduate School of Public Policy Studies at the University of Chicago, was recently appointed to a two-year term on the CBO’s health advisory panel. We spoke with Dr. Meltzer to learn more about his appointment.
Question: Can you explain the purpose of your role as a health adviser for the Congressional Budget Office?
Answer: The general purpose of having health advisors to the CBO is to provide thorough advice on issues relevant to public policies that are being considered. It is a tool to help clarify CBO’s thinking before they make public statements.
Q: Why is your role as a hospitalist beneficial to the advisory board?
A: Obviously, a lot of policy takes place in the hospital setting—both in terms of costs and interventions, which affect people's outcomes. My training as a hospitalist shapes how I think about that. Being a hospitalist makes me aware of some of the challenges in coordinating this care. This is something that I bring to the CBO.
Q: What topics do you typically discuss during your meetings with the CBO?
A: While I can’t disclose what we specifically discuss in the CBO because of confidentiality reasons, I can say that the advice we give is mostly general in nature, but occasionally it can be about more specific issues at hand.
Q: What role does hospital medicine play within the CBO’s analysis of healthcare issues?
A: In general, a lot of the issues facing the healthcare system are about how to control healthcare costs while maintaining and controlling quality. Hospital medicine has been very involved in measuring and improving quality of care and the coordination of care in the inpatient and outpatient setting—a broad issue for the whole U.S. healthcare system.
Founded in 1974, the Congressional Budget Office (CBO) assists Congress by preparing objective, nonpartisan analyses to aid in budgetary decisions. To do this, the CBO turns to its panel of health and economic advisers to examine frontier research in healthcare policy and other issues facing the nation.
David Meltzer, MD, PhD, FHM, a hospitalist and associate professor in the Department of Medicine and the Graduate School of Public Policy Studies at the University of Chicago, was recently appointed to a two-year term on the CBO’s health advisory panel. We spoke with Dr. Meltzer to learn more about his appointment.
Question: Can you explain the purpose of your role as a health adviser for the Congressional Budget Office?
Answer: The general purpose of having health advisors to the CBO is to provide thorough advice on issues relevant to public policies that are being considered. It is a tool to help clarify CBO’s thinking before they make public statements.
Q: Why is your role as a hospitalist beneficial to the advisory board?
A: Obviously, a lot of policy takes place in the hospital setting—both in terms of costs and interventions, which affect people's outcomes. My training as a hospitalist shapes how I think about that. Being a hospitalist makes me aware of some of the challenges in coordinating this care. This is something that I bring to the CBO.
Q: What topics do you typically discuss during your meetings with the CBO?
A: While I can’t disclose what we specifically discuss in the CBO because of confidentiality reasons, I can say that the advice we give is mostly general in nature, but occasionally it can be about more specific issues at hand.
Q: What role does hospital medicine play within the CBO’s analysis of healthcare issues?
A: In general, a lot of the issues facing the healthcare system are about how to control healthcare costs while maintaining and controlling quality. Hospital medicine has been very involved in measuring and improving quality of care and the coordination of care in the inpatient and outpatient setting—a broad issue for the whole U.S. healthcare system.
Are You Ready to Work?
Physicians generally have little experience hunting for jobs. After more than a decade of education and training, graduating residents are in their late 20s—or older—when they begin searching for full-time work, and many struggle with the transition. The following seasonal tips will help you find your first hospitalist job. For more details, check out The Hospitalist's “Resident’s Corner.”
July-September
- Choose a mentor. Find an experienced hospitalist who can provide valuable feedback during your job search.
- Choose your senior-year electives carefully. Focus on areas of weakness, or areas that are pertinent to HM (e.g., infectious disease, cardiology, neurology, critical-care medicine).
- Create or update your curriculum vitae and cover letter. Edit your words carefully; spelling errors or typos in documents are costly.
- • Request letters of recommendation. Think hard about who you want before asking for a letter, as these typically carry a lot of weight in the interview selection process. Although program directors, chiefs of medicine, and hospitalists can be good choices, it is important to choose people who know you well, as they tend to generate a more personal and powerful letter.
October-December
- Start your job search by applying for desired positions. Hospitalists are in high demand; check out these sites for openings: SHM’s Career Center; classified ad sections in the Journal of Hospital Medicine; general medicine journals and The Hospitalist; and hospitals and HM groups of interest. Even if they are not advertising, contact them personally.
- Research potential employers. Prepare appropriate interview questions.
- Bring extra copies of your updated CV and look sharp. Shine your shoes. Is it time to replace the suit you used to apply for residency?
- Send a thank-you note or e-mail to the person(s) you interviewed with.
January-March
- When you receive an offer, it’s time to review the contract and negotiate terms. Don’t hesitate to ask for clarification of unclear points. You might want to have a lawyer review the contract.
- Register for your board examination.
- Apply for state medical licensure. This process varies by state, but it can take several months to complete, especially if you are applying in a state other than where you trained.
- Apply for hospital credentials.
April-June
- Moving to a different city or state can be exciting—and stressful. Talk to your new co-workers to get a feel for the city and recommendations for places to live. Some employers are very helpful with a move; some provide new hires with a real estate agent. Moving expenses often are covered as a condition of employment, but it depends on your contract.
- Consider taking a vacation to either further explore relocation options or to simply relax. You might need time to unwind as your residency concludes. Some future hospitalists like to use this time to intensify their board review; others cringe at the thought.
Dr. Grant is a hospitalist at the University of Michigan Health System in Ann Arbor. Dr. Warren-Marzola is a hospitalist at St. Luke’s Hospital in Toledo, Ohio. Both are members of SHM’s Young Physicians Committee.
Physicians generally have little experience hunting for jobs. After more than a decade of education and training, graduating residents are in their late 20s—or older—when they begin searching for full-time work, and many struggle with the transition. The following seasonal tips will help you find your first hospitalist job. For more details, check out The Hospitalist's “Resident’s Corner.”
July-September
- Choose a mentor. Find an experienced hospitalist who can provide valuable feedback during your job search.
- Choose your senior-year electives carefully. Focus on areas of weakness, or areas that are pertinent to HM (e.g., infectious disease, cardiology, neurology, critical-care medicine).
- Create or update your curriculum vitae and cover letter. Edit your words carefully; spelling errors or typos in documents are costly.
- • Request letters of recommendation. Think hard about who you want before asking for a letter, as these typically carry a lot of weight in the interview selection process. Although program directors, chiefs of medicine, and hospitalists can be good choices, it is important to choose people who know you well, as they tend to generate a more personal and powerful letter.
October-December
- Start your job search by applying for desired positions. Hospitalists are in high demand; check out these sites for openings: SHM’s Career Center; classified ad sections in the Journal of Hospital Medicine; general medicine journals and The Hospitalist; and hospitals and HM groups of interest. Even if they are not advertising, contact them personally.
- Research potential employers. Prepare appropriate interview questions.
- Bring extra copies of your updated CV and look sharp. Shine your shoes. Is it time to replace the suit you used to apply for residency?
- Send a thank-you note or e-mail to the person(s) you interviewed with.
January-March
- When you receive an offer, it’s time to review the contract and negotiate terms. Don’t hesitate to ask for clarification of unclear points. You might want to have a lawyer review the contract.
- Register for your board examination.
- Apply for state medical licensure. This process varies by state, but it can take several months to complete, especially if you are applying in a state other than where you trained.
- Apply for hospital credentials.
April-June
- Moving to a different city or state can be exciting—and stressful. Talk to your new co-workers to get a feel for the city and recommendations for places to live. Some employers are very helpful with a move; some provide new hires with a real estate agent. Moving expenses often are covered as a condition of employment, but it depends on your contract.
- Consider taking a vacation to either further explore relocation options or to simply relax. You might need time to unwind as your residency concludes. Some future hospitalists like to use this time to intensify their board review; others cringe at the thought.
Dr. Grant is a hospitalist at the University of Michigan Health System in Ann Arbor. Dr. Warren-Marzola is a hospitalist at St. Luke’s Hospital in Toledo, Ohio. Both are members of SHM’s Young Physicians Committee.
Physicians generally have little experience hunting for jobs. After more than a decade of education and training, graduating residents are in their late 20s—or older—when they begin searching for full-time work, and many struggle with the transition. The following seasonal tips will help you find your first hospitalist job. For more details, check out The Hospitalist's “Resident’s Corner.”
July-September
- Choose a mentor. Find an experienced hospitalist who can provide valuable feedback during your job search.
- Choose your senior-year electives carefully. Focus on areas of weakness, or areas that are pertinent to HM (e.g., infectious disease, cardiology, neurology, critical-care medicine).
- Create or update your curriculum vitae and cover letter. Edit your words carefully; spelling errors or typos in documents are costly.
- • Request letters of recommendation. Think hard about who you want before asking for a letter, as these typically carry a lot of weight in the interview selection process. Although program directors, chiefs of medicine, and hospitalists can be good choices, it is important to choose people who know you well, as they tend to generate a more personal and powerful letter.
October-December
- Start your job search by applying for desired positions. Hospitalists are in high demand; check out these sites for openings: SHM’s Career Center; classified ad sections in the Journal of Hospital Medicine; general medicine journals and The Hospitalist; and hospitals and HM groups of interest. Even if they are not advertising, contact them personally.
- Research potential employers. Prepare appropriate interview questions.
- Bring extra copies of your updated CV and look sharp. Shine your shoes. Is it time to replace the suit you used to apply for residency?
- Send a thank-you note or e-mail to the person(s) you interviewed with.
January-March
- When you receive an offer, it’s time to review the contract and negotiate terms. Don’t hesitate to ask for clarification of unclear points. You might want to have a lawyer review the contract.
- Register for your board examination.
- Apply for state medical licensure. This process varies by state, but it can take several months to complete, especially if you are applying in a state other than where you trained.
- Apply for hospital credentials.
April-June
- Moving to a different city or state can be exciting—and stressful. Talk to your new co-workers to get a feel for the city and recommendations for places to live. Some employers are very helpful with a move; some provide new hires with a real estate agent. Moving expenses often are covered as a condition of employment, but it depends on your contract.
- Consider taking a vacation to either further explore relocation options or to simply relax. You might need time to unwind as your residency concludes. Some future hospitalists like to use this time to intensify their board review; others cringe at the thought.
Dr. Grant is a hospitalist at the University of Michigan Health System in Ann Arbor. Dr. Warren-Marzola is a hospitalist at St. Luke’s Hospital in Toledo, Ohio. Both are members of SHM’s Young Physicians Committee.
Hospitals Look to Future with White House Deal
The 10-year, $155 billion revenue cut that the nation's hospitals agreed to this summer to help President Obama push his healthcare reform package through has elicited mixed reactions as stakeholders debate whether reimbursement cuts in the short term will pay off in the long run. And while some hospitalists worry that hospitals might cut support to HM groups, the head of the American Hospital Association (AHA) says the deal was a smart move—one that creates an opportunity for hospitalists to further prove their worth.
Rich Umbdenstock, FACHE, president and CEO of the AHA, says some estimates had hospitals absorbing north of $300 billion in cuts from Medicare reimbursement. “We think that overall, although they are significant reductions, they’re not nearly as onerous or as far-reaching as what the president and the House were proposing,” Umbdenstock says. “As tough as it will be for all of us to navigate this, we believe we have limited the impact to a manageable amount.”
Managing that deficit is an area in which HM leaders can help their respective institutions, Umbdenstock adds.
More than 90% of HM groups receive hospital support from their institutions, according to SHM’s 2007-2008 "Bi-Annual Survey on the State of the Hospital Medicine Movement." “It’s a reality that has some HM groups nervous that the cuts will reduce hospital subsidies. When this money disappears, hospitals are going to have to make some very difficult decisions,” says hospitalist Marc Westle, DO, FACP, CPE, president and managing partner of Asheville Hospital Group in North Carolina. “Something will have to give.”
Umbdenstock sees opportunity in the challenge. And while acknowledging that QI won’t be an HM-centric concern in the coming years, SHM and rank-and-file hospitalists can lead the charge. “We’ve got to get better at understanding what gives us the best positive impact, the best return on information,” Umbdenstock says. “Given the role hospitalists play, they’ll be an increasingly important constituency to further the understanding of where those efficiencies … can be found. They’re on our front lines.”
The White House and hospital groups agreed to $103 billion in savings from delayed increases in Medicare payments, $50 billion from cutting charity care compensation, and $2 billion from readmission rates. Healthcare economists already have argued that the agreement will have less impact than some fear. Mark Pauly, PhD, professor of healthcare management at The Wharton School at the University of Pennsylvania, says hospitals operating on thin margins might suffer from upfront costs, but are likely to profit more when health insurance creates more “paying customers in the long run.”
The 10-year, $155 billion revenue cut that the nation's hospitals agreed to this summer to help President Obama push his healthcare reform package through has elicited mixed reactions as stakeholders debate whether reimbursement cuts in the short term will pay off in the long run. And while some hospitalists worry that hospitals might cut support to HM groups, the head of the American Hospital Association (AHA) says the deal was a smart move—one that creates an opportunity for hospitalists to further prove their worth.
Rich Umbdenstock, FACHE, president and CEO of the AHA, says some estimates had hospitals absorbing north of $300 billion in cuts from Medicare reimbursement. “We think that overall, although they are significant reductions, they’re not nearly as onerous or as far-reaching as what the president and the House were proposing,” Umbdenstock says. “As tough as it will be for all of us to navigate this, we believe we have limited the impact to a manageable amount.”
Managing that deficit is an area in which HM leaders can help their respective institutions, Umbdenstock adds.
More than 90% of HM groups receive hospital support from their institutions, according to SHM’s 2007-2008 "Bi-Annual Survey on the State of the Hospital Medicine Movement." “It’s a reality that has some HM groups nervous that the cuts will reduce hospital subsidies. When this money disappears, hospitals are going to have to make some very difficult decisions,” says hospitalist Marc Westle, DO, FACP, CPE, president and managing partner of Asheville Hospital Group in North Carolina. “Something will have to give.”
Umbdenstock sees opportunity in the challenge. And while acknowledging that QI won’t be an HM-centric concern in the coming years, SHM and rank-and-file hospitalists can lead the charge. “We’ve got to get better at understanding what gives us the best positive impact, the best return on information,” Umbdenstock says. “Given the role hospitalists play, they’ll be an increasingly important constituency to further the understanding of where those efficiencies … can be found. They’re on our front lines.”
The White House and hospital groups agreed to $103 billion in savings from delayed increases in Medicare payments, $50 billion from cutting charity care compensation, and $2 billion from readmission rates. Healthcare economists already have argued that the agreement will have less impact than some fear. Mark Pauly, PhD, professor of healthcare management at The Wharton School at the University of Pennsylvania, says hospitals operating on thin margins might suffer from upfront costs, but are likely to profit more when health insurance creates more “paying customers in the long run.”
The 10-year, $155 billion revenue cut that the nation's hospitals agreed to this summer to help President Obama push his healthcare reform package through has elicited mixed reactions as stakeholders debate whether reimbursement cuts in the short term will pay off in the long run. And while some hospitalists worry that hospitals might cut support to HM groups, the head of the American Hospital Association (AHA) says the deal was a smart move—one that creates an opportunity for hospitalists to further prove their worth.
Rich Umbdenstock, FACHE, president and CEO of the AHA, says some estimates had hospitals absorbing north of $300 billion in cuts from Medicare reimbursement. “We think that overall, although they are significant reductions, they’re not nearly as onerous or as far-reaching as what the president and the House were proposing,” Umbdenstock says. “As tough as it will be for all of us to navigate this, we believe we have limited the impact to a manageable amount.”
Managing that deficit is an area in which HM leaders can help their respective institutions, Umbdenstock adds.
More than 90% of HM groups receive hospital support from their institutions, according to SHM’s 2007-2008 "Bi-Annual Survey on the State of the Hospital Medicine Movement." “It’s a reality that has some HM groups nervous that the cuts will reduce hospital subsidies. When this money disappears, hospitals are going to have to make some very difficult decisions,” says hospitalist Marc Westle, DO, FACP, CPE, president and managing partner of Asheville Hospital Group in North Carolina. “Something will have to give.”
Umbdenstock sees opportunity in the challenge. And while acknowledging that QI won’t be an HM-centric concern in the coming years, SHM and rank-and-file hospitalists can lead the charge. “We’ve got to get better at understanding what gives us the best positive impact, the best return on information,” Umbdenstock says. “Given the role hospitalists play, they’ll be an increasingly important constituency to further the understanding of where those efficiencies … can be found. They’re on our front lines.”
The White House and hospital groups agreed to $103 billion in savings from delayed increases in Medicare payments, $50 billion from cutting charity care compensation, and $2 billion from readmission rates. Healthcare economists already have argued that the agreement will have less impact than some fear. Mark Pauly, PhD, professor of healthcare management at The Wharton School at the University of Pennsylvania, says hospitals operating on thin margins might suffer from upfront costs, but are likely to profit more when health insurance creates more “paying customers in the long run.”
Dirty Laundry?
You do it hundreds of times a year: After a long day of rounds and face-to-face encounters with patients, you walk back to your office and hang up your lab coat. But should you put the same lab coat on tomorrow?
Maybe not, according to the American Medical Association (AMA), which sponsored a discussion forum last month on whether lab coats and certain articles of clothing should be banned to help prevent the spread of methicillin-resistant Staphylococcus aureus and clostridium difficile. The discussion comes two years after the British National Health System instituted a policy banning neckties, white coats, and long sleeves because of the clothing’s potential to spread hospital-acquired infections. The Centers for Disease Control and Prevention estimates more than 2 million Americans contract hospital-acquired infections every year; more than 5% of those cases result in death.
But is the risk for real?
Armando Paez, MD, a hospitalist and infectious-disease specialist at Tufts University’s School of Medicine in Boston, says there is little evidence to show clothing can help spread disease. Nevertheless, he says hospitals should consider laundry policies as a precautionary measure. “Short sleeves are good because you can wash your hands and forearms from patient to patient,” Dr. Paez says. “You can’t do that with the sleeve of a lab coat. … Unless they run a study to compare physicians not wearing white coats versus the ones who do, we will never know. But there are a lot of variables that would need to be controlled to run that experiment.”
Erik DeLue, MD, MBA, FHM, a medical director of the hospitalist program at Virtua Memorial Hospital in Mount Holly, N.J., sees hospitalists adopting scrubs in the future because they are easier to clean and maintain. “We give everyone three lab coats and I know that people aren’t washing them,” he says. “People wash their shirts every day; why do they wash their lab coats once or twice a week?”
Both hospitalists acknowledge that a physician in a lab coat is iconic and beneficial to the healthcare profession. Dr. Paez says his geriatric patients are especially receptive to professional dress. “Traditionally, the white coat still has a large effect on the patient’s mind,” he says.
That said, if the AMA decides to hang up the lab coats, both doctors say their services would follow the guidelines. “While we don’t have great evidence, it’s just common sense,” Dr. DeLue says.
You do it hundreds of times a year: After a long day of rounds and face-to-face encounters with patients, you walk back to your office and hang up your lab coat. But should you put the same lab coat on tomorrow?
Maybe not, according to the American Medical Association (AMA), which sponsored a discussion forum last month on whether lab coats and certain articles of clothing should be banned to help prevent the spread of methicillin-resistant Staphylococcus aureus and clostridium difficile. The discussion comes two years after the British National Health System instituted a policy banning neckties, white coats, and long sleeves because of the clothing’s potential to spread hospital-acquired infections. The Centers for Disease Control and Prevention estimates more than 2 million Americans contract hospital-acquired infections every year; more than 5% of those cases result in death.
But is the risk for real?
Armando Paez, MD, a hospitalist and infectious-disease specialist at Tufts University’s School of Medicine in Boston, says there is little evidence to show clothing can help spread disease. Nevertheless, he says hospitals should consider laundry policies as a precautionary measure. “Short sleeves are good because you can wash your hands and forearms from patient to patient,” Dr. Paez says. “You can’t do that with the sleeve of a lab coat. … Unless they run a study to compare physicians not wearing white coats versus the ones who do, we will never know. But there are a lot of variables that would need to be controlled to run that experiment.”
Erik DeLue, MD, MBA, FHM, a medical director of the hospitalist program at Virtua Memorial Hospital in Mount Holly, N.J., sees hospitalists adopting scrubs in the future because they are easier to clean and maintain. “We give everyone three lab coats and I know that people aren’t washing them,” he says. “People wash their shirts every day; why do they wash their lab coats once or twice a week?”
Both hospitalists acknowledge that a physician in a lab coat is iconic and beneficial to the healthcare profession. Dr. Paez says his geriatric patients are especially receptive to professional dress. “Traditionally, the white coat still has a large effect on the patient’s mind,” he says.
That said, if the AMA decides to hang up the lab coats, both doctors say their services would follow the guidelines. “While we don’t have great evidence, it’s just common sense,” Dr. DeLue says.
You do it hundreds of times a year: After a long day of rounds and face-to-face encounters with patients, you walk back to your office and hang up your lab coat. But should you put the same lab coat on tomorrow?
Maybe not, according to the American Medical Association (AMA), which sponsored a discussion forum last month on whether lab coats and certain articles of clothing should be banned to help prevent the spread of methicillin-resistant Staphylococcus aureus and clostridium difficile. The discussion comes two years after the British National Health System instituted a policy banning neckties, white coats, and long sleeves because of the clothing’s potential to spread hospital-acquired infections. The Centers for Disease Control and Prevention estimates more than 2 million Americans contract hospital-acquired infections every year; more than 5% of those cases result in death.
But is the risk for real?
Armando Paez, MD, a hospitalist and infectious-disease specialist at Tufts University’s School of Medicine in Boston, says there is little evidence to show clothing can help spread disease. Nevertheless, he says hospitals should consider laundry policies as a precautionary measure. “Short sleeves are good because you can wash your hands and forearms from patient to patient,” Dr. Paez says. “You can’t do that with the sleeve of a lab coat. … Unless they run a study to compare physicians not wearing white coats versus the ones who do, we will never know. But there are a lot of variables that would need to be controlled to run that experiment.”
Erik DeLue, MD, MBA, FHM, a medical director of the hospitalist program at Virtua Memorial Hospital in Mount Holly, N.J., sees hospitalists adopting scrubs in the future because they are easier to clean and maintain. “We give everyone three lab coats and I know that people aren’t washing them,” he says. “People wash their shirts every day; why do they wash their lab coats once or twice a week?”
Both hospitalists acknowledge that a physician in a lab coat is iconic and beneficial to the healthcare profession. Dr. Paez says his geriatric patients are especially receptive to professional dress. “Traditionally, the white coat still has a large effect on the patient’s mind,” he says.
That said, if the AMA decides to hang up the lab coats, both doctors say their services would follow the guidelines. “While we don’t have great evidence, it’s just common sense,” Dr. DeLue says.
Nice to Meet You
Susan Connelly of Fruitland Park, Fla., is a volunteer at her local community hospital who until recently had never heard of a hospitalist. One day, she entered a hospital room and, as she regularly did with patients she visited, asked if there was anything the man in the bed needed.
“I want to know where my doctor is,” the patient said.
“You mean your doctor hasn’t seen you?” Connelly asked.
“No,” he said. “I’m not even sure he knows I’m here.”
Somewhat incredulous, Connelly retrieved the hospital’s physician handbook and helped the patient look up his physician’s phone number. “I didn’t think too much about it,” she says. But the following week, when she appeared at the hospital to volunteer, a supervisor called her into the office. The supervisor asked Connelly about the incident and gently admonished her for encouraging the patient to call his primary-care physician (PCP), as “a hospitalist is working with him now.”
“A what? I had never even heard the term,” Connelly says. She asked her fellow volunteers, known as patient representatives at her hospital, if they had ever heard of a hospitalist. One had, but only because her husband had been admitted for a hospital stay. Concerned, Connelly wrote letters to the editors of two local newspapers. Both were published (see Figure 2, “Familiar Face Gone Missing,” p. 30).
—Robert Centor, MD, associate dean of medicine, University of Alabama at Birmingham
“If I am admitted to the hospital, my doctor will most likely ‘dump’ me on what is now called a ‘hospitalist,’ ” she wrote. “Information gathered [by the hospitalist] should be forwarded to your doctor; the key word is ‘should.’ Why develop this long-term relationship with a doctor, if when you really need him, he is not there for you and you are dealing with a stranger?”
Why indeed?
It might not happen with every new admission, but patient fears are a reality. The uncertainty of a hospital stay, a new physician, and new medications can take their toll on the human psyche. Patients are upset with their PCP, the hospital, the system; many times it’s the hospitalist who feels the brunt of their anger. Not only do hospitalists have to calm a patient worried about PCP disconnect, but they also have to reassure the patient that they will be attentive to their needs, provide a high quality of care during the hospital stay, and communicate with their PCP about diagnoses, medications, and follow-up care. Hospitalists should weave in some of the documented plusses a hospitalist brings to the table: shorter length of stays, greater patient access and availability, and improved quality of care.
Although some patients might view hospitalists as “strangers,” HM physicians can learn methods to ease patient anxiety and answer tough questions from patients about the role they play in hospital care.
Restore Confidence
Simple conversations can help hospitalists defuse patient dissatisfaction. When a patient asks why their PCP won’t be seeing them in the hospital, it’s best to begin with a reassuring approach. For example, introduce yourself and say you have reviewed the case with their PCP. You can include key information from their medical history and recent hospitalizations, if appropriate.
Robert Centor, MD, a hospitalist and associate dean of medicine at the University of Alabama at Birmingham, suggests a few other key behaviors for initial patient visits. He finds a way to make appropriate physical contact by taking a pulse, checking the heart and lungs, or patting a shoulder to clearly embody the role of the physician in charge.
“And pull up a chair,” he says. “If there is no chair, bring in a chair. But sit down—always.”
Dr. Centor also recommends a transparent approach, “especially in hospital medicine,” he explains. “Be explicit about what you’re thinking, what you’re doing, and why you’re doing it.”1
Transparency can protect you as it informs and comforts patients and their families. For instance, “hospitalized patients are probably hearing from every relative they have and half the friends they have,” Dr. Centor says. “If one of those people is a physician, they may be second-guessing you. You can overcome their wariness by remembering that this is all about bedside manner and the explanations you give them, including discharge instructions.”
Dr. Centor says your bedside manner needs to fit your personality. When you talk to a patient, use language that matches your personality. You can adopt someone else’s introductory script; just make sure to modify it to fit your work environment (see “Strategies to Ease Patient Concerns,” p. 29).
“What Is This?”
Earlier this year, CJ Clarke of Leesburg, Fla., underwent a colonoscopy screening at a local doctor’s office. She had been kept on warfarin (Coumadin) to prevent complications, but after she bled for four days from a puncture sustained during the procedure, she went to the ED. She was admitted, but it wasn’t until the following afternoon that she learned that hospitalists—not her PCP— would be taking care of her.
“This totally unknown guy came in and said he would be filling in for my doctor and communicating with [my PCP],” Clarke says. “It was a weekend, and it turns out the first hospitalist was a substitute hospitalist, so then I got another hospitalist. The first one was subbing for the first hospitalist. I wasn’t exactly mad, but I thought, what is this?”
Clarke thought the first hospitalist was knowledgeable; she took comfort in that. “But the second one was extremely knowledgeable and explained the differences between Coumadin and heparin. He really knew his stuff. He talked to my cardiologist when she came in,” Clarke says. “The only thing that I was sorry about was that my primary didn’t seem to get the information very rapidly.”
Care coordination is a vital step in the discharge process, especially when patients think the flow of information between a hospital and a PCP is immediate and seamless. When Clarke was discharged and she returned home, she scheduled an appointment with her PCP. “When I first called, my [PCP] had not even heard I had been admitted,” Clarke says. But by the time she visited the PCP, “she knew everything. … I think it would have been good if sometime during that five-day hospitalization, she had been told—not afterward. Not that she would have come in, because that is not her policy, but just to know she knew.”
HM’s Role: Extended Education
Many HM groups have designated policies for educating patients and assuaging their fears. Because some PCPs might feel left out of the loop when hospitalists care for their patients, these strategies go beyond patient education.
One of the first steps is to involve PCPs in meaningful ways in their patients’ hospital care. When a patient is particularly angered by his PCP’s absence, invite the PCP to visit, or call the PCP more often and let the patient know you’re doing so. As proposed by Bob Wachter, MD, professor and chief of the division of hospital medicine at the University of California at San Francisco, a former SHM president, and author of the blog “Wachter’s World,” and Steven Pantilat, MD, FHM, professor of clinical medicine in the division of hospital medicine at UCSF, and a former SHM president, “the PCP can endorse the hospitalist model and the individual hospitalist, notice subtle findings that differ from the patient’s baseline, and help clarify patient preferences regarding difficult situations by drawing on their previous relationship with the patient. This visit may also benefit the PCP by providing insights into the patient’s illness, personality, or social support that he or she was unaware of previously.”2,3
Cogent Healthcare uses an outreach program to calm patient fears and connect with PCPs. The Brentwood, Tenn.-based hospitalist company distributes patient education pamphlets to the PCPs with whom they work, and distributes a flier on admission to show patients the photographs and names of their HM team (see “Make Patient Education A Priority,” p. 29).
Hospitalist training in this arena helps prepare physicians for a potentially uncomfortable work environment. “We need to stress in residency training the specific issue of helping make the patient feel comfortable when their own doctor is not seeing them in the hospital,” Dr. Centor says. “Most young hospitalists right out of their residencies have not experienced primary-care practice, and, so far, we don’t know how to get around that.”
Hospitalist groups also should consider broad initiatives to bring hospitalists together with patient representatives and other volunteers who work with patients. If volunteers are ignored in the educational outreach process, it could exacerbate patients’ negative reactions. Teach volunteers what hospitalists are, their benefit to care delivery, and their value in upholding the mission of quality HM. TH
Andrea Sattinger is a freelance writer based in North Carolina.
References
- Centor RM. A hospitalist inpatient system does not improve patient care outcomes. Arch Intern Med. 2008;168(12):1257-1258.
- Lo B. Ethical and policy implications of hospitalist systems. Dis Mon. 2002;48(4):281-290.
- Wachter RM, Pantilat SZ. The “continuity visit” and the hospitalist model of care. Dis Mon. 2002;48(4): 267-272.
Image Source: PETRI ARTTURI ASIKAINEN / GETTY IMAGES
Susan Connelly of Fruitland Park, Fla., is a volunteer at her local community hospital who until recently had never heard of a hospitalist. One day, she entered a hospital room and, as she regularly did with patients she visited, asked if there was anything the man in the bed needed.
“I want to know where my doctor is,” the patient said.
“You mean your doctor hasn’t seen you?” Connelly asked.
“No,” he said. “I’m not even sure he knows I’m here.”
Somewhat incredulous, Connelly retrieved the hospital’s physician handbook and helped the patient look up his physician’s phone number. “I didn’t think too much about it,” she says. But the following week, when she appeared at the hospital to volunteer, a supervisor called her into the office. The supervisor asked Connelly about the incident and gently admonished her for encouraging the patient to call his primary-care physician (PCP), as “a hospitalist is working with him now.”
“A what? I had never even heard the term,” Connelly says. She asked her fellow volunteers, known as patient representatives at her hospital, if they had ever heard of a hospitalist. One had, but only because her husband had been admitted for a hospital stay. Concerned, Connelly wrote letters to the editors of two local newspapers. Both were published (see Figure 2, “Familiar Face Gone Missing,” p. 30).
—Robert Centor, MD, associate dean of medicine, University of Alabama at Birmingham
“If I am admitted to the hospital, my doctor will most likely ‘dump’ me on what is now called a ‘hospitalist,’ ” she wrote. “Information gathered [by the hospitalist] should be forwarded to your doctor; the key word is ‘should.’ Why develop this long-term relationship with a doctor, if when you really need him, he is not there for you and you are dealing with a stranger?”
Why indeed?
It might not happen with every new admission, but patient fears are a reality. The uncertainty of a hospital stay, a new physician, and new medications can take their toll on the human psyche. Patients are upset with their PCP, the hospital, the system; many times it’s the hospitalist who feels the brunt of their anger. Not only do hospitalists have to calm a patient worried about PCP disconnect, but they also have to reassure the patient that they will be attentive to their needs, provide a high quality of care during the hospital stay, and communicate with their PCP about diagnoses, medications, and follow-up care. Hospitalists should weave in some of the documented plusses a hospitalist brings to the table: shorter length of stays, greater patient access and availability, and improved quality of care.
Although some patients might view hospitalists as “strangers,” HM physicians can learn methods to ease patient anxiety and answer tough questions from patients about the role they play in hospital care.
Restore Confidence
Simple conversations can help hospitalists defuse patient dissatisfaction. When a patient asks why their PCP won’t be seeing them in the hospital, it’s best to begin with a reassuring approach. For example, introduce yourself and say you have reviewed the case with their PCP. You can include key information from their medical history and recent hospitalizations, if appropriate.
Robert Centor, MD, a hospitalist and associate dean of medicine at the University of Alabama at Birmingham, suggests a few other key behaviors for initial patient visits. He finds a way to make appropriate physical contact by taking a pulse, checking the heart and lungs, or patting a shoulder to clearly embody the role of the physician in charge.
“And pull up a chair,” he says. “If there is no chair, bring in a chair. But sit down—always.”
Dr. Centor also recommends a transparent approach, “especially in hospital medicine,” he explains. “Be explicit about what you’re thinking, what you’re doing, and why you’re doing it.”1
Transparency can protect you as it informs and comforts patients and their families. For instance, “hospitalized patients are probably hearing from every relative they have and half the friends they have,” Dr. Centor says. “If one of those people is a physician, they may be second-guessing you. You can overcome their wariness by remembering that this is all about bedside manner and the explanations you give them, including discharge instructions.”
Dr. Centor says your bedside manner needs to fit your personality. When you talk to a patient, use language that matches your personality. You can adopt someone else’s introductory script; just make sure to modify it to fit your work environment (see “Strategies to Ease Patient Concerns,” p. 29).
“What Is This?”
Earlier this year, CJ Clarke of Leesburg, Fla., underwent a colonoscopy screening at a local doctor’s office. She had been kept on warfarin (Coumadin) to prevent complications, but after she bled for four days from a puncture sustained during the procedure, she went to the ED. She was admitted, but it wasn’t until the following afternoon that she learned that hospitalists—not her PCP— would be taking care of her.
“This totally unknown guy came in and said he would be filling in for my doctor and communicating with [my PCP],” Clarke says. “It was a weekend, and it turns out the first hospitalist was a substitute hospitalist, so then I got another hospitalist. The first one was subbing for the first hospitalist. I wasn’t exactly mad, but I thought, what is this?”
Clarke thought the first hospitalist was knowledgeable; she took comfort in that. “But the second one was extremely knowledgeable and explained the differences between Coumadin and heparin. He really knew his stuff. He talked to my cardiologist when she came in,” Clarke says. “The only thing that I was sorry about was that my primary didn’t seem to get the information very rapidly.”
Care coordination is a vital step in the discharge process, especially when patients think the flow of information between a hospital and a PCP is immediate and seamless. When Clarke was discharged and she returned home, she scheduled an appointment with her PCP. “When I first called, my [PCP] had not even heard I had been admitted,” Clarke says. But by the time she visited the PCP, “she knew everything. … I think it would have been good if sometime during that five-day hospitalization, she had been told—not afterward. Not that she would have come in, because that is not her policy, but just to know she knew.”
HM’s Role: Extended Education
Many HM groups have designated policies for educating patients and assuaging their fears. Because some PCPs might feel left out of the loop when hospitalists care for their patients, these strategies go beyond patient education.
One of the first steps is to involve PCPs in meaningful ways in their patients’ hospital care. When a patient is particularly angered by his PCP’s absence, invite the PCP to visit, or call the PCP more often and let the patient know you’re doing so. As proposed by Bob Wachter, MD, professor and chief of the division of hospital medicine at the University of California at San Francisco, a former SHM president, and author of the blog “Wachter’s World,” and Steven Pantilat, MD, FHM, professor of clinical medicine in the division of hospital medicine at UCSF, and a former SHM president, “the PCP can endorse the hospitalist model and the individual hospitalist, notice subtle findings that differ from the patient’s baseline, and help clarify patient preferences regarding difficult situations by drawing on their previous relationship with the patient. This visit may also benefit the PCP by providing insights into the patient’s illness, personality, or social support that he or she was unaware of previously.”2,3
Cogent Healthcare uses an outreach program to calm patient fears and connect with PCPs. The Brentwood, Tenn.-based hospitalist company distributes patient education pamphlets to the PCPs with whom they work, and distributes a flier on admission to show patients the photographs and names of their HM team (see “Make Patient Education A Priority,” p. 29).
Hospitalist training in this arena helps prepare physicians for a potentially uncomfortable work environment. “We need to stress in residency training the specific issue of helping make the patient feel comfortable when their own doctor is not seeing them in the hospital,” Dr. Centor says. “Most young hospitalists right out of their residencies have not experienced primary-care practice, and, so far, we don’t know how to get around that.”
Hospitalist groups also should consider broad initiatives to bring hospitalists together with patient representatives and other volunteers who work with patients. If volunteers are ignored in the educational outreach process, it could exacerbate patients’ negative reactions. Teach volunteers what hospitalists are, their benefit to care delivery, and their value in upholding the mission of quality HM. TH
Andrea Sattinger is a freelance writer based in North Carolina.
References
- Centor RM. A hospitalist inpatient system does not improve patient care outcomes. Arch Intern Med. 2008;168(12):1257-1258.
- Lo B. Ethical and policy implications of hospitalist systems. Dis Mon. 2002;48(4):281-290.
- Wachter RM, Pantilat SZ. The “continuity visit” and the hospitalist model of care. Dis Mon. 2002;48(4): 267-272.
Image Source: PETRI ARTTURI ASIKAINEN / GETTY IMAGES
Susan Connelly of Fruitland Park, Fla., is a volunteer at her local community hospital who until recently had never heard of a hospitalist. One day, she entered a hospital room and, as she regularly did with patients she visited, asked if there was anything the man in the bed needed.
“I want to know where my doctor is,” the patient said.
“You mean your doctor hasn’t seen you?” Connelly asked.
“No,” he said. “I’m not even sure he knows I’m here.”
Somewhat incredulous, Connelly retrieved the hospital’s physician handbook and helped the patient look up his physician’s phone number. “I didn’t think too much about it,” she says. But the following week, when she appeared at the hospital to volunteer, a supervisor called her into the office. The supervisor asked Connelly about the incident and gently admonished her for encouraging the patient to call his primary-care physician (PCP), as “a hospitalist is working with him now.”
“A what? I had never even heard the term,” Connelly says. She asked her fellow volunteers, known as patient representatives at her hospital, if they had ever heard of a hospitalist. One had, but only because her husband had been admitted for a hospital stay. Concerned, Connelly wrote letters to the editors of two local newspapers. Both were published (see Figure 2, “Familiar Face Gone Missing,” p. 30).
—Robert Centor, MD, associate dean of medicine, University of Alabama at Birmingham
“If I am admitted to the hospital, my doctor will most likely ‘dump’ me on what is now called a ‘hospitalist,’ ” she wrote. “Information gathered [by the hospitalist] should be forwarded to your doctor; the key word is ‘should.’ Why develop this long-term relationship with a doctor, if when you really need him, he is not there for you and you are dealing with a stranger?”
Why indeed?
It might not happen with every new admission, but patient fears are a reality. The uncertainty of a hospital stay, a new physician, and new medications can take their toll on the human psyche. Patients are upset with their PCP, the hospital, the system; many times it’s the hospitalist who feels the brunt of their anger. Not only do hospitalists have to calm a patient worried about PCP disconnect, but they also have to reassure the patient that they will be attentive to their needs, provide a high quality of care during the hospital stay, and communicate with their PCP about diagnoses, medications, and follow-up care. Hospitalists should weave in some of the documented plusses a hospitalist brings to the table: shorter length of stays, greater patient access and availability, and improved quality of care.
Although some patients might view hospitalists as “strangers,” HM physicians can learn methods to ease patient anxiety and answer tough questions from patients about the role they play in hospital care.
Restore Confidence
Simple conversations can help hospitalists defuse patient dissatisfaction. When a patient asks why their PCP won’t be seeing them in the hospital, it’s best to begin with a reassuring approach. For example, introduce yourself and say you have reviewed the case with their PCP. You can include key information from their medical history and recent hospitalizations, if appropriate.
Robert Centor, MD, a hospitalist and associate dean of medicine at the University of Alabama at Birmingham, suggests a few other key behaviors for initial patient visits. He finds a way to make appropriate physical contact by taking a pulse, checking the heart and lungs, or patting a shoulder to clearly embody the role of the physician in charge.
“And pull up a chair,” he says. “If there is no chair, bring in a chair. But sit down—always.”
Dr. Centor also recommends a transparent approach, “especially in hospital medicine,” he explains. “Be explicit about what you’re thinking, what you’re doing, and why you’re doing it.”1
Transparency can protect you as it informs and comforts patients and their families. For instance, “hospitalized patients are probably hearing from every relative they have and half the friends they have,” Dr. Centor says. “If one of those people is a physician, they may be second-guessing you. You can overcome their wariness by remembering that this is all about bedside manner and the explanations you give them, including discharge instructions.”
Dr. Centor says your bedside manner needs to fit your personality. When you talk to a patient, use language that matches your personality. You can adopt someone else’s introductory script; just make sure to modify it to fit your work environment (see “Strategies to Ease Patient Concerns,” p. 29).
“What Is This?”
Earlier this year, CJ Clarke of Leesburg, Fla., underwent a colonoscopy screening at a local doctor’s office. She had been kept on warfarin (Coumadin) to prevent complications, but after she bled for four days from a puncture sustained during the procedure, she went to the ED. She was admitted, but it wasn’t until the following afternoon that she learned that hospitalists—not her PCP— would be taking care of her.
“This totally unknown guy came in and said he would be filling in for my doctor and communicating with [my PCP],” Clarke says. “It was a weekend, and it turns out the first hospitalist was a substitute hospitalist, so then I got another hospitalist. The first one was subbing for the first hospitalist. I wasn’t exactly mad, but I thought, what is this?”
Clarke thought the first hospitalist was knowledgeable; she took comfort in that. “But the second one was extremely knowledgeable and explained the differences between Coumadin and heparin. He really knew his stuff. He talked to my cardiologist when she came in,” Clarke says. “The only thing that I was sorry about was that my primary didn’t seem to get the information very rapidly.”
Care coordination is a vital step in the discharge process, especially when patients think the flow of information between a hospital and a PCP is immediate and seamless. When Clarke was discharged and she returned home, she scheduled an appointment with her PCP. “When I first called, my [PCP] had not even heard I had been admitted,” Clarke says. But by the time she visited the PCP, “she knew everything. … I think it would have been good if sometime during that five-day hospitalization, she had been told—not afterward. Not that she would have come in, because that is not her policy, but just to know she knew.”
HM’s Role: Extended Education
Many HM groups have designated policies for educating patients and assuaging their fears. Because some PCPs might feel left out of the loop when hospitalists care for their patients, these strategies go beyond patient education.
One of the first steps is to involve PCPs in meaningful ways in their patients’ hospital care. When a patient is particularly angered by his PCP’s absence, invite the PCP to visit, or call the PCP more often and let the patient know you’re doing so. As proposed by Bob Wachter, MD, professor and chief of the division of hospital medicine at the University of California at San Francisco, a former SHM president, and author of the blog “Wachter’s World,” and Steven Pantilat, MD, FHM, professor of clinical medicine in the division of hospital medicine at UCSF, and a former SHM president, “the PCP can endorse the hospitalist model and the individual hospitalist, notice subtle findings that differ from the patient’s baseline, and help clarify patient preferences regarding difficult situations by drawing on their previous relationship with the patient. This visit may also benefit the PCP by providing insights into the patient’s illness, personality, or social support that he or she was unaware of previously.”2,3
Cogent Healthcare uses an outreach program to calm patient fears and connect with PCPs. The Brentwood, Tenn.-based hospitalist company distributes patient education pamphlets to the PCPs with whom they work, and distributes a flier on admission to show patients the photographs and names of their HM team (see “Make Patient Education A Priority,” p. 29).
Hospitalist training in this arena helps prepare physicians for a potentially uncomfortable work environment. “We need to stress in residency training the specific issue of helping make the patient feel comfortable when their own doctor is not seeing them in the hospital,” Dr. Centor says. “Most young hospitalists right out of their residencies have not experienced primary-care practice, and, so far, we don’t know how to get around that.”
Hospitalist groups also should consider broad initiatives to bring hospitalists together with patient representatives and other volunteers who work with patients. If volunteers are ignored in the educational outreach process, it could exacerbate patients’ negative reactions. Teach volunteers what hospitalists are, their benefit to care delivery, and their value in upholding the mission of quality HM. TH
Andrea Sattinger is a freelance writer based in North Carolina.
References
- Centor RM. A hospitalist inpatient system does not improve patient care outcomes. Arch Intern Med. 2008;168(12):1257-1258.
- Lo B. Ethical and policy implications of hospitalist systems. Dis Mon. 2002;48(4):281-290.
- Wachter RM, Pantilat SZ. The “continuity visit” and the hospitalist model of care. Dis Mon. 2002;48(4): 267-272.
Image Source: PETRI ARTTURI ASIKAINEN / GETTY IMAGES
Digital Dilemma
This spring, before Sentara Norfolk General Hospital in Virginia went live with eCare, its electronic health record (EHR) system, hospitalist Ryan Van Gomple, MD, would admit patients using the same system physicians have used for decades: hastily scrawled patient history notes, paper orders, and phone dictation. But eCare’s introduction—and subsequent tweaking in the past few months—has brought a radical transition to the 543-bed tertiary-care facility. Dr. Van Gomple and other hospitalists at institutions on similar systems can enter and access a patient’s data using desktop computers, handheld devices like Blackberrys or iPhones—even their personal laptops at home.
“One of the advantages is we can go back … not only with notes from the hospital stay; a lot of people are doing outpatient notes in the system, so you can start to piece together a total picture of a person’s medical care,” says Dr. Van Gomple, a hospitalist with Sentara Medical Group. “That’s one of the big goals of [EHR]—to have a streamlined system. One of the challenges is, How do you connect with different systems? That’s a great question.”
Dr. Van Gomple might not have the answer, but thanks to ambitious goals laid out by President Obama, the topic is in the national spotlight and already has nearly $20 billion in stimulus money scheduled for release in July 2010. Digitizing healthcare records to create a more efficient care delivery system—through improved record keeping, shortened patient length of stay (LOS), and increased ED throughput—isn’t a new idea. Hospitals have struggled for more than a decade with the EHR question, debating whether they should—not to mention how they would—create a computerized system to input patient records into a database that is accessible in real time to hospitalists, nurses, primary-care physicians, insurers, and so on. There have been long-stalled discussions on how to settle privacy concerns that arise from electronic records (see “EHR Upgrade Faces Privacy, Communication Obstacles,” p. 27). Still, a multi-billion-dollar federal pledge has created a moment in time to take EHR beyond the discussion phase.
The Office of the National Coordinator of Health Information Technology (ONCHIT) is empowered to shepherd this process. David Blumenthal, MD, MPP, the director of the Institute for Health Policy, a joint effort of Massachusetts General Hospital and Partners Healthcare System, has been named as ONCHIT’s head. Money to entice hospitals to invest in EHR is part of the American Recovery and Reinvestment Act of 2009. And with Congress hammering out the details of healthcare reform legislation, a sharper focus has been placed on the potential efficiencies EHR can offer.
Money and attention aren’t the only keys to this puzzle, however. IT advocates, medical information officers, and HM group leaders say the government spotlight is a wonderful springboard, but they also say physician involvement in implementing the EHR technology is a must and will spur more hospitals to adopt the systems. Less than 8% of U.S. hospitals have EHR in at least one unit, the New England Journal of Medicine reported earlier this year.1 Just 1.5% of hospitals have a comprehensive system in all of their units.
“There are so many barriers getting to where our country really needs to get,” says Dirk Stanley, MD, MPH, a hospitalist and chief medical informatics officer at Cooley Dickinson Hospital in Northampton, Mass. “One of the big issues is the meaningful use, and how do you actually set criteria for your using electronic health records the right way? If you look at the big picture, you’re talking about so many clinical practices. … How do you write criteria that are meaningful to all those different settings? The government has an enormous challenge.”
Efficiency: HM Cornerstone
David Yu, MD, FHM, works at a hospital with paperless capability and sees on a daily basis how streamlined health records have a practical effect on a hospitalist’s workload and efficiency. Dr. Yu, medical director of hospitalist services at 372-bed Decatur Memorial Hospital in Decatur, Ill., and clinical assistant professor of family and community medicine at Southern Illinois University School of Medicine in Carbondale, is one of EHR’s most passionate advocates.
Decatur Memorial uses GE Healthcare’s Centricity system, which allows hospitalists to “download automatically into our physical history with the click of a button,” says Dr. Yu, a member of Team Hospitalist. “As you’re downloading, you’re accessing the information. It’s literally the same as you driving to the patient’s primary-care physician’s office, pulling the chart, and looking at it.”
Dr. Yu and those who support EHR say it streamlines intakes, discharges, and handoffs, which in turn reduce throughput and length of stay—statistics often cited to prove HM’s value to the hospital administration. The rush for implementation takes on added urgency considering that less than half of 0.5% of hospitals are fully paperless, meaning they have interdepartmental systems that can communicate with each other, according to HIMMS Analytics.
Obama and other healthcare reform advocates envision a day not far in the future when all of America’s hospitals will be connected through a national health records system. Databases in hospitals and physician offices and other healthcare providers will communicate with each other. It will make such health records as X-rays and lab test results a portable commodity, which, in theory, will provide faster and more accurate information for both patients and their providers.
One of the economic stimulus plan’s most important features is its “clarity of purpose,” Dr. Blumenthal wrote in the New England Journal of Medicine earlier this year. “Congress apparently sees [health IT]—computers, software, Internet connection, telemedicine—not as an end in itself, but as a means of improving the quality of healthcare, the health of populations, and the efficiency of healthcare systems.”2
Proactive Approach
Obama has pushed EHR implementation as one of many solutions to the skyrocketing costs of healthcare, saying earlier this year that he is committed to “the immediate investments necessary to ensure that within five years, all of America’s medical records are computerized.” Even so, the EHR upgrade remains only a grand outline, one missing the details that will determine the future. There is time, of course. The first funding through the stimulus bill won’t be available until next summer.
Dr. Blumenthal’s office is crafting an interoperability plan in combination with a pair of still-forming advisory boards: a health information policy committee and a health standards committee. The stimulus bill also promises increased federal reimbursement payments for hospitals with meaningful use of certified EHR. First, the government has to define what is meaningful and, as Dr. Stanley points out, the definition will have different meanings to different sectors of the $2.2 trillion-per-year healthcare industry.
Once those definitions are set, there is a timetable for additional reimbursement and a one-time bonus of $2 million for institutions that implement “meaningful use.” There also will be escalating Medicare penalties for institutions that fail to show the kind of technological progress federal officials are looking for.
But even if those standards are set, it doesn’t guarantee hospitals will buy the technology that vendors are selling. Many in the HM field argue that the next step is the most important one.
“Physician adoption of electronic health records is the central, critical issue this industry is facing over the next few years,” says Todd Johnson, president of Salar Inc., a Baltimore-based firm that develops software applications for clinical documentation. “There are a lot of really bright people working on criteria that make electronic health records good tools. However, there doesn’t seem to be an organized body focused on the EHR adoption issues. Anybody can buy all these tools, but if you ultimately can’t get the right people to use them at the right time, the investment doesn’t yield much, right?”
Johnson, who thinks the federal focus on EHR technology is a main driver behind his firm’s 25% sales growth spurt in the first six months of 2009, says physicians have to be a driving force in the EMR implementation process or the system will fail. Take the industry’s classic cautionary tale: Cedars-Sinai Medical Center in Los Angeles. The oft-innovative institution made national headlines in 2002 when it scrapped a three-month-old, $34 million computerized physician order entry (CPOE) system after more than 400 doctors demanded it be shelved.
“The right thing to do is really steer the discussion to physician adoption,” Johnson says. “Make sure that physicians have a choice. Every hospital—and rightly so—wants to see the benefit of their investment in electronic medical records. If physicians don’t have a voice in what will or won’t work, purchasing decisions will be made without them. And that’s not a great thing. Hospital leadership needs to be cognizant of that.”
Dr. Stanley thinks hospitalists should take a proactive approach to EHR implementation at their hospitals. Many potential issues could be solved if hospitalists take an active role earlier in the process.
“As tedious as those early meetings are,” Dr. Stanley says, “that’s where the big planning and decisions get made. The problem is most people think of it as tedious and boring because they don’t appreciate the technology.”
What’s Ahead
Technology integration is the next step. A handful of companies offer complete EHR platforms, including industry leaders Epic, Meditech, Cerner Corp., GE Healthcare, and McKesson Corp. Specialty firms, such as Johnson’s Salar, offer ancillary and support software and hardware.
Kendall Rogers, MD, assistant professor at the University of New Mexico School of Medicine and chair of SHM’s IT Task Force, says the stimulus funding dedicated to technology will be better served if it focuses on incentives beyond hospitals. Dr. Rogers and others want to see guidelines to create incentives for IT vendors to offer user-friendly systems designed to further medical efficiency goals.
“If this needed technology was developed and proven, the needs for carrots and sticks for adoption would be far less,” Dr. Rogers and several of his peers wrote in an unpublished letter to the NEJM. “Rather than focusing primarily on adoption of systems that have serious limitations … a bill that requires improvements in existing technologies would have much more impact in improving the quality of healthcare.”
Even before that happens, full-scale implementation of these systems will be a costly project that requires a long-term relationship with a vendor. Dr. Van Gomple’s hospital system, Sentara Healthcare, has budgeted $235 million over 10 years for its EHR implementation, according to Bert Reese, senior vice president and chief information officer. His accountants tell him to expect roughly $50 million to be subsidized by the stimulus package. The money is helpful, but not enough for a hospital or system that still needs to find another $185 million.
“The stimulus is nice to get things going,” Reese says. “But if you as an organization think that will cover the cost, you’ll never get going.”
Reese says Sentara’s return on investment at full implementation—roughly five years from now—will be about $35 million per year in savings. He suggests organizations view the investment through a long-term profit goal in order to show the value over an extended timeframe. Otherwise, some C-suites will be scared off by the initial outlay, failing to see the value of efficiency, cost savings, and improved patient care.
“It’s not an IT project,” Reese says. “It’s a clinical project.” TH
Richard Quinn is a freelance writer based in New Jersey.
References
- Hamel MB, Drazen JM, Epstein AM. The growth of hospitalists and the changing face of primary care. N Engl J Med. 2009;360(11):1141-1143.
- Blumenthal D. Stimulating the adoption of health information technology. N Engl J Med. 2009;360(15):1477-1479.
- Liebovitz, D. Health care information technology: a cloud around the silver lining? Arch Intern Med. 2009;169(10):924-926.
Image Source: ILLUSTRATION / ALICIA BUELOW
This spring, before Sentara Norfolk General Hospital in Virginia went live with eCare, its electronic health record (EHR) system, hospitalist Ryan Van Gomple, MD, would admit patients using the same system physicians have used for decades: hastily scrawled patient history notes, paper orders, and phone dictation. But eCare’s introduction—and subsequent tweaking in the past few months—has brought a radical transition to the 543-bed tertiary-care facility. Dr. Van Gomple and other hospitalists at institutions on similar systems can enter and access a patient’s data using desktop computers, handheld devices like Blackberrys or iPhones—even their personal laptops at home.
“One of the advantages is we can go back … not only with notes from the hospital stay; a lot of people are doing outpatient notes in the system, so you can start to piece together a total picture of a person’s medical care,” says Dr. Van Gomple, a hospitalist with Sentara Medical Group. “That’s one of the big goals of [EHR]—to have a streamlined system. One of the challenges is, How do you connect with different systems? That’s a great question.”
Dr. Van Gomple might not have the answer, but thanks to ambitious goals laid out by President Obama, the topic is in the national spotlight and already has nearly $20 billion in stimulus money scheduled for release in July 2010. Digitizing healthcare records to create a more efficient care delivery system—through improved record keeping, shortened patient length of stay (LOS), and increased ED throughput—isn’t a new idea. Hospitals have struggled for more than a decade with the EHR question, debating whether they should—not to mention how they would—create a computerized system to input patient records into a database that is accessible in real time to hospitalists, nurses, primary-care physicians, insurers, and so on. There have been long-stalled discussions on how to settle privacy concerns that arise from electronic records (see “EHR Upgrade Faces Privacy, Communication Obstacles,” p. 27). Still, a multi-billion-dollar federal pledge has created a moment in time to take EHR beyond the discussion phase.
The Office of the National Coordinator of Health Information Technology (ONCHIT) is empowered to shepherd this process. David Blumenthal, MD, MPP, the director of the Institute for Health Policy, a joint effort of Massachusetts General Hospital and Partners Healthcare System, has been named as ONCHIT’s head. Money to entice hospitals to invest in EHR is part of the American Recovery and Reinvestment Act of 2009. And with Congress hammering out the details of healthcare reform legislation, a sharper focus has been placed on the potential efficiencies EHR can offer.
Money and attention aren’t the only keys to this puzzle, however. IT advocates, medical information officers, and HM group leaders say the government spotlight is a wonderful springboard, but they also say physician involvement in implementing the EHR technology is a must and will spur more hospitals to adopt the systems. Less than 8% of U.S. hospitals have EHR in at least one unit, the New England Journal of Medicine reported earlier this year.1 Just 1.5% of hospitals have a comprehensive system in all of their units.
“There are so many barriers getting to where our country really needs to get,” says Dirk Stanley, MD, MPH, a hospitalist and chief medical informatics officer at Cooley Dickinson Hospital in Northampton, Mass. “One of the big issues is the meaningful use, and how do you actually set criteria for your using electronic health records the right way? If you look at the big picture, you’re talking about so many clinical practices. … How do you write criteria that are meaningful to all those different settings? The government has an enormous challenge.”
Efficiency: HM Cornerstone
David Yu, MD, FHM, works at a hospital with paperless capability and sees on a daily basis how streamlined health records have a practical effect on a hospitalist’s workload and efficiency. Dr. Yu, medical director of hospitalist services at 372-bed Decatur Memorial Hospital in Decatur, Ill., and clinical assistant professor of family and community medicine at Southern Illinois University School of Medicine in Carbondale, is one of EHR’s most passionate advocates.
Decatur Memorial uses GE Healthcare’s Centricity system, which allows hospitalists to “download automatically into our physical history with the click of a button,” says Dr. Yu, a member of Team Hospitalist. “As you’re downloading, you’re accessing the information. It’s literally the same as you driving to the patient’s primary-care physician’s office, pulling the chart, and looking at it.”
Dr. Yu and those who support EHR say it streamlines intakes, discharges, and handoffs, which in turn reduce throughput and length of stay—statistics often cited to prove HM’s value to the hospital administration. The rush for implementation takes on added urgency considering that less than half of 0.5% of hospitals are fully paperless, meaning they have interdepartmental systems that can communicate with each other, according to HIMMS Analytics.
Obama and other healthcare reform advocates envision a day not far in the future when all of America’s hospitals will be connected through a national health records system. Databases in hospitals and physician offices and other healthcare providers will communicate with each other. It will make such health records as X-rays and lab test results a portable commodity, which, in theory, will provide faster and more accurate information for both patients and their providers.
One of the economic stimulus plan’s most important features is its “clarity of purpose,” Dr. Blumenthal wrote in the New England Journal of Medicine earlier this year. “Congress apparently sees [health IT]—computers, software, Internet connection, telemedicine—not as an end in itself, but as a means of improving the quality of healthcare, the health of populations, and the efficiency of healthcare systems.”2
Proactive Approach
Obama has pushed EHR implementation as one of many solutions to the skyrocketing costs of healthcare, saying earlier this year that he is committed to “the immediate investments necessary to ensure that within five years, all of America’s medical records are computerized.” Even so, the EHR upgrade remains only a grand outline, one missing the details that will determine the future. There is time, of course. The first funding through the stimulus bill won’t be available until next summer.
Dr. Blumenthal’s office is crafting an interoperability plan in combination with a pair of still-forming advisory boards: a health information policy committee and a health standards committee. The stimulus bill also promises increased federal reimbursement payments for hospitals with meaningful use of certified EHR. First, the government has to define what is meaningful and, as Dr. Stanley points out, the definition will have different meanings to different sectors of the $2.2 trillion-per-year healthcare industry.
Once those definitions are set, there is a timetable for additional reimbursement and a one-time bonus of $2 million for institutions that implement “meaningful use.” There also will be escalating Medicare penalties for institutions that fail to show the kind of technological progress federal officials are looking for.
But even if those standards are set, it doesn’t guarantee hospitals will buy the technology that vendors are selling. Many in the HM field argue that the next step is the most important one.
“Physician adoption of electronic health records is the central, critical issue this industry is facing over the next few years,” says Todd Johnson, president of Salar Inc., a Baltimore-based firm that develops software applications for clinical documentation. “There are a lot of really bright people working on criteria that make electronic health records good tools. However, there doesn’t seem to be an organized body focused on the EHR adoption issues. Anybody can buy all these tools, but if you ultimately can’t get the right people to use them at the right time, the investment doesn’t yield much, right?”
Johnson, who thinks the federal focus on EHR technology is a main driver behind his firm’s 25% sales growth spurt in the first six months of 2009, says physicians have to be a driving force in the EMR implementation process or the system will fail. Take the industry’s classic cautionary tale: Cedars-Sinai Medical Center in Los Angeles. The oft-innovative institution made national headlines in 2002 when it scrapped a three-month-old, $34 million computerized physician order entry (CPOE) system after more than 400 doctors demanded it be shelved.
“The right thing to do is really steer the discussion to physician adoption,” Johnson says. “Make sure that physicians have a choice. Every hospital—and rightly so—wants to see the benefit of their investment in electronic medical records. If physicians don’t have a voice in what will or won’t work, purchasing decisions will be made without them. And that’s not a great thing. Hospital leadership needs to be cognizant of that.”
Dr. Stanley thinks hospitalists should take a proactive approach to EHR implementation at their hospitals. Many potential issues could be solved if hospitalists take an active role earlier in the process.
“As tedious as those early meetings are,” Dr. Stanley says, “that’s where the big planning and decisions get made. The problem is most people think of it as tedious and boring because they don’t appreciate the technology.”
What’s Ahead
Technology integration is the next step. A handful of companies offer complete EHR platforms, including industry leaders Epic, Meditech, Cerner Corp., GE Healthcare, and McKesson Corp. Specialty firms, such as Johnson’s Salar, offer ancillary and support software and hardware.
Kendall Rogers, MD, assistant professor at the University of New Mexico School of Medicine and chair of SHM’s IT Task Force, says the stimulus funding dedicated to technology will be better served if it focuses on incentives beyond hospitals. Dr. Rogers and others want to see guidelines to create incentives for IT vendors to offer user-friendly systems designed to further medical efficiency goals.
“If this needed technology was developed and proven, the needs for carrots and sticks for adoption would be far less,” Dr. Rogers and several of his peers wrote in an unpublished letter to the NEJM. “Rather than focusing primarily on adoption of systems that have serious limitations … a bill that requires improvements in existing technologies would have much more impact in improving the quality of healthcare.”
Even before that happens, full-scale implementation of these systems will be a costly project that requires a long-term relationship with a vendor. Dr. Van Gomple’s hospital system, Sentara Healthcare, has budgeted $235 million over 10 years for its EHR implementation, according to Bert Reese, senior vice president and chief information officer. His accountants tell him to expect roughly $50 million to be subsidized by the stimulus package. The money is helpful, but not enough for a hospital or system that still needs to find another $185 million.
“The stimulus is nice to get things going,” Reese says. “But if you as an organization think that will cover the cost, you’ll never get going.”
Reese says Sentara’s return on investment at full implementation—roughly five years from now—will be about $35 million per year in savings. He suggests organizations view the investment through a long-term profit goal in order to show the value over an extended timeframe. Otherwise, some C-suites will be scared off by the initial outlay, failing to see the value of efficiency, cost savings, and improved patient care.
“It’s not an IT project,” Reese says. “It’s a clinical project.” TH
Richard Quinn is a freelance writer based in New Jersey.
References
- Hamel MB, Drazen JM, Epstein AM. The growth of hospitalists and the changing face of primary care. N Engl J Med. 2009;360(11):1141-1143.
- Blumenthal D. Stimulating the adoption of health information technology. N Engl J Med. 2009;360(15):1477-1479.
- Liebovitz, D. Health care information technology: a cloud around the silver lining? Arch Intern Med. 2009;169(10):924-926.
Image Source: ILLUSTRATION / ALICIA BUELOW
This spring, before Sentara Norfolk General Hospital in Virginia went live with eCare, its electronic health record (EHR) system, hospitalist Ryan Van Gomple, MD, would admit patients using the same system physicians have used for decades: hastily scrawled patient history notes, paper orders, and phone dictation. But eCare’s introduction—and subsequent tweaking in the past few months—has brought a radical transition to the 543-bed tertiary-care facility. Dr. Van Gomple and other hospitalists at institutions on similar systems can enter and access a patient’s data using desktop computers, handheld devices like Blackberrys or iPhones—even their personal laptops at home.
“One of the advantages is we can go back … not only with notes from the hospital stay; a lot of people are doing outpatient notes in the system, so you can start to piece together a total picture of a person’s medical care,” says Dr. Van Gomple, a hospitalist with Sentara Medical Group. “That’s one of the big goals of [EHR]—to have a streamlined system. One of the challenges is, How do you connect with different systems? That’s a great question.”
Dr. Van Gomple might not have the answer, but thanks to ambitious goals laid out by President Obama, the topic is in the national spotlight and already has nearly $20 billion in stimulus money scheduled for release in July 2010. Digitizing healthcare records to create a more efficient care delivery system—through improved record keeping, shortened patient length of stay (LOS), and increased ED throughput—isn’t a new idea. Hospitals have struggled for more than a decade with the EHR question, debating whether they should—not to mention how they would—create a computerized system to input patient records into a database that is accessible in real time to hospitalists, nurses, primary-care physicians, insurers, and so on. There have been long-stalled discussions on how to settle privacy concerns that arise from electronic records (see “EHR Upgrade Faces Privacy, Communication Obstacles,” p. 27). Still, a multi-billion-dollar federal pledge has created a moment in time to take EHR beyond the discussion phase.
The Office of the National Coordinator of Health Information Technology (ONCHIT) is empowered to shepherd this process. David Blumenthal, MD, MPP, the director of the Institute for Health Policy, a joint effort of Massachusetts General Hospital and Partners Healthcare System, has been named as ONCHIT’s head. Money to entice hospitals to invest in EHR is part of the American Recovery and Reinvestment Act of 2009. And with Congress hammering out the details of healthcare reform legislation, a sharper focus has been placed on the potential efficiencies EHR can offer.
Money and attention aren’t the only keys to this puzzle, however. IT advocates, medical information officers, and HM group leaders say the government spotlight is a wonderful springboard, but they also say physician involvement in implementing the EHR technology is a must and will spur more hospitals to adopt the systems. Less than 8% of U.S. hospitals have EHR in at least one unit, the New England Journal of Medicine reported earlier this year.1 Just 1.5% of hospitals have a comprehensive system in all of their units.
“There are so many barriers getting to where our country really needs to get,” says Dirk Stanley, MD, MPH, a hospitalist and chief medical informatics officer at Cooley Dickinson Hospital in Northampton, Mass. “One of the big issues is the meaningful use, and how do you actually set criteria for your using electronic health records the right way? If you look at the big picture, you’re talking about so many clinical practices. … How do you write criteria that are meaningful to all those different settings? The government has an enormous challenge.”
Efficiency: HM Cornerstone
David Yu, MD, FHM, works at a hospital with paperless capability and sees on a daily basis how streamlined health records have a practical effect on a hospitalist’s workload and efficiency. Dr. Yu, medical director of hospitalist services at 372-bed Decatur Memorial Hospital in Decatur, Ill., and clinical assistant professor of family and community medicine at Southern Illinois University School of Medicine in Carbondale, is one of EHR’s most passionate advocates.
Decatur Memorial uses GE Healthcare’s Centricity system, which allows hospitalists to “download automatically into our physical history with the click of a button,” says Dr. Yu, a member of Team Hospitalist. “As you’re downloading, you’re accessing the information. It’s literally the same as you driving to the patient’s primary-care physician’s office, pulling the chart, and looking at it.”
Dr. Yu and those who support EHR say it streamlines intakes, discharges, and handoffs, which in turn reduce throughput and length of stay—statistics often cited to prove HM’s value to the hospital administration. The rush for implementation takes on added urgency considering that less than half of 0.5% of hospitals are fully paperless, meaning they have interdepartmental systems that can communicate with each other, according to HIMMS Analytics.
Obama and other healthcare reform advocates envision a day not far in the future when all of America’s hospitals will be connected through a national health records system. Databases in hospitals and physician offices and other healthcare providers will communicate with each other. It will make such health records as X-rays and lab test results a portable commodity, which, in theory, will provide faster and more accurate information for both patients and their providers.
One of the economic stimulus plan’s most important features is its “clarity of purpose,” Dr. Blumenthal wrote in the New England Journal of Medicine earlier this year. “Congress apparently sees [health IT]—computers, software, Internet connection, telemedicine—not as an end in itself, but as a means of improving the quality of healthcare, the health of populations, and the efficiency of healthcare systems.”2
Proactive Approach
Obama has pushed EHR implementation as one of many solutions to the skyrocketing costs of healthcare, saying earlier this year that he is committed to “the immediate investments necessary to ensure that within five years, all of America’s medical records are computerized.” Even so, the EHR upgrade remains only a grand outline, one missing the details that will determine the future. There is time, of course. The first funding through the stimulus bill won’t be available until next summer.
Dr. Blumenthal’s office is crafting an interoperability plan in combination with a pair of still-forming advisory boards: a health information policy committee and a health standards committee. The stimulus bill also promises increased federal reimbursement payments for hospitals with meaningful use of certified EHR. First, the government has to define what is meaningful and, as Dr. Stanley points out, the definition will have different meanings to different sectors of the $2.2 trillion-per-year healthcare industry.
Once those definitions are set, there is a timetable for additional reimbursement and a one-time bonus of $2 million for institutions that implement “meaningful use.” There also will be escalating Medicare penalties for institutions that fail to show the kind of technological progress federal officials are looking for.
But even if those standards are set, it doesn’t guarantee hospitals will buy the technology that vendors are selling. Many in the HM field argue that the next step is the most important one.
“Physician adoption of electronic health records is the central, critical issue this industry is facing over the next few years,” says Todd Johnson, president of Salar Inc., a Baltimore-based firm that develops software applications for clinical documentation. “There are a lot of really bright people working on criteria that make electronic health records good tools. However, there doesn’t seem to be an organized body focused on the EHR adoption issues. Anybody can buy all these tools, but if you ultimately can’t get the right people to use them at the right time, the investment doesn’t yield much, right?”
Johnson, who thinks the federal focus on EHR technology is a main driver behind his firm’s 25% sales growth spurt in the first six months of 2009, says physicians have to be a driving force in the EMR implementation process or the system will fail. Take the industry’s classic cautionary tale: Cedars-Sinai Medical Center in Los Angeles. The oft-innovative institution made national headlines in 2002 when it scrapped a three-month-old, $34 million computerized physician order entry (CPOE) system after more than 400 doctors demanded it be shelved.
“The right thing to do is really steer the discussion to physician adoption,” Johnson says. “Make sure that physicians have a choice. Every hospital—and rightly so—wants to see the benefit of their investment in electronic medical records. If physicians don’t have a voice in what will or won’t work, purchasing decisions will be made without them. And that’s not a great thing. Hospital leadership needs to be cognizant of that.”
Dr. Stanley thinks hospitalists should take a proactive approach to EHR implementation at their hospitals. Many potential issues could be solved if hospitalists take an active role earlier in the process.
“As tedious as those early meetings are,” Dr. Stanley says, “that’s where the big planning and decisions get made. The problem is most people think of it as tedious and boring because they don’t appreciate the technology.”
What’s Ahead
Technology integration is the next step. A handful of companies offer complete EHR platforms, including industry leaders Epic, Meditech, Cerner Corp., GE Healthcare, and McKesson Corp. Specialty firms, such as Johnson’s Salar, offer ancillary and support software and hardware.
Kendall Rogers, MD, assistant professor at the University of New Mexico School of Medicine and chair of SHM’s IT Task Force, says the stimulus funding dedicated to technology will be better served if it focuses on incentives beyond hospitals. Dr. Rogers and others want to see guidelines to create incentives for IT vendors to offer user-friendly systems designed to further medical efficiency goals.
“If this needed technology was developed and proven, the needs for carrots and sticks for adoption would be far less,” Dr. Rogers and several of his peers wrote in an unpublished letter to the NEJM. “Rather than focusing primarily on adoption of systems that have serious limitations … a bill that requires improvements in existing technologies would have much more impact in improving the quality of healthcare.”
Even before that happens, full-scale implementation of these systems will be a costly project that requires a long-term relationship with a vendor. Dr. Van Gomple’s hospital system, Sentara Healthcare, has budgeted $235 million over 10 years for its EHR implementation, according to Bert Reese, senior vice president and chief information officer. His accountants tell him to expect roughly $50 million to be subsidized by the stimulus package. The money is helpful, but not enough for a hospital or system that still needs to find another $185 million.
“The stimulus is nice to get things going,” Reese says. “But if you as an organization think that will cover the cost, you’ll never get going.”
Reese says Sentara’s return on investment at full implementation—roughly five years from now—will be about $35 million per year in savings. He suggests organizations view the investment through a long-term profit goal in order to show the value over an extended timeframe. Otherwise, some C-suites will be scared off by the initial outlay, failing to see the value of efficiency, cost savings, and improved patient care.
“It’s not an IT project,” Reese says. “It’s a clinical project.” TH
Richard Quinn is a freelance writer based in New Jersey.
References
- Hamel MB, Drazen JM, Epstein AM. The growth of hospitalists and the changing face of primary care. N Engl J Med. 2009;360(11):1141-1143.
- Blumenthal D. Stimulating the adoption of health information technology. N Engl J Med. 2009;360(15):1477-1479.
- Liebovitz, D. Health care information technology: a cloud around the silver lining? Arch Intern Med. 2009;169(10):924-926.
Image Source: ILLUSTRATION / ALICIA BUELOW
Role Refinement
Physician assistants (PAs) and nurse practitioners (NPs), which I will refer to as non-physician providers (NPPs), are popular members of hospitalist practices and have a lot to offer. I think HM groups without NPPs should think about whether adding them would be valuable.
My experience suggests there are many different ways NPPs can contribute to an effective practice. But the optimal NPP role, one that is good for patient care, economically sound for the practice, and satisfying for both the NPP and the MD hospitalists, varies significantly from one practice to the next. I’ve worked with a number of practices that fail to achieve all these goals for a variety of reasons, but a common theme is that the MD hospitalists seem to think the NPPs have been provided for free. As a result, the MDs, and perhaps to some degree the NPPs, feel little or no obligation to develop the optimal NPP job description.
A popular role for NPPs is one very similar to that of the MD hospitalist (e.g., the NPP has a team of patients and rounds and admits daily). That might work well, but for reasons I’ve discussed previously (see “The 411 on NPPs,” September 2008, p. 61), many practices should at least consider other roles for NPPs. One alternative would be to have the NPP work an afternoon-to-night shift (e.g., 3 to 11 p.m.) to handle admissions and “crosscover.” Another option is for the NPP to essentially “own” a component of the practice, such as medical consults for orthopedic patients.
Whatever role is chosen, it must be one that provides the NPP career satisfaction. Over the last few years, I’ve had the pleasure of connecting with Ryan Genzink, PA-C, at various SHM meetings. He essentially is a career hospitalist, and I’ve found him to be a thoughtful guy. At HM09 in Chicago, he and I spoke for a while about NPP roles that provide value and career satisfaction. So I’ve invited him to share his thoughts here.
(Editor’s note: The following is written by Ryan Genzink, PA-C, of Hospitalists of West Michigan in Grand Rapids. He is the AAPA medical liaison to SHM.)
Dr. Nelson correctly observes that while NPPs can be beneficial to HM, there is no “one size fits all” model. However, I think finding the right model for your group sometimes is presented as being more difficult than it really needs to be. Over the years, I have had the opportunity to talk with a number of physicians, PAs, and NPs who work in HM. While models vary, those identified as successful seem to share some common elements.
My story is typical of a lot of PAs working in HM. When I was hired in 2000, my hospital was addressing a workforce shortage. Medical resident workloads were capped, private attending physicians wanted help admitting patients, and the ED was anxious to transfer admitted patients. The hospital was intent on not making our patients wait.
I joined a small group of PAs whose job description included addressing these issues. Like the residents we worked alongside, we took initial calls from the ED, performed histories and physicals, then staffed those with our attending physicians. As a new graduate, I was green and enthusiastic.
The hospitalists were fairly new to working with PAs, too. They had spent years teaching residents, but PAs had joined the group only a year prior. Even so, the group had developed a successful supervision model based on their experiences teaching residents. Patients I saw were cared for by attendings who reviewed the history, asked key questions, performed essential exam elements, and gave the final word on the treatment plan. Teaching naturally flowed from these interactions.
This model continues today. And like the interns who needed less attending input as they transitioned into chief residents, I also required less physician input over time. As our professional relationship grew, the hospitalists became more familiar with my work and exam skills, and I became proficient with our common treatment plans. We functioned together as a team. Of course, this process was no small investment on the part of the hospitalists I worked with. It took time—sometimes with detailed discussions of treatment protocols, or re-examining the patient together to make sure our exams were on the same page. Nonetheless, I think all involved agree the payoff was worth the effort. For our physicians, it made the transition from a resident-based program to one staffed with NPPs favorable. Granted, a residency program has different goals, but because the NPPs don’t rotate off service every six weeks, there is more time to develop collaborative, professional relationships. The investment the attending physicians made stuck.
As work volume increased, PAs in our group expanded into other roles. Our two academic rounding teams, each consisting of one hospitalist and a few residents, added a third team staffed with a hospitalist and a PA. When the residents left, all three teams were staffed with a physician and a PA. NPs later joined the group. And while NPs had slightly different state supervision rules, they functioned in the same roles as the PAs in our facility.
This team approach to rounding works well for our group. The hospitalists and NPPs work together to care for a set group of patients. The hospitalist and the NPP meet in the morning to divide the workload based on acuity, geographic location, and urgency. Sharing a common patient load helps with the common hospitalist dilemma of having to be in two places at the same time. I can see a patient who is ready for discharge (e.g., their ride is on the way), allowing my attending to dedicate his time to another patient’s family conference. In every case, the physician is involved. It is the extent of the involvement that varies. This model gives us flexibility and offers availability to our common patients.
Again, this is one of many successful models. Some, including Dr. Nelson, have suggested that a successful integration model might limit NPPs’ role in the group so that they can have ownership (e.g., post-op consult services). I think there is some merit to this, but this system also has potential unintended consequences.
When we look at what makes hospitalists successful at caring for post-operative patients, we often cite the experience gained from the wide variety of complex medical problems that we address on a daily basis. It is our frequent experience with patients with chronic heart failure that helps us identify the patient in early fluid overload. Our knowledge of diabetic ketoacidosis improves our routine diabetes management.
In my experience, rarely does a patient present with a single, narrowly defined problem. I think that limiting NPPs to the care of specific patient problems will result in limiting their effectiveness and possibly decrease their job satisfaction. I also think HM groups can err on the side of having unrealistic expectations for NPPs. Some groups have them perform the same role as an attending—with an NPP taking the spot of an off-service attending, and vice versa. This can work, if the NPP is experienced. Few would expect a new intern to perform like an attending. Conversely, restricting an NPP to collecting labs and paperwork is not an efficient use of resources.
As Dr. Nelson suggests, successful NPP integration depends on physician leaders being dedicated to the collaborative model and understanding that NPP success is tied to group success. And while admittedly not a perfect test, when in doubt about how an NPP could function in your group, I think asking if a resident would work in the same role is a good starting point. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelson flores.com). He is co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Physician assistants (PAs) and nurse practitioners (NPs), which I will refer to as non-physician providers (NPPs), are popular members of hospitalist practices and have a lot to offer. I think HM groups without NPPs should think about whether adding them would be valuable.
My experience suggests there are many different ways NPPs can contribute to an effective practice. But the optimal NPP role, one that is good for patient care, economically sound for the practice, and satisfying for both the NPP and the MD hospitalists, varies significantly from one practice to the next. I’ve worked with a number of practices that fail to achieve all these goals for a variety of reasons, but a common theme is that the MD hospitalists seem to think the NPPs have been provided for free. As a result, the MDs, and perhaps to some degree the NPPs, feel little or no obligation to develop the optimal NPP job description.
A popular role for NPPs is one very similar to that of the MD hospitalist (e.g., the NPP has a team of patients and rounds and admits daily). That might work well, but for reasons I’ve discussed previously (see “The 411 on NPPs,” September 2008, p. 61), many practices should at least consider other roles for NPPs. One alternative would be to have the NPP work an afternoon-to-night shift (e.g., 3 to 11 p.m.) to handle admissions and “crosscover.” Another option is for the NPP to essentially “own” a component of the practice, such as medical consults for orthopedic patients.
Whatever role is chosen, it must be one that provides the NPP career satisfaction. Over the last few years, I’ve had the pleasure of connecting with Ryan Genzink, PA-C, at various SHM meetings. He essentially is a career hospitalist, and I’ve found him to be a thoughtful guy. At HM09 in Chicago, he and I spoke for a while about NPP roles that provide value and career satisfaction. So I’ve invited him to share his thoughts here.
(Editor’s note: The following is written by Ryan Genzink, PA-C, of Hospitalists of West Michigan in Grand Rapids. He is the AAPA medical liaison to SHM.)
Dr. Nelson correctly observes that while NPPs can be beneficial to HM, there is no “one size fits all” model. However, I think finding the right model for your group sometimes is presented as being more difficult than it really needs to be. Over the years, I have had the opportunity to talk with a number of physicians, PAs, and NPs who work in HM. While models vary, those identified as successful seem to share some common elements.
My story is typical of a lot of PAs working in HM. When I was hired in 2000, my hospital was addressing a workforce shortage. Medical resident workloads were capped, private attending physicians wanted help admitting patients, and the ED was anxious to transfer admitted patients. The hospital was intent on not making our patients wait.
I joined a small group of PAs whose job description included addressing these issues. Like the residents we worked alongside, we took initial calls from the ED, performed histories and physicals, then staffed those with our attending physicians. As a new graduate, I was green and enthusiastic.
The hospitalists were fairly new to working with PAs, too. They had spent years teaching residents, but PAs had joined the group only a year prior. Even so, the group had developed a successful supervision model based on their experiences teaching residents. Patients I saw were cared for by attendings who reviewed the history, asked key questions, performed essential exam elements, and gave the final word on the treatment plan. Teaching naturally flowed from these interactions.
This model continues today. And like the interns who needed less attending input as they transitioned into chief residents, I also required less physician input over time. As our professional relationship grew, the hospitalists became more familiar with my work and exam skills, and I became proficient with our common treatment plans. We functioned together as a team. Of course, this process was no small investment on the part of the hospitalists I worked with. It took time—sometimes with detailed discussions of treatment protocols, or re-examining the patient together to make sure our exams were on the same page. Nonetheless, I think all involved agree the payoff was worth the effort. For our physicians, it made the transition from a resident-based program to one staffed with NPPs favorable. Granted, a residency program has different goals, but because the NPPs don’t rotate off service every six weeks, there is more time to develop collaborative, professional relationships. The investment the attending physicians made stuck.
As work volume increased, PAs in our group expanded into other roles. Our two academic rounding teams, each consisting of one hospitalist and a few residents, added a third team staffed with a hospitalist and a PA. When the residents left, all three teams were staffed with a physician and a PA. NPs later joined the group. And while NPs had slightly different state supervision rules, they functioned in the same roles as the PAs in our facility.
This team approach to rounding works well for our group. The hospitalists and NPPs work together to care for a set group of patients. The hospitalist and the NPP meet in the morning to divide the workload based on acuity, geographic location, and urgency. Sharing a common patient load helps with the common hospitalist dilemma of having to be in two places at the same time. I can see a patient who is ready for discharge (e.g., their ride is on the way), allowing my attending to dedicate his time to another patient’s family conference. In every case, the physician is involved. It is the extent of the involvement that varies. This model gives us flexibility and offers availability to our common patients.
Again, this is one of many successful models. Some, including Dr. Nelson, have suggested that a successful integration model might limit NPPs’ role in the group so that they can have ownership (e.g., post-op consult services). I think there is some merit to this, but this system also has potential unintended consequences.
When we look at what makes hospitalists successful at caring for post-operative patients, we often cite the experience gained from the wide variety of complex medical problems that we address on a daily basis. It is our frequent experience with patients with chronic heart failure that helps us identify the patient in early fluid overload. Our knowledge of diabetic ketoacidosis improves our routine diabetes management.
In my experience, rarely does a patient present with a single, narrowly defined problem. I think that limiting NPPs to the care of specific patient problems will result in limiting their effectiveness and possibly decrease their job satisfaction. I also think HM groups can err on the side of having unrealistic expectations for NPPs. Some groups have them perform the same role as an attending—with an NPP taking the spot of an off-service attending, and vice versa. This can work, if the NPP is experienced. Few would expect a new intern to perform like an attending. Conversely, restricting an NPP to collecting labs and paperwork is not an efficient use of resources.
As Dr. Nelson suggests, successful NPP integration depends on physician leaders being dedicated to the collaborative model and understanding that NPP success is tied to group success. And while admittedly not a perfect test, when in doubt about how an NPP could function in your group, I think asking if a resident would work in the same role is a good starting point. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelson flores.com). He is co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Physician assistants (PAs) and nurse practitioners (NPs), which I will refer to as non-physician providers (NPPs), are popular members of hospitalist practices and have a lot to offer. I think HM groups without NPPs should think about whether adding them would be valuable.
My experience suggests there are many different ways NPPs can contribute to an effective practice. But the optimal NPP role, one that is good for patient care, economically sound for the practice, and satisfying for both the NPP and the MD hospitalists, varies significantly from one practice to the next. I’ve worked with a number of practices that fail to achieve all these goals for a variety of reasons, but a common theme is that the MD hospitalists seem to think the NPPs have been provided for free. As a result, the MDs, and perhaps to some degree the NPPs, feel little or no obligation to develop the optimal NPP job description.
A popular role for NPPs is one very similar to that of the MD hospitalist (e.g., the NPP has a team of patients and rounds and admits daily). That might work well, but for reasons I’ve discussed previously (see “The 411 on NPPs,” September 2008, p. 61), many practices should at least consider other roles for NPPs. One alternative would be to have the NPP work an afternoon-to-night shift (e.g., 3 to 11 p.m.) to handle admissions and “crosscover.” Another option is for the NPP to essentially “own” a component of the practice, such as medical consults for orthopedic patients.
Whatever role is chosen, it must be one that provides the NPP career satisfaction. Over the last few years, I’ve had the pleasure of connecting with Ryan Genzink, PA-C, at various SHM meetings. He essentially is a career hospitalist, and I’ve found him to be a thoughtful guy. At HM09 in Chicago, he and I spoke for a while about NPP roles that provide value and career satisfaction. So I’ve invited him to share his thoughts here.
(Editor’s note: The following is written by Ryan Genzink, PA-C, of Hospitalists of West Michigan in Grand Rapids. He is the AAPA medical liaison to SHM.)
Dr. Nelson correctly observes that while NPPs can be beneficial to HM, there is no “one size fits all” model. However, I think finding the right model for your group sometimes is presented as being more difficult than it really needs to be. Over the years, I have had the opportunity to talk with a number of physicians, PAs, and NPs who work in HM. While models vary, those identified as successful seem to share some common elements.
My story is typical of a lot of PAs working in HM. When I was hired in 2000, my hospital was addressing a workforce shortage. Medical resident workloads were capped, private attending physicians wanted help admitting patients, and the ED was anxious to transfer admitted patients. The hospital was intent on not making our patients wait.
I joined a small group of PAs whose job description included addressing these issues. Like the residents we worked alongside, we took initial calls from the ED, performed histories and physicals, then staffed those with our attending physicians. As a new graduate, I was green and enthusiastic.
The hospitalists were fairly new to working with PAs, too. They had spent years teaching residents, but PAs had joined the group only a year prior. Even so, the group had developed a successful supervision model based on their experiences teaching residents. Patients I saw were cared for by attendings who reviewed the history, asked key questions, performed essential exam elements, and gave the final word on the treatment plan. Teaching naturally flowed from these interactions.
This model continues today. And like the interns who needed less attending input as they transitioned into chief residents, I also required less physician input over time. As our professional relationship grew, the hospitalists became more familiar with my work and exam skills, and I became proficient with our common treatment plans. We functioned together as a team. Of course, this process was no small investment on the part of the hospitalists I worked with. It took time—sometimes with detailed discussions of treatment protocols, or re-examining the patient together to make sure our exams were on the same page. Nonetheless, I think all involved agree the payoff was worth the effort. For our physicians, it made the transition from a resident-based program to one staffed with NPPs favorable. Granted, a residency program has different goals, but because the NPPs don’t rotate off service every six weeks, there is more time to develop collaborative, professional relationships. The investment the attending physicians made stuck.
As work volume increased, PAs in our group expanded into other roles. Our two academic rounding teams, each consisting of one hospitalist and a few residents, added a third team staffed with a hospitalist and a PA. When the residents left, all three teams were staffed with a physician and a PA. NPs later joined the group. And while NPs had slightly different state supervision rules, they functioned in the same roles as the PAs in our facility.
This team approach to rounding works well for our group. The hospitalists and NPPs work together to care for a set group of patients. The hospitalist and the NPP meet in the morning to divide the workload based on acuity, geographic location, and urgency. Sharing a common patient load helps with the common hospitalist dilemma of having to be in two places at the same time. I can see a patient who is ready for discharge (e.g., their ride is on the way), allowing my attending to dedicate his time to another patient’s family conference. In every case, the physician is involved. It is the extent of the involvement that varies. This model gives us flexibility and offers availability to our common patients.
Again, this is one of many successful models. Some, including Dr. Nelson, have suggested that a successful integration model might limit NPPs’ role in the group so that they can have ownership (e.g., post-op consult services). I think there is some merit to this, but this system also has potential unintended consequences.
When we look at what makes hospitalists successful at caring for post-operative patients, we often cite the experience gained from the wide variety of complex medical problems that we address on a daily basis. It is our frequent experience with patients with chronic heart failure that helps us identify the patient in early fluid overload. Our knowledge of diabetic ketoacidosis improves our routine diabetes management.
In my experience, rarely does a patient present with a single, narrowly defined problem. I think that limiting NPPs to the care of specific patient problems will result in limiting their effectiveness and possibly decrease their job satisfaction. I also think HM groups can err on the side of having unrealistic expectations for NPPs. Some groups have them perform the same role as an attending—with an NPP taking the spot of an off-service attending, and vice versa. This can work, if the NPP is experienced. Few would expect a new intern to perform like an attending. Conversely, restricting an NPP to collecting labs and paperwork is not an efficient use of resources.
As Dr. Nelson suggests, successful NPP integration depends on physician leaders being dedicated to the collaborative model and understanding that NPP success is tied to group success. And while admittedly not a perfect test, when in doubt about how an NPP could function in your group, I think asking if a resident would work in the same role is a good starting point. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelson flores.com). He is co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Only Fools Rush In
Irecently bought an Apple computer. This is newsworthy because I am a lifelong PC user, and because I suffer from a genetic inability to adopt new technologies. In fact, I’m so old-school that only the graceless act of brandishing my credit card kept me from being unceremoniously escorted out of the store by Apple “specialists” for scaring off iPod-yearning tweens. Even then I wasn’t allowed to linger post-purchase.
After racing home, I eagerly slipped the 24 inches of iMac splendor out of its slick, mod-ish packaging and set it up on my desk. Thoughts of newfound cachet danced in my head as I peeled off my black “dad” socks and Tevas. It was clear to me that, as advertised, this sleek beauty was going to transform me from a doddering old Luddite into one of those trendy hipsters so keyed into the pulse of society. And it promised to be easier … and better.
What followed reminded me that the gospel of technology could sometimes preach a false god. It took but a few keystrokes to find myself grappling with a new operating system—it looked like Windows but felt like closed doors. Things that I can do effortlessly on my PC—navigate through files, open Web browsers, access my Outlook account, PowerPoint—seemed to take extra steps, new steps, or unknown steps.
Eventually, I was able figure most of this out, but in the end I was left with the very un-sating realization that the Mac wasn’t really any better than my PC. It was cooler, that’s for sure—even my wife took a renewed interest in me. However, after that cool factor quickly chilled, I was left with the queasy feeling that I had just dropped thousands of dollars on a machine that didn’t really function any better than my old machine. And in some cases, it functioned worse.
Fool’s Gold?
Like my new iMac, electronic health records (EHR) are touted as the technological savior of healthcare—if you invest in the rhetoric coming out of Washington. As our legislators struggle to figure out ways to shoehorn 50 million more Americans into the “insured” category, stave off the growing epidemic of medical errors, and improve the general quality of care, digitizing our health records is a commonly noted panacea. And EHR, it’s promised, somehow will do all of this while conveniently reducing the skyrocketing costs of healthcare.
My hospital is on the verge of siphoning tens of millions of dollars of government stimulus funding and hospital capital into the purchase of a major EHR upgrade. This system aims to seamlessly integrate our inpatient and outpatient billing, documentation, lab, and ordering systems into one neatly packaged, computer-driven solution. I’m left wondering if my iMac experience is an augur of how this will play out on a grand stage.
In the spirit of full disclosure, I fully support automating healthcare as much as possible and sit on numerous committees at my institution charged with doing just that. Further, I believe it will be a salve to many of our efficiency, quality, and patient safety issues. However, I worry that in solving some problems, these new technological cure-alls will simply introduce other, unanticipated problems.
Several years ago, my hospital introduced several systems and technological applications aimed at improving quality, safety, and efficiency. One of these is called “rounds reports.” These very handy, printable documents are a terrific idea and a great example of simplified workflow. The doctor simply prints out a one-page summary of the patient’s 24-hour vital signs, medications, and labs, and uses that as the template for their daily note.
As promised, it’s a time-saver. The problem is that this new technology harbors insidious flaws that prey on the frailties of human nature by introducing new portals for error. For example, the report makes it simple to not reconcile medications daily. The time-honored and time-intensive manner of writing all the meds on a progress note is indeed cumbersome, but it has the added effect of forcing the provider to think about each medication—the utility, the dosage, the effects of the failing kidney on the dosage. Automation of this process removes this small but critical safety check. Sure, diligent providers can overcome this by paying close attention to the printout, but human nature dictates that we don’t always do it. In fact, we employed automation to save this type of time in the first place.
The rounds report also helpfully displays the vital signs and blood sugars for the past 24 hours, reducing the time the harried hospitalist has to spend looking these up and writing them down. However, the report doesn’t print out every vital sign and blood-sugar level; it provides a range. Again, it is possible to access these individual levels, but the post-EHR provider, lured by simplification, often doesn’t take the extra step to go to the separate program to gather these numbers. This shortcut enhances efficiency at the expense of having complete data, a scenario that can breed bad outcomes.
More Efficient Doesn’t Mean Better
Then there is the catch-22 of electronic imaging reports. It is impressive how quickly a chest X-ray gets read and reported electronically in my hospital. The downside, of course, is that today’s techno-doc can rely on the written report without reviewing the actual image. We’ve again, in not reviewing the films personally, removed an important safety check.
The point is that while mechanization offers great potential, it is easy to overlook the downside. Many physicians are not as tech-savvy as their kids and likely will struggle with these newfangled devices. For them, this will not simplify their workflow, but rather it will bog them down. These gizmos also are extremely expensive, and many small clinics and rural hospitals will struggle to afford these upgrades, even with taxpayer support. And let’s not overlook the myriad unforeseen hiccups these new systems will breed.
None of this is to say we shouldn’t embrace our “Jetsons”-like future. In fact, I’d counter that we must, and now is the opportune time. Still, I get nervous when I read stories of the endless EHR potential that omit or gloss over the probable limitations. The key will be to adopt these systems in ways that augment their strengths while mitigating their weaknesses. This must include achieving the delicate balance of usability, efficiency, and safety.
Otherwise, we might find that the technological apple will keep the doctors away. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
Irecently bought an Apple computer. This is newsworthy because I am a lifelong PC user, and because I suffer from a genetic inability to adopt new technologies. In fact, I’m so old-school that only the graceless act of brandishing my credit card kept me from being unceremoniously escorted out of the store by Apple “specialists” for scaring off iPod-yearning tweens. Even then I wasn’t allowed to linger post-purchase.
After racing home, I eagerly slipped the 24 inches of iMac splendor out of its slick, mod-ish packaging and set it up on my desk. Thoughts of newfound cachet danced in my head as I peeled off my black “dad” socks and Tevas. It was clear to me that, as advertised, this sleek beauty was going to transform me from a doddering old Luddite into one of those trendy hipsters so keyed into the pulse of society. And it promised to be easier … and better.
What followed reminded me that the gospel of technology could sometimes preach a false god. It took but a few keystrokes to find myself grappling with a new operating system—it looked like Windows but felt like closed doors. Things that I can do effortlessly on my PC—navigate through files, open Web browsers, access my Outlook account, PowerPoint—seemed to take extra steps, new steps, or unknown steps.
Eventually, I was able figure most of this out, but in the end I was left with the very un-sating realization that the Mac wasn’t really any better than my PC. It was cooler, that’s for sure—even my wife took a renewed interest in me. However, after that cool factor quickly chilled, I was left with the queasy feeling that I had just dropped thousands of dollars on a machine that didn’t really function any better than my old machine. And in some cases, it functioned worse.
Fool’s Gold?
Like my new iMac, electronic health records (EHR) are touted as the technological savior of healthcare—if you invest in the rhetoric coming out of Washington. As our legislators struggle to figure out ways to shoehorn 50 million more Americans into the “insured” category, stave off the growing epidemic of medical errors, and improve the general quality of care, digitizing our health records is a commonly noted panacea. And EHR, it’s promised, somehow will do all of this while conveniently reducing the skyrocketing costs of healthcare.
My hospital is on the verge of siphoning tens of millions of dollars of government stimulus funding and hospital capital into the purchase of a major EHR upgrade. This system aims to seamlessly integrate our inpatient and outpatient billing, documentation, lab, and ordering systems into one neatly packaged, computer-driven solution. I’m left wondering if my iMac experience is an augur of how this will play out on a grand stage.
In the spirit of full disclosure, I fully support automating healthcare as much as possible and sit on numerous committees at my institution charged with doing just that. Further, I believe it will be a salve to many of our efficiency, quality, and patient safety issues. However, I worry that in solving some problems, these new technological cure-alls will simply introduce other, unanticipated problems.
Several years ago, my hospital introduced several systems and technological applications aimed at improving quality, safety, and efficiency. One of these is called “rounds reports.” These very handy, printable documents are a terrific idea and a great example of simplified workflow. The doctor simply prints out a one-page summary of the patient’s 24-hour vital signs, medications, and labs, and uses that as the template for their daily note.
As promised, it’s a time-saver. The problem is that this new technology harbors insidious flaws that prey on the frailties of human nature by introducing new portals for error. For example, the report makes it simple to not reconcile medications daily. The time-honored and time-intensive manner of writing all the meds on a progress note is indeed cumbersome, but it has the added effect of forcing the provider to think about each medication—the utility, the dosage, the effects of the failing kidney on the dosage. Automation of this process removes this small but critical safety check. Sure, diligent providers can overcome this by paying close attention to the printout, but human nature dictates that we don’t always do it. In fact, we employed automation to save this type of time in the first place.
The rounds report also helpfully displays the vital signs and blood sugars for the past 24 hours, reducing the time the harried hospitalist has to spend looking these up and writing them down. However, the report doesn’t print out every vital sign and blood-sugar level; it provides a range. Again, it is possible to access these individual levels, but the post-EHR provider, lured by simplification, often doesn’t take the extra step to go to the separate program to gather these numbers. This shortcut enhances efficiency at the expense of having complete data, a scenario that can breed bad outcomes.
More Efficient Doesn’t Mean Better
Then there is the catch-22 of electronic imaging reports. It is impressive how quickly a chest X-ray gets read and reported electronically in my hospital. The downside, of course, is that today’s techno-doc can rely on the written report without reviewing the actual image. We’ve again, in not reviewing the films personally, removed an important safety check.
The point is that while mechanization offers great potential, it is easy to overlook the downside. Many physicians are not as tech-savvy as their kids and likely will struggle with these newfangled devices. For them, this will not simplify their workflow, but rather it will bog them down. These gizmos also are extremely expensive, and many small clinics and rural hospitals will struggle to afford these upgrades, even with taxpayer support. And let’s not overlook the myriad unforeseen hiccups these new systems will breed.
None of this is to say we shouldn’t embrace our “Jetsons”-like future. In fact, I’d counter that we must, and now is the opportune time. Still, I get nervous when I read stories of the endless EHR potential that omit or gloss over the probable limitations. The key will be to adopt these systems in ways that augment their strengths while mitigating their weaknesses. This must include achieving the delicate balance of usability, efficiency, and safety.
Otherwise, we might find that the technological apple will keep the doctors away. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
Irecently bought an Apple computer. This is newsworthy because I am a lifelong PC user, and because I suffer from a genetic inability to adopt new technologies. In fact, I’m so old-school that only the graceless act of brandishing my credit card kept me from being unceremoniously escorted out of the store by Apple “specialists” for scaring off iPod-yearning tweens. Even then I wasn’t allowed to linger post-purchase.
After racing home, I eagerly slipped the 24 inches of iMac splendor out of its slick, mod-ish packaging and set it up on my desk. Thoughts of newfound cachet danced in my head as I peeled off my black “dad” socks and Tevas. It was clear to me that, as advertised, this sleek beauty was going to transform me from a doddering old Luddite into one of those trendy hipsters so keyed into the pulse of society. And it promised to be easier … and better.
What followed reminded me that the gospel of technology could sometimes preach a false god. It took but a few keystrokes to find myself grappling with a new operating system—it looked like Windows but felt like closed doors. Things that I can do effortlessly on my PC—navigate through files, open Web browsers, access my Outlook account, PowerPoint—seemed to take extra steps, new steps, or unknown steps.
Eventually, I was able figure most of this out, but in the end I was left with the very un-sating realization that the Mac wasn’t really any better than my PC. It was cooler, that’s for sure—even my wife took a renewed interest in me. However, after that cool factor quickly chilled, I was left with the queasy feeling that I had just dropped thousands of dollars on a machine that didn’t really function any better than my old machine. And in some cases, it functioned worse.
Fool’s Gold?
Like my new iMac, electronic health records (EHR) are touted as the technological savior of healthcare—if you invest in the rhetoric coming out of Washington. As our legislators struggle to figure out ways to shoehorn 50 million more Americans into the “insured” category, stave off the growing epidemic of medical errors, and improve the general quality of care, digitizing our health records is a commonly noted panacea. And EHR, it’s promised, somehow will do all of this while conveniently reducing the skyrocketing costs of healthcare.
My hospital is on the verge of siphoning tens of millions of dollars of government stimulus funding and hospital capital into the purchase of a major EHR upgrade. This system aims to seamlessly integrate our inpatient and outpatient billing, documentation, lab, and ordering systems into one neatly packaged, computer-driven solution. I’m left wondering if my iMac experience is an augur of how this will play out on a grand stage.
In the spirit of full disclosure, I fully support automating healthcare as much as possible and sit on numerous committees at my institution charged with doing just that. Further, I believe it will be a salve to many of our efficiency, quality, and patient safety issues. However, I worry that in solving some problems, these new technological cure-alls will simply introduce other, unanticipated problems.
Several years ago, my hospital introduced several systems and technological applications aimed at improving quality, safety, and efficiency. One of these is called “rounds reports.” These very handy, printable documents are a terrific idea and a great example of simplified workflow. The doctor simply prints out a one-page summary of the patient’s 24-hour vital signs, medications, and labs, and uses that as the template for their daily note.
As promised, it’s a time-saver. The problem is that this new technology harbors insidious flaws that prey on the frailties of human nature by introducing new portals for error. For example, the report makes it simple to not reconcile medications daily. The time-honored and time-intensive manner of writing all the meds on a progress note is indeed cumbersome, but it has the added effect of forcing the provider to think about each medication—the utility, the dosage, the effects of the failing kidney on the dosage. Automation of this process removes this small but critical safety check. Sure, diligent providers can overcome this by paying close attention to the printout, but human nature dictates that we don’t always do it. In fact, we employed automation to save this type of time in the first place.
The rounds report also helpfully displays the vital signs and blood sugars for the past 24 hours, reducing the time the harried hospitalist has to spend looking these up and writing them down. However, the report doesn’t print out every vital sign and blood-sugar level; it provides a range. Again, it is possible to access these individual levels, but the post-EHR provider, lured by simplification, often doesn’t take the extra step to go to the separate program to gather these numbers. This shortcut enhances efficiency at the expense of having complete data, a scenario that can breed bad outcomes.
More Efficient Doesn’t Mean Better
Then there is the catch-22 of electronic imaging reports. It is impressive how quickly a chest X-ray gets read and reported electronically in my hospital. The downside, of course, is that today’s techno-doc can rely on the written report without reviewing the actual image. We’ve again, in not reviewing the films personally, removed an important safety check.
The point is that while mechanization offers great potential, it is easy to overlook the downside. Many physicians are not as tech-savvy as their kids and likely will struggle with these newfangled devices. For them, this will not simplify their workflow, but rather it will bog them down. These gizmos also are extremely expensive, and many small clinics and rural hospitals will struggle to afford these upgrades, even with taxpayer support. And let’s not overlook the myriad unforeseen hiccups these new systems will breed.
None of this is to say we shouldn’t embrace our “Jetsons”-like future. In fact, I’d counter that we must, and now is the opportune time. Still, I get nervous when I read stories of the endless EHR potential that omit or gloss over the probable limitations. The key will be to adopt these systems in ways that augment their strengths while mitigating their weaknesses. This must include achieving the delicate balance of usability, efficiency, and safety.
Otherwise, we might find that the technological apple will keep the doctors away. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
What Are They Doing (to Me) in D.C.?
Idon’t know about you, but sometimes I feel as if we are living in an era of information overload when it comes to all of the ideas that are spilling out of Washington and elsewhere under the sobriquet of “healthcare reform.” I thought we should take a few minutes and try to bring all these proposals into focus.
There is an ideal, almost a nirvana, that is being sought. This would include access with a very big “A” to include most, if not all, Americans, and would recognize key differences in patients (comorbidities), and would incentivize value (e.g., quality and cost), and promote evidence-based medicine (EBM) instead of the usual and customary.
That is a far cry from the system that we currently function in. The reality of today is much simpler, at least for the payment part of the system. A hospital or a doctor does something specific, such as a procedure or a visit, and whoever provides the care submits the bill. It is finite and usually involves a small group of individuals or facilities. Because it is simple and to the point, it allows small hospitals and the generally fragmented physician practices to be in the game.
Unfortunately, this system of payment and reward has not led us to the outcomes or performance that our country’s leaders or businesses, and more and more our patients, think we should be getting for our $2.2 trillion. Part of this is due to the fragmentation and variability in how healthcare currently is delivered. More unfortunate is the reality of the disconnect between patients and providers, and the complexity of care. In most healthcare communities, patients can wander through the course of their care to many providers and facilities, most of which have no common information system or business relationship.
One solution that is being considered in Washington is bundling. When policy wonks talk about bundling of payment for an episode of care, they are envisioning a world in which whoever is paying for care (Medicare, insurers, etc.) can pay one fee that would encompass the care provided by all providers, all facilities, and over a broad timeframe (e.g., hospitalization, then 30 days post-discharge). That might work for Mayo in southern Minnesota, Geisinger in northeast Pennsylvania, or Kaiser in Northern California, but just how would it work in the vast majority of places in the U.S.?
If a patient’s care involves two or three separate facilities or a number of providers in the hospital, and it spans as many as 30 days after discharge, how would you assign responsibility for flaws in the patient’s care or the need for additional services? And where is the patient responsibility in all of this?
Knowing all of this, is Congress really ready to write new regulations and pivot 180 degrees on the current system? Glenn Steele, CEO of Geisinger Health System and a recognized leader in a forward-thinking organization, has said, “We probably ought to have a system where we can be innovative, rather than just a new set of rules.”
Baby Steps
In some ways, healthcare reform already is moving forward. The Centers for Medicare and Medicaid Services (CMS) has enacted “never events” in an attempt to improve performance by withholding payment for incidents Medicare thinks just shouldn’t happen (e.g., wrong-side surgery, some hospital-acquired infections).
In addition, 14 communities are ready to perform three-year “comparative effectiveness” trials to attempt to coordinate care among disparate sectors of the healthcare continuum. The research model is looking for ways to deliver optimum care in less-organized sectors of healthcare.
In January, CMS announced site selections for the Acute Care Episode (ACE) demonstration project. ACE is a new, hospital-based demonstration that will test the use of a bundled payment for both hospital and physician services for a select set of inpatient episodes of care. The goal is to improve the quality of care delivered through Medicare fee-for-service. Baptist Health System in San Antonio; Oklahoma Heart Hospital LLC in Oklahoma City; Exempla Saint Joseph Hospital in Denver; Hillcrest Medical Center in Tulsa, Okla.; and Lovelace Health System in Albuquerque, N.M., will participate in the demonstration.
But even when we are just at the beginning of field-testing ideas to improve the delivery of care, all signs point to a major healthcare reform bill coming out of Congress this year. How can they know what will work when the demonstration projects are just starting? How can they anticipate the unintended consequences of wholesale reform? Well, that’s just what Congress can—and does—do. We will all be left to figure out the details on the fly.
The temperature seems to be getting turned up a notch with every healthcare blog and Web posting. When the public and legislators read what Atul Gawande, MD, MPH, wrote in The New Yorker and then it is quoted by the president, suddenly it seems that everyone knows that care is much more expensive in McAllen, Texas, than in nearby El Paso, albeit with worse outcomes. The solution is almost anything that purports to reduce unnecessary variability and ties payment to performance.
Prospective vs. Retrospective Payment
To allow yourself to have a broad context of payment reform, think of “prospective” and “retrospective” payment as two options for a new payment paradigm. Don’t roll your eyes; this stuff is material to how we earn our living.
A prospective system might drastically alter what we have now, by throwing out fee-for-service and no longer paying by the unit of the visit or the procedure, and instead using a global fee (e.g., bundling) that is geared more toward efficiency and effectiveness (i.e., use of resources and better outcomes).
On the other hand, a retrospective system might continue to pay a modified fee-for-service fee (i.e., lower than current reimbursement) with a “significant” bonus for performance (e.g., lower readmission rate, fewer visits to the ED) and improved measureable quality. Of course, this can be “new money” for quality or part of a holdback after upfront fees have been lowered.
In any event, we are probably entering an era in which hospitals and physicians will need to think of themselves as part of a “supply chain” and not just think “I did my job, so pay me.” And during this whole reshuffling of the healthcare deck, there might be calls to remove some of the inequities that have been cobbled onto the Medicare and insurance systems. For example, currently there are significant geographic disparities in Medicare reimbursement (e.g., surgery in Mississippi often is reimbursed at 50% of the same procedure in New York City or Los Angeles). And there are significant payment disparities between medical and surgical specialties and primary-care providers that the Resource-Based Relative Value Scale (RBRVS) system has certainly not corrected.
Integration Hurdles
The small percentage of hospitals, physicians, and patients who currently are in an integrated system probably will have a smoother ride into healthcare’s new future. It is easier for Kaiser or Geisinger or HealthPartners in Minnesota to take on all of the business challenges and risks of accountability for performance and rewards for efficiency. But what about most of the rest of us?
Well, it looks as if we will need to be linked together by contracts and business relationships, by information transfer and management, and we will need strong, forward-thinking, innovative leadership. And we’ll need some trust in each other and our institutions going forward. Equally important, our patients will need to step up and into this new world. If providers and facilities are required to perform better, then patients have to stay in their contracted systems. To have accountability, patients must participate actively.
Some of you might be old enough to remember the last time integration of physicians and hospitals was all the rage. In the 1990s, the driving force was to achieve “economies of scale” and to meet the challenges of managed care with an integrated entity. Most of these attempts were expensive failures.
In 2010, the drive to integration might be the radical reworking of a payment system that is based on a global fee, a system that produces the highest quality at the lowest cost.
One caveat is that significant integration might not be possible. Do hospitals have the expertise and capacity to employ physicians to efficiently deliver care? In this recession, is the capital available to purchase and implement the information systems crucial to integrated care?
Prepared for Change
I profess to have no expertise as a prognosticator, but I do expect some significant changes to come out of Washington in 2009. The common wisdom is that we are currently spending enough in the aggregate to provide all Americans with access to healthcare, and to get better performance and less variability. That seems to mean shaping a new system.
SHM supports changes in payment methodologies that improve the quality and value of healthcare services, align incentives, and promote better clinical outcomes. We believe that healthcare pricing and quality should be transparent to patients and purchasers. We have supported the PQRI, hospital value-based purchasing, and loosening of restrictions on gainsharing between facilities and providers.
Hospitalists are positioned well. We practice in groups and often are aligned with many others in our medical staffs, and with our hospitals’ roles in our communities. We already are thinking about the value equation and trying to balance resources and performance. We are young, adaptable, and less entrenched. And we are new and have less to lose.
I am confident we can be helpful in shaping the future and can thrive in most any new environment. But hold on tight: The future is getting here way ahead of schedule. TH
Dr. Wellikson is CEO of SHM.
Idon’t know about you, but sometimes I feel as if we are living in an era of information overload when it comes to all of the ideas that are spilling out of Washington and elsewhere under the sobriquet of “healthcare reform.” I thought we should take a few minutes and try to bring all these proposals into focus.
There is an ideal, almost a nirvana, that is being sought. This would include access with a very big “A” to include most, if not all, Americans, and would recognize key differences in patients (comorbidities), and would incentivize value (e.g., quality and cost), and promote evidence-based medicine (EBM) instead of the usual and customary.
That is a far cry from the system that we currently function in. The reality of today is much simpler, at least for the payment part of the system. A hospital or a doctor does something specific, such as a procedure or a visit, and whoever provides the care submits the bill. It is finite and usually involves a small group of individuals or facilities. Because it is simple and to the point, it allows small hospitals and the generally fragmented physician practices to be in the game.
Unfortunately, this system of payment and reward has not led us to the outcomes or performance that our country’s leaders or businesses, and more and more our patients, think we should be getting for our $2.2 trillion. Part of this is due to the fragmentation and variability in how healthcare currently is delivered. More unfortunate is the reality of the disconnect between patients and providers, and the complexity of care. In most healthcare communities, patients can wander through the course of their care to many providers and facilities, most of which have no common information system or business relationship.
One solution that is being considered in Washington is bundling. When policy wonks talk about bundling of payment for an episode of care, they are envisioning a world in which whoever is paying for care (Medicare, insurers, etc.) can pay one fee that would encompass the care provided by all providers, all facilities, and over a broad timeframe (e.g., hospitalization, then 30 days post-discharge). That might work for Mayo in southern Minnesota, Geisinger in northeast Pennsylvania, or Kaiser in Northern California, but just how would it work in the vast majority of places in the U.S.?
If a patient’s care involves two or three separate facilities or a number of providers in the hospital, and it spans as many as 30 days after discharge, how would you assign responsibility for flaws in the patient’s care or the need for additional services? And where is the patient responsibility in all of this?
Knowing all of this, is Congress really ready to write new regulations and pivot 180 degrees on the current system? Glenn Steele, CEO of Geisinger Health System and a recognized leader in a forward-thinking organization, has said, “We probably ought to have a system where we can be innovative, rather than just a new set of rules.”
Baby Steps
In some ways, healthcare reform already is moving forward. The Centers for Medicare and Medicaid Services (CMS) has enacted “never events” in an attempt to improve performance by withholding payment for incidents Medicare thinks just shouldn’t happen (e.g., wrong-side surgery, some hospital-acquired infections).
In addition, 14 communities are ready to perform three-year “comparative effectiveness” trials to attempt to coordinate care among disparate sectors of the healthcare continuum. The research model is looking for ways to deliver optimum care in less-organized sectors of healthcare.
In January, CMS announced site selections for the Acute Care Episode (ACE) demonstration project. ACE is a new, hospital-based demonstration that will test the use of a bundled payment for both hospital and physician services for a select set of inpatient episodes of care. The goal is to improve the quality of care delivered through Medicare fee-for-service. Baptist Health System in San Antonio; Oklahoma Heart Hospital LLC in Oklahoma City; Exempla Saint Joseph Hospital in Denver; Hillcrest Medical Center in Tulsa, Okla.; and Lovelace Health System in Albuquerque, N.M., will participate in the demonstration.
But even when we are just at the beginning of field-testing ideas to improve the delivery of care, all signs point to a major healthcare reform bill coming out of Congress this year. How can they know what will work when the demonstration projects are just starting? How can they anticipate the unintended consequences of wholesale reform? Well, that’s just what Congress can—and does—do. We will all be left to figure out the details on the fly.
The temperature seems to be getting turned up a notch with every healthcare blog and Web posting. When the public and legislators read what Atul Gawande, MD, MPH, wrote in The New Yorker and then it is quoted by the president, suddenly it seems that everyone knows that care is much more expensive in McAllen, Texas, than in nearby El Paso, albeit with worse outcomes. The solution is almost anything that purports to reduce unnecessary variability and ties payment to performance.
Prospective vs. Retrospective Payment
To allow yourself to have a broad context of payment reform, think of “prospective” and “retrospective” payment as two options for a new payment paradigm. Don’t roll your eyes; this stuff is material to how we earn our living.
A prospective system might drastically alter what we have now, by throwing out fee-for-service and no longer paying by the unit of the visit or the procedure, and instead using a global fee (e.g., bundling) that is geared more toward efficiency and effectiveness (i.e., use of resources and better outcomes).
On the other hand, a retrospective system might continue to pay a modified fee-for-service fee (i.e., lower than current reimbursement) with a “significant” bonus for performance (e.g., lower readmission rate, fewer visits to the ED) and improved measureable quality. Of course, this can be “new money” for quality or part of a holdback after upfront fees have been lowered.
In any event, we are probably entering an era in which hospitals and physicians will need to think of themselves as part of a “supply chain” and not just think “I did my job, so pay me.” And during this whole reshuffling of the healthcare deck, there might be calls to remove some of the inequities that have been cobbled onto the Medicare and insurance systems. For example, currently there are significant geographic disparities in Medicare reimbursement (e.g., surgery in Mississippi often is reimbursed at 50% of the same procedure in New York City or Los Angeles). And there are significant payment disparities between medical and surgical specialties and primary-care providers that the Resource-Based Relative Value Scale (RBRVS) system has certainly not corrected.
Integration Hurdles
The small percentage of hospitals, physicians, and patients who currently are in an integrated system probably will have a smoother ride into healthcare’s new future. It is easier for Kaiser or Geisinger or HealthPartners in Minnesota to take on all of the business challenges and risks of accountability for performance and rewards for efficiency. But what about most of the rest of us?
Well, it looks as if we will need to be linked together by contracts and business relationships, by information transfer and management, and we will need strong, forward-thinking, innovative leadership. And we’ll need some trust in each other and our institutions going forward. Equally important, our patients will need to step up and into this new world. If providers and facilities are required to perform better, then patients have to stay in their contracted systems. To have accountability, patients must participate actively.
Some of you might be old enough to remember the last time integration of physicians and hospitals was all the rage. In the 1990s, the driving force was to achieve “economies of scale” and to meet the challenges of managed care with an integrated entity. Most of these attempts were expensive failures.
In 2010, the drive to integration might be the radical reworking of a payment system that is based on a global fee, a system that produces the highest quality at the lowest cost.
One caveat is that significant integration might not be possible. Do hospitals have the expertise and capacity to employ physicians to efficiently deliver care? In this recession, is the capital available to purchase and implement the information systems crucial to integrated care?
Prepared for Change
I profess to have no expertise as a prognosticator, but I do expect some significant changes to come out of Washington in 2009. The common wisdom is that we are currently spending enough in the aggregate to provide all Americans with access to healthcare, and to get better performance and less variability. That seems to mean shaping a new system.
SHM supports changes in payment methodologies that improve the quality and value of healthcare services, align incentives, and promote better clinical outcomes. We believe that healthcare pricing and quality should be transparent to patients and purchasers. We have supported the PQRI, hospital value-based purchasing, and loosening of restrictions on gainsharing between facilities and providers.
Hospitalists are positioned well. We practice in groups and often are aligned with many others in our medical staffs, and with our hospitals’ roles in our communities. We already are thinking about the value equation and trying to balance resources and performance. We are young, adaptable, and less entrenched. And we are new and have less to lose.
I am confident we can be helpful in shaping the future and can thrive in most any new environment. But hold on tight: The future is getting here way ahead of schedule. TH
Dr. Wellikson is CEO of SHM.
Idon’t know about you, but sometimes I feel as if we are living in an era of information overload when it comes to all of the ideas that are spilling out of Washington and elsewhere under the sobriquet of “healthcare reform.” I thought we should take a few minutes and try to bring all these proposals into focus.
There is an ideal, almost a nirvana, that is being sought. This would include access with a very big “A” to include most, if not all, Americans, and would recognize key differences in patients (comorbidities), and would incentivize value (e.g., quality and cost), and promote evidence-based medicine (EBM) instead of the usual and customary.
That is a far cry from the system that we currently function in. The reality of today is much simpler, at least for the payment part of the system. A hospital or a doctor does something specific, such as a procedure or a visit, and whoever provides the care submits the bill. It is finite and usually involves a small group of individuals or facilities. Because it is simple and to the point, it allows small hospitals and the generally fragmented physician practices to be in the game.
Unfortunately, this system of payment and reward has not led us to the outcomes or performance that our country’s leaders or businesses, and more and more our patients, think we should be getting for our $2.2 trillion. Part of this is due to the fragmentation and variability in how healthcare currently is delivered. More unfortunate is the reality of the disconnect between patients and providers, and the complexity of care. In most healthcare communities, patients can wander through the course of their care to many providers and facilities, most of which have no common information system or business relationship.
One solution that is being considered in Washington is bundling. When policy wonks talk about bundling of payment for an episode of care, they are envisioning a world in which whoever is paying for care (Medicare, insurers, etc.) can pay one fee that would encompass the care provided by all providers, all facilities, and over a broad timeframe (e.g., hospitalization, then 30 days post-discharge). That might work for Mayo in southern Minnesota, Geisinger in northeast Pennsylvania, or Kaiser in Northern California, but just how would it work in the vast majority of places in the U.S.?
If a patient’s care involves two or three separate facilities or a number of providers in the hospital, and it spans as many as 30 days after discharge, how would you assign responsibility for flaws in the patient’s care or the need for additional services? And where is the patient responsibility in all of this?
Knowing all of this, is Congress really ready to write new regulations and pivot 180 degrees on the current system? Glenn Steele, CEO of Geisinger Health System and a recognized leader in a forward-thinking organization, has said, “We probably ought to have a system where we can be innovative, rather than just a new set of rules.”
Baby Steps
In some ways, healthcare reform already is moving forward. The Centers for Medicare and Medicaid Services (CMS) has enacted “never events” in an attempt to improve performance by withholding payment for incidents Medicare thinks just shouldn’t happen (e.g., wrong-side surgery, some hospital-acquired infections).
In addition, 14 communities are ready to perform three-year “comparative effectiveness” trials to attempt to coordinate care among disparate sectors of the healthcare continuum. The research model is looking for ways to deliver optimum care in less-organized sectors of healthcare.
In January, CMS announced site selections for the Acute Care Episode (ACE) demonstration project. ACE is a new, hospital-based demonstration that will test the use of a bundled payment for both hospital and physician services for a select set of inpatient episodes of care. The goal is to improve the quality of care delivered through Medicare fee-for-service. Baptist Health System in San Antonio; Oklahoma Heart Hospital LLC in Oklahoma City; Exempla Saint Joseph Hospital in Denver; Hillcrest Medical Center in Tulsa, Okla.; and Lovelace Health System in Albuquerque, N.M., will participate in the demonstration.
But even when we are just at the beginning of field-testing ideas to improve the delivery of care, all signs point to a major healthcare reform bill coming out of Congress this year. How can they know what will work when the demonstration projects are just starting? How can they anticipate the unintended consequences of wholesale reform? Well, that’s just what Congress can—and does—do. We will all be left to figure out the details on the fly.
The temperature seems to be getting turned up a notch with every healthcare blog and Web posting. When the public and legislators read what Atul Gawande, MD, MPH, wrote in The New Yorker and then it is quoted by the president, suddenly it seems that everyone knows that care is much more expensive in McAllen, Texas, than in nearby El Paso, albeit with worse outcomes. The solution is almost anything that purports to reduce unnecessary variability and ties payment to performance.
Prospective vs. Retrospective Payment
To allow yourself to have a broad context of payment reform, think of “prospective” and “retrospective” payment as two options for a new payment paradigm. Don’t roll your eyes; this stuff is material to how we earn our living.
A prospective system might drastically alter what we have now, by throwing out fee-for-service and no longer paying by the unit of the visit or the procedure, and instead using a global fee (e.g., bundling) that is geared more toward efficiency and effectiveness (i.e., use of resources and better outcomes).
On the other hand, a retrospective system might continue to pay a modified fee-for-service fee (i.e., lower than current reimbursement) with a “significant” bonus for performance (e.g., lower readmission rate, fewer visits to the ED) and improved measureable quality. Of course, this can be “new money” for quality or part of a holdback after upfront fees have been lowered.
In any event, we are probably entering an era in which hospitals and physicians will need to think of themselves as part of a “supply chain” and not just think “I did my job, so pay me.” And during this whole reshuffling of the healthcare deck, there might be calls to remove some of the inequities that have been cobbled onto the Medicare and insurance systems. For example, currently there are significant geographic disparities in Medicare reimbursement (e.g., surgery in Mississippi often is reimbursed at 50% of the same procedure in New York City or Los Angeles). And there are significant payment disparities between medical and surgical specialties and primary-care providers that the Resource-Based Relative Value Scale (RBRVS) system has certainly not corrected.
Integration Hurdles
The small percentage of hospitals, physicians, and patients who currently are in an integrated system probably will have a smoother ride into healthcare’s new future. It is easier for Kaiser or Geisinger or HealthPartners in Minnesota to take on all of the business challenges and risks of accountability for performance and rewards for efficiency. But what about most of the rest of us?
Well, it looks as if we will need to be linked together by contracts and business relationships, by information transfer and management, and we will need strong, forward-thinking, innovative leadership. And we’ll need some trust in each other and our institutions going forward. Equally important, our patients will need to step up and into this new world. If providers and facilities are required to perform better, then patients have to stay in their contracted systems. To have accountability, patients must participate actively.
Some of you might be old enough to remember the last time integration of physicians and hospitals was all the rage. In the 1990s, the driving force was to achieve “economies of scale” and to meet the challenges of managed care with an integrated entity. Most of these attempts were expensive failures.
In 2010, the drive to integration might be the radical reworking of a payment system that is based on a global fee, a system that produces the highest quality at the lowest cost.
One caveat is that significant integration might not be possible. Do hospitals have the expertise and capacity to employ physicians to efficiently deliver care? In this recession, is the capital available to purchase and implement the information systems crucial to integrated care?
Prepared for Change
I profess to have no expertise as a prognosticator, but I do expect some significant changes to come out of Washington in 2009. The common wisdom is that we are currently spending enough in the aggregate to provide all Americans with access to healthcare, and to get better performance and less variability. That seems to mean shaping a new system.
SHM supports changes in payment methodologies that improve the quality and value of healthcare services, align incentives, and promote better clinical outcomes. We believe that healthcare pricing and quality should be transparent to patients and purchasers. We have supported the PQRI, hospital value-based purchasing, and loosening of restrictions on gainsharing between facilities and providers.
Hospitalists are positioned well. We practice in groups and often are aligned with many others in our medical staffs, and with our hospitals’ roles in our communities. We already are thinking about the value equation and trying to balance resources and performance. We are young, adaptable, and less entrenched. And we are new and have less to lose.
I am confident we can be helpful in shaping the future and can thrive in most any new environment. But hold on tight: The future is getting here way ahead of schedule. TH
Dr. Wellikson is CEO of SHM.