User login
Delays in Pediatric Discharge
Inpatient pediatrics is undergoing a paradigm shift in at least 3 ways. First, more children with chronic disease are being cared for in the hospital over time.1 Second, previous inpatient conditions are treated at home with advancing technology such as peripherally‐inserted catheters.2 Third, there are new areas of growing specialization, such as hospital medicine, in which the practitioners deliver more efficient care.3, 4
Nationwide, there is increasing pressure to improve inpatient quality of care. The Institute of Medicine defines 6 aims for improvement, including timeliness (reducing waits and sometimes harmful delays for both those who receive and those who give care) and efficiency of care (avoiding waste, including waste of equipment, supplies, ideas, and energy).5 Reducing unnecessary stays in the hospital is a potential quality measure that hospitals may use to address the timeliness and efficiency of care delivered to hospitalized children.
Delays in discharge have been used as markers of unnecessary stays in the hospital for inpatient adult and pediatric care,6, 7 but these are limited to inpatient systems from almost 20 years ago. Current reasons why patients are delayed from discharge, if at all, are not well described. We undertook this study to describe delays in hospital discharges at a tertiary‐care children's hospital in terms of number of patients, length of days of delay, and type of delay. In addition, we sought to characterize the impact of discharge delays on overall length of stay (LOS) and costs.
Methods
Patient Population/Study Design
All children cared for on 2 pediatric medical teams at Primary Children's Medical Center during the month of August 2004 were eligible for the study. Two research assistants independently attended team rounds and collected data relating to: the reasons for ongoing hospitalization, pending items (eg, consultations, tests), and the plan of care for that day. The research assistants each attended daily team rounds for the entire month of August (1 for each team, switching to the opposite team after 2 weeks). This was combined with information available in the Patient Tracker, a software tool developed to improve communication between caregivers and improve discharge efficiency.8 This software tool details diagnoses, daily medical care plans, discharge criteria, and ongoing medical interventions while tracking daily changes in interventions and the medical care plan for each patient cared for on a pediatric medical team.
The research assistants subsequently presented their observations along with information from Patient Tracker to 2 experienced physicians (R.S. and B.S.) who independently determined if a delay occurred, the number of delay days extending discharge, and the cause of the delay, if present, categorized according to the taxonomy of the Delay Tool.6, 7 If there was not enough information for either of the physicians to identify and classify a delay, the electronic medical record of the patient was also reviewed. Discrepancies between physicians assigning delays were discussed until consensus was reached.
The study was approved by the Institutional Review Board of the University of Utah Health Sciences Center and Primary Children's Medical Center (PCMC).
Setting
PCMC is a 233‐bed tertiary‐care children's hospital, owned and operated by Intermountain Healthcare (a not‐for‐profit vertically integrated managed care organization) in the Intermountain West, which serves as both the primary hospital for Salt Lake County and as a tertiary‐care children's hospital for 5 states (UT, MT, WY, ID, and NV).9
Study Definitions
Delay and Length of Delay
Delays in discharge were measured using a validated and reliable instrument, the Delay Tool.6, 7 A discharge was classified as delayed if there was no medical reason for the patient to be in the hospital on a given day, identical to the definition used in the original studies to validate the tool. Delays were recorded as whole days, not fractions of days or hours, as described in the original validation of the tool. For example, if the medical team requested a consultation, and the consultant's opinion was rendered late, but the patient would have remained in the hospital anyway, then this period of time would not count as a delay. However, if the medical team did not receive a consultant's opinion within the standard time (24 hours as defined for this study and in validating studies for the Delay Tool), and the patient's sole reason for being in the hospital during that day was waiting for that opinion, then that period of time would count toward a delay due to a late consultative opinion. Delays of less than 1 day, due to the mechanics of discharging a patient from the hospital (providing prescriptions, follow up, communication, arranging home health, and transportation) were not measured in this study, to match the original methodology of the Delay Tool.
Type of Delay
Primary reason for delay was assigned according to the taxonomy of the Delay Tool.6, 7 Delays were categorized to 1 of the following: (1) test scheduling; (2) obtaining test results; (3) surgery; (4) consultation; (5) patient (eg, family unavailable for decision‐making); (6) physician responsibility; (7) education, training. or research; (8) discharge planning or scheduling; and (9) availability of outside care and resources. There are 166 subcategories that clarify why a delay occurred. For example, within the main category of obtaining test results (2), there are 3 subcategories of delays related to problem in executing the test (2.1), return of results is delayed (2.2), and test results not reviewed within standard time of return (2.3). Subcategories are further divided to provide detail on the cause of delay. For example, a delay categorized as a 2.1:1 [(2) obtaining test results; (2.1) problem in executing the test; and 2.1:1 test to be done by MD is delayed beyond day desired], or 2.3:1 [(2) obtaining test results; (2.3) test results not reviewed within standard time of return; and 2.3:1 delay because physician did not review results]) both relate to physician causes of delays within the general category of obtaining test results. Some delays had more than 1 cause. A secondary cause of delay was assigned if applicable; however, the number of days delayed was attributed to the primary cause for analysis purposes.
Exemptions to Delay and Special Populations
Certain subpopulations of patients presented unique issues that led to them being unlikely to be classified as having a delay. For example, patients with a diagnosis of new onset of type 1 diabetes are historically admitted for 3 days at our hospital, which includes a specific education program; delays were not considered until this minimum period had passed. Children with medically complex care (eg, multisystem disease, multiple specialists involved, multiple medications) were included in this study.10 However, these children with frequent hospital admission were often fragile at discharge, and could meet criteria for readmission even on the date of their discharge, hence assigning a delay day was usually not indicated because of easily justified ongoing medical need for hospitalization.
Study Variables
The LOS, total costs, and routine demographic and administrative data for each study patient were extracted from Intermountain Healthcare's Enterprise Data Warehouse (EDW). The EDW contains detailed data about the cost of providing health care. Costs were derived from the hospital's cost accounting program, the Standard Cost Master, which is a transaction‐based microcosting accounting system.1113
For patients whose LOS extended before August 1 or after August 31, total hospital costs were averaged per day, and only days falling inside the month of August were counted in calculating the impact the delays in discharge had on the total costs of hospitalization. Hospital days that extended outside of August were not counted in either the numerator for potential days of delay or in the denominator for total days in the hospital.
Analyses
Descriptive statistics were calculated for the number, length of days of delay, and type of delay. Interrater reliability to assign a delay was ascertained for the 2 physicians. Mean LOS, mean total costs, and standard deviations (SDs) were calculated. All analyses were performed using Statistical Analytical Software version 9.13 (SAS Institute, Cary, NC).
Results
During the 31 days of the study, 171 patients occupied hospital beds an average of 7.3 days on the 2 inpatient medical teams, for a total of 911 inpatient days. Seven patients were admitted prior to August 1; 6 of these were discharged during the month of August and 1 stayed through the entire month and was discharged in September. Three additional patients were admitted in August and discharged in September. There were 6 readmissions during the month of August, and 1 patient was excluded from the study because of lack of sufficient information. All patients with delays were able to be classified according to the Delay Tool taxonomy. Interrater reliability for the 2 study physicians was 98%.
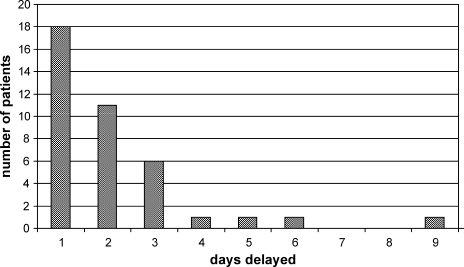
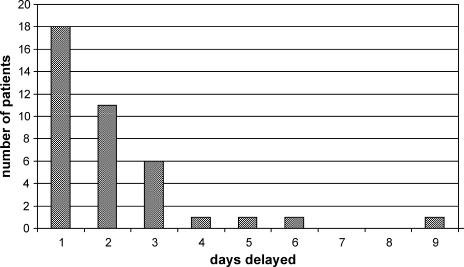
The characteristics between the patients who did and did not experience a delay in discharge are shown in Table 1. Thirty‐nine of 171 patients (22.8%), experienced at least 1 delay day. Eighteen of 39 patients had only 1 delay day (46.2%) and 11 patients experienced 2 delayed days (28.2%) (Figure 1). The average length of delay was 2.1 days.
| Nondelayed Patients (N = 132) | Delayed Patients (N = 39) | P Value | |
|---|---|---|---|
| |||
| Age (months), mean (SD)* | 22.6 (14.4) | 15.0 (14.6) | 0.009 |
| LOS during August (days), mean (SD)* | 4.64 (6.1) | 7.64 (7.15) | <0.001 |
| Total costs during August ($), mean (SD)* | 10,451 (19,254) | 14,341 (16,241) | 0.002 |
| Number of ICD‐9 CM diagnoses codes, mean (SD)* | 7.1 (7.4) | 8.5 (7.3) | 0.056 |
| Number of ICD‐9 CM procedure codes, mean (SD)* | 1.7 (3.8) | 1.6 (2.6) | 0.068 |
| Number of Patients with APR‐DRG SOI 3 (%) | 59 (44.7%) | 19 (48.7%) | 0.65 |
Delays attributed to physician responsibility accounted for 42.3% (16.5/39) of patient delays (conservative management or clinical decision‐making), with discharge planning delays accounting for 21.8% (family‐related, patient‐related, and hospital‐related problems), consultation for 14.1% (delay in obtaining or lack of follow‐up), test scheduling for 12.8%, and obtaining test results for 5.1% (ordering and weekend scheduling). There were no primary delays due to surgery, education and research, or unavailability of outside resources such as a skilled nursing bed. Four patients had a single additional secondary cause of delay assigned to them, related to physician responsibility, consultation, surgery and test scheduling; these were split, attributing 0.5 patients to each delay type (thus, the 17/39 patients delayed for physician responsibility was analyzed as 16.5/39) (Table 2).
| Delay Category | Number of Patients Experiencing Delays* | Percentage of All Patients Experiencing Delays (%) | Percentage of Study Patients Observed (%) | Total Delay Days | Average Length of Delays (days) | Percentage of Hospital Days That Were Delay Days (%) |
|---|---|---|---|---|---|---|
| ||||||
| 1. Scheduling | 5 | 12.8 | 2.92 | 16 | 3.20 | 1.76 |
| 2. Obtaining results | 2 | 5.1 | 1.17 | 3 | 1.50 | 0.33 |
| 3. Surgery | 0.5 | 1.3 | 0.29 | 1.5 | 3.00 | 0.16 |
| 4. Consultation | 5.5 | 14.1 | 3.22 | 10.5 | 1.91 | 1.15 |
| 5. Patient | 1 | 2.6 | 0.58 | 2 | 2.00 | 0.22 |
| 6. Physician | 16.5 | 42.3 | 9.65 | 33.5 | 2.03 | 3.68 |
| 7. Education | 0 | 0 | 0.00 | 0 | 0 | 0.00 |
| 8. Discharge | 8.5 | 21.8 | 4.97 | 15.5 | 1.82 | 1.70 |
| 9. Outside | 0 | 0 | 0.00 | 0 | 0 | 0.00 |
| Total | 39 | 100 | 22.81 | 82 | 2.10 | 9.00 |
There were 82 delay‐related hospital days of 911 total inpatient days on the 2 medical teams for August 2004 (9%). More than $170,000 in excess costs was incurred due to delay days from a total of approximately 1.9 million dollars in patient costs for the month (8.9%).
Discussion
This study finds that discharge delays in a tertiary care children's hospital are common; almost 1 in 4 patients experienced a medically unnecessary excess hospital stay of at least 1 day. The average length of a delay was 2.1 days, and overall, delays consumed 9% of pediatric hospital days and 8.9% of total costs. The most common reason for a delay was related to physician clinical care, including excessively conservative management and variability in clinical decision‐making.
Our study results are similar to the other 2 published studies that use the Delay Tool. In the adult and pediatric studies, between 10% and 30% of patients experienced a delay in discharge, with the average length of delay between 2.9 and 3 days.6, 7 Although both studies were conducted at teaching hospitals, what is particularly interesting is that they were conducted almost 20 years ago. During this period of time, there has been a shift in the inpatient pediatric patient population. In recent years, children who are cared for in the hospital have more chronic illnesses.1 In addition, there has been a shift in the types of conditions that may be cared for at home and those that now require inpatient stay.2 Despite this, delays continue at a similar proportion, but the cause of delays have shifted from scheduling and consultation to physician responsibility.
There is another tool in the literature which is more widely used, the Pediatric Appropriateness Evaluation Protocol (PAEP), which is based on the Appropriateness Evaluation Protocol for adults.1417 This tool is used to determine the appropriateness of ongoing hospitalization, not the cause of delay if ongoing hospitalization is inappropriate. The 3 areas that are evaluated (medical services, nursing and ancillary services, and patient's condition) have objective criteria that dictate if the hospitalization is appropriate or not (eg, parenteral (intravenous) therapy for at least 8 hours on that day, under nursing and ancillary services). The PAEP may be less sensitive given today's healthcare resource utilization climate. Many clinicians and families would agree that insertion of a peripheral central catheter is an acceptable form of outpatient treatment for many pediatric conditions. In conjunction with the Delay Tool, the PAEP could be used to determine if a delay occurred, then the Delay Tool used to categorize the cause of the delay. We choose to use expert clinician judgment to determine if a delay had occurred. We were more interested in why patients who are admitted (appropriately or inappropriately) cannot be discharged sooner, thus allowing for future intervention studies targeted to impact delays in discharge, as elucidated in this study. The Delay Tool specifically allowed us to categorize the reasons for delays. Given that the average LOS for patients in the nondelayed group was over 4 days, despite not using a tool such as the PAEP, we believe that these were likely to be appropriate admissions.
A recent study reported the first use of the Medical Care Appropriateness Protocol (MCAP) in a tertiary‐care children's hospital. The authors used the MCAP to determine the impact of an intervention on reducing inappropriate hospitals days for children. This tool is similarly labor‐intensive to the Delay Tool. Interestingly, this Canadian study found a high rate of inappropriate hospital days (47%), which may be in part attributable to a different outcome measurement tool and/or a different health care system.18
There are several limitations to our study that deserve mention. The Delay Tool requires clinician judgment regarding whether or not there was a delay in discharge for that day. We may have introduced some bias in our study, as hospitalist investigators assigned the delay and blinding to the attending physician specialty of record was not feasible. However, our results are similar to the other 2 published studies that have used this tool, and we specifically chose not to analyze or report results in terms of hospitalist and nonhospitalist attending physicians. The Delay Tool is not designed to differentiate shorter delays in terms of hours instead of days (eg, due to the inability for the patient to get a ride home). Shorter delays may be of particular importance depending on the occupancy rate of the hospital, the demand for beds, and other patient and hospital factors. We could not capture these shorter delays (although they did occur frequently) due to the original description of the Delay Tool. In addition, we would not have been able to report data on the impact on LOS and costs, as these are attributed to whole days in the hospital. However, if we had been able to differentiate shorter delays, this would bias our results to show a greater percentage of delays over smaller increments of time. Generalizability is an issue, given that this was a single‐center study. This study sample included over 80 different attending physicians participating in community pediatrician, subspecialty, and hospitalist practice groups. However, the patient population at PCMC is similar to other medium and large children's hospitals in the United States. The month observed may not reflect the entire year of hospitalizationsthere may be seasonal variations with delays depending on the volume and type of illness seen. The study was conducted in August, when there are newer house staff present. However, physician responsibility, which was the largest source of delays in our study, had little attribution to house staff. Most of the decisions were those of attending physicians, which would largely be unaffected by the time of year of the study. Finally, we were unable to assess the safety of the potential earlier discharge, as this was an observational study. However, in any future intervention studies examining processes to discharge patients sooner, measures of safety to the patient are a necessity. Finally, given the potential of ongoing admission, even on the date of discharge of our most fragile patients, this approach to discovering causes of delay may not apply to this important group, which is responsible for significant and growing resource utilization.
Despite these limitations, our findings demonstrate that in an era of children staying in the hospital less, and more medically‐complex children being admitted,10 a substantial number of children who are hospitalized at a children's hospital may have been discharged sooner. The majority of these decisions were directly related to physician responsibility. As consumers, providers, and hospitals work together to develop quality measures that are reflective of inpatient pediatric care, the Delay Tool may be able to highlight 2 aims of quality (ie, timeliness and efficiency of care) that could be used to assess the impact of interventions designed to safely discharge patients sooner. Interventions such as audit‐feedback,18 clinical guideline deployment,19 and hospitalist systems of care4 continue to hold the promise of earlier discharge; however, tools designed to measure inappropriate use of hospital days should be employed to demonstrate their effectiveness. Our study demonstrates ongoing waste in children's hospitals.
Conclusions
Almost 1 out of 4 patients in this 1‐month period could have been discharged sooner than they were. The impact of delays on costs and LOS are substantial and should provide strong incentives to develop effective interventions. Such interventions will need to address variations in physician criteria for discharge, more efficient discharge planning, and timely scheduling of consultation and diagnostic testing.
Acknowledgements
The authors thank Joni R. Beshanky and Harry Selker for their help and training in the use of the Delay Tool.
- .The transformation of child health in the United States: social disparities in child health persistent despite dramatic improvement in child health overall.Health Aff (Millwood).2004;23(5):9–25.
- , , , .Hospitalist care of medically complex children.Pediatr Res.2004;55(4):314A–315A.
- , , , .Pediatric hospitalists: a systematic review of the literature.Pediatrics.2006;117(5):1736–1744.
- , , , et al.Pediatric hospitalists: report of a leadership conference.Pediatrics.2006;117(4):1122–1130.
- Institute of Medicine.Crossing the Quality Chasm: A New Health System for the Twenty‐first Century.Washington, DC:National Academy Press;2001.
- , , .Using the Delay Tool to attribute causes for unnecessary pediatric hospital days.Med Care.1990;28(10):982–989.
- , , , .The epidemiology of delays in a teaching hospital. The development and use of a tool that detects unnecessary hospital days.Med Care.1989;27(2):112–129.
- , , , , .A tool for improving patient discharge process and hospital communication practices: the Patient Tracker.AMIA Annu Symp Proc.2007;11:493–497.
- , .Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101(4 Pt 2):805–811; discussion 811–802.
- , , .Hospitalist care of the medically complex child.Pediatr Clin North Am.2005;52(4):1165–1187.
- , , , et al.Epidemiology, complications, and cost of hospitalization in children with laboratory‐confirmed influenza infection.Pediatrics.2006;118(6):2409–2417.
- , , , , , .Clinical and economic outcomes of conventional amphotericin B‐associated nephrotoxicity.Clin Infect Dis.2002;35(12):e120–e127.
- , , , et al.Using a hospital information system to assess the effects of adverse drug events.Proc Annu Symp Comput Appl Med Care.1993;1993:161–165.
- , , .Appropriateness of hospitalization in a Canadian pediatric hospital.Pediatrics.1993;91(1):70–74.
- , , , .The appropriateness evaluation protocol: application in an Australian children's hospital.Aust Clin Rev.1991;11(4):123–131.
- , .Assessing the need to hospitalize children: pediatric appropriateness evaluation protocol.Pediatrics.1989;84(2):242–247.
- .Medically inappropriate hospital use in a pediatric population.N Engl J Med.1988;318(16):1033–1037.
- , , , , .Reducing inappropriate hospital use on a general pediatric inpatient unit.Pediatrics.2008;121(5):e1068–e1073.
- , .Reliable implementation of clinical pathways: what will it take—that is the question.J Pediatr.2008;152(3):303–304.
Inpatient pediatrics is undergoing a paradigm shift in at least 3 ways. First, more children with chronic disease are being cared for in the hospital over time.1 Second, previous inpatient conditions are treated at home with advancing technology such as peripherally‐inserted catheters.2 Third, there are new areas of growing specialization, such as hospital medicine, in which the practitioners deliver more efficient care.3, 4
Nationwide, there is increasing pressure to improve inpatient quality of care. The Institute of Medicine defines 6 aims for improvement, including timeliness (reducing waits and sometimes harmful delays for both those who receive and those who give care) and efficiency of care (avoiding waste, including waste of equipment, supplies, ideas, and energy).5 Reducing unnecessary stays in the hospital is a potential quality measure that hospitals may use to address the timeliness and efficiency of care delivered to hospitalized children.
Delays in discharge have been used as markers of unnecessary stays in the hospital for inpatient adult and pediatric care,6, 7 but these are limited to inpatient systems from almost 20 years ago. Current reasons why patients are delayed from discharge, if at all, are not well described. We undertook this study to describe delays in hospital discharges at a tertiary‐care children's hospital in terms of number of patients, length of days of delay, and type of delay. In addition, we sought to characterize the impact of discharge delays on overall length of stay (LOS) and costs.
Methods
Patient Population/Study Design
All children cared for on 2 pediatric medical teams at Primary Children's Medical Center during the month of August 2004 were eligible for the study. Two research assistants independently attended team rounds and collected data relating to: the reasons for ongoing hospitalization, pending items (eg, consultations, tests), and the plan of care for that day. The research assistants each attended daily team rounds for the entire month of August (1 for each team, switching to the opposite team after 2 weeks). This was combined with information available in the Patient Tracker, a software tool developed to improve communication between caregivers and improve discharge efficiency.8 This software tool details diagnoses, daily medical care plans, discharge criteria, and ongoing medical interventions while tracking daily changes in interventions and the medical care plan for each patient cared for on a pediatric medical team.
The research assistants subsequently presented their observations along with information from Patient Tracker to 2 experienced physicians (R.S. and B.S.) who independently determined if a delay occurred, the number of delay days extending discharge, and the cause of the delay, if present, categorized according to the taxonomy of the Delay Tool.6, 7 If there was not enough information for either of the physicians to identify and classify a delay, the electronic medical record of the patient was also reviewed. Discrepancies between physicians assigning delays were discussed until consensus was reached.
The study was approved by the Institutional Review Board of the University of Utah Health Sciences Center and Primary Children's Medical Center (PCMC).
Setting
PCMC is a 233‐bed tertiary‐care children's hospital, owned and operated by Intermountain Healthcare (a not‐for‐profit vertically integrated managed care organization) in the Intermountain West, which serves as both the primary hospital for Salt Lake County and as a tertiary‐care children's hospital for 5 states (UT, MT, WY, ID, and NV).9
Study Definitions
Delay and Length of Delay
Delays in discharge were measured using a validated and reliable instrument, the Delay Tool.6, 7 A discharge was classified as delayed if there was no medical reason for the patient to be in the hospital on a given day, identical to the definition used in the original studies to validate the tool. Delays were recorded as whole days, not fractions of days or hours, as described in the original validation of the tool. For example, if the medical team requested a consultation, and the consultant's opinion was rendered late, but the patient would have remained in the hospital anyway, then this period of time would not count as a delay. However, if the medical team did not receive a consultant's opinion within the standard time (24 hours as defined for this study and in validating studies for the Delay Tool), and the patient's sole reason for being in the hospital during that day was waiting for that opinion, then that period of time would count toward a delay due to a late consultative opinion. Delays of less than 1 day, due to the mechanics of discharging a patient from the hospital (providing prescriptions, follow up, communication, arranging home health, and transportation) were not measured in this study, to match the original methodology of the Delay Tool.
Type of Delay
Primary reason for delay was assigned according to the taxonomy of the Delay Tool.6, 7 Delays were categorized to 1 of the following: (1) test scheduling; (2) obtaining test results; (3) surgery; (4) consultation; (5) patient (eg, family unavailable for decision‐making); (6) physician responsibility; (7) education, training. or research; (8) discharge planning or scheduling; and (9) availability of outside care and resources. There are 166 subcategories that clarify why a delay occurred. For example, within the main category of obtaining test results (2), there are 3 subcategories of delays related to problem in executing the test (2.1), return of results is delayed (2.2), and test results not reviewed within standard time of return (2.3). Subcategories are further divided to provide detail on the cause of delay. For example, a delay categorized as a 2.1:1 [(2) obtaining test results; (2.1) problem in executing the test; and 2.1:1 test to be done by MD is delayed beyond day desired], or 2.3:1 [(2) obtaining test results; (2.3) test results not reviewed within standard time of return; and 2.3:1 delay because physician did not review results]) both relate to physician causes of delays within the general category of obtaining test results. Some delays had more than 1 cause. A secondary cause of delay was assigned if applicable; however, the number of days delayed was attributed to the primary cause for analysis purposes.
Exemptions to Delay and Special Populations
Certain subpopulations of patients presented unique issues that led to them being unlikely to be classified as having a delay. For example, patients with a diagnosis of new onset of type 1 diabetes are historically admitted for 3 days at our hospital, which includes a specific education program; delays were not considered until this minimum period had passed. Children with medically complex care (eg, multisystem disease, multiple specialists involved, multiple medications) were included in this study.10 However, these children with frequent hospital admission were often fragile at discharge, and could meet criteria for readmission even on the date of their discharge, hence assigning a delay day was usually not indicated because of easily justified ongoing medical need for hospitalization.
Study Variables
The LOS, total costs, and routine demographic and administrative data for each study patient were extracted from Intermountain Healthcare's Enterprise Data Warehouse (EDW). The EDW contains detailed data about the cost of providing health care. Costs were derived from the hospital's cost accounting program, the Standard Cost Master, which is a transaction‐based microcosting accounting system.1113
For patients whose LOS extended before August 1 or after August 31, total hospital costs were averaged per day, and only days falling inside the month of August were counted in calculating the impact the delays in discharge had on the total costs of hospitalization. Hospital days that extended outside of August were not counted in either the numerator for potential days of delay or in the denominator for total days in the hospital.
Analyses
Descriptive statistics were calculated for the number, length of days of delay, and type of delay. Interrater reliability to assign a delay was ascertained for the 2 physicians. Mean LOS, mean total costs, and standard deviations (SDs) were calculated. All analyses were performed using Statistical Analytical Software version 9.13 (SAS Institute, Cary, NC).
Results
During the 31 days of the study, 171 patients occupied hospital beds an average of 7.3 days on the 2 inpatient medical teams, for a total of 911 inpatient days. Seven patients were admitted prior to August 1; 6 of these were discharged during the month of August and 1 stayed through the entire month and was discharged in September. Three additional patients were admitted in August and discharged in September. There were 6 readmissions during the month of August, and 1 patient was excluded from the study because of lack of sufficient information. All patients with delays were able to be classified according to the Delay Tool taxonomy. Interrater reliability for the 2 study physicians was 98%.
The characteristics between the patients who did and did not experience a delay in discharge are shown in Table 1. Thirty‐nine of 171 patients (22.8%), experienced at least 1 delay day. Eighteen of 39 patients had only 1 delay day (46.2%) and 11 patients experienced 2 delayed days (28.2%) (Figure 1). The average length of delay was 2.1 days.

| Nondelayed Patients (N = 132) | Delayed Patients (N = 39) | P Value | |
|---|---|---|---|
| |||
| Age (months), mean (SD)* | 22.6 (14.4) | 15.0 (14.6) | 0.009 |
| LOS during August (days), mean (SD)* | 4.64 (6.1) | 7.64 (7.15) | <0.001 |
| Total costs during August ($), mean (SD)* | 10,451 (19,254) | 14,341 (16,241) | 0.002 |
| Number of ICD‐9 CM diagnoses codes, mean (SD)* | 7.1 (7.4) | 8.5 (7.3) | 0.056 |
| Number of ICD‐9 CM procedure codes, mean (SD)* | 1.7 (3.8) | 1.6 (2.6) | 0.068 |
| Number of Patients with APR‐DRG SOI 3 (%) | 59 (44.7%) | 19 (48.7%) | 0.65 |
Delays attributed to physician responsibility accounted for 42.3% (16.5/39) of patient delays (conservative management or clinical decision‐making), with discharge planning delays accounting for 21.8% (family‐related, patient‐related, and hospital‐related problems), consultation for 14.1% (delay in obtaining or lack of follow‐up), test scheduling for 12.8%, and obtaining test results for 5.1% (ordering and weekend scheduling). There were no primary delays due to surgery, education and research, or unavailability of outside resources such as a skilled nursing bed. Four patients had a single additional secondary cause of delay assigned to them, related to physician responsibility, consultation, surgery and test scheduling; these were split, attributing 0.5 patients to each delay type (thus, the 17/39 patients delayed for physician responsibility was analyzed as 16.5/39) (Table 2).
| Delay Category | Number of Patients Experiencing Delays* | Percentage of All Patients Experiencing Delays (%) | Percentage of Study Patients Observed (%) | Total Delay Days | Average Length of Delays (days) | Percentage of Hospital Days That Were Delay Days (%) |
|---|---|---|---|---|---|---|
| ||||||
| 1. Scheduling | 5 | 12.8 | 2.92 | 16 | 3.20 | 1.76 |
| 2. Obtaining results | 2 | 5.1 | 1.17 | 3 | 1.50 | 0.33 |
| 3. Surgery | 0.5 | 1.3 | 0.29 | 1.5 | 3.00 | 0.16 |
| 4. Consultation | 5.5 | 14.1 | 3.22 | 10.5 | 1.91 | 1.15 |
| 5. Patient | 1 | 2.6 | 0.58 | 2 | 2.00 | 0.22 |
| 6. Physician | 16.5 | 42.3 | 9.65 | 33.5 | 2.03 | 3.68 |
| 7. Education | 0 | 0 | 0.00 | 0 | 0 | 0.00 |
| 8. Discharge | 8.5 | 21.8 | 4.97 | 15.5 | 1.82 | 1.70 |
| 9. Outside | 0 | 0 | 0.00 | 0 | 0 | 0.00 |
| Total | 39 | 100 | 22.81 | 82 | 2.10 | 9.00 |
There were 82 delay‐related hospital days of 911 total inpatient days on the 2 medical teams for August 2004 (9%). More than $170,000 in excess costs was incurred due to delay days from a total of approximately 1.9 million dollars in patient costs for the month (8.9%).
Discussion
This study finds that discharge delays in a tertiary care children's hospital are common; almost 1 in 4 patients experienced a medically unnecessary excess hospital stay of at least 1 day. The average length of a delay was 2.1 days, and overall, delays consumed 9% of pediatric hospital days and 8.9% of total costs. The most common reason for a delay was related to physician clinical care, including excessively conservative management and variability in clinical decision‐making.
Our study results are similar to the other 2 published studies that use the Delay Tool. In the adult and pediatric studies, between 10% and 30% of patients experienced a delay in discharge, with the average length of delay between 2.9 and 3 days.6, 7 Although both studies were conducted at teaching hospitals, what is particularly interesting is that they were conducted almost 20 years ago. During this period of time, there has been a shift in the inpatient pediatric patient population. In recent years, children who are cared for in the hospital have more chronic illnesses.1 In addition, there has been a shift in the types of conditions that may be cared for at home and those that now require inpatient stay.2 Despite this, delays continue at a similar proportion, but the cause of delays have shifted from scheduling and consultation to physician responsibility.
There is another tool in the literature which is more widely used, the Pediatric Appropriateness Evaluation Protocol (PAEP), which is based on the Appropriateness Evaluation Protocol for adults.1417 This tool is used to determine the appropriateness of ongoing hospitalization, not the cause of delay if ongoing hospitalization is inappropriate. The 3 areas that are evaluated (medical services, nursing and ancillary services, and patient's condition) have objective criteria that dictate if the hospitalization is appropriate or not (eg, parenteral (intravenous) therapy for at least 8 hours on that day, under nursing and ancillary services). The PAEP may be less sensitive given today's healthcare resource utilization climate. Many clinicians and families would agree that insertion of a peripheral central catheter is an acceptable form of outpatient treatment for many pediatric conditions. In conjunction with the Delay Tool, the PAEP could be used to determine if a delay occurred, then the Delay Tool used to categorize the cause of the delay. We choose to use expert clinician judgment to determine if a delay had occurred. We were more interested in why patients who are admitted (appropriately or inappropriately) cannot be discharged sooner, thus allowing for future intervention studies targeted to impact delays in discharge, as elucidated in this study. The Delay Tool specifically allowed us to categorize the reasons for delays. Given that the average LOS for patients in the nondelayed group was over 4 days, despite not using a tool such as the PAEP, we believe that these were likely to be appropriate admissions.
A recent study reported the first use of the Medical Care Appropriateness Protocol (MCAP) in a tertiary‐care children's hospital. The authors used the MCAP to determine the impact of an intervention on reducing inappropriate hospitals days for children. This tool is similarly labor‐intensive to the Delay Tool. Interestingly, this Canadian study found a high rate of inappropriate hospital days (47%), which may be in part attributable to a different outcome measurement tool and/or a different health care system.18
There are several limitations to our study that deserve mention. The Delay Tool requires clinician judgment regarding whether or not there was a delay in discharge for that day. We may have introduced some bias in our study, as hospitalist investigators assigned the delay and blinding to the attending physician specialty of record was not feasible. However, our results are similar to the other 2 published studies that have used this tool, and we specifically chose not to analyze or report results in terms of hospitalist and nonhospitalist attending physicians. The Delay Tool is not designed to differentiate shorter delays in terms of hours instead of days (eg, due to the inability for the patient to get a ride home). Shorter delays may be of particular importance depending on the occupancy rate of the hospital, the demand for beds, and other patient and hospital factors. We could not capture these shorter delays (although they did occur frequently) due to the original description of the Delay Tool. In addition, we would not have been able to report data on the impact on LOS and costs, as these are attributed to whole days in the hospital. However, if we had been able to differentiate shorter delays, this would bias our results to show a greater percentage of delays over smaller increments of time. Generalizability is an issue, given that this was a single‐center study. This study sample included over 80 different attending physicians participating in community pediatrician, subspecialty, and hospitalist practice groups. However, the patient population at PCMC is similar to other medium and large children's hospitals in the United States. The month observed may not reflect the entire year of hospitalizationsthere may be seasonal variations with delays depending on the volume and type of illness seen. The study was conducted in August, when there are newer house staff present. However, physician responsibility, which was the largest source of delays in our study, had little attribution to house staff. Most of the decisions were those of attending physicians, which would largely be unaffected by the time of year of the study. Finally, we were unable to assess the safety of the potential earlier discharge, as this was an observational study. However, in any future intervention studies examining processes to discharge patients sooner, measures of safety to the patient are a necessity. Finally, given the potential of ongoing admission, even on the date of discharge of our most fragile patients, this approach to discovering causes of delay may not apply to this important group, which is responsible for significant and growing resource utilization.
Despite these limitations, our findings demonstrate that in an era of children staying in the hospital less, and more medically‐complex children being admitted,10 a substantial number of children who are hospitalized at a children's hospital may have been discharged sooner. The majority of these decisions were directly related to physician responsibility. As consumers, providers, and hospitals work together to develop quality measures that are reflective of inpatient pediatric care, the Delay Tool may be able to highlight 2 aims of quality (ie, timeliness and efficiency of care) that could be used to assess the impact of interventions designed to safely discharge patients sooner. Interventions such as audit‐feedback,18 clinical guideline deployment,19 and hospitalist systems of care4 continue to hold the promise of earlier discharge; however, tools designed to measure inappropriate use of hospital days should be employed to demonstrate their effectiveness. Our study demonstrates ongoing waste in children's hospitals.
Conclusions
Almost 1 out of 4 patients in this 1‐month period could have been discharged sooner than they were. The impact of delays on costs and LOS are substantial and should provide strong incentives to develop effective interventions. Such interventions will need to address variations in physician criteria for discharge, more efficient discharge planning, and timely scheduling of consultation and diagnostic testing.
Acknowledgements
The authors thank Joni R. Beshanky and Harry Selker for their help and training in the use of the Delay Tool.
Inpatient pediatrics is undergoing a paradigm shift in at least 3 ways. First, more children with chronic disease are being cared for in the hospital over time.1 Second, previous inpatient conditions are treated at home with advancing technology such as peripherally‐inserted catheters.2 Third, there are new areas of growing specialization, such as hospital medicine, in which the practitioners deliver more efficient care.3, 4
Nationwide, there is increasing pressure to improve inpatient quality of care. The Institute of Medicine defines 6 aims for improvement, including timeliness (reducing waits and sometimes harmful delays for both those who receive and those who give care) and efficiency of care (avoiding waste, including waste of equipment, supplies, ideas, and energy).5 Reducing unnecessary stays in the hospital is a potential quality measure that hospitals may use to address the timeliness and efficiency of care delivered to hospitalized children.
Delays in discharge have been used as markers of unnecessary stays in the hospital for inpatient adult and pediatric care,6, 7 but these are limited to inpatient systems from almost 20 years ago. Current reasons why patients are delayed from discharge, if at all, are not well described. We undertook this study to describe delays in hospital discharges at a tertiary‐care children's hospital in terms of number of patients, length of days of delay, and type of delay. In addition, we sought to characterize the impact of discharge delays on overall length of stay (LOS) and costs.
Methods
Patient Population/Study Design
All children cared for on 2 pediatric medical teams at Primary Children's Medical Center during the month of August 2004 were eligible for the study. Two research assistants independently attended team rounds and collected data relating to: the reasons for ongoing hospitalization, pending items (eg, consultations, tests), and the plan of care for that day. The research assistants each attended daily team rounds for the entire month of August (1 for each team, switching to the opposite team after 2 weeks). This was combined with information available in the Patient Tracker, a software tool developed to improve communication between caregivers and improve discharge efficiency.8 This software tool details diagnoses, daily medical care plans, discharge criteria, and ongoing medical interventions while tracking daily changes in interventions and the medical care plan for each patient cared for on a pediatric medical team.
The research assistants subsequently presented their observations along with information from Patient Tracker to 2 experienced physicians (R.S. and B.S.) who independently determined if a delay occurred, the number of delay days extending discharge, and the cause of the delay, if present, categorized according to the taxonomy of the Delay Tool.6, 7 If there was not enough information for either of the physicians to identify and classify a delay, the electronic medical record of the patient was also reviewed. Discrepancies between physicians assigning delays were discussed until consensus was reached.
The study was approved by the Institutional Review Board of the University of Utah Health Sciences Center and Primary Children's Medical Center (PCMC).
Setting
PCMC is a 233‐bed tertiary‐care children's hospital, owned and operated by Intermountain Healthcare (a not‐for‐profit vertically integrated managed care organization) in the Intermountain West, which serves as both the primary hospital for Salt Lake County and as a tertiary‐care children's hospital for 5 states (UT, MT, WY, ID, and NV).9
Study Definitions
Delay and Length of Delay
Delays in discharge were measured using a validated and reliable instrument, the Delay Tool.6, 7 A discharge was classified as delayed if there was no medical reason for the patient to be in the hospital on a given day, identical to the definition used in the original studies to validate the tool. Delays were recorded as whole days, not fractions of days or hours, as described in the original validation of the tool. For example, if the medical team requested a consultation, and the consultant's opinion was rendered late, but the patient would have remained in the hospital anyway, then this period of time would not count as a delay. However, if the medical team did not receive a consultant's opinion within the standard time (24 hours as defined for this study and in validating studies for the Delay Tool), and the patient's sole reason for being in the hospital during that day was waiting for that opinion, then that period of time would count toward a delay due to a late consultative opinion. Delays of less than 1 day, due to the mechanics of discharging a patient from the hospital (providing prescriptions, follow up, communication, arranging home health, and transportation) were not measured in this study, to match the original methodology of the Delay Tool.
Type of Delay
Primary reason for delay was assigned according to the taxonomy of the Delay Tool.6, 7 Delays were categorized to 1 of the following: (1) test scheduling; (2) obtaining test results; (3) surgery; (4) consultation; (5) patient (eg, family unavailable for decision‐making); (6) physician responsibility; (7) education, training. or research; (8) discharge planning or scheduling; and (9) availability of outside care and resources. There are 166 subcategories that clarify why a delay occurred. For example, within the main category of obtaining test results (2), there are 3 subcategories of delays related to problem in executing the test (2.1), return of results is delayed (2.2), and test results not reviewed within standard time of return (2.3). Subcategories are further divided to provide detail on the cause of delay. For example, a delay categorized as a 2.1:1 [(2) obtaining test results; (2.1) problem in executing the test; and 2.1:1 test to be done by MD is delayed beyond day desired], or 2.3:1 [(2) obtaining test results; (2.3) test results not reviewed within standard time of return; and 2.3:1 delay because physician did not review results]) both relate to physician causes of delays within the general category of obtaining test results. Some delays had more than 1 cause. A secondary cause of delay was assigned if applicable; however, the number of days delayed was attributed to the primary cause for analysis purposes.
Exemptions to Delay and Special Populations
Certain subpopulations of patients presented unique issues that led to them being unlikely to be classified as having a delay. For example, patients with a diagnosis of new onset of type 1 diabetes are historically admitted for 3 days at our hospital, which includes a specific education program; delays were not considered until this minimum period had passed. Children with medically complex care (eg, multisystem disease, multiple specialists involved, multiple medications) were included in this study.10 However, these children with frequent hospital admission were often fragile at discharge, and could meet criteria for readmission even on the date of their discharge, hence assigning a delay day was usually not indicated because of easily justified ongoing medical need for hospitalization.
Study Variables
The LOS, total costs, and routine demographic and administrative data for each study patient were extracted from Intermountain Healthcare's Enterprise Data Warehouse (EDW). The EDW contains detailed data about the cost of providing health care. Costs were derived from the hospital's cost accounting program, the Standard Cost Master, which is a transaction‐based microcosting accounting system.1113
For patients whose LOS extended before August 1 or after August 31, total hospital costs were averaged per day, and only days falling inside the month of August were counted in calculating the impact the delays in discharge had on the total costs of hospitalization. Hospital days that extended outside of August were not counted in either the numerator for potential days of delay or in the denominator for total days in the hospital.
Analyses
Descriptive statistics were calculated for the number, length of days of delay, and type of delay. Interrater reliability to assign a delay was ascertained for the 2 physicians. Mean LOS, mean total costs, and standard deviations (SDs) were calculated. All analyses were performed using Statistical Analytical Software version 9.13 (SAS Institute, Cary, NC).
Results
During the 31 days of the study, 171 patients occupied hospital beds an average of 7.3 days on the 2 inpatient medical teams, for a total of 911 inpatient days. Seven patients were admitted prior to August 1; 6 of these were discharged during the month of August and 1 stayed through the entire month and was discharged in September. Three additional patients were admitted in August and discharged in September. There were 6 readmissions during the month of August, and 1 patient was excluded from the study because of lack of sufficient information. All patients with delays were able to be classified according to the Delay Tool taxonomy. Interrater reliability for the 2 study physicians was 98%.
The characteristics between the patients who did and did not experience a delay in discharge are shown in Table 1. Thirty‐nine of 171 patients (22.8%), experienced at least 1 delay day. Eighteen of 39 patients had only 1 delay day (46.2%) and 11 patients experienced 2 delayed days (28.2%) (Figure 1). The average length of delay was 2.1 days.

| Nondelayed Patients (N = 132) | Delayed Patients (N = 39) | P Value | |
|---|---|---|---|
| |||
| Age (months), mean (SD)* | 22.6 (14.4) | 15.0 (14.6) | 0.009 |
| LOS during August (days), mean (SD)* | 4.64 (6.1) | 7.64 (7.15) | <0.001 |
| Total costs during August ($), mean (SD)* | 10,451 (19,254) | 14,341 (16,241) | 0.002 |
| Number of ICD‐9 CM diagnoses codes, mean (SD)* | 7.1 (7.4) | 8.5 (7.3) | 0.056 |
| Number of ICD‐9 CM procedure codes, mean (SD)* | 1.7 (3.8) | 1.6 (2.6) | 0.068 |
| Number of Patients with APR‐DRG SOI 3 (%) | 59 (44.7%) | 19 (48.7%) | 0.65 |
Delays attributed to physician responsibility accounted for 42.3% (16.5/39) of patient delays (conservative management or clinical decision‐making), with discharge planning delays accounting for 21.8% (family‐related, patient‐related, and hospital‐related problems), consultation for 14.1% (delay in obtaining or lack of follow‐up), test scheduling for 12.8%, and obtaining test results for 5.1% (ordering and weekend scheduling). There were no primary delays due to surgery, education and research, or unavailability of outside resources such as a skilled nursing bed. Four patients had a single additional secondary cause of delay assigned to them, related to physician responsibility, consultation, surgery and test scheduling; these were split, attributing 0.5 patients to each delay type (thus, the 17/39 patients delayed for physician responsibility was analyzed as 16.5/39) (Table 2).
| Delay Category | Number of Patients Experiencing Delays* | Percentage of All Patients Experiencing Delays (%) | Percentage of Study Patients Observed (%) | Total Delay Days | Average Length of Delays (days) | Percentage of Hospital Days That Were Delay Days (%) |
|---|---|---|---|---|---|---|
| ||||||
| 1. Scheduling | 5 | 12.8 | 2.92 | 16 | 3.20 | 1.76 |
| 2. Obtaining results | 2 | 5.1 | 1.17 | 3 | 1.50 | 0.33 |
| 3. Surgery | 0.5 | 1.3 | 0.29 | 1.5 | 3.00 | 0.16 |
| 4. Consultation | 5.5 | 14.1 | 3.22 | 10.5 | 1.91 | 1.15 |
| 5. Patient | 1 | 2.6 | 0.58 | 2 | 2.00 | 0.22 |
| 6. Physician | 16.5 | 42.3 | 9.65 | 33.5 | 2.03 | 3.68 |
| 7. Education | 0 | 0 | 0.00 | 0 | 0 | 0.00 |
| 8. Discharge | 8.5 | 21.8 | 4.97 | 15.5 | 1.82 | 1.70 |
| 9. Outside | 0 | 0 | 0.00 | 0 | 0 | 0.00 |
| Total | 39 | 100 | 22.81 | 82 | 2.10 | 9.00 |
There were 82 delay‐related hospital days of 911 total inpatient days on the 2 medical teams for August 2004 (9%). More than $170,000 in excess costs was incurred due to delay days from a total of approximately 1.9 million dollars in patient costs for the month (8.9%).
Discussion
This study finds that discharge delays in a tertiary care children's hospital are common; almost 1 in 4 patients experienced a medically unnecessary excess hospital stay of at least 1 day. The average length of a delay was 2.1 days, and overall, delays consumed 9% of pediatric hospital days and 8.9% of total costs. The most common reason for a delay was related to physician clinical care, including excessively conservative management and variability in clinical decision‐making.
Our study results are similar to the other 2 published studies that use the Delay Tool. In the adult and pediatric studies, between 10% and 30% of patients experienced a delay in discharge, with the average length of delay between 2.9 and 3 days.6, 7 Although both studies were conducted at teaching hospitals, what is particularly interesting is that they were conducted almost 20 years ago. During this period of time, there has been a shift in the inpatient pediatric patient population. In recent years, children who are cared for in the hospital have more chronic illnesses.1 In addition, there has been a shift in the types of conditions that may be cared for at home and those that now require inpatient stay.2 Despite this, delays continue at a similar proportion, but the cause of delays have shifted from scheduling and consultation to physician responsibility.
There is another tool in the literature which is more widely used, the Pediatric Appropriateness Evaluation Protocol (PAEP), which is based on the Appropriateness Evaluation Protocol for adults.1417 This tool is used to determine the appropriateness of ongoing hospitalization, not the cause of delay if ongoing hospitalization is inappropriate. The 3 areas that are evaluated (medical services, nursing and ancillary services, and patient's condition) have objective criteria that dictate if the hospitalization is appropriate or not (eg, parenteral (intravenous) therapy for at least 8 hours on that day, under nursing and ancillary services). The PAEP may be less sensitive given today's healthcare resource utilization climate. Many clinicians and families would agree that insertion of a peripheral central catheter is an acceptable form of outpatient treatment for many pediatric conditions. In conjunction with the Delay Tool, the PAEP could be used to determine if a delay occurred, then the Delay Tool used to categorize the cause of the delay. We choose to use expert clinician judgment to determine if a delay had occurred. We were more interested in why patients who are admitted (appropriately or inappropriately) cannot be discharged sooner, thus allowing for future intervention studies targeted to impact delays in discharge, as elucidated in this study. The Delay Tool specifically allowed us to categorize the reasons for delays. Given that the average LOS for patients in the nondelayed group was over 4 days, despite not using a tool such as the PAEP, we believe that these were likely to be appropriate admissions.
A recent study reported the first use of the Medical Care Appropriateness Protocol (MCAP) in a tertiary‐care children's hospital. The authors used the MCAP to determine the impact of an intervention on reducing inappropriate hospitals days for children. This tool is similarly labor‐intensive to the Delay Tool. Interestingly, this Canadian study found a high rate of inappropriate hospital days (47%), which may be in part attributable to a different outcome measurement tool and/or a different health care system.18
There are several limitations to our study that deserve mention. The Delay Tool requires clinician judgment regarding whether or not there was a delay in discharge for that day. We may have introduced some bias in our study, as hospitalist investigators assigned the delay and blinding to the attending physician specialty of record was not feasible. However, our results are similar to the other 2 published studies that have used this tool, and we specifically chose not to analyze or report results in terms of hospitalist and nonhospitalist attending physicians. The Delay Tool is not designed to differentiate shorter delays in terms of hours instead of days (eg, due to the inability for the patient to get a ride home). Shorter delays may be of particular importance depending on the occupancy rate of the hospital, the demand for beds, and other patient and hospital factors. We could not capture these shorter delays (although they did occur frequently) due to the original description of the Delay Tool. In addition, we would not have been able to report data on the impact on LOS and costs, as these are attributed to whole days in the hospital. However, if we had been able to differentiate shorter delays, this would bias our results to show a greater percentage of delays over smaller increments of time. Generalizability is an issue, given that this was a single‐center study. This study sample included over 80 different attending physicians participating in community pediatrician, subspecialty, and hospitalist practice groups. However, the patient population at PCMC is similar to other medium and large children's hospitals in the United States. The month observed may not reflect the entire year of hospitalizationsthere may be seasonal variations with delays depending on the volume and type of illness seen. The study was conducted in August, when there are newer house staff present. However, physician responsibility, which was the largest source of delays in our study, had little attribution to house staff. Most of the decisions were those of attending physicians, which would largely be unaffected by the time of year of the study. Finally, we were unable to assess the safety of the potential earlier discharge, as this was an observational study. However, in any future intervention studies examining processes to discharge patients sooner, measures of safety to the patient are a necessity. Finally, given the potential of ongoing admission, even on the date of discharge of our most fragile patients, this approach to discovering causes of delay may not apply to this important group, which is responsible for significant and growing resource utilization.
Despite these limitations, our findings demonstrate that in an era of children staying in the hospital less, and more medically‐complex children being admitted,10 a substantial number of children who are hospitalized at a children's hospital may have been discharged sooner. The majority of these decisions were directly related to physician responsibility. As consumers, providers, and hospitals work together to develop quality measures that are reflective of inpatient pediatric care, the Delay Tool may be able to highlight 2 aims of quality (ie, timeliness and efficiency of care) that could be used to assess the impact of interventions designed to safely discharge patients sooner. Interventions such as audit‐feedback,18 clinical guideline deployment,19 and hospitalist systems of care4 continue to hold the promise of earlier discharge; however, tools designed to measure inappropriate use of hospital days should be employed to demonstrate their effectiveness. Our study demonstrates ongoing waste in children's hospitals.
Conclusions
Almost 1 out of 4 patients in this 1‐month period could have been discharged sooner than they were. The impact of delays on costs and LOS are substantial and should provide strong incentives to develop effective interventions. Such interventions will need to address variations in physician criteria for discharge, more efficient discharge planning, and timely scheduling of consultation and diagnostic testing.
Acknowledgements
The authors thank Joni R. Beshanky and Harry Selker for their help and training in the use of the Delay Tool.
- .The transformation of child health in the United States: social disparities in child health persistent despite dramatic improvement in child health overall.Health Aff (Millwood).2004;23(5):9–25.
- , , , .Hospitalist care of medically complex children.Pediatr Res.2004;55(4):314A–315A.
- , , , .Pediatric hospitalists: a systematic review of the literature.Pediatrics.2006;117(5):1736–1744.
- , , , et al.Pediatric hospitalists: report of a leadership conference.Pediatrics.2006;117(4):1122–1130.
- Institute of Medicine.Crossing the Quality Chasm: A New Health System for the Twenty‐first Century.Washington, DC:National Academy Press;2001.
- , , .Using the Delay Tool to attribute causes for unnecessary pediatric hospital days.Med Care.1990;28(10):982–989.
- , , , .The epidemiology of delays in a teaching hospital. The development and use of a tool that detects unnecessary hospital days.Med Care.1989;27(2):112–129.
- , , , , .A tool for improving patient discharge process and hospital communication practices: the Patient Tracker.AMIA Annu Symp Proc.2007;11:493–497.
- , .Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101(4 Pt 2):805–811; discussion 811–802.
- , , .Hospitalist care of the medically complex child.Pediatr Clin North Am.2005;52(4):1165–1187.
- , , , et al.Epidemiology, complications, and cost of hospitalization in children with laboratory‐confirmed influenza infection.Pediatrics.2006;118(6):2409–2417.
- , , , , , .Clinical and economic outcomes of conventional amphotericin B‐associated nephrotoxicity.Clin Infect Dis.2002;35(12):e120–e127.
- , , , et al.Using a hospital information system to assess the effects of adverse drug events.Proc Annu Symp Comput Appl Med Care.1993;1993:161–165.
- , , .Appropriateness of hospitalization in a Canadian pediatric hospital.Pediatrics.1993;91(1):70–74.
- , , , .The appropriateness evaluation protocol: application in an Australian children's hospital.Aust Clin Rev.1991;11(4):123–131.
- , .Assessing the need to hospitalize children: pediatric appropriateness evaluation protocol.Pediatrics.1989;84(2):242–247.
- .Medically inappropriate hospital use in a pediatric population.N Engl J Med.1988;318(16):1033–1037.
- , , , , .Reducing inappropriate hospital use on a general pediatric inpatient unit.Pediatrics.2008;121(5):e1068–e1073.
- , .Reliable implementation of clinical pathways: what will it take—that is the question.J Pediatr.2008;152(3):303–304.
- .The transformation of child health in the United States: social disparities in child health persistent despite dramatic improvement in child health overall.Health Aff (Millwood).2004;23(5):9–25.
- , , , .Hospitalist care of medically complex children.Pediatr Res.2004;55(4):314A–315A.
- , , , .Pediatric hospitalists: a systematic review of the literature.Pediatrics.2006;117(5):1736–1744.
- , , , et al.Pediatric hospitalists: report of a leadership conference.Pediatrics.2006;117(4):1122–1130.
- Institute of Medicine.Crossing the Quality Chasm: A New Health System for the Twenty‐first Century.Washington, DC:National Academy Press;2001.
- , , .Using the Delay Tool to attribute causes for unnecessary pediatric hospital days.Med Care.1990;28(10):982–989.
- , , , .The epidemiology of delays in a teaching hospital. The development and use of a tool that detects unnecessary hospital days.Med Care.1989;27(2):112–129.
- , , , , .A tool for improving patient discharge process and hospital communication practices: the Patient Tracker.AMIA Annu Symp Proc.2007;11:493–497.
- , .Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101(4 Pt 2):805–811; discussion 811–802.
- , , .Hospitalist care of the medically complex child.Pediatr Clin North Am.2005;52(4):1165–1187.
- , , , et al.Epidemiology, complications, and cost of hospitalization in children with laboratory‐confirmed influenza infection.Pediatrics.2006;118(6):2409–2417.
- , , , , , .Clinical and economic outcomes of conventional amphotericin B‐associated nephrotoxicity.Clin Infect Dis.2002;35(12):e120–e127.
- , , , et al.Using a hospital information system to assess the effects of adverse drug events.Proc Annu Symp Comput Appl Med Care.1993;1993:161–165.
- , , .Appropriateness of hospitalization in a Canadian pediatric hospital.Pediatrics.1993;91(1):70–74.
- , , , .The appropriateness evaluation protocol: application in an Australian children's hospital.Aust Clin Rev.1991;11(4):123–131.
- , .Assessing the need to hospitalize children: pediatric appropriateness evaluation protocol.Pediatrics.1989;84(2):242–247.
- .Medically inappropriate hospital use in a pediatric population.N Engl J Med.1988;318(16):1033–1037.
- , , , , .Reducing inappropriate hospital use on a general pediatric inpatient unit.Pediatrics.2008;121(5):e1068–e1073.
- , .Reliable implementation of clinical pathways: what will it take—that is the question.J Pediatr.2008;152(3):303–304.
Copyright © 2009 Society of Hospital Medicine
World Mental Health Day: Preventing suicide
Increased engagement of men in mental health services is needed
Each year, the World Federation of Mental Health chooses a theme for World Mental Health Day, which is Oct. 10. This year’s theme is “Mental Health Promotion and Suicide Prevention.”
About 800,000 people die by suicide every year, according to the World Health Organization. Suicide is the second-leading cause of death among people aged 15-29 years.1
Most suicide occurs in low- and middle-income countries, the WHO reports. In addition, almost two-thirds of those deaths around the world occur in males, a recent study shows.2 The study, conducted by Danah Alothman and Andrew Fogarty, MBBS, of the NIHR Biomedical Research Center at the University of Nottingham (England), looked at sex-specific suicide rates for 182 countries in 2015.
They found that the highest difference between male:female suicide rates were in the Americas (median, 4:1/100,000), and the lowest were in Africa and Asia (median for both continents, 2.7:1/100,000).
“The implication is that as societies become richer and more educated, males have a higher risk of dying as a consequence of suicide relative to females,” they wrote in the Journal of Affective Disorders.
For clinicians who treat patients with mental illness, particularly those of us who practice in the Americas, this sex differential is concerning. We know that women are more likely to be diagnosed the depression.3 But perhaps this has something to do with the way men are socialized around the world. In other words, as John S. Ogrodniczuk, PhD, and John L. Oliffe, PhD, wrote,4 depression in men “often manifests as irritability; anger; hostile, aggressive, abusive behavior; risk taking, substance abuse; and escaping behavior.” They argue that the outward behavior shown by some men with depression might, in fact, “serve as a cover-up mechanism to hide the internal turmoil” they are experiencing. We certainly know that some men adhere to masculine norms such as stoicism, which in turn, heightens self-stigma. Unfortunately, men seek help for depression less often than do women.5 So one key question becomes: What can we as mental health professionals do to better meet the treatment needs of our male patients?
One example of a program that could hold promise in this area is one called Men at Risk. That program, developed by the nonprofit Centre for Suicide Prevention, in Grande Prairie, Alta., helps men who work in the oil, forestry, and agriculture sectors talk about their challenges and encourages them to let go of stigma.6
Factors other than male gender also might increase the likelihood of suicide. It has rightly been said that genetics and environment play a big role on the psyche of the individuals, and the act of suicide is no different when we discuss the etiologic factors that lead to perpetration of such an act. Genetic vulnerability is a factor that cannot be modified or altered in an easy way, hence, control of environmental factors is more pertinent.
Poverty and violence are two major detrimental factors that have reached alarming proportions and can lead people end their lives.
The developing countries, and now to a significant extent, developed countries, face terrorism that affect the human psyche and can lead to depression, psychosis, and substance abuse, and hence, increase the vulnerability toward the act of suicide. In our offices, we psychiatrists come across patients with borderline personality disorder, for example, who present to emergency departments with multiple and repeated suicidal attempts. There is a big role of genetics here – and role of specific interventions, such as dialectical behavior therapy. Pharmacologic treatment can play a vital role.
In order to make the world a safe place, joint global efforts are required. Enhanced security steps, improved immigration screening, and political will are essential to curb this heartbreaking act. Responsible reporting on the part of the media is needed to make suicide contagion less likely.7
Among other important measures are reducing access to guns and other firearms, and increasing health education about consumption of alcohol and other substances. We also need early identification and prompt treatment of mental illnesses; alleviation of poverty; mobilization of community supports; activation of multiple crisis lines; increased availability and affordability of psychotropic medications; reduction of waiting times for seeking treatment of mental illness; enhanced training of crisis workers; and refresher courses for psychiatrists, family physicians, and other allied mental health workers. Above all, strategies are needed to address the stigma associated with seeking help for mental health issues.
Suicide is a global public health issue, and it is of the utmost importance that a collaborative effort be placed in perspective by individual countries within their own health-related policies and parameters.
Good-quality data on suicide prevalence rates would be of the utmost help in understanding the magnitude of this grave problem. The WHO Mental Health Action Plan 2013-2020 indicates the commitment of member states to work toward the global target of reducing the suicide rate in countries by 10% by 2020.
Individual and collective efforts should become the priority to achieve this target going forward.
References
1. World Health Organization. Suicide. 2019 Sep 2.
2. Alothman D and A Fogarty. J Affect Disord. 2020 Jan 1. doi: 10.1016/j.jad.2019.08.093.
3. Albert PR. J Psychiatry Neurosci. 2015 Jul;40(4):219-21.
4. Ogrodniczuk JS and JL Oliffe. Can Fam Physician. 2011;57(2):153-5.
5. Seidler ZE et al. Clin Psychology Rev. 2016;49:106-18.
6. Ellwand O. Men at risk program helping men in Alberta trades, industry, agriculture struggling with mental health issues. Edmonton Sun. 2016 Mar 27.
7. American Association of Suicidology, et al. Recommendations for reporting on suicide.
Dr. Muhammad is clinical professor of psychiatry and consultant psychiatrist at Niagara Health Service, St. Catharines, Ont.
Increased engagement of men in mental health services is needed
Increased engagement of men in mental health services is needed
Each year, the World Federation of Mental Health chooses a theme for World Mental Health Day, which is Oct. 10. This year’s theme is “Mental Health Promotion and Suicide Prevention.”
About 800,000 people die by suicide every year, according to the World Health Organization. Suicide is the second-leading cause of death among people aged 15-29 years.1
Most suicide occurs in low- and middle-income countries, the WHO reports. In addition, almost two-thirds of those deaths around the world occur in males, a recent study shows.2 The study, conducted by Danah Alothman and Andrew Fogarty, MBBS, of the NIHR Biomedical Research Center at the University of Nottingham (England), looked at sex-specific suicide rates for 182 countries in 2015.
They found that the highest difference between male:female suicide rates were in the Americas (median, 4:1/100,000), and the lowest were in Africa and Asia (median for both continents, 2.7:1/100,000).
“The implication is that as societies become richer and more educated, males have a higher risk of dying as a consequence of suicide relative to females,” they wrote in the Journal of Affective Disorders.
For clinicians who treat patients with mental illness, particularly those of us who practice in the Americas, this sex differential is concerning. We know that women are more likely to be diagnosed the depression.3 But perhaps this has something to do with the way men are socialized around the world. In other words, as John S. Ogrodniczuk, PhD, and John L. Oliffe, PhD, wrote,4 depression in men “often manifests as irritability; anger; hostile, aggressive, abusive behavior; risk taking, substance abuse; and escaping behavior.” They argue that the outward behavior shown by some men with depression might, in fact, “serve as a cover-up mechanism to hide the internal turmoil” they are experiencing. We certainly know that some men adhere to masculine norms such as stoicism, which in turn, heightens self-stigma. Unfortunately, men seek help for depression less often than do women.5 So one key question becomes: What can we as mental health professionals do to better meet the treatment needs of our male patients?
One example of a program that could hold promise in this area is one called Men at Risk. That program, developed by the nonprofit Centre for Suicide Prevention, in Grande Prairie, Alta., helps men who work in the oil, forestry, and agriculture sectors talk about their challenges and encourages them to let go of stigma.6
Factors other than male gender also might increase the likelihood of suicide. It has rightly been said that genetics and environment play a big role on the psyche of the individuals, and the act of suicide is no different when we discuss the etiologic factors that lead to perpetration of such an act. Genetic vulnerability is a factor that cannot be modified or altered in an easy way, hence, control of environmental factors is more pertinent.
Poverty and violence are two major detrimental factors that have reached alarming proportions and can lead people end their lives.
The developing countries, and now to a significant extent, developed countries, face terrorism that affect the human psyche and can lead to depression, psychosis, and substance abuse, and hence, increase the vulnerability toward the act of suicide. In our offices, we psychiatrists come across patients with borderline personality disorder, for example, who present to emergency departments with multiple and repeated suicidal attempts. There is a big role of genetics here – and role of specific interventions, such as dialectical behavior therapy. Pharmacologic treatment can play a vital role.
In order to make the world a safe place, joint global efforts are required. Enhanced security steps, improved immigration screening, and political will are essential to curb this heartbreaking act. Responsible reporting on the part of the media is needed to make suicide contagion less likely.7
Among other important measures are reducing access to guns and other firearms, and increasing health education about consumption of alcohol and other substances. We also need early identification and prompt treatment of mental illnesses; alleviation of poverty; mobilization of community supports; activation of multiple crisis lines; increased availability and affordability of psychotropic medications; reduction of waiting times for seeking treatment of mental illness; enhanced training of crisis workers; and refresher courses for psychiatrists, family physicians, and other allied mental health workers. Above all, strategies are needed to address the stigma associated with seeking help for mental health issues.
Suicide is a global public health issue, and it is of the utmost importance that a collaborative effort be placed in perspective by individual countries within their own health-related policies and parameters.
Good-quality data on suicide prevalence rates would be of the utmost help in understanding the magnitude of this grave problem. The WHO Mental Health Action Plan 2013-2020 indicates the commitment of member states to work toward the global target of reducing the suicide rate in countries by 10% by 2020.
Individual and collective efforts should become the priority to achieve this target going forward.
References
1. World Health Organization. Suicide. 2019 Sep 2.
2. Alothman D and A Fogarty. J Affect Disord. 2020 Jan 1. doi: 10.1016/j.jad.2019.08.093.
3. Albert PR. J Psychiatry Neurosci. 2015 Jul;40(4):219-21.
4. Ogrodniczuk JS and JL Oliffe. Can Fam Physician. 2011;57(2):153-5.
5. Seidler ZE et al. Clin Psychology Rev. 2016;49:106-18.
6. Ellwand O. Men at risk program helping men in Alberta trades, industry, agriculture struggling with mental health issues. Edmonton Sun. 2016 Mar 27.
7. American Association of Suicidology, et al. Recommendations for reporting on suicide.
Dr. Muhammad is clinical professor of psychiatry and consultant psychiatrist at Niagara Health Service, St. Catharines, Ont.
Each year, the World Federation of Mental Health chooses a theme for World Mental Health Day, which is Oct. 10. This year’s theme is “Mental Health Promotion and Suicide Prevention.”
About 800,000 people die by suicide every year, according to the World Health Organization. Suicide is the second-leading cause of death among people aged 15-29 years.1
Most suicide occurs in low- and middle-income countries, the WHO reports. In addition, almost two-thirds of those deaths around the world occur in males, a recent study shows.2 The study, conducted by Danah Alothman and Andrew Fogarty, MBBS, of the NIHR Biomedical Research Center at the University of Nottingham (England), looked at sex-specific suicide rates for 182 countries in 2015.
They found that the highest difference between male:female suicide rates were in the Americas (median, 4:1/100,000), and the lowest were in Africa and Asia (median for both continents, 2.7:1/100,000).
“The implication is that as societies become richer and more educated, males have a higher risk of dying as a consequence of suicide relative to females,” they wrote in the Journal of Affective Disorders.
For clinicians who treat patients with mental illness, particularly those of us who practice in the Americas, this sex differential is concerning. We know that women are more likely to be diagnosed the depression.3 But perhaps this has something to do with the way men are socialized around the world. In other words, as John S. Ogrodniczuk, PhD, and John L. Oliffe, PhD, wrote,4 depression in men “often manifests as irritability; anger; hostile, aggressive, abusive behavior; risk taking, substance abuse; and escaping behavior.” They argue that the outward behavior shown by some men with depression might, in fact, “serve as a cover-up mechanism to hide the internal turmoil” they are experiencing. We certainly know that some men adhere to masculine norms such as stoicism, which in turn, heightens self-stigma. Unfortunately, men seek help for depression less often than do women.5 So one key question becomes: What can we as mental health professionals do to better meet the treatment needs of our male patients?
One example of a program that could hold promise in this area is one called Men at Risk. That program, developed by the nonprofit Centre for Suicide Prevention, in Grande Prairie, Alta., helps men who work in the oil, forestry, and agriculture sectors talk about their challenges and encourages them to let go of stigma.6
Factors other than male gender also might increase the likelihood of suicide. It has rightly been said that genetics and environment play a big role on the psyche of the individuals, and the act of suicide is no different when we discuss the etiologic factors that lead to perpetration of such an act. Genetic vulnerability is a factor that cannot be modified or altered in an easy way, hence, control of environmental factors is more pertinent.
Poverty and violence are two major detrimental factors that have reached alarming proportions and can lead people end their lives.
The developing countries, and now to a significant extent, developed countries, face terrorism that affect the human psyche and can lead to depression, psychosis, and substance abuse, and hence, increase the vulnerability toward the act of suicide. In our offices, we psychiatrists come across patients with borderline personality disorder, for example, who present to emergency departments with multiple and repeated suicidal attempts. There is a big role of genetics here – and role of specific interventions, such as dialectical behavior therapy. Pharmacologic treatment can play a vital role.
In order to make the world a safe place, joint global efforts are required. Enhanced security steps, improved immigration screening, and political will are essential to curb this heartbreaking act. Responsible reporting on the part of the media is needed to make suicide contagion less likely.7
Among other important measures are reducing access to guns and other firearms, and increasing health education about consumption of alcohol and other substances. We also need early identification and prompt treatment of mental illnesses; alleviation of poverty; mobilization of community supports; activation of multiple crisis lines; increased availability and affordability of psychotropic medications; reduction of waiting times for seeking treatment of mental illness; enhanced training of crisis workers; and refresher courses for psychiatrists, family physicians, and other allied mental health workers. Above all, strategies are needed to address the stigma associated with seeking help for mental health issues.
Suicide is a global public health issue, and it is of the utmost importance that a collaborative effort be placed in perspective by individual countries within their own health-related policies and parameters.
Good-quality data on suicide prevalence rates would be of the utmost help in understanding the magnitude of this grave problem. The WHO Mental Health Action Plan 2013-2020 indicates the commitment of member states to work toward the global target of reducing the suicide rate in countries by 10% by 2020.
Individual and collective efforts should become the priority to achieve this target going forward.
References
1. World Health Organization. Suicide. 2019 Sep 2.
2. Alothman D and A Fogarty. J Affect Disord. 2020 Jan 1. doi: 10.1016/j.jad.2019.08.093.
3. Albert PR. J Psychiatry Neurosci. 2015 Jul;40(4):219-21.
4. Ogrodniczuk JS and JL Oliffe. Can Fam Physician. 2011;57(2):153-5.
5. Seidler ZE et al. Clin Psychology Rev. 2016;49:106-18.
6. Ellwand O. Men at risk program helping men in Alberta trades, industry, agriculture struggling with mental health issues. Edmonton Sun. 2016 Mar 27.
7. American Association of Suicidology, et al. Recommendations for reporting on suicide.
Dr. Muhammad is clinical professor of psychiatry and consultant psychiatrist at Niagara Health Service, St. Catharines, Ont.
Vorinostat demonstrates consistent safety
Berlin, Germany—Vorinostat demonstrates safety and tolerability alone and in combination with other systemic treatments for a wide range of solid and hematologic malignancies, according to a study of collated data from the vorinostat clinical trial program.
Investigators presented the safety data in a poster at the ECCO 15 - 34th ESMO Multidisciplinary Congress. The data suggest that a supratherapeutic single dose (800 mg) of this orally active histone deacetylase inhibitor does not prolong ventricular repolarization to a significant degree. This is reassuring, since cardiac rhythm and EEG changes are thought to be a class effect of HDACs.
Lead author David Siegel, MD, from Hackensack University Medical Center, Hackensack, New Jersey, and his fellow researchers observed that the study data support the overall safety profile of vorinostat use in cancer patients.
They based their analysis on 18 phase 1 and phase 2 vorinostat trials that included 498 patients, 341 who received the agent as monotherapy and 157 treated with the drug in combination with other therapies.
Vorinostat is approved by the US Food and Drug Administration to treat relapsed or refractory cutaneous T-cell lymphoma and was dosed at the approved level of 400 mg/day for 156 of the 341 patients in the monotherapy cohort. In the combination group, vorinostat was given on weekly or 2-weekly schedules instead of continuous dosing.
In the monotherapy group, the most commonly reported treatment-related adverse events were fatigue (61.9%), nausea (55.7%), diarrhea (49.3%), and anorexia (48.1%). The most common grade 3/4 adverse events were fatigue (12.0%), thrombocytopenia (10.6%), dehydration (7.0%), decreased platelet count (5.3%), and anorexia (5.0%).
Seventy-one (20.8%) patients required dose modifications for toxicity and 38 (11.1%) discontinued study medication due to drug-related adverse events. Three drug-related adverse events led to death.
In the combination treatment cohort, nausea (48.4%), diarrhea (40.8%), fatigue (34.4%), and vomiting (31.2%) were the most commonly reported adverse events. The most common grade 3/4 adverse events were fatigue (13.4%), thrombocytopenia (9.6%), neutropenia (8.3%), diarrhea (5.7%), and nausea.
Dose modifications were required in 27 patients (17.2%). Discontinuation due to adverse events was necessary in 31 patients (19.7%), and 1 death was attributed to vorinostat combination treatment.
The QTc phase 1 substudy was randomized, partially blind, and placebo-controlled. None of the 22 evaluable patients included in the analysis experienced a QtcF change greater than 30 msec from their baseline scores. ![]()
Berlin, Germany—Vorinostat demonstrates safety and tolerability alone and in combination with other systemic treatments for a wide range of solid and hematologic malignancies, according to a study of collated data from the vorinostat clinical trial program.
Investigators presented the safety data in a poster at the ECCO 15 - 34th ESMO Multidisciplinary Congress. The data suggest that a supratherapeutic single dose (800 mg) of this orally active histone deacetylase inhibitor does not prolong ventricular repolarization to a significant degree. This is reassuring, since cardiac rhythm and EEG changes are thought to be a class effect of HDACs.
Lead author David Siegel, MD, from Hackensack University Medical Center, Hackensack, New Jersey, and his fellow researchers observed that the study data support the overall safety profile of vorinostat use in cancer patients.
They based their analysis on 18 phase 1 and phase 2 vorinostat trials that included 498 patients, 341 who received the agent as monotherapy and 157 treated with the drug in combination with other therapies.
Vorinostat is approved by the US Food and Drug Administration to treat relapsed or refractory cutaneous T-cell lymphoma and was dosed at the approved level of 400 mg/day for 156 of the 341 patients in the monotherapy cohort. In the combination group, vorinostat was given on weekly or 2-weekly schedules instead of continuous dosing.
In the monotherapy group, the most commonly reported treatment-related adverse events were fatigue (61.9%), nausea (55.7%), diarrhea (49.3%), and anorexia (48.1%). The most common grade 3/4 adverse events were fatigue (12.0%), thrombocytopenia (10.6%), dehydration (7.0%), decreased platelet count (5.3%), and anorexia (5.0%).
Seventy-one (20.8%) patients required dose modifications for toxicity and 38 (11.1%) discontinued study medication due to drug-related adverse events. Three drug-related adverse events led to death.
In the combination treatment cohort, nausea (48.4%), diarrhea (40.8%), fatigue (34.4%), and vomiting (31.2%) were the most commonly reported adverse events. The most common grade 3/4 adverse events were fatigue (13.4%), thrombocytopenia (9.6%), neutropenia (8.3%), diarrhea (5.7%), and nausea.
Dose modifications were required in 27 patients (17.2%). Discontinuation due to adverse events was necessary in 31 patients (19.7%), and 1 death was attributed to vorinostat combination treatment.
The QTc phase 1 substudy was randomized, partially blind, and placebo-controlled. None of the 22 evaluable patients included in the analysis experienced a QtcF change greater than 30 msec from their baseline scores. ![]()
Berlin, Germany—Vorinostat demonstrates safety and tolerability alone and in combination with other systemic treatments for a wide range of solid and hematologic malignancies, according to a study of collated data from the vorinostat clinical trial program.
Investigators presented the safety data in a poster at the ECCO 15 - 34th ESMO Multidisciplinary Congress. The data suggest that a supratherapeutic single dose (800 mg) of this orally active histone deacetylase inhibitor does not prolong ventricular repolarization to a significant degree. This is reassuring, since cardiac rhythm and EEG changes are thought to be a class effect of HDACs.
Lead author David Siegel, MD, from Hackensack University Medical Center, Hackensack, New Jersey, and his fellow researchers observed that the study data support the overall safety profile of vorinostat use in cancer patients.
They based their analysis on 18 phase 1 and phase 2 vorinostat trials that included 498 patients, 341 who received the agent as monotherapy and 157 treated with the drug in combination with other therapies.
Vorinostat is approved by the US Food and Drug Administration to treat relapsed or refractory cutaneous T-cell lymphoma and was dosed at the approved level of 400 mg/day for 156 of the 341 patients in the monotherapy cohort. In the combination group, vorinostat was given on weekly or 2-weekly schedules instead of continuous dosing.
In the monotherapy group, the most commonly reported treatment-related adverse events were fatigue (61.9%), nausea (55.7%), diarrhea (49.3%), and anorexia (48.1%). The most common grade 3/4 adverse events were fatigue (12.0%), thrombocytopenia (10.6%), dehydration (7.0%), decreased platelet count (5.3%), and anorexia (5.0%).
Seventy-one (20.8%) patients required dose modifications for toxicity and 38 (11.1%) discontinued study medication due to drug-related adverse events. Three drug-related adverse events led to death.
In the combination treatment cohort, nausea (48.4%), diarrhea (40.8%), fatigue (34.4%), and vomiting (31.2%) were the most commonly reported adverse events. The most common grade 3/4 adverse events were fatigue (13.4%), thrombocytopenia (9.6%), neutropenia (8.3%), diarrhea (5.7%), and nausea.
Dose modifications were required in 27 patients (17.2%). Discontinuation due to adverse events was necessary in 31 patients (19.7%), and 1 death was attributed to vorinostat combination treatment.
The QTc phase 1 substudy was randomized, partially blind, and placebo-controlled. None of the 22 evaluable patients included in the analysis experienced a QtcF change greater than 30 msec from their baseline scores. ![]()
Algorithm for Success
The use of a procalcitonin (PCT) algorithm reduced the usage of antibiotics in patients with lower-respiratory-tract infections (LTRI), according to a recent study that may highlight a new way for hospitalists to reduce costs.
The study found the mean duration of antibiotics exposure in the PCT group was lower than in a control group (5.7 days vs. 8.7 days). The researchers, who studied 1,359 patients at six tertiary-care hospitals in Switzerland, also reported less-frequent antibiotic-associated adverse effects, such as nausea, rashes or diarrhea, in the PCT group (JAMA. 2009;302(10):1059-1066).
Scott Flanders, MD, FHM, SHM president and director of the hospitalist program at the University of Michigan Health System in Ann Arbor, says if further review were to show more statistical impacts on costs savings, PCT usage would become more common.
"If you can reduce length-of-stay by half through treatment intervention, then this will easily pay for itself," says Dr. Flanders, who adds, "Hospitalists need to know and have at their fingertips the best avenues of treatment."
Devendra Amin, MD, director of critical-care services at Morton Plant Hospital in Clearwater, Fla., was one of the first physicians to use PCT tests after the Food and Drug Administration (FDA) approved wider usage last year. He says the overuse of antibiotics is a needless cost overrun that hospitalists using PCT tests could better control—and then tout as an example of their ability to reduce costs. Dr. Amin plans to team with a half-dozen of his health system's hospitalists next year to work on a study of the effectiveness of PCT in a community hospital setting.
"If everything else fits, it's another piece of information that's important to the puzzle," Dr. Amin says. "No single test in isolation is going to give you everything you want … but this can help."
The use of a procalcitonin (PCT) algorithm reduced the usage of antibiotics in patients with lower-respiratory-tract infections (LTRI), according to a recent study that may highlight a new way for hospitalists to reduce costs.
The study found the mean duration of antibiotics exposure in the PCT group was lower than in a control group (5.7 days vs. 8.7 days). The researchers, who studied 1,359 patients at six tertiary-care hospitals in Switzerland, also reported less-frequent antibiotic-associated adverse effects, such as nausea, rashes or diarrhea, in the PCT group (JAMA. 2009;302(10):1059-1066).
Scott Flanders, MD, FHM, SHM president and director of the hospitalist program at the University of Michigan Health System in Ann Arbor, says if further review were to show more statistical impacts on costs savings, PCT usage would become more common.
"If you can reduce length-of-stay by half through treatment intervention, then this will easily pay for itself," says Dr. Flanders, who adds, "Hospitalists need to know and have at their fingertips the best avenues of treatment."
Devendra Amin, MD, director of critical-care services at Morton Plant Hospital in Clearwater, Fla., was one of the first physicians to use PCT tests after the Food and Drug Administration (FDA) approved wider usage last year. He says the overuse of antibiotics is a needless cost overrun that hospitalists using PCT tests could better control—and then tout as an example of their ability to reduce costs. Dr. Amin plans to team with a half-dozen of his health system's hospitalists next year to work on a study of the effectiveness of PCT in a community hospital setting.
"If everything else fits, it's another piece of information that's important to the puzzle," Dr. Amin says. "No single test in isolation is going to give you everything you want … but this can help."
The use of a procalcitonin (PCT) algorithm reduced the usage of antibiotics in patients with lower-respiratory-tract infections (LTRI), according to a recent study that may highlight a new way for hospitalists to reduce costs.
The study found the mean duration of antibiotics exposure in the PCT group was lower than in a control group (5.7 days vs. 8.7 days). The researchers, who studied 1,359 patients at six tertiary-care hospitals in Switzerland, also reported less-frequent antibiotic-associated adverse effects, such as nausea, rashes or diarrhea, in the PCT group (JAMA. 2009;302(10):1059-1066).
Scott Flanders, MD, FHM, SHM president and director of the hospitalist program at the University of Michigan Health System in Ann Arbor, says if further review were to show more statistical impacts on costs savings, PCT usage would become more common.
"If you can reduce length-of-stay by half through treatment intervention, then this will easily pay for itself," says Dr. Flanders, who adds, "Hospitalists need to know and have at their fingertips the best avenues of treatment."
Devendra Amin, MD, director of critical-care services at Morton Plant Hospital in Clearwater, Fla., was one of the first physicians to use PCT tests after the Food and Drug Administration (FDA) approved wider usage last year. He says the overuse of antibiotics is a needless cost overrun that hospitalists using PCT tests could better control—and then tout as an example of their ability to reduce costs. Dr. Amin plans to team with a half-dozen of his health system's hospitalists next year to work on a study of the effectiveness of PCT in a community hospital setting.
"If everything else fits, it's another piece of information that's important to the puzzle," Dr. Amin says. "No single test in isolation is going to give you everything you want … but this can help."
Recession? What Recession?
The economy might be in the doldrums, but recruiters are looking for candidates to fill HM positions, says Mark Dotson, senior director of recruitment for Cogent Healthcare, a Brentwood, Tenn.-based company that manages HM programs nationwide. He recently spoke with The Hospitalist eWire about how hospitalists can take advantage of the bullish job market.
Question: What do you look for in HM job candidates?
Answer: We obviously look at their credentials, their training, and the focus of their training in inpatient medicine. We strive to look for physicians who are able to and interested in working in a team environment. They should have a good bedside manner and good communication skills. They should be able to show they have a team-based approach to their work.
Q: What alternative jobs are there in HM that hospitalists might not know about?
A: There are sometimes opportunities to chair a committee that hospitalists aren't aware of. There are also ways to get involved with more specialties by working with physicians on the hospital campus and building relationships with them.
Q: Has the current economic climate affected hospitalist recruiting?
A: Not so much. The demand is still there. But I do think more hospitalists aren’t looking to make a change, because they want stability in their workplace right now. Hospital medicine is a specialty that's growing, so there is stability. Hospitalists have to decide what’s best for their clinical skills and personal interests and not let the economy stop them. There are a hundred more opportunities out there waiting for them.
The economy might be in the doldrums, but recruiters are looking for candidates to fill HM positions, says Mark Dotson, senior director of recruitment for Cogent Healthcare, a Brentwood, Tenn.-based company that manages HM programs nationwide. He recently spoke with The Hospitalist eWire about how hospitalists can take advantage of the bullish job market.
Question: What do you look for in HM job candidates?
Answer: We obviously look at their credentials, their training, and the focus of their training in inpatient medicine. We strive to look for physicians who are able to and interested in working in a team environment. They should have a good bedside manner and good communication skills. They should be able to show they have a team-based approach to their work.
Q: What alternative jobs are there in HM that hospitalists might not know about?
A: There are sometimes opportunities to chair a committee that hospitalists aren't aware of. There are also ways to get involved with more specialties by working with physicians on the hospital campus and building relationships with them.
Q: Has the current economic climate affected hospitalist recruiting?
A: Not so much. The demand is still there. But I do think more hospitalists aren’t looking to make a change, because they want stability in their workplace right now. Hospital medicine is a specialty that's growing, so there is stability. Hospitalists have to decide what’s best for their clinical skills and personal interests and not let the economy stop them. There are a hundred more opportunities out there waiting for them.
The economy might be in the doldrums, but recruiters are looking for candidates to fill HM positions, says Mark Dotson, senior director of recruitment for Cogent Healthcare, a Brentwood, Tenn.-based company that manages HM programs nationwide. He recently spoke with The Hospitalist eWire about how hospitalists can take advantage of the bullish job market.
Question: What do you look for in HM job candidates?
Answer: We obviously look at their credentials, their training, and the focus of their training in inpatient medicine. We strive to look for physicians who are able to and interested in working in a team environment. They should have a good bedside manner and good communication skills. They should be able to show they have a team-based approach to their work.
Q: What alternative jobs are there in HM that hospitalists might not know about?
A: There are sometimes opportunities to chair a committee that hospitalists aren't aware of. There are also ways to get involved with more specialties by working with physicians on the hospital campus and building relationships with them.
Q: Has the current economic climate affected hospitalist recruiting?
A: Not so much. The demand is still there. But I do think more hospitalists aren’t looking to make a change, because they want stability in their workplace right now. Hospital medicine is a specialty that's growing, so there is stability. Hospitalists have to decide what’s best for their clinical skills and personal interests and not let the economy stop them. There are a hundred more opportunities out there waiting for them.
Budget Checkup
Editor’s note: Part one of a two-part series.
Why does a particular hospitalist practice require more than the typical amount of financial support from a hospital? This is one of the most common questions I am asked. This month and next, I will provide a thorough list of potential answers.
SHM’s “2007-2008 Bi-annual Survey on the State of the Hospital Medicine Movement” showed that hospitals pay an average of $97,400 per year in support per full-time hospitalist. I suspect that amount is higher now. Nevertheless, hospital executives and hospitalists should understand the reasons why the hospital support that is required for their practice might be more or less.
A comprehensive list of potential reasons would include dozens of factors, and my intent is only to highlight some of the most common and significant ones.
Documentation, Coding, Billing, and Collecting
This is an area in which many, if not most, practices have room for improvement. One very simple way to estimate how your group is doing on these things is to think about how you’re performing on the following tasks:
- Do the hospitalists really understand the documentation requirements for each CPT code, and is their performance in selecting CPT codes audited regularly (e.g., annually)?1
- Does the group have a reliable method of charge capture that minimizes problems like lost charges? Is there an established “chain of custody” of this information, from the hospitalist to the biller?
- Is there a rigorous review or audit of the biller’s performance? Does the group monitor metrics, such as days in accounts receivable, collection rate, etc.? Is there a periodic audit of the biller? An audit could be as simple as tracking down five to 10 billed encounters from six months prior for each doctor in the practice, and reviewing the status of each bill (e.g., paid, written off, or perhaps the bill has vanished or never made it into the billing system).
- Is revenue appropriately applied to the hospitalist cost center? For many hospital-employed hospitalists, payors might be including their professional fee payments on the same remittance advice as hospital inpatient payments (due to same tax ID number). The hospital’s business office might be unable or unwilling to break these payments into hospital and professional fee portions and apply them correctly. Hospital-employed hospitalists should know whether their collections are being applied to their revenue center accurately.
Payor Mix
The two factors that govern the amount of professional fee revenues a hospitalist practice will collect are the integrity of the billing process (described above) and the payor mix. The payor mix for most hospitalist practices is roughly 55% to 60% Medicare, 5% to 10% self-pay, 5% to 10% Medicaid, and commercial insurance for the rest.
A hospitalist practice that is significantly different from this example should expect professional fee collections to vary accordingly.
Hospitalist Fee Schedule
My experience is that very few hospitalists know their own fee schedule. The term “fee schedule” is generally used to mean the billed charge for each type of service provided. A hospitalist fee schedule usually fits on a single page, with a list of CPT codes (admits, consults, followups, etc.) down one column and the charge for that service in a second column to the right. It would be reasonable to post the fee schedule in hospitalists’ offices.
Groups that use electronic charge capture, in which the doctor enters into a computer the CPT code to bill for each patient daily, can often see the related charge for each code as it is entered.
Someone connected to the practice, often in the billing office, should review the fee schedule—at least annually—to ensure that services aren’t being billed below the rate allowed by payors.
Negotiated Rates Paid by Commercial Insurance
Some hospitalist groups are able to negotiate higher payments than the typical rates paid by commercial payors. Because commercial insurance is a relatively small portion of most hospitalists’ payor mix, this might not have a large impact on the overall practice finances. So my sense is that most groups don’t pursue this opportunity.
Groups in markets with significant managed care are an exception. They usually are aggressive in negotiations for commercial payor rates.
Some hospital-employed HM groups might end up with lower commercial rates than they could have. Here is how it might happen: A hospital negotiates with Aetna to pay rates for hospital services (the bills submitted by the hospital, not the physician bills) that are attractive to the hospital. To make this proposal more palatable to Aetna, the hospital says it will accept lower rates for its employed physicians, including hospitalists. So the hospitalists’ collections end up lower, and the support paid by the hospital to the hospitalist group is correspondingly higher. The hospital ends up fine in this scenario, because it is being paid an attractive rate by Aetna for hospital services, but the hospitalist practice appears to be underperforming financially.
It is worth knowing if this is an issue at your practice, but in most cases it won’t explain larger problems in the hospitalist budget or amount of support required from the hospital.
Accounting Issues
Budgets and financial statements can be confusing, and revenues and expenses might not be what you expect. For example, in my practice, auditors told our accountants that we needed to accrue an extra month of salary into this year’s budget. So when looking at our fiscal year-end financial statement, the salary expense is for 13 months instead of 12 months. This quirk made it appear that we required more than the budgeted amount of support from our hospital, when in fact we performed better than budget this year.
I certainly can’t explain all the reasons for unusual accounting issues, and I still struggle to understand why accrual accounting is better than cash-basis accounting. My best advice is to have the lead hospitalist in your group get to know the accountant who handles your budget and financial statements. The accountant should explain all of these issues clearly.
In next month’s column, I’ll review how a hospitalist practice’s internal operations, such as staffing and scheduling, can have a major influence on the budget and the amount of support required from the hospital. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program.” This column represents his views and is not intended to reflect an official position of SHM.
Reference
- Centers for Medicare and Medicaid Services. Improper medicare fee-for-service payments report, November 2006: long report. CMS Web site. Available at: www.cms.hhs.gov/apps/er_report/preview_er_report_print.asp?from=public&which=long&reportID=5. Accessed Sept. 1, 2009.
Editor’s note: Part one of a two-part series.
Why does a particular hospitalist practice require more than the typical amount of financial support from a hospital? This is one of the most common questions I am asked. This month and next, I will provide a thorough list of potential answers.
SHM’s “2007-2008 Bi-annual Survey on the State of the Hospital Medicine Movement” showed that hospitals pay an average of $97,400 per year in support per full-time hospitalist. I suspect that amount is higher now. Nevertheless, hospital executives and hospitalists should understand the reasons why the hospital support that is required for their practice might be more or less.
A comprehensive list of potential reasons would include dozens of factors, and my intent is only to highlight some of the most common and significant ones.
Documentation, Coding, Billing, and Collecting
This is an area in which many, if not most, practices have room for improvement. One very simple way to estimate how your group is doing on these things is to think about how you’re performing on the following tasks:
- Do the hospitalists really understand the documentation requirements for each CPT code, and is their performance in selecting CPT codes audited regularly (e.g., annually)?1
- Does the group have a reliable method of charge capture that minimizes problems like lost charges? Is there an established “chain of custody” of this information, from the hospitalist to the biller?
- Is there a rigorous review or audit of the biller’s performance? Does the group monitor metrics, such as days in accounts receivable, collection rate, etc.? Is there a periodic audit of the biller? An audit could be as simple as tracking down five to 10 billed encounters from six months prior for each doctor in the practice, and reviewing the status of each bill (e.g., paid, written off, or perhaps the bill has vanished or never made it into the billing system).
- Is revenue appropriately applied to the hospitalist cost center? For many hospital-employed hospitalists, payors might be including their professional fee payments on the same remittance advice as hospital inpatient payments (due to same tax ID number). The hospital’s business office might be unable or unwilling to break these payments into hospital and professional fee portions and apply them correctly. Hospital-employed hospitalists should know whether their collections are being applied to their revenue center accurately.
Payor Mix
The two factors that govern the amount of professional fee revenues a hospitalist practice will collect are the integrity of the billing process (described above) and the payor mix. The payor mix for most hospitalist practices is roughly 55% to 60% Medicare, 5% to 10% self-pay, 5% to 10% Medicaid, and commercial insurance for the rest.
A hospitalist practice that is significantly different from this example should expect professional fee collections to vary accordingly.
Hospitalist Fee Schedule
My experience is that very few hospitalists know their own fee schedule. The term “fee schedule” is generally used to mean the billed charge for each type of service provided. A hospitalist fee schedule usually fits on a single page, with a list of CPT codes (admits, consults, followups, etc.) down one column and the charge for that service in a second column to the right. It would be reasonable to post the fee schedule in hospitalists’ offices.
Groups that use electronic charge capture, in which the doctor enters into a computer the CPT code to bill for each patient daily, can often see the related charge for each code as it is entered.
Someone connected to the practice, often in the billing office, should review the fee schedule—at least annually—to ensure that services aren’t being billed below the rate allowed by payors.
Negotiated Rates Paid by Commercial Insurance
Some hospitalist groups are able to negotiate higher payments than the typical rates paid by commercial payors. Because commercial insurance is a relatively small portion of most hospitalists’ payor mix, this might not have a large impact on the overall practice finances. So my sense is that most groups don’t pursue this opportunity.
Groups in markets with significant managed care are an exception. They usually are aggressive in negotiations for commercial payor rates.
Some hospital-employed HM groups might end up with lower commercial rates than they could have. Here is how it might happen: A hospital negotiates with Aetna to pay rates for hospital services (the bills submitted by the hospital, not the physician bills) that are attractive to the hospital. To make this proposal more palatable to Aetna, the hospital says it will accept lower rates for its employed physicians, including hospitalists. So the hospitalists’ collections end up lower, and the support paid by the hospital to the hospitalist group is correspondingly higher. The hospital ends up fine in this scenario, because it is being paid an attractive rate by Aetna for hospital services, but the hospitalist practice appears to be underperforming financially.
It is worth knowing if this is an issue at your practice, but in most cases it won’t explain larger problems in the hospitalist budget or amount of support required from the hospital.
Accounting Issues
Budgets and financial statements can be confusing, and revenues and expenses might not be what you expect. For example, in my practice, auditors told our accountants that we needed to accrue an extra month of salary into this year’s budget. So when looking at our fiscal year-end financial statement, the salary expense is for 13 months instead of 12 months. This quirk made it appear that we required more than the budgeted amount of support from our hospital, when in fact we performed better than budget this year.
I certainly can’t explain all the reasons for unusual accounting issues, and I still struggle to understand why accrual accounting is better than cash-basis accounting. My best advice is to have the lead hospitalist in your group get to know the accountant who handles your budget and financial statements. The accountant should explain all of these issues clearly.
In next month’s column, I’ll review how a hospitalist practice’s internal operations, such as staffing and scheduling, can have a major influence on the budget and the amount of support required from the hospital. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program.” This column represents his views and is not intended to reflect an official position of SHM.
Reference
- Centers for Medicare and Medicaid Services. Improper medicare fee-for-service payments report, November 2006: long report. CMS Web site. Available at: www.cms.hhs.gov/apps/er_report/preview_er_report_print.asp?from=public&which=long&reportID=5. Accessed Sept. 1, 2009.
Editor’s note: Part one of a two-part series.
Why does a particular hospitalist practice require more than the typical amount of financial support from a hospital? This is one of the most common questions I am asked. This month and next, I will provide a thorough list of potential answers.
SHM’s “2007-2008 Bi-annual Survey on the State of the Hospital Medicine Movement” showed that hospitals pay an average of $97,400 per year in support per full-time hospitalist. I suspect that amount is higher now. Nevertheless, hospital executives and hospitalists should understand the reasons why the hospital support that is required for their practice might be more or less.
A comprehensive list of potential reasons would include dozens of factors, and my intent is only to highlight some of the most common and significant ones.
Documentation, Coding, Billing, and Collecting
This is an area in which many, if not most, practices have room for improvement. One very simple way to estimate how your group is doing on these things is to think about how you’re performing on the following tasks:
- Do the hospitalists really understand the documentation requirements for each CPT code, and is their performance in selecting CPT codes audited regularly (e.g., annually)?1
- Does the group have a reliable method of charge capture that minimizes problems like lost charges? Is there an established “chain of custody” of this information, from the hospitalist to the biller?
- Is there a rigorous review or audit of the biller’s performance? Does the group monitor metrics, such as days in accounts receivable, collection rate, etc.? Is there a periodic audit of the biller? An audit could be as simple as tracking down five to 10 billed encounters from six months prior for each doctor in the practice, and reviewing the status of each bill (e.g., paid, written off, or perhaps the bill has vanished or never made it into the billing system).
- Is revenue appropriately applied to the hospitalist cost center? For many hospital-employed hospitalists, payors might be including their professional fee payments on the same remittance advice as hospital inpatient payments (due to same tax ID number). The hospital’s business office might be unable or unwilling to break these payments into hospital and professional fee portions and apply them correctly. Hospital-employed hospitalists should know whether their collections are being applied to their revenue center accurately.
Payor Mix
The two factors that govern the amount of professional fee revenues a hospitalist practice will collect are the integrity of the billing process (described above) and the payor mix. The payor mix for most hospitalist practices is roughly 55% to 60% Medicare, 5% to 10% self-pay, 5% to 10% Medicaid, and commercial insurance for the rest.
A hospitalist practice that is significantly different from this example should expect professional fee collections to vary accordingly.
Hospitalist Fee Schedule
My experience is that very few hospitalists know their own fee schedule. The term “fee schedule” is generally used to mean the billed charge for each type of service provided. A hospitalist fee schedule usually fits on a single page, with a list of CPT codes (admits, consults, followups, etc.) down one column and the charge for that service in a second column to the right. It would be reasonable to post the fee schedule in hospitalists’ offices.
Groups that use electronic charge capture, in which the doctor enters into a computer the CPT code to bill for each patient daily, can often see the related charge for each code as it is entered.
Someone connected to the practice, often in the billing office, should review the fee schedule—at least annually—to ensure that services aren’t being billed below the rate allowed by payors.
Negotiated Rates Paid by Commercial Insurance
Some hospitalist groups are able to negotiate higher payments than the typical rates paid by commercial payors. Because commercial insurance is a relatively small portion of most hospitalists’ payor mix, this might not have a large impact on the overall practice finances. So my sense is that most groups don’t pursue this opportunity.
Groups in markets with significant managed care are an exception. They usually are aggressive in negotiations for commercial payor rates.
Some hospital-employed HM groups might end up with lower commercial rates than they could have. Here is how it might happen: A hospital negotiates with Aetna to pay rates for hospital services (the bills submitted by the hospital, not the physician bills) that are attractive to the hospital. To make this proposal more palatable to Aetna, the hospital says it will accept lower rates for its employed physicians, including hospitalists. So the hospitalists’ collections end up lower, and the support paid by the hospital to the hospitalist group is correspondingly higher. The hospital ends up fine in this scenario, because it is being paid an attractive rate by Aetna for hospital services, but the hospitalist practice appears to be underperforming financially.
It is worth knowing if this is an issue at your practice, but in most cases it won’t explain larger problems in the hospitalist budget or amount of support required from the hospital.
Accounting Issues
Budgets and financial statements can be confusing, and revenues and expenses might not be what you expect. For example, in my practice, auditors told our accountants that we needed to accrue an extra month of salary into this year’s budget. So when looking at our fiscal year-end financial statement, the salary expense is for 13 months instead of 12 months. This quirk made it appear that we required more than the budgeted amount of support from our hospital, when in fact we performed better than budget this year.
I certainly can’t explain all the reasons for unusual accounting issues, and I still struggle to understand why accrual accounting is better than cash-basis accounting. My best advice is to have the lead hospitalist in your group get to know the accountant who handles your budget and financial statements. The accountant should explain all of these issues clearly.
In next month’s column, I’ll review how a hospitalist practice’s internal operations, such as staffing and scheduling, can have a major influence on the budget and the amount of support required from the hospital. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program.” This column represents his views and is not intended to reflect an official position of SHM.
Reference
- Centers for Medicare and Medicaid Services. Improper medicare fee-for-service payments report, November 2006: long report. CMS Web site. Available at: www.cms.hhs.gov/apps/er_report/preview_er_report_print.asp?from=public&which=long&reportID=5. Accessed Sept. 1, 2009.
Spanish Flu Redux?
Apocalypse, pestilence, death. As I head back to work after a late-summer vacation, those words are on the tip of my tongue. Now before your mind drifts too far afield, this is not a synopsis of the time spent with family, or even my in-laws—although some have used similar words to describe my mother’s cooking. Rather, these are the descriptors of my vacation reading.
Summer Reading
I started the week relaxing contentedly with Cormac McCarthy’s “The Road.” I chose this book in part because I noticed that it soon will be released as a movie, but mostly because it had won the dustiest-book-in-my-office-reading-pile award. This tale of a young boy and his father traversing a post-apocalyptic America was shocking and surreal. I couldn’t help but interchange images of my 2-year-old son, Greyson, and me out on that road fighting for our existence. In my personal fictional account, I continuously, and heroically, MacGyver my way across a burned-out and treacherous landscape with death-defying adeptness—all the while Grey unknowingly totters, drooling and muttering in tow.
Reality, of course, would paint us in substantially different roles, with mine involving the lion’s share of muttering and drooling, leaving Grey wishing the apocalyptic dealer had dealt him his mother instead.
Next up, “The Last Town on Earth,” by Thomas Mullen. I don’t recall how this book got into my reading pile, but I’m glad it did. The story is set in the fictional city of Commonwealth in 1918. The small, isolated mill town makes the drastic decision to stanch the tide of Spanish flu by cutting itself off from civilization through a self-imposed quarantine.
It is here, on p. 98, that I was sidetracked by a family member’s question—“Do you think this swine flu will be as bad as the Spanish flu?” I was asked shortly after being inquired about my reading choice. “Of course not,” I replied knowingly, moments before realizing I didn’t know. In fact, I didn’t have the faintest idea—not because it’s tough to divine the future, but because I realized I had little more than a passing knowledge of the famous flu that raked the world early last century. And with that, I was off on my final vacation reading session—a quest to slake my thirst for influenza knowledge.
Flu Pandemic
The Spanish flu pandemic of 1918-1920 was the first of three to hit in the 20th century. It took its name not from its site of origin (debated but generally felt to be the U.S., Kansas specifically), but rather from the fact that Spain, a neutral country in World War I, had the most uncensored lines of communication, so the most credible news of the disease came from that country. This provided the false impression that Spain was the only—or at least most dramatically—affected country. Like today’s swine flu, the Spanish flu was an H1N1 influenza. To sate your inner microbiologist, this means the virus exhibits the first of 16 subtypes of hemagglutinin (H) and nine subtypes of neuraminidase (N). Generally, only H1, H2, H3, and N1 and N2 affect humans, and tend to cause mild disease in otherwise healthy populations, killing the immunocompromised, the very young, and the very old. This typically results in a case-fatality rate of about 0.1% and 250,000 to 500,000 deaths worldwide annually.
The Spanish flu, however, was different. For reasons that are not entirely clear, the Spanish flu struck in two waves. The first wave, in the spring of 1918, induced typical flu-like illness with generally mild outcomes, except for the immunocompromised. The second wave was unusual for two reasons. First, it began in the late summer of 1918, rather than the typical winter pattern seen in North America. Second, it was much more deadly, inducing what has been termed a cytokine storm. This immunological avalanche produced more severe disease in the immunopotent young, healthy populations—resulting in its unprecedented mortality in this cohort. In fact, upward of 99% of all Spanish flu deaths were in people younger than 65.
In the end, the pandemic left a broad swath of destruction in its wake. It is estimated that 500 million people—one-third of the world’s population at the time—were infected. The mortality rate was 10% to 20%, resulting in 50 million to 100 million deaths. Put another way, the Spanish flu killed 5% of humanity.
It did so rapidly. Nearly 1 million people died per week in the first 25 weeks of the second wave. To put it in perspective, it took HIV 25 years to reach that number. Thus, historians have termed the Spanish flu “the greatest medical holocaust in history.”
And then as quickly as it commenced, it abated. For example, in Philadelphia, there were about 5,000 flu deaths in one week in October 1918, yet a month later, the virus had nearly disappeared from the city. It’s not clear why this happened, but prevailing theories postulate that either the medical community got better at managing its mortal complications (e.g., bacterial pneumonia), or the bug itself mutated to a less virulent strain.
Is the Swine Flu our Spanish Flu?
On June 11, 2009, the World Health Organization (WHO) declared that the current H1N1 flu virus had reached pandemic status. This novel H1N1 serotype appears to be a direct descendent of the Spanish H1N1 subtype, but the new strain also combines genetic material culled from swine and birds reassorted in a manner that results in limited innate human defenses. And like the Spanish variant, it appears this new strain is hitting earlier in the year than usual and disproportionately affecting the young, with about two-thirds of U.S. deaths coming in the 25- to 64-year-old demographic.
So can we expect hundreds of millions of deaths from swine flu? Probably not. The WHO has been cautious to note that the upgrade to pandemic status was based on the rapidity and ease of spread, not the lethality of the virus. Furthermore, the Centers for Disease Control and Prevention (CDC)—which publishes a wonderful weekly update called FluView (www.cdc.gov/flu/weekly/)—notes that while the number of doctor’s visits for influenza-like illnesses through mid-August is unusually high, the rates of hospitalizations and proportion of deaths attributed to pneumonia and influenza are low and within normal limits for this time of year. Further, the virus continues in its original form, meaning it has not mutated, become more resistant to antiviral drugs, or been altered from the viruses selected for the 2009 vaccine.
So while we certainly must brace for the worst, I feel comfortable in the answer I provided my family member. I also am confident that Grey won’t be quarantined or left to roam the barren Earth anytime soon. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
Apocalypse, pestilence, death. As I head back to work after a late-summer vacation, those words are on the tip of my tongue. Now before your mind drifts too far afield, this is not a synopsis of the time spent with family, or even my in-laws—although some have used similar words to describe my mother’s cooking. Rather, these are the descriptors of my vacation reading.
Summer Reading
I started the week relaxing contentedly with Cormac McCarthy’s “The Road.” I chose this book in part because I noticed that it soon will be released as a movie, but mostly because it had won the dustiest-book-in-my-office-reading-pile award. This tale of a young boy and his father traversing a post-apocalyptic America was shocking and surreal. I couldn’t help but interchange images of my 2-year-old son, Greyson, and me out on that road fighting for our existence. In my personal fictional account, I continuously, and heroically, MacGyver my way across a burned-out and treacherous landscape with death-defying adeptness—all the while Grey unknowingly totters, drooling and muttering in tow.
Reality, of course, would paint us in substantially different roles, with mine involving the lion’s share of muttering and drooling, leaving Grey wishing the apocalyptic dealer had dealt him his mother instead.
Next up, “The Last Town on Earth,” by Thomas Mullen. I don’t recall how this book got into my reading pile, but I’m glad it did. The story is set in the fictional city of Commonwealth in 1918. The small, isolated mill town makes the drastic decision to stanch the tide of Spanish flu by cutting itself off from civilization through a self-imposed quarantine.
It is here, on p. 98, that I was sidetracked by a family member’s question—“Do you think this swine flu will be as bad as the Spanish flu?” I was asked shortly after being inquired about my reading choice. “Of course not,” I replied knowingly, moments before realizing I didn’t know. In fact, I didn’t have the faintest idea—not because it’s tough to divine the future, but because I realized I had little more than a passing knowledge of the famous flu that raked the world early last century. And with that, I was off on my final vacation reading session—a quest to slake my thirst for influenza knowledge.
Flu Pandemic
The Spanish flu pandemic of 1918-1920 was the first of three to hit in the 20th century. It took its name not from its site of origin (debated but generally felt to be the U.S., Kansas specifically), but rather from the fact that Spain, a neutral country in World War I, had the most uncensored lines of communication, so the most credible news of the disease came from that country. This provided the false impression that Spain was the only—or at least most dramatically—affected country. Like today’s swine flu, the Spanish flu was an H1N1 influenza. To sate your inner microbiologist, this means the virus exhibits the first of 16 subtypes of hemagglutinin (H) and nine subtypes of neuraminidase (N). Generally, only H1, H2, H3, and N1 and N2 affect humans, and tend to cause mild disease in otherwise healthy populations, killing the immunocompromised, the very young, and the very old. This typically results in a case-fatality rate of about 0.1% and 250,000 to 500,000 deaths worldwide annually.
The Spanish flu, however, was different. For reasons that are not entirely clear, the Spanish flu struck in two waves. The first wave, in the spring of 1918, induced typical flu-like illness with generally mild outcomes, except for the immunocompromised. The second wave was unusual for two reasons. First, it began in the late summer of 1918, rather than the typical winter pattern seen in North America. Second, it was much more deadly, inducing what has been termed a cytokine storm. This immunological avalanche produced more severe disease in the immunopotent young, healthy populations—resulting in its unprecedented mortality in this cohort. In fact, upward of 99% of all Spanish flu deaths were in people younger than 65.
In the end, the pandemic left a broad swath of destruction in its wake. It is estimated that 500 million people—one-third of the world’s population at the time—were infected. The mortality rate was 10% to 20%, resulting in 50 million to 100 million deaths. Put another way, the Spanish flu killed 5% of humanity.
It did so rapidly. Nearly 1 million people died per week in the first 25 weeks of the second wave. To put it in perspective, it took HIV 25 years to reach that number. Thus, historians have termed the Spanish flu “the greatest medical holocaust in history.”
And then as quickly as it commenced, it abated. For example, in Philadelphia, there were about 5,000 flu deaths in one week in October 1918, yet a month later, the virus had nearly disappeared from the city. It’s not clear why this happened, but prevailing theories postulate that either the medical community got better at managing its mortal complications (e.g., bacterial pneumonia), or the bug itself mutated to a less virulent strain.
Is the Swine Flu our Spanish Flu?
On June 11, 2009, the World Health Organization (WHO) declared that the current H1N1 flu virus had reached pandemic status. This novel H1N1 serotype appears to be a direct descendent of the Spanish H1N1 subtype, but the new strain also combines genetic material culled from swine and birds reassorted in a manner that results in limited innate human defenses. And like the Spanish variant, it appears this new strain is hitting earlier in the year than usual and disproportionately affecting the young, with about two-thirds of U.S. deaths coming in the 25- to 64-year-old demographic.
So can we expect hundreds of millions of deaths from swine flu? Probably not. The WHO has been cautious to note that the upgrade to pandemic status was based on the rapidity and ease of spread, not the lethality of the virus. Furthermore, the Centers for Disease Control and Prevention (CDC)—which publishes a wonderful weekly update called FluView (www.cdc.gov/flu/weekly/)—notes that while the number of doctor’s visits for influenza-like illnesses through mid-August is unusually high, the rates of hospitalizations and proportion of deaths attributed to pneumonia and influenza are low and within normal limits for this time of year. Further, the virus continues in its original form, meaning it has not mutated, become more resistant to antiviral drugs, or been altered from the viruses selected for the 2009 vaccine.
So while we certainly must brace for the worst, I feel comfortable in the answer I provided my family member. I also am confident that Grey won’t be quarantined or left to roam the barren Earth anytime soon. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
Apocalypse, pestilence, death. As I head back to work after a late-summer vacation, those words are on the tip of my tongue. Now before your mind drifts too far afield, this is not a synopsis of the time spent with family, or even my in-laws—although some have used similar words to describe my mother’s cooking. Rather, these are the descriptors of my vacation reading.
Summer Reading
I started the week relaxing contentedly with Cormac McCarthy’s “The Road.” I chose this book in part because I noticed that it soon will be released as a movie, but mostly because it had won the dustiest-book-in-my-office-reading-pile award. This tale of a young boy and his father traversing a post-apocalyptic America was shocking and surreal. I couldn’t help but interchange images of my 2-year-old son, Greyson, and me out on that road fighting for our existence. In my personal fictional account, I continuously, and heroically, MacGyver my way across a burned-out and treacherous landscape with death-defying adeptness—all the while Grey unknowingly totters, drooling and muttering in tow.
Reality, of course, would paint us in substantially different roles, with mine involving the lion’s share of muttering and drooling, leaving Grey wishing the apocalyptic dealer had dealt him his mother instead.
Next up, “The Last Town on Earth,” by Thomas Mullen. I don’t recall how this book got into my reading pile, but I’m glad it did. The story is set in the fictional city of Commonwealth in 1918. The small, isolated mill town makes the drastic decision to stanch the tide of Spanish flu by cutting itself off from civilization through a self-imposed quarantine.
It is here, on p. 98, that I was sidetracked by a family member’s question—“Do you think this swine flu will be as bad as the Spanish flu?” I was asked shortly after being inquired about my reading choice. “Of course not,” I replied knowingly, moments before realizing I didn’t know. In fact, I didn’t have the faintest idea—not because it’s tough to divine the future, but because I realized I had little more than a passing knowledge of the famous flu that raked the world early last century. And with that, I was off on my final vacation reading session—a quest to slake my thirst for influenza knowledge.
Flu Pandemic
The Spanish flu pandemic of 1918-1920 was the first of three to hit in the 20th century. It took its name not from its site of origin (debated but generally felt to be the U.S., Kansas specifically), but rather from the fact that Spain, a neutral country in World War I, had the most uncensored lines of communication, so the most credible news of the disease came from that country. This provided the false impression that Spain was the only—or at least most dramatically—affected country. Like today’s swine flu, the Spanish flu was an H1N1 influenza. To sate your inner microbiologist, this means the virus exhibits the first of 16 subtypes of hemagglutinin (H) and nine subtypes of neuraminidase (N). Generally, only H1, H2, H3, and N1 and N2 affect humans, and tend to cause mild disease in otherwise healthy populations, killing the immunocompromised, the very young, and the very old. This typically results in a case-fatality rate of about 0.1% and 250,000 to 500,000 deaths worldwide annually.
The Spanish flu, however, was different. For reasons that are not entirely clear, the Spanish flu struck in two waves. The first wave, in the spring of 1918, induced typical flu-like illness with generally mild outcomes, except for the immunocompromised. The second wave was unusual for two reasons. First, it began in the late summer of 1918, rather than the typical winter pattern seen in North America. Second, it was much more deadly, inducing what has been termed a cytokine storm. This immunological avalanche produced more severe disease in the immunopotent young, healthy populations—resulting in its unprecedented mortality in this cohort. In fact, upward of 99% of all Spanish flu deaths were in people younger than 65.
In the end, the pandemic left a broad swath of destruction in its wake. It is estimated that 500 million people—one-third of the world’s population at the time—were infected. The mortality rate was 10% to 20%, resulting in 50 million to 100 million deaths. Put another way, the Spanish flu killed 5% of humanity.
It did so rapidly. Nearly 1 million people died per week in the first 25 weeks of the second wave. To put it in perspective, it took HIV 25 years to reach that number. Thus, historians have termed the Spanish flu “the greatest medical holocaust in history.”
And then as quickly as it commenced, it abated. For example, in Philadelphia, there were about 5,000 flu deaths in one week in October 1918, yet a month later, the virus had nearly disappeared from the city. It’s not clear why this happened, but prevailing theories postulate that either the medical community got better at managing its mortal complications (e.g., bacterial pneumonia), or the bug itself mutated to a less virulent strain.
Is the Swine Flu our Spanish Flu?
On June 11, 2009, the World Health Organization (WHO) declared that the current H1N1 flu virus had reached pandemic status. This novel H1N1 serotype appears to be a direct descendent of the Spanish H1N1 subtype, but the new strain also combines genetic material culled from swine and birds reassorted in a manner that results in limited innate human defenses. And like the Spanish variant, it appears this new strain is hitting earlier in the year than usual and disproportionately affecting the young, with about two-thirds of U.S. deaths coming in the 25- to 64-year-old demographic.
So can we expect hundreds of millions of deaths from swine flu? Probably not. The WHO has been cautious to note that the upgrade to pandemic status was based on the rapidity and ease of spread, not the lethality of the virus. Furthermore, the Centers for Disease Control and Prevention (CDC)—which publishes a wonderful weekly update called FluView (www.cdc.gov/flu/weekly/)—notes that while the number of doctor’s visits for influenza-like illnesses through mid-August is unusually high, the rates of hospitalizations and proportion of deaths attributed to pneumonia and influenza are low and within normal limits for this time of year. Further, the virus continues in its original form, meaning it has not mutated, become more resistant to antiviral drugs, or been altered from the viruses selected for the 2009 vaccine.
So while we certainly must brace for the worst, I feel comfortable in the answer I provided my family member. I also am confident that Grey won’t be quarantined or left to roam the barren Earth anytime soon. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
The Time to Act Is Now
It should come as no surprise to most hospitalists that healthcare-associated infections (HAIs) are among the leading causes of death in the U.S. The Centers for Disease Control and Prevention (CDC) estimates that from 5% to 10% of hospitalized patients develops an HAI, which leads to nearly 100,000 deaths every year.
The big four infection categories are catheter-associated urinary tract infections (CAUTIs), ventilator-associated pneumonia (as well as non-ventilator-associated hospital-acquired pneumonia), central-line-associated bloodstream infections, and surgical-site infections. In addition, Clostridium difficile (C-diff) and methicillin-resistant Staphylococcus aureus (MRSA) infections add to the burden.
While the toll on patients is substantial, the financial burden is equally staggering. It is estimated that HAIs lead to $28 billion to $33 billion in excess healthcare costs each year. So what does this have to do with hospitalists? Everything.
National Efforts to Curb HAIs
The morbidity, mortality, and financial consequences of HAIs have not been lost on patients, payors, and policymakers; each group is demanding action. The Department of Health and Human Services (HHS) is coordinating a national effort addressing HAIs; it aims to bring together many of HHS’ agencies (CDC, Agency for Healthcare Research and Quality, National Institutes of Health, Centers for Medicare and Medicaid Services, etc.) and engage patients, payors, and care providers. As a show of support for these efforts, Congress provided HHS with more than $200 million to target HAIs. After much work, HHS released its action plan to prevent HAIs in January.
SHM was one of the organizations asked to comment on the prevention plan. We did.
- We supported HHS’ focus on process measures (rather than outcomes), which recognizes the inevitability of some HAIs;
- We asked to be more involved in the process of developing and implementing the action plan; and
- We specifically asked for hospitalist representation on the Healthcare Infection Control Practices Advisory Committee (HICPAC), which develops the guidelines and prioritizes national efforts targeting HAI prevention.
Wish granted. In June, I was invited to HHS’ offices in Washington, D.C., along with key stakeholders to hear details of the final plan of action and discuss implementation. The plan (www.hhs.gov/ophs/initiatives/hai/infection.html) addresses key HAIs, establishes baseline rates, and proposes five-year national targets for reductions in infections.
The National Quality Foundation (NQF) has endorsed most of the metrics. The targeted reductions seem reasonable, and they are mostly in line with current evidence on best practices.
Of note, HHS dropped ventilator-associated pneumonia as a target area because of feedback from stakeholders (e.g., SHM) who argued that current definitions of the condition were inadequate to allow accurate measurement of targeted performance improvement efforts. Also of note, SHM was offered a HICPAC seat, which will enhance our ability to further impact the development and evaluation of current and future metrics.
I was invited back to Washington in July to meet with Don Wright, MD, MPH, FAACP, HHS’ principal deputy assistant secretary for health. It’s obvious to me that HHS realizes that any effective campaign to reduce HAI incidence will require engaging hospitalists, and, interestingly, HHS has even heard from other professional societies that hospitalists are a key group to target if you plan to implement hospitalwide interventions that span the ED, hospital wards, ICUs, surgical patients, pediatrics, or any other nook or cranny in the hospital. Hospitalists and SHM appear to be at ground zero in the national effort to combat HAIs.
The Hospitalist’s Role
There are few medical conditions that impact more of SHM’s big tent of membership the way HAIs do. HAIs affect administrators, internists, family practitioners, pediatricians, physician assistants and nurse practitioners, nurses, residents, students, community practitioners, academics, and large management companies … the list goes on. Not surprisingly, efforts to combat HAIs will require teams composed of many of the groups highlighted above working together to create systems-based approaches in their own hospitals—in joint efforts to reduce the rate of preventable HAIs.
Take the most common HAI as an example: catheter-associated urinary tract infections. These infections affect patients in every hospital unit and are familiar to every care provider, regardless of background or practice setting. Administrators should care about CAUTIs in part because CMS no longer pays for a CAUTI when it complicates a hospitalization, but also because these infections adversely affect patient satisfaction. Efforts to reduce CAUTIs will need to address inappropriate catheter insertion, provide alternatives to catheter use (e.g., bladder scans), develop best practices for maintenance of necessary catheters, and facilitate timely removal of catheters no longer needed. Dealing with all of these issues will take a team-based systems approach.
I will not be surprised if hospitalists end up leading these initiatives across the country. Hospitalists will need to share best practices, collaborate in local or national initiatives, provide feedback to SHM and policymakers about what works and what doesn’t, and educate patients about HAIs and prevention. Every hospitalist in the country needs to understand the reasons HAIs develop, know strategies to prevent them, and work to implement these strategies in their hospitals.
Future Directions
Given the urgency, what can you expect next? The action plan is finalized, so HHS is turning its attention to implementation. HHS has reached out to SHM to see how we can get the word out to our members. Dissemination strategies include publication of key messages in The Hospitalist, the Journal of Hospital Medicine, Webinars, e-mail announcements, and presentations at our annual meeting.
And while HHS’ plan of action highlights the metrics, it does not provide detailed strategies to combat HAIs. Prevention tools will need to be developed, tested, and, if effective, disseminated. HHS has asked SHM to help in tool development and dissemination.
HHS will continue to work with CMS to align payment policies that incentivize prevention efforts, and SHM will need to follow these developments closely. In addition, AHRQ is dedicating substantial funds to support the development and dissemination of best practices to prevent HAIs.
HHS acknowledges we still have much to learn about HAIs and their prevention. I expect many hospitalists, as well as SHM, will be at the center of these initiatives. Healthcare-associated infections are a problem that can no longer be ignored. Prevention efforts need to be ramped up. Hospitalists around the country need to prepare to lead and champion these efforts. It is time to act. TH
Dr. Flanders is president of SHM.
It should come as no surprise to most hospitalists that healthcare-associated infections (HAIs) are among the leading causes of death in the U.S. The Centers for Disease Control and Prevention (CDC) estimates that from 5% to 10% of hospitalized patients develops an HAI, which leads to nearly 100,000 deaths every year.
The big four infection categories are catheter-associated urinary tract infections (CAUTIs), ventilator-associated pneumonia (as well as non-ventilator-associated hospital-acquired pneumonia), central-line-associated bloodstream infections, and surgical-site infections. In addition, Clostridium difficile (C-diff) and methicillin-resistant Staphylococcus aureus (MRSA) infections add to the burden.
While the toll on patients is substantial, the financial burden is equally staggering. It is estimated that HAIs lead to $28 billion to $33 billion in excess healthcare costs each year. So what does this have to do with hospitalists? Everything.
National Efforts to Curb HAIs
The morbidity, mortality, and financial consequences of HAIs have not been lost on patients, payors, and policymakers; each group is demanding action. The Department of Health and Human Services (HHS) is coordinating a national effort addressing HAIs; it aims to bring together many of HHS’ agencies (CDC, Agency for Healthcare Research and Quality, National Institutes of Health, Centers for Medicare and Medicaid Services, etc.) and engage patients, payors, and care providers. As a show of support for these efforts, Congress provided HHS with more than $200 million to target HAIs. After much work, HHS released its action plan to prevent HAIs in January.
SHM was one of the organizations asked to comment on the prevention plan. We did.
- We supported HHS’ focus on process measures (rather than outcomes), which recognizes the inevitability of some HAIs;
- We asked to be more involved in the process of developing and implementing the action plan; and
- We specifically asked for hospitalist representation on the Healthcare Infection Control Practices Advisory Committee (HICPAC), which develops the guidelines and prioritizes national efforts targeting HAI prevention.
Wish granted. In June, I was invited to HHS’ offices in Washington, D.C., along with key stakeholders to hear details of the final plan of action and discuss implementation. The plan (www.hhs.gov/ophs/initiatives/hai/infection.html) addresses key HAIs, establishes baseline rates, and proposes five-year national targets for reductions in infections.
The National Quality Foundation (NQF) has endorsed most of the metrics. The targeted reductions seem reasonable, and they are mostly in line with current evidence on best practices.
Of note, HHS dropped ventilator-associated pneumonia as a target area because of feedback from stakeholders (e.g., SHM) who argued that current definitions of the condition were inadequate to allow accurate measurement of targeted performance improvement efforts. Also of note, SHM was offered a HICPAC seat, which will enhance our ability to further impact the development and evaluation of current and future metrics.
I was invited back to Washington in July to meet with Don Wright, MD, MPH, FAACP, HHS’ principal deputy assistant secretary for health. It’s obvious to me that HHS realizes that any effective campaign to reduce HAI incidence will require engaging hospitalists, and, interestingly, HHS has even heard from other professional societies that hospitalists are a key group to target if you plan to implement hospitalwide interventions that span the ED, hospital wards, ICUs, surgical patients, pediatrics, or any other nook or cranny in the hospital. Hospitalists and SHM appear to be at ground zero in the national effort to combat HAIs.
The Hospitalist’s Role
There are few medical conditions that impact more of SHM’s big tent of membership the way HAIs do. HAIs affect administrators, internists, family practitioners, pediatricians, physician assistants and nurse practitioners, nurses, residents, students, community practitioners, academics, and large management companies … the list goes on. Not surprisingly, efforts to combat HAIs will require teams composed of many of the groups highlighted above working together to create systems-based approaches in their own hospitals—in joint efforts to reduce the rate of preventable HAIs.
Take the most common HAI as an example: catheter-associated urinary tract infections. These infections affect patients in every hospital unit and are familiar to every care provider, regardless of background or practice setting. Administrators should care about CAUTIs in part because CMS no longer pays for a CAUTI when it complicates a hospitalization, but also because these infections adversely affect patient satisfaction. Efforts to reduce CAUTIs will need to address inappropriate catheter insertion, provide alternatives to catheter use (e.g., bladder scans), develop best practices for maintenance of necessary catheters, and facilitate timely removal of catheters no longer needed. Dealing with all of these issues will take a team-based systems approach.
I will not be surprised if hospitalists end up leading these initiatives across the country. Hospitalists will need to share best practices, collaborate in local or national initiatives, provide feedback to SHM and policymakers about what works and what doesn’t, and educate patients about HAIs and prevention. Every hospitalist in the country needs to understand the reasons HAIs develop, know strategies to prevent them, and work to implement these strategies in their hospitals.
Future Directions
Given the urgency, what can you expect next? The action plan is finalized, so HHS is turning its attention to implementation. HHS has reached out to SHM to see how we can get the word out to our members. Dissemination strategies include publication of key messages in The Hospitalist, the Journal of Hospital Medicine, Webinars, e-mail announcements, and presentations at our annual meeting.
And while HHS’ plan of action highlights the metrics, it does not provide detailed strategies to combat HAIs. Prevention tools will need to be developed, tested, and, if effective, disseminated. HHS has asked SHM to help in tool development and dissemination.
HHS will continue to work with CMS to align payment policies that incentivize prevention efforts, and SHM will need to follow these developments closely. In addition, AHRQ is dedicating substantial funds to support the development and dissemination of best practices to prevent HAIs.
HHS acknowledges we still have much to learn about HAIs and their prevention. I expect many hospitalists, as well as SHM, will be at the center of these initiatives. Healthcare-associated infections are a problem that can no longer be ignored. Prevention efforts need to be ramped up. Hospitalists around the country need to prepare to lead and champion these efforts. It is time to act. TH
Dr. Flanders is president of SHM.
It should come as no surprise to most hospitalists that healthcare-associated infections (HAIs) are among the leading causes of death in the U.S. The Centers for Disease Control and Prevention (CDC) estimates that from 5% to 10% of hospitalized patients develops an HAI, which leads to nearly 100,000 deaths every year.
The big four infection categories are catheter-associated urinary tract infections (CAUTIs), ventilator-associated pneumonia (as well as non-ventilator-associated hospital-acquired pneumonia), central-line-associated bloodstream infections, and surgical-site infections. In addition, Clostridium difficile (C-diff) and methicillin-resistant Staphylococcus aureus (MRSA) infections add to the burden.
While the toll on patients is substantial, the financial burden is equally staggering. It is estimated that HAIs lead to $28 billion to $33 billion in excess healthcare costs each year. So what does this have to do with hospitalists? Everything.
National Efforts to Curb HAIs
The morbidity, mortality, and financial consequences of HAIs have not been lost on patients, payors, and policymakers; each group is demanding action. The Department of Health and Human Services (HHS) is coordinating a national effort addressing HAIs; it aims to bring together many of HHS’ agencies (CDC, Agency for Healthcare Research and Quality, National Institutes of Health, Centers for Medicare and Medicaid Services, etc.) and engage patients, payors, and care providers. As a show of support for these efforts, Congress provided HHS with more than $200 million to target HAIs. After much work, HHS released its action plan to prevent HAIs in January.
SHM was one of the organizations asked to comment on the prevention plan. We did.
- We supported HHS’ focus on process measures (rather than outcomes), which recognizes the inevitability of some HAIs;
- We asked to be more involved in the process of developing and implementing the action plan; and
- We specifically asked for hospitalist representation on the Healthcare Infection Control Practices Advisory Committee (HICPAC), which develops the guidelines and prioritizes national efforts targeting HAI prevention.
Wish granted. In June, I was invited to HHS’ offices in Washington, D.C., along with key stakeholders to hear details of the final plan of action and discuss implementation. The plan (www.hhs.gov/ophs/initiatives/hai/infection.html) addresses key HAIs, establishes baseline rates, and proposes five-year national targets for reductions in infections.
The National Quality Foundation (NQF) has endorsed most of the metrics. The targeted reductions seem reasonable, and they are mostly in line with current evidence on best practices.
Of note, HHS dropped ventilator-associated pneumonia as a target area because of feedback from stakeholders (e.g., SHM) who argued that current definitions of the condition were inadequate to allow accurate measurement of targeted performance improvement efforts. Also of note, SHM was offered a HICPAC seat, which will enhance our ability to further impact the development and evaluation of current and future metrics.
I was invited back to Washington in July to meet with Don Wright, MD, MPH, FAACP, HHS’ principal deputy assistant secretary for health. It’s obvious to me that HHS realizes that any effective campaign to reduce HAI incidence will require engaging hospitalists, and, interestingly, HHS has even heard from other professional societies that hospitalists are a key group to target if you plan to implement hospitalwide interventions that span the ED, hospital wards, ICUs, surgical patients, pediatrics, or any other nook or cranny in the hospital. Hospitalists and SHM appear to be at ground zero in the national effort to combat HAIs.
The Hospitalist’s Role
There are few medical conditions that impact more of SHM’s big tent of membership the way HAIs do. HAIs affect administrators, internists, family practitioners, pediatricians, physician assistants and nurse practitioners, nurses, residents, students, community practitioners, academics, and large management companies … the list goes on. Not surprisingly, efforts to combat HAIs will require teams composed of many of the groups highlighted above working together to create systems-based approaches in their own hospitals—in joint efforts to reduce the rate of preventable HAIs.
Take the most common HAI as an example: catheter-associated urinary tract infections. These infections affect patients in every hospital unit and are familiar to every care provider, regardless of background or practice setting. Administrators should care about CAUTIs in part because CMS no longer pays for a CAUTI when it complicates a hospitalization, but also because these infections adversely affect patient satisfaction. Efforts to reduce CAUTIs will need to address inappropriate catheter insertion, provide alternatives to catheter use (e.g., bladder scans), develop best practices for maintenance of necessary catheters, and facilitate timely removal of catheters no longer needed. Dealing with all of these issues will take a team-based systems approach.
I will not be surprised if hospitalists end up leading these initiatives across the country. Hospitalists will need to share best practices, collaborate in local or national initiatives, provide feedback to SHM and policymakers about what works and what doesn’t, and educate patients about HAIs and prevention. Every hospitalist in the country needs to understand the reasons HAIs develop, know strategies to prevent them, and work to implement these strategies in their hospitals.
Future Directions
Given the urgency, what can you expect next? The action plan is finalized, so HHS is turning its attention to implementation. HHS has reached out to SHM to see how we can get the word out to our members. Dissemination strategies include publication of key messages in The Hospitalist, the Journal of Hospital Medicine, Webinars, e-mail announcements, and presentations at our annual meeting.
And while HHS’ plan of action highlights the metrics, it does not provide detailed strategies to combat HAIs. Prevention tools will need to be developed, tested, and, if effective, disseminated. HHS has asked SHM to help in tool development and dissemination.
HHS will continue to work with CMS to align payment policies that incentivize prevention efforts, and SHM will need to follow these developments closely. In addition, AHRQ is dedicating substantial funds to support the development and dissemination of best practices to prevent HAIs.
HHS acknowledges we still have much to learn about HAIs and their prevention. I expect many hospitalists, as well as SHM, will be at the center of these initiatives. Healthcare-associated infections are a problem that can no longer be ignored. Prevention efforts need to be ramped up. Hospitalists around the country need to prepare to lead and champion these efforts. It is time to act. TH
Dr. Flanders is president of SHM.
Measures of Success
The headlines are harrowing: corporate layoffs; foreclosures on the rise; 401(k) retirement plans halved; government bailouts adding to the national debt. The worst economic downturn since the Great Depression has generated some unexpected outcomes, yet not all of them are bad for hospitalists. Below, four vignettes highlight HM groups that have achieved success despite—or in some cases because of—these troubled times.
A Better Business Agreement
It has taken nearly two years—and sometimes as many as four meetings a week—but Rajeev Alexander, MD, and his colleagues are nearing the finish line of an evolving business arrangement. The new arrangement has come about due to the economic downturn, which forced Oregon Medical Group (OMG), a multispecialty physician group serving hospitals in the Eugene/Springfield area and the HM group’s employer since 1988, to want to divest themselves of the hospitalist group. Now, after a lengthy negotiation, Dr. Alexander’s group of eight hospitalists is busier than ever.
Through what were essentially multiple quasi-buyouts, divestitures, and mergers, Dr. Alexander’s hospitalist group “spun off” from OMG and affiliated with PeaceHealth, a nonprofit health system serving seven hospitals in Oregon, Washington, and Alaska. The new contract means Dr. Alexander’s group is directly employed by Sacred Heart Hospital, a 541-bed PeaceHealth-owned facility in Eugene.
The new contract included a non-compete clause with OMG, which currently employs five hospitalists, yet Dr. Alexander’s group has maintained its patient volume. Compensation held steady and employee benefits improved. During an independent and slow-moving negotiation, Dr. Alexander’s group has merged with another HM service that originally was employed by PeaceHealth. The two HM groups (technically competitors) now practice in the same hospital and are ironing out the terms of the merger. At the moment, the groups have created a mutually respectful joint governance council.
“We’ve tackled the thorniest of problems,” Dr. Alexander says, “first, creating a combined work schedule to distribute patients and divide the work. Those of us on the governance council figured if we could get the docs to actually work together and share patients and communicate with each other as if they were one group, then the momentum for an actual administrative/contractual merger would feel inevitable.”
Although negotiations are expected to last through the end of the year to finalize such details as compensation, recruiting, and a group mission statement, the medical staff at Sacred Heart considers the merger a “done deal” and has thrown its support behind the effort. “Community outpatient docs have been clamoring for our services, and we have been having to hand out numbers and ask them to wait in line, so to speak,” Dr. Alexander says.
Dr. Alexander says he’s learned some lessons through the extensive negotiation process:
- Stay positive. In any business venture, absolutely nothing is impossible, even dodging a noncompete clause.
- Release your preconceptions. Conspiracy theories might abound, but most hospital administrators have the best of intentions. As highly regulated organizations, hospitals might simply be following their own bylaws and fulfilling responsibilities to stakeholders. Seek out at least one administrator whom you can trust, and with whom you can communicate effectively. A mutual understanding of intentions and objectives makes the process more successful for all concerned.
- Look beyond politics. Your trust and respect for administrators and fellow physicians will go a long way toward overcoming obstacles.
- Stick to your plan. Adhere to your goal of remaining independent, if that is important to you. “Our group resisted being funneled into becoming employed by a very large national hospitalist chain,” Dr. Alexander says, “and I would encourage physicians in other parts of the country to stick to their commitments as well.”
- Trust the negotiation process. Even if all goes well, what you’re shooting for at the beginning might not be exactly what you get after negotiations are over. This does not mean you’ve failed, or that hospital administration tricked you or failed to deliver on promises. It simply means you have created a negotiated settlement; both sides have come to a new appreciation for the other’s requirements and have made necessary and respectful accommodations.
Rural Rewards
Based in Traverse City, Hospitalists of Northwest Michigan (HNM) services four community hospitals and continues to witness solid growth. Since 2000, the group has grown from nine to nearly 40 providers, and from 2005 to 2008, patient encounters doubled. “In these hard economic times, hospitals are inviting us in because we provide value to the hospitals through leadership, increased hospital revenues, and improved recruiting and retention of specialists,” says Troy W. Ahlstrom, MD, president of HNM. “We continue to see healthy growth in patient volume as we align patient care goals with the needs of the hospitals and surrounding communities we serve.”
HNM, which established a service at the regional medical center and then assumed management of HM programs at three other rural hospitals, soon will add a fifth service to its ledger. HNM also began a pediatric program at the regional referral center, and the group is exploring the possibility of providing a network of pediatric care throughout the region.
Having grown up in the region, David Friar, MD, CEO of HNM, not only has a better understanding of the needs of rural hospitals, but also a personal investment in his group’s success. “These are our communities. We don’t view the hospitals as just a place to make a profit, but a place where our neighbors work and our families get their care,” he says.
Drs. Ahlstrom and Friar offer the following advice for achieving success in these economic times:
- Optimize receipts. Concern over compliance audits leads many hospitals to sacrifice group receipts by encouraging undercoding. “We’ve found hospitals do a poor job of negotiating the provider portion of third-party payer contracts and frequently lose provider charges because they focus on the much larger facility fees,” says Dr. Ahlstrom. The group’s receipts increased more than 30% when they began using an outside billing firm and adopted productivity incentives to encourage providers to practice better documentation and charge capture. Improving documentation also supports a hospital’s ability to accurately code its patients, which allows a hospital to bill for a more profitable diagnosis-related group (DRG), and improve its case-mix index. With these changes, Hospitalists of Northwest Michigan has increased provider pay and grown their practice while improving the hospitals’ profitability.
- Encourage frugality. The cost-plus model is popular, but it doesn’t incentivize programs to contain costs. In contrast, the fixed-price model encourages hospitalists to find cheaper ways to provide good care. “Because the money we save goes to us, we’ve all found creative ways to provide quality care for a third less money than similar cost-plus programs,” Dr. Ahlstrom says.
- Align incentives. Hospitals live or die on thin margins, Dr. Ahlstrom says. His group trains its employees to ask: What can I do to make the hospital stronger? “What’s good for the hospital is good for us, so we work with the hospitals, not for the hospitals,” Dr. Friar says.
At its smaller hospitals, HNM incentivized orthopedic admissions so that more surgical cases would stay local. Hospitalists were trained to perform stress tests so the hospital can provide testing on weekends. The group pays hospitalists a bonus for each admission, so when the ED calls, the hospitalists say, “Thanks! I’ll be right there.” The group also increased staffing on weekends.
The end result: It improves the hospital’s bottom line by shortening length of stay, and improving quality of care, patient satisfaction, and group morale.
“When we align the incentives, everybody wins,” says Dr. Friar. “The system has more capacity, the patients get better care, and the hospitalists no longer feel that weekend shifts are a huge burden.”
- Build “system-ness.” Sharing providers between hospitals has helped HNM build a cohesive system of quality care. What began as a way to cover shifts has created an interinstitutional camaraderie that allows for the easier flow of patients, improved communication, and widespread use of best-practice models. Sharing such resources as billing, credentialing, benefits, recruiting, and payroll has helped the group stay competitive, Dr. Ahlstrom says.
Growth in a Down Economy
Jude R. Alexander, MD, president of Inpatient Specialists in Rockville, Md., a bedroom community about 12 miles northwest of Washington, D.C., has continued to grow his group despite the down economy. Hospital admissions in the D.C. area decreased sharply in the second half of 2008, and patient volume rebounded slowly in the first half of 2009.
Inpatient Specialists initially downsized its staff, then it used flex physicians to meet demand as volume increased.
Despite national hospital trends of budget shortfalls, downgraded bond ratings, and increases in uninsured patients, two of Inpatient Specialists’ client hospitals chose to invest in the HM program. Dr. Alexander credits the vote of confidence to his group’s track record of optimal resource utilization, which has inherent cost savings in the millions.
Dr. Alexander also recommends HM groups in tough economic circumstances should:
- Maintain good relationships with partner hospitals;
- Run a lean business;
- Focus on excellent customer service to patients, their families, and their PCPs; and
- Build strong alliances with employed physicians by eliciting and giving constructive feedback.
“Following this basic strategy, Inpatient Specialists has experienced 7% growth in patient volume in the past 12 months,” Dr. Alexander says. “We’ve expanded to 40 full-time equivalent hospitalists, and 40 part-time employees.” Inpatient Specialists has its eye on geographic expansion, as well. The group is targeting services throughout the Capitol region—Maryland, Virginia, and the District of Columbia.
Bankruptcy to Profitability in One Year
In 2007, a few months after the 99-bed Auburn Memorial Hospital in Auburn, N.Y., was forced into bankruptcy, James Leyhane, MD, and his hospitalist group were displeased that they weren’t in control of their own program. Physicians had started leaving the hospital; Dr. Leyhane himself had interviewed at another hospital. “Our CEO approached me to ask what would make it right,” Dr. Leyhane recalls. “I said, ‘We’d need to be employed by the hospital.’ ”
The hospital and the private, six-physician internal-medicine group that employed the program entered bids on the HM group. In March 2008, the HM group became contractually employed by the hospital. Dr. Leyhane was given full control as hospitalist director of Auburn Memorial Hospitalists.
As a result of the new alignment, two major shifts took place. First, the hospital CEO more aggressively recruited subspecialists and surgeons. With the HM group now affiliated with the hospital, recruiting surgeons to Auburn Memorial became much easier. Second, more primary-care physicians (PCPs) began sending their patients to Auburn Memorial.
“We were all shocked at how quickly the administration was able to recruit new subspecialists to the area,” Dr. Leyhane says. “That helped get the profitable procedures back to the hospital.”
The biggest surprise came at the end of 2008. Patient volume had risen 11.5% higher than the hospital’s best-case predictions. “As a result of our emerging from under the umbrella of one physician group, the outlying physicians became less fearful that they might lose their patients to that group,” Dr. Leyhane says. “And in good faith, we still maintain a coverage arrangement with that IM group.”
Thus, what was first seen as a bad thing turned into a very good thing for both the hospitalist group and the hospital. Auburn Memorial posted a $3.1 million profit in 2008 (see Table 1).
Dr. Leyhane suggests HM group leaders facing similar financial crunches talk to area subspecialists and find out what it would take to get them affiliated with their institution.
“In our case, a stable hospitalist program was definitely one of their top requests,” Dr. Leyhane says, adding it also would be beneficial to include PCPs in the “what do you want from our hospital?” conversation. TH
Andrea M. Sattinger is a freelance writer based in North Carolina.
Image Source: COLIN ANDERSON/GETTYIMAGES
The headlines are harrowing: corporate layoffs; foreclosures on the rise; 401(k) retirement plans halved; government bailouts adding to the national debt. The worst economic downturn since the Great Depression has generated some unexpected outcomes, yet not all of them are bad for hospitalists. Below, four vignettes highlight HM groups that have achieved success despite—or in some cases because of—these troubled times.
A Better Business Agreement
It has taken nearly two years—and sometimes as many as four meetings a week—but Rajeev Alexander, MD, and his colleagues are nearing the finish line of an evolving business arrangement. The new arrangement has come about due to the economic downturn, which forced Oregon Medical Group (OMG), a multispecialty physician group serving hospitals in the Eugene/Springfield area and the HM group’s employer since 1988, to want to divest themselves of the hospitalist group. Now, after a lengthy negotiation, Dr. Alexander’s group of eight hospitalists is busier than ever.
Through what were essentially multiple quasi-buyouts, divestitures, and mergers, Dr. Alexander’s hospitalist group “spun off” from OMG and affiliated with PeaceHealth, a nonprofit health system serving seven hospitals in Oregon, Washington, and Alaska. The new contract means Dr. Alexander’s group is directly employed by Sacred Heart Hospital, a 541-bed PeaceHealth-owned facility in Eugene.
The new contract included a non-compete clause with OMG, which currently employs five hospitalists, yet Dr. Alexander’s group has maintained its patient volume. Compensation held steady and employee benefits improved. During an independent and slow-moving negotiation, Dr. Alexander’s group has merged with another HM service that originally was employed by PeaceHealth. The two HM groups (technically competitors) now practice in the same hospital and are ironing out the terms of the merger. At the moment, the groups have created a mutually respectful joint governance council.
“We’ve tackled the thorniest of problems,” Dr. Alexander says, “first, creating a combined work schedule to distribute patients and divide the work. Those of us on the governance council figured if we could get the docs to actually work together and share patients and communicate with each other as if they were one group, then the momentum for an actual administrative/contractual merger would feel inevitable.”
Although negotiations are expected to last through the end of the year to finalize such details as compensation, recruiting, and a group mission statement, the medical staff at Sacred Heart considers the merger a “done deal” and has thrown its support behind the effort. “Community outpatient docs have been clamoring for our services, and we have been having to hand out numbers and ask them to wait in line, so to speak,” Dr. Alexander says.
Dr. Alexander says he’s learned some lessons through the extensive negotiation process:
- Stay positive. In any business venture, absolutely nothing is impossible, even dodging a noncompete clause.
- Release your preconceptions. Conspiracy theories might abound, but most hospital administrators have the best of intentions. As highly regulated organizations, hospitals might simply be following their own bylaws and fulfilling responsibilities to stakeholders. Seek out at least one administrator whom you can trust, and with whom you can communicate effectively. A mutual understanding of intentions and objectives makes the process more successful for all concerned.
- Look beyond politics. Your trust and respect for administrators and fellow physicians will go a long way toward overcoming obstacles.
- Stick to your plan. Adhere to your goal of remaining independent, if that is important to you. “Our group resisted being funneled into becoming employed by a very large national hospitalist chain,” Dr. Alexander says, “and I would encourage physicians in other parts of the country to stick to their commitments as well.”
- Trust the negotiation process. Even if all goes well, what you’re shooting for at the beginning might not be exactly what you get after negotiations are over. This does not mean you’ve failed, or that hospital administration tricked you or failed to deliver on promises. It simply means you have created a negotiated settlement; both sides have come to a new appreciation for the other’s requirements and have made necessary and respectful accommodations.
Rural Rewards
Based in Traverse City, Hospitalists of Northwest Michigan (HNM) services four community hospitals and continues to witness solid growth. Since 2000, the group has grown from nine to nearly 40 providers, and from 2005 to 2008, patient encounters doubled. “In these hard economic times, hospitals are inviting us in because we provide value to the hospitals through leadership, increased hospital revenues, and improved recruiting and retention of specialists,” says Troy W. Ahlstrom, MD, president of HNM. “We continue to see healthy growth in patient volume as we align patient care goals with the needs of the hospitals and surrounding communities we serve.”
HNM, which established a service at the regional medical center and then assumed management of HM programs at three other rural hospitals, soon will add a fifth service to its ledger. HNM also began a pediatric program at the regional referral center, and the group is exploring the possibility of providing a network of pediatric care throughout the region.
Having grown up in the region, David Friar, MD, CEO of HNM, not only has a better understanding of the needs of rural hospitals, but also a personal investment in his group’s success. “These are our communities. We don’t view the hospitals as just a place to make a profit, but a place where our neighbors work and our families get their care,” he says.
Drs. Ahlstrom and Friar offer the following advice for achieving success in these economic times:
- Optimize receipts. Concern over compliance audits leads many hospitals to sacrifice group receipts by encouraging undercoding. “We’ve found hospitals do a poor job of negotiating the provider portion of third-party payer contracts and frequently lose provider charges because they focus on the much larger facility fees,” says Dr. Ahlstrom. The group’s receipts increased more than 30% when they began using an outside billing firm and adopted productivity incentives to encourage providers to practice better documentation and charge capture. Improving documentation also supports a hospital’s ability to accurately code its patients, which allows a hospital to bill for a more profitable diagnosis-related group (DRG), and improve its case-mix index. With these changes, Hospitalists of Northwest Michigan has increased provider pay and grown their practice while improving the hospitals’ profitability.
- Encourage frugality. The cost-plus model is popular, but it doesn’t incentivize programs to contain costs. In contrast, the fixed-price model encourages hospitalists to find cheaper ways to provide good care. “Because the money we save goes to us, we’ve all found creative ways to provide quality care for a third less money than similar cost-plus programs,” Dr. Ahlstrom says.
- Align incentives. Hospitals live or die on thin margins, Dr. Ahlstrom says. His group trains its employees to ask: What can I do to make the hospital stronger? “What’s good for the hospital is good for us, so we work with the hospitals, not for the hospitals,” Dr. Friar says.
At its smaller hospitals, HNM incentivized orthopedic admissions so that more surgical cases would stay local. Hospitalists were trained to perform stress tests so the hospital can provide testing on weekends. The group pays hospitalists a bonus for each admission, so when the ED calls, the hospitalists say, “Thanks! I’ll be right there.” The group also increased staffing on weekends.
The end result: It improves the hospital’s bottom line by shortening length of stay, and improving quality of care, patient satisfaction, and group morale.
“When we align the incentives, everybody wins,” says Dr. Friar. “The system has more capacity, the patients get better care, and the hospitalists no longer feel that weekend shifts are a huge burden.”
- Build “system-ness.” Sharing providers between hospitals has helped HNM build a cohesive system of quality care. What began as a way to cover shifts has created an interinstitutional camaraderie that allows for the easier flow of patients, improved communication, and widespread use of best-practice models. Sharing such resources as billing, credentialing, benefits, recruiting, and payroll has helped the group stay competitive, Dr. Ahlstrom says.
Growth in a Down Economy
Jude R. Alexander, MD, president of Inpatient Specialists in Rockville, Md., a bedroom community about 12 miles northwest of Washington, D.C., has continued to grow his group despite the down economy. Hospital admissions in the D.C. area decreased sharply in the second half of 2008, and patient volume rebounded slowly in the first half of 2009.
Inpatient Specialists initially downsized its staff, then it used flex physicians to meet demand as volume increased.
Despite national hospital trends of budget shortfalls, downgraded bond ratings, and increases in uninsured patients, two of Inpatient Specialists’ client hospitals chose to invest in the HM program. Dr. Alexander credits the vote of confidence to his group’s track record of optimal resource utilization, which has inherent cost savings in the millions.
Dr. Alexander also recommends HM groups in tough economic circumstances should:
- Maintain good relationships with partner hospitals;
- Run a lean business;
- Focus on excellent customer service to patients, their families, and their PCPs; and
- Build strong alliances with employed physicians by eliciting and giving constructive feedback.
“Following this basic strategy, Inpatient Specialists has experienced 7% growth in patient volume in the past 12 months,” Dr. Alexander says. “We’ve expanded to 40 full-time equivalent hospitalists, and 40 part-time employees.” Inpatient Specialists has its eye on geographic expansion, as well. The group is targeting services throughout the Capitol region—Maryland, Virginia, and the District of Columbia.
Bankruptcy to Profitability in One Year
In 2007, a few months after the 99-bed Auburn Memorial Hospital in Auburn, N.Y., was forced into bankruptcy, James Leyhane, MD, and his hospitalist group were displeased that they weren’t in control of their own program. Physicians had started leaving the hospital; Dr. Leyhane himself had interviewed at another hospital. “Our CEO approached me to ask what would make it right,” Dr. Leyhane recalls. “I said, ‘We’d need to be employed by the hospital.’ ”
The hospital and the private, six-physician internal-medicine group that employed the program entered bids on the HM group. In March 2008, the HM group became contractually employed by the hospital. Dr. Leyhane was given full control as hospitalist director of Auburn Memorial Hospitalists.
As a result of the new alignment, two major shifts took place. First, the hospital CEO more aggressively recruited subspecialists and surgeons. With the HM group now affiliated with the hospital, recruiting surgeons to Auburn Memorial became much easier. Second, more primary-care physicians (PCPs) began sending their patients to Auburn Memorial.
“We were all shocked at how quickly the administration was able to recruit new subspecialists to the area,” Dr. Leyhane says. “That helped get the profitable procedures back to the hospital.”
The biggest surprise came at the end of 2008. Patient volume had risen 11.5% higher than the hospital’s best-case predictions. “As a result of our emerging from under the umbrella of one physician group, the outlying physicians became less fearful that they might lose their patients to that group,” Dr. Leyhane says. “And in good faith, we still maintain a coverage arrangement with that IM group.”
Thus, what was first seen as a bad thing turned into a very good thing for both the hospitalist group and the hospital. Auburn Memorial posted a $3.1 million profit in 2008 (see Table 1).
Dr. Leyhane suggests HM group leaders facing similar financial crunches talk to area subspecialists and find out what it would take to get them affiliated with their institution.
“In our case, a stable hospitalist program was definitely one of their top requests,” Dr. Leyhane says, adding it also would be beneficial to include PCPs in the “what do you want from our hospital?” conversation. TH
Andrea M. Sattinger is a freelance writer based in North Carolina.
Image Source: COLIN ANDERSON/GETTYIMAGES
The headlines are harrowing: corporate layoffs; foreclosures on the rise; 401(k) retirement plans halved; government bailouts adding to the national debt. The worst economic downturn since the Great Depression has generated some unexpected outcomes, yet not all of them are bad for hospitalists. Below, four vignettes highlight HM groups that have achieved success despite—or in some cases because of—these troubled times.
A Better Business Agreement
It has taken nearly two years—and sometimes as many as four meetings a week—but Rajeev Alexander, MD, and his colleagues are nearing the finish line of an evolving business arrangement. The new arrangement has come about due to the economic downturn, which forced Oregon Medical Group (OMG), a multispecialty physician group serving hospitals in the Eugene/Springfield area and the HM group’s employer since 1988, to want to divest themselves of the hospitalist group. Now, after a lengthy negotiation, Dr. Alexander’s group of eight hospitalists is busier than ever.
Through what were essentially multiple quasi-buyouts, divestitures, and mergers, Dr. Alexander’s hospitalist group “spun off” from OMG and affiliated with PeaceHealth, a nonprofit health system serving seven hospitals in Oregon, Washington, and Alaska. The new contract means Dr. Alexander’s group is directly employed by Sacred Heart Hospital, a 541-bed PeaceHealth-owned facility in Eugene.
The new contract included a non-compete clause with OMG, which currently employs five hospitalists, yet Dr. Alexander’s group has maintained its patient volume. Compensation held steady and employee benefits improved. During an independent and slow-moving negotiation, Dr. Alexander’s group has merged with another HM service that originally was employed by PeaceHealth. The two HM groups (technically competitors) now practice in the same hospital and are ironing out the terms of the merger. At the moment, the groups have created a mutually respectful joint governance council.
“We’ve tackled the thorniest of problems,” Dr. Alexander says, “first, creating a combined work schedule to distribute patients and divide the work. Those of us on the governance council figured if we could get the docs to actually work together and share patients and communicate with each other as if they were one group, then the momentum for an actual administrative/contractual merger would feel inevitable.”
Although negotiations are expected to last through the end of the year to finalize such details as compensation, recruiting, and a group mission statement, the medical staff at Sacred Heart considers the merger a “done deal” and has thrown its support behind the effort. “Community outpatient docs have been clamoring for our services, and we have been having to hand out numbers and ask them to wait in line, so to speak,” Dr. Alexander says.
Dr. Alexander says he’s learned some lessons through the extensive negotiation process:
- Stay positive. In any business venture, absolutely nothing is impossible, even dodging a noncompete clause.
- Release your preconceptions. Conspiracy theories might abound, but most hospital administrators have the best of intentions. As highly regulated organizations, hospitals might simply be following their own bylaws and fulfilling responsibilities to stakeholders. Seek out at least one administrator whom you can trust, and with whom you can communicate effectively. A mutual understanding of intentions and objectives makes the process more successful for all concerned.
- Look beyond politics. Your trust and respect for administrators and fellow physicians will go a long way toward overcoming obstacles.
- Stick to your plan. Adhere to your goal of remaining independent, if that is important to you. “Our group resisted being funneled into becoming employed by a very large national hospitalist chain,” Dr. Alexander says, “and I would encourage physicians in other parts of the country to stick to their commitments as well.”
- Trust the negotiation process. Even if all goes well, what you’re shooting for at the beginning might not be exactly what you get after negotiations are over. This does not mean you’ve failed, or that hospital administration tricked you or failed to deliver on promises. It simply means you have created a negotiated settlement; both sides have come to a new appreciation for the other’s requirements and have made necessary and respectful accommodations.
Rural Rewards
Based in Traverse City, Hospitalists of Northwest Michigan (HNM) services four community hospitals and continues to witness solid growth. Since 2000, the group has grown from nine to nearly 40 providers, and from 2005 to 2008, patient encounters doubled. “In these hard economic times, hospitals are inviting us in because we provide value to the hospitals through leadership, increased hospital revenues, and improved recruiting and retention of specialists,” says Troy W. Ahlstrom, MD, president of HNM. “We continue to see healthy growth in patient volume as we align patient care goals with the needs of the hospitals and surrounding communities we serve.”
HNM, which established a service at the regional medical center and then assumed management of HM programs at three other rural hospitals, soon will add a fifth service to its ledger. HNM also began a pediatric program at the regional referral center, and the group is exploring the possibility of providing a network of pediatric care throughout the region.
Having grown up in the region, David Friar, MD, CEO of HNM, not only has a better understanding of the needs of rural hospitals, but also a personal investment in his group’s success. “These are our communities. We don’t view the hospitals as just a place to make a profit, but a place where our neighbors work and our families get their care,” he says.
Drs. Ahlstrom and Friar offer the following advice for achieving success in these economic times:
- Optimize receipts. Concern over compliance audits leads many hospitals to sacrifice group receipts by encouraging undercoding. “We’ve found hospitals do a poor job of negotiating the provider portion of third-party payer contracts and frequently lose provider charges because they focus on the much larger facility fees,” says Dr. Ahlstrom. The group’s receipts increased more than 30% when they began using an outside billing firm and adopted productivity incentives to encourage providers to practice better documentation and charge capture. Improving documentation also supports a hospital’s ability to accurately code its patients, which allows a hospital to bill for a more profitable diagnosis-related group (DRG), and improve its case-mix index. With these changes, Hospitalists of Northwest Michigan has increased provider pay and grown their practice while improving the hospitals’ profitability.
- Encourage frugality. The cost-plus model is popular, but it doesn’t incentivize programs to contain costs. In contrast, the fixed-price model encourages hospitalists to find cheaper ways to provide good care. “Because the money we save goes to us, we’ve all found creative ways to provide quality care for a third less money than similar cost-plus programs,” Dr. Ahlstrom says.
- Align incentives. Hospitals live or die on thin margins, Dr. Ahlstrom says. His group trains its employees to ask: What can I do to make the hospital stronger? “What’s good for the hospital is good for us, so we work with the hospitals, not for the hospitals,” Dr. Friar says.
At its smaller hospitals, HNM incentivized orthopedic admissions so that more surgical cases would stay local. Hospitalists were trained to perform stress tests so the hospital can provide testing on weekends. The group pays hospitalists a bonus for each admission, so when the ED calls, the hospitalists say, “Thanks! I’ll be right there.” The group also increased staffing on weekends.
The end result: It improves the hospital’s bottom line by shortening length of stay, and improving quality of care, patient satisfaction, and group morale.
“When we align the incentives, everybody wins,” says Dr. Friar. “The system has more capacity, the patients get better care, and the hospitalists no longer feel that weekend shifts are a huge burden.”
- Build “system-ness.” Sharing providers between hospitals has helped HNM build a cohesive system of quality care. What began as a way to cover shifts has created an interinstitutional camaraderie that allows for the easier flow of patients, improved communication, and widespread use of best-practice models. Sharing such resources as billing, credentialing, benefits, recruiting, and payroll has helped the group stay competitive, Dr. Ahlstrom says.
Growth in a Down Economy
Jude R. Alexander, MD, president of Inpatient Specialists in Rockville, Md., a bedroom community about 12 miles northwest of Washington, D.C., has continued to grow his group despite the down economy. Hospital admissions in the D.C. area decreased sharply in the second half of 2008, and patient volume rebounded slowly in the first half of 2009.
Inpatient Specialists initially downsized its staff, then it used flex physicians to meet demand as volume increased.
Despite national hospital trends of budget shortfalls, downgraded bond ratings, and increases in uninsured patients, two of Inpatient Specialists’ client hospitals chose to invest in the HM program. Dr. Alexander credits the vote of confidence to his group’s track record of optimal resource utilization, which has inherent cost savings in the millions.
Dr. Alexander also recommends HM groups in tough economic circumstances should:
- Maintain good relationships with partner hospitals;
- Run a lean business;
- Focus on excellent customer service to patients, their families, and their PCPs; and
- Build strong alliances with employed physicians by eliciting and giving constructive feedback.
“Following this basic strategy, Inpatient Specialists has experienced 7% growth in patient volume in the past 12 months,” Dr. Alexander says. “We’ve expanded to 40 full-time equivalent hospitalists, and 40 part-time employees.” Inpatient Specialists has its eye on geographic expansion, as well. The group is targeting services throughout the Capitol region—Maryland, Virginia, and the District of Columbia.
Bankruptcy to Profitability in One Year
In 2007, a few months after the 99-bed Auburn Memorial Hospital in Auburn, N.Y., was forced into bankruptcy, James Leyhane, MD, and his hospitalist group were displeased that they weren’t in control of their own program. Physicians had started leaving the hospital; Dr. Leyhane himself had interviewed at another hospital. “Our CEO approached me to ask what would make it right,” Dr. Leyhane recalls. “I said, ‘We’d need to be employed by the hospital.’ ”
The hospital and the private, six-physician internal-medicine group that employed the program entered bids on the HM group. In March 2008, the HM group became contractually employed by the hospital. Dr. Leyhane was given full control as hospitalist director of Auburn Memorial Hospitalists.
As a result of the new alignment, two major shifts took place. First, the hospital CEO more aggressively recruited subspecialists and surgeons. With the HM group now affiliated with the hospital, recruiting surgeons to Auburn Memorial became much easier. Second, more primary-care physicians (PCPs) began sending their patients to Auburn Memorial.
“We were all shocked at how quickly the administration was able to recruit new subspecialists to the area,” Dr. Leyhane says. “That helped get the profitable procedures back to the hospital.”
The biggest surprise came at the end of 2008. Patient volume had risen 11.5% higher than the hospital’s best-case predictions. “As a result of our emerging from under the umbrella of one physician group, the outlying physicians became less fearful that they might lose their patients to that group,” Dr. Leyhane says. “And in good faith, we still maintain a coverage arrangement with that IM group.”
Thus, what was first seen as a bad thing turned into a very good thing for both the hospitalist group and the hospital. Auburn Memorial posted a $3.1 million profit in 2008 (see Table 1).
Dr. Leyhane suggests HM group leaders facing similar financial crunches talk to area subspecialists and find out what it would take to get them affiliated with their institution.
“In our case, a stable hospitalist program was definitely one of their top requests,” Dr. Leyhane says, adding it also would be beneficial to include PCPs in the “what do you want from our hospital?” conversation. TH
Andrea M. Sattinger is a freelance writer based in North Carolina.
Image Source: COLIN ANDERSON/GETTYIMAGES
How should a patient with a new-onset seizure be managed?
Case
A 42-year-old man is brought to the hospital by his family after a reported seizure. The patient was found on the floor, unresponsive, and suffering convulsions lasting less than a minute. He suffered no apparent trauma before or during the event. He has no history of seizures. His mental status quickly improved; he experienced oriented lucidity with slight drowsiness. His neurological exam is nonfocal, and his vital signs and laboratory values are normal. A noncontrast head computed tomogram (CT) is normal.
What is the appropriate approach to diagnosis and management for this patient with a new-onset seizure?
Overview
A patient with a first seizure presents a dilemma. Underlying causes for seizure are potentially life-threatening, and must be identified if present. A patient whose first seizure is unprovoked is at risk for future seizures (i.e., epilepsy). However, long-term therapy with anticonvulsant medication has morbidity, side effects, and expense. Advising a patient on whether to drive has public safety and legal implications, as well as major lifestyle changes for the patient.
Seizures may be focal (limited to one area of the brain) or generalized (involving both hemispheres). For the most part, focal (also known as partial) seizures do not impair consciousness; generalized seizures do. Approximately 70% of first seizures are partial focal seizures.1 Such provoking causes as head trauma, stroke, alcohol withdrawal, brain tumors, and infections can be identified in about one-third of cases.1
Electroencephalogram (EEG) and computed tomogram (CT) of the brain should be obtained, but insufficient evidence exists to recommend other testing, which should be pursued according to the clinical context.2
Unprovoked seizures recur in about 25% to 50% of patients, resulting in a diagnosis of epilepsy.1,2,4-7
Therapy is unnecessary in patients whose seizures will not recur, but reliably identifying these patients is a challenge. Whether antiepileptic drug (AED) therapy should be initiated in patients with a first unprovoked seizure is controversial and will be reviewed below.
Review of the Data
History: No test or finding can reliably differentiate unwitnessed seizures from other events (e.g., syncope).2 History from a reliable observer often is necessary to determine whether the event actually was a seizure.2 In as many as 50% of patients with a “first” seizure, thorough history will likely reveal previously unrecognized seizures.1 Although most epilepsy syndromes begin in childhood or adolescence, a significant number of patients will experience their first seizure in adulthood.2
A thorough neurologic examination should be performed. In a minority of cases, an exam will suggest a focal lesion. An impaired level of consciousness might represent a post-ictal state or delirium.2
Diagnostic evaluation: If the history suggests a seizure, an EEG should be obtained. Although the EEG will be normal in 50% of patients following a first seizure, an abnormal EEG provides useful information about seizure type and the likelihood of recurrence.2 In nearly a quarter of patients, the EEG will show epileptiform abnormalities that predict future seizures.2
Generally, an EEG should be obtained as soon as feasible, once a seizure is suspected. Some evidence in children suggests that EEG yield is higher in the 24 hours after a first seizure.
A noncontrast head CT or magnetic resonance imaging (MRI) reveals a significant abnormality about 10% of the time.2 A CT or MRI should be obtained. Few studies have compared CT to MRI in terms of yield in determining first seizure etiology, and those that do compare the two suffer from selection bias.2 Although CT or MRI are appropriate in evaluating a patient with a first seizure, the MRI’s greater resolution might provide a higher diagnostic yield in terms of seizure etiology, and, therefore, some experts recommend MRI over CT in nonemergent cases.2
Insufficient data exist to support or refute diagnostic testing beyond brain imaging and EEG. Although electrolyte abnormalities, hypoglycemia, and infections might infrequently cause seizures, such routine blood tests as complete blood count (CBC) and chemistry panels are rarely helpful.
As many as 15% of patients with a seizure will have minor abnormalities on routine lab tests, but the abnormalities do not appear to be the cause of the seizure.2
Lumbar puncture (LP) is categorically recommended only in patients in whom there is a clinical suspicion for infection as a seizure etiology. Reviews suggest that signs and symptoms of infection are typically present in patients with meningitis or another infectious cause for seizure; LP generally has limited utility in other noninfectious causes of seizure.2
The utility of toxicology testing in a first seizure has not been studied widely. Testing urine or blood for the presence of alcohol, cocaine, methamphetamines, benzodiazepines, or drug metabolites could be useful in the appropriate clinical setting.2
It is unclear whether a patient with a first seizure requires hospitalization. If initial testing in the ED rules out serious causes of seizures, the yield for hospitalization is likely to be low. In clinical practice, however, hospitalization is common and often necessary to complete such diagnostic testing as EEG and MRI.
Medical therapy: Patients with suspected epilepsy (e.g., those whose presenting seizure is, in retrospect, not their first seizure) should begin antiepileptic drug therapy (AED).1
Typically, a broad-spectrum AED—one that is effective against both partial and generalized seizures—should be used as initial therapy for epilepsy. These include valproate, lamotrigine, topiramate, zonisamide, and levetiracetam (see Table 1, above). Valproate has the longest history of effectiveness; levetiracetam has fewer drug interactions, and randomized trials support its efficacy.1
Checking blood electrolytes and liver enzymes is recommended before beginning AED treatment. Significant hepatic or renal dysfunction might necessitate dosing adjustments in many AEDs.2
Inpatient consultation with a neurologist might be helpful, although insufficient evidence exists that such consultation improves patient outcomes or makes care more cost-efficient. A neurologist should follow up on patients with a first seizure after hospital discharge.2
Patients with a first seizure that likely was provoked by a reversible condition (e.g., hypotension, hypoglycemia, infection) should generally not begin AED therapy. This also includes patients with multiple seizures in a brief period of time (less than 24 hours), all attributed to the same reversible cause.1
The decision to begin AED therapy after a first unprovoked seizure is controversial. Estimates of the likelihood of seizure recurrence range from 25% at two years to 50% at one year (in the absence of AED therapy).1-2,4-7 The decision to start AED therapy after a first seizure must therefore be individualized for each patient.
Patients at high risk for recurrent seizures should begin AED therapy.1 However, no test or prognostic tool reliably identifies these patients, and initiating therapy carries side effects and places psychological, financial, and social burdens on the patient. The prevailing clinical practice, therefore, has been watchful waiting, with a second seizure constituting proof of high risk for recurrence—and need for AED therapy. Three-quarters of patients with two or more unprovoked seizures likely will go on to have recurrent seizures.6
On the other hand, in patients believed to be at high risk for seizure recurrence, a more aggressive approach of initiating AED therapy after the first seizure is reasonable. A number of risk factors increasing risk for seizure recurrence have been identified (see Table 2, left).1,2 It is justified to initiate AED therapy if any of these factors are present, even after a single seizure. Still, it’s important to note that most people with risk factors will not benefit from AEDs, as only about 40% will have a seizure in the following two years.1
Early initiation of AED therapy might be appropriate for patients with occupations or hobbies in which seizures could be life-threatening (e.g., scuba divers, truck drivers).2
Low-risk patients still have a roughly 20% to 30% risk of seizure recurrence within three years.1 A second seizure that occurs while driving or while engaged in any hazardous activity could lead to serious injury.
Patients should be advised of this small but inescapable risk and instructed to contact their department of motor vehicles for specific legal restrictions, which vary by state. Once three seizure-free years have passed after a patient’s initial seizure, the chance of a recurrence falls to around 10% to 20%.6-7
Back to the Case
Our 42-year-old patient with a first seizure had normal findings on examination, laboratory studies, and brain imaging. An EEG showed epileptiform discharges in a spike and wave pattern. The attending hospitalist counseled him on his elevated risk of future seizures; the patient then elected to begin AED therapy, citing a fear of losing his driving privileges. Levetiracetam was started, which he tolerated despite mild sedation.
A year later, he suffered another seizure at his home. With regular followup and titration of his AED, he remained seizure-free for the next five years.
Bottom Line
Most patients with a single unprovoked seizure can be managed with watchful waiting, counseling, and neurological followup. Initiation of AED therapy is appropriate for patients with a high risk of seizure recurrence, or for whom another seizure could pose personal or social harm. TH
Dr. Hoffman is a hospitalist at Emory University School of Medicine in Atlanta.
References
- French JA, Pedley TA. Clinical practice: Initial management of epilepsy. N Engl J Med. 2008;359:166-176.
- Krumholz A, Wiebe S, Gronseth G, et al. Practice Parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2007;69:1996-2007.
- Schachter SC. Antiepileptic drug therapy: general treatment principles and application for special patient populations. Epilepsia. 1999;40(9):S20-25.
- Hauser WA, Rich SS, Annegers JF, et al. Seizure recurrence after a first unprovoked seizure: an extended follow-up. Neurology. 1990;40:1163-1170.
- Marson A, Jacoby A, Johnson A, et al. Immediate versus deferred antiepileptic drug treatment for epilepsy and single seizures: a randomized controlled trial. Lancet. 2005;365: 2007-2013.
- Hauser WA, Rich SS, Lee JR, Annegers JF, Anderson VE. Risk of recurrent seizures after two unprovoked seizures. N Engl J Med. 1998;338:429-434.
- Berg AT. Risk of recurrence after a first unprovoked seizure. Epilepsia. 2008;49:S13-18.
- Kim LG, Johnson TL, Marson AG, et al. Prediction of risk of seizure recurrence after a single seizure and early epilepsy: further results from the MESS trial. Lancet Neurology. 2006;5(4):317-322.
Case
A 42-year-old man is brought to the hospital by his family after a reported seizure. The patient was found on the floor, unresponsive, and suffering convulsions lasting less than a minute. He suffered no apparent trauma before or during the event. He has no history of seizures. His mental status quickly improved; he experienced oriented lucidity with slight drowsiness. His neurological exam is nonfocal, and his vital signs and laboratory values are normal. A noncontrast head computed tomogram (CT) is normal.
What is the appropriate approach to diagnosis and management for this patient with a new-onset seizure?
Overview
A patient with a first seizure presents a dilemma. Underlying causes for seizure are potentially life-threatening, and must be identified if present. A patient whose first seizure is unprovoked is at risk for future seizures (i.e., epilepsy). However, long-term therapy with anticonvulsant medication has morbidity, side effects, and expense. Advising a patient on whether to drive has public safety and legal implications, as well as major lifestyle changes for the patient.
Seizures may be focal (limited to one area of the brain) or generalized (involving both hemispheres). For the most part, focal (also known as partial) seizures do not impair consciousness; generalized seizures do. Approximately 70% of first seizures are partial focal seizures.1 Such provoking causes as head trauma, stroke, alcohol withdrawal, brain tumors, and infections can be identified in about one-third of cases.1
Electroencephalogram (EEG) and computed tomogram (CT) of the brain should be obtained, but insufficient evidence exists to recommend other testing, which should be pursued according to the clinical context.2
Unprovoked seizures recur in about 25% to 50% of patients, resulting in a diagnosis of epilepsy.1,2,4-7
Therapy is unnecessary in patients whose seizures will not recur, but reliably identifying these patients is a challenge. Whether antiepileptic drug (AED) therapy should be initiated in patients with a first unprovoked seizure is controversial and will be reviewed below.
Review of the Data
History: No test or finding can reliably differentiate unwitnessed seizures from other events (e.g., syncope).2 History from a reliable observer often is necessary to determine whether the event actually was a seizure.2 In as many as 50% of patients with a “first” seizure, thorough history will likely reveal previously unrecognized seizures.1 Although most epilepsy syndromes begin in childhood or adolescence, a significant number of patients will experience their first seizure in adulthood.2
A thorough neurologic examination should be performed. In a minority of cases, an exam will suggest a focal lesion. An impaired level of consciousness might represent a post-ictal state or delirium.2
Diagnostic evaluation: If the history suggests a seizure, an EEG should be obtained. Although the EEG will be normal in 50% of patients following a first seizure, an abnormal EEG provides useful information about seizure type and the likelihood of recurrence.2 In nearly a quarter of patients, the EEG will show epileptiform abnormalities that predict future seizures.2
Generally, an EEG should be obtained as soon as feasible, once a seizure is suspected. Some evidence in children suggests that EEG yield is higher in the 24 hours after a first seizure.
A noncontrast head CT or magnetic resonance imaging (MRI) reveals a significant abnormality about 10% of the time.2 A CT or MRI should be obtained. Few studies have compared CT to MRI in terms of yield in determining first seizure etiology, and those that do compare the two suffer from selection bias.2 Although CT or MRI are appropriate in evaluating a patient with a first seizure, the MRI’s greater resolution might provide a higher diagnostic yield in terms of seizure etiology, and, therefore, some experts recommend MRI over CT in nonemergent cases.2
Insufficient data exist to support or refute diagnostic testing beyond brain imaging and EEG. Although electrolyte abnormalities, hypoglycemia, and infections might infrequently cause seizures, such routine blood tests as complete blood count (CBC) and chemistry panels are rarely helpful.
As many as 15% of patients with a seizure will have minor abnormalities on routine lab tests, but the abnormalities do not appear to be the cause of the seizure.2
Lumbar puncture (LP) is categorically recommended only in patients in whom there is a clinical suspicion for infection as a seizure etiology. Reviews suggest that signs and symptoms of infection are typically present in patients with meningitis or another infectious cause for seizure; LP generally has limited utility in other noninfectious causes of seizure.2
The utility of toxicology testing in a first seizure has not been studied widely. Testing urine or blood for the presence of alcohol, cocaine, methamphetamines, benzodiazepines, or drug metabolites could be useful in the appropriate clinical setting.2
It is unclear whether a patient with a first seizure requires hospitalization. If initial testing in the ED rules out serious causes of seizures, the yield for hospitalization is likely to be low. In clinical practice, however, hospitalization is common and often necessary to complete such diagnostic testing as EEG and MRI.
Medical therapy: Patients with suspected epilepsy (e.g., those whose presenting seizure is, in retrospect, not their first seizure) should begin antiepileptic drug therapy (AED).1
Typically, a broad-spectrum AED—one that is effective against both partial and generalized seizures—should be used as initial therapy for epilepsy. These include valproate, lamotrigine, topiramate, zonisamide, and levetiracetam (see Table 1, above). Valproate has the longest history of effectiveness; levetiracetam has fewer drug interactions, and randomized trials support its efficacy.1
Checking blood electrolytes and liver enzymes is recommended before beginning AED treatment. Significant hepatic or renal dysfunction might necessitate dosing adjustments in many AEDs.2
Inpatient consultation with a neurologist might be helpful, although insufficient evidence exists that such consultation improves patient outcomes or makes care more cost-efficient. A neurologist should follow up on patients with a first seizure after hospital discharge.2
Patients with a first seizure that likely was provoked by a reversible condition (e.g., hypotension, hypoglycemia, infection) should generally not begin AED therapy. This also includes patients with multiple seizures in a brief period of time (less than 24 hours), all attributed to the same reversible cause.1
The decision to begin AED therapy after a first unprovoked seizure is controversial. Estimates of the likelihood of seizure recurrence range from 25% at two years to 50% at one year (in the absence of AED therapy).1-2,4-7 The decision to start AED therapy after a first seizure must therefore be individualized for each patient.
Patients at high risk for recurrent seizures should begin AED therapy.1 However, no test or prognostic tool reliably identifies these patients, and initiating therapy carries side effects and places psychological, financial, and social burdens on the patient. The prevailing clinical practice, therefore, has been watchful waiting, with a second seizure constituting proof of high risk for recurrence—and need for AED therapy. Three-quarters of patients with two or more unprovoked seizures likely will go on to have recurrent seizures.6
On the other hand, in patients believed to be at high risk for seizure recurrence, a more aggressive approach of initiating AED therapy after the first seizure is reasonable. A number of risk factors increasing risk for seizure recurrence have been identified (see Table 2, left).1,2 It is justified to initiate AED therapy if any of these factors are present, even after a single seizure. Still, it’s important to note that most people with risk factors will not benefit from AEDs, as only about 40% will have a seizure in the following two years.1
Early initiation of AED therapy might be appropriate for patients with occupations or hobbies in which seizures could be life-threatening (e.g., scuba divers, truck drivers).2
Low-risk patients still have a roughly 20% to 30% risk of seizure recurrence within three years.1 A second seizure that occurs while driving or while engaged in any hazardous activity could lead to serious injury.
Patients should be advised of this small but inescapable risk and instructed to contact their department of motor vehicles for specific legal restrictions, which vary by state. Once three seizure-free years have passed after a patient’s initial seizure, the chance of a recurrence falls to around 10% to 20%.6-7
Back to the Case
Our 42-year-old patient with a first seizure had normal findings on examination, laboratory studies, and brain imaging. An EEG showed epileptiform discharges in a spike and wave pattern. The attending hospitalist counseled him on his elevated risk of future seizures; the patient then elected to begin AED therapy, citing a fear of losing his driving privileges. Levetiracetam was started, which he tolerated despite mild sedation.
A year later, he suffered another seizure at his home. With regular followup and titration of his AED, he remained seizure-free for the next five years.
Bottom Line
Most patients with a single unprovoked seizure can be managed with watchful waiting, counseling, and neurological followup. Initiation of AED therapy is appropriate for patients with a high risk of seizure recurrence, or for whom another seizure could pose personal or social harm. TH
Dr. Hoffman is a hospitalist at Emory University School of Medicine in Atlanta.
References
- French JA, Pedley TA. Clinical practice: Initial management of epilepsy. N Engl J Med. 2008;359:166-176.
- Krumholz A, Wiebe S, Gronseth G, et al. Practice Parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2007;69:1996-2007.
- Schachter SC. Antiepileptic drug therapy: general treatment principles and application for special patient populations. Epilepsia. 1999;40(9):S20-25.
- Hauser WA, Rich SS, Annegers JF, et al. Seizure recurrence after a first unprovoked seizure: an extended follow-up. Neurology. 1990;40:1163-1170.
- Marson A, Jacoby A, Johnson A, et al. Immediate versus deferred antiepileptic drug treatment for epilepsy and single seizures: a randomized controlled trial. Lancet. 2005;365: 2007-2013.
- Hauser WA, Rich SS, Lee JR, Annegers JF, Anderson VE. Risk of recurrent seizures after two unprovoked seizures. N Engl J Med. 1998;338:429-434.
- Berg AT. Risk of recurrence after a first unprovoked seizure. Epilepsia. 2008;49:S13-18.
- Kim LG, Johnson TL, Marson AG, et al. Prediction of risk of seizure recurrence after a single seizure and early epilepsy: further results from the MESS trial. Lancet Neurology. 2006;5(4):317-322.
Case
A 42-year-old man is brought to the hospital by his family after a reported seizure. The patient was found on the floor, unresponsive, and suffering convulsions lasting less than a minute. He suffered no apparent trauma before or during the event. He has no history of seizures. His mental status quickly improved; he experienced oriented lucidity with slight drowsiness. His neurological exam is nonfocal, and his vital signs and laboratory values are normal. A noncontrast head computed tomogram (CT) is normal.
What is the appropriate approach to diagnosis and management for this patient with a new-onset seizure?
Overview
A patient with a first seizure presents a dilemma. Underlying causes for seizure are potentially life-threatening, and must be identified if present. A patient whose first seizure is unprovoked is at risk for future seizures (i.e., epilepsy). However, long-term therapy with anticonvulsant medication has morbidity, side effects, and expense. Advising a patient on whether to drive has public safety and legal implications, as well as major lifestyle changes for the patient.
Seizures may be focal (limited to one area of the brain) or generalized (involving both hemispheres). For the most part, focal (also known as partial) seizures do not impair consciousness; generalized seizures do. Approximately 70% of first seizures are partial focal seizures.1 Such provoking causes as head trauma, stroke, alcohol withdrawal, brain tumors, and infections can be identified in about one-third of cases.1
Electroencephalogram (EEG) and computed tomogram (CT) of the brain should be obtained, but insufficient evidence exists to recommend other testing, which should be pursued according to the clinical context.2
Unprovoked seizures recur in about 25% to 50% of patients, resulting in a diagnosis of epilepsy.1,2,4-7
Therapy is unnecessary in patients whose seizures will not recur, but reliably identifying these patients is a challenge. Whether antiepileptic drug (AED) therapy should be initiated in patients with a first unprovoked seizure is controversial and will be reviewed below.
Review of the Data
History: No test or finding can reliably differentiate unwitnessed seizures from other events (e.g., syncope).2 History from a reliable observer often is necessary to determine whether the event actually was a seizure.2 In as many as 50% of patients with a “first” seizure, thorough history will likely reveal previously unrecognized seizures.1 Although most epilepsy syndromes begin in childhood or adolescence, a significant number of patients will experience their first seizure in adulthood.2
A thorough neurologic examination should be performed. In a minority of cases, an exam will suggest a focal lesion. An impaired level of consciousness might represent a post-ictal state or delirium.2
Diagnostic evaluation: If the history suggests a seizure, an EEG should be obtained. Although the EEG will be normal in 50% of patients following a first seizure, an abnormal EEG provides useful information about seizure type and the likelihood of recurrence.2 In nearly a quarter of patients, the EEG will show epileptiform abnormalities that predict future seizures.2
Generally, an EEG should be obtained as soon as feasible, once a seizure is suspected. Some evidence in children suggests that EEG yield is higher in the 24 hours after a first seizure.
A noncontrast head CT or magnetic resonance imaging (MRI) reveals a significant abnormality about 10% of the time.2 A CT or MRI should be obtained. Few studies have compared CT to MRI in terms of yield in determining first seizure etiology, and those that do compare the two suffer from selection bias.2 Although CT or MRI are appropriate in evaluating a patient with a first seizure, the MRI’s greater resolution might provide a higher diagnostic yield in terms of seizure etiology, and, therefore, some experts recommend MRI over CT in nonemergent cases.2
Insufficient data exist to support or refute diagnostic testing beyond brain imaging and EEG. Although electrolyte abnormalities, hypoglycemia, and infections might infrequently cause seizures, such routine blood tests as complete blood count (CBC) and chemistry panels are rarely helpful.
As many as 15% of patients with a seizure will have minor abnormalities on routine lab tests, but the abnormalities do not appear to be the cause of the seizure.2
Lumbar puncture (LP) is categorically recommended only in patients in whom there is a clinical suspicion for infection as a seizure etiology. Reviews suggest that signs and symptoms of infection are typically present in patients with meningitis or another infectious cause for seizure; LP generally has limited utility in other noninfectious causes of seizure.2
The utility of toxicology testing in a first seizure has not been studied widely. Testing urine or blood for the presence of alcohol, cocaine, methamphetamines, benzodiazepines, or drug metabolites could be useful in the appropriate clinical setting.2
It is unclear whether a patient with a first seizure requires hospitalization. If initial testing in the ED rules out serious causes of seizures, the yield for hospitalization is likely to be low. In clinical practice, however, hospitalization is common and often necessary to complete such diagnostic testing as EEG and MRI.
Medical therapy: Patients with suspected epilepsy (e.g., those whose presenting seizure is, in retrospect, not their first seizure) should begin antiepileptic drug therapy (AED).1
Typically, a broad-spectrum AED—one that is effective against both partial and generalized seizures—should be used as initial therapy for epilepsy. These include valproate, lamotrigine, topiramate, zonisamide, and levetiracetam (see Table 1, above). Valproate has the longest history of effectiveness; levetiracetam has fewer drug interactions, and randomized trials support its efficacy.1
Checking blood electrolytes and liver enzymes is recommended before beginning AED treatment. Significant hepatic or renal dysfunction might necessitate dosing adjustments in many AEDs.2
Inpatient consultation with a neurologist might be helpful, although insufficient evidence exists that such consultation improves patient outcomes or makes care more cost-efficient. A neurologist should follow up on patients with a first seizure after hospital discharge.2
Patients with a first seizure that likely was provoked by a reversible condition (e.g., hypotension, hypoglycemia, infection) should generally not begin AED therapy. This also includes patients with multiple seizures in a brief period of time (less than 24 hours), all attributed to the same reversible cause.1
The decision to begin AED therapy after a first unprovoked seizure is controversial. Estimates of the likelihood of seizure recurrence range from 25% at two years to 50% at one year (in the absence of AED therapy).1-2,4-7 The decision to start AED therapy after a first seizure must therefore be individualized for each patient.
Patients at high risk for recurrent seizures should begin AED therapy.1 However, no test or prognostic tool reliably identifies these patients, and initiating therapy carries side effects and places psychological, financial, and social burdens on the patient. The prevailing clinical practice, therefore, has been watchful waiting, with a second seizure constituting proof of high risk for recurrence—and need for AED therapy. Three-quarters of patients with two or more unprovoked seizures likely will go on to have recurrent seizures.6
On the other hand, in patients believed to be at high risk for seizure recurrence, a more aggressive approach of initiating AED therapy after the first seizure is reasonable. A number of risk factors increasing risk for seizure recurrence have been identified (see Table 2, left).1,2 It is justified to initiate AED therapy if any of these factors are present, even after a single seizure. Still, it’s important to note that most people with risk factors will not benefit from AEDs, as only about 40% will have a seizure in the following two years.1
Early initiation of AED therapy might be appropriate for patients with occupations or hobbies in which seizures could be life-threatening (e.g., scuba divers, truck drivers).2
Low-risk patients still have a roughly 20% to 30% risk of seizure recurrence within three years.1 A second seizure that occurs while driving or while engaged in any hazardous activity could lead to serious injury.
Patients should be advised of this small but inescapable risk and instructed to contact their department of motor vehicles for specific legal restrictions, which vary by state. Once three seizure-free years have passed after a patient’s initial seizure, the chance of a recurrence falls to around 10% to 20%.6-7
Back to the Case
Our 42-year-old patient with a first seizure had normal findings on examination, laboratory studies, and brain imaging. An EEG showed epileptiform discharges in a spike and wave pattern. The attending hospitalist counseled him on his elevated risk of future seizures; the patient then elected to begin AED therapy, citing a fear of losing his driving privileges. Levetiracetam was started, which he tolerated despite mild sedation.
A year later, he suffered another seizure at his home. With regular followup and titration of his AED, he remained seizure-free for the next five years.
Bottom Line
Most patients with a single unprovoked seizure can be managed with watchful waiting, counseling, and neurological followup. Initiation of AED therapy is appropriate for patients with a high risk of seizure recurrence, or for whom another seizure could pose personal or social harm. TH
Dr. Hoffman is a hospitalist at Emory University School of Medicine in Atlanta.
References
- French JA, Pedley TA. Clinical practice: Initial management of epilepsy. N Engl J Med. 2008;359:166-176.
- Krumholz A, Wiebe S, Gronseth G, et al. Practice Parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2007;69:1996-2007.
- Schachter SC. Antiepileptic drug therapy: general treatment principles and application for special patient populations. Epilepsia. 1999;40(9):S20-25.
- Hauser WA, Rich SS, Annegers JF, et al. Seizure recurrence after a first unprovoked seizure: an extended follow-up. Neurology. 1990;40:1163-1170.
- Marson A, Jacoby A, Johnson A, et al. Immediate versus deferred antiepileptic drug treatment for epilepsy and single seizures: a randomized controlled trial. Lancet. 2005;365: 2007-2013.
- Hauser WA, Rich SS, Lee JR, Annegers JF, Anderson VE. Risk of recurrent seizures after two unprovoked seizures. N Engl J Med. 1998;338:429-434.
- Berg AT. Risk of recurrence after a first unprovoked seizure. Epilepsia. 2008;49:S13-18.
- Kim LG, Johnson TL, Marson AG, et al. Prediction of risk of seizure recurrence after a single seizure and early epilepsy: further results from the MESS trial. Lancet Neurology. 2006;5(4):317-322.