User login
The Official Newspaper of the American Association for Thoracic Surgery
Restrictive transfusion strategy safe in cardiac surgery
ANAHEIM, CALIF. – Waiting to transfuse heart surgery patients until their hemoglobin drops below 7.5 g/dL is just as safe as transfusing them when their hemoglobin drops below 9.5 g/dL, and it saves a lot of blood, according to the TRICS III randomized, noninferiority trial of nearly 5,000 patients undergoing cardiac surgery with cardiopulmonary bypass.
Cardiac surgeons have been moving to more restrictive transfusion policies following reports of worse postoperative survival when patients are transfused. However, there are concerns about safety and uncertainty over whether it’s the transfusions themselves that are problematic or whether transfused patients do worse because they are sicker to begin with. The Transfusion Requirements in Cardiac Surgery (TRICS) III trial removes some of the doubt: “A restrictive transfusion strategy is as effective and safe as a liberal strategy in patients undergoing cardiac surgery,” said lead investigator C. David Mazer, MD, a professor in the department of anesthesia at the University of Toronto.
Overall, 11.4% in the restrictive-threshold group and 12.5% in the liberal-threshold group met the study’s composite primary outcome of death from any cause, MI, stroke, and new-onset renal failure with dialysis (P less than .001 for noninferiority). There were no statistically significant between-group differences in the individual components of the composite outcome. Mortality was 3% in the restrictive group and 3.6% in the liberal group, a 15% reduction for the restrictive group.
About 52% of the patients in the restrictive arm, compared with 72.6% in the liberal arm, were transfused. When transfused, patients in the restrictive arm received a median of 2 units of red cells; liberal-arm patients received a median of 3 units. The overall cost difference was roughly $3 million, Dr. Mazer said at the American Heart Association scientific sessions.
There were no statistically significant differences in secondary outcomes. Restrictive patients were on mechanical ventilation for a median of 0.38 days and in the ICU for a median of 2.1 days; patients in the liberal arm were ventilated for a median of 0.36 days and in the ICU for a median of 1.9 days. The median hospital stay was 8 days in both groups.
Unexpectedly, patients 75 years and older did better with the restrictive transfusion strategy, with a 30% lower risk of the composite outcome. “Many people think the older you are, the higher your hemoglobin should be, and the more liberal you should be with transfusions. We didn’t find that. [It] challenges current beliefs and may be considered to be hypothesis generating; at a minimum, it highlights that a restrictive transfusion strategy appears to be safe in elderly patients,” Dr. Mazer said.
The participants were a mean of 72 years old, and 35% were women. The majority in both arms underwent coronary artery bypass surgery, valve surgery, or both. Heart transplants were excluded from the study. The trial was conducted in 19 countries, including China and India, but “the results were remarkably consistent independent of where the sites were,” he said.
Results of the TRICS III trial were published simultaneously with Dr. Mazer’s presentation (N Engl J Med. 2017 Nov 12. doi: 10.1056/NEJMoa1711818).
The trial was funded by the Canadian Institutes of Health Research, among others. Dr. Mazer reported personal fees from Amgen, Boehringer Ingelheim, Octapharma, and Pharmascience, as well as grants and personal fees from Fresenius Kabi.
This is an extremely important study. There have been multiple other trials, and, unfortunately, results have been quite equivocal. It’s incumbent upon us to figure out the best transfusion strategy, especially in cardiac surgery, since it is associated with a large amount of blood utilization. Also, there’ve been projections for a significant lack of blood supply in the future.
While the overall results showed no significant difference in outcomes between the groups, there was a numerical benefit evident in the restrictive group for the composite outcome, as well as all components of the main primary outcome except MI. This is not entirely unexpected, but we are really looking at the short-term effects here. I’m hoping that the longer-term outcomes will be evaluated, because they are extremely important.
Frank Sellke, MD , is chief of cardiothoracic surgery at Brown University in Providence, R.I. He made his comments after the study was presented at the American Heart Association scientific sessions. He was not involved with the work.
This is an extremely important study. There have been multiple other trials, and, unfortunately, results have been quite equivocal. It’s incumbent upon us to figure out the best transfusion strategy, especially in cardiac surgery, since it is associated with a large amount of blood utilization. Also, there’ve been projections for a significant lack of blood supply in the future.
While the overall results showed no significant difference in outcomes between the groups, there was a numerical benefit evident in the restrictive group for the composite outcome, as well as all components of the main primary outcome except MI. This is not entirely unexpected, but we are really looking at the short-term effects here. I’m hoping that the longer-term outcomes will be evaluated, because they are extremely important.
Frank Sellke, MD , is chief of cardiothoracic surgery at Brown University in Providence, R.I. He made his comments after the study was presented at the American Heart Association scientific sessions. He was not involved with the work.
This is an extremely important study. There have been multiple other trials, and, unfortunately, results have been quite equivocal. It’s incumbent upon us to figure out the best transfusion strategy, especially in cardiac surgery, since it is associated with a large amount of blood utilization. Also, there’ve been projections for a significant lack of blood supply in the future.
While the overall results showed no significant difference in outcomes between the groups, there was a numerical benefit evident in the restrictive group for the composite outcome, as well as all components of the main primary outcome except MI. This is not entirely unexpected, but we are really looking at the short-term effects here. I’m hoping that the longer-term outcomes will be evaluated, because they are extremely important.
Frank Sellke, MD , is chief of cardiothoracic surgery at Brown University in Providence, R.I. He made his comments after the study was presented at the American Heart Association scientific sessions. He was not involved with the work.
ANAHEIM, CALIF. – Waiting to transfuse heart surgery patients until their hemoglobin drops below 7.5 g/dL is just as safe as transfusing them when their hemoglobin drops below 9.5 g/dL, and it saves a lot of blood, according to the TRICS III randomized, noninferiority trial of nearly 5,000 patients undergoing cardiac surgery with cardiopulmonary bypass.
Cardiac surgeons have been moving to more restrictive transfusion policies following reports of worse postoperative survival when patients are transfused. However, there are concerns about safety and uncertainty over whether it’s the transfusions themselves that are problematic or whether transfused patients do worse because they are sicker to begin with. The Transfusion Requirements in Cardiac Surgery (TRICS) III trial removes some of the doubt: “A restrictive transfusion strategy is as effective and safe as a liberal strategy in patients undergoing cardiac surgery,” said lead investigator C. David Mazer, MD, a professor in the department of anesthesia at the University of Toronto.
Overall, 11.4% in the restrictive-threshold group and 12.5% in the liberal-threshold group met the study’s composite primary outcome of death from any cause, MI, stroke, and new-onset renal failure with dialysis (P less than .001 for noninferiority). There were no statistically significant between-group differences in the individual components of the composite outcome. Mortality was 3% in the restrictive group and 3.6% in the liberal group, a 15% reduction for the restrictive group.
About 52% of the patients in the restrictive arm, compared with 72.6% in the liberal arm, were transfused. When transfused, patients in the restrictive arm received a median of 2 units of red cells; liberal-arm patients received a median of 3 units. The overall cost difference was roughly $3 million, Dr. Mazer said at the American Heart Association scientific sessions.
There were no statistically significant differences in secondary outcomes. Restrictive patients were on mechanical ventilation for a median of 0.38 days and in the ICU for a median of 2.1 days; patients in the liberal arm were ventilated for a median of 0.36 days and in the ICU for a median of 1.9 days. The median hospital stay was 8 days in both groups.
Unexpectedly, patients 75 years and older did better with the restrictive transfusion strategy, with a 30% lower risk of the composite outcome. “Many people think the older you are, the higher your hemoglobin should be, and the more liberal you should be with transfusions. We didn’t find that. [It] challenges current beliefs and may be considered to be hypothesis generating; at a minimum, it highlights that a restrictive transfusion strategy appears to be safe in elderly patients,” Dr. Mazer said.
The participants were a mean of 72 years old, and 35% were women. The majority in both arms underwent coronary artery bypass surgery, valve surgery, or both. Heart transplants were excluded from the study. The trial was conducted in 19 countries, including China and India, but “the results were remarkably consistent independent of where the sites were,” he said.
Results of the TRICS III trial were published simultaneously with Dr. Mazer’s presentation (N Engl J Med. 2017 Nov 12. doi: 10.1056/NEJMoa1711818).
The trial was funded by the Canadian Institutes of Health Research, among others. Dr. Mazer reported personal fees from Amgen, Boehringer Ingelheim, Octapharma, and Pharmascience, as well as grants and personal fees from Fresenius Kabi.
ANAHEIM, CALIF. – Waiting to transfuse heart surgery patients until their hemoglobin drops below 7.5 g/dL is just as safe as transfusing them when their hemoglobin drops below 9.5 g/dL, and it saves a lot of blood, according to the TRICS III randomized, noninferiority trial of nearly 5,000 patients undergoing cardiac surgery with cardiopulmonary bypass.
Cardiac surgeons have been moving to more restrictive transfusion policies following reports of worse postoperative survival when patients are transfused. However, there are concerns about safety and uncertainty over whether it’s the transfusions themselves that are problematic or whether transfused patients do worse because they are sicker to begin with. The Transfusion Requirements in Cardiac Surgery (TRICS) III trial removes some of the doubt: “A restrictive transfusion strategy is as effective and safe as a liberal strategy in patients undergoing cardiac surgery,” said lead investigator C. David Mazer, MD, a professor in the department of anesthesia at the University of Toronto.
Overall, 11.4% in the restrictive-threshold group and 12.5% in the liberal-threshold group met the study’s composite primary outcome of death from any cause, MI, stroke, and new-onset renal failure with dialysis (P less than .001 for noninferiority). There were no statistically significant between-group differences in the individual components of the composite outcome. Mortality was 3% in the restrictive group and 3.6% in the liberal group, a 15% reduction for the restrictive group.
About 52% of the patients in the restrictive arm, compared with 72.6% in the liberal arm, were transfused. When transfused, patients in the restrictive arm received a median of 2 units of red cells; liberal-arm patients received a median of 3 units. The overall cost difference was roughly $3 million, Dr. Mazer said at the American Heart Association scientific sessions.
There were no statistically significant differences in secondary outcomes. Restrictive patients were on mechanical ventilation for a median of 0.38 days and in the ICU for a median of 2.1 days; patients in the liberal arm were ventilated for a median of 0.36 days and in the ICU for a median of 1.9 days. The median hospital stay was 8 days in both groups.
Unexpectedly, patients 75 years and older did better with the restrictive transfusion strategy, with a 30% lower risk of the composite outcome. “Many people think the older you are, the higher your hemoglobin should be, and the more liberal you should be with transfusions. We didn’t find that. [It] challenges current beliefs and may be considered to be hypothesis generating; at a minimum, it highlights that a restrictive transfusion strategy appears to be safe in elderly patients,” Dr. Mazer said.
The participants were a mean of 72 years old, and 35% were women. The majority in both arms underwent coronary artery bypass surgery, valve surgery, or both. Heart transplants were excluded from the study. The trial was conducted in 19 countries, including China and India, but “the results were remarkably consistent independent of where the sites were,” he said.
Results of the TRICS III trial were published simultaneously with Dr. Mazer’s presentation (N Engl J Med. 2017 Nov 12. doi: 10.1056/NEJMoa1711818).
The trial was funded by the Canadian Institutes of Health Research, among others. Dr. Mazer reported personal fees from Amgen, Boehringer Ingelheim, Octapharma, and Pharmascience, as well as grants and personal fees from Fresenius Kabi.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Overall, 11.4% in the restrictive-threshold group, versus 12.5% in the liberal-threshold group, met the study’s composite primary outcome of death from any cause, myocardial infarction, stroke, or new-onset renal failure with dialysis.
Data source: TRICS III, a randomized, noninferiority trial with almost 5,000 participants
Disclosures: TRICS III was funded by the Canadian Institutes of Health Research, among others. The lead investigator reported personal fees from Amgen, Boehringer Ingelheim, Octapharma, and Pharmascience, as well as grants and personal fees from Fresenius Kabi.
Prescribers mostly ignore clopidogrel pharmacogenomic profiling
ANAHEIM, CALIF. – The boxed warning recommending pharmacogenomic testing of patients receiving clopidogrel to identify reduced metabolizers seems to be playing to a largely deaf audience.
Even when handed information on whether each clopidogrel-treated patient was a poor metabolizer of the drug, treating physicians usually did not switch them to a different antiplatelet drug, ticagrelor, that would be fully effective despite the patient’s reduced-metabolizer status. And clinicians who started patients on ticagrelor did not usually switch those with a good clopidogrel-metabolizing profile to the safer drug, clopidogrel, after learning that clopidogrel would be fully effective.
“Routine reporting of pharmacogenomics test results for acute coronary syndrome patients treated with P2Y12-inhibitor therapy had an uncertain yield and little impact on P2Y12-inhibitor switching,” E. Magnus Ohman, MBBS, said at the American Heart Association scientific sessions.
The study’s design gave each participating clinician free rein on whether to prescribe clopidogrel or ticagrelor (Brilinta) initially, and switching between the drugs was possible at any time after the initial prescription. At the trial’s start, 1,704 patients (56%) were on ticagrelor and 1,333 (44%) were on clopidogrel.
Pharmacogenomic testing showed that 34% of all patients were ultrametabolizers and 38% were extensive metabolizers. Patients in either of these categories metabolize enough clopidogrel into the active form to get full benefit from the drug and derive no additional efficacy benefit from switching to another P2Y12 inhibitor, such as ticagrelor or prasugrel (Effient) – drugs unaffected by metabolizer status. Testing also identified 25% of patients as intermediate metabolizers, who carry one loss-of-function allele for the CYP2C19 gene, and 3% were reduced metabolizers, who are homozygous for loss-of-function alleles. Standard practice is not to treat intermediate or reduced metabolizers with clopidogrel because they would not get an adequate antiplatelet effect; instead, these patients are usually treated with ticagrelor or with prasugrel when it’s an option.
After receiving the results regarding the clopidogrel-metabolizing status for each patient, attending physicians switched the drugs prescribed for only 7% of all patients: 9% of patients initially on ticagrelor and 4% of those initially on clopidogrel, reported Dr. Ohman, professor of medicine at Duke University in Durham, N.C. In addition, Dr. Ohman and his associates asked each participating physician who made a switch about his or her reasons for doing so. Of the patients who switched from clopidogrel to ticagrelor, only 23 were switched because of their pharmacogenomic results; this represents fewer than half of those who switched and only 2% of all patients who took clopidogrel. Only one patient changed from ticagrelor to clopidogrel based on pharmacogenomic results, representing 0.06% of all patients on ticagrelor.
“We believed the findings do not support the utility of mandatory testing in this context, as most did not act on the information,” Dr. Ohman said.
A major reason for the inertia, Dr. Gurbel suggested, may be the absence of any compelling data proving whether there’s any effect on clinical outcomes for switching reduced metabolizers off of clopidogrel or switching good metabolizers onto it.
“We have no large-scale, prospective data supporting pharmacogenomic-based personalization” of clopidogrel treatment leading to improved outcomes, but “we need to get over that,” he said. “It’s a challenge to get funding for this.” But “the answer is not to give ticagrelor or prasugrel to everyone because then the bleeding rate is too high.”
The findings Dr. Ohman reported came from the Study to Compare the Safety of Rivaroxaban Versus Acetylsalicylic Acid in Addition to Either Clopidogrel or Ticagrelor Therapy in Participants With Acute Coronary Syndrome (GEMINI-ACS-1), which had the primary goal of comparing the safety in acute coronary syndrome patients of a reduced dosage of rivaroxaban plus either clopidogrel or ticagrelor with the safety of aspirin plus one of these P2Y12 inhibitors. The primary endpoint was the rate of clinically significant bleeding events during a year of treatment. The study ran at 371 centers in 21 countries and showed similar bleeding rates in both treatment arms (Lancet. 2017 May 6; 389[10081]:1799-808).
The analysis also showed that patients identified as reduced metabolizers were fivefold more likely to be switched than patients identified as ultra metabolizers, and intermediate metabolizes had a 50% higher switching rate than ultra metabolizers. The rates of both ischemic and major bleeding outcomes were roughly similar across the spectrum of metabolizers, but Dr. Ohman cautioned that the trial was not designed to assess this. Dr. Gurbel urged the investigators to report on outcomes analyzed not just by metabolizer status but also by the treatment they received.
The boxed warning that clopidogrel received in 2010 regarding poor metabolizers led to “regulatory guidance” during design of the GEMINI-ACS-1 trial requiring routine pharmacogenomic testing for clopidogrel-metabolizing status, Dr. Ohman explained.
The trial was funded by Janssen and Bayer, the two companies that jointly market rivaroxaban (Xarelto). Dr. Ohman has been a consultant to Bayer and several other companies including AstraZeneca, the company that markets ticagrelor (Brilinta). He has also received research funding from Janssen, as well as Daiichi Sankyo and Gilead Sciences. Dr. Gurbel holds patents on platelet-function testing methods.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ANAHEIM, CALIF. – The boxed warning recommending pharmacogenomic testing of patients receiving clopidogrel to identify reduced metabolizers seems to be playing to a largely deaf audience.
Even when handed information on whether each clopidogrel-treated patient was a poor metabolizer of the drug, treating physicians usually did not switch them to a different antiplatelet drug, ticagrelor, that would be fully effective despite the patient’s reduced-metabolizer status. And clinicians who started patients on ticagrelor did not usually switch those with a good clopidogrel-metabolizing profile to the safer drug, clopidogrel, after learning that clopidogrel would be fully effective.
“Routine reporting of pharmacogenomics test results for acute coronary syndrome patients treated with P2Y12-inhibitor therapy had an uncertain yield and little impact on P2Y12-inhibitor switching,” E. Magnus Ohman, MBBS, said at the American Heart Association scientific sessions.
The study’s design gave each participating clinician free rein on whether to prescribe clopidogrel or ticagrelor (Brilinta) initially, and switching between the drugs was possible at any time after the initial prescription. At the trial’s start, 1,704 patients (56%) were on ticagrelor and 1,333 (44%) were on clopidogrel.
Pharmacogenomic testing showed that 34% of all patients were ultrametabolizers and 38% were extensive metabolizers. Patients in either of these categories metabolize enough clopidogrel into the active form to get full benefit from the drug and derive no additional efficacy benefit from switching to another P2Y12 inhibitor, such as ticagrelor or prasugrel (Effient) – drugs unaffected by metabolizer status. Testing also identified 25% of patients as intermediate metabolizers, who carry one loss-of-function allele for the CYP2C19 gene, and 3% were reduced metabolizers, who are homozygous for loss-of-function alleles. Standard practice is not to treat intermediate or reduced metabolizers with clopidogrel because they would not get an adequate antiplatelet effect; instead, these patients are usually treated with ticagrelor or with prasugrel when it’s an option.
After receiving the results regarding the clopidogrel-metabolizing status for each patient, attending physicians switched the drugs prescribed for only 7% of all patients: 9% of patients initially on ticagrelor and 4% of those initially on clopidogrel, reported Dr. Ohman, professor of medicine at Duke University in Durham, N.C. In addition, Dr. Ohman and his associates asked each participating physician who made a switch about his or her reasons for doing so. Of the patients who switched from clopidogrel to ticagrelor, only 23 were switched because of their pharmacogenomic results; this represents fewer than half of those who switched and only 2% of all patients who took clopidogrel. Only one patient changed from ticagrelor to clopidogrel based on pharmacogenomic results, representing 0.06% of all patients on ticagrelor.
“We believed the findings do not support the utility of mandatory testing in this context, as most did not act on the information,” Dr. Ohman said.
A major reason for the inertia, Dr. Gurbel suggested, may be the absence of any compelling data proving whether there’s any effect on clinical outcomes for switching reduced metabolizers off of clopidogrel or switching good metabolizers onto it.
“We have no large-scale, prospective data supporting pharmacogenomic-based personalization” of clopidogrel treatment leading to improved outcomes, but “we need to get over that,” he said. “It’s a challenge to get funding for this.” But “the answer is not to give ticagrelor or prasugrel to everyone because then the bleeding rate is too high.”
The findings Dr. Ohman reported came from the Study to Compare the Safety of Rivaroxaban Versus Acetylsalicylic Acid in Addition to Either Clopidogrel or Ticagrelor Therapy in Participants With Acute Coronary Syndrome (GEMINI-ACS-1), which had the primary goal of comparing the safety in acute coronary syndrome patients of a reduced dosage of rivaroxaban plus either clopidogrel or ticagrelor with the safety of aspirin plus one of these P2Y12 inhibitors. The primary endpoint was the rate of clinically significant bleeding events during a year of treatment. The study ran at 371 centers in 21 countries and showed similar bleeding rates in both treatment arms (Lancet. 2017 May 6; 389[10081]:1799-808).
The analysis also showed that patients identified as reduced metabolizers were fivefold more likely to be switched than patients identified as ultra metabolizers, and intermediate metabolizes had a 50% higher switching rate than ultra metabolizers. The rates of both ischemic and major bleeding outcomes were roughly similar across the spectrum of metabolizers, but Dr. Ohman cautioned that the trial was not designed to assess this. Dr. Gurbel urged the investigators to report on outcomes analyzed not just by metabolizer status but also by the treatment they received.
The boxed warning that clopidogrel received in 2010 regarding poor metabolizers led to “regulatory guidance” during design of the GEMINI-ACS-1 trial requiring routine pharmacogenomic testing for clopidogrel-metabolizing status, Dr. Ohman explained.
The trial was funded by Janssen and Bayer, the two companies that jointly market rivaroxaban (Xarelto). Dr. Ohman has been a consultant to Bayer and several other companies including AstraZeneca, the company that markets ticagrelor (Brilinta). He has also received research funding from Janssen, as well as Daiichi Sankyo and Gilead Sciences. Dr. Gurbel holds patents on platelet-function testing methods.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ANAHEIM, CALIF. – The boxed warning recommending pharmacogenomic testing of patients receiving clopidogrel to identify reduced metabolizers seems to be playing to a largely deaf audience.
Even when handed information on whether each clopidogrel-treated patient was a poor metabolizer of the drug, treating physicians usually did not switch them to a different antiplatelet drug, ticagrelor, that would be fully effective despite the patient’s reduced-metabolizer status. And clinicians who started patients on ticagrelor did not usually switch those with a good clopidogrel-metabolizing profile to the safer drug, clopidogrel, after learning that clopidogrel would be fully effective.
“Routine reporting of pharmacogenomics test results for acute coronary syndrome patients treated with P2Y12-inhibitor therapy had an uncertain yield and little impact on P2Y12-inhibitor switching,” E. Magnus Ohman, MBBS, said at the American Heart Association scientific sessions.
The study’s design gave each participating clinician free rein on whether to prescribe clopidogrel or ticagrelor (Brilinta) initially, and switching between the drugs was possible at any time after the initial prescription. At the trial’s start, 1,704 patients (56%) were on ticagrelor and 1,333 (44%) were on clopidogrel.
Pharmacogenomic testing showed that 34% of all patients were ultrametabolizers and 38% were extensive metabolizers. Patients in either of these categories metabolize enough clopidogrel into the active form to get full benefit from the drug and derive no additional efficacy benefit from switching to another P2Y12 inhibitor, such as ticagrelor or prasugrel (Effient) – drugs unaffected by metabolizer status. Testing also identified 25% of patients as intermediate metabolizers, who carry one loss-of-function allele for the CYP2C19 gene, and 3% were reduced metabolizers, who are homozygous for loss-of-function alleles. Standard practice is not to treat intermediate or reduced metabolizers with clopidogrel because they would not get an adequate antiplatelet effect; instead, these patients are usually treated with ticagrelor or with prasugrel when it’s an option.
After receiving the results regarding the clopidogrel-metabolizing status for each patient, attending physicians switched the drugs prescribed for only 7% of all patients: 9% of patients initially on ticagrelor and 4% of those initially on clopidogrel, reported Dr. Ohman, professor of medicine at Duke University in Durham, N.C. In addition, Dr. Ohman and his associates asked each participating physician who made a switch about his or her reasons for doing so. Of the patients who switched from clopidogrel to ticagrelor, only 23 were switched because of their pharmacogenomic results; this represents fewer than half of those who switched and only 2% of all patients who took clopidogrel. Only one patient changed from ticagrelor to clopidogrel based on pharmacogenomic results, representing 0.06% of all patients on ticagrelor.
“We believed the findings do not support the utility of mandatory testing in this context, as most did not act on the information,” Dr. Ohman said.
A major reason for the inertia, Dr. Gurbel suggested, may be the absence of any compelling data proving whether there’s any effect on clinical outcomes for switching reduced metabolizers off of clopidogrel or switching good metabolizers onto it.
“We have no large-scale, prospective data supporting pharmacogenomic-based personalization” of clopidogrel treatment leading to improved outcomes, but “we need to get over that,” he said. “It’s a challenge to get funding for this.” But “the answer is not to give ticagrelor or prasugrel to everyone because then the bleeding rate is too high.”
The findings Dr. Ohman reported came from the Study to Compare the Safety of Rivaroxaban Versus Acetylsalicylic Acid in Addition to Either Clopidogrel or Ticagrelor Therapy in Participants With Acute Coronary Syndrome (GEMINI-ACS-1), which had the primary goal of comparing the safety in acute coronary syndrome patients of a reduced dosage of rivaroxaban plus either clopidogrel or ticagrelor with the safety of aspirin plus one of these P2Y12 inhibitors. The primary endpoint was the rate of clinically significant bleeding events during a year of treatment. The study ran at 371 centers in 21 countries and showed similar bleeding rates in both treatment arms (Lancet. 2017 May 6; 389[10081]:1799-808).
The analysis also showed that patients identified as reduced metabolizers were fivefold more likely to be switched than patients identified as ultra metabolizers, and intermediate metabolizes had a 50% higher switching rate than ultra metabolizers. The rates of both ischemic and major bleeding outcomes were roughly similar across the spectrum of metabolizers, but Dr. Ohman cautioned that the trial was not designed to assess this. Dr. Gurbel urged the investigators to report on outcomes analyzed not just by metabolizer status but also by the treatment they received.
The boxed warning that clopidogrel received in 2010 regarding poor metabolizers led to “regulatory guidance” during design of the GEMINI-ACS-1 trial requiring routine pharmacogenomic testing for clopidogrel-metabolizing status, Dr. Ohman explained.
The trial was funded by Janssen and Bayer, the two companies that jointly market rivaroxaban (Xarelto). Dr. Ohman has been a consultant to Bayer and several other companies including AstraZeneca, the company that markets ticagrelor (Brilinta). He has also received research funding from Janssen, as well as Daiichi Sankyo and Gilead Sciences. Dr. Gurbel holds patents on platelet-function testing methods.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Physicians switched P2Y12 inhibitors for only 2% of patients on clopidogrel and only 0.06% on ticagrelor on the basis of their pharmacogenomic results.
Data source: GEMINI-ACS-1, a multicenter, prospective trial with 3,037 patients.
Disclosures: The GEMINI-ACS-1 trial was funded by Janssen and Bayer, the two companies that jointly market rivaroxaban (Xarelto). Dr. Ohman has been a consultant to Bayer and several other companies, including AstraZeneca, the company that markets ticagrelor (Brilinta). He has also received research funding from Janssen, as well as Daiichi Sankyo and Gilead Sciences. Dr. Gurbel holds patents on platelet-function testing methods.
Heart transplantation: Preop LVAD erases adverse impact of pulmonary hypertension
COLORADO SPRINGS – Reconsideration of the role of pulmonary hypertension in heart transplant outcomes is appropriate in the emerging era of the use of left ventricular assist devices (LVADs) as bridge to transplant, according to Ann C. Gaffey, MD, of the University of Pennsylvania, Philadelphia.
“Pulmonary hypertension secondary to congestive heart failure more than likely can be reversed to the values acceptable for heart transplant by the use of an LVAD. For bridge-to-transplant patients, pretransplant pulmonary hypertension does not affect recipient outcomes post transplantation,” she said at the annual meeting of the Western Thoracic Surgical Association.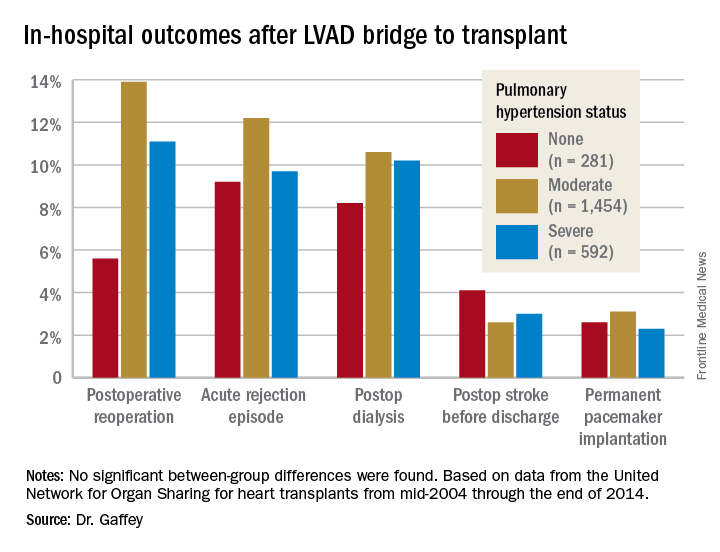
Vasodilators are prescribed in an effort to reduce PH; however, 40% of patients with PH are unresponsive to the medications and have therefore been excluded from consideration as potential candidates for a donor heart.
But the growing use of LVADs as a bridge to transplant has changed all that, Dr. Gaffey said. As supporting evidence, she presented a retrospective analysis of the United Network for Organ Sharing database on adult heart transplants from mid-2004 through the end of 2014.
The review turned up 3,951 heart transplant recipients who had been bridged to transplant with an LVAD. Dr. Gaffey and her coinvestigators divided them into three groups: 281 patients without pretransplant PH; 1,454 with moderate PH as defined by 1-3 Wood units; and 592 with severe PH and more than 3 Wood units.
The three groups didn’t differ in terms of age, sex, wait-list time, or the prevalence of diabetes or renal, liver, or cerebrovascular disease. Nor did their donors differ in age, sex, left ventricular function, or allograft ischemic time.
Key in-hospital outcomes were similar between the groups with no, mild, and severe PH.
Moreover, there was no between-group difference in the rate of rejection at 1 year. Five-year survival rates were closely similar in the three groups, in the mid-70s.
Audience member Nahush A. Mokadam, MD, rose to praise Dr. Gaffey’s report.
“This is a great and important study. I think as a group we have been too conservative with pulmonary hypertension, so thank you for shining a good light on it,” said Dr. Mokadam of the University of Washington, Seattle.
Dr. Gaffey reported having no financial conflicts regarding the study, which was conducted free of commercial support.
COLORADO SPRINGS – Reconsideration of the role of pulmonary hypertension in heart transplant outcomes is appropriate in the emerging era of the use of left ventricular assist devices (LVADs) as bridge to transplant, according to Ann C. Gaffey, MD, of the University of Pennsylvania, Philadelphia.
“Pulmonary hypertension secondary to congestive heart failure more than likely can be reversed to the values acceptable for heart transplant by the use of an LVAD. For bridge-to-transplant patients, pretransplant pulmonary hypertension does not affect recipient outcomes post transplantation,” she said at the annual meeting of the Western Thoracic Surgical Association.
Vasodilators are prescribed in an effort to reduce PH; however, 40% of patients with PH are unresponsive to the medications and have therefore been excluded from consideration as potential candidates for a donor heart.
But the growing use of LVADs as a bridge to transplant has changed all that, Dr. Gaffey said. As supporting evidence, she presented a retrospective analysis of the United Network for Organ Sharing database on adult heart transplants from mid-2004 through the end of 2014.
The review turned up 3,951 heart transplant recipients who had been bridged to transplant with an LVAD. Dr. Gaffey and her coinvestigators divided them into three groups: 281 patients without pretransplant PH; 1,454 with moderate PH as defined by 1-3 Wood units; and 592 with severe PH and more than 3 Wood units.
The three groups didn’t differ in terms of age, sex, wait-list time, or the prevalence of diabetes or renal, liver, or cerebrovascular disease. Nor did their donors differ in age, sex, left ventricular function, or allograft ischemic time.
Key in-hospital outcomes were similar between the groups with no, mild, and severe PH.
Moreover, there was no between-group difference in the rate of rejection at 1 year. Five-year survival rates were closely similar in the three groups, in the mid-70s.
Audience member Nahush A. Mokadam, MD, rose to praise Dr. Gaffey’s report.
“This is a great and important study. I think as a group we have been too conservative with pulmonary hypertension, so thank you for shining a good light on it,” said Dr. Mokadam of the University of Washington, Seattle.
Dr. Gaffey reported having no financial conflicts regarding the study, which was conducted free of commercial support.
COLORADO SPRINGS – Reconsideration of the role of pulmonary hypertension in heart transplant outcomes is appropriate in the emerging era of the use of left ventricular assist devices (LVADs) as bridge to transplant, according to Ann C. Gaffey, MD, of the University of Pennsylvania, Philadelphia.
“Pulmonary hypertension secondary to congestive heart failure more than likely can be reversed to the values acceptable for heart transplant by the use of an LVAD. For bridge-to-transplant patients, pretransplant pulmonary hypertension does not affect recipient outcomes post transplantation,” she said at the annual meeting of the Western Thoracic Surgical Association.
Vasodilators are prescribed in an effort to reduce PH; however, 40% of patients with PH are unresponsive to the medications and have therefore been excluded from consideration as potential candidates for a donor heart.
But the growing use of LVADs as a bridge to transplant has changed all that, Dr. Gaffey said. As supporting evidence, she presented a retrospective analysis of the United Network for Organ Sharing database on adult heart transplants from mid-2004 through the end of 2014.
The review turned up 3,951 heart transplant recipients who had been bridged to transplant with an LVAD. Dr. Gaffey and her coinvestigators divided them into three groups: 281 patients without pretransplant PH; 1,454 with moderate PH as defined by 1-3 Wood units; and 592 with severe PH and more than 3 Wood units.
The three groups didn’t differ in terms of age, sex, wait-list time, or the prevalence of diabetes or renal, liver, or cerebrovascular disease. Nor did their donors differ in age, sex, left ventricular function, or allograft ischemic time.
Key in-hospital outcomes were similar between the groups with no, mild, and severe PH.
Moreover, there was no between-group difference in the rate of rejection at 1 year. Five-year survival rates were closely similar in the three groups, in the mid-70s.
Audience member Nahush A. Mokadam, MD, rose to praise Dr. Gaffey’s report.
“This is a great and important study. I think as a group we have been too conservative with pulmonary hypertension, so thank you for shining a good light on it,” said Dr. Mokadam of the University of Washington, Seattle.
Dr. Gaffey reported having no financial conflicts regarding the study, which was conducted free of commercial support.
AT THE WTSA ANNUAL MEETING
Key clinical point:
Major finding: It’s time to reconsider the practice of excluding patients with pulmonary hypertension from consideration for a donor heart.
Data source: A retrospective analysis of the United Network for Organ Sharing database including outcomes out to 5 years on 3,951 heart transplant recipients who had been bridged to transplant with an LVAD, most of whom had moderate or severe pulmonary hypertension before transplant.
Disclosures: This study was conducted free of commercial support. The presenter reported having no relevant financial conflicts of interest.
Keep PCI patients on aspirin for noncardiac surgery
ANAHEIM, CALIF. – For every 1,000 patients with a history of percutaneous coronary intervention undergoing noncardiac surgery, perioperative aspirin would prevent 59 myocardial infarctions but cause 8 major/life-threatening bleeds, according to a substudy of the POISE-2 trial presented at the American Heart Association scientific sessions.
For patients with previous PCI undergoing noncardiac surgery, “I think aspirin will be more likely to benefit them than harm them,” so long as they are not having an operation where bleeding would be devastating.” These include “delicate neurosurgery in which, if you bleed into your spine, you end up paralyzed,” said lead investigator Michelle Graham, MD, an interventional cardiologist and professor of cardiology at the University of Alberta, Edmonton.
The original multisite POISE-2 trial (Perioperative Ischemic Evaluation 2) evaluated the effect of perioperative aspirin for noncardiac surgery. Patients were randomized to receive 200 mg aspirin or placebo within 4 hours of surgery and then 100 mg aspirin or placebo in the early postoperative period. There was no significant effect on the composite rate of death or myocardial infarction, but an increased risk of serious bleeding (N Engl J Med. 2014 Apr 17;370[16]:1494-503).
The new substudy focused on the 470 patients with previous PCIs, because such patients are known to have a higher risk for postop complications. More than half received bare-metal stents and a quarter got drug-eluting stents; in most of the rest, the stent type was not known. The median duration from PCI to noncardiac surgery was 64 months, ranging from 34 to 113 months. Patients with bare-metal stents placed within 6 weeks or drug-eluting stents within a year, were excluded.
Overall, 234 patients were randomized to the aspirin group, and 236 to placebo. Among those who came in on chronic, daily aspirin therapy – as almost all of the PCI subjects did – those who were randomized to perioperative aspirin stayed on daily 100 mg aspirin for a week postop, and then flipped back to whatever dose they were on at home. Likewise, placebo patients resumed their home aspirin after 1 week.
The results were very different from the main trial. At 30 days’ follow-up, just 6% of patients in the aspirin arm reached the primary endpoint of death or MI, versus 11.5% in the placebo group, a statistically significant 50% reduction.
This difference was driven almost entirely by a reduction in MIs. Whereas 5.1% of patients in the aspirin arm had MIs, 11% of the placebo group did, a significant 64% reduction. Meanwhile, the risk of major or life-threatening bleeding was not only similar between groups, but also to the overall trial, noted in 5.6% of aspirin and 4.2% of placebo subjects.
Over 75% of the participants were men, almost 60% were undergoing a major surgery, 30% had diabetes, and many had hypertension. Very few were on direct oral anticoagulants. The two arms were well matched, with a median age of about 68 years.
Simultaneously with Dr. Graham’s presentation, the results were published online (Ann Intern Med. 2017 Nov 14; doi: 10.7326/M17-2341)
The work was funded mostly by the Canadian Institutes of Health Research. Bayer supplied the aspirin. Dr. Graham has no industry disclosures.
ANAHEIM, CALIF. – For every 1,000 patients with a history of percutaneous coronary intervention undergoing noncardiac surgery, perioperative aspirin would prevent 59 myocardial infarctions but cause 8 major/life-threatening bleeds, according to a substudy of the POISE-2 trial presented at the American Heart Association scientific sessions.
For patients with previous PCI undergoing noncardiac surgery, “I think aspirin will be more likely to benefit them than harm them,” so long as they are not having an operation where bleeding would be devastating.” These include “delicate neurosurgery in which, if you bleed into your spine, you end up paralyzed,” said lead investigator Michelle Graham, MD, an interventional cardiologist and professor of cardiology at the University of Alberta, Edmonton.
The original multisite POISE-2 trial (Perioperative Ischemic Evaluation 2) evaluated the effect of perioperative aspirin for noncardiac surgery. Patients were randomized to receive 200 mg aspirin or placebo within 4 hours of surgery and then 100 mg aspirin or placebo in the early postoperative period. There was no significant effect on the composite rate of death or myocardial infarction, but an increased risk of serious bleeding (N Engl J Med. 2014 Apr 17;370[16]:1494-503).
The new substudy focused on the 470 patients with previous PCIs, because such patients are known to have a higher risk for postop complications. More than half received bare-metal stents and a quarter got drug-eluting stents; in most of the rest, the stent type was not known. The median duration from PCI to noncardiac surgery was 64 months, ranging from 34 to 113 months. Patients with bare-metal stents placed within 6 weeks or drug-eluting stents within a year, were excluded.
Overall, 234 patients were randomized to the aspirin group, and 236 to placebo. Among those who came in on chronic, daily aspirin therapy – as almost all of the PCI subjects did – those who were randomized to perioperative aspirin stayed on daily 100 mg aspirin for a week postop, and then flipped back to whatever dose they were on at home. Likewise, placebo patients resumed their home aspirin after 1 week.
The results were very different from the main trial. At 30 days’ follow-up, just 6% of patients in the aspirin arm reached the primary endpoint of death or MI, versus 11.5% in the placebo group, a statistically significant 50% reduction.
This difference was driven almost entirely by a reduction in MIs. Whereas 5.1% of patients in the aspirin arm had MIs, 11% of the placebo group did, a significant 64% reduction. Meanwhile, the risk of major or life-threatening bleeding was not only similar between groups, but also to the overall trial, noted in 5.6% of aspirin and 4.2% of placebo subjects.
Over 75% of the participants were men, almost 60% were undergoing a major surgery, 30% had diabetes, and many had hypertension. Very few were on direct oral anticoagulants. The two arms were well matched, with a median age of about 68 years.
Simultaneously with Dr. Graham’s presentation, the results were published online (Ann Intern Med. 2017 Nov 14; doi: 10.7326/M17-2341)
The work was funded mostly by the Canadian Institutes of Health Research. Bayer supplied the aspirin. Dr. Graham has no industry disclosures.
ANAHEIM, CALIF. – For every 1,000 patients with a history of percutaneous coronary intervention undergoing noncardiac surgery, perioperative aspirin would prevent 59 myocardial infarctions but cause 8 major/life-threatening bleeds, according to a substudy of the POISE-2 trial presented at the American Heart Association scientific sessions.
For patients with previous PCI undergoing noncardiac surgery, “I think aspirin will be more likely to benefit them than harm them,” so long as they are not having an operation where bleeding would be devastating.” These include “delicate neurosurgery in which, if you bleed into your spine, you end up paralyzed,” said lead investigator Michelle Graham, MD, an interventional cardiologist and professor of cardiology at the University of Alberta, Edmonton.
The original multisite POISE-2 trial (Perioperative Ischemic Evaluation 2) evaluated the effect of perioperative aspirin for noncardiac surgery. Patients were randomized to receive 200 mg aspirin or placebo within 4 hours of surgery and then 100 mg aspirin or placebo in the early postoperative period. There was no significant effect on the composite rate of death or myocardial infarction, but an increased risk of serious bleeding (N Engl J Med. 2014 Apr 17;370[16]:1494-503).
The new substudy focused on the 470 patients with previous PCIs, because such patients are known to have a higher risk for postop complications. More than half received bare-metal stents and a quarter got drug-eluting stents; in most of the rest, the stent type was not known. The median duration from PCI to noncardiac surgery was 64 months, ranging from 34 to 113 months. Patients with bare-metal stents placed within 6 weeks or drug-eluting stents within a year, were excluded.
Overall, 234 patients were randomized to the aspirin group, and 236 to placebo. Among those who came in on chronic, daily aspirin therapy – as almost all of the PCI subjects did – those who were randomized to perioperative aspirin stayed on daily 100 mg aspirin for a week postop, and then flipped back to whatever dose they were on at home. Likewise, placebo patients resumed their home aspirin after 1 week.
The results were very different from the main trial. At 30 days’ follow-up, just 6% of patients in the aspirin arm reached the primary endpoint of death or MI, versus 11.5% in the placebo group, a statistically significant 50% reduction.
This difference was driven almost entirely by a reduction in MIs. Whereas 5.1% of patients in the aspirin arm had MIs, 11% of the placebo group did, a significant 64% reduction. Meanwhile, the risk of major or life-threatening bleeding was not only similar between groups, but also to the overall trial, noted in 5.6% of aspirin and 4.2% of placebo subjects.
Over 75% of the participants were men, almost 60% were undergoing a major surgery, 30% had diabetes, and many had hypertension. Very few were on direct oral anticoagulants. The two arms were well matched, with a median age of about 68 years.
Simultaneously with Dr. Graham’s presentation, the results were published online (Ann Intern Med. 2017 Nov 14; doi: 10.7326/M17-2341)
The work was funded mostly by the Canadian Institutes of Health Research. Bayer supplied the aspirin. Dr. Graham has no industry disclosures.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: For every 1,000 patients with a history of percutaneous coronary intervention undergoing noncardiac surgery, perioperative aspirin would prevent 59 myocardial infarctions but cause 8 major/life-threatening bleeds.
Data source: POISE-2, a randomized trial of 470 PCI patients.
Disclosures: The work was funded mostly by the Canadian Institutes of Health Research. Bayer supplied the aspirin. The lead investigator has no industry disclosures.
VIDEO: Regionalized STEMI care slashes in-hospital mortality
ANAHEIM, CALIF. – An American Heart Association program aimed at streamlining care of patients with ST-elevation MI resulted in a dramatic near-halving of in-hospital mortality, compared with STEMI patients treated in hospitals not participating in the project, James G. Jollis, MD, reported at the American Heart Association scientific sessions.
He presented the results of the STEMI ACCELERATOR 2 study, which involved 12 participating metropolitan regions across the United States, 132 percutaneous coronary intervention–capable hospitals, and 946 emergency medical services agencies. The ACCELERATOR 2 program entailed regional implementation of a structured STEMI care plan in which EMS personnel were trained to obtain prehospital ECGs and to activate cardiac catheterization labs prior to hospital arrival, bypassing the emergency department when appropriate.
Key elements of the project, which was part of the AHA’s Mission: Lifeline program, included having participating hospitals measure their performance of key processes and send that information as well as patient outcome data to the National Cardiovascular Data Registry’s ACTION–Get With The Guidelines registry. The hospitals in turn received quarterly feedback reports containing blinded hospital comparisons.
Dr. Jollis and his coinvestigators worked to obtain buy-in from local stakeholders, organize regional leadership, and help in drafting a central regional STEMI plan featuring prespecified treatment protocols.
The STEMI ACCELERATOR 2 study was carried out in 2015-2017, during which 10,730 patients with STEMI were transported directly to participating hospitals with PCI capability.
The primary study outcome was the change from the first to the final quarter of the study in the proportion of EMS-transported patients with a time from first medical contact to treatment in the cath lab of 90 minutes or less. This improved significantly, from 67% at baseline to 74% in the final quarter. Nine of the 12 participating regions reduced their time from first medical contact to treatment in the cath lab, and eight reached the national of goal of having 75% of STEMI patients treated within 90 minutes.
The other key time-to-care measures improved, too: At baseline, only 38% of patients had a time from first medical contact to cath lab activation of 20 minutes or less; by the final quarter, this figure had climbed to 56%. That’s an important metric, as evidenced by the study finding that in-hospital mortality occurred in 4.5% of patients with a time from first medical contact to cath lab activation of more than 20 minutes, compared with 2.2% in those with a time of 20 minutes or less.
Also, the proportion of patients who spent 20 minutes or less in the emergency department improved from 33% to 43%.
In-hospital mortality improved from 4.4% in the baseline quarter to 2.3% in the final quarter. No similar improvement in in-hospital mortality occurred in a comparison group of 22,651 STEMI patients treated at hospitals not involved in ACCELERATOR 2.
A significant reduction in the rate of in-hospital congestive heart failure occurred in the ACCELERATOR 2 centers, from 7.4% at baseline to 5.0%. In contrast, stroke, cardiogenic shock, and major bleeding rates were unchanged over time.
The ACCELERATOR 2 model of emergency cardiovascular care is designed to be highly generalizable, according to Dr. Jollis.
“This study supports the implementation of regionally coordinated systems across the United States to abort heart attacks, save lives, and enable heart attack victims to return to their families and productive lives,” he said.
The ACCELERATOR 2 operations manual – essentially a blueprint for organizing a regional STEMI system of care – is available gratis.
Dr. Allen, a cardiologist at the University of Colorado, Denver, said the ACCELERATOR 2 model has been successful because it is consistent with a fundamental principle of implementation science as described by Carolyn Clancy, MD, Executive in Charge at the Veterans Health Affairs Administration, who has said it’s a matter of making the right thing to do the easy thing to do.
Gregg C. Fonarow, MD, founder of the Get With the Guidelines program, predicted that the success of this program will lead to a ramping up of efforts to regionalize and coordinate STEMI care across the country. “I hope and anticipate that the AHA will take and run with the ACCELERATOR 2 model and adopt this into Mission: Lifeline, hoping to make this the standard approach to further improving care and outcomes in these patients,” said Dr. Fonarow, professor and cochief of cardiology at the University of California, Los Angeles, in a video interview.
Simultaneous with his presentation at the AHA conference, the results of STEMI ACCELERATOR 2 were published online in Circulation (2017 Nov 14; doi: 0.1161/CIRCULATIONAHA.117.032446).
The trial was sponsored by research and educational grants from AstraZeneca and The Medicines Company. Dr. Jollis reported having no financial conflicts of interest.
ANAHEIM, CALIF. – An American Heart Association program aimed at streamlining care of patients with ST-elevation MI resulted in a dramatic near-halving of in-hospital mortality, compared with STEMI patients treated in hospitals not participating in the project, James G. Jollis, MD, reported at the American Heart Association scientific sessions.
He presented the results of the STEMI ACCELERATOR 2 study, which involved 12 participating metropolitan regions across the United States, 132 percutaneous coronary intervention–capable hospitals, and 946 emergency medical services agencies. The ACCELERATOR 2 program entailed regional implementation of a structured STEMI care plan in which EMS personnel were trained to obtain prehospital ECGs and to activate cardiac catheterization labs prior to hospital arrival, bypassing the emergency department when appropriate.
Key elements of the project, which was part of the AHA’s Mission: Lifeline program, included having participating hospitals measure their performance of key processes and send that information as well as patient outcome data to the National Cardiovascular Data Registry’s ACTION–Get With The Guidelines registry. The hospitals in turn received quarterly feedback reports containing blinded hospital comparisons.
Dr. Jollis and his coinvestigators worked to obtain buy-in from local stakeholders, organize regional leadership, and help in drafting a central regional STEMI plan featuring prespecified treatment protocols.
The STEMI ACCELERATOR 2 study was carried out in 2015-2017, during which 10,730 patients with STEMI were transported directly to participating hospitals with PCI capability.
The primary study outcome was the change from the first to the final quarter of the study in the proportion of EMS-transported patients with a time from first medical contact to treatment in the cath lab of 90 minutes or less. This improved significantly, from 67% at baseline to 74% in the final quarter. Nine of the 12 participating regions reduced their time from first medical contact to treatment in the cath lab, and eight reached the national of goal of having 75% of STEMI patients treated within 90 minutes.
The other key time-to-care measures improved, too: At baseline, only 38% of patients had a time from first medical contact to cath lab activation of 20 minutes or less; by the final quarter, this figure had climbed to 56%. That’s an important metric, as evidenced by the study finding that in-hospital mortality occurred in 4.5% of patients with a time from first medical contact to cath lab activation of more than 20 minutes, compared with 2.2% in those with a time of 20 minutes or less.
Also, the proportion of patients who spent 20 minutes or less in the emergency department improved from 33% to 43%.
In-hospital mortality improved from 4.4% in the baseline quarter to 2.3% in the final quarter. No similar improvement in in-hospital mortality occurred in a comparison group of 22,651 STEMI patients treated at hospitals not involved in ACCELERATOR 2.
A significant reduction in the rate of in-hospital congestive heart failure occurred in the ACCELERATOR 2 centers, from 7.4% at baseline to 5.0%. In contrast, stroke, cardiogenic shock, and major bleeding rates were unchanged over time.
The ACCELERATOR 2 model of emergency cardiovascular care is designed to be highly generalizable, according to Dr. Jollis.
“This study supports the implementation of regionally coordinated systems across the United States to abort heart attacks, save lives, and enable heart attack victims to return to their families and productive lives,” he said.
The ACCELERATOR 2 operations manual – essentially a blueprint for organizing a regional STEMI system of care – is available gratis.
Dr. Allen, a cardiologist at the University of Colorado, Denver, said the ACCELERATOR 2 model has been successful because it is consistent with a fundamental principle of implementation science as described by Carolyn Clancy, MD, Executive in Charge at the Veterans Health Affairs Administration, who has said it’s a matter of making the right thing to do the easy thing to do.
Gregg C. Fonarow, MD, founder of the Get With the Guidelines program, predicted that the success of this program will lead to a ramping up of efforts to regionalize and coordinate STEMI care across the country. “I hope and anticipate that the AHA will take and run with the ACCELERATOR 2 model and adopt this into Mission: Lifeline, hoping to make this the standard approach to further improving care and outcomes in these patients,” said Dr. Fonarow, professor and cochief of cardiology at the University of California, Los Angeles, in a video interview.
Simultaneous with his presentation at the AHA conference, the results of STEMI ACCELERATOR 2 were published online in Circulation (2017 Nov 14; doi: 0.1161/CIRCULATIONAHA.117.032446).
The trial was sponsored by research and educational grants from AstraZeneca and The Medicines Company. Dr. Jollis reported having no financial conflicts of interest.
ANAHEIM, CALIF. – An American Heart Association program aimed at streamlining care of patients with ST-elevation MI resulted in a dramatic near-halving of in-hospital mortality, compared with STEMI patients treated in hospitals not participating in the project, James G. Jollis, MD, reported at the American Heart Association scientific sessions.
He presented the results of the STEMI ACCELERATOR 2 study, which involved 12 participating metropolitan regions across the United States, 132 percutaneous coronary intervention–capable hospitals, and 946 emergency medical services agencies. The ACCELERATOR 2 program entailed regional implementation of a structured STEMI care plan in which EMS personnel were trained to obtain prehospital ECGs and to activate cardiac catheterization labs prior to hospital arrival, bypassing the emergency department when appropriate.
Key elements of the project, which was part of the AHA’s Mission: Lifeline program, included having participating hospitals measure their performance of key processes and send that information as well as patient outcome data to the National Cardiovascular Data Registry’s ACTION–Get With The Guidelines registry. The hospitals in turn received quarterly feedback reports containing blinded hospital comparisons.
Dr. Jollis and his coinvestigators worked to obtain buy-in from local stakeholders, organize regional leadership, and help in drafting a central regional STEMI plan featuring prespecified treatment protocols.
The STEMI ACCELERATOR 2 study was carried out in 2015-2017, during which 10,730 patients with STEMI were transported directly to participating hospitals with PCI capability.
The primary study outcome was the change from the first to the final quarter of the study in the proportion of EMS-transported patients with a time from first medical contact to treatment in the cath lab of 90 minutes or less. This improved significantly, from 67% at baseline to 74% in the final quarter. Nine of the 12 participating regions reduced their time from first medical contact to treatment in the cath lab, and eight reached the national of goal of having 75% of STEMI patients treated within 90 minutes.
The other key time-to-care measures improved, too: At baseline, only 38% of patients had a time from first medical contact to cath lab activation of 20 minutes or less; by the final quarter, this figure had climbed to 56%. That’s an important metric, as evidenced by the study finding that in-hospital mortality occurred in 4.5% of patients with a time from first medical contact to cath lab activation of more than 20 minutes, compared with 2.2% in those with a time of 20 minutes or less.
Also, the proportion of patients who spent 20 minutes or less in the emergency department improved from 33% to 43%.
In-hospital mortality improved from 4.4% in the baseline quarter to 2.3% in the final quarter. No similar improvement in in-hospital mortality occurred in a comparison group of 22,651 STEMI patients treated at hospitals not involved in ACCELERATOR 2.
A significant reduction in the rate of in-hospital congestive heart failure occurred in the ACCELERATOR 2 centers, from 7.4% at baseline to 5.0%. In contrast, stroke, cardiogenic shock, and major bleeding rates were unchanged over time.
The ACCELERATOR 2 model of emergency cardiovascular care is designed to be highly generalizable, according to Dr. Jollis.
“This study supports the implementation of regionally coordinated systems across the United States to abort heart attacks, save lives, and enable heart attack victims to return to their families and productive lives,” he said.
The ACCELERATOR 2 operations manual – essentially a blueprint for organizing a regional STEMI system of care – is available gratis.
Dr. Allen, a cardiologist at the University of Colorado, Denver, said the ACCELERATOR 2 model has been successful because it is consistent with a fundamental principle of implementation science as described by Carolyn Clancy, MD, Executive in Charge at the Veterans Health Affairs Administration, who has said it’s a matter of making the right thing to do the easy thing to do.
Gregg C. Fonarow, MD, founder of the Get With the Guidelines program, predicted that the success of this program will lead to a ramping up of efforts to regionalize and coordinate STEMI care across the country. “I hope and anticipate that the AHA will take and run with the ACCELERATOR 2 model and adopt this into Mission: Lifeline, hoping to make this the standard approach to further improving care and outcomes in these patients,” said Dr. Fonarow, professor and cochief of cardiology at the University of California, Los Angeles, in a video interview.
Simultaneous with his presentation at the AHA conference, the results of STEMI ACCELERATOR 2 were published online in Circulation (2017 Nov 14; doi: 0.1161/CIRCULATIONAHA.117.032446).
The trial was sponsored by research and educational grants from AstraZeneca and The Medicines Company. Dr. Jollis reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The in-hospital mortality rate of STEMI patients dropped from 4.4% in the baseline quarter to 2.3% in the final quarter of a study that examined the impact of introducing regionalized STEMI care.
Data source: This was a prospective study of an intervention that involved implementation of regionalized STEMI care in a dozen U.S. metropolitan areas.
Disclosures: The study was sponsored by research and educational grants from AstraZeneca and The Medicines Company. The presenter reported having no financial conflicts of interest.
Bicarb, acetylcysteine during angiography don’t protect kidneys
Periprocedural administration of intravenous sodium bicarbonate did not improve outcomes compared with standard sodium chloride in patients with impaired kidney function undergoing angiography, according to results of a randomized study of 5,177 patients.
In addition, there was no benefit for oral acetylcysteine administration over placebo for mitigating those same postangiography risks, Steven D. Weisbord, MD, said at the American Heart Association scientific sessions in Anaheim, Calif.
Hypothetically, both sodium bicarbonate and acetylcysteine could help prevent acute kidney injury associated with contrast material used during angiography, said Dr. Weisbord of the University of Pittsburgh.
However, multiple studies of the two agents have yielded “inconsistent results … consequently, equipoise exists regarding these interventions, despite their widespread use in clinical practice,” Dr. Weisbord said.
To provide more definitive evidence, Dr. Weisbord and his colleagues conducted PRESERVE, a multicenter, randomized, controlled trial comprising 5,177 patients scheduled for angiography who were at high risk of renal complications. Using a 2-by-2 factorial design, patients were randomized to receive intravenous 1.26% sodium bicarbonate or intravenous 0.9% sodium chloride, and to 5 days of oral acetylcysteine or oral placebo.
They found no significant differences between arms in the study’s composite primary endpoint of death, need for dialysis, or persistent increase in serum creatinine by 50% or more.
That composite endpoint occurred in 4.4% of patients receiving sodium bicarbonate, and similarly in 4.7% of patients receiving sodium chloride.
Likewise, the endpoint occurred in 4.6% of patients in the acetylcysteine group and 4.5% of the placebo group, Dr. Weisbord reported.
The investigators had planned to enroll 7,680 patients, but the sponsor of the trial stopped the study after enrollment of 5,177 based on the results showing no significant benefit of either treatment, he noted.
There are a few reasons why results of PRESERVE might show a lack of benefit for these agents, in contrast to some previous studies suggesting both the treatments might reduce risk of contrast-associated renal complications in high-risk patients.
Notably, “most of these interventions have been underpowered,” Dr. Weisbord noted.
Also, most previous trials used a primary endpoint of increase in blood creatinine level within days of the angiography. By contrast, the primary endpoint of the current study was a composite of serious adverse events “that are recognized sequelae of acute kidney injury,” he added.
Although subsequent investigations could shed new light on the controversy, the findings of PRESERVE support the “strong likelihood that these interventions are not clinically effective” in preventing acute kidney injury or longer-term adverse outcomes after angiography, he concluded.
The PRESERVE results were published simultaneously with Dr. Weisbord’s presentation (N Engl J Med. 2017 Nov 12. doi: 10.1056/NEJMoa1710933).
The study was supported by the U.S. Department of Veterans Affairs Office of Research and Development and the National Health and Medical Research Council of Australia. Dr. Weisbord reported receiving personal fees from Durect outside the submitted work.
Periprocedural administration of intravenous sodium bicarbonate did not improve outcomes compared with standard sodium chloride in patients with impaired kidney function undergoing angiography, according to results of a randomized study of 5,177 patients.
In addition, there was no benefit for oral acetylcysteine administration over placebo for mitigating those same postangiography risks, Steven D. Weisbord, MD, said at the American Heart Association scientific sessions in Anaheim, Calif.
Hypothetically, both sodium bicarbonate and acetylcysteine could help prevent acute kidney injury associated with contrast material used during angiography, said Dr. Weisbord of the University of Pittsburgh.
However, multiple studies of the two agents have yielded “inconsistent results … consequently, equipoise exists regarding these interventions, despite their widespread use in clinical practice,” Dr. Weisbord said.
To provide more definitive evidence, Dr. Weisbord and his colleagues conducted PRESERVE, a multicenter, randomized, controlled trial comprising 5,177 patients scheduled for angiography who were at high risk of renal complications. Using a 2-by-2 factorial design, patients were randomized to receive intravenous 1.26% sodium bicarbonate or intravenous 0.9% sodium chloride, and to 5 days of oral acetylcysteine or oral placebo.
They found no significant differences between arms in the study’s composite primary endpoint of death, need for dialysis, or persistent increase in serum creatinine by 50% or more.
That composite endpoint occurred in 4.4% of patients receiving sodium bicarbonate, and similarly in 4.7% of patients receiving sodium chloride.
Likewise, the endpoint occurred in 4.6% of patients in the acetylcysteine group and 4.5% of the placebo group, Dr. Weisbord reported.
The investigators had planned to enroll 7,680 patients, but the sponsor of the trial stopped the study after enrollment of 5,177 based on the results showing no significant benefit of either treatment, he noted.
There are a few reasons why results of PRESERVE might show a lack of benefit for these agents, in contrast to some previous studies suggesting both the treatments might reduce risk of contrast-associated renal complications in high-risk patients.
Notably, “most of these interventions have been underpowered,” Dr. Weisbord noted.
Also, most previous trials used a primary endpoint of increase in blood creatinine level within days of the angiography. By contrast, the primary endpoint of the current study was a composite of serious adverse events “that are recognized sequelae of acute kidney injury,” he added.
Although subsequent investigations could shed new light on the controversy, the findings of PRESERVE support the “strong likelihood that these interventions are not clinically effective” in preventing acute kidney injury or longer-term adverse outcomes after angiography, he concluded.
The PRESERVE results were published simultaneously with Dr. Weisbord’s presentation (N Engl J Med. 2017 Nov 12. doi: 10.1056/NEJMoa1710933).
The study was supported by the U.S. Department of Veterans Affairs Office of Research and Development and the National Health and Medical Research Council of Australia. Dr. Weisbord reported receiving personal fees from Durect outside the submitted work.
Periprocedural administration of intravenous sodium bicarbonate did not improve outcomes compared with standard sodium chloride in patients with impaired kidney function undergoing angiography, according to results of a randomized study of 5,177 patients.
In addition, there was no benefit for oral acetylcysteine administration over placebo for mitigating those same postangiography risks, Steven D. Weisbord, MD, said at the American Heart Association scientific sessions in Anaheim, Calif.
Hypothetically, both sodium bicarbonate and acetylcysteine could help prevent acute kidney injury associated with contrast material used during angiography, said Dr. Weisbord of the University of Pittsburgh.
However, multiple studies of the two agents have yielded “inconsistent results … consequently, equipoise exists regarding these interventions, despite their widespread use in clinical practice,” Dr. Weisbord said.
To provide more definitive evidence, Dr. Weisbord and his colleagues conducted PRESERVE, a multicenter, randomized, controlled trial comprising 5,177 patients scheduled for angiography who were at high risk of renal complications. Using a 2-by-2 factorial design, patients were randomized to receive intravenous 1.26% sodium bicarbonate or intravenous 0.9% sodium chloride, and to 5 days of oral acetylcysteine or oral placebo.
They found no significant differences between arms in the study’s composite primary endpoint of death, need for dialysis, or persistent increase in serum creatinine by 50% or more.
That composite endpoint occurred in 4.4% of patients receiving sodium bicarbonate, and similarly in 4.7% of patients receiving sodium chloride.
Likewise, the endpoint occurred in 4.6% of patients in the acetylcysteine group and 4.5% of the placebo group, Dr. Weisbord reported.
The investigators had planned to enroll 7,680 patients, but the sponsor of the trial stopped the study after enrollment of 5,177 based on the results showing no significant benefit of either treatment, he noted.
There are a few reasons why results of PRESERVE might show a lack of benefit for these agents, in contrast to some previous studies suggesting both the treatments might reduce risk of contrast-associated renal complications in high-risk patients.
Notably, “most of these interventions have been underpowered,” Dr. Weisbord noted.
Also, most previous trials used a primary endpoint of increase in blood creatinine level within days of the angiography. By contrast, the primary endpoint of the current study was a composite of serious adverse events “that are recognized sequelae of acute kidney injury,” he added.
Although subsequent investigations could shed new light on the controversy, the findings of PRESERVE support the “strong likelihood that these interventions are not clinically effective” in preventing acute kidney injury or longer-term adverse outcomes after angiography, he concluded.
The PRESERVE results were published simultaneously with Dr. Weisbord’s presentation (N Engl J Med. 2017 Nov 12. doi: 10.1056/NEJMoa1710933).
The study was supported by the U.S. Department of Veterans Affairs Office of Research and Development and the National Health and Medical Research Council of Australia. Dr. Weisbord reported receiving personal fees from Durect outside the submitted work.
FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The composite primary endpoint of death, need for dialysis, or persistent increase in serum creatinine was similar regardless of which treatments the patients received.
Data source: PRESERVE, a randomized study using a 2-by-2 factorial design to evaluate intravenous sodium bicarbonate versus sodium chloride and acetylcysteine versus placebo in 5,177 patients at high risk of renal complications.
Disclosures: PRESERVE was supported by the U.S. Department of Veterans Affairs Office of Research and Development and the National Health and Medical Research Council of Australia. Dr. Weisbord reported receiving personal fees from Durect outside the submitted work.
Gut bacteria influenced response to checkpoint inhibitors
The gut microbome may influence responses to immune checkpoint inhibitors, based on results from two studies, and one of the investigators is now gearing up for the next step - evaluating in a clinical trial whether altering the microflora will actually improve responses.
In the first study, investigators carried out a series of experiments using fecal microbiome samples from patients with metastatic melanoma embarking on therapy with a PD-1 (programmed cell death protein 1) inhibitor.
“In melanoma patients, there were differential signals in the gut microbiome of responders versus nonresponders, and I think the clincher was when we transplanted fecal samples from responders to nonresponders in germ-free mice, essentially reconstituting the microbiome and showing that it equally affected the systemic immunity and antitumor immunity when we implanted tumors, as well as response to checkpoint blockade,” lead author Jennifer A. Wargo, MD, MMSc, of the University of Texas MD Anderson Cancer Center in Houston, said in an interview.
Dr. Wargo and her colleagues first collected buccal and fecal microbiome samples from 112 patients with metastatic melanoma before they began therapy with a PD-1 inhibitor. After performing taxonomic profiling on all samples, they found that there was a clustering effect by response status in the gut microbiome, but not the oral microbiome, and because changes in the oral microbiome did not appear to be related to treatment response, they focused on the gut.
When Dr. Wargo and her colleagues studied the posttherapy microbiomes of 43 patients (30 responders and 13 nonresponders) according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1), they found that the responders had a significantly higher degree of alpha diversity, a measure of species diversity within a specific environment, compared with nonresponders (P less than .01). In addition, responders had a relative abundance of Ruminococcaceae, commonly occurring gut microbes that break down complex carbohydrates, the investigators reported (Science. 2017 Nov. 2. doi: 10.1126/science.aan4236).
They found that patients whose microbiomes were diverse in general, and in particular were enriched with Faecalibacterium and Clostridiales species, were more likely to respond to immunotherapy with a PD-1 inhibitor and have a longer duration of progression-free survival. In contrast, patients whose microbiomes were more enriched with Bacteroidales species were more likely to be nonresponders.
To get a better understanding of the mechanisms whereby gut bacteria may influence response to PD-1 inhibitors, they performed metagenomic analysis on samples from 14 responders and 11 nonresponders, and found that responders had micro-organisms predominantly associated with anabolic functions that may support host immunity, whereas nonresponders had microbiomes where catabolic functions were more common.
The investigators next performed immune profiling, and found that both systemic immunity and local immunity in the tumor microenvironment in responders were associated with the aforementioned favorable gut microbiome.
The researchers then transplanted feces from the human donors into germ-free mice and then injected tumor cells into the mice, and found that tumor growth was significantly reduced, and response to PD-1 inhibition was significantly enhanced, in mice who received feces from responders.
“An obvious next step is to run a clinical trial to test the hypothesis that by modulating the microbiome, you can actually enhance responses to therapy,” Dr. Wargo said. Details of the clinical trial are still being worked out, but will likely involve fecal transfers and other mechanisms for modulating the microbiome in hopes of improving responses to PD-1 inhibitors.
“It’s going to be a very biomarker-heavy trial,” she said. “We’re going to look, certainly, for changes in the microbiome, and will also do a lot of profiling in the blood, the tumor, and in the microbiome to see if there are changes that occur by modulating that microbiome. Then of course we’ll look for differences in response rates in patients as well.”
Bacteria also affect epithelial cancers
In a separate study, also published in Science, investigators led by Bertrand Routy, MD, of the Gustave Roussy Cancer Institute in Villejuif, France, reported that patients with non–small cell lung cancer and urothelial carcinoma who had previously used systemic antibiotics had reduced survival when treated with a PD-1 inhibitor, compared with patients who had never taken antibiotics (Science. 2017 Nov. 2 doi: 10.1126/science.aan3706).
Analysis of the gut microbiome in these patients showed that higher levels of Akkermansia muciniphila were associated with the best clinical outcomes, with the species detectable in the microbiome of 69% of patients who had partial responses to anti–PD-1 therapy, and in 58% of those with stable disease. In contrast, the bacterium was detectable in only 34% of patients who experienced disease progression.
As in the experiments by Dr. Wargo and her associates, when the French investigators first treated mice with antibiotics and then gave them oral supplements containing the bacteria, the supplements restored response to PD-1 blockade,
“We conclude from the study that the gut microbiome markedly influences the outcome of PD-1 blockade in mice and patients,” Dr. Routy and his associates wrote.
They acknowledged that the mechanism whereby a common organism such as Akkermansia muciniphila might have an immunomodulatory effect is still unknown,
“Irrespective of these remaining questions, our findings suggest that the microbiome governs the cancer-immune set point of cancer-bearing individuals and offer[s] novel avenues for manipulating the gut ecosystem to circumvent primary resistance to [immune checkpoint inhibitors],” they wrote.
The study by Dr. Wargo and her colleagues was supported by contributions to the University of Texas MD Anderson Melanoma Moon Shots program. Dr. Wargo is supported by the Binational Science Foundation, Melanoma Research Alliance, Stand Up to Cancer, and the MDACC Melanoma Moon Shots Program. The work by Dr. Routy and his associates was supported by the Goustave Roussy Cancer Institute and McGill University. Coauthors were supported by the National Cancer Institute of France and other agencies and philanthropies.
The gut microbome may influence responses to immune checkpoint inhibitors, based on results from two studies, and one of the investigators is now gearing up for the next step - evaluating in a clinical trial whether altering the microflora will actually improve responses.
In the first study, investigators carried out a series of experiments using fecal microbiome samples from patients with metastatic melanoma embarking on therapy with a PD-1 (programmed cell death protein 1) inhibitor.
“In melanoma patients, there were differential signals in the gut microbiome of responders versus nonresponders, and I think the clincher was when we transplanted fecal samples from responders to nonresponders in germ-free mice, essentially reconstituting the microbiome and showing that it equally affected the systemic immunity and antitumor immunity when we implanted tumors, as well as response to checkpoint blockade,” lead author Jennifer A. Wargo, MD, MMSc, of the University of Texas MD Anderson Cancer Center in Houston, said in an interview.
Dr. Wargo and her colleagues first collected buccal and fecal microbiome samples from 112 patients with metastatic melanoma before they began therapy with a PD-1 inhibitor. After performing taxonomic profiling on all samples, they found that there was a clustering effect by response status in the gut microbiome, but not the oral microbiome, and because changes in the oral microbiome did not appear to be related to treatment response, they focused on the gut.
When Dr. Wargo and her colleagues studied the posttherapy microbiomes of 43 patients (30 responders and 13 nonresponders) according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1), they found that the responders had a significantly higher degree of alpha diversity, a measure of species diversity within a specific environment, compared with nonresponders (P less than .01). In addition, responders had a relative abundance of Ruminococcaceae, commonly occurring gut microbes that break down complex carbohydrates, the investigators reported (Science. 2017 Nov. 2. doi: 10.1126/science.aan4236).
They found that patients whose microbiomes were diverse in general, and in particular were enriched with Faecalibacterium and Clostridiales species, were more likely to respond to immunotherapy with a PD-1 inhibitor and have a longer duration of progression-free survival. In contrast, patients whose microbiomes were more enriched with Bacteroidales species were more likely to be nonresponders.
To get a better understanding of the mechanisms whereby gut bacteria may influence response to PD-1 inhibitors, they performed metagenomic analysis on samples from 14 responders and 11 nonresponders, and found that responders had micro-organisms predominantly associated with anabolic functions that may support host immunity, whereas nonresponders had microbiomes where catabolic functions were more common.
The investigators next performed immune profiling, and found that both systemic immunity and local immunity in the tumor microenvironment in responders were associated with the aforementioned favorable gut microbiome.
The researchers then transplanted feces from the human donors into germ-free mice and then injected tumor cells into the mice, and found that tumor growth was significantly reduced, and response to PD-1 inhibition was significantly enhanced, in mice who received feces from responders.
“An obvious next step is to run a clinical trial to test the hypothesis that by modulating the microbiome, you can actually enhance responses to therapy,” Dr. Wargo said. Details of the clinical trial are still being worked out, but will likely involve fecal transfers and other mechanisms for modulating the microbiome in hopes of improving responses to PD-1 inhibitors.
“It’s going to be a very biomarker-heavy trial,” she said. “We’re going to look, certainly, for changes in the microbiome, and will also do a lot of profiling in the blood, the tumor, and in the microbiome to see if there are changes that occur by modulating that microbiome. Then of course we’ll look for differences in response rates in patients as well.”
Bacteria also affect epithelial cancers
In a separate study, also published in Science, investigators led by Bertrand Routy, MD, of the Gustave Roussy Cancer Institute in Villejuif, France, reported that patients with non–small cell lung cancer and urothelial carcinoma who had previously used systemic antibiotics had reduced survival when treated with a PD-1 inhibitor, compared with patients who had never taken antibiotics (Science. 2017 Nov. 2 doi: 10.1126/science.aan3706).
Analysis of the gut microbiome in these patients showed that higher levels of Akkermansia muciniphila were associated with the best clinical outcomes, with the species detectable in the microbiome of 69% of patients who had partial responses to anti–PD-1 therapy, and in 58% of those with stable disease. In contrast, the bacterium was detectable in only 34% of patients who experienced disease progression.
As in the experiments by Dr. Wargo and her associates, when the French investigators first treated mice with antibiotics and then gave them oral supplements containing the bacteria, the supplements restored response to PD-1 blockade,
“We conclude from the study that the gut microbiome markedly influences the outcome of PD-1 blockade in mice and patients,” Dr. Routy and his associates wrote.
They acknowledged that the mechanism whereby a common organism such as Akkermansia muciniphila might have an immunomodulatory effect is still unknown,
“Irrespective of these remaining questions, our findings suggest that the microbiome governs the cancer-immune set point of cancer-bearing individuals and offer[s] novel avenues for manipulating the gut ecosystem to circumvent primary resistance to [immune checkpoint inhibitors],” they wrote.
The study by Dr. Wargo and her colleagues was supported by contributions to the University of Texas MD Anderson Melanoma Moon Shots program. Dr. Wargo is supported by the Binational Science Foundation, Melanoma Research Alliance, Stand Up to Cancer, and the MDACC Melanoma Moon Shots Program. The work by Dr. Routy and his associates was supported by the Goustave Roussy Cancer Institute and McGill University. Coauthors were supported by the National Cancer Institute of France and other agencies and philanthropies.
The gut microbome may influence responses to immune checkpoint inhibitors, based on results from two studies, and one of the investigators is now gearing up for the next step - evaluating in a clinical trial whether altering the microflora will actually improve responses.
In the first study, investigators carried out a series of experiments using fecal microbiome samples from patients with metastatic melanoma embarking on therapy with a PD-1 (programmed cell death protein 1) inhibitor.
“In melanoma patients, there were differential signals in the gut microbiome of responders versus nonresponders, and I think the clincher was when we transplanted fecal samples from responders to nonresponders in germ-free mice, essentially reconstituting the microbiome and showing that it equally affected the systemic immunity and antitumor immunity when we implanted tumors, as well as response to checkpoint blockade,” lead author Jennifer A. Wargo, MD, MMSc, of the University of Texas MD Anderson Cancer Center in Houston, said in an interview.
Dr. Wargo and her colleagues first collected buccal and fecal microbiome samples from 112 patients with metastatic melanoma before they began therapy with a PD-1 inhibitor. After performing taxonomic profiling on all samples, they found that there was a clustering effect by response status in the gut microbiome, but not the oral microbiome, and because changes in the oral microbiome did not appear to be related to treatment response, they focused on the gut.
When Dr. Wargo and her colleagues studied the posttherapy microbiomes of 43 patients (30 responders and 13 nonresponders) according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1), they found that the responders had a significantly higher degree of alpha diversity, a measure of species diversity within a specific environment, compared with nonresponders (P less than .01). In addition, responders had a relative abundance of Ruminococcaceae, commonly occurring gut microbes that break down complex carbohydrates, the investigators reported (Science. 2017 Nov. 2. doi: 10.1126/science.aan4236).
They found that patients whose microbiomes were diverse in general, and in particular were enriched with Faecalibacterium and Clostridiales species, were more likely to respond to immunotherapy with a PD-1 inhibitor and have a longer duration of progression-free survival. In contrast, patients whose microbiomes were more enriched with Bacteroidales species were more likely to be nonresponders.
To get a better understanding of the mechanisms whereby gut bacteria may influence response to PD-1 inhibitors, they performed metagenomic analysis on samples from 14 responders and 11 nonresponders, and found that responders had micro-organisms predominantly associated with anabolic functions that may support host immunity, whereas nonresponders had microbiomes where catabolic functions were more common.
The investigators next performed immune profiling, and found that both systemic immunity and local immunity in the tumor microenvironment in responders were associated with the aforementioned favorable gut microbiome.
The researchers then transplanted feces from the human donors into germ-free mice and then injected tumor cells into the mice, and found that tumor growth was significantly reduced, and response to PD-1 inhibition was significantly enhanced, in mice who received feces from responders.
“An obvious next step is to run a clinical trial to test the hypothesis that by modulating the microbiome, you can actually enhance responses to therapy,” Dr. Wargo said. Details of the clinical trial are still being worked out, but will likely involve fecal transfers and other mechanisms for modulating the microbiome in hopes of improving responses to PD-1 inhibitors.
“It’s going to be a very biomarker-heavy trial,” she said. “We’re going to look, certainly, for changes in the microbiome, and will also do a lot of profiling in the blood, the tumor, and in the microbiome to see if there are changes that occur by modulating that microbiome. Then of course we’ll look for differences in response rates in patients as well.”
Bacteria also affect epithelial cancers
In a separate study, also published in Science, investigators led by Bertrand Routy, MD, of the Gustave Roussy Cancer Institute in Villejuif, France, reported that patients with non–small cell lung cancer and urothelial carcinoma who had previously used systemic antibiotics had reduced survival when treated with a PD-1 inhibitor, compared with patients who had never taken antibiotics (Science. 2017 Nov. 2 doi: 10.1126/science.aan3706).
Analysis of the gut microbiome in these patients showed that higher levels of Akkermansia muciniphila were associated with the best clinical outcomes, with the species detectable in the microbiome of 69% of patients who had partial responses to anti–PD-1 therapy, and in 58% of those with stable disease. In contrast, the bacterium was detectable in only 34% of patients who experienced disease progression.
As in the experiments by Dr. Wargo and her associates, when the French investigators first treated mice with antibiotics and then gave them oral supplements containing the bacteria, the supplements restored response to PD-1 blockade,
“We conclude from the study that the gut microbiome markedly influences the outcome of PD-1 blockade in mice and patients,” Dr. Routy and his associates wrote.
They acknowledged that the mechanism whereby a common organism such as Akkermansia muciniphila might have an immunomodulatory effect is still unknown,
“Irrespective of these remaining questions, our findings suggest that the microbiome governs the cancer-immune set point of cancer-bearing individuals and offer[s] novel avenues for manipulating the gut ecosystem to circumvent primary resistance to [immune checkpoint inhibitors],” they wrote.
The study by Dr. Wargo and her colleagues was supported by contributions to the University of Texas MD Anderson Melanoma Moon Shots program. Dr. Wargo is supported by the Binational Science Foundation, Melanoma Research Alliance, Stand Up to Cancer, and the MDACC Melanoma Moon Shots Program. The work by Dr. Routy and his associates was supported by the Goustave Roussy Cancer Institute and McGill University. Coauthors were supported by the National Cancer Institute of France and other agencies and philanthropies.
FROM SCIENCE
Key clinical point: Modulating the gut microbome may improve responses to immune checkpoint inhibitors in patients with advanced melanoma, non–small cell lung cancer, and urothelial carcinoma.
Major finding: Responders to a checkpoint inhibitor had a significantly higher degree of alpha diversity, a measure of species diversity within a specific environment, compared with nonresponders (P less than .01).
Data source: A series of studies using microbiome samples from cancer patients receiving immune checkpoint inhibitors.
Disclosures: The study by Dr. Wargo and her colleagues was supported by contributions to the University of Texas MD Anderson Melanoma Moon Shots Program. Dr. Wargo is supported by the Binational Science Foundation, Melanoma Research Alliance, Stand Up to Cancer, and the MDACC Melanoma Moon Shots Program. The work by Dr. Routy and his colleagues was supported by the Goustave Roussy Cancer Institute and McGill University. Coauthors were supported by the National Cancer Institute of France and other agencies and philanthropies.
VIDEO: U.S. hypertension guidelines reset threshold to 130/80 mm Hg
ANAHEIM, CALIF. – Thirty million Americans became hypertensive overnight on Nov. 13 with the introduction of new high blood pressure guidelines from the American College of Cardiology and American Heart Association.
That happened by resetting the definition of adult hypertension from the long-standing threshold of 140/90 mm Hg to a blood pressure at or above 130/80 mm Hg, a change that jumps the U.S. adult prevalence of hypertension from roughly 32% to 46%. Nearly half of all U.S. adults now have hypertension, bringing the total national hypertensive population to a staggering 103 million.
Goal is to transform care
But the new guidelines (J Am Coll Cardiol. 2017 Nov 13. doi: 10.1016/j.jacc.2017.11.005) for preventing, detecting, evaluating, and managing adult hypertension do lots more than just shake up the epidemiology of high blood pressure. With 106 total recommendations, the guidelines seek to transform every aspect of blood pressure in American medical practice, starting with how it’s measured and stretching to redefine applications of medical systems to try to ensure that every person with a blood pressure that truly falls outside the redefined limits gets a comprehensive package of interventions.
Many of these are “seismic changes,” said Lawrence J. Appel, MD. He particularly cited as seismic the new classification of stage 1 hypertension as a pressure at or above 130/80 mm Hg, the emphasis on using some form of out-of-office blood pressure measurement to confirm a diagnosis, the use of risk assessment when deciding whether to treat certain patients with drugs, and the same blood pressure goal of less than 130/80 mm Hg for all hypertensives, regardless of age, as long as they remain ambulatory and community dwelling.
One goal for all adults
“The systolic blood pressure goal for older people has gone from 140 mm Hg to 150 mm Hg and now to 130 mm Hg in the space of 2-3 years,” commented Dr. Appel, professor of epidemiology at Johns Hopkins University in Baltimore and not involved in the guideline-writing process.
In fact, the guidelines simplified the treatment goal all around, to less than 130/80 mm Hg for patients with diabetes, those with chronic kidney disease, and the elderly; that goal remains the same for all adults.
“It will be clearer and easier now that everyone should be less than 130/80 mm Hg. You won’t need to remember a second target,” said Sandra J. Taler, MD, a nephrologist and professor of medicine at the Mayo Clinic in Rochester, Minn., and a member of the guidelines task force.
“Some people may be upset that we changed the rules on them. They had normal blood pressure yesterday, and today it’s high. But it’s a good awakening, especially for using lifestyle interventions,” Dr. Taler said in an interview.
Preferred intervention: Lifestyle, not drugs
Lifestyle optimization is repeatedly cited as the cornerstone of intervention for everyone, including those with elevated blood pressure with a systolic pressure of 120-129 mm Hg, and as the only endorsed intervention for patients with hypertension of 130-139 mm Hg but below a 10% risk for a cardiovascular disease event during the next 10 years on the American College of Cardiology’s online risk calculator. The guidelines list six lifestyle goals: weight loss, following a DASH diet, reducing sodium, enhancing potassium, 90-150 min/wk of physical activity, and moderate alcohol intake.
Team-based care essential
The guidelines also put unprecedented emphasis on using a team-based management approach, which means having nurses, nurse practitioners, pharmacists, dietitians, and other clinicians, allowing for more frequent and focused care. Dr. Whelton and others cited in particular the VA Health System and Kaiser-Permanente as operating team-based and system-driven blood pressure management programs that have resulted in control rates for more than 90% of hypertensive patients. The team-based approach is also a key in the Target:BP program that the American Heart Association and American Medical Association founded. Target:BP will be instrumental in promoting implementation of the new guidelines, Dr. Carey said. Another systems recommendation is that every patient with hypertension should have a “clear, detailed, and current evidence-based plan of care.”
“Using nurse practitioners, physician assistants, and pharmacists has been shown to improve blood pressure levels,” and health systems that use this approach have had “great success,” commented Donald M. Lloyd-Jones, MD, professor and chairman of preventive medicine at Northwestern University in Chicago and not part of the guidelines task force. Some systems have used this approach to achieve high levels of blood pressure control. Now that financial penalties and incentives from payers also exist to push for higher levels of blood pressure control, the alignment of financial and health incentives should result in big changes, Dr. Lloyd-Jones predicted in a video interview.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ANAHEIM, CALIF. – Thirty million Americans became hypertensive overnight on Nov. 13 with the introduction of new high blood pressure guidelines from the American College of Cardiology and American Heart Association.
That happened by resetting the definition of adult hypertension from the long-standing threshold of 140/90 mm Hg to a blood pressure at or above 130/80 mm Hg, a change that jumps the U.S. adult prevalence of hypertension from roughly 32% to 46%. Nearly half of all U.S. adults now have hypertension, bringing the total national hypertensive population to a staggering 103 million.
Goal is to transform care
But the new guidelines (J Am Coll Cardiol. 2017 Nov 13. doi: 10.1016/j.jacc.2017.11.005) for preventing, detecting, evaluating, and managing adult hypertension do lots more than just shake up the epidemiology of high blood pressure. With 106 total recommendations, the guidelines seek to transform every aspect of blood pressure in American medical practice, starting with how it’s measured and stretching to redefine applications of medical systems to try to ensure that every person with a blood pressure that truly falls outside the redefined limits gets a comprehensive package of interventions.
Many of these are “seismic changes,” said Lawrence J. Appel, MD. He particularly cited as seismic the new classification of stage 1 hypertension as a pressure at or above 130/80 mm Hg, the emphasis on using some form of out-of-office blood pressure measurement to confirm a diagnosis, the use of risk assessment when deciding whether to treat certain patients with drugs, and the same blood pressure goal of less than 130/80 mm Hg for all hypertensives, regardless of age, as long as they remain ambulatory and community dwelling.
One goal for all adults
“The systolic blood pressure goal for older people has gone from 140 mm Hg to 150 mm Hg and now to 130 mm Hg in the space of 2-3 years,” commented Dr. Appel, professor of epidemiology at Johns Hopkins University in Baltimore and not involved in the guideline-writing process.
In fact, the guidelines simplified the treatment goal all around, to less than 130/80 mm Hg for patients with diabetes, those with chronic kidney disease, and the elderly; that goal remains the same for all adults.
“It will be clearer and easier now that everyone should be less than 130/80 mm Hg. You won’t need to remember a second target,” said Sandra J. Taler, MD, a nephrologist and professor of medicine at the Mayo Clinic in Rochester, Minn., and a member of the guidelines task force.
“Some people may be upset that we changed the rules on them. They had normal blood pressure yesterday, and today it’s high. But it’s a good awakening, especially for using lifestyle interventions,” Dr. Taler said in an interview.
Preferred intervention: Lifestyle, not drugs
Lifestyle optimization is repeatedly cited as the cornerstone of intervention for everyone, including those with elevated blood pressure with a systolic pressure of 120-129 mm Hg, and as the only endorsed intervention for patients with hypertension of 130-139 mm Hg but below a 10% risk for a cardiovascular disease event during the next 10 years on the American College of Cardiology’s online risk calculator. The guidelines list six lifestyle goals: weight loss, following a DASH diet, reducing sodium, enhancing potassium, 90-150 min/wk of physical activity, and moderate alcohol intake.
Team-based care essential
The guidelines also put unprecedented emphasis on using a team-based management approach, which means having nurses, nurse practitioners, pharmacists, dietitians, and other clinicians, allowing for more frequent and focused care. Dr. Whelton and others cited in particular the VA Health System and Kaiser-Permanente as operating team-based and system-driven blood pressure management programs that have resulted in control rates for more than 90% of hypertensive patients. The team-based approach is also a key in the Target:BP program that the American Heart Association and American Medical Association founded. Target:BP will be instrumental in promoting implementation of the new guidelines, Dr. Carey said. Another systems recommendation is that every patient with hypertension should have a “clear, detailed, and current evidence-based plan of care.”
“Using nurse practitioners, physician assistants, and pharmacists has been shown to improve blood pressure levels,” and health systems that use this approach have had “great success,” commented Donald M. Lloyd-Jones, MD, professor and chairman of preventive medicine at Northwestern University in Chicago and not part of the guidelines task force. Some systems have used this approach to achieve high levels of blood pressure control. Now that financial penalties and incentives from payers also exist to push for higher levels of blood pressure control, the alignment of financial and health incentives should result in big changes, Dr. Lloyd-Jones predicted in a video interview.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ANAHEIM, CALIF. – Thirty million Americans became hypertensive overnight on Nov. 13 with the introduction of new high blood pressure guidelines from the American College of Cardiology and American Heart Association.
That happened by resetting the definition of adult hypertension from the long-standing threshold of 140/90 mm Hg to a blood pressure at or above 130/80 mm Hg, a change that jumps the U.S. adult prevalence of hypertension from roughly 32% to 46%. Nearly half of all U.S. adults now have hypertension, bringing the total national hypertensive population to a staggering 103 million.
Goal is to transform care
But the new guidelines (J Am Coll Cardiol. 2017 Nov 13. doi: 10.1016/j.jacc.2017.11.005) for preventing, detecting, evaluating, and managing adult hypertension do lots more than just shake up the epidemiology of high blood pressure. With 106 total recommendations, the guidelines seek to transform every aspect of blood pressure in American medical practice, starting with how it’s measured and stretching to redefine applications of medical systems to try to ensure that every person with a blood pressure that truly falls outside the redefined limits gets a comprehensive package of interventions.
Many of these are “seismic changes,” said Lawrence J. Appel, MD. He particularly cited as seismic the new classification of stage 1 hypertension as a pressure at or above 130/80 mm Hg, the emphasis on using some form of out-of-office blood pressure measurement to confirm a diagnosis, the use of risk assessment when deciding whether to treat certain patients with drugs, and the same blood pressure goal of less than 130/80 mm Hg for all hypertensives, regardless of age, as long as they remain ambulatory and community dwelling.
One goal for all adults
“The systolic blood pressure goal for older people has gone from 140 mm Hg to 150 mm Hg and now to 130 mm Hg in the space of 2-3 years,” commented Dr. Appel, professor of epidemiology at Johns Hopkins University in Baltimore and not involved in the guideline-writing process.
In fact, the guidelines simplified the treatment goal all around, to less than 130/80 mm Hg for patients with diabetes, those with chronic kidney disease, and the elderly; that goal remains the same for all adults.
“It will be clearer and easier now that everyone should be less than 130/80 mm Hg. You won’t need to remember a second target,” said Sandra J. Taler, MD, a nephrologist and professor of medicine at the Mayo Clinic in Rochester, Minn., and a member of the guidelines task force.
“Some people may be upset that we changed the rules on them. They had normal blood pressure yesterday, and today it’s high. But it’s a good awakening, especially for using lifestyle interventions,” Dr. Taler said in an interview.
Preferred intervention: Lifestyle, not drugs
Lifestyle optimization is repeatedly cited as the cornerstone of intervention for everyone, including those with elevated blood pressure with a systolic pressure of 120-129 mm Hg, and as the only endorsed intervention for patients with hypertension of 130-139 mm Hg but below a 10% risk for a cardiovascular disease event during the next 10 years on the American College of Cardiology’s online risk calculator. The guidelines list six lifestyle goals: weight loss, following a DASH diet, reducing sodium, enhancing potassium, 90-150 min/wk of physical activity, and moderate alcohol intake.
Team-based care essential
The guidelines also put unprecedented emphasis on using a team-based management approach, which means having nurses, nurse practitioners, pharmacists, dietitians, and other clinicians, allowing for more frequent and focused care. Dr. Whelton and others cited in particular the VA Health System and Kaiser-Permanente as operating team-based and system-driven blood pressure management programs that have resulted in control rates for more than 90% of hypertensive patients. The team-based approach is also a key in the Target:BP program that the American Heart Association and American Medical Association founded. Target:BP will be instrumental in promoting implementation of the new guidelines, Dr. Carey said. Another systems recommendation is that every patient with hypertension should have a “clear, detailed, and current evidence-based plan of care.”
“Using nurse practitioners, physician assistants, and pharmacists has been shown to improve blood pressure levels,” and health systems that use this approach have had “great success,” commented Donald M. Lloyd-Jones, MD, professor and chairman of preventive medicine at Northwestern University in Chicago and not part of the guidelines task force. Some systems have used this approach to achieve high levels of blood pressure control. Now that financial penalties and incentives from payers also exist to push for higher levels of blood pressure control, the alignment of financial and health incentives should result in big changes, Dr. Lloyd-Jones predicted in a video interview.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS
Targeting PCSK9 inhibitors to reap most benefit
ANAHEIM, CALIF. – Patients with symptomatic peripheral artery disease or a high-risk history of MI got the biggest bang for the buck from aggressive LDL cholesterol lowering with evolocumab in two new prespecified subgroup analyses from the landmark FOURIER trial presented at the American Heart Association scientific sessions.
“At the end of the day, not all of our patients with ASCVD [atherosclerotic cardiovascular disease] can have these expensive medications. These subgroup analyses will help clinicians to target use of PCSK9 inhibitors to the patients who will benefit the most,” Lynne T. Braun, PhD, commented in her role as discussant of the two secondary analyses, presented back to back in a late-breaking science session. Dr. Braun is a professor in the department of internal medicine at Rush University, Chicago.
The FOURIER trial included 27,564 high-risk patients with prior MI, stroke, and/or symptomatic peripheral arterial disease (PAD) who had an LDL cholesterol level of 70 mg/dL or more on high- or moderate-intensity statin therapy. They were randomized in double-blind fashion to add-on subcutaneous evolocumab (Repatha) at either 140 mg every 2 weeks or 420 mg/month or to placebo, for a median of 2.5 years of follow-up. The evolocumab group experienced a 59% reduction in LDL cholesterol, compared with the controls on background statin therapy plus placebo, down to a mean LDL cholesterol level of just 30 mg/dL.
As previously reported, the risk of the primary composite endpoint – comprising cardiovascular death, MI, stroke, unstable angina, or coronary revascularization – was reduced by 15% in the evolocumab group at 3 years. The secondary endpoint of cardiovascular death, MI, or stroke was reduced by 20%, from 9.9% to 7.9% (N Engl J Med. 2017;376:1713-22).
Evolocumab tamed PAD
At the AHA scientific sessions, Marc P. Bonaca, MD, presented a secondary analysis restricted to the 3,642 FOURIER participants with symptomatic PAD. The goal was to answer two unresolved questions: Does LDL cholesterol lowering beyond what’s achievable with a statin further reduce PAD patients’ cardiovascular risk? And does it reduce their risk of major adverse leg events (MALE), defined as a composite of acute limb ischemia, major amputation, and urgent revascularization?
The rate of the composite endpoint comprising cardiovascular death, MI, or stroke was 13% over 3 years in PAD patients randomized to placebo, which was 81% greater than the 7.6% rate in placebo-treated participants with a baseline history of stroke or MI but no PAD, in an analysis adjusted for demographics, cardiovascular risk factors, kidney function, body mass index, and prior revascularization.
The event rate was even higher in patients with PAD plus a history of MI or stroke, at 14.9%. Evolocumab reduced that risk by 27%, compared with placebo in patients with PAD, for an absolute risk reduction of 3.5% and a number-needed-to-treat (NNT) of 29 for 2.5 years.
The benefit of evolocumab was even more pronounced in the subgroup of 1,505 patients with baseline PAD but no prior MI or stroke: a 43% relative risk reduction, from 10.3% to 5.5%, for an absolute risk reduction of 4.8% and a NNT of 21.
A linear relationship was seen between the MALE rate during follow-up and LDL cholesterol level after 1 month of therapy, down to an LDL cholesterol level of less than 10 mg/dL. The clinically relevant composite endpoint of MACE (major adverse cardiovascular events – a composite of cardiovascular death, MI, and stroke) or MALE in patients with baseline PAD but no history of MI or stroke occurred in 12.8% of controls and 6.5% of the evolocumab group. This translated to a 48% relative risk reduction, a 6.3% absolute risk reduction, and a NNT of 16. The event curves in the evolocumab and control arms separated quite early, within the first 90 days of treatment.
The take home message: “LDL reduction to very low levels should be considered in patients with PAD, regardless of their history of MI or stroke, to reduce the risk of MACE [major adverse cardiovascular event] and MALE,” declared Dr. Bonaca of Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Spotting the patients with a history of MI who’re at highest risk
Marc S. Sabatine, MD, presented the subanalysis involving the 22,351 FOURIER patients with a prior MI. He and his coinvestigators identified three high-risk features within this group: an MI within the past 2 years, a history of two or more MIs, and residual multivessel CAD. Each of these three features was individually associated with a 34%-90% increased risk of MACE during follow-up. All told, 63% of FOURIER participants with prior MI had one or more of the high-risk features.
The use of evolocumab in patients with at least one of the three high-risk features was associated with a 22% relative risk reduction and an absolute 2.5% risk reduction, compared with placebo. The event curves diverged at about 6 months, and the gap between them steadily widened during follow-up. Extrapolating from this pattern, it’s likely that evolocumab would achieve an absolute 5% risk reduction in MACE, compared with placebo over 5 years, with an NNT of 20, according to Dr. Sabatine, professor of medicine at Harvard Medical School and chairman of the Thrombolysis in Myocardial Infarction (TIMI) Study Group.
Lingering questions
Dr. Braun was particularly impressed that the absolute risk reduction in MACE was even larger in patients with baseline PAD but no history of stroke or MI than in PAD patients with such a history. She added that, while she recognizes the value of selecting objectively assessable hard clinical MACE as the primary endpoint in FOURIER, her own patients care even more about other outcomes.
“What my patients with PAD care most about is whether profound LDL lowering translates to less claudication, improved quality of life, and greater physical activity tolerance. These were prespecified secondary outcomes in FOURIER, and I look forward to future reports addressing those issues,” she said.
Another unanswered question involves the mechanism by which intensive LDL cholesterol lowering results in fewer MACE and MALE events in high-risk subgroups. The possibilities include the plaque regression that was documented in the GLAGOV trial, an anti-inflammatory plaque-stabilizing effect being exerted through PCSK9 inhibition, or perhaps the PCSK9 inhibitors’ ability to moderately lower lipoprotein(a) cholesterol levels.
Simultaneous with Dr. Bonaca’s presentation at the AHA, the FOURIER PAD analysis was published online in Circulation (2017 Nov 13; doi: 10.1161/CIRCULATIONAHA.117.032235).
The FOURIER trial was sponsored by Amgen. Dr. Bonaca and Dr. Sabatine reported receiving research grants from and serving as consultants to Amgen and other companies.
ANAHEIM, CALIF. – Patients with symptomatic peripheral artery disease or a high-risk history of MI got the biggest bang for the buck from aggressive LDL cholesterol lowering with evolocumab in two new prespecified subgroup analyses from the landmark FOURIER trial presented at the American Heart Association scientific sessions.
“At the end of the day, not all of our patients with ASCVD [atherosclerotic cardiovascular disease] can have these expensive medications. These subgroup analyses will help clinicians to target use of PCSK9 inhibitors to the patients who will benefit the most,” Lynne T. Braun, PhD, commented in her role as discussant of the two secondary analyses, presented back to back in a late-breaking science session. Dr. Braun is a professor in the department of internal medicine at Rush University, Chicago.
The FOURIER trial included 27,564 high-risk patients with prior MI, stroke, and/or symptomatic peripheral arterial disease (PAD) who had an LDL cholesterol level of 70 mg/dL or more on high- or moderate-intensity statin therapy. They were randomized in double-blind fashion to add-on subcutaneous evolocumab (Repatha) at either 140 mg every 2 weeks or 420 mg/month or to placebo, for a median of 2.5 years of follow-up. The evolocumab group experienced a 59% reduction in LDL cholesterol, compared with the controls on background statin therapy plus placebo, down to a mean LDL cholesterol level of just 30 mg/dL.
As previously reported, the risk of the primary composite endpoint – comprising cardiovascular death, MI, stroke, unstable angina, or coronary revascularization – was reduced by 15% in the evolocumab group at 3 years. The secondary endpoint of cardiovascular death, MI, or stroke was reduced by 20%, from 9.9% to 7.9% (N Engl J Med. 2017;376:1713-22).
Evolocumab tamed PAD
At the AHA scientific sessions, Marc P. Bonaca, MD, presented a secondary analysis restricted to the 3,642 FOURIER participants with symptomatic PAD. The goal was to answer two unresolved questions: Does LDL cholesterol lowering beyond what’s achievable with a statin further reduce PAD patients’ cardiovascular risk? And does it reduce their risk of major adverse leg events (MALE), defined as a composite of acute limb ischemia, major amputation, and urgent revascularization?
The rate of the composite endpoint comprising cardiovascular death, MI, or stroke was 13% over 3 years in PAD patients randomized to placebo, which was 81% greater than the 7.6% rate in placebo-treated participants with a baseline history of stroke or MI but no PAD, in an analysis adjusted for demographics, cardiovascular risk factors, kidney function, body mass index, and prior revascularization.
The event rate was even higher in patients with PAD plus a history of MI or stroke, at 14.9%. Evolocumab reduced that risk by 27%, compared with placebo in patients with PAD, for an absolute risk reduction of 3.5% and a number-needed-to-treat (NNT) of 29 for 2.5 years.
The benefit of evolocumab was even more pronounced in the subgroup of 1,505 patients with baseline PAD but no prior MI or stroke: a 43% relative risk reduction, from 10.3% to 5.5%, for an absolute risk reduction of 4.8% and a NNT of 21.
A linear relationship was seen between the MALE rate during follow-up and LDL cholesterol level after 1 month of therapy, down to an LDL cholesterol level of less than 10 mg/dL. The clinically relevant composite endpoint of MACE (major adverse cardiovascular events – a composite of cardiovascular death, MI, and stroke) or MALE in patients with baseline PAD but no history of MI or stroke occurred in 12.8% of controls and 6.5% of the evolocumab group. This translated to a 48% relative risk reduction, a 6.3% absolute risk reduction, and a NNT of 16. The event curves in the evolocumab and control arms separated quite early, within the first 90 days of treatment.
The take home message: “LDL reduction to very low levels should be considered in patients with PAD, regardless of their history of MI or stroke, to reduce the risk of MACE [major adverse cardiovascular event] and MALE,” declared Dr. Bonaca of Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Spotting the patients with a history of MI who’re at highest risk
Marc S. Sabatine, MD, presented the subanalysis involving the 22,351 FOURIER patients with a prior MI. He and his coinvestigators identified three high-risk features within this group: an MI within the past 2 years, a history of two or more MIs, and residual multivessel CAD. Each of these three features was individually associated with a 34%-90% increased risk of MACE during follow-up. All told, 63% of FOURIER participants with prior MI had one or more of the high-risk features.
The use of evolocumab in patients with at least one of the three high-risk features was associated with a 22% relative risk reduction and an absolute 2.5% risk reduction, compared with placebo. The event curves diverged at about 6 months, and the gap between them steadily widened during follow-up. Extrapolating from this pattern, it’s likely that evolocumab would achieve an absolute 5% risk reduction in MACE, compared with placebo over 5 years, with an NNT of 20, according to Dr. Sabatine, professor of medicine at Harvard Medical School and chairman of the Thrombolysis in Myocardial Infarction (TIMI) Study Group.
Lingering questions
Dr. Braun was particularly impressed that the absolute risk reduction in MACE was even larger in patients with baseline PAD but no history of stroke or MI than in PAD patients with such a history. She added that, while she recognizes the value of selecting objectively assessable hard clinical MACE as the primary endpoint in FOURIER, her own patients care even more about other outcomes.
“What my patients with PAD care most about is whether profound LDL lowering translates to less claudication, improved quality of life, and greater physical activity tolerance. These were prespecified secondary outcomes in FOURIER, and I look forward to future reports addressing those issues,” she said.
Another unanswered question involves the mechanism by which intensive LDL cholesterol lowering results in fewer MACE and MALE events in high-risk subgroups. The possibilities include the plaque regression that was documented in the GLAGOV trial, an anti-inflammatory plaque-stabilizing effect being exerted through PCSK9 inhibition, or perhaps the PCSK9 inhibitors’ ability to moderately lower lipoprotein(a) cholesterol levels.
Simultaneous with Dr. Bonaca’s presentation at the AHA, the FOURIER PAD analysis was published online in Circulation (2017 Nov 13; doi: 10.1161/CIRCULATIONAHA.117.032235).
The FOURIER trial was sponsored by Amgen. Dr. Bonaca and Dr. Sabatine reported receiving research grants from and serving as consultants to Amgen and other companies.
ANAHEIM, CALIF. – Patients with symptomatic peripheral artery disease or a high-risk history of MI got the biggest bang for the buck from aggressive LDL cholesterol lowering with evolocumab in two new prespecified subgroup analyses from the landmark FOURIER trial presented at the American Heart Association scientific sessions.
“At the end of the day, not all of our patients with ASCVD [atherosclerotic cardiovascular disease] can have these expensive medications. These subgroup analyses will help clinicians to target use of PCSK9 inhibitors to the patients who will benefit the most,” Lynne T. Braun, PhD, commented in her role as discussant of the two secondary analyses, presented back to back in a late-breaking science session. Dr. Braun is a professor in the department of internal medicine at Rush University, Chicago.
The FOURIER trial included 27,564 high-risk patients with prior MI, stroke, and/or symptomatic peripheral arterial disease (PAD) who had an LDL cholesterol level of 70 mg/dL or more on high- or moderate-intensity statin therapy. They were randomized in double-blind fashion to add-on subcutaneous evolocumab (Repatha) at either 140 mg every 2 weeks or 420 mg/month or to placebo, for a median of 2.5 years of follow-up. The evolocumab group experienced a 59% reduction in LDL cholesterol, compared with the controls on background statin therapy plus placebo, down to a mean LDL cholesterol level of just 30 mg/dL.
As previously reported, the risk of the primary composite endpoint – comprising cardiovascular death, MI, stroke, unstable angina, or coronary revascularization – was reduced by 15% in the evolocumab group at 3 years. The secondary endpoint of cardiovascular death, MI, or stroke was reduced by 20%, from 9.9% to 7.9% (N Engl J Med. 2017;376:1713-22).
Evolocumab tamed PAD
At the AHA scientific sessions, Marc P. Bonaca, MD, presented a secondary analysis restricted to the 3,642 FOURIER participants with symptomatic PAD. The goal was to answer two unresolved questions: Does LDL cholesterol lowering beyond what’s achievable with a statin further reduce PAD patients’ cardiovascular risk? And does it reduce their risk of major adverse leg events (MALE), defined as a composite of acute limb ischemia, major amputation, and urgent revascularization?
The rate of the composite endpoint comprising cardiovascular death, MI, or stroke was 13% over 3 years in PAD patients randomized to placebo, which was 81% greater than the 7.6% rate in placebo-treated participants with a baseline history of stroke or MI but no PAD, in an analysis adjusted for demographics, cardiovascular risk factors, kidney function, body mass index, and prior revascularization.
The event rate was even higher in patients with PAD plus a history of MI or stroke, at 14.9%. Evolocumab reduced that risk by 27%, compared with placebo in patients with PAD, for an absolute risk reduction of 3.5% and a number-needed-to-treat (NNT) of 29 for 2.5 years.
The benefit of evolocumab was even more pronounced in the subgroup of 1,505 patients with baseline PAD but no prior MI or stroke: a 43% relative risk reduction, from 10.3% to 5.5%, for an absolute risk reduction of 4.8% and a NNT of 21.
A linear relationship was seen between the MALE rate during follow-up and LDL cholesterol level after 1 month of therapy, down to an LDL cholesterol level of less than 10 mg/dL. The clinically relevant composite endpoint of MACE (major adverse cardiovascular events – a composite of cardiovascular death, MI, and stroke) or MALE in patients with baseline PAD but no history of MI or stroke occurred in 12.8% of controls and 6.5% of the evolocumab group. This translated to a 48% relative risk reduction, a 6.3% absolute risk reduction, and a NNT of 16. The event curves in the evolocumab and control arms separated quite early, within the first 90 days of treatment.
The take home message: “LDL reduction to very low levels should be considered in patients with PAD, regardless of their history of MI or stroke, to reduce the risk of MACE [major adverse cardiovascular event] and MALE,” declared Dr. Bonaca of Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Spotting the patients with a history of MI who’re at highest risk
Marc S. Sabatine, MD, presented the subanalysis involving the 22,351 FOURIER patients with a prior MI. He and his coinvestigators identified three high-risk features within this group: an MI within the past 2 years, a history of two or more MIs, and residual multivessel CAD. Each of these three features was individually associated with a 34%-90% increased risk of MACE during follow-up. All told, 63% of FOURIER participants with prior MI had one or more of the high-risk features.
The use of evolocumab in patients with at least one of the three high-risk features was associated with a 22% relative risk reduction and an absolute 2.5% risk reduction, compared with placebo. The event curves diverged at about 6 months, and the gap between them steadily widened during follow-up. Extrapolating from this pattern, it’s likely that evolocumab would achieve an absolute 5% risk reduction in MACE, compared with placebo over 5 years, with an NNT of 20, according to Dr. Sabatine, professor of medicine at Harvard Medical School and chairman of the Thrombolysis in Myocardial Infarction (TIMI) Study Group.
Lingering questions
Dr. Braun was particularly impressed that the absolute risk reduction in MACE was even larger in patients with baseline PAD but no history of stroke or MI than in PAD patients with such a history. She added that, while she recognizes the value of selecting objectively assessable hard clinical MACE as the primary endpoint in FOURIER, her own patients care even more about other outcomes.
“What my patients with PAD care most about is whether profound LDL lowering translates to less claudication, improved quality of life, and greater physical activity tolerance. These were prespecified secondary outcomes in FOURIER, and I look forward to future reports addressing those issues,” she said.
Another unanswered question involves the mechanism by which intensive LDL cholesterol lowering results in fewer MACE and MALE events in high-risk subgroups. The possibilities include the plaque regression that was documented in the GLAGOV trial, an anti-inflammatory plaque-stabilizing effect being exerted through PCSK9 inhibition, or perhaps the PCSK9 inhibitors’ ability to moderately lower lipoprotein(a) cholesterol levels.
Simultaneous with Dr. Bonaca’s presentation at the AHA, the FOURIER PAD analysis was published online in Circulation (2017 Nov 13; doi: 10.1161/CIRCULATIONAHA.117.032235).
The FOURIER trial was sponsored by Amgen. Dr. Bonaca and Dr. Sabatine reported receiving research grants from and serving as consultants to Amgen and other companies.
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS
Direct oral anticoagulants okay during AF device placement
ANAHEIM, CALIF. – Whether direct oral anticoagulants are continued or interrupted for device placement in atrial fibrillation patients, the risk of device pocket hematoma or stroke is very low, based on results of the BRUISE CONTROL–2 trial in more than 600 subjects.
Either strategy is reasonable depending on the clinical scenario, coprincipal investigator David Birnie, MD, said in presenting the results at the American Heart Association scientific sessions.
When atrial fibrillation (AF) patients on direct oral anticoagulants (DOACs) present for device surgery, there’s concern that keeping them on the drugs will increase the bleeding risk, but that taking them off will increase the stroke risk. “We sought to resolve this dilemma,” said Dr. Birnie, an electrophysiologist and director of the arrhythmia service at the University of Ottawa Heart Institute.
The subjects were on dabigatran, rivaroxaban, or apixaban, about a third in each group; 328 were randomized to continue their daily dosing, including on the day of surgery. The other 334 were randomized to interrupted treatment. For rivaroxaban and apixaban, that meant taking their last dose 2 days before surgery. Dabigatran patients discontinued the drug 1-2 days beforehand, depending on glomerular filtration rate. Patients resumed treatment about 24 hours after surgery. CHA2DS2-VASc scores were a mean of 3.9 in both arms, and at least 2 in all participants.
The rate of clinically significant hematoma – the primary outcome in the study, defined as a hematoma requiring prolonged hospitalization, interrupted postoperative anticoagulation, or reoperation to evacuate – was identical in both arms, 2.1% (seven patients each). There were two ischemic strokes, one in each arm. There was one delayed cardiac tamponade in the continuation arm and one pericardial effusion in the interrupted arm. The three deaths in the trial were not related to device placement.
So, what to do depends on the clinical scenario, Dr. Birnie said in an interview. If someone needs urgent placement and there’s no time to wait for DOAC washout, “it’s quite reasonable to go ahead.” Also, “if somebody is at extremely high risk for stroke, then it’s very reasonable to continue the drug.”
On the other hand, “if someone has a much lower stroke risk, then the risk-benefit ratio is probably in the opposite direction, so temporarily discontinuing the drug is the right thing to do,” he said.
Dr. Birnie cautioned that although continued DOAC may reduce the risk of thromboembolism, “this study was not designed with power to answer this.”
“We are already putting these findings into practice” in Ottawa, he said. “Our protocol” – as in many places – “ was always to stop anticoagulation for 2 or 3 days, but now, for very high-risk patients – high-risk AF, unstable temporary pacing, that type of thing – we are very comfortable continuing it,” he said. The study follows up a previous randomized trial by Dr. Birnie and his colleagues that pitted continued warfarin against heparin bridging for AF device placement. There were far fewer device pocket hematomas with uninterrupted warfarin (N Engl J Med. 2013 May 30;368[22]:2084-93).
The team wanted to repeat the study using DOACs, since their use has grown substantially, with the majority of AF patients now on them.
The arms in BRUISE CONTROL–2 (Strategy of Continued Versus Interrupted Novel Oral Anticoagulant at Time of Device Surgery in Patients With Moderate to High Risk of Arterial Thromboembolic Events) were well matched, with a mean age of about 74 years; men made up more than 70% of the subjects in both arms. About 17% of the participants were on chronic aspirin therapy and about 4% were on clopidogrel, in each arm. The uninterrupted DOAC group went about 14 hours between their last preop and first postop DOAC dose. The interrupted group went about 72 hours.
BRUISE CONTROL–2 was funded by the Heart and Stroke Foundation of Canada, Boehringer Ingelheim, Bayer, Pfizer, and Bristol-Myers Squibb, among others. Dr. Birnie had no relevant financial disclosures.
ANAHEIM, CALIF. – Whether direct oral anticoagulants are continued or interrupted for device placement in atrial fibrillation patients, the risk of device pocket hematoma or stroke is very low, based on results of the BRUISE CONTROL–2 trial in more than 600 subjects.
Either strategy is reasonable depending on the clinical scenario, coprincipal investigator David Birnie, MD, said in presenting the results at the American Heart Association scientific sessions.
When atrial fibrillation (AF) patients on direct oral anticoagulants (DOACs) present for device surgery, there’s concern that keeping them on the drugs will increase the bleeding risk, but that taking them off will increase the stroke risk. “We sought to resolve this dilemma,” said Dr. Birnie, an electrophysiologist and director of the arrhythmia service at the University of Ottawa Heart Institute.
The subjects were on dabigatran, rivaroxaban, or apixaban, about a third in each group; 328 were randomized to continue their daily dosing, including on the day of surgery. The other 334 were randomized to interrupted treatment. For rivaroxaban and apixaban, that meant taking their last dose 2 days before surgery. Dabigatran patients discontinued the drug 1-2 days beforehand, depending on glomerular filtration rate. Patients resumed treatment about 24 hours after surgery. CHA2DS2-VASc scores were a mean of 3.9 in both arms, and at least 2 in all participants.
The rate of clinically significant hematoma – the primary outcome in the study, defined as a hematoma requiring prolonged hospitalization, interrupted postoperative anticoagulation, or reoperation to evacuate – was identical in both arms, 2.1% (seven patients each). There were two ischemic strokes, one in each arm. There was one delayed cardiac tamponade in the continuation arm and one pericardial effusion in the interrupted arm. The three deaths in the trial were not related to device placement.
So, what to do depends on the clinical scenario, Dr. Birnie said in an interview. If someone needs urgent placement and there’s no time to wait for DOAC washout, “it’s quite reasonable to go ahead.” Also, “if somebody is at extremely high risk for stroke, then it’s very reasonable to continue the drug.”
On the other hand, “if someone has a much lower stroke risk, then the risk-benefit ratio is probably in the opposite direction, so temporarily discontinuing the drug is the right thing to do,” he said.
Dr. Birnie cautioned that although continued DOAC may reduce the risk of thromboembolism, “this study was not designed with power to answer this.”
“We are already putting these findings into practice” in Ottawa, he said. “Our protocol” – as in many places – “ was always to stop anticoagulation for 2 or 3 days, but now, for very high-risk patients – high-risk AF, unstable temporary pacing, that type of thing – we are very comfortable continuing it,” he said. The study follows up a previous randomized trial by Dr. Birnie and his colleagues that pitted continued warfarin against heparin bridging for AF device placement. There were far fewer device pocket hematomas with uninterrupted warfarin (N Engl J Med. 2013 May 30;368[22]:2084-93).
The team wanted to repeat the study using DOACs, since their use has grown substantially, with the majority of AF patients now on them.
The arms in BRUISE CONTROL–2 (Strategy of Continued Versus Interrupted Novel Oral Anticoagulant at Time of Device Surgery in Patients With Moderate to High Risk of Arterial Thromboembolic Events) were well matched, with a mean age of about 74 years; men made up more than 70% of the subjects in both arms. About 17% of the participants were on chronic aspirin therapy and about 4% were on clopidogrel, in each arm. The uninterrupted DOAC group went about 14 hours between their last preop and first postop DOAC dose. The interrupted group went about 72 hours.
BRUISE CONTROL–2 was funded by the Heart and Stroke Foundation of Canada, Boehringer Ingelheim, Bayer, Pfizer, and Bristol-Myers Squibb, among others. Dr. Birnie had no relevant financial disclosures.
ANAHEIM, CALIF. – Whether direct oral anticoagulants are continued or interrupted for device placement in atrial fibrillation patients, the risk of device pocket hematoma or stroke is very low, based on results of the BRUISE CONTROL–2 trial in more than 600 subjects.
Either strategy is reasonable depending on the clinical scenario, coprincipal investigator David Birnie, MD, said in presenting the results at the American Heart Association scientific sessions.
When atrial fibrillation (AF) patients on direct oral anticoagulants (DOACs) present for device surgery, there’s concern that keeping them on the drugs will increase the bleeding risk, but that taking them off will increase the stroke risk. “We sought to resolve this dilemma,” said Dr. Birnie, an electrophysiologist and director of the arrhythmia service at the University of Ottawa Heart Institute.
The subjects were on dabigatran, rivaroxaban, or apixaban, about a third in each group; 328 were randomized to continue their daily dosing, including on the day of surgery. The other 334 were randomized to interrupted treatment. For rivaroxaban and apixaban, that meant taking their last dose 2 days before surgery. Dabigatran patients discontinued the drug 1-2 days beforehand, depending on glomerular filtration rate. Patients resumed treatment about 24 hours after surgery. CHA2DS2-VASc scores were a mean of 3.9 in both arms, and at least 2 in all participants.
The rate of clinically significant hematoma – the primary outcome in the study, defined as a hematoma requiring prolonged hospitalization, interrupted postoperative anticoagulation, or reoperation to evacuate – was identical in both arms, 2.1% (seven patients each). There were two ischemic strokes, one in each arm. There was one delayed cardiac tamponade in the continuation arm and one pericardial effusion in the interrupted arm. The three deaths in the trial were not related to device placement.
So, what to do depends on the clinical scenario, Dr. Birnie said in an interview. If someone needs urgent placement and there’s no time to wait for DOAC washout, “it’s quite reasonable to go ahead.” Also, “if somebody is at extremely high risk for stroke, then it’s very reasonable to continue the drug.”
On the other hand, “if someone has a much lower stroke risk, then the risk-benefit ratio is probably in the opposite direction, so temporarily discontinuing the drug is the right thing to do,” he said.
Dr. Birnie cautioned that although continued DOAC may reduce the risk of thromboembolism, “this study was not designed with power to answer this.”
“We are already putting these findings into practice” in Ottawa, he said. “Our protocol” – as in many places – “ was always to stop anticoagulation for 2 or 3 days, but now, for very high-risk patients – high-risk AF, unstable temporary pacing, that type of thing – we are very comfortable continuing it,” he said. The study follows up a previous randomized trial by Dr. Birnie and his colleagues that pitted continued warfarin against heparin bridging for AF device placement. There were far fewer device pocket hematomas with uninterrupted warfarin (N Engl J Med. 2013 May 30;368[22]:2084-93).
The team wanted to repeat the study using DOACs, since their use has grown substantially, with the majority of AF patients now on them.
The arms in BRUISE CONTROL–2 (Strategy of Continued Versus Interrupted Novel Oral Anticoagulant at Time of Device Surgery in Patients With Moderate to High Risk of Arterial Thromboembolic Events) were well matched, with a mean age of about 74 years; men made up more than 70% of the subjects in both arms. About 17% of the participants were on chronic aspirin therapy and about 4% were on clopidogrel, in each arm. The uninterrupted DOAC group went about 14 hours between their last preop and first postop DOAC dose. The interrupted group went about 72 hours.
BRUISE CONTROL–2 was funded by the Heart and Stroke Foundation of Canada, Boehringer Ingelheim, Bayer, Pfizer, and Bristol-Myers Squibb, among others. Dr. Birnie had no relevant financial disclosures.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The rate of clinically significant hematoma was identical in both arms, at 2.1% (seven patients each).
Data source: BRUISE CONTROL-2, a randomized trial with more than 600 subjects.
Disclosures: The work was funded by the Heart and Stroke Foundation of Canada, Boehringer Ingelheim, Bayer, Pfizer, and Bristol-Myers Squibb, among others. The presenter had no relevant financial disclosures.















