User login
Epilepsy
In epilepsy, brain-responsive stimulation passes long-term tests
Two new long-term studies, one an extension trial and the other an analysis of real-world experience, show that Both studies showed that the benefit from the devices increased over time.
That accruing benefit may be because of improved protocols as clinicians gain experience with the device or because of network remodeling that occurs over time as seizures are controlled. “I think it’s both,” said Martha Morrell, MD, a clinical professor of neurology at Stanford (Calif.) University and chief medical officer at NeuroPace, the company that has marketed the device since it gained FDA approval in 2013.
In both studies, the slope of improvement over time was similar, but the real-world study showed greater improvement at the beginning of treatment. “I think the slopes represent physiological changes, but the fact that [the real-world study] starts with better outcomes is, I think, directly attributable to learning. When the long-term study was started in 2004, this had never been done before, and we had to make a highly educated guess about what we should do, and the initial stimulatory parameters were programmed in a way that’s very similar to what was used for movement disorders,” Dr. Morrell said in an interview.
The long-term treatment study appeared online July 20 in the journal Neurology, while the real-world analysis was published July 13 in Epilepsia.
An alternative option
Medications can effectively treat some seizures, but 30%-40% of patients must turn to other options for control. Surgery can sometimes be curative, but is not suitable for some patients. Other stimulation devices include vagus nerve stimulation (VNS), which sends pulses from a chest implant to the vagus nerve, reducing epileptic attacks through an unknown mechanism. Deep brain stimulation (DBS) places electrodes that deliver stimulation to the anterior nucleus of the thalamus, which can spread initially localized seizures.
The RNS device consists of a neurostimulator implanted cranially and connected to leads that are placed based on the individual patient’s seizure focus or foci. It also continuously monitors brain activity and delivers stimulation only when its signal suggests the beginning of a seizure.
That capacity for recording is a key benefit because the information can be stored and analyzed, according to Vikram Rao, MD, PhD, a coinvestigator in the real-world trial and an associate professor and the epilepsy division chief at the University of California, San Francisco, which was one of the trial centers. “You know more precisely than we previously did how many seizures a patient is having. Many of our patients are not able to quantify their seizures with perfect accuracy, so we’re better quantifying their seizure burden,” Dr. Rao said in an interview.
The ability to monitor patients can also improve clinical management. Dr. Morrell recounted an elderly patient who for many years has driven 5 hours for appointments. Recently she was able to review his data from the RNS System remotely. She determined that he was doing fine and, after a telephone consultation, told him he didn’t need to come in for a scheduled visit.
Real-world analysis
In the real-world analysis, researchers led by Babak Razavi, PhD, and Casey Halpern, MD, at Stanford University conducted a chart review of 150 patients at eight centers who underwent treatment with the RNS system between 2013 and 2018. All patients were followed at least 1 year, with a mean of 2.3 years. Patients had a median of 7.7 disabling seizures per month. The mean value was 52 and the numbers ranged from 0.1 to 3,000. A total of 60% had abnormal brain MRI findings.
At 1 year, subjects achieved a mean 67% decrease in seizure frequency (interquartile range, 50%-94%). At 2 years, that grew to 77%; at 3 or more years, 84%. There was no significant difference in seizure reduction at 1 year according to age, age at epilepsy onset, duration of epilepsy, location of seizure foci, presence of brain MRI abnormalities, prior intracranial monitoring, prior epilepsy surgery, or prior VNS treatment. When patients who underwent a resection at the time of RNS placement were excluded, the results were similar. There were no significant differences in outcome by center.
A total of 11.3% of patients experienced a device-related serious adverse event, and 4% developed infections. The rate of infection was not significantly different between patients who had the neurostimulator and leads implanted alone (3.0%) and patients who had intracranial EEG diagnostic monitoring (ICM) electrodes removed at the same time (6.1%; P = .38).
Although about one-third of the patients who started the long-term study dropped out before completion, most were because the participants moved away from treatment centers, according to Dr. Morrell, and other evidence points squarely to patient satisfaction. “At the end of the battery’s longevity, the neurostimulator needs to be replaced. It’s an outpatient, 45-minute procedure. Over 90% of patients chose to have it replaced. It’s not the answer for everybody, but the substantial majority of patients choose to continue,” she said.
Extension trial
The open-label extension trial, led by Dileep Nair, MD, of the Cleveland Clinic Foundation and Dr. Morrell, followed 230 of the 256 patients who participated in 2-year phase 3 study or feasibility studies, extending device usage to 9 years. A total of 162 completed follow-up (mean, 7.5 years). The median reduction of seizure frequency was 58% at the end of year 3, and 75% by year 9 (P < .0001; Wilcoxon signed rank). Although patient population enrichment could have explained this observation, other analyses confirmed that the improvement was real.
Nearly 75% had at least a 50% reduction in seizure frequency; 35% had a 90% or greater reduction in seizure frequency. Some patients (18.4%) had at least a full year with no seizures, and 62% who had a 1-year seizure-free period experienced no seizures at the latest follow-up. Overall, 21% had no seizures in the last 6 months of follow-up.
For those with a seizure-free period of more than 1 year, the average duration was 3.2 years (range, 1.04-9.6 years). There was no difference in response among patients based on previous antiseizure medication use or previous epilepsy surgery, VNS treatment, or intracranial monitoring, and there were no differences by patient age at enrollment, age of seizure onset, brain imaging abnormality, seizure onset locality, or number of foci.
The researchers noted improvement in overall Quality of Life in Epilepsy Inventory–89 scores at 1 year (mean, +3.2; P < .0001), which continued through year 9 (mean, +1.9; P < .05). Improvements were also seen in epilepsy targeted (mean, +4.5; P < .001) and cognitive domains (mean, +2.5; P = .005). Risk of infection was 4.1% per procedure, and 12.1% of subjects overall experienced a serious device-related implant infection. Of 35 infections, 16 led to device removal.
The extension study was funded by NeuroPace. NeuroPace supported data entry and institutional review board submission for the real-world trial. Dr. Morrell owns stock and is an employee of NeuroPace. Dr Rao has received support from and/or consulted for NeuroPace.
SOURCE: Nair DR et al. Neurology. 2020 Jul 20. doi: 10.1212/WNL.0000000000010154. Razavi B et al. Epilepsia. 2020 Jul 13. doi: 10.1111/epi.16593.
Two new long-term studies, one an extension trial and the other an analysis of real-world experience, show that Both studies showed that the benefit from the devices increased over time.
That accruing benefit may be because of improved protocols as clinicians gain experience with the device or because of network remodeling that occurs over time as seizures are controlled. “I think it’s both,” said Martha Morrell, MD, a clinical professor of neurology at Stanford (Calif.) University and chief medical officer at NeuroPace, the company that has marketed the device since it gained FDA approval in 2013.
In both studies, the slope of improvement over time was similar, but the real-world study showed greater improvement at the beginning of treatment. “I think the slopes represent physiological changes, but the fact that [the real-world study] starts with better outcomes is, I think, directly attributable to learning. When the long-term study was started in 2004, this had never been done before, and we had to make a highly educated guess about what we should do, and the initial stimulatory parameters were programmed in a way that’s very similar to what was used for movement disorders,” Dr. Morrell said in an interview.
The long-term treatment study appeared online July 20 in the journal Neurology, while the real-world analysis was published July 13 in Epilepsia.
An alternative option
Medications can effectively treat some seizures, but 30%-40% of patients must turn to other options for control. Surgery can sometimes be curative, but is not suitable for some patients. Other stimulation devices include vagus nerve stimulation (VNS), which sends pulses from a chest implant to the vagus nerve, reducing epileptic attacks through an unknown mechanism. Deep brain stimulation (DBS) places electrodes that deliver stimulation to the anterior nucleus of the thalamus, which can spread initially localized seizures.
The RNS device consists of a neurostimulator implanted cranially and connected to leads that are placed based on the individual patient’s seizure focus or foci. It also continuously monitors brain activity and delivers stimulation only when its signal suggests the beginning of a seizure.
That capacity for recording is a key benefit because the information can be stored and analyzed, according to Vikram Rao, MD, PhD, a coinvestigator in the real-world trial and an associate professor and the epilepsy division chief at the University of California, San Francisco, which was one of the trial centers. “You know more precisely than we previously did how many seizures a patient is having. Many of our patients are not able to quantify their seizures with perfect accuracy, so we’re better quantifying their seizure burden,” Dr. Rao said in an interview.
The ability to monitor patients can also improve clinical management. Dr. Morrell recounted an elderly patient who for many years has driven 5 hours for appointments. Recently she was able to review his data from the RNS System remotely. She determined that he was doing fine and, after a telephone consultation, told him he didn’t need to come in for a scheduled visit.
Real-world analysis
In the real-world analysis, researchers led by Babak Razavi, PhD, and Casey Halpern, MD, at Stanford University conducted a chart review of 150 patients at eight centers who underwent treatment with the RNS system between 2013 and 2018. All patients were followed at least 1 year, with a mean of 2.3 years. Patients had a median of 7.7 disabling seizures per month. The mean value was 52 and the numbers ranged from 0.1 to 3,000. A total of 60% had abnormal brain MRI findings.
At 1 year, subjects achieved a mean 67% decrease in seizure frequency (interquartile range, 50%-94%). At 2 years, that grew to 77%; at 3 or more years, 84%. There was no significant difference in seizure reduction at 1 year according to age, age at epilepsy onset, duration of epilepsy, location of seizure foci, presence of brain MRI abnormalities, prior intracranial monitoring, prior epilepsy surgery, or prior VNS treatment. When patients who underwent a resection at the time of RNS placement were excluded, the results were similar. There were no significant differences in outcome by center.
A total of 11.3% of patients experienced a device-related serious adverse event, and 4% developed infections. The rate of infection was not significantly different between patients who had the neurostimulator and leads implanted alone (3.0%) and patients who had intracranial EEG diagnostic monitoring (ICM) electrodes removed at the same time (6.1%; P = .38).
Although about one-third of the patients who started the long-term study dropped out before completion, most were because the participants moved away from treatment centers, according to Dr. Morrell, and other evidence points squarely to patient satisfaction. “At the end of the battery’s longevity, the neurostimulator needs to be replaced. It’s an outpatient, 45-minute procedure. Over 90% of patients chose to have it replaced. It’s not the answer for everybody, but the substantial majority of patients choose to continue,” she said.
Extension trial
The open-label extension trial, led by Dileep Nair, MD, of the Cleveland Clinic Foundation and Dr. Morrell, followed 230 of the 256 patients who participated in 2-year phase 3 study or feasibility studies, extending device usage to 9 years. A total of 162 completed follow-up (mean, 7.5 years). The median reduction of seizure frequency was 58% at the end of year 3, and 75% by year 9 (P < .0001; Wilcoxon signed rank). Although patient population enrichment could have explained this observation, other analyses confirmed that the improvement was real.
Nearly 75% had at least a 50% reduction in seizure frequency; 35% had a 90% or greater reduction in seizure frequency. Some patients (18.4%) had at least a full year with no seizures, and 62% who had a 1-year seizure-free period experienced no seizures at the latest follow-up. Overall, 21% had no seizures in the last 6 months of follow-up.
For those with a seizure-free period of more than 1 year, the average duration was 3.2 years (range, 1.04-9.6 years). There was no difference in response among patients based on previous antiseizure medication use or previous epilepsy surgery, VNS treatment, or intracranial monitoring, and there were no differences by patient age at enrollment, age of seizure onset, brain imaging abnormality, seizure onset locality, or number of foci.
The researchers noted improvement in overall Quality of Life in Epilepsy Inventory–89 scores at 1 year (mean, +3.2; P < .0001), which continued through year 9 (mean, +1.9; P < .05). Improvements were also seen in epilepsy targeted (mean, +4.5; P < .001) and cognitive domains (mean, +2.5; P = .005). Risk of infection was 4.1% per procedure, and 12.1% of subjects overall experienced a serious device-related implant infection. Of 35 infections, 16 led to device removal.
The extension study was funded by NeuroPace. NeuroPace supported data entry and institutional review board submission for the real-world trial. Dr. Morrell owns stock and is an employee of NeuroPace. Dr Rao has received support from and/or consulted for NeuroPace.
SOURCE: Nair DR et al. Neurology. 2020 Jul 20. doi: 10.1212/WNL.0000000000010154. Razavi B et al. Epilepsia. 2020 Jul 13. doi: 10.1111/epi.16593.
Two new long-term studies, one an extension trial and the other an analysis of real-world experience, show that Both studies showed that the benefit from the devices increased over time.
That accruing benefit may be because of improved protocols as clinicians gain experience with the device or because of network remodeling that occurs over time as seizures are controlled. “I think it’s both,” said Martha Morrell, MD, a clinical professor of neurology at Stanford (Calif.) University and chief medical officer at NeuroPace, the company that has marketed the device since it gained FDA approval in 2013.
In both studies, the slope of improvement over time was similar, but the real-world study showed greater improvement at the beginning of treatment. “I think the slopes represent physiological changes, but the fact that [the real-world study] starts with better outcomes is, I think, directly attributable to learning. When the long-term study was started in 2004, this had never been done before, and we had to make a highly educated guess about what we should do, and the initial stimulatory parameters were programmed in a way that’s very similar to what was used for movement disorders,” Dr. Morrell said in an interview.
The long-term treatment study appeared online July 20 in the journal Neurology, while the real-world analysis was published July 13 in Epilepsia.
An alternative option
Medications can effectively treat some seizures, but 30%-40% of patients must turn to other options for control. Surgery can sometimes be curative, but is not suitable for some patients. Other stimulation devices include vagus nerve stimulation (VNS), which sends pulses from a chest implant to the vagus nerve, reducing epileptic attacks through an unknown mechanism. Deep brain stimulation (DBS) places electrodes that deliver stimulation to the anterior nucleus of the thalamus, which can spread initially localized seizures.
The RNS device consists of a neurostimulator implanted cranially and connected to leads that are placed based on the individual patient’s seizure focus or foci. It also continuously monitors brain activity and delivers stimulation only when its signal suggests the beginning of a seizure.
That capacity for recording is a key benefit because the information can be stored and analyzed, according to Vikram Rao, MD, PhD, a coinvestigator in the real-world trial and an associate professor and the epilepsy division chief at the University of California, San Francisco, which was one of the trial centers. “You know more precisely than we previously did how many seizures a patient is having. Many of our patients are not able to quantify their seizures with perfect accuracy, so we’re better quantifying their seizure burden,” Dr. Rao said in an interview.
The ability to monitor patients can also improve clinical management. Dr. Morrell recounted an elderly patient who for many years has driven 5 hours for appointments. Recently she was able to review his data from the RNS System remotely. She determined that he was doing fine and, after a telephone consultation, told him he didn’t need to come in for a scheduled visit.
Real-world analysis
In the real-world analysis, researchers led by Babak Razavi, PhD, and Casey Halpern, MD, at Stanford University conducted a chart review of 150 patients at eight centers who underwent treatment with the RNS system between 2013 and 2018. All patients were followed at least 1 year, with a mean of 2.3 years. Patients had a median of 7.7 disabling seizures per month. The mean value was 52 and the numbers ranged from 0.1 to 3,000. A total of 60% had abnormal brain MRI findings.
At 1 year, subjects achieved a mean 67% decrease in seizure frequency (interquartile range, 50%-94%). At 2 years, that grew to 77%; at 3 or more years, 84%. There was no significant difference in seizure reduction at 1 year according to age, age at epilepsy onset, duration of epilepsy, location of seizure foci, presence of brain MRI abnormalities, prior intracranial monitoring, prior epilepsy surgery, or prior VNS treatment. When patients who underwent a resection at the time of RNS placement were excluded, the results were similar. There were no significant differences in outcome by center.
A total of 11.3% of patients experienced a device-related serious adverse event, and 4% developed infections. The rate of infection was not significantly different between patients who had the neurostimulator and leads implanted alone (3.0%) and patients who had intracranial EEG diagnostic monitoring (ICM) electrodes removed at the same time (6.1%; P = .38).
Although about one-third of the patients who started the long-term study dropped out before completion, most were because the participants moved away from treatment centers, according to Dr. Morrell, and other evidence points squarely to patient satisfaction. “At the end of the battery’s longevity, the neurostimulator needs to be replaced. It’s an outpatient, 45-minute procedure. Over 90% of patients chose to have it replaced. It’s not the answer for everybody, but the substantial majority of patients choose to continue,” she said.
Extension trial
The open-label extension trial, led by Dileep Nair, MD, of the Cleveland Clinic Foundation and Dr. Morrell, followed 230 of the 256 patients who participated in 2-year phase 3 study or feasibility studies, extending device usage to 9 years. A total of 162 completed follow-up (mean, 7.5 years). The median reduction of seizure frequency was 58% at the end of year 3, and 75% by year 9 (P < .0001; Wilcoxon signed rank). Although patient population enrichment could have explained this observation, other analyses confirmed that the improvement was real.
Nearly 75% had at least a 50% reduction in seizure frequency; 35% had a 90% or greater reduction in seizure frequency. Some patients (18.4%) had at least a full year with no seizures, and 62% who had a 1-year seizure-free period experienced no seizures at the latest follow-up. Overall, 21% had no seizures in the last 6 months of follow-up.
For those with a seizure-free period of more than 1 year, the average duration was 3.2 years (range, 1.04-9.6 years). There was no difference in response among patients based on previous antiseizure medication use or previous epilepsy surgery, VNS treatment, or intracranial monitoring, and there were no differences by patient age at enrollment, age of seizure onset, brain imaging abnormality, seizure onset locality, or number of foci.
The researchers noted improvement in overall Quality of Life in Epilepsy Inventory–89 scores at 1 year (mean, +3.2; P < .0001), which continued through year 9 (mean, +1.9; P < .05). Improvements were also seen in epilepsy targeted (mean, +4.5; P < .001) and cognitive domains (mean, +2.5; P = .005). Risk of infection was 4.1% per procedure, and 12.1% of subjects overall experienced a serious device-related implant infection. Of 35 infections, 16 led to device removal.
The extension study was funded by NeuroPace. NeuroPace supported data entry and institutional review board submission for the real-world trial. Dr. Morrell owns stock and is an employee of NeuroPace. Dr Rao has received support from and/or consulted for NeuroPace.
SOURCE: Nair DR et al. Neurology. 2020 Jul 20. doi: 10.1212/WNL.0000000000010154. Razavi B et al. Epilepsia. 2020 Jul 13. doi: 10.1111/epi.16593.
FROM EPILEPSIA AND FROM NEUROLOGY
Epilepsy after TBI linked to worse 12-month outcomes
findings from an analysis of a large, prospective database suggest. “We found that patients essentially have a 10-times greater risk of developing posttraumatic epilepsy and seizures at 12 months [post injury] if the presenting Glasgow Coma Scale GCS) is less than 8,” said lead author John F. Burke, MD, PhD, University of California, San Francisco, in presenting the findings as part of the virtual annual meeting of the American Association of Neurological Surgeons.
Assessing risk factors
While posttraumatic epilepsy represents an estimated 20% of all cases of symptomatic epilepsy, many questions remain on those most at risk and on the long-term effects of posttraumatic epilepsy on TBI outcomes. To probe those issues, Dr. Burke and colleagues turned to the multicenter TRACK-TBI database, which has prospective, longitudinal data on more than 2,700 patients with traumatic brain injuries and is considered the largest source of prospective data on posttraumatic epilepsy.
Using the criteria of no previous epilepsy and having 12 months of follow-up, the team identified 1,493 patients with TBI. In addition, investigators identified 182 orthopedic controls (included and prospectively followed because they have injuries but not specifically head trauma) and 210 controls who are friends of the patients and who do not have injuries but allow researchers to control for socioeconomic and environmental factors.
Of the 1,493 patients with TBI, 41 (2.7%) were determined to have posttraumatic epilepsy, assessed according to a National Institute of Neurological Disorders and Stroke epilepsy screening questionnaire, which is designed to identify patients with posttraumatic epilepsy symptoms. There were no reports of epilepsy symptoms using the screening tool among the controls. Dr. Burke noted that the 2.7% was in agreement with historical reports.
In comparing patients with TBI who did and did not have posttraumatic epilepsy, no differences were observed in the groups in terms of gender, although there was a trend toward younger age among those with PTE (mean age, 35.4 years with posttraumatic injury vs. 41.5 without; P = .05).
A major risk factor for the development of posttraumatic epilepsy was presenting GCS scores. Among those with scores of less than 8, indicative of severe injury, the rate of posttraumatic epilepsy was 6% at 6 months and 12.5% at 12 months. In contrast, those with TBI presenting with GCS scores between 13 and 15, indicative of minor injury, had an incidence of posttraumatic epilepsy of 0.9% at 6 months and 1.4% at 12 months.
Imaging findings in the two groups showed that hemorrhage detected on CT imaging was associated with a significantly higher risk for posttraumatic epilepsy (P < .001).
“The main takeaway is that any hemorrhage in the brain is a major risk factor for developing seizures,” Dr. Burke said. “Whether it is subdural, epidural blood, subarachnoid or contusion, any blood confers a very [high] risk for developing seizures.”
Posttraumatic epilepsy was linked to poorer longer-term outcomes even for patients with lesser injury: Among those with TBI and GCS of 13-15, the mean Glasgow Outcome Scale Extended (GOSE) score at 12 months among those without posttraumatic epilepsy was 7, indicative of a good recovery with minor defects, whereas the mean GOSE score for those with PTE was 4.6, indicative of moderate to severe disability (P < .001).
“It was surprising to us that PTE-positive patients had a very significant decrease in GOSE, compared to PTE-negative patients,” Dr. Burke said. “There was a nearly 2-point drop in the GOSE and that was extremely significant.”
A multivariate analysis showed there was still a significant independent risk for a poor GOSE score with posttraumatic epilepsy after controlling for GCS score, head CT findings, and age (P < .001).
The authors also looked at mood outcomes using the Brief Symptom Inventory–18, which showed significant worse effect in those with posttraumatic epilepsy after multivariate adjustment (P = .01). Additionally, a highly significant worse effect in cognitive outcomes on the Rivermead cognitive metric was observed with posttraumatic epilepsy (P = .001).
“On all metrics tested, posttraumatic epilepsy worsened outcomes,” Dr. Burke said.
He noted that the study has some key limitations, including the 12-month follow-up. A previous study showed a linear increase in posttraumatic follow-up up to 30 years. “The fact that we found 41 patients at 12 months indicates there are probably more that are out there who are going to develop seizures, but because we don’t have the follow-up we can’t look at that.”
Although the screening questionnaires are effective, “the issue is these people are not being seen by an epileptologist or having scalp EEG done, and we need a more accurate way to do this,” he said. A new study, TRACK-TBI EPI, will address those limitations and a host of other issues with a 5-year follow-up.
Capturing the nuances of brain injury
Commenting on the study as a discussant, neurosurgeon Uzma Samadani, MD, PhD, of the Minneapolis Veterans Affairs Medical Center and CentraCare in Minneapolis, suggested that the future work should focus on issues including the wide-ranging mechanisms that could explain the seizure activity.
“For example, it’s known that posttraumatic epilepsy or seizures can be triggered by abnormal conductivity due to multiple different mechanisms associated with brain injury, such as endocrine dysfunction, cortical-spreading depression, and many others,” said Dr. Samadani, who has been a researcher on the TRACK-TBI study.
Factors ranging from genetic differences to comorbid conditions such as alcoholism can play a role in brain injury susceptibility, Dr. Samadani added. Furthermore, outcome measures currently available simply may not capture the unknown nuances of brain injury.
“We have to ask, are these an all-or-none phenomena, or is aberrant electrical activity after brain injury a continuum of dysfunction?” Dr. Samadani speculated.
“I would caution that we are likely underestimating the non–easily measurable consequences of brain injury,” she said. “And the better we can quantitate susceptibility, classify the nature of injury and target acute management, the less posttraumatic epilepsy/aberrant electrical activity our patients will have.”
Dr. Burke and Dr. Samadani disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
findings from an analysis of a large, prospective database suggest. “We found that patients essentially have a 10-times greater risk of developing posttraumatic epilepsy and seizures at 12 months [post injury] if the presenting Glasgow Coma Scale GCS) is less than 8,” said lead author John F. Burke, MD, PhD, University of California, San Francisco, in presenting the findings as part of the virtual annual meeting of the American Association of Neurological Surgeons.
Assessing risk factors
While posttraumatic epilepsy represents an estimated 20% of all cases of symptomatic epilepsy, many questions remain on those most at risk and on the long-term effects of posttraumatic epilepsy on TBI outcomes. To probe those issues, Dr. Burke and colleagues turned to the multicenter TRACK-TBI database, which has prospective, longitudinal data on more than 2,700 patients with traumatic brain injuries and is considered the largest source of prospective data on posttraumatic epilepsy.
Using the criteria of no previous epilepsy and having 12 months of follow-up, the team identified 1,493 patients with TBI. In addition, investigators identified 182 orthopedic controls (included and prospectively followed because they have injuries but not specifically head trauma) and 210 controls who are friends of the patients and who do not have injuries but allow researchers to control for socioeconomic and environmental factors.
Of the 1,493 patients with TBI, 41 (2.7%) were determined to have posttraumatic epilepsy, assessed according to a National Institute of Neurological Disorders and Stroke epilepsy screening questionnaire, which is designed to identify patients with posttraumatic epilepsy symptoms. There were no reports of epilepsy symptoms using the screening tool among the controls. Dr. Burke noted that the 2.7% was in agreement with historical reports.
In comparing patients with TBI who did and did not have posttraumatic epilepsy, no differences were observed in the groups in terms of gender, although there was a trend toward younger age among those with PTE (mean age, 35.4 years with posttraumatic injury vs. 41.5 without; P = .05).
A major risk factor for the development of posttraumatic epilepsy was presenting GCS scores. Among those with scores of less than 8, indicative of severe injury, the rate of posttraumatic epilepsy was 6% at 6 months and 12.5% at 12 months. In contrast, those with TBI presenting with GCS scores between 13 and 15, indicative of minor injury, had an incidence of posttraumatic epilepsy of 0.9% at 6 months and 1.4% at 12 months.
Imaging findings in the two groups showed that hemorrhage detected on CT imaging was associated with a significantly higher risk for posttraumatic epilepsy (P < .001).
“The main takeaway is that any hemorrhage in the brain is a major risk factor for developing seizures,” Dr. Burke said. “Whether it is subdural, epidural blood, subarachnoid or contusion, any blood confers a very [high] risk for developing seizures.”
Posttraumatic epilepsy was linked to poorer longer-term outcomes even for patients with lesser injury: Among those with TBI and GCS of 13-15, the mean Glasgow Outcome Scale Extended (GOSE) score at 12 months among those without posttraumatic epilepsy was 7, indicative of a good recovery with minor defects, whereas the mean GOSE score for those with PTE was 4.6, indicative of moderate to severe disability (P < .001).
“It was surprising to us that PTE-positive patients had a very significant decrease in GOSE, compared to PTE-negative patients,” Dr. Burke said. “There was a nearly 2-point drop in the GOSE and that was extremely significant.”
A multivariate analysis showed there was still a significant independent risk for a poor GOSE score with posttraumatic epilepsy after controlling for GCS score, head CT findings, and age (P < .001).
The authors also looked at mood outcomes using the Brief Symptom Inventory–18, which showed significant worse effect in those with posttraumatic epilepsy after multivariate adjustment (P = .01). Additionally, a highly significant worse effect in cognitive outcomes on the Rivermead cognitive metric was observed with posttraumatic epilepsy (P = .001).
“On all metrics tested, posttraumatic epilepsy worsened outcomes,” Dr. Burke said.
He noted that the study has some key limitations, including the 12-month follow-up. A previous study showed a linear increase in posttraumatic follow-up up to 30 years. “The fact that we found 41 patients at 12 months indicates there are probably more that are out there who are going to develop seizures, but because we don’t have the follow-up we can’t look at that.”
Although the screening questionnaires are effective, “the issue is these people are not being seen by an epileptologist or having scalp EEG done, and we need a more accurate way to do this,” he said. A new study, TRACK-TBI EPI, will address those limitations and a host of other issues with a 5-year follow-up.
Capturing the nuances of brain injury
Commenting on the study as a discussant, neurosurgeon Uzma Samadani, MD, PhD, of the Minneapolis Veterans Affairs Medical Center and CentraCare in Minneapolis, suggested that the future work should focus on issues including the wide-ranging mechanisms that could explain the seizure activity.
“For example, it’s known that posttraumatic epilepsy or seizures can be triggered by abnormal conductivity due to multiple different mechanisms associated with brain injury, such as endocrine dysfunction, cortical-spreading depression, and many others,” said Dr. Samadani, who has been a researcher on the TRACK-TBI study.
Factors ranging from genetic differences to comorbid conditions such as alcoholism can play a role in brain injury susceptibility, Dr. Samadani added. Furthermore, outcome measures currently available simply may not capture the unknown nuances of brain injury.
“We have to ask, are these an all-or-none phenomena, or is aberrant electrical activity after brain injury a continuum of dysfunction?” Dr. Samadani speculated.
“I would caution that we are likely underestimating the non–easily measurable consequences of brain injury,” she said. “And the better we can quantitate susceptibility, classify the nature of injury and target acute management, the less posttraumatic epilepsy/aberrant electrical activity our patients will have.”
Dr. Burke and Dr. Samadani disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
findings from an analysis of a large, prospective database suggest. “We found that patients essentially have a 10-times greater risk of developing posttraumatic epilepsy and seizures at 12 months [post injury] if the presenting Glasgow Coma Scale GCS) is less than 8,” said lead author John F. Burke, MD, PhD, University of California, San Francisco, in presenting the findings as part of the virtual annual meeting of the American Association of Neurological Surgeons.
Assessing risk factors
While posttraumatic epilepsy represents an estimated 20% of all cases of symptomatic epilepsy, many questions remain on those most at risk and on the long-term effects of posttraumatic epilepsy on TBI outcomes. To probe those issues, Dr. Burke and colleagues turned to the multicenter TRACK-TBI database, which has prospective, longitudinal data on more than 2,700 patients with traumatic brain injuries and is considered the largest source of prospective data on posttraumatic epilepsy.
Using the criteria of no previous epilepsy and having 12 months of follow-up, the team identified 1,493 patients with TBI. In addition, investigators identified 182 orthopedic controls (included and prospectively followed because they have injuries but not specifically head trauma) and 210 controls who are friends of the patients and who do not have injuries but allow researchers to control for socioeconomic and environmental factors.
Of the 1,493 patients with TBI, 41 (2.7%) were determined to have posttraumatic epilepsy, assessed according to a National Institute of Neurological Disorders and Stroke epilepsy screening questionnaire, which is designed to identify patients with posttraumatic epilepsy symptoms. There were no reports of epilepsy symptoms using the screening tool among the controls. Dr. Burke noted that the 2.7% was in agreement with historical reports.
In comparing patients with TBI who did and did not have posttraumatic epilepsy, no differences were observed in the groups in terms of gender, although there was a trend toward younger age among those with PTE (mean age, 35.4 years with posttraumatic injury vs. 41.5 without; P = .05).
A major risk factor for the development of posttraumatic epilepsy was presenting GCS scores. Among those with scores of less than 8, indicative of severe injury, the rate of posttraumatic epilepsy was 6% at 6 months and 12.5% at 12 months. In contrast, those with TBI presenting with GCS scores between 13 and 15, indicative of minor injury, had an incidence of posttraumatic epilepsy of 0.9% at 6 months and 1.4% at 12 months.
Imaging findings in the two groups showed that hemorrhage detected on CT imaging was associated with a significantly higher risk for posttraumatic epilepsy (P < .001).
“The main takeaway is that any hemorrhage in the brain is a major risk factor for developing seizures,” Dr. Burke said. “Whether it is subdural, epidural blood, subarachnoid or contusion, any blood confers a very [high] risk for developing seizures.”
Posttraumatic epilepsy was linked to poorer longer-term outcomes even for patients with lesser injury: Among those with TBI and GCS of 13-15, the mean Glasgow Outcome Scale Extended (GOSE) score at 12 months among those without posttraumatic epilepsy was 7, indicative of a good recovery with minor defects, whereas the mean GOSE score for those with PTE was 4.6, indicative of moderate to severe disability (P < .001).
“It was surprising to us that PTE-positive patients had a very significant decrease in GOSE, compared to PTE-negative patients,” Dr. Burke said. “There was a nearly 2-point drop in the GOSE and that was extremely significant.”
A multivariate analysis showed there was still a significant independent risk for a poor GOSE score with posttraumatic epilepsy after controlling for GCS score, head CT findings, and age (P < .001).
The authors also looked at mood outcomes using the Brief Symptom Inventory–18, which showed significant worse effect in those with posttraumatic epilepsy after multivariate adjustment (P = .01). Additionally, a highly significant worse effect in cognitive outcomes on the Rivermead cognitive metric was observed with posttraumatic epilepsy (P = .001).
“On all metrics tested, posttraumatic epilepsy worsened outcomes,” Dr. Burke said.
He noted that the study has some key limitations, including the 12-month follow-up. A previous study showed a linear increase in posttraumatic follow-up up to 30 years. “The fact that we found 41 patients at 12 months indicates there are probably more that are out there who are going to develop seizures, but because we don’t have the follow-up we can’t look at that.”
Although the screening questionnaires are effective, “the issue is these people are not being seen by an epileptologist or having scalp EEG done, and we need a more accurate way to do this,” he said. A new study, TRACK-TBI EPI, will address those limitations and a host of other issues with a 5-year follow-up.
Capturing the nuances of brain injury
Commenting on the study as a discussant, neurosurgeon Uzma Samadani, MD, PhD, of the Minneapolis Veterans Affairs Medical Center and CentraCare in Minneapolis, suggested that the future work should focus on issues including the wide-ranging mechanisms that could explain the seizure activity.
“For example, it’s known that posttraumatic epilepsy or seizures can be triggered by abnormal conductivity due to multiple different mechanisms associated with brain injury, such as endocrine dysfunction, cortical-spreading depression, and many others,” said Dr. Samadani, who has been a researcher on the TRACK-TBI study.
Factors ranging from genetic differences to comorbid conditions such as alcoholism can play a role in brain injury susceptibility, Dr. Samadani added. Furthermore, outcome measures currently available simply may not capture the unknown nuances of brain injury.
“We have to ask, are these an all-or-none phenomena, or is aberrant electrical activity after brain injury a continuum of dysfunction?” Dr. Samadani speculated.
“I would caution that we are likely underestimating the non–easily measurable consequences of brain injury,” she said. “And the better we can quantitate susceptibility, classify the nature of injury and target acute management, the less posttraumatic epilepsy/aberrant electrical activity our patients will have.”
Dr. Burke and Dr. Samadani disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AANS 2020
FDA approves new treatment for Dravet syndrome
Dravet syndrome is a rare childhood-onset epilepsy characterized by frequent, drug-resistant convulsive seizures that may contribute to intellectual disability and impairments in motor control, behavior, and cognition, as well as an increased risk of sudden unexpected death in epilepsy (SUDEP).
Dravet syndrome takes a “tremendous toll on both patients and their families. Fintepla offers an additional effective treatment option for the treatment of seizures associated with Dravet syndrome,” Billy Dunn, MD, director, Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research, said in a news release.
The FDA approved fenfluramine for Dravet syndrome based on the results of two randomized, double-blind, placebo-controlled phase 3 trials involving children ages 2 to 18 years with Dravet syndrome.
In both studies, children treated with fenfluramine experienced significantly greater reductions in the frequency of convulsive seizures than did their peers who received placebo. These reductions occurred within 3 to 4 weeks, and remained generally consistent over the 14- to 15-week treatment periods, the FDA said.
“There remains a huge unmet need for the many Dravet syndrome patients who continue to experience frequent severe seizures even while taking one or more of the currently available antiseizure medications,” Joseph Sullivan, MD, who worked on the fenfluramine for Dravet syndrome studies, said in a news release.
Given the “profound reductions” in convulsive seizure frequency seen in the clinical trials, combined with the “ongoing, robust safety monitoring,” fenfluramine offers “an extremely important treatment option for Dravet syndrome patients,” said Dr. Sullivan, director of the Pediatric Epilepsy Center of Excellence at the University of California San Francisco (UCSF) Benioff Children’s Hospital.
Fenfluramine is an anorectic agent that was used to treat obesity until it was removed from the market in 1997 over reports of increased risk of valvular heart disease when prescribed in higher doses and most often when prescribed with phentermine. The combination of the two drugs was known as fen-phen.
In the clinical trials of Dravet syndrome, the most common adverse reactions were decreased appetite; somnolence, sedation, lethargy; diarrhea; constipation; abnormal echocardiogram; fatigue, malaise, asthenia; ataxia, balance disorder, gait disturbance; increased blood pressure; drooling, salivary hypersecretion; pyrexia; upper respiratory tract infection; vomiting; decreased weight; fall; and status epilepticus.
The Fintepla label has a boxed warning stating that the drug is associated with valvular heart disease (VHD) and pulmonary arterial hypertension (PAH). Due to these risks, patients must undergo echocardiography before treatment, every 6 months during treatment, and once 3 to 6 months after treatment is stopped.
If signs of VHD, PAH, or other cardiac abnormalities are present, clinicians should weigh the benefits and risks of continuing treatment with Fintepla, the FDA said.
Fintepla is available only through a risk evaluation and mitigation strategy (REMS) program, which requires physicians who prescribe the drug and pharmacies that dispense it to be certified in the Fintepla REMS and that patients be enrolled in the program.
As part of the REMS requirements, prescribers and patients must adhere to the required cardiac monitoring to receive the drug.
Fintepla will be available to certified prescribers in the United States in July. Zogenix is launching Zogenix Central, a comprehensive support service that will provide ongoing product assistance to patients, caregivers, and their medical teams. Further information is available online.
This article first appeared on Medscape.com.
Dravet syndrome is a rare childhood-onset epilepsy characterized by frequent, drug-resistant convulsive seizures that may contribute to intellectual disability and impairments in motor control, behavior, and cognition, as well as an increased risk of sudden unexpected death in epilepsy (SUDEP).
Dravet syndrome takes a “tremendous toll on both patients and their families. Fintepla offers an additional effective treatment option for the treatment of seizures associated with Dravet syndrome,” Billy Dunn, MD, director, Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research, said in a news release.
The FDA approved fenfluramine for Dravet syndrome based on the results of two randomized, double-blind, placebo-controlled phase 3 trials involving children ages 2 to 18 years with Dravet syndrome.
In both studies, children treated with fenfluramine experienced significantly greater reductions in the frequency of convulsive seizures than did their peers who received placebo. These reductions occurred within 3 to 4 weeks, and remained generally consistent over the 14- to 15-week treatment periods, the FDA said.
“There remains a huge unmet need for the many Dravet syndrome patients who continue to experience frequent severe seizures even while taking one or more of the currently available antiseizure medications,” Joseph Sullivan, MD, who worked on the fenfluramine for Dravet syndrome studies, said in a news release.
Given the “profound reductions” in convulsive seizure frequency seen in the clinical trials, combined with the “ongoing, robust safety monitoring,” fenfluramine offers “an extremely important treatment option for Dravet syndrome patients,” said Dr. Sullivan, director of the Pediatric Epilepsy Center of Excellence at the University of California San Francisco (UCSF) Benioff Children’s Hospital.
Fenfluramine is an anorectic agent that was used to treat obesity until it was removed from the market in 1997 over reports of increased risk of valvular heart disease when prescribed in higher doses and most often when prescribed with phentermine. The combination of the two drugs was known as fen-phen.
In the clinical trials of Dravet syndrome, the most common adverse reactions were decreased appetite; somnolence, sedation, lethargy; diarrhea; constipation; abnormal echocardiogram; fatigue, malaise, asthenia; ataxia, balance disorder, gait disturbance; increased blood pressure; drooling, salivary hypersecretion; pyrexia; upper respiratory tract infection; vomiting; decreased weight; fall; and status epilepticus.
The Fintepla label has a boxed warning stating that the drug is associated with valvular heart disease (VHD) and pulmonary arterial hypertension (PAH). Due to these risks, patients must undergo echocardiography before treatment, every 6 months during treatment, and once 3 to 6 months after treatment is stopped.
If signs of VHD, PAH, or other cardiac abnormalities are present, clinicians should weigh the benefits and risks of continuing treatment with Fintepla, the FDA said.
Fintepla is available only through a risk evaluation and mitigation strategy (REMS) program, which requires physicians who prescribe the drug and pharmacies that dispense it to be certified in the Fintepla REMS and that patients be enrolled in the program.
As part of the REMS requirements, prescribers and patients must adhere to the required cardiac monitoring to receive the drug.
Fintepla will be available to certified prescribers in the United States in July. Zogenix is launching Zogenix Central, a comprehensive support service that will provide ongoing product assistance to patients, caregivers, and their medical teams. Further information is available online.
This article first appeared on Medscape.com.
Dravet syndrome is a rare childhood-onset epilepsy characterized by frequent, drug-resistant convulsive seizures that may contribute to intellectual disability and impairments in motor control, behavior, and cognition, as well as an increased risk of sudden unexpected death in epilepsy (SUDEP).
Dravet syndrome takes a “tremendous toll on both patients and their families. Fintepla offers an additional effective treatment option for the treatment of seizures associated with Dravet syndrome,” Billy Dunn, MD, director, Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research, said in a news release.
The FDA approved fenfluramine for Dravet syndrome based on the results of two randomized, double-blind, placebo-controlled phase 3 trials involving children ages 2 to 18 years with Dravet syndrome.
In both studies, children treated with fenfluramine experienced significantly greater reductions in the frequency of convulsive seizures than did their peers who received placebo. These reductions occurred within 3 to 4 weeks, and remained generally consistent over the 14- to 15-week treatment periods, the FDA said.
“There remains a huge unmet need for the many Dravet syndrome patients who continue to experience frequent severe seizures even while taking one or more of the currently available antiseizure medications,” Joseph Sullivan, MD, who worked on the fenfluramine for Dravet syndrome studies, said in a news release.
Given the “profound reductions” in convulsive seizure frequency seen in the clinical trials, combined with the “ongoing, robust safety monitoring,” fenfluramine offers “an extremely important treatment option for Dravet syndrome patients,” said Dr. Sullivan, director of the Pediatric Epilepsy Center of Excellence at the University of California San Francisco (UCSF) Benioff Children’s Hospital.
Fenfluramine is an anorectic agent that was used to treat obesity until it was removed from the market in 1997 over reports of increased risk of valvular heart disease when prescribed in higher doses and most often when prescribed with phentermine. The combination of the two drugs was known as fen-phen.
In the clinical trials of Dravet syndrome, the most common adverse reactions were decreased appetite; somnolence, sedation, lethargy; diarrhea; constipation; abnormal echocardiogram; fatigue, malaise, asthenia; ataxia, balance disorder, gait disturbance; increased blood pressure; drooling, salivary hypersecretion; pyrexia; upper respiratory tract infection; vomiting; decreased weight; fall; and status epilepticus.
The Fintepla label has a boxed warning stating that the drug is associated with valvular heart disease (VHD) and pulmonary arterial hypertension (PAH). Due to these risks, patients must undergo echocardiography before treatment, every 6 months during treatment, and once 3 to 6 months after treatment is stopped.
If signs of VHD, PAH, or other cardiac abnormalities are present, clinicians should weigh the benefits and risks of continuing treatment with Fintepla, the FDA said.
Fintepla is available only through a risk evaluation and mitigation strategy (REMS) program, which requires physicians who prescribe the drug and pharmacies that dispense it to be certified in the Fintepla REMS and that patients be enrolled in the program.
As part of the REMS requirements, prescribers and patients must adhere to the required cardiac monitoring to receive the drug.
Fintepla will be available to certified prescribers in the United States in July. Zogenix is launching Zogenix Central, a comprehensive support service that will provide ongoing product assistance to patients, caregivers, and their medical teams. Further information is available online.
This article first appeared on Medscape.com.
Most adult epilepsy-related deaths could be avoided
The research shows that such avoidable deaths “remain common and have not declined over time, despite advances in treatment,” Gashirai Mbizvo, MBChB, PhD, clinical research fellow, Muir Maxwell Epilepsy Center, the University of Edinburgh, Scotland, told a press briefing.
The findings were presented at the Congress of the European Academy of Neurology (EAN) 2020, which is being conducted as a virtual/online meeting because of the COVID-19 pandemic.
As his PhD dissertation, Dr. Mbizvo is investigating the rates, causes, and risk factors for epilepsy-related deaths and the percentage of these that are potentially avoidable.
The National Health Service of Scotland contains various linked administrative data sets. Each resident of Scotland has a unique identifier that facilitates investigations across the health system.
Dr. Mbizvo investigated adults and adolescents aged 16 years and older who died because of epilepsy during 2009-2016. He compared this group to patients of similar age who were living with epilepsy to identify risk factors that might help focus resources. During the study period, 2,149 epilepsy-related deaths occurred. Nearly 60% involved at least one seizure-related hospital admission.
Heavy burden
Of the patients who died because of epilepsy, 24% were seen in an outpatient neurologic clinic. “So there’s this heavy burden of admissions not translating to neurology follow-up,” said Dr. Mbizvo.
During the study period, there was no reduction in mortality “despite advances in medical care,” said Dr. Mbizvo.
Younger people with epilepsy were found to be more likely to die. The standardized mortality rate was 6/100,000 (95% confidence interval, 2.3-9.7) among those aged 16-24 years. By contrast, among those aged 45-54 years, the rate was 2/100,000 (95% CI, 1.1-2.1); it was lower in older age groups.
“The overall mortality is not reducing; people are dying young, and neurologists are really not getting involved,” Dr. Mbizvo said.
Among the almost 600 deaths of those aged 16-54 years, 58% were from Scotland’s “most deprived areas,” he noted.
From medical records and antiepileptic drug (AED) use, Dr. Mbizvo looked for risk factors that may have contributed to these epilepsy-related deaths. The most common cause of death in the group aged 16- 54 years was sudden unexpected death in epilepsy (SUDEP), followed by respiratory disorders, such as aspiration pneumonia.
“We think this should be avoidable, in the sense that these are people that could perhaps be targeted early with, for example, antibiotics,” said Dr. Mbizvo.
The next most common cause of death was circulatory disease, largely cardiac arrest.
“The idea is that electroexcitation – an abnormality in the brain – and the heart are related, and maybe that’s translating to a risk of death,” said Dr. Mbizvo.
Worrisome group
Mental and behavioral disorders, largely alcohol related, were the next most common cause of death.
“This is a group I worry about,” said Dr. Mbizvo. “I think they’re seen in the acute services and discharged as alcohol-withdrawal seizures. It’s possible that some have epilepsy and are never referred to a neurologist, and this may translate into increased mortality.”
Dr. Mbizvo is analyzing how these results differ from what is seen in the general population of Scotland among those younger than 75 years.
The top cause of death in the general population is neoplasm of the lungs. Aspiration of the lung is near the top for those who died from epilepsy, but the mechanisms leading to lung-related deaths in these populations may differ, said Dr. Mbizvo.
By applying coding methodology from fields unrelated to epilepsy where this approach has been tried, he determined that 78% of epilepsy-related deaths among those younger than 55 years were potentially avoidable.
“As a method, this is still in its infancy and will require validation, but we see this as a start,” Dr. Mbizvo said.
He provided examples from medical records that illustrate avoidable factors that could contribute to death. These included cases in which patients were discharged with the wrong dose of AED and in which patients drowned in a bath after having not been appropriately educated about seizure safety.
Can’t plug in
Patients with a first seizure are typically referred quickly to an appropriate service, but Dr. Mbizvo is concerned about those with chronic, stable epilepsy. “These people may at some point decompensate, and there’s no channel to plug them back into neurology services to make it easy for them to access a neurologist,” he said.
Currently, experts tell discharged patients to call if a problem occurs, but the system “is rather ad hoc,” said Dr. Mbizvo.
Because of the COVID-19 crisis, the use of telemedicine is increasing. This is helping to improve the system. “We may be able to build a virtual community for people who are on antiepileptic drugs and who suddenly begin to experience seizures again, to enable them to quickly get help, alongside a defined pathway to an epilepsy specialist,” said Dr. Mbizvo.
He hopes to develop a risk index for epilepsy patients similar to one used in cardiology that assesses risks such as smoking, high cholesterol level, and obesity. Although such a risk score might be similar to the SUDEP risk indices being developed, it will take into account death from any epilepsy-related cause, said Dr. Mbizvo. “Having not yet completed the analysis, I’m not sure which aspects will confer the greatest risk,” he said.
He added that, anecdotally, he has noticed a slight trend toward high mortality among patients with epilepsy who present multiple times at emergency departments in a year.
If this trend is statistically valid, “it could help create a traffic light flagging system on A&Es [accident and emergency departments] in which individuals with epilepsy who, for example, have two or more attendances to A&E in a year become flagged as high risk of death and are plugged into a rapid access epilepsy specialist clinic,” he said.
For their part, neurologists should recognize drug-resistant epilepsy early and refer such patients for assessment for resective surgery. If successful, such surgery reduces the risk for premature mortality, said Dr. Mbizvo.
Patients should not become discouraged by drug resistance, either. Research shows that, with careful reassessment of epilepsy type and drug changes, some patients whose condition is thought to be intractable could experience significant improvement in seizure frequency or seizures could be stopped.
“We need to talk to our patients more about the importance of adherence and encourage them to be honest with us if they don’t like the drugs we’re giving them and, as a result, are not taking them as recommended,” Dr. Mbizvo said.
Physicians also need to screen for mood disorders, especially suicidal ideation. Increasingly, specialists are recognizing mental health as an important area of epilepsy care.
They should also conduct a “safety briefing” perhaps twice a year in which they discuss, for example, SUDEP risk, driving concerns, showering instead of bathing, ensuring that a life guard is present at a swimming pool, and other measures.
Commenting on the study, Josemir W. (Ley) Sander, MD, PhD, professor of neurology and clinical epilepsy at University College London, said he welcomes any effort that highlights the problem of premature death among people with epilepsy and that offers possible ways to mitigate it.
Although the study “shows that premature death among people with epilepsy is a major issue,” many health care providers are not fully aware of the extent of this problem, said Dr. Sander. “For many, epilepsy is just a benign condition in which people have seizures,” he said. A risk score that could identify those at high risk for death and establishing preventive measures “would go a long way to decrease the burden of epilepsy,” he noted.
The study was supported by Epilepsy Research UK and the Juliet Bergqvist Memorial Fund. Dr. Mbizvo and Dr. Sander have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The research shows that such avoidable deaths “remain common and have not declined over time, despite advances in treatment,” Gashirai Mbizvo, MBChB, PhD, clinical research fellow, Muir Maxwell Epilepsy Center, the University of Edinburgh, Scotland, told a press briefing.
The findings were presented at the Congress of the European Academy of Neurology (EAN) 2020, which is being conducted as a virtual/online meeting because of the COVID-19 pandemic.
As his PhD dissertation, Dr. Mbizvo is investigating the rates, causes, and risk factors for epilepsy-related deaths and the percentage of these that are potentially avoidable.
The National Health Service of Scotland contains various linked administrative data sets. Each resident of Scotland has a unique identifier that facilitates investigations across the health system.
Dr. Mbizvo investigated adults and adolescents aged 16 years and older who died because of epilepsy during 2009-2016. He compared this group to patients of similar age who were living with epilepsy to identify risk factors that might help focus resources. During the study period, 2,149 epilepsy-related deaths occurred. Nearly 60% involved at least one seizure-related hospital admission.
Heavy burden
Of the patients who died because of epilepsy, 24% were seen in an outpatient neurologic clinic. “So there’s this heavy burden of admissions not translating to neurology follow-up,” said Dr. Mbizvo.
During the study period, there was no reduction in mortality “despite advances in medical care,” said Dr. Mbizvo.
Younger people with epilepsy were found to be more likely to die. The standardized mortality rate was 6/100,000 (95% confidence interval, 2.3-9.7) among those aged 16-24 years. By contrast, among those aged 45-54 years, the rate was 2/100,000 (95% CI, 1.1-2.1); it was lower in older age groups.
“The overall mortality is not reducing; people are dying young, and neurologists are really not getting involved,” Dr. Mbizvo said.
Among the almost 600 deaths of those aged 16-54 years, 58% were from Scotland’s “most deprived areas,” he noted.
From medical records and antiepileptic drug (AED) use, Dr. Mbizvo looked for risk factors that may have contributed to these epilepsy-related deaths. The most common cause of death in the group aged 16- 54 years was sudden unexpected death in epilepsy (SUDEP), followed by respiratory disorders, such as aspiration pneumonia.
“We think this should be avoidable, in the sense that these are people that could perhaps be targeted early with, for example, antibiotics,” said Dr. Mbizvo.
The next most common cause of death was circulatory disease, largely cardiac arrest.
“The idea is that electroexcitation – an abnormality in the brain – and the heart are related, and maybe that’s translating to a risk of death,” said Dr. Mbizvo.
Worrisome group
Mental and behavioral disorders, largely alcohol related, were the next most common cause of death.
“This is a group I worry about,” said Dr. Mbizvo. “I think they’re seen in the acute services and discharged as alcohol-withdrawal seizures. It’s possible that some have epilepsy and are never referred to a neurologist, and this may translate into increased mortality.”
Dr. Mbizvo is analyzing how these results differ from what is seen in the general population of Scotland among those younger than 75 years.
The top cause of death in the general population is neoplasm of the lungs. Aspiration of the lung is near the top for those who died from epilepsy, but the mechanisms leading to lung-related deaths in these populations may differ, said Dr. Mbizvo.
By applying coding methodology from fields unrelated to epilepsy where this approach has been tried, he determined that 78% of epilepsy-related deaths among those younger than 55 years were potentially avoidable.
“As a method, this is still in its infancy and will require validation, but we see this as a start,” Dr. Mbizvo said.
He provided examples from medical records that illustrate avoidable factors that could contribute to death. These included cases in which patients were discharged with the wrong dose of AED and in which patients drowned in a bath after having not been appropriately educated about seizure safety.
Can’t plug in
Patients with a first seizure are typically referred quickly to an appropriate service, but Dr. Mbizvo is concerned about those with chronic, stable epilepsy. “These people may at some point decompensate, and there’s no channel to plug them back into neurology services to make it easy for them to access a neurologist,” he said.
Currently, experts tell discharged patients to call if a problem occurs, but the system “is rather ad hoc,” said Dr. Mbizvo.
Because of the COVID-19 crisis, the use of telemedicine is increasing. This is helping to improve the system. “We may be able to build a virtual community for people who are on antiepileptic drugs and who suddenly begin to experience seizures again, to enable them to quickly get help, alongside a defined pathway to an epilepsy specialist,” said Dr. Mbizvo.
He hopes to develop a risk index for epilepsy patients similar to one used in cardiology that assesses risks such as smoking, high cholesterol level, and obesity. Although such a risk score might be similar to the SUDEP risk indices being developed, it will take into account death from any epilepsy-related cause, said Dr. Mbizvo. “Having not yet completed the analysis, I’m not sure which aspects will confer the greatest risk,” he said.
He added that, anecdotally, he has noticed a slight trend toward high mortality among patients with epilepsy who present multiple times at emergency departments in a year.
If this trend is statistically valid, “it could help create a traffic light flagging system on A&Es [accident and emergency departments] in which individuals with epilepsy who, for example, have two or more attendances to A&E in a year become flagged as high risk of death and are plugged into a rapid access epilepsy specialist clinic,” he said.
For their part, neurologists should recognize drug-resistant epilepsy early and refer such patients for assessment for resective surgery. If successful, such surgery reduces the risk for premature mortality, said Dr. Mbizvo.
Patients should not become discouraged by drug resistance, either. Research shows that, with careful reassessment of epilepsy type and drug changes, some patients whose condition is thought to be intractable could experience significant improvement in seizure frequency or seizures could be stopped.
“We need to talk to our patients more about the importance of adherence and encourage them to be honest with us if they don’t like the drugs we’re giving them and, as a result, are not taking them as recommended,” Dr. Mbizvo said.
Physicians also need to screen for mood disorders, especially suicidal ideation. Increasingly, specialists are recognizing mental health as an important area of epilepsy care.
They should also conduct a “safety briefing” perhaps twice a year in which they discuss, for example, SUDEP risk, driving concerns, showering instead of bathing, ensuring that a life guard is present at a swimming pool, and other measures.
Commenting on the study, Josemir W. (Ley) Sander, MD, PhD, professor of neurology and clinical epilepsy at University College London, said he welcomes any effort that highlights the problem of premature death among people with epilepsy and that offers possible ways to mitigate it.
Although the study “shows that premature death among people with epilepsy is a major issue,” many health care providers are not fully aware of the extent of this problem, said Dr. Sander. “For many, epilepsy is just a benign condition in which people have seizures,” he said. A risk score that could identify those at high risk for death and establishing preventive measures “would go a long way to decrease the burden of epilepsy,” he noted.
The study was supported by Epilepsy Research UK and the Juliet Bergqvist Memorial Fund. Dr. Mbizvo and Dr. Sander have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The research shows that such avoidable deaths “remain common and have not declined over time, despite advances in treatment,” Gashirai Mbizvo, MBChB, PhD, clinical research fellow, Muir Maxwell Epilepsy Center, the University of Edinburgh, Scotland, told a press briefing.
The findings were presented at the Congress of the European Academy of Neurology (EAN) 2020, which is being conducted as a virtual/online meeting because of the COVID-19 pandemic.
As his PhD dissertation, Dr. Mbizvo is investigating the rates, causes, and risk factors for epilepsy-related deaths and the percentage of these that are potentially avoidable.
The National Health Service of Scotland contains various linked administrative data sets. Each resident of Scotland has a unique identifier that facilitates investigations across the health system.
Dr. Mbizvo investigated adults and adolescents aged 16 years and older who died because of epilepsy during 2009-2016. He compared this group to patients of similar age who were living with epilepsy to identify risk factors that might help focus resources. During the study period, 2,149 epilepsy-related deaths occurred. Nearly 60% involved at least one seizure-related hospital admission.
Heavy burden
Of the patients who died because of epilepsy, 24% were seen in an outpatient neurologic clinic. “So there’s this heavy burden of admissions not translating to neurology follow-up,” said Dr. Mbizvo.
During the study period, there was no reduction in mortality “despite advances in medical care,” said Dr. Mbizvo.
Younger people with epilepsy were found to be more likely to die. The standardized mortality rate was 6/100,000 (95% confidence interval, 2.3-9.7) among those aged 16-24 years. By contrast, among those aged 45-54 years, the rate was 2/100,000 (95% CI, 1.1-2.1); it was lower in older age groups.
“The overall mortality is not reducing; people are dying young, and neurologists are really not getting involved,” Dr. Mbizvo said.
Among the almost 600 deaths of those aged 16-54 years, 58% were from Scotland’s “most deprived areas,” he noted.
From medical records and antiepileptic drug (AED) use, Dr. Mbizvo looked for risk factors that may have contributed to these epilepsy-related deaths. The most common cause of death in the group aged 16- 54 years was sudden unexpected death in epilepsy (SUDEP), followed by respiratory disorders, such as aspiration pneumonia.
“We think this should be avoidable, in the sense that these are people that could perhaps be targeted early with, for example, antibiotics,” said Dr. Mbizvo.
The next most common cause of death was circulatory disease, largely cardiac arrest.
“The idea is that electroexcitation – an abnormality in the brain – and the heart are related, and maybe that’s translating to a risk of death,” said Dr. Mbizvo.
Worrisome group
Mental and behavioral disorders, largely alcohol related, were the next most common cause of death.
“This is a group I worry about,” said Dr. Mbizvo. “I think they’re seen in the acute services and discharged as alcohol-withdrawal seizures. It’s possible that some have epilepsy and are never referred to a neurologist, and this may translate into increased mortality.”
Dr. Mbizvo is analyzing how these results differ from what is seen in the general population of Scotland among those younger than 75 years.
The top cause of death in the general population is neoplasm of the lungs. Aspiration of the lung is near the top for those who died from epilepsy, but the mechanisms leading to lung-related deaths in these populations may differ, said Dr. Mbizvo.
By applying coding methodology from fields unrelated to epilepsy where this approach has been tried, he determined that 78% of epilepsy-related deaths among those younger than 55 years were potentially avoidable.
“As a method, this is still in its infancy and will require validation, but we see this as a start,” Dr. Mbizvo said.
He provided examples from medical records that illustrate avoidable factors that could contribute to death. These included cases in which patients were discharged with the wrong dose of AED and in which patients drowned in a bath after having not been appropriately educated about seizure safety.
Can’t plug in
Patients with a first seizure are typically referred quickly to an appropriate service, but Dr. Mbizvo is concerned about those with chronic, stable epilepsy. “These people may at some point decompensate, and there’s no channel to plug them back into neurology services to make it easy for them to access a neurologist,” he said.
Currently, experts tell discharged patients to call if a problem occurs, but the system “is rather ad hoc,” said Dr. Mbizvo.
Because of the COVID-19 crisis, the use of telemedicine is increasing. This is helping to improve the system. “We may be able to build a virtual community for people who are on antiepileptic drugs and who suddenly begin to experience seizures again, to enable them to quickly get help, alongside a defined pathway to an epilepsy specialist,” said Dr. Mbizvo.
He hopes to develop a risk index for epilepsy patients similar to one used in cardiology that assesses risks such as smoking, high cholesterol level, and obesity. Although such a risk score might be similar to the SUDEP risk indices being developed, it will take into account death from any epilepsy-related cause, said Dr. Mbizvo. “Having not yet completed the analysis, I’m not sure which aspects will confer the greatest risk,” he said.
He added that, anecdotally, he has noticed a slight trend toward high mortality among patients with epilepsy who present multiple times at emergency departments in a year.
If this trend is statistically valid, “it could help create a traffic light flagging system on A&Es [accident and emergency departments] in which individuals with epilepsy who, for example, have two or more attendances to A&E in a year become flagged as high risk of death and are plugged into a rapid access epilepsy specialist clinic,” he said.
For their part, neurologists should recognize drug-resistant epilepsy early and refer such patients for assessment for resective surgery. If successful, such surgery reduces the risk for premature mortality, said Dr. Mbizvo.
Patients should not become discouraged by drug resistance, either. Research shows that, with careful reassessment of epilepsy type and drug changes, some patients whose condition is thought to be intractable could experience significant improvement in seizure frequency or seizures could be stopped.
“We need to talk to our patients more about the importance of adherence and encourage them to be honest with us if they don’t like the drugs we’re giving them and, as a result, are not taking them as recommended,” Dr. Mbizvo said.
Physicians also need to screen for mood disorders, especially suicidal ideation. Increasingly, specialists are recognizing mental health as an important area of epilepsy care.
They should also conduct a “safety briefing” perhaps twice a year in which they discuss, for example, SUDEP risk, driving concerns, showering instead of bathing, ensuring that a life guard is present at a swimming pool, and other measures.
Commenting on the study, Josemir W. (Ley) Sander, MD, PhD, professor of neurology and clinical epilepsy at University College London, said he welcomes any effort that highlights the problem of premature death among people with epilepsy and that offers possible ways to mitigate it.
Although the study “shows that premature death among people with epilepsy is a major issue,” many health care providers are not fully aware of the extent of this problem, said Dr. Sander. “For many, epilepsy is just a benign condition in which people have seizures,” he said. A risk score that could identify those at high risk for death and establishing preventive measures “would go a long way to decrease the burden of epilepsy,” he noted.
The study was supported by Epilepsy Research UK and the Juliet Bergqvist Memorial Fund. Dr. Mbizvo and Dr. Sander have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM EAN 2020
Neurologists’ pay gets a boost, most happy with career choice
findings from the newly released Medscape Neurologist Compensation Report 2020 show.
Neurologists’ average annual income this year rose to $280,000, up from $267,000 last year. More than half of neurologists (53%) feel fairly compensated, similar to last year’s percentage.
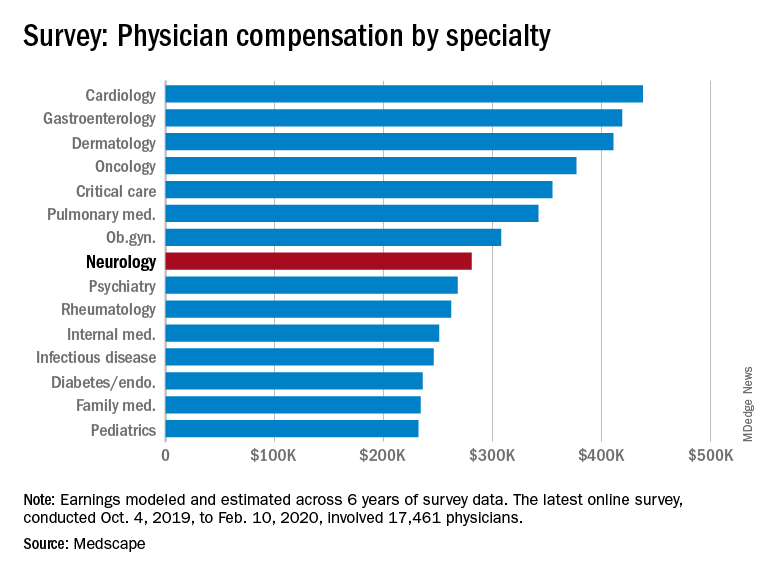
Neurologists are below the middle earners of all physician specialties. At $280,000 in annual compensation for patient care, neurologists rank ninth from the bottom, just below allergists/immunologists ($301,000) but ahead of psychiatrists ($268,000), rheumatologists ($262,000), and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in over 30 specialties.
COVID-19 impact
An important caveat is that the data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, data show that since the start of the crisis, physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they had closed their practices, at least temporarily.
There continues to be a gender pay gap in neurology, with male neurologists earning about 26% more than their female peers ($299,000 vs. $237,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
More than half of all physicians (56%) say they receive an incentive bonus. Neurologists report that they are eligible for an annual incentive bonus of $35,000. Average annual incentive bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one third of physicians overall who receive incentive bonuses say the prospect of receiving the bonus has encouraged them to work longer hours. A higher percentage of neurologists (41%) say their potential bonus influenced them to increase their work hours.
Fifty-eight percent of neurologists achieve more than three quarters of their potential annual incentive bonus. On average, neurologists achieve about two thirds of their potential bonus, the same proportion as for physicians overall.
However, COVID-19 may change that. Experts who were interviewed recently by Medscape noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male neurologists spend 37.7 hours per week seeing patients, somewhat more hours per week than female neurologists (36.1 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, neurologists spend 16.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5), infectious disease physicians (18.5), and psychiatrists (18.3). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a neurologist? Being good at what they do/finding answers, diagnoses tops the list (33%), followed by making the world a better place/helping others (26%), relationships with and gratitude from patients (18%), and making good money at a job they like (11%). A few cited teaching (5%) and pride in their profession (4%).
The most challenging part of practicing neurology is having to follow so many rules and regulations (26%). Other challenges include having to work long hours (18%), dealing with difficult patients (17%), trouble getting fair reimbursement (13%), and working with electronic health records (10%).
Despite the challenges, if they had to do it all over again, 73% of neurologists would still choose medicine as a career, and 86% would again choose neurology.
Other key findings in the latest report regarding neurologists include the following:
- At 18%, neurologists rank near the middle among physicians with regard to losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- 29% of neurologists say they use physician assistants (PAs) to treat patients in their practices, and 53% use nurse practitioners (NPs); 38% use neither for patient care. Of neurologists who work with PAs and NPs in their offices, 49% say these employees have helped boost profitability.
- Two-thirds of neurologists say they will continue taking new and current Medicare/Medicaid patients; none say they will not take new Medicare patients; and 26% are undecided.
- Neurologists participate in various payment methods; 78% are reimbursed via insurance, 35% have fee-for-service arrangements, and 28% are in accountable care organizations.
- Nearly 40% of neurologists expect to participate in the merit-based incentive payment system option, and 10% expect to participate in alternative payment models.
This article first appeared on Medscape.com.
findings from the newly released Medscape Neurologist Compensation Report 2020 show.
Neurologists’ average annual income this year rose to $280,000, up from $267,000 last year. More than half of neurologists (53%) feel fairly compensated, similar to last year’s percentage.

Neurologists are below the middle earners of all physician specialties. At $280,000 in annual compensation for patient care, neurologists rank ninth from the bottom, just below allergists/immunologists ($301,000) but ahead of psychiatrists ($268,000), rheumatologists ($262,000), and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in over 30 specialties.
COVID-19 impact
An important caveat is that the data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, data show that since the start of the crisis, physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they had closed their practices, at least temporarily.
There continues to be a gender pay gap in neurology, with male neurologists earning about 26% more than their female peers ($299,000 vs. $237,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
More than half of all physicians (56%) say they receive an incentive bonus. Neurologists report that they are eligible for an annual incentive bonus of $35,000. Average annual incentive bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one third of physicians overall who receive incentive bonuses say the prospect of receiving the bonus has encouraged them to work longer hours. A higher percentage of neurologists (41%) say their potential bonus influenced them to increase their work hours.
Fifty-eight percent of neurologists achieve more than three quarters of their potential annual incentive bonus. On average, neurologists achieve about two thirds of their potential bonus, the same proportion as for physicians overall.
However, COVID-19 may change that. Experts who were interviewed recently by Medscape noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male neurologists spend 37.7 hours per week seeing patients, somewhat more hours per week than female neurologists (36.1 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, neurologists spend 16.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5), infectious disease physicians (18.5), and psychiatrists (18.3). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a neurologist? Being good at what they do/finding answers, diagnoses tops the list (33%), followed by making the world a better place/helping others (26%), relationships with and gratitude from patients (18%), and making good money at a job they like (11%). A few cited teaching (5%) and pride in their profession (4%).
The most challenging part of practicing neurology is having to follow so many rules and regulations (26%). Other challenges include having to work long hours (18%), dealing with difficult patients (17%), trouble getting fair reimbursement (13%), and working with electronic health records (10%).
Despite the challenges, if they had to do it all over again, 73% of neurologists would still choose medicine as a career, and 86% would again choose neurology.
Other key findings in the latest report regarding neurologists include the following:
- At 18%, neurologists rank near the middle among physicians with regard to losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- 29% of neurologists say they use physician assistants (PAs) to treat patients in their practices, and 53% use nurse practitioners (NPs); 38% use neither for patient care. Of neurologists who work with PAs and NPs in their offices, 49% say these employees have helped boost profitability.
- Two-thirds of neurologists say they will continue taking new and current Medicare/Medicaid patients; none say they will not take new Medicare patients; and 26% are undecided.
- Neurologists participate in various payment methods; 78% are reimbursed via insurance, 35% have fee-for-service arrangements, and 28% are in accountable care organizations.
- Nearly 40% of neurologists expect to participate in the merit-based incentive payment system option, and 10% expect to participate in alternative payment models.
This article first appeared on Medscape.com.
findings from the newly released Medscape Neurologist Compensation Report 2020 show.
Neurologists’ average annual income this year rose to $280,000, up from $267,000 last year. More than half of neurologists (53%) feel fairly compensated, similar to last year’s percentage.

Neurologists are below the middle earners of all physician specialties. At $280,000 in annual compensation for patient care, neurologists rank ninth from the bottom, just below allergists/immunologists ($301,000) but ahead of psychiatrists ($268,000), rheumatologists ($262,000), and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in over 30 specialties.
COVID-19 impact
An important caveat is that the data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, data show that since the start of the crisis, physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they had closed their practices, at least temporarily.
There continues to be a gender pay gap in neurology, with male neurologists earning about 26% more than their female peers ($299,000 vs. $237,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
More than half of all physicians (56%) say they receive an incentive bonus. Neurologists report that they are eligible for an annual incentive bonus of $35,000. Average annual incentive bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one third of physicians overall who receive incentive bonuses say the prospect of receiving the bonus has encouraged them to work longer hours. A higher percentage of neurologists (41%) say their potential bonus influenced them to increase their work hours.
Fifty-eight percent of neurologists achieve more than three quarters of their potential annual incentive bonus. On average, neurologists achieve about two thirds of their potential bonus, the same proportion as for physicians overall.
However, COVID-19 may change that. Experts who were interviewed recently by Medscape noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male neurologists spend 37.7 hours per week seeing patients, somewhat more hours per week than female neurologists (36.1 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, neurologists spend 16.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5), infectious disease physicians (18.5), and psychiatrists (18.3). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a neurologist? Being good at what they do/finding answers, diagnoses tops the list (33%), followed by making the world a better place/helping others (26%), relationships with and gratitude from patients (18%), and making good money at a job they like (11%). A few cited teaching (5%) and pride in their profession (4%).
The most challenging part of practicing neurology is having to follow so many rules and regulations (26%). Other challenges include having to work long hours (18%), dealing with difficult patients (17%), trouble getting fair reimbursement (13%), and working with electronic health records (10%).
Despite the challenges, if they had to do it all over again, 73% of neurologists would still choose medicine as a career, and 86% would again choose neurology.
Other key findings in the latest report regarding neurologists include the following:
- At 18%, neurologists rank near the middle among physicians with regard to losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- 29% of neurologists say they use physician assistants (PAs) to treat patients in their practices, and 53% use nurse practitioners (NPs); 38% use neither for patient care. Of neurologists who work with PAs and NPs in their offices, 49% say these employees have helped boost profitability.
- Two-thirds of neurologists say they will continue taking new and current Medicare/Medicaid patients; none say they will not take new Medicare patients; and 26% are undecided.
- Neurologists participate in various payment methods; 78% are reimbursed via insurance, 35% have fee-for-service arrangements, and 28% are in accountable care organizations.
- Nearly 40% of neurologists expect to participate in the merit-based incentive payment system option, and 10% expect to participate in alternative payment models.
This article first appeared on Medscape.com.
Frontal lobe glucose abnormalities may indicate increased SUDEP risk
, new research suggests.
“The data provide initial evidence that hypometabolism in certain parts of the frontal cortex may be associated with higher SUDEP risk,” said lead author Maysaa M. Basha, MD, associate professor of neurology and director of the Adult Comprehensive Epilepsy Program, Wayne State University/Detroit Medical Center, in Michigan.
If this research is validated, “it potentially can be used to screen patients for higher SUDEP risk,” she said. The idea is to identify those at high risk and then reduce that risk with more aggressive management of seizures or closer monitoring in certain cases, she added.
The research is being presented online as part of the 2020 American Academy of Neurology (AAN) Science Highlights.
Hypometabolism
Dr. Basha and colleagues were encouraged to pursue this new line of research after a pilot [18F]fluorodeoxyglucose positron-emission tomography (FDG-PET) study revealed frontal lobe hypometabolism among patients who subsequently died.
“We wanted to determine if such a metabolic abnormality is associated with SUDEP risk,” said Dr. Basha. She noted that no PET studies have addressed this question, only MRI studies.
In this new study, researchers aimed to identify specific patterns of objectively detected brain glucose metabolic abnormalities in patients with refractory focal epilepsy who were at risk for SUDEP.
The study included 80 patients (45 female patients) aged 16 to 61 years (mean age, 37 years) who underwent FDG-PET as part of their presurgical evaluation for epilepsy surgery. Patients with large brain lesions, such as an infarct or a large tumor, were excluded from the study; such lesions can affect the accuracy of an objective PET analysis, explained Dr. Basha.
The researchers assessed risk for SUDEP using the seven-item SUDEP inventory (SUDEP-7), which was developed as a marker of clinical SUDEP risk. The 0- to 10-point scale is used to evaluate the frequency of tonic-clonic and other seizures, the duration of epilepsy, the use of antiepileptic drugs, and intellectual disability.
The researchers calculated SUDEP-7 inventory scores as closely as possible to FDG-PET assessments. The mean score in the patient population was 3.6.
The investigators divided participants into two subgroups: 22 patients had a SUDEP score of 5 or greater; and 58 had a score of less than 5 (higher scores indicate higher risk for SUDEP).
The researchers compared PET scans of each of these subgroups to PET scans from healthy adults to determine whether they showed common areas of metabolic abnormality. For this, they used an image analytic software program called Statistical Parametric Mapping, which compares group values of metabolic activity measured in small units of the brain (voxels) with statistical methods.
The analysis showed that the higher-risk group displayed a common pattern of hypometabolism in certain brain areas.
“The epilepsy patient subgroup with high SUDEP risk showed areas of decreased metabolism, as compared to the control group, in portions of the frontal cortex,” said Dr. Basha. “The statistically most significant decreases were in the right frontal lobe area—both lateral convexity and medial cortex.”
Dr. Basha added that these group abnormalities were “remarkably similar” to the individual metabolic abnormalities found in the four SUDEP patients in the previous pilot study who underwent PET scanning and who subsequently died.
A similar group analysis showed that the group at low SUDEP risk displayed no common metabolic abnormalities.
MRI findings were normal for 40 patients.
Dr. Basha and colleagues believe that “this is the first PET study assessing the metabolic correlates of SUDEP risk on the group level.”
Common feature
Interictal glucose hypometabolism is “common in and around epileptic foci,” noted Dr. Basha. However, this could extend into nonepileptic regions—for example, to remote connected regions where seizures can spread from the primary focus and into subcortical gray matter structures, such the thalamus.
Some of these metabolic abnormalities may indicate subtle, microscopic, structural abnormalities in the affected brain, said Dr. Basha.
Abnormalities that are induced by epilepsy and that result from purely metabolic changes could be partly or fully reversed if seizures are controlled on a long-term basis, she said. “Some metabolic abnormalities can be reversed after better seizure control with antiepileptic drugs, epileptic surgery, or other antiepileptic treatment,” she said.
It’s “quite possible” that the same brain pattern would be evident in children with epilepsy, although her team has not performed the same analysis in a younger pediatric group, said Dr. Basha. She noted that it would be unethical to administer PET scans, which involve radiation, to young, healthy control persons.
It’s too early to recommend that all epilepsy patients undergo FDG-PET scanning to see whether this pattern of brain glucose hypometabolism is present, said Dr. Basha. “But if this is proven to be a good biomarker, the next step would be a prospective study” to see whether this brain marker is a true signal of SUDEP risk.
“I don’t think our single study would do that, but ultimately, that would be the goal,” she added.
One more piece of the SUDEP puzzle
Commenting on the study, William Davis Gaillard, MD, president of the American Epilepsy Society and chief of neurology, Children’s National Medical Center, Chevy Chase, Maryland, said this new information provides one more piece of the SUDEP puzzle but doesn’t complete the picture.
The study authors assessed PET scans of a group of patients and found common abnormalities that implicate the right medial frontal cortex. “That’s a pretty reasonable method” of investigation, said Dr. Gaillard.
“The challenge is that they’re looking at people they believe have a risk of SUDEP as opposed to people who died,” said Dr. Gaillard.
But he agreed that the results might signal “a biomarker” that “allows you to identify who’s at high risk, and then you may be able to intervene to save them.”
It’s not clear that people with frontal lobe epilepsy are at greater risk for SUDEP than those with temporal lobe epilepsy, he said.
“What you don’t know is whether this represents people with a seizure focus in that area or this represents a common network implicated in people with diverse forms of focal epilepsy; so you need to do some more work,” he said.
Dr. Gaillard pointed out that other research has implicated regions other than the mesial frontal cortex in SUDEP risk. These regions include the insula, the amygdala, the hippocampus, and the brain stem.
He also noted that the SUDEP-7, which has not been thoroughly validated, is designed for use only in adults.
In his own practice, he asks patients about the frequency of tonic-clonic seizures and whether they occur at night. The number of antiepileptic medications a patient takes reflects the difficulty of controlling seizures and may not be “an independent variable for risk,” said Dr. Gaillard.
“It’s clear one needs a better assessment and better idea of who is at risk,” he said.
The researchers have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Basha A et al. AAN 2020. Abstract P5.001.
, new research suggests.
“The data provide initial evidence that hypometabolism in certain parts of the frontal cortex may be associated with higher SUDEP risk,” said lead author Maysaa M. Basha, MD, associate professor of neurology and director of the Adult Comprehensive Epilepsy Program, Wayne State University/Detroit Medical Center, in Michigan.
If this research is validated, “it potentially can be used to screen patients for higher SUDEP risk,” she said. The idea is to identify those at high risk and then reduce that risk with more aggressive management of seizures or closer monitoring in certain cases, she added.
The research is being presented online as part of the 2020 American Academy of Neurology (AAN) Science Highlights.
Hypometabolism
Dr. Basha and colleagues were encouraged to pursue this new line of research after a pilot [18F]fluorodeoxyglucose positron-emission tomography (FDG-PET) study revealed frontal lobe hypometabolism among patients who subsequently died.
“We wanted to determine if such a metabolic abnormality is associated with SUDEP risk,” said Dr. Basha. She noted that no PET studies have addressed this question, only MRI studies.
In this new study, researchers aimed to identify specific patterns of objectively detected brain glucose metabolic abnormalities in patients with refractory focal epilepsy who were at risk for SUDEP.
The study included 80 patients (45 female patients) aged 16 to 61 years (mean age, 37 years) who underwent FDG-PET as part of their presurgical evaluation for epilepsy surgery. Patients with large brain lesions, such as an infarct or a large tumor, were excluded from the study; such lesions can affect the accuracy of an objective PET analysis, explained Dr. Basha.
The researchers assessed risk for SUDEP using the seven-item SUDEP inventory (SUDEP-7), which was developed as a marker of clinical SUDEP risk. The 0- to 10-point scale is used to evaluate the frequency of tonic-clonic and other seizures, the duration of epilepsy, the use of antiepileptic drugs, and intellectual disability.
The researchers calculated SUDEP-7 inventory scores as closely as possible to FDG-PET assessments. The mean score in the patient population was 3.6.
The investigators divided participants into two subgroups: 22 patients had a SUDEP score of 5 or greater; and 58 had a score of less than 5 (higher scores indicate higher risk for SUDEP).
The researchers compared PET scans of each of these subgroups to PET scans from healthy adults to determine whether they showed common areas of metabolic abnormality. For this, they used an image analytic software program called Statistical Parametric Mapping, which compares group values of metabolic activity measured in small units of the brain (voxels) with statistical methods.
The analysis showed that the higher-risk group displayed a common pattern of hypometabolism in certain brain areas.
“The epilepsy patient subgroup with high SUDEP risk showed areas of decreased metabolism, as compared to the control group, in portions of the frontal cortex,” said Dr. Basha. “The statistically most significant decreases were in the right frontal lobe area—both lateral convexity and medial cortex.”
Dr. Basha added that these group abnormalities were “remarkably similar” to the individual metabolic abnormalities found in the four SUDEP patients in the previous pilot study who underwent PET scanning and who subsequently died.
A similar group analysis showed that the group at low SUDEP risk displayed no common metabolic abnormalities.
MRI findings were normal for 40 patients.
Dr. Basha and colleagues believe that “this is the first PET study assessing the metabolic correlates of SUDEP risk on the group level.”
Common feature
Interictal glucose hypometabolism is “common in and around epileptic foci,” noted Dr. Basha. However, this could extend into nonepileptic regions—for example, to remote connected regions where seizures can spread from the primary focus and into subcortical gray matter structures, such the thalamus.
Some of these metabolic abnormalities may indicate subtle, microscopic, structural abnormalities in the affected brain, said Dr. Basha.
Abnormalities that are induced by epilepsy and that result from purely metabolic changes could be partly or fully reversed if seizures are controlled on a long-term basis, she said. “Some metabolic abnormalities can be reversed after better seizure control with antiepileptic drugs, epileptic surgery, or other antiepileptic treatment,” she said.
It’s “quite possible” that the same brain pattern would be evident in children with epilepsy, although her team has not performed the same analysis in a younger pediatric group, said Dr. Basha. She noted that it would be unethical to administer PET scans, which involve radiation, to young, healthy control persons.
It’s too early to recommend that all epilepsy patients undergo FDG-PET scanning to see whether this pattern of brain glucose hypometabolism is present, said Dr. Basha. “But if this is proven to be a good biomarker, the next step would be a prospective study” to see whether this brain marker is a true signal of SUDEP risk.
“I don’t think our single study would do that, but ultimately, that would be the goal,” she added.
One more piece of the SUDEP puzzle
Commenting on the study, William Davis Gaillard, MD, president of the American Epilepsy Society and chief of neurology, Children’s National Medical Center, Chevy Chase, Maryland, said this new information provides one more piece of the SUDEP puzzle but doesn’t complete the picture.
The study authors assessed PET scans of a group of patients and found common abnormalities that implicate the right medial frontal cortex. “That’s a pretty reasonable method” of investigation, said Dr. Gaillard.
“The challenge is that they’re looking at people they believe have a risk of SUDEP as opposed to people who died,” said Dr. Gaillard.
But he agreed that the results might signal “a biomarker” that “allows you to identify who’s at high risk, and then you may be able to intervene to save them.”
It’s not clear that people with frontal lobe epilepsy are at greater risk for SUDEP than those with temporal lobe epilepsy, he said.
“What you don’t know is whether this represents people with a seizure focus in that area or this represents a common network implicated in people with diverse forms of focal epilepsy; so you need to do some more work,” he said.
Dr. Gaillard pointed out that other research has implicated regions other than the mesial frontal cortex in SUDEP risk. These regions include the insula, the amygdala, the hippocampus, and the brain stem.
He also noted that the SUDEP-7, which has not been thoroughly validated, is designed for use only in adults.
In his own practice, he asks patients about the frequency of tonic-clonic seizures and whether they occur at night. The number of antiepileptic medications a patient takes reflects the difficulty of controlling seizures and may not be “an independent variable for risk,” said Dr. Gaillard.
“It’s clear one needs a better assessment and better idea of who is at risk,” he said.
The researchers have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Basha A et al. AAN 2020. Abstract P5.001.
, new research suggests.
“The data provide initial evidence that hypometabolism in certain parts of the frontal cortex may be associated with higher SUDEP risk,” said lead author Maysaa M. Basha, MD, associate professor of neurology and director of the Adult Comprehensive Epilepsy Program, Wayne State University/Detroit Medical Center, in Michigan.
If this research is validated, “it potentially can be used to screen patients for higher SUDEP risk,” she said. The idea is to identify those at high risk and then reduce that risk with more aggressive management of seizures or closer monitoring in certain cases, she added.
The research is being presented online as part of the 2020 American Academy of Neurology (AAN) Science Highlights.
Hypometabolism
Dr. Basha and colleagues were encouraged to pursue this new line of research after a pilot [18F]fluorodeoxyglucose positron-emission tomography (FDG-PET) study revealed frontal lobe hypometabolism among patients who subsequently died.
“We wanted to determine if such a metabolic abnormality is associated with SUDEP risk,” said Dr. Basha. She noted that no PET studies have addressed this question, only MRI studies.
In this new study, researchers aimed to identify specific patterns of objectively detected brain glucose metabolic abnormalities in patients with refractory focal epilepsy who were at risk for SUDEP.
The study included 80 patients (45 female patients) aged 16 to 61 years (mean age, 37 years) who underwent FDG-PET as part of their presurgical evaluation for epilepsy surgery. Patients with large brain lesions, such as an infarct or a large tumor, were excluded from the study; such lesions can affect the accuracy of an objective PET analysis, explained Dr. Basha.
The researchers assessed risk for SUDEP using the seven-item SUDEP inventory (SUDEP-7), which was developed as a marker of clinical SUDEP risk. The 0- to 10-point scale is used to evaluate the frequency of tonic-clonic and other seizures, the duration of epilepsy, the use of antiepileptic drugs, and intellectual disability.
The researchers calculated SUDEP-7 inventory scores as closely as possible to FDG-PET assessments. The mean score in the patient population was 3.6.
The investigators divided participants into two subgroups: 22 patients had a SUDEP score of 5 or greater; and 58 had a score of less than 5 (higher scores indicate higher risk for SUDEP).
The researchers compared PET scans of each of these subgroups to PET scans from healthy adults to determine whether they showed common areas of metabolic abnormality. For this, they used an image analytic software program called Statistical Parametric Mapping, which compares group values of metabolic activity measured in small units of the brain (voxels) with statistical methods.
The analysis showed that the higher-risk group displayed a common pattern of hypometabolism in certain brain areas.
“The epilepsy patient subgroup with high SUDEP risk showed areas of decreased metabolism, as compared to the control group, in portions of the frontal cortex,” said Dr. Basha. “The statistically most significant decreases were in the right frontal lobe area—both lateral convexity and medial cortex.”
Dr. Basha added that these group abnormalities were “remarkably similar” to the individual metabolic abnormalities found in the four SUDEP patients in the previous pilot study who underwent PET scanning and who subsequently died.
A similar group analysis showed that the group at low SUDEP risk displayed no common metabolic abnormalities.
MRI findings were normal for 40 patients.
Dr. Basha and colleagues believe that “this is the first PET study assessing the metabolic correlates of SUDEP risk on the group level.”
Common feature
Interictal glucose hypometabolism is “common in and around epileptic foci,” noted Dr. Basha. However, this could extend into nonepileptic regions—for example, to remote connected regions where seizures can spread from the primary focus and into subcortical gray matter structures, such the thalamus.
Some of these metabolic abnormalities may indicate subtle, microscopic, structural abnormalities in the affected brain, said Dr. Basha.
Abnormalities that are induced by epilepsy and that result from purely metabolic changes could be partly or fully reversed if seizures are controlled on a long-term basis, she said. “Some metabolic abnormalities can be reversed after better seizure control with antiepileptic drugs, epileptic surgery, or other antiepileptic treatment,” she said.
It’s “quite possible” that the same brain pattern would be evident in children with epilepsy, although her team has not performed the same analysis in a younger pediatric group, said Dr. Basha. She noted that it would be unethical to administer PET scans, which involve radiation, to young, healthy control persons.
It’s too early to recommend that all epilepsy patients undergo FDG-PET scanning to see whether this pattern of brain glucose hypometabolism is present, said Dr. Basha. “But if this is proven to be a good biomarker, the next step would be a prospective study” to see whether this brain marker is a true signal of SUDEP risk.
“I don’t think our single study would do that, but ultimately, that would be the goal,” she added.
One more piece of the SUDEP puzzle
Commenting on the study, William Davis Gaillard, MD, president of the American Epilepsy Society and chief of neurology, Children’s National Medical Center, Chevy Chase, Maryland, said this new information provides one more piece of the SUDEP puzzle but doesn’t complete the picture.
The study authors assessed PET scans of a group of patients and found common abnormalities that implicate the right medial frontal cortex. “That’s a pretty reasonable method” of investigation, said Dr. Gaillard.
“The challenge is that they’re looking at people they believe have a risk of SUDEP as opposed to people who died,” said Dr. Gaillard.
But he agreed that the results might signal “a biomarker” that “allows you to identify who’s at high risk, and then you may be able to intervene to save them.”
It’s not clear that people with frontal lobe epilepsy are at greater risk for SUDEP than those with temporal lobe epilepsy, he said.
“What you don’t know is whether this represents people with a seizure focus in that area or this represents a common network implicated in people with diverse forms of focal epilepsy; so you need to do some more work,” he said.
Dr. Gaillard pointed out that other research has implicated regions other than the mesial frontal cortex in SUDEP risk. These regions include the insula, the amygdala, the hippocampus, and the brain stem.
He also noted that the SUDEP-7, which has not been thoroughly validated, is designed for use only in adults.
In his own practice, he asks patients about the frequency of tonic-clonic seizures and whether they occur at night. The number of antiepileptic medications a patient takes reflects the difficulty of controlling seizures and may not be “an independent variable for risk,” said Dr. Gaillard.
“It’s clear one needs a better assessment and better idea of who is at risk,” he said.
The researchers have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Basha A et al. AAN 2020. Abstract P5.001.
Patients with epilepsy may underreport seizures, survey finds
according to survey results presented online as part of the 2020 American Academy of Neurology Science Highlights.
Clinicians, for their part, may underestimate the number of seizures that go unreported. This disconnect may contribute to complacency about epilepsy treatment regimens among patients, caregivers, and health care professionals (HCPs), despite continuations in seizures. “Improved reporting of all seizure occurrences and more frequent discussion of potential treatment changes, initiated by all groups, may be needed to optimize treatment outcomes,” said Patricia E. Penovich, MD, a neurologist at Minnesota Epilepsy Group in St. Paul, and colleagues.
To evaluate treatment complacency among adult patients with epilepsy, caregivers, and HCPs, Dr. Penovich and collaborators analyzed data from the STEP survey (Seize the Truth about Epilepsy Perceptions), which was conducted between February and March 2019. In all, 400 adults with epilepsy, 201 caregivers, and 258 HCPs completed the survey. The HCPs included 96 epileptologists, 112 general neurologists, and 50 nurse practitioners or physician assistants.
Patients had an average epilepsy duration of 16 years, and 58% were on at least their third antiepileptic drug (AED). In the past year, 52% of patients had 1-9 seizures, and 31% had 10 or more seizures. “Patients estimated reporting 45% of their seizures to their HCPs, and for the seizures not reported, 57% provided reasoning that they were not serious enough to mention,” reported Dr. Penovich and colleagues. “Alternatively, HCPs estimated that patients report 73% of seizures.”
Survey participants most frequently selected HCPs as the ones to initiate conversations about changing AEDs or increasing dosage. “Patient-initiated discussions were reported by 39% of patients for changing AEDs and 27% of patients for increasing AED dosage; 25% of patients reported they were likely to ask their HCP about changing treatments in the next 12 months,” the authors said. Discussion of vagus nerve stimulation was reported by 21% of HCPs, and 10% reported discussion of responsive neurostimulation. HCPs also discussed surgical options such as hemispherectomy (3%), corpus callosotomy and multiple subpial transection (4%), lobe resection (8%), and lesionectomy (11%).
Among patients with 13 or more seizures per year, 27% reported referral to an epilepsy center. Most survey participants – 61% of patients and HCPs and 68% of caregivers – “reported a desire for a treatment map that tells patients to see an epileptologist/specialist as soon as they have symptoms,” the researchers said.
“What we would like to think is that we are getting the whole scoop and the honest scoop” about seizure activity, Dr. Penovich said in an interview. “What this shows us is that that’s probably not always true. Some health care providers understand that the patients do not tell them everything,” but the degree of seizure underreporting may be surprising.
Dr. Penovich has seen this phenomenon in practice. In some cases, caregivers return to the office to explain that a patient did not report all of their seizures. Other patients may omit entire days of seizures in their diaries as an oversight. In addition, patients may not report seizures to avoid having a driver’s license revoked. In some instances, clinicians may not take the time to discuss seizure activity in detail.
Having an accurate picture of seizure activity is an “important part of working with our patients, particularly when we are trying to get them to the point of being seizure free,” said Dr. Penovich.
Failing a first or second AED indicates a greater likelihood that medication will not stop a patient’s seizures, “but it does not mean that you will not be controlled,” Dr. Penovich said. More medications, surgical options, and investigative treatments have become available. Still, AED trials should not prevent a timely referral to an epilepsy center. “You don’t need to go through 10 or 15 before you get referred” to an epilepsy center, she said.
The STEP survey was conducted by Kantar Health on behalf of SK Life Science. Dr. Penovich has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from SK Life Science, Neurelis, GW Pharmaceuticals, Engage Therapeutics, and UCB Pharma. A coauthor was employed by Kantar Health. Other coauthors disclosed compensation from SK Life Science and various pharmaceutical companies.
SOURCE: Penovich PE et al. AAN 2020, Abstract S59.007.
according to survey results presented online as part of the 2020 American Academy of Neurology Science Highlights.
Clinicians, for their part, may underestimate the number of seizures that go unreported. This disconnect may contribute to complacency about epilepsy treatment regimens among patients, caregivers, and health care professionals (HCPs), despite continuations in seizures. “Improved reporting of all seizure occurrences and more frequent discussion of potential treatment changes, initiated by all groups, may be needed to optimize treatment outcomes,” said Patricia E. Penovich, MD, a neurologist at Minnesota Epilepsy Group in St. Paul, and colleagues.
To evaluate treatment complacency among adult patients with epilepsy, caregivers, and HCPs, Dr. Penovich and collaborators analyzed data from the STEP survey (Seize the Truth about Epilepsy Perceptions), which was conducted between February and March 2019. In all, 400 adults with epilepsy, 201 caregivers, and 258 HCPs completed the survey. The HCPs included 96 epileptologists, 112 general neurologists, and 50 nurse practitioners or physician assistants.
Patients had an average epilepsy duration of 16 years, and 58% were on at least their third antiepileptic drug (AED). In the past year, 52% of patients had 1-9 seizures, and 31% had 10 or more seizures. “Patients estimated reporting 45% of their seizures to their HCPs, and for the seizures not reported, 57% provided reasoning that they were not serious enough to mention,” reported Dr. Penovich and colleagues. “Alternatively, HCPs estimated that patients report 73% of seizures.”
Survey participants most frequently selected HCPs as the ones to initiate conversations about changing AEDs or increasing dosage. “Patient-initiated discussions were reported by 39% of patients for changing AEDs and 27% of patients for increasing AED dosage; 25% of patients reported they were likely to ask their HCP about changing treatments in the next 12 months,” the authors said. Discussion of vagus nerve stimulation was reported by 21% of HCPs, and 10% reported discussion of responsive neurostimulation. HCPs also discussed surgical options such as hemispherectomy (3%), corpus callosotomy and multiple subpial transection (4%), lobe resection (8%), and lesionectomy (11%).
Among patients with 13 or more seizures per year, 27% reported referral to an epilepsy center. Most survey participants – 61% of patients and HCPs and 68% of caregivers – “reported a desire for a treatment map that tells patients to see an epileptologist/specialist as soon as they have symptoms,” the researchers said.
“What we would like to think is that we are getting the whole scoop and the honest scoop” about seizure activity, Dr. Penovich said in an interview. “What this shows us is that that’s probably not always true. Some health care providers understand that the patients do not tell them everything,” but the degree of seizure underreporting may be surprising.
Dr. Penovich has seen this phenomenon in practice. In some cases, caregivers return to the office to explain that a patient did not report all of their seizures. Other patients may omit entire days of seizures in their diaries as an oversight. In addition, patients may not report seizures to avoid having a driver’s license revoked. In some instances, clinicians may not take the time to discuss seizure activity in detail.
Having an accurate picture of seizure activity is an “important part of working with our patients, particularly when we are trying to get them to the point of being seizure free,” said Dr. Penovich.
Failing a first or second AED indicates a greater likelihood that medication will not stop a patient’s seizures, “but it does not mean that you will not be controlled,” Dr. Penovich said. More medications, surgical options, and investigative treatments have become available. Still, AED trials should not prevent a timely referral to an epilepsy center. “You don’t need to go through 10 or 15 before you get referred” to an epilepsy center, she said.
The STEP survey was conducted by Kantar Health on behalf of SK Life Science. Dr. Penovich has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from SK Life Science, Neurelis, GW Pharmaceuticals, Engage Therapeutics, and UCB Pharma. A coauthor was employed by Kantar Health. Other coauthors disclosed compensation from SK Life Science and various pharmaceutical companies.
SOURCE: Penovich PE et al. AAN 2020, Abstract S59.007.
according to survey results presented online as part of the 2020 American Academy of Neurology Science Highlights.
Clinicians, for their part, may underestimate the number of seizures that go unreported. This disconnect may contribute to complacency about epilepsy treatment regimens among patients, caregivers, and health care professionals (HCPs), despite continuations in seizures. “Improved reporting of all seizure occurrences and more frequent discussion of potential treatment changes, initiated by all groups, may be needed to optimize treatment outcomes,” said Patricia E. Penovich, MD, a neurologist at Minnesota Epilepsy Group in St. Paul, and colleagues.
To evaluate treatment complacency among adult patients with epilepsy, caregivers, and HCPs, Dr. Penovich and collaborators analyzed data from the STEP survey (Seize the Truth about Epilepsy Perceptions), which was conducted between February and March 2019. In all, 400 adults with epilepsy, 201 caregivers, and 258 HCPs completed the survey. The HCPs included 96 epileptologists, 112 general neurologists, and 50 nurse practitioners or physician assistants.
Patients had an average epilepsy duration of 16 years, and 58% were on at least their third antiepileptic drug (AED). In the past year, 52% of patients had 1-9 seizures, and 31% had 10 or more seizures. “Patients estimated reporting 45% of their seizures to their HCPs, and for the seizures not reported, 57% provided reasoning that they were not serious enough to mention,” reported Dr. Penovich and colleagues. “Alternatively, HCPs estimated that patients report 73% of seizures.”
Survey participants most frequently selected HCPs as the ones to initiate conversations about changing AEDs or increasing dosage. “Patient-initiated discussions were reported by 39% of patients for changing AEDs and 27% of patients for increasing AED dosage; 25% of patients reported they were likely to ask their HCP about changing treatments in the next 12 months,” the authors said. Discussion of vagus nerve stimulation was reported by 21% of HCPs, and 10% reported discussion of responsive neurostimulation. HCPs also discussed surgical options such as hemispherectomy (3%), corpus callosotomy and multiple subpial transection (4%), lobe resection (8%), and lesionectomy (11%).
Among patients with 13 or more seizures per year, 27% reported referral to an epilepsy center. Most survey participants – 61% of patients and HCPs and 68% of caregivers – “reported a desire for a treatment map that tells patients to see an epileptologist/specialist as soon as they have symptoms,” the researchers said.
“What we would like to think is that we are getting the whole scoop and the honest scoop” about seizure activity, Dr. Penovich said in an interview. “What this shows us is that that’s probably not always true. Some health care providers understand that the patients do not tell them everything,” but the degree of seizure underreporting may be surprising.
Dr. Penovich has seen this phenomenon in practice. In some cases, caregivers return to the office to explain that a patient did not report all of their seizures. Other patients may omit entire days of seizures in their diaries as an oversight. In addition, patients may not report seizures to avoid having a driver’s license revoked. In some instances, clinicians may not take the time to discuss seizure activity in detail.
Having an accurate picture of seizure activity is an “important part of working with our patients, particularly when we are trying to get them to the point of being seizure free,” said Dr. Penovich.
Failing a first or second AED indicates a greater likelihood that medication will not stop a patient’s seizures, “but it does not mean that you will not be controlled,” Dr. Penovich said. More medications, surgical options, and investigative treatments have become available. Still, AED trials should not prevent a timely referral to an epilepsy center. “You don’t need to go through 10 or 15 before you get referred” to an epilepsy center, she said.
The STEP survey was conducted by Kantar Health on behalf of SK Life Science. Dr. Penovich has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from SK Life Science, Neurelis, GW Pharmaceuticals, Engage Therapeutics, and UCB Pharma. A coauthor was employed by Kantar Health. Other coauthors disclosed compensation from SK Life Science and various pharmaceutical companies.
SOURCE: Penovich PE et al. AAN 2020, Abstract S59.007.
FROM AAN 2020
Are patients with epilepsy at increased risk of COVID-19 infection?
Chronic conditions such as lung disease, diabetes, and heart disease frequently receive attention for increasing the risk of complications for people who contract the coronavirus. Meanwhile, many members of the epilepsy community continue to wonder how the virus affects them. To address these concerns, the Epilepsy Foundation has released information that answers many common questions that people with epilepsy have about how COVID-19 can impact their health.
Perhaps the most pressing of these questions is: Does epilepsy increase the risk or severity of the coronavirus?
“The most common thing we’re hearing from patients in my practice is their proactive concern for being at increased risk for getting the coronavirus,” confirmed Selim Benbadis, MD, division director, epilepsy, EEG, and sleep medicine at the University of South Florida in Tampa. “Epilepsy patients are not at increased risk for complications from the coronavirus because epilepsy does not affect the immune system.”
In other words, people who have epilepsy face the same health challenges as people who do not have the condition and are otherwise healthy. For this reason, people who have epilepsy should exercise the same habits and preventative measures that healthy people would typically take, such as social distancing; avoiding contact with sick people; washing hands regularly; disinfecting surfaces regularly; and avoiding touching hands, eyes, nose and mouth.
However, as Dr. Benbadis explained, the high fever associated with coronavirus can trigger seizures. The increased risk is another reason people who have epilepsy should do their best to avoid getting sick.
Seizure medications do not increase COVID-19 risk but other conditions can
Similarly, epilepsy medications do not increase the risk of contracting the disease.
“The medications patients take to treat their epilepsy do not affect their immune system,” said Andrew Wilner, MD, associate professor of neurology at the University of Tennessee Health Science Center, Memphis. There are a few exceptions – such as adrenocorticotropic hormone and everolimus – but doctors rarely use these drugs to treat epilepsy.
However, there are some situations and conditions that may pose a risk for people who contact the coronavirus. For instance, people who have problems swallowing their food and tend to suck food down their windpipes are more likely to develop pneumonia. Also, much like the general population, having diabetes, heart disease, or lung problems increase the chances of developing complications from the virus.
The best ways to avoid additional risks in epilepsy
Because of the pandemic, people who have epilepsy may have found that many of their doctors’ appointments have been canceled. Many clinics and medical practices have done this in order minimize exposing people who have acute illnesses to the virus. By focusing more on patients with acute conditions, doctors and nurses can better tend to patients with acute problems. As a result, practices have shifted to providing patient care using telemedicine as much as possible.
“Telemedicine services have surged, and I’ve been saying for years that telemedicine was going to grow,” Dr. Benbadis said. “It’s more convenient, and I believe that we’re going to see increased use of telemedicine long after the coronavirus pandemic is over.”
Aside from communicating with their doctors, the Epilepsy Foundation and Dr. Wilner stress that the best way for people who have epilepsy to stay healthy is by taking their medications on a regular basis exactly as prescribed.
“Taking mediation correctly and regularly is the best strategy for epilepsy patients to avoid unnecessary hospitalizations,” Dr. Wilner said. “If they have breakthrough seizures and get sent to the emergency room, then they risk being exposed to the virus in the ER.”
Also, because ERs are more crowded than usual, the Epilepsy Foundation encourages people who suspect they have the coronavirus to call their doctor’s office first. The goal is to try to make sure that people who have severe or life-threatening symptoms have access to treatment in the ER.
As with the general population, the first thing that epilepsy patients who suspect they have the coronavirus should do is call his or her doctor’s office. The health care professional taking the call will ask the patient a series of questions to determine whether the patient has COVID-19 or another condition or needs to seek emergency medical attention.
Fever, cough, and trouble breathing fall among the most commonly reported symptoms of the coronavirus. In many cases, health care providers recommend that people with mild versions of these symptoms stay at home.
Helpful tips
The Epilepsy Foundation offers tips on signs to look for when trying to figure out when a seizure requires an ER visit. These are:
- Seizures in which awareness is lost for more than 5 minutes and no reversal medications are available.
- Seizures with an unusual pattern or duration.
- Seizures that cannot be treated safely at home or are not responding to rescue medication even after the medication has had enough time to work.
- Seizures that occur after a severe blow to the head.
Additionally, while COVID-19 can cause death and sudden death in patients, the virus does not cause sudden unexpected death in epilepsy (SUDEP). Because SUDEP is extremely rare, Dr. Benbadis said that there is no information to suggest that contracting the coronavirus will increase the risk,
Finally, no shortages of seizures medications have been reported as a result of COVID-19. However, there were shortages of generic levetiracetam immediate-release and levetiracetam extended-release medications prior to and during COVID-19. Experts expect the shortage to continue.
Overall, people who have epilepsy should be able to stay healthy – provided they exercise healthy and preventative habits.
“The majority of epilepsy patients should be reassured that if they continue their usual care, take their meds as directed, get adequate sleep, nutritious diet, they’re not at any increased risk compared to the general population,” said Dr. Wilner.
Dr. Benbadis reported the following disclosures: consultant for Bioserenity (DigiTrace), Brain Sentinel, Cavion, Ceribell, Eisai, Greenwich, LivaNova, Neuropace, SK biopharmaceuticals, Sunovion; speakers bureau for Eisai, Greenwich, LivaNova, Sunovion; Florida Medical Director of Stratus/Alliance; Member: Epilepsy Study Consortium; grant support from Cavion, LivaNova, Greenwich, SK biopharmaceuticals, Sunovion, Takeda, UCB, Xenon; royalties as an author or editor for Emedicine-Medscape-WebMD, UpToDate; editorial board for the Epilepsy.com (Epilepsy Foundation) controversy section, Emedicine-Medscape-WebMD, Epileptic Disorders, Epilepsy and Behavior, and Expert Review of Neurotherapeutics. Dr. Wilner reports Medical Advisory Board of Accordant Health Services, Greensboro, S.C., and book royalties: “The Locum Life: A Physician’s Guide to Locum Tenens,” Lulu Press.
Chronic conditions such as lung disease, diabetes, and heart disease frequently receive attention for increasing the risk of complications for people who contract the coronavirus. Meanwhile, many members of the epilepsy community continue to wonder how the virus affects them. To address these concerns, the Epilepsy Foundation has released information that answers many common questions that people with epilepsy have about how COVID-19 can impact their health.
Perhaps the most pressing of these questions is: Does epilepsy increase the risk or severity of the coronavirus?
“The most common thing we’re hearing from patients in my practice is their proactive concern for being at increased risk for getting the coronavirus,” confirmed Selim Benbadis, MD, division director, epilepsy, EEG, and sleep medicine at the University of South Florida in Tampa. “Epilepsy patients are not at increased risk for complications from the coronavirus because epilepsy does not affect the immune system.”
In other words, people who have epilepsy face the same health challenges as people who do not have the condition and are otherwise healthy. For this reason, people who have epilepsy should exercise the same habits and preventative measures that healthy people would typically take, such as social distancing; avoiding contact with sick people; washing hands regularly; disinfecting surfaces regularly; and avoiding touching hands, eyes, nose and mouth.
However, as Dr. Benbadis explained, the high fever associated with coronavirus can trigger seizures. The increased risk is another reason people who have epilepsy should do their best to avoid getting sick.
Seizure medications do not increase COVID-19 risk but other conditions can
Similarly, epilepsy medications do not increase the risk of contracting the disease.
“The medications patients take to treat their epilepsy do not affect their immune system,” said Andrew Wilner, MD, associate professor of neurology at the University of Tennessee Health Science Center, Memphis. There are a few exceptions – such as adrenocorticotropic hormone and everolimus – but doctors rarely use these drugs to treat epilepsy.
However, there are some situations and conditions that may pose a risk for people who contact the coronavirus. For instance, people who have problems swallowing their food and tend to suck food down their windpipes are more likely to develop pneumonia. Also, much like the general population, having diabetes, heart disease, or lung problems increase the chances of developing complications from the virus.
The best ways to avoid additional risks in epilepsy
Because of the pandemic, people who have epilepsy may have found that many of their doctors’ appointments have been canceled. Many clinics and medical practices have done this in order minimize exposing people who have acute illnesses to the virus. By focusing more on patients with acute conditions, doctors and nurses can better tend to patients with acute problems. As a result, practices have shifted to providing patient care using telemedicine as much as possible.
“Telemedicine services have surged, and I’ve been saying for years that telemedicine was going to grow,” Dr. Benbadis said. “It’s more convenient, and I believe that we’re going to see increased use of telemedicine long after the coronavirus pandemic is over.”
Aside from communicating with their doctors, the Epilepsy Foundation and Dr. Wilner stress that the best way for people who have epilepsy to stay healthy is by taking their medications on a regular basis exactly as prescribed.
“Taking mediation correctly and regularly is the best strategy for epilepsy patients to avoid unnecessary hospitalizations,” Dr. Wilner said. “If they have breakthrough seizures and get sent to the emergency room, then they risk being exposed to the virus in the ER.”
Also, because ERs are more crowded than usual, the Epilepsy Foundation encourages people who suspect they have the coronavirus to call their doctor’s office first. The goal is to try to make sure that people who have severe or life-threatening symptoms have access to treatment in the ER.
As with the general population, the first thing that epilepsy patients who suspect they have the coronavirus should do is call his or her doctor’s office. The health care professional taking the call will ask the patient a series of questions to determine whether the patient has COVID-19 or another condition or needs to seek emergency medical attention.
Fever, cough, and trouble breathing fall among the most commonly reported symptoms of the coronavirus. In many cases, health care providers recommend that people with mild versions of these symptoms stay at home.
Helpful tips
The Epilepsy Foundation offers tips on signs to look for when trying to figure out when a seizure requires an ER visit. These are:
- Seizures in which awareness is lost for more than 5 minutes and no reversal medications are available.
- Seizures with an unusual pattern or duration.
- Seizures that cannot be treated safely at home or are not responding to rescue medication even after the medication has had enough time to work.
- Seizures that occur after a severe blow to the head.
Additionally, while COVID-19 can cause death and sudden death in patients, the virus does not cause sudden unexpected death in epilepsy (SUDEP). Because SUDEP is extremely rare, Dr. Benbadis said that there is no information to suggest that contracting the coronavirus will increase the risk,
Finally, no shortages of seizures medications have been reported as a result of COVID-19. However, there were shortages of generic levetiracetam immediate-release and levetiracetam extended-release medications prior to and during COVID-19. Experts expect the shortage to continue.
Overall, people who have epilepsy should be able to stay healthy – provided they exercise healthy and preventative habits.
“The majority of epilepsy patients should be reassured that if they continue their usual care, take their meds as directed, get adequate sleep, nutritious diet, they’re not at any increased risk compared to the general population,” said Dr. Wilner.
Dr. Benbadis reported the following disclosures: consultant for Bioserenity (DigiTrace), Brain Sentinel, Cavion, Ceribell, Eisai, Greenwich, LivaNova, Neuropace, SK biopharmaceuticals, Sunovion; speakers bureau for Eisai, Greenwich, LivaNova, Sunovion; Florida Medical Director of Stratus/Alliance; Member: Epilepsy Study Consortium; grant support from Cavion, LivaNova, Greenwich, SK biopharmaceuticals, Sunovion, Takeda, UCB, Xenon; royalties as an author or editor for Emedicine-Medscape-WebMD, UpToDate; editorial board for the Epilepsy.com (Epilepsy Foundation) controversy section, Emedicine-Medscape-WebMD, Epileptic Disorders, Epilepsy and Behavior, and Expert Review of Neurotherapeutics. Dr. Wilner reports Medical Advisory Board of Accordant Health Services, Greensboro, S.C., and book royalties: “The Locum Life: A Physician’s Guide to Locum Tenens,” Lulu Press.
Chronic conditions such as lung disease, diabetes, and heart disease frequently receive attention for increasing the risk of complications for people who contract the coronavirus. Meanwhile, many members of the epilepsy community continue to wonder how the virus affects them. To address these concerns, the Epilepsy Foundation has released information that answers many common questions that people with epilepsy have about how COVID-19 can impact their health.
Perhaps the most pressing of these questions is: Does epilepsy increase the risk or severity of the coronavirus?
“The most common thing we’re hearing from patients in my practice is their proactive concern for being at increased risk for getting the coronavirus,” confirmed Selim Benbadis, MD, division director, epilepsy, EEG, and sleep medicine at the University of South Florida in Tampa. “Epilepsy patients are not at increased risk for complications from the coronavirus because epilepsy does not affect the immune system.”
In other words, people who have epilepsy face the same health challenges as people who do not have the condition and are otherwise healthy. For this reason, people who have epilepsy should exercise the same habits and preventative measures that healthy people would typically take, such as social distancing; avoiding contact with sick people; washing hands regularly; disinfecting surfaces regularly; and avoiding touching hands, eyes, nose and mouth.
However, as Dr. Benbadis explained, the high fever associated with coronavirus can trigger seizures. The increased risk is another reason people who have epilepsy should do their best to avoid getting sick.
Seizure medications do not increase COVID-19 risk but other conditions can
Similarly, epilepsy medications do not increase the risk of contracting the disease.
“The medications patients take to treat their epilepsy do not affect their immune system,” said Andrew Wilner, MD, associate professor of neurology at the University of Tennessee Health Science Center, Memphis. There are a few exceptions – such as adrenocorticotropic hormone and everolimus – but doctors rarely use these drugs to treat epilepsy.
However, there are some situations and conditions that may pose a risk for people who contact the coronavirus. For instance, people who have problems swallowing their food and tend to suck food down their windpipes are more likely to develop pneumonia. Also, much like the general population, having diabetes, heart disease, or lung problems increase the chances of developing complications from the virus.
The best ways to avoid additional risks in epilepsy
Because of the pandemic, people who have epilepsy may have found that many of their doctors’ appointments have been canceled. Many clinics and medical practices have done this in order minimize exposing people who have acute illnesses to the virus. By focusing more on patients with acute conditions, doctors and nurses can better tend to patients with acute problems. As a result, practices have shifted to providing patient care using telemedicine as much as possible.
“Telemedicine services have surged, and I’ve been saying for years that telemedicine was going to grow,” Dr. Benbadis said. “It’s more convenient, and I believe that we’re going to see increased use of telemedicine long after the coronavirus pandemic is over.”
Aside from communicating with their doctors, the Epilepsy Foundation and Dr. Wilner stress that the best way for people who have epilepsy to stay healthy is by taking their medications on a regular basis exactly as prescribed.
“Taking mediation correctly and regularly is the best strategy for epilepsy patients to avoid unnecessary hospitalizations,” Dr. Wilner said. “If they have breakthrough seizures and get sent to the emergency room, then they risk being exposed to the virus in the ER.”
Also, because ERs are more crowded than usual, the Epilepsy Foundation encourages people who suspect they have the coronavirus to call their doctor’s office first. The goal is to try to make sure that people who have severe or life-threatening symptoms have access to treatment in the ER.
As with the general population, the first thing that epilepsy patients who suspect they have the coronavirus should do is call his or her doctor’s office. The health care professional taking the call will ask the patient a series of questions to determine whether the patient has COVID-19 or another condition or needs to seek emergency medical attention.
Fever, cough, and trouble breathing fall among the most commonly reported symptoms of the coronavirus. In many cases, health care providers recommend that people with mild versions of these symptoms stay at home.
Helpful tips
The Epilepsy Foundation offers tips on signs to look for when trying to figure out when a seizure requires an ER visit. These are:
- Seizures in which awareness is lost for more than 5 minutes and no reversal medications are available.
- Seizures with an unusual pattern or duration.
- Seizures that cannot be treated safely at home or are not responding to rescue medication even after the medication has had enough time to work.
- Seizures that occur after a severe blow to the head.
Additionally, while COVID-19 can cause death and sudden death in patients, the virus does not cause sudden unexpected death in epilepsy (SUDEP). Because SUDEP is extremely rare, Dr. Benbadis said that there is no information to suggest that contracting the coronavirus will increase the risk,
Finally, no shortages of seizures medications have been reported as a result of COVID-19. However, there were shortages of generic levetiracetam immediate-release and levetiracetam extended-release medications prior to and during COVID-19. Experts expect the shortage to continue.
Overall, people who have epilepsy should be able to stay healthy – provided they exercise healthy and preventative habits.
“The majority of epilepsy patients should be reassured that if they continue their usual care, take their meds as directed, get adequate sleep, nutritious diet, they’re not at any increased risk compared to the general population,” said Dr. Wilner.
Dr. Benbadis reported the following disclosures: consultant for Bioserenity (DigiTrace), Brain Sentinel, Cavion, Ceribell, Eisai, Greenwich, LivaNova, Neuropace, SK biopharmaceuticals, Sunovion; speakers bureau for Eisai, Greenwich, LivaNova, Sunovion; Florida Medical Director of Stratus/Alliance; Member: Epilepsy Study Consortium; grant support from Cavion, LivaNova, Greenwich, SK biopharmaceuticals, Sunovion, Takeda, UCB, Xenon; royalties as an author or editor for Emedicine-Medscape-WebMD, UpToDate; editorial board for the Epilepsy.com (Epilepsy Foundation) controversy section, Emedicine-Medscape-WebMD, Epileptic Disorders, Epilepsy and Behavior, and Expert Review of Neurotherapeutics. Dr. Wilner reports Medical Advisory Board of Accordant Health Services, Greensboro, S.C., and book royalties: “The Locum Life: A Physician’s Guide to Locum Tenens,” Lulu Press.
Artisanal CBD may provide less seizure control than pharmaceutical CBD
preliminary results of a small study indicate.
Given the widespread use of artisanal CBD products, Nathan T. Cohen, MD, pediatric epilepsy fellow, Children’s National Hospital, Washington, DC, and his colleagues wanted to know how these products differ from pharmaceutical grade CBD with respect to seizure control.
“One of the challenges or questions we have is whether there is any information that would guide us and suggest patients transition from artisanal to pharmaceutical grade CBD,” Dr. Cohen, who is lead author of the study, told Medscape Medical News.
The findings were released February 27 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology. The AAN canceled the meeting and released abstracts and access to presenters for press coverage. The study received no outside funding.
In addition to helping relieve anxiety and stress, CBD, one of many constituents of Cannabis sativa, has antiseizure properties. The US Food and Drug Administration (FDA) has approved a pharmaceutical CBD (Epidiolex, GW Pharmaceuticals) for the management of seizures associated with Lennox-Gastaut syndrome (LGS) and Dravet syndrome.
This purified oral CBD prescription product does not contain tetrahydrocannabinol (THC), the component of marijuana that produces a “high.”
Popular products
Artisanal CBD, which has been around since the late 1970s, is manufactured using variable amounts of CBD and THC. Artisanal products, which typically come in the form of oils that are swallowed, are available in dispensaries and elsewhere, depending on the legal status in individual states.
These artisanal formulations are popular among patients with epilepsy and their families. On the basis of the advertising he sees, Dr. Cohen estimates there are at least 100 artisanal CBD products, but he was quick to stress he’s not an expert on artisanal CBD.
He noted that some families are “searching for an alternative treatment” to help control their child’s seizures, and if the seizure syndrome isn’t LGS or Dravet, “then technically, they don’t qualify for prescription-strength CBD,” said Dr. Cohen.
The current study was a retrospective chart review and included patients with epilepsy who underwent treatment with artisanal or pharmaceutical CBD for whom serum CBD levels were available.
In addition to CBD levels, the researchers had information on patients’ date of birth, gender, epilepsy diagnosis, artisanal or pharmaceutical CBD dose, seizure history, and side effects, among other things.
The analysis included 31 patients (48% female; mean age, about 10 years). Of these, 32% had LGS, 6% had Dravet, and the rest had other epilepsy syndromes.
Of the total, 22 patients participated in a pharmaceutical CBD expanded-access program. The remaining nine patients received artisanal CBD.
The mean serum CBD level was 30.1 ng/mL in the artisanal group and 124 ng/mL in the pharmaceutical group.
Dr. Cohen noted that artisanal products contain lower amounts of CBD because they’re not purified, and they may contain other compounds derived from marijuana.
At the last follow-up, which was a median of 11.8 months, patients who took artisanal CBD had a 70% increase in overall seizures. Dr. Cohen pointed out that some of the hundreds of compounds in marijuana could be “pro-convulsant.”
Some seizure free
The prescription CBD group experienced a 39% reduction in seizures. “Some of these kids had up to hundreds of seizures a day and went down to tens, and some kids became seizure free,” said Dr. Cohen.
Because the study was “looking back in time,” the investigators couldn’t determine whether age, type of epilepsy, or other factors affected seizure control in the two groups, said Dr. Cohen. “One of the limitations of a retrospective study is that we’re not able to control for those factors,” he said.
Eleven patients—all in the prescription CBD group—reported adverse effects, including somnolence, emesis, diarrhea, and diminished appetite; six discontinued CBD because of side effects.
Dr. Cohen said he’s not aware of any study that has compared artisanal products “head to head” with pharmaceutical grade CBD. “The whole point of this study was to ask the question, Is there a difference between the groups?, and these new data would suggest that there may be.”
The results appear to support giving encouragement to patients to transition from artisanal to pharmaceutical CBD if appropriate. “Anytime you’re giving your child a medication that has not been produced under the stringent guidelines that all pharmaceutical FDA-approved medications undergo, you don’t know exactly what’s in the product, and not knowing is a potential issue,” said Dr. Cohen.
The findings need to be studied in a more controlled setting “to make sure they’re valid,” said Dr. Cohen. Because this is “a very hot topic,” he’s keen to see what further research his colleagues would be interested in pursuing.
Commenting on the research, Joseph Sirven, MD, a neurologist in Scottsdale, Arizona, said this is an important study.
“It highlights one of the most common questions that I receive almost on a daily basis in my neurology practice,” he said.
Most people think that dispensary-based CBD is the same as prescription-based CBD, said Dr. Sirven. “Technically and theoretically, they certainly could be; however, what this study highlights is that in practice, they are not the same.”
He stressed that prescription CBD has to meet certain quality standards. “That means that whatever the ingredient list states about the concentration of CBD in the product has to be within the product, which is why the FDA approved it. It is, in essence, a quality control issue.”
A dispensary-based product does not need to meet such stringent standards and so “is subject to whatever the manufacturer chooses to put in the product,” said Dr. Sirven.
The study received no outside funding. Drs. Cohen and Sirven reported no relevant financial relationships.
This article first appeared on Medscape.com.
preliminary results of a small study indicate.
Given the widespread use of artisanal CBD products, Nathan T. Cohen, MD, pediatric epilepsy fellow, Children’s National Hospital, Washington, DC, and his colleagues wanted to know how these products differ from pharmaceutical grade CBD with respect to seizure control.
“One of the challenges or questions we have is whether there is any information that would guide us and suggest patients transition from artisanal to pharmaceutical grade CBD,” Dr. Cohen, who is lead author of the study, told Medscape Medical News.
The findings were released February 27 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology. The AAN canceled the meeting and released abstracts and access to presenters for press coverage. The study received no outside funding.
In addition to helping relieve anxiety and stress, CBD, one of many constituents of Cannabis sativa, has antiseizure properties. The US Food and Drug Administration (FDA) has approved a pharmaceutical CBD (Epidiolex, GW Pharmaceuticals) for the management of seizures associated with Lennox-Gastaut syndrome (LGS) and Dravet syndrome.
This purified oral CBD prescription product does not contain tetrahydrocannabinol (THC), the component of marijuana that produces a “high.”
Popular products
Artisanal CBD, which has been around since the late 1970s, is manufactured using variable amounts of CBD and THC. Artisanal products, which typically come in the form of oils that are swallowed, are available in dispensaries and elsewhere, depending on the legal status in individual states.
These artisanal formulations are popular among patients with epilepsy and their families. On the basis of the advertising he sees, Dr. Cohen estimates there are at least 100 artisanal CBD products, but he was quick to stress he’s not an expert on artisanal CBD.
He noted that some families are “searching for an alternative treatment” to help control their child’s seizures, and if the seizure syndrome isn’t LGS or Dravet, “then technically, they don’t qualify for prescription-strength CBD,” said Dr. Cohen.
The current study was a retrospective chart review and included patients with epilepsy who underwent treatment with artisanal or pharmaceutical CBD for whom serum CBD levels were available.
In addition to CBD levels, the researchers had information on patients’ date of birth, gender, epilepsy diagnosis, artisanal or pharmaceutical CBD dose, seizure history, and side effects, among other things.
The analysis included 31 patients (48% female; mean age, about 10 years). Of these, 32% had LGS, 6% had Dravet, and the rest had other epilepsy syndromes.
Of the total, 22 patients participated in a pharmaceutical CBD expanded-access program. The remaining nine patients received artisanal CBD.
The mean serum CBD level was 30.1 ng/mL in the artisanal group and 124 ng/mL in the pharmaceutical group.
Dr. Cohen noted that artisanal products contain lower amounts of CBD because they’re not purified, and they may contain other compounds derived from marijuana.
At the last follow-up, which was a median of 11.8 months, patients who took artisanal CBD had a 70% increase in overall seizures. Dr. Cohen pointed out that some of the hundreds of compounds in marijuana could be “pro-convulsant.”
Some seizure free
The prescription CBD group experienced a 39% reduction in seizures. “Some of these kids had up to hundreds of seizures a day and went down to tens, and some kids became seizure free,” said Dr. Cohen.
Because the study was “looking back in time,” the investigators couldn’t determine whether age, type of epilepsy, or other factors affected seizure control in the two groups, said Dr. Cohen. “One of the limitations of a retrospective study is that we’re not able to control for those factors,” he said.
Eleven patients—all in the prescription CBD group—reported adverse effects, including somnolence, emesis, diarrhea, and diminished appetite; six discontinued CBD because of side effects.
Dr. Cohen said he’s not aware of any study that has compared artisanal products “head to head” with pharmaceutical grade CBD. “The whole point of this study was to ask the question, Is there a difference between the groups?, and these new data would suggest that there may be.”
The results appear to support giving encouragement to patients to transition from artisanal to pharmaceutical CBD if appropriate. “Anytime you’re giving your child a medication that has not been produced under the stringent guidelines that all pharmaceutical FDA-approved medications undergo, you don’t know exactly what’s in the product, and not knowing is a potential issue,” said Dr. Cohen.
The findings need to be studied in a more controlled setting “to make sure they’re valid,” said Dr. Cohen. Because this is “a very hot topic,” he’s keen to see what further research his colleagues would be interested in pursuing.
Commenting on the research, Joseph Sirven, MD, a neurologist in Scottsdale, Arizona, said this is an important study.
“It highlights one of the most common questions that I receive almost on a daily basis in my neurology practice,” he said.
Most people think that dispensary-based CBD is the same as prescription-based CBD, said Dr. Sirven. “Technically and theoretically, they certainly could be; however, what this study highlights is that in practice, they are not the same.”
He stressed that prescription CBD has to meet certain quality standards. “That means that whatever the ingredient list states about the concentration of CBD in the product has to be within the product, which is why the FDA approved it. It is, in essence, a quality control issue.”
A dispensary-based product does not need to meet such stringent standards and so “is subject to whatever the manufacturer chooses to put in the product,” said Dr. Sirven.
The study received no outside funding. Drs. Cohen and Sirven reported no relevant financial relationships.
This article first appeared on Medscape.com.
preliminary results of a small study indicate.
Given the widespread use of artisanal CBD products, Nathan T. Cohen, MD, pediatric epilepsy fellow, Children’s National Hospital, Washington, DC, and his colleagues wanted to know how these products differ from pharmaceutical grade CBD with respect to seizure control.
“One of the challenges or questions we have is whether there is any information that would guide us and suggest patients transition from artisanal to pharmaceutical grade CBD,” Dr. Cohen, who is lead author of the study, told Medscape Medical News.
The findings were released February 27 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology. The AAN canceled the meeting and released abstracts and access to presenters for press coverage. The study received no outside funding.
In addition to helping relieve anxiety and stress, CBD, one of many constituents of Cannabis sativa, has antiseizure properties. The US Food and Drug Administration (FDA) has approved a pharmaceutical CBD (Epidiolex, GW Pharmaceuticals) for the management of seizures associated with Lennox-Gastaut syndrome (LGS) and Dravet syndrome.
This purified oral CBD prescription product does not contain tetrahydrocannabinol (THC), the component of marijuana that produces a “high.”
Popular products
Artisanal CBD, which has been around since the late 1970s, is manufactured using variable amounts of CBD and THC. Artisanal products, which typically come in the form of oils that are swallowed, are available in dispensaries and elsewhere, depending on the legal status in individual states.
These artisanal formulations are popular among patients with epilepsy and their families. On the basis of the advertising he sees, Dr. Cohen estimates there are at least 100 artisanal CBD products, but he was quick to stress he’s not an expert on artisanal CBD.
He noted that some families are “searching for an alternative treatment” to help control their child’s seizures, and if the seizure syndrome isn’t LGS or Dravet, “then technically, they don’t qualify for prescription-strength CBD,” said Dr. Cohen.
The current study was a retrospective chart review and included patients with epilepsy who underwent treatment with artisanal or pharmaceutical CBD for whom serum CBD levels were available.
In addition to CBD levels, the researchers had information on patients’ date of birth, gender, epilepsy diagnosis, artisanal or pharmaceutical CBD dose, seizure history, and side effects, among other things.
The analysis included 31 patients (48% female; mean age, about 10 years). Of these, 32% had LGS, 6% had Dravet, and the rest had other epilepsy syndromes.
Of the total, 22 patients participated in a pharmaceutical CBD expanded-access program. The remaining nine patients received artisanal CBD.
The mean serum CBD level was 30.1 ng/mL in the artisanal group and 124 ng/mL in the pharmaceutical group.
Dr. Cohen noted that artisanal products contain lower amounts of CBD because they’re not purified, and they may contain other compounds derived from marijuana.
At the last follow-up, which was a median of 11.8 months, patients who took artisanal CBD had a 70% increase in overall seizures. Dr. Cohen pointed out that some of the hundreds of compounds in marijuana could be “pro-convulsant.”
Some seizure free
The prescription CBD group experienced a 39% reduction in seizures. “Some of these kids had up to hundreds of seizures a day and went down to tens, and some kids became seizure free,” said Dr. Cohen.
Because the study was “looking back in time,” the investigators couldn’t determine whether age, type of epilepsy, or other factors affected seizure control in the two groups, said Dr. Cohen. “One of the limitations of a retrospective study is that we’re not able to control for those factors,” he said.
Eleven patients—all in the prescription CBD group—reported adverse effects, including somnolence, emesis, diarrhea, and diminished appetite; six discontinued CBD because of side effects.
Dr. Cohen said he’s not aware of any study that has compared artisanal products “head to head” with pharmaceutical grade CBD. “The whole point of this study was to ask the question, Is there a difference between the groups?, and these new data would suggest that there may be.”
The results appear to support giving encouragement to patients to transition from artisanal to pharmaceutical CBD if appropriate. “Anytime you’re giving your child a medication that has not been produced under the stringent guidelines that all pharmaceutical FDA-approved medications undergo, you don’t know exactly what’s in the product, and not knowing is a potential issue,” said Dr. Cohen.
The findings need to be studied in a more controlled setting “to make sure they’re valid,” said Dr. Cohen. Because this is “a very hot topic,” he’s keen to see what further research his colleagues would be interested in pursuing.
Commenting on the research, Joseph Sirven, MD, a neurologist in Scottsdale, Arizona, said this is an important study.
“It highlights one of the most common questions that I receive almost on a daily basis in my neurology practice,” he said.
Most people think that dispensary-based CBD is the same as prescription-based CBD, said Dr. Sirven. “Technically and theoretically, they certainly could be; however, what this study highlights is that in practice, they are not the same.”
He stressed that prescription CBD has to meet certain quality standards. “That means that whatever the ingredient list states about the concentration of CBD in the product has to be within the product, which is why the FDA approved it. It is, in essence, a quality control issue.”
A dispensary-based product does not need to meet such stringent standards and so “is subject to whatever the manufacturer chooses to put in the product,” said Dr. Sirven.
The study received no outside funding. Drs. Cohen and Sirven reported no relevant financial relationships.
This article first appeared on Medscape.com.
Antiepileptic drugs may not independently impair cognition
according to research published online ahead of print Feb. 3 in Neurology. Optimizing AED therapy to reduce or prevent seizures is thus unlikely to affect cognition, according to the investigators.
Patients who take AEDs commonly report cognitive problems, but investigations into the cognitive effects of AEDs have yielded inconsistent results. “We were also interested in this association, as we often treat complex patients taking multiple or high-dose AEDs, and our patients often report cognitive dysfunction,” said Emma Foster, MBBS, an epilepsy fellow at Alfred Health and the Royal Melbourne Hospital in Victoria, Australia. “We were particularly interested to examine how much AEDs affect cognition relative to other factors. We commonly see patients in our tertiary epilepsy care unit who have had severe epilepsy for a long time or who have psychiatric disorders, and these factors may also contribute to cognitive dysfunction.”
Researchers analyzed patients admitted for video EEG monitoring
For their study, Dr. Foster and colleagues prospectively enrolled patients admitted to the Royal Melbourne Hospital’s video EEG monitoring unit between January 2009 and December 2016. Patients were included in the study if they were age 18 years or older, had been admitted for diagnostic or surgical evaluation, and had complete data for the relevant variables. Patients were prescribed AED monotherapy or polytherapy.
The researchers based epilepsy diagnoses on the 2014 International League Against Epilepsy criteria. Diagnoses of psychogenic nonepileptic seizures (PNES) were based on a consensus of epileptologists at weekly multidisciplinary clinical meetings, which was supported by evaluation of all available data. Some patients received a diagnosis of comorbid epilepsy and PNES. If data were insufficient to support a diagnosis of epilepsy or PNES, the admission was considered nondiagnostic.
All participants underwent neuropsychologic and neuropsychiatric screening. Researchers assessed patients’ objective, global cognitive function using the Neuropsychiatry Unit Cognitive Assessment Tool (NUCOG), a validated instrument. Patients responded to the Quality of Life in Epilepsy inventory (QOLIE-89) to provide a measure of subjective cognitive function. They also responded to the Hospital Anxiety and Depression Scale (HADS) to screen for mood disorders.
Dr. Foster and colleagues measured seizure frequency through patient self-report. Patients averaged their seizure frequency during the 12-month period before admission to the video EEG unit. They categorized it according to a 12-point system in which 0 denotes patients who are seizure-free and not taking AEDs and 12 denotes patients in status epilepticus. Patients with PNES used the same scale to report event frequency, although the system was not designed for this purpose.
Almost half of patients were prescribed polypharmacy
The researchers included 331 patients in their analysis. The population’s mean age was 39.3 years, and about 62% of patients were female. Approximately 47% of patients had epilepsy, 25.7% had PNES, 6.6% had comorbid epilepsy and PNES, and 20.5% had a nondiagnostic outcome. Among patients with epilepsy, most (54.5%) had temporal lobe epilepsy, followed by extratemporal focal epilepsy (32.1%) and generalized epilepsy (13.5%). The mean number of AEDs prescribed on admission was 1.6, and mean seizure or event frequency score was 7.2, which indicated 1-3 seizures per month. Mean HADS depression score was within the normal range (5.7), and mean HADS anxiety score was in the borderline range (8.2).
Approximately 45% of patients were prescribed AED polypharmacy on admission, 25.1% were prescribed AED monotherapy, and 29.9% were prescribed no AED. Levetiracetam, valproate, and carbamazepine were the most frequently prescribed AEDs. Most patients with epilepsy (73.1%) were on polypharmacy, compared with 17.6% of patients with PNES, 63.6% of patients with epilepsy and PNES, and 8.8% of nondiagnostic patients.
Older age and greater seizure frequency predicted impaired objective cognitive function. Comorbid epilepsy and PNES appeared to predict impaired objective cognitive function as well, but the data were inconclusive. No AED was a significant predictor of objective cognitive function. Higher depression and anxiety scores and greater seizure frequency predicted impaired subjective cognitive function. No AED predicted subjective cognitive function.
Future studies could address particular cognitive domains
Previous studies have suggested that treatment with topiramate predicts objective or subjective cognitive function, but Dr. Foster and colleagues did not observe this result. The current findings suggest that topiramate may have a less significant effect on cognition than the literature suggests, they wrote. In addition, more evidence is needed to fully understand the effects of clobazam, valproate, phenytoin, and gabapentin because the analysis was underpowered for these drugs.
Although NUCOG assesses global cognitive function reliably, its ability to measure particular cognitive subdomains is limited. “We aim to conduct future research investigating the complex associations between different cognitive functions, including processing speed, and specific AEDs in this heterogeneous population,” said Dr. Foster.
Despite the study’s large sample size, the researchers could not explore potential interactions between various predictor variables. “Epilepsy may interact with the aging process or with other medical conditions associated with aging, such as hypertension and diabetes, and this may increase the risk of cognitive decline,” said Dr. Foster. “Older age may also be associated with reduced capacity to metabolize drugs, increased sensitivity to the cognitive and neurological effects of drugs, less cognitive reserve, and increased likelihood of taking multiple medications, which, along with AEDs, may exert a cognitive effect.”
The current findings may reduce concerns about the effects of AEDs on cognitive function and encourage neurologists to pursue the proper dosing for optimal seizure control, wrote the authors. “However, it is possible that some individuals may be more susceptible than others to AED-related cognitive dysfunction,” said Dr. Foster. “We do not have a robust way to predict who these patients will be, and it is still good practice to make patients aware that some people experience adverse cognitive effects from AEDs. However, it needs to be emphasized that it is unlikely to be the sole reason for their cognitive impairment. Other issues, such as poor seizure control or unrecognized or undertreated mood disorders, are even more important factors for impaired cognition.”
Patients who report cognitive problems should be screened for mood disorders, Dr. Foster continued. “It would also be important to consider whether the patients’ cognitive complaints arise from subtle clinical or subclinical seizure activity and subsequent postictal periods. To investigate this [question] further, clinicians may arrange for prolonged EEG monitoring. This [monitoring] could be done in an ambulatory setting or during an inpatient admission.”
The study was conducted without external funding. Dr. Foster and other investigators reported research funding from professional associations and pharmaceutical companies that was unrelated to the study.
SOURCE: Foster E et al. Neurology. 2020 Feb 3. doi: 10.1212/WNL.0000000000009061.
according to research published online ahead of print Feb. 3 in Neurology. Optimizing AED therapy to reduce or prevent seizures is thus unlikely to affect cognition, according to the investigators.
Patients who take AEDs commonly report cognitive problems, but investigations into the cognitive effects of AEDs have yielded inconsistent results. “We were also interested in this association, as we often treat complex patients taking multiple or high-dose AEDs, and our patients often report cognitive dysfunction,” said Emma Foster, MBBS, an epilepsy fellow at Alfred Health and the Royal Melbourne Hospital in Victoria, Australia. “We were particularly interested to examine how much AEDs affect cognition relative to other factors. We commonly see patients in our tertiary epilepsy care unit who have had severe epilepsy for a long time or who have psychiatric disorders, and these factors may also contribute to cognitive dysfunction.”
Researchers analyzed patients admitted for video EEG monitoring
For their study, Dr. Foster and colleagues prospectively enrolled patients admitted to the Royal Melbourne Hospital’s video EEG monitoring unit between January 2009 and December 2016. Patients were included in the study if they were age 18 years or older, had been admitted for diagnostic or surgical evaluation, and had complete data for the relevant variables. Patients were prescribed AED monotherapy or polytherapy.
The researchers based epilepsy diagnoses on the 2014 International League Against Epilepsy criteria. Diagnoses of psychogenic nonepileptic seizures (PNES) were based on a consensus of epileptologists at weekly multidisciplinary clinical meetings, which was supported by evaluation of all available data. Some patients received a diagnosis of comorbid epilepsy and PNES. If data were insufficient to support a diagnosis of epilepsy or PNES, the admission was considered nondiagnostic.
All participants underwent neuropsychologic and neuropsychiatric screening. Researchers assessed patients’ objective, global cognitive function using the Neuropsychiatry Unit Cognitive Assessment Tool (NUCOG), a validated instrument. Patients responded to the Quality of Life in Epilepsy inventory (QOLIE-89) to provide a measure of subjective cognitive function. They also responded to the Hospital Anxiety and Depression Scale (HADS) to screen for mood disorders.
Dr. Foster and colleagues measured seizure frequency through patient self-report. Patients averaged their seizure frequency during the 12-month period before admission to the video EEG unit. They categorized it according to a 12-point system in which 0 denotes patients who are seizure-free and not taking AEDs and 12 denotes patients in status epilepticus. Patients with PNES used the same scale to report event frequency, although the system was not designed for this purpose.
Almost half of patients were prescribed polypharmacy
The researchers included 331 patients in their analysis. The population’s mean age was 39.3 years, and about 62% of patients were female. Approximately 47% of patients had epilepsy, 25.7% had PNES, 6.6% had comorbid epilepsy and PNES, and 20.5% had a nondiagnostic outcome. Among patients with epilepsy, most (54.5%) had temporal lobe epilepsy, followed by extratemporal focal epilepsy (32.1%) and generalized epilepsy (13.5%). The mean number of AEDs prescribed on admission was 1.6, and mean seizure or event frequency score was 7.2, which indicated 1-3 seizures per month. Mean HADS depression score was within the normal range (5.7), and mean HADS anxiety score was in the borderline range (8.2).
Approximately 45% of patients were prescribed AED polypharmacy on admission, 25.1% were prescribed AED monotherapy, and 29.9% were prescribed no AED. Levetiracetam, valproate, and carbamazepine were the most frequently prescribed AEDs. Most patients with epilepsy (73.1%) were on polypharmacy, compared with 17.6% of patients with PNES, 63.6% of patients with epilepsy and PNES, and 8.8% of nondiagnostic patients.
Older age and greater seizure frequency predicted impaired objective cognitive function. Comorbid epilepsy and PNES appeared to predict impaired objective cognitive function as well, but the data were inconclusive. No AED was a significant predictor of objective cognitive function. Higher depression and anxiety scores and greater seizure frequency predicted impaired subjective cognitive function. No AED predicted subjective cognitive function.
Future studies could address particular cognitive domains
Previous studies have suggested that treatment with topiramate predicts objective or subjective cognitive function, but Dr. Foster and colleagues did not observe this result. The current findings suggest that topiramate may have a less significant effect on cognition than the literature suggests, they wrote. In addition, more evidence is needed to fully understand the effects of clobazam, valproate, phenytoin, and gabapentin because the analysis was underpowered for these drugs.
Although NUCOG assesses global cognitive function reliably, its ability to measure particular cognitive subdomains is limited. “We aim to conduct future research investigating the complex associations between different cognitive functions, including processing speed, and specific AEDs in this heterogeneous population,” said Dr. Foster.
Despite the study’s large sample size, the researchers could not explore potential interactions between various predictor variables. “Epilepsy may interact with the aging process or with other medical conditions associated with aging, such as hypertension and diabetes, and this may increase the risk of cognitive decline,” said Dr. Foster. “Older age may also be associated with reduced capacity to metabolize drugs, increased sensitivity to the cognitive and neurological effects of drugs, less cognitive reserve, and increased likelihood of taking multiple medications, which, along with AEDs, may exert a cognitive effect.”
The current findings may reduce concerns about the effects of AEDs on cognitive function and encourage neurologists to pursue the proper dosing for optimal seizure control, wrote the authors. “However, it is possible that some individuals may be more susceptible than others to AED-related cognitive dysfunction,” said Dr. Foster. “We do not have a robust way to predict who these patients will be, and it is still good practice to make patients aware that some people experience adverse cognitive effects from AEDs. However, it needs to be emphasized that it is unlikely to be the sole reason for their cognitive impairment. Other issues, such as poor seizure control or unrecognized or undertreated mood disorders, are even more important factors for impaired cognition.”
Patients who report cognitive problems should be screened for mood disorders, Dr. Foster continued. “It would also be important to consider whether the patients’ cognitive complaints arise from subtle clinical or subclinical seizure activity and subsequent postictal periods. To investigate this [question] further, clinicians may arrange for prolonged EEG monitoring. This [monitoring] could be done in an ambulatory setting or during an inpatient admission.”
The study was conducted without external funding. Dr. Foster and other investigators reported research funding from professional associations and pharmaceutical companies that was unrelated to the study.
SOURCE: Foster E et al. Neurology. 2020 Feb 3. doi: 10.1212/WNL.0000000000009061.
according to research published online ahead of print Feb. 3 in Neurology. Optimizing AED therapy to reduce or prevent seizures is thus unlikely to affect cognition, according to the investigators.
Patients who take AEDs commonly report cognitive problems, but investigations into the cognitive effects of AEDs have yielded inconsistent results. “We were also interested in this association, as we often treat complex patients taking multiple or high-dose AEDs, and our patients often report cognitive dysfunction,” said Emma Foster, MBBS, an epilepsy fellow at Alfred Health and the Royal Melbourne Hospital in Victoria, Australia. “We were particularly interested to examine how much AEDs affect cognition relative to other factors. We commonly see patients in our tertiary epilepsy care unit who have had severe epilepsy for a long time or who have psychiatric disorders, and these factors may also contribute to cognitive dysfunction.”
Researchers analyzed patients admitted for video EEG monitoring
For their study, Dr. Foster and colleagues prospectively enrolled patients admitted to the Royal Melbourne Hospital’s video EEG monitoring unit between January 2009 and December 2016. Patients were included in the study if they were age 18 years or older, had been admitted for diagnostic or surgical evaluation, and had complete data for the relevant variables. Patients were prescribed AED monotherapy or polytherapy.
The researchers based epilepsy diagnoses on the 2014 International League Against Epilepsy criteria. Diagnoses of psychogenic nonepileptic seizures (PNES) were based on a consensus of epileptologists at weekly multidisciplinary clinical meetings, which was supported by evaluation of all available data. Some patients received a diagnosis of comorbid epilepsy and PNES. If data were insufficient to support a diagnosis of epilepsy or PNES, the admission was considered nondiagnostic.
All participants underwent neuropsychologic and neuropsychiatric screening. Researchers assessed patients’ objective, global cognitive function using the Neuropsychiatry Unit Cognitive Assessment Tool (NUCOG), a validated instrument. Patients responded to the Quality of Life in Epilepsy inventory (QOLIE-89) to provide a measure of subjective cognitive function. They also responded to the Hospital Anxiety and Depression Scale (HADS) to screen for mood disorders.
Dr. Foster and colleagues measured seizure frequency through patient self-report. Patients averaged their seizure frequency during the 12-month period before admission to the video EEG unit. They categorized it according to a 12-point system in which 0 denotes patients who are seizure-free and not taking AEDs and 12 denotes patients in status epilepticus. Patients with PNES used the same scale to report event frequency, although the system was not designed for this purpose.
Almost half of patients were prescribed polypharmacy
The researchers included 331 patients in their analysis. The population’s mean age was 39.3 years, and about 62% of patients were female. Approximately 47% of patients had epilepsy, 25.7% had PNES, 6.6% had comorbid epilepsy and PNES, and 20.5% had a nondiagnostic outcome. Among patients with epilepsy, most (54.5%) had temporal lobe epilepsy, followed by extratemporal focal epilepsy (32.1%) and generalized epilepsy (13.5%). The mean number of AEDs prescribed on admission was 1.6, and mean seizure or event frequency score was 7.2, which indicated 1-3 seizures per month. Mean HADS depression score was within the normal range (5.7), and mean HADS anxiety score was in the borderline range (8.2).
Approximately 45% of patients were prescribed AED polypharmacy on admission, 25.1% were prescribed AED monotherapy, and 29.9% were prescribed no AED. Levetiracetam, valproate, and carbamazepine were the most frequently prescribed AEDs. Most patients with epilepsy (73.1%) were on polypharmacy, compared with 17.6% of patients with PNES, 63.6% of patients with epilepsy and PNES, and 8.8% of nondiagnostic patients.
Older age and greater seizure frequency predicted impaired objective cognitive function. Comorbid epilepsy and PNES appeared to predict impaired objective cognitive function as well, but the data were inconclusive. No AED was a significant predictor of objective cognitive function. Higher depression and anxiety scores and greater seizure frequency predicted impaired subjective cognitive function. No AED predicted subjective cognitive function.
Future studies could address particular cognitive domains
Previous studies have suggested that treatment with topiramate predicts objective or subjective cognitive function, but Dr. Foster and colleagues did not observe this result. The current findings suggest that topiramate may have a less significant effect on cognition than the literature suggests, they wrote. In addition, more evidence is needed to fully understand the effects of clobazam, valproate, phenytoin, and gabapentin because the analysis was underpowered for these drugs.
Although NUCOG assesses global cognitive function reliably, its ability to measure particular cognitive subdomains is limited. “We aim to conduct future research investigating the complex associations between different cognitive functions, including processing speed, and specific AEDs in this heterogeneous population,” said Dr. Foster.
Despite the study’s large sample size, the researchers could not explore potential interactions between various predictor variables. “Epilepsy may interact with the aging process or with other medical conditions associated with aging, such as hypertension and diabetes, and this may increase the risk of cognitive decline,” said Dr. Foster. “Older age may also be associated with reduced capacity to metabolize drugs, increased sensitivity to the cognitive and neurological effects of drugs, less cognitive reserve, and increased likelihood of taking multiple medications, which, along with AEDs, may exert a cognitive effect.”
The current findings may reduce concerns about the effects of AEDs on cognitive function and encourage neurologists to pursue the proper dosing for optimal seizure control, wrote the authors. “However, it is possible that some individuals may be more susceptible than others to AED-related cognitive dysfunction,” said Dr. Foster. “We do not have a robust way to predict who these patients will be, and it is still good practice to make patients aware that some people experience adverse cognitive effects from AEDs. However, it needs to be emphasized that it is unlikely to be the sole reason for their cognitive impairment. Other issues, such as poor seizure control or unrecognized or undertreated mood disorders, are even more important factors for impaired cognition.”
Patients who report cognitive problems should be screened for mood disorders, Dr. Foster continued. “It would also be important to consider whether the patients’ cognitive complaints arise from subtle clinical or subclinical seizure activity and subsequent postictal periods. To investigate this [question] further, clinicians may arrange for prolonged EEG monitoring. This [monitoring] could be done in an ambulatory setting or during an inpatient admission.”
The study was conducted without external funding. Dr. Foster and other investigators reported research funding from professional associations and pharmaceutical companies that was unrelated to the study.
SOURCE: Foster E et al. Neurology. 2020 Feb 3. doi: 10.1212/WNL.0000000000009061.
FROM NEUROLOGY



