User login
Epilepsy
‘No justification’ for suicide warning on all antiseizure meds
, new research shows. “There appears to be no justification for the FDA to label every new antiseizure medication with a warning that it may increase risk of suicidality,” said study investigator Michael R. Sperling, MD, professor of neurology, Thomas Jefferson University, Philadelphia.
“How many patients are afraid of their medication and do not take it because of the warning – and are consequently at risk because of that? We do not know, but have anecdotal experience that this is certainly an issue,” Dr. Sperling, who is director of the Jefferson Comprehensive Epilepsy Center, added.
The study was published online August 2 in JAMA Neurology.
Blanket warning
In 2008, the FDA issued an alert stating that antiseizure medications increase suicidality. The alert was based on pooled data from placebo-controlled clinical trials that included 11 antiseizure medications – carbamazepine, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, valproate, and zonisamide.
The meta-analytic review showed that, compared with placebo, antiseizure medications nearly doubled suicide risk among patients treated for epilepsy, psychiatric disorders, and other diseases. As a result of the FDA study, all antiseizure medications that have been approved since 2008 carry a warning for suicidality.
However, subsequent analyses did not show the same results, Dr. Sperling and colleagues noted.
“Pivotal” antiseizure medication epilepsy trials since 2008 have evaluated suicidality prospectively. Since 2011, trials have included the validated Columbia Suicidality Severity Rating Scale, they noted.
Meta analysis showed no increased risk
Dr. Sperling and colleagues conducted a meta-analysis of 17 randomized placebo-controlled epilepsy trials of five antiseizure medications approved since 2008. These antiseizure medications were eslicarbazepine, perampanel, brivaracetam, cannabidiol, and cenobamate. The trials involved 5,996 patients, including 4,000 who were treated with antiseizure medications and 1,996 who were treated with placebo.
Confining the analysis to epilepsy trials avoids potential confounders, such as possible differences in suicidality risks between different diseases, the researchers noted.
They found no evidence of increased risk for suicidal ideation (overall risk ratio, antiseizure medications vs. placebo: 0.75; 95% confidence interval: 0.35-1.60) or suicide attempt (risk ratio, 0.75; 95% CI: 0.30-1.87) overall or for any individual antiseizure medication.
Suicidal ideation occurred in 12 of 4,000 patients treated with antiseizure medications (0.30%), versus 7 of 1,996 patients treated with placebo (0.35%) (P = .74). Three patients who were treated with antiseizure medications attempted suicide; no patients who were treated with placebo attempted suicide (P = .22). There were no completed suicides.
“There is no current evidence that the five antiseizure medications evaluated in this study increase suicidality in epilepsy and merit a suicidality class warning,” the investigators wrote. When prescribed for epilepsy, “evidence does not support the FDA’s labeling practice of a blanket assumption of increased suicidality,” said Dr. Sperling.
“Our findings indicate the nonspecific suicide warning for all epilepsy drugs is simply not justifiable,” he said. “The results are not surprising. Different drugs affect cells in different ways. So there’s no reason to expect that every drug would increase suicide risk for every patient,” Dr. Sperling said in a statement.
“It’s important to recognize that epilepsy has many causes – perinatal injury, stroke, tumor, head trauma, developmental malformations, genetic causes, and others – and these underlying etiologies may well contribute to the presence of depression and suicidality in this population,” he said in an interview. “Psychodynamic influences also may occur as a consequence of having seizures. This is a complicated area, and drugs are simply one piece of the puzzle,” he added.
Dr. Sperling said the FDA has accomplished “one useful thing with its warning – it highlighted that physicians and other health care providers must pay attention to their patients’ psychological state, ask questions, and treat accordingly.”
The study had no specific funding. Dr. Sperling has received grants from Eisai, Medtronic, Neurelis, SK Life Science, Sunovion, Takeda, Xenon, Cerevel Therapeutics, UCB Pharma, and Engage Pharma; personal fees from Neurelis, Medscape, Neurology Live, International Medical Press, UCB Pharma, Eisai, Oxford University Press, and Projects in Knowledge. He has also consulted for Medtronic outside the submitted work; payments went to Thomas Jefferson University. A complete list of authors’ disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
, new research shows. “There appears to be no justification for the FDA to label every new antiseizure medication with a warning that it may increase risk of suicidality,” said study investigator Michael R. Sperling, MD, professor of neurology, Thomas Jefferson University, Philadelphia.
“How many patients are afraid of their medication and do not take it because of the warning – and are consequently at risk because of that? We do not know, but have anecdotal experience that this is certainly an issue,” Dr. Sperling, who is director of the Jefferson Comprehensive Epilepsy Center, added.
The study was published online August 2 in JAMA Neurology.
Blanket warning
In 2008, the FDA issued an alert stating that antiseizure medications increase suicidality. The alert was based on pooled data from placebo-controlled clinical trials that included 11 antiseizure medications – carbamazepine, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, valproate, and zonisamide.
The meta-analytic review showed that, compared with placebo, antiseizure medications nearly doubled suicide risk among patients treated for epilepsy, psychiatric disorders, and other diseases. As a result of the FDA study, all antiseizure medications that have been approved since 2008 carry a warning for suicidality.
However, subsequent analyses did not show the same results, Dr. Sperling and colleagues noted.
“Pivotal” antiseizure medication epilepsy trials since 2008 have evaluated suicidality prospectively. Since 2011, trials have included the validated Columbia Suicidality Severity Rating Scale, they noted.
Meta analysis showed no increased risk
Dr. Sperling and colleagues conducted a meta-analysis of 17 randomized placebo-controlled epilepsy trials of five antiseizure medications approved since 2008. These antiseizure medications were eslicarbazepine, perampanel, brivaracetam, cannabidiol, and cenobamate. The trials involved 5,996 patients, including 4,000 who were treated with antiseizure medications and 1,996 who were treated with placebo.
Confining the analysis to epilepsy trials avoids potential confounders, such as possible differences in suicidality risks between different diseases, the researchers noted.
They found no evidence of increased risk for suicidal ideation (overall risk ratio, antiseizure medications vs. placebo: 0.75; 95% confidence interval: 0.35-1.60) or suicide attempt (risk ratio, 0.75; 95% CI: 0.30-1.87) overall or for any individual antiseizure medication.
Suicidal ideation occurred in 12 of 4,000 patients treated with antiseizure medications (0.30%), versus 7 of 1,996 patients treated with placebo (0.35%) (P = .74). Three patients who were treated with antiseizure medications attempted suicide; no patients who were treated with placebo attempted suicide (P = .22). There were no completed suicides.
“There is no current evidence that the five antiseizure medications evaluated in this study increase suicidality in epilepsy and merit a suicidality class warning,” the investigators wrote. When prescribed for epilepsy, “evidence does not support the FDA’s labeling practice of a blanket assumption of increased suicidality,” said Dr. Sperling.
“Our findings indicate the nonspecific suicide warning for all epilepsy drugs is simply not justifiable,” he said. “The results are not surprising. Different drugs affect cells in different ways. So there’s no reason to expect that every drug would increase suicide risk for every patient,” Dr. Sperling said in a statement.
“It’s important to recognize that epilepsy has many causes – perinatal injury, stroke, tumor, head trauma, developmental malformations, genetic causes, and others – and these underlying etiologies may well contribute to the presence of depression and suicidality in this population,” he said in an interview. “Psychodynamic influences also may occur as a consequence of having seizures. This is a complicated area, and drugs are simply one piece of the puzzle,” he added.
Dr. Sperling said the FDA has accomplished “one useful thing with its warning – it highlighted that physicians and other health care providers must pay attention to their patients’ psychological state, ask questions, and treat accordingly.”
The study had no specific funding. Dr. Sperling has received grants from Eisai, Medtronic, Neurelis, SK Life Science, Sunovion, Takeda, Xenon, Cerevel Therapeutics, UCB Pharma, and Engage Pharma; personal fees from Neurelis, Medscape, Neurology Live, International Medical Press, UCB Pharma, Eisai, Oxford University Press, and Projects in Knowledge. He has also consulted for Medtronic outside the submitted work; payments went to Thomas Jefferson University. A complete list of authors’ disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
, new research shows. “There appears to be no justification for the FDA to label every new antiseizure medication with a warning that it may increase risk of suicidality,” said study investigator Michael R. Sperling, MD, professor of neurology, Thomas Jefferson University, Philadelphia.
“How many patients are afraid of their medication and do not take it because of the warning – and are consequently at risk because of that? We do not know, but have anecdotal experience that this is certainly an issue,” Dr. Sperling, who is director of the Jefferson Comprehensive Epilepsy Center, added.
The study was published online August 2 in JAMA Neurology.
Blanket warning
In 2008, the FDA issued an alert stating that antiseizure medications increase suicidality. The alert was based on pooled data from placebo-controlled clinical trials that included 11 antiseizure medications – carbamazepine, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, valproate, and zonisamide.
The meta-analytic review showed that, compared with placebo, antiseizure medications nearly doubled suicide risk among patients treated for epilepsy, psychiatric disorders, and other diseases. As a result of the FDA study, all antiseizure medications that have been approved since 2008 carry a warning for suicidality.
However, subsequent analyses did not show the same results, Dr. Sperling and colleagues noted.
“Pivotal” antiseizure medication epilepsy trials since 2008 have evaluated suicidality prospectively. Since 2011, trials have included the validated Columbia Suicidality Severity Rating Scale, they noted.
Meta analysis showed no increased risk
Dr. Sperling and colleagues conducted a meta-analysis of 17 randomized placebo-controlled epilepsy trials of five antiseizure medications approved since 2008. These antiseizure medications were eslicarbazepine, perampanel, brivaracetam, cannabidiol, and cenobamate. The trials involved 5,996 patients, including 4,000 who were treated with antiseizure medications and 1,996 who were treated with placebo.
Confining the analysis to epilepsy trials avoids potential confounders, such as possible differences in suicidality risks between different diseases, the researchers noted.
They found no evidence of increased risk for suicidal ideation (overall risk ratio, antiseizure medications vs. placebo: 0.75; 95% confidence interval: 0.35-1.60) or suicide attempt (risk ratio, 0.75; 95% CI: 0.30-1.87) overall or for any individual antiseizure medication.
Suicidal ideation occurred in 12 of 4,000 patients treated with antiseizure medications (0.30%), versus 7 of 1,996 patients treated with placebo (0.35%) (P = .74). Three patients who were treated with antiseizure medications attempted suicide; no patients who were treated with placebo attempted suicide (P = .22). There were no completed suicides.
“There is no current evidence that the five antiseizure medications evaluated in this study increase suicidality in epilepsy and merit a suicidality class warning,” the investigators wrote. When prescribed for epilepsy, “evidence does not support the FDA’s labeling practice of a blanket assumption of increased suicidality,” said Dr. Sperling.
“Our findings indicate the nonspecific suicide warning for all epilepsy drugs is simply not justifiable,” he said. “The results are not surprising. Different drugs affect cells in different ways. So there’s no reason to expect that every drug would increase suicide risk for every patient,” Dr. Sperling said in a statement.
“It’s important to recognize that epilepsy has many causes – perinatal injury, stroke, tumor, head trauma, developmental malformations, genetic causes, and others – and these underlying etiologies may well contribute to the presence of depression and suicidality in this population,” he said in an interview. “Psychodynamic influences also may occur as a consequence of having seizures. This is a complicated area, and drugs are simply one piece of the puzzle,” he added.
Dr. Sperling said the FDA has accomplished “one useful thing with its warning – it highlighted that physicians and other health care providers must pay attention to their patients’ psychological state, ask questions, and treat accordingly.”
The study had no specific funding. Dr. Sperling has received grants from Eisai, Medtronic, Neurelis, SK Life Science, Sunovion, Takeda, Xenon, Cerevel Therapeutics, UCB Pharma, and Engage Pharma; personal fees from Neurelis, Medscape, Neurology Live, International Medical Press, UCB Pharma, Eisai, Oxford University Press, and Projects in Knowledge. He has also consulted for Medtronic outside the submitted work; payments went to Thomas Jefferson University. A complete list of authors’ disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Short-term approach is best for seizure prevention after intracerebral hemorrhage
(sICH), new research shows.
Investigators created a model that simulated common clinical scenarios to compare four antiseizure drug strategies – conservative, moderate, aggressive, and risk-guided. They used the 2HELPS2B score as a risk stratification tool to guide clinical decisions.
The investigators found that the short-term, early-seizure prophylaxis strategies “dominated” long-term therapy under most clinical scenarios, underscoring the importance of early discontinuation of antiseizure drug therapy.
“The main message here was that strategies that involved long-term antiseizure drug prescription (moderate and aggressive) fail to provide better outcomes in most clinical scenarios, when compared with strategies using short-term prophylaxis (conservative and risk-guided),” senior investigator Lidia M.V.R. Moura, MD, MPH, assistant professor of neurology, Harvard Medical School, Boston, said in an interview.
The study was published online July 26 in JAMA Neurology.
Common complication
“Acute asymptomatic seizures [early seizures ≤7 days after stroke] are a common complication of sICH,” the authors noted.
Potential safety concerns have prompted recommendations against the use of antiseizure medications for primary prophylaxis. However, approximately 40% of U.S. patients with sICH do receive prophylactic levetiracetam before seizure development. For these patients, the duration of prophylaxis varies widely.
“Because seizure risk is a key determinant of which patient groups might benefit most from different prophylaxis strategies, validated tools for predicting early ... and late ... seizure risks could aid physicians in treatment decisions. However, no clinical trials or prospective studies have evaluated the net benefit of various strategies after sICH,” the investigators noted.
“Our patients who were survivors of an intracerebral hemorrhage motivated us to conduct the study,” said Dr. Moura, who is also director of the MGH NeuroValue Laboratory. “Some would come to the clinic with a long list of medications; some of them were taking antiseizure drugs for many years, but they never had a documented seizure.” These patients did not know why they had been taking an antiseizure drug for so long.
“In these conversations, we noted so much variability in indications and variability in patient access to specialty care to make treatment decisions. We noted that the evidence behind our current guidelines on seizure management was limited,” she added.
Dr. Moura and colleagues were “committed to improve outcome for people with neurological conditions by leveraging research methods that can help guide providers and systems, especially when data from clinical trials is lacking,” so they “decided to compare different strategies head to head using available data and generate evidence that could be used in situations with many trade-offs in risks and benefits.”
To investigate, the researchers used a simulation model and decision analysis to compare four treatment strategies on the basis of type of therapy (primary vs. secondary prophylaxis), timing of event (early vs. late seizures), and duration of therapy (1-week [short-term] versus indefinite [long-term] therapy).
These four strategies were as follows:
- Conservative: short-term (7-day) secondary early-seizure prophylaxis with long-term therapy after late seizure
- Moderate: long-term secondary early-seizure prophylaxis or late-seizure therapy
- Aggressive: long-term primary prophylaxis
- Risk-guided: short-term secondary early-seizure prophylaxis among low-risk patients (2HELPS2b score, 0), short-term primary prophylaxis among patients at higher risk (2HELPS2B score ≥1), and long-term secondary therapy for late seizure
The decision tree’s outcome measure was the number of expected quality-adjusted life-years.
Primary prophylaxis was defined as “treatment initiated immediately on hospital admission.” Secondary prophylaxis was defined as “treatment started after a seizure” and was subdivided into secondary early-seizure prophylaxis, defined as treatment started after a seizure occurring in the first 7 days after the stroke, or secondary late-seizure therapy, defined as treatment started or restarted after a seizure occurring after the first poststroke week.
Incorporate early-risk stratification tool
The researchers created four common clinical scenarios and then applied the decision-making model to each. They found that the preferred strategies differed, depending on the particular scenario.
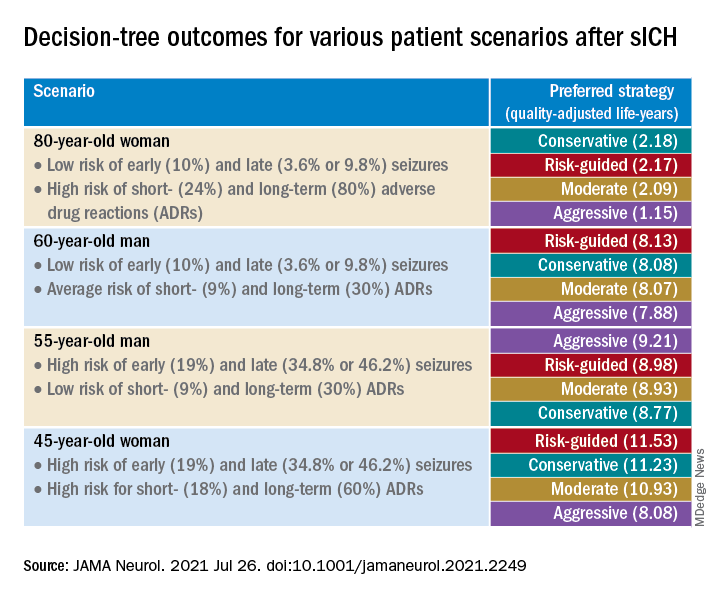
Sensitivity analyses revealed that short-term strategies, including the conservative and risk-guided approaches, were preferable in most cases, with the risk-guided strategy performing comparably or even better than alternative strategies in most cases.
“Our findings suggest that a strategy that incorporates an early-seizure risk stratification tool [2HELPS2B] is favored over alternative strategies in most settings,” Dr. Moura commented.
“Current services with rapidly available EEG may consider using a 1-hour screening with EEG upon admission for all patients presenting with sICH to risk-stratify those patients, using the 2HELPS2B tool,” she continued. “If EEG is unavailable for early-seizure risk stratification, the conservative strategy seems most reasonable.”
‘Potential fallacies’
Commenting on the study, José Biller, MD, professor and chairman, department of neurology, Loyola University Chicago, Maywood, Ill., called it a “well-written and intriguing contribution [to the field], with potential fallacies.”
The bottom line, he said, is that only a randomized, long-term, prospective, multicenter, high-quality study with larger cohorts can prove or disprove the investigators’ assumption.
The authors acknowledged that a limitation of the study was the use of published literature to obtain data to estimate model parameters and that they did not account for other possible factors that might modify some parameter estimates.
Nevertheless, Dr. Moura said the findings have important practical implications because they “highlight the importance of discontinuing antiseizure medications that were started during a hospitalization for sICH in patients that only had an early seizure.”
It is “of great importance for all providers to reassess the indication of antiseizure medications. Those drugs are not free of risks and can impact the patient’s health and quality of life,” she added.
The study was supported by grants from the National Institutes of Health. Dr. Moura reported receiving funding from the Centers for Disease Control and Prevention, the NIH, and the Epilepsy Foundation of America (Epilepsy Learning Healthcare System) as the director of the data coordinating center. Dr. Biller is the editor-in-chief of the Journal of Stroke and Cerebrovascular Diseases and a section editor of UpToDate.
A version of this article first appeared on Medscape.com.
(sICH), new research shows.
Investigators created a model that simulated common clinical scenarios to compare four antiseizure drug strategies – conservative, moderate, aggressive, and risk-guided. They used the 2HELPS2B score as a risk stratification tool to guide clinical decisions.
The investigators found that the short-term, early-seizure prophylaxis strategies “dominated” long-term therapy under most clinical scenarios, underscoring the importance of early discontinuation of antiseizure drug therapy.
“The main message here was that strategies that involved long-term antiseizure drug prescription (moderate and aggressive) fail to provide better outcomes in most clinical scenarios, when compared with strategies using short-term prophylaxis (conservative and risk-guided),” senior investigator Lidia M.V.R. Moura, MD, MPH, assistant professor of neurology, Harvard Medical School, Boston, said in an interview.
The study was published online July 26 in JAMA Neurology.
Common complication
“Acute asymptomatic seizures [early seizures ≤7 days after stroke] are a common complication of sICH,” the authors noted.
Potential safety concerns have prompted recommendations against the use of antiseizure medications for primary prophylaxis. However, approximately 40% of U.S. patients with sICH do receive prophylactic levetiracetam before seizure development. For these patients, the duration of prophylaxis varies widely.
“Because seizure risk is a key determinant of which patient groups might benefit most from different prophylaxis strategies, validated tools for predicting early ... and late ... seizure risks could aid physicians in treatment decisions. However, no clinical trials or prospective studies have evaluated the net benefit of various strategies after sICH,” the investigators noted.
“Our patients who were survivors of an intracerebral hemorrhage motivated us to conduct the study,” said Dr. Moura, who is also director of the MGH NeuroValue Laboratory. “Some would come to the clinic with a long list of medications; some of them were taking antiseizure drugs for many years, but they never had a documented seizure.” These patients did not know why they had been taking an antiseizure drug for so long.
“In these conversations, we noted so much variability in indications and variability in patient access to specialty care to make treatment decisions. We noted that the evidence behind our current guidelines on seizure management was limited,” she added.
Dr. Moura and colleagues were “committed to improve outcome for people with neurological conditions by leveraging research methods that can help guide providers and systems, especially when data from clinical trials is lacking,” so they “decided to compare different strategies head to head using available data and generate evidence that could be used in situations with many trade-offs in risks and benefits.”
To investigate, the researchers used a simulation model and decision analysis to compare four treatment strategies on the basis of type of therapy (primary vs. secondary prophylaxis), timing of event (early vs. late seizures), and duration of therapy (1-week [short-term] versus indefinite [long-term] therapy).
These four strategies were as follows:
- Conservative: short-term (7-day) secondary early-seizure prophylaxis with long-term therapy after late seizure
- Moderate: long-term secondary early-seizure prophylaxis or late-seizure therapy
- Aggressive: long-term primary prophylaxis
- Risk-guided: short-term secondary early-seizure prophylaxis among low-risk patients (2HELPS2b score, 0), short-term primary prophylaxis among patients at higher risk (2HELPS2B score ≥1), and long-term secondary therapy for late seizure
The decision tree’s outcome measure was the number of expected quality-adjusted life-years.
Primary prophylaxis was defined as “treatment initiated immediately on hospital admission.” Secondary prophylaxis was defined as “treatment started after a seizure” and was subdivided into secondary early-seizure prophylaxis, defined as treatment started after a seizure occurring in the first 7 days after the stroke, or secondary late-seizure therapy, defined as treatment started or restarted after a seizure occurring after the first poststroke week.
Incorporate early-risk stratification tool
The researchers created four common clinical scenarios and then applied the decision-making model to each. They found that the preferred strategies differed, depending on the particular scenario.

Sensitivity analyses revealed that short-term strategies, including the conservative and risk-guided approaches, were preferable in most cases, with the risk-guided strategy performing comparably or even better than alternative strategies in most cases.
“Our findings suggest that a strategy that incorporates an early-seizure risk stratification tool [2HELPS2B] is favored over alternative strategies in most settings,” Dr. Moura commented.
“Current services with rapidly available EEG may consider using a 1-hour screening with EEG upon admission for all patients presenting with sICH to risk-stratify those patients, using the 2HELPS2B tool,” she continued. “If EEG is unavailable for early-seizure risk stratification, the conservative strategy seems most reasonable.”
‘Potential fallacies’
Commenting on the study, José Biller, MD, professor and chairman, department of neurology, Loyola University Chicago, Maywood, Ill., called it a “well-written and intriguing contribution [to the field], with potential fallacies.”
The bottom line, he said, is that only a randomized, long-term, prospective, multicenter, high-quality study with larger cohorts can prove or disprove the investigators’ assumption.
The authors acknowledged that a limitation of the study was the use of published literature to obtain data to estimate model parameters and that they did not account for other possible factors that might modify some parameter estimates.
Nevertheless, Dr. Moura said the findings have important practical implications because they “highlight the importance of discontinuing antiseizure medications that were started during a hospitalization for sICH in patients that only had an early seizure.”
It is “of great importance for all providers to reassess the indication of antiseizure medications. Those drugs are not free of risks and can impact the patient’s health and quality of life,” she added.
The study was supported by grants from the National Institutes of Health. Dr. Moura reported receiving funding from the Centers for Disease Control and Prevention, the NIH, and the Epilepsy Foundation of America (Epilepsy Learning Healthcare System) as the director of the data coordinating center. Dr. Biller is the editor-in-chief of the Journal of Stroke and Cerebrovascular Diseases and a section editor of UpToDate.
A version of this article first appeared on Medscape.com.
(sICH), new research shows.
Investigators created a model that simulated common clinical scenarios to compare four antiseizure drug strategies – conservative, moderate, aggressive, and risk-guided. They used the 2HELPS2B score as a risk stratification tool to guide clinical decisions.
The investigators found that the short-term, early-seizure prophylaxis strategies “dominated” long-term therapy under most clinical scenarios, underscoring the importance of early discontinuation of antiseizure drug therapy.
“The main message here was that strategies that involved long-term antiseizure drug prescription (moderate and aggressive) fail to provide better outcomes in most clinical scenarios, when compared with strategies using short-term prophylaxis (conservative and risk-guided),” senior investigator Lidia M.V.R. Moura, MD, MPH, assistant professor of neurology, Harvard Medical School, Boston, said in an interview.
The study was published online July 26 in JAMA Neurology.
Common complication
“Acute asymptomatic seizures [early seizures ≤7 days after stroke] are a common complication of sICH,” the authors noted.
Potential safety concerns have prompted recommendations against the use of antiseizure medications for primary prophylaxis. However, approximately 40% of U.S. patients with sICH do receive prophylactic levetiracetam before seizure development. For these patients, the duration of prophylaxis varies widely.
“Because seizure risk is a key determinant of which patient groups might benefit most from different prophylaxis strategies, validated tools for predicting early ... and late ... seizure risks could aid physicians in treatment decisions. However, no clinical trials or prospective studies have evaluated the net benefit of various strategies after sICH,” the investigators noted.
“Our patients who were survivors of an intracerebral hemorrhage motivated us to conduct the study,” said Dr. Moura, who is also director of the MGH NeuroValue Laboratory. “Some would come to the clinic with a long list of medications; some of them were taking antiseizure drugs for many years, but they never had a documented seizure.” These patients did not know why they had been taking an antiseizure drug for so long.
“In these conversations, we noted so much variability in indications and variability in patient access to specialty care to make treatment decisions. We noted that the evidence behind our current guidelines on seizure management was limited,” she added.
Dr. Moura and colleagues were “committed to improve outcome for people with neurological conditions by leveraging research methods that can help guide providers and systems, especially when data from clinical trials is lacking,” so they “decided to compare different strategies head to head using available data and generate evidence that could be used in situations with many trade-offs in risks and benefits.”
To investigate, the researchers used a simulation model and decision analysis to compare four treatment strategies on the basis of type of therapy (primary vs. secondary prophylaxis), timing of event (early vs. late seizures), and duration of therapy (1-week [short-term] versus indefinite [long-term] therapy).
These four strategies were as follows:
- Conservative: short-term (7-day) secondary early-seizure prophylaxis with long-term therapy after late seizure
- Moderate: long-term secondary early-seizure prophylaxis or late-seizure therapy
- Aggressive: long-term primary prophylaxis
- Risk-guided: short-term secondary early-seizure prophylaxis among low-risk patients (2HELPS2b score, 0), short-term primary prophylaxis among patients at higher risk (2HELPS2B score ≥1), and long-term secondary therapy for late seizure
The decision tree’s outcome measure was the number of expected quality-adjusted life-years.
Primary prophylaxis was defined as “treatment initiated immediately on hospital admission.” Secondary prophylaxis was defined as “treatment started after a seizure” and was subdivided into secondary early-seizure prophylaxis, defined as treatment started after a seizure occurring in the first 7 days after the stroke, or secondary late-seizure therapy, defined as treatment started or restarted after a seizure occurring after the first poststroke week.
Incorporate early-risk stratification tool
The researchers created four common clinical scenarios and then applied the decision-making model to each. They found that the preferred strategies differed, depending on the particular scenario.

Sensitivity analyses revealed that short-term strategies, including the conservative and risk-guided approaches, were preferable in most cases, with the risk-guided strategy performing comparably or even better than alternative strategies in most cases.
“Our findings suggest that a strategy that incorporates an early-seizure risk stratification tool [2HELPS2B] is favored over alternative strategies in most settings,” Dr. Moura commented.
“Current services with rapidly available EEG may consider using a 1-hour screening with EEG upon admission for all patients presenting with sICH to risk-stratify those patients, using the 2HELPS2B tool,” she continued. “If EEG is unavailable for early-seizure risk stratification, the conservative strategy seems most reasonable.”
‘Potential fallacies’
Commenting on the study, José Biller, MD, professor and chairman, department of neurology, Loyola University Chicago, Maywood, Ill., called it a “well-written and intriguing contribution [to the field], with potential fallacies.”
The bottom line, he said, is that only a randomized, long-term, prospective, multicenter, high-quality study with larger cohorts can prove or disprove the investigators’ assumption.
The authors acknowledged that a limitation of the study was the use of published literature to obtain data to estimate model parameters and that they did not account for other possible factors that might modify some parameter estimates.
Nevertheless, Dr. Moura said the findings have important practical implications because they “highlight the importance of discontinuing antiseizure medications that were started during a hospitalization for sICH in patients that only had an early seizure.”
It is “of great importance for all providers to reassess the indication of antiseizure medications. Those drugs are not free of risks and can impact the patient’s health and quality of life,” she added.
The study was supported by grants from the National Institutes of Health. Dr. Moura reported receiving funding from the Centers for Disease Control and Prevention, the NIH, and the Epilepsy Foundation of America (Epilepsy Learning Healthcare System) as the director of the data coordinating center. Dr. Biller is the editor-in-chief of the Journal of Stroke and Cerebrovascular Diseases and a section editor of UpToDate.
A version of this article first appeared on Medscape.com.
Can a supplement that mimics the keto diet reduce seizures?
early research suggests. However, at least one expert has concerns.
In an open-label feasibility study, researchers assessed a liquid supplement known as K.Vita (Vitaflo International), which contains both decanoic acid and octanoic acid.
Although the study was small, the findings are promising, said coinvestigator Matthew Walker, MD, PhD, University College London Institute of Neurology, department of clinical and experimental epilepsy.
“The dietary supplement was reasonably well tolerated and while we weren’t specifically looking for efficacy here, we did see some patients had quite dramatic results in terms of reduced seizures,” Dr. Walker said.
Unlike the ketogenic diet, this dietary supplement is “very easy” to follow, involves only minor dietary modifications, and doesn’t require the intervention of a dietitian, he added.
The findings were published online July 23, 2021, in Brain Communications.
Key ingredients
In the ketogenic diet, the body uses body fat as its primary fuel source. The switch from carbohydrates to fat for body fuel results in built-up ketones.
Previous research shows the ketogenic diet is effective in reducing seizures in some patients with epilepsy. However, many patients find it difficult to tolerate, especially for extended periods. Dr. Walker also noted that ketones may have other long-term side effects, including osteoporosis.
He added that his team was keen to learn what elements of the ketogenic diet affect seizures. “Interestingly, we found that one of the fats used in the ketogenic diet, decanoic acid, has quite marked antiseizure effects,” Dr. Walker said.
Previous research has shown that decanoic acid, a medium-chain triglyceride–derived fatty acid, can cross the blood-brain barrier and decrease excitatory neurotransmission and network excitability in vitro.
Dr. Walker noted that ketones are necessary in order to reduce seizures.
“Rather than have a very high-fat, low-carbohydrate diet that causes ketones, we thought ‘why don’t we use a diet in which we just use mainly this fat, this decanoic acid, and avoid ketosis,’ ” he said.
The researchers then went to work developing the K.Vita dietary supplement, which mainly contains decanoic acid but also another fat, octanoic acid.
Assessing feasibility
The feasibility study included 61 patients (59% female) who began taking the supplement. Of these, 35 were children (aged 3-18 years) and 26 were adults. The children had Dravet syndrome or another genetically driven form of epilepsy, while most of the adults had a focal epilepsy.
All participants had failed multiple antiseizure medications – a median of 3 for children and 10 for adults who completed the trial. Of the 61 original participants, 20 (19 children and 1 adult) had tried the ketogenic diet but had stopped it for various reasons, including noncompliance and lack of efficacy.
The liquid supplement was introduced gradually. The amount administered was based on weight in the children and was a standard amount in adults, with the target being 240 mL.
Participants consumed the supplement in equal servings taken at regular intervals as part of a meal or snack. They could take it alone or mix it with yogurt or another food.
Patients with feeding tubes took the supplement immediately before or after or mixed into an enteral feed, with a water flush afterward.
Researchers provided patients and caregivers with guidance on excluding highly refined sugary foods and beverages. Starchy foods such as bread, pasta, rice, and potatoes were not restricted.
The study consisted of three visits: baseline, 5 weeks, and 12 weeks, in addition to regular phone and email contact. Participants were also asked to keep a seizure diary.
Highly acceptable to patients
Overall, the study withdrawal rate was 33%. After a protocol change involving a slower introduction of the supplement, there were fewer withdrawals, Dr. Walker reported. He noted that the proportion of participants who completed the study (41 of 61) is “much better than with most studies of adults following the ketogenic diet.”
The most frequently reported gastrointestinal symptoms with the supplement were bloating and constipation, but these were predominantly mild and tended to decrease over time. This, said Dr. Walker, contrasts to the ketogenic diet where side effects tend to persist.
There was no significant change in body weight or body mass index. “We did not see weight gain as a problem at all,” Dr. Walker said.
Of 15 caregivers and 19 adults who returned an acceptability questionnaire, 84% agreed or strongly agreed the supplement had a good flavor (strawberry); 88% liked the appearance and color; 77% liked the texture and consistency; and 88% agreed or strongly agreed it was easy to take.
About one-third of adults and two-thirds of caregivers said they believed the supplement reduced seizures.
50% seizure reduction
Only three children and one adult became ketotic. This is typically classified as a beta-hydroxybutyrate (BHB) greater than 1 mmol/L (10.4 mg/dL). The BHB levels detected were markedly lower than those observed in individuals following a ketogenic diet, the investigators note.
Of the 41 participants, 19 completed the diaries. There were also data from physician recordings, so researchers were able to retrieve seizure frequencies for 32 of the 41 (78%). Of these 32 patients, 14 (44%) had a 50% or greater reduction in seizures. Overall, children and adults “responded similarly,” Dr. Walker said.
He acknowledged the study numbers are small and emphasized that larger studies are needed to determine efficacy. He also hopes for a future randomized controlled trial comparing K.Vita with another supplement that contains different types of fats.
Interestingly, the product has already “passed” the regulatory approval process in the United Kingdom, so it can be labeled as a medicinal food and should be available for use at the beginning of 2022, Dr. Walker said.
Study concerns
Asked to comment on the findings, Daniel Goldenholz, MD, PhD, instructor in the department of neurology, Beth Israel Deaconess Medical Center, Boston, said the supplement may be helpful, but he has concerns about the study.
Many patients with epilepsy are “desperate” for therapies that will help treat their seizures, said Dr. Goldenholz, who was not involved with the research. “If there’s a dietary therapy that has the potential for being helpful, I’m loving that. I need that. My patients are begging for something that works.” It is “really exciting” that researchers are working on that goal, Dr. Goldenholz added.
However, he noted that it is too soon to start talking to patients about this new product. He also pointed out that a significant fraction of the study participants dropped out, many because they couldn’t tolerate the supplement. In addition, others didn’t produce a seizure diary.
Dr. Goldenholz and colleagues have published several studies showing that patients with no intervention at all can sometimes show a reduction in seizures compared with their baseline results.
“We found sizable 50% reductions attributable entirely to the natural fluctuations in seizure rates, rather than any therapy at all, he said.
Dr. Goldenholz added that he hopes to see future studies on this topic, and on similar therapies “with sufficient data and more reliable metrics for efficacy.”
The study was funded by Vitaflo International. Dr. Walker reports having received grants from Vitaflo International and personal fees from UCB Pharma, Eisai, and Sage. In addition, along with colleagues, he has a patent (Nutritional product) pending.
A version of this article first appeared on Medscape.com.
early research suggests. However, at least one expert has concerns.
In an open-label feasibility study, researchers assessed a liquid supplement known as K.Vita (Vitaflo International), which contains both decanoic acid and octanoic acid.
Although the study was small, the findings are promising, said coinvestigator Matthew Walker, MD, PhD, University College London Institute of Neurology, department of clinical and experimental epilepsy.
“The dietary supplement was reasonably well tolerated and while we weren’t specifically looking for efficacy here, we did see some patients had quite dramatic results in terms of reduced seizures,” Dr. Walker said.
Unlike the ketogenic diet, this dietary supplement is “very easy” to follow, involves only minor dietary modifications, and doesn’t require the intervention of a dietitian, he added.
The findings were published online July 23, 2021, in Brain Communications.
Key ingredients
In the ketogenic diet, the body uses body fat as its primary fuel source. The switch from carbohydrates to fat for body fuel results in built-up ketones.
Previous research shows the ketogenic diet is effective in reducing seizures in some patients with epilepsy. However, many patients find it difficult to tolerate, especially for extended periods. Dr. Walker also noted that ketones may have other long-term side effects, including osteoporosis.
He added that his team was keen to learn what elements of the ketogenic diet affect seizures. “Interestingly, we found that one of the fats used in the ketogenic diet, decanoic acid, has quite marked antiseizure effects,” Dr. Walker said.
Previous research has shown that decanoic acid, a medium-chain triglyceride–derived fatty acid, can cross the blood-brain barrier and decrease excitatory neurotransmission and network excitability in vitro.
Dr. Walker noted that ketones are necessary in order to reduce seizures.
“Rather than have a very high-fat, low-carbohydrate diet that causes ketones, we thought ‘why don’t we use a diet in which we just use mainly this fat, this decanoic acid, and avoid ketosis,’ ” he said.
The researchers then went to work developing the K.Vita dietary supplement, which mainly contains decanoic acid but also another fat, octanoic acid.
Assessing feasibility
The feasibility study included 61 patients (59% female) who began taking the supplement. Of these, 35 were children (aged 3-18 years) and 26 were adults. The children had Dravet syndrome or another genetically driven form of epilepsy, while most of the adults had a focal epilepsy.
All participants had failed multiple antiseizure medications – a median of 3 for children and 10 for adults who completed the trial. Of the 61 original participants, 20 (19 children and 1 adult) had tried the ketogenic diet but had stopped it for various reasons, including noncompliance and lack of efficacy.
The liquid supplement was introduced gradually. The amount administered was based on weight in the children and was a standard amount in adults, with the target being 240 mL.
Participants consumed the supplement in equal servings taken at regular intervals as part of a meal or snack. They could take it alone or mix it with yogurt or another food.
Patients with feeding tubes took the supplement immediately before or after or mixed into an enteral feed, with a water flush afterward.
Researchers provided patients and caregivers with guidance on excluding highly refined sugary foods and beverages. Starchy foods such as bread, pasta, rice, and potatoes were not restricted.
The study consisted of three visits: baseline, 5 weeks, and 12 weeks, in addition to regular phone and email contact. Participants were also asked to keep a seizure diary.
Highly acceptable to patients
Overall, the study withdrawal rate was 33%. After a protocol change involving a slower introduction of the supplement, there were fewer withdrawals, Dr. Walker reported. He noted that the proportion of participants who completed the study (41 of 61) is “much better than with most studies of adults following the ketogenic diet.”
The most frequently reported gastrointestinal symptoms with the supplement were bloating and constipation, but these were predominantly mild and tended to decrease over time. This, said Dr. Walker, contrasts to the ketogenic diet where side effects tend to persist.
There was no significant change in body weight or body mass index. “We did not see weight gain as a problem at all,” Dr. Walker said.
Of 15 caregivers and 19 adults who returned an acceptability questionnaire, 84% agreed or strongly agreed the supplement had a good flavor (strawberry); 88% liked the appearance and color; 77% liked the texture and consistency; and 88% agreed or strongly agreed it was easy to take.
About one-third of adults and two-thirds of caregivers said they believed the supplement reduced seizures.
50% seizure reduction
Only three children and one adult became ketotic. This is typically classified as a beta-hydroxybutyrate (BHB) greater than 1 mmol/L (10.4 mg/dL). The BHB levels detected were markedly lower than those observed in individuals following a ketogenic diet, the investigators note.
Of the 41 participants, 19 completed the diaries. There were also data from physician recordings, so researchers were able to retrieve seizure frequencies for 32 of the 41 (78%). Of these 32 patients, 14 (44%) had a 50% or greater reduction in seizures. Overall, children and adults “responded similarly,” Dr. Walker said.
He acknowledged the study numbers are small and emphasized that larger studies are needed to determine efficacy. He also hopes for a future randomized controlled trial comparing K.Vita with another supplement that contains different types of fats.
Interestingly, the product has already “passed” the regulatory approval process in the United Kingdom, so it can be labeled as a medicinal food and should be available for use at the beginning of 2022, Dr. Walker said.
Study concerns
Asked to comment on the findings, Daniel Goldenholz, MD, PhD, instructor in the department of neurology, Beth Israel Deaconess Medical Center, Boston, said the supplement may be helpful, but he has concerns about the study.
Many patients with epilepsy are “desperate” for therapies that will help treat their seizures, said Dr. Goldenholz, who was not involved with the research. “If there’s a dietary therapy that has the potential for being helpful, I’m loving that. I need that. My patients are begging for something that works.” It is “really exciting” that researchers are working on that goal, Dr. Goldenholz added.
However, he noted that it is too soon to start talking to patients about this new product. He also pointed out that a significant fraction of the study participants dropped out, many because they couldn’t tolerate the supplement. In addition, others didn’t produce a seizure diary.
Dr. Goldenholz and colleagues have published several studies showing that patients with no intervention at all can sometimes show a reduction in seizures compared with their baseline results.
“We found sizable 50% reductions attributable entirely to the natural fluctuations in seizure rates, rather than any therapy at all, he said.
Dr. Goldenholz added that he hopes to see future studies on this topic, and on similar therapies “with sufficient data and more reliable metrics for efficacy.”
The study was funded by Vitaflo International. Dr. Walker reports having received grants from Vitaflo International and personal fees from UCB Pharma, Eisai, and Sage. In addition, along with colleagues, he has a patent (Nutritional product) pending.
A version of this article first appeared on Medscape.com.
early research suggests. However, at least one expert has concerns.
In an open-label feasibility study, researchers assessed a liquid supplement known as K.Vita (Vitaflo International), which contains both decanoic acid and octanoic acid.
Although the study was small, the findings are promising, said coinvestigator Matthew Walker, MD, PhD, University College London Institute of Neurology, department of clinical and experimental epilepsy.
“The dietary supplement was reasonably well tolerated and while we weren’t specifically looking for efficacy here, we did see some patients had quite dramatic results in terms of reduced seizures,” Dr. Walker said.
Unlike the ketogenic diet, this dietary supplement is “very easy” to follow, involves only minor dietary modifications, and doesn’t require the intervention of a dietitian, he added.
The findings were published online July 23, 2021, in Brain Communications.
Key ingredients
In the ketogenic diet, the body uses body fat as its primary fuel source. The switch from carbohydrates to fat for body fuel results in built-up ketones.
Previous research shows the ketogenic diet is effective in reducing seizures in some patients with epilepsy. However, many patients find it difficult to tolerate, especially for extended periods. Dr. Walker also noted that ketones may have other long-term side effects, including osteoporosis.
He added that his team was keen to learn what elements of the ketogenic diet affect seizures. “Interestingly, we found that one of the fats used in the ketogenic diet, decanoic acid, has quite marked antiseizure effects,” Dr. Walker said.
Previous research has shown that decanoic acid, a medium-chain triglyceride–derived fatty acid, can cross the blood-brain barrier and decrease excitatory neurotransmission and network excitability in vitro.
Dr. Walker noted that ketones are necessary in order to reduce seizures.
“Rather than have a very high-fat, low-carbohydrate diet that causes ketones, we thought ‘why don’t we use a diet in which we just use mainly this fat, this decanoic acid, and avoid ketosis,’ ” he said.
The researchers then went to work developing the K.Vita dietary supplement, which mainly contains decanoic acid but also another fat, octanoic acid.
Assessing feasibility
The feasibility study included 61 patients (59% female) who began taking the supplement. Of these, 35 were children (aged 3-18 years) and 26 were adults. The children had Dravet syndrome or another genetically driven form of epilepsy, while most of the adults had a focal epilepsy.
All participants had failed multiple antiseizure medications – a median of 3 for children and 10 for adults who completed the trial. Of the 61 original participants, 20 (19 children and 1 adult) had tried the ketogenic diet but had stopped it for various reasons, including noncompliance and lack of efficacy.
The liquid supplement was introduced gradually. The amount administered was based on weight in the children and was a standard amount in adults, with the target being 240 mL.
Participants consumed the supplement in equal servings taken at regular intervals as part of a meal or snack. They could take it alone or mix it with yogurt or another food.
Patients with feeding tubes took the supplement immediately before or after or mixed into an enteral feed, with a water flush afterward.
Researchers provided patients and caregivers with guidance on excluding highly refined sugary foods and beverages. Starchy foods such as bread, pasta, rice, and potatoes were not restricted.
The study consisted of three visits: baseline, 5 weeks, and 12 weeks, in addition to regular phone and email contact. Participants were also asked to keep a seizure diary.
Highly acceptable to patients
Overall, the study withdrawal rate was 33%. After a protocol change involving a slower introduction of the supplement, there were fewer withdrawals, Dr. Walker reported. He noted that the proportion of participants who completed the study (41 of 61) is “much better than with most studies of adults following the ketogenic diet.”
The most frequently reported gastrointestinal symptoms with the supplement were bloating and constipation, but these were predominantly mild and tended to decrease over time. This, said Dr. Walker, contrasts to the ketogenic diet where side effects tend to persist.
There was no significant change in body weight or body mass index. “We did not see weight gain as a problem at all,” Dr. Walker said.
Of 15 caregivers and 19 adults who returned an acceptability questionnaire, 84% agreed or strongly agreed the supplement had a good flavor (strawberry); 88% liked the appearance and color; 77% liked the texture and consistency; and 88% agreed or strongly agreed it was easy to take.
About one-third of adults and two-thirds of caregivers said they believed the supplement reduced seizures.
50% seizure reduction
Only three children and one adult became ketotic. This is typically classified as a beta-hydroxybutyrate (BHB) greater than 1 mmol/L (10.4 mg/dL). The BHB levels detected were markedly lower than those observed in individuals following a ketogenic diet, the investigators note.
Of the 41 participants, 19 completed the diaries. There were also data from physician recordings, so researchers were able to retrieve seizure frequencies for 32 of the 41 (78%). Of these 32 patients, 14 (44%) had a 50% or greater reduction in seizures. Overall, children and adults “responded similarly,” Dr. Walker said.
He acknowledged the study numbers are small and emphasized that larger studies are needed to determine efficacy. He also hopes for a future randomized controlled trial comparing K.Vita with another supplement that contains different types of fats.
Interestingly, the product has already “passed” the regulatory approval process in the United Kingdom, so it can be labeled as a medicinal food and should be available for use at the beginning of 2022, Dr. Walker said.
Study concerns
Asked to comment on the findings, Daniel Goldenholz, MD, PhD, instructor in the department of neurology, Beth Israel Deaconess Medical Center, Boston, said the supplement may be helpful, but he has concerns about the study.
Many patients with epilepsy are “desperate” for therapies that will help treat their seizures, said Dr. Goldenholz, who was not involved with the research. “If there’s a dietary therapy that has the potential for being helpful, I’m loving that. I need that. My patients are begging for something that works.” It is “really exciting” that researchers are working on that goal, Dr. Goldenholz added.
However, he noted that it is too soon to start talking to patients about this new product. He also pointed out that a significant fraction of the study participants dropped out, many because they couldn’t tolerate the supplement. In addition, others didn’t produce a seizure diary.
Dr. Goldenholz and colleagues have published several studies showing that patients with no intervention at all can sometimes show a reduction in seizures compared with their baseline results.
“We found sizable 50% reductions attributable entirely to the natural fluctuations in seizure rates, rather than any therapy at all, he said.
Dr. Goldenholz added that he hopes to see future studies on this topic, and on similar therapies “with sufficient data and more reliable metrics for efficacy.”
The study was funded by Vitaflo International. Dr. Walker reports having received grants from Vitaflo International and personal fees from UCB Pharma, Eisai, and Sage. In addition, along with colleagues, he has a patent (Nutritional product) pending.
A version of this article first appeared on Medscape.com.
FROM BRAIN COMMUNICATION
Noted ability of Mozart’s music to reduce seizures explained?
– and now researchers believe they know why.
Investigators conducting new research found that the acoustic characteristics of Mozart’s Sonata for Two Pianos in D Major (K448) suppresses brain activity in patients with epilepsy, while a piece by the 18th century classical composer Franz Joseph Haydn did not have this effect.
Listening to this Mozart sonata and perhaps other musical pieces may eventually become a treatment for preventing epileptic seizures, said study investigator Ivan Rektor, MD, CSc, Epilepsy Centre at the Hospital St. Anne and professor at the Central European Institute of Technology, Masaryk University, Brno, Czech Republic.
“This research into the impact of listening to music could lead to the development of a music-related type of palliative neurostimulation therapy,” said Dr. Rektor.
The findings were presented at the 2021 Congress of the European Academy of Neurology and published online in the European Journal of Neurology.
Clinically controversial?
Epilepsy affects 6 million people in Europe. Furthermore, estimates show that about 15 million Europeans have had at least one seizure at some time in their lives. In addition, about 30% of patients with epilepsy are not adequately treated with antiseizure medications.
Researchers have been studying the impact of Mozart’s music on brain-wave activity since the 1990s. Various studies report a reduction in epileptiform discharges in patients with epileptic seizures, coma, and refractory nonconvulsive status.
A 2012 meta-analysis of 12 publications involving patients with epilepsy showed an overall reduction in the number of interictal epileptic discharges (IEDs) or abnormal electrical brain waves in 84% of participants who listened to Mozart’s music. A more recent meta-analysis also showed a significant reduction in epileptic seizures and IEDs.
American researchers also found Mozart’s music regulated abnormal interictal epileptiform activity (IEA), especially in those with a high baseline rate of interictal spikes.
However, the methodological quality of some of this research “has been limited,” Dr. Rektor noted. He added that use of music therapy in clinical practice is still considered “controversial.”
The new study included 18 treatment-resistant patients with epilepsy (50% men) who ranged in age from 19 to 55 years. Participants had intracerebral electrodes implanted in the brain before undergoing surgery.
Of the total study population, 15 had temporal lobe epilepsy and three had extratemporal epilepsy. Eleven were affected on the left side, six on the right side, and one bi-temporally. Duration of epilepsy ranged from 8 to 40 years.
Patients listened to the Mozart piece intermittently on one day and to Haydn’s “Surprise” Symphony No. 94 the next day. Researchers counted the number of ED discharges before, during, and after the patients listened to the music.
Surprising finding
Results showed that exposure to the Mozart piece was associated with a 32% reduction in IEDs, from 28 EDs pre-exposure to 19 during exposure. However, IEDs rose to 21 post-exposure.
Overall, the Haydn piece was associated with an increase in IEDs, from 23 pre-exposure to 26 during and post-exposure.
“We saw a clear decrease in epileptic spikes while listening and after listening to Mozart, while there was an increase in spikes while listening to Haydn,” Dr. Rektor said.
He added that all 18 patients responded “more or less” to the music and that the results were statistically significant.
Dr. Rektor noted that the investigators were not surprised by the Mozart effect but were somewhat taken aback by the opposite effect from listening to Haydn.
The impact differed between men and women. The Mozart piece had a larger effect on women. In addition, the Haydn piece led to a decrease in spikes in women but led to “a clear” increase in men, Dr. Rektor reported.
In an effort to explore why the two classical pieces had such different effects, the researchers examined the acoustic properties. They worked with acoustic engineers to examine three musical properties that might influence the number of spikes: rhythm (tempo or beats per minute), dynamics (energy), and timbre (how harsh or unpleasant, how noisy, and how many “high-frequency” parts the music has).
“We observed that K448 [Mozart’s piece] has a more harmonic spectrum and its spectral content doesn’t change quickly, which probably has a positive effect on epilepsy patients,” said Dr. Rektor.
Specific features of the music had a slightly different effect on men and women. Men were more sensitive to dissonance and high-frequency parts while women were more sensitive to energy.
A new theory
Researchers previously hypothesized that the Mozart effect in epilepsy was connected to the emotional impact of music. The neurotransmitter dopamine, which plays a role in the brain’s reward system, is released when listening to music. However, the new research seems to challenge that theory. The majority of the participants did not express a strong preference for classical music.
“We believe emotions didn’t play an important role in these patients,” Dr. Rektor said, adding that the impact was instead mostly related to acoustic signals.
The team also found that the reduction in IEDs was larger in the lateral temporal lobe, the part of the brain involved in translating acoustic signals, rather than in the mesiotemporal limbic region, which plays an important role in the emotional response to music.
Comparing men with women, there’s an “overlap” of brain activation in most brain areas. However, some areas are more activated in men and others in women, said Dr. Rektor.
While the Mozart Sonata for two pianos in D Major has become the “gold standard” in this type of research, Dr. Rektor said “it’s very probable” that other classical compositions with similar acoustic properties have the same effect in epilepsy.
The investigators are testing other musical pieces, both classical and nonclassical. The ultimate aim is to develop individualized musical patterns based on these acoustic features.
“If it works, we would like to use it as a noninvasive neurostimulation method,” Dr. Rektor said.
‘Inspiring research’
Commenting on the study, session chair Marte Bjørk, MD, PhD, associate professor, department of clinical medicine, University of Bergen, Norway, called it “inspiring.” She noted that she recently had a patient whose temporal lobe seizures were consistently triggered by music played on a children’s TV program. “So I have no doubt that music can be important for some patients,” Dr. Bjørk said.
She questioned whether factors other than gender may predict response to music.
The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– and now researchers believe they know why.
Investigators conducting new research found that the acoustic characteristics of Mozart’s Sonata for Two Pianos in D Major (K448) suppresses brain activity in patients with epilepsy, while a piece by the 18th century classical composer Franz Joseph Haydn did not have this effect.
Listening to this Mozart sonata and perhaps other musical pieces may eventually become a treatment for preventing epileptic seizures, said study investigator Ivan Rektor, MD, CSc, Epilepsy Centre at the Hospital St. Anne and professor at the Central European Institute of Technology, Masaryk University, Brno, Czech Republic.
“This research into the impact of listening to music could lead to the development of a music-related type of palliative neurostimulation therapy,” said Dr. Rektor.
The findings were presented at the 2021 Congress of the European Academy of Neurology and published online in the European Journal of Neurology.
Clinically controversial?
Epilepsy affects 6 million people in Europe. Furthermore, estimates show that about 15 million Europeans have had at least one seizure at some time in their lives. In addition, about 30% of patients with epilepsy are not adequately treated with antiseizure medications.
Researchers have been studying the impact of Mozart’s music on brain-wave activity since the 1990s. Various studies report a reduction in epileptiform discharges in patients with epileptic seizures, coma, and refractory nonconvulsive status.
A 2012 meta-analysis of 12 publications involving patients with epilepsy showed an overall reduction in the number of interictal epileptic discharges (IEDs) or abnormal electrical brain waves in 84% of participants who listened to Mozart’s music. A more recent meta-analysis also showed a significant reduction in epileptic seizures and IEDs.
American researchers also found Mozart’s music regulated abnormal interictal epileptiform activity (IEA), especially in those with a high baseline rate of interictal spikes.
However, the methodological quality of some of this research “has been limited,” Dr. Rektor noted. He added that use of music therapy in clinical practice is still considered “controversial.”
The new study included 18 treatment-resistant patients with epilepsy (50% men) who ranged in age from 19 to 55 years. Participants had intracerebral electrodes implanted in the brain before undergoing surgery.
Of the total study population, 15 had temporal lobe epilepsy and three had extratemporal epilepsy. Eleven were affected on the left side, six on the right side, and one bi-temporally. Duration of epilepsy ranged from 8 to 40 years.
Patients listened to the Mozart piece intermittently on one day and to Haydn’s “Surprise” Symphony No. 94 the next day. Researchers counted the number of ED discharges before, during, and after the patients listened to the music.
Surprising finding
Results showed that exposure to the Mozart piece was associated with a 32% reduction in IEDs, from 28 EDs pre-exposure to 19 during exposure. However, IEDs rose to 21 post-exposure.
Overall, the Haydn piece was associated with an increase in IEDs, from 23 pre-exposure to 26 during and post-exposure.
“We saw a clear decrease in epileptic spikes while listening and after listening to Mozart, while there was an increase in spikes while listening to Haydn,” Dr. Rektor said.
He added that all 18 patients responded “more or less” to the music and that the results were statistically significant.
Dr. Rektor noted that the investigators were not surprised by the Mozart effect but were somewhat taken aback by the opposite effect from listening to Haydn.
The impact differed between men and women. The Mozart piece had a larger effect on women. In addition, the Haydn piece led to a decrease in spikes in women but led to “a clear” increase in men, Dr. Rektor reported.
In an effort to explore why the two classical pieces had such different effects, the researchers examined the acoustic properties. They worked with acoustic engineers to examine three musical properties that might influence the number of spikes: rhythm (tempo or beats per minute), dynamics (energy), and timbre (how harsh or unpleasant, how noisy, and how many “high-frequency” parts the music has).
“We observed that K448 [Mozart’s piece] has a more harmonic spectrum and its spectral content doesn’t change quickly, which probably has a positive effect on epilepsy patients,” said Dr. Rektor.
Specific features of the music had a slightly different effect on men and women. Men were more sensitive to dissonance and high-frequency parts while women were more sensitive to energy.
A new theory
Researchers previously hypothesized that the Mozart effect in epilepsy was connected to the emotional impact of music. The neurotransmitter dopamine, which plays a role in the brain’s reward system, is released when listening to music. However, the new research seems to challenge that theory. The majority of the participants did not express a strong preference for classical music.
“We believe emotions didn’t play an important role in these patients,” Dr. Rektor said, adding that the impact was instead mostly related to acoustic signals.
The team also found that the reduction in IEDs was larger in the lateral temporal lobe, the part of the brain involved in translating acoustic signals, rather than in the mesiotemporal limbic region, which plays an important role in the emotional response to music.
Comparing men with women, there’s an “overlap” of brain activation in most brain areas. However, some areas are more activated in men and others in women, said Dr. Rektor.
While the Mozart Sonata for two pianos in D Major has become the “gold standard” in this type of research, Dr. Rektor said “it’s very probable” that other classical compositions with similar acoustic properties have the same effect in epilepsy.
The investigators are testing other musical pieces, both classical and nonclassical. The ultimate aim is to develop individualized musical patterns based on these acoustic features.
“If it works, we would like to use it as a noninvasive neurostimulation method,” Dr. Rektor said.
‘Inspiring research’
Commenting on the study, session chair Marte Bjørk, MD, PhD, associate professor, department of clinical medicine, University of Bergen, Norway, called it “inspiring.” She noted that she recently had a patient whose temporal lobe seizures were consistently triggered by music played on a children’s TV program. “So I have no doubt that music can be important for some patients,” Dr. Bjørk said.
She questioned whether factors other than gender may predict response to music.
The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– and now researchers believe they know why.
Investigators conducting new research found that the acoustic characteristics of Mozart’s Sonata for Two Pianos in D Major (K448) suppresses brain activity in patients with epilepsy, while a piece by the 18th century classical composer Franz Joseph Haydn did not have this effect.
Listening to this Mozart sonata and perhaps other musical pieces may eventually become a treatment for preventing epileptic seizures, said study investigator Ivan Rektor, MD, CSc, Epilepsy Centre at the Hospital St. Anne and professor at the Central European Institute of Technology, Masaryk University, Brno, Czech Republic.
“This research into the impact of listening to music could lead to the development of a music-related type of palliative neurostimulation therapy,” said Dr. Rektor.
The findings were presented at the 2021 Congress of the European Academy of Neurology and published online in the European Journal of Neurology.
Clinically controversial?
Epilepsy affects 6 million people in Europe. Furthermore, estimates show that about 15 million Europeans have had at least one seizure at some time in their lives. In addition, about 30% of patients with epilepsy are not adequately treated with antiseizure medications.
Researchers have been studying the impact of Mozart’s music on brain-wave activity since the 1990s. Various studies report a reduction in epileptiform discharges in patients with epileptic seizures, coma, and refractory nonconvulsive status.
A 2012 meta-analysis of 12 publications involving patients with epilepsy showed an overall reduction in the number of interictal epileptic discharges (IEDs) or abnormal electrical brain waves in 84% of participants who listened to Mozart’s music. A more recent meta-analysis also showed a significant reduction in epileptic seizures and IEDs.
American researchers also found Mozart’s music regulated abnormal interictal epileptiform activity (IEA), especially in those with a high baseline rate of interictal spikes.
However, the methodological quality of some of this research “has been limited,” Dr. Rektor noted. He added that use of music therapy in clinical practice is still considered “controversial.”
The new study included 18 treatment-resistant patients with epilepsy (50% men) who ranged in age from 19 to 55 years. Participants had intracerebral electrodes implanted in the brain before undergoing surgery.
Of the total study population, 15 had temporal lobe epilepsy and three had extratemporal epilepsy. Eleven were affected on the left side, six on the right side, and one bi-temporally. Duration of epilepsy ranged from 8 to 40 years.
Patients listened to the Mozart piece intermittently on one day and to Haydn’s “Surprise” Symphony No. 94 the next day. Researchers counted the number of ED discharges before, during, and after the patients listened to the music.
Surprising finding
Results showed that exposure to the Mozart piece was associated with a 32% reduction in IEDs, from 28 EDs pre-exposure to 19 during exposure. However, IEDs rose to 21 post-exposure.
Overall, the Haydn piece was associated with an increase in IEDs, from 23 pre-exposure to 26 during and post-exposure.
“We saw a clear decrease in epileptic spikes while listening and after listening to Mozart, while there was an increase in spikes while listening to Haydn,” Dr. Rektor said.
He added that all 18 patients responded “more or less” to the music and that the results were statistically significant.
Dr. Rektor noted that the investigators were not surprised by the Mozart effect but were somewhat taken aback by the opposite effect from listening to Haydn.
The impact differed between men and women. The Mozart piece had a larger effect on women. In addition, the Haydn piece led to a decrease in spikes in women but led to “a clear” increase in men, Dr. Rektor reported.
In an effort to explore why the two classical pieces had such different effects, the researchers examined the acoustic properties. They worked with acoustic engineers to examine three musical properties that might influence the number of spikes: rhythm (tempo or beats per minute), dynamics (energy), and timbre (how harsh or unpleasant, how noisy, and how many “high-frequency” parts the music has).
“We observed that K448 [Mozart’s piece] has a more harmonic spectrum and its spectral content doesn’t change quickly, which probably has a positive effect on epilepsy patients,” said Dr. Rektor.
Specific features of the music had a slightly different effect on men and women. Men were more sensitive to dissonance and high-frequency parts while women were more sensitive to energy.
A new theory
Researchers previously hypothesized that the Mozart effect in epilepsy was connected to the emotional impact of music. The neurotransmitter dopamine, which plays a role in the brain’s reward system, is released when listening to music. However, the new research seems to challenge that theory. The majority of the participants did not express a strong preference for classical music.
“We believe emotions didn’t play an important role in these patients,” Dr. Rektor said, adding that the impact was instead mostly related to acoustic signals.
The team also found that the reduction in IEDs was larger in the lateral temporal lobe, the part of the brain involved in translating acoustic signals, rather than in the mesiotemporal limbic region, which plays an important role in the emotional response to music.
Comparing men with women, there’s an “overlap” of brain activation in most brain areas. However, some areas are more activated in men and others in women, said Dr. Rektor.
While the Mozart Sonata for two pianos in D Major has become the “gold standard” in this type of research, Dr. Rektor said “it’s very probable” that other classical compositions with similar acoustic properties have the same effect in epilepsy.
The investigators are testing other musical pieces, both classical and nonclassical. The ultimate aim is to develop individualized musical patterns based on these acoustic features.
“If it works, we would like to use it as a noninvasive neurostimulation method,” Dr. Rektor said.
‘Inspiring research’
Commenting on the study, session chair Marte Bjørk, MD, PhD, associate professor, department of clinical medicine, University of Bergen, Norway, called it “inspiring.” She noted that she recently had a patient whose temporal lobe seizures were consistently triggered by music played on a children’s TV program. “So I have no doubt that music can be important for some patients,” Dr. Bjørk said.
She questioned whether factors other than gender may predict response to music.
The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EAN 2021
Risk factors identified for late seizure relapse after epilepsy surgery
according to a new study on the factors most associated with seizure recurrence in drug-resistant epilepsy.
“As our study analyzed late seizure relapse, our results are not applicable for short‐term seizure control. Vice versa, results for short‐term outcomes should not be transferred to long‐term outcomes,” Stephan Petrik of the Epilepsy Center at the University of Freiburg (Germany) and colleagues wrote. The study was published in the May 2021 issue of Epilepsia.
To assess the variables that increase risk of late seizure recurrence following surgery, the researchers retrospectively studied the medical records of patients who underwent resective epilepsy surgery at the University Hospital Freiburg (Germany) between 1999 and 2015. Of the 1,278 initial patients, a group of 99 participants (7.7%) with seizure relapses after at least 2 years of complete seizure freedom were matched with controls experiencing long-term seizure freedom. The two groups had similar mean durations of epilepsy from onset to surgery: 13.9 years in the relapse group and 13.0 years in the control group.
The mean follow-up was 9.7 years (standard deviation, 4.0; range, 2.9-18.5) in the relapse group and 8.2 years (SD, 3.5; range, 2.2-18.3) in the control group. The mean time to late seizure recurrence was 56.6 months, and two-thirds of patients relapsed in the 5 years after surgery. Twenty of the relapse patients only experienced a single seizure, and 41% of the patients who reported more than one seizure had a frequency of less than one per month.
The type of resection had no discernible impact on outcomes, although anterior temporal lobe resection did trend toward being associated with recurrence (odds ratio, 2.7; 95% confidence interval, 0.93-8.89; P = .06). Incomplete resection was significantly associated with late relapse but did not seem to affect timing: the mean duration of seizure freedom was 56.5 months with complete resection and 58.5 months with incomplete resection (P = .62). Additional preoperative PET scans were performed on 45% of patients in the relapse group, compared with 29% in the control group.
After multivariate analysis, predictors for late relapse included incomplete resection (OR, 3.81; 95% CI, 1.79-8.53; P < .001); the existence of additional, potentially epileptogenic lesions in the contralateral hemisphere on presurgical MRI (OR, 3.36; 95% CI, 1.18-10.62; P = .03); epilepsy onset during the first year of life (OR, 4.24; 95% CI, 1.4-15.89; P = .02); and preoperative PET scans being performed (OR, 2.47; 95% CI, 1.25-4.97; P = .01). Though use of preoperative and postoperative antiepileptic drugs (AEDs) was higher in the relapse group, along with complete withdrawal being more common in the control group (68%, compared with 51%), neither was deemed significant in multivariate analysis.
What to do about seizure relapse risk factors
“This is one of the best analyses of the factors that contribute to late seizure relapse,” Gregory K. Bergey, MD, director of the Johns Hopkins Epilepsy Center in Baltimore, said in an interview. “Am I surprised by their results? Not necessarily.”
What did jump out, he said, was AED use not being a predictor of recurrence, as well as all the patients with late relapse having lesional epilepsy. “As they point out, you can have relapse with nonlesional epilepsy, but very often it happens in the first year or 2. If someone is 2 years out and doesn’t have a lesion, they’re probably more likely to remain seizure free.”
Despite the researchers’ comprehensive review of risk factors, the question remains: What to do with this information?
“They’ve done a very good job of identifying that 7.7% of 1,200 who are at risk of a late relapse,” he said. “Now, take those patients with high-risk factors and launch a trial where you keep medicines the same or do something that would alter that outcome.”
“The problem is,” he added, “that’s a 10-year study. It’s easy for me to sit here and call for one of those. But still, as valuable as this was, it’s a retrospective study. Now you have to say, what are the implications of this? What can we do in the prospective fashion?”
The authors acknowledged their study’s other limitations, including a lack of information on the reasons for an incomplete resection, a notable decrease in follow-up visits more than 5 years after surgery, and potential selection bias. They added, however, that “matching by age at surgery, gender, and time to relapse/last follow‐up” should have helped reduce any significant bias.
No potential conflicts of interest were disclosed.
according to a new study on the factors most associated with seizure recurrence in drug-resistant epilepsy.
“As our study analyzed late seizure relapse, our results are not applicable for short‐term seizure control. Vice versa, results for short‐term outcomes should not be transferred to long‐term outcomes,” Stephan Petrik of the Epilepsy Center at the University of Freiburg (Germany) and colleagues wrote. The study was published in the May 2021 issue of Epilepsia.
To assess the variables that increase risk of late seizure recurrence following surgery, the researchers retrospectively studied the medical records of patients who underwent resective epilepsy surgery at the University Hospital Freiburg (Germany) between 1999 and 2015. Of the 1,278 initial patients, a group of 99 participants (7.7%) with seizure relapses after at least 2 years of complete seizure freedom were matched with controls experiencing long-term seizure freedom. The two groups had similar mean durations of epilepsy from onset to surgery: 13.9 years in the relapse group and 13.0 years in the control group.
The mean follow-up was 9.7 years (standard deviation, 4.0; range, 2.9-18.5) in the relapse group and 8.2 years (SD, 3.5; range, 2.2-18.3) in the control group. The mean time to late seizure recurrence was 56.6 months, and two-thirds of patients relapsed in the 5 years after surgery. Twenty of the relapse patients only experienced a single seizure, and 41% of the patients who reported more than one seizure had a frequency of less than one per month.
The type of resection had no discernible impact on outcomes, although anterior temporal lobe resection did trend toward being associated with recurrence (odds ratio, 2.7; 95% confidence interval, 0.93-8.89; P = .06). Incomplete resection was significantly associated with late relapse but did not seem to affect timing: the mean duration of seizure freedom was 56.5 months with complete resection and 58.5 months with incomplete resection (P = .62). Additional preoperative PET scans were performed on 45% of patients in the relapse group, compared with 29% in the control group.
After multivariate analysis, predictors for late relapse included incomplete resection (OR, 3.81; 95% CI, 1.79-8.53; P < .001); the existence of additional, potentially epileptogenic lesions in the contralateral hemisphere on presurgical MRI (OR, 3.36; 95% CI, 1.18-10.62; P = .03); epilepsy onset during the first year of life (OR, 4.24; 95% CI, 1.4-15.89; P = .02); and preoperative PET scans being performed (OR, 2.47; 95% CI, 1.25-4.97; P = .01). Though use of preoperative and postoperative antiepileptic drugs (AEDs) was higher in the relapse group, along with complete withdrawal being more common in the control group (68%, compared with 51%), neither was deemed significant in multivariate analysis.
What to do about seizure relapse risk factors
“This is one of the best analyses of the factors that contribute to late seizure relapse,” Gregory K. Bergey, MD, director of the Johns Hopkins Epilepsy Center in Baltimore, said in an interview. “Am I surprised by their results? Not necessarily.”
What did jump out, he said, was AED use not being a predictor of recurrence, as well as all the patients with late relapse having lesional epilepsy. “As they point out, you can have relapse with nonlesional epilepsy, but very often it happens in the first year or 2. If someone is 2 years out and doesn’t have a lesion, they’re probably more likely to remain seizure free.”
Despite the researchers’ comprehensive review of risk factors, the question remains: What to do with this information?
“They’ve done a very good job of identifying that 7.7% of 1,200 who are at risk of a late relapse,” he said. “Now, take those patients with high-risk factors and launch a trial where you keep medicines the same or do something that would alter that outcome.”
“The problem is,” he added, “that’s a 10-year study. It’s easy for me to sit here and call for one of those. But still, as valuable as this was, it’s a retrospective study. Now you have to say, what are the implications of this? What can we do in the prospective fashion?”
The authors acknowledged their study’s other limitations, including a lack of information on the reasons for an incomplete resection, a notable decrease in follow-up visits more than 5 years after surgery, and potential selection bias. They added, however, that “matching by age at surgery, gender, and time to relapse/last follow‐up” should have helped reduce any significant bias.
No potential conflicts of interest were disclosed.
according to a new study on the factors most associated with seizure recurrence in drug-resistant epilepsy.
“As our study analyzed late seizure relapse, our results are not applicable for short‐term seizure control. Vice versa, results for short‐term outcomes should not be transferred to long‐term outcomes,” Stephan Petrik of the Epilepsy Center at the University of Freiburg (Germany) and colleagues wrote. The study was published in the May 2021 issue of Epilepsia.
To assess the variables that increase risk of late seizure recurrence following surgery, the researchers retrospectively studied the medical records of patients who underwent resective epilepsy surgery at the University Hospital Freiburg (Germany) between 1999 and 2015. Of the 1,278 initial patients, a group of 99 participants (7.7%) with seizure relapses after at least 2 years of complete seizure freedom were matched with controls experiencing long-term seizure freedom. The two groups had similar mean durations of epilepsy from onset to surgery: 13.9 years in the relapse group and 13.0 years in the control group.
The mean follow-up was 9.7 years (standard deviation, 4.0; range, 2.9-18.5) in the relapse group and 8.2 years (SD, 3.5; range, 2.2-18.3) in the control group. The mean time to late seizure recurrence was 56.6 months, and two-thirds of patients relapsed in the 5 years after surgery. Twenty of the relapse patients only experienced a single seizure, and 41% of the patients who reported more than one seizure had a frequency of less than one per month.
The type of resection had no discernible impact on outcomes, although anterior temporal lobe resection did trend toward being associated with recurrence (odds ratio, 2.7; 95% confidence interval, 0.93-8.89; P = .06). Incomplete resection was significantly associated with late relapse but did not seem to affect timing: the mean duration of seizure freedom was 56.5 months with complete resection and 58.5 months with incomplete resection (P = .62). Additional preoperative PET scans were performed on 45% of patients in the relapse group, compared with 29% in the control group.
After multivariate analysis, predictors for late relapse included incomplete resection (OR, 3.81; 95% CI, 1.79-8.53; P < .001); the existence of additional, potentially epileptogenic lesions in the contralateral hemisphere on presurgical MRI (OR, 3.36; 95% CI, 1.18-10.62; P = .03); epilepsy onset during the first year of life (OR, 4.24; 95% CI, 1.4-15.89; P = .02); and preoperative PET scans being performed (OR, 2.47; 95% CI, 1.25-4.97; P = .01). Though use of preoperative and postoperative antiepileptic drugs (AEDs) was higher in the relapse group, along with complete withdrawal being more common in the control group (68%, compared with 51%), neither was deemed significant in multivariate analysis.
What to do about seizure relapse risk factors
“This is one of the best analyses of the factors that contribute to late seizure relapse,” Gregory K. Bergey, MD, director of the Johns Hopkins Epilepsy Center in Baltimore, said in an interview. “Am I surprised by their results? Not necessarily.”
What did jump out, he said, was AED use not being a predictor of recurrence, as well as all the patients with late relapse having lesional epilepsy. “As they point out, you can have relapse with nonlesional epilepsy, but very often it happens in the first year or 2. If someone is 2 years out and doesn’t have a lesion, they’re probably more likely to remain seizure free.”
Despite the researchers’ comprehensive review of risk factors, the question remains: What to do with this information?
“They’ve done a very good job of identifying that 7.7% of 1,200 who are at risk of a late relapse,” he said. “Now, take those patients with high-risk factors and launch a trial where you keep medicines the same or do something that would alter that outcome.”
“The problem is,” he added, “that’s a 10-year study. It’s easy for me to sit here and call for one of those. But still, as valuable as this was, it’s a retrospective study. Now you have to say, what are the implications of this? What can we do in the prospective fashion?”
The authors acknowledged their study’s other limitations, including a lack of information on the reasons for an incomplete resection, a notable decrease in follow-up visits more than 5 years after surgery, and potential selection bias. They added, however, that “matching by age at surgery, gender, and time to relapse/last follow‐up” should have helped reduce any significant bias.
No potential conflicts of interest were disclosed.
FROM EPILEPSIA
More reassurance for certain antiseizure drugs in pregnancy
Further evidence supporting the safety of two antiseizure medications in pregnancy has come from a new study. Most of the women with epilepsy in the study took either lamotrigine or levetiracetam, or a combination of the two, during their pregnancy.
However, a secondary analysis suggested a possible signal of exposure-dependent effects on child outcomes – worse outcomes with higher exposure levels – with levetiracetam.
The results were presented at the American Academy of Neurology’s 2021 annual meeting.
Additional reassurance
“Our new study adds confidence to the use of lamotrigine and levetiracetam during pregnancy, adding larger numbers with a new cohort. In addition, it provides some preliminary data on some of the other new antiseizure medications, and it is the first study to address the effects of clearance in pregnancy to better assess exposure,” said lead investigator, Kimford J. Meador, MD. “Overall, I am reassured by this data, but there is still a lot that is unknown,” he added.
“Our main results show no difference in verbal index or general conceptual ability scores in children born to women with epilepsy compared to children born to healthy women. This is a big positive message,” Dr. Meador said.
In terms of secondary analysis focusing on exposure levels (dose and blood levels of antiseizure medications), there was no overall signal of harm when looking at the whole group, but when the researchers analyzed the data on individual drugs, they found a “slight signal” toward reduced verbal index scores with increasing exposure levels with levetiracetam. No differences were seen on general conceptual ability.
“In the secondary analysis, there was a marginal signal for exposure levels with levetiracetam, with increased blood levels of the drug associated with reduced verbal index scores,” reported Dr. Meador, professor of neurology and neurological sciences at Stanford (Calif.) University. “We saw some signal in the children when they were 2 years old, and this was still there but not as striking at 3 years old.”
He said these secondary results should be interpreted with extreme caution. “We don’t want to overemphasize these secondary findings, as the primary outcome showed no difference, and there was no effect on exposure levels when looking at all the drugs together. I don’t want to oversimplify this, as I am still not sure whether this is a real association or not,” Dr. Meador commented.
He explained that conducting neurobehavioral tests on 2- and 3-year-olds was very difficult. “It is more of an art form than science, and as the children get older these signals often dissipate. We will know more by the time they are 6, when these tests become easier to conduct,” he said. He also noted that the results would need to be replicated in a different cohort.
“I don’t think these results would change how we manage women during pregnancy in terms of using levetiracetam. It is still a safe drug during pregnancy,” Dr. Meador said.
He pointed out that data on safety in pregnancy is only available for very few antiseizure drugs. “There are over 30 antiseizure medications, but we have adequate data in pregnancy on only a handful. We have data suggesting lamotrigine, levetiracetam, and carbamazepine appear to be relatively safe, and evidence showing phenobarbital and valproate are not safe.”
Antiseizure medications as a class are among the most commonly prescribed teratogenic drugs given to women of childbearing age, Dr. Meador noted. They are used not only for epilepsy but also for many other psychiatric and pain indications, so these results are applicable to quite a broad population, he added.
He pointed out that previous studies did not assess exposure using blood levels, which is important, as clearance of drug increases during pregnancy but varies across antiseizure medications and across individuals on the same drug. “Thus, it is unclear if these changes could obscure exposure-dependent effects. Our present studies assessed blood levels to better measure fetal exposure.”
Advice for pregnant patients with epilepsy
Dr. Meador explained that risk for adverse effects with antiseizure medication always needs to be balanced with risk for seizures if the medication was not used.
“In women planning a pregnancy, we recommend that they plan ahead with their physician to try and use the safest antiseizure medication and gain good control beforehand and then maintain the same blood levels of whichever drug is being used during pregnancy,” Dr. Meador said. “At present, lamotrigine and levetiracetam are the two safest drugs to use in pregnancy. They both look generally very safe compared with some other epilepsy drugs – such as valproate, which poses a serious risk to cognitive and behavioral development.”
He also advised that women should be taking folic acid regularly, as this has been shown to be related to improved cognitive and behavioral outcomes. “Since half of pregnancies are not planned, it is important to take these actions before pregnancy,” he added.
The current study involved 289 women with epilepsy and 89 women without epilepsy, all of whom enrolled in the study during pregnancy. Use of antiseizure medications was recorded. Of the women with epilepsy, 74% were on monotherapy, with 43% on lamotrigine and 37% on levetiracetam. There were 4% who took no drug and 22% took more than one drug. Of those who took more than one drug, close to half took a combination of lamotrigine and levetiracetam. Levels of medications in the blood of the women with epilepsy were measured in the third trimester.
Assessment of neurobehavioral development
For the current analysis, the children were evaluated at age 3 with a series of cognitive and developmental tests that measured vocabulary, listening comprehension, number recall, and pattern recognition, and results were adjusted for mother’s IQ, education level, age at enrollment, postbirth average BAI (Beck Anxiety Inventory score), and child’s ethnicity, sex, and breastfeeding status.
The primary outcome showed that verbal Index scores at age 3 did not differ for children of women with epilepsy versus those for children of women without epilepsy (LS mean 102.7 vs. 102.1).
Antiseizure medication exposure as evident by the maximum third trimester blood levels was not related to verbal index scores (n = 265; adjusted parameter estimate, -1.9; 95% confidence interval, -6.8 to 3.1).
General conceptual ability scores also did not differ between the two groups: 105.1 for children of women with epilepsy versus 103.5 for children of healthy women.
In terms of exposure levels, the third trimester maximum observed ratio of antiseizure medication blood levels was not significantly associated with adjusted general conceptual ability scores for children of women with epilepsy; neither were monotherapies or polytherapies evaluated separately, Dr. Meador reported.
However, when the verbal index scores for the main antiepileptic drug groups were analyzed separately, exposure level to levetiracetam was the only one that was significant, with a P value of .028. But Dr. Meador again stressed that this finding should be interpreted with caution given that it is a secondary exploratory analysis without control for multiple comparisons.
The researchers plan to assess these children at older ages where evaluations are more sensitive to ultimate outcomes.
“Information on use in pregnancy for most antiseizure medications is still unknown, so further studies to assess risks for the newer antiseizure medications are needed,” Dr. Meador added. “Further, additional research is needed on the underlying mechanisms including genetic predispositions, since teratogens act on a susceptible genotype.”
The study was supported by the National Institutes of Health, the National Institute of Neurological Disorders and Stroke, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
A version of this article first appeared on Medscape.com.
Further evidence supporting the safety of two antiseizure medications in pregnancy has come from a new study. Most of the women with epilepsy in the study took either lamotrigine or levetiracetam, or a combination of the two, during their pregnancy.
However, a secondary analysis suggested a possible signal of exposure-dependent effects on child outcomes – worse outcomes with higher exposure levels – with levetiracetam.
The results were presented at the American Academy of Neurology’s 2021 annual meeting.
Additional reassurance
“Our new study adds confidence to the use of lamotrigine and levetiracetam during pregnancy, adding larger numbers with a new cohort. In addition, it provides some preliminary data on some of the other new antiseizure medications, and it is the first study to address the effects of clearance in pregnancy to better assess exposure,” said lead investigator, Kimford J. Meador, MD. “Overall, I am reassured by this data, but there is still a lot that is unknown,” he added.
“Our main results show no difference in verbal index or general conceptual ability scores in children born to women with epilepsy compared to children born to healthy women. This is a big positive message,” Dr. Meador said.
In terms of secondary analysis focusing on exposure levels (dose and blood levels of antiseizure medications), there was no overall signal of harm when looking at the whole group, but when the researchers analyzed the data on individual drugs, they found a “slight signal” toward reduced verbal index scores with increasing exposure levels with levetiracetam. No differences were seen on general conceptual ability.
“In the secondary analysis, there was a marginal signal for exposure levels with levetiracetam, with increased blood levels of the drug associated with reduced verbal index scores,” reported Dr. Meador, professor of neurology and neurological sciences at Stanford (Calif.) University. “We saw some signal in the children when they were 2 years old, and this was still there but not as striking at 3 years old.”
He said these secondary results should be interpreted with extreme caution. “We don’t want to overemphasize these secondary findings, as the primary outcome showed no difference, and there was no effect on exposure levels when looking at all the drugs together. I don’t want to oversimplify this, as I am still not sure whether this is a real association or not,” Dr. Meador commented.
He explained that conducting neurobehavioral tests on 2- and 3-year-olds was very difficult. “It is more of an art form than science, and as the children get older these signals often dissipate. We will know more by the time they are 6, when these tests become easier to conduct,” he said. He also noted that the results would need to be replicated in a different cohort.
“I don’t think these results would change how we manage women during pregnancy in terms of using levetiracetam. It is still a safe drug during pregnancy,” Dr. Meador said.
He pointed out that data on safety in pregnancy is only available for very few antiseizure drugs. “There are over 30 antiseizure medications, but we have adequate data in pregnancy on only a handful. We have data suggesting lamotrigine, levetiracetam, and carbamazepine appear to be relatively safe, and evidence showing phenobarbital and valproate are not safe.”
Antiseizure medications as a class are among the most commonly prescribed teratogenic drugs given to women of childbearing age, Dr. Meador noted. They are used not only for epilepsy but also for many other psychiatric and pain indications, so these results are applicable to quite a broad population, he added.
He pointed out that previous studies did not assess exposure using blood levels, which is important, as clearance of drug increases during pregnancy but varies across antiseizure medications and across individuals on the same drug. “Thus, it is unclear if these changes could obscure exposure-dependent effects. Our present studies assessed blood levels to better measure fetal exposure.”
Advice for pregnant patients with epilepsy
Dr. Meador explained that risk for adverse effects with antiseizure medication always needs to be balanced with risk for seizures if the medication was not used.
“In women planning a pregnancy, we recommend that they plan ahead with their physician to try and use the safest antiseizure medication and gain good control beforehand and then maintain the same blood levels of whichever drug is being used during pregnancy,” Dr. Meador said. “At present, lamotrigine and levetiracetam are the two safest drugs to use in pregnancy. They both look generally very safe compared with some other epilepsy drugs – such as valproate, which poses a serious risk to cognitive and behavioral development.”
He also advised that women should be taking folic acid regularly, as this has been shown to be related to improved cognitive and behavioral outcomes. “Since half of pregnancies are not planned, it is important to take these actions before pregnancy,” he added.
The current study involved 289 women with epilepsy and 89 women without epilepsy, all of whom enrolled in the study during pregnancy. Use of antiseizure medications was recorded. Of the women with epilepsy, 74% were on monotherapy, with 43% on lamotrigine and 37% on levetiracetam. There were 4% who took no drug and 22% took more than one drug. Of those who took more than one drug, close to half took a combination of lamotrigine and levetiracetam. Levels of medications in the blood of the women with epilepsy were measured in the third trimester.
Assessment of neurobehavioral development
For the current analysis, the children were evaluated at age 3 with a series of cognitive and developmental tests that measured vocabulary, listening comprehension, number recall, and pattern recognition, and results were adjusted for mother’s IQ, education level, age at enrollment, postbirth average BAI (Beck Anxiety Inventory score), and child’s ethnicity, sex, and breastfeeding status.
The primary outcome showed that verbal Index scores at age 3 did not differ for children of women with epilepsy versus those for children of women without epilepsy (LS mean 102.7 vs. 102.1).
Antiseizure medication exposure as evident by the maximum third trimester blood levels was not related to verbal index scores (n = 265; adjusted parameter estimate, -1.9; 95% confidence interval, -6.8 to 3.1).
General conceptual ability scores also did not differ between the two groups: 105.1 for children of women with epilepsy versus 103.5 for children of healthy women.
In terms of exposure levels, the third trimester maximum observed ratio of antiseizure medication blood levels was not significantly associated with adjusted general conceptual ability scores for children of women with epilepsy; neither were monotherapies or polytherapies evaluated separately, Dr. Meador reported.
However, when the verbal index scores for the main antiepileptic drug groups were analyzed separately, exposure level to levetiracetam was the only one that was significant, with a P value of .028. But Dr. Meador again stressed that this finding should be interpreted with caution given that it is a secondary exploratory analysis without control for multiple comparisons.
The researchers plan to assess these children at older ages where evaluations are more sensitive to ultimate outcomes.
“Information on use in pregnancy for most antiseizure medications is still unknown, so further studies to assess risks for the newer antiseizure medications are needed,” Dr. Meador added. “Further, additional research is needed on the underlying mechanisms including genetic predispositions, since teratogens act on a susceptible genotype.”
The study was supported by the National Institutes of Health, the National Institute of Neurological Disorders and Stroke, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
A version of this article first appeared on Medscape.com.
Further evidence supporting the safety of two antiseizure medications in pregnancy has come from a new study. Most of the women with epilepsy in the study took either lamotrigine or levetiracetam, or a combination of the two, during their pregnancy.
However, a secondary analysis suggested a possible signal of exposure-dependent effects on child outcomes – worse outcomes with higher exposure levels – with levetiracetam.
The results were presented at the American Academy of Neurology’s 2021 annual meeting.
Additional reassurance
“Our new study adds confidence to the use of lamotrigine and levetiracetam during pregnancy, adding larger numbers with a new cohort. In addition, it provides some preliminary data on some of the other new antiseizure medications, and it is the first study to address the effects of clearance in pregnancy to better assess exposure,” said lead investigator, Kimford J. Meador, MD. “Overall, I am reassured by this data, but there is still a lot that is unknown,” he added.
“Our main results show no difference in verbal index or general conceptual ability scores in children born to women with epilepsy compared to children born to healthy women. This is a big positive message,” Dr. Meador said.
In terms of secondary analysis focusing on exposure levels (dose and blood levels of antiseizure medications), there was no overall signal of harm when looking at the whole group, but when the researchers analyzed the data on individual drugs, they found a “slight signal” toward reduced verbal index scores with increasing exposure levels with levetiracetam. No differences were seen on general conceptual ability.
“In the secondary analysis, there was a marginal signal for exposure levels with levetiracetam, with increased blood levels of the drug associated with reduced verbal index scores,” reported Dr. Meador, professor of neurology and neurological sciences at Stanford (Calif.) University. “We saw some signal in the children when they were 2 years old, and this was still there but not as striking at 3 years old.”
He said these secondary results should be interpreted with extreme caution. “We don’t want to overemphasize these secondary findings, as the primary outcome showed no difference, and there was no effect on exposure levels when looking at all the drugs together. I don’t want to oversimplify this, as I am still not sure whether this is a real association or not,” Dr. Meador commented.
He explained that conducting neurobehavioral tests on 2- and 3-year-olds was very difficult. “It is more of an art form than science, and as the children get older these signals often dissipate. We will know more by the time they are 6, when these tests become easier to conduct,” he said. He also noted that the results would need to be replicated in a different cohort.
“I don’t think these results would change how we manage women during pregnancy in terms of using levetiracetam. It is still a safe drug during pregnancy,” Dr. Meador said.
He pointed out that data on safety in pregnancy is only available for very few antiseizure drugs. “There are over 30 antiseizure medications, but we have adequate data in pregnancy on only a handful. We have data suggesting lamotrigine, levetiracetam, and carbamazepine appear to be relatively safe, and evidence showing phenobarbital and valproate are not safe.”
Antiseizure medications as a class are among the most commonly prescribed teratogenic drugs given to women of childbearing age, Dr. Meador noted. They are used not only for epilepsy but also for many other psychiatric and pain indications, so these results are applicable to quite a broad population, he added.
He pointed out that previous studies did not assess exposure using blood levels, which is important, as clearance of drug increases during pregnancy but varies across antiseizure medications and across individuals on the same drug. “Thus, it is unclear if these changes could obscure exposure-dependent effects. Our present studies assessed blood levels to better measure fetal exposure.”
Advice for pregnant patients with epilepsy
Dr. Meador explained that risk for adverse effects with antiseizure medication always needs to be balanced with risk for seizures if the medication was not used.
“In women planning a pregnancy, we recommend that they plan ahead with their physician to try and use the safest antiseizure medication and gain good control beforehand and then maintain the same blood levels of whichever drug is being used during pregnancy,” Dr. Meador said. “At present, lamotrigine and levetiracetam are the two safest drugs to use in pregnancy. They both look generally very safe compared with some other epilepsy drugs – such as valproate, which poses a serious risk to cognitive and behavioral development.”
He also advised that women should be taking folic acid regularly, as this has been shown to be related to improved cognitive and behavioral outcomes. “Since half of pregnancies are not planned, it is important to take these actions before pregnancy,” he added.
The current study involved 289 women with epilepsy and 89 women without epilepsy, all of whom enrolled in the study during pregnancy. Use of antiseizure medications was recorded. Of the women with epilepsy, 74% were on monotherapy, with 43% on lamotrigine and 37% on levetiracetam. There were 4% who took no drug and 22% took more than one drug. Of those who took more than one drug, close to half took a combination of lamotrigine and levetiracetam. Levels of medications in the blood of the women with epilepsy were measured in the third trimester.
Assessment of neurobehavioral development
For the current analysis, the children were evaluated at age 3 with a series of cognitive and developmental tests that measured vocabulary, listening comprehension, number recall, and pattern recognition, and results were adjusted for mother’s IQ, education level, age at enrollment, postbirth average BAI (Beck Anxiety Inventory score), and child’s ethnicity, sex, and breastfeeding status.
The primary outcome showed that verbal Index scores at age 3 did not differ for children of women with epilepsy versus those for children of women without epilepsy (LS mean 102.7 vs. 102.1).
Antiseizure medication exposure as evident by the maximum third trimester blood levels was not related to verbal index scores (n = 265; adjusted parameter estimate, -1.9; 95% confidence interval, -6.8 to 3.1).
General conceptual ability scores also did not differ between the two groups: 105.1 for children of women with epilepsy versus 103.5 for children of healthy women.
In terms of exposure levels, the third trimester maximum observed ratio of antiseizure medication blood levels was not significantly associated with adjusted general conceptual ability scores for children of women with epilepsy; neither were monotherapies or polytherapies evaluated separately, Dr. Meador reported.
However, when the verbal index scores for the main antiepileptic drug groups were analyzed separately, exposure level to levetiracetam was the only one that was significant, with a P value of .028. But Dr. Meador again stressed that this finding should be interpreted with caution given that it is a secondary exploratory analysis without control for multiple comparisons.
The researchers plan to assess these children at older ages where evaluations are more sensitive to ultimate outcomes.
“Information on use in pregnancy for most antiseizure medications is still unknown, so further studies to assess risks for the newer antiseizure medications are needed,” Dr. Meador added. “Further, additional research is needed on the underlying mechanisms including genetic predispositions, since teratogens act on a susceptible genotype.”
The study was supported by the National Institutes of Health, the National Institute of Neurological Disorders and Stroke, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
A version of this article first appeared on Medscape.com.
From AAN 2021
COVID-19 linked to novel epileptic seizures
, new research shows. In a retrospective study of more than 900 patients admitted to the hospital with COVID-19, those without a known history of epilepsy had three times greater odds of experiencing novel seizures than those with a known history of epilepsy.
In addition, among patients with new-onset seizures, hospital stays were about 15 days longer – and mortality rates were significantly higher.
“We’re finding that there are many neurological consequences that can happen with COVID-19 infections, and it’s important for clinicians to keep that in mind as they monitor people long term,” said study investigator Neeraj Singh, MD, neurologist and epileptologist with Northwell Health System, Great Neck, New York.
Dr. Singh noted that although seizures “might not be the most common thing we see in people with COVID-19, they seem to be new seizures and not just a seizure we knew would happen in someone with epilepsy.”
“So there’s definitely a need now for more prospective research and following people over time to fully understand all the different things that might be newly a problem for them in the long term,” he added.
Dr. Singh and Hardik Bhaskar, an undergraduate student at Hunter College, New York, presented the study findings at the American Academy of Neurology’s 2021 annual meeting.
Largest sample to date
“This study explores the relationship between the incidences of COVID-19 infections and [novel] epileptic seizures in the largest sample to date in a single New York–based hospital system,” the investigators noted. Novel seizures included both new-onset and breakthrough seizures.
Dr. Singh told meeting attendees that the “early epicenter” of the COVID pandemic was in New York and occurred from Feb. 29, 2020 to June 1, 2020. Patients with COVID-19 “had multiple neurological sequelae, including seizures, strokes, and encephalopathy,” he said.
However, the effects of COVID-19 on individuals with epilepsy “remain unclear,” Dr. Singh said.
For their study, the researchers assessed 917 patients in 13 New York City metropolitan hospitals. All participants had received a confirmed positive test result on PCR for COVID and had received an antiepileptic medication upon admission. The patients were admitted between Feb. 14 and June 14, 2020.
For the study, the patients were first divided into two groups: those with a history of epilepsy (n = 451), and those without such a history (n = 466).
The first group was further divided on the basis of those who presented with breakthrough seizures and those who presented without them. The second group was further divided on the basis of those who presented with new-onset seizures and those who presented without them.
Significant adverse outcomes
Results showed that 27% of the patients without a history of epilepsy experienced a novel/new-onset seizure and that 11% of the patients with a history of epilepsy experienced a novel/breakthrough seizure (odds ratio, 3.15; P < .0001).
In addition, participants with new-onset seizures had a longer stay in the hospital (mean, 26.9 days) than the subgroup with a history of epilepsy and no breakthrough seizures (10.9 days) and the subgroup with a history of epilepsy who did experience breakthrough seizures (12.8 days; P < .0001 for both comparisons).
In the group of patients with a history of epilepsy, there were no significant differences in lengths of stay between those with and those without breakthrough seizures (P = .68).
Although mortality rates did not differ significantly between the full group with a history of epilepsy versus the full group without epilepsy (23% vs. 25%; OR, 0.9), the mortality rate was significantly higher among patients who experienced novel seizures than among those who did not experience such seizures (29% vs. 23%; OR, 1.4; P = .045).
Mr. Bhaskar noted that there are “many hypotheses for the mechanism by which COVID-19 might cause seizures.” Those mechanisms include proinflammatory cytokine storms, which may increase the rate of apoptosis, neuronal necrosis, and glutamate concentrations and may disrupt the blood-brain barrier. Another hypothesis is that SARS-CoV-2 infection may lead to hypoxia and abnormal coagulation, resulting in stroke and a subsequent increase in the risk for seizures.
Interestingly, “the presence of antiepileptic medications in patients with epilepsy may confer a protective effect against breakthrough seizures,” Dr. Singh said. “However, some subclinical seizures may be misdiagnosed as encephalopathy when patients present with COVID-19 infections.”
He added that further research is needed into the mechanisms linking these infections and new-onset seizures and to “identify subclinical seizures in encephalopathic patients.”
Asked during the question-and-answer session whether the investigators had assessed differences by demographics, such as age or sex, Dr. Singh said, “We have not subdivided them that way yet,” but he said he would like to do so in the future. He also plans to look further into which specific medications were used by the participants.
The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. In a retrospective study of more than 900 patients admitted to the hospital with COVID-19, those without a known history of epilepsy had three times greater odds of experiencing novel seizures than those with a known history of epilepsy.
In addition, among patients with new-onset seizures, hospital stays were about 15 days longer – and mortality rates were significantly higher.
“We’re finding that there are many neurological consequences that can happen with COVID-19 infections, and it’s important for clinicians to keep that in mind as they monitor people long term,” said study investigator Neeraj Singh, MD, neurologist and epileptologist with Northwell Health System, Great Neck, New York.
Dr. Singh noted that although seizures “might not be the most common thing we see in people with COVID-19, they seem to be new seizures and not just a seizure we knew would happen in someone with epilepsy.”
“So there’s definitely a need now for more prospective research and following people over time to fully understand all the different things that might be newly a problem for them in the long term,” he added.
Dr. Singh and Hardik Bhaskar, an undergraduate student at Hunter College, New York, presented the study findings at the American Academy of Neurology’s 2021 annual meeting.
Largest sample to date
“This study explores the relationship between the incidences of COVID-19 infections and [novel] epileptic seizures in the largest sample to date in a single New York–based hospital system,” the investigators noted. Novel seizures included both new-onset and breakthrough seizures.
Dr. Singh told meeting attendees that the “early epicenter” of the COVID pandemic was in New York and occurred from Feb. 29, 2020 to June 1, 2020. Patients with COVID-19 “had multiple neurological sequelae, including seizures, strokes, and encephalopathy,” he said.
However, the effects of COVID-19 on individuals with epilepsy “remain unclear,” Dr. Singh said.
For their study, the researchers assessed 917 patients in 13 New York City metropolitan hospitals. All participants had received a confirmed positive test result on PCR for COVID and had received an antiepileptic medication upon admission. The patients were admitted between Feb. 14 and June 14, 2020.
For the study, the patients were first divided into two groups: those with a history of epilepsy (n = 451), and those without such a history (n = 466).
The first group was further divided on the basis of those who presented with breakthrough seizures and those who presented without them. The second group was further divided on the basis of those who presented with new-onset seizures and those who presented without them.
Significant adverse outcomes
Results showed that 27% of the patients without a history of epilepsy experienced a novel/new-onset seizure and that 11% of the patients with a history of epilepsy experienced a novel/breakthrough seizure (odds ratio, 3.15; P < .0001).
In addition, participants with new-onset seizures had a longer stay in the hospital (mean, 26.9 days) than the subgroup with a history of epilepsy and no breakthrough seizures (10.9 days) and the subgroup with a history of epilepsy who did experience breakthrough seizures (12.8 days; P < .0001 for both comparisons).
In the group of patients with a history of epilepsy, there were no significant differences in lengths of stay between those with and those without breakthrough seizures (P = .68).
Although mortality rates did not differ significantly between the full group with a history of epilepsy versus the full group without epilepsy (23% vs. 25%; OR, 0.9), the mortality rate was significantly higher among patients who experienced novel seizures than among those who did not experience such seizures (29% vs. 23%; OR, 1.4; P = .045).
Mr. Bhaskar noted that there are “many hypotheses for the mechanism by which COVID-19 might cause seizures.” Those mechanisms include proinflammatory cytokine storms, which may increase the rate of apoptosis, neuronal necrosis, and glutamate concentrations and may disrupt the blood-brain barrier. Another hypothesis is that SARS-CoV-2 infection may lead to hypoxia and abnormal coagulation, resulting in stroke and a subsequent increase in the risk for seizures.
Interestingly, “the presence of antiepileptic medications in patients with epilepsy may confer a protective effect against breakthrough seizures,” Dr. Singh said. “However, some subclinical seizures may be misdiagnosed as encephalopathy when patients present with COVID-19 infections.”
He added that further research is needed into the mechanisms linking these infections and new-onset seizures and to “identify subclinical seizures in encephalopathic patients.”
Asked during the question-and-answer session whether the investigators had assessed differences by demographics, such as age or sex, Dr. Singh said, “We have not subdivided them that way yet,” but he said he would like to do so in the future. He also plans to look further into which specific medications were used by the participants.
The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. In a retrospective study of more than 900 patients admitted to the hospital with COVID-19, those without a known history of epilepsy had three times greater odds of experiencing novel seizures than those with a known history of epilepsy.
In addition, among patients with new-onset seizures, hospital stays were about 15 days longer – and mortality rates were significantly higher.
“We’re finding that there are many neurological consequences that can happen with COVID-19 infections, and it’s important for clinicians to keep that in mind as they monitor people long term,” said study investigator Neeraj Singh, MD, neurologist and epileptologist with Northwell Health System, Great Neck, New York.
Dr. Singh noted that although seizures “might not be the most common thing we see in people with COVID-19, they seem to be new seizures and not just a seizure we knew would happen in someone with epilepsy.”
“So there’s definitely a need now for more prospective research and following people over time to fully understand all the different things that might be newly a problem for them in the long term,” he added.
Dr. Singh and Hardik Bhaskar, an undergraduate student at Hunter College, New York, presented the study findings at the American Academy of Neurology’s 2021 annual meeting.
Largest sample to date
“This study explores the relationship between the incidences of COVID-19 infections and [novel] epileptic seizures in the largest sample to date in a single New York–based hospital system,” the investigators noted. Novel seizures included both new-onset and breakthrough seizures.
Dr. Singh told meeting attendees that the “early epicenter” of the COVID pandemic was in New York and occurred from Feb. 29, 2020 to June 1, 2020. Patients with COVID-19 “had multiple neurological sequelae, including seizures, strokes, and encephalopathy,” he said.
However, the effects of COVID-19 on individuals with epilepsy “remain unclear,” Dr. Singh said.
For their study, the researchers assessed 917 patients in 13 New York City metropolitan hospitals. All participants had received a confirmed positive test result on PCR for COVID and had received an antiepileptic medication upon admission. The patients were admitted between Feb. 14 and June 14, 2020.
For the study, the patients were first divided into two groups: those with a history of epilepsy (n = 451), and those without such a history (n = 466).
The first group was further divided on the basis of those who presented with breakthrough seizures and those who presented without them. The second group was further divided on the basis of those who presented with new-onset seizures and those who presented without them.
Significant adverse outcomes
Results showed that 27% of the patients without a history of epilepsy experienced a novel/new-onset seizure and that 11% of the patients with a history of epilepsy experienced a novel/breakthrough seizure (odds ratio, 3.15; P < .0001).
In addition, participants with new-onset seizures had a longer stay in the hospital (mean, 26.9 days) than the subgroup with a history of epilepsy and no breakthrough seizures (10.9 days) and the subgroup with a history of epilepsy who did experience breakthrough seizures (12.8 days; P < .0001 for both comparisons).
In the group of patients with a history of epilepsy, there were no significant differences in lengths of stay between those with and those without breakthrough seizures (P = .68).
Although mortality rates did not differ significantly between the full group with a history of epilepsy versus the full group without epilepsy (23% vs. 25%; OR, 0.9), the mortality rate was significantly higher among patients who experienced novel seizures than among those who did not experience such seizures (29% vs. 23%; OR, 1.4; P = .045).
Mr. Bhaskar noted that there are “many hypotheses for the mechanism by which COVID-19 might cause seizures.” Those mechanisms include proinflammatory cytokine storms, which may increase the rate of apoptosis, neuronal necrosis, and glutamate concentrations and may disrupt the blood-brain barrier. Another hypothesis is that SARS-CoV-2 infection may lead to hypoxia and abnormal coagulation, resulting in stroke and a subsequent increase in the risk for seizures.
Interestingly, “the presence of antiepileptic medications in patients with epilepsy may confer a protective effect against breakthrough seizures,” Dr. Singh said. “However, some subclinical seizures may be misdiagnosed as encephalopathy when patients present with COVID-19 infections.”
He added that further research is needed into the mechanisms linking these infections and new-onset seizures and to “identify subclinical seizures in encephalopathic patients.”
Asked during the question-and-answer session whether the investigators had assessed differences by demographics, such as age or sex, Dr. Singh said, “We have not subdivided them that way yet,” but he said he would like to do so in the future. He also plans to look further into which specific medications were used by the participants.
The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From AAN 2021
Neurologic drug prices jump 50% in five years
, new research shows. Results of the retrospective study also showed that most of the increased costs for these agents were due to rising costs for neuroimmunology drugs, mainly for those used to treat multiple sclerosis (MS).
“The same brand name medication in 2017 cost approximately 50% more than in 2013,” said Adam de Havenon, MD, assistant professor of neurology, University of Utah, Salt Lake City.
“An analogy would be if you bought an iPhone 5 in 2013 for $500, and then in 2017, you were asked to pay $750 for the exact same iPhone 5,” Dr. de Havenon added.
The study findings were published online March 10 in the journal Neurology.
$26 billion in payments
Both neurologists and patients are concerned about the high cost of prescription drugs for neurologic diseases, and Medicare Part D data indicate that these drugs are the most expensive component of neurologic care, the researchers noted. In addition, out-of-pocket costs have increased significantly for patients with neurologic disease such as Parkinson’s disease, epilepsy, and MS.
To understand trends in payments for neurologic drugs, Dr. de Havenon and colleagues analyzed Medicare Part D claims filed from 2013 to 2017. The payments include costs paid by Medicare, the patient, government subsidies, and other third-party payers.
In addition to examining more current Medicare Part D data than previous studies, the current analysis examined all medications prescribed by neurologists that consistently remained branded or generic during the 5-year study period, said Dr. de Havenon. This approach resulted in a large number of claims and a large total cost.
To calculate the percentage change in annual payment claims, the researchers used 2013 prices as a reference point. They identified drugs named in 2013 claims and classified them as generic, brand-name only, or brand-name with generic equivalent. Researchers also divided the drugs by neurologic subspecialty.
The analysis included 520 drugs, all of which were available in each year of the study period. Of these drugs, 322 were generic, 61 were brand-name only, and 137 were brand-name with a generic equivalent. There were 90.7 million total claims.
Results showed total payments amounted to $26.65 billion. Yearly total payments increased from $4.05 billion in 2013 to $6.09 billion in 2017, representing a 50.4% increase, even after adjusting for inflation. Total claims increased by 7.6% – from 17.1 million in 2013 to 18.4 million in 2017.
From 2013 to 2017, claim payments increased by 0.6% for generic drugs, 42.4% for brand-name only drugs, and 45% for brand-name drugs with generic equivalents. The proportion of claims increased from 81.9% to 88% for generic drugs and from 4.9% to 6.2% for brand-name only drugs.
However, the proportion of claims for brand-name drugs with generic equivalents decreased from 13.3% to 5.8%.
Treatment barrier
Neuroimmunologic drugs, most of which were prescribed for MS, had exceptional cost, the researchers noted. These drugs accounted for more than 50% of payments but only 4.3% of claims. Claim payment for these drugs increased by 46.9% during the study period, from $3,337 to $4,902.
When neuroimmunologic drugs were removed from the analysis there was still significant increase in claim payments for brand-name only drugs (50.4%) and brand-name drugs with generic equivalents (45.6%).
Although neuroimmunologic medicines, including monoclonal antibodies, are more expensive to produce, this factor alone does not explain their exceptional cost, said Dr. de Havenon. “The high cost of brand-name drugs in this speciality is likely because the market bears it,” he added. “In other words, MS is a disabling disease and the medications work, so historically the Centers for Medicare & Medicaid Services have been willing to tolerate the high cost of these primarily brand-name medications.”
Several countries have controlled drug costs by negotiating with pharmaceutical companies and through legislation, Dr. de Havenon noted.
“My intent with this article was to raise awareness on the topic, which I struggle with frequently as a clinician. I know I want my patients to have a medication, but the cost prevents it,” he said.
‘Unfettered’ price-setting
Commenting on the findings, Robert J. Fox, MD, vice chair for research at the Neurological Institute of the Cleveland Clinic, said the study “brings into clear light” what neurologists, particularly those who treat MS, have long suspected but did not really know. These neurologists “are typically distanced from the payment aspects of the medications they prescribe,” said Dr. Fox, who was not involved with the research.
Although a particular strength of the study was its comprehensiveness, the researchers excluded infusion claims – which account for a large portion of total patient care costs for many disorders, he noted.
Drugs for MS historically have been expensive, ostensibly because of their high cost of development. In addition, the large and continued price increase that occurs long after these drugs have been approved remains unexplained, said Dr. Fox.
He noted that the study findings might not directly affect clinical practice because neurologists will continue prescribing medications they think are best for their patients. “Instead, I think this is a lesson to lawmakers about the massive error in the Medicare Modernization Act of 2003, where the federal government was prohibited from negotiating drug prices. If the seller is unfettered in setting a price, then no one should be surprised when the price rises,” Dr. Fox said.
Because many new drugs and new generic formulations for treating MS have become available during the past year, “repeating these types of economic studies for the period 2020-2025 will help us understand if generic competition – as well as new laws if they are passed – alter price,” he concluded.
The study was funded by the American Academy of Neurology, which publishes Neurology. Dr. de Havenon has received clinical research funding from AMAG Pharmaceuticals and Regeneron Pharmaceuticals. Dr. Fox receives consulting fees from many pharmaceutical companies involved in the development of therapies for MS.
A version of this article first appeared on Medscape.com.
, new research shows. Results of the retrospective study also showed that most of the increased costs for these agents were due to rising costs for neuroimmunology drugs, mainly for those used to treat multiple sclerosis (MS).
“The same brand name medication in 2017 cost approximately 50% more than in 2013,” said Adam de Havenon, MD, assistant professor of neurology, University of Utah, Salt Lake City.
“An analogy would be if you bought an iPhone 5 in 2013 for $500, and then in 2017, you were asked to pay $750 for the exact same iPhone 5,” Dr. de Havenon added.
The study findings were published online March 10 in the journal Neurology.
$26 billion in payments
Both neurologists and patients are concerned about the high cost of prescription drugs for neurologic diseases, and Medicare Part D data indicate that these drugs are the most expensive component of neurologic care, the researchers noted. In addition, out-of-pocket costs have increased significantly for patients with neurologic disease such as Parkinson’s disease, epilepsy, and MS.
To understand trends in payments for neurologic drugs, Dr. de Havenon and colleagues analyzed Medicare Part D claims filed from 2013 to 2017. The payments include costs paid by Medicare, the patient, government subsidies, and other third-party payers.
In addition to examining more current Medicare Part D data than previous studies, the current analysis examined all medications prescribed by neurologists that consistently remained branded or generic during the 5-year study period, said Dr. de Havenon. This approach resulted in a large number of claims and a large total cost.
To calculate the percentage change in annual payment claims, the researchers used 2013 prices as a reference point. They identified drugs named in 2013 claims and classified them as generic, brand-name only, or brand-name with generic equivalent. Researchers also divided the drugs by neurologic subspecialty.
The analysis included 520 drugs, all of which were available in each year of the study period. Of these drugs, 322 were generic, 61 were brand-name only, and 137 were brand-name with a generic equivalent. There were 90.7 million total claims.
Results showed total payments amounted to $26.65 billion. Yearly total payments increased from $4.05 billion in 2013 to $6.09 billion in 2017, representing a 50.4% increase, even after adjusting for inflation. Total claims increased by 7.6% – from 17.1 million in 2013 to 18.4 million in 2017.
From 2013 to 2017, claim payments increased by 0.6% for generic drugs, 42.4% for brand-name only drugs, and 45% for brand-name drugs with generic equivalents. The proportion of claims increased from 81.9% to 88% for generic drugs and from 4.9% to 6.2% for brand-name only drugs.
However, the proportion of claims for brand-name drugs with generic equivalents decreased from 13.3% to 5.8%.
Treatment barrier
Neuroimmunologic drugs, most of which were prescribed for MS, had exceptional cost, the researchers noted. These drugs accounted for more than 50% of payments but only 4.3% of claims. Claim payment for these drugs increased by 46.9% during the study period, from $3,337 to $4,902.
When neuroimmunologic drugs were removed from the analysis there was still significant increase in claim payments for brand-name only drugs (50.4%) and brand-name drugs with generic equivalents (45.6%).
Although neuroimmunologic medicines, including monoclonal antibodies, are more expensive to produce, this factor alone does not explain their exceptional cost, said Dr. de Havenon. “The high cost of brand-name drugs in this speciality is likely because the market bears it,” he added. “In other words, MS is a disabling disease and the medications work, so historically the Centers for Medicare & Medicaid Services have been willing to tolerate the high cost of these primarily brand-name medications.”
Several countries have controlled drug costs by negotiating with pharmaceutical companies and through legislation, Dr. de Havenon noted.
“My intent with this article was to raise awareness on the topic, which I struggle with frequently as a clinician. I know I want my patients to have a medication, but the cost prevents it,” he said.
‘Unfettered’ price-setting
Commenting on the findings, Robert J. Fox, MD, vice chair for research at the Neurological Institute of the Cleveland Clinic, said the study “brings into clear light” what neurologists, particularly those who treat MS, have long suspected but did not really know. These neurologists “are typically distanced from the payment aspects of the medications they prescribe,” said Dr. Fox, who was not involved with the research.
Although a particular strength of the study was its comprehensiveness, the researchers excluded infusion claims – which account for a large portion of total patient care costs for many disorders, he noted.
Drugs for MS historically have been expensive, ostensibly because of their high cost of development. In addition, the large and continued price increase that occurs long after these drugs have been approved remains unexplained, said Dr. Fox.
He noted that the study findings might not directly affect clinical practice because neurologists will continue prescribing medications they think are best for their patients. “Instead, I think this is a lesson to lawmakers about the massive error in the Medicare Modernization Act of 2003, where the federal government was prohibited from negotiating drug prices. If the seller is unfettered in setting a price, then no one should be surprised when the price rises,” Dr. Fox said.
Because many new drugs and new generic formulations for treating MS have become available during the past year, “repeating these types of economic studies for the period 2020-2025 will help us understand if generic competition – as well as new laws if they are passed – alter price,” he concluded.
The study was funded by the American Academy of Neurology, which publishes Neurology. Dr. de Havenon has received clinical research funding from AMAG Pharmaceuticals and Regeneron Pharmaceuticals. Dr. Fox receives consulting fees from many pharmaceutical companies involved in the development of therapies for MS.
A version of this article first appeared on Medscape.com.
, new research shows. Results of the retrospective study also showed that most of the increased costs for these agents were due to rising costs for neuroimmunology drugs, mainly for those used to treat multiple sclerosis (MS).
“The same brand name medication in 2017 cost approximately 50% more than in 2013,” said Adam de Havenon, MD, assistant professor of neurology, University of Utah, Salt Lake City.
“An analogy would be if you bought an iPhone 5 in 2013 for $500, and then in 2017, you were asked to pay $750 for the exact same iPhone 5,” Dr. de Havenon added.
The study findings were published online March 10 in the journal Neurology.
$26 billion in payments
Both neurologists and patients are concerned about the high cost of prescription drugs for neurologic diseases, and Medicare Part D data indicate that these drugs are the most expensive component of neurologic care, the researchers noted. In addition, out-of-pocket costs have increased significantly for patients with neurologic disease such as Parkinson’s disease, epilepsy, and MS.
To understand trends in payments for neurologic drugs, Dr. de Havenon and colleagues analyzed Medicare Part D claims filed from 2013 to 2017. The payments include costs paid by Medicare, the patient, government subsidies, and other third-party payers.
In addition to examining more current Medicare Part D data than previous studies, the current analysis examined all medications prescribed by neurologists that consistently remained branded or generic during the 5-year study period, said Dr. de Havenon. This approach resulted in a large number of claims and a large total cost.
To calculate the percentage change in annual payment claims, the researchers used 2013 prices as a reference point. They identified drugs named in 2013 claims and classified them as generic, brand-name only, or brand-name with generic equivalent. Researchers also divided the drugs by neurologic subspecialty.
The analysis included 520 drugs, all of which were available in each year of the study period. Of these drugs, 322 were generic, 61 were brand-name only, and 137 were brand-name with a generic equivalent. There were 90.7 million total claims.
Results showed total payments amounted to $26.65 billion. Yearly total payments increased from $4.05 billion in 2013 to $6.09 billion in 2017, representing a 50.4% increase, even after adjusting for inflation. Total claims increased by 7.6% – from 17.1 million in 2013 to 18.4 million in 2017.
From 2013 to 2017, claim payments increased by 0.6% for generic drugs, 42.4% for brand-name only drugs, and 45% for brand-name drugs with generic equivalents. The proportion of claims increased from 81.9% to 88% for generic drugs and from 4.9% to 6.2% for brand-name only drugs.
However, the proportion of claims for brand-name drugs with generic equivalents decreased from 13.3% to 5.8%.
Treatment barrier
Neuroimmunologic drugs, most of which were prescribed for MS, had exceptional cost, the researchers noted. These drugs accounted for more than 50% of payments but only 4.3% of claims. Claim payment for these drugs increased by 46.9% during the study period, from $3,337 to $4,902.
When neuroimmunologic drugs were removed from the analysis there was still significant increase in claim payments for brand-name only drugs (50.4%) and brand-name drugs with generic equivalents (45.6%).
Although neuroimmunologic medicines, including monoclonal antibodies, are more expensive to produce, this factor alone does not explain their exceptional cost, said Dr. de Havenon. “The high cost of brand-name drugs in this speciality is likely because the market bears it,” he added. “In other words, MS is a disabling disease and the medications work, so historically the Centers for Medicare & Medicaid Services have been willing to tolerate the high cost of these primarily brand-name medications.”
Several countries have controlled drug costs by negotiating with pharmaceutical companies and through legislation, Dr. de Havenon noted.
“My intent with this article was to raise awareness on the topic, which I struggle with frequently as a clinician. I know I want my patients to have a medication, but the cost prevents it,” he said.
‘Unfettered’ price-setting
Commenting on the findings, Robert J. Fox, MD, vice chair for research at the Neurological Institute of the Cleveland Clinic, said the study “brings into clear light” what neurologists, particularly those who treat MS, have long suspected but did not really know. These neurologists “are typically distanced from the payment aspects of the medications they prescribe,” said Dr. Fox, who was not involved with the research.
Although a particular strength of the study was its comprehensiveness, the researchers excluded infusion claims – which account for a large portion of total patient care costs for many disorders, he noted.
Drugs for MS historically have been expensive, ostensibly because of their high cost of development. In addition, the large and continued price increase that occurs long after these drugs have been approved remains unexplained, said Dr. Fox.
He noted that the study findings might not directly affect clinical practice because neurologists will continue prescribing medications they think are best for their patients. “Instead, I think this is a lesson to lawmakers about the massive error in the Medicare Modernization Act of 2003, where the federal government was prohibited from negotiating drug prices. If the seller is unfettered in setting a price, then no one should be surprised when the price rises,” Dr. Fox said.
Because many new drugs and new generic formulations for treating MS have become available during the past year, “repeating these types of economic studies for the period 2020-2025 will help us understand if generic competition – as well as new laws if they are passed – alter price,” he concluded.
The study was funded by the American Academy of Neurology, which publishes Neurology. Dr. de Havenon has received clinical research funding from AMAG Pharmaceuticals and Regeneron Pharmaceuticals. Dr. Fox receives consulting fees from many pharmaceutical companies involved in the development of therapies for MS.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
EEG data may help aid diagnosis, treatment of focal epilepsy
, new research suggests. Findings from a large longitudinal study show that seizure onset in patients with focal epilepsy follows circadian, multiday, and annual cycles.
“Although daily and multiday rhythms have previously been identified, the extent to which these nonrandom rhythms exist in a larger cohort has been unclear,” said study investigator Joline Marie Fan, MD, a clinical fellow at the University of California, San Francisco. “This means that a patient with epilepsy may have a unique combination of seizure rhythms that can inform the days and timing of his or her highest seizure risk,” she added.
The study was published online Feb. 8 in JAMA Neurology.
Distinct chronotypes
Clinicians and patients alike have long observed cyclical patterns in the onset of epileptic seizures. However, such patterns have rarely been measured in a quantitative way.
Previous studies have examined seizure cycles using inpatient seizure monitoring and patients’ seizure diaries, but the duration of these recordings and their accuracy have been limited. Within the past decade, the advent of cEEG has allowed researchers to observe the cyclical pattern of interictal epileptiform activity, but the numbers of patients involved in such studies have been limited.
To investigate seizure chronotypes in greater detail, the researchers examined retrospective data for 222 adults with medically refractory focal epilepsy who took part in clinical trials of the NeuroPace responsive neurostimulation (RNS) system.
After implantation in the brain, this system monitors the seizure focus or foci continuously and delivers stimulation to stop seizures. Participants also kept seizure diaries and classified their seizures as simple motor, simple other, complex partial, and generalized tonic-clonic.
Dr. Fan’s group examined three subpopulations of patients to investigate three durations of seizure cycles. They examined self-reported disabling seizures, electrographic seizures, and interictal epileptiform activity. Because patients did not record the time of their disabling seizures, the investigators examined them only in multidien and circannual cycles.
To examine circannual seizure cycles, the investigators included 194 patients who kept continuous seizure diaries for 2 or more years and who reported 24 or more days in which disabling seizures occurred.
To examine multidien seizure cycles, they included 186 participants who reported 24 or more days with disabling seizures over a period of 6 or more months during which the RNS system collected cEEG data. They included 85 patients who had 48 hours or more in which electrographic seizure counts were above zero during 6 or more months of cEEG data collection to examine circadian seizure cycles.
Phase-locking value (PLV) was used to determine the strength of a cycle (i.e., the degree of consistency with which seizures occur during certain phases of a cycle). A PLV of 0 represents a uniform distribution of events during various phases of a cycle; a PLV of 1 indicates that all events occur exactly at the same phase of a cycle.
The population’s median age was 35 years, and the sample included approximately equal numbers of men and women. Patients’ focal epilepsies included mesiotemporal (57.2%), frontal (14.0%), neocortical-temporal (9.9%), parietal (4.1%), occipital (1.4%), and multifocal (13.5%). The data included 1,118 patient-years of cEEG, 754,108 electrographic seizures, and 313,995 self-reported seizures.
The prevalence of statistically significant circannual seizure cycles in this population was 12%. The prevalence of multidien seizure cycles was 60%, and the prevalence of circadian seizure cycles was 89%. Multidien cycles (mean PLV, 0.34) and circadian cycles (mean PLV, 0.34) were stronger than were circannual cycles (mean PLV, 0.17).
Among patients with circannual seizure cycles, there was a weak to moderate tendency for seizures to occur during one of the four seasons. There was no overall trend toward seizure onset in one season among this group.
Among patients with multidien seizure cycles, investigators identified five patterns of interictal epileptiform activity fluctuations. One pattern had irregular periodicity, and the others reached peak periodicity at 7, 15, 20, and 30 days. For some patients, one or more periodicities occurred. For most patients, electrographic or self-reported seizures tended to occur on the rising phase of the interictal epileptiform activity cycle. Interictal epileptiform activity increased on days around seizures.
Results showed there were five main seizure peak times among patients with circadian seizure cycles: midnight, 3:00 a.m., 9:00 a.m., 2:00 p.m., and 6:00 p.m. These findings corroborate the observations of previous investigations, the researchers noted. Hourly interictal epileptiform activity peaked during the night, regardless of peak seizure time.
“Although the neurostimulation device offers us a unique opportunity to investigate electrographic seizure activity quantitatively, the generalizability of our study is limited to the patient cohort that we studied,” said Dr. Fan. “The study findings are limited to patients with neurostimulation devices used for intractable focal epilepsies.”
The results support patients’ impressions that their seizures occur in a cyclical pattern.
“Ultimately, these findings will be helpful for developing models to aid with seizure forecasting and prediction in order to help reduce the uncertainty of seizure timing for patients with epilepsy,” said Dr. Fan.
“Other implications include optimizing the timing for patients to be admitted into the hospital for seizure characterization based on their seizure chronotype, or possibly tailoring a medication regimen in accordance with a patient’s seizure cycles,” she added.
Need for more research
Commenting on the findings, Tobias Loddenkemper, MD, professor of neurology at Harvard Medical School, Boston, noted that the study is “one of the largest longitudinal seizure pattern analyses, based on the gold standard of intracranially recorded epileptic seizures.”
The research, he added, extends neurologists’ understanding of seizure patterns over time, expands knowledge about seizure chronotypes, and emphasizes a relationship between interictal epileptiform activity and seizures.
The strengths of the study include the recording of seizures with intracranial EEG, its large number of participants, and the long duration of recordings, Dr. Loddenkemper said.
However, he said, it is important to note that self-reports are not always reliable. The results may also reflect the influence of potential confounders of seizure patterns, such as seizure triggers, treatment, stimulation, or sleep-wake, circadian, or hormonal cycles, he added.
“In the short term, validation studies, as well as confirmatory studies with less invasive sensors, may be needed,” said Dr. Loddenkemper.
“This could potentially include a trial that confirms findings prospectively, utilizing results from video EEG monitoring admissions. In the long term, seizure detection and prediction, as well as interventional chronotherapeutic trials, may be enabled, predicting seizures in individual patients and treating at times of greatest seizure susceptibility.”
The study was supported by grants to some of the authors from the Wyss Center for Bio and Neuroengineering, the Ernest Gallo Foundation, the Swiss National Science Foundation, and the Velux Stiftung. Dr. Fan has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Findings from a large longitudinal study show that seizure onset in patients with focal epilepsy follows circadian, multiday, and annual cycles.
“Although daily and multiday rhythms have previously been identified, the extent to which these nonrandom rhythms exist in a larger cohort has been unclear,” said study investigator Joline Marie Fan, MD, a clinical fellow at the University of California, San Francisco. “This means that a patient with epilepsy may have a unique combination of seizure rhythms that can inform the days and timing of his or her highest seizure risk,” she added.
The study was published online Feb. 8 in JAMA Neurology.
Distinct chronotypes
Clinicians and patients alike have long observed cyclical patterns in the onset of epileptic seizures. However, such patterns have rarely been measured in a quantitative way.
Previous studies have examined seizure cycles using inpatient seizure monitoring and patients’ seizure diaries, but the duration of these recordings and their accuracy have been limited. Within the past decade, the advent of cEEG has allowed researchers to observe the cyclical pattern of interictal epileptiform activity, but the numbers of patients involved in such studies have been limited.
To investigate seizure chronotypes in greater detail, the researchers examined retrospective data for 222 adults with medically refractory focal epilepsy who took part in clinical trials of the NeuroPace responsive neurostimulation (RNS) system.
After implantation in the brain, this system monitors the seizure focus or foci continuously and delivers stimulation to stop seizures. Participants also kept seizure diaries and classified their seizures as simple motor, simple other, complex partial, and generalized tonic-clonic.
Dr. Fan’s group examined three subpopulations of patients to investigate three durations of seizure cycles. They examined self-reported disabling seizures, electrographic seizures, and interictal epileptiform activity. Because patients did not record the time of their disabling seizures, the investigators examined them only in multidien and circannual cycles.
To examine circannual seizure cycles, the investigators included 194 patients who kept continuous seizure diaries for 2 or more years and who reported 24 or more days in which disabling seizures occurred.
To examine multidien seizure cycles, they included 186 participants who reported 24 or more days with disabling seizures over a period of 6 or more months during which the RNS system collected cEEG data. They included 85 patients who had 48 hours or more in which electrographic seizure counts were above zero during 6 or more months of cEEG data collection to examine circadian seizure cycles.
Phase-locking value (PLV) was used to determine the strength of a cycle (i.e., the degree of consistency with which seizures occur during certain phases of a cycle). A PLV of 0 represents a uniform distribution of events during various phases of a cycle; a PLV of 1 indicates that all events occur exactly at the same phase of a cycle.
The population’s median age was 35 years, and the sample included approximately equal numbers of men and women. Patients’ focal epilepsies included mesiotemporal (57.2%), frontal (14.0%), neocortical-temporal (9.9%), parietal (4.1%), occipital (1.4%), and multifocal (13.5%). The data included 1,118 patient-years of cEEG, 754,108 electrographic seizures, and 313,995 self-reported seizures.
The prevalence of statistically significant circannual seizure cycles in this population was 12%. The prevalence of multidien seizure cycles was 60%, and the prevalence of circadian seizure cycles was 89%. Multidien cycles (mean PLV, 0.34) and circadian cycles (mean PLV, 0.34) were stronger than were circannual cycles (mean PLV, 0.17).
Among patients with circannual seizure cycles, there was a weak to moderate tendency for seizures to occur during one of the four seasons. There was no overall trend toward seizure onset in one season among this group.
Among patients with multidien seizure cycles, investigators identified five patterns of interictal epileptiform activity fluctuations. One pattern had irregular periodicity, and the others reached peak periodicity at 7, 15, 20, and 30 days. For some patients, one or more periodicities occurred. For most patients, electrographic or self-reported seizures tended to occur on the rising phase of the interictal epileptiform activity cycle. Interictal epileptiform activity increased on days around seizures.
Results showed there were five main seizure peak times among patients with circadian seizure cycles: midnight, 3:00 a.m., 9:00 a.m., 2:00 p.m., and 6:00 p.m. These findings corroborate the observations of previous investigations, the researchers noted. Hourly interictal epileptiform activity peaked during the night, regardless of peak seizure time.
“Although the neurostimulation device offers us a unique opportunity to investigate electrographic seizure activity quantitatively, the generalizability of our study is limited to the patient cohort that we studied,” said Dr. Fan. “The study findings are limited to patients with neurostimulation devices used for intractable focal epilepsies.”
The results support patients’ impressions that their seizures occur in a cyclical pattern.
“Ultimately, these findings will be helpful for developing models to aid with seizure forecasting and prediction in order to help reduce the uncertainty of seizure timing for patients with epilepsy,” said Dr. Fan.
“Other implications include optimizing the timing for patients to be admitted into the hospital for seizure characterization based on their seizure chronotype, or possibly tailoring a medication regimen in accordance with a patient’s seizure cycles,” she added.
Need for more research
Commenting on the findings, Tobias Loddenkemper, MD, professor of neurology at Harvard Medical School, Boston, noted that the study is “one of the largest longitudinal seizure pattern analyses, based on the gold standard of intracranially recorded epileptic seizures.”
The research, he added, extends neurologists’ understanding of seizure patterns over time, expands knowledge about seizure chronotypes, and emphasizes a relationship between interictal epileptiform activity and seizures.
The strengths of the study include the recording of seizures with intracranial EEG, its large number of participants, and the long duration of recordings, Dr. Loddenkemper said.
However, he said, it is important to note that self-reports are not always reliable. The results may also reflect the influence of potential confounders of seizure patterns, such as seizure triggers, treatment, stimulation, or sleep-wake, circadian, or hormonal cycles, he added.
“In the short term, validation studies, as well as confirmatory studies with less invasive sensors, may be needed,” said Dr. Loddenkemper.
“This could potentially include a trial that confirms findings prospectively, utilizing results from video EEG monitoring admissions. In the long term, seizure detection and prediction, as well as interventional chronotherapeutic trials, may be enabled, predicting seizures in individual patients and treating at times of greatest seizure susceptibility.”
The study was supported by grants to some of the authors from the Wyss Center for Bio and Neuroengineering, the Ernest Gallo Foundation, the Swiss National Science Foundation, and the Velux Stiftung. Dr. Fan has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Findings from a large longitudinal study show that seizure onset in patients with focal epilepsy follows circadian, multiday, and annual cycles.
“Although daily and multiday rhythms have previously been identified, the extent to which these nonrandom rhythms exist in a larger cohort has been unclear,” said study investigator Joline Marie Fan, MD, a clinical fellow at the University of California, San Francisco. “This means that a patient with epilepsy may have a unique combination of seizure rhythms that can inform the days and timing of his or her highest seizure risk,” she added.
The study was published online Feb. 8 in JAMA Neurology.
Distinct chronotypes
Clinicians and patients alike have long observed cyclical patterns in the onset of epileptic seizures. However, such patterns have rarely been measured in a quantitative way.
Previous studies have examined seizure cycles using inpatient seizure monitoring and patients’ seizure diaries, but the duration of these recordings and their accuracy have been limited. Within the past decade, the advent of cEEG has allowed researchers to observe the cyclical pattern of interictal epileptiform activity, but the numbers of patients involved in such studies have been limited.
To investigate seizure chronotypes in greater detail, the researchers examined retrospective data for 222 adults with medically refractory focal epilepsy who took part in clinical trials of the NeuroPace responsive neurostimulation (RNS) system.
After implantation in the brain, this system monitors the seizure focus or foci continuously and delivers stimulation to stop seizures. Participants also kept seizure diaries and classified their seizures as simple motor, simple other, complex partial, and generalized tonic-clonic.
Dr. Fan’s group examined three subpopulations of patients to investigate three durations of seizure cycles. They examined self-reported disabling seizures, electrographic seizures, and interictal epileptiform activity. Because patients did not record the time of their disabling seizures, the investigators examined them only in multidien and circannual cycles.
To examine circannual seizure cycles, the investigators included 194 patients who kept continuous seizure diaries for 2 or more years and who reported 24 or more days in which disabling seizures occurred.
To examine multidien seizure cycles, they included 186 participants who reported 24 or more days with disabling seizures over a period of 6 or more months during which the RNS system collected cEEG data. They included 85 patients who had 48 hours or more in which electrographic seizure counts were above zero during 6 or more months of cEEG data collection to examine circadian seizure cycles.
Phase-locking value (PLV) was used to determine the strength of a cycle (i.e., the degree of consistency with which seizures occur during certain phases of a cycle). A PLV of 0 represents a uniform distribution of events during various phases of a cycle; a PLV of 1 indicates that all events occur exactly at the same phase of a cycle.
The population’s median age was 35 years, and the sample included approximately equal numbers of men and women. Patients’ focal epilepsies included mesiotemporal (57.2%), frontal (14.0%), neocortical-temporal (9.9%), parietal (4.1%), occipital (1.4%), and multifocal (13.5%). The data included 1,118 patient-years of cEEG, 754,108 electrographic seizures, and 313,995 self-reported seizures.
The prevalence of statistically significant circannual seizure cycles in this population was 12%. The prevalence of multidien seizure cycles was 60%, and the prevalence of circadian seizure cycles was 89%. Multidien cycles (mean PLV, 0.34) and circadian cycles (mean PLV, 0.34) were stronger than were circannual cycles (mean PLV, 0.17).
Among patients with circannual seizure cycles, there was a weak to moderate tendency for seizures to occur during one of the four seasons. There was no overall trend toward seizure onset in one season among this group.
Among patients with multidien seizure cycles, investigators identified five patterns of interictal epileptiform activity fluctuations. One pattern had irregular periodicity, and the others reached peak periodicity at 7, 15, 20, and 30 days. For some patients, one or more periodicities occurred. For most patients, electrographic or self-reported seizures tended to occur on the rising phase of the interictal epileptiform activity cycle. Interictal epileptiform activity increased on days around seizures.
Results showed there were five main seizure peak times among patients with circadian seizure cycles: midnight, 3:00 a.m., 9:00 a.m., 2:00 p.m., and 6:00 p.m. These findings corroborate the observations of previous investigations, the researchers noted. Hourly interictal epileptiform activity peaked during the night, regardless of peak seizure time.
“Although the neurostimulation device offers us a unique opportunity to investigate electrographic seizure activity quantitatively, the generalizability of our study is limited to the patient cohort that we studied,” said Dr. Fan. “The study findings are limited to patients with neurostimulation devices used for intractable focal epilepsies.”
The results support patients’ impressions that their seizures occur in a cyclical pattern.
“Ultimately, these findings will be helpful for developing models to aid with seizure forecasting and prediction in order to help reduce the uncertainty of seizure timing for patients with epilepsy,” said Dr. Fan.
“Other implications include optimizing the timing for patients to be admitted into the hospital for seizure characterization based on their seizure chronotype, or possibly tailoring a medication regimen in accordance with a patient’s seizure cycles,” she added.
Need for more research
Commenting on the findings, Tobias Loddenkemper, MD, professor of neurology at Harvard Medical School, Boston, noted that the study is “one of the largest longitudinal seizure pattern analyses, based on the gold standard of intracranially recorded epileptic seizures.”
The research, he added, extends neurologists’ understanding of seizure patterns over time, expands knowledge about seizure chronotypes, and emphasizes a relationship between interictal epileptiform activity and seizures.
The strengths of the study include the recording of seizures with intracranial EEG, its large number of participants, and the long duration of recordings, Dr. Loddenkemper said.
However, he said, it is important to note that self-reports are not always reliable. The results may also reflect the influence of potential confounders of seizure patterns, such as seizure triggers, treatment, stimulation, or sleep-wake, circadian, or hormonal cycles, he added.
“In the short term, validation studies, as well as confirmatory studies with less invasive sensors, may be needed,” said Dr. Loddenkemper.
“This could potentially include a trial that confirms findings prospectively, utilizing results from video EEG monitoring admissions. In the long term, seizure detection and prediction, as well as interventional chronotherapeutic trials, may be enabled, predicting seizures in individual patients and treating at times of greatest seizure susceptibility.”
The study was supported by grants to some of the authors from the Wyss Center for Bio and Neuroengineering, the Ernest Gallo Foundation, the Swiss National Science Foundation, and the Velux Stiftung. Dr. Fan has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Physicians react: Doctors worry about patients reading their clinical notes
Patients will soon be able to read the notes that physicians make during an episode of care, as well as information about diagnostic testing and imaging results, tests for STDs, fetal ultrasounds, and cancer biopsies. This open access is raising concerns among physicians.
As part of the 21st Century Cures Act, patients have the right to see their medical notes. Known as Open Notes, the policy will go into effect on April 5, 2021. The Department of Health & Human Services recently changed the original start date, which was to be Nov. 2, 2020.
The mandate has some physicians worrying about potential legal risks and possible violation of doctor-patient confidentiality. When asked to share their views on the new Open Notes mandate, many physicians expressed their concerns but also cited some of the positive effects that could come from this.
Potentially more legal woes for physicians?
A key concern raised by one physician commenter is that patients could misunderstand legitimate medical terminology or even put a physician in legal crosshairs. For example, a medical term such as “spontaneous abortion” could be misconstrued by patients. A physician might write notes with the idea that a patient is reading them and thus might alter those notes in a way that creates legal trouble.
“This layers another level of censorship and legal liability onto physicians, who in attempting to be [politically correct], may omit critical information or have to use euphemisms in order to avoid conflict,” one physician said.
She also questioned whether notes might now have to be run through legal counsel before being posted to avoid potential liability.
Another doctor questioned how physicians would be able to document patients suspected of faking injuries for pain medication, for example. Could such documentation lead to lawsuits for the doctor?
As one physician noted, some patients “are drug seekers. Some refuse to aid in their own care. Some are malingerers. Not documenting that is bad medicine.”
The possibility of violating doctor-patient confidentiality laws, particularly for teenagers, could be another negative effect of Open Notes, said one physician.
“Won’t this violate the statutes that teenagers have the right to confidential evaluations?” the commenter mused. “If charts are to be immediately available, then STDs and pregnancies they weren’t ready to talk about will now be suddenly known by their parents.”
One doctor has already faced this issue. “I already ran into this problem once,” he noted. “Now I warn those on their parents’ insurance before I start the visit. I have literally had a patient state, ‘well then we are done,’ and leave without being seen due to it.”
Another physician questioned the possibility of having to write notes differently than they do now, especially if the patients have lower reading comprehension abilities.
One physician who uses Open Notes said he receives patient requests for changes that have little to do with the actual diagnosis and relate to ancillary issues. He highlighted patients who “don’t want psych diagnosis in their chart or are concerned a diagnosis will raise their insurance premium, so they ask me to delete it.”
Will Open Notes erode patient communication?
One physician questioned whether it would lead to patients being less open and forthcoming about their medical concerns with doctors.
“The main problem I see is the patient not telling me the whole story, or worse, telling me the story, and then asking me not to document it (as many have done in the past) because they don’t want their spouse, family, etc. to read the notes and they have already given their permission for them to do so, for a variety of reasons,” he commented. “This includes topics of STDs, infidelity, depression, suicidal thoughts, and other symptoms the patient doesn’t want their family to read about.”
Some physicians envision positive developments
Many physicians are unconcerned by the new mandate. “I see some potential good in this, such as improving doctor-patient communication and more scrupulous charting,” one physician said.
A doctor working in the U.S. federal health care system noted that open access has been a part of that system for decades.
“Since health care providers work in this unveiled setting for their entire career, they usually know how to write appropriate clinical notes and what information needs to be included in them,” he wrote. “Now it’s time for the rest of the medical community to catch up to a reality that we have worked within for decades now.
“The world did not end, malpractice complaints did not increase, and physician/patient relationships were not damaged. Living in the information age, archaic practices like private notes were surely going to end at some point.”
One doctor who has been using Open Notes has had experiences in which the patient noted an error in the medical chart that needed correcting. “I have had one patient correct me on a timeline in the HPI which was helpful and I made the requested correction in that instance,” he said.
Another physician agreed. “I’ve had patients add or correct valuable information I’ve missed. Good probably outweighs the bad if we set limits on behaviors expressed by the personality disordered group. The majority of people don’t seem to care and still ask me ‘what would you do’ or ‘tell me what to do.’ It’s all about patient/physician trust.”
Another talked about how Open Notes should have little or no impact. “Here’s a novel concept – talking to our patients,” he commented. “There is nothing in every one of my chart notes that has not already been discussed with my patients and I dictate (speech to text) my findings and plan in front of them. So, if they are reviewing my office notes, it will only serve to reinforce what we have already discussed.”
“I don’t intend to change anything,” he added. “Chances are if they were to see a test result before I have a chance to discuss it with them, they will have already ‘Googled’ its meaning and we can have more meaningful interaction if they have a basic understanding of the test.”
“I understand that this is anxiety provoking, but in general I think it is appropriate for patients to have access to their notes,” said another physician. “If physicians write lousy notes that say they did things they didn’t do, that fail to actually state a diagnosis and a plan (and they often do), that is the doc’s problem, not the patient’s.”
A version of this article first appeared on Medscape.com.
Patients will soon be able to read the notes that physicians make during an episode of care, as well as information about diagnostic testing and imaging results, tests for STDs, fetal ultrasounds, and cancer biopsies. This open access is raising concerns among physicians.
As part of the 21st Century Cures Act, patients have the right to see their medical notes. Known as Open Notes, the policy will go into effect on April 5, 2021. The Department of Health & Human Services recently changed the original start date, which was to be Nov. 2, 2020.
The mandate has some physicians worrying about potential legal risks and possible violation of doctor-patient confidentiality. When asked to share their views on the new Open Notes mandate, many physicians expressed their concerns but also cited some of the positive effects that could come from this.
Potentially more legal woes for physicians?
A key concern raised by one physician commenter is that patients could misunderstand legitimate medical terminology or even put a physician in legal crosshairs. For example, a medical term such as “spontaneous abortion” could be misconstrued by patients. A physician might write notes with the idea that a patient is reading them and thus might alter those notes in a way that creates legal trouble.
“This layers another level of censorship and legal liability onto physicians, who in attempting to be [politically correct], may omit critical information or have to use euphemisms in order to avoid conflict,” one physician said.
She also questioned whether notes might now have to be run through legal counsel before being posted to avoid potential liability.
Another doctor questioned how physicians would be able to document patients suspected of faking injuries for pain medication, for example. Could such documentation lead to lawsuits for the doctor?
As one physician noted, some patients “are drug seekers. Some refuse to aid in their own care. Some are malingerers. Not documenting that is bad medicine.”
The possibility of violating doctor-patient confidentiality laws, particularly for teenagers, could be another negative effect of Open Notes, said one physician.
“Won’t this violate the statutes that teenagers have the right to confidential evaluations?” the commenter mused. “If charts are to be immediately available, then STDs and pregnancies they weren’t ready to talk about will now be suddenly known by their parents.”
One doctor has already faced this issue. “I already ran into this problem once,” he noted. “Now I warn those on their parents’ insurance before I start the visit. I have literally had a patient state, ‘well then we are done,’ and leave without being seen due to it.”
Another physician questioned the possibility of having to write notes differently than they do now, especially if the patients have lower reading comprehension abilities.
One physician who uses Open Notes said he receives patient requests for changes that have little to do with the actual diagnosis and relate to ancillary issues. He highlighted patients who “don’t want psych diagnosis in their chart or are concerned a diagnosis will raise their insurance premium, so they ask me to delete it.”
Will Open Notes erode patient communication?
One physician questioned whether it would lead to patients being less open and forthcoming about their medical concerns with doctors.
“The main problem I see is the patient not telling me the whole story, or worse, telling me the story, and then asking me not to document it (as many have done in the past) because they don’t want their spouse, family, etc. to read the notes and they have already given their permission for them to do so, for a variety of reasons,” he commented. “This includes topics of STDs, infidelity, depression, suicidal thoughts, and other symptoms the patient doesn’t want their family to read about.”
Some physicians envision positive developments
Many physicians are unconcerned by the new mandate. “I see some potential good in this, such as improving doctor-patient communication and more scrupulous charting,” one physician said.
A doctor working in the U.S. federal health care system noted that open access has been a part of that system for decades.
“Since health care providers work in this unveiled setting for their entire career, they usually know how to write appropriate clinical notes and what information needs to be included in them,” he wrote. “Now it’s time for the rest of the medical community to catch up to a reality that we have worked within for decades now.
“The world did not end, malpractice complaints did not increase, and physician/patient relationships were not damaged. Living in the information age, archaic practices like private notes were surely going to end at some point.”
One doctor who has been using Open Notes has had experiences in which the patient noted an error in the medical chart that needed correcting. “I have had one patient correct me on a timeline in the HPI which was helpful and I made the requested correction in that instance,” he said.
Another physician agreed. “I’ve had patients add or correct valuable information I’ve missed. Good probably outweighs the bad if we set limits on behaviors expressed by the personality disordered group. The majority of people don’t seem to care and still ask me ‘what would you do’ or ‘tell me what to do.’ It’s all about patient/physician trust.”
Another talked about how Open Notes should have little or no impact. “Here’s a novel concept – talking to our patients,” he commented. “There is nothing in every one of my chart notes that has not already been discussed with my patients and I dictate (speech to text) my findings and plan in front of them. So, if they are reviewing my office notes, it will only serve to reinforce what we have already discussed.”
“I don’t intend to change anything,” he added. “Chances are if they were to see a test result before I have a chance to discuss it with them, they will have already ‘Googled’ its meaning and we can have more meaningful interaction if they have a basic understanding of the test.”
“I understand that this is anxiety provoking, but in general I think it is appropriate for patients to have access to their notes,” said another physician. “If physicians write lousy notes that say they did things they didn’t do, that fail to actually state a diagnosis and a plan (and they often do), that is the doc’s problem, not the patient’s.”
A version of this article first appeared on Medscape.com.
Patients will soon be able to read the notes that physicians make during an episode of care, as well as information about diagnostic testing and imaging results, tests for STDs, fetal ultrasounds, and cancer biopsies. This open access is raising concerns among physicians.
As part of the 21st Century Cures Act, patients have the right to see their medical notes. Known as Open Notes, the policy will go into effect on April 5, 2021. The Department of Health & Human Services recently changed the original start date, which was to be Nov. 2, 2020.
The mandate has some physicians worrying about potential legal risks and possible violation of doctor-patient confidentiality. When asked to share their views on the new Open Notes mandate, many physicians expressed their concerns but also cited some of the positive effects that could come from this.
Potentially more legal woes for physicians?
A key concern raised by one physician commenter is that patients could misunderstand legitimate medical terminology or even put a physician in legal crosshairs. For example, a medical term such as “spontaneous abortion” could be misconstrued by patients. A physician might write notes with the idea that a patient is reading them and thus might alter those notes in a way that creates legal trouble.
“This layers another level of censorship and legal liability onto physicians, who in attempting to be [politically correct], may omit critical information or have to use euphemisms in order to avoid conflict,” one physician said.
She also questioned whether notes might now have to be run through legal counsel before being posted to avoid potential liability.
Another doctor questioned how physicians would be able to document patients suspected of faking injuries for pain medication, for example. Could such documentation lead to lawsuits for the doctor?
As one physician noted, some patients “are drug seekers. Some refuse to aid in their own care. Some are malingerers. Not documenting that is bad medicine.”
The possibility of violating doctor-patient confidentiality laws, particularly for teenagers, could be another negative effect of Open Notes, said one physician.
“Won’t this violate the statutes that teenagers have the right to confidential evaluations?” the commenter mused. “If charts are to be immediately available, then STDs and pregnancies they weren’t ready to talk about will now be suddenly known by their parents.”
One doctor has already faced this issue. “I already ran into this problem once,” he noted. “Now I warn those on their parents’ insurance before I start the visit. I have literally had a patient state, ‘well then we are done,’ and leave without being seen due to it.”
Another physician questioned the possibility of having to write notes differently than they do now, especially if the patients have lower reading comprehension abilities.
One physician who uses Open Notes said he receives patient requests for changes that have little to do with the actual diagnosis and relate to ancillary issues. He highlighted patients who “don’t want psych diagnosis in their chart or are concerned a diagnosis will raise their insurance premium, so they ask me to delete it.”
Will Open Notes erode patient communication?
One physician questioned whether it would lead to patients being less open and forthcoming about their medical concerns with doctors.
“The main problem I see is the patient not telling me the whole story, or worse, telling me the story, and then asking me not to document it (as many have done in the past) because they don’t want their spouse, family, etc. to read the notes and they have already given their permission for them to do so, for a variety of reasons,” he commented. “This includes topics of STDs, infidelity, depression, suicidal thoughts, and other symptoms the patient doesn’t want their family to read about.”
Some physicians envision positive developments
Many physicians are unconcerned by the new mandate. “I see some potential good in this, such as improving doctor-patient communication and more scrupulous charting,” one physician said.
A doctor working in the U.S. federal health care system noted that open access has been a part of that system for decades.
“Since health care providers work in this unveiled setting for their entire career, they usually know how to write appropriate clinical notes and what information needs to be included in them,” he wrote. “Now it’s time for the rest of the medical community to catch up to a reality that we have worked within for decades now.
“The world did not end, malpractice complaints did not increase, and physician/patient relationships were not damaged. Living in the information age, archaic practices like private notes were surely going to end at some point.”
One doctor who has been using Open Notes has had experiences in which the patient noted an error in the medical chart that needed correcting. “I have had one patient correct me on a timeline in the HPI which was helpful and I made the requested correction in that instance,” he said.
Another physician agreed. “I’ve had patients add or correct valuable information I’ve missed. Good probably outweighs the bad if we set limits on behaviors expressed by the personality disordered group. The majority of people don’t seem to care and still ask me ‘what would you do’ or ‘tell me what to do.’ It’s all about patient/physician trust.”
Another talked about how Open Notes should have little or no impact. “Here’s a novel concept – talking to our patients,” he commented. “There is nothing in every one of my chart notes that has not already been discussed with my patients and I dictate (speech to text) my findings and plan in front of them. So, if they are reviewing my office notes, it will only serve to reinforce what we have already discussed.”
“I don’t intend to change anything,” he added. “Chances are if they were to see a test result before I have a chance to discuss it with them, they will have already ‘Googled’ its meaning and we can have more meaningful interaction if they have a basic understanding of the test.”
“I understand that this is anxiety provoking, but in general I think it is appropriate for patients to have access to their notes,” said another physician. “If physicians write lousy notes that say they did things they didn’t do, that fail to actually state a diagnosis and a plan (and they often do), that is the doc’s problem, not the patient’s.”
A version of this article first appeared on Medscape.com.

