User login
Aesthetics abound for the aging face
At the MedscapeLive’s Women’s and Pediatric Dermatology Seminar, Jacqueline Watchmaker, MD, a dermatologist in Scottsdale, Ariz., provided an overview of current options, along with advice on how to keep patients’ expectations realistic and how to properly choose the best candidates for the best procedures.
“One of the most common concerns patients come to me with are wrinkles on the upper face,” but this is far from their only concern, Dr. Watchmaker said. Wrinkles and sagging of the lower face, areas under the eyes, nasolabial folds, marionette lines, and the neck also draw concern. Uneven coloration is another common concern, she said.
“So, what can we do for all of this?” she asked. The options are plentiful. Wrinkles of the upper face are easy to address with neuromodulators, she said, and soft-tissue fillers help the jawline and cheek areas.
“For the lower face, skin tightening devices really shine,” she added. And lasers can help correct uneven coloration. Surgery, of course, can also produce good results, but many patients want to stick with noninvasive or minimally invasive procedures.
Case: 83-year-old woman
Dr. Watchmaker discussed an 83-year old patient, who had malar mounds and accentuation of the infraorbital hollowness resulting from changes in subcutaneous fat and ligament laxity. She also had uneven coloration from photo damage, wrinkles on the upper face, linear appearance of zygoma related to underlying bony changes and fat compartment descent, and nasolabial folds and jowls related to decreased bony compartments, ligament laxity, and shifting of fat. She was naive to any cosmetic procedure.
Despite her age, this patient had no wrinkling on the upper forehead. Dr. Watchmaker did not inject neuromodulator in the upper forehead, as this patient also had a slightly heavy eyelid. “If you inject too much, it can cause some drooping of the eyelid and eyebrow,” she said.
For filler, she used a combination of high G (firmness, support) hyaluronic acid filler, a medium G acid filler, and a low G filler. The result: The woman’s face became more balanced, the mid-face volumization lifted the lower face, and the glabellar and periocular lines were softer, although still present. “It’s important to counsel patients that neuromodulators won’t make the lines go away the first time, but they will be softened.”
Practice tips
It’s important to titrate neuromodulators to fit the patient, Dr. Watchmaker said. Ask: What are their goals: Reversal of static lines? Softening wrinkles? Maintaining current status? “There’s not one dosing regimen,” and both dosing and frequency of neuromodulators can be titrated to fit each patient’s aesthetic goals, she said. For older patients who want to soften or maintain appearance, she suggested treatment every 4-6 months. And some patients just want to maintain the status quo, she noted.
Ideal candidates
For neuromodulators and fillers, who is an ideal candidate? “I think it’s anyone who has realistic expectations,” she said. Patients need to know how many treatments are needed and how much it will cost. For patients with extensive wrinkling and sagging, she said, she does extensive counseling about what results to expect “because I don’t want them to feel like they wasted their time or their money.”
She also suggests a surgical consult, as some may opt for that route after learning about the options and expected results.
Skin tightening
Both radiofrequency and microfocused ultrasound are noninvasive and additional options. Radiofrequency uses radio waves, with electromagnetic energy to stimulate heat. Ultrasound uses ultrasound waves to stimulate heat. Both approaches cause collagen contraction, neocollagenesis, and skin tightening.
These procedures do well for the lower face, Dr. Watchmaker said, but “I am relatively unimpressed for how well they do for the upper face.” Ideal candidates have mild to moderate skin laxity and want to avoid surgery. She also tells patients that collagen isn’t made overnight. “You won’t see much for 3-6 months after.” The good news? Usually the treatments need to be repeated only every 1.5-2 years, she said.
Lasers
“There are so many lasers out there,” said Dr. Watchmaker, who groups them into three categories: those used for wrinkles, dyschromia, and erythema. Her picks: ablative lasers (CO2 and erbium) and erbium-doped YAG 1550 nm laser for rhytids. Thulium 1927 and QS and picosecond lasers are her picks for dyschromia, and for erythema, pulsed dye and KTP lasers.
Some laser treatments are not a “walk in the park,” as she warns patients. For example, after treatment with ablative lasers, there is pain, post-procedure redness, and crusting.
Take-home points
A combination of noninvasive and minimally invasive procedures can produce appearance-improving results. That’s more likely if dermatologists choose ideal candidates, personalize the treatment, and set realistic expectations. “We have a finite number of tools,” she said, but they can be used in a variety of ways.
At the interactive panel discussion following her presentation, Dr. Watchmaker was asked what she tells patients about sun protection. “I talk a lot about sunscreens,’’ she said, always urging patients to use them. While the options for rejuvenation are numerous, taking care of the skin is still crucial.
Dr. Watchmaker had no disclosures. MedscapeLive and this news organization are owned by the same parent company.
At the MedscapeLive’s Women’s and Pediatric Dermatology Seminar, Jacqueline Watchmaker, MD, a dermatologist in Scottsdale, Ariz., provided an overview of current options, along with advice on how to keep patients’ expectations realistic and how to properly choose the best candidates for the best procedures.
“One of the most common concerns patients come to me with are wrinkles on the upper face,” but this is far from their only concern, Dr. Watchmaker said. Wrinkles and sagging of the lower face, areas under the eyes, nasolabial folds, marionette lines, and the neck also draw concern. Uneven coloration is another common concern, she said.
“So, what can we do for all of this?” she asked. The options are plentiful. Wrinkles of the upper face are easy to address with neuromodulators, she said, and soft-tissue fillers help the jawline and cheek areas.
“For the lower face, skin tightening devices really shine,” she added. And lasers can help correct uneven coloration. Surgery, of course, can also produce good results, but many patients want to stick with noninvasive or minimally invasive procedures.
Case: 83-year-old woman
Dr. Watchmaker discussed an 83-year old patient, who had malar mounds and accentuation of the infraorbital hollowness resulting from changes in subcutaneous fat and ligament laxity. She also had uneven coloration from photo damage, wrinkles on the upper face, linear appearance of zygoma related to underlying bony changes and fat compartment descent, and nasolabial folds and jowls related to decreased bony compartments, ligament laxity, and shifting of fat. She was naive to any cosmetic procedure.
Despite her age, this patient had no wrinkling on the upper forehead. Dr. Watchmaker did not inject neuromodulator in the upper forehead, as this patient also had a slightly heavy eyelid. “If you inject too much, it can cause some drooping of the eyelid and eyebrow,” she said.
For filler, she used a combination of high G (firmness, support) hyaluronic acid filler, a medium G acid filler, and a low G filler. The result: The woman’s face became more balanced, the mid-face volumization lifted the lower face, and the glabellar and periocular lines were softer, although still present. “It’s important to counsel patients that neuromodulators won’t make the lines go away the first time, but they will be softened.”
Practice tips
It’s important to titrate neuromodulators to fit the patient, Dr. Watchmaker said. Ask: What are their goals: Reversal of static lines? Softening wrinkles? Maintaining current status? “There’s not one dosing regimen,” and both dosing and frequency of neuromodulators can be titrated to fit each patient’s aesthetic goals, she said. For older patients who want to soften or maintain appearance, she suggested treatment every 4-6 months. And some patients just want to maintain the status quo, she noted.
Ideal candidates
For neuromodulators and fillers, who is an ideal candidate? “I think it’s anyone who has realistic expectations,” she said. Patients need to know how many treatments are needed and how much it will cost. For patients with extensive wrinkling and sagging, she said, she does extensive counseling about what results to expect “because I don’t want them to feel like they wasted their time or their money.”
She also suggests a surgical consult, as some may opt for that route after learning about the options and expected results.
Skin tightening
Both radiofrequency and microfocused ultrasound are noninvasive and additional options. Radiofrequency uses radio waves, with electromagnetic energy to stimulate heat. Ultrasound uses ultrasound waves to stimulate heat. Both approaches cause collagen contraction, neocollagenesis, and skin tightening.
These procedures do well for the lower face, Dr. Watchmaker said, but “I am relatively unimpressed for how well they do for the upper face.” Ideal candidates have mild to moderate skin laxity and want to avoid surgery. She also tells patients that collagen isn’t made overnight. “You won’t see much for 3-6 months after.” The good news? Usually the treatments need to be repeated only every 1.5-2 years, she said.
Lasers
“There are so many lasers out there,” said Dr. Watchmaker, who groups them into three categories: those used for wrinkles, dyschromia, and erythema. Her picks: ablative lasers (CO2 and erbium) and erbium-doped YAG 1550 nm laser for rhytids. Thulium 1927 and QS and picosecond lasers are her picks for dyschromia, and for erythema, pulsed dye and KTP lasers.
Some laser treatments are not a “walk in the park,” as she warns patients. For example, after treatment with ablative lasers, there is pain, post-procedure redness, and crusting.
Take-home points
A combination of noninvasive and minimally invasive procedures can produce appearance-improving results. That’s more likely if dermatologists choose ideal candidates, personalize the treatment, and set realistic expectations. “We have a finite number of tools,” she said, but they can be used in a variety of ways.
At the interactive panel discussion following her presentation, Dr. Watchmaker was asked what she tells patients about sun protection. “I talk a lot about sunscreens,’’ she said, always urging patients to use them. While the options for rejuvenation are numerous, taking care of the skin is still crucial.
Dr. Watchmaker had no disclosures. MedscapeLive and this news organization are owned by the same parent company.
At the MedscapeLive’s Women’s and Pediatric Dermatology Seminar, Jacqueline Watchmaker, MD, a dermatologist in Scottsdale, Ariz., provided an overview of current options, along with advice on how to keep patients’ expectations realistic and how to properly choose the best candidates for the best procedures.
“One of the most common concerns patients come to me with are wrinkles on the upper face,” but this is far from their only concern, Dr. Watchmaker said. Wrinkles and sagging of the lower face, areas under the eyes, nasolabial folds, marionette lines, and the neck also draw concern. Uneven coloration is another common concern, she said.
“So, what can we do for all of this?” she asked. The options are plentiful. Wrinkles of the upper face are easy to address with neuromodulators, she said, and soft-tissue fillers help the jawline and cheek areas.
“For the lower face, skin tightening devices really shine,” she added. And lasers can help correct uneven coloration. Surgery, of course, can also produce good results, but many patients want to stick with noninvasive or minimally invasive procedures.
Case: 83-year-old woman
Dr. Watchmaker discussed an 83-year old patient, who had malar mounds and accentuation of the infraorbital hollowness resulting from changes in subcutaneous fat and ligament laxity. She also had uneven coloration from photo damage, wrinkles on the upper face, linear appearance of zygoma related to underlying bony changes and fat compartment descent, and nasolabial folds and jowls related to decreased bony compartments, ligament laxity, and shifting of fat. She was naive to any cosmetic procedure.
Despite her age, this patient had no wrinkling on the upper forehead. Dr. Watchmaker did not inject neuromodulator in the upper forehead, as this patient also had a slightly heavy eyelid. “If you inject too much, it can cause some drooping of the eyelid and eyebrow,” she said.
For filler, she used a combination of high G (firmness, support) hyaluronic acid filler, a medium G acid filler, and a low G filler. The result: The woman’s face became more balanced, the mid-face volumization lifted the lower face, and the glabellar and periocular lines were softer, although still present. “It’s important to counsel patients that neuromodulators won’t make the lines go away the first time, but they will be softened.”
Practice tips
It’s important to titrate neuromodulators to fit the patient, Dr. Watchmaker said. Ask: What are their goals: Reversal of static lines? Softening wrinkles? Maintaining current status? “There’s not one dosing regimen,” and both dosing and frequency of neuromodulators can be titrated to fit each patient’s aesthetic goals, she said. For older patients who want to soften or maintain appearance, she suggested treatment every 4-6 months. And some patients just want to maintain the status quo, she noted.
Ideal candidates
For neuromodulators and fillers, who is an ideal candidate? “I think it’s anyone who has realistic expectations,” she said. Patients need to know how many treatments are needed and how much it will cost. For patients with extensive wrinkling and sagging, she said, she does extensive counseling about what results to expect “because I don’t want them to feel like they wasted their time or their money.”
She also suggests a surgical consult, as some may opt for that route after learning about the options and expected results.
Skin tightening
Both radiofrequency and microfocused ultrasound are noninvasive and additional options. Radiofrequency uses radio waves, with electromagnetic energy to stimulate heat. Ultrasound uses ultrasound waves to stimulate heat. Both approaches cause collagen contraction, neocollagenesis, and skin tightening.
These procedures do well for the lower face, Dr. Watchmaker said, but “I am relatively unimpressed for how well they do for the upper face.” Ideal candidates have mild to moderate skin laxity and want to avoid surgery. She also tells patients that collagen isn’t made overnight. “You won’t see much for 3-6 months after.” The good news? Usually the treatments need to be repeated only every 1.5-2 years, she said.
Lasers
“There are so many lasers out there,” said Dr. Watchmaker, who groups them into three categories: those used for wrinkles, dyschromia, and erythema. Her picks: ablative lasers (CO2 and erbium) and erbium-doped YAG 1550 nm laser for rhytids. Thulium 1927 and QS and picosecond lasers are her picks for dyschromia, and for erythema, pulsed dye and KTP lasers.
Some laser treatments are not a “walk in the park,” as she warns patients. For example, after treatment with ablative lasers, there is pain, post-procedure redness, and crusting.
Take-home points
A combination of noninvasive and minimally invasive procedures can produce appearance-improving results. That’s more likely if dermatologists choose ideal candidates, personalize the treatment, and set realistic expectations. “We have a finite number of tools,” she said, but they can be used in a variety of ways.
At the interactive panel discussion following her presentation, Dr. Watchmaker was asked what she tells patients about sun protection. “I talk a lot about sunscreens,’’ she said, always urging patients to use them. While the options for rejuvenation are numerous, taking care of the skin is still crucial.
Dr. Watchmaker had no disclosures. MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Can dietary tweaks improve some skin diseases?
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
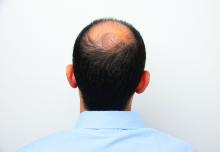
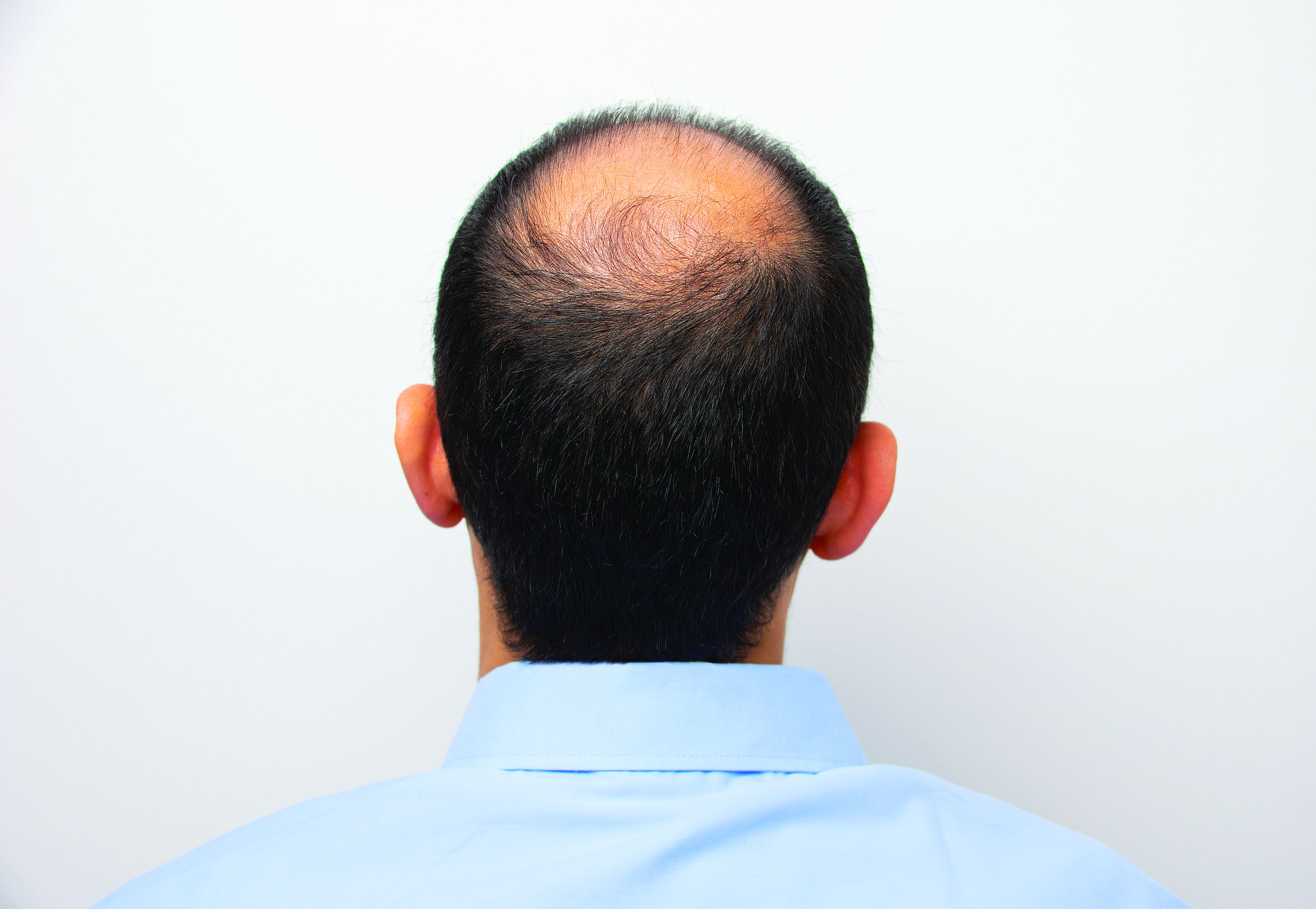
Hair disorder treatments are evolving
“No matter who the patient is, whether a child, adolescent, or adult, the key to figuring out hair disease is getting a good history,” Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said at the Medscape Live Women’s and Pediatric Dermatology Seminar.
. She also urged physicians and other health care providers to use the electronic medical record and to be thorough in documenting information – noting nutrition, hair care habits, supplement use, and other details.
Lab tests should be selected based on that history, she said. For instance, low iron stores can be associated with hair shedding; and thyroid function studies might be needed.
Other highlights of her presentation included comments on different types of alopecia, and some new treatment approaches:
Androgenetic alopecia. In a meta-analysis and systematic review published in 2017, all treatments tested (2% and 5% minoxidil in men, 1 mg finasteride in men, 2% minoxidil in women, and low-level laser light therapy in men) were superior to placebo. Several photobiomodulation (PBM) devices (also known as low-level laser light) for home use have been cleared for androgenetic alopecia by the Food and Drug Administration; a clinician’s guide, published in 2018, provides information on these devices.
Hair and hormones. Combination therapy for female-pattern hair loss – low-dose minoxidil and spironolactone – is important to know about, she said, adding there are data from an observational pilot study supporting this treatment. Women should not become pregnant while on this treatment, Dr. Hordinsky cautioned.
PRP (platelet rich plasma). This treatment for hair loss can be costly, she cautioned, as it’s viewed as a cosmetic technique, “but it actually can work rather well.”
Hair regrowth measures. Traditionally, measures center on global assessment, the patient’s self-assessment, investigator assessment, and an independent photo review. Enter the dermatoscope. “We can now get pictures as a baseline. Patients can see, and also see the health of their scalp,” and if treatments make it look better or worse, she noted.
Alopecia areata (AA). Patients and families need to be made aware that this is an autoimmune disease that can recur, and if it does recur, the extent of hair loss is not predictable. According to Dr. Hordinsky, the most widely used tool to halt disease activity has been treatment with a corticosteroid (topical, intralesional, oral, or even intravenous corticosteroids).
Clinical trials and publications from 2018 to 2020 have triggered interest in off-label use and further studies of JAK inhibitors for treating AA, which include baricitinib, ruxolitinib, and tofacitinib. At the American Academy of Dermatology meeting in March 2022, results of the ALLEGRO phase 2b/3 trial found that the JAK inhibitor ritlecitinib (50 mg or 20 mg daily, with or without a 200-mg loading dose), was efficacious in adults and adolescents with AA, compared with placebo, with no safety concerns noted. “This looks to be very, very promising,” she said, “and also very safe.” Two phase 3 trials of baricitinib also presented at the same meeting found it was superior to placebo for hair regrowth in adults with severe AA at 36 weeks. (On June 13, shortly after Dr. Hordinsky spoke at the meeting, the FDA approved baricitinib for treating AA in adults, making this the first systemic treatment to be approved for AA).
Research on topical JAK inhibitors for AA has been disappointing, Dr. Hordinsky said.
Alopecia areata and atopic dermatitis. For patients with both AA and AD, dupilumab may provide relief, she said. She referred to a recently published phase 2a trial in patients with AA (including some with both AA and AD), which found that Severity of Alopecia Tool (SALT) scores improved after 48 weeks of treatment, with higher response rates among those with baseline IgE levels of 200 IU/mL or higher. “If your patient has both, and their immunoglobulin-E level is greater than 200, then they may be a good candidate for dupilumab and both diseases may respond,” she said.
Scalp symptoms. It can be challenging when patients complain of itch, pain, or burning on the scalp, but have no obvious skin disease, Dr. Hordinsky said. Her tips: Some of these patients may be experiencing scalp symptoms secondary to a neuropathy; others may have mast cell degranulation, but for others, the basis of the symptoms may be unclear. Special nerve studies may be needed. For relief, a trial of antihistamines or topical or oral gabapentin may be needed, she said.
Frontal fibrosing alopecia (FFA). This condition, first described in postmenopausal women, is now reported in men and in younger women. While sunscreen has been suspected, there are no good data that have proven that link, she said. Cosmetics are also considered a possible culprit. For treatment, “the first thing we try to do is treat the inflammation,” Dr. Hordinsky said. Treatment options include topical high-potency corticosteroids, intralesional steroids, and topical nonsteroid anti-inflammatory creams (tier 1); hydroxychloroquine, low-dose antibiotics, and acitretin (tier 2); and cyclosporin and mycophenolate mofetil (tier 3).
In an observational study of mostly women with FFA, she noted, treatment with dutasteride was more effective than commonly used systemic treatments.
“Don’t forget to address the psychosocial needs of the hair loss patient,” Dr. Hordinsky advised. “Hair loss patients are very distressed, and you have to learn how to be fast and nimble and address those needs.” Working with a behavioral health specialist or therapist can help, she said.
She also recommended directing patients to appropriate organizations such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, as well as conferences, such as the upcoming NAAF conference in Washington. “These organizations do give good information that should complement what you are doing.”
Medscape Live and this news organization are owned by the same parent company. Dr. Hordinsky reported no disclosures.
“No matter who the patient is, whether a child, adolescent, or adult, the key to figuring out hair disease is getting a good history,” Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said at the Medscape Live Women’s and Pediatric Dermatology Seminar.
. She also urged physicians and other health care providers to use the electronic medical record and to be thorough in documenting information – noting nutrition, hair care habits, supplement use, and other details.
Lab tests should be selected based on that history, she said. For instance, low iron stores can be associated with hair shedding; and thyroid function studies might be needed.
Other highlights of her presentation included comments on different types of alopecia, and some new treatment approaches:
Androgenetic alopecia. In a meta-analysis and systematic review published in 2017, all treatments tested (2% and 5% minoxidil in men, 1 mg finasteride in men, 2% minoxidil in women, and low-level laser light therapy in men) were superior to placebo. Several photobiomodulation (PBM) devices (also known as low-level laser light) for home use have been cleared for androgenetic alopecia by the Food and Drug Administration; a clinician’s guide, published in 2018, provides information on these devices.
Hair and hormones. Combination therapy for female-pattern hair loss – low-dose minoxidil and spironolactone – is important to know about, she said, adding there are data from an observational pilot study supporting this treatment. Women should not become pregnant while on this treatment, Dr. Hordinsky cautioned.
PRP (platelet rich plasma). This treatment for hair loss can be costly, she cautioned, as it’s viewed as a cosmetic technique, “but it actually can work rather well.”
Hair regrowth measures. Traditionally, measures center on global assessment, the patient’s self-assessment, investigator assessment, and an independent photo review. Enter the dermatoscope. “We can now get pictures as a baseline. Patients can see, and also see the health of their scalp,” and if treatments make it look better or worse, she noted.
Alopecia areata (AA). Patients and families need to be made aware that this is an autoimmune disease that can recur, and if it does recur, the extent of hair loss is not predictable. According to Dr. Hordinsky, the most widely used tool to halt disease activity has been treatment with a corticosteroid (topical, intralesional, oral, or even intravenous corticosteroids).
Clinical trials and publications from 2018 to 2020 have triggered interest in off-label use and further studies of JAK inhibitors for treating AA, which include baricitinib, ruxolitinib, and tofacitinib. At the American Academy of Dermatology meeting in March 2022, results of the ALLEGRO phase 2b/3 trial found that the JAK inhibitor ritlecitinib (50 mg or 20 mg daily, with or without a 200-mg loading dose), was efficacious in adults and adolescents with AA, compared with placebo, with no safety concerns noted. “This looks to be very, very promising,” she said, “and also very safe.” Two phase 3 trials of baricitinib also presented at the same meeting found it was superior to placebo for hair regrowth in adults with severe AA at 36 weeks. (On June 13, shortly after Dr. Hordinsky spoke at the meeting, the FDA approved baricitinib for treating AA in adults, making this the first systemic treatment to be approved for AA).
Research on topical JAK inhibitors for AA has been disappointing, Dr. Hordinsky said.
Alopecia areata and atopic dermatitis. For patients with both AA and AD, dupilumab may provide relief, she said. She referred to a recently published phase 2a trial in patients with AA (including some with both AA and AD), which found that Severity of Alopecia Tool (SALT) scores improved after 48 weeks of treatment, with higher response rates among those with baseline IgE levels of 200 IU/mL or higher. “If your patient has both, and their immunoglobulin-E level is greater than 200, then they may be a good candidate for dupilumab and both diseases may respond,” she said.
Scalp symptoms. It can be challenging when patients complain of itch, pain, or burning on the scalp, but have no obvious skin disease, Dr. Hordinsky said. Her tips: Some of these patients may be experiencing scalp symptoms secondary to a neuropathy; others may have mast cell degranulation, but for others, the basis of the symptoms may be unclear. Special nerve studies may be needed. For relief, a trial of antihistamines or topical or oral gabapentin may be needed, she said.
Frontal fibrosing alopecia (FFA). This condition, first described in postmenopausal women, is now reported in men and in younger women. While sunscreen has been suspected, there are no good data that have proven that link, she said. Cosmetics are also considered a possible culprit. For treatment, “the first thing we try to do is treat the inflammation,” Dr. Hordinsky said. Treatment options include topical high-potency corticosteroids, intralesional steroids, and topical nonsteroid anti-inflammatory creams (tier 1); hydroxychloroquine, low-dose antibiotics, and acitretin (tier 2); and cyclosporin and mycophenolate mofetil (tier 3).
In an observational study of mostly women with FFA, she noted, treatment with dutasteride was more effective than commonly used systemic treatments.
“Don’t forget to address the psychosocial needs of the hair loss patient,” Dr. Hordinsky advised. “Hair loss patients are very distressed, and you have to learn how to be fast and nimble and address those needs.” Working with a behavioral health specialist or therapist can help, she said.
She also recommended directing patients to appropriate organizations such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, as well as conferences, such as the upcoming NAAF conference in Washington. “These organizations do give good information that should complement what you are doing.”
Medscape Live and this news organization are owned by the same parent company. Dr. Hordinsky reported no disclosures.
“No matter who the patient is, whether a child, adolescent, or adult, the key to figuring out hair disease is getting a good history,” Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said at the Medscape Live Women’s and Pediatric Dermatology Seminar.
. She also urged physicians and other health care providers to use the electronic medical record and to be thorough in documenting information – noting nutrition, hair care habits, supplement use, and other details.
Lab tests should be selected based on that history, she said. For instance, low iron stores can be associated with hair shedding; and thyroid function studies might be needed.
Other highlights of her presentation included comments on different types of alopecia, and some new treatment approaches:
Androgenetic alopecia. In a meta-analysis and systematic review published in 2017, all treatments tested (2% and 5% minoxidil in men, 1 mg finasteride in men, 2% minoxidil in women, and low-level laser light therapy in men) were superior to placebo. Several photobiomodulation (PBM) devices (also known as low-level laser light) for home use have been cleared for androgenetic alopecia by the Food and Drug Administration; a clinician’s guide, published in 2018, provides information on these devices.
Hair and hormones. Combination therapy for female-pattern hair loss – low-dose minoxidil and spironolactone – is important to know about, she said, adding there are data from an observational pilot study supporting this treatment. Women should not become pregnant while on this treatment, Dr. Hordinsky cautioned.
PRP (platelet rich plasma). This treatment for hair loss can be costly, she cautioned, as it’s viewed as a cosmetic technique, “but it actually can work rather well.”
Hair regrowth measures. Traditionally, measures center on global assessment, the patient’s self-assessment, investigator assessment, and an independent photo review. Enter the dermatoscope. “We can now get pictures as a baseline. Patients can see, and also see the health of their scalp,” and if treatments make it look better or worse, she noted.
Alopecia areata (AA). Patients and families need to be made aware that this is an autoimmune disease that can recur, and if it does recur, the extent of hair loss is not predictable. According to Dr. Hordinsky, the most widely used tool to halt disease activity has been treatment with a corticosteroid (topical, intralesional, oral, or even intravenous corticosteroids).
Clinical trials and publications from 2018 to 2020 have triggered interest in off-label use and further studies of JAK inhibitors for treating AA, which include baricitinib, ruxolitinib, and tofacitinib. At the American Academy of Dermatology meeting in March 2022, results of the ALLEGRO phase 2b/3 trial found that the JAK inhibitor ritlecitinib (50 mg or 20 mg daily, with or without a 200-mg loading dose), was efficacious in adults and adolescents with AA, compared with placebo, with no safety concerns noted. “This looks to be very, very promising,” she said, “and also very safe.” Two phase 3 trials of baricitinib also presented at the same meeting found it was superior to placebo for hair regrowth in adults with severe AA at 36 weeks. (On June 13, shortly after Dr. Hordinsky spoke at the meeting, the FDA approved baricitinib for treating AA in adults, making this the first systemic treatment to be approved for AA).
Research on topical JAK inhibitors for AA has been disappointing, Dr. Hordinsky said.
Alopecia areata and atopic dermatitis. For patients with both AA and AD, dupilumab may provide relief, she said. She referred to a recently published phase 2a trial in patients with AA (including some with both AA and AD), which found that Severity of Alopecia Tool (SALT) scores improved after 48 weeks of treatment, with higher response rates among those with baseline IgE levels of 200 IU/mL or higher. “If your patient has both, and their immunoglobulin-E level is greater than 200, then they may be a good candidate for dupilumab and both diseases may respond,” she said.
Scalp symptoms. It can be challenging when patients complain of itch, pain, or burning on the scalp, but have no obvious skin disease, Dr. Hordinsky said. Her tips: Some of these patients may be experiencing scalp symptoms secondary to a neuropathy; others may have mast cell degranulation, but for others, the basis of the symptoms may be unclear. Special nerve studies may be needed. For relief, a trial of antihistamines or topical or oral gabapentin may be needed, she said.
Frontal fibrosing alopecia (FFA). This condition, first described in postmenopausal women, is now reported in men and in younger women. While sunscreen has been suspected, there are no good data that have proven that link, she said. Cosmetics are also considered a possible culprit. For treatment, “the first thing we try to do is treat the inflammation,” Dr. Hordinsky said. Treatment options include topical high-potency corticosteroids, intralesional steroids, and topical nonsteroid anti-inflammatory creams (tier 1); hydroxychloroquine, low-dose antibiotics, and acitretin (tier 2); and cyclosporin and mycophenolate mofetil (tier 3).
In an observational study of mostly women with FFA, she noted, treatment with dutasteride was more effective than commonly used systemic treatments.
“Don’t forget to address the psychosocial needs of the hair loss patient,” Dr. Hordinsky advised. “Hair loss patients are very distressed, and you have to learn how to be fast and nimble and address those needs.” Working with a behavioral health specialist or therapist can help, she said.
She also recommended directing patients to appropriate organizations such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, as well as conferences, such as the upcoming NAAF conference in Washington. “These organizations do give good information that should complement what you are doing.”
Medscape Live and this news organization are owned by the same parent company. Dr. Hordinsky reported no disclosures.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Atopic dermatitis: Options abound, and more are coming
, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
More and more treatment options are available and even more are in the pipeline, said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of dermatology at the University of California, San Diego and Rady Children’s Hospital. As he put it: “We got pills, injections, things to smear on the skin.”
Those options are welcome and needed, as AD affects up to 20% of children and up to 10% of adults. The course is variable, as is severity, and quality of life is impacted.
Besides new treatment options, there is a new understanding about comorbidities, environmental effects, and triggers, Dr. Eichenfield said. Among the potential comorbidities health care providers should be aware of are allergies, such as food allergies; asthma; rhinitis; mental health issues (depression, anxiety, ADHD, learning disabilities, or in adults, substance abuse); bone health; skin infections; immune disorders such as alopecia areata or urticaria; and cardiovascular issues that could affect adults.
Environmental effects can play a role in aggravating AD, as providers learned after visits for AD increased after Northern California wildfires and also in other areas with high air pollution, Dr. Eichenfield said. “I actually discuss this with my families,” when making them aware of factors that may affect AD, he noted.
Dr. Eichenfield provided an overview of available treatment options, and what treatments may be coming next. Among the highlights:
Topical ruxolitinib: A JAK1,2 inhibitor in a cream formulation, it is now approved for patients with mild to moderate AD aged 12 years and older in the United States. Of the two strengths studied, the higher strength, 1.5%, was approved, Dr. Eichenfield said. How well did it work? In two phase 3 studies in patients aged 12 and older, of those on 1.5%, 53% were clear or almost clear at 8 weeks, versus 11% in the control group given the vehicle; 52% had at least a 4-point reduction in itch from baseline, versus 15.4% on vehicle. Quality of life improved in up to 73.2% of those given the medication versus 19.7% of those on the vehicle. There was a marked and quick improvement in itch, as early as 12 hours, and safety measures also look good, he said.
Topical tapinarof: Approved in May 2022, for adults with plaque psoriasis, phase 3 trials began in September, 2021, for adults and children with AD, according to the manufacturer. Activation of the aryl hydrocarbon receptor mediates its anti-inflammatory properties.
Topical roflumilast: A potent PDE-4 inhibitor, phase 3 AD studies are underway. It appears to be well tolerated, Dr. Eichenfield said.
Dupilumab: An IL-4/13 blocker, this biologic produced an itch reduction of 50% and EASI of 80%, improved quality of life, and reduced anxiety and depression. The drug “led the revolution in systemic therapy for atopic dermatitis,” he said. First approved for treating AD in patients aged 18 years and up in 2017, approval for patients 12 years and up followed in March 2019, then for age 6 years and up May 2020.
At the meeting on June 3, Dr. Eichenfield said that approval in children 5 years and under was imminent, and on June 7, the FDA approved dupilumab for use in children aged 6 months to 5 years. In a phase 3, 16-week trial, 28% of children treated with dupilumab added on to low-potency topical corticosteroids met the endpoint of clear or nearly clear skin, compared with 4% of those on the corticosteroids alone (P < .0001).
Tralokinumab: There is no approved indication yet for adolescents, but the injected biologic, an interleukin-13 antagonist, is approved for adults with moderate to severe AD who are not well-controlled with topicals, or who cannot use topicals.
Oral JAK inhibitors: These include abrocitinib and upadacitinib, both approved by the FDA in January 2022 for treating moderate to severe AD, and baricitinib (the latter not in the United States). “For AD, you probably won’t see it in the U.S.,” Dr. Eichenfield said, referring to baricitinib. However, it might get approved for alopecia areata, he noted.
Upadacitinib is approved for adolescents 12 and older with AD. Abrocitinib is approved for adults 18 and older with AD.
Regarding safety and tolerance concerns with oral JAK inhibitors, Dr. Eichenfield cites headache, acne, nausea, and upper respiratory tract infections as relatively common, while herpes zoster, venous thromboembolism, and lab anomalies (neutropenia, elevated CPK) are uncommon.
As the options for AD treatments increase, and expectations by families and clinicians change, Dr. Eichenfield said he often focuses on “bucket duty” – whether a specific patient should be in the topical bucket or the systemic one. It’s a decision that will continue to be crucial, he said.
When presented with treatment options, patients – and parents – often worry about side effects, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas Medical Center, Little Rock, who also spoke at the meeting. She gently tells them: “The worst side effect you can have is probably not treating the disease itself.”
Medscape Live and this news organization are owned by the same parent company. Dr. Eichenfield is a consultant or investigator for numerous companies that manufacture treatments for AD, but based his discussion on evidence-based recommendations and public presentations or publications.
, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
More and more treatment options are available and even more are in the pipeline, said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of dermatology at the University of California, San Diego and Rady Children’s Hospital. As he put it: “We got pills, injections, things to smear on the skin.”
Those options are welcome and needed, as AD affects up to 20% of children and up to 10% of adults. The course is variable, as is severity, and quality of life is impacted.
Besides new treatment options, there is a new understanding about comorbidities, environmental effects, and triggers, Dr. Eichenfield said. Among the potential comorbidities health care providers should be aware of are allergies, such as food allergies; asthma; rhinitis; mental health issues (depression, anxiety, ADHD, learning disabilities, or in adults, substance abuse); bone health; skin infections; immune disorders such as alopecia areata or urticaria; and cardiovascular issues that could affect adults.
Environmental effects can play a role in aggravating AD, as providers learned after visits for AD increased after Northern California wildfires and also in other areas with high air pollution, Dr. Eichenfield said. “I actually discuss this with my families,” when making them aware of factors that may affect AD, he noted.
Dr. Eichenfield provided an overview of available treatment options, and what treatments may be coming next. Among the highlights:
Topical ruxolitinib: A JAK1,2 inhibitor in a cream formulation, it is now approved for patients with mild to moderate AD aged 12 years and older in the United States. Of the two strengths studied, the higher strength, 1.5%, was approved, Dr. Eichenfield said. How well did it work? In two phase 3 studies in patients aged 12 and older, of those on 1.5%, 53% were clear or almost clear at 8 weeks, versus 11% in the control group given the vehicle; 52% had at least a 4-point reduction in itch from baseline, versus 15.4% on vehicle. Quality of life improved in up to 73.2% of those given the medication versus 19.7% of those on the vehicle. There was a marked and quick improvement in itch, as early as 12 hours, and safety measures also look good, he said.
Topical tapinarof: Approved in May 2022, for adults with plaque psoriasis, phase 3 trials began in September, 2021, for adults and children with AD, according to the manufacturer. Activation of the aryl hydrocarbon receptor mediates its anti-inflammatory properties.
Topical roflumilast: A potent PDE-4 inhibitor, phase 3 AD studies are underway. It appears to be well tolerated, Dr. Eichenfield said.
Dupilumab: An IL-4/13 blocker, this biologic produced an itch reduction of 50% and EASI of 80%, improved quality of life, and reduced anxiety and depression. The drug “led the revolution in systemic therapy for atopic dermatitis,” he said. First approved for treating AD in patients aged 18 years and up in 2017, approval for patients 12 years and up followed in March 2019, then for age 6 years and up May 2020.
At the meeting on June 3, Dr. Eichenfield said that approval in children 5 years and under was imminent, and on June 7, the FDA approved dupilumab for use in children aged 6 months to 5 years. In a phase 3, 16-week trial, 28% of children treated with dupilumab added on to low-potency topical corticosteroids met the endpoint of clear or nearly clear skin, compared with 4% of those on the corticosteroids alone (P < .0001).
Tralokinumab: There is no approved indication yet for adolescents, but the injected biologic, an interleukin-13 antagonist, is approved for adults with moderate to severe AD who are not well-controlled with topicals, or who cannot use topicals.
Oral JAK inhibitors: These include abrocitinib and upadacitinib, both approved by the FDA in January 2022 for treating moderate to severe AD, and baricitinib (the latter not in the United States). “For AD, you probably won’t see it in the U.S.,” Dr. Eichenfield said, referring to baricitinib. However, it might get approved for alopecia areata, he noted.
Upadacitinib is approved for adolescents 12 and older with AD. Abrocitinib is approved for adults 18 and older with AD.
Regarding safety and tolerance concerns with oral JAK inhibitors, Dr. Eichenfield cites headache, acne, nausea, and upper respiratory tract infections as relatively common, while herpes zoster, venous thromboembolism, and lab anomalies (neutropenia, elevated CPK) are uncommon.
As the options for AD treatments increase, and expectations by families and clinicians change, Dr. Eichenfield said he often focuses on “bucket duty” – whether a specific patient should be in the topical bucket or the systemic one. It’s a decision that will continue to be crucial, he said.
When presented with treatment options, patients – and parents – often worry about side effects, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas Medical Center, Little Rock, who also spoke at the meeting. She gently tells them: “The worst side effect you can have is probably not treating the disease itself.”
Medscape Live and this news organization are owned by the same parent company. Dr. Eichenfield is a consultant or investigator for numerous companies that manufacture treatments for AD, but based his discussion on evidence-based recommendations and public presentations or publications.
, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
More and more treatment options are available and even more are in the pipeline, said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of dermatology at the University of California, San Diego and Rady Children’s Hospital. As he put it: “We got pills, injections, things to smear on the skin.”
Those options are welcome and needed, as AD affects up to 20% of children and up to 10% of adults. The course is variable, as is severity, and quality of life is impacted.
Besides new treatment options, there is a new understanding about comorbidities, environmental effects, and triggers, Dr. Eichenfield said. Among the potential comorbidities health care providers should be aware of are allergies, such as food allergies; asthma; rhinitis; mental health issues (depression, anxiety, ADHD, learning disabilities, or in adults, substance abuse); bone health; skin infections; immune disorders such as alopecia areata or urticaria; and cardiovascular issues that could affect adults.
Environmental effects can play a role in aggravating AD, as providers learned after visits for AD increased after Northern California wildfires and also in other areas with high air pollution, Dr. Eichenfield said. “I actually discuss this with my families,” when making them aware of factors that may affect AD, he noted.
Dr. Eichenfield provided an overview of available treatment options, and what treatments may be coming next. Among the highlights:
Topical ruxolitinib: A JAK1,2 inhibitor in a cream formulation, it is now approved for patients with mild to moderate AD aged 12 years and older in the United States. Of the two strengths studied, the higher strength, 1.5%, was approved, Dr. Eichenfield said. How well did it work? In two phase 3 studies in patients aged 12 and older, of those on 1.5%, 53% were clear or almost clear at 8 weeks, versus 11% in the control group given the vehicle; 52% had at least a 4-point reduction in itch from baseline, versus 15.4% on vehicle. Quality of life improved in up to 73.2% of those given the medication versus 19.7% of those on the vehicle. There was a marked and quick improvement in itch, as early as 12 hours, and safety measures also look good, he said.
Topical tapinarof: Approved in May 2022, for adults with plaque psoriasis, phase 3 trials began in September, 2021, for adults and children with AD, according to the manufacturer. Activation of the aryl hydrocarbon receptor mediates its anti-inflammatory properties.
Topical roflumilast: A potent PDE-4 inhibitor, phase 3 AD studies are underway. It appears to be well tolerated, Dr. Eichenfield said.
Dupilumab: An IL-4/13 blocker, this biologic produced an itch reduction of 50% and EASI of 80%, improved quality of life, and reduced anxiety and depression. The drug “led the revolution in systemic therapy for atopic dermatitis,” he said. First approved for treating AD in patients aged 18 years and up in 2017, approval for patients 12 years and up followed in March 2019, then for age 6 years and up May 2020.
At the meeting on June 3, Dr. Eichenfield said that approval in children 5 years and under was imminent, and on June 7, the FDA approved dupilumab for use in children aged 6 months to 5 years. In a phase 3, 16-week trial, 28% of children treated with dupilumab added on to low-potency topical corticosteroids met the endpoint of clear or nearly clear skin, compared with 4% of those on the corticosteroids alone (P < .0001).
Tralokinumab: There is no approved indication yet for adolescents, but the injected biologic, an interleukin-13 antagonist, is approved for adults with moderate to severe AD who are not well-controlled with topicals, or who cannot use topicals.
Oral JAK inhibitors: These include abrocitinib and upadacitinib, both approved by the FDA in January 2022 for treating moderate to severe AD, and baricitinib (the latter not in the United States). “For AD, you probably won’t see it in the U.S.,” Dr. Eichenfield said, referring to baricitinib. However, it might get approved for alopecia areata, he noted.
Upadacitinib is approved for adolescents 12 and older with AD. Abrocitinib is approved for adults 18 and older with AD.
Regarding safety and tolerance concerns with oral JAK inhibitors, Dr. Eichenfield cites headache, acne, nausea, and upper respiratory tract infections as relatively common, while herpes zoster, venous thromboembolism, and lab anomalies (neutropenia, elevated CPK) are uncommon.
As the options for AD treatments increase, and expectations by families and clinicians change, Dr. Eichenfield said he often focuses on “bucket duty” – whether a specific patient should be in the topical bucket or the systemic one. It’s a decision that will continue to be crucial, he said.
When presented with treatment options, patients – and parents – often worry about side effects, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas Medical Center, Little Rock, who also spoke at the meeting. She gently tells them: “The worst side effect you can have is probably not treating the disease itself.”
Medscape Live and this news organization are owned by the same parent company. Dr. Eichenfield is a consultant or investigator for numerous companies that manufacture treatments for AD, but based his discussion on evidence-based recommendations and public presentations or publications.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Biologics, Women, and Pregnancy: What’s Known?
As the and the child’s development.
“I get asked a lot about fertility,” Vivian Shi, MD, associate professor of dermatology at the University of Arkansas, Little Rock, said at MedscapeLive’s Women’s and Pediatric Dermatology Seminar. Patients want to know, she said, if they go on a specific drug, whether it will affect their chances of conceiving and what else they need to know about safety.
She told the audience what she tells her patients: The answers are not complete but are evolving at a steady pace.
“Putting this talk together was kind of like a scavenger hunt,” said Dr. Shi, who gathered data from pregnancy exposure registries, published research, the Food and Drug Administration, and other sources on biologics. As more studies emerge each year, she said, recommendations will become stronger for considering treatment by certain biological drugs, taking into account effects on fertility, pregnancy, lactation, and the infant.
Among the biologics commonly used in dermatology are:
- Tumor necrosis factor (TNF) inhibitors (etanercept, adalimumab, infliximab, certolizumab).
- Interleukin (IL)–12 and -23 antagonist (ustekinumab).
- IL-17 antagonists (ixekizumab, secukinumab, brodalumab).
- IL-23 antagonists (risankizumab, tildrakizumab, guselkumab).
- IL-4, -13 antagonist (dupilumab) and IL-13 antagonist (tralokinumab).
- CD20-directed cytolytic antibody (rituximab).
To help with decision-making, Dr. Shi discussed the relatively new FDA labeling regulations as well as pregnancy exposure registries, research studies, and recommendations.
FDA pregnancy risk summaries
Under the previous system of classification of drugs in pregnancy, the FDA rated drugs as A, B, C, D, X. These categories ranged from showing no risks to the fetus to clear risk, but were oversimplistic and confusing, Dr. Shi said. Category C was especially confusing, as a drug with no animal or human data was put in the same category as a drug with adverse fetal effects on animals, she noted.
However, effective June 30, 2015, the FDA replaced pregnancy categories with risk summaries by medication. As of June, 2020, all prescription drugs were to remove pregnancy letter labeling. The risk summaries note human data when they are available and also note when no data are available. This information, Dr. Shi said, originates from many sources, including studies published in the medical literature, postmarketing studies conducted by companies, and pregnancy exposure registries, conducted by some companies and others. The FDA does not endorse any specific registries, but does post a list of such registries. Another helpful resource, she said, is Mother to Baby, a service of the nonprofit Organization of Teratology Information Specialists (OTIS).
Known, not known
Citing published literature, Dr. Shi said that TNF inhibitors have the most robust safety data from preconception to after birth. Less is known, she said, about the reproductive safety effects of other biologics used for dermatologic conditions, as they are newer than the anti-TNF medicines.
She reviewed a variety of research studies evaluating the safety of biologics during pregnancy and beyond. Highlights include results from a large registry, the Psoriasis Longitudinal Assessment and Registry (PSOLAR), of 298 pregnancies in about 220 women from 2007 to 2019, looking at 13 different biologics. The overall and live-birth outcomes in the women on biologics for psoriasis were similar to those for the general population and the rate of congenital anomalies was 0.8%, researchers reported in 2021, lower than the generally cited annual figure of U.S. births.
Studies evaluating biologics for nondermatologic conditions suggest safety. A prospective cohort study of women who took adalimumab in pregnancy (for rheumatoid arthritis or Crohn’s disease) found no increased risk for birth defects. In another study looking at women who were breastfeeding, researchers found no increased risk of infections or delay in developmental milestones in the children of women taking biologics for inflammatory bowel disease, compared with those not on the medications.
A report using data from the World Health Organization concludes that dupilumab appears to be safe during pregnancy, based on an evaluation of 36 pregnancy-related reports among more than 37,000 unique adverse event reports related to dupilumab in a global database.
Recommendations about biologic use from different organizations don’t always mesh, Dr. Shi said, noting that European guidelines tend to be stricter, as some reviews show.
If a mother is exposed to any biologic therapy other than certolizumab during the third trimester, after 27 weeks, Dr. Shi said, “you want to consider avoiding a live vaccine for the first 6 months of the baby’s life.” It turns out, she said, the only recommended live vaccine during that period is the rotavirus vaccine, and she suggests doctors recommend postponing that one until the babies are older if women have been on biologics other than certolizumab.
Her other take-home messages: TNF inhibitors have the most robust safety data from before conception through lactation. Under current guidelines, certolizumab is viewed as the safest to use throughout pregnancy. Dr. Shi’s message to her colleagues fielding the same questions she gets from patients: “There is more data coming out every year. Ultimately, we will have better information to inform our patients.”
At the conference, Lawrence F. Eichenfield, MD, a course director and professor of dermatology and pediatrics at the University of California, San Diego and Rady Children’s Hospital San Diego, encouraged Dr. Shi to write up her presentation as a resource for other dermatologists – which she said is in progress.
Medscape Live and this news organization are owned by the same parent company. Dr. Shi disclosed consulting and investigative and research funding from several pharmaceutical firms, but not directly related to the content of her presentation.
As the and the child’s development.
“I get asked a lot about fertility,” Vivian Shi, MD, associate professor of dermatology at the University of Arkansas, Little Rock, said at MedscapeLive’s Women’s and Pediatric Dermatology Seminar. Patients want to know, she said, if they go on a specific drug, whether it will affect their chances of conceiving and what else they need to know about safety.
She told the audience what she tells her patients: The answers are not complete but are evolving at a steady pace.
“Putting this talk together was kind of like a scavenger hunt,” said Dr. Shi, who gathered data from pregnancy exposure registries, published research, the Food and Drug Administration, and other sources on biologics. As more studies emerge each year, she said, recommendations will become stronger for considering treatment by certain biological drugs, taking into account effects on fertility, pregnancy, lactation, and the infant.
Among the biologics commonly used in dermatology are:
- Tumor necrosis factor (TNF) inhibitors (etanercept, adalimumab, infliximab, certolizumab).
- Interleukin (IL)–12 and -23 antagonist (ustekinumab).
- IL-17 antagonists (ixekizumab, secukinumab, brodalumab).
- IL-23 antagonists (risankizumab, tildrakizumab, guselkumab).
- IL-4, -13 antagonist (dupilumab) and IL-13 antagonist (tralokinumab).
- CD20-directed cytolytic antibody (rituximab).
To help with decision-making, Dr. Shi discussed the relatively new FDA labeling regulations as well as pregnancy exposure registries, research studies, and recommendations.
FDA pregnancy risk summaries
Under the previous system of classification of drugs in pregnancy, the FDA rated drugs as A, B, C, D, X. These categories ranged from showing no risks to the fetus to clear risk, but were oversimplistic and confusing, Dr. Shi said. Category C was especially confusing, as a drug with no animal or human data was put in the same category as a drug with adverse fetal effects on animals, she noted.
However, effective June 30, 2015, the FDA replaced pregnancy categories with risk summaries by medication. As of June, 2020, all prescription drugs were to remove pregnancy letter labeling. The risk summaries note human data when they are available and also note when no data are available. This information, Dr. Shi said, originates from many sources, including studies published in the medical literature, postmarketing studies conducted by companies, and pregnancy exposure registries, conducted by some companies and others. The FDA does not endorse any specific registries, but does post a list of such registries. Another helpful resource, she said, is Mother to Baby, a service of the nonprofit Organization of Teratology Information Specialists (OTIS).
Known, not known
Citing published literature, Dr. Shi said that TNF inhibitors have the most robust safety data from preconception to after birth. Less is known, she said, about the reproductive safety effects of other biologics used for dermatologic conditions, as they are newer than the anti-TNF medicines.
She reviewed a variety of research studies evaluating the safety of biologics during pregnancy and beyond. Highlights include results from a large registry, the Psoriasis Longitudinal Assessment and Registry (PSOLAR), of 298 pregnancies in about 220 women from 2007 to 2019, looking at 13 different biologics. The overall and live-birth outcomes in the women on biologics for psoriasis were similar to those for the general population and the rate of congenital anomalies was 0.8%, researchers reported in 2021, lower than the generally cited annual figure of U.S. births.
Studies evaluating biologics for nondermatologic conditions suggest safety. A prospective cohort study of women who took adalimumab in pregnancy (for rheumatoid arthritis or Crohn’s disease) found no increased risk for birth defects. In another study looking at women who were breastfeeding, researchers found no increased risk of infections or delay in developmental milestones in the children of women taking biologics for inflammatory bowel disease, compared with those not on the medications.
A report using data from the World Health Organization concludes that dupilumab appears to be safe during pregnancy, based on an evaluation of 36 pregnancy-related reports among more than 37,000 unique adverse event reports related to dupilumab in a global database.
Recommendations about biologic use from different organizations don’t always mesh, Dr. Shi said, noting that European guidelines tend to be stricter, as some reviews show.
If a mother is exposed to any biologic therapy other than certolizumab during the third trimester, after 27 weeks, Dr. Shi said, “you want to consider avoiding a live vaccine for the first 6 months of the baby’s life.” It turns out, she said, the only recommended live vaccine during that period is the rotavirus vaccine, and she suggests doctors recommend postponing that one until the babies are older if women have been on biologics other than certolizumab.
Her other take-home messages: TNF inhibitors have the most robust safety data from before conception through lactation. Under current guidelines, certolizumab is viewed as the safest to use throughout pregnancy. Dr. Shi’s message to her colleagues fielding the same questions she gets from patients: “There is more data coming out every year. Ultimately, we will have better information to inform our patients.”
At the conference, Lawrence F. Eichenfield, MD, a course director and professor of dermatology and pediatrics at the University of California, San Diego and Rady Children’s Hospital San Diego, encouraged Dr. Shi to write up her presentation as a resource for other dermatologists – which she said is in progress.
Medscape Live and this news organization are owned by the same parent company. Dr. Shi disclosed consulting and investigative and research funding from several pharmaceutical firms, but not directly related to the content of her presentation.
As the and the child’s development.
“I get asked a lot about fertility,” Vivian Shi, MD, associate professor of dermatology at the University of Arkansas, Little Rock, said at MedscapeLive’s Women’s and Pediatric Dermatology Seminar. Patients want to know, she said, if they go on a specific drug, whether it will affect their chances of conceiving and what else they need to know about safety.
She told the audience what she tells her patients: The answers are not complete but are evolving at a steady pace.
“Putting this talk together was kind of like a scavenger hunt,” said Dr. Shi, who gathered data from pregnancy exposure registries, published research, the Food and Drug Administration, and other sources on biologics. As more studies emerge each year, she said, recommendations will become stronger for considering treatment by certain biological drugs, taking into account effects on fertility, pregnancy, lactation, and the infant.
Among the biologics commonly used in dermatology are:
- Tumor necrosis factor (TNF) inhibitors (etanercept, adalimumab, infliximab, certolizumab).
- Interleukin (IL)–12 and -23 antagonist (ustekinumab).
- IL-17 antagonists (ixekizumab, secukinumab, brodalumab).
- IL-23 antagonists (risankizumab, tildrakizumab, guselkumab).
- IL-4, -13 antagonist (dupilumab) and IL-13 antagonist (tralokinumab).
- CD20-directed cytolytic antibody (rituximab).
To help with decision-making, Dr. Shi discussed the relatively new FDA labeling regulations as well as pregnancy exposure registries, research studies, and recommendations.
FDA pregnancy risk summaries
Under the previous system of classification of drugs in pregnancy, the FDA rated drugs as A, B, C, D, X. These categories ranged from showing no risks to the fetus to clear risk, but were oversimplistic and confusing, Dr. Shi said. Category C was especially confusing, as a drug with no animal or human data was put in the same category as a drug with adverse fetal effects on animals, she noted.
However, effective June 30, 2015, the FDA replaced pregnancy categories with risk summaries by medication. As of June, 2020, all prescription drugs were to remove pregnancy letter labeling. The risk summaries note human data when they are available and also note when no data are available. This information, Dr. Shi said, originates from many sources, including studies published in the medical literature, postmarketing studies conducted by companies, and pregnancy exposure registries, conducted by some companies and others. The FDA does not endorse any specific registries, but does post a list of such registries. Another helpful resource, she said, is Mother to Baby, a service of the nonprofit Organization of Teratology Information Specialists (OTIS).
Known, not known
Citing published literature, Dr. Shi said that TNF inhibitors have the most robust safety data from preconception to after birth. Less is known, she said, about the reproductive safety effects of other biologics used for dermatologic conditions, as they are newer than the anti-TNF medicines.
She reviewed a variety of research studies evaluating the safety of biologics during pregnancy and beyond. Highlights include results from a large registry, the Psoriasis Longitudinal Assessment and Registry (PSOLAR), of 298 pregnancies in about 220 women from 2007 to 2019, looking at 13 different biologics. The overall and live-birth outcomes in the women on biologics for psoriasis were similar to those for the general population and the rate of congenital anomalies was 0.8%, researchers reported in 2021, lower than the generally cited annual figure of U.S. births.
Studies evaluating biologics for nondermatologic conditions suggest safety. A prospective cohort study of women who took adalimumab in pregnancy (for rheumatoid arthritis or Crohn’s disease) found no increased risk for birth defects. In another study looking at women who were breastfeeding, researchers found no increased risk of infections or delay in developmental milestones in the children of women taking biologics for inflammatory bowel disease, compared with those not on the medications.
A report using data from the World Health Organization concludes that dupilumab appears to be safe during pregnancy, based on an evaluation of 36 pregnancy-related reports among more than 37,000 unique adverse event reports related to dupilumab in a global database.
Recommendations about biologic use from different organizations don’t always mesh, Dr. Shi said, noting that European guidelines tend to be stricter, as some reviews show.
If a mother is exposed to any biologic therapy other than certolizumab during the third trimester, after 27 weeks, Dr. Shi said, “you want to consider avoiding a live vaccine for the first 6 months of the baby’s life.” It turns out, she said, the only recommended live vaccine during that period is the rotavirus vaccine, and she suggests doctors recommend postponing that one until the babies are older if women have been on biologics other than certolizumab.
Her other take-home messages: TNF inhibitors have the most robust safety data from before conception through lactation. Under current guidelines, certolizumab is viewed as the safest to use throughout pregnancy. Dr. Shi’s message to her colleagues fielding the same questions she gets from patients: “There is more data coming out every year. Ultimately, we will have better information to inform our patients.”
At the conference, Lawrence F. Eichenfield, MD, a course director and professor of dermatology and pediatrics at the University of California, San Diego and Rady Children’s Hospital San Diego, encouraged Dr. Shi to write up her presentation as a resource for other dermatologists – which she said is in progress.
Medscape Live and this news organization are owned by the same parent company. Dr. Shi disclosed consulting and investigative and research funding from several pharmaceutical firms, but not directly related to the content of her presentation.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR