User login
What’s new in hepatitis C: Four themes that dominated at the Liver Meeting
BOSTON – Treatment of persons who inject drugs, updates in pangenotypic direct-acting antiviral therapy, the benefits of sustained virologic response, and preemptive therapy in donor-positive organ transplantation topped the list of notable hepatitis C–related abstracts this year at the annual meeting of the American Association for the Study of Liver Diseases.
That’s according to Marc Ghany, MD, of the liver diseases branch of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, who gave a hepatitis C debrief to attendees on the final day of the meeting. Here are some of the meeting highlights as summarized by Dr. Ghany in this well-attended last-day session.
Treatment of HCV in people who inject drugs
Emerging data suggest it is feasible to treat hepatitis C virus (HCV) infection in persons who inject drugs (PWIDs); however, overcoming adherence issues remains a challenge, Dr. Ghany told attendees.
According to one study presented at AASLD by Dhiman and coauthors (Abstract 0165), decentralized care of PWIDs using direct-acting antiviral (DAA) therapy was safe and effective, even in those with cirrhosis. Authors demonstrated an “impressive” rate of sustained virologic response at 12 weeks (SVR12) of 91% by a modified intention-to-treat analysis, Dr. Ghany said; however, treatment interruptions were frequent and reduced the overall SVR rate in the study to 78%.
Other studies at the meeting looked at strategies to improve DAA efficacy in this population of patients at high risk of nonadherence, including use of a digital medicine program (Abstract 1554) and a model of care in which an internist-addiction medicine specialist evaluated opiate-dependent patients for HCV infection in a hepatology clinic (Abstract 1589).
Reinfection remains a focus of research in PWIDs. At this meeting, Janjua and coauthors reported that DAA-treated PWIDs in British Columbia had a threefold higher rate of reinfection versus non-PWIDs; however, there were no detected reinfections among PWIDs who had received uninterrupted opioid agonist therapy. “These data suggested that opioid agonist therapy should be given before and after HCV treatment in persons who inject drugs to prevent the infection,” Dr. Ghany said in his presentation.
Updates on pangenotypic DAA therapy
Jonas and coauthors (Abstract 1551) reported on the safety and efficacy of glecaprevir/pibrentasvir for 8 weeks in children with chronic HCV infection enrolled in the ongoing phase 2/3 DORA study. The SVR12 was high, according to Dr. Ghany, at 96% overall, and consistent across age cohorts from 3 to less than 12 years of age.
“In the near future, we should have a safe and effective regimen (approved) for children 3 years or older,” Dr. Ghany said. “I think this will serve us well, as we try to eliminate HCV in children, who number up to 5 million cases worldwide.”
A short course of glecaprevir/pibrentasvir is approved for patients with HCV and compensated cirrhosis, and data to support that was presented last year at The Liver Meeting; however, data were not presented on patients with genotype 3, the most difficult-to-treat genotype, Dr. Ghany said. That gap was filled at this year’s meeting with a report (Abstract LP9) showing SVR12 rates of 98.4% per protocol and 95.2% in intention-to-treat analysis.
Relationship of SVR to clinical outcomes
While the impact of sustained virologic response (SVR) on all-cause mortality is clear in patients with HCV, less is known about the effect of SVR on liver-related mortality and other outcomes, Dr. Ghany said. In one study presented here (Abstract 0039), based on analysis of a Veterans Affairs database of patients with chronic HCV infection, SVR was linked to a significant reduction in liver-related mortality, while in another report (Abstract 0037), SVR was associated with significant reductions in acute coronary syndromes, end-stage renal disease, and ischemic stroke.
Similarly, a multinational, propensity score–matched analysis (Abstract 0040) demonstrated that SVR had an impact on 5-year overall survival and liver-related survival in patients with HCV-related hepatocellular carcinoma (HCC). “For HCC patients who are candidates for HCC therapy, consideration should also be given to treating these individuals (with DAA therapy) because of the impact on overall survival,” Dr. Ghany said.
Preemptive DAA therapy in organ transplantation
Exciting new data show that preemptive therapy, given for short durations, appears to either prevent or cure HCV infection after organ transplant, said Dr. Ghany.
A retrospective analysis by Wijarnpreecha and colleagues (Abstract 0003) showed that 12 or 24 weeks of direct-acting antiviral (DAA) therapy resulted in an SVR12 for 24 out of 24 HCV-seropositive to HCV-seronegative liver transplants, while Durand and colleagues (Abstract 0042) showed that just 4 weeks of pre- and postexposure DAA prophylaxis resulted in SVR12s for 9 out of 9 HCV donor-positive, recipient-negative kidney transplants. Finally, Feld and coauthors (Abstract 0038) showed that preemptive ezetimibe with DAA therapy for 7 days prevented or rapidly cured infection in an experience that included 16 HCV-positive organ donors and 25 HCV-negative recipients.
“While these data are very encouraging, I think we do need to have long-term follow-up of these patients for graft survival, as well as the effect on wait times,” Dr. Ghany said.
Dr. Ghany reported no disclosures related to his presentation.
BOSTON – Treatment of persons who inject drugs, updates in pangenotypic direct-acting antiviral therapy, the benefits of sustained virologic response, and preemptive therapy in donor-positive organ transplantation topped the list of notable hepatitis C–related abstracts this year at the annual meeting of the American Association for the Study of Liver Diseases.
That’s according to Marc Ghany, MD, of the liver diseases branch of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, who gave a hepatitis C debrief to attendees on the final day of the meeting. Here are some of the meeting highlights as summarized by Dr. Ghany in this well-attended last-day session.
Treatment of HCV in people who inject drugs
Emerging data suggest it is feasible to treat hepatitis C virus (HCV) infection in persons who inject drugs (PWIDs); however, overcoming adherence issues remains a challenge, Dr. Ghany told attendees.
According to one study presented at AASLD by Dhiman and coauthors (Abstract 0165), decentralized care of PWIDs using direct-acting antiviral (DAA) therapy was safe and effective, even in those with cirrhosis. Authors demonstrated an “impressive” rate of sustained virologic response at 12 weeks (SVR12) of 91% by a modified intention-to-treat analysis, Dr. Ghany said; however, treatment interruptions were frequent and reduced the overall SVR rate in the study to 78%.
Other studies at the meeting looked at strategies to improve DAA efficacy in this population of patients at high risk of nonadherence, including use of a digital medicine program (Abstract 1554) and a model of care in which an internist-addiction medicine specialist evaluated opiate-dependent patients for HCV infection in a hepatology clinic (Abstract 1589).
Reinfection remains a focus of research in PWIDs. At this meeting, Janjua and coauthors reported that DAA-treated PWIDs in British Columbia had a threefold higher rate of reinfection versus non-PWIDs; however, there were no detected reinfections among PWIDs who had received uninterrupted opioid agonist therapy. “These data suggested that opioid agonist therapy should be given before and after HCV treatment in persons who inject drugs to prevent the infection,” Dr. Ghany said in his presentation.
Updates on pangenotypic DAA therapy
Jonas and coauthors (Abstract 1551) reported on the safety and efficacy of glecaprevir/pibrentasvir for 8 weeks in children with chronic HCV infection enrolled in the ongoing phase 2/3 DORA study. The SVR12 was high, according to Dr. Ghany, at 96% overall, and consistent across age cohorts from 3 to less than 12 years of age.
“In the near future, we should have a safe and effective regimen (approved) for children 3 years or older,” Dr. Ghany said. “I think this will serve us well, as we try to eliminate HCV in children, who number up to 5 million cases worldwide.”
A short course of glecaprevir/pibrentasvir is approved for patients with HCV and compensated cirrhosis, and data to support that was presented last year at The Liver Meeting; however, data were not presented on patients with genotype 3, the most difficult-to-treat genotype, Dr. Ghany said. That gap was filled at this year’s meeting with a report (Abstract LP9) showing SVR12 rates of 98.4% per protocol and 95.2% in intention-to-treat analysis.
Relationship of SVR to clinical outcomes
While the impact of sustained virologic response (SVR) on all-cause mortality is clear in patients with HCV, less is known about the effect of SVR on liver-related mortality and other outcomes, Dr. Ghany said. In one study presented here (Abstract 0039), based on analysis of a Veterans Affairs database of patients with chronic HCV infection, SVR was linked to a significant reduction in liver-related mortality, while in another report (Abstract 0037), SVR was associated with significant reductions in acute coronary syndromes, end-stage renal disease, and ischemic stroke.
Similarly, a multinational, propensity score–matched analysis (Abstract 0040) demonstrated that SVR had an impact on 5-year overall survival and liver-related survival in patients with HCV-related hepatocellular carcinoma (HCC). “For HCC patients who are candidates for HCC therapy, consideration should also be given to treating these individuals (with DAA therapy) because of the impact on overall survival,” Dr. Ghany said.
Preemptive DAA therapy in organ transplantation
Exciting new data show that preemptive therapy, given for short durations, appears to either prevent or cure HCV infection after organ transplant, said Dr. Ghany.
A retrospective analysis by Wijarnpreecha and colleagues (Abstract 0003) showed that 12 or 24 weeks of direct-acting antiviral (DAA) therapy resulted in an SVR12 for 24 out of 24 HCV-seropositive to HCV-seronegative liver transplants, while Durand and colleagues (Abstract 0042) showed that just 4 weeks of pre- and postexposure DAA prophylaxis resulted in SVR12s for 9 out of 9 HCV donor-positive, recipient-negative kidney transplants. Finally, Feld and coauthors (Abstract 0038) showed that preemptive ezetimibe with DAA therapy for 7 days prevented or rapidly cured infection in an experience that included 16 HCV-positive organ donors and 25 HCV-negative recipients.
“While these data are very encouraging, I think we do need to have long-term follow-up of these patients for graft survival, as well as the effect on wait times,” Dr. Ghany said.
Dr. Ghany reported no disclosures related to his presentation.
BOSTON – Treatment of persons who inject drugs, updates in pangenotypic direct-acting antiviral therapy, the benefits of sustained virologic response, and preemptive therapy in donor-positive organ transplantation topped the list of notable hepatitis C–related abstracts this year at the annual meeting of the American Association for the Study of Liver Diseases.
That’s according to Marc Ghany, MD, of the liver diseases branch of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, who gave a hepatitis C debrief to attendees on the final day of the meeting. Here are some of the meeting highlights as summarized by Dr. Ghany in this well-attended last-day session.
Treatment of HCV in people who inject drugs
Emerging data suggest it is feasible to treat hepatitis C virus (HCV) infection in persons who inject drugs (PWIDs); however, overcoming adherence issues remains a challenge, Dr. Ghany told attendees.
According to one study presented at AASLD by Dhiman and coauthors (Abstract 0165), decentralized care of PWIDs using direct-acting antiviral (DAA) therapy was safe and effective, even in those with cirrhosis. Authors demonstrated an “impressive” rate of sustained virologic response at 12 weeks (SVR12) of 91% by a modified intention-to-treat analysis, Dr. Ghany said; however, treatment interruptions were frequent and reduced the overall SVR rate in the study to 78%.
Other studies at the meeting looked at strategies to improve DAA efficacy in this population of patients at high risk of nonadherence, including use of a digital medicine program (Abstract 1554) and a model of care in which an internist-addiction medicine specialist evaluated opiate-dependent patients for HCV infection in a hepatology clinic (Abstract 1589).
Reinfection remains a focus of research in PWIDs. At this meeting, Janjua and coauthors reported that DAA-treated PWIDs in British Columbia had a threefold higher rate of reinfection versus non-PWIDs; however, there were no detected reinfections among PWIDs who had received uninterrupted opioid agonist therapy. “These data suggested that opioid agonist therapy should be given before and after HCV treatment in persons who inject drugs to prevent the infection,” Dr. Ghany said in his presentation.
Updates on pangenotypic DAA therapy
Jonas and coauthors (Abstract 1551) reported on the safety and efficacy of glecaprevir/pibrentasvir for 8 weeks in children with chronic HCV infection enrolled in the ongoing phase 2/3 DORA study. The SVR12 was high, according to Dr. Ghany, at 96% overall, and consistent across age cohorts from 3 to less than 12 years of age.
“In the near future, we should have a safe and effective regimen (approved) for children 3 years or older,” Dr. Ghany said. “I think this will serve us well, as we try to eliminate HCV in children, who number up to 5 million cases worldwide.”
A short course of glecaprevir/pibrentasvir is approved for patients with HCV and compensated cirrhosis, and data to support that was presented last year at The Liver Meeting; however, data were not presented on patients with genotype 3, the most difficult-to-treat genotype, Dr. Ghany said. That gap was filled at this year’s meeting with a report (Abstract LP9) showing SVR12 rates of 98.4% per protocol and 95.2% in intention-to-treat analysis.
Relationship of SVR to clinical outcomes
While the impact of sustained virologic response (SVR) on all-cause mortality is clear in patients with HCV, less is known about the effect of SVR on liver-related mortality and other outcomes, Dr. Ghany said. In one study presented here (Abstract 0039), based on analysis of a Veterans Affairs database of patients with chronic HCV infection, SVR was linked to a significant reduction in liver-related mortality, while in another report (Abstract 0037), SVR was associated with significant reductions in acute coronary syndromes, end-stage renal disease, and ischemic stroke.
Similarly, a multinational, propensity score–matched analysis (Abstract 0040) demonstrated that SVR had an impact on 5-year overall survival and liver-related survival in patients with HCV-related hepatocellular carcinoma (HCC). “For HCC patients who are candidates for HCC therapy, consideration should also be given to treating these individuals (with DAA therapy) because of the impact on overall survival,” Dr. Ghany said.
Preemptive DAA therapy in organ transplantation
Exciting new data show that preemptive therapy, given for short durations, appears to either prevent or cure HCV infection after organ transplant, said Dr. Ghany.
A retrospective analysis by Wijarnpreecha and colleagues (Abstract 0003) showed that 12 or 24 weeks of direct-acting antiviral (DAA) therapy resulted in an SVR12 for 24 out of 24 HCV-seropositive to HCV-seronegative liver transplants, while Durand and colleagues (Abstract 0042) showed that just 4 weeks of pre- and postexposure DAA prophylaxis resulted in SVR12s for 9 out of 9 HCV donor-positive, recipient-negative kidney transplants. Finally, Feld and coauthors (Abstract 0038) showed that preemptive ezetimibe with DAA therapy for 7 days prevented or rapidly cured infection in an experience that included 16 HCV-positive organ donors and 25 HCV-negative recipients.
“While these data are very encouraging, I think we do need to have long-term follow-up of these patients for graft survival, as well as the effect on wait times,” Dr. Ghany said.
Dr. Ghany reported no disclosures related to his presentation.
REPORTING FROM THE LIVER MEETING 2019
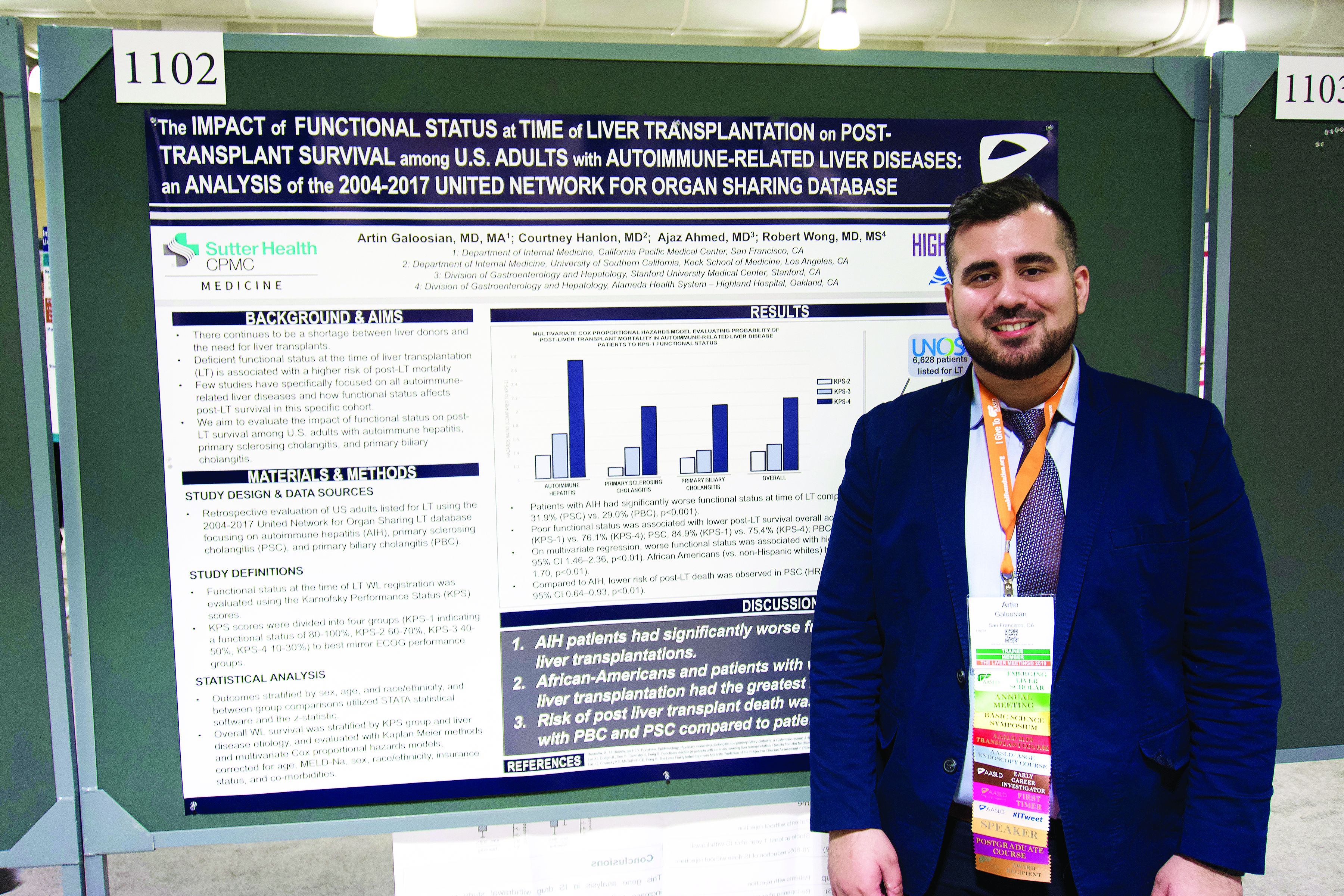
Autoimmune liver disease: Karnofsky score predicts transplant survival
BOSTON – The Karnofsky Performance Status is predictive of 5-year survival among patients with autoimmune-related liver disease who undergo a transplant, based on a retrospective look at more than 6,500 patients.
The analysis also showed that African American patients had a 33% higher mortality risk than non-Hispanic white patients, reported lead author Artin Galoosian, MD, of California Pacific Medical Center in San Francisco, who presented findings at the annual meeting of the American Association for the Study of Liver Diseases.
According to Dr. Galoosian, previous research has shown that Karnofsky scores are a quick and reliable means of predicting survival with liver transplant, but minimal research has evaluated this clinical tool specifically for patients with autoimmune-related liver diseases, which prompted the present study.
Drawing data from the United Network for Organ Sharing (UNOS; 2004-2017), the investigators evaluated performance status and survival in 6,628 patients who underwent liver transplant for one of three diseases: autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC), or primary biliary cholangitis (PBC). Karnofsky scores were divided into quartiles 1 through 4, from best to worst functional status. The investigators used Kaplan-Meier methods and multivariate Cox proportional hazard ratios to determine relationships between disease etiology, Karnofsky score, and survival; in addition, they evaluated the impact of demographic factors on outcomes.
The population was predominantly non-Hispanic white (73.0%) with smaller proportions of African American (13.4%) and Hispanic patients (11.5%). Of the three diseases, PBC was most common (38.2%), followed by PSC (32.1%), then AIH (29.7%).
Across all etiologies, Karnofsky status was significantly associated with survival; a score of 4 came with a 90% increased risk of posttransplant death, compared with a score of 1. Patients with AIH were most likely to have poor pretransplant functional status, as 39.1% of these patients had a Karnofsky score of 4, compared with 31.9% of patients with PSC and 29.0% of patients with PBC. AIH was also associated with a significantly higher risk of posttransplant death; relative risks for PSC and PBC were 20% and 17% lower, respectively.
Five years after surgery, 84.9% of AIH patients with a Karnofsky score of 1 were alive, compared with 76.1% of patients who had a score of 4. A similar association with functional status was found for PSC (84.9% vs. 75.4%), while PBC had a narrower survival margin (88.7% vs. 86.9%).
Analysis also revealed a wide survival gap between patients of different ethnic backgrounds. Compared with white patients, African American patients had a 33% higher risk of dying on the wait list or after transplant.
“[This gap] could reflect a multitude of issues, one being delayed referral to a hepatologist and being listed for transplant much later, so [patients] tend to be more sick,” Dr. Galoosian said.
He also offered some insight into clinical relevance.
“A broader implication of this research could be in the primary care setting,” Dr. Galoosian said. “[Clinicians need to be] aware that someone’s functional status has a broader impact on their health and be aware that ethnic minorities need to be more vigilantly up to date on their health care maintenance and more vigilantly connected to social workers if needed, in terms of getting the resources that they need to help break the [chain] of worse outcomes.”
The investigators disclosed relationships with Gilead, Salix, and AbbVie.
SOURCE: Galoosian A et al. The Liver Meeting 2019. Abstract 1102.
BOSTON – The Karnofsky Performance Status is predictive of 5-year survival among patients with autoimmune-related liver disease who undergo a transplant, based on a retrospective look at more than 6,500 patients.
The analysis also showed that African American patients had a 33% higher mortality risk than non-Hispanic white patients, reported lead author Artin Galoosian, MD, of California Pacific Medical Center in San Francisco, who presented findings at the annual meeting of the American Association for the Study of Liver Diseases.
According to Dr. Galoosian, previous research has shown that Karnofsky scores are a quick and reliable means of predicting survival with liver transplant, but minimal research has evaluated this clinical tool specifically for patients with autoimmune-related liver diseases, which prompted the present study.
Drawing data from the United Network for Organ Sharing (UNOS; 2004-2017), the investigators evaluated performance status and survival in 6,628 patients who underwent liver transplant for one of three diseases: autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC), or primary biliary cholangitis (PBC). Karnofsky scores were divided into quartiles 1 through 4, from best to worst functional status. The investigators used Kaplan-Meier methods and multivariate Cox proportional hazard ratios to determine relationships between disease etiology, Karnofsky score, and survival; in addition, they evaluated the impact of demographic factors on outcomes.
The population was predominantly non-Hispanic white (73.0%) with smaller proportions of African American (13.4%) and Hispanic patients (11.5%). Of the three diseases, PBC was most common (38.2%), followed by PSC (32.1%), then AIH (29.7%).
Across all etiologies, Karnofsky status was significantly associated with survival; a score of 4 came with a 90% increased risk of posttransplant death, compared with a score of 1. Patients with AIH were most likely to have poor pretransplant functional status, as 39.1% of these patients had a Karnofsky score of 4, compared with 31.9% of patients with PSC and 29.0% of patients with PBC. AIH was also associated with a significantly higher risk of posttransplant death; relative risks for PSC and PBC were 20% and 17% lower, respectively.
Five years after surgery, 84.9% of AIH patients with a Karnofsky score of 1 were alive, compared with 76.1% of patients who had a score of 4. A similar association with functional status was found for PSC (84.9% vs. 75.4%), while PBC had a narrower survival margin (88.7% vs. 86.9%).
Analysis also revealed a wide survival gap between patients of different ethnic backgrounds. Compared with white patients, African American patients had a 33% higher risk of dying on the wait list or after transplant.
“[This gap] could reflect a multitude of issues, one being delayed referral to a hepatologist and being listed for transplant much later, so [patients] tend to be more sick,” Dr. Galoosian said.
He also offered some insight into clinical relevance.
“A broader implication of this research could be in the primary care setting,” Dr. Galoosian said. “[Clinicians need to be] aware that someone’s functional status has a broader impact on their health and be aware that ethnic minorities need to be more vigilantly up to date on their health care maintenance and more vigilantly connected to social workers if needed, in terms of getting the resources that they need to help break the [chain] of worse outcomes.”
The investigators disclosed relationships with Gilead, Salix, and AbbVie.
SOURCE: Galoosian A et al. The Liver Meeting 2019. Abstract 1102.
BOSTON – The Karnofsky Performance Status is predictive of 5-year survival among patients with autoimmune-related liver disease who undergo a transplant, based on a retrospective look at more than 6,500 patients.
The analysis also showed that African American patients had a 33% higher mortality risk than non-Hispanic white patients, reported lead author Artin Galoosian, MD, of California Pacific Medical Center in San Francisco, who presented findings at the annual meeting of the American Association for the Study of Liver Diseases.
According to Dr. Galoosian, previous research has shown that Karnofsky scores are a quick and reliable means of predicting survival with liver transplant, but minimal research has evaluated this clinical tool specifically for patients with autoimmune-related liver diseases, which prompted the present study.
Drawing data from the United Network for Organ Sharing (UNOS; 2004-2017), the investigators evaluated performance status and survival in 6,628 patients who underwent liver transplant for one of three diseases: autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC), or primary biliary cholangitis (PBC). Karnofsky scores were divided into quartiles 1 through 4, from best to worst functional status. The investigators used Kaplan-Meier methods and multivariate Cox proportional hazard ratios to determine relationships between disease etiology, Karnofsky score, and survival; in addition, they evaluated the impact of demographic factors on outcomes.
The population was predominantly non-Hispanic white (73.0%) with smaller proportions of African American (13.4%) and Hispanic patients (11.5%). Of the three diseases, PBC was most common (38.2%), followed by PSC (32.1%), then AIH (29.7%).
Across all etiologies, Karnofsky status was significantly associated with survival; a score of 4 came with a 90% increased risk of posttransplant death, compared with a score of 1. Patients with AIH were most likely to have poor pretransplant functional status, as 39.1% of these patients had a Karnofsky score of 4, compared with 31.9% of patients with PSC and 29.0% of patients with PBC. AIH was also associated with a significantly higher risk of posttransplant death; relative risks for PSC and PBC were 20% and 17% lower, respectively.
Five years after surgery, 84.9% of AIH patients with a Karnofsky score of 1 were alive, compared with 76.1% of patients who had a score of 4. A similar association with functional status was found for PSC (84.9% vs. 75.4%), while PBC had a narrower survival margin (88.7% vs. 86.9%).
Analysis also revealed a wide survival gap between patients of different ethnic backgrounds. Compared with white patients, African American patients had a 33% higher risk of dying on the wait list or after transplant.
“[This gap] could reflect a multitude of issues, one being delayed referral to a hepatologist and being listed for transplant much later, so [patients] tend to be more sick,” Dr. Galoosian said.
He also offered some insight into clinical relevance.
“A broader implication of this research could be in the primary care setting,” Dr. Galoosian said. “[Clinicians need to be] aware that someone’s functional status has a broader impact on their health and be aware that ethnic minorities need to be more vigilantly up to date on their health care maintenance and more vigilantly connected to social workers if needed, in terms of getting the resources that they need to help break the [chain] of worse outcomes.”
The investigators disclosed relationships with Gilead, Salix, and AbbVie.
SOURCE: Galoosian A et al. The Liver Meeting 2019. Abstract 1102.
REPORTING FROM THE LIVER MEETING 2019
AASLD debrief: Five drugs show promise in NAFLD (and two do not)
BOSTON – For treatment of nonalcoholic fatty liver disease, cotadutide, licogliflozin, tropifexor, saroglitazar, and PF-05221304 are just a few of the drugs with promising data, Kathleen E. Corey, MD, MPH, said at the annual meeting of the American Association for the Study of Liver Diseases.
By contrast, selonsertib and emricasan did not achieve their endpoints in studies described here at the meeting, “but we have a lot to learn from them,” said Dr. Corey, director of the Mass General Fatty Liver Clinic and assistant professor at Harvard Medical School, Boston.
“This is an exciting time,” Dr. Corey said in a special debriefing oral session held on the final day of the conference. “There are many novel mechanisms of action out there, as well as some known mechanisms of action, with a considerable amount of promise.”
Cotadutide (MEDI0382)
Narha and coauthors (Abstract 35) described the effects of cotadutide, a GLP-1/glucagon receptor dual agonist on biomarkers of nonalcoholic steatohepatitis (NASH) at 26 weeks in overweight or obese patients with type 2 diabetes mellitus. In the randomized, phase 2b study, cotadutide produced superior reductions versus liraglutide, the GLP-1 receptor agonist, in alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and body weight, which investigators said supported prospective trials of the drug for a potential indication in NASH.
“The adverse events were fairly typical for what we see with the GLP-1s – GI side effects that usually over 8 weeks improve,” Dr. Corey told attendees at the debrief session.
Licogliflozin (LIK066)
Interim analysis of a 12-week, randomized, placebo-controlled, phase 2a study showed that this SGLT1/2 inhibitor produced “robust” decreases in ALT and improvements in markers of hepatic and metabolic health in patients with NASH, according to Zhang and coauthors (Abstract L07).
Some 67% of those who received licogliflozin had at least a 30% decrease in their liver fat, while decreases in weight and hemoglobin A1c were also reported, according to Dr. Corey. “It was associated with diarrhea in about 97%, but this was considered mild, and certainly, we’re seeing good metabolic effects overall,” she said.
Tropifexor
Treatment for 12 weeks with this potent FXR agonist resulted in robust, dose-dependent reductions in hepatic fat and serum ALT in patients with fibrotic NASH, according to investigators in a phase 2 randomized, placebo-controlled trial known as FLIGHT-FXR (Abstract L04).
A total of 65% of patients achieved a 30% or greater reduction in liver fat, and decreases in weight and insulin resistance were reported. “Similar to other FXRs, they did have this concerning although potentially manageable increase in low-density lipoprotein (LDL)-cholesterol, and the adverse event of pruritis,” said Dr. Corey.
Saroglitazar
Gawrieh and coauthors presented results from EVIDENCES IV, a phase 2, double-blind, randomized, placebo-controlled study of saroglitazar, a novel dual peroxisome proliferator activated receptor (PPAR) alpha/gamma agonist, in patients with NAFLD or NASH (Abstract LO10).
The investigators found that 41% of patients achieved a 30% or greater relative reduction in liver fat, as well as reductions in hemoglobin A1c and lipids, but the treatment was “weight neutral,” Dr. Corey said, adding that no serious adverse events were reported.
PF-05221304
This liver-targeted acetyl-CoA carboxylase inhibitor (ACCI) demonstrated robust reduction in liver fat and ALT in a 16-week phase 2a, dose-ranging study in adults with NAFLD, according to Amin and coinvestigators (Abstract 31).
There was a “dramatic” decrease in liver fat in this study, said Dr. Corey, with 90% of treated patients experiencing a 30% or greater decrease. Side effects included a “significant” increase in triglycerides, she added, as well as transient increases in ALT and AST.
Selonsertib and emricasan
One agent not meeting study endpoints was selonsertib, an apoptosis signal-regulating kinase 1 (ASK1) inhibitor. While safe and well tolerated, the drug was nevertheless not effective as monotherapy in phase 3 double-blind, placebo-controlled trials including patients with advanced fibrosis due to NASH, investigators said (Abstract 64). Currently, the agent is being evaluated in combination with firsocostat – an ACCI – in a phase 2 study called ATLAS, according to the authors.
Emricasan, an oral pan-caspase inhibitor that suppresses apoptosis, did not improve fibrosis or resolve NASH in a multicenter, double-blind, placebo-controlled randomized trial, and may have even worsened histology, according to Dr. Corey. Investigators said further evaluation of the mechanisms underlying findings could provide insights into the role of necro-apoptosis in NASH pathophysiology (Abstract 61).
Dr. Corey provided disclosures related to BMS, Novo Nordisk, Boehringer Ingelheim, and Gilead.
BOSTON – For treatment of nonalcoholic fatty liver disease, cotadutide, licogliflozin, tropifexor, saroglitazar, and PF-05221304 are just a few of the drugs with promising data, Kathleen E. Corey, MD, MPH, said at the annual meeting of the American Association for the Study of Liver Diseases.
By contrast, selonsertib and emricasan did not achieve their endpoints in studies described here at the meeting, “but we have a lot to learn from them,” said Dr. Corey, director of the Mass General Fatty Liver Clinic and assistant professor at Harvard Medical School, Boston.
“This is an exciting time,” Dr. Corey said in a special debriefing oral session held on the final day of the conference. “There are many novel mechanisms of action out there, as well as some known mechanisms of action, with a considerable amount of promise.”
Cotadutide (MEDI0382)
Narha and coauthors (Abstract 35) described the effects of cotadutide, a GLP-1/glucagon receptor dual agonist on biomarkers of nonalcoholic steatohepatitis (NASH) at 26 weeks in overweight or obese patients with type 2 diabetes mellitus. In the randomized, phase 2b study, cotadutide produced superior reductions versus liraglutide, the GLP-1 receptor agonist, in alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and body weight, which investigators said supported prospective trials of the drug for a potential indication in NASH.
“The adverse events were fairly typical for what we see with the GLP-1s – GI side effects that usually over 8 weeks improve,” Dr. Corey told attendees at the debrief session.
Licogliflozin (LIK066)
Interim analysis of a 12-week, randomized, placebo-controlled, phase 2a study showed that this SGLT1/2 inhibitor produced “robust” decreases in ALT and improvements in markers of hepatic and metabolic health in patients with NASH, according to Zhang and coauthors (Abstract L07).
Some 67% of those who received licogliflozin had at least a 30% decrease in their liver fat, while decreases in weight and hemoglobin A1c were also reported, according to Dr. Corey. “It was associated with diarrhea in about 97%, but this was considered mild, and certainly, we’re seeing good metabolic effects overall,” she said.
Tropifexor
Treatment for 12 weeks with this potent FXR agonist resulted in robust, dose-dependent reductions in hepatic fat and serum ALT in patients with fibrotic NASH, according to investigators in a phase 2 randomized, placebo-controlled trial known as FLIGHT-FXR (Abstract L04).
A total of 65% of patients achieved a 30% or greater reduction in liver fat, and decreases in weight and insulin resistance were reported. “Similar to other FXRs, they did have this concerning although potentially manageable increase in low-density lipoprotein (LDL)-cholesterol, and the adverse event of pruritis,” said Dr. Corey.
Saroglitazar
Gawrieh and coauthors presented results from EVIDENCES IV, a phase 2, double-blind, randomized, placebo-controlled study of saroglitazar, a novel dual peroxisome proliferator activated receptor (PPAR) alpha/gamma agonist, in patients with NAFLD or NASH (Abstract LO10).
The investigators found that 41% of patients achieved a 30% or greater relative reduction in liver fat, as well as reductions in hemoglobin A1c and lipids, but the treatment was “weight neutral,” Dr. Corey said, adding that no serious adverse events were reported.
PF-05221304
This liver-targeted acetyl-CoA carboxylase inhibitor (ACCI) demonstrated robust reduction in liver fat and ALT in a 16-week phase 2a, dose-ranging study in adults with NAFLD, according to Amin and coinvestigators (Abstract 31).
There was a “dramatic” decrease in liver fat in this study, said Dr. Corey, with 90% of treated patients experiencing a 30% or greater decrease. Side effects included a “significant” increase in triglycerides, she added, as well as transient increases in ALT and AST.
Selonsertib and emricasan
One agent not meeting study endpoints was selonsertib, an apoptosis signal-regulating kinase 1 (ASK1) inhibitor. While safe and well tolerated, the drug was nevertheless not effective as monotherapy in phase 3 double-blind, placebo-controlled trials including patients with advanced fibrosis due to NASH, investigators said (Abstract 64). Currently, the agent is being evaluated in combination with firsocostat – an ACCI – in a phase 2 study called ATLAS, according to the authors.
Emricasan, an oral pan-caspase inhibitor that suppresses apoptosis, did not improve fibrosis or resolve NASH in a multicenter, double-blind, placebo-controlled randomized trial, and may have even worsened histology, according to Dr. Corey. Investigators said further evaluation of the mechanisms underlying findings could provide insights into the role of necro-apoptosis in NASH pathophysiology (Abstract 61).
Dr. Corey provided disclosures related to BMS, Novo Nordisk, Boehringer Ingelheim, and Gilead.
BOSTON – For treatment of nonalcoholic fatty liver disease, cotadutide, licogliflozin, tropifexor, saroglitazar, and PF-05221304 are just a few of the drugs with promising data, Kathleen E. Corey, MD, MPH, said at the annual meeting of the American Association for the Study of Liver Diseases.
By contrast, selonsertib and emricasan did not achieve their endpoints in studies described here at the meeting, “but we have a lot to learn from them,” said Dr. Corey, director of the Mass General Fatty Liver Clinic and assistant professor at Harvard Medical School, Boston.
“This is an exciting time,” Dr. Corey said in a special debriefing oral session held on the final day of the conference. “There are many novel mechanisms of action out there, as well as some known mechanisms of action, with a considerable amount of promise.”
Cotadutide (MEDI0382)
Narha and coauthors (Abstract 35) described the effects of cotadutide, a GLP-1/glucagon receptor dual agonist on biomarkers of nonalcoholic steatohepatitis (NASH) at 26 weeks in overweight or obese patients with type 2 diabetes mellitus. In the randomized, phase 2b study, cotadutide produced superior reductions versus liraglutide, the GLP-1 receptor agonist, in alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and body weight, which investigators said supported prospective trials of the drug for a potential indication in NASH.
“The adverse events were fairly typical for what we see with the GLP-1s – GI side effects that usually over 8 weeks improve,” Dr. Corey told attendees at the debrief session.
Licogliflozin (LIK066)
Interim analysis of a 12-week, randomized, placebo-controlled, phase 2a study showed that this SGLT1/2 inhibitor produced “robust” decreases in ALT and improvements in markers of hepatic and metabolic health in patients with NASH, according to Zhang and coauthors (Abstract L07).
Some 67% of those who received licogliflozin had at least a 30% decrease in their liver fat, while decreases in weight and hemoglobin A1c were also reported, according to Dr. Corey. “It was associated with diarrhea in about 97%, but this was considered mild, and certainly, we’re seeing good metabolic effects overall,” she said.
Tropifexor
Treatment for 12 weeks with this potent FXR agonist resulted in robust, dose-dependent reductions in hepatic fat and serum ALT in patients with fibrotic NASH, according to investigators in a phase 2 randomized, placebo-controlled trial known as FLIGHT-FXR (Abstract L04).
A total of 65% of patients achieved a 30% or greater reduction in liver fat, and decreases in weight and insulin resistance were reported. “Similar to other FXRs, they did have this concerning although potentially manageable increase in low-density lipoprotein (LDL)-cholesterol, and the adverse event of pruritis,” said Dr. Corey.
Saroglitazar
Gawrieh and coauthors presented results from EVIDENCES IV, a phase 2, double-blind, randomized, placebo-controlled study of saroglitazar, a novel dual peroxisome proliferator activated receptor (PPAR) alpha/gamma agonist, in patients with NAFLD or NASH (Abstract LO10).
The investigators found that 41% of patients achieved a 30% or greater relative reduction in liver fat, as well as reductions in hemoglobin A1c and lipids, but the treatment was “weight neutral,” Dr. Corey said, adding that no serious adverse events were reported.
PF-05221304
This liver-targeted acetyl-CoA carboxylase inhibitor (ACCI) demonstrated robust reduction in liver fat and ALT in a 16-week phase 2a, dose-ranging study in adults with NAFLD, according to Amin and coinvestigators (Abstract 31).
There was a “dramatic” decrease in liver fat in this study, said Dr. Corey, with 90% of treated patients experiencing a 30% or greater decrease. Side effects included a “significant” increase in triglycerides, she added, as well as transient increases in ALT and AST.
Selonsertib and emricasan
One agent not meeting study endpoints was selonsertib, an apoptosis signal-regulating kinase 1 (ASK1) inhibitor. While safe and well tolerated, the drug was nevertheless not effective as monotherapy in phase 3 double-blind, placebo-controlled trials including patients with advanced fibrosis due to NASH, investigators said (Abstract 64). Currently, the agent is being evaluated in combination with firsocostat – an ACCI – in a phase 2 study called ATLAS, according to the authors.
Emricasan, an oral pan-caspase inhibitor that suppresses apoptosis, did not improve fibrosis or resolve NASH in a multicenter, double-blind, placebo-controlled randomized trial, and may have even worsened histology, according to Dr. Corey. Investigators said further evaluation of the mechanisms underlying findings could provide insights into the role of necro-apoptosis in NASH pathophysiology (Abstract 61).
Dr. Corey provided disclosures related to BMS, Novo Nordisk, Boehringer Ingelheim, and Gilead.
REPORTING FROM THE LIVER MEETING 2019
TARGET-NASH: One-third of NAFLD, NASH patients lost weight, but not all kept it off
BOSTON – While about one-third of overweight patients with nonalcoholic fatty liver disease (NAFLD) or nonalcoholic steatohepatitis (NASH) slimmed down after a weight-loss intervention, about 20% gained weight back within the next few years, according to results from a large, long-term observational cohort study.
Effectively, that meant about one-quarter of overweight subjects enrolled in the TARGET-NASH study were able to lose weight and keep it off, said investigator Miguel H. Malespin, MD, a transplant hepatologist at Tampa General Hospital.
These findings illustrate how challenging lifestyle interventions can be for patients with NAFLD and NASH, and have clinical implications for identifying those individuals who are more or less likely to respond to weight-loss intervention as an initial treatment, Dr. Malespin said.
“I think we as providers need to better identify what are the barriers for weight loss for these patients, and try to use data, such as this data from TARGET-NASH, to help our clinical judgment and to learn strategies to be able to help these patients optimize their weight loss,” he added in an interview.
With no approved pharmacologic treatments for NAFLD and NASH, weight reduction remains one of the mainstays of treatment, Dr. Malespin said.
However, there are few studies evaluating structured programs designed to achieve such weight loss, and the long-term sustainability of this approach remains in question, according to Dr. Malespin, who presented findings of TARGET-NASH at the annual meeting of the American Association for the Study of Liver Diseases.
To date, more than 4,500 patients have been enrolled in TARGET-NASH at 59 U.S. centers. The present analysis, which Dr. Malespin described in an oral abstract presentation, included 2,037 patients with a body mass index of at least 25 kg/m2 and no decompensated cirrhosis, bariatric surgery, weight-loss medication use, or cancer diagnoses.
In sum, 34% of patients had 5% or more weight loss over about 3 years of follow-up, while about 10% had a 10% or greater weight loss, Dr. Malespin reported.
It took patients about 18 months, on average, to achieve 5% or greater weight loss. Those who achieved weight loss tended to be older, according to the investigator, and were about 1.5 times as likely to be obese class II or III, as opposed to just being overweight.
Patients were significantly more likely to lose weight, Dr. Malespin and coauthors found, if they had cirrhosis or comorbid medical conditions such as diabetes, osteoarthritis, or cardiovascular disease.
“All these factors essentially imply that these patients are hopefully being seen by multiple providers,” Dr. Malespin said, “so hopefully they’re getting the message [on the need for lifestyle modifications] from multiple different providers, and this is contributing to why we’re seeing some of this weight loss.”
However, weight loss did not persist in all patients, with 20.2% regaining weight back up to baseline or greater, according to the report. The median time to weight regain was 24-31 months, depending on initial weight class.
TARGET-NASH, sponsored by TARGET PharmaSolutions, is a 5-year longitudinal observational study with an enrollment goal of 15,000 patients with NAFLD or NASH and an estimated study completion date of 2021, according to a ClinicalTrials.gov listing.
Dr. Malespin reported disclosures with Gilead (speaker board), Intercept (speaker board, advisory committee), and TARGET PharmaSolutions (research grant paid to institution).
SOURCE: Malespin MH et al. The Liver Meeting 2019, Abstract 240.
BOSTON – While about one-third of overweight patients with nonalcoholic fatty liver disease (NAFLD) or nonalcoholic steatohepatitis (NASH) slimmed down after a weight-loss intervention, about 20% gained weight back within the next few years, according to results from a large, long-term observational cohort study.
Effectively, that meant about one-quarter of overweight subjects enrolled in the TARGET-NASH study were able to lose weight and keep it off, said investigator Miguel H. Malespin, MD, a transplant hepatologist at Tampa General Hospital.
These findings illustrate how challenging lifestyle interventions can be for patients with NAFLD and NASH, and have clinical implications for identifying those individuals who are more or less likely to respond to weight-loss intervention as an initial treatment, Dr. Malespin said.
“I think we as providers need to better identify what are the barriers for weight loss for these patients, and try to use data, such as this data from TARGET-NASH, to help our clinical judgment and to learn strategies to be able to help these patients optimize their weight loss,” he added in an interview.
With no approved pharmacologic treatments for NAFLD and NASH, weight reduction remains one of the mainstays of treatment, Dr. Malespin said.
However, there are few studies evaluating structured programs designed to achieve such weight loss, and the long-term sustainability of this approach remains in question, according to Dr. Malespin, who presented findings of TARGET-NASH at the annual meeting of the American Association for the Study of Liver Diseases.
To date, more than 4,500 patients have been enrolled in TARGET-NASH at 59 U.S. centers. The present analysis, which Dr. Malespin described in an oral abstract presentation, included 2,037 patients with a body mass index of at least 25 kg/m2 and no decompensated cirrhosis, bariatric surgery, weight-loss medication use, or cancer diagnoses.
In sum, 34% of patients had 5% or more weight loss over about 3 years of follow-up, while about 10% had a 10% or greater weight loss, Dr. Malespin reported.
It took patients about 18 months, on average, to achieve 5% or greater weight loss. Those who achieved weight loss tended to be older, according to the investigator, and were about 1.5 times as likely to be obese class II or III, as opposed to just being overweight.
Patients were significantly more likely to lose weight, Dr. Malespin and coauthors found, if they had cirrhosis or comorbid medical conditions such as diabetes, osteoarthritis, or cardiovascular disease.
“All these factors essentially imply that these patients are hopefully being seen by multiple providers,” Dr. Malespin said, “so hopefully they’re getting the message [on the need for lifestyle modifications] from multiple different providers, and this is contributing to why we’re seeing some of this weight loss.”
However, weight loss did not persist in all patients, with 20.2% regaining weight back up to baseline or greater, according to the report. The median time to weight regain was 24-31 months, depending on initial weight class.
TARGET-NASH, sponsored by TARGET PharmaSolutions, is a 5-year longitudinal observational study with an enrollment goal of 15,000 patients with NAFLD or NASH and an estimated study completion date of 2021, according to a ClinicalTrials.gov listing.
Dr. Malespin reported disclosures with Gilead (speaker board), Intercept (speaker board, advisory committee), and TARGET PharmaSolutions (research grant paid to institution).
SOURCE: Malespin MH et al. The Liver Meeting 2019, Abstract 240.
BOSTON – While about one-third of overweight patients with nonalcoholic fatty liver disease (NAFLD) or nonalcoholic steatohepatitis (NASH) slimmed down after a weight-loss intervention, about 20% gained weight back within the next few years, according to results from a large, long-term observational cohort study.
Effectively, that meant about one-quarter of overweight subjects enrolled in the TARGET-NASH study were able to lose weight and keep it off, said investigator Miguel H. Malespin, MD, a transplant hepatologist at Tampa General Hospital.
These findings illustrate how challenging lifestyle interventions can be for patients with NAFLD and NASH, and have clinical implications for identifying those individuals who are more or less likely to respond to weight-loss intervention as an initial treatment, Dr. Malespin said.
“I think we as providers need to better identify what are the barriers for weight loss for these patients, and try to use data, such as this data from TARGET-NASH, to help our clinical judgment and to learn strategies to be able to help these patients optimize their weight loss,” he added in an interview.
With no approved pharmacologic treatments for NAFLD and NASH, weight reduction remains one of the mainstays of treatment, Dr. Malespin said.
However, there are few studies evaluating structured programs designed to achieve such weight loss, and the long-term sustainability of this approach remains in question, according to Dr. Malespin, who presented findings of TARGET-NASH at the annual meeting of the American Association for the Study of Liver Diseases.
To date, more than 4,500 patients have been enrolled in TARGET-NASH at 59 U.S. centers. The present analysis, which Dr. Malespin described in an oral abstract presentation, included 2,037 patients with a body mass index of at least 25 kg/m2 and no decompensated cirrhosis, bariatric surgery, weight-loss medication use, or cancer diagnoses.
In sum, 34% of patients had 5% or more weight loss over about 3 years of follow-up, while about 10% had a 10% or greater weight loss, Dr. Malespin reported.
It took patients about 18 months, on average, to achieve 5% or greater weight loss. Those who achieved weight loss tended to be older, according to the investigator, and were about 1.5 times as likely to be obese class II or III, as opposed to just being overweight.
Patients were significantly more likely to lose weight, Dr. Malespin and coauthors found, if they had cirrhosis or comorbid medical conditions such as diabetes, osteoarthritis, or cardiovascular disease.
“All these factors essentially imply that these patients are hopefully being seen by multiple providers,” Dr. Malespin said, “so hopefully they’re getting the message [on the need for lifestyle modifications] from multiple different providers, and this is contributing to why we’re seeing some of this weight loss.”
However, weight loss did not persist in all patients, with 20.2% regaining weight back up to baseline or greater, according to the report. The median time to weight regain was 24-31 months, depending on initial weight class.
TARGET-NASH, sponsored by TARGET PharmaSolutions, is a 5-year longitudinal observational study with an enrollment goal of 15,000 patients with NAFLD or NASH and an estimated study completion date of 2021, according to a ClinicalTrials.gov listing.
Dr. Malespin reported disclosures with Gilead (speaker board), Intercept (speaker board, advisory committee), and TARGET PharmaSolutions (research grant paid to institution).
SOURCE: Malespin MH et al. The Liver Meeting 2019, Abstract 240.
REPORTING FROM THE LIVER MEETING 2019
ED-based HCV screening found feasible, linkage low
BOSTON – ED-based screening is a feasible method of detecting hepatitis C virus (HCV) in high-risk populations, but linkage to care remains low, according to investigators.
An HCV screening program involving three Seattle hospitals and more than 4,000 patients showed that linkage to care was lowest among patients who were younger, homeless, or used injection drugs, reported lead author Charles S. Landis, MD, PhD, of the University of Washington, Seattle.
“In the U.S., rates of acute HCV infections are increasing in younger patients and in areas disproportionally affected by the opiate epidemic,” Dr. Landis said in a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “In order to achieve a goal of elimination, HCV screening, appropriate linkage to care, and treatment will need to be directed toward younger, marginalized, and underserved populations.”
Dr. Landis explained that EDs are suitable for HCV screening because users of emergency services are disproportionately affected by HCV, compared with patients in primary and specialty care settings. Despite this, linkage to care remains historically higher in primary and specialty care settings at approximately 70%, compared with 30% via the ED, Dr. Landis said.
Historically, EDs have been resistant to HCV screening programs, Dr. Landis said, but with the model used in the present study, which relied upon a full-time staff member in each ED who was employed by the infectious disease or hepatology department, no ED resources were needed.
Participants were willing adults who had reliable contact information. Patients were excluded if they were non–English speaking, incarcerated, enrolled or expected to enroll in another clinical study which excludes coenrollment, planned to move out of the region in the next 6 months, admitted to the ED with an acute life-threatening illness, or admitted to the ED for sexual assault. The program had three objectives: Screening, linkage to care, and treatment, all of which were coordinated by the aforementioned case manager.
To date, 4,182 patients have been screened, 936 have been enrolled, 95 have tested positive for HCV RNA, 32 have been linked with care, and 19 have been treated.
“So you can see, a lot of squeeze for a just a little bit of juice here,” Dr. Landis said, referring to the relatively low number of treated patients, compared with how many were screened.
The prevalence of HCV infection based on RNA testing was 2%, though one hospital had a rate of 5%. “This [prevalence] compares to, but is maybe slightly less than, the prevalence seen in others studies based in the emergency department,” Dr. Landis said. “The thought is, not all emergency departments are equal in terms of the patient population that they serve.”
Data analysis showed that the overall linkage to care was 36%. “This is still suboptimal, from my perspective,” Dr. Landis said, “but it does compare with several other ED-based studies.”
A closer look at the data showed that linkage was not uniform across the population. Among patients with homes, linkage to care was 59%, compared with 20% for patients who were homeless (P = .02).
“Ultimately, we need to tailor our approaches for linking homeless patients differently than patients who are not homeless,” Dr. Landis said.
Patients who reported no injection-drug use had a linkage to care of 50%, which was numerically higher than the rate of 20% among users of injection drugs; this difference was not statistically significant, which Dr. Landis attributed to insufficient population size. Similarly, younger patients showed numerical trends toward lower linkage to care.
“Future work will attempt to optimize linkage to care strategies based on patient demographic factors, such as active injection drug use or homelessness,” Dr. Landis said.
During discussion, a conference attendee from the United States expressed skepticism of the program’s merits.
“I may be a glass-half-empty person, but is it worth all this effort?” the attendee asked. “In all honesty, you treated a few dozen [patients] for 180,000 visits [per year]. I’m really not sure it’s worth those efforts, and I’m wondering if those efforts could be placed in different areas, especially for a higher yield.”
“Point well taken,” Dr. Landis said. “I think that was the purpose of the study, to see if the emergency department is a place to screen and link patients to care, and we’re trying to optimize that. Remember, there were 4,000 patients, but for many of those, it took literally a minute to screen them.”
An attendee from Australia offered a slightly more positive take on the findings, followed by a suggestion to improve linkage in marginalized populations.
“I’m not sure I’d be pessimistic,” the attendee said. “I think you ought to be commended for getting that number of people to link, because it is very difficult when we are looking at linking people from a hospital-based setting who actually live in the community and suffer from homelessness and mental health issues and incarceration and a whole range of other things. ... Maybe we need to change our idea of having these centralized silos where people are referred, and go out into the community, much like [tuberculosis] clinics used to do, and track people down.”
The study was funded by Gilead. The investigators disclosed additional relationships with HighTide Therapeutics, Intercept, AbbVie, and others.
SOURCE: Landis CS et al. The Liver Meeting 2019, Abstract 168.
BOSTON – ED-based screening is a feasible method of detecting hepatitis C virus (HCV) in high-risk populations, but linkage to care remains low, according to investigators.
An HCV screening program involving three Seattle hospitals and more than 4,000 patients showed that linkage to care was lowest among patients who were younger, homeless, or used injection drugs, reported lead author Charles S. Landis, MD, PhD, of the University of Washington, Seattle.
“In the U.S., rates of acute HCV infections are increasing in younger patients and in areas disproportionally affected by the opiate epidemic,” Dr. Landis said in a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “In order to achieve a goal of elimination, HCV screening, appropriate linkage to care, and treatment will need to be directed toward younger, marginalized, and underserved populations.”
Dr. Landis explained that EDs are suitable for HCV screening because users of emergency services are disproportionately affected by HCV, compared with patients in primary and specialty care settings. Despite this, linkage to care remains historically higher in primary and specialty care settings at approximately 70%, compared with 30% via the ED, Dr. Landis said.
Historically, EDs have been resistant to HCV screening programs, Dr. Landis said, but with the model used in the present study, which relied upon a full-time staff member in each ED who was employed by the infectious disease or hepatology department, no ED resources were needed.
Participants were willing adults who had reliable contact information. Patients were excluded if they were non–English speaking, incarcerated, enrolled or expected to enroll in another clinical study which excludes coenrollment, planned to move out of the region in the next 6 months, admitted to the ED with an acute life-threatening illness, or admitted to the ED for sexual assault. The program had three objectives: Screening, linkage to care, and treatment, all of which were coordinated by the aforementioned case manager.
To date, 4,182 patients have been screened, 936 have been enrolled, 95 have tested positive for HCV RNA, 32 have been linked with care, and 19 have been treated.
“So you can see, a lot of squeeze for a just a little bit of juice here,” Dr. Landis said, referring to the relatively low number of treated patients, compared with how many were screened.
The prevalence of HCV infection based on RNA testing was 2%, though one hospital had a rate of 5%. “This [prevalence] compares to, but is maybe slightly less than, the prevalence seen in others studies based in the emergency department,” Dr. Landis said. “The thought is, not all emergency departments are equal in terms of the patient population that they serve.”
Data analysis showed that the overall linkage to care was 36%. “This is still suboptimal, from my perspective,” Dr. Landis said, “but it does compare with several other ED-based studies.”
A closer look at the data showed that linkage was not uniform across the population. Among patients with homes, linkage to care was 59%, compared with 20% for patients who were homeless (P = .02).
“Ultimately, we need to tailor our approaches for linking homeless patients differently than patients who are not homeless,” Dr. Landis said.
Patients who reported no injection-drug use had a linkage to care of 50%, which was numerically higher than the rate of 20% among users of injection drugs; this difference was not statistically significant, which Dr. Landis attributed to insufficient population size. Similarly, younger patients showed numerical trends toward lower linkage to care.
“Future work will attempt to optimize linkage to care strategies based on patient demographic factors, such as active injection drug use or homelessness,” Dr. Landis said.
During discussion, a conference attendee from the United States expressed skepticism of the program’s merits.
“I may be a glass-half-empty person, but is it worth all this effort?” the attendee asked. “In all honesty, you treated a few dozen [patients] for 180,000 visits [per year]. I’m really not sure it’s worth those efforts, and I’m wondering if those efforts could be placed in different areas, especially for a higher yield.”
“Point well taken,” Dr. Landis said. “I think that was the purpose of the study, to see if the emergency department is a place to screen and link patients to care, and we’re trying to optimize that. Remember, there were 4,000 patients, but for many of those, it took literally a minute to screen them.”
An attendee from Australia offered a slightly more positive take on the findings, followed by a suggestion to improve linkage in marginalized populations.
“I’m not sure I’d be pessimistic,” the attendee said. “I think you ought to be commended for getting that number of people to link, because it is very difficult when we are looking at linking people from a hospital-based setting who actually live in the community and suffer from homelessness and mental health issues and incarceration and a whole range of other things. ... Maybe we need to change our idea of having these centralized silos where people are referred, and go out into the community, much like [tuberculosis] clinics used to do, and track people down.”
The study was funded by Gilead. The investigators disclosed additional relationships with HighTide Therapeutics, Intercept, AbbVie, and others.
SOURCE: Landis CS et al. The Liver Meeting 2019, Abstract 168.
BOSTON – ED-based screening is a feasible method of detecting hepatitis C virus (HCV) in high-risk populations, but linkage to care remains low, according to investigators.
An HCV screening program involving three Seattle hospitals and more than 4,000 patients showed that linkage to care was lowest among patients who were younger, homeless, or used injection drugs, reported lead author Charles S. Landis, MD, PhD, of the University of Washington, Seattle.
“In the U.S., rates of acute HCV infections are increasing in younger patients and in areas disproportionally affected by the opiate epidemic,” Dr. Landis said in a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “In order to achieve a goal of elimination, HCV screening, appropriate linkage to care, and treatment will need to be directed toward younger, marginalized, and underserved populations.”
Dr. Landis explained that EDs are suitable for HCV screening because users of emergency services are disproportionately affected by HCV, compared with patients in primary and specialty care settings. Despite this, linkage to care remains historically higher in primary and specialty care settings at approximately 70%, compared with 30% via the ED, Dr. Landis said.
Historically, EDs have been resistant to HCV screening programs, Dr. Landis said, but with the model used in the present study, which relied upon a full-time staff member in each ED who was employed by the infectious disease or hepatology department, no ED resources were needed.
Participants were willing adults who had reliable contact information. Patients were excluded if they were non–English speaking, incarcerated, enrolled or expected to enroll in another clinical study which excludes coenrollment, planned to move out of the region in the next 6 months, admitted to the ED with an acute life-threatening illness, or admitted to the ED for sexual assault. The program had three objectives: Screening, linkage to care, and treatment, all of which were coordinated by the aforementioned case manager.
To date, 4,182 patients have been screened, 936 have been enrolled, 95 have tested positive for HCV RNA, 32 have been linked with care, and 19 have been treated.
“So you can see, a lot of squeeze for a just a little bit of juice here,” Dr. Landis said, referring to the relatively low number of treated patients, compared with how many were screened.
The prevalence of HCV infection based on RNA testing was 2%, though one hospital had a rate of 5%. “This [prevalence] compares to, but is maybe slightly less than, the prevalence seen in others studies based in the emergency department,” Dr. Landis said. “The thought is, not all emergency departments are equal in terms of the patient population that they serve.”
Data analysis showed that the overall linkage to care was 36%. “This is still suboptimal, from my perspective,” Dr. Landis said, “but it does compare with several other ED-based studies.”
A closer look at the data showed that linkage was not uniform across the population. Among patients with homes, linkage to care was 59%, compared with 20% for patients who were homeless (P = .02).
“Ultimately, we need to tailor our approaches for linking homeless patients differently than patients who are not homeless,” Dr. Landis said.
Patients who reported no injection-drug use had a linkage to care of 50%, which was numerically higher than the rate of 20% among users of injection drugs; this difference was not statistically significant, which Dr. Landis attributed to insufficient population size. Similarly, younger patients showed numerical trends toward lower linkage to care.
“Future work will attempt to optimize linkage to care strategies based on patient demographic factors, such as active injection drug use or homelessness,” Dr. Landis said.
During discussion, a conference attendee from the United States expressed skepticism of the program’s merits.
“I may be a glass-half-empty person, but is it worth all this effort?” the attendee asked. “In all honesty, you treated a few dozen [patients] for 180,000 visits [per year]. I’m really not sure it’s worth those efforts, and I’m wondering if those efforts could be placed in different areas, especially for a higher yield.”
“Point well taken,” Dr. Landis said. “I think that was the purpose of the study, to see if the emergency department is a place to screen and link patients to care, and we’re trying to optimize that. Remember, there were 4,000 patients, but for many of those, it took literally a minute to screen them.”
An attendee from Australia offered a slightly more positive take on the findings, followed by a suggestion to improve linkage in marginalized populations.
“I’m not sure I’d be pessimistic,” the attendee said. “I think you ought to be commended for getting that number of people to link, because it is very difficult when we are looking at linking people from a hospital-based setting who actually live in the community and suffer from homelessness and mental health issues and incarceration and a whole range of other things. ... Maybe we need to change our idea of having these centralized silos where people are referred, and go out into the community, much like [tuberculosis] clinics used to do, and track people down.”
The study was funded by Gilead. The investigators disclosed additional relationships with HighTide Therapeutics, Intercept, AbbVie, and others.
SOURCE: Landis CS et al. The Liver Meeting 2019, Abstract 168.
REPORTING FROM THE LIVER MEETING 2019
Heavy metals linked with autoimmune liver disease
BOSTON – Exposure to heavy metals from natural and man-made sources may contribute to development of autoimmune liver disease, according to a recent U.K. study involving more than 3,500 patients.
Coal mines were particularly implicated, as they accounted for 39% of the risk of developing primary biliary cholangitis (PBC), reported lead author Jessica Dyson, MBBS, of Newcastle (England) University, and colleagues.
“We know that the etiology of autoimmune liver disease remains unclear, but we’re increasingly coming to understand that it’s likely to be a complex interplay between genetic and environmental factors,” Dr. Dyson said during a presentation at the annual meeting of the American Association for the Study of Liver Diseases. Showing a map of England, she pointed out how three autoimmune liver diseases – PBC, primary sclerosing cholangitis (PSC), and autoimmune hepatitis (AIH) – each have unique clusters of distribution. “This implies that environmental exposure may have a role in disease pathogenesis.”
To investigate this possibility, Dr. Dyson and colleagues used structural equation modeling to look for associations between the above three autoimmune liver diseases, socioeconomic status, and environmental factors. Specific environmental factors included soil concentrations of heavy metals (cadmium, arsenic, lead, manganese, and iron), coal mines, lead mines, quarries, urban areas, traffic, stream pH, and landfills.
The study was conducted in the northeast of England, where migration rates are low, Dr. Dyson said. From this region, the investigators identified patients with PBC (n = 2,150), AIH (n = 963), and PSC (n = 472). Conceptual models were used to examine relationships between covariates and prevalence of disease, with good models exhibiting a root-mean-square error of association less than 0.05 and a 95% covariate significance. After adjusting for population density, comparative fit was used to measure variation within each model.
The best model for PBC revealed the aforementioned link with coal mines, proximity to which accounted for 39% of the pathogenesis of PBC. High levels of cadmium in soil had an interactive role with coal mines, and itself directly contributed 22% of the risk of PBC; however, Dr. Dyson noted that, while many cadmium-rich areas had high rates of PBC, not all did.
“This demonstrates the complexity of causality of disease, and we certainly can’t say that cadmium, in its own right, is a direct cause and effect,” Dr. Dyson said. “But I think [cadmium] certainly potentially is one of the factors at play.”
For AIH, coal mines contributed less (6%), although cadmium still accounted for 22% of variation of disease, as did alkaline areas. Finally, a significant link was found between PSC and regions with high arsenic levels.
“To conclude, our data suggest that heavy metals may be risk factors for autoimmune liver disease,” Dr. Dyson said. “There are a number of exposure routes that may be pertinent to patients, from heavy metals occurring via natural sources, and also via virtue of human activity, such as burning of fossil fuels, heavy-metal production, and pesticides.” Dr. Dyson emphasized this latter route, as some rural areas, where pesticide use is common, had high prevalence rates of autoimmune liver disease.
Dr. Dyson went on to put her findings in context. “Heavy metals are a well-recognized cause of immune dysregulation and epithelial injury and are actually actively transported into the bile, and that may be particularly relevant in terms of cholangiopathies. And this leads us to the possibility of interventions to reduce toxic exposure that may modify risk of disease.”
Looking to the future, Dr. Dyson described plans to build on this research with measurements of heavy metals in tissues, serum, and urine.
The investigators reported no relevant disclosures.
SOURCE: Dyson J et al. The Liver Meeting 2019, Abstract 48.
BOSTON – Exposure to heavy metals from natural and man-made sources may contribute to development of autoimmune liver disease, according to a recent U.K. study involving more than 3,500 patients.
Coal mines were particularly implicated, as they accounted for 39% of the risk of developing primary biliary cholangitis (PBC), reported lead author Jessica Dyson, MBBS, of Newcastle (England) University, and colleagues.
“We know that the etiology of autoimmune liver disease remains unclear, but we’re increasingly coming to understand that it’s likely to be a complex interplay between genetic and environmental factors,” Dr. Dyson said during a presentation at the annual meeting of the American Association for the Study of Liver Diseases. Showing a map of England, she pointed out how three autoimmune liver diseases – PBC, primary sclerosing cholangitis (PSC), and autoimmune hepatitis (AIH) – each have unique clusters of distribution. “This implies that environmental exposure may have a role in disease pathogenesis.”
To investigate this possibility, Dr. Dyson and colleagues used structural equation modeling to look for associations between the above three autoimmune liver diseases, socioeconomic status, and environmental factors. Specific environmental factors included soil concentrations of heavy metals (cadmium, arsenic, lead, manganese, and iron), coal mines, lead mines, quarries, urban areas, traffic, stream pH, and landfills.
The study was conducted in the northeast of England, where migration rates are low, Dr. Dyson said. From this region, the investigators identified patients with PBC (n = 2,150), AIH (n = 963), and PSC (n = 472). Conceptual models were used to examine relationships between covariates and prevalence of disease, with good models exhibiting a root-mean-square error of association less than 0.05 and a 95% covariate significance. After adjusting for population density, comparative fit was used to measure variation within each model.
The best model for PBC revealed the aforementioned link with coal mines, proximity to which accounted for 39% of the pathogenesis of PBC. High levels of cadmium in soil had an interactive role with coal mines, and itself directly contributed 22% of the risk of PBC; however, Dr. Dyson noted that, while many cadmium-rich areas had high rates of PBC, not all did.
“This demonstrates the complexity of causality of disease, and we certainly can’t say that cadmium, in its own right, is a direct cause and effect,” Dr. Dyson said. “But I think [cadmium] certainly potentially is one of the factors at play.”
For AIH, coal mines contributed less (6%), although cadmium still accounted for 22% of variation of disease, as did alkaline areas. Finally, a significant link was found between PSC and regions with high arsenic levels.
“To conclude, our data suggest that heavy metals may be risk factors for autoimmune liver disease,” Dr. Dyson said. “There are a number of exposure routes that may be pertinent to patients, from heavy metals occurring via natural sources, and also via virtue of human activity, such as burning of fossil fuels, heavy-metal production, and pesticides.” Dr. Dyson emphasized this latter route, as some rural areas, where pesticide use is common, had high prevalence rates of autoimmune liver disease.
Dr. Dyson went on to put her findings in context. “Heavy metals are a well-recognized cause of immune dysregulation and epithelial injury and are actually actively transported into the bile, and that may be particularly relevant in terms of cholangiopathies. And this leads us to the possibility of interventions to reduce toxic exposure that may modify risk of disease.”
Looking to the future, Dr. Dyson described plans to build on this research with measurements of heavy metals in tissues, serum, and urine.
The investigators reported no relevant disclosures.
SOURCE: Dyson J et al. The Liver Meeting 2019, Abstract 48.
BOSTON – Exposure to heavy metals from natural and man-made sources may contribute to development of autoimmune liver disease, according to a recent U.K. study involving more than 3,500 patients.
Coal mines were particularly implicated, as they accounted for 39% of the risk of developing primary biliary cholangitis (PBC), reported lead author Jessica Dyson, MBBS, of Newcastle (England) University, and colleagues.
“We know that the etiology of autoimmune liver disease remains unclear, but we’re increasingly coming to understand that it’s likely to be a complex interplay between genetic and environmental factors,” Dr. Dyson said during a presentation at the annual meeting of the American Association for the Study of Liver Diseases. Showing a map of England, she pointed out how three autoimmune liver diseases – PBC, primary sclerosing cholangitis (PSC), and autoimmune hepatitis (AIH) – each have unique clusters of distribution. “This implies that environmental exposure may have a role in disease pathogenesis.”
To investigate this possibility, Dr. Dyson and colleagues used structural equation modeling to look for associations between the above three autoimmune liver diseases, socioeconomic status, and environmental factors. Specific environmental factors included soil concentrations of heavy metals (cadmium, arsenic, lead, manganese, and iron), coal mines, lead mines, quarries, urban areas, traffic, stream pH, and landfills.
The study was conducted in the northeast of England, where migration rates are low, Dr. Dyson said. From this region, the investigators identified patients with PBC (n = 2,150), AIH (n = 963), and PSC (n = 472). Conceptual models were used to examine relationships between covariates and prevalence of disease, with good models exhibiting a root-mean-square error of association less than 0.05 and a 95% covariate significance. After adjusting for population density, comparative fit was used to measure variation within each model.
The best model for PBC revealed the aforementioned link with coal mines, proximity to which accounted for 39% of the pathogenesis of PBC. High levels of cadmium in soil had an interactive role with coal mines, and itself directly contributed 22% of the risk of PBC; however, Dr. Dyson noted that, while many cadmium-rich areas had high rates of PBC, not all did.
“This demonstrates the complexity of causality of disease, and we certainly can’t say that cadmium, in its own right, is a direct cause and effect,” Dr. Dyson said. “But I think [cadmium] certainly potentially is one of the factors at play.”
For AIH, coal mines contributed less (6%), although cadmium still accounted for 22% of variation of disease, as did alkaline areas. Finally, a significant link was found between PSC and regions with high arsenic levels.
“To conclude, our data suggest that heavy metals may be risk factors for autoimmune liver disease,” Dr. Dyson said. “There are a number of exposure routes that may be pertinent to patients, from heavy metals occurring via natural sources, and also via virtue of human activity, such as burning of fossil fuels, heavy-metal production, and pesticides.” Dr. Dyson emphasized this latter route, as some rural areas, where pesticide use is common, had high prevalence rates of autoimmune liver disease.
Dr. Dyson went on to put her findings in context. “Heavy metals are a well-recognized cause of immune dysregulation and epithelial injury and are actually actively transported into the bile, and that may be particularly relevant in terms of cholangiopathies. And this leads us to the possibility of interventions to reduce toxic exposure that may modify risk of disease.”
Looking to the future, Dr. Dyson described plans to build on this research with measurements of heavy metals in tissues, serum, and urine.
The investigators reported no relevant disclosures.
SOURCE: Dyson J et al. The Liver Meeting 2019, Abstract 48.
REPORTING FROM THE LIVER MEETING 2019
Hepatitis B debrief: Key themes that emerged at AASLD
BOSTON – Some of the most notable abstracts presented here at the annual meeting of the American Association for the Study of Liver Diseases dealt with key topics including the natural history of hepatitis B virus, novel treatment approaches, and prevention, according to Marc Ghany, MD.
During a special hepatitis debriefing session held on the last day of the conference, Dr. Ghany reviewed his key selections in hepatitis B virus (HBV) research, including the following:
Natural history
Steatohepatitis may worsen HBV-related liver injury, according to results of an analysis of liver biopsies from adult patients enrolled in a North American cohort study (Abstract 162). Investigators found that steatohepatitis was associated with a 1.6-fold increased risk of advanced fibrosis.
“For all patients with hepatitis B, I think it’s important to screen and manage metabolic abnormalities to prevent liver disease progression,” said Dr. Ghany, who is with the Liver Diseases Branch of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md.
Risk of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B was evaluated in two notable studies, Dr. Ghany said, including one that found no difference in risk of HCC development among white patients who received long-term entecavir versus those who received long-term tenofovir (Abstract 454). This stands in contrast to a previous and controversial finding, according to investigators, that HCC incidence was lower in Asian patients treated with tenofovir versus those treated with entecavir.
In the other study, investigators found that dual-antiplatelet therapy (DAPT) was associated with a lower risk of HCC versus aspirin monotherapy in patients with chronic hepatitis B (Abstract 934). Antiplatelet therapy was associated with a near 20% reduction in HCC risk, while DAPT as compared with aspirin monotherapy was linked to a near 30% reduction. “These are very provocative findings,” Dr. Ghany said. “Of course they need to be confirmed, but if so, may open new avenues of HCC chemoprevention.”
Novel therapies
Several new and promising drugs are undergoing clinical trials, including JNJ-64530440 (JNJ-0440), a novel class N capsid assembly modulator. In phase 1a data presented here at The Liver Meeting, the treatment was safe, well tolerated, and resulted in potent inhibition of viral replication (Abstract 0089). “We await further studies on its effect on functional cure,” Dr. Ghany told attendees.
Another treatment to watch is GSK3389404 (GSK404), a liver-targeted antisense oligonucleotide; in a phase 2 placebo-controlled study in patients with chronic hepatitis B on stable nucleos(t)ide therapy, this treatment had acceptable safety and produced dose-dependent declines in hepatitis B surface antigen (HBsAg), according to investigators (Abstract 0695). Dr. Ghany said this constitutes “proof of principle” that antisense oligonucleotides can decrease HBsAg levels.
In a phase 2 randomized, placebo-controlled study in virally suppressed adults with chronic hepatitis B, the toll-like receptor 8 (TLR8) agonist GS-9688 was safe and well tolerated, and resulted in dose-dependent pharmacodynamic changes, with 5% of patients experiencing a 1 log10 IU/mL or greater decline in HBsAg levels or an HBsAg loss by week 24 (Abstract 0697). This is a “promising approach” that merits further study, according to Dr. Ghany.
Prevention: Vaccination and screening
A trivalent HBV vaccine is superior to monovalent vaccine, according to results from the double-blind, randomized, controlled, phase 3 PROTECT study presented here at the meeting (Abstract LP13). Known as Sci-B-Vac, the mammalian cell-derived trivalent vaccine had higher response rates versus the recombinant monovalent vaccine Engerix-B in difficult-to-vaccinate populations, according to Dr. Ghany.
A separate report based on a national health insurance cohort study in Korea demonstrated that regular follow-up, that is to say, every 3-6 months, significantly reduced liver cancer–related mortality (Abstract 0159). Patients compliant with screening in the study not only had a 44% reduction in risk of death from HCC, but also were more likely to receive curative treatments (23.1% versus 15.1%). “Notwithstanding the limitations or cohort studies, I think these data reinforce the need to screen patients with chronic hepatitis B,” Dr. Ghany said.
He provided no disclosures in his presentation.
BOSTON – Some of the most notable abstracts presented here at the annual meeting of the American Association for the Study of Liver Diseases dealt with key topics including the natural history of hepatitis B virus, novel treatment approaches, and prevention, according to Marc Ghany, MD.
During a special hepatitis debriefing session held on the last day of the conference, Dr. Ghany reviewed his key selections in hepatitis B virus (HBV) research, including the following:
Natural history
Steatohepatitis may worsen HBV-related liver injury, according to results of an analysis of liver biopsies from adult patients enrolled in a North American cohort study (Abstract 162). Investigators found that steatohepatitis was associated with a 1.6-fold increased risk of advanced fibrosis.
“For all patients with hepatitis B, I think it’s important to screen and manage metabolic abnormalities to prevent liver disease progression,” said Dr. Ghany, who is with the Liver Diseases Branch of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md.
Risk of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B was evaluated in two notable studies, Dr. Ghany said, including one that found no difference in risk of HCC development among white patients who received long-term entecavir versus those who received long-term tenofovir (Abstract 454). This stands in contrast to a previous and controversial finding, according to investigators, that HCC incidence was lower in Asian patients treated with tenofovir versus those treated with entecavir.
In the other study, investigators found that dual-antiplatelet therapy (DAPT) was associated with a lower risk of HCC versus aspirin monotherapy in patients with chronic hepatitis B (Abstract 934). Antiplatelet therapy was associated with a near 20% reduction in HCC risk, while DAPT as compared with aspirin monotherapy was linked to a near 30% reduction. “These are very provocative findings,” Dr. Ghany said. “Of course they need to be confirmed, but if so, may open new avenues of HCC chemoprevention.”
Novel therapies
Several new and promising drugs are undergoing clinical trials, including JNJ-64530440 (JNJ-0440), a novel class N capsid assembly modulator. In phase 1a data presented here at The Liver Meeting, the treatment was safe, well tolerated, and resulted in potent inhibition of viral replication (Abstract 0089). “We await further studies on its effect on functional cure,” Dr. Ghany told attendees.
Another treatment to watch is GSK3389404 (GSK404), a liver-targeted antisense oligonucleotide; in a phase 2 placebo-controlled study in patients with chronic hepatitis B on stable nucleos(t)ide therapy, this treatment had acceptable safety and produced dose-dependent declines in hepatitis B surface antigen (HBsAg), according to investigators (Abstract 0695). Dr. Ghany said this constitutes “proof of principle” that antisense oligonucleotides can decrease HBsAg levels.
In a phase 2 randomized, placebo-controlled study in virally suppressed adults with chronic hepatitis B, the toll-like receptor 8 (TLR8) agonist GS-9688 was safe and well tolerated, and resulted in dose-dependent pharmacodynamic changes, with 5% of patients experiencing a 1 log10 IU/mL or greater decline in HBsAg levels or an HBsAg loss by week 24 (Abstract 0697). This is a “promising approach” that merits further study, according to Dr. Ghany.
Prevention: Vaccination and screening
A trivalent HBV vaccine is superior to monovalent vaccine, according to results from the double-blind, randomized, controlled, phase 3 PROTECT study presented here at the meeting (Abstract LP13). Known as Sci-B-Vac, the mammalian cell-derived trivalent vaccine had higher response rates versus the recombinant monovalent vaccine Engerix-B in difficult-to-vaccinate populations, according to Dr. Ghany.
A separate report based on a national health insurance cohort study in Korea demonstrated that regular follow-up, that is to say, every 3-6 months, significantly reduced liver cancer–related mortality (Abstract 0159). Patients compliant with screening in the study not only had a 44% reduction in risk of death from HCC, but also were more likely to receive curative treatments (23.1% versus 15.1%). “Notwithstanding the limitations or cohort studies, I think these data reinforce the need to screen patients with chronic hepatitis B,” Dr. Ghany said.
He provided no disclosures in his presentation.
BOSTON – Some of the most notable abstracts presented here at the annual meeting of the American Association for the Study of Liver Diseases dealt with key topics including the natural history of hepatitis B virus, novel treatment approaches, and prevention, according to Marc Ghany, MD.
During a special hepatitis debriefing session held on the last day of the conference, Dr. Ghany reviewed his key selections in hepatitis B virus (HBV) research, including the following:
Natural history
Steatohepatitis may worsen HBV-related liver injury, according to results of an analysis of liver biopsies from adult patients enrolled in a North American cohort study (Abstract 162). Investigators found that steatohepatitis was associated with a 1.6-fold increased risk of advanced fibrosis.
“For all patients with hepatitis B, I think it’s important to screen and manage metabolic abnormalities to prevent liver disease progression,” said Dr. Ghany, who is with the Liver Diseases Branch of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md.
Risk of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B was evaluated in two notable studies, Dr. Ghany said, including one that found no difference in risk of HCC development among white patients who received long-term entecavir versus those who received long-term tenofovir (Abstract 454). This stands in contrast to a previous and controversial finding, according to investigators, that HCC incidence was lower in Asian patients treated with tenofovir versus those treated with entecavir.
In the other study, investigators found that dual-antiplatelet therapy (DAPT) was associated with a lower risk of HCC versus aspirin monotherapy in patients with chronic hepatitis B (Abstract 934). Antiplatelet therapy was associated with a near 20% reduction in HCC risk, while DAPT as compared with aspirin monotherapy was linked to a near 30% reduction. “These are very provocative findings,” Dr. Ghany said. “Of course they need to be confirmed, but if so, may open new avenues of HCC chemoprevention.”
Novel therapies
Several new and promising drugs are undergoing clinical trials, including JNJ-64530440 (JNJ-0440), a novel class N capsid assembly modulator. In phase 1a data presented here at The Liver Meeting, the treatment was safe, well tolerated, and resulted in potent inhibition of viral replication (Abstract 0089). “We await further studies on its effect on functional cure,” Dr. Ghany told attendees.
Another treatment to watch is GSK3389404 (GSK404), a liver-targeted antisense oligonucleotide; in a phase 2 placebo-controlled study in patients with chronic hepatitis B on stable nucleos(t)ide therapy, this treatment had acceptable safety and produced dose-dependent declines in hepatitis B surface antigen (HBsAg), according to investigators (Abstract 0695). Dr. Ghany said this constitutes “proof of principle” that antisense oligonucleotides can decrease HBsAg levels.
In a phase 2 randomized, placebo-controlled study in virally suppressed adults with chronic hepatitis B, the toll-like receptor 8 (TLR8) agonist GS-9688 was safe and well tolerated, and resulted in dose-dependent pharmacodynamic changes, with 5% of patients experiencing a 1 log10 IU/mL or greater decline in HBsAg levels or an HBsAg loss by week 24 (Abstract 0697). This is a “promising approach” that merits further study, according to Dr. Ghany.
Prevention: Vaccination and screening
A trivalent HBV vaccine is superior to monovalent vaccine, according to results from the double-blind, randomized, controlled, phase 3 PROTECT study presented here at the meeting (Abstract LP13). Known as Sci-B-Vac, the mammalian cell-derived trivalent vaccine had higher response rates versus the recombinant monovalent vaccine Engerix-B in difficult-to-vaccinate populations, according to Dr. Ghany.
A separate report based on a national health insurance cohort study in Korea demonstrated that regular follow-up, that is to say, every 3-6 months, significantly reduced liver cancer–related mortality (Abstract 0159). Patients compliant with screening in the study not only had a 44% reduction in risk of death from HCC, but also were more likely to receive curative treatments (23.1% versus 15.1%). “Notwithstanding the limitations or cohort studies, I think these data reinforce the need to screen patients with chronic hepatitis B,” Dr. Ghany said.
He provided no disclosures in his presentation.
REPORTING FROM THE LIVER MEETING 2019
HVPG predicts clinical benefit after sustained virologic response
BOSTON – For patients with hepatitis C virus infection who achieve sustained virologic response to interferon-free therapy, changes in hepatic venous pressure gradient (HVPG) predict clinical benefit, according to investigators.
This finding will allow investigators to use HVPG as a surrogate endpoint for etiologic therapies, which could accelerate future research, reported lead author Mattias Mandorfer, MD, PhD, of the Medical University of Vienna and colleagues.
“Sustained virologic response to interferon-free therapies ameliorates portal hypertension,” Dr. Mandorfer said during a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “[Previous research has shown that] nearly two-thirds of patients with pretreatment clinically significant portal hypertension had an HVPG decrease above or equal to 10%, which denotes a clinically meaningful change according to current recommendations. However, evidence is limited to studies evaluating the impact of HVPG response to nonselective beta-blockers, and nonselective beta-blockers have a completely different mode of action than etiological therapies. Accordingly, it is unclear whether a decrease in HVPG after the cure of hepatitis C translates into the same clinical benefit.”
To find out, the investigators enrolled 90 patients with hepatitis C virus who had an elevated HVPG of 6 mm Hg or higher prior to sustained virologic response. Before and after interferon-free therapy, patients underwent paired HVPG measurement. In addition, to evaluate noninvasive methods of HVPG assessment, transient elastography and von Willebrand factor to platelet count ratio testing were performed.
Analysis showed that HVPG measurements after, but not before, interferon-free therapy predicted liver decompensation. Specifically, HVPG was associated with an 18% increased risk of hepatic decompensation per mm Hg. After 3 years, 40.1% of patients with posttherapy HVPG measurements of 16 mm Hg or more developed hepatic decompensation, an event that occurred in none of the patients with a posttherapy HVPG of 9 mm Hg or less. Among patients who had a baseline HVPG of 10 mm Hg or more, which is considered a clinically significant level of portal hypertension, a decrease in HVPG of least 10% after therapy was associated with a similar level of protection against decompensation, compared with those who had no such decrease (2.5% vs. 31.8%).
While the two noninvasive methods (transient elastography and von Willebrand factor to platelet count ratio) were able to detect clinically significant portal hypertension (at least 10 mm Hg), they were not accurate enough to detect the protective 10% drop in HVPG.
“These results support the concept of applying HVPG as a surrogate endpoint for interventions that primarily aim at decreasing intrahepatic resistance (e.g., etiological therapies),” the investigators concluded in their abstract.
Jaime Bosch, MD, PhD, of the University of Barcelona provided some expert insight into the findings.
“The significance of the work is very important,” Dr. Bosch said in a public comment. “This provides, for the first time, firm evidence that HVPG can be taken as a surrogate endpoint ... for studies involving portal hypertension and cirrhosis in general.”
In an interview, Dr. Bosch elaborated on this statement. “The problem is, it takes a long time to get rid of cirrhosis [after sustained virologic response], and meanwhile, as long as portal hypertension remains, there is a risk for decompensation, so the patients cannot be said to be cured. They are cured of the infection, of the consequences of the infection, but it may take 10 years or more [to resolve cirrhosis], so the patient needs clinical surveillance and treatment after curing the cause of the disease.
“An academic consequence of these findings is that they’ve proved that decreasing HVPG by means of achieving sustained virologic response is followed by an improvement in prognosis. ... And when you can influence prognosis, and the influence in prognosis is reflected by a measurement independent from the way that we achieve this effect on the measurement, it means that this measurement is robust and now has to be used as a surrogate marker of resolution of cirrhosis.”
The study was funded by the Medical Scientific Fund of the city of Vienna. The investigators disclosed relationships with AbbVie, Bristol-Myers Squibb, Gilead, and others.
SOURCE: Mandorfer M et al. The Liver Meeting 2019, Abstract 146.
BOSTON – For patients with hepatitis C virus infection who achieve sustained virologic response to interferon-free therapy, changes in hepatic venous pressure gradient (HVPG) predict clinical benefit, according to investigators.
This finding will allow investigators to use HVPG as a surrogate endpoint for etiologic therapies, which could accelerate future research, reported lead author Mattias Mandorfer, MD, PhD, of the Medical University of Vienna and colleagues.
“Sustained virologic response to interferon-free therapies ameliorates portal hypertension,” Dr. Mandorfer said during a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “[Previous research has shown that] nearly two-thirds of patients with pretreatment clinically significant portal hypertension had an HVPG decrease above or equal to 10%, which denotes a clinically meaningful change according to current recommendations. However, evidence is limited to studies evaluating the impact of HVPG response to nonselective beta-blockers, and nonselective beta-blockers have a completely different mode of action than etiological therapies. Accordingly, it is unclear whether a decrease in HVPG after the cure of hepatitis C translates into the same clinical benefit.”
To find out, the investigators enrolled 90 patients with hepatitis C virus who had an elevated HVPG of 6 mm Hg or higher prior to sustained virologic response. Before and after interferon-free therapy, patients underwent paired HVPG measurement. In addition, to evaluate noninvasive methods of HVPG assessment, transient elastography and von Willebrand factor to platelet count ratio testing were performed.
Analysis showed that HVPG measurements after, but not before, interferon-free therapy predicted liver decompensation. Specifically, HVPG was associated with an 18% increased risk of hepatic decompensation per mm Hg. After 3 years, 40.1% of patients with posttherapy HVPG measurements of 16 mm Hg or more developed hepatic decompensation, an event that occurred in none of the patients with a posttherapy HVPG of 9 mm Hg or less. Among patients who had a baseline HVPG of 10 mm Hg or more, which is considered a clinically significant level of portal hypertension, a decrease in HVPG of least 10% after therapy was associated with a similar level of protection against decompensation, compared with those who had no such decrease (2.5% vs. 31.8%).
While the two noninvasive methods (transient elastography and von Willebrand factor to platelet count ratio) were able to detect clinically significant portal hypertension (at least 10 mm Hg), they were not accurate enough to detect the protective 10% drop in HVPG.
“These results support the concept of applying HVPG as a surrogate endpoint for interventions that primarily aim at decreasing intrahepatic resistance (e.g., etiological therapies),” the investigators concluded in their abstract.
Jaime Bosch, MD, PhD, of the University of Barcelona provided some expert insight into the findings.
“The significance of the work is very important,” Dr. Bosch said in a public comment. “This provides, for the first time, firm evidence that HVPG can be taken as a surrogate endpoint ... for studies involving portal hypertension and cirrhosis in general.”
In an interview, Dr. Bosch elaborated on this statement. “The problem is, it takes a long time to get rid of cirrhosis [after sustained virologic response], and meanwhile, as long as portal hypertension remains, there is a risk for decompensation, so the patients cannot be said to be cured. They are cured of the infection, of the consequences of the infection, but it may take 10 years or more [to resolve cirrhosis], so the patient needs clinical surveillance and treatment after curing the cause of the disease.
“An academic consequence of these findings is that they’ve proved that decreasing HVPG by means of achieving sustained virologic response is followed by an improvement in prognosis. ... And when you can influence prognosis, and the influence in prognosis is reflected by a measurement independent from the way that we achieve this effect on the measurement, it means that this measurement is robust and now has to be used as a surrogate marker of resolution of cirrhosis.”
The study was funded by the Medical Scientific Fund of the city of Vienna. The investigators disclosed relationships with AbbVie, Bristol-Myers Squibb, Gilead, and others.
SOURCE: Mandorfer M et al. The Liver Meeting 2019, Abstract 146.
BOSTON – For patients with hepatitis C virus infection who achieve sustained virologic response to interferon-free therapy, changes in hepatic venous pressure gradient (HVPG) predict clinical benefit, according to investigators.
This finding will allow investigators to use HVPG as a surrogate endpoint for etiologic therapies, which could accelerate future research, reported lead author Mattias Mandorfer, MD, PhD, of the Medical University of Vienna and colleagues.
“Sustained virologic response to interferon-free therapies ameliorates portal hypertension,” Dr. Mandorfer said during a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “[Previous research has shown that] nearly two-thirds of patients with pretreatment clinically significant portal hypertension had an HVPG decrease above or equal to 10%, which denotes a clinically meaningful change according to current recommendations. However, evidence is limited to studies evaluating the impact of HVPG response to nonselective beta-blockers, and nonselective beta-blockers have a completely different mode of action than etiological therapies. Accordingly, it is unclear whether a decrease in HVPG after the cure of hepatitis C translates into the same clinical benefit.”
To find out, the investigators enrolled 90 patients with hepatitis C virus who had an elevated HVPG of 6 mm Hg or higher prior to sustained virologic response. Before and after interferon-free therapy, patients underwent paired HVPG measurement. In addition, to evaluate noninvasive methods of HVPG assessment, transient elastography and von Willebrand factor to platelet count ratio testing were performed.
Analysis showed that HVPG measurements after, but not before, interferon-free therapy predicted liver decompensation. Specifically, HVPG was associated with an 18% increased risk of hepatic decompensation per mm Hg. After 3 years, 40.1% of patients with posttherapy HVPG measurements of 16 mm Hg or more developed hepatic decompensation, an event that occurred in none of the patients with a posttherapy HVPG of 9 mm Hg or less. Among patients who had a baseline HVPG of 10 mm Hg or more, which is considered a clinically significant level of portal hypertension, a decrease in HVPG of least 10% after therapy was associated with a similar level of protection against decompensation, compared with those who had no such decrease (2.5% vs. 31.8%).
While the two noninvasive methods (transient elastography and von Willebrand factor to platelet count ratio) were able to detect clinically significant portal hypertension (at least 10 mm Hg), they were not accurate enough to detect the protective 10% drop in HVPG.
“These results support the concept of applying HVPG as a surrogate endpoint for interventions that primarily aim at decreasing intrahepatic resistance (e.g., etiological therapies),” the investigators concluded in their abstract.
Jaime Bosch, MD, PhD, of the University of Barcelona provided some expert insight into the findings.
“The significance of the work is very important,” Dr. Bosch said in a public comment. “This provides, for the first time, firm evidence that HVPG can be taken as a surrogate endpoint ... for studies involving portal hypertension and cirrhosis in general.”
In an interview, Dr. Bosch elaborated on this statement. “The problem is, it takes a long time to get rid of cirrhosis [after sustained virologic response], and meanwhile, as long as portal hypertension remains, there is a risk for decompensation, so the patients cannot be said to be cured. They are cured of the infection, of the consequences of the infection, but it may take 10 years or more [to resolve cirrhosis], so the patient needs clinical surveillance and treatment after curing the cause of the disease.
“An academic consequence of these findings is that they’ve proved that decreasing HVPG by means of achieving sustained virologic response is followed by an improvement in prognosis. ... And when you can influence prognosis, and the influence in prognosis is reflected by a measurement independent from the way that we achieve this effect on the measurement, it means that this measurement is robust and now has to be used as a surrogate marker of resolution of cirrhosis.”
The study was funded by the Medical Scientific Fund of the city of Vienna. The investigators disclosed relationships with AbbVie, Bristol-Myers Squibb, Gilead, and others.
SOURCE: Mandorfer M et al. The Liver Meeting 2019, Abstract 146.
REPORTING FROM THE LIVER MEETING 2019
Pediatric study characterizes recurrent PSC
BOSTON – Children who have recurrence of primary sclerosing cholangitis after liver transplant tend to be younger and have more rapidly progressive disease, based on an international retrospective analysis.
Within 5 years of transplant, the probability of primary sclerosing cholangitis (PSC) recurrence in pediatric patients is 26%, reported lead author Mercedes Martinez, MD, of Columbia University, New York, and colleagues.
“The aim of our study was to identify risk factors for primary sclerosing cholangitis recurrence following transplant,” Dr. Martinez said during a presentation at the annual meeting of the American Association for the Study of Liver Diseases. This may be the largest pediatric study evaluating recurrent PSC to date, she added.
The investigators drew data from 35 centers around the world via the Pediatric PSC Consortium database. Recurrence was defined by cholestatic biochemistry with nonanastomotic biliary strictures and beading of bile ducts on cholangiography. Recurrences caused by hepatic artery thrombosis or chronic rejection were excluded, as were any cases that recurred within 6 months of transplant.
The final analysis included 149 patients with a median age at diagnosis and liver transplant of 12 years and 15.4 years, respectively. Of these, 31 patients had recurrence after a median of 3.3 years. A closer look at the data showed that recurrence was linked with younger median age at time of transplant (13.2 vs. 16.2 years). In cases of recurrence, PSC was generally more aggressive prior to transplant, with a shorter interval between diagnosis and transplant (1.6 vs. 4.1 years), higher total bilirubin (7.8 vs. 3.8 mg/dL), and higher ALT (118 vs. 62 U/L). Furthermore, almost half of the patients (45%) who had recurrence also had pretransplant autoimmune hepatitis overlap, compared with approximately one-quarter of the patients (27%) who did not have recurrence, although this trend was not statistically significant (P = .06).
Recurrent PSC was also associated with poorer outcomes; almost half of those with recurrence (48%) were relisted for liver transplant, developed portal hypertension, or died within 2 years of diagnosis. Mean rejection rates were higher in recurrent versus nonrecurrent cases (3 vs. 1); recurrent cases also had shorter time until rejection (3 vs. 6 months) and greater prevalence of rejection that was refractory to steroids (23% vs. 12%). Moreover, a significantly greater proportion of patients with recurrence had Epstein-Barr viremia (41% vs. 21%).
Dr. Martinez noted that ongoing therapy involving mammalian target of rapamycin inhibition was associated with lower rates of recurrence and suggested that this deserves further investigation; however, owing to small population size, she urged a cautious interpretation of this finding.
“We have to do prospective research,” Dr. Martinez said, emphasizing that tissue immunophenotyping was needed, as a better understanding of underlying immune processes and disease subtypes may open doors to more effective therapies.
The investigators disclosed relationships with Gilead, Merck, Novartis, and others.
SOURCE: Martinez M et al. The Liver Meeting 2019, Abstract 44.
BOSTON – Children who have recurrence of primary sclerosing cholangitis after liver transplant tend to be younger and have more rapidly progressive disease, based on an international retrospective analysis.
Within 5 years of transplant, the probability of primary sclerosing cholangitis (PSC) recurrence in pediatric patients is 26%, reported lead author Mercedes Martinez, MD, of Columbia University, New York, and colleagues.
“The aim of our study was to identify risk factors for primary sclerosing cholangitis recurrence following transplant,” Dr. Martinez said during a presentation at the annual meeting of the American Association for the Study of Liver Diseases. This may be the largest pediatric study evaluating recurrent PSC to date, she added.
The investigators drew data from 35 centers around the world via the Pediatric PSC Consortium database. Recurrence was defined by cholestatic biochemistry with nonanastomotic biliary strictures and beading of bile ducts on cholangiography. Recurrences caused by hepatic artery thrombosis or chronic rejection were excluded, as were any cases that recurred within 6 months of transplant.
The final analysis included 149 patients with a median age at diagnosis and liver transplant of 12 years and 15.4 years, respectively. Of these, 31 patients had recurrence after a median of 3.3 years. A closer look at the data showed that recurrence was linked with younger median age at time of transplant (13.2 vs. 16.2 years). In cases of recurrence, PSC was generally more aggressive prior to transplant, with a shorter interval between diagnosis and transplant (1.6 vs. 4.1 years), higher total bilirubin (7.8 vs. 3.8 mg/dL), and higher ALT (118 vs. 62 U/L). Furthermore, almost half of the patients (45%) who had recurrence also had pretransplant autoimmune hepatitis overlap, compared with approximately one-quarter of the patients (27%) who did not have recurrence, although this trend was not statistically significant (P = .06).
Recurrent PSC was also associated with poorer outcomes; almost half of those with recurrence (48%) were relisted for liver transplant, developed portal hypertension, or died within 2 years of diagnosis. Mean rejection rates were higher in recurrent versus nonrecurrent cases (3 vs. 1); recurrent cases also had shorter time until rejection (3 vs. 6 months) and greater prevalence of rejection that was refractory to steroids (23% vs. 12%). Moreover, a significantly greater proportion of patients with recurrence had Epstein-Barr viremia (41% vs. 21%).
Dr. Martinez noted that ongoing therapy involving mammalian target of rapamycin inhibition was associated with lower rates of recurrence and suggested that this deserves further investigation; however, owing to small population size, she urged a cautious interpretation of this finding.
“We have to do prospective research,” Dr. Martinez said, emphasizing that tissue immunophenotyping was needed, as a better understanding of underlying immune processes and disease subtypes may open doors to more effective therapies.
The investigators disclosed relationships with Gilead, Merck, Novartis, and others.
SOURCE: Martinez M et al. The Liver Meeting 2019, Abstract 44.
BOSTON – Children who have recurrence of primary sclerosing cholangitis after liver transplant tend to be younger and have more rapidly progressive disease, based on an international retrospective analysis.
Within 5 years of transplant, the probability of primary sclerosing cholangitis (PSC) recurrence in pediatric patients is 26%, reported lead author Mercedes Martinez, MD, of Columbia University, New York, and colleagues.
“The aim of our study was to identify risk factors for primary sclerosing cholangitis recurrence following transplant,” Dr. Martinez said during a presentation at the annual meeting of the American Association for the Study of Liver Diseases. This may be the largest pediatric study evaluating recurrent PSC to date, she added.
The investigators drew data from 35 centers around the world via the Pediatric PSC Consortium database. Recurrence was defined by cholestatic biochemistry with nonanastomotic biliary strictures and beading of bile ducts on cholangiography. Recurrences caused by hepatic artery thrombosis or chronic rejection were excluded, as were any cases that recurred within 6 months of transplant.
The final analysis included 149 patients with a median age at diagnosis and liver transplant of 12 years and 15.4 years, respectively. Of these, 31 patients had recurrence after a median of 3.3 years. A closer look at the data showed that recurrence was linked with younger median age at time of transplant (13.2 vs. 16.2 years). In cases of recurrence, PSC was generally more aggressive prior to transplant, with a shorter interval between diagnosis and transplant (1.6 vs. 4.1 years), higher total bilirubin (7.8 vs. 3.8 mg/dL), and higher ALT (118 vs. 62 U/L). Furthermore, almost half of the patients (45%) who had recurrence also had pretransplant autoimmune hepatitis overlap, compared with approximately one-quarter of the patients (27%) who did not have recurrence, although this trend was not statistically significant (P = .06).
Recurrent PSC was also associated with poorer outcomes; almost half of those with recurrence (48%) were relisted for liver transplant, developed portal hypertension, or died within 2 years of diagnosis. Mean rejection rates were higher in recurrent versus nonrecurrent cases (3 vs. 1); recurrent cases also had shorter time until rejection (3 vs. 6 months) and greater prevalence of rejection that was refractory to steroids (23% vs. 12%). Moreover, a significantly greater proportion of patients with recurrence had Epstein-Barr viremia (41% vs. 21%).
Dr. Martinez noted that ongoing therapy involving mammalian target of rapamycin inhibition was associated with lower rates of recurrence and suggested that this deserves further investigation; however, owing to small population size, she urged a cautious interpretation of this finding.
“We have to do prospective research,” Dr. Martinez said, emphasizing that tissue immunophenotyping was needed, as a better understanding of underlying immune processes and disease subtypes may open doors to more effective therapies.
The investigators disclosed relationships with Gilead, Merck, Novartis, and others.
SOURCE: Martinez M et al. The Liver Meeting 2019, Abstract 44.
REPORTING FROM THE LIVER MEETING 2019
Bezafibrate beats placebo in pruritus of chronic cholestasis: The FITCH trial
BOSTON – Bezafibrate was superior to placebo for ameliorating pruritus in patients with chronic cholestatic liver diseases, investigators reported at the annual meeting of the American Association for the Study of Liver Diseases.
Improvements in itch were reported by four times as many patients treated with the peroxisome proliferator-activated receptor (PPAR) agonist, compared with those treated with placebo, according to results of the FITCH (Fibrates for cholestatic ITCH) trial.
That finding from FITCH is very encouraging for patients with this “vexing” clinical issue, which can be highly distressing and is a common feature of cholestatic liver diseases, said Michael R. Charlton, MBBS, FRCP, director of the Transplant Institute and hepatology chief at the University of Chicago.
“It’s generally a misery-making condition,” Dr. Charlton said in a podium discussion of the FITCH study results at the Liver Meeting 2019. “I had a patient tell me that they felt like the subject of Edvard Munch’s ‘Scream’ painting.”
As of this meeting, bezafibrate should be considered superior to placebo for treatment of pruritus in cholangiopathies and should be “considered as first-line treatment” for pruritus in primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC), added Dr. Charlton, who was not involved in the study.
Investigators in FITCH recruited a total of 74 patients – all enrolled in the Netherlands or Spain – with cholestasis-induced pruritus who reported itch with an intensity of least 5 out of 10 on a visual analogue scale (VAS). Of the 70 patients who completed the trial, 44 had PSC, 24 had PBC, and 2 had secondary sclerosing cholangitis. Patients were randomly allocated to receive bezafibrate 400 mg once daily or placebo for 21 days.
The hypothesis was that PPAR agonist treatment would relieve itch by alleviating hepatobiliary inflammation and reducing formation of a biliary itch factor, according to the investigators, led by Elsemieke de Vries, MD, of the department of gastroenterology and hepatology, Amsterdam University Medical Centers.
“Guideline-approved pharmacological strategies show limited efficacy and can provoke serious side effects,” Dr. de Vries and coauthors said in the published abstract on the study.
The primary study endpoint, a 50% reduction in pruritus VAS score, was achieved in 45% of patients in the bezafibrate treatment arm (17 of 38 patients) versus just 11% in the placebo arm (4 of 36 patients; P = .003), according to updated results presented at the meeting.
The mean VAS score, comparable at baseline, was significantly lower in the bezafibrate group vs. the placebo group at day 21 (P < .001), the results showed.
Authors of the FITCH study reported disclosures related to Intercept Pharmaceuticals, Gilead, Takeda, Tillotts, Pliant, and Dr. Falk GmbH.
SOURCE: de Vries E et al. The Liver Meeting 2019. Abstract 13.
BOSTON – Bezafibrate was superior to placebo for ameliorating pruritus in patients with chronic cholestatic liver diseases, investigators reported at the annual meeting of the American Association for the Study of Liver Diseases.
Improvements in itch were reported by four times as many patients treated with the peroxisome proliferator-activated receptor (PPAR) agonist, compared with those treated with placebo, according to results of the FITCH (Fibrates for cholestatic ITCH) trial.
That finding from FITCH is very encouraging for patients with this “vexing” clinical issue, which can be highly distressing and is a common feature of cholestatic liver diseases, said Michael R. Charlton, MBBS, FRCP, director of the Transplant Institute and hepatology chief at the University of Chicago.
“It’s generally a misery-making condition,” Dr. Charlton said in a podium discussion of the FITCH study results at the Liver Meeting 2019. “I had a patient tell me that they felt like the subject of Edvard Munch’s ‘Scream’ painting.”
As of this meeting, bezafibrate should be considered superior to placebo for treatment of pruritus in cholangiopathies and should be “considered as first-line treatment” for pruritus in primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC), added Dr. Charlton, who was not involved in the study.
Investigators in FITCH recruited a total of 74 patients – all enrolled in the Netherlands or Spain – with cholestasis-induced pruritus who reported itch with an intensity of least 5 out of 10 on a visual analogue scale (VAS). Of the 70 patients who completed the trial, 44 had PSC, 24 had PBC, and 2 had secondary sclerosing cholangitis. Patients were randomly allocated to receive bezafibrate 400 mg once daily or placebo for 21 days.
The hypothesis was that PPAR agonist treatment would relieve itch by alleviating hepatobiliary inflammation and reducing formation of a biliary itch factor, according to the investigators, led by Elsemieke de Vries, MD, of the department of gastroenterology and hepatology, Amsterdam University Medical Centers.
“Guideline-approved pharmacological strategies show limited efficacy and can provoke serious side effects,” Dr. de Vries and coauthors said in the published abstract on the study.
The primary study endpoint, a 50% reduction in pruritus VAS score, was achieved in 45% of patients in the bezafibrate treatment arm (17 of 38 patients) versus just 11% in the placebo arm (4 of 36 patients; P = .003), according to updated results presented at the meeting.
The mean VAS score, comparable at baseline, was significantly lower in the bezafibrate group vs. the placebo group at day 21 (P < .001), the results showed.
Authors of the FITCH study reported disclosures related to Intercept Pharmaceuticals, Gilead, Takeda, Tillotts, Pliant, and Dr. Falk GmbH.
SOURCE: de Vries E et al. The Liver Meeting 2019. Abstract 13.
BOSTON – Bezafibrate was superior to placebo for ameliorating pruritus in patients with chronic cholestatic liver diseases, investigators reported at the annual meeting of the American Association for the Study of Liver Diseases.
Improvements in itch were reported by four times as many patients treated with the peroxisome proliferator-activated receptor (PPAR) agonist, compared with those treated with placebo, according to results of the FITCH (Fibrates for cholestatic ITCH) trial.
That finding from FITCH is very encouraging for patients with this “vexing” clinical issue, which can be highly distressing and is a common feature of cholestatic liver diseases, said Michael R. Charlton, MBBS, FRCP, director of the Transplant Institute and hepatology chief at the University of Chicago.
“It’s generally a misery-making condition,” Dr. Charlton said in a podium discussion of the FITCH study results at the Liver Meeting 2019. “I had a patient tell me that they felt like the subject of Edvard Munch’s ‘Scream’ painting.”
As of this meeting, bezafibrate should be considered superior to placebo for treatment of pruritus in cholangiopathies and should be “considered as first-line treatment” for pruritus in primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC), added Dr. Charlton, who was not involved in the study.
Investigators in FITCH recruited a total of 74 patients – all enrolled in the Netherlands or Spain – with cholestasis-induced pruritus who reported itch with an intensity of least 5 out of 10 on a visual analogue scale (VAS). Of the 70 patients who completed the trial, 44 had PSC, 24 had PBC, and 2 had secondary sclerosing cholangitis. Patients were randomly allocated to receive bezafibrate 400 mg once daily or placebo for 21 days.
The hypothesis was that PPAR agonist treatment would relieve itch by alleviating hepatobiliary inflammation and reducing formation of a biliary itch factor, according to the investigators, led by Elsemieke de Vries, MD, of the department of gastroenterology and hepatology, Amsterdam University Medical Centers.
“Guideline-approved pharmacological strategies show limited efficacy and can provoke serious side effects,” Dr. de Vries and coauthors said in the published abstract on the study.
The primary study endpoint, a 50% reduction in pruritus VAS score, was achieved in 45% of patients in the bezafibrate treatment arm (17 of 38 patients) versus just 11% in the placebo arm (4 of 36 patients; P = .003), according to updated results presented at the meeting.
The mean VAS score, comparable at baseline, was significantly lower in the bezafibrate group vs. the placebo group at day 21 (P < .001), the results showed.
Authors of the FITCH study reported disclosures related to Intercept Pharmaceuticals, Gilead, Takeda, Tillotts, Pliant, and Dr. Falk GmbH.
SOURCE: de Vries E et al. The Liver Meeting 2019. Abstract 13.
REPORTING FROM THE LIVER MEETING 2019
Key clinical point: Bezafibrate was superior to placebo for improving pruritus in chronic cholestatic liver diseases.
Major finding: A 50% reduction in pruritus visual analogue scale (VAS) score was achieved in 45% of patients in the bezafibrate treatment arm versus 11% in the placebo arm (P = .003).
Study details: Report on the randomized, placebo-controlled FITCH trial including 74 patients with cholestasis-induced pruritus.
Disclosures: Authors of the FITCH study reported disclosures related to Intercept Pharmaceuticals, Gilead, Takeda, Tillotts, Pliant, and Dr. Falk GmbH.
Source: de Vries E et al. The Liver Meeting 2019. Abstract 13.