User login
Sleep: The new frontier in cardiovascular prevention
MUNICH – Getting less than 6 hours of sleep nightly on a regular basis or waking up multiple times was independently associated with increased risk of subclinical atherosclerosis in the Spanish PESA study, Fernando Dominguez, MD, reported at the annual congress of the European Society of Cardiology.
Moreover, a graded response was evident in PESA (Progression of Early Subclinical Atherosclerosis): The more times an individual typically awoke per night, the greater the number of atherosclerotic carotid or femoral artery territories documented on three-dimensional vascular ultrasound, added Dr. Dominguez of the Spanish National Center for Cardiovascular Research in Madrid.
the cardiologist said.
The cross-sectional PESA study, whose principal investigator was Valentin Fuster, MD, PhD, included 3,974 middle-aged Madrid bank employees free of known heart disease or history of stroke who wore a waistband activity monitor for a week to record sleep quantity and quality. They also underwent three-dimensional vascular ultrasound and measurement of coronary artery calcium.
PESA was one of several large studies presented at the meeting that focused on deviations from normal sleep as a marker for increased risk of cardiovascular disease and/or mortality. Of note, however, PESA was the only one to use activity monitoring technology to track sleep.
“It was essential to use objectively measured sleep variables, because they showed huge disparity with patients’ self-reports on sleep questionnaires,” Dr. Dominguez explained.
Indeed, while 10.7% of PESA participants self-reported sleeping less than 6 hours per night on the Sleep Habits Questionnaire, actigraphy showed the true rate was 27.1%.
Based on actigraphic findings, subjects were divided into tertiles based upon average hours of sleep per night, ranging from less than 6 to more than 8. They were also grouped in quintiles based upon their extent of fragmented sleep.
Subjects with short sleep were significantly older and more likely to have high blood pressure, a higher body mass index, and metabolic syndrome than those who averaged 7-8 hours of sleep. Individuals in the top quintile for sleep awakening were older and had higher prevalences of smoking and hypertension than those in the lowest quintile.
In multivariate analyses adjusted for these differences as well as for physical activity, depression, obstructive sleep apnea, daily calorie consumption, alcohol intake, and other potential confounders, subjects who slept less than 6 hours per night had a 27% greater volume of noncoronary plaque than those who slept 7-8 hours. They also had 21% more vascular territories laden with subclinical atherosclerosis. The risk of subclinical noncoronary atherosclerosis was greater among women who averaged less than 6 hours of sleep per night, representing a 48% relative risk increase in plaque volume, versus 21% in men.
At the other extreme, women who slept more than 8 hours per night had an 83% increased plaque volume, while men who slept that much had no increase in risk, compared with men who slept for 7-8 hours.
Subjects in the top quintile for sleep fragmentation had 34% more vascular territories affected by atherosclerosis than those in the lowest quintile. Their noncoronary plaque burden was 23% greater as well.
An 11-study meta-analysis
Epameinondas Fountas, MD, of the Onassis Cardiac Surgery Center in Athens, presented a meta-analysis of 11 prospective studies of the relationship between daily sleep duration and cardiovascular disease morbidity and mortality published within the past 5 years, reflecting burgeoning interest in this hot-button topic. Collectively, the meta-analysis totaled 1,000,541 adults without baseline cardiovascular disease who were followed for an average of 9.3 years.
In an analysis adjusted for numerous known cardiovascular risk factors, the Greek investigators found that short sleep duration as defined by a self-reported average of less than 6 hours per night was independently associated with a statistically significant and clinically meaningful 11% increase in the risk of diagnosis of fatal or nonfatal cardiovascular disease, compared with individuals who averaged 6-8 hours nightly. Moreover, those who averaged more than 8 hours of sleep per night were also at risk: they averaged a 32% increased risk in fatal or nonfatal cardiovascular events compared to normal 6- to 8-hour sleepers. Thus, 6-8 hours of sleep per night appears to be the sweet spot in terms of cardioprotection.
“Our message to patients is simple: Sleep well, not too long, nor too short, and be active,” Dr. Fountas said.
Numerous investigators have highlighted the pathophysiologic changes related to sleep deprivation that likely boost cardiovascular risk. These include activation of the sympathetic nervous system, increased inflammation, and disrupted glucose metabolism, he noted.
Swedes weigh in
Moa Bengtsson, a combined medical/PhD student at the University of Gothenburg (Sweden), presented a prospective study of 798 men who were 50 years old in 1993, when they underwent a physical examination and completed extensive lifestyle questionnaires that included average self-reported sleep duration. Among the 759 men still available for evaluation after 21 years, or nearly 15,000 person-years of followup, those who reported sleeping an average of 5 hours or less per night back at age 50 were 93% more likely to have experienced a major cardiovascular event by age 71 -- acute MI, stroke, coronary revascularization, heart failure hospitalization, or cardiovascular death -- compared with those who averaged 7-8 hours of shut eye.
The short sleepers had a higher prevalence of obesity, diabetes, hypertension, smoking, and physical inactivity than the men who slept 7-8 hours per night. However, these and other confounders were adjusted for in the multivariate analysis.
To place sleep abnormalities in context, Ms. Bengtssen observed that short sleep in the Gothenburg men was numerically a stronger independent risk factor for future cardiovascular events than obesity, which was associated with an 82% increase in risk, or even smoking, with a 70% increase in risk.
Men who averaged either 6 hours of sleep per night or more than 8 hours were not at increased cardiovascular risk over 21 years of followup, compared with those who slept 7-8 hours.
Like the other investigators, she noted that the studies presented at the meeting, despite their extensive adjustments for potential confounders, don’t prove a direct causal relationship between short sleep and increased cardiovascular risk. An informative next step in research, albeit a challenging one, would be to show whether improved long-term sleep habits favorably alter cardiovascular risk.
All three study investigators reported having no financial conflicts regarding their research, which was conducted free of commercial support.
MUNICH – Getting less than 6 hours of sleep nightly on a regular basis or waking up multiple times was independently associated with increased risk of subclinical atherosclerosis in the Spanish PESA study, Fernando Dominguez, MD, reported at the annual congress of the European Society of Cardiology.
Moreover, a graded response was evident in PESA (Progression of Early Subclinical Atherosclerosis): The more times an individual typically awoke per night, the greater the number of atherosclerotic carotid or femoral artery territories documented on three-dimensional vascular ultrasound, added Dr. Dominguez of the Spanish National Center for Cardiovascular Research in Madrid.
the cardiologist said.
The cross-sectional PESA study, whose principal investigator was Valentin Fuster, MD, PhD, included 3,974 middle-aged Madrid bank employees free of known heart disease or history of stroke who wore a waistband activity monitor for a week to record sleep quantity and quality. They also underwent three-dimensional vascular ultrasound and measurement of coronary artery calcium.
PESA was one of several large studies presented at the meeting that focused on deviations from normal sleep as a marker for increased risk of cardiovascular disease and/or mortality. Of note, however, PESA was the only one to use activity monitoring technology to track sleep.
“It was essential to use objectively measured sleep variables, because they showed huge disparity with patients’ self-reports on sleep questionnaires,” Dr. Dominguez explained.
Indeed, while 10.7% of PESA participants self-reported sleeping less than 6 hours per night on the Sleep Habits Questionnaire, actigraphy showed the true rate was 27.1%.
Based on actigraphic findings, subjects were divided into tertiles based upon average hours of sleep per night, ranging from less than 6 to more than 8. They were also grouped in quintiles based upon their extent of fragmented sleep.
Subjects with short sleep were significantly older and more likely to have high blood pressure, a higher body mass index, and metabolic syndrome than those who averaged 7-8 hours of sleep. Individuals in the top quintile for sleep awakening were older and had higher prevalences of smoking and hypertension than those in the lowest quintile.
In multivariate analyses adjusted for these differences as well as for physical activity, depression, obstructive sleep apnea, daily calorie consumption, alcohol intake, and other potential confounders, subjects who slept less than 6 hours per night had a 27% greater volume of noncoronary plaque than those who slept 7-8 hours. They also had 21% more vascular territories laden with subclinical atherosclerosis. The risk of subclinical noncoronary atherosclerosis was greater among women who averaged less than 6 hours of sleep per night, representing a 48% relative risk increase in plaque volume, versus 21% in men.
At the other extreme, women who slept more than 8 hours per night had an 83% increased plaque volume, while men who slept that much had no increase in risk, compared with men who slept for 7-8 hours.
Subjects in the top quintile for sleep fragmentation had 34% more vascular territories affected by atherosclerosis than those in the lowest quintile. Their noncoronary plaque burden was 23% greater as well.
An 11-study meta-analysis
Epameinondas Fountas, MD, of the Onassis Cardiac Surgery Center in Athens, presented a meta-analysis of 11 prospective studies of the relationship between daily sleep duration and cardiovascular disease morbidity and mortality published within the past 5 years, reflecting burgeoning interest in this hot-button topic. Collectively, the meta-analysis totaled 1,000,541 adults without baseline cardiovascular disease who were followed for an average of 9.3 years.
In an analysis adjusted for numerous known cardiovascular risk factors, the Greek investigators found that short sleep duration as defined by a self-reported average of less than 6 hours per night was independently associated with a statistically significant and clinically meaningful 11% increase in the risk of diagnosis of fatal or nonfatal cardiovascular disease, compared with individuals who averaged 6-8 hours nightly. Moreover, those who averaged more than 8 hours of sleep per night were also at risk: they averaged a 32% increased risk in fatal or nonfatal cardiovascular events compared to normal 6- to 8-hour sleepers. Thus, 6-8 hours of sleep per night appears to be the sweet spot in terms of cardioprotection.
“Our message to patients is simple: Sleep well, not too long, nor too short, and be active,” Dr. Fountas said.
Numerous investigators have highlighted the pathophysiologic changes related to sleep deprivation that likely boost cardiovascular risk. These include activation of the sympathetic nervous system, increased inflammation, and disrupted glucose metabolism, he noted.
Swedes weigh in
Moa Bengtsson, a combined medical/PhD student at the University of Gothenburg (Sweden), presented a prospective study of 798 men who were 50 years old in 1993, when they underwent a physical examination and completed extensive lifestyle questionnaires that included average self-reported sleep duration. Among the 759 men still available for evaluation after 21 years, or nearly 15,000 person-years of followup, those who reported sleeping an average of 5 hours or less per night back at age 50 were 93% more likely to have experienced a major cardiovascular event by age 71 -- acute MI, stroke, coronary revascularization, heart failure hospitalization, or cardiovascular death -- compared with those who averaged 7-8 hours of shut eye.
The short sleepers had a higher prevalence of obesity, diabetes, hypertension, smoking, and physical inactivity than the men who slept 7-8 hours per night. However, these and other confounders were adjusted for in the multivariate analysis.
To place sleep abnormalities in context, Ms. Bengtssen observed that short sleep in the Gothenburg men was numerically a stronger independent risk factor for future cardiovascular events than obesity, which was associated with an 82% increase in risk, or even smoking, with a 70% increase in risk.
Men who averaged either 6 hours of sleep per night or more than 8 hours were not at increased cardiovascular risk over 21 years of followup, compared with those who slept 7-8 hours.
Like the other investigators, she noted that the studies presented at the meeting, despite their extensive adjustments for potential confounders, don’t prove a direct causal relationship between short sleep and increased cardiovascular risk. An informative next step in research, albeit a challenging one, would be to show whether improved long-term sleep habits favorably alter cardiovascular risk.
All three study investigators reported having no financial conflicts regarding their research, which was conducted free of commercial support.
MUNICH – Getting less than 6 hours of sleep nightly on a regular basis or waking up multiple times was independently associated with increased risk of subclinical atherosclerosis in the Spanish PESA study, Fernando Dominguez, MD, reported at the annual congress of the European Society of Cardiology.
Moreover, a graded response was evident in PESA (Progression of Early Subclinical Atherosclerosis): The more times an individual typically awoke per night, the greater the number of atherosclerotic carotid or femoral artery territories documented on three-dimensional vascular ultrasound, added Dr. Dominguez of the Spanish National Center for Cardiovascular Research in Madrid.
the cardiologist said.
The cross-sectional PESA study, whose principal investigator was Valentin Fuster, MD, PhD, included 3,974 middle-aged Madrid bank employees free of known heart disease or history of stroke who wore a waistband activity monitor for a week to record sleep quantity and quality. They also underwent three-dimensional vascular ultrasound and measurement of coronary artery calcium.
PESA was one of several large studies presented at the meeting that focused on deviations from normal sleep as a marker for increased risk of cardiovascular disease and/or mortality. Of note, however, PESA was the only one to use activity monitoring technology to track sleep.
“It was essential to use objectively measured sleep variables, because they showed huge disparity with patients’ self-reports on sleep questionnaires,” Dr. Dominguez explained.
Indeed, while 10.7% of PESA participants self-reported sleeping less than 6 hours per night on the Sleep Habits Questionnaire, actigraphy showed the true rate was 27.1%.
Based on actigraphic findings, subjects were divided into tertiles based upon average hours of sleep per night, ranging from less than 6 to more than 8. They were also grouped in quintiles based upon their extent of fragmented sleep.
Subjects with short sleep were significantly older and more likely to have high blood pressure, a higher body mass index, and metabolic syndrome than those who averaged 7-8 hours of sleep. Individuals in the top quintile for sleep awakening were older and had higher prevalences of smoking and hypertension than those in the lowest quintile.
In multivariate analyses adjusted for these differences as well as for physical activity, depression, obstructive sleep apnea, daily calorie consumption, alcohol intake, and other potential confounders, subjects who slept less than 6 hours per night had a 27% greater volume of noncoronary plaque than those who slept 7-8 hours. They also had 21% more vascular territories laden with subclinical atherosclerosis. The risk of subclinical noncoronary atherosclerosis was greater among women who averaged less than 6 hours of sleep per night, representing a 48% relative risk increase in plaque volume, versus 21% in men.
At the other extreme, women who slept more than 8 hours per night had an 83% increased plaque volume, while men who slept that much had no increase in risk, compared with men who slept for 7-8 hours.
Subjects in the top quintile for sleep fragmentation had 34% more vascular territories affected by atherosclerosis than those in the lowest quintile. Their noncoronary plaque burden was 23% greater as well.
An 11-study meta-analysis
Epameinondas Fountas, MD, of the Onassis Cardiac Surgery Center in Athens, presented a meta-analysis of 11 prospective studies of the relationship between daily sleep duration and cardiovascular disease morbidity and mortality published within the past 5 years, reflecting burgeoning interest in this hot-button topic. Collectively, the meta-analysis totaled 1,000,541 adults without baseline cardiovascular disease who were followed for an average of 9.3 years.
In an analysis adjusted for numerous known cardiovascular risk factors, the Greek investigators found that short sleep duration as defined by a self-reported average of less than 6 hours per night was independently associated with a statistically significant and clinically meaningful 11% increase in the risk of diagnosis of fatal or nonfatal cardiovascular disease, compared with individuals who averaged 6-8 hours nightly. Moreover, those who averaged more than 8 hours of sleep per night were also at risk: they averaged a 32% increased risk in fatal or nonfatal cardiovascular events compared to normal 6- to 8-hour sleepers. Thus, 6-8 hours of sleep per night appears to be the sweet spot in terms of cardioprotection.
“Our message to patients is simple: Sleep well, not too long, nor too short, and be active,” Dr. Fountas said.
Numerous investigators have highlighted the pathophysiologic changes related to sleep deprivation that likely boost cardiovascular risk. These include activation of the sympathetic nervous system, increased inflammation, and disrupted glucose metabolism, he noted.
Swedes weigh in
Moa Bengtsson, a combined medical/PhD student at the University of Gothenburg (Sweden), presented a prospective study of 798 men who were 50 years old in 1993, when they underwent a physical examination and completed extensive lifestyle questionnaires that included average self-reported sleep duration. Among the 759 men still available for evaluation after 21 years, or nearly 15,000 person-years of followup, those who reported sleeping an average of 5 hours or less per night back at age 50 were 93% more likely to have experienced a major cardiovascular event by age 71 -- acute MI, stroke, coronary revascularization, heart failure hospitalization, or cardiovascular death -- compared with those who averaged 7-8 hours of shut eye.
The short sleepers had a higher prevalence of obesity, diabetes, hypertension, smoking, and physical inactivity than the men who slept 7-8 hours per night. However, these and other confounders were adjusted for in the multivariate analysis.
To place sleep abnormalities in context, Ms. Bengtssen observed that short sleep in the Gothenburg men was numerically a stronger independent risk factor for future cardiovascular events than obesity, which was associated with an 82% increase in risk, or even smoking, with a 70% increase in risk.
Men who averaged either 6 hours of sleep per night or more than 8 hours were not at increased cardiovascular risk over 21 years of followup, compared with those who slept 7-8 hours.
Like the other investigators, she noted that the studies presented at the meeting, despite their extensive adjustments for potential confounders, don’t prove a direct causal relationship between short sleep and increased cardiovascular risk. An informative next step in research, albeit a challenging one, would be to show whether improved long-term sleep habits favorably alter cardiovascular risk.
All three study investigators reported having no financial conflicts regarding their research, which was conducted free of commercial support.
REPORTING FROM THE ESC CONGRESS 2018
Data support revising ASCVD cardiovascular risk threshold
MUNICH – Revising the threshold for actionable high cardiovascular risk from the current 7.5% or greater risk of an event within 10 years as defined in American College of Cardiology/American Heart Association guidelines using the Atherosclerotic Cardiovascular Disease (ASCVD ) Risk Calculator to a 10% or greater 10-year risk would provide the optimal balance of sensitivity and specificity for discriminating future risk of cardiovascular events, according to Robert S. Rosenman, MD.
“I think this is very important from a public health policy perspective,” Dr. Rosenman, a cardiologist who is professor of medicine at Mount Sinai School of Medicine in New York, said at the annual congress of the European Society of Cardiology.
He elaborated: “This would eliminate 11.4 million people who are currently candidates for a statin but may not be getting the benefits of statin therapy. We feel that this information is actually quite important for the primary prevention population because there’s been a lot of pushback from our primary care physician colleagues about the overtreatment of low-risk individuals” under the current guidelines (Circulation. 2014 Jun 24;129[25 Suppl 2]:S49-73).
Dr. Rosenman and his coinvestigators conducted a secondary analysis of data on 21,343 adults in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. All participants were free of a baseline history of heart disease or stroke. During a median 8.5 years of follow-up, 1,717 of them experienced adjudicated coronary heart disease or stroke events.
In multivariate analyses adjusted for standard cardiovascular risk factors, socioeconomic and demographic factors, and the use of statins and/or antihypertensive drugs, the higher the baseline 10-year predicted risk using the ACC/AHA ASCVD Risk Calculator based on the Pooled Cohort risk equations, the higher the incidence rate of cardiovascular events. No surprise there.
What was impressive, however, was that the optimal combination of sensitivity and specificity as captured in a statistic known as Youden’s index occurred at a 10-year predicted risk of 10%-12%. The biggest net improvement obtained through reclassification resulted from moving the threshold for elevated 10-year cardiovascular risk warranting statin therapy from 7.5% or greater to 10% or more, rather than using thresholds of 15% or 20%.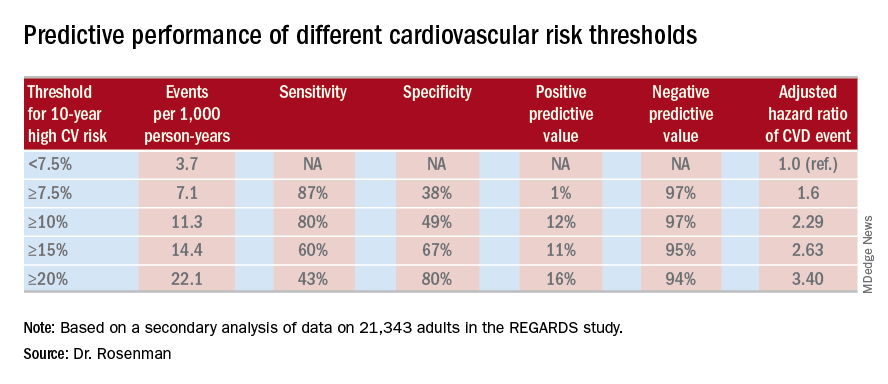
He cited data from the 2011-2014 National Health and Nutrition Examination Survey in support of his estimate that switching to a 10% threshold from the current 7.5% threshold would reduce the number of Americans deemed at high cardiovascular risk from 57.1 million to 45.8 million.
“This cutoff value of 10%, by the way, is the same cutoff value used in the recently published ACC/AHA guideline on hypertension. And it’s also the same cutoff value used for antiplatelet therapy in looking at the benefit/risk ratio. So this value of 10% is, I think, really the right number. Our study is the first effort that has been shown to validate that number, and it brings the cutoff values in the various guidelines in line,” the cardiologist observed.
Asked if these new findings are likely to result in a revision of the ACC/AHA cardiovascular risk assessment guidelines, Dr. Rosenman replied that the guidelines are under revision, with the draft update now circulating for comment. So the timing is dicey: His study is now in prepublication peer review, but hasn’t yet been published and thus may not carry persuasive weight.
“Hopefully, the guideline panel is going to make an adjustment to make the 10% figure in line with the blood pressure guidelines,” he said.
The new analysis of the REGARDS study was funded by a collaboration between Amgen, Mount Sinai School of Medicine, and the University of Alabama. Dr. Rosenman reported receiving research funding from and serving as an advisor to Amgen and a handful of other companies.
MUNICH – Revising the threshold for actionable high cardiovascular risk from the current 7.5% or greater risk of an event within 10 years as defined in American College of Cardiology/American Heart Association guidelines using the Atherosclerotic Cardiovascular Disease (ASCVD ) Risk Calculator to a 10% or greater 10-year risk would provide the optimal balance of sensitivity and specificity for discriminating future risk of cardiovascular events, according to Robert S. Rosenman, MD.
“I think this is very important from a public health policy perspective,” Dr. Rosenman, a cardiologist who is professor of medicine at Mount Sinai School of Medicine in New York, said at the annual congress of the European Society of Cardiology.
He elaborated: “This would eliminate 11.4 million people who are currently candidates for a statin but may not be getting the benefits of statin therapy. We feel that this information is actually quite important for the primary prevention population because there’s been a lot of pushback from our primary care physician colleagues about the overtreatment of low-risk individuals” under the current guidelines (Circulation. 2014 Jun 24;129[25 Suppl 2]:S49-73).
Dr. Rosenman and his coinvestigators conducted a secondary analysis of data on 21,343 adults in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. All participants were free of a baseline history of heart disease or stroke. During a median 8.5 years of follow-up, 1,717 of them experienced adjudicated coronary heart disease or stroke events.
In multivariate analyses adjusted for standard cardiovascular risk factors, socioeconomic and demographic factors, and the use of statins and/or antihypertensive drugs, the higher the baseline 10-year predicted risk using the ACC/AHA ASCVD Risk Calculator based on the Pooled Cohort risk equations, the higher the incidence rate of cardiovascular events. No surprise there.
What was impressive, however, was that the optimal combination of sensitivity and specificity as captured in a statistic known as Youden’s index occurred at a 10-year predicted risk of 10%-12%. The biggest net improvement obtained through reclassification resulted from moving the threshold for elevated 10-year cardiovascular risk warranting statin therapy from 7.5% or greater to 10% or more, rather than using thresholds of 15% or 20%.
He cited data from the 2011-2014 National Health and Nutrition Examination Survey in support of his estimate that switching to a 10% threshold from the current 7.5% threshold would reduce the number of Americans deemed at high cardiovascular risk from 57.1 million to 45.8 million.
“This cutoff value of 10%, by the way, is the same cutoff value used in the recently published ACC/AHA guideline on hypertension. And it’s also the same cutoff value used for antiplatelet therapy in looking at the benefit/risk ratio. So this value of 10% is, I think, really the right number. Our study is the first effort that has been shown to validate that number, and it brings the cutoff values in the various guidelines in line,” the cardiologist observed.
Asked if these new findings are likely to result in a revision of the ACC/AHA cardiovascular risk assessment guidelines, Dr. Rosenman replied that the guidelines are under revision, with the draft update now circulating for comment. So the timing is dicey: His study is now in prepublication peer review, but hasn’t yet been published and thus may not carry persuasive weight.
“Hopefully, the guideline panel is going to make an adjustment to make the 10% figure in line with the blood pressure guidelines,” he said.
The new analysis of the REGARDS study was funded by a collaboration between Amgen, Mount Sinai School of Medicine, and the University of Alabama. Dr. Rosenman reported receiving research funding from and serving as an advisor to Amgen and a handful of other companies.
MUNICH – Revising the threshold for actionable high cardiovascular risk from the current 7.5% or greater risk of an event within 10 years as defined in American College of Cardiology/American Heart Association guidelines using the Atherosclerotic Cardiovascular Disease (ASCVD ) Risk Calculator to a 10% or greater 10-year risk would provide the optimal balance of sensitivity and specificity for discriminating future risk of cardiovascular events, according to Robert S. Rosenman, MD.
“I think this is very important from a public health policy perspective,” Dr. Rosenman, a cardiologist who is professor of medicine at Mount Sinai School of Medicine in New York, said at the annual congress of the European Society of Cardiology.
He elaborated: “This would eliminate 11.4 million people who are currently candidates for a statin but may not be getting the benefits of statin therapy. We feel that this information is actually quite important for the primary prevention population because there’s been a lot of pushback from our primary care physician colleagues about the overtreatment of low-risk individuals” under the current guidelines (Circulation. 2014 Jun 24;129[25 Suppl 2]:S49-73).
Dr. Rosenman and his coinvestigators conducted a secondary analysis of data on 21,343 adults in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. All participants were free of a baseline history of heart disease or stroke. During a median 8.5 years of follow-up, 1,717 of them experienced adjudicated coronary heart disease or stroke events.
In multivariate analyses adjusted for standard cardiovascular risk factors, socioeconomic and demographic factors, and the use of statins and/or antihypertensive drugs, the higher the baseline 10-year predicted risk using the ACC/AHA ASCVD Risk Calculator based on the Pooled Cohort risk equations, the higher the incidence rate of cardiovascular events. No surprise there.
What was impressive, however, was that the optimal combination of sensitivity and specificity as captured in a statistic known as Youden’s index occurred at a 10-year predicted risk of 10%-12%. The biggest net improvement obtained through reclassification resulted from moving the threshold for elevated 10-year cardiovascular risk warranting statin therapy from 7.5% or greater to 10% or more, rather than using thresholds of 15% or 20%.
He cited data from the 2011-2014 National Health and Nutrition Examination Survey in support of his estimate that switching to a 10% threshold from the current 7.5% threshold would reduce the number of Americans deemed at high cardiovascular risk from 57.1 million to 45.8 million.
“This cutoff value of 10%, by the way, is the same cutoff value used in the recently published ACC/AHA guideline on hypertension. And it’s also the same cutoff value used for antiplatelet therapy in looking at the benefit/risk ratio. So this value of 10% is, I think, really the right number. Our study is the first effort that has been shown to validate that number, and it brings the cutoff values in the various guidelines in line,” the cardiologist observed.
Asked if these new findings are likely to result in a revision of the ACC/AHA cardiovascular risk assessment guidelines, Dr. Rosenman replied that the guidelines are under revision, with the draft update now circulating for comment. So the timing is dicey: His study is now in prepublication peer review, but hasn’t yet been published and thus may not carry persuasive weight.
“Hopefully, the guideline panel is going to make an adjustment to make the 10% figure in line with the blood pressure guidelines,” he said.
The new analysis of the REGARDS study was funded by a collaboration between Amgen, Mount Sinai School of Medicine, and the University of Alabama. Dr. Rosenman reported receiving research funding from and serving as an advisor to Amgen and a handful of other companies.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point:
Major finding: Redefining the threshold for high 10-year cardiovascular risk from the current 7.5% to 10% would reduce the number of Americans warranting statin therapy by 11.4 million.
Study details: This was a secondary analysis of data on 21,343 adults in the REGARDS study, 1,717 of whom experienced coronary heart disease or stroke events during a median 8.5 years of prospective follow-up.
Disclosures: The new analysis of the REGARDS study was funded by a collaboration between Amgen, Mount Sinai School of Medicine, and the University of Alabama. The presenter reported ties to Amgen and a handful of other companies.
Fat attenuation index boosts coronary CT prognostication
MUNICH – The perivascular fat attenuation index, a new measure of coronary plaque inflammation using data collected by a conventional coronary CT scan, identified an elevated risk for future cardiovascular death that was independent of standard risk factors in both derivation and validation studies with prospective data from more than 3,900 patients.
Patients with a high fat attenuation index (FAI) in the perivascular fat surrounding their right coronary artery had a five- to ninefold higher risk of cardiac mortality during 4-6 years of follow-up than did those with lower scores, after adjustment for conventional risk factors and standard findings from the coronary CT, Charalambos Antoniades, MD, said at the annual congress of the European Society of Cardiology.
The FAI is a “powerful, novel technology for cardiovascular disease risk stratification. It has striking prognostic value for cardiac death and nonfatal MI over and above other risk scores and state-of-the-art interpretation of coronary CT angiography,” said Dr. Antoniades, professor of cardiovascular medicine at the University of Oxford (England). He also highlighted that the data he reported suggested a protective effect in patients with a high FAI who received treatment with aspirin or a statin, and he suggested that FAI might be a way to better target an anti-inflammatory agent such as canakinumab (Ilaris), which showed cardiovascular protective effects in the CANTOS trial (N Engl J Med. 2017 Sep 21;377[12]:1119-31).
Another key feature of the FAI analysis is that it uses data collected by “any standard” coronary CT angiogram, Dr. Antoniades said. He is a founder of, shareholder in, and chief scientific officer of Caristo Diagnostics, the company developing the software that uses coronary CT data to calculate the FAI.
Dr. Antoniades and his associates derived the FAI using data collected from 1,872 patients who underwent planned coronary CT angiography at a clinic in Erlangen, Germany, during 2005-2009. The researchers correlated FAI scores with cardiovascular disease outcomes during a median follow-up of 72 months. They then validated the FAI using data collected from 2,040 patients who underwent a planned coronary CT exam at the Cleveland Clinic during 2008-2016 and then had a median follow-up of 54 months. The researchers called this overall post-hoc analysis of prospectively collected CT and outcomes data the Cardiovascular Risk Prediction using Computed Tomography (CRISP-CT) study.
Using the derivation data, FAI measurements taken from fat around the proximal right coronary artery that met or exceeded the specified cutoff value of –70.1 Hounsfield units were tied to a 9.04-fold greater rate of cardiac death during follow-up, compared with patients with a lower FAI, and after adjustment for several demographic, clinical, and CT-angiography variables. When the researchers ran this analysis using data from the validation patients and the same cutoff value they found that an elevated FAI linked with a 5.6-fold higher rate of cardiac death. Concurrently with Dr. Antoniades’ talk the results appeared in an online article that was later published (Lancet. 2018 Sep 15;392[10151]:929-39).
Calculating a patient’s FAI offers the prospect for “better use of CT information,” Dr. Antoniades said during the discussion of his report. After a coronary CT scan and conventional data processing, about half the patients have no finding that warrants intervention, but about 10% of these patients actually have an inflamed coronary plaque that is at high risk for rupture that could trigger a cardiac event. The FAI provides a way to use coronary CT angiography to identify these at-risk patients, he explained.
MUNICH – The perivascular fat attenuation index, a new measure of coronary plaque inflammation using data collected by a conventional coronary CT scan, identified an elevated risk for future cardiovascular death that was independent of standard risk factors in both derivation and validation studies with prospective data from more than 3,900 patients.
Patients with a high fat attenuation index (FAI) in the perivascular fat surrounding their right coronary artery had a five- to ninefold higher risk of cardiac mortality during 4-6 years of follow-up than did those with lower scores, after adjustment for conventional risk factors and standard findings from the coronary CT, Charalambos Antoniades, MD, said at the annual congress of the European Society of Cardiology.
The FAI is a “powerful, novel technology for cardiovascular disease risk stratification. It has striking prognostic value for cardiac death and nonfatal MI over and above other risk scores and state-of-the-art interpretation of coronary CT angiography,” said Dr. Antoniades, professor of cardiovascular medicine at the University of Oxford (England). He also highlighted that the data he reported suggested a protective effect in patients with a high FAI who received treatment with aspirin or a statin, and he suggested that FAI might be a way to better target an anti-inflammatory agent such as canakinumab (Ilaris), which showed cardiovascular protective effects in the CANTOS trial (N Engl J Med. 2017 Sep 21;377[12]:1119-31).
Another key feature of the FAI analysis is that it uses data collected by “any standard” coronary CT angiogram, Dr. Antoniades said. He is a founder of, shareholder in, and chief scientific officer of Caristo Diagnostics, the company developing the software that uses coronary CT data to calculate the FAI.
Dr. Antoniades and his associates derived the FAI using data collected from 1,872 patients who underwent planned coronary CT angiography at a clinic in Erlangen, Germany, during 2005-2009. The researchers correlated FAI scores with cardiovascular disease outcomes during a median follow-up of 72 months. They then validated the FAI using data collected from 2,040 patients who underwent a planned coronary CT exam at the Cleveland Clinic during 2008-2016 and then had a median follow-up of 54 months. The researchers called this overall post-hoc analysis of prospectively collected CT and outcomes data the Cardiovascular Risk Prediction using Computed Tomography (CRISP-CT) study.
Using the derivation data, FAI measurements taken from fat around the proximal right coronary artery that met or exceeded the specified cutoff value of –70.1 Hounsfield units were tied to a 9.04-fold greater rate of cardiac death during follow-up, compared with patients with a lower FAI, and after adjustment for several demographic, clinical, and CT-angiography variables. When the researchers ran this analysis using data from the validation patients and the same cutoff value they found that an elevated FAI linked with a 5.6-fold higher rate of cardiac death. Concurrently with Dr. Antoniades’ talk the results appeared in an online article that was later published (Lancet. 2018 Sep 15;392[10151]:929-39).
Calculating a patient’s FAI offers the prospect for “better use of CT information,” Dr. Antoniades said during the discussion of his report. After a coronary CT scan and conventional data processing, about half the patients have no finding that warrants intervention, but about 10% of these patients actually have an inflamed coronary plaque that is at high risk for rupture that could trigger a cardiac event. The FAI provides a way to use coronary CT angiography to identify these at-risk patients, he explained.
MUNICH – The perivascular fat attenuation index, a new measure of coronary plaque inflammation using data collected by a conventional coronary CT scan, identified an elevated risk for future cardiovascular death that was independent of standard risk factors in both derivation and validation studies with prospective data from more than 3,900 patients.
Patients with a high fat attenuation index (FAI) in the perivascular fat surrounding their right coronary artery had a five- to ninefold higher risk of cardiac mortality during 4-6 years of follow-up than did those with lower scores, after adjustment for conventional risk factors and standard findings from the coronary CT, Charalambos Antoniades, MD, said at the annual congress of the European Society of Cardiology.
The FAI is a “powerful, novel technology for cardiovascular disease risk stratification. It has striking prognostic value for cardiac death and nonfatal MI over and above other risk scores and state-of-the-art interpretation of coronary CT angiography,” said Dr. Antoniades, professor of cardiovascular medicine at the University of Oxford (England). He also highlighted that the data he reported suggested a protective effect in patients with a high FAI who received treatment with aspirin or a statin, and he suggested that FAI might be a way to better target an anti-inflammatory agent such as canakinumab (Ilaris), which showed cardiovascular protective effects in the CANTOS trial (N Engl J Med. 2017 Sep 21;377[12]:1119-31).
Another key feature of the FAI analysis is that it uses data collected by “any standard” coronary CT angiogram, Dr. Antoniades said. He is a founder of, shareholder in, and chief scientific officer of Caristo Diagnostics, the company developing the software that uses coronary CT data to calculate the FAI.
Dr. Antoniades and his associates derived the FAI using data collected from 1,872 patients who underwent planned coronary CT angiography at a clinic in Erlangen, Germany, during 2005-2009. The researchers correlated FAI scores with cardiovascular disease outcomes during a median follow-up of 72 months. They then validated the FAI using data collected from 2,040 patients who underwent a planned coronary CT exam at the Cleveland Clinic during 2008-2016 and then had a median follow-up of 54 months. The researchers called this overall post-hoc analysis of prospectively collected CT and outcomes data the Cardiovascular Risk Prediction using Computed Tomography (CRISP-CT) study.
Using the derivation data, FAI measurements taken from fat around the proximal right coronary artery that met or exceeded the specified cutoff value of –70.1 Hounsfield units were tied to a 9.04-fold greater rate of cardiac death during follow-up, compared with patients with a lower FAI, and after adjustment for several demographic, clinical, and CT-angiography variables. When the researchers ran this analysis using data from the validation patients and the same cutoff value they found that an elevated FAI linked with a 5.6-fold higher rate of cardiac death. Concurrently with Dr. Antoniades’ talk the results appeared in an online article that was later published (Lancet. 2018 Sep 15;392[10151]:929-39).
Calculating a patient’s FAI offers the prospect for “better use of CT information,” Dr. Antoniades said during the discussion of his report. After a coronary CT scan and conventional data processing, about half the patients have no finding that warrants intervention, but about 10% of these patients actually have an inflamed coronary plaque that is at high risk for rupture that could trigger a cardiac event. The FAI provides a way to use coronary CT angiography to identify these at-risk patients, he explained.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point:
Major finding: Patients with a fat attenuation index at or above the selected cutoff had a five- to ninefold increased rate of cardiac death.
Study details: The CRISP-CT study, which ran a post-hoc analysis of CT angiography data from 3,912 patients.
Disclosures: Dr. Antoniades is a founder of, shareholder in, and chief scientific officer of Caristo Diagnostics, the company developing fat attenuation index software.
PURE Healthy Diet Score validated
MUNICH – A formula for scoring diet quality that during its development phase significantly correlated with overall survival received validation when tested using three independent, large data sets that together included almost 80,000 people.

With these new findings the PURE Healthy Diet Score had now shown consistent, significant correlations with overall survival and the incidence of MI and stroke in a total of about 218,000 people from 50 countries who had been followed in any of four separate studies. This new validation is especially notable because the optimal diet identified by the scoring system diverged from current American diet recommendations in two important ways: Optimal food consumption included three daily servings of full-fat dairy and 1.5 servings daily of unprocessed red meat Andrew Mente, PhD, reported at the annual congress of the European Society of Cardiology. He explained this finding as possibly related to the global scope of the study, which included many people from low- or middle-income countries where average diets are usually low in important nutrients.
The PURE Healthy Diet Score should now be “considered for broad, global dietary recommendations,” Dr. Mente said in a video interview. Testing a diet profile in a large, randomized trial would be ideal, but also difficult to run. Until then, the only alternative for defining an evidence-based optimal diet is observational data, as in the current study. The PURE Healthy Diet Score “is ready for routine use,” said Dr. Mente, a clinical epidemiologist at McMaster University in Hamilton, Canada.
Dr. Mente and his associates developed the Pure Healthy Diet Score with data taken from 138,527 people enrolled in the Prospective Urban Rural Epidemiology (PURE) study. They published a pair of reports in 2017 with their initial findings that also included some of their first steps toward developing the score (Lancet. 2017 Nov 4; 380[10107]:2037-49; 380[10107]:2050-62). The PURE analysis identified seven food groups for which daily intake levels significantly linked with survival: fruits, vegetables, nuts, legumes, dairy, red meat, and fish. Based on this, they devised a scoring formula that gives a person a rating of 1-5 for each of these seven food types, from the lowest quintile of consumption, which scores 1, to the highest quintile, which scores 5. The result is a score than can range from 7 to 35. They then divided the PURE participants into quintiles based on their intakes of all seven food types and found the highest survival rate among people in the quintile with the highest intake level for all of the food groups.
The best-outcome quintile consumed on average about eight servings of fruits and vegetables daily, 2.5 servings of legumes and nuts, three servings of full-fat daily, 1.5 servings of unprocessed red meat, and 0.3 servings of fish (or about two servings of fish weekly). Energy consumption in the best-outcome quintile received 54% of calories as carbohydrates, 28% as fat, and 18% as protein. In contrast, the worst-outcomes quintile received 69% of calories from carbohydrates, 19% from fat, and 12% from protein.
In a model that adjusted for all measured confounders the people in PURE with the best-outcome diet had a statistically significant, 25% reduced all-cause mortality, compared with people in the quintile with the worst diet.
To validate the formula the researchers used data collected from three other trials run by their group at McMaster University:
- The ONTARGET and TRANSCEND studies (N Engl J Med. 2008 Apr 10;358[15]:1547-58), which together included diet and outcomes data for 31,546 patients with vascular disease. Diet analysis and scoring showed that enrolled people in the quintile with the highest score had a statistically significant 24% relative reduction in mortality, compared with the quintile with the worst score after adjusting for measured confounders.
- The INTERHEART study (Lancet. 2004 Sep 11;364[9438]:937-52), which had data for 27,098 people and showed that the primary outcome of incident MI was a statistically significant 22% lower after adjustment in the quintile with the best diet score, compared with the quintile with the worst score.
- The INTERSTROKE study (Lancet. 2016 Aug 20;388[10046]:761-75), with data for 20,834 people, showed that the rate of stroke was a statistically significant 25% lower after adjustment in the quintile with the highest diet score, compared with those with the lowest score.
Dr. Mente had no financial disclosures.
Dr. Mente and his associates have validated the PURE Healthy Diet Score. However, it remains unclear whether the score captures all of the many facets of diet, and it’s also uncertain whether the score is sensitive to changes in diet.
Another issue with the quintile analysis that the researchers used to derive the formula was that the spread between the median scores of the bottom, worst-outcome quartile and the top, best-outcome quartile was only 7 points on a scale that ranged from 7 to 35. The small magnitude of the difference in scores between the bottom and top quintiles might limit the discriminatory power of this scoring system.
Eva Prescott, MD, is a cardiologist at Bispebjerg Hospital in Copenhagen. She has been an advisor to AstraZeneca, NovoNordisk, and Sanofi. She made these comments as designated discussant for the report.
Dr. Mente and his associates have validated the PURE Healthy Diet Score. However, it remains unclear whether the score captures all of the many facets of diet, and it’s also uncertain whether the score is sensitive to changes in diet.
Another issue with the quintile analysis that the researchers used to derive the formula was that the spread between the median scores of the bottom, worst-outcome quartile and the top, best-outcome quartile was only 7 points on a scale that ranged from 7 to 35. The small magnitude of the difference in scores between the bottom and top quintiles might limit the discriminatory power of this scoring system.
Eva Prescott, MD, is a cardiologist at Bispebjerg Hospital in Copenhagen. She has been an advisor to AstraZeneca, NovoNordisk, and Sanofi. She made these comments as designated discussant for the report.
Dr. Mente and his associates have validated the PURE Healthy Diet Score. However, it remains unclear whether the score captures all of the many facets of diet, and it’s also uncertain whether the score is sensitive to changes in diet.
Another issue with the quintile analysis that the researchers used to derive the formula was that the spread between the median scores of the bottom, worst-outcome quartile and the top, best-outcome quartile was only 7 points on a scale that ranged from 7 to 35. The small magnitude of the difference in scores between the bottom and top quintiles might limit the discriminatory power of this scoring system.
Eva Prescott, MD, is a cardiologist at Bispebjerg Hospital in Copenhagen. She has been an advisor to AstraZeneca, NovoNordisk, and Sanofi. She made these comments as designated discussant for the report.
MUNICH – A formula for scoring diet quality that during its development phase significantly correlated with overall survival received validation when tested using three independent, large data sets that together included almost 80,000 people.

With these new findings the PURE Healthy Diet Score had now shown consistent, significant correlations with overall survival and the incidence of MI and stroke in a total of about 218,000 people from 50 countries who had been followed in any of four separate studies. This new validation is especially notable because the optimal diet identified by the scoring system diverged from current American diet recommendations in two important ways: Optimal food consumption included three daily servings of full-fat dairy and 1.5 servings daily of unprocessed red meat Andrew Mente, PhD, reported at the annual congress of the European Society of Cardiology. He explained this finding as possibly related to the global scope of the study, which included many people from low- or middle-income countries where average diets are usually low in important nutrients.
The PURE Healthy Diet Score should now be “considered for broad, global dietary recommendations,” Dr. Mente said in a video interview. Testing a diet profile in a large, randomized trial would be ideal, but also difficult to run. Until then, the only alternative for defining an evidence-based optimal diet is observational data, as in the current study. The PURE Healthy Diet Score “is ready for routine use,” said Dr. Mente, a clinical epidemiologist at McMaster University in Hamilton, Canada.
Dr. Mente and his associates developed the Pure Healthy Diet Score with data taken from 138,527 people enrolled in the Prospective Urban Rural Epidemiology (PURE) study. They published a pair of reports in 2017 with their initial findings that also included some of their first steps toward developing the score (Lancet. 2017 Nov 4; 380[10107]:2037-49; 380[10107]:2050-62). The PURE analysis identified seven food groups for which daily intake levels significantly linked with survival: fruits, vegetables, nuts, legumes, dairy, red meat, and fish. Based on this, they devised a scoring formula that gives a person a rating of 1-5 for each of these seven food types, from the lowest quintile of consumption, which scores 1, to the highest quintile, which scores 5. The result is a score than can range from 7 to 35. They then divided the PURE participants into quintiles based on their intakes of all seven food types and found the highest survival rate among people in the quintile with the highest intake level for all of the food groups.
The best-outcome quintile consumed on average about eight servings of fruits and vegetables daily, 2.5 servings of legumes and nuts, three servings of full-fat daily, 1.5 servings of unprocessed red meat, and 0.3 servings of fish (or about two servings of fish weekly). Energy consumption in the best-outcome quintile received 54% of calories as carbohydrates, 28% as fat, and 18% as protein. In contrast, the worst-outcomes quintile received 69% of calories from carbohydrates, 19% from fat, and 12% from protein.
In a model that adjusted for all measured confounders the people in PURE with the best-outcome diet had a statistically significant, 25% reduced all-cause mortality, compared with people in the quintile with the worst diet.
To validate the formula the researchers used data collected from three other trials run by their group at McMaster University:
- The ONTARGET and TRANSCEND studies (N Engl J Med. 2008 Apr 10;358[15]:1547-58), which together included diet and outcomes data for 31,546 patients with vascular disease. Diet analysis and scoring showed that enrolled people in the quintile with the highest score had a statistically significant 24% relative reduction in mortality, compared with the quintile with the worst score after adjusting for measured confounders.
- The INTERHEART study (Lancet. 2004 Sep 11;364[9438]:937-52), which had data for 27,098 people and showed that the primary outcome of incident MI was a statistically significant 22% lower after adjustment in the quintile with the best diet score, compared with the quintile with the worst score.
- The INTERSTROKE study (Lancet. 2016 Aug 20;388[10046]:761-75), with data for 20,834 people, showed that the rate of stroke was a statistically significant 25% lower after adjustment in the quintile with the highest diet score, compared with those with the lowest score.
Dr. Mente had no financial disclosures.
MUNICH – A formula for scoring diet quality that during its development phase significantly correlated with overall survival received validation when tested using three independent, large data sets that together included almost 80,000 people.

With these new findings the PURE Healthy Diet Score had now shown consistent, significant correlations with overall survival and the incidence of MI and stroke in a total of about 218,000 people from 50 countries who had been followed in any of four separate studies. This new validation is especially notable because the optimal diet identified by the scoring system diverged from current American diet recommendations in two important ways: Optimal food consumption included three daily servings of full-fat dairy and 1.5 servings daily of unprocessed red meat Andrew Mente, PhD, reported at the annual congress of the European Society of Cardiology. He explained this finding as possibly related to the global scope of the study, which included many people from low- or middle-income countries where average diets are usually low in important nutrients.
The PURE Healthy Diet Score should now be “considered for broad, global dietary recommendations,” Dr. Mente said in a video interview. Testing a diet profile in a large, randomized trial would be ideal, but also difficult to run. Until then, the only alternative for defining an evidence-based optimal diet is observational data, as in the current study. The PURE Healthy Diet Score “is ready for routine use,” said Dr. Mente, a clinical epidemiologist at McMaster University in Hamilton, Canada.
Dr. Mente and his associates developed the Pure Healthy Diet Score with data taken from 138,527 people enrolled in the Prospective Urban Rural Epidemiology (PURE) study. They published a pair of reports in 2017 with their initial findings that also included some of their first steps toward developing the score (Lancet. 2017 Nov 4; 380[10107]:2037-49; 380[10107]:2050-62). The PURE analysis identified seven food groups for which daily intake levels significantly linked with survival: fruits, vegetables, nuts, legumes, dairy, red meat, and fish. Based on this, they devised a scoring formula that gives a person a rating of 1-5 for each of these seven food types, from the lowest quintile of consumption, which scores 1, to the highest quintile, which scores 5. The result is a score than can range from 7 to 35. They then divided the PURE participants into quintiles based on their intakes of all seven food types and found the highest survival rate among people in the quintile with the highest intake level for all of the food groups.
The best-outcome quintile consumed on average about eight servings of fruits and vegetables daily, 2.5 servings of legumes and nuts, three servings of full-fat daily, 1.5 servings of unprocessed red meat, and 0.3 servings of fish (or about two servings of fish weekly). Energy consumption in the best-outcome quintile received 54% of calories as carbohydrates, 28% as fat, and 18% as protein. In contrast, the worst-outcomes quintile received 69% of calories from carbohydrates, 19% from fat, and 12% from protein.
In a model that adjusted for all measured confounders the people in PURE with the best-outcome diet had a statistically significant, 25% reduced all-cause mortality, compared with people in the quintile with the worst diet.
To validate the formula the researchers used data collected from three other trials run by their group at McMaster University:
- The ONTARGET and TRANSCEND studies (N Engl J Med. 2008 Apr 10;358[15]:1547-58), which together included diet and outcomes data for 31,546 patients with vascular disease. Diet analysis and scoring showed that enrolled people in the quintile with the highest score had a statistically significant 24% relative reduction in mortality, compared with the quintile with the worst score after adjusting for measured confounders.
- The INTERHEART study (Lancet. 2004 Sep 11;364[9438]:937-52), which had data for 27,098 people and showed that the primary outcome of incident MI was a statistically significant 22% lower after adjustment in the quintile with the best diet score, compared with the quintile with the worst score.
- The INTERSTROKE study (Lancet. 2016 Aug 20;388[10046]:761-75), with data for 20,834 people, showed that the rate of stroke was a statistically significant 25% lower after adjustment in the quintile with the highest diet score, compared with those with the lowest score.
Dr. Mente had no financial disclosures.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point:
Major finding: The highest-scoring quintiles had about 25% fewer deaths, MIs, and strokes, compared with the lowest-scoring quintiles.
Study details: The PURE Healthy Diet Score underwent validation using three independent data sets with a total of 79,478 people.
Disclosures: Dr. Mente had no financial disclosures.
GARFIELD-AF registry: DOACs cut mortality 19%
MUNICH – Treatment of real-world patients newly diagnosed with atrial fibrillation using a direct oral anticoagulant led to benefits that tracked the advantages previously seen in randomized, controlled trials of these drugs, based on findings from more than 26,000 patients enrolled in a global registry.
Atrial fibrillation patients enrolled in the GARFIELD-AF(Global Anticoagulant Registry in the Field) study who started treatment with a direct oral anticoagulant (DOAC) had a 19% relative risk reduction in all-cause mortality during 2 years of follow-up, compared with patients on an oral vitamin K antagonist (VKA) regimen (such as warfarin), a statistically significant difference after adjustment for 30 demographic, clinical, and registry variables, A. John Camm, MD, said at the annual congress of the European Society of Cardiology. The analysis also showed trends toward lower rates of stroke or systemic thrombosis as well as major bleeding events when patients received a DOAC, compared with those on VKA, but these differences were not statistically significant, reported Dr. Camm, a professor of clinical cardiology at St. George’s University of London.
The analyses run by Dr. Camm and his associates also confirmed the superiority of oral anticoagulation. There was an adjusted 17% relative risk reduction in all-cause mortality during 2-year follow-up in patients on any form of oral anticoagulation, compared with patients who did not receive anticoagulation, a statistically significant difference. The comparison of patients on any oral anticoagulant with those not on treatment also showed a significant lowering of stroke or systemic embolism, as well as a 36% relative increase in the risk for a major bleeding episode that was close to statistical significance.
These findings in a registry of patients undergoing routine care “suggest that the effectiveness of oral anticoagulants in randomized clinical trials can be translated to the broad cross section of patients treated in everyday practice,” Dr. Camm said. However, he highlighted two important qualifications to the findings.
First, the analysis focused on the type of anticoagulation patients received at the time they entered the GARFIELD-AF registry and did not account for possible changes in treatment after that. Second, the analysis did not adjust for additional potential confounding variables, which Dr. Camm was certain existed and affected the findings.
“I’m concerned that a confounder we have not been able to account for is the quality of medical care that patients received,” he noted. “The substantial reduction in mortality [using a DOAC, compared with a VKA] is not simply due to reductions in stroke or major bleeding. We must look at other explanations, such as differences in quality of care and access to care.”
The analyses have also not yet looked at outcomes based on the specific DOAC a patient received – apixaban, dabigatran, edoxaban, or rivaroxaban – something that Dr. Camm said is in the works.
GARFIELD-AF enrolled nearly 35,000 patients with newly diagnosed atrial fibrillation and at least one stroke risk factor in 35 countries from April 2013 to September 2016. The analysis winnowed this down to 26,742 patients who also had a CHA2DS2-VASc score of at least 2 (which identifies patients with a high thrombotic risk) and had complete enrollment and follow-up data.
GARFIELD-AF was funded in part by Bayer. Dr. Camm reported being an adviser to Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Pfizer/Bristol-Myers Squibb.
MUNICH – Treatment of real-world patients newly diagnosed with atrial fibrillation using a direct oral anticoagulant led to benefits that tracked the advantages previously seen in randomized, controlled trials of these drugs, based on findings from more than 26,000 patients enrolled in a global registry.
Atrial fibrillation patients enrolled in the GARFIELD-AF(Global Anticoagulant Registry in the Field) study who started treatment with a direct oral anticoagulant (DOAC) had a 19% relative risk reduction in all-cause mortality during 2 years of follow-up, compared with patients on an oral vitamin K antagonist (VKA) regimen (such as warfarin), a statistically significant difference after adjustment for 30 demographic, clinical, and registry variables, A. John Camm, MD, said at the annual congress of the European Society of Cardiology. The analysis also showed trends toward lower rates of stroke or systemic thrombosis as well as major bleeding events when patients received a DOAC, compared with those on VKA, but these differences were not statistically significant, reported Dr. Camm, a professor of clinical cardiology at St. George’s University of London.
The analyses run by Dr. Camm and his associates also confirmed the superiority of oral anticoagulation. There was an adjusted 17% relative risk reduction in all-cause mortality during 2-year follow-up in patients on any form of oral anticoagulation, compared with patients who did not receive anticoagulation, a statistically significant difference. The comparison of patients on any oral anticoagulant with those not on treatment also showed a significant lowering of stroke or systemic embolism, as well as a 36% relative increase in the risk for a major bleeding episode that was close to statistical significance.
These findings in a registry of patients undergoing routine care “suggest that the effectiveness of oral anticoagulants in randomized clinical trials can be translated to the broad cross section of patients treated in everyday practice,” Dr. Camm said. However, he highlighted two important qualifications to the findings.
First, the analysis focused on the type of anticoagulation patients received at the time they entered the GARFIELD-AF registry and did not account for possible changes in treatment after that. Second, the analysis did not adjust for additional potential confounding variables, which Dr. Camm was certain existed and affected the findings.
“I’m concerned that a confounder we have not been able to account for is the quality of medical care that patients received,” he noted. “The substantial reduction in mortality [using a DOAC, compared with a VKA] is not simply due to reductions in stroke or major bleeding. We must look at other explanations, such as differences in quality of care and access to care.”
The analyses have also not yet looked at outcomes based on the specific DOAC a patient received – apixaban, dabigatran, edoxaban, or rivaroxaban – something that Dr. Camm said is in the works.
GARFIELD-AF enrolled nearly 35,000 patients with newly diagnosed atrial fibrillation and at least one stroke risk factor in 35 countries from April 2013 to September 2016. The analysis winnowed this down to 26,742 patients who also had a CHA2DS2-VASc score of at least 2 (which identifies patients with a high thrombotic risk) and had complete enrollment and follow-up data.
GARFIELD-AF was funded in part by Bayer. Dr. Camm reported being an adviser to Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Pfizer/Bristol-Myers Squibb.
MUNICH – Treatment of real-world patients newly diagnosed with atrial fibrillation using a direct oral anticoagulant led to benefits that tracked the advantages previously seen in randomized, controlled trials of these drugs, based on findings from more than 26,000 patients enrolled in a global registry.
Atrial fibrillation patients enrolled in the GARFIELD-AF(Global Anticoagulant Registry in the Field) study who started treatment with a direct oral anticoagulant (DOAC) had a 19% relative risk reduction in all-cause mortality during 2 years of follow-up, compared with patients on an oral vitamin K antagonist (VKA) regimen (such as warfarin), a statistically significant difference after adjustment for 30 demographic, clinical, and registry variables, A. John Camm, MD, said at the annual congress of the European Society of Cardiology. The analysis also showed trends toward lower rates of stroke or systemic thrombosis as well as major bleeding events when patients received a DOAC, compared with those on VKA, but these differences were not statistically significant, reported Dr. Camm, a professor of clinical cardiology at St. George’s University of London.
The analyses run by Dr. Camm and his associates also confirmed the superiority of oral anticoagulation. There was an adjusted 17% relative risk reduction in all-cause mortality during 2-year follow-up in patients on any form of oral anticoagulation, compared with patients who did not receive anticoagulation, a statistically significant difference. The comparison of patients on any oral anticoagulant with those not on treatment also showed a significant lowering of stroke or systemic embolism, as well as a 36% relative increase in the risk for a major bleeding episode that was close to statistical significance.
These findings in a registry of patients undergoing routine care “suggest that the effectiveness of oral anticoagulants in randomized clinical trials can be translated to the broad cross section of patients treated in everyday practice,” Dr. Camm said. However, he highlighted two important qualifications to the findings.
First, the analysis focused on the type of anticoagulation patients received at the time they entered the GARFIELD-AF registry and did not account for possible changes in treatment after that. Second, the analysis did not adjust for additional potential confounding variables, which Dr. Camm was certain existed and affected the findings.
“I’m concerned that a confounder we have not been able to account for is the quality of medical care that patients received,” he noted. “The substantial reduction in mortality [using a DOAC, compared with a VKA] is not simply due to reductions in stroke or major bleeding. We must look at other explanations, such as differences in quality of care and access to care.”
The analyses have also not yet looked at outcomes based on the specific DOAC a patient received – apixaban, dabigatran, edoxaban, or rivaroxaban – something that Dr. Camm said is in the works.
GARFIELD-AF enrolled nearly 35,000 patients with newly diagnosed atrial fibrillation and at least one stroke risk factor in 35 countries from April 2013 to September 2016. The analysis winnowed this down to 26,742 patients who also had a CHA2DS2-VASc score of at least 2 (which identifies patients with a high thrombotic risk) and had complete enrollment and follow-up data.
GARFIELD-AF was funded in part by Bayer. Dr. Camm reported being an adviser to Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Pfizer/Bristol-Myers Squibb.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point:
Major finding: Direct oral anticoagulant–treated patients had a 19% relative reduction in all-cause death, compared with patients on a vitamin K antagonist.
Study details: The GARFIELD-AF registry, which included 26,742 patients with newly diagnosed atrial fibrillation.
Disclosures: GARFIELD-AF was funded in part by Bayer. Dr. Camm has been an adviser to Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Pfizer/Bristol-Myers Squibb.
Drug-coated balloons shown noninferior to DES in thin coronaries
MUNICH – for preventing the clinical consequences of restenosis during 12 months following coronary intervention, according to results from a prospective, randomized, multicenter trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Drug-coated balloons are already used to treat in-stent coronary restenosis. The findings of the current study establish the tested DCB as noninferior to a DES for treating coronary stenoses in narrow arteries less than 3 mm in diameter, Raban V. Jeger, MD, said at the annual congress of the European Society of Cardiology. The DCB approach avoids placing a metal stent in a narrow coronary and thus has no long-term risk for in-stent thrombosis, said Dr. Jeger, a professor of cardiology at Basel (Switzerland) University Hospital. Dr. Jeger acknowledged that the tested DCB is more expensive than the second-generation DES used as the comparator in most of the control patients, “but I think the benefit to patients is worth” the added cost, he said when discussing his report.
The BASKET-SMALL 2 (NCT01574534) study enrolled 758 patients at 14 centers in Switzerland, Germany, and Austria. The trial limited enrollment to patients who were scheduled to undergo percutaneous coronary intervention for stenosis in a coronary artery that was at least 2.0 mm and less than 3.0 mm in diameter and had first undergone successful predilatation without any flow-limiting dissections or residual stenosis, a step in the DCB procedure that adds to the procedure’s cost.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study randomized patients to treatment with either a balloon coated with paclitaxel/iopromide (SeQuent Please) or a DES. The first quarter of patients randomized into the DES arm received a first-generation, paclitaxel-eluting DES (Taxus Element); the remaining patients in the comparator arm received a second-generation everolimus-eluting DES (Xience). The DCB tested is not approved for U.S. marketing.
The primary endpoint was the combined rate of cardiac death, nonfatal MI, or target vessel revascularization during 12 months of follow-up. In the intention-to-treat analysis, this occurred in 7.33% of the DCB patients and in 7.45% of the DES patients, a difference that was not statistically significant and that met the prespecified criterion for noninferiority of the DCB. Concurrently with Dr. Jeger’s report at the congress, the results also appeared in an article published in The Lancet (Lancet. 2018 Sep 8;392[10190]:849-56).
One limitation of the study was that the first 25% of patients enrolled into the DES arm received a first-generation DES, while the remaining 75% received a second-generation device. Analysis of the primary endpoint by DES type showed that events occurred more than twice as often in the patients who received a first-generation DES, and their inclusion may have affected the comparator group’s results.
Coronary arteries that need percutaneous intervention and are less than 3 mm in diameter constitute about a third of all target vessels, and they are especially common among women and in patients with diabetes, Dr. Jeger said. Despite this, women made up about a quarter of the study enrollment, and about a third had diabetes. He also noted that a key aspect of adopting the DCB approach into routine practice is that operators would need to have the “courage” to accept some amount of recoil and “minor” dissections after DCB treatment and not feel compelled to correct these with a stent.
Other features of the BASKET-SMALL 2 trial also have raised concerns about the immediate clinical implications of the results, said Roxana Mehran, MD, a professor of medicine at Icahn School of Medicine at Mount Sinai, New York, and the congress’s designated discussant for the report.
The study began in 2012, which means it took more than 5 years to enroll and suggests that the study may have a selection bias. Dr. Mehran also questioned whether it was really a small vessel study, with an enrollment criterion of less than 3 mm in diameter. A future study should be done in “truly” small vessels, those thinner than 2.5 mm, she said.
Dr. Mehran agreed it’s attractive to speculate that, by using a DCB and avoiding stent placement, fewer patients will eventually have very-late adverse events, but this must be proven with longer follow-up and in larger numbers of patients, she said.
Treating thin coronary arteries is a problem because they have a higher risk for in-stent restenosis, although usually we will put a stent in arteries that are at least 2.5 mm wide and sometimes in coronaries as narrow as 2.25 mm. That’s using the narrowest stent we have available. Sometimes in vessels this size, if the result from initial balloon angioplasty looks good on angiography, we accept that outcome and do not place a stent.
Steen Dalby Kristensen, MD , is a professor of cardiology at Aarhus University in Skejby, Denmark. He had no relevant disclosures. He made these comments in a video interview.
Treating thin coronary arteries is a problem because they have a higher risk for in-stent restenosis, although usually we will put a stent in arteries that are at least 2.5 mm wide and sometimes in coronaries as narrow as 2.25 mm. That’s using the narrowest stent we have available. Sometimes in vessels this size, if the result from initial balloon angioplasty looks good on angiography, we accept that outcome and do not place a stent.
Steen Dalby Kristensen, MD , is a professor of cardiology at Aarhus University in Skejby, Denmark. He had no relevant disclosures. He made these comments in a video interview.
Treating thin coronary arteries is a problem because they have a higher risk for in-stent restenosis, although usually we will put a stent in arteries that are at least 2.5 mm wide and sometimes in coronaries as narrow as 2.25 mm. That’s using the narrowest stent we have available. Sometimes in vessels this size, if the result from initial balloon angioplasty looks good on angiography, we accept that outcome and do not place a stent.
Steen Dalby Kristensen, MD , is a professor of cardiology at Aarhus University in Skejby, Denmark. He had no relevant disclosures. He made these comments in a video interview.
MUNICH – for preventing the clinical consequences of restenosis during 12 months following coronary intervention, according to results from a prospective, randomized, multicenter trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Drug-coated balloons are already used to treat in-stent coronary restenosis. The findings of the current study establish the tested DCB as noninferior to a DES for treating coronary stenoses in narrow arteries less than 3 mm in diameter, Raban V. Jeger, MD, said at the annual congress of the European Society of Cardiology. The DCB approach avoids placing a metal stent in a narrow coronary and thus has no long-term risk for in-stent thrombosis, said Dr. Jeger, a professor of cardiology at Basel (Switzerland) University Hospital. Dr. Jeger acknowledged that the tested DCB is more expensive than the second-generation DES used as the comparator in most of the control patients, “but I think the benefit to patients is worth” the added cost, he said when discussing his report.
The BASKET-SMALL 2 (NCT01574534) study enrolled 758 patients at 14 centers in Switzerland, Germany, and Austria. The trial limited enrollment to patients who were scheduled to undergo percutaneous coronary intervention for stenosis in a coronary artery that was at least 2.0 mm and less than 3.0 mm in diameter and had first undergone successful predilatation without any flow-limiting dissections or residual stenosis, a step in the DCB procedure that adds to the procedure’s cost.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study randomized patients to treatment with either a balloon coated with paclitaxel/iopromide (SeQuent Please) or a DES. The first quarter of patients randomized into the DES arm received a first-generation, paclitaxel-eluting DES (Taxus Element); the remaining patients in the comparator arm received a second-generation everolimus-eluting DES (Xience). The DCB tested is not approved for U.S. marketing.
The primary endpoint was the combined rate of cardiac death, nonfatal MI, or target vessel revascularization during 12 months of follow-up. In the intention-to-treat analysis, this occurred in 7.33% of the DCB patients and in 7.45% of the DES patients, a difference that was not statistically significant and that met the prespecified criterion for noninferiority of the DCB. Concurrently with Dr. Jeger’s report at the congress, the results also appeared in an article published in The Lancet (Lancet. 2018 Sep 8;392[10190]:849-56).
One limitation of the study was that the first 25% of patients enrolled into the DES arm received a first-generation DES, while the remaining 75% received a second-generation device. Analysis of the primary endpoint by DES type showed that events occurred more than twice as often in the patients who received a first-generation DES, and their inclusion may have affected the comparator group’s results.
Coronary arteries that need percutaneous intervention and are less than 3 mm in diameter constitute about a third of all target vessels, and they are especially common among women and in patients with diabetes, Dr. Jeger said. Despite this, women made up about a quarter of the study enrollment, and about a third had diabetes. He also noted that a key aspect of adopting the DCB approach into routine practice is that operators would need to have the “courage” to accept some amount of recoil and “minor” dissections after DCB treatment and not feel compelled to correct these with a stent.
Other features of the BASKET-SMALL 2 trial also have raised concerns about the immediate clinical implications of the results, said Roxana Mehran, MD, a professor of medicine at Icahn School of Medicine at Mount Sinai, New York, and the congress’s designated discussant for the report.
The study began in 2012, which means it took more than 5 years to enroll and suggests that the study may have a selection bias. Dr. Mehran also questioned whether it was really a small vessel study, with an enrollment criterion of less than 3 mm in diameter. A future study should be done in “truly” small vessels, those thinner than 2.5 mm, she said.
Dr. Mehran agreed it’s attractive to speculate that, by using a DCB and avoiding stent placement, fewer patients will eventually have very-late adverse events, but this must be proven with longer follow-up and in larger numbers of patients, she said.
MUNICH – for preventing the clinical consequences of restenosis during 12 months following coronary intervention, according to results from a prospective, randomized, multicenter trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Drug-coated balloons are already used to treat in-stent coronary restenosis. The findings of the current study establish the tested DCB as noninferior to a DES for treating coronary stenoses in narrow arteries less than 3 mm in diameter, Raban V. Jeger, MD, said at the annual congress of the European Society of Cardiology. The DCB approach avoids placing a metal stent in a narrow coronary and thus has no long-term risk for in-stent thrombosis, said Dr. Jeger, a professor of cardiology at Basel (Switzerland) University Hospital. Dr. Jeger acknowledged that the tested DCB is more expensive than the second-generation DES used as the comparator in most of the control patients, “but I think the benefit to patients is worth” the added cost, he said when discussing his report.
The BASKET-SMALL 2 (NCT01574534) study enrolled 758 patients at 14 centers in Switzerland, Germany, and Austria. The trial limited enrollment to patients who were scheduled to undergo percutaneous coronary intervention for stenosis in a coronary artery that was at least 2.0 mm and less than 3.0 mm in diameter and had first undergone successful predilatation without any flow-limiting dissections or residual stenosis, a step in the DCB procedure that adds to the procedure’s cost.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study randomized patients to treatment with either a balloon coated with paclitaxel/iopromide (SeQuent Please) or a DES. The first quarter of patients randomized into the DES arm received a first-generation, paclitaxel-eluting DES (Taxus Element); the remaining patients in the comparator arm received a second-generation everolimus-eluting DES (Xience). The DCB tested is not approved for U.S. marketing.
The primary endpoint was the combined rate of cardiac death, nonfatal MI, or target vessel revascularization during 12 months of follow-up. In the intention-to-treat analysis, this occurred in 7.33% of the DCB patients and in 7.45% of the DES patients, a difference that was not statistically significant and that met the prespecified criterion for noninferiority of the DCB. Concurrently with Dr. Jeger’s report at the congress, the results also appeared in an article published in The Lancet (Lancet. 2018 Sep 8;392[10190]:849-56).
One limitation of the study was that the first 25% of patients enrolled into the DES arm received a first-generation DES, while the remaining 75% received a second-generation device. Analysis of the primary endpoint by DES type showed that events occurred more than twice as often in the patients who received a first-generation DES, and their inclusion may have affected the comparator group’s results.
Coronary arteries that need percutaneous intervention and are less than 3 mm in diameter constitute about a third of all target vessels, and they are especially common among women and in patients with diabetes, Dr. Jeger said. Despite this, women made up about a quarter of the study enrollment, and about a third had diabetes. He also noted that a key aspect of adopting the DCB approach into routine practice is that operators would need to have the “courage” to accept some amount of recoil and “minor” dissections after DCB treatment and not feel compelled to correct these with a stent.
Other features of the BASKET-SMALL 2 trial also have raised concerns about the immediate clinical implications of the results, said Roxana Mehran, MD, a professor of medicine at Icahn School of Medicine at Mount Sinai, New York, and the congress’s designated discussant for the report.
The study began in 2012, which means it took more than 5 years to enroll and suggests that the study may have a selection bias. Dr. Mehran also questioned whether it was really a small vessel study, with an enrollment criterion of less than 3 mm in diameter. A future study should be done in “truly” small vessels, those thinner than 2.5 mm, she said.
Dr. Mehran agreed it’s attractive to speculate that, by using a DCB and avoiding stent placement, fewer patients will eventually have very-late adverse events, but this must be proven with longer follow-up and in larger numbers of patients, she said.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: Drug-coated balloon treatment worked as well as drug-eluting stents in thin coronaries.
Major finding: Twelve-month MACE occurred in 7.33% of balloon-treated patients and in 7.45% of stent-treated patients.
Study details: BASKET-SMALL 2, an international, multicenter randomized trial with 758 patients.
Disclosures: The investigator-initiated study received partial funding from B. Braun, the company that markets the drug-coated balloon (SeQuent Please) tested in the study. Dr. Jeger has received research funding from B. Braun. Dr. Mehran has been a consultant to Abbott, Bayer, BSC, and CSL Behring and has received research funding from Abbott, Astra Zeneca, Bayer, BCC, DSI, and Janssen.
Coronary CT FFR sharpens patient assessment in two studies
MUNICH – Noninvasive assessment of fractional flow reserve (FFR) within coronary arteries using data collected by CT angiography again has been shown to provide important additional diagnostic information that better guides patient management.
“The value of FFRCT is to reduce the number of patients who go to the cath lab. For patients with a stenosis of 60% that is not likely to have functional significance we can avoid catheterization and treat the patient medically. FFRCT is a valuable technology, but my concern is that currently it costs about $1,400 for this test,” commented Todd C. Villines, MD, a cardiologist at Georgetown University in Washington who was a discussant for the study. “Given the cost, we need to better define the patients on whom we use FFRCT and integrate it into clinical decision making,” Dr. Villines said in an interview.
Perhaps the best demonstration of the potential role for FFRCT came from a single-center study at Aarhus (Denmark) University with 3,674 patients with stable chest pain who underwent CCTA as their initial assessment for suspected coronary artery disease between May 2014 and December 2016. More than two-thirds of these patients had coronary stenoses of less than 30% and had no further assessment or treatment, and 11% had at least one coronary stenosis of at least 70% on CCTA and then had follow-up testing by either conventional angiography or myocardial perfusion imaging. The report at the congress focused on the 697 patients with an inconclusive result based on CCTA alone and at least one stenosis of 30%-69% who underwent FFRCT analysis, and focused specifically on 677 patients with a useful FFRCT result.
Of these patients, 410 (61% of this subgroup) had no coronary lesion that created a FFRCT of 0.8 or less. All received treatment with optimal medical therapy only, and after a median follow-up had a 3.9% incidence of the primary endpoint, the combined rate of all-cause death, nonfatal MI, hospitalization for unstable angina, or unplanned revascularization. This 3.9% rate was not significantly different from the 2.8% rate seen during follow-up of the patients with no coronary stenosis of 30% or greater.
The remaining 267 patients (39% of the subgroup) with a FFRCT that showed 80% or less flow reserve either received optimal medical therapy (112 patients, 42% of this group) or angiography by coronary catheterization (155 patients, 58% of this group).
The second report used data collected from 5,083 patients entered into a multinational registry, ADVANCE, with symptoms suggestive of coronary artery disease and results from CCTA that suggested coronary stenosis. The collaborating researchers then used the CCTA results to generate a FFR analysis for 4,893 (96%) of the patients, and the analysis was usable for 4,737 of them. The FFRCT results led to reclassification of the management strategy for 67% of the patients, the primary endpoint for this analysis, reported Timothy A. Fairbairn, MD, a cardiologist at the Liverpool (England) Heart and Chest Hospital.
One limitation of this study was the relatively brief, 90-day follow-up, but it is the first real-world, multicenter assessment of the utility and safety of FFRCT.
These findings highlight what a “disruptive technology” FFRCT represents, commented Dr. Villines. He also noted that the reclassifications triggered by the FFRCT analysis led to fewer patients undergoing invasive angiography, a good outcome from a cost-effectiveness perspective.
Concurrently with Dr. Fairbairn’s report the results from ADVANCE also appeared in an article published online (Euro Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy530).
A third FFRCT study reported at the session, the Computed Tomographic Evaluation of Atherosclerotic Determinants of Myocardial Ischemia (CREDENCE) study, enrolled 612 patients with suspected coronary artery disease who had been referred for and underwent invasive coronary angiography with FFR evaluation at 13 international centers, including several in the United States. All 612 patients also had assessment by CCTA and FFRCT, and also some type of functional myocardial perfusion assessment using positron emission tomography, single-photon emission CT, or coronary MR.
The Aarhus University study received no commercial funding. Dr. Nørgaard has received research funding from Edwards; Siemens; and HeartFlow, the company that markets FFR analysis for coronary CT angiography data. The ADVANCE registry was sponsored by HeartFlow. Dr. Fairbairn has been a speaker for Heartflow. Dr. Stuijfzand and Dr. Villines had no relevant disclosures.
MUNICH – Noninvasive assessment of fractional flow reserve (FFR) within coronary arteries using data collected by CT angiography again has been shown to provide important additional diagnostic information that better guides patient management.
“The value of FFRCT is to reduce the number of patients who go to the cath lab. For patients with a stenosis of 60% that is not likely to have functional significance we can avoid catheterization and treat the patient medically. FFRCT is a valuable technology, but my concern is that currently it costs about $1,400 for this test,” commented Todd C. Villines, MD, a cardiologist at Georgetown University in Washington who was a discussant for the study. “Given the cost, we need to better define the patients on whom we use FFRCT and integrate it into clinical decision making,” Dr. Villines said in an interview.
Perhaps the best demonstration of the potential role for FFRCT came from a single-center study at Aarhus (Denmark) University with 3,674 patients with stable chest pain who underwent CCTA as their initial assessment for suspected coronary artery disease between May 2014 and December 2016. More than two-thirds of these patients had coronary stenoses of less than 30% and had no further assessment or treatment, and 11% had at least one coronary stenosis of at least 70% on CCTA and then had follow-up testing by either conventional angiography or myocardial perfusion imaging. The report at the congress focused on the 697 patients with an inconclusive result based on CCTA alone and at least one stenosis of 30%-69% who underwent FFRCT analysis, and focused specifically on 677 patients with a useful FFRCT result.
Of these patients, 410 (61% of this subgroup) had no coronary lesion that created a FFRCT of 0.8 or less. All received treatment with optimal medical therapy only, and after a median follow-up had a 3.9% incidence of the primary endpoint, the combined rate of all-cause death, nonfatal MI, hospitalization for unstable angina, or unplanned revascularization. This 3.9% rate was not significantly different from the 2.8% rate seen during follow-up of the patients with no coronary stenosis of 30% or greater.
The remaining 267 patients (39% of the subgroup) with a FFRCT that showed 80% or less flow reserve either received optimal medical therapy (112 patients, 42% of this group) or angiography by coronary catheterization (155 patients, 58% of this group).
The second report used data collected from 5,083 patients entered into a multinational registry, ADVANCE, with symptoms suggestive of coronary artery disease and results from CCTA that suggested coronary stenosis. The collaborating researchers then used the CCTA results to generate a FFR analysis for 4,893 (96%) of the patients, and the analysis was usable for 4,737 of them. The FFRCT results led to reclassification of the management strategy for 67% of the patients, the primary endpoint for this analysis, reported Timothy A. Fairbairn, MD, a cardiologist at the Liverpool (England) Heart and Chest Hospital.
One limitation of this study was the relatively brief, 90-day follow-up, but it is the first real-world, multicenter assessment of the utility and safety of FFRCT.
These findings highlight what a “disruptive technology” FFRCT represents, commented Dr. Villines. He also noted that the reclassifications triggered by the FFRCT analysis led to fewer patients undergoing invasive angiography, a good outcome from a cost-effectiveness perspective.
Concurrently with Dr. Fairbairn’s report the results from ADVANCE also appeared in an article published online (Euro Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy530).
A third FFRCT study reported at the session, the Computed Tomographic Evaluation of Atherosclerotic Determinants of Myocardial Ischemia (CREDENCE) study, enrolled 612 patients with suspected coronary artery disease who had been referred for and underwent invasive coronary angiography with FFR evaluation at 13 international centers, including several in the United States. All 612 patients also had assessment by CCTA and FFRCT, and also some type of functional myocardial perfusion assessment using positron emission tomography, single-photon emission CT, or coronary MR.
The Aarhus University study received no commercial funding. Dr. Nørgaard has received research funding from Edwards; Siemens; and HeartFlow, the company that markets FFR analysis for coronary CT angiography data. The ADVANCE registry was sponsored by HeartFlow. Dr. Fairbairn has been a speaker for Heartflow. Dr. Stuijfzand and Dr. Villines had no relevant disclosures.
MUNICH – Noninvasive assessment of fractional flow reserve (FFR) within coronary arteries using data collected by CT angiography again has been shown to provide important additional diagnostic information that better guides patient management.
“The value of FFRCT is to reduce the number of patients who go to the cath lab. For patients with a stenosis of 60% that is not likely to have functional significance we can avoid catheterization and treat the patient medically. FFRCT is a valuable technology, but my concern is that currently it costs about $1,400 for this test,” commented Todd C. Villines, MD, a cardiologist at Georgetown University in Washington who was a discussant for the study. “Given the cost, we need to better define the patients on whom we use FFRCT and integrate it into clinical decision making,” Dr. Villines said in an interview.
Perhaps the best demonstration of the potential role for FFRCT came from a single-center study at Aarhus (Denmark) University with 3,674 patients with stable chest pain who underwent CCTA as their initial assessment for suspected coronary artery disease between May 2014 and December 2016. More than two-thirds of these patients had coronary stenoses of less than 30% and had no further assessment or treatment, and 11% had at least one coronary stenosis of at least 70% on CCTA and then had follow-up testing by either conventional angiography or myocardial perfusion imaging. The report at the congress focused on the 697 patients with an inconclusive result based on CCTA alone and at least one stenosis of 30%-69% who underwent FFRCT analysis, and focused specifically on 677 patients with a useful FFRCT result.
Of these patients, 410 (61% of this subgroup) had no coronary lesion that created a FFRCT of 0.8 or less. All received treatment with optimal medical therapy only, and after a median follow-up had a 3.9% incidence of the primary endpoint, the combined rate of all-cause death, nonfatal MI, hospitalization for unstable angina, or unplanned revascularization. This 3.9% rate was not significantly different from the 2.8% rate seen during follow-up of the patients with no coronary stenosis of 30% or greater.
The remaining 267 patients (39% of the subgroup) with a FFRCT that showed 80% or less flow reserve either received optimal medical therapy (112 patients, 42% of this group) or angiography by coronary catheterization (155 patients, 58% of this group).
The second report used data collected from 5,083 patients entered into a multinational registry, ADVANCE, with symptoms suggestive of coronary artery disease and results from CCTA that suggested coronary stenosis. The collaborating researchers then used the CCTA results to generate a FFR analysis for 4,893 (96%) of the patients, and the analysis was usable for 4,737 of them. The FFRCT results led to reclassification of the management strategy for 67% of the patients, the primary endpoint for this analysis, reported Timothy A. Fairbairn, MD, a cardiologist at the Liverpool (England) Heart and Chest Hospital.
One limitation of this study was the relatively brief, 90-day follow-up, but it is the first real-world, multicenter assessment of the utility and safety of FFRCT.
These findings highlight what a “disruptive technology” FFRCT represents, commented Dr. Villines. He also noted that the reclassifications triggered by the FFRCT analysis led to fewer patients undergoing invasive angiography, a good outcome from a cost-effectiveness perspective.
Concurrently with Dr. Fairbairn’s report the results from ADVANCE also appeared in an article published online (Euro Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy530).
A third FFRCT study reported at the session, the Computed Tomographic Evaluation of Atherosclerotic Determinants of Myocardial Ischemia (CREDENCE) study, enrolled 612 patients with suspected coronary artery disease who had been referred for and underwent invasive coronary angiography with FFR evaluation at 13 international centers, including several in the United States. All 612 patients also had assessment by CCTA and FFRCT, and also some type of functional myocardial perfusion assessment using positron emission tomography, single-photon emission CT, or coronary MR.
The Aarhus University study received no commercial funding. Dr. Nørgaard has received research funding from Edwards; Siemens; and HeartFlow, the company that markets FFR analysis for coronary CT angiography data. The ADVANCE registry was sponsored by HeartFlow. Dr. Fairbairn has been a speaker for Heartflow. Dr. Stuijfzand and Dr. Villines had no relevant disclosures.
REPORTING FROM THE ESC CONGRESS 2018
Home telemonitoring for heart failure cuts mortality
MUNICH – A comprehensive home telemonitoring program paid off big for selected patients with heart failure in a large, German nationwide masked randomization trial.
First, TIM-HF2 didn’t rely on passive monitoring of the patients’ daily electronically submitted home data. Instead, the data went straight to a central telemonitoring center staffed 24/7 by physicians and nurses with heart failure expertise. There, the information was immediately analyzed using proprietary telemedical analytic software known as the Fontane system. The software employs individually tailored, self-adapting algorithms in order to alert staff when trouble is brewing.
But the telemonitoring intervention doesn’t merely detect early clinical deterioration. It’s also a vehicle for ongoing patient education, outpatient adjustment of drugs, management of major comorbid conditions, and hospital admissions as needed. The patient’s local primary care physician was also plugged into the remote monitoring system and kept abreast of the patient’s condition.
Second, TIM-HF2 focused on a carefully selected subgroup of heart failure patients whom prior studies suggested were particularly likely to benefit from home telemedical management. All participants were NYHA class II or III with a left ventricular ejection fraction of 45% or less, a hospitalization for heart failure within 12 months prior to randomization, and free of moderate or severe depression as evidenced by a baseline Patient Health Questionnaire-9 score of 9 or less, explained Dr. Koehler, head of the center for cardiovascular telemedicine at Charite University in Berlin.
Why exclude patients with depression?
“In this concept, with wholistic remote patient management, we need an active patient who is able to measure every day, who is able to communicate with the telemedical center, and who is able to communicate in this network created between the telemedical center and local caregivers. If someone is really depressed, unable to act, lying in bed saying it makes no sense to take drugs or do anything, then we cannot help. That is for us, I think, the most important thing. We’ve seen it now in two trials,” according to the cardiologist.
The all-cause mortality rate was 7.86 per 100 person-years in the home-telemonitoring group versus 11.34 in usual-care controls. Patients in the active intervention arm lost a mean of 17.8 days per year because of unplanned cardiovascular hospital admissions, compared with 24.2 days per year in controls.
Importantly, outcomes were equally good in the remote patient-management group regardless of whether patients were among the 40% of participants living in urban Germany or the 60% in rural areas. Thus, the telemonitoring intervention eliminated the geographic disparity in health care outcomes which is a prominent issue in Germany, as well as the United States.
A formal cost-benefit analysis of the TIM-HF2 results is in the works, Dr. Koehler said.
Simultaneous with his presentation in Munich, the TIM-HF2 study was published online in the Lancet.
In an accompanying editorial, two prominent heart failure experts – John F.G. Cleland, MD, of the University of Glasgow and Robin A. Clark, MD, of Flinders University in Adelaide – hailed TIM-HF2 as a major advance and indicated in sharp terms that it’s time for guideline writers to sit up and take notice.
“Despite much clinical skepticism and feeble support from most guidelines, in our view the growing weight of evidence suggests that home telemonitoring does reduce mortality for patients with heart failure, and this effect might be substantial. These and other trials also show that the emphasis placed on hospital admission for heart failure might be misplaced, at least from a patient’s perspective, because the proportion of days lost due to hospital admission is small, compared with those lost to death,” the physicians wrote in the editorial.
They also noted that, even though the between-group difference in the number of days during which patients were hospitalized for cardiovascular causes was relatively small, it’s clear that home telemonitoring triggered some potentially life-saving hospitalizations. “Home telemonitoring puts the patient back in the center of health care, ensuring that they know what the health professional is trying to achieve and that they agree with those aims. Ultimately, patients and their families are a large and relatively untapped health care resource,” they wrote.
The TIM-HF 2 trial was funded by the German Federal Ministry of Education and Research. Dr. Koehler reported receiving speaking and/or consultant fees from Novartis, Abbott, and Medtronic.
bjancin@mdedge.com
SOURCE: Koehler F et al. ESC Congress 2018. Lancet. 2018 Sep 22;392(10152):1047-57.
MUNICH – A comprehensive home telemonitoring program paid off big for selected patients with heart failure in a large, German nationwide masked randomization trial.
First, TIM-HF2 didn’t rely on passive monitoring of the patients’ daily electronically submitted home data. Instead, the data went straight to a central telemonitoring center staffed 24/7 by physicians and nurses with heart failure expertise. There, the information was immediately analyzed using proprietary telemedical analytic software known as the Fontane system. The software employs individually tailored, self-adapting algorithms in order to alert staff when trouble is brewing.
But the telemonitoring intervention doesn’t merely detect early clinical deterioration. It’s also a vehicle for ongoing patient education, outpatient adjustment of drugs, management of major comorbid conditions, and hospital admissions as needed. The patient’s local primary care physician was also plugged into the remote monitoring system and kept abreast of the patient’s condition.
Second, TIM-HF2 focused on a carefully selected subgroup of heart failure patients whom prior studies suggested were particularly likely to benefit from home telemedical management. All participants were NYHA class II or III with a left ventricular ejection fraction of 45% or less, a hospitalization for heart failure within 12 months prior to randomization, and free of moderate or severe depression as evidenced by a baseline Patient Health Questionnaire-9 score of 9 or less, explained Dr. Koehler, head of the center for cardiovascular telemedicine at Charite University in Berlin.
Why exclude patients with depression?
“In this concept, with wholistic remote patient management, we need an active patient who is able to measure every day, who is able to communicate with the telemedical center, and who is able to communicate in this network created between the telemedical center and local caregivers. If someone is really depressed, unable to act, lying in bed saying it makes no sense to take drugs or do anything, then we cannot help. That is for us, I think, the most important thing. We’ve seen it now in two trials,” according to the cardiologist.
The all-cause mortality rate was 7.86 per 100 person-years in the home-telemonitoring group versus 11.34 in usual-care controls. Patients in the active intervention arm lost a mean of 17.8 days per year because of unplanned cardiovascular hospital admissions, compared with 24.2 days per year in controls.
Importantly, outcomes were equally good in the remote patient-management group regardless of whether patients were among the 40% of participants living in urban Germany or the 60% in rural areas. Thus, the telemonitoring intervention eliminated the geographic disparity in health care outcomes which is a prominent issue in Germany, as well as the United States.
A formal cost-benefit analysis of the TIM-HF2 results is in the works, Dr. Koehler said.
Simultaneous with his presentation in Munich, the TIM-HF2 study was published online in the Lancet.
In an accompanying editorial, two prominent heart failure experts – John F.G. Cleland, MD, of the University of Glasgow and Robin A. Clark, MD, of Flinders University in Adelaide – hailed TIM-HF2 as a major advance and indicated in sharp terms that it’s time for guideline writers to sit up and take notice.
“Despite much clinical skepticism and feeble support from most guidelines, in our view the growing weight of evidence suggests that home telemonitoring does reduce mortality for patients with heart failure, and this effect might be substantial. These and other trials also show that the emphasis placed on hospital admission for heart failure might be misplaced, at least from a patient’s perspective, because the proportion of days lost due to hospital admission is small, compared with those lost to death,” the physicians wrote in the editorial.
They also noted that, even though the between-group difference in the number of days during which patients were hospitalized for cardiovascular causes was relatively small, it’s clear that home telemonitoring triggered some potentially life-saving hospitalizations. “Home telemonitoring puts the patient back in the center of health care, ensuring that they know what the health professional is trying to achieve and that they agree with those aims. Ultimately, patients and their families are a large and relatively untapped health care resource,” they wrote.
The TIM-HF 2 trial was funded by the German Federal Ministry of Education and Research. Dr. Koehler reported receiving speaking and/or consultant fees from Novartis, Abbott, and Medtronic.
bjancin@mdedge.com
SOURCE: Koehler F et al. ESC Congress 2018. Lancet. 2018 Sep 22;392(10152):1047-57.
MUNICH – A comprehensive home telemonitoring program paid off big for selected patients with heart failure in a large, German nationwide masked randomization trial.
First, TIM-HF2 didn’t rely on passive monitoring of the patients’ daily electronically submitted home data. Instead, the data went straight to a central telemonitoring center staffed 24/7 by physicians and nurses with heart failure expertise. There, the information was immediately analyzed using proprietary telemedical analytic software known as the Fontane system. The software employs individually tailored, self-adapting algorithms in order to alert staff when trouble is brewing.
But the telemonitoring intervention doesn’t merely detect early clinical deterioration. It’s also a vehicle for ongoing patient education, outpatient adjustment of drugs, management of major comorbid conditions, and hospital admissions as needed. The patient’s local primary care physician was also plugged into the remote monitoring system and kept abreast of the patient’s condition.
Second, TIM-HF2 focused on a carefully selected subgroup of heart failure patients whom prior studies suggested were particularly likely to benefit from home telemedical management. All participants were NYHA class II or III with a left ventricular ejection fraction of 45% or less, a hospitalization for heart failure within 12 months prior to randomization, and free of moderate or severe depression as evidenced by a baseline Patient Health Questionnaire-9 score of 9 or less, explained Dr. Koehler, head of the center for cardiovascular telemedicine at Charite University in Berlin.
Why exclude patients with depression?
“In this concept, with wholistic remote patient management, we need an active patient who is able to measure every day, who is able to communicate with the telemedical center, and who is able to communicate in this network created between the telemedical center and local caregivers. If someone is really depressed, unable to act, lying in bed saying it makes no sense to take drugs or do anything, then we cannot help. That is for us, I think, the most important thing. We’ve seen it now in two trials,” according to the cardiologist.
The all-cause mortality rate was 7.86 per 100 person-years in the home-telemonitoring group versus 11.34 in usual-care controls. Patients in the active intervention arm lost a mean of 17.8 days per year because of unplanned cardiovascular hospital admissions, compared with 24.2 days per year in controls.
Importantly, outcomes were equally good in the remote patient-management group regardless of whether patients were among the 40% of participants living in urban Germany or the 60% in rural areas. Thus, the telemonitoring intervention eliminated the geographic disparity in health care outcomes which is a prominent issue in Germany, as well as the United States.
A formal cost-benefit analysis of the TIM-HF2 results is in the works, Dr. Koehler said.
Simultaneous with his presentation in Munich, the TIM-HF2 study was published online in the Lancet.
In an accompanying editorial, two prominent heart failure experts – John F.G. Cleland, MD, of the University of Glasgow and Robin A. Clark, MD, of Flinders University in Adelaide – hailed TIM-HF2 as a major advance and indicated in sharp terms that it’s time for guideline writers to sit up and take notice.
“Despite much clinical skepticism and feeble support from most guidelines, in our view the growing weight of evidence suggests that home telemonitoring does reduce mortality for patients with heart failure, and this effect might be substantial. These and other trials also show that the emphasis placed on hospital admission for heart failure might be misplaced, at least from a patient’s perspective, because the proportion of days lost due to hospital admission is small, compared with those lost to death,” the physicians wrote in the editorial.
They also noted that, even though the between-group difference in the number of days during which patients were hospitalized for cardiovascular causes was relatively small, it’s clear that home telemonitoring triggered some potentially life-saving hospitalizations. “Home telemonitoring puts the patient back in the center of health care, ensuring that they know what the health professional is trying to achieve and that they agree with those aims. Ultimately, patients and their families are a large and relatively untapped health care resource,” they wrote.
The TIM-HF 2 trial was funded by the German Federal Ministry of Education and Research. Dr. Koehler reported receiving speaking and/or consultant fees from Novartis, Abbott, and Medtronic.
bjancin@mdedge.com
SOURCE: Koehler F et al. ESC Congress 2018. Lancet. 2018 Sep 22;392(10152):1047-57.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: Comprehensive home-telemonitoring program for heart failure saves lives.
Major finding: All-cause mortality was reduced by 30% at 1 year with a comprehensive home-telemonitoring program.
Study details: This prospective, masked randomization study included 1,538 German heart failure patients.
Disclosures: The TIM-HF2 trial was funded by the German Federal Ministry of Education and Research.
Source: Koehler F. ESC Congress 2018. Lancet. 2018 Sep 22;392(10152):1047-57.
Rivaroxaban bonus: Early unmasking of occult GI cancers
MUNICH – The increased risk of major GI bleeding documented with dual-antiplatelet therapy using rivaroxaban and aspirin when compared with aspirin alone for vascular protection in the previously reported massive COMPASS trial may turn out to be a blessing in disguise.
“Among COMPASS patients with vascular disease receiving long-term antithrombotic therapy, more than 1 in 5 new diagnoses of cancer are preceded by bleeding. GI and GU bleeding are powerful and relatively specific predictors of new GI and GU cancer diagnoses, respectively, and more than 75% of these cancers diagnosed after bleeding are diagnosed within 6 months of the bleed,” John W. Eikelboom, MD, reported at the annual congress of the European Society of Cardiology.
These findings strongly suggest that rivaroxaban (Xarelto), a direct-acting oral anticoagulant (DOAC), may be unmasking occult GI and GU cancers earlier than the malignancies would otherwise have declared themselves.
“Although overall cancer rates were similar in the three treatment groups [rivaroxaban at 2.5 mg twice daily plus aspirin at 100 mg/day, rivaroxaban monotherapy at 5 mg twice daily, or aspirin alone at 100 mg/day], the early increase in GI bleeding with rivaroxaban-based therapy resulted in earlier diagnosis of GI cancer in these patients. By reducing major cardiovascular events and mortality, the combination of rivaroxaban and aspirin already produces a clear net benefit, and by unmasking GI cancers at an earlier stage, the combination could potentially lead to the added benefit of improved GI cancer outcomes,” commented Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont., and lead investigator of the previously published COMPASS trial (N Engl J Med. 2017 Oct 5;377[14]:1319-30).
This possibility that unmasking of occult GI cancer might result in improved survival deserves to be a high research priority in light of the enormous death toll caused by colorectal cancer. Planned longer-term follow-up of the COMPASS participants should be helpful in this regard, he added.
This new COMPASS finding effectively makes a silk purse out of a sow’s ear. When the primary outcomes of COMPASS were announced, many physicians reasoned that if the other commercially available DOACs also outperform warfarin but don’t pose a significantly increased threat of serious bleeding events, why not preferentially turn to them rather than rivaroxaban in patients with high HAS-BLED scores? But now rivaroxaban’s increased GI bleeding risk has begun to look like a potentially important advantage.
As previously reported, COMPASS participants on rivaroxaban plus aspirin had a 3.1% major bleeding rate as defined according to modified International Society on Thrombosis and Hemostasis criteria, for a statistically significant 70% increase in risk, compared with the 1.9% rate in patients on aspirin alone. Rivaroxaban monotherapy also was associated with increased bleeding risk. Of note, most of the excess in bleeding involved GI bleeding, and it was front-loaded during the first year of the trial. Also, reassuringly, there was no increased incidence of intracranial or fatal bleeding in patients on rivaroxaban.
A total of 1,082 patients were diagnosed with a new cancer during 23 months of follow-up. Nearly a quarter (23%) of the new GI cancers and 45% of the new GU cancers were diagnosed after bleeding from those sites.
The incidence of GI cancer diagnosed after GI bleeding was 7.8%, whereas patients with no prior GI bleeding had a mere 0.9% rate of newly diagnosed GI cancer during the study period. Thus, roughly 1 out of every 12 cases of GI bleeding was associated with diagnosis of a new GI cancer, and GI bleeding was associated with a 13-fold increased risk of subsequent GI cancer diagnosis. Put another way, the number of cases of GI bleeding occurring in patients on rivaroxaban that needed to be investigated in order to find one new GI cancer was 12.
Similarly, 13% of COMPASS participants who developed GU bleeding were subsequently diagnosed with a new GU cancer, compared with just 0.3% of subjects without GU bleeding. That translates into an 83.4-fold increased risk of GU cancer in patients with GU bleeding.
In contrast, the incidence of non-GI cancer following a GI bleed was 1.5%, while the rate was 1.0% in patients with no prior GI bleeding.
“That relationship was much weaker, with an odds ratio of 1.77, indicating that the relationship between GI bleeding and GI cancer is not only very strong but it’s rather specific,” according to Dr. Eikelboom.
Of GI cancers associated with a prior major GI bleed, 77% were diagnosed within 6 months following the bleed, as were nearly 89% of GU cancers. Another 9%-10% were diagnosed 6-12 months after the bleeding event.
“The implication for clinical practice is certainly that in patients who have GI or GU bleeding while receiving antithrombotic therapy, we should conduct a vigorous search for underlying cancer in the same organ system,” he concluded.
Discussant Lars C. Wallentin, MD, concurred. He called it a wake-up call for cardiologists to broaden their horizons and recognize that their elderly patients with vascular disease also are at substantial competing risk for major noncardiovascular diseases – and that his colleagues may have an important role to play in earlier cancer diagnoses.
He and his coinvestigators in the RE-LY (Randomized Evaluation of Long-Term Anticoagulant Therapy) study made a similar point in their investigation of 18,113 patients with atrial fibrillation on oral anticoagulation for stroke protection. In that randomized trial of dabigatran (Pradaxa) versus warfarin, roughly 1 in 12 major GI bleeding events was found to be related to an occult colorectal or gastric cancer (Clin Gastroenterol Hepatol. 2017 May;15[5]:682-90).
“The data are very consistent. The message to cardiologists is that no bleeding should be disregarded in patients on oral anticoagulation,” declared Dr. Wallentin, professor of cardiology at Uppsala (Sweden) University.
COMPASS was sponsored by Bayer. Dr. Eikelboom reported receiving research grants from that company and more than half a dozen others.
MUNICH – The increased risk of major GI bleeding documented with dual-antiplatelet therapy using rivaroxaban and aspirin when compared with aspirin alone for vascular protection in the previously reported massive COMPASS trial may turn out to be a blessing in disguise.
“Among COMPASS patients with vascular disease receiving long-term antithrombotic therapy, more than 1 in 5 new diagnoses of cancer are preceded by bleeding. GI and GU bleeding are powerful and relatively specific predictors of new GI and GU cancer diagnoses, respectively, and more than 75% of these cancers diagnosed after bleeding are diagnosed within 6 months of the bleed,” John W. Eikelboom, MD, reported at the annual congress of the European Society of Cardiology.
These findings strongly suggest that rivaroxaban (Xarelto), a direct-acting oral anticoagulant (DOAC), may be unmasking occult GI and GU cancers earlier than the malignancies would otherwise have declared themselves.
“Although overall cancer rates were similar in the three treatment groups [rivaroxaban at 2.5 mg twice daily plus aspirin at 100 mg/day, rivaroxaban monotherapy at 5 mg twice daily, or aspirin alone at 100 mg/day], the early increase in GI bleeding with rivaroxaban-based therapy resulted in earlier diagnosis of GI cancer in these patients. By reducing major cardiovascular events and mortality, the combination of rivaroxaban and aspirin already produces a clear net benefit, and by unmasking GI cancers at an earlier stage, the combination could potentially lead to the added benefit of improved GI cancer outcomes,” commented Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont., and lead investigator of the previously published COMPASS trial (N Engl J Med. 2017 Oct 5;377[14]:1319-30).
This possibility that unmasking of occult GI cancer might result in improved survival deserves to be a high research priority in light of the enormous death toll caused by colorectal cancer. Planned longer-term follow-up of the COMPASS participants should be helpful in this regard, he added.
This new COMPASS finding effectively makes a silk purse out of a sow’s ear. When the primary outcomes of COMPASS were announced, many physicians reasoned that if the other commercially available DOACs also outperform warfarin but don’t pose a significantly increased threat of serious bleeding events, why not preferentially turn to them rather than rivaroxaban in patients with high HAS-BLED scores? But now rivaroxaban’s increased GI bleeding risk has begun to look like a potentially important advantage.
As previously reported, COMPASS participants on rivaroxaban plus aspirin had a 3.1% major bleeding rate as defined according to modified International Society on Thrombosis and Hemostasis criteria, for a statistically significant 70% increase in risk, compared with the 1.9% rate in patients on aspirin alone. Rivaroxaban monotherapy also was associated with increased bleeding risk. Of note, most of the excess in bleeding involved GI bleeding, and it was front-loaded during the first year of the trial. Also, reassuringly, there was no increased incidence of intracranial or fatal bleeding in patients on rivaroxaban.
A total of 1,082 patients were diagnosed with a new cancer during 23 months of follow-up. Nearly a quarter (23%) of the new GI cancers and 45% of the new GU cancers were diagnosed after bleeding from those sites.
The incidence of GI cancer diagnosed after GI bleeding was 7.8%, whereas patients with no prior GI bleeding had a mere 0.9% rate of newly diagnosed GI cancer during the study period. Thus, roughly 1 out of every 12 cases of GI bleeding was associated with diagnosis of a new GI cancer, and GI bleeding was associated with a 13-fold increased risk of subsequent GI cancer diagnosis. Put another way, the number of cases of GI bleeding occurring in patients on rivaroxaban that needed to be investigated in order to find one new GI cancer was 12.
Similarly, 13% of COMPASS participants who developed GU bleeding were subsequently diagnosed with a new GU cancer, compared with just 0.3% of subjects without GU bleeding. That translates into an 83.4-fold increased risk of GU cancer in patients with GU bleeding.
In contrast, the incidence of non-GI cancer following a GI bleed was 1.5%, while the rate was 1.0% in patients with no prior GI bleeding.
“That relationship was much weaker, with an odds ratio of 1.77, indicating that the relationship between GI bleeding and GI cancer is not only very strong but it’s rather specific,” according to Dr. Eikelboom.
Of GI cancers associated with a prior major GI bleed, 77% were diagnosed within 6 months following the bleed, as were nearly 89% of GU cancers. Another 9%-10% were diagnosed 6-12 months after the bleeding event.
“The implication for clinical practice is certainly that in patients who have GI or GU bleeding while receiving antithrombotic therapy, we should conduct a vigorous search for underlying cancer in the same organ system,” he concluded.
Discussant Lars C. Wallentin, MD, concurred. He called it a wake-up call for cardiologists to broaden their horizons and recognize that their elderly patients with vascular disease also are at substantial competing risk for major noncardiovascular diseases – and that his colleagues may have an important role to play in earlier cancer diagnoses.
He and his coinvestigators in the RE-LY (Randomized Evaluation of Long-Term Anticoagulant Therapy) study made a similar point in their investigation of 18,113 patients with atrial fibrillation on oral anticoagulation for stroke protection. In that randomized trial of dabigatran (Pradaxa) versus warfarin, roughly 1 in 12 major GI bleeding events was found to be related to an occult colorectal or gastric cancer (Clin Gastroenterol Hepatol. 2017 May;15[5]:682-90).
“The data are very consistent. The message to cardiologists is that no bleeding should be disregarded in patients on oral anticoagulation,” declared Dr. Wallentin, professor of cardiology at Uppsala (Sweden) University.
COMPASS was sponsored by Bayer. Dr. Eikelboom reported receiving research grants from that company and more than half a dozen others.
MUNICH – The increased risk of major GI bleeding documented with dual-antiplatelet therapy using rivaroxaban and aspirin when compared with aspirin alone for vascular protection in the previously reported massive COMPASS trial may turn out to be a blessing in disguise.
“Among COMPASS patients with vascular disease receiving long-term antithrombotic therapy, more than 1 in 5 new diagnoses of cancer are preceded by bleeding. GI and GU bleeding are powerful and relatively specific predictors of new GI and GU cancer diagnoses, respectively, and more than 75% of these cancers diagnosed after bleeding are diagnosed within 6 months of the bleed,” John W. Eikelboom, MD, reported at the annual congress of the European Society of Cardiology.
These findings strongly suggest that rivaroxaban (Xarelto), a direct-acting oral anticoagulant (DOAC), may be unmasking occult GI and GU cancers earlier than the malignancies would otherwise have declared themselves.
“Although overall cancer rates were similar in the three treatment groups [rivaroxaban at 2.5 mg twice daily plus aspirin at 100 mg/day, rivaroxaban monotherapy at 5 mg twice daily, or aspirin alone at 100 mg/day], the early increase in GI bleeding with rivaroxaban-based therapy resulted in earlier diagnosis of GI cancer in these patients. By reducing major cardiovascular events and mortality, the combination of rivaroxaban and aspirin already produces a clear net benefit, and by unmasking GI cancers at an earlier stage, the combination could potentially lead to the added benefit of improved GI cancer outcomes,” commented Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont., and lead investigator of the previously published COMPASS trial (N Engl J Med. 2017 Oct 5;377[14]:1319-30).
This possibility that unmasking of occult GI cancer might result in improved survival deserves to be a high research priority in light of the enormous death toll caused by colorectal cancer. Planned longer-term follow-up of the COMPASS participants should be helpful in this regard, he added.
This new COMPASS finding effectively makes a silk purse out of a sow’s ear. When the primary outcomes of COMPASS were announced, many physicians reasoned that if the other commercially available DOACs also outperform warfarin but don’t pose a significantly increased threat of serious bleeding events, why not preferentially turn to them rather than rivaroxaban in patients with high HAS-BLED scores? But now rivaroxaban’s increased GI bleeding risk has begun to look like a potentially important advantage.
As previously reported, COMPASS participants on rivaroxaban plus aspirin had a 3.1% major bleeding rate as defined according to modified International Society on Thrombosis and Hemostasis criteria, for a statistically significant 70% increase in risk, compared with the 1.9% rate in patients on aspirin alone. Rivaroxaban monotherapy also was associated with increased bleeding risk. Of note, most of the excess in bleeding involved GI bleeding, and it was front-loaded during the first year of the trial. Also, reassuringly, there was no increased incidence of intracranial or fatal bleeding in patients on rivaroxaban.
A total of 1,082 patients were diagnosed with a new cancer during 23 months of follow-up. Nearly a quarter (23%) of the new GI cancers and 45% of the new GU cancers were diagnosed after bleeding from those sites.
The incidence of GI cancer diagnosed after GI bleeding was 7.8%, whereas patients with no prior GI bleeding had a mere 0.9% rate of newly diagnosed GI cancer during the study period. Thus, roughly 1 out of every 12 cases of GI bleeding was associated with diagnosis of a new GI cancer, and GI bleeding was associated with a 13-fold increased risk of subsequent GI cancer diagnosis. Put another way, the number of cases of GI bleeding occurring in patients on rivaroxaban that needed to be investigated in order to find one new GI cancer was 12.
Similarly, 13% of COMPASS participants who developed GU bleeding were subsequently diagnosed with a new GU cancer, compared with just 0.3% of subjects without GU bleeding. That translates into an 83.4-fold increased risk of GU cancer in patients with GU bleeding.
In contrast, the incidence of non-GI cancer following a GI bleed was 1.5%, while the rate was 1.0% in patients with no prior GI bleeding.
“That relationship was much weaker, with an odds ratio of 1.77, indicating that the relationship between GI bleeding and GI cancer is not only very strong but it’s rather specific,” according to Dr. Eikelboom.
Of GI cancers associated with a prior major GI bleed, 77% were diagnosed within 6 months following the bleed, as were nearly 89% of GU cancers. Another 9%-10% were diagnosed 6-12 months after the bleeding event.
“The implication for clinical practice is certainly that in patients who have GI or GU bleeding while receiving antithrombotic therapy, we should conduct a vigorous search for underlying cancer in the same organ system,” he concluded.
Discussant Lars C. Wallentin, MD, concurred. He called it a wake-up call for cardiologists to broaden their horizons and recognize that their elderly patients with vascular disease also are at substantial competing risk for major noncardiovascular diseases – and that his colleagues may have an important role to play in earlier cancer diagnoses.
He and his coinvestigators in the RE-LY (Randomized Evaluation of Long-Term Anticoagulant Therapy) study made a similar point in their investigation of 18,113 patients with atrial fibrillation on oral anticoagulation for stroke protection. In that randomized trial of dabigatran (Pradaxa) versus warfarin, roughly 1 in 12 major GI bleeding events was found to be related to an occult colorectal or gastric cancer (Clin Gastroenterol Hepatol. 2017 May;15[5]:682-90).
“The data are very consistent. The message to cardiologists is that no bleeding should be disregarded in patients on oral anticoagulation,” declared Dr. Wallentin, professor of cardiology at Uppsala (Sweden) University.
COMPASS was sponsored by Bayer. Dr. Eikelboom reported receiving research grants from that company and more than half a dozen others.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point:
Major finding: Among patients on rivaroxaban, 1 in 12 GI bleeding events was associated with an occult GI cancer.
Study details: This was a secondary analysis looking at cancers in COMPASS, a randomized trial of more than 27,000 patients on rivaroxaban and/or aspirin for vascular prevention.
Disclosures: The presenter reported receiving research grants from Bayer, which sponsored the COMPASS trial.
Two red flags spell trouble ahead in spontaneous coronary artery dissection
MUNICH –
Women whose spontaneous coronary artery dissection (SCAD) occurred during the peripartum period were at 2.8-fold increased risk of in-hospital major adverse events in a multivariate analysis, while those with a background connective tissue disorder were at 8.7-fold increased risk for major adverse cardiovascular events within 30 days of hospitalization in the Canadian SCAD (CanSCAD) study, Jacqueline Saw, MD, reported at the annual congress of the European Society of Cardiology.
CanSCAD is an ongoing, rigorous, prospective, multicenter, observational study of 750 patients with SCAD documented on angiography and confirmed in a core lab. To put the study in perspective, the worldwide medical literature published over the last decade contains fewer than 1,300 other cases of this seriously underdiagnosed, poorly understood disorder, noted Dr. Saw, CanSCAD principal investigator and a cardiologist at the University of British Columbia, Vancouver. Because much remains unclear about SCAD, a condition mistakenly considered to be rare in the past, the Canadian study was undertaken to shed light on predisposing and precipitating factors, optimal management, and clinical outcomes. Although Dr. Saw could present only the in-hospital and 30-day outcomes, follow-up will continue at 6, 12, 24, and 36 months.
SCAD is a nontraumatic, noniatrogenic, nonatherosclerotic separation of the coronary artery wall by intramural hematoma, creating a false lumen which compresses the true arterial lumen. This compromises blood flow with resultant myocardial ischemia or infarction. Intimal tear may or may not be present.
CanSCAD underscored that this is predominantly a disease affecting relatively young women: 89% of SCAD participants were female, 55% of whom were postmenopausal. The mean age at presentation was 52 years, and only 9% of subjects were older than age 65. Seventy percent of subjects presented with non–ST-elevation MI, the other 30% with STEMI. The predominant symptom was chest pain in 92% of patients. The average length of hospital stay was 4 days.
In terms of precipitating factors, half of patients cited high or severe emotional stress, with 41% of subjects scoring 20 or higher on the Perceived Stress Scale. About 30% of patients cited unusually intense physical stress, such as lifting more than 50 pounds, as a precipitating factor.
Of note, one-third of patients had no cardiovascular risk factors.
The in-hospital major adverse event rate – a composite of all-cause mortality, stroke, recurrent MI, cardiogenic shock, heart failure, cardiac arrest, repeat or unplanned revascularization, and heart transplantation – was 8.8%. Mortality through 1 month was reassuringly low, at 0.1%. Nonetheless, 4.9% of patients experienced recurrent symptoms necessitating emergency room visits within 30 days post discharge, and 2.5% required hospitalization because of their chest pain.
Patients who presented with SCAD during the peripartum period were more severely affected. Although they accounted for only 4.5% of subjects, their in-hospital major adverse event rate was 20.6%, compared with 8.2% in the others. They had a 17.6% prevalence of a left ventricular ejection fraction below 35%, as did only 3.1% of patients without peripartum SCAD. They were more than twice as likely to have elevated cardiac troponin levels. Moreover, peripartum SCAD was independently associated with a 2.9-fold increased risk of major adverse cardiovascular events at 30 days, a composite of all-cause mortality, stroke, recurrent MI, heart failure, or revascularization.
The other independent predictor of 30-day major adverse cardiovascular events in a multivariate logistic regression analysis was having a connective tissue disorder, present in 3.6% of participants.
Management was conservative, with no percutaneous coronary intervention used in 84% of patients. Outcomes were worse in the subgroup who underwent PCI, but Dr. Saw cautioned against making much of that.
“Keep in mind that the patients who undergo PCI are typically the higher-risk cohort with ongoing ischemia and chest pain, so there will be some bias there,” according to the cardiologist.
Session chair Patrick W. Serruys, MD, of Erasmus University, Rotterdam, the Netherlands, commented, “It seems like there is a critical period of 30 days, more or less, and beyond that time the situation can be considered as settled and a wait-and-see attitude is fine. It looked like patients with peripartum dissection or connective tissue disease should potentially be kept in hospital for at least 15 days, and maybe 30 days, because I see your cumulative adverse event curve plateauing around 15 days.”
“Are you doing that in your practice?” asked Dr. Serruys.
“It’s true that conservatively managed patients should remain in hospital for typically about 4 days. For patients with a high-risk presentation we do advocate staying in hospital for longer periods,” Dr. Saw replied. “It would be great to keep them for 15 days, although typically if their chest pain has settled by 10 days they can be discharged home.”
Audience members were eager to hear her recommendations regarding dual-antiplatelet therapy. She explained that in her practice patients are generally discharged on aspirin and clopidogrel and typically continue the clopidogrel for at least a month.
“When we follow them in the office at 1 month, if their chest pain has settled, we would discontinue DAPT,” Dr. Saw said.
The ongoing CanSCAD study is sponsored by the Canadian Institutes of Health Research, the Stroke Foundation of Canada, the National Institutes of Health, Abbott Vascular, Boston Scientific, AstraZeneca, and Servier. Dr. Saw reported serving as a consultant to Abbott Vascular and Boston Scientific.
MUNICH –
Women whose spontaneous coronary artery dissection (SCAD) occurred during the peripartum period were at 2.8-fold increased risk of in-hospital major adverse events in a multivariate analysis, while those with a background connective tissue disorder were at 8.7-fold increased risk for major adverse cardiovascular events within 30 days of hospitalization in the Canadian SCAD (CanSCAD) study, Jacqueline Saw, MD, reported at the annual congress of the European Society of Cardiology.
CanSCAD is an ongoing, rigorous, prospective, multicenter, observational study of 750 patients with SCAD documented on angiography and confirmed in a core lab. To put the study in perspective, the worldwide medical literature published over the last decade contains fewer than 1,300 other cases of this seriously underdiagnosed, poorly understood disorder, noted Dr. Saw, CanSCAD principal investigator and a cardiologist at the University of British Columbia, Vancouver. Because much remains unclear about SCAD, a condition mistakenly considered to be rare in the past, the Canadian study was undertaken to shed light on predisposing and precipitating factors, optimal management, and clinical outcomes. Although Dr. Saw could present only the in-hospital and 30-day outcomes, follow-up will continue at 6, 12, 24, and 36 months.
SCAD is a nontraumatic, noniatrogenic, nonatherosclerotic separation of the coronary artery wall by intramural hematoma, creating a false lumen which compresses the true arterial lumen. This compromises blood flow with resultant myocardial ischemia or infarction. Intimal tear may or may not be present.
CanSCAD underscored that this is predominantly a disease affecting relatively young women: 89% of SCAD participants were female, 55% of whom were postmenopausal. The mean age at presentation was 52 years, and only 9% of subjects were older than age 65. Seventy percent of subjects presented with non–ST-elevation MI, the other 30% with STEMI. The predominant symptom was chest pain in 92% of patients. The average length of hospital stay was 4 days.
In terms of precipitating factors, half of patients cited high or severe emotional stress, with 41% of subjects scoring 20 or higher on the Perceived Stress Scale. About 30% of patients cited unusually intense physical stress, such as lifting more than 50 pounds, as a precipitating factor.
Of note, one-third of patients had no cardiovascular risk factors.
The in-hospital major adverse event rate – a composite of all-cause mortality, stroke, recurrent MI, cardiogenic shock, heart failure, cardiac arrest, repeat or unplanned revascularization, and heart transplantation – was 8.8%. Mortality through 1 month was reassuringly low, at 0.1%. Nonetheless, 4.9% of patients experienced recurrent symptoms necessitating emergency room visits within 30 days post discharge, and 2.5% required hospitalization because of their chest pain.
Patients who presented with SCAD during the peripartum period were more severely affected. Although they accounted for only 4.5% of subjects, their in-hospital major adverse event rate was 20.6%, compared with 8.2% in the others. They had a 17.6% prevalence of a left ventricular ejection fraction below 35%, as did only 3.1% of patients without peripartum SCAD. They were more than twice as likely to have elevated cardiac troponin levels. Moreover, peripartum SCAD was independently associated with a 2.9-fold increased risk of major adverse cardiovascular events at 30 days, a composite of all-cause mortality, stroke, recurrent MI, heart failure, or revascularization.
The other independent predictor of 30-day major adverse cardiovascular events in a multivariate logistic regression analysis was having a connective tissue disorder, present in 3.6% of participants.
Management was conservative, with no percutaneous coronary intervention used in 84% of patients. Outcomes were worse in the subgroup who underwent PCI, but Dr. Saw cautioned against making much of that.
“Keep in mind that the patients who undergo PCI are typically the higher-risk cohort with ongoing ischemia and chest pain, so there will be some bias there,” according to the cardiologist.
Session chair Patrick W. Serruys, MD, of Erasmus University, Rotterdam, the Netherlands, commented, “It seems like there is a critical period of 30 days, more or less, and beyond that time the situation can be considered as settled and a wait-and-see attitude is fine. It looked like patients with peripartum dissection or connective tissue disease should potentially be kept in hospital for at least 15 days, and maybe 30 days, because I see your cumulative adverse event curve plateauing around 15 days.”
“Are you doing that in your practice?” asked Dr. Serruys.
“It’s true that conservatively managed patients should remain in hospital for typically about 4 days. For patients with a high-risk presentation we do advocate staying in hospital for longer periods,” Dr. Saw replied. “It would be great to keep them for 15 days, although typically if their chest pain has settled by 10 days they can be discharged home.”
Audience members were eager to hear her recommendations regarding dual-antiplatelet therapy. She explained that in her practice patients are generally discharged on aspirin and clopidogrel and typically continue the clopidogrel for at least a month.
“When we follow them in the office at 1 month, if their chest pain has settled, we would discontinue DAPT,” Dr. Saw said.
The ongoing CanSCAD study is sponsored by the Canadian Institutes of Health Research, the Stroke Foundation of Canada, the National Institutes of Health, Abbott Vascular, Boston Scientific, AstraZeneca, and Servier. Dr. Saw reported serving as a consultant to Abbott Vascular and Boston Scientific.
MUNICH –
Women whose spontaneous coronary artery dissection (SCAD) occurred during the peripartum period were at 2.8-fold increased risk of in-hospital major adverse events in a multivariate analysis, while those with a background connective tissue disorder were at 8.7-fold increased risk for major adverse cardiovascular events within 30 days of hospitalization in the Canadian SCAD (CanSCAD) study, Jacqueline Saw, MD, reported at the annual congress of the European Society of Cardiology.
CanSCAD is an ongoing, rigorous, prospective, multicenter, observational study of 750 patients with SCAD documented on angiography and confirmed in a core lab. To put the study in perspective, the worldwide medical literature published over the last decade contains fewer than 1,300 other cases of this seriously underdiagnosed, poorly understood disorder, noted Dr. Saw, CanSCAD principal investigator and a cardiologist at the University of British Columbia, Vancouver. Because much remains unclear about SCAD, a condition mistakenly considered to be rare in the past, the Canadian study was undertaken to shed light on predisposing and precipitating factors, optimal management, and clinical outcomes. Although Dr. Saw could present only the in-hospital and 30-day outcomes, follow-up will continue at 6, 12, 24, and 36 months.
SCAD is a nontraumatic, noniatrogenic, nonatherosclerotic separation of the coronary artery wall by intramural hematoma, creating a false lumen which compresses the true arterial lumen. This compromises blood flow with resultant myocardial ischemia or infarction. Intimal tear may or may not be present.
CanSCAD underscored that this is predominantly a disease affecting relatively young women: 89% of SCAD participants were female, 55% of whom were postmenopausal. The mean age at presentation was 52 years, and only 9% of subjects were older than age 65. Seventy percent of subjects presented with non–ST-elevation MI, the other 30% with STEMI. The predominant symptom was chest pain in 92% of patients. The average length of hospital stay was 4 days.
In terms of precipitating factors, half of patients cited high or severe emotional stress, with 41% of subjects scoring 20 or higher on the Perceived Stress Scale. About 30% of patients cited unusually intense physical stress, such as lifting more than 50 pounds, as a precipitating factor.
Of note, one-third of patients had no cardiovascular risk factors.
The in-hospital major adverse event rate – a composite of all-cause mortality, stroke, recurrent MI, cardiogenic shock, heart failure, cardiac arrest, repeat or unplanned revascularization, and heart transplantation – was 8.8%. Mortality through 1 month was reassuringly low, at 0.1%. Nonetheless, 4.9% of patients experienced recurrent symptoms necessitating emergency room visits within 30 days post discharge, and 2.5% required hospitalization because of their chest pain.
Patients who presented with SCAD during the peripartum period were more severely affected. Although they accounted for only 4.5% of subjects, their in-hospital major adverse event rate was 20.6%, compared with 8.2% in the others. They had a 17.6% prevalence of a left ventricular ejection fraction below 35%, as did only 3.1% of patients without peripartum SCAD. They were more than twice as likely to have elevated cardiac troponin levels. Moreover, peripartum SCAD was independently associated with a 2.9-fold increased risk of major adverse cardiovascular events at 30 days, a composite of all-cause mortality, stroke, recurrent MI, heart failure, or revascularization.
The other independent predictor of 30-day major adverse cardiovascular events in a multivariate logistic regression analysis was having a connective tissue disorder, present in 3.6% of participants.
Management was conservative, with no percutaneous coronary intervention used in 84% of patients. Outcomes were worse in the subgroup who underwent PCI, but Dr. Saw cautioned against making much of that.
“Keep in mind that the patients who undergo PCI are typically the higher-risk cohort with ongoing ischemia and chest pain, so there will be some bias there,” according to the cardiologist.
Session chair Patrick W. Serruys, MD, of Erasmus University, Rotterdam, the Netherlands, commented, “It seems like there is a critical period of 30 days, more or less, and beyond that time the situation can be considered as settled and a wait-and-see attitude is fine. It looked like patients with peripartum dissection or connective tissue disease should potentially be kept in hospital for at least 15 days, and maybe 30 days, because I see your cumulative adverse event curve plateauing around 15 days.”
“Are you doing that in your practice?” asked Dr. Serruys.
“It’s true that conservatively managed patients should remain in hospital for typically about 4 days. For patients with a high-risk presentation we do advocate staying in hospital for longer periods,” Dr. Saw replied. “It would be great to keep them for 15 days, although typically if their chest pain has settled by 10 days they can be discharged home.”
Audience members were eager to hear her recommendations regarding dual-antiplatelet therapy. She explained that in her practice patients are generally discharged on aspirin and clopidogrel and typically continue the clopidogrel for at least a month.
“When we follow them in the office at 1 month, if their chest pain has settled, we would discontinue DAPT,” Dr. Saw said.
The ongoing CanSCAD study is sponsored by the Canadian Institutes of Health Research, the Stroke Foundation of Canada, the National Institutes of Health, Abbott Vascular, Boston Scientific, AstraZeneca, and Servier. Dr. Saw reported serving as a consultant to Abbott Vascular and Boston Scientific.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: SCAD occurring during the peripartum period or in patients with connective tissue disease identifies subgroups at high risk for major adverse cardiovascular events within 30 days.
Major finding: Patients with spontaneous coronary artery dissection and comorbid connective tissue disease were at 8.7-fold increased risk of major adverse cardiovascular events within 30 days.
Study details: CanSCAD is an ongoing, prospective, multicenter, observational study in 750 patients with confirmed spontaneous coronary artery dissection.
Disclosures: CanSCAD is sponsored by the Canadian Institutes of Health Research, the Stroke Foundation of Canada, the National Institutes of Health, Abbott Vascular, Boston Scientific, AstraZeneca, and Servier.



























