User login
Hydroquinone, found in skin-lightening agents worldwide, linked with increased skin cancer risk
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
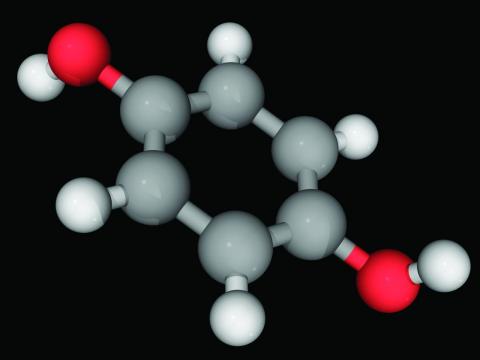
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Study suggests psoriasis and PsA are underdiagnosed in underserved groups
, a study based on national registry data suggests.
“Using the All of Us dataset, we identified lower rates of psoriasis and psoriatic arthritis in participants with skin of color, lower education levels, and no health insurance,” lead author Megan M. Tran said in her oral presentation at the annual meeting of the Society for Investigative Dermatology.
“This suggests psoriasis and psoriatic arthritis underdiagnosis in these underserved populations, possibly due to limited dermatologic care access,” added Ms. Tran, a second-year medical student at Brown University in Providence, R.I.
Ms. Tran and colleagues used the ongoing National Institutes of Health All of Us Research Program registry that contains a large proportion of participants from groups in the United States who have historically been underrepresented in biomedical research, she said in her talk.
Of the 329,038 participants with data in version 5 (released this past March) of the All of Us database, 150,158 (45.6%) had skin of color, and 251,597 (76.5%) had available electronic health records (EHRs).
Underserved groups need better access to health care
Linking data from EHRs, surveys, and physical measurements at enrollment, the researchers used several variables to estimate psoriasis and psoriatic arthritis (PsA) prevalence, and they used multivariate logistic regression to adjust for the variables. They found:
- Twenty-two percent of patients with psoriasis had PsA. Odds of psoriasis and PsA were lower among Black (psoriasis odds ratio [OR], 0.32, 95% confidence interval [CI], 0.28-0.36; PsA OR, 0.20, 95% CI, 0.15-0.26) and Hispanic participants (psoriasis OR, 0.77, 95% CI, 0.71-0.84; PsA OR, 0.74, 95% CI, 0.61-0.89) compared with White participants.
- Psoriasis prevalence increased linearly with age (topping off at age 70 and older [OR, 3.35, 95% CI, 2.91-3.88], with 18-29 years as the reference). The same trend was found with PsA (70 years and above [OR, 4.41, 95% CI, 3.07-6.55] compared with those aged 18-29 years).
- Psoriasis prevalence increased linearly with body mass index (BMI 40 and above [OR, 1.71, 95% CI, 1.54-1.90], with 20-24.9 as the reference). The same trend was found with PsA (BMI 40 and above [OR, 2.09, 95% CI, 1.68-2.59], with 20-24.9 as the reference).
- Former smokers were at increased risk for disease, compared with people who had never smoked (psoriasis OR, 1.30, 95% CI, 1.22-1.39; PsA OR, 2.15, 95% CI, 1.33-3.78).
- Lower odds were found in uninsured adults (psoriasis OR, 0.43, 95% CI, 0.35-0.52; PsA OR, 0.37, 95% CI, 0.22-0.58) compared with those who were insured, and in those with less than a high school degree (psoriasis OR, 0.72, 95% CI, 0.63-0.82; PsA OR, 0.65, 95% CI, 0.47-0.87) compared with those with a college degree.
“The All of Us research program has demonstrated to be a valuable resource to gain unique dermatologic insights on diverse participant populations,” Ms. Tran said.
“There needs to be improvement in access to quality dermatologic care, as this may help to reduce underdiagnosis of psoriasis and psoriatic arthritis,” she added. Access can be increased in various ways, including “outreach to underserved communities, equitable distribution of resources, and increased awareness of clinical variations in skin of color.”
Laura Korb Ferris, MD, PhD, professor of dermatology and director of clinical trials for the department of dermatology at University of Pittsburgh Medical Center, said the study is interesting.
“Because All of Us uses electronic health records to identify cases, while these findings could suggest that these patients are less likely to develop psoriasis and psoriatic arthritis, it more likely shows that they are less likely to receive care for these conditions,” she told this news organization.
“This is concerning, as psoriasis is associated with other comorbidities such as cardiovascular disease and depression, and psoriatic arthritis if left untreated can cause irreversible joint damage that limits function,” she explained in an email. “Both conditions profoundly impact a patient’s quality of life.
“It is important to know whether the diagnoses are simply being missed in these patients or are being neglected,” noted Dr. Ferris, who was not involved in the study and was asked to comment on the results. “It is also important to find strategies to improve diagnosis and treatment, improve quality of life, and allow for interventions to improve long-term sequelae of these diseases and their comorbid conditions.”
The NIH All of Us Research Program, which aims to build a diverse database from at least 1 million adult participants in the United States as a part of the agency’s precision medicine initiative, is open to researchers and to the public. Researchers can access All of Us data and tools to conduct studies at the All of Us Research Hub, and adults who live in the United States can contribute their health data at the All of Us Research Program website and at participating health care provider organizations.
Ms. Tran, study coauthors, and Dr. Ferris reported no relevant relationships. The All of Us Research Program is supported by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
, a study based on national registry data suggests.
“Using the All of Us dataset, we identified lower rates of psoriasis and psoriatic arthritis in participants with skin of color, lower education levels, and no health insurance,” lead author Megan M. Tran said in her oral presentation at the annual meeting of the Society for Investigative Dermatology.
“This suggests psoriasis and psoriatic arthritis underdiagnosis in these underserved populations, possibly due to limited dermatologic care access,” added Ms. Tran, a second-year medical student at Brown University in Providence, R.I.
Ms. Tran and colleagues used the ongoing National Institutes of Health All of Us Research Program registry that contains a large proportion of participants from groups in the United States who have historically been underrepresented in biomedical research, she said in her talk.
Of the 329,038 participants with data in version 5 (released this past March) of the All of Us database, 150,158 (45.6%) had skin of color, and 251,597 (76.5%) had available electronic health records (EHRs).
Underserved groups need better access to health care
Linking data from EHRs, surveys, and physical measurements at enrollment, the researchers used several variables to estimate psoriasis and psoriatic arthritis (PsA) prevalence, and they used multivariate logistic regression to adjust for the variables. They found:
- Twenty-two percent of patients with psoriasis had PsA. Odds of psoriasis and PsA were lower among Black (psoriasis odds ratio [OR], 0.32, 95% confidence interval [CI], 0.28-0.36; PsA OR, 0.20, 95% CI, 0.15-0.26) and Hispanic participants (psoriasis OR, 0.77, 95% CI, 0.71-0.84; PsA OR, 0.74, 95% CI, 0.61-0.89) compared with White participants.
- Psoriasis prevalence increased linearly with age (topping off at age 70 and older [OR, 3.35, 95% CI, 2.91-3.88], with 18-29 years as the reference). The same trend was found with PsA (70 years and above [OR, 4.41, 95% CI, 3.07-6.55] compared with those aged 18-29 years).
- Psoriasis prevalence increased linearly with body mass index (BMI 40 and above [OR, 1.71, 95% CI, 1.54-1.90], with 20-24.9 as the reference). The same trend was found with PsA (BMI 40 and above [OR, 2.09, 95% CI, 1.68-2.59], with 20-24.9 as the reference).
- Former smokers were at increased risk for disease, compared with people who had never smoked (psoriasis OR, 1.30, 95% CI, 1.22-1.39; PsA OR, 2.15, 95% CI, 1.33-3.78).
- Lower odds were found in uninsured adults (psoriasis OR, 0.43, 95% CI, 0.35-0.52; PsA OR, 0.37, 95% CI, 0.22-0.58) compared with those who were insured, and in those with less than a high school degree (psoriasis OR, 0.72, 95% CI, 0.63-0.82; PsA OR, 0.65, 95% CI, 0.47-0.87) compared with those with a college degree.
“The All of Us research program has demonstrated to be a valuable resource to gain unique dermatologic insights on diverse participant populations,” Ms. Tran said.
“There needs to be improvement in access to quality dermatologic care, as this may help to reduce underdiagnosis of psoriasis and psoriatic arthritis,” she added. Access can be increased in various ways, including “outreach to underserved communities, equitable distribution of resources, and increased awareness of clinical variations in skin of color.”
Laura Korb Ferris, MD, PhD, professor of dermatology and director of clinical trials for the department of dermatology at University of Pittsburgh Medical Center, said the study is interesting.
“Because All of Us uses electronic health records to identify cases, while these findings could suggest that these patients are less likely to develop psoriasis and psoriatic arthritis, it more likely shows that they are less likely to receive care for these conditions,” she told this news organization.
“This is concerning, as psoriasis is associated with other comorbidities such as cardiovascular disease and depression, and psoriatic arthritis if left untreated can cause irreversible joint damage that limits function,” she explained in an email. “Both conditions profoundly impact a patient’s quality of life.
“It is important to know whether the diagnoses are simply being missed in these patients or are being neglected,” noted Dr. Ferris, who was not involved in the study and was asked to comment on the results. “It is also important to find strategies to improve diagnosis and treatment, improve quality of life, and allow for interventions to improve long-term sequelae of these diseases and their comorbid conditions.”
The NIH All of Us Research Program, which aims to build a diverse database from at least 1 million adult participants in the United States as a part of the agency’s precision medicine initiative, is open to researchers and to the public. Researchers can access All of Us data and tools to conduct studies at the All of Us Research Hub, and adults who live in the United States can contribute their health data at the All of Us Research Program website and at participating health care provider organizations.
Ms. Tran, study coauthors, and Dr. Ferris reported no relevant relationships. The All of Us Research Program is supported by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
, a study based on national registry data suggests.
“Using the All of Us dataset, we identified lower rates of psoriasis and psoriatic arthritis in participants with skin of color, lower education levels, and no health insurance,” lead author Megan M. Tran said in her oral presentation at the annual meeting of the Society for Investigative Dermatology.
“This suggests psoriasis and psoriatic arthritis underdiagnosis in these underserved populations, possibly due to limited dermatologic care access,” added Ms. Tran, a second-year medical student at Brown University in Providence, R.I.
Ms. Tran and colleagues used the ongoing National Institutes of Health All of Us Research Program registry that contains a large proportion of participants from groups in the United States who have historically been underrepresented in biomedical research, she said in her talk.
Of the 329,038 participants with data in version 5 (released this past March) of the All of Us database, 150,158 (45.6%) had skin of color, and 251,597 (76.5%) had available electronic health records (EHRs).
Underserved groups need better access to health care
Linking data from EHRs, surveys, and physical measurements at enrollment, the researchers used several variables to estimate psoriasis and psoriatic arthritis (PsA) prevalence, and they used multivariate logistic regression to adjust for the variables. They found:
- Twenty-two percent of patients with psoriasis had PsA. Odds of psoriasis and PsA were lower among Black (psoriasis odds ratio [OR], 0.32, 95% confidence interval [CI], 0.28-0.36; PsA OR, 0.20, 95% CI, 0.15-0.26) and Hispanic participants (psoriasis OR, 0.77, 95% CI, 0.71-0.84; PsA OR, 0.74, 95% CI, 0.61-0.89) compared with White participants.
- Psoriasis prevalence increased linearly with age (topping off at age 70 and older [OR, 3.35, 95% CI, 2.91-3.88], with 18-29 years as the reference). The same trend was found with PsA (70 years and above [OR, 4.41, 95% CI, 3.07-6.55] compared with those aged 18-29 years).
- Psoriasis prevalence increased linearly with body mass index (BMI 40 and above [OR, 1.71, 95% CI, 1.54-1.90], with 20-24.9 as the reference). The same trend was found with PsA (BMI 40 and above [OR, 2.09, 95% CI, 1.68-2.59], with 20-24.9 as the reference).
- Former smokers were at increased risk for disease, compared with people who had never smoked (psoriasis OR, 1.30, 95% CI, 1.22-1.39; PsA OR, 2.15, 95% CI, 1.33-3.78).
- Lower odds were found in uninsured adults (psoriasis OR, 0.43, 95% CI, 0.35-0.52; PsA OR, 0.37, 95% CI, 0.22-0.58) compared with those who were insured, and in those with less than a high school degree (psoriasis OR, 0.72, 95% CI, 0.63-0.82; PsA OR, 0.65, 95% CI, 0.47-0.87) compared with those with a college degree.
“The All of Us research program has demonstrated to be a valuable resource to gain unique dermatologic insights on diverse participant populations,” Ms. Tran said.
“There needs to be improvement in access to quality dermatologic care, as this may help to reduce underdiagnosis of psoriasis and psoriatic arthritis,” she added. Access can be increased in various ways, including “outreach to underserved communities, equitable distribution of resources, and increased awareness of clinical variations in skin of color.”
Laura Korb Ferris, MD, PhD, professor of dermatology and director of clinical trials for the department of dermatology at University of Pittsburgh Medical Center, said the study is interesting.
“Because All of Us uses electronic health records to identify cases, while these findings could suggest that these patients are less likely to develop psoriasis and psoriatic arthritis, it more likely shows that they are less likely to receive care for these conditions,” she told this news organization.
“This is concerning, as psoriasis is associated with other comorbidities such as cardiovascular disease and depression, and psoriatic arthritis if left untreated can cause irreversible joint damage that limits function,” she explained in an email. “Both conditions profoundly impact a patient’s quality of life.
“It is important to know whether the diagnoses are simply being missed in these patients or are being neglected,” noted Dr. Ferris, who was not involved in the study and was asked to comment on the results. “It is also important to find strategies to improve diagnosis and treatment, improve quality of life, and allow for interventions to improve long-term sequelae of these diseases and their comorbid conditions.”
The NIH All of Us Research Program, which aims to build a diverse database from at least 1 million adult participants in the United States as a part of the agency’s precision medicine initiative, is open to researchers and to the public. Researchers can access All of Us data and tools to conduct studies at the All of Us Research Hub, and adults who live in the United States can contribute their health data at the All of Us Research Program website and at participating health care provider organizations.
Ms. Tran, study coauthors, and Dr. Ferris reported no relevant relationships. The All of Us Research Program is supported by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Low-level light therapy cap shows subtle effects on CCCA
though the treatment effects from a small prospective trial appear to be subtle.
Central centrifugal cicatricial alopecia (CCCA) is a form of scarring hair loss with unknown etiology and no known cure that affects mainly women of African descent.
“The low-level light therapy (LLLT) cap does indeed seem to help with symptoms and mild regrowth in CCCA,” senior study author Amy J. McMichael, MD, told this news organization. “The dual-wavelength cap we used appears to have anti-inflammatory properties, and that makes sense for a primarily inflammatory scarring from of alopecia.
“Quality of life improved with the treatment and there were no reported side effects,” added Dr. McMichael, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
The REVIAN RED cap (REVIAN Inc.) used in the study contains 119 light-emitting diodes (LEDs) arrayed on the cap’s interior surface that emit orange (620 nm) and red (660 nm) light.
The hypothesis for how the dual-wavelength lights work is that light is absorbed by the chromophore cytochrome c oxidase in the mitochondrial membrane. This induces the release of nitric oxide and the production of adenosine triphosphate (ATP), which leads to vasodilation, cytokine regulation, and increased transcription and release of growth factors.
LLLT is approved to treat androgenetic alopecia, the authors wrote, but has not been studied as a treatment for CCCA.
To assess the effects of LLLT on CCCA, Dr. McMichael and her colleagues at Wake Forest followed the condition’s progress in five Black women over their 6-month course of treatment. Four participants completed the study.
At baseline, all participants had been on individual stable CCCA treatment regimens for at least 3 months. They continued those treatments along with LLLT therapy throughout the study. The women ranged in age from 38 to 69 years, had had CCCA for an average of 12 years, and their disease severity ranged from stage IIB to IVA.
They were instructed to wear the REVIAN RED cap with the LEDs activated for 10 minutes each day.
At 2, 4, and 6 months, participants self-assessed their symptoms, a clinician evaluated the condition’s severity, and digital photographs were taken.
At 6 months:
- Three patients showed improved Dermatology Life Quality Index (DLQI).
- Three patients showed decreased loss of follicular openings and breakage.
- A dermoscopic image of the scalp of one patient revealed short, regrowing vellus hairs and minimal interfollicular and perifollicular scale.
- No patients reported side effects.
Small study raises big questions
“I hope this study will lead to a larger study that will look at the long-term outcomes of CCCA,” Dr. McMichael said. “This is a nice treatment that does not require application of something to the scalp that may affect hair styling, and it has no systemic side effects.”
Dr. McMichael acknowledges that the small sample size, participants continuing with their individual stable treatments while also undergoing light therapy, and the lack of patients with stage I disease, are weaknesses in the study.
“However, the strength is that none of the patients had side effects or stopped using the treatment due to difficulty with the system,” she added.
Dr. McMichael said she would like to investigate the effects of longer use of the cap and whether the cap can be used to prevent CCCA.
Chesahna Kindred, MD, assistant professor of dermatology at Howard University, Washington, D.C., and founder of Kindred Hair & Skin Center in Columbia, Md., told this news organization that she uses LLLT in her practice.
“I find that LLLT is mildly helpful, or at least does not worsen, androgenetic alopecia,” she said.
“Interestingly, while all four patients had stable disease upon initiating the study, it appears as though two of the four worsened after the use of LLLT, one improved, and one remained relatively stable,” noted Dr. Kindred, who was not involved in the study. “This is important because once there is complete destruction of the follicle, CCCA is difficult to improve.
“Given that there are several options to address inflammation and follicular damage in CCCA, more studies are needed before I would incorporate LLLT into my regular treatment algorithms,” she added.
“Studies like this are important and remind us to not lump all forms of hair loss together,” she said.
REVIAN Inc. provided the caps, but the study received no additional funding. Dr. McMichael and Dr. Kindred report relevant financial relationships with the pharmaceutical industry. Study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
though the treatment effects from a small prospective trial appear to be subtle.
Central centrifugal cicatricial alopecia (CCCA) is a form of scarring hair loss with unknown etiology and no known cure that affects mainly women of African descent.
“The low-level light therapy (LLLT) cap does indeed seem to help with symptoms and mild regrowth in CCCA,” senior study author Amy J. McMichael, MD, told this news organization. “The dual-wavelength cap we used appears to have anti-inflammatory properties, and that makes sense for a primarily inflammatory scarring from of alopecia.
“Quality of life improved with the treatment and there were no reported side effects,” added Dr. McMichael, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
The REVIAN RED cap (REVIAN Inc.) used in the study contains 119 light-emitting diodes (LEDs) arrayed on the cap’s interior surface that emit orange (620 nm) and red (660 nm) light.
The hypothesis for how the dual-wavelength lights work is that light is absorbed by the chromophore cytochrome c oxidase in the mitochondrial membrane. This induces the release of nitric oxide and the production of adenosine triphosphate (ATP), which leads to vasodilation, cytokine regulation, and increased transcription and release of growth factors.
LLLT is approved to treat androgenetic alopecia, the authors wrote, but has not been studied as a treatment for CCCA.
To assess the effects of LLLT on CCCA, Dr. McMichael and her colleagues at Wake Forest followed the condition’s progress in five Black women over their 6-month course of treatment. Four participants completed the study.
At baseline, all participants had been on individual stable CCCA treatment regimens for at least 3 months. They continued those treatments along with LLLT therapy throughout the study. The women ranged in age from 38 to 69 years, had had CCCA for an average of 12 years, and their disease severity ranged from stage IIB to IVA.
They were instructed to wear the REVIAN RED cap with the LEDs activated for 10 minutes each day.
At 2, 4, and 6 months, participants self-assessed their symptoms, a clinician evaluated the condition’s severity, and digital photographs were taken.
At 6 months:
- Three patients showed improved Dermatology Life Quality Index (DLQI).
- Three patients showed decreased loss of follicular openings and breakage.
- A dermoscopic image of the scalp of one patient revealed short, regrowing vellus hairs and minimal interfollicular and perifollicular scale.
- No patients reported side effects.
Small study raises big questions
“I hope this study will lead to a larger study that will look at the long-term outcomes of CCCA,” Dr. McMichael said. “This is a nice treatment that does not require application of something to the scalp that may affect hair styling, and it has no systemic side effects.”
Dr. McMichael acknowledges that the small sample size, participants continuing with their individual stable treatments while also undergoing light therapy, and the lack of patients with stage I disease, are weaknesses in the study.
“However, the strength is that none of the patients had side effects or stopped using the treatment due to difficulty with the system,” she added.
Dr. McMichael said she would like to investigate the effects of longer use of the cap and whether the cap can be used to prevent CCCA.
Chesahna Kindred, MD, assistant professor of dermatology at Howard University, Washington, D.C., and founder of Kindred Hair & Skin Center in Columbia, Md., told this news organization that she uses LLLT in her practice.
“I find that LLLT is mildly helpful, or at least does not worsen, androgenetic alopecia,” she said.
“Interestingly, while all four patients had stable disease upon initiating the study, it appears as though two of the four worsened after the use of LLLT, one improved, and one remained relatively stable,” noted Dr. Kindred, who was not involved in the study. “This is important because once there is complete destruction of the follicle, CCCA is difficult to improve.
“Given that there are several options to address inflammation and follicular damage in CCCA, more studies are needed before I would incorporate LLLT into my regular treatment algorithms,” she added.
“Studies like this are important and remind us to not lump all forms of hair loss together,” she said.
REVIAN Inc. provided the caps, but the study received no additional funding. Dr. McMichael and Dr. Kindred report relevant financial relationships with the pharmaceutical industry. Study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
though the treatment effects from a small prospective trial appear to be subtle.
Central centrifugal cicatricial alopecia (CCCA) is a form of scarring hair loss with unknown etiology and no known cure that affects mainly women of African descent.
“The low-level light therapy (LLLT) cap does indeed seem to help with symptoms and mild regrowth in CCCA,” senior study author Amy J. McMichael, MD, told this news organization. “The dual-wavelength cap we used appears to have anti-inflammatory properties, and that makes sense for a primarily inflammatory scarring from of alopecia.
“Quality of life improved with the treatment and there were no reported side effects,” added Dr. McMichael, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
The REVIAN RED cap (REVIAN Inc.) used in the study contains 119 light-emitting diodes (LEDs) arrayed on the cap’s interior surface that emit orange (620 nm) and red (660 nm) light.
The hypothesis for how the dual-wavelength lights work is that light is absorbed by the chromophore cytochrome c oxidase in the mitochondrial membrane. This induces the release of nitric oxide and the production of adenosine triphosphate (ATP), which leads to vasodilation, cytokine regulation, and increased transcription and release of growth factors.
LLLT is approved to treat androgenetic alopecia, the authors wrote, but has not been studied as a treatment for CCCA.
To assess the effects of LLLT on CCCA, Dr. McMichael and her colleagues at Wake Forest followed the condition’s progress in five Black women over their 6-month course of treatment. Four participants completed the study.
At baseline, all participants had been on individual stable CCCA treatment regimens for at least 3 months. They continued those treatments along with LLLT therapy throughout the study. The women ranged in age from 38 to 69 years, had had CCCA for an average of 12 years, and their disease severity ranged from stage IIB to IVA.
They were instructed to wear the REVIAN RED cap with the LEDs activated for 10 minutes each day.
At 2, 4, and 6 months, participants self-assessed their symptoms, a clinician evaluated the condition’s severity, and digital photographs were taken.
At 6 months:
- Three patients showed improved Dermatology Life Quality Index (DLQI).
- Three patients showed decreased loss of follicular openings and breakage.
- A dermoscopic image of the scalp of one patient revealed short, regrowing vellus hairs and minimal interfollicular and perifollicular scale.
- No patients reported side effects.
Small study raises big questions
“I hope this study will lead to a larger study that will look at the long-term outcomes of CCCA,” Dr. McMichael said. “This is a nice treatment that does not require application of something to the scalp that may affect hair styling, and it has no systemic side effects.”
Dr. McMichael acknowledges that the small sample size, participants continuing with their individual stable treatments while also undergoing light therapy, and the lack of patients with stage I disease, are weaknesses in the study.
“However, the strength is that none of the patients had side effects or stopped using the treatment due to difficulty with the system,” she added.
Dr. McMichael said she would like to investigate the effects of longer use of the cap and whether the cap can be used to prevent CCCA.
Chesahna Kindred, MD, assistant professor of dermatology at Howard University, Washington, D.C., and founder of Kindred Hair & Skin Center in Columbia, Md., told this news organization that she uses LLLT in her practice.
“I find that LLLT is mildly helpful, or at least does not worsen, androgenetic alopecia,” she said.
“Interestingly, while all four patients had stable disease upon initiating the study, it appears as though two of the four worsened after the use of LLLT, one improved, and one remained relatively stable,” noted Dr. Kindred, who was not involved in the study. “This is important because once there is complete destruction of the follicle, CCCA is difficult to improve.
“Given that there are several options to address inflammation and follicular damage in CCCA, more studies are needed before I would incorporate LLLT into my regular treatment algorithms,” she added.
“Studies like this are important and remind us to not lump all forms of hair loss together,” she said.
REVIAN Inc. provided the caps, but the study received no additional funding. Dr. McMichael and Dr. Kindred report relevant financial relationships with the pharmaceutical industry. Study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Vitamin D supplements during pregnancy may protect infants from atopic eczema
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SID 2022