User login
PIANO study provides insight on safety of biologics in pregnancy
MAUI, HAWAII – The consensus among gastroenterologists with expertise in inflammatory bowel disease is that continuation of biologics or immunomodulators in affected women throughout pregnancy and breastfeeding poses no increased risks to the fetus – and therein lies a message for rheumatologists and obstetricians, Dr. Uma Mahadevan said at the 2016 Rheumatology Winter Clinical Symposium.
“The risk of uncontrolled disease must be weighed against the risk of medical therapy. And this is something that is often missed,” according to Dr. Mahadevan, professor of medicine and co–medical director of the Center for Colitis and Crohn’s Disease at the University of California, San Francisco.
Gastroenterologists – at least, those whose practices focus on inflammatory bowel disease (IBD) – have led the way within medicine in terms of establishing the safety of biologics and immunomodulators such as azathioprine in pregnant women with chronic inflammatory diseases and their babies. And having accomplished that, they have been ahead of the curve in terms of continuing such therapy throughout pregnancy and breastfeeding. That’s because active Crohn’s disease and ulcerative colitis are particularly common in women during their childbearing years. And a disease flare during pregnancy is associated with a markedly increased risk of preterm birth and other adverse outcomes.
Gastroenterologists’ longstanding interest in the safety to mother and fetus of continued use of effective, potent medications throughout pregnancy was the impetus for the ongoing prospective U.S. Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes (PIANO) study, now in its ninth year, with an enrollment of roughly 1,500 women with IBD. The study has included comparisons of outcomes of women on different medications during pregnancy versus unmedicated women.
In multiple publications, Dr. Mahadevan and other PIANO investigators have established that increased IBD activity adversely affects pregnancy outcomes, and that stabilization of disease and effective maintenance therapy throughout pregnancy is important. The PIANO group has demonstrated that IBD medication exposure well into the third trimester in patients in sustained remission was not associated with an increase in congenital anomalies, spontaneous abortions, intrauterine growth restriction, or low birth weight.
To the surprise of many gastroenterologists, the PIANO study has shown that women with Crohn’s disease generally have smoother pregnancies than do those with ulcerative colitis, who tend to get sicker and have more complications.
Since PIANO data show an increased rate of preterm birth and low birth weight in IBD patients on combination therapy with azathioprine plus a biologic throughout pregnancy, Dr. Mahadevan and others try to discontinue the azathioprine, even though the need for combination therapy is a marker for patients with more severe disease.
Anti-TNF-alpha use during third trimester
Of particular interest to rheumatologists, who rely heavily on many of the same biologic agents gastroenterologists use to treat IBD, the use of anti–tumor necrosis factor–alpha biologics in the third trimester was not associated with an increase in preterm birth or maternal disease activity in the third trimester or the first 4 months post partum. When women on certolizumab pegol (Cimzia) during the third trimester were excluded from the analysis, since this biologic uniquely does not cross the placenta at all, the most recent PIANO data show a modest yet statistically significant 35% increase in infections in infants at age 12 months whose mothers were on other biologics in the third trimester. But Dr. Mahadevan said she doesn’t yet consider this finding definitive.
“It’s still a small group of patients, and every year when we update the results the infant infection risk goes back and forth from statistically significant to nonsignificant. I think there’s a signal here; we just need to keep collecting more data,” she said.
Particularly reassuring is the finding that the offspring of PIANO participants who had in utero exposure to biologics and immunomodulators didn’t have any developmental delay, compared with unexposed babies, according to the validated Ages and Stages Questionnaire at ages 1, 2, 3, and 4 years and Denver Childhood Developmental Score at months 4, 9, and 12.
“These kids do great later in life. Actually, they have better scores than unexposed kids. Not to say that biologics make your kid smarter. It probably has to do with better IBD control,” Dr. Mahadevan said.
Effects while breastfeeding
Breastfeeding while on biologics or azathioprine didn’t adversely affect infant growth, infection rate, or developmental milestones. More specifically, levels of biologics in the mothers, babies, or cord serum were not associated with the likelihood of a neonatal intensive care unit stay, an increase in infant infections, or achievement of developmental milestones.
“Almost all the agents are detectable in breast milk, but only at the nanogram level. We tell all our patients on biologics they can breastfeed. It doesn’t matter when their last dose was, don’t worry about it,” the gastroenterologist said.
Importance of preconception counseling
Key practical lessons she has learned in taking care of large numbers of patients with severe IBD referred to her tertiary center include the importance of preconception counseling. A woman should stop methotrexate at least 3 months prior to conception. Providing information on medication safety and the risks of poorly controlled disease helps in adherence.
It’s best to communicate with the patient’s obstetrician about the importance of continuing her IBD therapy during pregnancy before she becomes pregnant.
“It’s better to have this discussion ahead of time and have a plan in place. Once a patient is pregnant it’s very difficult if her doula or someone else has told them to stop a medication to convince them to continue it,” said Dr. Mahadevan.
All women with IBD should be followed as high-risk pregnancies. Mode of delivery is at the discretion of the obstetrician unless the patient has an open rectovaginal fistula; even if it’s inactive, cesarean delivery is preferable in that situation, she said.
Steps taken in the third trimester
In the third trimester, she routinely sends a letter to the patient’s pediatrician requesting no live virus vaccines in the coming baby’s first 6 months – in the United States, that’s the rotavirus vaccine – if the infant was exposed in utero to a biologic other than certolizumab pegol, but that all other vaccines can be given on schedule. She also asks that the pediatrician monitor an exposed baby for infections.
“That being said, there have been 20-plus exposures to rotavirus vaccine in the first 6 months of life recorded in the PIANO registry in the infants of mothers on biologics and we haven’t seen any adverse events. So maybe the CDC is overstating the risk, but at this point the rule is still no live virus vaccines,” she said.
She tries to time her last dose of biologic agents during pregnancy as follows: at week 30-32 for infliximab or vedolizumab and week 36-38 for adalimumab or golimumab. As for certolizumab pegol, “they can take that on their way to labor and delivery,” she quipped.
“I give the next dose of a biologic agent soon after delivery, often while the patient is still in the hospital, 24 hours after vaginal delivery and 48 hours after a C-section, assuming no infection,” Dr. Mahadevan said.
The elements of her approach to management of the pregnant patient with IBD are in accord with a recent report, The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy, in which she participated (Gastroenterology. 2016 Mar;150[3]:734-57).
Rheumatologists’ habits with biologics in pregnancy
Dr. John J. Cush rose from the audience to observe that the situation in rheumatology with regard to biologics in pregnancy is quite a bit different from what’s going on in gastroenterology.
“I think a lot of rheumatologists don’t let their patients get pregnant on biologics for fear of what may happen. And that’s because they don’t know the data. I think you’ve shown very clearly that you can get pregnant on biologics and do well. But for many of us who do allow our patients to get pregnant on biologic monotherapy, the practice is to stop it once they get pregnant. The idea is we want them to be in a very deep remission to increase the odds of getting pregnant and having a successful pregnancy, but then we stop the drug and we assume the disease state is going to stay the same. Often, though, it doesn’t stay the same, it gets worse. Yet this is a common practice in rheumatology. What’s your response?” asked Dr. Cush, professor of medicine and rheumatology at Baylor University Medical Center, Dallas, and director of clinical rheumatology at the Baylor Research Institute.
“I think in IBD it’s very clear that patients with active disease in pregnancy do much, much worse,” Dr. Mahadevan replied. “They have preterm birth, they get very sick, they’re hospitalized and placed on steroids. So for us, the benefit is very clear. I don’t know the data in rheumatoid arthritis – whether active disease leads to increased complication rates – but I do know from colleagues that in the postpartum period women with poorly controlled rheumatoid arthritis can’t take care of their baby because their hands are so damaged. And that’s a big deal.
“So when you see that the drugs are not associated with an increased risk of birth defects and on monotherapy there’s no increase in infections and other complications, you’d think that in the right patient continuing treatment until the late third trimester would be the way to go, especially since if you’ve put the patient on a biologic it must mean she has severe disease,” the gastroenterologist observed.
Dr. Roy Fleischmann of the University of Texas, Dallas, asked why gastroenterologists don’t put all IBD patients of childbearing age on certolizumab pegol if they need biologic therapy, since it doesn’t cross the placenta.
“Maybe IBD is different, but our biologics don’t necessarily have the longest persistence,” Dr. Mahadevan replied. “If you start a woman on certolizumab pegol at age 20 the chances of her still being on it at 28 are probably pretty low.”
Conference director Dr. Arthur Kavanaugh, professor of medicine at the University of California, San Diego, asked if the message regarding management of IBD in pregnancy being put forth by Dr. Mahadevan and the IBD in Pregnancy Consensus Group has gained wide acceptance by gastroenterologists across the country.
“I think the people who do IBD as a concentrated practice, whether in private or academic practice, are very aware of this literature,” she said.
“In general, IBD has become more and more centered among a group of people who want to take care of IBD. If you look at the big private practices, they have a hepatitis C person, an IBD person, and everyone else just wants to scope. I think the message is getting across to non-IBD gastroenterologists, but there is some confusion because the Europeans are very firm about stopping biologics at 22 weeks’ gestation if the patient is in deep remission and we in North America continue treatment,” said Dr. Mahadevan.
Her work with the PIANO study is funded by the Crohn’s and Colitis Foundation of America. In addition, Dr. Mahadevan disclosed ties to more than half a dozen pharmaceutical companies.
MAUI, HAWAII – The consensus among gastroenterologists with expertise in inflammatory bowel disease is that continuation of biologics or immunomodulators in affected women throughout pregnancy and breastfeeding poses no increased risks to the fetus – and therein lies a message for rheumatologists and obstetricians, Dr. Uma Mahadevan said at the 2016 Rheumatology Winter Clinical Symposium.
“The risk of uncontrolled disease must be weighed against the risk of medical therapy. And this is something that is often missed,” according to Dr. Mahadevan, professor of medicine and co–medical director of the Center for Colitis and Crohn’s Disease at the University of California, San Francisco.
Gastroenterologists – at least, those whose practices focus on inflammatory bowel disease (IBD) – have led the way within medicine in terms of establishing the safety of biologics and immunomodulators such as azathioprine in pregnant women with chronic inflammatory diseases and their babies. And having accomplished that, they have been ahead of the curve in terms of continuing such therapy throughout pregnancy and breastfeeding. That’s because active Crohn’s disease and ulcerative colitis are particularly common in women during their childbearing years. And a disease flare during pregnancy is associated with a markedly increased risk of preterm birth and other adverse outcomes.
Gastroenterologists’ longstanding interest in the safety to mother and fetus of continued use of effective, potent medications throughout pregnancy was the impetus for the ongoing prospective U.S. Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes (PIANO) study, now in its ninth year, with an enrollment of roughly 1,500 women with IBD. The study has included comparisons of outcomes of women on different medications during pregnancy versus unmedicated women.
In multiple publications, Dr. Mahadevan and other PIANO investigators have established that increased IBD activity adversely affects pregnancy outcomes, and that stabilization of disease and effective maintenance therapy throughout pregnancy is important. The PIANO group has demonstrated that IBD medication exposure well into the third trimester in patients in sustained remission was not associated with an increase in congenital anomalies, spontaneous abortions, intrauterine growth restriction, or low birth weight.
To the surprise of many gastroenterologists, the PIANO study has shown that women with Crohn’s disease generally have smoother pregnancies than do those with ulcerative colitis, who tend to get sicker and have more complications.
Since PIANO data show an increased rate of preterm birth and low birth weight in IBD patients on combination therapy with azathioprine plus a biologic throughout pregnancy, Dr. Mahadevan and others try to discontinue the azathioprine, even though the need for combination therapy is a marker for patients with more severe disease.
Anti-TNF-alpha use during third trimester
Of particular interest to rheumatologists, who rely heavily on many of the same biologic agents gastroenterologists use to treat IBD, the use of anti–tumor necrosis factor–alpha biologics in the third trimester was not associated with an increase in preterm birth or maternal disease activity in the third trimester or the first 4 months post partum. When women on certolizumab pegol (Cimzia) during the third trimester were excluded from the analysis, since this biologic uniquely does not cross the placenta at all, the most recent PIANO data show a modest yet statistically significant 35% increase in infections in infants at age 12 months whose mothers were on other biologics in the third trimester. But Dr. Mahadevan said she doesn’t yet consider this finding definitive.
“It’s still a small group of patients, and every year when we update the results the infant infection risk goes back and forth from statistically significant to nonsignificant. I think there’s a signal here; we just need to keep collecting more data,” she said.
Particularly reassuring is the finding that the offspring of PIANO participants who had in utero exposure to biologics and immunomodulators didn’t have any developmental delay, compared with unexposed babies, according to the validated Ages and Stages Questionnaire at ages 1, 2, 3, and 4 years and Denver Childhood Developmental Score at months 4, 9, and 12.
“These kids do great later in life. Actually, they have better scores than unexposed kids. Not to say that biologics make your kid smarter. It probably has to do with better IBD control,” Dr. Mahadevan said.
Effects while breastfeeding
Breastfeeding while on biologics or azathioprine didn’t adversely affect infant growth, infection rate, or developmental milestones. More specifically, levels of biologics in the mothers, babies, or cord serum were not associated with the likelihood of a neonatal intensive care unit stay, an increase in infant infections, or achievement of developmental milestones.
“Almost all the agents are detectable in breast milk, but only at the nanogram level. We tell all our patients on biologics they can breastfeed. It doesn’t matter when their last dose was, don’t worry about it,” the gastroenterologist said.
Importance of preconception counseling
Key practical lessons she has learned in taking care of large numbers of patients with severe IBD referred to her tertiary center include the importance of preconception counseling. A woman should stop methotrexate at least 3 months prior to conception. Providing information on medication safety and the risks of poorly controlled disease helps in adherence.
It’s best to communicate with the patient’s obstetrician about the importance of continuing her IBD therapy during pregnancy before she becomes pregnant.
“It’s better to have this discussion ahead of time and have a plan in place. Once a patient is pregnant it’s very difficult if her doula or someone else has told them to stop a medication to convince them to continue it,” said Dr. Mahadevan.
All women with IBD should be followed as high-risk pregnancies. Mode of delivery is at the discretion of the obstetrician unless the patient has an open rectovaginal fistula; even if it’s inactive, cesarean delivery is preferable in that situation, she said.
Steps taken in the third trimester
In the third trimester, she routinely sends a letter to the patient’s pediatrician requesting no live virus vaccines in the coming baby’s first 6 months – in the United States, that’s the rotavirus vaccine – if the infant was exposed in utero to a biologic other than certolizumab pegol, but that all other vaccines can be given on schedule. She also asks that the pediatrician monitor an exposed baby for infections.
“That being said, there have been 20-plus exposures to rotavirus vaccine in the first 6 months of life recorded in the PIANO registry in the infants of mothers on biologics and we haven’t seen any adverse events. So maybe the CDC is overstating the risk, but at this point the rule is still no live virus vaccines,” she said.
She tries to time her last dose of biologic agents during pregnancy as follows: at week 30-32 for infliximab or vedolizumab and week 36-38 for adalimumab or golimumab. As for certolizumab pegol, “they can take that on their way to labor and delivery,” she quipped.
“I give the next dose of a biologic agent soon after delivery, often while the patient is still in the hospital, 24 hours after vaginal delivery and 48 hours after a C-section, assuming no infection,” Dr. Mahadevan said.
The elements of her approach to management of the pregnant patient with IBD are in accord with a recent report, The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy, in which she participated (Gastroenterology. 2016 Mar;150[3]:734-57).
Rheumatologists’ habits with biologics in pregnancy
Dr. John J. Cush rose from the audience to observe that the situation in rheumatology with regard to biologics in pregnancy is quite a bit different from what’s going on in gastroenterology.
“I think a lot of rheumatologists don’t let their patients get pregnant on biologics for fear of what may happen. And that’s because they don’t know the data. I think you’ve shown very clearly that you can get pregnant on biologics and do well. But for many of us who do allow our patients to get pregnant on biologic monotherapy, the practice is to stop it once they get pregnant. The idea is we want them to be in a very deep remission to increase the odds of getting pregnant and having a successful pregnancy, but then we stop the drug and we assume the disease state is going to stay the same. Often, though, it doesn’t stay the same, it gets worse. Yet this is a common practice in rheumatology. What’s your response?” asked Dr. Cush, professor of medicine and rheumatology at Baylor University Medical Center, Dallas, and director of clinical rheumatology at the Baylor Research Institute.
“I think in IBD it’s very clear that patients with active disease in pregnancy do much, much worse,” Dr. Mahadevan replied. “They have preterm birth, they get very sick, they’re hospitalized and placed on steroids. So for us, the benefit is very clear. I don’t know the data in rheumatoid arthritis – whether active disease leads to increased complication rates – but I do know from colleagues that in the postpartum period women with poorly controlled rheumatoid arthritis can’t take care of their baby because their hands are so damaged. And that’s a big deal.
“So when you see that the drugs are not associated with an increased risk of birth defects and on monotherapy there’s no increase in infections and other complications, you’d think that in the right patient continuing treatment until the late third trimester would be the way to go, especially since if you’ve put the patient on a biologic it must mean she has severe disease,” the gastroenterologist observed.
Dr. Roy Fleischmann of the University of Texas, Dallas, asked why gastroenterologists don’t put all IBD patients of childbearing age on certolizumab pegol if they need biologic therapy, since it doesn’t cross the placenta.
“Maybe IBD is different, but our biologics don’t necessarily have the longest persistence,” Dr. Mahadevan replied. “If you start a woman on certolizumab pegol at age 20 the chances of her still being on it at 28 are probably pretty low.”
Conference director Dr. Arthur Kavanaugh, professor of medicine at the University of California, San Diego, asked if the message regarding management of IBD in pregnancy being put forth by Dr. Mahadevan and the IBD in Pregnancy Consensus Group has gained wide acceptance by gastroenterologists across the country.
“I think the people who do IBD as a concentrated practice, whether in private or academic practice, are very aware of this literature,” she said.
“In general, IBD has become more and more centered among a group of people who want to take care of IBD. If you look at the big private practices, they have a hepatitis C person, an IBD person, and everyone else just wants to scope. I think the message is getting across to non-IBD gastroenterologists, but there is some confusion because the Europeans are very firm about stopping biologics at 22 weeks’ gestation if the patient is in deep remission and we in North America continue treatment,” said Dr. Mahadevan.
Her work with the PIANO study is funded by the Crohn’s and Colitis Foundation of America. In addition, Dr. Mahadevan disclosed ties to more than half a dozen pharmaceutical companies.
MAUI, HAWAII – The consensus among gastroenterologists with expertise in inflammatory bowel disease is that continuation of biologics or immunomodulators in affected women throughout pregnancy and breastfeeding poses no increased risks to the fetus – and therein lies a message for rheumatologists and obstetricians, Dr. Uma Mahadevan said at the 2016 Rheumatology Winter Clinical Symposium.
“The risk of uncontrolled disease must be weighed against the risk of medical therapy. And this is something that is often missed,” according to Dr. Mahadevan, professor of medicine and co–medical director of the Center for Colitis and Crohn’s Disease at the University of California, San Francisco.
Gastroenterologists – at least, those whose practices focus on inflammatory bowel disease (IBD) – have led the way within medicine in terms of establishing the safety of biologics and immunomodulators such as azathioprine in pregnant women with chronic inflammatory diseases and their babies. And having accomplished that, they have been ahead of the curve in terms of continuing such therapy throughout pregnancy and breastfeeding. That’s because active Crohn’s disease and ulcerative colitis are particularly common in women during their childbearing years. And a disease flare during pregnancy is associated with a markedly increased risk of preterm birth and other adverse outcomes.
Gastroenterologists’ longstanding interest in the safety to mother and fetus of continued use of effective, potent medications throughout pregnancy was the impetus for the ongoing prospective U.S. Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes (PIANO) study, now in its ninth year, with an enrollment of roughly 1,500 women with IBD. The study has included comparisons of outcomes of women on different medications during pregnancy versus unmedicated women.
In multiple publications, Dr. Mahadevan and other PIANO investigators have established that increased IBD activity adversely affects pregnancy outcomes, and that stabilization of disease and effective maintenance therapy throughout pregnancy is important. The PIANO group has demonstrated that IBD medication exposure well into the third trimester in patients in sustained remission was not associated with an increase in congenital anomalies, spontaneous abortions, intrauterine growth restriction, or low birth weight.
To the surprise of many gastroenterologists, the PIANO study has shown that women with Crohn’s disease generally have smoother pregnancies than do those with ulcerative colitis, who tend to get sicker and have more complications.
Since PIANO data show an increased rate of preterm birth and low birth weight in IBD patients on combination therapy with azathioprine plus a biologic throughout pregnancy, Dr. Mahadevan and others try to discontinue the azathioprine, even though the need for combination therapy is a marker for patients with more severe disease.
Anti-TNF-alpha use during third trimester
Of particular interest to rheumatologists, who rely heavily on many of the same biologic agents gastroenterologists use to treat IBD, the use of anti–tumor necrosis factor–alpha biologics in the third trimester was not associated with an increase in preterm birth or maternal disease activity in the third trimester or the first 4 months post partum. When women on certolizumab pegol (Cimzia) during the third trimester were excluded from the analysis, since this biologic uniquely does not cross the placenta at all, the most recent PIANO data show a modest yet statistically significant 35% increase in infections in infants at age 12 months whose mothers were on other biologics in the third trimester. But Dr. Mahadevan said she doesn’t yet consider this finding definitive.
“It’s still a small group of patients, and every year when we update the results the infant infection risk goes back and forth from statistically significant to nonsignificant. I think there’s a signal here; we just need to keep collecting more data,” she said.
Particularly reassuring is the finding that the offspring of PIANO participants who had in utero exposure to biologics and immunomodulators didn’t have any developmental delay, compared with unexposed babies, according to the validated Ages and Stages Questionnaire at ages 1, 2, 3, and 4 years and Denver Childhood Developmental Score at months 4, 9, and 12.
“These kids do great later in life. Actually, they have better scores than unexposed kids. Not to say that biologics make your kid smarter. It probably has to do with better IBD control,” Dr. Mahadevan said.
Effects while breastfeeding
Breastfeeding while on biologics or azathioprine didn’t adversely affect infant growth, infection rate, or developmental milestones. More specifically, levels of biologics in the mothers, babies, or cord serum were not associated with the likelihood of a neonatal intensive care unit stay, an increase in infant infections, or achievement of developmental milestones.
“Almost all the agents are detectable in breast milk, but only at the nanogram level. We tell all our patients on biologics they can breastfeed. It doesn’t matter when their last dose was, don’t worry about it,” the gastroenterologist said.
Importance of preconception counseling
Key practical lessons she has learned in taking care of large numbers of patients with severe IBD referred to her tertiary center include the importance of preconception counseling. A woman should stop methotrexate at least 3 months prior to conception. Providing information on medication safety and the risks of poorly controlled disease helps in adherence.
It’s best to communicate with the patient’s obstetrician about the importance of continuing her IBD therapy during pregnancy before she becomes pregnant.
“It’s better to have this discussion ahead of time and have a plan in place. Once a patient is pregnant it’s very difficult if her doula or someone else has told them to stop a medication to convince them to continue it,” said Dr. Mahadevan.
All women with IBD should be followed as high-risk pregnancies. Mode of delivery is at the discretion of the obstetrician unless the patient has an open rectovaginal fistula; even if it’s inactive, cesarean delivery is preferable in that situation, she said.
Steps taken in the third trimester
In the third trimester, she routinely sends a letter to the patient’s pediatrician requesting no live virus vaccines in the coming baby’s first 6 months – in the United States, that’s the rotavirus vaccine – if the infant was exposed in utero to a biologic other than certolizumab pegol, but that all other vaccines can be given on schedule. She also asks that the pediatrician monitor an exposed baby for infections.
“That being said, there have been 20-plus exposures to rotavirus vaccine in the first 6 months of life recorded in the PIANO registry in the infants of mothers on biologics and we haven’t seen any adverse events. So maybe the CDC is overstating the risk, but at this point the rule is still no live virus vaccines,” she said.
She tries to time her last dose of biologic agents during pregnancy as follows: at week 30-32 for infliximab or vedolizumab and week 36-38 for adalimumab or golimumab. As for certolizumab pegol, “they can take that on their way to labor and delivery,” she quipped.
“I give the next dose of a biologic agent soon after delivery, often while the patient is still in the hospital, 24 hours after vaginal delivery and 48 hours after a C-section, assuming no infection,” Dr. Mahadevan said.
The elements of her approach to management of the pregnant patient with IBD are in accord with a recent report, The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy, in which she participated (Gastroenterology. 2016 Mar;150[3]:734-57).
Rheumatologists’ habits with biologics in pregnancy
Dr. John J. Cush rose from the audience to observe that the situation in rheumatology with regard to biologics in pregnancy is quite a bit different from what’s going on in gastroenterology.
“I think a lot of rheumatologists don’t let their patients get pregnant on biologics for fear of what may happen. And that’s because they don’t know the data. I think you’ve shown very clearly that you can get pregnant on biologics and do well. But for many of us who do allow our patients to get pregnant on biologic monotherapy, the practice is to stop it once they get pregnant. The idea is we want them to be in a very deep remission to increase the odds of getting pregnant and having a successful pregnancy, but then we stop the drug and we assume the disease state is going to stay the same. Often, though, it doesn’t stay the same, it gets worse. Yet this is a common practice in rheumatology. What’s your response?” asked Dr. Cush, professor of medicine and rheumatology at Baylor University Medical Center, Dallas, and director of clinical rheumatology at the Baylor Research Institute.
“I think in IBD it’s very clear that patients with active disease in pregnancy do much, much worse,” Dr. Mahadevan replied. “They have preterm birth, they get very sick, they’re hospitalized and placed on steroids. So for us, the benefit is very clear. I don’t know the data in rheumatoid arthritis – whether active disease leads to increased complication rates – but I do know from colleagues that in the postpartum period women with poorly controlled rheumatoid arthritis can’t take care of their baby because their hands are so damaged. And that’s a big deal.
“So when you see that the drugs are not associated with an increased risk of birth defects and on monotherapy there’s no increase in infections and other complications, you’d think that in the right patient continuing treatment until the late third trimester would be the way to go, especially since if you’ve put the patient on a biologic it must mean she has severe disease,” the gastroenterologist observed.
Dr. Roy Fleischmann of the University of Texas, Dallas, asked why gastroenterologists don’t put all IBD patients of childbearing age on certolizumab pegol if they need biologic therapy, since it doesn’t cross the placenta.
“Maybe IBD is different, but our biologics don’t necessarily have the longest persistence,” Dr. Mahadevan replied. “If you start a woman on certolizumab pegol at age 20 the chances of her still being on it at 28 are probably pretty low.”
Conference director Dr. Arthur Kavanaugh, professor of medicine at the University of California, San Diego, asked if the message regarding management of IBD in pregnancy being put forth by Dr. Mahadevan and the IBD in Pregnancy Consensus Group has gained wide acceptance by gastroenterologists across the country.
“I think the people who do IBD as a concentrated practice, whether in private or academic practice, are very aware of this literature,” she said.
“In general, IBD has become more and more centered among a group of people who want to take care of IBD. If you look at the big private practices, they have a hepatitis C person, an IBD person, and everyone else just wants to scope. I think the message is getting across to non-IBD gastroenterologists, but there is some confusion because the Europeans are very firm about stopping biologics at 22 weeks’ gestation if the patient is in deep remission and we in North America continue treatment,” said Dr. Mahadevan.
Her work with the PIANO study is funded by the Crohn’s and Colitis Foundation of America. In addition, Dr. Mahadevan disclosed ties to more than half a dozen pharmaceutical companies.
EXPERT ANALYSIS FROM RWCS 2016
A dermatologist’s skin tips for rheumatologists
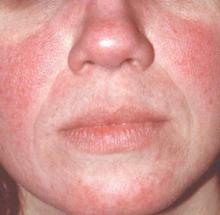
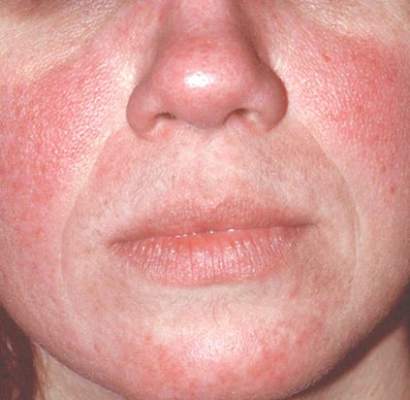
MAUI, HAWAII – Before concluding that a patient’s erythematous facial butterfly rash is an open and shut case of acute cutaneous lupus erythematosus, consider this, Dr. George M. Martin advised: 10-15 million Americans have acne rosacea.
“The 1% of those acne rosacea patients with the most severe disease are very often walking around misdiagnosed as having the butterfly rash of lupus,” Dr. Martin said at the 2016 Rheumatology Winter Clinical Symposium.
Indeed, rosacea – particularly the erythrotelangiectatic subtype – is the skin disease most often confused with acute cutaneous lupus erythematosus (ACLE), noted Dr. Martin, symposium codirector and a dermatologist practicing on Maui.
That’s a particularly common mistake in the event an individual with severe acne rosacea also happens to have the musculoskeletal symptoms of fibromyalgia, another very common disorder.
One clue useful in separating erythrotelangiectatic rosacea from ACLE is that “usually patients with the butterfly rash of acute lupus are sick. Rosacea patients are out there playing tennis and having a good time with a little bit of flush,” he said.
Other causes of a butterfly rash that get mistaken for ACLE are sebhorrheic dermatitis, contact dermatitis, and a photosensitizing drug eruption.
A biopsy may be necessary to establish a diagnosis, especially if the possibility of fibromyalgia is muddying the waters, according to Dr. Martin.
But cutaneous clues can also be helpful in deciding whether a butterfly rash is due to ACLE or cutaneous dermatomyositis. In lupus the rash typically spares the nasolabial folds, while the facial rash of dermatomyositis involves them. On the hands, the rash of ACLE spares the knuckles, whereas one-third of patients with dermatomyositis have the scaly red lesions known as Gottron’s papules over their knuckles. Cutaneous dermatomyositis has a predilection for the scalp, knees, and elbows.
A key distinction is that patients will report that the skin lesions of dermatomyositis are very itchy or have a burning sensation; that’s uncommon in cutaneous LE.
Also, prominent nailfold microhemorrhages at the distal cuticle are a telltale clue of dermatomyositis or possibly systemic sclerosis but aren’t part of the clinical picture in cutaneous LE. These microvascular changes are actually hemosiderin-containing deposits.
A skin biopsy is unhelpful in distinguishing cutaneous LE from cutaneous dermatomyositis. They basically have the same histopathologic findings, an interface dermatitis. Moreover, direct immunofluorescence and antinuclear antibody testing can be positive in both.
Dermatomyositis is more difficult to treat successfully than is cutaneous lupus. Antimalarials are often ineffective. But dapsone at 100-200 mg/day often works well in dermatomyositis, and it’s a relatively inexpensive drug, Dr. Martin said.
The hallmarks of subacute cutaneous lupus erythematosus (SCLE) are photosensitivity and small erythematous papules that expand into scaly patches and plaques that resemble psoriasis. SCLE is most common in women up to about age 40. It’s a disorder that predominates from the neck down. If more inflammation is present on the patient’s face than on the neck, shoulders, and upper torso, it’s not SCLE.
SCLE can be either drug induced or idiopathic; the two forms don’t differ clinically or histopathologically. In a patient older than age 50, SCLE is most commonly a drug-related eruption. In a 2011 review, Dr. Richard D. Sontheimer and his coinvestigators reported that antihypertensive agents accounted for one-third of cases of drug-induced SCLE, antifungals for one-quarter, chemotherapeutic agents for less than 9%, and antihistamines for 8% (Br J Dermatol. 2011 Mar;164[3]:465-72).
Dr. Sontheimer, professor of dermatology at the University of Utah in Salt Lake City, recently told Dr. Martin that his soon-to-be-published follow-up study of new cases since 2011 shows big changes in the culprit drugs. The proportion associated with chemotherapeutic agents, proton pump inhibitors, or biologics has jumped sharply. And with proton pump inhibitors now available over the counter, that class of medication is likely to continue to grow in importance as a source of drug-induced SCLE.
Dr. Martin reported serving on scientific advisory boards for and/or as a consultant to nine pharmaceutical companies.
MAUI, HAWAII – Before concluding that a patient’s erythematous facial butterfly rash is an open and shut case of acute cutaneous lupus erythematosus, consider this, Dr. George M. Martin advised: 10-15 million Americans have acne rosacea.
“The 1% of those acne rosacea patients with the most severe disease are very often walking around misdiagnosed as having the butterfly rash of lupus,” Dr. Martin said at the 2016 Rheumatology Winter Clinical Symposium.
Indeed, rosacea – particularly the erythrotelangiectatic subtype – is the skin disease most often confused with acute cutaneous lupus erythematosus (ACLE), noted Dr. Martin, symposium codirector and a dermatologist practicing on Maui.
That’s a particularly common mistake in the event an individual with severe acne rosacea also happens to have the musculoskeletal symptoms of fibromyalgia, another very common disorder.
One clue useful in separating erythrotelangiectatic rosacea from ACLE is that “usually patients with the butterfly rash of acute lupus are sick. Rosacea patients are out there playing tennis and having a good time with a little bit of flush,” he said.
Other causes of a butterfly rash that get mistaken for ACLE are sebhorrheic dermatitis, contact dermatitis, and a photosensitizing drug eruption.
A biopsy may be necessary to establish a diagnosis, especially if the possibility of fibromyalgia is muddying the waters, according to Dr. Martin.
But cutaneous clues can also be helpful in deciding whether a butterfly rash is due to ACLE or cutaneous dermatomyositis. In lupus the rash typically spares the nasolabial folds, while the facial rash of dermatomyositis involves them. On the hands, the rash of ACLE spares the knuckles, whereas one-third of patients with dermatomyositis have the scaly red lesions known as Gottron’s papules over their knuckles. Cutaneous dermatomyositis has a predilection for the scalp, knees, and elbows.
A key distinction is that patients will report that the skin lesions of dermatomyositis are very itchy or have a burning sensation; that’s uncommon in cutaneous LE.
Also, prominent nailfold microhemorrhages at the distal cuticle are a telltale clue of dermatomyositis or possibly systemic sclerosis but aren’t part of the clinical picture in cutaneous LE. These microvascular changes are actually hemosiderin-containing deposits.
A skin biopsy is unhelpful in distinguishing cutaneous LE from cutaneous dermatomyositis. They basically have the same histopathologic findings, an interface dermatitis. Moreover, direct immunofluorescence and antinuclear antibody testing can be positive in both.
Dermatomyositis is more difficult to treat successfully than is cutaneous lupus. Antimalarials are often ineffective. But dapsone at 100-200 mg/day often works well in dermatomyositis, and it’s a relatively inexpensive drug, Dr. Martin said.
The hallmarks of subacute cutaneous lupus erythematosus (SCLE) are photosensitivity and small erythematous papules that expand into scaly patches and plaques that resemble psoriasis. SCLE is most common in women up to about age 40. It’s a disorder that predominates from the neck down. If more inflammation is present on the patient’s face than on the neck, shoulders, and upper torso, it’s not SCLE.
SCLE can be either drug induced or idiopathic; the two forms don’t differ clinically or histopathologically. In a patient older than age 50, SCLE is most commonly a drug-related eruption. In a 2011 review, Dr. Richard D. Sontheimer and his coinvestigators reported that antihypertensive agents accounted for one-third of cases of drug-induced SCLE, antifungals for one-quarter, chemotherapeutic agents for less than 9%, and antihistamines for 8% (Br J Dermatol. 2011 Mar;164[3]:465-72).
Dr. Sontheimer, professor of dermatology at the University of Utah in Salt Lake City, recently told Dr. Martin that his soon-to-be-published follow-up study of new cases since 2011 shows big changes in the culprit drugs. The proportion associated with chemotherapeutic agents, proton pump inhibitors, or biologics has jumped sharply. And with proton pump inhibitors now available over the counter, that class of medication is likely to continue to grow in importance as a source of drug-induced SCLE.
Dr. Martin reported serving on scientific advisory boards for and/or as a consultant to nine pharmaceutical companies.
MAUI, HAWAII – Before concluding that a patient’s erythematous facial butterfly rash is an open and shut case of acute cutaneous lupus erythematosus, consider this, Dr. George M. Martin advised: 10-15 million Americans have acne rosacea.
“The 1% of those acne rosacea patients with the most severe disease are very often walking around misdiagnosed as having the butterfly rash of lupus,” Dr. Martin said at the 2016 Rheumatology Winter Clinical Symposium.
Indeed, rosacea – particularly the erythrotelangiectatic subtype – is the skin disease most often confused with acute cutaneous lupus erythematosus (ACLE), noted Dr. Martin, symposium codirector and a dermatologist practicing on Maui.
That’s a particularly common mistake in the event an individual with severe acne rosacea also happens to have the musculoskeletal symptoms of fibromyalgia, another very common disorder.
One clue useful in separating erythrotelangiectatic rosacea from ACLE is that “usually patients with the butterfly rash of acute lupus are sick. Rosacea patients are out there playing tennis and having a good time with a little bit of flush,” he said.
Other causes of a butterfly rash that get mistaken for ACLE are sebhorrheic dermatitis, contact dermatitis, and a photosensitizing drug eruption.
A biopsy may be necessary to establish a diagnosis, especially if the possibility of fibromyalgia is muddying the waters, according to Dr. Martin.
But cutaneous clues can also be helpful in deciding whether a butterfly rash is due to ACLE or cutaneous dermatomyositis. In lupus the rash typically spares the nasolabial folds, while the facial rash of dermatomyositis involves them. On the hands, the rash of ACLE spares the knuckles, whereas one-third of patients with dermatomyositis have the scaly red lesions known as Gottron’s papules over their knuckles. Cutaneous dermatomyositis has a predilection for the scalp, knees, and elbows.
A key distinction is that patients will report that the skin lesions of dermatomyositis are very itchy or have a burning sensation; that’s uncommon in cutaneous LE.
Also, prominent nailfold microhemorrhages at the distal cuticle are a telltale clue of dermatomyositis or possibly systemic sclerosis but aren’t part of the clinical picture in cutaneous LE. These microvascular changes are actually hemosiderin-containing deposits.
A skin biopsy is unhelpful in distinguishing cutaneous LE from cutaneous dermatomyositis. They basically have the same histopathologic findings, an interface dermatitis. Moreover, direct immunofluorescence and antinuclear antibody testing can be positive in both.
Dermatomyositis is more difficult to treat successfully than is cutaneous lupus. Antimalarials are often ineffective. But dapsone at 100-200 mg/day often works well in dermatomyositis, and it’s a relatively inexpensive drug, Dr. Martin said.
The hallmarks of subacute cutaneous lupus erythematosus (SCLE) are photosensitivity and small erythematous papules that expand into scaly patches and plaques that resemble psoriasis. SCLE is most common in women up to about age 40. It’s a disorder that predominates from the neck down. If more inflammation is present on the patient’s face than on the neck, shoulders, and upper torso, it’s not SCLE.
SCLE can be either drug induced or idiopathic; the two forms don’t differ clinically or histopathologically. In a patient older than age 50, SCLE is most commonly a drug-related eruption. In a 2011 review, Dr. Richard D. Sontheimer and his coinvestigators reported that antihypertensive agents accounted for one-third of cases of drug-induced SCLE, antifungals for one-quarter, chemotherapeutic agents for less than 9%, and antihistamines for 8% (Br J Dermatol. 2011 Mar;164[3]:465-72).
Dr. Sontheimer, professor of dermatology at the University of Utah in Salt Lake City, recently told Dr. Martin that his soon-to-be-published follow-up study of new cases since 2011 shows big changes in the culprit drugs. The proportion associated with chemotherapeutic agents, proton pump inhibitors, or biologics has jumped sharply. And with proton pump inhibitors now available over the counter, that class of medication is likely to continue to grow in importance as a source of drug-induced SCLE.
Dr. Martin reported serving on scientific advisory boards for and/or as a consultant to nine pharmaceutical companies.
EXPERT ANALYSIS FROM RWCS 2016
Superhigh ferritin spells trouble in macrophage activation syndrome
MAUI, HAWAII – The use of new classification criteria for macrophage activation syndrome specific to systemic juvenile idiopathic arthritis will help to avoid missing patients with the criteria that hematologists have used to diagnose the condition, which they call hemophagocytic lymphohistiocytosis, according to Dr. Anne M. Stevens, a pediatric rheumatologist at the University of Washington, Seattle.
“The problem is, if we try to use the HLH [hemophagocytic lymphohistiocytosis] criteria, we’re going to miss a lot of patients with systemic juvenile idiopathic arthritis–related macrophage activation syndrome, so we’re not going to treat them aggressively and many of them will die,” she said at the 2016 Rheumatology Winter Clinical Symposium.
Indeed, roughly one-third of patients with macrophage activation syndrome (MAS) arising as a complication of systemic juvenile idiopathic arthritis (SJIA) don’t meet the HLH diagnostic criteria. And the mortality of untreated MAS is up to 28%, Dr. Stevens noted.
MAS isn’t a new entity. It has become a more frequent topic of discussion lately because the pathogenesis of this cytokine storm is better understood, and biologic agents addressing the underlying disease mechanisms are in clinical trials. But MAS was first described nearly 40 years ago at a pediatric rheumatology conference in Park City, Utah.
Recently, an international consensus panel convened as a collaboration between the European League Against Rheumatism, the American College of Rheumatology, and the Paediatric Rheumatology International Trials Organisation has developed a new set of classification criteria for MAS complicating SJIA (Arthritis Rheumatol. 2016 Mar;68[3]:566-76).
In a validation study, the criteria had a sensitivity of 73% and a specificity of 99% for the diagnosis.
Under the 2016 criteria, a patient with known or suspected SJIA is classified as having MAS if he or she is febrile, has a serum ferritin level in excess of 684 ng/mL, and meets any two of the following four criteria: a platelet count of 181 x 109/L or less, triglycerides greater than 156 mg/dL, a serum aspartate aminotransferase level in excess of 48 U/L, or a fibrinogen level of 360 mg/dL or lower.
The specific cutoff threshold values for these laboratory measures are mainly of interest for research purposes in assuring appropriate patient enrollment in clinical trials. Practically speaking, it’s time to suspect MAS – a true medical emergency – in a child with SJIA whose fever has become persistent all day every day, rather than the quotidian fever that spikes in the evening characteristic of SJIA, along with hemodynamic instability, altered mental status, a rash, organomegaly, relative or progressive cytopenias, a very high ferritin level, and/or a relatively low or falling erythrocyte sedimentation rate.
A child with MAS superimposed on SJIA will look septic, according to Dr. Stevens. And if the ESR was 75 mm/hr yesterday and has “improved” to 45 mm/hr today, yet the child looks sicker or the other blood parameters are falling, that’s not an encouraging sign.
A very high serum ferritin level in patients with SJIA-related MAS is a red flag for increased mortality risk, as demonstrated in a study by physicians at Seattle Children’s Hospital. They retrospectively looked at 171 patients with a serum ferritin of 1,000 ng/dL and showed that the mortality risk climbed in parallel with the peak ferritin level. Children with a peak serum ferritin greater than 3,000 ng/mL had a 4.3-fold increased risk of mortality and a 2.5-fold greater risk of ICU admission than did those with a ferritin level of 1,000-3,000 ng/mL (Pediatr Crit Care Med. 2011 Nov;12[6]:e233-6).
Based upon these data, Dr. Stevens said she considers a serum ferritin of 1,000 ng/mL or more worthy of immediate attention.
The current standard of care in treating SJIA-related MAS is high-dose pulsed methylprednisolone at 30 mg up to 1 g/kg and IV cyclosporine at 5 mg/kg per day divided into two doses.
“There are good data to show that this will turn around half of affected kids,” according to the pediatric rheumatologist.
Second-line therapy targets interleukin-1: either IV anakinra (Kineret) at 2 mg/kg per day titrated up to 10 mg/kg, or canakinumab (Ilaris) at 4 mg/kg titrated up to 12.5 mg/kg every 4 weeks.
However, the current thinking is that what tips patients with SJIA into MAS is high levels of free interleukin-18. For that reason, several different anti–IL-18 agents are now under study, including tadekinig alfa, an IL-18 binding protein, and rituximab (Rituxan) for patients with Epstein-Barr virus–related MAS.
Dr. Stevens reported having no relevant financial conflicts.
MAUI, HAWAII – The use of new classification criteria for macrophage activation syndrome specific to systemic juvenile idiopathic arthritis will help to avoid missing patients with the criteria that hematologists have used to diagnose the condition, which they call hemophagocytic lymphohistiocytosis, according to Dr. Anne M. Stevens, a pediatric rheumatologist at the University of Washington, Seattle.
“The problem is, if we try to use the HLH [hemophagocytic lymphohistiocytosis] criteria, we’re going to miss a lot of patients with systemic juvenile idiopathic arthritis–related macrophage activation syndrome, so we’re not going to treat them aggressively and many of them will die,” she said at the 2016 Rheumatology Winter Clinical Symposium.
Indeed, roughly one-third of patients with macrophage activation syndrome (MAS) arising as a complication of systemic juvenile idiopathic arthritis (SJIA) don’t meet the HLH diagnostic criteria. And the mortality of untreated MAS is up to 28%, Dr. Stevens noted.
MAS isn’t a new entity. It has become a more frequent topic of discussion lately because the pathogenesis of this cytokine storm is better understood, and biologic agents addressing the underlying disease mechanisms are in clinical trials. But MAS was first described nearly 40 years ago at a pediatric rheumatology conference in Park City, Utah.
Recently, an international consensus panel convened as a collaboration between the European League Against Rheumatism, the American College of Rheumatology, and the Paediatric Rheumatology International Trials Organisation has developed a new set of classification criteria for MAS complicating SJIA (Arthritis Rheumatol. 2016 Mar;68[3]:566-76).
In a validation study, the criteria had a sensitivity of 73% and a specificity of 99% for the diagnosis.
Under the 2016 criteria, a patient with known or suspected SJIA is classified as having MAS if he or she is febrile, has a serum ferritin level in excess of 684 ng/mL, and meets any two of the following four criteria: a platelet count of 181 x 109/L or less, triglycerides greater than 156 mg/dL, a serum aspartate aminotransferase level in excess of 48 U/L, or a fibrinogen level of 360 mg/dL or lower.
The specific cutoff threshold values for these laboratory measures are mainly of interest for research purposes in assuring appropriate patient enrollment in clinical trials. Practically speaking, it’s time to suspect MAS – a true medical emergency – in a child with SJIA whose fever has become persistent all day every day, rather than the quotidian fever that spikes in the evening characteristic of SJIA, along with hemodynamic instability, altered mental status, a rash, organomegaly, relative or progressive cytopenias, a very high ferritin level, and/or a relatively low or falling erythrocyte sedimentation rate.
A child with MAS superimposed on SJIA will look septic, according to Dr. Stevens. And if the ESR was 75 mm/hr yesterday and has “improved” to 45 mm/hr today, yet the child looks sicker or the other blood parameters are falling, that’s not an encouraging sign.
A very high serum ferritin level in patients with SJIA-related MAS is a red flag for increased mortality risk, as demonstrated in a study by physicians at Seattle Children’s Hospital. They retrospectively looked at 171 patients with a serum ferritin of 1,000 ng/dL and showed that the mortality risk climbed in parallel with the peak ferritin level. Children with a peak serum ferritin greater than 3,000 ng/mL had a 4.3-fold increased risk of mortality and a 2.5-fold greater risk of ICU admission than did those with a ferritin level of 1,000-3,000 ng/mL (Pediatr Crit Care Med. 2011 Nov;12[6]:e233-6).
Based upon these data, Dr. Stevens said she considers a serum ferritin of 1,000 ng/mL or more worthy of immediate attention.
The current standard of care in treating SJIA-related MAS is high-dose pulsed methylprednisolone at 30 mg up to 1 g/kg and IV cyclosporine at 5 mg/kg per day divided into two doses.
“There are good data to show that this will turn around half of affected kids,” according to the pediatric rheumatologist.
Second-line therapy targets interleukin-1: either IV anakinra (Kineret) at 2 mg/kg per day titrated up to 10 mg/kg, or canakinumab (Ilaris) at 4 mg/kg titrated up to 12.5 mg/kg every 4 weeks.
However, the current thinking is that what tips patients with SJIA into MAS is high levels of free interleukin-18. For that reason, several different anti–IL-18 agents are now under study, including tadekinig alfa, an IL-18 binding protein, and rituximab (Rituxan) for patients with Epstein-Barr virus–related MAS.
Dr. Stevens reported having no relevant financial conflicts.
MAUI, HAWAII – The use of new classification criteria for macrophage activation syndrome specific to systemic juvenile idiopathic arthritis will help to avoid missing patients with the criteria that hematologists have used to diagnose the condition, which they call hemophagocytic lymphohistiocytosis, according to Dr. Anne M. Stevens, a pediatric rheumatologist at the University of Washington, Seattle.
“The problem is, if we try to use the HLH [hemophagocytic lymphohistiocytosis] criteria, we’re going to miss a lot of patients with systemic juvenile idiopathic arthritis–related macrophage activation syndrome, so we’re not going to treat them aggressively and many of them will die,” she said at the 2016 Rheumatology Winter Clinical Symposium.
Indeed, roughly one-third of patients with macrophage activation syndrome (MAS) arising as a complication of systemic juvenile idiopathic arthritis (SJIA) don’t meet the HLH diagnostic criteria. And the mortality of untreated MAS is up to 28%, Dr. Stevens noted.
MAS isn’t a new entity. It has become a more frequent topic of discussion lately because the pathogenesis of this cytokine storm is better understood, and biologic agents addressing the underlying disease mechanisms are in clinical trials. But MAS was first described nearly 40 years ago at a pediatric rheumatology conference in Park City, Utah.
Recently, an international consensus panel convened as a collaboration between the European League Against Rheumatism, the American College of Rheumatology, and the Paediatric Rheumatology International Trials Organisation has developed a new set of classification criteria for MAS complicating SJIA (Arthritis Rheumatol. 2016 Mar;68[3]:566-76).
In a validation study, the criteria had a sensitivity of 73% and a specificity of 99% for the diagnosis.
Under the 2016 criteria, a patient with known or suspected SJIA is classified as having MAS if he or she is febrile, has a serum ferritin level in excess of 684 ng/mL, and meets any two of the following four criteria: a platelet count of 181 x 109/L or less, triglycerides greater than 156 mg/dL, a serum aspartate aminotransferase level in excess of 48 U/L, or a fibrinogen level of 360 mg/dL or lower.
The specific cutoff threshold values for these laboratory measures are mainly of interest for research purposes in assuring appropriate patient enrollment in clinical trials. Practically speaking, it’s time to suspect MAS – a true medical emergency – in a child with SJIA whose fever has become persistent all day every day, rather than the quotidian fever that spikes in the evening characteristic of SJIA, along with hemodynamic instability, altered mental status, a rash, organomegaly, relative or progressive cytopenias, a very high ferritin level, and/or a relatively low or falling erythrocyte sedimentation rate.
A child with MAS superimposed on SJIA will look septic, according to Dr. Stevens. And if the ESR was 75 mm/hr yesterday and has “improved” to 45 mm/hr today, yet the child looks sicker or the other blood parameters are falling, that’s not an encouraging sign.
A very high serum ferritin level in patients with SJIA-related MAS is a red flag for increased mortality risk, as demonstrated in a study by physicians at Seattle Children’s Hospital. They retrospectively looked at 171 patients with a serum ferritin of 1,000 ng/dL and showed that the mortality risk climbed in parallel with the peak ferritin level. Children with a peak serum ferritin greater than 3,000 ng/mL had a 4.3-fold increased risk of mortality and a 2.5-fold greater risk of ICU admission than did those with a ferritin level of 1,000-3,000 ng/mL (Pediatr Crit Care Med. 2011 Nov;12[6]:e233-6).
Based upon these data, Dr. Stevens said she considers a serum ferritin of 1,000 ng/mL or more worthy of immediate attention.
The current standard of care in treating SJIA-related MAS is high-dose pulsed methylprednisolone at 30 mg up to 1 g/kg and IV cyclosporine at 5 mg/kg per day divided into two doses.
“There are good data to show that this will turn around half of affected kids,” according to the pediatric rheumatologist.
Second-line therapy targets interleukin-1: either IV anakinra (Kineret) at 2 mg/kg per day titrated up to 10 mg/kg, or canakinumab (Ilaris) at 4 mg/kg titrated up to 12.5 mg/kg every 4 weeks.
However, the current thinking is that what tips patients with SJIA into MAS is high levels of free interleukin-18. For that reason, several different anti–IL-18 agents are now under study, including tadekinig alfa, an IL-18 binding protein, and rituximab (Rituxan) for patients with Epstein-Barr virus–related MAS.
Dr. Stevens reported having no relevant financial conflicts.
EXPERT ANALYSIS FROM RWCS 2016
Imaging can predict who’ll progress from nonspecific symptoms to arthritis
MAUI, HAWAII – Recent evidence indicates that subclinical joint inflammation on ultrasound or MRI in patients having a positive cyclic citrullinated peptide antibody test but only nonspecific musculoskeletal symptoms predicts sharply increased risk of progression to full-blown diagnosable inflammatory arthritis within a matter of months. And this creates a new dilemma for rheumatologists.
The quandary is this: What are you going to do about it? After all, in the current era, the traditional treatment pyramid has been turned upside down, and early and aggressive therapy of arthritis is now recognized as best care.
“These are patients I have come back on a monthly basis instead of every 3 or 6 months. I’m hoping I’m going to be able to pick up diagnosable arthritis either by my physical exam or by the CDAI [Clinical Disease Activity Index score] or SDAI [Simplified Disease Activity Index score] so that I can initiate therapy as quickly as I possibly can to try to have a bigger impact. But I don’t initiate therapy just based on this imaging information,” Dr. Orrin M. Troum said at the 2016 Rheumatology Winter Clinical Symposium.
He highlighted two recent European studies that illustrate the prognostic power of contemporary joint imaging technologies performed at a point when patients don’t yet meet diagnostic criteria for arthritis. Both prospective studies were presented at the 2015 annual meeting of the American College of Rheumatology and have since been published.
Dr. Jackie L. Nam of the University of Leeds (England) reported on 136 consecutive anti–cyclic citrullinated peptide (anti-CCP) antibody–positive patients who presented with nonspecific musculoskeletal symptoms and no clinical synovitis. They underwent baseline ultrasound evaluation using power Doppler and grayscale imaging of 32 joints and were then prospectively followed for a median of 18.3 months.
At baseline ultrasound, 21% of patients had one or more erosions, 96% of patients had positive grayscale findings in one or more joints, and 30% were positive on power Doppler.
Forty-two percent of patients developed inflammatory arthritis after a median of 8.6 months. Patients with a baseline power Doppler score of 2 or more on a standard 0-3 scale in any joint had a 55% risk of developing inflammatory arthritis, compared with 4% if their power Doppler score was 0 or 1. A grayscale score of at least 2 was associated with a 26% likelihood of developing inflammatory arthritis, while a 0 or 1 on gray scale conferred a 3% risk. Progression to inflammatory arthritis occurred earlier in patients with a power Doppler score of 2 or 3, as well, at a median of 7.1 months (Ann Rheum Dis. 2016 Jan 22. doi: 10.1136/annrheumdis-2015-208235).
The other longitudinal study that grabbed Dr. Troum’s attention was a Dutch report on 150 patients who presented with nonspecific aches and pains. They underwent baseline serologic testing along with unilateral imaging of metacarpophalangeal, wrist, and metatarsophalangeal joints using 1.5-Tesla MRI. The imaging study was able to detect erosions as well as osteitis – that is, an inflammatory infiltrate in the bone marrow, as opposed to edema, which is watery fluid.
During a minimum of 6 months and median of 75 weeks of follow-up, 30 patients developed clinical arthritis. Twenty-six of the 30 did so before 20 weeks of follow-up had elapsed. The strongest risk factors for progression to arthritis were anti-CCP antibody positivity, with a hazard ratio of 6.4, and subclinical MRI inflammation, with a hazard ratio of 5.1.
The 1-year rate of progression to arthritis was 31% with MRI-detected subclinical inflammation alone, 71% in patients who were both MRI- and anti-CCP antibody–positive, and 3% in those who were both MRI- and anti-CCP antibody–negative (Ann Rheum Dis. 2015 Nov 27. doi: 10.1136/annrheumdis-2015-208138).
Dr. Roy Fleischmann rose from the audience to challenge Dr. Troum: Don’t these baseline imaging–positive, anti-CCP antibody–positive patients already have disease? Isn’t this the time you want to treat? he asked.
“It would ideally be the time to treat, yes,” replied Dr. Troum. “If it was me, I probably would treat myself.”
“With what, Xanax?” quipped Dr. Fleischmann of the University of Texas, Dallas.
“With methotrexate, probably,” Dr. Troum said.
He added that these were preliminary studies with relatively small numbers of patients. Before changing his practice regarding patients with these subclinical findings, he’d like to see more data and an estimate of the number needed to treat.
“It could be as many as two-thirds of the population that you’d be overtreating, people who were never going to develop anything,” Dr. Troum observed.
He reported having no financial conflicts of interest regarding his presentation.
MAUI, HAWAII – Recent evidence indicates that subclinical joint inflammation on ultrasound or MRI in patients having a positive cyclic citrullinated peptide antibody test but only nonspecific musculoskeletal symptoms predicts sharply increased risk of progression to full-blown diagnosable inflammatory arthritis within a matter of months. And this creates a new dilemma for rheumatologists.
The quandary is this: What are you going to do about it? After all, in the current era, the traditional treatment pyramid has been turned upside down, and early and aggressive therapy of arthritis is now recognized as best care.
“These are patients I have come back on a monthly basis instead of every 3 or 6 months. I’m hoping I’m going to be able to pick up diagnosable arthritis either by my physical exam or by the CDAI [Clinical Disease Activity Index score] or SDAI [Simplified Disease Activity Index score] so that I can initiate therapy as quickly as I possibly can to try to have a bigger impact. But I don’t initiate therapy just based on this imaging information,” Dr. Orrin M. Troum said at the 2016 Rheumatology Winter Clinical Symposium.
He highlighted two recent European studies that illustrate the prognostic power of contemporary joint imaging technologies performed at a point when patients don’t yet meet diagnostic criteria for arthritis. Both prospective studies were presented at the 2015 annual meeting of the American College of Rheumatology and have since been published.
Dr. Jackie L. Nam of the University of Leeds (England) reported on 136 consecutive anti–cyclic citrullinated peptide (anti-CCP) antibody–positive patients who presented with nonspecific musculoskeletal symptoms and no clinical synovitis. They underwent baseline ultrasound evaluation using power Doppler and grayscale imaging of 32 joints and were then prospectively followed for a median of 18.3 months.
At baseline ultrasound, 21% of patients had one or more erosions, 96% of patients had positive grayscale findings in one or more joints, and 30% were positive on power Doppler.
Forty-two percent of patients developed inflammatory arthritis after a median of 8.6 months. Patients with a baseline power Doppler score of 2 or more on a standard 0-3 scale in any joint had a 55% risk of developing inflammatory arthritis, compared with 4% if their power Doppler score was 0 or 1. A grayscale score of at least 2 was associated with a 26% likelihood of developing inflammatory arthritis, while a 0 or 1 on gray scale conferred a 3% risk. Progression to inflammatory arthritis occurred earlier in patients with a power Doppler score of 2 or 3, as well, at a median of 7.1 months (Ann Rheum Dis. 2016 Jan 22. doi: 10.1136/annrheumdis-2015-208235).
The other longitudinal study that grabbed Dr. Troum’s attention was a Dutch report on 150 patients who presented with nonspecific aches and pains. They underwent baseline serologic testing along with unilateral imaging of metacarpophalangeal, wrist, and metatarsophalangeal joints using 1.5-Tesla MRI. The imaging study was able to detect erosions as well as osteitis – that is, an inflammatory infiltrate in the bone marrow, as opposed to edema, which is watery fluid.
During a minimum of 6 months and median of 75 weeks of follow-up, 30 patients developed clinical arthritis. Twenty-six of the 30 did so before 20 weeks of follow-up had elapsed. The strongest risk factors for progression to arthritis were anti-CCP antibody positivity, with a hazard ratio of 6.4, and subclinical MRI inflammation, with a hazard ratio of 5.1.
The 1-year rate of progression to arthritis was 31% with MRI-detected subclinical inflammation alone, 71% in patients who were both MRI- and anti-CCP antibody–positive, and 3% in those who were both MRI- and anti-CCP antibody–negative (Ann Rheum Dis. 2015 Nov 27. doi: 10.1136/annrheumdis-2015-208138).
Dr. Roy Fleischmann rose from the audience to challenge Dr. Troum: Don’t these baseline imaging–positive, anti-CCP antibody–positive patients already have disease? Isn’t this the time you want to treat? he asked.
“It would ideally be the time to treat, yes,” replied Dr. Troum. “If it was me, I probably would treat myself.”
“With what, Xanax?” quipped Dr. Fleischmann of the University of Texas, Dallas.
“With methotrexate, probably,” Dr. Troum said.
He added that these were preliminary studies with relatively small numbers of patients. Before changing his practice regarding patients with these subclinical findings, he’d like to see more data and an estimate of the number needed to treat.
“It could be as many as two-thirds of the population that you’d be overtreating, people who were never going to develop anything,” Dr. Troum observed.
He reported having no financial conflicts of interest regarding his presentation.
MAUI, HAWAII – Recent evidence indicates that subclinical joint inflammation on ultrasound or MRI in patients having a positive cyclic citrullinated peptide antibody test but only nonspecific musculoskeletal symptoms predicts sharply increased risk of progression to full-blown diagnosable inflammatory arthritis within a matter of months. And this creates a new dilemma for rheumatologists.
The quandary is this: What are you going to do about it? After all, in the current era, the traditional treatment pyramid has been turned upside down, and early and aggressive therapy of arthritis is now recognized as best care.
“These are patients I have come back on a monthly basis instead of every 3 or 6 months. I’m hoping I’m going to be able to pick up diagnosable arthritis either by my physical exam or by the CDAI [Clinical Disease Activity Index score] or SDAI [Simplified Disease Activity Index score] so that I can initiate therapy as quickly as I possibly can to try to have a bigger impact. But I don’t initiate therapy just based on this imaging information,” Dr. Orrin M. Troum said at the 2016 Rheumatology Winter Clinical Symposium.
He highlighted two recent European studies that illustrate the prognostic power of contemporary joint imaging technologies performed at a point when patients don’t yet meet diagnostic criteria for arthritis. Both prospective studies were presented at the 2015 annual meeting of the American College of Rheumatology and have since been published.
Dr. Jackie L. Nam of the University of Leeds (England) reported on 136 consecutive anti–cyclic citrullinated peptide (anti-CCP) antibody–positive patients who presented with nonspecific musculoskeletal symptoms and no clinical synovitis. They underwent baseline ultrasound evaluation using power Doppler and grayscale imaging of 32 joints and were then prospectively followed for a median of 18.3 months.
At baseline ultrasound, 21% of patients had one or more erosions, 96% of patients had positive grayscale findings in one or more joints, and 30% were positive on power Doppler.
Forty-two percent of patients developed inflammatory arthritis after a median of 8.6 months. Patients with a baseline power Doppler score of 2 or more on a standard 0-3 scale in any joint had a 55% risk of developing inflammatory arthritis, compared with 4% if their power Doppler score was 0 or 1. A grayscale score of at least 2 was associated with a 26% likelihood of developing inflammatory arthritis, while a 0 or 1 on gray scale conferred a 3% risk. Progression to inflammatory arthritis occurred earlier in patients with a power Doppler score of 2 or 3, as well, at a median of 7.1 months (Ann Rheum Dis. 2016 Jan 22. doi: 10.1136/annrheumdis-2015-208235).
The other longitudinal study that grabbed Dr. Troum’s attention was a Dutch report on 150 patients who presented with nonspecific aches and pains. They underwent baseline serologic testing along with unilateral imaging of metacarpophalangeal, wrist, and metatarsophalangeal joints using 1.5-Tesla MRI. The imaging study was able to detect erosions as well as osteitis – that is, an inflammatory infiltrate in the bone marrow, as opposed to edema, which is watery fluid.
During a minimum of 6 months and median of 75 weeks of follow-up, 30 patients developed clinical arthritis. Twenty-six of the 30 did so before 20 weeks of follow-up had elapsed. The strongest risk factors for progression to arthritis were anti-CCP antibody positivity, with a hazard ratio of 6.4, and subclinical MRI inflammation, with a hazard ratio of 5.1.
The 1-year rate of progression to arthritis was 31% with MRI-detected subclinical inflammation alone, 71% in patients who were both MRI- and anti-CCP antibody–positive, and 3% in those who were both MRI- and anti-CCP antibody–negative (Ann Rheum Dis. 2015 Nov 27. doi: 10.1136/annrheumdis-2015-208138).
Dr. Roy Fleischmann rose from the audience to challenge Dr. Troum: Don’t these baseline imaging–positive, anti-CCP antibody–positive patients already have disease? Isn’t this the time you want to treat? he asked.
“It would ideally be the time to treat, yes,” replied Dr. Troum. “If it was me, I probably would treat myself.”
“With what, Xanax?” quipped Dr. Fleischmann of the University of Texas, Dallas.
“With methotrexate, probably,” Dr. Troum said.
He added that these were preliminary studies with relatively small numbers of patients. Before changing his practice regarding patients with these subclinical findings, he’d like to see more data and an estimate of the number needed to treat.
“It could be as many as two-thirds of the population that you’d be overtreating, people who were never going to develop anything,” Dr. Troum observed.
He reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM RWCS 2016
The year in gout brings a controversial new drug
MAUI, HAWAII – The recently approved uric acid–lowering drug lesinurad received a lukewarm reception at best when introduced during a ‘highlights of the year in gout’ session presented at the 2016 Rheumatology Winter Clinical Symposium.
Panelist Martin J. Bergman presented a dispassionate overview of the data from four pivotal randomized trials which in late December 2015 resulted in Food and Drug Administration approval of lesinurad (Zurampic) at 200 mg/day in combination with a xanthine oxidase inhibitor, but not as monotherapy at 400 mg/day.
After highlighting the drug’s safety concerns, including the black box warning about lesinurad’s risk of acute renal failure and its numerous potential drug interactions, Dr. Bergman opened the floor to discussion. An audience member immediately shot up his hand and asked, “Isn’t this drug a crappy drug?”
Dr. Bergman, chief of rheumatology at Taylor Hospital in Ridley Park, Pa., answered diplomatically: “It’s not the strongest drug, it’s not the best. Is this going to be something which revolutionizes the care of gout? I don’t think so. But it does give us a way to get to the treatment goal of a serum uric acid below 6.0 mg/dL in gout patients unable to get there on allopurinol or febuxostat [Uloric] alone. “
Faculty member Dr. Eric M. Ruderman was blunt in his appraisal of lesinurad: “I really don’t understand the place for this drug.”
“In the trial of combination therapy with febuxostat [the 324-patient CRYSTAL study] it didn’t meet the primary endpoint at the 200 mg/day dose. It’s amazing to me that the FDA will approve a drug when one of the pivotal trials didn’t meet the primary endpoint at the dose they approved. That’s bizarre. And in the trials with allopurinol [CLEAR 1 and 2, with a total of 1,213 patients] they didn’t use maximum-dose allopurinol. So I don’t see where this drug adds anything to our treatment paradigm,” said Dr. Ruderman, professor of medicine at Northwestern University in Chicago.
In the pivotal clinical trials, the 400 mg/day dose was more effective than 200 mg/day, but it was also associated with a doubling of serum creatinine in 1 in every 12 treated patients, as compared with a 1%-2% incidence at 200 mg. That’s why the FDA didn’t approve the higher dose.
Lesinurad is a selective inhibitor of uric acid resorption which acts in the proximal tubule on URAT1, an inhibitor of uric acid transport.
Arhalofenate, a promising investigational gout drug, shares the same mechanism of action, but in addition it blocks release of interleukin-1beta. In a 239-patient, phase IIb trial presented at the 2015 annual meeting of the American College of Rheumatology, arhalofenate effectively reduced the rate of gout flares while lowering serum uric acid levels, and most notably it did so with no treatment-related serious adverse events and no cases of elevated serum creatinine. This is a drug to keep an eye on, according to Dr. Bergman.
Copanelist Dr. Orrin M. Troum of the University of Southern California, Los Angeles, presented highlights of other significant recent studies in the field of gout, some of them quite surprising:
• Colchicine reduces cardiovascular events in gout patients. A comparison between 501 Medicare gout patients on colchicine and an equal number of matched gout patients not on colchicine showed that during a median 16.5 months of follow-up, the colchicine users had an adjusted 49% reduction in the composite endpoint of acute MI, stroke, or TIA. They also had a 73% reduction in all-cause mortality, according to Dr. Daniel H. Solomon, professor of medicine at Harvard Medical School, Boston, and coinvestigators (Ann Rheum Dis. 2015 Nov 18. doi: 10.1136/annrheumdis-2015-207984).
“Once my gout patients stop clutching their chest when they see the price of colchicine, which actually increases their cardiovascular risk, they are very excited when I tell them about this study,” Dr. Bergman quipped. “This study controlled for other comorbidities and for serum uric acid levels. Those relative risk reductions are not to be sneezed at.”
• Treating gout improves survival. In a prospective case-matched cohort study, Taiwanese investigators compared 764 gout patients on urate-lowering therapy with an equal number of matched gout patients who did not take a urate-lowering drug. During 6.5 years of follow-up, the group on urate-lowering medication had a 71% lower risk of cardiovascular mortality and a 53% reduction in all-cause mortality, compared with gout patients not on urate-lowering therapy. Moreover, in a separate analysis comparing 1,189 gout patients not taking urate-lowering therapy and three times as many matched controls without gout, the gout patients had a 2.43-fold greater rate of cardiovascular mortality and a 1.45-fold increased risk of all-cause mortality (J Rheumatol. 2015 Sep;42[9]:1694-701).
• Gout is associated with reduced risk of Alzheimer’s disease. Using a U.K. electronic medical record database to track nearly 60,000 patients with gout and 239,000 matched controls, investigators determined that the incidence of Alzheimer’s disease during a median 5 years of follow-up was reduced by 24% in an analysis extensively adjusted for smoking, alcohol intake, medications, comorbid conditions, social deprivation, and other potential confounders. The researchers concluded based upon this and other evidence that uric acid appears to be neuroprotective (Ann Rheum Dis. 2016 Mar;75[3]:547-51).
• Sleep apnea is an independent risk factor for gout. Patients newly diagnosed with sleep apnea had a 50% greater risk of developing gout in the next year, compared with BMI-matched controls without sleep apnea in a population-based study conducted by investigators in Boston and the United Kingdom. The study included 9,865 patients with a new physician diagnosis of sleep apnea and nearly 44,000 matched controls. The incidence of newly diagnosed gout was 8.4 per 1,000 person-years in the group with sleep apnea and 4.8 per 1,000 person-years in the comparison group.
In a multivariate analysis adjusted for numerous potential confounders, new-onset sleep apnea remained an independent predictor of increased risk for gout. The results raise the testable hypothesis that effective treatment of sleep apnea might reduce the risk of hyperuricemia and gout flares (Arthritis Rheumatol. 2015 Dec;67[12]:3298-302).
• Gout linked to increased risk of septic arthritis. In a population-based study, investigators at Boston University and Massachusetts General Hospital turned to the U.K. Health Improvement Network general practice database, where they identified 72,073 new-onset gout patients and 358,342 matched controls without gout. The incidence rate of a septic arthritis diagnosis during follow-up was 0.24 cases per 1,000 person-years in the gout group and 0.09 per 1,000 person-years in controls. In a multivariate regression analysis, gout patients were at 2.6-fold greater risk of septic arthritis (Rheumatology [Oxford]. 2015 Nov;54[11]:2095-9).
• Gout is associated with increased risk of new-onset atrial fibrillation. A cohort study conducted using a U.S. commercial health insurance database identified 70,015 patients with gout and 210,045 with osteoarthritis. During a mean 2 years of follow-up, newly diagnosed atrial fibrillation occurred at a rate of 7.19 cases per 1,000 person-years in the gout group and 5.87 per 1,000 in the osteoarthritis patients. In a multivariate regression analysis, patients with gout had a 13% increased risk of new-onset atrial fibrillation, compared with the osteoarthritis group (Ann Rheum Dis. 2015 Aug 31. doi: 10.1136/annrheumdis-2015-208161).
• Genetic screening test enables patients to avoid allopurinol-induced severe cutaneous adverse reactions. In a prospective cohort study, Taiwanese investigators performed screening for the HLA-B*58:01 allele in 2,926 patients of Han Chinese descent who had an indication for treatment with allopurinol. Those who tested positive – 571 patients, or 19.6% – received some alternative drug, while those who were HLA-B*58:01-negative were placed on allopurinol. All subjects were interviewed once weekly for the next 2 months, and hospital admissions for adverse drug reactions were monitored nationwide. Not a single study participant developed an allopurinol-induced severe cutaneous adverse reaction. Based upon historical incidence, seven cases would have been expected in the study population (BMJ. 2015 Sep 23;351:h4848. doi: 10.1136/bmj.h4848).
Dr. Bergman and Dr. Troum reported having no financial conflicts regarding their presentation.
MAUI, HAWAII – The recently approved uric acid–lowering drug lesinurad received a lukewarm reception at best when introduced during a ‘highlights of the year in gout’ session presented at the 2016 Rheumatology Winter Clinical Symposium.
Panelist Martin J. Bergman presented a dispassionate overview of the data from four pivotal randomized trials which in late December 2015 resulted in Food and Drug Administration approval of lesinurad (Zurampic) at 200 mg/day in combination with a xanthine oxidase inhibitor, but not as monotherapy at 400 mg/day.
After highlighting the drug’s safety concerns, including the black box warning about lesinurad’s risk of acute renal failure and its numerous potential drug interactions, Dr. Bergman opened the floor to discussion. An audience member immediately shot up his hand and asked, “Isn’t this drug a crappy drug?”
Dr. Bergman, chief of rheumatology at Taylor Hospital in Ridley Park, Pa., answered diplomatically: “It’s not the strongest drug, it’s not the best. Is this going to be something which revolutionizes the care of gout? I don’t think so. But it does give us a way to get to the treatment goal of a serum uric acid below 6.0 mg/dL in gout patients unable to get there on allopurinol or febuxostat [Uloric] alone. “
Faculty member Dr. Eric M. Ruderman was blunt in his appraisal of lesinurad: “I really don’t understand the place for this drug.”
“In the trial of combination therapy with febuxostat [the 324-patient CRYSTAL study] it didn’t meet the primary endpoint at the 200 mg/day dose. It’s amazing to me that the FDA will approve a drug when one of the pivotal trials didn’t meet the primary endpoint at the dose they approved. That’s bizarre. And in the trials with allopurinol [CLEAR 1 and 2, with a total of 1,213 patients] they didn’t use maximum-dose allopurinol. So I don’t see where this drug adds anything to our treatment paradigm,” said Dr. Ruderman, professor of medicine at Northwestern University in Chicago.
In the pivotal clinical trials, the 400 mg/day dose was more effective than 200 mg/day, but it was also associated with a doubling of serum creatinine in 1 in every 12 treated patients, as compared with a 1%-2% incidence at 200 mg. That’s why the FDA didn’t approve the higher dose.
Lesinurad is a selective inhibitor of uric acid resorption which acts in the proximal tubule on URAT1, an inhibitor of uric acid transport.
Arhalofenate, a promising investigational gout drug, shares the same mechanism of action, but in addition it blocks release of interleukin-1beta. In a 239-patient, phase IIb trial presented at the 2015 annual meeting of the American College of Rheumatology, arhalofenate effectively reduced the rate of gout flares while lowering serum uric acid levels, and most notably it did so with no treatment-related serious adverse events and no cases of elevated serum creatinine. This is a drug to keep an eye on, according to Dr. Bergman.
Copanelist Dr. Orrin M. Troum of the University of Southern California, Los Angeles, presented highlights of other significant recent studies in the field of gout, some of them quite surprising:
• Colchicine reduces cardiovascular events in gout patients. A comparison between 501 Medicare gout patients on colchicine and an equal number of matched gout patients not on colchicine showed that during a median 16.5 months of follow-up, the colchicine users had an adjusted 49% reduction in the composite endpoint of acute MI, stroke, or TIA. They also had a 73% reduction in all-cause mortality, according to Dr. Daniel H. Solomon, professor of medicine at Harvard Medical School, Boston, and coinvestigators (Ann Rheum Dis. 2015 Nov 18. doi: 10.1136/annrheumdis-2015-207984).
“Once my gout patients stop clutching their chest when they see the price of colchicine, which actually increases their cardiovascular risk, they are very excited when I tell them about this study,” Dr. Bergman quipped. “This study controlled for other comorbidities and for serum uric acid levels. Those relative risk reductions are not to be sneezed at.”
• Treating gout improves survival. In a prospective case-matched cohort study, Taiwanese investigators compared 764 gout patients on urate-lowering therapy with an equal number of matched gout patients who did not take a urate-lowering drug. During 6.5 years of follow-up, the group on urate-lowering medication had a 71% lower risk of cardiovascular mortality and a 53% reduction in all-cause mortality, compared with gout patients not on urate-lowering therapy. Moreover, in a separate analysis comparing 1,189 gout patients not taking urate-lowering therapy and three times as many matched controls without gout, the gout patients had a 2.43-fold greater rate of cardiovascular mortality and a 1.45-fold increased risk of all-cause mortality (J Rheumatol. 2015 Sep;42[9]:1694-701).
• Gout is associated with reduced risk of Alzheimer’s disease. Using a U.K. electronic medical record database to track nearly 60,000 patients with gout and 239,000 matched controls, investigators determined that the incidence of Alzheimer’s disease during a median 5 years of follow-up was reduced by 24% in an analysis extensively adjusted for smoking, alcohol intake, medications, comorbid conditions, social deprivation, and other potential confounders. The researchers concluded based upon this and other evidence that uric acid appears to be neuroprotective (Ann Rheum Dis. 2016 Mar;75[3]:547-51).
• Sleep apnea is an independent risk factor for gout. Patients newly diagnosed with sleep apnea had a 50% greater risk of developing gout in the next year, compared with BMI-matched controls without sleep apnea in a population-based study conducted by investigators in Boston and the United Kingdom. The study included 9,865 patients with a new physician diagnosis of sleep apnea and nearly 44,000 matched controls. The incidence of newly diagnosed gout was 8.4 per 1,000 person-years in the group with sleep apnea and 4.8 per 1,000 person-years in the comparison group.
In a multivariate analysis adjusted for numerous potential confounders, new-onset sleep apnea remained an independent predictor of increased risk for gout. The results raise the testable hypothesis that effective treatment of sleep apnea might reduce the risk of hyperuricemia and gout flares (Arthritis Rheumatol. 2015 Dec;67[12]:3298-302).
• Gout linked to increased risk of septic arthritis. In a population-based study, investigators at Boston University and Massachusetts General Hospital turned to the U.K. Health Improvement Network general practice database, where they identified 72,073 new-onset gout patients and 358,342 matched controls without gout. The incidence rate of a septic arthritis diagnosis during follow-up was 0.24 cases per 1,000 person-years in the gout group and 0.09 per 1,000 person-years in controls. In a multivariate regression analysis, gout patients were at 2.6-fold greater risk of septic arthritis (Rheumatology [Oxford]. 2015 Nov;54[11]:2095-9).
• Gout is associated with increased risk of new-onset atrial fibrillation. A cohort study conducted using a U.S. commercial health insurance database identified 70,015 patients with gout and 210,045 with osteoarthritis. During a mean 2 years of follow-up, newly diagnosed atrial fibrillation occurred at a rate of 7.19 cases per 1,000 person-years in the gout group and 5.87 per 1,000 in the osteoarthritis patients. In a multivariate regression analysis, patients with gout had a 13% increased risk of new-onset atrial fibrillation, compared with the osteoarthritis group (Ann Rheum Dis. 2015 Aug 31. doi: 10.1136/annrheumdis-2015-208161).
• Genetic screening test enables patients to avoid allopurinol-induced severe cutaneous adverse reactions. In a prospective cohort study, Taiwanese investigators performed screening for the HLA-B*58:01 allele in 2,926 patients of Han Chinese descent who had an indication for treatment with allopurinol. Those who tested positive – 571 patients, or 19.6% – received some alternative drug, while those who were HLA-B*58:01-negative were placed on allopurinol. All subjects were interviewed once weekly for the next 2 months, and hospital admissions for adverse drug reactions were monitored nationwide. Not a single study participant developed an allopurinol-induced severe cutaneous adverse reaction. Based upon historical incidence, seven cases would have been expected in the study population (BMJ. 2015 Sep 23;351:h4848. doi: 10.1136/bmj.h4848).
Dr. Bergman and Dr. Troum reported having no financial conflicts regarding their presentation.
MAUI, HAWAII – The recently approved uric acid–lowering drug lesinurad received a lukewarm reception at best when introduced during a ‘highlights of the year in gout’ session presented at the 2016 Rheumatology Winter Clinical Symposium.
Panelist Martin J. Bergman presented a dispassionate overview of the data from four pivotal randomized trials which in late December 2015 resulted in Food and Drug Administration approval of lesinurad (Zurampic) at 200 mg/day in combination with a xanthine oxidase inhibitor, but not as monotherapy at 400 mg/day.
After highlighting the drug’s safety concerns, including the black box warning about lesinurad’s risk of acute renal failure and its numerous potential drug interactions, Dr. Bergman opened the floor to discussion. An audience member immediately shot up his hand and asked, “Isn’t this drug a crappy drug?”
Dr. Bergman, chief of rheumatology at Taylor Hospital in Ridley Park, Pa., answered diplomatically: “It’s not the strongest drug, it’s not the best. Is this going to be something which revolutionizes the care of gout? I don’t think so. But it does give us a way to get to the treatment goal of a serum uric acid below 6.0 mg/dL in gout patients unable to get there on allopurinol or febuxostat [Uloric] alone. “
Faculty member Dr. Eric M. Ruderman was blunt in his appraisal of lesinurad: “I really don’t understand the place for this drug.”
“In the trial of combination therapy with febuxostat [the 324-patient CRYSTAL study] it didn’t meet the primary endpoint at the 200 mg/day dose. It’s amazing to me that the FDA will approve a drug when one of the pivotal trials didn’t meet the primary endpoint at the dose they approved. That’s bizarre. And in the trials with allopurinol [CLEAR 1 and 2, with a total of 1,213 patients] they didn’t use maximum-dose allopurinol. So I don’t see where this drug adds anything to our treatment paradigm,” said Dr. Ruderman, professor of medicine at Northwestern University in Chicago.
In the pivotal clinical trials, the 400 mg/day dose was more effective than 200 mg/day, but it was also associated with a doubling of serum creatinine in 1 in every 12 treated patients, as compared with a 1%-2% incidence at 200 mg. That’s why the FDA didn’t approve the higher dose.
Lesinurad is a selective inhibitor of uric acid resorption which acts in the proximal tubule on URAT1, an inhibitor of uric acid transport.
Arhalofenate, a promising investigational gout drug, shares the same mechanism of action, but in addition it blocks release of interleukin-1beta. In a 239-patient, phase IIb trial presented at the 2015 annual meeting of the American College of Rheumatology, arhalofenate effectively reduced the rate of gout flares while lowering serum uric acid levels, and most notably it did so with no treatment-related serious adverse events and no cases of elevated serum creatinine. This is a drug to keep an eye on, according to Dr. Bergman.
Copanelist Dr. Orrin M. Troum of the University of Southern California, Los Angeles, presented highlights of other significant recent studies in the field of gout, some of them quite surprising:
• Colchicine reduces cardiovascular events in gout patients. A comparison between 501 Medicare gout patients on colchicine and an equal number of matched gout patients not on colchicine showed that during a median 16.5 months of follow-up, the colchicine users had an adjusted 49% reduction in the composite endpoint of acute MI, stroke, or TIA. They also had a 73% reduction in all-cause mortality, according to Dr. Daniel H. Solomon, professor of medicine at Harvard Medical School, Boston, and coinvestigators (Ann Rheum Dis. 2015 Nov 18. doi: 10.1136/annrheumdis-2015-207984).
“Once my gout patients stop clutching their chest when they see the price of colchicine, which actually increases their cardiovascular risk, they are very excited when I tell them about this study,” Dr. Bergman quipped. “This study controlled for other comorbidities and for serum uric acid levels. Those relative risk reductions are not to be sneezed at.”
• Treating gout improves survival. In a prospective case-matched cohort study, Taiwanese investigators compared 764 gout patients on urate-lowering therapy with an equal number of matched gout patients who did not take a urate-lowering drug. During 6.5 years of follow-up, the group on urate-lowering medication had a 71% lower risk of cardiovascular mortality and a 53% reduction in all-cause mortality, compared with gout patients not on urate-lowering therapy. Moreover, in a separate analysis comparing 1,189 gout patients not taking urate-lowering therapy and three times as many matched controls without gout, the gout patients had a 2.43-fold greater rate of cardiovascular mortality and a 1.45-fold increased risk of all-cause mortality (J Rheumatol. 2015 Sep;42[9]:1694-701).
• Gout is associated with reduced risk of Alzheimer’s disease. Using a U.K. electronic medical record database to track nearly 60,000 patients with gout and 239,000 matched controls, investigators determined that the incidence of Alzheimer’s disease during a median 5 years of follow-up was reduced by 24% in an analysis extensively adjusted for smoking, alcohol intake, medications, comorbid conditions, social deprivation, and other potential confounders. The researchers concluded based upon this and other evidence that uric acid appears to be neuroprotective (Ann Rheum Dis. 2016 Mar;75[3]:547-51).
• Sleep apnea is an independent risk factor for gout. Patients newly diagnosed with sleep apnea had a 50% greater risk of developing gout in the next year, compared with BMI-matched controls without sleep apnea in a population-based study conducted by investigators in Boston and the United Kingdom. The study included 9,865 patients with a new physician diagnosis of sleep apnea and nearly 44,000 matched controls. The incidence of newly diagnosed gout was 8.4 per 1,000 person-years in the group with sleep apnea and 4.8 per 1,000 person-years in the comparison group.
In a multivariate analysis adjusted for numerous potential confounders, new-onset sleep apnea remained an independent predictor of increased risk for gout. The results raise the testable hypothesis that effective treatment of sleep apnea might reduce the risk of hyperuricemia and gout flares (Arthritis Rheumatol. 2015 Dec;67[12]:3298-302).
• Gout linked to increased risk of septic arthritis. In a population-based study, investigators at Boston University and Massachusetts General Hospital turned to the U.K. Health Improvement Network general practice database, where they identified 72,073 new-onset gout patients and 358,342 matched controls without gout. The incidence rate of a septic arthritis diagnosis during follow-up was 0.24 cases per 1,000 person-years in the gout group and 0.09 per 1,000 person-years in controls. In a multivariate regression analysis, gout patients were at 2.6-fold greater risk of septic arthritis (Rheumatology [Oxford]. 2015 Nov;54[11]:2095-9).
• Gout is associated with increased risk of new-onset atrial fibrillation. A cohort study conducted using a U.S. commercial health insurance database identified 70,015 patients with gout and 210,045 with osteoarthritis. During a mean 2 years of follow-up, newly diagnosed atrial fibrillation occurred at a rate of 7.19 cases per 1,000 person-years in the gout group and 5.87 per 1,000 in the osteoarthritis patients. In a multivariate regression analysis, patients with gout had a 13% increased risk of new-onset atrial fibrillation, compared with the osteoarthritis group (Ann Rheum Dis. 2015 Aug 31. doi: 10.1136/annrheumdis-2015-208161).
• Genetic screening test enables patients to avoid allopurinol-induced severe cutaneous adverse reactions. In a prospective cohort study, Taiwanese investigators performed screening for the HLA-B*58:01 allele in 2,926 patients of Han Chinese descent who had an indication for treatment with allopurinol. Those who tested positive – 571 patients, or 19.6% – received some alternative drug, while those who were HLA-B*58:01-negative were placed on allopurinol. All subjects were interviewed once weekly for the next 2 months, and hospital admissions for adverse drug reactions were monitored nationwide. Not a single study participant developed an allopurinol-induced severe cutaneous adverse reaction. Based upon historical incidence, seven cases would have been expected in the study population (BMJ. 2015 Sep 23;351:h4848. doi: 10.1136/bmj.h4848).
Dr. Bergman and Dr. Troum reported having no financial conflicts regarding their presentation.
EXPERT ANALYSIS FROM RWCS 2016
Small fiber neuropathy common, vexing in sarcoidosis
MAUI, HAWAII – Small fiber neuropathy is a common and underappreciated expression of systemic sarcoidosis, Dr. Alvin F. Wells observed at the 2016 Rheumatology Winter Clinical Symposium.
Small fiber neuropathy (SFN) occurs in roughly 40% of patients with sarcoidosis. Affected patients present with painful neuropathic symptoms and/or dysautonomia. SFN is often mistaken for fibromyalgia syndrome. And it poses a special therapeutic challenge.
“It can be very, very difficult to treat. It’s generally resistant to methotrexate, even to corticosteroids, our treatment mainstay in sarcoidosis,” said Dr. Wells, director of the Rheumatology and Immunotherapy Center in Franklin, Wisc.
He credited researchers at the Cleveland Clinic with bringing intravenous immunoglobulin (IVIG) to wider attention as an effective treatment for refractory SFN associated with sarcoidosis (Respir Med. 2011 Jan;105[1]:101-5).
“Here you’re treating it more like an immune-mediated neuropathy,” Dr. Wells noted.”It’s expensive therapy, but when these patients have a positive biopsy and they’ve failed other types of treatment, these data show IVIG can achieve a good response.”
Indeed, the Cleveland Clinic physicians reported excellent success with IVIG dosed at 2 g/kg initially, then 1 g/kg in 2 weeks, followed by maintenance dosing at 1 g/kg every 4 weeks.
This sarcoidosis-associated neuropathy involves both myelinated and nonmyelinated small nerve fibers. The diagnosis is established by epidermal nerve fiber density testing. This entails taking three 3-mm skin punch biopsies, one each at the lateral proximal and distal thigh, the third 10 cm proximal to the lateral malleolus. Specimens obtained from these sites in normal individuals feature a rich density of small nerve fibers; in patients with SNF-associated sarcoidosis, there is a notable paucity of the fibers, the rheumatologist explained.
Treating organ involvement besides SNF
Studies have consistently shown that the lungs and thoracic lymph nodes are the organs most commonly involved in sarcoidosis, affecting more than 90% of patients. Indeed, respiratory symptoms are most often the complaint that brings patients in seeking medical attention. The skin is involved in about 30% of cases, the eyes in 20%-25%, and the liver or heart in roughly 20% each.
Osteoarticular involvement is uncommon. Moroccan investigators for a study presented at the 2015 European League Against Rheumatism (EULAR) meeting in Rome concluded that when osteoarticular sarcoidosis occurs, it most often takes the form of an inflammatory chronic polyarthritis (Ann Rheum Dis. 2015;74[Suppl2]:786). Bone involvement is rare but damaging and mostly affects small distal bones, Dr. Wells said.
With the exception of sarcoidosis-associated SNF, the other types of organ involvement typically respond well to corticosteroids.
“Dosing depends upon disease severity. Most of us use 1 mg/kg to get ocular disease under control,” according to the rheumatologist. “The disease is very organ-specific. So if someone has eye disease, we throw everything at them, including the kitchen sink, to make sure they don’t go blind.”
“Steroids are the mainstay,” Dr. Wells emphasized. “The question to ask is, ‘What can I find that’s steroid-sparing and yet keeps the same kind of clinical response?’ ”
It’s virtually all off-label therapy. There is a dearth of randomized, blinded, placebo-controlled treatment trials in sarcoidosis. Many, many agents have been tried as second-, third-, and fourth-line therapy, including various disease-modifying antirheumatic drugs, tumor necrosis factor inhibitors, and phosphodiesterase-4 inhibitors. Dr. Wells’ favorites are methotrexate, azathioprine, and mycophenolate mofetil. However, hydroxychloroquine works well for skin disease, chloroquine helps combat hypercalcemia, and pentoxifylline and thalidomide can be helpful in cases of treatment-resistant lupus pernio.
A study presented at the 2015 EULAR meeting in Rome showed high-dose methotrexate in the 25-30 mg/week range was significantly more effective than mycophenolate mofetil in preventing relapses of neurosarcoidosis (Ann Rheum Dis. 2015;74[Suppl2]:859). This study has caused Dr. Wells to change his own practice.
He noted that the oral phosphodiesterase-4 inhibitor apremilast (Otezla) at 20 mg/day added to background therapy achieved “really dramatic results” for refractory chronic cutaneous sarcoidosis in a 15-patient, open-label, phase II study (Arch Dermatol. 2012 Feb;148[2]:262-4). A definitive randomized, placebo-controlled trial is certainly warranted, he added.
A question often asked by patients with extrathoracic sarcoid is, “Am I at greater risk of mortality than if I just had pulmonary disease?” The answer is no, Dr. Wells said, citing a study presented by investigators from Barcelona at the 2015 EULAR meeting (Ann Rheum Dis. 2015;74[Suppl2]:404). Mortality during a mean follow-up of 107 months was 11% in patients with extrapulmonary involvement and similar at 14% in patients whose sarcoidosis was limited to the lungs.
He reported serving as a consultant to roughly a dozen pharmaceutical companies.
MAUI, HAWAII – Small fiber neuropathy is a common and underappreciated expression of systemic sarcoidosis, Dr. Alvin F. Wells observed at the 2016 Rheumatology Winter Clinical Symposium.
Small fiber neuropathy (SFN) occurs in roughly 40% of patients with sarcoidosis. Affected patients present with painful neuropathic symptoms and/or dysautonomia. SFN is often mistaken for fibromyalgia syndrome. And it poses a special therapeutic challenge.
“It can be very, very difficult to treat. It’s generally resistant to methotrexate, even to corticosteroids, our treatment mainstay in sarcoidosis,” said Dr. Wells, director of the Rheumatology and Immunotherapy Center in Franklin, Wisc.
He credited researchers at the Cleveland Clinic with bringing intravenous immunoglobulin (IVIG) to wider attention as an effective treatment for refractory SFN associated with sarcoidosis (Respir Med. 2011 Jan;105[1]:101-5).
“Here you’re treating it more like an immune-mediated neuropathy,” Dr. Wells noted.”It’s expensive therapy, but when these patients have a positive biopsy and they’ve failed other types of treatment, these data show IVIG can achieve a good response.”
Indeed, the Cleveland Clinic physicians reported excellent success with IVIG dosed at 2 g/kg initially, then 1 g/kg in 2 weeks, followed by maintenance dosing at 1 g/kg every 4 weeks.
This sarcoidosis-associated neuropathy involves both myelinated and nonmyelinated small nerve fibers. The diagnosis is established by epidermal nerve fiber density testing. This entails taking three 3-mm skin punch biopsies, one each at the lateral proximal and distal thigh, the third 10 cm proximal to the lateral malleolus. Specimens obtained from these sites in normal individuals feature a rich density of small nerve fibers; in patients with SNF-associated sarcoidosis, there is a notable paucity of the fibers, the rheumatologist explained.
Treating organ involvement besides SNF
Studies have consistently shown that the lungs and thoracic lymph nodes are the organs most commonly involved in sarcoidosis, affecting more than 90% of patients. Indeed, respiratory symptoms are most often the complaint that brings patients in seeking medical attention. The skin is involved in about 30% of cases, the eyes in 20%-25%, and the liver or heart in roughly 20% each.
Osteoarticular involvement is uncommon. Moroccan investigators for a study presented at the 2015 European League Against Rheumatism (EULAR) meeting in Rome concluded that when osteoarticular sarcoidosis occurs, it most often takes the form of an inflammatory chronic polyarthritis (Ann Rheum Dis. 2015;74[Suppl2]:786). Bone involvement is rare but damaging and mostly affects small distal bones, Dr. Wells said.
With the exception of sarcoidosis-associated SNF, the other types of organ involvement typically respond well to corticosteroids.
“Dosing depends upon disease severity. Most of us use 1 mg/kg to get ocular disease under control,” according to the rheumatologist. “The disease is very organ-specific. So if someone has eye disease, we throw everything at them, including the kitchen sink, to make sure they don’t go blind.”
“Steroids are the mainstay,” Dr. Wells emphasized. “The question to ask is, ‘What can I find that’s steroid-sparing and yet keeps the same kind of clinical response?’ ”
It’s virtually all off-label therapy. There is a dearth of randomized, blinded, placebo-controlled treatment trials in sarcoidosis. Many, many agents have been tried as second-, third-, and fourth-line therapy, including various disease-modifying antirheumatic drugs, tumor necrosis factor inhibitors, and phosphodiesterase-4 inhibitors. Dr. Wells’ favorites are methotrexate, azathioprine, and mycophenolate mofetil. However, hydroxychloroquine works well for skin disease, chloroquine helps combat hypercalcemia, and pentoxifylline and thalidomide can be helpful in cases of treatment-resistant lupus pernio.
A study presented at the 2015 EULAR meeting in Rome showed high-dose methotrexate in the 25-30 mg/week range was significantly more effective than mycophenolate mofetil in preventing relapses of neurosarcoidosis (Ann Rheum Dis. 2015;74[Suppl2]:859). This study has caused Dr. Wells to change his own practice.
He noted that the oral phosphodiesterase-4 inhibitor apremilast (Otezla) at 20 mg/day added to background therapy achieved “really dramatic results” for refractory chronic cutaneous sarcoidosis in a 15-patient, open-label, phase II study (Arch Dermatol. 2012 Feb;148[2]:262-4). A definitive randomized, placebo-controlled trial is certainly warranted, he added.
A question often asked by patients with extrathoracic sarcoid is, “Am I at greater risk of mortality than if I just had pulmonary disease?” The answer is no, Dr. Wells said, citing a study presented by investigators from Barcelona at the 2015 EULAR meeting (Ann Rheum Dis. 2015;74[Suppl2]:404). Mortality during a mean follow-up of 107 months was 11% in patients with extrapulmonary involvement and similar at 14% in patients whose sarcoidosis was limited to the lungs.
He reported serving as a consultant to roughly a dozen pharmaceutical companies.
MAUI, HAWAII – Small fiber neuropathy is a common and underappreciated expression of systemic sarcoidosis, Dr. Alvin F. Wells observed at the 2016 Rheumatology Winter Clinical Symposium.
Small fiber neuropathy (SFN) occurs in roughly 40% of patients with sarcoidosis. Affected patients present with painful neuropathic symptoms and/or dysautonomia. SFN is often mistaken for fibromyalgia syndrome. And it poses a special therapeutic challenge.
“It can be very, very difficult to treat. It’s generally resistant to methotrexate, even to corticosteroids, our treatment mainstay in sarcoidosis,” said Dr. Wells, director of the Rheumatology and Immunotherapy Center in Franklin, Wisc.
He credited researchers at the Cleveland Clinic with bringing intravenous immunoglobulin (IVIG) to wider attention as an effective treatment for refractory SFN associated with sarcoidosis (Respir Med. 2011 Jan;105[1]:101-5).
“Here you’re treating it more like an immune-mediated neuropathy,” Dr. Wells noted.”It’s expensive therapy, but when these patients have a positive biopsy and they’ve failed other types of treatment, these data show IVIG can achieve a good response.”
Indeed, the Cleveland Clinic physicians reported excellent success with IVIG dosed at 2 g/kg initially, then 1 g/kg in 2 weeks, followed by maintenance dosing at 1 g/kg every 4 weeks.
This sarcoidosis-associated neuropathy involves both myelinated and nonmyelinated small nerve fibers. The diagnosis is established by epidermal nerve fiber density testing. This entails taking three 3-mm skin punch biopsies, one each at the lateral proximal and distal thigh, the third 10 cm proximal to the lateral malleolus. Specimens obtained from these sites in normal individuals feature a rich density of small nerve fibers; in patients with SNF-associated sarcoidosis, there is a notable paucity of the fibers, the rheumatologist explained.
Treating organ involvement besides SNF
Studies have consistently shown that the lungs and thoracic lymph nodes are the organs most commonly involved in sarcoidosis, affecting more than 90% of patients. Indeed, respiratory symptoms are most often the complaint that brings patients in seeking medical attention. The skin is involved in about 30% of cases, the eyes in 20%-25%, and the liver or heart in roughly 20% each.
Osteoarticular involvement is uncommon. Moroccan investigators for a study presented at the 2015 European League Against Rheumatism (EULAR) meeting in Rome concluded that when osteoarticular sarcoidosis occurs, it most often takes the form of an inflammatory chronic polyarthritis (Ann Rheum Dis. 2015;74[Suppl2]:786). Bone involvement is rare but damaging and mostly affects small distal bones, Dr. Wells said.
With the exception of sarcoidosis-associated SNF, the other types of organ involvement typically respond well to corticosteroids.
“Dosing depends upon disease severity. Most of us use 1 mg/kg to get ocular disease under control,” according to the rheumatologist. “The disease is very organ-specific. So if someone has eye disease, we throw everything at them, including the kitchen sink, to make sure they don’t go blind.”
“Steroids are the mainstay,” Dr. Wells emphasized. “The question to ask is, ‘What can I find that’s steroid-sparing and yet keeps the same kind of clinical response?’ ”
It’s virtually all off-label therapy. There is a dearth of randomized, blinded, placebo-controlled treatment trials in sarcoidosis. Many, many agents have been tried as second-, third-, and fourth-line therapy, including various disease-modifying antirheumatic drugs, tumor necrosis factor inhibitors, and phosphodiesterase-4 inhibitors. Dr. Wells’ favorites are methotrexate, azathioprine, and mycophenolate mofetil. However, hydroxychloroquine works well for skin disease, chloroquine helps combat hypercalcemia, and pentoxifylline and thalidomide can be helpful in cases of treatment-resistant lupus pernio.
A study presented at the 2015 EULAR meeting in Rome showed high-dose methotrexate in the 25-30 mg/week range was significantly more effective than mycophenolate mofetil in preventing relapses of neurosarcoidosis (Ann Rheum Dis. 2015;74[Suppl2]:859). This study has caused Dr. Wells to change his own practice.
He noted that the oral phosphodiesterase-4 inhibitor apremilast (Otezla) at 20 mg/day added to background therapy achieved “really dramatic results” for refractory chronic cutaneous sarcoidosis in a 15-patient, open-label, phase II study (Arch Dermatol. 2012 Feb;148[2]:262-4). A definitive randomized, placebo-controlled trial is certainly warranted, he added.
A question often asked by patients with extrathoracic sarcoid is, “Am I at greater risk of mortality than if I just had pulmonary disease?” The answer is no, Dr. Wells said, citing a study presented by investigators from Barcelona at the 2015 EULAR meeting (Ann Rheum Dis. 2015;74[Suppl2]:404). Mortality during a mean follow-up of 107 months was 11% in patients with extrapulmonary involvement and similar at 14% in patients whose sarcoidosis was limited to the lungs.
He reported serving as a consultant to roughly a dozen pharmaceutical companies.
EXPERT ANALYSIS FROM RWCS 2016
How to beat apremilast-induced diarrhea
MAUI, HAWAII – If you’re going to prescribe apremilast for psoriasis or psoriatic arthritis – and more and more physicians are doing so because of the drug’s exceptional safety profile – you’d better get familiar with the oral phosphodiesterase-4 inhibitor’s gastrointestinal side effects, Dr. George M. Martin advised at the 2016 Rheumatology Winter Clinical Symposium.
“One of the biggest hurdles we have to deal with when we prescribe apremilast is the fact that there are these GI side effects,” said Dr. Martin, a dermatologist practicing in Maui and codirector of the rheumatology symposium.
Celgene, which markets apremilast (Otezla), sponsored an analysis of the pattern of diarrhea that emerged in the pooled results of the phase III ESTEEM 1 and 2 trials of apremilast at 30 mg twice daily for psoriasis and the PALACE 1-3 phase III psoriatic arthritis trials.
Diarrhea occurred in 16%-18% of patients on apremilast, a rate roughly threefold greater than in placebo-treated controls. Diarrhea onset was usually within the first 14 days of therapy. When it occurred, the duration was typically about 2 weeks.
“This you can relay to your patients so they’re not surprised if it happens,” the dermatologist said.
It’s a secretory diarrhea, and it is believed to be a classwide effect for the phosphodiesterase-4 (PDE-4) inhibitors. For example, roflumilast (Daliresp), an oral PDE-4 inhibitor used in the treatment of chronic obstructive pulmonary disease, has the same diarrhea issues. The mechanism has been worked out: The drug increases intracellular cyclic adenosine monophosphate, with resultant activation of chloride channels in crypts in the small bowel, which in turn leads to secretion of chloride ions. It takes the large bowel a couple of weeks to adapt. Caffeine causes diarrhea in some individuals through a similar mechanism.
Apremilast-related diarrhea often responds to the time-tested OTC remedies, including bismuth salicylate or fiber supplements. Alternatively, Dr. Martin said he is a fan of the oral prescription agent crofelemer (Fulyzaq) because of its exceptional safety, tolerability, and effectiveness. Plus, many residents of the garden islands of Hawaii like the idea of using a botanical derived from the latexlike sap – known as ‘dragon’s blood – of a South American tree. Crofelemer’s approved indication is the treatment of diarrhea associated with anti-HIV agents.
Diphenoxylate/atropine (Lomotil) is another effective prescription option.
Nausea and/or vomiting occurred in 15%-17% of apremilast-treated patients in the phase III trials. As with diarrhea, if nausea and/or vomiting is going to happen, it occurs early, within the first week or two. Dr. Martin said he finds in his own practice that the nausea/vomiting is less bothersome for patients than the diarrhea. Drug discontinuation due to any GI side effects is rarely necessary.
The nausea/vomiting is usually readily managed by encouraging affected patients to make sure that they’re well hydrated, take their apremilast with food, and eat smaller, more frequent meals. OTC diphenhydramine (Benadryl) is often effective, as are the usual prescription antiemetic agents.
Pharmaceutical industry data indicate apremilast has quickly captured a 17% share of the market for systemic psoriasis therapies. There is a good reason for that, according to Dr. Martin: “Dermatologists have historically been risk averse. And apremilast is arguably the safest systemic agent we have to treat psoriasis. The beauty of apremilast is it requires no laboratory monitoring. That makes it attractive to dermatologists who are concerned about systemic therapy. It’s why there has been a huge jump in adoption of apremilast.”
Apremilast is comparable to methotrexate in terms of efficacy as reflected in week 16 PASI-75 response rates of about 35%, meaning 35% of treated patients obtain at least a 75% improvement in Psoriasis Area and Severity Index scores, he continued. Apremilast is particularly effective for scalp and nail psoriasis, making it a good option for patients who have psoriasis at those sites but not extensive involvement elsewhere, which might call for the use of a more potent biologic agent.
Surveys indicate that 20% of dermatologists write 80% of all prescriptions for biologic agents used to treat psoriasis. The thinking was that apremilast would appeal to the 80% of dermatologists who have steered clear of the biologics, and that after becoming comfortable with apremilast, they might become more receptive to using biologics for their patients with an inadequate response to the oral PDE-4 inhibitor. That hasn’t happened yet.
“We’re not seeing apremilast function as the gateway drug we thought it would be. It’s just going to take some time for those prescribers either to refer their patients who aren’t getting a good response to the next doctor who’s more adept at treating with biologic agents, or perhaps they themselves will get more involved,” Dr. Martin predicted.
He reported serving on scientific advisory boards for, and/or as a consultant to, nine pharmaceutical companies.
MAUI, HAWAII – If you’re going to prescribe apremilast for psoriasis or psoriatic arthritis – and more and more physicians are doing so because of the drug’s exceptional safety profile – you’d better get familiar with the oral phosphodiesterase-4 inhibitor’s gastrointestinal side effects, Dr. George M. Martin advised at the 2016 Rheumatology Winter Clinical Symposium.
“One of the biggest hurdles we have to deal with when we prescribe apremilast is the fact that there are these GI side effects,” said Dr. Martin, a dermatologist practicing in Maui and codirector of the rheumatology symposium.
Celgene, which markets apremilast (Otezla), sponsored an analysis of the pattern of diarrhea that emerged in the pooled results of the phase III ESTEEM 1 and 2 trials of apremilast at 30 mg twice daily for psoriasis and the PALACE 1-3 phase III psoriatic arthritis trials.
Diarrhea occurred in 16%-18% of patients on apremilast, a rate roughly threefold greater than in placebo-treated controls. Diarrhea onset was usually within the first 14 days of therapy. When it occurred, the duration was typically about 2 weeks.
“This you can relay to your patients so they’re not surprised if it happens,” the dermatologist said.
It’s a secretory diarrhea, and it is believed to be a classwide effect for the phosphodiesterase-4 (PDE-4) inhibitors. For example, roflumilast (Daliresp), an oral PDE-4 inhibitor used in the treatment of chronic obstructive pulmonary disease, has the same diarrhea issues. The mechanism has been worked out: The drug increases intracellular cyclic adenosine monophosphate, with resultant activation of chloride channels in crypts in the small bowel, which in turn leads to secretion of chloride ions. It takes the large bowel a couple of weeks to adapt. Caffeine causes diarrhea in some individuals through a similar mechanism.
Apremilast-related diarrhea often responds to the time-tested OTC remedies, including bismuth salicylate or fiber supplements. Alternatively, Dr. Martin said he is a fan of the oral prescription agent crofelemer (Fulyzaq) because of its exceptional safety, tolerability, and effectiveness. Plus, many residents of the garden islands of Hawaii like the idea of using a botanical derived from the latexlike sap – known as ‘dragon’s blood – of a South American tree. Crofelemer’s approved indication is the treatment of diarrhea associated with anti-HIV agents.
Diphenoxylate/atropine (Lomotil) is another effective prescription option.
Nausea and/or vomiting occurred in 15%-17% of apremilast-treated patients in the phase III trials. As with diarrhea, if nausea and/or vomiting is going to happen, it occurs early, within the first week or two. Dr. Martin said he finds in his own practice that the nausea/vomiting is less bothersome for patients than the diarrhea. Drug discontinuation due to any GI side effects is rarely necessary.
The nausea/vomiting is usually readily managed by encouraging affected patients to make sure that they’re well hydrated, take their apremilast with food, and eat smaller, more frequent meals. OTC diphenhydramine (Benadryl) is often effective, as are the usual prescription antiemetic agents.
Pharmaceutical industry data indicate apremilast has quickly captured a 17% share of the market for systemic psoriasis therapies. There is a good reason for that, according to Dr. Martin: “Dermatologists have historically been risk averse. And apremilast is arguably the safest systemic agent we have to treat psoriasis. The beauty of apremilast is it requires no laboratory monitoring. That makes it attractive to dermatologists who are concerned about systemic therapy. It’s why there has been a huge jump in adoption of apremilast.”
Apremilast is comparable to methotrexate in terms of efficacy as reflected in week 16 PASI-75 response rates of about 35%, meaning 35% of treated patients obtain at least a 75% improvement in Psoriasis Area and Severity Index scores, he continued. Apremilast is particularly effective for scalp and nail psoriasis, making it a good option for patients who have psoriasis at those sites but not extensive involvement elsewhere, which might call for the use of a more potent biologic agent.
Surveys indicate that 20% of dermatologists write 80% of all prescriptions for biologic agents used to treat psoriasis. The thinking was that apremilast would appeal to the 80% of dermatologists who have steered clear of the biologics, and that after becoming comfortable with apremilast, they might become more receptive to using biologics for their patients with an inadequate response to the oral PDE-4 inhibitor. That hasn’t happened yet.
“We’re not seeing apremilast function as the gateway drug we thought it would be. It’s just going to take some time for those prescribers either to refer their patients who aren’t getting a good response to the next doctor who’s more adept at treating with biologic agents, or perhaps they themselves will get more involved,” Dr. Martin predicted.
He reported serving on scientific advisory boards for, and/or as a consultant to, nine pharmaceutical companies.
MAUI, HAWAII – If you’re going to prescribe apremilast for psoriasis or psoriatic arthritis – and more and more physicians are doing so because of the drug’s exceptional safety profile – you’d better get familiar with the oral phosphodiesterase-4 inhibitor’s gastrointestinal side effects, Dr. George M. Martin advised at the 2016 Rheumatology Winter Clinical Symposium.
“One of the biggest hurdles we have to deal with when we prescribe apremilast is the fact that there are these GI side effects,” said Dr. Martin, a dermatologist practicing in Maui and codirector of the rheumatology symposium.
Celgene, which markets apremilast (Otezla), sponsored an analysis of the pattern of diarrhea that emerged in the pooled results of the phase III ESTEEM 1 and 2 trials of apremilast at 30 mg twice daily for psoriasis and the PALACE 1-3 phase III psoriatic arthritis trials.
Diarrhea occurred in 16%-18% of patients on apremilast, a rate roughly threefold greater than in placebo-treated controls. Diarrhea onset was usually within the first 14 days of therapy. When it occurred, the duration was typically about 2 weeks.
“This you can relay to your patients so they’re not surprised if it happens,” the dermatologist said.
It’s a secretory diarrhea, and it is believed to be a classwide effect for the phosphodiesterase-4 (PDE-4) inhibitors. For example, roflumilast (Daliresp), an oral PDE-4 inhibitor used in the treatment of chronic obstructive pulmonary disease, has the same diarrhea issues. The mechanism has been worked out: The drug increases intracellular cyclic adenosine monophosphate, with resultant activation of chloride channels in crypts in the small bowel, which in turn leads to secretion of chloride ions. It takes the large bowel a couple of weeks to adapt. Caffeine causes diarrhea in some individuals through a similar mechanism.
Apremilast-related diarrhea often responds to the time-tested OTC remedies, including bismuth salicylate or fiber supplements. Alternatively, Dr. Martin said he is a fan of the oral prescription agent crofelemer (Fulyzaq) because of its exceptional safety, tolerability, and effectiveness. Plus, many residents of the garden islands of Hawaii like the idea of using a botanical derived from the latexlike sap – known as ‘dragon’s blood – of a South American tree. Crofelemer’s approved indication is the treatment of diarrhea associated with anti-HIV agents.
Diphenoxylate/atropine (Lomotil) is another effective prescription option.
Nausea and/or vomiting occurred in 15%-17% of apremilast-treated patients in the phase III trials. As with diarrhea, if nausea and/or vomiting is going to happen, it occurs early, within the first week or two. Dr. Martin said he finds in his own practice that the nausea/vomiting is less bothersome for patients than the diarrhea. Drug discontinuation due to any GI side effects is rarely necessary.
The nausea/vomiting is usually readily managed by encouraging affected patients to make sure that they’re well hydrated, take their apremilast with food, and eat smaller, more frequent meals. OTC diphenhydramine (Benadryl) is often effective, as are the usual prescription antiemetic agents.
Pharmaceutical industry data indicate apremilast has quickly captured a 17% share of the market for systemic psoriasis therapies. There is a good reason for that, according to Dr. Martin: “Dermatologists have historically been risk averse. And apremilast is arguably the safest systemic agent we have to treat psoriasis. The beauty of apremilast is it requires no laboratory monitoring. That makes it attractive to dermatologists who are concerned about systemic therapy. It’s why there has been a huge jump in adoption of apremilast.”
Apremilast is comparable to methotrexate in terms of efficacy as reflected in week 16 PASI-75 response rates of about 35%, meaning 35% of treated patients obtain at least a 75% improvement in Psoriasis Area and Severity Index scores, he continued. Apremilast is particularly effective for scalp and nail psoriasis, making it a good option for patients who have psoriasis at those sites but not extensive involvement elsewhere, which might call for the use of a more potent biologic agent.
Surveys indicate that 20% of dermatologists write 80% of all prescriptions for biologic agents used to treat psoriasis. The thinking was that apremilast would appeal to the 80% of dermatologists who have steered clear of the biologics, and that after becoming comfortable with apremilast, they might become more receptive to using biologics for their patients with an inadequate response to the oral PDE-4 inhibitor. That hasn’t happened yet.
“We’re not seeing apremilast function as the gateway drug we thought it would be. It’s just going to take some time for those prescribers either to refer their patients who aren’t getting a good response to the next doctor who’s more adept at treating with biologic agents, or perhaps they themselves will get more involved,” Dr. Martin predicted.
He reported serving on scientific advisory boards for, and/or as a consultant to, nine pharmaceutical companies.
EXPERT ANALYSIS FROM RWCS 2016
How to beat apremilast-induced diarrhea
MAUI, HAWAII – If you’re going to prescribe apremilast for psoriasis or psoriatic arthritis – and more and more physicians are doing so because of the drug’s exceptional safety profile – you’d better get familiar with the oral phosphodiesterase-4 inhibitor’s gastrointestinal side effects, Dr. George M. Martin advised at the 2016 Rheumatology Winter Clinical Symposium.
“One of the biggest hurdles we have to deal with when we prescribe apremilast is the fact that there are these GI side effects,” said Dr. Martin, a dermatologist practicing in Maui and codirector of the rheumatology symposium.
Celgene, which markets apremilast (Otezla), sponsored an analysis of the pattern of diarrhea that emerged in the pooled results of the phase III ESTEEM 1 and 2 trials of apremilast at 30 mg twice daily for psoriasis and the PALACE 1-3 phase III psoriatic arthritis trials.
Diarrhea occurred in 16%-18% of patients on apremilast, a rate roughly threefold greater than in placebo-treated controls. Diarrhea onset was usually within the first 14 days of therapy. When it occurred, the duration was typically about 2 weeks.
“This you can relay to your patients so they’re not surprised if it happens,” the dermatologist said.
It’s a secretory diarrhea, and it is believed to be a classwide effect for the phosphodiesterase-4 (PDE-4) inhibitors. For example, roflumilast (Daliresp), an oral PDE-4 inhibitor used in the treatment of chronic obstructive pulmonary disease, has the same diarrhea issues. The mechanism has been worked out: The drug increases intracellular cyclic adenosine monophosphate, with resultant activation of chloride channels in crypts in the small bowel, which in turn leads to secretion of chloride ions. It takes the large bowel a couple of weeks to adapt. Caffeine causes diarrhea in some individuals through a similar mechanism.
Apremilast-related diarrhea often responds to the time-tested OTC remedies, including bismuth salicylate or fiber supplements. Alternatively, Dr. Martin said he is a fan of the oral prescription agent crofelemer (Fulyzaq) because of its exceptional safety, tolerability, and effectiveness. Plus, many residents of the garden islands of Hawaii like the idea of using a botanical derived from the latexlike sap – known as ‘dragon’s blood – of a South American tree. Crofelemer’s approved indication is the treatment of diarrhea associated with anti-HIV agents.
Diphenoxylate/atropine (Lomotil) is another effective prescription option.
Nausea and/or vomiting occurred in 15%-17% of apremilast-treated patients in the phase III trials. As with diarrhea, if nausea and/or vomiting is going to happen, it occurs early, within the first week or two. Dr. Martin said he finds in his own practice that the nausea/vomiting is less bothersome for patients than the diarrhea. Drug discontinuation due to any GI side effects is rarely necessary.
The nausea/vomiting is usually readily managed by encouraging affected patients to make sure that they’re well hydrated, take their apremilast with food, and eat smaller, more frequent meals. OTC diphenhydramine (Benadryl) is often effective, as are the usual prescription antiemetic agents.
Pharmaceutical industry data indicate apremilast has quickly captured a 17% share of the market for systemic psoriasis therapies. There is a good reason for that, according to Dr. Martin: “Dermatologists have historically been risk averse. And apremilast is arguably the safest systemic agent we have to treat psoriasis. The beauty of apremilast is it requires no laboratory monitoring. That makes it attractive to dermatologists who are concerned about systemic therapy. It’s why there has been a huge jump in adoption of apremilast.”
Apremilast is comparable to methotrexate in terms of efficacy as reflected in week 16 PASI-75 response rates of about 35%, meaning 35% of treated patients obtain at least a 75% improvement in Psoriasis Area and Severity Index scores, he continued. Apremilast is particularly effective for scalp and nail psoriasis, making it a good option for patients who have psoriasis at those sites but not extensive involvement elsewhere, which might call for the use of a more potent biologic agent.
Surveys indicate that 20% of dermatologists write 80% of all prescriptions for biologic agents used to treat psoriasis. The thinking was that apremilast would appeal to the 80% of dermatologists who have steered clear of the biologics, and that after becoming comfortable with apremilast, they might become more receptive to using biologics for their patients with an inadequate response to the oral PDE-4 inhibitor. That hasn’t happened yet.
“We’re not seeing apremilast function as the gateway drug we thought it would be. It’s just going to take some time for those prescribers either to refer their patients who aren’t getting a good response to the next doctor who’s more adept at treating with biologic agents, or perhaps they themselves will get more involved,” Dr. Martin predicted.
He reported serving on scientific advisory boards for, and/or as a consultant to, nine pharmaceutical companies.
MAUI, HAWAII – If you’re going to prescribe apremilast for psoriasis or psoriatic arthritis – and more and more physicians are doing so because of the drug’s exceptional safety profile – you’d better get familiar with the oral phosphodiesterase-4 inhibitor’s gastrointestinal side effects, Dr. George M. Martin advised at the 2016 Rheumatology Winter Clinical Symposium.
“One of the biggest hurdles we have to deal with when we prescribe apremilast is the fact that there are these GI side effects,” said Dr. Martin, a dermatologist practicing in Maui and codirector of the rheumatology symposium.
Celgene, which markets apremilast (Otezla), sponsored an analysis of the pattern of diarrhea that emerged in the pooled results of the phase III ESTEEM 1 and 2 trials of apremilast at 30 mg twice daily for psoriasis and the PALACE 1-3 phase III psoriatic arthritis trials.
Diarrhea occurred in 16%-18% of patients on apremilast, a rate roughly threefold greater than in placebo-treated controls. Diarrhea onset was usually within the first 14 days of therapy. When it occurred, the duration was typically about 2 weeks.
“This you can relay to your patients so they’re not surprised if it happens,” the dermatologist said.
It’s a secretory diarrhea, and it is believed to be a classwide effect for the phosphodiesterase-4 (PDE-4) inhibitors. For example, roflumilast (Daliresp), an oral PDE-4 inhibitor used in the treatment of chronic obstructive pulmonary disease, has the same diarrhea issues. The mechanism has been worked out: The drug increases intracellular cyclic adenosine monophosphate, with resultant activation of chloride channels in crypts in the small bowel, which in turn leads to secretion of chloride ions. It takes the large bowel a couple of weeks to adapt. Caffeine causes diarrhea in some individuals through a similar mechanism.
Apremilast-related diarrhea often responds to the time-tested OTC remedies, including bismuth salicylate or fiber supplements. Alternatively, Dr. Martin said he is a fan of the oral prescription agent crofelemer (Fulyzaq) because of its exceptional safety, tolerability, and effectiveness. Plus, many residents of the garden islands of Hawaii like the idea of using a botanical derived from the latexlike sap – known as ‘dragon’s blood – of a South American tree. Crofelemer’s approved indication is the treatment of diarrhea associated with anti-HIV agents.
Diphenoxylate/atropine (Lomotil) is another effective prescription option.
Nausea and/or vomiting occurred in 15%-17% of apremilast-treated patients in the phase III trials. As with diarrhea, if nausea and/or vomiting is going to happen, it occurs early, within the first week or two. Dr. Martin said he finds in his own practice that the nausea/vomiting is less bothersome for patients than the diarrhea. Drug discontinuation due to any GI side effects is rarely necessary.
The nausea/vomiting is usually readily managed by encouraging affected patients to make sure that they’re well hydrated, take their apremilast with food, and eat smaller, more frequent meals. OTC diphenhydramine (Benadryl) is often effective, as are the usual prescription antiemetic agents.
Pharmaceutical industry data indicate apremilast has quickly captured a 17% share of the market for systemic psoriasis therapies. There is a good reason for that, according to Dr. Martin: “Dermatologists have historically been risk averse. And apremilast is arguably the safest systemic agent we have to treat psoriasis. The beauty of apremilast is it requires no laboratory monitoring. That makes it attractive to dermatologists who are concerned about systemic therapy. It’s why there has been a huge jump in adoption of apremilast.”
Apremilast is comparable to methotrexate in terms of efficacy as reflected in week 16 PASI-75 response rates of about 35%, meaning 35% of treated patients obtain at least a 75% improvement in Psoriasis Area and Severity Index scores, he continued. Apremilast is particularly effective for scalp and nail psoriasis, making it a good option for patients who have psoriasis at those sites but not extensive involvement elsewhere, which might call for the use of a more potent biologic agent.
Surveys indicate that 20% of dermatologists write 80% of all prescriptions for biologic agents used to treat psoriasis. The thinking was that apremilast would appeal to the 80% of dermatologists who have steered clear of the biologics, and that after becoming comfortable with apremilast, they might become more receptive to using biologics for their patients with an inadequate response to the oral PDE-4 inhibitor. That hasn’t happened yet.
“We’re not seeing apremilast function as the gateway drug we thought it would be. It’s just going to take some time for those prescribers either to refer their patients who aren’t getting a good response to the next doctor who’s more adept at treating with biologic agents, or perhaps they themselves will get more involved,” Dr. Martin predicted.
He reported serving on scientific advisory boards for, and/or as a consultant to, nine pharmaceutical companies.
MAUI, HAWAII – If you’re going to prescribe apremilast for psoriasis or psoriatic arthritis – and more and more physicians are doing so because of the drug’s exceptional safety profile – you’d better get familiar with the oral phosphodiesterase-4 inhibitor’s gastrointestinal side effects, Dr. George M. Martin advised at the 2016 Rheumatology Winter Clinical Symposium.
“One of the biggest hurdles we have to deal with when we prescribe apremilast is the fact that there are these GI side effects,” said Dr. Martin, a dermatologist practicing in Maui and codirector of the rheumatology symposium.
Celgene, which markets apremilast (Otezla), sponsored an analysis of the pattern of diarrhea that emerged in the pooled results of the phase III ESTEEM 1 and 2 trials of apremilast at 30 mg twice daily for psoriasis and the PALACE 1-3 phase III psoriatic arthritis trials.
Diarrhea occurred in 16%-18% of patients on apremilast, a rate roughly threefold greater than in placebo-treated controls. Diarrhea onset was usually within the first 14 days of therapy. When it occurred, the duration was typically about 2 weeks.
“This you can relay to your patients so they’re not surprised if it happens,” the dermatologist said.
It’s a secretory diarrhea, and it is believed to be a classwide effect for the phosphodiesterase-4 (PDE-4) inhibitors. For example, roflumilast (Daliresp), an oral PDE-4 inhibitor used in the treatment of chronic obstructive pulmonary disease, has the same diarrhea issues. The mechanism has been worked out: The drug increases intracellular cyclic adenosine monophosphate, with resultant activation of chloride channels in crypts in the small bowel, which in turn leads to secretion of chloride ions. It takes the large bowel a couple of weeks to adapt. Caffeine causes diarrhea in some individuals through a similar mechanism.
Apremilast-related diarrhea often responds to the time-tested OTC remedies, including bismuth salicylate or fiber supplements. Alternatively, Dr. Martin said he is a fan of the oral prescription agent crofelemer (Fulyzaq) because of its exceptional safety, tolerability, and effectiveness. Plus, many residents of the garden islands of Hawaii like the idea of using a botanical derived from the latexlike sap – known as ‘dragon’s blood – of a South American tree. Crofelemer’s approved indication is the treatment of diarrhea associated with anti-HIV agents.
Diphenoxylate/atropine (Lomotil) is another effective prescription option.
Nausea and/or vomiting occurred in 15%-17% of apremilast-treated patients in the phase III trials. As with diarrhea, if nausea and/or vomiting is going to happen, it occurs early, within the first week or two. Dr. Martin said he finds in his own practice that the nausea/vomiting is less bothersome for patients than the diarrhea. Drug discontinuation due to any GI side effects is rarely necessary.
The nausea/vomiting is usually readily managed by encouraging affected patients to make sure that they’re well hydrated, take their apremilast with food, and eat smaller, more frequent meals. OTC diphenhydramine (Benadryl) is often effective, as are the usual prescription antiemetic agents.
Pharmaceutical industry data indicate apremilast has quickly captured a 17% share of the market for systemic psoriasis therapies. There is a good reason for that, according to Dr. Martin: “Dermatologists have historically been risk averse. And apremilast is arguably the safest systemic agent we have to treat psoriasis. The beauty of apremilast is it requires no laboratory monitoring. That makes it attractive to dermatologists who are concerned about systemic therapy. It’s why there has been a huge jump in adoption of apremilast.”
Apremilast is comparable to methotrexate in terms of efficacy as reflected in week 16 PASI-75 response rates of about 35%, meaning 35% of treated patients obtain at least a 75% improvement in Psoriasis Area and Severity Index scores, he continued. Apremilast is particularly effective for scalp and nail psoriasis, making it a good option for patients who have psoriasis at those sites but not extensive involvement elsewhere, which might call for the use of a more potent biologic agent.
Surveys indicate that 20% of dermatologists write 80% of all prescriptions for biologic agents used to treat psoriasis. The thinking was that apremilast would appeal to the 80% of dermatologists who have steered clear of the biologics, and that after becoming comfortable with apremilast, they might become more receptive to using biologics for their patients with an inadequate response to the oral PDE-4 inhibitor. That hasn’t happened yet.
“We’re not seeing apremilast function as the gateway drug we thought it would be. It’s just going to take some time for those prescribers either to refer their patients who aren’t getting a good response to the next doctor who’s more adept at treating with biologic agents, or perhaps they themselves will get more involved,” Dr. Martin predicted.
He reported serving on scientific advisory boards for, and/or as a consultant to, nine pharmaceutical companies.
EXPERT ANALYSIS FROM RWCS 2016
Getting a leg up on bone comorbidities in lupus
MAUI, HAWAII – Patients with systemic lupus erythematosus (SLE) really need to be placed on bone protection therapy as soon as they start on corticosteroids because their risks of steroid-related osteoporosis and osteonecrosis are so high, Dr. Dafna D. Gladman advised at the 2016 Rheumatology Winter Clinical Symposium.
Investigators for new studies of large cohorts of SLE patients seen at the University of Toronto Lupus Clinic have examined numerous potential predictors of bone comorbidities, but only one independent risk factor emerged: a high cumulative dose of corticosteroids, said Dr. Gladman, professor of medicine and codirector of the clinic.
“The dose of steroids is certainly important. It’s relevant to patient management because we obviously want to try to minimize the amount of steroids that patients with lupus get,” the rheumatologist observed.
The Toronto experience underscores just how common and serious these bone comorbidities are.
Among 1,729 SLE patients in the clinic database, 13.6% developed symptomatic osteonecrosis as defined by clinical symptoms plus positive imaging findings. Overall, 86% were female. The mean age at diagnosis of SLE was 26.6 years, with a mean 8.2-year interval from SLE diagnosis to the first episode of osteonecrosis. The 235 patients with osteonecrosis had a collective 382 affected joints at the time of their first osteonecrosis diagnosis, with an additional 160 joints becoming osteonecrotic later.
Particularly noteworthy was the finding that fully 47% of patients had more than one site involved at the time of their first osteonecrotic event, according to Dr. Gladman.
By far the most frequently affected joints were the hips, followed by knees. Surgery was often required in order to manage the injuries. Indeed, surgery was performed on more than half of osteonecrotic hips, 30% of affected wrists, and 20% of knees.
In a multivariate regression analysis involving 162 SLE patients with osteonecrosis and an equal number of matched controls who had SLE but not osteonecrosis, the only independent predictor of osteonecrosis was a high cumulative dose of corticosteroids; in the osteonecrosis group, it averaged 31 g. Factors that didn’t pan out as predictors included patient age, gender, race, smoking status, current or past use of antimalarial drugs, duration of immunosuppressive therapy, disease severity as reflected by the adjusted mean SLE Disease Activity Index score 3 years prior to osteonecrosis or the last clinic visit, total serum cholesterol, and a history of Raynaud’s, vasculitis, or renal or CNS involvement.
Turning to osteoporosis in SLE patients, Dr. Gladman said that among 286 patients who underwent bone mineral density (BMD) measurement at the time they were first seen in the clinic, 31.5% had an abnormal result. Among the 173 premenopausal females, 17% had a BMD below the lower limit of normal for their age. So did 27% of men below age 50. In postmenopausal women, the prevalences of osteoporosis and osteopenia were 12% and 43%, respectively. One of 10 men over age 50 had osteoporosis, while 8 had low BMD.
Twenty patients had a symptomatic fragility fracture at the time of their BMD test, and, of note, only half of them had an abnormal BMD.
“So the BMD does not actually identify all those patients who are at risk for the adverse outcome of osteoporosis, which will be a fragility fracture,” Dr. Gladman said.
She reported having no financial conflicts of interest regarding her presentation.
MAUI, HAWAII – Patients with systemic lupus erythematosus (SLE) really need to be placed on bone protection therapy as soon as they start on corticosteroids because their risks of steroid-related osteoporosis and osteonecrosis are so high, Dr. Dafna D. Gladman advised at the 2016 Rheumatology Winter Clinical Symposium.
Investigators for new studies of large cohorts of SLE patients seen at the University of Toronto Lupus Clinic have examined numerous potential predictors of bone comorbidities, but only one independent risk factor emerged: a high cumulative dose of corticosteroids, said Dr. Gladman, professor of medicine and codirector of the clinic.
“The dose of steroids is certainly important. It’s relevant to patient management because we obviously want to try to minimize the amount of steroids that patients with lupus get,” the rheumatologist observed.
The Toronto experience underscores just how common and serious these bone comorbidities are.
Among 1,729 SLE patients in the clinic database, 13.6% developed symptomatic osteonecrosis as defined by clinical symptoms plus positive imaging findings. Overall, 86% were female. The mean age at diagnosis of SLE was 26.6 years, with a mean 8.2-year interval from SLE diagnosis to the first episode of osteonecrosis. The 235 patients with osteonecrosis had a collective 382 affected joints at the time of their first osteonecrosis diagnosis, with an additional 160 joints becoming osteonecrotic later.
Particularly noteworthy was the finding that fully 47% of patients had more than one site involved at the time of their first osteonecrotic event, according to Dr. Gladman.
By far the most frequently affected joints were the hips, followed by knees. Surgery was often required in order to manage the injuries. Indeed, surgery was performed on more than half of osteonecrotic hips, 30% of affected wrists, and 20% of knees.
In a multivariate regression analysis involving 162 SLE patients with osteonecrosis and an equal number of matched controls who had SLE but not osteonecrosis, the only independent predictor of osteonecrosis was a high cumulative dose of corticosteroids; in the osteonecrosis group, it averaged 31 g. Factors that didn’t pan out as predictors included patient age, gender, race, smoking status, current or past use of antimalarial drugs, duration of immunosuppressive therapy, disease severity as reflected by the adjusted mean SLE Disease Activity Index score 3 years prior to osteonecrosis or the last clinic visit, total serum cholesterol, and a history of Raynaud’s, vasculitis, or renal or CNS involvement.
Turning to osteoporosis in SLE patients, Dr. Gladman said that among 286 patients who underwent bone mineral density (BMD) measurement at the time they were first seen in the clinic, 31.5% had an abnormal result. Among the 173 premenopausal females, 17% had a BMD below the lower limit of normal for their age. So did 27% of men below age 50. In postmenopausal women, the prevalences of osteoporosis and osteopenia were 12% and 43%, respectively. One of 10 men over age 50 had osteoporosis, while 8 had low BMD.
Twenty patients had a symptomatic fragility fracture at the time of their BMD test, and, of note, only half of them had an abnormal BMD.
“So the BMD does not actually identify all those patients who are at risk for the adverse outcome of osteoporosis, which will be a fragility fracture,” Dr. Gladman said.
She reported having no financial conflicts of interest regarding her presentation.
MAUI, HAWAII – Patients with systemic lupus erythematosus (SLE) really need to be placed on bone protection therapy as soon as they start on corticosteroids because their risks of steroid-related osteoporosis and osteonecrosis are so high, Dr. Dafna D. Gladman advised at the 2016 Rheumatology Winter Clinical Symposium.
Investigators for new studies of large cohorts of SLE patients seen at the University of Toronto Lupus Clinic have examined numerous potential predictors of bone comorbidities, but only one independent risk factor emerged: a high cumulative dose of corticosteroids, said Dr. Gladman, professor of medicine and codirector of the clinic.
“The dose of steroids is certainly important. It’s relevant to patient management because we obviously want to try to minimize the amount of steroids that patients with lupus get,” the rheumatologist observed.
The Toronto experience underscores just how common and serious these bone comorbidities are.
Among 1,729 SLE patients in the clinic database, 13.6% developed symptomatic osteonecrosis as defined by clinical symptoms plus positive imaging findings. Overall, 86% were female. The mean age at diagnosis of SLE was 26.6 years, with a mean 8.2-year interval from SLE diagnosis to the first episode of osteonecrosis. The 235 patients with osteonecrosis had a collective 382 affected joints at the time of their first osteonecrosis diagnosis, with an additional 160 joints becoming osteonecrotic later.
Particularly noteworthy was the finding that fully 47% of patients had more than one site involved at the time of their first osteonecrotic event, according to Dr. Gladman.
By far the most frequently affected joints were the hips, followed by knees. Surgery was often required in order to manage the injuries. Indeed, surgery was performed on more than half of osteonecrotic hips, 30% of affected wrists, and 20% of knees.
In a multivariate regression analysis involving 162 SLE patients with osteonecrosis and an equal number of matched controls who had SLE but not osteonecrosis, the only independent predictor of osteonecrosis was a high cumulative dose of corticosteroids; in the osteonecrosis group, it averaged 31 g. Factors that didn’t pan out as predictors included patient age, gender, race, smoking status, current or past use of antimalarial drugs, duration of immunosuppressive therapy, disease severity as reflected by the adjusted mean SLE Disease Activity Index score 3 years prior to osteonecrosis or the last clinic visit, total serum cholesterol, and a history of Raynaud’s, vasculitis, or renal or CNS involvement.
Turning to osteoporosis in SLE patients, Dr. Gladman said that among 286 patients who underwent bone mineral density (BMD) measurement at the time they were first seen in the clinic, 31.5% had an abnormal result. Among the 173 premenopausal females, 17% had a BMD below the lower limit of normal for their age. So did 27% of men below age 50. In postmenopausal women, the prevalences of osteoporosis and osteopenia were 12% and 43%, respectively. One of 10 men over age 50 had osteoporosis, while 8 had low BMD.
Twenty patients had a symptomatic fragility fracture at the time of their BMD test, and, of note, only half of them had an abnormal BMD.
“So the BMD does not actually identify all those patients who are at risk for the adverse outcome of osteoporosis, which will be a fragility fracture,” Dr. Gladman said.
She reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM RWCS 2016
Mongersen could be an impressive new treatment for inflammatory bowel disease
MAUI, HAWAII – Oral mongersen shows promise of being an ideal treatment for inflammatory bowel disease (IBD) if the promising phase II results are confirmed in phase III clinical trials, Dr. Uma Mahadevan, AGAF, said at the 2016 Rheumatology Winter Clinical Symposium.*
This perfect drug for IBD would be something that’s effective, safe, and well tolerated; orally administered; shows long-term efficacy; and can be dosed as needed. Preliminary indications are that mongersen may check off those boxes, according to Dr. Mahadevan, professor of medicine and joint medical director of the Center for Colitis and Crohn’s Disease at the University of California, San Francisco.
An Italian double-blind, placebo-controlled, phase II study of mongersen in Crohn’s disease generated enormous attention among IBD patients and gastroenterologists, with reported day 15 clinical remission rates of 55% and 65% at 40 and 160 mg/day, respectively (N Engl J Med. 2015 Mar 19;372:1104-13).
“We haven’t seen numbers like that in a Crohn’s trial since the very first Remicade [infliximab] trial. So there’s a lot of excitement about it. The study mechanics are a little suspect, but if the data in the current phase III trials are half as good, mongersen may be what we’re looking for,” Dr. Mahadevan said.
Mongersen is an oligonucleotide that inhibits ileal and colonic SMAD7, a protein that prevents transforming growth factor beta1–mediated suppression of inflammatory genes.
Why was a gastroenterologist providing an IBD update at a rheumatology conference? Dr. Mahadevan was invited in order to provide the conference’s annual “Outside the Box” feature, in which a nonrheumatologist expert shares the latest thinking about a disease relevant to rheumatology.
IBD fills the bill for several reasons. For one, many of the extraintestinal manifestations of IBD overlap with rheumatologic conditions. These include uveitis, arthritis, and arthralgias; osteopenia and osteoporosis; psoriasiform skin lesions; and thromboembolism. It’s not unusual for these extraintestinal manifestations to bring a patient to a nongastroenterologist before the diagnosis of IBD has been made.
Also, gastroenterologists and rheumatologists use a lot of the same drugs.
“The only drug gastroenterologists use for IBD that didn’t come from rheumatology first is vedolizumab [Entyvio],” she observed.
Ustekinumab (Stelara), which Dr. Mahadevan and other IBD experts have been using off-label for refractory Crohn’s disease with what she termed “very good” results, is expected to receive an Food and Drug Administration indication for Crohn’s disease by year’s end on the strength of impressive phase III data reported last fall.
“When approved, ustekinumab should be a first-line therapy, parallel to the TNF [tumor necrosis factor] inhibitors,” she predicted.
Another reason it’s worthwhile for rheumatologists to be up to date on IBD is that in many parts of the country, some gastroenterologists in community practice are uncomfortable using TNF inhibitors, which are now standard therapy for IBD. They may refer those patients to their local rheumatologist. But be advised: the dosing is very different from in rheumatology.
“For example, you start adalimumab [Humira] at 40 mg every other week for rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis, but we [gastroenterologists] do a loading dose of four 40-mg injections on day 1, then 80 mg 2 weeks later, and then maintenance dosing at 40 mg every 2 weeks. Sometimes in our patient population you’re not going to get a response unless you use a high dose like that,” the gastroenterologist explained.
The incidence of IBD is climbing. It was estimated at 5-7 cases per 100,000 population in the late 1990s, but it’s now believed to be 7-12 per 100,000. The peak age of onset is still in 15- to 30-year-olds, but now physicians are seeing a strong second peak later in life, among individuals in their 60s and 70s. And while Caucasians and Ashkenazi Jews have traditionally been thought of as the groups at increased risk for IBD, treatment centers are now springing up in India, China, and other countries where IBD was formerly thought to be less common.
“What is happening? Is it that because of better medical care we’re now picking up more cases outside of North America and Europe? Or is it a change in the environment – the microbiome, the Westernized diet, pollution? We really don’t know. But this is where a lot of study is going,” according to Dr. Mahadevan.
Something nongastroenterologists need to know about the clinical care of IBD patients is that even though TNF inhibitors are now considered standard therapy for patients with more severe disease and these biologic agents are routinely used in IBD centers of excellence, their penetration into community practice has been slow. Although high-dose anti-TNF therapy is supposed to be employed in ulcerative colitis patients who are refractory to corticosteroid induction therapy or who are steroid dependent, it’s fairly common in some parts of the country for a patient to undergo colectomy without ever having received an anti-TNF biologic.
“If you’re in a surgery-dominated area, the surgeons were very influenced by a Cleveland Clinic paper that said patients who received infliximab had more complications after surgery. There were a lot of caveats to that paper, but colorectal surgeons don’t want you to use a TNF inhibitor if they feel the patient is going to surgery,” she said.
In addition to mongersen, other novel agents targeting new pathways in IBD include the oral Janus-associated kinase inhibitor tofacitinib (Xeljanz), under study for both ulcerative colitis and Crohn’s disease; ozanimod, an oral sphingosine 1–phosphate 1 and 5 receptor modulator in clinical trials for ulcerative colitis; and the humanized monoclonal antibody etrolizumab, a selective anti-integrin agent.
Dr. Mahadevan reported serving as a consultant to and/or research grant recipient from more than half a dozen pharmaceutical companies.
*Changes were made to this story on March 24, 2016.
MAUI, HAWAII – Oral mongersen shows promise of being an ideal treatment for inflammatory bowel disease (IBD) if the promising phase II results are confirmed in phase III clinical trials, Dr. Uma Mahadevan, AGAF, said at the 2016 Rheumatology Winter Clinical Symposium.*
This perfect drug for IBD would be something that’s effective, safe, and well tolerated; orally administered; shows long-term efficacy; and can be dosed as needed. Preliminary indications are that mongersen may check off those boxes, according to Dr. Mahadevan, professor of medicine and joint medical director of the Center for Colitis and Crohn’s Disease at the University of California, San Francisco.
An Italian double-blind, placebo-controlled, phase II study of mongersen in Crohn’s disease generated enormous attention among IBD patients and gastroenterologists, with reported day 15 clinical remission rates of 55% and 65% at 40 and 160 mg/day, respectively (N Engl J Med. 2015 Mar 19;372:1104-13).
“We haven’t seen numbers like that in a Crohn’s trial since the very first Remicade [infliximab] trial. So there’s a lot of excitement about it. The study mechanics are a little suspect, but if the data in the current phase III trials are half as good, mongersen may be what we’re looking for,” Dr. Mahadevan said.
Mongersen is an oligonucleotide that inhibits ileal and colonic SMAD7, a protein that prevents transforming growth factor beta1–mediated suppression of inflammatory genes.
Why was a gastroenterologist providing an IBD update at a rheumatology conference? Dr. Mahadevan was invited in order to provide the conference’s annual “Outside the Box” feature, in which a nonrheumatologist expert shares the latest thinking about a disease relevant to rheumatology.
IBD fills the bill for several reasons. For one, many of the extraintestinal manifestations of IBD overlap with rheumatologic conditions. These include uveitis, arthritis, and arthralgias; osteopenia and osteoporosis; psoriasiform skin lesions; and thromboembolism. It’s not unusual for these extraintestinal manifestations to bring a patient to a nongastroenterologist before the diagnosis of IBD has been made.
Also, gastroenterologists and rheumatologists use a lot of the same drugs.
“The only drug gastroenterologists use for IBD that didn’t come from rheumatology first is vedolizumab [Entyvio],” she observed.
Ustekinumab (Stelara), which Dr. Mahadevan and other IBD experts have been using off-label for refractory Crohn’s disease with what she termed “very good” results, is expected to receive an Food and Drug Administration indication for Crohn’s disease by year’s end on the strength of impressive phase III data reported last fall.
“When approved, ustekinumab should be a first-line therapy, parallel to the TNF [tumor necrosis factor] inhibitors,” she predicted.
Another reason it’s worthwhile for rheumatologists to be up to date on IBD is that in many parts of the country, some gastroenterologists in community practice are uncomfortable using TNF inhibitors, which are now standard therapy for IBD. They may refer those patients to their local rheumatologist. But be advised: the dosing is very different from in rheumatology.
“For example, you start adalimumab [Humira] at 40 mg every other week for rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis, but we [gastroenterologists] do a loading dose of four 40-mg injections on day 1, then 80 mg 2 weeks later, and then maintenance dosing at 40 mg every 2 weeks. Sometimes in our patient population you’re not going to get a response unless you use a high dose like that,” the gastroenterologist explained.
The incidence of IBD is climbing. It was estimated at 5-7 cases per 100,000 population in the late 1990s, but it’s now believed to be 7-12 per 100,000. The peak age of onset is still in 15- to 30-year-olds, but now physicians are seeing a strong second peak later in life, among individuals in their 60s and 70s. And while Caucasians and Ashkenazi Jews have traditionally been thought of as the groups at increased risk for IBD, treatment centers are now springing up in India, China, and other countries where IBD was formerly thought to be less common.
“What is happening? Is it that because of better medical care we’re now picking up more cases outside of North America and Europe? Or is it a change in the environment – the microbiome, the Westernized diet, pollution? We really don’t know. But this is where a lot of study is going,” according to Dr. Mahadevan.
Something nongastroenterologists need to know about the clinical care of IBD patients is that even though TNF inhibitors are now considered standard therapy for patients with more severe disease and these biologic agents are routinely used in IBD centers of excellence, their penetration into community practice has been slow. Although high-dose anti-TNF therapy is supposed to be employed in ulcerative colitis patients who are refractory to corticosteroid induction therapy or who are steroid dependent, it’s fairly common in some parts of the country for a patient to undergo colectomy without ever having received an anti-TNF biologic.
“If you’re in a surgery-dominated area, the surgeons were very influenced by a Cleveland Clinic paper that said patients who received infliximab had more complications after surgery. There were a lot of caveats to that paper, but colorectal surgeons don’t want you to use a TNF inhibitor if they feel the patient is going to surgery,” she said.
In addition to mongersen, other novel agents targeting new pathways in IBD include the oral Janus-associated kinase inhibitor tofacitinib (Xeljanz), under study for both ulcerative colitis and Crohn’s disease; ozanimod, an oral sphingosine 1–phosphate 1 and 5 receptor modulator in clinical trials for ulcerative colitis; and the humanized monoclonal antibody etrolizumab, a selective anti-integrin agent.
Dr. Mahadevan reported serving as a consultant to and/or research grant recipient from more than half a dozen pharmaceutical companies.
*Changes were made to this story on March 24, 2016.
MAUI, HAWAII – Oral mongersen shows promise of being an ideal treatment for inflammatory bowel disease (IBD) if the promising phase II results are confirmed in phase III clinical trials, Dr. Uma Mahadevan, AGAF, said at the 2016 Rheumatology Winter Clinical Symposium.*
This perfect drug for IBD would be something that’s effective, safe, and well tolerated; orally administered; shows long-term efficacy; and can be dosed as needed. Preliminary indications are that mongersen may check off those boxes, according to Dr. Mahadevan, professor of medicine and joint medical director of the Center for Colitis and Crohn’s Disease at the University of California, San Francisco.
An Italian double-blind, placebo-controlled, phase II study of mongersen in Crohn’s disease generated enormous attention among IBD patients and gastroenterologists, with reported day 15 clinical remission rates of 55% and 65% at 40 and 160 mg/day, respectively (N Engl J Med. 2015 Mar 19;372:1104-13).
“We haven’t seen numbers like that in a Crohn’s trial since the very first Remicade [infliximab] trial. So there’s a lot of excitement about it. The study mechanics are a little suspect, but if the data in the current phase III trials are half as good, mongersen may be what we’re looking for,” Dr. Mahadevan said.
Mongersen is an oligonucleotide that inhibits ileal and colonic SMAD7, a protein that prevents transforming growth factor beta1–mediated suppression of inflammatory genes.
Why was a gastroenterologist providing an IBD update at a rheumatology conference? Dr. Mahadevan was invited in order to provide the conference’s annual “Outside the Box” feature, in which a nonrheumatologist expert shares the latest thinking about a disease relevant to rheumatology.
IBD fills the bill for several reasons. For one, many of the extraintestinal manifestations of IBD overlap with rheumatologic conditions. These include uveitis, arthritis, and arthralgias; osteopenia and osteoporosis; psoriasiform skin lesions; and thromboembolism. It’s not unusual for these extraintestinal manifestations to bring a patient to a nongastroenterologist before the diagnosis of IBD has been made.
Also, gastroenterologists and rheumatologists use a lot of the same drugs.
“The only drug gastroenterologists use for IBD that didn’t come from rheumatology first is vedolizumab [Entyvio],” she observed.
Ustekinumab (Stelara), which Dr. Mahadevan and other IBD experts have been using off-label for refractory Crohn’s disease with what she termed “very good” results, is expected to receive an Food and Drug Administration indication for Crohn’s disease by year’s end on the strength of impressive phase III data reported last fall.
“When approved, ustekinumab should be a first-line therapy, parallel to the TNF [tumor necrosis factor] inhibitors,” she predicted.
Another reason it’s worthwhile for rheumatologists to be up to date on IBD is that in many parts of the country, some gastroenterologists in community practice are uncomfortable using TNF inhibitors, which are now standard therapy for IBD. They may refer those patients to their local rheumatologist. But be advised: the dosing is very different from in rheumatology.
“For example, you start adalimumab [Humira] at 40 mg every other week for rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis, but we [gastroenterologists] do a loading dose of four 40-mg injections on day 1, then 80 mg 2 weeks later, and then maintenance dosing at 40 mg every 2 weeks. Sometimes in our patient population you’re not going to get a response unless you use a high dose like that,” the gastroenterologist explained.
The incidence of IBD is climbing. It was estimated at 5-7 cases per 100,000 population in the late 1990s, but it’s now believed to be 7-12 per 100,000. The peak age of onset is still in 15- to 30-year-olds, but now physicians are seeing a strong second peak later in life, among individuals in their 60s and 70s. And while Caucasians and Ashkenazi Jews have traditionally been thought of as the groups at increased risk for IBD, treatment centers are now springing up in India, China, and other countries where IBD was formerly thought to be less common.
“What is happening? Is it that because of better medical care we’re now picking up more cases outside of North America and Europe? Or is it a change in the environment – the microbiome, the Westernized diet, pollution? We really don’t know. But this is where a lot of study is going,” according to Dr. Mahadevan.
Something nongastroenterologists need to know about the clinical care of IBD patients is that even though TNF inhibitors are now considered standard therapy for patients with more severe disease and these biologic agents are routinely used in IBD centers of excellence, their penetration into community practice has been slow. Although high-dose anti-TNF therapy is supposed to be employed in ulcerative colitis patients who are refractory to corticosteroid induction therapy or who are steroid dependent, it’s fairly common in some parts of the country for a patient to undergo colectomy without ever having received an anti-TNF biologic.
“If you’re in a surgery-dominated area, the surgeons were very influenced by a Cleveland Clinic paper that said patients who received infliximab had more complications after surgery. There were a lot of caveats to that paper, but colorectal surgeons don’t want you to use a TNF inhibitor if they feel the patient is going to surgery,” she said.
In addition to mongersen, other novel agents targeting new pathways in IBD include the oral Janus-associated kinase inhibitor tofacitinib (Xeljanz), under study for both ulcerative colitis and Crohn’s disease; ozanimod, an oral sphingosine 1–phosphate 1 and 5 receptor modulator in clinical trials for ulcerative colitis; and the humanized monoclonal antibody etrolizumab, a selective anti-integrin agent.
Dr. Mahadevan reported serving as a consultant to and/or research grant recipient from more than half a dozen pharmaceutical companies.
*Changes were made to this story on March 24, 2016.
EXPERT ANALYSIS FROM RWCS 2016