User login
Society for Laproendoscopic Surgeons (SLS): Minimally Invasive Surgery Week
Thumbs-up on laparoscopic splenectomy for malignancies
LAS VEGAS – Laparoscopic splenectomy for hematologic malignancies involving the spleen is an effective surgical strategy, although the operator must expect a higher rate of conversion to laparotomy than when laparoscopic splenectomy is performed for benign hematologic diseases, Dr. Roberta Gelmini said at the annual Minimally Invasive Surgery Week.
The rate of postoperative complications, including wound infection, abdominal abscess, hemiperitoneum, and splenic vein thrombosis, is low and similar to that associated with laparoscopic splenectomy for benign hematologic diseases. The one exception is the complication of pleural effusion, which is seven- to eightfold more frequent following laparoscopic splenectomy for malignancies, probably reflecting affected patients’ larger spleen size and higher conversion rate, observed Dr. Gelmini, professor of surgery at the University of Modena and Reggio Emilia (Italy).
She presented a retrospective analysis of a series of 126 consecutive elective laparoscopic splenectomies performed in adults. Fifty-five were performed for malignant diseases, 71 for benign hematologic conditions. The two groups were well matched except that the patients with benign hematologic diseases were younger and thinner, with a mean age of 39 and a body mass index of 24.4 kg/m2, as compared with 55 years and 25.9 kg/m2 in patients undergoing laparoscopic splenectomy for hematologic malignancies.
The No. 1 indication for splenectomy in patients with benign disease was idiopathic thrombocytopenic purpura, accounting for 61% of cases. In patients with malignancies, non-Hodgkin’s lymphoma was the indication for splenectomy in two-thirds of cases.
As part of their preoperative work-up, all patients underwent ultrasound to establish spleen size and vessel diameter. Bipolar splenic length was significantly greater in the group with malignancies: a mean of 17 cm, compared with 13.4 cm in patients with benign disease.
With regard to key intraoperative findings, the mean 148-minute operative time in patients with malignancies was 22 minutes longer than in patients with benign disease. The conversion rate was 18.2% in patients with malignancies, compared with 5.6% when laparoscopic splenectomy was undertaken for benign disease. But the incidence of intraoperative blood loss greater than 500 mL was similar in the two groups, as was the transfusion rate, Dr. Gelmini reported at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
Four patients underwent conversion to laparotomy or mini-laparotomy for malignancies because of a difficult splenic hilum dissection.
Pleural effusion occurred in 7% of patients operated upon for benign disease, compared with 56% of those with hematologic malignancies. Otherwise the complication rates in the two groups were similar.
Time to refeeding was similar in the two patient groups. Mean postoperative length of hospital stay was 5.3 days in patients who underwent laparoscopic splenectomy for benign disease and similar at 5.8 days for those with malignancies.
“Our data suggest that the nature of the disease does not significantly influence the postoperative outcome,” the surgeon observed.
She added that the results of her series were quite similar to those in an earlier report by surgeons at the University of Genoa (Italy) in terms of conversion rate and complications in 38 patients who underwent laparoscopic splenectomy for benign hematologic diseases and 25 with hematologic malignancies (Tumori 2004;90:229-32).
Dr. Gelmini reported having no financial conflicts regarding her presentation.
LAS VEGAS – Laparoscopic splenectomy for hematologic malignancies involving the spleen is an effective surgical strategy, although the operator must expect a higher rate of conversion to laparotomy than when laparoscopic splenectomy is performed for benign hematologic diseases, Dr. Roberta Gelmini said at the annual Minimally Invasive Surgery Week.
The rate of postoperative complications, including wound infection, abdominal abscess, hemiperitoneum, and splenic vein thrombosis, is low and similar to that associated with laparoscopic splenectomy for benign hematologic diseases. The one exception is the complication of pleural effusion, which is seven- to eightfold more frequent following laparoscopic splenectomy for malignancies, probably reflecting affected patients’ larger spleen size and higher conversion rate, observed Dr. Gelmini, professor of surgery at the University of Modena and Reggio Emilia (Italy).
She presented a retrospective analysis of a series of 126 consecutive elective laparoscopic splenectomies performed in adults. Fifty-five were performed for malignant diseases, 71 for benign hematologic conditions. The two groups were well matched except that the patients with benign hematologic diseases were younger and thinner, with a mean age of 39 and a body mass index of 24.4 kg/m2, as compared with 55 years and 25.9 kg/m2 in patients undergoing laparoscopic splenectomy for hematologic malignancies.
The No. 1 indication for splenectomy in patients with benign disease was idiopathic thrombocytopenic purpura, accounting for 61% of cases. In patients with malignancies, non-Hodgkin’s lymphoma was the indication for splenectomy in two-thirds of cases.
As part of their preoperative work-up, all patients underwent ultrasound to establish spleen size and vessel diameter. Bipolar splenic length was significantly greater in the group with malignancies: a mean of 17 cm, compared with 13.4 cm in patients with benign disease.
With regard to key intraoperative findings, the mean 148-minute operative time in patients with malignancies was 22 minutes longer than in patients with benign disease. The conversion rate was 18.2% in patients with malignancies, compared with 5.6% when laparoscopic splenectomy was undertaken for benign disease. But the incidence of intraoperative blood loss greater than 500 mL was similar in the two groups, as was the transfusion rate, Dr. Gelmini reported at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
Four patients underwent conversion to laparotomy or mini-laparotomy for malignancies because of a difficult splenic hilum dissection.
Pleural effusion occurred in 7% of patients operated upon for benign disease, compared with 56% of those with hematologic malignancies. Otherwise the complication rates in the two groups were similar.
Time to refeeding was similar in the two patient groups. Mean postoperative length of hospital stay was 5.3 days in patients who underwent laparoscopic splenectomy for benign disease and similar at 5.8 days for those with malignancies.
“Our data suggest that the nature of the disease does not significantly influence the postoperative outcome,” the surgeon observed.
She added that the results of her series were quite similar to those in an earlier report by surgeons at the University of Genoa (Italy) in terms of conversion rate and complications in 38 patients who underwent laparoscopic splenectomy for benign hematologic diseases and 25 with hematologic malignancies (Tumori 2004;90:229-32).
Dr. Gelmini reported having no financial conflicts regarding her presentation.
LAS VEGAS – Laparoscopic splenectomy for hematologic malignancies involving the spleen is an effective surgical strategy, although the operator must expect a higher rate of conversion to laparotomy than when laparoscopic splenectomy is performed for benign hematologic diseases, Dr. Roberta Gelmini said at the annual Minimally Invasive Surgery Week.
The rate of postoperative complications, including wound infection, abdominal abscess, hemiperitoneum, and splenic vein thrombosis, is low and similar to that associated with laparoscopic splenectomy for benign hematologic diseases. The one exception is the complication of pleural effusion, which is seven- to eightfold more frequent following laparoscopic splenectomy for malignancies, probably reflecting affected patients’ larger spleen size and higher conversion rate, observed Dr. Gelmini, professor of surgery at the University of Modena and Reggio Emilia (Italy).
She presented a retrospective analysis of a series of 126 consecutive elective laparoscopic splenectomies performed in adults. Fifty-five were performed for malignant diseases, 71 for benign hematologic conditions. The two groups were well matched except that the patients with benign hematologic diseases were younger and thinner, with a mean age of 39 and a body mass index of 24.4 kg/m2, as compared with 55 years and 25.9 kg/m2 in patients undergoing laparoscopic splenectomy for hematologic malignancies.
The No. 1 indication for splenectomy in patients with benign disease was idiopathic thrombocytopenic purpura, accounting for 61% of cases. In patients with malignancies, non-Hodgkin’s lymphoma was the indication for splenectomy in two-thirds of cases.
As part of their preoperative work-up, all patients underwent ultrasound to establish spleen size and vessel diameter. Bipolar splenic length was significantly greater in the group with malignancies: a mean of 17 cm, compared with 13.4 cm in patients with benign disease.
With regard to key intraoperative findings, the mean 148-minute operative time in patients with malignancies was 22 minutes longer than in patients with benign disease. The conversion rate was 18.2% in patients with malignancies, compared with 5.6% when laparoscopic splenectomy was undertaken for benign disease. But the incidence of intraoperative blood loss greater than 500 mL was similar in the two groups, as was the transfusion rate, Dr. Gelmini reported at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
Four patients underwent conversion to laparotomy or mini-laparotomy for malignancies because of a difficult splenic hilum dissection.
Pleural effusion occurred in 7% of patients operated upon for benign disease, compared with 56% of those with hematologic malignancies. Otherwise the complication rates in the two groups were similar.
Time to refeeding was similar in the two patient groups. Mean postoperative length of hospital stay was 5.3 days in patients who underwent laparoscopic splenectomy for benign disease and similar at 5.8 days for those with malignancies.
“Our data suggest that the nature of the disease does not significantly influence the postoperative outcome,” the surgeon observed.
She added that the results of her series were quite similar to those in an earlier report by surgeons at the University of Genoa (Italy) in terms of conversion rate and complications in 38 patients who underwent laparoscopic splenectomy for benign hematologic diseases and 25 with hematologic malignancies (Tumori 2004;90:229-32).
Dr. Gelmini reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM MINIMALLY INVASIVE SURGERY WEEK
Laparoscopic Splenectomy Underutilized in the U.S.
LAS VEGAS – Laparoscopic splenectomy has become the preferred surgical approach in the literature, but the number of such procedures performed in the United States remains relatively low, according to Dr. John Afthinos.
National data for 2005-2010 showed a total of 37,006 elective total splenectomies were performed. Only 4,938 of them, or 13.3%, began as laparoscopic procedures, of which 40% had to be converted to open splenectomies, he reported at the annual Minimally Invasive Surgery Week.
“Laparoscopic splenectomy remains underutilized,” Dr. Afthinos commented. “This is one of the lowest recorded rates of laparoscopic completion of any advanced procedure that we know of.”
For example, other investigators have demonstrated that laparoscopic colectomies and bariatric surgical procedures are typically completed laparoscopically in 85%-95% of cases, noted Dr. Afthinos of Staten Island (N.Y.) University Hospital.
The laparoscopic approach has convincingly been shown to result in less pain, shorter hospital length of stay, faster recovery, and improved cosmetic results, compared with the open surgical versions of various operations.
“In the U.S., laparoscopic splenectomy is not well incorporated into the armamentarium of the average general surgeon. We can speculate that the underutilization of this approach around the country prevents development of the familiarity and skill that you need to perform the operation safely without conversion,” he said at the meeting, presented by the Society of Laparoscopic Surgeons and affiliated societies.
Dr. Afthinos’s data were drawn from the Nationwide Inpatient Sample maintained by the U.S. Agency for Healthcare Research and Quality. In this database, patients who had a laparoscopic splenectomy had the shortest average hospital length of stay: 5.6 days, compared with 7.5 in those who underwent open splenectomy and 7.1 for conversion procedures. The overall morbidity rate was significantly lower in the laparoscopic group, too: 7.4%, compared with 10.4% in the open splenectomy group.
In a multivariate analysis, he and his coworkers identified three independent risk factors for conversion from laparoscopic to open splenectomy: hemorrhage, with a 3.23-fold increased risk; splenomegaly, with a 1.3-fold increased risk; and autoimmune hemolytic anemia, with an associated 1.36-fold elevated risk of conversion.
Dr. Catalin Vasilescu of Carol Davila University in Bucharest, Romania, rose from the audience to voice his incredulity at the American data: “I am really surprised at your national conversion rate of well over 30%. That is really a problem. Earlier at this meeting, we presented 520 laparoscopic splenectomy patients with a conversion rate over 20 years of 4%-5%,” the Romanian general surgeon said.
“How do you explain this? I have a hypothesis: Perhaps there are so many surgeons each performing very few of these procedures, instead of referring patients to an experienced center,” he said.
“It was surprising to us, too,” Dr. Afthinos replied. “I mean, I was expecting the conversion rate to be high, but not that high.”
He said Dr. Vasilescu was right on the mark. In addition, he explained, it’s important to understand that there are vast rural areas of the United States, and some rural patients are reluctant to travel hundreds of miles to undergo laparoscopic splenectomy at a large experienced center, especially in the winter or if their family can’t come along for support. And a rural general surgeon is not going to risk a patient’s life if he or she isn’t comfortable with a laparoscopic approach.
There is hope that the situation will improve, however, as more fellowship-trained minimally invasive surgeons enter clinical practice, according to Dr. Afthinos. He cited a recent report from a Columbus, Ohio, general surgery practice that after a fellowship-trained minimally invasive surgeon joined the practice, the group – excluding their fellowship-trained recent hire – increased its rate of various advanced procedures being performed laparoscopically from 12% to 48%. The five established surgeons indicated they found mentoring by a colleague with minimally invasive surgery training was a better way to learn the procedures than via weekend courses, videos, traveling proctors, and other methods (Surg. Endosc. 2013;27:1267-72).
Dr. Afthinos reported having no relevant financial conflicts.
LAS VEGAS – Laparoscopic splenectomy has become the preferred surgical approach in the literature, but the number of such procedures performed in the United States remains relatively low, according to Dr. John Afthinos.
National data for 2005-2010 showed a total of 37,006 elective total splenectomies were performed. Only 4,938 of them, or 13.3%, began as laparoscopic procedures, of which 40% had to be converted to open splenectomies, he reported at the annual Minimally Invasive Surgery Week.
“Laparoscopic splenectomy remains underutilized,” Dr. Afthinos commented. “This is one of the lowest recorded rates of laparoscopic completion of any advanced procedure that we know of.”
For example, other investigators have demonstrated that laparoscopic colectomies and bariatric surgical procedures are typically completed laparoscopically in 85%-95% of cases, noted Dr. Afthinos of Staten Island (N.Y.) University Hospital.
The laparoscopic approach has convincingly been shown to result in less pain, shorter hospital length of stay, faster recovery, and improved cosmetic results, compared with the open surgical versions of various operations.
“In the U.S., laparoscopic splenectomy is not well incorporated into the armamentarium of the average general surgeon. We can speculate that the underutilization of this approach around the country prevents development of the familiarity and skill that you need to perform the operation safely without conversion,” he said at the meeting, presented by the Society of Laparoscopic Surgeons and affiliated societies.
Dr. Afthinos’s data were drawn from the Nationwide Inpatient Sample maintained by the U.S. Agency for Healthcare Research and Quality. In this database, patients who had a laparoscopic splenectomy had the shortest average hospital length of stay: 5.6 days, compared with 7.5 in those who underwent open splenectomy and 7.1 for conversion procedures. The overall morbidity rate was significantly lower in the laparoscopic group, too: 7.4%, compared with 10.4% in the open splenectomy group.
In a multivariate analysis, he and his coworkers identified three independent risk factors for conversion from laparoscopic to open splenectomy: hemorrhage, with a 3.23-fold increased risk; splenomegaly, with a 1.3-fold increased risk; and autoimmune hemolytic anemia, with an associated 1.36-fold elevated risk of conversion.
Dr. Catalin Vasilescu of Carol Davila University in Bucharest, Romania, rose from the audience to voice his incredulity at the American data: “I am really surprised at your national conversion rate of well over 30%. That is really a problem. Earlier at this meeting, we presented 520 laparoscopic splenectomy patients with a conversion rate over 20 years of 4%-5%,” the Romanian general surgeon said.
“How do you explain this? I have a hypothesis: Perhaps there are so many surgeons each performing very few of these procedures, instead of referring patients to an experienced center,” he said.
“It was surprising to us, too,” Dr. Afthinos replied. “I mean, I was expecting the conversion rate to be high, but not that high.”
He said Dr. Vasilescu was right on the mark. In addition, he explained, it’s important to understand that there are vast rural areas of the United States, and some rural patients are reluctant to travel hundreds of miles to undergo laparoscopic splenectomy at a large experienced center, especially in the winter or if their family can’t come along for support. And a rural general surgeon is not going to risk a patient’s life if he or she isn’t comfortable with a laparoscopic approach.
There is hope that the situation will improve, however, as more fellowship-trained minimally invasive surgeons enter clinical practice, according to Dr. Afthinos. He cited a recent report from a Columbus, Ohio, general surgery practice that after a fellowship-trained minimally invasive surgeon joined the practice, the group – excluding their fellowship-trained recent hire – increased its rate of various advanced procedures being performed laparoscopically from 12% to 48%. The five established surgeons indicated they found mentoring by a colleague with minimally invasive surgery training was a better way to learn the procedures than via weekend courses, videos, traveling proctors, and other methods (Surg. Endosc. 2013;27:1267-72).
Dr. Afthinos reported having no relevant financial conflicts.
LAS VEGAS – Laparoscopic splenectomy has become the preferred surgical approach in the literature, but the number of such procedures performed in the United States remains relatively low, according to Dr. John Afthinos.
National data for 2005-2010 showed a total of 37,006 elective total splenectomies were performed. Only 4,938 of them, or 13.3%, began as laparoscopic procedures, of which 40% had to be converted to open splenectomies, he reported at the annual Minimally Invasive Surgery Week.
“Laparoscopic splenectomy remains underutilized,” Dr. Afthinos commented. “This is one of the lowest recorded rates of laparoscopic completion of any advanced procedure that we know of.”
For example, other investigators have demonstrated that laparoscopic colectomies and bariatric surgical procedures are typically completed laparoscopically in 85%-95% of cases, noted Dr. Afthinos of Staten Island (N.Y.) University Hospital.
The laparoscopic approach has convincingly been shown to result in less pain, shorter hospital length of stay, faster recovery, and improved cosmetic results, compared with the open surgical versions of various operations.
“In the U.S., laparoscopic splenectomy is not well incorporated into the armamentarium of the average general surgeon. We can speculate that the underutilization of this approach around the country prevents development of the familiarity and skill that you need to perform the operation safely without conversion,” he said at the meeting, presented by the Society of Laparoscopic Surgeons and affiliated societies.
Dr. Afthinos’s data were drawn from the Nationwide Inpatient Sample maintained by the U.S. Agency for Healthcare Research and Quality. In this database, patients who had a laparoscopic splenectomy had the shortest average hospital length of stay: 5.6 days, compared with 7.5 in those who underwent open splenectomy and 7.1 for conversion procedures. The overall morbidity rate was significantly lower in the laparoscopic group, too: 7.4%, compared with 10.4% in the open splenectomy group.
In a multivariate analysis, he and his coworkers identified three independent risk factors for conversion from laparoscopic to open splenectomy: hemorrhage, with a 3.23-fold increased risk; splenomegaly, with a 1.3-fold increased risk; and autoimmune hemolytic anemia, with an associated 1.36-fold elevated risk of conversion.
Dr. Catalin Vasilescu of Carol Davila University in Bucharest, Romania, rose from the audience to voice his incredulity at the American data: “I am really surprised at your national conversion rate of well over 30%. That is really a problem. Earlier at this meeting, we presented 520 laparoscopic splenectomy patients with a conversion rate over 20 years of 4%-5%,” the Romanian general surgeon said.
“How do you explain this? I have a hypothesis: Perhaps there are so many surgeons each performing very few of these procedures, instead of referring patients to an experienced center,” he said.
“It was surprising to us, too,” Dr. Afthinos replied. “I mean, I was expecting the conversion rate to be high, but not that high.”
He said Dr. Vasilescu was right on the mark. In addition, he explained, it’s important to understand that there are vast rural areas of the United States, and some rural patients are reluctant to travel hundreds of miles to undergo laparoscopic splenectomy at a large experienced center, especially in the winter or if their family can’t come along for support. And a rural general surgeon is not going to risk a patient’s life if he or she isn’t comfortable with a laparoscopic approach.
There is hope that the situation will improve, however, as more fellowship-trained minimally invasive surgeons enter clinical practice, according to Dr. Afthinos. He cited a recent report from a Columbus, Ohio, general surgery practice that after a fellowship-trained minimally invasive surgeon joined the practice, the group – excluding their fellowship-trained recent hire – increased its rate of various advanced procedures being performed laparoscopically from 12% to 48%. The five established surgeons indicated they found mentoring by a colleague with minimally invasive surgery training was a better way to learn the procedures than via weekend courses, videos, traveling proctors, and other methods (Surg. Endosc. 2013;27:1267-72).
Dr. Afthinos reported having no relevant financial conflicts.
AT MINIMALLY INVASIVE SURGERY WEEK
Laparoscopic splenectomy underutilized in the U.S.
LAS VEGAS – Laparoscopic splenectomy has become the preferred surgical approach in the literature, but the number of such procedures performed in the United States remains relatively low, according to Dr. John Afthinos.
National data for 2005-2010 showed a total of 37,006 elective total splenectomies were performed. Only 4,938 of them, or 13.3%, began as laparoscopic procedures, of which 40% had to be converted to open splenectomies, he reported at the annual Minimally Invasive Surgery Week.
“Laparoscopic splenectomy remains underutilized,” Dr. Afthinos commented. “This is one of the lowest recorded rates of laparoscopic completion of any advanced procedure that we know of.”
For example, other investigators have demonstrated that laparoscopic colectomies and bariatric surgical procedures are typically completed laparoscopically in 85%-95% of cases, noted Dr. Afthinos of Staten Island (N.Y.) University Hospital.
The laparoscopic approach has convincingly been shown to result in less pain, shorter hospital length of stay, faster recovery, and improved cosmetic results, compared with the open surgical versions of various operations.
“In the U.S., laparoscopic splenectomy is not well incorporated into the armamentarium of the average general surgeon. We can speculate that the underutilization of this approach around the country prevents development of the familiarity and skill that you need to perform the operation safely without conversion,” he said at the meeting, presented by the Society of Laparoscopic Surgeons and affiliated societies.
Dr. Afthinos’s data were drawn from the Nationwide Inpatient Sample maintained by the U.S. Agency for Healthcare Research and Quality. In this database, patients who had a laparoscopic splenectomy had the shortest average hospital length of stay: 5.6 days, compared with 7.5 in those who underwent open splenectomy and 7.1 for conversion procedures. The overall morbidity rate was significantly lower in the laparoscopic group, too: 7.4%, compared with 10.4% in the open splenectomy group.
In a multivariate analysis, he and his coworkers identified three independent risk factors for conversion from laparoscopic to open splenectomy: hemorrhage, with a 3.23-fold increased risk; splenomegaly, with a 1.3-fold increased risk; and autoimmune hemolytic anemia, with an associated 1.36-fold elevated risk of conversion.
Dr. Catalin Vasilescu of Carol Davila University in Bucharest, Romania, rose from the audience to voice his incredulity at the American data: “I am really surprised at your national conversion rate of well over 30%. That is really a problem. Earlier at this meeting, we presented 520 laparoscopic splenectomy patients with a conversion rate over 20 years of 4%-5%,” the Romanian general surgeon said.
“How do you explain this? I have a hypothesis: Perhaps there are so many surgeons each performing very few of these procedures, instead of referring patients to an experienced center,” he said.
“It was surprising to us, too,” Dr. Afthinos replied. “I mean, I was expecting the conversion rate to be high, but not that high.”
He said Dr. Vasilescu was right on the mark. In addition, he explained, it’s important to understand that there are vast rural areas of the United States, and some rural patients are reluctant to travel hundreds of miles to undergo laparoscopic splenectomy at a large experienced center, especially in the winter or if their family can’t come along for support. And a rural general surgeon is not going to risk a patient’s life if he or she isn’t comfortable with a laparoscopic approach.
There is hope that the situation will improve, however, as more fellowship-trained minimally invasive surgeons enter clinical practice, according to Dr. Afthinos. He cited a recent report from a Columbus, Ohio, general surgery practice that after a fellowship-trained minimally invasive surgeon joined the practice, the group – excluding their fellowship-trained recent hire – increased its rate of various advanced procedures being performed laparoscopically from 12% to 48%. The five established surgeons indicated they found mentoring by a colleague with minimally invasive surgery training was a better way to learn the procedures than via weekend courses, videos, traveling proctors, and other methods (Surg. Endosc. 2013;27:1267-72).
Dr. Afthinos reported having no relevant financial conflicts.
LAS VEGAS – Laparoscopic splenectomy has become the preferred surgical approach in the literature, but the number of such procedures performed in the United States remains relatively low, according to Dr. John Afthinos.
National data for 2005-2010 showed a total of 37,006 elective total splenectomies were performed. Only 4,938 of them, or 13.3%, began as laparoscopic procedures, of which 40% had to be converted to open splenectomies, he reported at the annual Minimally Invasive Surgery Week.
“Laparoscopic splenectomy remains underutilized,” Dr. Afthinos commented. “This is one of the lowest recorded rates of laparoscopic completion of any advanced procedure that we know of.”
For example, other investigators have demonstrated that laparoscopic colectomies and bariatric surgical procedures are typically completed laparoscopically in 85%-95% of cases, noted Dr. Afthinos of Staten Island (N.Y.) University Hospital.
The laparoscopic approach has convincingly been shown to result in less pain, shorter hospital length of stay, faster recovery, and improved cosmetic results, compared with the open surgical versions of various operations.
“In the U.S., laparoscopic splenectomy is not well incorporated into the armamentarium of the average general surgeon. We can speculate that the underutilization of this approach around the country prevents development of the familiarity and skill that you need to perform the operation safely without conversion,” he said at the meeting, presented by the Society of Laparoscopic Surgeons and affiliated societies.
Dr. Afthinos’s data were drawn from the Nationwide Inpatient Sample maintained by the U.S. Agency for Healthcare Research and Quality. In this database, patients who had a laparoscopic splenectomy had the shortest average hospital length of stay: 5.6 days, compared with 7.5 in those who underwent open splenectomy and 7.1 for conversion procedures. The overall morbidity rate was significantly lower in the laparoscopic group, too: 7.4%, compared with 10.4% in the open splenectomy group.
In a multivariate analysis, he and his coworkers identified three independent risk factors for conversion from laparoscopic to open splenectomy: hemorrhage, with a 3.23-fold increased risk; splenomegaly, with a 1.3-fold increased risk; and autoimmune hemolytic anemia, with an associated 1.36-fold elevated risk of conversion.
Dr. Catalin Vasilescu of Carol Davila University in Bucharest, Romania, rose from the audience to voice his incredulity at the American data: “I am really surprised at your national conversion rate of well over 30%. That is really a problem. Earlier at this meeting, we presented 520 laparoscopic splenectomy patients with a conversion rate over 20 years of 4%-5%,” the Romanian general surgeon said.
“How do you explain this? I have a hypothesis: Perhaps there are so many surgeons each performing very few of these procedures, instead of referring patients to an experienced center,” he said.
“It was surprising to us, too,” Dr. Afthinos replied. “I mean, I was expecting the conversion rate to be high, but not that high.”
He said Dr. Vasilescu was right on the mark. In addition, he explained, it’s important to understand that there are vast rural areas of the United States, and some rural patients are reluctant to travel hundreds of miles to undergo laparoscopic splenectomy at a large experienced center, especially in the winter or if their family can’t come along for support. And a rural general surgeon is not going to risk a patient’s life if he or she isn’t comfortable with a laparoscopic approach.
There is hope that the situation will improve, however, as more fellowship-trained minimally invasive surgeons enter clinical practice, according to Dr. Afthinos. He cited a recent report from a Columbus, Ohio, general surgery practice that after a fellowship-trained minimally invasive surgeon joined the practice, the group – excluding their fellowship-trained recent hire – increased its rate of various advanced procedures being performed laparoscopically from 12% to 48%. The five established surgeons indicated they found mentoring by a colleague with minimally invasive surgery training was a better way to learn the procedures than via weekend courses, videos, traveling proctors, and other methods (Surg. Endosc. 2013;27:1267-72).
Dr. Afthinos reported having no relevant financial conflicts.
LAS VEGAS – Laparoscopic splenectomy has become the preferred surgical approach in the literature, but the number of such procedures performed in the United States remains relatively low, according to Dr. John Afthinos.
National data for 2005-2010 showed a total of 37,006 elective total splenectomies were performed. Only 4,938 of them, or 13.3%, began as laparoscopic procedures, of which 40% had to be converted to open splenectomies, he reported at the annual Minimally Invasive Surgery Week.
“Laparoscopic splenectomy remains underutilized,” Dr. Afthinos commented. “This is one of the lowest recorded rates of laparoscopic completion of any advanced procedure that we know of.”
For example, other investigators have demonstrated that laparoscopic colectomies and bariatric surgical procedures are typically completed laparoscopically in 85%-95% of cases, noted Dr. Afthinos of Staten Island (N.Y.) University Hospital.
The laparoscopic approach has convincingly been shown to result in less pain, shorter hospital length of stay, faster recovery, and improved cosmetic results, compared with the open surgical versions of various operations.
“In the U.S., laparoscopic splenectomy is not well incorporated into the armamentarium of the average general surgeon. We can speculate that the underutilization of this approach around the country prevents development of the familiarity and skill that you need to perform the operation safely without conversion,” he said at the meeting, presented by the Society of Laparoscopic Surgeons and affiliated societies.
Dr. Afthinos’s data were drawn from the Nationwide Inpatient Sample maintained by the U.S. Agency for Healthcare Research and Quality. In this database, patients who had a laparoscopic splenectomy had the shortest average hospital length of stay: 5.6 days, compared with 7.5 in those who underwent open splenectomy and 7.1 for conversion procedures. The overall morbidity rate was significantly lower in the laparoscopic group, too: 7.4%, compared with 10.4% in the open splenectomy group.
In a multivariate analysis, he and his coworkers identified three independent risk factors for conversion from laparoscopic to open splenectomy: hemorrhage, with a 3.23-fold increased risk; splenomegaly, with a 1.3-fold increased risk; and autoimmune hemolytic anemia, with an associated 1.36-fold elevated risk of conversion.
Dr. Catalin Vasilescu of Carol Davila University in Bucharest, Romania, rose from the audience to voice his incredulity at the American data: “I am really surprised at your national conversion rate of well over 30%. That is really a problem. Earlier at this meeting, we presented 520 laparoscopic splenectomy patients with a conversion rate over 20 years of 4%-5%,” the Romanian general surgeon said.
“How do you explain this? I have a hypothesis: Perhaps there are so many surgeons each performing very few of these procedures, instead of referring patients to an experienced center,” he said.
“It was surprising to us, too,” Dr. Afthinos replied. “I mean, I was expecting the conversion rate to be high, but not that high.”
He said Dr. Vasilescu was right on the mark. In addition, he explained, it’s important to understand that there are vast rural areas of the United States, and some rural patients are reluctant to travel hundreds of miles to undergo laparoscopic splenectomy at a large experienced center, especially in the winter or if their family can’t come along for support. And a rural general surgeon is not going to risk a patient’s life if he or she isn’t comfortable with a laparoscopic approach.
There is hope that the situation will improve, however, as more fellowship-trained minimally invasive surgeons enter clinical practice, according to Dr. Afthinos. He cited a recent report from a Columbus, Ohio, general surgery practice that after a fellowship-trained minimally invasive surgeon joined the practice, the group – excluding their fellowship-trained recent hire – increased its rate of various advanced procedures being performed laparoscopically from 12% to 48%. The five established surgeons indicated they found mentoring by a colleague with minimally invasive surgery training was a better way to learn the procedures than via weekend courses, videos, traveling proctors, and other methods (Surg. Endosc. 2013;27:1267-72).
Dr. Afthinos reported having no relevant financial conflicts.
AT MINIMALLY INVASIVE SURGERY WEEK
Key clinical point: American general surgeons lag far behind their foreign colleagues in their rate of laparoscopically completed splenectomies.
Major finding: Only 13.3% of elective splenectomies performed in the United States started out as laparoscopic procedures – and of those, 40% were converted to open splenectomy.
Data source: A retrospective study of the Nationwide Inpatient Sample for 2005-2010, during which 37,006 elective total splenectomies were performed.
Disclosures: The presenter reported having no relevant financial conflicts.
Study finds lap approach to bariatric revision safe, effective
LAS VEGAS– As the volume of bariatric surgery climbs sharply in response to the obesity epidemic, the need for revision procedures due to weight regain is also on the rise.
Laparoscopic revision bariatric surgery, while more technically challenging, is safe and effective, and it entails less morbidity than typically seen with open revisions, Dr. Rana M. Ballo said at the annual Minimally Invasive Surgery Week.
Dr. Ballo, a fifth-year general surgery resident at Rush University Medical Center in Chicago, presented a retrospective single-center study involving 227 patients who during 2001-2011 underwent laparoscopic revision of bariatric procedures that had failed because of weight regain. The goal of the revision bariatric surgery was to restore the restrictive component and/or add a malabsorptive component in order to improve long-term weight loss.
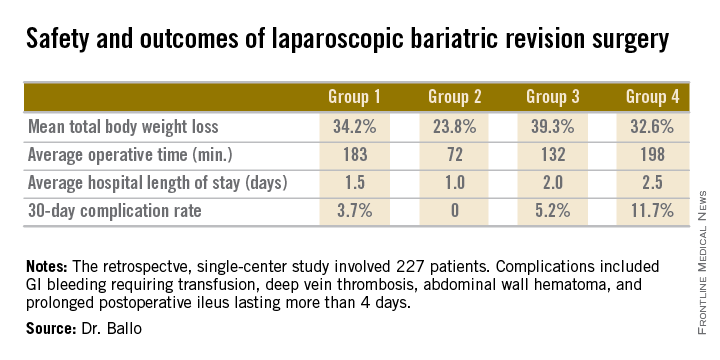
The patients fell into four groups. Group 1 consisted of 53 patients who initially had a laparoscopic adjustable gastric band procedure which was converted to a gastric bypass. Group 2, the largest group, initially had a Roux-en-Y gastric bypass in which the pouch eventually became dilated, which is the No. 1 cause of failure of this operation in the literature; their revision surgery entailed laparoscopic pouch reduction. The 38 patients who comprised Group 3 had a Roux-en-Y gastric bypass with subsequent pouch reduction and elongation of the biliopancreatic limb. Group 4 consisted of 17 patients who initially had a vertical banded gastroplasty – a procedure with a restrictive component only – and subsequently underwent laparoscopic conversion to a gastric bypass.
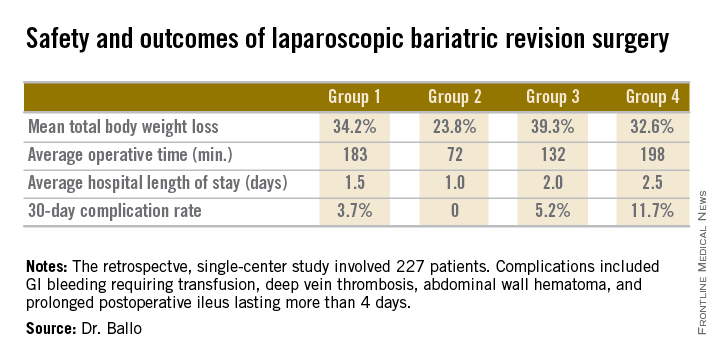
After a median follow-up of 3.9 years, Group 2 had significantly less total body weight loss than the rest of the groups, all of which had similar weight losses. On the other hand, Group 2 also had a shorter operative time and briefer average hospital length of stay than the other three groups, and it was the only group with zero complications. Still, the lengths of stay and 30-day morbidity rates across the board in this laparoscopic revision series were impressively low in comparison to those previously reported in series involving open revision, Dr. Ballo noted at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
There was no mortality in this study. This is one of the largest series reported to date of laparoscopic revision of failed bariatric surgery. Most prior studies have involved 30-100 patients, according to Dr. Ballo.
In the 1990s, roughly 13,000 bariatric procedures were performed annually in the United States. Today that figure is in excess of 200,000 annually.
Dr. Ballo reported having no financial conflicts with regard to this study.
LAS VEGAS– As the volume of bariatric surgery climbs sharply in response to the obesity epidemic, the need for revision procedures due to weight regain is also on the rise.
Laparoscopic revision bariatric surgery, while more technically challenging, is safe and effective, and it entails less morbidity than typically seen with open revisions, Dr. Rana M. Ballo said at the annual Minimally Invasive Surgery Week.
Dr. Ballo, a fifth-year general surgery resident at Rush University Medical Center in Chicago, presented a retrospective single-center study involving 227 patients who during 2001-2011 underwent laparoscopic revision of bariatric procedures that had failed because of weight regain. The goal of the revision bariatric surgery was to restore the restrictive component and/or add a malabsorptive component in order to improve long-term weight loss.
The patients fell into four groups. Group 1 consisted of 53 patients who initially had a laparoscopic adjustable gastric band procedure which was converted to a gastric bypass. Group 2, the largest group, initially had a Roux-en-Y gastric bypass in which the pouch eventually became dilated, which is the No. 1 cause of failure of this operation in the literature; their revision surgery entailed laparoscopic pouch reduction. The 38 patients who comprised Group 3 had a Roux-en-Y gastric bypass with subsequent pouch reduction and elongation of the biliopancreatic limb. Group 4 consisted of 17 patients who initially had a vertical banded gastroplasty – a procedure with a restrictive component only – and subsequently underwent laparoscopic conversion to a gastric bypass.

After a median follow-up of 3.9 years, Group 2 had significantly less total body weight loss than the rest of the groups, all of which had similar weight losses. On the other hand, Group 2 also had a shorter operative time and briefer average hospital length of stay than the other three groups, and it was the only group with zero complications. Still, the lengths of stay and 30-day morbidity rates across the board in this laparoscopic revision series were impressively low in comparison to those previously reported in series involving open revision, Dr. Ballo noted at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
There was no mortality in this study. This is one of the largest series reported to date of laparoscopic revision of failed bariatric surgery. Most prior studies have involved 30-100 patients, according to Dr. Ballo.
In the 1990s, roughly 13,000 bariatric procedures were performed annually in the United States. Today that figure is in excess of 200,000 annually.
Dr. Ballo reported having no financial conflicts with regard to this study.
LAS VEGAS– As the volume of bariatric surgery climbs sharply in response to the obesity epidemic, the need for revision procedures due to weight regain is also on the rise.
Laparoscopic revision bariatric surgery, while more technically challenging, is safe and effective, and it entails less morbidity than typically seen with open revisions, Dr. Rana M. Ballo said at the annual Minimally Invasive Surgery Week.
Dr. Ballo, a fifth-year general surgery resident at Rush University Medical Center in Chicago, presented a retrospective single-center study involving 227 patients who during 2001-2011 underwent laparoscopic revision of bariatric procedures that had failed because of weight regain. The goal of the revision bariatric surgery was to restore the restrictive component and/or add a malabsorptive component in order to improve long-term weight loss.
The patients fell into four groups. Group 1 consisted of 53 patients who initially had a laparoscopic adjustable gastric band procedure which was converted to a gastric bypass. Group 2, the largest group, initially had a Roux-en-Y gastric bypass in which the pouch eventually became dilated, which is the No. 1 cause of failure of this operation in the literature; their revision surgery entailed laparoscopic pouch reduction. The 38 patients who comprised Group 3 had a Roux-en-Y gastric bypass with subsequent pouch reduction and elongation of the biliopancreatic limb. Group 4 consisted of 17 patients who initially had a vertical banded gastroplasty – a procedure with a restrictive component only – and subsequently underwent laparoscopic conversion to a gastric bypass.

After a median follow-up of 3.9 years, Group 2 had significantly less total body weight loss than the rest of the groups, all of which had similar weight losses. On the other hand, Group 2 also had a shorter operative time and briefer average hospital length of stay than the other three groups, and it was the only group with zero complications. Still, the lengths of stay and 30-day morbidity rates across the board in this laparoscopic revision series were impressively low in comparison to those previously reported in series involving open revision, Dr. Ballo noted at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
There was no mortality in this study. This is one of the largest series reported to date of laparoscopic revision of failed bariatric surgery. Most prior studies have involved 30-100 patients, according to Dr. Ballo.
In the 1990s, roughly 13,000 bariatric procedures were performed annually in the United States. Today that figure is in excess of 200,000 annually.
Dr. Ballo reported having no financial conflicts with regard to this study.
AT MINIMALLY INVASIVE SURGERY WEEK
Key clinical point: Laparoscopic revision of failed bariatric surgery is safe and effective.
Major finding: Patients experienced a mean total body weight loss of 24%-39% at a median of 3.9 years after laparoscopic revision of failed bariatric surgery. The weight loss depended upon the primary procedure and type of revision.
Data source: This was a retrospective single-center series involving 227 patients who underwent laparoscopic revisional bariatric surgery in response to weight regain.
Disclosures: The presenter reported having no financial conflicts regarding this study.
Rethinking the postop patient-surgeon visit
LAS VEGAS– Telephone follow-up by a midlevel provider after laparoscopic inguinal hernia repair is a possible alternative to the traditional surgeon-patient clinic visit, a pilot study indicates.
“It is feasible and seems effective. It is well received by patients, and it’s especially attractive for patients traveling long distances to receive their medical care. It may well prove to be the most efficient method for follow-up after laparoscopic inguinal hernia repair. It frees up clinic time: More than 80% of patients in our study were spared a clinic visit, and this allowed us to increase the number of our outpatient encounters,” Dr. Dan Eisenberg said at the annual Minimally Invasive Surgery Week.
He presented a prospective study of 62 consecutive patients who underwent laparoscopic inguinal hernia repair at the Veterans Affairs Palo Alto (Calif.) Health Care System and agreed to follow-up by a physician assistant 2-3 weeks after surgery in lieu of the traditional face-to-face clinic visit with the surgeon. The phone interview involved a predetermined nine-question script. A single “yes” answer prompted an appointment for a clinic visit.
Of the 62 patients, 3 were lost to follow-up. Because of a scheduling error, another four showed up at the VA clinic for a follow-up visit before the planned phone call. Of the remaining 55 patients, 50 (91%) were satisfied with their telephone follow-up experience.
Five patients were seen face to face at the clinic as a result of their telephone follow-up. Three did so because of self-limited groin discomfort, one for a large seroma, and one for early hernia recurrence treated by the total extraperitoneal approach, reported Dr. Eisenberg, a general surgeon at the Palo Alto VA.
Session chair Vincenzo Neri voiced a misgiving about the study.
“The only problem I see is that it contributes to the dehumanization of surgery,” commented Dr. Neri, professor and director of the division of general surgery at the University of Foggia (Italy). “The follow-up contact that you have in the clinic when you actually see the patient can be important because so many things can happen to the patient that he has no awareness of. Your way, the follow-up is basically gone.”
Dr. Eisenberg was quick to concur that the patient-surgeon relationship is basic to clinical medicine, and that the postop clinic visit is a fundamental part of this relationship.
“It is unfortunate to see that in the U.S., external constraints are changing the way we practice medicine,” he added, “but these external pressures are demanding more time efficiency and more resource efficiency, ultimately culminating, hopefully, in cost efficiency. The VA system is single payer. At the Palo Alto VA, we’ve noticed an increase in resource constraint limiting clinic access, and financial constraints going along with it.”
The Palo Alto VA Health Care System serves an enormous geographic area running north to the Oregon border and east into Nevada. The average roundtrip distance to the VA hospital for the study participants was 122 miles, and they were happy to forgo the journey.
“In Bay Area traffic, that corresponds to 3 to 3 1/2 hours on the road,” Dr. Eisenberg noted at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
His future research plans include randomizing patients to telephone follow-up or a face-to-face clinic visit after laparoscopic inguinal hernia repair in order to quantify the impact of the novel alternative on clinic flow and patient satisfaction. He also plans to extend the practice of telephone follow-up by a midlevel provider to other surgical procedures. He and his coworkers have already applied it to patients after laparoscopic cholecystectomy, where it also appears to be safe and efficient.
“It raises the question of how much further we can push this. Maybe it doesn’t have to be just for outpatient surgery,” according to Dr. Eisenberg.
Dr. Eisenberg reported having no financial conflicts with regard to the study, which was funded by the Department of Veterans Affairs.
The nine yes/no telephone follow-up questions
Do you feel unwell?
Are you requiring frequent analgesics?
Are you having trouble returning to your normal activities?
Do you have fever or chills?
Is there increasing redness or swelling at the incision site?
Do you have testicular swelling or pain?
Are you having trouble tolerating a regular diet?
Do you have any concerns?
Would you like a face-to-face clinic visit?
A “yes” answer to any of the above triggers a clinic visit.
Source: Dr. Eisenberg
LAS VEGAS– Telephone follow-up by a midlevel provider after laparoscopic inguinal hernia repair is a possible alternative to the traditional surgeon-patient clinic visit, a pilot study indicates.
“It is feasible and seems effective. It is well received by patients, and it’s especially attractive for patients traveling long distances to receive their medical care. It may well prove to be the most efficient method for follow-up after laparoscopic inguinal hernia repair. It frees up clinic time: More than 80% of patients in our study were spared a clinic visit, and this allowed us to increase the number of our outpatient encounters,” Dr. Dan Eisenberg said at the annual Minimally Invasive Surgery Week.
He presented a prospective study of 62 consecutive patients who underwent laparoscopic inguinal hernia repair at the Veterans Affairs Palo Alto (Calif.) Health Care System and agreed to follow-up by a physician assistant 2-3 weeks after surgery in lieu of the traditional face-to-face clinic visit with the surgeon. The phone interview involved a predetermined nine-question script. A single “yes” answer prompted an appointment for a clinic visit.
Of the 62 patients, 3 were lost to follow-up. Because of a scheduling error, another four showed up at the VA clinic for a follow-up visit before the planned phone call. Of the remaining 55 patients, 50 (91%) were satisfied with their telephone follow-up experience.
Five patients were seen face to face at the clinic as a result of their telephone follow-up. Three did so because of self-limited groin discomfort, one for a large seroma, and one for early hernia recurrence treated by the total extraperitoneal approach, reported Dr. Eisenberg, a general surgeon at the Palo Alto VA.
Session chair Vincenzo Neri voiced a misgiving about the study.
“The only problem I see is that it contributes to the dehumanization of surgery,” commented Dr. Neri, professor and director of the division of general surgery at the University of Foggia (Italy). “The follow-up contact that you have in the clinic when you actually see the patient can be important because so many things can happen to the patient that he has no awareness of. Your way, the follow-up is basically gone.”
Dr. Eisenberg was quick to concur that the patient-surgeon relationship is basic to clinical medicine, and that the postop clinic visit is a fundamental part of this relationship.
“It is unfortunate to see that in the U.S., external constraints are changing the way we practice medicine,” he added, “but these external pressures are demanding more time efficiency and more resource efficiency, ultimately culminating, hopefully, in cost efficiency. The VA system is single payer. At the Palo Alto VA, we’ve noticed an increase in resource constraint limiting clinic access, and financial constraints going along with it.”
The Palo Alto VA Health Care System serves an enormous geographic area running north to the Oregon border and east into Nevada. The average roundtrip distance to the VA hospital for the study participants was 122 miles, and they were happy to forgo the journey.
“In Bay Area traffic, that corresponds to 3 to 3 1/2 hours on the road,” Dr. Eisenberg noted at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
His future research plans include randomizing patients to telephone follow-up or a face-to-face clinic visit after laparoscopic inguinal hernia repair in order to quantify the impact of the novel alternative on clinic flow and patient satisfaction. He also plans to extend the practice of telephone follow-up by a midlevel provider to other surgical procedures. He and his coworkers have already applied it to patients after laparoscopic cholecystectomy, where it also appears to be safe and efficient.
“It raises the question of how much further we can push this. Maybe it doesn’t have to be just for outpatient surgery,” according to Dr. Eisenberg.
Dr. Eisenberg reported having no financial conflicts with regard to the study, which was funded by the Department of Veterans Affairs.
The nine yes/no telephone follow-up questions
Do you feel unwell?
Are you requiring frequent analgesics?
Are you having trouble returning to your normal activities?
Do you have fever or chills?
Is there increasing redness or swelling at the incision site?
Do you have testicular swelling or pain?
Are you having trouble tolerating a regular diet?
Do you have any concerns?
Would you like a face-to-face clinic visit?
A “yes” answer to any of the above triggers a clinic visit.
Source: Dr. Eisenberg
LAS VEGAS– Telephone follow-up by a midlevel provider after laparoscopic inguinal hernia repair is a possible alternative to the traditional surgeon-patient clinic visit, a pilot study indicates.
“It is feasible and seems effective. It is well received by patients, and it’s especially attractive for patients traveling long distances to receive their medical care. It may well prove to be the most efficient method for follow-up after laparoscopic inguinal hernia repair. It frees up clinic time: More than 80% of patients in our study were spared a clinic visit, and this allowed us to increase the number of our outpatient encounters,” Dr. Dan Eisenberg said at the annual Minimally Invasive Surgery Week.
He presented a prospective study of 62 consecutive patients who underwent laparoscopic inguinal hernia repair at the Veterans Affairs Palo Alto (Calif.) Health Care System and agreed to follow-up by a physician assistant 2-3 weeks after surgery in lieu of the traditional face-to-face clinic visit with the surgeon. The phone interview involved a predetermined nine-question script. A single “yes” answer prompted an appointment for a clinic visit.
Of the 62 patients, 3 were lost to follow-up. Because of a scheduling error, another four showed up at the VA clinic for a follow-up visit before the planned phone call. Of the remaining 55 patients, 50 (91%) were satisfied with their telephone follow-up experience.
Five patients were seen face to face at the clinic as a result of their telephone follow-up. Three did so because of self-limited groin discomfort, one for a large seroma, and one for early hernia recurrence treated by the total extraperitoneal approach, reported Dr. Eisenberg, a general surgeon at the Palo Alto VA.
Session chair Vincenzo Neri voiced a misgiving about the study.
“The only problem I see is that it contributes to the dehumanization of surgery,” commented Dr. Neri, professor and director of the division of general surgery at the University of Foggia (Italy). “The follow-up contact that you have in the clinic when you actually see the patient can be important because so many things can happen to the patient that he has no awareness of. Your way, the follow-up is basically gone.”
Dr. Eisenberg was quick to concur that the patient-surgeon relationship is basic to clinical medicine, and that the postop clinic visit is a fundamental part of this relationship.
“It is unfortunate to see that in the U.S., external constraints are changing the way we practice medicine,” he added, “but these external pressures are demanding more time efficiency and more resource efficiency, ultimately culminating, hopefully, in cost efficiency. The VA system is single payer. At the Palo Alto VA, we’ve noticed an increase in resource constraint limiting clinic access, and financial constraints going along with it.”
The Palo Alto VA Health Care System serves an enormous geographic area running north to the Oregon border and east into Nevada. The average roundtrip distance to the VA hospital for the study participants was 122 miles, and they were happy to forgo the journey.
“In Bay Area traffic, that corresponds to 3 to 3 1/2 hours on the road,” Dr. Eisenberg noted at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
His future research plans include randomizing patients to telephone follow-up or a face-to-face clinic visit after laparoscopic inguinal hernia repair in order to quantify the impact of the novel alternative on clinic flow and patient satisfaction. He also plans to extend the practice of telephone follow-up by a midlevel provider to other surgical procedures. He and his coworkers have already applied it to patients after laparoscopic cholecystectomy, where it also appears to be safe and efficient.
“It raises the question of how much further we can push this. Maybe it doesn’t have to be just for outpatient surgery,” according to Dr. Eisenberg.
Dr. Eisenberg reported having no financial conflicts with regard to the study, which was funded by the Department of Veterans Affairs.
The nine yes/no telephone follow-up questions
Do you feel unwell?
Are you requiring frequent analgesics?
Are you having trouble returning to your normal activities?
Do you have fever or chills?
Is there increasing redness or swelling at the incision site?
Do you have testicular swelling or pain?
Are you having trouble tolerating a regular diet?
Do you have any concerns?
Would you like a face-to-face clinic visit?
A “yes” answer to any of the above triggers a clinic visit.
Source: Dr. Eisenberg
AT MINIMALLY INVASIVE SURGERY WEEK
Key clinical point: Scripted telephone follow-up by a midlevel provider after laparoscopic inguinal hernia repair is a safe, effective, and resource-sparing alternative to the traditional face-to-face surgeon-patient follow-up visit.
Major finding: Fifty of 55 patients who underwent laparoscopic inguinal hernia repair were safely able to be spared a follow-up clinic visit as a result of telephone follow-up by a physician assistant several weeks after surgery.
Data source: This was a prospective observational study in which patients who had laparoscopic repair of an inguinal hernia agreed to a scripted telephone follow-up by a physician assistant instead of returning to the clinic for the traditional surgeon-patient face-to-face encounter.
Disclosures: The presenter reported having no financial conflicts with regard to the study, which was funded by the Department of Veterans Affairs.
When to use mesh in laparoscopic hiatal hernia repair
LAS VEGAS – Routine use of mesh reinforcement when performing laparoscopic repair of hiatal hernia defects 5 cm or larger in diameter is associated with a low recurrence rate, Dr. Chetan V. Aher reported at the annual Minimally Invasive Surgery Week.
His coinvestigators had shown in an earlier randomized controlled trial that mesh reinforcement of primary cruroplasty in patients with a hernia of 8 cm or greater was associated with no recurrences. Repair with simple cruroplasty was associated with a 22% recurrence rate (Arch. Surg. 2002;137:649-52).
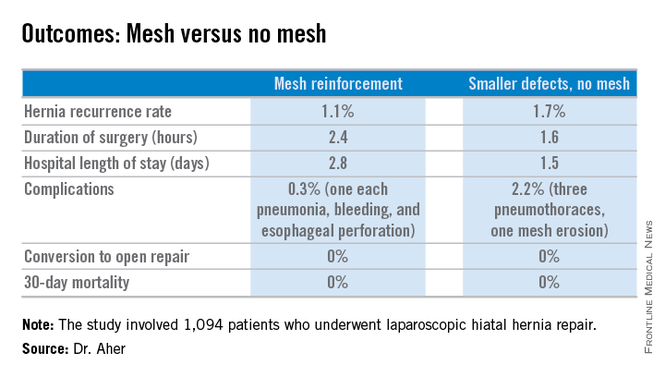
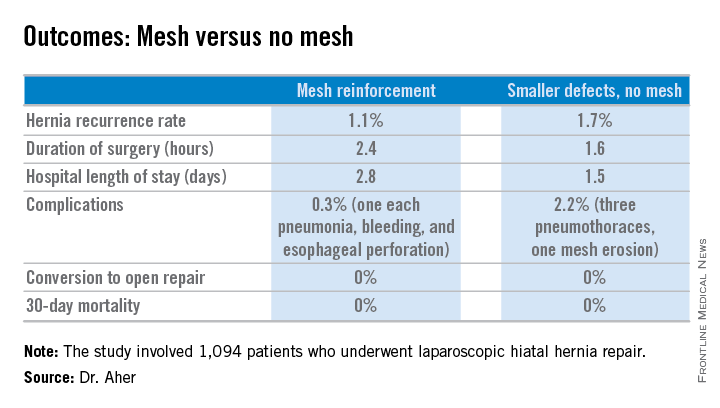
However, Dr. Aher and his coinvestigators subsequently observed a high recurrence rate following mesh-free simple cruroplasty for defects in the 5- to 8-cm range. He presented a case series involving 1,094 laparoscopic hiatal hernia repairs performed since he and his colleagues changed their practice by lowering their threshold for polytetrafluoroethylene mesh reinforcement to defects of at least 5 cm from their prior standard of 8 cm or more.
Hernias were less than 5 cm in diameter in 84% of the patients, so mesh wasn’t used for those repairs. In the remaining 178 patients – those with hernias of at least 5 cm – PTFE mesh was utilized to circumferentially reinforce the cruroplasty.
During a mean follow-up of 3.1 years, the hernia recurrence rate was 1.7% in the group with hernia defects of less than 5 cm and similar at 1.1% in those who received mesh reinforcement because their hernias were larger, reported Dr. Aher of Rush University Medical Center in Chicago.
Operative time and length of stay were longer in the mesh reinforcement group (see chart).
“There’s more dissection when using mesh, and obviously the placement of the mesh takes a little longer,” he noted at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
All repairs were performed using cruroplasty with interrupted nonabsorbable sutures approximating the right and left bundles of the right crura.
Laparoscopic repair has become the standard approach in the primary repair of hiatal hernias. In a 2010 survey of members of the Society of Gastrointestinal and Endoscopic Surgeons conducted by Dr. Aher’s colleagues, respondents indicated they laparoscopically performed 77% of their mesh-reinforced repairs. However, the survey results underscored a lack of consensus within the surgical community regarding mesh usage. Biologic mesh was used by 28% of surgeons; 25% used PTFE (polytetrafluoroethylene), and 21% polypropylene. Mesh placement practices also varied widely: 14% of surgeons utilized anterior placement, 34% posterior, and only 10% circumferential (Surg. Endosc. 2010;24:1017-24).
Asked how he counsels patients about the competing risks of mesh erosion and hernia recurrence in the absence of mesh reinforcement, Dr. Aher pointed to the 22% recurrence risk with large hernias in the earlier randomized trial.
“I would counsel my own family that if you have a large hernia, the risk of mesh erosion is very low and the risk of undergoing a recurrent operation if there is no mesh reinforcement is, I think, overall higher. So I would say they should get the mesh reinforcement,” he concluded.
Dr. Aher reported having no financial conflicts regarding this study.
LAS VEGAS – Routine use of mesh reinforcement when performing laparoscopic repair of hiatal hernia defects 5 cm or larger in diameter is associated with a low recurrence rate, Dr. Chetan V. Aher reported at the annual Minimally Invasive Surgery Week.
His coinvestigators had shown in an earlier randomized controlled trial that mesh reinforcement of primary cruroplasty in patients with a hernia of 8 cm or greater was associated with no recurrences. Repair with simple cruroplasty was associated with a 22% recurrence rate (Arch. Surg. 2002;137:649-52).

However, Dr. Aher and his coinvestigators subsequently observed a high recurrence rate following mesh-free simple cruroplasty for defects in the 5- to 8-cm range. He presented a case series involving 1,094 laparoscopic hiatal hernia repairs performed since he and his colleagues changed their practice by lowering their threshold for polytetrafluoroethylene mesh reinforcement to defects of at least 5 cm from their prior standard of 8 cm or more.
Hernias were less than 5 cm in diameter in 84% of the patients, so mesh wasn’t used for those repairs. In the remaining 178 patients – those with hernias of at least 5 cm – PTFE mesh was utilized to circumferentially reinforce the cruroplasty.
During a mean follow-up of 3.1 years, the hernia recurrence rate was 1.7% in the group with hernia defects of less than 5 cm and similar at 1.1% in those who received mesh reinforcement because their hernias were larger, reported Dr. Aher of Rush University Medical Center in Chicago.
Operative time and length of stay were longer in the mesh reinforcement group (see chart).
“There’s more dissection when using mesh, and obviously the placement of the mesh takes a little longer,” he noted at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
All repairs were performed using cruroplasty with interrupted nonabsorbable sutures approximating the right and left bundles of the right crura.
Laparoscopic repair has become the standard approach in the primary repair of hiatal hernias. In a 2010 survey of members of the Society of Gastrointestinal and Endoscopic Surgeons conducted by Dr. Aher’s colleagues, respondents indicated they laparoscopically performed 77% of their mesh-reinforced repairs. However, the survey results underscored a lack of consensus within the surgical community regarding mesh usage. Biologic mesh was used by 28% of surgeons; 25% used PTFE (polytetrafluoroethylene), and 21% polypropylene. Mesh placement practices also varied widely: 14% of surgeons utilized anterior placement, 34% posterior, and only 10% circumferential (Surg. Endosc. 2010;24:1017-24).
Asked how he counsels patients about the competing risks of mesh erosion and hernia recurrence in the absence of mesh reinforcement, Dr. Aher pointed to the 22% recurrence risk with large hernias in the earlier randomized trial.
“I would counsel my own family that if you have a large hernia, the risk of mesh erosion is very low and the risk of undergoing a recurrent operation if there is no mesh reinforcement is, I think, overall higher. So I would say they should get the mesh reinforcement,” he concluded.
Dr. Aher reported having no financial conflicts regarding this study.
LAS VEGAS – Routine use of mesh reinforcement when performing laparoscopic repair of hiatal hernia defects 5 cm or larger in diameter is associated with a low recurrence rate, Dr. Chetan V. Aher reported at the annual Minimally Invasive Surgery Week.
His coinvestigators had shown in an earlier randomized controlled trial that mesh reinforcement of primary cruroplasty in patients with a hernia of 8 cm or greater was associated with no recurrences. Repair with simple cruroplasty was associated with a 22% recurrence rate (Arch. Surg. 2002;137:649-52).

However, Dr. Aher and his coinvestigators subsequently observed a high recurrence rate following mesh-free simple cruroplasty for defects in the 5- to 8-cm range. He presented a case series involving 1,094 laparoscopic hiatal hernia repairs performed since he and his colleagues changed their practice by lowering their threshold for polytetrafluoroethylene mesh reinforcement to defects of at least 5 cm from their prior standard of 8 cm or more.
Hernias were less than 5 cm in diameter in 84% of the patients, so mesh wasn’t used for those repairs. In the remaining 178 patients – those with hernias of at least 5 cm – PTFE mesh was utilized to circumferentially reinforce the cruroplasty.
During a mean follow-up of 3.1 years, the hernia recurrence rate was 1.7% in the group with hernia defects of less than 5 cm and similar at 1.1% in those who received mesh reinforcement because their hernias were larger, reported Dr. Aher of Rush University Medical Center in Chicago.
Operative time and length of stay were longer in the mesh reinforcement group (see chart).
“There’s more dissection when using mesh, and obviously the placement of the mesh takes a little longer,” he noted at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
All repairs were performed using cruroplasty with interrupted nonabsorbable sutures approximating the right and left bundles of the right crura.
Laparoscopic repair has become the standard approach in the primary repair of hiatal hernias. In a 2010 survey of members of the Society of Gastrointestinal and Endoscopic Surgeons conducted by Dr. Aher’s colleagues, respondents indicated they laparoscopically performed 77% of their mesh-reinforced repairs. However, the survey results underscored a lack of consensus within the surgical community regarding mesh usage. Biologic mesh was used by 28% of surgeons; 25% used PTFE (polytetrafluoroethylene), and 21% polypropylene. Mesh placement practices also varied widely: 14% of surgeons utilized anterior placement, 34% posterior, and only 10% circumferential (Surg. Endosc. 2010;24:1017-24).
Asked how he counsels patients about the competing risks of mesh erosion and hernia recurrence in the absence of mesh reinforcement, Dr. Aher pointed to the 22% recurrence risk with large hernias in the earlier randomized trial.
“I would counsel my own family that if you have a large hernia, the risk of mesh erosion is very low and the risk of undergoing a recurrent operation if there is no mesh reinforcement is, I think, overall higher. So I would say they should get the mesh reinforcement,” he concluded.
Dr. Aher reported having no financial conflicts regarding this study.
AT MINIMALLY INVASIVE SURGERY WEEK
Key clinical point: Using hernia defect size to guide selective use of mesh reinforcement in laparoscopic hiatal hernia repair results in a low recurrence rate and excellent safety.
Major finding: The hernia recurrence rate was 1.7% in patients who underwent primary cruroplasty for hernias less than 5 cm in diameter and 1.1% in those who received mesh reinforcement because their hernias exceeded that size.
Data source: This was a retrospective study of 1,094 patients who underwent laparoscopic hiatal hernia repair since the investigators changed their threshold for utilizing mesh reinforcement from 8- to 5-cm hernia defects.
Disclosures: The presenter reported having no financial conflicts.
Ovarian cancer often arises from precursor endometriosis
LAS VEGAS – Gynecologists, general surgeons, and primary care physicians now share an unprecedented opportunity to put a major dent in the incidence of ovarian cancer, according to Dr. Farr R. Nezhat.
Mounting evidence suggests that identification and complete surgical removal of endometriosis reduce the risk of several histologic types of ovarian cancer. So when a woman visits her primary care physician for pelvic pain or vaginal bleeding that might be due to endometrial pathology, or a general surgeon finds asymptomatic endometriosis during pelvic surgery, these encounters provide an opportunity for preventive intervention, explained Dr. Nezhat, professor of ob.gyn. and director of minimally invasive surgery and gynecologic robotics at Mount Sinai Medical Center, New York.
The latest thinking about the pathophysiology of ovarian cancer, he noted, is that there are two different types of the malignancy. One type, which likely arises from endometriosis as the precursor lesion, is characterized by low-grade serous, clear cell, and endometrioid carcinomas, which tend to present at an earlier stage and are more indolent. They are associated with mutations in the PTEN, BCL2, and ARID1A genes.
A pooled analysis of 13 ovarian cancer case-control studies conducted by investigators in the Ovarian Cancer Association Consortium made the point that women with endometriosis are at increased risk of specific subtypes of the malignancy. The analysis, which included 7,911 women with invasive ovarian cancer, 1,907 others with borderline ovarian cancer, and more than 13,000 controls, concluded that women with a self-reported history of endometriosis had a 3.05-fold increased risk of clear cell invasive ovarian cancer, compared with controls, a 2.04-fold increased risk of endometrioid ovarian cancer, and a 2.11-fold greater likelihood of low-grade serous ovarian cancer.
In contrast, no association was apparent between endometriosis and the risk of high-grade serous or mucinous invasive ovarian cancer or borderline tumors. Thus, the pathogenesis of low- and high-grade serous ovarian cancers may differ (Lancet Oncol. 2012;13:385-94).
Dr. Nezhat cited as another influential study a Swedish national registry case-control study involving all Swedes with a first-time hospital discharge diagnosis of endometriosis during 1969-2007. The cases in this study were all 220 Swedish women diagnosed with epithelial ovarian cancer at least 1 year after their endometriosis was diagnosed. Each was matched with two controls with no ovarian cancer diagnosis before the date of the case’s cancer diagnosis.
This was the first published study to demonstrate that treatment of endometriosis has a salutary impact on subsequent risk of ovarian cancer. Complete surgical removal of all visible endometriotic tissue was associated with a 63% reduction in the risk of ovarian cancer in a univariate analysis and a 70% relative risk reduction in a multivariate analysis. One-sided oophorectomy involving the endometriosis-involved ovary was similarly associated with a 58% risk reduction for ovarian cancer in a univariate analysis and an 81% reduction in risk in a multivariate analysis (Acta Obstet. Gynecol. Scand. 2013:92:546-54).
An earlier study in which Dr. Nezhat was senior author highlighted that different histologic types of early-stage ovarian carcinoma feature distinctive patterns of clinical symptoms. The study included 76 consecutive patients with FIGO stage I ovarian carcinoma, of which 54 – that is, more than two-thirds – were nonserous, which is a much higher proportion than is seen in women diagnosed with stage III and IV disease.
Most patients with serous papillary carcinoma in this series presented with an asymptomatic pelvic mass. In contrast, most of those with endometrioid or clear cell carcinoma presented with pelvic pain or abnormal vaginal bleeding with or without a pelvic mass (Fertil. Steril. 2007;88:906-10).
Endometrioisis is a pervasive condition. Dr. Nezhat said the endometriosis patients he considers to be at possible increased risk for ovarian cancer include those with longstanding endometriosis, a history of infertility, endometriosis diagnosed at an early age, as well as those with ovarian endometriomas. Eventually it will be possible to pin down more precisely the ovarian cancer risk of an individual with endometriosis through screening for genetic mutations, but the evidence base isn’t yet sufficient to introduce this into everyday practice, he said.
One audience member said it’s her practice and that of many of her gynecologic colleagues that when they incidentally find a patient has asymptomatic endometriosis, for example, during surgery for ectopic pregnancy, they will often leave it in place, even if it is quite severe. Is it time to rethink that practice and instead remove all visible endometriosis, even if the patient is asymptomatic? she asked.
“The short answer is, Yes,” Dr. Nezhat replied. “The most important thing is that when you do surgery, remove it all or else do biopsies to make sure you’re not leaving early ovarian cancer behind. Draining endometriomas is not adequate.”
He reported having no relevant financial conflicts.
LAS VEGAS – Gynecologists, general surgeons, and primary care physicians now share an unprecedented opportunity to put a major dent in the incidence of ovarian cancer, according to Dr. Farr R. Nezhat.
Mounting evidence suggests that identification and complete surgical removal of endometriosis reduce the risk of several histologic types of ovarian cancer. So when a woman visits her primary care physician for pelvic pain or vaginal bleeding that might be due to endometrial pathology, or a general surgeon finds asymptomatic endometriosis during pelvic surgery, these encounters provide an opportunity for preventive intervention, explained Dr. Nezhat, professor of ob.gyn. and director of minimally invasive surgery and gynecologic robotics at Mount Sinai Medical Center, New York.
The latest thinking about the pathophysiology of ovarian cancer, he noted, is that there are two different types of the malignancy. One type, which likely arises from endometriosis as the precursor lesion, is characterized by low-grade serous, clear cell, and endometrioid carcinomas, which tend to present at an earlier stage and are more indolent. They are associated with mutations in the PTEN, BCL2, and ARID1A genes.
A pooled analysis of 13 ovarian cancer case-control studies conducted by investigators in the Ovarian Cancer Association Consortium made the point that women with endometriosis are at increased risk of specific subtypes of the malignancy. The analysis, which included 7,911 women with invasive ovarian cancer, 1,907 others with borderline ovarian cancer, and more than 13,000 controls, concluded that women with a self-reported history of endometriosis had a 3.05-fold increased risk of clear cell invasive ovarian cancer, compared with controls, a 2.04-fold increased risk of endometrioid ovarian cancer, and a 2.11-fold greater likelihood of low-grade serous ovarian cancer.
In contrast, no association was apparent between endometriosis and the risk of high-grade serous or mucinous invasive ovarian cancer or borderline tumors. Thus, the pathogenesis of low- and high-grade serous ovarian cancers may differ (Lancet Oncol. 2012;13:385-94).
Dr. Nezhat cited as another influential study a Swedish national registry case-control study involving all Swedes with a first-time hospital discharge diagnosis of endometriosis during 1969-2007. The cases in this study were all 220 Swedish women diagnosed with epithelial ovarian cancer at least 1 year after their endometriosis was diagnosed. Each was matched with two controls with no ovarian cancer diagnosis before the date of the case’s cancer diagnosis.
This was the first published study to demonstrate that treatment of endometriosis has a salutary impact on subsequent risk of ovarian cancer. Complete surgical removal of all visible endometriotic tissue was associated with a 63% reduction in the risk of ovarian cancer in a univariate analysis and a 70% relative risk reduction in a multivariate analysis. One-sided oophorectomy involving the endometriosis-involved ovary was similarly associated with a 58% risk reduction for ovarian cancer in a univariate analysis and an 81% reduction in risk in a multivariate analysis (Acta Obstet. Gynecol. Scand. 2013:92:546-54).
An earlier study in which Dr. Nezhat was senior author highlighted that different histologic types of early-stage ovarian carcinoma feature distinctive patterns of clinical symptoms. The study included 76 consecutive patients with FIGO stage I ovarian carcinoma, of which 54 – that is, more than two-thirds – were nonserous, which is a much higher proportion than is seen in women diagnosed with stage III and IV disease.
Most patients with serous papillary carcinoma in this series presented with an asymptomatic pelvic mass. In contrast, most of those with endometrioid or clear cell carcinoma presented with pelvic pain or abnormal vaginal bleeding with or without a pelvic mass (Fertil. Steril. 2007;88:906-10).
Endometrioisis is a pervasive condition. Dr. Nezhat said the endometriosis patients he considers to be at possible increased risk for ovarian cancer include those with longstanding endometriosis, a history of infertility, endometriosis diagnosed at an early age, as well as those with ovarian endometriomas. Eventually it will be possible to pin down more precisely the ovarian cancer risk of an individual with endometriosis through screening for genetic mutations, but the evidence base isn’t yet sufficient to introduce this into everyday practice, he said.
One audience member said it’s her practice and that of many of her gynecologic colleagues that when they incidentally find a patient has asymptomatic endometriosis, for example, during surgery for ectopic pregnancy, they will often leave it in place, even if it is quite severe. Is it time to rethink that practice and instead remove all visible endometriosis, even if the patient is asymptomatic? she asked.
“The short answer is, Yes,” Dr. Nezhat replied. “The most important thing is that when you do surgery, remove it all or else do biopsies to make sure you’re not leaving early ovarian cancer behind. Draining endometriomas is not adequate.”
He reported having no relevant financial conflicts.
LAS VEGAS – Gynecologists, general surgeons, and primary care physicians now share an unprecedented opportunity to put a major dent in the incidence of ovarian cancer, according to Dr. Farr R. Nezhat.
Mounting evidence suggests that identification and complete surgical removal of endometriosis reduce the risk of several histologic types of ovarian cancer. So when a woman visits her primary care physician for pelvic pain or vaginal bleeding that might be due to endometrial pathology, or a general surgeon finds asymptomatic endometriosis during pelvic surgery, these encounters provide an opportunity for preventive intervention, explained Dr. Nezhat, professor of ob.gyn. and director of minimally invasive surgery and gynecologic robotics at Mount Sinai Medical Center, New York.
The latest thinking about the pathophysiology of ovarian cancer, he noted, is that there are two different types of the malignancy. One type, which likely arises from endometriosis as the precursor lesion, is characterized by low-grade serous, clear cell, and endometrioid carcinomas, which tend to present at an earlier stage and are more indolent. They are associated with mutations in the PTEN, BCL2, and ARID1A genes.
A pooled analysis of 13 ovarian cancer case-control studies conducted by investigators in the Ovarian Cancer Association Consortium made the point that women with endometriosis are at increased risk of specific subtypes of the malignancy. The analysis, which included 7,911 women with invasive ovarian cancer, 1,907 others with borderline ovarian cancer, and more than 13,000 controls, concluded that women with a self-reported history of endometriosis had a 3.05-fold increased risk of clear cell invasive ovarian cancer, compared with controls, a 2.04-fold increased risk of endometrioid ovarian cancer, and a 2.11-fold greater likelihood of low-grade serous ovarian cancer.
In contrast, no association was apparent between endometriosis and the risk of high-grade serous or mucinous invasive ovarian cancer or borderline tumors. Thus, the pathogenesis of low- and high-grade serous ovarian cancers may differ (Lancet Oncol. 2012;13:385-94).
Dr. Nezhat cited as another influential study a Swedish national registry case-control study involving all Swedes with a first-time hospital discharge diagnosis of endometriosis during 1969-2007. The cases in this study were all 220 Swedish women diagnosed with epithelial ovarian cancer at least 1 year after their endometriosis was diagnosed. Each was matched with two controls with no ovarian cancer diagnosis before the date of the case’s cancer diagnosis.
This was the first published study to demonstrate that treatment of endometriosis has a salutary impact on subsequent risk of ovarian cancer. Complete surgical removal of all visible endometriotic tissue was associated with a 63% reduction in the risk of ovarian cancer in a univariate analysis and a 70% relative risk reduction in a multivariate analysis. One-sided oophorectomy involving the endometriosis-involved ovary was similarly associated with a 58% risk reduction for ovarian cancer in a univariate analysis and an 81% reduction in risk in a multivariate analysis (Acta Obstet. Gynecol. Scand. 2013:92:546-54).
An earlier study in which Dr. Nezhat was senior author highlighted that different histologic types of early-stage ovarian carcinoma feature distinctive patterns of clinical symptoms. The study included 76 consecutive patients with FIGO stage I ovarian carcinoma, of which 54 – that is, more than two-thirds – were nonserous, which is a much higher proportion than is seen in women diagnosed with stage III and IV disease.
Most patients with serous papillary carcinoma in this series presented with an asymptomatic pelvic mass. In contrast, most of those with endometrioid or clear cell carcinoma presented with pelvic pain or abnormal vaginal bleeding with or without a pelvic mass (Fertil. Steril. 2007;88:906-10).
Endometrioisis is a pervasive condition. Dr. Nezhat said the endometriosis patients he considers to be at possible increased risk for ovarian cancer include those with longstanding endometriosis, a history of infertility, endometriosis diagnosed at an early age, as well as those with ovarian endometriomas. Eventually it will be possible to pin down more precisely the ovarian cancer risk of an individual with endometriosis through screening for genetic mutations, but the evidence base isn’t yet sufficient to introduce this into everyday practice, he said.
One audience member said it’s her practice and that of many of her gynecologic colleagues that when they incidentally find a patient has asymptomatic endometriosis, for example, during surgery for ectopic pregnancy, they will often leave it in place, even if it is quite severe. Is it time to rethink that practice and instead remove all visible endometriosis, even if the patient is asymptomatic? she asked.
“The short answer is, Yes,” Dr. Nezhat replied. “The most important thing is that when you do surgery, remove it all or else do biopsies to make sure you’re not leaving early ovarian cancer behind. Draining endometriomas is not adequate.”
He reported having no relevant financial conflicts.
EXPERT ANALYSIS FROM MINIMALLY INVASIVE SURGERY WEEK
Paradigm shift: Prophylactic salpingectomy for ovarian cancer risk reduction
LAS VEGAS – Removing the fallopian tubes at the time of pelvic surgeries as a potential means of reducing ovarian cancer risk appears to be a movement that’s picking up steam in clinical practice.
A recent survey of 234 U.S. gynecologists showed prophylactic bilateral salpingectomy is catching on when performed in conjunction with hysterectomy, but far less so for tubal sterilization, Dr. Austin Findley observed at the annual Minimally Invasive Surgery Week.
A total of 54% of respondents indicated they routinely perform salpingectomy at the time of hysterectomy in an effort to reduce the risk of ovarian cancer as well as to avoid the need for reoperations. However, only 7% of the gynecologic surgeons said they perform salpingectomy for tubal sterilization, even though 58% of respondents stated they believe the procedure is the most effective form of tubal sterilization (J. Minim. Invasive Gynecol. 2013;20:517-21).
“In my experience at various hospitals, I think these numbers are a pretty accurate reflection of what folks are doing,” commented Dr. Findley of Wright State University in Dayton, Ohio.
The prophylactic salpingectomy movement is an outgrowth of the tubal hypothesis of ovarian cancer.
“There is now increasing and dramatic evidence to suggest that most ovarian cancers actually originate in the distal fallopian tubes. I think this is a concept most people are unaware of or are just becoming accustomed to. The tubal hypothesis represents a major paradigm shift in the way we think about ovarian cancers. The previous belief that excessive ovulation is a cause of ovarian cancer is no longer regarded as accurate,” he explained at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
Ovarian cancer is the No. 1 cause of mortality from gynecologic malignancy, accounting for more than 14,000 deaths per year, according to National Cancer Institute data. The lifetime risk of the malignancy is 1.3%, with the average age at diagnosis being 63 years.
Only 10%-15% of ovarian cancers occur in women at high risk for the malignancy because they carry a BRCA mutation or other predisposing gene. The vast majority of ovarian cancer deaths are caused by high-grade serous tumors that have been shown to be strongly associated with precursor lesions in the distal fallopian tubes of women at low risk for the malignancy.
There is no proven-effective screening program or risk-reduction method for these low-risk women. However, with 600,000 hysterectomies and 700,000 tubal sterilizations being performed annually in the United States, prophylactic salpingectomy has been advocated as an attractive opportunity to potentially reduce ovarian cancer risk. Other common pelvic surgeries in which it might be used for this purpose include excision of endometriosis and laparoscopy for pelvic pain. It also has recently been shown to be feasible and safe post partum at cesarean or vaginal delivery (Obstet. Gynecol. 2014 [doi: 10.1097/01.AOG.0000447427.80479.ae]).
But the key word here is “potentially.” It must be emphasized that at present the ovarian cancer prevention benefit of prophylactic salpingectomy remains hypothetical; in theory, the procedure should reduce ovarian cancer risk, but there is not yet persuasive evidence that it actually does, Dr. Findley emphasized at the meeting, presented by the Society of Laparoendoscopic Surgeons and affiliated societies.
In contrast, one well-established ancillary benefit of prophylactic salpingectomy is that it eliminates the need for future reoperation for salpingectomy. This was demonstrated in a large Danish cohort study including close to 10,000 women undergoing hysterectomy and a similar number undergoing sterilization procedures. Among the nearly two-thirds of hysterectomy patients who had both fallopian tubes retained, there was a 2.13-fold increased likelihood of subsequent salpingectomy, compared with nonhysterectomized women.
Similarly, Danish women who underwent a sterilization procedure with retention of the fallopian tubes – typically tubal ligation with clips – were 2.42 times more likely to undergo subsequent salpingectomy, most often because of the development of hydrosalpinx, infection, ectopic pregnancy, or other complications (BMJ Open 2013;3 [doi:10.1136/bmjopen-2013-002845]).
The most commonly cited potential risk of prophylactic salpingectomy – decreased ovarian function – now appears to be a nonissue. This was demonstrated in a recent retrospective Italian study (Gynecol. Oncol. 2013;129:448-51) as well as in a pilot randomized controlled trial conducted by Dr. Findley and his coworkers (Fertil. Steril. 2013;100:1704-8), which appears to have answered many skeptics’ concerns. Indeed, Dr. Findley’s coinvestigator Dr. Matthew Siedhoff said he has recently been approached by researchers interested in collaborating in a larger confirmatory randomized trial, but all parties eventually agreed it was a no-go.
“It’s a little hard to demonstrate equipoise for a larger randomized controlled trial. We’re beyond that now, given that prophylactic salpingectomy really doesn’t seem to make a difference as far as ovarian function,” according to Dr. Siedhoff, director of the division of advanced laparoscopy and pelvic pain at the University of North Carolina, Chapel Hill.
Another oft-expressed reservation about salpingectomy as a means of reducing ovarian cancer risk in women seeking sterilization is that salpingectomy’s irreversibility may lead to “tubal regret” on the part of patients who later change their mind about further pregnancies. However, Dr. Findley cited a recent editorial whose authors criticized colleagues who made that claim. The editorialists argued that the tubal regret concern indicates surgeons weren’t really listening to their patients’ true desires during the informed consent conversation.
“We should not have started thinking about salpingectomy for female sterilization only once a decrease in ovarian cancer risk became part of the equation,” they declared (Obstet. Gynecol. 2014;124:596-9).
Dr. Findley noted that Canadian gynecologists are leading the way forward regarding prophylactic salpingectomy as a potential method of ovarian cancer prevention. The Society of Gynecologic Oncology of Canada in a 2011 policy statement recommended patient/physician discussion of the risks and benefits of bilateral salpingectomy for patients undergoing hysterectomy or requesting permanent sterilization. The Society of Gynecologic Oncology followed suit with a similar clinical practice statement in late 2013.
Additionally, the Canadian group declared that a national ovarian cancer prevention study focused on fallopian tube removal should be a top priority.
Gynecologic oncologists in British Columbia recently reported the eye-catching results of a province-wide educational initiative targeting gynecologists and their patients. In 2010, all British Columbia gynecologists had to attend a course on the role of the fallopian tubes in the development of ovarian cancer, during which they were advised to consider performing bilateral salpingectomy for ovarian cancer risk reduction.
Surgical practice changed dramatically in British Columbia in response. In 2009 – the year prior to the physician education initiative – salpingectomy was utilized in just 0.3% of permanent sterilization procedures. In 2010, it was 11.4%. By 2011, it was 33.3%.
Similarly, only 7% of hysterectomies performed in British Columbia in 2009 were accompanied by bilateral salpingectomy. This figure climbed to 23% in 2010 and jumped further to 35% in 2011. Meanwhile the rate of hysterectomy with bilateral salpingo-oophorectomy remained steady over time at 44% (Am. J. Obstet. Gynecol. 2014;210:471.e1-11).
This project was conducted in collaboration with the B.C. Cancer Agency, which maintains comprehensive province-wide registries. Over time, it will be possible to demonstrate whether prophylactic salpingectomy is indeed associated with a reduction in the incidence of ovarian cancer. “I think this study demonstrated that there’s a lack of awareness on this issue, but also [that there’s] potential effectiveness of introducing an educational initiative like this in changing our practice patterns. As we start talking more about this issue amongst our colleagues and our patients, we’re more likely to see a practice pattern shift in the United States as well,” Dr. Findley commented.
He reported having no financial conflicts with regard to his presentation.
The practice of salpingectomy for ovarian cancer risk reduction has quietly gained momentum in the gynecology world, however, it has not been well advertised in the patient community, despite steadily increasing amounts of data to support its plausibility as a risk-reducing strategy. Recent surveys reveal that physicians are slowly changing practices and including “prophylactic” salpingectomy during benign gynecologic surgeries, including at the time of hysterectomy, tubal sterilization (including at the time of cesarean section), and at the time of surgery for other benign gynecologic conditions, such as laparoscopy for endometriosis.
While the change in practice is encouraging, the supporting hypothesis is still in its infancy. The historical theory of the etiology of ovarian cancer states that ovulation events led to an increased risk of ovarian cancer. The theory of “incessant ovulation” suggested that the epithelium of the ovary is sensitive to the number of events of ovulation, which may in turn act as a promoting factor in the carcinogenic process (Clinical Gynecologic Oncology, 8th ed.; Epithelial Ovarian Cancer (Chapter 11) [Maryland Heights, Mo.: Mosby, 2012]). This was supported by epidemiologic data that noted that women who used oral contraceptives, had multiple pregnancies, breastfed, and underwent late menarche and early menopause were at decreased risk of developing ovarian cancer (Cancer Causes Control 2007;18:517 ; Am. J. Epidemiol. 1992;136:1184-203; Int. J. Epidemiol. 2000;29:799-802). The hypothesis was adopted, as the epidemiology of ovulation was supportive.
The weakness of the incessant ovulation theory has been our inability to identify precursor lesions. In almost all other gynecologic malignancies, a precursor lesion has been identified and supports a theory of carcinogenesis. In patients with ovarian cancer, over 80% are diagnosed with advanced stage, and this is where the new theory of the pathogenesis of ovarian cancer originating in the fimbriated end of the fallopian tube begins to have credibility. Serous tubal intraepithelial carcinoma (STIC) lesions are the proposed precursor lesions to high-grade serous carcinomas. STIC lesions exhibit histologic features of morphologic atypia (increased nuclear/cytoplasmic ratio, prominent nucleoli, increased proliferation with an intact basement membrane, variably stratified fallopian tube epithelium with nuclear pleomorphism) and have evidence of TP53 mutations (J. Pathol. 2012;226:421-6 ). STIC lesions were first described as a potential precursor to fallopian tube serous carcinoma in the 1950s, however, it was not proposed as a precursor to extra-fallopian tube serous pelvic cancers until the 2000s (Am. J. Obstet. Gynecol. 1950;59:58-67). One of the suggested pathogeneses of this evolving hypothesis stipulates that TP53 mutations are associated with telomere shortening, one of the main genetic manifestations in cancer development, leading to chromosomal instability, gene expression reprogramming, and tumor progression (Am. J. Surg. Pathol. 2010;34:829-3). The finding of TP-53 mutations in STIC further supports the STIC precursor hypothesis, as identical mutations have been reported in concurrent high-grade serous carcinomas, providing evidence that supports the clonal relationship of the two lesions (J. Pathol. 2012;226:421-6 ). The theory further stipulates that STIC cells can exfoliate and disseminate to the ovary and peritoneal surfaces prior to becoming invasive, and subsequently demonstrating invasion at the distant sites. In addition, this theory can explain the development of primary peritoneal high-grade serous cancer, a disease essentially identical to high-grade serous ovarian cancer, although the etiology of this disease is largely unknown.
Interest in the STIC to extra–fallopian tube serous cancers hypothesis was enhanced by the histopathologic evaluation of the ovaries and fallopian tubes of BRCA-positive women undergoing prophylactic bilateral salpingo-oophorectomy. In this population, women were diagnosed with a serous cancer (up to 17%), and roughly 80% occurred in the fallopian tube (Gynecol. Oncol. 2002;87:52-6 ). STIC was subsequently described to occur not only in BRCA-positive women, but in sporadic cases of serous cancer as well (Am. J. Surg. Pathol. 2007;31:161-9).Additionally, up to 60%-70% of sporadic high-grade serous cancers (ovarian, primary peritoneal) have been reported to have STIC lesions on final pathology (Int. J. Gynecol. Cancer 2009;19:58-64 ). The finding of a STIC lesion is not routinely noted in pathology reports however, possibly due to the lack of serial sectioning of tubes and ovaries in the general population, when no germline mutation is present.
While the majority of the data supporting STIC as a potential precursor lesion to ovarian cancer is from the BRCA literature, the application of the theory can be and has been extrapolated to women at baseline ovarian cancer risk. As described in the article presented, there appears to be a paradigm shift in benign gynecology practice towards prophylactic salpingectomy for ovarian cancer risk reduction. The appropriate application of the prophylactic salpingectomy should be as described – at the time of benign hysterectomies, tubal sterilizations, and can be performed at the time of surgeries for other benign conditions (endometriosis, pelvic masses, diagnostic laparoscopies).
The data from this paradigm shift in practice will contribute significantly to answering some of the many questions surrounding this hypothesis, including the incidence of STIC in the baseline risk population, as well as answer the question of whether this practice will actually reduce the ovarian cancer incidence in the years to come. Additionally, investigation into the efficacy of ovarian cancer risk reduction of prophylactic salpingectomy in the high-risk patients (those with germline mutations) who undergo ovarian conservation at the time of salpingectomy is imperative. These women are currently counseled to undergo prophylactic bilateral salpingo-oophorectomy at the age of 35 or at the time of childbearing completion. As data support that oophorectomy for benign disease in women under the age of 50 increases all-cause mortality (Obstet. Gynecol. 2009;113:1027-37), the impact that prophylactic salpingectomy with ovarian conservation has in this population could be monumental, as this represents a group of women subjected to the sequelae of early surgical menopause. Furthermore, given the current economic climate of modern medicine, additional investigation into the cost-effectiveness of salpingectomy as a risk-reducing option in both women with increased risk (germline mutation) and in the general population, is indicated.
In conclusion, the practice of prophylactic salpingectomy is still in its infancy. The early paradigm shift will certainly contribute to the existing literature and potentially improve our ability to reduce risk of ovarian cancer, without compromising the overall health of our patients through surgical castration. The current hypothesis of STIC as the primary site for ovarian cancer carcinogenesis is certainly plausible and may allow for improved screening modalities and targeted therapies, which may lead to improved outcomes for our patients.
Caroline C. Billingsley, M.D., and Larry J. Copeland, M.D., who are gynecologic oncologists at Ohio State University, Columbus, wrote this commentary.
The practice of salpingectomy for ovarian cancer risk reduction has quietly gained momentum in the gynecology world, however, it has not been well advertised in the patient community, despite steadily increasing amounts of data to support its plausibility as a risk-reducing strategy. Recent surveys reveal that physicians are slowly changing practices and including “prophylactic” salpingectomy during benign gynecologic surgeries, including at the time of hysterectomy, tubal sterilization (including at the time of cesarean section), and at the time of surgery for other benign gynecologic conditions, such as laparoscopy for endometriosis.
While the change in practice is encouraging, the supporting hypothesis is still in its infancy. The historical theory of the etiology of ovarian cancer states that ovulation events led to an increased risk of ovarian cancer. The theory of “incessant ovulation” suggested that the epithelium of the ovary is sensitive to the number of events of ovulation, which may in turn act as a promoting factor in the carcinogenic process (Clinical Gynecologic Oncology, 8th ed.; Epithelial Ovarian Cancer (Chapter 11) [Maryland Heights, Mo.: Mosby, 2012]). This was supported by epidemiologic data that noted that women who used oral contraceptives, had multiple pregnancies, breastfed, and underwent late menarche and early menopause were at decreased risk of developing ovarian cancer (Cancer Causes Control 2007;18:517 ; Am. J. Epidemiol. 1992;136:1184-203; Int. J. Epidemiol. 2000;29:799-802). The hypothesis was adopted, as the epidemiology of ovulation was supportive.
The weakness of the incessant ovulation theory has been our inability to identify precursor lesions. In almost all other gynecologic malignancies, a precursor lesion has been identified and supports a theory of carcinogenesis. In patients with ovarian cancer, over 80% are diagnosed with advanced stage, and this is where the new theory of the pathogenesis of ovarian cancer originating in the fimbriated end of the fallopian tube begins to have credibility. Serous tubal intraepithelial carcinoma (STIC) lesions are the proposed precursor lesions to high-grade serous carcinomas. STIC lesions exhibit histologic features of morphologic atypia (increased nuclear/cytoplasmic ratio, prominent nucleoli, increased proliferation with an intact basement membrane, variably stratified fallopian tube epithelium with nuclear pleomorphism) and have evidence of TP53 mutations (J. Pathol. 2012;226:421-6 ). STIC lesions were first described as a potential precursor to fallopian tube serous carcinoma in the 1950s, however, it was not proposed as a precursor to extra-fallopian tube serous pelvic cancers until the 2000s (Am. J. Obstet. Gynecol. 1950;59:58-67). One of the suggested pathogeneses of this evolving hypothesis stipulates that TP53 mutations are associated with telomere shortening, one of the main genetic manifestations in cancer development, leading to chromosomal instability, gene expression reprogramming, and tumor progression (Am. J. Surg. Pathol. 2010;34:829-3). The finding of TP-53 mutations in STIC further supports the STIC precursor hypothesis, as identical mutations have been reported in concurrent high-grade serous carcinomas, providing evidence that supports the clonal relationship of the two lesions (J. Pathol. 2012;226:421-6 ). The theory further stipulates that STIC cells can exfoliate and disseminate to the ovary and peritoneal surfaces prior to becoming invasive, and subsequently demonstrating invasion at the distant sites. In addition, this theory can explain the development of primary peritoneal high-grade serous cancer, a disease essentially identical to high-grade serous ovarian cancer, although the etiology of this disease is largely unknown.
Interest in the STIC to extra–fallopian tube serous cancers hypothesis was enhanced by the histopathologic evaluation of the ovaries and fallopian tubes of BRCA-positive women undergoing prophylactic bilateral salpingo-oophorectomy. In this population, women were diagnosed with a serous cancer (up to 17%), and roughly 80% occurred in the fallopian tube (Gynecol. Oncol. 2002;87:52-6 ). STIC was subsequently described to occur not only in BRCA-positive women, but in sporadic cases of serous cancer as well (Am. J. Surg. Pathol. 2007;31:161-9).Additionally, up to 60%-70% of sporadic high-grade serous cancers (ovarian, primary peritoneal) have been reported to have STIC lesions on final pathology (Int. J. Gynecol. Cancer 2009;19:58-64 ). The finding of a STIC lesion is not routinely noted in pathology reports however, possibly due to the lack of serial sectioning of tubes and ovaries in the general population, when no germline mutation is present.
While the majority of the data supporting STIC as a potential precursor lesion to ovarian cancer is from the BRCA literature, the application of the theory can be and has been extrapolated to women at baseline ovarian cancer risk. As described in the article presented, there appears to be a paradigm shift in benign gynecology practice towards prophylactic salpingectomy for ovarian cancer risk reduction. The appropriate application of the prophylactic salpingectomy should be as described – at the time of benign hysterectomies, tubal sterilizations, and can be performed at the time of surgeries for other benign conditions (endometriosis, pelvic masses, diagnostic laparoscopies).
The data from this paradigm shift in practice will contribute significantly to answering some of the many questions surrounding this hypothesis, including the incidence of STIC in the baseline risk population, as well as answer the question of whether this practice will actually reduce the ovarian cancer incidence in the years to come. Additionally, investigation into the efficacy of ovarian cancer risk reduction of prophylactic salpingectomy in the high-risk patients (those with germline mutations) who undergo ovarian conservation at the time of salpingectomy is imperative. These women are currently counseled to undergo prophylactic bilateral salpingo-oophorectomy at the age of 35 or at the time of childbearing completion. As data support that oophorectomy for benign disease in women under the age of 50 increases all-cause mortality (Obstet. Gynecol. 2009;113:1027-37), the impact that prophylactic salpingectomy with ovarian conservation has in this population could be monumental, as this represents a group of women subjected to the sequelae of early surgical menopause. Furthermore, given the current economic climate of modern medicine, additional investigation into the cost-effectiveness of salpingectomy as a risk-reducing option in both women with increased risk (germline mutation) and in the general population, is indicated.
In conclusion, the practice of prophylactic salpingectomy is still in its infancy. The early paradigm shift will certainly contribute to the existing literature and potentially improve our ability to reduce risk of ovarian cancer, without compromising the overall health of our patients through surgical castration. The current hypothesis of STIC as the primary site for ovarian cancer carcinogenesis is certainly plausible and may allow for improved screening modalities and targeted therapies, which may lead to improved outcomes for our patients.
Caroline C. Billingsley, M.D., and Larry J. Copeland, M.D., who are gynecologic oncologists at Ohio State University, Columbus, wrote this commentary.
The practice of salpingectomy for ovarian cancer risk reduction has quietly gained momentum in the gynecology world, however, it has not been well advertised in the patient community, despite steadily increasing amounts of data to support its plausibility as a risk-reducing strategy. Recent surveys reveal that physicians are slowly changing practices and including “prophylactic” salpingectomy during benign gynecologic surgeries, including at the time of hysterectomy, tubal sterilization (including at the time of cesarean section), and at the time of surgery for other benign gynecologic conditions, such as laparoscopy for endometriosis.
While the change in practice is encouraging, the supporting hypothesis is still in its infancy. The historical theory of the etiology of ovarian cancer states that ovulation events led to an increased risk of ovarian cancer. The theory of “incessant ovulation” suggested that the epithelium of the ovary is sensitive to the number of events of ovulation, which may in turn act as a promoting factor in the carcinogenic process (Clinical Gynecologic Oncology, 8th ed.; Epithelial Ovarian Cancer (Chapter 11) [Maryland Heights, Mo.: Mosby, 2012]). This was supported by epidemiologic data that noted that women who used oral contraceptives, had multiple pregnancies, breastfed, and underwent late menarche and early menopause were at decreased risk of developing ovarian cancer (Cancer Causes Control 2007;18:517 ; Am. J. Epidemiol. 1992;136:1184-203; Int. J. Epidemiol. 2000;29:799-802). The hypothesis was adopted, as the epidemiology of ovulation was supportive.
The weakness of the incessant ovulation theory has been our inability to identify precursor lesions. In almost all other gynecologic malignancies, a precursor lesion has been identified and supports a theory of carcinogenesis. In patients with ovarian cancer, over 80% are diagnosed with advanced stage, and this is where the new theory of the pathogenesis of ovarian cancer originating in the fimbriated end of the fallopian tube begins to have credibility. Serous tubal intraepithelial carcinoma (STIC) lesions are the proposed precursor lesions to high-grade serous carcinomas. STIC lesions exhibit histologic features of morphologic atypia (increased nuclear/cytoplasmic ratio, prominent nucleoli, increased proliferation with an intact basement membrane, variably stratified fallopian tube epithelium with nuclear pleomorphism) and have evidence of TP53 mutations (J. Pathol. 2012;226:421-6 ). STIC lesions were first described as a potential precursor to fallopian tube serous carcinoma in the 1950s, however, it was not proposed as a precursor to extra-fallopian tube serous pelvic cancers until the 2000s (Am. J. Obstet. Gynecol. 1950;59:58-67). One of the suggested pathogeneses of this evolving hypothesis stipulates that TP53 mutations are associated with telomere shortening, one of the main genetic manifestations in cancer development, leading to chromosomal instability, gene expression reprogramming, and tumor progression (Am. J. Surg. Pathol. 2010;34:829-3). The finding of TP-53 mutations in STIC further supports the STIC precursor hypothesis, as identical mutations have been reported in concurrent high-grade serous carcinomas, providing evidence that supports the clonal relationship of the two lesions (J. Pathol. 2012;226:421-6 ). The theory further stipulates that STIC cells can exfoliate and disseminate to the ovary and peritoneal surfaces prior to becoming invasive, and subsequently demonstrating invasion at the distant sites. In addition, this theory can explain the development of primary peritoneal high-grade serous cancer, a disease essentially identical to high-grade serous ovarian cancer, although the etiology of this disease is largely unknown.
Interest in the STIC to extra–fallopian tube serous cancers hypothesis was enhanced by the histopathologic evaluation of the ovaries and fallopian tubes of BRCA-positive women undergoing prophylactic bilateral salpingo-oophorectomy. In this population, women were diagnosed with a serous cancer (up to 17%), and roughly 80% occurred in the fallopian tube (Gynecol. Oncol. 2002;87:52-6 ). STIC was subsequently described to occur not only in BRCA-positive women, but in sporadic cases of serous cancer as well (Am. J. Surg. Pathol. 2007;31:161-9).Additionally, up to 60%-70% of sporadic high-grade serous cancers (ovarian, primary peritoneal) have been reported to have STIC lesions on final pathology (Int. J. Gynecol. Cancer 2009;19:58-64 ). The finding of a STIC lesion is not routinely noted in pathology reports however, possibly due to the lack of serial sectioning of tubes and ovaries in the general population, when no germline mutation is present.
While the majority of the data supporting STIC as a potential precursor lesion to ovarian cancer is from the BRCA literature, the application of the theory can be and has been extrapolated to women at baseline ovarian cancer risk. As described in the article presented, there appears to be a paradigm shift in benign gynecology practice towards prophylactic salpingectomy for ovarian cancer risk reduction. The appropriate application of the prophylactic salpingectomy should be as described – at the time of benign hysterectomies, tubal sterilizations, and can be performed at the time of surgeries for other benign conditions (endometriosis, pelvic masses, diagnostic laparoscopies).
The data from this paradigm shift in practice will contribute significantly to answering some of the many questions surrounding this hypothesis, including the incidence of STIC in the baseline risk population, as well as answer the question of whether this practice will actually reduce the ovarian cancer incidence in the years to come. Additionally, investigation into the efficacy of ovarian cancer risk reduction of prophylactic salpingectomy in the high-risk patients (those with germline mutations) who undergo ovarian conservation at the time of salpingectomy is imperative. These women are currently counseled to undergo prophylactic bilateral salpingo-oophorectomy at the age of 35 or at the time of childbearing completion. As data support that oophorectomy for benign disease in women under the age of 50 increases all-cause mortality (Obstet. Gynecol. 2009;113:1027-37), the impact that prophylactic salpingectomy with ovarian conservation has in this population could be monumental, as this represents a group of women subjected to the sequelae of early surgical menopause. Furthermore, given the current economic climate of modern medicine, additional investigation into the cost-effectiveness of salpingectomy as a risk-reducing option in both women with increased risk (germline mutation) and in the general population, is indicated.
In conclusion, the practice of prophylactic salpingectomy is still in its infancy. The early paradigm shift will certainly contribute to the existing literature and potentially improve our ability to reduce risk of ovarian cancer, without compromising the overall health of our patients through surgical castration. The current hypothesis of STIC as the primary site for ovarian cancer carcinogenesis is certainly plausible and may allow for improved screening modalities and targeted therapies, which may lead to improved outcomes for our patients.
Caroline C. Billingsley, M.D., and Larry J. Copeland, M.D., who are gynecologic oncologists at Ohio State University, Columbus, wrote this commentary.
LAS VEGAS – Removing the fallopian tubes at the time of pelvic surgeries as a potential means of reducing ovarian cancer risk appears to be a movement that’s picking up steam in clinical practice.
A recent survey of 234 U.S. gynecologists showed prophylactic bilateral salpingectomy is catching on when performed in conjunction with hysterectomy, but far less so for tubal sterilization, Dr. Austin Findley observed at the annual Minimally Invasive Surgery Week.
A total of 54% of respondents indicated they routinely perform salpingectomy at the time of hysterectomy in an effort to reduce the risk of ovarian cancer as well as to avoid the need for reoperations. However, only 7% of the gynecologic surgeons said they perform salpingectomy for tubal sterilization, even though 58% of respondents stated they believe the procedure is the most effective form of tubal sterilization (J. Minim. Invasive Gynecol. 2013;20:517-21).
“In my experience at various hospitals, I think these numbers are a pretty accurate reflection of what folks are doing,” commented Dr. Findley of Wright State University in Dayton, Ohio.
The prophylactic salpingectomy movement is an outgrowth of the tubal hypothesis of ovarian cancer.
“There is now increasing and dramatic evidence to suggest that most ovarian cancers actually originate in the distal fallopian tubes. I think this is a concept most people are unaware of or are just becoming accustomed to. The tubal hypothesis represents a major paradigm shift in the way we think about ovarian cancers. The previous belief that excessive ovulation is a cause of ovarian cancer is no longer regarded as accurate,” he explained at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
Ovarian cancer is the No. 1 cause of mortality from gynecologic malignancy, accounting for more than 14,000 deaths per year, according to National Cancer Institute data. The lifetime risk of the malignancy is 1.3%, with the average age at diagnosis being 63 years.
Only 10%-15% of ovarian cancers occur in women at high risk for the malignancy because they carry a BRCA mutation or other predisposing gene. The vast majority of ovarian cancer deaths are caused by high-grade serous tumors that have been shown to be strongly associated with precursor lesions in the distal fallopian tubes of women at low risk for the malignancy.
There is no proven-effective screening program or risk-reduction method for these low-risk women. However, with 600,000 hysterectomies and 700,000 tubal sterilizations being performed annually in the United States, prophylactic salpingectomy has been advocated as an attractive opportunity to potentially reduce ovarian cancer risk. Other common pelvic surgeries in which it might be used for this purpose include excision of endometriosis and laparoscopy for pelvic pain. It also has recently been shown to be feasible and safe post partum at cesarean or vaginal delivery (Obstet. Gynecol. 2014 [doi: 10.1097/01.AOG.0000447427.80479.ae]).
But the key word here is “potentially.” It must be emphasized that at present the ovarian cancer prevention benefit of prophylactic salpingectomy remains hypothetical; in theory, the procedure should reduce ovarian cancer risk, but there is not yet persuasive evidence that it actually does, Dr. Findley emphasized at the meeting, presented by the Society of Laparoendoscopic Surgeons and affiliated societies.
In contrast, one well-established ancillary benefit of prophylactic salpingectomy is that it eliminates the need for future reoperation for salpingectomy. This was demonstrated in a large Danish cohort study including close to 10,000 women undergoing hysterectomy and a similar number undergoing sterilization procedures. Among the nearly two-thirds of hysterectomy patients who had both fallopian tubes retained, there was a 2.13-fold increased likelihood of subsequent salpingectomy, compared with nonhysterectomized women.
Similarly, Danish women who underwent a sterilization procedure with retention of the fallopian tubes – typically tubal ligation with clips – were 2.42 times more likely to undergo subsequent salpingectomy, most often because of the development of hydrosalpinx, infection, ectopic pregnancy, or other complications (BMJ Open 2013;3 [doi:10.1136/bmjopen-2013-002845]).
The most commonly cited potential risk of prophylactic salpingectomy – decreased ovarian function – now appears to be a nonissue. This was demonstrated in a recent retrospective Italian study (Gynecol. Oncol. 2013;129:448-51) as well as in a pilot randomized controlled trial conducted by Dr. Findley and his coworkers (Fertil. Steril. 2013;100:1704-8), which appears to have answered many skeptics’ concerns. Indeed, Dr. Findley’s coinvestigator Dr. Matthew Siedhoff said he has recently been approached by researchers interested in collaborating in a larger confirmatory randomized trial, but all parties eventually agreed it was a no-go.
“It’s a little hard to demonstrate equipoise for a larger randomized controlled trial. We’re beyond that now, given that prophylactic salpingectomy really doesn’t seem to make a difference as far as ovarian function,” according to Dr. Siedhoff, director of the division of advanced laparoscopy and pelvic pain at the University of North Carolina, Chapel Hill.
Another oft-expressed reservation about salpingectomy as a means of reducing ovarian cancer risk in women seeking sterilization is that salpingectomy’s irreversibility may lead to “tubal regret” on the part of patients who later change their mind about further pregnancies. However, Dr. Findley cited a recent editorial whose authors criticized colleagues who made that claim. The editorialists argued that the tubal regret concern indicates surgeons weren’t really listening to their patients’ true desires during the informed consent conversation.
“We should not have started thinking about salpingectomy for female sterilization only once a decrease in ovarian cancer risk became part of the equation,” they declared (Obstet. Gynecol. 2014;124:596-9).
Dr. Findley noted that Canadian gynecologists are leading the way forward regarding prophylactic salpingectomy as a potential method of ovarian cancer prevention. The Society of Gynecologic Oncology of Canada in a 2011 policy statement recommended patient/physician discussion of the risks and benefits of bilateral salpingectomy for patients undergoing hysterectomy or requesting permanent sterilization. The Society of Gynecologic Oncology followed suit with a similar clinical practice statement in late 2013.
Additionally, the Canadian group declared that a national ovarian cancer prevention study focused on fallopian tube removal should be a top priority.
Gynecologic oncologists in British Columbia recently reported the eye-catching results of a province-wide educational initiative targeting gynecologists and their patients. In 2010, all British Columbia gynecologists had to attend a course on the role of the fallopian tubes in the development of ovarian cancer, during which they were advised to consider performing bilateral salpingectomy for ovarian cancer risk reduction.
Surgical practice changed dramatically in British Columbia in response. In 2009 – the year prior to the physician education initiative – salpingectomy was utilized in just 0.3% of permanent sterilization procedures. In 2010, it was 11.4%. By 2011, it was 33.3%.
Similarly, only 7% of hysterectomies performed in British Columbia in 2009 were accompanied by bilateral salpingectomy. This figure climbed to 23% in 2010 and jumped further to 35% in 2011. Meanwhile the rate of hysterectomy with bilateral salpingo-oophorectomy remained steady over time at 44% (Am. J. Obstet. Gynecol. 2014;210:471.e1-11).
This project was conducted in collaboration with the B.C. Cancer Agency, which maintains comprehensive province-wide registries. Over time, it will be possible to demonstrate whether prophylactic salpingectomy is indeed associated with a reduction in the incidence of ovarian cancer. “I think this study demonstrated that there’s a lack of awareness on this issue, but also [that there’s] potential effectiveness of introducing an educational initiative like this in changing our practice patterns. As we start talking more about this issue amongst our colleagues and our patients, we’re more likely to see a practice pattern shift in the United States as well,” Dr. Findley commented.
He reported having no financial conflicts with regard to his presentation.
LAS VEGAS – Removing the fallopian tubes at the time of pelvic surgeries as a potential means of reducing ovarian cancer risk appears to be a movement that’s picking up steam in clinical practice.
A recent survey of 234 U.S. gynecologists showed prophylactic bilateral salpingectomy is catching on when performed in conjunction with hysterectomy, but far less so for tubal sterilization, Dr. Austin Findley observed at the annual Minimally Invasive Surgery Week.
A total of 54% of respondents indicated they routinely perform salpingectomy at the time of hysterectomy in an effort to reduce the risk of ovarian cancer as well as to avoid the need for reoperations. However, only 7% of the gynecologic surgeons said they perform salpingectomy for tubal sterilization, even though 58% of respondents stated they believe the procedure is the most effective form of tubal sterilization (J. Minim. Invasive Gynecol. 2013;20:517-21).
“In my experience at various hospitals, I think these numbers are a pretty accurate reflection of what folks are doing,” commented Dr. Findley of Wright State University in Dayton, Ohio.
The prophylactic salpingectomy movement is an outgrowth of the tubal hypothesis of ovarian cancer.
“There is now increasing and dramatic evidence to suggest that most ovarian cancers actually originate in the distal fallopian tubes. I think this is a concept most people are unaware of or are just becoming accustomed to. The tubal hypothesis represents a major paradigm shift in the way we think about ovarian cancers. The previous belief that excessive ovulation is a cause of ovarian cancer is no longer regarded as accurate,” he explained at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
Ovarian cancer is the No. 1 cause of mortality from gynecologic malignancy, accounting for more than 14,000 deaths per year, according to National Cancer Institute data. The lifetime risk of the malignancy is 1.3%, with the average age at diagnosis being 63 years.
Only 10%-15% of ovarian cancers occur in women at high risk for the malignancy because they carry a BRCA mutation or other predisposing gene. The vast majority of ovarian cancer deaths are caused by high-grade serous tumors that have been shown to be strongly associated with precursor lesions in the distal fallopian tubes of women at low risk for the malignancy.
There is no proven-effective screening program or risk-reduction method for these low-risk women. However, with 600,000 hysterectomies and 700,000 tubal sterilizations being performed annually in the United States, prophylactic salpingectomy has been advocated as an attractive opportunity to potentially reduce ovarian cancer risk. Other common pelvic surgeries in which it might be used for this purpose include excision of endometriosis and laparoscopy for pelvic pain. It also has recently been shown to be feasible and safe post partum at cesarean or vaginal delivery (Obstet. Gynecol. 2014 [doi: 10.1097/01.AOG.0000447427.80479.ae]).
But the key word here is “potentially.” It must be emphasized that at present the ovarian cancer prevention benefit of prophylactic salpingectomy remains hypothetical; in theory, the procedure should reduce ovarian cancer risk, but there is not yet persuasive evidence that it actually does, Dr. Findley emphasized at the meeting, presented by the Society of Laparoendoscopic Surgeons and affiliated societies.
In contrast, one well-established ancillary benefit of prophylactic salpingectomy is that it eliminates the need for future reoperation for salpingectomy. This was demonstrated in a large Danish cohort study including close to 10,000 women undergoing hysterectomy and a similar number undergoing sterilization procedures. Among the nearly two-thirds of hysterectomy patients who had both fallopian tubes retained, there was a 2.13-fold increased likelihood of subsequent salpingectomy, compared with nonhysterectomized women.
Similarly, Danish women who underwent a sterilization procedure with retention of the fallopian tubes – typically tubal ligation with clips – were 2.42 times more likely to undergo subsequent salpingectomy, most often because of the development of hydrosalpinx, infection, ectopic pregnancy, or other complications (BMJ Open 2013;3 [doi:10.1136/bmjopen-2013-002845]).
The most commonly cited potential risk of prophylactic salpingectomy – decreased ovarian function – now appears to be a nonissue. This was demonstrated in a recent retrospective Italian study (Gynecol. Oncol. 2013;129:448-51) as well as in a pilot randomized controlled trial conducted by Dr. Findley and his coworkers (Fertil. Steril. 2013;100:1704-8), which appears to have answered many skeptics’ concerns. Indeed, Dr. Findley’s coinvestigator Dr. Matthew Siedhoff said he has recently been approached by researchers interested in collaborating in a larger confirmatory randomized trial, but all parties eventually agreed it was a no-go.
“It’s a little hard to demonstrate equipoise for a larger randomized controlled trial. We’re beyond that now, given that prophylactic salpingectomy really doesn’t seem to make a difference as far as ovarian function,” according to Dr. Siedhoff, director of the division of advanced laparoscopy and pelvic pain at the University of North Carolina, Chapel Hill.
Another oft-expressed reservation about salpingectomy as a means of reducing ovarian cancer risk in women seeking sterilization is that salpingectomy’s irreversibility may lead to “tubal regret” on the part of patients who later change their mind about further pregnancies. However, Dr. Findley cited a recent editorial whose authors criticized colleagues who made that claim. The editorialists argued that the tubal regret concern indicates surgeons weren’t really listening to their patients’ true desires during the informed consent conversation.
“We should not have started thinking about salpingectomy for female sterilization only once a decrease in ovarian cancer risk became part of the equation,” they declared (Obstet. Gynecol. 2014;124:596-9).
Dr. Findley noted that Canadian gynecologists are leading the way forward regarding prophylactic salpingectomy as a potential method of ovarian cancer prevention. The Society of Gynecologic Oncology of Canada in a 2011 policy statement recommended patient/physician discussion of the risks and benefits of bilateral salpingectomy for patients undergoing hysterectomy or requesting permanent sterilization. The Society of Gynecologic Oncology followed suit with a similar clinical practice statement in late 2013.
Additionally, the Canadian group declared that a national ovarian cancer prevention study focused on fallopian tube removal should be a top priority.
Gynecologic oncologists in British Columbia recently reported the eye-catching results of a province-wide educational initiative targeting gynecologists and their patients. In 2010, all British Columbia gynecologists had to attend a course on the role of the fallopian tubes in the development of ovarian cancer, during which they were advised to consider performing bilateral salpingectomy for ovarian cancer risk reduction.
Surgical practice changed dramatically in British Columbia in response. In 2009 – the year prior to the physician education initiative – salpingectomy was utilized in just 0.3% of permanent sterilization procedures. In 2010, it was 11.4%. By 2011, it was 33.3%.
Similarly, only 7% of hysterectomies performed in British Columbia in 2009 were accompanied by bilateral salpingectomy. This figure climbed to 23% in 2010 and jumped further to 35% in 2011. Meanwhile the rate of hysterectomy with bilateral salpingo-oophorectomy remained steady over time at 44% (Am. J. Obstet. Gynecol. 2014;210:471.e1-11).
This project was conducted in collaboration with the B.C. Cancer Agency, which maintains comprehensive province-wide registries. Over time, it will be possible to demonstrate whether prophylactic salpingectomy is indeed associated with a reduction in the incidence of ovarian cancer. “I think this study demonstrated that there’s a lack of awareness on this issue, but also [that there’s] potential effectiveness of introducing an educational initiative like this in changing our practice patterns. As we start talking more about this issue amongst our colleagues and our patients, we’re more likely to see a practice pattern shift in the United States as well,” Dr. Findley commented.
He reported having no financial conflicts with regard to his presentation.
EXPERT ANALYSIS FROM MINIMALLY INVASIVE SURGERY WEEK
Morcellation shows favorable risk/benefit ratio in decision analysis
LAS VEGAS – Morcellation during laparoscopic hysterectomy or myomectomy for presumed large fibroids provides slightly better 5-year overall survival and higher quality of life scores than abdominal hysterectomy, according to a new study.
The strength of this soon-to-be-published decision analysis lies in its balance and comprehensive nature. It utilized the best-available published literature to estimate the mortality risk stemming from tissue dissemination of occult leiomyosarcoma through power morcellation – an issue of hot controversy – but it also incorporated the increased risks of procedure-related morbidity and mortality associated with the alternative to morcellation in patients with large fibroids: that is, abdominal hysterectomy, Dr. Matthew Siedhoff explained at the annual Minimally Invasive Surgery Week.
The decision analysis was undertaken in the wake of the Food and Drug Administration’s April 2014 safety warning citing a 1 in 350 risk of occult malignancy in women undergoing hysterectomies or myomectomies for removal of presumed fibroids and that laparoscopic surgeries involving power morcellation performed in women with unsuspected uterine sarcomas may spread cancerous tissue, potentially shortening survival. The attendant publicity has caused considerable alarm and confusion among patients and the general public. The FDA is currently deliberating possible actions ranging from a black box warning to outright banning of power morcellation.
Meanwhile, several prominent medical centers, including Brigham and Women’s Hospital, Massachusetts General Hospital, both in Boston, and the Cleveland Clinic, have banned the use of power morcellation – precipitously, in Dr. Siedhoff’s view.
Also since the FDA safety statement, Johnson & Johnson has taken its power morcellator off the market and several large insurance companies have announced plans to halt reimbursement when power morcellation is used in gynecologic surgery. However, exactly how that would happen is unclear because there is no billing code associated with power morcellation, and it would be arduous for insurers to actually read through all operative reports.
“This decision analysis is a tool for surgeons and patients to help make informed decisions. It balances the FDA analysis, which pretty much just emphasizes morcellation risk to the exclusion of all the known benefits of laparoscopy. The analysis argues that reducing the risk associated with morcellation – perhaps through the use of specimen containment or preoperative leiomyosarcoma diagnosis – is a better approach than abandoning minimally invasive gynecologic surgery for fibroids. That would be throwing the baby out with the bathwater,” declared Dr. Siedhoff, an ob.gyn. and director of the division of advanced laparoscopy and pelvic pain at the University of North Carolina, Chapel Hill.
A decision analysis entails probability modeling of outcomes based upon consensus event rates obtained from studies in the published literature.
“It’s helpful to do a decision analysis when you can’t do a randomized controlled trial, which you certainly can’t for this issue,” he observed at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
The decision analysis was carried out by Dr. Siedhoff and coinvestigators at the University of North Carolina at Chapel Hill, with department of obstetrics and gynecology chair Dr. Daniel Clarke-Peterson as senior author. The researchers assumed two hypothetical cohorts of 100,000 women undergoing hysterectomy for fibroids large enough that surgical options were limited to laparoscopic hysterectomy with morcellation or abdominal hysterectomy. The primary outcomes were 5-year overall mortality and quality of life as measured in quality-adjusted life-years (QALYs).
The analysis assumed that among the 100,000 women in each group there would be 120 FIGO Stage I or II occult leiomyosarcomas, with an associated 59% 5-year mortality from cancer. It was further assumed that intraperitoneal dissemination of tumor via morcellation would in effect boost those cancers to FIGO Stage III, with 72% mortality at 5 years. This would result in 86 deaths from leiomyosarcoma over 5 years in the morcellation group, compared with 71 in women who underwent abdominal hysterectomy. However, this was counterbalanced by more hysterectomy-related deaths in the abdominal hysterectomy group: 32, compared with 12 in women undergoing morcellation. The final 5-year tally: 98 deaths overall in the morcellation group and 103 in the abdominal hysterectomy group.
QALYs are calculated by estimating how much a given adverse event – for example, venous thromboembolism, along with its attendant treatment and potential further complications – would diminish a theoretical year of otherwise perfect health. The total QALYs in the group of 100,000 women undergoing morcellation was estimated at 499,171, compared with 490,711 over 5 years in the abdominal hysterectomy group. That’s because with the exception of vaginal cuff dehiscence, all of the other complications assessed in the decision analysis, including wound infections, transfusions, hernias, and venous thromboembolisms, were more frequent in the abdominal hysterectomy group. The forthcoming final publication will include the citations on which all of the event probabilities were based.
An alternative and perhaps more readily grasped way of expressing the QALY results is that patients undergoing laparoscopic hysterectomy enjoyed an additional 0.85 QALY, or roughly 1 extra month of life in perfect health over a 5-year time period, Dr. Siedhoff explained.
The decision analysis didn’t include the well-established facts that laparoscopic hysterectomy entails less postoperative pain, a shorter average hospital length of stay, and faster return to daily activities.
Dr. Siedhoff was quick to assert that the true incidence of occult leiomyosarcoma in women undergoing surgery for presumed fibroids is unknown. An American College of Obstetricians and Gynecologist’s position statement issued earlier this year quoted an estimate of 1 in 500. The FDA cited a figure of 1 in 350. But when the North Carolina researchers examined the 10 studies published during 1990-2013, upon which the FDA based its estimate, the investigators felt compelled to reject 6 of them because of poor quality. For example, several studies included morcellation in patients with preoperative known or suspected sarcoma, even though morcellation should absolutely never be done in that situation. Based upon a weighted analysis of the remaining four highest-quality studies, the investigators came up with an estimate of 12 cases/10,000 women.
Noting that the largest of the studies in the FDA analysis included just 1,584 women with 2 cases of leiomyosarcoma, Dr. Siedhoff said, “I think it’s important to point out that really important decisions are being made on awfully small numerators and denominators. The truth of the matter is we have no idea what the true number is. It could be twice as high as our estimate or half as low.”
He admitted that he has been personally affected by the rancorous tone of recent public debate regarding morcellation safety, in which the procedure’s defenders often are demonized.
“It’s been confusing to me why in the Wall Street Journal they talk about these evil doctors who want to use this technique, as if it somehow benefits us. It’s not easier to do laparoscopic surgery, and it’s certainly not easier to morcellate tissue. The only reason that we’re talking about this is because we care about the outcomes for our patients,” the gynecologic surgeon said at the meeting presented by the Society of Laparoendoscopic Surgeons and affiliated societies. “One of the things that has been most difficult about all this,” he continued, “is the way that the information has moved from some very vocal people who feel strongly about this issue to the level of the lay person that you see in the elevator, or worse yet, your own patient. I think it’s almost like a game of telephone, so that by the time it gets down to a person who’s not a surgeon, the message is ‘morcellation causes cancer.’”Not only is the true prevalence of occult malignancy in women undergoing laparoscopic surgery for removal of fibroids unclear, but the data on the adverse impact of morcellation in this situation is sketchy as well. To date, it consists of two single-center retrospective studies. The more recent report, from Brigham and Women’s Hospital, involved 19 patients who underwent morcellation and 39 who had a total abdominal hysterectomy, all found to have leiomyosarcoma. The cancer recurrence rate was significantly higher in women who had morcellation, by a margin of 74% to 51%. However, there was no significant difference in overall survival (Cancer 2014 [doi:10.1002/cncr.28844]).
In contrast, an earlier Korean study involving a consecutive series comprised of 25 patients with occult leiomyosarcoma who underwent morcellation and 31 with total abdominal hysterectomy found a significant difference in 5-year overall survival: 46% in the morcellation group, and what Dr. Siedhoff deemed an unusually favorable 73% in the total abdominal hysterectomy patients (Gynecol. Oncol. 2011;122:255-9).
There is a great unmet need for a reliable preoperative method to distinguish leiomyosarcomas from benign fibroids. Imaging is of limited value. Endometrial biopsy is rarely positive. No biomarkers have been identified. Clinical factors that increase the likelihood of leiomyosarcoma include rapid growth, African American ethnicity, older age, a history of pelvic radiation, and the presence of the retinoblastoma gene.
In his own practice, Dr. Siedhoff sometimes uses specimen retrieval bags when performing morcellation, but finds the currently available versions to be cumbersome and a challenge to work with. Besides, he noted, there is to date no evidence that they are actually effective in reducing leiomyosarcoma recurrence risk. He was a member of an AAGL task force which in May issued a position statement on morcellation, which noted, “Use of morcellation within specimen retrieval pouches for containment of benign or malignant uterine tissue requires significant skill and experience, and use of specimen retrieval pouches should be further investigated for safety and outcomes in a controlled setting.”
Dr. Siedhoff reported having no financial conflicts regarding the decision analysis, conducted with university funds.
LAS VEGAS – Morcellation during laparoscopic hysterectomy or myomectomy for presumed large fibroids provides slightly better 5-year overall survival and higher quality of life scores than abdominal hysterectomy, according to a new study.
The strength of this soon-to-be-published decision analysis lies in its balance and comprehensive nature. It utilized the best-available published literature to estimate the mortality risk stemming from tissue dissemination of occult leiomyosarcoma through power morcellation – an issue of hot controversy – but it also incorporated the increased risks of procedure-related morbidity and mortality associated with the alternative to morcellation in patients with large fibroids: that is, abdominal hysterectomy, Dr. Matthew Siedhoff explained at the annual Minimally Invasive Surgery Week.

The decision analysis was undertaken in the wake of the Food and Drug Administration’s April 2014 safety warning citing a 1 in 350 risk of occult malignancy in women undergoing hysterectomies or myomectomies for removal of presumed fibroids and that laparoscopic surgeries involving power morcellation performed in women with unsuspected uterine sarcomas may spread cancerous tissue, potentially shortening survival. The attendant publicity has caused considerable alarm and confusion among patients and the general public. The FDA is currently deliberating possible actions ranging from a black box warning to outright banning of power morcellation.
Meanwhile, several prominent medical centers, including Brigham and Women’s Hospital, Massachusetts General Hospital, both in Boston, and the Cleveland Clinic, have banned the use of power morcellation – precipitously, in Dr. Siedhoff’s view.
Also since the FDA safety statement, Johnson & Johnson has taken its power morcellator off the market and several large insurance companies have announced plans to halt reimbursement when power morcellation is used in gynecologic surgery. However, exactly how that would happen is unclear because there is no billing code associated with power morcellation, and it would be arduous for insurers to actually read through all operative reports.
“This decision analysis is a tool for surgeons and patients to help make informed decisions. It balances the FDA analysis, which pretty much just emphasizes morcellation risk to the exclusion of all the known benefits of laparoscopy. The analysis argues that reducing the risk associated with morcellation – perhaps through the use of specimen containment or preoperative leiomyosarcoma diagnosis – is a better approach than abandoning minimally invasive gynecologic surgery for fibroids. That would be throwing the baby out with the bathwater,” declared Dr. Siedhoff, an ob.gyn. and director of the division of advanced laparoscopy and pelvic pain at the University of North Carolina, Chapel Hill.
A decision analysis entails probability modeling of outcomes based upon consensus event rates obtained from studies in the published literature.
“It’s helpful to do a decision analysis when you can’t do a randomized controlled trial, which you certainly can’t for this issue,” he observed at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
The decision analysis was carried out by Dr. Siedhoff and coinvestigators at the University of North Carolina at Chapel Hill, with department of obstetrics and gynecology chair Dr. Daniel Clarke-Peterson as senior author. The researchers assumed two hypothetical cohorts of 100,000 women undergoing hysterectomy for fibroids large enough that surgical options were limited to laparoscopic hysterectomy with morcellation or abdominal hysterectomy. The primary outcomes were 5-year overall mortality and quality of life as measured in quality-adjusted life-years (QALYs).
The analysis assumed that among the 100,000 women in each group there would be 120 FIGO Stage I or II occult leiomyosarcomas, with an associated 59% 5-year mortality from cancer. It was further assumed that intraperitoneal dissemination of tumor via morcellation would in effect boost those cancers to FIGO Stage III, with 72% mortality at 5 years. This would result in 86 deaths from leiomyosarcoma over 5 years in the morcellation group, compared with 71 in women who underwent abdominal hysterectomy. However, this was counterbalanced by more hysterectomy-related deaths in the abdominal hysterectomy group: 32, compared with 12 in women undergoing morcellation. The final 5-year tally: 98 deaths overall in the morcellation group and 103 in the abdominal hysterectomy group.
QALYs are calculated by estimating how much a given adverse event – for example, venous thromboembolism, along with its attendant treatment and potential further complications – would diminish a theoretical year of otherwise perfect health. The total QALYs in the group of 100,000 women undergoing morcellation was estimated at 499,171, compared with 490,711 over 5 years in the abdominal hysterectomy group. That’s because with the exception of vaginal cuff dehiscence, all of the other complications assessed in the decision analysis, including wound infections, transfusions, hernias, and venous thromboembolisms, were more frequent in the abdominal hysterectomy group. The forthcoming final publication will include the citations on which all of the event probabilities were based.
An alternative and perhaps more readily grasped way of expressing the QALY results is that patients undergoing laparoscopic hysterectomy enjoyed an additional 0.85 QALY, or roughly 1 extra month of life in perfect health over a 5-year time period, Dr. Siedhoff explained.
The decision analysis didn’t include the well-established facts that laparoscopic hysterectomy entails less postoperative pain, a shorter average hospital length of stay, and faster return to daily activities.
Dr. Siedhoff was quick to assert that the true incidence of occult leiomyosarcoma in women undergoing surgery for presumed fibroids is unknown. An American College of Obstetricians and Gynecologist’s position statement issued earlier this year quoted an estimate of 1 in 500. The FDA cited a figure of 1 in 350. But when the North Carolina researchers examined the 10 studies published during 1990-2013, upon which the FDA based its estimate, the investigators felt compelled to reject 6 of them because of poor quality. For example, several studies included morcellation in patients with preoperative known or suspected sarcoma, even though morcellation should absolutely never be done in that situation. Based upon a weighted analysis of the remaining four highest-quality studies, the investigators came up with an estimate of 12 cases/10,000 women.
Noting that the largest of the studies in the FDA analysis included just 1,584 women with 2 cases of leiomyosarcoma, Dr. Siedhoff said, “I think it’s important to point out that really important decisions are being made on awfully small numerators and denominators. The truth of the matter is we have no idea what the true number is. It could be twice as high as our estimate or half as low.”
He admitted that he has been personally affected by the rancorous tone of recent public debate regarding morcellation safety, in which the procedure’s defenders often are demonized.
“It’s been confusing to me why in the Wall Street Journal they talk about these evil doctors who want to use this technique, as if it somehow benefits us. It’s not easier to do laparoscopic surgery, and it’s certainly not easier to morcellate tissue. The only reason that we’re talking about this is because we care about the outcomes for our patients,” the gynecologic surgeon said at the meeting presented by the Society of Laparoendoscopic Surgeons and affiliated societies. “One of the things that has been most difficult about all this,” he continued, “is the way that the information has moved from some very vocal people who feel strongly about this issue to the level of the lay person that you see in the elevator, or worse yet, your own patient. I think it’s almost like a game of telephone, so that by the time it gets down to a person who’s not a surgeon, the message is ‘morcellation causes cancer.’”Not only is the true prevalence of occult malignancy in women undergoing laparoscopic surgery for removal of fibroids unclear, but the data on the adverse impact of morcellation in this situation is sketchy as well. To date, it consists of two single-center retrospective studies. The more recent report, from Brigham and Women’s Hospital, involved 19 patients who underwent morcellation and 39 who had a total abdominal hysterectomy, all found to have leiomyosarcoma. The cancer recurrence rate was significantly higher in women who had morcellation, by a margin of 74% to 51%. However, there was no significant difference in overall survival (Cancer 2014 [doi:10.1002/cncr.28844]).
In contrast, an earlier Korean study involving a consecutive series comprised of 25 patients with occult leiomyosarcoma who underwent morcellation and 31 with total abdominal hysterectomy found a significant difference in 5-year overall survival: 46% in the morcellation group, and what Dr. Siedhoff deemed an unusually favorable 73% in the total abdominal hysterectomy patients (Gynecol. Oncol. 2011;122:255-9).
There is a great unmet need for a reliable preoperative method to distinguish leiomyosarcomas from benign fibroids. Imaging is of limited value. Endometrial biopsy is rarely positive. No biomarkers have been identified. Clinical factors that increase the likelihood of leiomyosarcoma include rapid growth, African American ethnicity, older age, a history of pelvic radiation, and the presence of the retinoblastoma gene.
In his own practice, Dr. Siedhoff sometimes uses specimen retrieval bags when performing morcellation, but finds the currently available versions to be cumbersome and a challenge to work with. Besides, he noted, there is to date no evidence that they are actually effective in reducing leiomyosarcoma recurrence risk. He was a member of an AAGL task force which in May issued a position statement on morcellation, which noted, “Use of morcellation within specimen retrieval pouches for containment of benign or malignant uterine tissue requires significant skill and experience, and use of specimen retrieval pouches should be further investigated for safety and outcomes in a controlled setting.”
Dr. Siedhoff reported having no financial conflicts regarding the decision analysis, conducted with university funds.
LAS VEGAS – Morcellation during laparoscopic hysterectomy or myomectomy for presumed large fibroids provides slightly better 5-year overall survival and higher quality of life scores than abdominal hysterectomy, according to a new study.
The strength of this soon-to-be-published decision analysis lies in its balance and comprehensive nature. It utilized the best-available published literature to estimate the mortality risk stemming from tissue dissemination of occult leiomyosarcoma through power morcellation – an issue of hot controversy – but it also incorporated the increased risks of procedure-related morbidity and mortality associated with the alternative to morcellation in patients with large fibroids: that is, abdominal hysterectomy, Dr. Matthew Siedhoff explained at the annual Minimally Invasive Surgery Week.

The decision analysis was undertaken in the wake of the Food and Drug Administration’s April 2014 safety warning citing a 1 in 350 risk of occult malignancy in women undergoing hysterectomies or myomectomies for removal of presumed fibroids and that laparoscopic surgeries involving power morcellation performed in women with unsuspected uterine sarcomas may spread cancerous tissue, potentially shortening survival. The attendant publicity has caused considerable alarm and confusion among patients and the general public. The FDA is currently deliberating possible actions ranging from a black box warning to outright banning of power morcellation.
Meanwhile, several prominent medical centers, including Brigham and Women’s Hospital, Massachusetts General Hospital, both in Boston, and the Cleveland Clinic, have banned the use of power morcellation – precipitously, in Dr. Siedhoff’s view.
Also since the FDA safety statement, Johnson & Johnson has taken its power morcellator off the market and several large insurance companies have announced plans to halt reimbursement when power morcellation is used in gynecologic surgery. However, exactly how that would happen is unclear because there is no billing code associated with power morcellation, and it would be arduous for insurers to actually read through all operative reports.
“This decision analysis is a tool for surgeons and patients to help make informed decisions. It balances the FDA analysis, which pretty much just emphasizes morcellation risk to the exclusion of all the known benefits of laparoscopy. The analysis argues that reducing the risk associated with morcellation – perhaps through the use of specimen containment or preoperative leiomyosarcoma diagnosis – is a better approach than abandoning minimally invasive gynecologic surgery for fibroids. That would be throwing the baby out with the bathwater,” declared Dr. Siedhoff, an ob.gyn. and director of the division of advanced laparoscopy and pelvic pain at the University of North Carolina, Chapel Hill.
A decision analysis entails probability modeling of outcomes based upon consensus event rates obtained from studies in the published literature.
“It’s helpful to do a decision analysis when you can’t do a randomized controlled trial, which you certainly can’t for this issue,” he observed at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
The decision analysis was carried out by Dr. Siedhoff and coinvestigators at the University of North Carolina at Chapel Hill, with department of obstetrics and gynecology chair Dr. Daniel Clarke-Peterson as senior author. The researchers assumed two hypothetical cohorts of 100,000 women undergoing hysterectomy for fibroids large enough that surgical options were limited to laparoscopic hysterectomy with morcellation or abdominal hysterectomy. The primary outcomes were 5-year overall mortality and quality of life as measured in quality-adjusted life-years (QALYs).
The analysis assumed that among the 100,000 women in each group there would be 120 FIGO Stage I or II occult leiomyosarcomas, with an associated 59% 5-year mortality from cancer. It was further assumed that intraperitoneal dissemination of tumor via morcellation would in effect boost those cancers to FIGO Stage III, with 72% mortality at 5 years. This would result in 86 deaths from leiomyosarcoma over 5 years in the morcellation group, compared with 71 in women who underwent abdominal hysterectomy. However, this was counterbalanced by more hysterectomy-related deaths in the abdominal hysterectomy group: 32, compared with 12 in women undergoing morcellation. The final 5-year tally: 98 deaths overall in the morcellation group and 103 in the abdominal hysterectomy group.
QALYs are calculated by estimating how much a given adverse event – for example, venous thromboembolism, along with its attendant treatment and potential further complications – would diminish a theoretical year of otherwise perfect health. The total QALYs in the group of 100,000 women undergoing morcellation was estimated at 499,171, compared with 490,711 over 5 years in the abdominal hysterectomy group. That’s because with the exception of vaginal cuff dehiscence, all of the other complications assessed in the decision analysis, including wound infections, transfusions, hernias, and venous thromboembolisms, were more frequent in the abdominal hysterectomy group. The forthcoming final publication will include the citations on which all of the event probabilities were based.
An alternative and perhaps more readily grasped way of expressing the QALY results is that patients undergoing laparoscopic hysterectomy enjoyed an additional 0.85 QALY, or roughly 1 extra month of life in perfect health over a 5-year time period, Dr. Siedhoff explained.
The decision analysis didn’t include the well-established facts that laparoscopic hysterectomy entails less postoperative pain, a shorter average hospital length of stay, and faster return to daily activities.
Dr. Siedhoff was quick to assert that the true incidence of occult leiomyosarcoma in women undergoing surgery for presumed fibroids is unknown. An American College of Obstetricians and Gynecologist’s position statement issued earlier this year quoted an estimate of 1 in 500. The FDA cited a figure of 1 in 350. But when the North Carolina researchers examined the 10 studies published during 1990-2013, upon which the FDA based its estimate, the investigators felt compelled to reject 6 of them because of poor quality. For example, several studies included morcellation in patients with preoperative known or suspected sarcoma, even though morcellation should absolutely never be done in that situation. Based upon a weighted analysis of the remaining four highest-quality studies, the investigators came up with an estimate of 12 cases/10,000 women.
Noting that the largest of the studies in the FDA analysis included just 1,584 women with 2 cases of leiomyosarcoma, Dr. Siedhoff said, “I think it’s important to point out that really important decisions are being made on awfully small numerators and denominators. The truth of the matter is we have no idea what the true number is. It could be twice as high as our estimate or half as low.”
He admitted that he has been personally affected by the rancorous tone of recent public debate regarding morcellation safety, in which the procedure’s defenders often are demonized.
“It’s been confusing to me why in the Wall Street Journal they talk about these evil doctors who want to use this technique, as if it somehow benefits us. It’s not easier to do laparoscopic surgery, and it’s certainly not easier to morcellate tissue. The only reason that we’re talking about this is because we care about the outcomes for our patients,” the gynecologic surgeon said at the meeting presented by the Society of Laparoendoscopic Surgeons and affiliated societies. “One of the things that has been most difficult about all this,” he continued, “is the way that the information has moved from some very vocal people who feel strongly about this issue to the level of the lay person that you see in the elevator, or worse yet, your own patient. I think it’s almost like a game of telephone, so that by the time it gets down to a person who’s not a surgeon, the message is ‘morcellation causes cancer.’”Not only is the true prevalence of occult malignancy in women undergoing laparoscopic surgery for removal of fibroids unclear, but the data on the adverse impact of morcellation in this situation is sketchy as well. To date, it consists of two single-center retrospective studies. The more recent report, from Brigham and Women’s Hospital, involved 19 patients who underwent morcellation and 39 who had a total abdominal hysterectomy, all found to have leiomyosarcoma. The cancer recurrence rate was significantly higher in women who had morcellation, by a margin of 74% to 51%. However, there was no significant difference in overall survival (Cancer 2014 [doi:10.1002/cncr.28844]).
In contrast, an earlier Korean study involving a consecutive series comprised of 25 patients with occult leiomyosarcoma who underwent morcellation and 31 with total abdominal hysterectomy found a significant difference in 5-year overall survival: 46% in the morcellation group, and what Dr. Siedhoff deemed an unusually favorable 73% in the total abdominal hysterectomy patients (Gynecol. Oncol. 2011;122:255-9).
There is a great unmet need for a reliable preoperative method to distinguish leiomyosarcomas from benign fibroids. Imaging is of limited value. Endometrial biopsy is rarely positive. No biomarkers have been identified. Clinical factors that increase the likelihood of leiomyosarcoma include rapid growth, African American ethnicity, older age, a history of pelvic radiation, and the presence of the retinoblastoma gene.
In his own practice, Dr. Siedhoff sometimes uses specimen retrieval bags when performing morcellation, but finds the currently available versions to be cumbersome and a challenge to work with. Besides, he noted, there is to date no evidence that they are actually effective in reducing leiomyosarcoma recurrence risk. He was a member of an AAGL task force which in May issued a position statement on morcellation, which noted, “Use of morcellation within specimen retrieval pouches for containment of benign or malignant uterine tissue requires significant skill and experience, and use of specimen retrieval pouches should be further investigated for safety and outcomes in a controlled setting.”
Dr. Siedhoff reported having no financial conflicts regarding the decision analysis, conducted with university funds.
AT MINIMALLY INVASIVE SURGERY WEEK
Key clinical point: Despite the much-publicized risk of intraperitoneal dissemination of tumor during morcellation in women undergoing laparoscopic hysterectomy for fibroids, the projected 5-year overall survival is better than with abdominal hysterectomy.
Major finding: In a hypothetical population of 200,000 women undergoing hysterectomy for presumed fibroids, laparoscopic surgery with morcellation would result in a projected 98 deaths from all causes during 5 years of follow-up and abdominal hysterectomy would result in 103 deaths.
Data source: This was a decision analysis in which the probabilities of various surgical outcomes based upon studies in the published literature were applied to a hypothetical cohort of 200,000 women undergoing hysterectomy for removal of fibroids, half laparoscopically with morcellation and half via abdominal hysterectomy.
Disclosures: The presenter reported having no financial conflicts of interest regarding the decision analysis study.