User login
Held every five years 15th International Thyroid Congress
Wide variation in clinical management of thyroid nodules seen in first-ever survey
LAKE BUENA VISTA, FLA. – When making diagnostic and treatment decisions about thyroid nodules, many endocrinologists are not following current clinical practice guidelines, according to a recent survey. Further, according to a recent international survey of endocrinologists, there is wide regional variation in the use of molecular testing and calcitonin levels.
Dr. Nicole Vietor of the department of endocrinology at Walter Reed National Military Medical Center, Bethesda, Md., and her collaborators contacted members of the American Thyroid Association (ATA), the Endocrine Society (TES), and the American Association of Clinical Endocrinologists (AACE). Members of these societies were contacted directly by investigators and asked to complete a web-based survey regarding their practices for diagnosing and managing thyroid nodules.
The survey consisted of 36 questions, with an index case with variations presented to respondents, who then answered questions about diagnostic and management decisionmaking practices. The hypothetical index patient was a 52 year old woman with an incidental finding of a 1.5 cm right thyroid nodule. The patient was healthy and without risk factors; the patient’s nodule was not palpable on physical exam and she had no cervical lymphadenopathy.
Almost all respondents (99.4%) would order a thyroid-stimulating hormone for initial lab testing. Other commonly ordered exams included free T4 levels and thyroid peroxidase antibody requested by 41.5% and 24.3% of respondents, respectively. Fewer than 15% of respondents would have ordered any further lab exams.
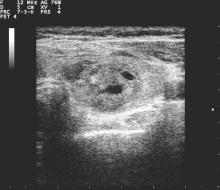
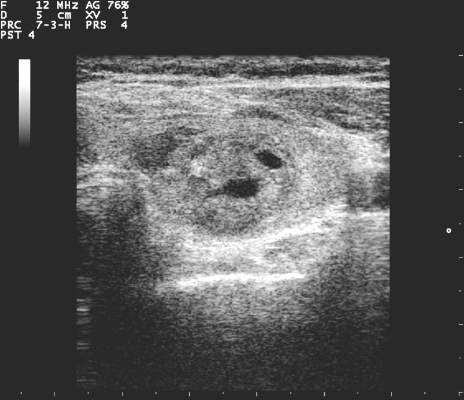
All but 1.5% of respondents would order an anatomic or functional test for this index patient, with 57.2% ordering a thyroid ultrasound to be performed in radiology and 52.1% ordering an ultrasound in clinic (multiple responses were permitted). Cervical lymph nodes were included in the initial ultrasound assessment by 68.5% of respondents. Overall, more than half (56.6%) of thyroid ultrasounds were performed by endocrinologists, and about a third (31.9%) done by radiologists.
Practice variation from guidelines became apparent when respondents were asked how various nodule characteristics affected the decision to perform a fine needle aspiration (FNA). For a 1.5-cm solid hypoechoic nodule, 93.8% of respondents would perform FNA, while two thirds (67.0%) would perform an FNA for a 0.7-cm hypoechoic nodule with microcalcifications.
When performing FNAs, 83.3% of respondents use ultrasound to guide the biopsy, with most operators performing two (19.2%), three (28.5%), or four (23.0%) passes per nodule.
Of the 897 respondents, 80.5% were TES members, 56.5% were AACE members, and 44.5% were ATA members; most respondents belonged to more than one society. Almost two thirds of respondents (63.0%) were from North America, while 12.2% were from Europe, 10.8% were from Latin America, 6.5% were from Asia, 5.6% were from the Middle East or Africa, and just 1.9% were from Oceania. More men (60.2%) than women responded.
The AACE issued clinical practice guidelines for the management of thyroid nodules in 2010, as did the ATA in 2009 and 2015.
“In summary, management of a thyroid nodule is highly variable and differs from societal guidelines in multiple areas,” wrote Dr. Vietor and her colleagues in the presentation abstract.
On Twitter @karioakes
LAKE BUENA VISTA, FLA. – When making diagnostic and treatment decisions about thyroid nodules, many endocrinologists are not following current clinical practice guidelines, according to a recent survey. Further, according to a recent international survey of endocrinologists, there is wide regional variation in the use of molecular testing and calcitonin levels.
Dr. Nicole Vietor of the department of endocrinology at Walter Reed National Military Medical Center, Bethesda, Md., and her collaborators contacted members of the American Thyroid Association (ATA), the Endocrine Society (TES), and the American Association of Clinical Endocrinologists (AACE). Members of these societies were contacted directly by investigators and asked to complete a web-based survey regarding their practices for diagnosing and managing thyroid nodules.
The survey consisted of 36 questions, with an index case with variations presented to respondents, who then answered questions about diagnostic and management decisionmaking practices. The hypothetical index patient was a 52 year old woman with an incidental finding of a 1.5 cm right thyroid nodule. The patient was healthy and without risk factors; the patient’s nodule was not palpable on physical exam and she had no cervical lymphadenopathy.
Almost all respondents (99.4%) would order a thyroid-stimulating hormone for initial lab testing. Other commonly ordered exams included free T4 levels and thyroid peroxidase antibody requested by 41.5% and 24.3% of respondents, respectively. Fewer than 15% of respondents would have ordered any further lab exams.
All but 1.5% of respondents would order an anatomic or functional test for this index patient, with 57.2% ordering a thyroid ultrasound to be performed in radiology and 52.1% ordering an ultrasound in clinic (multiple responses were permitted). Cervical lymph nodes were included in the initial ultrasound assessment by 68.5% of respondents. Overall, more than half (56.6%) of thyroid ultrasounds were performed by endocrinologists, and about a third (31.9%) done by radiologists.
Practice variation from guidelines became apparent when respondents were asked how various nodule characteristics affected the decision to perform a fine needle aspiration (FNA). For a 1.5-cm solid hypoechoic nodule, 93.8% of respondents would perform FNA, while two thirds (67.0%) would perform an FNA for a 0.7-cm hypoechoic nodule with microcalcifications.
When performing FNAs, 83.3% of respondents use ultrasound to guide the biopsy, with most operators performing two (19.2%), three (28.5%), or four (23.0%) passes per nodule.
Of the 897 respondents, 80.5% were TES members, 56.5% were AACE members, and 44.5% were ATA members; most respondents belonged to more than one society. Almost two thirds of respondents (63.0%) were from North America, while 12.2% were from Europe, 10.8% were from Latin America, 6.5% were from Asia, 5.6% were from the Middle East or Africa, and just 1.9% were from Oceania. More men (60.2%) than women responded.
The AACE issued clinical practice guidelines for the management of thyroid nodules in 2010, as did the ATA in 2009 and 2015.
“In summary, management of a thyroid nodule is highly variable and differs from societal guidelines in multiple areas,” wrote Dr. Vietor and her colleagues in the presentation abstract.
On Twitter @karioakes
LAKE BUENA VISTA, FLA. – When making diagnostic and treatment decisions about thyroid nodules, many endocrinologists are not following current clinical practice guidelines, according to a recent survey. Further, according to a recent international survey of endocrinologists, there is wide regional variation in the use of molecular testing and calcitonin levels.
Dr. Nicole Vietor of the department of endocrinology at Walter Reed National Military Medical Center, Bethesda, Md., and her collaborators contacted members of the American Thyroid Association (ATA), the Endocrine Society (TES), and the American Association of Clinical Endocrinologists (AACE). Members of these societies were contacted directly by investigators and asked to complete a web-based survey regarding their practices for diagnosing and managing thyroid nodules.
The survey consisted of 36 questions, with an index case with variations presented to respondents, who then answered questions about diagnostic and management decisionmaking practices. The hypothetical index patient was a 52 year old woman with an incidental finding of a 1.5 cm right thyroid nodule. The patient was healthy and without risk factors; the patient’s nodule was not palpable on physical exam and she had no cervical lymphadenopathy.
Almost all respondents (99.4%) would order a thyroid-stimulating hormone for initial lab testing. Other commonly ordered exams included free T4 levels and thyroid peroxidase antibody requested by 41.5% and 24.3% of respondents, respectively. Fewer than 15% of respondents would have ordered any further lab exams.
All but 1.5% of respondents would order an anatomic or functional test for this index patient, with 57.2% ordering a thyroid ultrasound to be performed in radiology and 52.1% ordering an ultrasound in clinic (multiple responses were permitted). Cervical lymph nodes were included in the initial ultrasound assessment by 68.5% of respondents. Overall, more than half (56.6%) of thyroid ultrasounds were performed by endocrinologists, and about a third (31.9%) done by radiologists.
Practice variation from guidelines became apparent when respondents were asked how various nodule characteristics affected the decision to perform a fine needle aspiration (FNA). For a 1.5-cm solid hypoechoic nodule, 93.8% of respondents would perform FNA, while two thirds (67.0%) would perform an FNA for a 0.7-cm hypoechoic nodule with microcalcifications.
When performing FNAs, 83.3% of respondents use ultrasound to guide the biopsy, with most operators performing two (19.2%), three (28.5%), or four (23.0%) passes per nodule.
Of the 897 respondents, 80.5% were TES members, 56.5% were AACE members, and 44.5% were ATA members; most respondents belonged to more than one society. Almost two thirds of respondents (63.0%) were from North America, while 12.2% were from Europe, 10.8% were from Latin America, 6.5% were from Asia, 5.6% were from the Middle East or Africa, and just 1.9% were from Oceania. More men (60.2%) than women responded.
The AACE issued clinical practice guidelines for the management of thyroid nodules in 2010, as did the ATA in 2009 and 2015.
“In summary, management of a thyroid nodule is highly variable and differs from societal guidelines in multiple areas,” wrote Dr. Vietor and her colleagues in the presentation abstract.
On Twitter @karioakes
AT THE 15TH INTERNATIONAL THYROID CONGRESS
Key clinical point: A first-ever international survey of endocrinologists showed wide variation in clinical management of thyroid nodules.
Major finding: Respondents reported performing more fine needle aspiration (FNA) biopsies of thyroid nodules than recommended by practice guidelines.
Data source: Web-based survey of 897 members of three different professional organizations.
Disclosures: No disclosures were identified.
Core needle biopsy proves sensitive for first-line thyroid nodule diagnosis
LAKE BUENA VISTA, FLA. – The nondiagnostic result rate was significantly lower with core needle biopsy than with fine needle aspiration as a first-line biopsy method for newly detected thyroid nodules in a comparative study.
In 631 pairs of initially detected thyroid nodules that were matched based on propensity score analysis, the nondiagnostic result rate was 1.4% when core needle biopsy (CNB) was used, compared with 8.1% with ultrasound-guided fine-needle aspiration. The indeterminate result rate was 5.1% vs. 8.1% with the two approaches, respectively, and the differences between the groups were statistically significant, Dr. Hyun Kyung Lim of Soonchunhyang University Seoul Hospital, Korea, reported at the International Thyroid Congress.
The nondiagnostic rate with CNB was also significantly lower than with fine-needle aspiration for nodules with calcifications, posterior location, or diameter less than 1 cm, Dr. Lim said at the meeting at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
No difference was seen between the groups with respect to diagnostic performance based on degree of clinician experience.
The complication rate was higher with CNB than with fine -needle aspiration (3.6% vs. 1.6%), but complications were minor, Dr. Lim said.
Core needle biopsy has been suggested as a complementary tool for the diagnosis of thyroid nodules when the results of fine-needle aspiration are inconclusive, and the approach has been shown to be both safe and accurate for biopsy. However, its role as a first-line approach for thyroid nodule biopsy has been controversial and few studies have evaluated it as a first-line tool, she noted.
The current findings suggest that CNB is a safe and highly sensitive first-line biopsy method for such nodules, she concluded.
Dr. Lim reported having no disclosures.
LAKE BUENA VISTA, FLA. – The nondiagnostic result rate was significantly lower with core needle biopsy than with fine needle aspiration as a first-line biopsy method for newly detected thyroid nodules in a comparative study.
In 631 pairs of initially detected thyroid nodules that were matched based on propensity score analysis, the nondiagnostic result rate was 1.4% when core needle biopsy (CNB) was used, compared with 8.1% with ultrasound-guided fine-needle aspiration. The indeterminate result rate was 5.1% vs. 8.1% with the two approaches, respectively, and the differences between the groups were statistically significant, Dr. Hyun Kyung Lim of Soonchunhyang University Seoul Hospital, Korea, reported at the International Thyroid Congress.
The nondiagnostic rate with CNB was also significantly lower than with fine-needle aspiration for nodules with calcifications, posterior location, or diameter less than 1 cm, Dr. Lim said at the meeting at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
No difference was seen between the groups with respect to diagnostic performance based on degree of clinician experience.
The complication rate was higher with CNB than with fine -needle aspiration (3.6% vs. 1.6%), but complications were minor, Dr. Lim said.
Core needle biopsy has been suggested as a complementary tool for the diagnosis of thyroid nodules when the results of fine-needle aspiration are inconclusive, and the approach has been shown to be both safe and accurate for biopsy. However, its role as a first-line approach for thyroid nodule biopsy has been controversial and few studies have evaluated it as a first-line tool, she noted.
The current findings suggest that CNB is a safe and highly sensitive first-line biopsy method for such nodules, she concluded.
Dr. Lim reported having no disclosures.
LAKE BUENA VISTA, FLA. – The nondiagnostic result rate was significantly lower with core needle biopsy than with fine needle aspiration as a first-line biopsy method for newly detected thyroid nodules in a comparative study.
In 631 pairs of initially detected thyroid nodules that were matched based on propensity score analysis, the nondiagnostic result rate was 1.4% when core needle biopsy (CNB) was used, compared with 8.1% with ultrasound-guided fine-needle aspiration. The indeterminate result rate was 5.1% vs. 8.1% with the two approaches, respectively, and the differences between the groups were statistically significant, Dr. Hyun Kyung Lim of Soonchunhyang University Seoul Hospital, Korea, reported at the International Thyroid Congress.
The nondiagnostic rate with CNB was also significantly lower than with fine-needle aspiration for nodules with calcifications, posterior location, or diameter less than 1 cm, Dr. Lim said at the meeting at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
No difference was seen between the groups with respect to diagnostic performance based on degree of clinician experience.
The complication rate was higher with CNB than with fine -needle aspiration (3.6% vs. 1.6%), but complications were minor, Dr. Lim said.
Core needle biopsy has been suggested as a complementary tool for the diagnosis of thyroid nodules when the results of fine-needle aspiration are inconclusive, and the approach has been shown to be both safe and accurate for biopsy. However, its role as a first-line approach for thyroid nodule biopsy has been controversial and few studies have evaluated it as a first-line tool, she noted.
The current findings suggest that CNB is a safe and highly sensitive first-line biopsy method for such nodules, she concluded.
Dr. Lim reported having no disclosures.
AT THE INTERNATIONAL THYROID CONGRESS
Key clinical point: The nondiagnostic result rate was significantly lower with core needle biopsy than with fine-needle aspiration as a first-line biopsy method for newly detected thyroid nodules in a comparative study.
Major finding: The nondiagnostic result rate was 1.4% with core needle biopsy vs. 8.1% with fine-needle aspiration.
Data source: A comparative study in 631 propensity score–matched pairs of thyroid nodules.
Disclosures: Dr. Lim reported having no disclosures.
Iron deficiency may explain persistent hypothyroidism symptoms
LAKE BUENA VISTA, FLA. – Between 30% and 50% of hypothyroid patients with persistent symptoms despite adequate levothyroxine therapy may have covert iron deficiency, findings from a small study suggest.
The findings cast “a dark shadow of doubt on the validity of the studies on the effect of T3 therapy in these patients,” Dr. Esa Soppi reported in a poster at the International Thyroid Congress.
Study subjects were women with a history of overt hypothyroidism who had persistent symptoms after appropriate and ongoing treatment with L-T4. L-T4 dosing was adjusted as necessary to achieve a thyroid-stimulating hormone concentration of 1-2 mU/L, and diabetes, B12-vitamin deficiency, celiac disease, hypercalcemia, and vitamin D deficiency were ruled out as causes for the persistent symptoms.
Further, none of the patients had anemia, and red cell indices were within the reference range.
Five of the women had serum ferritin of less than 15 mcg/L, and two of those had serum iron, transferrin, or soluble transferrin receptor concentration or transferrin saturation out of range, suggesting iron deficiency. The remaining 20 women had a serum ferritin concentration between 15 mcg/L and 60 mcg/L, Dr. Soppi of Eira Hospital, Helsinki, noted at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association , European Thyroid Association, and Latin American Thyroid Society.
Four of the five women with serum ferritin less than 15 mcg/L, and 14 of the 20 with less than 15-60 mcg/L became symptom free when treated with oral iron substitution therapy for 6-12 months, Dr. Soppi said.
“All patients were advised to take their thyroxine dose at the fasting state in the morning and start breakfast 30 minutes later. The interval between the iron and thyroxine was at least 4 hours. The response was observed at a serum ferritin concentration approaching 70-100 mcg/L,” Dr. Soppi wrote, noting that in one patient – a 28-year-old woman with type 1 diabetes and hypothyroidism – all symptoms of fatigue, failure to thrive, and lethargy experienced before the start of the iron therapy disappeared after about 4 months of oral iron therapy at a dose of 100 mg twice daily.
However, another patient – an 18-year-old woman with hypothyroidism after total thyroidectomy performed because of a suspected thyroid malignancy – was found to have no malignancy; disabling tiredness, and failure to thrive emerged after the thyroidectomy and persisted despite iron therapy given at 100 mg twice daily.
“Iron deficiency is as common as hypothyroidism and its symptoms resemble those of hypothyroidism. However, the diagnosis of iron deficiency without anemia is extremely challenging since all indicators of iron status may be ‘normal.’ A clinical suspicion is key to diagnosis of covert iron deficiency,” Dr. Soppi wrote, noting that the serum ferritin concentration may be helpful, and restoration of ferritin above 100 mcg/L seems to ameliorate symptoms in about two-thirds of patients, and that it is not currently known why some iron-deficient patients fail to respond to restoration of their functional iron stores.
LAKE BUENA VISTA, FLA. – Between 30% and 50% of hypothyroid patients with persistent symptoms despite adequate levothyroxine therapy may have covert iron deficiency, findings from a small study suggest.
The findings cast “a dark shadow of doubt on the validity of the studies on the effect of T3 therapy in these patients,” Dr. Esa Soppi reported in a poster at the International Thyroid Congress.
Study subjects were women with a history of overt hypothyroidism who had persistent symptoms after appropriate and ongoing treatment with L-T4. L-T4 dosing was adjusted as necessary to achieve a thyroid-stimulating hormone concentration of 1-2 mU/L, and diabetes, B12-vitamin deficiency, celiac disease, hypercalcemia, and vitamin D deficiency were ruled out as causes for the persistent symptoms.
Further, none of the patients had anemia, and red cell indices were within the reference range.
Five of the women had serum ferritin of less than 15 mcg/L, and two of those had serum iron, transferrin, or soluble transferrin receptor concentration or transferrin saturation out of range, suggesting iron deficiency. The remaining 20 women had a serum ferritin concentration between 15 mcg/L and 60 mcg/L, Dr. Soppi of Eira Hospital, Helsinki, noted at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association , European Thyroid Association, and Latin American Thyroid Society.
Four of the five women with serum ferritin less than 15 mcg/L, and 14 of the 20 with less than 15-60 mcg/L became symptom free when treated with oral iron substitution therapy for 6-12 months, Dr. Soppi said.
“All patients were advised to take their thyroxine dose at the fasting state in the morning and start breakfast 30 minutes later. The interval between the iron and thyroxine was at least 4 hours. The response was observed at a serum ferritin concentration approaching 70-100 mcg/L,” Dr. Soppi wrote, noting that in one patient – a 28-year-old woman with type 1 diabetes and hypothyroidism – all symptoms of fatigue, failure to thrive, and lethargy experienced before the start of the iron therapy disappeared after about 4 months of oral iron therapy at a dose of 100 mg twice daily.
However, another patient – an 18-year-old woman with hypothyroidism after total thyroidectomy performed because of a suspected thyroid malignancy – was found to have no malignancy; disabling tiredness, and failure to thrive emerged after the thyroidectomy and persisted despite iron therapy given at 100 mg twice daily.
“Iron deficiency is as common as hypothyroidism and its symptoms resemble those of hypothyroidism. However, the diagnosis of iron deficiency without anemia is extremely challenging since all indicators of iron status may be ‘normal.’ A clinical suspicion is key to diagnosis of covert iron deficiency,” Dr. Soppi wrote, noting that the serum ferritin concentration may be helpful, and restoration of ferritin above 100 mcg/L seems to ameliorate symptoms in about two-thirds of patients, and that it is not currently known why some iron-deficient patients fail to respond to restoration of their functional iron stores.
LAKE BUENA VISTA, FLA. – Between 30% and 50% of hypothyroid patients with persistent symptoms despite adequate levothyroxine therapy may have covert iron deficiency, findings from a small study suggest.
The findings cast “a dark shadow of doubt on the validity of the studies on the effect of T3 therapy in these patients,” Dr. Esa Soppi reported in a poster at the International Thyroid Congress.
Study subjects were women with a history of overt hypothyroidism who had persistent symptoms after appropriate and ongoing treatment with L-T4. L-T4 dosing was adjusted as necessary to achieve a thyroid-stimulating hormone concentration of 1-2 mU/L, and diabetes, B12-vitamin deficiency, celiac disease, hypercalcemia, and vitamin D deficiency were ruled out as causes for the persistent symptoms.
Further, none of the patients had anemia, and red cell indices were within the reference range.
Five of the women had serum ferritin of less than 15 mcg/L, and two of those had serum iron, transferrin, or soluble transferrin receptor concentration or transferrin saturation out of range, suggesting iron deficiency. The remaining 20 women had a serum ferritin concentration between 15 mcg/L and 60 mcg/L, Dr. Soppi of Eira Hospital, Helsinki, noted at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association , European Thyroid Association, and Latin American Thyroid Society.
Four of the five women with serum ferritin less than 15 mcg/L, and 14 of the 20 with less than 15-60 mcg/L became symptom free when treated with oral iron substitution therapy for 6-12 months, Dr. Soppi said.
“All patients were advised to take their thyroxine dose at the fasting state in the morning and start breakfast 30 minutes later. The interval between the iron and thyroxine was at least 4 hours. The response was observed at a serum ferritin concentration approaching 70-100 mcg/L,” Dr. Soppi wrote, noting that in one patient – a 28-year-old woman with type 1 diabetes and hypothyroidism – all symptoms of fatigue, failure to thrive, and lethargy experienced before the start of the iron therapy disappeared after about 4 months of oral iron therapy at a dose of 100 mg twice daily.
However, another patient – an 18-year-old woman with hypothyroidism after total thyroidectomy performed because of a suspected thyroid malignancy – was found to have no malignancy; disabling tiredness, and failure to thrive emerged after the thyroidectomy and persisted despite iron therapy given at 100 mg twice daily.
“Iron deficiency is as common as hypothyroidism and its symptoms resemble those of hypothyroidism. However, the diagnosis of iron deficiency without anemia is extremely challenging since all indicators of iron status may be ‘normal.’ A clinical suspicion is key to diagnosis of covert iron deficiency,” Dr. Soppi wrote, noting that the serum ferritin concentration may be helpful, and restoration of ferritin above 100 mcg/L seems to ameliorate symptoms in about two-thirds of patients, and that it is not currently known why some iron-deficient patients fail to respond to restoration of their functional iron stores.
AT THE INTERNATIONAL THYROID CONGRESS
Key clinical point: Between 30% and 50% of hypothyroid patients with persistent symptoms despite adequate levothyroxine therapy may have covert iron deficiency, findings from a small study suggest.
Major finding: Four of five women with serum ferritin less than 15 mcg/L, and 14 of 20 with less than 15-60 mcg/L became symptom free when treated with oral iron substitution therapy for 6-12 months.
Data source: A prospective study of 25 women.
Disclosures: Dr. Soppi reported having no disclosures.
LT4 therapy for SCH may improve pregnancy outcomes
LAKE BUENA VISTA, FLA. – Levothyroxine therapy during pregnancy in women with subclinical hypothyroidism was associated with a decrease in low birth weight and low Apgar scores but not with a significant decrease in pregnancy loss in a large retrospective cohort study.
In 79 pregnant women with subclinical hypothyroidism (SCH) who received therapy with levothyroxine (LT4), and 285 with SCH who did not receive LT4 therapy, the frequency of low birth weight (less than 2,500 g) was 1.3% vs. 10%, respectively, and the frequency of Apgar scores of 7 or less at 5 minutes was 0% vs. 6.9%, Dr. S. Maraka reported at the International Thyroid Congress.
The rate of pregnancy loss was clinically, but not significantly, lower in the treated group (5.1% vs. 8.8%), and there was no difference between the groups with respect to 11 other maternal and neonatal outcomes, Dr. Maraka of the Mayo Clinic, Rochester, Minn., said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
Study subjects were women evaluated at the Mayo Clinic, Rochester, between January 2011 and December 2013, who had SCH during pregnancy. SCH was defined as thyroid stimulating hormone levels greater than 2.5 mIU/L during the first trimester, or greater than 3 mIU/L but no more than 10 mIU/L during the second and third trimesters. Those with a twin pregnancy or who used medications that might affect thyroid function were excluded.
The treated and untreated groups were similar with regard to age, history of pregnancy loss, and smoking status, but the treated group had higher body mass index and higher TSH levels, Dr. Maraka noted.
The findings that LT4 may improve pregnancy outcome in women with SCH are important because SCH has been associated with an increased risk of adverse pregnancy outcomes in some studies and it has been unclear whether LT4 therapy improves outcomes.
However, the association seen in the current study, which involves the largest cohort reporting pregnancy outcomes of women with SCH treated vs. untreated with LT4 therapy, requires confirmation in randomized trials before widespread use of LT4 therapy for SCH can be recommended, she concluded.
Dr. Maraka reported having no disclosures.
LAKE BUENA VISTA, FLA. – Levothyroxine therapy during pregnancy in women with subclinical hypothyroidism was associated with a decrease in low birth weight and low Apgar scores but not with a significant decrease in pregnancy loss in a large retrospective cohort study.
In 79 pregnant women with subclinical hypothyroidism (SCH) who received therapy with levothyroxine (LT4), and 285 with SCH who did not receive LT4 therapy, the frequency of low birth weight (less than 2,500 g) was 1.3% vs. 10%, respectively, and the frequency of Apgar scores of 7 or less at 5 minutes was 0% vs. 6.9%, Dr. S. Maraka reported at the International Thyroid Congress.
The rate of pregnancy loss was clinically, but not significantly, lower in the treated group (5.1% vs. 8.8%), and there was no difference between the groups with respect to 11 other maternal and neonatal outcomes, Dr. Maraka of the Mayo Clinic, Rochester, Minn., said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
Study subjects were women evaluated at the Mayo Clinic, Rochester, between January 2011 and December 2013, who had SCH during pregnancy. SCH was defined as thyroid stimulating hormone levels greater than 2.5 mIU/L during the first trimester, or greater than 3 mIU/L but no more than 10 mIU/L during the second and third trimesters. Those with a twin pregnancy or who used medications that might affect thyroid function were excluded.
The treated and untreated groups were similar with regard to age, history of pregnancy loss, and smoking status, but the treated group had higher body mass index and higher TSH levels, Dr. Maraka noted.
The findings that LT4 may improve pregnancy outcome in women with SCH are important because SCH has been associated with an increased risk of adverse pregnancy outcomes in some studies and it has been unclear whether LT4 therapy improves outcomes.
However, the association seen in the current study, which involves the largest cohort reporting pregnancy outcomes of women with SCH treated vs. untreated with LT4 therapy, requires confirmation in randomized trials before widespread use of LT4 therapy for SCH can be recommended, she concluded.
Dr. Maraka reported having no disclosures.
LAKE BUENA VISTA, FLA. – Levothyroxine therapy during pregnancy in women with subclinical hypothyroidism was associated with a decrease in low birth weight and low Apgar scores but not with a significant decrease in pregnancy loss in a large retrospective cohort study.
In 79 pregnant women with subclinical hypothyroidism (SCH) who received therapy with levothyroxine (LT4), and 285 with SCH who did not receive LT4 therapy, the frequency of low birth weight (less than 2,500 g) was 1.3% vs. 10%, respectively, and the frequency of Apgar scores of 7 or less at 5 minutes was 0% vs. 6.9%, Dr. S. Maraka reported at the International Thyroid Congress.
The rate of pregnancy loss was clinically, but not significantly, lower in the treated group (5.1% vs. 8.8%), and there was no difference between the groups with respect to 11 other maternal and neonatal outcomes, Dr. Maraka of the Mayo Clinic, Rochester, Minn., said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
Study subjects were women evaluated at the Mayo Clinic, Rochester, between January 2011 and December 2013, who had SCH during pregnancy. SCH was defined as thyroid stimulating hormone levels greater than 2.5 mIU/L during the first trimester, or greater than 3 mIU/L but no more than 10 mIU/L during the second and third trimesters. Those with a twin pregnancy or who used medications that might affect thyroid function were excluded.
The treated and untreated groups were similar with regard to age, history of pregnancy loss, and smoking status, but the treated group had higher body mass index and higher TSH levels, Dr. Maraka noted.
The findings that LT4 may improve pregnancy outcome in women with SCH are important because SCH has been associated with an increased risk of adverse pregnancy outcomes in some studies and it has been unclear whether LT4 therapy improves outcomes.
However, the association seen in the current study, which involves the largest cohort reporting pregnancy outcomes of women with SCH treated vs. untreated with LT4 therapy, requires confirmation in randomized trials before widespread use of LT4 therapy for SCH can be recommended, she concluded.
Dr. Maraka reported having no disclosures.
AT ITC 2015
Key clinical point: LT4 therapy during pregnancy in women with subclinical hypothyroidism was associated with a decrease in low birth weight and low Apgar scores, but not with a decrease in pregnancy loss in a large retrospective cohort study.
Major finding: The frequency of low birth weight was 1.3% vs. 10%, and the frequency of Apgar scores of 7 or less at 5 minutes was 0% vs. 6.9% in the treated vs. untreated patients.
Data source: A retrospective cohort study of 364 women.
Disclosures: Dr. Maraka reported having no disclosures.
ITC 2015: Review IDs features to aid thyroid lymphoma diagnosis
LAKE BUENA VISTA, FLA. – Rapidly enlarging thyroid masses with compressive symptoms may signal thyroid lymphoma, according to findings from a review of cases at the Mayo Clinic.
Radiologically, these masses tend to present as large, unilateral, thyroid-centered masses that are hypoechoic on ultrasound and that expand into adjacent soft tissue, Dr. Anu Sharma reported at the International Thyroid Congress.
The findings are based on a review of 75 patients with biopsy-proven thyroid lymphoma – a relatively rare disease, accounting for between 1% and 5% of all thyroid malignancies, and less than 1% of all lymphomas – who presented to the Mayo Clinic between 2000 and 2014.
“Thyroid lymphoma can sometimes present very similar to anaplastic carcinoma, and we wanted to see if there are any unique identification factors that you can use to increase your suspicion of thyroid lymphoma,” Dr. Sharma of the Mayo Clinic, Rochester, Minn., said.
Indeed, rapid enlargement and compressive symptoms are also common presenting features of anaplastic carcinoma, she said.
Of the 75 cases included in the review – compromising all cases presenting during the study period – 70.7% involved primary thyroid lymphoma. A neck mass was present in 88% of cases, dysphagia in 45%, and hoarseness in 37%.
The typical presentation included a solid, hypoechoic mass with mildly increased vascularity, no internal calcifications, and edge characteristics that ranged from well-defined (80%) to ill-defined (20%). Median tumor volume was 64 cm3, Dr. Sharma said.
This differs from anaplastic carcinoma in that most patients with anaplastic carcinoma have ill-defined edges, she noted.
Another difference between thyroid lymphoma and anaplastic carcinoma as noted in this study involves necrosis; none of the patients in the current study had areas of necrosis, whereas 78% of anaplastic carcinoma patients in another study had areas of necrosis, she explained.
The patients in the current study had a median age of 67 years, although the ages varied widely. About half (50.7%) were men, and 54.7% had a history of Hashimoto’s thyroiditis. Fifty-seven of the patients had an ultrasound before treatment.
The first diagnostic procedure performed was fine needle aspiration (FNA) in 65 subjects, and the FNA biopsies were abnormal in 69% of those, with 42% suggesting a specific lymphoma subtype. The subtype diagnosis was accurate, based on final tissue analysis, in 89% of those.
“While this is quite impressive, all patients who had FNA ended up having further tissue biopsy for subtype confirmation and for treatment, and this is important, because the subtype of the lymphoma is important in determining the type of treatment uses as well as determining prognosis,” she said.
The diagnosis was confirmed by core biopsy in 46.7% of cases, by incisional biopsy in 9.3%, by partial or total thyroidectomy in 25.3%, and by lymph node biopsy in 13.3%; percentages total 94.6% due to downward rounding. Histologic subtypes included diffuse large B-cell lymphoma (DLBCL) in 73.3% of cases, follicular lymphoma in 5.3%, mucosa-associated lymphoid tissue (MALT) in 10.7%, MALT/DLBCL in 2.6%, T-cell lymphoma in 2.6%, and Hodgkin’s lymphoma in 1.3%; percentages total 95.8% rather than 100% due to downward rounding.
In addition to rapid enlargement of a neck mass with compressive symptoms, findings that should raise suspicion of thyroid lymphoma include a history of Hashimoto’s thyroiditis and the ultrasound findings characterized by this study, Dr. Sharma said.
“Once you have that increased suspicion, you should move toward going to core biopsy rather than FNA to save the patient from having two diagnostic steps rather than one,” she concluded.
Dr. Sharma reported having no disclosures.
LAKE BUENA VISTA, FLA. – Rapidly enlarging thyroid masses with compressive symptoms may signal thyroid lymphoma, according to findings from a review of cases at the Mayo Clinic.
Radiologically, these masses tend to present as large, unilateral, thyroid-centered masses that are hypoechoic on ultrasound and that expand into adjacent soft tissue, Dr. Anu Sharma reported at the International Thyroid Congress.
The findings are based on a review of 75 patients with biopsy-proven thyroid lymphoma – a relatively rare disease, accounting for between 1% and 5% of all thyroid malignancies, and less than 1% of all lymphomas – who presented to the Mayo Clinic between 2000 and 2014.
“Thyroid lymphoma can sometimes present very similar to anaplastic carcinoma, and we wanted to see if there are any unique identification factors that you can use to increase your suspicion of thyroid lymphoma,” Dr. Sharma of the Mayo Clinic, Rochester, Minn., said.
Indeed, rapid enlargement and compressive symptoms are also common presenting features of anaplastic carcinoma, she said.
Of the 75 cases included in the review – compromising all cases presenting during the study period – 70.7% involved primary thyroid lymphoma. A neck mass was present in 88% of cases, dysphagia in 45%, and hoarseness in 37%.
The typical presentation included a solid, hypoechoic mass with mildly increased vascularity, no internal calcifications, and edge characteristics that ranged from well-defined (80%) to ill-defined (20%). Median tumor volume was 64 cm3, Dr. Sharma said.
This differs from anaplastic carcinoma in that most patients with anaplastic carcinoma have ill-defined edges, she noted.
Another difference between thyroid lymphoma and anaplastic carcinoma as noted in this study involves necrosis; none of the patients in the current study had areas of necrosis, whereas 78% of anaplastic carcinoma patients in another study had areas of necrosis, she explained.
The patients in the current study had a median age of 67 years, although the ages varied widely. About half (50.7%) were men, and 54.7% had a history of Hashimoto’s thyroiditis. Fifty-seven of the patients had an ultrasound before treatment.
The first diagnostic procedure performed was fine needle aspiration (FNA) in 65 subjects, and the FNA biopsies were abnormal in 69% of those, with 42% suggesting a specific lymphoma subtype. The subtype diagnosis was accurate, based on final tissue analysis, in 89% of those.
“While this is quite impressive, all patients who had FNA ended up having further tissue biopsy for subtype confirmation and for treatment, and this is important, because the subtype of the lymphoma is important in determining the type of treatment uses as well as determining prognosis,” she said.
The diagnosis was confirmed by core biopsy in 46.7% of cases, by incisional biopsy in 9.3%, by partial or total thyroidectomy in 25.3%, and by lymph node biopsy in 13.3%; percentages total 94.6% due to downward rounding. Histologic subtypes included diffuse large B-cell lymphoma (DLBCL) in 73.3% of cases, follicular lymphoma in 5.3%, mucosa-associated lymphoid tissue (MALT) in 10.7%, MALT/DLBCL in 2.6%, T-cell lymphoma in 2.6%, and Hodgkin’s lymphoma in 1.3%; percentages total 95.8% rather than 100% due to downward rounding.
In addition to rapid enlargement of a neck mass with compressive symptoms, findings that should raise suspicion of thyroid lymphoma include a history of Hashimoto’s thyroiditis and the ultrasound findings characterized by this study, Dr. Sharma said.
“Once you have that increased suspicion, you should move toward going to core biopsy rather than FNA to save the patient from having two diagnostic steps rather than one,” she concluded.
Dr. Sharma reported having no disclosures.
LAKE BUENA VISTA, FLA. – Rapidly enlarging thyroid masses with compressive symptoms may signal thyroid lymphoma, according to findings from a review of cases at the Mayo Clinic.
Radiologically, these masses tend to present as large, unilateral, thyroid-centered masses that are hypoechoic on ultrasound and that expand into adjacent soft tissue, Dr. Anu Sharma reported at the International Thyroid Congress.
The findings are based on a review of 75 patients with biopsy-proven thyroid lymphoma – a relatively rare disease, accounting for between 1% and 5% of all thyroid malignancies, and less than 1% of all lymphomas – who presented to the Mayo Clinic between 2000 and 2014.
“Thyroid lymphoma can sometimes present very similar to anaplastic carcinoma, and we wanted to see if there are any unique identification factors that you can use to increase your suspicion of thyroid lymphoma,” Dr. Sharma of the Mayo Clinic, Rochester, Minn., said.
Indeed, rapid enlargement and compressive symptoms are also common presenting features of anaplastic carcinoma, she said.
Of the 75 cases included in the review – compromising all cases presenting during the study period – 70.7% involved primary thyroid lymphoma. A neck mass was present in 88% of cases, dysphagia in 45%, and hoarseness in 37%.
The typical presentation included a solid, hypoechoic mass with mildly increased vascularity, no internal calcifications, and edge characteristics that ranged from well-defined (80%) to ill-defined (20%). Median tumor volume was 64 cm3, Dr. Sharma said.
This differs from anaplastic carcinoma in that most patients with anaplastic carcinoma have ill-defined edges, she noted.
Another difference between thyroid lymphoma and anaplastic carcinoma as noted in this study involves necrosis; none of the patients in the current study had areas of necrosis, whereas 78% of anaplastic carcinoma patients in another study had areas of necrosis, she explained.
The patients in the current study had a median age of 67 years, although the ages varied widely. About half (50.7%) were men, and 54.7% had a history of Hashimoto’s thyroiditis. Fifty-seven of the patients had an ultrasound before treatment.
The first diagnostic procedure performed was fine needle aspiration (FNA) in 65 subjects, and the FNA biopsies were abnormal in 69% of those, with 42% suggesting a specific lymphoma subtype. The subtype diagnosis was accurate, based on final tissue analysis, in 89% of those.
“While this is quite impressive, all patients who had FNA ended up having further tissue biopsy for subtype confirmation and for treatment, and this is important, because the subtype of the lymphoma is important in determining the type of treatment uses as well as determining prognosis,” she said.
The diagnosis was confirmed by core biopsy in 46.7% of cases, by incisional biopsy in 9.3%, by partial or total thyroidectomy in 25.3%, and by lymph node biopsy in 13.3%; percentages total 94.6% due to downward rounding. Histologic subtypes included diffuse large B-cell lymphoma (DLBCL) in 73.3% of cases, follicular lymphoma in 5.3%, mucosa-associated lymphoid tissue (MALT) in 10.7%, MALT/DLBCL in 2.6%, T-cell lymphoma in 2.6%, and Hodgkin’s lymphoma in 1.3%; percentages total 95.8% rather than 100% due to downward rounding.
In addition to rapid enlargement of a neck mass with compressive symptoms, findings that should raise suspicion of thyroid lymphoma include a history of Hashimoto’s thyroiditis and the ultrasound findings characterized by this study, Dr. Sharma said.
“Once you have that increased suspicion, you should move toward going to core biopsy rather than FNA to save the patient from having two diagnostic steps rather than one,” she concluded.
Dr. Sharma reported having no disclosures.
AT ITC 2015
Key clinical point: Rapidly enlarging thyroid masses with compressive symptoms may signal thyroid lymphoma, according to findings from a review of cases at the Mayo Clinic.
Major finding: Typical presentation included a solid, hypoechoic mass with mildly increased vascularity, no internal calcifications, and edge characteristics that ranged from well-defined (80%) to ill-defined (20%).
Data source: A retrospective review of 75 cases.
Disclosures: Dr. Sharma reported having no disclosures.
Guidelines: Follow low-risk benign thyroid nodules conservatively
LAKE BUENA VISTA, FLA. – Observation is a reasonable option for managing patients with benign thyroid nodules that display initial low-suspicion ultrasound patterns.
Presented at the International Thyroid Congress, the American Thyroid Association’s new guidelines on the management of thyroid nodules offer specific ultrasound patterns that help distinguish benign nodules from developing malignancy (Thyroid. 2015 Oct. doi: 10.1089/thy.2015.0020]).
They reflect new data obtained from studies conducted in the years since 2009, when the last guidelines were issued, Dr. Susan Mandel said at the meeting, which was held by the American Thyroid Association, Asia-Oceania Thyroid Association , European Thyroid Association, and Latin American Thyroid Society.
“We now know that about 20% of cytologically benign thyroid nodules will grow,” said Dr. Mandel of the University of Pennsylvania, Philadelphia. But very few of those will become malignant. According to a newly published study of 553 patients, 200 must be followed to identify one cancer that would have been missed (Thyroid. 2015 Oct 25;10:1115-20).
The new guidelines reflect findings like these and attempt to balance sensible follow-up while avoiding overtreatment, Dr. Mandel said. Diagnosed thyroid cancers are on the rise in the United States, from about 37,000 in 2009 to about 63,000 last year. But this is not because the cancer itself is more prevalent. More likely, it is the result of the increasing use of sonography or other imaging studies, which allows for earlier diagnosis and treatment. These technologies have a downside as well, leading to overdiagnosis and overtreatment of benign nodules that, most likely, would never undergo malignant transformation, Dr. Mandel said.
Nodules are most likely to grow in younger patients and those with longer follow-up, “which makes sense, as thyroid nodules grow very slowly.” Predominately solid nodules are also more likely to grow than are cystic nodules. Size at baseline is a controversial topic. There is some evidence that subcentimeter nodules are more likely to grow, while larger nodules are less likely.
Worrisome initial ultrasound patterns, however, should throw up a red flag even if the nodule is cytologically negative at baseline, Dr. Mandel said. These patterns are:
• Marked hypoechogeniticy.
• Microcalcifications.
• Irregular, microlobulated margins.
• Taller-than-wide shape.
• Incomplete or peripheral calcification.
These nodules should undergo a repeat ultrasound and a repeat ultrasound-guided fine needle biopsy within 6-12 months of the initial assessment, the guidelines recommend.
For nodules of any state, if (1) there is sonographic evidence of growth (20% increase in at least 2 nodule dimensions with a minimal increase of 2 mm or (2) more than 50% change in volume) or (3) development of new suspicious sonographic features, there should be an additional fine-needle biopsy. “Although the risk of malignancy after two benign cytology results is virtually zero, routine repeat biopsy is not a viable or cost-effective option because of the low false negative rate,” the document notes.
Small nodules (less than 1 cm) that have very low-suspicion ultrasound patterns (including spongiform nodules) don’t require routine follow-up sonograms. Nor do nodules larger than 5 mm without high-suspicion findings.
The guidelines also offer recommendations on treatment. There is no need to offer routine therapy with thyroid stimulating hormone (TSH) for benign nodules. “Though modest responses to therapy can be detected, the potential harm outweighs benefit for most patients,” the document states.
If benign nodules grow, surgery could be an option if they become large (greater than 4 cm), or cause compression or structural symptoms – or if there is clinical concern, although the document does not specify exactly what that could be.
Patients with growing nodules that are benign after a second biopsy should be regularly monitored. Most asymptomatic nodules demonstrating modest growth should be followed without intervention,” it says.
Recurrent benign cystic thyroid nodules may be treated surgically or with ethanol ablation, but asymptotic cystic nodules may be followed conservatively There are no data to guide the use of thyroid hormone treatment for benign nodules, even if they do grow.
The guidelines also offer recommendations for initial serum and molecular studies, and treatment for follicular and differentiated thyroid cancers.
Dr. Mandel had no financial disclosures.
LAKE BUENA VISTA, FLA. – Observation is a reasonable option for managing patients with benign thyroid nodules that display initial low-suspicion ultrasound patterns.
Presented at the International Thyroid Congress, the American Thyroid Association’s new guidelines on the management of thyroid nodules offer specific ultrasound patterns that help distinguish benign nodules from developing malignancy (Thyroid. 2015 Oct. doi: 10.1089/thy.2015.0020]).
They reflect new data obtained from studies conducted in the years since 2009, when the last guidelines were issued, Dr. Susan Mandel said at the meeting, which was held by the American Thyroid Association, Asia-Oceania Thyroid Association , European Thyroid Association, and Latin American Thyroid Society.
“We now know that about 20% of cytologically benign thyroid nodules will grow,” said Dr. Mandel of the University of Pennsylvania, Philadelphia. But very few of those will become malignant. According to a newly published study of 553 patients, 200 must be followed to identify one cancer that would have been missed (Thyroid. 2015 Oct 25;10:1115-20).
The new guidelines reflect findings like these and attempt to balance sensible follow-up while avoiding overtreatment, Dr. Mandel said. Diagnosed thyroid cancers are on the rise in the United States, from about 37,000 in 2009 to about 63,000 last year. But this is not because the cancer itself is more prevalent. More likely, it is the result of the increasing use of sonography or other imaging studies, which allows for earlier diagnosis and treatment. These technologies have a downside as well, leading to overdiagnosis and overtreatment of benign nodules that, most likely, would never undergo malignant transformation, Dr. Mandel said.
Nodules are most likely to grow in younger patients and those with longer follow-up, “which makes sense, as thyroid nodules grow very slowly.” Predominately solid nodules are also more likely to grow than are cystic nodules. Size at baseline is a controversial topic. There is some evidence that subcentimeter nodules are more likely to grow, while larger nodules are less likely.
Worrisome initial ultrasound patterns, however, should throw up a red flag even if the nodule is cytologically negative at baseline, Dr. Mandel said. These patterns are:
• Marked hypoechogeniticy.
• Microcalcifications.
• Irregular, microlobulated margins.
• Taller-than-wide shape.
• Incomplete or peripheral calcification.
These nodules should undergo a repeat ultrasound and a repeat ultrasound-guided fine needle biopsy within 6-12 months of the initial assessment, the guidelines recommend.
For nodules of any state, if (1) there is sonographic evidence of growth (20% increase in at least 2 nodule dimensions with a minimal increase of 2 mm or (2) more than 50% change in volume) or (3) development of new suspicious sonographic features, there should be an additional fine-needle biopsy. “Although the risk of malignancy after two benign cytology results is virtually zero, routine repeat biopsy is not a viable or cost-effective option because of the low false negative rate,” the document notes.
Small nodules (less than 1 cm) that have very low-suspicion ultrasound patterns (including spongiform nodules) don’t require routine follow-up sonograms. Nor do nodules larger than 5 mm without high-suspicion findings.
The guidelines also offer recommendations on treatment. There is no need to offer routine therapy with thyroid stimulating hormone (TSH) for benign nodules. “Though modest responses to therapy can be detected, the potential harm outweighs benefit for most patients,” the document states.
If benign nodules grow, surgery could be an option if they become large (greater than 4 cm), or cause compression or structural symptoms – or if there is clinical concern, although the document does not specify exactly what that could be.
Patients with growing nodules that are benign after a second biopsy should be regularly monitored. Most asymptomatic nodules demonstrating modest growth should be followed without intervention,” it says.
Recurrent benign cystic thyroid nodules may be treated surgically or with ethanol ablation, but asymptotic cystic nodules may be followed conservatively There are no data to guide the use of thyroid hormone treatment for benign nodules, even if they do grow.
The guidelines also offer recommendations for initial serum and molecular studies, and treatment for follicular and differentiated thyroid cancers.
Dr. Mandel had no financial disclosures.
LAKE BUENA VISTA, FLA. – Observation is a reasonable option for managing patients with benign thyroid nodules that display initial low-suspicion ultrasound patterns.
Presented at the International Thyroid Congress, the American Thyroid Association’s new guidelines on the management of thyroid nodules offer specific ultrasound patterns that help distinguish benign nodules from developing malignancy (Thyroid. 2015 Oct. doi: 10.1089/thy.2015.0020]).
They reflect new data obtained from studies conducted in the years since 2009, when the last guidelines were issued, Dr. Susan Mandel said at the meeting, which was held by the American Thyroid Association, Asia-Oceania Thyroid Association , European Thyroid Association, and Latin American Thyroid Society.
“We now know that about 20% of cytologically benign thyroid nodules will grow,” said Dr. Mandel of the University of Pennsylvania, Philadelphia. But very few of those will become malignant. According to a newly published study of 553 patients, 200 must be followed to identify one cancer that would have been missed (Thyroid. 2015 Oct 25;10:1115-20).
The new guidelines reflect findings like these and attempt to balance sensible follow-up while avoiding overtreatment, Dr. Mandel said. Diagnosed thyroid cancers are on the rise in the United States, from about 37,000 in 2009 to about 63,000 last year. But this is not because the cancer itself is more prevalent. More likely, it is the result of the increasing use of sonography or other imaging studies, which allows for earlier diagnosis and treatment. These technologies have a downside as well, leading to overdiagnosis and overtreatment of benign nodules that, most likely, would never undergo malignant transformation, Dr. Mandel said.
Nodules are most likely to grow in younger patients and those with longer follow-up, “which makes sense, as thyroid nodules grow very slowly.” Predominately solid nodules are also more likely to grow than are cystic nodules. Size at baseline is a controversial topic. There is some evidence that subcentimeter nodules are more likely to grow, while larger nodules are less likely.
Worrisome initial ultrasound patterns, however, should throw up a red flag even if the nodule is cytologically negative at baseline, Dr. Mandel said. These patterns are:
• Marked hypoechogeniticy.
• Microcalcifications.
• Irregular, microlobulated margins.
• Taller-than-wide shape.
• Incomplete or peripheral calcification.
These nodules should undergo a repeat ultrasound and a repeat ultrasound-guided fine needle biopsy within 6-12 months of the initial assessment, the guidelines recommend.
For nodules of any state, if (1) there is sonographic evidence of growth (20% increase in at least 2 nodule dimensions with a minimal increase of 2 mm or (2) more than 50% change in volume) or (3) development of new suspicious sonographic features, there should be an additional fine-needle biopsy. “Although the risk of malignancy after two benign cytology results is virtually zero, routine repeat biopsy is not a viable or cost-effective option because of the low false negative rate,” the document notes.
Small nodules (less than 1 cm) that have very low-suspicion ultrasound patterns (including spongiform nodules) don’t require routine follow-up sonograms. Nor do nodules larger than 5 mm without high-suspicion findings.
The guidelines also offer recommendations on treatment. There is no need to offer routine therapy with thyroid stimulating hormone (TSH) for benign nodules. “Though modest responses to therapy can be detected, the potential harm outweighs benefit for most patients,” the document states.
If benign nodules grow, surgery could be an option if they become large (greater than 4 cm), or cause compression or structural symptoms – or if there is clinical concern, although the document does not specify exactly what that could be.
Patients with growing nodules that are benign after a second biopsy should be regularly monitored. Most asymptomatic nodules demonstrating modest growth should be followed without intervention,” it says.
Recurrent benign cystic thyroid nodules may be treated surgically or with ethanol ablation, but asymptotic cystic nodules may be followed conservatively There are no data to guide the use of thyroid hormone treatment for benign nodules, even if they do grow.
The guidelines also offer recommendations for initial serum and molecular studies, and treatment for follicular and differentiated thyroid cancers.
Dr. Mandel had no financial disclosures.
EXPERT ANALYSIS FROM ITC 2015
Even Subclinical Hypothyroidism Ups Risk for Metabolic Syndrome
LAKE BUENA VISTA, FLA. – Patients with low thyroid function may experience a “double whammy” of hypothyroidism and metabolic syndrome.
Even subclinical hypothyroidism affects many metabolic pathways that can contribute to deranged glucose and lipid metabolism, raising the risk of metabolic syndrome, according to Dr. Gabriela Brenta of the department of endocrinology at the Dr. Cesar Milstein Hospital in Buenos Aires. Though some mechanisms are incompletely understood, the association is clear enough to warrant screening all metabolic syndrome patients for hypothyroidism, she said.
Dr. Brenta described the recent work she and others have completed in the field. Basic science work revealed some early clues. For example, those who studied the effects of acute thyroid hormone withdrawal on patients with no thyroid gland found that these patients saw a rapid rise in insulin resistance. It’s known that even subclinical insulin resistance can lead to impaired glucose metabolism, making it logical to follow both normal and deranged metabolic pathways to help sort out the relationship between thyroid dysfunction and impaired glucose metabolism, she reported at the International Thyroid Congress.
Hypothyroidism can affect glucose homeostasis through multiple mechanisms, said Dr. Brenta. Firstly, hypothyroidism can lead to decreased hepatic gluconeogenesis and glycogenolysis. Hypothyroidism also can lead to reduced baseline plasma insulin levels and increased postglucose insulin secretion. In the peripheral tissues, hypothyroidism can interfere with glucose metabolism and disposal. All of these mechanisms can decrease hepatic glucose metabolism and lead to a postabsorptive hyperglycemia state, said Dr. Brenta, noting: “Insulin resistance is in some way the backbone of metabolic syndrome.”
Lipid metabolism is also affected by subclinical hypothyroidism, which can decrease expression of mRNA for LDL-C receptors, leading to LDL-C receptor down-regulation. With fewer receptors available, serum levels of LDL-C increase, with resultant increased susceptibility to oxidative effects and increased foam cell generation.
Dr. Brenta cited her earlier work showing that “triglyceride enrichment of LDL particles correlates with lower hepatic lipase activity” for individuals with subclinical hypothyroidism, with significantly lower hepatic lipase activity and a higher LDL-C to triglyceride ratio for those patients than for controls (Thyroid. 2007 May;17[5]:453-60). Overall, in hypothyroidism, “LDL particles are exposed to more substances that make them more atherogenic with decreased degradation and increased half-life,” said Dr. Brenta.
The increased risk for hypertension in both subclinical and overt hypothyroidism may be related, in part, to the fact that triiodothyronine deficiency can contribute to endothelial dysfunction. The relationship between subclinical hypothyroidism and hypertension was confirmed in a 2011 meta-analysis, said Dr. Brenta (Hypertens Res. 2011 Oct;34[10]:1098-105).
Though many factors contribute to obesity and thyroid function alone does not regulate body weight, a large population-based Danish study found that “even mild elevations of TSH are important for body weight,” said Dr. Brenta. The relationship is complex and bidirectional – a classic “chicken and egg” story – since obesity also may modulate TSH, she said; “however, we must not forget the ample literature on low levels of thyroid hormones reducing resting energy expenditure” (J Clin Endocrinol Metab. 2005 Jul;90[7]:4019-24).
Even though TSH tends to rise naturally through the lifespan, the association between elevated TSH and increased risk of metabolic syndrome held true even for older patients in one study, with “each one unit increase in TSH predicting a 3% increase in the odds of metabolic syndrome,” even after adjustment for age, BMI, and HOMA-IR status, among other variables, said Dr. Brenta (Clin Endocrinol [Oxf]. 2012 Jun;76[6]:911-8).
Advocating for universal screening for hypothyroidism among patients with metabolic syndrome, Dr. Brenta said that “hypothyroid disturbances are associated with an adverse metabolic profile, and even low normal TSH levels are associated with the metabolic traits of metabolic syndrome.”
The meeting was held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society. Dr. Brenta did not identify any conflicts of interest.
LAKE BUENA VISTA, FLA. – Patients with low thyroid function may experience a “double whammy” of hypothyroidism and metabolic syndrome.
Even subclinical hypothyroidism affects many metabolic pathways that can contribute to deranged glucose and lipid metabolism, raising the risk of metabolic syndrome, according to Dr. Gabriela Brenta of the department of endocrinology at the Dr. Cesar Milstein Hospital in Buenos Aires. Though some mechanisms are incompletely understood, the association is clear enough to warrant screening all metabolic syndrome patients for hypothyroidism, she said.
Dr. Brenta described the recent work she and others have completed in the field. Basic science work revealed some early clues. For example, those who studied the effects of acute thyroid hormone withdrawal on patients with no thyroid gland found that these patients saw a rapid rise in insulin resistance. It’s known that even subclinical insulin resistance can lead to impaired glucose metabolism, making it logical to follow both normal and deranged metabolic pathways to help sort out the relationship between thyroid dysfunction and impaired glucose metabolism, she reported at the International Thyroid Congress.
Hypothyroidism can affect glucose homeostasis through multiple mechanisms, said Dr. Brenta. Firstly, hypothyroidism can lead to decreased hepatic gluconeogenesis and glycogenolysis. Hypothyroidism also can lead to reduced baseline plasma insulin levels and increased postglucose insulin secretion. In the peripheral tissues, hypothyroidism can interfere with glucose metabolism and disposal. All of these mechanisms can decrease hepatic glucose metabolism and lead to a postabsorptive hyperglycemia state, said Dr. Brenta, noting: “Insulin resistance is in some way the backbone of metabolic syndrome.”
Lipid metabolism is also affected by subclinical hypothyroidism, which can decrease expression of mRNA for LDL-C receptors, leading to LDL-C receptor down-regulation. With fewer receptors available, serum levels of LDL-C increase, with resultant increased susceptibility to oxidative effects and increased foam cell generation.
Dr. Brenta cited her earlier work showing that “triglyceride enrichment of LDL particles correlates with lower hepatic lipase activity” for individuals with subclinical hypothyroidism, with significantly lower hepatic lipase activity and a higher LDL-C to triglyceride ratio for those patients than for controls (Thyroid. 2007 May;17[5]:453-60). Overall, in hypothyroidism, “LDL particles are exposed to more substances that make them more atherogenic with decreased degradation and increased half-life,” said Dr. Brenta.
The increased risk for hypertension in both subclinical and overt hypothyroidism may be related, in part, to the fact that triiodothyronine deficiency can contribute to endothelial dysfunction. The relationship between subclinical hypothyroidism and hypertension was confirmed in a 2011 meta-analysis, said Dr. Brenta (Hypertens Res. 2011 Oct;34[10]:1098-105).
Though many factors contribute to obesity and thyroid function alone does not regulate body weight, a large population-based Danish study found that “even mild elevations of TSH are important for body weight,” said Dr. Brenta. The relationship is complex and bidirectional – a classic “chicken and egg” story – since obesity also may modulate TSH, she said; “however, we must not forget the ample literature on low levels of thyroid hormones reducing resting energy expenditure” (J Clin Endocrinol Metab. 2005 Jul;90[7]:4019-24).
Even though TSH tends to rise naturally through the lifespan, the association between elevated TSH and increased risk of metabolic syndrome held true even for older patients in one study, with “each one unit increase in TSH predicting a 3% increase in the odds of metabolic syndrome,” even after adjustment for age, BMI, and HOMA-IR status, among other variables, said Dr. Brenta (Clin Endocrinol [Oxf]. 2012 Jun;76[6]:911-8).
Advocating for universal screening for hypothyroidism among patients with metabolic syndrome, Dr. Brenta said that “hypothyroid disturbances are associated with an adverse metabolic profile, and even low normal TSH levels are associated with the metabolic traits of metabolic syndrome.”
The meeting was held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society. Dr. Brenta did not identify any conflicts of interest.
LAKE BUENA VISTA, FLA. – Patients with low thyroid function may experience a “double whammy” of hypothyroidism and metabolic syndrome.
Even subclinical hypothyroidism affects many metabolic pathways that can contribute to deranged glucose and lipid metabolism, raising the risk of metabolic syndrome, according to Dr. Gabriela Brenta of the department of endocrinology at the Dr. Cesar Milstein Hospital in Buenos Aires. Though some mechanisms are incompletely understood, the association is clear enough to warrant screening all metabolic syndrome patients for hypothyroidism, she said.
Dr. Brenta described the recent work she and others have completed in the field. Basic science work revealed some early clues. For example, those who studied the effects of acute thyroid hormone withdrawal on patients with no thyroid gland found that these patients saw a rapid rise in insulin resistance. It’s known that even subclinical insulin resistance can lead to impaired glucose metabolism, making it logical to follow both normal and deranged metabolic pathways to help sort out the relationship between thyroid dysfunction and impaired glucose metabolism, she reported at the International Thyroid Congress.
Hypothyroidism can affect glucose homeostasis through multiple mechanisms, said Dr. Brenta. Firstly, hypothyroidism can lead to decreased hepatic gluconeogenesis and glycogenolysis. Hypothyroidism also can lead to reduced baseline plasma insulin levels and increased postglucose insulin secretion. In the peripheral tissues, hypothyroidism can interfere with glucose metabolism and disposal. All of these mechanisms can decrease hepatic glucose metabolism and lead to a postabsorptive hyperglycemia state, said Dr. Brenta, noting: “Insulin resistance is in some way the backbone of metabolic syndrome.”
Lipid metabolism is also affected by subclinical hypothyroidism, which can decrease expression of mRNA for LDL-C receptors, leading to LDL-C receptor down-regulation. With fewer receptors available, serum levels of LDL-C increase, with resultant increased susceptibility to oxidative effects and increased foam cell generation.
Dr. Brenta cited her earlier work showing that “triglyceride enrichment of LDL particles correlates with lower hepatic lipase activity” for individuals with subclinical hypothyroidism, with significantly lower hepatic lipase activity and a higher LDL-C to triglyceride ratio for those patients than for controls (Thyroid. 2007 May;17[5]:453-60). Overall, in hypothyroidism, “LDL particles are exposed to more substances that make them more atherogenic with decreased degradation and increased half-life,” said Dr. Brenta.
The increased risk for hypertension in both subclinical and overt hypothyroidism may be related, in part, to the fact that triiodothyronine deficiency can contribute to endothelial dysfunction. The relationship between subclinical hypothyroidism and hypertension was confirmed in a 2011 meta-analysis, said Dr. Brenta (Hypertens Res. 2011 Oct;34[10]:1098-105).
Though many factors contribute to obesity and thyroid function alone does not regulate body weight, a large population-based Danish study found that “even mild elevations of TSH are important for body weight,” said Dr. Brenta. The relationship is complex and bidirectional – a classic “chicken and egg” story – since obesity also may modulate TSH, she said; “however, we must not forget the ample literature on low levels of thyroid hormones reducing resting energy expenditure” (J Clin Endocrinol Metab. 2005 Jul;90[7]:4019-24).
Even though TSH tends to rise naturally through the lifespan, the association between elevated TSH and increased risk of metabolic syndrome held true even for older patients in one study, with “each one unit increase in TSH predicting a 3% increase in the odds of metabolic syndrome,” even after adjustment for age, BMI, and HOMA-IR status, among other variables, said Dr. Brenta (Clin Endocrinol [Oxf]. 2012 Jun;76[6]:911-8).
Advocating for universal screening for hypothyroidism among patients with metabolic syndrome, Dr. Brenta said that “hypothyroid disturbances are associated with an adverse metabolic profile, and even low normal TSH levels are associated with the metabolic traits of metabolic syndrome.”
The meeting was held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society. Dr. Brenta did not identify any conflicts of interest.
EXPERT ANALYSIS FROM ITC 2015
Even subclinical hypothyroidism ups risk for metabolic syndrome
LAKE BUENA VISTA, FLA. – Patients with low thyroid function may experience a “double whammy” of hypothyroidism and metabolic syndrome.
Even subclinical hypothyroidism affects many metabolic pathways that can contribute to deranged glucose and lipid metabolism, raising the risk of metabolic syndrome, according to Dr. Gabriela Brenta of the department of endocrinology at the Dr. Cesar Milstein Hospital in Buenos Aires. Though some mechanisms are incompletely understood, the association is clear enough to warrant screening all metabolic syndrome patients for hypothyroidism, she said.
Dr. Brenta described the recent work she and others have completed in the field. Basic science work revealed some early clues. For example, those who studied the effects of acute thyroid hormone withdrawal on patients with no thyroid gland found that these patients saw a rapid rise in insulin resistance. It’s known that even subclinical insulin resistance can lead to impaired glucose metabolism, making it logical to follow both normal and deranged metabolic pathways to help sort out the relationship between thyroid dysfunction and impaired glucose metabolism, she reported at the International Thyroid Congress.
Hypothyroidism can affect glucose homeostasis through multiple mechanisms, said Dr. Brenta. Firstly, hypothyroidism can lead to decreased hepatic gluconeogenesis and glycogenolysis. Hypothyroidism also can lead to reduced baseline plasma insulin levels and increased postglucose insulin secretion. In the peripheral tissues, hypothyroidism can interfere with glucose metabolism and disposal. All of these mechanisms can decrease hepatic glucose metabolism and lead to a postabsorptive hyperglycemia state, said Dr. Brenta, noting: “Insulin resistance is in some way the backbone of metabolic syndrome.”
Lipid metabolism is also affected by subclinical hypothyroidism, which can decrease expression of mRNA for LDL-C receptors, leading to LDL-C receptor down-regulation. With fewer receptors available, serum levels of LDL-C increase, with resultant increased susceptibility to oxidative effects and increased foam cell generation.
Dr. Brenta cited her earlier work showing that “triglyceride enrichment of LDL particles correlates with lower hepatic lipase activity” for individuals with subclinical hypothyroidism, with significantly lower hepatic lipase activity and a higher LDL-C to triglyceride ratio for those patients than for controls (Thyroid. 2007 May;17[5]:453-60). Overall, in hypothyroidism, “LDL particles are exposed to more substances that make them more atherogenic with decreased degradation and increased half-life,” said Dr. Brenta.
The increased risk for hypertension in both subclinical and overt hypothyroidism may be related, in part, to the fact that triiodothyronine deficiency can contribute to endothelial dysfunction. The relationship between subclinical hypothyroidism and hypertension was confirmed in a 2011 meta-analysis, said Dr. Brenta (Hypertens Res. 2011 Oct;34[10]:1098-105).
Though many factors contribute to obesity and thyroid function alone does not regulate body weight, a large population-based Danish study found that “even mild elevations of TSH are important for body weight,” said Dr. Brenta. The relationship is complex and bidirectional – a classic “chicken and egg” story – since obesity also may modulate TSH, she said; “however, we must not forget the ample literature on low levels of thyroid hormones reducing resting energy expenditure” (J Clin Endocrinol Metab. 2005 Jul;90[7]:4019-24).
Even though TSH tends to rise naturally through the lifespan, the association between elevated TSH and increased risk of metabolic syndrome held true even for older patients in one study, with “each one unit increase in TSH predicting a 3% increase in the odds of metabolic syndrome,” even after adjustment for age, BMI, and HOMA-IR status, among other variables, said Dr. Brenta (Clin Endocrinol [Oxf]. 2012 Jun;76[6]:911-8).
Advocating for universal screening for hypothyroidism among patients with metabolic syndrome, Dr. Brenta said that “hypothyroid disturbances are associated with an adverse metabolic profile, and even low normal TSH levels are associated with the metabolic traits of metabolic syndrome.”
The meeting was held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society. Dr. Brenta did not identify any conflicts of interest.
On Twitter @karioakes
LAKE BUENA VISTA, FLA. – Patients with low thyroid function may experience a “double whammy” of hypothyroidism and metabolic syndrome.
Even subclinical hypothyroidism affects many metabolic pathways that can contribute to deranged glucose and lipid metabolism, raising the risk of metabolic syndrome, according to Dr. Gabriela Brenta of the department of endocrinology at the Dr. Cesar Milstein Hospital in Buenos Aires. Though some mechanisms are incompletely understood, the association is clear enough to warrant screening all metabolic syndrome patients for hypothyroidism, she said.
Dr. Brenta described the recent work she and others have completed in the field. Basic science work revealed some early clues. For example, those who studied the effects of acute thyroid hormone withdrawal on patients with no thyroid gland found that these patients saw a rapid rise in insulin resistance. It’s known that even subclinical insulin resistance can lead to impaired glucose metabolism, making it logical to follow both normal and deranged metabolic pathways to help sort out the relationship between thyroid dysfunction and impaired glucose metabolism, she reported at the International Thyroid Congress.
Hypothyroidism can affect glucose homeostasis through multiple mechanisms, said Dr. Brenta. Firstly, hypothyroidism can lead to decreased hepatic gluconeogenesis and glycogenolysis. Hypothyroidism also can lead to reduced baseline plasma insulin levels and increased postglucose insulin secretion. In the peripheral tissues, hypothyroidism can interfere with glucose metabolism and disposal. All of these mechanisms can decrease hepatic glucose metabolism and lead to a postabsorptive hyperglycemia state, said Dr. Brenta, noting: “Insulin resistance is in some way the backbone of metabolic syndrome.”
Lipid metabolism is also affected by subclinical hypothyroidism, which can decrease expression of mRNA for LDL-C receptors, leading to LDL-C receptor down-regulation. With fewer receptors available, serum levels of LDL-C increase, with resultant increased susceptibility to oxidative effects and increased foam cell generation.
Dr. Brenta cited her earlier work showing that “triglyceride enrichment of LDL particles correlates with lower hepatic lipase activity” for individuals with subclinical hypothyroidism, with significantly lower hepatic lipase activity and a higher LDL-C to triglyceride ratio for those patients than for controls (Thyroid. 2007 May;17[5]:453-60). Overall, in hypothyroidism, “LDL particles are exposed to more substances that make them more atherogenic with decreased degradation and increased half-life,” said Dr. Brenta.
The increased risk for hypertension in both subclinical and overt hypothyroidism may be related, in part, to the fact that triiodothyronine deficiency can contribute to endothelial dysfunction. The relationship between subclinical hypothyroidism and hypertension was confirmed in a 2011 meta-analysis, said Dr. Brenta (Hypertens Res. 2011 Oct;34[10]:1098-105).
Though many factors contribute to obesity and thyroid function alone does not regulate body weight, a large population-based Danish study found that “even mild elevations of TSH are important for body weight,” said Dr. Brenta. The relationship is complex and bidirectional – a classic “chicken and egg” story – since obesity also may modulate TSH, she said; “however, we must not forget the ample literature on low levels of thyroid hormones reducing resting energy expenditure” (J Clin Endocrinol Metab. 2005 Jul;90[7]:4019-24).
Even though TSH tends to rise naturally through the lifespan, the association between elevated TSH and increased risk of metabolic syndrome held true even for older patients in one study, with “each one unit increase in TSH predicting a 3% increase in the odds of metabolic syndrome,” even after adjustment for age, BMI, and HOMA-IR status, among other variables, said Dr. Brenta (Clin Endocrinol [Oxf]. 2012 Jun;76[6]:911-8).
Advocating for universal screening for hypothyroidism among patients with metabolic syndrome, Dr. Brenta said that “hypothyroid disturbances are associated with an adverse metabolic profile, and even low normal TSH levels are associated with the metabolic traits of metabolic syndrome.”
The meeting was held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society. Dr. Brenta did not identify any conflicts of interest.
On Twitter @karioakes
LAKE BUENA VISTA, FLA. – Patients with low thyroid function may experience a “double whammy” of hypothyroidism and metabolic syndrome.
Even subclinical hypothyroidism affects many metabolic pathways that can contribute to deranged glucose and lipid metabolism, raising the risk of metabolic syndrome, according to Dr. Gabriela Brenta of the department of endocrinology at the Dr. Cesar Milstein Hospital in Buenos Aires. Though some mechanisms are incompletely understood, the association is clear enough to warrant screening all metabolic syndrome patients for hypothyroidism, she said.
Dr. Brenta described the recent work she and others have completed in the field. Basic science work revealed some early clues. For example, those who studied the effects of acute thyroid hormone withdrawal on patients with no thyroid gland found that these patients saw a rapid rise in insulin resistance. It’s known that even subclinical insulin resistance can lead to impaired glucose metabolism, making it logical to follow both normal and deranged metabolic pathways to help sort out the relationship between thyroid dysfunction and impaired glucose metabolism, she reported at the International Thyroid Congress.
Hypothyroidism can affect glucose homeostasis through multiple mechanisms, said Dr. Brenta. Firstly, hypothyroidism can lead to decreased hepatic gluconeogenesis and glycogenolysis. Hypothyroidism also can lead to reduced baseline plasma insulin levels and increased postglucose insulin secretion. In the peripheral tissues, hypothyroidism can interfere with glucose metabolism and disposal. All of these mechanisms can decrease hepatic glucose metabolism and lead to a postabsorptive hyperglycemia state, said Dr. Brenta, noting: “Insulin resistance is in some way the backbone of metabolic syndrome.”
Lipid metabolism is also affected by subclinical hypothyroidism, which can decrease expression of mRNA for LDL-C receptors, leading to LDL-C receptor down-regulation. With fewer receptors available, serum levels of LDL-C increase, with resultant increased susceptibility to oxidative effects and increased foam cell generation.
Dr. Brenta cited her earlier work showing that “triglyceride enrichment of LDL particles correlates with lower hepatic lipase activity” for individuals with subclinical hypothyroidism, with significantly lower hepatic lipase activity and a higher LDL-C to triglyceride ratio for those patients than for controls (Thyroid. 2007 May;17[5]:453-60). Overall, in hypothyroidism, “LDL particles are exposed to more substances that make them more atherogenic with decreased degradation and increased half-life,” said Dr. Brenta.
The increased risk for hypertension in both subclinical and overt hypothyroidism may be related, in part, to the fact that triiodothyronine deficiency can contribute to endothelial dysfunction. The relationship between subclinical hypothyroidism and hypertension was confirmed in a 2011 meta-analysis, said Dr. Brenta (Hypertens Res. 2011 Oct;34[10]:1098-105).
Though many factors contribute to obesity and thyroid function alone does not regulate body weight, a large population-based Danish study found that “even mild elevations of TSH are important for body weight,” said Dr. Brenta. The relationship is complex and bidirectional – a classic “chicken and egg” story – since obesity also may modulate TSH, she said; “however, we must not forget the ample literature on low levels of thyroid hormones reducing resting energy expenditure” (J Clin Endocrinol Metab. 2005 Jul;90[7]:4019-24).
Even though TSH tends to rise naturally through the lifespan, the association between elevated TSH and increased risk of metabolic syndrome held true even for older patients in one study, with “each one unit increase in TSH predicting a 3% increase in the odds of metabolic syndrome,” even after adjustment for age, BMI, and HOMA-IR status, among other variables, said Dr. Brenta (Clin Endocrinol [Oxf]. 2012 Jun;76[6]:911-8).
Advocating for universal screening for hypothyroidism among patients with metabolic syndrome, Dr. Brenta said that “hypothyroid disturbances are associated with an adverse metabolic profile, and even low normal TSH levels are associated with the metabolic traits of metabolic syndrome.”
The meeting was held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society. Dr. Brenta did not identify any conflicts of interest.
On Twitter @karioakes
EXPERT ANALYSIS FROM ITC 2015
High free thyroxine may increase risk of sudden cardiac death
LAKE BUENA VISTA, FLA. – High levels of free thyroxine (T4) can double the risk of sudden cardiac death, a large cohort study has determined.
Even people with free T4 in the upper range of normal may face an increased risk – up to 4% over 10 years, Dr. Layal Chaker said at the International Thyroid Conference.
The association remained significant, even when researchers controlled for independent cardiovascular risk factors, including hypertension and hyperlipidemia, said Dr. Chaker of Erasmus Medical Center, Rotterdam, the Netherlands.
The findings were based on a 9-year analysis of about 10,000 people included in the Rotterdam Elderly Study, conducted from 1990 to 2008. That study followed 15,000 people from middle age to old age, assessing cardiovascular and neurological diseases and their relationship to aging.
Dr. Chaker’s study comprised a subset of those who were without frank cardiovascular disease at baseline. She defined sudden cardiac death as a natural death from cardiac causes, heralded by an abrupt loss of consciousness within an hour of the onset of acute symptoms. Unwitnessed deaths were also included in the analysis. All of the death records were reviewed by two clinicians and a senior cardiologist.
The cohort comprised 10,318 subjects who had measurements of thyroid stimulating hormone (TSH) and free thyroxine (free T4); the mean age was 65 years.
Dr. Chaker stratified the group into tertiles based on the levels of these biomarkers. She conducted a multivariate analysis that controlled for age, sex, pulse, hypertension, cholesterol levels, diabetes, body mass index, smoking, and QT interval.
There were 261 sudden cardiac deaths by the end of follow-up.
There was no significant relationship between any level of TSH and sudden cardiac death. However, when she assessed the deaths by tertiles of free T4, she found a significant 40% increase in the risk among those whose levels ranged from 1.29 to 4 ng/L. The absolute 10-year risk rose from 1% at the lowest tertile to 7% in the highest.
Dr. Chaker then included only patients whose free T4 levels were in the euthyroid range of 0.85-1.95 ng/L. Among these, the risk of sudden cardiac death increased as free T4 increased (hazard ratio [HR], 2.25 for the highest level). The absolute 10-year risk rose from 1% at the lowest euthyroid level to 4% at 1.95 ng/L.
The reason for this finding isn’t completely clear, although other studies have shown a relationship between cardiac problems and thyroid function, she said.
“There may be some hemodynamic abnormalities that go along with even subclinical hyperthyroidism. High free T4 also has been associated with atrial fibrillation; both subclinical hyper- and hypothyroidism are associated with a prolongation of the QT interval.”
The meeting was held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society. Dr. Chaker had no financial disclosures.
On Twitter @Alz_Gal
LAKE BUENA VISTA, FLA. – High levels of free thyroxine (T4) can double the risk of sudden cardiac death, a large cohort study has determined.
Even people with free T4 in the upper range of normal may face an increased risk – up to 4% over 10 years, Dr. Layal Chaker said at the International Thyroid Conference.
The association remained significant, even when researchers controlled for independent cardiovascular risk factors, including hypertension and hyperlipidemia, said Dr. Chaker of Erasmus Medical Center, Rotterdam, the Netherlands.
The findings were based on a 9-year analysis of about 10,000 people included in the Rotterdam Elderly Study, conducted from 1990 to 2008. That study followed 15,000 people from middle age to old age, assessing cardiovascular and neurological diseases and their relationship to aging.
Dr. Chaker’s study comprised a subset of those who were without frank cardiovascular disease at baseline. She defined sudden cardiac death as a natural death from cardiac causes, heralded by an abrupt loss of consciousness within an hour of the onset of acute symptoms. Unwitnessed deaths were also included in the analysis. All of the death records were reviewed by two clinicians and a senior cardiologist.
The cohort comprised 10,318 subjects who had measurements of thyroid stimulating hormone (TSH) and free thyroxine (free T4); the mean age was 65 years.
Dr. Chaker stratified the group into tertiles based on the levels of these biomarkers. She conducted a multivariate analysis that controlled for age, sex, pulse, hypertension, cholesterol levels, diabetes, body mass index, smoking, and QT interval.
There were 261 sudden cardiac deaths by the end of follow-up.
There was no significant relationship between any level of TSH and sudden cardiac death. However, when she assessed the deaths by tertiles of free T4, she found a significant 40% increase in the risk among those whose levels ranged from 1.29 to 4 ng/L. The absolute 10-year risk rose from 1% at the lowest tertile to 7% in the highest.
Dr. Chaker then included only patients whose free T4 levels were in the euthyroid range of 0.85-1.95 ng/L. Among these, the risk of sudden cardiac death increased as free T4 increased (hazard ratio [HR], 2.25 for the highest level). The absolute 10-year risk rose from 1% at the lowest euthyroid level to 4% at 1.95 ng/L.
The reason for this finding isn’t completely clear, although other studies have shown a relationship between cardiac problems and thyroid function, she said.
“There may be some hemodynamic abnormalities that go along with even subclinical hyperthyroidism. High free T4 also has been associated with atrial fibrillation; both subclinical hyper- and hypothyroidism are associated with a prolongation of the QT interval.”
The meeting was held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society. Dr. Chaker had no financial disclosures.
On Twitter @Alz_Gal
LAKE BUENA VISTA, FLA. – High levels of free thyroxine (T4) can double the risk of sudden cardiac death, a large cohort study has determined.
Even people with free T4 in the upper range of normal may face an increased risk – up to 4% over 10 years, Dr. Layal Chaker said at the International Thyroid Conference.
The association remained significant, even when researchers controlled for independent cardiovascular risk factors, including hypertension and hyperlipidemia, said Dr. Chaker of Erasmus Medical Center, Rotterdam, the Netherlands.
The findings were based on a 9-year analysis of about 10,000 people included in the Rotterdam Elderly Study, conducted from 1990 to 2008. That study followed 15,000 people from middle age to old age, assessing cardiovascular and neurological diseases and their relationship to aging.
Dr. Chaker’s study comprised a subset of those who were without frank cardiovascular disease at baseline. She defined sudden cardiac death as a natural death from cardiac causes, heralded by an abrupt loss of consciousness within an hour of the onset of acute symptoms. Unwitnessed deaths were also included in the analysis. All of the death records were reviewed by two clinicians and a senior cardiologist.
The cohort comprised 10,318 subjects who had measurements of thyroid stimulating hormone (TSH) and free thyroxine (free T4); the mean age was 65 years.
Dr. Chaker stratified the group into tertiles based on the levels of these biomarkers. She conducted a multivariate analysis that controlled for age, sex, pulse, hypertension, cholesterol levels, diabetes, body mass index, smoking, and QT interval.
There were 261 sudden cardiac deaths by the end of follow-up.
There was no significant relationship between any level of TSH and sudden cardiac death. However, when she assessed the deaths by tertiles of free T4, she found a significant 40% increase in the risk among those whose levels ranged from 1.29 to 4 ng/L. The absolute 10-year risk rose from 1% at the lowest tertile to 7% in the highest.
Dr. Chaker then included only patients whose free T4 levels were in the euthyroid range of 0.85-1.95 ng/L. Among these, the risk of sudden cardiac death increased as free T4 increased (hazard ratio [HR], 2.25 for the highest level). The absolute 10-year risk rose from 1% at the lowest euthyroid level to 4% at 1.95 ng/L.
The reason for this finding isn’t completely clear, although other studies have shown a relationship between cardiac problems and thyroid function, she said.
“There may be some hemodynamic abnormalities that go along with even subclinical hyperthyroidism. High free T4 also has been associated with atrial fibrillation; both subclinical hyper- and hypothyroidism are associated with a prolongation of the QT interval.”
The meeting was held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society. Dr. Chaker had no financial disclosures.
On Twitter @Alz_Gal
AT ITC 2015
Key clinical point: Even subclinical hyperthyroidism may increase the risk of sudden cardiac death.
Major finding: High free thyroxine in euthyroid patients doubled the risk of sudden cardiac death.
Data source: A longitudinal cohort study comprising 10,318 subjects.
Disclosures: Dr. Chaker had no financial disclosures.
ITC: Study provides first evidence of paclitaxel benefit for anaplastic thyroid cancer
LAKE BUENA VISTA, FLA. – Weekly infusions of paclitaxel delayed progression in some patients with the very aggressive anaplastic thyroid cancer, a small prospective study determined.
The drug was most effective as adjuvant therapy for patients who had already undergone chemotherapy plus resection of the primary tumor, Dr. Naoyoshi Onoda reported in a poster session at the International Thyroid Conference. They survived for a median of 1 year (112-788 days) – an impressive feat considering that most patients with anaplastic thyroid cancer die within 6 months of diagnosis.
This finding suggests a place for paclitaxel as a standardized therapy for such patients, said Dr. Onoda of Osaka (Japan) City University. “We have objective data supporting standardized chemotherapy for the first time in the world.”
Anaplastic thyroid cancer is a very rare – but very aggressive – disease; there is no standardized treatment option. Dr. Onoda and his colleagues conducted a national prospective open-label study of weekly paclitaxel infusions in 56 patients with the malignancy.
The cohort was a median of 71 years old. All had stage IV disease: 10 were grade A, 18 grade B, 24 grade C, and four grade X. They received 80 mg/m2 infusions once a week. The median number of cycles was 2, although it ranged from 0-23 cycles.
Almost everyone (98%) experienced adverse events; the most common was anemia (77%). About a quarter (28%) experienced adverse events of at least grade 3, but there were no serious events and no deaths related to the study drug.
The objective response rate and the clinical benefit rate were 23% and 79%. The agent was not curative; at the last follow-up, no patient had achieved a complete response, and 43 of 56 in the study had died of their disease. “Overall, the median time to progression was only 47 days, and median overall survival just 227 days. That is so very short. But it’s a little bit longer than we had been seeing rates from reported cases,” he said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
The study was sponsored by the Prospective Clinical Study Committee of the Anaplastic Thyroid Carcinoma Research Consortium of Japan (ATCCJ). Dr. Onoda had no financial disclosures.
LAKE BUENA VISTA, FLA. – Weekly infusions of paclitaxel delayed progression in some patients with the very aggressive anaplastic thyroid cancer, a small prospective study determined.
The drug was most effective as adjuvant therapy for patients who had already undergone chemotherapy plus resection of the primary tumor, Dr. Naoyoshi Onoda reported in a poster session at the International Thyroid Conference. They survived for a median of 1 year (112-788 days) – an impressive feat considering that most patients with anaplastic thyroid cancer die within 6 months of diagnosis.
This finding suggests a place for paclitaxel as a standardized therapy for such patients, said Dr. Onoda of Osaka (Japan) City University. “We have objective data supporting standardized chemotherapy for the first time in the world.”
Anaplastic thyroid cancer is a very rare – but very aggressive – disease; there is no standardized treatment option. Dr. Onoda and his colleagues conducted a national prospective open-label study of weekly paclitaxel infusions in 56 patients with the malignancy.
The cohort was a median of 71 years old. All had stage IV disease: 10 were grade A, 18 grade B, 24 grade C, and four grade X. They received 80 mg/m2 infusions once a week. The median number of cycles was 2, although it ranged from 0-23 cycles.
Almost everyone (98%) experienced adverse events; the most common was anemia (77%). About a quarter (28%) experienced adverse events of at least grade 3, but there were no serious events and no deaths related to the study drug.
The objective response rate and the clinical benefit rate were 23% and 79%. The agent was not curative; at the last follow-up, no patient had achieved a complete response, and 43 of 56 in the study had died of their disease. “Overall, the median time to progression was only 47 days, and median overall survival just 227 days. That is so very short. But it’s a little bit longer than we had been seeing rates from reported cases,” he said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
The study was sponsored by the Prospective Clinical Study Committee of the Anaplastic Thyroid Carcinoma Research Consortium of Japan (ATCCJ). Dr. Onoda had no financial disclosures.
LAKE BUENA VISTA, FLA. – Weekly infusions of paclitaxel delayed progression in some patients with the very aggressive anaplastic thyroid cancer, a small prospective study determined.
The drug was most effective as adjuvant therapy for patients who had already undergone chemotherapy plus resection of the primary tumor, Dr. Naoyoshi Onoda reported in a poster session at the International Thyroid Conference. They survived for a median of 1 year (112-788 days) – an impressive feat considering that most patients with anaplastic thyroid cancer die within 6 months of diagnosis.
This finding suggests a place for paclitaxel as a standardized therapy for such patients, said Dr. Onoda of Osaka (Japan) City University. “We have objective data supporting standardized chemotherapy for the first time in the world.”
Anaplastic thyroid cancer is a very rare – but very aggressive – disease; there is no standardized treatment option. Dr. Onoda and his colleagues conducted a national prospective open-label study of weekly paclitaxel infusions in 56 patients with the malignancy.
The cohort was a median of 71 years old. All had stage IV disease: 10 were grade A, 18 grade B, 24 grade C, and four grade X. They received 80 mg/m2 infusions once a week. The median number of cycles was 2, although it ranged from 0-23 cycles.
Almost everyone (98%) experienced adverse events; the most common was anemia (77%). About a quarter (28%) experienced adverse events of at least grade 3, but there were no serious events and no deaths related to the study drug.
The objective response rate and the clinical benefit rate were 23% and 79%. The agent was not curative; at the last follow-up, no patient had achieved a complete response, and 43 of 56 in the study had died of their disease. “Overall, the median time to progression was only 47 days, and median overall survival just 227 days. That is so very short. But it’s a little bit longer than we had been seeing rates from reported cases,” he said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
The study was sponsored by the Prospective Clinical Study Committee of the Anaplastic Thyroid Carcinoma Research Consortium of Japan (ATCCJ). Dr. Onoda had no financial disclosures.
AT ITC 2015
Key clinical point: Paclitaxel may have use as a standardized treatment in anaplastic thyroid cancer.
Major finding: Weekly paclitaxel extended survival to a median of 1 year as adjuvant therapy for patients with resected anaplastic thyroid cancer.
Data source: The prospective open-label study involved 56 patients.
Disclosures: The study was sponsored by the Prospective Clinical Study Committee of the Anaplastic Thyroid Carcinoma Research Consortium of Japan (ATCCJ). Dr. Onoda had no financial disclosures.