User login
Be judicious with empiric antibiotics for febrile neutropenia
SAN FRANCISCO – Empiric antibiotic therapy for febrile neutropenia, a common and life-threatening complication of chemotherapy, hasn’t really changed much in 20 years, according to Alison Freifeld, MD, director of the section of oncology infectious diseases at the University of Nebraska, Omaha.
Antibiotic resistance has become a major problem over that time. Multidrug-resistant, gram-negative blood stream infections are not uncommon, particularly with extended-spectrum, beta-lactamase–producing Escherichia coli and Klebsiella pneumoniae. Carbapenemase-producing Enterobacteriaceae are also on the rise, among others.
“Our standard empiric antibiotics” – ceftazidime, cefepime, piperacillin/tazobactam, and carbapenems – “are generally not active against these organisms, putting us in a major dilemma about what to do” with patients who have them, Dr. Freifeld said.
“Our goal at the moment is to unpack this ship, take some of these loads of antibiotics off, and figure out how we can more effectively bridge the gap between risk factors and outcomes, with fewer and more stringently applied targeted antibiotics,” she said at ID Week, an annual scientific meeting on infectious diseases.
Dr. Freifeld shared her advice at the meeting on what to do as that plays out. The main driver is to protect the remaining potency of current antibiotics without sacrificing patient care while also keeping new options in reserve for the sickest patients, so “we do not overuse these precious commodities.”
For one thing, it’s okay to shorten treatment – traditionally around 2 weeks, until the absolute neutrophil count (ANC) tops 500 cells/mcg – once the fever abates and cultures turn negative, even if the ANC remains low.
A recent trial put the approach to the test. A total of 78 patients had their antibiotics stopped after they had been free of fever for 72 hours, with normal vital signs and no other signs of infection; 79 in the control group had usual care, continuing treatment until their ANC recovered.
Early withdrawal shortened treatment by about 3 days and there were no statistically significant differences in mortality, with one death in the short-arm group and three in the long-arm group. Over half of the patients in the short-arm group were neutropenic when antibiotics were discontinued.
Serious adverse events, meanwhile, were far less common in the short-arm group (18 vs. 38). The take-home lesson is that “interventions to shorten duration of empiric antibiotics are safe and effective and important to implement now,” Dr. Freifeld said (Lancet Haematol. 2017 Dec;4(12):e573-83).
Also, “use escalation and deescalation approaches,” she said. The basic idea is to begin with monotherapy – cefepime or piperacillin/tazobactam – in uncomplicated cases, bumped up as necessary, and, in complicated cases, to start with broad, multidrug regimens, deescalated as culture reports and other information comes in (Haematologica. 2013 Dec;98(12):1826-35).
Finally, fluoroquinolone prophylaxis, “once considered the wonder of the world,” Dr. Freifeld said, needs to be limited to the highest-risk patients, particularly those with neutropenia expected to last a week or more. It does seem to lower the rates of fever and bloodstream infections, but recent investigations have shown no mortality benefit, and fluoroquinolone prophylaxis makes patients more likely to be colonized by multidrug-resistant bacteria. Many centers have opted against it, even in higher-risk patients (J Infect. 2018 Jan;76(1):20-37).
Dr. Freifeld serves on a data adjudication committee for Merck, and reported research support from the company.
SAN FRANCISCO – Empiric antibiotic therapy for febrile neutropenia, a common and life-threatening complication of chemotherapy, hasn’t really changed much in 20 years, according to Alison Freifeld, MD, director of the section of oncology infectious diseases at the University of Nebraska, Omaha.
Antibiotic resistance has become a major problem over that time. Multidrug-resistant, gram-negative blood stream infections are not uncommon, particularly with extended-spectrum, beta-lactamase–producing Escherichia coli and Klebsiella pneumoniae. Carbapenemase-producing Enterobacteriaceae are also on the rise, among others.
“Our standard empiric antibiotics” – ceftazidime, cefepime, piperacillin/tazobactam, and carbapenems – “are generally not active against these organisms, putting us in a major dilemma about what to do” with patients who have them, Dr. Freifeld said.
“Our goal at the moment is to unpack this ship, take some of these loads of antibiotics off, and figure out how we can more effectively bridge the gap between risk factors and outcomes, with fewer and more stringently applied targeted antibiotics,” she said at ID Week, an annual scientific meeting on infectious diseases.
Dr. Freifeld shared her advice at the meeting on what to do as that plays out. The main driver is to protect the remaining potency of current antibiotics without sacrificing patient care while also keeping new options in reserve for the sickest patients, so “we do not overuse these precious commodities.”
For one thing, it’s okay to shorten treatment – traditionally around 2 weeks, until the absolute neutrophil count (ANC) tops 500 cells/mcg – once the fever abates and cultures turn negative, even if the ANC remains low.
A recent trial put the approach to the test. A total of 78 patients had their antibiotics stopped after they had been free of fever for 72 hours, with normal vital signs and no other signs of infection; 79 in the control group had usual care, continuing treatment until their ANC recovered.
Early withdrawal shortened treatment by about 3 days and there were no statistically significant differences in mortality, with one death in the short-arm group and three in the long-arm group. Over half of the patients in the short-arm group were neutropenic when antibiotics were discontinued.
Serious adverse events, meanwhile, were far less common in the short-arm group (18 vs. 38). The take-home lesson is that “interventions to shorten duration of empiric antibiotics are safe and effective and important to implement now,” Dr. Freifeld said (Lancet Haematol. 2017 Dec;4(12):e573-83).
Also, “use escalation and deescalation approaches,” she said. The basic idea is to begin with monotherapy – cefepime or piperacillin/tazobactam – in uncomplicated cases, bumped up as necessary, and, in complicated cases, to start with broad, multidrug regimens, deescalated as culture reports and other information comes in (Haematologica. 2013 Dec;98(12):1826-35).
Finally, fluoroquinolone prophylaxis, “once considered the wonder of the world,” Dr. Freifeld said, needs to be limited to the highest-risk patients, particularly those with neutropenia expected to last a week or more. It does seem to lower the rates of fever and bloodstream infections, but recent investigations have shown no mortality benefit, and fluoroquinolone prophylaxis makes patients more likely to be colonized by multidrug-resistant bacteria. Many centers have opted against it, even in higher-risk patients (J Infect. 2018 Jan;76(1):20-37).
Dr. Freifeld serves on a data adjudication committee for Merck, and reported research support from the company.
SAN FRANCISCO – Empiric antibiotic therapy for febrile neutropenia, a common and life-threatening complication of chemotherapy, hasn’t really changed much in 20 years, according to Alison Freifeld, MD, director of the section of oncology infectious diseases at the University of Nebraska, Omaha.
Antibiotic resistance has become a major problem over that time. Multidrug-resistant, gram-negative blood stream infections are not uncommon, particularly with extended-spectrum, beta-lactamase–producing Escherichia coli and Klebsiella pneumoniae. Carbapenemase-producing Enterobacteriaceae are also on the rise, among others.
“Our standard empiric antibiotics” – ceftazidime, cefepime, piperacillin/tazobactam, and carbapenems – “are generally not active against these organisms, putting us in a major dilemma about what to do” with patients who have them, Dr. Freifeld said.
“Our goal at the moment is to unpack this ship, take some of these loads of antibiotics off, and figure out how we can more effectively bridge the gap between risk factors and outcomes, with fewer and more stringently applied targeted antibiotics,” she said at ID Week, an annual scientific meeting on infectious diseases.
Dr. Freifeld shared her advice at the meeting on what to do as that plays out. The main driver is to protect the remaining potency of current antibiotics without sacrificing patient care while also keeping new options in reserve for the sickest patients, so “we do not overuse these precious commodities.”
For one thing, it’s okay to shorten treatment – traditionally around 2 weeks, until the absolute neutrophil count (ANC) tops 500 cells/mcg – once the fever abates and cultures turn negative, even if the ANC remains low.
A recent trial put the approach to the test. A total of 78 patients had their antibiotics stopped after they had been free of fever for 72 hours, with normal vital signs and no other signs of infection; 79 in the control group had usual care, continuing treatment until their ANC recovered.
Early withdrawal shortened treatment by about 3 days and there were no statistically significant differences in mortality, with one death in the short-arm group and three in the long-arm group. Over half of the patients in the short-arm group were neutropenic when antibiotics were discontinued.
Serious adverse events, meanwhile, were far less common in the short-arm group (18 vs. 38). The take-home lesson is that “interventions to shorten duration of empiric antibiotics are safe and effective and important to implement now,” Dr. Freifeld said (Lancet Haematol. 2017 Dec;4(12):e573-83).
Also, “use escalation and deescalation approaches,” she said. The basic idea is to begin with monotherapy – cefepime or piperacillin/tazobactam – in uncomplicated cases, bumped up as necessary, and, in complicated cases, to start with broad, multidrug regimens, deescalated as culture reports and other information comes in (Haematologica. 2013 Dec;98(12):1826-35).
Finally, fluoroquinolone prophylaxis, “once considered the wonder of the world,” Dr. Freifeld said, needs to be limited to the highest-risk patients, particularly those with neutropenia expected to last a week or more. It does seem to lower the rates of fever and bloodstream infections, but recent investigations have shown no mortality benefit, and fluoroquinolone prophylaxis makes patients more likely to be colonized by multidrug-resistant bacteria. Many centers have opted against it, even in higher-risk patients (J Infect. 2018 Jan;76(1):20-37).
Dr. Freifeld serves on a data adjudication committee for Merck, and reported research support from the company.
EXPERT ANALYSIS FROM IDWEEK 2018
It’s time for universal HCV screening in the ED
SAN FRANCISCO – Emergency departments are the ideal place to screen for hepatitis C infection, according to investigators from Vanderbilt University, Nashville, Tenn.
Current recommendations call for screening baby boomers born from 1945 to 1965 and patients with risk factors, especially injection drug use. The problem is that the guidelines don’t say, exactly, how and where that should be done, so uptake has been spotty. Also, people aren’t exactly forthcoming when it comes to admitting IV drug use.
Enter universal screening in the ED. Vanderbilt is one of several academic centers that have adopted the approach, and others are following suit. Across the board, they’ve found that HCV infection is more common than projections based on baby boomer and risk factor demographics suggest, and, even more importantly, the boomer/risk factor strategy misses a large number of active cases, said Cody A. Chastain, MD, assistant professor of infectious diseases at Vanderbilt, who led the ED screening initiative.
In short, universal screening in the ED would keep people from falling through the cracks.
From April 2017 to March 2018, every adult who had blood drawn at Vanderbilt’s tertiary care ED was asked by a nurse if they’d also like to be checked for HCV, so long as they were alert enough for the conversation. If they agreed, an additional phlebotomy tube was added to the draw, and sent off for testing. Fewer than 5% of patients opted out.
Antibody positive samples were automatically screened for active disease by HCV RNA. Results were entered into the medical record and shared with patients at discharge. Active cases were counseled and offered linkage to care, regardless of insurance status.
The initiative screened 11,637 patients; 1,008 (8.7%) were antibody positive, of whom 488 (48%) were RNA positive. Thirty-seven percent of the active cases were in non–baby boomers – most born after 1965 – with no known injection drug use. The baby boomer/risk factor model would have missed most of them.
Also, spontaneous clearance – antibody positive, RNA negative without HCV treatment – “is dramatically higher” than what’s thought. “The historic estimate of 20% clearly is not reflected” in the Vanderbilt results, nor in similar universal screening studies; “spontaneous clearance is about 50% or so,” Dr. Chastain said.
Even so, “virtually every study published in this space finds more cases of infection than traditional screening would find. [Our work] is just one more piece of data” to indicate the usefulness of the approach. “Emergency departments [are] ideal for hepatitis C screening,” he said at IDWeek, an annual scientific meeting on infectious diseases, where he presented the findings.
“This is well trodden territory; we’ve already addressed it with HIV. We recognized that HIV screening had a stigma and was a challenge, [so we] moved to universal screening” of all adults, at least once. It “drastically improved screening rates. I don’t see a rational reason” not to do this for hepatitis C. “There are very well-meaning people who engage in the cost effectiveness side of this discussion, but I don’t think it helps us in our efforts to control this epidemic from a public health standpoint,” Dr. Chastain said.
Vanderbilt continues to screen for HCV in the ED; the next step is to see how well efforts to link active cases with care are working. Many times during the study, Dr. Chastain said positive patients eventually revealed that they already knew they had HCV, but had been told there was nothing they could do about it, so they didn’t get care. Maybe they were told that because they didn’t have insurance.
Vanderbilt has dropped screening ED patients born before 1945 because the odds of picking up an unknown HCV infection proved to be tiny, and, in any case, patients are generally too comorbid for treatment. It’s made screening more efficient.
Dr. Chastain reported that he had no personal disclosures. The study was funded by Vanderbilt, which receives grants from pharmaceutical companies.
SOURCE: Chastain C et al. 2018 ID Week, Abstract 932.
SAN FRANCISCO – Emergency departments are the ideal place to screen for hepatitis C infection, according to investigators from Vanderbilt University, Nashville, Tenn.
Current recommendations call for screening baby boomers born from 1945 to 1965 and patients with risk factors, especially injection drug use. The problem is that the guidelines don’t say, exactly, how and where that should be done, so uptake has been spotty. Also, people aren’t exactly forthcoming when it comes to admitting IV drug use.
Enter universal screening in the ED. Vanderbilt is one of several academic centers that have adopted the approach, and others are following suit. Across the board, they’ve found that HCV infection is more common than projections based on baby boomer and risk factor demographics suggest, and, even more importantly, the boomer/risk factor strategy misses a large number of active cases, said Cody A. Chastain, MD, assistant professor of infectious diseases at Vanderbilt, who led the ED screening initiative.
In short, universal screening in the ED would keep people from falling through the cracks.
From April 2017 to March 2018, every adult who had blood drawn at Vanderbilt’s tertiary care ED was asked by a nurse if they’d also like to be checked for HCV, so long as they were alert enough for the conversation. If they agreed, an additional phlebotomy tube was added to the draw, and sent off for testing. Fewer than 5% of patients opted out.
Antibody positive samples were automatically screened for active disease by HCV RNA. Results were entered into the medical record and shared with patients at discharge. Active cases were counseled and offered linkage to care, regardless of insurance status.
The initiative screened 11,637 patients; 1,008 (8.7%) were antibody positive, of whom 488 (48%) were RNA positive. Thirty-seven percent of the active cases were in non–baby boomers – most born after 1965 – with no known injection drug use. The baby boomer/risk factor model would have missed most of them.
Also, spontaneous clearance – antibody positive, RNA negative without HCV treatment – “is dramatically higher” than what’s thought. “The historic estimate of 20% clearly is not reflected” in the Vanderbilt results, nor in similar universal screening studies; “spontaneous clearance is about 50% or so,” Dr. Chastain said.
Even so, “virtually every study published in this space finds more cases of infection than traditional screening would find. [Our work] is just one more piece of data” to indicate the usefulness of the approach. “Emergency departments [are] ideal for hepatitis C screening,” he said at IDWeek, an annual scientific meeting on infectious diseases, where he presented the findings.
“This is well trodden territory; we’ve already addressed it with HIV. We recognized that HIV screening had a stigma and was a challenge, [so we] moved to universal screening” of all adults, at least once. It “drastically improved screening rates. I don’t see a rational reason” not to do this for hepatitis C. “There are very well-meaning people who engage in the cost effectiveness side of this discussion, but I don’t think it helps us in our efforts to control this epidemic from a public health standpoint,” Dr. Chastain said.
Vanderbilt continues to screen for HCV in the ED; the next step is to see how well efforts to link active cases with care are working. Many times during the study, Dr. Chastain said positive patients eventually revealed that they already knew they had HCV, but had been told there was nothing they could do about it, so they didn’t get care. Maybe they were told that because they didn’t have insurance.
Vanderbilt has dropped screening ED patients born before 1945 because the odds of picking up an unknown HCV infection proved to be tiny, and, in any case, patients are generally too comorbid for treatment. It’s made screening more efficient.
Dr. Chastain reported that he had no personal disclosures. The study was funded by Vanderbilt, which receives grants from pharmaceutical companies.
SOURCE: Chastain C et al. 2018 ID Week, Abstract 932.
SAN FRANCISCO – Emergency departments are the ideal place to screen for hepatitis C infection, according to investigators from Vanderbilt University, Nashville, Tenn.
Current recommendations call for screening baby boomers born from 1945 to 1965 and patients with risk factors, especially injection drug use. The problem is that the guidelines don’t say, exactly, how and where that should be done, so uptake has been spotty. Also, people aren’t exactly forthcoming when it comes to admitting IV drug use.
Enter universal screening in the ED. Vanderbilt is one of several academic centers that have adopted the approach, and others are following suit. Across the board, they’ve found that HCV infection is more common than projections based on baby boomer and risk factor demographics suggest, and, even more importantly, the boomer/risk factor strategy misses a large number of active cases, said Cody A. Chastain, MD, assistant professor of infectious diseases at Vanderbilt, who led the ED screening initiative.
In short, universal screening in the ED would keep people from falling through the cracks.
From April 2017 to March 2018, every adult who had blood drawn at Vanderbilt’s tertiary care ED was asked by a nurse if they’d also like to be checked for HCV, so long as they were alert enough for the conversation. If they agreed, an additional phlebotomy tube was added to the draw, and sent off for testing. Fewer than 5% of patients opted out.
Antibody positive samples were automatically screened for active disease by HCV RNA. Results were entered into the medical record and shared with patients at discharge. Active cases were counseled and offered linkage to care, regardless of insurance status.
The initiative screened 11,637 patients; 1,008 (8.7%) were antibody positive, of whom 488 (48%) were RNA positive. Thirty-seven percent of the active cases were in non–baby boomers – most born after 1965 – with no known injection drug use. The baby boomer/risk factor model would have missed most of them.
Also, spontaneous clearance – antibody positive, RNA negative without HCV treatment – “is dramatically higher” than what’s thought. “The historic estimate of 20% clearly is not reflected” in the Vanderbilt results, nor in similar universal screening studies; “spontaneous clearance is about 50% or so,” Dr. Chastain said.
Even so, “virtually every study published in this space finds more cases of infection than traditional screening would find. [Our work] is just one more piece of data” to indicate the usefulness of the approach. “Emergency departments [are] ideal for hepatitis C screening,” he said at IDWeek, an annual scientific meeting on infectious diseases, where he presented the findings.
“This is well trodden territory; we’ve already addressed it with HIV. We recognized that HIV screening had a stigma and was a challenge, [so we] moved to universal screening” of all adults, at least once. It “drastically improved screening rates. I don’t see a rational reason” not to do this for hepatitis C. “There are very well-meaning people who engage in the cost effectiveness side of this discussion, but I don’t think it helps us in our efforts to control this epidemic from a public health standpoint,” Dr. Chastain said.
Vanderbilt continues to screen for HCV in the ED; the next step is to see how well efforts to link active cases with care are working. Many times during the study, Dr. Chastain said positive patients eventually revealed that they already knew they had HCV, but had been told there was nothing they could do about it, so they didn’t get care. Maybe they were told that because they didn’t have insurance.
Vanderbilt has dropped screening ED patients born before 1945 because the odds of picking up an unknown HCV infection proved to be tiny, and, in any case, patients are generally too comorbid for treatment. It’s made screening more efficient.
Dr. Chastain reported that he had no personal disclosures. The study was funded by Vanderbilt, which receives grants from pharmaceutical companies.
SOURCE: Chastain C et al. 2018 ID Week, Abstract 932.
REPORTING FROM IDWEEK 2018
Key clinical point: HCV infection is more common than traditionally thought; screening in the ED will keep people from falling through the cracks.
Major finding: Of patients screened; 8.7% were antibody positive, with 48% of these RNA positive; 37% of active cases were in non–baby boomers with no known injection drug use. Spontaneous remission appeared to top 50%.
Study details: Quality improvement initiative in Vanderbilt University’s tertiary care ED.
Disclosures: Dr. Chastain reported that he had no disclosures. The study was funded by Vanderbilt, which receives grants from pharmaceutical companies.
Source: Chastain C et al. 2018 ID Week, Abstract 932.
Three-drug combo proves effective against multidrug-resistant UTIs
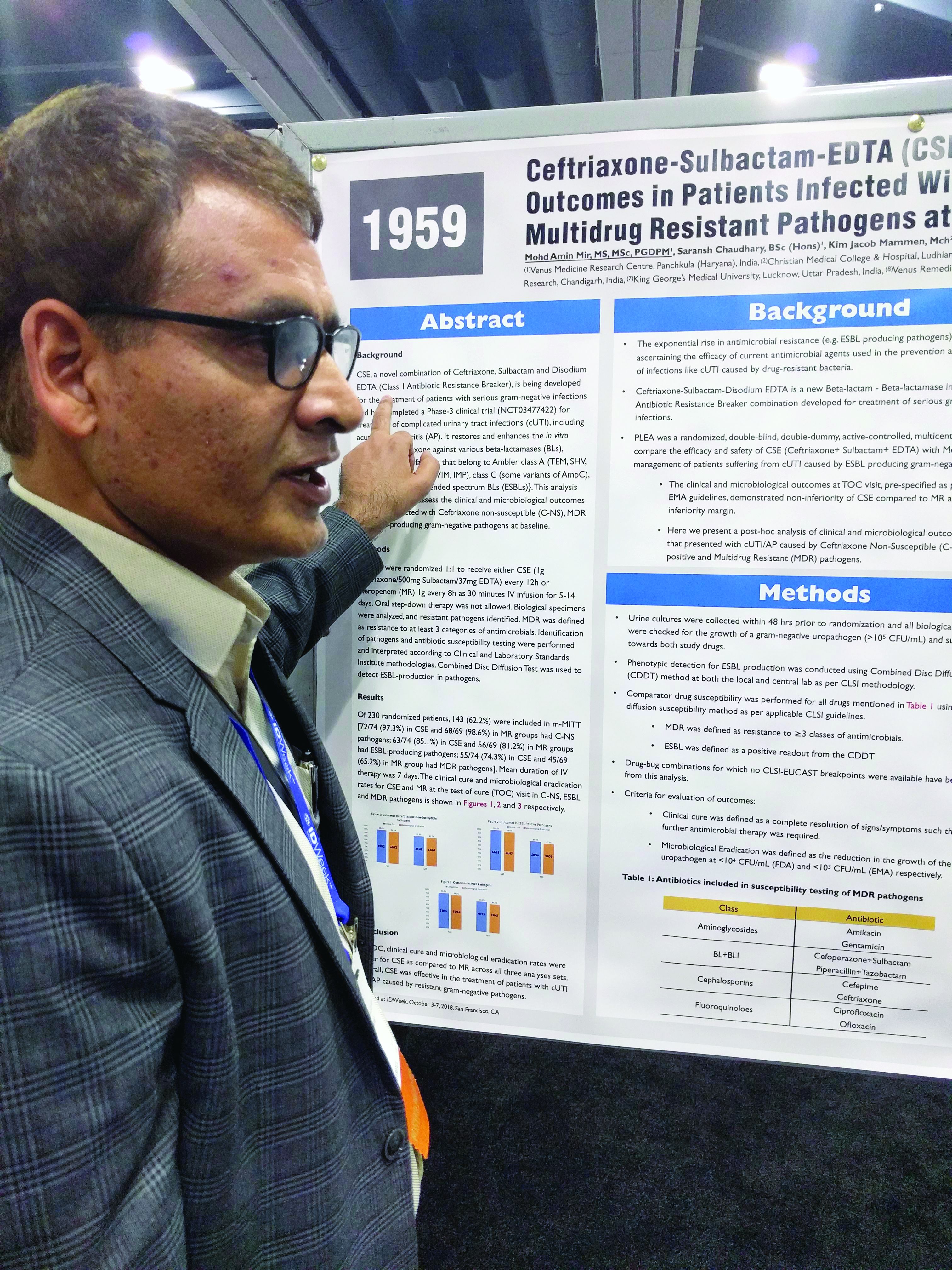
SAN FRANCISCO – A combination of ceftriaxone, a beta-lactamase inhibitor, and disodium ethylenediaminetetraacetic acid (EDTA) is superior to meropenem in the treatment of complicated urinary tract infections caused by extended-spectrum beta-lactamase (ESBL) gram-negative bacteria, according to a new study.
The post-hoc analysis also found that the three-drug combination – known as CSE – is noninferior to meropenem in multidrug-resistant (MDR) and ceftriaxone-nonsusceptible (C-NS) pathogens.
CSE is aimed at the growing problem of antibiotic resistance, particularly the mechanisms used by bacteria to counter beta-lactamase inhibitors. EDTA chelates zinc and calcium, and many of the resistance mechanisms rely on one or the other of these ions to function. In in vitro models, the combination of sulbactam and EDTA restores activity of ceftriaxone against various beta-lactamases.
Mohd Amin Mir, MD, head of clinical research at the Venus Medicine Research Center, Panchkula, India, and presenter of the study, said that, in the case of efflux pumps, “when there is EDTA present, it chelates the calcium, and that means there is no energy for the efflux pump to throw out the drug.”
The penems, which include meropenem, are a class of synthetic antibiotics with an unsaturated beta-lactam ring. Like other antibiotics, they are under assault from antibiotic resistance, especially beta-lactamase enzymes. “Penems are very precious drugs. The objective of developing [EDTA combinations] is to save the penems,” Dr. Mir said at an annual scientific meeting on infectious diseases.
The PLEA trial randomized 143 patients with complicated urinary tract infections or acute pyelonephritis to CSE (1 g ceftriaxone/500 mg sulbactam/37 mg EDTA) every 12 hours or 1 g meropenem (MR) as a 30-minute intravenous infusion over 30 minutes. Patients received treatment for 5-14 days.
The original study demonstrated that CSE is noninferior to meropenem at a 10% noninferiority margin. The researchers conducted a post-hoc analysis of patients who presented with complicated UTIs or acute pyelonephritis cases that were C-NS, ESBL-positive, or multidrug-resistant (MDR) pathogens. The researchers defined MDR as resistance to three or more categories of antimicrobial agents.
Of patients who received CSE, 97.3% had pathogens that were nonsusceptible to ceftriaxone, as did 98.6% of those who received MR; 85.1% of CSE and 81.2% of MR patients had an ESBL-producing pathogen; and 74.3% of infections in the CSE group were MDR, as were 65.2% of the MR group.
In all three resistant phenotypes, CSE at least trended to more favorable outcomes. In the MDR group, 96.4% of CSE patients achieved a clinical cure, compared with 88.9% in the MR group, and 94.5% achieved microbial eradication, compared with 86.75% in the MR group.
In the ESBL subgroup, 100% of patients in the CSE group achieved a clinical cure, compared with 89.3%, while 98.4% had complete eradication in the CSE group, compared with 87.5%. In the C-NS subgroup, 95.8% in the CSE group achieved a clinical cure, compared with 91.2%, and 94.4% achieved eradication, compared with 89.7% in the MR group.
In the ESBL subgroup, the lower bound of the 95% confidence interval of the between-group difference was greater than 0, indicating superiority of CSE over MR for both clinical cure and eradication. In the MDR and C-NS subgroups, CSE achieved noninferiority at a –10% margin.
CSE is currently commercially available in India, and the manufacturer is now seeking approval in Europe and the United States.
SOURCE: Mir MA et al. IDWeek 2018, Abstract 1959.
SAN FRANCISCO – A combination of ceftriaxone, a beta-lactamase inhibitor, and disodium ethylenediaminetetraacetic acid (EDTA) is superior to meropenem in the treatment of complicated urinary tract infections caused by extended-spectrum beta-lactamase (ESBL) gram-negative bacteria, according to a new study.
The post-hoc analysis also found that the three-drug combination – known as CSE – is noninferior to meropenem in multidrug-resistant (MDR) and ceftriaxone-nonsusceptible (C-NS) pathogens.
CSE is aimed at the growing problem of antibiotic resistance, particularly the mechanisms used by bacteria to counter beta-lactamase inhibitors. EDTA chelates zinc and calcium, and many of the resistance mechanisms rely on one or the other of these ions to function. In in vitro models, the combination of sulbactam and EDTA restores activity of ceftriaxone against various beta-lactamases.
Mohd Amin Mir, MD, head of clinical research at the Venus Medicine Research Center, Panchkula, India, and presenter of the study, said that, in the case of efflux pumps, “when there is EDTA present, it chelates the calcium, and that means there is no energy for the efflux pump to throw out the drug.”
The penems, which include meropenem, are a class of synthetic antibiotics with an unsaturated beta-lactam ring. Like other antibiotics, they are under assault from antibiotic resistance, especially beta-lactamase enzymes. “Penems are very precious drugs. The objective of developing [EDTA combinations] is to save the penems,” Dr. Mir said at an annual scientific meeting on infectious diseases.
The PLEA trial randomized 143 patients with complicated urinary tract infections or acute pyelonephritis to CSE (1 g ceftriaxone/500 mg sulbactam/37 mg EDTA) every 12 hours or 1 g meropenem (MR) as a 30-minute intravenous infusion over 30 minutes. Patients received treatment for 5-14 days.
The original study demonstrated that CSE is noninferior to meropenem at a 10% noninferiority margin. The researchers conducted a post-hoc analysis of patients who presented with complicated UTIs or acute pyelonephritis cases that were C-NS, ESBL-positive, or multidrug-resistant (MDR) pathogens. The researchers defined MDR as resistance to three or more categories of antimicrobial agents.
Of patients who received CSE, 97.3% had pathogens that were nonsusceptible to ceftriaxone, as did 98.6% of those who received MR; 85.1% of CSE and 81.2% of MR patients had an ESBL-producing pathogen; and 74.3% of infections in the CSE group were MDR, as were 65.2% of the MR group.
In all three resistant phenotypes, CSE at least trended to more favorable outcomes. In the MDR group, 96.4% of CSE patients achieved a clinical cure, compared with 88.9% in the MR group, and 94.5% achieved microbial eradication, compared with 86.75% in the MR group.
In the ESBL subgroup, 100% of patients in the CSE group achieved a clinical cure, compared with 89.3%, while 98.4% had complete eradication in the CSE group, compared with 87.5%. In the C-NS subgroup, 95.8% in the CSE group achieved a clinical cure, compared with 91.2%, and 94.4% achieved eradication, compared with 89.7% in the MR group.
In the ESBL subgroup, the lower bound of the 95% confidence interval of the between-group difference was greater than 0, indicating superiority of CSE over MR for both clinical cure and eradication. In the MDR and C-NS subgroups, CSE achieved noninferiority at a –10% margin.
CSE is currently commercially available in India, and the manufacturer is now seeking approval in Europe and the United States.
SOURCE: Mir MA et al. IDWeek 2018, Abstract 1959.
SAN FRANCISCO – A combination of ceftriaxone, a beta-lactamase inhibitor, and disodium ethylenediaminetetraacetic acid (EDTA) is superior to meropenem in the treatment of complicated urinary tract infections caused by extended-spectrum beta-lactamase (ESBL) gram-negative bacteria, according to a new study.
The post-hoc analysis also found that the three-drug combination – known as CSE – is noninferior to meropenem in multidrug-resistant (MDR) and ceftriaxone-nonsusceptible (C-NS) pathogens.
CSE is aimed at the growing problem of antibiotic resistance, particularly the mechanisms used by bacteria to counter beta-lactamase inhibitors. EDTA chelates zinc and calcium, and many of the resistance mechanisms rely on one or the other of these ions to function. In in vitro models, the combination of sulbactam and EDTA restores activity of ceftriaxone against various beta-lactamases.
Mohd Amin Mir, MD, head of clinical research at the Venus Medicine Research Center, Panchkula, India, and presenter of the study, said that, in the case of efflux pumps, “when there is EDTA present, it chelates the calcium, and that means there is no energy for the efflux pump to throw out the drug.”
The penems, which include meropenem, are a class of synthetic antibiotics with an unsaturated beta-lactam ring. Like other antibiotics, they are under assault from antibiotic resistance, especially beta-lactamase enzymes. “Penems are very precious drugs. The objective of developing [EDTA combinations] is to save the penems,” Dr. Mir said at an annual scientific meeting on infectious diseases.
The PLEA trial randomized 143 patients with complicated urinary tract infections or acute pyelonephritis to CSE (1 g ceftriaxone/500 mg sulbactam/37 mg EDTA) every 12 hours or 1 g meropenem (MR) as a 30-minute intravenous infusion over 30 minutes. Patients received treatment for 5-14 days.
The original study demonstrated that CSE is noninferior to meropenem at a 10% noninferiority margin. The researchers conducted a post-hoc analysis of patients who presented with complicated UTIs or acute pyelonephritis cases that were C-NS, ESBL-positive, or multidrug-resistant (MDR) pathogens. The researchers defined MDR as resistance to three or more categories of antimicrobial agents.
Of patients who received CSE, 97.3% had pathogens that were nonsusceptible to ceftriaxone, as did 98.6% of those who received MR; 85.1% of CSE and 81.2% of MR patients had an ESBL-producing pathogen; and 74.3% of infections in the CSE group were MDR, as were 65.2% of the MR group.
In all three resistant phenotypes, CSE at least trended to more favorable outcomes. In the MDR group, 96.4% of CSE patients achieved a clinical cure, compared with 88.9% in the MR group, and 94.5% achieved microbial eradication, compared with 86.75% in the MR group.
In the ESBL subgroup, 100% of patients in the CSE group achieved a clinical cure, compared with 89.3%, while 98.4% had complete eradication in the CSE group, compared with 87.5%. In the C-NS subgroup, 95.8% in the CSE group achieved a clinical cure, compared with 91.2%, and 94.4% achieved eradication, compared with 89.7% in the MR group.
In the ESBL subgroup, the lower bound of the 95% confidence interval of the between-group difference was greater than 0, indicating superiority of CSE over MR for both clinical cure and eradication. In the MDR and C-NS subgroups, CSE achieved noninferiority at a –10% margin.
CSE is currently commercially available in India, and the manufacturer is now seeking approval in Europe and the United States.
SOURCE: Mir MA et al. IDWeek 2018, Abstract 1959.
REPORTING FROM IDWEEK 2018
Key clinical point:
Major finding: The combination was noninferior in the context of different resistant subtypes.
Study details: Posthoc analysis of a randomized, controlled trial (n = 143).
Disclosures: The study was funded by Venus Medicine Research Center, which employs Dr. Mir.
Source: Mir MA et al. IDWeek 2018, Abstract 1959.
Daptomycin/fosfomycin: A new standard for MRSA bacteremia?
SAN FRANCISCO – Daptomycin plus fosfomycin is more effective than daptomycin alone for methicillin-resistant Staphylococcus aureus bacteremia, according to a multicenter, randomized trial from Spain.
“I think this is really an important study; I think it will change clinical practice for this infection” once it’s published, said lead investigator Miquel Pujol, MD, PhD, clinical head of infectious diseases at Bellvitge University Hospital in Barcelona.
The current standard for MRSA bacteremia is daptomycin (Cubicin) or vancomycin (Vancocin) monotherapy on both sides of the Atlantic, but mortality rates are way too high, more than 30% in some reviews. Dr. Pujol and his colleagues wanted to find something better.
Their lab work showed that daptomycin and fosfomycin (Monurol) were synergistic and rapidly bactericidal against MRSA, and anecdotal experience in Spain suggested the drugs improved bacteremia outcomes, so they decided to put the combination to the test.
They randomized 74 MRSA bacteremia patients to the combination, daptomycin 10mg/kg IV daily plus fosfomycin 2g IV q 6h. They randomized 81 other subjects to standard of care with daptomycin monotherapy, also at 10mg/kg IV daily. Treatment was 10-14 days for uncomplicated and 28-42 days for complicated bacteremia.
The open-label trial was conducted at 18 medical centers in Spain, where fosfomycin was discovered in dirt samples in the late 1960s and remains a matter of pride.
At day 7, 69 of the 74 combination patients (93.2%) were alive with clinical improvement, clearance of bacteremia, and no subsequent relapse, versus 62 of 81 patients (76.5%) on monotherapy (absolute difference 16.7%; 95% confidence interval, 5.4%-27.7%). Three people in the combination arm (4.1%) had died by day 7, versus six on monotherapy (7.4%).
Six weeks after the end of treatment at the test-of-cure visit, 40 of 74 combination patients (54.1%) were alive with resolution of all clinical signs and symptoms, negative blood cultures, and no previous or subsequent relapses; just 34 of 81 patients (42%) in the monotherapy arm hit that mark. The 12.1% difference was not statistically significant, nor was the difference in 12-week survival.
However, patients in the combination arm were 70% less likely to have complicated bacteremia at the test-of-cure visit (9.5% vs. 28.4%; relative risk 0.3; 95% CI, 0.2-0.7). There were no cases of persistent or recurrent infection in the combination arm, but nine persistent (11.1%) and five recurrent (6.2%) cases with daptomycin monotherapy. The differences were statistically significant.
The subjects all had at least one positive MRSA blood culture within 72 hours of randomization. Exclusion criteria included MRSA pneumonia, prosthetic valve endocarditis, end-stage liver disease, and moderate to severe heart failure.
There were no significant baseline differences between the groups. About half the subjects were men, and the mean age was about 73 years. The mean Charlson Comorbidity Index score was a bit under 4, and the mean Pitt bacteremia score a bit over 1. The leading source of infection was vascular catheter; acquisition was thought to be nosocomial in more than 40% of patients.
There were no discontinuations from drug side effects in the daptomycin arm, but there were five in the combination arm, including two for heart failure, two for respiratory insufficiency, and one for GI bleeding. Even so, the benefit outweighed the risk, Dr. Pujol said.
Intravenous fosfomycin is available in Europe, but the drug is approved in the United States only as an oral formulation. That could change soon; Nabriva Therapeutics plans to file its IV formulation (Contepo) for Food and Drug Administration approval in late 2018.
Though it is not standard of practice yet, the combination is increasingly being used in Spain for MRSA bacteremia, according to Dr. Pujol. “Patients probably need the combination [at least] initially, especially if they have complicated bacteremia” or fail monotherapy, he said at ID week, an annual scientific meeting on infectious diseases.
The work was funded by the Spanish government. Dr. Pujol said he had no relevant disclosures.
SOURCE: Pujol M et al. 2018 ID Week abstract LB3
SAN FRANCISCO – Daptomycin plus fosfomycin is more effective than daptomycin alone for methicillin-resistant Staphylococcus aureus bacteremia, according to a multicenter, randomized trial from Spain.
“I think this is really an important study; I think it will change clinical practice for this infection” once it’s published, said lead investigator Miquel Pujol, MD, PhD, clinical head of infectious diseases at Bellvitge University Hospital in Barcelona.
The current standard for MRSA bacteremia is daptomycin (Cubicin) or vancomycin (Vancocin) monotherapy on both sides of the Atlantic, but mortality rates are way too high, more than 30% in some reviews. Dr. Pujol and his colleagues wanted to find something better.
Their lab work showed that daptomycin and fosfomycin (Monurol) were synergistic and rapidly bactericidal against MRSA, and anecdotal experience in Spain suggested the drugs improved bacteremia outcomes, so they decided to put the combination to the test.
They randomized 74 MRSA bacteremia patients to the combination, daptomycin 10mg/kg IV daily plus fosfomycin 2g IV q 6h. They randomized 81 other subjects to standard of care with daptomycin monotherapy, also at 10mg/kg IV daily. Treatment was 10-14 days for uncomplicated and 28-42 days for complicated bacteremia.
The open-label trial was conducted at 18 medical centers in Spain, where fosfomycin was discovered in dirt samples in the late 1960s and remains a matter of pride.
At day 7, 69 of the 74 combination patients (93.2%) were alive with clinical improvement, clearance of bacteremia, and no subsequent relapse, versus 62 of 81 patients (76.5%) on monotherapy (absolute difference 16.7%; 95% confidence interval, 5.4%-27.7%). Three people in the combination arm (4.1%) had died by day 7, versus six on monotherapy (7.4%).
Six weeks after the end of treatment at the test-of-cure visit, 40 of 74 combination patients (54.1%) were alive with resolution of all clinical signs and symptoms, negative blood cultures, and no previous or subsequent relapses; just 34 of 81 patients (42%) in the monotherapy arm hit that mark. The 12.1% difference was not statistically significant, nor was the difference in 12-week survival.
However, patients in the combination arm were 70% less likely to have complicated bacteremia at the test-of-cure visit (9.5% vs. 28.4%; relative risk 0.3; 95% CI, 0.2-0.7). There were no cases of persistent or recurrent infection in the combination arm, but nine persistent (11.1%) and five recurrent (6.2%) cases with daptomycin monotherapy. The differences were statistically significant.
The subjects all had at least one positive MRSA blood culture within 72 hours of randomization. Exclusion criteria included MRSA pneumonia, prosthetic valve endocarditis, end-stage liver disease, and moderate to severe heart failure.
There were no significant baseline differences between the groups. About half the subjects were men, and the mean age was about 73 years. The mean Charlson Comorbidity Index score was a bit under 4, and the mean Pitt bacteremia score a bit over 1. The leading source of infection was vascular catheter; acquisition was thought to be nosocomial in more than 40% of patients.
There were no discontinuations from drug side effects in the daptomycin arm, but there were five in the combination arm, including two for heart failure, two for respiratory insufficiency, and one for GI bleeding. Even so, the benefit outweighed the risk, Dr. Pujol said.
Intravenous fosfomycin is available in Europe, but the drug is approved in the United States only as an oral formulation. That could change soon; Nabriva Therapeutics plans to file its IV formulation (Contepo) for Food and Drug Administration approval in late 2018.
Though it is not standard of practice yet, the combination is increasingly being used in Spain for MRSA bacteremia, according to Dr. Pujol. “Patients probably need the combination [at least] initially, especially if they have complicated bacteremia” or fail monotherapy, he said at ID week, an annual scientific meeting on infectious diseases.
The work was funded by the Spanish government. Dr. Pujol said he had no relevant disclosures.
SOURCE: Pujol M et al. 2018 ID Week abstract LB3
SAN FRANCISCO – Daptomycin plus fosfomycin is more effective than daptomycin alone for methicillin-resistant Staphylococcus aureus bacteremia, according to a multicenter, randomized trial from Spain.
“I think this is really an important study; I think it will change clinical practice for this infection” once it’s published, said lead investigator Miquel Pujol, MD, PhD, clinical head of infectious diseases at Bellvitge University Hospital in Barcelona.
The current standard for MRSA bacteremia is daptomycin (Cubicin) or vancomycin (Vancocin) monotherapy on both sides of the Atlantic, but mortality rates are way too high, more than 30% in some reviews. Dr. Pujol and his colleagues wanted to find something better.
Their lab work showed that daptomycin and fosfomycin (Monurol) were synergistic and rapidly bactericidal against MRSA, and anecdotal experience in Spain suggested the drugs improved bacteremia outcomes, so they decided to put the combination to the test.
They randomized 74 MRSA bacteremia patients to the combination, daptomycin 10mg/kg IV daily plus fosfomycin 2g IV q 6h. They randomized 81 other subjects to standard of care with daptomycin monotherapy, also at 10mg/kg IV daily. Treatment was 10-14 days for uncomplicated and 28-42 days for complicated bacteremia.
The open-label trial was conducted at 18 medical centers in Spain, where fosfomycin was discovered in dirt samples in the late 1960s and remains a matter of pride.
At day 7, 69 of the 74 combination patients (93.2%) were alive with clinical improvement, clearance of bacteremia, and no subsequent relapse, versus 62 of 81 patients (76.5%) on monotherapy (absolute difference 16.7%; 95% confidence interval, 5.4%-27.7%). Three people in the combination arm (4.1%) had died by day 7, versus six on monotherapy (7.4%).
Six weeks after the end of treatment at the test-of-cure visit, 40 of 74 combination patients (54.1%) were alive with resolution of all clinical signs and symptoms, negative blood cultures, and no previous or subsequent relapses; just 34 of 81 patients (42%) in the monotherapy arm hit that mark. The 12.1% difference was not statistically significant, nor was the difference in 12-week survival.
However, patients in the combination arm were 70% less likely to have complicated bacteremia at the test-of-cure visit (9.5% vs. 28.4%; relative risk 0.3; 95% CI, 0.2-0.7). There were no cases of persistent or recurrent infection in the combination arm, but nine persistent (11.1%) and five recurrent (6.2%) cases with daptomycin monotherapy. The differences were statistically significant.
The subjects all had at least one positive MRSA blood culture within 72 hours of randomization. Exclusion criteria included MRSA pneumonia, prosthetic valve endocarditis, end-stage liver disease, and moderate to severe heart failure.
There were no significant baseline differences between the groups. About half the subjects were men, and the mean age was about 73 years. The mean Charlson Comorbidity Index score was a bit under 4, and the mean Pitt bacteremia score a bit over 1. The leading source of infection was vascular catheter; acquisition was thought to be nosocomial in more than 40% of patients.
There were no discontinuations from drug side effects in the daptomycin arm, but there were five in the combination arm, including two for heart failure, two for respiratory insufficiency, and one for GI bleeding. Even so, the benefit outweighed the risk, Dr. Pujol said.
Intravenous fosfomycin is available in Europe, but the drug is approved in the United States only as an oral formulation. That could change soon; Nabriva Therapeutics plans to file its IV formulation (Contepo) for Food and Drug Administration approval in late 2018.
Though it is not standard of practice yet, the combination is increasingly being used in Spain for MRSA bacteremia, according to Dr. Pujol. “Patients probably need the combination [at least] initially, especially if they have complicated bacteremia” or fail monotherapy, he said at ID week, an annual scientific meeting on infectious diseases.
The work was funded by the Spanish government. Dr. Pujol said he had no relevant disclosures.
SOURCE: Pujol M et al. 2018 ID Week abstract LB3
REPORTING FROM ID WEEK 2018
Key clinical point:
Major finding: At day 93% of the combination patients were alive with clinical improvement, clearance of bacteremia, and no subsequent relapse, vs. 77% on monotherapy.
Study details: Randomized, open label trial in 155 patients with MRSA bacteremia.
Disclosures: The work was funded by the Spanish government. The lead investigator said he had no relevant disclosures.
Source: Pujol M et al. 2018 ID Week, Abstract LB3
Cholera, bacteriophage in an epic evolutionary struggle
SAN FRANCISCO – A new analysis of cholera strains suggests that bacteriophages – viruses that prey on bacteria – are engaged in an evolutionary arms race with the Vibrio cholerae bacteria, and the dynamic between the two organisms may be an important factor in determining which strain of cholera goes on to cause a pandemic.
The work, presented by Kim Seed, PhD, at IDWeek, an annual scientific meeting on infectious diseases, examined a defense mechanism in V. cholerae, called phage inducible chromosomal island like element (PLE), as well as a unique mechanism in the bacteriophage to counter it. The work adds insight into the cholera strains that could emerge to produce future epidemics, and could even inform the use of bacteriophages as prophylactic agents to counter V. cholerae infection
In her talk, Dr. Seed described the dynamics of the current cholera pandemic, which is the seventh in recorded history and began in the 1960s. Over the past 100 years, six previous strains arose and then vanished, yielding each time to a new strain that became the predominant cholera-causing agent.
“This pattern of evolution, this so-called disappearing act, drives my research – I’m trying to understand what factors promote the evolution of novel genetic variants, and what factors contribute to why those variants disappear,” said Dr. Seed.
That quest brought her to the Bay of Bengal and Bangladesh. Genetic studies have shown this region to be the epicenter of cholera strains. It appears that cholera strains evolve there and then invade other regions of the world as a result of human travel and activity. Go to places in Africa or Asia where there is a cholera outbreak, and you can find cholera bacteria in the water that has the potential to cause human disease – but it won’t be the strain that is causing disease nearby. “(The culprit) is these introduced strains that come from Southeast Asia,” said Dr. Seed.
So her team went to Bangladesh, and studied cholera bacteria isolated from patients at the International Centre for Diarrhoeal Disease Research. The current strain is antibiotic resistant, as has been well documented. But Dr. Seed was interested in bacteriophages – viruses that prey on bacteria – because they live in the water supply and can also be isolated from the stool of cholera-infected patients, and it seemed likely that they could be an important selective force.
Indeed, her team found only a few bacteriophages that prey on V. cholerae in the samples from this hospital, and one type predominated in samples collected between 2001 and 2017; a bacteriophage known as ICP1. “This set up a very nice dynamic to be able to study the molecular mechanisms by which co-evolution was occurring in this one specific phage and Vibrio cholerae,” said Dr. Seed.
Genetic analysis revealed a mobile genetic element in V. cholerae – PLE –that conferred specific resistance against ICP1. After an infection, one of the bacteriophage’s proteins leads to excision and transcription of PLE. That produces a predicted 25 proteins, which in turn interfere with ICP1 through an as yet undetermined mechanism. But it’s effective, completely shutting down bacteriophage replication.
That couldn’t be the end of the story, Dr. Seed reasoned. Otherwise the bacteriophage would die out entirely for lack of a vulnerable host. More searching revealed the biggest surprise of all – ICP1, even though it is a virus, contains a complete suite of CRISPR (clustered regularly interspaced short palindromic repeats) apparatus that directly targets the PLE sequence. CRISPR is currently all the rage as a potential tool for genetic modification. It was discovered in bacteria, as a sort of immune response against bacteriophages. The CRISPR DNA contains a guide sequence that is complementary to and binds viral DNA, and then recruits other proteins to destroy the viral blueprint.
But here, for the first and only time, Dr. Seed’s team found that a bacteriophage had turned the tables, somehow capturing a CRISPR system of its own and turning it against its host’s defense system. Soon after infection, PLE switches on in response to its bacteriophage trigger, but the ICP1 counters by activating its CRISPR system, which is effective enough to allow the bacteriophage to reproduce.
The researchers then examined historical samples, and found another surprise: The appearance of CRISPR in ICP1 predated the appearance of the PLE variant that it targeted in V. cholerae. A little more digging revealed older variants of PLE, now gone from the V. cholerae population. “This explains why ICP1 had to have CRISPR, so it could overcome these previously prevalent genetic variants,” said Dr. Seed.
All told, the researchers found five unique PLE variants dating back to 1931, and the co-evolution of V. cholerae and ICP1 no doubt stretch much farther into the dim past. More recently, they found that previous strains of V. cholerae that went extinct also had different variations of PLE, suggesting that it may have been a temporary evolutionary victory by ICP1 over a PLE variant that caused the demise of an existing V. cholerae strain. But each time, it seems the bacteria responded with a new PLE variant, prolonging the arms race.
The work has the potential to affect other bacterial diseases, since most bacteria have phages that prey on them. “I have no doubt that they are a strong presence and selective force on all pathogens. People haven’t done so much work on that yet, but I think it’s coming,” said Dr. Seed.
SOURCE: Seed K. et al. ID Week 2018. Abstract 954.
SAN FRANCISCO – A new analysis of cholera strains suggests that bacteriophages – viruses that prey on bacteria – are engaged in an evolutionary arms race with the Vibrio cholerae bacteria, and the dynamic between the two organisms may be an important factor in determining which strain of cholera goes on to cause a pandemic.
The work, presented by Kim Seed, PhD, at IDWeek, an annual scientific meeting on infectious diseases, examined a defense mechanism in V. cholerae, called phage inducible chromosomal island like element (PLE), as well as a unique mechanism in the bacteriophage to counter it. The work adds insight into the cholera strains that could emerge to produce future epidemics, and could even inform the use of bacteriophages as prophylactic agents to counter V. cholerae infection
In her talk, Dr. Seed described the dynamics of the current cholera pandemic, which is the seventh in recorded history and began in the 1960s. Over the past 100 years, six previous strains arose and then vanished, yielding each time to a new strain that became the predominant cholera-causing agent.
“This pattern of evolution, this so-called disappearing act, drives my research – I’m trying to understand what factors promote the evolution of novel genetic variants, and what factors contribute to why those variants disappear,” said Dr. Seed.
That quest brought her to the Bay of Bengal and Bangladesh. Genetic studies have shown this region to be the epicenter of cholera strains. It appears that cholera strains evolve there and then invade other regions of the world as a result of human travel and activity. Go to places in Africa or Asia where there is a cholera outbreak, and you can find cholera bacteria in the water that has the potential to cause human disease – but it won’t be the strain that is causing disease nearby. “(The culprit) is these introduced strains that come from Southeast Asia,” said Dr. Seed.
So her team went to Bangladesh, and studied cholera bacteria isolated from patients at the International Centre for Diarrhoeal Disease Research. The current strain is antibiotic resistant, as has been well documented. But Dr. Seed was interested in bacteriophages – viruses that prey on bacteria – because they live in the water supply and can also be isolated from the stool of cholera-infected patients, and it seemed likely that they could be an important selective force.
Indeed, her team found only a few bacteriophages that prey on V. cholerae in the samples from this hospital, and one type predominated in samples collected between 2001 and 2017; a bacteriophage known as ICP1. “This set up a very nice dynamic to be able to study the molecular mechanisms by which co-evolution was occurring in this one specific phage and Vibrio cholerae,” said Dr. Seed.
Genetic analysis revealed a mobile genetic element in V. cholerae – PLE –that conferred specific resistance against ICP1. After an infection, one of the bacteriophage’s proteins leads to excision and transcription of PLE. That produces a predicted 25 proteins, which in turn interfere with ICP1 through an as yet undetermined mechanism. But it’s effective, completely shutting down bacteriophage replication.
That couldn’t be the end of the story, Dr. Seed reasoned. Otherwise the bacteriophage would die out entirely for lack of a vulnerable host. More searching revealed the biggest surprise of all – ICP1, even though it is a virus, contains a complete suite of CRISPR (clustered regularly interspaced short palindromic repeats) apparatus that directly targets the PLE sequence. CRISPR is currently all the rage as a potential tool for genetic modification. It was discovered in bacteria, as a sort of immune response against bacteriophages. The CRISPR DNA contains a guide sequence that is complementary to and binds viral DNA, and then recruits other proteins to destroy the viral blueprint.
But here, for the first and only time, Dr. Seed’s team found that a bacteriophage had turned the tables, somehow capturing a CRISPR system of its own and turning it against its host’s defense system. Soon after infection, PLE switches on in response to its bacteriophage trigger, but the ICP1 counters by activating its CRISPR system, which is effective enough to allow the bacteriophage to reproduce.
The researchers then examined historical samples, and found another surprise: The appearance of CRISPR in ICP1 predated the appearance of the PLE variant that it targeted in V. cholerae. A little more digging revealed older variants of PLE, now gone from the V. cholerae population. “This explains why ICP1 had to have CRISPR, so it could overcome these previously prevalent genetic variants,” said Dr. Seed.
All told, the researchers found five unique PLE variants dating back to 1931, and the co-evolution of V. cholerae and ICP1 no doubt stretch much farther into the dim past. More recently, they found that previous strains of V. cholerae that went extinct also had different variations of PLE, suggesting that it may have been a temporary evolutionary victory by ICP1 over a PLE variant that caused the demise of an existing V. cholerae strain. But each time, it seems the bacteria responded with a new PLE variant, prolonging the arms race.
The work has the potential to affect other bacterial diseases, since most bacteria have phages that prey on them. “I have no doubt that they are a strong presence and selective force on all pathogens. People haven’t done so much work on that yet, but I think it’s coming,” said Dr. Seed.
SOURCE: Seed K. et al. ID Week 2018. Abstract 954.
SAN FRANCISCO – A new analysis of cholera strains suggests that bacteriophages – viruses that prey on bacteria – are engaged in an evolutionary arms race with the Vibrio cholerae bacteria, and the dynamic between the two organisms may be an important factor in determining which strain of cholera goes on to cause a pandemic.
The work, presented by Kim Seed, PhD, at IDWeek, an annual scientific meeting on infectious diseases, examined a defense mechanism in V. cholerae, called phage inducible chromosomal island like element (PLE), as well as a unique mechanism in the bacteriophage to counter it. The work adds insight into the cholera strains that could emerge to produce future epidemics, and could even inform the use of bacteriophages as prophylactic agents to counter V. cholerae infection
In her talk, Dr. Seed described the dynamics of the current cholera pandemic, which is the seventh in recorded history and began in the 1960s. Over the past 100 years, six previous strains arose and then vanished, yielding each time to a new strain that became the predominant cholera-causing agent.
“This pattern of evolution, this so-called disappearing act, drives my research – I’m trying to understand what factors promote the evolution of novel genetic variants, and what factors contribute to why those variants disappear,” said Dr. Seed.
That quest brought her to the Bay of Bengal and Bangladesh. Genetic studies have shown this region to be the epicenter of cholera strains. It appears that cholera strains evolve there and then invade other regions of the world as a result of human travel and activity. Go to places in Africa or Asia where there is a cholera outbreak, and you can find cholera bacteria in the water that has the potential to cause human disease – but it won’t be the strain that is causing disease nearby. “(The culprit) is these introduced strains that come from Southeast Asia,” said Dr. Seed.
So her team went to Bangladesh, and studied cholera bacteria isolated from patients at the International Centre for Diarrhoeal Disease Research. The current strain is antibiotic resistant, as has been well documented. But Dr. Seed was interested in bacteriophages – viruses that prey on bacteria – because they live in the water supply and can also be isolated from the stool of cholera-infected patients, and it seemed likely that they could be an important selective force.
Indeed, her team found only a few bacteriophages that prey on V. cholerae in the samples from this hospital, and one type predominated in samples collected between 2001 and 2017; a bacteriophage known as ICP1. “This set up a very nice dynamic to be able to study the molecular mechanisms by which co-evolution was occurring in this one specific phage and Vibrio cholerae,” said Dr. Seed.
Genetic analysis revealed a mobile genetic element in V. cholerae – PLE –that conferred specific resistance against ICP1. After an infection, one of the bacteriophage’s proteins leads to excision and transcription of PLE. That produces a predicted 25 proteins, which in turn interfere with ICP1 through an as yet undetermined mechanism. But it’s effective, completely shutting down bacteriophage replication.
That couldn’t be the end of the story, Dr. Seed reasoned. Otherwise the bacteriophage would die out entirely for lack of a vulnerable host. More searching revealed the biggest surprise of all – ICP1, even though it is a virus, contains a complete suite of CRISPR (clustered regularly interspaced short palindromic repeats) apparatus that directly targets the PLE sequence. CRISPR is currently all the rage as a potential tool for genetic modification. It was discovered in bacteria, as a sort of immune response against bacteriophages. The CRISPR DNA contains a guide sequence that is complementary to and binds viral DNA, and then recruits other proteins to destroy the viral blueprint.
But here, for the first and only time, Dr. Seed’s team found that a bacteriophage had turned the tables, somehow capturing a CRISPR system of its own and turning it against its host’s defense system. Soon after infection, PLE switches on in response to its bacteriophage trigger, but the ICP1 counters by activating its CRISPR system, which is effective enough to allow the bacteriophage to reproduce.
The researchers then examined historical samples, and found another surprise: The appearance of CRISPR in ICP1 predated the appearance of the PLE variant that it targeted in V. cholerae. A little more digging revealed older variants of PLE, now gone from the V. cholerae population. “This explains why ICP1 had to have CRISPR, so it could overcome these previously prevalent genetic variants,” said Dr. Seed.
All told, the researchers found five unique PLE variants dating back to 1931, and the co-evolution of V. cholerae and ICP1 no doubt stretch much farther into the dim past. More recently, they found that previous strains of V. cholerae that went extinct also had different variations of PLE, suggesting that it may have been a temporary evolutionary victory by ICP1 over a PLE variant that caused the demise of an existing V. cholerae strain. But each time, it seems the bacteria responded with a new PLE variant, prolonging the arms race.
The work has the potential to affect other bacterial diseases, since most bacteria have phages that prey on them. “I have no doubt that they are a strong presence and selective force on all pathogens. People haven’t done so much work on that yet, but I think it’s coming,” said Dr. Seed.
SOURCE: Seed K. et al. ID Week 2018. Abstract 954.
REPORTING FROM IDWEEK 2018
Key clinical point: Bacteriophages place selective pressure on bacteria that may influence the emergence of new pathogenic strains.
Major finding: Bacteriophages turn on their own CRISPR system to target cholera defensive genes.
Study details: Genetic analysis of an antibiotic resistant strain and attacking bacteriophage strains.
Disclosures: The National Institutes of Health and the Chan Zuckerberg Biohub provided research funding.
Source: Seed K et al. ID Week 2018. Abstract 954.
Swollen knee in a kid? Above 9, treat for Lyme
SAN FRANCISCO – There’s no need to wait for western blot results to differentiate Lyme arthritis from septic arthritis in children, as long as your lab, like many, uses the Liaison Borrelia burgdorferi assay, according to investigators at the University of Minnesota, Minneapolis.
Acute, isolated monoarthritis presents with a single swollen joint and pain whether it’s due to Lyme disease or infection, so it’s hard to tell them apart. Current guidelines recommend a two-tier approach to diagnose Lyme arthritis, an initial blood screen followed by western blot confirmation. Screening results come back in a few hours, but western blot confirmation can take days.
In the meantime, children are treated presumptively for the more concerning diagnosis – septic arthritis – which means hospitalization, surgical drainage, and IV antibiotics. Those who turn out to have Lyme are exposed to the risks and costs of unnecessary treatment and delays to proper diagnosis and doxycycline.
When “kids come in with a swollen knee, maybe 10% or 15% end up in the hospital being treated for septic arthritis that they never had. I wanted to see if we can diagnose Lyme arthritis more quickly,” said lead investigator Bazak Sharon, MD, a pediatric infectious disease specialist at the university’s Masonic Children’s Hospital.
Masonic and its affiliated health system use the Liaison Borrelia burgdorferi assay (DiaSorin) to screen for Lyme, and a careful parsing of the results seems to solve the problem.
Liaison is a chemiluminescence immunoassay that uses light to measure IgM and IgG antibodies to a B. burgdorferi surface protein in serum samples. Results are reported as relative light units (RLUs); below 0.9 RLUs is negative; 0.9-1.1 is equivocal, and over 1.1 is positive.
It’s where patients fall in the range of positivity that matters when it comes to differentiating Lyme from septic arthritis, Dr. Sharon said at ID Week, an annual scientific meeting on infectious diseases (Clin Vaccine Immunol. 2008 Dec;15[12]:1796-804).
He and his team reviewed 60 cases of acute, isolated monoarthritis culled from more than 700 children who presented with joint complaints from 2011 to 2016; 47 had Lyme arthritis confirmed by western blot; 13 had septic arthritis.
It turned out that “every single patient with a” Liaison value of 9 RLUs or higher was confirmed on western blot for Lyme. “Under 9, there was not a single case of Lyme arthritis,” Dr. Sharon said. Three other patients with acute arthritis also tested positive on the screen, but their RLU values were below 4; two turned out to be trauma related and one was ultimately diagnosed with juvenile idiopathic arthritis. Western blots were negative in all three.
The RLU number reported on the screening test “appears to correlate very well with Lyme arthritis. In an otherwise healthy child presenting with acute joint swelling, utilizing this screening test can confirm clinical suspicion of Lyme arthritis within hours, and prevent the potential harmful interventions accompanying a misdiagnosis of septic arthritis. Just do the screening. If it comes up above 9, you’ve got Lyme arthritis,” and don’t need to wait for western blot results to treat, Dr. Sharon said.
In other words, above 9, treat for Lyme.
The investigators plan to delve further into their results with sensitivity/specificity and other analyses before publishing. Ultimately, “my goal is to have a better diagnosis algorithm for kids who present with acute, isolated monoarthritis,” Dr. Sharon said.
There was no industry funding for the work, and the investigators didn’t have any disclosures.
SOURCE: Sharon B et al. 2018 ID Week abstract 286.
SAN FRANCISCO – There’s no need to wait for western blot results to differentiate Lyme arthritis from septic arthritis in children, as long as your lab, like many, uses the Liaison Borrelia burgdorferi assay, according to investigators at the University of Minnesota, Minneapolis.
Acute, isolated monoarthritis presents with a single swollen joint and pain whether it’s due to Lyme disease or infection, so it’s hard to tell them apart. Current guidelines recommend a two-tier approach to diagnose Lyme arthritis, an initial blood screen followed by western blot confirmation. Screening results come back in a few hours, but western blot confirmation can take days.
In the meantime, children are treated presumptively for the more concerning diagnosis – septic arthritis – which means hospitalization, surgical drainage, and IV antibiotics. Those who turn out to have Lyme are exposed to the risks and costs of unnecessary treatment and delays to proper diagnosis and doxycycline.
When “kids come in with a swollen knee, maybe 10% or 15% end up in the hospital being treated for septic arthritis that they never had. I wanted to see if we can diagnose Lyme arthritis more quickly,” said lead investigator Bazak Sharon, MD, a pediatric infectious disease specialist at the university’s Masonic Children’s Hospital.
Masonic and its affiliated health system use the Liaison Borrelia burgdorferi assay (DiaSorin) to screen for Lyme, and a careful parsing of the results seems to solve the problem.
Liaison is a chemiluminescence immunoassay that uses light to measure IgM and IgG antibodies to a B. burgdorferi surface protein in serum samples. Results are reported as relative light units (RLUs); below 0.9 RLUs is negative; 0.9-1.1 is equivocal, and over 1.1 is positive.
It’s where patients fall in the range of positivity that matters when it comes to differentiating Lyme from septic arthritis, Dr. Sharon said at ID Week, an annual scientific meeting on infectious diseases (Clin Vaccine Immunol. 2008 Dec;15[12]:1796-804).
He and his team reviewed 60 cases of acute, isolated monoarthritis culled from more than 700 children who presented with joint complaints from 2011 to 2016; 47 had Lyme arthritis confirmed by western blot; 13 had septic arthritis.
It turned out that “every single patient with a” Liaison value of 9 RLUs or higher was confirmed on western blot for Lyme. “Under 9, there was not a single case of Lyme arthritis,” Dr. Sharon said. Three other patients with acute arthritis also tested positive on the screen, but their RLU values were below 4; two turned out to be trauma related and one was ultimately diagnosed with juvenile idiopathic arthritis. Western blots were negative in all three.
The RLU number reported on the screening test “appears to correlate very well with Lyme arthritis. In an otherwise healthy child presenting with acute joint swelling, utilizing this screening test can confirm clinical suspicion of Lyme arthritis within hours, and prevent the potential harmful interventions accompanying a misdiagnosis of septic arthritis. Just do the screening. If it comes up above 9, you’ve got Lyme arthritis,” and don’t need to wait for western blot results to treat, Dr. Sharon said.
In other words, above 9, treat for Lyme.
The investigators plan to delve further into their results with sensitivity/specificity and other analyses before publishing. Ultimately, “my goal is to have a better diagnosis algorithm for kids who present with acute, isolated monoarthritis,” Dr. Sharon said.
There was no industry funding for the work, and the investigators didn’t have any disclosures.
SOURCE: Sharon B et al. 2018 ID Week abstract 286.
SAN FRANCISCO – There’s no need to wait for western blot results to differentiate Lyme arthritis from septic arthritis in children, as long as your lab, like many, uses the Liaison Borrelia burgdorferi assay, according to investigators at the University of Minnesota, Minneapolis.
Acute, isolated monoarthritis presents with a single swollen joint and pain whether it’s due to Lyme disease or infection, so it’s hard to tell them apart. Current guidelines recommend a two-tier approach to diagnose Lyme arthritis, an initial blood screen followed by western blot confirmation. Screening results come back in a few hours, but western blot confirmation can take days.
In the meantime, children are treated presumptively for the more concerning diagnosis – septic arthritis – which means hospitalization, surgical drainage, and IV antibiotics. Those who turn out to have Lyme are exposed to the risks and costs of unnecessary treatment and delays to proper diagnosis and doxycycline.
When “kids come in with a swollen knee, maybe 10% or 15% end up in the hospital being treated for septic arthritis that they never had. I wanted to see if we can diagnose Lyme arthritis more quickly,” said lead investigator Bazak Sharon, MD, a pediatric infectious disease specialist at the university’s Masonic Children’s Hospital.
Masonic and its affiliated health system use the Liaison Borrelia burgdorferi assay (DiaSorin) to screen for Lyme, and a careful parsing of the results seems to solve the problem.
Liaison is a chemiluminescence immunoassay that uses light to measure IgM and IgG antibodies to a B. burgdorferi surface protein in serum samples. Results are reported as relative light units (RLUs); below 0.9 RLUs is negative; 0.9-1.1 is equivocal, and over 1.1 is positive.
It’s where patients fall in the range of positivity that matters when it comes to differentiating Lyme from septic arthritis, Dr. Sharon said at ID Week, an annual scientific meeting on infectious diseases (Clin Vaccine Immunol. 2008 Dec;15[12]:1796-804).
He and his team reviewed 60 cases of acute, isolated monoarthritis culled from more than 700 children who presented with joint complaints from 2011 to 2016; 47 had Lyme arthritis confirmed by western blot; 13 had septic arthritis.
It turned out that “every single patient with a” Liaison value of 9 RLUs or higher was confirmed on western blot for Lyme. “Under 9, there was not a single case of Lyme arthritis,” Dr. Sharon said. Three other patients with acute arthritis also tested positive on the screen, but their RLU values were below 4; two turned out to be trauma related and one was ultimately diagnosed with juvenile idiopathic arthritis. Western blots were negative in all three.
The RLU number reported on the screening test “appears to correlate very well with Lyme arthritis. In an otherwise healthy child presenting with acute joint swelling, utilizing this screening test can confirm clinical suspicion of Lyme arthritis within hours, and prevent the potential harmful interventions accompanying a misdiagnosis of septic arthritis. Just do the screening. If it comes up above 9, you’ve got Lyme arthritis,” and don’t need to wait for western blot results to treat, Dr. Sharon said.
In other words, above 9, treat for Lyme.
The investigators plan to delve further into their results with sensitivity/specificity and other analyses before publishing. Ultimately, “my goal is to have a better diagnosis algorithm for kids who present with acute, isolated monoarthritis,” Dr. Sharon said.
There was no industry funding for the work, and the investigators didn’t have any disclosures.
SOURCE: Sharon B et al. 2018 ID Week abstract 286.
REPORTING FROM IDWEEK 2018
Key clinical point:
Major finding: There was not a single case of Lyme arthritis under 9 RLUs on the screening test.
Study details: Review of 60 children with acute, isolated monoarthritis, culled from more than 700 with joint complaints.
Disclosures: There was no industry funding for the work, and the investigators didn’t have any disclosures.
Source: Sharon B et al. 2018 ID Week abstract 286.
TB vaccine shows promise in previously infected
san francisco – A new The vaccine showed efficacy in young adults – an important finding because models suggest that inducing immunity in adolescents and young adults would be the fastest and most cost-effective approach to dealing with the global TB epidemic.
The study recruited adults who had previously been exposed to Mycobacterium tuberculosis, a population that receives no benefit from the long-standing bacillus Calmette-Guérin (BCG) vaccine. The overall efficacy of protection was 54%. “There isn’t any vaccine that’s been demonstrated to work in people who are already infected. It’s also the first vaccine to show this level of statistically significant protection in adults, and it’s adults who are the major transmitters of tuberculosis. The modeling has shown that even a vaccine that could protect infected adults at 20% vaccine efficacy would have a substantial impact on the epidemic and be cost effective,” said Ann Ginsberg, MD, PhD, chief medical officer at Aeras, which developed the vaccine and is now testing it in partnership with GlaxoSmithKline.
The results of the study were presented at ID Week 2018 and published in the New England Journal of Medicine (2018 Sep 25. doi: 10.1056/NEJMoa1803484).
The results address a major weakness of the BCG vaccine, which is that some studies have shown it offers little benefit to subjects who are already infected with the disease, which is the case for about a quarter of the world’s population, according to Dr. Ginsberg. The probable explanation is that previous infection with M. tuberculosis or a related bacteria is common in some populations and that this exposure grants some protection against progression to active disease.
The researchers tested the M72/AS01E vaccine, which includes two M. tuberculosis antigens that were identified from patients who had controlled their infection and also the AS01 adjuvant, which contains two immunostimulating agents and is a component of a developmental malaria vaccine and the recombinant zoster vaccine Shingrix.
In Kenya, South Africa, and Zambia, the researchers randomized 3,330 participants (mean age, 28.9 years; 43% female) to receive two doses 1 month apart of either vaccine or placebo. After a mean follow-up of 2.3 years, the protocol efficacy analysis showed that the vaccine had an efficacy rate of 54.0% (P = .04) for pulmonary tuberculosis.
The vaccine had greater efficacy in men (75.2%; P = .03) than it did in women (27.4%; P = .52) and among individuals aged 25 years or younger (84.4%; P = .01) than it did among older subjects (10.2%, P = .82).
The frequency of serious adverse events was similar between the vaccine (1.6%) and the placebo group (1.8%). Unsolicited reports of adverse events were more common in the vaccine group than the placebo group (67.4% vs. 45.4%, respectively), driven largely by more reports of injection site reactions and flu-like symptoms. Solicited reports of adverse events were highlighted by a greater frequency of injection site pain in the vaccine group (81.8% vs. 34.4%). A total of 24.3% of the vaccine recipients reported grade 3 pain, compared with 3.3% in the placebo arm. Rates of fatigue, headache, malaise, or myalgia were also higher in the vaccine group, as was fever.
All of the subjects in the vaccine group had seroconversion at month 2, and 99% remained seroconverted at 12 months.
Next, the researchers plan to conduct studies in HIV-infected individuals and to proceed with phase III trials.
The trial was funded by GlaxoSmithKline Biologicals and Aeras. Dr. Ginsberg is an employee of Aeras.
SOURCE: Ginsberg A et al. IDWeek 2018, Abstract 120
san francisco – A new The vaccine showed efficacy in young adults – an important finding because models suggest that inducing immunity in adolescents and young adults would be the fastest and most cost-effective approach to dealing with the global TB epidemic.
The study recruited adults who had previously been exposed to Mycobacterium tuberculosis, a population that receives no benefit from the long-standing bacillus Calmette-Guérin (BCG) vaccine. The overall efficacy of protection was 54%. “There isn’t any vaccine that’s been demonstrated to work in people who are already infected. It’s also the first vaccine to show this level of statistically significant protection in adults, and it’s adults who are the major transmitters of tuberculosis. The modeling has shown that even a vaccine that could protect infected adults at 20% vaccine efficacy would have a substantial impact on the epidemic and be cost effective,” said Ann Ginsberg, MD, PhD, chief medical officer at Aeras, which developed the vaccine and is now testing it in partnership with GlaxoSmithKline.
The results of the study were presented at ID Week 2018 and published in the New England Journal of Medicine (2018 Sep 25. doi: 10.1056/NEJMoa1803484).
The results address a major weakness of the BCG vaccine, which is that some studies have shown it offers little benefit to subjects who are already infected with the disease, which is the case for about a quarter of the world’s population, according to Dr. Ginsberg. The probable explanation is that previous infection with M. tuberculosis or a related bacteria is common in some populations and that this exposure grants some protection against progression to active disease.
The researchers tested the M72/AS01E vaccine, which includes two M. tuberculosis antigens that were identified from patients who had controlled their infection and also the AS01 adjuvant, which contains two immunostimulating agents and is a component of a developmental malaria vaccine and the recombinant zoster vaccine Shingrix.
In Kenya, South Africa, and Zambia, the researchers randomized 3,330 participants (mean age, 28.9 years; 43% female) to receive two doses 1 month apart of either vaccine or placebo. After a mean follow-up of 2.3 years, the protocol efficacy analysis showed that the vaccine had an efficacy rate of 54.0% (P = .04) for pulmonary tuberculosis.
The vaccine had greater efficacy in men (75.2%; P = .03) than it did in women (27.4%; P = .52) and among individuals aged 25 years or younger (84.4%; P = .01) than it did among older subjects (10.2%, P = .82).
The frequency of serious adverse events was similar between the vaccine (1.6%) and the placebo group (1.8%). Unsolicited reports of adverse events were more common in the vaccine group than the placebo group (67.4% vs. 45.4%, respectively), driven largely by more reports of injection site reactions and flu-like symptoms. Solicited reports of adverse events were highlighted by a greater frequency of injection site pain in the vaccine group (81.8% vs. 34.4%). A total of 24.3% of the vaccine recipients reported grade 3 pain, compared with 3.3% in the placebo arm. Rates of fatigue, headache, malaise, or myalgia were also higher in the vaccine group, as was fever.
All of the subjects in the vaccine group had seroconversion at month 2, and 99% remained seroconverted at 12 months.
Next, the researchers plan to conduct studies in HIV-infected individuals and to proceed with phase III trials.
The trial was funded by GlaxoSmithKline Biologicals and Aeras. Dr. Ginsberg is an employee of Aeras.
SOURCE: Ginsberg A et al. IDWeek 2018, Abstract 120
san francisco – A new The vaccine showed efficacy in young adults – an important finding because models suggest that inducing immunity in adolescents and young adults would be the fastest and most cost-effective approach to dealing with the global TB epidemic.
The study recruited adults who had previously been exposed to Mycobacterium tuberculosis, a population that receives no benefit from the long-standing bacillus Calmette-Guérin (BCG) vaccine. The overall efficacy of protection was 54%. “There isn’t any vaccine that’s been demonstrated to work in people who are already infected. It’s also the first vaccine to show this level of statistically significant protection in adults, and it’s adults who are the major transmitters of tuberculosis. The modeling has shown that even a vaccine that could protect infected adults at 20% vaccine efficacy would have a substantial impact on the epidemic and be cost effective,” said Ann Ginsberg, MD, PhD, chief medical officer at Aeras, which developed the vaccine and is now testing it in partnership with GlaxoSmithKline.
The results of the study were presented at ID Week 2018 and published in the New England Journal of Medicine (2018 Sep 25. doi: 10.1056/NEJMoa1803484).
The results address a major weakness of the BCG vaccine, which is that some studies have shown it offers little benefit to subjects who are already infected with the disease, which is the case for about a quarter of the world’s population, according to Dr. Ginsberg. The probable explanation is that previous infection with M. tuberculosis or a related bacteria is common in some populations and that this exposure grants some protection against progression to active disease.
The researchers tested the M72/AS01E vaccine, which includes two M. tuberculosis antigens that were identified from patients who had controlled their infection and also the AS01 adjuvant, which contains two immunostimulating agents and is a component of a developmental malaria vaccine and the recombinant zoster vaccine Shingrix.
In Kenya, South Africa, and Zambia, the researchers randomized 3,330 participants (mean age, 28.9 years; 43% female) to receive two doses 1 month apart of either vaccine or placebo. After a mean follow-up of 2.3 years, the protocol efficacy analysis showed that the vaccine had an efficacy rate of 54.0% (P = .04) for pulmonary tuberculosis.
The vaccine had greater efficacy in men (75.2%; P = .03) than it did in women (27.4%; P = .52) and among individuals aged 25 years or younger (84.4%; P = .01) than it did among older subjects (10.2%, P = .82).
The frequency of serious adverse events was similar between the vaccine (1.6%) and the placebo group (1.8%). Unsolicited reports of adverse events were more common in the vaccine group than the placebo group (67.4% vs. 45.4%, respectively), driven largely by more reports of injection site reactions and flu-like symptoms. Solicited reports of adverse events were highlighted by a greater frequency of injection site pain in the vaccine group (81.8% vs. 34.4%). A total of 24.3% of the vaccine recipients reported grade 3 pain, compared with 3.3% in the placebo arm. Rates of fatigue, headache, malaise, or myalgia were also higher in the vaccine group, as was fever.
All of the subjects in the vaccine group had seroconversion at month 2, and 99% remained seroconverted at 12 months.
Next, the researchers plan to conduct studies in HIV-infected individuals and to proceed with phase III trials.
The trial was funded by GlaxoSmithKline Biologicals and Aeras. Dr. Ginsberg is an employee of Aeras.
SOURCE: Ginsberg A et al. IDWeek 2018, Abstract 120
REPORTING FROM IDWEEK 2018
Key clinical point: The vaccine is the first to show efficacy in patients previously exposed to the TB bacterium.
Major finding: The vaccine had a protective efficacy of 54%.
Study details: Randomized, controlled trial with 3,330 participants.
Disclosures: The trial was funded by GlaxoSmithKline Biologicals and Aeras. Dr. Ginsberg is an employee of Aeras.
Source: Ginsberg A et al. IDWeek 2018, Abstract 120.
Stepdown to oral ciprofloxacin looks safe in gram-negative bloodstream infections
SAN FRANCISCO – In gram-negative bloodstream infections, in patients who are stable at 48 hours, are no longer feverish, and whose infections aren’t invasive, it may be safe to step down from IV antibiotics to oral ciprofloxacin (PO). That is the tentative conclusion from a new single-center, retrospective chart review.
The study adds to growing suspicion among practitioners that stepping down may be safe in gram-negative patients, as well as mounting evidence that shorter treatment durations may also be safe, according to Gregory Cook, PharmD, who presented the study at a poster session at an annual scientific meeting on infectious diseases. “We’re getting more aggressive” in backing off IV treatment, he said in an interview.
Oral medications are associated with shorter hospital stays and decreased costs.
Froedtert & the Medical College of Wisconsin, where the study was performed, switched some years ago from levofloxacin to ciprofloxacin for cost reasons. But ciprofloxacin has a lower bioavailability, and a recent study showed levofloxacin had less treatment failure at 90 days than ciprofloxacin. Levofloxacin is restricted at the institution and requires antibiotic stewardship approval for use, whereas ciprofloxacin can be used without approval.
But the researchers were concerned about bioavailability. “We like to think of ciprofloxacin as having excellent bioavailability, and it does, it has 80% bioavailability, but it’s still not exactly the same as levofloxacin. We wanted to look into this and see if we were doing our patients a disservice or not (by stepping down to ciprofloxacin),” said Dr. Cook, who is now the antimicrobial stewardship pharmacist at Children’s Hospital New Orleans. The results were reassuring. “Ultimately we were trying to see how our patients were doing on oral ciprofloxacin, and after 2-3 days of IV therapy, most of them did extremely well,” he said.
The researchers analyzed the records of 198 patients who presented with a monomicrobial, gram-negative bloodstream infection between January 2015 and January 2018, and who survived at least 5 days past blood culture collection. One hundred and three switched to PO within 5 days, while 95 remained on intravenous antibiotics for longer than 5 days. On average, patients in the PO group received IV antibiotics for 2 days, while the IV group averaged 15 days. Oral ciprofloxacin treatment length averaged 12 days.
The primary endpoint of treatment failure at 90 days, defined as recurrent infection or all-cause mortality, favored the PO group (1.9% versus 16.8%, P less than .01). This was likely because of patient selection, as those in the IV group tended to be more ill, according to Dr. Cook. More were immunosuppressed (41% IV versus 22% in PO group, P less than .01). There were more nonurinary sources of infection (41% in IV group, P less than .01; 65% urinary source in PO group). Thirty-four percent of the PO group had an infectious disease consult, compared with 60% of the IV group.
SOURCE: Gregory Cook et al. ID Week 2018. Abstract 39.
SAN FRANCISCO – In gram-negative bloodstream infections, in patients who are stable at 48 hours, are no longer feverish, and whose infections aren’t invasive, it may be safe to step down from IV antibiotics to oral ciprofloxacin (PO). That is the tentative conclusion from a new single-center, retrospective chart review.
The study adds to growing suspicion among practitioners that stepping down may be safe in gram-negative patients, as well as mounting evidence that shorter treatment durations may also be safe, according to Gregory Cook, PharmD, who presented the study at a poster session at an annual scientific meeting on infectious diseases. “We’re getting more aggressive” in backing off IV treatment, he said in an interview.
Oral medications are associated with shorter hospital stays and decreased costs.
Froedtert & the Medical College of Wisconsin, where the study was performed, switched some years ago from levofloxacin to ciprofloxacin for cost reasons. But ciprofloxacin has a lower bioavailability, and a recent study showed levofloxacin had less treatment failure at 90 days than ciprofloxacin. Levofloxacin is restricted at the institution and requires antibiotic stewardship approval for use, whereas ciprofloxacin can be used without approval.
But the researchers were concerned about bioavailability. “We like to think of ciprofloxacin as having excellent bioavailability, and it does, it has 80% bioavailability, but it’s still not exactly the same as levofloxacin. We wanted to look into this and see if we were doing our patients a disservice or not (by stepping down to ciprofloxacin),” said Dr. Cook, who is now the antimicrobial stewardship pharmacist at Children’s Hospital New Orleans. The results were reassuring. “Ultimately we were trying to see how our patients were doing on oral ciprofloxacin, and after 2-3 days of IV therapy, most of them did extremely well,” he said.
The researchers analyzed the records of 198 patients who presented with a monomicrobial, gram-negative bloodstream infection between January 2015 and January 2018, and who survived at least 5 days past blood culture collection. One hundred and three switched to PO within 5 days, while 95 remained on intravenous antibiotics for longer than 5 days. On average, patients in the PO group received IV antibiotics for 2 days, while the IV group averaged 15 days. Oral ciprofloxacin treatment length averaged 12 days.
The primary endpoint of treatment failure at 90 days, defined as recurrent infection or all-cause mortality, favored the PO group (1.9% versus 16.8%, P less than .01). This was likely because of patient selection, as those in the IV group tended to be more ill, according to Dr. Cook. More were immunosuppressed (41% IV versus 22% in PO group, P less than .01). There were more nonurinary sources of infection (41% in IV group, P less than .01; 65% urinary source in PO group). Thirty-four percent of the PO group had an infectious disease consult, compared with 60% of the IV group.
SOURCE: Gregory Cook et al. ID Week 2018. Abstract 39.
SAN FRANCISCO – In gram-negative bloodstream infections, in patients who are stable at 48 hours, are no longer feverish, and whose infections aren’t invasive, it may be safe to step down from IV antibiotics to oral ciprofloxacin (PO). That is the tentative conclusion from a new single-center, retrospective chart review.
The study adds to growing suspicion among practitioners that stepping down may be safe in gram-negative patients, as well as mounting evidence that shorter treatment durations may also be safe, according to Gregory Cook, PharmD, who presented the study at a poster session at an annual scientific meeting on infectious diseases. “We’re getting more aggressive” in backing off IV treatment, he said in an interview.
Oral medications are associated with shorter hospital stays and decreased costs.
Froedtert & the Medical College of Wisconsin, where the study was performed, switched some years ago from levofloxacin to ciprofloxacin for cost reasons. But ciprofloxacin has a lower bioavailability, and a recent study showed levofloxacin had less treatment failure at 90 days than ciprofloxacin. Levofloxacin is restricted at the institution and requires antibiotic stewardship approval for use, whereas ciprofloxacin can be used without approval.
But the researchers were concerned about bioavailability. “We like to think of ciprofloxacin as having excellent bioavailability, and it does, it has 80% bioavailability, but it’s still not exactly the same as levofloxacin. We wanted to look into this and see if we were doing our patients a disservice or not (by stepping down to ciprofloxacin),” said Dr. Cook, who is now the antimicrobial stewardship pharmacist at Children’s Hospital New Orleans. The results were reassuring. “Ultimately we were trying to see how our patients were doing on oral ciprofloxacin, and after 2-3 days of IV therapy, most of them did extremely well,” he said.
The researchers analyzed the records of 198 patients who presented with a monomicrobial, gram-negative bloodstream infection between January 2015 and January 2018, and who survived at least 5 days past blood culture collection. One hundred and three switched to PO within 5 days, while 95 remained on intravenous antibiotics for longer than 5 days. On average, patients in the PO group received IV antibiotics for 2 days, while the IV group averaged 15 days. Oral ciprofloxacin treatment length averaged 12 days.
The primary endpoint of treatment failure at 90 days, defined as recurrent infection or all-cause mortality, favored the PO group (1.9% versus 16.8%, P less than .01). This was likely because of patient selection, as those in the IV group tended to be more ill, according to Dr. Cook. More were immunosuppressed (41% IV versus 22% in PO group, P less than .01). There were more nonurinary sources of infection (41% in IV group, P less than .01; 65% urinary source in PO group). Thirty-four percent of the PO group had an infectious disease consult, compared with 60% of the IV group.
SOURCE: Gregory Cook et al. ID Week 2018. Abstract 39.
REPORTING FROM IDWEEK 2018
Key clinical point: Stepping down to oral ciprofloxacin at 48 hours is likely safe in stable patients.
Major finding: The 90-day treatment failure rate was 1.9% in patients switched to oral ciprofloxacin.
Study details: Retrospective analysis of 193 cases.
Disclosures: The study was not funded. Dr. Cook declared no financial conflicts of interest.
Source: ID Week 2018. Abstract 39.
In C. difficile, metronidazole may not benefit ICU patients on vancomycin
SAN FRANCISCO – , according to a review of 101 cases at the University of Maryland.
Adding metronidazole is a common move in ICUs when patients start circling the drain with C. difficile, in part because delivery to the gut doesn’t depend on gut motility. “At that point, you are throwing the kitchen sink at them, but it’s” based, like much in C. difficile management, on expert opinion, not evidence, said study lead Ana Vega, PharmD, a former resident at the university’s school of pharmacy in Baltimore, and now an infectious disease pharmacist at Jackson Memorial Hospital, Miami. The investigators wanted to plug the evidence gap. Forty-seven of the 101 patients in their review – all with signs of C. difficile sepsis – had IV metronidazole added to their vancomycin regimens. Thirty-day mortality was 14.9% in the combination group versus 7.4% in the monotherapy arm, and not significantly different (P = .338). There were also no significant differences in resolution rates or normalization of white blood cell counts and temperature.
“Our data question the utility of” of adding IV metronidazole to oral vancomycin in patients with severe disease. “It’s definitely something to think twice about because metronidazole isn’t benign. It makes people feel crummy; you can induce resistance; and it increases the risk of vancomycin-resistant Enterococci colonization,” already a risk with vancomycin, Dr. Vega said at an annual scientific meeting on infectious diseases.
“When you get to the point that you are trying combination therapy based on expert opinion, I think fecal transplants are something to consider” because the success rates are so high. “That would be my suggestion,” she said, even though “it’s much easier to write an order for a drug than to get a fecal transplant.”
The issue is far from resolved, and debate will continue. A similar review of ICU patients at Wake Forest University in Winston-Salem, N.C., did find a significant mortality benefit with combination therapy, regardless of C. difficile severity (Clin Infect Dis. 2015 Sep 15. doi: 10.1093/cid/civ409).
The Maryland investigators excluded patients with toxic megacolon and other life-threatening intra-abdominal complications requiring surgery, because combination therapy is more strongly recommended in fulminant disease. They were interested in people who were not quite ready for the operating room, when what to do is more in doubt.
Subjects were admitted to the ICU from April 2016 to April 2018 with positive C. difficile nucleic acid testing and an order for oral vancomycin. The only statistically significant baseline differences were that patients who got IV metronidazole had higher median white blood cell counts (18,400 versus 13,900 cells/mL; P = .035) and were more likely to receive higher than 500-mg doses of vancomycin (36.2% versus 7.4%; P less than .0001).
The Mean Acute Physiology and Chronic Health Evaluation II (APACHE II) score in the combination group was 23 versus 19 in the monotherapy arm (P = .247). There was no difference in the probability of receiving metronidazole based on the score.
The study again found no significant 30-day mortality differences among 76 patients matched by their APACHE II scores (15.8% in the combination arm versus 9.7%; P = .480).
Severe C. difficile infection was defined as either a white cell count above 15,000 or below 4,000 cells/mL, or a serum creatinine at least 1.5 times above baseline, plus at least one other sign of severe sepsis, such as a mean arterial pressure at or below 60 mm Hg. Metronidazole was started within 72 hours of the first vancomycin dose, and subjects on combination therapy were on both for at least 72 hours.
The mean age in the study was about 60 years old, and just over half of the subjects were men.
Dr. Vega said the investigators hope to expand their sample size and see if patients with more virulent strains of C. difficile do better on combination therapy.
There was no industry funding for the work, and the investigators didn’t have any relevant disclosures.
SOURCE: Vega AD et al. ID Week 2018, Abstract 488.
SAN FRANCISCO – , according to a review of 101 cases at the University of Maryland.
Adding metronidazole is a common move in ICUs when patients start circling the drain with C. difficile, in part because delivery to the gut doesn’t depend on gut motility. “At that point, you are throwing the kitchen sink at them, but it’s” based, like much in C. difficile management, on expert opinion, not evidence, said study lead Ana Vega, PharmD, a former resident at the university’s school of pharmacy in Baltimore, and now an infectious disease pharmacist at Jackson Memorial Hospital, Miami. The investigators wanted to plug the evidence gap. Forty-seven of the 101 patients in their review – all with signs of C. difficile sepsis – had IV metronidazole added to their vancomycin regimens. Thirty-day mortality was 14.9% in the combination group versus 7.4% in the monotherapy arm, and not significantly different (P = .338). There were also no significant differences in resolution rates or normalization of white blood cell counts and temperature.
“Our data question the utility of” of adding IV metronidazole to oral vancomycin in patients with severe disease. “It’s definitely something to think twice about because metronidazole isn’t benign. It makes people feel crummy; you can induce resistance; and it increases the risk of vancomycin-resistant Enterococci colonization,” already a risk with vancomycin, Dr. Vega said at an annual scientific meeting on infectious diseases.
“When you get to the point that you are trying combination therapy based on expert opinion, I think fecal transplants are something to consider” because the success rates are so high. “That would be my suggestion,” she said, even though “it’s much easier to write an order for a drug than to get a fecal transplant.”
The issue is far from resolved, and debate will continue. A similar review of ICU patients at Wake Forest University in Winston-Salem, N.C., did find a significant mortality benefit with combination therapy, regardless of C. difficile severity (Clin Infect Dis. 2015 Sep 15. doi: 10.1093/cid/civ409).
The Maryland investigators excluded patients with toxic megacolon and other life-threatening intra-abdominal complications requiring surgery, because combination therapy is more strongly recommended in fulminant disease. They were interested in people who were not quite ready for the operating room, when what to do is more in doubt.
Subjects were admitted to the ICU from April 2016 to April 2018 with positive C. difficile nucleic acid testing and an order for oral vancomycin. The only statistically significant baseline differences were that patients who got IV metronidazole had higher median white blood cell counts (18,400 versus 13,900 cells/mL; P = .035) and were more likely to receive higher than 500-mg doses of vancomycin (36.2% versus 7.4%; P less than .0001).
The Mean Acute Physiology and Chronic Health Evaluation II (APACHE II) score in the combination group was 23 versus 19 in the monotherapy arm (P = .247). There was no difference in the probability of receiving metronidazole based on the score.
The study again found no significant 30-day mortality differences among 76 patients matched by their APACHE II scores (15.8% in the combination arm versus 9.7%; P = .480).
Severe C. difficile infection was defined as either a white cell count above 15,000 or below 4,000 cells/mL, or a serum creatinine at least 1.5 times above baseline, plus at least one other sign of severe sepsis, such as a mean arterial pressure at or below 60 mm Hg. Metronidazole was started within 72 hours of the first vancomycin dose, and subjects on combination therapy were on both for at least 72 hours.
The mean age in the study was about 60 years old, and just over half of the subjects were men.
Dr. Vega said the investigators hope to expand their sample size and see if patients with more virulent strains of C. difficile do better on combination therapy.
There was no industry funding for the work, and the investigators didn’t have any relevant disclosures.
SOURCE: Vega AD et al. ID Week 2018, Abstract 488.
SAN FRANCISCO – , according to a review of 101 cases at the University of Maryland.
Adding metronidazole is a common move in ICUs when patients start circling the drain with C. difficile, in part because delivery to the gut doesn’t depend on gut motility. “At that point, you are throwing the kitchen sink at them, but it’s” based, like much in C. difficile management, on expert opinion, not evidence, said study lead Ana Vega, PharmD, a former resident at the university’s school of pharmacy in Baltimore, and now an infectious disease pharmacist at Jackson Memorial Hospital, Miami. The investigators wanted to plug the evidence gap. Forty-seven of the 101 patients in their review – all with signs of C. difficile sepsis – had IV metronidazole added to their vancomycin regimens. Thirty-day mortality was 14.9% in the combination group versus 7.4% in the monotherapy arm, and not significantly different (P = .338). There were also no significant differences in resolution rates or normalization of white blood cell counts and temperature.
“Our data question the utility of” of adding IV metronidazole to oral vancomycin in patients with severe disease. “It’s definitely something to think twice about because metronidazole isn’t benign. It makes people feel crummy; you can induce resistance; and it increases the risk of vancomycin-resistant Enterococci colonization,” already a risk with vancomycin, Dr. Vega said at an annual scientific meeting on infectious diseases.
“When you get to the point that you are trying combination therapy based on expert opinion, I think fecal transplants are something to consider” because the success rates are so high. “That would be my suggestion,” she said, even though “it’s much easier to write an order for a drug than to get a fecal transplant.”
The issue is far from resolved, and debate will continue. A similar review of ICU patients at Wake Forest University in Winston-Salem, N.C., did find a significant mortality benefit with combination therapy, regardless of C. difficile severity (Clin Infect Dis. 2015 Sep 15. doi: 10.1093/cid/civ409).
The Maryland investigators excluded patients with toxic megacolon and other life-threatening intra-abdominal complications requiring surgery, because combination therapy is more strongly recommended in fulminant disease. They were interested in people who were not quite ready for the operating room, when what to do is more in doubt.
Subjects were admitted to the ICU from April 2016 to April 2018 with positive C. difficile nucleic acid testing and an order for oral vancomycin. The only statistically significant baseline differences were that patients who got IV metronidazole had higher median white blood cell counts (18,400 versus 13,900 cells/mL; P = .035) and were more likely to receive higher than 500-mg doses of vancomycin (36.2% versus 7.4%; P less than .0001).
The Mean Acute Physiology and Chronic Health Evaluation II (APACHE II) score in the combination group was 23 versus 19 in the monotherapy arm (P = .247). There was no difference in the probability of receiving metronidazole based on the score.
The study again found no significant 30-day mortality differences among 76 patients matched by their APACHE II scores (15.8% in the combination arm versus 9.7%; P = .480).
Severe C. difficile infection was defined as either a white cell count above 15,000 or below 4,000 cells/mL, or a serum creatinine at least 1.5 times above baseline, plus at least one other sign of severe sepsis, such as a mean arterial pressure at or below 60 mm Hg. Metronidazole was started within 72 hours of the first vancomycin dose, and subjects on combination therapy were on both for at least 72 hours.
The mean age in the study was about 60 years old, and just over half of the subjects were men.
Dr. Vega said the investigators hope to expand their sample size and see if patients with more virulent strains of C. difficile do better on combination therapy.
There was no industry funding for the work, and the investigators didn’t have any relevant disclosures.
SOURCE: Vega AD et al. ID Week 2018, Abstract 488.
REPORTING FROM ID WEEK 2018
Key clinical point: The jury is still out on whether adding IV metronidazole helps C. difficile patients already on oral vancomycin in the ICU. Consider fecal transplant.
Major finding: Thirty-day mortality was 14.9% in the combination group versus 7.4% in the monotherapy arm (P = .338).
Study details: Review of 101 ICU patients with severe C. difficile infections
Disclosures: There was no industry funding for the work, and the investigators didn’t have any disclosures.
Source: Vega AD. ID Week 2018, Abstract 488.
ICU infections: Chlorhexidine wipes tame MRSA, CRE
according to a report presented at ID Week 2018.
The move prevented an estimated eight methicillin-resistant Staphylococcus aureus (MRSA) and three carbapenem-resistant Enterobacteriaceae (CRE) infections and saved the medical center more than $150,000 in the year following the November 2016 switch.
The goal was to address the rate of MRSA bacteremia, which was higher than national ICU averages. Contact precautions began to make less sense as MRSA became more common in the surrounding community, and “we just wanted to get rid of contact precautions,” said study lead Jason Moss, DO, an infectious disease fellow at the university.
Contact precautions are expensive, make patients feel isolated, and according to some studies, lead to worse outcomes, he said at the annual scientific meeting on infectious diseases.
Decolonization is not routine in most ICUs, but it’s gaining traction. Guidelines recommend chlorhexidine bathing with wipes to stop CRE transmission, and chlorhexidine is used to prevent central line–associated bloodstream infections (CLABSI).
A recent analysis of 17 trials found marked decreases in MRSA and CLABSI with decolonization and concluded that chlorhexidine bathing “appears to be of the most clinical benefit when infection rates are high for a given ICU population,” as was the case in Kentucky (Crit Care. 2016 Nov 23;20[1]:379).
When researchers compared the year before the change to the year after, “we were pretty surprised at how much the rates of infection and colonization decreased. There have been some people that have been doing this in the ICU, but probably not to our extent. If you want to get rid of contact precautions, this is a great process to do it with,” Dr. Moss said.
Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6 (P = .026). Infection rates fell from 3.9 isolates per 10,000 patient-days to 2 (P = .083). Combined rates of infections and colonizations fell from almost 18 isolates per 10,000 patient-days to fewer than 8 (P = .010).
Decolonization is now standard practice at the university. Every ICU patient gets a one-time povidone iodine nasal swab at admission, then daily baths with 2% chlorhexidine gluconate applied by impregnated wipe. It usually takes four or five wipes to do the entire body.
Spending on gowns fell from about $153,000 per year to just under $60,000, but spending on wipes went up from about $2,700 to $275,000, and spending on povidone iodine nasal swabs went up to more than $100,000.
When balanced against the money not spent on those 11 prevented infections, however, the program saved the medical center about $152,000 in its first year, according to Dr. Moss and his team.
There was no funding for the work, and the investigators had no disclosures.
SOURCE: Moss J et al. ID Week 2018, Abstract 32.
according to a report presented at ID Week 2018.
The move prevented an estimated eight methicillin-resistant Staphylococcus aureus (MRSA) and three carbapenem-resistant Enterobacteriaceae (CRE) infections and saved the medical center more than $150,000 in the year following the November 2016 switch.
The goal was to address the rate of MRSA bacteremia, which was higher than national ICU averages. Contact precautions began to make less sense as MRSA became more common in the surrounding community, and “we just wanted to get rid of contact precautions,” said study lead Jason Moss, DO, an infectious disease fellow at the university.
Contact precautions are expensive, make patients feel isolated, and according to some studies, lead to worse outcomes, he said at the annual scientific meeting on infectious diseases.
Decolonization is not routine in most ICUs, but it’s gaining traction. Guidelines recommend chlorhexidine bathing with wipes to stop CRE transmission, and chlorhexidine is used to prevent central line–associated bloodstream infections (CLABSI).
A recent analysis of 17 trials found marked decreases in MRSA and CLABSI with decolonization and concluded that chlorhexidine bathing “appears to be of the most clinical benefit when infection rates are high for a given ICU population,” as was the case in Kentucky (Crit Care. 2016 Nov 23;20[1]:379).
When researchers compared the year before the change to the year after, “we were pretty surprised at how much the rates of infection and colonization decreased. There have been some people that have been doing this in the ICU, but probably not to our extent. If you want to get rid of contact precautions, this is a great process to do it with,” Dr. Moss said.
Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6 (P = .026). Infection rates fell from 3.9 isolates per 10,000 patient-days to 2 (P = .083). Combined rates of infections and colonizations fell from almost 18 isolates per 10,000 patient-days to fewer than 8 (P = .010).
Decolonization is now standard practice at the university. Every ICU patient gets a one-time povidone iodine nasal swab at admission, then daily baths with 2% chlorhexidine gluconate applied by impregnated wipe. It usually takes four or five wipes to do the entire body.
Spending on gowns fell from about $153,000 per year to just under $60,000, but spending on wipes went up from about $2,700 to $275,000, and spending on povidone iodine nasal swabs went up to more than $100,000.
When balanced against the money not spent on those 11 prevented infections, however, the program saved the medical center about $152,000 in its first year, according to Dr. Moss and his team.
There was no funding for the work, and the investigators had no disclosures.
SOURCE: Moss J et al. ID Week 2018, Abstract 32.
according to a report presented at ID Week 2018.
The move prevented an estimated eight methicillin-resistant Staphylococcus aureus (MRSA) and three carbapenem-resistant Enterobacteriaceae (CRE) infections and saved the medical center more than $150,000 in the year following the November 2016 switch.
The goal was to address the rate of MRSA bacteremia, which was higher than national ICU averages. Contact precautions began to make less sense as MRSA became more common in the surrounding community, and “we just wanted to get rid of contact precautions,” said study lead Jason Moss, DO, an infectious disease fellow at the university.
Contact precautions are expensive, make patients feel isolated, and according to some studies, lead to worse outcomes, he said at the annual scientific meeting on infectious diseases.
Decolonization is not routine in most ICUs, but it’s gaining traction. Guidelines recommend chlorhexidine bathing with wipes to stop CRE transmission, and chlorhexidine is used to prevent central line–associated bloodstream infections (CLABSI).
A recent analysis of 17 trials found marked decreases in MRSA and CLABSI with decolonization and concluded that chlorhexidine bathing “appears to be of the most clinical benefit when infection rates are high for a given ICU population,” as was the case in Kentucky (Crit Care. 2016 Nov 23;20[1]:379).
When researchers compared the year before the change to the year after, “we were pretty surprised at how much the rates of infection and colonization decreased. There have been some people that have been doing this in the ICU, but probably not to our extent. If you want to get rid of contact precautions, this is a great process to do it with,” Dr. Moss said.
Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6 (P = .026). Infection rates fell from 3.9 isolates per 10,000 patient-days to 2 (P = .083). Combined rates of infections and colonizations fell from almost 18 isolates per 10,000 patient-days to fewer than 8 (P = .010).
Decolonization is now standard practice at the university. Every ICU patient gets a one-time povidone iodine nasal swab at admission, then daily baths with 2% chlorhexidine gluconate applied by impregnated wipe. It usually takes four or five wipes to do the entire body.
Spending on gowns fell from about $153,000 per year to just under $60,000, but spending on wipes went up from about $2,700 to $275,000, and spending on povidone iodine nasal swabs went up to more than $100,000.
When balanced against the money not spent on those 11 prevented infections, however, the program saved the medical center about $152,000 in its first year, according to Dr. Moss and his team.
There was no funding for the work, and the investigators had no disclosures.
SOURCE: Moss J et al. ID Week 2018, Abstract 32.
REPORTING FROM ID WEEK 2018
Key clinical point: For high rates of MRSA and CRE in the ICU, consider decolonization instead of contact precautions.
Major finding: Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6; infection rates fell from 3.9 isolates to 2 per 10,000 patient-days.
Study details: Review of ICU quality improvement initiative
Disclosures: There was no funding for the work, and the investigators had no disclosures.
Source: Moss J et al. ID Week 2018, Abstract 32.