User login
Heart Failure Society of America (HFSA): Annual Scientific Meeting
Heart failure device shown safe in PARACHUTE III
LAS VEGAS – Percutaneous ventricular restoration using the Parachute device in patients with ischemic dilated cardiomyopathy had a promisingly low 26% combined rate of all-cause mortality and heart failure hospitalization at 1 year in the PARACHUTE III trial, according to Dr. William T. Abraham
PARACHUTE III was a European postmarketing study. And while the composite endpoint of all-cause mortality and heart failure hospitalization was a prespecified secondary outcome in that study, it’s the primary endpoint in the ongoing pivotal U.S. phase III PARACHUTE IV trial headed by Dr. Abraham.
What’s encouraging about the PARACHUTE III result is that the 26% rate of the combined endpoint is in line with the PARACHUTE IV investigators’ assumption for outcomes in the ongoing U.S. trial, which will randomize roughly 500 patients to device therapy plus optimal medical therapy or optimal medical therapy alone, he reported at the annual meeting of the Heart Failure Society of America.
“The PARACHUTE III results are reassuring. In historical controls, the expected rate [of all-cause mortality and heart failure hospitalization] in this kind of population at 1 year is about 35%,” according to Dr. Abraham, professor of internal medicine, physiology, and cell biology and director of the division of cardiovascular medicine at Ohio State University, Columbus.
Plus, technical improvements in device design and percutaneous implantation technique have been made since PARACHUTE III and are incorporated in PARACHUTE IV. These improvements could drive the composite endpoint rate in the ongoing U.S. trial even lower, he continued. For example, while the 1-year major complication rate in the European postmarketing study was 11%, thus far it’s less than 8% in the U.S. experience.
The proprietary Parachute device is so named because, when deployed, it looks like an upside-down parachute. It is intended for patients with ischemic dilated cardiomyopathy secondary to anteroapical MI. The goal of this device therapy is to attenuate or reverse progression of heart failure in this high-risk population.
The Parachute device has two proposed mechanisms of action, the cardiologist explained. One involves ventricular restoration via ventricular partitioning to reduce wall stress in the upper chamber as well as fostering a return to a more normal, elliptical shape to the heart. Dr. Abraham termed the other mechanism a trampoline effect, in which the compliant device encourages replacement of eccentric apical wall motion with more synchronized wall motion throughout the cardiac cycle.
The device, comprised mainly of a fluoropolymer membrane and a nitinol frame, is implanted percutaneously retrograde across the aortic valve. It is placed in the apex of the left ventricle.
The European Union PARACHUTE III postmarketing study was a single-arm study including 100 consecutive symptomatic patients in 10 countries. Their baseline left ventricular ejection fraction was 28%. Device implantation was accomplished in 97 of the 100 patients, with a mean 94-minute procedure duration and 23 minutes of fluoroscopy time.
The primary study endpoint was a safety outcome: the 1-year rate of procedure- and/or device-related adverse events. The 7% rate included several cases of left ventricular perforation as well as aortic or mitral valve damage.
“The rates and distribution of these events are comparable to other structural heart disease interventions, such as transcatheter aortic valve replacement, and might be considered an acceptable postmarketing result,” Dr. Abraham commented.
There was a 3.2% stroke rate through 1 year and a 9.5% mortality rate.
Hemodynamic improvements were seen in both systolic and diastolic function. The left atrial volume index improved from 42.5 to 38.3 mL/m2, indicative of favorable remodeling. The improvement in systolic function was expressed in an increased contractility index.
Moreover, left ventricular end-systolic volume index improved over the course of the year from 84 to 70.5 mL/m2, while left ventricular end-diastolic volume index also improved, from 117.3 to 99.1 mL/m2.
Eighty percent of patients maintained or improved their 6-minute walk distance. On average, patients improved their walk distance by 25 m from a mean baseline of 372 m. Similarly, 80% of patients maintained or improved their baseline New York Heart Association functional class.
Discussant Dr. James C. Fang called the Parachute device “an important investigational therapeutic option. ” He urged cardiologists to enroll patients in PARACHUTE IV in order to learn whether the percutaneous device therapy will indeed enable patients to live longer and/or avoid hospitalizations for heart failure.
The improvements in myocardial contractility and left atrial volume documented in PARACHUTE III are particularly encouraging, added Dr. Fang, professor of internal medicine and chief of the division of cardiovascular medicine at the University of Utah, Salt Lake City.
Dr. Abraham reported serving as a consultant to CardioKinetix, which sponsors the PARACHUTE IV trial.
LAS VEGAS – Percutaneous ventricular restoration using the Parachute device in patients with ischemic dilated cardiomyopathy had a promisingly low 26% combined rate of all-cause mortality and heart failure hospitalization at 1 year in the PARACHUTE III trial, according to Dr. William T. Abraham
PARACHUTE III was a European postmarketing study. And while the composite endpoint of all-cause mortality and heart failure hospitalization was a prespecified secondary outcome in that study, it’s the primary endpoint in the ongoing pivotal U.S. phase III PARACHUTE IV trial headed by Dr. Abraham.
What’s encouraging about the PARACHUTE III result is that the 26% rate of the combined endpoint is in line with the PARACHUTE IV investigators’ assumption for outcomes in the ongoing U.S. trial, which will randomize roughly 500 patients to device therapy plus optimal medical therapy or optimal medical therapy alone, he reported at the annual meeting of the Heart Failure Society of America.
“The PARACHUTE III results are reassuring. In historical controls, the expected rate [of all-cause mortality and heart failure hospitalization] in this kind of population at 1 year is about 35%,” according to Dr. Abraham, professor of internal medicine, physiology, and cell biology and director of the division of cardiovascular medicine at Ohio State University, Columbus.
Plus, technical improvements in device design and percutaneous implantation technique have been made since PARACHUTE III and are incorporated in PARACHUTE IV. These improvements could drive the composite endpoint rate in the ongoing U.S. trial even lower, he continued. For example, while the 1-year major complication rate in the European postmarketing study was 11%, thus far it’s less than 8% in the U.S. experience.
The proprietary Parachute device is so named because, when deployed, it looks like an upside-down parachute. It is intended for patients with ischemic dilated cardiomyopathy secondary to anteroapical MI. The goal of this device therapy is to attenuate or reverse progression of heart failure in this high-risk population.
The Parachute device has two proposed mechanisms of action, the cardiologist explained. One involves ventricular restoration via ventricular partitioning to reduce wall stress in the upper chamber as well as fostering a return to a more normal, elliptical shape to the heart. Dr. Abraham termed the other mechanism a trampoline effect, in which the compliant device encourages replacement of eccentric apical wall motion with more synchronized wall motion throughout the cardiac cycle.
The device, comprised mainly of a fluoropolymer membrane and a nitinol frame, is implanted percutaneously retrograde across the aortic valve. It is placed in the apex of the left ventricle.
The European Union PARACHUTE III postmarketing study was a single-arm study including 100 consecutive symptomatic patients in 10 countries. Their baseline left ventricular ejection fraction was 28%. Device implantation was accomplished in 97 of the 100 patients, with a mean 94-minute procedure duration and 23 minutes of fluoroscopy time.
The primary study endpoint was a safety outcome: the 1-year rate of procedure- and/or device-related adverse events. The 7% rate included several cases of left ventricular perforation as well as aortic or mitral valve damage.
“The rates and distribution of these events are comparable to other structural heart disease interventions, such as transcatheter aortic valve replacement, and might be considered an acceptable postmarketing result,” Dr. Abraham commented.
There was a 3.2% stroke rate through 1 year and a 9.5% mortality rate.
Hemodynamic improvements were seen in both systolic and diastolic function. The left atrial volume index improved from 42.5 to 38.3 mL/m2, indicative of favorable remodeling. The improvement in systolic function was expressed in an increased contractility index.
Moreover, left ventricular end-systolic volume index improved over the course of the year from 84 to 70.5 mL/m2, while left ventricular end-diastolic volume index also improved, from 117.3 to 99.1 mL/m2.
Eighty percent of patients maintained or improved their 6-minute walk distance. On average, patients improved their walk distance by 25 m from a mean baseline of 372 m. Similarly, 80% of patients maintained or improved their baseline New York Heart Association functional class.
Discussant Dr. James C. Fang called the Parachute device “an important investigational therapeutic option. ” He urged cardiologists to enroll patients in PARACHUTE IV in order to learn whether the percutaneous device therapy will indeed enable patients to live longer and/or avoid hospitalizations for heart failure.
The improvements in myocardial contractility and left atrial volume documented in PARACHUTE III are particularly encouraging, added Dr. Fang, professor of internal medicine and chief of the division of cardiovascular medicine at the University of Utah, Salt Lake City.
Dr. Abraham reported serving as a consultant to CardioKinetix, which sponsors the PARACHUTE IV trial.
LAS VEGAS – Percutaneous ventricular restoration using the Parachute device in patients with ischemic dilated cardiomyopathy had a promisingly low 26% combined rate of all-cause mortality and heart failure hospitalization at 1 year in the PARACHUTE III trial, according to Dr. William T. Abraham
PARACHUTE III was a European postmarketing study. And while the composite endpoint of all-cause mortality and heart failure hospitalization was a prespecified secondary outcome in that study, it’s the primary endpoint in the ongoing pivotal U.S. phase III PARACHUTE IV trial headed by Dr. Abraham.
What’s encouraging about the PARACHUTE III result is that the 26% rate of the combined endpoint is in line with the PARACHUTE IV investigators’ assumption for outcomes in the ongoing U.S. trial, which will randomize roughly 500 patients to device therapy plus optimal medical therapy or optimal medical therapy alone, he reported at the annual meeting of the Heart Failure Society of America.
“The PARACHUTE III results are reassuring. In historical controls, the expected rate [of all-cause mortality and heart failure hospitalization] in this kind of population at 1 year is about 35%,” according to Dr. Abraham, professor of internal medicine, physiology, and cell biology and director of the division of cardiovascular medicine at Ohio State University, Columbus.
Plus, technical improvements in device design and percutaneous implantation technique have been made since PARACHUTE III and are incorporated in PARACHUTE IV. These improvements could drive the composite endpoint rate in the ongoing U.S. trial even lower, he continued. For example, while the 1-year major complication rate in the European postmarketing study was 11%, thus far it’s less than 8% in the U.S. experience.
The proprietary Parachute device is so named because, when deployed, it looks like an upside-down parachute. It is intended for patients with ischemic dilated cardiomyopathy secondary to anteroapical MI. The goal of this device therapy is to attenuate or reverse progression of heart failure in this high-risk population.
The Parachute device has two proposed mechanisms of action, the cardiologist explained. One involves ventricular restoration via ventricular partitioning to reduce wall stress in the upper chamber as well as fostering a return to a more normal, elliptical shape to the heart. Dr. Abraham termed the other mechanism a trampoline effect, in which the compliant device encourages replacement of eccentric apical wall motion with more synchronized wall motion throughout the cardiac cycle.
The device, comprised mainly of a fluoropolymer membrane and a nitinol frame, is implanted percutaneously retrograde across the aortic valve. It is placed in the apex of the left ventricle.
The European Union PARACHUTE III postmarketing study was a single-arm study including 100 consecutive symptomatic patients in 10 countries. Their baseline left ventricular ejection fraction was 28%. Device implantation was accomplished in 97 of the 100 patients, with a mean 94-minute procedure duration and 23 minutes of fluoroscopy time.
The primary study endpoint was a safety outcome: the 1-year rate of procedure- and/or device-related adverse events. The 7% rate included several cases of left ventricular perforation as well as aortic or mitral valve damage.
“The rates and distribution of these events are comparable to other structural heart disease interventions, such as transcatheter aortic valve replacement, and might be considered an acceptable postmarketing result,” Dr. Abraham commented.
There was a 3.2% stroke rate through 1 year and a 9.5% mortality rate.
Hemodynamic improvements were seen in both systolic and diastolic function. The left atrial volume index improved from 42.5 to 38.3 mL/m2, indicative of favorable remodeling. The improvement in systolic function was expressed in an increased contractility index.
Moreover, left ventricular end-systolic volume index improved over the course of the year from 84 to 70.5 mL/m2, while left ventricular end-diastolic volume index also improved, from 117.3 to 99.1 mL/m2.
Eighty percent of patients maintained or improved their 6-minute walk distance. On average, patients improved their walk distance by 25 m from a mean baseline of 372 m. Similarly, 80% of patients maintained or improved their baseline New York Heart Association functional class.
Discussant Dr. James C. Fang called the Parachute device “an important investigational therapeutic option. ” He urged cardiologists to enroll patients in PARACHUTE IV in order to learn whether the percutaneous device therapy will indeed enable patients to live longer and/or avoid hospitalizations for heart failure.
The improvements in myocardial contractility and left atrial volume documented in PARACHUTE III are particularly encouraging, added Dr. Fang, professor of internal medicine and chief of the division of cardiovascular medicine at the University of Utah, Salt Lake City.
Dr. Abraham reported serving as a consultant to CardioKinetix, which sponsors the PARACHUTE IV trial.
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: A percutaneous device known as the Parachute, when placed in the apex of the left ventricle, may slow or reverse progression of heart failure in patients with ischemic dilated cardiomyopathy.
Major finding: The 1-year rate of the composite endpoint of all-cause mortality and heart failure hospitalization following Parachute placement was 26%.
Data source: The PARACHUTE III study was a single-arm European postmarketing study involving 100 consecutive symptomatic patients.
Disclosures: PARACHUTE III as well as the ongoing pivotal U.S. PARACHUTE IV study were sponsored by CardioKinetix. The presenter serves as a consultant to the company and principal investigator of PARACHUTE IV.
Phase III outcomes bode well for novel hyperkalemia therapies
LAS VEGAS – Positive phase III results for two novel oral therapies for hyperkalemia received an enthusiastic reception at the annual meeting of the Heart Failure Society of America.
That’s because even though spironolactone and eplerenone (Inspra) have a well-established mortality benefit and a class I indication for the treatment of heart failure with reduced ejection fraction, these renin-angiotensin-aldosterone system (RAAS) inhibitors and mineralocorticoid receptor antagonists remain greatly underutilized on account of their associated substantial risk of hyperkalemia.
“I think the availability of new and safe therapies to treat hyperkalemia and maintain patients on a RAAS inhibitor opens up the possibility of further reductions of cardiovascular events and reductions in health care costs in these very high-risk patients,” said Dr. Bertram Pitt, professor of internal medicine at the University of Michigan, Ann Arbor.
The data we have so far is quite promising,” agreed Dr. Gregg C. Fonarow. “There is a large and unmet need for better treatments for hyperkalemia. Many patients end up on RAAS inhibitor doses below those identified in randomized controlled trials and the major guidelines as the target dose.”
“Moreover, I’m impressed that in the United States we find that two-thirds of patients who have a class I recommendation for a RAAS inhibitor according to the guidelines are not being treated with any dose of these agents at all. So I see a large potential benefit in removing that concern and providing a reliable way of greatly reducing the risk of hyperkalemia. That would really meet a very important unmet need,” according to Dr. Fonarow, professor of medicine, director of the Ahmanson-UCLA Cardiomyopathy Center, and cochief of the cardiology division at the University of California, Los Angeles.
The sole approved agent at present for treating hyperkalemia – sodium polystyrene sulfonate (Kayexalate) – is problematic. Approved by the Food and Drug Administration back in 1958 based upon weak data, this agent is nonselective, unpopular, and poorly tolerated, with loose stools or diarrhea a common complaint.
The two new agents with positive phase III results reported at the meeting are patiromer and sodium zirconium cyclosilicate, also known as ZS-9. Patiromer is a nonabsorbed polymer that exchanges calcium for potassium. Its site of action is the lumen of the colon, where potassium concentration is highest. ZS-9 is a highly stable, inert, insoluble, nonabsorbed, inorganic polymer whose selectivity for potassium is 80- to 125-fold greater than that of sodium polystyrene sulfonate.
Patiromer
Dr. Pitt presented a prespecified subgroup analysis of the pivotal, multinational, phase III randomized trial of patiromer. This analysis, intended for an audience of heart failure specialists, was restricted to patients with chronic kidney disease and hyperkalemia who were on a RAAS inhibitor. In all, 102 of them had heart failure; 141 did not. Chronic kidney disease was severe in 45% of subjects, as evident in their estimated glomerular filtration rate of less than 30 mL/min.
Mean serum potassium dropped by about 1 mEq/L over the course of 4 weeks of therapy, regardless of whether a patient had heart failure or not. The benefit occurred quickly, with a mean drop of 0.5 mEq/L seen during the first 3 days of therapy. At the 4-week mark, three-quarters of patients had a normal-range serum potassium of 3.8 mEq/L to less than 5.1 mEq/L. They were then randomized single-blind to continued patiromer or a switch to placebo for another 4 weeks. Serum potassium climbed in placebo-treated controls by an average of 0.8 mEq/L while remaining unchanged in patients on patiromer. Fifty-two percent of heart failure patients on placebo developed hyperkalemia as defined by a serum potassium level of 5.5 mEq/L or more, compared with 8% on patiromer.
The safety profile of patiromer was essentially that of placebo in the pivotal trial, with two exceptions: 3% of patients on active therapy developed mild, transient hypokalemia which was easily corrected, and 7% experienced mild nausea or diarrhea, Dr. Pitt reported.
Relypsa, the company developing the drug, announced that it submitted a New Drug Application to the Food and Drug Administration for patiromer in October, seeking an indication for hyperkalemia.
ZS-9
The ZS-9 phase III study involved 753 hyperkalemic patients, making it the largest-ever trial of a hyperkalemia therapy. Dr. Mohamed El-Shahawy presented a prespecified subgroup analysis focusing on the 300 participants with heart failure, although he noted that the results were no different in the larger group without heart failure.
During the acute phase of the study, when participants were randomized double-blind to one of four t.i.d. doses of ZS-9 or placebo, serum potassium fell in patients on ZS-9 in a dose-dependent fashion. A reduction in serum potassium was observed as early as 1 hour after the first dose. In the highest-dose group, at 10 g three times daily, mean serum potassium fell from 5.3 mEq/L to 4.5 mEq/L.
In the subsequent extended phase of the study, patients on ZS-9 t.i.d. in the acute phase were rerandomized to once-daily ZS-9 or placebo for 12 days. Patients on ZS-9 at 5 g or 10 g once daily maintained normokalemia, while those switched to placebo became hyperkalemic once again by day 12, according to Dr. El-Shahawy of the Academic Medical Research Institute, Los Angeles.
The side-effect profile of ZS-9 was similar to that of placebo, with no significant hypokalemia or GI side effects being noted, he added.
A large, long-term study of ZS-9 for maintenance of normokalemia is ongoing.
Dr. Fonarow noted that another, entirely different approach to reducing the risk of hyperkalemia involves the development of novel, nonsteroidal, cardioselective mineralocorticoid receptor antagonists.
He cited a promising phase II study of one such agent, known for now as BAY 94-8862. That study was conducted in patients with heart failure and mild to moderate chronic kidney disease. Patients assigned to the novel agent had significantly smaller rises in serum potassium than those randomized to spironolactone. Yet BAY 94-8862 reduced levels of B-type natriuretic peptide, amino-terminal brain natriuretic peptide, and albuminuria at least as much as did spironolactone, and with significantly lower rates of worsening renal failure (Eur. Heart J. 2013;34:2453-63).
Once new and better therapies for hyperkalemia enter clinical practice, Dr. Fonarow said, the priorities will be to get patients on RAAS inhibitor therapy who should be on it but now aren’t, as well as to augment dosing up to target levels in those now on suboptimal doses. But access to improved treatments for hyperkalemia also opens the door to another intriguing possibility.
“Could the ability to raise doses of RAAS inhibitors even higher than current target doses without causing hyperkalemia further enhance remodeling and improve clinical outcomes? That could indeed be the case. It’s something we’ll want to test in prospective trials,” he said.
Dr. Fonarow reported serving as a consultant to and/or advisory board member for Medtronic, Novartis, Johnson & Johnson, Bayer, and Amgen. Dr. El-Shahawy is on an advisory board for ZS Pharma and has received research grants from that company as well as Amgen, GlaxoSmithKline, Celgene, and Abbvie. Dr. Pitt reported serving as a consultant to Pfizer, Bayer, Lilly, Relypsa, and Sarfez.
LAS VEGAS – Positive phase III results for two novel oral therapies for hyperkalemia received an enthusiastic reception at the annual meeting of the Heart Failure Society of America.
That’s because even though spironolactone and eplerenone (Inspra) have a well-established mortality benefit and a class I indication for the treatment of heart failure with reduced ejection fraction, these renin-angiotensin-aldosterone system (RAAS) inhibitors and mineralocorticoid receptor antagonists remain greatly underutilized on account of their associated substantial risk of hyperkalemia.
“I think the availability of new and safe therapies to treat hyperkalemia and maintain patients on a RAAS inhibitor opens up the possibility of further reductions of cardiovascular events and reductions in health care costs in these very high-risk patients,” said Dr. Bertram Pitt, professor of internal medicine at the University of Michigan, Ann Arbor.
The data we have so far is quite promising,” agreed Dr. Gregg C. Fonarow. “There is a large and unmet need for better treatments for hyperkalemia. Many patients end up on RAAS inhibitor doses below those identified in randomized controlled trials and the major guidelines as the target dose.”
“Moreover, I’m impressed that in the United States we find that two-thirds of patients who have a class I recommendation for a RAAS inhibitor according to the guidelines are not being treated with any dose of these agents at all. So I see a large potential benefit in removing that concern and providing a reliable way of greatly reducing the risk of hyperkalemia. That would really meet a very important unmet need,” according to Dr. Fonarow, professor of medicine, director of the Ahmanson-UCLA Cardiomyopathy Center, and cochief of the cardiology division at the University of California, Los Angeles.
The sole approved agent at present for treating hyperkalemia – sodium polystyrene sulfonate (Kayexalate) – is problematic. Approved by the Food and Drug Administration back in 1958 based upon weak data, this agent is nonselective, unpopular, and poorly tolerated, with loose stools or diarrhea a common complaint.
The two new agents with positive phase III results reported at the meeting are patiromer and sodium zirconium cyclosilicate, also known as ZS-9. Patiromer is a nonabsorbed polymer that exchanges calcium for potassium. Its site of action is the lumen of the colon, where potassium concentration is highest. ZS-9 is a highly stable, inert, insoluble, nonabsorbed, inorganic polymer whose selectivity for potassium is 80- to 125-fold greater than that of sodium polystyrene sulfonate.
Patiromer
Dr. Pitt presented a prespecified subgroup analysis of the pivotal, multinational, phase III randomized trial of patiromer. This analysis, intended for an audience of heart failure specialists, was restricted to patients with chronic kidney disease and hyperkalemia who were on a RAAS inhibitor. In all, 102 of them had heart failure; 141 did not. Chronic kidney disease was severe in 45% of subjects, as evident in their estimated glomerular filtration rate of less than 30 mL/min.
Mean serum potassium dropped by about 1 mEq/L over the course of 4 weeks of therapy, regardless of whether a patient had heart failure or not. The benefit occurred quickly, with a mean drop of 0.5 mEq/L seen during the first 3 days of therapy. At the 4-week mark, three-quarters of patients had a normal-range serum potassium of 3.8 mEq/L to less than 5.1 mEq/L. They were then randomized single-blind to continued patiromer or a switch to placebo for another 4 weeks. Serum potassium climbed in placebo-treated controls by an average of 0.8 mEq/L while remaining unchanged in patients on patiromer. Fifty-two percent of heart failure patients on placebo developed hyperkalemia as defined by a serum potassium level of 5.5 mEq/L or more, compared with 8% on patiromer.
The safety profile of patiromer was essentially that of placebo in the pivotal trial, with two exceptions: 3% of patients on active therapy developed mild, transient hypokalemia which was easily corrected, and 7% experienced mild nausea or diarrhea, Dr. Pitt reported.
Relypsa, the company developing the drug, announced that it submitted a New Drug Application to the Food and Drug Administration for patiromer in October, seeking an indication for hyperkalemia.
ZS-9
The ZS-9 phase III study involved 753 hyperkalemic patients, making it the largest-ever trial of a hyperkalemia therapy. Dr. Mohamed El-Shahawy presented a prespecified subgroup analysis focusing on the 300 participants with heart failure, although he noted that the results were no different in the larger group without heart failure.
During the acute phase of the study, when participants were randomized double-blind to one of four t.i.d. doses of ZS-9 or placebo, serum potassium fell in patients on ZS-9 in a dose-dependent fashion. A reduction in serum potassium was observed as early as 1 hour after the first dose. In the highest-dose group, at 10 g three times daily, mean serum potassium fell from 5.3 mEq/L to 4.5 mEq/L.
In the subsequent extended phase of the study, patients on ZS-9 t.i.d. in the acute phase were rerandomized to once-daily ZS-9 or placebo for 12 days. Patients on ZS-9 at 5 g or 10 g once daily maintained normokalemia, while those switched to placebo became hyperkalemic once again by day 12, according to Dr. El-Shahawy of the Academic Medical Research Institute, Los Angeles.
The side-effect profile of ZS-9 was similar to that of placebo, with no significant hypokalemia or GI side effects being noted, he added.
A large, long-term study of ZS-9 for maintenance of normokalemia is ongoing.
Dr. Fonarow noted that another, entirely different approach to reducing the risk of hyperkalemia involves the development of novel, nonsteroidal, cardioselective mineralocorticoid receptor antagonists.
He cited a promising phase II study of one such agent, known for now as BAY 94-8862. That study was conducted in patients with heart failure and mild to moderate chronic kidney disease. Patients assigned to the novel agent had significantly smaller rises in serum potassium than those randomized to spironolactone. Yet BAY 94-8862 reduced levels of B-type natriuretic peptide, amino-terminal brain natriuretic peptide, and albuminuria at least as much as did spironolactone, and with significantly lower rates of worsening renal failure (Eur. Heart J. 2013;34:2453-63).
Once new and better therapies for hyperkalemia enter clinical practice, Dr. Fonarow said, the priorities will be to get patients on RAAS inhibitor therapy who should be on it but now aren’t, as well as to augment dosing up to target levels in those now on suboptimal doses. But access to improved treatments for hyperkalemia also opens the door to another intriguing possibility.
“Could the ability to raise doses of RAAS inhibitors even higher than current target doses without causing hyperkalemia further enhance remodeling and improve clinical outcomes? That could indeed be the case. It’s something we’ll want to test in prospective trials,” he said.
Dr. Fonarow reported serving as a consultant to and/or advisory board member for Medtronic, Novartis, Johnson & Johnson, Bayer, and Amgen. Dr. El-Shahawy is on an advisory board for ZS Pharma and has received research grants from that company as well as Amgen, GlaxoSmithKline, Celgene, and Abbvie. Dr. Pitt reported serving as a consultant to Pfizer, Bayer, Lilly, Relypsa, and Sarfez.
LAS VEGAS – Positive phase III results for two novel oral therapies for hyperkalemia received an enthusiastic reception at the annual meeting of the Heart Failure Society of America.
That’s because even though spironolactone and eplerenone (Inspra) have a well-established mortality benefit and a class I indication for the treatment of heart failure with reduced ejection fraction, these renin-angiotensin-aldosterone system (RAAS) inhibitors and mineralocorticoid receptor antagonists remain greatly underutilized on account of their associated substantial risk of hyperkalemia.
“I think the availability of new and safe therapies to treat hyperkalemia and maintain patients on a RAAS inhibitor opens up the possibility of further reductions of cardiovascular events and reductions in health care costs in these very high-risk patients,” said Dr. Bertram Pitt, professor of internal medicine at the University of Michigan, Ann Arbor.
The data we have so far is quite promising,” agreed Dr. Gregg C. Fonarow. “There is a large and unmet need for better treatments for hyperkalemia. Many patients end up on RAAS inhibitor doses below those identified in randomized controlled trials and the major guidelines as the target dose.”
“Moreover, I’m impressed that in the United States we find that two-thirds of patients who have a class I recommendation for a RAAS inhibitor according to the guidelines are not being treated with any dose of these agents at all. So I see a large potential benefit in removing that concern and providing a reliable way of greatly reducing the risk of hyperkalemia. That would really meet a very important unmet need,” according to Dr. Fonarow, professor of medicine, director of the Ahmanson-UCLA Cardiomyopathy Center, and cochief of the cardiology division at the University of California, Los Angeles.
The sole approved agent at present for treating hyperkalemia – sodium polystyrene sulfonate (Kayexalate) – is problematic. Approved by the Food and Drug Administration back in 1958 based upon weak data, this agent is nonselective, unpopular, and poorly tolerated, with loose stools or diarrhea a common complaint.
The two new agents with positive phase III results reported at the meeting are patiromer and sodium zirconium cyclosilicate, also known as ZS-9. Patiromer is a nonabsorbed polymer that exchanges calcium for potassium. Its site of action is the lumen of the colon, where potassium concentration is highest. ZS-9 is a highly stable, inert, insoluble, nonabsorbed, inorganic polymer whose selectivity for potassium is 80- to 125-fold greater than that of sodium polystyrene sulfonate.
Patiromer
Dr. Pitt presented a prespecified subgroup analysis of the pivotal, multinational, phase III randomized trial of patiromer. This analysis, intended for an audience of heart failure specialists, was restricted to patients with chronic kidney disease and hyperkalemia who were on a RAAS inhibitor. In all, 102 of them had heart failure; 141 did not. Chronic kidney disease was severe in 45% of subjects, as evident in their estimated glomerular filtration rate of less than 30 mL/min.
Mean serum potassium dropped by about 1 mEq/L over the course of 4 weeks of therapy, regardless of whether a patient had heart failure or not. The benefit occurred quickly, with a mean drop of 0.5 mEq/L seen during the first 3 days of therapy. At the 4-week mark, three-quarters of patients had a normal-range serum potassium of 3.8 mEq/L to less than 5.1 mEq/L. They were then randomized single-blind to continued patiromer or a switch to placebo for another 4 weeks. Serum potassium climbed in placebo-treated controls by an average of 0.8 mEq/L while remaining unchanged in patients on patiromer. Fifty-two percent of heart failure patients on placebo developed hyperkalemia as defined by a serum potassium level of 5.5 mEq/L or more, compared with 8% on patiromer.
The safety profile of patiromer was essentially that of placebo in the pivotal trial, with two exceptions: 3% of patients on active therapy developed mild, transient hypokalemia which was easily corrected, and 7% experienced mild nausea or diarrhea, Dr. Pitt reported.
Relypsa, the company developing the drug, announced that it submitted a New Drug Application to the Food and Drug Administration for patiromer in October, seeking an indication for hyperkalemia.
ZS-9
The ZS-9 phase III study involved 753 hyperkalemic patients, making it the largest-ever trial of a hyperkalemia therapy. Dr. Mohamed El-Shahawy presented a prespecified subgroup analysis focusing on the 300 participants with heart failure, although he noted that the results were no different in the larger group without heart failure.
During the acute phase of the study, when participants were randomized double-blind to one of four t.i.d. doses of ZS-9 or placebo, serum potassium fell in patients on ZS-9 in a dose-dependent fashion. A reduction in serum potassium was observed as early as 1 hour after the first dose. In the highest-dose group, at 10 g three times daily, mean serum potassium fell from 5.3 mEq/L to 4.5 mEq/L.
In the subsequent extended phase of the study, patients on ZS-9 t.i.d. in the acute phase were rerandomized to once-daily ZS-9 or placebo for 12 days. Patients on ZS-9 at 5 g or 10 g once daily maintained normokalemia, while those switched to placebo became hyperkalemic once again by day 12, according to Dr. El-Shahawy of the Academic Medical Research Institute, Los Angeles.
The side-effect profile of ZS-9 was similar to that of placebo, with no significant hypokalemia or GI side effects being noted, he added.
A large, long-term study of ZS-9 for maintenance of normokalemia is ongoing.
Dr. Fonarow noted that another, entirely different approach to reducing the risk of hyperkalemia involves the development of novel, nonsteroidal, cardioselective mineralocorticoid receptor antagonists.
He cited a promising phase II study of one such agent, known for now as BAY 94-8862. That study was conducted in patients with heart failure and mild to moderate chronic kidney disease. Patients assigned to the novel agent had significantly smaller rises in serum potassium than those randomized to spironolactone. Yet BAY 94-8862 reduced levels of B-type natriuretic peptide, amino-terminal brain natriuretic peptide, and albuminuria at least as much as did spironolactone, and with significantly lower rates of worsening renal failure (Eur. Heart J. 2013;34:2453-63).
Once new and better therapies for hyperkalemia enter clinical practice, Dr. Fonarow said, the priorities will be to get patients on RAAS inhibitor therapy who should be on it but now aren’t, as well as to augment dosing up to target levels in those now on suboptimal doses. But access to improved treatments for hyperkalemia also opens the door to another intriguing possibility.
“Could the ability to raise doses of RAAS inhibitors even higher than current target doses without causing hyperkalemia further enhance remodeling and improve clinical outcomes? That could indeed be the case. It’s something we’ll want to test in prospective trials,” he said.
Dr. Fonarow reported serving as a consultant to and/or advisory board member for Medtronic, Novartis, Johnson & Johnson, Bayer, and Amgen. Dr. El-Shahawy is on an advisory board for ZS Pharma and has received research grants from that company as well as Amgen, GlaxoSmithKline, Celgene, and Abbvie. Dr. Pitt reported serving as a consultant to Pfizer, Bayer, Lilly, Relypsa, and Sarfez.
EXPERT ANALYSIS FROM THE HFSA ANNUAL SCIENTIFIC MEETING
Who will get cancer treatment-induced cardiotoxicity?
LAS VEGAS – The search is on for predictors of which cancer patients will experience treatment-induced cardiotoxicity, and an initial report from the PREDICT study has identified several.
One predictor is the patient’s type of cancer. In PREDICT, patients with lymphoma had a twofold greater risk of developing treatment-related cardiotoxicity, compared with those with breast cancer. Moreover, those with a cancer diagnosis other than lymphoma or breast cancer had a fivefold greater risk than breast cancer patients, Dr. Daniel J. Lenihan reported at the annual meeting of the Heart Failure Society of America.
“It’s important to know that many of the studies on cardiotoxicity risk have been done in breast cancer patients. There are a lot of other cancer patients out there,” observed Dr. Lenihan, professor of medicine and director of clinical research in the division of cardiovascular medicine at Vanderbilt University in Nashville.
PREDICT is a prospective, community-based study of 597 cancer patients undergoing anthracycline-based chemotherapy in 24 community oncology programs. It is primarily a study of the effectiveness of using cardiac biomarkers to predict cardiotoxicity, along with an analysis of the results of various forms of treatment of the cardiotoxicity.
During up to 12 months of follow-up 11% of PREDICT participants experienced a cardiac event, most commonly symptomatic heart failure or a greater than 10% drop in left ventricular ejection fraction, which took a patient from normal range to below normal. Another impressive finding was the substantial burden of conventional cardiovascular risk factors present at baseline in patients scheduled for anthracycline-based chemotherapy. In a multivariate logistic regression analysis, the higher a cancer patient’s cardiovascular risk factor level, the greater the likelihood of chemotherapy-related cardiotoxicity.
A baseline B-type natriuretic peptide (BNP) level in excess of 100 pg/mL was a powerful predictor of a chemotherapy-related cardiac event, with an associated 2.1-fold increased risk. As a predictor of cardiotoxicity during the study period, baseline BNP had a sensitivity of 35%, a specificity of 85%, a positive predictive value of 22%, and – most importantly – a negative predictive value of 92%, according to Dr. Lenihan.
Similarly, using as cutoffs either a baseline BNP greater than 100 pg/mL or a troponin greater than 0.05 ng/mL had a sensitivity of 60%, a specificity of 50%, a positive predictive value of 13%, and a negative predictive value of 91%, he continued.
Myocardial imaging as a tool for predicting which patients will develop cardiotoxicity during or after cancer therapy is another active area of investigation. Other investigators have shown that myocardial strain imaging holds considerable promise (J. Am. Coll. Cardiol. 2014;63:2751-68); however, it’s not terribly practical because many echocardiography laboratories balk at the idea of routinely performing serial strain imaging studies in all cancer patients, Dr. Lenihan said.
Practical predictors of increased risk for cancer therapy-related cardiotoxicity are sorely needed in order to identify candidates for prophylaxis with an agent such as dexrazoxane, which has been shown in a meta-analysis to reduce the risk of clinical or subclinical heart failure by 71% (Cochrane Database Syst. Review 2008; April 16:CD003917).
Another promising preventive approach was displayed in the Spanish OVERCOME trial, involving 90 patients undergoing intensive chemotherapy for malignant hemopathies. Those randomized to combined prophylaxis with enalapril plus carvedilol had a 6.7% rate of the composite endpoint of death, heart failure, or an LVEF below 45% at 6 months, compared with 24.4% in controls (J. Am. Coll. Cardiol. 2013;61:2355-62).
Also, predictors of increased risk are helpful in identifying cancer therapy–related cardiotoxicity early in its course, when aggressive treatment with standard heart failure medications such as beta blockers and ACE inhibitors is most likely to be beneficial.
“Everybody in cardiology is used to the concept that time is muscle: don’t let ischemia persist. Have a strategy to resolve it as soon as possible. That paradigm really can also apply to chemotherapy-related injury: the longer you leave it alone, the more permanent it becomes. Being able to detect it at its earliest stage is critically important,” according to Dr. Lenihan.
He cited as an example a study of 201 consecutive patients with anthracycline-induced cardiomyopathy conducted at the European Institute of Oncology in Milan. The conventional dogma is that anthracycline-induced cardiomyopathy is typically permanent, but this study showed that’s not true.
When treatment with enalapril and carvedilol was initiated within the first couple of months following the end of chemotherapy, 64% of patients experienced complete recovery of their LVEF. When the heart failure medications were commenced 3-4 months after completing chemotherapy, the LVEF recovery rate dropped to 28%. No complete recovery of LVEF occurred in patients who began enalapril plus carvedilol after 6 months (J. Am. Coll. Cardiol. 2010;55:213-20).
“There is a real opportunity here to improve the care of cancer patients and prevent heart failure,” Dr. Lenihan concluded.
He reported receiving research support from Singulex, Millenium, and Acorda as well as serving as a consultant to Onyx and Roche.
LAS VEGAS – The search is on for predictors of which cancer patients will experience treatment-induced cardiotoxicity, and an initial report from the PREDICT study has identified several.
One predictor is the patient’s type of cancer. In PREDICT, patients with lymphoma had a twofold greater risk of developing treatment-related cardiotoxicity, compared with those with breast cancer. Moreover, those with a cancer diagnosis other than lymphoma or breast cancer had a fivefold greater risk than breast cancer patients, Dr. Daniel J. Lenihan reported at the annual meeting of the Heart Failure Society of America.
“It’s important to know that many of the studies on cardiotoxicity risk have been done in breast cancer patients. There are a lot of other cancer patients out there,” observed Dr. Lenihan, professor of medicine and director of clinical research in the division of cardiovascular medicine at Vanderbilt University in Nashville.
PREDICT is a prospective, community-based study of 597 cancer patients undergoing anthracycline-based chemotherapy in 24 community oncology programs. It is primarily a study of the effectiveness of using cardiac biomarkers to predict cardiotoxicity, along with an analysis of the results of various forms of treatment of the cardiotoxicity.
During up to 12 months of follow-up 11% of PREDICT participants experienced a cardiac event, most commonly symptomatic heart failure or a greater than 10% drop in left ventricular ejection fraction, which took a patient from normal range to below normal. Another impressive finding was the substantial burden of conventional cardiovascular risk factors present at baseline in patients scheduled for anthracycline-based chemotherapy. In a multivariate logistic regression analysis, the higher a cancer patient’s cardiovascular risk factor level, the greater the likelihood of chemotherapy-related cardiotoxicity.
A baseline B-type natriuretic peptide (BNP) level in excess of 100 pg/mL was a powerful predictor of a chemotherapy-related cardiac event, with an associated 2.1-fold increased risk. As a predictor of cardiotoxicity during the study period, baseline BNP had a sensitivity of 35%, a specificity of 85%, a positive predictive value of 22%, and – most importantly – a negative predictive value of 92%, according to Dr. Lenihan.
Similarly, using as cutoffs either a baseline BNP greater than 100 pg/mL or a troponin greater than 0.05 ng/mL had a sensitivity of 60%, a specificity of 50%, a positive predictive value of 13%, and a negative predictive value of 91%, he continued.
Myocardial imaging as a tool for predicting which patients will develop cardiotoxicity during or after cancer therapy is another active area of investigation. Other investigators have shown that myocardial strain imaging holds considerable promise (J. Am. Coll. Cardiol. 2014;63:2751-68); however, it’s not terribly practical because many echocardiography laboratories balk at the idea of routinely performing serial strain imaging studies in all cancer patients, Dr. Lenihan said.
Practical predictors of increased risk for cancer therapy-related cardiotoxicity are sorely needed in order to identify candidates for prophylaxis with an agent such as dexrazoxane, which has been shown in a meta-analysis to reduce the risk of clinical or subclinical heart failure by 71% (Cochrane Database Syst. Review 2008; April 16:CD003917).
Another promising preventive approach was displayed in the Spanish OVERCOME trial, involving 90 patients undergoing intensive chemotherapy for malignant hemopathies. Those randomized to combined prophylaxis with enalapril plus carvedilol had a 6.7% rate of the composite endpoint of death, heart failure, or an LVEF below 45% at 6 months, compared with 24.4% in controls (J. Am. Coll. Cardiol. 2013;61:2355-62).
Also, predictors of increased risk are helpful in identifying cancer therapy–related cardiotoxicity early in its course, when aggressive treatment with standard heart failure medications such as beta blockers and ACE inhibitors is most likely to be beneficial.
“Everybody in cardiology is used to the concept that time is muscle: don’t let ischemia persist. Have a strategy to resolve it as soon as possible. That paradigm really can also apply to chemotherapy-related injury: the longer you leave it alone, the more permanent it becomes. Being able to detect it at its earliest stage is critically important,” according to Dr. Lenihan.
He cited as an example a study of 201 consecutive patients with anthracycline-induced cardiomyopathy conducted at the European Institute of Oncology in Milan. The conventional dogma is that anthracycline-induced cardiomyopathy is typically permanent, but this study showed that’s not true.
When treatment with enalapril and carvedilol was initiated within the first couple of months following the end of chemotherapy, 64% of patients experienced complete recovery of their LVEF. When the heart failure medications were commenced 3-4 months after completing chemotherapy, the LVEF recovery rate dropped to 28%. No complete recovery of LVEF occurred in patients who began enalapril plus carvedilol after 6 months (J. Am. Coll. Cardiol. 2010;55:213-20).
“There is a real opportunity here to improve the care of cancer patients and prevent heart failure,” Dr. Lenihan concluded.
He reported receiving research support from Singulex, Millenium, and Acorda as well as serving as a consultant to Onyx and Roche.
LAS VEGAS – The search is on for predictors of which cancer patients will experience treatment-induced cardiotoxicity, and an initial report from the PREDICT study has identified several.
One predictor is the patient’s type of cancer. In PREDICT, patients with lymphoma had a twofold greater risk of developing treatment-related cardiotoxicity, compared with those with breast cancer. Moreover, those with a cancer diagnosis other than lymphoma or breast cancer had a fivefold greater risk than breast cancer patients, Dr. Daniel J. Lenihan reported at the annual meeting of the Heart Failure Society of America.
“It’s important to know that many of the studies on cardiotoxicity risk have been done in breast cancer patients. There are a lot of other cancer patients out there,” observed Dr. Lenihan, professor of medicine and director of clinical research in the division of cardiovascular medicine at Vanderbilt University in Nashville.
PREDICT is a prospective, community-based study of 597 cancer patients undergoing anthracycline-based chemotherapy in 24 community oncology programs. It is primarily a study of the effectiveness of using cardiac biomarkers to predict cardiotoxicity, along with an analysis of the results of various forms of treatment of the cardiotoxicity.
During up to 12 months of follow-up 11% of PREDICT participants experienced a cardiac event, most commonly symptomatic heart failure or a greater than 10% drop in left ventricular ejection fraction, which took a patient from normal range to below normal. Another impressive finding was the substantial burden of conventional cardiovascular risk factors present at baseline in patients scheduled for anthracycline-based chemotherapy. In a multivariate logistic regression analysis, the higher a cancer patient’s cardiovascular risk factor level, the greater the likelihood of chemotherapy-related cardiotoxicity.
A baseline B-type natriuretic peptide (BNP) level in excess of 100 pg/mL was a powerful predictor of a chemotherapy-related cardiac event, with an associated 2.1-fold increased risk. As a predictor of cardiotoxicity during the study period, baseline BNP had a sensitivity of 35%, a specificity of 85%, a positive predictive value of 22%, and – most importantly – a negative predictive value of 92%, according to Dr. Lenihan.
Similarly, using as cutoffs either a baseline BNP greater than 100 pg/mL or a troponin greater than 0.05 ng/mL had a sensitivity of 60%, a specificity of 50%, a positive predictive value of 13%, and a negative predictive value of 91%, he continued.
Myocardial imaging as a tool for predicting which patients will develop cardiotoxicity during or after cancer therapy is another active area of investigation. Other investigators have shown that myocardial strain imaging holds considerable promise (J. Am. Coll. Cardiol. 2014;63:2751-68); however, it’s not terribly practical because many echocardiography laboratories balk at the idea of routinely performing serial strain imaging studies in all cancer patients, Dr. Lenihan said.
Practical predictors of increased risk for cancer therapy-related cardiotoxicity are sorely needed in order to identify candidates for prophylaxis with an agent such as dexrazoxane, which has been shown in a meta-analysis to reduce the risk of clinical or subclinical heart failure by 71% (Cochrane Database Syst. Review 2008; April 16:CD003917).
Another promising preventive approach was displayed in the Spanish OVERCOME trial, involving 90 patients undergoing intensive chemotherapy for malignant hemopathies. Those randomized to combined prophylaxis with enalapril plus carvedilol had a 6.7% rate of the composite endpoint of death, heart failure, or an LVEF below 45% at 6 months, compared with 24.4% in controls (J. Am. Coll. Cardiol. 2013;61:2355-62).
Also, predictors of increased risk are helpful in identifying cancer therapy–related cardiotoxicity early in its course, when aggressive treatment with standard heart failure medications such as beta blockers and ACE inhibitors is most likely to be beneficial.
“Everybody in cardiology is used to the concept that time is muscle: don’t let ischemia persist. Have a strategy to resolve it as soon as possible. That paradigm really can also apply to chemotherapy-related injury: the longer you leave it alone, the more permanent it becomes. Being able to detect it at its earliest stage is critically important,” according to Dr. Lenihan.
He cited as an example a study of 201 consecutive patients with anthracycline-induced cardiomyopathy conducted at the European Institute of Oncology in Milan. The conventional dogma is that anthracycline-induced cardiomyopathy is typically permanent, but this study showed that’s not true.
When treatment with enalapril and carvedilol was initiated within the first couple of months following the end of chemotherapy, 64% of patients experienced complete recovery of their LVEF. When the heart failure medications were commenced 3-4 months after completing chemotherapy, the LVEF recovery rate dropped to 28%. No complete recovery of LVEF occurred in patients who began enalapril plus carvedilol after 6 months (J. Am. Coll. Cardiol. 2010;55:213-20).
“There is a real opportunity here to improve the care of cancer patients and prevent heart failure,” Dr. Lenihan concluded.
He reported receiving research support from Singulex, Millenium, and Acorda as well as serving as a consultant to Onyx and Roche.
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: An elevated baseline B-type natriuretic peptide or troponin level identifies cancer patients at increased risk of cardiotoxicity during anthracycline-based chemotherapy.
Major finding: Cancer patients with a BNP level greater than 100 pg/mL prior to going on anthracycline-based chemotherapy were 2.1-fold more likely to develop treatment-related cardiotoxicity.
Data source: The prospective PREDICT study includes roughly 600 patients undergoing anthracycline-based chemotherapy at 24 community cancer centers.
Disclosures: The study is sponsored by M.D. Anderson Cancer Center. The presenter reported serving as a consultant to Onyx and Roche.
Cancer treatment–induced cardiotoxicity greatly underappreciated
LAS VEGAS – When it comes to discussing the cardiotoxicity of cancer pharmacotherapies, it often seems that oncologists and cardiologists are on different planets.
“You will see wildly different toxicity numbers. … One trial will report 3% cardiotoxicity; another studying the same regimen will report 30%. It’s usually because they’re using vastly different definitions of toxicity,” Dr. Ronald M. Witteles said at the annual meeting of the Heart Failure Society of America.
“The typical clinical trial definition of a cardiac event in a cancer treatment trial is new symptomatic NYHA class III or IV heart failure or cardiac death; that’s it,” added Dr. Witteles, a cardiologist at Stanford (Calif.) University.
The consensus within the emerging field of cardio-oncology is that that simply isn’t good enough. A reasonable definition of cancer treatment–related cardiotoxicity must be far broader, encompassing a drop in left ventricular ejection fraction (LVEF) of greater than 10% from normal-range pretreatment to below the threshold of normal, regardless of whether that decline is accompanied by symptoms, as well as the development of an acute coronary syndrome or symptomatic arrhythmia, according to Dr. Daniel J. Lenihan, president of the International Cardioncology Society, an organization devoted to closer collaboration between the two medical specialties.
“Development of cardiomyopathy during cancer therapy is a lot more common than most people think,” emphasized Dr. Lenihan, professor of medicine and director of clinical research in the division of cardiovascular medicine at Vanderbilt University, Nashville, Tenn.
Dr. Witteles noted that the product labeling for doxorubicin quotes a preposterously low 1%-2% probability of developing impaired myocardial function at a total cumulative dose of 300 mg/m2, which is what’s typically used in treating lymphoma. That’s because the primary source of that estimate is 35-year-old data in which there was no routine measurement of LVEF and the diagnosis of impaired myocardial function was made by the treating oncologist on the basis of clinical signs and symptoms.
There are far better data available, but they’re not in the product label. Dr. Witteles cited as an example a more recent study featuring routine LVEF monitoring, where the incidence of a greater than 10% fall from normal to below normal at a cumulative dose of 300 mg/m2 wasn’t 1%-2%; it was 16% (Cancer 2003;97:2869-79).
He added that current evidence indicates that a fall in LVEF of more than 10%, taking a patient from normal range to below normal, occurs in 7%-8% of patients on an anthracycline such as doxorubicin at a cumulative dose of 240 mg/m2, as is typical in treating breast cancer. The incidence with trastuzumab (Herceptin) when prescribed after completion of anthracycline-based chemotherapy is about 25%; however, when trastuzumab is given in an anthracycline-free regimen, the rate drops to 9%-10%. A study by Dr. Witteles and his coworkers pegged the rate in cancer patients treated with sunitinib (Sutent) or other tyrosine kinase inhibitors at about 15% (JACC Heart Fail. 2013;1:72-8).
“Any way you slice it, that’s a whole lot of people,” he commented.
Dr. Lenihan reported receiving research support from Singulex, Millenium, and Acorda as well as serving as a consultant to Onyx and Roche. Dr. Witteles declared having no financial conflicts.
LAS VEGAS – When it comes to discussing the cardiotoxicity of cancer pharmacotherapies, it often seems that oncologists and cardiologists are on different planets.
“You will see wildly different toxicity numbers. … One trial will report 3% cardiotoxicity; another studying the same regimen will report 30%. It’s usually because they’re using vastly different definitions of toxicity,” Dr. Ronald M. Witteles said at the annual meeting of the Heart Failure Society of America.
“The typical clinical trial definition of a cardiac event in a cancer treatment trial is new symptomatic NYHA class III or IV heart failure or cardiac death; that’s it,” added Dr. Witteles, a cardiologist at Stanford (Calif.) University.
The consensus within the emerging field of cardio-oncology is that that simply isn’t good enough. A reasonable definition of cancer treatment–related cardiotoxicity must be far broader, encompassing a drop in left ventricular ejection fraction (LVEF) of greater than 10% from normal-range pretreatment to below the threshold of normal, regardless of whether that decline is accompanied by symptoms, as well as the development of an acute coronary syndrome or symptomatic arrhythmia, according to Dr. Daniel J. Lenihan, president of the International Cardioncology Society, an organization devoted to closer collaboration between the two medical specialties.
“Development of cardiomyopathy during cancer therapy is a lot more common than most people think,” emphasized Dr. Lenihan, professor of medicine and director of clinical research in the division of cardiovascular medicine at Vanderbilt University, Nashville, Tenn.
Dr. Witteles noted that the product labeling for doxorubicin quotes a preposterously low 1%-2% probability of developing impaired myocardial function at a total cumulative dose of 300 mg/m2, which is what’s typically used in treating lymphoma. That’s because the primary source of that estimate is 35-year-old data in which there was no routine measurement of LVEF and the diagnosis of impaired myocardial function was made by the treating oncologist on the basis of clinical signs and symptoms.
There are far better data available, but they’re not in the product label. Dr. Witteles cited as an example a more recent study featuring routine LVEF monitoring, where the incidence of a greater than 10% fall from normal to below normal at a cumulative dose of 300 mg/m2 wasn’t 1%-2%; it was 16% (Cancer 2003;97:2869-79).
He added that current evidence indicates that a fall in LVEF of more than 10%, taking a patient from normal range to below normal, occurs in 7%-8% of patients on an anthracycline such as doxorubicin at a cumulative dose of 240 mg/m2, as is typical in treating breast cancer. The incidence with trastuzumab (Herceptin) when prescribed after completion of anthracycline-based chemotherapy is about 25%; however, when trastuzumab is given in an anthracycline-free regimen, the rate drops to 9%-10%. A study by Dr. Witteles and his coworkers pegged the rate in cancer patients treated with sunitinib (Sutent) or other tyrosine kinase inhibitors at about 15% (JACC Heart Fail. 2013;1:72-8).
“Any way you slice it, that’s a whole lot of people,” he commented.
Dr. Lenihan reported receiving research support from Singulex, Millenium, and Acorda as well as serving as a consultant to Onyx and Roche. Dr. Witteles declared having no financial conflicts.
LAS VEGAS – When it comes to discussing the cardiotoxicity of cancer pharmacotherapies, it often seems that oncologists and cardiologists are on different planets.
“You will see wildly different toxicity numbers. … One trial will report 3% cardiotoxicity; another studying the same regimen will report 30%. It’s usually because they’re using vastly different definitions of toxicity,” Dr. Ronald M. Witteles said at the annual meeting of the Heart Failure Society of America.
“The typical clinical trial definition of a cardiac event in a cancer treatment trial is new symptomatic NYHA class III or IV heart failure or cardiac death; that’s it,” added Dr. Witteles, a cardiologist at Stanford (Calif.) University.
The consensus within the emerging field of cardio-oncology is that that simply isn’t good enough. A reasonable definition of cancer treatment–related cardiotoxicity must be far broader, encompassing a drop in left ventricular ejection fraction (LVEF) of greater than 10% from normal-range pretreatment to below the threshold of normal, regardless of whether that decline is accompanied by symptoms, as well as the development of an acute coronary syndrome or symptomatic arrhythmia, according to Dr. Daniel J. Lenihan, president of the International Cardioncology Society, an organization devoted to closer collaboration between the two medical specialties.
“Development of cardiomyopathy during cancer therapy is a lot more common than most people think,” emphasized Dr. Lenihan, professor of medicine and director of clinical research in the division of cardiovascular medicine at Vanderbilt University, Nashville, Tenn.
Dr. Witteles noted that the product labeling for doxorubicin quotes a preposterously low 1%-2% probability of developing impaired myocardial function at a total cumulative dose of 300 mg/m2, which is what’s typically used in treating lymphoma. That’s because the primary source of that estimate is 35-year-old data in which there was no routine measurement of LVEF and the diagnosis of impaired myocardial function was made by the treating oncologist on the basis of clinical signs and symptoms.
There are far better data available, but they’re not in the product label. Dr. Witteles cited as an example a more recent study featuring routine LVEF monitoring, where the incidence of a greater than 10% fall from normal to below normal at a cumulative dose of 300 mg/m2 wasn’t 1%-2%; it was 16% (Cancer 2003;97:2869-79).
He added that current evidence indicates that a fall in LVEF of more than 10%, taking a patient from normal range to below normal, occurs in 7%-8% of patients on an anthracycline such as doxorubicin at a cumulative dose of 240 mg/m2, as is typical in treating breast cancer. The incidence with trastuzumab (Herceptin) when prescribed after completion of anthracycline-based chemotherapy is about 25%; however, when trastuzumab is given in an anthracycline-free regimen, the rate drops to 9%-10%. A study by Dr. Witteles and his coworkers pegged the rate in cancer patients treated with sunitinib (Sutent) or other tyrosine kinase inhibitors at about 15% (JACC Heart Fail. 2013;1:72-8).
“Any way you slice it, that’s a whole lot of people,” he commented.
Dr. Lenihan reported receiving research support from Singulex, Millenium, and Acorda as well as serving as a consultant to Onyx and Roche. Dr. Witteles declared having no financial conflicts.
EXPERT ANALYSIS FROM THE HFSA ANNUAL SCIENTIFIC MEETING
Annual echo an option for cardiac allograft vasculopathy screening
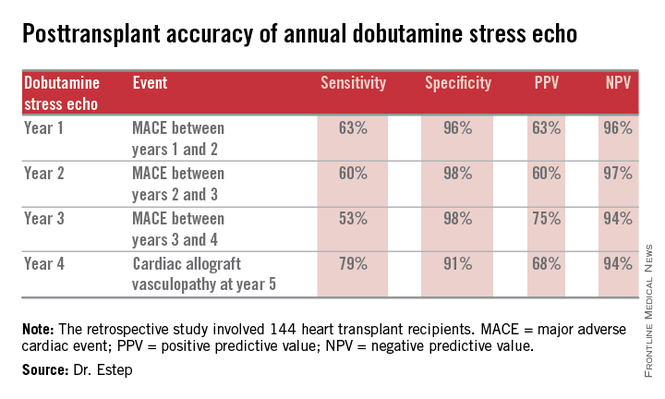
LAS VEGAS – The experience at one major heart transplantation center indicates that annual screening dobutamine stress echocardiography to detect cardiac allograft vasculopathy renders annual coronary angiography unnecessary.
“This noninvasive method has very good specificity and is associated with a negative predictive value of 94%-97%. It can be used in our experience in lieu of annual invasive coronary angiography,” Dr. Jerry D. Estep declared at the annual meeting of the Heart Failure Society of America.
Cardiac allograft vasculopathy (CAV) is a unique, highly aggressive form of CAD. After 3 years post transplant it becomes the No. 1 cause of cardiac retransplantation and mortality. Guidelines recommend consideration of annual screening coronary angiography to detect it early to institute aggressive countermeasures. That’s the practice at most transplant centers.
However, at Houston Methodist Hospital, where Dr. Estep is medical director of the heart transplant and LVAD program, annual dobutamine stress echocardiography (DSE) is used instead. Because there is a scarcity of published data on this noninvasive alternative approach, he presented a retrospective study of the Houston transplant center’s experience over a recent 5-year period.
The study included 144 heart transplant recipients who underwent screening DSE for CAV annually for the first 4 years post transplant and coronary angiography at year 5.
During years 1-4, DSE detected CAV in 19% of patients. They didn’t differ in terms of baseline characteristics from those who remained free of this serious complication.
The good news: Ninety-four percent of patients with normal DSEs during years 1-4 had no CAV upon angiography at year 5. Moreover, the 5% who did have CAV at year 5 after earlier negative DSEs had mild to moderate disease.
The investigators documented the performance of annual screening DSE in predicting the development of major adverse cardiac events, defined as readmission for acute coronary syndrome, heart failure, revascularization, repeat heart transplantation, or cardiac death.
Dr. Estep reported having no financial conflicts regarding this study.
Dr. Hossein Almassi, FCCP, comments: Among solid organ transplants, cardiac transplant is rather unique in its need for invasive biopsy and angiography in following up the cardiac allograft. The search for noninvasive monitoring tools has been ongoing for a number of years. The report by the Houston group is a positive development in the right direction awaiting further confirmation by other cardiac transplant centers.
Dr. Almassi specializes in cardiothoracic surgery at the Medical College of Wisconsin in Milwaukee, Wisconsin.
Dr. Hossein Almassi, FCCP, comments: Among solid organ transplants, cardiac transplant is rather unique in its need for invasive biopsy and angiography in following up the cardiac allograft. The search for noninvasive monitoring tools has been ongoing for a number of years. The report by the Houston group is a positive development in the right direction awaiting further confirmation by other cardiac transplant centers.
Dr. Almassi specializes in cardiothoracic surgery at the Medical College of Wisconsin in Milwaukee, Wisconsin.
Dr. Hossein Almassi, FCCP, comments: Among solid organ transplants, cardiac transplant is rather unique in its need for invasive biopsy and angiography in following up the cardiac allograft. The search for noninvasive monitoring tools has been ongoing for a number of years. The report by the Houston group is a positive development in the right direction awaiting further confirmation by other cardiac transplant centers.
Dr. Almassi specializes in cardiothoracic surgery at the Medical College of Wisconsin in Milwaukee, Wisconsin.
LAS VEGAS – The experience at one major heart transplantation center indicates that annual screening dobutamine stress echocardiography to detect cardiac allograft vasculopathy renders annual coronary angiography unnecessary.
“This noninvasive method has very good specificity and is associated with a negative predictive value of 94%-97%. It can be used in our experience in lieu of annual invasive coronary angiography,” Dr. Jerry D. Estep declared at the annual meeting of the Heart Failure Society of America.
Cardiac allograft vasculopathy (CAV) is a unique, highly aggressive form of CAD. After 3 years post transplant it becomes the No. 1 cause of cardiac retransplantation and mortality. Guidelines recommend consideration of annual screening coronary angiography to detect it early to institute aggressive countermeasures. That’s the practice at most transplant centers.
However, at Houston Methodist Hospital, where Dr. Estep is medical director of the heart transplant and LVAD program, annual dobutamine stress echocardiography (DSE) is used instead. Because there is a scarcity of published data on this noninvasive alternative approach, he presented a retrospective study of the Houston transplant center’s experience over a recent 5-year period.
The study included 144 heart transplant recipients who underwent screening DSE for CAV annually for the first 4 years post transplant and coronary angiography at year 5.
During years 1-4, DSE detected CAV in 19% of patients. They didn’t differ in terms of baseline characteristics from those who remained free of this serious complication.
The good news: Ninety-four percent of patients with normal DSEs during years 1-4 had no CAV upon angiography at year 5. Moreover, the 5% who did have CAV at year 5 after earlier negative DSEs had mild to moderate disease.
The investigators documented the performance of annual screening DSE in predicting the development of major adverse cardiac events, defined as readmission for acute coronary syndrome, heart failure, revascularization, repeat heart transplantation, or cardiac death.
Dr. Estep reported having no financial conflicts regarding this study.

LAS VEGAS – The experience at one major heart transplantation center indicates that annual screening dobutamine stress echocardiography to detect cardiac allograft vasculopathy renders annual coronary angiography unnecessary.
“This noninvasive method has very good specificity and is associated with a negative predictive value of 94%-97%. It can be used in our experience in lieu of annual invasive coronary angiography,” Dr. Jerry D. Estep declared at the annual meeting of the Heart Failure Society of America.
Cardiac allograft vasculopathy (CAV) is a unique, highly aggressive form of CAD. After 3 years post transplant it becomes the No. 1 cause of cardiac retransplantation and mortality. Guidelines recommend consideration of annual screening coronary angiography to detect it early to institute aggressive countermeasures. That’s the practice at most transplant centers.
However, at Houston Methodist Hospital, where Dr. Estep is medical director of the heart transplant and LVAD program, annual dobutamine stress echocardiography (DSE) is used instead. Because there is a scarcity of published data on this noninvasive alternative approach, he presented a retrospective study of the Houston transplant center’s experience over a recent 5-year period.
The study included 144 heart transplant recipients who underwent screening DSE for CAV annually for the first 4 years post transplant and coronary angiography at year 5.
During years 1-4, DSE detected CAV in 19% of patients. They didn’t differ in terms of baseline characteristics from those who remained free of this serious complication.
The good news: Ninety-four percent of patients with normal DSEs during years 1-4 had no CAV upon angiography at year 5. Moreover, the 5% who did have CAV at year 5 after earlier negative DSEs had mild to moderate disease.
The investigators documented the performance of annual screening DSE in predicting the development of major adverse cardiac events, defined as readmission for acute coronary syndrome, heart failure, revascularization, repeat heart transplantation, or cardiac death.
Dr. Estep reported having no financial conflicts regarding this study.

AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: Annual dobutamine stress echocardiography to screen heart transplant recipients for cardiac allograft vasculopathy is an excellent noninvasive alternative to the widely used practice of annual screening coronary angiography.
Major finding: Annual screening dobutamine stress echo during years 1-4 after heart transplant had a 94% negative predictive value for cardiac allograft vasculopathy at year 5.
Data source: A retrospective study of 144 heart transplant recipients at a major transplant center where screening for cardiac allograft vasculopathy is done noninvasively by annual dobutamine stress echocardiography rather than angiography, which is widely used elsewhere.
Disclosures: The presenter reported having no conflicts relevant to the study, which was free of commercial support.
Riociguat benefits subgroup of pulmonary hypertension patients
LAS VEGAS – Patients with pulmonary hypertension due to systolic left ventricular dysfunction show marked hemodynamic and quality of life improvements in response to oral riociguat if they have low baseline pulmonary vascular resistance, but not if their baseline pulmonary vascular resistance exceeds 240 dyn s/cm5, Dr. Marc J. Semigran reported at the annual meeting of the Heart Failure Society of America.
This was the key finding of a prespecified secondary analysis of the Left Ventricular Systolic Dysfunction Association with Pulmonary Hypertension Riociguat Trial (LEPHT).
LEPHT was a phase-IIb, double-blind, placebo-controlled study in which 201 affected patients were randomized to 16 weeks of oral riociguat or placebo. This previously reported study (Circulation 2013;128:502-11) did not meet its primary endpoint because patients on riociguat at 2 mg t.i.d. did not experience a significantly greater decrease in mean pulmonary artery pressure than with placebo. However, the investigators hypothesized that the response to riociguat would differ based upon baseline pulmonary vasomotor tone, and this hypothesis was testable in the secondary analysis, explained Dr. Semigran, medical director of the heart failure and transplantation program at Massachusetts General Hospital, Boston.
Riociguat (Adempas) is a first-in-class oral stimulator of soluble guanylate cyclase. It is approved for two indications: chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension, both of which are uncommon conditions. The LEPHT study was part of an ongoing program to win an additional indication for a much more common condition: pulmonary hypertension caused by systolic left ventricular dysfunction.
For the new secondary analysis, Dr. Semigran and coworkers divided the LEPHT population into two subgroups: the 92 patients with a low baseline pulmonary vascular resistance (PVR) of 240 dyn s/cm5, and 109 others with a PVR above that threshold. The two groups were well balanced in terms of baseline characteristics with two exceptions: ischemic etiology of heart failure and diabetes mellitus were both more common in the high PVR group.
While both groups showed a significant reduction in systemic vascular resistance over 16 weeks in response to riociguat at 2 mg t.i.d., only patients in the low baseline PVR group experienced significant reductions in pulmonary capillary wedge pressure (–6.55 mm Hg) and mean arterial pressure (–8.8 mm Hg). Moreover, improvements in stroke volume (+17 mL) and cardiac index (+0.6 L/min/m2) in response to riociguat were confined to the low-baseline-PVR group.
“The increase in cardiac index and decrease in pulmonary capillary wedge pressure seen in the low PVR group may reflect balanced pulmonary and systemic vasodilation, although a direct myocardial effect can’t be excluded based on the data at hand,” the cardiologist said.
Also, riociguat-treated patients in the low PVR group showed a mean 12-point improvement on the Minnesota Living With Heart Failure Questionnaire, while those with high baseline PVR didn’t show a significant change.
“I think a question for future consideration is, do the patients in the lower baseline PVR group have less advanced disease, making them better candidates for soluble guanylate cyclase stimulator therapy?” Dr. Semigran concluded.
The LEPHT study was sponsored by Bayer. As principal investigator in the trial, Dr. Semigran received a research grant from the company.
LAS VEGAS – Patients with pulmonary hypertension due to systolic left ventricular dysfunction show marked hemodynamic and quality of life improvements in response to oral riociguat if they have low baseline pulmonary vascular resistance, but not if their baseline pulmonary vascular resistance exceeds 240 dyn s/cm5, Dr. Marc J. Semigran reported at the annual meeting of the Heart Failure Society of America.
This was the key finding of a prespecified secondary analysis of the Left Ventricular Systolic Dysfunction Association with Pulmonary Hypertension Riociguat Trial (LEPHT).
LEPHT was a phase-IIb, double-blind, placebo-controlled study in which 201 affected patients were randomized to 16 weeks of oral riociguat or placebo. This previously reported study (Circulation 2013;128:502-11) did not meet its primary endpoint because patients on riociguat at 2 mg t.i.d. did not experience a significantly greater decrease in mean pulmonary artery pressure than with placebo. However, the investigators hypothesized that the response to riociguat would differ based upon baseline pulmonary vasomotor tone, and this hypothesis was testable in the secondary analysis, explained Dr. Semigran, medical director of the heart failure and transplantation program at Massachusetts General Hospital, Boston.
Riociguat (Adempas) is a first-in-class oral stimulator of soluble guanylate cyclase. It is approved for two indications: chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension, both of which are uncommon conditions. The LEPHT study was part of an ongoing program to win an additional indication for a much more common condition: pulmonary hypertension caused by systolic left ventricular dysfunction.
For the new secondary analysis, Dr. Semigran and coworkers divided the LEPHT population into two subgroups: the 92 patients with a low baseline pulmonary vascular resistance (PVR) of 240 dyn s/cm5, and 109 others with a PVR above that threshold. The two groups were well balanced in terms of baseline characteristics with two exceptions: ischemic etiology of heart failure and diabetes mellitus were both more common in the high PVR group.
While both groups showed a significant reduction in systemic vascular resistance over 16 weeks in response to riociguat at 2 mg t.i.d., only patients in the low baseline PVR group experienced significant reductions in pulmonary capillary wedge pressure (–6.55 mm Hg) and mean arterial pressure (–8.8 mm Hg). Moreover, improvements in stroke volume (+17 mL) and cardiac index (+0.6 L/min/m2) in response to riociguat were confined to the low-baseline-PVR group.
“The increase in cardiac index and decrease in pulmonary capillary wedge pressure seen in the low PVR group may reflect balanced pulmonary and systemic vasodilation, although a direct myocardial effect can’t be excluded based on the data at hand,” the cardiologist said.
Also, riociguat-treated patients in the low PVR group showed a mean 12-point improvement on the Minnesota Living With Heart Failure Questionnaire, while those with high baseline PVR didn’t show a significant change.
“I think a question for future consideration is, do the patients in the lower baseline PVR group have less advanced disease, making them better candidates for soluble guanylate cyclase stimulator therapy?” Dr. Semigran concluded.
The LEPHT study was sponsored by Bayer. As principal investigator in the trial, Dr. Semigran received a research grant from the company.
LAS VEGAS – Patients with pulmonary hypertension due to systolic left ventricular dysfunction show marked hemodynamic and quality of life improvements in response to oral riociguat if they have low baseline pulmonary vascular resistance, but not if their baseline pulmonary vascular resistance exceeds 240 dyn s/cm5, Dr. Marc J. Semigran reported at the annual meeting of the Heart Failure Society of America.
This was the key finding of a prespecified secondary analysis of the Left Ventricular Systolic Dysfunction Association with Pulmonary Hypertension Riociguat Trial (LEPHT).
LEPHT was a phase-IIb, double-blind, placebo-controlled study in which 201 affected patients were randomized to 16 weeks of oral riociguat or placebo. This previously reported study (Circulation 2013;128:502-11) did not meet its primary endpoint because patients on riociguat at 2 mg t.i.d. did not experience a significantly greater decrease in mean pulmonary artery pressure than with placebo. However, the investigators hypothesized that the response to riociguat would differ based upon baseline pulmonary vasomotor tone, and this hypothesis was testable in the secondary analysis, explained Dr. Semigran, medical director of the heart failure and transplantation program at Massachusetts General Hospital, Boston.
Riociguat (Adempas) is a first-in-class oral stimulator of soluble guanylate cyclase. It is approved for two indications: chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension, both of which are uncommon conditions. The LEPHT study was part of an ongoing program to win an additional indication for a much more common condition: pulmonary hypertension caused by systolic left ventricular dysfunction.
For the new secondary analysis, Dr. Semigran and coworkers divided the LEPHT population into two subgroups: the 92 patients with a low baseline pulmonary vascular resistance (PVR) of 240 dyn s/cm5, and 109 others with a PVR above that threshold. The two groups were well balanced in terms of baseline characteristics with two exceptions: ischemic etiology of heart failure and diabetes mellitus were both more common in the high PVR group.
While both groups showed a significant reduction in systemic vascular resistance over 16 weeks in response to riociguat at 2 mg t.i.d., only patients in the low baseline PVR group experienced significant reductions in pulmonary capillary wedge pressure (–6.55 mm Hg) and mean arterial pressure (–8.8 mm Hg). Moreover, improvements in stroke volume (+17 mL) and cardiac index (+0.6 L/min/m2) in response to riociguat were confined to the low-baseline-PVR group.
“The increase in cardiac index and decrease in pulmonary capillary wedge pressure seen in the low PVR group may reflect balanced pulmonary and systemic vasodilation, although a direct myocardial effect can’t be excluded based on the data at hand,” the cardiologist said.
Also, riociguat-treated patients in the low PVR group showed a mean 12-point improvement on the Minnesota Living With Heart Failure Questionnaire, while those with high baseline PVR didn’t show a significant change.
“I think a question for future consideration is, do the patients in the lower baseline PVR group have less advanced disease, making them better candidates for soluble guanylate cyclase stimulator therapy?” Dr. Semigran concluded.
The LEPHT study was sponsored by Bayer. As principal investigator in the trial, Dr. Semigran received a research grant from the company.
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Vagal stimulation advances for heart failure
LAS VEGAS – A novel device-based approach to the treatment of heart failure via vagal nerve stimulation resulted in improvements in objectively measurable cardiac function as well as in subjective heart failure symptoms in the ANTHEM-HF trial.
The improvements seen in ANTHEM-HF (Autonomic Neural Regulation Therapy to Enhance Myocardial Function in Heart Failure) were similar regardless of whether participants were randomized to stimulation of the left or right vagal nerve. That’s an important finding because left-sided vagal nerve stimulation (VNS) technology could readily be combined with implantable cardioverter-defibrillators and cardiac resynchronization therapy devices, which are routinely placed on the left side of the thorax, Dr. Inder S. Anand observed in presenting the study findings at the annual meeting of the Heart Failure Society of America.
“We believe that if this technology pans out and proves effective, it will be introduced into devices very easily. That’s the advantage of left-sided stimulation,” explained Dr. Anand, professor of medicine at the University of Minnesota, Minneapolis.
He was quick to add, however, that “There needs to be a lot more work before autonomic regulation therapy is ready for prime time.” That’s because ANTHEM-HF was a relatively small prospective study – just 60 patients – and it was uncontrolled and unblinded.
Left-sided VNS is a well-established treatment for epilepsy, with more than 100,000 patients having received devices in the last several decades. It’s also seeing increasing use in refractory depression.
The approach is still investigational for heart failure, where autonomic imbalance with increased sympathetic activity and decreased parasympathetic tone is associated with heart failure progression and worse clinical outcomes, the cardiologist explained.
In ANTHEM-HF, 60 patients on optimal medical therapy for heart failure with reduced ejection fraction were randomized to left- or right-sided implantation of the Cyberonics VNS device. Once activated, the VNS intensity was titrated over 10 weeks to the maximum tolerable current that remained below the threshold of heart rate change, side effects, and patient sensation. The chronic intermittent stimulation to the vagus nerve was delivered at 10 Hz, a 250 microsec pulse width, and an average stimulation current of 2.0 mA. The stimulation cycle was 14 seconds on, 66 seconds off.
The primary study endpoint was change in LVEF over the course of 6 months. From a baseline of 32.4% it improved significantly to 37.2%, which Dr. Anand deemed “very impressive” for just 6 months of therapy. The improvement was similar regardless of whether the VNS was left or right sided.
He said the study didn’t raise any safety concerns. The only serious treatment-related adverse event was a fatal perioperative embolic stroke in a recipient of a left-sided device.
The most common adverse events were hoarseness or other voice alterations, which occurred in 19 patients. Thirteen patients reported a new cough. These side effects mirror the experience in patients treated with the VNS device for epilepsy or depression.
Discussant Jagmeet P. Singh emphasized that “it’s important to interpret the ANTHEM-HF results with some degree of caution.” That’s because the usual variability within LVEF measurements can be in the 5% range, and the 4.8% improvement seen in this study wasn’t accompanied by a significant improvement in LV end systolic volume. Moreover, the improvements seen in secondary endpoints – 6-minute walk distance, NYHA class, and the Minnesota Living With Heart Failure qualify-of-life score – are all potentially open to bias since neither patients nor physicians were blinded.
Dr. Singh noted that the NECTAR-HF study, a blinded, sham-controlled clinical trial of VNS presented earlier at the annual congress of the European Society of Cardiology, was a negative study that showed no improvement in functional measures. While NECTAR-HF caused some skeptics to question the whole concept of VNS as a useful therapeutic strategy, the stimulation protocols used in NECTAR-HF and ANTHEM-HF were quite different, and it’s likely that the stimulation amplitude employed in NECTAR-HF wasn’t sufficient to affect the target subset of vagal nerve fibers, according to Dr. Singh, director of the cardiac resynchronization therapy program and the Holter laboratory at Massachusetts General Hospital, Boston.
He considers the ANTHEM-HF finding that left-sided VNS is comparable to right to be an important advance that “really moves the field forward.” Yet many critical questions about autonomic regulation therapy for heart failure remain unanswered. These include whether it truly is safe and effective, and if so, the optimal target dose and frequency of stimulation. Answers should be forthcoming from the ongoing INOVATE-HF study, a randomized, controlled, 650-patient study, sponsored by BioControl Medical. The trial features hard clinical endpoints and higher target-dose stimulation protocols than in prior studies.
“This trial will probably put an end to the debate as to whether vagal nerve stimulation has an impact on heart failure or not,” the cardiologist predicted.
“Strategies to nonpharmacologically modulate the autonomic nervous system is an area of immense interest and investigation, and it’s going to be extended,” he observed. “I think that this is the autonomic era, and so it’s really important that we get it right. I think we’ve learned from the renal denervation experience that we need to be really careful in selecting patients for any form of autonomic manipulation. I don’t think we should select patients just off their LVEF. We should have some parameter that can quantify autonomic dysregulation prior to initiation of therapy.”
It will also be important to be able to individualize VNS therapy, just as physicians now do with beta blockers and other pharmacotherapies, he added.
The ANTHEM-HF trial was sponsored by Cyberonics. Dr. Anand reported serving as a consultant to and/or recipient of research grants from Cyberonics, Amgen, Critical Diagnostics, Novartis, and Zensun. Dr. Singh has received research support and/or served on speakers bureaus for Boston Scientific, St. Jude Medical, and the Sorin Group.
LAS VEGAS – A novel device-based approach to the treatment of heart failure via vagal nerve stimulation resulted in improvements in objectively measurable cardiac function as well as in subjective heart failure symptoms in the ANTHEM-HF trial.
The improvements seen in ANTHEM-HF (Autonomic Neural Regulation Therapy to Enhance Myocardial Function in Heart Failure) were similar regardless of whether participants were randomized to stimulation of the left or right vagal nerve. That’s an important finding because left-sided vagal nerve stimulation (VNS) technology could readily be combined with implantable cardioverter-defibrillators and cardiac resynchronization therapy devices, which are routinely placed on the left side of the thorax, Dr. Inder S. Anand observed in presenting the study findings at the annual meeting of the Heart Failure Society of America.
“We believe that if this technology pans out and proves effective, it will be introduced into devices very easily. That’s the advantage of left-sided stimulation,” explained Dr. Anand, professor of medicine at the University of Minnesota, Minneapolis.
He was quick to add, however, that “There needs to be a lot more work before autonomic regulation therapy is ready for prime time.” That’s because ANTHEM-HF was a relatively small prospective study – just 60 patients – and it was uncontrolled and unblinded.
Left-sided VNS is a well-established treatment for epilepsy, with more than 100,000 patients having received devices in the last several decades. It’s also seeing increasing use in refractory depression.
The approach is still investigational for heart failure, where autonomic imbalance with increased sympathetic activity and decreased parasympathetic tone is associated with heart failure progression and worse clinical outcomes, the cardiologist explained.
In ANTHEM-HF, 60 patients on optimal medical therapy for heart failure with reduced ejection fraction were randomized to left- or right-sided implantation of the Cyberonics VNS device. Once activated, the VNS intensity was titrated over 10 weeks to the maximum tolerable current that remained below the threshold of heart rate change, side effects, and patient sensation. The chronic intermittent stimulation to the vagus nerve was delivered at 10 Hz, a 250 microsec pulse width, and an average stimulation current of 2.0 mA. The stimulation cycle was 14 seconds on, 66 seconds off.
The primary study endpoint was change in LVEF over the course of 6 months. From a baseline of 32.4% it improved significantly to 37.2%, which Dr. Anand deemed “very impressive” for just 6 months of therapy. The improvement was similar regardless of whether the VNS was left or right sided.
He said the study didn’t raise any safety concerns. The only serious treatment-related adverse event was a fatal perioperative embolic stroke in a recipient of a left-sided device.
The most common adverse events were hoarseness or other voice alterations, which occurred in 19 patients. Thirteen patients reported a new cough. These side effects mirror the experience in patients treated with the VNS device for epilepsy or depression.
Discussant Jagmeet P. Singh emphasized that “it’s important to interpret the ANTHEM-HF results with some degree of caution.” That’s because the usual variability within LVEF measurements can be in the 5% range, and the 4.8% improvement seen in this study wasn’t accompanied by a significant improvement in LV end systolic volume. Moreover, the improvements seen in secondary endpoints – 6-minute walk distance, NYHA class, and the Minnesota Living With Heart Failure qualify-of-life score – are all potentially open to bias since neither patients nor physicians were blinded.
Dr. Singh noted that the NECTAR-HF study, a blinded, sham-controlled clinical trial of VNS presented earlier at the annual congress of the European Society of Cardiology, was a negative study that showed no improvement in functional measures. While NECTAR-HF caused some skeptics to question the whole concept of VNS as a useful therapeutic strategy, the stimulation protocols used in NECTAR-HF and ANTHEM-HF were quite different, and it’s likely that the stimulation amplitude employed in NECTAR-HF wasn’t sufficient to affect the target subset of vagal nerve fibers, according to Dr. Singh, director of the cardiac resynchronization therapy program and the Holter laboratory at Massachusetts General Hospital, Boston.
He considers the ANTHEM-HF finding that left-sided VNS is comparable to right to be an important advance that “really moves the field forward.” Yet many critical questions about autonomic regulation therapy for heart failure remain unanswered. These include whether it truly is safe and effective, and if so, the optimal target dose and frequency of stimulation. Answers should be forthcoming from the ongoing INOVATE-HF study, a randomized, controlled, 650-patient study, sponsored by BioControl Medical. The trial features hard clinical endpoints and higher target-dose stimulation protocols than in prior studies.
“This trial will probably put an end to the debate as to whether vagal nerve stimulation has an impact on heart failure or not,” the cardiologist predicted.
“Strategies to nonpharmacologically modulate the autonomic nervous system is an area of immense interest and investigation, and it’s going to be extended,” he observed. “I think that this is the autonomic era, and so it’s really important that we get it right. I think we’ve learned from the renal denervation experience that we need to be really careful in selecting patients for any form of autonomic manipulation. I don’t think we should select patients just off their LVEF. We should have some parameter that can quantify autonomic dysregulation prior to initiation of therapy.”
It will also be important to be able to individualize VNS therapy, just as physicians now do with beta blockers and other pharmacotherapies, he added.
The ANTHEM-HF trial was sponsored by Cyberonics. Dr. Anand reported serving as a consultant to and/or recipient of research grants from Cyberonics, Amgen, Critical Diagnostics, Novartis, and Zensun. Dr. Singh has received research support and/or served on speakers bureaus for Boston Scientific, St. Jude Medical, and the Sorin Group.
LAS VEGAS – A novel device-based approach to the treatment of heart failure via vagal nerve stimulation resulted in improvements in objectively measurable cardiac function as well as in subjective heart failure symptoms in the ANTHEM-HF trial.
The improvements seen in ANTHEM-HF (Autonomic Neural Regulation Therapy to Enhance Myocardial Function in Heart Failure) were similar regardless of whether participants were randomized to stimulation of the left or right vagal nerve. That’s an important finding because left-sided vagal nerve stimulation (VNS) technology could readily be combined with implantable cardioverter-defibrillators and cardiac resynchronization therapy devices, which are routinely placed on the left side of the thorax, Dr. Inder S. Anand observed in presenting the study findings at the annual meeting of the Heart Failure Society of America.
“We believe that if this technology pans out and proves effective, it will be introduced into devices very easily. That’s the advantage of left-sided stimulation,” explained Dr. Anand, professor of medicine at the University of Minnesota, Minneapolis.
He was quick to add, however, that “There needs to be a lot more work before autonomic regulation therapy is ready for prime time.” That’s because ANTHEM-HF was a relatively small prospective study – just 60 patients – and it was uncontrolled and unblinded.
Left-sided VNS is a well-established treatment for epilepsy, with more than 100,000 patients having received devices in the last several decades. It’s also seeing increasing use in refractory depression.
The approach is still investigational for heart failure, where autonomic imbalance with increased sympathetic activity and decreased parasympathetic tone is associated with heart failure progression and worse clinical outcomes, the cardiologist explained.
In ANTHEM-HF, 60 patients on optimal medical therapy for heart failure with reduced ejection fraction were randomized to left- or right-sided implantation of the Cyberonics VNS device. Once activated, the VNS intensity was titrated over 10 weeks to the maximum tolerable current that remained below the threshold of heart rate change, side effects, and patient sensation. The chronic intermittent stimulation to the vagus nerve was delivered at 10 Hz, a 250 microsec pulse width, and an average stimulation current of 2.0 mA. The stimulation cycle was 14 seconds on, 66 seconds off.
The primary study endpoint was change in LVEF over the course of 6 months. From a baseline of 32.4% it improved significantly to 37.2%, which Dr. Anand deemed “very impressive” for just 6 months of therapy. The improvement was similar regardless of whether the VNS was left or right sided.
He said the study didn’t raise any safety concerns. The only serious treatment-related adverse event was a fatal perioperative embolic stroke in a recipient of a left-sided device.
The most common adverse events were hoarseness or other voice alterations, which occurred in 19 patients. Thirteen patients reported a new cough. These side effects mirror the experience in patients treated with the VNS device for epilepsy or depression.
Discussant Jagmeet P. Singh emphasized that “it’s important to interpret the ANTHEM-HF results with some degree of caution.” That’s because the usual variability within LVEF measurements can be in the 5% range, and the 4.8% improvement seen in this study wasn’t accompanied by a significant improvement in LV end systolic volume. Moreover, the improvements seen in secondary endpoints – 6-minute walk distance, NYHA class, and the Minnesota Living With Heart Failure qualify-of-life score – are all potentially open to bias since neither patients nor physicians were blinded.
Dr. Singh noted that the NECTAR-HF study, a blinded, sham-controlled clinical trial of VNS presented earlier at the annual congress of the European Society of Cardiology, was a negative study that showed no improvement in functional measures. While NECTAR-HF caused some skeptics to question the whole concept of VNS as a useful therapeutic strategy, the stimulation protocols used in NECTAR-HF and ANTHEM-HF were quite different, and it’s likely that the stimulation amplitude employed in NECTAR-HF wasn’t sufficient to affect the target subset of vagal nerve fibers, according to Dr. Singh, director of the cardiac resynchronization therapy program and the Holter laboratory at Massachusetts General Hospital, Boston.
He considers the ANTHEM-HF finding that left-sided VNS is comparable to right to be an important advance that “really moves the field forward.” Yet many critical questions about autonomic regulation therapy for heart failure remain unanswered. These include whether it truly is safe and effective, and if so, the optimal target dose and frequency of stimulation. Answers should be forthcoming from the ongoing INOVATE-HF study, a randomized, controlled, 650-patient study, sponsored by BioControl Medical. The trial features hard clinical endpoints and higher target-dose stimulation protocols than in prior studies.
“This trial will probably put an end to the debate as to whether vagal nerve stimulation has an impact on heart failure or not,” the cardiologist predicted.
“Strategies to nonpharmacologically modulate the autonomic nervous system is an area of immense interest and investigation, and it’s going to be extended,” he observed. “I think that this is the autonomic era, and so it’s really important that we get it right. I think we’ve learned from the renal denervation experience that we need to be really careful in selecting patients for any form of autonomic manipulation. I don’t think we should select patients just off their LVEF. We should have some parameter that can quantify autonomic dysregulation prior to initiation of therapy.”
It will also be important to be able to individualize VNS therapy, just as physicians now do with beta blockers and other pharmacotherapies, he added.
The ANTHEM-HF trial was sponsored by Cyberonics. Dr. Anand reported serving as a consultant to and/or recipient of research grants from Cyberonics, Amgen, Critical Diagnostics, Novartis, and Zensun. Dr. Singh has received research support and/or served on speakers bureaus for Boston Scientific, St. Jude Medical, and the Sorin Group.
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: Autonomic regulation therapy via a vagal nerve stimulation device shows promise for the treatment of heart failure with reduced ejection fraction.
Major finding: During 6 months of vagal nerve stimulation, patients’ mean LVEF rose significantly from 32.4% to 37.2%, with similar results regardless of whether the stimulation was left- or right-sided.
Data source: The ANTHEM-HF study was a prospective, unblinded, and uncontrolled study involving 60 patients who received vagal nerve stimulation for 6 months.
Disclosures: The study was sponsored by Cyberonics. The presenter has received research grants from and served as a consultant to that and other companies.
Four lifestyle changes might prevent 40% of heart failure
LAS VEGAS – Not smoking, maintaining a normal body weight, exercising regularly, and eating a healthy diet would avert roughly 40% of all cases of heart failure, Dr. Luc Djousse asserted at the annual meeting of the Heart Failure Society of America.
“Those four simple lifestyle changes offer a cost-effective opportunity for primary prevention of heart failure in the population,” observed Dr. Djousse, director of research in the division of aging at Brigham and Women’s Hospital, Boston.
The evidence to back his claim comes chiefly from well-conducted epidemiologic studies. For example, in the Physicians Health Study I, involving nearly 21,000 male physicians followed for a mean of 22 years, 40-year-olds who didn’t adhere to any of six key lifestyle factors – maintaining normal body weight, not smoking, regular exercise, moderate alcohol intake, consumption of fruits and vegetables, and eating breakfast cereals – had a lifetime heart failure risk of 21%.(JAMA 2009;302:394-400). “That’s right,” he asserted, “even if you’re a physician.”
In contrast, physicians who adhered to four or more of the six lifestyle factors cut their lifetime risk of heart failure by more than half, to 10.1%.
Similarly, a Finnish national population-based study of 18,346 men and 19,729 women who were ages 25-74, free of heart failure at baseline, and followed for a median of 14.1 years found that women who engaged in four healthy lifestyle behaviors -- not smoking, maintaining normal body weight, physical activity, and liberal intake of vegetables -- were an adjusted 81% less likely to develop heart failure than women who didn’t adhere to any of the four behaviors. Men who followed the four-fold path were 69% less likely to develop heart failure, again after adjusting for age, blood pressure, lipid levels, education, and history of heart disease (Circ. Heart Fail. 2011;4:607-12).
Dr. Djousse said that while Framingham Heart Study data show that hypertension accounts for 39% of the population-attributable risk for heart failure in men and 59% in women, analysis of Physicians Health Study data underscore the negative impact of unhealthy and modifiable lifestyle factors. In that analysis, smoking accounted for 20% of the population attributable risk for heart failure, being sedentary accounted for 12%, alcohol consumption of less than 1 drink per week 10%, and dietary shortcomings less than 10%.
In this analysis, individual dietary aspects made a relatively modest contribution to the incidence of heart failure compared to other modifiable lifestyle factors. Yet dietary issues are a neverending source of fascination both for the general public and physicians. So Dr. Djousse’s update on the evidence regarding a number of hot dietary topics received close attention:
Coffee: A meta-analysis of five prospective studies totalling more than 140,000 participants found a J-shaped dose-response relationship. Consumption of 4 cups per day was optimal, with an associated roughly 10% reduction in heart failure risk (Circ. Heart Fail. 2012;5:401-05). This information elicited a spontaneous cheer -- more of a roar, actually -- from Dr. Djousse’s large audience.
Red meat: In a population-based study of more than 37,000 Swedish men, consumption of at least 75 g/day of processed red meat, such as salami or prosciutto, was independently associated with a 28% increased risk of developing heart failure compared to that of men who ate less than 25 g/day. Intake of unprocessed red meat was unrelated to heart failure risk (Circ. Heart Fail. 2014;7:552-57).
Alcohol: A six-study meta-analysis by Dr. Djousse and coinvestigators concluded that light-to-moderate drinking was associated with a 23% reduction in the risk of heart failure compared to never drinkers. They defined moderate drinking as one to two drinks per day in men and one in women (Phys. Sportsmed. 2010; 38:84-89).
Fish: In a meta-analysis of seven prospective studies featuring more than 176,000 subjects and 5,480 incident cases of heart failure, regular fish consumption was associated with a 15% reduction in the risk of heart failure compared to the lowest category of fish intake (Clin. Nutr. 2012;31:846-53).
Chocolate: Among nearly 32,000 women participating in the prospective Swedish Mammography Cohort study, consumption of one or two servings of chocate per week was associated with a 32% reduction in the risk of incident heart failure in a multivariate analysis compared to no regular chocolate intake. However, no protective effect was seen with consumption or one or more daily servings (Circ. Heart Fail. 2010;3:612-16). Evidence from other studies suggests that dark chocolate, which is rich in cocoa flavinoids, is the type with the most cardioprotective effects, according to Dr. Djousse.
He reported receiving research support from Merck and GlaxoSmithKline and serving as a consultant to Bayer.
LAS VEGAS – Not smoking, maintaining a normal body weight, exercising regularly, and eating a healthy diet would avert roughly 40% of all cases of heart failure, Dr. Luc Djousse asserted at the annual meeting of the Heart Failure Society of America.
“Those four simple lifestyle changes offer a cost-effective opportunity for primary prevention of heart failure in the population,” observed Dr. Djousse, director of research in the division of aging at Brigham and Women’s Hospital, Boston.
The evidence to back his claim comes chiefly from well-conducted epidemiologic studies. For example, in the Physicians Health Study I, involving nearly 21,000 male physicians followed for a mean of 22 years, 40-year-olds who didn’t adhere to any of six key lifestyle factors – maintaining normal body weight, not smoking, regular exercise, moderate alcohol intake, consumption of fruits and vegetables, and eating breakfast cereals – had a lifetime heart failure risk of 21%.(JAMA 2009;302:394-400). “That’s right,” he asserted, “even if you’re a physician.”
In contrast, physicians who adhered to four or more of the six lifestyle factors cut their lifetime risk of heart failure by more than half, to 10.1%.
Similarly, a Finnish national population-based study of 18,346 men and 19,729 women who were ages 25-74, free of heart failure at baseline, and followed for a median of 14.1 years found that women who engaged in four healthy lifestyle behaviors -- not smoking, maintaining normal body weight, physical activity, and liberal intake of vegetables -- were an adjusted 81% less likely to develop heart failure than women who didn’t adhere to any of the four behaviors. Men who followed the four-fold path were 69% less likely to develop heart failure, again after adjusting for age, blood pressure, lipid levels, education, and history of heart disease (Circ. Heart Fail. 2011;4:607-12).
Dr. Djousse said that while Framingham Heart Study data show that hypertension accounts for 39% of the population-attributable risk for heart failure in men and 59% in women, analysis of Physicians Health Study data underscore the negative impact of unhealthy and modifiable lifestyle factors. In that analysis, smoking accounted for 20% of the population attributable risk for heart failure, being sedentary accounted for 12%, alcohol consumption of less than 1 drink per week 10%, and dietary shortcomings less than 10%.
In this analysis, individual dietary aspects made a relatively modest contribution to the incidence of heart failure compared to other modifiable lifestyle factors. Yet dietary issues are a neverending source of fascination both for the general public and physicians. So Dr. Djousse’s update on the evidence regarding a number of hot dietary topics received close attention:
Coffee: A meta-analysis of five prospective studies totalling more than 140,000 participants found a J-shaped dose-response relationship. Consumption of 4 cups per day was optimal, with an associated roughly 10% reduction in heart failure risk (Circ. Heart Fail. 2012;5:401-05). This information elicited a spontaneous cheer -- more of a roar, actually -- from Dr. Djousse’s large audience.
Red meat: In a population-based study of more than 37,000 Swedish men, consumption of at least 75 g/day of processed red meat, such as salami or prosciutto, was independently associated with a 28% increased risk of developing heart failure compared to that of men who ate less than 25 g/day. Intake of unprocessed red meat was unrelated to heart failure risk (Circ. Heart Fail. 2014;7:552-57).
Alcohol: A six-study meta-analysis by Dr. Djousse and coinvestigators concluded that light-to-moderate drinking was associated with a 23% reduction in the risk of heart failure compared to never drinkers. They defined moderate drinking as one to two drinks per day in men and one in women (Phys. Sportsmed. 2010; 38:84-89).
Fish: In a meta-analysis of seven prospective studies featuring more than 176,000 subjects and 5,480 incident cases of heart failure, regular fish consumption was associated with a 15% reduction in the risk of heart failure compared to the lowest category of fish intake (Clin. Nutr. 2012;31:846-53).
Chocolate: Among nearly 32,000 women participating in the prospective Swedish Mammography Cohort study, consumption of one or two servings of chocate per week was associated with a 32% reduction in the risk of incident heart failure in a multivariate analysis compared to no regular chocolate intake. However, no protective effect was seen with consumption or one or more daily servings (Circ. Heart Fail. 2010;3:612-16). Evidence from other studies suggests that dark chocolate, which is rich in cocoa flavinoids, is the type with the most cardioprotective effects, according to Dr. Djousse.
He reported receiving research support from Merck and GlaxoSmithKline and serving as a consultant to Bayer.
LAS VEGAS – Not smoking, maintaining a normal body weight, exercising regularly, and eating a healthy diet would avert roughly 40% of all cases of heart failure, Dr. Luc Djousse asserted at the annual meeting of the Heart Failure Society of America.
“Those four simple lifestyle changes offer a cost-effective opportunity for primary prevention of heart failure in the population,” observed Dr. Djousse, director of research in the division of aging at Brigham and Women’s Hospital, Boston.
The evidence to back his claim comes chiefly from well-conducted epidemiologic studies. For example, in the Physicians Health Study I, involving nearly 21,000 male physicians followed for a mean of 22 years, 40-year-olds who didn’t adhere to any of six key lifestyle factors – maintaining normal body weight, not smoking, regular exercise, moderate alcohol intake, consumption of fruits and vegetables, and eating breakfast cereals – had a lifetime heart failure risk of 21%.(JAMA 2009;302:394-400). “That’s right,” he asserted, “even if you’re a physician.”
In contrast, physicians who adhered to four or more of the six lifestyle factors cut their lifetime risk of heart failure by more than half, to 10.1%.
Similarly, a Finnish national population-based study of 18,346 men and 19,729 women who were ages 25-74, free of heart failure at baseline, and followed for a median of 14.1 years found that women who engaged in four healthy lifestyle behaviors -- not smoking, maintaining normal body weight, physical activity, and liberal intake of vegetables -- were an adjusted 81% less likely to develop heart failure than women who didn’t adhere to any of the four behaviors. Men who followed the four-fold path were 69% less likely to develop heart failure, again after adjusting for age, blood pressure, lipid levels, education, and history of heart disease (Circ. Heart Fail. 2011;4:607-12).
Dr. Djousse said that while Framingham Heart Study data show that hypertension accounts for 39% of the population-attributable risk for heart failure in men and 59% in women, analysis of Physicians Health Study data underscore the negative impact of unhealthy and modifiable lifestyle factors. In that analysis, smoking accounted for 20% of the population attributable risk for heart failure, being sedentary accounted for 12%, alcohol consumption of less than 1 drink per week 10%, and dietary shortcomings less than 10%.
In this analysis, individual dietary aspects made a relatively modest contribution to the incidence of heart failure compared to other modifiable lifestyle factors. Yet dietary issues are a neverending source of fascination both for the general public and physicians. So Dr. Djousse’s update on the evidence regarding a number of hot dietary topics received close attention:
Coffee: A meta-analysis of five prospective studies totalling more than 140,000 participants found a J-shaped dose-response relationship. Consumption of 4 cups per day was optimal, with an associated roughly 10% reduction in heart failure risk (Circ. Heart Fail. 2012;5:401-05). This information elicited a spontaneous cheer -- more of a roar, actually -- from Dr. Djousse’s large audience.
Red meat: In a population-based study of more than 37,000 Swedish men, consumption of at least 75 g/day of processed red meat, such as salami or prosciutto, was independently associated with a 28% increased risk of developing heart failure compared to that of men who ate less than 25 g/day. Intake of unprocessed red meat was unrelated to heart failure risk (Circ. Heart Fail. 2014;7:552-57).
Alcohol: A six-study meta-analysis by Dr. Djousse and coinvestigators concluded that light-to-moderate drinking was associated with a 23% reduction in the risk of heart failure compared to never drinkers. They defined moderate drinking as one to two drinks per day in men and one in women (Phys. Sportsmed. 2010; 38:84-89).
Fish: In a meta-analysis of seven prospective studies featuring more than 176,000 subjects and 5,480 incident cases of heart failure, regular fish consumption was associated with a 15% reduction in the risk of heart failure compared to the lowest category of fish intake (Clin. Nutr. 2012;31:846-53).
Chocolate: Among nearly 32,000 women participating in the prospective Swedish Mammography Cohort study, consumption of one or two servings of chocate per week was associated with a 32% reduction in the risk of incident heart failure in a multivariate analysis compared to no regular chocolate intake. However, no protective effect was seen with consumption or one or more daily servings (Circ. Heart Fail. 2010;3:612-16). Evidence from other studies suggests that dark chocolate, which is rich in cocoa flavinoids, is the type with the most cardioprotective effects, according to Dr. Djousse.
He reported receiving research support from Merck and GlaxoSmithKline and serving as a consultant to Bayer.
EXPERT ANALYSIS FROM THE HFSA ANNUAL SCIENTIFIC MEETING
Hospitalists Shine in Study of Heart Failure Outcomes
LAS VEGAS – When it comes to managing patients hospitalized for heart failure, academic hospitalists are the experts – and cardiologists, primary care physicians, and nephrologists are the students.
That’s the lesson Dr. Paul M. Ndunda drew from his retrospective cohort study of 1,735 patients hospitalized for heart failure at three community hospitals. The patients admitted by members of the three teaching hospitalist groups had the lowest inpatient mortality, mean length of stay, and 30-day readmission rates compared to the other admitting physicians.
Although these data aren’t risk adjusted and its certainly possible that the academic hospitalists culled patients with a more favorable risk profile, it’s also likely that the explanation for the outcome differences is multifactorial and that specialty-specific differences in the care delivery process played a role. Indeed, it would appear that the hospitalist model of management of heart failure patients holds lessons of value for other types of physicians, Dr. Ndunda asserted at the annual meeting of the Heart Failure Society of America.
It’s also noteworthy that the teaching hospitalist groups achieved patient outcomes that were by and large better than the nonteaching hospitalist groups, added Dr. Ndunda of the University of Kansas, Wichita.
The major caveat, he conceded, is that outcomes weren’t risk adjusted. This takes on added weight in light of the fact that the teaching hospitalists’ patients were significantly younger than other physicians’ patients were. They were also more likely to be discharged home.
Dr. Ndunda reported having no financial conflicts with regard to this study.
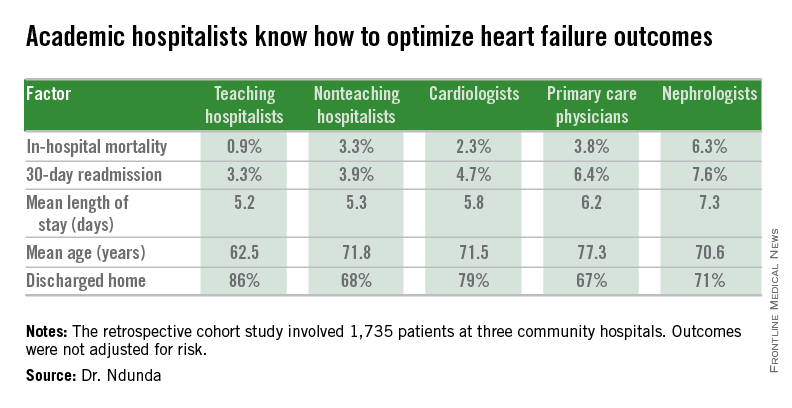
LAS VEGAS – When it comes to managing patients hospitalized for heart failure, academic hospitalists are the experts – and cardiologists, primary care physicians, and nephrologists are the students.
That’s the lesson Dr. Paul M. Ndunda drew from his retrospective cohort study of 1,735 patients hospitalized for heart failure at three community hospitals. The patients admitted by members of the three teaching hospitalist groups had the lowest inpatient mortality, mean length of stay, and 30-day readmission rates compared to the other admitting physicians.
Although these data aren’t risk adjusted and its certainly possible that the academic hospitalists culled patients with a more favorable risk profile, it’s also likely that the explanation for the outcome differences is multifactorial and that specialty-specific differences in the care delivery process played a role. Indeed, it would appear that the hospitalist model of management of heart failure patients holds lessons of value for other types of physicians, Dr. Ndunda asserted at the annual meeting of the Heart Failure Society of America.
It’s also noteworthy that the teaching hospitalist groups achieved patient outcomes that were by and large better than the nonteaching hospitalist groups, added Dr. Ndunda of the University of Kansas, Wichita.
The major caveat, he conceded, is that outcomes weren’t risk adjusted. This takes on added weight in light of the fact that the teaching hospitalists’ patients were significantly younger than other physicians’ patients were. They were also more likely to be discharged home.
Dr. Ndunda reported having no financial conflicts with regard to this study.

LAS VEGAS – When it comes to managing patients hospitalized for heart failure, academic hospitalists are the experts – and cardiologists, primary care physicians, and nephrologists are the students.
That’s the lesson Dr. Paul M. Ndunda drew from his retrospective cohort study of 1,735 patients hospitalized for heart failure at three community hospitals. The patients admitted by members of the three teaching hospitalist groups had the lowest inpatient mortality, mean length of stay, and 30-day readmission rates compared to the other admitting physicians.
Although these data aren’t risk adjusted and its certainly possible that the academic hospitalists culled patients with a more favorable risk profile, it’s also likely that the explanation for the outcome differences is multifactorial and that specialty-specific differences in the care delivery process played a role. Indeed, it would appear that the hospitalist model of management of heart failure patients holds lessons of value for other types of physicians, Dr. Ndunda asserted at the annual meeting of the Heart Failure Society of America.
It’s also noteworthy that the teaching hospitalist groups achieved patient outcomes that were by and large better than the nonteaching hospitalist groups, added Dr. Ndunda of the University of Kansas, Wichita.
The major caveat, he conceded, is that outcomes weren’t risk adjusted. This takes on added weight in light of the fact that the teaching hospitalists’ patients were significantly younger than other physicians’ patients were. They were also more likely to be discharged home.
Dr. Ndunda reported having no financial conflicts with regard to this study.

AT THE HFSA ANNUAL SCIENTIFIC MEETING
Hospitalists shine in study of heart failure outcomes
LAS VEGAS – When it comes to managing patients hospitalized for heart failure, academic hospitalists are the experts – and cardiologists, primary care physicians, and nephrologists are the students.
That’s the lesson Dr. Paul M. Ndunda drew from his retrospective cohort study of 1,735 patients hospitalized for heart failure at three community hospitals. The patients admitted by members of the three teaching hospitalist groups had the lowest inpatient mortality, mean length of stay, and 30-day readmission rates compared to the other admitting physicians.
Although these data aren’t risk adjusted and its certainly possible that the academic hospitalists culled patients with a more favorable risk profile, it’s also likely that the explanation for the outcome differences is multifactorial and that specialty-specific differences in the care delivery process played a role. Indeed, it would appear that the hospitalist model of management of heart failure patients holds lessons of value for other types of physicians, Dr. Ndunda asserted at the annual meeting of the Heart Failure Society of America.
It’s also noteworthy that the teaching hospitalist groups achieved patient outcomes that were by and large better than the nonteaching hospitalist groups, added Dr. Ndunda of the University of Kansas, Wichita.
The major caveat, he conceded, is that outcomes weren’t risk adjusted. This takes on added weight in light of the fact that the teaching hospitalists’ patients were significantly younger than other physicians’ patients were. They were also more likely to be discharged home.
Dr. Ndunda reported having no financial conflicts with regard to this study.
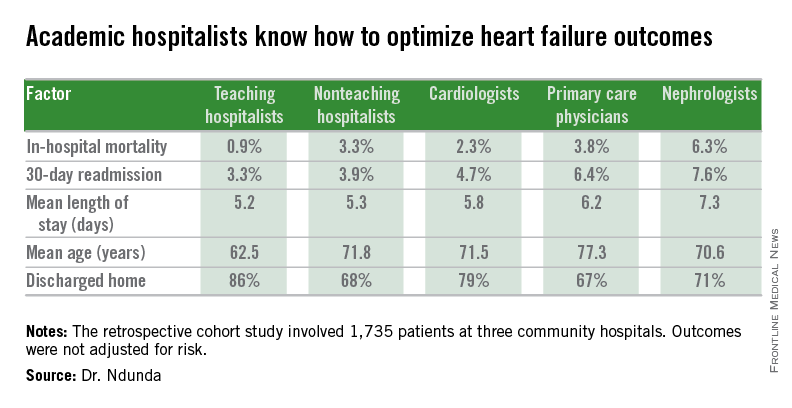
LAS VEGAS – When it comes to managing patients hospitalized for heart failure, academic hospitalists are the experts – and cardiologists, primary care physicians, and nephrologists are the students.
That’s the lesson Dr. Paul M. Ndunda drew from his retrospective cohort study of 1,735 patients hospitalized for heart failure at three community hospitals. The patients admitted by members of the three teaching hospitalist groups had the lowest inpatient mortality, mean length of stay, and 30-day readmission rates compared to the other admitting physicians.
Although these data aren’t risk adjusted and its certainly possible that the academic hospitalists culled patients with a more favorable risk profile, it’s also likely that the explanation for the outcome differences is multifactorial and that specialty-specific differences in the care delivery process played a role. Indeed, it would appear that the hospitalist model of management of heart failure patients holds lessons of value for other types of physicians, Dr. Ndunda asserted at the annual meeting of the Heart Failure Society of America.
It’s also noteworthy that the teaching hospitalist groups achieved patient outcomes that were by and large better than the nonteaching hospitalist groups, added Dr. Ndunda of the University of Kansas, Wichita.
The major caveat, he conceded, is that outcomes weren’t risk adjusted. This takes on added weight in light of the fact that the teaching hospitalists’ patients were significantly younger than other physicians’ patients were. They were also more likely to be discharged home.
Dr. Ndunda reported having no financial conflicts with regard to this study.

LAS VEGAS – When it comes to managing patients hospitalized for heart failure, academic hospitalists are the experts – and cardiologists, primary care physicians, and nephrologists are the students.
That’s the lesson Dr. Paul M. Ndunda drew from his retrospective cohort study of 1,735 patients hospitalized for heart failure at three community hospitals. The patients admitted by members of the three teaching hospitalist groups had the lowest inpatient mortality, mean length of stay, and 30-day readmission rates compared to the other admitting physicians.
Although these data aren’t risk adjusted and its certainly possible that the academic hospitalists culled patients with a more favorable risk profile, it’s also likely that the explanation for the outcome differences is multifactorial and that specialty-specific differences in the care delivery process played a role. Indeed, it would appear that the hospitalist model of management of heart failure patients holds lessons of value for other types of physicians, Dr. Ndunda asserted at the annual meeting of the Heart Failure Society of America.
It’s also noteworthy that the teaching hospitalist groups achieved patient outcomes that were by and large better than the nonteaching hospitalist groups, added Dr. Ndunda of the University of Kansas, Wichita.
The major caveat, he conceded, is that outcomes weren’t risk adjusted. This takes on added weight in light of the fact that the teaching hospitalists’ patients were significantly younger than other physicians’ patients were. They were also more likely to be discharged home.
Dr. Ndunda reported having no financial conflicts with regard to this study.

AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: Outcomes were significantly better among patients admitted for heart failure by academic hospitalists than in those admitted by primary care physicians, cardiologists, nonteaching hospitalists, or nephrologists.
Major finding: The unadjusted 30-day heart failure–specific readmission rate for patients admitted by academic hospitalists was 3.3% – significantly lower than other physicians’ rates.
Data source: This retrospective cohort study included 1,735 patients admitted for heart failure to three community hospitals.
Disclosures: The presenter reported having no financial conflicts with regard to this study.